UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form
Or
For the fiscal year ended
Or
Or
Date of event requiring this shell company report________________________
For the transition period from__________ to ___________
Commission File No.
(Exact name of Registrant as specified in its charter)
Can-Fite BioPharma Ltd., an Israeli Limited Company
(Translation of Registrant’s name into English)
(Jurisdiction of incorporation or organization)
(Address of principal executive offices)
Chief Executive, Financial and Operating Officer
Tel: +
Fax: +
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
Securities registered or to be registered pursuant
to Section 12(g) of the Act:
None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
| * | Ordinary shares not for trading, but only in connection with the registration of the American Depositary Shares. |
Indicate the number of outstanding
shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report (December
31, 2023):
Indicate by check mark if
the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
If this report is an annual
or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934. Yes ☐
Indicate by check mark whether
the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such a shorter period that the registrant was required to file such reports), and (2) has been subject to
such filing requirements for the past 90 days.
Indicate by check mark whether
the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T
(§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit
such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | ☒ | |
| Emerging growth company |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act ☐
Ϯ The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether
the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control
over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that
prepared or issued its audit report.
If securities are registered
pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant reflect the correction
of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| International Financial Reporting Standards | Other ☐ | |
| as issued by the International Accounting Standards Board ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. Item 17 ☐ Item 18 ☐
If this is an annual report,
indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No
TABLE OF CONTENTS
i
INTRODUCTION
Can-Fite is a clinical-stage biopharmaceutical company that develops orally bioavailable small molecule therapeutic products for the treatment of cancer, liver and inflammatory diseases and erectile dysfunction. We are also developing specific formulations of cannabis components for the treatment of cancer, inflammatory, and metabolic diseases. Our platform technology utilizes the Gi protein associated A3 adenosine receptor, or A3AR, as a therapeutic target. A3AR is highly expressed in pathological body cells such as inflammatory and cancer cells, and has a low expression in normal cells, suggesting that the receptor could be a specific target for pharmacological intervention. Our pipeline of drug candidates are synthetic, highly specific agonists and allosteric modulators targeting the A3AR.
Our ordinary shares have been trading on the Tel Aviv Stock Exchange, or TASE, under the symbol “CFBI” since October 2005. On October 2, 2012, our ADSs began trading over the counter, or OTC, in the United States under the symbol “CANFY” and on November 19, 2013, our ADSs began trading on the NYSE American under the symbol “CANF.”
Unless otherwise indicated, all references to the “Company,” “we,” “our” and “Can-Fite” refer to Can-Fite BioPharma Ltd. and its consolidated subsidiary. References to “ordinary shares”, “ADSs”, “warrants” and “share capital” refer to the ordinary shares, ADSs, warrants and share capital, respectively, of Can-Fite.
References to “U.S. dollars”, “dollars”, “USD”, and “$” are to currency of the United States of America, and references to “NIS” are to New Israeli Shekels. References to “ordinary shares” are to our ordinary shares, no par value. We report financial information under generally accepted accounting principles in the United States, or U.S. GAAP.
Unless otherwise indicated, U.S. dollar translations of NIS amounts presented in this Annual Report on Form 20-F for the year ended on December 31, 2023 are translated using the rate of NIS 3.627 to $1.00, the exchange rate reported by the Bank of Israel on December 29, 2023, U.S. dollar translations of NIS amounts presented in this Annual Report on Form 20-F for the year ended on December 31, 2022 are translated using the rate of NIS 3.519 to $1.00, the exchange rate reported by the Bank of Israel on December 30, 2022, and U.S. dollar translations of NIS amounts presented in this Annual Report on Form 20-F for the year ended on December 31, 2021 are translated using the rate of NIS 3.11 to $1.00, the exchange rate reported by the Bank of Israel on December 31, 2021.
On January 9, 2023, we effected a change in the ratio of our ADSs to ordinary shares from one (1) ADS representing thirty (30) ordinary shares to a new ratio of one (1) ADS representing three hundred (300) ordinary shares. For ADS holders, the ratio change had the same effect as a one-for-ten reverse ADS split. All ADS and related option and warrant information presented in this Annual Report on Form 20-F have been retroactively adjusted to reflect the reduced number of ADSs and the increase in the ADS price which resulted from this action. Unless otherwise indicated, in this Annual Report on Form 20-F fractional ADSs have been rounded to the nearest whole number.
ii
FORWARD LOOKING STATEMENTS
This Annual Report on Form 20-F contains forward-looking statements, about our expectations, beliefs or intentions regarding, among other things, our product development efforts, business, financial condition, results of operations, strategies or prospects. In addition, from time to time, we or our representatives have made or may make forward-looking statements, orally or in writing. Forward-looking statements can be identified by the use of forward-looking words such as “believe,” “expect,” “intend,” “plan,” “may,” “should” or “anticipate” or their negatives or other variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical or current matters. These forward-looking statements may be included in, but are not limited to, various filings made by us with the U.S. Securities and Exchange Commission, or the SEC, press releases or oral statements made by or with the approval of one of our authorized executive officers. Forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause our actual results to differ materially from any future results expressed or implied by the forward-looking statements. Many factors could cause our actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including, but not limited to, the factors summarized below.
This Annual Report on Form 20-F identifies important factors which could cause our actual results to differ materially from those indicated by the forward-looking statements, particularly those set forth under the heading “Risk Factors.” The risk factors included in this Annual Report on Form 20-F are not necessarily all of the important factors that could cause actual results to differ materially from those expressed in any of our forward-looking statements. Given these uncertainties, readers are cautioned not to place undue reliance on such forward-looking statements. Factors that could cause our actual results to differ materially from those expressed or implied in such forward-looking statements include, but are not limited to:
| ● | our history of losses and needs for additional capital to fund our operations and our inability to obtain additional capital on acceptable terms, or at all; |
| ● | uncertainties of cash flows and inability to meet working capital needs; |
| ● | the initiation, timing, progress and results of our preclinical studies, clinical trials and other product candidate development efforts; |
| ● | our ability to advance our product candidates into clinical trials or to successfully complete our preclinical studies or clinical trials; |
| ● | our receipt of regulatory approvals for our product candidates, and the timing of other regulatory filings and approvals; |
| ● | the clinical development, commercialization and market acceptance of our product candidates; |
| ● | our ability to establish and maintain strategic partnerships and other corporate collaborations; |
| ● | the implementation of our business model and strategic plans for our business and product candidates; |
| ● | the scope of protection we are able to establish and maintain for intellectual property rights covering our product candidates and our ability to operate our business without infringing the intellectual property rights of others; |
| ● | competitive companies, technologies and our industry; |
| ● | risks related to the resurgence of the COVID-19 pandemic and the Russian invasion of Ukraine; |
| ● | risks related to not satisfying the continued listing requirements of NYSE American; |
| ● | statements as to the impact of the political, economic and security situation in Israel on our business, including due to the current war between Israel and Hamas; and |
| ● | those factors referred to in “Item 3.D. Risk Factors,” “Item 4. Information on the Company,” and “Item 5. Operating and Financial Review and Prospects”, as well as in this annual report on Form 20-F generally. |
All forward-looking statements attributable to us or persons acting on our behalf speak only as of the date of this Annual Report on Form 20-F and are expressly qualified in their entirety by the cautionary statements included in this Annual Report on Form 20-F. We undertake no obligations to update or revise forward-looking statements to reflect events or circumstances that arise after the date made or to reflect the occurrence of unanticipated events. In evaluating forward-looking statements, you should consider these risks and uncertainties.
iii
EXPLANATORY NOTE
Market data and certain industry data and forecasts used throughout this Annual Report on Form 20-F were obtained from sources we believe to be reliable, including market research databases, publicly available information, reports of governmental agencies and industry publications and surveys. We have relied on certain data from third-party sources, including internal surveys, industry forecasts and market research, which we believe to be reliable based on our management’s knowledge of the industry. Forecasts are particularly likely to be inaccurate, especially over long periods of time. In addition, we do not necessarily know what assumptions regarding general economic growth were used in preparing the third-party forecasts we cite. Statements as to our market position are based on the most currently available data. While we are not aware of any misstatements regarding the industry data presented in this Annual Report on Form 20-F, our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” in this Annual Report on Form 20-F.
iv
PART I
ITEM 1. Identity of Directors, Senior Management and Advisers.
Not applicable.
ITEM 2. Offer Statistics and Expected Timetable.
Not applicable.
ITEM 3. Key Information.
A. [Reserved]
B. Capitalization and Indebtedness.
Not applicable.
C. Reasons for the Offer and Use of Proceeds.
Not applicable.
D. Risk Factors
You should carefully consider the risks we describe below, in addition to the other information set forth elsewhere in this Annual Report on Form 20-F, including our consolidated financial statements and the related notes beginning on page F-1, before deciding to invest in our ordinary shares and American Depositary Shares, or ADSs. These material risks could adversely impact our results of operations, possibly causing the trading price of our ordinary shares and ADSs to decline, and you could lose all or part of your investment.
Summary Risk Factors
The principal factors and uncertainties that make investing in our ordinary shares risky, include, among others:
Risks Related to Our Financial Position and Capital Requirements
| ● | We have incurred operating losses since our inception and anticipate that we will continue to incur substantial operating losses for the foreseeable future. |
| ● | We will need to raise additional capital to meet our business requirements in the future, and such capital raising may be costly or difficult to obtain and will dilute current shareholders’ ownership interests. |
1
Risks Related to Our Business and Regulatory Matters
| ● | We have not yet commercialized any products or technologies, and we may never become profitable. |
| ● | Our product candidates are at various stages of clinical and preclinical development and may never be commercialized. |
| ● | Results of earlier clinical trials may not be predictive of the results of later-stage clinical trials. |
| ● | We might be unable to develop product candidates that will achieve commercial success in a timely and cost-effective manner, or ever. |
| ● | Our current pipeline is based on our platform technology utilizing the Gi protein associated A3AR, as a potent therapeutic target and currently includes three molecules, Piclidenoson, Namodenoson and CF602 product candidates, of which Piclidenoson is the most advanced. Failure to develop these molecules will have a material adverse effect on us. |
| ● | Clinical trials are very expensive, time-consuming and difficult to design and implement, and, as a result, we may suffer delays or suspensions in future trials which would have a material adverse effect on our ability to generate revenues. |
| ● | The manufacture of our product candidates is a chemical synthesis process and if one of our materials suppliers encounters problems manufacturing our products, our business could suffer. |
| ● | We do not currently have sales, marketing or distribution capabilities or experience, and we are unable to effectively sell, market or distribute our product candidates now and we do not expect to be able to do so in the future. The failure to enter into agreements with third parties that are capable of performing these functions would have a material adverse effect on our business and results of operations. |
| ● | We depend on key members of our management and key consultants and will need to add and retain additional leading experts. Failure to retain our management and consulting team and add additional leading experts could have a material adverse effect on our business, results of operations or financial condition. |
| ● | Our product candidates will remain subject to ongoing regulatory requirements even if they receive marketing approval, and if we fail to comply with these requirements, we could lose these approvals, and the sales of any approved commercial products could be suspended. |
| ● | We may not be able to successfully grow and expand our business. Failure to manage our growth effectively will have a material adverse effect on our business, results of operations and financial condition. |
| ● | Our cannabinoid initiative is uncertain and may not yield commercial results and is subject to significant regulatory risks. |
| ● | We or the third parties upon whom we depend may be adversely affected by natural disasters and/or health epidemics, and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster. |
2
Risks Related to Our Intellectual Property
| ● | The expiry of a patent that we licensed from the National Institute of Health, or NIH, and the consequent loss of composition of matter exclusivity that we had by virtue of this license may diminish our proprietary position. |
| ● | We license from Leiden University intellectual property, which protects certain small molecules which target the A3AR, in furtherance of our platform technology, and we could lose our rights to this license if a dispute with Leiden University arises or if we fail to comply with the financial and other terms of the license. |
| ● | The failure to obtain or maintain patents, licensing agreements, including our current licensing agreements, and other intellectual property could impact our ability to compete effectively. |
| ● | International patent protection is particularly uncertain, and if we are involved in opposition proceedings in foreign countries, we may have to expend substantial sums and management resources. |
| ● | We may be unable to protect the intellectual property rights of the third parties from whom we license certain of our intellectual property or with whom we have entered into other strategic relationships. |
| ● | Under applicable U.S. and Israeli law, we may not be able to enforce covenants not to compete and therefore, may be unable to prevent our competitors from benefiting from the expertise of some of our former employees. In addition, employees may be entitled to seek compensation for their inventions irrespective of their agreements with us, which in turn could impact our future profitability. |
| ● | We may be subject to claims challenging the inventorship of our patents and other intellectual property. |
Risks Related to Our Industry
| ● | We expect the healthcare industry to face increased limitations on reimbursement as a result of healthcare reform, which could adversely affect third-party coverage of our products and how much or under what circumstances healthcare providers will prescribe or administer our products. |
| ● | Our employees, principal investigators, consultants, commercial partners or vendors may engage in misconduct or other improper activities, including non-compliance with regulatory standards. |
Risks Related to Our Operations in Israel
| ● | We conduct our operations in Israel and therefore our results may be adversely affected by political, economic and military instability in Israel and its region. |
| ● | Because a certain portion of our expenses is incurred in currencies other than U.S. dollars, our results of operations may be harmed by currency fluctuations and inflation. |
Risks Related to Our Ordinary Shares and ADSs
| ● | Our business, operating results and growth rates may be adversely affected by current or future unfavorable economic and market conditions and adverse developments with respect to financial institutions and associated liquidity risk. |
| ● | Our business could be negatively impacted by unsolicited takeover proposals, by shareholder activism or by proxy contests relating to the election of directors or other matters. |
3
| ● | Issuance of additional equity securities may adversely affect the market price of our ADSs or ordinary shares. |
| ● | The market price of our ordinary shares and ADSs is subject to fluctuation, which could result in substantial losses by our investors. |
| ● | We may not satisfy the NYSE American requirements for continued listing. If we cannot satisfy these requirements, the NYSE American could delist our securities. |
| ● | As a foreign private issuer, we are permitted to follow certain home country corporate governance practices instead of applicable SEC and NYSE American requirements, which may result in less protection than is accorded to investors under rules applicable to domestic issuers. |
Risks Related to Our Financial Position and Capital Requirements
We have incurred operating losses since our inception and anticipate that we will continue to incur substantial operating losses for the foreseeable future.
We are a clinical-stage biopharmaceutical company that develops orally bioavailable small molecule therapeutic products for the treatment of cancer, liver and inflammatory diseases and erectile dysfunction. Since our incorporation in 1994, we have been focused on research and development activities with a view to developing our product candidates, CF101, also known as Piclidenoson, CF102, also known as Namodenoson, and CF602. We have financed our operations primarily through the sale of equity securities (both in private placements and in public offerings on the TASE and NYSE American) and payments received under out-licensing agreements and have incurred losses in each year since our inception in 1994. We have historically incurred substantial net losses, including net losses of approximately $7.6 million in 2023, $10.1 million in 2022, and $12.6 million in 2021. As of December 31, 2023, we had an accumulated deficit of approximately $158.5 million. We do not know whether or when we will become profitable. To date, we have not commercialized any products or generated any revenues from product sales and accordingly we do not have a revenue stream to support our cost structure. Our losses have resulted principally from costs incurred in development and discovery activities. We expect to continue to incur losses for the foreseeable future, and these losses will likely increase as we:
| ● | initiate and manage pre-clinical development and clinical trials for our current and new product candidates; |
| ● | seek regulatory approvals for our product candidates; |
| ● | implement internal systems and infrastructures; |
| ● | seek to license additional technologies to develop; |
| ● | hire management and other personnel; and |
| ● | move towards commercialization. |
If our product candidates fail in clinical trials or do not gain regulatory clearance or approval, or if our product candidates do not achieve market acceptance, we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our inability to achieve and then maintain profitability would negatively affect our business, financial condition, results of operations and cash flows. Moreover, our prospects must be considered in light of the risks and uncertainties encountered by an early-stage company and in highly regulated and competitive markets, such as the biopharmaceutical market, where regulatory approval and market acceptance of our products are uncertain. There can be no assurance that our efforts will ultimately be successful or result in revenues or profits.
4
We will need to raise additional capital to meet our business requirements in the future, and such capital raising may be costly or difficult to obtain and will dilute current shareholders’ ownership interests.
As of December 31, 2023, we had cash and cash equivalents of $4.3 million and short-term deposits of $4.6 million. In January 2023, we raised approximately $7.5 million in gross proceeds (approximately $6.5 million net of issuance costs) from a registered direct offering and a concurrent private placement and subsequently in November 2023, we raised approximately $3.0 million in gross proceeds (approximately $2.61 million net of issuance costs) from a warrant repricing and exercise transaction. We believe that our existing financial resources will be sufficient to meet our requirements for the next twelve months from the date of issuance of this Annual Report on Form 20-F. We have expended and believe that we will continue to expend substantial resources for the foreseeable future developing our product candidates. These expenditures will include costs associated with research and development, manufacturing, conducting preclinical experiments and clinical trials and obtaining regulatory approvals, as well as commercializing any products approved for sale. Because the outcome of our planned and anticipated clinical trials is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development and commercialization of our product candidates. In addition, other unanticipated costs may arise. As a result of these and other factors currently unknown to us, we will require additional funds, through public or private equity or debt financings or other sources, such as strategic partnerships and alliances and licensing arrangements. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Our future capital requirements will depend on many factors, including the progress and results of our clinical trials, the duration and cost of discovery and preclinical development, and laboratory testing and clinical trials for our product candidates, the timing and outcome of regulatory review of our product candidates, the number and development requirements of other product candidates that we pursue, and the costs of activities, such as product marketing, sales, and distribution. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our anticipated clinical trials.
Our future capital requirements depend on many factors, including:
| ● | the level of research and development investment required to develop our product candidates; |
| ● | the failure to obtain regulatory approval or achieve commercial success of our product candidates, including Piclidenoson, Namodenoson and CF602; |
| ● | the results of our preclinical studies and clinical trials for our earlier stage product candidates, and any decisions to initiate clinical trials if supported by the preclinical results; |
| ● | the costs, timing and outcome of regulatory review of our product candidates that progress to clinical trials; |
| ● | our ability to partner or sub-license any of our product candidates; |
| ● | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our issued patents and defending intellectual property-related claims; |
| ● | the cost of commercialization activities if any of our product candidates are approved for sale, including marketing, sales and distribution costs; |
| ● | the cost of manufacturing our product candidates and any products we successfully commercialize; |
| ● | the timing, receipt and amount of sales of, or royalties on, our future products, if any; |
| ● | the expenses needed to attract and retain skilled personnel; |
| ● | any product liability or other lawsuits related to our products; |
5
| ● | the extent to which we acquire or invest in businesses, products or technologies and other strategic relationships; |
| ● | the costs of financing unanticipated working capital requirements and responding to competitive pressures; and |
| ● | maintaining minimum shareholders’ equity requirements and complying with other continue listing standards under the NYSE American Company Guide |
Additional funds may not be available when we need them, on terms that are acceptable to us, or at all. General market conditions may make it very difficult for us to seek financing from the capital markets and the Russian invasion of Ukraine and the current war between Israel and Hamas could impact the availability or cost of future financings. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate preclinical studies, clinical trials or other research and development activities for one or more of our product candidates or delay, limit, reduce or terminate our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize our product candidates.
We may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we issue, such as convertible notes and warrants, which may adversely impact our financial condition.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
We may seek additional capital through a combination of private and public equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of existing shareholders will be diluted, and the terms may include liquidation or other preferences that adversely affect shareholder rights. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take certain actions, such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us. If we are unable to raise additional funds through equity or debt financing when needed, we may be required to delay, limit, reduce or terminate our product development or commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Risks Related to Our Business and Regulatory Matters
We have not yet commercialized any products or technologies, and we may never become profitable.
We have not yet commercialized any products or technologies, and we may never be able to do so. We do not know when or if we will complete any of our product development efforts, obtain regulatory approval for any product candidates incorporating our technologies or successfully commercialize any approved products. Even if we are successful in developing products that are approved for marketing, we will not be successful unless these products gain market acceptance for appropriate indications at favorable reimbursement rates. The degree of market acceptance of these products will depend on a number of factors, including:
| ● | the timing of regulatory approvals in the countries, and for the uses, we seek; |
| ● | the competitive environment; |
| ● | the establishment and demonstration in the medical community of the safety and clinical efficacy of our products and their potential advantages over existing therapeutic products; |
6
| ● | our ability to enter into distribution and other strategic agreements with pharmaceutical and biotechnology companies with strong marketing and sales capabilities; |
| ● | the adequacy and success of distribution, sales and marketing efforts; and |
| ● | the pricing and reimbursement policies of government and third-party payors, such as insurance companies, health maintenance organizations and other plan administrators. |
Physicians, patients, thirty-party payors or the medical community in general may be unwilling to accept, utilize or recommend, and in the case of third-party payors, cover any of our products or products incorporating our technologies. As a result, we are unable to predict the extent of future losses or the time required to achieve profitability, if at all. Even if we successfully develop one or more products that incorporate our technologies, we may not become profitable.
Our product candidates are at various stages of clinical and preclinical development and may never be commercialized.
Our product candidates are at various stages of clinical development and may never be commercialized. The progress and results of any future pre-clinical testing or future clinical trials are uncertain, and the failure of our product candidates to receive regulatory approvals will have a material adverse effect on our business, operating results and financial condition to the extent we are unable to commercialize any products. None of our product candidates has received regulatory approval for commercial sale. In addition, we face the risks of failure inherent in developing therapeutic products. Our product candidates are not expected to be commercially available for several years, if at all.
In order to receive U.S. Food and Drug Administration, or FDA, approval or approval from foreign regulatory authorities to market a product candidate or to distribute our products, we must demonstrate thorough pre-clinical testing and thorough human clinical trials that the product candidate is safe and effective for its intended uses (e.g., treatment of a specific condition in a specific way subject to contraindications and other limitations). If the FDA, or foreign regulatory authorities, determine that data from our pre-clinical testing and clinical trials are not sufficient to support approval, the FDA, or foreign regulatory authorities, may require additional pre-clinical testing or clinical trials for our product candidates. Even if we comply with all FDA requests, the FDA may ultimately reject one or more of our New Drug Applications, or NDA, or grant approval for a narrowly intended use that is not commercially feasible. We might not obtain regulatory approval for our drug candidates in a timely manner, if at all. Failure to obtain FDA approval of any of our drug candidates in a timely manner or at all will severely undermine our business by reducing the number of salable products and, therefore, corresponding product revenues.
Results of earlier clinical trials may not be predictive of the results of later-stage clinical trials.
The results of preclinical studies and early clinical trials of product candidates may not be predictive of the results of later-stage clinical trials. Also, interim results, if at all, during a clinical trial do not necessarily predict final results. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy results despite having progressed through preclinical studies and initial clinical trials. For example, our former subsidiary OphthaliX Inc, or OphthaliX, announced top-line results of a Phase III study with Piclidenoson for dry-eye syndrome in which Piclidenoson did not meet the primary efficacy endpoint of complete clearing of corneal staining, nor the secondary efficacy endpoints, OphthaliX released top-line results from its Phase II clinical trial of Piclidenoson for the treatment of glaucoma in which no statistically significant differences were found between the Piclidenoson treated group and the placebo group in the primary endpoint of lowering intraocular pressure, or IOP. In addition, two Phase IIb studies in rheumatoid arthritis, utilizing Piclidenoson in combination with methotrexate, a generic drug commonly used for treating rheumatoid arthritis patients, or MTX, failed to reach their primary endpoints and we ended our Phase III ACROBAT study after the independent data monitoring committee, or IDMC recommended in a pre-planned interim analysis not to continue this study. A Phase II/III study of Piclidenoson for psoriasis did not meet its primary endpoint although positive data from further analysis of the Phase II/III study suggests Piclidenoson as a potential systemic therapy for patients with moderate-severe psoriasis. Furthermore, a Phase II study for advanced HCC in subjects with Child-Pugh B who failed Nexavar as a first line treatment did not meet its primary endpoint although it showed superiority in overall survival in the largest study subpopulation.
7
Many companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials due to adverse safety profiles or lack of efficacy, notwithstanding promising results in earlier studies. Any delay in, or termination or suspension of, our clinical trials will delay the requisite filings with the FDA, the EMA or other foreign regulatory authorities and, ultimately, our ability to commercialize our product candidates and generate product revenues. If the clinical trials do not support our product claims, the completion of development of such product candidates may be significantly delayed or abandoned, which will significantly impair our ability to generate product revenues and will materially adversely affect our results of operations.
This drug candidate development risk is heightened by any changes in the planned clinical trials compared to the completed clinical trials. As product candidates are developed from preclinical through early to late stage clinical trials towards approval and commercialization, it is customary that various aspects of the development program, such as manufacturing and methods of administration, are altered along the way in an effort to optimize processes and results. While these types of changes are common and are intended to optimize the product candidates for late stage clinical trials, approval and commercialization, such changes do carry the risk that they will not achieve these intended objectives.
Changes in our planned clinical trials or future clinical trials could cause our product candidates to perform differently, including causing toxicities, which could delay completion of our clinical trials, delay approval, if any, of our product candidates, and/or jeopardize our ability to commence product sales and generate revenues.
We might be unable to develop product candidates that will achieve commercial success in a timely and cost-effective manner, or ever.
Even if regulatory authorities approve our product candidates, they may not be commercially successful. Our product candidates may not be commercially successful because government agencies and other third-party payors may not cover the product or the coverage may be too limited to be commercially successful; physicians and others may not use or recommend our products, even following regulatory approval. A product approval, assuming one issues, may limit the uses for which the product may be distributed thereby adversely affecting the commercial viability of the product. Third parties may develop superior products or have proprietary rights that preclude us from marketing our products. We also expect that at least some of our product candidates will be expensive, if approved. Patient acceptance of and demand for any product candidates for which we obtain regulatory approval or license will depend largely on many factors, including but not limited to the extent, if any, of reimbursement of costs by government agencies and other third-party payors, pricing, the effectiveness of our marketing and distribution efforts, the safety and effectiveness of alternative products, and the prevalence and severity of side effects associated with our products. If physicians, government agencies and other third-party payors do not accept our products, we will not be able to generate significant revenue. In addition, government regulators and legislative bodies in the U.S. are considering numerous proposals that may result in limitations on the prices at which we could charge customers for our products if we have products that are ultimately approved for sale. At this time, we are unable to predict how these potential legislative changes might affect our business.
Our current pipeline is based on our platform technology utilizing the Gi protein associated A3AR, as a potent therapeutic target and currently includes three molecules, Piclidenoson, Namodenoson and CF602 product candidates, of which Piclidenoson is the most advanced. Failure to develop these molecules will have a material adverse effect on us.
Our current pipeline is based on a platform technology where we target the A3AR with highly selective ligands, or small signal triggering molecules that bind to specific cell surface receptors, such as the A3AR, including Piclidenoson, Namodenoson and CF602. A3ARs are structures found in cell surfaces that record and transfer messages from small molecules or ligands, such as Piclidenoson, Namodenoson and CF602 to the rest of the cell. Piclidenoson is the most advanced of our drug candidates. As such, we are currently dependent on only three molecules for our potential commercial success, and any safety or efficacy concerns related to such molecules would have a significant impact on our business. Failure to develop our drug candidates, in whole or in part, will have a material adverse effect on us.
8
Clinical trials are very expensive, time-consuming and difficult to design and implement, and, as a result, we may suffer delays or suspensions in future trials which would have a material adverse effect on our ability to generate revenues.
Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. Regulatory authorities, such as the FDA, may preclude or prohibit clinical trials from proceeding. Additionally, the clinical trial process is time-consuming, failure can occur at any stage of the trials, and we may encounter problems that cause us to abandon or repeat clinical trials. The commencement and completion of clinical trials may be delayed by several factors, including:
| ● | unforeseen safety issues; |
| ● | non-acceptance of an IND by the FDA; |
| ● | determination of dosing issues; |
| ● | lack of effectiveness or efficacy during clinical trials; |
| ● | inability to manufacture sufficient quantities of drug candidate; |
| ● | changes in formulation or manufacturing changes; |
| ● | failure of third-party suppliers to perform final manufacturing steps for the drug substance; |
| ● | slower than expected rates of patient recruitment and enrollment; |
| ● | inability to retain patients in clinical trials; |
| ● | lack of healthy volunteers and patients to conduct trials; |
| ● | inability to monitor patients adequately during or after treatment; |
| ● | failure to reach an agreement with contract research organizations or clinical trial sites; |
| ● | failure of institutional review boards, or IRBs, to approve our clinical trial protocols or suspension or termination of our clinical trial by the IRB, DSMB, or the FDA; |
| ● | failure of institutional review boards to approve our clinical trial protocols; |
| ● | inability or unwillingness of clinical investigators and institutional review boards to follow our clinical trial protocols; |
| ● | failure of clinical investigators or sites to maintain necessary licenses or permits or comply with good clinical practices, or GCP, or other regulatory requirements; |
| ● | debarment of a clinical investigator by FDA or other similar suspension or exclusion by a regulatory authority; and |
| ● | lack of sufficient funding to finance the clinical trials. |
We have experienced the risks involved with conducting clinical trials, including but not limited to, increased expense and delay and failure to meet end points of the trial. For example, OphthaliX, announced top-line results of a Phase III study with Piclidenoson for dry-eye syndrome in which Piclidenoson did not meet the primary efficacy endpoint of complete clearing of corneal staining, nor the secondary efficacy endpoints and OphthaliX released top-line results from its Phase II clinical trial of Piclidenoson for the treatment of glaucoma in which no statistically significant differences were found between the Piclidenoson treated group and the placebo group in the primary endpoint of lowering IOP. In addition, two Phase IIb studies in rheumatoid arthritis, utilizing Piclidenoson in combination with MTX failed to reach their primary end points and we ended our Phase III ACROBAT study after the IDMC recommended in a pre-planned interim analysis not to continue this study. A Phase II/III study of Piclidenoson for psoriasis did not meet its primary endpoint although positive data from further analysis of the Phase II/III study suggests Piclidenoson as a potential systemic therapy for patients with moderate-severe psoriasis. Furthermore, a Phase II study of Namodenoson for advanced HCC in subjects with Child-Pugh B who failed Nexavar as a first line treatment did not meet its primary endpoint although it showed superiority in overall survival in the largest study subpopulation.
9
In addition, we or regulatory authorities may suspend our clinical trials at any time if it appears that we are exposing participants to unacceptable health risks or if the regulatory authorities find deficiencies in our regulatory submissions or the conduct of these trials. Any suspension of clinical trials will delay possible regulatory approval, if any, increase costs, and adversely impact our ability to develop products and generate revenue.
We seek to partner with third-party collaborators with respect to the development and commercialization of Piclidenoson and for any other product candidate, and we may not succeed in establishing and maintaining collaborative relationships, which may significantly limit our ability to develop and commercialize our product candidates successfully, if at all.
Our business strategy relies in part on partnering with pharmaceutical companies to complement our internal development efforts. We will be competing with many other companies as we seek partners for Piclidenoson, Namodenoson and for any other product candidate and we may not be able to compete successfully against those companies. If we are not able to enter into collaboration arrangements for Piclidenoson, Namodenoson and for any other product candidate, we may be required to undertake and fund further development, clinical trials, manufacturing and commercialization activities solely at our own expense and risk. If we are unable to finance and/or successfully execute those expensive activities, or we delay such activities due to capital availability, our business could be materially and adversely affected, and potential future product launch could be materially delayed, be less successful, or we may be forced to discontinue clinical development of these product candidates. The process of establishing and maintaining collaborative relationships is difficult, time-consuming and involves significant uncertainty, including:
| ● | a collaboration partner may shift its priorities and resources away from our product candidates due to a change in business strategies, or a merger, acquisition, sale or downsizing; |
| ● | a collaboration partner may seek to renegotiate or terminate their relationships with us due to unsatisfactory clinical results, manufacturing issues, a change in business strategy, a change of control or other reasons; |
| ● | a collaboration partner may cease development in therapeutic areas which are the subject of our strategic collaboration |
| ● | a collaboration partner may not devote sufficient capital or resources towards our product candidates; |
| ● | a collaboration partner may change the success criteria for a drug candidate thereby delaying or ceasing development of such candidate; |
| ● | a significant delay in initiation of certain development activities by a collaboration partner will also delay payment of milestones tied to such activities, thereby impacting our ability to fund our own activities; |
| ● | a collaboration partner could develop a product that competes, either directly or indirectly, with our drug candidate; |
| ● | a collaboration partner with commercialization obligations may not commit sufficient financial or human resources to the marketing, distribution or sale of a product; |
| ● | a collaboration partner with manufacturing responsibilities may encounter regulatory, resource or quality issues and be unable to meet demand requirements; |
10
| ● | a partner may exercise a contractual right to terminate a strategic alliance; |
| ● | a dispute may arise between us and a partner concerning the research, development or commercialization of a drug candidate resulting in a delay in milestones, royalty payments or termination of an alliance and possibly resulting in costly litigation or arbitration which may divert management attention and resources; and |
| ● | a partner may use our products or technology in such a way as to invite litigation from a third party. |
Any collaborative partners we enter into agreements with in the future may shift their priorities and resources away from our product candidates or seek to renegotiate or terminate their relationships with us. If any collaborator fails to fulfill its responsibilities in a timely manner, or at all, our research, clinical development, manufacturing or commercialization efforts related to that collaboration could be delayed or terminated, or it may be necessary for us to assume responsibility for expenses or activities that would otherwise have been the responsibility of our collaborator. If we are unable to establish and maintain collaborative relationships on acceptable terms or to successfully transition terminated collaborative agreements, we may have to delay or discontinue further development of one or more of our product candidates, undertake development and commercialization activities at our own expense or find alternative sources of capital.
If we acquire or license additional technology or product candidates, we may incur a number of costs, may have integration difficulties and may experience other risks that could harm our business and results of operations.
We may acquire and license additional product candidates and technologies. Any product candidate or technology we license from others or acquire will likely require additional development efforts prior to commercial sale, including extensive pre-clinical or clinical testing, or both, and approval by the FDA and applicable foreign regulatory authorities, if any. All product candidates are prone to risks of failure inherent in pharmaceutical product development, including the possibility that the product candidate or product developed based on licensed technology will not be shown to be sufficiently safe and effective for approval by regulatory authorities. In addition, we cannot assure you that any product candidate that we develop based on acquired or licensed technology that is granted regulatory approval will be manufactured or produced economically, successfully commercialized or widely accepted in the marketplace. Moreover, integrating any newly acquired product candidates could be expensive and time-consuming. If we cannot effectively manage these aspects of our business strategy, our business may not succeed.
The manufacture of our product candidates is a chemical synthesis process and if one of our materials suppliers encounters problems manufacturing our products, our business could suffer.
The FDA and foreign regulators require manufacturers to register manufacturing facilities. The FDA and foreign regulators also inspect these facilities to confirm compliance with requirements that the FDA or foreign regulators establish. We do not intend to engage in the manufacture of our products other than for pre-clinical and clinical studies, but we or our materials suppliers may face manufacturing or quality control problems causing product production and shipment delays or a situation where we or the supplier may not be able to maintain compliance with the FDA’s or foreign regulators’ requirements necessary to continue manufacturing our drug substance. Drug manufacturers are subject to ongoing periodic unannounced inspections by the FDA, the U.S. Drug Enforcement Agency, or DEA, and corresponding foreign regulators to ensure strict compliance with requirements and other governmental regulations and corresponding foreign standards. Any failure to comply with DEA requirements or FDA or foreign regulatory requirements could adversely affect our clinical research activities and our ability to develop our product candidates, and delay possible regulatory approval.
We do not currently have sales, marketing or distribution capabilities or experience, and we are unable to effectively sell, market or distribute our product candidates now and we do not expect to be able to do so in the future. The failure to enter into agreements with third parties that are capable of performing these functions would have a material adverse effect on our business and results of operations.
We do not currently have, and we do not expect to develop, sales, marketing and distribution capabilities. If we are unable to enter into agreements with third parties to perform these functions, we will not be able to successfully market any of our platforms or product candidates. In order to successfully market any of our platform or product candidates, we must make arrangements with third parties to perform these services.
11
As we do not intend to develop a marketing and sales force with technical expertise and supporting distribution capabilities, we will be unable to market any of our product candidates directly. To promote any of our potential products through third parties, we will have to locate acceptable third parties for these functions and enter into agreements with them on acceptable terms, and we may not be able to do so. Any third-party arrangements we are able to enter into may result in lower revenues than we could achieve by directly marketing and selling our potential products. In addition, to the extent that we depend on third parties for marketing and distribution, any revenues we receive will depend upon the efforts of such third parties, as well as the terms of our agreements with such third parties, which cannot be predicted in most cases at this time. As a result, we might not be able to market and sell our products in the United States or overseas, which would have a material adverse effect on us.
We will to some extent rely on third parties to implement our manufacturing and supply strategies. Failure of these third parties in any respect could have a material adverse effect on our business, results of operations and financial condition.
If our current and future manufacturing and supply strategies are unsuccessful, then we may be unable to conduct and complete any future pre-clinical or clinical trials or commercialize our product candidates in a timely manner, if at all. Completion of any potential future pre-clinical or clinical trials and commercialization of our product candidates will require access to, or development of, facilities to manufacture a sufficient supply of our product candidates. We do not have the resources, facilities or experience to manufacture our product candidates for commercial purposes on our own and do not intend to develop or acquire facilities for the manufacture of product candidates for commercial purposes in the foreseeable future. We may rely on contract manufacturers to produce sufficient quantities of our product candidates necessary for any pre-clinical or clinical testing we undertake in the future. Such contract manufacturers may be the sole source of production and they may have limited experience at manufacturing, formulating, analyzing, filling and finishing our types of product candidates.
We also intend to rely on third parties to supply the requisite materials needed for the manufacturing of our active pharmaceutical ingredients, or API. There may be a limited supply of these requisite materials. We might not be able to enter into agreements that provide us assurance of availability of such components in the future from any supplier. Our potential suppliers may not be able to adequately supply us with the components necessary to successfully conduct our pre-clinical and clinical trials or to commercialize our product candidates. In particular, the any resurgence of COVID-19 globally could result in the inability of our suppliers to deliver components or raw materials on a timely basis or at all. If we cannot acquire an acceptable supply of the requisite materials to produce our product candidates, we will not be able to complete pre-clinical and clinical trials delaying possible regulatory approval, and adversely impacting our ability to develop products, and will not be able to market or commercialize our product candidates, if approved.
We depend on key members of our management and key consultants and will need to add and retain additional leading experts. Failure to retain our management and consulting team and add additional leading experts could have a material adverse effect on our business, results of operations or financial condition.
We are highly dependent on our executive officers and other key management and technical personnel. Our failure to retain our Executive Chairman and Chief Scientific Officer, Pnina Fishman, Ph.D., who has developed much of the technology we utilize today, or any other key management and technical personnel, could have a material adverse effect on our future operations. Our success is also dependent on our ability to attract, retain and motivate highly trained technical, and management personnel, among others, to continue the development and commercialization, if approved, of our current and future product candidates.
Our success also depends on our ability to attract, retain and motivate personnel required for the development, maintenance and expansion of our activities. There can be no assurance that we will be able to retain our existing personnel or attract additional qualified employees or consultants. The loss of key personnel or the inability to hire and retain additional qualified personnel in the future could have a material adverse effect on our business, financial condition and results of operation.
12
We face significant competition and continuous technological change, and developments by competitors may render our products or technologies obsolete or non-competitive. If we cannot successfully compete with new or existing products, our marketing and sales will suffer and we may not ever be profitable.
We will compete against fully integrated pharmaceutical and biotechnology companies and smaller companies that are collaborating with larger pharmaceutical companies, academic institutions, government agencies and other public and private research organizations. In addition, many of these competitors, either alone or together with their collaborative partners, operate larger research and development programs than we do, and have substantially greater financial resources than we do, as well as significantly greater experience in:
| ● | developing drugs; |
| ● | undertaking pre-clinical testing and human clinical trials; |
| ● | obtaining FDA approval, addressing various regulatory matters and other regulatory approvals of drugs; |
| ● | formulating and manufacturing drugs; and |
| ● | launching, marketing and selling drugs. |
If our competitors develop and commercialize products faster than we do, or develop and commercialize products that are superior to our product candidates, our commercial opportunities will be reduced or eliminated. The extent to which any of our product candidates achieve market acceptance will depend on competitive factors, many of which are beyond our control. Competition in the biotechnology and biopharmaceutical industry is intense and has been accentuated by the rapid pace of technology development. Our competitors include large integrated pharmaceutical companies, biotechnology companies that currently have drug and target discovery efforts, universities, and public and private research institutions. Almost all of these entities have substantially greater research and development capabilities and financial, scientific, manufacturing, marketing and sales resources than we do. These organizations also compete with us to:
| ● | attract parties for acquisitions, joint ventures or other collaborations; |
| ● | license proprietary technology that is competitive with the technology we are developing; |
| ● | attract funding; and |
| ● | attract and hire scientific talent and other qualified personnel. |
Our competitors may succeed in developing and commercializing products earlier and obtaining regulatory approvals from the FDA or foreign regulators more rapidly than we do. Our competitors may also develop products or technologies that are superior to those we are developing, and render our product candidates or technologies obsolete or non-competitive. If we cannot successfully compete with new or existing products, our marketing and sales will suffer and we may not ever be profitable.
Our competitors currently include companies with marketed products and/or an advanced research and development pipeline. The major competitors in the psoriasis therapeutic field include Amgen, J&J, Pfizer, Novartis, Abbvie, Eli Lilly, Bristol-Myers Squibb, UCB and more. Competitors in the HCC field include companies such as Bayer, Exelixis, Merck, Roche, Eisai, Astrazenca, Beigene, Novartis, and Bristol-Myers Squibb. Competitors in the nonalcoholic steatohepatisi, or NASH, field (also known as metabolic dysfunction-associated steatohepatitis, or MASH)) include companies such as Gilead, Genfit, Galmed, Madrigal, Akero, 89Bio, Viking, and Terns. Competitors in the erectile dysfunction field include Pfizer, Eli Lilly, Bayer, and Petros Pharmaceuticals. See “Item 4. Information on the Company—B. Business Overview—Competition.”
13
Moreover, several companies have reported the commencement of research projects related to the A3AR. Such companies include CV Therapeutics Inc. (which was acquired by Gilead), King Pharmaceuticals R&D Inv. (which was acquired by Pfizer), Hoechst Marion Roussel Inc. (which was acquired by Aventis), Novo Nordisk A/S and Inotek Pharmaceuticals. However, to the best of our knowledge, there is no approved drug currently on the market, which is similar to our A3AR agonists, nor are we aware of any allosteric modulator in the A3AR product pipeline similar to our allosteric modulator with respect to chemical profile and mechanism of action.
We may suffer losses from product liability claims if our product candidates cause harm to patients.
Any of our product candidates could cause adverse events. Although a pooled safety analysis from clinical trials encompassing more than 1,600 humans dosed with Piclidenoson through the completion of our Phase II rheumatoid arthritis and psoriasis trials indicated that Piclidenoson is generally well-tolerated at doses up to 4.0 mg administered twice daily for up to 12-48 weeks, there were incidences (less than or equal to 5%) of adverse events in eight completed and fully analyzed trials in inflammatory disease. Such adverse events included nausea, diarrhea, abdominal pain, vomiting, constipation, common bacterial and viral syndromes (such as tonsillitis, otitis and respiratory and urinary tract infections), abdominal pain, vomiting, myalgia, arthralgia, dizziness, headache and pruritus. We observed an even lower incidence (less than or equal to 2%) of serious adverse events, although only one type of event was reported in more than a single Piclidenoson-treated subject, which was exacerbation of chronic obstructive lung disease reported in two subjects. Notwithstanding the foregoing, the placebo group in such studies had a higher incidence of overall adverse events than the pooled Piclidenoson groups. In addition, in normal volunteers, Piclidenoson at doses 3-4-fold higher than those to be used in therapeutic trials, but not at therapeutic doses, was associated with prolongation of the electrocardiographic QT intervals. No new safety concerns have been identified and no novel or unexpected safety concerns have appeared over 48 weeks of treatment in more recent trials.
There is also a risk that certain adverse events may not be observed in clinical trials, but may nonetheless occur in the future. If any of these adverse events occur, they may render our product candidates ineffective or harmful in some patients, and our sales would suffer, materially adversely affecting our business, financial condition and results of operations.
In addition, potential adverse events caused by our product candidates could lead to product liability lawsuits. If product liability lawsuits are successfully brought against us, we may incur substantial liabilities and may be required to limit the marketing and commercialization of our product candidates. Our business exposes us to potential product liability risks, which are inherent in the testing, manufacturing, marketing and sale of pharmaceutical products. We may not be able to avoid product liability claims. Product liability insurance for the pharmaceutical and biotechnology industries is generally expensive, if available at all. If, at any time, we are unable to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims, we may be unable to clinically test, market or commercialize our product candidates. A successful product liability claim brought against us in excess of our insurance coverage, if any, may cause us to incur substantial liabilities, and, as a result, our business, liquidity and results of operations would be materially adversely affected.
Our product candidates will remain subject to ongoing regulatory requirements even if they receive marketing approval, and if we fail to comply with these requirements, we could lose these approvals, and the sales of any approved commercial products could be suspended.
Even if we receive regulatory approval to market a particular product candidate, the product will remain subject to extensive regulatory requirements, including requirements relating to manufacturing, labelling, packaging, adverse event reporting, storage, advertising, promotion, distribution and recordkeeping. Even if regulatory approval of a product is granted, the approval may be subject to limitations on the uses for which the product may be marketed or the conditions of approval, or may contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product, which could negatively impact us or our collaboration partners by reducing revenues or increasing expenses, and cause the approved product candidate not to be commercially viable. In addition, as clinical experience with a drug expands after approval, typically because it is used by a greater number and more diverse group of patients after approval than during clinical trials, side effects and other problems may be observed after approval that were not seen or anticipated during pre-approval clinical trials or other studies. Any adverse effects observed after the approval and marketing of a product candidate could result in limitations on the use of or withdrawal of any approved products from the marketplace. Absence of long-term safety data may also limit the approved uses of our products, if any. If we fail to comply with the regulatory requirements of the FDA and other applicable U.S. and foreign regulatory authorities, or previously unknown problems with any approved commercial products, manufacturers or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions or other setbacks, including the following:
| ● | Restrictions on the products, manufacturers or manufacturing process; |
| ● | Warning or other enforcement letters; |
14
| ● | Civil or criminal penalties, fines and injunctions; |
| ● | Product seizures or detentions; |
| ● | Import or export bans or restrictions; |
| ● | Voluntary or mandatory product recalls and related publicity requirements; |
| ● | Suspension or withdrawal of regulatory approvals; |
| ● | Total or partial suspension of production; and |
| ● | Refusal to approve pending applications for marketing approval of new products or supplements to approved applications. |
If we or our collaborators are slow or unable to adapt to changes in existing regulatory requirements or adoption of new regulatory requirements or policies, marketing approval for our product candidates may be lost or cease to be achievable, resulting in decreased revenue from milestones, product sales or royalties, which would have a material adverse effect on our results of operations.
We deal with hazardous materials and must comply with environmental, health and safety laws and regulations, which can be expensive and restrict how we do business.
Our activities and those of our third-party manufacturers on our behalf involve the controlled storage, use and disposal of hazardous materials, including corrosive, explosive and flammable chemicals and other hazardous compounds. We and our manufacturers are subject to U.S. federal, state, and local, and Israeli and other foreign laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of accidental contamination or injury from these materials. In addition, if we develop a manufacturing capacity, we may incur substantial costs to comply with environmental regulations and would be subject to the risk of accidental contamination or injury from the use of hazardous materials in our manufacturing process.
In the event of an accident, government authorities may curtail our use of these materials and interrupt our business operations. In addition, we could be liable for any civil damages that result, which may exceed our financial resources and may seriously harm our business. Although our Israeli insurance program covers certain unforeseen sudden pollutions, we do not maintain a separate insurance policy for any of the foregoing types of risks. In addition, although the general liability section of our life sciences policy covers certain unforeseen, sudden environmental issues, pollution in the United States and Canada is excluded from the policy. In the event of environmental discharge or contamination or an accident, we may be held liable for any resulting damages, and any liability could exceed our resources. In addition, we may be subject to liability and may be required to comply with new or existing environmental laws regulating pharmaceuticals or other medical products in the environment.
15
Environmental, social and corporate governance (ESG) issues, including those related to climate change and sustainability, may have an adverse effect on our business, financial condition and results of operations and damage our reputation.
There is an increasing focus from certain investors, customers, consumers, employees and other stakeholders concerning ESG matters. Additionally, public interest and legislative pressure related to public companies’ ESG practices continue to grow. If our ESG practices fail to meet regulatory requirements or investor, customer, consumer, employee or other shareholders’ evolving expectations and standards for responsible corporate citizenship in areas including environmental stewardship, support for local communities, Board of Director and employee diversity, human capital management, employee health and safety practices, product quality, supply chain management, corporate governance and transparency, our reputation, brand and employee retention may be negatively impacted, and our customers and suppliers may be unwilling to continue to do business with us.
Customers, consumers, investors and other shareholders are increasingly focusing on environmental issues, including climate change, energy and water use, plastic waste and other sustainability concerns. Concern over climate change may result in new or increased legal and regulatory requirements to reduce or mitigate impacts to the environment. Changing customer and consumer preferences or increased regulatory requirements may result in increased demands or requirements regarding plastics and packaging materials, including single-use and non-recyclable plastic products and packaging, other components of our products and their environmental impact on sustainability, or increased customer and consumer concerns or perceptions (whether accurate or inaccurate) regarding the effects of substances present in certain of our products. Complying with these demands or requirements could cause us to incur additional manufacturing, operating or product development costs.
If we do not adapt to or comply with new regulations, including the SEC’s newly adopted rules that would require companies to provide expanded climate-related disclosures in their periodic reporting, which may require us to incur significant additional costs to comply and impose increased oversight obligations on our management and board of directors, or fail to meet evolving investor, industry or stakeholder expectations and concerns regarding ESG issues, investors may reconsider their capital investment in our Company, we may become subject to penalties, and customers and consumers may choose to stop purchasing our products, if approved for commercialization, which could have a material adverse effect on our reputation, business or financial condition.
Our business and operations may be materially adversely affected in the event of computer system failures or security breaches.
Despite the implementation of security measures, our internal computer systems, and those of our contract research organizations, or CROs, and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, cyber-attacks, natural disasters, fire, terrorism, war, and telecommunication and electrical failures. If such an event were to occur and interrupt our operations, it could result in a material disruption of our drug development programs. For example, the loss of clinical trial data from ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, loss of trade secrets or inappropriate disclosure of confidential or proprietary information, including protected health information or personal data of employees or former employees, access to our clinical data, or disruption of the manufacturing process, we could incur liability and the further development of our drug candidates could be delayed. We may also be vulnerable to cyber-attacks by hackers or other malfeasance. This type of breach of our cybersecurity may compromise our confidential information and/or our financial information and adversely affect our business or result in legal proceedings. Further, these cybersecurity breaches may inflict reputational harm upon us that may result in decreased market value and erode public trust.
We may not be able to successfully grow and expand our business. Failure to manage our growth effectively will have a material adverse effect on our business, results of operations and financial condition.
We may not be able to successfully grow and expand. Successful implementation of our business plan will require management of growth, including potentially rapid and substantial growth, which will result in an increase in the level of responsibility for management personnel and place a strain on our human and capital resources. To manage growth effectively, we will be required to continue to implement and improve our operating and financial systems and controls to expand, train and manage our employee base. Our ability to manage our operations and growth effectively requires us to continue to expend funds to enhance our operational, financial and management controls, reporting systems and procedures and to attract and retain sufficient numbers of talented personnel. If we are unable to scale up and implement improvements to our control systems in an efficient or timely manner, or if we encounter deficiencies in existing systems and controls, then we will not be able to make available the products required to successfully commercialize our technology. Failure to attract and retain sufficient numbers of talented personnel will further strain our human resources and could impede our growth or result in ineffective growth. Moreover, the management, systems and controls currently in place or to be implemented may not be adequate for such growth, and the steps taken to hire personnel and to improve such systems and controls might not be sufficient. If we are unable to manage our growth effectively, it will have a material adverse effect on our business, results of operations and financial condition.
16
If we are unable to obtain adequate insurance, our financial condition could be adversely affected in the event of uninsured or inadequately insured loss or damage. Our ability to effectively recruit and retain qualified officers and directors could also be adversely affected if we experience difficulty in obtaining adequate directors’ and officers’ liability insurance.
We may not be able to obtain insurance policies on terms affordable to us that would adequately insure our business and property against damage, loss or claims by third parties. To the extent our business or property suffers any damages, losses or claims by third parties, which are not covered or adequately covered by insurance, our financial condition may be materially adversely affected.
We may be unable to maintain sufficient insurance as a public company to cover liability claims made against our officers and directors. Our insurance costs have increased for directors’ and officers’ liability insurance, and we may be required to incur further substantial increased costs to maintain the same or similar coverage or be forced to accept reduced coverage in future. If we are unable to adequately ensure our officers and directors, we may not be able to retain or recruit qualified officers and directors to manage us.
Our cannabinoid initiative is uncertain and may not yield commercial results and is subject to significant regulatory risks.
We are developing formulations of cannabis components for the treatment of diseases in which there is an overexpression of A3AR. While we believe there are substantial business opportunities for us in this field, there can be no assurance that our activities will be successful, or that any research and development and product testing efforts will result in commercially saleable products, or that the market will accept or respond positively to our products. In addition, our current and potential involvement in cannabis-related activity may expose us to legal and reputational risks. Such risks include:
| ● | Medical-use cannabis remains illegal under U.S. federal law, and therefore, strict enforcement of federal laws regarding medical -use cannabis would likely result in our inability to market any products; |
| ● | FDA has not approved a marketing application for the treatment of any disease or condition; |
| ● | Changes in laws, regulations and guidelines related to cannabis may result in significant additional compliance costs for us or limit our ability to operate in certain jurisdictions; |
| ● | Certain banks will not accept deposits from or provide other bank services to businesses involved with cannabis and U.S. federal money laundering laws make it a federal crime to engage in financial transactions involving the proceeds of some form of unlawful activity; and |
| ● | Third parties with whom we do business may perceive that they are exposed to reputational risk as a result of our cannabis-related business activities and may ultimately elect not to do business with us. |
Complying with laws and regulations relating to cannabinoids is evolving, complex and expensive, and may divert management’s attention and resources from other aspects of our business. Failure to maintain compliance with such laws and regulations may result in regulatory action that could have a material adverse effect on our business, results of operations and financial condition. The DEA, FDA or state agencies may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal proceedings.
17
We or the third parties upon whom we depend may be adversely affected by natural disasters and/or health epidemics, and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster.
Natural disasters could severely disrupt our operations and have a material adverse effect on our business, results of operations, financial condition and prospects. If a natural disaster, power outage, health epidemic or other event occurred that prevented us from using all or a significant portion of our office, manufacturing and/or lab spaces, that damaged critical infrastructure, such as the manufacturing facilities of our third-party contract manufacturers, CROs, clinical sites, third parties ongoing activities and schedules or that otherwise disrupted operations, it may be difficult or, in certain cases, impossible for us to continue our plans and business for a substantial period of time.
In late 2019, a novel strain of COVID-19, also known as coronavirus, was reported in Wuhan, China and began spreading to various parts of the world. Epidemics such as this can adversely impact our business and that of third parties with whom we engage as they can cause disruptions, such as travel bans, quarantines, and interruptions to access the trial sites and supply chain, which could result in material delays and complications with respect to our research and development programs and clinical trials. If there are future outbreaks of COVID-19 this may result in a period of business disruption in these and other areas impacting our business, including the establishment of contractual relationships with investigators enrolling subjects in our clinical trials, the continuity of care provided by these institutions to the subjects we seek to enroll and their ability to support industry-funded research as a means of caring for their subjects, supply of these sites with study materials, and the enrollment of subjects and their adherence with study requirements.
The disaster recovery and business continuity plans we have in place may prove inadequate in the event of a serious disaster or similar event. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity plans, which could have a material adverse effect on our business.
Risks Related to Our Intellectual Property
The expiry of a patent that we licensed from the National Institute of Health, or NIH, and the consequent loss of composition of matter exclusivity that we had by virtue of this license may diminish our proprietary position.
As a result of the expiry in June 2015 of a patent that provided composition of matter protection over Piclidenoson and Namodenoson, that we licensed from the NIH, we no longer enjoy composition of matter patent exclusivity relating to Piclidenoson and Namodenoson. Nevertheless, because Piclidenoson and Namodenoson may each be a new chemical entity, or NCE, following approval of an NDA, we, if we are the first applicant to obtain NDA approval, may be entitled to five years of data exclusivity in the United States with respect to such NCEs. Analogous data and market exclusivity provisions, of varying duration, may be available in Europe and other foreign jurisdictions. We also have rights under our pharmaceutical use issued patents with respect to Piclidenoson and Namodenoson and under our Piclidenoson manufacturing process patents, which provide patent exclusivity within our field of activity until the mid- to late-2020s. While we believe that we may be able to protect our exclusivity through such use patent portfolio and such period of exclusivity, the lack of composition of matter patent protection may diminish our ability to maintain a proprietary position for our intended uses of Piclidenoson or Namodenoson. Moreover, we cannot be certain that we will be the first applicant to obtain an FDA approval for any indication of Piclidenoson or Namodenoson and we cannot be certain that we will be entitled to NCE exclusivity. In addition, we have discontinued the prosecution of a family of pending patent applications under joint ownership of us and NIH pertaining to the use of A3AR agonists for the treatment of uveitis. Such diminution of our proprietary position could have a material adverse effect on our business, results of operation and financial condition.
We license from Leiden University intellectual property, which protects certain small molecules which target the A3AR, in furtherance of our platform technology, and we could lose our rights to this license if a dispute with Leiden University arises or if we fail to comply with the financial and other terms of the license.
We have licensed intellectual property from Leiden University pursuant to a license agreement. The license agreement imposes certain payment, reporting, confidentiality and other obligations on us. In the event that we were to breach any of the obligations and fail to cure, Leiden University would have the right to terminate the license agreement. In addition, Leiden University has the right to terminate the license agreement upon our bankruptcy, insolvency, or receivership. If any dispute arises with respect to our arrangements with Leiden University, such dispute may disrupt our operations and may have a material adverse impact on us if resolved in a manner that is unfavorable to us.
18
The failure to obtain or maintain patents, licensing agreements, including our current licensing agreements, and other intellectual property could impact our ability to compete effectively.
To compete effectively, we need to develop and maintain a proprietary position with regard to our own technologies, intellectual property, licensing agreements, product candidates and business. Legal standards relating to the validity and scope of claims in the biotechnology and biopharmaceutical fields are still evolving. Therefore, the degree of future protection for our proprietary rights in our core technologies and any products that might be made using these technologies is also uncertain. The risks and uncertainties that we face with respect to our patents and other proprietary rights include the following:
| ● | while some of our patents or patents that we in-licensed have issued, the pending patent applications we have filed may not result in issued patents or may take longer than we expect to result in issued patents; |
| ● | a third party may initiate an inter parties review, or IPR, proceedings in the U.S.; |
| ● | we may be subject to interference proceedings in the U.S.; |
| ● | a third party may initiate opposition proceedings in foreign countries; |
| ● | any patents that are issued may not provide meaningful protection; |
| ● | we may not be able to develop additional proprietary technologies that are patentable; |
| ● | other companies may challenge patents licensed or issued to us; |
| ● | other companies may independently develop similar or alternative technologies, or duplicate our technologies; |
| ● | other companies may design around patents we have in-licensed or developed; and |
| ● | enforcement of patents is complex, uncertain and expensive. |
If patent rights covering our products and methods are not sufficiently broad or not issued at all by the United States Patent and Trademark Office, or the USPTO, or by foreign patent offices, we may not have adequate protection against competitors with similar products and technologies. Furthermore, if the USPTO or foreign patent offices issue patents to us or our licensors, others may challenge the patents or design around the patents, or the patent office or the courts may invalidate the patents. Thus, any patents we own or license from third parties may not provide any protection against our competitors.
We cannot be certain that patents will be issued as a result of any pending applications, and we cannot be certain that any of our issued patents will give us adequate protection from competing products. For example, issued patents, including the patents in-licensed by us, may be circumvented or challenged, declared invalid or unenforceable, or narrowed in scope. In addition, since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that we were the first to make our inventions or to file patent applications covering those inventions.
It is also possible that others may obtain issued patents that could prevent us from commercializing our products or require us to obtain licenses requiring the payment of significant fees or royalties in order to enable us to conduct our business. As to those patents that we have in-licensed, our rights depend on maintaining our obligations to the licensor under the applicable license agreement, and we may be unable to do so.
In addition to patents and patent applications, we depend upon trade secrets and proprietary know-how to protect our proprietary technology. We require our employees, consultants, advisors and collaborators to enter into confidentiality agreements that prohibit the disclosure of confidential information to any other parties. We require our employees and consultants to disclose and assign to us their ideas, developments, discoveries and inventions. These agreements may not, however, provide adequate protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure.
19
Costly litigation may be necessary to protect our intellectual property rights and we may be subject to claims alleging the violation of the intellectual property rights of others.
We may face significant expense and liability as a result of litigation or other proceedings relating to patents and other intellectual property rights of others. In the event that another party has also filed a patent application or been issued a patent relating to an invention or technology claimed by us in pending applications, we may be required to participate in an interference proceeding declared by the USPTO to determine priority of invention, which could result in substantial uncertainties and costs for us, even if the eventual outcome were favorable to us. We, or our licensors, also could be required to participate in interference proceedings involving issued patents and pending applications of another entity. An adverse outcome in an interference proceeding could require us to cease using the technology or to license rights from prevailing third parties.
The cost to us of any patent litigation or other proceeding relating to our own or in-licensed patents or patent applications, even if resolved in our favor, could be substantial. Our ability to enforce our patent protection could be limited by our financial resources, and may be subject to lengthy delays. If we are unable to effectively enforce our proprietary rights, or if we are found to infringe the rights of others, we may be in breach of our License Agreement.
A third party may claim that we are using inventions claimed by their patents and may go to court to stop us from engaging in our normal operations and activities, such as research, development and the sale of any future products. Such lawsuits are expensive and would consume time and other resources. There is a risk that the court will decide that we are infringing the third party’s patents and will order us to stop the activities claimed by the patents, redesign our products or processes to avoid infringement or obtain licenses (which may not be available on commercially reasonable terms). In addition, there is a risk that a court will order us to pay the other party damages for having infringed their patents and possibly also their legal fees.
Moreover, there is no guarantee that any prevailing patent owner would offer us a license so that we could continue to engage in activities claimed by the patent, or that such a license, if made available to us, could be acquired on commercially acceptable terms. In addition, third parties may, in the future, assert other intellectual property infringement claims against us with respect to our product candidates, technologies or other matters.
We rely on confidentiality agreements that could be breached and may be difficult to enforce, which could result in third parties using our intellectual property to compete against us.
Although we believe that we take reasonable steps to protect our intellectual property, including the use of agreements relating to the non-disclosure of confidential information to third parties, as well as agreements that purport to require the disclosure and assignment to us of the rights to the ideas, developments, discoveries and inventions of our employees and consultants while we employ them, the agreements can be difficult and costly to enforce. Although we seek to obtain these types of agreements from our contractors, consultants, advisors and research collaborators, to the extent that employees and consultants utilize or independently develop intellectual property in connection with any of our projects, disputes may arise as to the intellectual property rights associated with our products. If a dispute arises, a court may determine that the right belongs to a third party. In addition, enforcement of our rights can be costly and unpredictable. We also rely on trade secrets and proprietary know-how that we seek to protect in part by confidentiality agreements with our employees, contractors, consultants, advisors or others. Despite the protective measures we employ, we still face the risk that:
| ● | these agreements may be breached; |
| ● | these agreements may not provide adequate remedies for the applicable type of breach; |
| ● | our trade secrets or proprietary know-how will otherwise become known; or |
| ● | our competitors will independently develop similar technology or proprietary information. |
20
International patent protection is particularly uncertain, and if we are involved in opposition proceedings in foreign countries, we may have to spend substantial sums and management resources.
Patent law outside the United States is different than in the United States. Further, the laws of some foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States, if at all. A failure to obtain sufficient intellectual property protection in any foreign country could materially and adversely affect our business, results of operations and future prospects. Moreover, we may participate in opposition proceedings to determine the validity of our foreign patents or our competitors’ foreign patents, which could result in substantial costs and divert management’s resources and attention.
Although most jurisdictions in which we have applied for, intend to apply for, or have been issued patents have patent protection laws similar to those of the United States, some of them do not. For example, we expect to do business in Brazil and India in the future. However, the Brazilian drug regulatory agency, ENVISA, has the authority to nullify patents on the basis of its perceived public interest and the Indian patent law does not allow patent protection for new uses of pharmaceuticals (many of our current patent applications are of such nature). Additionally, due to uncertainty in patent protection law, we have not filed applications in many countries where significant markets exist, including Indonesia, Pakistan, Russia, African countries and Taiwan.
We may be unable to protect the intellectual property rights of the third parties from whom we license certain of our intellectual property or with whom we have entered into other strategic relationships.
Certain of our intellectual property rights are currently licensed from Leiden University, and, in the future, we wish to continue to license intellectual property from Leiden University and/or other universities and/or strategic partners. Such third parties may determine not to protect the intellectual property rights that we license from them and we may be unable to defend such intellectual property rights on our own or we may have to undertake costly litigation to defend the intellectual property rights of such third parties. There can be no assurances that we will be able to obtain licenses to such third party intellectual property or otherwise have the right to use through similar strategic relationships. Any loss or limitations on use with respect to our right to use such intellectual property licensed from third parties or otherwise obtained from third parties with whom we have entered into strategic relationships could have a material adverse effect on our business, results of operations and financial condition.
Under applicable U.S. and Israeli law, we may not be able to enforce covenants not to compete and therefore, may be unable to prevent our competitors from benefiting from the expertise of some of our former employees. In addition, employees may be entitled to seek compensation for their inventions irrespective of their agreements with us, which in turn could impact our future profitability.
We generally enter into confidentiality and non-competition agreements with our employees and certain key consultants, or our employment and consulting agreements contain confidentiality and non-competition provisions. These agreements, to the extent they are in place and in effect, prohibit our employees and certain key consultants, if they cease working for us, from competing directly with us or working for our competitors or clients for a limited period of time and maintain confidentiality of our know-how and trade secrets, as long as they do not enter the public domain. We may be unable to enforce these agreements under the laws of the jurisdictions in which our employees work and it may be difficult for us to restrict our competitors from benefitting from the expertise our former employees or consultants developed while working for us. For example, Israeli courts have required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have been recognized by the courts, such as the secrecy of a company’s confidential commercial information or the protection of its intellectual property. If we cannot demonstrate that such interests will be harmed, we may be unable to prevent our competitors from benefiting from the expertise of our former employees or consultants and our ability to remain competitive may be diminished.
In addition, Chapter 8 to the Israeli Patents Law, 5727-1967, or the Patents Law, deals with inventions made in the course of an employee’s service and during his or her term of employment, whether or not the invention is patentable, or service inventions. Section 134 of the Patents Law provides that if there is no agreement that explicitly determines whether the employee is entitled to compensation for the service inventions and the extent and terms of such compensation, such determination will be made by the Compensation and Rewards Committee, a statutory committee of the Israeli Patents Office. Although our employees have agreed to assign to us service invention rights, we may face claims demanding remuneration in consideration for assigned inventions. As a consequence of such claims, we could be required to pay additional remuneration or royalties to our current and/or former employees, or be forced to litigate such claims, which could negatively affect our business.
21
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
| ● | Others may be able to make compounds that are the same as or similar to our product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed; |
| ● | We or our licensors or any future strategic partners might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed; |
| ● | We or our licensors or any future strategic partners might not have been the first to file patent applications covering certain of our inventions; |
| ● | Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
| ● | It is possible that our pending patent applications will not lead to issued patents; |
| ● | Issued patents that we own or have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or unenforceable, as a result of legal challenges by our competitors; |
| ● | Our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; and |
| ● | We may not develop additional proprietary technologies that are patentable. |
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an interest in our patents or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Risks Related to Our Industry
We are subject to government regulations and we may experience delays in obtaining required regulatory approvals in the United States and the regulatory authorities in foreign jurisdiction in which we intend to market our proposed product candidates, of which there can be no assurance.
Various aspects of our operations are subject to foreign, federal, state or local laws, and rules and regulations, any of which may change from time to time. We are not permitted to market our product candidates as prescription pharmaceutical products in the United States until we receive approval of a New Drug Application, or NDA, from the FDA or in any foreign countries until we receive the requisite approval from such countries. Costs arising out of any regulatory developments could be time-consuming and expensive and could divert management resources and attention and, consequently, could adversely affect our business operations and financial performance.
22
Delays in regulatory approval, limitations in regulatory approval and withdrawals of regulatory approval may have a material adverse effect on us. In the United States, the FDA generally requires the completion of clinical trials of each drug to establish its safety and efficacy and extensive pharmaceutical development to ensure its quality before an NDA is approved. If we experience significant delays in testing or receiving approvals or sign-offs to conduct clinical trials, our product development costs, or our ability to license product candidates, will increase. If the FDA grants regulatory approval to market a product, this approval will be limited to those disease states and conditions and populations for which the product has demonstrated, through clinical trials, to be safe and effective. Any product approvals that we receive in the future could also include significant restrictions on the use or marketing of our products. Product approvals, if granted, can be withdrawn for failure to comply with regulatory requirements or upon the occurrence of adverse events following commercial introduction of the products. Failure to comply with applicable FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production or injunction, as well as other regulatory action against our product candidates or us. If approval is withdrawn for a product, or if a product were seized or recalled, we would be unable to sell or license that product and our revenues would suffer. In addition, outside the United States, our ability to market any of our potential products is contingent upon receiving market application authorizations from the appropriate regulatory authorities and these foreign regulatory approval processes include all of the risks associated with the FDA approval process described above.
Our success depends on our receipt of the regulatory approvals described above, and the issuance of such regulatory approvals is uncertain and subject to a number of risks, including the following:
| ● | such authorities may disagree with the number, design, size, conduct or implementation of our clinical trials or any of our collaborators’ clinical trials; |
| ● | such authorities may disagree with our interpretation of data from preclinical studies or clinical trials or the use of results from studies that served as precursors to our current or future product candidates; |
| ● | the results of toxicology studies may not support the filing of an Investigational New Drug Application, or IND, or NDA for our product candidates; |
| ● | the FDA or comparable foreign regulatory authorities or Institutional Review Boards, or IRBs, may disagree with the design or implementation of our clinical trials; |
| ● | we may not be able to provide acceptable evidence of our product candidates’ safety and efficacy; |
| ● | the results of our clinical trials may not be satisfactory or may not meet the level of statistical or clinical significance required by the FDA, European Medicines Agency, or EMA, or other regulatory agencies for us to receive marketing approval for any of our product candidates; |
| ● | the dosing of our product candidates in a particular clinical trial may not be at an optimal level; |
| ● | patients in our clinical trials may suffer adverse effects for reasons that may or may not be related to our product candidates; |
| ● | the data collected from clinical trials may not be sufficient to support the submission of an NDA, or other submission to obtain regulatory approval in the United States or elsewhere; |
| ● | the FDA may require development of a Risk Evaluation and Mitigation Strategy, or REMS, as a condition of approval; |
| ● | the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and |
| ● | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval of our product candidates. |
23
The process of obtaining regulatory approvals is expensive, often takes many years, if approval is obtained at all, and can vary substantially based upon, among other things, the type, complexity and novelty of the product candidates involved, the jurisdiction in which regulatory approval is sought and the substantial discretion of the regulatory authorities. Changes in regulatory approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for a submitted product application may cause delays in the approval or rejection of an application. Regulatory approval obtained in one jurisdiction does not necessarily mean that a product candidate will receive regulatory approval in all jurisdictions in which we may seek approval, but the failure to obtain approval in one jurisdiction may negatively impact our ability to seek approval in a different jurisdiction.
Clinical testing is expensive, is difficult to design and implement, can take many years to complete and is uncertain as to outcome.
Our business model depends entirely on the successful development, regulatory approval and commercialization of our product candidates, which may never occur. Our product candidates are in the early stages of development and as of the date of this Annual Report on Form 20-F. We may not be successful in obtaining approval from the FDA or comparable foreign regulatory authorities to start or continue clinical trials for any of our product candidates. Moreover, there is no guarantee that we will receive approval to commence human clinical trials or that our clinical trials will be successful or that we will continue clinical development in support of an approval from the FDA or comparable foreign regulatory authorities for any indication. We note that most product candidates never reach the clinical development stage and even those that do commence clinical development have only a small chance of successfully completing clinical development and gaining regulatory approval. Success in early phases of pre-clinical and clinical trials does not ensure that later clinical trials will be successful, and interim results of a clinical trial do not necessarily predict final results. A failure of one or more of our clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates. Therefore, our business currently depends entirely on the successful development, regulatory approval and commercialization of our product candidates, which may never occur.
The results of previous clinical trials may not be predictive of future results, our progress in trials for one product candidate may not be indicative of progress in trials for other product candidates, and our trials may not be designed so as to support regulatory approval.
We currently have no products approved for sale and we cannot guarantee that we will ever have marketable products. Clinical failure can occur at any stage of clinical development. Clinical trials may produce negative or inconclusive results, and we or any of our current and future collaborators may decide, or regulators may require us, to conduct additional clinical or non-clinical testing. We will be required to demonstrate with substantial evidence through well-controlled clinical trials that our product candidates are safe and effective for use in a diverse population before we can obtain regulatory approvals for their commercial sale. Success in early clinical trials does not mean that future clinical trials will be successful because product candidates in later-stage clinical trials may fail to demonstrate sufficient safety and efficacy to the satisfaction of the FDA and other regulatory authorities despite having progressed through initial clinical trials. Product candidates that have shown promising results in early clinical trials may still suffer significant setbacks in subsequent clinical trials. Similarly, the outcome of non-clinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. Progress in trials of one product candidate does not indicate that we will make similar progress in additional trials for that product candidate or in trials for our other product candidates. A number of companies in the pharmaceutical industry, including those with greater resources and experience than us, have suffered significant setbacks in advanced clinical trials, even after obtaining promising results in earlier clinical trials.
The design of a clinical trial can determine whether its results will support approval of a product. We may be unable to design and/or execute a clinical trial to support regulatory approval. Flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced or completed. In addition, we or our investigators may have little control over whether subjects comply with important aspects of clinical trial protocols.
24
In some instances, there can be significant variability in safety and/or efficacy results between different trials of the same product candidate due to numerous factors, including changes in trial protocols, differences in size and type of the patient populations, adherence to the dosing regimen and other trial protocols, modifications in the formulation throughout the course of development and the rate of dropout among clinical trial participants. While we have not had any serious adverse events in our clinical trials to date that are believed to be related to our oral product candidates, we may need to change future trial designs in response to adverse events that occur during future clinical development. We do not know whether any Phase 2, Phase 3 or other clinical trials we or any of our collaborators may conduct will demonstrate consistent or adequate efficacy and safety to obtain regulatory approval to market our product candidates.
Even if we receive regulatory approval for any of our product candidates, we may not be able to successfully commercialize the product and the revenue that we generate from its sales, if any, may be limited.
If approved for marketing, the commercial success of our product candidates will depend upon each product’s acceptance by the medical community, including physicians, patients and health care payors. The degree of market acceptance for any of our product candidates will depend on a number of factors, including:
| ● | demonstration of clinical safety and efficacy; |
| ● | relative convenience, dosing burden and ease of administration; |
| ● | the prevalence and severity of any adverse effects; |
| ● | the willingness of physicians to prescribe our product candidates, and the target patient population to try new therapies; |
| ● | efficacy of our product candidates compared to competing products; |
| ● | the introduction of any new products that may in the future become available targeting indications for which our product candidates may be approved; |
| ● | new procedures or therapies that may reduce the incidences of any of the indications in which our product candidates may show utility; |
| ● | pricing and cost-effectiveness; |
| ● | the inclusion or omission of our product candidates in applicable therapeutic guidelines; |
| ● | the effectiveness of our own or any future collaborators’ sales and marketing strategies; |
| ● | limitations or warnings contained in approved labeling from regulatory authorities; |
| ● | our ability to obtain and maintain sufficient third-party coverage or reimbursement from government health care programs, including Medicare and Medicaid, private health insurers and other third-party payors or to receive the necessary pricing approvals from government bodies regulating the pricing and usage of therapeutics; and |
| ● | the willingness of patients to pay out-of-pocket in the absence of third-party coverage or reimbursement or government pricing approvals. |
If any of our product candidates are approved, but do not achieve an adequate level of acceptance by physicians, health care payors, and patients, we may not generate sufficient revenue and we may not be able to achieve or sustain profitability. Our efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources and may never be successful.
25
In addition, even if we obtain regulatory approvals, the timing or scope of any approvals may prohibit or reduce our ability to commercialize our product candidates successfully. For example, if the approval process takes too long, we may miss market opportunities and give other companies the ability to develop competing products or establish market dominance. Any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render our product candidates not commercially viable. For example, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request, may not approve the price we intend to charge for any of our product candidates, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve any of our product candidates with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that indication. Further, the FDA or comparable foreign regulatory authorities may place conditions on approvals or require risk management plans or a REMS to assure the safe use of the drug. Moreover, product approvals may be withdrawn for non-compliance with regulatory standards or if problems occur following the initial marketing of the product. Any of the foregoing scenarios could materially harm the commercial success of our product candidates.
Even if we obtain marketing approval for any of our product candidates, we will be subject to ongoing obligations and continued regulatory review, which may result in significant additional expense. Additionally, our product candidates could be subject to labeling and other restrictions and withdrawal from the market and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our product candidates.
Even if we obtain regulatory approval for any of our product candidates for an indication, the FDA or foreign equivalent may still impose significant restrictions on their indicated uses or marketing or the conditions of approval, or impose ongoing requirements for potentially costly and time-consuming post-approval studies, including Phase 4 clinical trials, and post-market surveillance to monitor safety and efficacy. Our product candidates will also be subject to ongoing regulatory requirements governing the manufacturing, labeling, packaging, storage, distribution, safety surveillance, advertising, promotion, recordkeeping and reporting of adverse events and other post-market information. These requirements include registration with the FDA, as well as continued compliance with current Good Clinical Practices regulations, or cGCPs, for any clinical trials that we conduct post-approval. In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with current Good Manufacturing Practices, or cGMPs, requirements relating to quality control, quality assurance and corresponding maintenance of records and documents.
The FDA has the authority to require a REMS as part of an NDA or after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria or requiring patient testing, monitoring and/or enrollment in a registry.
With respect to sales and marketing activities related to our product candidates, advertising and promotional materials must comply with FDA rules in addition to other applicable federal, state and local laws in the United States and similar legal requirements in other countries. In the United States, the distribution of product samples to physicians must comply with the requirements of the U.S. Prescription Drug Marketing Act. Application holders must obtain FDA approval for product and manufacturing changes, depending on the nature of the change. We may also be subject, directly or indirectly through our customers and partners, to various fraud and abuse laws, including, without limitation, the U.S. Anti-Kickback Statute, U.S. False Claims Act, and similar state laws, which impact, among other things, our proposed sales, marketing, and scientific/educational grant programs. If we participate in the U.S. Medicaid Drug Rebate Program, the Federal Supply Schedule of the U.S. Department of Veterans Affairs, or other government drug programs, we will be subject to complex laws and regulations regarding reporting and payment obligations. All of these activities are also potentially subject to U.S. federal and state consumer protection and unfair competition laws. Similar requirements exist in many of these areas in other countries.
26
In addition, if any of our product candidates are approved for a particular indication, our product labeling, advertising and promotion would be subject to regulatory requirements and continuing regulatory review. The FDA strictly regulates the promotional claims that may be made about prescription products. In particular, a product may not be promoted for uses that are not approved by the FDA as reflected in the product’s approved labeling. If we receive marketing approval for our product candidates, physicians may nevertheless legally prescribe our products to their patients in a manner that is inconsistent with the approved label. If we are found to have promoted such off-label uses, we may become subject to significant liability and government fines. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant sanctions. The federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees of permanent injunctions under which specified promotional conduct is changed or curtailed.
If we or a regulatory agency discover previously unknown problems with a product candidate, such as adverse events of unanticipated severity or frequency, problems with the facility where the product is manufactured, or we or our manufacturers fail to comply with applicable regulatory requirements, we may be subject to the following administrative or judicial sanctions:
| ● | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| ● | issuance of warning letters or untitled letters; |
| ● | clinical holds; |
| ● | injunctions or the imposition of civil or criminal penalties or monetary fines; |
| ● | suspension or withdrawal of regulatory approval; |
| ● | suspension of any ongoing clinical trials; |
| ● | refusal to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product license approvals; |
| ● | suspension or imposition of restrictions on operations, including costly new manufacturing requirements; or |
| ● | product seizure or detention or refusal to permit the import or export of product. |
The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and generate revenue. Adverse regulatory action, whether pre- or post-approval, can also potentially lead to product liability claims and increase our product liability exposure.
We also cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad, and compliance with such regulation may be expensive and consume substantial financial and management resources. If we or any future marketing collaborators or contract manufacturers are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies or are not able to maintain regulatory compliance, it could delay or prevent the promotion, marketing or sale of our products, which would adversely affect our business and results of operations.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction, but a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a product candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from those in the United States, including additional preclinical studies or clinical trials, as clinical studies conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval.
27
Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our product candidates in certain countries. If we fail to comply with the regulatory requirements in international markets and/ or to receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our product candidates will be harmed.
Even though we may apply for orphan drug designation for a product candidate, we may not be able to obtain orphan drug status or marketing exclusivity.
We believe that in some cases our dry powder drug products may qualify for the FDA’s orphan drug status. There is no guarantee that the FDA will grant any future application for orphan drug designation for any of our product candidates, which would make us ineligible for the additional exclusivity and other benefits of orphan drug designation.
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug available in the United States for this type of disease or condition will be recovered from sales of the product. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan product designation does not convey any advantage in or shorten the duration of regulatory review and approval process. In addition to the potential period of exclusivity, orphan designation makes a company eligible for grant funding of up to $500,000 per year for four years to defray costs of clinical trial expenses, tax credits for clinical research expenses and potential exemption from the FDA application user fee.
If a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan drug exclusivity, which means the FDA may not approve any other applications to market the same drug for the same indication for seven years, except in limited circumstances, such as (i) the drug’s orphan designation is revoked; (ii) its marketing approval is withdrawn; (iii) the orphan exclusivity holder consents to the approval of another applicant’s product; (iv) the orphan exclusivity holder is unable to assure the availability of a sufficient quantity of drug; or (v) a showing of clinical superiority to the product with orphan exclusivity by a competitor product. If a drug designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan drug exclusivity. There can be no assurance that we will receive orphan drug designation for any of our product candidates in the indications for which we think they might qualify, if we elect to seek such applications.
Current and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval for our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell our product candidates. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We do not know whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
28
In the United States, the Medicare Modernization Act, or MMA, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for drugs. In addition, this legislation authorized Medicare Part D prescription drug plans to use formularies where they can limit the number of drugs that will be covered in any therapeutic class. As a result of this legislation and the expansion of federal coverage of drug products, we expect that there will be additional pressure to contain and reduce costs. These cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for our product candidates and could seriously harm our business. While the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates, and any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010 or, collectively, the ACA, is a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. The ACA revised the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, the law imposed a significant annual fee on companies that manufacture or import branded prescription drug products.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. In 2011, the U.S. Congress enacted the Budget Control Act of 2011, or the Budget Control Act, which included provisions intended to reduce the federal deficit. The Budget Control Act resulted in the imposition of 2% reductions in Medicare payments to providers beginning in 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2027 absent additional congressional action. However, pursuant to the CARES Act, and subsequent legislation, these reductions were suspended from May 1, 2020 through March 31, 2022 due to the COVID-19 pandemic. In January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on customers for our drugs, if approved, and accordingly, our financial operations. If government spending is further reduced, anticipated budgetary shortfalls may also impact the ability of relevant agencies, such as the FDA, to continue to function at current levels, which may impact the ability of relevant agencies to timely review and approve research and development, manufacturing and marketing activities, which may delay our ability to develop, market and sell any product candidates we may develop. In addition, any significant spending reductions affecting Medicare, Medicaid or other publicly funded or subsidized health programs that may be implemented, or any significant taxes or fees that may be imposed on us, as part of any broader deficit reduction effort or legislative replacement to the Budget Control Act, could have an adverse impact on our anticipated product revenues.
There have been judicial and Congressional challenges to certain aspects of the ACA, and we expect such challenges to continue. In 2017, the U.S. Congress enacted the Tax Cuts and Jobs Act, or the 2017 Tax Act, which eliminated the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” On January 22, 2018, President Trump signed a continuing resolution on appropriations for fiscal year 2018 that delayed the implementation of certain fees mandated by the ACA, including the so-called “Cadillac” tax on certain high cost employer-sponsored insurance plans and the annual fee imposed on certain health insurance providers based on market share. The Bipartisan Budget Act of 2018, or the BBA, among other things, amends the ACA, effective January 1, 2019, to close the coverage gap in most Medicare drug plans. In July 2018, the Centers for Medicare and Medicaid Services, or CMS, published a final rule permitting further collections and payments to and from certain ACA qualified health plans and health insurance issuers under the ACA risk adjustment program in response to the outcome of federal district court litigation, regarding the method CMS uses to determine this risk adjustment. Litigation and legislation over the ACA are likely to continue, with unpredictable and uncertain results.
29
Moreover, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their commercial products. There have been several recent U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the cost of drugs under Medicare, and reform government program reimbursement methodologies for drugs. On September 24, 2020, the FDA released a final rule providing guidance for states to build and submit importation plans for drugs from Canada. Further, on November 20, 2020, the U.S. Department of Health and Human Services, or HHS, finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers. On August 16, 2022, President Biden signed the Inflation Reduction Act of 2022, which includes several provisions to lower prescription drug costs for people with Medicare, including price negotiation requirements for drugs covered under Medicare, rebate requirements when drug prices rise faster than inflation, and a cap on out-of-pocket spending for Medicare Part D enrollees. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
On November 20, 2020, the HHS Office of Inspector General finalized further modifications to the federal Anti-Kickback Statute. Under the final rules, the HHS Office of Inspector General added safe harbor protections under the Anti-Kickback Statute for certain coordinated care and value-based arrangements among clinicians, providers, and others, yet removed safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers. This rule (with exceptions) became effective January 19, 2021. We continue to evaluate what effect, if any, these rules will have on our business. CMS issued a final rule, effective on July 9, 2019, that requires direct-to-consumer advertisements of prescription drugs and biological products, for which payment is available through or under Medicare or Medicaid, to include in the advertisement the Wholesale Acquisition Cost, or list price, of that drug or biological product if it is equal to or greater than $35 for a monthly supply or usual course of treatment. Prescription drugs and biological products that are in violation of these requirements will be included on a public list. Any adopted health reform measure could reduce the ultimate demand for our products, if approved, or put pressure on our product pricing. Individual states in the United States have also become increasingly active in passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. We expect that additional state and federal healthcare reform measures will be adopted in the future.
The delivery of healthcare in the EU, including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than EU, law and policy. National governments and health service providers have different priorities and approaches to the delivery of health care and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most EU member states have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing EU and national regulatory burdens on those wishing to develop and market products, this could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to commercialize any products for which we obtain marketing approval. Both in the United States and in the EU, legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We do not know whether additional legislative changes will be enacted, or whether the regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be.
30
Any termination or suspension of, or delays in the commencement or completion of, any necessary studies of any of our product candidates for any indications could result in increased costs to us, delay or limit our ability to generate revenue and adversely affect our commercial prospects.
The commencement and completion of clinical studies can be delayed for a number of reasons, including delays related to:
| ● | the FDA or a comparable foreign regulatory authority failing to grant permission to proceed and placing the clinical study on hold; |
| ● | subjects for clinical testing failing to enroll or remain enrolled in our trials at the rate we expect; |
| ● | a facility manufacturing any of our product candidates being ordered by the FDA or other government or regulatory authorities to temporarily or permanently shut down due to violations of cGMP requirements or other applicable requirements, or cross-contaminations of product candidates in the manufacturing process; |
| ● | any changes to our manufacturing process that may be necessary or desired; |
| ● | subjects choosing an alternative treatment for the indications for which we are developing our product candidates, or participating in competing clinical studies; |
| ● | subjects experiencing severe or unexpected drug-related adverse effects; |
| ● | reports from clinical testing on similar technologies and products raising safety and/or efficacy concerns; |
| ● | third-party clinical investigators losing their license or permits necessary to perform our clinical trials, not performing our clinical trials on our anticipated schedule or employing methods consistent with the clinical trial protocol, cGMP requirements, or other third parties not performing data collection and analysis in a timely or accurate manner; |
| ● | inspections of clinical study sites by the FDA, comparable foreign regulatory authorities, or IRBs finding regulatory violations that require us to undertake corrective action, result in suspension or termination of one or more sites or the imposition of a clinical hold on the entire study, or that prohibit us from using some or all of the data in support of our marketing applications; |
| ● | third-party contractors becoming debarred or suspended or otherwise penalized by the FDA or other government or regulatory authorities for violations of regulatory requirements, in which case we may need to find a substitute contractor, and we may not be able to use some or any of the data produced by such contractors in support of our marketing applications; |
| ● | one or more IRBs refusing to approve, suspending or terminating the study at an investigational site, precluding enrollment of additional subjects, or withdrawing its approval of the trial, reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| ● | deviations of the clinical sites from trial protocols or dropping out of a trial; |
| ● | adding new clinical trial sites; |
| ● | the inability of the CRO to execute any clinical trials for any reason; and |
| ● | government or regulatory delays or “clinical holds” requiring suspension or termination of a trial. |
31
Product development costs for any of our product candidates will increase if we have delays in testing or approval or if we need to perform more or larger clinical studies than planned. Additionally, changes in regulatory requirements and policies may occur and we may need to amend study protocols to reflect these changes. Amendments may require us to resubmit our study protocols to the FDA, comparable foreign regulatory authorities, and IRBs for reexamination, which may impact the costs, timing or successful completion of that study. If we experience delays in completion of, or if we, the FDA or other regulatory authorities, the IRB, or other reviewing entities, or any of our clinical study sites suspend or terminate any of our clinical studies of any of our product candidates, its commercial prospects may be materially harmed and our ability to generate product revenues will be delayed. Any delays in completing our clinical trials will increase our costs, slow down our development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that cause, or lead to, termination or suspension of, or a delay in the commencement or completion of, clinical studies may also ultimately lead to the denial of regulatory approval of our product candidates. In addition, if one or more clinical studies are delayed, our competitors may be able to bring competing products to market before we do, and the commercial viability of any of our affected product candidates could be significantly reduced.
If we or any of our independent contractors, consultants, collaborators, manufacturers, or service providers fail to comply with healthcare and data privacy laws and regulations, we or they could be subject to enforcement actions, which could result in penalties and affect our ability to develop, market and sell our product candidates and may harm our reputation.
We are or may in the future be subject to federal, state, and foreign healthcare and data privacy laws and regulations pertaining to, among other things, fraud and abuse of patients’ rights. These laws and regulations include:
| ● | The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully soliciting, offering, receiving, or paying any remuneration, directly or indirectly, in cash or in kind, to induce or reward purchasing, ordering or arranging for or recommending the purchase or order of any item or service for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid. Liability may be established without a person or entity having actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it. This statute has been interpreted to apply broadly to arrangements between pharmaceutical manufacturers on the one hand and prescribers, patients, purchasers and formulary managers on the other. In addition, the ACA amended the Social Security Act to provide that the U.S. government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act, or the FCA. A conviction for violation of the Anti-Kickback Statute requires mandatory exclusion from participation in federal healthcare programs. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and those activities may be subject to scrutiny or penalty if they do not qualify for an exemption or safe harbor; |
| ● | The FCA prohibits, among other things, knowingly presenting, or causing to be presented claims for payment of government funds that are false or fraudulent, or knowingly making, using or causing to be made or used a false record or statement material to such a false or fraudulent claim, or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government. This statute also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the FCA and to share in any monetary recovery. The FCA prohibits anyone from knowingly presenting, conspiring to present, making a false statement in order to present, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services, including drugs, that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. This law also prohibits anyone from knowingly underpaying an obligation owed to a federal program. Increasingly, U.S. federal agencies are requiring nonmonetary remedial measures, such as corporate integrity agreements in FCA settlements. The U.S. Department of Justice announced in 2016 its intent to follow the “Yates Memo,” taking a far more aggressive approach in pursuing individuals as FCA defendants in addition to the corporations. On October 28, 2021, the Biden Administration announced that it would continue the policies set forth in the “Yates” memo. FCA liability is potentially significant in the healthcare industry because the statute provides for treble damages and mandatory penalties of $5,500 to $11,000 per false claim or statement ($12,537 to $25,076 per false claim or statement for penalties assessed after May 9, 2022 for violations occurring after November 2, 2015. Government enforcement agencies and private whistleblowers have investigated pharmaceutical companies for or asserted liability under the FCA for a variety of alleged promotional and marketing activities, such as providing free product to customers with the expectation that the customers would bill federal programs for the product; providing consulting fees and other benefits to physicians to induce them to prescribe products; engaging in promotion for “off-label” uses; and submitting inflated best price information to the Medicaid Rebate Program; |
32
| ● | The federal False Statements Statute prohibits knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry, in connection with the delivery of or payment for healthcare benefits, items, or services; |
| ● | The federal Civil Monetary Penalties Law authorizes the imposition of substantial civil monetary penalties against an entity, such as a pharmaceutical manufacturer, that engages in activities including, among others (1) knowingly presenting, or causing to be presented, a claim for services not provided as claimed or that is otherwise false or fraudulent in any way; (2) arranging for or contracting with an individual or entity that is excluded from participation in federal healthcare programs to provide items or services reimbursable by a federal healthcare program; (3) violations of the federal Anti-Kickback Statute; (4) knowingly offering or transferring remuneration to a Medicare or Medicaid beneficiary or recipient to influence the beneficiary’s or recipient’s selection of a particular provider, practitioner, or supplier for the order or receipt of any item or service; or (5) failing to report and return a known overpayment; |
| ● | The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of, or payment for, healthcare benefits, items or services; similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, which imposes requirements on certain types of people and entities relating to the privacy, security, and transmission of individually identifiable health information, and requires notification to affected individuals and regulatory authorities of certain breaches of security of individually identifiable health information; |
| ● | The federal Physician Payment Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program, to report annually to the Centers for Medicare & Medicaid Services information related to payments and other transfers of value to physicians, other healthcare providers and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members, which is published in a searchable form on an annual basis; |
| ● | State laws comparable to each of the above federal laws, such as, for example, anti-kickback and false claims laws that may be broader in scope and also apply to commercial insurers and other non-federal payor; |
| ● | Requirements for mandatory corporate regulatory compliance programs, and laws relating to patient data privacy and security. Other state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts; and |
33
| ● | In the European Union, the General Data Protection Regulation, or the GDPR, Regulation EU 2016/679, which was adopted in May 2016 and has become applicable on May 25, 2018. The GDPR is further intended to harmonize data protection requirements across the European Union member states by establishing new and expanded operational requirements for entities that collect, process or use personal data generated in the European Union, including consent requirements for disclosing the way personal information will be used, information retention requirements, and notification requirements in the event of a data breach. |
| ● | The California Consumer Privacy Act of 2018, or CCPA, effective as of January 1, 2020, gives California residents expanded rights to access and require deletion of their personal information, opt out of certain personal information sharing, and receive detailed information about how their personal information is used. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches, that is expected to increase data breach litigation. |
| ● | In addition, failure to comply with the Israeli Privacy Protection Law of 1981, and its regulations, as well as the guidelines of the Israeli Privacy Protection Authority, may expose us to administrative fines, civil claims (including class actions) and in certain cases criminal liability. Current pending legislation may result in a change of the current enforcement measures and sanctions. |
If our operations are found to be in violation of any such healthcare laws and regulations, we may be subject to penalties, including administrative, civil and criminal penalties, monetary damages, disgorgement, imprisonment, the curtailment or restructuring of our operations, loss of eligibility to obtain approvals from the FDA or foreign regulatory authorities, or exclusion from participation in government contracting, healthcare reimbursement or other government programs, including Medicare and Medicaid, any of which could adversely our financial results. Any action against us for an alleged or suspected violation could cause us to incur significant legal expenses and could divert our management’s attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with applicable laws and regulations may be costly to us in terms of money, time and resources.
Our employees, principal investigators, consultants, commercial partners or vendors may engage in misconduct or other improper activities, including non-compliance with regulatory standards.
We are also exposed to the risk of employees, independent contractors, principal investigators, consultants, commercial partners or vendors engaging in fraud or other misconduct. Misconduct by employees, independent contractors, principal investigators, consultants, commercial partners and vendors could include intentional failures to comply with EU regulations, to provide accurate information to the EMA or EU Member States authorities or to comply with manufacturing or quality standards we have or will have established. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices such as promotion of products by medical practitioners. The EU Member States in which we operate have different statutory provisions regulating the cooperation of pharmaceutical companies with healthcare professionals. In addition to these statutory provisions, codes of conduct issued by business associations or other non-statutory standards may be applicable to our activities. Both statutory provisions and non-statutory codes or standards restrict payments or other benefits provided to healthcare professionals, and in case of non-compliance, may result in severe sanctions such as bans, administrative fines, criminal fines or even imprisonment. The advertising of medicinal products for human use in the EU is regulated by Title VIII of European Directive 2001/83/EC. These provisions have been implemented into the law of the EU member States. Such laws inter alia restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Misconduct could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory sanctions and serious and irreparable harm to our reputation.
34
This could also apply with respect to data privacy. In the EU, the EU Directive 95/46/EEC was replaced by the GDPR on May 25, 2018. The GDPR as an EU regulation does not have to be implemented into Member States’ national law, but applies directly in all Member States since May 25, 2018. It applies to companies with an establishment in the European Economic Area (EEA) and to certain other companies not in the EEA that offer or provide goods or services to individuals located in the EEA or monitor individuals located in the EEA. The GDPR implements more stringent operational requirements for controllers of personal data, including, for example, expanded disclosures about how personal information is to be used, limitations on retention of information, increased requirements pertaining to health data and pseudonymized (i.e., key-coded) data, increased cyber security requirements, mandatory data breach notification requirements and higher standards for controllers to demonstrate that they have obtained a valid legal basis for certain data processing activities. The GDPR provides that EU Member States may continue to make their own further laws and regulations in relation to the processing of genetic, biometric or health data, which could result in continued or new differences between Member States, limit our ability to use and share personal data or could cause our costs to increase, and harm our business and financial condition. We are also subject to evolving and strict rules on the transfer of personal data out of the European Union to the United States. Further prospective revision of the Directive on privacy and electronic communications (Directive 2002/58/EC), or ePrivacy Directive, may affect our marketing communications.
Our actual or alleged failure to comply with this regulation, or to protect personal data, could result in enforcement actions and significant penalties against us, which could result in negative publicity, increase our operating costs, subject us to claims or other remedies and have a material adverse effect on our business, financial condition, and results of operations. It is not always possible to identify and deter misconduct by employees or other parties. The precautions we take to detect and prevent such activity may not protect us from legal or regulatory action resulting from a failure to comply with applicable laws or regulations. Misconduct by our employees, principal investigators, consultants, commercial partners or vendors could result in significant financial penalties, criminal sanctions, civil law claims and/or negative media coverage, and thus have a material adverse effect on our business, including through the imposition of significant fines or other sanctions, and our reputation. In particular, failure to comply with EU laws, including failure under the GDPR, ePrivacy Directive and other laws relating to the security of personal data may result in fines up to €20,000,000 or up to 4% of the total worldwide annual turnover of the preceding financial year, if greater, and other administrative penalties including criminal liability, which may be onerous and adversely affect our business, financial condition, results of operations and prospects. Failure to comply with the GDPR and related laws may also give risk to increase risk of private actions, including a new form of class action that is available under the GDPR.
Risks Related to Our Operations in Israel
We conduct our operations in Israel and therefore our results may be adversely affected by political, economic and military instability in Israel and its region.
Our headquarters are located in Israel and we conduct operations in Israel. Accordingly, political, economic and military conditions in the Middle East may affect our business directly. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its neighboring countries, as well as terrorist acts committed within Israel by hostile elements.
In particular, in October 2023, Hamas terrorists infiltrated Israel’s southern border from the Gaza Strip and conducted a series of attacks on civilian and military targets. Hamas also launched extensive rocket attacks on the Israeli population and industrial centers located along Israel’s border with the Gaza Strip and in other areas within the State of Israel. These attacks resulted in thousands of deaths and injuries, and Hamas additionally kidnapped many Israeli civilians and soldiers. Following the attack, Israel’s security cabinet declared war against Hamas and commenced a military campaign against Hamas and these terrorist organizations in parallel continued rocket and terror attacks. As a result of the events of October 7, 2023 whereby Hamas terrorists invaded southern Israel and launched thousands of rockets in a widespread terrorist attack on Israel, the Israeli government declared that the country was at war and the Israeli military began to call-up reservists for active duty, including our CEO who was called up for reserve service during which time he continued to perform his main work duties and has since been released from reserve service. Military service call ups that result in absences of personnel from us for an extended period of time may materially and adversely affect our business, prospects, financial condition and results of operations. As of the date hereof, we currently have five full-time employees, all of whom are located in Israel, and two external consultants, both of whom are located in the United States.
35
In addition, since the commencement of these events, there have been continued hostilities along Israel’s northern border with Lebanon (with the Hezbollah terror organization) and southern border (with the Houthi movement in Yemen). It is possible that hostilities with Hezbollah in Lebanon will escalate, and that other terrorist organizations, including Palestinian military organizations in the West Bank as well as other hostile countries, such as Iran, will join the hostilities. Such clashes may escalate in the future into a greater regional conflict. In addition, Iran has threatened to attack Israel and is widely believed to be developing nuclear weapons. Iran is also believed to have a strong influence among extremist groups in the region, such as Hamas in Gaza, Hezbollah in Lebanon, the Houthi movement in Yemen and various rebel militia groups in Syria and Iraq. These situations may potentially escalate in the future to more violent events which may affect Israel and us. Any hostilities, armed conflicts, terrorist activities involving Israel or the interruption or curtailment of trade between Israel and its trading partners, or any political instability in the region could adversely affect business conditions and our results of operations and could make it more difficult for us to raise capital and could adversely affect the market price of our ordinary share. An escalation of tensions or violence might result in a significant downturn in the economic or financial condition of Israel, which could have a material adverse effect on our operations in Israel and our business. Parties with whom we do business have sometimes declined to travel to Israel during periods of heightened unrest or tension, forcing us to make alternative arrangements when necessary in order to meet our business partners face to face. In addition, the political and security situation in Israel may result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in such agreements.
Since the war broke out on October 7, 2023, our operations have not been adversely affected by this situation, and we have not experienced disruptions to our clinical studies. Of the 58 clinical sites currently participating in our clinical studies, only 4 are located in Israel. Additionally, all of our manufacturing and supply of our drug candidates takes place outside of Israel. As such, our clinical and business development activities remain on track. However, the intensity and duration of Israel’s current war against Hamas is difficult to predict at this stage, as are such war’s economic implications on the Company’s business and operations and on Israel’s economy in general. if the war extends for a long period of time or expands to other fronts, such as Lebanon, Syria and the West Bank, our operations may be adversely affected.
Our commercial insurance does not cover losses that may occur as a result of events associated with the security situation in the Middle East. Although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained. Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflicts or political instability in the region would likely negatively affect business conditions and could harm our results of operations.
Finally, political conditions within Israel may affect our operations. Israel has held five general elections between 2019 and 2022, and prior to October 2023, the Israeli government pursued extensive changes to Israel’s judicial system, which sparked extensive political debate and unrest. To date, these initiatives have been substantially put on hold. Actual or perceived political instability in Israel or any negative changes in the political environment, may individually or in the aggregate adversely affect the Israeli economy and, in turn, our business, financial condition, results of operations and growth prospects.
Because a certain portion of our expenses is incurred in currencies other than U.S. dollars, our results of operations may be harmed by currency fluctuations and inflation.
Company’s functional and presentation currency is U.S. dollar. To date, we have not engaged in hedging transactions. Although the Israeli rate of inflation has not had a material adverse effect on our financial condition during 2021, 2022 or 2023 to date, we may, in the future, decide to enter into currency hedging transactions to decrease the risk of financial exposure from fluctuations in the exchange rates of the currencies mentioned above in relation to U.S. dollars. These measures, however, may not adequately protect us from material adverse effects.
36
It may be difficult to enforce a U.S. judgment against us and our officers and directors named in this Annual Report on Form 20-F in Israel or the United States, or to serve process on our officers and directors.
We are incorporated in Israel. All of our executive officers and directors listed in this Annual Report on Form 20-F reside outside of the United States, and all of our assets and most of the assets of our executive officers and directors are located outside of the United States. Therefore, a judgment obtained against us or most of our executive officers and all of our directors in the United States, including one based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not be enforced by an Israeli court. It also may be difficult for you to effect service of process on these persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel.
Your rights and responsibilities as a shareholder will be governed by Israeli law which may differ in some respects from the rights and responsibilities of shareholders of U.S. companies.
We are incorporated under Israeli law. The rights and responsibilities of the holders of our shareholders are governed by our Amended and Restated Articles of Association and Israeli law. These rights and responsibilities differ in some respects from the rights and responsibilities of shareholders in typical U.S.-based corporations. In particular, a shareholder of an Israeli company has a duty to act in good faith toward the company and other shareholders and to refrain from abusing its power in the company, including, among other things, in voting at the general meeting of shareholders on matters such as amendments to a company’s articles of association, increases in a company’s authorized share capital, mergers and acquisitions and interested party transactions requiring shareholder approval. In addition, a shareholder who knows that it possesses the power to determine the outcome of a shareholder vote or to appoint or prevent the appointment of a director or executive officer in the company has a duty of fairness toward the company. There is limited case law available to assist us in understanding the implications of these provisions that govern shareholders’ actions. These provisions may be interpreted to impose additional obligations and liabilities of our shareholders that are not typically imposed on shareholders of U.S. corporations.
Provisions of Israeli law may delay, prevent or otherwise impede a merger with, or an acquisition of, our Company, which could prevent a change of control, even when the terms of such a transaction are favorable to us and our shareholders.
Israeli corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to these types of transactions. For example, a merger may not be consummated unless at least 50 days have passed from the date that a merger proposal was filed by each merging company with the Israel Registrar of Companies and at least 30 days from the date that the shareholders of both merging companies approved the merger. In addition, a majority of each class of securities of the target company must approve a merger. Moreover, a full tender offer can only be completed if the acquirer receives at least 95% of the issued share capital; provided that, pursuant to an amendment to the Companies Law, 5759-1999, as amended, or the Israeli Companies Law, effective as of May 15, 2011, a majority of the offerees that do not have a personal interest in such tender offer shall have approved the tender offer; except that, if the total votes to reject the tender offer represent less than 2% of our issued and outstanding share capital, in the aggregate, approval by a majority of the offerees that do not have a personal interest in such tender offer is not required to complete the tender offer, and the shareholders, including those who indicated their acceptance of the tender offer, may, at any time within six months following the completion of the tender offer, petition the court to alter the consideration for the acquisition (unless the acquirer stipulated in the tender offer that a shareholder that accepts the offer may not seek appraisal rights and the acquirer or the company published all required information with respect to the tender offer prior to the tender offer’s response date).
Furthermore, Israeli tax considerations may make potential transactions unappealing to us or to our shareholders whose country of residence does not have a tax treaty with Israel exempting such shareholders from Israeli tax. For example, Israeli tax law does not recognize tax-free share exchanges to the same extent as U.S. tax law. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent on the fulfillment of numerous conditions, including, in some cases, requirement for a holding period of two years from the date of the transaction during which sales and dispositions of shares of the participating companies are restricted. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires, the tax becomes payable even if no actual disposition of the shares has occurred.
These and other similar provisions could delay, prevent or impede an acquisition of us or our merger with another company, even if such an acquisition or merger would be beneficial to us or to our shareholders. See “Description of the Offered Securities—Articles of Association.”
37
Inflation could adversely affect our business and results of operations.
While inflation in the United States and global markets has been relatively low in recent years, during 2021 and 2022, the economy in the United States and global markets encountered a material increase in the level of inflation. The impact of any resurgence of COVID-19, geopolitical developments such as the Russia-Ukraine conflict and the war between Israel and Hamas and global supply chain disruptions continue to increase uncertainty in the outlook of near-term and long-term economic activity, including whether inflation will continue and how long, and at what rate. Increases in inflation raise our costs for commodities, labor, materials and services and other costs required to grow and operate our business, and failure to secure these on reasonable terms may adversely impact our financial condition. Additionally, increases in inflation, along with the uncertainties surrounding COVID-19, including any potential resurgence thereof, geopolitical developments and global supply chain disruptions, have caused, and may in the future cause, global economic uncertainty and uncertainty about the interest rate environment, which may make it more difficult, costly or dilutive for us to secure additional financing. A failure to adequately respond to these risks could have a material adverse impact on our financial condition, results of operations or cash flows.
Risks Related to Our Ordinary Shares and ADSs
Our business, operating results and growth rates may be adversely affected by current or future unfavorable economic and market conditions and adverse developments with respect to financial institutions and associated liquidity risk.
Our business depends on the economic health of the global economies. If the conditions in the global economies remain uncertain or continue to be volatile, or if they deteriorate, including as a result of the impact of military conflict, such as the war between Russia and Ukraine and Israel and Hamas, terrorism or other geopolitical events, our business, operating results and financial condition may be materially adversely affected.
In addition, increases in inflation raise our costs for commodities, labor, materials and services and other costs required to grow and operate our business, and failure to secure these on reasonable terms may adversely impact our financial condition. Additionally, increases in inflation, along with the uncertainties surrounding COVID-19, geopolitical developments and global supply chain disruptions, have caused, and may in the future cause, global economic uncertainty and uncertainty about the interest rate environment, which may make it more difficult, costly or dilutive for us to secure additional financing. A failure to adequately respond to these risks could have a material adverse impact on our financial condition, results of operations or cash flows.
There can be no assurance that future credit and financial market instability and a deterioration in confidence in economic conditions will not occur. Our general business strategy may be adversely affected by any such economic downturn, liquidity shortages, volatile business environment or continued unpredictable and unstable market conditions. If the current equity and credit markets deteriorate, or if adverse developments are experienced by financial institutions, it may cause short-term liquidity risk and also make any necessary debt or equity financing more difficult, more costly, more onerous with respect to financial and operating covenants and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to alter our operating plans. In addition, there is a risk that one or more of our service providers, financial institutions, manufacturers, suppliers and other partners may be adversely affected by the foregoing risks, which could directly affect our ability to attain our operating goals on schedule and on budget.
There can be no assurance that we will not be a passive foreign investment company, or PFIC, for U.S. federal income tax purposes in 2024 or in any subsequent year. If we are a PFIC, there may be negative tax consequences for U.S. taxpayers that are holders of our ordinary shares, ADSs, Warrants, or Pre-funded Warrants.
We will be treated as a PFIC for U.S. federal income tax purposes in any taxable year in which either (i) at least 75% of our gross income is “passive income” or (ii) on average at least 50% of our assets by value produce passive income or are held for the production of passive income. Passive income for this purpose generally includes, among other things, certain dividends, interest, royalties, rents and gains from commodities and securities transactions and from the sale or exchange of property that gives rise to passive income. Passive income also includes amounts derived by reason of the temporary investment of funds, including those raised in a public offering. In determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
38
Based on our analysis of our income, assets, and operations, we may have been a PFIC for 2023. Because the PFIC determination is highly fact intensive, there can be no assurance that we will not be a PFIC in 2024 or in any other taxable year. Even if we determine that we are not a PFIC after the close of a taxable year, there can be no assurance that a court or the U.S. Internal Revenue Service, or IRS, will agree with our conclusion. If we were to be characterized as a PFIC for U.S. federal income tax purposes in any taxable year during which a U.S. shareholder owns our ordinary shares, ADSs, Warrants, or Pre-funded Warrants, then “excess distributions” to such U.S. shareholder, and any gain realized on the sale or other disposition of our ordinary shares, ADSs, Warrants, or Pre-funded Warrants, as applicable, will be subject to special rules. Under these rules: (i) the excess distribution or gain would be allocated ratably over the U.S. shareholder’s holding period for the ordinary shares (or ADSs, Warrants, or Pre-funded Warrants, as the case may be); (ii) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (iii) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. Certain of the adverse consequences of PFIC status can be mitigated if a U.S. shareholder makes an election to treat us as a “qualified electing fund,” or QEF, or makes a “mark-to-market” election. In addition, if the IRS, determines that we are a PFIC for a year with respect to which we have determined that we were not a PFIC, it may be too late for a U.S. shareholder to make a timely QEF or mark-to-market election. U.S. shareholders who hold our ordinary shares, ADSs, Warrants or Pre-funded Warrants during a period when we are a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC in subsequent years, subject to exceptions for U.S. shareholders who made a timely QEF or mark-to-market election (to the extent available). A U.S. shareholder can make a QEF election by completing the relevant portions of and filing IRS Form 8621 in accordance with the instructions thereto. Upon request, we intend to annually furnish U.S. shareholders with information needed in order to complete IRS Form 8621 (which form would be required to be filed with the IRS on an annual basis by the U.S. shareholder) and to make and maintain a valid QEF election for any year in which we are a PFIC. There is no assurance, however, that we will have timely knowledge of our status as a PFIC, or that the information that we provide will be adequate to allow U.S. shareholders to make a QEF election. For further discussion, see “Item 10.E—Additional Information—Taxation—Certain Material U.S. Federal Income Tax Consequences—Passive Foreign Investment Company.”
If a United States person is treated as owning at least 10% of our shares, such holder may be subject to adverse U.S. federal income tax consequences.
If a United States person is treated as owning (directly, indirectly or constructively) at least 10% of the value or voting power of our shares, such person may be treated as a “United States shareholder” with respect to each “controlled foreign corporation” in our group (if any). A United States shareholder of a controlled foreign corporation may be required to annually report and include in its U.S. taxable income its pro rata share of “Subpart F income,” “global intangible low-taxed income” and investments in U.S. property by controlled foreign corporations, whether or not we make any distributions, and may be subject to tax reporting obligations. An individual that is a United States shareholder with respect to a controlled foreign corporation generally would not be allowed certain tax deductions or foreign tax credits that would be allowed to a United States shareholder that is a U.S. corporation. A failure to comply with these reporting obligations may subject you to significant monetary penalties and may prevent the statute of limitations with respect to your U.S. federal income tax return for the year for which reporting was due from starting. We cannot provide any assurances that we will assist any shareholder in determining whether such shareholder is treated as a United States shareholder with respect to any “controlled foreign corporation” in our group (if any) or furnish to any United States shareholders information that may be necessary to comply with the aforementioned reporting and tax paying obligations. A United States investor should consult its tax advisors regarding the potential application of these rules to its investment in our securities.
39
Provisions of our charter documents and Israeli law may discourage, delay, prevent or otherwise impede a merger with, or an acquisition of, our Company, which could prevent a change of control, even when the terms of such a transaction are favorable to us and our shareholders.
Provisions in articles of association may discourage, delay, prevent or otherwise impede a merger, acquisition or other change in control of us that shareholders may consider favorable, including transactions in which they might otherwise receive a premium for their ADSs. On February 20, 2020, we amended our articles of association to establish a staggered board of directors, which divides the board into three groups, with directors in each group serving a three-year term. The existence of a staggered board can make it more difficult for shareholders to replace or remove incumbent members of our Board of Directors. As such, these provisions could also limit the price that investors might be willing to pay in the future for our ADSs, thereby depressing the market price of our ADSs. In addition, because our Board of Directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our shareholders to replace or remove our current management by making it more difficult for shareholders to replace members of our Board of Directors.
In addition, Israeli corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to these types of transactions. For example, a merger may not be consummated unless at least 50 days have passed from the date that a merger proposal was filed by each merging company with the Israel Registrar of Companies and at least 30 days from the date that the shareholders of both merging companies approved the merger. In addition, a majority of each class of securities of the target company must approve a merger. Moreover, a full tender offer can only be completed if the acquirer receives at least 95% of the issued share capital; provided that, pursuant to an amendment to the Companies Law, 5759-1999, as amended, or the Israeli Companies Law, effective as of May 15, 2011, a majority of the offerees that do not have a personal interest in such tender offer shall have approved the tender offer; except that, if the total votes to reject the tender offer represent less than 2% of our issued and outstanding share capital, in the aggregate, approval by a majority of the offerees that do not have a personal interest in such tender offer is not required to complete the tender offer, and the shareholders, including those who indicated their acceptance of the tender offer, may, at any time within six months following the completion of the tender offer, petition the court to alter the consideration for the acquisition (unless the acquirer stipulated in the tender offer that a shareholder that accepts the offer may not seek appraisal rights).
Furthermore, Israeli tax considerations may make potential transactions unappealing to us or to our shareholders whose country of residence does not have a tax treaty with Israel exempting such shareholders from Israeli tax. For example, Israeli tax law does not recognize tax-free share exchanges to the same extent as U.S. tax law. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent on the fulfillment of numerous conditions, including a holding period of two years from the date of the transaction during which sales and dispositions of shares of the participating companies are restricted. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires, the tax becomes payable even if no actual disposition of the shares has occurred.
Our business could be negatively impacted by unsolicited takeover proposals, by shareholder activism or by proxy contests relating to the election of directors or other matters.
Our business could be negatively affected as a result of an unsolicited takeover proposal, by shareholder activism or a proxy contest. During 2019, an activist shareholder sought to make changes to our Board of Directors, among other matters, which ultimately resulted in us entering into a settlement agreement with the shareholder, and for which considerable costs were incurred and absorbed significant time and attention by management and the Board of Directors. A future proxy contest, unsolicited takeover proposal, or other shareholder activism relating to the election of directors or other matters would most likely require us to incur significant legal fees and proxy solicitation expenses and require significant time and attention by management and our Board of Directors. The potential of a proxy contest, unsolicited takeover proposal, or other shareholder activism could interfere with our ability to execute our strategic plan, give rise to perceived uncertainties as to our future direction, result in the loss of potential business opportunities or make it more difficult to attract and retain qualified personnel, any of which could materially and adversely affect our business and operating results.
40
Issuance of additional equity securities may adversely affect the market price of our ADSs or ordinary shares.
We are currently authorized to issue 5,000,000,000 ordinary shares, no par value. As of the date of this Annual Report on Form 20-F, we had 1,497,128,493 ordinary shares issued and outstanding, 316,800,000 shares in abeyance and we had no preferred shares outstanding. As of the date of this Annual Report, we also had warrants to purchase 1,852,010,606 ordinary shares and options to purchase 82,477,000 ordinary shares outstanding, of which options to purchase 33,623,875 ordinary shares are currently fully vested or vest within the next 60 days.
To the extent that ADSs or ordinary shares are issued or options and warrants are exercised, holders of our ADSs and our ordinary shares will experience dilution. In addition, in the event of any future issuances of equity securities or securities convertible into or exchangeable for ADSs or ordinary shares, holders of our ADSs or our ordinary shares may experience dilution. We also consider from time to time various strategic alternatives that could involve issuances of additional ADSs or ordinary shares, including but not limited to acquisitions and business combinations, but do not currently have any definitive plans to enter into any of these transactions.
We have no plans to pay dividends on our ordinary shares, and you may not receive funds without selling our ADSs or ordinary shares.
We have not declared or paid any cash dividends on our ordinary shares, nor do we expect to pay any cash dividends on our ordinary shares for the foreseeable future. We currently intend to retain any additional future earnings to finance our operations and growth and for future stock repurchases and, therefore, we have no plans to pay cash dividends on our ordinary shares at this time. Any future determination to pay cash dividends on our ordinary shares will be at the discretion of our Board of Directors and will be dependent on our earnings, financial condition, operating results, capital requirements, any contractual restrictions, and other factors that our Board of Directors deems relevant. Accordingly, you may have to sell some or all of our ADSs or ordinary shares in order to generate cash from your investment. You may not receive a gain on your investment when you sell our ADSs or ordinary shares and may lose the entire amount of your investment.
The market price of our ordinary shares and ADSs is subject to fluctuation, which could result in substantial losses by our investors.
The stock market in general and the market price of our ordinary shares on the TASE and our ADSs on the NYSE American is subject to fluctuation, and changes in our share price may be unrelated to our operating performance. The market price of our ordinary shares and ADSs are and will be subject to a number of factors, including:
| ● | announcements of technological innovations or new products by us or others; |
| ● | announcements by us of significant strategic partnerships, out-licensing, in-licensing, joint ventures, acquisitions or capital commitments; |
| ● | expiration or terminations of licenses, research contracts or other collaboration agreements; |
| ● | public concern as to the safety of drugs we, our licensees or others develop; |
| ● | general market conditions; |
| ● | the volatility of market prices for shares of biotechnology companies generally; |
| ● | success of research and development projects; |
| ● | success in clinical and preclinical studies; |
| ● | departure of key personnel; |
41
| ● | developments concerning intellectual property rights or regulatory approvals; |
| ● | variations in our and our competitors’ results of operations; |
| ● | changes in earnings estimates or recommendations by securities analysts, if our ordinary shares or ADSs are covered by analysts; |
| ● | changes in government regulations or patent decisions; |
| ● | developments by our licensees; and |
| ● | general market conditions and other factors, including factors unrelated to our operating performance, such as natural disasters and political and economic instability, including wars, terrorism, political unrest, results of certain elections and votes, emergence of a pandemic, or other widespread health emergencies (or concerns over the possibility of such an emergency, including for example, any resurgence of the COVID-19 outbreak), boycotts, adoption or expansion of government trade restrictions, and other business restrictions. |
These factors and any corresponding price fluctuations may materially and adversely affect the market price of our ordinary shares and our ADSs and result in substantial losses by our investors.
Additionally, market prices for securities of biotechnology and pharmaceutical companies historically have been very volatile. The market for these securities has from time to time experienced significant price and volume fluctuations for reasons unrelated to the operating performance of any one company. See also Risk Factors—Risks Relating to Ownership of Our Ordinary Shares and ADSs “Our business, operating results and growth rates may be adversely affected by current or future unfavorable economic and market conditions and adverse developments with respect to financial institutions and associated liquidity risk.” In the past, following periods of market volatility, shareholders have often instituted securities class action litigation and we have been named in the past in a lawsuit requesting recognition as a class action, in which we ultimately prevailed. If we were involved in securities litigation, it could have a substantial cost and divert resources and attention of management from our business, even if we are successful.
Future sales of our ordinary shares or ADSs could reduce the market price of our ordinary shares and ADSs.
Substantial sales of our ordinary shares or our ADSs either on the TASE or on the NYSE American, as applicable, may cause the market price of our ordinary shares or our ADSs to decline.
Sales by us or our security-holders of substantial amounts of our ordinary shares or our ADSs, or the perception that these sales may occur in the future, could cause a reduction in the market price of our ordinary shares or our ADSs. The issuance of any additional ordinary shares or ADSs, or any securities that are exercisable for or convertible into our ordinary shares or our ADSs, may have an adverse effect on the market price of our ordinary shares or our ADSs, as applicable, and will have a dilutive effect on our shareholders.
We may not satisfy the NYSE American requirements for continued listing. If we cannot satisfy these requirements, the NYSE American could delist our securities.
Our ADSs are listed on the NYSE American under the symbol “CANF.” To continue to be listed on the NYSE American, we are required to satisfy a number of conditions, including maintaining a share price and shareholders’ equity above certain thresholds. If we are delisted from the NYSE American, trading in our securities may be conducted, if available, on the OTC Markets or, if available, via another market. In the event of such delisting, our shareholders would likely find it significantly more difficult to dispose of, or to obtain accurate quotations as to the value of our securities, and our ability to raise future capital through the sale of our securities could be severely limited. In addition, if our securities were delisted from the NYSE American, our ADSs could be considered a “penny stock” under the U.S. federal securities laws. Additional regulatory requirements apply to trading by broker-dealers of penny stocks that could result in the loss of an effective trading market for our securities. Moreover, if our ADSs were delisted from the NYSE American, we will no longer be exempt from certain provisions of the Israeli Securities Law, and therefore will have increased disclosure requirements.
42
ADS holders are not shareholders and do not have shareholder rights.
The Bank of New York Mellon, as Depositary, delivers our ADSs. Each ADS represents two of our ordinary shares. ADS holders will not be treated as shareholders and do not have the rights of shareholders. The Depositary will be the holder of the shares underlying our ADSs. Holders of ADSs will have ADS holder rights. A deposit agreement among us, the Depositary, ADS holders and the beneficial owners of ADSs sets out ADS holder rights as well as the rights and obligations of the Depositary. New York law governs the deposit agreement and our ADSs. Our shareholders have shareholder rights. Israeli law and our Amended and Restated Articles of Association govern shareholder rights. ADS holders do not have the same voting rights as our shareholders. Shareholders are entitled to our notices of general meetings and to attend and vote at our general meetings of shareholders. At a general meeting, every shareholder present (in person or by proxy, attorney or representative) and entitled to vote has one vote. This is subject to any other rights or restrictions which may be attached to any shares. ADS holders may instruct the Depositary how to vote the number of deposited shares their ADSs represent. Otherwise, you won’t be able to exercise your right to vote unless you withdraw the shares. However, you may not know about the meeting enough in advance to withdraw the shares. The Depositary will notify ADS holders of shareholders’ meetings and arrange to deliver our voting materials to them if we ask it to. Those materials will describe the matters to be voted on and explain how ADS holders may instruct the Depositary how to vote. For instructions to be valid, they must reach the Depositary by a date set by the Depositary. The Depositary will try, as far as practical, subject to the laws of Israel and our Amended and Restated Articles of Association or similar documents, to vote or to have its agents vote the shares or other deposited securities as instructed by ADS holders. The Depositary will only vote or attempt to vote as instructed. We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the Depositary to vote your shares. In addition, the Depositary and its agents are not responsible for failing to carry out voting instructions or for the matter of carrying out voting instructions. This means that you may not be able to exercise your right to vote and there may be nothing you can do if your shares are not voted as requested.
ADS holders do not have the same rights to receive dividends or other distributions as our shareholders. Subject to any special rights or restrictions attached to a share, the directors may determine that a dividend will be payable on a share and fix the amount, the time for payment and the method for payment (although we have never declared or paid any cash dividends on our ordinary shares and we do not anticipate paying any cash dividends in the foreseeable future). Dividends and other distributions payable to our shareholders with respect to our ordinary shares generally will be payable directly to them. Any dividends or distributions payable with respect to ordinary shares deposited in the ADS facility will be paid to the Depositary, which has agreed to pay to ADS holders the cash dividends or other distributions it or the custodian receives on shares or other deposited securities, after deducting its fees and expenses. ADS holders will receive these distributions in proportion to the number of ordinary shares their ADSs represent. In addition, there may be certain circumstances in which the Depositary may not pay ADS holder’s amounts distributed by us as a dividend or distribution.
Our ordinary shares and our ADSs are traded on different markets and this may result in price variations.
Our ordinary shares have traded on the TASE since October 2005 and our ADSs have been listed on the NYSE American since November 2013. Trading on these markets will take place in different currencies (U.S. dollars on the NYSE American and NIS on the TASE), and at different times (resulting from different time zones, different trading days and different public holidays in the United States and Israel). The trading prices of our securities on these two markets may differ due to these and other factors. Any decrease in the price of our securities on one of these markets could cause a decrease in the trading price of our securities on the other market.
43
We have incurred significant additional increased costs as a result of the listing of our ADSs for trading on the NYSE American, and our management is required to devote substantial time to new compliance initiatives as well as to compliance with ongoing U.S. and Israeli reporting requirements.
As a public company in the United States, we incur additional significant accounting, legal and other expenses that we did not incur before becoming a reporting company in the United States. We also incur costs associated with corporate governance requirements of the SEC and the NYSE American Company Guide, as well as requirements under Section 404 and other provisions of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act as a result of our ADSs being listed on the NYSE American. These rules and regulations have increased our legal and financial compliance costs, introduced new costs such as investor relations, stock exchange listing fees and shareholder reporting, and made some activities more time consuming and costly. Since we are no longer an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, and are no longer able to take advantage of certain exemptions from various reporting requirements that are applicable to public companies that are “emerging growth companies” and that were applicable to us prior to January 1, 2020, we may incur additional compliance costs in the future. The implementation and testing of such processes and systems may require us to hire outside consultants and incur other significant costs. Any future changes in the laws and regulations affecting public companies in the United States and Israel, including Section 404 and other provisions of the Sarbanes-Oxley Act, the rules and regulations adopted by the SEC and the NYSE American Company Guide, as well as applicable Israeli reporting requirements, for so long as they apply to us, may result in increased costs to us as we respond to such changes. These laws, rules and regulations could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors, our Board committees or as executive officers.
As a foreign private issuer, we are permitted to follow certain home country corporate governance practices instead of applicable SEC and NYSE American requirements, which may result in less protection than is accorded to investors under rules applicable to domestic issuers.
As a foreign private issuer, we will be permitted to follow certain home country corporate governance practices instead of those otherwise required under the NYSE American Company Guide for domestic issuers. For instance, we may follow home country practice in Israel with regard to, among other things, composition and function of the audit committee and other committees of our Board of Directors and certain general corporate governance matters. In addition, in certain instances we will follow our home country law, instead of the NYSE American Company Guide, which requires that we obtain shareholder approval for certain dilutive events, such as an issuance that will result in a change of control of the company, certain transactions other than a public offering, involving issuances of a 20% or more interest in the company and certain acquisitions of the stock or assets of another company. We comply with the director independence requirements of the NYSE American Company Guide, including the requirement that a majority of the Board of Directors be independent. Following our home country governance practices as opposed to the requirements that would otherwise apply to a U.S. company listed on the NYSE American may provide less protection than is accorded to investors under the NYSE American Company Guide applicable to domestic issuers.
In addition, as a foreign private issuer, we are exempt from the rules and regulations under the U.S. Securities Exchange Act of 1934, as amended, or the Exchange Act, related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as domestic companies whose securities are registered under the Exchange Act.
Because we became a reporting company under the Exchange Act by means of filing a Form 20-F, we may have difficulty attracting the attention of research analysts at major brokerage firms.
Because we did not become a reporting company by conducting an underwritten initial public offering in the United States, we may have difficulty attracting the attention of security analysts at major brokerage firms in order for them to provide coverage of our company. The failure to receive research coverage or support in the market for our shares will have an adverse effect on our ability to develop a liquid market for our ADSs.
44
If we are unable to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act as they apply to a foreign private issuer that is listed on a U.S. exchange, or our internal control over financial reporting is not effective, the reliability of our financial statements may be questioned and our share price and the ADS price may suffer.
We are subject to the requirements of the Sarbanes-Oxley Act. Section 404 of the Sarbanes-Oxley Act requires companies subject to the reporting requirements of the U.S. securities laws to do a comprehensive evaluation of its and its subsidiaries’ internal control over financial reporting. To comply with this statute, we must document and test our internal control procedures and our management and issue a report concerning our internal control over financial reporting. In addition, as long as we do not become an accelerated or large accelerated filer, we are exempt from the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act. Under this exemption, our auditor will not be required to attest to and report on our management’s assessment of our internal control over financial reporting until the date we are no longer a non-accelerated filer. We will need to prepare for compliance with Section 404 by strengthening, assessing and testing our system of internal controls to provide the basis for our report. However, the continuous process of strengthening our internal controls and complying with Section 404 is complicated and time-consuming. Furthermore, as our business continues to grow both domestically and internationally, our internal controls will become more complex and will require significantly more resources and attention to ensure our internal controls remain effective overall. During the course of the testing, our management may identify material weaknesses or significant deficiencies, which may not be remedied in a timely manner to meet the deadline imposed by the Sarbanes-Oxley Act. If our management cannot favorably assess the effectiveness of our internal control over financial reporting, or our independent registered public accounting firm identifies material weaknesses in our internal controls, investor confidence in our financial results may weaken, and the market price of our securities may suffer.
ITEM 4. Information on the Company
A. History and Development of the Company
Our legal name is Can-Fite BioPharma Ltd. and our commercial name is “Can-Fite.” We are a company limited by shares organized under the laws of the State of Israel. Our principal executive offices are located at 26 Ben Gurion Street, Ramat Gan 5257346 Israel. Our telephone number is +972 (3) 924-1114.
We were founded on September 11, 1994 by Pnina Fishman, Ph.D., our Executive Chairman and Chief Scientific Officer, and Ilan Cohn, Ph.D., our member of the Board of Directors, under the name Can-Fite Technologies Ltd. On January 7, 2001, we changed our name to Can-Fite BioPharma Ltd. We completed our initial public offering in Israel in October 2005 and our ordinary shares are traded on the TASE under the symbol “CFBI.” On October 2, 2012, our ADSs began trading over the counter in the United States under the symbol “CANFY” and on November 19, 2013, our ADSs began trading on the NYSE American under the symbol “CANF.”
In November 2011, through a series of transactions, we spun-off our activity in the ophthalmic field to our now former subsidiary, OphthaliX, a Delaware corporation and successor-in-interest to Denali Concrete Management, Inc., a Nevada corporation, whose common shares were traded in the United States on OTC under the symbol “OPLI.” In the spin-off transactions, we granted an exclusive license for the use of our Piclidenoson drug candidate in the ophthalmic field, or the License Agreement, to Eye-Fite Ltd., an Israel limited company, or Eye-Fite, and transferred our issued and outstanding ordinary shares in Eye-Fite to OphthaliX in exchange for an 86.7% interest in OphthaliX. In connection with the spin-off transactions, OphthaliX completed a series of private placement financing transactions. Following the spin-off transactions and the private placement financing transactions, we held approximately 82% interest in OphthaliX. In July 2016, OphthaliX released top-line results from its Phase II clinical trial of Piclidenoson for the treatment of glaucoma. In this trial, no statistically significant differences were found between the Piclidenoson treated group and the placebo group in the primary endpoint of lowering IOP. High IOP is a characteristic of glaucoma. Piclidenoson was found to have a favorable safety profile and was generally well-tolerated. Based on these overall results, OphthaliX saw no immediate path forward in glaucoma and ceased active business operations. Subsequently, on May 21, 2017, OphthaliX and a wholly owned private Israeli subsidiary of OphthaliX, Bufiduck Ltd, or the Merger Sub, and Wize Pharma Ltd., or Wize Israel, an Israeli company formerly listed on the TASE entered into an Agreement and Plan of Merger, or the Merger Agreement, providing for the merger of the Merger Sub with and into Wize Israel, with Wize Israel becoming a wholly-owned subsidiary of OphthaliX and the surviving corporation of the merger, or the Merger. On November 16, 2017, the Merger was completed. As a result of the Merger, our ownership of OphthaliX, immediately post-Merger, became approximately 8% of the outstanding shares of common stock. In addition, immediately prior to the Merger, OphthaliX sold on an “as is” basis to us all the ordinary shares of Eye-Fite in exchange for the irrevocable cancellation and waiver of all indebtedness owed by OphthaliX and Eye-Fite to us, including approximately $5 million of deferred payments owed by OphthaliX and Eye-Fite to us and, as part of the purchase of Eye-Fite, we also assumed certain accrued milestone payments in the amount of $175,000 under a license agreement previously entered into with NIH. In addition, that certain License Agreement granted to OphthaliX by us and a related services agreement was terminated.
45
Our capital expenditures for the years ended December 31, 2023, 2022 and 2021 were $2,000, $9,000 and $11,000, respectively. Our current capital expenditures are made solely within Israel and primarily consist of the acquisition of computers and related communications equipment. Such capital expenditures are financed internally.
We use our website (http://www.canfite.com) as a channel of distribution of Company information. The information we post on our website may be deemed material. Accordingly, investors should monitor the website, in addition to following our press releases, SEC filings and public conference calls and webcasts. The contents of our website are not, however, a part of this Annual Report on Form 20-F.
B. Business Overview
We are an advanced clinical-stage biopharmaceutical company that develops orally bioavailable small molecule therapeutic products for the treatment of cancer, liver and inflammatory diseases. Our platform technology utilizes the Gi protein associated A3 adenosine receptor, or A3AR, as a therapeutic target. A3AR is highly expressed in pathological body cells such as inflammatory and cancer cells, and has a low expression in normal cells, suggesting that the receptor could be a specific target for pharmacological intervention. Our pipeline of drug candidates are synthetic, highly specific agonists and allosteric modulators targeting the A3AR.
Our product pipeline is based on the research of Dr. Pnina Fishman, who investigated a clinical observation that tumor metastasis can be found in most body tissues, but are rarely found in muscle tissue, which constitutes approximately 60% of human body weight. Dr. Fishman’s research revealed that one reason that striated muscle tissue is resistant to tumor metastasis is that muscle cells release small molecules which bind with high selectivity to the A3AR. As part of her research, Dr. Fishman also discovered that A3ARs have significant expression in tumor and inflammatory cells, whereas normal cells have low or no expression of this receptor. The A3AR agonists and allosteric modulators, currently our pipeline of drug candidates, bind with high selectivity and affinity to the A3ARs and initiate down-stream signal transduction pathways resulting in apoptosis, or programmed cell death, of tumor and inflammatory cells and to the inhibition of inflammatory cytokines. Cytokines are proteins produced by cells that interact with cells of the immune system in order to regulate the body’s response to disease and infection. Overproduction or inappropriate production of certain cytokines by the body can result in disease. In addition, our product candidates also induce the production of positive cytokines such as granulocyte colony stimulating factor (G-CSF) and adiponectin which are responsible for the chemo-protective and liver-protective effects of the drugs on liver.
Our product candidates, CF101, CF102 and CF602, are being developed to treat oncological and inflammatory diseases, as well as erectile dysfunction. CF101, also known as Piclidenoson, is in an advanced stage of clinical development for the treatment of psoriasis. CF102, also known as Namodenoson, is being developed for the treatment of HCC and has orphan drug designation for this indication in the United States and Europe. Namodenoson was granted Fast Track designation by the FDA for patients with advanced HCC who failed first line treatment. Namodenoson is also being developed for the treatment of pancreatic cancer based on pre-clinical findings showing robust anti-pancreatic tumor growth. Due to the liver protective effect of Namidenoson, it is also being developed for the treatment of NASH (also known as MASH). , CF602 is our second generation allosteric drug candidate for the treatment of erectile dysfunction, which has shown efficacy in the treatment of erectile dysfunction in preclinical studies and we are investigating additional compounds, targeting A3AR, for the treatment of erectile dysfunction. Preclinical studies revealed that our drug candidates have potential to treat additional inflammatory diseases, such as Crohn’s disease, prostate cancer, oncological diseases, viral diseases, such as the JC virus, obesity and Lowe Syndrome.
We believe our pipeline of drug candidates represent a significant market opportunity. For instance, according to iHealthcareAnalyst, the psoriasis drug market is forecasted to be worth $11.3 billion by 2025. According to DelveInsight, the HCC drug market in the G8 countries (U.S., Germany, France, Italy, Spain, UK, Japan and China) is expected to reach $3.8 billion by 2027.
46
We have in-licensed an allosteric modulator of the A3AR, CF602 from Leiden University. In addition, we have out-licensed the following product candidates for indications that we are currently pursuing:
| ● | Piclidenoson for the treatment of (i) psoriasis to Cipher Pharmaceuticals, or Cipher, for Canada, (ii) psoriasis to Gebro Holding, or Gebro, for Spain, Switzerland and Austria, (iii) psoriasis to CMS Medical, or CMS, for China (including Hong Kong, Macao and Taiwan), (iv) psoriasis to Kyongbo Pharm Co. Ltd., or Kyongbo Pharm, for South Korea, (v) psoriasis to Ewopharma AG, or Ewopharma, for Central Eastern Europe, and (vi) osteoarthritis in companion animals including dogs and cats to Vetbiolix. |
| ● | Namodenoson for the treatment of (i) liver cancer and NASH to Chong Kun Dang Pharmaceuticals, or CKD, for South Korea, (ii) advanced liver cancer and NASH to CMS for China (including Hong Kong, Macao and Taiwan), and (iii) HCC, NASH and pancreatic cancer to Ewopharma, for Central Eastern Europe and Switzerland. |
Currently, (i) we are undertaking preparatory work for pivotal Phase III studies for Piclidenoson in the treatment of psoriasis, following meetings with the FDA & EMA, which we expect to commence in the second half of 2024 (ii) we are conducting a pivotal Phase III trial for Namodenoson in the treatment of advanced liver cancer which is enrolling patients, (iii) we are conducting a Phase IIb study of Namodenoson in the treatment of NASH which is enrolling patients, (iv) we are undertaking preparatory work for Phase IIa study with Namodenoson for the treatment of pancreatic cancer, (v) we are investigating additional compounds, targeting the A3 adenosine receptor, for the treatment of erectile dysfunction, and (vi) we are conducting pre-clinical studies with formulations of cannabis components for the treatment of diseases in which there is an overexpression of A3AR. Since inception, we have incurred significant losses in connection with our research and development.
Moreover, we believe characteristics of Piclidenoson, as exhibited in our clinical studies to date, including its good safety profile, clinical activity, simple and less frequent delivery through oral administration and its low cost of production, position it well against the competition in psoriasis markets, where treatments, when available, often include injectable drugs, many of which can be highly toxic, expensive and not always effective.
Like Piclidenoson, Namodenoson has a good safety profile, is orally administered and has a low cost of goods, which we believe may position it well in the HCC market, where no drug has yet been approved by the FDA for patients with advanced liver cancer disease defined as Child Pugh B7. In addition, pre-clinical studies show Namodenoson’s novel mechanism of action which entails de-regulation of three key signaling pathways which mediate the etiology and pathology of NAFLD/NASH and are responsible for the anti-inflammatory, anti-steatotic and anti-fibrotic effect in the liver. Most recently, pre-clinical data support Piclidenoson’s potential utilization for the treatment of Lowe Syndrome and Namodenoson’s potential utilization as an anti-obesity drug.
Nevertheless, other drugs on the market, new drugs under development (including drugs that are in more advanced stages of development in comparison to our drug candidates) and additional drugs that were originally intended for other purposes, but were found effective for purposes targeted by us, may all be competitive to the current drugs in our pipeline. In fact, some of these drugs are well established and accepted among patients and physicians in their respective markets, are orally bioavailable, can be efficiently produced and marketed, and are relatively safe. None of our product candidates have been approved for sale or marketing and, to date, there have been no commercial sales of any of our product candidates.
47
Our Strategy
Our strategy is to build a fully integrated biotechnology company that discovers, in-licenses and develops an innovative and effective small molecule drug portfolio of ligands that bind to a specific therapeutic target for the treatment of liver, oncological and inflammatory diseases as well as erectile dysfunction. We continue to develop and test our existing pipeline, while also testing other indications for our existing drugs and examining, from time to time, the potential of other small molecules that may fit our platform technology of utilizing small molecules to target the A3AR. We generally focus on drugs with global market potential and we seek to create global partnerships to effectively assist us in developing our portfolio and to market our products. Our approach allows us to:
| ● | continue to advance our clinical and preclinical pipeline; |
| ● | test our products for additional indications which fit our molecules’ mechanism of action; |
| ● | identify other small molecule drugs or ligands; |
| ● | focus on our product candidates closest to realizing their potential; and |
| ● | avoid dependency on a small number of small molecules and indications. |
Using this approach, we have successfully advanced our product candidates for a number of indications into various stages of clinical development. Specific elements of our current strategy include the following:
Successful development of our existing portfolio of small molecule orally bioavailable drugs for the treatment of various diseases. We intend to continue to develop our existing portfolio of small molecule orally bioavailable drugs, both for existing targeted diseases, as well as other potential indications. Our drug development will continue to focus on cancer, liver and inflammatory diseases. We intend to focus most prominently on advancing our product candidates that are in the most advanced stages, i.e., psoriasis with respect to Piclidenoson, and HCC, NASH and pancreatic cancer with respect to Namodenoson.
Use our expertise with our platform technology to evaluate in-licensing opportunities. We continuously seek attractive product candidates and innovative technologies to in-license or acquire. We intend to focus on product candidates that would be synergistic with our A3AR expertise. We believe that by pursuing selective acquisitions of technologies in businesses that complement our own, we will be able to enhance our competitiveness and strengthen our market position. We intend to utilize our expertise in A3AR and our pharmacological expertise to validate new classes of small molecule orally bioavailable drugs. We will then seek to grow our product candidate portfolio by attempting to in-license those various candidates and to develop them for a variety of indications.
Primarily develop products that target major global markets. Our existing product candidates are almost all directed at diseases that have major global markets. Our intent is to continue to develop products that target diseases that affect significant populations using our platform technology. We believe these arrangements will allow us to share the high development cost, minimize the risk of failure and enjoy our partners’ marketing capabilities, while also enabling us to treat a more significant number of persons. We believe further that this strategy will increase the likelihood of advancing clinical development and potential commercialization of our product candidates.
Commercialize our product candidates throughout-licensing arrangements. We have entered into several out-licensing arrangements with leading pharmaceutical companies in the Far East, Canada and Europe. We intend to continue to commercialize our product candidates throughout-licensing arrangements with third parties who may perform any or all of the following tasks: completing development, securing regulatory approvals, manufacturing, marketing and sales. We do not intend to develop our own manufacturing facilities or sales forces. If appropriate, we may enter into co-development and similar arrangements with respect to any product candidate with third parties or commercialize a product candidate ourselves. We believe these arrangements will allow us to share the high development cost, minimize the risk of failure and enjoy our partners’ marketing capabilities. We believe further that this strategy will increase the likelihood of advancing clinical development and potential commercialization of our product candidates.
48
Our Product Pipeline
The table below sets forth our current pipeline of product candidates, including the target indication and status of each.
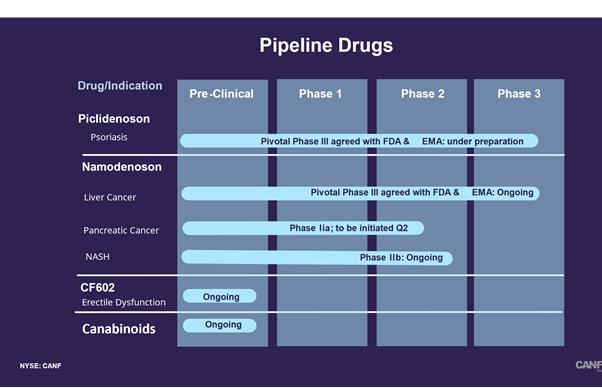
Piclidenoson (CF101)
Piclidenoson, our lead therapeutic product candidate, is in development for the treatment of inflammatory diseases. Piclidenoson is a highly-selective, orally bioavailable small molecule synthetic drug, which targets the A3AR. Based on our clinical studies to date, we believe that Piclidenoson has a favorable safety profile and significant anti-inflammatory effects as a result of its capability to inhibit the production of inflammatory cytokines, such as TNF-α, IL-6 and IL-1, chemokines and MMPs, by modulating key proteins such as NF-kB-;and PKB/AKT. Overall, these up-stream events result in apoptosis of inflammatory cells. See Figure 1 below. Piclidenoson’s anti-inflammatory effect is mediated via the A3AR, which is highly expressed in inflammatory cells.
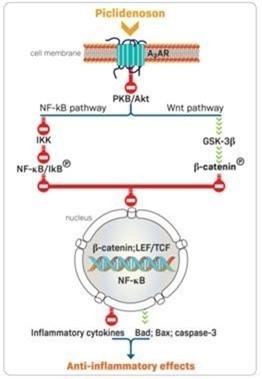
Figure 1: Piclidenoson anti-inflammatory mechanism of action
49
Psoriasis is an inflammatory hereditary disease that affects the skin. In psoriasis, immune cells move from the dermis to the epidermis, where they stimulate keratinocytes, or skin cells, to proliferate. DNA acts as an inflammatory stimulus to stimulate receptors which produce cytokines, such as IL-1, IL-6, and TNF-α, and antimicrobial peptides. These cytokines and antimicrobial peptides signal more inflammatory cells to arrive and produce further inflammation. In other words, psoriasis occurs when the immune system overreacts and mistakes the skin cells as a pathogen, and sends out faulty signals that speed up the growth cycle of skin cells. Normally, skin cells grow gradually and flake off approximately every four weeks. New skin cells grow to replace the outer layers of the skin as they shed. But in psoriasis, new skin cells move rapidly to the surface of the skin in days rather than weeks. They build up and form thick patches called plaques.
There are five types of psoriasis: plaque, guttate, inverse, pustular and erythrodermic. The most common form, plaque psoriasis, is commonly seen as red and white hues of scaly patches appearing on the top first layer of the epidermis, or skin. In plaque psoriasis, skin rapidly accumulates at these sites, which gives it a silvery-white appearance. Plaques frequently occur on the skin of the lower back, elbows and knees, but can affect any area, including the scalp, palms of hands, soles of feet and genitals. The plaques range in size from small to large. In contrast to eczema, psoriasis is more likely to be found on the outer side of the joint. Some patients, though, have no dermatological symptoms.
Psoriasis is a chronic recurring condition that varies in severity from minor localized patches to complete body coverage. Fingernails and toenails are frequently affected, known as psoriatic nail dystrophy, and can be seen as an isolated symptom. Psoriasis can also cause inflammation of the joints, which is known as psoriatic arthritis.
Pre-Clinical Studies with Piclidenoson
The information below is based on the various studies conducted with Piclidenoson, including preclinical studies. All of the studies were conducted by Can-Fite and/or by Can-Fite’s partners or affiliates.
Pre-clinical studies are a set of experiments carried out in animals to show that a certain drug does not evoke toxicity. Based on the animal studies and safety data, one can approach the FDA and request permission to conduct a Phase I study in human beings.
The toxicity of Piclidenoson has been evaluated following 28-day, 90-day, six-month and nine-month good laboratory practice repeated-dose toxicity studies in male and female mice (28-day, 90-day and six-month), dogs (single-dose only), and monkeys (28-day, 90-day and nine-month). Even though the dose of Piclidenoson in these studies was escalated to an exposure that is many folds higher than the dose used in human clinical studies, no toxic side effects were identified.
Effects on cardiovascular parameters were evaluated in conscious instrumented monkeys and anesthetized dogs. These studies demonstrated no significant cardiovascular risk.
Genotoxicity studies were conducted in bacterial and mammalian mutation assays in vitro (i.e., laboratory) and in an in vivo (i.e., animal) mouse micronucleus assay. These studies were all negative, indicating no deleterious action on cellular genetic material.
50
Reproductive toxicology studies that we completed in mice and rabbits did not reveal evidence of negative effects on male or female fertility. In mouse teratology studies, or studies for abnormalities of physiological developments, craniofacial and skeletal abnormalities were observed at doses greater than 10 mg/kg; however, no such effects were observed at 3 mg/kg demonstrating the safety of the drug in this concentration range. Teratogenicity, or any developmental anomaly in a fetus, was not observed in rabbits given doses (greater than 13 mg/kg) that induced severe maternal toxicity in such rabbits.
Studies of P450 enzymes, or enzymes that participate in the metabolism of drugs, showed that Piclidenoson caused no P450 enzyme inhibition, or increased drug activity, or induction, or reduced drug activity. Studies carried out with radiolabeled (C14) Piclidenoson in rats showed that the drug is excreted essentially unchanged. These studies also showed that the drug is widely distributed in all body parts, except the central nervous system.
In pre-clinical studies with skin cells, modeling psoriasis in humans, Piclidenoson destroyed pathological skin cells. We observed that in a cell culture of human HaCaT cells, incubated with Piclidenoson, cell apoptosis was induced with an increase in the caspase protein, known to mediate apoptotic responses.
Clinical Studies with Piclidenoson
The information below is based on the various studies conducted with Piclidenoson, including clinical studies in patients with autoimmune-inflammatory and ophthalmic diseases. All of the studies were conducted by Can-Fite and/or by Can-Fite’s partners or affiliates.
Phase I Clinical Studies of Piclidenoson
Piclidenoson has been studied comprehensively in normal volunteer trials to assess safety, pharmacokinetic metabolism and food interaction. Two Phase I studies in 40 healthy volunteers, single dose and repeated dose, indicated that Piclidenoson is rapidly absorbed (reaching a maximal concentration within one to two hours) with a half-life of eight to nine hours. Some mild adverse events (principally, increased heart rate) were observed at doses higher than single doses of 10.0 mg and twice-daily doses of 5.0 mg. Such increase in heart rate was not accompanied by any change in QT intervals. The drug showed linear kinetics, in that the concentration that results from the dose is proportional to the dose and the rate of elimination of the drug is proportional to the concentration, and low inter-subject variability, meaning that the same dose of the drug does not produce large differences in pharmacological responses in different individuals. A fed-fast Phase I study (with and without food) demonstrated that food causes some attenuation in Piclidenoson absorption; accordingly, Piclidenoson is administered to patients on an empty stomach in our trials. An additional Phase I study of the absorption, metabolism, excretion and mass balance of 4.0 mg (C14) Piclidenoson was conducted in six healthy male subjects and demonstrated that Piclidenoson was generally well-tolerated in this group.
Based on the findings from Phase I clinical studies, 4.0 mg twice daily, or BID, was selected as the upper limit for initial Phase II clinical trials.
Additionally, in preparation for Phase III studies of Piclidenoson to establish cardiac safety in humans prior to registration for marketing approval, we conducted a cardiodynamic trial that was a placebo-controlled crossover study using precise methodology to determine the effect of Piclidenoson on electrocardiograms of healthy volunteers. The primary objective of the trial was to assess whether Piclidenoson causes a delay in cardiac repolarization, as manifested by prolongation of the QT interval of the electrocardiogram. A drug-induced delay in cardiac repolarization creates an electrophysiological environment that can lead to the development of ventricular cardiac arrhythmias. In this study, Piclidenoson doses were up to 3-fold higher than the highest dose expected to be used in our registration-directed clinical trials. Trial results showed that our highest projected Piclidenoson dose had no clinically significant adverse electrocardiographic effects.
51
Phase II, Phase II/III and Phase III Clinical Studies of Piclidenoson
Piclidenoson has completed eleven Phase II studies, one Phase II/III study and three Phase III studies in different clinical indications including psoriasis, rheumatoid arthritis, glaucoma and dry eye syndrome, or DES, in approximately 1,700 patients. These studies indicate that Piclidenoson has a favorable safety profile at doses up to 4.0 mg BID for up to 48 weeks. In these studies, we did not observe a dose-response relationship between Piclidenoson and adverse events. Moreover, we did not observe any clinically significant changes in vital signs, electrocardiograms, blood chemistry or hematology. Furthermore, no new emergent safety signals have been observed in the completed or ongoing Phase III rheumatoid arthritis and psoriasis trials.
In June 2022, we announced positive top-line results from our Phase III COMFORT study of Piclidenoson in the treatment of moderate to serious psoriasis in which Piclidenoson met its primary endpoint. Previously, Piclidenoson given as a standalone therapy reached the primary endpoint in Phase II clinical studies in DES; however, a Phase III study of Piclidenoson for DES failed to reach the primary endpoint. We have observed positive data utilizing Piclidenoson as a standalone drug in a Phase IIa clinical study in rheumatoid arthritis. In this study, we also observed a significant direct correlation between A3AR expression prior to treatment and the patients’ responses to Piclidenoson. However, we did not fully attain the primary endpoint in this study as we did not observe a significant difference in responses between Piclidenoson and the placebo (which for this study was 0.1 mg of Piclidenoson). Moreover, two Phase IIb studies in rheumatoid arthritis utilizing Piclidenoson in combination with MTX, also failed to reach the primary endpoints. Based on this data, we believe that the failures in the Phase IIb studies in rheumatoid arthritis may have been due to low A3AR expression in the MTX-treated patients. A Phase IIb of Piclidenoson given as a standalone therapy in patients with A3AR expression levels above a certain threshold reached the primary endpoint in rheumatoid arthritis in December 2013. Piclidenoson has been tested in Phase II trials to establish dose and activity (first, orally administered capsules and then tablets in formulations of 1.0, 2.0 and 4.0 mg of Piclidenoson BID) in psoriasis (moderate to severe plaque psoriasis), rheumatoid arthritis and DES (moderate to severe). A Phase II/III study of Piclidenoson for psoriasis did not meet its primary endpoint although positive data from further analysis of the Phase II/III study suggested Piclidenoson as a potential systemic therapy for patients with moderate-severe psoriasis. In addition, a Phase II study of Piclidenoson for glaucoma showed no statistically significant differences between the Piclidenoson treated group and the placebo group in the primary endpoint of lowering IOP.
Psoriasis: The rationale for utilizing Piclidenoson to treat psoriasis stems from our pre-clinical pharmacology studies showing that Piclidenoson acts as an anti-inflammatory agent via the inhibition of inflammatory cytokines, including TNF-α, which plays a major role in the pathogenesis of psoriasis. In addition, the A3AR is over-expressed in the tissue and PBMCs of patients with psoriasis.
We completed an exploratory Phase II trial in ten European and Israeli medical centers involving 76 patients. This study was a randomized, double-blind, placebo controlled and included four cohorts of 1.0, 2.0, and 4.0 mg of Piclidenoson and a placebo for a 12-week period. The study objectives were efficacy and safety of daily doses of Piclidenoson administered orally in patients with moderate-to-severe plaque-type psoriasis and the efficacy endpoints were improvements in both the Psoriasis Area Sensitivity Index score, or PASI score, and the Physicians’ Global Assessment score, or PGA score. We concluded that Piclidenoson met such efficacy endpoints and was well-tolerated and effective in ameliorating disease manifestations in these patients. The patient group receiving 2.0 mg Piclidenoson BID showed progressive improvement over the course of the 12-week study in the PGA and PASI scores. Analysis of the mean change from baseline in the PASI score at week 12 revealed a statistically significant difference between the 2.0 mg Piclidenoson BID treated group and the placebo group (p<0.001 versus baseline and p=0.031 versus placebo). Analysis of the PGA score revealed that 23.5% of the patients treated with the 2.0 mg Piclidenoson BID achieved a score of 0 or 1, in comparison to 0% in the placebo group (p<0.05). The study also demonstrated linear improvement in patients in both PASI and PGA. See Figure 2. No drug-related serious adverse events were evident during the study.
52
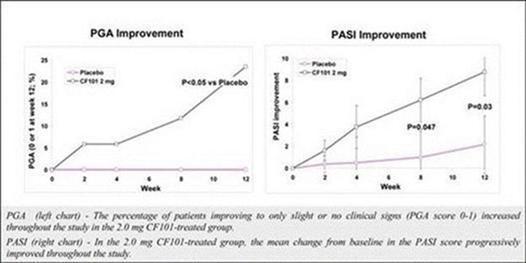
Figure 2: Psoriasis efficacy by PGA and PASI
Set forth below are representative pictures of a patient with plaque-type psoriasis on the upper and lower back treated with 2.0 mg Piclidenoson BID, both baseline and week 12.
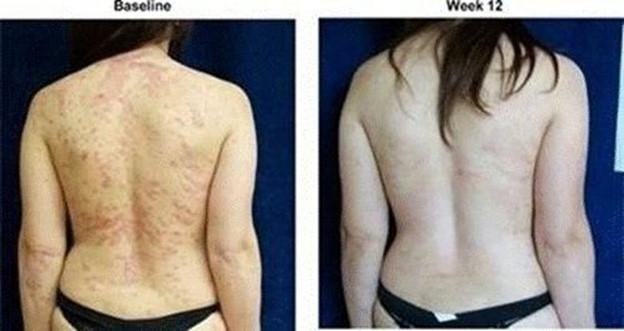
A comparison between baseline and week 12 of a patient treated with 2.0 mg CF101
53
In February 2015, we completed a Phase II/III randomized, double-blind, placebo-controlled, dose-finding study of the efficacy and safety of Piclidenoson administered daily orally in patients with moderate-to-severe plaque psoriasis. This clinical trial enrolled 326 patients in 17 clinical centers in the United States, Europe and Israel, of which 103 patients were enrolled in the first study cohort and were treated for 6 months and 223 patients were enrolled in the second study cohort and were treated for 8 months. The first study cohort was comprised of three arms with patients receiving: 1.0 mg of Piclidenoson; 2.0 mg of Piclidenoson; and placebo. All patients receiving placebo were switched to either 1.0 mg or 2.0 mg of Piclidenoson after 12 weeks. Based on a positive safety and efficacy interim analysis of the first 103 patients who completed 24 weeks of treatment in the trial, we decided to continue patient enrollment for the second stage of the study and the study protocol was amended to extend the Piclidenoson 2.0 mg BID and placebo administration for a period of 32 weeks. The positive clinical effects of the Piclidenoson 2.0 mg BID dose relative to a placebo were observed in a variety of standard psoriasis assessment parameters, including PASI 75 and PGA scores, with the responses accumulating steadily over the 24-week treatment period.
In March 2015, we announced the study did not meet its primary endpoint of a statistically significant improvement in the PASI 75 score relative to placebo after 12 weeks of treatment. Further analysis of the entire study period revealed that by 32 weeks of treatment with Piclidenoson, 33% of the patients achieved PASI 75 while the mean percent of improvement in PASI score was 57% (p<0.001). This was a statistically significant cumulative and linear improvement during weeks 16 to 32. Most significantly, by week 32 of the study, 20% of the study patients reached PASI 90, a result demonstrating a response rate of 90% clearing of skin lesions. PASI 90 is one of the most stringent and difficult to meet clinical endpoints for measuring responses to psoriasis treatments. Moreover, the PASI 90 subset analysis further suggests a higher and significant (p=0.026) Piclidenoson response rate of 27% among patients previously untreated with systemic psoriasis therapy compared to patients pre-treated with systemic drugs. We believe this presents the opportunity that Piclidenoson can be developed as a first-line systemic therapy for patients with moderate-severe psoriasis and for patients who do not want to be treated with the current systemic drugs due to safety issues.
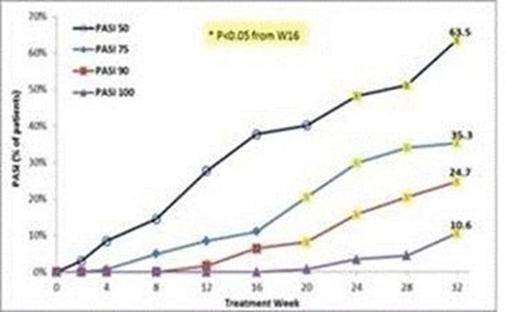
Figure 3: Linear Effect of Piclidenoson on PASI Scores through 32 Weeks of Treatment
In June 2022, we announced positive top-line results from our Phase III COMFORT study of Piclidenoson in the treatment of moderate to serious psoriasis in which Piclidenoson met its primary endpoint. The trial was a Phase III randomized, double-blind, placebo- and active-controlled study of the efficacy and safety of daily Piclidenoson (CF101) administered orally in patients with moderate-to-severe plaque psoriasis. The primary objectives of this study were to evaluate the efficacy of oral Piclidenoson 2 mg or 3 mg twice daily (BID) in patients with moderate-to-severe plaque psoriasis, compared with placebo, as determined by the proportion of subjects who achieve a PASI score response of ≥75% (PASI 75) at Week 16 (superiority); and evaluate the safety of oral Piclidenoson in this patient population. The secondary objectives of this study are to evaluate the efficacy of oral Piclidenoson 2 mg or 3 mg BID, compared with placebo, as determined by the proportion of subjects who achieve, respectively, PASI 50, Physician Global Assessment (PGA) score of 0 or 1, and improvement on the Psoriasis Disability Index (PDI) at Week 16 (superiority); evaluate the efficacy of oral Piclidenoson 2 mg or 3 mg BID, compared with Otezla (apremilast), as determined by the proportion of subjects who achieve PASI 75, PGA score of 0 or 1, PASI 50, and improvement in PDI at Weeks 16 and 32 (non-inferiority); and evaluate the efficacy and safety data for Piclidenoson through the extension period of up to 48 weeks of treatment.
54
The study data showed that patients treated with oral Piclidenoson 2 mg or 3 mg twice daily, had clinically equivalent efficacy responses. At week 16, patients receiving Piclidenoson 3mg demonstrated statistically significant improvement when compared with placebo, as measured by the PASI 75 response (representing a 75% reduction in psoriasis severity): Piclidenoson 3mg: 9.7% vs. placebo: 2.6% (P< 0.04). Secondary endpoint parameters at week 32 comparing Piclidenoson to the active control drug, Otezla, revealed inferiority with respect to PASI 75 (17% vs. 26.2%, respectively) and PASI 50 (34.1% vs. 49.5%, respectively), but revealed superiority of Piclidenoson as compared to Otezla in the Psoriasis Disability Index (PDI) (20.5% vs. 10.3%, respectively, P<0.05). A linear increase in the response of patients to Piclidenoson was achieved along the study period, on week 48 reaching PASI 50 in 90% of patients, PASI 90 in 10% of patients and PDI improvement in 60% of patients. Piclidenoson had an excellent safety profile overlapping that of the placebo treated patients, showing a better safety profile when compared to Otezla.
Further analysis of the study data showed that Piclidenoson had a significantly better tolerability profile than Otezla, as GI-related adverse events were 1% for Piclidenoson vs. 6% for Otezla, nervous system disorders were 0.7% for Piclidenoson, 9.9% for Otezla and 3.3% for the placebo. The discontinuation rate was significantly higher for Otezla vs. Piclidenoson. In the secondary endpoint of achieving a PASI 75 response (at week 32, in the whole patient population, Piclidenoson was inferior to Otezla; however, in a sub-group analysis of patients who had PASI>25 (more severe psoriasis) at baseline, Piclidenoson had a comparable response to Otezla.
During 2023, we submitted a market registration plan to the EMA and FDA for Piclidenoson in the treatment of moderate to severe psoriasis. Following positive responses from the FDA and EMA for its registration plan and pivotal Phase III study protocol for Piclidenoson in the treatment of moderate to severe psoriasis, we are preparing for study initiation. In addition, we received a positive response from the FDA on a pediatric study plan for the treatment of children suffering from psoriasis with Piclidenoson. Inclusion of adolescents for the psoriasis indication is expected to broaden the market. The FDA requested two Phase III safety and efficacy studies and to harmonize the requests of the EMA and the FDA, we plan to conduct two Phase III studies in parallel, including adolescent patients which is expected to commence in the second half of 2024. Upon positive conclusion of the Phase 3 program, we plan to submit a New Drug Application (NDA) to the FDA and Marketing Authorization Plan (MAA) to the EMA.
Rheumatoid Arthritis: We previously conducted a Phase IIa blinded to dose study in 74 patients with rheumatoid arthritis, randomized to receive Piclidenoson as a monotherapy in one of three doses-0.1 mg, 1.0 mg and 4.0 mg. The primary efficacy endpoint was ACR20 response at week 12, a criterion determined by the American College of Rheumatology that reflects 20% improvement in inflammation parameters. The study data revealed maximal response at the 1.0 mg group, showing 55.6% with ACR20, 33.3% with 50% improvement, or ACR50, and 11.5% with 70% improvement, or ACR70. Piclidenoson administered BID for 12 weeks resulted in improvement in signs and symptoms of rheumatoid arthritis and was well-tolerated.
Subsequently, two Phase IIb studies with Piclidenoson in combination with MTX were conducted. The study protocols were multicenter, randomized, double-blind, placebo-controlled, parallel-group and dose-finding to determine the safety and efficacy of daily Piclidenoson administered orally when added to weekly MTX in patients with active rheumatoid arthritis. The objectives of both studies were improvement in ACR20, ACR50, ACR70 and DAS28, or the Disease Activity Score of 28 Joints, and EULAR, or the European League Against Rheumatism, response criteria, as well as a positive safety profile. The trials’ primary endpoints were both ACR20. The first Phase IIb trial showed that the combined treatment had an excellent safety profile, but no significant ACR20 response was observed between the rheumatoid arthritis group treated with Piclidenoson and MTX and the group treated with MTX alone (the placebo group). However, the ACR50, ACR70 and the EULAR Good Values in the combined treatment group were higher than those of the MTX placebo group. The study also indicated that the 1.0 mg Piclidenoson dose was the most favorable dose, i.e., the dose yielded the highest ACR50 and EULAR Good Values as compared to the MTX placebo group. The most commonly reported adverse events in this study included nausea, dizziness, headache and common bacterial and viral infections and infestations. Following a decision of our Clinical Advisory Board in October 2007, an additional Phase IIb study was initiated. This study was conducted in medical centers in Europe and Israel and included 230 patients who received the drug orally BID (0.1 and 1.0 mg Piclidenoson tablets plus MTX versus a placebo, which was MTX alone) for 12 weeks. On April 30, 2009, we published preliminary results of the Phase IIb study, which were later confirmed as the final results, also indicating that the study’s objectives were not achieved. The most commonly reported adverse events in this study included nausea, myalgia and dizziness. The two Phase IIb studies failed to achieve the primary endpoint of ACR20. A cross study analysis of the three rheumatoid arthritis clinical studies revealed that in the first Phase IIa study, where Piclidenoson had been administered as a standalone drug, A3AR had been over-expressed in the patients’ PBMCs prior to Piclidenoson treatment, whereas A3AR had not been over-expressed in the Phase IIb patient population. We believe, based on the foregoing data, that there may be a direct and statistically significant correlation between A3AR over-expression at baseline and patients’ response to Piclidenoson, and that Piclidenoson should be administered as a standalone drug and not in combination with MTX. Furthermore, the correlation between A3AR expression levels prior to treatment and patients’ response to the drug suggest that the A3AR may be a predictive biomarker to be analyzed prior to Piclidenoson treatment.
55
Based on the results of the two Phase IIb studies, we conducted an additional Phase IIb clinical study with Piclidenoson as a stand-alone, monotherapy treatment and not in combination with MTX. The trial was a 12-week multicenter, randomized, double-blind, placebo-controlled, parallel-group study involving 79 patients to determine the safety and efficacy of Piclidenoson administered orally daily in patients with active rheumatoid arthritis and elevated baseline expression levels of the A3AR in PBMCs. Enrolled patients had high baseline A3AR biomarker expression (determined at 1.5-fold over a predetermined age-matched standard). This selection criteria was made following the findings during previous Phase IIa and IIb rheumatoid arthritis studies showing a positive correlation between A3AR expression at baseline and patients’ response to the drug, potentially rendering A3AR expression as a predictive biomarker. The primary objectives of this study were to determine the efficacy of oral Piclidenoson when administered daily as a standalone treatment for 12 weeks to patients with active rheumatoid arthritis and elevated baseline expression levels of the A3AR in the patients’ PBMCs, in comparison to a placebo treatment, and to assess the safety of daily oral Piclidenoson under the circumstances of the trial. In December 2013, we announced the results of the study in which Piclidenoson met all primary efficacy endpoints, showing statistically significant superiority over placebo in reducing signs and symptoms of rheumatoid arthritis as compared to the placebo. The treatment had an ACR20 response rate of 49% for Piclidenoson compared to 25% for placebo (p=0.035), an ACR50 response rate of 19% for Piclidenoson compared to 9% for placebo, and an ACR70 response rate of 11% for Piclidenoson compared to 3% for placebo. Similar to our observations in the previously reported Piclidenoson psoriasis trials, the response of patients with rheumatoid arthritis was cumulative over time, suggesting a consistent anti-inflammatory effect of Piclidenoson. Moreover, half of the rheumatoid arthritis patients treated with Piclidenoson showed clinically meaningful improvement. Piclidenoson was very well-tolerated and showed no evidence of immunosuppression, and there were no severe treatment-emergent adverse events during the study. A subgroup analysis of 16 patients with no prior systemic therapy showed a dramatic increase in the response showing ACR20 of 75%, ACR50 of 50%, and ACR70 of 50%. See Figure 7. We believe this may be related to the fact that in this patient population there is a full receptor expression since they had not been treated earlier with any systemic drugs.
At the end of 2017, we initiated the pivotal ACRobat Phase III trial of Piclidenoson to evaluate Piclidenoson as a first line treatment and replacement for MTX. The trial was a randomized, double-blind, active and placebo-controlled, parallel-group study in approximately 500 patients in Europe, Israel and Canada. The primary endpoint of ACRobat was low disease activity after 12 weeks of treatment in patients dosed with Piclidenoson compared to those dosed with MTX. Piclidenoson at 1.0 mg and 2.0 mg, or placebo, will be administered twice daily, and MTX or placebo will be administered once weekly. Secondary endpoints include disease activity remission at week 24, ACR 20/50/70 response rates, European League Against Rheumatism good and moderate response rates and change from baseline for disease activity and ACR responses. The total study duration was to be 24 weeks in order to provide more data on long term efficacy and safety. In October 2020, an independent data monitoring committee for an interim analysis recommended not to continue this study. Subsequently, we conducted a detailed analysis of the interim results which showed that although Piclidenoson efficacy was significantly superior to placebo, the study missed the primary endpoint which was non-inferiority vs. the comparator methotrexate. Accordingly, we decided to stop developing Piclidenoson for the treatment of rheumatoid arthritis to focus on other indications.
Additional Developments with Piclidenoson
Lowe Syndrome
In preclinical studies, Piclidenoson has been found to be effective in Lowe Syndrome, a rare genetic disease with no treatment available, and an estimated $100 million treatment market in the U.S. alone. Lowe Syndrome usually develops in the first year of life, causing brain abnormalities associated with intellectual disabilities and a life span shortened to less than 40 years.
The discovery of Piclidenoson’s efficacy in Lowe Syndrome was made by researchers at the University of Naples Federico II and The Telethon Institute of Genetics and Medicine in Italy after testing thousands of compounds in search of a treatment. Can-Fite and Fondazione Telethon signed an agreement outlining their collaboration for the development of Piclidenoson for the treatment of Lowe Syndrome. As a rare genetic disease in dire need of a treatment, Lowe Syndrome may qualify for an accelerated approval path, we plan to move into an advanced stage clinical study in this indication.
56
Topical Piclidenoson
In April 2022, we announced we are developing a topical psoriasis treatment with Piclidenoson. In a preclinical model, imiquimod-induced skin psoriasis, daily treatment with topical Piclidenoson significantly inhibited the disease as measured by PASI calculated based on observation of erythema, thickness, scaling, and a score of skin lesions.
Osteoarthritis
According to the Arthritis Foundation, osteoarthritis, or OA, is the most common arthritic disease. Currently, there is a shortage of effective drugs for treating OA patients. Piclidenoson has induced a significant anti-inflammatory effect in experimental animal models with respect to the treatment of OA. We have not yet filed an IND for this indication as Piclidenoson for the treatment of OA is not currently being clinically tested in the United States and there are no near-term plans to do so.
In November 2019, we announced that the U.S. Patent and Trademark Office, or the PTO, has issued to us Patent #10,265,337 titled “Use of A3 Adenosine Receptor Agonist in Osteoarthritis Treatment” for Piclidenoson for the treatment of osteoarthritis in mammals. We are evaluating potential partnerships with companies in the animal health pharmaceutical market that may in-license and develop Piclidenoson for the companion animal market, a substantial and rapidly growing global market.
In June 2021, we signed a development and commercialization agreement with Vetbiolix, a France-based veterinary biotech company, for the development of Piclidenoson for the treatment of osteoarthritis in companion animals including dogs and cats. During 2022, Vetbiolix completed dose-ranging pharmacokinetic (PK) studies in dogs and determined the optimal efficacy and safety dosage for a planned European multicentric clinical study. Piclidenoson was well tolerated, with the PK data proportional to dose. Pre-clinical studies were also conducted showing Piclidenoson has a very favorable safety profile. Based on these data, Vetbiolix is conducting a clinical study in dogs with osteoarthritis and results are expected during the second half of 2024. e.
Namodenoson (CF102)
Namodenoson is our second drug candidate and is under development for the treatment of HCC, hepatitis C virus, or HCV, or NAFLD, the precursor to NASH. Namodenoson is also a small, orally bioavailable molecule, and an A3AR agonist, with high affinity and selectivity to the A3AR. In comparison to the expression in adjacent normal liver tissue, the A3AR is over-expressed in tumor tissues of patients with HCC, and the over-expression is also reflected in the patients’ PBMCs. A3AR over-expression in the patients’ tumor cells and PBMCs is attributed to high expression of certain A3AR transcription factors. The binding of Namodenoson to the A3AR results in down-regulation, or a decrease in the quantity of a cellular component, such as the number of receptors on a cell’s surface, of certain A3AR transcription factors. Our studies have shown that this down-regulation leads to apoptosis of HCC cells. In our pre-clinical and clinical studies, Namodenoson demonstrated anti-cancer, anti-viral and liver protective effects. As a result, we believe that Namodenoson can be used to treat a variety of oncological and liver-related diseases and viruses.
In February 2012, the FDA granted an orphan drug status for the active moiety, or the part of the drug that is responsible for the physiological or pharmacological action of the drug substance, of Namodenoson for the treatment of HCC. Subsequently, in October 2015, the EMA granted Namodenoson orphan drug designation for the treatment of HCC.
57
An orphan drug designation is a special designation for drug approval and marketing. The special designation is granted to companies that develop a given drug for unique populations and for incurable and relatively rare diseases. The FDA orphan drug designation program provides orphan status to drugs and biologics, which are intended for the safe and effective treatment, diagnosis or prevention of rare diseases or disorders that affect fewer than 200,000 people in the United States and in the EU not more than 5 per 10,000. Orphan drug designations have enabled companies to achieve medical breakthroughs that may not have otherwise been achieved due to the economics of drug research and development as this status lessens some of the regulatory burdens, for approval, including statistical requirements for efficacy, safety and stability, in an effort to maintain development momentum. Orphan drug designation also results in additional marketing exclusivity and could result in certain financial incentives.
In September 2015, the FDA granted Fast Track designation to Namodenoson as a second line treatment to improve survival for patients with advanced HCC who have previously received Nexavar (sorafenib). Fast Track, aimed at getting important new drugs that meet an unmet need to patients earlier, is expected to expedite the development of Namodenoson. Drugs that receive Fast Track designation benefit from more frequent meetings and communications with the FDA to review the drug’s development plan to support approval. It also allows us to submit parts of the NDA on a rolling basis for review as data becomes available.
Israel’s Ministry of Health has previously approved Namodenoson for Compassionate Use for HCC.
Set forth below are general descriptions of the diseases with respect to which Namodenoson has underwent or is currently undergoing or being prepared for clinical trials.
HCC: HCC is an oncological disease characterized by malignant tumors that grow on the surface or inside of the liver. This type of tumor is refractory to chemotherapy and to other anti-cancer agents. HCC, like any other cancer, develops when there is a mutation to the cellular machinery that causes the cell to replicate at a higher rate and/or results in the cell avoiding apoptosis. Chronic infections of Hepatitis B and/or C can aid the development of HCC by repeatedly causing the body’s own immune system to attack the liver cells, some of which are infected by the virus. While this constant cycle of damage followed by repair can lead to mistakes during repair which in turn lead to carcinogenesis, this hypothesis is more applicable, at present, to HCV. Chronic HCV causes HCC through cirrhosis. In chronic Hepatitis B, however, the integration of the virus into infected cells can directly induce a non-cirrhotic liver to develop HCC. Alternatively, repeated consumption of large amounts of ethanol can have a similar effect.
Hepatitis C: HCV is an infectious disease affecting primarily the liver, caused by the Hepatitis C virus. The infection is often asymptomatic, but chronic infection can lead to scarring of the liver and ultimately to cirrhosis, which is generally apparent after many years, and chronic liver disease. The virus also increases the chance for HCC development. In some cases, those with cirrhosis will develop liver failure, liver cancer or life-threatening esophageal and gastric varices, or dilated submucosal veins, which can be life-threatening. HCV is spread primarily by blood-to-blood contact often associated with intravenous drug use, poorly sterilized medical equipment, transfusions, and erectile intercourse.
NAFLD/NASH: NASH (or MASH), also called “fatty liver disease,” is a condition in which fat builds up inside the liver causing inflammation. Prior to the presence of inflammation, the disease was simply referred to as NAFLD, the most common form of liver disorder in the United States. The accumulation of macroglobular fat inside the liver causes oxidative stress that reduces the efficiency of the liver and can lead to increased liver enzymes such as alanine aminotransferase and aspartate aminotransferase. Loss of liver efficiency and oxidative stress leads to inflammation, liver cell ballooning, and the development of NASH. Prolonged inflammation results in cirrhosis (scar tissue), liver failure, or liver cancer. There are currently no drugs approved for the treatment of NASH.
58
Pre-Clinical Studies with Namodenoson
In pre-clinical pharmacology studies, Namodenoson inhibited the growth of HCC via the induction of tumor cell apoptosis. In addition, in collaboration with leading virology labs, we observed that Namodenoson inhibited viral replication of HCV through the down-regulation of viral proteins. Both of these findings served as a basis to further explore development of this drug for HCC.
We conducted several pre-clinical studies demonstrating robust anti-inflammatory, anti-fibrogenic and anti-steatotic effects, supporting the development of Namodenoson for the NASH indication. Furthermore, the results indicated that Namodenoson was very well-tolerated.
In pre-clinical studies, we evaluated the toxicity, stability, metabolism and other safety parameters of Namodenoson at doses much higher than the doses that we currently administer to humans in our clinical trials of Namodenoson.
In preclinical studies, Namodenoson revealed its capability to act as an anti NAFLD/NASH agent and data has shown as follows:
| ● | In the STAM model, Namodenoson significantly decreased the non-alcoholic fatty liver disease (NAFLd) activity score, NAS, demonstrating anti-inflammatory and anti steatotic effects. |
| ● |
In the carbon tetrachloride (ccl4) model, Namodenoson reversed alanine aminotransferase (ALT) to normal values and significantly improved liver inflammation and fibrosis, as well as the adiponectin and leptin levels. |
| ● | Namodenoson mechanism of action entailed de-regulation of the Wnt/β-catenin pathway in the liver extracts of the ccl4 model mice and in the LX2 HScs, manifested by a decreasing the expression of phosphoinositide 3-kinase (PI3K), NF-κB. |
Clinical Studies of Namodenoson
The information discussed below is based on the various studies conducted by Can-Fite with Namodenoson, including clinical studies in patients with oncological and liver-related diseases and viruses.
59
Phase I Clinical Study
Namodenoson completed a Phase I double-blind, randomized, placebo-controlled, ascending single dose trial to evaluate the safety, tolerability, and pharmacokinetics of orally administered Namodenoson in healthy volunteers. The study was conducted in the United States under an open IND. Namodenoson was found to be safe and well-tolerated with a half-life time of 12 hours. See Figure 8.
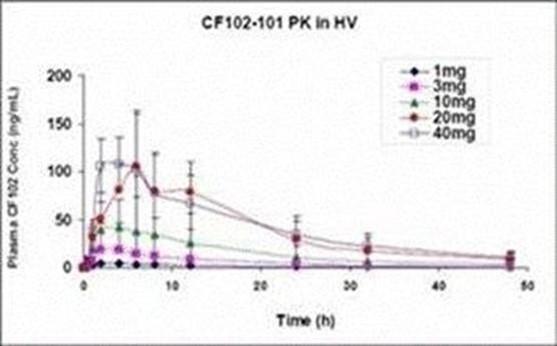
Figure 8: Half-life of orally administered Namodenoson - Phase I Clinical Study
Phase I/II and Phase II Clinical Studies
HCC
Namodenoson completed two Phase I/II studies in Israel, one in patients with HCC and another in patients with HCV. The HCC Phase I/II study was an open-label, dose-escalation study evaluating the safety, tolerability, pharmacokinetics and pharmacodynamics of orally administered Namodenoson in patients with advanced HCC. The primary objectives of the study were to determine the safety and tolerability, dose-limiting toxicities, maximum tolerated dose, and recommended Phase II dose of orally administered Namodenoson in patients with advanced HCC; and to assess the repeat-dose pharmacokinetics behavior of Namodenoson in those patients. The secondary objectives were to document any observed therapeutic effect of Namodenoson in patients with HCC and to evaluate the relationship between PBMCs and the A3AR expression at baseline, as a biomarker, and the effects of Namodenoson in patients with HCC. The study included 18 patients, nine of which were also carriers of HCV. The initial dose of Namodenoson was 1.0 mg BID, with planned dose escalations in subsequent cohorts to 5.0 and 25.0 mg BID. This Phase I/II study achieved its objectives, showing a good safety profile, or no material differences versus a placebo with respect to observed and patient-indicated side effects, for Namodenoson and a linear pharmacokinetic drug profile, with no dose-limiting toxicities at any dose level. The median overall survival time for the patients in this study was 7.8 months, which is encouraging data considering that (i) 67% of the patient population in the study had previously progressed on Nexavar, produced by Onyx Pharmaceuticals and Bayer, and that Namodenoson was a second line therapy for these patients and (ii) 28% of the patient population were Child-Pugh Class B patients (patients classified on the Child Pugh scoring system for chronic liver disease as having significantly impaired liver function) whose overall survival time is usually 3.5 to 5.5 months. Accordingly, we may also consider Namodenoson as a drug to be developed for this patient sub-population of Child-Pugh Class B patients. Namodenoson had no adverse effect on routine measures of liver function over a six-month period in 12 patients treated for at least that duration. These findings are consistent with our pre-clinical Namodenoson data which demonstrated a protective effect on normal liver tissue in an experimental model of liver inflammation. As such, Namodenoson may potentially be a safer alternative to patients with cirrhosis and/or hepatic impairment. The study also demonstrated a direct relationship between A3AR expression at baseline and patients’ response to Namodenoson, suggesting A3AR as a predictive biological marker. We also observed a decrease in the viral load of seven out of nine patients who were also carriers of HCV. The most commonly reported adverse events included loss of appetite, ascites, nausea, diarrhea, constipation and pain. However, many of these events are expected in a population of patients with advanced HCC. The most frequently reported drug-related adverse events included diarrhea, fatigue, loss of appetite, pain and weakness.
60
Our second Phase I/II study was a randomized, double-blind, placebo-controlled, dose-escalation study evaluating the safety, tolerability, biological activity, and pharmacokinetics of orally administered Namodenoson in 32 subjects with chronic HCV genotype 1. Eligible subjects were assigned in a 3:1 ratio (eight subjects in each cohort) to receive QD or BID treatment (1.0, 5.0 and 25.0 mg of Namodenoson) for 15 days with oral Namodenoson or with a placebo. Dose escalation occurred in four sequential cohorts. The study’s primary objectives were to determine the safety and tolerability of orally administered Namodenoson in patients with chronic HCV genotype 1, to assess the effects on HCV load during 15 days of treatment with Namodenoson and to assess the repeat-dose pharmacokinetic behavior of Namodenoson under the conditions of this trial. The secondary objective of this trial was to perform an exploratory evaluation of the relationship between A3AR in PBMCs at baseline and the clinical effects of Namodenoson on the study’s patients. Following the decrease in HCV load that had been observed in HCV patients treated with Namodenoson in the parallel HCC study and the good safety profile of Namodenoson, we received Israeli Institutional Review Board, or IRB, approval to extend the treatment period of the Phase I/II in patients with HCV to four months with the 1.0 mg dose vs. the placebo. The results of this Phase I/II HCV study demonstrated a good safety profile and a linear pharmacokinetic drug profile, however, no significant decrease in the viral load was observed. Notwithstanding, we did observe in the parallel HCC study that seven out of the nine patients with both HCC and HCV experienced a decrease in viral load and that these seven patients were treated with higher Namodenoson dosages than what was administered to the patients with chronic HCV genotype 1 only, and not HCC, possibly explaining the difference in results. The most commonly reported adverse events included loss of appetite, ascites, nausea, diarrhea, constipation and pain. However, many of these events are expected in a population of patients with advanced HCV. The most frequently reported drug-related adverse events included diarrhea, fatigue, loss of appetite, pain and weakness.
In 2019, we completed a Phase II study in HCC patients. In January 2013, as part of our preparatory work for such study, we announced that we believe that the optimal drug dose for the upcoming study is Namodenoson 25.0 mg. This dose was found to be the most effective dose out of the three dosages tested (1.0 mg, 5.0 mg and 25.0 mg) in the previous Phase I/II study. We filed a patent application protecting such optimal dose of Namodenoson for HCC. A publication summarizing the results of the Phase I/II study was published in “The Oncologist,” a leading oncology scientific journal.
The Phase II study was a randomized, double-blind, placebo controlled trial conducted in the United States, Europe and Israel to evaluate the efficacy and safety of Namodenoson as a second-line treatment for advanced HCC in subjects with Child-Pugh B who failed Nexavar as a first line treatment. Advanced HCC in patients with underlying cirrhosis is categorized into three subclasses based on the severity of cirrhosis, starting with Child Pugh A, or CPA, mostly treated with Nexavar and progressing to Child Pugh B, or CPB, and Child Pugh C, or CPC, for which there are no drugs on market with proven efficacy. In the study, we enrolled only patients with CPB stage liver cancer with CBP stage patients being further divided into three categories of increasing severity, namely CPB7, CPB8, and CPB9. These patients already failed first line Nexavar and were treated with Namodenoson (25mg), or placebo, as a second line treatment, twice daily, using a 2:1 randomization. The primary endpoint of the study was median overall survival. Secondary endpoints included progression free survival, partial response, and disease control rate. In March 2014, the study protocol was approved by the IRB at the Rabin Medical Center in Israel and in December 2014, we dosed the first patient at the study’s Israeli site. In the third quarter of 2017, we announced that we completed enrollment and randomization of all 78 patients and in March 2019, we announced top-line results.
While the study did not achieve the primary end point of overall survival in the whole population (n=78), superiority in overall survival was found in the largest study subpopulation of CPB7 (n=56) and in secondary end points in the whole population, including objective response measured by CT or MRI. Findings from the study include the following: (i) for the whole population (n=78), median overall survival was 4.1 months for Namodenoson vs. 4.3 months for placebo (HR: 0.82), (ii) pre-planned subpopulation analysis of the CPB7 patients (n=56), revealed that the Namodenoson treated group (n=34) showed median overall survival of 6.8 months vs 4.3 months in placebo (n=22) [HR: 0.77 (95% CI 0.49-1.40)]; similarly, for this subgroup of patients, progression free survival was 3.5 months for the Namodenoson treated group vs 1.9 (HR: 0.87) in the placebo group; (iv) 1-year survival in the CPB7 population was 44% for the Namodenoson treated group, as compared to 18% for patients dosed with placebo (p=0.028); (v) objective response in the whole patient population measured by CT or MRI, demonstrated that 9% treated by Namodenoson achieved partial response vs 0% in the placebo group, (vi) consistent with safety results from previously completed clinical trials, Namodenoson was generally well-tolerated, with no treated patients being withdrawn for toxicity and no cases of treatment-related deaths, (vii) disease control rate was 18.0% in the Namodenoson group vs 7.1% in the placebo group (p=0.013) after four months of treatment, (viii) 32.0% of patients treated with Namodenoson completed at least 12 months of treatment vs 14.3% who were treated with placebo (p=0.058), (vii) as of March 27, 2019, two patients in the Namodenoson group are ongoing after 30 months of treatment; these patients will continue to receive Namodenoson, and (ix) all nine patients with CBP9 cirrhosis, the most severe grade allowed into the trial, were randomly assigned to the Namodenoson treatment group (OS=3.5 months), a fact which has distorted the results in the whole population. Subgroup analyses using a variety of demographic and baseline disease characteristics, such as sex, performance status, and HCC disease status indicate the overall survival advantage of Namodenoson over placebo persists in the vast majority of the subgroups.
61
A paper titled “Namodenoson in Advanced Hepatocellular Carcinoma and Child-Pugh B Cirrhosis: Randomized Placebo-Controlled Clinical Trial” was published in the peer reviewed journal Cancers with respect to the Phase II HCC trial.
In February 2021, we announced that two patients continued to receive Namodenoson treatment from our Phase II study of HCC after nearly four years under an open label extension. Additional findings show disappearance of ascites, normal liver function and good quality of life. In one patient stable disease has been recorded with disappearance of peritoneal carcinomatosis. Namodenoson continued to demonstrate a good safety profile and was well tolerated with no severe adverse events reported. Furthermore, in December 2021, we reported that the last patient treated under the open label extension experienced a complete response and treatment is ongoing under a compassionate use program established in Romania in August 2022. Under treatment with Namodenoson, we reported that the patient survived seven years, during which time the clinical benefits of treatment have included the disappearance of ascites, normal liver function, and the disappearance of peritoneal carcinomatosis leading to complete clearance of all cancer lesions.
In October 2019, we held an End-of-Phase II Meeting with the FDA regarding our Phase II study of Namodenoson in the treatment of HCC. The purpose of the meeting was to review the Phase II study data with the FDA and to present our proposed Phase III study design to the regulatory agency. The FDA agreed with our proposed pivotal Phase III trial design to support an NDA submission and approval. Subsequently in June 2020, we concluded a meeting with the Scientific Advice Working Party of the EMA to discuss our planned Phase III study. Based on the input received from the FDA and EMA, we plan on conducting a Phase III, randomized, double blind, placebo controlled trial of Namodenoson, that will enroll approximately 450 patients with HCC and underlying CPB7 cirrhosis at multiple centers worldwide. Patients will be randomized to oral treatment with either Namodenoson 25 mg or matching placebo given twice daily. The primary efficacy endpoint of the trial is overall survival (OS), based on the favorable OS response seen in the Phase II trial in patients with HCC and CPB7 cirrhosis. Other oncology trial efficacy outcomes, such as tumor radiographic response rates and median progression-free survival, as well as standard safety parameters, will be assessed. The study is designed to support an NDA submission in the U.S. and a marketing authorization application, or MAA, in Europe. The Phase III study is currently open for enrollment.
In November 2019, we initiated a compassionate use program in Israel for Namodenoson in the treatment of HCC, the most common form of liver cancer. Our compassionate use program has enrolled and continues to treat patients. The compassionate use program, which enables liver cancer patients not enrolled in our clinical study to be treated with Namodenoson, is being administered by Dr. Salomon Stemmer, the Principal Investigator of the Company’s prior Phase II liver cancer study, and Professor at the Institute of Oncology, Rabin Medical Center, Israel. In August 2022, Namodenoson was approved for compassionate use in Romania.
NAFLD/NASH
We conducted a Phase II double-blind, placebo-controlled, dose-finding efficacy and safety study that enrolled 60 patients with NAFLD with or without NASH in three clinical sites in Israel including Hadassah Medical Center, Jerusalem; Rabin Medical Center, Petach Tikva; and Holy Family Hospital, Nazareth; with Prof. Rifaat Safadi as the Principal Investigator. Patients with evidence of an active inflammation were treated twice daily with 12.5 mg (n=21) or 25 mg (n=19) of oral Namodenoson vs. placebo (n=20). The patients were treated for 12 weeks and followed-up until week 16. The study’s end points included among others alanine aminotransferase (ALT) and aspartate aminotransferase (AST) blood level, % of liver fat, liver stiffness, serum adiponectin, leptin and patient’s weight loss. In April 2020 we announced top-line data and in June 2020 we announced our final data analysis. As a whole, the data show that Namodenoson at the 25 mg dose produced statistically significant results in all measures of efficacy, while also having a strong safety profile and well tolerated.
62
Therapeutically significant positive data and trends were found as follows:
Anti-Inflammatory Effect: Namodenoson significantly reduced two liver enzymes, AST and ALT, which are elevated in a damaged liver, and increased the anti-inflammatory cytokine adiponectin known also to act as an anti-fibrotic factor. Serum adiponectin levels increased in the 25 mg dose group by 220 ng/mL and the 12.5 mg dose group by 539 ng/mL (p=0.03). Adiponectin is a cytokine with robust anti-inflammatory and anti-fibrotic effects that is used as a biomarker in NAFLD/NASH trials. In addition, a dose response decrease compared to placebo was observed, indicating a reduction of hepatic inflammation was achieved: (i) % of patients who reached ALT normalization at follow up was 36.8% in the 25 mg dose vs. 10% in the placebo (p=0.038). In the 12.5 mg dose, 23.8% was recorded at follow up, (ii) ALT change from baseline (CFB) and % change from baseline (PCFB) - in the 25 mg group, CFB decreased by 15.4 U/L (p=0.066) and PCFB by 22% (p=0.079) compared to placebo (1.7 U/L, 3.0%, respectively); in the 12.5 mg group, a decrease CFB of 10.4 U/L and PCFB of 8.2% was recorded; and, (iii) AST CFB and PCFB - in the 25 mg group, CFB decreased by 8.1 U/L (p=0.03) and PCFB by 17.9% (p=0.05) compared to placebo (increase of 0.3 U/L, decrease of 1.3%, respectively). In the 12.5 mg group, a decrease in CFB of 7.4 U/L and PCFB of 8.1 % was recorded.
Reduction of Liver Steatosis: In the Namodenoson 25 mg treated group, the proportion of patients with high steatosis scores declined from 37.5% to 13.3% of the population, as compared to the placebo treated group in which the proportion of patients with high steatosis scores decreased from 37.5% to 35.3% of the population, with p=0.08. Steatosis was assessed by Controlled Attenuation Parameter (CAP) measurement of the FibroScan, a non-invasive marker of hepatic steatosis.
NASH - All Cases Resolved: 25% of patients randomized into the Namodenoson 25 mg dosed group had NASH at baseline, as compared to none in the placebo group, which comprised of patients who had NAFLD without NASH at baseline. Following 12 weeks of treatment, all NASH cases were resolved in patients treated with 25 mg of Namodenoson, as compared to new NASH that developed in the placebo group representing 5% of that population, with p<0.009. NASH was evaluated by FibroScan-AST (FAST) score, a noninvasive marker of NASH, the severe form of NAFLD (equivalent to biopsy findings of NAS≥4, F≥2), measured by FibroScan elastography, CAP and serum AST.
Decrease in Body weight: A linear decrease in body weight was recorded in the 25 mg and 12.5 mg Namodenoson groups.
A3 Adenosine Receptor (A3AR): The A3AR biomarker was stable, demonstrating the presence of the receptor after chronic treatment and reflecting the validity of the target.
Safety: Namodenoson continued to be safe and very well tolerated with no drug emergent severe adverse effects and no hepatotoxicity.
We are currently conducting a Phase IIb study Namodenoson in the treatment of NASH, having announced first patient enrollment in January 2022. The Phase IIb trial is a multicenter, randomized, double-blind, placebo-controlled study in subjects with biopsy-confirmed NASH. 140 subjects with NASH, as determined by a histological endpoint. Eligible subjects are randomly assigned in a 2:1 ratio to oral doses of Namodenoson 25 mg every 12 hours or a matching placebo for 36 weeks. The protocol was developed in conjunction with Dr. Scott Friedman, Chief of the Division of Liver Diseases at the Icahn School of Medicine at Mount Sinai in New York, and Dr. Stephen A. Harrison, Medical Director of Pinnacle Clinical Research, both of whom were involved in the design of Namodenoson’s Phase II study in NAFLD/NASH.
63
Pancreatic Cancer
In January 2023, we announced that in pre-clinical studies, Namodenoson significantly inhibits the growth of pancreatic carcinoma as a stand-alone treatment. In combination with the leading chemotherapy used in pancreatic cancer, gemcitabine, Namodenoson demonstrated a significant additive effect. These pre-clinical studies were conducted on advanced pancreatic carcinoma patient cells. Namodenoson’s molecular mechanism of action in pancreatic cancer involves the regulation of the NF-κB /IκB /STAT3-mediated pathway.
We are currently preparing for an exploratory Phase IIa study that is designed to allow treatment of patients with pancreatic carcinoma who failed first line therapy. This is a multicenter open-label trial in patients with advanced pancreatic adenocarcinoma whose disease has progressed on at least first line therapy or who refuse standard treatment. The trial will evaluate the safety, clinical activity, and pharmacokinetics (PK) of Namodenoson in this population. All patients will receive oral Namodenoson 25 mg administered twice daily for consecutive 28-day cycles. Patients will be evaluated regularly for safety. Approximately 20 evaluable patients will be enrolled. The primary objective of this trial is to characterize the safety profile of Namodenoson and the secondary objective is to evaluate the clinical activity as determined by the Objective Response Rate (ORR) using Response Evaluation Criteria in Solid Tumors (RECIST 1.1), Progression-Free Survival (PFS), Disease Control Rate (DCR), Duration of Response (DoR), and Overall Survival (OS).
Additional Developments with Namodenoson
Anti-Obesity
In January 2019, we announced new pre-clinical findings demonstrating that Namodenoson, inhibits lipid production and fat accumulation in adipocytes (lipid producing cells). More specifically, Namodenoson showed a significant decrease in lipid production and fat accumulation utilizing 3T3-L1 adipocytes, functioning as lipid producing cells and are also responsible for fat storage. Namodenoson was also shown to inhibit the proliferation of adipocytes, further hampering the expansion of fat producing cells. These findings, together with the safety profile of Namodenoson, support its potential utilization as an anti-obesity drug. A patent application for the utilization of Namodenoson as an anti-obesity drug has been filed. In January 2020, we announced further pre-clinical data generated at the Hadassah Medical Center by Dr. Rifaat Safadi’s lab that demonstrated that Namodenoson induces weight loss in experimental models and normalizes glucose levels.
In December 2023, we reported that new data on Namodenoson’s anti-obesity mechanism of action which demonstrated that treatment of fat cells (3T3-L1 adipocytes) with Namodenoson leads to modulation of proteins that increase adiponectin level. Adiponectin is a regulator of fat production in the cells, resulting in the inhibition of fat levels. Furthermore, Namodenoson reduced body weight in an experimental animal model of obesity induced by a high fat diet. In a Phase IIa NASH study, in patients treated with Namodenoson, a 2.1% weight loss was observed after 3 months of treatment (Safadi at Al) and a significant decrease in serum adiponectin levels was found.
JC Virus
In April 2011, we announced that, in laboratory study, Namodenoson inhibited the reproduction of the JC virus, a type of polyomavirus, which is dormant in approximately 70% to 90% of the world population. However, in patients treated with biological drugs, including monoclonal antibody therapeutics, such as anti-TNFs or anti-CD20, JC virus replication may occur, resulting in development of progressive multifocal leukoencephalopathy, or PML, which is characterized by progressive damage or inflammation of the white matter of the brain and, eventually, death. The ability of Namodenoson to suppress the JC virus culture, as indicated in the laboratory study, may indicate that it may be used for the treatment of PML as a combination therapy with biological drugs. As Namodenoson is already in various stages of clinical development for other indications, its efficacy for this new application may be tested in clinical trials.
64
CF602
The allosteric modulator, CF602, is our third drug candidate in its pipeline. CF602 is an orally bioavailable small molecule, which enhances the affinity of the natural ligand, adenosine, to its A3AR. The advantage of this molecule is its capability to target specific areas where adenosine levels are increased. Normal body cells and tissues are refractory to allosteric modulators. This approach complements the basic platform technology of Can-Fite, utilizing the Gi coupled protein A3AR as a potent target in inflammatory diseases. CF602 has demonstrated proof of concept for anti-inflammatory activity in in vitro and in vivo studies performed by us.
During clinical studies conducted with our product candidates, other than CF602, patients suffering from erectile dysfunction reported that they returned to normal functioning following the treatment with such drugs. We believe that these findings are correlated with our platform technology, which is the targeting of the A3AR. Adenosine, like nitric oxide, is a potent and short-lived vaso-relaxant that functions via intracellular signaling (in particular, through cAMP) to promote smooth muscle relaxation. Recent studies conducted by others show that adenosine functions to relax the corpus cavernosum and thereby promote penile erection.
CF602 was tested in an experimental animal model of diabetic rats, which similar to diabetic patients, suffer from erectile dysfunction. Erectile dysfunction was assessed by monitoring the ratio between intra-cavernosal pressure, or ICP, and mean arterial pressure, or MAP, as a physiological index of erectile function. The ICP/MAP for the CF602 treated group improved by 118% over the placebo group. This data is similar to that achieved earlier by sildenafil (Viagra) in preclinical studies. In addition, treatment with CF602 reversed smooth muscle and endothelial damage, in a dose dependent manner, leading to the improvement in erectile dysfunction.
Further studies of CF602 have revealed that CF602 restores the impaired vascular endothelial growth factor system in the penis of diabetes mellitus rats, thereby inducing an increase in nitric oxide resulting in significant improvement of penile erection compared to placebo. This mechanism of action is similar to that of sildenafil, with CF602 demonstrating effects on erection superior to that demonstrated by sildenafil in animal studies. Among the most important factors to affect erectile function is nitric oxide, which is released by endothelial cells that line the corpus cavernosum and control smooth muscle relaxation and vascular inflow. It has been well established that release of nitric oxide is diminished in diabetes.
In addition, CF602 induced a dose-dependent, linear effect in a diabetic mellitus rat model after treatment with one single dose of CF602. One hour after dosing, erectile function was measured. Statistically significant full recovery from erectile dysfunction took place in rats treated with a 500 µ/kg dose.
In March 2021, we announced new data from a preclinical study of CF602 in the treatment of erectile dysfunction, or ED, in a diabetes experimental model. The study evaluated the efficacy of topically applied CF602 in a 4-cohort study with diabetic Sprague-Dawley (SD) rats receiving placebo; 100nM CF602; 500nM CF602. Naïve rats served as a comparative negative control. ED was assessed by tracing ICP under cavernous nerve stimulation. Treatment with CF602 at a dose of 500 nM resulted in statistically significant improvement of erectile dysfunction compared to vehicle treated controls when measured in a two-way ANOVA followed by Bonferroni multiple comparisons post-hoc analysis (p<0.001). The improvement was even better than recorded for the naïve animals group. Efficacy was dose-dependent.
According to the American Diabetes Association, approximately 30 million children and adults have diabetes mellitus in the United States. It is estimated that 35-75% of men with diabetes mellitus suffer from erectile dysfunction.
In January 2017, a patent was granted to us by the USPTO covering A3AR ligands for use in the treatment of erectile dysfunction. The patent addresses methods for treating erectile dysfunction with different A3AR ligands including our erectile dysfunction drug candidate, CF602. With this new broader patent protection, we made a strategic decision to investigate additional compounds, owned by us, for the most effective and safest profile in this indication and we are seeking to partner development of CF602.
65
Cannabinoid-Based Pharmaceuticals
In September 2019, we entered into a collaboration agreement with Univo, a medical cannabis company, to identify and co-develop specific formulations of cannabis components for the treatment of diseases in which there is an overexpression of A3AR. Based on our recent scientific findings, we have filed patents for the use of cannabinoid-based drugs to treat cancer, autoimmune, inflammatory and metabolic diseases. Our most recent pre-clinical research revealed that cannabis derived CBD enriched fractions, supplied by Univo, inhibit the expansion of human fat cells (pre-adipocytes) by 60%, a result that points towards the potential anti-obesity effect of this agent. Although it is already documented that cannabis derived compounds possess anti-obesity effect, the novel data presented by us demonstrates the anti-obesity effect at low nano-molar concentrations. Low CBD concentrations are known to be safe and well accepted in humans. Previously, we announced pre-clinical findings demonstrating CBD’s robust anti-neoplastic effect in pre-clinical studies against liver cancer. The studies were carried out on human liver cancer cells and utilized cannabinoid fractions enriched for CBD, in nano and pico molar concentrations. Marked inhibition of Hep-3b, liver cancer cell proliferation was noted and was mediated via the A3 adenosine receptor. As of December 31, 2020, our collaboration agreement with Univo expired.
In July 2020, we completed the development of a biological cell-based in vitro assay which can identify clinically active cannabis derived compounds that bind to and activate A3AR, thus enabling the development of pharmaceuticals that use a specific cannabis derived compound to treat a variety of diseases.
In December 2020, we received approval from the Medical Cannabis Unit of Israel’s Ministry of Health to conduct pre-clinical studies on the effect of nanomolar concentrations of cannabinoid fractions on the proliferation and functionality of cancer, inflammatory and adipocyte cells (fat cells). This regulatory approval allows us to advance our cannabinoid program by evaluating the effect of cannabis fractions at nanomolar concentrations binding with A3AR.
In February 2021, we announced the completion of a set of pre-clinical studies demonstrating that cannabis derived compounds bind to A3AR, mediating therapeutic effects. CBD rich T3/C15 cannabis fraction inhibited the proliferation of LX-2 hepatic-stellate cells, the liver cell type mediating the development of fibrosis. This inhibitory response was neutralized by an antagonist to the A3AR, demonstrating that the anti-fibrotic effect was mediated via the A3AR.
In April 2021, we announced the completion of a set of pre-clinical studies demonstrating that a CBD rich T3/C15 cannabis fraction inhibited the growth of liver HEP-3b hepatocellular carcinoma cells via the A3AR by inhibiting Wnt- and NF-kappa B-related regulatory pathways. The Wnt signaling pathway is known to be highly active in controlling the growth of liver cancer cells. An A3AR antagonist, MRS1523 reversed this effect demonstrating that the inhibitory effect is mediated via our target, A3AR.
Commercial Biomarker Test
In March 2015, we completed the development of a commercial predictive biomarker blood test kit for A3AR. The biomarker test can be used at any molecular biology lab, where a small blood sample from a prospective patient would be tested and within just a few hours, results indicate if the patient would benefit from treatment with our drugs, which are currently in clinical trials for rheumatoid arthritis, psoriasis, and liver cancer.
The USPTO previously issued to us a patent for the utilization of A3AR as a biomarker to predict patient response to our drug Piclidenoson in autoimmune inflammatory indications.
In-Licensing Agreements
The following is a summary description of our in-licensing agreement with Leiden University. Our previously granted license with NIH expired in June 2015 with the expiration of certain patents. The description provided below does not purport to be complete and is qualified in its entirety by the complete agreement, which is attached as an exhibit to this Annual Report on Form 20-F
66
Leiden University Agreements
On November 2, 2009, we entered into a license agreement, or the Leiden University Agreement, with Leiden University. Leiden University is affiliated with NIH and is the joint owner with NIH of the patents licensed pursuant to the Leiden University Agreement. The Leiden University Agreement grants an exclusive license for the use of the patents of several compounds, including CF602, that comprise certain allosteric compound drugs, and for the use, sale, production and distribution of products derived from such patents in the territory, i.e., China and certain countries in Europe (Austria, Belgium, Denmark, France, Germany, Italy, Spain, Sweden, Switzerland, Holland and England). Subject to certain conditions, we may sublicense the Leiden University Agreement. However, the U.S. government has an irrevocable, royalty-free, paid-up right to practice the patent rights throughout the territory on behalf of itself or any foreign government or international organization pursuant to any existing or future treaty or agreement to which the U.S. government is a signatory and the U.S. government may require us to grant sublicenses when necessary to fulfill health or safety needs.
Pursuant to the Leiden University Agreement, we are committed to make the following payments: (i) a one-time concession commission of 25,000 Euros; (ii) annual royalties of 10,000 Euros until clinical trials commence; (iii) 2% to 3% of net sales value, as defined in the Leiden University Agreement, received by us; (iv) royalties of up to 850,000 Euros based on certain progress milestones in the clinical stages of the products which are the subject of the patent under the Leiden University Agreement; and (v) if we sublicense the agreement, we will provide Leiden University royalties at a rate of 2-3% of net sales value, as defined in the Leiden University Agreement, and 10% of certain consideration received for granting the sublicense. In the event that we transfer to a transferee the aspect of our business involving the Leiden University Agreement, we must pay to Leiden University an assignment royalty of 10% of the consideration received for the transfer of the agreement. However, a merger, consolidation or any other change in ownership will not be viewed as an assignment of the agreement. In addition, we have agreed to bear all costs associated with the prosecution of the patents and patent applications to which we are granted a license under the Leiden University Agreement. As of December 31, 2022, we have paid approximately 155,000 Euros in royalties to Leiden University in connection with the Leiden University Agreement.
The Leiden University Agreement expires when the last of the patents expires in each country of the territory, unless earlier terminated in accordance with the terms of the Leiden University Agreement. The last of such patents is set to expire on 2027. The termination rights of the parties include, but are not limited to, (i) the non-defaulting party’s right to terminate if the defaulting party does not cure within 90 days of written notice identifying the default and requesting remedy of the same; and (ii) Leiden University’s right to terminate if we become insolvent, have a receiver appointed over our assets or initiate a winding-up. In addition, Leiden University may terminate the agreement when it is determined, in consultation with NIH, that termination is necessary to alleviate health and safety needs and certain other similar circumstances.
Out-Licensing and Distribution Agreements
The following are summary descriptions of certain out-licensing and distribution agreements of Piclidenoson and Namodenoson for the indications we are currently investigating.
Cipher Pharmaceuticals Agreement
On March 20, 2015, we entered into a Distribution and Supply Agreement with Cipher granting Cipher the exclusive right to distribute Piclidenoson in Canada for the treatment of psoriasis and rheumatoid arthritis. In 2020, we ended our development of Piclidenoson for rheumatoid arthritis.
Under the Distribution and Supply Agreement, we are entitled to CAD 1.65 million upon execution of the agreement plus milestone payments upon receipt of regulatory approval by the Therapeutic Products Directorate of Health Canada, or Health Canada, for Piclidenoson and the first delivery of commercial launch quantities as follows (i) CAD 1 million upon the first approved indication for either psoriasis or rheumatoid arthritis, and (ii) CAD 1 million upon the second approved indication for either psoriasis or rheumatoid arthritis. In addition, following regulatory approval, we shall be entitled to a royalty of 16.5% of net sales of Piclidenoson in Canada and reimbursement for the cost of manufacturing Piclidenoson. We are also entitled to a royalty payment for any authorized generic of Piclidenoson that Cipher distributes in Canada. To date, we have received a total of $1.3 million from Cipher in an upfront payment.
67
We are responsible for supplying Cipher with finished product for distribution and conducting product development activities while Cipher is responsible for distributing, marketing and obtaining applicable regulatory approvals in Canada. The Distribution and Supply Agreement has an initial term of fifteen years, automatically renewable for additional five-year periods and may be terminated in certain limited circumstances including certain breaches of the agreement and failure to achieve certain minimum quantities of sales during the contract period.
The timeline to regulatory submissions to Health Canada will be determined by the completion of the remaining clinical trial program.
CKD Agreement
On October 25, 2016, we entered into an exclusive Distribution Agreement with CKD for the exclusive right to distribute Namodenoson for the treatment of liver cancer in South Korea, upon receipt of regulatory approvals. On February 25, 2019, the Distribution Agreement was amended to expand the exclusive right to distribute Namodenoson for the treatment of NASH in South Korea. The Distribution Agreement further provides that we will deliver finished product to CKD and grant CKD a right of first refusal to distribute Namodenoson for other indications for which we develop Namodenoson.
The Distribution Agreement provides for up to $3,000,000 in upfront and milestone payments payable with respect to the liver cancer indication and up to $6,000,000 with respect to the NASH indication. In addition, we are entitled to a transfer price of the higher of the manufacturing cost plus 10% or 23% of net sales of Namodenoson following commercial launch in South Korea. To date, we have received a total of $2,000,000 from CKD, $1,500,000 in upfront payments for the expansion of CKD’s existing agreement with us to include the rights to distribute Namodenoson for the treatment of NASH in South Korea, and a further $500,000 for a milestone payment received in the third quarter of 2017 upon receipt by CKD of a positive result from the preliminary review by the MFDS on obtaining orphan drug designation in South Korea.
The Distribution Agreement has an initial term of 10 years from first commercial sale of Namodenoson for the treatment of liver cancer or SL and is renewable for additional 3-year periods unless either party gives notice of termination at least 6 months prior to the then current term. The Distribution Agreement may be terminated by CKD upon 30 days prior written notice if we fail to successfully complete our ongoing Phase II clinical trial for Namodenoson and we may terminate the Distribution Agreement upon 30 days prior written notice if certain commercialization milestones are not met by CKD or certain minimum quantities of sales are not made during the contract period. In addition, either party may terminate the Distribution Agreement in the event of an uncured material breach or insolvency.
Gebro Agreement
On January 8, 2018, we entered into a Distribution and Supply Agreement with Gebro, granting Gebro the exclusive right to distribute Piclidenoson in Spain, Switzerland, Liechtenstein and Austria for the treatment of psoriasis and rheumatoid arthritis. In 2020, we ended our development of Piclidenoson for rheumatoid arthritis.
Under the Distribution and Supply Agreement, we are entitled to €1,500,000 upon execution of the agreement plus milestone payments upon achieving certain clinical, launch and sales milestones, as follows: (i) €300,000 upon initiation of the ACRobat Phase III clinical trial for the treatment of rheumatoid arthritis and €300,000 upon the initiation of the COMFORT Phase III clinical trial for the treatment of psoriasis, (ii) between €750,000 and €1,600,000 following first delivery of commercial launch quantities of Piclidenson for either the treatment of rheumatoid arthritis or psoriasis, and (iii) between €300,000 and up to €4,025,000 upon meeting certain net sales. In addition, following regulatory approval, we shall be entitled to double digit percentage royalties on net sales of Piclidenoson in the territories and payment for the manufacturing Piclidenoson. To date, we have received a total of €2,100,000 from Gebro in upfront and milestone payments.
We are initially responsible for supplying Gebro with finished product for distribution and obtaining EMA and Swissmedic marketing approval while Gebro is responsible for distributing, marketing and obtaining pricing and reimbursement approvals in the territories. The Distribution and Supply Agreement has an initial term of fifteen years, automatically renewable for additional five-year periods and may be terminated in certain limited circumstances including certain breaches of the agreement and failure to achieve certain minimum quantities of sales during the contract period.
68
CMS Medical Agreement
On August 6, 2018, we entered into a License, Collaboration and Distribution Agreement with CMS for the exclusive right to develop, manufacture and commercialize Piclidenoson for the treatment of rheumatoid arthritis and psoriasis and Namodenoson for the treatment of HCC and NASH in China (including Hong Kong, Macau and Taiwan).
Under the License, Collaboration and Distribution Agreement, we are entitled to $2,000,000 upon execution of the agreement plus milestone payments of up to $14,000,000 upon achieving certain regulatory milestones and payments of up to $58,500,000 upon achieving certain sales milestones, as follows: (i) $500,000 upon the granting of the marketing authorization of Piclidenoson in the United States for rheumatoid arthritis; (ii) $500,000 upon the granting of the marketing authorization of Piclidenoson in the European Union for rheumatoid arthritis; (iii) $500,000 upon the granting of the marketing authorization of Piclidenoson in the United States for psoriasis; (iv) $500,000 upon the granting of the marketing authorization of Piclidenoson in the European Union for psoriasis; (v) $500,000 upon the granting of the marketing authorization of Namodenoson in the United States for HCC; (vi) $500,000 upon the granting of the marketing authorization of Namodenoson in the European Union for HCC; (vii) $500,000 upon the granting of the marketing authorization of Namodenoson in the United States for NAFLD/NASH; (viii) $500,000 upon the granting of the marketing authorization of Namodenoson in the European Union for NAFLD/NASH; (ix) $2,500,000 upon the issuance of an imported drug license permitting the product to be imported into and marketed in China, or the IDL and granting of marketing authorization of Piclidenoson in China for rheumatoid arthritis; (x) $2,500,000 upon the issuance of the IDL and granting of marketing authorization of Piclidenoson in China for psoriasis; (xi) $2,500,000 upon the issuance of the IDL and granting of marketing authorization of Namodenoson in China for HCC; (xii) $2,500,000 upon the issuance of the IDL and granting of marketing authorization of Namodenoson in China for NAFLD/NASH; and (xiii) between $1,000,000 and up to $30,000,000 upon meeting certain net sales. In addition, following regulatory approval, we shall be entitled to double-digit percentage royalties on net sales of Piclidenoson and Namodenoson in the licensed territories. To date, we have received a total of $2,000,000 from CMS in upfront and milestone payments.
According to the agreement, CMS will be responsible for the development of Piclidenoson and Namodenoson to obtain regulatory approval in China and shall be further responsible for obtaining and maintaining regulatory approval in China for the indications described above. We may, at the option of CMS, supply finished product to CMS.
The License, Collaboration and Distribution Agreement shall continue in force unless earlier terminated and may be terminated in certain limited circumstances including certain breaches of the agreement and failure to achieve certain minimum quantities of sales during the contract period. Following expiration of the term of the agreement, the license granted shall become non-exclusive, fully paid, royalty free and irrevocable.
Kyongbo Pharm Agreement
In August 2019, we entered into a License and Distribution Agreement with Kyongbo Pharm. Under the terms of agreement, Kyongbo Pharm, in exchange for exclusive distribution rights to sell Piclidenoson in the treatment of psoriasis in South Korea, made a total upfront payment of $750,000 to us, with additional payments of up to $3,250,000 upon achievement of certain milestones. We will also be entitled to a transfer price for delivering finished product to Kyongbo Pharm. To date, we have received a total of $750,000 from Kyongbo Pharm in upfront and milestone payments.
Ewopharma Agreement
In March 2021, we signed an exclusive distribution agreement with Switzerland-based Ewopharma for Piclidenoson in the treatment of psoriasis and Namodenoson in the treatment of liver diseases namely, HCC, the most common form of liver cancer, and NASH. Under the terms of the distribution agreement, Ewopharma we received $2.25 million upfront and are entitled to up to an additional $40.45 million payable upon the achievement of regulatory and sales milestones plus 17.5% royalties on net sales. In exchange, Ewopharma will have the exclusive right to market and sell Piclidenoson in Central Eastern European (CEE) countries and Namodenoson in CEE countries and Switzerland. Ewopharma has the right to extend the distribution agreement to new indications that we may identify for its drug candidates. We will also be entitled to a transfer price for delivering finished product to Ewopharma. In January 2024, we entered into an amendment to the distribution agreement with Ewopharma to expand the distribution to include the indication of pancreatic cancer with respect to Namodenoson. To date, we have received a total of $2.25 million from Ewopharma in upfront, milestone and royalty payments.
69
Vetbiolix Agreement
In June 2021, we signed a development and commercialization agreement with Vetbiolix, a France-based veterinary biotech company, for the development of Piclidenoson for the treatment of osteoarthritis in companion animals including dogs and cats. Vetbiolix will have the exclusive right to Piclidenoson in the veterinary osteoarthritis market for two years, during which time Vetbiolix will conduct proof-of-concept studies and cover all associated costs. If the studies yield positive data and Vetbiolix exercises its option to obtain the license from Can-Fite, then Vetbiolix will be obligated to pay us upfront and milestone payments of €250,000, in addition to royalties on sales upon regulatory approval for veterinary use.
Total Revenues by Category of Activity and Geographic Markets
Historically, we have generated revenues from payments received pursuant to our out-licensing agreements with respect to Piclidenoson and Namodenoson. See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements.”
For the year ended December 31, 2023, we recorded the following revenues: (i) $0.13 million as a result of recognition of a portion of an advance payment received in January 2018 under the distribution agreement with Gebro; (ii) $0.19 million under the Distribution Agreement with CKD which was due to the recognition of a portion of the advance payment received in December 2016 and April 2019 under the Distribution Agreement with CKD; (iii) $0.08 million under the Distribution Agreement with Cipher; and (iv) $0.34 million under the Distribution Agreement with Ewopharma.
For the year ended December 31, 2022, we recorded the following revenues: (i) $0.15 million for the as a result of recognition of a portion of an advance payment received in January 2018 under the distribution agreement with Gebro; (ii) $0.20 million under the Distribution Agreement with CKD which was due to the recognition of a portion of the advance payment received in December 2016 and April 2019 under the Distribution Agreement with CKD; (iii) $0.09 million under the Distribution Agreement with Cipher; and (iv) $0.37 million under the Distribution Agreement with Ewopharma.
For the year ended December 31, 2021, we recorded the following revenues: (i) $0.19 million as a result of recognition of a portion of an advance payment received in January 2018 under the distribution agreement with Gebro; (ii) $0.20 million under the Distribution Agreement with CKD which was due to the recognition of a portion of the advance payment received in December 2016 and April 2019 under the Distribution Agreement with CKD; and (iii) $0.11 million under the Distribution Agreement with Cipher; and (iv) $0.35 million under the Distribution Agreement with Ewopharma.
We expect to generate future revenues through our current and potential future out-licensing arrangements with respect to Piclidenoson and Namodenoson based on the progress we make in our clinical trials.
Seasonality
Our business and operations are generally not affected by seasonal fluctuations or factors.
Raw Materials and Suppliers
We believe that the raw materials that we require to manufacture Piclidenoson, Namodenoson and CF602 are widely available from numerous suppliers and are generally considered to be generic industrial chemical supplies. We do not rely on a single or unique supplier for the current production of any therapeutic small molecule in our pipeline.
70
Manufacturing
We are currently manufacturing our API through a leading CRO. The relevant suppliers of our drug products are compliant with both current Good Manufacturing Practices, or cGMP, and current Good Laboratory Practices, or cGLP, and allow us to manufacture drug products for our current clinical trials. We anticipate that we will continue to rely on third parties to produce our drug products for clinical trials and commercialization.
There can be no assurance that our drug candidates, if approved, can be manufactured in sufficient commercial quantities, in compliance with regulatory requirements and at an acceptable cost. We and our contract manufacturers are, and will be, subject to extensive governmental regulation in connection with the manufacture of any pharmaceutical products or medical devices. We and our contract manufacturers must ensure that all of the processes, methods and equipment are compliant with cGMP for drugs on an ongoing basis, as mandated by the FDA and other regulatory authorities, and conduct extensive audits of vendors, contract laboratories and suppliers.
Contract Research Organizations
We outsource certain preclinical and clinical development activities to CROs, which in pre-clinical studies work according to cGMP and cGLP. We believe our clinical CROs comply with guidelines from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, which attempt to harmonize the FDA and the EMA regulations and guidelines. We create and implement the drug development plans and, during the preclinical and clinical phases of development, manage the CROs according to the specific requirements of the drug candidate under development.
Marketing and Sales
We do not currently have any marketing or sales capabilities. We intend to license to, or enter into strategic alliances with, larger companies in the pharmaceutical business, which are equipped to market and/or sell our products, if any, through their well-developed marketing capabilities and distribution networks. We intend to out-license some or all of our worldwide patent rights to more than one party to achieve the fullest development, marketing and distribution of any products we develop.
Intellectual Property
Our success depends in part on our ability to obtain and maintain proprietary protection for our product candidates, technology and know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other filing U.S. and foreign patent applications related to our proprietary technology, inventions and improvements that we believe are important to the development of our business. We also rely on trade secrets, know-how and continuing technological innovation to develop and maintain our proprietary position.
Patents
As of March 28, 2024, we owned or exclusively licensed (from Leiden University) 18 patent families that, collectively, contain approximately 209 issued patents and pending patent applications in various countries around the world relating to our two clinical candidates, Piclidenoson and Namodenoson, and our preclinical candidate, CF602. Patents related to our drug candidates may provide future competitive advantages by providing exclusivity related to the composition of matter, formulation and method of administration of the applicable compounds and could materially improve their value. The patent positions for our leading drug candidates are described below. In addition, we filed a patent application which is currently pending in various countries around the world concerning the use of cannabinoids for treatment of conditions associated with elevated expression of the A3 adenosine receptor.
With respect to our product candidates, we currently own patents and/or have patent applications pending in several countries around the world for the following families of patents:
| ● | A3AR ligands to treat viral diseases - a family of patents which pertain to use of substances that bind to the A3AR for the treatment of viral diseases, such as AIDS and hepatitis, and which inhibit viral replication. Such patents were granted in the United States, in Europe (by the EPO and validated in France, Germany, Italy, Switzerland and the United Kingdom), Australia, China, Israel, Japan, Singapore, Canada and Hong Kong. These patents have a filing date of January 1, 2002 and a priority date of January 16, 2001 and expired in January 2022, other than the U.S. patent that will expire in 2023. |
71
| ● | A3AR ligands to treat RA - a patent which pertains to the use of A3AR agonists for the treatment of inflammatory arthritis, in particular rheumatoid arthritis. This patent was granted in the United States and is set to expire in 2023. |
| ● | A3AR as a predictive and follow up biomarker - a family of patents and patent applications which pertain to a method of identifying inflammation, determining its severity, and determining and monitoring the efficacy of the anti-inflammatory treatment by determining the level of A3AR expression in white blood cells as a biological marker for inflammation. These patents were granted in the United States, Europe (by the EPO and validated in France, Germany, Italy, Spain, Switzerland and the United Kingdom), Australia, Israel, Japan, China, Mexico and Canada. The patents are set to expire in 2025. There is a patent application pending in Brazil that was refused and the refusal may be appealed until September 2025. Each of the patents and the patent application has a filing date of November 30, 2005 and a priority date of December 2, 2004. |
| ● | Specific dose to protect from psoriasis - a family of patents and patent applications which pertains to the use of a specific dose level of Piclidenoson (total daily dose of 4.0 mg) for the treatment of psoriasis. Such a patent was granted in Israel, Japan, the United States, South Korea and Europe (by the EPO and validated in in Austria, Belgium, Denmark, France, Germany, Italy, the Netherlands, Spain, Sweden, Switzerland and the United Kingdom). The patent is set to expire in 2030, and in 2031 in the U.S. There is a patent application pending in India with a filing date of September 6, 2010 and a priority date of September 6, 2009. |
| ● | Piclidenoson method of synthesis - a family of patents which pertain to the method for producing Piclidenoson. Such patents were granted in the United States, India, China, Japan, Israel and Europe (by the EPO and validated in Austria, Belgium, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Luxembourg, Monaco, the Netherlands, Norway, Poland, Portugal, Spain, Sweden, Switzerland, Turkey and the United Kingdom). These patents are set to expire in 2028 and in 2031 in the U.S. Each patent has a filing date of March 13, 2008 and a priority date of March 14, 2007. |
| ● | Osteoarthritis (OA) indication - a family of patents and patent applications which pertain to the use of A3AR agonists for the treatment of OA. Such patents were granted in Europe (by the EPO and validated in Austria, Belgium, Denmark, France, Germany, Italy, Spain, Sweden, Switzerland, Netherlands and the United Kingdom), U.S., Australia, Canada, South Korea, China, Israel, Japan and Mexico. The patents are set to expire in 2026. A patent application is pending in Brazil. These patents and patent applications have a filing date of November 29, 2006 and a priority date of November 30, 2005. |
| ● | Liver protection- a family of patents which pertains to the use of A3AR agonists for increasing liver cell division, intended to induce liver regeneration following injury or surgery. Such patents were granted in China, Israel, Japan, U.S. and Europe (by the EPO and validated in Austria, Belgium, Denmark, France, Germany, Italy, the Netherlands, Poland, Spain, Sweden, Switzerland, United Kingdom and Turkey). Each patent in this family has a filing date of October 22, 2008 and a priority date of October 15, 2007. |
| ● | Erectile dysfunction - a family of patents and patent applications which pertain to treatment of erectile dysfunction. This family includes granted patents in the United States, Australia, China, Hong Kong, Canada, South Korea, Israel, Mexico and Japan and patent applications in Brazil and Europe. The patents and patent applications have a filing date of August 8, 2013 with priority dates of August 8, 2012 and November 12, 2012. |
| ● | CAR T induced cytokine release syndrome - a family of patent applications which pertains to the use of A3AR ligands for managing cytokine release syndrome. This family includes patent applications in Israel and in the U.S., EP and HK claiming priority from this Israeli application. The US, EP and HK patent applications have a filing date of September 16, 2018 and the Israeli patent application has a filing date of September 17, 2017. |
72
| ● | NAFLD/NASH - a family of patents and patent applications which pertain to the use of A3AR ligands for treatment of ectopic fat accumulation. This family includes granted patents in U.S., Israel, Europe (by the EPO and validated in Austria, Belgium, Bulgaria, Croatia, Czechia, Denmark, Estonia, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, the Netherlands, North Macedonia, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, United Kingdom and Turkey), South Korea, Hong Kong, China, Japan and Mexico, and patent applications in Brazil and Canada. The patent applications have a filing date of November 22, 2016. |
| ● | Obesity - a family of patent applications which pertains to the use of A3AR ligand for reducing level of adipocytes and specifically, for treating obesity. This family includes patent applications in Australia, Brazil, Canada, China, Europe, Israel, Japan, Mexico, Korea U.S. and Hong Kong. These patent applications have a filing date of January 6, 2020. |
| ● | Cannabinoids - a family of patent applications pertaining to the use of cannabinoids for treating conditions and diseases that involve elevated expression of the A3AR. This family includes a patent application in Israel and in Australia, Brazil, Canada, China, Eurasia, Europe, India, Japan, Mexico, Korea, Singapore, South Africa, Thailand, and the U.S. claiming priority from this Israeli application. These patent applications have a filing date of January 14, 2021 while the Israeli patent application has a filing date of January 16, 2020. |
| ● | PD-1/PD-L1 - an Israeli patent application pertaining to the use of PD-1/PD-L1 axis inhibitor in combination with an A3AR. This Israeli patent application has a filing date of January 29, 2020. |
| ● | Treatment of advanced cancer - a family of patent applications that makes use of A3AR ligands, particularly Namodenoson, for the treatment of advanced solid cancer, including advanced liver cancer. This family includes patent applications in the US, Australia, Brazil, Canada, Turkey, Japan, South Korea and Mexico. The US application was filed on December 28, 2022 claiming priority from a provisional application that was filed on December 29, 2021. |
| ● | Treatment of joint inflammation __ an Israeli patent application that makes use of A3AR ligands for the local treatment of articular arthritis. This patent application was filed on January 9, 2023. |
| ● | Treatment of psoriasis __ an Israeli patent application that makes use of A3AR ligands for the treatment of psoriasis. This patent application was filed on June 29, 2022. |
| ● | Treatment of pancreatic cancer __ an Israeli patent application that makes use of A3AR ligands for the treatment of pancreatic cancer, including advanced forms. This patent application was filed on January 23, 2023. |
We currently hold an exclusive license from Leiden University of the Netherlands to a family of patents and patent applications that relate to the allosteric modulators of the A3AR, which includes the allosteric modulator CF602. This exclusive license relates to patents that were granted in the United States, China, Japan, South Korea, India and in Europe (validated in, Austria, Belgium, Denmark, France, Germany, Italy, the Netherlands, Spain, Sweden, Switzerland and United Kingdom). These granted patents are set to expire in 2028.
We believe that our owned and licensed patents provide broad and comprehensive coverage of our technology, and we intend to aggressively enforce our intellectual property rights if necessary to preserve such rights and to gain the benefit of our investment. However, as a result of the termination of the NIH license agreement between Can-Fite and NIH in June 2015 due to patent expiration, we no longer hold rights to a family of composition of matter patents relating to Piclidenoson and Namodenoson that were licensed from NIH. Nevertheless, because Piclidenoson or Namodenoson may be a NCE following approval of an NDA, we, if we are the first applicant to obtain NDA approval, may be entitled to five years of data exclusivity in the United States with respect to such NCEs. Analogous data and market exclusivity provisions, of varying duration, may be available in Europe and other foreign jurisdictions. However, we cannot be certain that we will be the first applicant to obtain an FDA approval for any indication of Piclidenoson or Namodenoson and we cannot be certain that we will be entitled to NCE exclusivity. In addition, we have discontinued the prosecution of a family of pending patent applications under joint ownership of Can-Fite and NIH pertaining to the use of A3AR agonists for the treatment of uveitis. Such diminution of our proprietary position could have a material adverse effect on our business, results of operation and financial condition.
73
The patent positions of companies like ours are generally uncertain and involve complex legal and factual questions. Our ability to maintain and solidify our proprietary position for our technology will depend on our success in obtaining effective claims and enforcing those claims once granted. We do not know whether any of our patent applications or those patent applications that we license will result in the issuance of any patents. Our issued patents and those that may issue in the future, or those licensed to us, may be challenged, narrowed, circumvented or found to be invalid or unenforceable, which could limit our ability to stop competitors from marketing related products or the length of term of patent protection that we may have for our products. Neither we nor our licensors can be certain that we were the first to invent the inventions claimed in our owned or licensed patents or patent applications. In addition, our competitors may independently develop similar technologies or duplicate any technology developed by us, and the rights granted under any issued patents may not provide us with any meaningful competitive advantages against these competitors. Furthermore, because of the extensive time required for development, testing and regulatory review of a potential product, before any of our products can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of the patent.
Trade Secrets
We may rely, in some circumstances, on trade secrets to protect our technology. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by confidentiality agreements and assignment of inventions agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, such agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors or others.
Scientific Advisory Board
We seek advice from our Scientific Advisory Board on scientific and medical matters generally. We call for Scientific Advisory Board meetings on an as-needed basis. The following table sets forth certain information with respect to our Scientific Advisory Board member.
| Name | Position/Institutional Affiliation | |
| Nabil Hanna, Ph.D. | Former Chief Science Officer of Biogen-Idec |
Clinical Advisory Board
Our Clinical Advisory Board, which consists of six members, an oncologist, dermatologist, and three hepatologists, who play an active role in consulting with us with respect to clinical drug development. We call for Clinical Advisory Board meetings on an as-needed basis. The following table sets forth certain information with respect to our Clinical Advisory Board members.
| Name | Position/Institutional Affiliation | |
| Dr. Kim Papp | Head, Probity Medical Research Inc., Ontario, Canada | |
| Dr. Salomon Stemmer | Davidoff Cancer Center, Rabin Medical Center-Beilinson Hospital, Petah Tikva and Sackler Faculty of Medicine, Tel Aviv | |
| Dr. Scott Friedman | Dean for Therapeutic Discovery and Chief of the Division of Liver Diseases at the Icahn School of Medicine at Mount Sinai in New York | |
| Dr. Arun Sanyal | Professor of Medicine, Physiology and Molecular Pathology at Virginia Commonwealth University School of Medicine | |
| Dr. Rifaat Safadi | Head of the Liver Unit, Gastroenterology and Liver Diseases, Division of Medicine at Hadassah Medical Center and Professor of Internal Medicine, Bowel, Liver Disease, and Metabolic Syndrome at Hadassah University in Israel | |
| Dr. Stephen Harrison | Dr. Harrison is currently a Visiting Professor of Hepatology at the Radcliffe Department of Medicine, University of Oxford. | |
| Dr. Ohad Etzion | Director, Department of Gastroenterology and Liver Diseases at Soroka Medical Center in Israel |
74
Competition
The pharmaceutical industry is characterized by rapidly evolving technology, intense competition and a highly risky, costly and lengthy research and development process. Adequate protection of intellectual property, successful product development, adequate funding and retention of skilled, experienced and professional personnel are among the many factors critical to success in the pharmaceutical industry.
Our technology platform is based on the finding that the A3AR is highly expressed in pathological cells, such as various tumor cell types and inflammatory cells. We believe that targeting the A3AR with synthetic and highly selective A3AR agonists, such as Piclidenoson and Namodenoson, and allosteric modulators, such as CF602, induces anti-cancer and anti-inflammatory effects. Currently, our drug candidates, Piclidenoson, Namodenoson, and CF602 are being developed to treat autoimmune inflammatory indications, oncology, and liver diseases as well as erectile dysfunction, including but not limited to psoriasis, HCC, and NASH. Preclinical studies have also indicated that our drug candidates have the potential to treat additional inflammatory diseases, such as Lowe Syndrome, Crohn’s disease, oncological diseases and viral diseases, such as the JC virus, and obesity.
Despite the competition, however, we believe that our drug candidates have unique characteristics and advantages over certain drugs currently available on the market and under development to treat these indications. We believe that our pipeline of drug candidates has exhibited a potential for therapeutic success with respect to the treatment of autoimmune-inflammatory, oncological and liver diseases. We believe that targeting the A3AR with synthetic and highly selective A3AR agonists, such as Piclidenoson and Namodenoson, and allosteric modulators, such as CF602, induces anti-cancer and anti-inflammatory effects.
We believe the characteristics of Piclidenoson, as exhibited in our clinical studies to date, including its good safety profile, clinical activity, simple and less frequent delivery through oral administration and its low cost of production, position it well against the competition in the autoimmune-inflammatory markets, including the psoriasis markets, where treatments, when available, often include injectable drugs, many of which can be highly toxic, expensive and not always effective. For example, while TNF inhibitor therapies transformed the treatment for many patients, a substantial percentage of patients (40% to 60%) do not respond to either a DMARD or biologic therapies (Simsek, 2010).
Pre-clinical pharmacology studies in different experimental animal models revealed that Piclidenoson acts as a DMARD, which, when coupled with its good safety profile, makes it competitive in the psoriasis market. Our recent findings suggest that Piclidenoson might offer a superior efficacy and tolerability profile, allowing patients to stay on drug longer and potentially leading to an improvement in response rate. Like Piclidenoson, Namodenoson has a good safety profile, is orally administered and has a low cost of production, which we believe positions it well in the HCC market, where only a handful of drugs have been approved by the FDA.
In addition, our human clinical data suggests that A3AR may be a biological marker in that high A3AR expression prior to treatment has been predictive of good patient response to our drug treatment. In fact, as a result of our research we have developed a simple blood assay to test for A3AR expression as a predictive biological marker. We hold a patent with respect to the intellectual property related to such assay and are currently analyzing A3AR expression levels in our Phase III psoriasis trial.
75
On the other hand, other drugs on the market, new drugs under development (including drugs that are in more advanced stages of development in comparison to our drug pipeline) and additional drugs that were originally intended for other purposes, but were found effective for purposes targeted by us, may all be competitive to the current drug candidates in our pipeline. In fact, some of these drugs are well established and accepted among patients and physicians in their respective markets, are orally bioavailable, can be efficiently produced and marketed, and are relatively safe. Moreover, other companies of various sizes engage in activities similar to ours. Most, if not all, of our competitors have substantially greater financial and other resources available to them. Competitors include companies with marketed products and/or an advanced research and development pipeline. The major competitors in the psoriasis therapeutic field include Amgen, J&J, Pfizer, Novartis, Abbvie, Eli Lilly, Bristol-Myers, UCB, and more. Competitors in the HCC field include companies such as Bayer, Exelixis, Merck, Roche, Eisai, Astrazenca, Beigene, Novartis, and Bristol-Myers. Competitors in the NASH field include companies such as Madrigal, Gilead, Genfit, Galmed, Madrigal, Akero, 89Bio, Viking, and Terns. Competitors in the erectile dysfunction field include Pfizer, Eli Lilly, Bayer, and Petros Pharmaceuticals.
Moreover, several companies have reported the commencement of research projects related to the A3AR. Such companies include CV Therapeutics Inc. (which was acquired by Gilead), King Pharmaceuticals R&D Inv. (which was acquired by Pfizer), Hoechst Marion Roussel Inc., Novo Nordisk A/S and Inotek Pharmaceuticals. However, to the best of our knowledge, there is no approved drug currently on the market which is similar to our A3AR agonists, nor are we aware of any allosteric modulator in the A3AR product pipeline similar to our allosteric modulator with respect to chemical profile and mechanism of action.
Piclidenoson for the Treatment of Psoriasis
Psoriasis is a skin condition that affects 2% to 3% of the general population according to the National Psoriasis Foundation. The disease is manifested by scaly plaques on the skin and in the severe form has a major effect on the physical and emotional well-being of the patients. Topical agents are typically used for mild disease, phototherapy for moderate disease, and systemic agents for severe disease. For moderate to severe cases, systemic biologic drugs, delivered via intravenous injection, or IV, have dominated the market. According to the National Psoriasis Foundation, common side effects of biologics include respiratory infections, flu-like symptoms, and injection site reactions while rare side effects include serious nervous system disorders, such as multiple sclerosis, seizures, or inflammation of the nerves of the eyes, blood disorders, and certain types of cancer. We believe a significant need remains for novel oral and safe drugs for patients who do not respond to existing therapies or for whom these therapies are unsuitable.
The psoriasis therapeutic market is dominated by biological drugs that are primarily administered via IV and have potential side effects. In January 2015, the FDA approved Cosentyx (secukinumab) by Novartis. In March 2016, the FDA approved Taltz (ixekizumab) by Eli Lilly. In April 2019, the FDA approved Skyrizi (risankizumab) by AbbVie. An oral small molecule inhibitor of phosphodiesterase 4, Otezla (Amgen), has gained sizable market share as a result in part due to its convenience of oral dose and comparable efficacy to the biologic drugs. Recently, a new oral inhibitor of TYK2 called SOTYKTU (Bristol) received U.S. FDA approval in September 2022. The psoriasis drug market is forecast to grow to $32 billion by 2026, according to estimate by Evaluate.
The current common treatments for psoriasis include topical and systemic drugs, steroids, immunosuppressive drugs such as Cyclosporine A by Novartis, MTX and biological drugs. Biological drugs, such as Enbrel (etanercept) by Amgen and Pfizer, Remicade (infliximab) by Centocor, Humira (adalimumab) by Abbvie, Stelara (ustekinumab) by Janssen, Otezla (aprelimast) byAmgen, Cosentyx (secukinumab) by Novartis and Taltz (ixekizumab) by Eli Lilly have significant side effects, are expensive and patients are often not responsive. For example, some of these drugs have received an FDA “black box” warning for increased risk of cancer in children and adolescents and risk of infection with Legionella and Listeria bacteria.
76
Namodenoson for the Treatment of HCC
According to the American Cancer Society, HCC is the fifth most common form of cancer death in the U.S., the most common form of liver cancer in adults and the third most common cause of cancer-related mortality worldwide, particularly in Asia. According to the American Cancer Society, more than 800,000 people are diagnosed with liver cancer each year throughout the world and more than 700,000 persons die from liver cancer each year. Despite several new approvals in the HCC market, including immunotherapy agents, this remains a significant unmet medical need where five year survival rates remain less than 20%. According to iHealthcareAnalyst, the HCC drug market is expected to reach $6.3 billion by 2029.
Several therapies are in advanced clinical development for HCC. Some drugs under development act as a single agent and some act in combination with Nexavar or approved checkpoint inhibitors pembrolizumab, atezolizumab, and/or nivolumab. Moreover, some are first line treatments while others are second line treatments. In addition, many existing approaches are used in the treatment of unresectable liver cancer, including alcohol injection, radiofrequency ablation, chemoembolization, cryoablation and radiation therapy.
Namodenoson for the Treatment of NASH (MASH)
Rates of NAFLD and NASH are increasing in the United States in concert with increasing rates of obesity and diabetes. In fact, NASH is now the third leading cause of liver transplant in the United States. It is estimated that 17-33% of Americans have fatty liver, with approximately one-third going on to develop NASH. NASH is believed to affect 2-5% of adult Americans. In March 2024, Madrigal Pharmaceuticals announced FDA approval of Rezdiffra (resmetirom) for the treatment of NASH with moderate to advanced liver fibrosis. By 2028, Vantage Market Research estimates the addressable pharmaceutical market for NASH will reach $21.9 billion in size.
Namodenoson for the Treatment of Pancreatic Cancer
Pancreatic cancer is increasing in most industrialized countries. The American Cancer Society estimates that in 2024 there will be 66,440 people in the U.S. diagnosed with pancreatic cancer. It also estimates 51,750 patients with pancreatic cancer will die in 2024. Pancreatic cancer accounts for about 3% of all cancers in the U.S. and about 8.5% of all cancer deaths. We face intense competition in the field of treating pancreatic cancer. There are dozens of startups, smaller biotech companies, big pharma, and several academic institutions and cancer centers all trying to improve the outcome for pancreatic cancer patients. There are several drugs already available and in the pipelines of pharmaceutical companies worldwide, not the least of which is the combination of the drugs of Abraxane® and gemcitabine. This is the primary FDA-approved combination of drugs for treating advanced pancreatic cancer. Recently, the FDA approved Onivyde® (Ipsen) in combination with 5FU/LV for the treatment of first-line metastatic pancreatic cancer. Onivyde® was previously approved in the second-line setting.
CF602 for the Treatment of Erectile Dysfunction
According to the Massachusetts Male Aging Study in 1994, 52% of the respondents between the ages of 40 and 70 years old reported some degree of erectile dysfunction.
The most popular class of drug to treat erectile dysfunction is the phosphodiesterase type 5, or PDE5, inhibitors. These drugs block the degradative action of cyclic guanosine monophosphate, or GMP, specific PDE5 on cyclic GMP in the smooth muscle cells lining the blood vessels supplying the corpus cavernosum of the penis. An erection is caused by increased blood flow into the penis resulting from the relaxation of penile arteries and corpus cavernosal smooth muscle. This response is mediated by the release of nitric oxide from nerve terminals and endothelial cells, which stimulates the synthesis of cyclic GMP in smooth muscle cells. The inhibition of PDE5 enhances erectile function by increasing the concentration of cyclic GMP in the corpus cavernosum and pulmonary arteries.
Unfortunately, the systemic side effects of PDE5 inhibitors include a decrease in sitting blood pressure. This has resulted in warnings and precautions and contraindications of use in patients already taking antihypertensive agents like nitrates or alpha-blockers. A study published in the American Journal of Medicine (Selvin E., et al., 2007) found that persons with a history of heart disease, hypertension, and diabetes had a higher probability of impotence. A second study published in the same journal (Shah NP., et al, 2015) notes that vascular erectile dysfunction is a powerful marker of increased cardiovascular risk. We believe a significant market opportunity exists targeting erectile dysfunction patients contraindicated for use of the market leading products, Viagra and Cialis.
77
Market Research Future estimates the value of the erectile dysfunction therapeutic market to reach approximately $6.1 billion by 2030.
Insurance
We maintain directors’ and officers’ liability insurance with coverage of $5.0 million per claim and $5.0 million in the aggregate.
We also maintain worldwide product and clinical trial liability insurance with coverage of approximately $5 million with respect to the Piclidenoson and Namodenoson drugs used in clinical trials. We also procure additional insurance for each specific clinical trial which covers a certain number of trial participants and which varies based on the particular clinical trial. Certain of such policies are based on the Declaration of Helsinki, which is a set of ethical principles regarding human experimentation developed for the medical community by the World Medical Association, and certain protocols of the Israeli Ministry of Health.
We procure cargo marine coverage when we ship substances for our clinical studies. Such insurance is custom-fit to the special requirements of the applicable shipment, such as temperature and/or climate sensitivity. If required, we ensure the substances to the extent they are stored in central depots and at clinical sites.
We believe that our insurance policies are adequate and customary for a business of our kind. However, because of the nature of our business, we cannot assure you that we will be able to maintain insurance on a commercially reasonable basis or at all, or that any future claims will not exceed our insurance coverage.
Environmental Matters
We are subject to various environmental, health and safety laws and regulations, including those governing air emissions, water and wastewater discharges, noise emissions, the use, management and disposal of hazardous, radioactive and biological materials and wastes and the cleanup of contaminated sites. We believe that our business, operations and facilities are being operated in compliance in all material respects with applicable environmental and health and safety laws and regulations. Our laboratory personnel in Israel have ongoing communication with the Israeli Ministry of Environmental Protection in order to verify compliance with relevant instructions and regulations. In addition, all of our laboratory personnel participate in instruction on the proper handling of chemicals, including hazardous substances before commencing employment, and during the course of their employment with us. In addition, all information with respect to any chemical substance that we use is filed and stored as a Material Safety Data Sheet, as required by applicable environmental regulations. Based on information currently available to us, we do not expect environmental costs and contingencies to have a material adverse effect on us. The operation of our testing facilities, however, entails risks in these areas. Significant expenditures could be required in the future if these facilities are required to comply with new or more stringent environmental or health and safety laws, regulations or requirements.
Government Regulation and Funding
We operate in a highly controlled regulatory environment. Stringent regulations establish requirements relating to analytical, toxicological and clinical standards and protocols in respect of the testing of pharmaceuticals. Regulations also cover research, development, manufacturing and reporting procedures, both pre- and post-approval. In many markets, especially in Europe, marketing and pricing strategies are subject to national legislation or administrative practices that include requirements to demonstrate not only the quality, safety and efficacy of a new product, but also its cost-effectiveness relating to other treatment options. Failure to comply with regulations can result in stringent sanctions, including product recalls, withdrawal of approvals, seizure of products and criminal prosecution.
78
Before obtaining regulatory approvals for the commercial sale of our product candidates, we must demonstrate through preclinical studies and clinical trials that our product candidates are safe and effective. Historically, the results from preclinical studies and early clinical trials often have not accurately predicted results of later clinical trials. In addition, a number of pharmaceutical products have shown promising results in clinical trials but subsequently failed to establish sufficient safety and efficacy results to obtain necessary regulatory approvals. We have incurred, and will continue to incur substantial expense for and devote a significant amount of time to, preclinical studies and clinical trials. Many factors can delay the commencement and rate of completion of clinical trials, including the inability to recruit patients at the expected rate, the inability to follow patients adequately after treatment, the failure to manufacture sufficient quantities of materials used for clinical trials, and the emergence of unforeseen safety issues and governmental and regulatory delays. If a product candidate fails to demonstrate safety and efficacy in clinical trials, this failure may delay development of other product candidates and hinder our ability to conduct related preclinical studies and clinical trials. Additionally, as a result of these failures, we may also be unable to obtain additional financing.
Governmental authorities in all major markets require that a new pharmaceutical product be approved or exempted from approval before it is marketed and have established high standards for technical appraisal which can result in an expensive and lengthy approval process. The time to obtain approval varies by country and some products are never approved. The lengthy process of conducting clinical trials, seeking approval and subsequent compliance with applicable statutes and regulations, if approval is obtained, are very costly and require the expenditure of substantial resources. These regulatory requirements impact our operations and differ from one country to another, so that securing the applicable regulatory approvals of one country does not imply the approval of another country. The approval procedures involve high costs and are manpower intensive, usually extend over many years and require highly skilled and professional resources.
A summary of the United States, European Union and Israeli regulatory processes follow below.
United States
In the United States, the Public Health Service Act (IPHS) and the Federal Food, Drug, and Cosmetic Act (FDCA), as amended, and the regulations promulgated thereunder, and other federal and state statutes and regulations govern, among other things, the safety and effectiveness standards for our products and the raw materials and components used in the production of, testing, manufacture, labeling, storage, record keeping, approval, advertising and promotion of our products on a product-by-product basis.
The failure to comply with the applicable requirements at any time during the product development process, including preclinical testing, clinical testing, the approval process or post-approval process, may subject an applicant to delays in the conduct of clinical trials, regulatory review and approval, and/or administrative or judicial sanctions. These sanctions may include, but are not limited to, the FDA’s refusal to allow an applicant to proceed with clinical testing, refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, warning letters, adverse publicity, customer notification, product recalls, product seizures, refusal to grant export or import approval, total or partial suspension of production or distribution, consent decrees, injunctions, fines, and civil or criminal investigations and penalties brought by the FDA or the U.S. Department of Justice, or other governmental entities.
The steps usually required to be taken before a new drug may be marketed in the U.S. generally include:
| ● | completion of preclinical laboratory tests and animal studies performed in accordance with the FDA’s current Good Laboratory Practices regulation; |
| ● | submission to the FDA of an IND, which must become effective before clinical trials may begin and must be updated annually or when significant changes are made; |
| ● | approval by an independent Institutional Review Board, or IRB, or ethics committee at each treatment site before the trial is commenced; |
| ● | performance of adequate and well controlled human clinical trials to establish the safety, purity and potency of the proposed drug product candidate for its intended purpose; |
79
| ● | submission of data supporting safety and efficacy as well as detailed information on the manufacture and composition of the product in clinical development and proposed labeling; |
| ● | preparation of and submission to the FDA of a New Drug Application, or NDA, after completion of all pivotal clinical trials; |
| ● | satisfactory completion of an FDA Advisory Committee review, if applicable; |
| ● | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the proposed product is produced to assess compliance with cGMP standards and to assure that the facilities, methods and controls are adequate to preserve the drug product’s continued safety, purity and potency, and of selected clinical investigation sites to assess compliance with Good Clinical Practices, or GCP; |
| ● | satisfactory completion of any FDA audits of the non-clinical and clinical trial sites to assure compliance with GCP requirement sand the integrity of clinical data in support of the NDA; |
| ● | payment of user fees and securing FDA approval of the NDA for the proposed indication for use; |
| ● | FDA review and approval of the NDA to permit commercial marketing of the product for particular indications for use in the United States.; and |
| ● | compliance with any post-approval requirements, including risk evaluation and mitigation strategies, or REMS, and any post-approval studies required by the FDA. |
Preclinical tests include in vitro and in vivo evaluation of the product candidate, its chemistry, formulation and stability, and animal studies to assess potential safety and efficacy. Certain preclinical tests must be conducted in compliance with good laboratory practice, or GLP, regulations. Violations of these regulations can, in some cases, lead to invalidation of the studies, requiring them to be replicated. After laboratory analysis and preclinical testing, a sponsor files an IND application including the results of the preclinical testing, manufacturing information and analytical data, with FDA. An IND is a request for authorization from FDA to administer an investigational new drug or biological product to humans to begin human testing. An IND becomes effective 30 days after FDA receives it, unless the FDA notifies the sponsor that the clinical trial is subject to a clinical hold. FDA also may impose a clinical hold at any time during a clinical trial. A sponsor may not proceed with a clinical trial that is subject to a clinical hold until the clinical hold has been lifted.
Clinical trials involve the administration of the investigational product candidate to healthy volunteers or patients with the disease to be treated under the supervision of a qualified principal investigator in accordance with CGP requirements. Clinical studies are conducted under protocols detailing, among other things, the objectives of the study, what types of patients may enter the study, schedules of tests and procedures, drugs, dosages, and length of study, as well as the parameters to be used in monitoring safety, and the efficacy criteria to be evaluated. A protocol for each clinical study and any subsequent protocol amendments must be submitted to the FDA as part of the IND process.
A sponsor who wishes to conduct a clinical trial outside the U.S. may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. If a clinical trial outside the U.S. is not conducted under an IND, the sponsor may submit data from the clinical trial to the FDA in support of an NDA so long as the clinical trial is conducted consistent with the spirit of GCP and in compliance with an international guideline for the ethical conduct of clinical research known as the Declaration of Helsinki and/or the laws and regulations of the country or countries in which the clinical trial is performed, whichever provides the greater protection to the participants in the clinical trial.
80
An IRB, either centrally or individually, must also review each clinical trial at each institution at which the clinical trial will be conducted. The IRB will consider, among other things, clinical trial design, patient informed consent, ethical factors, the safety of human subjects, the possible liability of the institution, and, where appropriate, the protection of privacy of the human subjects. An IRB must operate in compliance with the FDA regulations. The FDA, IRB, or the clinical trial sponsor, or the principal investigator may suspend or discontinue a clinical trial at any time for various reasons, including a finding that the clinical trial is not being conducted in accordance with FDA requirements or the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive GCP rules and the requirements for informed consent. Additionally, some clinical studies are overseen by an independent group of qualified experts organized by the clinical study sponsor, known as a data safety monitoring board or committee. This group recommends whether or not a trial may move forward at designated check points based on access to certain data from the study. The clinical study sponsor may also suspend or terminate a clinical trial based on evolving business objectives and/or competitive climate.
Typically, clinical trials are conducted in three sequential phases which are subject to numerous laws and regulatory requirements, including good clinical practice requirements, or GCP, which include adequate monitoring, reporting, record keeping and informed consent. In Phase I, small clinical trials are conducted, typically in healthy human volunteers, to determine the safety and proper dose ranges of product candidates. In Phase II, clinical trials are generally conducted in patients with the targeted disease or condition to identify possible adverse effects and safety risks, evaluate the efficacy of the product candidate for specific targeted indications and determine dose tolerance and optimate dosage. Multiple Phase II clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more costly Phase III clinical trials. In Phase III, clinical trials are conducted in an expanded population of patients with the targeted disease or condition to demonstrate that a dose range of the product candidate is potentially effective and has an acceptable safety profile. Phase III trials are designed to provide sufficient data for the statistically valid evidence of safety and efficacy to support approval, and if approved, the basis for product labeling. Post-approval studies, sometimes referred to as Phase IV trials, may be conducted, voluntarily or as a condition of approval, after initial product approval to obtain additional information about the drugs risks and benefits in patients with the targeted disease or condition. The time and expense required for us to perform this clinical testing can vary and is substantial. We cannot be certain that we will successfully complete Phase I, Phase II or Phase III testing of our product candidates within any specific time period, if at all. Furthermore, the FDA, the Institutional Review Board responsible for approving and monitoring the clinical trials at a given site, the Data Safety Monitoring Board, where one is used, or we may suspend the clinical trials at any time on various grounds, including a finding that subjects or patients are exposed to unacceptable health risk.
If the clinical data from these clinical trials (Phases I, II and III) are deemed to support the safety and effectiveness of the candidate product for its intended use, then we may proceed to seek to file with the FDA an NDA seeking approval to market a new drug for one or more specified intended uses. We have not completed our clinical trials for any candidate product for any intended use and therefore, we cannot ascertain whether the clinical data will support and justify filing an NDA. Nevertheless, if and when we are able to ascertain that the clinical data supports and justifies filing an NDA, we intend to make such appropriate filings.
The purpose of the NDA is to provide the FDA with sufficient information so that it can assess whether it ought to approve the candidate product for marketing in the U.S. for specific intended uses. Under the Prescription Drug User Fee Act, or PDUFA, the submission of an NDA requires the payment of substantial user fees, which the FDA adjusts on an annual basis, unless a waiver is granted. Moreover, no user fees are assessed on NDAs for products designated as orphan drugs, unless the product as a non-orphan indication for use.
The NDA normally contains, among other things, sections describing the chemistry, manufacturing, and controls, non-clinical pharmacology and toxicology, human pharmacokinetics and bioavailability, microbiology, the results of the clinical trials, and the proposed labeling which contains, among other things, the intended uses of the candidate product. FDA reviews the information submitted under the NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is compliant with cGMP requirements to assure and preserve the product’s identity, strength, quality and purity. Before accepting an NDA for filing, FDA conducts a preliminary review of the NDA, within 60 days of submission, to determine whether it is sufficiently complete to permit substantive review. Generally, under PDUFA guidelines, FDA has a goal of ten months from the date of acceptance of a standard NDA for a new molecular entity to review and act on the submission. The FDA does not always meet its PDUFA goal dates for standard and priority applications. The review process may often be significantly extended by FDA requests for additional information or clarification. The review process and the PDUFA goal date may be extended by three months if the FDA requests, or the applicant otherwise provides additional information or clarification regarding information already provided in the submission within the last three months before the PDUFA goal date.
81
The FDA may refer an application for a novel drug to an advisory committee. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions. Before approving an NDA, the FDA will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA may inspect one or more clinical trial sites to assure compliance with GCP requirements.
Manufacturers cannot take any action to market any new drug or biologic product in the United States until an appropriate marketing application has been approved by the FDA. The FDA has substantial discretion over the approval process and may disagree with a manufacturer’s interpretation of the data submitted. The process may be significantly extended by requests for additional information or clarification regarding information already provided. As part of this review, the FDA may refer the application to an appropriate advisory committee. An advisory committee is a panel comprised of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether an application should be approved and under what conditions. FDA is not bound by the recommendations of an advisory committee; however, it takes into consideration the advisory committee’s recommendations when making decisions., typically a panel of clinicians. As part of the approval process, FDA determines whether the manufacturer’s facilities and manufacturing processes are in compliance with cGMP requirements and sufficient to ensure consistent production of the product within required specifications. Prior to approving an NDA, FDA also may inspect one or more clinical trial sites to assure compliance with GCP requirements.
Following the completion of its evaluation of an NDA, FDA will issue an approval letter or a Complete Response Letter, or CRL. An approval letter authorizes commercialization of the drug for specified indications. A CRL informs the manufacturer that the review cycle for an application is complete and that the application is not ready for approval in its present form. A CRL typically identifies specific deficiencies in the NDA that may require additional clinical data or other significant requirements related to clinical trials or manufacturing. If FDA issues a CRL, the manufacturer must resubmit the NDA with all deficiencies addressed or withdraw the NDA. Even if all requested data and information are submitted, FDA ultimately may not approve the NDA.
Satisfaction of these and other regulatory requirements typically takes several years, and the actual time required may vary substantially based upon the type, complexity and novelty of the product. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly procedures on our activities. We cannot be certain that the FDA or other regulatory agencies will approve any of our products on a timely basis, if at all. Success in preclinical or early stage clinical trials does not assure success in later-stage clinical trials. Even if a product receives regulatory approval, the approval may be significantly limited to specific indications or uses and these limitations may adversely affect the commercial viability of the product. Delays in obtaining, or failures to obtain regulatory approvals, would have a material adverse effect on our business.
If the FDA approves a new product, it may limit the approved indications for use of the product. Additionally, the FDA may call for further clinical trials (i.e., Phase IV trials) and require additional data on safety and effectiveness. FDA also may impose other conditions on approval including the requirement for a risk evaluation and mitigation strategy, or REMS, designed to ensure the safe use of the drug. A REMS could include, among other things, medication guides, physician communication plans, restricted distribution methods, and patient registries. Any of these limitations on approval or marketing could restrict our ability to successfully commercialize products.
We are also required to gain separate approval for the use of an approved product as a treatment for indications other than those initially approved. In addition, side effects or adverse events that are reported during clinical trials can delay, impede or prevent marketing approval. Similarly, adverse events that are reported after marketing approval can result in additional limitations being placed on the product’s use and, potentially, withdrawal of the product from the market. Any adverse event, either before or after marketing approval, can result in product liability claims against us.
82
As an alternate path for FDA approval of new indications or new formulations of previously-approved products, a company may file a Section 505(b)(2) NDA, instead of a “stand-alone” or “full” NDA. Section 505(b)(2) of the FDCA, was enacted as part of the Drug Price Competition and Patent Term Restoration Act of 1984, otherwise known as the Hatch-Waxman Amendments. Section 505(b)(2) permits the submission of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. Some examples of products that may be allowed to follow a 505(b)(2) path to approval are drugs that have a new dosage form, strength, route of administration, formulation or indication. The Hatch-Waxman Amendments permit the applicant to rely upon certain published nonclinical or clinical studies conducted for an approved product or the FDA’s conclusions from prior review of such studies. The FDA may require companies to perform additional studies or measurements to support any changes from the approved product. The FDA may then approve the new product for all or some of the labeled indications for which the reference product has been approved, as well as for any new indication supported by the NDA. While references to nonclinical and clinical data not generated by the applicant or for which the applicant does not have a right of reference are allowed, all development, process, stability, qualification and validation data related to the manufacturing and quality of the new product must be included in an NDA submitted under Section 505(b)(2).
To the extent that the Section 505(b)(2) applicant is relying on the FDA’s conclusions regarding studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Orange Book publication. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. The Section 505(b)(2) application also will not be approved until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the reference product has expired. Thus, the Section 505(b)(2) applicant may invest a significant amount of time and expense in the development of its products only to be subject to significant delay and patent litigation before its products may be commercialized.
In addition to regulating and auditing human clinical trials, the FDA regulates and inspects equipment, facilities, laboratories and processes used in the manufacturing and testing of such products prior to providing approval to market a product. If, after receiving FDA approval, we make a material change in manufacturing equipment, location or process, additional regulatory review and approval may be required. We also must adhere to cGMP regulations and product-specific regulations enforced by the FDA through its facilities inspection program. The FDA also conducts regular, periodic visits to re-inspect our equipment, facilities, laboratories and processes following the initial approval. If, as a result of these inspections, the FDA determines that our equipment, facilities, laboratories or processes do not comply with applicable FDA regulations and conditions of product approval, the FDA may seek civil, criminal or administrative sanctions and/or remedies against us, including the suspension of our manufacturing operations.
We have currently received no approvals to market our products from the FDA or other foreign regulators.
The FDA is authorized to designate certain products for expedited review if they are intended to address an unmet medical need in the treatment of a serious or life-threatening disease or condition, or in the event of an emergency. These programs are fast track designation, breakthrough therapy designation and priority review designation.
Specifically, the FDA may designate a product for fast track review if it is intended, whether alone or in combination with one or more other products, for the treatment of a serious or life-threatening disease or condition, and it demonstrates the potential to address unmet medical needs for such a disease or condition. For fast track products, sponsors may have greater interactions with the FDA and the FDA may initiate review of sections of a fast track product’s application before the application is complete. This rolling review may be available if the FDA determines, after preliminary evaluation of clinical data submitted by the sponsor, that a fast track product may be effective. The sponsor must also provide, and the FDA must approve, a schedule for the submission of the remaining information and the sponsor must pay applicable user fees. However, the FDA’s time period goal for reviewing a fast track application does not begin until the last section of the application is submitted. In addition, the fast track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
83
In 2012, Congress enacted the Food and Drug Administration Safety and Innovation Act, or FDASIA. This law established a new regulatory scheme allowing for expedited review of products designated as “breakthrough therapies.” A product may be designated as a breakthrough therapy if it is intended, either alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA may take certain actions with respect to breakthrough therapies, including holding meetings with the sponsor throughout the development process; providing timely advice to the product sponsor regarding development and approval; involving more senior staff in the review process; assigning a cross-disciplinary project lead for the review team; and taking other steps to design the clinical trials in an efficient manner.
The FDA may also designate a product for priority review if it is a product that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. The FDA determines, on a case-by-case basis, whether the proposed product represents a significant improvement when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment-limiting product reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes, and evidence of safety and effectiveness in a new subpopulation. A priority designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA’s goal for taking action on a marketing application from ten months to six months.
The Secretary of Health and Human Services may additionally authorize unapproved drugs and biologics to be marketed in the event an actual or potential emergency has been designated by the U.S. government. After an emergency has been designated, the FDA may issue an Emergency Use Authorization, or EUA, for the use of a specific product based on criteria established by the FDCA. An EUA is product specific and is subject to specific conditions and restrictions. Once the emergency underlying the EUA ends, then the EUA terminates.
Once regulatory approval for marketing of a product or new indication for an existing product is obtained, the sponsor will be required to comply with post-approval regulatory requirements, including any post-approval requirements that the FDA may have imposed as a condition of approval. The sponsor will be required to report certain adverse reactions and production problems to the FDA, provide updated safety and efficacy information and comply with requirements concerning advertising and promotional labeling requirements. Manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMP regulations, which impose certain procedural and documentation requirements upon drug manufacturers. Accordingly, the sponsor and its third-party manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMP regulations and other regulatory requirements.
A product may also be subject to official lot release, meaning that the manufacturer is required to perform certain tests on each lot of the product before it is released for distribution. If the product is subject to official release, the manufacturer must submit samples of each lot, together with a release protocol showing a summary of the history of manufacture of the lot and the results of all of the manufacturer’s tests performed on the lot, to the FDA. The FDA may in addition perform certain confirmatory tests on lots of some products before releasing the lots for distribution. Finally, the FDA will conduct laboratory research related to the safety, purity, potency, and effectiveness of pharmaceutical products.
After an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
| ● | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| ● | fines, warning letters or holds on post-approval clinical trials; |
84
| ● | refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product license approvals; |
| ● | product seizure or detention, or refusal to permit the import or export of products; or |
| ● | injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs and biologics may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
Orphan Drug Designation
Pursuant to the Orphan Drug Act, FDA may grant special status, or orphan designation, to a drug intended to treat a rare disease or condition, which is a defined as a disease or condition that affects fewer than 200,000 individuals in the United States, or there is no reasonable expectation that the sales of the product will offset the cost of developing and making the drug available in the United States. A request for orphan drug designation must be filed before the NDA is filed. Following the grant of orphan designation, FDA will publicly disclose the identity of the therapeutic drug candidate and its potential orphan use. Orphan designation does not shorten the duration of the regulatory review and approval process. The fact that the FDA has designated a drug as an orphan drug for a particular intended use does not mean that the drug has been approved for marketing. Only after an NDA has been approved by the FDA is marketing appropriate.
If a drug candidate with orphan designation subsequently receives the first FDA approval for the disease or condition for which it has orphan designation, the drug is entitled to a seven-year period of market exclusivity subject to certain exceptions (e.g., clinical superiority of a subsequent product). This means that FDA may not approve another drug application authorizing another manufacturer to market the same drug for the same indication for seven years. This does not preclude competitors from receiving approval of the same product that has orphan exclusivity for a different indication or a different product for the same indication for which the orphan product has exclusivity. The orphan designation of a drug also provides the sponsor with certain financial incentives including tax credit, waiver of PDUFA fees, and access to certain grant funding for orphan products.
In February 2012, the FDA granted orphan drug status for the active moiety, or the part of the drug that is responsible for the physiological or pharmacological action of the drug substance, of Namodenoson for the treatment of HCC. Subsequently, in October 2015, the EMA granted Namodenoson orphan drug designation for the treatment of HCC.
Other U.S. Healthcare Laws and Compliance Requirements
For products distributed in the United States, we will also be subject to additional healthcare regulation and enforcement by the federal government and the states in which we conduct our business. Applicable federal and state healthcare laws and regulations include the following:
| ● | The federal anti-kickback law, which governs federal healthcare programs (e.g., Medicare, Medicaid), makes it illegal to solicit, offer, receive or pay any remuneration in exchange for, or to induce, the referral of business, including the purchase or prescription of a particular drug. Many states have similar laws that are not restricted to federal healthcare programs. A person or entity does not need to have actual knowledge of the federal anti-kickback statute or specific intent to violate it to have committed a violation; in addition, items or services resulting from a violation of the federal anti-kickback statute may constitute a false or fraudulent claim for purposes of the False Claims Act; |
85
| ● | The Ethics in Patient Referrals Act, commonly referred to as the Stark Law, and its corresponding regulations, prohibit physicians from referring patients for designated health services (including outpatient drugs) reimbursed under the Medicare or Medicaid programs to entities with which the physicians or their immediate family members have a financial relationship or an ownership interest, subject to narrow regulatory exceptions, and prohibits those entities from submitting claims to Medicare or Medicaid for payment of items or services provided to a referred beneficiary; |
| ● | Federal and state false claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented for payment to third party payers (including Medicare and Medicaid), claims for reimbursement, including claims for the sale of drugs or services, that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. If the government or a whistleblower were to allege that we violated these laws there could be a material adverse effect on us, including our stock price. Even an unsuccessful challenge could cause adverse publicity and be costly to respond to, which could have a materially adverse effect on our business, results of operations and financial condition; |
| ● | Health Insurance Portability and Accountability Act of 1996, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. This statute also prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items, or services; |
| ● | the federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for health care benefits, items or services; |
| ● | the Physician Payments Sunshine Act, created under the ACA, and its implementing regulations, which requires specified manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare & Medicaid Services, or CMS, information related to payments or other “transfers of value” made to physicians. All such reported information is publicly available; and |
| ● | Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our future business activities could be subject to challenge under one or more of such laws. Efforts to ensure that our business arrangements with third parties will comply with applicable laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other health care laws and regulations. A finding of liability under these laws can have significant adverse financial implications for us and can result in payment of large penalties and possible exclusion from federal healthcare programs. We will consult counsel concerning the potential application of these and other laws to our business and our sales, marketing and other activities and will make good faith efforts to comply with them. However, given their broad reach and the increasing attention given by law enforcement authorities, we cannot assure you that some of our activities will not be challenged or deemed to violate some of these laws.
86
Reimbursement
Sales of our product candidates in the United States may depend, in part, on the extent to which the costs of the product candidates may be covered by third-party payers, such as government health programs, commercial insurance and managed health care organizations. These third-party payers are increasingly challenging the prices charged for medical products and services. Additionally, the containment of health care costs has become a priority of federal and state governments, and the prices of drugs have been a focus in this effort. The United States government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. If these third-party payers do not consider our product candidates to be cost-effective compared to other available therapies, they may not cover our product candidates after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our product candidates on a profitable basis.
In order to secure coverage and reimbursement for any product that might be approved for sale, we may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA, EMA or other comparable regulatory approvals. Our product candidates may not be considered medically necessary or cost-effective. A payer’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Third-party reimbursement may not be sufficient to enable us to maintain price levels high enough to realize an appropriate return on our investment in product development.
Pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies. The conduct of such studies could be expensive and result in delays in our commercializing efforts. The EU provides options for its member states to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. EU member states may approve a specific price for a drug product or may instead adopt a system of direct or indirect controls on the profitability of the company placing the drug product on the market. Other member states allow companies to fix their own prices for drug products but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country. There can be no assurance that any country that has price controls or reimbursement limitations for drug products will allow favorable reimbursement and pricing arrangements for any of our products.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, emphasis on managed care in the United States has increased and we expect will continue to increase the pressure on drug pricing. Coverage policies, third-party reimbursement rates and drug pricing regulation may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Healthcare Reform
In the United States, there have been and continue to be a number of significant legislative initiatives to contain healthcare costs. The ACA was enacted in the United States in March 2010 and contains provisions that may reduce the profitability of drug products, including, for example, increased rebates for drugs subject to the Medicaid Drug Rebate Program, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies’ share of sales to federal health care programs.
87
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. These changes included aggregate reductions to Medicare payments to providers of 2% per fiscal year, effective April 1, 2013 and, due to subsequent legislative amendments to the statute, will stay in effect through 2027, unless additional Congressional action is taken; however, pursuant to the CARES Act, and subsequent legislation, these reductions were suspended from May 1, 2020 through March 31, 2022 due to the COVID-19 pandemic. In January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on customers for our drugs, if approved, and, accordingly, our financial operations.
Moreover, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their commercial products. There have been several recent U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, reduce the cost of drugs under Medicare, and reform government program reimbursement methodologies for drugs. The FDA released a final rule on September 24, 2020, effective November 30, 2020, providing guidance for states to build and submit importation plans for drugs from Canada. Further, on November 20, 2020, the U.S. Department of Health and Human Services, or HHS, finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers. On August 16, 2022, President Biden signed the Inflation Reduction Act of 2022, which includes several provisions to lower prescription drug costs for people with Medicare, including price negotiation requirements for drugs covered under Medicare, rebate requirements when drug prices rise faster than inflation, and a cap on out-of-pocket spending for Medicare Part D enrollees. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Although a number of these, and other proposed measures may require authorization through additional legislation to become effective, Congress has indicated that it will continue to seek new legislative measures to control drug costs.
CMS issued a final rule, effective on July 9, 2019, that requires direct-to-consumer advertisements of prescription drugs and biological products, for which payment is available through or under Medicare or Medicaid, to include in the advertisement the Wholesale Acquisition Cost, or list price, of that drug or biological product if it is equal to or greater than $35 for a monthly supply or usual course of treatment. Prescription drugs and biological products that are in violation of these requirements will be included on a public list. In addition, in December 2022, the Federal Trade Commission (FTC) released its Health Products Compliance Guidance that is intended to ensure health-related product claims, including drugs and biologics, are truthful, not misleading and supported by science. The FTC also updated its Guides Concerning the Use of Endorsements and Testimonials in Advertising that raises the growth of social media in marketing.
Any adopted health reform measure could reduce the ultimate demand for our products, if approved, or put pressure on our product pricing. Individual states in the United States have also become increasingly active in passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. We expect that additional state and federal healthcare reform measures will be adopted in the future.
We expect that additional state and federal healthcare reform measures, as well as legal changes by foreign governments, will be adopted in the future, any of which could limit the amounts that governments will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
88
European Union
In order to market any product outside of the United States, a company must also comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy that govern, among other things, clinical trials, marketing authorization, commercial sales and distribution of drug products. Whether or not it obtains FDA approval for a product, the company would need to obtain the necessary approvals by the comparable non-U.S. regulatory authorities before it can commence clinical trials or marketing of the product in those countries or jurisdictions. The approval process ultimately varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
Regulation and Marketing Authorization in the European Union
The European Medicines Agency, or EMA, is the scientific agency of the European Union, or EU, that coordinates the evaluation and monitoring of new and approved medicinal products such as drugs and biologics. It is responsible for the scientific evaluation of applications for EU marketing authorizations, as well as the development of technical guidance and the provision of scientific advice to sponsors.
The process governing approval of medicinal products in the European Union follows essentially the same lines as in the United States and, likewise, generally involves satisfactorily completing each of the following:
| ● | preclinical laboratory tests, animal studies and formulation studies all performed in accordance with the applicable E.U. Good Laboratory Practice regulations; |
| ● | submission to the relevant national authorities of a clinical trial application, or CTA, which must be approved before human clinical trials may begin; |
| ● | performance of adequate and well-controlled clinical trials to establish the safety and efficacy of the product for each proposed indication; |
| ● | submission to the relevant competent authorities of a Marketing Authorisation Application, or MAA, which includes the data supporting safety and efficacy as well as detailed information on the manufacture and composition of the product in clinical development and proposed labelling; |
| ● | satisfactory completion of an inspection by the relevant national authorities of the manufacturing facility or facilities, including those of third parties, at which the product is produced to assess compliance with strictly enforced current cGMP; |
| ● | potential audits of the non-clinical and clinical trial sites that generated the data in support of the MAA; and |
| ● | review and approval by the relevant competent authority of the MAA before any commercial marketing, sale or shipment of the product. |
Preclinical Studies
Preclinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as studies to evaluate toxicity in animal studies, in order to assess the potential safety and efficacy of the product. The conduct of the preclinical tests and formulation of the compounds for testing must comply with the relevant E.U. and/or Member States’ regulations and requirements. The results of the preclinical tests, together with relevant manufacturing information and analytical data, are submitted as part of the CTA.
89
Clinical Trial Approval
Clinical trials in the EU are regulated under Regulation (EU) 536/2014 (CTR), as amended. As opposed to the previous Directive 2001/20/EC (CTD), which as an EU directive was not directly applicable in the member states, the CTR has immediate effect for the whole EU and did not have to be transposed into national law. While national laws implementing the CTD varied to a great extent, the CTR provides for a significant further harmonization of the law governing clinical trials in the EU. After significant delay, the CTR became applicable on January 31, 2022. The CTR now harmonizes the assessment and supervision processes for clinical trials throughout the EU via the Clinical Trials Information System (CTIS), which includes a centralized EU portal and database for clinical trials. From January 31, 2023 onwards, clinical trial sponsors need to apply to start a clinical trial via CTIS. From January 31, 2025, any trials previously approved under the CTD that continue to run after such date, will need to comply with CTR and their sponsors must have recorded the required information on such trials in CTIS. The CTR provides inter alia:
| ● | Consistent rules for conducting clinical trials throughout the EU; |
| ● | Making information on the authorization, conduct and results of each clinical trial carried out in the EU publicly available; |
| ● | Harmonized electronic submission and assessment process for clinical trials conducted in multiple member states; |
| ● | Improved collaboration, information sharing and decision-making between and within member states; |
| ● | Increased transparency of information on clinical trials; and |
| ● | Higher standards of safety for all participants in EU clinical trials. |
The authorization of a clinical trial (Phase I-III) in an EU member state requires the submission of a CTA via the EU Portal. The application will be reviewed by the competent authorities of the member states where the trial is supposed to take place. The application and approval process is conducted by the member states under the cooperation system set forth in the CTR. Particularities under member states’ national law still apply to some extent. In general, the CTA should include, among other documents, the study protocol, results of the nonclinical studies and manufacturing information and analytical results. Also, the sponsor has to suggest one of the concerned member states as reporting member state. The CTR aims at speeding up the validation and review of clinical trial applications and therefore provides strict deadlines.
Marketing Authorization
Authorization to market a product in the member states of the European Union proceeds under one of four procedures: a centralized authorization procedure, a mutual recognition procedure, a decentralized procedure or a national procedure.
Centralized Authorization Procedure
The centralized procedure enables applicants to obtain a marketing authorization that is valid in all E.U. member states based on a single application. Certain medicinal products, including products developed by means of biotechnological processes, must undergo the centralized authorization procedure for marketing authorization which, if granted by the European Commission, is automatically valid in all 28 E.U. member states. The EMA and the European Commission administer this centralized authorization procedure pursuant to Regulation (EC) No 726/2004.
Pursuant to Regulation (EC) No 726/2004, this procedure is inter alia mandatory for:
| ● | medicinal products developed by means of one of the following biotechnological processes: |
| ● | recombinant DNA technology; |
90
| ● | controlled expression of genes coding for biologically active proteins in prokaryotes and eukaryotes including transformed mammalian cells; and |
| ● | hybridoma and monoclonal antibody methods; |
| ● | advanced therapy medicinal products as defined in Article 2 of Regulation (EC) No. 1394/2007 on advanced therapy medicinal products; |
| ● | medicinal products for human use containing a new active substance that, on the date of effectiveness of this regulation, was not authorized in the European Union, and for which the therapeutic indication is the treatment of any of the following diseases: |
| ● | acquired immune deficiency syndrome; |
| ● | cancer; |
| ● | neurodegenerative disorder; |
| ● | diabetes; |
| ● | auto-immune diseases and other immune dysfunctions; |
| ● | viral diseases; and |
| ● | medicinal products that are designated as orphan medicinal products pursuant to Regulation (EC) No 141/2000. |
The centralized authorization procedure is optional for other medicinal products if they contain a new active substance or if the applicant shows that the medicinal product concerned constitutes a significant therapeutic, scientific or technical innovation or that the granting of authorization is in the interest of patients in the European Union.
Administrative Procedure
Under the centralized authorization procedure, the EMA’s Committee for Human Medicinal Products, or CHMP, serves as the scientific committee that renders opinions about the safety, efficacy and quality of medicinal products for human use on behalf of the EMA. The CHMP is composed of experts nominated by each member state’s national authority for medicinal products, with expert appointed to act as Rapporteur for the co-ordination of the evaluation with the possible assistance of a further member of the Committee acting as a Co-Rapporteur. After approval, the Rapporteur(s) continue to monitor the product throughout its life cycle. The CHMP has 210 days to adopt an opinion as to whether a marketing authorization should be granted. The process usually takes longer in case additional information is requested, which triggers clock-stops in the procedural timelines. The process is complex and involves extensive consultation with the regulatory authorities of member states and a number of experts. When an application is submitted for a marketing authorization in respect of a drug that is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation, the applicant may pursuant to Article 14(9) Regulation (EC) No 726/20042, as amended, request an accelerated assessment procedure. If the CHMP accepts such request, the time-limit of 210 days will be reduced to 150 days but it is possible that the CHMP can revert to the standard time-limit for the centralized procedure if it considers that it is no longer appropriate to conduct an accelerated assessment. Once the procedure is completed, a European Public Assessment Report, or EPAR, is produced. If the opinion is negative, information is given as to the grounds on which this conclusion was reached. After the adoption of the CHMP opinion, a decision on the MAA must be adopted by the European Commission, after consulting the E.U. member states.
91
Conditional Approval
In specific circumstances, E.U. legislation (Article 14a Regulation (EC) No 726/2004, as amended and Regulation (EC) No 507/2006 on Conditional Marketing Authorisations for Medicinal Products for Human Use) enables applicants to obtain a conditional marketing authorization prior to obtaining the comprehensive clinical data required for an application for a full marketing authorization. Such conditional approvals may be granted for medicinal products intended for the treatment, prevention or medical diagnosis of seriously debilitating or life-threatening diseases (including medicines designated as orphan medicinal products) if (1) the risk-benefit balance of the product candidate is positive, (2) it is likely that the applicant will be in a position to provide the required comprehensive clinical trial data post-authorization, (3) the product fulfills unmet medical needs and (4) the benefit to public health of the immediate availability on the market of the medicinal product concerned outweighs the risk inherent in the fact that additional data are still required. A conditional marketing authorization may contain specific obligations to be fulfilled by the marketing authorization holder, including obligations with respect to the completion of ongoing or new studies with a view to confirming that the risk-benefit balance is favorable. Conditional marketing authorizations are valid for one year, and may be renewed annually, if the risk-benefit balance remains positive, and after an assessment of the need for additional or modified conditions and/or specific obligations. The timelines for the centralized procedure described above also apply with respect to the review by the CHMP of applications for a conditional marketing authorization.
Marketing Authorization under Exceptional Circumstances
Under Article 14(8) Regulation (EC) No 726/2004, as amended, products for which the applicant can demonstrate that comprehensive data (in line with the requirements laid down in Annex I of Directive 2001/83/EC, as amended) cannot be provided (for objective, verifiable reasons) might be eligible for marketing authorization under exceptional circumstances. This type of authorization is reviewed annually to reassess the risk-benefit balance. The fulfillment of any specific procedures/obligations imposed as part of the marketing authorization under exceptional circumstances is aimed at the provision of information on the safe and effective use of the product and will normally not lead to a standard marketing authorization.
Market Authorizations Granted by Authorities of E.U. Member States
In general, if the centralized procedure is not followed, there are three alternative procedures as prescribed in Directive 2001/83/EC, as amended:
| ● | The decentralized procedure allows applicants to file identical applications to several E.U. member states and receive simultaneous national approvals based on the recognition by E.U. member states of an assessment by a reference member state; |
| ● | The national procedure is only available for products intended to be authorized in a single E.U. member state; and |
| ● | A mutual recognition procedure similar to the decentralized procedure is available when a marketing authorization has already been obtained in at least one E.U. member state. |
A marketing authorization may be granted only to an applicant established in the European Union.
Pediatric Studies
Prior to obtaining a marketing authorization in the European Union, applicants have to demonstrate compliance with all measures included in an EMA-approved Paediatric Investigation Plan, or PIP, covering all subsets of the paediatric population, unless the EMA has granted a product-specific waiver, a class waiver, or a deferral for one or more of the measures included in the PIP. The respective requirements for all marketing authorization procedures are set forth in Regulation (EC) No 1902/2006, which is referred to as the Pediatric Regulation. This requirement also applies when a company wants to add a new indication, pharmaceutical form or route of administration for a medicine that is already authorized. The Pediatric Committee of the EMA, or PDCO, may grant deferrals for some medicines, allowing a company to delay development of the medicine in children until there is enough information to demonstrate its effectiveness and safety in adults. The PDCO may also grant waivers when development of a medicine in children is not needed or is not appropriate, such as for diseases that only affect the elderly population.
92
Before a marketing authorization application can be filed, or an existing marketing authorization can be amended, the EMA determines that companies actually comply with the agreed studies and measures listed in each relevant PIP.
Periods of Authorization and Renewals
A marketing authorization is valid for five years in principle and the marketing authorization may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the competent authority of the authorizing member state. To this end, the marketing authorization holder must provide the EMA and the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variations introduced since the marketing authorization was granted, at least nine months before the marketing authorization ceases to be valid. Once renewed, the marketing authorization is valid for an unlimited period, unless the European Commission decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal. Any authorization which is not followed by the actual placing of the drug on the E.U. market (in case of centralized procedure) or on the market of the authorizing member state within three years after authorization ceases to be valid (the so-called sunset clause).
Orphan Drug Designation and Exclusivity
Pursuant to Regulation (EC) No 141/2000, as amended, and Regulation (EC) No. 847/2000, as amended, the European Commission can grant such orphan medicinal product designation to products for which the sponsor can establish that it is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in 10,000 people in the European Union, or a life threatening, seriously debilitating or serious and chronic condition in the European Union and with regards to that without incentives it is unlikely that sales of the drug in the European Union would generate a sufficient return to justify the necessary investment. In addition, the sponsor must establish that there is no other satisfactory method approved in the European Union of diagnosing, preventing or treating the condition, or if such a method exists, the proposed orphan drug will be of significant benefit to patients.
Orphan drug designation is not a marketing authorization. It is a designation that provides a number of benefits, including fee reductions, regulatory assistance, and the possibility to apply for a centralized E.U. marketing authorization, as well as ten years of market exclusivity following a marketing authorization. During this market exclusivity period, neither the EMA, the European Commission nor the member states can accept an application or grant a marketing authorization for a “similar medicinal product.” A “similar medicinal product” is defined as a medicinal product containing a similar active substance or substances as those contained in an authorized orphan medicinal product and that is intended for the same therapeutic indication. The market exclusivity period for the authorized therapeutic indication may be reduced to six years if, at the end of the fifth year, it is established that the orphan designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity. In addition, a competing similar medicinal product may in limited circumstances be authorized prior to the expiration of the market exclusivity period, including if the marketing authorization holder has given his consent to the second applicant or the marketing authorization holder is unable to supply sufficient quantities of the product or if the competing product is shown to be safer, more effective or otherwise clinically superior to the already approved orphan drug. Furthermore, a product can lose orphan designation, and the related benefits, prior to us obtaining a marketing authorization if it is demonstrated that the orphan designation criteria are no longer met.
If the MAA of a medicinal product designated as an orphan drug includes the results of all studies conducted in compliance with an agreed PIP, and a corresponding statement is subsequently included in the marketing authorization granted, the ten-year period of market exclusivity will be extended by two additional years.
93
Regulatory Data Protection
E.U. legislation also provides for a system of regulatory data and market exclusivity. According to Article 14(11) of Regulation (EC) No 726/2004, as amended, and Article 10(1) of Directive 2001/83/EC, as amended, upon receiving marketing authorization, new chemical entities approved on the basis of a complete independent data package benefit from eight years of data exclusivity and ten years of market exclusivity. Data exclusivity prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a generic (abbreviated) application. During the ten-year period of market exclusivity, a generic marketing authorization can be submitted, and the innovator’s data may be referenced, but no generic medicinal product can be marketed until the expiration of the market exclusivity. The overall ten-year period will be extended to a maximum of 11 years if, during the first eight years of those ten years, the marketing authorization holder, or MAH, obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. Even if a compound is considered to be a new chemical entity and the innovator is able to gain the period of data exclusivity, another company nevertheless could also market another version of the drug if such company obtained marketing authorization based on an MAA with a complete independent data package of pharmaceutical tests, preclinical tests and clinical trials. However, products designated as orphan medicinal products enjoy, upon receiving marketing authorization, a period of ten years of orphan market exclusivity-see also Orphan Drug Designation and Exclusivity. Depending upon the timing and duration of the E.U. marketing authorization process, products may be eligible for up to five years’ supplementary protection certificates, or SPCs, pursuant to Regulation (EC) No 469/2009, as amended. A six-month additional extension is available in accordance with Regulation (EC) No 1901/2006 if the SPC relates to a medicinal product for children for which data has been submitted according to a Paediatric Investigation Plan (PIP). Such SPCs extend the rights under the basic patent for the drug (see below sub Patent Term Extension).
Regulatory Requirements After a Marketing Authorization has been Obtained
If we obtain authorization for a medicinal product in the European Union, we will be required to comply with a range of requirements applicable to the manufacturing, marketing, promotion and sale of medicinal products:
Pharmacovigilance and other requirements
We will, for example, have to comply with the E.U.’s stringent pharmacovigilance or safety reporting rules, pursuant to which post-authorization studies and additional monitoring obligations can be imposed. Other requirements relate, for example, to the manufacturing of products and APIs in accordance with good manufacturing practice standards. E.U. regulators may conduct inspections to verify our compliance with applicable requirements, and we will have to continue to expend time, money and effort to remain compliant. Non-compliance with E.U. requirements regarding safety monitoring or pharmacovigilance, and with requirements related to the development of products for the pediatric population, can also result in significant financial penalties in the European Union. Similarly, failure to comply with the E.U.’s requirements regarding the protection of individual personal data can also lead to significant penalties and sanctions. Individual E.U. member states may also impose various sanctions and penalties in case we do not comply with locally applicable requirements.
Manufacturing
The manufacturing of authorized drugs, for which a separate manufacturer’s license is mandatory, must be conducted in strict compliance with the EMA’s Good Manufacturing Practices, or cGMP, requirements and comparable requirements of other regulatory bodies in the European Union, which mandate the methods, facilities and controls used in manufacturing, processing and packing of drugs to assure their safety and identity. The EMA enforces its current cGMP requirements through mandatory registration of facilities and inspections of those facilities. The EMA may have a coordinating role for these inspections while the responsibility for carrying them out rests with the member states competent authority under whose responsibility the manufacturer falls. Failure to comply with these requirements could interrupt supply and result in delays, unanticipated costs and lost revenues, and could subject the applicant to potential legal or regulatory action, including but not limited to warning letters, suspension of manufacturing, seizure of product, injunctive action or possible civil and criminal penalties.
Marketing and Promotion
The marketing and promotion of authorized drugs, including industry-sponsored continuing medical education and advertising directed toward the prescribers of drugs and/or the general public, are strictly regulated in the European Union under Directive 2001/83/EC, as amended. The applicable regulations aim to ensure that information provided by holders of marketing authorizations regarding their products is truthful, balanced and accurately reflects the safety and efficacy claims authorized by the EMA or by the competent authority of the authorizing member state. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties.
94
Patent Term Extension
In order to compensate the patentee for delays in obtaining a marketing authorization for a patented product, a supplementary certificate, or SPC, may be granted extending the exclusivity period for that specific product by up to five years. Applications for SPCs must be made to the relevant patent office in each E.U. member state and the granted certificates are valid only in the member state of grant. An application has to be made by the patent owner within six months of the first marketing authorization being granted in the European Union (assuming the patent in question has not expired, lapsed or been revoked) or within six months of the grant of the patent (if the marketing authorization is granted first). In the context of SPCs, the term “product” means the active ingredient or combination of active ingredients for a medicinal product and the term “basic patent means a patent which protects a product as such, a process to obtain a product or an application of a product, and which is designated by its holder for the purpose of the procedure for grant of a certificate. The duration of an SPC is calculated as the difference between the patent’s filing date and the date of the first marketing authorization, minus five years, subject to a maximum term of five years.
A six month pediatric extension of an SPC may be obtained where the patentee has carried out an agreed pediatric investigation plan, the authorized product information includes information on the results of the studies and the product is authorized in all member states of the European Union.
United Kingdom
The withdrawal of the United Kingdom (U.K.) from the E.U. took effect on January 1, 2021, and there are 27 member states remaining in the E.U. As of January 1, 2021, the U.K. is a “third country” with regard to the EU (subject to the terms of the EU UK Trade Agreement) and EU law ceased to apply directly in the UK. However, the U.K. has retained the E.U. regulatory regime with certain modifications as standalone U.K. legislation. Therefore, the U.K. regulatory regime is currently similar to E.U. regulations, but the U.K. may adopt changed regulations that may diverge from the E.U. legislative regime for medicines and their research, development and commercialization. For a two-year period starting January 1, 2021, the U.K. has adopted transitional provisions, which inter alia apply to the importation of medicines into the U.K. and rely on certain EMA marketing authorization application procedures.
Clinical Testing in Israel
In order to conduct clinical testing on humans in Israel, special authorization must first be obtained from the ethics committee and general manager of the institution in which the clinical studies are scheduled to be conducted, as required under the Guidelines for Clinical Trials in Human Subjects implemented pursuant to the Israeli Public Health Regulations (Clinical Trials in Human Subjects), as amended from time to time, and other applicable legislation. These regulations also require authorization from the Israeli Ministry of Health, except in certain circumstances, and in the case of genetic trials, special fertility trials and similar trials, an additional authorization of the overseeing institutional ethics committee. The institutional ethics committee must, among other things, evaluate the anticipated benefits that are likely to be derived from the project to determine if it justifies the risks and inconvenience to be inflicted on the human subjects, and the committee must ensure that adequate protection exists for the rights and safety of the participants as well as the accuracy of the information gathered in the course of the clinical testing. Since we intend to perform a portion of the clinical studies on certain of our product candidates in Israel, we will be required to obtain authorization from the ethics committee and general manager of each institution in which we intend to conduct our clinical trials, and in most cases, from the Israeli Ministry of Health.
Israel Ministry of Health
Israel’s Ministry of Health, which regulates medical testing, has adopted protocols that correspond, generally, to those of the FDA and the EMA, making it comparatively straightforward for studies conducted in Israel to satisfy FDA and the European Medicines Agency requirements, thereby enabling medical technologies subjected to clinical trials in Israel to reach U.S. and EU commercial markets in an expedited fashion. Many members of Israel’s medical community have earned international prestige in their chosen fields of expertise and routinely collaborate, teach and lecture at leading medical centers throughout the world. Israel also has free trade agreements with the United States and the European Union.
95
Other Countries
In addition to regulations in the United States, the EU and Israel, we are subject to a variety of other regulations governing clinical trials and commercial sales and distribution of drugs in other countries. Whether or not our products receive approval from the FDA, approval of such products must be obtained by the comparable regulatory authorities of countries other than the United States before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials and product licensing vary greatly from country to country.
The requirements that we and our collaborators must satisfy to obtain regulatory approval by government agencies in other countries prior to commercialization of our products in such countries can be rigorous, costly and uncertain. In Canada and Australia, regulatory requirements and approval processes are similar in principle to those in the United States. For example, in Canada, pharmaceutical product candidates are regulated by the Food and Drugs Act and the rules and regulations promulgated thereunder, which are enforced by Health Canada. Before commencing clinical trials in Canada, an applicant must complete preclinical studies and file a clinical trial application with Health Canada. After filing a clinical trial application, the applicant must receive different clearance authorizations to proceed with Phase 1 clinical trials, which can then lead to Phase 2 and Phase 3 clinical trials. To obtain regulatory approval to commercialize a new drug in Canada, a new drug submission, or NDS, must be filed with Health Canada. If the NDS demonstrates that the product was developed in accordance with the regulatory authorities’ rules, regulations and guidelines and demonstrates favorable safety and efficacy and receives a favorable risk/benefit analysis, Health Canada issues a notice of compliance which allows the applicant to market the product. Facilities, procedures, operations and/or testing of products are subject to periodic inspection by Health Canada and the Health Products and Food Branch Inspectorate. In addition, Health Canada conducts pre-approval and post-approval reviews and plant inspections to determine whether systems are in compliance with the good manufacturing practices in Canada, Drug Establishment Licensing requirements and other provisions of the Food and Drug Regulations.
Foreign governments also have stringent post-approval requirements including those relating to manufacture, labeling, reporting, record keeping and marketing. Failure to substantially comply with these on-going requirements could lead to government action against the product, our company and/or our representatives.
Related Matters
From time to time, legislation is drafted, introduced and passed in governmental bodies that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA, the EMA, the Israeli Ministry of Health and other applicable regulatory bodies to which we are subject. In addition, regulations and guidance are often revised or reinterpreted by the national agency in ways that may significantly affect our business and our product candidates. It is impossible to predict whether such legislative changes will be enacted, whether FDA, EMA or Israeli Ministry of Health regulations, guidance or interpretations will change, or what the impact of such changes, if any, may be. We may need to adapt our business and product candidates and products to changes that occur in the future.
C. Organizational Structure
Our corporate structure consists of Can-Fite and our wholly owned subsidiary, Can-Fite Biopharma Europe, incorporated in France.
D. Property, Plants and Equipment
We are headquartered in Ramat Gan, Israel. We lease office space for NIS 4,000 ($1,102 based on December 31, 2023 exchange rate by Bank of Israel) per month. As of December 31, 2023, we did not have obligation to any lease payments.
96
ITEM 4A. Unresolved Staff Comments
Not Applicable.
ITEM 5. Operating and Financial Review and Prospects
The information in this section should be read in conjunction with our consolidated financial statements and related notes beginning on page F-1 and the related information included elsewhere in this Annual Report on Form 20-F. Our financial statements are prepared in accordance with U.S. GAAP. We maintain our accounting books and records in U.S. dollars and our functional currency is the U.S. dollar. Certain amounts presented herein may not sum due to rounding.
Overview
We are a clinical-stage biopharmaceutical company that develops orally bioavailable small molecule therapeutic products for the treatment of cancer, liver and inflammatory diseases and erectile dysfunction. We are also developing specific formulations of cannabis components for the treatment of cancer, inflammatory, autoimmune, and metabolic diseases. Our platform technology utilizes the Gi protein associated A3 adenosine receptor, or A3AR, as a therapeutic target. A3AR is highly expressed in pathological body cells such as inflammatory and cancer cells, and has a low expression in normal cells, suggesting that the receptor could be a specific target for pharmacological intervention. Our pipeline of drug candidates are synthetic, highly specific agonists and allosteric modulators targeting the A3AR.
Our product pipeline is based on the research of Dr. Pnina Fishman, who investigated a clinical observation that tumor metastasis can be found in most body tissues, but are rarely found in muscle tissue, which constitutes approximately 60% of human body weight. Dr. Fishman’s research revealed that one reason that striated muscle tissue is resistant to tumor metastasis is that muscle cells release small molecules which bind with high selectivity to the A3AR. As part of her research, Dr. Fishman also discovered that A3ARs have significant expression in tumor and inflammatory cells, whereas normal cells have low or no expression of this receptor. The A3AR agonists and allosteric modulators, currently our pipeline of drug candidates, bind with high selectivity and affinity to the A3ARs and upon binding to the receptor initiate down-stream signal transduction pathways resulting in apoptosis, or programmed cell death, of tumors and inflammatory cells and to the inhibition of inflammatory cytokines. Cytokines are proteins produced by cells that interact with cells of the immune system in order to regulate the body’s response to disease and infection. Overproduction or inappropriate production of certain cytokines by the body can result in disease. In addition, our product candidateds also induce the production of positive cytokines such as granulocyte colony stimulating factor (G-CSF) and adiponectin which are responsible for the chemo-protective and liver-protective effects of the drugs on liver
Our product candidates, CF101, CF102 and CF602, are being developed to treat oncological and inflammatory diseases, as well as erectile dysfunction. CF101, also known as Piclidenoson, is in an advanced stage of clinical development for the treatment of psoriasis. CF102, also known as Namodenoson, is being developed for the treatment of HCC and has orphan drug designation for this indication in the United States and Europe. Namodenoson was granted Fast Track designation by the FDA for patients with advanced HCC. who failed first line treatment. Namodenoson is also being developed for the treatment of pancreatic cancer based on pre-clinical findings showing robust anti-pancreatic tumor growth. Due to the liver protective effect of Namidenoson, it is also being developed for the treatment of NASH (also known as MASH). CF602 is our second generation allosteric drug candidate for the treatment of erectile dysfunction, which has shown efficacy in the treatment of erectile dysfunction in preclinical studies and we are investigating additional compounds, targeting A3AR, for the treatment of erectile dysfunction. Preclinical studies revealed that our drug candidates have potential to treat additional inflammatory diseases, such as Crohn’s disease, oncological diseases such as prostate cancer, viral diseases, such as the JC virus, obesity and Lowe Syndrome.
97
We believe our pipeline of drug candidates represent a significant market opportunity. For instance, according to iHealthcareAnalyst, the psoriasis drug market is forecasted to be worth $11.3 billion by 2025. According to DelveInsight, the HCC drug market in the G8 countries (U.S., Germany, France, Italy, Spain, UK, Japan and China) is expected to reach $3.8 billion by 2027.
We have in-licensed an allosteric modulator of the A3AR, CF602 from Leiden University. In addition, we have out-licensed the following product candidates for indications that we are currently pursuing:
| ● | Piclidenoson for the treatment of (i) psoriasis to Cipher Pharmaceuticals, or Cipher, for Canada, (ii) psoriasis to Gebro Holding, or Gebro, for Spain, Switzerland and Austria, (iii) psoriasis to CMS Medical, or CMS, for China (including Hong Kong, Macao and Taiwan), (iv) psoriasis to Kyongbo Pharm Co. Ltd., or Kyongbo Pharm, for South Korea, (v) psoriasis to Ewopharma AG, or Ewopharma, for Central Eastern Europe, and (vi) osteoarthritis in companion animals including dogs and cats to Vetbiolix. |
| ● | Namodenoson for the treatment of (i) liver cancer and NASH to Chong Kun Dang Pharmaceuticals, or CKD, for South Korea, (ii) advanced liver cancer and NAFLD/NASH to CMS for China (including Hong Kong, Macao and Taiwan), and (iii) HCC, NASH and pancreatic cancer to Ewopharma, for Central Eastern Europe and Switzerland. |
Currently, (i) we are undertaking preparatory work for pivotal Phase III studies for Piclidenoson in the treatment of psoriasis following meetings with the FDA & EMA, , which we expect to commence in the second half of 2024 (ii) we are conducting a pivotal Phase III trial for Namodenoson in the treatment of advanced liver cancer which is enrolling patients, (iii) we are conducting a Phase IIb study of Namodenoson in the treatment of NASH which is enrolling patients, (iv) we are undertaking preparatory work for Phase IIa study with Namodenoson for the treatment of pancreatic cancer, (v) we are investigating additional compounds, targeting the A3 adenosine receptor, for the treatment of erectile dysfunction, and (vi) we are conducting pre-clinical studies with formulations of cannabis components for the treatment of diseases in which there is an overexpression of A3AR. Since inception, we have incurred significant losses in connection with our research and development.
Moreover, we believe characteristics of Piclidenoson, as exhibited in our clinical studies to date, including its good safety profile, clinical activity, simple and less frequent delivery through oral administration and its low cost of production, position it well against the competition in psoriasis markets, where treatments, when available, often include injectable drugs, many of which can be highly toxic, expensive and not always effective.
Like Piclidenoson, Namodenoson has a good safety profile, is orally administered and has a low cost of goods, which we believe may position it well in the HCC market, where no drug has yet been approved by the FDA for patients with advanced liver cancer disease defined as Child Pugh B7. In addition, pre-clinical studies show Namodenoson’s novel mechanism of action which entails de-regulation of three key signaling pathways which mediate the etiology and pathology of NAFLD/NASH and are responsible for the anti-inflammatory, anti-steatotic and anti-fibrotic effect in the liver. Most recently, pre-clinical data support Piclidenoson’s potential utilization for the treatment of Lowe Syndrome and Namodenoson’s potential utilization as an anti-obesity drug.
Nevertheless, other drugs on the market, new drugs under development (including drugs that are in more advanced stages of development in comparison to our drug candidates) and additional drugs that were originally intended for other purposes, but were found effective for purposes targeted by us, may all be competitive to the current drugs in our pipeline. In fact, some of these drugs are well established and accepted among patients and physicians in their respective markets, are orally bioavailable, can be efficiently produced and marketed, and are relatively safe. None of our product candidates have been approved for sale or marketing and, to date, there have been no commercial sales of any of our product candidates.
Since inception, we have incurred significant losses in connection with our research and development. As of December 31, 2023, we had an accumulated deficit of approximately $158.5 million. Although we have recognized revenues in connection with our existing out-licensing agreements with, Cipher, CKD, Gebro and Ewopharma, and our historic out-licensing agreement with KD, CMS, Kyongbo and Seikagaku Corporation, or SKK, we expect to generate losses in connection with the research and development activities relating to our pipeline of drug candidates. Such research and development activities are budgeted to expand over time and will require further resources if we are to be successful. As a result, we expect to incur operating losses, which may be substantial over the next several years, and we will need to obtain additional funds to further develop or research and development programs.
98
We have funded our operations primarily through the sale of equity securities (both in private placements and in public offerings) and payments received under our existing out-licensing agreements with KD, Cipher, CKD Gebro, CMS, and Kyongbo and our historic out-licensing agreement with SKK. We expect to continue to fund our operations over the next several years through our existing cash resources, potential future milestone payments that we expect to receive from our licensees, interest earned on our investments, if any, and additional capital to be raised through public or private equity offerings or debt financings. As of December 31, 2023, we had approximately $4.3 million of cash and cash equivalents and $4.6 million of short-term deposits. A substantial part of this amount is designated for payments to be made in relation to the ongoing treatment of patients who are currently enrolled in the Company’s on-going trials. In November 2023, we raised approximately $3.0 million in gross proceeds (approximately $2.61 million net of issuance costs) from a warrant repricing transaction.
Revenues
Our revenues to date have been generated primarily from payments under our existing out-licensing agreements with, Cipher, CKD, Gebro, Kyongbo and Ewophrma, and our historic out-licensing agreement with Kwang Dong and SKK.
Under the Kwang Dong License Agreement, we are entitled to up-front and milestone payments of up to $1.5 million. In accordance with the Kwang Dong License Agreement, we received an up-front payment of $0.3 million and a payment of $0.048 million as consideration for Kwang Dong’s purchase of our ordinary shares in 2009 and a milestone payment of $0.2 million in 2010. Under the terms of the Kwang Dong License Agreement, in addition to the payments mentioned above, we are entitled to certain additional payments based on the sale of raw materials, subject to the terms and conditions of the respective agreements. To date, we have received a total of $0.5 million from Kwang Dong in an upfront payment. See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”.
Under the Distribution and Supply Agreement with Cipher we received CAD 1.65 million upon execution of the agreement and are entitled to milestone payments upon receipt of regulatory approval by Health Canada for Piclidenoson and the first delivery of commercial launch quantities as follows (i) CAD 1 million upon the first approved indication for either psoriasis or rheumatoid arthritis, and (ii) CAD 1 million upon the second approved indication for either psoriasis or rheumatoid arthritis. In addition, following regulatory approval, we shall be entitled to a royalty of 16.5% of net sales of Piclidenoson in Canada and reimbursement for the cost of manufacturing Piclidenoson. We are also entitled to a royalty payment for any authorized generic of Piclidenoson that Cipher distributes in Canada. To date, we have received a total of $1.3 million (CAD 1.65 million) from Cipher in an upfront payment. See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”.
The Distribution Agreement with CKD provides for up to $3,000,000 in upfront and milestone payments payable with respect to the liver cancer indication and up to $6,000,000 with respect to the NASH indication. In addition, we are entitled to a transfer price of the higher of (a) the manufacturing cost plus 10% or (b) 23% of net sales of Namodenoson following commercial launch in South Korea. To date, we have received a total of $2,000,000 from CKD, comprising $1,500,000 in upfront payments for the expansion of CKD’s existing agreement with us to include the rights to distribute Namodenoson for the treatment of NASH in South Korea, and a further $500,000 for a milestone payment received in the third quarter of 2017 upon receipt by CKD of a positive result from the preliminary review by the MFDS, on obtaining orphan drug designation in South Korea. See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”.
In January 2018, we entered into a Distribution and Supply Agreement with Gebro. The Distribution and Supply Agreement with Gebro provides that we are entitled to €1,500,000 upon execution of the agreement plus milestone payments upon achieving certain clinical, launch and sales milestones, as follows: (i) €300,000 upon initiation of the ACRobat Phase III clinical trial for the treatment of rheumatoid arthritis and €300,000 upon the initiation of the COMFORT Phase III clinical trial for the treatment of psoriasis, (ii) between €750,000 and €1,600,000 following first delivery of commercial launch quantities of Piclidenson for either the treatment of rheumatoid arthritis or psoriasis, and (iii) between €300,000 and up to €4,025,000 upon meeting certain net sales. In addition, following regulatory approval, we shall be entitled to double digit percentage royalties on net sales of Piclidenoson in the territories and payment for the manufacturing Piclidenoson. To date, we have received a total of €2,100,000 from Gebro in upfront and milestone payments. See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”.
99
In August 2018, we entered into a License, Collaboration and Distribution Agreement with CMS. Under the License, Collaboration and Distribution Agreement, we are entitled to $2,000,000 upon execution of the agreement plus milestone payments of up to $14,000,000 upon achieving certain regulatory milestones and payments of up to $58,500,000 upon achieving certain sales milestones. In addition, following regulatory approval, we shall be entitled to double-digit percentage royalties on net sales of Piclidenoson and Namodenoson in the licensed territories. To date, we have received a total of $2,000,000 from CMS in upfront and milestone payments. See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”.
In July 2019, we entered into a License and Distribution Agreement with Kyongbo Pharm. Under the terms of agreement, Kyongbo Pharm, in exchange for exclusive distribution rights to sell Piclidenoson in the treatment of psoriasis in South Korea, made a total upfront payment of $750,000 to us, with additional payments of up to $3,250,000 upon achievement of certain milestones. We will also be entitled to a transfer price for delivering finished product to Kyongbo Pharm. To date, we have received a total of $750,000 from Kyongbo Pharm in upfront and milestone payments.
In March 2021, we signed an exclusive distribution agreement with Switzerland-based Ewopharma for Piclidenoson in the treatment of psoriasis and Namodenoson in the treatment of liver diseases namely, HCC, the most common form of liver cancer, and NASH. Under the terms of the distribution agreement, Ewopharma we received $2.25 million upfront and are entitled to up to an additional $40.45 million payable upon the achievement of regulatory and sales milestones plus 17.5% royalties on net sales. We will also be entitled to a transfer price for delivering finished product to Ewopharma. In January 2024, we entered into an amendment to the distribution agreement with Ewopharma to expand the distribution to include the indication of pancreatic cancer with respect to Namodenoson. To date, we have received a total of $2,250,000 from Ewopharma in upfront, milestone and royalty payments.
Under the terminated SKK license agreement we received an aggregate of approximately $8.5 million from SKK. See “Item 4. Information on the Company—B. Business—Out-Licensing and Distribution Agreements”.
Certain payments we have received from SKK and KD have been subject to a 10% and 5% withholding tax in Japan and Korea, respectively, and certain payments we may receive in the future, if at all, may also be subject to the same withholding tax in Korea. Receipt of any milestone payment under our out-licensing agreements depends on many factors, some of which are beyond our control. We cannot assure you that we will receive any of these future payments. We expect our revenues for the next several years, if any, to be derived primarily from payments under our current out-license agreements and our public capital raising activities, as well as additional collaborations that we may enter into in the future with respect to our drug candidates.
Research and Development
Our research and development expenses consist primarily of salaries and related personnel expenses, fees paid to external service providers, up-front and milestone payments under our license agreements, patent-related legal fees, costs of preclinical studies and clinical trials, drug and laboratory supplies and costs for facilities and equipment. We charge all research and development expenses to operations as they are incurred. We expect our research and development expense to remain our primary expense in the near future as we continue to develop our products. Increases or decreases in research and development expenditures are attributable to the number and/or duration of the pre-clinical and clinical studies that we conduct.
100
The following table identifies our current major research and development projects:
| Project | Status | Expected or Recent Near Term Milestone | ||
| Piclidenoson | COMFORT Phase III study in psoriasis | Commencement of pivotal Phase III studies following meetings with FDA & EMA expected in second half of 2024. | ||
| Namodenoson | Phase III in HCC | Enrolling patients | ||
| Phase IIb study in NASH | Enrolling patients | |||
| Phase IIa study in pancreatic cancer | Preparatory work for Phase IIa study |
We record certain costs for each development project on a “direct cost” basis, as they are recorded to the project for which such costs are incurred. Such costs include, but are not limited to, CRO expenses, drug production for pre-clinical and clinical studies and other pre-clinical and clinical expenses. However, certain other costs, including but not limited to, salary expenses (including salaries for research and development personnel), facilities, depreciation, share-based compensation and other overhead costs are recorded on an “indirect cost” basis, i.e., they are shared among all of our projects and are not recorded to the project for which such costs are incurred. We do not allocate direct salaries to projects due to the fact that our project managers are generally involved in several projects at different stages of development, and the related salary expense is not significant to the overall cost of the applicable projects. In addition, indirect labor costs relating to our support of the research and development process, such as manufacturing, controls, pre-clinical analysis, laboratory testing and initial drug sample production, as well as rent and other administrative overhead costs, are shared by many different projects and have never been considered by management to be of significance in its decision-making process with respect to any specific project. Accordingly, such costs have not been specifically allocated to individual projects.
Set forth below is a summary of the gross direct costs allocated to our main projects on an individual basis, as well as the gross direct costs allocated to our less significant projects on an aggregate basis, for the years ended December 31, 2021, 2022 and 2023; and on an aggregate basis since project inception:
| (USD in thousands) | Total Costs | |||||||||||||||
| Year Ended December 31, | Since Project | |||||||||||||||
| 2021 | 2022 | 2023 | Inception | |||||||||||||
| Piclidenoson | 4,041 | 2,790 | 1,471 | 48,058 | ||||||||||||
| Namodenoson | 3,991 | 3,383 | 2,989 | 22,399 | ||||||||||||
| CF602 | 31 | 6 | - | 1,740 | ||||||||||||
| Other projects | - | - | - | 4,129 | ||||||||||||
| Total gross direct project costs (1) | 8,063 | 6,179 | 4,460 | 76,326 | ||||||||||||
| (1) | Does not include indirect project costs and overhead, such as payroll and related expenses (including stock-based compensation), facilities, depreciation and impairment of intellectual property, which are included in total research and development expenses in our financial statements. |
From our inception through December 31, 2023, we have incurred research and development expenses of approximately $146.2 million. We expect that a large percentage of our research and development expense in the future will be incurred in support of our current and future preclinical and clinical development projects. Due to the inherently unpredictable nature of preclinical and clinical development processes and given the early stage of our preclinical product development projects, we are unable to estimate with any certainty the costs we will incur in the continued development of the product candidates in our pipeline for potential commercialization. Clinical development timelines, the probability of success and development costs can differ materially from expectations. We expect to continue to test our product candidates in preclinical studies for toxicology, safety and efficacy, and to conduct additional clinical trials for each product candidate. If we are not able to enter into an out-licensing arrangement with respect to any product candidate prior to the commencement of later stage clinical trials, we may fund the trials for the product candidates ourselves.
101
While we are currently focused on advancing each of our product development projects, our future research and development expenses will depend on the clinical success of each product candidate, as well as ongoing assessments of each product candidate’s commercial potential. In addition, we cannot forecast with any degree of certainty which product candidates may be subject to future out-licensing arrangements, when such out-licensing arrangements will be secured, if at all, and to what degree such arrangements would affect our development plans and capital requirements.
As we obtain results from clinical trials, we may elect to discontinue or delay clinical trials for certain product candidates or projects in order to focus our resources on more promising product candidates or projects. Completion of clinical trials by us or our licensees may take several years or more, but the length of time generally varies according to the type, complexity, novelty and intended use of a product candidate.
The cost of clinical trials may vary significantly over the life of a project as a result of differences arising during clinical development, including, among others:
| ● | the number of sites included in the clinical trials; |
| ● | the length of time required to enroll suitable patients; |
| ● | the number of patients that participate in the clinical trials; |
| ● | the duration of patient follow-up; |
| ● | the development stage of the product candidate; and |
| ● | the efficacy and safety profile of the product candidate. |
We expect our research and development expenses to increase in the future from current levels as we continue the advancement of our clinical trials and preclinical product development and to the extent we in-license new product candidates. The lengthy process of completing clinical trials and seeking regulatory approval for our product candidates requires expenditure of substantial resources. Any failure or delay in completing clinical trials, or in obtaining regulatory approvals, could cause a delay in generating product revenue and cause our research and development expenses to increase and, in turn, have a material adverse effect on our operations. Because of the factors set forth above, we are not able to estimate with any certainty when we would recognize any net cash inflows from our projects.
General and Administrative Expenses
General and administrative expenses consist primarily of compensation for employees in executive and operational functions, including accounting, finance, legal, business development, investor relations, information technology and human resources. Other significant general and administration costs include facilities costs, professional fees for outside accounting and legal services, travel costs, insurance premiums and depreciation.
Financial Expense and Income
Financial expense and income consists of interest earned on our cash and cash equivalents; bank fees and other transactional costs; expense or income resulting from fluctuations of the NIS and other currencies, in which a portion of our assets and liabilities are denominated, against the U.S. dollar (our functional currency).
Critical Accounting Policies and Estimates
Our accounting policies and their effect on our financial condition and results of operations are more fully described in our audited consolidated financial statements included elsewhere in this Annual Report on Form 20-F. The preparation of financial statements in conformity with U.S. GAAP as issued by the Financial Accounting Standards Board (FASB) requires management to make estimates and assumptions that in certain circumstances affect the reported amounts of assets and liabilities, revenues and expenses and disclosure of contingent assets and liabilities. These estimates are prepared using our best judgment, after considering past and current events and economic conditions. While management believes the factors evaluated provide a meaningful basis for establishing and applying sound accounting policies, management cannot guarantee that the estimates will always be consistent with actual results. In addition, certain information relied upon by us in preparing such estimates includes internally generated financial and operating information, external market information, when available, and when necessary, information obtained from consultations with third party experts. Actual results could differ from these estimates and could have a material adverse effect on our reported results.
102
We believe that the accounting policies discussed below are critical to our financial results and to the understanding of our past and future performance, as these policies relate to the more significant areas involving management’s estimates and assumptions. We consider an accounting estimate to be critical if: (1) it requires us to make assumptions because information was not available at the time or it included matters that were highly uncertain at the time we were making our estimate; and (2) changes in the estimate could have a material impact on our financial condition or results of operations.
Functional and Presentation Currency
Our functional and presentation currency is the U.S. dollar since the USD is the primary currency of the economic environment in which we operate.
Principles of Consolidation
Our financial statements reflect the consolidation of the financial statements of companies that we control based on legal control or effective control. We fully consolidate into our financial statements the results of operations of companies that we control. Legal control exists when we have the power, directly or indirectly, to govern the financial and operating policies of an entity. The effect of potential voting rights that are exercisable at the balance sheet date are considered when assessing whether we have legal control. In addition, we consolidate on the basis of effective control even if we do not have voting control.
Revenue Recognition
We generate income from out-licensing and distribution agreements. See “Item 4. Information on the Company—B. Business—Out-Licensing and Distribution Agreements”. Such income comprises of upfront license fees, milestone payments and potential royalty payments.
We recognize revenue in accordance with ASC 606, “Revenue from Contracts with Customers” pursuant to which each required deliverable is evaluated to determine whether it qualifies as a separate unit of accounting based on whether the deliverable has “stand-alone value” to the customer. The arrangement’s consideration that is fixed or determinable is then allocated to each separate unit of accounting based on the relative selling price of each deliverable which is based on the Estimated Selling Price.
Our contracts generally include three contract obligations: (i) performing the research and development services through regulatory approval; (ii) delivery of an exclusive licensing to distribute the product, once available; and, (iii) participation in joint steering committee.
Our contracts also include development milestones payments and future sales-based royalties. Development milestones payments are recognized only to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with those milestones is subsequently resolved. As such sales based royalties are recognized only when the subsequent sale occurs. We have not yet received the required regulatory approvals for our products and we have not yet recognized any sales-based royalties.
Revenue from supply and distribution agreements with customers are recognized over time as we satisfy the performance obligations. We usually accept long-term upfront payment from our customers. Contract liabilities for those upfront payments are recognized as revenue over time. We have concluded that the performance obligation in the abovementioned agreements should be combined for a single performance obligation for accounting purposes.
Revenues from milestone payments:
Contingent payments related to milestones will be recognized upon satisfaction of the milestone and contingent payments related to royalties will be recognized in the period that the related sales have occurred.
Revenues from royalties:
Revenues from royalties will be recognized as they accrue in accordance with the terms of the relevant agreement.
103
Share-based Compensation
We account for share-based compensation arrangements in accordance with ASC 718, “Compensation - Stock Compensation” (“ASC 718”), which requires companies to estimate the fair value of equity-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods in the Company’s consolidated statement of comprehensive loss. We recognize compensation expenses for the value of its awards granted based on the vesting acceleration approach over the requisite service period of each of the awards, net of estimated forfeitures. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
We selected the binomial option pricing model as the most appropriate method for determining the estimated fair value of our share-based awards. The determination of the grant date fair value of options using an option pricing model is affected by estimates and assumptions regarding a number of complex and subjective variables. These variables include the expected volatility of our share price over the expected term of the options, share option exercise and forfeiture rate, risk-free interest rates, expected dividends and the price of our ordinary shares on the TASE. As our ordinary shares are publicly traded on the TASE, we do not need to estimate the fair value of our ordinary shares. Rather, we use the actual closing market price of our ordinary shares on the date of grant, as reported by the TASE although in the future may use the closing market price of our ADSs on the date of grant, as reported by the NYSE American.
If any of the assumptions used in the binomial option pricing model change significantly, share-based compensation for future awards may differ materially compared with the awards previously granted.
As for other service providers, the cost of the transactions is measured at the fair value of the goods or services received as consideration for equity instruments. In cases where the fair value of the goods or services received as consideration of equity instruments cannot be measured, they are measured by reference to the fair value of the equity instruments granted.
The cost of equity-settled transactions is recognized in statements of comprehensive loss, together with a corresponding increase in equity, during the period which the service are to be satisfied, ending on the date on which the relevant employees or other service providers become fully entitled to the award.
If we modify the conditions on which equity-instruments are granted, an additional expense is recognized for any modification that increases the total fair value of the share-based payment arrangement or is otherwise beneficial to the employee or other service provider at the modification date.
Recently Issued Accounting Pronouncements
In May 2021, the FASB issued ASU No. 2021-04, Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options. The ASU addresses the previous lack of specific guidance in the accounting standards codification related to modifications or exchanges of freestanding equity-classified written call options (such as warrants) by specifying the accounting for various modification scenarios. The ASU is effective for interim and annual periods beginning after December 15, 2021, with early adoption permitted for any periods after issuance to be applied as of the beginning of the fiscal year that includes the interim period. The Company adopted this standard effective January 1, 2022. The adoption of this standard did not have a material impact on the Company’s consolidated financial statements.
In August 2020, the FASB issued Accounting Standards Update No. 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (ASU 2020-06), which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts on an entity’s own equity. This guidance also eliminates the treasury stock method to calculate diluted earnings per share for convertible instruments and requires the use of the if-converted method. The Company’s adopted the standard effective January 1, 2022. Adoption of the new standard did not have a material impact on Company’s consolidated financial statements.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280), Improvements to Reportable Segment Disclosures, which expands annual and interim disclosure requirements for reportable segments, primarily through enhanced disclosures about significant segment expenses. In addition, it provides new segment disclosure requirements for entities with a single reportable segment. The guidance will be effective for the Company for annual periods beginning January 1, 2024 and for interim periods beginning January 1, 2025. Early adoption is permitted. The Company is currently evaluating the impact on its financial statement disclosures.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740), Improvements to Income Tax Disclosures, which requires disaggregated information about the effective tax rate reconciliation as well as information on income taxes paid. The guidance will be effective for the Company for annual periods beginning January 1, 2025, with early adoption permitted. The Company is currently evaluating the impact on its financial statement disclosures.
Recent Offerings
In February and March 2021, we issued 50,926,830 ordinary shares represented by 169,756 ADSs in exchange for exercise of warrants. Total consideration received by us was approximately $2,744,000.
104
On August 16, 2021, we sold to an institutional investor an aggregate of (i) 57,000,000 ordinary shares represented by 190,000 ADSs at $20.0 per ADS, and (ii) 93,000,000 ordinary shares represented by 310,000 ADSs issuable upon the exercise of pre-funded warrants, or Pre-funded Warrants, at a price of $19.99 per Pre-funded Warrant, in a registered direct offering. The Pre-funded Warrants were exercised in full during 2021. In addition, we issued to the investors unregistered warrants to purchase 150,000,000 ordinary shares represented by 500,000 ADSs in a private placement. The warrants are immediately exercisable and will expire three years from the effectiveness of an initial resale registration statement registering the ordinary shares issuable upon the exercise of the warrants. We paid on aggregate of $700,000 in placement agent fees and expenses and issued placement agent warrants in an amount equal to 7.0% of the aggregate number of ADSs sold in the offering (or warrants to purchase up to an aggregate of 35,000 ADSs), at an initial exercise price equal to $20.0 per ADS, on substantially the same terms as the investor warrants, except that the placement agent warrants expire on the earlier of (i) the third-year anniversary of the date on which an initial resale registration statement registering the ordinary shares (or the ADSs) issuable upon the exercise of the warrants becomes effective and (ii) August 11, 2026.
On December 20, 2021, we entered into a warrant exercise agreement, or the Exercise Agreement, with an institutional investor, or the Holder, of warrants issued in August 2021, or the Warrants, to purchase ordinary shares, represented by ADS, pursuant to which the Holder agreed to exercise in cash its Warrant to purchase up to an aggregate of 150,000,000 ordinary shares represented by 500,000 ADSs having an exercise price of $20.0 per ADS, resulting in gross proceeds of $10.0 million. Closing occurred on December 23, 2021. Under the Exercise Agreement, we also issued to the Holder new unregistered warrants to purchase up to 180,000,000 ordinary shares represented by 600,000 ADSs, or the Private Placement Warrants. The Private Placement Warrants are immediately exercisable, expire five years following the effectiveness of an initial resale registration statement registering the ADSs issuable upon the exercise of the warrants and have an exercise price of $5.5 per ADS (following a reduction from $20.0 per ADS as a result of the January 2023 offering), subject to adjustment as set forth therein. We paid an aggregate of $875,000 in placement agent fees and expenses and issued unregistered placement agent warrants to purchase 35,000 ADS on the same terms as the warrant.
On January 11, 2023, we sold to an institutional investor (i) 90,000,000 ordinary shares represented by 300,000 ADSs at a purchase price of $5.50 per ADS, and (ii) pre-funded warrants to Purchase up to 210,000,000 ordinary shares represented by 700,000 ADSs at an offering price of $5.499 per pre-funded warrant, in a registered direct offering. The pre-funded warrants were exercised in full. In a concurrent private placement, we also issued pre-funded warrants to purchase up to an aggregate of 109,091,100 ordinary shares represented by 363,637 ADS, at the same purchase price as in the registered direct offering. We have also issued unregistered Series A warrants to purchase up to an aggregate of 409,091,100 ordinary shares represented by 1,363,637 ADSs for an exercise price of $0.02 per share and Series B warrants to purchase up to an aggregate of 409,091,100 ordinary shares represented by 1,363,637 ADSs for an exercise price of $0.018 per share. In addition, we paid an aggregate of $973,000 in placement agent fees and expenses and issued 28,636,500 ordinary shares represented by 94,455 ADSs issuable upon the exercise of placement agent warrants for an exercise price of $0.02 per share. The offerings closed on January 13, 2023.
On November 21, 2023, we entered into an inducement offer letter agreement, or the Inducement Letter, with a certain holder, or the Holder, of certain of our existing warrants to purchase up to (i) 1,363,637 ADSs representing 409,091,100 ordinary shares issued in January 2023 at an exercise price of $6.00 per ADS, or the January 2023 Warrants, and (ii) 600,000 ADSs representing 180,000,000 ordinary shares issued in December 2021 at an exercise price of $5.50 per ADS, or the December 2021 Warrants and together with the January 2023 Warrants, the Existing Warrants. Pursuant to the Inducement Letter, the Holder agreed to exercise for cash its Existing Warrants to purchase an aggregate of 1,963,637 ADSs representing 589,091,100 ordinary shares at a reduced exercise price of $1.53 per ADS in consideration of our agreement to issue new warrants to purchase ADSs, or the New Warrants, as described below, to purchase up to an aggregate of 3,927,274 ADSs representing 1,178,182,200 ordinary shares, or the New Warrant Shares, at an exercise price of $1.75 per ADS (subject to adjustment as set forth therein). In addition, we also agreed to reduce the exercise price of certain series B warrants to purchase 1,363,637 ADSs representing 409,091,100 ordinary shares issued on January 13, 2023 and held by the Holder from $5.50 per ADS to $1.75 per ADS and extend the term of such series B warrants to twenty months from the closing date of the transactions contemplated by the Inducement Letter. We paid an aggregate of approximately $386,000 in placement agent fees and expenses and issued unregistered placement agent warrants to purchase 137,455 ADS on the same terms as the warrant.
105
A. Results of Operations
Comparison of the Year Ended December 31, 2023 to Year Ended December 31, 2022
Revenues
Revenues for the year ended December 31, 2023 were $0.74 million, a decrease of $0.07 million, or 8.6%, compared to $0.81 million for the year ended December 31, 2022. The decrease in revenues was mainly due to the recognition a lower portion of advance payments received under the Ewopharma distribution agreement entered in 2021 and a lower portion of advance payments received under distribution agreements from Gebro, Chong Kun Dung Pharmaceuticals, and Cipher Pharmaceuticals.
Research and development expenses
Research and development expenses for the year ended December 31, 2023 were $5.98 million, a decrease of $1.78 million, or 22.9%, compared to $7.76 million for the year ended December 31, 2022. Research and development expenses for the year ended December 31, 2023 comprised primarily of expenses associated with the completion of the Phase 3 study of Piclidenoson for the treatment of psoriasis and two ongoing studies for Namodenoson, a Phase 3 study in the treatment of advanced liver cancer and a Phase 2b study for NASH. The decrease is primarily due to a decrease in expenses associated with Piclidenosnon.
General and administrative expenses
General and administrative expenses were $2.95 million for the year ended December 31, 2023 a decrease of $0.19 million, or 6.05%, compared to $3.14 million for the year ended December 31, 2022. The decrease is primarily due to the decrease in directors and officer’s insurance policy premium. We expect that general and administrative expenses will remain at the same level through 2024.
Financial income (expenses), net
Financial income (expense), net for the year ended December 31, 2023 aggregated $0.56 million compared to financial expense, net of $(0.07) for the year ended December 31, 2022. The decrease in financial expense, net was mainly due to increase interest from deposits and reduction in expenses related to the revaluation of our short-term investment.
Comparison of the Year Ended December 31, 2022 to Year Ended December 31, 2021
Revenues
Revenues for the year ended December 31, 2022 were $0.81 million, a decrease of $0.04 million, or 5.1%, compared to $0.85 million for the year ended December 31, 2021. The decrease in revenues was mainly due to the recognition a higher portion of advance payments received under Ewopharma distribution agreement entered in 2021 which was set off by recognition of a lower portion of advance payments received under distribution agreements from Gebro, Chong Kun Dung Pharmaceuticals, and Cipher Pharmaceuticals.
Research and development expenses
Research and development expenses for the year ended December 31, 2022 were $7.76 million, a decrease of $2.09 million, or 21.2%, compared to $9.85 million for the year ended December 31, 2021. Research and development expenses for the year ended December 31, 2022 comprised primarily of expenses associated with the completion of the Phase III study of Piclidenoson for the treatment of psoriasis and two ongoing studies for Namodenoson, a Phase III study in the treatment of advanced liver cancer and a Phase IIb study for NASH. The decrease is primarily due to the wrap up of the Phase III study of Piclidenoson for the treatment of psoriasis in 2022. We expect that the research and development expenses will increase through 2023 and beyond.
General and administrative expenses
General and administrative expenses were $3.14 million for the year ended December 31, 2022 a decrease of $0.70 million, or 18%, compared to $3.84 million for the year ended December 31, 2021. The decrease is primarily due to the decrease in professional services and public and investor relations expenses. We expect that general and administrative expenses will remain at the same level through 2023.
106
Financial income (expenses), net
Financial income (expense), net for the year ended December 31, 2022 aggregated $(0.07) million compared to financial expense, net of $0.23 for the year ended December 31, 2021. The decrease in financial expense, net was mainly due to increase in the revaluation of our short-term investment.
B. Liquidity and Capital Resources
Since inception, we have funded our operations primarily through public (in Israel and US) and private offerings of our equity securities and payments received under our strategic licensing arrangements. As of December 31, 2023, we had approximately $8.9 million in cash, cash equivalents and short-term deposits, and have invested most of our available cash funds in ongoing cash accounts. Under Accounting Standard Codification (“ASC”) Subtopic 205-40, Presentation of Financial Statements—Going Concern (“ASC 205-40”), the Company has the responsibility to evaluate whether conditions and/or events raise substantial doubt about its ability to meet its obligations as they become due within one year after the date that the financial statements are issued. As required under ASC 205-40, management’s evaluation should initially not take into consideration the potential mitigating effects of management’s plans that have not been fully implemented as of the date the financial statements are issued.
For information regarding the revenues and expenses associated with our licensing agreements, see “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”, “Item 4. Information on the Company—B. Business Overview—In-Licensing Agreements” and “Item 5. Operating and Financial Review and Prospects—Revenues.”
Developing drugs, conducting clinical trials and commercializing products is expensive and we will need to raise substantial additional funds to achieve our strategic objectives. Although we believe our existing financial resources as of the date of issuance of this Annual Report on Form 20-F, will be sufficient to fund our projected cash requirements at least through the next twelve months, we will require significant additional financing to fund our operations. Additional financing may not be available on acceptable terms, if at all. Our future capital requirements will depend on many factors, including:
| ● | the level of research and development investment required to develop our product candidates; |
| ● | the failure to obtain regulatory approval or achieve commercial success of our product candidates, including Piclidenoson, Namodenoson and CF602; |
| ● | the results of our preclinical studies and clinical trials for our earlier stage product candidates, and any decisions to initiate clinical trials if supported by the preclinical results; |
| ● | the costs, timing and outcome of regulatory review of our product candidates that progress to clinical trials; |
| ● | our ability to partner or sub-license any of our product candidates; |
| ● | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our issued patents and defending intellectual property-related claims; |
| ● | the cost of commercialization activities if any of our product candidates are approved for sale, including marketing, sales and distribution costs; |
| ● | the cost of manufacturing our product candidates and any products we successfully commercialize; |
| ● | the timing, receipt and amount of sales of, or royalties on, our future products, if any; |
| ● | the expenses needed to attract and retain skilled personnel; |
107
| ● | any product liability or other lawsuits related to our products; |
| ● | the extent to which we acquire or invest in businesses, products or technologies and other strategic relationships; |
| ● | the costs of financing unanticipated working capital requirements and responding to competitive pressures; and |
| ● | maintaining minimum shareholders’ equity requirements and complying with other continue listing standards under the NYSE American Company Guide; and |
| ● | the impact of the COVID-19 outbreak, the Russian invasion of Ukraine and the war between Israel and Hamas, which may exacerbate the magnitude of the factors discussed above. |
Until we can generate significant continuing revenues, we expect to satisfy our future cash needs through payments received under our license agreements, debt or equity financings, or by out-licensing other product candidates. We cannot be certain that additional funding will be available to us on acceptable terms, or at all. If funds are not available, we may be required to delay, reduce the scope of, or eliminate one or more of our research or development programs or our commercialization efforts.
Cash Flows
The following tables sets forth selected cash flow information for the periods indicated:
| Year ended December 31, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| U.S. $ in thousands | ||||||||||||
| Cash used in operating activities: | $ | (8,440 | ) | $ | (10,801 | ) | $ | (9,858 | ) | |||
| Cash used in investing activities: | $ | 498 | $ | 9,502 | $ | (14,511 | ) | |||||
| Cash provided by financing activities: | $ | 9,144 | $ | - | $ | 20,457 | ||||||
| Exchange differences on balances of cash and cash equivalents | $ | 98 | $ | (113 | ) | $ | 34 | |||||
| $ | 1,300 | $ | (1,472 | ) | $ | (3,878 | ) | |||||
Net cash used in operating activities was $8.4 million for the year ended December 31, 2023, compared with net cash used in operating activities of $10.8 million and $9.85 million for the years ended December 31, 2022 and 2021, respectively. The $2.4 million decrease in the net cash used in operating activities during 2023, compared to 2022, was primarily the result of decrease in our operating loss of approximately $2 million.
Net cash provided by investing activities for the year ended December 31, 2023 was $0.5 million compared to net cash used in investing activities of $9.5 million and $14.5 million for the years ended December 31, 2022 and December 31, 2021, respectively. The decrease in net cash provided by investing activities for the year ended December 31, 2023 compared with 2022 was primarily due to a lower maturity of a short term deposit in 2023 compared with 2022.
Net cash provided by financing activities was $9.1 million for the year ended December 31, 2023, compared to no net cash provided by financing activities for the year ended December 31, 2022 and $20.4 million of net cash provided by financing activities for the year ended December 31, 2021. The increase in net cash provided by financing activities during 2023 compared to 2022 was due to an increase in issuance of shares and warrants, net of issuance expenses during 2023.
The following table summarizes our significant contractual obligations in U.S. dollars as of December 31, 2023:
| Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | ||||||||||||||||
| Leiden University milestones(1) | 33,181 | 11,060 | 22,121 | - | - | |||||||||||||||
| Car lease obligations | 46,101 | 23,520 | 22,581 | - | - | |||||||||||||||
| Total | 79,282 | 34,580 | 44,702 | - | - | |||||||||||||||
| (1) | The obligations above do not include a potential milestone payment of €50,000 upon the initiation of a Phase I study, €100,000 upon the initiation of a Phase II study, €200,000 upon the initiation of a Phase III study or €500,000 upon marketing approval by any regulatory authority. |
Other than as described above, we did not have any material commitments for capital expenditures, including any anticipated material acquisition of plant and equipment or interests in other companies, as of December 31, 2023.
108
C. Research and Development, Patents and Licenses, Etc.
For information concerning our research and development policies and a description of the amount spent during each of the last three fiscal years on company-sponsored research and development activities, see “Item 5. Operating and Financial Review and Prospects— Results of Operation.”
D. Trend Information
We are a development stage company and it is not possible for us to predict with any degree of accuracy the outcome of our research, development or commercialization efforts. As such, it is not possible for us to predict with any degree of accuracy any significant trends, uncertainties, demands, commitments or events that are reasonably likely to have a material effect on our net sales or revenues, income from continuing operations, profitability, liquidity or capital resources, or that would cause financial information to not necessarily be indicative of future operating results or financial condition. However, to the extent possible, certain trends, uncertainties, demands, commitments and events are in this “Operating and Financial Review and Prospects.”
E. Critical Accounting Estimates
We prepare our financial statements in accordance with U.S. GAAP. In doing so, we must make estimates and assumptions that affect our reported amounts of assets, liabilities and expenses, as well as related disclosure of contingent assets and liabilities. In some cases, we could reasonably have used different accounting policies and estimates. Changes in the accounting estimates are reasonably likely to occur from period to period. Accordingly, actual results could differ materially from our estimates. To the extent that there are material differences between these estimates and actual results, our financial condition or results of operations will be affected. Significant estimates include, but are not limited to, those related to deferred revenue, revenue recognition, stock-based compensation and fair value of marketable debt securities. For further significant accounting policies please see Note 2 to our audited consolidated financial statements of this annual report. We believe that our accounting policies contained therein are critical in fully understanding and evaluating our financial condition and operating results.
ITEM 6. Directors, Senior Management and Employees
A. Directors and Senior Management.
The following table sets forth our directors and senior management:
| Member | Age | Position | ||
| Pnina Fishman, Ph.D. | 74 | Chairman of the Board, Chief Scientific Officer | ||
| Motti Farbstein | 60 | Chief Executive, Financial and Operating Officer | ||
| Sari Fishman, Ph.D. | 51 | VP of Business Development | ||
| Ilan Cohn, Ph.D. | 67 | Director | ||
| Guy Regev(1)(2)(3)(4)(5) | 53 | Director | ||
| Abraham Sartani, M.D.(4)(5) | 76 | Director | ||
| Yoseph Bornstein(1)(2)(4)(5) | 65 | Director | ||
| Yaacov Goldman (1)(2)(3)(4)(5) | 67 | Director |
| (1) | Member of the Compensation Committee |
| (2) | Member of the Audit Committee |
| (3) | External Director under Israeli Law |
| (4) | Independent Director under Israeli Law |
| (5) | Independent Director under the NYSE American rules |
109
Pnina Fishman, Ph.D. Pnina Fishman, Ph.D. co-founded Can-Fite, has served as our Chief Scientific Officer since June 2023, served on our Board of Directors since September 2005 and since June 2023 has served as the Chairman of the Can-Fite Board of Directors. Dr. Fishman also served as our Chief Executive Officer from 2005 to June 2023. Dr. Fishman is the scientific founder of Can-Fite and was previously a professor of Life Sciences and headed the Laboratory of Clinical and Tumor Immunology at the Felsenstein Medical Research Institute, Rabin Medical Center, Israel. Dr. Fishman has authored or co-authored over 150 publications and presented the findings of her research at many major scientific meetings. Her past managerial experience included seven years as Chief Executive Officer of Mor Research Application, the technology transfer arm of Clalit Health Services, the largest healthcare provider in Israel. Mor Research Application was also the first clinical research organization in Israel. Dr. Fishman currently also serves as a member of the Board of Directors of F.D Consulting Ltd., Eye-Fite Ltd. and Ultratrend. Dr. Fishman holds a Ph.D. in Immunology from the Bar Ilan University in Ramat Gan, Israel.
Motti Farbstein. Motti Farbstein has been with Can-Fite since 2003. Mr. Farbstein has served as our Chief Executive Officer since June 2023. Mr. Farbstein served as our Chief Operating Officer from August 2003 until May 2005 and from that date onwards he served as Chief Operating and Financial Officer. Mr. Farbstein also serves as a director of Eye-Fite Ltd. since July 2011. Mr. Farbstein’s past managerial experience includes seven years as Vice President of Mor Research Application, a company that managed the commercialization of the intellectual property of all hospitals and research centers affiliated with Clalit Health Services, which is the largest healthcare provider in Israel and was Israel’s first clinical CRO. Mr. Farbstein also has extensive experience in the data management of clinical trials.
Sari Fishman, Ph.D. Sari Fishman, Ph.D. has served as our Director Clinical Affairs from 2004 to 2014, Director of Business Development from 2014 to 2017 and since 2017 serves as VP of Business Development. Dr. Fishman gained her Ph.D. at the Bar-Ilan University, Ramat-Gan, Israel. Dr. Sari Fishman is the daughter of Pnina Fishman, our Chief Scientific Officer and the Chairman of our Board of Directors .
Abraham Sartani, M.D. Abraham Sartani has served on our Board of Directors since 2001. Dr. Sartani has over 30 years of experience in the pharmaceuticals industry and currently acts as a consultant to pharmaceutical and medical device companies. Dr. Sartani is a member of a number of scientific and management societies and the author or co-author of numerous publications and patents in the urology, pain treatment and hypertension fields. Dr. Sartani previously served on the Board of Directors of Akkadeas Pharma Srl (formerly Arkadia Pharma) and was a co-founder. From 1985 until 2008, Dr. Sartani was the Vice-President of R&D and Licensing and Group coordinator of B&D of Recordati, a European specialty pharmaceutical company. Prior to joining Recordati, from 1980 until 1985, Dr. Sartani was employed at Farmitalia-Carlo Erba, serving in a number of capacities, including as the Medical Director for Europe. Currently, Dr. Sartani is a member of the board of directors of BLV Pharma Group Srl, a privately owned Italian food supplements company.
Ilan Cohn, Ph.D. Ilan Cohn, Ph.D. is a patent attorney and a founding partner at the patent attorney firm Cohn, de Vries, Stadler & Co. since December 2020. Previously, Dr. Cohen was a senior partner at Reinhold Cohn and Partners, where he has been an attorney since 1986. Dr. Cohn co-founded Can-Fite, served as its Chief Executive Officer until September 2004, served on our Board of Directors since 1994 and from May 2013 to June 2023 served as the Chairman of the Can-Fite Board of Directors. Dr. Cohn has also been a director of OphthaliX since November 21, 2011 and until control was transferred. Dr. Cohn is a patent attorney with many years of experience in the biopharmaceutical field. He has served on the Board of Directors of a number of life science companies, including Discovery Laboratories Inc. (formerly Ansan Pharmaceuticals), a U.S. public company. Dr. Cohn has also been involved in the past in management of venture capital funds focused on investments in the life sciences industry. Dr. Cohn served a number of years as a co-chairman of the Biotech Committee of the US-Israeli Science and Technology Commission. Dr. Cohn is also currently a member of the Board of Directors of I.C.R.C. Management Ltd, RedDress Medical Ltd. and Feelter Sales Tools Ltd. Dr. Cohn holds a Ph.D. in Biology from the Hebrew University of Jerusalem.
110
Guy Regev. Guy Regev has over fourteen years of experience in accounting, financial management and control and general management of commercial enterprises. He has served on our Board of Directors since July 2011 and has served as a member of our Audit Committee and Compensation Committee since February 2014. Mr. Regev has also been a director of OphthaliX since November 2011. Mr. Regev is currently the Chief Executive Officer of Gaon Holdings Ltd, a publicly traded Israeli holding company traded on the TASE which focuses on three areas of operation - Cleantech / Water, Financial Services, Retail/Trading. Mr. Regev is currently also the Chief Executive Officer of Middle East Tube Company Ltd a publicly traded Israeli company traded on the TASE which focuses on steel pipe manufacturing and galvanization services. Mr. Regev was the Chief Executive Officer of Shaked Global Group Ltd, a privately-held equity investment firm that provides value added capital to environmental-related companies and technologies. Prior to joining Shaked, from 2001 to 2008, Mr. Regev was Vice President of Commercial Business at Housing & Construction Holding, or HCH, Israel’s largest infrastructure company. His duties included being responsible for the consolidation and financial recovery of various business units within HCH. Prior to that, Mr. Regev carried several roles within the group including as a Chief Financial Officer and later the Chief Executive Officer of Blue-Green Ltd., the environmental services subsidiary of HCH. Between 1999 and 2001, Mr. Regev was a manager at Deloitte & Touche, Israel. Mr. Regev holds an LLB degree in Law (Israel) and is a licensed attorney and has been a licensed CPA since 1999. Mr. Regev is also a director of, The Green Way Ltd, Shtang Construction and Engineering Ltd, R.I.B.E. Consulting & Investment Ltd., Middle East Tube Company Ltd, Middle East Tube - Industries 2001 Ltd, Middle East Tubes - Galvanizing (1994) Ltd, I-Solar Greentech Ltd, Plassim Infrastructure Ltd, Plassim Advanced Solutions in Sanitation Ltd, Hakohav Valves Industries Metal (1987) Ltd, Metzerplas Agriculture Cooperative Ltd, B. Gaon Retail & Trading Ltd, Gaon Agro - Rimon Management Services Ltd, B. Gaon Business (2004) Ltd, Gaon Antan Investments Ltd, Or Asaf Investments Ltd, Hamashbir Holdings (1999) Ltd, and AHAVA Holdings LTD.
Yoseph Borenstein. Yoseph Bornstein has played key roles in the Israeli biomed industry during the past 35 years. Mr. Bornstein is a co-founder of Microbot Medical and has been a member of the Board of Directors since Microbot Israel was founded in November 2010. He is also serving as a compensation committee member and Audit committee member at Microbot Medical. Mr. Bornstein founded Shizim Ltd., a life science holding group in October 2000 and has served as its president since then. Mr. Bornstein is the Chairman of GCP Clinical Studies Ltd., a provider of clinical research services and educational programs in Israel since January 2002. He is the Chairman of Biotis Ltd., that supplies bio-pharmaceutical industry, since June 2000. In addition, he is the Chairman of Dolphin Medical Ltd, that supplies medical device industry, since April 2012. Mr. Bornstein is a co-founder and director of XACT Robotics, developing a novel platform technology for robotic needle steering in minimally invasive interventional procedures, and is the founder of ShizimXL & ShizimVS, Innovation Centers. In October 1992, Mr. Bornstein founded Pharmateam Ltd., an Israeli company that specialized in representing international pharmaceutical companies which was sold in 2000. Mr. Bornstein is also a founder of a number of other privately held life-science companies. Mr. Bornstein served as the Biotechnology Committee Chairman of the United States-Israel Science & Technology Commission, or the USISTC, from September 2002 to February 2005 as well as a consultant for USISTF from September 2002 to February 2005. He is also the founder of ILSI-Israel Life Science Industry Organization (who was integrated into Israel Advanced Technology Industries) and ITTN-Israel Tech Transfer Organization. Mr. Bornstein was the General Manager of Bristol-Myers Squibb (Israel), until 1992. Mr. Bornstein holds a Bachelor of Science degree in Agriculture from the Hebrew University of Jerusalem.
Yaacov Goldman. Yaacov Goldman has served as external director since August 2017. Mr. Goldman provides consulting services to companies in strategic-financial areas, through his wholly owned company, Maanit-Goldman Management & Investments (2002) Ltd. Mr. Goldman also serves as a director of Avgol Industries 1953 Ltd., Mivne Real Estate (K.D) Ltd., Prashkovsky Investments and Construction Ltd., Wearable Devices Ltd., and until May 2022, Fattal Propoerties (Europe). Mr. Goldman served as the Professional Secretary of the Peer Review Institute of the Certified Public Accountants Institute in Israel from October 2004 until September 2008. Commencing in 1981, Mr. Goldman worked for Kesselman & Kesselman (Israeli member firm of PricewaterhouseCoopers) for 19 years, and from 1991 until 2000, as a partner and then senior partner of such firm. From September 2000 until November 2001, Mr. Goldman served as managing director of Argoquest Holdings, LLC. Mr. Goldman holds a B.A. degree in Economics and Accounting from Tel Aviv University and is a Certified Public Accountant (Israel).
111
B. Compensation.
Compensation of Directors and Senior Management
The following table presents in the aggregate all compensation we paid to all of our office holders as a group
The term ‘office holder’ as defined in the Companies Law includes a general manager, chief business manager, deputy general manager, vice general manager, any other person fulfilling or assuming the responsibilities of any of the foregoing positions without regard to such person’s title, as well as a director, or a manager directly subordinate to the general manager or the chief executive officer. As of December 31, 2023, in addition to the six members of the Board of Directors, we consider two other individuals, including our Chief Executive Officer, who is also our Chief Financial Officer, and its VP Business Development to be office holders.
| Salaries, fees, commissions, bonuses and options (thousand USD) | ||||
| All office holders as a group, consisting of 8 persons | 1,811 | * | ||
| * | This amount includes approximately $0.1 million set aside or accrued to provide pension, severance, retirement or similar benefits or expenses, but does not include business travel, professional and business association dues and expenses reimbursed to office holders, and other benefits commonly reimbursed or paid by companies in our industry. |
The following table presents information regarding compensation reflected in our financial statements for five most highly compensated office holders, as of December 31, 2023.
| Salary | Bonus | Value of Options Granted(4) | Other(5) | Total | ||||||||||||||||
| Name and Position | (USD in thousands) | |||||||||||||||||||
| Pnina Fishman Executive Chairman and Chief Scientific Officer | 368 | (1) | 246 | 73 | 16 | 703 | ||||||||||||||
| Motti Farbstein Chief Executive, Financial and Operating Officer | 310 | (2) | 96 | 44 | 17 | 467 | ||||||||||||||
| Sari Fishman VP Business Development | 223 | (2) | 101 | 33 | 16 | 373 | ||||||||||||||
| Yaacov Goldman External Director | 39 | (3) | - | 21 | - | 60 | ||||||||||||||
| Guy Regev External Director | 39 | (3) | - | 21 | - | 60 | ||||||||||||||
| (1) | Amount represents consulting fee. |
| (2) | Salary includes gross salary plus payment of social benefits made by us on behalf of such person. Such benefits may include, to the extent applicable, payments, contributions and/or allocations for savings funds (e.g., managers’ life insurance policy), education funds (referred to in Hebrew as “keren hishtalmut”), pension, severance, risk insurances (e.g., life, or work disability insurance), payments for social security payments and tax gross-up payments, vacation, medical insurance and benefits, convalescence or recreation pay and other benefits and perquisites consistent with our policies. |
112
| (3) | Amount represents fees for Board service. |
| (4) | The value of options is the expense recorded in our financial statements for the period ended December 31, 2023 with respect to all options granted to such person. Assumptions and key variables used in the calculation of such amounts are discussed in Note 10 of our financial statements. |
| (5) | Amount represents cost of use of company car. |
Each director other than our Chief Executive Officer and Avraham Sartani, is entitled to the payment of annual fee of NIS 53,155 or $14,655 (based on the exchange rate reported by the Bank of Israel on December 31, 2023), and payment of NIS 3,552 or $979 (based on the exchange rate reported by the Bank of Israel on December 31, 2023) per meeting for participating in meetings of the Board and committees of the Board. The annual fee shall not exceed the annual fee of an expert external director set forth in the Companies Regulations (Rules regarding Compensation and Expenses of External Directors) 5760-2000 as adjusted by the Companies Regulations (Relief for Public Companies with Shares Listed for Trading on a Stock Market Outside of Israel), 5760-2000. The compensation awarded for participating in resolutions that are adopted without an actual convening (i.e., unanimous written resolutions) and for participating through telephone meetings will be reduced as follows: (1) for resolutions that will be adopted without an actual convening, the participation compensation will be reduced by 50%; and (2) for participation through telephone meetings, the participation compensation will be reduced by 40%. The participation compensation and the annual fee is inclusive of all expenses incurred by our directors in connection with their participation in a meeting held at our offices or with regard to resolutions resolved by written consent or teleconference. Avraham Sartani is entitled to a fee of $1,000 per meeting. In addition, our directors (other than our Chief Executive Officer and external directors) are entitled to reimbursement for expenses related to their participation at meetings taking place not at our offices and outside their respective residency area.
Employment and Consulting Agreements
We have entered into employment or consulting agreements with our directors, senior management and key service providers. All of these agreements contain customary provisions regarding noncompetition, confidentiality of information and assignment of proprietary information and inventions. However, the enforceability of the noncompetition provisions may be limited under applicable law.
The following are summary descriptions of certain agreements to which we are a party. The descriptions provided below do not purport to be complete and are qualified in their entirety by the complete agreements, which are attached as exhibits to this Annual Report on Form 20-F.
Service Management Agreement with F.D. Consulting: On June 27, 2002, we entered into a Service Management Agreement with F.D. Consulting, a company partially owned by Pnina Fishman, pursuant to which Dr. Fishman began serving as our Chief Scientific Officer and later became our Chief Executive Officer and is a member of our Board of Directors and continues to be retained through this agreement. F.D. Consulting’s current gross monthly fee is NIS 100,00 or $28,417 (based on the exchange rate reported by the Bank of Israel on December 31, 2022) which is linked to the Israeli CPI and fluctuates accordingly. Dr. Fishman, through F.D. Consulting, is also entitled to reimbursement for reasonable out-of-pocket expenses and use of a company automobile and mobile phone.
The term of F.D. Consulting’s service management agreement is indefinite, unless earlier terminated for cause by us or without cause by either party, subject to three months’ advanced notice.
113
Dr. Fishman is also entitled to receive options exercisable into our ordinary shares from time to time. As of the date of this Annual Report, Dr. Fishman has outstanding options to purchase an aggregate of 16,100,000 ordinary shares, of which (i) 200,000 options to purchase 200,000 ordinary shares have an exercise price of NIS 3.573 per ordinary share or $0.98 per ordinary share (based on the exchange rate reported by the Bank of Israel on December 31, 2023), fully vested as of the date of this Annual Report, (ii) 400,000 options to purchase 400,000 ordinary shares have an exercise price of NIS 2.344 per ordinary share or $0.64 per ordinary share (based on the exchange rate reported by the Bank of Israel on December 31, 2023), fully vested as of the date of this Annual Report , (iii) 2,500,000 options to purchase 2,500,000 ordinary shares have an exercise price of NIS 0.25 per ordinary share or $0.06 per ordinary share (based on the exchange rate reported by the Bank of Israel on December 31, 2023), vesting on a quarterly basis over four years commencing May 27, 2020, and expire on May 27, 2030, (iv) 2,500,000 options to purchase 2,500,000 ordinary shares have an exercise price of NIS 0.25 per ordinary share or $0.06 per ordinary share (based on the exchange rate reported by the Bank of Israel on December 31, 2023), vesting on a quarterly basis over four years commencing April 13, 2021, and expire on April 13, 2031, (v) 3,000,000 options to purchase 3,000,000 ordinary shares have an exercise price of NIS 0.25 per ordinary share or $0.06 per ordinary share (based on the exchange rate reported by the Bank of Israel on December 31, 2023), vesting on a quarterly basis over four years commencing September 5, 2022, and expire on July 16, 2032, and (vi) 7,500,000 options to purchase 7,500,000 ordinary shares have an exercise price of NIS 0.0261 per ordinary share or $0.007 per ordinary share (based on the exchange rate reported by the Bank of Israel on December 31, 2023), vesting on a quarterly basis over four years commencing May 1, 2023 and expire on April 30, 3033.
Employment and Non-Competition Agreement with Motti Farbstein: On September 1, 2003 we entered into an employment and non-competition agreement with Motti Farbstein pursuant to which Mr. Farbstein began serving as our Director of Clinical Operations and Administrative Affairs on September 1, 2003 and is currently serving as our Chief Executive, Financial and Operating Officer. Mr. Farbstein’s current gross monthly salary is NIS 80,000 or $22,057 (based on the exchange rate reported by the Bank of Israel on December 31, 2023). Mr. Farbstein is entitled to an allocation to a manager’s insurance policy equivalent to an amount up to 13-1/3% of his gross monthly salary, up to 2-1/2% of his gross monthly salary for disability insurance and 7-1/2% of his gross monthly salary for a study fund. The foregoing amounts are paid by us. Five percent of his gross monthly salary is deducted for the manager’s insurance policy and 2-1/2% is deducted for the study fund. Mr. Farbstein is also entitled to reimbursement for reasonable out-of-pocket expenses, including travel expenses, and use of a company automobile and mobile phone.
The term of Mr. Farbstein’s employment and non-competition agreement is indefinite, unless earlier terminated for just cause by either party, upon the death, disability or retirement age, or without cause by either party, subject to 60 days’ advanced notice.
Mr. Farbstein is also entitled to receive options exercisable into our ordinary shares from time to time. As of the date of this Annual Report , Mr. Farbstein has outstanding options to purchase an aggregate of 12,470,000 ordinary shares, of which (i) 10,000 options to purchase 10,000 ordinary shares at an exercise price of NIS 8.1205 per option or $2.23 per option (based on the exchange rate reported by the Bank of Israel on December 31, 2023), fully vested, (ii) 60,000 options to purchase 60,000 ordinary shares at an exercise price of NIS 4.317 per option or $1.19 per option (based on the exchange rate reported by the Bank of Israel on December 31, 2023), fully vested, (iii) 250,000 options to purchase 250,000 ordinary shares at an exercise price of NIS 2.513 per option or $0.69 per option (based on the exchange rate reported by the Bank of Israel on December 31, 2023), fully vested, (iv) 150,000 options to purchase 150,000 ordinary shares at an exercise price of NIS 2.344 per option or $0.64 per option (based on the exchange rate reported by the Bank of Israel on December 31, 2023), fully vested, (v) 1,000,000 options to purchase 1,000,000 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option (based on the exchange rate reported by the Bank of Israel on December 31, 2023), vesting on a quarterly basis over four years commencing May 27, 2020 and expire on May 27, 2030, (vi) 1,500,000 options to purchase 1,500,000 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option (based on the exchange rate reported by the Bank of Israel on December 31, 2023), vesting on a quarterly basis over four years commencing April 13, 2021 and expire on April 14, 2031; (vii) 2,000,000 options to purchase 2,000,000 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option (based on the exchange rate reported by the Bank of Israel on December 31, 2023), vesting on a quarterly basis over four years commencing July 7, 2022 and expire on July 6, 2032, and (viii) 7,500,000 options to purchase 7,500,000 ordinary shares have an exercise price of NIS 0.0261 per ordinary share or $0.007 per ordinary share (based on the exchange rate reported by the Bank of Israel on December 31, 2023), vesting on a quarterly basis over four years commencing May 1, 2023 and expire on April 30, 2033.
Consulting Agreement with BioStrategics: On September 27, 2005, we entered into a consulting agreement with BioStrategics through its President, Michael Silverman pursuant to which Dr. Silverman began serving as our Medical Director. Dr. Silverman has extensive experience in clinical development acquired through his involvement in clinical development in large pharmaceutical and small biopharmaceutical companies. He was involved in international clinical research, market-oriented strategic planning, and the challenges of managing research and development portfolios in various capacities at Sterling Winthrop Research Institute and subsequently at Sandoz Research Institute.
114
BioStrategics’ current fee is $400 per hour with a maximum daily fee of $2,600. In addition, BioStrategics is entitled to reimbursement for reasonable pre-approved expenses. The term of the consulting agreement is currently on a year-to-year basis, unless earlier terminated by either party upon 30 days’ prior written notice or immediately by either party if such termination is for cause.
Master Services Agreement with Accellient Partners: On May 10, 2010, we entered into a Master Services Agreement with Accellient Partners, a company owned by William Kerns, who currently serves as our current Vice President of Drug Development. Dr. Kerns has over 20 years of experience in Pharmaceutical Research and Development at SmithKline Beecham and Eisai Pharmaceuticals. As a Senior Executive he has participated in the development of drugs for over 100 Phase I studies and 13 NDA’s and/or Marketing Authorization Applications. Dr. Kerns has chaired an FDA committee on biomarkers and he is an expert in preclinical development and regulatory strategy.
According to the agreement, consulting services are provided by Accellient Partners’ personnel in accordance with individual work orders that are executed from time to time. Each individual work order defines the scope of work to be provided and sets forth the fees to be paid to Accellient Partners.
Beginning on May 10, 2012, the term of the master services agreement is on a month-to-month basis, unless terminated by us upon 30 days’ prior written notice, by us at any time if Accellient Partners commits a breach and fails to cure, or by Accellient Partners upon 30 days’ prior written notice if we commit a breach and fail to cure.
Cohn, De Vries, Stadler & Co.: Cohn, De Vriew, Stadler & Co. of which Ilan Cohn, Ph.D. is a partner provides intellectual property services to us in the ordinary course of business. Previously, Dr. Cohn was a partner with Reinhold Cohn and Partners that provided intellectual property services to us in the ordinary course of business.
C. Board Practices
General
According to the Israeli Companies Law, the management of our business is vested in our Board of Directors. Our Board of Directors may exercise all powers and may take all actions that are not specifically granted to our shareholders. Our executive officers are responsible for our day-to-day management and have individual responsibilities established by our Board of Directors. Executive officers are appointed by and serve at the discretion of our Board of Directors, subject to any applicable employment agreements we have entered into with the executive officers. See “Item 6. Directors, Senior Management and Employees—B. Compensation—Employment and Consulting Agreements.”
Election of Directors and Terms of Office
Our Amended and Restated Articles of Association provide that the maximum number of members of the Board of Directors is 13. The Board of Directors is presently comprised of six members.
In February 2020, a special general meeting of our shareholders approved an amendment to the our Amended and Restated Articles of Association, according to which the Board of Directors, excluding the external directors, if any (who shall be elected and serve in office in strict accordance with the provisions of the Companies Law, if so required by the Companies Law), shall consist of three classes of directors as nearly equal in number as practicable, which are appointed for fixed terms of office in accordance with the Israeli Companies Law and our Amended and Restated Articles of Association, as follows: (i) the term of office of the initial Class I directors expired at the first annual general meeting of our shareholders held in 2020 and when their successors are elected and qualified, (ii) the term of office of the initial Class II directors shall expire at the first annual general meeting of our shareholders following the annual general meeting of our shareholders referred to in clause (i) above and when their successors are elected and qualified, and (iii) the term of office of the initial Class III directors shall expire at the first annual general meeting of our shareholders following the annual general meeting of our shareholders referred to in clause (ii) above and when their successors are elected and qualified.
115
Directors (other than external directors), may be elected only in annual general meetings of our shareholders. At each annual general meeting of our shareholders, commencing with the annual general meeting of our shareholders held in 2021, each of the successors elected to replace the directors of a class whose term shall have expired at such annual general meeting of our shareholders shall be elected to hold office until the third annual general meeting of our shareholders next succeeding his or her election and until his or her respective successor shall have been elected and qualified. Notwithstanding anything to the contrary, each director shall serve until his or her successor is elected and qualified or until such earlier time as such director’s office is vacated.
If the number of directors (excluding external directors) that constitutes the Board of Directors is hereafter changed, the then-serving directors shall be re-designated to other classes and/or any newly created directorships or decrease in directorships shall be apportioned by the Board of Directors among the classes so as to make all classes as nearly equal in number as is practicable, provided that no decrease in the number of Directors constituting the Board of Directors shall shorten the term of any incumbent director.
Directors so elected may not be dismissed from office by the shareholders or by a general meeting of our shareholders prior to the expiration of their term of office. The directors do not receive any benefits upon the expiration of their term of office.
The three classes of directors are Class I Directors, Class II Directors and Class III Directors. Abraham Sartani serves as our Class I Director until the close of the annual meeting to be held in 2026; Dr. Pnina Fishman and Mr. Guy Regev serve as our Class III Directors until the close of the annual meeting to be held in 2025; and Ilan Cohn serves as our Class II Director until the close of the annual meeting to be held in 2024.
Any amendment, replacement or suspension of our Amended and Restated Articles of Association regarding the election of directors, as described above, require a majority of 65% of the voting power represented at the general meeting of our shareholders in person or by proxy and voting thereon, disregarding abstentions from the count of the voting power present and voting, provided that such majority constitutes more than 20% of our then issued and outstanding share capital.
A nominee for service as a director in a public company may not be elected without submitting a declaration to the company, prior to election, specifying that he or she has the requisite qualifications to serve as a director, independent director or external director (if required), as applicable, and the ability to devote the appropriate time to performing his or her duties as such.
A director, who ceases to meet the statutory requirements to serve as a director, external director or independent director, as applicable, must notify the company to that effect immediately and his or her service as a director will expire upon submission of such notice.
On June 30, 2023, Dr. Pnina Fishman was appointed as Chairman of the Board, and Ilan Cohn, the former Chairman of the Board, remained as one of our Class II directors. On August 7, 2023 at an annual general meeting of our shareholders, Yaacov Goldman was elected to serve for an additional three-year term ending July 24, 2026. On August 5, 2021, at a special meeting of our shareholders Yoseph Bornstein was elected to serve as one of our external directors for a three-year term ending July 29, 2024.
None of our directors or senior management has any family relationship with any other director or senior management except that Sari Fishman is the daughter of Pnina Fishman. None of our directors have service contracts that provide for benefits upon termination of his or her directorship with us, other than the payment of salary due, accrued and unpaid as of and through the date of termination. See “Item 6. Directors, Senior Management and Employees—B. Compensation—Employment and Consulting Agreements.”
Chairman of the Board. Under the Israeli Companies Law, without shareholder approval, a person cannot hold the role of both chairman of the Board of Directors and chief executive officer of a company. Furthermore, a person who is directly or indirectly subordinate to a chief executive officer of a company may not serve as the chairman of the Board of Directors of that company and the chairman of the Board of Directors may not otherwise serve in any other capacity in a company or in a subsidiary of that company other than as the chairman of the Board of Directors of such a subsidiary.
116
The Israeli Companies Law provides that an Israeli company may, under certain circumstances, exculpate an office holder from liability with respect to a breach of his duty of care toward the company if appropriate provisions allowing such exculpation are included in its articles of association. Our Amended and Restated Articles of Association permit us to maintain directors’ and officers’ liability insurance and to indemnify our directors and officers for actions performed on behalf of us, subject to specified limitations. We maintain a directors and officers insurance policy which covers the liability of our directors and officers as allowed under the Israeli Companies Law.
The term office holder is defined in the Israeli Companies Law as a director, general manager, chief business manager, deputy general manager, vice general manager, executive vice president, vice president, any other manager directly subordinate to the general manager, director, or any other person assuming the responsibilities of any of the foregoing positions, without regard to such person’s title.
External and Independent Directors
Under the Israeli Companies Law, the boards of directors of companies whose shares are publicly traded, either within or outside of Israel, are required to include at least two members who qualify as external directors.
External directors must be elected by a majority vote of the shares present and voting at a shareholders meeting, provided that either:
| ● | the majority of the shares that are voted at the meeting, shall include at least a majority of the shares held by non-controlling shareholders and shareholders who do not have a personal interest in the election of the external director (other than a personal interest not deriving from a relationship with a controlling shareholder) who voted at the meeting, excluding abstentions, vote in favor of the election of the external director; Anyone with a personal interest will be subject to the provisions of section 276, mutatis mutandis; or |
| ● | the total number of shares held by non-controlling, disinterested shareholders (as described in the preceding bullet point) that are voted against the election of the external director does not exceed 2% of the aggregate voting rights in the company. |
The term “controlling shareholder” means a shareholder with the ability to direct the activities of the company, other than by virtue of being an office holder. A shareholder is presumed to have “control” of the company and thus to be a controlling shareholder of the company if the shareholder holds 50% or more of the “means of control” of the company. “Means of control” is defined as (1) the right to vote at a general meeting of a company or a corresponding body of another corporation; or (2) the right to appoint directors of the corporation or its general manager. For the purpose of approving related-party transactions, the term also includes any shareholder that holds 25% or more of the voting rights of the company if the company has no shareholder that owns more than 50% of its voting rights. For the purpose of determining the holding percentage stated above, two or more shareholders who have a personal interest in a transaction that is brought for the company’s approval are deemed as joint holders.
A person may not serve as an external director of a company if (i) such person is a relative of a controlling shareholder of a company or (ii) at the date of such person’s appointment or within the prior two years, such person, such person’s relative, partner, employer or any entity under such person’s control or anyone to whom such person is subordinate, whether directly or indirectly, has or had any affiliation with (a) the company, (b) the controlling shareholder or his relative, at the time of such person’s appointment or (c) any entity that is either controlled by the company or by its controlling shareholder at the time of such appointment or during the prior two years. If a company does not have a controlling shareholder or a group of shareholders who have a control block entitling them to vote at least 25% of the votes in a shareholders meeting, then a person may not serve as an external director if, such person or such person’s relative, partner, employer or any entity under such person’s control, has or had, on or within the two years preceding the date of the person’s appointment to serve as an external director, any affiliation with the chairman of our Board of Directors, chief executive officer, a substantial shareholder who holds at least 5% of the issued and outstanding shares of the company or voting rights which entitle him to vote at least 5% of the votes in a shareholders meeting, or the chief financial officer of the company.
117
The term affiliation includes:
| ● | an employment relationship; |
| ● | a business or professional relationship even if not maintained on a regular basis (excluding insignificant relationships); |
| ● | control; and |
| ● | service as an office holder, excluding service as a director in a private company prior to the initial offering of its shares to the public if such director was appointed as a director of the private company in order to serve as an external director following the public offering. |
The term relative is defined as a spouse, sibling, parent, grandparent or descendant; a spouse’s sibling, parent or descendant; and the spouse of each of the foregoing persons.
In addition, no person may serve as an external director if that person’s professional activities create, or may create, a conflict of interest with that person’s responsibilities as a director or otherwise interfere with that person’s ability to serve as an external director or if the person is an employee of the Israel Securities Authority, or the ISA, or of the TASE. Furthermore, a person may not continue to serve as an external director if he or she received direct or indirect compensation from the company for his or her role as a director. This prohibition does not apply to compensation paid or given in accordance with regulations promulgated under the Israeli Companies Law or amounts paid pursuant to indemnification and/or exculpation contracts or commitments and insurance coverage. If, at the time an external director is appointed, all current members of the Board of Directors not otherwise affiliated with the company are of the same gender, then that external director must be of the other gender. In addition, a director of a company may not be elected as an external director of another company if, at that time, a director of the other company is acting as an external director of the first company.
Following the termination of an external director’s service on a Board of Directors, such former external director and his or her spouse and children may not be provided with a direct or indirect benefit by the company, its controlling shareholder or any entity under its controlling shareholder’s control. This includes engagement to serve as an executive officer or director of the company or a company controlled by its controlling shareholder, or employment by, or providing services to, any such company for consideration, either directly or indirectly, including through a corporation controlled by the former external director, for a period of two years (and for a period of one year with respect to relatives of the former external director).
The Israeli Companies Law provides that an external director must meet certain professional qualifications or have financial and accounting expertise and that at least one external director must have financial and accounting expertise. However, if at least one of our other directors (i) meets the independence requirements of the Securities Exchange Act of 1934, as amended, (ii) meets the standards of the NYSE American rules for membership on the audit committee and (iii) has financial and accounting expertise as defined in the Israeli Companies Law and applicable regulations, then neither of our external directors is required to possess financial and accounting expertise as long as both possess other requisite professional qualifications. Our Board of Directors is required to determine whether a director possesses financial and accounting expertise by examining whether, due to the director’s education, experience and qualifications, the director is highly proficient and knowledgeable with regard to business-accounting issues and financial statements, to the extent that the director is able to engage in a discussion concerning the presentation of financial information in our financial statements, among others. The regulations define a director with the requisite professional qualifications as a director who satisfies one of the following requirements: (i) the director holds an academic degree in either economics, business administration, accounting, law or public administration; (ii) the director either holds an academic degree in any other field or has completed another form of higher education in our primary field of business or in an area which is relevant to the office of an external director; or (iii) the director has at least five years of experience serving in any one of the following, or at least five years of cumulative experience serving in two or more of the following capacities: (a) a senior business management position in a corporation with a substantial scope of business; (b) a senior position in our primary field of business; or (c) a senior position in public administration. Yaacov Goldman, who is one of our external directors, meets the required qualifications and has financial and accounting expertise as required by the Israeli Companies Law, while Guy Regev, an independent director, also meets the required qualifications and has financial and accounting expertise as required by the Israeli Companies Law.
118
The Israeli Companies Law defines an independent director as a director who complies with the following and was appointed as such in accordance with Chapter 1 of Part 56 of the Israeli Companies Law: (1) the director complies with the qualification to serve as an external director as set out in Sections 240 (b)-(f) of the Israeli Companies Law and the audit committee has approved such compliance; and (2) the director has not served as a director of the company for more than nine consecutive years (which, for such purpose, does not include breaks in such service for periods of less than two year).
If an external directorship becomes vacant and there are less than two external directors on the Board of Directors at the time, then the Board of Directors is required under the Israeli Companies Law to call a shareholders’ meeting as soon as possible to appoint a replacement external director.
Each committee of the Board of Directors that is authorized to exercise the powers of the Board of Directors must include at least one external director, except that the audit committee and compensation committee must each include all external directors then serving on the Board of Directors. Under the Israeli Companies Law, external directors of a company are prohibited from receiving, directly or indirectly, any compensation for their services as external directors, other than compensation and reimbursement of expenses pursuant to applicable regulations promulgated under the Israeli Companies Law. Compensation of an external director is determined prior to his or her appointment and may not be changed during his or her term subject to certain exceptions.
Yoseph Borenstein and Yaacov Goldman serve as external directors on our Board of Directors pursuant to the provisions of the Israeli Companies Law. They both serve on our audit committee and our compensation committee. Our Board of Directors has determined that Yaacov Goldman possesses accounting and financial expertise, and that both of our external directors possess the requisite professional qualifications. In addition to our external directors, Guy Regev and Abraham Sartani serve as independent directors on our Board of Directors. Guy Regev also serves on our audit committee and our compensation committee.
Audit Committee
The Israeli Companies Law requires public companies to appoint an audit committee. The responsibilities of the audit committee include identifying irregularities in the management of our business and approving related party transactions as required by law. An audit committee must consist of at least three directors, including all of its external directors and a majority of independent directors. The chairman of the Board of Directors, any director employed by or otherwise providing services to the company, and a controlling shareholder or any relative of a controlling shareholder, may not be a member of the audit committee. An audit committee may not approve an action or a transaction with a controlling shareholder, or with an office holder, unless at the time of approval two external directors are serving as members of the audit committee and at least one of the external directors was present at the meeting in which an approval was granted.
Our audit committee is currently comprised of three independent non-executive directors. The audit committee is chaired by Yaacov Goldman, who serves as the audit committee financial expert, with Yoseph Borenstein and Guy Regev as members. Our audit committee meets at least four times a year and monitors the adequacy of our internal controls, accounting policies and financial reporting. It regularly reviews the results of the ongoing risk self-assessment process, which we undertake, and our interim and annual reports prior to their submission for approval by the full Board of Directors. The audit committee oversees the activities of the internal auditor, sets its annual tasks and goals and reviews its reports. The audit committee reviews the objectivity and independence of the external auditors and also considers the scope of their work and fees.
Our audit committee provides assistance to our Board of Directors in fulfilling its legal and fiduciary obligations in matters involving our accounting, auditing, financial reporting, internal control and legal compliance functions by pre-approving the services performed by our independent accountants and reviewing their reports regarding our accounting practices and systems of internal control over financial reporting. Our audit committee also oversees the audit efforts of our independent accountants and takes those actions that it deems necessary to satisfy itself that the accountants are independent of management.
119
Under the Israeli Companies Law, our audit committee is responsible for (i) determining whether there are deficiencies in the business management practices of our company, including in consultation with our internal auditor or the independent auditor, and making recommendations to the Board of Directors to improve such practices and amend such deficiencies, (ii) determining whether certain related party transactions (including transactions in which an office holder has a personal interest) should be deemed as material or extraordinary, and to approve such transactions (which may be approved according to certain criteria set out by our audit committee on an annual basis) (see “—Approval of Related Party Transactions under the Israeli Companies Law”); (iii) establishing procedures to be followed in respect of related party transactions with a controlling shareholder (where such are not extraordinary transactions), which may include, where applicable, the establishment of a competitive process for such transaction, under the supervision of the audit committee, or individual, or other committee or body selected by the audit committee, in accordance with criteria determined by the audit committee; (iv) determining procedures for approving certain related party transactions with a controlling shareholder, which having been determined by the audit committee not to be extraordinary transactions, were also determined by the audit committee not to be negligible transactions; (v) approving the working plan of the internal auditor, to examine such working plan before its submission to the Board and proposing amendments thereto, (vi) examining our internal controls and internal auditor’s performance, including whether the internal auditor has sufficient resources and tools to dispose of its responsibilities, (vii) examining the scope of our auditor’s work and compensation and submitting a recommendation with respect thereto to our Board of Directors or shareholders, depending on which of them is considering the appointment of our auditor, and (viii) establishing procedures for the handling of employees’ complaints as to the management of our business and the protection to be provided to such employees.
We have adopted a written charter for our audit committee, setting forth its responsibilities as outlined by the regulations of the SEC. In addition, our audit committee has adopted procedures for the receipt, retention and treatment of complaints we may receive regarding accounting, internal accounting controls or auditing matters and the submission by our employees of concerns regarding questionable accounting or auditing matters. In addition, SEC rules mandate that the audit committee of a listed issuer consist of at least three members, all of whom must be independent, as such term is defined by rules and regulations promulgated by the SEC. We are in compliance with the independence requirements of the SEC rules.
Any person who is not eligible to serve on the audit committee is further restricted from participating in its meetings and votes, unless the chairman of the audit committee determines that such person’s presence is necessary in order to present a certain matter; provided, however, that company employees who are not controlling shareholders or relatives of such shareholders may be present in the meetings, but not for actual voting, and likewise, company counsel and secretary who are not controlling shareholders or relatives of such shareholders may be present in the meetings and for actual voting if such presence is requested by the audit committee.
In addition to the above, all such committee’s members must apply with the following requirements:
| ● | All members shall be members of the Board of Directors of the company. |
| ● | At least one of the committee’s members shall have financial and accounting expertise and the rest of the committee’s members must have the ability to read and understand financial statements. |
Our company, through our audit committee, is in full compliance with the above requirements.
Financial Statement Examination Committee
Under the Israeli Companies Law, the Board of Directors of a public company must appoint a financial statement examination committee, which consists of members with accounting and financial expertise or the ability to read and understand financial statements. According to a resolution of our Board of Directors, the audit committee has been assigned the responsibilities and duties of a financial statements examination committee, as permitted under relevant regulations promulgated under the Israeli Companies Law. From time to time as necessary and required to approve our financial statements, the audit committee holds separate meetings, prior to the scheduled meetings of the entire Board of Directors regarding financial statement approval. The function of a financial statements examination committee is to discuss and provide recommendations to its Board of Directors (including the report of any deficiency found) with respect to the following issues: (i) estimations and assessments made in connection with the preparation of financial statements; (ii) internal controls related to the financial statements; (iii) completeness and propriety of the disclosure in the financial statements; (iv) the accounting policies adopted and the accounting treatments implemented in material matters of the company; and (v) value evaluations, including the assumptions and assessments on which evaluations are based and the supporting data in the financial statements. Our independent auditors and our internal auditors are invited to attend all meetings of audit committee when it is acting in the role of the financial statements examination committee.
120
Compensation Committee
Amendment no. 20 to the Israeli Companies Law was published on November 12, 2012 and became effective on December 12, 2012, or Amendment no. 20. In general, Amendment no. 20 requires public companies to appoint a compensation committee and to adopt a compensation policy with respect to its officers, or the Compensation Policy. In addition, Amendment no. 20 addresses the corporate approval process required for a public company’s engagement with its officers (with specific reference to a director, a non-director officer, a chief executive officer and controlling shareholders and their relatives who are employed by the company).
The compensation committee shall be nominated by the Board of Directors and be comprised of its members. The compensation committee must consist of at least three members. All of the external directors must serve on the compensation committee and constitute a majority of its members. The remaining members of the compensation committee must be directors who qualify to serve as members of the audit committee (including the fact that they are independent) and their compensation should be identical to the compensation paid to the external directors of the company.
Similar to the rules that apply to the audit committee, the compensation committee may not include the chairman of the board, or any director employed by the Company, by a controlling shareholder or by any entity controlled by a controlling shareholder, or any director providing services to the company, to a controlling shareholder or to any entity controlled by a controlling shareholder on a regular basis, or any director whose primary income is dependent on a controlling shareholder, and may not include a controlling shareholder or any of its relatives. Individuals who are not permitted to be compensation committee members may not participate in the committee’s meetings other than to present a particular issue; provided, however, that an employee that is not a controlling shareholder or relative may participate in the committee’s discussions, but not in any vote, and our legal counsel and corporate secretary may participate in the committee’s discussions and votes if requested by the committee.
The roles of the compensation committee are, among others, to: (i) recommend to the Board of Directors the Compensation Policy for office holders and recommend to the board once every three years the extension of a Compensation Policy that had been approved for a period of more than three years; (ii) recommend to the directors any update of the Compensation Policy, from time to time, and examine its implementation; (iii) decide whether to approve the terms of office and of employment of office holders that require approval of the compensation committee; and (iv) decide, in certain circumstances, whether to exempt the approval of terms of office of a chief executive officer from the requirement of shareholder approval.
The Compensation Policy requires the approval of the general meeting of shareholders with a “Special Majority”, which requires a majority of the shareholders of the company who are not either a controlling shareholder or an “interested party” in the proposed resolution, or that shareholders holding less than 2% of the voting power in the company voted against the proposed resolution at such meeting. However, under special circumstances, the Board of Directors may approve the compensation policy without shareholder approval, if the compensation committee and thereafter the Board of Directors decided, based on substantiated reasons after they have reviewed the Compensation Policy again, that the Compensation Policy is in the best interest of the company. The Compensation Policy is required to be brought before the shareholders of the Company once every three years for approval.
121
Under the Israeli Companies Law, our Compensation Policy must generally serve as the basis for corporate approvals with respect to the financial terms of employment or engagement of office holders, including exemption, insurance, indemnification or any monetary payment or obligation of payment in respect of employment or engagement. The Compensation Policy must relate to certain factors, including advancement of the company’s objective, the company’s business plan and its long term strategy, and creation of appropriate incentives for office holders. It must also consider, among other things, the company’s risk management, size and nature of its operations. The Compensation Policy must furthermore consider the following additional factors:
| ● | The knowledge, skills, expertise, and accomplishments of the relevant office holder; |
| ● | The office holder’s roles and responsibilities and prior compensation agreements with him or her; |
| ● | The relationship between the terms offered and the average compensation of the other employees of the company, including those employed through manpower companies; |
| ● | The impact of disparities in salary upon work relationships in the company; |
| ● | The possibility of reducing variable compensation at the discretion of the Board of Directors; |
| ● | The possibility of setting a limit on the exercise value of non-cash variable equity-based compensation; and |
| ● | As to severance compensation, the period of service of the office holder, the terms of his or her compensation during such service period, the company’s performance during that period of service, the person’s contributions towards the company’s achievement of its goals and the maximization of its profits, and the circumstances under which the person is leaving the company. |
The Compensation Policy must also include the following principles:
| ● | the link between variable compensation and the long term performance and measurable criteria; |
| ● | the relationship between variable and fixed compensation, and the ceiling for the value of variable compensation; |
| ● | the conditions under which an office holder would be required to repay compensation paid to him or her if it was later shown that the data upon which such compensation was based was inaccurate and was required to be restated in the company’s financial statements; |
| ● | the minimum holding or vesting period for variable, equity-based compensation; and |
| ● | maximum limits for severance compensation. |
The Compensation Policy was approved by the general meeting of shareholders on January 19, 2017 after discussions and recommendation of the compensation committee and approval by the Board of Directors. The Compensation Policy was amended by the general meeting of shareholders on June 7, 2021. Moreover, the approval of the compensation committee is required in order to approve terms of office and/or employment of office holders.
Yaacov Goldman is the chairman of our compensation committee. Guy Regev and Yoseph Bornstein serve as the other members of our compensation committee.
Under Amendment no. 27 to the Israeli Companies Law, which became effective as of February 17, 2016, the audit committee of an Israeli public company which has been established and conducts itself also in accordance with provisions governing the composition of the compensation committee as set forth in the Israeli Companies Law, may act in lieu of a compensation committee with respect to the responsibilities of a compensation committee which are set forth in the Israeli Companies Law.
122
Approval of Related Party Transactions under the Israeli Companies Law
Fiduciary duties of the office holders
The Israeli Companies Law imposes a duty of care and a duty of loyalty on all office holders of a company. The duty of care of an office holder is based on the duty of care set forth in connection with the tort of negligence under the Israeli Torts Ordinance (New Version) 5728-1968. This duty of care requires an office holder to act with the degree of proficiency with which a reasonable office holder in the same position would have acted under the same circumstances. The duty of care includes a duty to use reasonable means, in light of the circumstances, to obtain:
| ● | information on the advisability of a given action brought for his or her approval or performed by virtue of his or her position; and |
| ● | all other important information pertaining to these actions. |
The duty of loyalty requires an office holder to act in good faith and for the benefit of the company, and includes the duty to:
| ● | refrain from any act involving a conflict of interest between the performance of his or her duties in the company and his or her other duties or personal affairs; |
| ● | refrain from any activity that is competitive with the business of the company; |
| ● | refrain from exploiting any business opportunity of the company for the purpose of gaining a personal advantage for himself or herself or others; and |
| ● | disclose to the company any information or documents relating to our affairs which the office holder received as a result of his or her position as an office holder. |
We may approve an act performed in breach of the duty of loyalty of an office holder provided that the office holder acted in good faith, the act or its approval does not harm the company, and the office holder discloses his or her personal interest, as described below.
Disclosure of personal interests of an office holder and approval of acts and transactions
The Compensation Policy requires the approval of the general meeting of shareholders with a “Special Majority”, which requires a majority of the shareholders of the company who are not either a controlling shareholder or an “interested party” in the proposed resolution, or that shareholders holding less than 2% of the voting power in the company voted against the proposed resolution at such meeting. However, under special circumstances, the Board of Directors may approve the compensation policy without shareholder approval, if the compensation committee and thereafter the Board of Directors decided, based on substantiated reasons after they have reviewed the Compensation Policy again, that the Compensation Policy is in the best interest of the company. The Compensation Policy is required to be brought before the shareholders of the Company once every three years for approval.
The term personal interest is defined under the Israeli Companies Law to include the personal interest of a person in an action or in the business of a company, including the personal interest of such person’s relative or the interest of any corporation in which the person is an interested party, but excluding a personal interest stemming solely from the fact of holding shares in the company. A personal interest furthermore includes the personal interest of a person for whom the office holder holds a voting proxy or the interest of the office holder with respect to his or her vote on behalf of the shareholder for whom he or she holds a proxy even if such shareholder itself has no personal interest in the approval of the matter. An office holder is not, however, obliged to disclose a personal interest if it derives solely from the personal interest of his or her relative in a transaction that is not considered an extraordinary transaction.
123
Under the Israeli Companies Law, an extraordinary transaction which requires approval is defined as any of the following:
| ● | a transaction other than in the ordinary course of business; |
| ● | a transaction that is not on market terms; or |
| ● | a transaction that may have a material impact on our profitability, assets or liabilities. |
Under the Israeli Companies Law, once an office holder has complied with the disclosure requirement described above, a company may approve a transaction between the company and the office holder or a third party in which the office holder has a personal interest, or approve an action by the office holder that would otherwise be deemed a breach of duty of loyalty. However, a company may not approve a transaction or action that is adverse to our interest or that is not performed by the office holder in good faith.
Under the Israeli Companies Law, unless the articles of association of a company provide otherwise, a transaction with an office holder, a transaction with a third party in which the office holder has a personal interest, and an action of an office holder that would otherwise be deemed a breach of duty of loyalty requires approval by the Board of Directors. Our Amended and Restated Articles of Association do not provide otherwise. If the transaction or action considered is (i) an extraordinary transaction, (ii) an action of an office holder that would otherwise be deemed a breach of duty of loyalty and may have a material impact on a company’s profitability, assets or liabilities, (iii) an undertaking to indemnify or insure an office holder who is not a director, or (iv) for matters considered an undertaking concerning the terms of compensation of an office holder who is not a director, including, an undertaking to indemnify or insure such office holder, then approval by the audit committee is required prior to approval by the Board of Directors. Arrangements regarding the compensation, indemnification or insurance of a director require the approval of the audit committee, Board of Directors and shareholders, in that order.
A director who has a personal interest in a matter that is considered at a meeting of the Board of Directors or the audit committee may generally not be present at the meeting or vote on the matter, unless a majority of the directors or members of the audit committee have a personal interest in the matter or the chairman of the audit committee or Board of Directors, as applicable, determines that he or she should be present to present the transaction that is subject to approval. If a majority of the directors have a personal interest in the matter, such matter would also require approval of the shareholders of the company.
Disclosure of personal interests of a controlling shareholder and approval of transactions
Under the Israeli Companies Law, the disclosure requirements that apply to an office holder also apply to a controlling shareholder of a public company. See “— Audit Committee” for a definition of controlling shareholder. Extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, including a private placement in which a controlling shareholder has a personal interest, as well as transactions for the provision of services whether directly or indirectly by a controlling shareholder or his or her relative, or a company such controlling shareholder controls, and transactions concerning the terms of engagement of a controlling shareholder or a controlling shareholder’s relative, whether as an office holder or an employee, require the approval of the audit committee, the Board of Directors and a majority of the shares voted by the shareholders of the company participating and voting on the matter in a shareholders’ meeting. In addition, such shareholder approval must fulfill one of the following requirements:
| ● | at least a majority of the shares held by shareholders who have no personal interest in the transaction and are voting at the meeting must be voted in favor of approving the transaction, excluding abstentions; or |
| ● | the shares voted by shareholders who have no personal interest in the transaction who vote against the transaction represent no more than 2% of the voting rights in the company. |
To the extent that any such transaction with a controlling shareholder is for a period extending beyond three years, approval is required once every three years, unless the audit committee determines that the duration of the transaction is reasonable given the circumstances related thereto.
124
Duties of shareholders
Under the Israeli Companies Law, a shareholder has a duty to refrain from abusing its power in the company and to act in good faith and in an acceptable manner in exercising its rights and performing its obligations to the company and other shareholders, including, among other things, voting at general meetings of shareholders on the following matters:
| ● | an amendment to the articles of association; |
| ● | an increase in our authorized share capital; |
| ● | a merger; and |
| ● | the approval of related party transactions and acts of office holders that require shareholder approval. |
A shareholder also has a general duty to refrain from discriminating against other shareholders.
The remedies generally available upon a breach of contract will also apply to a breach of the above mentioned duties, and in the event of discrimination against other shareholders, additional remedies are available to the injured shareholder.
In addition, any controlling shareholder, any shareholder that knows that its vote can determine the outcome of a shareholder vote and any shareholder that, under a company’s articles of association, has the power to appoint or prevent the appointment of an office holder, or has another power with respect to a company, is under a duty to act with fairness towards the company. The Israeli Companies Law does not describe the substance of this duty except to state that the remedies generally available upon a breach of contract will also apply in the event of a breach of the duty to act with fairness, taking the shareholder’s position in the company into account.
Exculpation, Insurance and Indemnification of Directors and Officers
Under the Israeli Companies Law, a company may not exculpate an office holder from liability for a breach of the duty of loyalty. An Israeli company may exculpate an office holder in advance from liability to us, in whole or in part, for damages caused to us as a result of a breach of duty of care but only if a provision authorizing such exculpation is included in its articles of association. Our Amended and Restated Articles of Association include such a provision. We may not exculpate in advance a director from liability arising out of a prohibited dividend or distribution to shareholders.
Under the Israeli Companies Law and the Israeli Securities Law, a company may indemnify, or undertake in advance to indemnify, an office holder, provided its articles of association include a provision authorizing such indemnification, for the following liabilities and expenses imposed on an office holder or incurred by office holder due to acts performed by him or her as an office holder:
| ● | Financial liability incurred by or imposed on him or her in favor of another person pursuant to a judgment, including a settlement or arbitrator’s award approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the Board of Directors, can be foreseen based on our activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the Board of Directors as reasonable under the circumstances, and such undertaking shall detail the abovementioned foreseen events and amount or criteria; |
| ● | Reasonable litigation expenses, including attorneys’ fees, incurred by the office holder as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no indictment was filed against such office holder as a result of such investigation or proceeding; and (ii) no financial liability was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent or as a monetary sanction; |
125
| ● | Reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by us, on our behalf, or by a third party, or in connection with criminal proceedings in which the office holder was acquitted, or as a result of a conviction for an offense that does not require proof of criminal intent; and |
| ● | Expenses, including reasonable litigation expenses and legal fees, incurred by an office holder in relation to an administrative proceeding instituted against such office holder, or certain compensation payments required to be made to an injured party, pursuant to certain provisions of the Israeli Securities Law. |
Under the Israeli Companies Law, a company may insure an office holder against the following liabilities incurred for acts performed by him or her as an office holder if and to the extent provided in the Company’s articles of association:
| ● | a breach of the duty of loyalty to us, provided that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm us; |
| ● | a breach of duty of care to us or to a third party; and |
| ● | a financial liability imposed on the office holder in favor of a third party. |
Subject to the provisions of the Israeli Companies Law and the Israeli Securities Law, we may also enter into a contract to insure an office holder, in respect of expenses, including reasonable litigation expenses and legal fees, incurred by an office holder in relation to an administrative proceeding instituted against such office holder or payment required to be made to an injured party, pursuant to certain provisions of the Israeli Securities Law.
Nevertheless, under the Israeli Companies Law, a company may not indemnify, exculpate or insure an office holder against any of the following:
| ● | a breach of fiduciary duty, except for indemnification and insurance for a breach of the duty of loyalty to us in the event office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice us; |
| ● | a breach of duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office holder; |
| ● | an act or omission committed with intent to derive unlawful personal benefit; or |
| ● | a fine, monetary sanction, penalty or forfeit levied against the office holder. |
Under the Israeli Companies Law, exculpation, indemnification and insurance of office holders require the approval of the compensation committee, Board of Directors and, in certain circumstances, the shareholders. Our Amended and Restated Articles of Association permit us to exculpate, indemnify and insure our office holders to the fullest extent permitted by the Israeli Companies Law.
Approval of Compensation to Our Officers
The Israeli Companies Law prescribes that compensation to officers must be approved by a company’s Board of Directors after obtaining the approval of the compensation committee.
As detailed above, our compensation committee consists of three independent directors: Yoseph Borenstein, Yaacov Goldman and Guy Regev. The responsibilities of the compensation committee are to set our overall policy on executive remuneration and to decide the specific remuneration, benefits and terms of employment for directors, officers and the Chief Executive Officer.
126
The objectives of the compensation committee’s policies are that such individuals should receive compensation which is appropriate given their performance, level of responsibility and experience. Compensation packages should also allow us to attract and retain executives of the necessary caliber while, at the same time, motivating them to achieve the highest level of corporate performance in line with the best interests of shareholders. In order to determine the elements and level of remuneration appropriate to each executive director, the compensation committee reviews surveys on executive pay, obtains external professional advice and considers individual performance.
Internal Auditor
Under the Israeli Companies Law, the Board of Directors must appoint an internal auditor, nominated by the audit committee. The role of the internal auditor is to examine, among other matters, whether our actions comply with the law and orderly business procedure. Under the Israeli Companies Law, an internal auditor may not be:
| ● | a person (or a relative of a person) who holds more than 5% of our ordinary shares; |
| ● | a person (or a relative of a person) who has the power to appoint a director or the general manager of the company; |
| ● | an executive officer or director of the company (or a relative thereof); or |
| ● | a member of our independent accounting firm, or anyone on his or her behalf. |
We comply with the requirement of the Israeli Companies Law relating to internal auditors. Our internal auditors examine whether our various activities comply with the law and orderly business procedure. Our current internal auditor is Brightman Almagor Zohar & Co. (A Firm in the Deloitte Global Network).
D. Employees.
As of December 31, 2023, we had five employees, two of whom were employed in management and administration, two of whom were employed in research and development and one of whom was employed in business development. All of these employees were located in Israel.
While none of our employees are party to any collective bargaining agreements, certain provisions of the collective bargaining agreements between the Histadrut (General Federation of Labor in Israel) and the Coordination Bureau of Economic Organizations (including the Industrialists’ Associations) are applicable to our employees by order of the Israel Ministry of Labor. These provisions primarily concern the length of the workday, minimum daily wages for professional workers, pension fund benefits for all employees, insurance for work-related accidents, procedures for dismissing employees, determination of severance pay and other conditions of employment. We generally provide our employees with benefits and working conditions beyond the required minimums. We have never experienced any employment-related work stoppages and believe our relationship with our employees is good.
127
E. Share Ownership.
The following table sets forth information regarding the beneficial ownership of our outstanding ordinary shares as of March 28, 2024 by the members of our senior management and Board of Directors individually and as a group. The beneficial ownership of ordinary shares is based on the 1,497,128,493 ordinary shares outstanding as of March 28, 2024 and is determined in accordance with the rules of the SEC and generally includes any ordinary shares over which a person exercises sole or shared voting or investment power. For purposes of the table below, we deem shares subject to options or warrants that are currently exercisable or exercisable within 60 days of March 28, 2024, to be outstanding and to be beneficially owned by the person holding the options or warrants for the purposes of computing the percentage ownership of that person but we do not treat them as outstanding for the purpose of computing the percentage ownership of any other person.
| Name of Beneficial Owner | Number of Ordinary Shares* | Percentage of Class** | ||||||
| Senior Management and Directors | ||||||||
| Pnina Fishman, Ph.D. | 8,238,433 | (1) | ** | |||||
| Motti Farbstein | 5,346,133 | (2) | ** | |||||
| Sari Fishman, Ph.D. | 4,431,250 | (3) | ** | |||||
| Ilan Cohn, Ph.D. | 2,369,067 | (4) | ** | |||||
| Guy Regev | 2,259,740 | (5) | ** | |||||
| Abraham Sartani, M.D. | 2,235,500 | (6) | ** | |||||
| Yaacov Goldman | 2,235,500 | (7) | ** | |||||
| Yoseph Borenstein | 2,037,500 | (8) | ** | |||||
| Senior Management and Directors as a group (8 persons) | 29,153,123 | 1.6 | ||||||
| Holders of more than 5% of our voting securities | 196,183,800 | (9) | ||||||
| Armistice Capital, LLC | ||||||||
| * | U.S. dollar translations of NIS amounts presented in the footnotes to this table are translated using the exchange rate reported by the Bank of Israel on December 31, 2023. |
| ** | Denotes less than 1% |
| (1) | Represents (i) 263,433 ordinary shares, (ii) 200,000 options to purchase 200,000 ordinary shares at an exercise price of NIS 3.573 per option or $0.98 per option and expire on October 22, 2025, (iii) 400,000 options to purchase 400,000 ordinary shares at an exercise price of NIS 2.344 per option or $0.64 per option and expire on January 7, 2029, (iv) 2,500,000 options to purchase 2,500,000 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on May 27, 2030; (v) 1,875,000 options to purchase 1,875,000 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on April 13, 2031; (vi) 1,125,000 options to purchase 1,125,000 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on July 16, 2032 and (v) 1,875,000 options to purchase 1,875,000 ordinary shares at an exercise price of NIS 0.0261 per option or $0.007 per option and expire on April 30, 2033. Excludes 8,125,000 options to purchase 8,125,000 ordinary shares that vest in more than 60 days from March 28, 2024. |
| (2) | Represents (i) 1,133 ordinary shares, (ii) 10,000 options to purchase 10,000 ordinary shares at an exercise price of NIS 8.1205 per option or $2.23 per option and expire on March 18, 2025, (iii) 60,000 options to purchase 60,000 ordinary shares at an exercise price of NIS 4.317 per option or $1.19 per option and expire on February 18, 2026, (iv) 250,000 options to purchase 250,000 ordinary shares at an exercise price of NIS 2.513 per option or $0.69 per option and expire on December 28, 2027, (v) 150,000 options to purchase 150,000 ordinary shares at an exercise price of NIS 2.344 per option or $0.64 per option and expire on January 7, 2029, and (vi) 1,000,000 options to purchase 1,000,000 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on May 27, 2030; (vii) 1,125,000 options to purchase 1,125,000 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on April 13, 2031; (viii) 875,000 options to purchase 875,000 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on July 16, 2032 and (x) 1,875,000 options to purchase 1,875,000 ordinary shares at an exercise price of NIS 0.0261 per option or $0.007 per option and expire on April 30, 2033. Excludes 7,125,000 options to purchase 7,125,000 ordinary shares that vest in more than 60 days from March 28, 2024. |
128
| (3) | Represents (i) 10,000 options to purchase 10,000 ordinary shares at an exercise price of NIS 8.1205 per option or $2.23 per option and expire on March 18, 2025, (ii) 40,000 options to purchase 40,000 ordinary shares at an exercise price of NIS 4.317 per option or $1.19 per option and expire on February 18, 2026, (iii) 80,000 options to purchase 80,000 ordinary shares at an exercise price of NIS 3.662 per option or $1.00 per option and expire on March 30, 2027, (iv) 150,000 options to purchase 150,000 ordinary shares at an exercise price of NIS 2.513 per option or $0.69 per option and expire on December 30, 2027, (v) 120,000 options to purchase 120,000 ordinary shares at an exercise price of NIS 2.344 per option or $0.64 per option and expire on January 7, 2029, (vi) 1,000,000 options to purchase 1,000,000 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on May 27, 2030 and (vii) 1,125,000 options to purchase 1,125,000 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on April 13, 2031; (viii) 656,250 options to purchase 656,250 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on July 16, 2032 and and (x) 1,250,000 options to purchase 1,250,000 ordinary shares at an exercise price of NIS 0.0261 per option or $0.007 per option and expire on April 30, 2033. Excludes 4,968,750 options to purchase 4,968,750 ordinary shares that vest in more than 60 days from March 28, 2024. |
| (4) | Represents (i) 133,567 ordinary shares, (ii) 48,000 options to purchase 48,000 ordinary shares at an exercise price of NIS 2.926 per option or $0.80 per option and expire on November 8, 2027, (iii) 562,500 options to purchase 562,500 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on June 14, 2030 and (iv) 1,625,000 options to purchase 1,625,000 ordinary shares at an exercise price of NIS 0.0261 per option or $0.007 per option and expire on April 30, 2033. Excludes 4,912,500 options to purchase 4,912,500 ordinary shares that vest in more than 60 days from March 28, 2024. |
| (5) | Represents (i) 24,240 ordinary shares, (ii) 48,000 options to purchase 48,000 ordinary shares at an exercise price of NIS 2.926 per option or $0.80 per option and expire on November 28, 2027, and (iii) 562,500 options to purchase 562,500 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on June 14, 2030 and (iv) 1,625,000 options to purchase 1,625,000 ordinary shares at an exercise price of NIS 0.0261 per option or $0.007 per option and expire on April 30, 2033. Excludes 4,912,500 options to purchase 4,912,500 ordinary shares that vest in more than 60 days from March 28, 2024. |
| (6) | Represents (i) 48,000 options to purchase 48,000 ordinary shares at an exercise price of NIS 2.926 per option or $0.80 per option and expire on November 8, 2027, and (ii) 562,500 options are exercisable into 562,500 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on June 14, 2030 and (iii) 1,625,000 options to purchase 1,625,000 ordinary shares at an exercise price of NIS 0.0261 per option or $0.007 per option and expire on April 30, 2033. Excludes 4,912,500 options to purchase 4,912,500 ordinary shares that vest in more than 60 days from March 28, 2024. |
| (7) | Represents (i) 48,000 options to purchase 48,000 ordinary shares at an exercise price of NIS 2.926 per option or $0.80 per option and expire on November 8, 2027, (ii) 562,500 options to purchase 562,500 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on June 14, 2030 and (iii) 1,625,000 options to purchase 1,625,000 ordinary shares at an exercise price of NIS 0.0261 per option or $0.007 per option and expire on April 30, 2033. Excludes 4,912,500 options to purchase 4,912,500 ordinary shares that vest in more than 60 days from March 28, 2024. |
|
(8) |
Represents (i) 412,500 options to purchase 412,500 ordinary shares at an exercise price of NIS 0.25 per option or $0.06 per option and expire on June 22, 2031 and (ii) 1,625,000 options to purchase 1,625,000 ordinary shares at an exercise price of NIS 0.0261 per option or $0.007 per option and expire on April 30, 2033. Excludes 5,062,500 options to purchase 5,062,500 ordinary shares that vest in more than 60 days from March 28, 2024. |
| (9) | Based on information contained in a Schedule 13G filed with the SEC on February 14, 2024. Armistice Capital, LLC (“Armistice Capital”) is the investment manager of Armistice Capital Master Fund Ltd. (the "Master Fund"), the direct holder of the shares, and pursuant to an Investment Management Agreement, Armistice Capital exercises voting and investment power over our securities held by the Master Fund and thus may be deemed to beneficially own the securities of the Issuer held by the Master Fund. Steven Boyd, as the managing member of Armistice Capital, may be deemed to beneficially own our securities held by the Master Fund. The Master Fund specifically disclaims beneficial ownership of the securities of the Issuer directly held by it by virtue of its inability to vote or dispose of such securities as a result of its Investment Management Agreement with Armistice Capital. |
The Bank of New York Mellon, or BNY, is the holder of record for our ADR program, pursuant to which each ADS represents 300 ordinary shares. As of March 20, 2024, BNY held 937,170,720 ordinary shares representing approximately 51.6% of the outstanding ordinary shares at that date. Certain of these ordinary shares were held by brokers or other nominees.
As a result, the number of holders of record or registered holders in the United States is not representative of the number of beneficial holders or of the residence of beneficial holders.
129
Share Option Plans
We maintain the following share option plans for our and our subsidiary’s employees, directors and consultants. In addition to the discussion below, see Note 10 of our consolidated financial statements, included elsewhere in this Annual Report on Form 20-F.
Our Board of Directors administers our share option plans and has the authority to designate all terms of the options granted under our plans including the grantees, exercise prices, grant dates, vesting schedules and expiration dates, which may be no more than ten years after the grant date. Options may not be granted with an exercise price of less than the fair market value of our ordinary shares on the date of grant, unless otherwise determined by our Board of Directors.
As of December 31, 2023, options to purchase an aggregate of 82,477,000 ordinary shares, are outstanding pursuant to the 2003 and 2013 share option plans.
2003 Share Option Plan
Under the 2003 Plan we granted options during the period between 2003 and 2013, at exercise prices between NIS 0.25 and NIS 31.175 per ordinary share, no par value. There are no options to purchase ordinary shares available to be granted under the 2003 Plan. As of December 31, 2023, no options were outstanding. Options granted to Israeli employees were in accordance with section 102 of the Income Tax Ordinance, 1961, or the Tax Ordinance, under the capital gains route set forth in section 102(b)(2) of the Tax Ordinance. The options are non-transferable.
The option term is for a period of ten years from the grant date. The options were granted for no consideration. The options vest over a four or two year period. As of December 31, 2023, no options were fully vested.
2013 Share Option Plan
Under the 2013 Plan we granted options at exercise prices between NIS 0.25 and NIS 12 per ordinary share, no par value. As of December 31, 2023, no options were available to be granted under the 2013 Plan. As of December 31, 2023, 82,477,000 options to purchase 82,477,000 ordinary shares were outstanding. Options granted to Israeli employees were in accordance with the Tax Ordinance under the capital gains route set forth in section 102(b)(2) of the Tax Ordinance. The options are non-transferable.
The option term is for a period of ten years from the grant date. The options were granted for no consideration. The options vest over a four-year period. As of December 31, 2023, options to purchase 24,280,125 ordinary shares were fully vested.
2023 Share Option Plan
As of December 31, 2023, options to purchase up to 100,000,000 ordinary shares were available to be granted under the 2013 Plan. As of December 31, 2023, no options to ordinary shares were outstanding. Options granted to Israeli employees were in accordance with the Tax Ordinance under the capital gains route set forth in section 102(b)(2) of the Tax Ordinance. The options are non-transferable.
The option term is for a period of ten years from the grant date. The options were granted for no consideration. The options vest over a four year period. As of December 31, 2023, no options were fully vested.
F. Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation.
None.
130
ITEM 7. Major Shareholders and Related Party Transactions
A. Major Shareholders.
Except as set forth in “Item 6. Directors, Senior Management and Employees—E. Share Ownership,” to the best of our knowledge, no other person who we know beneficially owns 5.0% or more of the Company’s ordinary shares outstanding as of March 28, 2024. None of our shareholders has different voting rights from other shareholders. Other than as described herein, to the best of our knowledge, we are not owned or controlled, directly or indirectly, by another corporation, by any foreign government or by any natural person or legal persons, severally or jointly, and we are not aware of any arrangement that may, at a subsequent date, result in a change of control of our Company.
To our knowledge, other than as disclosed in the table above, our other filings with the SEC and this Annual Report on Form 20-F, there has been no significant change in the percentage ownership held by any major shareholder since January 1, 2021.
B. Related Party Transactions.
The following is a description of the transactions with related parties to which we, or our subsidiary, are party, and which were in effect since January 1, 2023. The descriptions provided below are summaries of the terms of such agreements, do not purport to be complete and are qualified in their entirety by the complete agreements.
We believe that we have executed all of our transactions with related parties on terms no less favorable to us than those we could have obtained from unaffiliated third parties. We are required by Israeli law to ensure that all future transactions between us and our officers, directors and principal shareholders and their affiliates are approved by a majority of our Board of Directors, including a majority of the independent and disinterested members of our Board of Directors, and that they are on terms no less favorable to us than those that we could obtain from unaffiliated third parties.
Employment and Consulting Agreements
We have or have had employment, consulting or related agreements with each member of our senior management. See “Item 6. Directors, Senior Management and Employees—Compensation”.
One of the Company’s directors is a senior partner in the patent firm which represents the Company in intellectual property and commercial matters (the “Service Provider”). The Service Provider charges the Company for services it renders on an hourly basis. The aggregate amount of these expenses was approximately $237,000, $218,000 and $241,000 in 2023, 2022 and 2021.
We employ Zivit Harpaz as Director of Regulatory and Clinical Operations. For fiscal years 2023, 2022 and 2021, Ms. Harpaz received salary, bonus and benefits totaling approximately $262,000, $278,000 and $262,000, respectively. During fiscal years 2023, 2022 and 2021, we awarded to Ms. Harpaz options to purchase 3,000,000, 1,250,000 and 1,000,000 ordinary shares, respectively. Ms. Harpaz is the daughter of Pnina Fishman.
Options
We have granted options to purchase our ordinary shares to certain of our senior management and directors. See “Item 6. B.—Compensation” and “Item 6. Directors, Senior Management and Employees—Share Ownership”. We describe our option plans under “Item 6. Directors, Senior Management and Employees—Share Ownership”.
Indemnification Agreements
Our Amended and Restated Articles of Association permit us to exculpate, indemnify and insure our directors and officeholders to the fullest extent permitted by the Israeli Companies Law. We have obtained directors’ and officers’ insurance for each of our officers and directors and have entered into indemnification agreements with all of our current officers and directors.
C. Interests of Experts and Counsel.
Not applicable.
131
ITEM 8. Financial Information
A. Consolidated Financial Statements and Other Financial Information
See “Item 18. Financial Statements” for a list of all financial statements filed as part of this Annual Report on Form 20-F.
Legal Matters
We are not involved in any legal or arbitration proceedings that may have or have had in the recent past, significant effects on our financial position or profitability.
Dividend Policy
We have never declared or paid cash dividends to our shareholders. Currently we do not intend to pay cash dividends. We intend to reinvest any earnings in developing and expanding our business. Any future determination relating to our dividend policy will be at the discretion of our Board of Directors and will depend on a number of factors, including future earnings, our financial condition, operating results, contractual restrictions, capital requirements, business prospects, applicable Israeli Companies Law and other factors our Board of Directors may deem relevant.
B. Significant Changes
See “Note 16:—Subsequent Events” to our consolidated financial statements included in this Annual Report beginning on page F-1 for a discussion of significant events that have occurred since December 31, 2023.
ITEM 9. The Offer and Listing
A. Offer and Listing Details
Ordinary Shares
Our ordinary shares have been trading on the TASE under the symbol “CFBI” since October 2005.
ADSs
On October 2, 2012, our ADSs began trading OTC in the United States under the symbol “CANFY” and on November 19, 2013, our ADSs began trading on the NYSE American under the symbol “CANF.” One ADS represents three hundred (300) ordinary shares. See “Item 12. Description of Securities Other Than Equity Securities—D. American Depositary Shares” for a description of the rights attaching to our ADSs. See “Item 12. Description of Securities Other Than Equity Securities—D. American Depositary Shares” for a description of the rights attaching to our ADSs.
B. Plan of Distribution.
Not applicable.
C. Markets.
See “—Offer and Listing Details” above.
D. Selling Shareholders.
Not applicable.
E. Dilution.
Not applicable.
132
F. Expenses of the Issue.
Not applicable.
ITEM 10. Additional Information
A. Share Capital.
Not applicable.
B. Memorandum and Articles of Association.
Our number with the Israeli Registrar of Companies is 512022153. Our purpose is set forth in Section 3 of our Articles of Association and includes every lawful purpose.
On February 22, 2023, our shareholders approved the cancellation of the par value of our ordinary shares such that our authorized share capital is equal to NIS 1,250,000,000 divided into 5,000,000,000 ordinary shares with no par value. Our ordinary shares that are fully paid for are issued in registered form and may be freely transferred under our Amended and Restated Articles of Association, unless the transfer is restricted or prohibited by applicable law or the rules of a stock exchange on which the shares are traded. The ownership or voting of our ordinary shares by non-residents of Israel is not restricted in any way by our Amended and Restated Articles of Association or the laws of the State of Israel, except for ownership by nationals of some countries that are, or have been, in a state of war with Israel.
Pursuant to the Israeli Companies Law and our Amended and Restated Articles of Association, our Board of Directors may exercise all powers and take all actions that are not required under law or under our Amended and Restated Articles of Association to be exercised or taken by our shareholders, including the power to borrow money for company purposes.
Our Amended and Restated Articles of Association enable us to increase or reduce our share capital. Any such changes are subject to the provisions of the Israeli Companies Law and must be approved by a resolution duly passed by our shareholders at a general or special meeting by voting on such change in the capital. In addition, transactions that have the effect of reducing capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings and profits and an issuance of shares for less than their nominal value, require a resolution of our Board of Directors and court approval.
Dividends
We may declare a dividend to be paid to the holders of our ordinary shares in proportion to their respective shareholdings. Under the Israeli Companies Law, dividend distributions are determined by the Board of Directors and do not require the approval of the shareholders of a company unless such company’s articles of association provide otherwise. Our Amended and Restated Articles of Association do not require shareholder approval of a dividend distribution and provide that dividend distributions may be determined by our Board of Directors.
Pursuant to the Israeli Companies Law, we may only distribute dividends from our profits accrued over the previous two years, as defined in the Israeli Companies Law, according to our then last reviewed or audited financial reports, or we may distribute dividends with court approval. In each case, we are only permitted to pay a dividend if there is no reasonable concern that payment of the dividend will prevent us from satisfying our existing and foreseeable obligations as they become due.
133
Election of Directors
Our Amended and Restated Articles of Association provide that the maximum number of members of the Board of Directors is 13. The Board of Directors is presently comprised of six members.
In February 2020, a special general meeting of our shareholders approved an amendment to the our Amended and Restated Articles of Association, according to which the Board of Directors, excluding the external directors, if any (who shall be elected and serve in office in strict accordance with the provisions of the Companies Law, if so required by the Companies Law), shall consist of three classes of directors as nearly equal in number as practicable, which are appointed for fixed terms of office in accordance with the Israeli Companies Law and our Amended and Restated Articles of Association, as follows: (i) the term of office of the initial Class I directors shall expire at the first annual general meeting of our shareholders held in 2020 and when their successors are elected and qualified, (ii) the term of office of the initial Class II directors shall expire at the first annual general meeting of our shareholders following the annual general meeting of our shareholders referred to in clause (i) above and when their successors are elected and qualified, and (iii) the term of office of the initial Class III directors shall expire at the first annual general meeting of our shareholders following the annual general meeting of our shareholders referred to in clause (ii) above and when their successors are elected and qualified.
Directors (other than external directors), may be elected only in annual general meetings of our shareholders. At each annual general meeting of our shareholders, commencing with the annual general meeting of our shareholders held in 2020, each of the successors elected to replace the directors of a class whose term shall have expired at such annual general meeting of our shareholders shall be elected to hold office until the third annual general meeting of our shareholders next succeeding his or her election and until his or her respective successor shall have been elected and qualified. Notwithstanding anything to the contrary, each director shall serve until his or her successor is elected and qualified or until such earlier time as such director’s office is vacated.
If the number of directors (excluding external directors) that constitutes the Board of Directors is hereafter changed, the then-serving directors shall be re-designated to other classes and/or any newly created directorships or decrease in directorships shall be apportioned by the Board of Directors among the classes so as to make all classes as nearly equal in number as is practicable, provided that no decrease in the number of Directors constituting the Board of Directors shall shorten the term of any incumbent director.
Directors so elected may not be dismissed from office by the shareholders or by a general meeting of our shareholders prior to the expiration of their term of office. The directors do not receive any benefits upon the expiration of their term of office.
The three classes of directors are Class I Directors, Class II Directors and Class III Directors. Abraham Sartani serves as our Class I Director until the close of the annual meeting to be held in 2026; Dr. Pnina Fishman and Mr. Guy Regev serve as our Class III Directors until the close of the annual meeting to be held in 2025; and Ilan Cohn serves as our Class II Director until the close of the annual meeting to be held in 2024.
Any amendment, replacement or suspension of our Amended and Restated Articles of Association regarding the election of directors, as described above, require a majority of 65% of the voting power represented at the general meeting of our shareholders in person or by proxy and voting thereon, disregarding abstentions from the count of the voting power present and voting, provided that such majority constitutes more than 20% of our then issued and outstanding share capital.
A nominee for service as a director in a public company may not be elected without submitting a declaration to the company, prior to election, specifying that he or she has the requisite qualifications to serve as a director, independent director or external director (if required), as applicable, and the ability to devote the appropriate time to performing his or her duties as such.
A director, who ceases to meet the statutory requirements to serve as a director, external director or independent director, as applicable, must notify the company to that effect immediately and his or her service as a director will expire upon submission of such notice.
See “Item 6. Directors, Senior Management and Employees—C. Board Practices—External Directors.”
134
Shareholder Meetings
Under Israeli Companies Law, we are required to hold an annual general meeting of our shareholders once every calendar year that must be no later than 15 months after the date of the previous annual general meeting. All meetings other than the annual general meeting of shareholders are referred to as special meetings. Our Board of Directors may call special meetings whenever it sees fit, at such time and place, within or outside of Israel, as it may determine. In addition, the Israeli Companies Law and our Amended and Restated Articles of Association provide that our Board of Directors is required to convene a special meeting upon the written request of (i) any two of our directors or one quarter of our Board of Directors or (ii) one or more shareholders holding, in the aggregate, either (1) 5% of our outstanding shares and 1% of our outstanding voting power or (2) 5% of our outstanding voting power.
Subject to the provisions of the Israeli Companies Law and the regulations promulgated thereunder, shareholders entitled to participate and vote at general meetings are the shareholders of record on a date to be decided by the Board of Directors, which may be between four and forty days prior to the date of the meeting. Furthermore, the Israeli Companies Law and our Amended and Restated Articles of Association require that resolutions regarding the following matters must be passed at a general meeting of our shareholders:
| ● | amendments to our Amended and Restated Articles of Association; |
| ● | appointment or termination of our auditors; |
| ● | appointment of directors and appointment and dismissal of external directors; |
| ● | approval of acts and transactions requiring general meeting approval pursuant to the Israeli Companies Law; |
| ● | director compensation, indemnification and change of the principal executive officer; |
| ● | increases or reductions of our authorized share capital; |
| ● | a merger; and |
| ● | the exercise of our Board of Director’s powers by a general meeting, if our Board of Directors is unable to exercise its powers and the exercise of any of its powers is required for our proper management. |
The Israeli Companies Law requires that a notice of any annual or special shareholders meeting be provided at least 21 days prior to the meeting and if the agenda of the meeting includes the appointment or removal of directors, the approval of transactions with office holders or interested or related parties, or an approval of a merger, notice must be provided at least 35 days prior to the meeting.
The Israeli Companies Law does not allow shareholders of publicly traded companies to approve corporate matters by written consent. Consequently, our Amended and Restated Articles of Association does not allow shareholders to approve corporate matters by written consent.
Pursuant to our Amended and Restated Articles of Association, holders of our ordinary shares have one vote for each ordinary share held on all matters submitted to a vote before the shareholders at a general meeting.
Quorum
The quorum required for our general meetings of shareholders consists of at least two shareholders present in person, by proxy or written ballot who hold or represent between them at least 25% of the total outstanding voting rights.
A meeting adjourned for lack of a quorum is adjourned to the same day in the following week at the same time and place or on a later date if so specified in the summons or notice of the meeting. At the reconvened meeting, any number of our shareholders present in person or by proxy shall constitute a lawful quorum.
135
Resolutions
Our Amended and Restated Articles of Association provide that all resolutions of our shareholders require a simple majority vote, unless otherwise required by applicable law.
Israeli law provides that a shareholder of a public company may vote in a meeting and in a class meeting by means of a written ballot in which the shareholder indicates how he or she votes on resolutions relating to the following matters:
| ● | an appointment or removal of directors; |
| ● | an approval of transactions with office holders or interested or related parties; |
| ● | an approval of a merger or any other matter in respect of which there is a provision in the articles of association providing that decisions of the general meeting may also be passed by written ballot; |
| ● | authorizing the chairman of the Board of Directors or his relative to act as our chief executive officer or act with such authority; or authorize our chief executive officer or his relative to act as the chairman of the Board of Directors or act with such authority; and |
| ● | other matters which may be prescribed by Israel’s Minister of Justice. |
The provision allowing the vote by written ballot does not apply where the voting power of the controlling shareholder is sufficient to determine the vote. Our Amended and Restated Articles of Association provide that our Board of Directors may prevent voting by means of a written ballot and this determination is required to be stated in the notice convening the general meeting.
The Israeli Companies Law provides that a shareholder, in exercising his or her rights and performing his or her obligations toward the company and its other shareholders, must act in good faith and in a customary manner, and avoid abusing his or her power. This is required when voting at general meetings on matters such as changes to the articles of association, increasing our registered capital, mergers and approval of related party transactions. A shareholder also has a general duty to refrain from depriving any other shareholder of its rights as a shareholder. In addition, any controlling shareholder, any shareholder who knows that its vote can determine the outcome of a shareholder vote and any shareholder who, under such company’s articles of association, can appoint or prevent the appointment of an office holder, is required to act with fairness towards the company. The Israeli Companies Law does not describe the substance of this duty except to state that the remedies generally available upon a breach of contract will also apply to a breach of the duty to act with fairness, and, to the best of our knowledge, there is no binding case law that addresses this subject directly.
Under the Israeli Companies Law, unless provided otherwise in a company’s articles of association, a resolution at a shareholders meeting requires approval by a simple majority of the voting rights represented at the meeting, in person, by proxy or written ballot, and voting on the resolution. A resolution for the voluntary winding up of the company requires the approval of holders of 75% of the voting rights represented at the meeting, in person, by proxy or by written ballot and voting on the resolution.
In the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of our ordinary shares in proportion to their shareholdings. This right, as well as the right to receive dividends, may be affected by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future.
Access to Corporate Records
Under the Israeli Companies Law, all shareholders of a company generally have the right to review minutes of our general meetings, its shareholders register and principal shareholders register, articles of association, financial statements and any document it is required by law to file publicly with the Israeli Companies Registrar and the Israel Securities Authority. Any of our shareholders may request access to review any document in our possession that relates to any action or transaction with a related party, interested party or office holder that requires shareholder approval under the Israeli Companies Law. We may deny a request to review a document if we determine that the request was not made in good faith, that the document contains a commercial secret or a patent or that the document’s disclosure may otherwise prejudice our interests.
136
Acquisitions under Israeli Law
Full Tender Offer
A person wishing to acquire shares of a public Israeli company and who would as a result hold over 90% of the target company’s issued and outstanding share capital is required by the Israeli Companies Law to make a tender offer to all of our shareholders for the purchase of all of the issued and outstanding shares of the company. A person wishing to acquire shares of a public Israeli company and who would as a result hold over 90% of the issued and outstanding share capital of a certain class of shares is required to make a tender offer to all of the shareholders who hold shares of the same class for the purchase of all of the issued and outstanding shares of the same class. If the shareholders who do not accept the offer hold less than 5% of the issued and outstanding share capital of the company or of the applicable class, all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law (provided that a majority of the offerees that do not have a personal interest in such tender offer shall have approved the tender offer except that if the total votes to reject the tender offer represent less than 2% of the company’s issued and outstanding share capital, in the aggregate, approval by a majority of the offerees that do not have a personal interest in such tender offer is not required to complete the tender offer). However, a shareholder that had its shares so transferred may petition the court within six months from the date of acceptance of the full tender offer, whether or not such shareholder agreed to the tender or not, to determine whether the tender offer was for less than fair value and whether the fair value should be paid as determined by the court unless the acquirer stipulated in the tender offer that a shareholder that accepts the offer may not seek appraisal rights. If the shareholders who did not accept the tender offer hold 5% or more of the issued and outstanding share capital of the company or of the applicable class, the acquirer may not acquire shares of the company that will increase its holdings to more than 90% of our issued and outstanding share capital or of the applicable class from shareholders who accepted the tender offer.
Special Tender Offer
The Israeli Companies Law provides that an acquisition of shares of a public Israeli company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of 25% or more of the voting rights in the company, unless one of the exemptions in the Israeli Companies Law is met. This rule does not apply if there is already another holder of at least 25% of the voting rights in the company. Similarly, the Israeli Companies Law provides that an acquisition of shares in a public company must be made by means of a tender offer if as a result of the acquisition the purchaser would become a holder of 45% or more of the voting rights in the company, if there is no other shareholder of the company who holds 45% or more of the voting rights in the company, unless one of the exemptions in the Israeli Companies Law is met.
A special tender offer must be extended to all shareholders of a company but the offeror is not required to purchase shares representing more than 5% of the voting power attached to our outstanding shares, regardless of how many shares are tendered by shareholders. A special tender offer may be consummated only if (i) at least 5% of the voting power attached to our outstanding shares will be acquired by the offeror and (ii) the number of shares tendered in the offer exceeds the number of shares whose holders objected to the offer.
If a special tender offer is accepted, then the purchaser or any person or entity controlling it or under common control with the purchaser or such controlling person or entity may not make a subsequent tender offer for the purchase of shares of the target company and may not enter into a merger with the target company for a period of one year from the date of the offer, unless the purchaser or such person or entity undertook to effect such an offer or merger in the initial special tender offer.
Merger
The Israeli Companies Law permits merger transactions if approved by each party’s Board of Directors and, unless certain requirements described under the Israeli Companies Law are met, a majority of each party’s shares voted on the proposed merger at a shareholders’ meeting called with at least 35 days’ prior notice.
For purposes of the shareholder vote, unless a court rules otherwise, the merger will not be deemed approved if a majority of the shares represented at the shareholders meeting that are held by parties other than the other party to the merger, or by any person who holds 25% or more of the outstanding shares or the right to appoint 25% or more of the directors of the other party, vote against the merger. If the transaction would have been approved but for the separate approval of each class or the exclusion of the votes of certain shareholders as provided above, a court may still approve the merger upon the request of holders of at least 25% of the voting rights of a company, if the court holds that the merger is fair and reasonable, taking into account the value of the parties to the merger and the consideration offered to the shareholders.
137
Upon the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of any of the parties to the merger, and may further give instructions to secure the rights of creditors.
In addition, a merger may not be completed unless at least 50 days have passed from the date that a proposal for approval of the merger was filed by each party with the Israeli Registrar of Companies and 30 days have passed from the date the merger was approved by the shareholders of each party.
Antitakeover Measures
The Israeli Companies Law allows us to create and issue shares having rights different from those attached to our ordinary shares, including shares providing certain preferred rights, distributions or other matters and shares having preemptive rights. As of the date of this Annual Report on Form 20-F, we do not have any authorized or issued shares other than our ordinary shares. In the future, if we do create and issue a class of shares other than ordinary shares, such class of shares, depending on the specific rights that may be attached to them, may delay or prevent a takeover or otherwise prevent our shareholders from realizing a potential premium over the market value of their ordinary shares. The authorization of a new class of shares will require an amendment to our Amended and Restated Articles of Association which requires the prior approval of the holders of a majority of our shares at a general meeting. In addition, the rules and regulations of the TASE also limit the terms permitted with respect to a new class of shares and prohibit any such new class of shares from having voting rights. Shareholders voting in such meeting will be subject to the restrictions provided in the Israeli Companies Law as described above.
Borrowing Powers
Under the Israeli Companies Law and our Amended and Restated Articles of Association, our Board of Directors may exercise all powers and take all actions that are not required under law or under our amended and restated articles of association to be exercised or taken by our shareholders or other corporate bodies, including the power to borrow money for company purposes.
Changes in Capital
Our Amended and Restated Articles of Association enable us to increase or reduce our share capital. Any such changes are subject to the provisions of the Israeli Companies Law and must be approved by a resolution duly passed by our shareholders at a general meeting by voting on such change in the capital. In addition, transactions that have the effect of reducing capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings or profits and, in certain circumstances, an issuance of shares for less than their nominal value, require the approval of both our Board of Directors and an Israeli court.
C. Material Contracts.
The following are summary descriptions of certain material agreements to which we are a party. The descriptions provided below do not purport to be complete and are qualified in their entirety by the complete agreements, which may be attached as exhibits to this Annual Report on Form 20-F.
License Agreements
See “Item 4. Information on the Company—B. Business Overview—Out-Licensing and Distribution Agreements”.
138
Employment and Consulting Agreements
See “Item 6. Directors, Senior Management and Employees—B. Compensation—Employment and Consulting Agreements”.
D. Exchange Controls
There are no Israeli government laws, decrees or regulations that restrict or that affect our export or import of capital or the remittance of dividends, interest or other payments to non-resident holders of our securities, including the availability of cash and cash equivalents for use by us and our wholly-owned subsidiary, except or otherwise as set forth under “Item 10. Additional Information—E. Taxation.”
E. Taxation
The following description is not intended to constitute a complete analysis of all tax consequences relating to the acquisition, ownership and disposition of our ordinary shares, ADSs, Warrants or Pre-funded Warrants. You should consult your own tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign, or other taxing jurisdiction.
Certain Israeli Tax Considerations
The following is a summary of the material Israeli tax laws applicable to us. This section also contains a discussion of material Israeli tax consequences concerning the ownership and disposition of our ordinary shares. This summary does not discuss all the aspects of Israeli tax law that may be relevant to a particular investor in light of his or her personal investment circumstances or to some types of investors subject to special treatment under Israeli law. Examples of this kind of investor include residents of Israel or traders in securities who are subject to special tax regimes not covered in this discussion. Because certain parts of this discussion are based on new tax legislation that has not yet been subject to judicial or administrative interpretation, we cannot assure you that the appropriate tax authorities or the courts will accept the views expressed in this discussion. The discussion does not cover all possible tax consequences.
You are urged to consult your own tax advisor as to the Israeli and other tax consequences of the purchase, ownership and disposition of our ADSs, including, in particular, the effect of any non-Israeli, state or local taxes.
General Corporate Tax Structure in Israel
Israeli companies are generally subject to corporate tax, which has decreased in recent years, from a rate of 26.5% in 2014 and 2015 to 25% in 2016 and to 24% in 2017 to 23% for 2018, 2019, 2020, 2021, 2022 and 2023. However, the effective corporate tax rate payable by a company that derives income from an Approved Enterprise, a Privileged Enterprise or a Preferred Enterprise (as discussed below) may be considerably less. Capital gains generated by an Israeli company are generally subject to tax at the corporate tax rate.
In 2006, transfer pricing regulations came into force, following the introduction of Section 85A of the Israeli Tax Ordinance under Amendment 132. The transfer pricing rules require that cross-border transactions between related parties (as defined in Section 85A of the Israeli Tax Ordinance) be carried out implementing an arms’ length principle based on a transfer pricing study and reported and taxed accordingly.
Law for the Encouragement of Capital Investments, 1959:
The Law for Encouragement of Capital Investments, 1959 (the “Investment Law”) provides tax benefits for Israeli companies meeting certain requirements and criteria. The Investment Law has undergone certain amendments and reforms in recent years.
139
The Israeli parliament enacted a reform to the Investment Law, effective January 2011 (which was amended in August 2013). According to the reform, a flat rate tax applies to Preferred Income of companies eligible for the “Preferred Enterprise” status. In order to be eligible for Preferred Enterprise status, a company must meet minimum requirements to establish that it contributes to the country’s economic growth and is a competitive factor for the gross domestic product.
Benefits granted to a Preferred Enterprise include reduced tax rates. As part of the Economic Efficiency Law (Legislative Amendments for Accomplishment of Budgetary Targets for Budget Years 2017-2018), 5777-2016, the tax rate is 16% for all areas other than Development Area A (which was 7.5% from 2017 onward).
Pre-Ruling from the Israeli Income Tax Authorities
In connection with the spin-off, we received a pre-ruling decision from the Israeli Income Tax Authority which confirms: (i) that the grant of the license to Eye-Fite is not liable for tax pursuant to the provisions of section 104a to the Income Tax Ordinance (New Version), 1961, or the Ordinance; (ii) that OphthaliX is considered the receiving company pursuant to section 103c(7)(b) to the Ordinance; (iii) that the sale of Eye-Fite shares to OphthaliX as consideration for OphthaliX shares does not create liability for tax pursuant to the provisions of section 103t to the Ordinance, or change in structure; and (iv) the date for the change in structure was determined. According to the tax pre-ruling, the date of change in structure shall also be the date of exchange of shares with respect to the spin-off and notification to the tax assessor. We and Eye-Fite presented to the tax assessor and the merger and spin-off department of the tax assessor the forms required by the Ordinance and the regulations thereunder. The tax pre-ruling further provides that the grant of a license to Eye-Fite as consideration for the issuance of Eye-Fite shares to us does not create liability for tax pursuant to the provisions of section 104a to the Ordinance.
According to the pre-ruling, we must not sell more than 10% of our common stock holdings in OphthaliX issued in connection with the change in structure for at least two years from the date of the change (i.e., November 21, 2011), OphthaliX must not sell more than 10% of its ordinary share holdings in Eye-Fite received in connection with the change in structure for at least two years from the date of the change and Eye-Fite must retain the assets received from us in connection with the change in structure for at least two years from the date of the change.
The shares of Eye-Fite which were transferred to OphthaliX in connection with the change in structure will be held in escrow. The sale of these shares will be deemed as a sale by an Israeli company and will be taxed accordingly. The trustee will withhold tax at the source.
The shares of OphthaliX which were transferred to us in connection with the change in structure will be held in escrow. The sale of these shares will be deemed as a sale by an Israeli company and will be taxed accordingly. The trustee will withhold tax at the source.
Any dividend distributed by Eye-Fite to OphthaliX will be taxed in Israel in accordance with paragraph 125b(5) of the Israeli Tax Ordinance.
A description of the terms of the pre-ruling is also included in the notes to the financial statements.
Tax Benefits and Grants for Research and Development
According to section 20A of the Israeli tax Ordinance, under certain conditions, a tax deduction for research and development expenditures, including capital expenditures, for the year in which they are incurred. These expenses must relate to scientific research and development projects in the fields of industry, agriculture, transportation or energy and must be approved by the Office of the Chief Scientist, or the OCS, of the relevant Israeli government ministry, determined by the field of research. Furthermore, the research and development must be for the promotion of the company and carried out by or on behalf of the company seeking such tax deduction. The amount of such deductible expenses is reduced by the sum of any funds received through government grants for the funding of the scientific research and development projects. No deduction under these research and development deduction rules is allowed if such deduction is related to an expense invested in an asset depreciable under the general depreciation rules of the Tax Ordinance. Expenditures not so approved are deductible in equal amounts over three years.
On a yearly basis, we evaluate the applicability of the above tax deduction for research and development expenditures and, based on our evaluation, determine whether to apply to the OCS for approval of a tax deduction. There can be no assurance that any application for a tax deduction will be accepted.
140
Taxation of our Shareholders
Capital Gains Taxes Applicable to Non-Israeli Resident Shareholders. Shareholders that are not Israeli residents are generally exempt from Israeli capital gains tax on any gains derived from the sale, exchange or disposition of our ordinary shares, provided that such shareholders did not acquire their shares prior to our initial public offering on the TASE and such gains were not derived from a permanent establishment or business activity of such shareholders in Israel. However, non-Israeli corporations will not be entitled to the foregoing exemptions if they are managed and controlled from Israel (then they are deemed as Israeli Resident).
In addition, under the U.S.-Israel Income Tax Treaty, 1995, or the U.S.-Israel Tax Treaty, the sale, exchange or disposition of our ordinary shares by a shareholder who is a U.S. resident (for purposes of the U.S.-Israel Tax Treaty) holding the shares as a capital asset is exempt from Israeli capital gains tax unless either (i) the shareholder holds, directly or indirectly, shares representing 10% or more of our voting capital during any part of the 12-month period preceding such sale, exchange or disposition or (ii) the capital gains arising from such sale are attributable to a ‘permanent establishment’ of the shareholder located in Israel. In either case, the sale, exchange or disposition of the shares would be subject to Israeli tax, at the applicable rate; however, under the U.S.-Israel Tax Treaty, the U.S. resident would be permitted to claim tax credit IL tax paid with respect to the sale, exchange or disposition, subject to the limitations in U.S. laws applicable to foreign tax credits. The U.S.-Israel Tax Treaty does not relate to U.S. state or local taxes.
Shareholders may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid withholding at source at the time of sale.
Taxation of Non-Israeli Shareholders on Receipt of Dividends. Non-residents of Israel are generally subject to Israeli income tax on the receipt of dividends paid on our ordinary shares at the rate of 25%, which tax will be withheld at the source, unless a different rate is provided in a tax treaty between Israel and the shareholder’s country of residence. With respect to a person who is a “substantial shareholder” at the time receiving the dividend or on any date in the 12 months preceding such date, the applicable tax rate is 30%. A “substantial shareholder” is generally a person who alone, or together with his relative or another person who collaborates with him on a permanent basis, holds, directly or indirectly, at least 10% of any of the “means of control” of the corporation. “Means of control” generally include the right to vote, receive profits, nominate a director or an officer, receive assets upon liquidation, or order someone who holds any of the aforesaid rights how to act, and all regardless of the source of such right.
Under the U.S.-Israel Tax Treaty, the maximum rate of tax withheld in Israel on dividends paid to a holder of our ordinary shares who is a U.S. resident (for purposes of the U.S.-Israel Tax Treaty) is 25%. However, generally, the maximum rate of withholding tax on dividends paid shall be 12.5% in the event that the U.S. corporation holding 10% or more of our outstanding voting capital throughout the tax year in which the dividend is distributed as well as the previous tax year, and no more than 25% of the gross income of our income in such prior taxable year (if any) consists of interest or dividends (excluding interest derived from the conduct of a banking, insurance, or financing business or interest received from subsidiary corporations, 50 percent or more of the outstanding shares of the voting stock of which is owned us at the time such dividends or interest is received). Note that 12.5% tax on dividend shall not apply in the event the dividend derives from income that is entitled to the reduced tax rate applicable to an approved enterprise under Israel’s Encouragement of Capital Investments Law (1959) (In such case, 15% tax rate shall apply).
A non-resident of Israel who receives dividends from which tax was withheld is generally exempt from the duty to file tax returns in Israel in respect of such income, provided such income was not derived from a business conducted in Israel by the taxpayer, and the taxpayer has no other taxable sources of income in Israel.
Taxation of Israeli Shareholders on Receipt of Dividends
Residents of Israel are generally subject to Israeli income tax on the receipt of dividends paid on our ordinary shares at the rate of 25%, which tax will be withheld at the source. With respect to a person who is a “substantial shareholder” at the time of receiving the dividend or on any date within the 12 months preceding such date, the applicable tax rate is 30%.
141
Additional taxation of high income
Section 121B of the Israeli Tax ordnance applies additional tax at a rate of 3% on individuals who produced annual income (of all sources) surpasses 698,280 ILS (for 2023).
Certain Material U.S. Federal Income Tax Consequences
The following is a general summary of certain material U.S. federal income tax consequences relating to the purchase, ownership and disposition of our ordinary shares, ADSs, Warrants, and Pre-funded Warrants by U.S. Holders (as defined below) that hold such ordinary shares, ADSs, Warrants, or Pre-funded Warrants as capital assets (generally, property held for investment). This summary is based on the Internal Revenue Code of 1986, as amended, or the Code, the regulations of the U.S. Department of the Treasury issued pursuant to the Code, or the Treasury Regulations, administrative and judicial interpretations thereof, and the U.S.-Israel Income Tax Treaty, all as in effect on the date hereof and all of which are subject to change, possibly with retroactive effect, or to different interpretation. No ruling has been sought from the IRS, with respect to any United States federal income tax consequences described below, and there can be no assurance that the IRS or a court will not take a contrary position. This summary does not address all of the tax considerations that may be relevant to specific U.S. Holders in light of their particular circumstances or to U.S. Holders subject to special treatment under U.S. federal income tax law, including but not limited to, (1) a bank, insurance company, regulated investment company, or other financial institution or “financial services entity”; (2) a broker or dealer in securities or foreign currency; (3) a person who acquired our ordinary shares, ADSs, Warrants, or Pre-funded Warrants in connection with employment or other performance of services; (4) a U.S. Holder that is subject to the U.S. alternative minimum tax; (5) a U.S. Holder that holds our ordinary shares, ADSs, Warrants, or Pre-funded Warrants as a hedge or as part of a hedging, straddle, conversion or constructive sale transaction or other risk-reduction transaction for U.S. federal income tax purposes; (6) a retirement plan or tax-exempt entity; (7) real estate investment trusts or grantor trusts; (8) a U.S. Holder that expatriates out of the United States or a former long-term resident of the United States; or (9) a U.S. Holder having a functional currency other than the U.S. dollar. This discussion does not address the U.S. federal income tax treatment of a U.S. Holder that owns, directly or constructively, at any time, ordinary shares or ADSs representing 10% or more of our voting power or value (including by treating U.S. Holders of Warrants, Pre-funded Warrants, or other options to acquire our ordinary shares or ADSs as owning such ordinary shares or ADSs). Additionally, this summary does not address any U.S. state or local or non-U.S. tax considerations or any U.S. federal estate, gift or alternative minimum tax considerations or any U.S. federal tax consequences other than U.S. federal income tax consequences. In addition, this discussion assumes that a U.S. Holder will not be entitled to a fractional share upon the exercise of a Warrant or Pre-funded Warrant.
As used in this summary, the term “U.S. Holder” means a beneficial owner of our ordinary shares, ADSs, Warrants, or Pre-funded Warrants that is, for U.S. federal income tax purposes, (i) an individual citizen or resident of the United States, (ii) a corporation, or other entity taxable as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state thereof, or the District of Columbia, (iii) an estate the income of which is subject to U.S. federal income tax regardless of its source or (iv) a trust with respect to which a court within the United States is able to exercise primary supervision over its administration and one or more U.S. persons have the authority to control all of its substantial decisions, or that has a valid election in effect under applicable Treasury Regulations to be treated as a “United States person.”
If an entity or arrangement treated as a partnership for U.S. federal income tax purposes holds our ordinary shares, ADSs, Warrants, or Pre-funded Warrants, the tax treatment of such partnership and each person or entity treated as a partner thereof will generally depend upon the status and activities of the partnership and such partner. A holder that is treated as a partnership for U.S. federal income tax purposes should consult its own tax advisor regarding the U.S. federal income tax considerations applicable to it and its partners of the purchase, ownership and disposition of its ordinary shares, ADSs, Warrants, or Pre-funded Warrants.
The discussions under “— Distributions” and under “— Sale, Exchange or Other Disposition of Ordinary Shares, ADSs, Warrants, and Pre-funded Warrants” below assume that we will not be treated as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. Based on our analysis of our income, assets, and operations, we believe that we were not a PFIC for 2023. Because the PFIC determination is highly fact intensive, there can be no assurance that we will not be a PFIC for 2024 or for any other taxable year. For a discussion of the rules that would apply if we are treated as a PFIC, see the discussion under “— Passive Foreign Investment Company.”
This summary is not intended to be, and should not be considered to be, legal or tax advice. Investors should be aware that this summary does not address the tax consequences to investors who are not U.S. Holders. Investors should consult their own tax advisors as to the particular tax considerations applicable to them relating to the purchase, ownership and disposition of their ordinary shares, ADSs, Warrants, or Pre-funded Warrants, including the applicability of U.S. federal, state and local tax laws and non-U.S. tax laws.
142
Tax Characterization of Pre-funded Warrants.
Although the appropriate characterization of Pre-funded Warrants under the tax law is unsettled, it is likely that the Pre-funded Warrants will be treated as a class of our ordinary shares for U.S. federal income tax purposes. However, it is possible that the IRS could treat the Pre-funded Warrants as warrants to acquire our ADSs. If the Pre-funded Warrants are not treated as a class of our ordinary shares for U.S. federal income tax purposes and are instead treated as warrants to acquire our ADSs, then the U.S. federal income tax treatment of Pre-Funded Warrants generally should be the same as the treatment of Warrants as described below and the holding period of an ADS acquired pursuant to the exercise of a Pre-funded Warrant would not include the period during which the Pre-funded Warrant was held. U.S. Holders should consult their own tax advisors regarding the U.S. federal income tax consequences of an investment in our Pre-funded Warrants.
Ownership of ADSs
For U.S. federal income tax purposes, we expect that a holder of ADSs generally should be treated as the owner of the ordinary shares represented by such ADSs. As a result, gain or loss is generally not expected to be recognized on account of exchanges of ordinary shares for ADSs, or of ADSs for ordinary shares.
Distributions.
We have no current plans to pay dividends. To the extent we pay any dividends, a U.S. Holder will be required to include in gross income as a taxable dividend the amount of any distributions made on the ordinary shares or ADSs, including the amount of any Israeli taxes withheld, to the extent that those distributions are paid out of our current and/or accumulated earnings and profits as determined for U.S. federal income tax purposes. Any distributions in excess of our earnings and profits will be applied against and will reduce the U.S. Holder’s tax basis in its ordinary shares or ADSs and to the extent they exceed that tax basis, will be treated as gain from the sale or exchange of those ordinary shares or ADSs. If we were to pay dividends, we expect to pay such dividends in NIS with respect to the ordinary shares and in U.S. dollars with respect to ADSs. A dividend paid in NIS, including the amount of any Israeli taxes withheld, will be includible in a U.S. Holder’s income as a U.S. dollar amount calculated by reference to the exchange rate in effect on the date such dividend is received, regardless of whether the payment is in fact converted into U.S. dollars. If the dividend is converted to U.S. dollars on the date of receipt, a U.S. Holder generally will not recognize a foreign currency gain or loss. However, if the U.S. Holder converts the NIS into U.S. dollars on a later date, the U.S. Holder must include, in computing its income, any gain or loss resulting from any exchange rate fluctuations. The gain or loss will be equal to the difference between (i) the U.S. dollar value of the amount included in income when the dividend was received and (ii) the amount received on the conversion of the NIS into U.S. dollars. Such gain or loss will generally be ordinary income or loss and United States source for U.S. foreign tax credit purposes. U.S. Holders should consult their own tax advisors regarding the tax consequences to them if we pay dividends in NIS or any other non-U.S. currency.
Subject to certain significant conditions and limitations, including potential limitations under the U.S.-Israel Tax Treaty, any Israeli taxes paid on or withheld from distributions from us and not refundable to a U.S. Holder may be credited against the investor’s U.S. federal income tax liability or, alternatively, may be deducted from the investor’s taxable income. The election to credit or deduct foreign taxes is made on a year-by-year basis and applies to all foreign taxes paid by a U.S. Holder or withheld from a U.S. Holder that year. Dividends paid on the ordinary shares generally will constitute income from sources outside the United States and be categorized as “passive category income” or, in the case of some U.S. Holders, as “general category income” for U.S. foreign tax credit purposes.
As a result of recent changes to the U.S. foreign tax credit rules, a withholding tax may need to satisfy certain additional requirements in order to be considered a creditable tax for a U.S. Holder. We have not determined whether these requirements have been met and, accordingly, no assurance can be given that any withholding tax on dividends paid by us will be creditable. Because the rules governing foreign tax credits are complex, U.S. Holders should consult their own tax advisor regarding the availability of foreign tax credits in their particular circumstances.
143
Dividends paid on the ordinary shares and ADSs will not be eligible for the “dividends-received” deduction generally allowed to corporate U.S. Holders with respect to dividends received from U.S. corporations.
Certain distributions treated as dividends that are received by an individual U.S. Holder from “qualified foreign corporations” generally qualify for a 20% tax rate so long as certain holding period and other requirements are met. A non-U.S. corporation (other than a corporation that is treated as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be considered to be a qualified foreign corporation (i) if it is eligible for the benefits of a comprehensive tax treaty with the United States which the Secretary of Treasury of the United States determines is satisfactory for purposes of this provision and which includes an exchange of information program, or (ii) with respect to any dividend it pays on stock (or ADSs in respect of such stock) which is readily tradable on an established securities market in the United States. Dividends paid by us in a taxable year in which we are not a PFIC and with respect to which we were not a PFIC in the preceding taxable year are expected to be eligible for the 20% tax rate, although we can offer no assurances in this regard. However, any dividend paid by us in a taxable year in which we are a PFIC or were a PFIC in the preceding taxable year will be subject to tax at regular ordinary income rates. Because the PFIC determination is highly fact intensive, there can be no assurance that we will not be a PFIC in 2024 or in any other taxable year. The additional 3.8% “net investment income tax” (described below) may apply to dividends received by certain U.S. Holders who meet certain modified adjusted gross income thresholds.
Adjustments with respect to Warrants and Pre-funded Warrants.
The terms of the Warrants and Pre-funded Warrants provide for an adjustment to the number of ordinary shares for which the warrant may be exercised or to the exercise price of the warrant in certain events. An adjustment that has the effect of preventing dilution generally is not taxable. However, the U.S. Holders of the Warrants or Pre-funded Warrants would be treated as receiving a constructive distribution from us if, for example, the adjustment increases the warrant holders’ proportionate interest in our assets or earnings and profits (e.g., through a decrease in the exercise price of the Warrants or Pre-funded Warrants) as a result of a distribution of cash to the holders of our ordinary shares or ADSs, which is taxable to the U.S. Holders of such ordinary shares or ADSs as described under “—Distributions” above. Such constructive distribution would be subject to tax as described under that section in the same manner as if the U.S. Holders of the Warrants or Pre-funded Warrants received a cash distribution from us equal to the fair market value of such increased interest. U.S. Holders of Warrants and Pre-funded Warrants are urged to consult their own tax advisors on these issues.
Sale, Exchange or Other Disposition of Ordinary Shares, ADSs, Warrants, and Pre-funded Warrants.
Subject to the discussion under “— Passive Foreign Investment Company” below, a U.S. Holder generally will recognize capital gain or loss upon the sale, exchange or other taxable disposition of our ordinary shares, ADSs, Warrants, or Pre-funded Warrants in an amount equal to the difference between the amount realized on the sale, exchange or other disposition and the U.S. Holder’s adjusted tax basis in such securities. This capital gain or loss will be long-term capital gain or loss if the U.S. Holder’s holding period in our securities exceeds one year. Preferential tax rates for long-term capital gain (currently, with a maximum rate of 20%) will apply to individual U.S. Holders. The deductibility of capital losses is subject to limitations. The gain or loss will generally be income or loss from sources within the United States for U.S. foreign tax credit purposes, subject to certain exceptions in the U.S.-Israel Tax Treaty. The additional 3.8% “net investment income tax” (described below) may apply to gains recognized upon the sale, exchange, or other taxable disposition of our securities by certain U.S. Holders who meet certain modified adjusted gross income thresholds.
U.S. Holders should consult their own tax advisors regarding the U.S. federal income tax consequences of receiving currency other than U.S. dollars upon the disposition of their ordinary shares, ADSs, Warrants, or Pre-funded Warrants.
144
Exercise or Lapse of a Warrant and Pre-funded Warrants.
Subject to the discussion under “—Passive Foreign Investment Company” below, a U.S. Holder generally will not recognize gain or loss upon the exercise of a Warrant or Pre-funded Warrant for cash. An ADS acquired pursuant to the exercise of a Warrant or Pre-funded Warrant for cash generally will have a tax basis equal to the U.S. Holder’s tax basis in the Warrant or Pre-funded Warrant, increased by the amount paid to exercise the Warrant or Pre-funded Warrant. The holding period of an ADS acquired pursuant to the exercise of a Warrant generally would begin on the day after the date of exercise of the Warrant. Subject to the discussion above regarding the tax characterization of the Pre-funded Warrants, the holding period of a Pre-funded Warrant should carry over to an ADS acquired pursuant to the exercise of a Pre-funded Warrant. If a Warrant or Pre-funded Warrant is allowed to lapse unexercised, a U.S. Holder generally will recognize a capital loss equal to such holder’s tax basis in the warrant.
The tax consequences of a cashless exercise of Warrants are unclear and could differ from the consequences described above. It is possible that a cashless exercise could be a taxable event. U.S. Holders should consult their own tax advisors regarding the U.S. federal income tax consequences of the cashless exercise of Warrants, including with respect to whether the exercise is a taxable event, and their holding period and tax basis in the ADSs received.
Passive Foreign Investment Company
In general, a corporation organized outside the United States will be treated as a PFIC for U.S. federal income tax purposes in any taxable year in which either (i) at least 75% of its gross income is “passive income” or (ii) on average at least 50% of its assets (by value) produce passive income or are held for the production of passive income. Passive income for this purpose generally includes, among other things, certain dividends, interest, royalties, rents and gains from commodities and securities transactions and from the sale or exchange of property that gives rise to passive income. Passive income also includes amounts derived by reason of the temporary investment of funds, including those raised in the public offering. Assets that produce or are held for the production of passive income may include cash, even if held as working capital or raised in a public offering, as well as marketable securities and other assets that may produce passive income. In determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
Under the tests described above, whether or not we are a PFIC will be determined annually based upon the composition of our income and the composition and valuation of our assets, all of which are subject to change.
Based on our analysis of our income, assets, and operations, we believe that we were not a PFIC for 2023. Because the PFIC determination is highly fact intensive, there can be no assurance that we will not be a PFIC in 2024 or in any other taxable year. Even if we determine that we are not a PFIC after the close of a taxable year, there can be no assurance that a court or the IRS will agree with our conclusion.
Default PFIC Rules. If we are a PFIC for any tax year, a U.S. Holder who does not make a timely QEF election or a mark-to-market election, referred to in this disclosure as a “Non-Electing U.S. Holder,” will be subject to special rules with respect to (i) any “excess distribution” (generally, the portion of any distributions received by the Non-Electing U.S. Holder on the ordinary shares or ADSs (or warrants, to the extent applicable) in a taxable year in excess of 125% of the average annual distributions received by the Non-Electing U.S. Holder in the three preceding taxable years, or, if shorter, the Non-Electing U.S. Holder’s holding period for the ordinary shares or ADSs), and (ii) any gain realized on the sale or other disposition of ordinary shares, ADSs, Warrants, or Pre-funded Warrants. Under these rules:
| ● | the excess distribution or gain would be allocated ratably over the Non-Electing U.S. Holder’s holding period for such ordinary shares, ADSs, Warrants, or Pre-funded Warrants; |
145
| ● | the amount allocated to the current taxable year and any year prior to us becoming a PFIC would be taxed as ordinary income; and |
| ● | the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. |
If a Non-Electing U.S. Holder who is an individual dies while owning our ordinary shares, ADSs, Warrants, or Pre-funded Warrants, the Non-Electing U.S. Holder’s successor would be ineligible to receive a step-up in tax basis of such ordinary shares, ADSs, Warrants, or Pre-funded Warrants. Non-Electing U.S. Holders should consult their tax advisors regarding the application of the “net investment income tax” (described below) to their specific situation.
To the extent a distribution on our ordinary shares or ADSs (or warrants, to the extent applicable) does not constitute an excess distribution to a Non-Electing U.S. Holder, such Non-Electing U.S. Holder generally will be required to include the amount of such distribution in gross income as a dividend to the extent of our current and/or accumulated earnings and profits (as determined for U.S. federal income tax purposes) that are not allocated to excess distributions. The tax consequences of such distributions are discussed above under “— Taxation of U.S. Holders — Distributions.” Each U.S. Holder is encouraged to consult its own tax advisor with respect to the appropriate U.S. federal income tax treatment of any distribution on our ordinary shares or ADSs (or warrants, to the extent applicable).
If we are treated as a PFIC for any taxable year during the holding period of a Non-Electing U.S. Holder, we will continue to be treated as a PFIC for all succeeding years during which the Non-Electing U.S. Holder is treated as a direct or indirect Non-Electing U.S. Holder even if we are not a PFIC for such years. A U.S. Holder is encouraged to consult its tax advisor with respect to any available elections that may be applicable in such a situation, including the “deemed sale” election of Code Section 1298(b)(1) (which will be taxed under the adverse tax rules described above).
We may invest in the equity of foreign corporations that are PFICs or may own subsidiaries that own PFICs. If we are classified as a PFIC, under attribution rules U.S. Holders will be subject to the PFIC rules with respect to their indirect ownership interests in such PFICs, such that a disposition of the shares of the PFIC or receipt by us of a distribution from the PFIC generally will be treated as a deemed disposition of such shares or the deemed receipt of such distribution by the U.S. Holder, subject to taxation under the PFIC rules. There can be no assurance that a U.S. Holder will be able to make a QEF election or a mark-to-market election with respect to PFICs in which we invest. Each U.S. Holder is encouraged to consult its own tax advisor with respect to tax consequences of an investment by us in a corporation that is a PFIC.
QEF Election. Certain adverse consequences of PFIC status can be mitigated for holders of our ordinary shares, ADSs, and Pre-funded Warrants if a U.S. Holder makes a QEF election. A U.S. Holder may not make a QEF election with respect to our Warrants. A U.S. Holder who makes a timely QEF election, referred to in this disclosure as an “Electing U.S. Holder,” with respect to us must report for U.S. federal income tax purposes his pro rata share of our ordinary earnings and net capital gain, if any, for our taxable year that ends with or within the taxable year of the Electing U.S. Holder. The “net capital gain” of a PFIC is the excess, if any, of the PFIC’s net long-term capital gains over its net short-term capital losses. The amount so included in income generally will be treated as ordinary income to the extent of such Electing U.S. Holder’s allocable share of the PFIC’s ordinary earnings and as long-term capital gain to the extent of such Electing U.S. Holder’s allocable share of the PFIC’s net capital gains. Such Electing U.S. Holder generally will be required to translate such income into U.S. dollars based on the average exchange rate for the PFIC’s taxable year with respect to the PFIC’s functional currency. Such income generally will be treated as income from sources outside the United States for U.S. foreign tax credit purposes. Amounts previously included in income by such Electing U.S. Holder under the QEF rules generally will not be subject to tax when they are distributed to such Electing U.S. Holder. The Electing U.S. Holder’s tax basis in our ordinary shares, ADSs, or Pre-funded Warrants generally will increase by any amounts so included under the QEF rules and decrease by any amounts not included in income when distributed.
An Electing U.S. Holder will be subject to U.S. federal income tax on such amounts for each taxable year in which we are a PFIC, regardless of whether such amounts are actually distributed to such Electing U.S. Holder. However, an Electing U.S. Holder may, subject to certain limitations, elect to defer payment of current U.S. federal income tax on such amounts, subject to an interest charge. If an Electing U.S. Holder is an individual, any such interest will be treated as non-deductible “personal interest.”
146
Any net operating losses or net capital losses of a PFIC will not pass through to the Electing U.S. Holder and will not offset any ordinary earnings or net capital gain of a PFIC recognized by Electing U.S. Holder in subsequent years.
So long as an Electing U.S. Holder’s QEF election with respect to us is in effect with respect to the entire holding period for our ordinary shares, ADSs, or Pre-funded Warrants, any gain or loss recognized by such Electing U.S. Holder on the sale, exchange or other disposition of such shares, ADSs, or Pre-funded Warrants generally will be long-term capital gain or loss if such Electing U.S. Holder has held such shares, ADSs, or Pre-funded Warrants for more than one year at the time of such sale, exchange or other disposition. Preferential tax rates for long-term capital gain (currently, a maximum rate of 20%) will apply to individual U.S. Holders. The deductibility of capital losses is subject to limitations.
In general, a U.S. Holder must make a QEF election on or before the due date for filing its income tax return for the first year to which the QEF election is to apply. A U.S. Holder makes a QEF election by completing the relevant portions of and filing IRS Form 8621 in accordance with the instructions thereto. Upon request, we intend to annually furnish U.S. Holders with information needed in order to complete IRS Form 8621 (which form would be required to be filed with the IRS on an annual basis by the U.S. Holder) and to make and maintain a valid QEF election for any year in which we are a PFIC. There is no assurance, however, that we will have timely knowledge of our status as a PFIC, or that the information that we provide will be adequate to allow U.S. Holders to make a QEF election. A QEF election will not apply to any taxable year during which we are not a PFIC, but will remain in effect with respect to any subsequent taxable year in which we become a PFIC.
A U.S. Holder may not make a QEF election with respect to our Warrants. As a result, if a U.S. Holder sells or otherwise disposes of such Warrants (other than upon exercise thereof), any gain recognized generally will be subject to special tax and interest charge rules treating the gain as an excess distribution, as described above, if we were a PFIC at any time during the period the U.S. Holder held the Warrants. If a U.S. Holder that exercises such Warrants properly makes a QEF election with respect to the newly acquired ADSs (or has previously made a QEF election with respect to our ADSs), the QEF election will apply to the newly acquired ADSs, but the adverse tax consequences attributable to the period prior to exercise of the Warrants, adjusted to take into account the current income inclusions resulting from the QEF election, will continue to apply with respect to such newly acquired ADSs, unless the U.S. Holder makes a “purging election” that creates a deemed sale of such ADSs at their fair market value. The gain recognized by the purging election will be subject to the special tax and interest charge rules treating the gain as an excess distribution, as described above.
Each U.S. Holder should consult its own tax advisor with respect to the advisability of, the tax consequences of, and the procedures for making a QEF election with respect to us.
Mark-to-Market Election. Alternatively, if our ordinary shares or ADSs are treated as “marketable stock,” a U.S. Holder would be allowed to make a “mark-to-market” election with respect to our ordinary shares or ADSs, provided the U.S. Holder completes and files IRS Form 8621 in accordance with the relevant instructions and related Treasury Regulations. If the election is made, the U.S. Holder generally would include as ordinary income in each taxable year the excess, if any, of the fair market value of our ordinary shares or ADSs at the end of the taxable year over such holder’s adjusted tax basis in such shares or ADSs. The U.S. Holder would also be permitted an ordinary loss in respect of the excess, if any, of the U.S. Holder’s adjusted tax basis in our ordinary shares or ADSs over their fair market value at the end of the taxable year, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. A U.S. Holder’s tax basis in our ordinary shares or ADSs would be adjusted to reflect any such income or loss amount. Gain realized on the sale, exchange or other disposition of our ordinary shares or ADSs would be treated as ordinary income, and any loss realized on the sale, exchange or other disposition of our ordinary shares or ADSs would be treated as ordinary loss to the extent that such loss does not exceed the net mark-to-market gains previously included in income by the U.S. Holder, and any loss in excess of such amount will be treated as capital loss. Amounts treated as ordinary income will not be eligible for the favorable tax rates applicable to qualified dividend income or long-term capital gains.
147
Generally, stock will be considered marketable stock if it is “regularly traded” on a “qualified exchange” within the meaning of applicable Treasury Regulations. A class of stock is regularly traded on an exchange during any calendar year during which such class of stock is traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. To be marketable stock, our ordinary shares and ADSs must be regularly traded on a qualifying exchange (i) in the United States that is registered with the SEC or a national market system established pursuant to the Exchange Act or (ii) outside the United States that is properly regulated and meets certain trading, listing, financial disclosure and other requirements. Our ordinary shares should constitute “marketable stock” as long as they remain listed on the TASE and are regularly traded. Our ADSs will be listed on the OTC and/or the NYSE American. While we believe that our ordinary shares and ADSs may be treated as marketable stock for purposes of the PFIC rules so long as they are listed on the TASE and the OTC and/or the NYSE American, as applicable, and are regularly traded, the IRS has not provided a list of the exchanges that meet the foregoing requirements and thus no assurance can be provided that our ordinary shares and/or ADSs will be (or will remain) treated as marketable stock for purposes of the PFIC rules.
A mark-to-market election will not apply to our ordinary shares or ADSs held by a U.S. Holder for any taxable year during which we are not a PFIC, but will remain in effect with respect to any subsequent taxable year in which we become a PFIC. Such election will not apply to any PFIC subsidiary that we own. Each U.S. Holder is encouraged to consult its own tax advisor with respect to the availability and tax consequences of a mark-to-market election with respect to our ordinary shares and ADSs.
In addition, U.S. Holders should consult their tax advisors regarding the IRS information reporting and filing obligations that may arise as a result of the ownership of ordinary shares in a PFIC, including IRS Form 8621, Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund.
The U.S. federal income tax rules relating to PFICs, QEF elections, and mark-to market elections are complex. U.S. Holders are urged to consult their own tax advisors with respect to the purchase, ownership and disposition of our ordinary shares, ADSs, Warrants, or Pre-funded Warrants, any elections available with respect to such shares, ADSs, Warrants, or Pre-funded Warrants, and the IRS information reporting obligations with respect to the purchase, ownership and disposition of our ordinary shares, ADSs, Warrants, or Pre-funded Warrants.
Certain Reporting Requirements
Certain U.S. Holders may be required to file IRS Form 926, Return by U.S. Transferor of Property to a Foreign Corporation, and IRS Form 5471, Information Return of U.S. Persons With Respect to Certain Foreign Corporations, reporting transfers of cash or other property to us and information relating to the U.S. Holder and us. Substantial penalties may be imposed upon a U.S. Holder that fails to comply. See also the discussion regarding Form 8621, Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund, above.
In addition, certain U.S. Holders must report information on IRS Form 8938, Statement of Specified Foreign Financial Assets, with respect to their investments in certain “specified foreign financial assets,” which would include an investment in our securities, if the aggregate value of all of those assets exceeds $50,000 on the last day of the taxable year (and in some circumstances, a higher threshold). This reporting requirement applies to individuals and certain U.S. entities.
U.S. Holders who fail to report required information could become subject to substantial penalties. U.S. Holders should consult their tax advisors regarding the possible implications of these reporting requirements arising from their investment in our securities.
148
Backup Withholding Tax and Information Reporting Requirements
Generally, information reporting requirements will apply to distributions on our ordinary shares or ADSs (or warrants, to the extent applicable) or proceeds on the disposition of our securities paid within the United States (and, in certain) proceeds on the disposition of our securities paid within the United States (and, in certain cases, outside the United States) to U.S. Holders other than certain exempt recipients, such as corporations. Furthermore, backup withholding (currently at 24%) may apply to such amounts if the U.S. Holder fails to (i) provide a correct taxpayer identification number, (ii) report interest and dividends required to be shown on its U.S. federal income tax return, or (iii) make other appropriate certifications in the required manner. U.S. Holders who are required to establish their exempt status generally must provide such certification on IRS Form W-9.
Backup withholding is not an additional tax. Amounts withheld as backup withholding from a payment may be credited against a U.S. Holder’s U.S. federal income tax liability and such U.S. Holder may obtain a refund of any excess amounts withheld by filing the appropriate claim for refund with the IRS and furnishing any required information in a timely manner.
Tax on Net Investment Income
Certain U.S. persons, including individuals, estates and trusts are generally subject to an additional 3.8% Medicare tax. For individuals, the additional Medicare tax applies to the lesser of (i) “net investment income” or (ii) the excess of “modified adjusted gross income” over $200,000 ($250,000 if married and filing jointly or $125,000 if married and filing separately). “Net investment income” generally equals the taxpayer’s gross investment income reduced by the deductions that are allocable to such income. Investment income generally includes passive income such as interest, dividends, annuities, royalties, rents, and capital gains. U.S. Holders are urged to consult their own tax advisors regarding the implications of the additional Medicare tax resulting from their ownership and disposition of our securities.
Tax Consequences for Non-U.S. Holders of Ordinary Shares, ADSs, Warrants, or Pre-funded Warrants
Except as provided below, an individual, corporation, estate or trust that is not a U.S. Holder, referred to below as a non-U.S. Holder, generally will not be subject to U.S. federal income or withholding tax on the payment of dividends on, and the proceeds from the disposition of, our ordinary shares, ADSs, Warrants, or Pre-funded Warrants.
A non-U.S. Holder may be subject to U.S. federal income tax on a dividend paid on our ordinary shares or ADSs (or warrants, to the extent applicable) or gain from the disposition of our ordinary shares, ADSs, Warrants, or Pre-funded Warrants if: (1) such item is effectively connected with the conduct by the non-U.S. Holder of a trade or business in the United States and, if required by an applicable income tax treaty is attributable to a permanent establishment or fixed place of business in the United States; or (2) in the case of a disposition of our ordinary shares, ADSs, Warrants, or Pre-funded Warrants, the individual non-U.S. Holder is present in the United States for 183 days or more in the taxable year of the disposition and other specified conditions are met.
In general, non-U.S. Holders will not be subject to backup withholding with respect to the payment of dividends on our ordinary shares or ADSs (or warrants, to the extent applicable) if payment is made through a paying agent, or office of a foreign broker outside the United States. However, if payment is made in the United States or by a U.S. related person, non-U.S. Holders may be subject to backup withholding, unless the non-U.S. Holder provides an applicable IRS Form W-8 (or a substantially similar form) certifying its foreign status, or otherwise establishes an exemption.
The amount of any backup withholding from a payment to a non-U.S. Holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
149
The discussion above is a general summary and is not intended to constitute a complete analysis of all tax consequences relating to the purchase, ownership and disposition of our ordinary shares, ADSs, Warrants or Pre-funded Warrants. It does not cover all tax matters that may be of importance to a U.S. Holder. U.S. Holders should consult their own tax advisors concerning the tax consequences relating to the purchase, ownership and disposition of our ordinary shares, ADSs, Warrants or Pre-funded Warrants.
F. Dividends and Paying Agents.
Not applicable.
G. Statements by Experts.
Not applicable.
H. Documents on Display.
We are subject to the information reporting requirements of the Exchange Act, applicable to foreign private issuers and under those requirements will file reports with the SEC. The SEC maintains an Internet website that contains reports and other information regarding issuers that file electronically with the SEC. You may read and copy this annual report, including the related exhibits and schedules, and any document we file with the SEC at http://www.sec.gov.
As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. However, we file with the SEC, within four months after the end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm, and submit to the SEC, on a Form 6-K, unaudited quarterly financial information for the first three quarters of each fiscal year within 60 days after the end of each such quarter, or such applicable time as required by the SEC.
In addition, because our ordinary shares are traded on the TASE, we have filed Hebrew language periodic and immediate reports with, and furnish information to, the TASE and the ISA, as required under Chapter Six of the Israel Securities Law. Copies of our filings with the ISA can be retrieved electronically through the MAGNA distribution site of the ISA (www.magna.isa.gov.il) and the TASE website (www.maya.tase.co.il).
We maintain a corporate website at www.canfite.com. Information contained on, or that can be accessed through, our website does not constitute a part of this Annual Report on Form 20-F. We have included our website address in this Annual Report on Form 20-F solely as an inactive textual reference.
I. Subsidiary Information.
Not applicable.
J. Annual Report to Security Holders.
Not applicable.
ITEM 11. Quantitative and Qualitative Disclosures About Market Risk
Market risk is the risk of loss related to changes in market prices, including interest rates and foreign exchange rates, of financial instruments that may adversely impact our consolidated financial position, results of operations or cash flows.
150
Interest Rate Risk
We do not anticipate undertaking any significant long-term borrowings. At present, our investments consist primarily of cash and cash equivalents. We may invest in investment-grade marketable securities with maturities of up to three years, including commercial paper, money market funds, and government/non-government debt securities. The primary objective of our investment activities is to preserve principal while maximizing the income that we receive from our investments without significantly increasing risk and loss. Our investments are exposed to market risk due to fluctuation in interest rates, which may affect our interest income and the fair market value of our investments, if any. We manage this exposure by performing ongoing evaluations of our investments. Due to the short-term maturities, if any, of our investments to date, their carrying value has always approximated their fair value. If we decide to invest in investments other than cash and cash equivalents, it will be our policy to hold such investments to maturity in order to limit our exposure to interest rate fluctuations.
Foreign Currency Exchange Risk
Our foreign currency exposures give rise to market risk associated with exchange rate movements of the U.S. dollar, our functional and reporting currency, mainly against the NIS and the euro. Although the U.S. dollar is our functional currency, a portion of our expenses are denominated in both NIS and euro and currently all of our revenues are denominated in dollars. Our U.S. dollar and euro expenses consist principally of payments made to sub-contractors and consultants for preclinical studies, clinical trials and other research and development activities, Our NIS expenses consist principally of salary related payments. We anticipate that a sizable portion of our expenses will continue to be denominated in currencies other than the NIS. If the U.S. dollar fluctuates significantly against either the NIS or the euro, it may have a negative impact on our results of operations. To date, fluctuations in the exchange rates have not materially affected our results of operations or financial condition for the periods under review.
To date, we have not engaged in hedging transactions. In the future, we may enter into currency hedging transactions to decrease the risk of financial exposure from fluctuations in the exchange rates of our principal operating currencies. These measures, however, may not adequately protect us from the material adverse effects of such fluctuations.
ITEM 12. Description of Securities Other Than Equity Securities
A. Debt Securities.
Not applicable.
B. Warrants and Rights.
Not applicable.
C. Other Securities.
Not applicable.
D. American Depositary Shares
The Bank of New York Mellon, as Depositary, will register and deliver American Depositary Shares, or ADSs. Each ADS will represent three hundred (300) ordinary shares (or a right to receive three hundred (300) ordinary shares) deposited with the principal Tel Aviv office of Bank Hapoalim, as custodian for the Depositary. Each ADS will also represent any other securities, cash or other property which may be held by the Depositary. The Depositary’s corporate trust office at which the ADSs will be administered is located at 101 Barclay Street, New York, New York 10286. The Bank of New York Mellon’s principal executive office is located at One Wall Street, New York, New York 10286.
The form of the deposit agreement for our ADSs and the form of American Depositary Receipt that represents an ADS have been incorporated by reference as exhibits to this Annual Report on Form 20-F.
151
Fees and Expenses
| Persons depositing or withdrawing shares or ADS holders must pay: | For: | ||
| $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) | ● | Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property | |
| ● | Cancellation of ADSs for the purpose of withdrawal, including if the Deposit Agreement terminates | ||
| $.05 (or less) per ADS | ● | Any cash distribution to ADS holders | |
| A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs | ● | Distribution of securities distributed to holders of deposited securities which are distributed by the Depositary to ADS holders | |
| $.05 (or less) per ADSs per calendar year | ● | Depositary services | |
| Registration or transfer fees | ● | Transfer and registration of shares on our share register to or from the name of the Depositary or its agent when you deposit or withdraw shares | |
| Expenses of the Depositary | ● | Cable, telex and facsimile transmissions (when expressly provided in the Deposit Agreement) | |
| ● | Converting foreign currency to U.S. dollars | ||
| Taxes and other governmental charges the Depositary or the custodian have to pay on any ADS or share underlying an ADS, for example, stock transfer taxes, stamp duty or withholding taxes | ● | As necessary | |
| Any charges incurred by the Depositary or its agents for servicing the deposited securities | ● | As necessary | |
The Depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The Depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The Depositary may collect its annual fee for depositary services by deduction from cash distributions, by directly billing investors or by charging the book-entry system accounts of participants acting for them. The Depositary may generally refuse to provide fee-attracting services until its fees for those services are paid.
From time to time, the Depositary may make payments to us to reimburse us for expenses and/or share revenue with us from the fees collected from ADS holders, or waive fees and expenses for services provided, generally relating to costs and expenses arising out of the establishment and maintenance of the ADS program. In performing its duties under the Deposit Agreement, the Depositary may use brokers, dealers or other service providers that are affiliates of the Depositary and that may earn or share fees or commissions.
152
PART II
ITEM 13. Defaults, Dividend Arrearages and Delinquencies
Not applicable.
ITEM 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
Not applicable.
ITEM 15. Controls and Procedures
Disclosure controls and procedures
Our management, including our chief executive officer, or CEO, who is also our chief financial officer, or CFO, is responsible for establishing and maintaining our disclosure controls and procedures (within the meaning of Rule 13a-15(e) of the Exchange Act). These controls and procedures were designed to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC and that such information is accumulated and communicated to our management, including our CEO and CFO, as appropriate, to allow timely decisions regarding required disclosure. We evaluated these disclosure controls and procedures under the supervision of our CEO and CFO as of December 31, 2023. Based upon that evaluation, our management, including our CEO and CFO, concluded that our disclosure controls and procedures as of December 31, 2023 were effective.
Management’s annual report on internal control over financial reporting
Our management, including our CEO, and our CFO, is responsible for establishing and maintaining adequate internal control over our financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act of 1934, as amended. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Internal control over financial reporting includes policies and procedures that:
| ● | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and asset dispositions; |
| ● | provide reasonable assurance that transactions are recorded as necessary to permit the preparation of our financial statements in accordance with generally accepted accounting principles; |
| ● | provide reasonable assurance that receipts and expenditures are made only in accordance with authorizations of our management and board of directors (as appropriate); and |
| ● | provide reasonable assurance regarding the prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on our financial statements. |
Due to its inherent limitations, internal controls over financial reporting may not prevent or detect misstatements. In addition, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, including our CEO, and our CFO, we assessed the effectiveness of our internal control over financial reporting as of December 31, 20223 based on the framework for Internal Control-Integrated Framework set forth by The Committee of Sponsoring Organizations of the Treadway Commission (COSO) (2013).
Based on our assessment and this framework, our management concluded that our internal control over financial reporting were effective as of December 31, 2023.
153
Attestation Report of Registered Public Accounting Firm
Not applicable.
Changes in internal control over financial reporting
There were no changes in our internal control over financial reporting, other than as described above, that occurred during the year ended December 31, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 16. [RESERVED]
ITEM 16A. Audit Committee Financial Expert
Our Board of Directors has determined that Guy Regev and Yaacov Goldman are audit committee financial experts, as defined by applicable SEC regulations. Messrs. Regev and Goldman qualified as an “independent director,” as that term is defined under NYSE American rules.
ITEM 16B. Code of Ethics
We have adopted a code of ethics, referred to as a Code of Business Conduct, applicable to our directors, officers and all other employees. Our code of ethics is publicly available on our website at www.canfite.com. If we make any amendment to the code of ethics or grant any waivers, including any implicit waiver, from a provision of the code of ethics, which applies to our chief executive officer, chief financial officer, chief accounting officer or controller, or persons performing similar functions, we will disclose the nature of such amendment or waiver on our website.
ITEM 16C. Principal Accountant Fees and Services
The following table sets forth, for each of the years indicated, the fees billed by our independent registered public accounting firm.
| Year Ended December 31, | ||||||||
| 2022 | 2023 | |||||||
| Services Rendered | (USD in thousands) | |||||||
| Audit (1) | 143 | 169 | ||||||
| Audit related services | - | - | ||||||
| Tax | - | - | ||||||
| All other fees | - | - | ||||||
| Total | 143 | 169 | ||||||
| (1) | Audit fees consist of services that would normally be provided in connection with statutory and regulatory filings or engagements, including services that generally only the independent accountant can reasonably provide. |
Audit Committee Pre-Approval Policies and Procedures
Our audit committee’s specific responsibilities in carrying out its oversight of the quality and integrity of the accounting, auditing and reporting practices of us include the approval of audit and non-audit services to be provided by the external auditor. The audit committee approves in advance the particular services or categories of services to be provided to us during the following yearly period and also sets forth a specific budget for such audit and non-audit services. Additional non-audit services may be pre-approved by the audit committee.
ITEM 16D. Exemptions from the Listing Standards for Audit Committees
Not applicable.
ITEM 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
Not applicable.
154
ITEM 16F. Change in Registrant’s Certifying Accountant
Not applicable.
ITEM 16G. Corporate Governance
We are a foreign private issuer whose ordinary shares are listed on the NYSE American. As such, we are required to comply with U.S. federal securities laws, including the Sarbanes-Oxley Act, and the NYSE American rules, including the NYSE American corporate governance requirements. The NYSE American rules provide that foreign private issuers may follow home country practice in lieu of certain qualitative listing requirements subject to certain exceptions and except to the extent that such exemptions would be contrary to U.S. federal securities laws, so long as the foreign issuer discloses that it does not follow such listing requirement and describes the home country practice followed in its reports filed with the SEC. Below is a concise summary of the significant ways in which our corporate governance practices differ from the corporate governance requirements of NYSE American applicable to domestic U.S. listed companies:
| ● | The NYSE American rules recommend that an issuer have a quorum requirement for shareholders meetings of at least one-third of the outstanding shares of the issuer’s common voting stock. We have chosen to follow home country practice with respect to the quorum requirements of our shareholders meeting and our adjourned shareholders meeting. Our Amended and Restated Articles of Association, as permitted under the Israeli Companies Law and Israeli practice, provide that the quorum requirements for a shareholders meeting are the presence of at least two shareholders who represent at least 25% of the outstanding shares of the issuer’s common voting stock, and in the event of an adjourned meeting, the presence of a minimum of two shareholders present in person. |
| ● | We have chosen to follow our home country practice in lieu of the requirements of the NYSE American rules relating to shareholder approval required prior to the issuance of securities (i) when a stock option or purchase plan is to be established or materially amended or other equity compensation arrangement made or materially amended, pursuant to which stock may be acquired by officers, directors, employees or consultants and (ii) in connection with a transaction, other than a public offering, involving the issuance or potential issuance by the Company of ordinary shares (or their equivalent) equal to 20% or more of the ordinary shares or 20% voting power outstanding before the issuance for or at a price less than the greater of book or market value of the shares. We follow the provisions of the Israeli Companies Law with regard to transactions with our affiliates, i.e., our controlling shareholder and our directors and officers, including private placement transactions. |
ITEM 16H. Mine Safety Disclosure
Not applicable.
ITEM 16I. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
ITEM 16J.
Pursuant to applicable SEC transition guidance, the disclosure required by Item 16J will only be applicable to the Company from the fiscal year ending on December 31, 2024.
ITEM 16K. CYBERSECURITY
We have developed and maintain a cybersecurity risk management program, consisting of cybersecurity policies, procedures, compliance and awareness programs to mitigate risk and to ensure compliance with security, availability and confidentiality trust principles. The cybersecurity process has been integrated into our overall risk management system and process, and is solely internally managed. Management is responsible for identifying risks that threaten achievement of the control activities stated in the management’s description of the services organizations systems. Management has implemented a process for identifying relevant risks that could affect the organization’s ability to provide secure and reliable service to its users. The risk assessment occurs annually, or as business needs change, and covers identification of risks that could act against the company’s objectives as well as specific risks related to a compromise to the security of data. See “Item 3.D Risk Factors— Risks Related to Our Business and Regulatory Matters— Our business and operations may be materially adversely affected in the event of computer system failures or security breaches.”
The oversight of cybersecurity threats is undertaken by our Chief Executive Officer who and is supported by outside IT consultants.. Our audit committee is responsible for cybersecurity oversight and monitoring risk. Management informs the audit committee of such risk by committee meetings.
As of the date of this report, we are not aware of any material risks from cybersecurity threats that have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations or financial condition.
155
PART III
ITEM 17. Financial Statements
We have responded to Item 18 in lieu of responding to this item.
ITEM 18. Financial Statements
Please refer to the financial statements beginning on page F-1.
ITEM 19. Exhibits
Index to Exhibits
156
157
| * | Filed Herewith. |
| # | Management contract or compensatory plan. |
| † | Certain confidential information contained in this exhibit was omitted by means of redacting a portion of the text and replacing it with [...]. This exhibit has been filed separately with the Commission without the redaction pursuant to a Confidential Treatment Request under Rule 24b-2 of the Securities Exchange Act of 1934 and Rule 406 of the Securities Act. |
| (1) | Incorporated herein by reference to the Annual Report on Form 20-F filed with the SEC on March 30, 2023. |
| (2) | Incorporated herein by reference to the Registration Statement on Form 8-A filed with the SEC on November 15, 2013. |
| (3) | Incorporated herein by reference to Amendment No. 1 to the Registration Statement on Form 20-F filed with the SEC on September 10, 2013. |
| (4) | Incorporated herein by reference to Annual Report on Form 20-F filed with the SEC on March 27, 2015. |
| (5) | Incorporated herein by reference to the Annual Report on Form 20- F filed with the SEC on March 30, 2017. |
| (6) | Incorporated herein by reference to the Report on Form 6-K filed with the SEC on January 20, 2017. |
| (7) | Incorporated herein by reference to Registration Statement on Form F-1 filed with the SEC on February 15, 2019. |
| (8) | Incorporated herein by reference to Registration Statement on Form F-1 filed with the SEC on May 3, 2019. |
| (9) | Incorporated herein by reference to Report on Form 6-K filed with the SEC on May 22, 2019. |
| (10) | Incorporated herein by reference to Registration Statement on Form F-1 filed with the SEC on May 28, 2019. |
| (11) | Incorporated herein by reference to Registration Statement on Form F-1 filed with the SEC on October 17, 2019. |
| (12) | Incorporated herein by reference to the Report on Form 6-K filed with the SEC on January 10, 2020. |
| (13) | Incorporated herein by reference to the Report on Form 6-K filed with the SEC on February 12, 2020. |
| (14) | Incorporated herein by reference to the Report on Form 6-K filed with the SEC on June 12, 2020. |
| (15) | Incorporated herein by reference to the Report on Form 6-K filed with the SEC on July 8, 2020. |
| (16) | Incorporated herein by reference to the Report on Form 6-K filed with the SEC on August 16, 2021. |
| (17) | Incorporated herein by reference to the Report on Form 6-K filed with the SEC on December 23, 2021. |
| (18) | Incorporated herein by reference to the Annual Report on Form 20- F filed with the SEC on March 24, 2022. |
| (19) | Incorporated herein by reference to the Report on Form 6-K filed with the SEC on January 13, 2023. |
| (20) | Incorporated herein by reference to the Report on Form 6-K filed with the SEC on January 13, 2023. |
| (21) | Incorporated herein by reference to the Report on Form 6-K filed with the SEC on November 22, 2023. |
158
SIGNATURES
The Registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
| CAN-FITE BIOPHARMA LTD. | ||
| Date: March 28, 2024 | By: | /s/ Motti Farbstein |
| Motti Farbstein | ||
Chief Executive Officer and Chief Financial Officer | ||
159
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2023
INDEX
- - - - - - - - - - -
F-1
 |
Kost Forer Gabbay & Kasierer 144 Menachem Begin Road, Building A Tel-Aviv 6492102, Israel |
Tel: +972-3-6232525 Fax: +972-3-5622555 ey.com |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of
CAN-FITE BIOPHARMA LTD.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Can-Fite Biopharma Ltd. and its subsidiary (the “Company”) as of December 31, 2023 and 2022, the related consolidated statements of comprehensive loss, changes in shareholders’ equity and cash flows for each of the three years in the period ended December 31, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
F-2
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
| Liquidity and Capital Resources | ||
| Description of the Matter | As discussed in Note 1 to the consolidated financial statements, the Company has incurred operating losses and negative cash-flow from operations since inception. The Company’s operations are dependent on its ability to raise additional funds. This dependency will continue until the Company will be able to completely finance its operations by generating revenue from its product candidates. Management has concluded that, based on its current projections and plans, the Company will be able to satisfy its liquidity requirements for more than one year from the date these financial statements were issued. | |
| We identified the assessment of liquidity and the Company’s ability to continue as a going concern as a critical audit matter due to the subjective judgments required of management to conclude the Company would have sufficient liquidity to sustain itself for at least a year beyond the date of the issuance of the consolidated financial statements. This in turn led to a high degree of auditor subjectivity and judgment to evaluate the audit evidence supporting the liquidity conclusions. | ||
| How We Addressed the Matter in Our Audit | Addressing the matter involved performing procedures and evaluating audit evidence in connection with our overall opinion on the consolidated financial statements. Our audit procedures to evaluate the significant judgments made by management included, among others, testing the reasonableness of forecasted research and development costs, comparison of prior period forecasts to actual results, evaluating the fluctuations in forecasted clinical trial expenses and payments thereof as compared to historic amounts and the underlying management assumptions, evaluating the reasonableness of the amounts and timing of payments of forecasted general and administrative expenses and other income, net, and considering positive and negative evidence impacting management’s forecasts. In addition we assessed the adequacy of the company’s going concern disclosures included in note 1 to the consolidated financial statements. |
/s/
A Member of EY Global
We have served as the Company’s auditor since at least 2001, but we are unable to determine the specific year.
March 28, 2024
F-3
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
(U.S. dollars in thousands except for share and per share data)
| December 31, | ||||||||||
| Note | 2023 | 2022 | ||||||||
| ASSETS | ||||||||||
| CURRENT ASSETS: | ||||||||||
| Cash and cash equivalents | $ | $ | ||||||||
| Short-term deposits | ||||||||||
| Prepaid expenses, and other current assets | 3 | |||||||||
| Short-term investment | 4 | |||||||||
| Total current assets | ||||||||||
| NON-CURRENT ASSETS: | ||||||||||
| Operating lease right of use assets | 11 | |||||||||
| Property, plant and equipment, net | 5 | |||||||||
| Total non-current assets | ||||||||||
| Total assets | $ | $ | ||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-4
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
(U.S. dollars in thousands except for share and per share data)
| December 31, | ||||||||||
| Note | 2023 | 2022 | ||||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||||
| CURRENT LIABILITIES: | ||||||||||
| Trade payables | $ | $ | ||||||||
| Current maturity of operating lease liability | 11 | |||||||||
| Deferred revenues | 8 | |||||||||
| Other accounts payable | 6 | |||||||||
| Total current liabilities | ||||||||||
| NON-CURRENT LIABILITIES: | ||||||||||
| Long-term operating lease liability | 12 | |||||||||
| Deferred revenues | 8 | |||||||||
| Total non-current liabilities | ||||||||||
| CONTINGENT LIABILITIES AND COMMITMENTS | 9 | |||||||||
| SHAREHOLDERS’ EQUITY: | 10 | |||||||||
| Ordinary shares of par value - Authorized: | ||||||||||
| Additional paid-in capital | ||||||||||
| Accumulated other comprehensive income | ||||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||||
| Total shareholders’ equity | ||||||||||
| Total liabilities and shareholders’ equity | $ | $ | ||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-5
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(U.S. dollars in thousands except for share and per share data)
| Year ended December 31, | ||||||||||||||
| Note | 2023 | 2022 | 2021 | |||||||||||
| Revenues | 8 | $ | $ | $ | ||||||||||
| Research and development expenses | ( | ) | ( | ) | ( | ) | ||||||||
| General and administrative expenses | ( | ) | ( | ) | ( | ) | ||||||||
| Operating loss | ( | ) | ( | ) | ( | ) | ||||||||
| Total financial income (expense), net | 13 | ( | ) | |||||||||||
| Loss before taxes on income | ( | ) | ( | ) | ( | ) | ||||||||
| Taxes on income | 14 | |||||||||||||
| Net loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||||
| Deemed dividend | ( | ) | ||||||||||||
| Total comprehensive loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||||
| $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-6
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
(U.S. dollars in thousands except for share and per share data)
| Ordinary shares | Additional paid-in | Accumulated other comprehensive | Accumulated | Total shareholders’ | ||||||||||||||||||||
| Number | Amount | capital | Income | deficit | equity | |||||||||||||||||||
| Balance as of January 1, 2021 | $ | $ | $ | | $ | ( | ) | $ | ||||||||||||||||
| Issuance of share capital due to warrants exercise, net of issuance expenses of $ | ||||||||||||||||||||||||
| Issuance of share capital and warrants, net of issuance expenses of $ | ||||||||||||||||||||||||
| Issuance of share capital in exchange for services (Note 10b(10)) | ||||||||||||||||||||||||
| dividends related to the warrants exercise (Note 10b(12)) | - | ( | ) | |||||||||||||||||||||
| Share-based payments | - | |||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||
| Balance as of December 31, 2021 | ( | ) | ||||||||||||||||||||||
| Share-based payments | - | |||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||
| Balance as of December 31, 2022 | $ | $ | ( | ) | $ | |||||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-7
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
(U.S. dollars in thousands except for share and per share data)
| Ordinary shares | Additional paid-in | Accumulated other comprehensive | Accumulated | Total shareholders’ | ||||||||||||||||||||
| Number | Amount | capital | Income | deficit | equity | |||||||||||||||||||
| Balance as of January 1, 2023 | $ | | $ | ( | ) | $ | ||||||||||||||||||
| Issuance of ordinary shares and warrants, net of issuance costs of $ | ||||||||||||||||||||||||
| Issuance of ordinary shares due to exercise of warrants net of issuance expenses of $ | ||||||||||||||||||||||||
| Share-based payments | - | |||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||
| Balance as of December 31, 2023 | $ | $ | ( | ) | $ | |||||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-8
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
(U.S. dollars in thousands except for share and per share data)
| Year ended December 31, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Adjustments required to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation of property, plant and equipment | ||||||||||||
| Reduction in the carrying amount of operating lease right of use asset | ||||||||||||
| Share-based payment | ||||||||||||
| Changes in fair value of short-term investment | ( | ) | ( | ) | ||||||||
| Financial expenses (income), net | ( | ) | ( | ) | ||||||||
| Change in prepaid expenses, and other current assets | ( | ) | ||||||||||
| Decrease in operating lease liability | ( | ) | ( | ) | ( | ) | ||||||
| Increase (decrease) in trade payable | ( | ) | ( | ) | ||||||||
| Increase (decrease) in deferred revenues | ( | ) | ( | ) | ||||||||
| Increase (decrease) in other accounts payable | ( | ) | ||||||||||
| Net cash used in operating activities | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Cash flows from investing activities: | ||||||||||||
| Purchase of property, plant and equipment | ( | ) | ( | ) | ( | ) | ||||||
| Investment in (maturity) of short-term deposits, net | ( | ) | ||||||||||
| Net cash used in investing activities | ( | ) | ||||||||||
| Cash flows from financing activities: | ||||||||||||
| Issuance of ordinary shares and warrants, net of issuance expenses | ||||||||||||
| Net cash provided by financing activities | ||||||||||||
| Exchange differences on balances of cash and cash equivalents | ( | ) | ||||||||||
| Increase (decrease) in cash and cash equivalents | ( | ) | ( | ) | ||||||||
| Cash and cash equivalents at the beginning of the year | ||||||||||||
| Cash and cash equivalents at the end of the year | ||||||||||||
| Non-cash activities: | ||||||||||||
| Lease liabilities arising from obtaining right-of-use-assets | $ | $ | $ | |||||||||
| Deemed Dividend | $ | $ | $ | |||||||||
| Supplemental disclosure of cash flow information: | ||||||||||||
| Cash paid during the year for interest | ||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-9
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 1:- GENERAL
| a. | Company description: |
Can-Fite Biopharma Ltd. (the “Company”) was incorporated and started to operate in September 1994 as a private Israeli company. Can-Fite is a clinical-stage biopharmaceutical company focused on developing orally bioavailable small molecule therapeutic products for the treatment of psoriasis, liver cancer, NASH and erectile dysfunction. Its platform technology utilizes the Gi protein associated A3AR as a therapeutic target. A3AR is highly expressed in patholological body cells such as inflammatory and cancer cells, and has a low expression in normal cells, suggesting that the receptor could be a specific target for pharmacological intervention. The Company’s pipeline of drug candidates are synthetic, highly specific agonists and allosteric modulators at the A3AR.
The Company’s ordinary shares
have been publicly traded on the Tel-Aviv Stock Exchange since October 2005 under the symbol “CFBI” and the Company’s
American Depositary Shares (“ADSs”) began public trading on the over the counter market in the U.S. in October 2012 and since
November 2013 the Company’s ADSs have been publicly traded on the NYSE American under the symbol “CANF”. Each ADS represents
| b. | Under Accounting Standard Codification (“ASC”) Subtopic 205-40, Presentation of Financial Statements—Going Concern (“ASC 205-40”), the Company has the responsibility to evaluate whether conditions and/or events raise substantial doubt about its ability to meet its obligations as they become due within one year after the date that the financial statements are issued. As required under ASC 205-40, management’s evaluation should initially not take into consideration the potential mitigating effects of management’s plans that have not been fully implemented as of the date the financial statements are issued. The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. |
Evaluation of Substantial Doubt Raised
In performing the first step of the evaluation, the Company concluded that the following conditions raised substantial doubt about its ability to continue as a going concern:
| - | History of net losses of $ |
| - | Net operating cash outflow of $ |
| - | Reliance on additional financing in order to execute its research and development plans. |
Consideration of Management’s Plans
In performing
the second step of this assessment, the Company is required to evaluate whether it is probable that the Company’s plans will be
effectively implemented within one year after the financial statements are issued and whether it is probable those plans will alleviate
the substantial doubt raised about the Company’s ability to continue as a going concern. As of December 31, 2023, the Company had
$
The Company has approved a plan, to improve its available cash balances, liquidity and cash flows generated from operations. The Company is prepared to implement the following actions as required by business and market conditions: reducing non-essential expenses to conserve cash and improve its liquidity position, deferral and reprioritization of certain research and development programs that would involve reduced program spend until additional financing will be obtained in order to strengthen liquidity and to preserve key research and development, commercial and functional roles.
F-10
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 1:- GENERAL (Cont.)
Management Assessment of Ability to Continue as a Going Concern
The Company has a history of operating losses and negative cash flows from operations. However, despite these conditions, the Company believes management’s plans, as described more fully above, will provide sufficient liquidity to meet its financial obligations.
Therefore, management concluded these plans alleviate the substantial doubt that was raised about the Company’s ability to continue as a going concern for at least twelve months from the date that the consolidated financial statements were issued.
Future Plans and Considerations
Although not considered for purposes of the Company’s assessment of whether substantial doubt was alleviated, the Company has plans to improve operating cash flows by entering into strategic partnerships with other companies that can provide access to additional customers and new markets. The Company may also seek to raise additional funds through the issuance of debt and/or equity securities or otherwise.
The Company’s plans are subject to inherent risks and uncertainties. Accordingly, there can be no assurance that the Company’s plans can be effectively implemented and, therefore, that the conditions can be effectively mitigated.
Until such time, if ever, that the Company can generate revenue sufficient to achieve profitability, the Company expects to finance its operations through equity or debt financings, which may not be available to the Company on the timing needed or on terms that the Company deems to be favorable. To the extent that the Company raises additional capital through the sale of equity or debt securities, the ownership interest of its stockholders will be diluted. If the Company is unable to maintain sufficient financial resources, its business, financial condition and results of operations will be materially and adversely affected.
F-11
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES
The consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States (“U.S. GAAP”).
| a. | Use of estimates: |
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates, judgments and assumptions. The Company’s management believes that the estimates, judgments and assumptions used are reasonable based upon information available at the time they are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements, and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
| b. | Principles of consolidation: |
The consolidated financial statements include the accounts of the Company and its subsidiary. Intercompany accounts and transactions have been eliminated.
| c. | Segment Information |
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. The Company and its chief operating decision-maker view the Company’s operations and manage its business in one operating segment which is the research and development of the Company’s drug candidates.
| c. | Functional currency, reporting currency and foreign currency: |
| 1. | Functional currency and reporting currency: |
The functional currency of the Company is the U.S. dollar. Accordingly, monetary accounts maintained in currencies other than the U.S. dollar are re-measured into U.S. dollars in accordance with Accounting Standard Codification (“ASC”) No. 830 “Foreign Currency Matters.” All transaction gains and losses of the re-measured monetary balance sheet items are reflected in the consolidated statements of comprehensive loss as financial income or expenses, as appropriate.
| 2. | Transactions, assets and liabilities in foreign currency: |
Transactions and balances denominated in U.S. dollars are presented at their original amounts. Monetary accounts denominated in currencies other than the dollar are re-measured into dollars in accordance with ASC No. 830, “Foreign Currency Matters”. All transaction gains and losses from the re-measurement of monetary balance sheet items are reflected in the consolidated statement of comprehensive loss as financial income or expenses, as appropriate.
| d. | Cash and cash equivalents: |
Cash equivalents are short-term, highly liquid investments that are readily convertible to cash with a maturity of three months or less at the date of acquisition, to be cash equivalents.
| e. | Short-term bank deposits:
Short-term bank deposits are deposits with maturities of more than three months and less than one year. As of December 31, 2023 and 2022, the Company’s bank deposits are denominated mainly in U.S. dollars and bears yearly interest at weighted average rates of |
F-12
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| f. | Prepaid expenses, and other current assets |
Prepaid expenses are composed mainly from prepayments to suppliers and from active pharmaceutical ingredients and clinical trial drug-capsules which are expensed when designate to a clinical trial and no longer have an alternative future use.
| g. | Operating lease: |
In accordance with ASU No. 2016-02, “Leases (Topic 842)”, the Company determines if an arrangement is a lease and the classification of that lease at inception based on: (1) whether the contract involves the use of a distinct identified asset, (2) whether the Company obtains the right to substantially all the economic benefits from the use of the asset throughout the period, and (3) whether the Company has a right to direct the use of the asset. The Company elected to not recognize a lease liability and a right-of-use (“ROU”) asset for leases with a term of twelve months or less. The Company also elected the practical expedient to not separate lease and non-lease components for its leases.
ROU assets represent the right to use an underlying asset for the lease term and lease liabilities represent the obligation to make minimum lease payments arising from the lease.
ROU assets are initially measured at amounts, which represents the discounted present value of the lease payments over the lease, plus any initial direct costs incurred. The lease liability is initially measured at lease commencement date based on the discounted present value of minimum lease payments over the lease term. The implicit rate within the operating leases is generally not determinable, therefore the Company uses its Incremental Borrowing Rate (“IBR”) based on the information available at commencement date in determining the present value of lease payments. The Company’s IBR is estimated to approximate the interest rate for collateralized borrowing with similar terms and payments and in economic environments where the leased asset is located. Certain leases include options to extend or terminate the lease. An option to extend the lease is considered in connection with determining the ROU asset and lease liability when it is reasonably certain that the Company will exercise that option. An option to terminate is considered unless it is reasonably certain that the Company will not exercise the option.
| h. | Property, plant and equipment: |
| % | Mainly % | |||||
| Laboratory equipment | ||||||
| Computers, office furniture and equipment | ||||||
| i. | Impairment of long-lived assets: |
Property and equipment are reviewed for impairment in accordance with ASC 360, “Property, Plant and Equipment,” whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated by the assets. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. As of December 31, 2023, and 2022, no impairment indicators have been identified.
F-13
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| j. | Revenue recognition: |
The Company recognizes revenue in accordance with ASC Topic 606, Revenue from contracts with customers (“ASC 606”) and determines revenue recognition through the following steps:
| 1. | Identification of the contract, or contracts, with a customer; |
| 2. | Identification of the performance obligations in the contract; |
| 3. | Determination of the transaction price; |
| 4. | Allocation of the transaction price to the performance obligations in the contract; and |
| 5. | Recognition of revenue when, or as, the performance obligations are satisfied. |
The Company generates revenues from supply and distribution agreements. The consideration under these agreements comprises of upfront fees, milestone payments and potential royalty payments.
Revenue from supply and distribution agreements with customers is recognized when the control over the goods or services is transferred to the customer. The transaction price is the amount of the consideration that is expected to be received based on the contract terms, excluding amounts collected on behalf of third parties (such as taxes).
Revenue from supply and distribution agreements with customers are recognized over time as the Company satisfies the performance obligations. The Company usually accepts long-term upfront payment from its customers. Contract liabilities for those upfront payments are recognized as revenue over time.
The Company’s contracts generally include three contract obligations: (i) performing the research and development services through regulatory approval; (ii) delivery of an exclusive licensing to distribute the product, once available; and, (iii) participation in joint steering committee.
The Company has concluded that the abovementioned contracts contain a single performance obligation satisfied over time. Consequently, revenue from these contracts is recorded based on the term of the research and development services.
The Company’s contracts also include development milestones payments and future sales-based royalties. Development milestones payments are recognized only to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with those milestones is subsequently resolved. Sales based royalties are recognized only when the subsequent sale occurs. As of December 31, 2023, the Company has not yet received the required regulatory approvals for its products and has not yet recognized any sales-based royalties.
The prepayments terms from the Company’s contracts with customers do not include a significant financing component as the primary purpose of these payments is not to receive financing from the customers.
F-14
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
Revenue Recognition – Contract Balances
Contract liabilities, due to the upfront
payments, include amounts received from customers for which revenue has not yet been recognized. As of December 31, 2023 and December
31, 2022 contract liabilities amounted to $
Deferred revenue that is anticipated to be recognized during the succeeding 12-months period is recorded as current deferred revenue and the remaining portion is recorded as non-current deferred revenue.
| Year ended December 31, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Cipher Pharmaceuticals (Canada) | % | % | % | |||||||||
| Chong Kun Dang Pharmaceuticals Corp. (South Korea) | % | % | % | |||||||||
| Gebro Holding GmbH (Austria) | % | % | % | |||||||||
| Ewopharma AG (Switzerland) | % | % | % | |||||||||
| Total | % | % | % | |||||||||
| k. | Research and development expenditures: |
Research and development costs are expensed as incurred. Research and development costs include payroll and personnel expenses, consulting costs, external contract research and development expenses, raw materials, drug product manufacturing costs, and allocated overhead including depreciation and amortization, rent, and utilities. Research and development are generally expensed as incurred.
| l. | Fair value measurement: |
The Company applies ASC 820, “Fair Value Measurements and Disclosures” (“ASC 820”). Under this standard, fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (i.e., the “exit price”) in an orderly transaction between market participants at the measurement date.
In determining fair value, the Company uses various valuation approaches. ASC 820 establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs that market participants would use in pricing the asset or liability developed based on market data obtained from sources independent from the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. The hierarchy is broken down into three levels based on the inputs as follows:
Level 1 - Valuations based on quoted prices in active markets for identical assets that the Company has the ability to access. Valuation adjustments and block discounts are not applied to Level 1 instruments. Since valuations are based on quoted prices that are readily and regularly available in an active market, valuation of these products does not entail a significant degree of judgment.
F-15
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
Level 2 - Valuations based on one or more quoted prices in markets that are not active or for which all significant inputs are observable, either directly or indirectly.
Level 3 - Valuations based on inputs that are unobservable and significant to the overall fair value measurement.
The carrying amounts of cash and cash equivalents, other accounts receivable and prepaid expenses, trade payables and other accounts payable and accrued expenses approximate their fair value due to the short-term maturity of such instruments. The Company has an investment in marketable equity security classified within level 1 in the fair value hierarchy.
| m. | Concentration of Credit Risk: |
Financial instruments that potentially subject the Company to credit risk primarily consist of cash and cash equivalents and short-term deposits. For cash and cash equivalents, the Company is exposed to credit risk in the event of default by the financial institutions to the extent of the amounts recorded on the accompanying consolidated balance sheets exceed federally insured limits. The Company places its cash and cash equivalents and short-term deposits with financial institutions with high-quality credit ratings and has not experienced any losses in such accounts.
| n. | Legal and other contingencies |
The Company accounts for its contingent liabilities in accordance with ASC 450, Contingencies (“ASC 450”). A provision is recorded when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated. With respect to legal matters, the Company reviews the status of each matter and assesses its potential financial exposure. If the potential loss from any claim or legal proceeding is considered probable and the amount can be reasonably estimated, the Company accrues a liability for the estimated loss. As of December 31, 2023, and 2022, the Company is not a party to any litigation that could have a material adverse effect on the Company’s business, financial position, results of operations or cash flows. Legal costs incurred in connection with loss contingencies are expensed as incurred.
| o. | Severance pay: |
The Company’s liability for severance
pay is pursuant to Section 14 of the Israeli Severance Compensation Act, 1963 (“Section 14”), pursuant to which all the Company’s
employees are included under Section 14, and are entitled only to monthly deposits, at a rate of
Severance pay expense for the year
ended December 31, 2023, 2022 and 2021 amounted to $
| p. | Share-based payment transactions: |
The Company accounts for share-based compensation in accordance with ASC 718, “Compensation - Stock Compensation” (“ASC 718”), which requires companies to estimate the fair value of equity-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods in the Company’s consolidated statement of comprehensive loss, based on acceleration method.
F-16
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
The Company recognizes compensation expenses for the value of its awards granted based on the vesting attribution approach over the requisite service period of each of the awards, net of estimated forfeitures. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
The Company estimates the fair value of share options granted using the Binomial option pricing model. The option-pricing model requires a number of assumptions, of which the most significant are the expected stock price, volatility, early exercise factor and the expected option term. Expected volatility was calculated based upon historical volatility of the Company and early exercise factor was calculated based statistical studies in the U.S market. The risk-free interest rate is based on the yield from government bonds with an equivalent term. The Company has historically not paid dividends and has no foreseeable plans to pay dividends.
| q. | Taxes on income: |
The Company accounts for income taxes in accordance with ASC No. 740, “Income Taxes”, (“ASC 740”) which prescribes the use of the liability method whereby deferred tax asset and liability account balances are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse.
The Company provides a valuation allowance, if necessary, to reduce deferred tax assets to their estimated realizable value. As of December 31, 2023, and 2022, a full valuation allowance was provided by the Company.
ASC 740 contains a two-step approach
to recognizing and measuring a liability for uncertain tax positions. The first step is to evaluate the tax position taken or expected
to be taken in a tax return by determining if the weight of available evidence indicates that it is more likely than not that, on an evaluation
of the technical merits, the tax position will be sustained on audit, including resolution of any related appeals or litigation processes.
The second step is to measure the tax benefit as the largest amount that is more than
| r. | Warrants to purchase ordinary shares: |
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance. The assessment considers whether the warrants are freestanding financial instruments, meet the definition of a liability under ASC 480, are indexed to the Company’s own stock and whether the warrants are eligible for equity classification under ASC 815-40. This assessment is conducted at the time of warrant issuance and as of each subsequent reporting period end date while the warrants are outstanding.
Warrants that meet all the criteria for equity classification, are required to be recorded as a component of additional paid-in capital. Warrants that do not meet all the criteria for equity classification, are required to be recorded as liabilities at their initial fair value on the date of issuance and remeasured to fair value through earnings at each balance sheet date thereafter.
| s. | Basic and diluted net loss per share: |
Basic and diluted net loss per share is calculated based on the weighted average number of ordinary shares outstanding during each year. The Company include in the basic net loss per share calculation, pre-funded warrants that are exercisable for a nominal consideration. Diluted net loss per share is calculated based on the weighted average number of ordinary shares outstanding during each year, plus dilutive potential in accordance with ASC 260, “Earnings per Share”.
F-17
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 2:- SIGNIFICANT ACCOUNTING POLICIES (Cont.)
| Year ended December 31, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Options | ||||||||||||
| Warrants | ||||||||||||
| Total | ||||||||||||
| t. | Recently adopted and recently issued accounting pronouncements: |
In May 2021, the FASB issued ASU No. 2021-04, Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options. The ASU addresses the previous lack of specific guidance in the accounting standards codification related to modifications or exchanges of freestanding equity-classified written call options (such as warrants) by specifying the accounting for various modification scenarios. The ASU is effective for interim and annual periods beginning after December 15, 2021, with early adoption permitted for any periods after issuance to be applied as of the beginning of the fiscal year that includes the interim period. The Company adopted this standard effective January 1, 2022. The adoption of this standard did not have a material impact on the Company’s consolidated financial statements.
In August 2020, the FASB issued Accounting Standards Update No. 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (ASU 2020-06), which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts on an entity’s own equity. This guidance also eliminates the treasury stock method to calculate diluted earnings per share for convertible instruments and requires the use of the if-converted method. The Company’s adopted the standard effective January 1, 2022. Adoption of the new standard did not have a material impact on the financial statements.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280), Improvements to Reportable Segment Disclosures, which expands annual and interim disclosure requirements for reportable segments, primarily through enhanced disclosures about significant segment expenses. In addition, it provides new segment disclosure requirements for entities with a single reportable segment. The guidance will be effective for the Company for annual periods beginning January 1, 2024 and for interim periods beginning January 1, 2025. Early adoption is permitted. The Company is currently evaluating the impact on its financial statement disclosures.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740), Improvements to Income Tax Disclosures, which requires disaggregated information about the effective tax rate reconciliation as well as information on income taxes paid. The guidance will be effective for the Company for annual periods beginning January 1, 2025, with early adoption permitted. The Company is currently evaluating the impact on its financial statement disclosures.
F-18
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 3:- PREPAID EXPENSES AND OTHER CURRENT ASSETS
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Government authorities | $ | $ | ||||||
| Prepaid expenses and others | ||||||||
| $ | $ | |||||||
NOTE 4:- SHORT-TERM INVESTMENT
As of December 31, 2023 and 2022,
the Company holds
NOTE 5:- PROPERTY, PLANT AND EQUIPMENT, NET
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Cost: | ||||||||
| Laboratory equipment | $ | $ | ||||||
| Computers, office furniture and equipment | ||||||||
| Leasehold improvements | ||||||||
| Accumulated depreciation: | ||||||||
| Laboratory equipment | ||||||||
| Computers, office furniture and equipment | ||||||||
| Leasehold improvements | ||||||||
| Property and equipment, net | $ | $ | ||||||
Depreciation
expenses for the year ended December 31, 2023, 2022 and 2021 amounted to $
NOTE 6:- OTHER ACCOUNTS PAYABLE
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Employees and payroll accruals | $ | $ | ||||||
| Accrued expenses | ||||||||
| $ | $ | |||||||
F-19
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 7:- FAIR VALUE MEASUREMENTS
In accordance with ASC 820 “Fair Value Measurements and Disclosures”, the Company measures its short-term investment at fair value. Short-term investments are classified within Level 1 as the valuation inputs are valuations based on quoted prices in active markets for identical assets.
| December 31, 2023 | ||||||||||||||||
| Fair value measurements | ||||||||||||||||
| Description | Fair value | Level 1 | Level 2 | Level 3 | ||||||||||||
| Short-term investment | $ | $ | $ | $ | ||||||||||||
| December 31, 2022 | ||||||||||||||||
| Fair value measurements | ||||||||||||||||
| Description | Fair value | Level 1 | Level 2 | Level 3 | ||||||||||||
| Short-term investment | $ | $ | $ | $ | ||||||||||||
NOTE 8:- REVENUES
Cipher Pharmaceuticals
In March 2015, the Company signed a distribution agreement with Cipher Pharmaceuticals (“Cipher”). As part of the distribution agreement, Cipher will distribute Can-Fite’s lead drug candidate, Piclidenoson for the treatment of psoriasis and rheumatoid arthritis in the Canadian market upon receipt of regulatory approvals.
Under the terms of the agreement, Cipher
made an upfront payment of $
Under the agreement, the Company shall be responsible for conducting product development activities including management of the clinical studies required in order to secure regulatory approvals and shall use commercially reasonable efforts in conducting such activities. In addition, the Company agreed to form a joint steering committee with Cipher which will oversee the progress of the clinical studies.
As of December 31, 2023, the Company estimates that such services will be completed by December 31, 2027 and therefore revenues from such up-front payment are recognized over such period.
F-20
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 8:- REVENUES (Cont.)
Chong Kun Dang Pharmaceuticals Corp.
In October 2016, the Company signed a distribution agreement with Chong Kun Dang Pharmaceuticals Corp. (“CKD”). As part of the distribution agreement, CKD will have exclusive rights to distribute Namodenoson for the treatment of liver cancer in the South Korean market upon receipt of regulatory approvals. On February 25, 2019, distribution agreement with CKD was amended to expand the exclusive right to distribute Namodenoson for the treatment of NASH in addition to liver cancer in South Korea.
Under the agreement, the Company shall be responsible for conducting product development activities including management of the clinical studies required in order to secure regulatory approvals and shall use commercially reasonable efforts in conducting such activities.
As of December 31, 2023, the Company
received an amount of $
As of December 31, 2023, the Company estimates that such services will be completed by June 30, 2027 and therefore revenues from such up-front payment are recognized over such period.
Gebro Holding GmBH
On January 8, 2018, the Company entered into a Distribution and Supply Agreement with Gebro Holding GmBH (“Gebro”), granting Gebro the exclusive right to distribute Piclidenoson in Spain, Switzerland, Liechtenstein and Austria for the treatment of psoriasis and rheumatoid arthritis.
As of December 31, 2023, the Company
received an amount of €
As of December 31, 2023, the Company estimates that such services will be completed by December 31, 2027 and therefore revenues from such up-front payment are recognized over such period.
F-21
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 8:- REVENUES (Cont.)
CMS Medical Venture Investment Limited
On August 6, 2018, the Company entered into a License, Collaboration and Distribution Agreement with CMS Medical Venture Investment Limited (“CMS Medical”) for the commercialization of Piclidenoson for the treatment of rheumatoid arthritis, psoriasis and Namodenoson for the treatment of advanced liver cancer and NAFLD/NASH in China (including Hong Kong, Macao and Taiwan).
As of December 31, 2023, the Company received an amount of $2,000 for up-front payment which was previously recognized in revenues.
Kyongbo Pharm Co., Ltd.
On July 31, 2019, the Company signed a distribution agreement with Kyongbo Pharm Co., Ltd. (“Kyongbo Pharm”), to distribute Piclidenoson, for the treatment of psoriasis in South Korea, upon receipt of regulatory approvals.
In 2019, the Company received an amount of $750 for up-front payment which recognized as revenues upon the Company providing access to information and clinical study data.
Ewopharma
As of December 31, 2023, the Company
received an amount of $
As of December 31, 2023, the Company estimates that such services will be completed by December 31, 2027 and therefore revenues from such up-front payment are recognized over such period.
On January 30, 2024, subsequent to the balance sheet date, the Company entered into an amendment to its distribution agreement with Ewopharma, and to add the rights to the Company’s pancreatic cancer indication for an additional up-font and milestones payments.
F-22
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 8:- REVENUES (Cont.)
Vetbiolix
As of December 31, 2023 and 2022, the Company did not receive payment related to such agreement.
NOTE 9:- CONTINGENT LIABILITIES AND COMMITMENTS
| a. | Liabilities to pay royalties: |
| According to the patent license agreement that the Company entered into with Leiden University in the Netherlands on November 2, 2009, which is affiliated with the National Institutes of Health (NIH), the Company was granted an exclusive license for the use of the patents of several compounds, including CF602 in certain territories. |
The Company is committed to pay royalties as follows:
| 1) | A one-time concession commission of € |
| 2) | Annual royalties of € |
| 3) |
| 4) |
| 5) | If the agreement is sublicensed to another company, the Company will provide Leiden University royalties at a rate of |
As of December 31, 2023 and 2022, no accrual has been recorded with respect to Leiden University.
F-23
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 10:- SHAREHOLDERS’ EQUITY
| a. | On February 22, 2023, the Company’s shareholders approved a change in the Company’s ordinary shares par value from NIS |
All ordinary shares have equal rights for all intent and purposes and each ordinary share confers its holder:
| 1. | The right to be invited and participate in all the Company’s general meetings, both annual and regular, and the right to one vote per ordinary share owned in all votes and in all Company’s general meeting participated. |
| 2. | The right to receive dividends if and when declared and the right to receive bonus shares if and when distributed. |
| 3. | The right to participate in the distribution of the Company’s assets upon liquidation. |
| b. | Issuance of ordinary shares and warrants: |
| 1. | In February and March 2021, the Company issued |
| 2. | In March 2021, the Company issued |
| 3. | On August 16, 2021, |
The Company has also sold to the investor,
in a concurrent private placement, unregistered warrants to purchase up to an aggregate of
During the year ended December 31,
2021, the Company received a total of $
The Company also paid on aggregate
of $
F-24
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 10:- SHAREHOLDERS’ EQUITY (Cont.)
| 4. |
Under the Exercise Agreement, the Company
also agreed to issue to the holder new unregistered warrants to purchase up to
Pursuant to the terms of the Exercise Agreements, the holder agreed to exercise the warrants while receiving a-120% warrants coverage, thereby creating a benefit to this warrant holder. As such, the Company recorded a deemed dividend in the amount of $2,590.
| 5. | On January 11, 2023, |
In addition, the Company entered into
a securities purchase agreement (the “PIPE Purchase Agreement,” and together with the RD Purchase Agreement, the “Purchase
Agreements”) pursuant to which the Company agreed to sell and issue in a concurrent private placement (the “PIPE Offering,”
and together with the Registered Direct Offering, the “Offerings”) unregistered Pre-funded Warrants to purchase up to
Moreover, the Company has also issued
a placement agent warrants (the “Placement Agent Warrants”) on substantially the same terms as the Series A Warrants to purchase
up to
As a results of the aforementioned
issuance, the Company incurred an aggregate amount of $
The Company received total consideration
of $
The Company accounted for the aforementioned warrants as freestanding instrument classified as part of the Company’s permanent equity in accordance with ASC-480 and ASC-815-40.
F-25
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 10:- SHAREHOLDERS’ EQUITY (Cont.)
The Company has also agreed to amend
certain warrants to purchase up to an aggregate of
The Company accounted for the reduction in the warrants exercise price as issuance costs to be recorded in the Company’s additional paid in capital in accordance with ASC-815-40, following the adoption of ASU 2021-04.
| 6. | During the year ended December 31, 2023, certain investors exercised their pre-funded warrants and purchased
|
| 7. | On November 22, 2023, the Company entered into an inducement offer to purchase ordinary shares with a certain existing investor, according to which, the Company reduced the exercise price of previously issued warrants to purchase ordinary shares from $ |
In consideration for the immediate
exercise of the warrants for cash, the Company issued new unregistered warrants to purchase
Moreover, the Company
has also issued a placement agent warrants (the “Placement Agent Warrants”) to purchase up to
As part of the aforementioned agreement,
the Company incurred an aggregate issuance costs of $
The Company accounted for the aforementioned warrants as freestanding instrument classified at part of the Company’s permanent equity in accordance with ASC-480 and ASC-815-40.
The Company accounted for the reduction in the warrants exercise price as issuance costs to be recorded in the Company’s additional paid in capital in accordance with ASC 815-40, following the adoption of ASU 2021-04.
F-26
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 10:- SHAREHOLDERS’ EQUITY (Cont.)
| c. | Warrants to purchase ordinary share: |
| Issuance date | Number of outstanding Warrants | Exercise price per warrant | ||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
As of December 31, 2023 and 2022, all of the Company’s outstanding warrants are classified as part of the Company’s equity.
| a. | Share options plan: |
On November 28, 2013, the board of
directors approved the adoption of the 2013 Share Option Plan (the “2013 Plan”). Under the Company’s 2013 Plan, in May 2023,
the Company’s Board of Directors approved to increase number of ordinary shares reserved for issuance to
On August 30, 2023, the Company’s board
of directors approved the adoption on a new 2023 Share Option Plan (the “2023 Plan”) and approved the reserve of
Under the Company’s Plans, the Company
may grant its officers, directors, employees and consultants, share options. Each share option granted shall be exercisable at such times
and terms and conditions as the Board of Directors may specify in the applicable option agreement, provided that no option will be granted
with a term in excess of
As of December 31, 2023,
| b. |
| Year ended December 31, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Research and development | $ | $ | $ | |||||||||
| General and administrative | ||||||||||||
F-27
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 10:- SHAREHOLDERS’ EQUITY (Cont.)
| c. | Share-based payment transactions granted by the Company: |
| Description | 2023 | 2022 | 2021 | |||||||||
| Risk-free interest rate | % | % | % | |||||||||
| Expected volatility | % | % | | % | ||||||||
| Dividend yield | % | % | % | |||||||||
| Contractual life (in years) | ||||||||||||
| Early Exercise Multiple (Suboptimal Factor) | ||||||||||||
| Number of options | Weighted average exercise price | Weighted average remaining contractual terms (in years) | Aggregate intrinsic value | |||||||||||||
| Outstanding at December 31, 2022 | ||||||||||||||||
| Granted | ||||||||||||||||
| Forfeited/expired | ( | ) | - | |||||||||||||
| Outstanding at December 31, 2023 | ||||||||||||||||
| Vested and expected to vest at December 31, 2023 | ||||||||||||||||
| Exercisable at December 31, 2023 | ||||||||||||||||
| d. | The weighted average remaining contractual life for the shares subject to options outstanding as of December 31, 2023, 2022 and 2021 was |
| e. | The weighted average grant date fair value in 2023 was $ |
F-28
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 11- LEASES:
The Company has lease contracts for
motor vehicles used in its operations. Leases of motor vehicles have lease terms of
| December 31, 2023 | December 31, 2022 | |||||||
| weighted average remaining lease term (years) | ||||||||
| weighted average discount rate | % | % | ||||||
| Year ended December 31, 2023 | Year ended December 31, 2022 | |||||||
| Components of lease expense: | ||||||||
| Operating lease cost | $ | | $ | | ||||
| Year ended December 31, 2023 | Year ended December 31, 2022 | |||||||
| Supplemental cash flow information | ||||||||
| Cash paid for amounts included in the measurement of lease liabilities | $ | | $ | | ||||
| Supplemental non-cash information related to lease liabilities arising from obtaining ROU assets | $ | $ | - | |||||
| 2023 | $ | |||
| 2024 | $ | |||
| 2025 | $ | |||
| Total operating lease payments | $ | |||
| Less: imputed interest | $ | ( | ) | |
| Present value of lease liability | $ |
F-29
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 12:- FINANCE INCOME (EXPENSE), NET
| Year ended December 31, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Interest income | $ | $ | $ | |||||||||
| Bank commisions | ( | ) | ( | ) | ( | ) | ||||||
| Exchange rate fluctuations and others | ( | ) | ||||||||||
| short-term investment revaluation | ( | ) | ||||||||||
| Total financial income (expenses), net | $ | $ | ( | ) | $ | |||||||
NOTE 13:- TAXES ON INCOME
| a. | Corporate tax rates: |
Israeli taxation:
Corporate tax rate in Israel in 2023,
2022 and 2021 was
Law for the Encouragement of Capital Investments, 1959:
The Law for Encouragement of Capital Investments, 1959 (the “Investment Law”) provides tax benefits for Israeli companies meeting certain requirements and criteria. The Investment Law has undergone certain amendments and reforms in recent years.
The Israeli parliament enacted a reform to the Investment Law, effective January 2011 (which was amended in August 2013). According to the reform, a flat rate tax applies to Preferred Income of companies eligible for the “Preferred Enterprise” status. In order to be eligible for Preferred Enterprise status, a company must meet minimum requirements to establish that it contributes to the country’s economic growth and is a competitive factor for the gross domestic product.
Benefits granted to a Preferred Enterprise
include reduced tax rates. As part of the Economic Efficiency Law (Legislative Amendments for Accomplishment of Budgetary Targets for
Budget Years 2017-2018), 5777-2016, the tax rate is
| b. | Final tax assessments: |
The Company’s tax return up until the year ended December 31, 2018 are considered final.
| c. | Net operating carryforward losses for tax purposes and other temporary differences: |
As of December 31, 2023, the Company
had carryforward losses amounting to approximately $
F-30
CAN-FITE BIOPHARMA LTD. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands except for share and per share data)
NOTE 13:- TAXES ON INCOME (Cont.)
| d. | Deferred income taxes: |
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carry forward | $ | $ | ||||||
| Temporary differences mainly relating to Research and Development | ||||||||
| Deferred tax asset before valuation allowance | ||||||||
| Valuation allowance | ( | ) | ( | ) | ||||
| Deferred tax asset, net | $ | $ | ||||||
| e. | Reconciliation of the theoretical tax expense to the actual tax expense: |
The main reconciling item between the statutory tax rate of the Company and the effective tax rate is the recognition of valuation allowance in respect of deferred taxes relating to accumulated net operating losses carried forward due to the uncertainty of the realization of such deferred taxes and the company’s preferred enterprise tax rate.
NOTE 14:- TRANSACTIONS WITH RELATED PARTIES
One of the Company’s directors
is a senior partner in the patent firm which represents the Company in intellectual property and commercial matters (the “Service
Provider”). The Service Provider charges the Company for services it renders on an hourly basis. The aggregate amount of these expenses
was approximately $
F-31