FALSEFY0001131399P7YP3Y0.31252.500011313992023-01-012023-12-310001131399dei:BusinessContactMember2023-01-012023-12-310001131399gsk:AmericanDepositarySharesMember2023-01-012023-12-310001131399gsk:ThreePointZeroNotesDue2024Member2023-01-012023-12-310001131399gsk:ThreePointSixTwoFiveNotesDue2025Member2023-01-012023-12-310001131399gsk:ThreePointEightSevenFiveNotesDue2028Member2023-01-012023-12-310001131399gsk:ThreePointThreeSevenFiveNotesDue2029Member2023-01-012023-12-310001131399gsk:SixPointThreeSevenFiveNotesDue2038Member2023-01-012023-12-310001131399gsk:FourPointTwoZeroZeroNotesDue2043Member2023-01-012023-12-3100011313992023-12-31xbrli:sharesiso4217:GBP00011313992022-01-012022-12-3100011313992021-01-012021-12-31iso4217:GBPxbrli:shares00011313992022-12-310001131399ifrs-full:IssuedCapitalMember2020-12-310001131399ifrs-full:SharePremiumMember2020-12-310001131399ifrs-full:RetainedEarningsMember2020-12-310001131399ifrs-full:OtherReservesMember2020-12-310001131399ifrs-full:EquityAttributableToOwnersOfParentMember2020-12-310001131399ifrs-full:NoncontrollingInterestsMember2020-12-3100011313992020-12-310001131399ifrs-full:RetainedEarningsMember2021-01-012021-12-310001131399ifrs-full:EquityAttributableToOwnersOfParentMember2021-01-012021-12-310001131399ifrs-full:NoncontrollingInterestsMember2021-01-012021-12-310001131399ifrs-full:OtherReservesMember2021-01-012021-12-310001131399ifrs-full:IssuedCapitalMember2021-01-012021-12-310001131399ifrs-full:SharePremiumMember2021-01-012021-12-310001131399ifrs-full:IssuedCapitalMember2021-12-310001131399ifrs-full:SharePremiumMember2021-12-310001131399ifrs-full:RetainedEarningsMember2021-12-310001131399ifrs-full:OtherReservesMember2021-12-310001131399ifrs-full:EquityAttributableToOwnersOfParentMember2021-12-310001131399ifrs-full:NoncontrollingInterestsMember2021-12-3100011313992021-12-310001131399ifrs-full:RetainedEarningsMember2022-01-012022-12-310001131399ifrs-full:EquityAttributableToOwnersOfParentMember2022-01-012022-12-310001131399ifrs-full:NoncontrollingInterestsMember2022-01-012022-12-310001131399ifrs-full:OtherReservesMember2022-01-012022-12-310001131399ifrs-full:SharePremiumMember2022-01-012022-12-310001131399ifrs-full:IssuedCapitalMember2022-12-310001131399ifrs-full:SharePremiumMember2022-12-310001131399ifrs-full:RetainedEarningsMember2022-12-310001131399ifrs-full:OtherReservesMember2022-12-310001131399ifrs-full:EquityAttributableToOwnersOfParentMember2022-12-310001131399ifrs-full:NoncontrollingInterestsMember2022-12-310001131399ifrs-full:RetainedEarningsMember2023-01-012023-12-310001131399ifrs-full:OtherReservesMember2023-01-012023-12-310001131399ifrs-full:EquityAttributableToOwnersOfParentMember2023-01-012023-12-310001131399ifrs-full:NoncontrollingInterestsMember2023-01-012023-12-310001131399ifrs-full:IssuedCapitalMember2023-01-012023-12-310001131399ifrs-full:SharePremiumMember2023-01-012023-12-310001131399ifrs-full:IssuedCapitalMember2023-12-310001131399ifrs-full:SharePremiumMember2023-12-310001131399ifrs-full:RetainedEarningsMember2023-12-310001131399ifrs-full:OtherReservesMember2023-12-310001131399ifrs-full:EquityAttributableToOwnersOfParentMember2023-12-310001131399ifrs-full:NoncontrollingInterestsMember2023-12-31gsk:therapeuticArea0001131399ifrs-full:BottomOfRangeMembergsk:FreeholdBuildingsMember2023-01-012023-12-310001131399gsk:FreeholdBuildingsMemberifrs-full:TopOfRangeMember2023-01-012023-12-310001131399ifrs-full:BottomOfRangeMembergsk:LeaseholdLandAndBuildingsMember2023-01-012023-12-310001131399ifrs-full:TopOfRangeMembergsk:LeaseholdLandAndBuildingsMember2023-01-012023-12-310001131399ifrs-full:BottomOfRangeMembergsk:PlantAndMachineryMember2023-01-012023-12-310001131399gsk:PlantAndMachineryMemberifrs-full:TopOfRangeMember2023-01-012023-12-310001131399ifrs-full:BottomOfRangeMembergsk:EquipmentAndVehiclesMember2023-01-012023-12-310001131399gsk:EquipmentAndVehiclesMemberifrs-full:TopOfRangeMember2023-01-012023-12-310001131399ifrs-full:TopOfRangeMember2023-12-310001131399ifrs-full:BottomOfRangeMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMember2023-01-012023-12-310001131399ifrs-full:BottomOfRangeMembergsk:PharmaceuticalsMember2023-01-012023-12-31xbrli:pure0001131399ifrs-full:TopOfRangeMembergsk:PharmaceuticalsMember2023-01-012023-12-310001131399gsk:RemainingBookValueMemberifrs-full:BottomOfRangeMembergsk:PharmaceuticalsMember2023-01-012023-12-310001131399gsk:RemainingBookValueMemberifrs-full:TopOfRangeMembergsk:PharmaceuticalsMember2023-01-012023-12-310001131399ifrs-full:BottomOfRangeMembergsk:VaccinesMember2023-01-012023-12-310001131399ifrs-full:TopOfRangeMembergsk:VaccinesMember2023-01-012023-12-310001131399gsk:ERPSoftwareSystemsMemberifrs-full:BottomOfRangeMember2023-01-012023-12-310001131399gsk:ERPSoftwareSystemsMemberifrs-full:TopOfRangeMember2023-01-012023-12-310001131399ifrs-full:BottomOfRangeMembergsk:OtherComputerSoftwareMember2023-01-012023-12-310001131399gsk:OtherComputerSoftwareMemberifrs-full:TopOfRangeMember2023-01-012023-12-310001131399gsk:EstimatedRebatesDiscountsOrAllowancePayableToCustomerMembergsk:USPharmaceuticalsAndVaccinesMember2023-01-012023-12-310001131399gsk:EstimatedRebatesDiscountsOrAllowancePayableToCustomerMembergsk:USPharmaceuticalsAndVaccinesMember2022-01-012022-12-310001131399gsk:EstimatedRebatesDiscountsOrAllowancePayableToCustomerMembergsk:USPharmaceuticalsAndVaccinesMember2023-12-310001131399gsk:EstimatedRebatesDiscountsOrAllowancePayableToCustomerMembergsk:USPharmaceuticalsAndVaccinesMember2022-12-310001131399gsk:ActuarialAssumptionPercentageRateChangeMember2023-01-012023-12-310001131399gsk:ActuarialAssumptionPercentageRateChangeMember2022-01-012022-12-310001131399ifrs-full:BusinessCombinationsMember2023-12-310001131399ifrs-full:BusinessCombinationsMember2022-12-310001131399gsk:ShionogiViiVHealthcareJointVentureMember2023-12-310001131399gsk:ShionogiViiVHealthcareJointVentureMember2022-12-31gsk:pensionSchemegsk:country0001131399gsk:ActuarialAssumptionPercentageRateChangeMembergsk:SensitivityAnalysisForActuarialAssumptionsMember2023-01-012023-12-310001131399gsk:SensitivityAnalysisForActuarialAssumptionsMembergsk:ActuarialAssumptionOfDiscountRatesAtZeroPointSevenFivePercentMember2023-01-012023-12-310001131399currency:USD2023-01-012023-12-310001131399currency:USD2022-01-012022-12-310001131399currency:USD2021-01-012021-12-310001131399currency:EUR2023-01-012023-12-310001131399currency:EUR2022-01-012022-12-310001131399currency:EUR2021-01-012021-12-310001131399currency:JPY2023-01-012023-12-310001131399currency:JPY2022-01-012022-12-310001131399currency:JPY2021-01-012021-12-310001131399currency:USD2023-12-310001131399currency:USD2022-12-310001131399currency:USD2021-12-310001131399currency:EUR2023-12-310001131399currency:EUR2022-12-310001131399currency:EUR2021-12-310001131399currency:JPY2023-12-310001131399currency:JPY2022-12-310001131399currency:JPY2021-12-31gsk:Segments0001131399gsk:CommercialOperationsMember2023-01-012023-12-310001131399gsk:CommercialOperationsMember2022-01-012022-12-310001131399gsk:CommercialOperationsMember2021-01-012021-12-31gsk:productGroup0001131399gsk:ShinglesMembergsk:CommercialOperationsMember2023-01-012023-12-310001131399gsk:ShinglesMembergsk:CommercialOperationsMember2022-01-012022-12-310001131399gsk:ShinglesMembergsk:CommercialOperationsMember2021-01-012021-12-310001131399gsk:MeningitisMembergsk:CommercialOperationsMember2023-01-012023-12-310001131399gsk:MeningitisMembergsk:CommercialOperationsMember2022-01-012022-12-310001131399gsk:MeningitisMembergsk:CommercialOperationsMember2021-01-012021-12-310001131399gsk:CommercialOperationsMembergsk:RSVArexvyMember2023-01-012023-12-310001131399gsk:CommercialOperationsMembergsk:RSVArexvyMember2022-01-012022-12-310001131399gsk:CommercialOperationsMembergsk:RSVArexvyMember2021-01-012021-12-310001131399gsk:InfluenzaMembergsk:CommercialOperationsMember2023-01-012023-12-310001131399gsk:InfluenzaMembergsk:CommercialOperationsMember2022-01-012022-12-310001131399gsk:InfluenzaMembergsk:CommercialOperationsMember2021-01-012021-12-310001131399gsk:CommercialOperationsMembergsk:EstablishedVaccinesMember2023-01-012023-12-310001131399gsk:CommercialOperationsMembergsk:EstablishedVaccinesMember2022-01-012022-12-310001131399gsk:CommercialOperationsMembergsk:EstablishedVaccinesMember2021-01-012021-12-310001131399gsk:MeningitisInfluenzaShinglesEstablishedVaccinesRSVArexvyMembergsk:CommercialOperationsMember2023-01-012023-12-310001131399gsk:MeningitisInfluenzaShinglesEstablishedVaccinesRSVArexvyMembergsk:CommercialOperationsMember2022-01-012022-12-310001131399gsk:MeningitisInfluenzaShinglesEstablishedVaccinesRSVArexvyMembergsk:CommercialOperationsMember2021-01-012021-12-310001131399gsk:CommercialOperationsMembergsk:PandemicVaccinesMember2023-01-012023-12-310001131399gsk:CommercialOperationsMembergsk:PandemicVaccinesMember2022-01-012022-12-310001131399gsk:CommercialOperationsMembergsk:PandemicVaccinesMember2021-01-012021-12-310001131399gsk:CommercialOperationsMembergsk:VaccinesMember2023-01-012023-12-310001131399gsk:CommercialOperationsMembergsk:VaccinesMember2022-01-012022-12-310001131399gsk:CommercialOperationsMembergsk:VaccinesMember2021-01-012021-12-310001131399gsk:CommercialOperationsMembergsk:HIVMember2023-01-012023-12-310001131399gsk:CommercialOperationsMembergsk:HIVMember2022-01-012022-12-310001131399gsk:CommercialOperationsMembergsk:HIVMember2021-01-012021-12-310001131399gsk:ImmunoInflammationRespiratoryAndOtherMembergsk:CommercialOperationsMember2023-01-012023-12-310001131399gsk:ImmunoInflammationRespiratoryAndOtherMembergsk:CommercialOperationsMember2022-01-012022-12-310001131399gsk:ImmunoInflammationRespiratoryAndOtherMembergsk:CommercialOperationsMember2021-01-012021-12-310001131399gsk:CommercialOperationsMembergsk:OncologyMember2023-01-012023-12-310001131399gsk:CommercialOperationsMembergsk:OncologyMember2022-01-012022-12-310001131399gsk:CommercialOperationsMembergsk:OncologyMember2021-01-012021-12-310001131399gsk:HIVOncologyImmunoInflammationRespiratoryAndOtherMember2023-01-012023-12-310001131399gsk:HIVOncologyImmunoInflammationRespiratoryAndOtherMember2022-01-012022-12-310001131399gsk:HIVOncologyImmunoInflammationRespiratoryAndOtherMember2021-01-012021-12-310001131399gsk:CommercialOperationsMembergsk:PandemicMember2023-01-012023-12-310001131399gsk:CommercialOperationsMembergsk:PandemicMember2022-01-012022-12-310001131399gsk:CommercialOperationsMembergsk:PandemicMember2021-01-012021-12-310001131399gsk:SpecialtyMedicineMembergsk:CommercialOperationsMember2023-01-012023-12-310001131399gsk:SpecialtyMedicineMembergsk:CommercialOperationsMember2022-01-012022-12-310001131399gsk:SpecialtyMedicineMembergsk:CommercialOperationsMember2021-01-012021-12-310001131399gsk:CommercialOperationsMembergsk:RespiratoryMember2023-01-012023-12-310001131399gsk:CommercialOperationsMembergsk:RespiratoryMember2022-01-012022-12-310001131399gsk:CommercialOperationsMembergsk:RespiratoryMember2021-01-012021-12-310001131399gsk:CommercialOperationsMembergsk:OtherGeneralMedicinesMember2023-01-012023-12-310001131399gsk:CommercialOperationsMembergsk:OtherGeneralMedicinesMember2022-01-012022-12-310001131399gsk:CommercialOperationsMembergsk:OtherGeneralMedicinesMember2021-01-012021-12-310001131399gsk:CommercialOperationsMembergsk:GeneralMedicinesMember2023-01-012023-12-310001131399gsk:CommercialOperationsMembergsk:GeneralMedicinesMember2022-01-012022-12-310001131399gsk:CommercialOperationsMembergsk:GeneralMedicinesMember2021-01-012021-12-31gsk:wholesalers0001131399country:USgsk:Wholesalers1Member2023-01-012023-12-310001131399country:USgsk:Wholesalers1Member2022-01-012022-12-310001131399country:USgsk:Wholesalers1Member2021-01-012021-12-310001131399country:USgsk:Wholesalers2Member2023-01-012023-12-310001131399country:USgsk:Wholesalers2Member2022-01-012022-12-310001131399country:USgsk:Wholesalers2Member2021-01-012021-12-310001131399country:USgsk:Wholesalers3Member2023-01-012023-12-310001131399country:USgsk:Wholesalers3Member2022-01-012022-12-310001131399country:USgsk:Wholesalers3Member2021-01-012021-12-310001131399ifrs-full:OperatingSegmentsMembergsk:CommercialOperationsMember2023-01-012023-12-310001131399ifrs-full:OperatingSegmentsMembergsk:CommercialOperationsMember2022-01-012022-12-310001131399ifrs-full:OperatingSegmentsMembergsk:CommercialOperationsMember2021-01-012021-12-310001131399ifrs-full:OperatingSegmentsMembergsk:ResearchAndDevelopmentMember2023-01-012023-12-310001131399ifrs-full:OperatingSegmentsMembergsk:ResearchAndDevelopmentMember2022-01-012022-12-310001131399ifrs-full:OperatingSegmentsMembergsk:ResearchAndDevelopmentMember2021-01-012021-12-310001131399ifrs-full:OperatingSegmentsMember2023-01-012023-12-310001131399ifrs-full:OperatingSegmentsMember2022-01-012022-12-310001131399ifrs-full:OperatingSegmentsMember2021-01-012021-12-310001131399ifrs-full:UnallocatedAmountsMember2023-01-012023-12-310001131399ifrs-full:UnallocatedAmountsMember2022-01-012022-12-310001131399ifrs-full:UnallocatedAmountsMember2021-01-012021-12-310001131399ifrs-full:MaterialReconcilingItemsMember2023-01-012023-12-310001131399ifrs-full:MaterialReconcilingItemsMember2022-01-012022-12-310001131399ifrs-full:MaterialReconcilingItemsMember2021-01-012021-12-310001131399ifrs-full:OperatingSegmentsMembergsk:CommercialOperationsMember2023-12-310001131399ifrs-full:OperatingSegmentsMembergsk:CommercialOperationsMember2022-12-310001131399ifrs-full:OperatingSegmentsMembergsk:ResearchAndDevelopmentMember2023-12-310001131399ifrs-full:OperatingSegmentsMembergsk:ResearchAndDevelopmentMember2022-12-310001131399ifrs-full:OperatingSegmentsMember2023-12-310001131399ifrs-full:OperatingSegmentsMember2022-12-310001131399ifrs-full:UnallocatedAmountsMember2023-12-310001131399ifrs-full:UnallocatedAmountsMember2022-12-310001131399gsk:ShionogiViivHealthcareMember2023-12-310001131399gsk:ShionogiViivHealthcareMember2022-12-310001131399gsk:PfizerMember2023-12-310001131399gsk:PfizerMember2022-12-310001131399gsk:CustomerMembercountry:GB2023-01-012023-12-310001131399gsk:CustomerMembercountry:GB2022-01-012022-12-310001131399gsk:CustomerMembercountry:GB2021-01-012021-12-310001131399gsk:CustomerMembercountry:US2023-01-012023-12-310001131399gsk:CustomerMembercountry:US2022-01-012022-12-310001131399gsk:CustomerMembercountry:US2021-01-012021-12-310001131399gsk:CustomerMembergsk:RestOfWorldMember2023-01-012023-12-310001131399gsk:CustomerMembergsk:RestOfWorldMember2022-01-012022-12-310001131399gsk:CustomerMembergsk:RestOfWorldMember2021-01-012021-12-310001131399gsk:CustomerMember2023-01-012023-12-310001131399gsk:CustomerMember2022-01-012022-12-310001131399gsk:CustomerMember2021-01-012021-12-310001131399country:GB2023-12-310001131399country:GB2022-12-310001131399country:US2023-12-310001131399country:US2022-12-310001131399country:BE2023-12-310001131399country:BE2022-12-310001131399gsk:RestOfWorldMember2023-12-310001131399gsk:RestOfWorldMember2022-12-310001131399gsk:GileadSciencesIncMember2022-02-15iso4217:USD0001131399gsk:HaleonPlcMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMember2023-01-012023-12-310001131399gsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:AffinivaxMember2023-01-012023-12-310001131399gsk:LiquidationOrDisposalOfOverseasSubsidiaryMember2023-01-012023-12-310001131399gsk:LiquidationOrDisposalOfOverseasSubsidiaryMember2022-01-012022-12-310001131399gsk:LiquidationOrDisposalOfOverseasSubsidiaryMember2021-01-012021-12-310001131399gsk:LiquidationOrDisposalOfInterestInAssociatesMember2023-01-012023-12-310001131399gsk:LiquidationOrDisposalOfInterestInAssociatesMember2022-01-012022-12-310001131399ifrs-full:ParentMember2023-01-012023-12-310001131399ifrs-full:ParentMember2022-01-012022-12-310001131399ifrs-full:ParentMember2021-01-012021-12-310001131399ifrs-full:SubsidiariesMember2023-01-012023-12-310001131399ifrs-full:SubsidiariesMember2022-01-012022-12-310001131399ifrs-full:SubsidiariesMember2021-01-012021-12-310001131399gsk:ReportingAccountantWorkPerformedInConnectionWithConsumerHealthcareDemergerMember2023-01-012023-12-310001131399gsk:ReportingAccountantWorkPerformedInConnectionWithConsumerHealthcareDemergerMember2022-01-012022-12-310001131399gsk:ReportingAccountantWorkPerformedInConnectionWithConsumerHealthcareDemergerMember2021-01-012021-12-310001131399ifrs-full:PensionDefinedBenefitPlansMember2023-01-012023-12-310001131399ifrs-full:PensionDefinedBenefitPlansMember2022-01-012022-12-310001131399ifrs-full:PensionDefinedBenefitPlansMember2021-01-012021-12-310001131399gsk:ShareValuePlanMember2023-01-012023-12-310001131399gsk:ShareValuePlanMember2022-01-012022-12-310001131399gsk:ShareValuePlanMember2021-01-012021-12-310001131399gsk:PerformanceSharePlansMember2023-01-012023-12-310001131399gsk:PerformanceSharePlansMember2022-01-012022-12-310001131399gsk:PerformanceSharePlansMember2021-01-012021-12-310001131399gsk:ShareOptionPlanMember2023-01-012023-12-310001131399gsk:ShareOptionPlanMember2022-01-012022-12-310001131399gsk:ShareOptionPlanMember2021-01-012021-12-310001131399gsk:CashSettledAndOtherPlansMember2023-01-012023-12-310001131399gsk:CashSettledAndOtherPlansMember2022-01-012022-12-310001131399gsk:CashSettledAndOtherPlansMember2021-01-012021-12-310001131399gsk:ManufacturingMember2023-01-012023-12-31gsk:numberOfEmployees0001131399gsk:ManufacturingMember2022-01-012022-12-310001131399gsk:ManufacturingMember2021-01-012021-12-310001131399gsk:SellingGeneralAndAdministrationMember2023-01-012023-12-310001131399gsk:SellingGeneralAndAdministrationMember2022-01-012022-12-310001131399gsk:SellingGeneralAndAdministrationMember2021-01-012021-12-310001131399gsk:ResearchAndDevelopmentMember2023-01-012023-12-310001131399gsk:ResearchAndDevelopmentMember2022-01-012022-12-310001131399gsk:ResearchAndDevelopmentMember2021-01-012021-12-310001131399srt:MinimumMember2023-01-012023-12-31gsk:company0001131399gsk:SeparationPreparationProgrammeMember2023-01-012023-12-310001131399gsk:SeparationPreparationProgrammeMember2022-01-012022-12-310001131399gsk:SeparationPreparationProgrammeMember2021-01-012021-12-310001131399gsk:SignificantAcquisitionsMember2023-01-012023-12-310001131399gsk:LegacyProgrammesMember2023-01-012023-12-310001131399gsk:SignificantAcquisitionsMember2022-01-012022-12-310001131399gsk:LegacyProgrammesMember2022-01-012022-12-310001131399gsk:CostOfMajorRestructuringMember2023-01-012023-12-310001131399gsk:CostOfMajorRestructuringMember2022-01-012022-12-310001131399gsk:CostOfMajorRestructuringMember2021-01-012021-12-310001131399ifrs-full:AssociatesMember2023-01-012023-12-310001131399ifrs-full:AssociatesMember2022-01-012022-12-310001131399ifrs-full:AssociatesMember2021-01-012021-12-310001131399ifrs-full:JointVenturesMember2023-01-012023-12-310001131399ifrs-full:JointVenturesMember2022-01-012022-12-310001131399ifrs-full:JointVenturesMember2021-01-012021-12-310001131399gsk:InnovivaIncMember2021-05-012021-05-310001131399gsk:InnovivaIncMember2021-01-012021-12-310001131399gsk:OtherAssociatesMember2023-01-012023-12-310001131399gsk:OtherAssociatesMember2022-01-012022-12-310001131399gsk:OtherAssociatesMember2021-01-012021-12-310001131399country:GB2023-01-012023-12-310001131399country:GB2022-01-012022-12-310001131399country:GB2021-01-012021-12-310001131399ifrs-full:ForeignCountriesMember2023-01-012023-12-310001131399ifrs-full:ForeignCountriesMember2022-01-012022-12-310001131399ifrs-full:ForeignCountriesMember2021-01-012021-12-310001131399gsk:IncreaseInTheHeadlineRateOfTaxMembercountry:GB2022-01-012022-12-310001131399gsk:IncreaseInTheHeadlineRateOfTaxMembercountry:GB2023-01-012023-12-310001131399gsk:OverseasMember2023-12-310001131399gsk:OverseasMember2022-12-310001131399gsk:AcceleratedCapitalAllowancesMember2021-12-310001131399ifrs-full:IntangibleAssetsOtherThanGoodwillMember2021-12-310001131399ifrs-full:ContingentConsiderationMember2021-12-310001131399gsk:IntraGroupProfitMember2021-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMember2021-12-310001131399ifrs-full:UnusedTaxLossesMember2021-12-310001131399gsk:ShareOptionAndAwardSchemesMember2021-12-310001131399ifrs-full:OtherTemporaryDifferencesMember2021-12-310001131399gsk:AcceleratedCapitalAllowancesMember2022-01-012022-12-310001131399ifrs-full:IntangibleAssetsOtherThanGoodwillMember2022-01-012022-12-310001131399ifrs-full:ContingentConsiderationMember2022-01-012022-12-310001131399gsk:IntraGroupProfitMember2022-01-012022-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMember2022-01-012022-12-310001131399ifrs-full:UnusedTaxLossesMember2022-01-012022-12-310001131399gsk:ShareOptionAndAwardSchemesMember2022-01-012022-12-310001131399ifrs-full:OtherTemporaryDifferencesMember2022-01-012022-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMembergsk:AcceleratedCapitalAllowancesMember2022-01-012022-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMemberifrs-full:IntangibleAssetsOtherThanGoodwillMember2022-01-012022-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMemberifrs-full:ContingentConsiderationMember2022-01-012022-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMembergsk:IntraGroupProfitMember2022-01-012022-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMembergsk:PensionsAndOtherPostEmploymentBenefitsMember2022-01-012022-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMemberifrs-full:UnusedTaxLossesMember2022-01-012022-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMembergsk:ShareOptionAndAwardSchemesMember2022-01-012022-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMemberifrs-full:OtherTemporaryDifferencesMember2022-01-012022-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMember2022-01-012022-12-310001131399gsk:AcceleratedCapitalAllowancesMember2022-12-310001131399ifrs-full:IntangibleAssetsOtherThanGoodwillMember2022-12-310001131399ifrs-full:ContingentConsiderationMember2022-12-310001131399gsk:IntraGroupProfitMember2022-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMember2022-12-310001131399ifrs-full:UnusedTaxLossesMember2022-12-310001131399gsk:ShareOptionAndAwardSchemesMember2022-12-310001131399ifrs-full:OtherTemporaryDifferencesMember2022-12-310001131399gsk:AcceleratedCapitalAllowancesMember2023-01-012023-12-310001131399ifrs-full:IntangibleAssetsOtherThanGoodwillMember2023-01-012023-12-310001131399ifrs-full:ContingentConsiderationMember2023-01-012023-12-310001131399gsk:IntraGroupProfitMember2023-01-012023-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMember2023-01-012023-12-310001131399ifrs-full:UnusedTaxLossesMember2023-01-012023-12-310001131399gsk:ShareOptionAndAwardSchemesMember2023-01-012023-12-310001131399ifrs-full:OtherTemporaryDifferencesMember2023-01-012023-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMembergsk:AcceleratedCapitalAllowancesMember2023-01-012023-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMemberifrs-full:IntangibleAssetsOtherThanGoodwillMember2023-01-012023-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMemberifrs-full:ContingentConsiderationMember2023-01-012023-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMembergsk:IntraGroupProfitMember2023-01-012023-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMembergsk:PensionsAndOtherPostEmploymentBenefitsMember2023-01-012023-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMemberifrs-full:UnusedTaxLossesMember2023-01-012023-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMembergsk:ShareOptionAndAwardSchemesMember2023-01-012023-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMemberifrs-full:OtherTemporaryDifferencesMember2023-01-012023-12-310001131399gsk:OriginationAndReversalOfTemporaryDifferencesMember2023-01-012023-12-310001131399gsk:AcceleratedCapitalAllowancesMember2023-12-310001131399ifrs-full:IntangibleAssetsOtherThanGoodwillMember2023-12-310001131399ifrs-full:ContingentConsiderationMember2023-12-310001131399gsk:IntraGroupProfitMember2023-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMember2023-12-310001131399ifrs-full:UnusedTaxLossesMember2023-12-310001131399gsk:ShareOptionAndAwardSchemesMember2023-12-310001131399ifrs-full:OtherTemporaryDifferencesMember2023-12-310001131399gsk:WithinTenYearsMember2023-01-012023-12-310001131399gsk:WithinTenYearsMember2022-01-012022-12-310001131399ifrs-full:LaterThanTenYearsMember2023-01-012023-12-310001131399ifrs-full:LaterThanTenYearsMember2022-01-012022-12-310001131399gsk:AvailableIndefinitelyMember2023-01-012023-12-310001131399gsk:AvailableIndefinitelyMember2022-01-012022-12-310001131399gsk:FirstInterimMember2023-01-012023-12-310001131399gsk:FirstInterimMember2022-01-012022-12-310001131399gsk:FirstInterimMember2021-01-012021-12-310001131399gsk:SecondInterimMember2023-01-012023-12-310001131399gsk:SecondInterimMember2022-01-012022-12-310001131399gsk:SecondInterimMember2021-01-012021-12-310001131399gsk:ThirdInterimMember2023-01-012023-12-310001131399gsk:ThirdInterimMember2022-01-012022-12-310001131399gsk:ThirdInterimMember2021-01-012021-12-310001131399gsk:FourthInterimMember2023-01-012023-12-310001131399gsk:FourthInterimMember2022-01-012022-12-310001131399gsk:FourthInterimMember2021-01-012021-12-310001131399gsk:FourthInterimMember2022-12-310001131399gsk:FourthInterimMember2022-10-012022-12-310001131399gsk:DemergerOfConsumerHealthcareBusinessMember2022-01-012022-12-310001131399ifrs-full:LandAndBuildingsMemberifrs-full:GrossCarryingAmountMember2021-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:GrossCarryingAmountMember2021-12-310001131399ifrs-full:ConstructionInProgressMemberifrs-full:GrossCarryingAmountMember2021-12-310001131399ifrs-full:GrossCarryingAmountMember2021-12-310001131399ifrs-full:LandAndBuildingsMemberifrs-full:GrossCarryingAmountMember2022-01-012022-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:GrossCarryingAmountMember2022-01-012022-12-310001131399ifrs-full:ConstructionInProgressMemberifrs-full:GrossCarryingAmountMember2022-01-012022-12-310001131399ifrs-full:GrossCarryingAmountMember2022-01-012022-12-310001131399ifrs-full:LandAndBuildingsMemberifrs-full:GrossCarryingAmountMember2022-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:GrossCarryingAmountMember2022-12-310001131399ifrs-full:ConstructionInProgressMemberifrs-full:GrossCarryingAmountMember2022-12-310001131399ifrs-full:GrossCarryingAmountMember2022-12-310001131399ifrs-full:LandAndBuildingsMemberifrs-full:GrossCarryingAmountMember2023-01-012023-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:GrossCarryingAmountMember2023-01-012023-12-310001131399ifrs-full:ConstructionInProgressMemberifrs-full:GrossCarryingAmountMember2023-01-012023-12-310001131399ifrs-full:GrossCarryingAmountMember2023-01-012023-12-310001131399ifrs-full:LandAndBuildingsMemberifrs-full:GrossCarryingAmountMember2023-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:GrossCarryingAmountMember2023-12-310001131399ifrs-full:ConstructionInProgressMemberifrs-full:GrossCarryingAmountMember2023-12-310001131399ifrs-full:GrossCarryingAmountMember2023-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:LandAndBuildingsMember2021-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:AccumulatedDepreciationAndAmortisationMember2021-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ConstructionInProgressMember2021-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMember2021-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:LandAndBuildingsMember2022-01-012022-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:AccumulatedDepreciationAndAmortisationMember2022-01-012022-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ConstructionInProgressMember2022-01-012022-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMember2022-01-012022-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:LandAndBuildingsMember2022-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:AccumulatedDepreciationAndAmortisationMember2022-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ConstructionInProgressMember2022-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMember2022-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:LandAndBuildingsMember2023-01-012023-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:AccumulatedDepreciationAndAmortisationMember2023-01-012023-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ConstructionInProgressMember2023-01-012023-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMember2023-01-012023-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:LandAndBuildingsMember2023-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:AccumulatedDepreciationAndAmortisationMember2023-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ConstructionInProgressMember2023-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMember2023-12-310001131399ifrs-full:AccumulatedImpairmentMemberifrs-full:LandAndBuildingsMember2021-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:AccumulatedImpairmentMember2021-12-310001131399ifrs-full:AccumulatedImpairmentMemberifrs-full:ConstructionInProgressMember2021-12-310001131399ifrs-full:AccumulatedImpairmentMember2021-12-310001131399ifrs-full:AccumulatedImpairmentMemberifrs-full:LandAndBuildingsMember2022-01-012022-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:AccumulatedImpairmentMember2022-01-012022-12-310001131399ifrs-full:AccumulatedImpairmentMemberifrs-full:ConstructionInProgressMember2022-01-012022-12-310001131399ifrs-full:AccumulatedImpairmentMember2022-01-012022-12-310001131399ifrs-full:AccumulatedImpairmentMemberifrs-full:LandAndBuildingsMember2021-01-012021-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:AccumulatedImpairmentMember2021-01-012021-12-310001131399ifrs-full:AccumulatedImpairmentMemberifrs-full:ConstructionInProgressMember2021-01-012021-12-310001131399ifrs-full:AccumulatedImpairmentMember2021-01-012021-12-310001131399ifrs-full:AccumulatedImpairmentMemberifrs-full:LandAndBuildingsMember2022-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:AccumulatedImpairmentMember2022-12-310001131399ifrs-full:AccumulatedImpairmentMemberifrs-full:ConstructionInProgressMember2022-12-310001131399ifrs-full:AccumulatedImpairmentMember2022-12-310001131399ifrs-full:AccumulatedImpairmentMemberifrs-full:LandAndBuildingsMember2023-01-012023-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:AccumulatedImpairmentMember2023-01-012023-12-310001131399ifrs-full:AccumulatedImpairmentMemberifrs-full:ConstructionInProgressMember2023-01-012023-12-310001131399ifrs-full:AccumulatedImpairmentMember2023-01-012023-12-310001131399ifrs-full:AccumulatedImpairmentMemberifrs-full:LandAndBuildingsMember2023-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:AccumulatedImpairmentMember2023-12-310001131399ifrs-full:AccumulatedImpairmentMemberifrs-full:ConstructionInProgressMember2023-12-310001131399ifrs-full:AccumulatedImpairmentMember2023-12-310001131399ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:LandAndBuildingsMember2022-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2022-12-310001131399ifrs-full:ConstructionInProgressMemberifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2022-12-310001131399ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2022-12-310001131399ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:LandAndBuildingsMember2023-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMemberifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2023-12-310001131399ifrs-full:ConstructionInProgressMemberifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2023-12-310001131399ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2023-12-310001131399ifrs-full:LandAndBuildingsMember2021-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMember2021-12-310001131399ifrs-full:ConstructionInProgressMember2021-12-310001131399ifrs-full:LandAndBuildingsMember2022-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMember2022-12-310001131399ifrs-full:ConstructionInProgressMember2022-12-310001131399ifrs-full:LandAndBuildingsMember2023-12-310001131399gsk:PropertyPlantAndEquipmentAndVehiclesMember2023-12-310001131399ifrs-full:ConstructionInProgressMember2023-12-310001131399gsk:NetBookValueMember2023-01-012023-12-310001131399ifrs-full:CostOfSalesMember2023-01-012023-12-310001131399ifrs-full:CostOfSalesMember2022-01-012022-12-310001131399gsk:ResearchAndDevelopmentMember2023-01-012023-12-310001131399gsk:ResearchAndDevelopmentMember2022-01-012022-12-310001131399ifrs-full:SellingGeneralAndAdministrativeExpenseMember2023-01-012023-12-310001131399ifrs-full:SellingGeneralAndAdministrativeExpenseMember2022-01-012022-12-310001131399gsk:MajorRestructuringProgrammesMember2023-01-012023-12-310001131399gsk:MajorRestructuringProgrammesMember2022-01-012022-12-310001131399ifrs-full:CostOfSalesMemberifrs-full:AccumulatedImpairmentMember2023-01-012023-12-310001131399ifrs-full:AccumulatedImpairmentMembergsk:ResearchAndDevelopmentMember2023-01-012023-12-310001131399ifrs-full:SellingGeneralAndAdministrativeExpenseMemberifrs-full:AccumulatedImpairmentMember2023-01-012023-12-310001131399ifrs-full:CostOfSalesMemberifrs-full:AccumulatedImpairmentMember2022-01-012022-12-310001131399ifrs-full:ComputerSoftwareMember2023-01-012023-12-310001131399ifrs-full:ComputerSoftwareMember2022-01-012022-12-310001131399gsk:LowerCarbonPropellantMemberifrs-full:GrossCarryingAmountMember2023-12-310001131399ifrs-full:RightofuseAssetsMemberifrs-full:LandAndBuildingsMember2021-12-310001131399gsk:PlantAndEquipmentMemberifrs-full:RightofuseAssetsMember2021-12-310001131399ifrs-full:VehiclesMemberifrs-full:RightofuseAssetsMember2021-12-310001131399ifrs-full:RightofuseAssetsMember2021-12-310001131399ifrs-full:RightofuseAssetsMemberifrs-full:LandAndBuildingsMember2022-01-012022-12-310001131399gsk:PlantAndEquipmentMemberifrs-full:RightofuseAssetsMember2022-01-012022-12-310001131399ifrs-full:VehiclesMemberifrs-full:RightofuseAssetsMember2022-01-012022-12-310001131399ifrs-full:RightofuseAssetsMember2022-01-012022-12-310001131399ifrs-full:RightofuseAssetsMemberifrs-full:LandAndBuildingsMember2022-12-310001131399gsk:PlantAndEquipmentMemberifrs-full:RightofuseAssetsMember2022-12-310001131399ifrs-full:VehiclesMemberifrs-full:RightofuseAssetsMember2022-12-310001131399ifrs-full:RightofuseAssetsMember2022-12-310001131399ifrs-full:RightofuseAssetsMemberifrs-full:LandAndBuildingsMember2023-01-012023-12-310001131399gsk:PlantAndEquipmentMemberifrs-full:RightofuseAssetsMember2023-01-012023-12-310001131399ifrs-full:VehiclesMemberifrs-full:RightofuseAssetsMember2023-01-012023-12-310001131399ifrs-full:RightofuseAssetsMember2023-01-012023-12-310001131399ifrs-full:RightofuseAssetsMemberifrs-full:LandAndBuildingsMember2023-12-310001131399gsk:PlantAndEquipmentMemberifrs-full:RightofuseAssetsMember2023-12-310001131399ifrs-full:VehiclesMemberifrs-full:RightofuseAssetsMember2023-12-310001131399ifrs-full:RightofuseAssetsMember2023-12-310001131399gsk:CommercialOperationsMember2023-12-310001131399gsk:CommercialOperationsMember2022-12-310001131399gsk:ResearchAndDevelopmentMember2023-12-310001131399gsk:ResearchAndDevelopmentMember2022-12-310001131399gsk:CommercialOperationsMember2023-12-310001131399gsk:ResearchAndDevelopmentMember2023-12-310001131399gsk:CommercialOperationsMember2022-12-310001131399gsk:ResearchAndDevelopmentMember2022-12-310001131399ifrs-full:ComputerSoftwareMemberifrs-full:GrossCarryingAmountMember2021-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMemberifrs-full:GrossCarryingAmountMember2021-12-310001131399gsk:BrandsWithIndefiniteUsefulLifeMemberifrs-full:GrossCarryingAmountMember2021-12-310001131399ifrs-full:ComputerSoftwareMemberifrs-full:GrossCarryingAmountMember2022-01-012022-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMemberifrs-full:GrossCarryingAmountMember2022-01-012022-12-310001131399gsk:BrandsWithIndefiniteUsefulLifeMemberifrs-full:GrossCarryingAmountMember2022-01-012022-12-310001131399ifrs-full:ComputerSoftwareMemberifrs-full:GrossCarryingAmountMember2022-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMemberifrs-full:GrossCarryingAmountMember2022-12-310001131399gsk:BrandsWithIndefiniteUsefulLifeMemberifrs-full:GrossCarryingAmountMember2022-12-310001131399ifrs-full:ComputerSoftwareMemberifrs-full:GrossCarryingAmountMember2023-01-012023-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMemberifrs-full:GrossCarryingAmountMember2023-01-012023-12-310001131399gsk:BrandsWithIndefiniteUsefulLifeMemberifrs-full:GrossCarryingAmountMember2023-01-012023-12-310001131399ifrs-full:ComputerSoftwareMemberifrs-full:GrossCarryingAmountMember2023-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMemberifrs-full:GrossCarryingAmountMember2023-12-310001131399gsk:BrandsWithIndefiniteUsefulLifeMemberifrs-full:GrossCarryingAmountMember2023-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerSoftwareMember2021-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMembergsk:LicencesAndFranchisesAmortisedBrandsMember2021-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMembergsk:BrandsWithIndefiniteUsefulLifeMember2021-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerSoftwareMember2022-01-012022-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMembergsk:LicencesAndFranchisesAmortisedBrandsMember2022-01-012022-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMembergsk:BrandsWithIndefiniteUsefulLifeMember2022-01-012022-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerSoftwareMember2022-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMembergsk:LicencesAndFranchisesAmortisedBrandsMember2022-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMembergsk:BrandsWithIndefiniteUsefulLifeMember2022-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerSoftwareMember2023-01-012023-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMembergsk:LicencesAndFranchisesAmortisedBrandsMember2023-01-012023-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMembergsk:BrandsWithIndefiniteUsefulLifeMember2023-01-012023-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerSoftwareMember2023-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMembergsk:LicencesAndFranchisesAmortisedBrandsMember2023-12-310001131399ifrs-full:AccumulatedDepreciationAndAmortisationMembergsk:BrandsWithIndefiniteUsefulLifeMember2023-12-310001131399ifrs-full:ComputerSoftwareMemberifrs-full:AccumulatedImpairmentMember2021-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMemberifrs-full:AccumulatedImpairmentMember2021-12-310001131399ifrs-full:AccumulatedImpairmentMembergsk:BrandsWithIndefiniteUsefulLifeMember2021-12-310001131399ifrs-full:ComputerSoftwareMemberifrs-full:AccumulatedImpairmentMember2022-01-012022-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMemberifrs-full:AccumulatedImpairmentMember2022-01-012022-12-310001131399ifrs-full:AccumulatedImpairmentMembergsk:BrandsWithIndefiniteUsefulLifeMember2022-01-012022-12-310001131399ifrs-full:ComputerSoftwareMemberifrs-full:AccumulatedImpairmentMember2022-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMemberifrs-full:AccumulatedImpairmentMember2022-12-310001131399ifrs-full:AccumulatedImpairmentMembergsk:BrandsWithIndefiniteUsefulLifeMember2022-12-310001131399ifrs-full:ComputerSoftwareMemberifrs-full:AccumulatedImpairmentMember2023-01-012023-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMemberifrs-full:AccumulatedImpairmentMember2023-01-012023-12-310001131399ifrs-full:AccumulatedImpairmentMembergsk:BrandsWithIndefiniteUsefulLifeMember2023-01-012023-12-310001131399ifrs-full:ComputerSoftwareMemberifrs-full:AccumulatedImpairmentMember2023-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMemberifrs-full:AccumulatedImpairmentMember2023-12-310001131399ifrs-full:AccumulatedImpairmentMembergsk:BrandsWithIndefiniteUsefulLifeMember2023-12-310001131399ifrs-full:ComputerSoftwareMemberifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2022-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMemberifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2022-12-310001131399gsk:BrandsWithIndefiniteUsefulLifeMemberifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2022-12-310001131399ifrs-full:ComputerSoftwareMemberifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2023-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMemberifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2023-12-310001131399gsk:BrandsWithIndefiniteUsefulLifeMemberifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2023-12-310001131399ifrs-full:ComputerSoftwareMember2021-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMember2021-12-310001131399gsk:BrandsWithIndefiniteUsefulLifeMember2021-12-310001131399ifrs-full:ComputerSoftwareMember2022-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMember2022-12-310001131399gsk:BrandsWithIndefiniteUsefulLifeMember2022-12-310001131399ifrs-full:ComputerSoftwareMember2023-12-310001131399gsk:LicencesAndFranchisesAmortisedBrandsMember2023-12-310001131399gsk:BrandsWithIndefiniteUsefulLifeMember2023-12-310001131399ifrs-full:InternallyGeneratedMember2023-12-310001131399ifrs-full:InternallyGeneratedMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:TesaroAssetsMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:TesaroAssetsMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:MeningitisPortfolioMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:MeningitisPortfolioMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:MomelotinibMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:MomelotinibMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:CamlipixantMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:CamlipixantMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:AffinivaxAssetsMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:AffinivaxAssetsMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:DolutegravirIncludingCabotegravirMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:DolutegravirIncludingCabotegravirMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:IteosAssetsMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:IteosAssetsMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:AlectorAssetsMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:AlectorAssetsMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:BenlystaMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:BenlystaMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:ShingrixMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:ShingrixMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:OkairosMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:OkairosMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:BMSAssetsMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:BMSAssetsMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:CureVacAssetsMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:CureVacAssetsMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:SperoMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:SperoMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:RSVMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:RSVMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:RelvarBreoAnoroMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:RelvarBreoAnoroMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:StiefelTradeNameMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:StiefelTradeNameMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:WaveLifeSciencesMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:WaveLifeSciencesMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:UCBMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:UCBMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:ArrowheadMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:ArrowheadMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:DTMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:DTMember2022-12-310001131399gsk:FluarixFluLavalMemberifrs-full:LicencesAndFranchisesMember2023-12-310001131399gsk:FluarixFluLavalMemberifrs-full:LicencesAndFranchisesMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:VirAssetsMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:VirAssetsMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:OthersMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMembergsk:OthersMember2022-12-310001131399ifrs-full:LicencesAndFranchisesMember2023-12-310001131399ifrs-full:LicencesAndFranchisesMember2022-12-310001131399ifrs-full:JointVenturesMember2022-12-310001131399ifrs-full:AssociatesMember2022-12-310001131399ifrs-full:JointVenturesMember2021-12-310001131399ifrs-full:AssociatesMember2021-12-310001131399ifrs-full:AssociatesMember2023-01-012023-12-310001131399ifrs-full:AssociatesMember2022-01-012022-12-310001131399ifrs-full:JointVenturesMember2023-12-310001131399ifrs-full:AssociatesMember2023-12-310001131399gsk:CurrentEquityInvestmentsMeasuredAsFVTPLMembergsk:CurrentEquityInvestmentsMember2022-12-310001131399gsk:CurrentEquityInvestmentsMeasuredAsFVTPLMembergsk:CurrentEquityInvestmentsMember2021-12-310001131399gsk:CurrentEquityInvestmentsMeasuredAsFVTPLMembergsk:CurrentEquityInvestmentsMember2023-01-012023-12-310001131399gsk:CurrentEquityInvestmentsMeasuredAsFVTPLMembergsk:CurrentEquityInvestmentsMember2022-01-012022-12-310001131399gsk:CurrentEquityInvestmentsMeasuredAsFVTPLMembergsk:CurrentEquityInvestmentsMember2023-12-310001131399gsk:GlaxoGroupLimitedMembergsk:InvestmentInHaleonPlcMember2023-01-012023-12-310001131399gsk:GlaxoGroupLimitedMembergsk:InvestmentInHaleonPlcMember2022-01-012022-12-310001131399gsk:ScottishLimitedPartnershipsMembergsk:InvestmentInHaleonPlcMember2023-01-012023-12-310001131399gsk:ScottishLimitedPartnershipsMembergsk:InvestmentInHaleonPlcMember2022-01-012022-12-310001131399gsk:InvestmentInHaleonPlcMembergsk:GSKScottishLimitedPartnershipNo.2Member2023-01-012023-12-310001131399gsk:InvestmentInHaleonPlcMembergsk:GSKScottishLimitedPartnershipNo.2Member2022-01-012022-12-310001131399gsk:GSKScottishLimitedPartnershipNo.3Membergsk:InvestmentInHaleonPlcMember2023-01-012023-12-310001131399gsk:GSKScottishLimitedPartnershipNo.3Membergsk:InvestmentInHaleonPlcMember2022-01-012022-12-310001131399gsk:EsopTrustsMembergsk:InvestmentInHaleonPlcMember2023-01-012023-12-310001131399gsk:EsopTrustsMembergsk:InvestmentInHaleonPlcMember2022-01-012022-12-310001131399gsk:OtherInvestmentsNoncurrentMemberifrs-full:InvestmentsInEquityInstrumentsMeasuredAtFairValueThroughOtherComprehensiveIncomeMember2022-12-310001131399gsk:InvestmentsMeasuredAtFairValueThroughProfitOrLossMembergsk:OtherInvestmentsNoncurrentMember2022-12-310001131399gsk:OtherInvestmentsNoncurrentMember2022-12-310001131399gsk:OtherInvestmentsNoncurrentMemberifrs-full:InvestmentsInEquityInstrumentsMeasuredAtFairValueThroughOtherComprehensiveIncomeMember2021-12-310001131399gsk:InvestmentsMeasuredAtFairValueThroughProfitOrLossMembergsk:OtherInvestmentsNoncurrentMember2021-12-310001131399gsk:OtherInvestmentsNoncurrentMember2021-12-310001131399gsk:OtherInvestmentsNoncurrentMemberifrs-full:InvestmentsInEquityInstrumentsMeasuredAtFairValueThroughOtherComprehensiveIncomeMember2023-01-012023-12-310001131399gsk:InvestmentsMeasuredAtFairValueThroughProfitOrLossMembergsk:OtherInvestmentsNoncurrentMember2023-01-012023-12-310001131399gsk:OtherInvestmentsNoncurrentMember2023-01-012023-12-310001131399gsk:OtherInvestmentsNoncurrentMemberifrs-full:InvestmentsInEquityInstrumentsMeasuredAtFairValueThroughOtherComprehensiveIncomeMember2022-01-012022-12-310001131399gsk:InvestmentsMeasuredAtFairValueThroughProfitOrLossMembergsk:OtherInvestmentsNoncurrentMember2022-01-012022-12-310001131399gsk:OtherInvestmentsNoncurrentMember2022-01-012022-12-310001131399gsk:OtherInvestmentsNoncurrentMemberifrs-full:InvestmentsInEquityInstrumentsMeasuredAtFairValueThroughOtherComprehensiveIncomeMember2023-12-310001131399gsk:InvestmentsMeasuredAtFairValueThroughProfitOrLossMembergsk:OtherInvestmentsNoncurrentMember2023-12-310001131399gsk:OtherInvestmentsNoncurrentMember2023-12-310001131399gsk:EquityInvestmentsMeasuredAtFairValueThroughOtherComprehensiveIncomeMember2023-01-012023-12-310001131399gsk:EquityInvestmentsMeasuredAtFairValueThroughProfitOrLossMember2023-01-012023-12-310001131399gsk:EquityInvestmentsMeasuredAtFairValueThroughOtherComprehensiveIncomeMember2022-01-012022-12-310001131399gsk:EquityInvestmentsMeasuredAtFairValueThroughProfitOrLossMember2022-01-012022-12-310001131399gsk:CrisprTherapeuticsMember2023-12-310001131399gsk:CrisprTherapeuticsMember2022-12-310001131399gsk:VirBiotechnologyIncMember2023-12-310001131399gsk:VirBiotechnologyIncMember2022-12-310001131399gsk:NimbusTherapeuticsLLCMember2023-12-310001131399gsk:NimbusTherapeuticsLLCMember2022-12-310001131399gsk:EquityInvestmentsMeasuredAtFairValueThroughOtherComprehensiveIncomeMember2023-12-310001131399gsk:EquityInvestmentsMeasuredAtFairValueThroughOtherComprehensiveIncomeMember2022-12-310001131399gsk:InvestmentsMeasuredAtFairValueThroughProfitOrLossMembergsk:OtherInvestmentsNoncurrentMembergsk:SROneCapitalFundIBLPMember2023-12-310001131399gsk:InvestmentsMeasuredAtFairValueThroughProfitOrLossMembergsk:OtherInvestmentsNoncurrentMembergsk:SROneCapitalFundIBLPMember2022-12-310001131399gsk:OtherNonCurrentReceivablesMember2023-12-310001131399gsk:OtherNonCurrentReceivablesMember2022-12-310001131399gsk:OtherNonCurrentReceivablesMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399gsk:OtherNonCurrentReceivablesMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399gsk:RemainingBalanceMembergsk:OtherNonCurrentReceivablesMember2023-12-310001131399gsk:RemainingBalanceMembergsk:OtherNonCurrentReceivablesMember2022-12-310001131399gsk:AssociatesAndJointVenturesMember2022-12-310001131399ifrs-full:FinancialInstrumentsCreditimpairedMemberifrs-full:TradeReceivablesMember2023-12-310001131399ifrs-full:FinancialInstrumentsCreditimpairedMemberifrs-full:TradeReceivablesMember2022-12-310001131399ifrs-full:TradeReceivablesMember2023-12-310001131399ifrs-full:TradeReceivablesMember2022-12-310001131399gsk:OtherReceivableMember2023-12-310001131399gsk:OtherReceivableMember2022-12-310001131399ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMemberifrs-full:TradeReceivablesMember2023-12-310001131399ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMemberifrs-full:TradeReceivablesMember2022-12-310001131399gsk:OtherReceivableMember2023-01-012023-12-310001131399gsk:OtherReceivableMember2022-01-012022-12-310001131399gsk:ConsumerHealthcareBusinessMember2023-01-012023-12-310001131399ifrs-full:AssetsAndLiabilitiesClassifiedAsHeldForSaleMember2023-12-310001131399ifrs-full:AssetsAndLiabilitiesClassifiedAsHeldForSaleMember2022-12-310001131399gsk:AssociatesAndJointVenturesMember2023-12-310001131399gsk:CurrencyRiskTenPercentageIncreaseInSalesForecastsOrSalesMultipleAppliedMember2023-01-012023-12-310001131399gsk:CurrencyRiskTenPercentageIncreaseInSalesForecastsOrSalesMultipleAppliedMember2022-01-012022-12-310001131399gsk:CurrencyRiskFifteenPercentageIncreaseInSalesForecastsOrSalesMultipleAppliedMember2023-01-012023-12-310001131399gsk:CurrencyRiskFifteenPercentageIncreaseInSalesForecastsOrSalesMultipleAppliedMember2022-01-012022-12-310001131399gsk:CurrencyRiskTenPercentageDecreaseInSalesForecastsOrSalesMultipleAppliedMember2023-01-012023-12-310001131399gsk:CurrencyRiskTenPercentageDecreaseInSalesForecastsOrSalesMultipleAppliedMember2022-01-012022-12-310001131399gsk:CurrencyRiskFifteenPercentageDecreaseInSalesForecastsOrSalesMultipleAppliedMember2023-01-012023-12-310001131399gsk:CurrencyRiskFifteenPercentageDecreaseInSalesForecastsOrSalesMultipleAppliedMember2022-01-012022-12-310001131399ifrs-full:DiscountRateMeasurementInputMember2023-01-012023-12-310001131399ifrs-full:DiscountRateMeasurementInputMember2022-01-012022-12-310001131399gsk:CurrencyRiskOneFiftyBasisPointsIncreaseInDiscountRateMember2023-01-012023-12-310001131399gsk:CurrencyRiskOneFiftyBasisPointsIncreaseInDiscountRateMember2022-01-012022-12-310001131399gsk:CurrencyRiskOneFiftyBasisPointsDecreaseInDiscountRateMember2023-01-012023-12-310001131399gsk:CurrencyRiskOneFiftyBasisPointsDecreaseInDiscountRateMember2022-01-012022-12-310001131399gsk:CurrencyRiskTenCentAppreciationOfUSDollarMember2023-01-012023-12-310001131399gsk:CurrencyRiskTenCentAppreciationOfUSDollarMember2022-01-012022-12-310001131399gsk:CurrencyRiskFifteenCentAppreciationOfUsDollarMember2023-01-012023-12-310001131399gsk:CurrencyRiskFifteenCentAppreciationOfUsDollarMember2022-01-012022-12-310001131399gsk:CurrencyRiskTenCentDepreciationOfUSDollarMember2023-01-012023-12-310001131399gsk:CurrencyRiskTenCentDepreciationOfUSDollarMember2022-01-012022-12-310001131399gsk:CurrencyRiskFifteenCentDepreciationOfUsDollarMember2023-01-012023-12-310001131399gsk:CurrencyRiskFifteenCentDepreciationOfUsDollarMember2022-01-012022-12-310001131399gsk:CurrencyRiskTenCentAppreciationOfEuroMember2023-01-012023-12-310001131399gsk:CurrencyRiskTenCentAppreciationOfEuroMember2022-01-012022-12-310001131399gsk:CurrencyRiskFifteenCentAppreciationOfEuroMember2023-01-012023-12-310001131399gsk:CurrencyRiskFifteenCentAppreciationOfEuroMember2022-01-012022-12-310001131399gsk:CurrencyRiskTenCentDepreciationOfEuroMember2023-01-012023-12-310001131399gsk:CurrencyRiskTenCentDepreciationOfEuroMember2022-01-012022-12-310001131399gsk:CurrencyRiskFifteenCentDepreciationOfEuroMember2023-01-012023-12-310001131399gsk:CurrencyRiskFifteenCentDepreciationOfEuroMember2022-01-012022-12-310001131399gsk:Debt1Member2023-12-310001131399gsk:Debt1Member2022-12-310001131399gsk:ZeroPointOneTwoFivePercentEuropeanMediumTermNoteTwoThousandTwentyThreeMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:ZeroPointOneTwoFivePercentEuropeanMediumTermNoteTwoThousandTwentyThreeMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:ZeroPointOneTwoFivePercentEuropeanMediumTermNoteTwoThousandTwentyThreeMember2022-12-310001131399gsk:ZeroPointZeroPercentEuropeanMediumTermNoteTwoThousandTwentyThreeMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:ZeroPointZeroPercentEuropeanMediumTermNoteTwoThousandTwentyThreeMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:ZeroPointZeroPercentEuropeanMediumTermNoteTwoThousandTwentyThreeMember2022-12-310001131399gsk:ZeroPointFiveThreeFourPercentUsMediumTermNoteTwoThousandTwentyThreeMember2023-12-310001131399gsk:ZeroPointFiveThreeFourPercentUsMediumTermNoteTwoThousandTwentyThreeMembergsk:NewYorkStockExchangeMember2023-12-310001131399gsk:ZeroPointFiveThreeFourPercentUsMediumTermNoteTwoThousandTwentyThreeMembergsk:NewYorkStockExchangeMember2022-12-310001131399gsk:ThreePercentUSMediumTermNoteTwoThousandTwentyFourMember2023-12-310001131399gsk:ThreePercentUSMediumTermNoteTwoThousandTwentyFourMembergsk:NewYorkStockExchangeMember2023-12-310001131399gsk:ThreePercentUSMediumTermNoteTwoThousandTwentyFourMembergsk:NewYorkStockExchangeMember2022-12-310001131399gsk:OnePointThreeSevenFivePercentEuroMediumTermNoteTwoThousandTwentyFourMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointThreeSevenFivePercentEuroMediumTermNoteTwoThousandTwentyFourMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointThreeSevenFivePercentEuroMediumTermNoteTwoThousandTwentyFourMember2022-12-310001131399gsk:FourPointZeroZeroPercentEuroMediumTermNoteTwoThousandTwentyFiveMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:FourPointZeroZeroPercentEuroMediumTermNoteTwoThousandTwentyFiveMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:FourPointZeroZeroPercentEuroMediumTermNoteTwoThousandTwentyFiveMember2022-12-310001131399gsk:ThreePointSixTwoFivePercentUSMediumTermNoteTwoThousandTwentyFiveMember2023-12-310001131399gsk:ThreePointSixTwoFivePercentUSMediumTermNoteTwoThousandTwentyFiveMembergsk:NewYorkStockExchangeMember2023-12-310001131399gsk:ThreePointSixTwoFivePercentUSMediumTermNoteTwoThousandTwentyFiveMembergsk:NewYorkStockExchangeMember2022-12-310001131399gsk:OnePointZeroZeroPercentEuroMediumTermNoteTwoThousandTwentySixMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointZeroZeroPercentEuroMediumTermNoteTwoThousandTwentySixMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointZeroZeroPercentEuroMediumTermNoteTwoThousandTwentySixMember2022-12-310001131399gsk:OnePointTwoFivePercentageEuroMediumTermNoteTwoThousandTwentySixMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointTwoFivePercentageEuroMediumTermNoteTwoThousandTwentySixMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointTwoFivePercentageEuroMediumTermNoteTwoThousandTwentySixMember2022-12-310001131399gsk:ThreePointZeroPercentEuroMediumTermNoteTwoThousandTwentySevenMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:ThreePointZeroPercentEuroMediumTermNoteTwoThousandTwentySevenMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:ThreePointZeroPercentEuroMediumTermNoteTwoThousandTwentySevenMember2022-12-310001131399gsk:ThreePointThreeSevenFivePercentEuroMediumTermNoteTwoThousandTwentySevenMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:ThreePointThreeSevenFivePercentEuroMediumTermNoteTwoThousandTwentySevenMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:ThreePointThreeSevenFivePercentEuroMediumTermNoteTwoThousandTwentySevenMember2022-12-310001131399gsk:ThreePointEightSevenFivePercentageUSMediumTermNoteTwoThousandTwentyEightMember2023-12-310001131399gsk:ThreePointEightSevenFivePercentageUSMediumTermNoteTwoThousandTwentyEightMembergsk:NewYorkStockExchangeMember2023-12-310001131399gsk:ThreePointEightSevenFivePercentageUSMediumTermNoteTwoThousandTwentyEightMembergsk:NewYorkStockExchangeMember2022-12-310001131399gsk:ZeroPointEightEightThreePercentEuroMediumTermNoteTwoThousandTwentyEightMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:ZeroPointEightEightThreePercentEuroMediumTermNoteTwoThousandTwentyEightMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:ZeroPointEightEightThreePercentEuroMediumTermNoteTwoThousandTwentyEightMember2022-12-310001131399gsk:OnePointTwoFiveEuroMediumTermNoteTwoThousandTwentyEightMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointTwoFiveEuroMediumTermNoteTwoThousandTwentyEightMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointTwoFiveEuroMediumTermNoteTwoThousandTwentyEightMember2022-12-310001131399gsk:ThreePointThreeSevenFivePercentUsMediumTermNoteTwoThousandTwentyNineMember2023-12-310001131399gsk:ThreePointThreeSevenFivePercentUsMediumTermNoteTwoThousandTwentyNineMembergsk:NewYorkStockExchangeMember2023-12-310001131399gsk:ThreePointThreeSevenFivePercentUsMediumTermNoteTwoThousandTwentyNineMembergsk:NewYorkStockExchangeMember2022-12-310001131399gsk:OnePointThreeSevenFivePercentEuroMediumTermNotesTwoThousandTwentyNineMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointThreeSevenFivePercentEuroMediumTermNotesTwoThousandTwentyNineMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointThreeSevenFivePercentEuroMediumTermNotesTwoThousandTwentyNineMember2022-12-310001131399gsk:OnePointSevenFiveZeroPercentageEuroMediumTermNoteTwoThousandThirtyMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointSevenFiveZeroPercentageEuroMediumTermNoteTwoThousandThirtyMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointSevenFiveZeroPercentageEuroMediumTermNoteTwoThousandThirtyMember2022-12-310001131399gsk:ThreePointOneTwoFivePercentEuroMediumTermNoteTwoThousandThirtyTwoMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:ThreePointOneTwoFivePercentEuroMediumTermNoteTwoThousandThirtyTwoMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:ThreePointOneTwoFivePercentEuroMediumTermNoteTwoThousandThirtyTwoMember2022-12-310001131399gsk:FivePointTwoFivePercentEuroMediumTermNoteTwoThousandThirtyThreeMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:FivePointTwoFivePercentEuroMediumTermNoteTwoThousandThirtyThreeMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:FivePointTwoFivePercentEuroMediumTermNoteTwoThousandThirtyThreeMember2022-12-310001131399gsk:FivePointThreeSevenFivePercentUsMediumTermNoteTwoThousandThirtyFourMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:FivePointThreeSevenFivePercentUsMediumTermNoteTwoThousandThirtyFourMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:FivePointThreeSevenFivePercentUsMediumTermNoteTwoThousandThirtyFourMember2022-12-310001131399gsk:OnePointSixTwoFiveEuroMediumTermNoteTwoThousandTwentyFiveMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointSixTwoFiveEuroMediumTermNoteTwoThousandTwentyFiveMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:OnePointSixTwoFiveEuroMediumTermNoteTwoThousandTwentyFiveMember2022-12-310001131399gsk:SixPointThreeSevenFivePercentUsMediumTermNoteTwoThousandThirtyEightMember2023-12-310001131399gsk:SixPointThreeSevenFivePercentUsMediumTermNoteTwoThousandThirtyEightMembergsk:NewYorkStockExchangeMember2023-12-310001131399gsk:SixPointThreeSevenFivePercentUsMediumTermNoteTwoThousandThirtyEightMembergsk:NewYorkStockExchangeMember2022-12-310001131399gsk:SixPointThreeSevenFivePercentEuroMediumTermNoteTwoThousandThirtyNineMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:SixPointThreeSevenFivePercentEuroMediumTermNoteTwoThousandThirtyNineMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:SixPointThreeSevenFivePercentEuroMediumTermNoteTwoThousandThirtyNineMember2022-12-310001131399gsk:FivePointTwoFivePercentEuroTermNoteTwoThousandFortyTwoMember2023-12-310001131399gsk:FivePointTwoFivePercentEuroTermNoteTwoThousandFortyTwoMembergsk:LondonStockExchangeMember2023-12-310001131399gsk:FivePointTwoFivePercentEuroTermNoteTwoThousandFortyTwoMembergsk:LondonStockExchangeMember2022-12-310001131399gsk:FourPointTwoPercentUsMediumTermNoteTwoThousandFortyThreeMember2023-12-310001131399gsk:NewYorkStockExchangeMembergsk:FourPointTwoPercentUsMediumTermNoteTwoThousandFortyThreeMember2023-12-310001131399gsk:NewYorkStockExchangeMembergsk:FourPointTwoPercentUsMediumTermNoteTwoThousandFortyThreeMember2022-12-310001131399gsk:FourPointTwoFivePercentEuroMediumTermNoteTwoThousandFortyFiveMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:FourPointTwoFivePercentEuroMediumTermNoteTwoThousandFortyFiveMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:FourPointTwoFivePercentEuroMediumTermNoteTwoThousandFortyFiveMember2022-12-310001131399gsk:OtherLongtermBorrowingsMember2023-12-310001131399gsk:OtherLongtermBorrowingsMember2022-12-310001131399ifrs-full:FloatingInterestRateMember2023-12-310001131399ifrs-full:FloatingInterestRateMember2022-12-310001131399ifrs-full:FixedInterestRateMember2023-12-310001131399ifrs-full:FixedInterestRateMember2022-12-310001131399gsk:NonInterestBearingMember2023-12-310001131399gsk:NonInterestBearingMember2022-12-310001131399gsk:UsCommercialPaperProgramMember2023-12-310001131399gsk:UsCommercialPaperProgramMember2022-12-310001131399gsk:EuroCommercialPaperProgramMember2023-12-31iso4217:EUR0001131399gsk:EuroCommercialPaperProgramMember2022-12-310001131399ifrs-full:LiquidityRiskMemberifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMember2023-12-310001131399ifrs-full:LiquidityRiskMemberifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMember2023-01-012023-12-310001131399ifrs-full:LiquidityRiskMemberifrs-full:NotLaterThanOneYearMember2023-12-310001131399ifrs-full:LiquidityRiskMemberifrs-full:NotLaterThanOneYearMember2023-01-012023-12-310001131399gsk:CommercialPaperDebtMember2023-01-012023-12-310001131399gsk:CommercialPaperDebtMember2022-01-012022-12-310001131399gsk:ShortTermNotesMember2023-12-310001131399gsk:ShortTermNotesMember2022-12-310001131399ifrs-full:LaterThanFiveYearsMember2023-12-310001131399ifrs-full:LaterThanFiveYearsMember2022-12-310001131399gsk:LondonStockExchangeMembergsk:FivePointTwoFivePercentEuroMediumTermNoteTwoThousandThirtyThreeMember2023-01-012023-12-310001131399gsk:FivePointTwoFivePercentEuroMediumTermNoteTwoThousandThirtyThreeRepaidLoanMember2023-12-310001131399gsk:LondonStockExchangeMembergsk:SixPointThreeSevenFivePercentEuroMediumTermNoteTwoThousandThirtyNineMember2023-01-012023-12-310001131399gsk:SixPointThreeSevenFivePercentEuroMediumTermNoteTwoThousandThirtyNineRepaidLoanMember2023-12-310001131399ifrs-full:BottomOfRangeMemberifrs-full:LaterThanFiveYearsMember2023-01-012023-12-310001131399ifrs-full:TopOfRangeMemberifrs-full:LaterThanFiveYearsMember2023-01-012023-12-310001131399gsk:LetterOfCreditHeldMember2023-01-012023-12-310001131399gsk:LetterOfCreditHeldMember2022-01-012022-12-310001131399ifrs-full:NotLaterThanOneYearMember2023-12-310001131399ifrs-full:NotLaterThanOneYearMember2022-12-310001131399ifrs-full:LaterThanOneYearAndNotLaterThanTwoYearsMember2023-12-310001131399ifrs-full:LaterThanOneYearAndNotLaterThanTwoYearsMember2022-12-310001131399ifrs-full:LaterThanTwoYearsAndNotLaterThanThreeYearsMember2023-12-310001131399ifrs-full:LaterThanTwoYearsAndNotLaterThanThreeYearsMember2022-12-310001131399ifrs-full:LaterThanThreeYearsAndNotLaterThanFourYearsMember2023-12-310001131399ifrs-full:LaterThanThreeYearsAndNotLaterThanFourYearsMember2022-12-310001131399ifrs-full:LaterThanFourYearsAndNotLaterThanFiveYearsMember2023-12-310001131399ifrs-full:LaterThanFourYearsAndNotLaterThanFiveYearsMember2022-12-310001131399gsk:UkDefinedBenefitAndDefinedContributionPlansMember2023-01-012023-12-310001131399gsk:UkDefinedBenefitAndDefinedContributionPlansMember2022-01-012022-12-310001131399gsk:UkDefinedBenefitAndDefinedContributionPlansMember2021-01-012021-12-310001131399gsk:UsDefinedBenefitAndDefinedContributionPlansMember2023-01-012023-12-310001131399gsk:UsDefinedBenefitAndDefinedContributionPlansMember2022-01-012022-12-310001131399gsk:UsDefinedBenefitAndDefinedContributionPlansMember2021-01-012021-12-310001131399gsk:OtherOverseasPensionPlansMember2023-01-012023-12-310001131399gsk:OtherOverseasPensionPlansMember2022-01-012022-12-310001131399gsk:OtherOverseasPensionPlansMember2021-01-012021-12-310001131399gsk:UnfundedPostRetirementPensionPlansMember2023-01-012023-12-310001131399gsk:UnfundedPostRetirementPensionPlansMember2022-01-012022-12-310001131399gsk:UnfundedPostRetirementPensionPlansMember2021-01-012021-12-310001131399gsk:FundedDefinedBenefitPensionPlansMember2023-01-012023-12-310001131399gsk:FundedDefinedBenefitPensionPlansMember2022-01-012022-12-310001131399gsk:FundedDefinedBenefitPensionPlansMember2021-01-012021-12-310001131399gsk:UnfundedDefinedBenefitPensionPlanMember2023-01-012023-12-310001131399gsk:UnfundedDefinedBenefitPensionPlanMember2022-01-012022-12-310001131399gsk:UnfundedDefinedBenefitPensionPlanMember2021-01-012021-12-310001131399gsk:DefinedBenefitSchemesMember2023-01-012023-12-310001131399gsk:DefinedBenefitSchemesMember2022-01-012022-12-310001131399gsk:DefinedBenefitSchemesMember2021-01-012021-12-310001131399gsk:DefinedContributionPensionSchemeMember2023-01-012023-12-310001131399gsk:DefinedContributionPensionSchemeMember2022-01-012022-12-310001131399gsk:DefinedContributionPensionSchemeMember2021-01-012021-12-310001131399gsk:DefinedBenefitPensionAndPostretirementHealthcareSchemeMember2023-01-012023-12-310001131399gsk:DefinedBenefitPensionAndPostretirementHealthcareSchemeMember2022-01-012022-12-310001131399gsk:DefinedBenefitPensionAndPostretirementHealthcareSchemeMember2021-01-012021-12-310001131399gsk:CurrentPeriodMembercountry:GB2023-01-012023-12-310001131399country:USgsk:CurrentPeriodMember2023-01-012023-12-310001131399gsk:ProjectedPeriodMembercountry:GB2023-01-012023-12-310001131399gsk:ProjectedPeriodMembercountry:US2023-01-012023-12-310001131399country:US2023-01-012023-12-310001131399gsk:UKDefinedBenefitPlansMember2022-04-012022-04-010001131399country:GB2021-12-310001131399gsk:RestOfWorldMember2021-12-310001131399country:US2021-12-310001131399country:US2022-01-012022-12-310001131399country:US2021-01-012021-12-310001131399gsk:RestOfWorldMember2023-01-012023-12-310001131399gsk:RestOfWorldMember2022-01-012022-12-310001131399gsk:RestOfWorldMember2021-01-012021-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMembercountry:GB2023-01-012023-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMembercountry:US2023-01-012023-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMembergsk:RestOfWorldMember2023-01-012023-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMemberifrs-full:PensionDefinedBenefitPlansMember2023-01-012023-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMembergsk:PostRetirementBenefitsMember2023-01-012023-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMembercountry:GB2022-01-012022-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMembercountry:US2022-01-012022-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMembergsk:RestOfWorldMember2022-01-012022-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMemberifrs-full:PensionDefinedBenefitPlansMember2022-01-012022-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMembergsk:PostRetirementBenefitsMember2022-01-012022-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMembercountry:GB2021-01-012021-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMembercountry:US2021-01-012021-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMembergsk:RestOfWorldMember2021-01-012021-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMemberifrs-full:PensionDefinedBenefitPlansMember2021-01-012021-12-310001131399gsk:ContinuingOperationsDefinedBenefitPensionAndPostretirementHealthcareSchemeMembergsk:PostRetirementBenefitsMember2021-01-012021-12-310001131399ifrs-full:PensionDefinedBenefitPlansMembergsk:NoncurrentAssetsOtherMember2023-12-310001131399ifrs-full:PensionDefinedBenefitPlansMembergsk:NoncurrentAssetsOtherMember2022-12-310001131399ifrs-full:PensionDefinedBenefitPlansMembergsk:NoncurrentAssetsOtherMember2021-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMemberifrs-full:PensionDefinedBenefitPlansMember2023-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMemberifrs-full:PensionDefinedBenefitPlansMember2022-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMemberifrs-full:PensionDefinedBenefitPlansMember2021-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMembergsk:PostRetirementBenefitsMember2023-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMembergsk:PostRetirementBenefitsMember2022-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMembergsk:PostRetirementBenefitsMember2021-12-310001131399gsk:GroupMember2023-12-310001131399gsk:NoncurrentAssetsOtherMembercountry:GB2023-12-310001131399country:USgsk:NoncurrentAssetsOtherMember2023-12-310001131399gsk:RestOfWorldMembergsk:NoncurrentAssetsOtherMember2023-12-310001131399gsk:GroupMembergsk:NoncurrentAssetsOtherMember2023-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMembercountry:GB2023-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMembercountry:US2023-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMembergsk:RestOfWorldMember2023-12-310001131399gsk:GroupMembergsk:PensionsAndOtherPostEmploymentBenefitsMember2023-12-310001131399gsk:GroupMember2023-01-012023-12-310001131399ifrs-full:OtherAssetsMembercountry:GB2023-12-310001131399ifrs-full:OtherAssetsMembercountry:GB2022-12-310001131399ifrs-full:OtherAssetsMembercountry:GB2021-12-310001131399gsk:GroupMember2022-12-310001131399gsk:NoncurrentAssetsOtherMembercountry:GB2022-12-310001131399country:USgsk:NoncurrentAssetsOtherMember2022-12-310001131399gsk:RestOfWorldMembergsk:NoncurrentAssetsOtherMember2022-12-310001131399gsk:GroupMembergsk:NoncurrentAssetsOtherMember2022-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMembercountry:GB2022-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMembercountry:US2022-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMembergsk:RestOfWorldMember2022-12-310001131399gsk:GroupMembergsk:PensionsAndOtherPostEmploymentBenefitsMember2022-12-310001131399gsk:GroupMember2022-01-012022-12-310001131399gsk:GroupMember2021-12-310001131399gsk:NoncurrentAssetsOtherMembercountry:GB2021-12-310001131399country:USgsk:NoncurrentAssetsOtherMember2021-12-310001131399gsk:RestOfWorldMembergsk:NoncurrentAssetsOtherMember2021-12-310001131399gsk:GroupMembergsk:NoncurrentAssetsOtherMember2021-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMembercountry:GB2021-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMembercountry:US2021-12-310001131399gsk:PensionsAndOtherPostEmploymentBenefitsMembergsk:RestOfWorldMember2021-12-310001131399gsk:GroupMembergsk:PensionsAndOtherPostEmploymentBenefitsMember2021-12-310001131399gsk:GroupMember2021-01-012021-12-310001131399ifrs-full:PlanAssetsMembercountry:GB2020-12-310001131399country:USifrs-full:PlanAssetsMember2020-12-310001131399gsk:RestOfWorldMemberifrs-full:PlanAssetsMember2020-12-310001131399ifrs-full:PensionDefinedBenefitPlansMemberifrs-full:PlanAssetsMember2020-12-310001131399ifrs-full:PlanAssetsMembergsk:PostRetirementBenefitsMember2020-12-310001131399ifrs-full:PlanAssetsMembercountry:GB2021-01-012021-12-310001131399country:USifrs-full:PlanAssetsMember2021-01-012021-12-310001131399gsk:RestOfWorldMemberifrs-full:PlanAssetsMember2021-01-012021-12-310001131399ifrs-full:PensionDefinedBenefitPlansMemberifrs-full:PlanAssetsMember2021-01-012021-12-310001131399ifrs-full:PlanAssetsMembergsk:PostRetirementBenefitsMember2021-01-012021-12-310001131399ifrs-full:PlanAssetsMembercountry:GB2021-12-310001131399country:USifrs-full:PlanAssetsMember2021-12-310001131399gsk:RestOfWorldMemberifrs-full:PlanAssetsMember2021-12-310001131399ifrs-full:PensionDefinedBenefitPlansMemberifrs-full:PlanAssetsMember2021-12-310001131399ifrs-full:PlanAssetsMembergsk:PostRetirementBenefitsMember2021-12-310001131399ifrs-full:PlanAssetsMembercountry:GB2022-01-012022-12-310001131399country:USifrs-full:PlanAssetsMember2022-01-012022-12-310001131399gsk:RestOfWorldMemberifrs-full:PlanAssetsMember2022-01-012022-12-310001131399ifrs-full:PensionDefinedBenefitPlansMemberifrs-full:PlanAssetsMember2022-01-012022-12-310001131399ifrs-full:PlanAssetsMembergsk:PostRetirementBenefitsMember2022-01-012022-12-310001131399ifrs-full:PlanAssetsMembercountry:GB2022-12-310001131399country:USifrs-full:PlanAssetsMember2022-12-310001131399gsk:RestOfWorldMemberifrs-full:PlanAssetsMember2022-12-310001131399ifrs-full:PensionDefinedBenefitPlansMemberifrs-full:PlanAssetsMember2022-12-310001131399ifrs-full:PlanAssetsMembergsk:PostRetirementBenefitsMember2022-12-310001131399ifrs-full:PlanAssetsMembercountry:GB2023-01-012023-12-310001131399country:USifrs-full:PlanAssetsMember2023-01-012023-12-310001131399gsk:RestOfWorldMemberifrs-full:PlanAssetsMember2023-01-012023-12-310001131399ifrs-full:PensionDefinedBenefitPlansMemberifrs-full:PlanAssetsMember2023-01-012023-12-310001131399ifrs-full:PlanAssetsMembergsk:PostRetirementBenefitsMember2023-01-012023-12-310001131399ifrs-full:PlanAssetsMembercountry:GB2023-12-310001131399country:USifrs-full:PlanAssetsMember2023-12-310001131399gsk:RestOfWorldMemberifrs-full:PlanAssetsMember2023-12-310001131399ifrs-full:PensionDefinedBenefitPlansMemberifrs-full:PlanAssetsMember2023-12-310001131399ifrs-full:PlanAssetsMembergsk:PostRetirementBenefitsMember2023-12-310001131399gsk:GlaxoSmithKlineConsumerHealthcareHoldingsLimitedMembergsk:UKDefinedBenefitPlansMembergsk:SLPPartneshipsMember2020-12-3100011313992020-01-012020-12-310001131399gsk:HaleonMember2022-03-012022-03-3100011313992022-03-012022-03-31gsk:partnership0001131399gsk:HaleonMember2023-12-310001131399gsk:GlaxoWelcomeMembercountry:GB2023-12-310001131399gsk:GlaxoSmithKlineConsumerHealthcareHoldingsLimitedMembergsk:UKDefinedBenefitPlansMembergsk:SLPPartneshipsMember2023-01-012023-12-310001131399gsk:UKDefinedBenefitPlansMembergsk:SLPPartneshipsMember2023-12-310001131399gsk:UKDefinedBenefitPlansMembergsk:SLPPartneshipsMember2022-12-310001131399gsk:UKDefinedBenefitPlansMembergsk:SLPPartneshipsMember2021-12-310001131399gsk:UKDefinedBenefitPlansMembergsk:SLPPartneshipsMembergsk:HaleonMember2023-12-310001131399gsk:GSKLPLtdMember2023-01-012023-12-310001131399gsk:PostRetirementBenefitsMember2022-01-012022-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:GB2020-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:US2020-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:RestOfWorldMember2020-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMemberifrs-full:PensionDefinedBenefitPlansMember2020-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:PostRetirementBenefitsMember2020-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:GB2021-01-012021-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:US2021-01-012021-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:RestOfWorldMember2021-01-012021-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMemberifrs-full:PensionDefinedBenefitPlansMember2021-01-012021-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:PostRetirementBenefitsMember2021-01-012021-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:GB2021-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:US2021-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:RestOfWorldMember2021-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMemberifrs-full:PensionDefinedBenefitPlansMember2021-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:PostRetirementBenefitsMember2021-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:GB2022-01-012022-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:US2022-01-012022-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:RestOfWorldMember2022-01-012022-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMemberifrs-full:PensionDefinedBenefitPlansMember2022-01-012022-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:PostRetirementBenefitsMember2022-01-012022-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:GB2022-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:US2022-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:RestOfWorldMember2022-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMemberifrs-full:PensionDefinedBenefitPlansMember2022-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:PostRetirementBenefitsMember2022-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:GB2023-01-012023-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:US2023-01-012023-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:RestOfWorldMember2023-01-012023-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMemberifrs-full:PensionDefinedBenefitPlansMember2023-01-012023-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:PostRetirementBenefitsMember2023-01-012023-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:GB2023-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembercountry:US2023-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:RestOfWorldMember2023-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMemberifrs-full:PensionDefinedBenefitPlansMember2023-12-310001131399ifrs-full:PresentValueOfDefinedBenefitObligationMembergsk:PostRetirementBenefitsMember2023-12-310001131399ifrs-full:WhollyOrPartlyFundedDefinedBenefitPlansMember2023-12-310001131399ifrs-full:WhollyOrPartlyFundedDefinedBenefitPlansMember2022-12-310001131399ifrs-full:WhollyOrPartlyFundedDefinedBenefitPlansMember2021-12-310001131399ifrs-full:WhollyUnfundedDefinedBenefitPlansMember2023-12-310001131399ifrs-full:WhollyUnfundedDefinedBenefitPlansMember2022-12-310001131399ifrs-full:WhollyUnfundedDefinedBenefitPlansMember2021-12-310001131399gsk:TwoThousandThirtyOneAndThereafterMembercountry:US2023-12-310001131399country:USifrs-full:WhollyUnfundedDefinedBenefitPlansMember2023-01-012023-12-310001131399country:USifrs-full:WhollyUnfundedDefinedBenefitPlansMember2022-01-012022-12-310001131399country:USifrs-full:WhollyUnfundedDefinedBenefitPlansMember2021-01-012021-12-310001131399gsk:PostRetirementBenefitsMember2023-01-012023-12-310001131399gsk:PostRetirementBenefitsMember2021-01-012021-12-310001131399gsk:DefinedBenefitObligationActiveMemberifrs-full:PensionDefinedBenefitPlansMember2023-12-310001131399gsk:DefinedBenefitObligationActiveMemberifrs-full:PensionDefinedBenefitPlansMember2022-12-310001131399gsk:DefinedBenefitObligationActiveMemberifrs-full:PensionDefinedBenefitPlansMember2021-12-310001131399gsk:DefinedBenefitObligationRetiredMemberifrs-full:PensionDefinedBenefitPlansMember2023-12-310001131399gsk:DefinedBenefitObligationRetiredMemberifrs-full:PensionDefinedBenefitPlansMember2022-12-310001131399gsk:DefinedBenefitObligationRetiredMemberifrs-full:PensionDefinedBenefitPlansMember2021-12-310001131399ifrs-full:PensionDefinedBenefitPlansMembergsk:DefinedBenefitObligationDeferredMember2023-12-310001131399ifrs-full:PensionDefinedBenefitPlansMembergsk:DefinedBenefitObligationDeferredMember2022-12-310001131399ifrs-full:PensionDefinedBenefitPlansMembergsk:DefinedBenefitObligationDeferredMember2021-12-310001131399ifrs-full:PensionDefinedBenefitPlansMember2023-12-310001131399ifrs-full:PensionDefinedBenefitPlansMember2022-12-310001131399ifrs-full:PensionDefinedBenefitPlansMember2021-12-310001131399gsk:DefinedBenefitObligationActiveMembergsk:PostRetirementBenefitsMember2023-12-310001131399gsk:DefinedBenefitObligationActiveMembergsk:PostRetirementBenefitsMember2022-12-310001131399gsk:DefinedBenefitObligationActiveMembergsk:PostRetirementBenefitsMember2021-12-310001131399gsk:DefinedBenefitObligationRetiredMembergsk:PostRetirementBenefitsMember2023-12-310001131399gsk:DefinedBenefitObligationRetiredMembergsk:PostRetirementBenefitsMember2022-12-310001131399gsk:DefinedBenefitObligationRetiredMembergsk:PostRetirementBenefitsMember2021-12-310001131399gsk:DefinedBenefitObligationDeferredMembergsk:PostRetirementBenefitsMember2023-12-310001131399gsk:DefinedBenefitObligationDeferredMembergsk:PostRetirementBenefitsMember2022-12-310001131399gsk:DefinedBenefitObligationDeferredMembergsk:PostRetirementBenefitsMember2021-12-310001131399gsk:PostRetirementBenefitsMember2023-12-310001131399gsk:PostRetirementBenefitsMember2022-12-310001131399gsk:PostRetirementBenefitsMember2021-12-31utr:Y0001131399gsk:PostRetirementBenefitMember2023-01-012023-12-310001131399gsk:PostRetirementBenefitMember2022-01-012022-12-310001131399gsk:PostRetirementBenefitMember2021-01-012021-12-310001131399gsk:ActuarialAssumptionOfIncreaseInDiscountRatesOneMember2023-01-012023-12-310001131399gsk:ActuarialAssumptionOfDecreaseInDiscountRatesOneMember2023-01-012023-12-310001131399gsk:ActuarialAssumptionOfIncreaseInDiscountRatesTwoMember2023-01-012023-12-310001131399gsk:ActuarialAssumptionOfDecreaseInDiscountRatesTwoMember2023-01-012023-12-310001131399gsk:ActuarialAssumptionOfIncreaseInExpectedRatesOfInflationOneMember2023-01-012023-12-310001131399gsk:ActuarialAssumptionOfDecreaseInExpectedRatesOfInflationOneMember2023-01-012023-12-310001131399gsk:ActuarialAssumptionOfDecreaseInExpectedRatesOfInflationTwoMember2023-01-012023-12-310001131399gsk:ActuarialAssumptionOfIncreaseInExpectedRatesOfInflationTwoMember2023-01-012023-12-310001131399gsk:ActuarialAssumptionOfLifeExpectancyRatesMember2023-01-012023-12-310001131399gsk:ActuarialAssumptionForFutureHealthcareInflationMember2023-01-012023-12-310001131399gsk:LegalAndOtherDisputesMember2022-12-310001131399ifrs-full:RestructuringProvisionMember2022-12-310001131399gsk:EmployeeRelatedProvisionsMember2022-12-310001131399gsk:OtherProvisionMember2022-12-310001131399gsk:LegalAndOtherDisputesMember2023-01-012023-12-310001131399ifrs-full:RestructuringProvisionMember2023-01-012023-12-310001131399gsk:EmployeeRelatedProvisionsMember2023-01-012023-12-310001131399gsk:OtherProvisionMember2023-01-012023-12-310001131399gsk:LegalAndOtherDisputesMember2023-12-310001131399ifrs-full:RestructuringProvisionMember2023-12-310001131399gsk:EmployeeRelatedProvisionsMember2023-12-310001131399gsk:OtherProvisionMember2023-12-31gsk:restructuringProgramme0001131399gsk:MajorRestructuringProvisionsMember2023-01-012023-12-310001131399gsk:MajorRestructuringProvisionsMember2022-01-012022-12-310001131399gsk:DisabledEmployeesMember2023-12-310001131399gsk:DisabledEmployeesMember2022-12-310001131399gsk:ShionogiViivHealthcareMember2020-12-310001131399gsk:AffinivaxMember2020-12-310001131399gsk:NovartisMember2020-12-310001131399gsk:OtherBusinessMember2020-12-310001131399gsk:ShionogiViivHealthcareMember2021-01-012021-12-310001131399gsk:AffinivaxMember2021-01-012021-12-310001131399gsk:NovartisMember2021-01-012021-12-310001131399gsk:OtherBusinessMember2021-01-012021-12-310001131399gsk:ShionogiViivHealthcareMember2021-12-310001131399gsk:AffinivaxMember2021-12-310001131399gsk:NovartisMember2021-12-310001131399gsk:OtherBusinessMember2021-12-310001131399gsk:ShionogiViivHealthcareMember2022-01-012022-12-310001131399gsk:AffinivaxMember2022-01-012022-12-310001131399gsk:NovartisMember2022-01-012022-12-310001131399gsk:OtherBusinessMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMember2022-12-310001131399gsk:AffinivaxMember2022-12-310001131399gsk:NovartisMember2022-12-310001131399gsk:OtherBusinessMember2022-12-310001131399gsk:ShionogiViivHealthcareMember2023-01-012023-12-310001131399gsk:AffinivaxMember2023-01-012023-12-310001131399gsk:NovartisMember2023-01-012023-12-310001131399gsk:OtherBusinessMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMember2023-12-310001131399gsk:AffinivaxMember2023-12-310001131399gsk:NovartisMember2023-12-310001131399gsk:OtherBusinessMember2023-12-310001131399ifrs-full:ContingentConsiderationMember2023-12-310001131399ifrs-full:ContingentConsiderationMember2022-12-310001131399gsk:CommercialisedProductsMembergsk:NovartisVaccinesMember2023-12-310001131399gsk:CommercialisedProductsMembergsk:NovartisVaccinesMember2022-12-310001131399gsk:PipelineAssetsMembergsk:NovartisVaccinesMember2023-12-310001131399gsk:PipelineAssetsMembergsk:NovartisVaccinesMember2022-12-31gsk:payment0001131399ifrs-full:ContingentConsiderationMembergsk:AffinivaxMember2023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskTenPercentageIncreaseInSalesForecastsMember2023-01-012023-12-310001131399gsk:OtherRiskTenPercentageIncreaseInSalesForecastsMembergsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskTenPercentageIncreaseInSalesForecastsMember2022-01-012022-12-310001131399gsk:OtherRiskTenPercentageIncreaseInSalesForecastsMembergsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskFifteenPercentageIncreaseInSalesForecastsMember2023-01-012023-12-310001131399gsk:OtherRiskFifteenPercentageIncreaseInSalesForecastsMembergsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskFifteenPercentageIncreaseInSalesForecastsMember2022-01-012022-12-310001131399gsk:OtherRiskFifteenPercentageIncreaseInSalesForecastsMembergsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskTenPercentageDecreaseInSalesForecastsMember2023-01-012023-12-310001131399gsk:OtherRiskTenPercentageDecreaseInSalesForecastsMembergsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskTenPercentageDecreaseInSalesForecastsMember2022-01-012022-12-310001131399gsk:OtherRiskTenPercentageDecreaseInSalesForecastsMembergsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskFifteenPercentageDecreaseInSalesForecastsMember2023-01-012023-12-310001131399gsk:OtherRiskFifteenPercentageDecreaseInSalesForecastsMembergsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskFifteenPercentageDecreaseInSalesForecastsMember2022-01-012022-12-310001131399gsk:OtherRiskFifteenPercentageDecreaseInSalesForecastsMembergsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskOnePercentageIncreaseInDiscountRateMember2023-01-012023-12-310001131399gsk:OtherRiskOnePercentageIncreaseInDiscountRateMembergsk:AffinivaxMember2023-01-012023-12-310001131399gsk:OtherRiskOnePercentageIncreaseInDiscountRateMembergsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskOnePercentageIncreaseInDiscountRateMember2022-01-012022-12-310001131399gsk:OtherRiskOnePercentageIncreaseInDiscountRateMembergsk:AffinivaxMember2022-01-012022-12-310001131399gsk:OtherRiskOnePercentageIncreaseInDiscountRateMembergsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskOnePointFivePercentageIncreaseInDiscountRateMember2023-01-012023-12-310001131399gsk:OtherRiskOnePointFivePercentageIncreaseInDiscountRateMembergsk:AffinivaxMember2023-01-012023-12-310001131399gsk:OtherRiskOnePointFivePercentageIncreaseInDiscountRateMembergsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskOnePointFivePercentageIncreaseInDiscountRateMember2022-01-012022-12-310001131399gsk:OtherRiskOnePointFivePercentageIncreaseInDiscountRateMembergsk:AffinivaxMember2022-01-012022-12-310001131399gsk:OtherRiskOnePointFivePercentageIncreaseInDiscountRateMembergsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskOnePercentageDecreaseInDiscountRateMember2023-01-012023-12-310001131399gsk:OtherRiskOnePercentageDecreaseInDiscountRateMembergsk:AffinivaxMember2023-01-012023-12-310001131399gsk:OtherRiskOnePercentageDecreaseInDiscountRateMembergsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskOnePercentageDecreaseInDiscountRateMember2022-01-012022-12-310001131399gsk:OtherRiskOnePercentageDecreaseInDiscountRateMembergsk:AffinivaxMember2022-01-012022-12-310001131399gsk:OtherRiskOnePercentageDecreaseInDiscountRateMembergsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskOnePointFivePercentageDecreaseInDiscountRateMember2023-01-012023-12-310001131399gsk:AffinivaxMembergsk:OtherRiskOnePointFivePercentageDecreaseInDiscountRateMember2023-01-012023-12-310001131399gsk:NovartisVaccinesMembergsk:OtherRiskOnePointFivePercentageDecreaseInDiscountRateMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:OtherRiskOnePointFivePercentageDecreaseInDiscountRateMember2022-01-012022-12-310001131399gsk:AffinivaxMembergsk:OtherRiskOnePointFivePercentageDecreaseInDiscountRateMember2022-01-012022-12-310001131399gsk:NovartisVaccinesMembergsk:OtherRiskOnePointFivePercentageDecreaseInDiscountRateMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskTenCentAppreciationOfUSDollarMember2023-01-012023-12-310001131399gsk:AffinivaxMembergsk:CurrencyRiskTenCentAppreciationOfUSDollarMember2023-01-012023-12-310001131399gsk:CurrencyRiskTenCentAppreciationOfUSDollarMembergsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskTenCentAppreciationOfUSDollarMember2022-01-012022-12-310001131399gsk:AffinivaxMembergsk:CurrencyRiskTenCentAppreciationOfUSDollarMember2022-01-012022-12-310001131399gsk:CurrencyRiskTenCentAppreciationOfUSDollarMembergsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskFifteenCentAppreciationOfUsDollarMember2023-01-012023-12-310001131399gsk:AffinivaxMembergsk:CurrencyRiskFifteenCentAppreciationOfUsDollarMember2023-01-012023-12-310001131399gsk:NovartisVaccinesMembergsk:CurrencyRiskFifteenCentAppreciationOfUsDollarMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskFifteenCentAppreciationOfUsDollarMember2022-01-012022-12-310001131399gsk:AffinivaxMembergsk:CurrencyRiskFifteenCentAppreciationOfUsDollarMember2022-01-012022-12-310001131399gsk:NovartisVaccinesMembergsk:CurrencyRiskFifteenCentAppreciationOfUsDollarMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskTenCentDepreciationOfUSDollarMember2023-01-012023-12-310001131399gsk:AffinivaxMembergsk:CurrencyRiskTenCentDepreciationOfUSDollarMember2023-01-012023-12-310001131399gsk:CurrencyRiskTenCentDepreciationOfUSDollarMembergsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskTenCentDepreciationOfUSDollarMember2022-01-012022-12-310001131399gsk:AffinivaxMembergsk:CurrencyRiskTenCentDepreciationOfUSDollarMember2022-01-012022-12-310001131399gsk:CurrencyRiskTenCentDepreciationOfUSDollarMembergsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskFifteenCentDepreciationOfUsDollarMember2023-01-012023-12-310001131399gsk:CurrencyRiskFifteenCentDepreciationOfUsDollarMembergsk:AffinivaxMember2023-01-012023-12-310001131399gsk:CurrencyRiskFifteenCentDepreciationOfUsDollarMembergsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskFifteenCentDepreciationOfUsDollarMember2022-01-012022-12-310001131399gsk:CurrencyRiskFifteenCentDepreciationOfUsDollarMembergsk:AffinivaxMember2022-01-012022-12-310001131399gsk:CurrencyRiskFifteenCentDepreciationOfUsDollarMembergsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskTenCentAppreciationOfEuroMember2023-01-012023-12-310001131399gsk:CurrencyRiskTenCentAppreciationOfEuroMembergsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskTenCentAppreciationOfEuroMember2022-01-012022-12-310001131399gsk:CurrencyRiskTenCentAppreciationOfEuroMembergsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskFifteenCentAppreciationOfEuroMember2023-01-012023-12-310001131399gsk:NovartisVaccinesMembergsk:CurrencyRiskFifteenCentAppreciationOfEuroMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskFifteenCentAppreciationOfEuroMember2022-01-012022-12-310001131399gsk:NovartisVaccinesMembergsk:CurrencyRiskFifteenCentAppreciationOfEuroMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskTenCentDepreciationOfEuroMember2023-01-012023-12-310001131399gsk:CurrencyRiskTenCentDepreciationOfEuroMembergsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskTenCentDepreciationOfEuroMember2022-01-012022-12-310001131399gsk:CurrencyRiskTenCentDepreciationOfEuroMembergsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskFifteenCentDepreciationOfEuroMember2023-01-012023-12-310001131399gsk:CurrencyRiskFifteenCentDepreciationOfEuroMembergsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:ShionogiViivHealthcareMembergsk:CurrencyRiskFifteenCentDepreciationOfEuroMember2022-01-012022-12-310001131399gsk:CurrencyRiskFifteenCentDepreciationOfEuroMembergsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:NovartisVaccinesMember2023-01-012023-12-310001131399gsk:NovartisVaccinesMember2022-01-012022-12-310001131399gsk:CarbonRemovalProjectsMember2023-12-310001131399gsk:LowerCarbonPropellantMember2023-12-310001131399ifrs-full:OrdinarySharesMembergsk:ShareConsolidationAsAResultOfConsumerHealthcareBusinessDemergerMember2022-07-180001131399ifrs-full:OrdinarySharesMemberifrs-full:IssuedCapitalMember2020-12-310001131399ifrs-full:OrdinarySharesMemberifrs-full:IssuedCapitalMember2021-01-012021-12-310001131399ifrs-full:OrdinarySharesMemberifrs-full:IssuedCapitalMember2021-12-310001131399ifrs-full:OrdinarySharesMemberifrs-full:IssuedCapitalMember2022-01-012022-12-310001131399ifrs-full:OrdinarySharesMemberifrs-full:IssuedCapitalMember2022-12-310001131399ifrs-full:OrdinarySharesMemberifrs-full:IssuedCapitalMember2023-01-012023-12-310001131399ifrs-full:OrdinarySharesMemberifrs-full:IssuedCapitalMember2023-12-310001131399gsk:EmployeeShareOwnershipPlanMember2023-12-310001131399gsk:EmployeeShareOwnershipPlanHeldForFutureExerciseMember2023-12-310001131399gsk:ExecutiveSupplementalSavingsPlanMember2023-12-310001131399ifrs-full:TreasurySharesMember2023-12-310001131399gsk:FreeIssueMember2023-12-310001131399gsk:AssociatesAndJointVenturesMember2023-12-310001131399gsk:AssociatesAndJointVenturesMember2022-12-310001131399gsk:AssociatesAndJointVenturesMember2021-12-310001131399gsk:FairValueReserveMember2020-12-310001131399gsk:FairValueReserveMember2021-01-012021-12-310001131399gsk:FairValueReserveMember2021-12-310001131399gsk:FairValueReserveMember2022-01-012022-12-310001131399gsk:FairValueReserveMember2022-12-310001131399gsk:FairValueReserveMember2023-01-012023-12-310001131399gsk:FairValueReserveMember2023-12-310001131399ifrs-full:DiscontinuedOperationsMembergsk:DemergerMembergsk:ConsumerHealthcareBusinessMember2023-01-012023-12-310001131399gsk:EsopTrustMember2020-12-310001131399ifrs-full:ReserveOfCashFlowHedgesMember2020-12-310001131399ifrs-full:MiscellaneousOtherReservesMember2020-12-310001131399gsk:EsopTrustMember2021-01-012021-12-310001131399ifrs-full:ReserveOfCashFlowHedgesMember2021-01-012021-12-310001131399ifrs-full:MiscellaneousOtherReservesMember2021-01-012021-12-310001131399gsk:EsopTrustMember2021-12-310001131399ifrs-full:ReserveOfCashFlowHedgesMember2021-12-310001131399ifrs-full:MiscellaneousOtherReservesMember2021-12-310001131399gsk:EsopTrustMember2022-01-012022-12-310001131399ifrs-full:ReserveOfCashFlowHedgesMember2022-01-012022-12-310001131399ifrs-full:MiscellaneousOtherReservesMember2022-01-012022-12-310001131399gsk:EsopTrustMember2022-12-310001131399ifrs-full:ReserveOfCashFlowHedgesMember2022-12-310001131399ifrs-full:MiscellaneousOtherReservesMember2022-12-310001131399gsk:EsopTrustMember2023-01-012023-12-310001131399ifrs-full:ReserveOfCashFlowHedgesMember2023-01-012023-12-310001131399ifrs-full:MiscellaneousOtherReservesMember2023-01-012023-12-310001131399gsk:EsopTrustMember2023-12-310001131399ifrs-full:ReserveOfCashFlowHedgesMember2023-12-310001131399ifrs-full:MiscellaneousOtherReservesMember2023-12-310001131399gsk:NonDistributableMergerAndPreMergerReservesMember2023-12-310001131399gsk:NonDistributableMergerAndPreMergerReservesMember2022-12-310001131399gsk:NonDistributableMergerAndPreMergerReservesMember2021-12-310001131399ifrs-full:CapitalRedemptionReserveMember2023-12-310001131399ifrs-full:CapitalRedemptionReserveMember2022-12-310001131399ifrs-full:CapitalRedemptionReserveMember2021-12-310001131399gsk:ViiVHealthCareMember2023-01-012023-12-310001131399gsk:ViiVHealthCareMember2022-01-012022-12-310001131399gsk:ViiVHealthCareMember2021-01-012021-12-310001131399gsk:ViiVHealthCareMember2023-12-310001131399gsk:ViiVHealthCareMember2022-12-310001131399gsk:ViiVHealthCareMember2021-12-310001131399gsk:IndexVenturesMember2023-01-012023-12-310001131399gsk:IndexVenturesMember2022-01-012022-12-310001131399gsk:MedicxiVenturesILimitedPartnerMember2023-01-012023-12-310001131399gsk:MedicxiVenturesILimitedPartnerMember2022-01-012022-12-310001131399gsk:QuraTherapeuticsLLCMemberifrs-full:TopOfRangeMember2023-12-012023-12-310001131399gsk:BellusMember2023-04-18iso4217:USDxbrli:shares0001131399gsk:TwoThousandTwentyThreeAcquisitionMember2023-12-310001131399ifrs-full:IntangibleAssetsMaterialToEntityMembergsk:AffinivaxIncMember2023-01-012023-12-310001131399ifrs-full:GoodwillMembergsk:AffinivaxIncMember2023-01-012023-12-310001131399gsk:DeferredTaxationMembergsk:AffinivaxIncMember2023-01-012023-12-310001131399ifrs-full:BusinessCombinationsMember2023-01-012023-12-310001131399gsk:TwoThousandAndTwentyThreeBusinessDisposalsMember2023-01-012023-12-310001131399gsk:SierraOncologyIncMember2022-07-010001131399gsk:SierraOncologyIncMember2022-07-012022-07-010001131399gsk:AffinivaxIncMember2022-08-150001131399gsk:AffinivaxIncMember2022-08-152022-08-150001131399gsk:SierraOncologyIncMember2022-01-012022-12-310001131399gsk:AffinivaxIncMember2022-01-012022-12-310001131399gsk:SierraOncologyMembergsk:TwoThousandTwentyTwoAcquisitionMember2022-12-310001131399gsk:AffinivaxMembergsk:TwoThousandTwentyTwoAcquisitionMember2022-12-310001131399gsk:TwoThousandTwentyTwoAcquisitionMember2022-12-310001131399gsk:GlaxoSaudiArabiaLimitedMember2022-11-24iso4217:SAR0001131399gsk:HaleonPlcMembergsk:GskAggregateHoldingMember2022-07-182022-07-180001131399gsk:ConsumerHealthcareBusinessMember2022-07-182022-07-180001131399gsk:HaleonPlcMembergsk:GskShareholdersMember2022-07-182022-07-180001131399gsk:HaleonPlcMembergsk:GskAndGsksConsolidatedEsotTrustsMember2022-07-182022-07-180001131399gsk:HaleonPlcMembergsk:ScottishLimitedPartnershipsMember2022-07-182022-07-180001131399gsk:HaleonPlcMembergsk:PfizerMember2022-07-182022-07-180001131399gsk:GskHoldingMembergsk:ConsumerHealthcareBusinessMember2022-07-182022-07-1800011313992022-07-180001131399gsk:HaleonPlcMembergsk:GskAggregateHoldingMember2022-07-180001131399gsk:HaleonPlcMembergsk:GskHoldingMember2022-07-182022-07-180001131399gsk:ConsumerHealthcareBusinessMember2022-07-1800011313992022-07-182022-07-180001131399ifrs-full:DiscontinuedOperationsMember2022-01-012022-12-310001131399ifrs-full:DiscontinuedOperationsMember2021-01-012021-12-310001131399ifrs-full:BusinessCombinationsMembergsk:CashFlowsInTwoThousandAndTwentyTwoMember2022-01-012022-12-310001131399gsk:TwoThousandAndTwentyTwoDisposalsDemergerMemberifrs-full:BusinessCombinationsMember2022-01-012022-12-310001131399ifrs-full:BusinessCombinationsMembergsk:TwoThousandTwentyTwoBusinessDisposalsMember2022-01-012022-12-310001131399gsk:TwoThousandAndTwentyTwoBusinessAcquisitionsMember2023-01-012023-12-310001131399ifrs-full:BusinessCombinationsMember2022-01-012022-12-310001131399gsk:TwoThousandAndTwentyOneBusinessDisposalsMemberifrs-full:BusinessCombinationsMember2022-01-012022-12-310001131399gsk:TwoThousandAndTwentyOneBusinessDisposalsMember2021-01-012021-12-310001131399gsk:TwoThousandAndTwentyOneBusinessDisposalsMember2021-12-310001131399gsk:InnovivaIncMemberifrs-full:BusinessCombinationsMembergsk:AssociatesAndJointVenturesTwoThousandAndTwentyOneDisposalsMember2021-05-202021-05-200001131399gsk:InnovivaIncMemberifrs-full:BusinessCombinationsMembergsk:AssociatesAndJointVenturesTwoThousandAndTwentyOneDisposalsMember2021-05-200001131399gsk:TwoThousandAndTwentyOneBusinessDisposalsMember2021-01-012021-12-310001131399gsk:AssociatesAndJointVenturesTwoThousandAndTwentyOneDisposalsMember2021-01-012021-12-310001131399gsk:LiquidInvestmentsMember2022-12-310001131399gsk:LiquidInvestmentsMember2023-01-012023-12-310001131399gsk:LiquidInvestmentsMember2023-12-310001131399gsk:CashAndCashEquivalentMember2022-12-310001131399gsk:CashAndCashEquivalentMember2023-01-012023-12-310001131399gsk:CashAndCashEquivalentMember2023-12-310001131399gsk:BankOverdrafts1Member2022-12-310001131399gsk:BankOverdrafts1Member2023-01-012023-12-310001131399gsk:BankOverdrafts1Member2023-12-310001131399gsk:LiquidInvestmentsCashAndCashEquivalentsMember2022-12-310001131399gsk:LiquidInvestmentsCashAndCashEquivalentsMember2023-01-012023-12-310001131399gsk:LiquidInvestmentsCashAndCashEquivalentsMember2023-12-310001131399ifrs-full:ShorttermBorrowingsMembergsk:CommercialPaperDebtMember2022-12-310001131399ifrs-full:ShorttermBorrowingsMembergsk:CommercialPaperDebtMember2023-01-012023-12-310001131399ifrs-full:ShorttermBorrowingsMembergsk:CommercialPaperDebtMember2023-12-310001131399ifrs-full:ShorttermBorrowingsMembergsk:EuropeanUsMediumTermNotesAndBankFacilitiesMember2022-12-310001131399ifrs-full:ShorttermBorrowingsMembergsk:EuropeanUsMediumTermNotesAndBankFacilitiesMember2023-01-012023-12-310001131399ifrs-full:ShorttermBorrowingsMembergsk:EuropeanUsMediumTermNotesAndBankFacilitiesMember2023-12-310001131399ifrs-full:ShorttermBorrowingsMemberifrs-full:LeaseLiabilitiesMember2022-12-310001131399ifrs-full:ShorttermBorrowingsMemberifrs-full:LeaseLiabilitiesMember2023-01-012023-12-310001131399ifrs-full:ShorttermBorrowingsMemberifrs-full:LeaseLiabilitiesMember2023-12-310001131399gsk:OtherCurrentDebtMemberifrs-full:ShorttermBorrowingsMember2022-12-310001131399gsk:OtherCurrentDebtMemberifrs-full:ShorttermBorrowingsMember2023-01-012023-12-310001131399gsk:OtherCurrentDebtMemberifrs-full:ShorttermBorrowingsMember2023-12-310001131399ifrs-full:ShorttermBorrowingsMember2022-12-310001131399ifrs-full:ShorttermBorrowingsMember2023-01-012023-12-310001131399ifrs-full:ShorttermBorrowingsMember2023-12-310001131399gsk:EuropeanUsMediumTermNotesAndBankFacilitiesMemberifrs-full:LongtermBorrowingsMember2022-12-310001131399gsk:EuropeanUsMediumTermNotesAndBankFacilitiesMemberifrs-full:LongtermBorrowingsMember2023-01-012023-12-310001131399gsk:EuropeanUsMediumTermNotesAndBankFacilitiesMemberifrs-full:LongtermBorrowingsMember2023-12-310001131399ifrs-full:LongtermBorrowingsMemberifrs-full:LeaseLiabilitiesMember2022-12-310001131399ifrs-full:LongtermBorrowingsMemberifrs-full:LeaseLiabilitiesMember2023-01-012023-12-310001131399ifrs-full:LongtermBorrowingsMemberifrs-full:LeaseLiabilitiesMember2023-12-310001131399ifrs-full:LongtermBorrowingsMember2022-12-310001131399ifrs-full:LongtermBorrowingsMember2023-01-012023-12-310001131399ifrs-full:LongtermBorrowingsMember2023-12-310001131399gsk:NetDebtMember2022-12-310001131399gsk:NetDebtMember2023-01-012023-12-310001131399gsk:NetDebtMember2023-12-310001131399gsk:DerivativeFinancialInstrumentsMembergsk:AccruedInterestMember2022-12-310001131399gsk:DerivativeFinancialInstrumentsMembergsk:AccruedInterestMember2023-01-012023-12-310001131399gsk:DerivativeFinancialInstrumentsMembergsk:AccruedInterestMember2023-12-310001131399gsk:DerivativeFinancialInstrumentsMemberifrs-full:AssetsHeldToHedgeLiabilitiesArisingFromFinancingActivitiesMember2022-12-310001131399gsk:DerivativeFinancialInstrumentsMemberifrs-full:AssetsHeldToHedgeLiabilitiesArisingFromFinancingActivitiesMember2023-01-012023-12-310001131399gsk:DerivativeFinancialInstrumentsMemberifrs-full:AssetsHeldToHedgeLiabilitiesArisingFromFinancingActivitiesMember2023-12-310001131399gsk:DerivativeFinancialInstrumentsMember2022-12-310001131399gsk:DerivativeFinancialInstrumentsMember2023-01-012023-12-310001131399gsk:DerivativeFinancialInstrumentsMember2023-12-310001131399gsk:LiquidInvestmentsMember2021-12-310001131399gsk:LiquidInvestmentsMember2022-01-012022-12-310001131399gsk:CashAndCashEquivalentMember2021-12-310001131399gsk:CashAndCashEquivalentMember2022-01-012022-12-310001131399gsk:BankOverdrafts1Member2021-12-310001131399gsk:BankOverdrafts1Member2022-01-012022-12-310001131399gsk:LiquidInvestmentsAttributedToContinuingOperationsMember2021-12-310001131399gsk:LiquidInvestmentsAttributedToContinuingOperationsMember2022-01-012022-12-310001131399gsk:LiquidInvestmentsAttributedToContinuingOperationsMember2022-12-310001131399gsk:LiquidInvestmentsAttributedToDiscontinuedOperationsMember2021-12-310001131399gsk:LiquidInvestmentsAttributedToDiscontinuedOperationsMember2022-01-012022-12-310001131399gsk:LiquidInvestmentsAttributedToDiscontinuedOperationsMember2022-12-310001131399gsk:LiquidInvestmentsCashAndCashEquivalentsMember2021-12-310001131399gsk:LiquidInvestmentsCashAndCashEquivalentsMember2022-01-012022-12-310001131399ifrs-full:ShorttermBorrowingsMembergsk:CommercialPaperDebtMember2021-12-310001131399ifrs-full:ShorttermBorrowingsMembergsk:CommercialPaperDebtMember2022-01-012022-12-310001131399ifrs-full:ShorttermBorrowingsMembergsk:EuropeanUsMediumTermNotesAndBankFacilitiesMember2021-12-310001131399ifrs-full:ShorttermBorrowingsMembergsk:EuropeanUsMediumTermNotesAndBankFacilitiesMember2022-01-012022-12-310001131399ifrs-full:ShorttermBorrowingsMemberifrs-full:LeaseLiabilitiesMember2021-12-310001131399ifrs-full:ShorttermBorrowingsMemberifrs-full:LeaseLiabilitiesMember2022-01-012022-12-310001131399gsk:OtherCurrentDebtMemberifrs-full:ShorttermBorrowingsMember2021-12-310001131399gsk:OtherCurrentDebtMemberifrs-full:ShorttermBorrowingsMember2022-01-012022-12-310001131399gsk:ShortTermBorrowingsAttributedToContinuingOperationsMemberifrs-full:ShorttermBorrowingsMember2021-12-310001131399gsk:ShortTermBorrowingsAttributedToContinuingOperationsMemberifrs-full:ShorttermBorrowingsMember2022-01-012022-12-310001131399gsk:ShortTermBorrowingsAttributedToContinuingOperationsMemberifrs-full:ShorttermBorrowingsMember2022-12-310001131399ifrs-full:ShorttermBorrowingsMembergsk:ShortTermBorrowingsAttributedToDiscontinuedOperationsMember2021-12-310001131399ifrs-full:ShorttermBorrowingsMembergsk:ShortTermBorrowingsAttributedToDiscontinuedOperationsMember2022-01-012022-12-310001131399ifrs-full:ShorttermBorrowingsMembergsk:ShortTermBorrowingsAttributedToDiscontinuedOperationsMember2022-12-310001131399ifrs-full:ShorttermBorrowingsMember2021-12-310001131399ifrs-full:ShorttermBorrowingsMember2022-01-012022-12-310001131399gsk:EuropeanUsMediumTermNotesAndBankFacilitiesMemberifrs-full:LongtermBorrowingsMember2021-12-310001131399gsk:EuropeanUsMediumTermNotesAndBankFacilitiesMemberifrs-full:LongtermBorrowingsMember2022-01-012022-12-310001131399ifrs-full:LongtermBorrowingsMemberifrs-full:LeaseLiabilitiesMember2021-12-310001131399ifrs-full:LongtermBorrowingsMemberifrs-full:LeaseLiabilitiesMember2022-01-012022-12-310001131399gsk:LongTermBorrowingsContinuingOperationsMemberifrs-full:LongtermBorrowingsMember2021-12-310001131399gsk:LongTermBorrowingsContinuingOperationsMemberifrs-full:LongtermBorrowingsMember2022-01-012022-12-310001131399gsk:LongTermBorrowingsContinuingOperationsMemberifrs-full:LongtermBorrowingsMember2022-12-310001131399ifrs-full:LongtermBorrowingsMembergsk:LongTermBorrowingsDiscontinuedOperationsMember2021-12-310001131399ifrs-full:LongtermBorrowingsMembergsk:LongTermBorrowingsDiscontinuedOperationsMember2022-01-012022-12-310001131399ifrs-full:LongtermBorrowingsMembergsk:LongTermBorrowingsDiscontinuedOperationsMember2022-12-310001131399ifrs-full:LongtermBorrowingsMember2021-12-310001131399ifrs-full:LongtermBorrowingsMember2022-01-012022-12-310001131399gsk:NetDebtMember2021-12-310001131399gsk:NetDebtMember2022-01-012022-12-310001131399gsk:DerivativeFinancialInstrumentsMembergsk:AccruedInterestMember2021-12-310001131399gsk:DerivativeFinancialInstrumentsMembergsk:AccruedInterestMember2022-01-012022-12-310001131399gsk:DerivativeFinancialInstrumentsMemberifrs-full:AssetsHeldToHedgeLiabilitiesArisingFromFinancingActivitiesMember2021-12-310001131399gsk:DerivativeFinancialInstrumentsMemberifrs-full:AssetsHeldToHedgeLiabilitiesArisingFromFinancingActivitiesMember2022-01-012022-12-310001131399gsk:DerivativeFinancialInstrumentsMember2021-12-310001131399gsk:DerivativeFinancialInstrumentsMember2022-01-012022-12-310001131399ifrs-full:LiquidityRiskMember2023-12-310001131399ifrs-full:LiquidityRiskMembergsk:UsCommercialPaperProgramMember2023-12-310001131399ifrs-full:LiquidityRiskMembergsk:UsCommercialPaperProgramMember2022-12-310001131399ifrs-full:LiquidityRiskMembergsk:EuroCommercialPaperProgrammeMember2023-12-310001131399ifrs-full:LiquidityRiskMembergsk:EuroCommercialPaperProgrammeMember2022-12-310001131399ifrs-full:CreditRiskMember2023-12-310001131399ifrs-full:CreditRiskMember2022-12-310001131399ifrs-full:CreditRiskMembergsk:WholesalerInTheUsMemberifrs-full:ExternalCreditGradesMember2023-12-310001131399ifrs-full:CreditRiskMembergsk:WholesalerInTheUsMemberifrs-full:ExternalCreditGradesMember2022-12-310001131399ifrs-full:CreditRiskMembergsk:LowerThanBBBBaa3Member2023-12-310001131399ifrs-full:CreditRiskMembergsk:LowerThanBBBBaa3Member2022-12-310001131399gsk:SaudiBritishBankMembercountry:SA2023-12-310001131399gsk:HalkBankMembercountry:GB2023-12-310001131399country:NGgsk:UnitedBankForAfricaZenithBankAccessBankAndStanbicIbtcBankMember2023-12-310001131399country:BRgsk:BancoBradescoItauUniBancoBancoDoBrasilAndCaixaEconomicaFederalMember2023-12-310001131399gsk:BancoDeLaProduccionMembercountry:EC2023-12-310001131399ifrs-full:CreditRiskMembergsk:BbbOrBaaMember2023-12-310001131399gsk:BBB-OrBaaThreeRatedMemberifrs-full:CreditRiskMember2023-12-310001131399gsk:StateBankOfIndiaMembergsk:BBB-OrBaaThreeRatedMemberifrs-full:CreditRiskMember2023-12-310001131399ifrs-full:CreditRiskMembergsk:AAAOrAaaMember2023-12-310001131399ifrs-full:CreditRiskMembergsk:AaOrAaMember2023-12-310001131399ifrs-full:CreditRiskMembergsk:AorAMember2023-12-310001131399ifrs-full:CreditRiskMembergsk:BBPlusOrBaOneMember2023-12-310001131399ifrs-full:CreditRiskMembergsk:AAAOrAaaMember2022-12-310001131399ifrs-full:CreditRiskMembergsk:AaOrAaMember2022-12-310001131399ifrs-full:CreditRiskMembergsk:AorAMember2022-12-310001131399ifrs-full:CreditRiskMembergsk:BbbOrBaaMember2022-12-310001131399ifrs-full:CreditRiskMembergsk:BBPlusOrBaOneMember2022-12-310001131399ifrs-full:NotLaterThanThreeMonthsMemberifrs-full:CreditRiskMember2023-12-310001131399ifrs-full:NotLaterThanThreeMonthsMemberifrs-full:CreditRiskMembergsk:ViiVHealthCareMember2023-12-310001131399ifrs-full:NotLaterThanThreeMonthsMemberifrs-full:CreditRiskMembergsk:ViiVHealthCareMember2023-01-012023-12-310001131399gsk:NonUsaMemberifrs-full:TopOfRangeMembergsk:WholesaleAndRetailCreditRiskMember2023-01-012023-12-310001131399country:USgsk:WholesaleAndRetailCreditRiskMembergsk:InLineWithOtherPharmaceuticalCompaniesMember2023-01-012023-12-310001131399country:USgsk:WholesaleAndRetailCreditRiskMembergsk:InLineWithOtherPharmaceuticalCompaniesMember2022-01-012022-12-310001131399gsk:DueFromThreeWholesalersMembergsk:WholesaleAndRetailCreditRiskMember2023-12-310001131399gsk:DueFromThreeWholesalersMembercountry:USgsk:WholesaleAndRetailCreditRiskMember2023-01-012023-12-310001131399gsk:DueFromThreeWholesalersMembergsk:WholesaleAndRetailCreditRiskMember2022-12-310001131399gsk:DueFromThreeWholesalersMembercountry:USgsk:WholesaleAndRetailCreditRiskMember2022-01-012022-12-310001131399ifrs-full:CreditRiskMembergsk:GroupCreditEnhancementsAndCreditInsuranceMember2023-12-310001131399ifrs-full:CreditRiskMembergsk:GroupCreditEnhancementsAndCreditInsuranceMember2022-12-310001131399ifrs-full:FinancialAssetsAtAmortisedCostCategoryMembergsk:NoncurrentAssetsOtherMember2023-12-310001131399ifrs-full:FinancialAssetsAtAmortisedCostCategoryMembergsk:NoncurrentAssetsOtherMember2022-12-310001131399gsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2023-12-310001131399gsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2022-12-310001131399ifrs-full:FinancialAssetsAtAmortisedCostCategoryMembergsk:LiquidInvestmentsMember2023-12-310001131399ifrs-full:FinancialAssetsAtAmortisedCostCategoryMembergsk:LiquidInvestmentsMember2022-12-310001131399gsk:CashAndCashEquivalentsFinancialAssetsMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2023-12-310001131399gsk:CashAndCashEquivalentsFinancialAssetsMemberifrs-full:FinancialAssetsAtAmortisedCostCategoryMember2022-12-310001131399gsk:OtherFinancialInvestmentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310001131399gsk:OtherFinancialInvestmentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2022-12-310001131399gsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310001131399gsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2022-12-310001131399ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:CurrentEquityAndOtherInvestmentsMember2023-12-310001131399ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:CurrentEquityAndOtherInvestmentsMember2022-12-310001131399gsk:NoncurrentAssetsOtherMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399gsk:NoncurrentAssetsOtherMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399gsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399gsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2023-12-310001131399ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2022-12-310001131399gsk:CashAndCashEquivalentsFinancialAssetsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399gsk:CashAndCashEquivalentsFinancialAssetsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399gsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMember2023-12-310001131399gsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMember2022-12-310001131399ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembergsk:BondsInDesignatedHedgingRelationshipMember2023-12-310001131399ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembergsk:BondsInDesignatedHedgingRelationshipMember2022-12-310001131399ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembergsk:OtherBondsMember2023-12-310001131399ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembergsk:OtherBondsMember2022-12-310001131399ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembergsk:BankLoansAndOverdraftsMember2023-12-310001131399ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembergsk:BankLoansAndOverdraftsMember2022-12-310001131399ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembergsk:CommercialPaperInADesignatedHedgingRelationshipMember2023-12-310001131399ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMembergsk:CommercialPaperInADesignatedHedgingRelationshipMember2022-12-310001131399gsk:OtherCommercialPaperMemberifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMember2023-12-310001131399gsk:OtherCommercialPaperMemberifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMember2022-12-310001131399gsk:OtherBorrowingMemberifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMember2023-12-310001131399gsk:OtherBorrowingMemberifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMember2022-12-310001131399ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMember2023-12-310001131399ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMember2022-12-310001131399gsk:TradeAndOtherPayableMemberifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMember2023-12-310001131399gsk:TradeAndOtherPayableMemberifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMember2022-12-310001131399ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMemberifrs-full:OtherProvisionsMember2023-12-310001131399ifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMemberifrs-full:OtherProvisionsMember2022-12-310001131399gsk:OtherNonCurrentLiabilitiesMemberifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMember2023-12-310001131399gsk:OtherNonCurrentLiabilitiesMemberifrs-full:FinancialLiabilitiesAtAmortisedCostCategoryMember2022-12-310001131399gsk:ContingentConsiderationLiabilitiesMembergsk:FinancialLiabilitiesAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399gsk:ContingentConsiderationLiabilitiesMembergsk:FinancialLiabilitiesAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399gsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMembergsk:FinancialLiabilitiesAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399gsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMembergsk:FinancialLiabilitiesAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:OtherFinancialInvestmentsMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMembergsk:OtherFinancialInvestmentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:OtherFinancialInvestmentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:OtherFinancialInvestmentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:TradeAndOtherReceivableMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMembergsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:CurrentEquityAndOtherInvestmentsMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:CurrentEquityAndOtherInvestmentsMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMemberifrs-full:Level3OfFairValueHierarchyMembergsk:CurrentEquityAndOtherInvestmentsMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:CurrentEquityAndOtherInvestmentsMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:NoncurrentAssetsOtherMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:NoncurrentAssetsOtherMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:NoncurrentAssetsOtherMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:NoncurrentAssetsOtherMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:TradeAndOtherReceivableMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMembergsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:CashAndCashEquivalentsFinancialAssetsMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMembergsk:CashAndCashEquivalentsFinancialAssetsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:CashAndCashEquivalentsFinancialAssetsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:CashAndCashEquivalentsFinancialAssetsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level1OfFairValueHierarchyMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399ifrs-full:AtFairValueMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMemberifrs-full:Level1OfFairValueHierarchyMembergsk:ContingentConsiderationLiabilitiesMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:ContingentConsiderationLiabilitiesMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:ContingentConsiderationLiabilitiesMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:ContingentConsiderationLiabilitiesMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMemberifrs-full:Level1OfFairValueHierarchyMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMemberifrs-full:Level1OfFairValueHierarchyMembergsk:FinancialLiabilitiesAtFairValueThroughOtherComprehensiveIncomeMember2023-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMembergsk:FinancialLiabilitiesAtFairValueThroughOtherComprehensiveIncomeMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMembergsk:FinancialLiabilitiesAtFairValueThroughOtherComprehensiveIncomeMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMembergsk:FinancialLiabilitiesAtFairValueThroughOtherComprehensiveIncomeMember2023-12-310001131399ifrs-full:AtFairValueMembergsk:OtherFinancialInvestmentsMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMembergsk:OtherFinancialInvestmentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:OtherFinancialInvestmentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:OtherFinancialInvestmentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:TradeAndOtherReceivableMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMembergsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:OtherFinancialInvestmentsMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMembergsk:OtherFinancialInvestmentsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:OtherFinancialInvestmentsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:OtherFinancialInvestmentsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:NoncurrentAssetsOtherMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:NoncurrentAssetsOtherMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:NoncurrentAssetsOtherMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:NoncurrentAssetsOtherMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:TradeAndOtherReceivableMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMembergsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:TradeAndOtherReceivableMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:CashAndCashEquivalentsFinancialAssetsMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMembergsk:CashAndCashEquivalentsFinancialAssetsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:CashAndCashEquivalentsFinancialAssetsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:CashAndCashEquivalentsFinancialAssetsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMemberifrs-full:Level1OfFairValueHierarchyMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMemberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level1OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMemberifrs-full:Level1OfFairValueHierarchyMembergsk:ContingentConsiderationLiabilitiesMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:ContingentConsiderationLiabilitiesMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:ContingentConsiderationLiabilitiesMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:ContingentConsiderationLiabilitiesMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMemberifrs-full:Level1OfFairValueHierarchyMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:HeldForTradingDerivativesThatAreNotInADesignatedAndEffectiveHedgingRelationshipMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMemberifrs-full:Level1OfFairValueHierarchyMembergsk:FinancialLiabilitiesAtFairValueThroughOtherComprehensiveIncomeMember2022-12-310001131399ifrs-full:AtFairValueMemberifrs-full:Level2OfFairValueHierarchyMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMembergsk:FinancialLiabilitiesAtFairValueThroughOtherComprehensiveIncomeMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMembergsk:FinancialLiabilitiesAtFairValueThroughOtherComprehensiveIncomeMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:AtFairValueMembergsk:DerivativesDesignatedAndEffectiveAsHedgingInstrumentsMembergsk:FinancialLiabilitiesAtFairValueThroughOtherComprehensiveIncomeMember2022-12-310001131399ifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:Level3OfFairValueHierarchyMember2021-12-310001131399ifrs-full:Level3OfFairValueHierarchyMember2023-01-012023-12-310001131399ifrs-full:Level3OfFairValueHierarchyMember2022-01-012022-12-310001131399ifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399gsk:ShionogiViiVHealthcareJointVentureMemberifrs-full:Level3OfFairValueHierarchyMember2023-01-012023-12-310001131399gsk:ShionogiViiVHealthcareJointVentureMemberifrs-full:Level3OfFairValueHierarchyMember2022-01-012022-12-310001131399gsk:NovartisVaccinesBusinessMemberifrs-full:Level3OfFairValueHierarchyMember2023-01-012023-12-310001131399gsk:NovartisVaccinesBusinessMemberifrs-full:Level3OfFairValueHierarchyMember2022-01-012022-12-310001131399gsk:AffinivaxAcquisitionMemberifrs-full:Level3OfFairValueHierarchyMember2023-01-012023-12-310001131399gsk:AffinivaxAcquisitionMemberifrs-full:Level3OfFairValueHierarchyMember2022-01-012022-12-310001131399gsk:FinancialLiabilitiesOneMember2023-01-012023-12-310001131399gsk:FinancialLiabilitiesOneMember2022-01-012022-12-310001131399gsk:ShionogiViiVHealthcareJointVentureMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399gsk:ShionogiViiVHealthcareJointVentureMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399gsk:NovartisVaccinesBusinessMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399gsk:NovartisVaccinesBusinessMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399gsk:AffinivaxMemberifrs-full:Level3OfFairValueHierarchyMember2023-12-310001131399gsk:AffinivaxMemberifrs-full:Level3OfFairValueHierarchyMember2022-12-310001131399ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossCategoryMembergsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Member2023-12-310001131399gsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Memberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2023-12-310001131399gsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Memberifrs-full:FinancialLiabilitiesAtAmortisedCostMember2023-12-310001131399gsk:FinancialInstrumentsMembergsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Member2023-12-310001131399gsk:NonFinancialInstrumentsMembergsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Member2023-12-310001131399gsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Member2023-12-310001131399ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossCategoryMembergsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Member2022-12-310001131399gsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Memberifrs-full:FinancialAssetsAtFairValueThroughOtherComprehensiveIncomeCategoryMember2022-12-310001131399gsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Memberifrs-full:FinancialLiabilitiesAtAmortisedCostMember2022-12-310001131399gsk:FinancialInstrumentsMembergsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Member2022-12-310001131399gsk:NonFinancialInstrumentsMembergsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Member2022-12-310001131399gsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Member2022-12-310001131399gsk:AmortizedCostMembergsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Member2023-12-310001131399gsk:AmortizedCostMembergsk:TradeAndOtherReceivablesOtherNoncurrentAssetsAndAssetsHeldForSaleInScopeOfIFRS9Member2022-12-310001131399ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:TradeAndOtherPayablesOtherProvisionsAndOtherNoncurrentLiabilitiesInScopeOfIfrs9Member2023-12-310001131399gsk:TradeAndOtherPayablesOtherProvisionsAndOtherNoncurrentLiabilitiesInScopeOfIfrs9Memberifrs-full:FinancialLiabilitiesAtAmortisedCostMember2023-12-310001131399gsk:TradeAndOtherPayablesOtherProvisionsAndOtherNoncurrentLiabilitiesInScopeOfIfrs9Membergsk:FinancialInstrumentsMember2023-12-310001131399gsk:NonFinancialInstrumentsMembergsk:TradeAndOtherPayablesOtherProvisionsAndOtherNoncurrentLiabilitiesInScopeOfIfrs9Member2023-12-310001131399gsk:TradeAndOtherPayablesOtherProvisionsAndOtherNoncurrentLiabilitiesInScopeOfIfrs9Member2023-12-310001131399ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossCategoryMembergsk:TradeAndOtherPayablesOtherProvisionsAndOtherNoncurrentLiabilitiesInScopeOfIfrs9Member2022-12-310001131399gsk:TradeAndOtherPayablesOtherProvisionsAndOtherNoncurrentLiabilitiesInScopeOfIfrs9Memberifrs-full:FinancialLiabilitiesAtAmortisedCostMember2022-12-310001131399gsk:TradeAndOtherPayablesOtherProvisionsAndOtherNoncurrentLiabilitiesInScopeOfIfrs9Membergsk:FinancialInstrumentsMember2022-12-310001131399gsk:NonFinancialInstrumentsMembergsk:TradeAndOtherPayablesOtherProvisionsAndOtherNoncurrentLiabilitiesInScopeOfIfrs9Member2022-12-310001131399gsk:TradeAndOtherPayablesOtherProvisionsAndOtherNoncurrentLiabilitiesInScopeOfIfrs9Member2022-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:LaterThanOneYearMemberifrs-full:CashFlowHedgesMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:LaterThanOneYearMemberifrs-full:CashFlowHedgesMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossDesignatedUponInitialRecognitionCategoryMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2023-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossDesignatedUponInitialRecognitionCategoryMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2022-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:LaterThanOneYearMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:NetInvestmentHedgesMember2023-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:LaterThanOneYearMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:NetInvestmentHedgesMember2022-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:NetInvestmentHedgesMember2023-12-310001131399ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossDesignatedUponInitialRecognitionCategoryMembergsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2023-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMembergsk:NetInvestmentHedgesMember2022-12-310001131399ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossDesignatedUponInitialRecognitionCategoryMembergsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2022-12-310001131399ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2023-12-310001131399ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossDesignatedUponInitialRecognitionCategoryMember2023-12-310001131399ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossMandatorilyMeasuredAtFairValueCategoryMember2022-12-310001131399ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossDesignatedUponInitialRecognitionCategoryMember2022-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossClassifiedAsHeldForTradingCategoryMember2023-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossClassifiedAsHeldForTradingCategoryMember2022-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossThatMeetDefinitionOfHeldForTradingCategoryMember2023-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossThatMeetDefinitionOfHeldForTradingCategoryMember2022-12-310001131399gsk:EmbeddedAndOtherDerivativesMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossClassifiedAsHeldForTradingCategoryMember2023-12-310001131399gsk:EmbeddedAndOtherDerivativesMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossThatMeetDefinitionOfHeldForTradingCategoryMember2023-12-310001131399gsk:EmbeddedAndOtherDerivativesMemberifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossClassifiedAsHeldForTradingCategoryMember2022-12-310001131399gsk:EmbeddedAndOtherDerivativesMemberifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossThatMeetDefinitionOfHeldForTradingCategoryMember2022-12-310001131399ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossClassifiedAsHeldForTradingCategoryMember2023-12-310001131399ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossThatMeetDefinitionOfHeldForTradingCategoryMember2023-12-310001131399ifrs-full:FinancialAssetsAtFairValueThroughProfitOrLossClassifiedAsHeldForTradingCategoryMember2022-12-310001131399ifrs-full:FinancialLiabilitiesAtFairValueThroughProfitOrLossThatMeetDefinitionOfHeldForTradingCategoryMember2022-12-310001131399gsk:NetInvestmentHedgedMembergsk:BondsInDesignatedHedgingRelationshipMember2023-12-310001131399gsk:NetInvestmentHedgedMembergsk:BondsInDesignatedHedgingRelationshipMember2022-12-310001131399ifrs-full:CashFlowHedgesMembergsk:ForeignExchangeRiskMember2023-01-012023-12-310001131399ifrs-full:CashFlowHedgesMembergsk:ForeignExchangeRiskMember2022-01-012022-12-310001131399ifrs-full:HedgesOfNetInvestmentInForeignOperationsMembergsk:ForeignExchangeRiskMember2023-01-012023-12-310001131399ifrs-full:HedgesOfNetInvestmentInForeignOperationsMembergsk:ForeignExchangeRiskMember2022-01-012022-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMembergsk:BuyForeignCurrencyLessThanThreeMonthsMember2023-01-012023-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMembergsk:BuyForeignCurrencyLessThanThreeMonthsMember2023-12-310001131399gsk:BuyForeignCurrencyThreeToSixMonthsMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2023-01-012023-12-310001131399gsk:BuyForeignCurrencyThreeToSixMonthsMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2023-12-310001131399gsk:BuyForeignCurrencyOverSixMonthsMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2023-01-012023-12-310001131399gsk:BuyForeignCurrencyOverSixMonthsMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2023-12-310001131399gsk:SellForeignCurrencyLessThanThreeMonthsMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2023-01-012023-12-310001131399gsk:SellForeignCurrencyLessThanThreeMonthsMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2023-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2023-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2023-01-012023-12-310001131399gsk:SellForeignCurrencyLessThanThreeMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2023-01-012023-12-310001131399gsk:SellForeignCurrencyLessThanThreeMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2023-12-310001131399currency:JPYgsk:SellForeignCurrencyLessThanThreeMonthsMembergsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2023-01-012023-12-310001131399currency:JPYgsk:SellForeignCurrencyLessThanThreeMonthsMembergsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2023-12-310001131399gsk:SellForeignCurrencyLessThanThreeMonthsMembergsk:ForeignExchangeContractsMembercurrency:USDgsk:NetInvestmentHedgesMember2023-01-012023-12-310001131399gsk:SellForeignCurrencyLessThanThreeMonthsMembergsk:ForeignExchangeContractsMembercurrency:USDgsk:NetInvestmentHedgesMember2023-12-310001131399currency:CADgsk:ForeignExchangeContractsMembergsk:SellForeignCurrencyOverSixMonthsMembergsk:NetInvestmentHedgesMember2023-01-012023-12-310001131399currency:CADgsk:ForeignExchangeContractsMembergsk:SellForeignCurrencyOverSixMonthsMembergsk:NetInvestmentHedgesMember2023-12-310001131399currency:SGDgsk:ForeignExchangeContractsMembergsk:SellForeignCurrencyOverSixMonthsMembergsk:NetInvestmentHedgesMember2023-01-012023-12-310001131399currency:SGDgsk:ForeignExchangeContractsMembergsk:SellForeignCurrencyOverSixMonthsMembergsk:NetInvestmentHedgesMember2023-12-310001131399gsk:BorrowingsLessThanThreeMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2023-12-310001131399gsk:BorrowingsLessThanThreeMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2023-01-012023-12-310001131399currency:JPYgsk:BorrowingsOverSixMonthsMembergsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2023-12-310001131399currency:JPYgsk:BorrowingsOverSixMonthsMembergsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2023-01-012023-12-310001131399gsk:BorrowingsOverSixMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2023-12-310001131399gsk:BorrowingsOverSixMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2023-01-012023-12-310001131399gsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2023-12-310001131399gsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2023-01-012023-12-310001131399ifrs-full:CashFlowHedgesMembergsk:VariabilityInCashFlowsFromAHighlyProbableForecastTransactionMember2023-01-012023-12-310001131399gsk:NetInvestmentHedgesMember2023-01-012023-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMembergsk:BuyForeignCurrencyLessThanThreeMonthsMember2022-01-012022-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMembergsk:BuyForeignCurrencyLessThanThreeMonthsMember2022-12-310001131399gsk:BuyForeignCurrencyThreeToSixMonthsMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2022-01-012022-12-310001131399gsk:BuyForeignCurrencyThreeToSixMonthsMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2022-12-310001131399gsk:BuyForeignCurrencyOverSixMonthsMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2022-01-012022-12-310001131399gsk:BuyForeignCurrencyOverSixMonthsMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2022-12-310001131399gsk:SellForeignCurrencyLessThanThreeMonthsMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2022-01-012022-12-310001131399gsk:SellForeignCurrencyLessThanThreeMonthsMembergsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2022-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2022-12-310001131399gsk:ForeignExchangeContractsMemberifrs-full:CashFlowHedgesMember2022-01-012022-12-310001131399gsk:SellForeignCurrencyLessThanThreeMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2022-01-012022-12-310001131399gsk:SellForeignCurrencyLessThanThreeMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2022-12-310001131399currency:JPYgsk:SellForeignCurrencyLessThanThreeMonthsMembergsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2022-01-012022-12-310001131399currency:JPYgsk:SellForeignCurrencyLessThanThreeMonthsMembergsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2022-12-310001131399currency:CADgsk:ForeignExchangeContractsMembergsk:SellForeignCurrencyOverSixMonthsMembergsk:NetInvestmentHedgesMember2022-01-012022-12-310001131399currency:CADgsk:ForeignExchangeContractsMembergsk:SellForeignCurrencyOverSixMonthsMembergsk:NetInvestmentHedgesMember2022-12-310001131399currency:SGDgsk:ForeignExchangeContractsMembergsk:SellForeignCurrencyOverSixMonthsMembergsk:NetInvestmentHedgesMember2022-01-012022-12-310001131399currency:SGDgsk:ForeignExchangeContractsMembergsk:SellForeignCurrencyOverSixMonthsMembergsk:NetInvestmentHedgesMember2022-12-310001131399gsk:BorrowingsLessThanThreeMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2022-12-310001131399gsk:BorrowingsLessThanThreeMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2022-01-012022-12-310001131399gsk:BorrowingThreeToSixMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2022-12-310001131399gsk:BorrowingThreeToSixMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2022-01-012022-12-310001131399gsk:BorrowingsOverSixMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2022-12-310001131399gsk:BorrowingsOverSixMonthsMembercurrency:EURgsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2022-01-012022-12-310001131399gsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2022-12-310001131399gsk:ForeignExchangeContractsMembergsk:NetInvestmentHedgesMember2022-01-012022-12-310001131399ifrs-full:CashFlowHedgesMembergsk:VariabilityInCashFlowsFromAHighlyProbableForecastTransactionMember2022-01-012022-12-310001131399ifrs-full:CashFlowHedgesMembergsk:VariabilityInCashFlowsFromForeignExchangeExposureArisingOnEuroDenominatedCouponPaymentsRelatingToDebtIssuedMember2022-01-012022-12-310001131399gsk:NetInvestmentHedgesMember2022-01-012022-12-310001131399gsk:ForeignExchangeContractsMember2023-12-310001131399gsk:ForeignExchangeContractsMember2022-12-310001131399ifrs-full:CashFlowHedgesMembergsk:VariabilityInCashFlowsFromAHighlyProbableForecastTransactionMember2023-12-310001131399ifrs-full:CashFlowHedgesMembergsk:VariabilityInCashFlowsFromForeignExchangeExposureArisingOnEuroDenominatedCouponPaymentsRelatingToDebtIssuedMember2023-12-310001131399ifrs-full:CashFlowHedgesMembergsk:VariabilityInCashFlowsFromForeignExchangeExposureArisingOnEuroDenominatedCouponPaymentsRelatingToDebtIssuedMember2023-01-012023-12-310001131399ifrs-full:HedgesOfNetInvestmentInForeignOperationsMember2023-12-310001131399ifrs-full:HedgesOfNetInvestmentInForeignOperationsMember2023-01-012023-12-310001131399ifrs-full:CashFlowHedgesMembergsk:VariabilityInCashFlowsFromAHighlyProbableForecastTransactionMember2022-12-310001131399ifrs-full:CashFlowHedgesMembergsk:VariabilityInCashFlowsFromForeignExchangeExposureArisingOnEuroDenominatedCouponPaymentsRelatingToDebtIssuedMember2022-12-310001131399ifrs-full:HedgesOfNetInvestmentInForeignOperationsMember2022-12-310001131399ifrs-full:HedgesOfNetInvestmentInForeignOperationsMember2022-01-012022-12-310001131399ifrs-full:InterestRateSwapContractMember2023-12-310001131399ifrs-full:InterestRateSwapContractMember2022-12-310001131399gsk:PreHedgingOfLongTermInterestRatesMembergsk:MaturedInThePastPeriodMember2023-12-310001131399gsk:PreHedgingOfLongTermInterestRatesMembergsk:MaturedInThePastPeriodMember2023-01-012023-12-310001131399gsk:PreHedgingOfLongTermInterestRatesMembergsk:MaturedInThePastPeriodMember2022-12-310001131399gsk:PreHedgingOfLongTermInterestRatesMembergsk:MaturedInThePastPeriodMember2022-01-012022-12-310001131399gsk:TradeAndOtherReceivableMember2023-12-310001131399gsk:DerivativeFinancialAssetsMember2023-12-310001131399gsk:TradesAndOtherPayablesMember2023-12-310001131399gsk:DerivativeFinancialLiabilitiesMember2023-12-310001131399gsk:TradeAndOtherReceivableMember2022-12-310001131399gsk:DerivativeFinancialAssetsMember2022-12-310001131399gsk:TradesAndOtherPayablesMember2022-12-310001131399gsk:DerivativeFinancialLiabilitiesMember2022-12-310001131399ifrs-full:NotLaterThanOneYearMembergsk:FloatingAndFixedRateDebtMember2023-12-310001131399ifrs-full:NotLaterThanOneYearMembergsk:FloatingAndFixedRateDebtMember2022-12-310001131399ifrs-full:LaterThanOneYearAndNotLaterThanTwoYearsMembergsk:FloatingAndFixedRateDebtMember2023-12-310001131399ifrs-full:LaterThanOneYearAndNotLaterThanTwoYearsMembergsk:FloatingAndFixedRateDebtMember2022-12-310001131399ifrs-full:LaterThanTwoYearsAndNotLaterThanThreeYearsMembergsk:FloatingAndFixedRateDebtMember2023-12-310001131399ifrs-full:LaterThanTwoYearsAndNotLaterThanThreeYearsMembergsk:FloatingAndFixedRateDebtMember2022-12-310001131399ifrs-full:LaterThanThreeYearsAndNotLaterThanFourYearsMembergsk:FloatingAndFixedRateDebtMember2023-12-310001131399ifrs-full:LaterThanThreeYearsAndNotLaterThanFourYearsMembergsk:FloatingAndFixedRateDebtMember2022-12-310001131399ifrs-full:LaterThanFourYearsAndNotLaterThanFiveYearsMembergsk:FloatingAndFixedRateDebtMember2023-12-310001131399ifrs-full:LaterThanFourYearsAndNotLaterThanFiveYearsMembergsk:FloatingAndFixedRateDebtMember2022-12-310001131399ifrs-full:LaterThanFiveYearsAndNotLaterThanTenYearsMembergsk:FloatingAndFixedRateDebtMember2023-12-310001131399ifrs-full:LaterThanFiveYearsAndNotLaterThanTenYearsMembergsk:FloatingAndFixedRateDebtMember2022-12-310001131399ifrs-full:LaterThanTenYearsMembergsk:FloatingAndFixedRateDebtMember2023-12-310001131399ifrs-full:LaterThanTenYearsMembergsk:FloatingAndFixedRateDebtMember2022-12-310001131399gsk:CurrencyRiskTenYenAppreciationOfYenMember2023-01-012023-12-310001131399gsk:CurrencyRiskTenYenAppreciationOfYenMember2022-01-012022-12-310001131399gsk:CurrencyRiskFifteenYenAppreciationOfYenMember2023-01-012023-12-310001131399gsk:CurrencyRiskFifteenYenAppreciationOfYenMember2022-01-012022-12-310001131399gsk:CurrencyRiskTenYenDepreciationOfYenMember2023-01-012023-12-310001131399gsk:CurrencyRiskTenYenDepreciationOfYenMember2022-01-012022-12-310001131399gsk:CurrencyRiskFifteenYenDepreciationOfYenMember2023-01-012023-12-310001131399gsk:CurrencyRiskFifteenYenDepreciationOfYenMember2022-01-012022-12-310001131399gsk:InterestRateSensitivityMembergsk:InterestRateRiskOnePercentMovementInEUROrSterlingInterestRatesMember2023-01-012023-12-310001131399gsk:InterestRateSensitivityMembergsk:InterestRateRiskOnePointFivePercentMovementInEUROrSterlingInterestRatesMember2023-01-012023-12-310001131399gsk:InterestRateSensitivityMembergsk:InterestRateRiskOnePointFivePercentMovementInUSDInterestRatesMember2022-01-012022-12-310001131399gsk:InterestRateSensitivityMembergsk:InterestRateRiskOnePercentMovementInUSDInterestRatesMember2022-01-012022-12-310001131399gsk:InterestRateRiskOnePercentIncreaseInSterlingInterestRatesMember2023-01-012023-12-310001131399gsk:InterestRateRiskOnePercentIncreaseInSterlingInterestRatesMember2022-01-012022-12-310001131399gsk:InterestRateRiskOnePointFivePercentIncreaseInSterlingInterestRatesMember2023-01-012023-12-310001131399gsk:InterestRateRiskOnePointFivePercentIncreaseInSterlingInterestRatesMember2022-01-012022-12-310001131399gsk:InterestRateRiskOnePercentIncreaseInUSDollarInterestRatesMember2023-01-012023-12-310001131399gsk:InterestRateRiskOnePercentIncreaseInUSDollarInterestRatesMember2022-01-012022-12-310001131399gsk:InterestRateRiskOnePointFivePercentIncreaseInUsDollarInterestRatesMember2023-01-012023-12-310001131399gsk:InterestRateRiskOnePointFivePercentIncreaseInUsDollarInterestRatesMember2022-01-012022-12-310001131399gsk:InterestRateRiskOnePercentIncreaseInEuroInterestRatesMember2023-01-012023-12-310001131399gsk:InterestRateRiskOnePercentIncreaseInEuroInterestRatesMember2022-01-012022-12-310001131399gsk:InterestRateRiskOnePointFivePercentIncreaseInEuroInterestRatesMember2023-01-012023-12-310001131399gsk:InterestRateRiskOnePointFivePercentIncreaseInEuroInterestRatesMember2022-01-012022-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:NotLaterThanOneYearMember2023-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:NotLaterThanOneYearMember2023-01-012023-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:LaterThanOneYearAndNotLaterThanTwoYearsMember2023-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:LaterThanOneYearAndNotLaterThanTwoYearsMember2023-01-012023-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:LaterThanTwoYearsAndNotLaterThanThreeYearsMember2023-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:LaterThanTwoYearsAndNotLaterThanThreeYearsMember2023-01-012023-12-310001131399ifrs-full:LaterThanThreeYearsAndNotLaterThanFourYearsMembergsk:NonDerivativesFinancialLiabilitiesMember2023-12-310001131399ifrs-full:LaterThanThreeYearsAndNotLaterThanFourYearsMembergsk:NonDerivativesFinancialLiabilitiesMember2023-01-012023-12-310001131399ifrs-full:LaterThanFourYearsAndNotLaterThanFiveYearsMembergsk:NonDerivativesFinancialLiabilitiesMember2023-12-310001131399ifrs-full:LaterThanFourYearsAndNotLaterThanFiveYearsMembergsk:NonDerivativesFinancialLiabilitiesMember2023-01-012023-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:LaterThanFiveYearsAndNotLaterThanTenYearsMember2023-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:LaterThanFiveYearsAndNotLaterThanTenYearsMember2023-01-012023-12-310001131399ifrs-full:LaterThanTenYearsMembergsk:NonDerivativesFinancialLiabilitiesMember2023-12-310001131399ifrs-full:LaterThanTenYearsMembergsk:NonDerivativesFinancialLiabilitiesMember2023-01-012023-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMember2023-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMember2023-01-012023-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:NotLaterThanOneYearMember2022-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:NotLaterThanOneYearMember2022-01-012022-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:LaterThanOneYearAndNotLaterThanTwoYearsMember2022-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:LaterThanOneYearAndNotLaterThanTwoYearsMember2022-01-012022-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:LaterThanTwoYearsAndNotLaterThanThreeYearsMember2022-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:LaterThanTwoYearsAndNotLaterThanThreeYearsMember2022-01-012022-12-310001131399ifrs-full:LaterThanThreeYearsAndNotLaterThanFourYearsMembergsk:NonDerivativesFinancialLiabilitiesMember2022-12-310001131399ifrs-full:LaterThanThreeYearsAndNotLaterThanFourYearsMembergsk:NonDerivativesFinancialLiabilitiesMember2022-01-012022-12-310001131399ifrs-full:LaterThanFourYearsAndNotLaterThanFiveYearsMembergsk:NonDerivativesFinancialLiabilitiesMember2022-12-310001131399ifrs-full:LaterThanFourYearsAndNotLaterThanFiveYearsMembergsk:NonDerivativesFinancialLiabilitiesMember2022-01-012022-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:LaterThanFiveYearsAndNotLaterThanTenYearsMember2022-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMemberifrs-full:LaterThanFiveYearsAndNotLaterThanTenYearsMember2022-01-012022-12-310001131399ifrs-full:LaterThanTenYearsMembergsk:NonDerivativesFinancialLiabilitiesMember2022-12-310001131399ifrs-full:LaterThanTenYearsMembergsk:NonDerivativesFinancialLiabilitiesMember2022-01-012022-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMember2022-12-310001131399gsk:NonDerivativesFinancialLiabilitiesMember2022-01-012022-12-310001131399gsk:ForeignExchangeForwardContractsAndSwapsMemberifrs-full:DerivativesMemberifrs-full:NotLaterThanOneYearMember2023-12-310001131399gsk:ForeignExchangeForwardContractsAndSwapsMemberifrs-full:DerivativesMemberifrs-full:NotLaterThanOneYearMember2022-12-310001131399gsk:ForeignExchangeForwardContractsAndSwapsMemberifrs-full:DerivativesMember2023-12-310001131399gsk:ForeignExchangeForwardContractsAndSwapsMemberifrs-full:DerivativesMember2022-12-310001131399gsk:SavingsRelatedShareOptionSchemesMember2023-01-012023-12-310001131399gsk:ShareValuePlanMemberifrs-full:TopOfRangeMember2023-01-012023-12-310001131399gsk:ShareValuePlanMember2020-12-310001131399gsk:ShareValuePlanMembergsk:AmericanDepositarySharesMember2020-12-310001131399gsk:ShareValuePlanMembergsk:AmericanDepositarySharesMember2021-01-012021-12-310001131399gsk:ShareValuePlanMember2021-12-310001131399gsk:ShareValuePlanMembergsk:AmericanDepositarySharesMember2021-12-310001131399gsk:ShareValuePlanMembergsk:AmericanDepositarySharesMember2022-01-012022-12-310001131399gsk:ShareValuePlanMember2022-12-310001131399gsk:ShareValuePlanMembergsk:AmericanDepositarySharesMember2022-12-310001131399gsk:ShareValuePlanMembergsk:AmericanDepositarySharesMember2023-01-012023-12-310001131399gsk:ShareValuePlanMember2023-12-310001131399gsk:ShareValuePlanMembergsk:AmericanDepositarySharesMember2023-12-3100011313992020-01-012021-12-31gsk:performanceMeasure0001131399gsk:PerformanceSharePlansMember2020-01-012021-12-310001131399gsk:FreeCashFlowMember2020-01-012021-12-310001131399gsk:TsrMember2020-01-012021-12-310001131399gsk:RAndDNewProductPerformanceMember2020-01-012021-12-310001131399gsk:PipelineProgressMember2020-01-012021-12-3100011313992022-01-012023-12-310001131399gsk:PerformanceSharePlansMember2022-01-012023-12-310001131399gsk:TsrMember2022-01-012023-12-310001131399gsk:PipelineProgressMember2022-01-012023-12-310001131399gsk:ProfitMeasureMember2022-01-012023-12-310001131399gsk:SaleMeasureMember2022-01-012023-12-310001131399gsk:ESGEnvironmentMember2022-01-012023-12-310001131399gsk:PerformanceSharePlansMember2023-12-310001131399gsk:PerformanceSharePlansMembergsk:AmericanDepositarySharesMember2023-01-012023-12-310001131399gsk:PerformanceSharePlansMembergsk:AmericanDepositarySharesMember2023-12-310001131399gsk:ShareOptionsAndSavingsRelatedOptionsMember2023-01-012023-12-310001131399gsk:ShareOptionsAndSavingsRelatedOptionsMember2022-01-012022-12-310001131399gsk:ShareOptionsAndSavingsRelatedOptionsMember2021-01-012021-12-310001131399gsk:SavingsRelatedShareOptionSchemesMember2023-12-310001131399gsk:EmployeeShareOwnershipPlanMember2023-12-310001131399gsk:EmployeeShareOwnershipPlanMember2022-12-310001131399ifrs-full:BottomOfRangeMembergsk:ShareValuePlanMember2023-01-012023-12-310001131399gsk:GlaxoGroupLimitedMembergsk:EnglandMember2023-01-012023-12-310001131399gsk:GlaxoOperationsUkLimitedMembergsk:EnglandMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:GlaxoWellcomeUkLimitedMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:GlaxoSmithKlineCapitalPLCMember2023-01-012023-12-310001131399gsk:GlaxoSmithKlineExportLimitedMembergsk:EnglandMember2023-01-012023-12-310001131399gsk:GlaxoSmithKlineFinancePLCMembergsk:EnglandMember2023-01-012023-12-310001131399gsk:GlaxoSmithKlineHoldingsLimitedMembergsk:EnglandMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:GlaxosmithklineIhcLimitedMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:GlaxosmithklineIntellectualPropertyNo.2LimitedMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:GlaxosmithklineIntellectualPropertyNo.3LimitedMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:GlaxosmithklineIntellectualPropertyNo.4LimitedMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:GlaxosmithklineIntellectualPropertyDevelopmentLimitedMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:GlaxosmithklineIntellectualPropertyLimitedMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:GlaxoSmithKlineResearchAndDevelopmentLimitedMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:GlaxoSmithKlineServicesUnlimitedMember2023-01-012023-12-310001131399gsk:GlaxoSmithKlineUnitedKingdomLimitedMembergsk:EnglandMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:GlaxosmithklineUsTradingLimitedMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:SetFirstLimitedMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:SmithKlineBeechamLimitedMember2023-01-012023-12-310001131399gsk:EnglandMembergsk:ViivHealthcareFinanceLimitedMember2023-01-012023-12-310001131399gsk:ViivHealthcareUnitedKingdomNo.3LimitedMembergsk:EnglandMember2023-01-012023-12-310001131399gsk:ViiVHealthCareUnitedKingdomLimitedMembergsk:EnglandMember2023-01-012023-12-310001131399gsk:GlaxosmithklineAgMembercountry:CH2023-01-012023-12-310001131399gsk:GlaxoWellcomeProductionS.A.SMembercountry:FR2023-01-012023-12-310001131399gsk:GlaxosmithklineB.v.Membercountry:NL2023-01-012023-12-310001131399country:BEgsk:GlaxoSmithKlineBiologicalsSAMember2023-01-012023-12-310001131399gsk:GlaxoSmithKlineGmbHAndCoKGMembercountry:DE2023-01-012023-12-310001131399country:ITgsk:GlaxoSmithKlineManufacturingSpAMember2023-01-012023-12-310001131399gsk:GlaxosmithklinePharmaGmbhMembercountry:AT2023-01-012023-12-310001131399country:BEgsk:GlaxoSmithKlinePharmaceuticalsSAMember2023-01-012023-12-310001131399gsk:GlaxoSmithKlineSAMembercountry:ES2023-01-012023-12-310001131399country:ITgsk:GlaxoSmithKlineSPAMember2023-01-012023-12-310001131399gsk:GlaxosmithklineSingleMemberA.e.b.e.Membercountry:GR2023-01-012023-12-310001131399country:IEgsk:GlaxoSmithKlineTradingServicesLimitedMember2023-01-012023-12-310001131399gsk:GskCapitalB.v.Membercountry:NL2023-01-012023-12-310001131399gsk:GSKServicesSPZOOMembercountry:PL2023-01-012023-12-310001131399gsk:GSKVaccinesGmbHMembercountry:DE2023-01-012023-12-310001131399country:ITgsk:GSKVaccinesSRLMember2023-01-012023-12-310001131399gsk:JscGlaxosmithklineTradingMembercountry:RU2023-01-012023-12-310001131399country:FRgsk:LaboratoireGlaxoSmithKlineMember2023-01-012023-12-310001131399gsk:LaboratoriosViiVHealthCareSLMembercountry:ES2023-01-012023-12-310001131399country:DEgsk:ViivHealthcareGmbhMember2023-01-012023-12-310001131399country:ITgsk:ViivHealthcareSRLItalyMember2023-01-012023-12-310001131399gsk:ViiVHealthcareSASMembercountry:FR2023-01-012023-12-310001131399gsk:GskNo.1ScottishLimitedPartnershipMembergsk:ScotlandMember2023-01-012023-12-310001131399gsk:AffinivaxIncMembercountry:US2023-01-012023-12-310001131399gsk:CorixaCorporationMembercountry:US2023-01-012023-12-310001131399gsk:GlaxoSmithKlineCapitalIncMembercountry:US2023-01-012023-12-310001131399country:USgsk:GlaxoSmithKlineHoldingsIncMember2023-01-012023-12-310001131399country:USgsk:GlaxoSmithKlineLLCMember2023-01-012023-12-310001131399country:USgsk:GskEquityInvestmentsLimitedMember2023-01-012023-12-310001131399gsk:HumanGenomeSciencesIncMembercountry:US2023-01-012023-12-310001131399gsk:StiefelLaboratoriesIncMembercountry:US2023-01-012023-12-310001131399gsk:TesaroMembercountry:US2023-01-012023-12-310001131399gsk:ViiVHealthcareCompanyMembercountry:US2023-01-012023-12-310001131399gsk:GlaxoSaudiArabiaLimitedMembercountry:SA2023-01-012023-12-310001131399country:SGgsk:GlaxoWellcomeManufacturingPteLtdMember2023-01-012023-12-310001131399country:THgsk:GlaxosmithklineThailandLimitedMember2023-01-012023-12-310001131399gsk:GlaxoSmithKlineAustraliaPtyLtdMembercountry:AU2023-01-012023-12-310001131399country:BRgsk:GlaxoSmithKlineBrasilLimitadaMember2023-01-012023-12-310001131399gsk:GlaxosmithklineFarEastB.v.Membercountry:TW2023-01-012023-12-310001131399country:TRgsk:GlaxosmithklineIlaclariSanayiVeTicaretA.s.Member2023-01-012023-12-310001131399gsk:GlaxoSmithKlineIncMembercountry:CA2023-01-012023-12-310001131399country:JPgsk:GlaxoSmithKlineKKMember2023-01-012023-12-310001131399gsk:GlaxoSmithKlineKoreaLimitedMembercountry:KR2023-01-012023-12-310001131399gsk:GlaxoSmithKlineLimitedMembercountry:HK2023-01-012023-12-310001131399gsk:GlaxosmithklineMexicoS.a.DeC.v.Membercountry:MX2023-01-012023-12-310001131399gsk:GlaxoSmithKlinePakistanLimitedMembercountry:PK2023-01-012023-12-310001131399country:INgsk:GlaxosmithklinePharmaceuticalsLimitedIndiaMember2023-01-012023-12-310001131399gsk:GskEnterpriseManagementCoLtdMembercountry:CN2023-01-012023-12-310001131399gsk:GskPharmaVietnamCompanyLimitedMembercountry:VN2023-01-012023-12-310001131399gsk:IDBiomedicalCorporationOfQuebecMembercountry:CA2023-01-012023-12-310001131399gsk:ViivHealthcareK.kMembercountry:JP2023-01-012023-12-310001131399gsk:ViivHealthcareUlcMembercountry:CA2023-01-012023-12-310001131399gsk:CoreqMember2017-06-300001131399gsk:CoreqMember2020-12-02gsk:member0001131399gsk:CoreqMember2021-08-050001131399gsk:RSVMember2022-06-07gsk:patent0001131399gsk:RSVMember2023-08-020001131399gsk:RSVMember2023-11-300001131399gsk:RSVMemberifrs-full:CommencementOfMajorLitigationMembercountry:NL2024-01-262024-03-01gsk:hearing0001131399gsk:AvandiaMember2023-01-012023-12-31gsk:classAction0001131399gsk:ZantacMember2019-01-012022-12-31gsk:defendantgsk:cancer0001131399gsk:ZantacMember2022-01-252022-01-250001131399gsk:ZantacMember2020-01-012023-12-310001131399gsk:ZantacMember2022-12-062022-12-06gsk:casegsk:plaintiff0001131399gsk:ZantacMembercountry:US2020-01-012023-12-310001131399gsk:ZantacMemberstpr:DE2020-01-012023-12-310001131399ifrs-full:CommencementOfMajorLitigationMembergsk:ZantacMember2024-01-222024-01-240001131399gsk:ZantacMember2023-10-110001131399gsk:ZantacMemberstpr:IL2023-01-012023-12-310001131399gsk:ZantacMembergsk:OntarioAndQuebecMember2020-01-012020-12-310001131399ifrs-full:CommencementOfMajorLitigationMembergsk:ZantacMembercountry:CA2021-01-012024-02-210001131399gsk:ZofranMember2015-01-012019-12-310001131399gsk:ZofranMember2021-06-012021-06-010001131399gsk:ZofranMembercountry:CA2023-01-012023-12-310001131399srt:MinimumMembergsk:GSKKoreaMember2020-08-012020-08-31gsk:employee0001131399gsk:GSKKoreaMember2023-02-012023-02-28iso4217:KRW0001131399gsk:GSKKoreaMember2023-07-012023-07-300001131399gsk:GSKGroupMembergsk:OrangeBookChallengeMember2023-11-012023-11-300001131399gsk:OrangeBookChallengeMember2023-11-012023-11-300001131399gsk:GSKGroupMembergsk:OrangeBookChallengeMember2023-11-300001131399gsk:ZejulaRoyaltyDisputeMember2012-10-012012-10-31gsk:agreement0001131399gsk:ZejulaRoyaltyDisputeMember2023-04-052023-04-050001131399gsk:AiolosBioIncMemberifrs-full:MajorBusinessCombinationMember2024-01-092024-01-090001131399srt:MaximumMembergsk:AiolosBioIncMemberifrs-full:MajorBusinessCombinationMember2024-01-090001131399gsk:HaleonPlcMemberifrs-full:OtherDisposalsOfAssetsMember2024-01-170001131399gsk:HaleonPlcMemberifrs-full:OtherDisposalsOfAssetsMember2024-01-172024-01-170001131399gsk:HaleonPlcMemberifrs-full:OtherDisposalsOfAssetsMember2024-01-180001131399gsk:HaleonPlcMemberifrs-full:OtherDisposalsOfAssetsMember2024-01-182024-01-18
As filed with the Securities and Exchange Commission on March 5, 2024
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
☐ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☐ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of event requiring this shell company report
For the transition period from to
Commission file number 1-15170
GSK plc
(Exact name of Registrant as specified in its charter)
England
(Jurisdiction of incorporation or organization)
980 Great West Road, Brentford, Middlesex TW8 9GS England
(Address of principal executive offices)
Victoria Whyte
Company Secretary
GSK plc
980 Great West Road
Brentford, TW8 9GS
England
+44 20 8047 5000
company.secretary@gsk.com
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act: | | | | | | | | |
Title of Each Class | Trading Symbol(s) | Name of Each Exchange On Which Registered |
America Depositary Shares, each representing 2 Ordinary Shares, Par value 31 1⁄4 pence | GSK | New York Stock Exchange |
3.000% Notes due 2024 | GSK/24 | New York Stock Exchange |
3.625% Notes due 2025 | GSK/25 | New York Stock Exchange |
3.875% Notes due 2028 | GSK/28 | New York Stock Exchange |
3.375% Notes due 2029 | GSK/29 | New York Stock Exchange |
6.375% Notes due 2038 | GSK/38 | New York Stock Exchange |
4.200% Notes due 2043 | GSK/43 | New York Stock Exchange |
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
(Title of class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
(Title of class)
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
Ordinary Shares of Par value 31 1⁄4 pence each 4,312,145,983
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
☒ Yes ☐ No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
☐ Yes ☒ No
Note – Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
☒ Yes ☐ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “accelerated filer,” “large accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
Large accelerated filer ☒ Accelerated filer ☐ Non-accelerated filer ☐ Emerging growth company ☐
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13 (a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| | | | | | | | |
| U.S. GAAP ☐ | International Financial Reporting Standards as issued by the International Accounting Standards Board ☒ | Other ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 ☐ Item 18 ☐
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
☐ Yes ☒ No
Auditor Firm - Deloitte LLP - PCAOB ID No 1147

20F Item Page and caption references in this document* 1 Identity of Directors, Senior Management and Advisers Not applicable 2 Offer Statistics and Expected Timetable Not applicable 3 Key information A. [Reserved] B. Capitalisation and Indebtedness Not applicable C. Reasons for offer and use of proceeds Not applicable D. Risk Factors 241 - 244, 254 - 260 4 Information on the Company A. History and development of the company 86-87, 90, 97, 214 - 218, 266, 267, 298 B. Business overview 1 - 28, 31 - 40, 44 - 53, 57 - 69, 172 - 174, 214 - 218, 251 - 260 C. Organisational structure 240, 287 - 295 D. Property, plant and equipment 87, 174, 185 - 186 4A Unresolved staff comments Not applicable 5 Operating and Financial Review and Prospects A. Operating results 8 - 10, 70 - 97 B. Liquidity and capital resources 86 - 91, 93 - 94, 97, 193, 195 - 196, 209, 221 - 237 C. Research and development, patents and licences, etc. 14 - 28, 247 - 253 D. Trend information 70 - 97 E. Critical accounting estimates Not applicable 6 A. Directors, Senior Managers and Employees A. Directors and senior management 99 - 105 B. Compensation 127 - 148, 238 - 239 C. Board practices 3, 98 - 128, 147, 149 - 150, 273 - 274 D. Employees 177, 197 - 205, 246 E. Share ownership 130, 134, 135, 144 - 145, 238 - 239 F. Disclosure of a registrant’s action to recover erroneously awarded compensation Not applicable 7 Major Shareholders and Related Party Transactions A. Major shareholders 149, 261 - 263 B. Related party transactions 213, 270 - 271 C. Interests of experts and counsel Not applicable 8 Financial Information A. Consolidated Financial Statements and Other Financial Information 151 - 244, 263, 273 B. Significant Changes 241 - 244 9 The Offer and Listing A. Offer and listing details 262 B. Plan of distribution Not applicable C. Markets 261, 262 D. Selling shareholders Not applicable E. Dilution Not applicable F. Expenses of the issue Not applicable Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Form 20-F Cross Reference Guide

10 Additional information A. Share Capital Not applicable B. Articles of Association of GSK plc 267 - 270 C. Material contracts 270 - 271 D. Exchange controls 261 E. Taxation 265 - 266 F. Dividends and paying agents Not applicable G. Statement by experts Not applicable H. Documents on display 264 I. Subsidiary information Not applicable J. Annual Report to Security Holders Not applicable 11 Quantitative and Qualitative Disclosures about Market Risk 93 - 94, 221 - 237 12 Description of Securities Other Than Equity Securities A. Debt securities Not applicable B. Warrants and Rights Not applicable C. Other Securities Not applicable D. American Depository Shares 271 - 272 13 Defaults, Dividend Arrearages and Delinquencies Not applicable 14 Material Modifications to the Rights of Security Holders and Use of Proceeds Not applicable 15 Controls and Procedures 122 - 123, 277 - 279 16 [Reserved] 16 A. Audit Committee Financial Expert 100, 110, 121, 277 16 B. Code of Ethics 126, 273 16 C. Principal Accountant Fees and Services 126, 176, 275 16 D. Exemptions from the Listing Standards for Audit Committees Not applicable 16 E. Purchase of Equity Securities by the Issuer and Affiliated Purchasers Not applicable 16 F. Change in Registrant’s Certifying Accountant Not applicable 16 G. Corporate Governance 280 - 286 16 H. Mine Safety Disclosure Not applicable 16 I. Disclosure regarding Foreign Jurisdictions that Prevent Inspections Not applicable 16 J. Insider Trading Policies Not applicable 16 K. Cybersecurity Disclosures 55, 104, 121 - 122, 259, 272 - 273 17 Financial Statements See Item 8 above 18 Financial Statements Not applicable 19 Exhibits Exhibit Index Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Form 20-F Cross Reference Guide continued

At GSK, we unite science, technology and talent to get ahead of disease together. We aim to positively impact the health of 2.5 billion people by the end of the decade, as a successful, growing company where people can thrive. Contents Strategic report Ahead Together 01 Chair’s statement 02 CEO’s statement 04 Business model 06 Our external environment 08 Prevention 11 Our culture and people 12 Research and development 14 Commercial operations: 29 Performance: Vaccines 31 Performance: Specialty Medicines 35 Performance: General Medicines 38 Operations: Manufacturing and supply 41 Responsible business 43 Risk management and disclosure statements 54 Climate-related financial disclosures 57 Nature-related financial disclosures 65 Employees by gender 69 Group financial review 70 Corporate governance The Board and GSK Leadership Team 99 Chair’s governance statement 106 Corporate governance architecture 108 Ahead Together – Board oversight 111 Continuous engagements and key decisions 113 Board committee reports 116 Remuneration Committee Chair’s annual statement 127 Annual report on remuneration 130 Directors’ report 149 Financial statements Directors’ statement of responsibilities 152 Independent auditor’s report 154 Financial statements 158 Notes to the financial statements 162 Investor information Number of employees 246 Product development pipeline 247 Products, competition and intellectual property 251 Risk factors 254 Share capital and control 261 Dividends 263 Financial calendar 2024 264 Annual General Meeting 2024 264 Tax information for shareholders 265 Additional information 267 Shareholder services and contacts 276 US law and regulation 277 Corporate governance comparison 280 Group companies 287 Glossary of terms 296 Cautionary statement See page 298 for the cautionary statement regarding forward-looking statements. Non-IFRS measures We use a number of adjusted, non-International Financial Reporting Standards (IFRS) measures to report the performance of our business. Total reported results represent the Group’s overall performance under IFRS. Adjusted results and other non-IFRS measures may be considered in addition to, but not as a substitute for or superior to, information presented in accordance with IFRS. Adjusted results and other non-IFRS measures are defined on pages 72 and 73 and reconciliations to the nearest IFRS measures are on pages 82 to 84. Websites Information on our website or any other website referenced in this Annual Report on Form 20-F is not incorporated into this Annual Report on Form 20-F and should not be considered to be a part of this Annual Report on Form 20-F. We have included any references to the website as an inactive textual reference only.

We are a focused biopharma company with strong momentum and big ambitions. We prevent and treat disease with vaccines, specialty and general medicines. We focus on the science of the immune system and the use of new platform and data technologies, investing in four core therapeutic areas (infectious diseases, HIV, respiratory/ immunology and oncology). Our Ahead Together strategy means intervening early to prevent and change the course of disease, helping to protect people and support healthcare systems. We’re confident in our future. With our strong momentum and improving outlook for sustained growth through the decade, we're confident in our ability to deliver human health impact at scale, worldwide. We’re committed to getting ahead of issues that matter for society and for the sustainability of our company, too – including access to healthcare, diversity, equity and inclusion, and the health of our planet. We're sector leaders in ESG performance, making an impact on some of society’s most urgent challenges. Our purpose puts our people at the heart of our success. Core to our Ahead Together ambition is to make GSK a place where talented people thrive. Our culture of being ambitious for patients, accountable for impact and doing the right thing is the foundation for how, together, we deliver for our patients, shareholders and GSK people. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F 1

The programme of change Emma and her team are delivering is fundamentally improving GSK’s competitiveness: sharpening operational execution and cost discipline; strengthening the pipeline; enhancing the Group’s capital allocation capacity; and shifting GSK’s culture to combine high integrity with performance. As is also clear from this report, GSK is developing a distinctive role and voice in prevention of disease, offering clear benefits to patients, healthcare systems and wider society. Strategic progress In 2023, we saw further evidence of the success of this transformation. Operationally, GSK is performing better – and crucially more consistently and competitively – than at any point in the last 20 years. Group sales and operating profits grew strongly in 2023. Growth is being driven by very strong performance across all areas of the business, especially Vaccines and Specialty Medicines, including in HIV and respiratory, where the company has built significant leadership positions and competitive advantage. The exceptional launch of the world’s first RSV vaccine, Arexvy, in the US was a clear stand out achievement for the year. Cost discipline across the Group continues to improve. Following a period of necessary investment in product launches, management is now focused on delivering further improvements in operating margin over the coming years. As I have previously discussed, the demerger of Haleon in 2022 fundamentally reset and strengthened GSK’s balance sheet. During 2023 we monetised £1.8 billion of our holding in Haleon to enable further investment in the pipeline and the future growth of the company. We have also confirmed our commitment to shareholder returns through a progressive dividend policy. The Board agreed to pay shareholders an increased dividend of 58p per share for 2023, up 3p per share1 on a comparable basis. R&D progress Executing the company’s late-stage pipeline and strengthening our earlier- stage R&D and technological capabilities, remains the company’s number one priority. This continues to receive significant attention from the Board, including through our Science Committee, which undertook detailed reviews during 2023 of several research areas, including vaccines & RNA technology, antimicrobial resistance (AMR). oligonucleotides, antibody-drug conjugates (ADCs) and liver disease (NASH). Improving R&D productivity is inevitably a long-term programme. But I was pleased to see good progress made during the year, both organically and through targeted business development. In total we deployed approximately £2 billion to R&D business development, including acquisitions and partnerships during the year. As Emma sets out in her letter on pages 4 to 5, GSK now has significant and potentially very valuable late- stage R&D programmes in vaccines/ infectious diseases, HIV, respiratory and specific areas of oncology. Successful progression of these programmes is vital to support the Group’s growth outlook in the second half of the decade and beyond. (1) GSK group dividend in 2022 was 55p, this is GSK related only and excludes the dividend related to Consumer Healthcare in H1 2022. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Chair’s statement 2 In 2023 GSK continued to make significant progress on its strategic transformation.

Culture and responsibility I believe that one of the strongest drivers of GSK’s long-term performance is the culture shift that Emma and her team are driving. We are seeing significant change here, with the focus on developing a clear purpose, strengthening leadership, and embedding business-relevant values and behaviours. Ensuring environmental, social and governance considerations are also properly embedded into our strategy remains very important. I was delighted to see the company ranked first in the sector in S&P’s 2023 assessment of Corporate Sustainability. GSK also continues to lead in our approach to ensuring global access to our products and in developing new medicines and vaccines for diseases such as malaria and TB which disproportionately affect the poorest countries in the world. 2023 was also the second year of operation of our new remuneration policy. This is designed to support achievement of outperformance across strategic, financial and ESG goals, and I believe it is helping to drive the strong performance culture and deep commitment to responsibility that is evident at GSK. Shareholder returns The Board remains focused on delivering strong shareholder returns and valuation for GSK over the long term. It is clear from the extensive meetings and discussions I have had with shareholders over the year, that they recognise the significant performance improvements that have been delivered. The uncertainty around Zantac (ranitidine) litigation has clearly impacted GSK’s share price performance over the 18 months. We continue to vigorously defend ourselves against the remaining claims in the US, including the ongoing proceedings in Delaware and hope to see greater clarity on the litigation during 2024. Board evolution The composition and maturity of the Board continues to improve to ensure we have the relevant skills and experience to provide good oversight and support, and constructively challenge management as GSK’s business develops as a pure biopharma company. I was pleased to welcome Wendy Becker to the Board in October. Wendy is a highly experienced non-executive director and brings excellent business, technology and life sciences experience. She will also succeed Urs Rohner as Chair of the Remuneration Committee when he steps down at the May 2024 AGM. I would like to thank Urs for his contribution to the GSK Board, particularly the development of our new remuneration policy, approved in 2022, to incentivise and reward management performance. He has been a consistent and determined supporter of GSK and has provided huge support to Emma and I. I was also pleased to welcome Julie Brown as Chief Financial Officer (CFO) in May last year. Julie brings huge experience in life sciences and as a CFO of large UK-based companies. The GSK Board now has excellent, in many cases world-leading, experience and expertise including in human genetics, vaccines, respiratory and infectious disease; advanced technologies including in AI and ML; biopharma commercial and financial expertise and US payer, HCP and patient understanding. GSK is performing better than it has done for many years and has an increasingly positive outlook, and this is due to the energy, commitment and leadership of Emma and her team in support of the company’s ambitious programme of change. Finally, I would also like to thank all of our people, partners, customers and shareholders for their support and commitment through the last year and I look forward to another year of progress in 2024 for GSK. Sir Jonathan Symonds Chair Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Chair's statement continued 3

The excellent performance we delivered in 2023 provides us with clear momentum and we expect to deliver another year of meaningful growth in 2024, as we continue to focus on prevention and changing the course of disease. Delivering on our commitments In 2021, we set out a series of commitments to shareholders, including for a 'step-change' in performance, following the significant transformation in GSK’s structure, strategy, capital allocation and culture. Since then, we have delivered 10 quarters of consecutive sales growth (excluding COVID-19 solutions), and around two-thirds of sales are now generated from Vaccines and Specialty Medicines, a key strategic priority. At the same time, we have continued to strengthen our pipeline. We now have 71 vaccines and medicines in clinical development and the majority of the late-stage assets we highlighted in 2021 have moved forward positively. Since 2021, we have also added multiple new opportunities through targeted business development, securing more than 16 acquisitions and alliances for innovative assets and new technologies. We have achieved all of this whilst maintaining a continued sharp focus on operating margins and cash flow – mindful of the need to both invest for the future and to deliver attractive returns to shareholders. Strong 2023 performance Our performance for 2023 demonstrated this progress, with sales excluding COVID-19 solutions growing double-digit levels at AER and CER. Total and Adjusted operating profit reflected strong growth at AER and CER. A clear highlight for the year was the exceptional launch of Arexvy, the world’s first vaccine for RSV, which contributed £1.2 billion of sales in its first year. More than 10% of American adults aged 60 years and older have now been vaccinated against RSV, and over two-thirds of those have been vaccinated with Arexvy. Over time we expect Arexvy to generate annual sales of more than £3 billion and 2024 sales to be driven by further penetration, initial roll out of the vaccine in Europe and Japan and expansion of Arexvy’s indication to at risk individuals aged 50-59 years. Our shingles vaccine, Shingrix, also delivered another very strong performance in 2023, with £3.4 billion of sales. In Specialty Medicines, our HIV business grew strongly, up 12% AER, 13% CER, driven by acceleration in our oral two-drug and long-acting injectable regimens for treatment and prevention. We also saw good progress in respiratory with our market-leading IL-5, Nucala, up 16% AER, 18% CER. Lupus treatment Benlysta was also a major contributor up 18% AER, 19% CER. Overall, sales from new products launched since 2017 contributed more than £11 billion. This level of performance helped us to deliver Total net cash inflow from operating activities of £6.8 billion and free cash flow of £3.4 billion. As a consequence of this performance and momentum, we were also pleased to increase the dividend for the year to 58 pence per share. Pipeline strengthening In R&D, we continued to make progress in 2023 both organically and through business development, as set out on pages 14 to 28. We delivered four major product approvals during the year: Arexvy; Apretude in HIV prevention; Ojjarra for myelofibrosis and Jemperli in first-line endometrial cancer. With 18 assets now in phase III or registrational studies, we are looking forward to further significant late- stage R&D milestones in 2024. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F CEO’s statement 4 GSK is delivering on its commitments and performing to a new standard.

Targeted business development also continued to strengthen the pipeline and support future growth. Our activity in 2023 included the acquisition of Bellus Health and Aiolos Bio1, which both further strengthen our respiratory pipeline, and the signing of licence agreements with Janssen and Hansoh Pharma, in infectious diseases and oncology. Building trust We are committed to making GSK a place where talented people can thrive, with a culture where we are all ambitious for patients, accountable for impact and do the right thing. It was very positive that engagement scores remain high, at 81%, in our latest employee engagement survey. Operating responsibly remains core to GSK. We aim to continue delivering sector-leading ESG performance, as recognised in our latest ranking as sector leaders of the S&P’s Global Corporate Sustainability Assessment. This reflects strong progress across our six core ESG areas: Access to healthcare, Global health and health security, Environment, Diversity, Equity and Inclusion, Ethical standards and Product governance. (1) Closed in early 2024. We have long-term goals and key metrics in place for all these areas, and our overall performance rating for 2023 was ‘on track,’ based on 95% of metrics being met or exceeded. Highlights for the year included, moving to phase III development for our low-carbon Ventolin inhaler programme, achieving our leadership diversity aspirations two years early, and Gavi confirming the roll out of our malaria vaccine, Mosquirix, in up to 12 countries in Africa. Further details are set out on pages 43 to 53 and in our published standalone ESG Performance Report. Clear momentum as we look ahead In conclusion, GSK has strong momentum and improving outlooks. As a standalone biopharma company, with expertise in developing innovative vaccines and medicines, we have enormous opportunity to prevent and change the course of disease for hundreds of millions of people. All of this bodes well. Equally, we also know there is much to be done. We remain very focused on delivering this potential – and more – at continued pace for patients, for shareholders and for our people. Finally, as ever, it is our people who fuel this momentum and I want to thank them for all they have achieved during 2023. I am very optimistic for the future and excited by what we can achieve, to get ahead of disease, together. Emma Walmsley Chief Executive Officer Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F CEO's statement continued 5

We unite science, technology and talent to get ahead of disease together for health impact, shareholder returns and thriving people. Central to our success are our people: experts in science, technology, manufacturing, regulation, intellectual property and commercialisation... 70,200 >75 22,000 GSK people countries worldwide suppliers working directly with GSK £6.2bn 37 4 R&D investment in 2023 – up 13% AER, 14% CER1 manufacturing sites global R&D centres in the US, UK, Belgium and Italy ...who are identifying, researching, developing and testing ground-breaking discoveries, and manufacturing and commercialising... Vaccines Our broad vaccines portfolio targets infectious diseases at every stage of life, helping to protect people from meningitis, shingles, RSV, flu, polio and many more. Specialty Medicines Our specialty medicines prevent and treat diseases, from HIV and respiratory diseases, to immune- inflammation diseases like lupus, to cancer. Many are first or best-in-class. General Medicines We have a portfolio of more than 150 primary care medicines, including our inhaled medicines for asthma and COPD, and antibiotics for infections. ...products that prevent and treat disease, improving the health of millions of people around the world in our core therapeutic areas... Infectious diseases Our infectious diseases portfolio is the broadest in the industry and, including HIV, accounts for two thirds of our pipeline. HIV We are leaders in HIV, focused on ending the global epidemic. We have an industry-leading pipeline, driven by patient insights. Respiratory/ immunology We’re pushing the frontiers of respiratory science and harnessing the science of the immune system to transform patient outcomes in areas of unmet need, based on decades of innovative research. Oncology We have an emerging portfolio focused on blood and women's cancers, and are seeking to make transformative breakthroughs in immuno-oncology. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Business model 6 (1) Total R&D expenditure includes intangible asset amortisation and impairments plus immaterial amounts of major restructuring and other costs.

...powered by technology... Pipeline We are leveraging new platform and data technology at every step of the R&D process to be faster, more effective, and more predictive in discovering and developing innovative new medicines and vaccines. Performance We use technology to enable more productive and efficient manufacturing processes, supply chain reliability and returns on investment. People and productivity Technology is also core to how we work. We ensure our people have the tools, analytical capabilities and resources to make data-driven decisions and do their best work. ...steered by our long-term priorities... Innovation We develop and launch new medicines and vaccines where they are needed, with better and faster R&D. Performance Our bold ambitions for patients are reflected in our growth strategy. Trust We focus on issues where we can have the greatest impact and reduce pressure on health systems including tackling health challenges and inequities, protecting the environment and taking action on diversity, equity and inclusion. ...and creating value for: Patients Shareholders Society 2.3bn 58.00p £1.3bn packs of medicines and doses of vaccines delivered per share dividend corporate income tax paid; in addition we pay duties, levies, transactional and employment taxes The economy Disease prevention and earlier intervention to improve health can lessen pressure on health systems and support economic productivity. Our people We support all our people to grow, be well and do work that really matters. Reinvestment The returns we make enable us to reinvest in discovering and developing new vaccines and medicines so we can continue getting ahead of disease. Company directors are required by law to promote the success of their organisation for the benefit of both shareholders and their wider stakeholders, including employees, suppliers and the community. + Our business model is supported by our ESG strategy, described on page 44 + Our strategy is supported by a robust framework for monitoring and managing risk, see page 55 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Business model continued 7
Five major themes influenced our external environment in 2023. Here, we set out what they mean for us and how we are responding. Economic growth shows resilience but pressure continues on public finances The global economy proved to be more resilient than expected in 2023. But the recovery remained relatively fragile and uneven, with prospects diverging between regions. Many countries continued to grapple with persistent inflation, driven by factors including tight labour markets. Several major central banks responded by increasing interest rates, adding to the burden of rising costs for consumers and businesses. Despite sticky inflation and a consequent tightening of monetary policy, the global economy continued to expand, albeit at a slower rate. There were notable pockets of strength. America’s economy grew in 2023, buoyed by sustained consumer spending and robust government spending associated with infrastructure investment legislation passed in 2021 and 2022. But this resilience was not always mirrored elsewhere. For example, forecasts for the eurozone were revised downwards, as the region continued to feel the impact of weaker demand and higher costs. Amid rising levels of debt and political volatility, global growth prospects remain tepid. Public spending – including on health – remains under strain. Governments face unprecedented pressure on their finances due to a string of economic shocks, sustained sluggish growth and higher debt. Higher interest rates are now making it more challenging to service those debts. This is compelling governments to make tough choices about where to direct spending. Geopolitical tensions fuel shifting alliances Fragmentation and regionalisation continued to grow in 2023, with ongoing conflicts in Ukraine and the Middle East focusing ever more attention on political alliances. Tensions between China and the US remained, with new export controls and investment screening mechanisms emerging on both sides, particularly focused on critical minerals, AI, semiconductors and biotechnologies. But there were signs of relations improving between the two nations, with their presidents meeting for the first time in a year on the sidelines of the Asia-Pacific Economic Cooperation summit. New alliances also emerged, potentially shifting the weight and influence of various blocs. A summit in August saw the BRICS group of countries widen its membership, for the first time since 2010, inviting six further countries, including Saudi Arabia and Iran, to join. As countries look to diversify and de- risk their supply chains in strategic sectors including biopharmaceuticals, many are looking towards India as an alternative supplier to China. Yet against this backdrop, activity in China's biopharmaceutical sector is resilient, recognising the acceleration of Chinese innovation and growth potential. More low and middle-income countries capitalised on global policy forums, such as the UN General Assembly, to set the agenda on issues related to health, new technologies and industrial development. With more diverse voices on global platforms, inequality is seen as a critical issue where governments must collectively make progress. In healthcare, there are debates around the best measure of widening access, with attention on equitable distribution of the infrastructure, capability and know- how to make health products, while protecting intellectual property rights and efficient supply chains. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Our external environment 8 3% Global growth was forecast to slow to 3% in 2023.1 >25% In August 2023, six further countries were invited to join the BRICS group. The combined economies of an enlarged group would be worth more than $28.5 trillion – more than a quarter of the world's economy.2 (1) https://www.imf.org/en/Blogs/Articles/2023/10/10/resilient-global-economy-still-limping-along-with-growing-divergences. (2) https://www.bbc.co.uk/news/world-66525474

Changing demographics create opportunity for innovation and prevention Pressures on health systems continued into 2023 amid ongoing efforts to recover and rebuild in the aftermath of the COVID-19 pandemic. Populations are ageing, bringing more complex health needs. Chronic diseases are taking an increasing toll while infectious diseases remain a significant threat. The individual impact of changing demographics and disease patterns extends to societies and economies at large. Poor health is a significant drag on economic growth. Every year, poor health costs around 15% of global real GDP from premature deaths and lost productive potential among working age people1. In the UK alone, 131 million working days are estimated to be lost each year due to illness.2 Despite the potential to improve individual outcomes and boost economic productivity through investing in health, particularly through prevention and earlier intervention, governments continued to look for cost savings in health systems. The US progressed implementation of the Inflation Reduction Act (IRA). This included selecting the first 10 drugs for potential price cuts under a new programme enabling Medicare to negotiate the price of some of the costliest medicines. While this could potentially limit future innovation and access to currently available medicines, the IRA does bring meaningful benefits to certain Medicare patients, such as access to vaccines without having to bear part of the cost. The EU also took forward legislation that could test pharmaceutical innovation and competitiveness. Meanwhile, the UK agreed a five-year deal aimed at reducing medicine costs for the NHS by setting an annual limit on the allowed growth in sales value of branded medicines. Even as governments sought ways to cut medicine costs, they continued to look to the biopharma industry to be a driver of innovation and economic growth, with the US President's State of the Union address underlining an appetite for more and better treatments, particularly in cancer. This highlights the potential for the biopharma industry to be a partner in recovery, harnessing science and technology to provide solutions that help prevent and change the course of disease and bring value to individuals, health systems and societies. Balancing potential of tech and data with appropriate use Rapid advances in science and technology continue to shape the life sciences sector and R&D. Established technologies such as small molecules and vaccines remain key. Emerging technologies, such as MAPS and DNA/RNA therapeutics, including oligonucleotides, are gaining ground and building market share. Major biopharma companies continue to increase their focus on artificial intelligence and machine learning (AI/ ML) to accelerate drug discovery. Progress hinges on diverse patient data being available for computational research, in particular genomic data, linked to health information held in clinical records. Revolutions in data and technological capabilities open up new possibilities for patients through advances in drug discovery, as well as enhancing manufacturing and supply of medicines. But the possibilities for improving health outcomes need to be balanced with appropriate regulation that supports innovation and ensures responsible use by those who develop the technology, as well as those who use and apply it. During the year, the debate around regulation of AI gathered pace as governments stepped up their efforts to examine the technology’s promise and risks. In the first legislation of its kind, the EU passed the AI Act in June 2023, taking a stringent approach that does not consider context-specific use of AI in healthcare. The US and the UK continue to consider how to place appropriate guardrails around the use of AI, while supporting innovation and considering implications for specific sectors. At a landmark summit in November 2023, the UK, EU, US, Australia and China all agreed to work together on AI safety research. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Our external environment continued 9 1.6bn Societies are ageing, bringing different health challenges. The number of people aged 65 years or older worldwide is projected to more than double, rising from 761 million in 2021 to 1.6 billion in 2050.3 $45bn In the last five years, biopharma has entered into collaborations with AI companies which are estimated to be worth more than $45 billion.4 (1) www.mckinsey.com/industries/healthcare/our-insights/how-prioritizing-health-could-help-rebuild-economies (2) www.cbi.org.uk/media-centre/articles/boosting-workforce-health-can-help-the-uk-achieve-economic-growth-ambitions-says-cbi/ (3) www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2023/01/WSR_2023_Chapter_Key_Messages.pdf - Chapter Key Messages_WSR 2023 (un.org) (4) www.accenture.com/content/dam/accenture/final/accenture-com/document-2/Accenture-Tech_Vision_Biopharma_Accenture-Merging %20atoms%20and%20bits%20in%20Life%20Sciences_V14.pdf

Pressure increasing on climate and nature action Economic pressures and political realignments are influencing how countries approach global challenges that need collective action, including climate change and nature loss. The Intergovernmental Panel on Climate Change issued a 'final warning' in March 2023 to keep the 1.5°C degrees target within reach,1 setting out the urgency for sufficient and swift climate action. Some regions see the need for climate action as an opportunity to use green policies as a lever for growth. For example, the European Commission set out a Green Deal industrial plan to make Europe a centre for clean technology and innovation. But as policy makers tackled rising inflation and increased living costs, climate targets came under pressure. The UK softened its net zero policies and EU environment ministers did not increase their target for reducing greenhouse gas emissions, after opposition from some member countries. At the international climate conference COP28 in Dubai, countries committed to transition away from fossil fuels and to triple renewable energy capacity. It also saw the climate-health agenda given more prominence than ever before, with 123 governments endorsing the COP28 Declaration on Climate and Health. Companies continue to take action to reduce their climate impact and protect their business model, taking steps to ensure their products and supply chains remain resilient to the consequences of climate change. Scientific evidence of the link between climate change and human health means we continue to see high expectations of the healthcare sector to both reduce carbon emissions and respond to the health impacts of climate change. During the year, biopharma companies stepped up their commitments, including to strengthen locally led adaptation and health resilience programmes for vulnerable communities affected by climate change. There's also a growing focus on limiting nature loss. The Taskforce on Nature-related Financial Disclosures released its final recommendations in 2023, providing a risk management and disclosure framework for organisations to report and act on evolving nature-related risks. See the Responsible Business section on page 43 and Nature-related Financial Disclosures on page 57. Our position In a challenging economic and political landscape, it's critical that we invest in a pipeline of vaccines and medicines that prevent and change the course of disease, to meet changing and unmet healthcare needs. At the same time, we have to work with governments, regulators and industry partners to make sure these medicines and vaccines can reach patients at scale, bringing value to both the people who need them and to payers. Scientific innovation is a critical lever to improve health, boost productivity and economic growth, and ease the strain on health systems. We continue to work with our peers and governments to make sure that the policy and regulatory environment stimulates and sustains innovation. This includes, for example, advocating for appropriate IP protections; a balanced regulatory framework that supports the discovery and delivery of vaccines and medicines developed through emerging technologies; and reinforcing the importance of global, diversified supply chains. As the pricing environment becomes tougher, we believe we’re well placed to offer a differentiated, high-value pipeline across prevention and treatment of disease. This is built on using transformational new technology and techniques to make our R&D faster and smarter. Demand for data and real-world evidence to support continued reimbursement of new products is likely to increase. We continue to work with payers to design innovative solutions that manage their risk and uncertainty, while also recognising the full health, social and economic value of innovative medicines and vaccines. Populations are ageing, infectious diseases are still spreading and chronic diseases are taking a greater toll. All of this is creating unsustainable pressure on health systems. More than ever, we believe that getting ahead of disease is the best investment – for patients, carers, communities, health systems and economies. We’ll continue to work with governments, payers and partners to move towards new models of care that enable earlier action to prevent, diagnose and treat disease. Together, we have an opportunity to rethink health – not just to treat sickness, but to invest in keeping people well. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Our external environment continued 10 100 In further evidence of a growing investor interest in nature, ‘Nature Action 100’ aims to mobilise institutional investors to establish a common agenda for corporate action on nature. It will focus on eight sectors, including biotechnology and pharmaceuticals. (1) https://sciencebasedtargets.org/blog/ ipcc-releases-final-warning-to-keep-1-5- c-within-reach For more on why and how prevention underpins our purpose to get ahead of disease, see page 11
Prevention is at the heart of getting ahead of disease – preventing ill health in the first place and stopping disease in its tracks. Why is prevention important? Health systems are stretched and health needs are evolving as demographics and disease patterns change. >3 million premature deaths among people under 75 could have been avoided through better prevention and healthcare interventions across OECD countries in 2019. This amounts to over a quarter of all deaths. (source: OECD) $1 trillion loss in productivity each year in the G20 from preventable conditions among people aged 50-64. (source: ilcuk) $7 trillion In the US alone, health spending is projected to reach almost $7 trillion by 2030. (source: CMS) Prevention and earlier intervention offer a solution to these challenges, helping to improve people's health outcomes – and bring benefits to health systems and economies. $12 trillion could be added to global GDP by 2040 by improving health. Around half of the annual economic benefits would come from a larger and healthier workforce. (source: McKinsey) What does prevention mean to us? Preventing and changing the course of disease is at the heart of what we mean by getting ahead of disease together. By harnessing our science and technology, we have an opportunity to prevent disease in the first place, as well as change the course of a disease – helping to prevent or slow progression of an illness and limit long-term complications. Prevention is a focus across our pipeline and portfolio including: Vaccines We’ve built one of the broadest vaccine portfolios in the industry to help protect people at all stages of life, from childhood to older age. With our wide range of vaccine technologies like MAPS, mRNA and adjuvants, we can take a targeted approach, allowing us to develop tailored vaccines for different diseases and individuals – see page 16. HIV For decades, we’ve transformed the lives of people living with HIV by making breakthroughs in treatment and prevention. We’re focusing research on novel treatment options that allow people living with HIV to take fewer drugs or take them much less often, and we’ve also developed a long-acting regimen that can prevent HIV – see page 20. Severe asthma Our decades of experience in respiratory care have led us to create treatments that could bring patients closer than ever before to remission for severe asthma. This could free them from exacerbations (attacks) that cause cumulative lung damage and could potentially avoid hospitalisation – removing the need for oral corticosteroids, stabilising lung function and controlling symptoms – see page 21. Hepatitis B Using the latest AI/ML techniques, our scientists have identified biomarkers to help work out which treatment combinations fit which patients. This potentially increases the likelihood of achieving 'functional cure' – when the virus is no longer present in the blood, and liver functions have normalised, stopping any future damage – see page 17. We believe that preventing and getting ahead of disease is the best investment for everyone – for patients, carers, communities, health systems and economies. We want to work with patients, policy makers and our peers to stop disease in its tracks, creating the right conditions to champion prevention and enable timely, proactive access to preventative interventions. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F At GSK, we believe prevention is the best medicine 11

Our purpose – to unite science, technology and talent to get Ahead of disease Together – puts our people at the heart of our success. Our culture We are committed to making GSK a place where people can thrive, with a culture where we are all ambitious for patients, accountable for impact, and do the right thing. This means we support our people to do things better and faster, focusing on what matters most. It means setting clear objectives, creating accountability for results and giving everyone the support and space they need to succeed. It means doing everything responsibly with integrity and care, because people and patients around the world count on us. Our culture is embedded in everything we do from our recruitment and onboarding, training and development, to our assessments of performance and promotion. Our Code sets out our culture as well as the commitments GSK and our people make so we can deliver on our ambition in the right way. Our people sign up to The Code annually and personally commit ‘I’m in’. + See The Code on gsk.com1 Helping people thrive Making GSK a place where people thrive is core to our Ahead Together ambition. While thriving is different for each individual, there are common themes that matter to everyone. Firstly, a belief in our purpose and a desire to live our culture and contribute to delivering our ambition. Secondly, feeling included and able to be yourself with opportunities to keep growing, with the support, feedback and space needed to succeed. And finally, feeling good, with positive mental, physical, financial and social wellbeing. This means GSK should be a place where people feel welcome and valued, in an environment (including our policies, workplaces and ways of working) that enables and supports them to deliver at their best. Welcoming and developing outstanding people We are committed to developing outstanding people and giving them opportunities to grow. We expect all our people to have an agreed development plan, regardless of grade or role, based on a conversation to understand what space and support they need to succeed. We continue to invest in learning and development initiatives which everyone can access through our Keep Growing Campus, our training and knowledge sharing platform. Digital and technology remain core to our purpose and delivery of our ambitions. We have built our people's skills in this area with global events such as DataCon, where all employees can experience immersive sessions to see first- hand how to apply digital, data and tech tools including generative AI to become more digitally fluent. This year, more than 7,000 employees took part from every business unit and 28 countries. In our Data Academy, employees can access resources and online training. We've run programmes to develop our senior leaders' leadership skills in the digital age. We've also piloted a career hub using AI to match employees with mentors, projects and potential job opportunities. We will scale this up in 2024. In 2023, we enhanced our onboarding experience for new joiners by introducing monthly live virtual sessions with our CEO and other senior leaders. By having access to senior global leaders from the beginning of their career with us, we aim to provide a more intimate connection to GSK and the patients we serve, creating emotional connection with our purpose, strategy and culture, to complement ongoing local onboarding activities. Supporting our people managers Our people managers play a crucial role in helping their teams to thrive and connecting the contributions the team makes to the patient and GSK's broader impact. We expect people managers to motivate, focus, care for and develop their teams and we deliver training anchored in these four areas. In 2023 all of our VPs were invited to attend a four day in-person event called Leading Leaders, a programme to help leaders bring out the best in their teams and foster the culture we need to succeed together. We also continue to invest in growing the next generation of senior leaders to support our talent and succession needs through bespoke development interventions, equipping them with leadership skills for the future. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Our culture and people 12 (1) https://www.gsk.com/en-gb/company/governance/compliance/#the-code

Maintaining momentum on diversity, equity and inclusion We are continuing our focus on building a more diverse organisation and an equitable and inclusive culture so that everyone feels welcome, valued and included. By taking steps to ensure equal opportunity and non-discrimination, we are delivering on our ambition to make our leadership and teams more diverse and inclusive. We support development for all with numerous offerings for our employees, including an award-winning leadership development programme, Accelerating Difference. Also, all our people complete a mandatory DEI module as part of our annual training, this year focused on how to create an inclusive workplace so all our people can thrive. For more details on our DEI aspirations, see the Responsible Business section on page 50. Health, wellbeing and volunteering Our health and wellbeing benefits support people through different life stages and are fair and inclusive. These include: a global minimum standard of 18 weeks’ parental leave for primary and secondary carers for all forms of family, a global minimum standard for care of a family member for end of life or serious health emergencies, insured benefits to include same-sex partners wherever possible, and mental health training – available to everyone. We have also enhanced our financial wellbeing support for employees by introducing the ‘nudge’ financial education platform in over 50 countries, helping people manage their finances and achieve their financial goals. In 2023 we reignited volunteering across the company, focused on our ambition and charitable investment themes (Health for people, Health for the planet, Innovators for the future). All employees can volunteer for one or two days each year by taking part in team-based hands-on ‘Together Days’ or through skills-based volunteering. A smaller number of people can volunteer up to four days each year for selected skills-based volunteering projects. Performance with Choice Performance with Choice, our approach to hybrid working for those in office-based roles (about a quarter of our people), allows the right balance of on-site and remote working. We are clear in our expectations that people take accountability to spend enough time together in person, while maintaining flexibility, to help us continue to build our sense of community and connectedness, enable development and achieve our Ahead Together ambitions. Data from our annual employee survey shows broad support for our approach and expectations. Recognising and rewarding our people Sharing our success and recognising and rewarding our people equitably, not just on the progress we have made but how we have made it, continues to be an important part of our culture. In addition to our bonus scheme that rewards performance across the company, each year we award 10% of our people with extra ‘Ahead Together’ awards for delivering exceptional performance in line with being accountable for their impact, ambitious for patients and doing the right thing. And we identify 5% of people as having missed performance for those not delivering on their objectives or living the culture. How our people experience GSK To ensure we continue to listen to our people, we regularly measure their experience of GSK as a place to work. This includes an annual survey for all employees featuring questions on engagement, confidence, inclusivity, our culture focus areas and trust priorities. We are proud that our engagement levels remained high at 81% in 2023. We also continue to see high scores with positive upward trends in confidence in delivery of our strategy and our culture focus areas – ambitious for patients, accountability for impact and doing the right thing – as well as measures of inclusion. In 2023 we expanded analysis of the survey to understand differences in employee experience across diverse characteristics. We continue to make good progress in creating a culture and workplace where people feel a sense of belonging and can thrive. To measure the effectiveness of our global managers, their teams provide feedback through an annual One80 survey and managers receive anonymised aggregate feedback. In 2023, 78% of our managers were rated as highly effective by their teams. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Our culture and people continued 13

Research and development We combine the science of the immune system with technology and outstanding talent to find new ways to prevent and treat the most challenging diseases, better and faster. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F 14

Highlights 71 vaccines and medicines in the pipeline 18 in phase III/registration 4 major approvals – Arexvy, the world’s first RSV vaccine for older adults, approved in the US, EU and Japan – Apretude, long-acting preventative treatment for HIV, approved as the first and only HIV prevention option in Europe – Ojjaara/Omjjara approved in the US, EU and UK as the first and only treatment for both newly diagnosed and previously treated myelofibrosis patients with anaemia – Jemperli approved in the US, EU and UK as the only frontline immuno-oncology treatment, in combination with chemotherapy, for patients with dMMR/MSI-H primary advanced or recurrent endometrial cancer – Shingrix vaccine for shingles approved for people at risk over 18 in Japan and positive data from first efficacy trial in adults aged 50 and over in China – Positive phase III data for our MenABCWY vaccine candidate, supporting filing in 2024 – US FDA Fast-Track designation for gonorrhoea vaccine candidate – Targeted business development including acquisition of Bellus Health and Aiolos Bio1 (respiratory), licence agreements with Janssen (infectious diseases) and Hansoh Pharma (oncology) Our R&D approach Our R&D purpose is to unite science, technology and talent to get ahead of disease. This is how we discover and develop the vaccines and medicines that will transform people’s lives. In 2023, our R&D expenditure was £6.2 billion, up 13% AER and 14% CER on 2022, driven by investment across the portfolio. We’ve also strengthened our pipeline and technology capabilities through business development, seeking out new, differentiated opportunities in diseases with high patient need. We now have 19 vaccines and 52 medicines in development, many with the potential to be first-in-class or best-in-class. In a revolutionary era of science and technology, we’re making the most of rapid advances to drive the discovery and development of vaccines and medicines. Across our pipeline, we consider not just how we can prevent disease in the first place, but also intervene and treat earlier to change its course, preventing or slowing progression of an illness and limiting longer-term complications. Focusing on execution, technology and culture Our priorities in R&D are: – execution, to accelerate our pipeline, including with business development, to deliver innovative vaccines and medicines, see page 16 – technology, to deliver more innovation, better and faster, using new platform and data technologies that speed discovery and development and improve the chance of success, see page 25 – culture, to create an agile, innovative environment that’s ambitious for patients and attracts the best people, scientists and partners, see page 27. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development 15 For more on why and how prevention underpins our purpose to get ahead of disease, see page 11 (1) Closed in early 2024.

Execution Pipeline acceleration and business development for transformational vaccines and medicines. Our broad portfolio and pipeline, based on the science of the immune system and the use of new platform and data technologies, continues to strengthen, with key milestones across our core therapeutic areas in 2023. This positive momentum, together with further business development, underpins our confidence in delivering our growth strategy for the medium and long term. Across all phases, our pipeline now has 71 vaccines and medicines. More than 70% modulate the immune system and a similar proportion are based on genetic evidence. In 2023, we began eight phase I programmes, moved 14 assets into phase II and three into phase III. Since 2016 our development cycle times have shortened by 20%, or 3.7 years, with a median of 9.6 years, compared to the industry's 11.4 years. We’re investing heavily in our late-stage pipeline to drive growth in line with our therapeutic area strategies. We rigorously evaluate our early-stage portfolio to back the right programmes to maximise our impact on health and unlock pipeline value. Reflecting our progress in 2023, we are now planning for at least 12 major product launches from 2025. In 2023, we reinforced our status as a world leader in infectious diseases. We gained approvals in the US, EU and Japan for our world-first respiratory syncytial virus (RSV) vaccine for older adults, Arexvy, and in Japan for our shingles vaccine, Shingrix, for people at risk over 18. In HIV, we’re reshaping treatment and prevention by delivering long-acting regimens, such as Apretude, approved in Europe for HIV prevention in 2023. In oncology, we’re optimising our portfolio, focusing on blood and women's cancers, and breakthroughs in immuno-oncology. In 2023, there were approvals for Ojjaara, the first treatment specifically indicated for myelofibrosis patients with anaemia and Jemperli, our frontline treatment for endometrial cancer. We also had positive phase III results for Blenrep, our treatment for multiple myeloma. Business development is a critical contributor to growth, creating extra value for patients, partners and shareholders. Major deals include our acquisition of Bellus Health and Aiolos Bio1 and new collaborations including with Janssen and Hansoh Pharma which we believe will bolster our existing strengths across our therapeutic areas. We focus on four therapeutic areas: – infectious diseases, see below – HIV, see page 20 – respiratory/immunology, see page 21 – oncology, see page 23. Infectious diseases Infectious diseases affect everyone, everywhere, putting a major strain on societies and healthcare systems. Our combined expertise in vaccines and medicines means we can focus on both prevention and treatment of infectious diseases, resulting in significant public health benefits, reduced deaths and increased productivity. Two thirds of the vaccines and medicines in our pipeline address infectious diseases (including HIV), and we’re a world leader in this area. – Infectious diseases are responsible for an estimated one in six deaths globally. – Around one billion people are infected every year by viruses like RSV, influenza virus and SARS-CoV-2 and many need hospital treatment. – Millions more struggle with bacterial and fungal infections or live with chronic viral conditions like hepatitis B (hep B) and HIV. – Vaccine-preventable diseases impose significant medical and economic costs related to treatment and to cover resulting productivity losses. For over 70 years, we’ve pioneered novel research methods and technologies to help protect people against infectious diseases including: chronic infections (hepatitis B, HIV, shingles), seasonal infections (RSV, influenza), common childhood diseases (measles), rare but devastating conditions (meningitis) and a range of bacterial infections made more challenging by antimicrobial resistance (AMR); as well as diseases which predominantly affect lower- income countries (malaria, TB, rotavirus). Of the more than 2.5 billion people we reach this decade, a significant majority will be through our infectious disease portfolio, which is the broadest in the industry. In 2023, key highlights have included approvals for Arexvy, our world-first RSV vaccine for adults aged 60 and above, and positive phase III data for our pentavalent meningitis vaccine candidate. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development continued 16 (1) Closed in early 2024.

Tackling RSV with the world's first Arexvy vaccine – Around 470,000 older people each year face hospital stays because of RSV. – People with underlying conditions like chronic obstructive pulmonary disease (COPD), asthma, diabetes and heart disease are at increased risk of the severe outcomes of RSV, such as pneumonia. – Around 14,000 people a year die from RSV in the US, and in the EU the figure is closer to 20,000. In 2023, Arexvy was approved in the US, EU, Japan and several other countries for the prevention of lower respiratory tract disease caused by RSV in adults aged 60 and older. This followed positive phase III data published in the New England Journal of Medicine which showed exceptional efficacy in older people, including those with certain underlying medical conditions, and against severe RSV. In 2023, we reported data from season two of our ongoing phase III trial showing vaccine efficacy over two complete RSV seasons. The clinical development programme will continue to evaluate longer-term follow-up and the optimal timing for potential revaccination. In the US, following approval by the US Food and Drug Administration (FDA), the US Centers for Disease Control and Prevention’s Advisory Committee on Immunisation Practices recommended that adults 60 years of age and older may receive a single dose of the vaccine using shared clinical decision-making. Arexvy is now available across the US, Canada and multiple European countries. Regulatory reviews in other countries are ongoing, with approvals and launches expected throughout 2024 and beyond. In 2023, we also reported positive preliminary data from a clinical trial in people aged 50 to 59 at increased risk of RSV showing non-inferior immune responses compared to adults aged 60 and older. Based on these data, in February 2024, the US FDA accepted a regulatory application under Priority Review to extend the vaccine's indication for adults aged 50-59 at increased risk. Regulatory submissions for adults aged 50-59 were also accepted by the European Medicines Agency and the Japanese Ministry of Health, Labour and Welfare. In 2024, we expect to generate further data in people aged 18 and older at increased risk of RSV, as well as from trials exploring co-administration with other adult vaccines including for shingles and pneumococcal disease. Expanding the use of our shingles vaccine – One in three people develop shingles in their lifetime, sometimes with serious consequences like long-term nerve pain and loss of vision. Shingrix, our vaccine to protect people from shingles, has launched in 40 countries for people over 50 and for people over 18 at increased risk of shingles. Shingrix was specifically designed to combine one of our adjuvants with an antigen selected to enhance a protective immune response, based on our understanding of the virus that causes shingles. This formulation helps overcome the natural age-related decline in immunity that can make protecting older people from infectious diseases challenging. In 2023, Shingrix was approved in Japan for the prevention of shingles in people over 18 at increased risk, for instance due to immune suppression or immune deficiency. The vaccine has been approved in Japan for people aged 50 and older since 2018. The latest approval followed six clinical trials with people aged 18 or older at increased risk of shingles, including those who had undergone stem cell transplants or kidney transplants, or who had blood cancer, solid tumours or HIV. A regulatory application for this patient group was also accepted for review by the China National Medical Products Administration in February 2024. In 2023, we reported data from the first-ever efficacy trial of Shingrix in China, which demonstrated 100% vaccine efficacy. These results come from the phase IV trial (ZOSTER-076), which evaluated the efficacy and safety of the vaccine in preventing shingles in adults aged 50 and older. Progressing towards a 5-in-1 meningitis vaccine – Around 1.2 million people contract invasive meningococcal disease (IMD) each year, and one in six people diagnosed with it will die. – At least one in five IMD survivors will have long-term disabilities including brain damage, deafness and nervous system problems. Our meningitis ACWY vaccine Menveo and meningitis B vaccine Bexsero together protect against most forms of IMD. Our first-generation 5-in-1 vaccine candidate combines these vaccines, aiming to protect against the serotypes that cause most disease globally in a single vaccine. In 2023, we presented preliminary phase III data to the European Society for Paediatric Infectious Diseases showing the vaccine candidate performed statistically as well as Bexsero and Menveo in people aged 10 to 25. It’s currently the only investigational 5-in-1 vaccine with data to show immunological effectiveness against 110 diverse meningitis B invasive strains in a trial. Multivalent vaccines of this kind have the potential to support the WHO's strategy to eradicate meningitis by 2030. We also have a second generation 5-in-1 vaccine in phase II development, which aims to improve protection against B strains in broader age groups. Trials for our investigational medicine for chronic hepatitis B (CHB) – Around 300 million people are living with CHB. – Only about 10% of these people have a diagnosis, 5% receive treatment and almost a million die each year. – Currently, patients take nucleoside/nucleotide analogues (NA), often for life, because they suppress the virus but rarely clear it. For 35 years, we’ve been a leader in hepatitis B vaccination. Bepirovirsen, our triple-action antisense oligonucleotide, has the potential to be the cornerstone of functional cure for patients with CHB. It could eliminate the need for continued therapy, ultimately reducing the long-term risk of developing liver complications. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development continued 17

Bepirovirsen is the only single agent in phase III development that has shown a clinically meaningful functional cure response for patients with CHB receiving NA therapy, as demonstrated in the B-Clear and B-Sure clinical trials. As well as developing bepirovirsen in our phase III trials for patients receiving NAs, we are also exploring potential sequential therapy options with the aim of helping more patients achieve functional cure. In October 2023 we announced an exclusive licence agreement for a phase II small interfering RNA-based therapeutic, originally developed by Arrowhead Pharmaceuticals. This provides a further opportunity to develop a potential novel sequential regimen to benefit a broader group of patients and potentially drive higher functional cure rates. Other infectious diseases Pneumococcal disease – Pneumococcal disease is the name for any illness caused by the Streptococcus pneumoniae bacterium, which is a leading cause of acute bacterial diseases and an important area of growing antimicrobial resistance. – Multiple licensed pneumococcal vaccines are available, however the burden of pneumococcal disease remains significant. – In the US alone, it is estimated that pneumococcal pneumonia causes 150,000 hospitalisations every year. – The WHO estimates that about one million children die of pneumococcal disease every year. Our novel 24-valent vaccine candidate (currently in phase II development) and 30 plus-valent pneumococcal vaccine candidate (currently in pre-clinical development), added to our pipeline through our 2022 acquisition of Affinivax, both incorporate innovative MAPS platform technology. MAPS potentially enables higher antibody responses against more disease-causing serotypes for broader and stronger protection (see page 25). We continue to examine potential acceleration options in the 24- and 30-plus valent programmes for infants and adults. Herpes simplex virus – Genital herpes is a chronic sexually transmitted infection caused by herpes simplex type 1 (HSV-1) and herpes simplex type 2 (HSV-2) viruses. – Worldwide, an estimated 683 million people aged 15 to 49 are living with HSV-2 or genital HSV-1 infection. – Many patients suffer frequent outbreaks along with psychological morbidity, stigma and a threefold increase in the risk of acquiring HIV. GSK 3943104 is our candidate against HSV that contains HSV antigens complemented with an adjuvant, designed to stimulate immune responses in people already infected with HSV. Following the successful completion of a phase I first- time-in-humans study, a phase II first-time-in-patients proof of concept trial started in late 2023 and is assessing two formulations in adults with a history of genital herpes outbreaks. If successful, we hope that this could help better control symptomatic outbreaks and viral shedding while mitigating the associated emotional burden and improving quality of life for people living with genital herpes. Influenza – Influenza remains one of the world’s greatest public health challenges. – Every year, there are an estimated one billion cases around the world, many resulting in severe illness and death. Our adjuvanted pandemic influenza vaccine has been extensively studied and consists of egg-based antigen and pandemic adjuvant AS03. We have agreements with the US, Canada, Europe and the WHO to provide at least 200 million doses of pandemic influenza vaccine in the event of a global health emergency. Egg-based influenza vaccines are the backbone of worldwide efforts to limit the impact of seasonal influenza. Different platforms and technologies will continue to be needed in the future and we’re committed to playing our part in meeting an important patient need. We’re exploring opportunities to develop mRNA-based influenza vaccines through our collaboration with CureVac. Building on positive phase I results for modified monovalent mRNA vaccine candidates that target COVID-19 and monovalent flu, we’re developing a next-generation multivalent mRNA flu vaccine to protect against multiple influenza virus strains. Phase I/II trials are underway. COVID-19 Now that the acute phase of the COVID-19 pandemic is over, our focus is on next-generation platforms and combination vaccines that have the potential to protect against multiple seasonal respiratory viruses. In 2023, our COVID-19 mRNA development programme with CureVac progressed to a phase II clinical trial and we recently reported positive interim data for both the monovalent and bivalent vaccine candidates. Human papillomavirus Human papillomavirus (HPV) is a common sexually transmitted infection affecting around 14 million people a year in the US alone. It often has no symptoms but can cause genital warts and several types of cancer. HPV is associated with nearly all (99%) cases of cervical cancer, which is the fourth most common cancer among women globally and causes an estimated 342,000 deaths each year. HPV also accounts for about 5% of all cancers worldwide, including 90% of anal cancers and 70% of oropharyngeal cancers. We’re working with Innovax on a next-generation adjuvanted vaccine to protect against more types of HPV. Antibiotics and antimicrobial resistance Antimicrobial resistance (AMR) is one of the world’s top 10 health threats. It’s estimated that, without action, AMR, including antifungal resistance, could contribute to 10 million deaths per year by 2050 and cause an economic loss of Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development continued 18

£100 trillion. Across our medicines and vaccines pipeline, we have more than 30 projects relevant to AMR, 12 of them targeting pathogens deemed ‘critical’ or ‘urgent’ by the WHO. Moving towards a potential treatment for uncomplicated urinary tract infections and urogenital gonorrhoea – Over half of women are affected by uncomplicated urinary tract infections (uUTI) in their lifetime, with over a quarter suffering recurring disease. – Despite concern over rising resistance to existing treatments, there’s been no new class of antibiotics in uUTI for over two decades. Our investigational antibiotic gepotidacin is a novel mechanism triazaacenaphthylene antibiotic for uUTI and gonorrhoea, discovered and developed by us, and part-funded by our partnership with the US Biomedical Advanced Research and Development Authority (BARDA). In 2023, positive phase III data showed it has the potential to be the first in a new class of oral antibiotics for uUTI in over 20 years. In the EAGLE-2 and EAGLE-3 phase III trials, which were stopped early for efficacy in November 2022 following a planned interim analysis, gepotidacin performed as well as nitrofurantoin, an existing first-line treatment for uUTI. In the EAGLE-3 trial, gepotidacin demonstrated statistically significant superiority over nitrofurantoin. Treating complicated urinary tract infections with tebipenem Through our partnership with Spero Therapeutics, Inc., we have an exclusive licence agreement for tebipenem HBr, a late-stage oral carbapenem antibiotic with the potential to treat complicated urinary tract infections (cUTIs). In December 2023, the first patient was dosed in PIVOT-PO, our pivotal phase III trial for tebipenem. If approved, tebipenem HBr will address an unmet medical need for a novel oral antibiotic as an alternative to intravenous hospital therapy for drug-resistant cUTIs. Vulvovaginal candidiasis In 2023, we also signed an exclusive licence agreement with Scynexis to develop and further commercialise Brexafemme, a US FDA-approved first-in-class antifungal treatment for vulvovaginal candidiasis (VVC) and for reducing the incidence of recurrent VVC. Brexafemme complements gepotidacin and tebipenem, and reinforces our commitment to developing new antibiotic and antifungal treatments in areas of high unmet medical need. Fast-tracking our gonorrhoea vaccine – Gonorrhoea is the second-most prevalent bacterial sexually transmitted infection worldwide, with an estimated 82 million new cases each year. – AMR to gonorrhoea has increased over the past 80 years, rendering many classes of antibiotics to treat the disease ineffective and making a vaccine even more important to the global effort to tackle AMR. Our investigational Neisseria gonorrhoeae (NgG) vaccine, based on our generalised modules for membrane antigens (GMMA) technology, aims to protect people aged 16 and older. Currently in an ongoing phase I/II efficacy trial, NgG received a Fast-Track designation from the US FDA in 2023, accelerating its path to FDA submission. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development continued 19

HIV For decades, we’ve transformed the lives of people living with HIV by making breakthroughs in treatment and prevention. Our work to develop long-acting injectable medicines means that many only need therapy a few times a year, instead of once a day. – The WHO estimates there were approximately 1.3 million new HIV infections globally in 2022, with the burden greatest in sub-Saharan Africa. – In the US, about two-thirds of people living with HIV are virally suppressed and there were more than 36,000 new diagnoses in 2021. – There remains a pressing need for new approaches to treatment and prevention. We work on HIV through ViiV Healthcare, which we majority own, with Pfizer and Shionogi as shareholders. ViiV Healthcare is the only company that is 100% focused on the treatment and prevention of HIV. Our goal is to leave no person living with HIV behind. We’ve focused research on transforming the experience of people living with HIV through novel treatment options that allow them to take fewer drugs or take them much less often. We’ve also developed a long-acting regimen that can prevent HIV. Transforming patients’ lives with long-acting regimens Cabenuva (cabotegravir; rilpivirine) is the world’s first and only complete long-acting injectable regimen to treat HIV. It means some patients have treatment only six times a year instead of taking medicine orally every day. Our SOLAR study data, announced in 2023, showed Cabenuva is as effective as daily Biktarvy tablets for treating HIV. The 12- month findings also showed that nine out of ten participants switching from Biktarvy to Cabenuva preferred the long- acting regimen. Apretude (long-acting cabotegravir), launched in 2022, is the world’s first and only long-acting injectable pre-exposure prophylaxis (PrEP) to reduce the risk of sexually transmitted HIV. Two large phase III studies demonstrated that Apretude was superior to daily oral PrEP (TDF/FTC) in men and women. And, in the open label phase, when given the choice, the majority of study participants chose Apretude over oral TDF/FTC. The European Commission authorised Apretude in 2023 in injectable and tablet form. This followed a positive opinion from the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP). Apretude is also approved in Australia and South Africa, among many others. Looking to the future of long-acting treatment and prevention Through a new formulation (reformulated CAB), we’re now focused on progressing to injectable doses every four months, doubling today’s interval for cabotegravir for treatment and PrEP, which would halve visits to the clinic to three times a year. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development continued 20

We aim to make this a reality for prevention by 2026, with a registrational study starting in 2024. For treatment, we aim to deliver by 2027, by evaluating possible combinations of reformulated CAB with rilpivirine or our broadly neutralising antibody, N6LS. In 2023, we completed a study that combined N6LS with Halozyme’s recombinant hyaluronidase (PH20) technology, which allows delivery of a larger volume of drug through subcutaneous dosing. This showed it’s possible to deliver a single subcutaneous dose that’s well-tolerated and can last up to four months. Beyond this, six-monthly doses are our goal by the end of the decade by partnering our new integrase inhibitors, VH184 or VH310, with new-mechanism-of-action agents such as capsid inhibitors. Moving towards self-injected long-acting treatment Our other main aim is to develop the world’s first long- acting treatment that people living with HIV can inject themselves. This will allow individuals to dose at home and reduce the number of clinic visits. We are targeting dosing every two to three months, with efficacy and tolerability similar to Cabenuva. In short, our goal is to develop new agents for HIV treatment and prevention that reduce the burden of treatment and allow people to have improved quality of life. Respiratory/immunology We’ve been leaders in delivering medicines that help manage asthma and COPD for over 50 years. Our research looks to harness the science of the immune system to develop medicines that reduce signs and symptoms of disease, address treatment resistance and slow the progression of immune-mediated conditions. These include lupus, severe asthma with an eosinophilic phenotype and other inflammatory diseases. We help millions of people with respiratory and immune conditions worldwide with our current portfolio. Advancing the science and treatment of IL-5 mediated diseases For more than 25 years we have been leaders in researching the roles that eosinophils (a type of white blood cell) and interleukin-5 (IL-5) play in health and disease. – Eosinophil-driven diseases are associated with heightened levels of eosinophils. When eosinophils infiltrate certain tissues, they can cause inflammation and organ damage which, over time, can affect patients’ day- to-day life. – IL-5 is the major cytokine responsible for the proliferation, activation and survival of eosinophils, making it a proven treatment target for patients with higher levels of eosinophils. – IL-5 mediated conditions encompass a range of diseases for which there have been few, if any, effective treatments. These include respiratory conditions like severe asthma with an eosinophilic phenotype, COPD and chronic rhinosinusitis with nasal polyps (CRSwNP), and rarer conditions like eosinophilic granulomatosis with polyangiitis (EGPA) or hypereosinophilic syndrome (HES). Our research aims to redefine treatment goals across these conditions, going beyond optimal management of daily symptoms, to modify the course of disease. This could slow or halt disease progression, reduce the risk of organ damage and even mean some people could achieve clinical remission. Nucala is a first-in-class anti-IL5-biologic (monoclonal antibody) that targets and directly inhibits IL-5. It is the only treatment in the US and Europe with indications in four IL-5 mediated diseases: severe asthma with an eosinophilic phenotype, CRSwNP, EGPA and HES. In 2023, the Japanese Ministry of Labour, Health and Welfare accepted for review a supplementary new drug application for Nucala to treat CRSwNP in adults. This submission is based on data from the pivotal phase III MERIT trial studying the safety and efficacy of Nucala in people with CRSwNP. In January 2024, the China National Medical Products Administration approved Nucala as an add-on maintenance treatment for severe asthma with an eosinophilic phenotype. Nucala is the first targeted IL-5 treatment in China for adult and adolescent patients with the condition. Depemokimab is our novel monoclonal antibody developed for its affinity for IL-5 and long-acting inhibition of the IL-5 pathological process, which includes suppression of eosinophil activity. It is the first potential ultra-long-acting anti-IL-5 biologic that treats a range of IL-5 mediated diseases. Our phase III programme continues to make progress across diseases including severe asthma, CRSwNP, HES and EGPA. Currently, approved IL-5 inhibitors are dosed every four or eight weeks, while depemokimab is designed to be administered every six months, addressing the challenges commonly associated with more frequent dosing including adherence anxiety and emotional burden. Reaching a broader range of asthma patients In early 2024, we acquired Aiolos Bio, Inc. The acquisition adds AIO-001, a phase II-ready, long-acting antibody that targets the clinically validated TSLP pathway to our respiratory pipeline. This could redefine the standard of care for asthma patients with dosing every six months. AIO-001 has the potential to expand our reach to a broader portion of asthma patients, including the 40% of severe asthma patients with low T2 inflammation where treatment options are still needed. In addition to the treatment of adult patients with asthma, AIO-001 also has the potential for additional indications including chronic rhinosinusitis with nasal polyps. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development continued 21

Progress towards a treatment for refractory chronic cough with camlipixant – Approximately 28 million people suffer from chronic cough, with about 10 million worldwide suffering from refractory chronic cough (RCC) for over a year. – RCC is a cough that persists for more than eight weeks and doesn’t respond to treatment for an underlying condition or is otherwise unexplained. – For decades there have been no effective treatments for RCC, with patients often suffering from depression, urinary incontinence, rib fractures and loss of sleep. In 2023, we acquired Bellus Health, which included camlipixant, a potential best-in-disease and highly selective oral P2X3 antagonist currently in phase III development as a first-line treatment for adults with RCC. Current clinical data show that by selectively inhibiting P2X3 receptors, camlipixant may reduce cough frequency for patients suffering from RCC with a relatively low incidence of dysgeusia. This is the taste disturbance associated with other medicines that broadly target the P2X2/3 receptor. We expect data in 2025 from the phase III CALM development programme, evaluating the efficacy and safety of camlipixant. Treating systemic sclerosis with Benlysta We continue to work to realise the full potential of Benlysta, our anti-B Lymphocyte stimulator (BLyS) monoclonal antibody, so that people affected by a range of immune- mediated conditions beyond lupus and lupus nephritis (LN) can benefit from its targeted mode of action, and reassuring safety profile. Systemic sclerosis (SSc) is a rare autoimmune disease that causes atypical growth of connective tissues and can affect the musculoskeletal system, heart, lungs, kidneys, skin and other organs. Interstitial lung disease (ILD), marked by inflammation and scar tissue build-up in the lungs, affects as many as half of people living with SSc. Current treatment options are limited. In 2023, the US FDA granted Orphan Drug Designation (ODD) to Benlysta as a potential treatment for SSc. The ODD is a special status granted to support development and evaluation of potential medicines to treat, diagnose or prevent rare diseases or disorders affecting fewer than 200,000 people in the US. We began a phase II/III trial for SSc-associated ILD in 2023. We will be exploring other potential studies in a wider range of potential indications in 2024. Benlysta remains the first and only approved biologic for both systemic lupus erythematosus (SLE) and LN in more than 50 years. Its robust efficacy and long-term safety have been recognised in updated recommendations from the European Alliance of Associations for Rheumatology (EULAR) for the management of SLE and LN, endorsing earlier use in the treatment pathway. We plan a phase IV study in early 2024 to further inform the proactive management of lupus to prevent organ damage. Benlysta has been approved for use in over 75 countries to treat adults with SLE. This has been extended to include children aged five and older with SLE in the US, Japan, the EEA countries, the UK and over 15 other countries. Benlysta is currently approved to treat adults with LN in the US, all EEA countries, the UK and over 15 further countries. In the US, this indication includes children aged five and older with LN, and reviews for this continue in other countries. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development continued 22

Oncology Cancer, one of the world’s major causes of death, is a field where patients’ needs are still widely unmet and treatment options remain limited. We have an emerging portfolio in oncology that is focused on seeking solutions for blood and women's cancers, and making transformative break- throughs in immuno-oncology. Ojjaara (momelotinib), Blenrep (belantamab mafodotin), Jemperli (dostarlimab) and Zejula (niraparib) are the strong foundation of our work in blood and women's cancers. Our goal is to realise the full potential of our existing medicines, as well as expand our portfolio in areas of high unmet need. In 2023, we received approval in the US for Ojjaara our treatment for myelofibrosis. Omjjaara was then approved in the EU and UK in January 2024. We also received approval in the US, EU and UK for our immuno-oncology therapy Jemperli plus chemotherapy as a first-line treatment for endometrial cancer patients with a certain biomarker. We continue to evaluate dostarlimab in studies that further reinforce our ambition for it to become the backbone of our ongoing immuno-oncology research and development programme. Blood cancers Ojjaara: helping myelofibrosis patients with anaemia – Myelofibrosis (MF) is a rare blood cancer affecting around 25,000 people in the US. – Nearly all MF patients will eventually develop anaemia, requiring regular blood transfusions and leading over 30% to stop treatment with established therapies. – In addition to anaemia, patients can experience debilitating symptoms like night sweats, fatigue and bone pain, as well as an enlarged spleen (splenomegaly), bringing pain and inflammation and frequent infection risk. Ojjaara, taken orally once a day, is the only medicine specifically indicated for newly diagnosed and previously treated MF patients with anaemia. It treats anaemia, along with the constitutional symptoms and enlarged spleen that accompany the disease. This means it potentially offers a new standard of care for patients, as established treatments can further exacerbate anaemia. In September 2023, the FDA granted broad, line-agnostic approval for Ojjaara for the treatment of primary or secondary MF in adults with anaemia, regardless of previous MF therapy. This was followed by a positive CHMP opinion in November 2023 and approval by the European Commission, as well as MHRA approval, in January 2024. We’ve also submitted a new drug application in Japan. Blenrep: our treatment for multiple myeloma – Multiple myeloma is the third most common blood cancer globally and is generally considered treatable but not curable. – Approximately 176,000 new cases of multiple myeloma are diagnosed globally each year. – Research into new therapies is needed, as multiple myeloma commonly becomes refractory to available treatments. Blenrep is our antibody-drug conjugate treatment for relapsed/refractory multiple myeloma. Our DREAMM (Driving Excellence in Approaches to Multiple Myeloma) clinical development programme continues to evaluate the potential of Blenrep to address unmet need in early lines of treatment and in combination with novel therapies and standard of care treatments. In November 2023, we announced positive phase III results from the DREAMM-7 trial, showing potential for Blenrep combination therapy to benefit patients in earlier treatment lines. Interim analysis of DREAMM-7 showed that patients receiving Blenrep in combination with bortezomib and dexamethasone (BorDex) lived longer without their disease progressing than those receiving daratumumab plus BorDex, an existing standard of care combination therapy. We are sharing this data with health authorities and the scientific community as we await the results from DREAMM-8, another phase III combination trial exploring Blenrep’s potential in earlier therapy lines. Also during 2023, health authorities continued to review existing monotherapy indications for Blenrep in later therapy lines based on the results of previous studies. This included in December 2023, the EMA recommending against renewal of the conditional marketing authorisation for its existing fourth line and later monotherapy indication. Women's cancers Jemperli: a backbone immuno-oncology therapy – Endometrial, or uterine, cancer is the sixth most common cancer in women worldwide, with an estimated 417,000 new cases and 97,370 deaths in 2020. – About 30% of endometrial cancer cases have a biomarker known as dMMR/MSI-H. – Patients with this type of endometrial cancer have faced significant unmet need and typically experience poor long-term outcomes with standard of care chemotherapy. In 2023, Jemperli became the only immuno-oncology treatment approved in the US, EU and UK in the frontline setting in combination with chemotherapy for patients with mismatch repair deficient or microsatellite instability-high (dMMR/MSI-H) primary advanced or recurrent endometrial cancer. In the RUBY trial supporting these approvals, Jemperli plus chemotherapy showed a 71% reduction in the risk of disease progression or death compared to chemotherapy alone. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development continued 23

In 2023 we announced two additional positive data readouts for the RUBY phase III trial. In part 1 of the trial, Jemperli plus chemotherapy showed statistically significant and clinically meaningful overall survival benefit compared to chemotherapy in the overall population of patients with primary advanced or recurrent endometrial cancer. Jemperli is the only immuno-oncology combination regimen to achieve this. Part 2 of the RUBY trial, which evaluated Jemperli plus chemotherapy followed by Jemperli plus Zejula for the treatment of primary advanced or recurrent endometrial cancer, demonstrated significantly improved progression-free survival compared to chemotherapy alone in both the overall and mismatch repair proficient/ microsatellite stable (MMRp/MSS) patient populations. Jemperli is also approved as a stand-alone treatment for certain types of endometrial cancer. Earlier in 2023, the FDA converted the accelerated approval for Jemperli as a second-line treatment into a full approval as a monotherapy for adult patients with dMMR recurrent or advanced endometrial cancer, as determined by an FDA-approved test, that has progressed on, or following, a prior platinum-containing regimen in any setting, and who aren’t candidates for surgery or radiation. The European Commission's conditional approval for Jemperli as a monotherapy for adult patients in the same patient population was also converted to full approval. Zejula: our PARP inhibitor for ovarian cancer and beyond We continue to develop Zejula in multiple pivotal trials, assessing activity in gynaecologic cancers and other solid tumours and evaluating several potential combinations of Zejula with other therapeutics. Aiming to address the unmet medical needs of patients, the ongoing development programme includes the FIRST phase III trial assessing the potential for niraparib in combination with dostarlimab in first-line ovarian cancer maintenance and the ZEAL phase III trial evaluating niraparib in combination with standard of care for the maintenance treatment of first-line advanced non-small cell lung cancer. In addition, based on promising early clinical data for niraparib in glioblastoma in November 2023, we are exploring next steps for its clinical development in this type of cancer. Other cancers Colorectal cancer – Cancers that start in the colon or in the rectum, both of which are distinct sections of the large intestine, are classified as colorectal cancers. – Colorectal cancer is the second leading cause of cancer- related death and the third most common cancer worldwide, accounting for approximately 10% of all cancer cases. – In 2020, it was estimated that worldwide, there were more than 1.9 million new cases of colorectal cancer and more than 930,000 deaths. In January 2023, the US FDA granted dostarlimab Fast- Track designation for the treatment of dMMR/MSI-H locally advanced rectal cancer. We also started our AZUR clinical trial programme studying dostarlimab in certain colorectal cancer indications. AZUR-1 is a global, open-label, phase II clinical trial to investigate the efficacy and safety of dostarlimab as monotherapy – replacing chemotherapy, radiation and/or surgery – for treatment-naïve patients with dMMR/MSI-H locally advanced rectal cancer. If successful, there’s potential to transform the treatment of some patients with locally advanced rectal cancer. The trial aims to confirm results generated in a separate ongoing investigator-initiated trial by researchers at Memorial Sloan Kettering Cancer Center. In 2023, this trial reported that all participants treated with dostarlimab achieved clinical complete responses, enabling them to avoid surgery, chemotherapy and radiotherapy. We also began our AZUR-2 trial, a phase III trial that evaluates the efficacy of perioperative dostarlimab monotherapy compared with standard of care adjuvant chemotherapy in patients with high-risk early stage dMMR/ MSI-H colon cancer. If approved, this could give patients a new chemotherapy-free option that reduces the risk of disease progression through dostarlimab treatment in both neoadjuvant and adjuvant settings. Lung cancer – Lung cancer is the second most common cancer globally and the most common cancer in men. – In 2020, there were more than 2.2 million new cases of lung cancer worldwide. – The majority of lung cancers fall into a category called non-small cell lung cancer (NSCLC). While this form of lung cancer progresses more slowly, 40% of NSCLC cases will have spread beyond the lungs by diagnosis. In 2023, we published data from our phase II PERLA clinical trial showing a favourable numerical trend in overall survival results for dostarlimab plus chemotherapy compared to pembrolizumab plus chemotherapy in first-line metastatic NSCLC. Data from the PERLA trial supports our ambition for dostarlimab to become a backbone immuno-oncology therapy when used alone and in combination with standard of care and future novel cancer therapies, including targets along the CD226 axis. We have access to antibodies targeting all three known CD226 checkpoints – CD96, PVRIG and TIGIT. Our goal of studying these immune checkpoints in combination with dostarlimab is aimed at increasing the proportion of patients who respond to therapy and improving the durability of response. In 2023, our CD226 axis development programme continued with several early-phase trials underway, including GALAXIES Lung-201, our phase II platform study in first-line metastatic NSCLC that combines dostarlimab with belrestotug, our TIGIT antibody partnered with iTeos Therapeutics. GALAXIES Lung-201 will also explore a triplet combination with dostarlimab, belrestotug, and GSK6097608, our CD96 antibody. In addition, our two phase III trials in NSCLC continued in 2023 with readouts expected in 2024: Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development continued 24

– COSTAR Lung, our phase III, randomised, open-label three-arm trial is comparing investigational compound cobolimab plus dostarlimab plus docetaxel to dostarlimab plus docetaxel to docetaxel alone in patients with advanced NSCLC who have progressed on prior anti-PD-L1 therapy and chemotherapy. – ZEAL, our phase III, randomised, double-blind trial is assessing niraparib in combination with standard of care for the maintenance treatment of first-line advanced NSCLC. Pipeline growth through business development In October 2023, we announced an exclusive licence agreement with the Chinese company Hansoh Pharma for HS-20089, its B7-H4-targeted ADC. This supports our work in developing treatments for ovarian and endometrial cancers, as well as solid tumours. The B7-H4 surface antigen is over-expressed in ovarian and endometrial cancers and often associated with poor prognosis. As well as targeting B7-H4, HS-20089 uses clinically validated ADC technologies such as topoisomerase inhibitor payload (TOPOi). This is a validated mechanism of action in approved anti-cancer medicines and a proven standard of care in treating breast and ovarian cancers. In December 2023, we added to our oncology portfolio of clinical-stage ADCs by entering a second exclusive licensing agreement with Hansoh Pharma for HS-20093. HS-20093, a B7-H3 targeted ADC also utilising a clinically validated TOPOi payload, has shown promising initial clinical activity in lung cancer with potential to address unmet medical need in broader solid tumour indications including colorectal cancer. Technology New platform and data technology are fundamentally transforming how we discover and develop vaccines and medicines, speeding up discovery and development and improving the chance of success. Technology makes us more effective at every stage of the discovery and development process, so that we progress vaccines and medicines that are the first or best of their kind. Our early investment in these capabilities is already leading to differentiated, high-impact vaccines and medicines including a new vaccine for RSV, long-acting HIV prevention, and the prospect of a functional cure for chronic hepatitis B. We combine the power of genetic data and genomic insights with the speed and scale of AI to make better predictions and increase the probability of new vaccines and medicines becoming available for patients. Our AI team – one of the largest in the industry – works with our genomics team to improve how we select disease targets, determine the best technology approach, and identify groups of patients where a treatment might work best. We’re not doing this alone. We partner with the world’s best minds across academia and the tech and biotech industries – from large companies to small start-ups. This collaboration leads to new ways of thinking, so that together we can strive for the most innovative solutions for patients. Using platform technologies to discover and develop novel vaccines and medicines One of the major challenges in addressing diseases where no vaccines or medicines currently exist is that they are difficult to treat with small molecules or biologics. We’re overcoming this challenge by investing in both our own innovation and in external collaborations to develop a range of platform technologies. With platform technology, we pair disease targets with the best treatment modalities, addressing diseases once thought to be too difficult to target with drug discovery. These expand our ability to identify novel vaccine and medicine options to prevent or treat these diseases. We are investing in platform technologies including: Multiple antigen presenting system (MAPS), which allows us to develop multivalent vaccines for complex bacterial infections by introducing T-cell mediated, disease-specific anti-protein immunity. This potentially enables broader coverage against certain disease types and higher immunogenicity than current vaccines, as well as higher antibody responses. We are developing MAPS through our 2022 acquisition of Affinivax. We’ve mainly directed MAPS at preventing pneumococcal disease, and it’s part of our 24-valent pneumococcal vaccine candidate in phase II development (see page 18). This platform also shows promise against other pathogens, including those that cause hospital-acquired infections. mRNA, which enables protein synthesis in the human body, carrying the information required for cells to produce proteins. By using mRNA technology for vaccine development, specific proteins, or antigens, can be produced by the body’s own cells and elicit both humoral and immune responses, enabling the human immune system to prevent or fight disease. We’re developing mRNA in-house in parallel with our collaboration with CureVac, a biopharmaceutical company developing therapies based on mRNA. We’re currently developing RNA vaccines based on CureVac’s second-generation mRNA backbone, with monovalent and bivalent COVID-19 vaccine candidates in phase II. A multivalent seasonal influenza vaccine candidate to protect against multiple strains is also in phase I/II (see page 18). Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development continued 25

Small molecule design, paired with our own small molecule generative AI tools. Our system has the advantage of using known chemical reactions and building blocks to create large 'virtual libraries' of potential drug molecules for specific biological targets. The molecules comprising these virtual libraries have the advantage of being easily synthesised and free from many of the known problems associated with small molecule drugs since they are filtered by a series of machine-learned molecular property models based on GSK historical data and clean public sources. Oligonucleotides, which are short strands of DNA or RNA that can reduce, restore or modulate RNA through several mechanisms, giving them a unique capability to address a wide range of genomic targets in multiple therapeutic areas for the first time. Oligonucleotides currently in our pipeline include bepirovirsen for chronic hepatitis B; and GSK 4532990, a phase II programme for non-alcoholic steatohepatitis (NASH). We also have two collaborations to build a leading oligonucleotide platform: – In 2022, we entered a collaboration with Wave Life Sciences, which pairs our genetic expertise with Wave’s PRISM, the only oligonucleotide platform offering three RNA-targeting modalities (editing, splicing and silencing, including siRNA and antisense). The collaboration helps us accelerate drug discovery for newly identified targets, by matching them to the best therapeutic modality. – In 2023, we announced a partnership with Elsie Biotechnologies, Inc. The collaboration combines our expertise in DNA encoded library technologies with Elsie's drug discovery platform. Throughout the collaboration, we can exercise an option on a non- exclusive licence from Elsie for its discovery platform and P(V) chemistry technologies to use in our own oligonucleotide drug discovery research. Monoclonal antibodies, are produced by a single clone of cells or cell lines and consist of identical antibody molecules that are meant to modulate a patient’s immune system. We have all the platforms needed to make best-in-class monoclonal antibodies (like Nucala), bispecific antibodies, and antibody-drug conjugates (like Blenrep). We are also developing generative design capabilities based on increased use of next generation sequencing as well as public and proprietary protein structure tools. The structures designed using these tools are then realised using highly automated antibody synthesis, isolation, and purification processes. Adjuvants, substances that enhance the body’s immune response to antigens, which we use in Arexvy and Shingrix, our vaccines for RSV and shingles, and our HSV vaccine candidate, GSK 3943104. We are also working with Xiamen Innovax Biotech on a next-generation adjuvanted vaccine to protect against more types of HPV. Using genetic data to better understand disease and choose the right solutions for the right patients With data technology, we combine AI/ML with human genetics and functional genomics to understand patients, human biology and disease mechanisms. This makes us better at choosing and prioritising targets, designing trials and bringing new vaccines and medicines to patients. The combined power of biology and technology is profound and is reshaping the way science is done. For example, we now generate more data in one quarter than in our company’s 300-year history and, by the end of 2024, we aim to bring predictive, real-time insight to inform 90% of our progression and development decisions in our research. Combining AI, genetic and genomics for unexpected possibilities We have built in-house teams dedicated to genomics and AI, including at our key R&D sites in London, Tel Aviv, San Francisco, Seattle, Philadelphia and Boston. Their expertise helps us collect more data, generate more ideas and arrive at unexpected possibilities. They’re bringing us closer to finding vaccines and medicines for diseases that once felt outside our reach, making our research process faster, more effective, and more predictable. We have invested to build a world-class research data platform, which includes one of the world’s most comprehensive large language models on genetic disease. It brings over 700 billion data points into a single place to map gene expression and function activity. This enables our scientists to run experiments and get answers to questions in a matter of hours, a process that once took weeks or months. Genetics and genomics We are using a combination of genetics, functional genomics and genetic engineering techniques like CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) to enable us to screen and validate hundreds of genetic targets in parallel, instead of one at a time. Through the screening process we can discover causal genes through genomics and link to biomarkers that may predict disease. In 2023, we had 53 targets with strong genetic evidence in our pipeline, an increase from 45 in 2022. Applying data tech to our clinical research At the clinical stage of development, AI/ML and genomics are helping us assess how certain patient profiles might respond, so we’ll be able to make sure we have the right people in the right trials. This offers the potential to have shorter, less expensive clinical trials with greater chances of success. An example of this is our research on our antisense oligonucleotide bepirovirsen for chronic hepatitis B. Using ML, we developed algorithms that helped us categorise patients into five distinct subtypes based on their response to treatment. This almost doubled our ability to correctly predict future patient outcomes, compared to using the traditional methods. This is significant because it will help Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development continued 26

inform sequential and combination therapy options, potentially leading to better outcomes and ultimately helping more people living with hepatitis B experience functional cure. Collaborating to change the course of disease for patients By working with others we achieve more, better and faster to address disease areas of high unmet need and for as many people as possible. Our collaborations with UK Biobank, 23andMe and FinnGen have given us access to large genetic datasets to deepen understanding of diseases and improve drug discovery. We’re also a founding partner of Our Future Health, a UK initiative setting out to recruit up to five million people to capture genetic and medical information. And we work with Genes & Health and Discover Me South Africa to make sure we have a diverse genetic representation of diseases. In January 2024, we also announced we'd joined the Alliance for Genomic Discovery, further expanding our access to diverse genetic datasets. Other collaborations in functional genomics give us insights to help select targets that are more likely to become medicines. We continue to work with genomics research centres like the Broad Institute, affiliated with MIT and Harvard University in Boston, and the Altius Institute in Seattle. In the UK, our partners include the consortium Open Targets, which we co-founded. This work complements technology and biology projects underway at the Laboratory for Genomic Research, which we co-founded in 2019 with researchers at the University of California in San Francisco. These projects are automating and advancing CRISPR for new discoveries of disease mechanisms for immunology, oncology and neurology. Other collaborations are helping make advances in multiple fields of human health. – With King’s College London, we’re using tumour models alongside digital pathology and AI to develop personalised immuno-oncology treatments for solid cancers. – With PathAI, we’re working to accelerate R&D in oncology and NASH. – We established the Oxford-GSK Institute of Molecular and Computational Medicine (IMCM) with Oxford University in 2021. It combines human genetics with functional genomics and ML to focus on diseases including amyotrophic lateral sclerosis (ALS), Alzheimer’s and Parkinson’s. – Our work with precision medicine company Tempus has focused on using data to further enable clinical trial designs and target selections in oncology.	 Culture We create an agile, innovative environment that’s ambitious for patients and attracts the best people, scientists and partners To get ahead of disease, we need the best people – scientists, researchers, trial specialists, technologists and more – and an environment where they can thrive and make the most of their expertise, inside the company or as partners. Our R&D people work together in an inclusive environment to foster new ideas and make connections, including our scientists, technologists and data engineers working side by side. 27% of our R&D leadership team started their roles in the past two years, bringing 56 years of combined experience, adding to our leadership and delivering against key priorities. Our culture unites us in being ambitious for patients and accountable for impact, and always doing the right thing. This culture encourages teams to focus on what matters most, take smart risks and make informed decisions at pace. It also helps them take ownership of objectives, seize opportunities and solve problems together. To support this, in 2023 we’ve taken steps to focus even more intently on our core therapeutic areas, strengthen decision-making with clearer ownership and simplified, agile governance, and embed technology more deeply in our work. We’ve created three research units dedicated to vaccines and infectious diseases, respiratory and immunology, and oncology. Reporting directly to the Chief Scientific Officer, they use their expertise to pick the right targets for the right patients, leading clinical development through to phase II and making recommendations on phase III programmes. These research teams complement our ongoing research in HIV, through ViiV Healthcare. Close collaboration between R&D, commercial, manufacturing and medical leaders makes sure we match scientific potential with unmet patient need to maximise our impact on disease and deliver competitive commercial value. We’ve also created one research technologies organisation, bringing together platform and data groups to create a scaled engine for identifying and progressing targets for ourselves and our partners. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development continued 27
Pipeline overview We have 71 assets in development, of which 18 are late-stage. Phase III/Registration Arexvy (Recombinant protein, adjuvanted)1 RSV older adults (50-59 YoA)3 gepotidacin (BTI inhibitor)1 Uncomplicated UTI2 bepirovirsen (Antisense oligonucleotide)1 Chronic HBV infection2 Bexsero (Recombinant protein, OMV) Meningitis B (infants US) MenABCWY vaccine (Recombinant protein, OMV, conjugated vaccine) MenABCWY, 1stGen tebipenem pivoxil (Antibacterial carbapenem)1 Complicated UTI ibrexafungerp (Antifungal glucan synthase inhibitor)1 Invasive candidiasis Nucala (Anti-IL5 antibody) COPD depemokimab (Long-acting anti-IL5 antibody)1 Asthma2 latozinemab (Anti-sortilin antibody)1 Frontotemporal dementia2,4 camlipixant (P2X3 receptor antagonist) Refractory chronic cough Low carbon version of MDI5, Ventolin (Beta 2 adrenergic receptor agonist) Asthma6 Ojjaara/Omjjara (JAK1, JAK2 and ACVR1 inhibitor)3,7 Jemperli (Anti-PD-1 antibody)1 Endometrial cancer2 Zejula (PARP inhibitor)1 Ovarian cancer2 Blenrep (Anti-BCMA ADC)1 Multiple myeloma cobolimab (Anti-TIM-3 antibody)1 Non-small cell lung cancer linerixibat (IBAT inhibitor) Cholestatic pruritus in primary biliary cholangitis Phase II 3437949 (Recombinant protein, adjuvanted)1 Malaria fractional dose 4406371 (live, attenuated) MMRV new strain 3536852 (GMMA)1 Shigella 3528869 (Viral vector with recombinant protein, adjuvanted)1 Chronic HBV infection2,8 4023393 (Recombinant protein, OMV, conjugated vaccine) MenABCWY, 2ndGen8 4178116 (Live, attenuated) Varicella new strain 5101956 (MAPS)1 Adult pneumococcal disease, 24-valent 5101955 (MAPS)1 Paediatric pneumococcal disease, 24-valent 4106647 (Recombinant protein, adjuvanted)1 Human papillomavirus8 4348413 (GMMA) Gonorrhoea8 4382276 (mRNA)1 Seasonal flu 4396687 (mRNA)1 COVID-19 3993129 (Adjuvanted recombinant subunit) Cytomegalovirus8 3943104 (Recombinant protein, adjuvanted)1 Therapeutic herpes simplex virus8 5637608 (Hepatitis B virus-targeted siRNA)1 Chronic HBV infection 4077164 (Bivalent GMMA)1 Invasive non-typhoidal salmonella2 ganfeborole 3036656 (Leucyl t-RNA synthetase inhibitor)1 Tuberculosis sanfetrinem cilexetil (Serine beta lactamase inhibitor)1 Tuberculosis alpibectir BVL-GSK098 (Ethionamide booster)1 Tuberculosis 3810109 (Broadly neutralizing antibody)1 HIV 3739937 (Maturation inhibitor) HIV 4004280 (Capsid protein inhibitor) HIV 4011499 (Capsid protein inhibitor) HIV 4524184 (Integrase inhibitor)1 HIV9 Benlysta (Anti-BLys antibody) Systemic sclerosis associated interstitial lung disease 3858279 (Anti-CCL17 antibody)1 Osteoarthritis pain2 1070806 (Anti-IL18 antibody) Atopic dermatitis 4527226 (Anti-sortilin antibody)1 Alzheimer’s disease belrestotug (Anti-TIGIT antibody)1 Non-small cell lung cancer2 4532990 (HSD17B13 siRNA)1 Non-alcoholic steatohepatitis Phase I 3536867 (Bivalent conjugate)1 Salmonella (typhoid + paratyphoid A) 2556286 (Mtb cholesterol dependent inhibitor)1 Tuberculosis 3186899 (CRK-12 inhibitor)1,10 Visceral leishmaniasis 3494245 (Proteasome inhibitor)1 Visceral leishmaniasis 3772701 (P. falciparum whole cell inhibitor)1 Malaria 4024484 (P. falciparum whole cell inhibitor)1 Malaria 3882347 (FimH antagonist)1 Uncomplicated UTI 3923868 (PI4K beta inhibitor) Viral COPD exacerbations 3965193 (PAPD5/PAPD7 inhibitor) Chronic HBV infection8 5251738 (TLR8 agonist)1 Chronic HBV infection cabotegravir (Integrase inhibitor) HIV 3888130 (Anti-IL7 antibody)1 Autoimmune disease 3915393 (TG2 inhibitor)1 Pulmonary fibrosis 3862995 (Anti-IL33 antibody) COPD 5462688 (RNA-editing oligonucleotide)1 Alpha-1 antitrypsin deficiency 4347859 (Interferon pathway modulator) Systemic lupus erythematosus 4381562 (Anti-PVRIG antibody)1 Cancer 6097608 (Anti-CD96 antibody)1 Cancer XMT-205611 (STING agonist ADC)1 Cancer belantamab (Anti-BCMA antibody) Multiple myeloma 4524101 (DNA polymerase theta inhibitor)1 Cancer8 5733584 (ADC-targeting B7-H4)1 Gynecologic malignancies 4172239 (DNMT1 inhibitor)1 Sickle cell disease Assets are ordered by therapy area within each phase: infectious diseases, HIV, respiratory/immunology, oncology and opportunity driven. Only the most advanced indications are shown for each asset. (1) In-licence or other alliance relationship with third party (2) Additional indications or candidates also under investigation (3) In registration (4) Phase III trial in patients with progranulin gene mutation (5) Metered dose inhaler (6) Phase III start expected in 2024 (7) Approved in US and EU (8) In phase I/II study (9) Phase II study start imminent (10)Transition activities underway to enable further progression by partner (11) GSK has an exclusive global licence option to co-develop and commercialise the candidate RSV: respiratory syncytial virus; UTI: urinary tract infection; HBV: hepatitis B virus; ADC: Antibody drug conjugate; COPD: chronic obstructive pulmonary disease; MMRV: measles, mumps, rubella & varicella; OMV: outer membrane vasicle; siRNA: small interfering RNA GMMA: generalised modules for membrane antigens; YoA years of age Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Research and development continued 28

Commercial operations In 2023 we delivered strong and sustained performance momentum, with successful commercial launches, supported by our integrated global supply chain. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F 29

Highlights £30.3bn total sales +3% AER +5% CER Strong operational performance In 2023 we've continued to focus on operational performance, with strong growth across all product areas and regions. This builds on good progress in 2022 and demonstrates strong, sustained performance momentum. Strong performance in 2023 was driven by a continued step-change in commercial execution. This was underpinned by a focus on leadership, developing outstanding people, and building meaningful connections with healthcare professionals (HCPs) and patients – supported by data and technology – to give us strong insights into how we can best meet their needs. For details on our performance and drivers of growth see: – Vaccines performance, page 31 – Specialty Medicines performance, page 35 – General Medicines performance, page 38. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Commercial operations 30

Our broad vaccines portfolio targets infectious diseases at every stage of life, helping to protect people from meningitis, shingles, RSV, flu, polio and many more. Turnover £9.9bn +24% AER, +25% CER l Established £3.3bn l Shingles £3.4bn l Meningitis £1.3bn l RSV £1.2bn l Influenza £504m l Pandemic £150m Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Performance: Vaccines 31 Double-digit growth for Vaccines Successful launch of Arexvy in the US Continued strong uptake of Shingrix in International and Europe
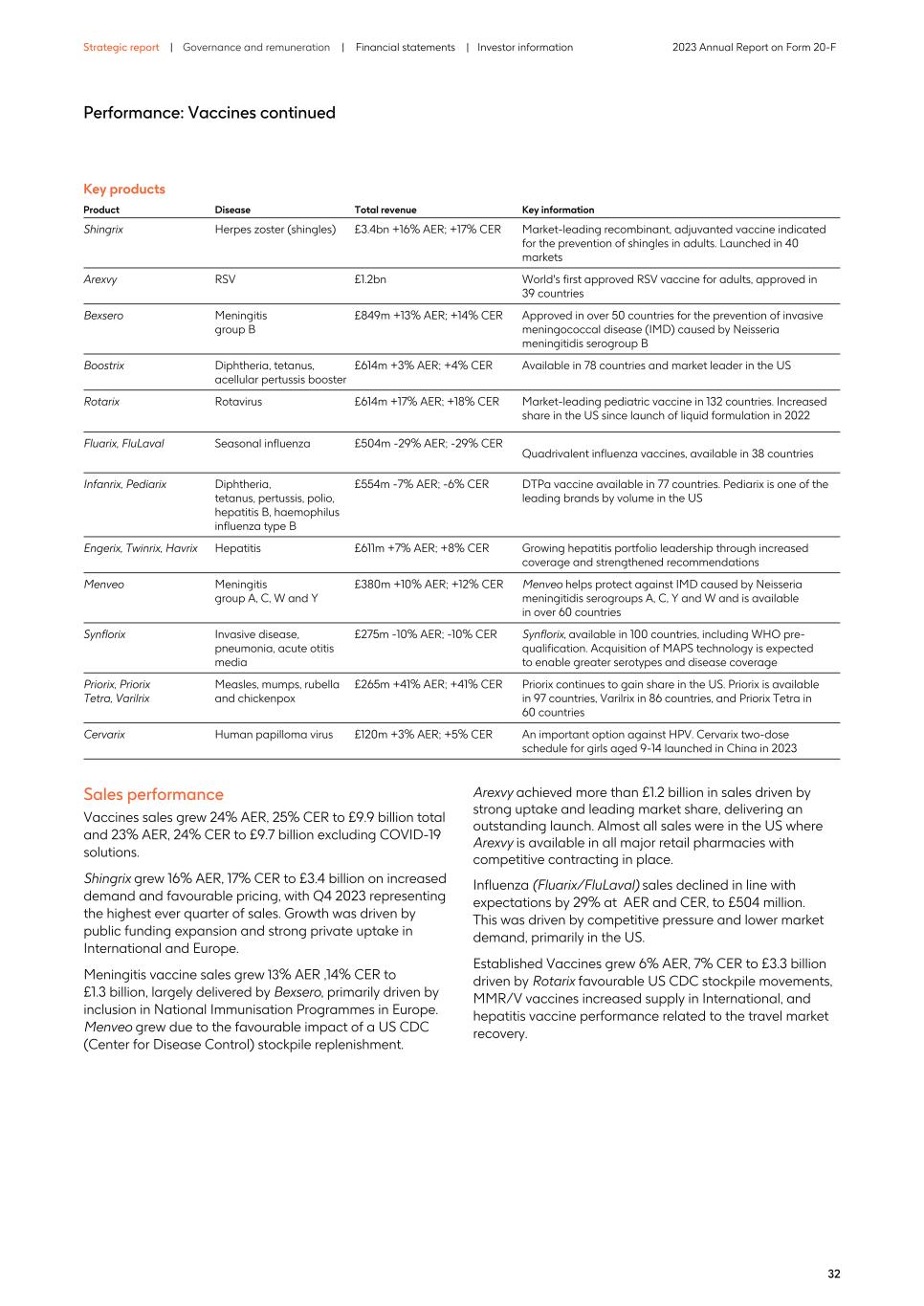
Key products Product Disease Total revenue Key information Shingrix Herpes zoster (shingles) £3.4bn +16% AER; +17% CER Market-leading recombinant, adjuvanted vaccine indicated for the prevention of shingles in adults. Launched in 40 markets Arexvy RSV £1.2bn World's first approved RSV vaccine for adults, approved in 39 countries Bexsero Meningitis group B £849m +13% AER; +14% CER Approved in over 50 countries for the prevention of invasive meningococcal disease (IMD) caused by Neisseria meningitidis serogroup B Boostrix Diphtheria, tetanus, acellular pertussis booster £614m +3% AER; +4% CER Available in 78 countries and market leader in the US Rotarix Rotavirus £614m +17% AER; +18% CER Market-leading pediatric vaccine in 132 countries. Increased share in the US since launch of liquid formulation in 2022 Fluarix, FluLaval Seasonal influenza £504m -29% AER; -29% CER Quadrivalent influenza vaccines, available in 38 countries Infanrix, Pediarix Diphtheria, tetanus, pertussis, polio, hepatitis B, haemophilus influenza type B £554m -7% AER; -6% CER DTPa vaccine available in 77 countries. Pediarix is one of the leading brands by volume in the US Engerix, Twinrix, Havrix Hepatitis £611m +7% AER; +8% CER Growing hepatitis portfolio leadership through increased coverage and strengthened recommendations Menveo Meningitis group A, C, W and Y £380m +10% AER; +12% CER Menveo helps protect against IMD caused by Neisseria meningitidis serogroups A, C, Y and W and is available in over 60 countries Synflorix Invasive disease, pneumonia, acute otitis media £275m -10% AER; -10% CER Synflorix, available in 100 countries, including WHO pre- qualification. Acquisition of MAPS technology is expected to enable greater serotypes and disease coverage Priorix, Priorix Tetra, Varilrix Measles, mumps, rubella and chickenpox £265m +41% AER; +41% CER Priorix continues to gain share in the US. Priorix is available in 97 countries, Varilrix in 86 countries, and Priorix Tetra in 60 countries Cervarix Human papilloma virus £120m +3% AER; +5% CER An important option against HPV. Cervarix two-dose schedule for girls aged 9-14 launched in China in 2023 Sales performance Vaccines sales grew 24% AER, 25% CER to £9.9 billion total and 23% AER, 24% CER to £9.7 billion excluding COVID-19 solutions. Shingrix grew 16% AER, 17% CER to £3.4 billion on increased demand and favourable pricing, with Q4 2023 representing the highest ever quarter of sales. Growth was driven by public funding expansion and strong private uptake in International and Europe. Meningitis vaccine sales grew 13% AER ,14% CER to £1.3 billion, largely delivered by Bexsero, primarily driven by inclusion in National Immunisation Programmes in Europe. Menveo grew due to the favourable impact of a US CDC (Center for Disease Control) stockpile replenishment. Arexvy achieved more than £1.2 billion in sales driven by strong uptake and leading market share, delivering an outstanding launch. Almost all sales were in the US where Arexvy is available in all major retail pharmacies with competitive contracting in place. Influenza (Fluarix/FluLaval) sales declined in line with expectations by 29% at AER and CER, to £504 million. This was driven by competitive pressure and lower market demand, primarily in the US. Established Vaccines grew 6% AER, 7% CER to £3.3 billion driven by Rotarix favourable US CDC stockpile movements, MMR/V vaccines increased supply in International, and hepatitis vaccine performance related to the travel market recovery. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Performance: Vaccines continued 32

Our strategy for growth Our portfolio of more than 20 marketed vaccines is one of the industry’s broadest. We deliver approximately 1.5 million doses of our vaccines every day; and 4 out of 10 infants born each year receive at least one GSK vaccine. Our vaccines portfolio targets infectious diseases at every stage of life, helping to protect people from RSV, meningitis, shingles, flu, polio and many more. Vaccines are critical to delivering our growth plans. Our focus is on strong execution in key markets with Shingrix and our existing portfolio, and on delivering the value of our pipeline with new launches, particularly our world-first RSV vaccine, Arexvy, so we can bring our vaccines to as many patients as possible. Vaccines are complex and highly technical to develop and manufacture. This helps to protect our portfolio from potential disruption from new technologies. There’s no established generic industry and vaccines don’t generally face the so-called ‘patent cliff’. This longer lifecycle means vaccines can remain in use for decades after their initial authorisation. For example, Boostrix, Infanrix, Priorix and Engerix remain important parts of our portfolio in terms of contribution to performance. Our established platform technologies, and the new platforms we’re building, such as the MAPS and mRNA technologies, are a key part of our vaccines growth strategy and are enabling us to tackle the most complex diseases from birth throughout adulthood (see page 25). Drivers of growth across the portfolio Our launch of Arexvy supports our market leadership ambition and has multi-billion-pound sales potential. Approximately 6 million of the 83 million US adults aged 60 and older at risk have been vaccinated with Arexvy. Launches are also underway across Europe and Canada, and the vaccine has been approved in Japan and several other countries. We’re strengthening relationships with retailers, given our expertise in the older adult population through Shingrix. We’re also drawing on our expertise in respiratory diseases and the experience of our primary care sales force. With further approvals and launches expected in 2024, and increasing awareness of the impact of RSV on adults at increased risk, we look forward to seeing the impact this vaccine will have on helping to prevent the severe consequences of RSV globally. Shingrix continued to grow and is now available in 40 countries, with less than 4% penetration in the majority of those markets. In the US 35% of the 120 million adults recommended to receive Shingrix have now been vaccinated. 70 million people are already protected with Shingrix and our ambition is to vaccinate more than 100 million people by 2026. To support this, in 2023 we entered into an exclusive agreement with Chongqing Zhifei Biological Products, Ltd. (Zhifei) with a value of £2.5 billion for an initial three-year period to co-promote Shingrix in China. Zhifei will import and distribute Shingrix in China, promoting the vaccine through its network of over 30,000 vaccination points. The partnership will significantly extend the availability of Shingrix, supporting the rapid expansion of patient access to the vaccine and future potential indications. We continue to lead the meningitis market driven by Bexsero (MenB) and Menveo (MenACWY), as we prepare for the transition to our pentavalent MenABCWY vaccine that combines these established vaccines. Continuing to invest in Bexsero remains integral to strengthening our leadership by securing key National Immunisation Programmes (NIP) in countries like Germany and Switzerland. We’ll do this by building our real-world evidence base, and by helping to improve immunisation rates globally, focusing on the US adolescent population. To improve our competitiveness, we’ll look to drive future growth with multiple lifecycle innovations in the coming years, including launching Menveo in a convenient liquid formulation in additional countries. Our established vaccines remain a key priority for growth, representing a third of our total vaccines business. Our core vaccines continue to grow strongly as we seek to maximise uptake in those who need them. We achieve this by prioritising specific segments for growth opportunity, such as a return to travel and strengthened recommendations for hepatitis in the adult segment, and increasing awareness of the importance of vaccination. We’re also working to maintain our strong performance in key markets by making sure we resource our teams for success and that we can deliver against our supply commitments. Meeting the needs of ageing populations by prioritising prevention By focusing on prevention, we can reduce the burden of disease and create a healthier, thriving world. Vaccination is a critical element for prevention of infectious diseases, especially for children and older adults. From the age of around 50, our immune system starts to decline and becomes less effective, leading to increased risk from infectious diseases. We focus our efforts on helping to keep older adults healthy, moving from ‘sick care’ to true healthcare by prioritising prevention and making adult immunisation the standard of care. With the help of vaccination, adults can remain active, healthy participants in society and the economy – prolonging productivity, contributing to local economies and reducing healthcare costs. To improve uptake of adult immunisation, we are working to build the investment case for vaccination, ease access, and increase belief in the importance of vaccines. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Performance: Vaccines continued 33

To support healthcare professionals to routinely initiate vaccination conversations with their patients and build broader belief in the importance of vaccination, we held Vaccine Virtual Days 2023. These bring together healthcare professionals and experts from the international vaccine community to discuss and present important updates, data and trends in adult immunisation. We’ve also continued our series of Vaccinology Master Classes, helping to better equip healthcare professionals for conversations with their patients about vaccines. Adult immunisation rates in the US still haven’t recovered fully after the COVID-19 pandemic. In 2023, we commissioned a report, published with the IQVIA Institute for Human Data Science and the Global Coalition on Aging. It estimated that around 100 million fewer doses of some adult vaccines (excluding COVID-19 solutions) were administered in 2021 and 2022 than anticipated. To help address this, we launched the COiMMUNITY Initiative in the US which commits $1 million in grant funding to national, state and local non-profit organisations to address long-term barriers to immunisation, particularly among older adults susceptible to declining immune systems. This year we commissioned research that spotlights hyperlocal factors contributing to – or inhibiting – adult immunisation uptake in five diverse, geographically representative cities across the world. This research builds upon existing global frameworks and progresses vital initiatives, such as the UN Decade on Ageing and WHO Age-Friendly Cities Network. As part of COiMMUNITY, we’re also supporting public health efforts by making data on vaccination trends available through the Vaccine Track platform and sharing tools and resources with healthcare organisations to help them address gaps in adult immunisation. The COiMMUNITY initiative builds on recent regulatory and industry changes in the US that make vaccines more available and easier to access for Medicare and Medicaid beneficiaries and support community vaccine infrastructure. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Performance: Vaccines continued 34

We continue to be global leaders in infectious diseases, respiratory and HIV medicines and have an emerging portfolio of cancer medicines. Turnover £10.2bn -9% AER, -8% CER l HIV £6.4bn l Respiratory/immunology and other £3.0bn l Oncology £731m l Pandemic £44m Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Performance: Specialty Medicines 35 Total Specialty Medicines decreased -9% AER, -8% CER due to lower sales of Pandemic. Specialty Medicines growth (excluding COVID-19 solutions) of 14% AER, 15% CER Continued growth momentum in HIV Growth acceleration in both oncology and respiratory/immunology
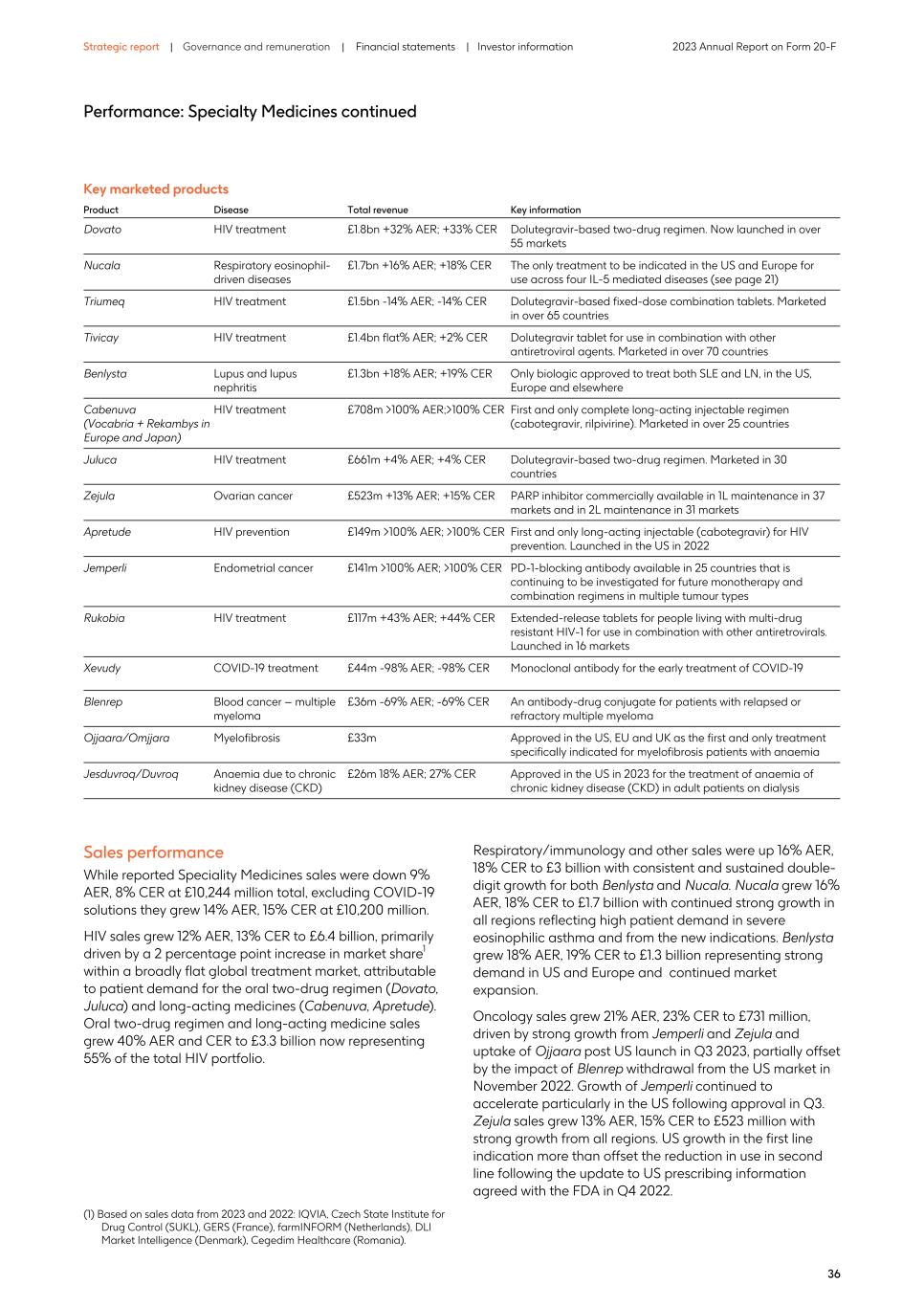
Key marketed products Product Disease Total revenue Key information Dovato HIV treatment £1.8bn +32% AER; +33% CER Dolutegravir-based two-drug regimen. Now launched in over 55 markets Nucala Respiratory eosinophil- driven diseases £1.7bn +16% AER; +18% CER The only treatment to be indicated in the US and Europe for use across four IL-5 mediated diseases (see page 21) Triumeq HIV treatment £1.5bn -14% AER; -14% CER Dolutegravir-based fixed-dose combination tablets. Marketed in over 65 countries Tivicay HIV treatment £1.4bn flat% AER; +2% CER Dolutegravir tablet for use in combination with other antiretroviral agents. Marketed in over 70 countries Benlysta Lupus and lupus nephritis £1.3bn +18% AER; +19% CER Only biologic approved to treat both SLE and LN, in the US, Europe and elsewhere Cabenuva (Vocabria + Rekambys in Europe and Japan) HIV treatment £708m >100% AER;>100% CER First and only complete long-acting injectable regimen (cabotegravir, rilpivirine). Marketed in over 25 countries Juluca HIV treatment £661m +4% AER; +4% CER Dolutegravir-based two-drug regimen. Marketed in 30 countries Zejula Ovarian cancer £523m +13% AER; +15% CER PARP inhibitor commercially available in 1L maintenance in 37 markets and in 2L maintenance in 31 markets Apretude HIV prevention £149m >100% AER; >100% CER First and only long-acting injectable (cabotegravir) for HIV prevention. Launched in the US in 2022 Jemperli Endometrial cancer £141m >100% AER; >100% CER PD-1-blocking antibody available in 25 countries that is continuing to be investigated for future monotherapy and combination regimens in multiple tumour types Rukobia HIV treatment £117m +43% AER; +44% CER Extended-release tablets for people living with multi-drug resistant HIV-1 for use in combination with other antiretrovirals. Launched in 16 markets Xevudy COVID-19 treatment £44m -98% AER; -98% CER Monoclonal antibody for the early treatment of COVID-19 Blenrep Blood cancer – multiple myeloma £36m -69% AER; -69% CER An antibody-drug conjugate for patients with relapsed or refractory multiple myeloma Ojjaara/Omjjara Myelofibrosis £33m Approved in the US, EU and UK as the first and only treatment specifically indicated for myelofibrosis patients with anaemia Jesduvroq/Duvroq Anaemia due to chronic kidney disease (CKD) £26m 18% AER; 27% CER Approved in the US in 2023 for the treatment of anaemia of chronic kidney disease (CKD) in adult patients on dialysis Sales performance While reported Speciality Medicines sales were down 9% AER, 8% CER at £10,244 million total, excluding COVID-19 solutions they grew 14% AER, 15% CER at £10,200 million. HIV sales grew 12% AER, 13% CER to £6.4 billion, primarily driven by a 2 percentage point increase in market share1 within a broadly flat global treatment market, attributable to patient demand for the oral two-drug regimen (Dovato, Juluca) and long-acting medicines (Cabenuva, Apretude). Oral two-drug regimen and long-acting medicine sales grew 40% AER and CER to £3.3 billion now representing 55% of the total HIV portfolio. (1) Based on sales data from 2023 and 2022: IQVIA, Czech State Institute for Drug Control (SUKL), GERS (France), farmINFORM (Netherlands), DLI Market Intelligence (Denmark), Cegedim Healthcare (Romania). Respiratory/immunology and other sales were up 16% AER, 18% CER to £3 billion with consistent and sustained double- digit growth for both Benlysta and Nucala. Nucala grew 16% AER, 18% CER to £1.7 billion with continued strong growth in all regions reflecting high patient demand in severe eosinophilic asthma and from the new indications. Benlysta grew 18% AER, 19% CER to £1.3 billion representing strong demand in US and Europe and continued market expansion. Oncology sales grew 21% AER, 23% CER to £731 million, driven by strong growth from Jemperli and Zejula and uptake of Ojjaara post US launch in Q3 2023, partially offset by the impact of Blenrep withdrawal from the US market in November 2022. Growth of Jemperli continued to accelerate particularly in the US following approval in Q3. Zejula sales grew 13% AER, 15% CER to £523 million with strong growth from all regions. US growth in the first line indication more than offset the reduction in use in second line following the update to US prescribing information agreed with the FDA in Q4 2022. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Performance: Specialty Medicines continued 36

Our strategy for growth Our portfolio of Specialty Medicines focuses on four therapeutic areas: infectious diseases, HIV, respiratory/ immunology and oncology. We are reinforcing our strength and leadership in infectious diseases, respiratory and HIV, and building our emerging capabilities in oncology to drive growth. 34% of sales come from Specialty Medicines, which we expect to provide durable and profitable growth over the next five years. We drive growth by accelerating our pipeline as well as prioritising business development, targeting acquisitions and partnerships to strengthen and complement our core therapy areas, and helping to deliver above and beyond our current long-term outlooks. Our acquisition of Bellus Health, announced in April 2023, for example, builds on our respiratory expertise and complements our broader respiratory pipeline. We’re increasingly confident that this will be a major source of new long-term growth. Drivers of growth across the portfolio In HIV, our strategy for growth is built on our innovative portfolio of medicines that are transforming HIV treatment and prevention with strong competitive execution. Launched in 2019, our dolutegravir-based oral two-drug regimen, Dovato, continues to perform strongly, enabling people living with HIV to remain virally suppressed with fewer medicines. Our long-acting portfolio of medicines are central to our growth and are delivering strong results as they launch across our markets. Cabenuva, the world’s first and only complete long-acting regimen for HIV treatment is available in the US, Europe, Japan, China and Australia. Two-monthly Cabenuva addresses the challenges associated with daily oral therapy, including fear of disclosure, adherence anxiety and pill fatigue. Apretude is the world’s only long-acting medicine for HIV prevention offering superior efficacy to daily oral prevention (FTC/TDF tablets) and two-monthly dosing. In 2023 Apretude expanded beyond the US with approval in Europe and several sub-Saharan Africa countries as an important lever to end the global epidemic. In respiratory/immunology, our market-leading medicines Nucala and Benlysta continued to deliver double-digit growth. Nucala, the only targeted biologic therapy approved for use across four IL-5 mediated diseases (eosinophil disease), continues to drive growth. Consistent evidence across multiple indications combined with market-leading safety data reinforce Nucala as the biologic of choice for HCPs. The severe asthma market continues to grow in the US and in other markets, which offer opportunities for Nucala to help more patients. Benlysta remains the only biologic approved for both systemic lupus erythematosus and lupus nephritis In 2023, Benlysta saw consistent growth across all major markets, with over 14,000 US patients starting therapy in 2023. We’re focused on helping to identify and treat patients earlier, before lupus progresses and organ damage occurs (see page 22). In oncology, Jemperli continues to demonstrate its potential as the backbone of our ongoing immuno-oncology-based research and development programme. Used alone and in combination with standard of care and future novel cancer therapies, it has the potential to transform patients’ lives across multiple tumour types, including endometrial cancer. In 2023, Jemperli plus chemotherapy was approved in the US, EU and UK as the first and only immuno-oncology regimen for the treatment of frontline primary advanced or recurrent dMMR/MSI-H endometrial cancer. These approvals have been a significant driver of performance and sales growth in oncology. Ojjaara/Omjjara, a JAK- and ACVR1-inhibitor, acquired through the purchase of Sierra Oncology in April 2022, is now approved in the US, EU and UK to treat myelofibrosis with anaemia. This makes Ojjaara the only medicine specifically indicated for both newly diagnosed and previously treated myelofibrosis patients with anaemia that addresses the anaemia, constitutional symptoms and splenomegaly (enlarged spleen) that are the hallmarks of this complex blood cancer. The line-agnostic label was broader than anticipated, expanding the opportunity to reach more patients with a novel treatment option. Additional regulatory filings were initiated in 2023, with an aim in 2024 to expand access to patients in other markets. In ovarian cancer, Zejula continues to provide a significant opportunity for first-line maintenance therapy, reaching more than 15,000 patients every month. We’re working to develop other combination therapies with Zejula in women's cancers and other solid tumours. To ensure we focus on areas where we can make the biggest impact for patients, we’ve withdrawn our filing for Jesduvroq in the EU, and will stop filing in other markets because other medicines are already available for patients living with anaemia of CKD. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Performance: Specialty Medicines continued 37

From antibiotics to inhaled medicines for asthma and COPD, we have over 150 general medicine products, many of them leaders in their class, making life better for millions of people worldwide. Turnover £10.2bn +1% AER, +5% CER l Respiratory £6.8bn l Other General Medicines £3.4bn Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Performance: General Medicines 38 Growth driven by both respiratory and other general medicines Ongoing strong demand for Trelegy in all regions; Anoro in Europe and International Continued post pandemic recovery of the antibiotic market in Europe and International regions

Key marketed products Product Disease Total revenue Key information Trelegy Ellipta COPD, asthma £2.2bn +27% AER; +29% CER Most prescribed single inhaler triple therapy (SITT) worldwide, reaching an estimated 8.6 million patients since launch Seretide/Advair Asthma, COPD £1.1bn -2% AER; +1% CER One of the market-leading ICS/LABA1 treatments worldwide by sales value Relvar/Breo Ellipta Asthma, COPD £1.1bn -4% AER; -2% CER One of the leading ICS/LABA treatments worldwide by sales value Ventolin Asthma, COPD £749m -3% AER; — CER Global market-leading SABA2 reliever by sales value Augmentin Common bacterial infections £628m +9% AER; +17% CER Global leader in oral antibiotics by sales value, available in over 95 countries Anoro Ellipta COPD £557m +15% AER; +16% CER Global market leader in the LAMA/LABA3 class by volume (unit sales), approved in over 70 countries Avodart & Duodart Benign prostatic hyperplasia (BPH) £345m +5% AER; +7% CER Market leaders by sales value in the global dutasteride and dutasteride+tamsulosin FDC4 market respectively, and approved in over 101 and 88 countries respectively Avamys/Veramyst Allergic rhinitis £299m -7% AER; -4% CER Global leader in the inhaled corticosteroids prescription class by sales value Dermovate, Betnovate, Cutivate, Eumovate Inflammatory skin conditions £195m -3% AER, +6% CER Global leader in topical corticosteroids across 60 markets globally by value of sales, excluding the US (1) ICS/LABA: inhaled corticosteroid/long-acting beta agonists (2) SABA: short-acting beta agonist (3) LABA/LAMA: long-acting beta agonists/long-acting muscarinic antagonists (4) FDC: fixed-dose combination Key information source IQVIA Sales performance General Medicines sales grew 1% AER, 5% CER to £10.2 billion, reflecting growth of Trelegy and the single inhaler triple therapy class across all regions, and of Anoro in Europe and International. Trelegy grew 27% AER, 29% CER to £2.2 billion with growth delivered across all regions, reflecting increased patient demand, growth of the SITT market and penetration of the class. Seretide/Advair sales decreased 2% AER but increased 1% CER at £1.1 billion, primarily reflecting favourable US pricing. However this was offset by generic erosion impacts in Europe and certain International markets. Other General Medicines decreased 5% AER, but grew 2% CER at £3.4 billion reflecting ongoing post pandemic demand for anti-infectives in Europe and International, and certain third party manufacturing arrangements. Overall growth in this product group continues to be impacted by ongoing generic competition. Our strategy for impact Our General Medicines portfolio includes medicines that are typically prescribed in primary care. In 2023, General Medicines contributed over one third of GSK's sales, helping to fund growth and investment in R&D and returns to shareholders. We expect our combination of more than 150 products, several of which are market leaders, to have a positive impact on the lives of hundreds of millions of patients over the next 10 years. We supply our products in more than 100 countries, and they comprise over 80% of our total medicines and vaccines supply volume. Every day, these medicines improve health and make life better for millions of people all over the world. Together, respiratory and infectious diseases therapeutics generate 73% of our General Medicines revenue. With expected growth from Trelegy, Anoro and the established products portfolio in emerging markets, we are committed to positively impacting more lives every day. We focus investment in our brands that are growing strongly to maximise returns, while managing the expected decline of other products in mature markets as they lose their exclusivity. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Performance: General Medicines continued 39

Drivers of growth across the portfolio Our main sources of growth in General Medicines in 2023 were Trelegy, Anoro and Augmentin. Trelegy, our SITT for asthma and COPD, delivered sales of over £2 billion for the first time in 2023. Trelegy has continued to accelerate strongly, with growth in all regions including the US, and is the third biggest growth driver in our portfolio. Trelegy is licensed in 60 countries for COPD, with dual indications for asthma and COPD in 19 countries, including the US and Japan1. We received several new approvals in 2023, further expanding Trelegy’s availability to asthma patients in Turkey, Hong Kong, Bahrain and Kuwait.2 Trelegy is the number one SITT globally, selling over 21 million packs – more than twice the volume of the nearest competitor3. Trelegy is the market leader in our two largest markets, the US and Japan, with market shares significantly exceeding the next-largest competitor (83% and 67%, respectively)5. In November 2022, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommended triple therapy over ICS/LABA for exacerbating patients. This has helped to continue the growth of the SITT market which, six years after first launch, is still growing at 41% year on year. We expect Trelegy to be a key driver of growth in General Medicines in the coming years. Anoro is approved in approximately 70 countries to treat symptomatic COPD. Anoro remains the global market leader in the LAMA/LABA class by volume (unit sales)4, with continued growth in global sales (excluding US). Anoro has a robust clinical data profile, which includes head-to- head data in the LAMA/LABA class and versus other common initial maintenance therapy options, such as LAMA. Augmentin is a global leader in oral antibiotics by sales value4 and is available in 95 countries. It has reached over 2.65 billion patients since launching more than 40 years ago and continues to grow strongly across regions. Augmentin grew 9% AER, 17% CER to £628 million with strong ongoing demand across all regions. Since its launch in 1969, Ventolin remains an important medicine for patients in more than 100 countries. A significant proportion of our carbon emissions come from our Ventolin metered dose inhalers (MDIs). We have started an R&D programme to redevelop our Ventolin MDIs with a lower global warming potential (GWP) propellant, which is now in clinical assessment. If successful, this could reduce greenhouse gas emissions from our rescue MDIs by approximately 90%. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Performance: General Medicines continued 40 (1) Regulatory Data on File. Latest update 25 August 2023 for Asthma and July 2022 for COPD (2) Regulatory Data on File, Latest update 25 August 2023 (3) IQVIA Patient Volume Data as of 6 October 2023 (4) Source: IQVIA (5) Based on internal analysis by GSK using sales data from the following source: IQVIA MIDAS® global monthly data, December 2023, reflecting estimates of real-world activity: US, JP. Measure: volume sales (units); ATC: R03L3. All rights reserved. Copyright IQVIA

Our global supply chain is critical to the successful manufacture and supply of our vaccines and medicines. It enables us to deliver reliable, high-quality products to meet patients’ needs and maintain our performance. In 2023, we made significant progress in bringing together our vaccines and medicines supply chains to create one global supply chain. This integration helps drive efficiency and ensures we have the capacity and capabilities, including the best digital and technology capabilities, to deliver our new products. Our global network of 37 vaccines and medicines manufacturing sites delivered more than 500 million vaccine doses and 1.8 billion packs of medicines to help make a positive impact on the health of millions of people. Investing for future productivity We are investing in our manufacturing and supply chain to increase productivity and efficiency. In 2023 we opened a $100 million adjuvant manufacturing facility in Hamilton, in the US. It means we can produce the QS-21 adjuvant in-house, contributing to our RSV, shingles, malaria and cervical cancer vaccines. In late 2022, we opened a manufacturing and testing facility at Jurong in Singapore to produce a cytotoxic agent for antibody drug conjugates needed for next-generation cancer treatments. And at our Tuas site, also in Singapore, we’ve begun building a new vaccine manufacturing facility for our hepatitis B vaccines which will feature the latest advanced technology and be sustainable by design. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Operations: Manufacturing and supply 41

At Ware in the UK, we opened a new oral solid dose facility, bringing together R&D and supply chain specialists to use new technologies and rapid knowledge transfer to deliver new medicines faster and more efficiently. And at our Wavre site in Belgium, we’ve started building a €250 million freeze-drying centre, using automation and robotics, to create more capacity for adult vaccines. At Barnard Castle in the UK, our multi-million-pound Q Block smart manufacturing facility started commercial production in 2023, and immunology products are now being shipped to patients around the world. The facility uses digital technology and robotics to make production more efficient. We are exceeding targets in our ongoing programme of productivity and efficiency improvements. This year, the programme delivered £101 million in savings across Medicines and Vaccines. Site productivity increased by 9.3% during the year. Promoting quality, safety and reliability Quality, safety and reliable supply are critical to meeting patients’ needs, and to creating competitive advantage. Our reliability remains strong, with an on-time, in-full (OTIF) measure of 99.3% for Specialty Medicines, 98.4% for General Medicines and 92.3% for Vaccines. Our deviation rates improved for Medicines and increased marginally for Vaccines with clear action plans for improvement in 2024. For information on product governance, see the Responsible Business section on page 53. We’ve also received external recognition. In 2023, we featured in Gartner’s Top 25 Supply Chain companies, based on financial metrics, ESG criteria, and opinion from industry analysts and experts. Supporting innovation Our global supply chain plays a central role in bringing our innovations to patients as quickly, efficiently and effectively as possible. The teams are involved early in product development, working with R&D to make sure that what works in clinical trials can be produced commercially at scale. In 2023 we supplied our RSV vaccine Arexvy in record time to more than 20 countries, including the US, the EU and Canada, following regulatory approvals. We are also bringing on additional capacity to deliver Jemperli to more patients around the world following regulatory approvals in the US and Europe. And we worked with external manufacturing partners to deliver supply chain excellence for the US launch of Ojjaara. Embracing technology and data By harnessing the power of technology and data, we are transforming our manufacturing and supply chain. By identifying and implementing the best digital and technology capabilities, we can unlock growth for patients, shareholders and our people. We are also working to industrialise new platform technologies such as oligonucleotides in medicines and mRNA and MAPS in vaccines. As MAPS clinical trials continue at the new Binney Street site in Cambridge, US, we plan to scale up production and bring MAPS to market. We’re using digital twins to simulate processes, anticipate issues and use what we learn to accelerate manufacturing. The technology helps increase production yields for both our vaccines and medicines. We’re also investing in automation and robotics at our sites, improving ergonomics, increasing efficiency and helping us to deliver more medicines and vaccines to patients around the world. Increasing our environmental sustainability Our manufacturing sites have a key role in our contribution to a net zero, nature positive, healthier planet, and environmental sustainability is a fundamental part of our global supply chain strategy. See our Responsible Business section on page 43 for more information on carbon emissions, water use and waste. We’re also investing in plans to improve natural habitats, protect biodiversity and improve soil and water quality near our sites. + For more on our approach to sustainability and progress made at our sites, see our ESG Performance Report Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Operations: Manufacturing and supply continued 42

Responsible business ESG is embedded in our strategy. It helps us deliver our purpose and supports our sustainable performance and long-term growth. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F 43

Our approach We are a global biopharma company with a purpose to unite science, technology and talent to get ahead of disease together. To deliver our purpose, we need to consider ESG impacts across everything we do, from the lab to the patient. That’s why ESG is embedded in our strategy and supports our sustainable performance and long-term growth. It helps us to build trust with and generate value for our stakeholders, reduce risk to our operations and create positive social impact. We have identified six ESG focus areas that address what is most material to our business and the issues that matter the most to our stakeholders. These focus areas are core to our strategy and are where we can have the greatest positive impact on some of society’s most urgent challenges, including those set out in the UN Sustainable Development Goals (UN SDGs). They are: – Access to healthcare – Global health and health security – Environment – Diversity, equity and inclusion (DEI) – Ethical standards – Product governance. These focus areas were informed by our most recent materiality assessment in 2022, which reaffirmed that the most material issues for our business were well aligned with our six ESG focus areas. We recognise that being a responsible business is not a static requirement. This means that we will continue to evolve our approach in response to the rapidly changing operating environment and strive for continuous improvement to ensure we maintain strong ESG performance. Our ESG Performance Rating Our ESG Performance Rating helps us integrate ESG into the delivery of our strategy and allows us to measure and verify the progress we are making. The rating is one of our corporate KPIs and measures progress against key metrics aligned to each of our six focus areas. In 2023, this included 22 metrics, which are summarised in our ESG Performance Report. We continue to evolve our ESG Performance Rating to ensure it meets the expectations of our stakeholders. The executive leadership team and the Board, via the Corporate Responsibility Committee, review the metrics that make up this Rating each year to ensure they are sufficiently challenging and ambitious. This year, we have removed two metrics, relating to Access and Ethical standards, and added one relating to anti-microbial resistance (AMR). We met one of our 2022 metrics relating to Access by developing and publishing pricing and access principles. We have also removed one of our Ethical standards metrics that tracks the number of employees leaving GSK for misconduct. Increases or decreases in this number could indicate either a higher/ lower number of breaches or stronger/weaker enforcement of our processes, so setting a threshold is not an effective measure for success in upholding our standards. We continue to monitor this data internally and publish it externally. We have three additional metrics which provide a strong measure of our commitment to ethical standards. We have added a metric within Global health and health security, focused on AMR. AMR is an urgent public health threat, and we have seen increased stakeholder interest in our approach. We updated our biodiversity target as we achieved it in 2022. Our new target focuses on deforestation free sourcing of paper and palm oil. How we assess performance The GSK Leadership Team (GLT) is accountable for delivering progress against the metrics and regularly reviews performance along with the Board’s Corporate Responsibility Committee (CRC). Each individual metric is assessed as either: on track (metric met or exceeded); on track with work to do (at least 80% of metric has been achieved); or off track (metric missed by more than 20%). In addition, in order to calculate the overall ESG Performance Rating, performance across all metrics is aggregated to a single score to illustrate whether we are on track, on track with work to do, or off track. This rating is defined below: On track: 70% or more of all metrics are on track On track with work to do: more than 50% of all metrics are either on track, or on track with work to do Off track: more than 50% of all metrics are off track 2023 ESG Performance Rating Our 2023 ESG Performance Rating is on track, based on 95% of all performance metrics being met or exceeded. + For full details of progress against our six focus areas, our ESG Performance Rating and 22 metrics and independent limited assurance reports, see our ESG Performance Report Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Responsible business continued 44

External benchmarking Detailed below is how we perform in key ESG ratings that we are frequently asked about by investors: – Access to Medicines: Ranked 1st in the Access to Medicines Index in 2022 and an industry leader in the 2021 Antimicrobial Resistance Benchmark – S&P Corporate Sustainability Assessment: Ranked 1st in the pharmaceuticals industry with a score of 84 (as of 24 November 2023) and included in the DJSI World and Europe indices – FTSE4Good: Member of FTSE4Good Index since 2004 – CDP: A- in Climate change, A- in Water security, B in Forests (palm oil) and B in Forests (timber) – Sustainalytics: Low risk rating – MSCI: AA rating – Moody's Analytics: ESG Overall Score of 62 (out of 100; sector average 38) – ISS Corporate Rating: B+ rating Access We aim to positively impact the health of 2.5 billion people by the end of 2030. We will do this by making our vaccines and medicines available as widely as possible, through responsible pricing, strategic access programmes and partnerships. Our commitment Make our products available at value-based prices that are sustainable for our business and implement access strategies that increase the use of our medicines and vaccines to treat and protect underserved people Our ESG Performance Rating metric – Progress towards our 2030 goal of reaching 1.3 billion people in lower income countries with our products Progress in 2023 Putting the right value on innovation We set responsible prices in line with the benefits we bring to patients and health systems, measured by clinical, economic and social outcomes. We compare our offer to what is already available for patients and we generate evidence from clinical trials to establish the added value provided by our medicines and vaccines. We adjust our pricing in line with the socio-economic status of a country to ensure affordability and availability. We operate under robust pricing approvals, developing access plans informed by payers. We also work to create stability and predictability for payers and our business, engaging proactively on upcoming product launches for budget planning, and adjusting prices to account for inflation. In the US in 2023, our combined average net price (after discounts, rebates or other allowances) for our pharmaceutical and vaccines portfolio increased by 0.4%, while the average list price increased by 3.2%, compared with 5.4% (list) for the industry. Over the past five years, the average net price for our products increased by 0.3% annually, while the average list price rose by 3.3%, compared with 4.7% (list) for the industry. Providing access for patients in lower income countries We collaborate with global health partners, including NGOs and generic manufacturers, to increase our reach to patients in lower income countries. In 2023, we reached 89 million people with our vaccines and antiretrovirals in lower income countries. Vaccines We reserve our lowest vaccine prices for Gavi, the vaccine alliance, and similar organisations. We have partnered with Gavi since its foundation in 2000 and have supplied more than one billion vaccine doses to date at our lowest prices to the lowest income countries. In 2023, through our partnership we significantly increased our supply to deliver around 5 million doses of Cervarix, a critical vaccine in lower income countries for addressing cervical cancer. In 2023, we supplied around 41 million doses of our pneumococcal vaccine, Synflorix, to eight Gavi-eligible countries at our lowest price. Our vaccine against rotavirus, Rotarix, reaches children across 25 Gavi-eligible countries and four former Gavi countries. We have offered vaccines to civil society organisations serving refugees and working in other emergency situations through the Humanitarian Mechanism since 2017. We are also a long-standing supplier of oral polio vaccines through UNICEF and, in 2023 alone, supplied around 130 million doses to help eradicate polio. Neglected tropical diseases In 2023, we donated 615 million albendazole tablets to help tackle lymphatic filariasis (LF), soil transmitted helminths and echinococcosis, taking the total we have donated to over 11 billion. We remain committed to supplying albendazole to endemic countries until LF is eliminated everywhere. So far, LF has been eliminated in 19 countries including Bangladesh and Lao PDR, who announced elimination of the disease in 2023 – significant milestones in our collaborative effort to get ahead of disease together. The number of tablets we are donating is declining each year, given the gradual eradication of the neglected tropical diseases (NTDs) that the medicine is targeting. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Responsible business continued 45

The programme has benefited over 935 million people since it began, according to WHO data. HIV In 2023, Aurobindo, Cipla and Viatris, three generic manufacturers, signed sub-licences of ViiV Healthcare’s licence with the Medicines Patent Pool (MPP) to develop, manufacture and supply generic versions of cabotegravir long-acting for HIV pre-exposure prophylaxis (cabotegravir LA for PrEP) in 90 countries, subject to obtaining regulatory approvals. ViiV Healthcare also works with global health agencies, NGOs, governments and community partners to plan for and support the introduction of ViiV-manufactured cabotegravir LA for PrEP introduction into national programmes. In late 2023, our first orders of cabotegravir LA for PrEP were delivered to a global partner for programmatic use in low- and middle-income countries. ViiV Healthcare also has voluntary licensing agreements with 15 generic manufacturers to produce and sell low-cost single or fixed-dose combination products containing our HIV medicine dolutegravir for adults. These agreements cover 95 low- and middle-income countries, with one direct licence and the others via the MPP. There are similar agreements with 14 generic manufacturers for children, covering 123 countries, as well as separate agreements to enable greater access to dolutegravir in certain upper middle-income countries. In total, around 24 million people living with HIV across 128 countries had access to a generic product containing dolutegravir by the end of 2023. This is more than 90% of people living with HIV on antiretrovirals in generic-accessible low- and middle-income countries. Malaria To date, over two million children in Ghana, Kenya and Malawi have been reached with at least one dose of Mosquirix (RTS,S/AS01E) through the WHO-coordinated Malaria Vaccine Implementation Programme. Developed by GSK and our partners, Mosquirix is a significant scientific breakthrough – it is the world’s first malaria vaccine and first vaccine against any human parasite. In July 2023, Gavi announced that up to nine more African countries are to be allocated doses of Mosquirix from early 2024. We have committed to supply a total of 18 million doses to Gavi-eligible countries between 2023 and 2025, with a plan to produce 15 million doses annually from 2026 to 2028. In 2023, a landmark study by the London School of Hygiene & Tropical Medicine showed that combining Mosquirix with antimalarial drugs in areas of Africa with seasonal malaria reduced malaria cases and deaths in young children over a period of five years. These findings confirm the potential of seasonal vaccination to provide a high level of protection over the first five years of life, when this protection is much needed. Helping to strengthen healthcare systems In 2023, GSK and ViiV Healthcare joined forces with The Global Fund to pledge $7.5 million over three years to create the Gender Equality Fund, which will support community- based and -led organisations that are working to deliver lasting changes in health policies and programmes focusing on TB, HIV and malaria for women and girls in all their diversity. The Bill & Melinda Gates Foundation has committed to match this donation. We also renewed our partnership with Save the Children for another five years. Building on learnings over the last decade, we are focusing our partnership on reducing the number of ‘zero dose’ children – those who have never received a vaccine – in Ethiopia and Nigeria, which represent more than a third of the zero-dosed children in Africa. + For full details of our progress in our six focus areas, please see our ESG Performance Report Global health and health security We want to help address the biggest health challenges faced by people around the world. Our commitment To develop novel products and technologies to treat and prevent priority diseases, including pandemic threats Our ESG Performance Rating metrics – Progress six Global Health pipeline assets to address priority WHO diseases – Progress eight active R&D projects that address pathogens prioritised by WHO and CDC as posing the highest level of concern due to drug resistance (critical and/or urgent threats) Progress in 2023 Global health R&D In 2022, with ViiV Healthcare, we announced an investment of £1 billion over 10 years to accelerate global health R&D. By the end of 2023, we had invested 21%1 of this and progressed 11 Global Health pipeline assets to address priority WHO diseases, including climate-aggravated diseases that have a disproportionate impact on lower income countries. Promising avenues for tuberculosis prevention and treatment GSK is committed to tackling tuberculosis (TB), one of the world’s deadliest diseases. We have developed a promising candidate vaccine, M72/AS01E, up to proof of concept (phase IIb). Building on our long-standing, successful history of working with external partners we have partnered with the Bill and Melinda Gates Medical Research Institute (MRI) for its further development. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Responsible business continued 46 (1) Budget phasing is not linear across the 10 year period.

Gates MRI is well positioned to lead the large and complex phase III study required. In June 2023, Wellcome and the Bill and Melinda Gates Foundation announced funding of up to $550 million for phase III trials. If these trials are successful, M72/AS01E could be the first new vaccine to help prevent pulmonary TB in over a century. Breakthroughs in malaria research and treatment In August 2023, we announced that GSK scientists had discovered a strain of a naturally occurring bacterium that could potentially help eradicate the disease. The Tres Cantos 1 (TC1) strain of the Delftia tsuruhatensis bacterium significantly reduces the load of P. falciparum malaria parasites in mosquitoes. This could potentially inhibit transmission of the parasite to humans. We continue to pursue this ground-breaking research while engaging with global health institutions and partners to identify the most effective and sustainable approach for development and mobilisation if successful. Supporting innovation through capacity and capability building Through our Africa Open Lab initiative, launched in 2014, we support early-career scientists based in sub-Saharan Africa focusing on infectious diseases that disproportionately affect sub-Saharan populations, such as malaria, TB and AMR. In 2023, we agreed grants to ten researchers in six countries in sub-Saharan Africa and announced a further call for proposals in November. We are also working with African academic institutions to provide grantees with supplemental training in areas including epidemiology, statistics and clinical research. Strengthening health security There are many factors that can jeopardise our health security – from new and emerging infectious diseases to the rise of AMR. Our primary contribution to strengthening health security is through our innovation to prevent and mitigate infectious disease. We have more than 30 R&D projects across medicines and vaccines that are relevant to AMR, ranging from early- to late-stage development, with 12 R&D projects targeting pathogens deemed ‘critical’ or ‘urgent’ by the WHO and the US Centers for Disease Control and Prevention. These include gepotidacin, which could be the first novel oral antibiotic treatment for uncomplicated urinary tract infections (UTIs) in over 20 years. Positive phase III data from the EAGLE-2 and EAGLE-3 trials were presented at the European Congress of Clinical Microbiology and Infectious Diseases in Copenhagen in April 2023. In March 2023, we announced an exclusive licence agreement with Scynexis for Brexafemme (ibrexafungerp tablets), a first-in-class antifungal for the treatment of vulvovaginal candidiasis (VVC) and for reduction in the incidence of recurrent VVC. Progressing vaccines against enteric diseases to reduce the burden of antimicrobial resistance Antimicrobial resistance (AMR) is a major threat to health globally, and it is particularly prevalent in low-resource settings. We continue to progress candidate vaccines against several enteric diseases which contribute to the burden of AMR, including invasive non-typhoidal salmonella, klebsiella, shigella, typhoid and paratyphoid fever. In 2023, it was announced that we are partnering with LimmaTech Biologics for the further development of a candidate vaccine against shigellosis, while we continue to develop another candidate vaccine against the disease which uses our vaccine platform technology, GMMA. Currently, there are no vaccines to help prevent shigellosis, a disease which causes 600,000 deaths each year. See page 15 for more about our R&D pipeline. + For full details of our progress in our six focus areas, please see our ESG Performance Report Environment Climate change and nature loss are an urgent threat to human health, as well as a risk to business resilience. To get ahead of disease and to help ensure long-term business success, we need to take action on climate and nature. Our commitment Commit to a net zero, nature positive, healthier planet with ambitious goals set for 2030 and 2045 Our ESG Performance Rating metrics1 – Operational emissions reduction (Scope 1 and 2 market- based emissions) – Industrialisation of low-carbon Ventolin initiated, and clinical and non-clinical data available to support regulatory submissions – Percentage of carbon offset volume in project pipeline – Average of the percentage of GSK sites and suppliers compliant with wastewater active pharmaceutical ingredient limits and the percentage of suppliers that are compliant with the AMR Industry Alliance Common Antibiotic Manufacturing Framework and discharge limits – Percentage of paper and palm oil deforestation free – Operational waste and material reduction at our sites Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Responsible business continued 47 (1) These metrics are related to the ESG Performance Rating outlined in our ESG Performance Report 2023. We also measure and report performance against our public environmental sustainability targets, which we publish on gsk.com

Progress in 2023 Climate We have a clear pathway to a net zero impact on climate with ambitious goals for 2030 and 2045. In 2023, the Science Based Targets initiative (SBTi) approved GSK’s net zero target for 2045 in line with its Corporate Net-Zero Standard, the world’s only framework for corporate net zero target setting in line with climate science. Our value chain carbon footprint1 is made up of: – Scope 1 and 2 emissions from our own operations (7%) – Scope 3 emissions from our supply chain (31%) – Scope 3 emissions from patients using our products (57%), mostly metered-dose inhalers (MDIs) – Scope 3 emissions from logistics (4%) – Scope 3 emissions from the disposal of our products (1%) Targets2 – 80% absolute reduction in greenhouse gas emissions from a 2020 baseline, across all scopes, and investment in nature-based solutions for the remaining 20% of our footprint by 2030 – 100% imported renewable electricity by 2025 and 100% renewable electricity (imported and generated) by 2030 (Scope 2) – Net zero greenhouse gas emissions across our full value chain by 2045: 90% absolute reduction in emissions from a 2020 baseline, across all scopes, and all residual emissions neutralised Performance In 2023, we reduced our Scope 1 and 2 carbon emissions by 10% compared with 2022, and by 27% compared with our 2020 baseline. This was primarily from energy efficiency measures and increasing the amount of renewable electricity we use. As a member of the RE100 initiative, we have committed to reach 100% of our imported electricity from renewable sources by 2025 and 100% of all electricity we generate and import from renewable sources by 2030. In 2023, we reached 83% imported renewable electricity, an increase of 10% from 2022. We signed a power purchase agreement to source renewable electricity to cover 50% of our electricity demand for our sites in Europe from mid-2026. Two additional wind turbines and the new solar farm at our manufacturing facility in Irvine, Scotland began generating renewable energy. Our overall Scope 3 emissions are 10% lower than our baseline year of 2020, although there was a 4% increase in 2022 (our latest available data) compared to 2021. This was primarily driven by higher sales of metered dose inhaler (MDI) products. Although overall Scope 3 emissions increased from 2021 to 2022, in the same period, we reduced upstream Scope 3 emissions from our suppliers. The goods and services we buy to make our medicines and vaccines, and additional upstream emissions, account for approximately 31% of our total emissions footprint. In 2023, our supply chain emissions fell by 2%. The use of our medicines and vaccines makes up 57% of our total footprint. Most of this is from the propellant used in MDIs for asthma and chronic obstructive pulmonary disease (COPD). GSK’s rescue MDI medication, Ventolin (salbutamol) is an essential medicine prescribed to approximately 35 million people with respiratory conditions worldwide. Patient use of the inhaler, due to the current propellant, accounts for just under half (48%) of our carbon footprint. We are investing in a low-carbon programme with the potential to reduce greenhouse gas emissions from the inhaler by 90% by transitioning to a next generation, lower carbon propellant. Phase III trials will begin in 2024 and, if successful, regulatory submissions will start in 2025. This is to supplement our existing low carbon dry powder inhalers. See pages 57 to 65 for our disclosure on climate risk and resilience in line with the Task Force on Climate-related Financial Disclosures (TCFD) framework. Nature In 2023, we shared more detail on our plan for contributing to a nature-positive world, in line with the goal of the Global Biodiversity Framework to halt and reverse biodiversity loss by 2030. It sets out how we approach nature through four focus areas – freshwater, land, oceans and atmosphere – including the biodiversity of living species across these areas.3 We aim to deliver our contribution in three ways: avoiding or reducing our impact on nature, protecting and restoring nature, and helping to accelerate collaborative action. This approach is aligned with the work of the Taskforce on Nature-related Financial Disclosures (TNFD) and the Science Based Targets Network (SBTN). In May 2023 we were selected to be part of the first group of companies to participate in the initial target validation process with SBTN to set validated science-based targets for nature, starting with targets for freshwater and land, followed by targets for oceans and biodiversity. We have already started to implement the final TNFD recommendations in our 2023 disclosure, which you can read on page 65. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Responsible business continued 48 (1) Based on 2022 data (2) The target boundary includes biogenic land-related emissions and removals from bioenergy feedstocks (3) We previously reported our Nature targets grouped by water, waste and materials, and biodiversity. In 2023, we updated our target categories to align with the four areas of nature as defined by TNFD and SBTN, with underlying targets on waste and materials. The targets remain the same

Freshwater We continue to work towards our existing water targets. Targets – Achieve good water stewardship at 100% of our sites by 2025 – Reduce overall water use in our operations by 20% by 2030 – Be water neutral in our own operations and at key suppliers in water-stressed regions by 2030 – Zero impact API levels1 for all sites and key suppliers by 2030 Performance We achieved our overall water reduction target in 2022. In 2023, we reduced overall water use in our operations by an additional 1% compared with 2022 and by 6% in sites in high water stress regions. This is a decrease of 24% for overall water use and 11% for sites in high water stress regions against our 2020 baseline. For our sites and key suppliers located in water-stressed areas, we are developing catchment-level water replenishment, restoration and regeneration projects, including partnering with NGOs to deliver our water neutrality target. In 2023, 87% of all sites and key suppliers were compliant with AMR Alliance and API Wastewater discharge limits. This is down from 94% in 2022, primarily due to a scope expansion. This is driven by us expanding our scope to include more API suppliers which led to a decrease in the percentage of key suppliers that were confirmed to be within Wastewater API discharge limits. Our work to strengthen responsible manufacturing of antibiotics was highlighted as an example of good practice in a 2023 report on the issue from the Access to Medicine Foundation’s AMR Benchmark. Land We continue to deliver on our existing land targets. Targets – Positive impact on biodiversity at all sites2 by 2030 – 100% of agricultural and forestry-derived materials sustainably sourced and deforestation free by 2030 Performance During 2023 we completed baseline assessments for six of our sites, meaning we have now assessed all our sites, using the Natural England Biodiversity Net Positive methodology. In parallel, we have plans in place to improve biodiversity at nine of our manufacturing sites from 2022. We set out ambitious new Sustainable Sourcing Standards for suppliers who provide us with materials that are highly dependent on nature, like lactose, gelatine and soy. We have roadmaps in place to achieve 100% sustainably sourced paper packaging and palm oil by 2025. In 2023, 86% of our paper packaging was derived from certified sources or from recycled raw materials and 98% of our core palm oil materials were certified by third-parties as being from sustainable sources. While working with suppliers is a key part of our goal to reduce our impact on nature, where appropriate we will also look at opportunities to reduce or avoid the use of some natural materials, including through process efficiencies and synthetic alternatives. For example, we are working on a process improvement to deliver a significant yield increase, reducing our nature impact and improving supply resilience. Oceans We continue to deliver on our existing ocean target (set out below), and will apply the relevant science-based methodology on oceans when it becomes available. Target – 100% of marine-derived materials sustainably sourced by 2030 Performance Our impacts and dependencies on oceans come primarily from marine-derived materials that are a critical part of manufacturing vaccines and medicines. For example, we use horseshoe crab blood, which is an important substance that is required by some regulators to be used in pharmaceutical and biomedical quality control processes to ensure the quality and safety of medicines, vaccines and devices. We continue to make progress on volume reductions, and we are advancing a pilot across five of our sites to test the use of non-animal alternatives. At the same time, we are engaging with regulators to support wider uptake of these alternatives. While we make progress on reducing volumes and moving to synthetic alternatives, we are working with our suppliers to improve sustainability. Our new Sustainable Sourcing Standards include a specific Marine Sustainable Sourcing Standard which outlines the requirements that our suppliers of marine-derived materials must adhere to. As part of this, we conducted physical site audits of key suppliers in 2023. Atmosphere Air pollution is a significant risk to human health, particularly for patients with respiratory conditions like asthma and COPD. Performance Our approach to air pollution includes reducing pollutants linked to burning of fossil fuels that will be addressed via our SBTi-aligned climate targets (set out on page 48), as well as looking more broadly at our air pollution footprint. We are members of the Alliance for Clean Air through the Clean Air Fund and the World Economic Forum. We have done an initial assessment to establish an air pollution footprint in our operations and our supply chain. We are creating reduction plans that are aligned to our pathway to net zero and which aim to have a positive impact on air quality. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Responsible business continued 49 (1) Below the predicted no-effect level (2) GSK-owned sites

Waste and materials The overuse of natural resources and the generation of waste and pollution are key drivers of climate change and nature loss. Targets Our approach to product stewardship means that we consider and aim to address impacts on nature and climate at every stage of the product lifecycle, from discovery, design, sourcing and manufacturing through to product use and disposal. We have set a target to help accelerate the adoption of this approach: – 25% environmental impact reduction for our products and packaging by 2030 We have also set targets to reduce operational and supply chain waste: – Zero operational waste1, including eliminating single use plastics2 by 2030 – 10% waste reduction from supply chain by 2030 Product stewardship Our approach to product stewardship across both new and existing products is built on a scientific method for environmental footprinting called Life Cycle Assessment (LCA). Since 2022 we have completed an LCA analysis of 22 products using the LCA methodology which has enabled us to identify where we need to improve the manufacturing design, to assess potential savings from design changes and provide product-level information to key customers on specific products. 100% of GSK sites are now manufacturing PVC-free secondary and tertiary packaging. Waste In 2023, we reduced operational waste by 1% since last year, a total of 21% since 2020. We increased the amount of materials recovered by circular routes by 53%. We have maintained zero operational waste to landfill and we continue to build on our long-standing operational waste management programme to identify opportunities to find more beneficial uses for waste. + For full details of our progress in our six focus areas, please see our ESG + Performance Report Diversity, equity and inclusion We want to be an inclusive business where all our people can thrive, which ensures diversity in our clinical trials and supports diverse communities. Our commitment Create a diverse, equitable and inclusive workplace; enhance recruitment of diverse patient populations in our clinical trials; and support diverse communities Our ESG Performance Rating metrics – 100% of phase III trials initiated in 2023 will have proactive plans in place designed to enrol appropriately diverse trial participants, consistent with disease epidemiology – Improve year-on-year spend with US-based certified diverse-owned suppliers – Update towards 2025 people aspirations through fair and equitable opportunities: – aspire to have women hold at least 45% of VP-and- above roles globally by the end of 2025 – aspire to have at least 30% ethnically diverse leaders in our roles at VP-and-above in the US by the end of 2025, and increase the percentage of Black or African American, and Hispanic or Latinx VP-and-above leaders year on year – aspire to have at least 18% ethnically diverse leaders in our roles at VP-and-above in the UK by the end of 2025, and increase the percentage of Black VP-and- above leaders year on year Progress in 2023 Clinical trial diversity We continue to make progress in advancing clinical trial diversity. We met our objective of 100% of the phase III interventional trials initiated in 2023 having proactive diversity plans. We also are challenging ourselves to actively monitor patient recruitment in real time to ensure that we reach our diversity goals. In February 2023, we published a study of 17 years of GSK and ViiV Healthcare US clinical trial diversity data. It showed that enrolling participants to clinical trials based on real- world disease epidemiology data, rather than census data, would ensure that those trials reflect the populations affected by different diseases. By publicly sharing this research, we hope to advance the discussion around clinical trial diversity and improve how the pharmaceutical sector approaches the issue of clinical trial diversity. Supporting diversity in our supply chains By engaging with and mentoring small and diverse-owned businesses in our supply chain, we can help them identify potential areas for growth. In 2023, we increased our spend annually with US-based certified diverse-owned suppliers. This year, we expanded our successful US supplier diversity programme to the UK. Groups which benefit from this programme include women, ethnic minorities, members of the LGBTQ+ community, people with disabilities and military veterans, as well as small businesses in high- unemployment, low income communities. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Responsible business continued 50 (1) Including a 20% reduction in routine hazardous and non-hazardous waste (2) Where regulatory obligations allow, and excluding plastics which are critical to product discovery and development and health & safety

Ensuring diversity in our workplaces We are fundamentally committed to equal employment opportunity and non-discrimination for all employees and we want all our leadership to reflect our GSK people and our people to reflect the communities we work and hire in. At the end of 2023, women held 45% of VP-and-above roles globally, compared with 42% in 2022. Women made up 48% of all employees in 2023, and 50% of all management roles. In the UK at the end of 2023, we had 18.4% ethnically diverse leaders at VP-and-above, compared with 14.3% in 2022. We had 1.9% Black leaders at VP-and-above compared with 1.6% in 2022. In the US, at the end of 2023, we had 35.7% ethnically diverse leaders at VP-and-above, compared with 31.3% in 2022. We had 8.1% Black or African American leaders at VP-and-above compared with 8.6% in 2022. We had 6.4% Hispanic or Latinx leaders at VP-and- above compared with 6.4% in 2022. We remain committed to the application of fair and equitable pay practices to ensure equal opportunities and equal pay for equal work. Our 2023 gender pay gap for all permanent UK-based GSK employees is -0.50% (mean), compared to the national average of 13.2%. We are also publishing our second UK ethnicity pay gap comparing the average pay of our White and Ethnically Diverse employees. Our 2023 UK ethnicity pay gap for all permanent UK-based GSK employees is -0.74% (mean), compared with 0.06% in 2022. In addition, within our 2023 UK ethnicity pay gap report we are also sharing the pay gaps comparing the average pay of our White employees with those in the ethnic groupings of Black, Mixed, Asian and Other. This is with reference to the UK government’s recently published guidance to provide a more granular view. This year, we added Disability Confidence training into our First Line Leader training, aimed at all our people managers. This training is designed to develop inclusive leaders that are able to promote disability confidence within their teams. We continue to work to make sure that our LGBTQ+ colleagues feel welcome, valued and included. We were once more recognised as a Gold employer in Stonewall’s Top Global Employers Index. We also relaunched our Mental Health Matters training. Available globally, it is designed to help our people spot the signs of poor mental health, know how to start a conversation with others, and signpost resources to support everyone’s wellbeing. Supporting diverse innovators for the future In the UK, we launched a £6 million, ten-year STEM equity programme, targeting 11–25-year-old girls and young women, black people and people from low socio-economic backgrounds. The programme includes nationwide STEM mentoring, delivered in partnership with established mentoring organisations. In its first three years, we aim to reach approximately 4,000 young people through this programme. + For full details of our progress in our six focus areas, please see our ESG Performance Report Ethical standards Our culture guides our people to behave in an ethical way, to do the right thing and Speak Up about any concerns they have. We expect everyone who works for us to live up to this, and we expect the same of our suppliers. Our commitment Promote ethical behaviour across our business by supporting our employees to do the right thing and working with suppliers that share our standards and operate in a responsible way Our ESG Performance Rating metrics – 100% of employees and complementary workers complete GSK’s 2023 mandatory training – Percentage of employees who believe they ‘can and do Speak Up if things don’t feel right’ is above the general industry benchmark1 – 80% of direct high-risk suppliers that achieve GSK’s minimum EcoVadis score or have an improvement plan in place Progress in 2023 Supporting GSK people to do the right thing Our Code of Conduct (The Code) reflects our purpose to unite science, technology and talent to get ahead of disease together. It sets out the commitments we make as a company and to each other to deliver on our purpose and ambition. The Code is supported by additional global policies and standards. We also have an accompanying global mandatory learning curriculum, Living our Code, which all our people are required to complete. In 2023, 100% of our employees and 99% of complementary workers completed this training where due by year-end. We also have anti-bribery and corruption (ABAC) training for our people in certain high-risk roles or geographic regions. This helps them identify and mitigate any potential ABAC risk – especially in third-party relationships – and to recognise, report and manage conflicts of interest. In 2023, 100% of employees and 99% of complementary workers completed this training. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Responsible business continued 51 (1) The general industry benchmark is 66% according to 2023 research by KornFerry

Reporting and investigating concerns In 2023, we saw an overall decrease in the number of employees who had concerns raised against them, employees disciplined for policy violations and open cases at year end. This is reflective of several factors including external geopolitical and economic issues affecting some countries which changes the nature of concerns raised and, internally, our continued emphasis on appropriate management and closure of cases. Our commitment to human rights We are signatories to the UN Global Compact and our Human Rights Position Statement lays out our commitment to the UN Guiding Principles on Business and Human Rights. We have a cross-business Human Rights Steering Group, which reports to the GLT and Board’s Corporate Responsibility Committee, and drives progress on human rights impacts and risks across the business. In 2023, we carried out human rights training for priority suppliers, aimed at ensuring a good understanding of human rights and labour principles and aligned with international standards. We also continued our human rights training for procurement and third-party engagement leads, to better equip them to spot human rights issues when visiting suppliers. We conduct audits and site visits covering Environment, Health and Safety (EHS) and labour rights for our priority suppliers.1 Some of the top issues identified during supplier visits in 2023 related to policy, wages and compliance. All observations have action plans in place to drive improvement. We are committed to fair and equitable pay, ensuring that all employees globally receive pay that is competitive in their local markets and sufficient to support a sustainable standard of living. In 2023, the Fair Wage Network certified GSK as a Living Wage employer, after it reviewed the global gap analysis we conducted in 2022. It confirmed that all GSK workers are paid at or above the living wage in their relevant markets. We have also developed a consistent approach to how GSK will manage global fair wage analysis annually, as well as a methodology for the Fair Wage Network to use to continue to assess us. Working with third parties We expect our third parties to comply with applicable laws and regulations and to adopt, at minimum our ABAC and labour rights principles and, where relevant, to comply with our standards on quality, patient safety, health and safety, and the environment. In 2023, we performed over 7,500 assessments of our high-risk third parties across 17 risk areas. Across the organisation, we give additional support on EHS risks to our largest suppliers, including those who supply globally medically-critical products, as well as those who are critical to our R&D, and those largest by spend.2 We visit sites, in person or virtually, to help suppliers better understand and control their EHS risks. This year, we conducted 73 physical visits across 63 priority suppliers.3 We conducted 47 supplier audits following industry standard Pharmaceutical Supply Chain Initiative guidelines. We trained more than 1,000 supplier employees on EHS, strengthened EHS contractual obligations and have worked with suppliers to help them improve their EcoVadis scores. Using data responsibly Data is an essential foundation to realising our ambitions for patients. Advances in artificial intelligence (AI) and machine learning (ML) technologies present tremendous opportunities, but the technologies must be approached correctly, responsibly and ethically. Increases in the volume of data processed through AI/ML use have resulted in a greater focus on data governance and the ethical use of personal information, over and above compliance with data privacy laws. We take our responsibility for data privacy seriously and we exercise high standards of integrity in dealing with personal information. Our Digital and Privacy Governance Board oversees our overall data ethics and privacy operating model, supported by digital and privacy legal experts and compliance professionals. We monitor and mitigate new and emerging cyber threats to protect ourselves from cyber security risks. We have additional governance boards that oversee the use of our data in the research, development, manufacture and supply of our products to ensure we follow regulations and meet ethical obligations. In 2023, we created a cross-functional AI Governance Council to oversee our AI strategy and to ensure responsible adoption of AI/ML. This is complemented by an internal policy to ensure AI/ML adoption is safe and aligned with GSK's culture by establishing AI Principles underpinned by the ethical standards set out in the GSK Code. Political engagement At GSK, we seek to contribute to public policy debate, especially in relation to life sciences and healthcare. We are committed to the highest ethical standards and legislative requirements in all of our political engagements. We do not make corporate political contributions, nor do we sponsor party political meetings anywhere around the world. + For full details of our progress in our six focus areas, please see our ESG Performance Report Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Responsible business continued 52 (1) Our largest suppliers, including those who supply globally medically critical products, are critical to our R&D, and those largest by spend (2) GSK maintains a list of globally medically critical products. These are drug products approved to treat a life-threatening disease or medical condition for which there is no other adequately available alternative and of which GSK is the only provider (3) Our EHS priority suppliers are API suppliers who are, or will be, medically-, R&D-, or revenue-critical to GSK, or are high spend suppliers

Product governance Our commitment We commit to maintaining robust quality and safety processes, and using data and new technologies responsibly Our ESG Performance Rating metrics – Average number of critical and major findings per inspection by FDA/MHRA/EMA regulators – Percentage of inspections from all regulators with no critical findings or official action indicated – Number of FDA warning letters – Total number of Class I/II external product recalls across all markets – Register and disclose all human subject research of GSK products. Specifically, register protocol summaries for studies initiated in 2023; and disclose results summaries for studies with results due in 2023 Progress in 2023 Maintaining quality across GSK We have a detailed and specific quality framework that describes how we comply with regulatory requirements and other standards across our markets. This addresses global and local regulations across manufacturing and distribution processes, and is based on principles defined by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Our GSK quality function is responsible for managing quality and for ensuring a quality mindset is embedded throughout the organisation at all levels. It brings together an extensive global network of quality and compliance professionals within each of our business units, from site level to senior management. Our quality management depends upon comprehensive and ongoing patient safety and quality process training. The Quality Management System details the training required by GSK people, including induction, hygiene, safety and technical skills training, as well as good distribution and manufacturing practice training. Employees who carry out specific, quality-critical or sensitive activities are subject to additional training as necessary. Inspections, recalls and audit In 2023, we had 114 regulatory inspections at our manufacturing sites and local operating companies, compared with 122 in 2022. We received zero warning letters from the United States Food and Drugs Administration (FDA) or critical findings from the Medicines Healthcare products Regulatory Agency (MHRA) and European Medicines Agency (EMA) regulators in 2023. We respond to and learn from all inspection findings, taking the necessary action to address them. Throughout 2023, we had two Class I product recalls and there were fewer Class II recalls compared with 2022.2 If necessary to protect patients, we will not hesitate to recall products voluntarily. Quality management along our supply chains In 2023, we conducted 1,081 quality audits of contract manufacturers and suppliers to verify that they comply with GSK standards. We have a comprehensive quality oversight model that is aligned to our Quality Management System. It uses a risk-based approach to assess, qualify, manage and monitor our third-party suppliers on an ongoing basis, driving continuous performance. Pharmacovigilance at all times We have a well-established and rigorous worldwide system to monitor and review the safety of our products throughout clinical development and after regulatory approval. We expect our partners to meet the same high standards of safety and governance. We conduct reviews of third-party safety systems, monitoring of contractual obligations and fostering collaboration through the lifecycle of the relationship. Tackling counterfeit medicines and vaccines Falsified products put the health of patients at risk and threaten our brand and reputation. We report all cases of confirmed counterfeit products to the WHO and to relevant regulatory authorities. We actively participate in legal proceedings against illegal actors, and support customs and local authorities with regular training. We also monitor online marketplaces and social media to request takedowns of sites illicitly selling prescription-only medicines. Clinical data transparency As part of our commitment to transparency, we have made 7,988 protocol summaries and 6,734 summaries of results available since the GSK trial register was set up in 2004. We have also listed 2,669 clinical trials for data sharing via www.vivli.org. + For full details of our progress against our six focus areas, please see our ESG Performance Report Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Responsible business continued 53 (1) We consider any observations from the US FDA as major (2) Class I recalls are triggered by a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death. Class II recalls address the use of or exposure to a violative product which may cause temporary or medically reversible adverse health consequences, or where the probability of serious adverse health consequences is remote. Class III recalls relate to the use of or exposure to a violative product which is not likely to cause adverse health consequences

Risk management and disclosure statements In this section Risk management 55 Climate-related financial disclosures 57 Nature-related financial disclosures 65 Employees by gender 69 2023 Annual Report on Form 20-F 54

We can only deliver our bold ambitions for patients if we maintain a well-embedded risk management and internal control framework overseen and evaluated by our Board. Controls and guidance to manage risk effectively Our well-embedded risk management and internal control framework gives our Board the ability to evaluate and oversee how the company manages principal and emerging risks in line with our strategy and long-term priorities. Our company-wide policy sets out the requirements, roles and responsibilities for the management and governance of risks and controls, as well as supporting guidance on the essential elements of our internal control framework. We routinely evaluate our risk management and internal control framework for improvements. Board oversight setting the 'tone from the top' The Board oversees our system of risk management and internal control and establishes our risk appetite, supported by the Audit & Risk Committee (ARC). The Corporate Responsibility Committee (CRC) and Science Committee further assess the effectiveness of risk management strategies that fall within their defined remits. Both the ARC and the Board oversee our cyber security risks. For more details on the Board and its committees’ responsibilities and remit, see page 108. Our Risk Oversight and Compliance Council (ROCC), co-chaired by our Group General Counsel and our Chief Compliance Officer, helps the ARC, CRC and Science Committee to oversee the risks, and the strategies used to address them. Also, risk management and compliance boards (RMCBs) across the Group promote the ‘tone from the top’, establish our risk culture and oversee the effectiveness of risk management activities, while also communicating information about internal controls. Management is held accountable for delivering on its objectives in line with the established risk appetite pertaining to principal risks. The Disclosure Committee has the responsibility for considering the materiality of information and determining the disclosure of this information in a timely way. An enterprise risk owner is responsible for each principal risk, overseen by a GLT member. Risk owners report risk and mitigation to ROCC and the appropriate Board committee each quarter. Significant risks or issues can also be escalated to the GLT, RMCB, or appropriate risk governance forum (e.g., Global Safety Board) throughout the year as needed. Legal & Compliance support these efforts by advising on our business strategies, activities, risks and controls. Audit & Assurance provides assessments of the adequacy and effectiveness of our framework. Considering the likelihood, impact and timescale of risks Our enterprise risk assessment methodology is the mechanism by which we assess all risk, including our principal risks. Our enterprise risk assessment methodology considers the likelihood and impact of risks, and the timescale over which a risk could occur based on the most probable scenario and considering our existing internal controls. Our impact assessments include considerations across patient safety, quality and supply; environment, health and safety; legal; people; regulatory; reputation; strategic objectives; and finance, incorporating materiality thresholds. As well as considering current and evolving risks, we evaluate emerging risks that could affect our ability to achieve our long-term priorities over the three-year horizon. We also define risks as ‘emerging’ if we need to know more about how likely they are to materialise, or what impact they would have if they did. We further evaluate emerging risks and their impact on the company to assess whether they should be elevated to a principal risk. Our risk management and compliance boards at all levels identify emerging risks on an ongoing basis, and ROCC discusses evolving and emerging risks at each meeting. At the same time, we scan the risk horizon throughout the year to identify external trends that may be opportunities and/or emerging risks and monitor our business activities and internal environment. ROCC conducts an annual risk review to assess principal and emerging risks for the company. This review is supported by extensive analysis of external trends and insights, senior-level interviews and recommendations from risk management and compliance boards and risk owners. ROCC shares this annual review with the ARC and Board for assessment and agreement, forming the basis for the following year’s risk management focus. Our business strategy, results of operations and financial condition have not been materially affected by risks from cyber security threats, including as a result of previous cyber security incidents, but we cannot provide assurance that they will not be materially affected in the future by such risks and any future material incidents. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Risk management 55

Our risk management and internal control framework Our risk management and internal control framework is aligned to industry standards and legal and regulatory requirements. It defines the essential elements we expect and helps us to identify, assess, manage, report and oversee risk relevant to our business activities. This framework helps to ensure our risks are proportionately managed in line with our risk appetite throughout the year in a timely and transparent manner to support our strategic objectives. For our principal risks, which include information and cyber security, we define enterprise risk plans that include a description of the risk, its context, our assessment, risk appetite, how we will treat the risk, and the actions businesses will take in line with our internal control framework to mitigate the risk. These plans enable our Board committees to assess the effectiveness of our risk management strategies. We report on our principal risks and emerging risks to ROCC and the respective Board committees every quarter, to drive more dynamic, data- driven discussions, agile risk management strategies and oversight. We report on existing control measures, implementation, emerging risks, external insights and key risk indicators with risk reporting thresholds aligned to risk appetite. We include risks and mitigations associated with relevant events around us, such as geopolitical tensions. Our Code sets out the overarching expectations for our employees and complementary workers. We aim to do the right thing with integrity and care as part of our culture. Our risk management framework complements our culture and Speak Up processes in making sure that we identify and mitigate risks effectively. We monitor our most important risks and take action to address issues. Our annual confirmation exercise with General Managers, Site Directors, senior leaders and GLT, checks that key risks are well managed, and that actions are in place to address gaps. Our principal risks include controls for responding to problems within their risk plans. We also have business continuity planning embedded in our framework and our critical processes, so we can continue business operations in the event of a crisis. + ARC report – see page 121 + Internal control framework – see page 122 + Legal proceedings – see page 241 + Environment – see page 47 + Climate-related financial disclosures – see page 57 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Risk management continued 56

Our climate-related financial disclosures are consistent with the recommendations and recommended disclosures of the Task Force on Climate-related Financial Disclosures (TCFD) including the TCFD all-sector guidance, and in compliance with the requirements of LR 9.8.6R.(8) (UK Listing Rules). The disclosures are in compliance with the Companies (Strategic Report) (Climate-related Financial Disclosure) Regulations 2022 of the Company Act 2006. In 2023 we have updated our risk assessments to reflect changes in the supply chain and the progression of our sustainability transformation programme. Governance The board’s oversight of climate-related risks and opportunities Board The Board considers climate-related matters throughout the year. This includes assessing risk management processes, challenging and endorsing the business plan and budgets, including overseeing major capital expenditures, acquisitions and divestments. In 2023, the Board approved progression to the next phase of development of the low carbon Ventolin programme. The Corporate Responsibility Committee (CRC) exercises oversight, provides guidance and reviews our ESG performance, including climate-related risks and opportunities, and environmental performance against targets. The CRC receives quarterly updates on environmental sustainability, including climate. Regular attendees include the CEO, and the President Global Supply Chain. See page 108 for further details of the Board architecture. In 2020 the CRC reviewed and approved GSK’s twin goals on climate and nature. Following the demerger of the consumer healthcare business in July 2022, the CRC approved that GSK would submit updated refreshed targets to the Science Based Targets initiative (SBTi) that are aligned to a 1.5°C pathway, and to align to the SBTi Net Zero Standard, to reduce carbon emissions by 80% by 2030 and 90% by 2045. In 2023 the CRC met five times and discussed climate- related issues on three separate occasions with management. It focused on: – progress in delivering against our climate ambitions including low carbon Ventolin and Nature Plan updates – implications of the geopolitical landscape – key milestones and decisions required to achieve net zero targets – mid-year performance for key environmental metrics, including climate-related metrics, as part of reviewing the interim ESG Performance Rating for 2023 – approved our climate disclosure statement and final ESG Performance Rating for 2022 and other public environmental reporting and disclosures Management’s role in assessing and managing climate- related risks and opportunities GSK Leadership Team (GLT) The GLT meets regularly, giving members an opportunity to discuss strategic, financial and reputational matters. The President, Global Supply Chain, a GLT member, has management responsibility for environmental sustainability, which includes climate change. He is responsible for governance and oversight of risks and opportunities and makes sure there is an effective framework to manage the risks and opportunities across each of our business units, along with delivering on our commitments to a net zero, nature positive, healthier planet, with ambitious goals set for 2030 and 2045 across our entire value chain. In 2023 GLT reviewed and discussed the mid-year performance for key environmental metrics, including climate-related ones (see page 47) as part of reviewing GSK’s ESG Performance Rating. GSK Sustainability Council The Sustainability Council, held quarterly, is attended by senior leaders from across the business. Members include leaders from procurement, finance, HR, compliance, R&D, manufacturing and corporate affairs. The Council is co- chaired by the President Global Supply Chain and the VP Sustainability and supported by the global Sustainability team and external third parties, who provide specialist expertise and advice to the business. In 2023 the Council: – approved the annual targets for the climate Key Performance Indicators (KPI) of the sustainability programme – reviewed monthly performance and escalations of any potential concerns or issues – approved the annual climate risk review and approach for risk disclosure – agreed that the newly formed ESG Reporting Hub would be accountable for assurance of environmental data in 2023 – reviewed progress of the core programmes to improve the sustainability of our supply chain – reviewed progress towards securing a portfolio of carbon credits in support of our 2030 commitment Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Climate-related financial disclosures 57

Other business support The Sustainability Council is supported in assessing and managing climate-related risks and opportunities by: – the sustainability programme steering team, which is chaired by the VP Sustainability that meets monthly and co-ordinates the sustainability programme and associated workstreams. This team monitors programme performance and the progress of the enablers required to deliver the sustainability programme – sustainability councils within each business, which meet quarterly to review their business unit performance and delivery against the company sustainability ambition. These are chaired by senior leaders within the business who also attend the GSK sustainability council – the Metered Dose Inhaler steering team, which is attended by senior leaders from across the commercial, supply chain, regulatory and R&D businesses aligned to our respiratory business. This team is chaired by the President Global Supply Chain, who also chairs the Sustainability Council and is the decision-making body for the programme to reduce the climate impact of metered dose inhalers which contribute to approximately 50% of GSK’s total GHG emissions – the Capital Allocations Board (CAB), which is chaired by the CFO and includes the Group Financial Controller, reviews climate-related capital expenditure as part of its annual planning and capital allocation process – the ESG Reporting Hub, which was established in 2023, provides oversight and assurance of ESG performance data, including carbon emissions data – The carbon offset programme steering committee, which includes the Group Financial Controller and the VP Sustainability, who also attends Sustainability Council, reviews the due diligence outcomes of potential carbon offset projects, the performance of established investments and makes new investment decisions – A cross-functional team from the Sustainability, Finance, Supply Chain and Procurement functions performs an annual review of climate risks to monitor previously identified climate risk and escalate new or emerging climate risks to the Sustainability Council – Results of climate scenario modelling are shared with business unit Risk Management Control Boards (RMCB) Strategy The climate-related risks and opportunities we have identified over the short, medium, and long term Climate-related risks and opportunities are considered in three different time horizons: 1. short term (less than three years) aligning with financial planning timeframes 2. medium term (three to ten years) aligning with long-term business forecasting timeframes 3. long term (more than ten years) to enable us to explore the uncertainties in changes to weather, disease patterns and societal responses to climate change across the globe We have identified and prioritised these climate-related risks and opportunities: Risks: – changes to regulations governing the supply of high global warming potential (GWP) substances by the EU, UK and US governments could restrict our ability to manufacture metered dose inhalers – future regulatory policy responses to address climate change could lead to the imposition of carbon taxes by countries where we manufacture and source goods from third parties – increasing levels of water stress could lead to interruptions to supply of water to our and third-party supply sites – increasing frequency and impact of extreme weather events that could disrupt to GSK and third-party supplier sites – nature-based projects might not deliver sufficient volumes of carbon credits to offset 2 million tonnes CO2e per year from 2030, requiring us to buy additional credits at higher cost Opportunities: – At COP28 in 2023, more than 70 countries committed to provide low-carbon healthcare systems. This could lead to increasing demand for low-carbon medicines and vaccines – Several reports exploring the impact of climate change and health have shown that climate change affects water- and vector-borne diseases. This could lead to increasing demand for new medicines and vaccines The processes for identifying and assessing climate-related risks and opportunities are set out in the Risk Management section. We will continue to monitor for emerging risks and new data to include in future assessments. The impact of climate-related risks and opportunities on our business, strategy and financial planning Our commitment to work towards a net zero, nature positive, healthier planet with ambitious goals set for 2030 and 2045 is embedded in our strategic long-term priorities, always considering the social, environmental and governance impacts of everything we do from laboratory to patient. Our near-term carbon reduction target is an 80% reduction in Scope 1 & 2 and Scope 3 carbon emissions by 2030. Our long-term carbon reduction target is a 90% reduction in Scope 1 & 2 and Scope 3 carbon emissions by 2045. Both targets are measured against a 2020 baseline. These targets are aligned to the 1.5°C pathway and were approved by the Science Based Targets initiative (SBTi) during 2023. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Climate-related financial disclosures continued 58

Transition plan We are taking action to reduce emissions across our full value chain, prioritising the highest-impact areas. We will invest around £1 billion from 2020-30 to deliver emissions reductions and removals to achieve our targets though the activities outlined below. Beyond 2030 we expect we will be left with the harder- to-tackle emissions from across our supply chain, our own operations, logistics, and disposal. In many cases, addressing these residual emissions is likely to depend on technologies, infrastructure and regulatory frameworks that require broad public/private collaboration. So our decarbonisation plan is interdependent with the broader economic transition and follows a similar timeframe. Our progress in reducing carbon emissions can be found on page 48. Direct operations In order to continue reducing Scope 1 & 2 emissions across our operations by 2030, we are focusing on: – maximising energy efficiency in our sites through our long-standing energy efficiency programme – transitioning to 100% imported renewable electricity by 2025 by investing in power purchase agreements, supplemented by the purchase of energy attribute certificates – increasing the use of electric vehicles by our sales fleet Risks and uncertainties In some markets where we operate, such as Singapore, accessing renewable electricity will be challenging because of the limited generation capacity and the market boundary rules governing imported electricity. There are uncertainties in the transition to renewable heat. High-temperature heat produced by electricity is not generally commercially available today. Biogas can replace natural gas without introducing major changes to facilities but is not widely available in the locations where we operate. The use of biomass as fuel could introduce issues of land use change and impacts on local air quality. The transition to 100% electric vehicles by 2030 could be restricted by vehicle availability, lack of charging infrastructure and sourcing of key materials for battery production. Supply chain Our Sustainable Procurement Programme requires our suppliers to disclose emissions and set carbon reduction targets aligned with a 1.5°C reduction pathway. We also work with suppliers, particularly those with the largest footprint, to encourage them to adopt new sustainability measures. Supply chain emissions are a shared challenge across our sector, and we are working with our peers on collaborative initiatives such as: – the Activate programme to help Active Pharmaceutical Ingredients (API) suppliers accelerate decarbonisation initiatives – the Energize programme to encourage the use of renewable energy throughout the pharmaceutical sector’s supply chain – the Manufacture 2030 initiative to encourage suppliers to measure, manage and reduce their emissions – the Pharma LCA consortium is a group of eight global pharmaceutical that have come together via the Pharmaceutical Environment Group with support from the Sustainable Markets Initiative to co-develop a shared way of measuring and reporting environmental product footprints Risks and uncertainties Pharmaceutical manufacturing processes are highly regulated by different agencies across the world which may slow down the implementation of some decarbonisation initiatives. Our supply chains are complex and can involve several intermediate stages of production that are highly product- specific. Our volume demand on specific materials is quite low which can reduce our ability to influence where we only purchase a small share of a supplier's production. Many suppliers are based in regions where renewable electricity and heat is less available than elsewhere. Measuring Scope 3 emissions is complex and challenging and there is a lack of primary data from suppliers. Methodologies involve using spend-based estimates mixed in with activity-based data, industry average data and extrapolations based on subjective choices and judgments. As data systems, processes and controls mature and more primary data becomes available, there may be the need to restate reported emissions data in the future. Product impact The use of our products makes up 57% of our carbon footprint. Patient use of GSK’s rescue metered dose inhaler (MDI) medication, Ventolin (salbutamol), accounts for just under half (48%) of our carbon footprint. We are investing in an R&D programme and a large factory upgrade project to redevelop this inhaler by transitioning to a lower-carbon propellant. Recent data from early clinical trials has supported the decision to progress to phase III and dosing of first patients is planned in the first half of 2024. If successful, regulatory submissions will begin in 2025. Risks and uncertainties Metered dose inhalers are complex devices, and any new medical propellant must meet a specific range of technical performance characteristics to be safe and efficacious for patients. We are engaging with medical regulators such as the US Food and Drug Administration (FDA), European Medicines Agency (EMA) and the UK Medicines and Healthcare Products Regulatory Agency (MHRA) on how advances in pharmaceutical product design can reduce the environmental impact of medicines. Carbon credits While we are focused on emissions reductions to meet our carbon targets, we are also investing in high quality nature Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Climate-related financial disclosures continued 59

protection and restoration projects that support our net- zero and nature positive goals and deliver co-benefits to human health to generate carbon credits to offset annually the 20% of our baseline value chain carbon footprint from 2030. The volume of credits required will taper down to 10% as we continue to reduce our emissions, aiming to achieve net zero emissions across our full value chain by 2045. Our criteria for high quality projects include avoidance of harm, transparency, additionality, permanence, mitigation of leakage, project monitoring, reporting and verification of claims and avoidance of double counting. For our 2030 target we are prioritising carbon removal credits, but we will also secure a proportion of carbon avoidance and reductions credits in recognition of their critical role in conserving existing carbon stocks and protecting nature. For our 2045 Net Zero target, we will aim to secure only carbon removal credits. Risks and uncertainties We recognise that this is a fast-moving field, and that methodologies and guidelines will likely evolve as we implement our plans. We commit to remaining flexible and transparent about our progress and learning. There is a risk that the nature-based projects do not deliver sufficient volumes of carbon credits to meet our needs in a given year and that we may need to purchase of more credits at higher cost. Climate scenarios We use climate scenarios to inform management about climate risks, reporting the results to Risk Management Control Boards (RMCB) in the business as well as to the Sustainability Council. We have developed modelling tools with the support of third parties that enable us to model the impacts of physical and transition risks where our sites and supply chains are located. For example we have modelled the probability of an interruption from an extreme weather event at our key sites and supplier sites and the subsequent financial impact of that interruption assuming the inventory levels carried under existing business continuity plans. We have modelled the impact of future carbon taxes, such as direct taxes on energy-related emissions, emissions trading schemes and taxes from carbon border adjustment mechanisms assuming we deliver our carbon reduction glidepath to 2030 and beyond. In 2022, we reviewed and updated our climate scenarios first developed in 2019. We intend to review the scenarios again in 2025 to make sure they'll remain up to date.. Net zero scenario (SSP 1 – RCP 1.9) This scenario sets out a narrow but achievable pathway for the global energy sector to achieve net zero CO2 emissions by 20501. It does not rely on emissions reduction from outside the energy sector to achieve its goal. The scenario is consistent with limiting the global temperature rise to 1.5°C without a temperature overshoot. Net zero means huge declines in the use of coal, oil and gas and a shift to renewable energy sources. Low-carbon scenario (SSP 1 – RCP 2.6) In this scenario, all current net zero pledges are achieved in full and there are extensive efforts to realise near-term emissions reductions; advanced economies reach net zero emissions by 2050, China around 2060, and all other countries by 2070 at the latest2. The scenario is consistent with limiting the global temperature rise to below 2°C. With some level of net negative emissions after 2070, the temperature rise could be reduced to 1.5°C in 2100. Current trajectory scenario (SSP2 – RCP4.5) This scenario sets out to show to what extent announced ambitions and targets are on the path to deliver the emissions reductions required to achieve net zero emissions by 20503. The temperature rise will exceed 2°C by 2100, with a more noticeable shift to happen in the latter half of the century. A net zero pledge for emissions within the scenario does not necessarily mean that CO2 emissions from the energy sector need to reach net zero, but there is an allocation for carbon offsetting within the pledges. Breach of planetary boundaries scenarios (SSP 5 – RCP 8.5) This scenario is not aligned to any of the pledges laid out in the Paris Agreement and is one where countries are unable to meet the United Nations Sustainable Development Goals. This scenario will have the most severe physical consequences for the planet. The temperature rise will exceed 4°C by 2100, leading to high loss of biodiversity and species extinction. Risk management Our processes for identifying and assessing climate-related risks The nature of the risks and opportunities from climate change depends not only on the physical aspects of climate change, but also regulatory and commercial changes in the markets in which we operate, including pressures to reduce the climate impact of our metered dose inhaler medicines. Our risk management policies are designed to address all types of risks, including the Group principal risks and uncertainties. Climate risk management follows the same policy and framework. Risks from climate change at Group level fall under the governance of the CRC with the support of the Sustainability Council. Individual risks from climate change are raised with appropriate business unit or functional Risk Management Control Boards to make sure we integrate these risks into business risk management processes. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Climate-related financial disclosures continued 60 (1) IEA Net Zero emissions scenario, https://www.iea.org/reports/global- energy-and-climate-model/net-zero-emissions-by-2050-scenario-nze last accessed 17 November 2022 (2) IEA World Energy Outlook 2021, Chapter 2, p94, download report from https://www.iea.org/reports/world-energy-outlook-2021/overview, last accessed 17 November 2022 (3) IEA Announced Pledges, https://www.iea.org/reports/global-energy- and-climate-model/announced-pledges-scenario-aps last accessed 17 November 2022

A specific and dedicated environmental sustainability risk management plan was put in place in 2020. The risk management plan covers expectations that we are addressing our impact on the environment, and that the environment has increasing impacts on operational resilience, such as access to energy, water and the natural resources used in products, along with any anticipated cost increases from regulatory changes or environmental taxes. We review developments in policy and regulations at global and national level, receiving quarterly monitoring reports. We have procedures to identify risks from climate change when factors evolve, for example to assess the climate impact of merger and acquisition activity, or the construction of new buildings. We use a shadow carbon price of $100 per tonne CO2e to inform decision-making on investments in major capital expenditure to understand the implications on potential carbon offset costs for the carbon emissions from our value chain in 2030. This value is based on the recommendation by the Carbon Pricing Leadership Coalition that concluded in 2017 that the explicit carbon price level required to drive change to restrict temperature increases to below 1.5°C is at least US$50–100/tCO2 by 20301. We monitor the value used for internal carbon pricing against estimates for the future costs of carbon credits. Our processes for managing climate-related risk For the purposes of this disclosure, we differentiate between 'physical' and 'transition' climate-related risks. Physical risks are typically identified at the asset or project level and are managed depending on the level of risk assessed. We use climate scenario analysis to model the potential impacts of our prioritised physical risks which helps us understand the resilience of our supply chains against climate change. Transition risks are typically risks associated with changes to regulations or societal expectations during the transition to a lower-carbon economy. They are identified at enterprise level and at market level. We manage transition risks through our investment decisions, our sustainability transformation programme and our procedures. For example, we manage risks which may arise from product claims based on environmental performance by using external accreditation processes and organisations to review the evidence used to support these claims. Our Communications and Government Affairs team manages corporate reputation by identifying and monitoring of climate-related issues and undertaking both proactive and reactive engagement with relevant stakeholder groups to communicate our position Details of how we manage our prioritised risks are in the Risk Table How our processes for identifying, assessing and managing climate related risks are integrated into overall risk management On an annual basis, a cross-functional team from Sustainability, Finance, Supply Chain and Procurement functions reviews climate risks. Climate-related risks are considered from a strategic and operational perspective to make sure we maintain a comprehensive view of the different types of climate risks we face and the different time horizons in which they may affect us. The team review previously identified climate risks, plus new or emerging risks and opportunities, and make recommendations in a paper to the Sustainability Council. Risk assessment papers are prepared for the prioritised risks, considering the likelihood and financial impact on us of each risk under different climate scenarios. Each risk and opportunity is analysed to understand how we are managing them, the metrics and targets being used and the potential impact on our total profit using a low (<£100 million), medium (£100 million – £250 million) or high (£250 million) threshold. The impact assessments are approved by the VP Sustainability and a Finance VP from our Global Supply Chain business unit. The results are shared with Business Unit Risk Management and Compliance Boards (RMCB) and the Finance RMCB to make sure risks are both contextualised with other business risks and managed appropriately. This allows management to take a holistic view and optimise risk mitigation responses, to ensure that responses to climate-related risks are properly integrated into the relevant business unit and function activities. The resilience of our strategy, considering different climate- related scenarios, including a 2°C or lower scenario We used the climate scenarios described above to stress test the resilience of the organisation by considering the impacts of potential physical and transition risks and opportunities on the locations where we operate as described in the table below. The modelling did not identify any material impact to our business resilience. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Climate-related financial disclosures continued 61 (1) Report of the High-Level Commission on Carbon Prices, Carbon Pricing Leadership Coalition, 2017, p10, https:// www.carbonpricingleadership.org/report-of-the-highlevel-commission- on-carbon-prices/
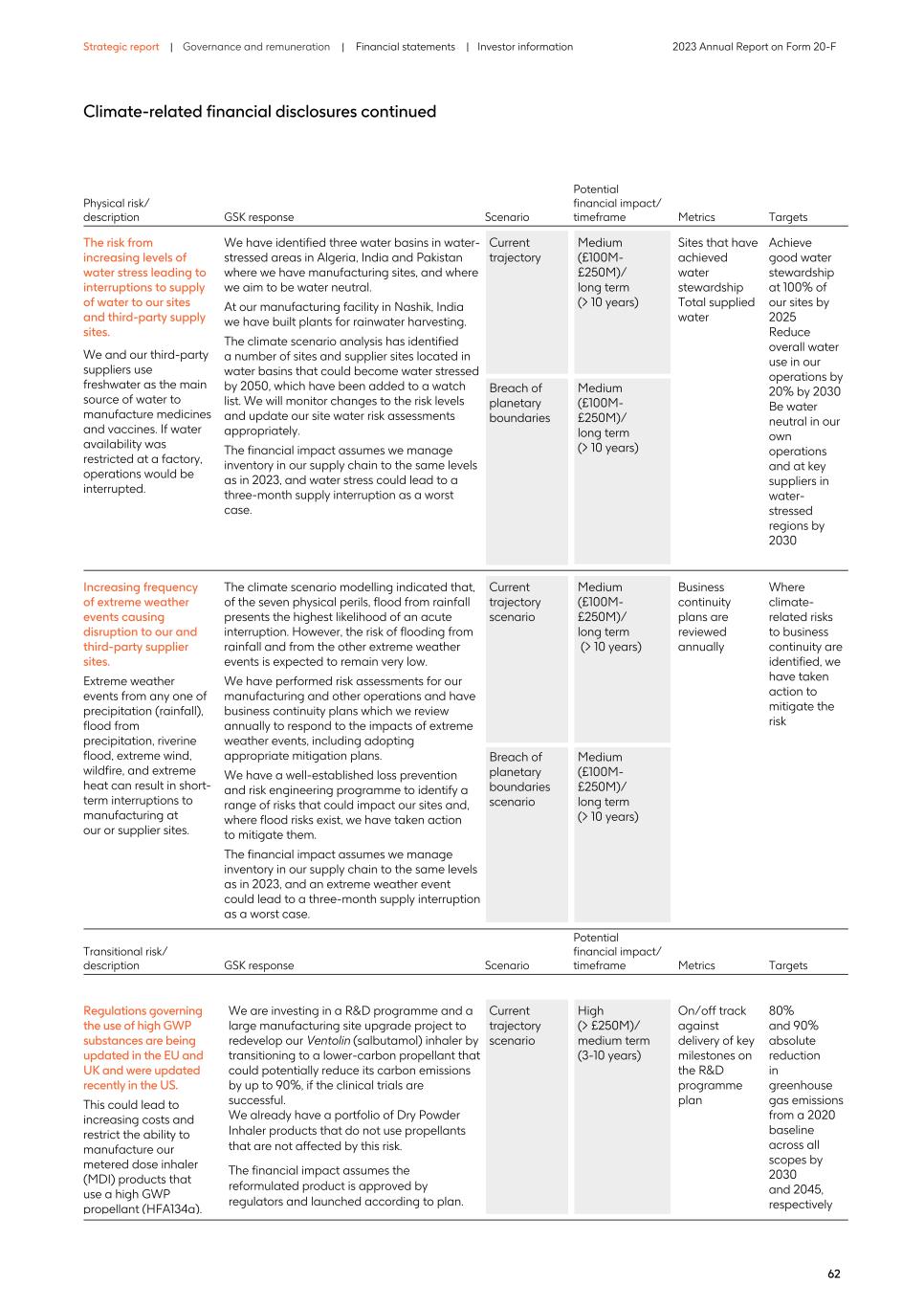
Physical risk/ description GSK response Scenario Potential financial impact/ timeframe Metrics Targets The risk from increasing levels of water stress leading to interruptions to supply of water to our sites and third-party supply sites. We and our third-party suppliers use freshwater as the main source of water to manufacture medicines and vaccines. If water availability was restricted at a factory, operations would be interrupted. We have identified three water basins in water- stressed areas in Algeria, India and Pakistan where we have manufacturing sites, and where we aim to be water neutral. At our manufacturing facility in Nashik, India we have built plants for rainwater harvesting. The climate scenario analysis has identified a number of sites and supplier sites located in water basins that could become water stressed by 2050, which have been added to a watch list. We will monitor changes to the risk levels and update our site water risk assessments appropriately. The financial impact assumes we manage inventory in our supply chain to the same levels as in 2023, and water stress could lead to a three-month supply interruption as a worst case. Current trajectory Medium (£100M- £250M)/ long term (> 10 years) Sites that have achieved water stewardship Total supplied water Achieve good water stewardship at 100% of our sites by 2025 Reduce overall water use in our operations by 20% by 2030 Be water neutral in our own operations and at key suppliers in water- stressed regions by 2030 Breach of planetary boundaries Medium (£100M- £250M)/ long term (> 10 years) Increasing frequency of extreme weather events causing disruption to our and third-party supplier sites. Extreme weather events from any one of precipitation (rainfall), flood from precipitation, riverine flood, extreme wind, wildfire, and extreme heat can result in short- term interruptions to manufacturing at our or supplier sites. The climate scenario modelling indicated that, of the seven physical perils, flood from rainfall presents the highest likelihood of an acute interruption. However, the risk of flooding from rainfall and from the other extreme weather events is expected to remain very low. We have performed risk assessments for our manufacturing and other operations and have business continuity plans which we review annually to respond to the impacts of extreme weather events, including adopting appropriate mitigation plans. We have a well-established loss prevention and risk engineering programme to identify a range of risks that could impact our sites and, where flood risks exist, we have taken action to mitigate them. The financial impact assumes we manage inventory in our supply chain to the same levels as in 2023, and an extreme weather event could lead to a three-month supply interruption as a worst case. Current trajectory scenario Medium (£100M- £250M)/ long term (> 10 years) Business continuity plans are reviewed annually Where climate- related risks to business continuity are identified, we have taken action to mitigate the risk Breach of planetary boundaries scenario Medium (£100M- £250M)/ long term (> 10 years) Transitional risk/ description GSK response Scenario Potential financial impact/ timeframe Metrics Targets Regulations governing the use of high GWP substances are being updated in the EU and UK and were updated recently in the US. This could lead to increasing costs and restrict the ability to manufacture our metered dose inhaler (MDI) products that use a high GWP propellant (HFA134a). We are investing in a R&D programme and a large manufacturing site upgrade project to redevelop our Ventolin (salbutamol) inhaler by transitioning to a lower-carbon propellant that could potentially reduce its carbon emissions by up to 90%, if the clinical trials are successful. We already have a portfolio of Dry Powder Inhaler products that do not use propellants that are not affected by this risk. The financial impact assumes the reformulated product is approved by regulators and launched according to plan. Current trajectory scenario High (> £250M)/ medium term (3-10 years) On/off track against delivery of key milestones on the R&D programme plan 80% and 90% absolute reduction in greenhouse gas emissions from a 2020 baseline across all scopes by 2030 and 2045, respectively Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Climate-related financial disclosures continued 62
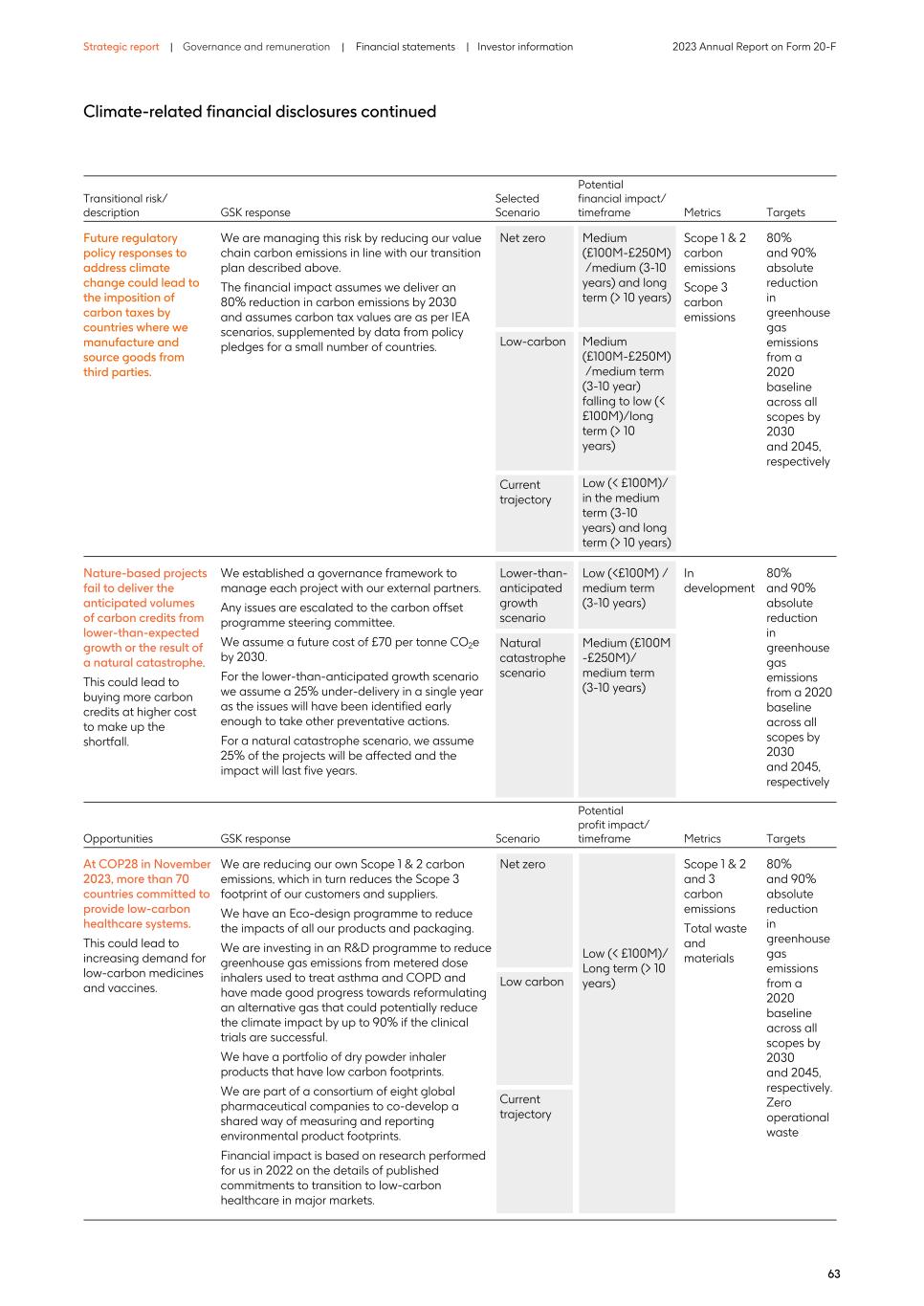
Transitional risk/ description GSK response Selected Scenario Potential financial impact/ timeframe Metrics Targets Future regulatory policy responses to address climate change could lead to the imposition of carbon taxes by countries where we manufacture and source goods from third parties. We are managing this risk by reducing our value chain carbon emissions in line with our transition plan described above. The financial impact assumes we deliver an 80% reduction in carbon emissions by 2030 and assumes carbon tax values are as per IEA scenarios, supplemented by data from policy pledges for a small number of countries. Net zero Medium (£100M-£250M) /medium (3-10 years) and long term (> 10 years) Scope 1 & 2 carbon emissions Scope 3 carbon emissions 80% and 90% absolute reduction in greenhouse gas emissions from a 2020 baseline across all scopes by 2030 and 2045, respectively Low-carbon Medium (£100M-£250M) /medium term (3-10 year) falling to low (< £100M)/long term (> 10 years) Current trajectory Low (< £100M)/ in the medium term (3-10 years) and long term (> 10 years) Nature-based projects fail to deliver the anticipated volumes of carbon credits from lower-than-expected growth or the result of a natural catastrophe. This could lead to buying more carbon credits at higher cost to make up the shortfall. We established a governance framework to manage each project with our external partners. Any issues are escalated to the carbon offset programme steering committee. We assume a future cost of £70 per tonne CO2e by 2030. For the lower-than-anticipated growth scenario we assume a 25% under-delivery in a single year as the issues will have been identified early enough to take other preventative actions. For a natural catastrophe scenario, we assume 25% of the projects will be affected and the impact will last five years. Lower-than- anticipated growth scenario Low (<£100M) / medium term (3-10 years) In development 80% and 90% absolute reduction in greenhouse gas emissions from a 2020 baseline across all scopes by 2030 and 2045, respectively Natural catastrophe scenario Medium (£100M -£250M)/ medium term (3-10 years) Opportunities GSK response Scenario Potential profit impact/ timeframe Metrics Targets At COP28 in November 2023, more than 70 countries committed to provide low-carbon healthcare systems. This could lead to increasing demand for low-carbon medicines and vaccines. We are reducing our own Scope 1 & 2 carbon emissions, which in turn reduces the Scope 3 footprint of our customers and suppliers. We have an Eco-design programme to reduce the impacts of all our products and packaging. We are investing in an R&D programme to reduce greenhouse gas emissions from metered dose inhalers used to treat asthma and COPD and have made good progress towards reformulating an alternative gas that could potentially reduce the climate impact by up to 90% if the clinical trials are successful. We have a portfolio of dry powder inhaler products that have low carbon footprints. We are part of a consortium of eight global pharmaceutical companies to co-develop a shared way of measuring and reporting environmental product footprints. Financial impact is based on research performed for us in 2022 on the details of published commitments to transition to low-carbon healthcare in major markets. Net zero Low (< £100M)/ Long term (> 10 years) Scope 1 & 2 and 3 carbon emissions Total waste and materials 80% and 90% absolute reduction in greenhouse gas emissions from a 2020 baseline across all scopes by 2030 and 2045, respectively. Zero operational waste Low carbon Current trajectory Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Climate-related financial disclosures continued 63

Metrics and targets The metrics we use to assess climate-related risks and opportunities in line with our strategy and risk management process. a. Disclose the metrics used by the organisation to assess climate risks and opportunities in line with its strategy and risk management process We have considered the key metrics following the TCFD guidance of Tables A1.1 and A1.2 as well as the metrics consistent with cross-industry, climate-related metrics. Based on that, our strategic metrics are: – Scope 1 & 2 emissions (market-based and location-based approach), described in the table below – Scope 3 emissions, described in the table below – % renewably sourced electricity, described in the table below – Total supplied water, described in the table below – Total waste and materials, described in the table below – ESG composite metric, as part of our senior leaders‘ remuneration policy – see page 137 – Sites that have achieved water stewardship, described in the table below Our ESG Performance Report includes more metrics used to support the strategic metrics listed above. b. Disclose Scope 1, 2 and if applicable Scope 3 GHG emissions and related risks In energy and carbon emissions, see table below: – Scope 1 emissions from energy – Scope 1 emissions from other sources – Scope 2 emissions (market-based) – Scope 2 emissions (location-based) – Scope 3 emissions metrics – Scope 1 & 2 emissions intensity metrics Prioritised physical and transition risks are included in the Risk Table above. c. Describe the targets used by the organisation to manage climate- related risks and opportunities and performance against targets Our targets (measured against a 2020 baseline where applicable) are: – 80% absolute reduction in greenhouse gas emissions from a 2020 baseline, across all scopes, and investment in nature-based solutions for the remaining 20% of our footprint by 2030 – Net zero greenhouse gas emissions across our full value chain by 2045: 90% absolute reduction in emissions from a 2020 baseline, across all scopes, and all residual emissions neutralised – 100% renewable electricity by 2025 (Scope 2) – Achieve good water stewardship at 100% of our sites by 2025 – Reduce overall water use in our operations by 20% in 2030 – Zero operational waste1, including eliminating single use plastics2 by 2030 – Be water neutral in our own operations and at key suppliers in water-stressed regions by 2030 The performance against our targets is on page 48 .3 (1) Including a 20% reduction in routine hazardous and non-hazardous waste (2) Where regulatory obligations allow, and excluding plastics which are critical to product discovery and development and health & safety (3) See Basis of Reporting 2023 in the ESG resources section of GSK.com (https://www.gsk.com/en-gb/responsibility/esg-resources/) for detailed methodologies for measuring and reporting all GSK environmental KPIs We commit to a net zero, nature positive, healthier planet, with ambitious goals set for 2030 and 2045 across our entire value chain. We report progress in reducing Scope 1 & 2 carbon emissions, Scope 3 carbon emissions, energy use, water, waste annually towards these targets on page 48, in our ESG Performance Report and in our public responses to the CDP Climate, Water and Forest questionnaires.	 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Climate-related financial disclosures continued 64
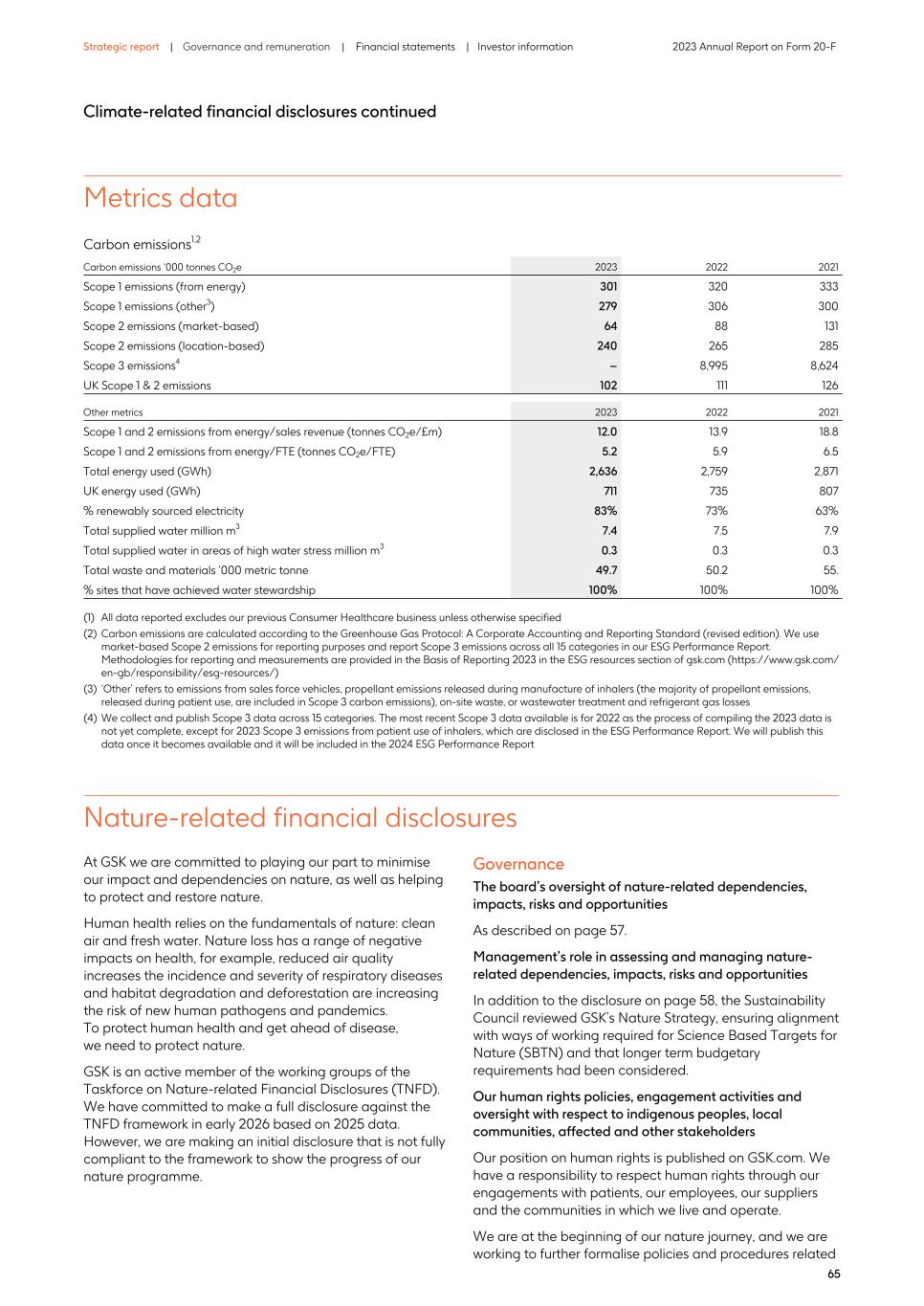
Metrics data Carbon emissions1,2 Carbon emissions ‘000 tonnes CO2e 2023 2022 2021 Scope 1 emissions (from energy) 301 320 333 Scope 1 emissions (other3) 279 306 300 Scope 2 emissions (market-based) 64 88 131 Scope 2 emissions (location-based) 240 265 285 Scope 3 emissions4 – 8,995 8,624 UK Scope 1 & 2 emissions 102 111 126 Other metrics 2023 2022 2021 Scope 1 and 2 emissions from energy/sales revenue (tonnes CO2e/£m) 12.0 13.9 18.8 Scope 1 and 2 emissions from energy/FTE (tonnes CO2e/FTE) 5.2 5.9 6.5 Total energy used (GWh) 2,636 2,759 2,871 UK energy used (GWh) 711 735 807 % renewably sourced electricity 83% 73% 63% Total supplied water million m3 7.4 7.5 7.9 Total supplied water in areas of high water stress million m3 0.3 0.3 0.3 Total waste and materials ‘000 metric tonne 49.7 50.2 55. % sites that have achieved water stewardship 100% 100% 100% (1) All data reported excludes our previous Consumer Healthcare business unless otherwise specified (2) Carbon emissions are calculated according to the Greenhouse Gas Protocol: A Corporate Accounting and Reporting Standard (revised edition). We use market-based Scope 2 emissions for reporting purposes and report Scope 3 emissions across all 15 categories in our ESG Performance Report. Methodologies for reporting and measurements are provided in the Basis of Reporting 2023 in the ESG resources section of gsk.com (https://www.gsk.com/ en-gb/responsibility/esg-resources/) (3) ‘Other’ refers to emissions from sales force vehicles, propellant emissions released during manufacture of inhalers (the majority of propellant emissions, released during patient use, are included in Scope 3 carbon emissions), on-site waste, or wastewater treatment and refrigerant gas losses (4) We collect and publish Scope 3 data across 15 categories. The most recent Scope 3 data available is for 2022 as the process of compiling the 2023 data is not yet complete, except for 2023 Scope 3 emissions from patient use of inhalers, which are disclosed in the ESG Performance Report. We will publish this data once it becomes available and it will be included in the 2024 ESG Performance Report Nature-related financial disclosures At GSK we are committed to playing our part to minimise our impact and dependencies on nature, as well as helping to protect and restore nature. Human health relies on the fundamentals of nature: clean air and fresh water. Nature loss has a range of negative impacts on health, for example, reduced air quality increases the incidence and severity of respiratory diseases and habitat degradation and deforestation are increasing the risk of new human pathogens and pandemics. To protect human health and get ahead of disease, we need to protect nature. GSK is an active member of the working groups of the Taskforce on Nature-related Financial Disclosures (TNFD). We have committed to make a full disclosure against the TNFD framework in early 2026 based on 2025 data. However, we are making an initial disclosure that is not fully compliant to the framework to show the progress of our nature programme. Governance The board’s oversight of nature-related dependencies, impacts, risks and opportunities As described on page 57. Management’s role in assessing and managing nature- related dependencies, impacts, risks and opportunities In addition to the disclosure on page 58, the Sustainability Council reviewed GSK’s Nature Strategy, ensuring alignment with ways of working required for Science Based Targets for Nature (SBTN) and that longer term budgetary requirements had been considered. Our human rights policies, engagement activities and oversight with respect to indigenous peoples, local communities, affected and other stakeholders Our position on human rights is published on GSK.com. We have a responsibility to respect human rights through our engagements with patients, our employees, our suppliers and the communities in which we live and operate. We are at the beginning of our nature journey, and we are working to further formalise policies and procedures related Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Climate-related financial disclosures continued 65

to stakeholders’ engagement and human rights specifically in relation to our assessment of impacts and our action on nature. – Protecting and restoring nature is a key part of our climate and nature strategy. As nature investments are always context dependant, it is key for us to work with expert partners and NGOs to ensure project implementation includes local experts and local communities – Before we make decisions on protection and restoration projects, we run a human rights assessment as part of our broader due diligence. The assessment allows us to understand the local context and history, the process that partners use or plan to use to engage and involve local communities (including Free, Prior and Informed Consent (FPIC) and grievance mechanisms) and the how benefits will be shared – The connection between nature projects and health benefits has not been consistently included in nature projects and we have worked with third-party experts to develop and publish a toolkit to enable project developers and investors to do that Strategy The nature-related dependencies, impacts, risks and opportunities we have identified over the short, medium and long term Impacts and dependencies Water Water is essential for the production of our vaccines and medicines. We have mapped our water footprint and calculated the volume of water we use in our value chain and in our own operations and have improved our understanding as to where in the world we have the biggest impact on water. Our primary operational impact on water availability is through our own manufacturing sites that are located in areas of water stress. Using water risk data from the World Resources Institute and the World Wildlife Fund, we have identified five sites located in water-stressed areas across Algeria, India and Pakistan, which face increasing water availability and quality risks. Releases of Active Pharmaceutical Ingredients are a priority focus for us regarding water quality.1 Pharmaceutical residues may sometimes pass into the environment as part of the normal biological process following patient use. To a lesser extent, pharmaceuticals can also enter the environment from unused medical products or factory discharges. There are concerns that long-term exposure to pharmaceuticals in the environment can pose a risk to environmental species, including aquatic life. The presence of antibiotics in the environment, and its potential impact on driving antibiotic resistance as well as reducing microbial biodiversity, is a growing concern for many stakeholders and an active area of research. While clinical and agricultural practices are generally recognised as the dominant sources of antibiotics entering the environment, unregulated manufacturing practices may also contribute to anti-microbial resistance2. Land Our primary dependency on land is due to the natural materials we source, some of which derive from agricultural commodities, a key driver of deforestation and land use change, globally. The supply chains for some of these commodities are often long and complex and may be many tiers removed from our direct engagement. Our operational land holdings are relatively small, although two of our R&D sites, one in Belgium and one in Spain, are located in Key Biodiversity Areas. Oceans Our impacts and dependencies on oceans come primarily from marine-derived materials that are a critical part of manufacturing vaccines and medicines. This includes, for example, horseshoe crab blood which is an important substance that is required by some regulators to be used in pharmaceutical and biomedical quality control processes to ensure the quality and safety of medicines, vaccines and devices. Atmosphere As a leader in medicines and vaccines for respiratory health, we want to play our part in improving air quality. We have done an initial assessment to establish an air pollution footprint in our operations and our supply chain. This showed that, directly, we are having a relatively low impact on air quality, and that the largest proportion of our emissions sits in our supply chain. Waste and Materials Our approach to product stewardship means that we consider and aim to address impacts on nature and climate at every stage of the product lifecycle, from discovery, design, sourcing and manufacturing through to product use and disposal. We have set a target to help accelerate the adoption of this approach. The effect nature-related dependencies, impacts, risks and opportunities have on our business model, value chain, strategy and financial planning, as well as any transition plans or analysis in place. We are committed to have a net positive impact on nature by 2030 by reducing our environmental impacts across water, waste and materials, biodiversity and by investing in nature protection and restoration. We set targets in 2020 with a focus on the realms of nature, as well as supportive targets on waste and materials. We report progress against our nature plan and targets annually. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Nature-related financial disclosures continued 66 (1) For more information see our public policy: https://www.gsk.com/ media/8867/gsk-position-on-pharmaceuticals-in-the-environment- march-2022.pdf (2) Read more about our position on antimicrobial resistance in our public policy

In 2023 we were selected to be one of the first group of companies to work with the Science Based Targets Network (SBTN) in the pilot to develop and set validated science-based targets for nature, starting with targets for freshwater and land, followed by targets for oceans and biodiversity. These targets will focus on locations across our value chain where nature is particularly under pressure. We aim to have pilot science-based targets for nature in 2024. The resilience of our strategy to nature-related risks and opportunities, taking into consideration different scenarios We manage organisational resilience to nature related risks through the implementation of our sustainability programme. Our delivery plan will evolve as external guidance continues to evolve, The locations of our direct operations that meet the criteria for priority locations Freshwater We have identified three initial water basins in water- stressed areas where we have manufacturing sites, including across India, Pakistan and Algeria, which we have prioritised for investment in water neutrality to achieve a measurable and positive impact in water-stressed basins on availability, quality and accessibility. Land Our operational land holdings are relatively small, although two of our R&D sites, one in Belgium and one in Spain, are located in Key Biodiversity Areas. In 2021, we piloted our approach to biodiversity with a baseline assessment and action plans at three sites to improving habitats, protecting species and improving soil and water quality. We have now commenced biodiversity uplift projects at our three largest R&D facilities – Stevenage in the UK and Upper Providence and Upper Merion in Pennsylvania in the US. We are addressing 12 critical agricultural, forestry and marine-derived materials. We have engaged with associated suppliers and external independent experts to map the full supply chains involved, understand existing sustainability standards, identify gaps and establish improvement plans. Oceans We committed to restore mangroves in Indonesia, through community-led projects. Mangroves play a crucial role in climate regulation and climate change mitigation because of their carbon sequestration potential. Mangroves make the local population more resilient to flooding, improve the local fish ecosystem, water quality and contribute to the health and livelihood of local communities. Risk & impact management Our processes for identifying, assessing and prioritising nature-related dependencies, impacts, risks and opportunities in our direct operations and value chain Since 2020 we have deepened our understanding of our full value chain nature impacts and dependencies and continued to align with evolving practices and guidance. We are following the TNFD LEAP (Locate, Evaluate, Assess and Prepare) methodology to better understand our nature-related risks and opportunities and are involved in the pilot working with the Science Based Targets Network (SBTN) to set validated science-based targets for nature, starting with targets for freshwater and land, followed by targets for oceans and biodiversity. Our processes for managing nature-related dependencies, impacts, risks and opportunities We set targets in 2020 with a focus on the realms of nature, as well as supportive targets on waste and materials. We report progress against our nature plan and targets annually. Water Across all of our sites, we maintain high quality water infrastructure to ensure there is no leakage, and we reduce our overall water use through water-efficiency projects, including behaviour change programmes and introducing water-efficient cleaning procedures. Today, all GSK sites complete a GSK water stewardship assessment, aligned to the Alliance for Water Stewardship (AWS) standard, and implement action plans to comply with our standard. For our sites located in water-stressed areas, we aim to secure certification under the AWS standard. Land While we work on avoiding or reducing impact by assessing opportunities to improve efficiency, material changes or switching to alternatives, we have set ambitious standards for suppliers who provide us with materials that are highly dependent on nature, such as sugar, paper, palm oil, lactose, gelatine and soy. These standards, developed in collaboration with third- party experts, aim to support these suppliers to assess, improve, and verify their approach to addressing a range of nature impacts – and associated climate and social impacts – including land use, water stewardship and biodiversity. As a first stage, we are addressing the 12 most critical materials, including paper and palm oil. We have roadmaps in place with an aim to achieve 100% sustainable sourced paper and palm oil by 2025. We have engaged with associated suppliers to map the full supply chains involved, understand existing sustainability standards, identify gaps and establish action plans. We are committed to having positive impact on biodiversity at all our operational sites. We used the Integrated Biodiversity Assessment Tool (IBAT) and have worked with ecological experts to complete mapping and baseline biodiversity assessments for 80% of our sites. We are now implementing biodiversity action plans across our estate with an aim to improve habitats, protect species and improve soil and water quality. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Nature-related financial disclosures continued 67

Oceans To reduce our impact on oceans, we are implementing our Marine Sustainable Sourcing Standard which outlines the specific requirements that our suppliers of marine-derived materials must adhere to. As part of our approach to product stewardship, we are working to reduce the volume of marine-derived materials, for example, through process efficiencies. In the longer term, we are seeking to transition to alternatives to marine- derived materials, wherever possible from both a technical and regulatory perspective. Atmosphere The outcome of an initial air quality assessment highlighted opportunities for reductions in emissions linked to on-site electricity generation and use of solid fuels, car use and move to electric fleet, as well as indicating opportunities in our value chain for the sourcing of plastic and glass products. We are creating reduction plans around these key areas that are aligned to our pathway to net zero and which aim to have a positive impact on air quality. We are conducting an additional air quality assessment, working with Stockholm Environment Institute (SEI) and the University of York, broadening the suite of air pollutants to be taken into consideration to understand their impact across our value chain and their connection to human health. To help accelerate collective action on air pollution, we are members of the Alliance for Clean Air through the Clean Air Fund (CAF) and the World Economic Forum, which aims to drive corporate action on clean air to accelerate climate action and create healthy communities around the world. The collective measurement of direct and value chain emissions across the Clean Air Fund membership aims to build a picture of the activities that give rise to poor air quality globally and intends to enable policy makers and industries to make informed decisions, considering the broader global impacts on health from poor air quality. Waste and materials Embedding our approach to product stewardship to reduce our impact on nature means working to minimise the waste and materials used, and the waste and pollution generated, from delivering our medicines and vaccines across the full product lifecycle. We have already achieved zero operational waste to landfill and we continue to build on our long-standing operational waste management programme to identify opportunities to achieve more beneficial use from waste. However, there is a risk that circular routes of recovery for all our waste streams may still not exist by 2030. For our supply chain, we’re working on a waste footprint assessment to help with supplier engagement on waste reduction, and on product design so we can build in circularity and reduce waste by design. How our processes for identifying, assessing, prioritising and monitoring nature-related risks are integrated into and inform our overall risk management processes We are a part of the first group of companies to be working with the Science Based Targets Network (SBTN) to set validated science-based targets for nature, starting with targets for freshwater and land, followed by targets for oceans and biodiversity. These targets will focus on locations across our value chain where nature is particularly under pressure. We aim to have science-based targets for nature in 2024. We continue to work towards our existing targets while we work through the SBTN pilot. Our delivery plan will continue to evolve as we go through SBTN target validation, as external guidance continues to evolve, and our data is developed, primarily through greater supply chain traceability. Metrics and targets We report performance against our existing targets using metrics for water use and waste and materials see table on page 65. Realm Key performance indicator Freshwater Average of the percentage of GSK sites and suppliers compliant with wastewater active pharmaceutical ingredient limits and the percentage of suppliers that are compliant with the AMR Industry Alliance Common Antibiotic Manufacturing Framework and discharge limits Land The percentage of paper and palm oil that is deforestation free Waste and materials The reduction in routine operational hazardous and non-hazardous waste Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Nature-related financial disclosures continued 68

GSK sets the following targets for managing our nature commitments: Focus area Target Freshwater – 100% of our sites to achieve good water stewardship by 2025 and reduce overall water use by 20% by 2030 – Water neutral in operations and with key suppliers in water-stressed regions by 2030 – Zero impact active pharmaceutical ingredient levels1 for all our sites and key suppliers by 20302 Land – Positive impact on biodiversity at all sites3 by 2030 – 100% of agricultural and forestry derived materials sustainably sourced and deforestation free by 20302,4 Oceans – 100% of marine-derived materials sustainably sourced by 2030 Atmosphere – 100% renewable electricity by 2025 (Scope 2)2 – 80% reduction in carbon emissions across our full value chain by 20302 – Net zero carbon emissions across our full value chain by 20452 Waste and materials – Zero operational waste5 10, including eliminating single use plastics6 by 20302 – 10% waste reduction from supply chain by 2030 – 25% environmental impact reduction for our products and packaging by 2030 (1) Below the predicted no-effect level (2) Linked with the remuneration of our senior leaders (3) GSK sites (4) Target updated in December 2021 to reflect priority materials (5) Including a 20% reduction in routine hazardous and non-hazardous waste (6) Where regulatory obligations allow, and excluding plastics which are critical to product discovery and development and health & safety Employees by gender Male Female Total Board1 7 5 12 Management1,2 8,682 8,788 17,470 All employees3 36,510 33,702 70,212 (1) Headcounts as of 31 December 2023 (2) Senior managers as defined in the Companies Act 2006 (Strategic Report and Directors’ Report) Regulations 2013 (3) ‘Total’ calculated as full-time equivalent employees (FTEs) as of 31 December 2023. ‘Male’ and ‘female’ calculated by applying ‘all employees’ gender diversity percentages to ‘total’ FTE number Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Nature-related financial disclosures continued 69

Group financial review In this section Financial performance summary 71 Reporting framework 72 Financial performance 76 Adjusting items 82 Cash generation and conversion 86 Financial position and resources 87 Approach to tax 92 Treasury policies 93 Capital allocation 94 Critical accounting policies 95 Liquidity and capital resources 97 2023 Annual Report on Form 20-F 70

Financial performance summary The Total results of the Group are set out below. 2023 2022 Growth £m % of turnover £m % of turnover £% CER% Turnover 30,328 100 29,324 100 3 5 Cost of sales (8,565) (28.2) (9,554) (32.6) (10) (10) Gross profit 21,763 71.8 19,770 67.4 10 13 Selling, general and administration (9,385) (30.9) (8,372) (28.6) 12 14 Research and development (6,223) (20.5) (5,488) (18.7) 13 14 Royalty income 953 3.1 758 2.6 26 26 Other operating income/(expense) (363) (1.3) (235) (0.8) Operating profit 6,745 22.2 6,433 21.9 5 10 Net finance costs (677) (803) Share of after tax profits/(losses) of associates and joint ventures (5) (2) Profit/(loss) on disposal of interest in associates and joint ventures 1 – Profit before taxation 6,064 5,628 8 14 Taxation (756) (707) Profit after taxation from continuing operations 5,308 4,921 8 14 Profit after taxation from discontinued operations and other gains/(losses) from the demerger – 3,049 Remeasurement of discontinued operations distributed to shareholders on demerger – 7,651 Profit after taxation from discontinued operations – 10,700 (100) (100) Total profit after taxation for the year 5,308 15,621 Profit attributable to non-controlling interests from continuing operations 380 460 Profit attributable to shareholders from continuing operations 4,928 4,461 Profit attributable to non-controlling interests from discontinued operations – 205 Profit attributable to shareholders from discontinued operations – 10,495 5,308 15,621 (66) (64) Total profit attributable to non-controlling interests 380 665 Total profit attributable to shareholders 4,928 14,956 5,308 15,621 (66) (64) Earnings per share from continuing operations (pence) 121.6p 110.8p 10 16 Earnings per share from discontinued operations (pence) – 260.6p (100) (100) Total earnings per share (pence) 121.6p 371.4p (67) (65) Earnings per ADS from continuing operations (US$) 3.02 2.75 Earnings per ADS from discontinued operations (US$) – 6.46 Total earnings per ADS (US$) 3.02 9.21 The Adjusted results for the Group are set out below. Reconciliations between Total results and Adjusted results for 2023 and 2022 are set out on pages 82 to 83. 2023 2022 Growth £m % of turnover £m % of turnover £% CER% Turnover 30,328 100 29,324 100 3 5 Cost of sales (7,716) (25.4) (8,741) (29.8) (12) (11) Selling, general and administration (9,029) (29.8) (8,128) (27.7) 11 13 Research and development (5,750) (19.0) (5,062) (17.3) 14 14 Royalty income 953 3.2 758 2.6 26 26 Adjusted operating profit 8,786 29.0 8,151 27.8 8 12 Adjusted profit attributable to non-controlling interest 572 595 Adjusted profit attributable to shareholders 6,283 5,625 Adjusted profit after taxation 6,855 6,220 10 15 Adjusted earnings per share (p) 155.1p 139.7p 11 16 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review 71

Reporting framework Total and Adjusted results The Group financial review discusses the operating and financial performance of the Group, its cash flows and financial position and our resources. The results for each year are compared primarily with the results of the preceding year. Total results Total reported results represent the Group’s overall performance. GSK also uses a number of adjusted, non-IFRS, measures to report the performance of its business. Adjusted results and other non-IFRS measures may be considered in addition to, but not as a substitute for or superior to, information presented in accordance with IFRS. Adjusted results are defined below and other non-IFRS measures are defined on page 73. GSK believes that Adjusted results, when considered together with Total results, provide investors, analysts and other stakeholders with helpful complementary information to understand better the financial performance and position of the Group from period to period, and allow the Group’s performance to be more easily compared against the majority of its peer companies. These measures are also used by management for planning and reporting purposes and when determining compensation. They may not be directly comparable with similarly described measures used by other companies. GSK encourages investors and analysts not to rely on any single financial measure but to review GSK’s Annual Reports, including the financial statements and notes, in their entirety. Adjusted results Adjusted results exclude the profits from discontinued operations from the Consumer Healthcare business (see details on page 216) and the following items in relation to our continuing operations from Total results, together with the tax effects of all of these items: – amortisation of intangible assets (excluding computer software and capitalised development costs) – impairment of intangible assets (excluding computer software) and goodwill – Major restructuring costs, which include impairments of tangible assets and computer software, (under specific Board approved programmes that are structural, of a significant scale and where the costs of individual or related projects exceed £25 million) including integration costs following material acquisitions – transaction-related accounting or other adjustments related to significant acquisitions – proceeds and costs of disposals of associates, products and businesses; significant settlement income; significant legal charges (net of insurance recoveries) and expenses on the settlement of litigation and government investigations; other operating income other than royalty income, and other items Costs for all other ordinary course smaller scale restructuring and legal charges and expenses are retained within both Total and Adjusted results. As Adjusted results include the benefits of Major restructuring programmes but exclude significant costs (such as amortisation of intangible assets except for computer software and capitalised development costs, significant legal, major restructuring and transaction items), they should not be regarded as a complete picture of the Group’s financial performance, which is presented in its Total results. The exclusion of other Adjusting items may result in Adjusted earnings being materially higher or lower than Total earnings. In particular, when significant impairments, restructuring charges and legal costs are excluded, Adjusted earnings will be higher than Total earnings. GSK has undertaken a number of Major restructuring programmes in response to significant changes in the Group’s trading environment or overall strategy or following material acquisitions. Within the Pharmaceuticals sector, the highly regulated manufacturing operations and supply chains and long lifecycle of the business mean that restructuring programmes, particularly those that involve the rationalisation or closure of manufacturing or R&D sites are likely to take several years to complete. Costs, both cash and non-cash, of these programmes are provided for as individual elements are approved and meet the accounting recognition criteria. As a result, charges may be incurred over a number of years following the initiation of a Major restructuring programme. Significant legal charges and expenses are those arising from the settlement of litigation or government investigations that are not in the normal course and are materially larger than more regularly occurring individual matters. They also include certain major legacy matters. Reconciliations between Total and Adjusted results, providing further information on the key Adjusting items for 2023, 2022 and 2021, are set out on pages 82 to 84. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 72
Historical record of Adjusting items The reconciliations between Total and Adjusted operating profit from continuing operations over the last three years can be summarised as follows: 2023 £m 2022 £m 2021 £m Total operating profit from continuing operations 6,745 6,433 4,357 Intangible amortisation 719 739 761 Intangible impairment 398 296 347 Major restructuring 382 321 424 Transaction-related items 572 1,750 1,143 Divestments, significant legal and other items (30) (1,388) (539) Adjusted results 8,786 8,151 6,493 The analysis of the impact of transaction-related items on operating profit for each of the last three years is as follows: 2023 £m 2022 £m 2021 £m Contingent consideration on former Shionogi-ViiV Healthcare JV (including Shionogi preferential dividends) 934 1,431 1,026 ViiV Healthcare put options and Pfizer preferential dividends (245) 85 48 Contingent consideration on former Novartis Vaccines business (187) 193 27 Contingent consideration on acquisition of Affinivax 44 17 – Other adjustments 26 24 42 Transaction-related items 572 1,750 1,143 Full reconciliations between Total and Adjusted results for 2021–2023 including continuing and discontinued operations are set out on pages 82 to 84. Further explanations on the Adjusting items for 2023 are reported on page 85. Other non-IFRS measures Free cash flow Free cash flow is defined as the net cash inflow/outflow from continuing operating activities less capital expenditure on property, plant and equipment and intangible assets, contingent consideration payments, net finance costs, and dividends paid to non-controlling interests, contributions from non-controlling interests plus proceeds from the sale of property, plant and equipment and intangible assets, and dividends received from joint ventures and associates (all attributable to continuing operations). It is used by management for planning and reporting purposes and in discussions with and presentations to investment analysts and rating agencies. Free cash flow growth is calculated on a reported basis. A reconciliation of net cash inflow from continuing operations to free cash flow from continuing operations is set out on page 86. Working capital Working capital represents inventory and trade receivables less trade payables. CER and AER growth In order to illustrate underlying performance, it is the Group’s practice to discuss its results in terms of constant exchange rate (CER) growth. This represents growth calculated as if the exchange rates used to determine the results of overseas companies in Sterling had remained unchanged from those used in the comparative period. CER% represents growth at constant exchange rates. £% or AER% represents growth at actual exchange rates. Return on capital employed Return on capital employed is calculated as total profit before taxation as a percentage of average net assets over the year. Total net debt Net debt is defined as total borrowings less cash, cash equivalents, liquid investments, and short-term loans to third parties that are subject to an insignificant risk of change in value. Please see Note 30 ‘Net Debt’ for the calculation of net debt. Total Operating Margin Total operating margin is operating profit divided by turnover. Adjusted Operating Margin Adjusted operating margin is Adjusted operating profit divided by turnover. Compound Annual Growth Rate (CAGR) CAGR is defined as the compound annual growth rate and shows the annualised average rate of revenue growth between a number of given years, assuming growth takes place at an exponentially compounded rate. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued Reporting framework continued 73
Non-controlling interests in ViiV Healthcare Trading profit allocations As ViiV Healthcare is a subsidiary of the Group, 100% of its operating results (turnover, operating profit, profit after tax) are included within the Group income statement and then a portion of the earnings is allocated to the non-controlling interests owned by the other shareholders, in line with their respective equity shareholdings (Pfizer, Inc. (Pfizer) 11.7% and Shionogi & Co. Ltd (Shionogi) 10%). Each of the shareholders, including GSK, is also entitled to preferential dividends determined by the performance of certain products that each shareholder contributed. As the relative performance of these products changes over time, the proportion of the overall earnings allocated to each shareholder also changes. In particular, the increasing proportion of sales of dolutegravir- and cabotegravir-containing products has a favourable impact on the proportion of the preferential dividends that is allocated to GSK. Adjusting items are allocated to shareholders based on their equity interests. GSK was entitled to approximately 84% of the Total earnings and 83% of the Adjusted earnings of ViiV Healthcare for 2023. Remeasurements of the liabilities for the preferential dividends allocated to Pfizer and Shionogi are included within other operating income/(expenses). Acquisition-related arrangements As consideration for the acquisition of Shionogi’s interest in the former Shionogi-ViiV Healthcare joint venture in 2012, Shionogi received the 10% equity stake in ViiV Healthcare and ViiV Healthcare also agreed to pay additional future cash consideration to Shionogi, contingent on the future sales performance of the products being developed by that joint venture, dolutegravir and cabotegravir. Under IFRS 3 `Business combinations’, GSK was required to provide for the estimated fair value of this contingent consideration at the time of acquisition and is required to update the liability to the latest estimate of fair value at each subsequent period end. The liability for the contingent consideration recognised in the balance sheet at the date of acquisition was £659 million. Subsequent re-measurements are reflected within other operating income/(expenses) and within Adjusting items in the income statement in each period. Cash payments to settle the contingent consideration are made to Shionogi by ViiV Healthcare each quarter, based on the actual sales performance and other income of the relevant products in the previous quarter. These payments reduce the balance sheet liability and hence are not recorded in the income statement, but are included in the cash flow. The cash payments made to Shionogi by ViiV Healthcare in 2023 were £1,106 million. As the liability is required to be recorded at the fair value of estimated future payments, there is a significant timing difference between the charges that are recorded in the Total income statement to reflect movements in the fair value of the liability and the actual cash payments made to settle the liability. The cash payments are reflected in the cash flow statement partly in operating cash flows and partly within investing activities. The tax relief on these payments is reflected in the Group’s Adjusting items as part of the tax charge. The part of each payment relating to the original estimate of the fair value of the contingent consideration on the acquisition of the Shionogi-ViiV Healthcare joint venture in 2012 of £659 million is reported within investing activities in the cash flow statement and the part of each payment relating to the increase in the liability since the acquisition is reported within operating cash flows. Movements in contingent consideration payable to Shionogi were as follows: 2023 £m 2022 £m Contingent consideration at beginning of the year 5,890 5,559 Remeasurement through income statement and other movements 934 1,431 Cash payments: operating cash flows (1,106) (1,031) Cash payments: investing activities – (69) Contingent consideration at end of the year 5,718 5,890 Of the contingent consideration payable (on a post-tax basis) to Shionogi at 31 December 2023, £1,017 million (31 December 2022: £940 million) is expected to be paid within one year. Exit rights Pfizer may request an IPO of ViiV Healthcare at any time and if either GSK does not consent to such IPO or an offering is not completed within nine months, Pfizer could require GSK to acquire its shareholding. Under the original agreements, GSK had the unconditional right, so long as it made no subsequent distribution to its shareholders, to withhold its consent to the exercise of the Pfizer put option and, as a result, in accordance with IFRS, GSK did not recognise a liability for the put option on its balance sheet. However, during Q1 2016, GSK notified Pfizer that it had irrevocably given up this right and accordingly recognised the liability for the put option on the Group’s balance sheet during Q1 2016 at an initial value of £1,070 million. Consistent with this revised treatment, at the end of Q1 2016 GSK also recognised liabilities for the future preferential dividends anticipated to become payable to Pfizer and Shionogi on the Group’s balance sheet. Pfizer has the right to require GSK to acquire its shareholding in ViiV Healthcare in certain circumstances at any time. A put option liability is therefore recorded on the Group’s balance sheet as a current liability. It is measured on the gross redemption basis derived from an internal valuation of the ViiV Healthcare business. The closing balances of the liabilities related to Pfizer’s shareholding are as follows: 2023 £m 2022 £m Pfizer put option 848 1,093 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued Reporting framework continued 74

Under the original agreements, Shionogi could also have requested GSK to acquire its shareholding in ViiV Healthcare in six-month windows commencing in 2017, 2020 and 2022. GSK had the unconditional right, so long as it made no subsequent distribution to its shareholders, to withhold its consent to the exercise of the Shionogi put option and, as a result, GSK did not recognise a liability for the put option on its balance sheet. However, during Q1 2016, GSK notified Shionogi that it had irrevocably given up this right and accordingly recognised the liability for the put option on the Group’s balance sheet during Q1 2016 at an initial value of £926 million. In Q4 2016, Shionogi irrevocably agreed to waive its put option and, as a result, GSK de-recognised the liability for this put option on the Group’s balance sheet directly to equity. The value of the liability was £1,244 million when it was de-recognised. GSK also has a call option over Shionogi’s shareholding in ViiV Healthcare, which under the original agreements was exercisable in six-month windows commencing in 2027, 2030 and 2032. GSK has now irrevocably agreed to waive the first two exercise windows, but the last six-month window in 2032 remains. As this call option is at fair value, it has no value for accounting purposes. Reporting definitions COVID-19 solutions COVID-19 solutions include the sales of pandemic adjuvant and other COVID-19 solutions including vaccine manufacturing and Xevudy and the associated costs but does not include reinvestment in R&D. This categorisation is used by management and we believe is helpful to investors through providing clarity on the results of the Group by showing the contribution to growth from COVID-19 solutions. Turnover excluding COVID-19 solutions Turnover excluding COVID-19 solutions excludes the impact of sales of pandemic adjuvant within Vaccines and Xevudy within Specialty Medicines related to the COVID-19 pandemic. Management believes that the exclusion of the impact of these COVID-19 solutions sales aids comparability in the reporting periods and understanding of GSK's growth including by region versus prior periods.. General Medicines General medicines are usually prescribed in the primary care or community settings by general healthcare practitioners. For GSK, this includes medicines in inhaled respiratory, dermatology, antibiotics and other diseases. Specialty Medicines Specialty Medicines are typically prescription medicines used to treat complex or rare chronic conditions. For GSK, this comprises medicines in infectious diseases, HIV, Oncology, Respiratory/Immunology and Other. Share Consolidation Following completion of the Consumer Healthcare business demerger on 18 July 2022, GSK plc Ordinary shares were consolidated to maintain share price comparability before and after demerger. Shareholders received 4 new Ordinary shares with a nominal value of 31¼ pence each for every 5 existing Ordinary shares which had a nominal value of 25 pence each. Earnings per share, diluted earnings per share, adjusted earnings per share and dividends per share were retrospectively adjusted to reflect the Share Consolidation in all the periods presented. Earnings per share Earnings per share has been retrospectively adjusted for the Share Consolidation on 18 July 2022, applying a ratio of 4 new Ordinary shares for every 5 existing Ordinary shares. Total Earnings per share Unless otherwise stated, Total earnings per share refers to Total basic earnings per share. RAR (Returns and Rebates) GSK sells to customers, both commercial and government mandated contracts, with reimbursement arrangements that include rebates, chargebacks and a right of return for certain pharmaceutical products principally in the US. Revenue recognition reflects gross-to-net sales adjustments as a result. These adjustments are known as the RAR accruals and are a source of significant estimation, uncertainty and fluctuation which can have a material impact on reported revenue from one accounting period to the next. Total Operating Margin Total Operating margin is Total operating profit divided by turnover. Adjusted Operating Margin Adjusted operating margin is Adjusted operating profit divided by turnover. Discontinued operations Consumer Healthcare was presented as a discontinued operation from Q2 2022. The demerger of Consumer Healthcare was completed on 18 July 2022. The Group Income Statement and Group Cash Flow Statement distinguish discontinued operations from continuing operations. Percentage points Percentage points of growth which is abbreviated to ppts. Non-controlling interest Non-controlling interest is the equity in a subsidiary not attributable, directly or indirectly, to a parent. Brand names and partner acknowledgements Brand names appearing in italics throughout this document are trademarks of GSK or associated companies or used under licence by the Group. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued Reporting framework continued 75
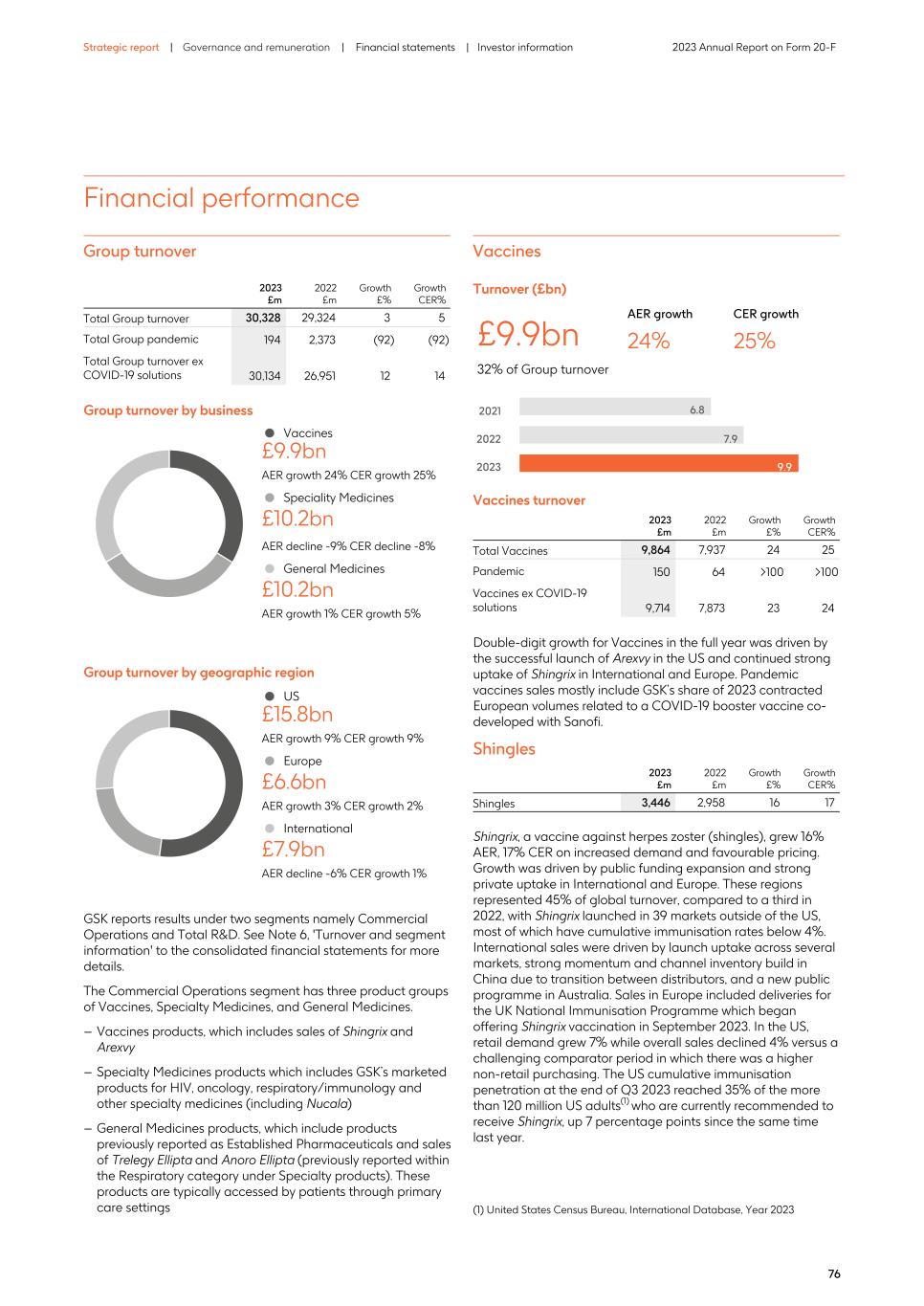
Financial performance Group turnover 2023 £m 2022 £m Growth £% Growth CER% Total Group turnover 30,328 29,324 3 5 Total Group pandemic 194 2,373 (92) (92) Total Group turnover ex COVID-19 solutions 30,134 26,951 12 14 Group turnover by business l Vaccines £9.9bn AER growth 24% CER growth 25% l Speciality Medicines £10.2bn AER decline -9% CER decline -8% l General Medicines £10.2bn AER growth 1% CER growth 5% Group turnover by geographic region l US £15.8bn AER growth 9% CER growth 9% l Europe £6.6bn AER growth 3% CER growth 2% l International £7.9bn AER decline -6% CER growth 1% GSK reports results under two segments namely Commercial Operations and Total R&D. See Note 6, 'Turnover and segment information' to the consolidated financial statements for more details. The Commercial Operations segment has three product groups of Vaccines, Specialty Medicines, and General Medicines. – Vaccines products, which includes sales of Shingrix and Arexvy – Specialty Medicines products which includes GSK’s marketed products for HIV, oncology, respiratory/immunology and other specialty medicines (including Nucala) – General Medicines products, which include products previously reported as Established Pharmaceuticals and sales of Trelegy Ellipta and Anoro Ellipta (previously reported within the Respiratory category under Specialty products). These products are typically accessed by patients through primary care settings Vaccines Turnover (£bn) £9.9bn AER growth CER growth 24% 25% 32% of Group turnover 6.8 7.9 9.9 2021 2022 2023 Vaccines turnover 2023 £m 2022 £m Growth £% Growth CER% Total Vaccines 9,864 7,937 24 25 Pandemic 150 64 >100 >100 Vaccines ex COVID-19 solutions 9,714 7,873 23 24 Double-digit growth for Vaccines in the full year was driven by the successful launch of Arexvy in the US and continued strong uptake of Shingrix in International and Europe. Pandemic vaccines sales mostly include GSK’s share of 2023 contracted European volumes related to a COVID-19 booster vaccine co- developed with Sanofi. Shingles 2023 £m 2022 £m Growth £% Growth CER% Shingles 3,446 2,958 16 17 Shingrix, a vaccine against herpes zoster (shingles), grew 16% AER, 17% CER on increased demand and favourable pricing. Growth was driven by public funding expansion and strong private uptake in International and Europe. These regions represented 45% of global turnover, compared to a third in 2022, with Shingrix launched in 39 markets outside of the US, most of which have cumulative immunisation rates below 4%. International sales were driven by launch uptake across several markets, strong momentum and channel inventory build in China due to transition between distributors, and a new public programme in Australia. Sales in Europe included deliveries for the UK National Immunisation Programme which began offering Shingrix vaccination in September 2023. In the US, retail demand grew 7% while overall sales declined 4% versus a challenging comparator period in which there was a higher non-retail purchasing. The US cumulative immunisation penetration at the end of Q3 2023 reached 35% of the more than 120 million US adults(1) who are currently recommended to receive Shingrix, up 7 percentage points since the same time last year. (1) United States Census Bureau, International Database, Year 2023 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F 76
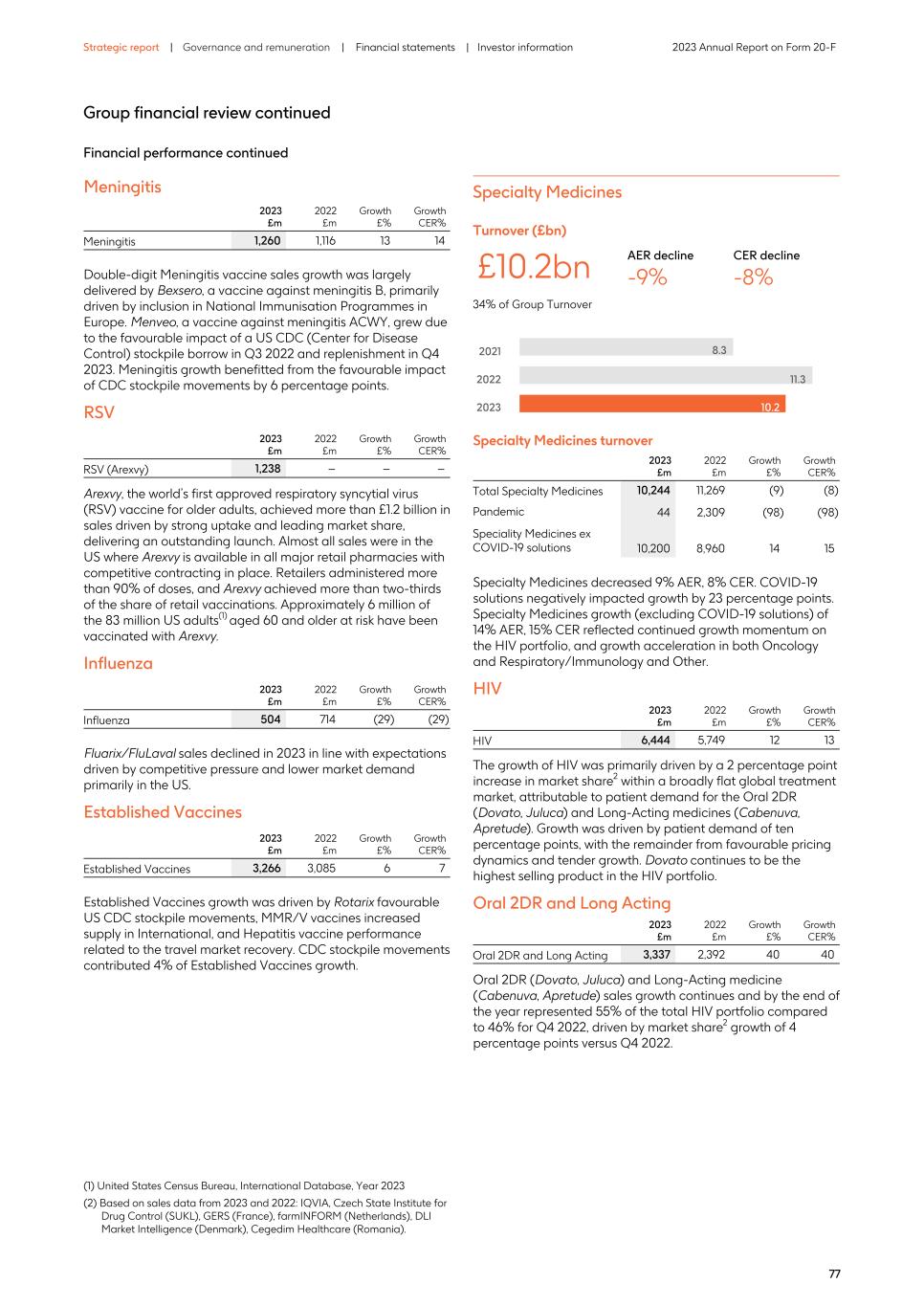
Meningitis 2023 £m 2022 £m Growth £% Growth CER% Meningitis 1,260 1,116 13 14 Double-digit Meningitis vaccine sales growth was largely delivered by Bexsero, a vaccine against meningitis B, primarily driven by inclusion in National Immunisation Programmes in Europe. Menveo, a vaccine against meningitis ACWY, grew due to the favourable impact of a US CDC (Center for Disease Control) stockpile borrow in Q3 2022 and replenishment in Q4 2023. Meningitis growth benefitted from the favourable impact of CDC stockpile movements by 6 percentage points. RSV 2023 £m 2022 £m Growth £% Growth CER% RSV (Arexvy) 1,238 – – – Arexvy, the world’s first approved respiratory syncytial virus (RSV) vaccine for older adults, achieved more than £1.2 billion in sales driven by strong uptake and leading market share, delivering an outstanding launch. Almost all sales were in the US where Arexvy is available in all major retail pharmacies with competitive contracting in place. Retailers administered more than 90% of doses, and Arexvy achieved more than two-thirds of the share of retail vaccinations. Approximately 6 million of the 83 million US adults(1) aged 60 and older at risk have been vaccinated with Arexvy. Influenza 2023 £m 2022 £m Growth £% Growth CER% Influenza 504 714 (29) (29) Fluarix/FluLaval sales declined in 2023 in line with expectations driven by competitive pressure and lower market demand primarily in the US. Established Vaccines 2023 £m 2022 £m Growth £% Growth CER% Established Vaccines 3,266 3,085 6 7 Established Vaccines growth was driven by Rotarix favourable US CDC stockpile movements, MMR/V vaccines increased supply in International, and Hepatitis vaccine performance related to the travel market recovery. CDC stockpile movements contributed 4% of Established Vaccines growth. (1) United States Census Bureau, International Database, Year 2023 (2) Based on sales data from 2023 and 2022: IQVIA, Czech State Institute for Drug Control (SUKL), GERS (France), farmINFORM (Netherlands), DLI Market Intelligence (Denmark), Cegedim Healthcare (Romania). Specialty Medicines Turnover (£bn) £10.2bn AER decline CER decline -9% -8% 34% of Group Turnover 8.3 11.3 10.2 2021 2022 2023 Specialty Medicines turnover 2023 £m 2022 £m Growth £% Growth CER% Total Specialty Medicines 10,244 11,269 (9) (8) Pandemic 44 2,309 (98) (98) Speciality Medicines ex COVID-19 solutions 10,200 8,960 14 15 Specialty Medicines decreased 9% AER, 8% CER. COVID-19 solutions negatively impacted growth by 23 percentage points. Specialty Medicines growth (excluding COVID-19 solutions) of 14% AER, 15% CER reflected continued growth momentum on the HIV portfolio, and growth acceleration in both Oncology and Respiratory/Immunology and Other. HIV 2023 £m 2022 £m Growth £% Growth CER% HIV 6,444 5,749 12 13 The growth of HIV was primarily driven by a 2 percentage point increase in market share2 within a broadly flat global treatment market, attributable to patient demand for the Oral 2DR (Dovato, Juluca) and Long-Acting medicines (Cabenuva, Apretude). Growth was driven by patient demand of ten percentage points, with the remainder from favourable pricing dynamics and tender growth. Dovato continues to be the highest selling product in the HIV portfolio. Oral 2DR and Long Acting 2023 £m 2022 £m Growth £% Growth CER% Oral 2DR and Long Acting 3,337 2,392 40 40 Oral 2DR (Dovato, Juluca) and Long-Acting medicine (Cabenuva, Apretude) sales growth continues and by the end of the year represented 55% of the total HIV portfolio compared to 46% for Q4 2022, driven by market share2 growth of 4 percentage points versus Q4 2022. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued Financial performance continued 77
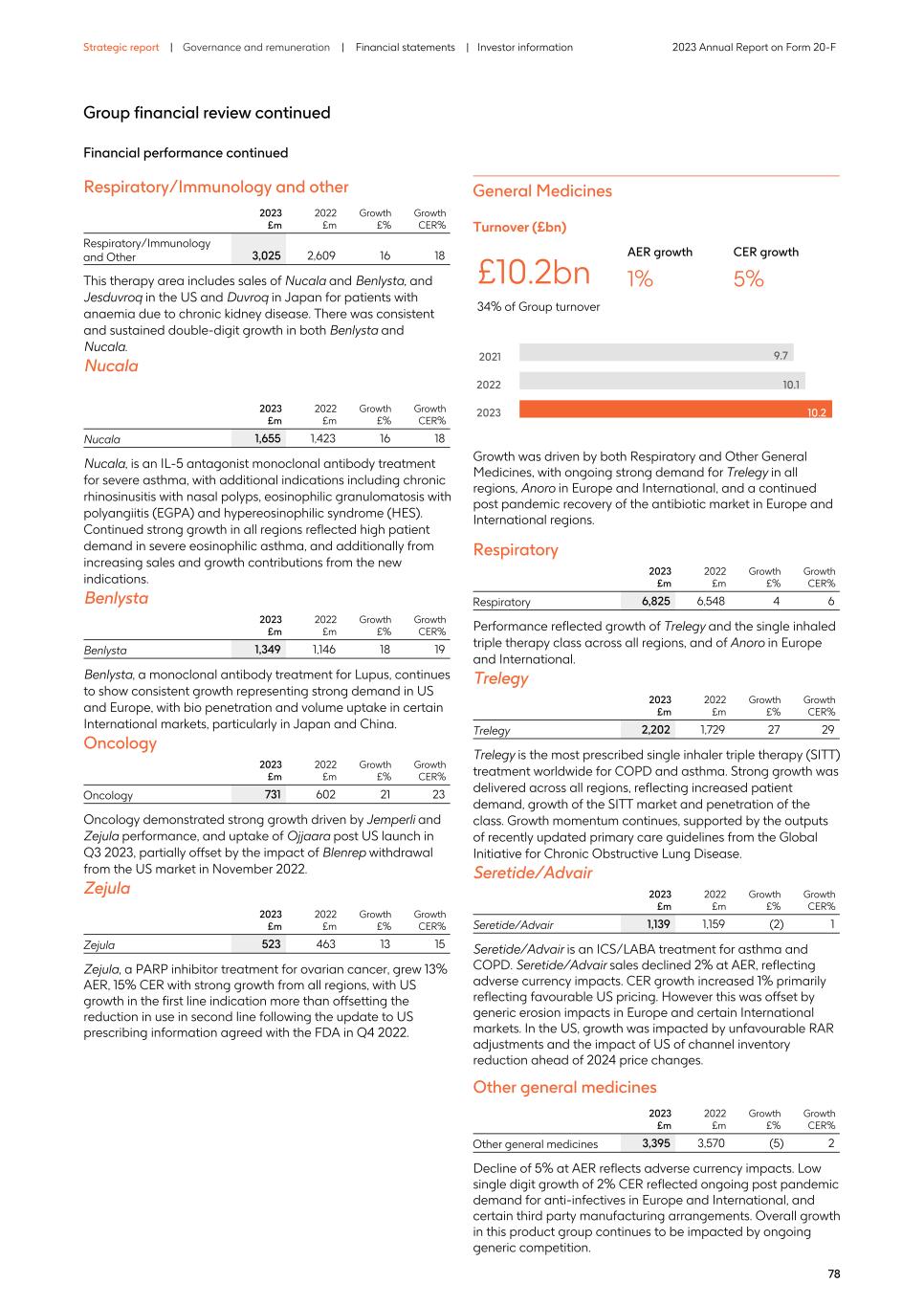
Respiratory/Immunology and other 2023 £m 2022 £m Growth £% Growth CER% Respiratory/Immunology and Other 3,025 2,609 16 18 This therapy area includes sales of Nucala and Benlysta, and Jesduvroq in the US and Duvroq in Japan for patients with anaemia due to chronic kidney disease. There was consistent and sustained double-digit growth in both Benlysta and Nucala. Nucala 2023 £m 2022 £m Growth £% Growth CER% Nucala 1,655 1,423 16 18 Nucala, is an IL-5 antagonist monoclonal antibody treatment for severe asthma, with additional indications including chronic rhinosinusitis with nasal polyps, eosinophilic granulomatosis with polyangiitis (EGPA) and hypereosinophilic syndrome (HES). Continued strong growth in all regions reflected high patient demand in severe eosinophilic asthma, and additionally from increasing sales and growth contributions from the new indications. Benlysta 2023 £m 2022 £m Growth £% Growth CER% Benlysta 1,349 1,146 18 19 Benlysta, a monoclonal antibody treatment for Lupus, continues to show consistent growth representing strong demand in US and Europe, with bio penetration and volume uptake in certain International markets, particularly in Japan and China. Oncology 2023 £m 2022 £m Growth £% Growth CER% Oncology 731 602 21 23 Oncology demonstrated strong growth driven by Jemperli and Zejula performance, and uptake of Ojjaara post US launch in Q3 2023, partially offset by the impact of Blenrep withdrawal from the US market in November 2022. Zejula 2023 £m 2022 £m Growth £% Growth CER% Zejula 523 463 13 15 Zejula, a PARP inhibitor treatment for ovarian cancer, grew 13% AER, 15% CER with strong growth from all regions, with US growth in the first line indication more than offsetting the reduction in use in second line following the update to US prescribing information agreed with the FDA in Q4 2022. General Medicines Turnover (£bn) £10.2bn AER growth CER growth 1% 5% 34% of Group turnover 9.7 10.1 10.2 2021 2022 2023 Growth was driven by both Respiratory and Other General Medicines, with ongoing strong demand for Trelegy in all regions, Anoro in Europe and International, and a continued post pandemic recovery of the antibiotic market in Europe and International regions. Respiratory 2023 £m 2022 £m Growth £% Growth CER% Respiratory 6,825 6,548 4 6 Performance reflected growth of Trelegy and the single inhaled triple therapy class across all regions, and of Anoro in Europe and International. Trelegy 2023 £m 2022 £m Growth £% Growth CER% Trelegy 2,202 1,729 27 29 Trelegy is the most prescribed single inhaler triple therapy (SITT) treatment worldwide for COPD and asthma. Strong growth was delivered across all regions, reflecting increased patient demand, growth of the SITT market and penetration of the class. Growth momentum continues, supported by the outputs of recently updated primary care guidelines from the Global Initiative for Chronic Obstructive Lung Disease. Seretide/Advair 2023 £m 2022 £m Growth £% Growth CER% Seretide/Advair 1,139 1,159 (2) 1 Seretide/Advair is an ICS/LABA treatment for asthma and COPD. Seretide/Advair sales declined 2% at AER, reflecting adverse currency impacts. CER growth increased 1% primarily reflecting favourable US pricing. However this was offset by generic erosion impacts in Europe and certain International markets. In the US, growth was impacted by unfavourable RAR adjustments and the impact of US of channel inventory reduction ahead of 2024 price changes. Other general medicines 2023 £m 2022 £m Growth £% Growth CER% Other general medicines 3,395 3,570 (5) 2 Decline of 5% at AER reflects adverse currency impacts. Low single digit growth of 2% CER reflected ongoing post pandemic demand for anti-infectives in Europe and International, and certain third party manufacturing arrangements. Overall growth in this product group continues to be impacted by ongoing generic competition. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued Financial performance continued 78
Turnover by regions US 2023 £m 2022 £m Growth £% Growth CER% Total 15,820 14,542 9 9 Pandemic 10 828 (99) (99) Ex COVID-19 solutions 15,810 13,714 15 16 Sales growth was adversely impacted by 6 percentage points AER, 7 percentage points CER due to decreased sales of Xevudy. Vaccines grew strongly driven by Arexvy launch uptake and leading market share, partly offset by competition and lower market demand for Influenza vaccines. Growth benefitted from favourable US CDC stockpile movements by 4 percentage points. Specialty Medicines grew driven by a strong HIV performance, Benlysta and Nucala continued growth, and strong Oncology growth despite partial offset from the impact of the withdrawal of Blenrep in November 2022. General Medicines growth was largely driven by Trelegy from increased patient demand and growth of the SITT market, partially offset by Established Respiratory and Other General Medicines. Europe 2023 £m 2022 £m Growth £% Growth CER% Total 6,564 6,348 3 2 Pandemic sales 133 513 (74) (75) Ex COVID-19 solutions 6,431 5,835 10 8 COVID-19 solutions impacted growth by 7 percentage points AER, 6 percentage points CER. Excluding the impact of COVID-19 solutions, Europe delivered strong growth of 10% AER, 8% CER. Vaccines growth reflected Shingrix national immunisation programme initiation in the UK and launch uptake across several markets, together with Bexsero national immunisation campaigns in France and Spain, and ongoing travel vaccine recovery. Specialty Medicines double digit growth was driven by growth in HIV, Oncology, Benlysta and Nucala including the impact of new indication launches. General Medicines low single digit growth was maintained. International 2023 £m 2022 £m Growth £% Growth CER% Total 7,944 8,434 (6) 1 Pandemic sales 51 1,032 (95) (95) Ex COVID-19 solutions 7,893 7,402 7 15 COVID-19 solutions impacted growth by 13 percentage points AER, 14 percentage points CER. Excluding the impact of COVID-19 solutions, International continued to grow by 7% AER, 15% CER, with strong growth across all product groups. Vaccines double digit growth was driven by Shingrix launch uptake across several markets, strong momentum and channel inventory build in China, and a new public programme in Australia. Established and Meningitis vaccines also contributed to the growth. Specialty Medicines grew in HIV, Nucala, Benlysta and Zejula. General Medicines growth was driven by Trelegy and growth across Established Respiratory. Other General Medicines growth was driven by Augmentin on strong post pandemic antibiotic demand. Cost of sales 2023 £m 2022 £m Growth £% Growth CER% Total cost of sales (8,565) (9,554) (10) (10) % of sales 28.2% 32.6% (4.3) (4.6) Adjusted cost of sales (7,716) (8,741) (12) (11) % of sales 25.4% 29.8% (4.4) (4.6) Total and Adjusted cost of sales as a percentage of sales decreased primarily reflecting lower sales of lower margin Xevudy compared to 2022. Excluding Xevudy, the year benefitted from an increasing margin contribution from Vaccines sales, particularly the launch of Arexvy in Q3 2023 in the US and Shingrix outside the US. In addition, Specialty Medicines, particularly HIV, contributed to the improved margin, as well as continued operational efficiencies. This was partly offset by adverse inventory provision adjustments in the year as well as inflationary impact on input costs. Selling, general and administration 2023 £m 2022 £m Growth £% Growth CER% Total selling, general and administration (9,385) (8,372) 12 14 % of sales 30.9% 28.6% 2.4 2.3 Adjusted selling, general and administration (9,029) (8,128) 11 13 % of sales 29.8% 27.7% 2.1 1.9 Growth in Total and Adjusted SG&A in 2023 primarily reflected increased investment for growth in Vaccines, including disease awareness, launch and global market expansion for Arexvy, and investment behind global market expansion and disease awareness for Shingrix. In Specialty Medicines, increased investment was targeted behind long-acting injectables in HIV and the launch of Ojjaara for myelofibrosis in Oncology. This was partly offset by the continuing benefit of restructuring and tight control of ongoing costs. 2023 also reflected the Zejula royalty dispute in Q1 2023. Total SG&A also included an increase in significant legal costs (see details on page 85). Research and development 2023 £m 2022 £m Growth £% Growth CER% Total research and development (6,223) (5,488) 13 14 % of sales 20.5% 18.7% 1.8 1.5 Adjusted research and development (5,750) (5,062) 14 14 % of sales 19.0% 17.3% 1.7 1.4 R&D operating expense growth in 2023 was driven by investment across the portfolio. In the late stage, increased investment in Vaccines was driven by continued acceleration and progression of the pipeline including RSV, pneumococcal, mRNA and therapeutic HSV vaccines. Respiratory/Immunology investment continued in depemokimab in the Phase III programmes in asthma and nasal polyps together with camlipixant a new asset for refractory chronic cough, Nucala in COPD, paediatric Benlysta and CCL 17 in osteo arthritic pain. This was offset by decreased expense in the completion of the clinical programme for otilimab. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued Financial performance continued 79
Infectious Diseases investment in bepirovirsen for treatment of chronic hepatitis B increased to support both monotherapy and combination programmes. Investment in key assets in oncology continued such as Jemperli and Ojjaara but were offset by reduction in the terminated Cell and Gene Therapy programme. In the early-stages, investment increased in IL18 for atopic dermatitis, and in the HIV portfolio, focused on next generation long-acting treatments and preventative medicines. Total R&D included higher impairment charges compared with 2022. Royalty income 2023 £m 2022 £m Growth £% Growth CER% Total royalty income 953 758 26 26 Adjusted royalty income 953 758 26 26 Growth in Total and Adjusted royalty income primarily related to Gardasil royalties, which were £472 million in 2023, as well as Kesimpta and Biktarvy royalties. The overwhelming majority of the income from Gardasil royalties ceased at the end of 2023. Other operating income/(expense) 2023 £m 2022 £m Growth £% Growth CER% Other operating income/ (expenses) (363) (235) (54) (54) Other operating expenses reflected a charge of £546 million (2022: £1,726 million) arising from the remeasurement of contingent consideration liabilities and the liabilities for the Pfizer put option, and a fair value loss of £17 million (2022: £229 million gain) on the retained stake in Haleon plc, partly offset by £200 million (2022: £306 million) of other net income primarily related to equity investments and milestone income (including £49 million dividends received from the retained investment in Haleon plc). In Q1 2022 upfront income of £0.9 billion was received from the settlement with Gilead Sciences Inc. Operating profit 2023 £m 2022 £m Growth £% Growth CER% Total operating profit 6,745 6,433 5 10 % of sales 22.2% 21.9% 0.3 1.0 Adjusted operating profit 8,786 8,151 8 12 % of sales 29.0% 27.8% 1.2 1.8 Total operating profit margin was higher in 2023 due to profitable growth across the portfolio as well as favourable movements in contingent consideration liabilities, partly offset by an unfavourable comparison due to the £0.9 billion upfront income received from the settlement with Gilead Sciences Inc. in Q1 2022. Adjusted operating profit benefitted from strong sales, favourable product mix and increased royalty income partly offset by increased investment behind product launches and in R&D. It also included increased legal charges primarily relating to the Zejula royalty dispute. In 2023 the adverse impact of lower sales of COVID-19 solutions was 5 percentage points of Total operating profit growth at AER (6 percentage points at CER), with an impact on Total operating profit margin of 0.5 percentage points. In 2023 the adverse impact of lower sales of COVID-19 solutions was 4 percentage points of Adjusted operating profit growth, with an impact on Adjusted operating profit margin of 0.4 percentage points. Adjusted operating profit by business 2023 £m 2022 £m Growth £% Growth CER% Commercial operations 14,656 13,590 8 10 % of sales 48.3% 46.3% 2 2 R&D (5,607) (5,060) (11) (11) Commercial Operations Adjusted operating profit benefitted from strong sales and favourable product mix (with minimal Xevudy sales) and increased royalty income, partly offset by increased investment in growth and launch assets as well as an increase in legal provisions in 2023. The R&D segment operating expenses growth was driven by progression of the late stage in Vaccines, Respiratory/ Immunology and Infectious Diseases. This included pneumococcal and mRNA programmes together with the newly acquired camlipixant and ongoing investment in key programmes such as depemokimab and bepirovirsen. Net finance costs 2023 £m 2022 £m Growth £% Growth CER% Total net finance cost 677 803 (16) (15) Adjusted finance cost 669 791 (15) (15) Total net finance costs were £677 million compared with £803 million in 2022. Adjusted net finance costs were £669 million compared with £791 million in 2022. The decrease was mainly driven by the net savings from maturing bonds including the Sterling Notes repurchase in Q4 2022 and higher interest income on cash, partly offset by higher interest on short-term financing. Share of after tax profits of associates and joint ventures The share of after tax loss of associates and joint ventures was £5 million (2022: £2 million share of loss) Profit on disposal of interest in associates In 2023, the Group also reported a profit on disposal of interests in associates and joint ventures of £1 million. Profit before tax Taking account of net finance costs, the share of profits or losses of associates and profit or loss on disposal of interest in associates, profit before taxation was £6,064 million compared with £5,628 million in 2022 .Taxation 2023 £m 2022 £m UK current year charge 207 200 Rest of world current year charge 1,371 1,351 Charge/(credit) in respect of prior periods 43 (60) Total current taxation 1,621 1,491 Total deferred taxation (865) (784) Taxation on total profits 756 707 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued Financial performance continued 80
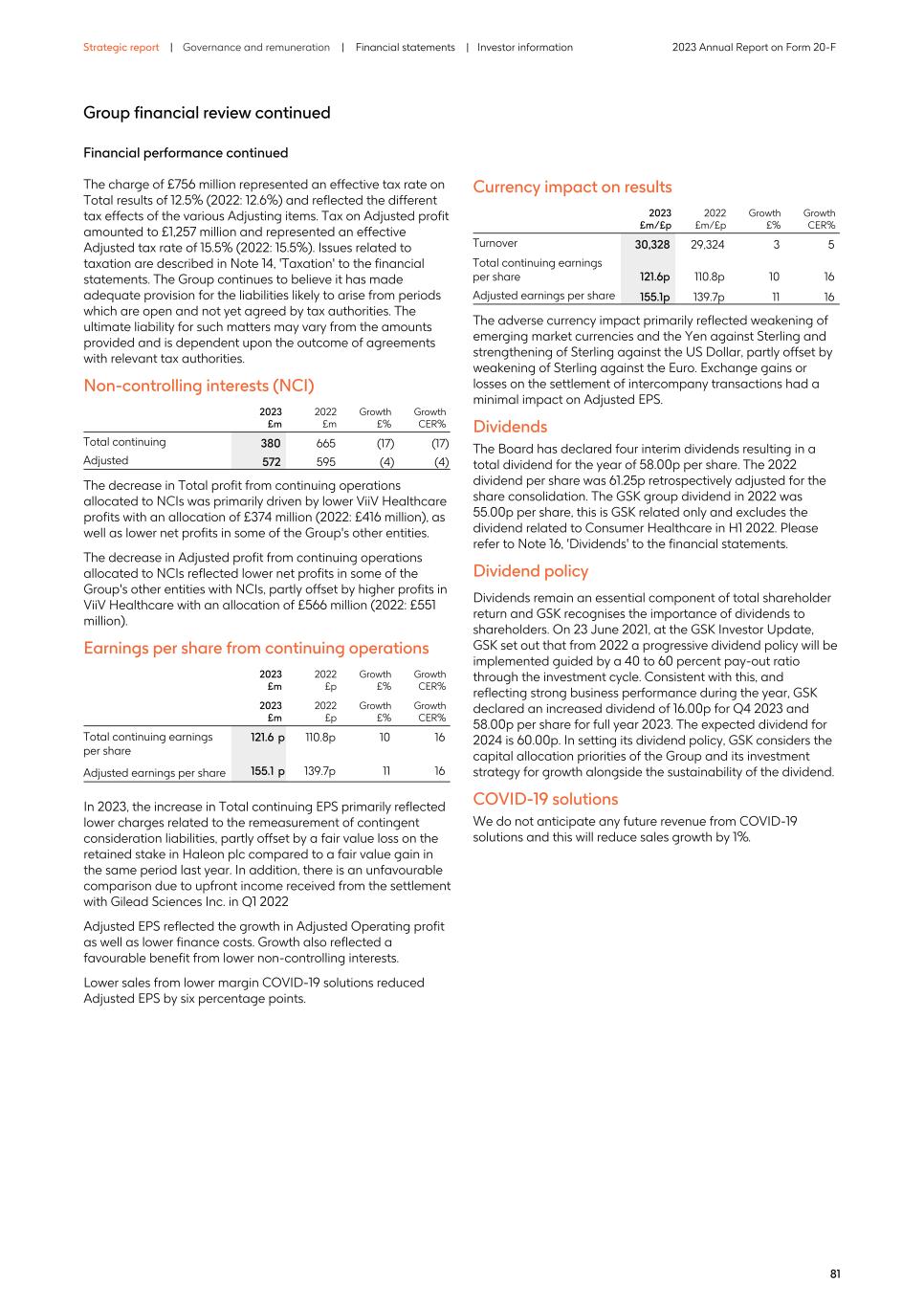
The charge of £756 million represented an effective tax rate on Total results of 12.5% (2022: 12.6%) and reflected the different tax effects of the various Adjusting items. Tax on Adjusted profit amounted to £1,257 million and represented an effective Adjusted tax rate of 15.5% (2022: 15.5%). Issues related to taxation are described in Note 14, 'Taxation' to the financial statements. The Group continues to believe it has made adequate provision for the liabilities likely to arise from periods which are open and not yet agreed by tax authorities. The ultimate liability for such matters may vary from the amounts provided and is dependent upon the outcome of agreements with relevant tax authorities. Non-controlling interests (NCI) 2023 £m 2022 £m Growth £% Growth CER% Total continuing 380 665 (17) (17) Adjusted 572 595 (4) (4) The decrease in Total profit from continuing operations allocated to NCIs was primarily driven by lower ViiV Healthcare profits with an allocation of £374 million (2022: £416 million), as well as lower net profits in some of the Group's other entities. The decrease in Adjusted profit from continuing operations allocated to NCIs reflected lower net profits in some of the Group's other entities with NCIs, partly offset by higher profits in ViiV Healthcare with an allocation of £566 million (2022: £551 million). Earnings per share from continuing operations 2023 £m 2022 £p Growth £% Growth CER% 2023 £m 2022 £p Growth £% Growth CER% Total continuing earnings per share 121.6 p 110.8p 10 16 Adjusted earnings per share 155.1 p 139.7p 11 16 In 2023, the increase in Total continuing EPS primarily reflected lower charges related to the remeasurement of contingent consideration liabilities, partly offset by a fair value loss on the retained stake in Haleon plc compared to a fair value gain in the same period last year. In addition, there is an unfavourable comparison due to upfront income received from the settlement with Gilead Sciences Inc. in Q1 2022 Adjusted EPS reflected the growth in Adjusted Operating profit as well as lower finance costs. Growth also reflected a favourable benefit from lower non-controlling interests. Lower sales from lower margin COVID-19 solutions reduced Adjusted EPS by six percentage points. Currency impact on results 2023 £m/£p 2022 £m/£p Growth £% Growth CER% Turnover 30,328 29,324 3 5 Total continuing earnings per share 121.6 p 110.8p 10 16 Adjusted earnings per share 155.1 p 139.7p 11 16 The adverse currency impact primarily reflected weakening of emerging market currencies and the Yen against Sterling and strengthening of Sterling against the US Dollar, partly offset by weakening of Sterling against the Euro. Exchange gains or losses on the settlement of intercompany transactions had a minimal impact on Adjusted EPS. Dividends The Board has declared four interim dividends resulting in a total dividend for the year of 58.00p per share. The 2022 dividend per share was 61.25p retrospectively adjusted for the share consolidation. The GSK group dividend in 2022 was 55.00p per share, this is GSK related only and excludes the dividend related to Consumer Healthcare in H1 2022. Please refer to Note 16, 'Dividends' to the financial statements. Dividend policy Dividends remain an essential component of total shareholder return and GSK recognises the importance of dividends to shareholders. On 23 June 2021, at the GSK Investor Update, GSK set out that from 2022 a progressive dividend policy will be implemented guided by a 40 to 60 percent pay-out ratio through the investment cycle. Consistent with this, and reflecting strong business performance during the year, GSK declared an increased dividend of 16.00p for Q4 2023 and 58.00p per share for full year 2023. The expected dividend for 2024 is 60.00p. In setting its dividend policy, GSK considers the capital allocation priorities of the Group and its investment strategy for growth alongside the sustainability of the dividend. COVID-19 solutions We do not anticipate any future revenue from COVID-19 solutions and this will reduce sales growth by 1%. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued Financial performance continued 81
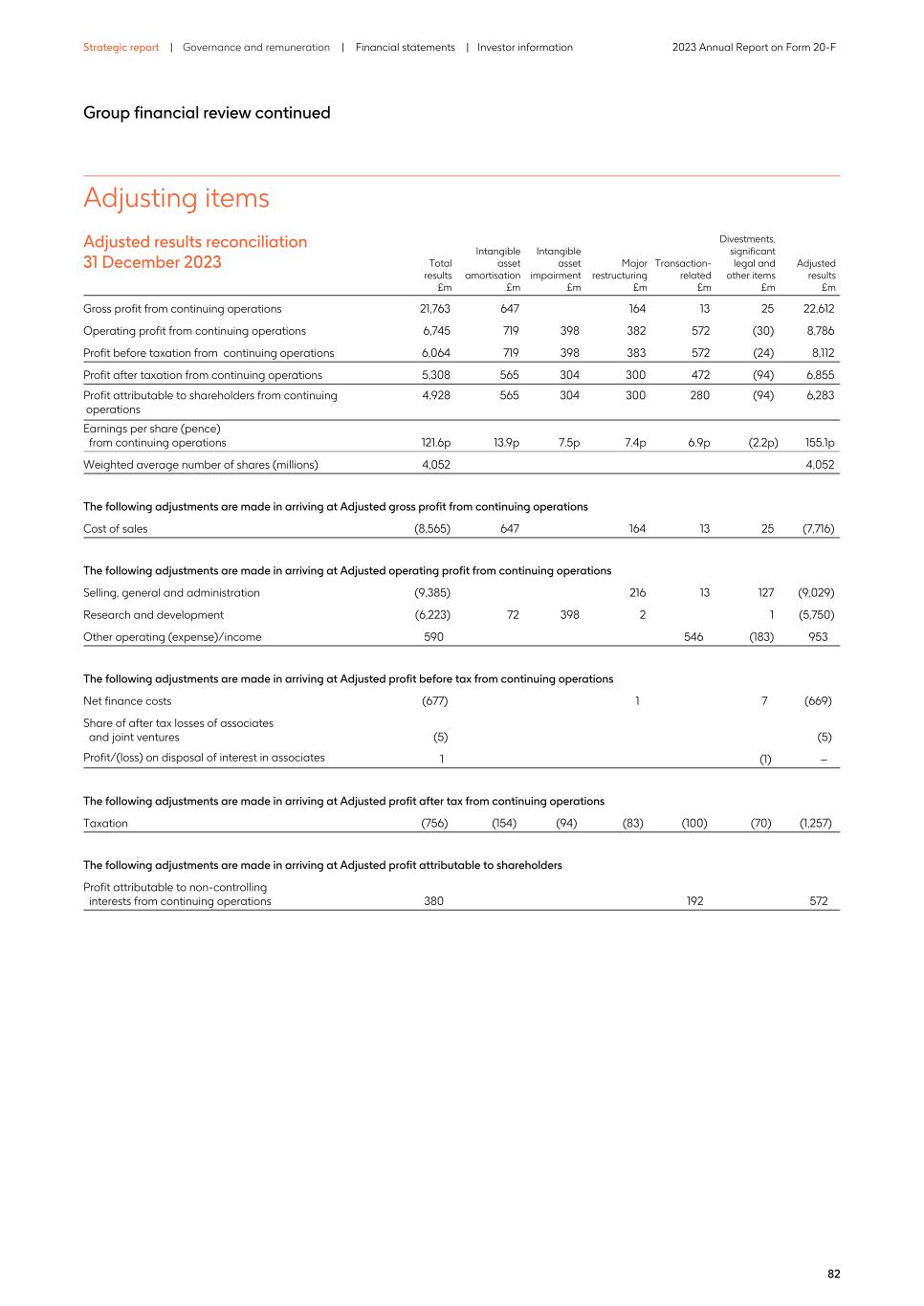
Adjusting items Adjusted results reconciliation 31 December 2023 Total results £m Intangible asset amortisation £m Intangible asset impairment £m Major restructuring £m Transaction- related £m Divestments, significant legal and other items £m Adjusted results £m Gross profit from continuing operations 21,763 647 164 13 25 22,612 Operating profit from continuing operations 6,745 719 398 382 572 (30) 8,786 Profit before taxation from continuing operations 6,064 719 398 383 572 (24) 8,112 Profit after taxation from continuing operations 5,308 565 304 300 472 (94) 6,855 Profit attributable to shareholders from continuing operations 4,928 565 304 300 280 (94) 6,283 Earnings per share (pence) from continuing operations 121.6p 13.9p 7.5p 7.4p 6.9p (2.2p) 155.1p Weighted average number of shares (millions) 4,052 4,052 The following adjustments are made in arriving at Adjusted gross profit from continuing operations Cost of sales (8,565) 647 164 13 25 (7,716) The following adjustments are made in arriving at Adjusted operating profit from continuing operations Selling, general and administration (9,385) 216 13 127 (9,029) Research and development (6,223) 72 398 2 1 (5,750) Other operating (expense)/income 590 546 (183) 953 The following adjustments are made in arriving at Adjusted profit before tax from continuing operations Net finance costs (677) 1 7 (669) Share of after tax losses of associates and joint ventures (5) (5) Profit/(loss) on disposal of interest in associates 1 (1) – The following adjustments are made in arriving at Adjusted profit after tax from continuing operations Taxation (756) (154) (94) (83) (100) (70) (1,257) The following adjustments are made in arriving at Adjusted profit attributable to shareholders Profit attributable to non-controlling interests from continuing operations 380 192 572 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 82
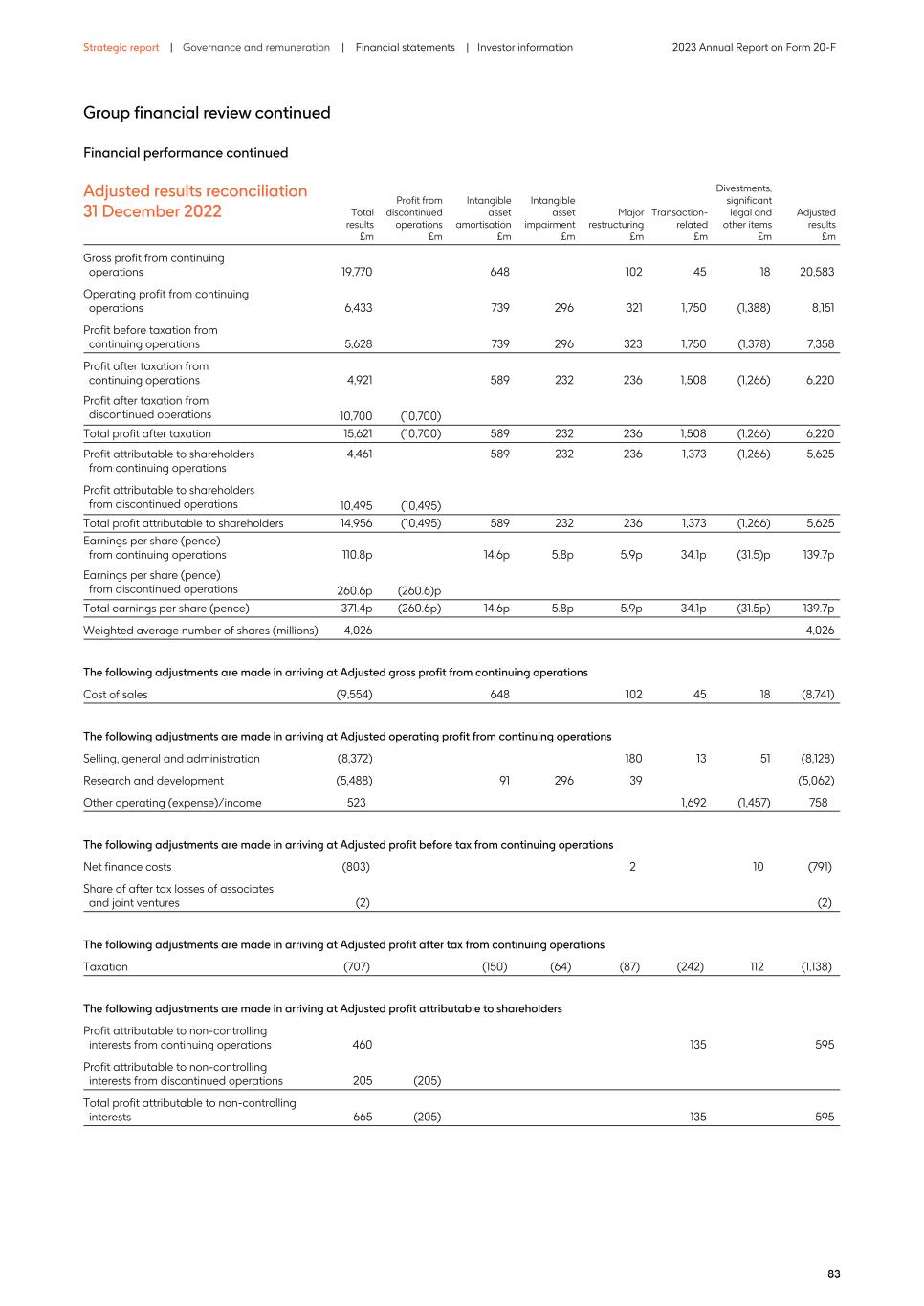
Adjusted results reconciliation 31 December 2022 Total results £m Profit from discontinued operations £m Intangible asset amortisation £m Intangible asset impairment £m Major restructuring £m Transaction- related £m Divestments, significant legal and other items £m Adjusted results £m Gross profit from continuing operations 19,770 648 102 45 18 20,583 Operating profit from continuing operations 6,433 739 296 321 1,750 (1,388) 8,151 Profit before taxation from continuing operations 5,628 739 296 323 1,750 (1,378) 7,358 Profit after taxation from continuing operations 4,921 589 232 236 1,508 (1,266) 6,220 Profit after taxation from discontinued operations 10,700 (10,700) Total profit after taxation 15,621 (10,700) 589 232 236 1,508 (1,266) 6,220 Profit attributable to shareholders from continuing operations 4,461 589 232 236 1,373 (1,266) 5,625 Profit attributable to shareholders from discontinued operations 10,495 (10,495) Total profit attributable to shareholders 14,956 (10,495) 589 232 236 1,373 (1,266) 5,625 Earnings per share (pence) from continuing operations 110.8 p 14.6 p 5.8 p 5.9 p 34.1 p (31.5) p 139.7 p Earnings per share (pence) from discontinued operations 260.6 p (260.6) p Total earnings per share (pence) 371.4p (260.6p) 14.6p 5.8p 5.9p 34.1p (31.5p) 139.7p Weighted average number of shares (millions) 4,026 4,026 The following adjustments are made in arriving at Adjusted gross profit from continuing operations Cost of sales (9,554) 648 102 45 18 (8,741) The following adjustments are made in arriving at Adjusted operating profit from continuing operations Selling, general and administration (8,372) 180 13 51 (8,128) Research and development (5,488) 91 296 39 (5,062) Other operating (expense)/income 523 1,692 (1,457) 758 The following adjustments are made in arriving at Adjusted profit before tax from continuing operations Net finance costs (803) 2 10 (791) Share of after tax losses of associates and joint ventures (2) (2) The following adjustments are made in arriving at Adjusted profit after tax from continuing operations Taxation (707) (150) (64) (87) (242) 112 (1,138) The following adjustments are made in arriving at Adjusted profit attributable to shareholders Profit attributable to non-controlling interests from continuing operations 460 135 595 Profit attributable to non-controlling interests from discontinued operations 205 (205) Total profit attributable to non-controlling interests 665 (205) 135 595 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued Financial performance continued 83
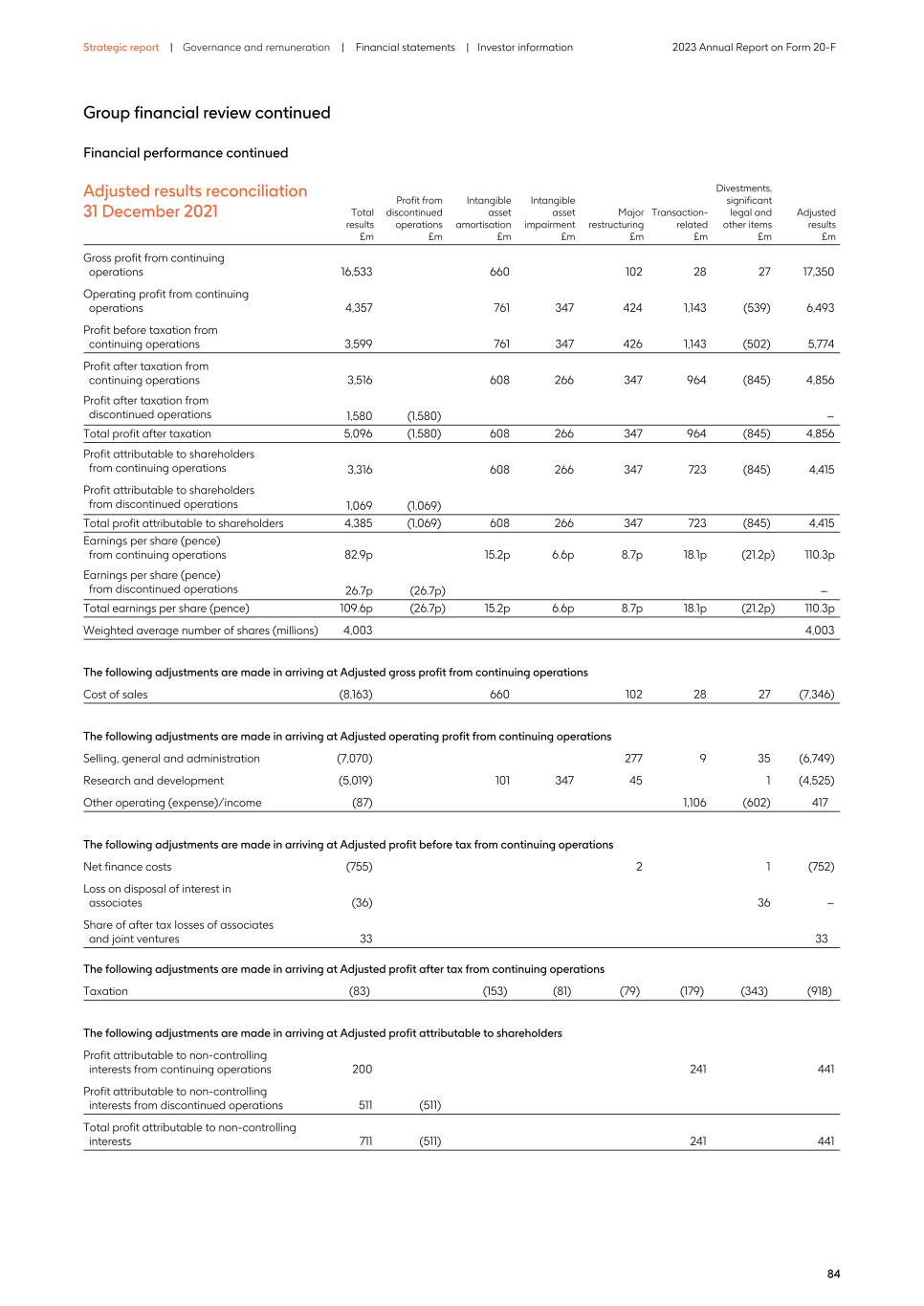
Adjusted results reconciliation 31 December 2021 Total results £m Profit from discontinued operations £m Intangible asset amortisation £m Intangible asset impairment £m Major restructuring £m Transaction- related £m Divestments, significant legal and other items £m Adjusted results £m Gross profit from continuing operations 16,533 660 102 28 27 17,350 Operating profit from continuing operations 4,357 761 347 424 1,143 (539) 6,493 Profit before taxation from continuing operations 3,599 761 347 426 1,143 (502) 5,774 Profit after taxation from continuing operations 3,516 608 266 347 964 (845) 4,856 Profit after taxation from discontinued operations 1,580 (1,580) – Total profit after taxation 5,096 (1,580) 608 266 347 964 (845) 4,856 Profit attributable to shareholders from continuing operations 3,316 608 266 347 723 (845) 4,415 Profit attributable to shareholders from discontinued operations 1,069 (1,069) Total profit attributable to shareholders 4,385 (1,069) 608 266 347 723 (845) 4,415 Earnings per share (pence) from continuing operations 82.9p 15.2p 6.6p 8.7p 18.1p (21.2p) 110.3p Earnings per share (pence) from discontinued operations 26.7p (26.7p) – Total earnings per share (pence) 109.6p (26.7p) 15.2p 6.6p 8.7p 18.1p (21.2p) 110.3p Weighted average number of shares (millions) 4,003 4,003 The following adjustments are made in arriving at Adjusted gross profit from continuing operations Cost of sales (8,163) 660 102 28 27 (7,346) The following adjustments are made in arriving at Adjusted operating profit from continuing operations Selling, general and administration (7,070) 277 9 35 (6,749) Research and development (5,019) 101 347 45 1 (4,525) Other operating (expense)/income (87) 1,106 (602) 417 The following adjustments are made in arriving at Adjusted profit before tax from continuing operations Net finance costs (755) 2 1 (752) Loss on disposal of interest in associates (36) 36 – Share of after tax losses of associates and joint ventures 33 33 The following adjustments are made in arriving at Adjusted profit after tax from continuing operations Taxation (83) (153) (81) (79) (179) (343) (918) The following adjustments are made in arriving at Adjusted profit attributable to shareholders Profit attributable to non-controlling interests from continuing operations 200 241 441 Profit attributable to non-controlling interests from discontinued operations 511 (511) Total profit attributable to non-controlling interests 711 (511) 241 441 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued Financial performance continued 84
Intangible asset amortisation See page 188 for description and information on Intangible asset amortisation. Intangible asset impairment See page 188 for description and information on Intangible asset impairment. No individual intangible asset accounted for a material impairment. Major restructuring and integration Within the Pharmaceuticals sector, the highly regulated manufacturing operations and supply chains and long life cycle of the business mean that restructuring programmes, particularly those that involve the rationalisation or closure of manufacturing or R&D sites are likely to take several years to complete. Major restructuring costs are those related to specific Board- approved Major restructuring programmes and are excluded from Adjusted results. Major restructuring programmes, including integration costs following material acquisitions, are those that are structural and are of a significant scale where the costs of individual or related projects exceed £25 million. Other ordinary course smaller-scale restructuring costs are retained within Total and Adjusted results. Total Major restructuring charges incurred in 2023 were £382 million (2022: £321 million), analysed as follows: 2023 2022 Cash £m Non- cash £m Total £m Cash £m Non- cash £m Total £m Separation preparation restructuring programme 199 117 316 177 110 287 Significant acquisitions 65 1 66 20 – 20 Legacy programmes (1) 1 – 9 5 14 263 119 382 206 115 321 The Separation Preparation programme incurred cash charges of £199 million primarily from the restructuring of some commercial and administrative functions as well as Global Supply Chain. The non-cash charges of £117 million primarily reflected the write-down of assets in administrative and manufacturing locations. The benefit in the year 2023 from restructuring programmes was £0.2 billion, primarily relating to the Separation Preparation restructuring programme. The programme is now largely complete and has delivered its target of £1.1 billion of annual savings, with total costs still expected at £2.4 billion, with slightly higher cash charges of £1.7 billion but lower non-cash charges of £0.7 billion. Costs of significant acquisitions relate to integration costs of Sierra Oncology Inc (Sierra) and Affinivax Inc. (Affinivax) which were acquired in Q3 2022 and BELLUS Health Inc. acquired in Q2 2023. Transaction-related adjustments Transaction-related adjustments from continuing operations resulted in a net charge of £572 million (2022: £1,750 million), the majority of which related to charges/(credits) for the remeasurement of contingent consideration liabilities, the liabilities for the Pfizer put option, and Pfizer and Shionogi preferential dividends in ViiV Healthcare. Charge/(credit) 2023 £m 2022 £m Contingent consideration on former Shionogi-ViiV Healthcare Joint Venture (including Shionogi preferential dividends) 934 1,431 ViiV Healthcare put options and Pfizer preferential dividends (245) 85 Contingent consideration on former Novartis Vaccines business (187) 193 Contingent consideration on acquisition of Affinivax 44 17 Other adjustments 26 24 Total transaction-related charges 572 1,750 The £934 million charge relating to the contingent consideration for the former Shionogi-ViiV Healthcare joint venture represented an increase in the valuation of the contingent consideration due to Shionogi, driven by £534 million from updated future sales forecasts and exchange rates, and the unwind of the discount for £400 million. The £245 million credit relating to the ViiV Healthcare put option and Pfizer preferential dividends represented a reduction in the valuation of the put option as a result of updated exchange rates, sales forecasts and cash balances. The ViiV Healthcare contingent consideration liability is fair valued under IFRS. An explanation of the accounting for the non- controlling interests in ViiV Healthcare is set out on page 74. The £187 million credit relating to the contingent consideration on the former Novartis Vaccines business primarily relates to changes to future sales forecasts. The £44 million charge relating to the contingent consideration on the acquisition of Affinivax primarily relates to the unwind of the discount. Divestments, significant legal charges and other items Divestments, significant legal charges, and other items primarily included £200 million of net income from dividends and milestones related to investments, including £49 million of dividends received from the retained investment in Haleon plc, partly offset by £17 million fair value losses on the investment in Haleon plc. Legal charges provide for all significant legal matters, including Zantac, and are not broken out separately by litigation or investigation. Significant legal charges in the year primarily reflected increased legal charges for Zantac of which the vast majority relate to the prospective legal costs for the defence of the litigation. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued Financial performance continued 85
Cash generation and conversion A summary of the consolidated cash flow statement is set out below. 2023 £m 2022 £m Total net cash inflow from operating activities 6,768 7,403 Total net cash (outflow) from investing activities (1,595) (8,772) Total net cash inflow/(outflow) from financing activities (5,641) 823 Decrease in cash and bank overdrafts (468) (546) Cash and bank overdrafts at beginning of year 3,425 3,819 Exchange adjustments (99) 152 Decrease in cash and bank overdrafts (468) (546) Cash and bank overdrafts at end of year 2,858 3,425 Cash and bank overdrafts at end of year comprise: Cash and cash equivalents 2,936 3,723 Overdrafts (78) (298) 2,858 3,425 Reconciliation of net cash inflow from continuing operating activities to free cash inflow A reconciliation of net cash inflow from operating activities, which is the closest equivalent IFRS measure to free cash flow, is shown below. 2023 £m 2022 £m Net cash inflow from continuing operating activities 6,768 6,634 Purchase of property, plant and equipment (1,314) (1,143) Purchase of intangible assets (1,030) (1,115) Proceeds from sale of property, plant and equipment 28 146 Proceeds from sale of intangible assets 12 196 Net finance costs (651) (784) Dividends and disposal proceeds from joint ventures and associates 12 6 Contingent consideration paid (reported in investing activities) (11) (79) Contribution from non-controlling interests 7 8 Distributions to non-controlling interests (412) (521) Free cash inflow 3,409 3,348 Net cash inflow from continuing operating activities was £6,768 million (2022: £6,634 million). The increase primarily reflected higher adjusted operating profit, a favourable comparison on the timing of net Xevudy related receipts and payments, and lower pension contributions, partly offset by an unfavourable comparison due to the upfront income from the settlement with Gilead received in Q1 2022, an increase in trade receivables due to higher sales including the launch of Arexvy, lower payable balances reflecting increased investment in 2022, higher inventory and corporation tax payments. Capital expenditure and financial investment Cash payments for tangible and intangible fixed assets amounted to £2,344 million (2022: £2,258 million) and disposals realised £40 million (2022: £342 million). Cash payments to acquire equity investments amounted to £123 million (2022: £143 million) and sales of equity investments realised £1,832 million (2022: £238 million). Free cash flow Free cash flow is the amount of cash generated by the Group after meeting our obligations for contingent consideration, interest, tax and dividends paid to non-controlling interests, and after capital expenditure on property, plant and equipment and intangible assets. 2023 £m 2022 £m Free cash inflow 3,409 3,348 Total cash payments to Shionogi in relation to the ViiV Healthcare contingent consideration liability in the year were £1,106 million (2022: £1,100 million), all of which was recognised in cash flows from operating activities. These payments are deductible for tax purposes. Future cash flow Over the long term, we expect that future cash generated from operations will be sufficient to fund our operating and debt servicing costs, normal levels of capital expenditure, obligations under existing licensing agreements, expenditure arising from restructuring programmes and other routine outflows including tax, pension contributions and dividends, subject to the ‘Risk Factors’ discussed on pages 254 to 260. We may from time to time have additional demands for finance, such as for acquisitions. We have access to multiple sources of liquidity from short and long-term capital markets and financial institutions for such needs, in addition to the cash flow from operations. The Group, has in its opinion, sufficient working capital to meet its present requirements. Please refer to "Group financial review 2022" in Item 5.A of the GSK Annual Report on Form 20-F for the year ended 31 December 2022 for a discussion of 2022 financial results compared to 2021. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 86
Financial position and resources 2023 £m 2022 £m Assets Non-current assets Property, plant and equipment 9,020 8,933 Right of use assets 937 687 Goodwill 6,811 7,046 Other intangible assets 14,768 14,318 Investments in associates and joint ventures 55 74 Other investments 1,137 1,467 Deferred tax assets 6,049 5,658 Other non-current assets 1,584 1,194 Total non-current assets 40,361 39,377 Current assets Inventories 5,498 5,146 Current tax recoverable 373 405 Trade and other receivables 7,385 7,053 Derivative financial instruments 130 190 Current equity investments 2,204 4,087 Liquid investments 42 67 Cash and cash equivalents 2,936 3,723 Assets held for sale 76 98 Total current assets 18,644 20,769 Total assets 59,005 60,146 Liabilities Current liabilities Short-term borrowings (2,813) (3,952) Contingent consideration liabilities (1,053) (1,289) Trade and other payables (15,844) (16,263) Derivative financial instruments (114) (183) Current tax payable (500) (471) Short-term provisions (744) (652) Total current liabilities (21,068) (22,810) Non-current liabilities Long-term borrowings (15,205) (17,035) Corporation tax payable (75) (127) Deferred tax liabilities (311) (289) Pensions and other post-employment benefits (2,340) (2,579) Other provisions (495) (532) Contingent consideration liabilities (5,609) (5,779) Other non-current liabilities (1,107) (899) Total non-current liabilities (25,142) (27,240) Total liabilities (46,210) (50,050) Net assets 12,795 10,096 Total equity 12,795 10,096 Property, plant and equipment Our business is science-based, technology-intensive and highly regulated by governmental authorities. We allocate significant financial resources to the renewal and maintenance of our property, plant, equipment and vehicles to minimise risks of interruption to production and to ensure compliance with regulatory standards. A number of our processes use hazardous materials. The total cost of our property, plant and equipment at 31 December 2023 was £19,279 million, with a net book value of £9,020 million. Of this, land and buildings represented £2,895 million, plant, equipment and vehicles £4,033 million and assets in construction £2,092 million. In 2023, we invested £1,295 million in new property, plant and equipment. This was mainly related to a large number of projects for the renewal, improvement and expansion of facilities at various worldwide sites to support new product development and launches as well as to improve the efficiency of existing supply chains. Property is mainly held freehold. New investment is financed from our liquid resources. At 31 December 2023, we had contractual commitments for future capital expenditure of £762 million. We believe that our property and plant facilities are adequate for our current requirements. Right of use assets Right of use assets amounted to £937 million at 31 December 2023 compared with £687 million at 31 December 2022. The increase in the year reflected the impact of additions through business combinations of £1 million and other additions of £499 million partly offset by depreciation of £190 million, disposals and impairments amounting to £30 million. Goodwill Goodwill decreased to £6,811 million at 31 December 2023, from £7,046 million primarily as a result of an exchange rate loss of £313 million, partially offset by an increase of £109 million from acquisitions-related transactions. Other intangible assets Other intangible assets include the cost of intangibles acquired from third parties and computer software. The net book value of other intangible assets as at 31 December 2023 was £14,768 million (2022: £14,318 million). The increase primarily reflected additions, net of disposals and write-offs of £2,476 million partly offset by impairment losses, net of reversals and amortisation of £1,630 million and exchange rate losses of £431 million. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 87

Investments in associates and joint ventures We held investments in associates and joint ventures with a carrying value at 31 December 2023 of £55 million (2022: £74 million). See Note 21, 'Investments in associates and joint ventures' to the financial statements, for more details. Current equity investments Current equity investments amounted to £2,204 million at 31 December 2023 (2022: £4,087 million). Current equity investments comprise equity investments which the Group holds with the intention to sell and which it may sell in the short term. Where acquired with this intention, they are measured at fair value through the profit and loss (FVTPL). They are initially recorded at fair value plus transaction costs and then remeasured at subsequent reporting dates to fair value. Unrealised gains and losses are recognised in the income statement. The investment of £2,204 million (2022: £4,087 million) represents the shares held in Haleon plc after the demerger. During 2023, disposals of Haleon plc shares resulted in gross proceeds of £1,863 million (2022: £nil). Other investments At 31 December 2023 we held other investments with a carrying value of £1,137 million (2022: £1,467 million). The most significant of these investments held at 31 December 2023 were in Crispr Therapeutics AG, Vir Biotechnology Inc. and SR One Capital Fund I-B, LP. These investments had a fair value at 31 December 2023 of £158 million (2022: £109 million), £67 million (2022: £180 million) and £102 million (2022: £211 million) respectively. The other investments included equity stakes in companies with which we have research collaborations, and which provide access to biotechnology developments of potential interest and interests in companies that arise from business divestments. Derivative financial instruments: assets We held current derivative financial assets at fair value of £130 million (2022: £190 million). The majority of these financial instruments related to foreign exchange contracts both designated and not designated as accounting hedges. Inventories Inventories amounted to £5,498 million (2022: £5,146) at 31 December 2023. Trade and other receivables Trade and other receivables amounted to £7,385 million (2022: £7,053 million) at 31 December 2023. The increase is mainly driven by Arexvy sales in the US. Deferred tax assets Deferred tax assets amounted to £6,049 million (2022: £5,658 million) at 31 December 2023. Derivative financial instruments: liabilities We held current derivative financial liabilities at fair value of £114 million (2022: £183 million). This is primarily related to foreign exchange contracts both designated and not designated as accounting hedges. Trade and other payables At 31 December 2023, trade and other payables were £15,844 million compared with £16,263 million at 31 December 2022. The decrease was primarily driven by lower accruals relating to profit share collaborations partly offset by higher customer return and rebates accruals. See Note 29, 'Trade and other payables' to the financial statements. Provisions We carried deferred tax provisions and other short-term and non-current provisions of £1,550 million at 31 December 2023 (2022: £1,473 million). Other provisions at the year-end included £267 million (2022: £218 million) related to legal and other disputes and £282 million (2022: £351 million) related to Major restructuring programmes. Provision has been made for legal and other disputes, indemnified disposal liabilities, employee related liabilities and the costs of the restructuring programme to the extent that at the balance sheet date a legal or constructive obligation existed and could be reliably estimated. Pensions and other post-employment benefits We account for pension and other post-employment arrangements in accordance with IAS 19. The net deficits were £763 million (2022: £1,356 million) on pension arrangements and £943 million (2022: £994 million) on unfunded post- employment liabilities. See Note 31, 'Pensions and other post- employment benefits' to the financial statements. Other non-current liabilities Other non-current liabilities amounted to £1,107 million at 31 December 2023 (2022: £899 million). Contingent consideration liabilities Contingent consideration amounted to £6,662 million at 31 December 2023 (2022: £7,068 million), of which £5,718 million (2022: £5,890 million) represented the estimated present value of amounts payable to Shionogi relating to ViiV Healthcare, £516 million (2022: £501 million) represented the estimated present value of contingent consideration payable to the former shareholders of Affinivax and £424 million (2022: £673 million) represented the estimated present value of contingent consideration payable to Novartis related to the Vaccines acquisition. The liability due to Shionogi was £267 million in respect of preferential dividends. An explanation of the accounting for the non-controlling interests in ViiV Healthcare is set out on page 74. Of the total contingent consideration payable (on a post-tax basis) at 31 December 2023, £1,017 million (2022: £940 million) is expected to be paid within one year to Shionogi. The consideration payable is expected to be paid over a number of years. As a result, the total estimated liabilities are discounted to their present values, on a post-tax basis using post-tax discount rates. The Shionogi-ViiV Healthcare contingent consideration liability is discounted at 8%, the Affinivax contingent consideration liability is discounted at 8.5%, and the Novartis Vaccines contingent consideration liability is discounted partly at 7.5% and partly at 8.5%. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 88
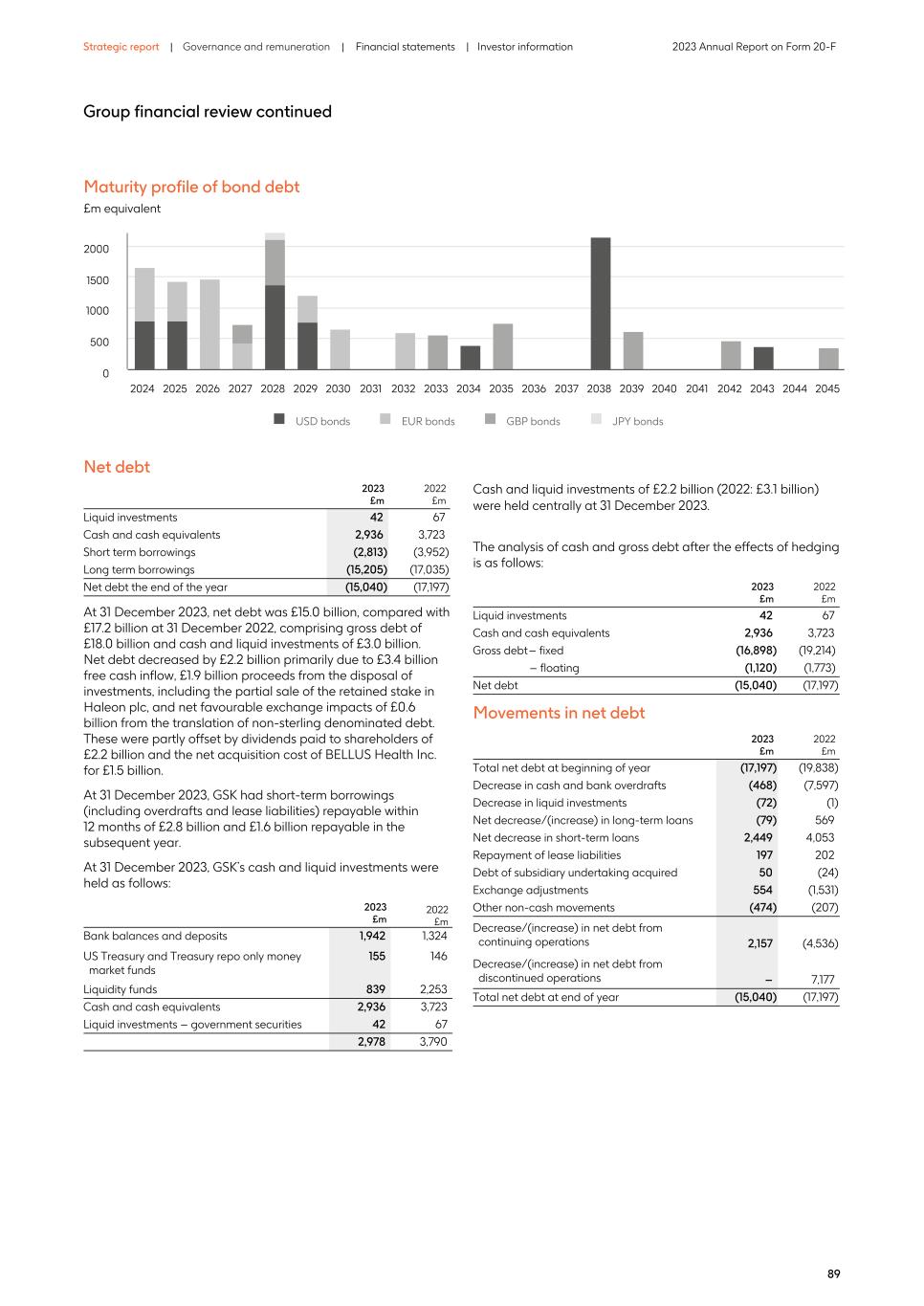
Maturity profile of bond debt £m equivalent USD bonds EUR bonds GBP bonds JPY bonds 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 2034 2035 2036 2037 2038 2039 2040 2041 2042 2043 2044 2045 0 500 1000 1500 2000 Net debt 2023 £m 2022 £m Liquid investments 42 67 Cash and cash equivalents 2,936 3,723 Short term borrowings (2,813) (3,952) Long term borrowings (15,205) (17,035) Net debt the end of the year (15,040) (17,197) At 31 December 2023, net debt was £15.0 billion, compared with £17.2 billion at 31 December 2022, comprising gross debt of £18.0 billion and cash and liquid investments of £3.0 billion. Net debt decreased by £2.2 billion primarily due to £3.4 billion free cash inflow, £1.9 billion proceeds from the disposal of investments, including the partial sale of the retained stake in Haleon plc, and net favourable exchange impacts of £0.6 billion from the translation of non-sterling denominated debt. These were partly offset by dividends paid to shareholders of £2.2 billion and the net acquisition cost of BELLUS Health Inc. for £1.5 billion. At 31 December 2023, GSK had short-term borrowings (including overdrafts and lease liabilities) repayable within 12 months of £2.8 billion and £1.6 billion repayable in the subsequent year. At 31 December 2023, GSK’s cash and liquid investments were held as follows: 2023 £m 2022 £m Bank balances and deposits 1,942 1,324 US Treasury and Treasury repo only money market funds 155 146 Liquidity funds 839 2,253 Cash and cash equivalents 2,936 3,723 Liquid investments – government securities 42 67 2,978 3,790 Cash and liquid investments of £2.2 billion (2022: £3.1 billion) were held centrally at 31 December 2023. The analysis of cash and gross debt after the effects of hedging is as follows: 2023 £m 2022 £m Liquid investments 42 67 Cash and cash equivalents 2,936 3,723 Gross debt – fixed (16,898) (19,214) – floating (1,120) (1,773) Net debt (15,040) (17,197) Movements in net debt 2023 £m 2022 £m Total net debt at beginning of year (17,197) (19,838) Decrease in cash and bank overdrafts (468) (7,597) Decrease in liquid investments (72) (1) Net decrease/(increase) in long-term loans (79) 569 Net decrease in short-term loans 2,449 4,053 Repayment of lease liabilities 197 202 Debt of subsidiary undertaking acquired 50 (24) Exchange adjustments 554 (1,531) Other non-cash movements (474) (207) Decrease/(increase) in net debt from continuing operations 2,157 (4,536) Decrease/(increase) in net debt from discontinued operations – 7,177 Total net debt at end of year (15,040) (17,197) Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 89
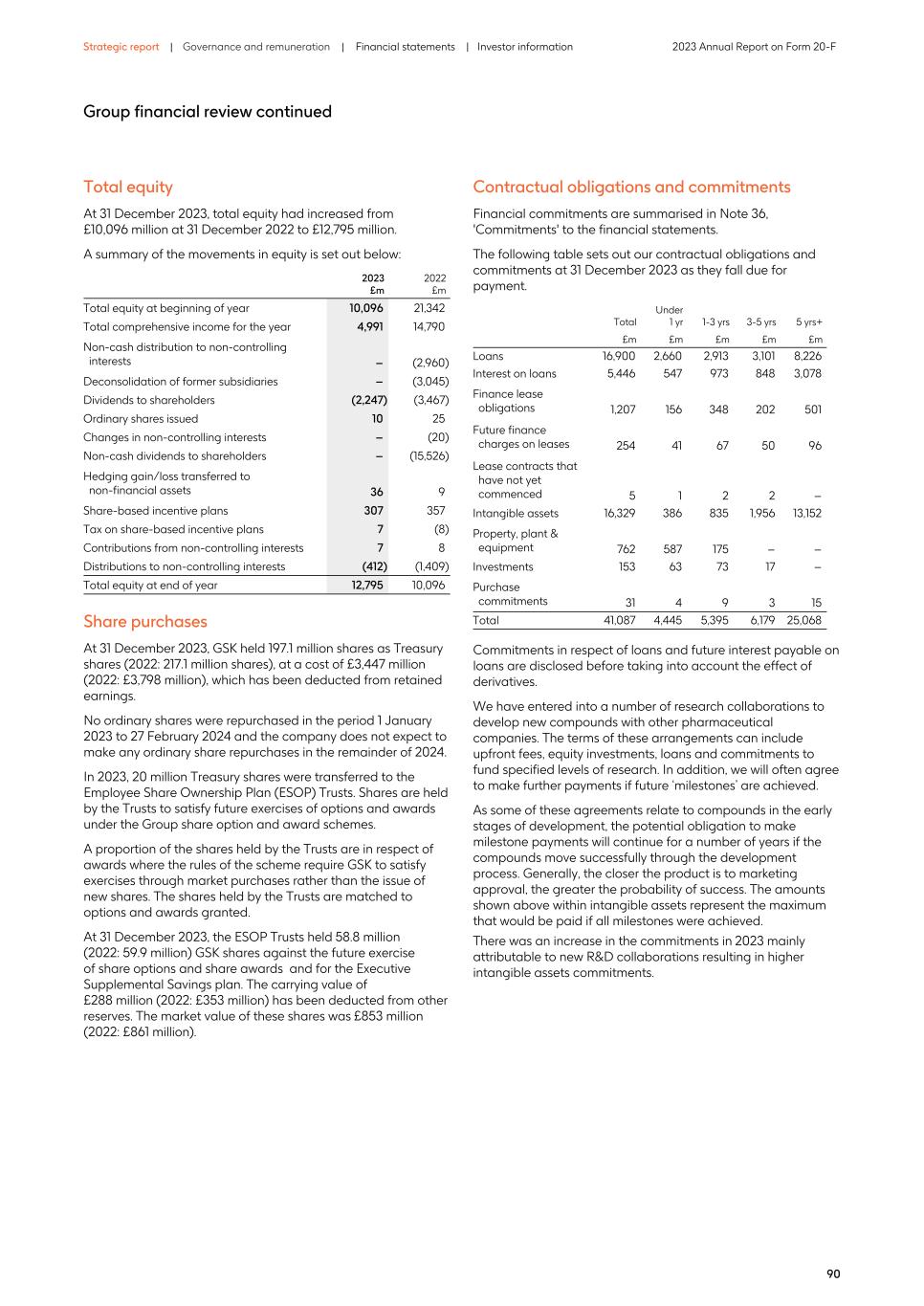
Total equity At 31 December 2023, total equity had increased from £10,096 million at 31 December 2022 to £12,795 million. A summary of the movements in equity is set out below: 2023 £m 2022 £m Total equity at beginning of year 10,096 21,342 Total comprehensive income for the year 4,991 14,790 Non-cash distribution to non-controlling interests – (2,960) Deconsolidation of former subsidiaries – (3,045) Dividends to shareholders (2,247) (3,467) Ordinary shares issued 10 25 Changes in non-controlling interests – (20) Non-cash dividends to shareholders – (15,526) Hedging gain/loss transferred to non-financial assets 36 9 Share-based incentive plans 307 357 Tax on share-based incentive plans 7 (8) Contributions from non-controlling interests 7 8 Distributions to non-controlling interests (412) (1,409) Total equity at end of year 12,795 10,096 Share purchases At 31 December 2023, GSK held 197.1 million shares as Treasury shares (2022: 217.1 million shares), at a cost of £3,447 million (2022: £3,798 million), which has been deducted from retained earnings. No ordinary shares were repurchased in the period 1 January 2023 to 27 February 2024 and the company does not expect to make any ordinary share repurchases in the remainder of 2024. In 2023, 20 million Treasury shares were transferred to the Employee Share Ownership Plan (ESOP) Trusts. Shares are held by the Trusts to satisfy future exercises of options and awards under the Group share option and award schemes. A proportion of the shares held by the Trusts are in respect of awards where the rules of the scheme require GSK to satisfy exercises through market purchases rather than the issue of new shares. The shares held by the Trusts are matched to options and awards granted. At 31 December 2023, the ESOP Trusts held 58.8 million (2022: 59.9 million) GSK shares against the future exercise of share options and share awards and for the Executive Supplemental Savings plan. The carrying value of £288 million (2022: £353 million) has been deducted from other reserves. The market value of these shares was £853 million (2022: £861 million). Contractual obligations and commitments Financial commitments are summarised in Note 36, 'Commitments' to the financial statements. The following table sets out our contractual obligations and commitments at 31 December 2023 as they fall due for payment. Total Under 1 yr 1-3 yrs 3-5 yrs 5 yrs+ £m £m £m £m £m Loans 16,900 2,660 2,913 3,101 8,226 Interest on loans 5,446 547 973 848 3,078 Finance lease obligations 1,207 156 348 202 501 Future finance charges on leases 254 41 67 50 96 Lease contracts that have not yet commenced 5 1 2 2 – Intangible assets 16,329 386 835 1,956 13,152 Property, plant & equipment 762 587 175 – – Investments 153 63 73 17 – Purchase commitments 31 4 9 3 15 Total 41,087 4,445 5,395 6,179 25,068 Commitments in respect of loans and future interest payable on loans are disclosed before taking into account the effect of derivatives. We have entered into a number of research collaborations to develop new compounds with other pharmaceutical companies. The terms of these arrangements can include upfront fees, equity investments, loans and commitments to fund specified levels of research. In addition, we will often agree to make further payments if future ‘milestones’ are achieved. As some of these agreements relate to compounds in the early stages of development, the potential obligation to make milestone payments will continue for a number of years if the compounds move successfully through the development process. Generally, the closer the product is to marketing approval, the greater the probability of success. The amounts shown above within intangible assets represent the maximum that would be paid if all milestones were achieved. There was an increase in the commitments in 2023 mainly attributable to new R&D collaborations resulting in higher intangible assets commitments. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 90
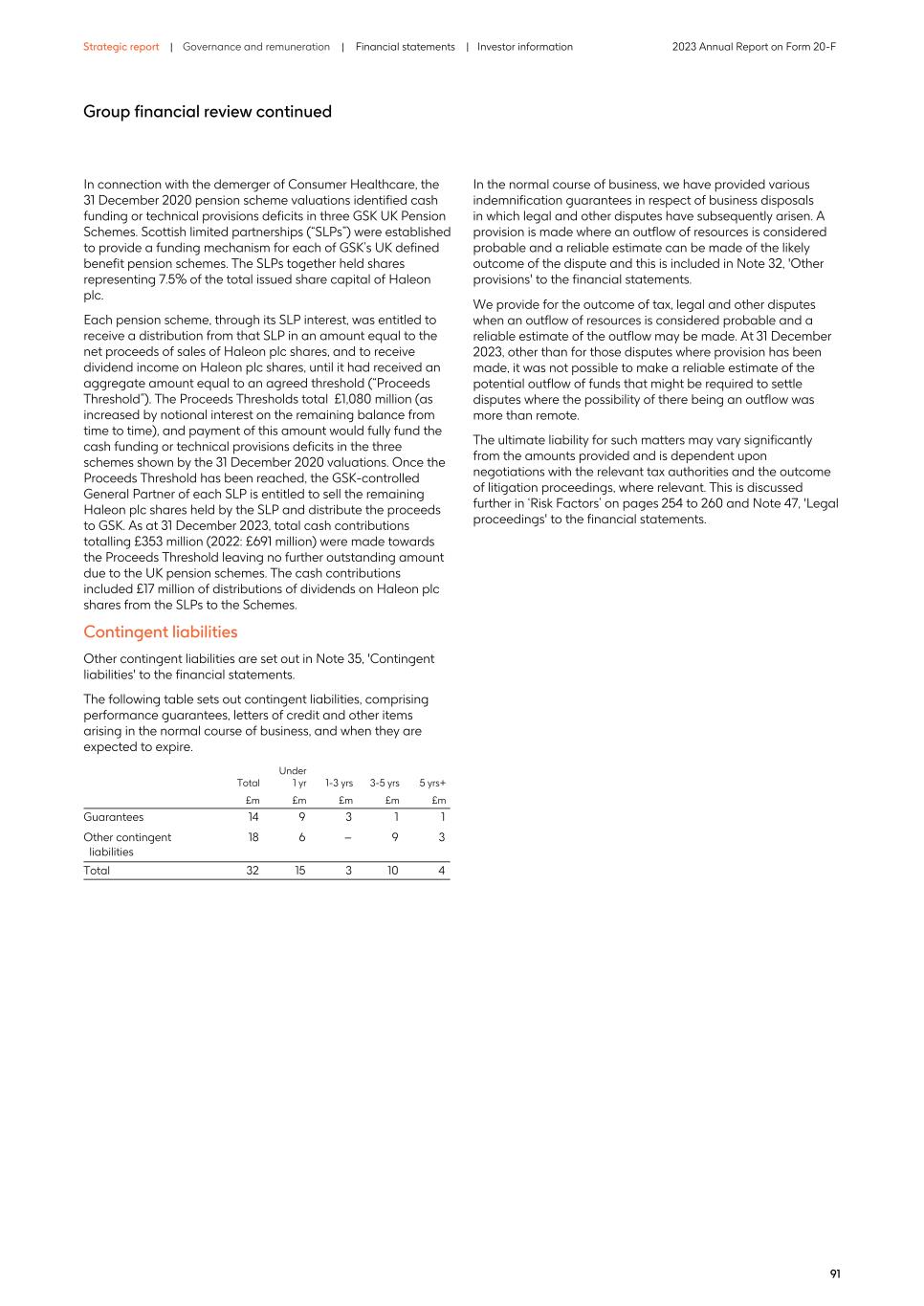
In connection with the demerger of Consumer Healthcare, the 31 December 2020 pension scheme valuations identified cash funding or technical provisions deficits in three GSK UK Pension Schemes. Scottish limited partnerships (“SLPs”) were established to provide a funding mechanism for each of GSK’s UK defined benefit pension schemes. The SLPs together held shares representing 7.5% of the total issued share capital of Haleon plc. Each pension scheme, through its SLP interest, was entitled to receive a distribution from that SLP in an amount equal to the net proceeds of sales of Haleon plc shares, and to receive dividend income on Haleon plc shares, until it had received an aggregate amount equal to an agreed threshold (“Proceeds Threshold”). The Proceeds Thresholds total £1,080 million (as increased by notional interest on the remaining balance from time to time), and payment of this amount would fully fund the cash funding or technical provisions deficits in the three schemes shown by the 31 December 2020 valuations. Once the Proceeds Threshold has been reached, the GSK-controlled General Partner of each SLP is entitled to sell the remaining Haleon plc shares held by the SLP and distribute the proceeds to GSK. As at 31 December 2023, total cash contributions totalling £353 million (2022: £691 million) were made towards the Proceeds Threshold leaving no further outstanding amount due to the UK pension schemes. The cash contributions included £17 million of distributions of dividends on Haleon plc shares from the SLPs to the Schemes. Contingent liabilities Other contingent liabilities are set out in Note 35, 'Contingent liabilities' to the financial statements. The following table sets out contingent liabilities, comprising performance guarantees, letters of credit and other items arising in the normal course of business, and when they are expected to expire. Total Under 1 yr 1-3 yrs 3-5 yrs 5 yrs+ £m £m £m £m £m Guarantees 14 9 3 1 1 Other contingent liabilities 18 6 – 9 3 Total 32 15 3 10 4 In the normal course of business, we have provided various indemnification guarantees in respect of business disposals in which legal and other disputes have subsequently arisen. A provision is made where an outflow of resources is considered probable and a reliable estimate can be made of the likely outcome of the dispute and this is included in Note 32, 'Other provisions' to the financial statements. We provide for the outcome of tax, legal and other disputes when an outflow of resources is considered probable and a reliable estimate of the outflow may be made. At 31 December 2023, other than for those disputes where provision has been made, it was not possible to make a reliable estimate of the potential outflow of funds that might be required to settle disputes where the possibility of there being an outflow was more than remote. The ultimate liability for such matters may vary significantly from the amounts provided and is dependent upon negotiations with the relevant tax authorities and the outcome of litigation proceedings, where relevant. This is discussed further in ‘Risk Factors’ on pages 254 to 260 and Note 47, 'Legal proceedings' to the financial statements. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 91

Approach to tax Business makes a major contribution to the public purse through its tax contribution. This includes direct taxes (such as corporate income tax) and indirect taxes (such as VAT and customs duties) as well as other taxes (such as employment taxes and property taxes). It is therefore important that companies explain their approach to tax. This helps inform dialogue about tax and tax policy. We are supportive of efforts to ensure companies are appropriately transparent about how their tax affairs are managed. As part of that, our Tax Strategy is set out in detail within the Public policies section of our website. We support the exchange of country-by-country reporting (CBCR) data between tax authorities as, validated against existing information held on taxpayers, it will support their ability to ensure multinational groups pay the right amount of tax in the right places. As a global biopharmaceutical company, we have a substantial business and employment presence in many countries around the world and pay a significant amount of tax. This includes corporate income tax and other business taxes, and tax associated with our employees. We also collect a significant amount of tax on behalf of governments along our supply chain, including from our employees. We are subject to taxation throughout our supply chain. The worldwide nature of our operations means that our cross- border supply routes, necessary to ensure supplies of medicines into numerous countries, can result in conflicting claims from tax authorities as to the profits to be taxed in individual countries. This can lead to double taxation (with profits taxed in more than one country). Profits are recognised in territories by reference to the activities performed there and the value they generate. To ensure the profits recognised in jurisdictions are aligned to the activity undertaken there, and in line with current OECD guidelines, we base our transfer pricing policy on the arm’s length principle and support our transfer prices with economic analysis and reports. We do not engage in artificial tax arrangements – those without business or commercial substance. We do not seek to avoid tax by the use of ‘tax havens’ or transactions we would not fully disclose to a tax authority. We have a zero-tolerance approach to tax evasion and the facilitation of tax evasion. Tax risk in all countries in which we operate is managed through robust internal policies, processes, training and compliance programmes. Our Board of Directors and the Audit & Risk Committee are responsible for approving our tax policies and risk management arrangements as part of our wider internal control framework. We seek to maintain open and constructive relationships with tax authorities worldwide, meeting regularly to discuss our tax affairs and real time business updates wherever possible. We also monitor government debate on tax policy in our key jurisdictions so that we can understand and share an informed point of view regarding any potential future changes in tax law, in support of a transparent and sustainable tax system. Where relevant, we provide pragmatic and constructive business input to tax policy makers either directly or through industry trade bodies, advocating reform to support economic growth and job creation as well as the needs of our patients and other key stakeholders. In 2023, the Group corporate tax charge was £756 million (2022: £707 million) on profits before tax of £6,064 million (2022: £5,628 million) representing an effective tax rate of 12.5% (2022: 12.6%). We made cash tax payments of £1,328 million in the year (2022: £1,310 million). In addition to the taxes we pay on our profits, we pay duties, levies, transactional and employment taxes. The Group’s Total tax rate for 2023 of 12.5% (2022: 12.6%) was lower than the Adjusted tax rate reflecting the different tax effects of various Adjusting items. Our Adjusted tax rate for 2023 was 15.5% (2022: 15.5%). The rate has benefited from innovation incentives available in key territories in which we operate, such as the UK and Belgium Patent Box regimes. During 2023 the UK Government enacted legislation introducing a global minimum corporate income tax rate, to have effect from 2024 in line with the Organisation for Economic Co-operation and Development’s (OECD) Pillar Two model framework. We anticipate that the rules will restrict our ability to benefit from innovation incentives and consequentially our effective Adjusted tax rate is forecast to increase to around 17% for 2024. Further details about our corporate tax charges for the year are set out in Note 14 'Taxation' to the financial statements. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 92

Treasury policies We report in Sterling and pay dividends out of Sterling cash flows. The role of Treasury is to monitor and manage the Group’s external and internal funding requirements and financial risks in support of our strategic objectives. GSK operates on a global basis, primarily through subsidiary companies, and we manage our capital to ensure that our subsidiaries are able to operate as going concerns and to optimise returns to shareholders through an appropriate balance of debt and equity. Treasury activities are governed by policies approved annually by the Board of Directors, and most recently on 11 October 2023. A Treasury Management Group (TMG) meeting, chaired by our Chief Financial Officer, takes place on a regular basis to review Treasury activities. Its members receive management information relating to these activities. Treasury operations The objective of GSK’s Treasury activities is to minimise the post-tax net cost of financial operations and reduce its volatility in order to benefit earnings and cash flows. GSK uses a variety of financial instruments to finance its operations and derivative financial instruments to manage market risks from these operations. Derivatives principally comprise foreign exchange forward contracts and swaps which are used to swap borrowings and liquid assets into currencies required for Group purposes, as well as interest rate swaps which are used to manage exposure to financial risks from changes in interest rates. Derivatives are used exclusively for hedging purposes in relation to underlying business activities and not as trading or speculative instruments. Capital management GSK’s financial strategy, implemented through the Group’s financial architecture, supports GSK’s strategic priorities and is regularly reviewed by the Board. We manage the capital structure of the Group through an appropriate mix of debt and equity. We continue to manage our financial policies to a credit profile that particularly targets ratings of at least A2/A (Moody's/S&P), through the cycle. Liquidity risk management GSK’s policy is to borrow centrally in order to meet anticipated funding requirements. Our cash flow forecasts and funding requirements are monitored by the TMG on a regular basis. Our strategy is to diversify liquidity sources using a range of facilities and to maintain broad access to financial markets. Each day, we sweep cash to or from a number of global subsidiaries to central treasury accounts for liquidity management purposes. Interest rate risk management GSK’s objective is to minimise the effective net interest cost and to balance the mix of debt at fixed and floating interest rates over time. The policy on interest rate risk management limits the net amount of floating rate debt to a specific cap, reviewed and agreed no less than annually by the Board. Foreign exchange risk management Our objective is to minimise the exposure of overseas operating subsidiaries to transaction risk by matching local currency income with local currency costs where possible. Foreign currency transaction exposures arising on external and internal trade flows are selectively hedged. GSK’s internal trading transactions are matched centrally and we manage inter- company payment terms to reduce foreign currency risk. Where possible, we manage the cash surpluses or borrowing requirements of subsidiary companies centrally using forward contracts to hedge future repayments back into the originating currency. In order to reduce foreign currency translation exposure, we seek to denominate borrowings in the currencies of our principal assets and cash flows. These are primarily denominated in US Dollars, Euros and Sterling. Borrowings can be swapped into other currencies as required. Borrowings denominated in, or swapped into, foreign currencies that match investments in overseas Group assets may be treated as a hedge against the relevant assets. Forward contracts in major currencies are also used to reduce exposure to the Group’s investment in overseas Group assets. The TMG reviews the ratio of borrowings to assets for major currencies regularly. Commodity risk management Our objective is to minimise income statement volatility arising from fluctuations in commodity prices, where practical and cost effective to do so. The TMG is authorised to approve the execution of certain financial derivatives to hedge commodity price exposures. Counterparty risk management We set global counterparty limits for each of our banking and investment counterparties based on long-term credit ratings from Moody’s and Standard and Poor’s. Usage of these limits is actively monitored and any breach of these limits would be reported to the Chief Financial Officer immediately. In addition, relationship banks and their credit ratings are reviewed regularly so that, when changes in ratings occur, changes can be made to investment levels or to authority limits as appropriate. All banking counterparty limits are reviewed at least annually. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 93

Capital allocation framework to support investment and returns Priority is to invest for growth, coupled with attractive shareholder returns (1) GSK group dividend in 2022; GSK related only and excludes dividend related to Consumer Healthcare in H1-2022; FY 2022 dividend 61.25p/share Our capital allocation framework to support investment and returns Our capital allocation framework means our first priority remains to invest in the business, with capital allocated towards development of the pipeline, both organic and targeted business development. We also remain committed to delivering attractive returns to shareholders and pursuing a progressive dividend policy, guided by a 40 to 60 percent pay-out ratio through the investment cycle. In setting its dividend policy, GSK considers the priorities of the Group and its investment strategy for growth, alongside the sustainability of the dividend. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 94
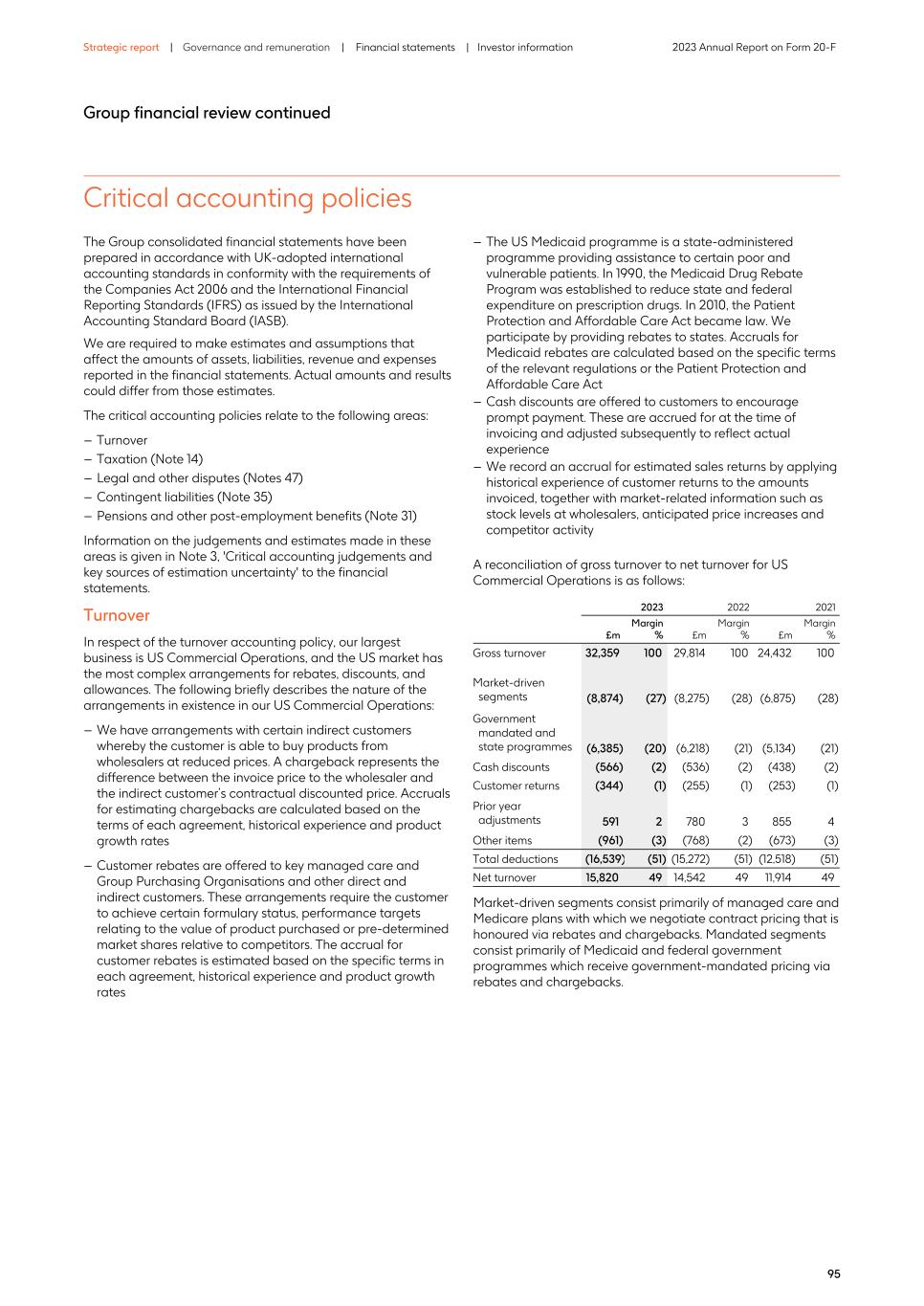
Critical accounting policies The Group consolidated financial statements have been prepared in accordance with UK-adopted international accounting standards in conformity with the requirements of the Companies Act 2006 and the International Financial Reporting Standards (IFRS) as issued by the International Accounting Standard Board (IASB). We are required to make estimates and assumptions that affect the amounts of assets, liabilities, revenue and expenses reported in the financial statements. Actual amounts and results could differ from those estimates. The critical accounting policies relate to the following areas: – Turnover – Taxation (Note 14) – Legal and other disputes (Notes 47) – Contingent liabilities (Note 35) – Pensions and other post-employment benefits (Note 31) Information on the judgements and estimates made in these areas is given in Note 3, 'Critical accounting judgements and key sources of estimation uncertainty' to the financial statements. Turnover In respect of the turnover accounting policy, our largest business is US Commercial Operations, and the US market has the most complex arrangements for rebates, discounts, and allowances. The following briefly describes the nature of the arrangements in existence in our US Commercial Operations: – We have arrangements with certain indirect customers whereby the customer is able to buy products from wholesalers at reduced prices. A chargeback represents the difference between the invoice price to the wholesaler and the indirect customer’s contractual discounted price. Accruals for estimating chargebacks are calculated based on the terms of each agreement, historical experience and product growth rates – Customer rebates are offered to key managed care and Group Purchasing Organisations and other direct and indirect customers. These arrangements require the customer to achieve certain formulary status, performance targets relating to the value of product purchased or pre-determined market shares relative to competitors. The accrual for customer rebates is estimated based on the specific terms in each agreement, historical experience and product growth rates – The US Medicaid programme is a state-administered programme providing assistance to certain poor and vulnerable patients. In 1990, the Medicaid Drug Rebate Program was established to reduce state and federal expenditure on prescription drugs. In 2010, the Patient Protection and Affordable Care Act became law. We participate by providing rebates to states. Accruals for Medicaid rebates are calculated based on the specific terms of the relevant regulations or the Patient Protection and Affordable Care Act – Cash discounts are offered to customers to encourage prompt payment. These are accrued for at the time of invoicing and adjusted subsequently to reflect actual experience – We record an accrual for estimated sales returns by applying historical experience of customer returns to the amounts invoiced, together with market-related information such as stock levels at wholesalers, anticipated price increases and competitor activity A reconciliation of gross turnover to net turnover for US Commercial Operations is as follows: 2023 2022 2021 £m Margin % £m Margin % £m Margin % Gross turnover 32,359 100 29,814 100 24,432 100 Market-driven segments (8,874) (27) (8,275) (28) (6,875) (28) Government mandated and state programmes (6,385) (20) (6,218) (21) (5,134) (21) Cash discounts (566) (2) (536) (2) (438) (2) Customer returns (344) (1) (255) (1) (253) (1) Prior year adjustments 591 2 780 3 855 4 Other items (961) (3) (768) (2) (673) (3) Total deductions (16,539) (51) (15,272) (51) (12,518) (51) Net turnover 15,820 49 14,542 49 11,914 49 Market-driven segments consist primarily of managed care and Medicare plans with which we negotiate contract pricing that is honoured via rebates and chargebacks. Mandated segments consist primarily of Medicaid and federal government programmes which receive government-mandated pricing via rebates and chargebacks. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 95

Overall sales deduction as a percentage of sales is consistent year over year with sales growth coming primarily from Trelegy, Arexvy and Specialty Products including HIV. Deductions within the year were split approximately as follows: General Medicines 67%, Specialty Medicines 21% and Vaccines 12%. At 31 December 2023, the total accrual for discounts, rebates, allowances and returns for US Commercial Operations amounted to £5,951 million (2022: £5,855 million). A monthly process is operated to monitor inventory levels at wholesalers for any abnormal movements. This process uses gross sales volumes, prescription volumes based on third party data sources and information received from key wholesalers. The aim of this is to maintain inventories at a consistent level from year to year based on the pattern of consumption. On this basis, US Commercial Operations inventory levels at wholesalers and in other distribution channels at 31 December 2023 were estimated to amount to approximately four weeks of turnover. This calculation uses third party information, the accuracy of which cannot be totally verified, but is believed to be sufficiently reliable for this purpose. Legal and other disputes In respect of the accounting policy for legal and other disputes, the following briefly describes the process by which we determine the level of provision that is necessary. In accordance with the requirements of IAS 37, ‘Provisions, contingent liabilities and contingent assets’, we provide for anticipated settlement costs where an outflow of resources is considered probable and a reliable estimate may be made of the likely outcome of the dispute and legal and other expenses arising from claims against the Group. We may become involved in significant legal proceedings, in respect of which it is not possible to meaningfully assess whether the outcome will result in a probable outflow, or to quantify or reliably estimate the liability, if any, that could result from ultimate resolution of the proceedings. In these cases, appropriate disclosure about such cases would be included in the Annual Report, but no provision would be made. This position could change over time and, therefore, there can be no assurance that any losses that result from the outcome of any legal proceedings will not exceed by a material amount the amount of the provisions reported in the Group’s financial statements. Like many pharmaceutical companies, we are faced with various complex product liability, anti-trust and patent litigation, as well as investigations of our operations conducted by various governmental regulatory agencies. Throughout the year, the General Counsel of the Group, as head of the Group’s legal function, supported by the Senior Vice President and Head of Global Litigation for the Group, who is responsible for all litigation and government investigations, routinely brief the Chief Executive Officer, the Chief Financial Officer and the Board of Directors on the significant litigation pending against the Group and governmental investigations of the Group. These meetings, as appropriate, detail the status of significant litigation and government investigations and review matters such as the number of claims notified to us, information on potential claims not yet notified, assessment of the validity of claims, progress made in settling claims, recent settlement levels and potential reimbursement by insurers. The meetings also include an assessment of whether or not there is sufficient information available for us to be able to make a reliable estimate of the potential outcomes of the disputes. Often, external counsel assisting us with various litigation matters and investigations will also assist in the briefing of the Board and senior management. Following these discussions, for those matters where it is possible to make a reliable estimate of the amount of a provision, if any, that may be required, the level of provision for legal and other disputes is reviewed and adjusted as appropriate. These matters are discussed further in Note 47, 'Legal proceedings' to the financial statements. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 96

Liquidity and capital resources Loans: At 31 December 2023 the Group had £16.9 billion of borrowings of which £2.7 billion was repayable within one year and £14.2 billion was payable after one year. Interest payable on these loans amounted to £5.4 billion of which £0.5 billion was payable within one year and £4.9 billion was payable after more than one year. Commitments in respect of loans and future interest payable on loans are disclosed before taking into account the effect of derivatives. Intangible assets commitments: At 31 December 2023, the Group had intangible assets commitments of £16.3 billion. Of these, £0.4 billion fall due within one year and £15.9 billion fall due after more than one year. The commitments include milestone payments, which are dependent on successful clinical development or on meeting specified sales targets, and which represent the maximum that would be paid if all milestones, however unlikely, are achieved. The amounts are not risk- adjusted or discounted. Refer to Contractual obligations and commitments on page 90 for more details. Finance Lease obligations: 31 December 2023 the Group had £1.2 billion of finance lease obligations of which £0.2 billion was payable within one year and £1.1 billion was payable after one year. Property, plant and equipment: At 31 December 2023 the Group had property, plant and equipment commitments of £0.8 billion of which £0.6 billion was payable within one year and £0.2 billion was payable after one year. Purchases commitments: At 31 December 2023 the Group had £0.03 billion of purchase commitments most of which was payable after one year. Future finance charges on leases: At 31 December 2023 the Group had £0.3 billion of future finance charges most of which was payable after one year. Investments: At 31 December 2023 the Group had £0.2 billion of investments commitments most of which £0.1 billion was payable within one year and £0.1 billion was payable after one year. Pensions: In 2022, GSK reached an agreement with the trustees of the UK pension schemes to make additional contributions of £1,080 million, to eliminate the pension deficit identified at the 31 December 2020 actuarial funding valuation. Prior to the Consumer Healthcare demerger, GSK agreed to collateralise this commitment and accelerate funding with additional contributions (Refer to Note 31 'Pensions and other post- employment benefits'). At 31 December 2023, £nil (2022: £345 million) additional contributions were unpaid. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Group financial review continued 97

Corporate governance In this section The Board and GSK Leadership Team 99 Chair’s governance statement 106 Corporate governance architecture 108 Ahead Together – Board oversight 111 Continuous engagement and key decisions 113 Board committee reports 116 Remuneration Committee Chair's annual statement 127 Annual report on remuneration 130 Directors’ report 149 2023 Annual Report on Form 20-F 98

Sir Jonathan Symonds, CBE Non-Executive Chair Age: 64 Nationality: British Appointed: 1 September 2019 Skills and experience Jon has extensive international financial, life sciences and governance experience. Jon served as an Independent Non-Executive Director of HSBC Holdings plc from April 2014 and as Chairman of the Group Audit Committee from 1 September 2014 and Deputy Group Chairman from August 2018, until his retirement from the Board in February 2020. He was previously Chairman of HSBC Bank plc, Chief Financial Officer of Novartis AG, Partner and Managing Director of Goldman Sachs, Chief Financial Officer of AstraZeneca plc, and a Partner at KPMG. Jon is a Fellow of the Institute of Chartered Accountants in England and Wales. External appointments Non-Executive Director, Genomics England Limited having previously served as its Chairman; Non-Executive Chair, Energy Aspects; Member, European Round Table for Industry; Senior Advisor to Chatham House. Dame Emma Walmsley Chief Executive Officer Age: 54 Nationality: British Appointed: 1 January 2017 Chief Executive Officer from 1 April 2017 Skills and experience Before being appointed as GSK’s CEO, Emma was the CEO of GSK Consumer Healthcare, a joint venture between GSK and Novartis, from its creation in March 2015. Emma joined GSK in 2010 from L’Oreal, having worked there for 17 years in a variety of roles in Paris, London, New York and Shanghai. Emma was previously a Non-Executive Director of Diageo plc. Emma’s position as an Independent Director of Microsoft, Inc., further supplements the technology and cyber security experience she brings to the Board. Emma holds an MA in Classics and Modern Languages from Oxford University. External appointments Independent Director, Microsoft, Inc. Julie Brown Chief Financial Officer Age: 61 Nationality: British Appointed: 3 April 2023 Chief Financial Officer from 1 May 2023 Skills and experience Julie has an extensive financial and life sciences background, having been the Group CFO of Smith & Nephew from 2013 to 2017 and serving as a Non-Executive Director and Audit Chair of Roche Holding AG from 2016 to 2022. Before this, Julie was Interim Group CFO of AstraZeneca plc, having worked in a wide range of commercial, strategic and financial positions across three continents over a 25 year period. Julie was also Chief Operating Officer and CFO and Executive Director of Burberry Group plc from 2017 to 2023, where her responsibilities included Finance, Transformation, Information Technology and oversight of cyber security. Julie is a Fellow of the Institute of Chartered Accountants and the Institute of Tax. External appointments Co-Chair, CFO Leadership Network, Accounting for Sustainability (part of the King Charles III Charitable Fund Group of Companies); Patron, Oxford University Women in Business; Non- Executive Director and Chair of the Audit Committee, Diageo plc (effective 5 August 2024). Elizabeth (Liz) McKee Anderson Independent Non-Executive Director Age: 66 Nationality: American Appointed: 1 September 2022 Skills and experience Liz brings significant experience in commercial biopharmaceuticals and is a seasoned biotech board member. Her significant experience in commercial biopharmaceuticals, both operationally and at Board level, as well as her deep understanding of the biotechnology sector and application of technology, are invaluable to GSK as a pure biopharma company. Before her current roles, Liz served as Worldwide Vice President and commercial leader in infectious diseases and vaccines and also for immunology and oncology at Janssen Pharmaceuticals, and as Vice President and General Manager at Wyeth Vaccines. Liz was also previously a Board member of Huntsworth Plc and a Board Member and Chair of the Science, Technology and Investment Committee of Bavarian Nordic A/S. Liz has a degree in Engineering and Technical Management and an MBA in Finance. External appointments Board Member, BioMarin Pharmaceutical, Inc; Board Member, Revolution Medicines, Inc; Board Member, Insmed, Inc; Trustee, The Wistar Institute; Director, Aro Biotherapeutics Company, a private company. Key Committee Chair Corporate Responsibility Science Nominations & Corporate Governance Audit & Risk Remuneration Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F The Board 99 S N AC R A R N

Charles Bancroft Senior Independent Non-Executive Director Age: 64 Nationality: American Appointed: 1 May 2020 Senior Independent Non-Executive Director from 18 July 2022 Skills and experience Charlie has a wealth of financial and management experience in global biopharma. Charlie retired from a successful career at Bristol Myers Squibb (BMS) in March 2020 where he held a number of leadership roles in commercial, strategy and finance. Beginning his career at BMS in 1984, he held positions of increasing responsibility within the finance organisation and had commercial operational responsibility for Latin America, Middle East, Africa, Canada, Japan and several Pacific Rim countries. He was appointed Chief Financial Officer in 2010, Chief Financial Officer and Executive Vice President, Global Business Operations in 2016 and Executive Vice President and Head of Integration and Strategy & Business Development in 2019. As Chief Financial Officer, Charlie had line management responsibility for Information Technology, including cyber security. Charlie successfully steered BMS through a period of strategic transformation, including its $74 billion acquisition of Celgene. Charlie also served as a member of the Board of Colgate-Palmolive Company from 2017 until March 2020. External appointments Board Member, Kodiak Sciences Inc; Board Member, BioVector Inc; Advisory Board Member, Drexel University’s LeBow College of Business. The Board determined that Charlie has recent and relevant financial experience and agreed that he has the appropriate qualifications and background to be an audit committee financial expert. Dr Hal Barron Non-Executive Director Age: 61 Nationality: American Appointed: 1 January 2018 Chief Scientific Officer and President, R&D from 1 April 2018 Transitioned to the role of Non-Executive Director on 1 August 2022 Skills and experience Hal has had a distinguished career in biosciences, with a strong track record of research and development (R&D). He joined the Board of GSK in 2018 as Chief Scientific Officer and President, R&D, where he brought a new approach to R&D which focused on science related to the immune system, the use of human genetics and advanced technologies to help identify the next generation of transformational medicines. In August 2022, he transitioned to a Non-Independent Non-Executive Director, with additional responsibilities to support R&D. Before joining GSK, Hal was President, R&D at Calico LLC (California Life Company), an Alphabet-funded company that uses advanced technologies to increase understanding of lifespan biology. Hal was previously Executive Vice President, Head of Global Product Development, and Chief Medical Officer of Roche, responsible for all the products in the combined portfolio of Roche and Genentech. At Genentech, he was Senior Vice President of Development and Chief Medical Officer. Hal was a Non-Executive Director and Chair of the Science & Technology Committee at Juno Therapeutics, Inc until March 2018, when it was acquired by Celgene Corporation. He previously served as a Non-Executive Board Director of GRAIL, Inc and an Advisory Board Member of Verily Life Sciences LLC. External appointments CEO and Board Co-Chair, Altos Labs Inc; Associate Adjunct Professor, Epidemiology & Biostatistics, University of California, San Francisco. Dr Anne Beal Independent Non-Executive Director Age: 61 Nationality: American Appointed: 6 May 2021 Skills and experience Anne brings extensive healthcare experience to the Board as a physician and entrepreneur, and combines this with a passion for patient advocacy. She is a recognised health policy expert in the development of global and national programmes for improving healthcare access for all patient groups and for ensuring the voice of patients is reflected in research programmes. Before her current roles, Anne spent six years at Harvard Medical School and Massachusetts General Hospital, where she was an instructor in paediatrics. She has also held leadership roles at the Commonwealth Fund and the Aetna Foundation. Anne was previously Deputy Executive Director and Chief Engagement Officer for The Patient-Centered Outcomes Research Institute in the US and Chief Patient Officer and Global Head of Patient Solutions at Sanofi. External appointments Founder and CEO, AbsoluteJOI Skincare; Board Member, AcademyHealth; Board Member, Prolacta Bioscience. Key Committee Chair Corporate Responsibility Science Nominations & Corporate Governance Audit & Risk Remuneration Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F The Board continued 100 A N R S RC N S N AC R

Wendy Becker Independent Non-Executive Director Age: 58 Nationality: American Appointed: 1 October 2023 Skills and experience Wendy is a highly experienced Non-Executive Director and has held significant leadership positions in a wide range of global businesses in public, private and non-profit sectors. She possesses a wealth of strategic and consumer marketing expertise in particular across the technology and life sciences sectors. Wendy has strong executive management experience, having been Chief Executive Officer at Jack Wills Limited, Group Chief Marketing Officer at Vodafone Group plc and Partner at McKinsey & Company. Wendy’s interest in science, healthcare and medical research dates to her time at McKinsey, where she worked with a range of healthcare clients in the US and Europe. This was furthered during the years that she served on the Board of Cancer Research UK. More recently, Wendy spent time as a Non-Executive Director of NHS England and as Chair of the British Heart Foundation. Wendy has held several Non-Executive Director roles, amongst others, as Chair of the Remuneration Committees of Great Portland Estates plc and Ocado Group plc and as a member of the Remuneration and Audit Committees of Whitbread plc. Through her current and prior roles in technology companies, Wendy adds to the Board’s experience in cyber security. External appointments Chair of Logitech International S.A.; Board member and Chair of the Compensation Committee, Sony Group Corporation; Senior Independent Director and Chair of the Remuneration Committee, Oxford Nanopore Technologies plc; Member of the governing bodies of the University of Oxford. Dr Harry (Hal) C Dietz Independent Non-Executive Director and Scientific & Medical Expert Age: 65 Nationality: American Appointed: 1 January 2022 Skills and experience Hal brings extensive experience in the field of human genetics which is central to GSK’s approach to R&D. He is a former President of the American Society of Human Genetics and is recognised as the world’s leading authority on the genetic disorder known as Marfan Syndrome. He also brings experience in developing novel therapies, particularly in relation to disease-modifying treatments for fibrotic and neurodegenerative diseases. In total, Hal has authored 282 original publications in peer-reviewed journals during his career. As a physician scientist, he has dedicated his entire career to the care and study of individuals with heritable connective tissue disorders with primary perturbations of extracellular matrix homeostasis and function. His lab has identified the genes for many of these conditions, for which he uses model systems to explain disease mechanisms. Hal has received many prestigious awards including the Curt Stern Award from the American Society of Human Genetics, the Colonel Harland Sanders Lifetime Achievement Award in Medical Genetics, the Taubman Prize for excellence in translational medical science, the Harrington Prize from the American Society for Clinical Investigation and the Harrington Discovery Institute, the Pasarow Award in Cardiovascular Research, the InBev-Baillet Latour Health Prize from Belgium, and the Research Achievement Award from the American Heart Association. He is an inductee of the American Society for Clinical Investigation, the American Association for the Advancement of Science, the Association of American Physicians, the National Academy of Medicine, and the National Academy of Sciences. External appointments Victor A. McKusick Professor of Paediatrics, Medicine, and Molecular Biology & Genetics in the Department of Genetic Medicine, The Johns Hopkins University School of Medicine; Investigator, Howard Hughes Medical Institute; Non-Executive Board Director, Altius Institute for Biomedical Sciences; Independent Chair, GSK’s Human Genetics Scientific Advisory Board. Key Committee Chair Corporate Responsibility Science Nominations & Corporate Governance Audit & Risk Remuneration Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F The Board continued 101 S S N AC R A R

Dr Jesse Goodman Independent Non-Executive Director and Scientific & Medical Expert Age: 72 Nationality: American Appointed: 1 January 2016 Skills and experience Jesse brings scientific and public health expertise to the Board’s deliberations. He has a wealth of experience spanning science, medicine, vaccines, regulation and public health, and has a proven record in addressing pressing public health needs in both the academic and federal sectors. Jesse previously served in senior leadership positions at the US Food and Drug Administration (FDA), including most recently as the FDA’s Chief Scientist and previously as Deputy Commissioner for Science and Public Health and as Director of the Center for Biologics Evaluation and Research (CBER). Jesse played a leadership role in developing the FDA’s Regulatory Science and Medical Countermeasures Initiatives and has worked collaboratively with industry, academia, government and global public health and regulatory partners to prepare for and respond to major public health threats, including emerging infectious diseases, disasters and terrorism. He led the FDA’s response to West Nile Virus and to the 2009 H1N1 influenza pandemic and served on the Senior Leadership Team for the 2010 White House Medical Countermeasure Review. Jesse was previously a member of both the Scientific Advisory Committee and the Regulatory and Legal Working Group of the Coalition for Epidemic Preparedness Innovations (CEPI). External appointments Professor of Medicine and Attending Physician, Infectious Diseases, Georgetown University and directs the Georgetown University Center on Medical Product Access, Safety and Stewardship (COMPASS); Board Member (formerly President), United States Pharmacopeia (USP); Board Member, Scientific Counselors for Infectious Diseases, Centers for Disease Control and Prevention (CDC); Board Member, Intellia Therapeutics Inc; Member, US National Academy of Medicine; Board Member, Adaptive Phage Therapeutics, Inc. Dr Jeannie Lee Independent Non-Executive Director and Scientific & Medical Expert Age: 59 Nationality: American Appointed: 4 March 2024 Skills and experience Jeannie is a pioneer in the field of RNA Biology and its application to drug development and therapeutics. In addition to senior leadership positions held at both Harvard Medical School and the Massachusetts General Hospital, Jeannie co-founded Translate Bio and Fulcrum Therapeutics, two biotech companies specialising in RNA and epigenetic therapies Jeannie is a Member of the National Academy of Sciences, the National Academy of Medicine, a Harrington Rare Disease Scholar of the Harrington Discovery Institute, a recipient of the Lurie Prize from the Foundation for the National Institutes of Health, an awardee of the Centennial Prize from the Genetics Society of America, the 2010 Molecular Biology Prize and the 2020 Cozzarelli Prize from the National Academy of Sciences, US, and a Fellow of the American Association for the Advancement of Science. She has also served on the Board of the Genetics Society of America. External appointments Endowed Chair in Molecular Biology and Professor of Genetics (and Pathology), Harvard Medical School; Acting Chair of the Department of Molecular Biology, Massachusetts General Hospital; Member of Scientific Advisory Board, Skyhawk Therapeutics, Inc.; Founder of and advisor to Fulcrum Therapeutics, Inc. Key Committee Chair Corporate Responsibility Science Nominations & Corporate Governance Audit & Risk Remuneration Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F The Board continued 102 S C S C C S N A R

Urs Rohner Independent Non-Executive Director Age: 64 Nationality: Swiss Appointed: 1 January 2015 Skills and experience Urs has a broad business, banking and legal background and extensive senior level experience at multinational companies. Urs has served as Chairman on a number of Boards, most recently for Credit Suisse Group from 2011 until April 2021. Before joining Credit Suisse in 2004, Urs served as Chairman of the Executive Board and CEO of ProSieben and ProSiebenSat.1 Media AG. This followed a number of years in private practice at major law firms in Switzerland and the US, having been admitted to the bars of the canton of Zurich in Switzerland in 1986 and the state of New York in the US in 1990. As a founding partner and Chair of Vega Cyber Associates AG, he brings current technology and cybersecurity experience to the Board, further supplemented by digital transformation during his time as Chair of Credit Suisse. External appointments Member, International Advisory Board, Investcorp; Chair, Vega Cyber Associates AG. Dr Vishal Sikka Independent Non-Executive Director Age: 56 Nationality: American Appointed: 18 July 2022 Skills and experience Vishal has a distinguished background in technology, particularly in Artificial Intelligence (AI) and Machine Learning (ML), which are central to GSK’s approach to R&D. He also brings a deep understanding of cyber security to the Board. He is the founder and CEO of Vianai Systems, Inc, a Silicon Valley-based company that provides advanced technological software and services in AI and ML to large enterprises around the world. Before founding Vianai Systems in 2019, Vishal served as CEO of Infosys Limited, where he led an innovative strategy to help clients renew existing IT landscapes, using automation, design thinking and next-generation technologies to transform customer experiences. He also served as a member of the Executive Board of SAP SE, prior to which he was its Chief Technology Officer. Vishal has a PhD in AI from Stanford University and has co-authored several research abstracts related to AI, technology and database management. External appointments Founder and CEO, Vianai Systems, Inc; Board Member, Oracle Corporation; Member, Supervisory Board, BMW AG; Member of the Advisory Board of Stanford University's AI Center (Center for Human-Centered Artificial Intelligence). Key Committee Chair Corporate Responsibility Science Nominations & Corporate Governance Audit & Risk Remuneration Directors departing during 2023 Iain Mackay 14 January 2019 to 1 May 2023 Stepped down from the Board on 1 May and retired from the company on 31 December 2023. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F The Board continued 103 R A N C S N AC R
Skills and experience Emma Walmsley Chief Executive Officer Emma joined GSK in 2010 and the GLT in 2011. See Board biographies on pages 99 to 103. Julie Brown Chief Financial Officer Julie joined GSK and the GLT in 2023. See Board biographies on pages 99 to 103. Diana Conrad Chief People Officer Diana was appointed Chief People Officer and member of the GLT in April 2019. She was previously Senior Vice President, HR, Pharmaceuticals R&D from 2016 where she played a key strategic role as leader of the R&D people and culture agenda to support its transformation. Diana joined GSK Canada’s HR team in 2000 where she held several roles of increasing responsibility before becoming Senior Vice President, HR for Consumer Healthcare in 2009. Prior to joining GSK, she held HR roles in companies including GE Capital, Gennum Corporation and Zenon Environmental Laboratories. Diana has an Honours Bachelor of Arts from McMaster University in Canada. James Ford SVP & Group General Counsel, Legal and Compliance James joined the GLT in 2018, when he was appointed Senior Vice President and Group General Counsel, later taking responsibility for Compliance, Corporate Security and Investigations in 2021. He joined GSK in 1995 and has served as General Counsel Consumer Healthcare, General Counsel Global Pharmaceuticals, Vice President of Corporate Legal and was Acting Head of Global Ethics and Compliance. Prior to GSK, James was a solicitor at Clifford Chance and DLA. He holds a law degree from the University of East Anglia and a Diploma in Competition Law from King's College. He is qualified as a solicitor in England and Wales and is an attorney at the New York State Bar. James is based in London and has practised law and lived in the US, Singapore and Hong Kong. James was co-chair of the US-based Civil Justice Reform Group 2019-2022, and is a director of the European General Counsel Association and the Association of Corporate Counsel. Sally Jackson SVP, Global Communications and CEO Office Sally joined the GLT in March 2019 as Senior Vice President, Global Communications and CEO Office. She leads our Communications and Government Affairs function globally and is also the CEO’s Chief of Staff. Prior to this, Sally was Senior Vice President, Office of the CEO and CFO and she previously served as Head of Investor Relations. She joined GSK in 2001. Sally holds a degree in Natural Sciences from the University of Cambridge. Luke Miels Chief Commercial Officer Luke joined GSK and the GLT in 2017. As Chief Commercial Officer he is responsible for our commercial portfolio of medicines and vaccines. Luke also co-chairs the Portfolio Investment Board with Tony Wood and is a member of the ViiV Healthcare Board. Outside of GSK, Luke is a member of the Singapore Economic Development Board. He previously worked for AstraZeneca as Executive Vice President of their European business and, prior to that, was Executive Vice President of Global Product and Portfolio Strategy, Global Medical Affairs and Corporate Affairs. Before that, he was head of Asia for Roche, based in Shanghai and then Singapore. Prior to that he held roles of increasing seniority at Roche and Sanofi-Aventis in the US, Europe and Asia. Luke holds a Bachelor of Science degree in Biology from Flinders University in Adelaide and a MBA from the Macquarie University, Sydney. Shobie Ramakrishnan Chief Digital and Technology Officer Shobie joined the GLT in 2021 when she was appointed Chief Digital and Technology Officer. She joined GSK in 2018 and has deep and broad experience in both biotech and hi-tech companies and, most recently, has led Digital and Technology for GSK’s Global Commercial organisation, transforming the company’s capabilities in digital, data and analytics and playing a pivotal role in establishing a more agile commercial operating model. Before joining GSK, Shobie held senior technology leadership roles in organisations including AstraZeneca, Salesforce, Genentech and Roche. She is a Non-Executive Director at Deliveroo. She is Board Member Emeritus at SustainableIT.org and was formerly a member of the board of directors at Remediant. Shobie holds a Bachelor’s degree in Electronics Engineering from Vellore Institute of Technology, University of Madras, India. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F GSK Leadership Team (GLT) 104
Skills and experience David Redfern President, Corporate Development David joined the GLT as Chief Strategy Officer in 2008 and is responsible for corporate development and strategic planning. Previously, he was Senior Vice President, Northern Europe with responsibility for GSK’s pharmaceutical businesses in that region and, before that, he was Senior Vice President for Central and Eastern Europe. He joined GSK in 1994. David was appointed Chairman of the Board of ViiV Healthcare Limited in 2011 and a Non-Executive Director of the Aspen Pharmacare Holdings Limited Board in 2015. He has a Bachelor of Science degree from Bristol University and is a Chartered Accountant. Regis Simard President, Global Supply Chain Regis joined the GLT in 2018, when he became President, Pharmaceuticals Supply Chain. He is responsible for the manufacturing and supply of GSK’s medicines and vaccines. In addition, he leads Quality and Environment, Health, Safety and Sustainability at a corporate level. Regis joined GSK in 2005 as a Site Director in France, rising to become Senior Vice President of Global Pharmaceuticals Manufacturing before his current role. Previously, he held senior positions at Sony, Konica Minolta and Tyco Healthcare. He is a member of the Board of ViiV Healthcare. He is a mechanical engineer and holds an MBA. Phil Thomson President, Global Affairs Phil joined the GLT in 2011. He was appointed President, Global Affairs in 2017, and has responsibility for the Group’s strategic approach to stakeholder engagement, reputation and policy development. Previously, Phil was Senior Vice President, Communications and Government Affairs. He joined Glaxo Wellcome as a commercial trainee in 1996. Phil holds a degree in English, History and Russian Studies from Durham University. Deborah Waterhouse CEO, ViiV Healthcare and President, GSK Global Health Deborah was appointed to the GLT in January 2020. She became Chief Executive Officer of ViiV Healthcare in April 2017. In addition to ViiV, Deborah also leads GSK’s Global Health organisation. Deborah joined GSK in 1996 and, prior to ViiV, was the Senior Vice President of Primary Care within GSK’s US business. She has a strong track record of performance in both specialty and primary care. Deborah led the HIV business in the UK before heading the HIV Centre of Excellence for Pharma Europe and held roles as General Manager of Australia and New Zealand and Senior Vice President for Central and Eastern Europe. Deborah is a Non-Executive Director of Schroders plc and holds a degree in Economic History and English Literature from Liverpool University. Tony Wood Chief Scientific Officer Tony was appointed Chief Scientific Officer (CSO), Head of R&D and a member of GLT on 1 August 2022, following his appointment as CSO designate on 19 January 2022. He joined GSK from Pfizer in 2017 as Senior Vice President, Medicinal Science and Technology, responsible for all science and technology platforms driving the delivery of new innovation. Tony has led large-scale global organisations in drug discovery and development in multiple therapeutic areas, including immunology, oncology and infectious diseases. During his time at Pfizer, Tony was responsible for the invention of a new antiretroviral medication used to treat HIV infection. He is a Fellow of the Academy of Medical Sciences, an Honorary Fellow of the Royal Society of Chemistry (RSC), the highest honour given by the RSC, and a Fellow of the Royal Society of Biology. Tony has a BSc in chemistry and PhD in organic synthesis from the University of Newcastle, and was a postdoctoral fellow at Imperial College, London. He is also currently a visiting professor at IMCM Oxford. GLT members departing during 2023 (1) Iain Mackay was a member of the GLT and CFO until 1 May 2023. He stepped down from the Board on 1 May and retired from the company on 31 December 2023. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F GSK Leadership Team (GLT) continued 105
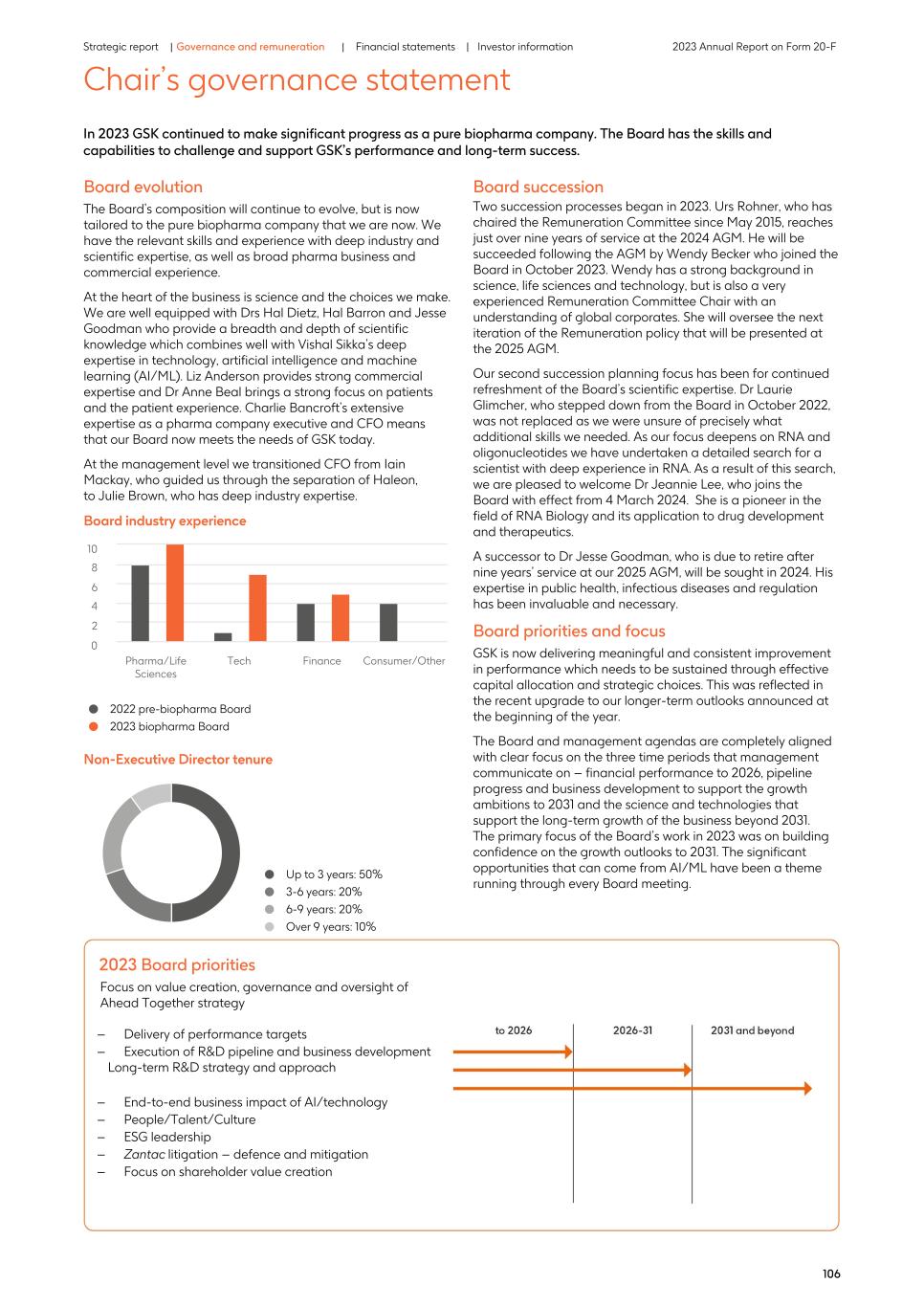
Board evolution The Board’s composition will continue to evolve, but is now tailored to the pure biopharma company that we are now. We have the relevant skills and experience with deep industry and scientific expertise, as well as broad pharma business and commercial experience. At the heart of the business is science and the choices we make. We are well equipped with Drs Hal Dietz, Hal Barron and Jesse Goodman who provide a breadth and depth of scientific knowledge which combines well with Vishal Sikka’s deep expertise in technology, artificial intelligence and machine learning (AI/ML). Liz Anderson provides strong commercial expertise and Dr Anne Beal brings a strong focus on patients and the patient experience. Charlie Bancroft’s extensive expertise as a pharma company executive and CFO means that our Board now meets the needs of GSK today. At the management level we transitioned CFO from Iain Mackay, who guided us through the separation of Haleon, to Julie Brown, who has deep industry expertise. Board industry experience Pharma/Life Sciences Tech Finance Consumer/Other 0 2 4 6 8 10 l 2022 pre-biopharma Board l 2023 biopharma Board Non-Executive Director tenure l Up to 3 years: 50% l 3-6 years: 20% l 6-9 years: 20% l Over 9 years: 10% 2023 Board priorities Focus on value creation, governance and oversight of Ahead Together strategy – Delivery of performance targets – Execution of R&D pipeline and business development Long-term R&D strategy and approach – End-to-end business impact of AI/technology – People/Talent/Culture – ESG leadership – Zantac litigation – defence and mitigation – Focus on shareholder value creation Board succession Two succession processes began in 2023. Urs Rohner, who has chaired the Remuneration Committee since May 2015, reaches just over nine years of service at the 2024 AGM. He will be succeeded following the AGM by Wendy Becker who joined the Board in October 2023. Wendy has a strong background in science, life sciences and technology, but is also a very experienced Remuneration Committee Chair with an understanding of global corporates. She will oversee the next iteration of the Remuneration policy that will be presented at the 2025 AGM. Our second succession planning focus has been for continued refreshment of the Board’s scientific expertise. Dr Laurie Glimcher, who stepped down from the Board in October 2022, was not replaced as we were unsure of precisely what additional skills we needed. As our focus deepens on RNA and oligonucleotides we have undertaken a detailed search for a scientist with deep experience in RNA. As a result of this search, we are pleased to welcome Dr Jeannie Lee, who joins the Board with effect from 4 March 2024. She is a pioneer in the field of RNA Biology and its application to drug development and therapeutics. A successor to Dr Jesse Goodman, who is due to retire after nine years’ service at our 2025 AGM, will be sought in 2024. His expertise in public health, infectious diseases and regulation has been invaluable and necessary. Board priorities and focus GSK is now delivering meaningful and consistent improvement in performance which needs to be sustained through effective capital allocation and strategic choices. This was reflected in the recent upgrade to our longer-term outlooks announced at the beginning of the year. The Board and management agendas are completely aligned with clear focus on the three time periods that management communicate on – financial performance to 2026, pipeline progress and business development to support the growth ambitions to 2031 and the science and technologies that support the long-term growth of the business beyond 2031. The primary focus of the Board’s work in 2023 was on building confidence on the growth outlooks to 2031. The significant opportunities that can come from AI/ML have been a theme running through every Board meeting. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Chair’s governance statement In 2023 GSK continued to make significant progress as a pure biopharma company. The Board has the skills and capabilities to challenge and support GSK’s performance and long-term success. 106

The Board supported the modular communication plan for 2023, which included deep insights into vaccines and infectious disease, HIV, respiratory, immunology and oncology. The Board reviewed all of the strategies and priorities prior to communication to the market. In terms of business development, the Board and Science Committee work alongside Emma and the management team to understand the scientific rationale, competitiveness of the asset under consideration and potential returns and value creation. This was a significant activity of the Board in 2023. Board visits are an important element of our Board programme. In March the Board spent three days visiting our Vaccines site in Wavre, Belgium. Board members had a deep immersion in the vaccines business and the work at the site and were inspired by the passion and commitment of the group of around 150 employees they spent time with during the visit. Similarly, the Board will be holding its March meeting in 2024 in North Carolina for an immersive briefing on our HIV business. R&D progress and Technology The longer-term future of the company comes from deep sustainable productivity of internal and externally sourced R&D and from our investment in technology. The path we set out on five years ago was routed in our commitment to transform our productivity through the use of technology. Last year the Board’s R&D updates centred on antibody drug conjugates, Oligonucleotides, AMR, Vaccines and RNA and liver disease. These discussions were supported and validated by prior deep-dives by the Science Committee. Embracing the potential of AI/ML in every part of the business is crucial to our medium and long-term success. We deliberately have a wealth of tech experience on the Board ranging from Dr Hal Barron’s R&D experience at Verily and Google, to Vishal Sikka’s unique tech vantage point and expertise in AI and ML. Our CEO also brings unique insights from her role at Microsoft, along with my own experience of the use of technology in biotechs and through the UK’s national genomics programmes. Collectively the deep appreciation of the tremendous potential that technology can unlock give us the reassurance to execute with confidence. While our biggest investment has of course been in R&D, every part of GSK now has technology built into optimising their priorities. Culture & responsibility The Board receives regular briefings on our people, talent and culture. At every Board interaction, wherever we are, the Board meets between 50 to 100 members of local employee talent. This enables us to get a first hand impression of our culture and the mood of employees and to hear their views of the company. Similarly, wherever I go in GSK, and this year my travels included the US, China, the Middle East and Europe, I take the opportunity to meet with local employees at all levels in small groups. It is impressive to hear those I meet all talk with pride in our purpose and our mission towards prevention and improved human health. ESG continues to be right at the very heart of GSK and its ambition. We are particularly proud of the progress that we are making in DEI in terms of our people, and in the diversity of our clinical trials. Shareholder perspectives and engagement The Board and I believe in the importance of maintaining a continuous level of engagement with shareholders. During the year I continued to meet with a range of investors; combined they represented approximately 30% of our share register. This year all our Non-Executive Board members attended our Annual Governance Meeting to hear shareholders views first hand. The feedback shareholders provide is invaluable to the shaping of the Board’s work. We appreciate the clarity and efficiency that direct engagement brings and we continue to welcome the opportunity to engage with investors directly on all aspects of GSK and the Board’s work. We welcome the approach taken by the Financial Reporting Council in its updated UK Corporate Governance Code to encourage Boards to be bolder in choosing the right approach for their business and explaining why it is important to do so if necessary. Shareholder value We have made progress in 2023 but there is, and will always be, more to do. The key to improved returns is consistency of performance, and consistency in communication. During 2023, I believe management delivered on both, but this all has to be translated into sustained shareholder value creation. We are acutely aware that has not happened yet. In terms of the Zantac litigation, the Board is deeply involved in the overall strategy with the CEO and General Counsel. In addition, every quarter the Audit & Risk Committee reviews the disclosures with our Auditor to ensure that they are complete, fair and that the accounting judgments are appropriate. I believe 2023 was a year of significant progress across all of the time periods to 2026, 2026 to 2031 and beyond 2031. We have a clear and aligned work programme for 2024. The Board is very different to what it was two years ago. I am really delighted not just with the progress Emma and the management team have made, but the performance of the Board too. I encourage you to read my Board colleagues' committee updates (which follow on pages 116 to 126) and provide greater detail on their work during 2023. Thank you for your continued support and I look forward to connecting with you during the year, whether at our Annual General Meeting in May, or otherwise. Sir Jonathan Symonds Chair Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Chair’s governance statement continued 107
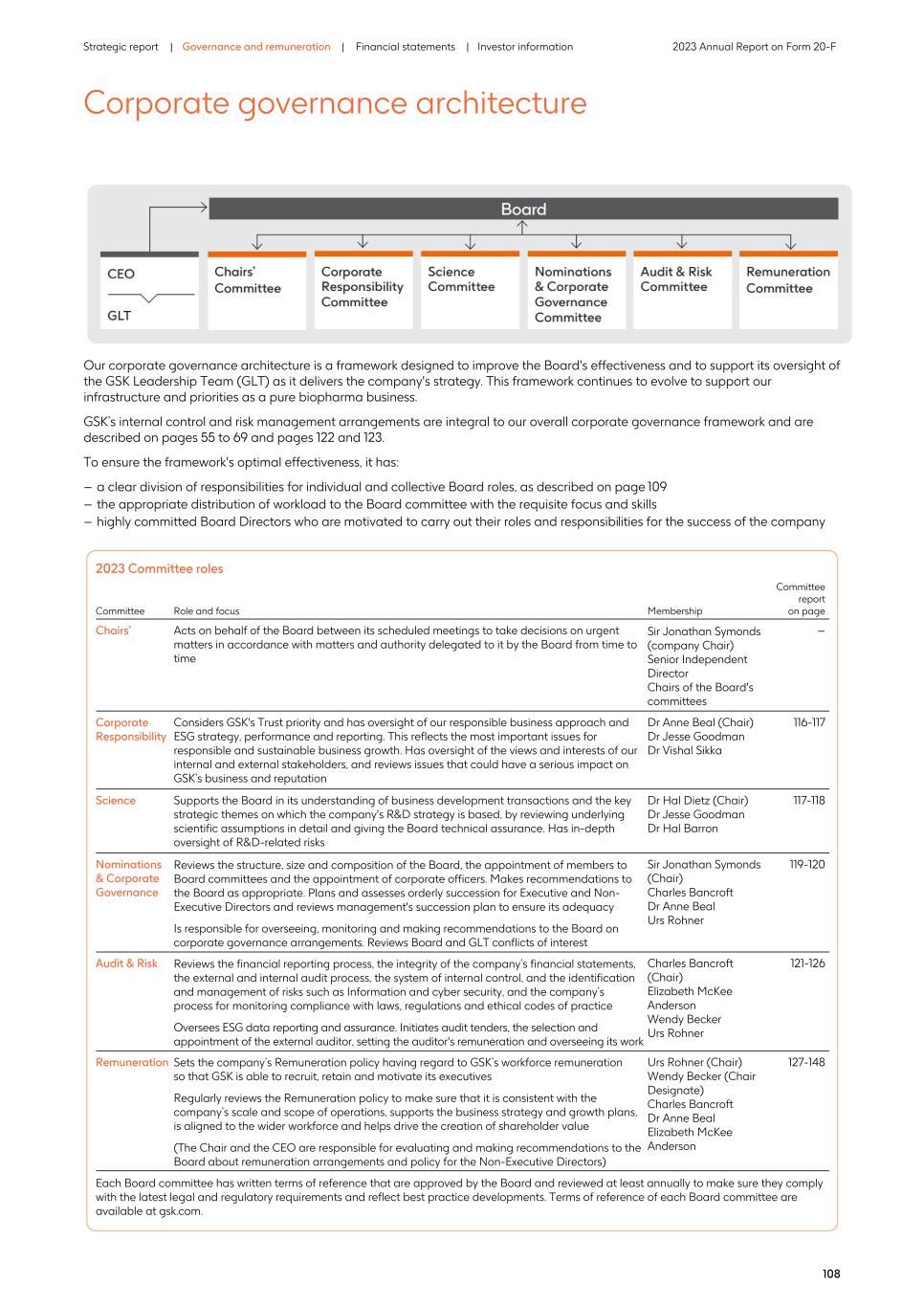
Our corporate governance architecture is a framework designed to improve the Board's effectiveness and to support its oversight of the GSK Leadership Team (GLT) as it delivers the company's strategy. This framework continues to evolve to support our infrastructure and priorities as a pure biopharma business. GSK’s internal control and risk management arrangements are integral to our overall corporate governance framework and are described on pages 55 to 69 and pages 122 and 123. To ensure the framework's optimal effectiveness, it has: – a clear division of responsibilities for individual and collective Board roles, as described on page 109 – the appropriate distribution of workload to the Board committee with the requisite focus and skills – highly committed Board Directors who are motivated to carry out their roles and responsibilities for the success of the company Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Corporate governance architecture 108 2023 Committee roles Committee Role and focus Membership Committee report on page Chairs’ Acts on behalf of the Board between its scheduled meetings to take decisions on urgent matters in accordance with matters and authority delegated to it by the Board from time to time Sir Jonathan Symonds (company Chair) Senior Independent Director Chairs of the Board's committees – Corporate Responsibility Considers GSK's Trust priority and has oversight of our responsible business approach and ESG strategy, performance and reporting. This reflects the most important issues for responsible and sustainable business growth. Has oversight of the views and interests of our internal and external stakeholders, and reviews issues that could have a serious impact on GSK’s business and reputation Dr Anne Beal (Chair) Dr Jesse Goodman Dr Vishal Sikka 116-117 Science Supports the Board in its understanding of business development transactions and the key strategic themes on which the company's R&D strategy is based, by reviewing underlying scientific assumptions in detail and giving the Board technical assurance. Has in-depth oversight of R&D-related risks Dr Hal Dietz (Chair) Dr Jesse Goodman Dr Hal Barron 117-118 Nominations & Corporate Governance Reviews the structure, size and composition of the Board, the appointment of members to Board committees and the appointment of corporate officers. Makes recommendations to the Board as appropriate. Plans and assesses orderly succession for Executive and Non- Executive Directors and reviews management's succession plan to ensure its adequacy Is responsible for overseeing, monitoring and making recommendations to the Board on corporate governance arrangements. Reviews Board and GLT conflicts of interest Sir Jonathan Symonds (Chair) Charles Bancroft Dr Anne Beal Urs Rohner 119-120 Audit & Risk Reviews the financial reporting process, the integrity of the company’s financial statements, the external and internal audit process, the system of internal control, and the identification and management of risks such as Information and cyber security, and the company’s process for monitoring compliance with laws, regulations and ethical codes of practice Oversees ESG data reporting and assurance. Initiates audit tenders, the selection and appointment of the external auditor, setting the auditor's remuneration and overseeing its work Charles Bancroft (Chair) Elizabeth McKee Anderson Wendy Becker Urs Rohner 121-126 Remuneration Sets the company’s Remuneration policy having regard to GSK’s workforce remuneration so that GSK is able to recruit, retain and motivate its executives Regularly reviews the Remuneration policy to make sure that it is consistent with the company’s scale and scope of operations, supports the business strategy and growth plans, is aligned to the wider workforce and helps drive the creation of shareholder value (The Chair and the CEO are responsible for evaluating and making recommendations to the Board about remuneration arrangements and policy for the Non-Executive Directors) Urs Rohner (Chair) Wendy Becker (Chair Designate) Charles Bancroft Dr Anne Beal Elizabeth McKee Anderson 127-148 Each Board committee has written terms of reference that are approved by the Board and reviewed at least annually to make sure they comply with the latest legal and regulatory requirements and reflect best practice developments. Terms of reference of each Board committee are available at gsk.com.

Leadership Chair Jonathan Symonds – leads and manages the business of the Board – provides direction and focus – ensures a clear structure for the Board and its committees to operate effectively – maintains a dialogue with shareholders about the governance of the company – sets the Board agenda and ensures sufficient time is allocated to promote effective debate to support sound decision-making – ensures the Board receives accurate, timely and clear information – meets regularly with each Non-Executive Director to discuss individual contributions and performance, and training and development needs – shares peer feedback that is provided as part of the Board evaluation process – meets regularly with all the Non-Executive Directors independently of the Executive Directors + The Chair’s role description is available at gsk.com Chief Executive Officer Emma Walmsley – manages the Group and its business – develops the Group’s strategic direction for the Board's consideration and approval – implements the agreed strategy – is supported by the GLT – maintains a continuous dialogue with shareholders in respect of the company’s performance + The Chief Executive Officer’s role description is available at gsk.com Independent oversight and rigorous challenge Senior Independent Director Charles Bancroft – acts as a sounding board for the Chair and a trusted intermediary for other Directors – together with the Non-Executive Directors, leads the annual review of the Chair’s performance, taking into account the views of the Executive Directors – discusses the results of the Chair’s effectiveness review with the Chair – leads the search and appointment process and makes the recommendation to the Board for a new Chair – acts as an additional point of contact for shareholders. Maintains an understanding of their issues and concerns through meetings with shareholders and briefings from the Company Secretary and Investor Relations + GSK's Senior Independent Non-Executive Director’s role description is available at gsk.com Non-Executive Directors – provide a strong independent element to the Board – constructively support and challenge management and scrutinise its performance in achieving agreed deliverables – shape proposals about strategy and offer specialist advice to management – each has a letter of appointment setting out the terms and conditions of their directorship – devote such time as is necessary to the proper performance of their duties – are expected to attend all meetings as required Independence statement The Board considers all its Non-Executive Directors who are identified on pages 99 to 103 – except Dr Hal Barron – to be independent after being assessed against Provision 10 of the Financial Reporting Council's (FRC) UK Corporate Governance Code (Code) + GSK's Non-Executive Directors' role description is available at gsk.com Company Secretary Victoria Whyte – is secretary to the Board and all Board committees – supports the Board and Committee Chairs to plan future agendas and annual programmes – ensures information is made available to Board members in a timely fashion – supports the Chair to design and deliver Board inductions – coordinates continuing business awareness and training for the Non-Executive Directors – undertakes internal Board and committee evaluations at the Chair's request – advises the Directors on Board practice and procedures and corporate governance matters – chairs the Group's Disclosure Committee – operates a Board-approved appointments policy that reflects the Board and external appointment requirements of the Code – is a point of contact for shareholders on all corporate governance matters Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Corporate governance architecture continued 109
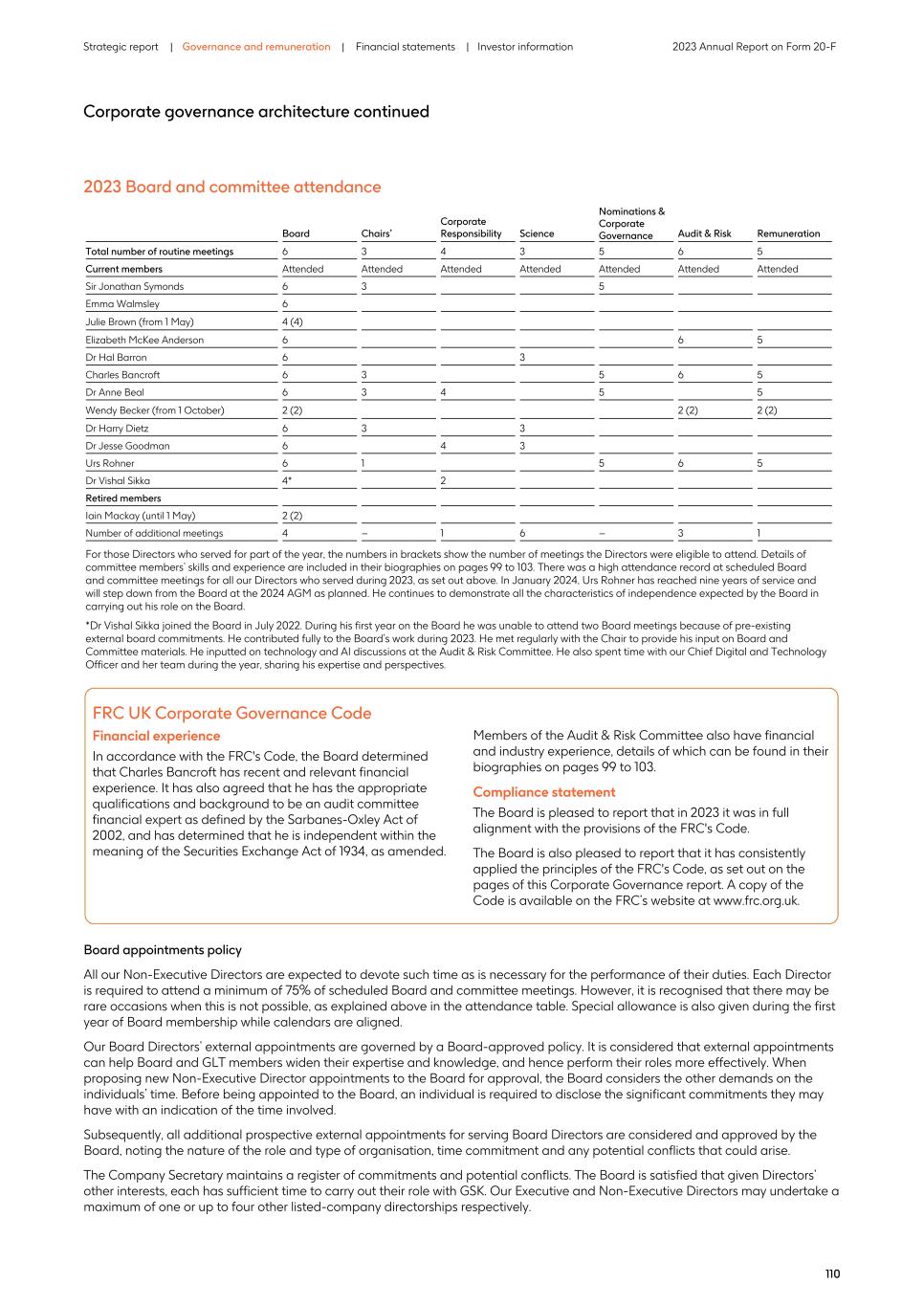
2023 Board and committee attendance FRC UK Corporate Governance Code Financial experience In accordance with the FRC's Code, the Board determined that Charles Bancroft has recent and relevant financial experience. It has also agreed that he has the appropriate qualifications and background to be an audit committee financial expert as defined by the Sarbanes-Oxley Act of 2002, and has determined that he is independent within the meaning of the Securities Exchange Act of 1934, as amended. Members of the Audit & Risk Committee also have financial and industry experience, details of which can be found in their biographies on pages 99 to 103. Compliance statement The Board is pleased to report that in 2023 it was in full alignment with the provisions of the FRC's Code. The Board is also pleased to report that it has consistently applied the principles of the FRC's Code, as set out on the pages of this Corporate Governance report. A copy of the Code is available on the FRC’s website at www.frc.org.uk. Board appointments policy All our Non-Executive Directors are expected to devote such time as is necessary for the performance of their duties. Each Director is required to attend a minimum of 75% of scheduled Board and committee meetings. However, it is recognised that there may be rare occasions when this is not possible, as explained above in the attendance table. Special allowance is also given during the first year of Board membership while calendars are aligned. Our Board Directors’ external appointments are governed by a Board-approved policy. It is considered that external appointments can help Board and GLT members widen their expertise and knowledge, and hence perform their roles more effectively. When proposing new Non-Executive Director appointments to the Board for approval, the Board considers the other demands on the individuals’ time. Before being appointed to the Board, an individual is required to disclose the significant commitments they may have with an indication of the time involved. Subsequently, all additional prospective external appointments for serving Board Directors are considered and approved by the Board, noting the nature of the role and type of organisation, time commitment and any potential conflicts that could arise. The Company Secretary maintains a register of commitments and potential conflicts. The Board is satisfied that given Directors’ other interests, each has sufficient time to carry out their role with GSK. Our Executive and Non-Executive Directors may undertake a maximum of one or up to four other listed-company directorships respectively. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Corporate governance architecture continued 110 Board Chairs’ Corporate Responsibility Science Nominations & Corporate Governance Audit & Risk Remuneration Total number of routine meetings 6 3 4 3 5 6 5 Current members Attended Attended Attended Attended Attended Attended Attended Sir Jonathan Symonds 6 3 5 Emma Walmsley 6 Julie Brown (from 1 May) 4 (4) Elizabeth McKee Anderson 6 6 5 Dr Hal Barron 6 3 Charles Bancroft 6 3 5 6 5 Dr Anne Beal 6 3 4 5 5 Wendy Becker (from 1 October) 2 (2) 2 (2) 2 (2) Dr Harry Dietz 6 3 3 Dr Jesse Goodman 6 4 3 Urs Rohner 6 1 5 6 5 Dr Vishal Sikka 4* 2 Retired members Iain Mackay (until 1 May) 2 (2) Number of additional meetings 4 – 1 6 – 3 1 For those Directors who served for part of the year, the numbers in brackets show the number of meetings the Directors were eligible to attend. Details of committee members’ skills and experience are included in their biographies on pages 99 to 103. There was a high attendance record at scheduled Board and committee meetings for all our Directors who served during 2023, as set out above. In January 2024, Urs Rohner has reached nine years of service and will step down from the Board at the 2024 AGM as planned. He continues to demonstrate all the characteristics of independence expected by the Board in carrying out his role on the Board. *Dr Vishal Sikka joined the Board in July 2022. During his first year on the Board he was unable to attend two Board meetings because of pre-existing external board commitments. He contributed fully to the Board’s work during 2023. He met regularly with the Chair to provide his input on Board and Committee materials. He inputted on technology and AI discussions at the Audit & Risk Committee. He also spent time with our Chief Digital and Technology Officer and her team during the year, sharing his expertise and perspectives.
The Board carries out its responsibilities through an annual programme of meetings The Board seeks to optimise its effectiveness by setting its annual meeting programme to focus on priorities agreed for the year to support delivery of the company's short-, medium- and long-term strategy. The Board and its committees' programmes of work are set to complement each other and avoid unnecessary duplication. During the year the Board received papers and presentations and actively discussed progress with management and our people. These materials and discussions help the Board make effective decisions, and contribute to its oversight of business performance and ensure good governance. The key areas the Board considered in 2023 are highlighted below: Areas of focus in 2023 The Board's work in 2023 included: Building momentum as a pure biopharma company Overseeing GSK as a pure biopharma business and delivery of performance included: – setting and approving the Board's 2023-2024 priorities – discussing and scrutinising strategic plans for GSK and assessing the potential to upgrade our longer-term outlook – scrutinising updates on R&D strategy, progress and progression of the company's pipeline – discussing GSK's overall commercial strategy and in particular for China – discussing end-to-end business opportunities and the impact of AI and other advanced technologies for performance and patients Ahead Together – further strengthening the fundamentals of value creation Overseeing the fundamentals of commercial execution, cost-base management, capital allocation, pipeline and culture included: – receiving regular reports from the CEO, CFO and CSO including the assessment of delivery of performance targets – receiving updates on R&D strategy, approach and pipeline progress – assessing the product area strategy reports on Vaccines, Speciality Care (including HIV), Oncology and General Medicines – reviewing GSK's capital allocation priorities to ensure investment for growth to deliver improved returns for shareholders – evaluating business development transactions, acquisitions and strategic partnerships with third parties including BELLUS Health, Zhifei, Hansoh, Aiolos Bio, Arrowhead Pharmaceuticals and Janssen Pharmaceuticals – scrutinising the Group's financial performance, shareholder value creation and development of Investor Relations Roadmap – reviewing Zantac litigation strategy – approving the monetisation of the retained shares in Haleon post demerger of the Consumer Healthcare business Enhancing ESG leadership Overseeing culture and embedding ESG at our core included: – assessing ESG performance and reviewed plans for low-carbon Ventolin, including clinical and non-clinical data available to support regulatory submissions – approving the ESG Performance Report – oversight of the company's Pricing and Access Policy principles – reviewing stakeholder perception research Regular corporate governance oversight The Board’s programme of governance included: – reviewing the quarterly financial results, dividend proposals, earnings guidance, investor materials, results announcements and 2022 Annual Report and Form 20F and receiving related reports from the external auditor – setting the annual budget and the forward-looking three-year plan and long-range forecast – conducting an annual review of the enterprise risk responsibility framework and enterprise-wide risks – undertaking an annual Board evaluation and implementing its agreed outcomes – receiving reports on Board committee work and reviewing and continuing to evolve the Board’s governance architecture – evaluating the CEO’s 2023 performance, and setting her 2024 objectives – reviewing culture, talent and succession plans annually – engaging with GSK's stakeholders and people to gather and understand their views about the company’s activities, operations and culture – reviewing the employee pulse survey results – receiving reports on wider corporate governance and regulatory developments, and the Company Secretary’s report – approving the company's modern slavery statement and gender pay gap positioning Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Ahead Together – Board oversight 111

Board performance The Board evaluates its performance, and that of its committees, every year. The evaluation is normally carried out externally every third year. The most recent external evaluation was facilitated in 2022 by Jan Hall of No 4, a business advisory company that does not have any other connection with GSK. The 2023 Board and committee evaluation was conducted internally by the Company Secretary who: – provided a questionnaire to Board members – drew together responses and themes from the responses to discuss outcomes and recommendations with each Committee Chair – following discussion with each committee and the Board as a whole, identified areas of focus and improvement for the Board and committees, which are set out below Action points After due consideration and discussion, the following action points to further improve performance in 2024 were agreed: – the key priorities for the Board's focus and programme of meetings for the year ahead – given the fundamental importance of culture, the Board wished to ensure it too fully embodied GSK's culture and would therefore undertake the culture training provided to employees – additional opportunities to increase informal engagement between the Board and management – the removal of unnecessary duplication in the Board and its committees' work would be continued. This programme aims to further simplify papers and create time to have deeper discussions in meetings Board committee evaluations The review of the Board committees focused on potential opportunities to further support GSK's momentum as a pure biopharma company, to help remove duplication and support the delivery of the Board's priorities identified for 2024. In addition, each committee reviewed its committee members' tenure, expertise and diversity. Each committee was considered to have operated effectively and the following enhancements were agreed: – Corporate Responsibility Committee: has a wide remit and was performing well. Consideration would be given to additional routes to identify potential emerging issues within the Committee's area of responsibility for its review. In addition, the Committee would continue to seek external perspectives to provide challenge – Science Committee: was working effectively. Opportunities to further enhance effectiveness were considered. In particular the Committee's 2024 programme would focus on R&D's Tech strategy. The capacity to undertake more deep dives on specific areas of R&D activity and to input earlier into new projects would be explored – Nominations & Corporate Governance Committee: was working effectively. A successor to Dr Jesse Goodman was being sought ahead of his retirement from the Board in 2025. The Committee would undertake a review of the Board and committee architecture and membership in 2024 to ensure it remained aligned to Board priorities – Audit & Risk Committee: was considered to be effective. The work to appropriately streamline material reviewed by the Committee has made good progress and will continue as an area of opportunity. In the year ahead the Committee will also continue to give focus on tech, cyber security and the use of AI – Remuneration Committee: had operated effectively during 2023 despite a challenging environment. The focus for 2024 would be to determine the right business imperatives for GSK's next remuneration policy to ensure it was globally competitive and rewarded delivery of outperformance Chair's evaluation The Senior Independent Director (SID) carried out the Chair's evaluation. He sought feedback on the Chair's performance from the Directors individually and collectively. From this review, they concluded that the Chair was leading the Board appropriately and effectively. The Chair and SID discussed the results of the review. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Ahead Together – Board oversight continued 112

Prioritising continuous engagement Our stakeholders rightly have high expectations of us, and the company's dynamic operating environment presents many challenges and opportunities. As a Board we aim to make sure that being commercially successful is balanced and aligned with meeting our stakeholders’ expectations, upholding our reputation, maintaining our licence to operate and building trust. We engage with or are briefed about our stakeholders' views to make sure we identify and respond to their expectations effectively and appropriately. How we engage with our main stakeholder groups – including patients, shareholders, consumers, customers and our people – across the company is covered in the pages of the Strategic report. Patients and our people are two stakeholders at the heart of our culture, with all our people ambitious for patients, accountable for outcomes and committed to doing the right thing. Our culture is described on pages 12 and 13 of the Strategic report. The influence and importance of different stakeholder groups can vary, depending on the matter being considered. Certain stakeholders’ interests can be in conflict, meaning that we, as a Board, need to make balanced judgements. Continuous stakeholder engagement and feedback helps us identify emerging issues. It also enables us to make decisions in the context of what is relevant and important to each of them. Our principal Board committees, and the GLT, undertake engagement on the Board’s behalf according to their remit. This means that they can build a detailed understanding of how our actions or plans are affecting or might affect stakeholders. These insights are then shared with the Board. In particular, the Board receives briefings on stakeholders’ perspectives from the work of the Corporate Responsibility Committee, which is discussed on pages 116 and 117. Board members regularly receive: – the CEO’s Board report – a specific external stakeholder insights report. This provides strategic insights based on an analysis of key developments, achievements and risks affecting our reputation and the perceptions of all our external stakeholders – a regular investor relations report which summarises investor perceptions – regular corporate governance, litigation and regulatory updates The Board also learns of stakeholders' views through: Engagement and feedback events: such as quarterly investor results calls, the Annual General Meeting, employee survey reports, the Board’s workforce engagement activities, and from experts presenting at Board or committee meetings. The Chair also holds regular investor check-in meetings, which the SID, Charlie Bancroft, sometimes joins, and is available for individual meetings with investors. Other opportunities: Board members also gain wider stakeholder views during the annual strategy meeting with the GLT, as part of the yearly review of strategy, budget and planning processes. This also includes a review of specific aspects of the company’s policies or strategy. In addition, Board members are encouraged to meet individually with employees, shareholders and other key stakeholders during their induction, and then on an ongoing basis. They are encouraged to report to the Board on such experiences where relevant and material. Engaging with our people We have well-established and strong engagement mechanisms with our employees, which are described on pages 12 and 13, and which the Board monitors regularly. Four key governance channels help the Board understand what our people are thinking: – regular Board updates from our Chief People Officer and the CEO on culture and talent – feedback from an annual employee engagement survey, including questions on engagement, confidence and inclusivity – a range of pulse surveys of different-sized employee groups to help check sentiment on a quicker and more frequent basis, and to provide valuable insights on the impact of major initiatives, events or communications – direct engagement by the Board Workforce engagement: Before the company's demerger, the Board reviewed its formal workforce engagement arrangements. It was decided to move from a specific Workforce Engagement Director model and to apply an ‘alternative arrangement’ to the three methods set out in the FRC’s Code. Given that the new GSK Board was recently refreshed in terms of tenure, with more than half the independent Non-Executive Directors having served for less than three years, and given GSK's renewed purpose and focus as a global biopharma company, it was considered important to adopt a collective Board engagement model. This was agreed to be the most effective approach to ensure newer Board members meet employees and hear their views. This new model operated in 2023 through: – direct in-person receptions with local employees during Board site visits, including in Wavre, Belgium (as one of our two global Vaccines hubs), Boston, US, and our global headquarters in Brentford – the Chair's site visits, including to the Wavre and Singapore Vaccine manufacturing sites, and the Philadelphia Commercial site – the Chair's attendance at management meetings, including China Commercial employees, the Commercial Core Leadership team in the UK, China regional general managers and Commercial talent and Saudi Arabia general management team – the Chair and Corporate Responsibility Committee Chair convene and attend ongoing meetings with leaders of the company's employee resource groups to talk about how they experience GSK, how they think the DEI agenda and ambitions are progressing and sharing their suggestions to further enhance our DEI agenda – utilising a variety of bespoke engagements that have enabled a broad and open dialogue and facilitated first- hand engagement discussions between the NEDs and our people individually and as part of small groups, encompassing perspectives on our strategy, purpose and Ahead Together culture, and DEI Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Continuous engagement and key decisions 113

Engaging with our shareholders As a Board we aim to directly engage with and be directly accountable to institutional investors and private retail shareholders. We do this in several ways, including regular communications, the Annual Governance Meeting, our Annual General Meeting, and through the work of our Investor Relations team, the Chair, Jonathan Symonds, and our Company Secretary, Victoria Whyte. Our SID, Charlie Bancroft, is another point of contact for our shareholders. Each quarter, our CEO, Emma Walmsley, and CFO, Julie Brown, give results presentations to institutional investors, analysts and the media by webcast. They are also regularly joined by the CSO, the Chief Commercial Officer (CCO), and CEO, ViiV. They are able to provide investors with more detailed insights into their specific areas of responsibility. Through regular meetings, they each have an ongoing and active dialogue with institutional shareholders about the company's performance, plans and objectives. In 2023, – CEO: 103 engagements, representing 38% of the company's share register – The current and previous CFO: 60 and 51 engagements, comprising 33% and 31% of the register – CSO: 90 engagements, representing 31% of the register – CCO: 80 engagements, representing 39% of the register – CEO, ViiV: 61 engagements with 39% of the register Our Chair maintains a consistent dialogue with shareholders too – including fund and portfolio managers – and regularly engages with governance and ESG professionals. During 2023 and up to the date of publication of this Annual Report, Jon held over 30 individual engagements with a range of institutional shareholders, which make up approximately 30% of the company’s share register. This enables him to gain a current understanding of shareholders' views, insights and perspectives of the company. He also discusses the continual evolution of the many aspects of Board governance, performance oversight and succession. This year our Chair, CEO and the rest of the Board and key GLT members focused on communicating the strong ongoing performance of GSK as a global biopharma business, the successful launch of Arexvy, the world's first RSV vaccine, and progressing our pipeline across the core therapy areas of infectious diseases, HIV, respiratory/immunology.and oncology. Annual Governance Meeting This year’s hybrid meeting was held in central London. Institutional shareholders, key investment industry bodies and proxy advisory firms were invited. 15 representatives of various institutional shareholders and proxy advisers attended the event, comprising approximately 25% of the company's share register. The meeting had a new format to make it as interactive as possible. It began with Jon sharing with investors the Board's priorities and focus for 2023 and beyond, with Charlie then providing his reflections on the year. Jon, Charlie and our Non- Executive Directors then held an informal and open discussion of those issues on shareholders' minds, which helped foster a richer dialogue. The key themes covered included the: – Board changes and succession planning arrangements – work of and challenges for the Board over the last year – company's current and future momentum and excellent execution of our key priorities – harnessing of digital, technology and talent, driven by our Ahead Together purpose – positive signs of the influence of our culture of being ambitious for patients, accountable for impact and doing the right thing The meeting and its new format were well received and shareholder feedback was shared with the full Board. Annual General Meeting We were pleased to hold the company's hybrid AGM at the Sofitel Heathrow in May 2023. 72 shareholders joined the meeting in person and 49 shareholders joined virtually via the Lumi platform to watch or listen to updates from our Chair and the CEO, and to vote. Shareholders were able to ask questions during the meeting in person and virtually. All our proposed resolutions were approved by shareholders, with majorities ranging from 89% to 99%. Our hybrid AGM this year will be held at a new venue, Royal Lancaster Hotel in Central London, which is located close to our new global headquarters. For more details see page 264. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Continuous engagement and key decisions continued 114
Key decisions In its decision-making, the Board focuses on GSK's priorities as a pure biopharma company with strong momentum and big ambitions, whilst balancing the interests of our stakeholders. Selected examples of some of the key decisions taken by the Board in 2023 and January 2024 to drive our purpose, momentum and strategy include: Decision How the Board/Committee regarded stakeholder interests Stakeholder Groups Progressive dividend policy The Board and Audit & Risk Committee considered the application of the progressive dividend policy in line with capital allocation priorities The Board recognises the importance of dividends to shareholders. In December, the Audit & Risk Committee and the Board considered how GSK's progressive dividend policy should best be applied in line with the agreed capital allocation priorities of the Group and its investment strategy for growth alongside the sustainability of the dividend This resulted in an increased dividend of 16p for Q4 2023 (Q4 2022: 13.75p) and 58p for the full year 2023 (2022: 61.25p). The expected dividend for 2024 is 60p Investors, patients and our workforce Capital allocation framework The Board considered an updated capital allocation framework to best support growth and sustainable returns to shareholders The Board approved an updated capital allocation framework, with the priority of investing in the business, focussed towards development of the pipeline through both the organic R&D portfolio, and targeted business development. This will be achieved through an increased focus on ROI for these investments Ultimately, the Board determined that the updated framework would continue to support investing in growth and delivering sustainable returns to shareholders, underpinned by a strong balance sheet. It is also consistent with GSK's strategic priorities and supports the company's commitment to deliver long-term profitable growth Patients, employees and investors Business development The Science Committee considered the scientific merits of business development opportunities and where relevant for late stage assets commercial reviews, prior to the Board's review and approval The Board, with support from the Science Committee and commercial reviews for late stage assets, reviewed many business development opportunities during the year. Those leading to concluded transactions included: – licence agreements with Hansoh Pharma for two antibody-drug conjugates with potential across several solid tumour indications to support our work in developing cancer treatments – agreement with Chongqing Zhifei Biological Products to co-promote Shingrix in China, which will significantly extend the availability of the vaccine and support patient access – acquisitions of BELLUS Health and Aiolos Bio to expand and strengthen GSK's respiratory portfolio These deals were considered in the context of their potential to help GSK deliver transformational medicines to patients and drive growth through accelerating the pipeline Patients and employees Artificial intelligence and workforce culture The Board considered the approach to and impact of adopting AI on an end-to- end basis across the business The Board reviewed and provided feedback on the strategy to integrate and responsibly scale AI across the business to accelerate the pipeline, amplify performance and drive productivity The Board recognises the significant potential of AI, particularly in the context of interpreting datasets to develop medicines with a higher probability of success. However, with support from the Audit & Risk Committee, the Board also considered the associated risks of AI, as described on pages 121 and 122. The Board approved the establishment of the AI Governance Council, co-chaired by the General Counsel and CDTO to help manage these risks across the Group Close attention was also paid to the impact of adopting AI on the workforce, including wellbeing gains enabled through increased efficiency and the benefits of further upskilling and building AI capabilities Patients, employees and investors Low-carbon Ventolin strategy The Corporate Responsibility Committee and Board reviewed plans for progression to the next phase of development of the low- carbon Ventolin programme During the year, the Corporate Responsibility Committee endorsed and the Board reviewed and approved plans to progress the transition from a metered dose inhaler to new-generation low-carbon inhalers, to significantly contribute to GSK's carbon reduction targets for 2030 and 2045. Phase III trials will begin in 2024 and, if successful, the programme has the potential to reduce greenhouse gas emissions from use of the inhaler by approximately 90% The Board and Corporate Responsibility Committee carefully considered the needs of patients who rely on Ventolin, the complexity of the clinical development process as well as the investment required in new manufacturing facilities. If successful, the programme could lead to regulatory submissions in 2025, supporting the health of asthma and COPD patients and making a significant positive impact on GSK's transition to a more environmentally sustainable future Patients, employees and investors Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Continuous engagement and key decisions continued 115

Corporate Responsibility Committee report Dr Anne Beal Corporate Responsibility Committee I am pleased to present this report, which is my second as Chair of the Corporate Responsibility Committee (the Committee). This is the first full year for GSK operating as a global biopharma company, with a renewed purpose to unite science, technology and talent to get ahead of disease together. To deliver this purpose, the company needs to consider ESG impacts across everything it does. The Committee oversees the six ESG focus areas that address what is most material to the business and the issues that matter the most to stakeholders. As we worked through our programme of activities this year, my Committee’s focus was to ask management fundamental questions concerning: – how well the company is performing against and making an impact on the six ESG areas embedded in the company’s strategy – how this supports our sustainable performance and long- term growth – how further improvements can be identified and implemented To support this, we undertook a number of ESG performance deep-dives. Access The Committee reviewed progress towards the company’s aim to improve the health of 2.5 billion people by 2030 through ensuring access to our vaccines and medicines, including reaching 1.3 billion people in lower-middle income countries (L/LMICs). In particular, we discussed: – the flexible and tailored operating model to driving access in L/LMICs, depending on need – working with partners with the right capabilities and geographical footprint to deliver interventions, which may include donations, affordable supply and licensing, to make sure people have access to the vaccines and medicines they need – investing £1 billion over 10 years in our Global Health R&D pipeline and contributing to building resilient health systems Global Health & Security Anti-Microbial Resistance (AMR): The Committee reviewed the external AMR landscape and trends, which are a major threat globally, and considered the company’s holistic and innovative investment approach to addressing this AMR threat. We were pleased to note that this approach has resulted in the largest relevant AMR vaccine R&D pipeline in the industry. We discussed with management the steps needed to help leverage this leadership position, in conjunction with the support and expertise of the Science Committee. This included growing our business development strategy and improving pathogen surveillance capabilities. Given AMR is an urgent public threat and stakeholders are increasingly interested in GSK’s approach to it, we agreed that it was appropriate to include AMR as one of our ESG Performance Rating metrics for the first time. Environment Carbon reduction plan: There is a very strong case for making the transition to low-carbon inhalers. Salbutamol is an essential rescue/reliever medicine, and GSK's Ventolin (salbutamol) metered dose inhaler (MDI) is used by 35 million patients globally. Use of the inhaler, due to the high global warming potential (GWP) of the current propellent, accounts for half of GSK’s carbon footprint. Management updated the Committee about developing proposed plans to transition the inhaler to a next-generation low-carbon propellant which, if successful, will significantly contribute to GSK's carbon reduction targets for 2030 and 2045. However, developing this low-carbon inhaler is complex and involves clinical and non-clinical programmes, as well as establishing new manufacturing facilities. Having examined these and other key considerations behind investing in a low-carbon transition programme – which could reduce greenhouse gas emissions from the inhaler by 90% – we endorsed management’s R&D MDI transition programme investment case to the Board, submitted after the read-outs from latest early clinical data had been received and evaluated. This supported the Board’s decision in November to progress to phase III trials in 2024. If these trials are successful, they could lead to regulatory submissions in 2025. Nature plan review: The Committee received an update on current performance against the company’s Nature positive goal by 2030, which will be achieved by reducing the company’s environmental impacts across water, waste and materials, and biodiversity and by investing in protecting and restoring nature. The Committee was satisfied that these Nature goals and targets remain appropriate and industry leading. We also noted that standards for assessing and verifying companies' nature approach continued to strengthen. We were pleased that GSK was actively helping to shape this environment as a source of competitive advantage. Science Based Targets Network for Nature (SBTN) set the first science- based targets for a nature framework to validate companies’ nature targets, which was similar to the regulatory approach previously adopted for climate-based targets. Given the relative maturity of our Nature positive programme, the company is pleased to be selected in the first group of 17 companies globally to go through the target validation process, to accredit our nature targets when the SBTN methodology was finalised. Additionally, GSK has also committed to disclosing our arrangements against the Taskforce on Nature-related Financial Disclosures framework in our 2025 Annual Report. Diversity, Equity and Inclusion Delivery against People DEI aspiration: The Committee heard from the Chief People Officer (CPO) on progress over the company’s workstreams to drive increased leadership diversity, build a diverse talent pipeline and foster an inclusive culture. We were pleased with the excellent progress that had been made to date and discussed the challenges and opportunities to maintain future progress in these areas. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Board committee reports 116

Clinical trial diversity: The Chief Scientific Officer (CSO) outlined the approach to broadening clinical trial diversity and how this connects with our patient impact. The Committee strongly agreed that the company’s clinical trials must be accessible and inclusive to ensure our clinical development programmes reflect the diversity of the patient populations impacted by the disease under study including, but not limited to, age, race, ethnicity, sex and gender. In doing so, we noted the importance of reflecting epidemiological profile rather than census profile as the benchmark from which to plan appropriate patient representation and of working through community groups to build trust, awareness and participation in clinical trials. ESG Performance Rating The ESG Performance Rating (Rating) helps us integrate ESG into the delivery of our strategy and allows us to measure and verify the progress we are making. This is the second year that the Rating has been used and the Committee continues to oversee its evolution to make sure it meets the expectations of key stakeholders. We discussed with management the rationale for removing one of the ESG metrics relating to Access. We also scrutinised how the new AMR metric was formulated, reviewed, and set, to make sure that it was a suitably stretching and strategically relevant metric. We were joined by the CPO and CSO to discuss the status of the five DEI metrics and any other measures needed to progress performance against these metrics. Separately, the Committee was kept informed of the work being led by the CFO to assure the data underlying the ESG metrics and Rating which has been overseen by the Audit & Risk Committee. We monitored and evaluated the company’s progress against these metrics and the Rating at the half and full year. We then recommended to the Board publishing a final 'on track' ESG Performance Rating alongside the other ESG disclosures in this Annual Report and our ESG Performance Report. For more details, see page 44 of the Strategic report and in the ESG Performance Report – both of which are available at gsk.com. Dr Anne Beal Corporate Responsibility Committee Chair 27 February 2024 Science Committee report Dr Hal Dietz Science Committee I am pleased to present my first report as Chair of the Science Committee (the Committee) on our activities during 2023. I joined the Committee in January 2022, and succeeded Dr Jesse Goodman as Chair on 1 January 2023. Jesse had been Chair of the Committee since it was created seven years ago and made an outstanding contribution to defining and implementing the Committee’s role. He remains an important member of the Committee and offers vital insights to our work. The Committee’s key activities in 2023 were split into three important areas: – pipeline reviews: monitoring of GSK’s pipeline – business development: undertaking technical reviews and assurance of the underlying science of potential business development transactions – scientific deep-dives: discussing and analysing the key scientific and technology themes which drive the company’s R&D strategy Pipeline progress During 2023 the Committee continued to monitor the progress of R&D. Our CSO, Dr Tony Wood, provided regular updates on progress across the company’s four therapeutic areas: infectious diseases, HIV, respiratory/immunology and oncology. A particular pipeline highlight during 2023 was the launch of Arexvy, GSK’s world-first RSV vaccine for older adults. During the year, the vaccine gained approvals in the US, EU, Japan and several other countries. Arexvy marked a turning point in efforts to reduce the burden of the RSV, a respiratory virus which has evaded prevention or therapeutic advances for over 60 years. It also heralds the next wave of vaccine innovation at GSK. In oncology, Jemperli, in combination with chemotherapy, received approval in the US and EU as the first new frontline treatment option in decades for patients with dMMR/MSI-H primary advanced or recurrent endometrial cancer. These approvals reinforced the potential of Jemperli to redefine cancer treatment as the backbone of immuno-oncology therapy. A number of other key regulatory milestones were also achieved during the year: – Shingrix vaccine for shingles approved for people at risk over 18 in Japan and positive data from first efficacy trial in China – Apretude, a long-acting preventative treatment for HIV, approved as the first and only HIV prevention option in Europe – Ojjaara/Omjjara, approved in the US, EU and UK as the first and only treatment for both newly diagnosed and previously treated myelofibrosis patients with anaemia – gonorrhoea vaccine candidate received US FDA fast-track designation Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Board committee reports continued 117

The Committee was pleased with the progress made to accelerate the pipeline during the year, both in terms of investment in the late-stage pipeline to drive growth, as well as through rigorous decision-making with the early stage pipeline to maximise its potential impact on patients. R&D leadership changes Continuing to accelerate the pipeline relies on attracting the best people and fostering a culture that is ambitious for patients, accountable for impact and always does the right thing. That is why the Committee was pleased with the R&D leadership changes made this year, including creating three dedicated Research Units: vaccines and infectious diseases, respiratory and immunology, and oncology. HIV research operates as part of our global specialist HIV company, ViiV Healthcare. These changes mean the company can better focus on its core therapeutic areas and more easily identify the targets that will have the best outcomes for patients. Supporting these new teams is a single research technologies organisation, which brings together platform and data groups to integrate technology more effectively across GSK's workflow. Business development transactions Since the demerger, the CSO and his team have worked hard to accelerate business development to complement GSK’s organic pipeline. This called upon our Committee to devote nearly twice as much time in our meetings to critically vet business development proposals and transactions, compared with last year. Transactions reviewed by the Committee during the year include: BELLUS Health: the acquisition of BELLUS, a late-stage biopharmaceutical company working to better the lives of patients suffering from refractory chronic cough (RCC). The acquisition provided GSK with access to camlipixant, a potential best-in-class treatment in phase III development for the first-line treatment of RCC. This acquisition aligned to GSK’s expertise in and prioritisation of respiratory medicines. Hansoh Pharma: two exclusive licence agreements for antibody-drug conjugates: HS-20089, with best-in-class potential in ovarian and endometrial cancer and HS-20093, with promising initial clinical activity in lung cancer with the potential to address unmet medical need in broader solid tumour indications. Aiolos Bio: acquisition of Aiolos, which closed in February 2024, adds AIO-001 to GSK's respiratory biologics portfolio. AIO-001 is a phase II-ready long-acting antibody that could redefine the standard of care for asthma patients, with dosing every six months. It also has the potential to expand the company's reach to a broader range of asthma patients. Arrowhead Pharmaceuticals and Janssen Pharmaceuticals: the transfer of exclusive worldwide rights to further the development and commercialisation of an investigational therapeutic to treat chronic hepatitis B. GSK plans to evaluate this drug in a sequential regimen with bepirovirsen (GSK’s investigational antisense oligonucleotide) for the treatment of chronic hepatitis B. The transaction has the potential to redefine the treatment paradigm for chronic hepatitis B by enabling more patients to achieve functional cure. As a Committee, we are confident that these transactions have strong scientific justification and look forward to seeing them develop in the next few years. Deep-dives into innovative science During the year the Committee has continued to undertake scientific deep-dives into some of the highly innovative technologies currently being explored by the CSO and his team. Deep-dives undertaken in 2023 included, but were not limited to, both liver disease and oligonucleotide strategy. GSK’s expertise in infectious disease, immunology and human genetics has driven research into chronic hepatitis B. In 2023, new data presented for bepirovirsen has improved understanding of the heterogeneous nature of hepatitis B infections. Insights – from the B-Clear and B-Together phase IIb trials for bepirovirsen – will help GSK progress towards a comprehensive functional cure for people living with chronic hepatitis B, a common cause of chronic liver disease. Our deep-dive into oligonucleotide-based therapeutic strategies positions GSK to achieve leadership in this field. Oligonucleotides have a unique ability to address a wide range of genomic targets across many therapeutic areas, which means they offer enormous potential to help patients with diseases that have historically been difficult to treat. The company’s collaboration with Wave Life Sciences, initiated in 2022, brought together Wave’s PRISMTM platform and GSK’s expertise in genetics and genomics to progress up to eight preclinical programmes. The collaboration also granted GSK the exclusive licence for Wave’s preclinical programme to treat alpha-1 antitrypsin deficiency, complementing GSK’s own clinical-phase oligonucleotides, including bepirovirsen. Committee changes Since I became Chair, there have been no changes to the Committee’s composition during 2023. I look forward to welcoming as a member of the Committee Dr Jeannie Lee who joins the Board on 4 March 2024. Work is also underway to appoint a successor to Dr Jesse Goodman, who is due to retire from the Board in 2025. I look forward to providing an update on this appointment next year. Dr Hal Dietz Science Committee Chair 27 February 2024 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Board committee reports continued 118

Nominations & Corporate Governance Committee report Jonathan Symonds Nominations & Corporate Governance Committee I am pleased to present my fifth report as Chair of the Nominations & Corporate Governance Committee (the Committee). Evolving the Board and pipeline of talent In my Corporate governance statement on page 106, I discussed the important Board appointment processes that have been undertaken recently. Julie Brown, our new CFO, was appointed in September 2022 and joined the Board in 2023. Wendy Becker, our new Remuneration Committee Chair designate, was appointed and joined the Board in the second half of 2023. A transition process is underway to enable Wendy to succeed Urs Rohner as Remuneration Committee Chair at the close of the 2024 AGM. Finally, we welcome Dr Jeannie Lee, who joins the Board with effect from 4 March 2024 as part of our ongoing Board succession arrangements. These appointments are tailored to the biopharma company we now are. The Committee seeks to follow best practice in all the searches it makes and appointments it recommends to the Board, agreeing the criteria for each role, the most appropriate diverse interview panel and considering a comprehensive and diverse longlist of candidates. Shortlisted candidates are interviewed and assessed against the chosen criteria. Due diligence is then undertaken before the Committee makes its final recommendation. Executive search firms are appointed according to the company’s procurement policy and based on their expertise relative to each role. The Committee only engages search firms that are signatories to the Voluntary Code of Conduct of Executive Search Firms on gender diversity and best practice. The Committee worked with a number of executive search firms in 2023. They also provided additional consultancy services to the company: Korn Ferry (general recruitment, executive search and assessment services and other HR-related services); Egon Zehnder (executive search, assessment and coaching services to specific senior executives); Russell Reynolds (executive search services). The Committee reviewed the potential for conflicts of interest and judged that there were appropriate safeguards against such conflicts. I look forward to reporting on the Committee's continued work and progress to evolve the Board further in next year's report. The Committee also continues to review our diverse talent and succession pipelines and the development plans for key management roles and their successors. During the year, we undertook a deep-dive of the emerging senior talent that the GLT had identified – people who were exceeding expectations or exceptionally talented, and who have the potential to take on a GLT role in the future. This included reviewing the strategic approach to talent development planning. The Board seeks to meet with these individuals at employee receptions and through other Board engagement opportunities. Board and GLT diversity We are committed to the diversity of our Board and its committees, just as GSK is committed to equal opportunities for all employees at every level of the company. The Board and management seek to support and encourage a diverse and inclusive culture throughout the company. An effective Board includes a range and balance of skills, experience and knowledge as well as diversity of ethnicity, gender, sexual orientation, professional and social-economic background, disability, age and independence, with individuals who are prepared to challenge each other collaboratively. This mix is complemented by a diversity of personal Board attributes, including character, intellect, judgement, honesty and courage. The Committee is responsible for developing measurable objectives, in line with the relevant regulatory and best practice targets, and monitoring their progress – which is part of implementing the Board’s diversity policy (Policy). This includes gender and ethnicity diversity targets, and applying it to our Board committees. As a minimum, we seek to align our Policy objectives with the Financial Conduct Authority (FCA), FTSE Women Leaders Review and Parker Review diversity targets (Regulatory and Best Practice Targets) and ensure that they are consistent with our public DEI aspirations. We currently meet or exceed our policy objectives and the Regulatory and Best Practice Targets shown on the next page. Board and GLT diversity data collection This year, diversity data has been gathered directly on a self- identified basis as follows: – Board members: using a questionnaire – GLT members: individual election held on GSK's HR database All diversity data published in the following section of the report are as at 31 December 2023 and the date of publication. We also continue to oversee the developing pipeline of direct reports to the GLT by gender and from ethnically diverse backgrounds. Full details of GSK’s representation of women and ethnically diverse leaders is covered on page 51, as part of the diversity of our global workforce. The pleasing progress against our DEI commitments, including gender and ethnicity, is illustrated in our ESG Performance Report on gsk.com. This good progress has been boosted since introducing a DEI measure in 2022 as part of the Annual bonus arrangements for our Executive Directors and other GLT members. Sir Jonathan Symonds Nominations & Corporate Governance Committee Chair 27 February 2024 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Board committee reports continued 119
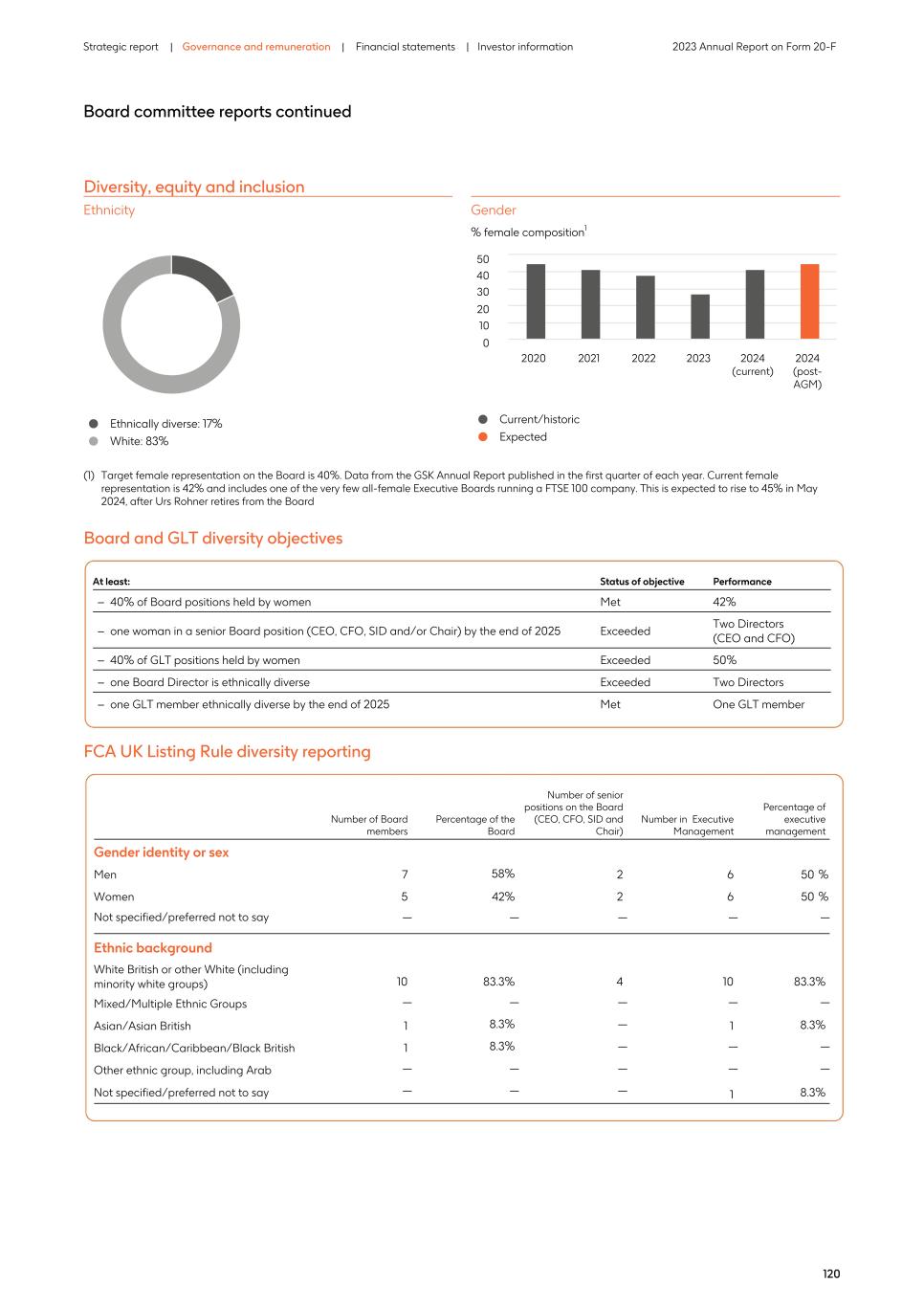
Diversity, equity and inclusion Ethnicity Gender % female composition1 l Ethnically diverse: 17% l White: 83% 2020 2021 2022 2023 2024 (current) 2024 (post- AGM) 0 10 20 30 40 50 l Current/historic l Expected (1) Target female representation on the Board is 40%. Data from the GSK Annual Report published in the first quarter of each year. Current female representation is 42% and includes one of the very few all-female Executive Boards running a FTSE 100 company. This is expected to rise to 45% in May 2024, after Urs Rohner retires from the Board Board and GLT diversity objectives FCA UK Listing Rule diversity reporting Number of Board members Percentage of the Board Number of senior positions on the Board (CEO, CFO, SID and Chair) Number in Executive Management Percentage of executive management Gender identity or sex Men 7 58% 2 6 50 % Women 5 42% 2 6 50 % Not specified/preferred not to say — — — — — Ethnic background White British or other White (including minority white groups) 10 83.3% 4 10 83.3% Mixed/Multiple Ethnic Groups — — — — — Asian/Asian British 1 8.3% — 1 8.3% Black/African/Caribbean/Black British 1 8.3% — — — Other ethnic group, including Arab — — — — — Not specified/preferred not to say — — — 1 8.3% Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Board committee reports continued 120 At least: Status of objective Performance – 40% of Board positions held by women Met 42% – one woman in a senior Board position (CEO, CFO, SID and/or Chair) by the end of 2025 Exceeded Two Directors (CEO and CFO) – 40% of GLT positions held by women Exceeded 50% – one Board Director is ethnically diverse Exceeded Two Directors – one GLT member ethnically diverse by the end of 2025 Met One GLT member

Audit & Risk Committee report Charles Bancroft Audit & Risk Committee I am pleased to present this report, which is my third as Chair of the Audit & Risk Committee (the Committee), and in the following pages I aim to share insights into the activities undertaken or overseen by the Committee during the year. 2023 was GSK’s first full year as a focused global biopharma company. The Committee reviewed key operational features and risks of the governance platform that underpin our purpose to unite science, technology and talent to get ahead of disease together. I am also pleased to report on progress against the Committee's expanded remit to oversee and review ESG data assurance. This Committee continues to have primary oversight for the Zantac litigation through regular legally privileged updates and review of the related accounting, disclosure and communication requirements. The Committee also reviews the current indicative timeline of cases. I report and summarise the key matters for the Board for its awareness, input or decision. We continue to defend all claims brought vigorously based on the science. Based on the work the Committee has done or inspected, GSK continues to exhibit a strong compliance culture, with a consistent tone and engagement from the top that runs throughout the organisation. Our financial reporting and controls framework remains robust and required no fundamental changes during the year. Science Strategic vaccine partnership in China: In Q3 2023, the company entered into an exclusive agreement with Chongqing Zhifei Biological Products, Ltd (Zhifei) to co-promote Shingrix in China to support accelerated market penetration for our innovative vaccine. The Committee scrutinised this arrangement before it was concluded. A significant amount of time and resources were devoted to due diligence of this arrangement, and key controls were introduced to ensure our compliance expectations would be met to mitigate risks. The Committee was satisfied that the ongoing governance framework – with monthly reviews and monitoring arrangements – was appropriate. Experienced compliance personnel were also allocated to support the partnership. Chief Patient Officer: A new role of Chief Patient Officer was established from the beginning of 2024. The Committee assessed the controls and governance arrangements for this new role. The Chief Patient Officer's primary role is to provide medical leadership as part of one overarching GSK asset and disease strategy. The Chief Patient Officer reports to the Chief Commercial Officer to ensure patients treated with GSK products benefit from robust, compliant scientific information, in line with our commitment to patients. We also satisfied ourselves that key areas of medical ethics, safety and execution of clinical trials have clear lines of escalation to our Chief Medical Officer. This further enhanced the company’s Internal Control Framework and Independent Business Monitoring protocols. Technology Data privacy and ethics: This is a rapidly evolving principal risk for the Committee’s oversight. The number of privacy laws and regulations, often based on the EU General Data Protection Regulations, is increasing in a number of territories around the world. Consequently, the Committee was interested to further understand the regulatory approaches being adopted in some of our biggest markets, including the US, India and China, and how they may affect GSK’s operations, including our R&D operations. The Committee receives regular reports on the robust and integrated governance framework GSK operates to monitor and govern the use of data generally. GSK's framework is made up of specialist governance boards that include representative members from relevant internal functions. This framework has been further augmented with a team from the Legal and Compliance function with expertise to advise on global digital, privacy and cyber security matters. The Committee discussed the tenets of the new enhanced flexible data privacy model being introduced. This is expected to comprise global privacy principles and standardised global controls meeting the EU standards. The reward would be flexibility to adopt different standards where local laws are incompatible with GSK's standardised global controls, provided they meet GSK's global privacy principles. Information and cyber security: This is a risk factor for GSK and an area that remains a standing agenda item which is discussed at each of our scheduled meetings. The Chief Digital and Technology Officer (CDTO), Chief Information and Security Officer (CISO) and Chief Compliance Officer (CCO) present updates on information and cyber security, as well as assessments of the status of their associated key risk indicators. The CDTO’s skills and experience, especially those related to cyber security, are set out on page 104. Our CISO has spent his career building and leading technology teams across several functional areas, including cyber security and IT infrastructure for digital communications and healthcare companies. He was also responsible for establishing the cyber security function for Haleon plc prior to its demerger. Our CCO focuses on ensuring that a consistent and cohesive approach to information and cyber security operates across all aspects of the business and enterprise risk management. The CCO is also responsible for the Risk Analytics and Monitoring organisation. He has previous experience in creating a dedicated Global Risk Office that combines enterprise risk management and reporting activities for GSK. During the year, the Committee reviewed progress against the first full year of our updated multi-year Cyber Security Plan (Plan) which was benchmarked against the National Institute of Standards and Technology Cyber Security Framework (NIST- CSF). At the end of 2023, to help validate how the company’s capabilities had improved, the Committee examined the results of an internal NIST assessment that was undertaken jointly by our Tech and Audit & Assurance functions. In 2024, building on this assessment, the Committee will review the scheduled external NIST review by specialist independent cyber experts. I look forward to providing an update on the results of this independent review in my report next year. The Committee has also been closely following the development, finalisation and introduction of the Securities and Exchange Commission’s (SEC) new cyber security rules (Rules) effective from the end of 2023. We are satisfied that the Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Board committee reports continued 121

company, as a foreign private issuer, has taken the necessary governance steps to ensure compliance with these requirements. The Committee assesses the adequacy of GSK's insurance risk coverage arrangements annually, including the information and cyber security risk, as part of its insurance risk programme review. I then communicate the Committee’s recommendation to the Board before implementation. Also on our agenda was cyber security training for GLT – which included cyber security simulation exercises and learnings – and for the wider workforce. We discussed mandatory training for new joiners and regular phishing-simulation exercises. I highlighted previously the relevant cyber security expertise my Board colleague, Dr Vishal Sikka, brings to our deliberations. We are looking to further strengthen our oversight in this area by running bespoke cyber incident training sessions for all Directors. Their cyber-related experience is included in the Board biographies on pages 99 to 103. Artificial Intelligence (AI): The rapid advancement of AI, in particular generative technology and its potential application across the company, presents significant opportunities to drive innovation, growth and productivity and, in doing so, to accelerate our purpose. To this end, the Board is reviewing opportunities to scale use of AI for potential competitive advantage. Balanced against this is the awareness that there is a lack of harmonisation from new and emerging regulations that govern ethical and responsible use of AI. These new trends may impact the risk profile of our Research practices, Scientific and patient engagement, Data ethics and privacy, and Information and cyber security principal risks, and could have a future impact on GSK’s value chain. Therefore, these developments were being monitored very closely by management and the Committee. To this end, the Board approved the establishment of the AI Governance Council (Council), co-chaired by the General Counsel and CDTO and comprising cross-functional experts. In particular, the Council is assessing business activities against the current risk environment through our internal control framework. Importantly, the Council is fully connected to the key data management boards for data ethics and privacy and information and cyber security. I am pleased to report that the Council has approved and implemented a suite of written standards, controls for adopting new AI tools, and training tailored for developers, procurers and users of AI. The Committee and our auditor have a shared goal to leverage technology as appropriate. We were particularly interested in and discussed with the auditor how they can use AI to deliver increased effectiveness and efficiency in their audit. We are also keen to better understand the opportunities for GSK to use scalable AI-enabled innovations to improve the speed and performance of its tracking and detection capabilities. This is a key part of staying ahead of the increasingly sophisticated threats to the Group and our third parties. This will be an area we continue to focus on. ESG data assurance The Committee oversaw the creation and implementation in Q3 2023 of a new dedicated ESG data assurance hub in our Finance organisation. This formally established a consistent approach to governance, processes and controls which have been developed to further improve assurance of ESG data in support of the company’s performance against key ESG metrics. Meanwhile, our Corporate Responsibility Committee, on behalf of the Board, continues to oversee ESG strategy, performance assessment and reporting. The hub's initial focus is on environmental data. Later, as its processes and capabilities develop, the hub will look at social and governance areas too. Soon the Committee will also oversee the development and implementation of technology solutions to automate information gathering and to supplement the level of process and control standards that surround ESG performance data. Looking forward, the Committee will continue to review upcoming regulations that might affect our future ESG assurance and reporting obligations, which have been highlighted by the hub’s horizon-scanning activities. In particular, the Committee discussed the initial results of the ongoing impact assessment that is underway for the Corporate Sustainability Reporting Directive. This directive could become partially effective from our 2025 reporting year, and would be fully effective at a consolidated reporting level for GSK by 2028. We are also aware that reporting arrangements to reflect the published SEC Climate regulations are expected to become effective from the 2026 financial year. Internal control framework The Board recognises its obligation to present a fair, balanced and understandable assessment of GSK’s current position and prospects. It is accountable for evaluating and approving the effectiveness of GSK’s internal controls, including financial, operational and compliance controls, and risk management processes. We ensure the reliability of our financial reporting, and compliance with laws and regulations, through our internal control framework. This is a comprehensive enterprise-wide risk management model, which supports the Board to identify, evaluate and manage the Group’s principal and emerging risks, as required by the FRC’s Code. The framework is designed to manage the risk of GSK not achieving its business objectives. A fit-for-purpose framework – complemented by our corporate culture and Speak Up processes – ensures that the risks associated with our business activities are actively and effectively controlled in line with our agreed risk appetite. We believe GSK’s framework provides reasonable, but not absolute, assurance against material misstatement or loss. The Board mandates the Group’s Risk Oversight and Compliance Council (ROCC) of senior leaders to support the Committee in overseeing risk management and internal control activities. It also provides the business with a framework for risk management and escalation of significant risks. Risk management and compliance boards (RMCBs) across the Group promote the ‘tone from the top’ and establish our risk culture, and ensure effective oversight of internal controls and risk management processes. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Board committee reports continued 122

Each principal risk has an assigned risk owner, drawn from senior management, who is accountable for managing the principal risk with oversight from a GLT member, which includes setting and implementing risk mitigation plans. Risk owners report quarterly on their respective risk management approach and progress at the ROCC and the appropriate Board committee. Our Compliance function assists the ROCC and RMCBs. Compliance is responsible for advancing enterprise- wide risk management and for developing risk-based and ethically sound working practices. It also actively promotes ethical behaviours by enabling all employees to operate in line with our culture and comply with applicable laws and regulations. Our Audit & Assurance (A&A) function provides independent assurance to senior management and the Board on the effectiveness of risk management Group-wide, in line with an agreed assurance plan. This helps senior management and the Board to meet their oversight and advisory responsibilities to fulfil GSK’s strategic objectives and build trust with patients and other stakeholders. A&A has a dual reporting line to the CFO and the Committee. As a Committee we receive regular reports from principal risk owners, Compliance and A&A on areas of significant risk to the Group and on related internal controls. These reports assess the internal control environment within each principal risk area, including enhancements to strengthen controls. Once we have considered these reports, the Committee reports annually to the Board on the effectiveness of GSK’s internal controls. In 2023, through the authority delegated to the Committee, the Board conducted a robust assessment of the Group’s principal risks. This assessment in line with the FRC’s Code included consideration of the nature and extent of risk the Board is willing to take to achieve GSK’s strategic objectives. The Board, via the Committee, also oversaw the effectiveness of our internal control environment and risk management processes across the Group for the whole year, up to the approval date of this Annual Report. More detail about the review of the Group’s risk management approach is further discussed in the Risk management section of the strategic report on pages 55 to 69. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Board committee reports continued 123

Significant issues relating to the financial statements In considering GSK’s quarterly financial results announcements and the financial results in the 2023 Annual Report, the Committee reviewed the significant issues and management judgements in determining those results. It reviewed management papers setting out the key areas of risk, actions taken to quantify the effects of the relevant issues, and judgements made by management on the appropriate accounting required to address those issues in the financial statements. The significant issues considered in relation to the financial statements for the year ended 31 December 2023 are set out in the following table, with a summary of the financial outcomes where appropriate. The Committee and the external auditor have discussed the significant issues addressed by the Committee during the year and the areas of particular audit focus, as described in the Independent Auditor’s Report on pages 154 to 157. Significant issues considered by the Committee in relation to the financial statements How the issue was addressed by the Committee Going concern basis for the preparation of the financial statements The Committee considered the outcome of management’s half-yearly and year-end reviews of current and forecast net debt positions and the various financing facilities and options available to the Group. The Committee also considered management’s review of the impacts of both the current economic environment and climate change. Following consideration of these assessments, which included stress testing and viability scenarios, sources of liquidity and funding, forecasts and estimates, the Committee confirmed that the application of the going concern basis for the preparation of the financial statements continued to be appropriate. Revenue recognition, including returns and rebates (RAR) accruals The Committee reviewed management’s approach to the timing of recognition of revenue and accruals for customer returns and rebates. The RAR accrual for US Commercial Operations was £6 billion at 31 December 2023 and the Committee reviewed the basis on which the accrual had been made and concurred with management’s judgements on the amounts involved. A fuller description of the process operated in US Commercial Operations in determining the level of accrual necessary is set out in ‘Critical accounting policies’ on pages 169 and 170. Provisions for legal matters, including investigations into the Group’s commercial practices The Committee received detailed reports on actual and potential litigation from both internal and external legal counsel including the Zantac litigation, together with a number of detailed updates on investigations into the Group’s commercial practices. Management outlined the levels of provision and corresponding disclosure considered necessary in respect of potential adverse litigation outcomes and also those areas where it was not yet possible to determine if a provision was necessary, or its amount. At 31 December 2023, the provision for legal matters was £0.3 billion; see Note 32 to the financial statements, ‘Other provisions’ for more details. Provisions for uncertain tax positions The Committee considered current tax disputes and areas of potential risk and concurred with management’s judgement on the levels of tax contingencies required. At 31 December 2023, a tax payable liability of £0.6 billion, including provisions for uncertain tax positions, was recognised on the Group’s balance sheet. Impairments of intangible assets The Committee reviewed management’s process for reviewing and testing goodwill and other intangible assets for potential impairment. The Committee accepted management’s judgements on the intangible assets that required writing down and the resulting impairment losses of £421 million in 2023. See Note 20 to the financial statements, ‘Other intangible assets’ for more details. Valuation of contingent consideration in relation to ViiV Healthcare The Committee considered management’s judgement that it was necessary to increase the liability to pay contingent consideration primarily as a result of updated exchange rate assumptions as well as increases in sales forecasts and the unwind of the discount. After cash payments of nearly £1.1 billion in the year, at 31 December 2023, the Group's balance sheet included a contingent consideration liability of £5.7 billion in relation to ViiV Healthcare. See Note 33 to the financial statements, ‘Contingent consideration liabilities’ for more details. ViiV Healthcare put option The Committee reviewed and agreed the accounting for the Pfizer put option and concurred with management’s judgement on the valuation of the put option of £0.8 billion at 31 December 2023. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Board committee reports continued 124
Effectiveness and quality of external audit process The Committee is committed to making sure that GSK receives a high-quality and effective external audit. In evaluating Deloitte’s performance during 2022, prior to making a recommendation on its reappointment in early 2023, the Committee reviewed the effectiveness of its performance against the criteria which it agreed with management at the beginning of 2022. The detailed criteria used to judge Deloitte's effectiveness as external auditor are available at gsk.com. These are based on the audit approach and strategy, ensuring a high-quality independent audit, effective partnership and value for money. The Committee monitors engagements with external stakeholders relevant to our areas of oversight, including the FRC and Securities and Exchange Commission. We sought to ensure that Deloitte would deliver a smooth, thorough and efficiently executed audit for 2023 and so considered: – the overall quality of the audit – the independence of Deloitte – whether Deloitte showed an appropriate level of challenge and scepticism in its work Deloitte’s length of tenure was not taken into account when assessing its independence and objectivity, given it only commenced its role as auditor in 2018. However, the Committee did consider how effectively it had assumed its role as auditor. The Committee also considered feedback on the 2023 external audit, through a survey of Committee members and the financial management team at corporate and business unit level. The survey covered the: – effectiveness of the auditor’s challenge – integrity of Deloitte – transparency of its reporting to management and the Committee – the auditor's effective use of technology – clarity of the auditor’s communications and ways of working – quality of the audit team’s leadership – skills and experience of the audit team As Committee Chair, I regularly meet independently with the audit partner. We also meet with the auditor privately at the end of each Committee meeting to discuss progress, as appropriate. Having reviewed the above feedback, and noted any areas of improvement to be implemented by the audit team for 2024, the Committee was satisfied with the: – effectiveness of the auditor and the external audit process – auditor’s independence, qualifications, objectivity, expertise and resources We agreed to recommend to the Board Deloitte's reappointment at the next AGM, and did so free from the influence of any third party. Auditor’s reappointment External auditor External auditor appointment Last tender May–December 2016 Transition year 2017 First shareholder approval of current auditor May 2018 First audited Annual Report and 20-F Year ending 31 December 2018 New lead audit engagement partner 2023 Next audit tender required by regulations 2025/2026 (to take effect from 2028) There were no contractual or similar obligations restricting the Group’s choice of external auditor. Audit partner rotation The external auditor is required to rotate the audit engagement partner for GSK every five years. Our previous audit partner stepped down in March 2023 after the audit of GSK’s financial statements for 2022 was concluded. After a robust review process by the Committee, together with the former CFO, the new audit partner was selected. The Committee approved the appointment with effect from the start of the 2023 financial year. We were satisfied that Deloitte managed an orderly handover to the new audit engagement partner. This resulted in a seamless transition and maintenance of high levels of audit quality and effectiveness throughout the reporting year. Audit tender The Committee considers that, during 2023, the company complied with the mandatory audit processes and audit committee responsibility provisions of the Competition and Markets Authority Statutory Audit Services Order 2014. As Deloitte continues to maintain its independence and objectivity, and the Committee remains satisfied with its performance, GSK does not intend to tender the external auditor contract before the end of the current required period of 10 years identified above and considers that this is in the best interests of shareholders. The Committee was mindful that there were appointments of a new CFO for GSK and audit partner for Deloitte during the 2023 financial year, which is helpful in further mitigating the risks of any over-familiarity between the company and the auditor. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Board committee reports continued 125
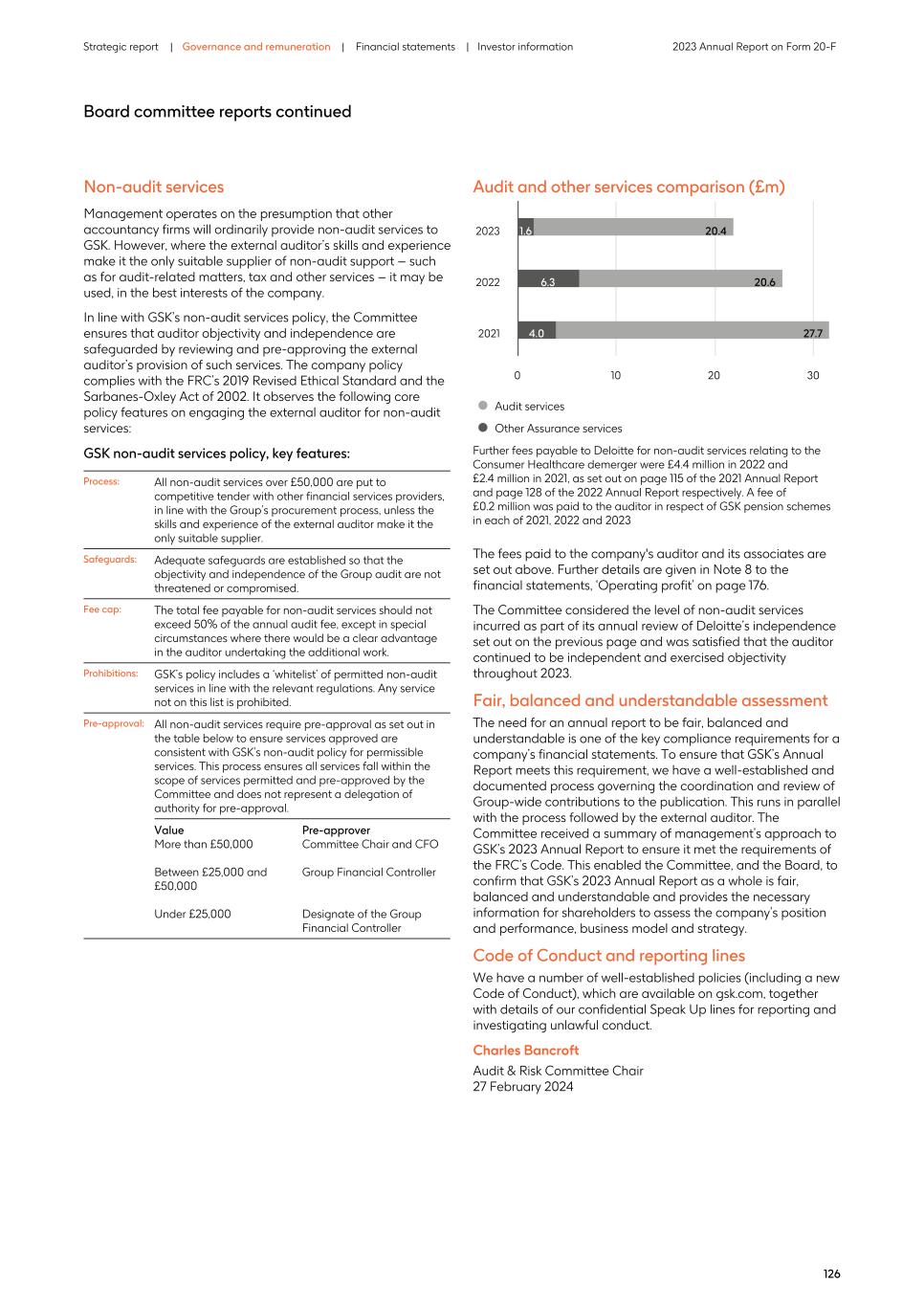
Non-audit services Management operates on the presumption that other accountancy firms will ordinarily provide non-audit services to GSK. However, where the external auditor’s skills and experience make it the only suitable supplier of non-audit support – such as for audit-related matters, tax and other services – it may be used, in the best interests of the company. In line with GSK’s non-audit services policy, the Committee ensures that auditor objectivity and independence are safeguarded by reviewing and pre-approving the external auditor’s provision of such services. The company policy complies with the FRC’s 2019 Revised Ethical Standard and the Sarbanes-Oxley Act of 2002. It observes the following core policy features on engaging the external auditor for non-audit services: GSK non-audit services policy, key features: Process: All non-audit services over £50,000 are put to competitive tender with other financial services providers, in line with the Group’s procurement process, unless the skills and experience of the external auditor make it the only suitable supplier. Safeguards: Adequate safeguards are established so that the objectivity and independence of the Group audit are not threatened or compromised. Fee cap: The total fee payable for non-audit services should not exceed 50% of the annual audit fee, except in special circumstances where there would be a clear advantage in the auditor undertaking the additional work. Prohibitions: GSK’s policy includes a ‘whitelist’ of permitted non-audit services in line with the relevant regulations. Any service not on this list is prohibited. Pre-approval: All non-audit services require pre-approval as set out in the table below to ensure services approved are consistent with GSK’s non-audit policy for permissible services. This process ensures all services fall within the scope of services permitted and pre-approved by the Committee and does not represent a delegation of authority for pre-approval. Value More than £50,000 Between £25,000 and £50,000 Under £25,000 Pre-approver Committee Chair and CFO Group Financial Controller Designate of the Group Financial Controller Audit and other services comparison (£m) 1.6 6.3 4.0 20.4 20.6 27.7 2023 2022 2021 0 10 20 30 l Audit services l Other Assurance services Further fees payable to Deloitte for non-audit services relating to the Consumer Healthcare demerger were £4.4 million in 2022 and £2.4 million in 2021, as set out on page 115 of the 2021 Annual Report and page 128 of the 2022 Annual Report respectively. A fee of £0.2 million was paid to the auditor in respect of GSK pension schemes in each of 2021, 2022 and 2023 The fees paid to the company's auditor and its associates are set out above. Further details are given in Note 8 to the financial statements, ‘Operating profit’ on page 176. The Committee considered the level of non-audit services incurred as part of its annual review of Deloitte’s independence set out on the previous page and was satisfied that the auditor continued to be independent and exercised objectivity throughout 2023. Fair, balanced and understandable assessment The need for an annual report to be fair, balanced and understandable is one of the key compliance requirements for a company’s financial statements. To ensure that GSK’s Annual Report meets this requirement, we have a well-established and documented process governing the coordination and review of Group-wide contributions to the publication. This runs in parallel with the process followed by the external auditor. The Committee received a summary of management’s approach to GSK’s 2023 Annual Report to ensure it met the requirements of the FRC’s Code. This enabled the Committee, and the Board, to confirm that GSK’s 2023 Annual Report as a whole is fair, balanced and understandable and provides the necessary information for shareholders to assess the company’s position and performance, business model and strategy. Code of Conduct and reporting lines We have a number of well-established policies (including a new Code of Conduct), which are available on gsk.com, together with details of our confidential Speak Up lines for reporting and investigating unlawful conduct. Charles Bancroft Audit & Risk Committee Chair 27 February 2024 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Board committee reports continued 126

Dear Shareholder, On behalf of the Remuneration Committee, I am pleased to present our Remuneration Report for 2023. This includes my Annual Statement, explaining the Committee’s work this year and our Annual Report on remuneration for 2023. Context for 2023 remuneration and outcomes As outlined earlier in this report, GSK delivered very strong performance in 2023, with sales, adjusted operating profit and adjusted EPS all growing at double-digit levels for the year (excluding COVID-19 solutions). There was also strong pipeline progress with four major product approvals, including the world's first RSV vaccine, Arexvy. Overall, the company is delivering the step change in performance in the commitments it has previously made to shareholders. 2023 was the second year of operation of the company's new Remuneration policy. This is a fundamental part of the architecture of GSK post-separation to ensure we build a performance culture and generate sustained delivery of shareholder value. Our policy better links executive remuneration to delivery of outperformance, with the Annual Bonus opportunity significantly reduced for below target performance, and increased for exceptional outperformance. Under the new scheme, the increase in the Bonus opportunity does not increase the potential cash reward for executives, as any incremental award is delivered in the form of shares, deferred for three years. 2023 Annual Bonus It is against this delivery that the Committee reviewed the Bonus outcomes for the CEO and CFO. In terms of the two financial measures, the company delivered sales growth and adjusted operating profit growth (both excluding COVID-19 solutions) which were significantly higher than both the guidance the company provided at the start of the year and market expectations. This very strong performance led to an overall payout under the financial elements of the Bonus of 190% of salary. The 2023 targets were set after consideration of analyst consensus, and the Committee is comfortable that the payout is representative of very strong performance. The Committee also reviewed performance against the non- financial measures previously disclosed, together with executives’ delivery against their specific individual strategic and operational measures. When this performance was combined the overall payout against a maximum of 300% was 288% of salary for the CEO (of which 188% of salary is delivered in deferred shares) and 264% of salary for the CFO (197.5% of salary, after proration for the period she was employed in 2023; of which 99% of salary is delivered in deferred shares). Long-term incentive (LTI) awards 69.95% of the grant under the 2021 Performance Share Plan (PSP) award vested based on performance to the end of 2023. The award vested in three out of four measures. There was full vesting of the Pipeline Progress measure and almost full vesting under our Innovation Sales measure (20% and 19.95% respectively). The Cash Flow measure also vested in full (30%). We remain disappointed that we have not yet achieved vesting under our Relative TSR measure. In part this reflects the adverse share price reaction to Zantac litigation in the period, but we also recognise there have been relative concerns on the strength of the company’s pipeline. We are confident that the progress we are making to develop our portfolio, together with our improving longer-term outlooks for growth, will be increasingly reflected in GSK’s valuation. Summary of incentive outcomes Following a review of contextual factors including previous payouts, the Committee believes that the outcomes appropriately reflect performance in the round having considered the experience of all stakeholders including shareholders and our employees. The incentive awards in relation to 2023 were all made in accordance with the 2022 Remuneration policy. I also confirm that following careful review the Committee did not deem it necessary to exercise discretion. Remuneration policy implementation for 2024 Annual Bonus and LTI The Committee has determined that no changes will be made to our Bonus and LTI measures for 2024. The total sales growth and adjusted operating profit growth targets exclude the commercial benefit from COVID-19 solutions. Annual Bonus measures will continue to be based on: – annual total sales growth (30%) – annual adjusted operating profit growth (30%) – personal performance against strategic and operational measures (30%) – ESG: diversity, equity and inclusion (DEI) (10%) PSP measures will remain as: – relative TSR (30%) – total sales growth over three years (20%) – adjusted operating profit growth over three years (20%) – pipeline progress (20%) – ESG: environment composite scorecard (10%) The performance targets were also calibrated to consider a number of internal and external reference points, in particular analyst consensus. These were used to challenge the metrics and with input from our Science and Corporate Responsibility committees where relevant. The Committee is therefore satisfied that the targets set for 2024 are suitably stretching. Salary The Committee noted that a 4% increase has been agreed for the wider workforce in the UK. After careful consideration, including a review of the market and the CEO and CFO’s competitive positioning, it was agreed that they should each receive salary increases of 4% for 2024. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Remuneration report Committee Chair's annual statement 127

Workforce fairness In setting executive pay it is important that the Committee does so with a good understanding of the Group’s wider workforce approach to pay, with an emphasis on fairness and equity. To that end, on an annual basis, I meet with senior Human Resources Leaders from across the company to understand their perspectives on pay and GSK’s remuneration arrangements for the wider workforce globally. This year was the fifth such annual meeting held. Details of this important check are given on page 114. Board changes As announced in September 2022, Julie Brown joined the Board on 1 May 2023 as CFO, at which point Iain Mackay stepped down from the Board. Details of the joining and leaving arrangements for this transition were described in last year’s report. Remuneration Committee Chair succession Finally, I will be retiring as a Non-Executive Director of GSK at the 2024 AGM and this will therefore be my final report as Chair of the Committee. I was delighted to welcome Wendy Becker, who joined the Committee on 1 October 2023. Since then, Wendy and I have been working on a smooth transition and handover before she succeeds me as Committee Chair in May 2024. She has a wealth of experience chairing remuneration committees and is looking forward to chairing the Committee and leading our 2025 Policy review. The Committee is planning to undertake a review of the effectiveness of our remuneration arrangements in advance of the scheduled Policy renewal at the 2025 AGM. Wendy and Jonathan Symonds, our Chair, are looking forward to engaging with investors to ensure we are clear on your perspectives as we work to update our Remuneration policy. Thank you I would like to take this opportunity to thank both my fellow Committee members and shareholders for your support and engagement during my tenure as Committee Chair. I welcome all further feedback and look forward to receiving your support for this report at our Annual General Meeting on 8 May 2024. Urs Rohner Remuneration Committee Chair 27 February 2024 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Committee Chair's annual statement continued 128

2023 Total Remuneration Emma Walmsley, CEO 2023 2022 £0m £2m £4m £6m £8m £10m £12m £14m Julie Brown, CFO (from 1 May 2023)(1) 2023 £0m £2m £4m £6m £8m (1) See details of CFO joining arrangements on page 130 l Fixed pay – salary, benefits, pensions and other l Performance pay – annual bonus and vested LTIs 2023 Pay for performance 2023 Annual bonus outcome Financial measures Target Delivered Total sales growth* 7.0% Exceeded Adjusted operating profit growth* 11.0% Exceeded Non-Financial measures Overall Strategic and Operational measures ESG DEI Emma Walmsley Exceeded Met in full Julie Brown Exceeded Met in full 2023 Annual bonus delivery 65% 35% 50%50% l Shares deferred for 3 years (1)For service from employment on 3 April 2023l Cash 2021 PSP outcome Adjusted free cash flow 30% of 30% Innovation sales 19.95% of 20% Pipeline progress 20% of 20% l Vested l Lapsed 2024 Remuneration implementation Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Remuneration at a glance 129 Pay for Performance Bonus and LTI measures remain as follows Alignment to strategy Annual bonus LTIs (PSP) Total sales growth* In line with sales growth ambitions 30% 20% Adjusted operating profit growth* In line with adjusted operating profit growth ambitions 30% 20% Strategic and operational Individual accountability for delivery of our strategy and public ambitions 30% – Pipeline Emphasis on Innovation – rewards acceleration and strengthening of pipeline – 20% Relative total shareholder return Alignment with shareholders – 30% ESG ambitions Nature and Climate ambitions 2024 – DEI Priorities – 10% 10% – *excluding COVID-19 solutions CEO Overall bonus 288% of salary CFO(1) Overall bonus 197.5% of salary Overall vesting 69.95% Relative TSR 0% of 30% Base salary 4% increase for UK employees and Executive Directors Benefits and pensions No changes
Fixed pay Pay for performance Salary Total remunerationPension Annual Bonus LTI awards (2021 PSP award vesting) Benefits Read more on page 131 pages 132 and 133 pages 134 and 135 below 2023 Total remuneration (audited) The following sections from this page to page 148 provide details of each element of 2023 ‘Total remuneration’ and how the Committee implemented the company’s shareholder-approved 2022 Remuneration policy during the year in terms of fixed and performance pay. 2023 Total remuneration (audited) Emma Walmsley, CEO Julie Brown, CFO (from 1 May 2023) Iain Mackay, former CFO (to 1 May 2023) 2023 £000 2022 £000 2023 £000 2022 £000 2023 £000 2022 £000 Fixed pay Salary 1,310 1,260 635 — 305 915 Benefits 212 131 50 — 82 291 Pension 94 253 44 — 22 183 Other(1) — — 2,411 — — — Total fixed pay 1,616 1,644 3,140 — 409 1,389 Pay for performance Annual bonus(2) (3) 3,774 3,143 1,687 — 728 2,082 Vesting of PSP LTI awards(4) (5) (6) 7,328 3,662 — — 5,294 1,854 Total pay for performance 11,102 6,805 1,687 — 6,022 3,936 Total remuneration 12,718 8,449 4,827 — 6,431 5,325 (1) Other: Represents the sum paid to Julie Brown, the CFO, as part of her Buyout arrangements in relation to leaving Burberry, as set out in full on page 149 of the 2022 Annual Report. In setting the Buyout arrangements, which are staged over a two year period, the Committee sought to ensure she was compensated on a like-for-like basis as far as possible. In fulfilment of these arrangements, the CFO purchased 22,500 shares in June 2023 (2) Deferred Annual Bonus Plan (DABP): The mandatory DABP bonus deferrals for 2022 and 2023 are set out on page 144 (3) Annual bonus: The 2023 bonus payment for Iain Mackay represents bonus earned in respect of the period from 1 January to 1 May 2023. Details of the bonus paid in respect of the remainder of the year can be found in the Leaving Directors section on page 142. The 2023 bonus payment for Julie Brown represents bonus earned in respect of the period from 1 May to 31 December 2023. Bonus for the full period of her employment in 2023 is shown on page 132 (4) 2020 PSP vesting in 2023: The Total remuneration figure for the CEO in 2022 included vesting of the top-up award made in May 2020 which did not vest until May 2023. The final actual value received has been updated, bringing the total value to £3.662 million (previously reported as £3.666 million) (5) 2021 PSP vesting in 2024: For the CEO, the figure has been valued based on the vesting prices on 9 February 2024 of £16.60. The share price on 10 February 2021, the date of grant, was £12.77. Of the vested amounts for the Executive Directors, 23.1% of the value was attributable to share price appreciation over the performance period. The Committee did not exercise any discretion in relation to the vesting of the awards or share price changes (6) The PSP vesting value for the former CFO is unreduced and is an illustrative amount as the award will not vest until January 2025 in accordance with the terms of the Executive and Senior Management Recoupment Policy (Recoupment Policy). His award will then remain subject to the two-year holding period which started from the original vesting date of the award in February 2024. The actual value received will be updated in the 2024 Annual Report. Further details of the former CFO’s leaving arrangements are set out in the Leaving Directors section on page 142 (7) The CEO and CFO each contribute the maximum of £250 and £125 a month into the Share Save plan and to buy shares under the Share Reward plan respectively. Further details of these HM Revenue & Customs (HMRC) approved all-employee plans are set out on page 135 (8) Malus and clawback: The Committee may in specific circumstances, and in line with stated principles, apply malus/clawback, as it determines appropriate. Following due consideration by the Committee, there has been no recovery of sums paid (clawback) or reduction of outstanding awards or vesting levels (malus) applied during 2023 in respect of any of the CEO, CFO or the former CFO Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration 130 =++
Fixed pay (audited) Salary The Committee is very aware of the sensitivity amongst stakeholders to levels of pay. Before setting or reviewing salary, it considered the average increases awarded to employees below Executive Directors and the multiplier effect of increases in base salaries on total remuneration opportunity. The Committee considered the wider economic context, individual performance and market positioning of the increases awarded. The table below sets out the base salaries and increases agreed for 2023 and 2024 of the Executive Directors compared to increases of the UK workforce. 2023 effective date % change Salary £000 2024 2023 2024 2023 2022 UK employees 1 April 4 5 Emma Walmsley 1 January 4 4 1,363 1,310 1,260 Julie Brown 1 May 4 4 990 952 — Julie Brown's salary on the announcement of her appointment in September 2022 was set in line with her predecessor, given her extensive experience as a CFO. Her salary upon joining was increased to reflect the increases awarded to UK employees and the CEO in early 2023. Benefits This table provides an analysis of total benefits (grossed up for tax) received by the Executive Directors in 2023 and 2022. The UK remuneration reporting regulations require the company to add into each Executive Director’s total benefits all items which are deemed by tax authorities to be a taxable benefit for them. These include employee benefits as well as business-related services provided to employees to assist or enable them to carry out their role, which a tax authority has deemed to be a taxable “benefit” to the individual. Because these are business expenses, the company meets the tax which arises on them and therefore the items are shown grossed up for tax. Benefits £000 2023 2022 Emma Walmsley Benefits available to employees 118 66 Business-related services 94 65 Total benefits 212 131 Julie Brown Benefits available to employees 25 — Business-related services 25 — Total benefits 50 — Pensions From 1 January 2023, pension arrangements for Executive Directors were aligned to the wider workforce. They received GSK pension contributions or cash supplements of 7% of base salary and matching contributions of up to 3% on the first £26,666 of salary to 31 March 2023 and on the first £66,666 of salary for the rest of 2023. The table shows the breakdown of the pension values included in 2023 Total remuneration on page 130. They are calculated as set out in the UK Large and Medium-sized Companies and Groups (Accounts and Reports) Regulations 2008 (as amended) (Remuneration regulations). Pension remuneration values Emma Walmsley Julie Brown (from May 2023) 2023 2022 2023 2022 UK defined contribution 6 3 — — Employer cash contributions 88 250 44 — Pension 94 253 44 — Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued 2023 Total remuneration (audited) continued 131
Pay for performance (audited) Annual Bonus l Financial Measures: 60% l Non-Financial Measures: 40% 2023 Annual Bonus performance The following table shows the Annual Bonuses earned compared to the bonus opportunity for 2023: 2023 Bonus opportunity 2023 Bonus outcome Target (% of salary) Maximum (% of salary) 2023 salary £000 Paid as (£000) % of Maximum Bonus % of Salary Cash Shares (DABP Award)Bonus Emma Walmsley 100 300 1,310 96 288 1,310 2,463 Julie Brown 952 66 197 940 940 Details of the mandatory deferral by Executive Directors into the DABP for the 2023 bonus are set out on page 144. See page 142 for details of Iain Mackay's 2023 bonus arrangements following his retirement from the company. Julie Brown's bonus has been pro-rated to reflect the period for which she was employed in 2023. The table on page 130 provides the details of her bonus from 1 May when she became an Executive Director. 2023 Financial measures Total sales growth Adjusted operating profit growth These targets were set following consideration of analyst consensus as well as internal budgets. Threshold and maximum performance was at 1% below and 5% above target growth respectively. The total sales growth and adjusted operating profit growth targets and outcomes for the purposes of the Annual Bonus calculation are based on CER and excluding the commercial benefit from COVID-19 solutions. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued 132 Total sales growth 30% Adjusted operating profit growth 30% Strategic and operational measures 30% ESG: DEI 10% Annual Bonus+ + + =
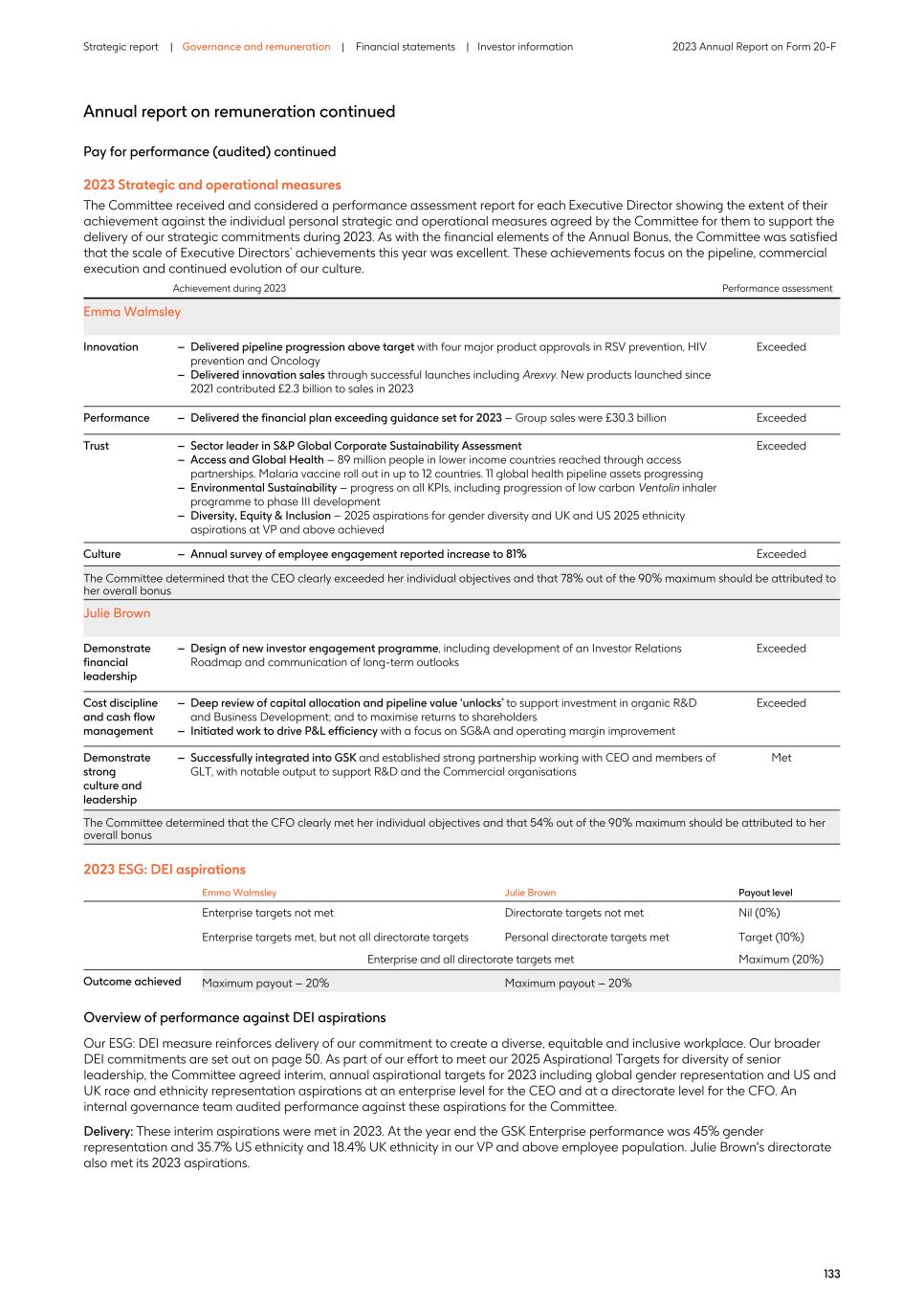
2023 Strategic and operational measures The Committee received and considered a performance assessment report for each Executive Director showing the extent of their achievement against the individual personal strategic and operational measures agreed by the Committee for them to support the delivery of our strategic commitments during 2023. As with the financial elements of the Annual Bonus, the Committee was satisfied that the scale of Executive Directors’ achievements this year was excellent. These achievements focus on the pipeline, commercial execution and continued evolution of our culture. Achievement during 2023 Performance assessment Emma Walmsley Innovation – Delivered pipeline progression above target with four major product approvals in RSV prevention, HIV prevention and Oncology – Delivered innovation sales through successful launches including Arexvy. New products launched since 2021 contributed £2.3 billion to sales in 2023 Exceeded Performance – Delivered the financial plan exceeding guidance set for 2023 – Group sales were £30.3 billion Exceeded Trust – Sector leader in S&P Global Corporate Sustainability Assessment – Access and Global Health – 89 million people in lower income countries reached through access partnerships. Malaria vaccine roll out in up to 12 countries. 11 global health pipeline assets progressing – Environmental Sustainability – progress on all KPIs, including progression of low carbon Ventolin inhaler programme to phase III development – Diversity, Equity & Inclusion – 2025 aspirations for gender diversity and UK and US 2025 ethnicity aspirations at VP and above achieved Exceeded Culture – Annual survey of employee engagement reported increase to 81% Exceeded The Committee determined that the CEO clearly exceeded her individual objectives and that 78% out of the 90% maximum should be attributed to her overall bonus Julie Brown Demonstrate financial leadership – Design of new investor engagement programme, including development of an Investor Relations Roadmap and communication of long-term outlooks Exceeded Cost discipline and cash flow management – Deep review of capital allocation and pipeline value ‘unlocks’ to support investment in organic R&D and Business Development; and to maximise returns to shareholders – Initiated work to drive P&L efficiency with a focus on SG&A and operating margin improvement Exceeded Demonstrate strong culture and leadership – Successfully integrated into GSK and established strong partnership working with CEO and members of GLT, with notable output to support R&D and the Commercial organisations Met The Committee determined that the CFO clearly met her individual objectives and that 54% out of the 90% maximum should be attributed to her overall bonus 2023 ESG: DEI aspirations Emma Walmsley Julie Brown Payout level Enterprise targets not met Directorate targets not met Nil (0%) Enterprise targets met, but not all directorate targets Personal directorate targets met Target (10%) Enterprise and all directorate targets met Maximum (20%) Outcome achieved Maximum payout – 20% Maximum payout – 20% Overview of performance against DEI aspirations Our ESG: DEI measure reinforces delivery of our commitment to create a diverse, equitable and inclusive workplace. Our broader DEI commitments are set out on page 50. As part of our effort to meet our 2025 Aspirational Targets for diversity of senior leadership, the Committee agreed interim, annual aspirational targets for 2023 including global gender representation and US and UK race and ethnicity representation aspirations at an enterprise level for the CEO and at a directorate level for the CFO. An internal governance team audited performance against these aspirations for the Committee. Delivery: These interim aspirations were met in 2023. At the year end the GSK Enterprise performance was 45% gender representation and 35.7% US ethnicity and 18.4% UK ethnicity in our VP and above employee population. Julie Brown's directorate also met its 2023 aspirations. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued Pay for performance (audited) continued 133
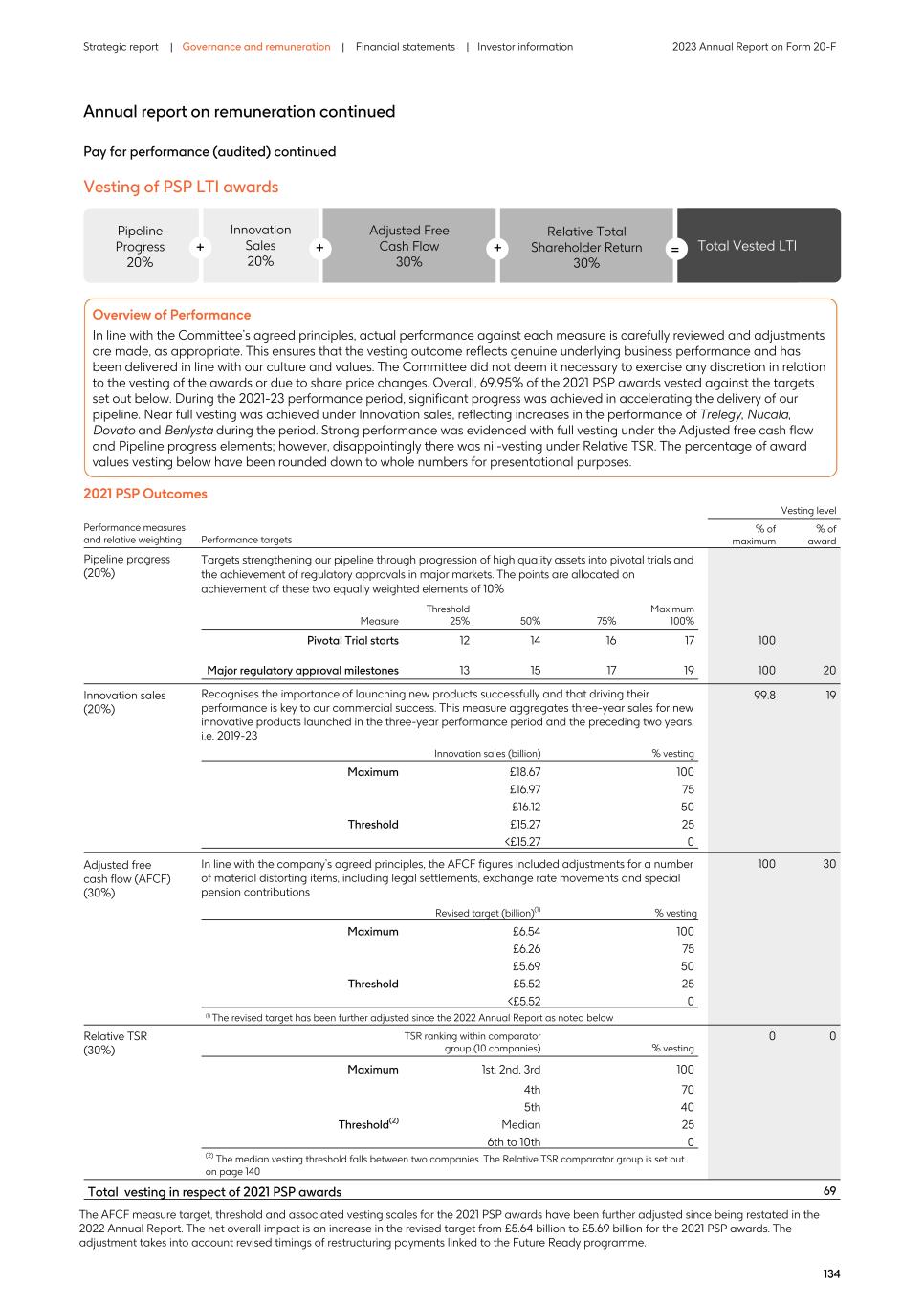
Vesting of PSP LTI awards Overview of Performance In line with the Committee’s agreed principles, actual performance against each measure is carefully reviewed and adjustments are made, as appropriate. This ensures that the vesting outcome reflects genuine underlying business performance and has been delivered in line with our culture and values. The Committee did not deem it necessary to exercise any discretion in relation to the vesting of the awards or due to share price changes. Overall, 69.95% of the 2021 PSP awards vested against the targets set out below. During the 2021-23 performance period, significant progress was achieved in accelerating the delivery of our pipeline. Near full vesting was achieved under Innovation sales, reflecting increases in the performance of Trelegy, Nucala, Dovato and Benlysta during the period. Strong performance was evidenced with full vesting under the Adjusted free cash flow and Pipeline progress elements; however, disappointingly there was nil-vesting under Relative TSR. The percentage of award values vesting below have been rounded down to whole numbers for presentational purposes. 2021 PSP Outcomes Vesting level Performance measures and relative weighting Performance targets % of maximum % of award Pipeline progress (20%) Targets strengthening our pipeline through progression of high quality assets into pivotal trials and the achievement of regulatory approvals in major markets. The points are allocated on achievement of these two equally weighted elements of 10% Measure Threshold 25% 50% 75% Maximum 100% Pivotal Trial starts 12 14 16 17 100 20Major regulatory approval milestones 13 15 17 19 100 Innovation sales (20%) Recognises the importance of launching new products successfully and that driving their performance is key to our commercial success. This measure aggregates three-year sales for new innovative products launched in the three-year performance period and the preceding two years, i.e. 2019-23 99.8 19 Innovation sales (billion) % vesting Maximum £18.67 100 £16.97 75 £16.12 50 Threshold £15.27 25 <£15.27 0 Adjusted free cash flow (AFCF) (30%) In line with the company’s agreed principles, the AFCF figures included adjustments for a number of material distorting items, including legal settlements, exchange rate movements and special pension contributions 100 30 Revised target (billion)(1) % vesting Maximum £6.54 100 £6.26 75 £5.69 50 Threshold £5.52 25 <£5.52 0 (1) The revised target has been further adjusted since the 2022 Annual Report as noted below Relative TSR (30%) TSR ranking within comparator group (10 companies) % vesting 0 0 Maximum 1st, 2nd, 3rd 100 4th 70 5th 40 Threshold(2) Median 25 6th to 10th 0 (2) The median vesting threshold falls between two companies. The Relative TSR comparator group is set out on page 140 Total vesting in respect of 2021 PSP awards 69 The AFCF measure target, threshold and associated vesting scales for the 2021 PSP awards have been further adjusted since being restated in the 2022 Annual Report. The net overall impact is an increase in the revised target from £5.64 billion to £5.69 billion for the 2021 PSP awards. The adjustment takes into account revised timings of restructuring payments linked to the Future Ready programme. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued Pay for performance (audited) continued 134 Pipeline Progress 20% Innovation Sales 20% Adjusted Free Cash Flow 30% Relative Total Shareholder Return 30% Total Vested LTI+ + + =
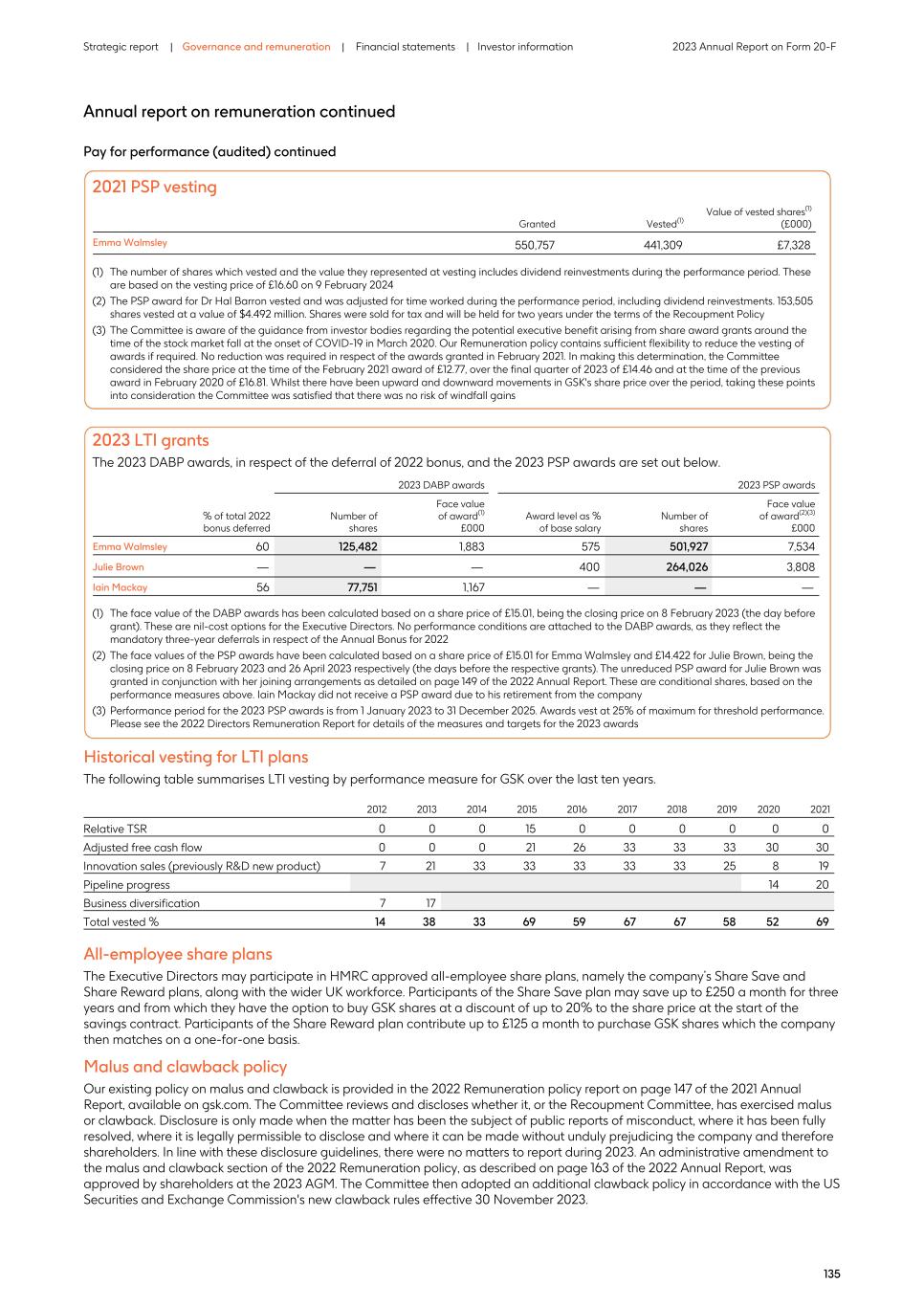
2021 PSP vesting Granted Vested(1) Value of vested shares(1) (£000) Emma Walmsley 550,757 441,309 £7,328 (1) The number of shares which vested and the value they represented at vesting includes dividend reinvestments during the performance period. These are based on the vesting price of £16.60 on 9 February 2024 (2) The PSP award for Dr Hal Barron vested and was adjusted for time worked during the performance period, including dividend reinvestments. 153,505 shares vested at a value of $4.492 million. Shares were sold for tax and will be held for two years under the terms of the Recoupment Policy (3) The Committee is aware of the guidance from investor bodies regarding the potential executive benefit arising from share award grants around the time of the stock market fall at the onset of COVID-19 in March 2020. Our Remuneration policy contains sufficient flexibility to reduce the vesting of awards if required. No reduction was required in respect of the awards granted in February 2021. In making this determination, the Committee considered the share price at the time of the February 2021 award of £12.77, over the final quarter of 2023 of £14.46 and at the time of the previous award in February 2020 of £16.81. Whilst there have been upward and downward movements in GSK's share price over the period, taking these points into consideration the Committee was satisfied that there was no risk of windfall gains 2023 LTI grants The 2023 DABP awards, in respect of the deferral of 2022 bonus, and the 2023 PSP awards are set out below. 2023 DABP awards 2023 PSP awards % of total 2022 bonus deferred Number of shares Face value of award(1) £000 Award level as % of base salary Number of shares Face value of award(2)(3) £000 Emma Walmsley 60 125,482 1,883 575 501,927 7,534 Julie Brown — — — 400 264,026 3,808 Iain Mackay 56 77,751 1,167 — — — (1) The face value of the DABP awards has been calculated based on a share price of £15.01, being the closing price on 8 February 2023 (the day before grant). These are nil-cost options for the Executive Directors. No performance conditions are attached to the DABP awards, as they reflect the mandatory three-year deferrals in respect of the Annual Bonus for 2022 (2) The face values of the PSP awards have been calculated based on a share price of £15.01 for Emma Walmsley and £14.422 for Julie Brown, being the closing price on 8 February 2023 and 26 April 2023 respectively (the days before the respective grants). The unreduced PSP award for Julie Brown was granted in conjunction with her joining arrangements as detailed on page 149 of the 2022 Annual Report. These are conditional shares, based on the performance measures above. Iain Mackay did not receive a PSP award due to his retirement from the company (3) Performance period for the 2023 PSP awards is from 1 January 2023 to 31 December 2025. Awards vest at 25% of maximum for threshold performance. Please see the 2022 Directors Remuneration Report for details of the measures and targets for the 2023 awards Historical vesting for LTI plans The following table summarises LTI vesting by performance measure for GSK over the last ten years. 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 Relative TSR 0 0 0 15 0 0 0 0 0 0 Adjusted free cash flow 0 0 0 21 26 33 33 33 30 30 Innovation sales (previously R&D new product) 7 21 33 33 33 33 33 25 8 19 Pipeline progress 14 20 Business diversification 7 17 Total vested % 14 38 33 69 59 67 67 58 52 69 All-employee share plans The Executive Directors may participate in HMRC approved all-employee share plans, namely the company’s Share Save and Share Reward plans, along with the wider UK workforce. Participants of the Share Save plan may save up to £250 a month for three years and from which they have the option to buy GSK shares at a discount of up to 20% to the share price at the start of the savings contract. Participants of the Share Reward plan contribute up to £125 a month to purchase GSK shares which the company then matches on a one-for-one basis. Malus and clawback policy Our existing policy on malus and clawback is provided in the 2022 Remuneration policy report on page 147 of the 2021 Annual Report, available on gsk.com. The Committee reviews and discloses whether it, or the Recoupment Committee, has exercised malus or clawback. Disclosure is only made when the matter has been the subject of public reports of misconduct, where it has been fully resolved, where it is legally permissible to disclose and where it can be made without unduly prejudicing the company and therefore shareholders. In line with these disclosure guidelines, there were no matters to report during 2023. An administrative amendment to the malus and clawback section of the 2022 Remuneration policy, as described on page 163 of the 2022 Annual Report, was approved by shareholders at the 2023 AGM. The Committee then adopted an additional clawback policy in accordance with the US Securities and Exchange Commission's new clawback rules effective 30 November 2023. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued Pay for performance (audited) continued 135
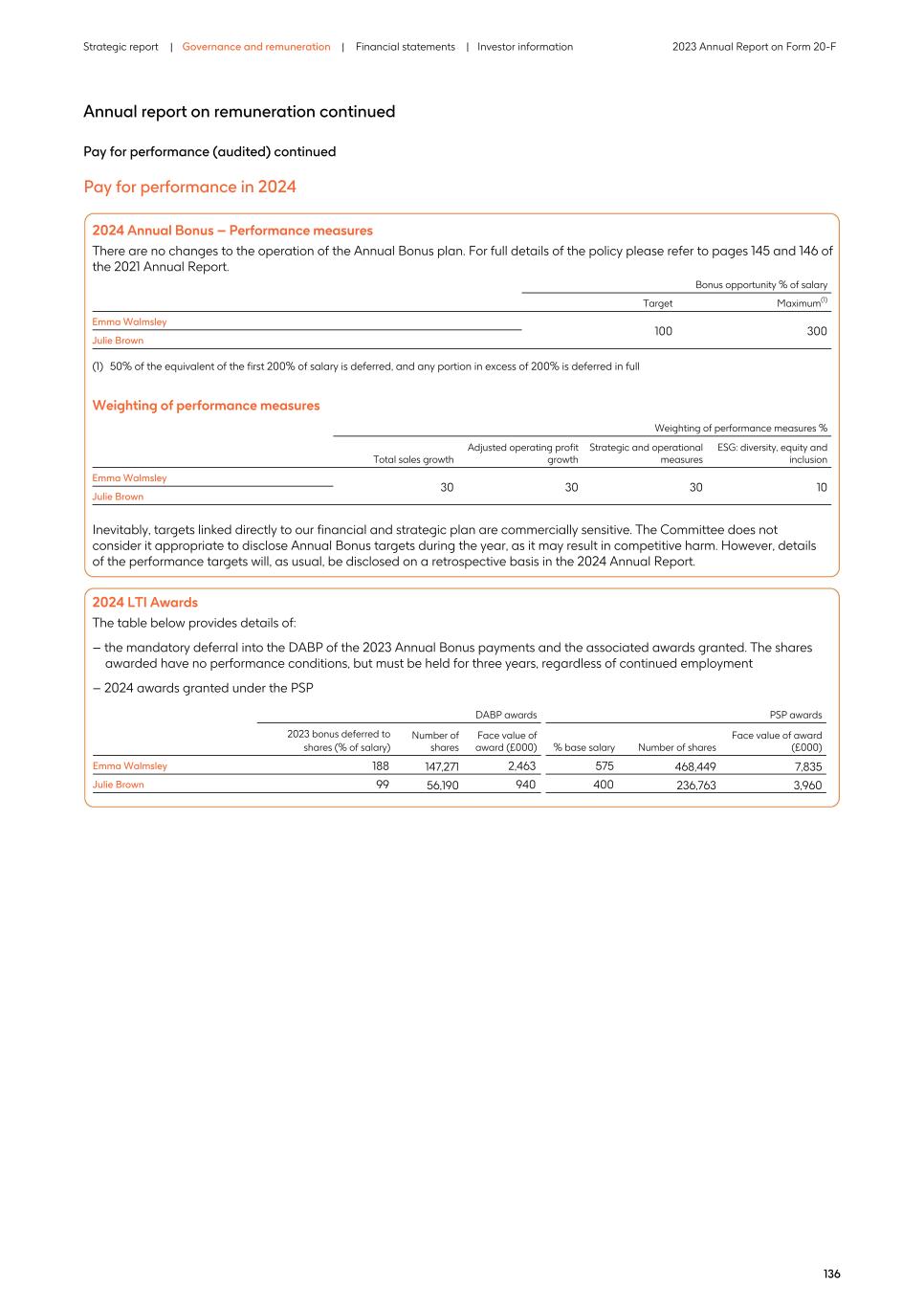
Pay for performance in 2024 2024 Annual Bonus – Performance measures There are no changes to the operation of the Annual Bonus plan. For full details of the policy please refer to pages 145 and 146 of the 2021 Annual Report. Bonus opportunity % of salary Target Maximum(1) Emma Walmsley 100 300 Julie Brown (1) 50% of the equivalent of the first 200% of salary is deferred, and any portion in excess of 200% is deferred in full Weighting of performance measures Weighting of performance measures % Total sales growth Adjusted operating profit growth Strategic and operational measures ESG: diversity, equity and inclusion Emma Walmsley 30 30 30 10 Julie Brown Inevitably, targets linked directly to our financial and strategic plan are commercially sensitive. The Committee does not consider it appropriate to disclose Annual Bonus targets during the year, as it may result in competitive harm. However, details of the performance targets will, as usual, be disclosed on a retrospective basis in the 2024 Annual Report. 2024 LTI Awards The table below provides details of: – the mandatory deferral into the DABP of the 2023 Annual Bonus payments and the associated awards granted. The shares awarded have no performance conditions, but must be held for three years, regardless of continued employment – 2024 awards granted under the PSP DABP awards PSP awards 2023 bonus deferred to shares (% of salary) Number of shares Face value of award (£000) % base salary Number of shares Face value of award (£000) Emma Walmsley 188 147,271 2,463 575 468,449 7,835 Julie Brown 99 56,190 940 400 236,763 3,960 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued Pay for performance (audited) continued 136
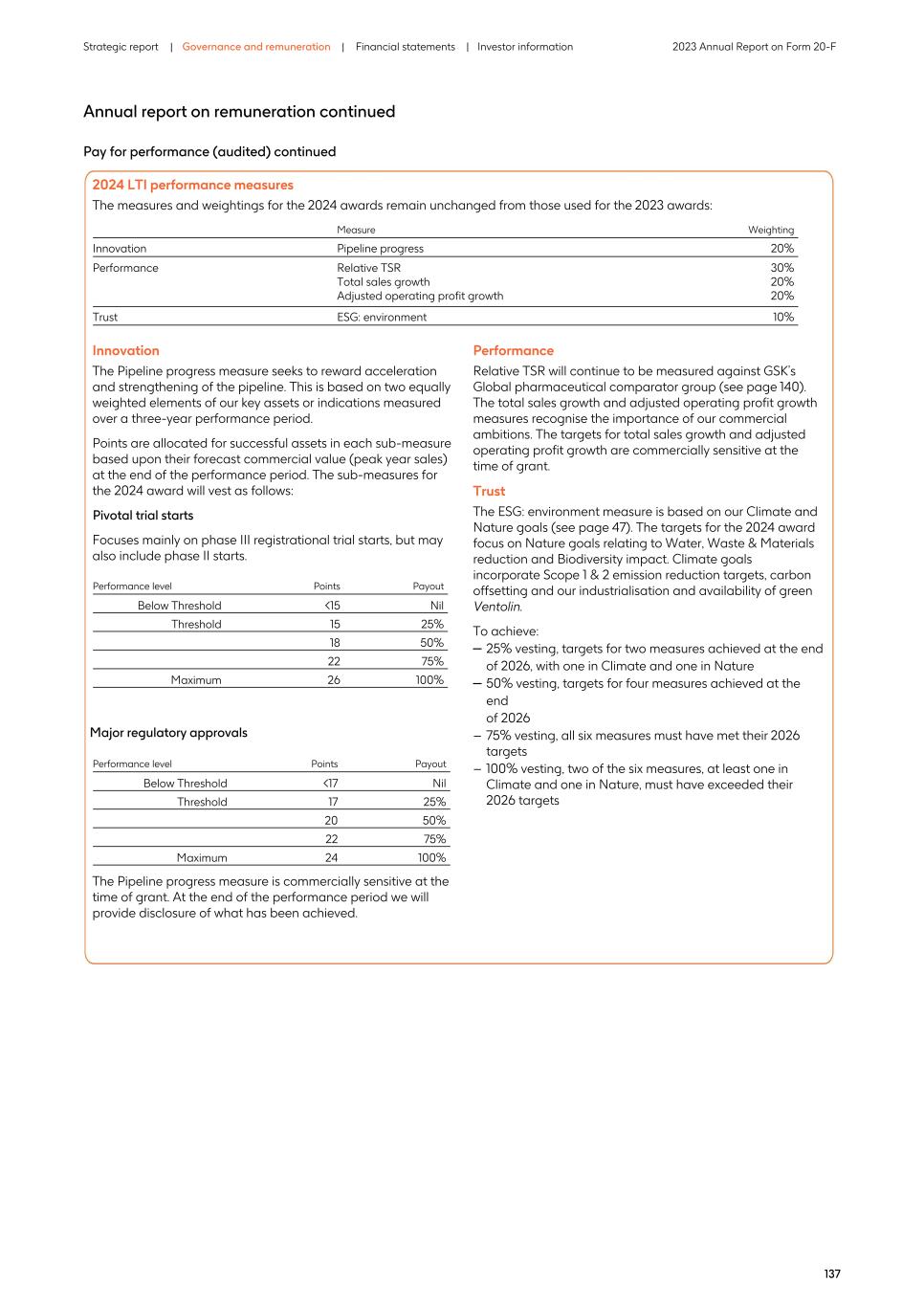
2024 LTI performance measures The measures and weightings for the 2024 awards remain unchanged from those used for the 2023 awards: Measure Weighting Innovation Pipeline progress 20% Performance Relative TSR Total sales growth Adjusted operating profit growth 30% 20% 20% Trust ESG: environment 10% Innovation The Pipeline progress measure seeks to reward acceleration and strengthening of the pipeline. This is based on two equally weighted elements of our key assets or indications measured over a three-year performance period. Points are allocated for successful assets in each sub-measure based upon their forecast commercial value (peak year sales) at the end of the performance period. The sub-measures for the 2024 award will vest as follows: Pivotal trial starts Focuses mainly on phase III registrational trial starts, but may also include phase II starts. Performance level Points Payout Below Threshold <15 Nil Threshold 15 25% 18 50% 22 75% Maximum 26 100% Major regulatory approvals Performance level Points Payout Below Threshold <17 Nil Threshold 17 25% 20 50% 22 75% Maximum 24 100% The Pipeline progress measure is commercially sensitive at the time of grant. At the end of the performance period we will provide disclosure of what has been achieved. Performance Relative TSR will continue to be measured against GSK’s Global pharmaceutical comparator group (see page 140). The total sales growth and adjusted operating profit growth measures recognise the importance of our commercial ambitions. The targets for total sales growth and adjusted operating profit growth are commercially sensitive at the time of grant. Trust The ESG: environment measure is based on our Climate and Nature goals (see page 47). The targets for the 2024 award focus on Nature goals relating to Water, Waste & Materials reduction and Biodiversity impact. Climate goals incorporate Scope 1 & 2 emission reduction targets, carbon offsetting and our industrialisation and availability of green Ventolin. To achieve: – 25% vesting, targets for two measures achieved at the end of 2026, with one in Climate and one in Nature – 50% vesting, targets for four measures achieved at the end of 2026 – 75% vesting, all six measures must have met their 2026 targets – 100% vesting, two of the six measures, at least one in Climate and one in Nature, must have exceeded their 2026 targets Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued Pay for performance (audited) continued 137
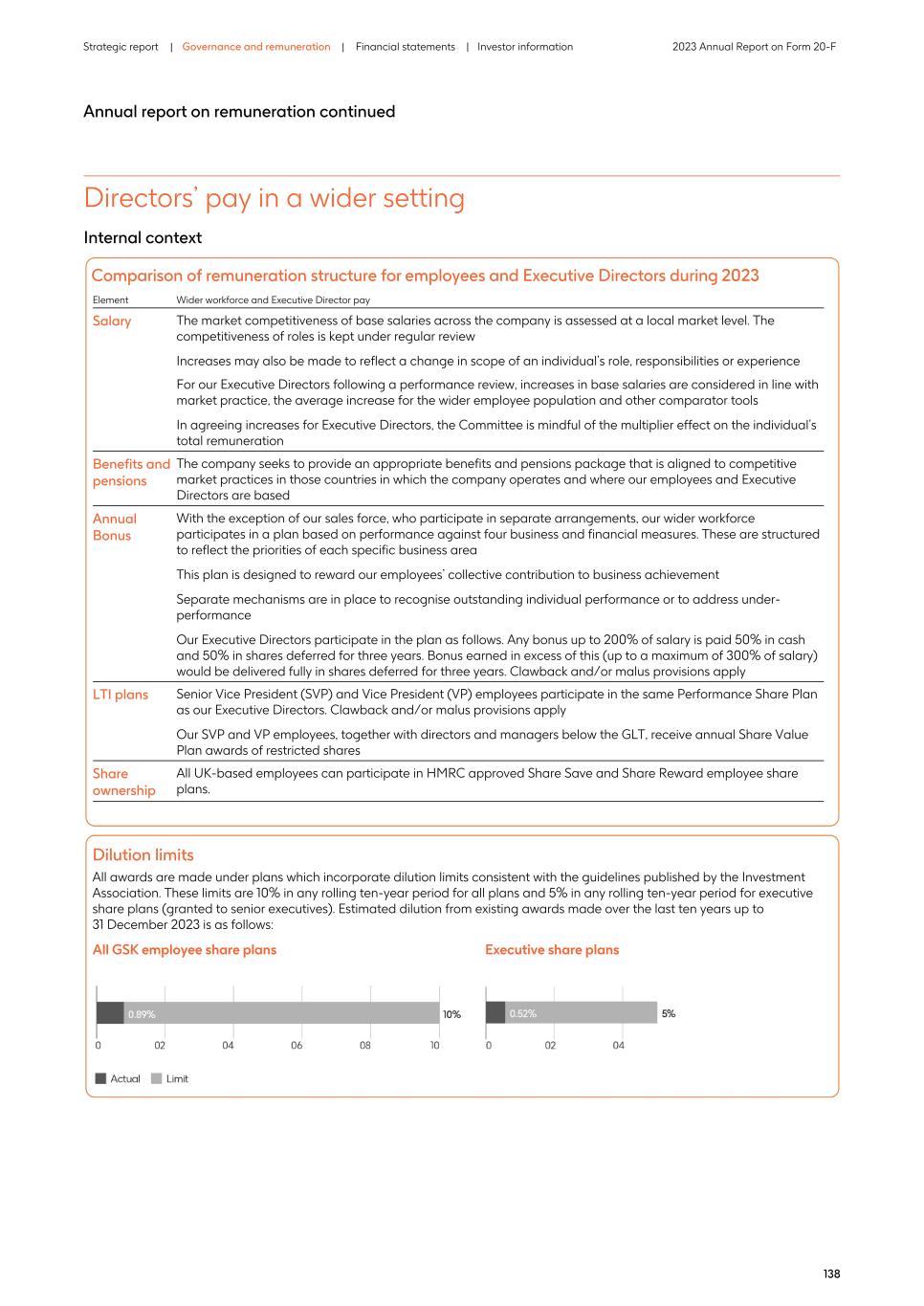
Directors’ pay in a wider setting Internal context Comparison of remuneration structure for employees and Executive Directors during 2023 Element Wider workforce and Executive Director pay Salary The market competitiveness of base salaries across the company is assessed at a local market level. The competitiveness of roles is kept under regular review Increases may also be made to reflect a change in scope of an individual’s role, responsibilities or experience For our Executive Directors following a performance review, increases in base salaries are considered in line with market practice, the average increase for the wider employee population and other comparator tools In agreeing increases for Executive Directors, the Committee is mindful of the multiplier effect on the individual’s total remuneration Benefits and pensions The company seeks to provide an appropriate benefits and pensions package that is aligned to competitive market practices in those countries in which the company operates and where our employees and Executive Directors are based Annual Bonus With the exception of our sales force, who participate in separate arrangements, our wider workforce participates in a plan based on performance against four business and financial measures. These are structured to reflect the priorities of each specific business area This plan is designed to reward our employees’ collective contribution to business achievement Separate mechanisms are in place to recognise outstanding individual performance or to address under- performance Our Executive Directors participate in the plan as follows. Any bonus up to 200% of salary is paid 50% in cash and 50% in shares deferred for three years. Bonus earned in excess of this (up to a maximum of 300% of salary) would be delivered fully in shares deferred for three years. Clawback and/or malus provisions apply LTI plans Senior Vice President (SVP) and Vice President (VP) employees participate in the same Performance Share Plan as our Executive Directors. Clawback and/or malus provisions apply Our SVP and VP employees, together with directors and managers below the GLT, receive annual Share Value Plan awards of restricted shares Share ownership All UK-based employees can participate in HMRC approved Share Save and Share Reward employee share plans. Dilution limits All awards are made under plans which incorporate dilution limits consistent with the guidelines published by the Investment Association. These limits are 10% in any rolling ten-year period for all plans and 5% in any rolling ten-year period for executive share plans (granted to senior executives). Estimated dilution from existing awards made over the last ten years up to 31 December 2023 is as follows: All GSK employee share plans Executive share plans Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued 138
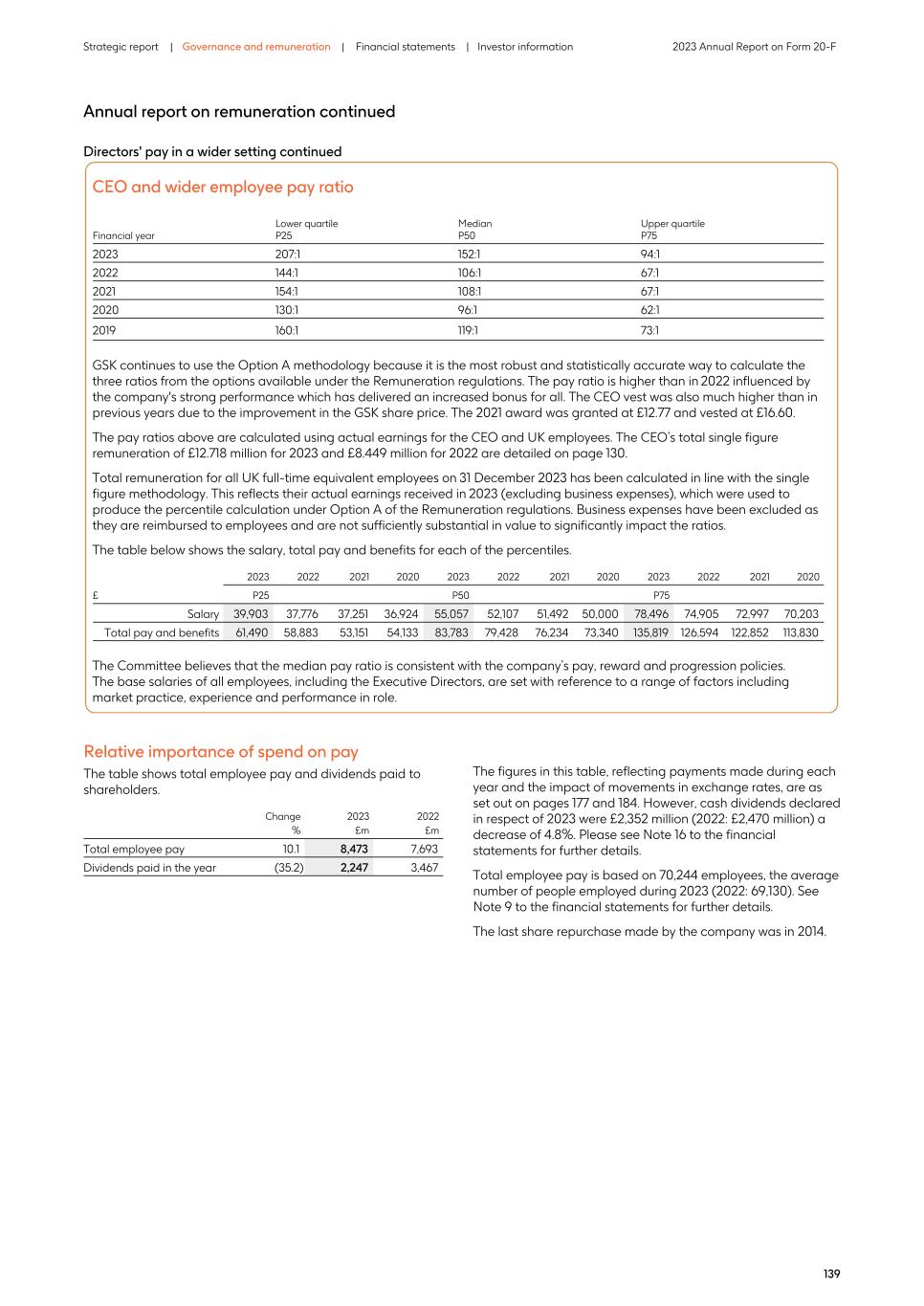
CEO and wider employee pay ratio Financial year Lower quartile P25 Median P50 Upper quartile P75 2023 207:1 152:1 94:1 2022 144:1 106:1 67:1 2021 154:1 108:1 67:1 2020 130:1 96:1 62:1 2019 160:1 119:1 73:1 GSK continues to use the Option A methodology because it is the most robust and statistically accurate way to calculate the three ratios from the options available under the Remuneration regulations. The pay ratio is higher than in 2022 influenced by the company's strong performance which has delivered an increased bonus for all. The CEO vest was also much higher than in previous years due to the improvement in the GSK share price. The 2021 award was granted at £12.77 and vested at £16.60. The pay ratios above are calculated using actual earnings for the CEO and UK employees. The CEO’s total single figure remuneration of £12.718 million for 2023 and £8.449 million for 2022 are detailed on page 130. Total remuneration for all UK full-time equivalent employees on 31 December 2023 has been calculated in line with the single figure methodology. This reflects their actual earnings received in 2023 (excluding business expenses), which were used to produce the percentile calculation under Option A of the Remuneration regulations. Business expenses have been excluded as they are reimbursed to employees and are not sufficiently substantial in value to significantly impact the ratios. The table below shows the salary, total pay and benefits for each of the percentiles. 2023 2022 2021 2020 2023 2022 2021 2020 2023 2022 2021 2020 £ P25 P50 P75 Salary 39,903 37,776 37,251 36,924 55,057 52,107 51,492 50,000 78,496 74,905 72,997 70,203 Total pay and benefits 61,490 58,883 53,151 54,133 83,783 79,428 76,234 73,340 135,819 126,594 122,852 113,830 The Committee believes that the median pay ratio is consistent with the company’s pay, reward and progression policies. The base salaries of all employees, including the Executive Directors, are set with reference to a range of factors including market practice, experience and performance in role. Relative importance of spend on pay The table shows total employee pay and dividends paid to shareholders. Change % 2023 £m 2022 £m Total employee pay 10.1 8,473 7,693 Dividends paid in the year (35.2) 2,247 3,467 The figures in this table, reflecting payments made during each year and the impact of movements in exchange rates, are as set out on pages 177 and 184. However, cash dividends declared in respect of 2023 were £2,352 million (2022: £2,470 million) a decrease of 4.8%. Please see Note 16 to the financial statements for further details. Total employee pay is based on 70,244 employees, the average number of people employed during 2023 (2022: 69,130). See Note 9 to the financial statements for further details. The last share repurchase made by the company was in 2014. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued Directors' pay in a wider setting continued 139
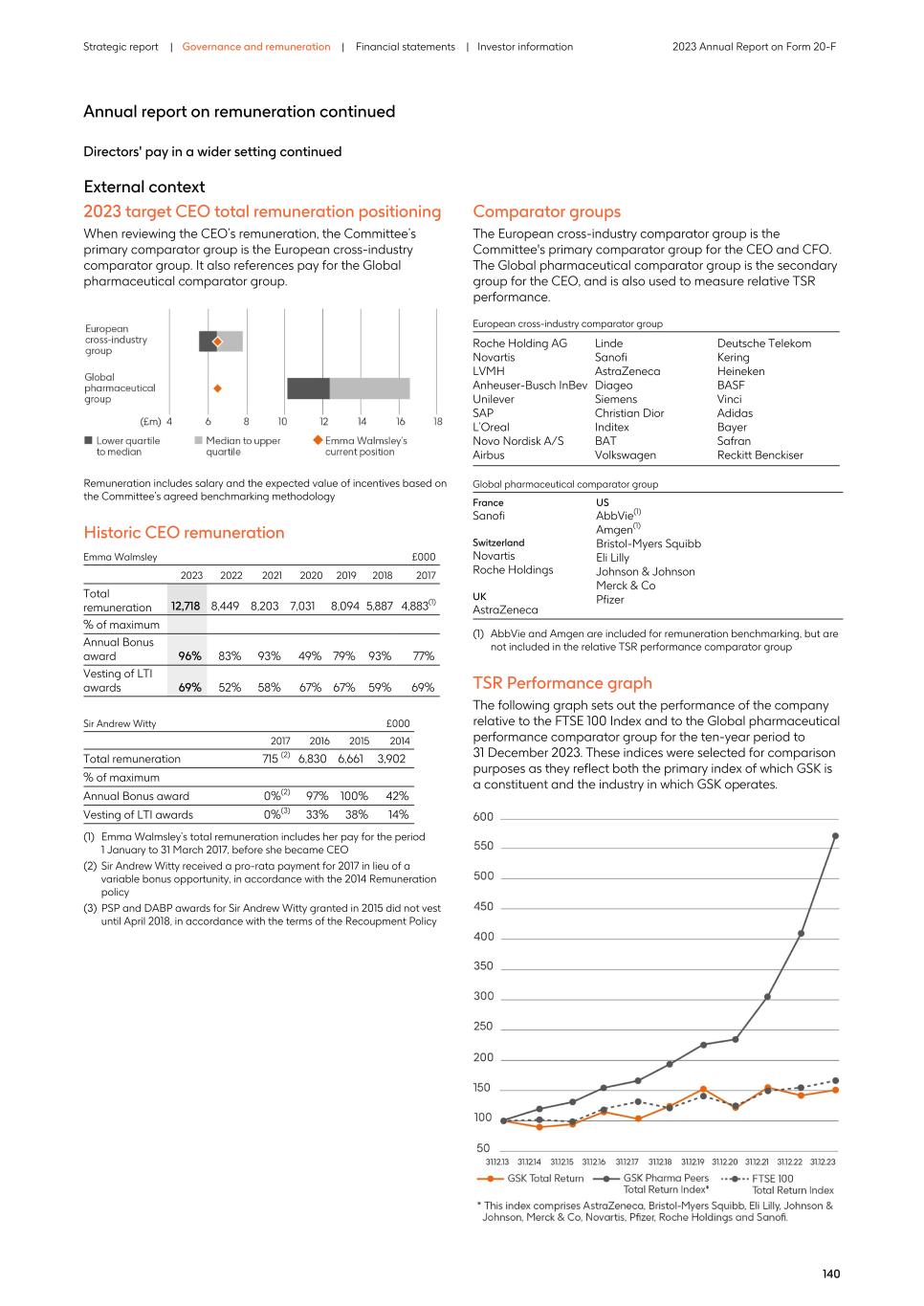
External context 2023 target CEO total remuneration positioning When reviewing the CEO’s remuneration, the Committee’s primary comparator group is the European cross-industry comparator group. It also references pay for the Global pharmaceutical comparator group. Remuneration includes salary and the expected value of incentives based on the Committee’s agreed benchmarking methodology Historic CEO remuneration Emma Walmsley £000 2023 2022 2021 2020 2019 2018 2017 Total remuneration 12,718 8,449 8,203 7,031 8,094 5,887 4,883(1) % of maximum Annual Bonus award 96% 83% 93% 49% 79% 93% 77% Vesting of LTI awards 69% 52% 58% 67% 67% 59% 69% Sir Andrew Witty £000 2017 2016 2015 2014 Total remuneration 715 (2) 6,830 6,661 3,902 % of maximum Annual Bonus award 0%(2) 97% 100% 42% Vesting of LTI awards 0%(3) 33% 38% 14% (1) Emma Walmsley’s total remuneration includes her pay for the period 1 January to 31 March 2017, before she became CEO (2) Sir Andrew Witty received a pro-rata payment for 2017 in lieu of a variable bonus opportunity, in accordance with the 2014 Remuneration policy (3) PSP and DABP awards for Sir Andrew Witty granted in 2015 did not vest until April 2018, in accordance with the terms of the Recoupment Policy Comparator groups The European cross-industry comparator group is the Committee's primary comparator group for the CEO and CFO. The Global pharmaceutical comparator group is the secondary group for the CEO, and is also used to measure relative TSR performance. European cross-industry comparator group Roche Holding AG Novartis LVMH Anheuser-Busch InBev Unilever SAP L’Oreal Novo Nordisk A/S Airbus Linde Sanofi AstraZeneca Diageo Siemens Christian Dior Inditex BAT Volkswagen Deutsche Telekom Kering Heineken BASF Vinci Adidas Bayer Safran Reckitt Benckiser Global pharmaceutical comparator group France Sanofi Switzerland Novartis Roche Holdings UK AstraZeneca US AbbVie(1) Amgen(1) Bristol-Myers Squibb Eli Lilly Johnson & Johnson Merck & Co Pfizer (1) AbbVie and Amgen are included for remuneration benchmarking, but are not included in the relative TSR performance comparator group TSR Performance graph The following graph sets out the performance of the company relative to the FTSE 100 Index and to the Global pharmaceutical performance comparator group for the ten-year period to 31 December 2023. These indices were selected for comparison purposes as they reflect both the primary index of which GSK is a constituent and the industry in which GSK operates. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued Directors' pay in a wider setting continued 140
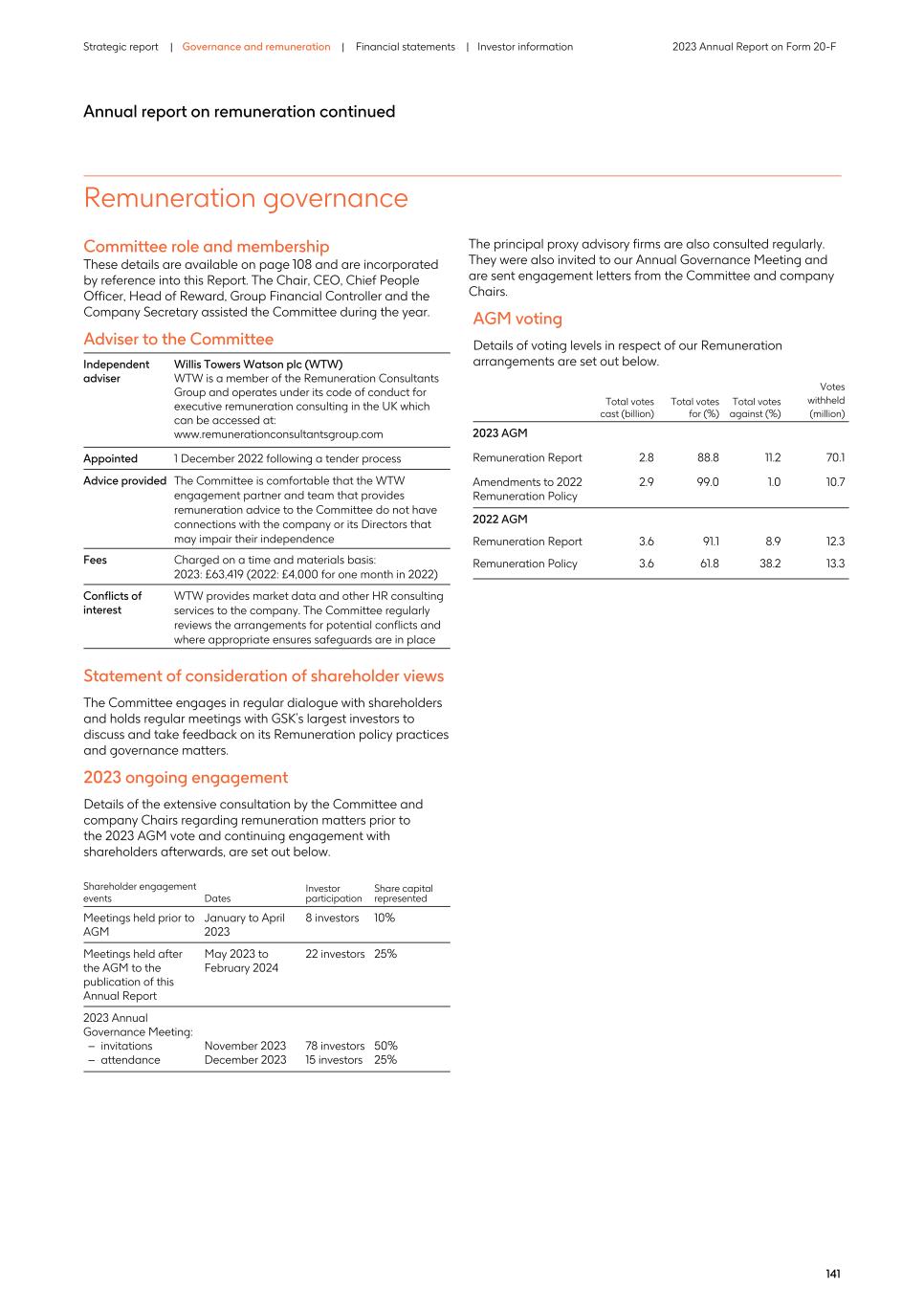
Remuneration governance Committee role and membership These details are available on page 108 and are incorporated by reference into this Report. The Chair, CEO, Chief People Officer, Head of Reward, Group Financial Controller and the Company Secretary assisted the Committee during the year. Adviser to the Committee Independent adviser Willis Towers Watson plc (WTW) WTW is a member of the Remuneration Consultants Group and operates under its code of conduct for executive remuneration consulting in the UK which can be accessed at: www.remunerationconsultantsgroup.com Appointed 1 December 2022 following a tender process Advice provided The Committee is comfortable that the WTW engagement partner and team that provides remuneration advice to the Committee do not have connections with the company or its Directors that may impair their independence Fees Charged on a time and materials basis: 2023: £63,419 (2022: £4,000 for one month in 2022) Conflicts of interest WTW provides market data and other HR consulting services to the company. The Committee regularly reviews the arrangements for potential conflicts and where appropriate ensures safeguards are in place Statement of consideration of shareholder views The Committee engages in regular dialogue with shareholders and holds regular meetings with GSK’s largest investors to discuss and take feedback on its Remuneration policy practices and governance matters. 2023 ongoing engagement Details of the extensive consultation by the Committee and company Chairs regarding remuneration matters prior to the 2023 AGM vote and continuing engagement with shareholders afterwards, are set out below. Shareholder engagement events Dates Investor participation Share capital represented Meetings held prior to AGM January to April 2023 8 investors 10% Meetings held after the AGM to the publication of this Annual Report May 2023 to February 2024 22 investors 25% 2023 Annual Governance Meeting: – invitations – attendance November 2023 December 2023 78 investors 15 investors 50% 25% The principal proxy advisory firms are also consulted regularly. They were also invited to our Annual Governance Meeting and are sent engagement letters from the Committee and company Chairs. AGM voting Details of voting levels in respect of our Remuneration arrangements are set out below. Total votes cast (billion) Total votes for (%) Total votes against (%) Votes withheld (million) 2023 AGM Remuneration Report 2.8 88.8 11.2 70.1 Amendments to 2022 Remuneration Policy 2.9 99.0 1.0 10.7 2022 AGM Remuneration Report 3.6 91.1 8.9 12.3 Remuneration Policy 3.6 61.8 38.2 13.3 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued 141

Committee focus during 2023 Items discussed Remuneration policy – Prepared, agreed and proposed administrative amendments to the 2022 Remuneration Policy – Continued engagement with shareholders and reviewed and considered shareholder and proxy advisor feedback Fixed Pay – Considered Executive Director and GLT performance, benchmarking competitiveness against GSK comparator groups – Reviewed GLT and Company Secretary salary recommendations for 2023 – Executive Director salary review recommendations for 2023 and 2024 – Reviewed company Chair’s fees for 2023 and 2024 Pay for Performance Annual Bonus – Executive Director and GLT 2022 bonus recommendations and set 2023 Executive Directors’ bonus objectives LTI plans – Considered the LTI performance outcomes and award vesting for the CEO, Executive Directors, GLT and below – Confirmed LTI grants for Executive Directors, GLT and below Governance and other areas of focus – Remuneration considerations and Committee programme for 2023 and 2024 – Committee evaluation and Annual Review of its Terms of Reference – Approved 2022 Remuneration report – Confirmed 2023 Group Budget for remuneration purposes – Considered AGM and Remuneration report feedback, the external remuneration environment and performance target disclosure for incentive plans – Agreed Committee's key messages for Annual Governance Meeting – Committee Chair consulted with employee representatives on wider workforce pay practices and pay generally Leaving Directors To support the CFO succession and transition process, as announced in September 2022 and set out in the 2022 Annual Report, after stepping down from the Board Iain Mackay continued to receive remuneration until he left GSK on 31 December 2023. This was in line with the current Remuneration policy. His base salary was not increased during 2023. Whilst serving as an Executive Director (until 1 May 2023) he received total benefits of £82,000 (comprising £67,000 for benefits that are available to employees and £15,000 for business-related services). The value of his pension until May 2023 totalled £22,000 (comprising £2,000 UK defined contribution and £20,000 employer cash contributions). See page 131 for further explanation. As an employee to the end of 2023, he remained eligible to receive a bonus under the Executive bonus plan for 2023 based on delivery of the measures described on page 132. This was reviewed by the Committee and determined to be £2,196,810 in total for the year, comprising £915,335 in cash and £1,281,475 delivered as GSK shares deferred for three years under the DABP plan. The bonus value in respect of the period he served as an Executive Director was £728,000. He was not eligible for and therefore did not receive any further PSP awards in 2023 given he was due to leave GSK. Vesting of his existing LTI awards will be in accordance with the Recoupment Policy. With regard to the 2021 PSP award of 278,363 ordinary shares, this will not vest until January 2025. 69.95% of the award (223,045 shares inclusive of dividends) will vest in accordance with the performance described on page 130. The illustrative unreduced value for this award is disclosed in the Single figure table on page 135 and the value at the time of vesting will be updated in the 2024 Annual Report. Since his executive service contract ended on 31 December 2023, he will be required to satisfy the post-employment share ownership requirement as set out on page 144. Payments (audited): to past Directors for loss of office No payments were made to past Directors in 2023 No loss of office payments were made during 2023 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued Remuneration governance continued 142
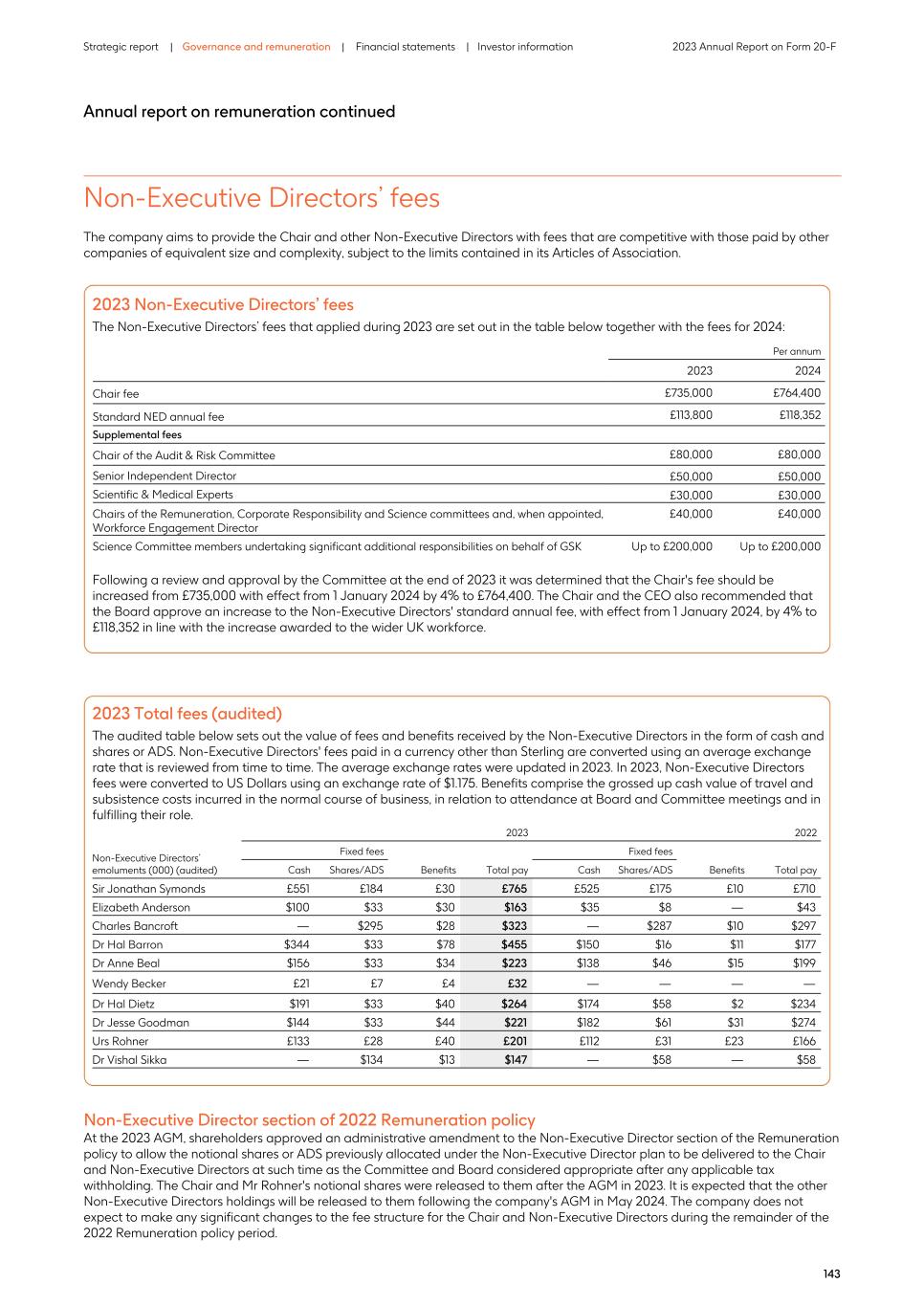
Non-Executive Directors’ fees The company aims to provide the Chair and other Non-Executive Directors with fees that are competitive with those paid by other companies of equivalent size and complexity, subject to the limits contained in its Articles of Association. 2023 Non-Executive Directors’ fees The Non-Executive Directors’ fees that applied during 2023 are set out in the table below together with the fees for 2024: Per annum 2023 2024 Chair fee £735,000 £764,400 Standard NED annual fee £113,800 £118,352 Supplemental fees Chair of the Audit & Risk Committee £80,000 £80,000 Senior Independent Director £50,000 £50,000 Scientific & Medical Experts £30,000 £30,000 Chairs of the Remuneration, Corporate Responsibility and Science committees and, when appointed, Workforce Engagement Director £40,000 £40,000 Science Committee members undertaking significant additional responsibilities on behalf of GSK Up to £200,000 Up to £200,000 Following a review and approval by the Committee at the end of 2023 it was determined that the Chair's fee should be increased from £735,000 with effect from 1 January 2024 by 4% to £764,400. The Chair and the CEO also recommended that the Board approve an increase to the Non-Executive Directors' standard annual fee, with effect from 1 January 2024, by 4% to £118,352 in line with the increase awarded to the wider UK workforce. 2023 Total fees (audited) The audited table below sets out the value of fees and benefits received by the Non-Executive Directors in the form of cash and shares or ADS. Non-Executive Directors' fees paid in a currency other than Sterling are converted using an average exchange rate that is reviewed from time to time. The average exchange rates were updated in 2023. In 2023, Non-Executive Directors fees were converted to US Dollars using an exchange rate of $1.175. Benefits comprise the grossed up cash value of travel and subsistence costs incurred in the normal course of business, in relation to attendance at Board and Committee meetings and in fulfilling their role. Non-Executive Directors’ emoluments (000) (audited) 2023 2022 Fixed fees Fixed fees Cash Shares/ADS Benefits Total pay Cash Shares/ADS Benefits Total pay Sir Jonathan Symonds £551 £184 £30 £765 £525 £175 £10 £710 Elizabeth Anderson $100 $33 $30 $163 $35 $8 — $43 Charles Bancroft — $295 $28 $323 — $287 $10 $297 Dr Hal Barron $344 $33 $78 $455 $150 $16 $11 $177 Dr Anne Beal $156 $33 $34 $223 $138 $46 $15 $199 Wendy Becker £21 £7 £4 £32 — — — — Dr Hal Dietz $191 $33 $40 $264 $174 $58 $2 $234 Dr Jesse Goodman $144 $33 $44 $221 $182 $61 $31 $274 Urs Rohner £133 £28 £40 £201 £112 £31 £23 £166 Dr Vishal Sikka — $134 $13 $147 — $58 — $58 Non-Executive Director section of 2022 Remuneration policy At the 2023 AGM, shareholders approved an administrative amendment to the Non-Executive Director section of the Remuneration policy to allow the notional shares or ADS previously allocated under the Non-Executive Director plan to be delivered to the Chair and Non-Executive Directors at such time as the Committee and Board considered appropriate after any applicable tax withholding. The Chair and Mr Rohner's notional shares were released to them after the AGM in 2023. It is expected that the other Non-Executive Directors holdings will be released to them following the company's AGM in May 2024. The company does not expect to make any significant changes to the fee structure for the Chair and Non-Executive Directors during the remainder of the 2022 Remuneration policy period. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued 143
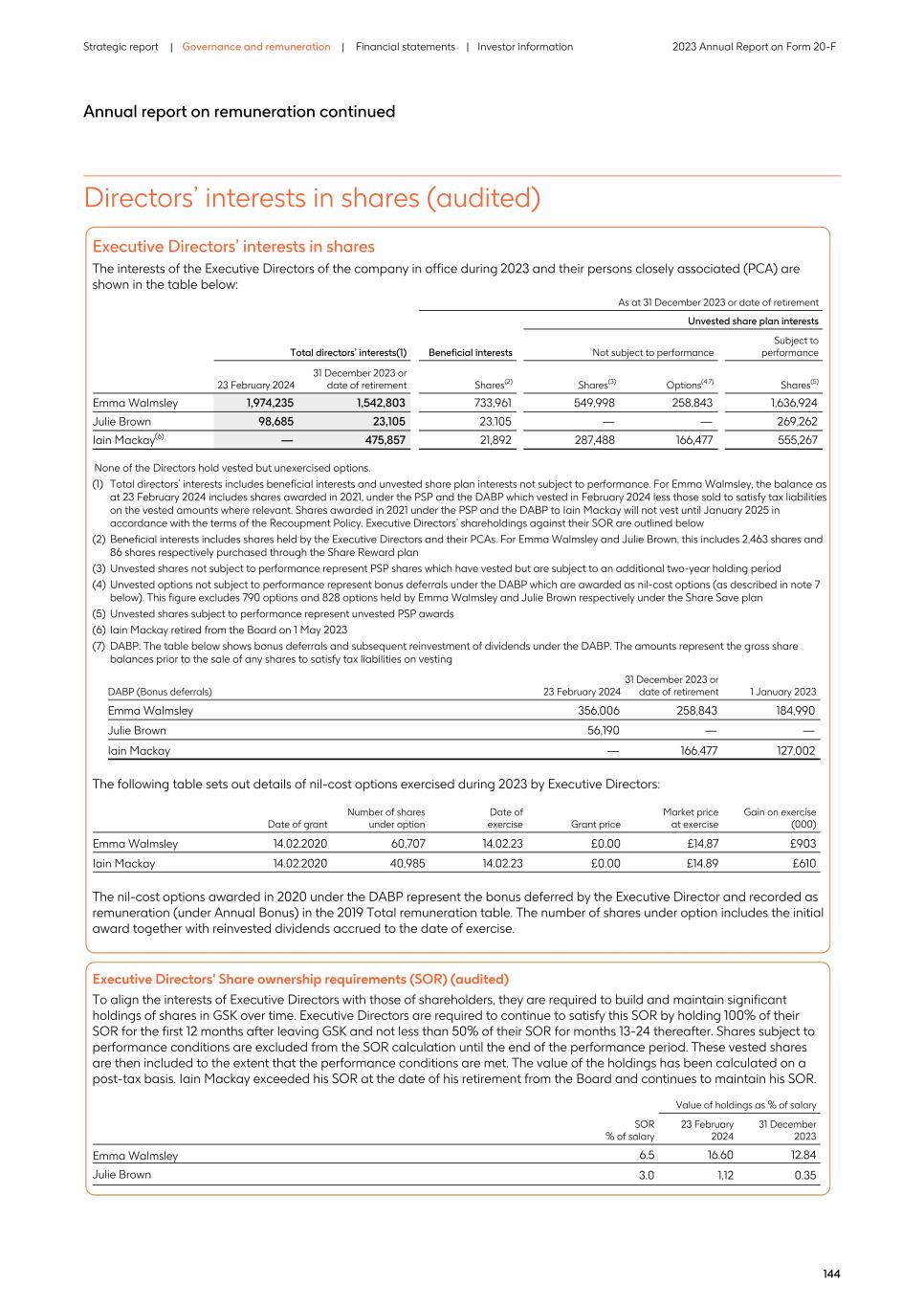
Directors’ interests in shares (audited) Executive Directors’ interests in shares The interests of the Executive Directors of the company in office during 2023 and their persons closely associated (PCA) are shown in the table below: As at 31 December 2023 or date of retirement Unvested share plan interests Total directors’ interests(1) Beneficial interests Not subject to performance Subject to performance 23 February 2024 31 December 2023 or date of retirement Shares(2) Shares(3) Options(4,7) Shares(5) Emma Walmsley 1,974,235 1,542,803 733,961 549,998 258,843 1,636,924 Julie Brown 98,685 23,105 23,105 — — 269,262 Iain Mackay(6) — 475,857 21,892 287,488 166,477 555,267 None of the Directors hold vested but unexercised options. (1) Total directors’ interests includes beneficial interests and unvested share plan interests not subject to performance. For Emma Walmsley, the balance as at 23 February 2024 includes shares awarded in 2021, under the PSP and the DABP which vested in February 2024 less those sold to satisfy tax liabilities on the vested amounts where relevant. Shares awarded in 2021 under the PSP and the DABP to Iain Mackay will not vest until January 2025 in accordance with the terms of the Recoupment Policy. Executive Directors’ shareholdings against their SOR are outlined below (2) Beneficial interests includes shares held by the Executive Directors and their PCAs. For Emma Walmsley and Julie Brown, this includes 2,463 shares and 86 shares respectively purchased through the Share Reward plan (3) Unvested shares not subject to performance represent PSP shares which have vested but are subject to an additional two-year holding period (4) Unvested options not subject to performance represent bonus deferrals under the DABP which are awarded as nil-cost options (as described in note 7 below). This figure excludes 790 options and 828 options held by Emma Walmsley and Julie Brown respectively under the Share Save plan (5) Unvested shares subject to performance represent unvested PSP awards (6) Iain Mackay retired from the Board on 1 May 2023 (7) DABP: The table below shows bonus deferrals and subsequent reinvestment of dividends under the DABP. The amounts represent the gross share balances prior to the sale of any shares to satisfy tax liabilities on vesting DABP (Bonus deferrals) 23 February 2024 31 December 2023 or date of retirement 1 January 2023 Emma Walmsley 356,006 258,843 184,990 Julie Brown 56,190 — — Iain Mackay — 166,477 127,002 The following table sets out details of nil-cost options exercised during 2023 by Executive Directors: Date of grant Number of shares under option Date of exercise Grant price Market price at exercise Gain on exercise (000) Emma Walmsley 14.02.2020 60,707 14.02.23 £0.00 £14.87 £903 Iain Mackay 14.02.2020 40,985 14.02.23 £0.00 £14.89 £610 The nil-cost options awarded in 2020 under the DABP represent the bonus deferred by the Executive Director and recorded as remuneration (under Annual Bonus) in the 2019 Total remuneration table. The number of shares under option includes the initial award together with reinvested dividends accrued to the date of exercise. Executive Directors' Share ownership requirements (SOR) (audited) To align the interests of Executive Directors with those of shareholders, they are required to build and maintain significant holdings of shares in GSK over time. Executive Directors are required to continue to satisfy this SOR by holding 100% of their SOR for the first 12 months after leaving GSK and not less than 50% of their SOR for months 13-24 thereafter. Shares subject to performance conditions are excluded from the SOR calculation until the end of the performance period. These vested shares are then included to the extent that the performance conditions are met. The value of the holdings has been calculated on a post-tax basis. Iain Mackay exceeded his SOR at the date of his retirement from the Board and continues to maintain his SOR. Value of holdings as % of salary SOR % of salary 23 February 2024 31 December 2023 Emma Walmsley 6.5 16.60 12.84 Julie Brown 3.0 1.12 0.35 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued 144

Non-Executive Directors’ interests in shares The interests of the Non-Executive Directors in office during 2023 and their persons closely associated (PCA) are shown in the table below: Prior NED share allocation plan Total directors’ interests as at(2) Number of shares/ADS NED SOR 23 February 2024(1) 23 February 2024 31 December 2023 Beneficial interests at 31 December 2023(4) Dividends reinvested after year end 31 December 2023 Elected & allocated during the year(5) 1 January 2023 Shares Sir Jonathan Symonds Met 68,207 64,707 64,707 — — 1,047 34,391 Wendy Becker In progress 478 478 478 — — — — Urs Rohner Met 17,362 17,362 17,362 — — 564 18,519 ADS Elizabeth Anderson In progress 1,179 1,171 1,171 — — — — Charles Bancroft Met 23,564 22,809 7,005 709 15,804 240 15,564 Dr Hal Barron Met 641,269(3) 753,357 530,020 — — — — Dr Anne Beal In progress 2,821 2,734 934 80 1,800 23 1,777 Dr Hal Dietz In progress 2,605 2,527 934 71 1,593 18 1,575 Dr Jesse Goodman Met 14,120 13,548 934 566 12,614 238 12,375 Vishal Sikka Met 4,454 4,422 4,422 — — — — (1) NED Share Ownership Requirements: Since July 2022, the company has operated a minimum Non-Executive Director share ownership requirement (NED SOR) of at least one times the standard NED annual fee (or the Chair’s fee) to be maintained until after retirement. from the Board. The Chair and Non-Executive Directors have transitioned from the previous NED share allocation plan (NED Plan) to purchasing shares and ADSs in the market from their net fees. They all spend a minimum of 25% of their net fees in purchasing GSK shares or ADSs in the market (2) Total directors’ interests include beneficial interests and any notional shares/ADS received as all or part of their fees under the previously operated NED Plan. Dividends received on notional shares/ADS under the prior NED Plan during the year and in January 2024 were converted into notional shares/ADS as at 11 January 2024. For Dr Hal Barron, this includes the PSP award that vested in February 2024, see page 135 (3) The Total interests for Dr Barron have reduced since 31 December 2023 following the vesting of PSP and DABP awards granted to him in his former executive capacity as CSO. Details of the vesting level for the 2021 PSP is shown on page 135 and the DABP vest relates to the deferral of shares from the 2021 annual bonus. In addition, on vesting, shares are sold to meet an executive's tax liabilities. Details of his transition from CSO to a Non- Executive Director are given on page 135 of the 2022 Annual Report (4) Beneficial interests includes shares/ADS held by the Non-Executive Directors and their PCAs (5) Notional shares/ADS allocated during the year under the NED plan relates to dividends reinvested during the year Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued Directors interest in shares (audited) continued 145

Percentage change in remuneration of Directors 2023 percentage change 2022 percentage change 2021 percentage change 2020 percentage change Salary/ fees % Benefits % Bonus % Salary/ fees % Benefits % Bonus % Salary/ fees % Benefits % Bonus % Salary/ fees % Benefits % Bonus % UK employees(1) 7.1 0.92 34.8 3.0 2.3 44.81 2.0 0.0 4.85 2.5 — 11.0 Executive Directors(2,3) Emma Walmsley 4.0 61.8 20.1 3.0 (2.2) 38.2 2.0 (5.0) 94.6 8.0 (26.6) (33.4) Julie Brown — — — — — — — — — — — — Non-Executive Directors(2,5) Sir Jonathan Symonds 5.0 200.0 — 0.0 233.3 — 0.0 50.0 — 201.7 0.0 — Elizabeth McKee Anderson 209.3 — — — — — — — — — — — Charles Bancroft 2.8 180.0 — 36.7 100.0 — 156.1 — — — — — Dr Hal Barron(4) 127.1 609.1 — — — — — — — — — — Dr Anne Beal 2.7 126.7 — 121.7 — — — — — — — — Wendy Becker — — — — — — — — — — — — Dr Hal Dietz (3.4) 1900.0 — — — — — — — — — — Dr Jesse Goodman (27.2) 41.9 — 11.0 34.8 — (5.6) 0.0 — (12.5) (65.2) — Urs Rohner 12.6 73.9 — 5.9 109.1 — (5.6) 175.0 — 16.3 (69.2) — Dr Vishal Sikka 131.0 — — — — — — — — — — — Retired Executive Directors(2) Iain Mackay (66.7) (71.8) (65.0) 3.0 20.2 32.4 2.0 56.1 94.2 5.6 (11.5) (31.6) (1) This table is provided in accordance with Schedule 8 of The Companies (Directors’ Remuneration Policy and Directors’ Remuneration Report) Regulations 2020. The UK employee population was considered to be the most relevant comparison as it most closely reflects the economic environment encountered by the Executive Directors (2) Percentage changes have been calculated based on the 2023 Total remuneration table on page 130 for Executive Directors and the 2023 Total fees table on page 143 for Non-Executive Directors (3) Further information on Executive Directors’ salary and benefits can be found on page 131 (4) Dr Hal Barron transitioned to a Non-Executive Director role on 1 August 2022 (5) Fees of Non-Executive Directors include fees received as cash and in the form of shares or ADS Directors and Senior Management Further information is provided on compensation and interests of Directors and Senior Management as a group (the group). For this purpose, the group is defined as the Executive and Non-Executive Directors, other members of the GLT and the Company Secretary. For the financial year 2023, the following table sets out aggregate remuneration for the group for the periods during which they served in that capacity. Remuneration for 2023 £ Total compensation paid 37,406,891 Aggregate increase in accrued pension benefits (net of inflation) 6,403 Aggregate payments to defined contribution schemes 1,314,332 During 2023, members of the group were awarded shares and ADS under the company’s various LTI plans, as set out in the table below. To align the interests of Senior Management with those of shareholders, Executive Directors and GLT members are required to build and maintain significant holdings of shares in GSK over time. GLT members are required to hold shares to an equivalent multiple of two times their base salary, and must continue to satisfy these share ownership requirements for a minimum of 12 months after leaving GSK. Awards Dividend reinvestment awards Awarded during 2023 Shares ADS Shares ADS Performance Share Plan 2,278,202 64,427 258,760 4,236 Deferred Investment Awards(1,2) — — 11,694 328 Share Value Plan(2) 10,050 — — — (1) Notional shares and ADS (2) Executive Directors are not eligible to receive Deferred Investment Awards or participate in the Share Value Plan Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued 146
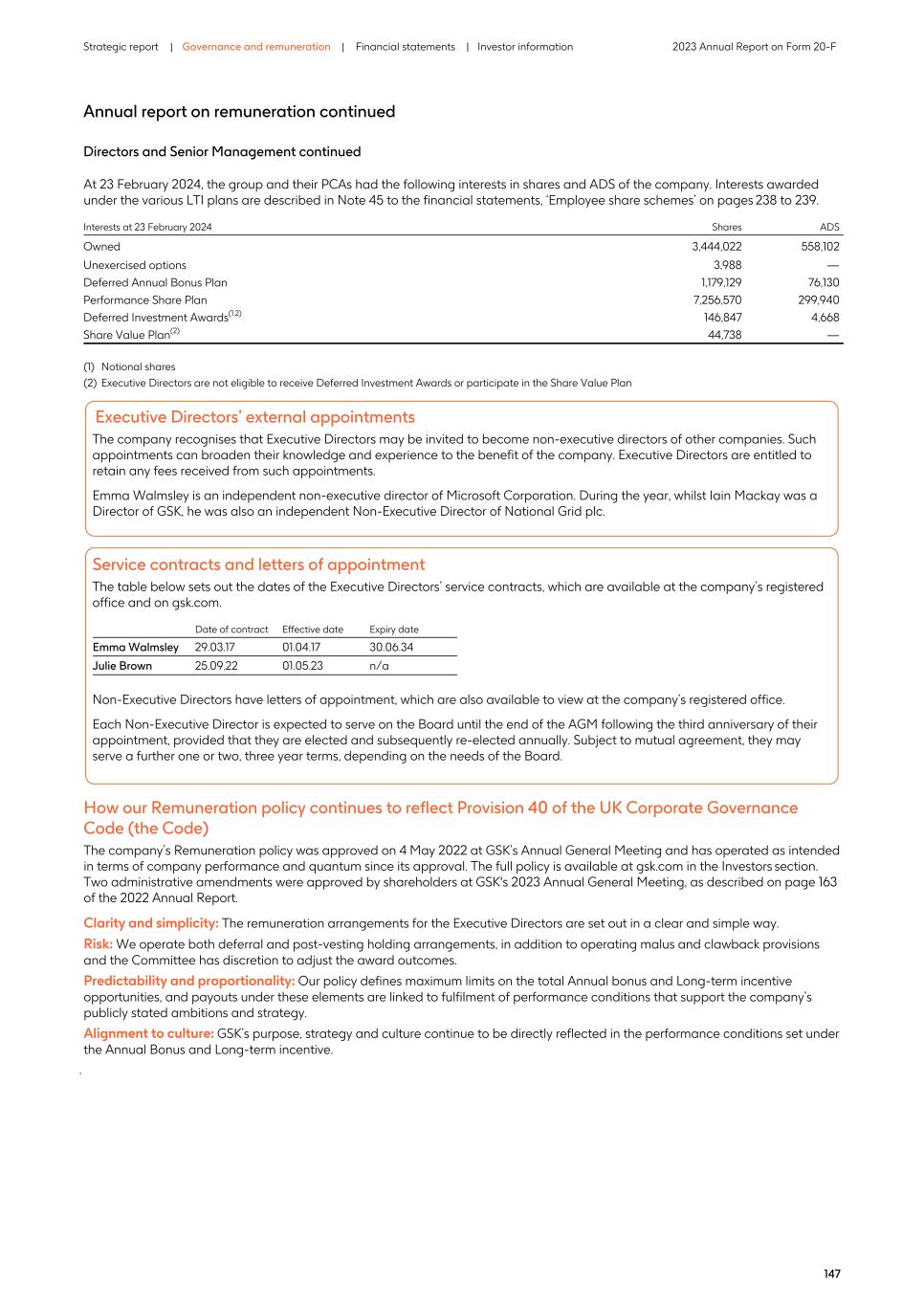
At 23 February 2024, the group and their PCAs had the following interests in shares and ADS of the company. Interests awarded under the various LTI plans are described in Note 45 to the financial statements, ‘Employee share schemes’ on pages 238 to 239. Interests at 23 February 2024 Shares ADS Owned 3,444,022 558,102 Unexercised options 3,988 — Deferred Annual Bonus Plan 1,179,129 76,130 Performance Share Plan 7,256,570 299,940 Deferred Investment Awards(1,2) 146,847 4,668 Share Value Plan(2) 44,738 — (1) Notional shares (2) Executive Directors are not eligible to receive Deferred Investment Awards or participate in the Share Value Plan Executive Directors’ external appointments The company recognises that Executive Directors may be invited to become non-executive directors of other companies. Such appointments can broaden their knowledge and experience to the benefit of the company. Executive Directors are entitled to retain any fees received from such appointments. Emma Walmsley is an independent non-executive director of Microsoft Corporation. During the year, whilst Iain Mackay was a Director of GSK, he was also an independent Non-Executive Director of National Grid plc. Service contracts and letters of appointment The table below sets out the dates of the Executive Directors’ service contracts, which are available at the company’s registered office and on gsk.com. Date of contract Effective date Expiry date Emma Walmsley 29.03.17 01.04.17 30.06.34 Julie Brown 25.09.22 01.05.23 n/a Non-Executive Directors have letters of appointment, which are also available to view at the company’s registered office. Each Non-Executive Director is expected to serve on the Board until the end of the AGM following the third anniversary of their appointment, provided that they are elected and subsequently re-elected annually. Subject to mutual agreement, they may serve a further one or two, three year terms, depending on the needs of the Board. How our Remuneration policy continues to reflect Provision 40 of the UK Corporate Governance Code (the Code) The company’s Remuneration policy was approved on 4 May 2022 at GSK’s Annual General Meeting and has operated as intended in terms of company performance and quantum since its approval. The full policy is available at gsk.com in the Investors section. Two administrative amendments were approved by shareholders at GSK's 2023 Annual General Meeting, as described on page 163 of the 2022 Annual Report. Clarity and simplicity: The remuneration arrangements for the Executive Directors are set out in a clear and simple way. Risk: We operate both deferral and post-vesting holding arrangements, in addition to operating malus and clawback provisions and the Committee has discretion to adjust the award outcomes. Predictability and proportionality: Our policy defines maximum limits on the total Annual bonus and Long-term incentive opportunities, and payouts under these elements are linked to fulfilment of performance conditions that support the company’s publicly stated ambitions and strategy. Alignment to culture: GSK’s purpose, strategy and culture continue to be directly reflected in the performance conditions set under the Annual Bonus and Long-term incentive. . Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Annual report on remuneration continued Directors and Senior Management continued 147

Operation and scope of Remuneration policy It is intended that the Remuneration policy (Policy) for GSK’s Executive and Non-Executive Directors will operate for a period of three years from the date of approval at the company’s Annual General Meeting on 4 May 2022. The Committee wrote the Policy principally in relation to the remuneration arrangements for the Executive Directors, whilst taking into account the possible recruitment of a replacement or an additional Executive Director during the operation of the Policy. The Committee intends the Policy to operate for the period set out above in its entirety. However, it may after due consideration seek to change the Policy during this period, but only if it believes it is appropriate to do so for the long-term success of the company, after consultation with shareholders and having sought shareholder approval at a general meeting. The Committee reserves the right to make any remuneration payments and/or payments for loss of office (including exercising any discretions available to it in connection with such payments) notwithstanding that they are not in line with the Policy where the terms of the payment were agreed: (i) before the AGM on 7 May 2014 (the date the company’s first shareholder-approved Directors’ Remuneration policy came into effect); (ii) before the Policy came into effect, provided that the terms of the payment were consistent with the shareholder-approved Remuneration policy in force at the time they were agreed; or (iii) at a time when the relevant individual was not a Director of the company and, in the opinion of the Committee, the payment was not in consideration for the individual becoming a Director of the company. For these purposes ‘payments’ includes the Committee satisfying awards of variable remuneration and, in relation to an award over shares or ADS, the terms of the payment are ‘agreed’ at the time the award is granted. Performance Share Plan (PSP) awards are subject to the terms of the PSP plan rules under which the award has been granted. The Committee may adjust or amend awards only in accordance with the provisions of the plan rules. This includes making adjustments to reflect one-off corporate events, such as a change in the company’s capital structure. The Committee may also make minor amendments to the Policy (for regulatory, exchange control, tax or administrative purposes or to take account of a change in legislation) without obtaining shareholder approval for such amendments. Basis of preparation The Annual report on remuneration has been prepared in accordance with the Companies Act 2006 and The Large and Medium-sized Companies and Groups (Accounts and Reports) (Amendment) Regulations 2013 (the Regulations). In accordance with the Regulations, the following parts of the Annual report on remuneration are subject to audit: total remuneration figures for Executive Directors including further details for each element of remuneration (salary, benefits, pension, Annual Bonus and Long-term incentive awards); Non- Executive Directors’ fees and emoluments received in the year; Directors’ interests in shares, including interests in GSK share plans; payments to past Directors; payments for loss of office; and share ownership requirements and holdings, for which the opinion thereon is expressed in pages 154-157. The remaining sections of the Annual report on remuneration are not subject to audit nor are the pages referred to from within the audited sections. The Annual report on remuneration has been approved by the Board of Directors and signed on its behalf by: Urs Rohner Remuneration Committee Chair 27 February 2024 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F 148
Directors' powers GSK Directors’ powers are determined by UK legislation and our Articles of Association, which contain rules about their appointment and replacement. They provide that Directors may be appointed by an ordinary resolution of the members or by a resolution of the Board. If appointed by the Board, the Director must retire at the next Annual General Meeting to be elected by shareholders Our Articles also provide that all Directors are required to seek re-election annually at our Annual General Meeting in accordance with the FRC's Code. A Director will then cease to be a Director if he or she: – becomes bankrupt – ceases to be a Director by virtue of the Companies Act or the Articles – suffers mental or physical ill health and the Board resolves that he or she shall cease to be a Director – has missed Directors’ meetings for a continuous period of six months without permission and the Board resolves that he or she shall cease to be a Director – is otherwise prohibited from being a Director by law – resigns, or offers to resign and the Board accepts that offer – is required to resign by the Board Directors’ conflicts of interest All Directors have a duty under the Companies Act 2006 to avoid a situation in which they have, or could have, a direct or indirect conflict of interest or possible conflict with the company. Our Articles provide a general power for the Board to authorise such conflicts. The Board reviews any new potential or actual conflict, which is recorded by the Company Secretary. Directors are not counted in the quorum for the authorisation of their own actual or potential conflicts. The Nominations & Corporate Governance Committee reviews the Register of Potential Conflicts on an annual basis which the Board subsequently approves. On a continuing basis, the Directors are responsible for informing the Company Secretary of any such new actual or potential conflicts that may arise or if there are any changes in circumstances that may affect an authorisation previously given. Even when provided with authorisation, a Director is not absolved from his or her statutory duty to promote the success of the company. If an actual conflict arises post-authorisation, the Board may choose to exclude the Director from receipt of the relevant information and participation in the debate, or suspend the Director from the Board, or, as a last resort, require the Director to resign. The Nominations & Corporate Governance Committee reviewed the Register of Potential Conflict authorisations (the Register of Potential Conflicts) in January 2024. The Committee reported to the Board that the conflicts had been appropriately authorised and that the process for authorisation continued to operate effectively. The Committee then recommended the approval of the Register of Potential Conflicts to the Board which it subsequently approved. Except as described in Note 40 to the financial statements, ‘Related party transactions’, during or at the end of the financial year no Director or Person Closely Associated had any material interest in any contract of significance with a Group company. Our Articles prohibit a Director from voting on any resolution concerning his or her appointment or the terms or termination of his or her appointment. Independent advice The company has an agreed procedure for Directors to take independent legal and/or financial advice at the company’s expense where they deem it necessary. Indemnification of Directors Qualifying third party indemnity provisions (as defined in the Companies Act 2006) are in force for the benefit of Directors and former Directors who held office during 2023 and up to the approval and signature of the Annual Report. Change of control and essential contracts We do not have contracts or other arrangements which individually are fundamental to the ability of the business to operate effectively. Neither is the company party to any material agreements that would take effect, be altered, or terminate upon a change of control following a takeover bid. We do not have agreements with any Director that would provide compensation for loss of office or employment resulting from a takeover, except that provisions of the company’s share plans may cause options and awards granted under such plans to vest on a takeover. Details of the termination provisions in the Executive Directors’ service contracts are given in the full version of the company’s 2022 Remuneration policy which is available on gsk.com in the Investors section. Content of the Directors’ report For the purposes of the UK Companies Act 2006, the Directors’ report of GSK plc for the year ended 31 December 2023 comprises: Directors’ report Section Pages Corporate governance report 98 to 150 Employee engagement 113 Directors’ statements of responsibilities 152 and 153 Investor information 245 to 295 The Strategic report sets out those matters required to be disclosed in the Directors’ report which are considered to be of strategic importance: Strategic report Section Pages Risk management objectives and policies 55 to 69 and 254 to 260 Likely future developments of the company 1 to 97 Research and development activities 15 to 28 Business relationships 44 to 53 Diversity 50 and 51 Provision of information to and consultations with employees 12, 13, 50 and 51 Carbon emissions 47 to 50 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Directors' report 149
The following information is also incorporated into the Directors’ report: Location in Annual Report Interest capitalised Financial statements, Notes 17 and 20 Particulars of important post-balance sheet events of the company or its subsidiaries Financial statements, Note 48 Publication of unaudited financial information Group financial review Details of any long-term incentive schemes Remuneration report Waiver of emoluments by a Director Not applicable Waiver of future emoluments by a Director Not applicable Non pre-emptive issues of equity for cash Not applicable Non pre-emptive issues of equity for cash by any unlisted major subsidiary undertaking Not applicable Parent company participation in a placing by a listed subsidiary Not applicable Provision of services by a controlling shareholder Not applicable Shareholder waiver of dividends Financial statements, Notes 16 and 45 Shareholder waiver of future dividends Financial statements, Notes 16 and 45 Agreements with controlling shareholders Not applicable The Directors’ report – has been drawn up and presented in accordance with and in reliance upon English company law and the liabilities of the Directors in connection with that Report shall be subject to the limitations and restrictions provided by such law. – was approved by the Board of Directors on 27 February 2024 and signed on its behalf by: Sir Jonathan Symonds Chair 27 February 2024 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Directors' report continued 150

Financial statements In this section Directors’ statement of responsibilities 152 Report of the Independent Registered Public Accounting Firm - Deloitte LLP 154 Financial statements 158 Notes to the financial statements 162 2023 Annual Report on Form 20-F 151
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Directors' statement of responsibilities |
| | | |
The Directors are responsible for preparing the Annual Report, the Remuneration report and the Group and parent company financial statements in accordance with applicable law and regulations.
UK company law requires the Directors to prepare financial statements for each financial year. The Directors are required to prepare the Group consolidated financial statements in accordance with UK-adopted international accounting standards in conformity with the requirements of the Companies Act 2006 and the International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB). The Directors have elected to prepare the parent company financial statements in accordance with United Kingdom Accounting Standards and applicable law (United Kingdom Generally Accepted Accounting Practice) (Financial Reporting Standard 101 Reduced Disclosure Framework). Under company law the Directors must not approve the financial statements unless they are satisfied that they give a true and fair view of the state of affairs of the Group and its profit or loss for that period. In preparing the financial statements, the Directors are required to:
–select suitable accounting policies and then apply them consistently;
–make judgements and accounting estimates that are reasonable and prudent;
–state that the Group financial statements comply with IFRS, as issued by the IASB and in conformity with the requirements of the Companies Act 2006;
–state with regard to the parent company financial statements that applicable UK Accounting Standards have been followed, subject to any material departures disclosed and explained in the parent company financial statements; and
–prepare the financial statements on a going concern basis unless it is inappropriate to presume that the Group and the parent company will continue in business.
In preparing the Group financial statements, International Accounting Standard 1 requires that directors properly select and apply accounting policies; present information, including accounting policies, in a manner that provides relevant, reliable, comparable and understandable information; provide additional disclosures when compliance with the specific requirements in IFRS Standards are insufficient to enable users to understand the impact of particular transactions, other events and conditions on the entity’s financial position and financial performance; and make an assessment of the company’s ability to continue as a going concern.
The Directors are responsible for keeping adequate accounting records that are sufficient to show and explain the company’s transactions and disclose with reasonable accuracy at any time the financial position of the Group and to enable them to ensure that the Group financial statements and the Remuneration report comply with the Companies Act 2006. They are also responsible for safeguarding the assets of the Group and hence for taking reasonable steps for the prevention and detection of fraud and other irregularities.
The Group financial statements for the year ended 31 December 2023, comprising principal statements and supporting notes, are set out in the ‘Financial statements’ on pages 158 to 244 of this report.
The responsibilities of the auditor in relation to the financial statements are set out in the Independent Auditor’s report on pages 154 to 157.
The financial statements for the year ended 31 December 2023 are included in the Annual Report, which is published in printed form and made available on our website. The Directors are responsible for the maintenance and integrity of the corporate and financial information included on the company’s website. Legislation in the United Kingdom governing the preparation and dissemination of financial statements may differ from legislation in other jurisdictions.
Each of the current Directors, whose names and functions are listed in the Corporate Governance section of the Annual Report 2023 confirms that, to the best of his or her knowledge:
–the Group financial statements, which have been prepared in accordance with the applicable set of accounting standards and in conformity with the requirements of Companies Act 2006, give a true and fair view of the assets, liabilities, financial position and profit of the Group;
–the strategic report and risk sections of the Annual Report, which represent the management report, include a fair review of the development and performance of the business and the position of the company and the Group taken as a whole, together with a description of the principal risks and uncertainties that it faces; and
–the Annual Report and financial statement, taken as a whole, are fair, balanced and understandable and provide the information necessary for shareholders to assess the company’s position and performance, business model and strategy.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Directors' statement of responsibilities continued |
| | | |
Disclosure of information to auditor
The Directors in office at the date of this Annual Report have each confirmed that:
–so far as he or she is aware, there is no relevant audit information of which the company’s auditor is unaware; and
–he or she has taken all the steps that he or she ought to have taken as a Director to make himself or herself aware of any relevant audit information and to establish that the company’s auditor is aware of that information.
This confirmation is given and should be interpreted in accordance with the provisions of section 418 of the Companies Act 2006.
Going concern basis
Pages 71 to 97 and pages 57 to 64 contain information on the performance of the Group, its financial position, cash flows, net debt position, borrowing facilities and climate related risks. Further information, including Treasury risk management policies, exposures to market and credit risk and hedging activities, is given in Note 44, 'Financial instruments, and related disclosures' to the financial statements. Having assessed the principal risks and other matters considered in connection with the viability statement, the Directors considered it appropriate to adopt the going concern basis of accounting in preparing the financial statements.
Internal control
The Board, through the Audit & Risk Committee, has reviewed the assessment of risks and the internal control framework that operates in GSK and has considered the effectiveness of the system of internal control in operation in the Group for the year covered by this Annual Report and up to the date of its approval by the Board of Directors. Further detail on the review of internal controls is set out in the Governance report on page 122.
The 2018 UK Corporate Governance Code
The Board considers that GSK plc applies the principles and complies with the provisions of the UK Corporate Governance Code maintained by the Financial Reporting Council, as described in the Corporate Governance section on pages 110 to 126. The Board further considers that the Annual Report, taken as a whole, is fair, balanced and understandable, and provides the information necessary for shareholders to assess the Group’s position and performance, business model and strategy.
As required by the Financial Conduct Authority’s Listing Rules, the auditor has considered the Directors’ statement of compliance in relation to those points of the UK Corporate Governance Code which are specified for their review.
Annual Report
The Annual Report for the year ended 31 December 2023, comprising the Report of the Directors, the Remuneration report, the Financial statements and Additional information for investors, has been approved by the Board of Directors and signed on its behalf by
Sir Jonathan Symonds
Chair
27 February 2024
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Report of Independent Registered Public Accounting Firm |
| | | |
| | |
| Report on the audit of the financial statements |
To the shareholders and the Board of Directors of GSK plc
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of GSK plc and subsidiaries (the “Group”) as at 31 December 2023 and 2022, the related consolidated income statements, statements of comprehensive income, statements of changes in equity, and cash flow statements, for each of the three years in the period ended 31 December 2023, and the related notes, included on pages 158 to 244 (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Group as at 31 December 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended 31 December 2023, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Group’s internal control over financial reporting as at 31 December 2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated 5 March 2024, expressed an unqualified opinion on the Group’s internal control over financial reporting.
Basis for Opinion
These financial statements are the responsibility of the Group’s management. Our responsibility is to express an opinion on the Group’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Group in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current-period audit of the financial statements that were communicated or required to be communicated to the audit committee and that (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical
audit matters or on the accounts or disclosures to which they relate.
Valuation of the ViiV Healthcare Shionogi contingent consideration liability
Accounts impacted: Contingent consideration liabilities and Other operating expense
Refer to Notes 33 to the financial statements
Critical Audit Matter Description
The Group has completed a number of significant transactions which resulted in the recognition of material contingent consideration liabilities, which are a key source of estimation uncertainty. The most significant of these liabilities was the ViiV Healthcare Shionogi Contingent Consideration Liability (ViiV CCL).
The Group completed the acquisition of the remaining 50% interest in the Shionogi-ViiV Healthcare joint venture in 2012. Upon completion, the Group recognised a contingent consideration liability for the fair value of the expected future payments to be made to Shionogi. As at 31 December 2023 the liability was valued at £5,718 million.
We identified the ViiV CCL as a critical audit matter because of the significant estimates and assumptions relating to the sales forecasts used in valuing the ViiV CCL and the sensitivity of the valuation to these inputs. The most significant of these relate to sales forecasts in the United States (US) on certain products in the treatment and prevention portfolio. Such forecasts are based on an assessment of the expected launch dates for pipeline assets, the ability to shift market practice and prescriber behavior towards long-acting injectable treatments and 2-drug regimens, the size of the long-acting prevention market and subsequent sales volumes. There is incremental challenge in forecasting sales associated with recently launched products due to the lack of historical actual data. The sales forecasts also required significant audit effort to perform appropriate audit procedures to challenge and evaluate the reasonableness of those forecasts.
How the Critical Audit Matter Was Addressed in the Audit
We performed the following audit procedures, amongst others, related primarily to the sales forecasts:
–Tested the controls over the key inputs and assumptions used in the valuation of the contingent consideration liability, including review controls over the sales forecasts of the treatment product portfolio used to value the ViiV CCL;
–Obtained the Group’s assessment of the key inputs and assumptions used in the sales forecasts and challenged the reasonableness of these, including through enquiries of key individuals from the senior leadership team, commercial strategy team and key personnel involved in the budgeting and forecasting process, and inspection of supporting evidence;
–Challenged the US volume assumptions made by the Group to estimate sales forecasts. This involved benchmarking forecast market share data against external data, such as total prescription volumes and new patient prescription volumes, in order to assess for any sources of contradictory evidence;
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Independent Auditor's report continued |
| Report on the audit of the financial statements continued | | | |
–Challenged the reasonableness of US pricing assumptions by the Group, by comparing the forecasted Returns and Rebates rate by product against the current rate, and assessing the forecasted Returns and Rebates against comparable products and expected changes in payer policy;
–Considered the results of clinical studies undertaken in the year by the Group and key competitors in order to assess whether these are corroborative or contradictory to assumptions used in the product portfolio sales forecasts in the US;
–Benchmarked the Group’s sales forecasts against those included in reports from 9 analysts and considered sales forecasts on both a total ViiV basis and an individual product basis, assessing against identified contradictory data; and
–Together with our valuations specialists, assessed the reasonableness of the overall valuation methodology, including benchmarking the discount rate used and testing the valuation model for mechanical accuracy.
Valuation of US Returns and Rebates (RAR) accruals
Accounts impacted: Turnover and Trade and other payables
Refer to Notes 29 to the financial statements
Critical Audit Matter Description
In the US, the Group sells to customers under various commercial and government mandated contracts and reimbursement arrangements that include rebates, chargebacks and a right of return for certain pharmaceutical products. As such, revenue recognition reflects gross-to-net sales adjustments. These adjustments are known as the Returns and Rebates (RAR) accruals and are a source of significant estimation uncertainty which could have a material impact on reported revenue.
In the US Commercial Operations in 2023, £16,539 million of RAR deductions were made to gross revenue of £32,359 million, resulting in net revenue of £15,820 million. The balance sheet accrual at 31 December 2023 for the US Commercial business amounted to £5,951 million.
The four most significant payer channels (also referred to as buying groups) to which the RAR accrual relates are managed healthcare organisations, Medicaid, Ryan White and Medicare Part D.
The two main causes of significant estimation uncertainty are:
–The utilisation rate, which is the portion of total sales that will be made into each payer channel, estimated in recording the accruals. The utilisation assumption is the most challenging of the key assumptions used to derive the accrual given that it is influenced by market demand and other factors outside the control of the Group; and
–The time lag between the point of sale and the point at which exact rebate amounts are known to the Group upon receipt of a claim. Those payer channels with the longest time lag result in a greater accrued period, and therefore, a greater level of estimation uncertainty in estimating the period-end accrual.
The level of estimation uncertainty is also impacted by significant shifts in channel mix driven by changes in the competitive landscape, including competitor and generic product launches, changes in government legislation and other macroeconomic factors. As such, we focus on the utilisation assumptions for those products where we deem the level of estimation uncertainty to be the most significant.
We also focus on the period-end adjustments made to the RAR accruals. These adjustments reflected updates made to the initial assumptions included within the forecasted RAR rates and, in our view, present the greatest opportunity for fraud in revenue recognition (notwithstanding the existence of internal controls).
How the Critical Audit Matter Was Addressed in the Audit
We performed the following audit procedures, amongst others, related to estimates in the RAR accruals:
–Tested the key controls over the estimation of RAR accruals including the controls associated with the forecasting of utilisation rates process and the month-end accrual review controls;
–Challenged assumptions for a selection of utilisation rates, focusing on certain products where we concluded the accrual is most sensitive to these assumptions. Our challenge included comparison to historical utilisation rates, consideration of historical accuracy and assessment of how market changes such as the impact of competition, new product launches, changes in government legislation and macroeconomic factors are appropriately reflected in the RAR accruals;
–Supplemented this with substantive analytical procedures by developing an independent expectation of the accrual balance for each of the key segments, based on historical claims received adjusted to reflect market changes in the period including an assessment of the time lag between the initial point of sale and the claim receipt. We then compared this independent expectation to those recorded to evaluate the appropriateness of the year ending accrual position;
–Considered the historical accuracy of estimates and evaluated whether forecast assumptions had been appropriately updated in a selection of cases where the actual rebate claims differed to the amount accrued;
–Evaluated the appropriateness of, and completeness of, period-end adjustments to the liability made as part of the ongoing review of the estimated accrual; and
–Performed audit procedures over the actual rebate payments made in the year by agreeing to the relevant contract to assess whether the rebate payments were in line with the contractual terms.
Valuation of other intangible assets
Accounts impacted: Other intangible assets, Cost of sales, Research and development, and Selling, general and administration
Refer to Notes 20 and 41 to the financial statements
Critical Audit Matter Description
As at 31 December 2023, the Group held £14,166 million of other intangible assets (including licenses, patents, trademarks, and trade names, but excluding goodwill and computer software). This includes £1,438 million of intangible assets acquired as part of the acquisition of Bellus Health during the year.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Independent Auditor's report continued |
| Report on the audit of the financial statements continued | | | |
Intangible assets which are in-development and not available for use should be tested at least annually for impairment irrespective of whether an indication of impairment exists.
When the carrying amount of an individual intangible asset, or cash-generating unit to which an intangible asset belongs, exceeds its recoverable amount, an impairment occurs. Recoverability of an intangible asset is derived from certain assumptions and estimates of future trading performance which create significant estimation uncertainty.
The underlying assumptions include forecast sales pricing, volume, growth rates and probability of technical and regulatory success of ongoing clinical trials. This includes assumptions on timing of cash flows determined by anticipated launch year, peak year sales, subsequent sales erosion due to generic product competition and profit margin levels. In addition, due to the impact of uncertainty driven by ongoing global macroeconomic volatility, the valuation of intangible assets will also be affected by discount rate assumptions made by the Group.
During 2023, impairment charges of £398 million were recorded. These were primarily full impairments due to cessation of research and development dictated by negative clinical trial readouts or lack of commercial attractiveness.
We identified the valuation of other intangible assets as a critical audit matter due to the inherent judgements involved in estimating future cash flows. Auditing such assumptions and estimates required extensive audit effort to challenge and evaluate the reasonableness of forecasts and management judgements.
How the Critical Audit Matter Was Addressed in the Audit
We performed the following audit procedures, amongst others, over the forecast sales pricing, volume, growth rates, probability of technical and regulatory success, profit margin levels, and discount rates used in the assessment of the valuation of other intangible assets, such as those acquired as part of the Group’s acquisition of Bellus Health:
–Tested review controls over the key inputs and assumptions used in the valuation of other intangible assets. The controls encompass review of the valuation models, which contain a number of assumptions such as the probability of technical and regulatory success, launch dates plus other revenue and cost assumptions;
–Inquired with key individuals from the corporate development team, commercial forecasting leads, and key personnel involved in the assets research and development process. We used the outcome of these inquiries to evaluate the Group’s evidence to support key assumptions such as overall sales forecasts, peak year sales (including anticipated market share, volume and uptake alongside price points where required), foreseeable competitive landscape, growth rates, probability of regulatory and technical success and margins;
–Evaluated the key inputs and assumptions applied in estimating sales and profit margin forecasts, including benchmarking of forecasts against external market data. This included independent market research of therapeutic area price points, price growth rates, and anticipated competitor market landscape, currently and at the time of forecast regulatory approval, plus assessment of any sources of contradictory evidence;
–Compared the forecast sales and profit margin levels to the Plan data (asset by asset internal forecasts) approved by the GSK Leadership Team and the Board of Directors, where the
in-development intangible asset is forecast to launch within the next 3-year period;
–Assessed the historical accuracy of sales forecasts by performing retrospective reviews across marketed assets within the business;
–Engaged our fair valuation specialists to assess the reasonableness of discount rates and valuation methodology applied as well as performing mechanical accuracy checks; and
–Considered whether events or transactions that occurred after the balance sheet date, but before the reporting date, affect the conclusions reached on the carrying values of the assets and associated disclosures.
Valuation of uncertain tax positions, including transfer pricing
Accounts impacted: Corporation tax payable, Deferred tax liabilities and Taxation charge
Refer to Notes 14 to the financial statements
Critical Audit Matter Description
The Group operates in numerous jurisdictions and there are open tax and transfer pricing matters and exposures with UK, US and overseas tax authorities that give rise to uncertain tax positions. There is a wide range of possible outcomes for provisions and contingencies. Certain judgements in respect of estimates of tax exposures and contingencies are required in order to assess the adequacy of tax provisions, which are sometimes complex as a result of the considerations required over multiple tax laws and regulations.
At 31 December 2023, the Group has recorded provisions of £584 million in respect of uncertain tax positions.
How the Critical Audit Matter Was Addressed in the Audit
With the support of our tax specialists, we assessed the appropriateness of the uncertain tax provisions, focused on those jurisdictions where the Group has the greatest potential exposure and where the highest level of judgement is required, by performing the following audit procedures amongst others:
–Tested key controls over preparation, review and reporting of judgmental tax balances and transactions, which include provisions for uncertain tax provisions;
–Assessed the assumptions and judgements that are required to determine the range of possible outcomes for recognition and measurement of provisions for uncertain tax positions in compliance with the requirements of IFRIC 23 Uncertainty over Income Tax Treatments;
–Involved our transfer pricing specialists to evaluate the transfer pricing methodology of the Group and associated approach to provision recognition and measurement; and
–Considered evidence such as the actual results from the recent tax authority audits and enquiries, third-party tax advice obtained by the Group and our tax specialists’ own knowledge of market practice in relevant jurisdictions.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Independent Auditor's report continued |
| Report on the audit of the financial statements continued | | | |
Valuation of the contingent liabilities and significant legal proceedings
Accounts impacted: Contingent liabilities and Other operating expense
Refer to Notes 35 and 47 to the financial statements
Critical Audit Matter Description
The Group operates in an environment where it is subject to significant legal and administrative proceedings, including product liability, intellectual property, tax, anti-trust, consumer fraud and governmental regulations.
The Group is currently exposed to a number of regulatory and litigation matters. The Group’s provision for these matters is £267 million at 31 December 2023. Other matters are disclosed as contingent liabilities where the criteria for recognising a provision under IAS 37 Provisions, Contingent Liabilities and Contingent Assets are not met, including the Zantac litigation described in Note 47.
Significant judgement is required by the Group in determining whether, under IAS 37 Provisions, Contingent Liabilities and Contingent Assets, in particular in relation to the Zantac litigation, as to:
–Whether the outcome will result in a probable outflow, particularly where the outcome of litigation is uncertain and subject to additional court proceedings;
–The determination of a reliable estimate can be made of the amounts of the obligation; and
–The nature and extent of any contingent liabilities and underlying significant estimation uncertainties disclosed.
How the Critical Audit Matter Was Addressed in the Audit
We performed the following audit procedures:
–Tested the Group’s controls over the completeness of provisions, the robustness of the provision against the requirements of IAS 37, the appropriateness of judgements used to determine a ‘best estimate’ and completeness and accuracy of data used in the process;
–Evaluated the assessment of the provisions, associated probabilities, and potential outcomes in accordance with IAS 37;
–Evaluated whether the methodology, data and significant judgements and assumptions used in the valuation of the provisions are appropriate in the context of the applicable financial reporting framework;
–Inquired with and inspected correspondence from the Group’s internal and external counsel to assess the litigation matters and evaluate the Group’s significant judgements and assumptions;
–Where no provision was made for expected or actual trial outcomes or settlements, evaluated the Group’s conclusion supportive and contradictory evidence and the requirements of IAS 37, particularly with respect to the Zantac litigation;
–Read board minutes and settlement agreements to understand and corroborate management’s approach in respect of the litigation and agreed the terms and conditions of such arrangements to the payments made to evaluate the provisions already recorded and whether there is a requirement for additional provisions;
–In respect of the Zantac litigation, inspected the evidence presented in relevant scientific studies and the outcomes of other product liability litigation in the same jurisdictions alongside the entity’s assessment of possible outcomes of each ongoing trial and expectation of which trials will go ahead as per the schedule of future trials; and
–Evaluated whether the disclosures made in the financial statements appropriately reflect the facts and critical accounting judgements.
/s/ Deloitte LLP
London, United Kingdom
5 March 2024
The first accounting period we audited was 31 December 2018.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Consolidated income statement |
for the year ended 31 December 2023 | | | |
| | | | | | | | | | | | | | |
| Notes | 2023
£m | 2022
£m | 2021
£m |
| Turnover | 6 | 30,328 | 29,324 | 24,696 |
| Cost of sales | | (8,565) | | (9,554) | | (8,163) | |
| Gross profit | | 21,763 | 19,770 | 16,533 |
| Selling, general and administration | | (9,385) | | (8,372) | | (7,070) | |
| Research and development | | (6,223) | | (5,488) | | (5,019) | |
| Royalty income | | 953 | 758 | 417 |
| Other operating income/(expense) | 7 | (363) | | (235) | | (504) | |
| Operating profit | 8 | 6,745 | 6,433 | 4,357 |
| Finance income | 11 | 115 | 76 | 14 |
| Finance expense | 12 | (792) | | (879) | | (769) | |
| Share of after tax profit/(loss) of associates and joint ventures | 13 | (5) | | (2) | | 33 |
| Profit/(loss) on disposal of interests in associates and joint ventures | 13 | 1 | – | (36) | |
| Profit before taxation | | 6,064 | 5,628 | 3,599 |
| Taxation | 14 | (756) | | (707) | | (83) | |
| Profit after taxation from continuing operations | | 5,308 | 4,921 | 3,516 |
| Profit after taxation from discontinued operations and other gains/(losses) from the demerger | | – | 3,049 | 1,580 |
| Re-measurement of discontinued operations distributed to shareholders on demerger | | – | 7,651 | – |
| Profit after taxation from discontinued operations | | – | 10,700 | 1,580 |
| Total profit after taxation for the year | | 5,308 | 15,621 | 5,096 |
| Profit attributable to non-controlling interests from continuing operations | | 380 | 460 | 200 |
| Profit attributable to shareholders from continuing operations | | 4,928 | 4,461 | 3,316 |
| Profit attributable to non-controlling interests from discontinued operations | | – | 205 | 511 |
| Profit attributable to shareholders from discontinued operations | | – | 10,495 | 1,069 |
| | 5,308 | 15,621 | 5,096 |
| Total profit attributable to non-controlling interests | | 380 | 665 | 711 |
| Total profit attributable to shareholders | | 4,928 | 14,956 | 4,385 |
| | 5,308 | 15,621 | 5,096 |
| Basic earnings per share (pence) from continuing operations | 15 | 121.6 p | 110.8 p | 82.9 p |
| Basic earnings per share (pence) from discontinued operations | | – | 260.6 p | 26.7 p |
| Total basic earnings per share (pence) | | 121.6 p | 371.4 p | 109.6 p |
| Diluted earnings per share (pence) from continued operations | 15 | 119.9 p | 109.2 p | 81.8 p |
| Diluted earnings per share (pence) from discontinued operations | | – | 257.0 p | 26.4 p |
Total diluted earnings per share (pence) | | 119.9 p | 366.2 p | 108.2 p |
Consolidated statement of comprehensive income
for the year ended 31 December 2023
| | | | | | | | | | | | | | |
| Notes | 2023
£m | 2022
£m | 2021
£m |
| Total profit for the year | | 5,308 | 15,621 | 5,096 |
| Other comprehensive income/(expense) for the year | | | | |
| Items that may be reclassified subsequently to continuing operations income statement: | | | | |
| Exchange movements on overseas net assets and net investment hedges | 38 | (22) | | 113 | (339) | |
Reclassification of exchange movements on liquidation or disposal of overseas subsidiaries associates | 38 | (34) | | 2 | (25) | |
| Fair value movements on cash flow hedges | | (1) | | (18) | | 5 |
| Deferred tax on fair value movements on cash flow hedges | | 1 | 9 | (8) | |
| Reclassification of cash flow hedges to income statement | | 4 | 14 | 12 |
| | (52) | | 120 | (355) | |
| Items that will not be reclassified to continuing operations income statement: | | | | |
| Exchange movements on overseas net assets of non-controlling interests | 38 | (25) | | (28) | | (20) | |
| Fair value movements on equity investments | | (244) | | (754) | | (911) | |
| Tax on fair value movements on equity investments | | 14 | 56 | 131 |
| Fair value movements on cash flow hedges | | (40) | | (6) | | – |
| Remeasurement gains/(losses) on defined benefit plans | | 71 | (786) | | 940 |
| Tax on remeasurement losses/(gains) on defined benefit plans | | (41) | | 211 | (223) | |
| | (265) | | (1,307) | | (83) | |
| Other comprehensive income /(expense) for the year from continuing operations | 38 | (317) | | (1,187) | | (438) | |
| Other comprehensive income for the year from discontinued operations | | – | 356 | 101 |
| Total comprehensive income for the year | | 4,991 | 14,790 | 4,759 |
| Total comprehensive income for the year attributable to: | | | | |
| Shareholders | | 4,636 | 14,153 | 4,068 |
| Non-controlling interests | | 355 | 637 | 691 |
| Total comprehensive income for the year | | 4,991 | 14,790 | 4,759 |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
Consolidated balance sheet |
as at 31 December 2023 | | | |
| | | | | | | | | | | |
| Notes | 2023
£m | 2022
£m |
| Non-current assets | | | |
| Property, plant and equipment | 17 | 9,020 | | 8,933 | |
| Right of use assets | 18 | 937 | | 687 | |
| Goodwill | 19 | 6,811 | | 7,046 | |
| Other intangible assets | 20 | 14,768 | | 14,318 | |
| Investments in associates and joint ventures | 21 | 55 | | 74 | |
| Other investments | 23 | 1,137 | | 1,467 | |
| Deferred tax assets | 14 | 6,049 | | 5,658 | |
| Other non-current assets | 24 | 1,584 | | 1,194 | |
| Total non-current assets | | 40,361 | | 39,377 | |
| | | |
| Current assets | | | |
| Inventories | 25 | 5,498 | | 5,146 | |
| Current tax recoverable | 14 | 373 | | 405 | |
| Trade and other receivables | 26 | 7,385 | | 7,053 | |
| Derivative financial instruments | 44 | 130 | | 190 | |
| Current equity investments | 22 | 2,204 | | 4,087 | |
| Liquid investments | 30 | 42 | | 67 | |
| Cash and cash equivalents | 27 | 2,936 | | 3,723 | |
| Assets held for sale | 28 | 76 | | 98 | |
| Total current assets | | 18,644 | | 20,769 | |
| Total assets | | 59,005 | | 60,146 | |
| | | |
| Current liabilities | | | |
| Short-term borrowings | 30 | (2,813) | | (3,952) | |
| Contingent consideration liabilities | 33 | (1,053) | | (1,289) | |
| Trade and other payables | 29 | (15,844) | | (16,263) | |
| Derivative financial instruments | 44 | (114) | | (183) | |
| Current tax payable | 14 | (500) | | (471) | |
| Short-term provisions | 32 | (744) | | (652) | |
| Total current liabilities | | (21,068) | | (22,810) | |
| | | |
| Non-current liabilities | | | |
| Long-term borrowings | 30 | (15,205) | | (17,035) | |
| Corporation tax payable | 14 | (75) | | (127) | |
| Deferred tax liabilities | 14 | (311) | | (289) | |
| Pensions and other post-employment benefits | 31 | (2,340) | | (2,579) | |
| Other provisions | 32 | (495) | | (532) | |
| Contingent consideration liabilities | 33 | (5,609) | | (5,779) | |
| Other non-current liabilities | 34 | (1,107) | | (899) | |
| Total non-current liabilities | | (25,142) | | (27,240) | |
| Total liabilities | | (46,210) | | (50,050) | |
| Net assets | | 12,795 | | 10,096 | |
| | | |
| Equity | | | |
| Share capital | 37 | 1,348 | | 1,347 | |
| Share premium account | 37 | 3,451 | | 3,440 | |
| Retained earnings | 38 | 7,239 | | 4,363 | |
| Other reserves | 38 | 1,309 | | 1,448 | |
| Shareholders’ equity | | 13,347 | | 10,598 | |
| Non-controlling interests | | (552) | | (502) | |
| Total equity | | 12,795 | | 10,096 | |
The financial statements on pages 158 to 244 were approved by the Board on 27 February, 2024 and signed on its behalf by
Sir Jonathan Symonds
Chair
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
Consolidated statement of changes in equity |
for the year ended 31 December 2023 | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| Shareholders’ equity | | |
| Share
capital
£m | Share
premium
£m | Retained
earnings
£m | Other
reserves*
£m | Total
£m | Non-controlling interests
£m | Total
equity
£m |
At 31 December 2020 | 1,346 | | 3,281 | | 6,755 | | 3,205 | | 14,587 | | 6,221 | | 20,808 | |
| Profit for the year | – | | – | | 4,385 | | – | | 4,385 | | 711 | | 5,096 | |
| Other comprehensive income/(expense) for the year | – | | – | | 454 | | (771) | | (317) | | (20) | | (337) | |
| Total comprehensive income/(expense) for the year | – | | – | | 4,839 | | (771) | | 4,068 | | 691 | | 4,759 | |
| Distributions to non-controlling interests | – | | – | | – | | – | | – | | (642) | | (642) | |
| Contributions from non-controlling interests | – | | – | | – | | – | | – | | 7 | | 7 | |
| Dividends to shareholders | – | | – | | (3,999) | | – | | (3,999) | | – | | (3,999) | |
| Shares issued | 1 | | 20 | | – | | – | | 21 | | – | | 21 | |
Realised after tax profits on disposal of equity
investments | – | | – | | 132 | | (132) | | – | | – | | – | |
Share of associates and joint ventures realised profits
on disposal of equity investments | – | | – | | 7 | | (7) | | – | | – | | – | |
| Write-down of shares held by ESOP Trusts | – | | – | | (168) | | 168 | | – | | – | | – | |
| Share-based incentive plans | – | | – | | 367 | | – | | 367 | | – | | 367 | |
| Transaction with non-controlling interests | – | | – | | – | | – | | – | | 10 | | 10 | |
| Tax on share-based incentive plans | – | | – | | 11 | | – | | 11 | | – | | 11 | |
At 31 December 2021 | 1,347 | | 3,301 | | 7,944 | | 2,463 | | 15,055 | | 6,287 | | 21,342 | |
| Profit for the year | – | | – | | 14,956 | | – | | 14,956 | | 665 | | 15,621 | |
| Other comprehensive income/(expense) for the year | – | | – | | (89) | | (714) | | (803) | | (28) | | (831) | |
| Total comprehensive income/(expense) for the year | – | | – | | 14,867 | | (714) | | 14,153 | | 637 | | 14,790 | |
| Distributions to non-controlling interests | – | | – | | – | | – | | – | | (1,409) | | (1,409) | |
| Non-cash distribution to non-controlling interests | – | | – | | – | | – | | – | | (2,960) | | (2,960) | |
| Contributions from non-controlling interests | – | | – | | – | | – | | – | | 8 | | 8 | |
| Changes to non-controlling interests | – | | – | | – | | – | | – | | (20) | | (20) | |
| Deconsolidation of former subsidiaries | – | | – | | – | | – | | – | | (3,045) | | (3,045) | |
| Dividends to shareholders | – | | – | | (3,467) | | – | | (3,467) | | – | | (3,467) | |
| Non-cash dividend to shareholders | – | | – | | (15,526) | | – | | (15,526) | | – | | (15,526) | |
Realised after tax losses on disposal or liquidation of
equity investments | – | | – | | 14 | | (14) | | – | | – | | – | |
Share of associates and joint ventures realised profits
on disposal of equity investments | – | | – | | 7 | | (7) | | – | | – | | – | |
| Shares issued | – | | 25 | | – | | – | | 25 | | – | | 25 | |
| Write-down of shares held by ESOP Trusts | – | | – | | (911) | | 911 | | – | | – | | – | |
| Shares acquired by ESOP Trusts | – | | 114 | | 1,086 | | (1,200) | | – | | – | | – | |
| Share-based incentive plans | – | | – | | 357 | | – | | 357 | | – | | 357 | |
| Tax on share-based incentive plans | – | | – | | (8) | | – | | (8) | | – | | (8) | |
Hedging gain after taxation transferred to
non-financial assets | – | | – | | – | | 9 | | 9 | | – | | 9 | |
At 31 December 2022 | 1,347 | | 3,440 | | 4,363 | | 1,448 | | 10,598 | | (502) | | 10,096 | |
| Profit for the year | – | | – | | 4,928 | | – | | 4,928 | | 380 | | 5,308 | |
| Other comprehensive income/(expense) for the year | – | | – | | (45) | | (247) | | (292) | | (25) | | (317) | |
| Total comprehensive income/(expense) for the year | – | | – | | 4,883 | | (247) | | 4,636 | | 355 | | 4,991 | |
| Distributions to non-controlling interests | – | | – | | – | | – | | – | | (412) | | (412) | |
| Contributions from non-controlling interests | – | | – | | – | | – | | – | | 7 | | 7 | |
| Dividends to shareholders | – | | – | | (2,247) | | – | | (2,247) | | – | | (2,247) | |
Realised after tax losses on disposal or liquidation of
equity investments | – | | – | | (26) | | 26 | | – | | – | | – | |
Share of associates and joint ventures realised profits
on disposal of equity investments | – | | – | | (7) | | 7 | | – | | – | | – | |
| Shares issued | 1 | | 9 | | – | | – | | 10 | | – | | 10 | |
| Write-down of shares held by ESOP Trusts | – | | – | | (324) | | 324 | | – | | – | | – | |
| Shares acquired by ESOP Trusts | – | | 2 | | 283 | | (285) | | – | | – | | – | |
| Share-based incentive plans | – | | – | | 307 | | – | | 307 | | – | | 307 | |
Hedging gain/(loss) after taxation transferred to
non-financial assets | – | | – | | – | | 36 | | 36 | | – | | 36 | |
| Tax on share-based incentive plans | – | | – | | 7 | | – | | 7 | | – | | 7 | |
At 31 December 2023 | 1,348 | | 3,451 | | 7,239 | | 1,309 | | 13,347 | | (552) | | 12,795 | |
*an analysis of Other reserves is presented as part of Note 38, ‘Movements in equity’.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
Consolidated cash flow statement |
for the year ended 31 December 2023 | | | |
| | | | | | | | | | | | | | |
| Notes | 2023
£m | 2022
£m | 2021
£m |
| Cash flow from operating activities | | | | |
| Profit after taxation from continuing operations for the year | | 5,308 | | 4,921 | | 3,516 | |
| Adjustments reconciling profit after tax to operating cash flows | 42 | 2,788 | | 3,023 | | 3,733 | |
| Cash generated from operations attributable to continuing operations | | 8,096 | | 7,944 | | 7,249 | |
| Taxation paid | | (1,328) | | (1,310) | | (972) | |
| Net cash inflow/(outflow) from continuing operating activities | | 6,768 | | 6,634 | | 6,277 | |
| Cash generated from operations attributable to discontinued operations | | – | | 932 | | 1,994 | |
| Taxation paid from discontinued operations | | – | | (163) | | (319) | |
| Net operating cash flows attributable to discontinued operations | | – | | 769 | | 1,675 | |
| Total net cash inflow/(outflow) from operating activities | | 6,768 | | 7,403 | | 7,952 | |
| | | | |
| Cash flow from investing activities | | | | |
| Purchase of property, plant and equipment | | (1,314) | | (1,143) | | (950) | |
| Proceeds from sale of property, plant and equipment | | 28 | | 146 | | 132 | |
| Purchase of intangible assets | | (1,030) | | (1,115) | | (1,704) | |
| Proceeds from sale of intangible assets | | 12 | | 196 | | 641 | |
| Purchase of equity investments | | (123) | | (143) | | (162) | |
| (Increase)/decrease in liquid investments | | 72 | | 1 | | 18 | |
| Purchase of businesses, net of cash acquired | 41 | (1,457) | | (3,108) | | – | |
| Proceeds from sale of equity investments | | 1,832 | | 238 | | 202 | |
| Contingent consideration paid | | (11) | | (79) | | (114) | |
| Disposal of businesses | 41 | 49 | | (43) | | (17) | |
| Investments in associates and joint ventures | | – | | (1) | | (1) | |
| Proceeds from disposal of associates and joint ventures | | 1 | | – | | 277 | |
| Interest received | | 115 | | 64 | | 14 | |
| Dividend and distributions from investments | | 220 | | – | | – | |
| Dividends from associates and joint ventures | | 11 | | 6 | | 9 | |
| Net cash inflow/(outflow) from continuing investing activities | | (1,595) | | (4,981) | | (1,655) | |
| Net investing cash flows attributable to discontinued operations | | – | | (3,791) | | (122) | |
| Total net cash inflow/(outflow) from investing activities | | (1,595) | | (8,772) | | (1,777) | |
| | | | |
| Cash flow from financing activities | | | | |
| Issue of share capital | 37 | 10 | | 25 | | 21 | |
| Repayment of long-term loans | | (144) | | (1,594) | | – | |
| Issue of long-term notes | | 223 | | 1,025 | | – | |
| Repayment of short-term loans | | (2,116) | | (5,074) | | (2,304) | |
| Net increase in/(repayment of) other short-term loans | | (333) | | 1,021 | | 301 | |
| Repayment of lease liabilities | | (197) | | (202) | | (181) | |
| Interest paid | | (766) | | (848) | | (772) | |
| Dividends paid to shareholders | | (2,247) | | (3,467) | | (3,999) | |
| Distributions to non-controlling interests | | (412) | | (521) | | (239) | |
| Contributions from non-controlling interests | | 7 | | 8 | | 7 | |
| Other financing items | | 334 | | 376 | | 40 | |
| Net cash inflow/(outflow) from continuing financing activities | | (5,641) | | (9,251) | | (7,126) | |
| Net financing cash flows attributable to discontinued operations | | – | | 10,074 | | (463) | |
| Total net cash inflow/(outflow) from financing activities | | (5,641) | | 823 | | (7,589) | |
| Increase/(decrease) in cash and bank overdrafts | 43 | (468) | | (546) | | (1,414) | |
| | | | |
| Cash and bank overdrafts at the beginning of year | | 3,425 | | 3,819 | | 5,262 | |
| Exchange adjustments | | (99) | | 152 | | (29) | |
| Increase/(decrease) in cash and bank overdrafts in the year | | (468) | | (546) | | (1,414) | |
| Cash and bank overdrafts at the end of year | | 2,858 | | 3,425 | | 3,819 | |
| | | | |
| Cash and bank overdrafts at end of year comprise: | | | | |
| Cash and cash equivalents | | 2,936 | | 3,723 | | 4,274 | |
| Overdrafts | | (78) | | (298) | | (455) | |
| | 2,858 | | 3,425 | | 3,819 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements |
| | | |
| | |
| 1. Presentation of the financial statements |
Description of business
GSK is a global biopharma group which prevents and treats disease with vaccines, specialty and general medicines. GSK focuses on the science of the immune system and the use of new platform and data technologies, investing in four core therapeutic areas: infectious diseases, HIV, respiratory/immunology and oncology.
Compliance with applicable law and IFRS
The financial statements have been prepared in accordance with UK-adopted international accounting standards in conformity with the requirements of the Companies Act 2006 and the International Financial Reporting Standards as issued by the IASB.
Composition of financial statements
The consolidated financial statements are drawn up in Sterling, the functional currency of GSK plc, and in accordance with IFRS accounting presentation. The financial statements comprise:
–Consolidated income statement
–Consolidated statement of comprehensive income
–Consolidated balance sheet
–Consolidated statement of changes in equity
–Consolidated cash flow statement
–Notes to the financial statements.
Composition of the Group
A list of the subsidiaries and associates which, in the opinion of the Directors, principally affected the amount of profit or net assets of the Group is given in Note 46, ‘Principal Group companies’.
Financial period
These financial statements cover the financial year from 1 January to 31 December 2023, with comparative figures for the financial years from 1 January to 31 December 2022 and, where appropriate, from 1 January to 31 December 2021.
Accounting principles and policies
The financial statements have been prepared using the historical cost convention modified by the revaluation of certain items, as stated in the accounting policies, and on a going concern basis.
The financial statements have been prepared in accordance with the Group’s accounting policies approved by the Board and described in Note 2, ‘Accounting principles and policies’. Information on the application of these accounting policies, including areas of estimation and judgement is given in Note 3, ‘Critical accounting judgements and key sources of estimation uncertainty’.
The preparation of the financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
In preparing the consolidated financial statements, the Group has considered the impact of both physical and transitional climate change risks, as well as the plans to mitigate against these, on the current valuation of assets and liabilities; particularly in the context of the risks identified in the Task Force on Climate-related Financial Disclosures (“TCFD”).
The Group does not believe that there is a material impact to judgements and estimates in relation to climate-related risks and, as a result, the valuation of the assets or liabilities have not been significantly impacted as at 31 December 2023. The Group has reviewed the recoverable values of property, plant and equipment, inventories, goodwill and intangible assets as those are the material balances impacted by climate-related risks, and the Group’s transition plans to mitigate those risks.
One of the climate-related risks identified relates to metered-dose inhalers (MDI). The Group is addressing this risk by transitioning to a lower-carbon propellant. The transition is not expected to have a material impact on the recoverable amount, or estimated useful lives, of related property, plant and equipment. See Note 17 'Property, plant and equipment' for further details.
Whilst there is currently no significant medium-term impact expected, the Group is aware of the ever-changing risks attached to climate change and continues to assess the impact on judgements and estimates, and on the preparation of the consolidated financial statements.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| | | |
| | |
| 2. Accounting principles and policies |
Consolidation
The consolidated financial statements include:
–the assets and liabilities, and the results and cash flows, of the company and its subsidiaries, including ESOP Trusts
–the Group’s share of the results and net assets of associates and joint ventures
–the Group’s share of assets, liabilities, revenue and expenses of joint operations.
The financial statements of entities consolidated are made up to 31 December each year.
Entities over which the Group has the power to direct the relevant activities so as to affect the returns to the Group, generally through control over the financial and operating policies, are accounted for as subsidiaries.
Where the Group has the ability to exercise joint control over, and rights to, the net assets of entities, the entities are accounted for as joint ventures. Where the Group has the ability to exercise joint control over an arrangement, but has rights to specified assets and obligations for specified liabilities of the arrangement, the arrangement is accounted for as a joint operation. Where the Group has the ability to exercise significant influence over entities, they are accounted for as associates. The results and assets and liabilities of associates and joint ventures are incorporated into the consolidated financial statements using the equity method of accounting. The assets, liabilities, revenue and expenses of joint operations are included in the consolidated financial statements in accordance with the Group’s rights and obligations. Interests acquired in entities are consolidated from the date the Group acquires control and interests sold are de-consolidated from the date control ceases.
Transactions and balances between subsidiaries are eliminated and no profit before tax is taken on sales between subsidiaries until the products are sold to customers outside the Group. The relevant proportion of profits on transactions with joint ventures, joint operations and associates is also deferred until the products are sold to third parties. Transactions with non-controlling interests are recorded directly in equity. Deferred tax relief on unrealised intra-Group profit is accounted for only to the extent that it is considered recoverable.
Business combinations
Business combinations are accounted for using the acquisition accounting method. Identifiable assets, liabilities and contingent liabilities acquired are measured at fair value at acquisition date. The consideration transferred is measured at fair value and includes the fair value of any contingent consideration.
The fair value of contingent consideration liabilities is reassessed at each balance sheet date with changes recognised in the income statement. Payments of contingent consideration reduce the balance sheet liability and as a result are not recorded in the income statement.
The part of each payment relating to the original estimate of the fair value of the contingent consideration on acquisition is reported within investing activities in the cash flow statement and the part of each payment relating to the increase in the liability since the acquisition date is reported within operating cash flows.
Where the consideration transferred, together with the non-controlling interest, exceeds the fair value of the net assets, liabilities and contingent liabilities acquired, the excess is recorded as goodwill. The costs of effecting an acquisition are charged to the income statement in the period in which they are incurred.
Goodwill is capitalised as a separate item in the case of subsidiaries and as part of the cost of investment in the case of joint ventures and associates. Goodwill is denominated in the currency of the operation acquired.
Where the cost of acquisition is below the Group’s interest in the net assets acquired, the difference is recognised directly in the income statement.
Where not all of the equity of a subsidiary is acquired the non-controlling interest is recognised either at fair value or at the non-controlling interest’s share of the net assets of the subsidiary, on a case-by-case basis. Changes in the Group’s ownership percentage of subsidiaries are accounted for within equity.
Foreign currency translation
Foreign currency transactions are booked in the functional currency of the Group company at the exchange rate ruling on the date of transaction. Foreign currency monetary assets and liabilities are retranslated into the functional currency at rates of exchange ruling at the balance sheet date. Exchange differences are included in the income statement.
On consolidation, assets and liabilities, including related goodwill, of overseas subsidiaries, associates and joint ventures, are translated into Sterling at rates of exchange ruling at the balance sheet date. The results and cash flows of overseas subsidiaries, associates and joint ventures are translated into sterling using average rates of exchange.
Exchange adjustments arising when the opening net assets and the profits for the year retained by overseas subsidiaries, associates and joint ventures are translated into Sterling, less exchange differences arising on related foreign currency borrowings which hedge the Group’s net investment in these operations, are taken to a separate component of equity within Retained Earnings.
When translating into Sterling the assets, liabilities, results and cash flows of overseas subsidiaries, associates and joint ventures which are reported in currencies of hyper-inflationary economies, adjustments are made where material to reflect current price levels. Any loss on net monetary assets is charged to the consolidated income statement.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 2. Accounting principles and policies continued | | | |
Revenue
Turnover
The Group receives revenue for supply of goods to external customers against orders received. The majority of contracts that GSK enters into relate to sales orders containing single performance obligations for the delivery of pharmaceutical and vaccine products. The average duration of a sales order is less than 12 months.
Product revenue is recognised when control of the goods is passed to the customer. The point at which control passes is determined by each customer arrangement, but generally occurs on delivery to the customer.
Product revenue represents net invoice value including fixed and variable consideration. Variable consideration arises on the sale of goods as a result of discounts and allowances given and accruals for estimated future returns and rebates. Revenue is not recognised in full until it is highly probable that a significant reversal in the amount of cumulative revenue recognised will not occur. The methodology and assumptions used to estimate rebates and returns are monitored and adjusted regularly in the light of contractual and legal obligations, historical trends, past experience and projected market conditions. Estimates associated with returns and rebates are revisited at each reporting date or when they are resolved and revenue is adjusted accordingly. Please refer to Note 3, 'Critical accounting judgements and key sources of estimation uncertainty' for the details on rebates, discounts and allowances.
The Group has entered into collaborative agreements, typically with other pharmaceutical or biotechnology companies to develop, produce and market drug candidates and vaccines that do not qualify as joint arrangements. When GSK has control over the commercialisation activities, the Group recognises turnover and cost of sales on a gross basis. Profit sharing amounts and royalties due to the counterparty are recorded within cost of sales. Cost of sales includes net recoveries of cost of £45 million (2022: cost of £1,635 million; 2021: cost of £640 million) from profit sharing arrangements and royalties due to the counterparty. When the counterparty controls the commercialisation activities and records the sale, the Group is not the principal in the customer contract and instead records its share of gross profit as co-promotion income, on a net basis, within turnover. The nature of co-promotion activities is such that the Group records no costs of sales. Commercial Operations turnover includes co-promotion revenue of £1 million (2022: £3 million; 2021: £7 million). Reimbursements to and from the counterparty under collaboration agreements for ‘selling, general and administration’ and ‘research and development’ costs are recorded net in the respective lines in the consolidated income statement.
Other operating income and royalty income
GSK enters into development and marketing collaborations and out-licences of the Group’s compounds or products to other parties. These contracts give rise to fixed and variable consideration from upfront payments, development milestones, sales-based milestones and royalties.
Income dependent on the achievement of a development milestone is recognised when it is highly probable that a significant reversal in the amount of cumulative revenue recognised will not occur, which is usually when the related event occurs. Sales-based milestone income is recognised when it is highly probable that the sales threshold will be reached.
Sales-based royalties on a licence of intellectual property are not recognised until the relevant product sale occurs.
For all revenue, if the time between the recognition of revenue and payment from the customer is expected to be more than one year and the impact is material, the amount of consideration is discounted using appropriate discount rates.
Value added tax and other sales taxes are excluded from revenue.
Expenditure
Expenditure is recognised in respect of goods and services received when supplied in accordance with contractual terms. Provision is made when an obligation exists for a future liability in respect of a past event and where the amount of the obligation can be reliably estimated. Manufacturing start-up costs between validation and the achievement of normal production are expensed as incurred.
Advertising and promotion expenditure is charged to the income statement as incurred.
Shipment costs on inter-company transfers are charged to cost of sales; distribution costs on sales to customers are included in selling, general and administration expenditure.
Restructuring costs are recognised and provided for, where appropriate, in respect of the direct expenditure of a business reorganisation where the plans are sufficiently detailed and well advanced, and where appropriate communication to those affected has been undertaken.
Software as a service (SaaS) configuration costs are expensed as they are incurred where the software being configured is controlled by the SaaS provider.
Research and development
Research and development expenditure is charged to the income statement in the period in which it is incurred. Development expenditure is capitalised when the criteria for recognising an asset are met, usually when a regulatory filing has been made in a major market and approval is considered highly probable. Property, plant and equipment used for research and development is capitalised and depreciated in accordance with the Group’s policy.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 2. Accounting principles and policies continued | | | |
Legal and other disputes
Provision is made for the anticipated settlement costs of legal or other disputes against the Group where an outflow of resources is considered probable and a reliable estimate can be made of the likely outcome. In respect of product liability claims related to certain products, provision is made when there is sufficient history of claims made and settlements to enable management to make a reliable estimate of the provision required to cover asserted and unasserted claims.
In certain cases, an incurred but not reported (IBNR) actuarial technique is used to determine this estimate. In addition, provision is made for legal or other expenses arising from claims received or other disputes.
The Group may become involved in legal proceedings, in respect of which it is not possible to meaningfully assess whether the outcome will result in a probable outflow, or to quantify or reliably estimate the liability. In these cases, appropriate disclosure about such cases is included but no provision is made.
Costs associated with claims made by the Group against third parties are charged to the income statement as they are incurred.
Pensions and other post-employment benefits
The costs of providing pensions under defined benefit schemes are calculated using the projected unit credit method and spread over the period during which benefit is expected to be derived from the employees’ services, consistent with the advice of qualified actuaries.
Pension obligations are measured as the present value of estimated future cash flows discounted at rates reflecting the yields of high-quality corporate bonds. Pension scheme assets are measured at fair value at the balance sheet date.
The costs of other post-employment liabilities are calculated in a similar way to defined benefit pension schemes and spread over the period during which benefit is expected to be derived from the employees’ services, in accordance with the advice of qualified actuaries.
The service cost of providing retirement benefits to employees during the year, together with the cost of any curtailment, is charged to operating profit in the year.
Actuarial gains and losses and the effect of changes in actuarial assumptions are recognised in the statement of comprehensive income in the year in which they arise.
The Group’s contributions to defined contribution plans are charged to the income statement as incurred.
Employee share plans
Incentives in the form of shares are provided to employees under share option and share award schemes.
The fair values of these options and awards are calculated at their grant dates using a Black-Scholes option pricing model and charged to the income statement over the relevant vesting periods.
The Group provides finance to ESOP Trusts to purchase company shares to meet the obligation to provide shares when employees exercise their options or awards. Costs of running the ESOP Trusts are charged to the income statement.
Shares held by the ESOP Trusts are deducted from other reserves. A transfer is made between other reserves and retained earnings over the vesting periods of the related share options or awards to reflect the ultimate proceeds receivable from employees on exercise.
Property, plant and equipment
Property, plant and equipment (PP&E) is stated at the cost of purchase or construction, less provisions for depreciation and impairment. Financing costs are capitalised within the cost of qualifying assets in construction.
Depreciation is calculated to write off the cost less residual value of PP&E, excluding freehold land, using the straight-line basis over the expected useful life. Residual values and lives are reviewed, and where appropriate adjusted annually. The normal expected useful lives of the major categories of PP&E are:
| | | | | |
| Freehold buildings | 20 to 50 years |
| Leasehold land and buildings | Lease term or 20 to 50 years |
| Plant and machinery | 10 to 20 years |
| Equipment and vehicles | 3 to 10 years |
On disposal of PP&E, the cost and related accumulated depreciation and impairments are removed from the financial statements and the net amount, less any proceeds, is taken to the income statement.
Leases
The Group recognises right of use assets under lease arrangements in which it is the lessee, except for short-term leases (defined as leases with a lease term of 12 months or less) and leases of low value assets. Rights to use assets owned by third parties under lease agreements are capitalised at the inception of the lease and recognised on the consolidated balance sheet.
The corresponding liability to the lessor is recognised as a lease obligation within short and long-term borrowings. The carrying amount is subsequently increased to reflect interest on the lease liability and reduced by lease payments made.
For calculating the discounted lease liability on leases with annual payments of £2 million or more, the implicit rate in the lease is used. If this is not available, the incremental borrowing rate with a lease specific adjustment is used. If neither of these is available, and for leases with annual payments of less than £2 million, the incremental borrowing rate is used. The incremental borrowing rate is the rate of interest at which GSK would have been able to borrow for a similar term and with a similar security the funds necessary to obtain a similar asset in a similar market.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 2. Accounting principles and policies continued | | | |
Finance costs are charged to the income statement so as to produce a constant periodic rate of charge on the remaining balance of the obligations for each accounting period.
Variable rents are not part of the lease liability and the right of use asset. These payments are charged to the income statement as incurred. Lease rental costs for short-term and low-value leases which are not capitalised are also charged to the income statement as incurred.
Non-lease components are accounted for separately from the lease components in plant and equipment leases but are not separately accounted for in land and buildings or vehicle leases.
If modifications or reassessments of lease obligations occur, the lease liability and right of use asset are remeasured.
Right of use assets where title is expected to pass to GSK at a point in the future are depreciated on a basis consistent with similar owned assets. In other cases, right of use assets are depreciated over the shorter of the useful life of the asset or the lease term.
Goodwill
Goodwill is stated at cost less impairments. Goodwill is deemed to have an indefinite useful life and is tested for impairment at least annually.
Where the fair value of the interest acquired in an entity’s assets, liabilities and contingent liabilities exceeds the consideration paid, this excess is recognised immediately as a gain in the income statement.
Other intangible assets
Intangible assets are stated at cost less provisions for amortisation and impairments.
Licences, patents, know-how and marketing rights separately acquired or acquired as part of a business combination are amortised over their estimated useful lives, generally not exceeding 30 years, using the straight-line basis, from the time they are available for use. The estimated useful lives for determining the amortisation charge take into account patent lives (exclusivity period), where applicable, as well as the value obtained from periods of non-exclusivity. For Pharmaceutical intangible assets, depending on the characteristics, competitive environment and estimated long-term profits of the asset, between 80% to 90% of the book value is amortised over the exclusivity period on a straight-line basis and the remaining book value is amortised over a non-exclusivity period of 5-15 years on a straight-line basis. For Vaccines intangible assets, cost is usually amortised over the exclusivity period plus 10 years, or 30 years if no exclusivity period is granted, on a straight-line basis. Asset lives are reviewed, and where appropriate adjusted, annually.
Contingent milestone payments are recognised at the point that the contingent event becomes probable. Any development costs incurred by the Group and associated with acquired licences, patents, know-how or marketing rights are written off to the income statement when incurred, unless the criteria for recognition of an internally generated intangible asset are met, usually when a regulatory filing has been made in a major market and approval is considered highly probable.
Acquired in process R&D and marketed products are valued independently as part of the fair value of businesses acquired from third parties where they have a value which is substantial and long term and where the assets either are contractual or legal in nature or can be sold separately from the rest of the businesses acquired.
The costs of acquiring and developing computer software for internal use and internet sites for external use are capitalised as intangible fixed assets where the software or site supports a significant business system and the expenditure leads to the creation of a durable asset controlled by the Group. ERP systems software is amortised over seven to ten years and other computer software over three to five years using the straight-line basis.
Impairment of non-current assets
The carrying values of all non-current assets are reviewed for impairment, either on a stand-alone basis or as part of a larger cash generating unit, when there is an indication that the assets might be impaired. Additionally, goodwill and intangible assets which are not yet available for use are tested for impairment annually. Any provision for impairment is charged to the income statement in the year concerned.
Impairments of goodwill are not reversed. Impairment losses on other non-current assets are only reversed if there has been a change in estimates used to determine recoverable amounts and only to the extent that the revised recoverable amounts do not exceed the carrying values that would have existed, net of depreciation or amortisation, had no impairments been recognised.
Investments in associates, joint ventures and joint operations
Investments in associates and joint ventures are carried in the consolidated balance sheet at the Group’s share of their net assets at date of acquisition and of their post-acquisition retained profits or losses and other comprehensive income together with any goodwill arising on the acquisition. The Group recognises the assets, liabilities, revenue and expenses of joint operations in accordance with its rights and obligations.
Inventories
Inventories are included in the financial statements at the lower of cost (including raw materials, direct labour, other direct costs and related production overheads) and net realisable value. Cost is generally determined on a first in, first out basis. Pre-launch inventory is held as an asset when there is a high probability of regulatory approval for the product. Before that point a provision is made against the carrying value to reduce it to its recoverable amount; the provision is then reversed at the point when a high probability of regulatory approval is determined.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 2. Accounting principles and policies continued | | | |
Financial instruments
Financial assets
Financial assets are measured at amortised cost, fair value through other comprehensive income (FVTOCI) or fair value through profit or loss (FVTPL). The measurement basis is determined by reference to both the business model for managing the financial asset and the contractual cash flow characteristics of the financial asset. For financial assets other than trade receivables a 12-month expected credit loss (ECL) allowance is recorded on initial recognition. If there is subsequent evidence of a significant increase in the credit risk of an asset, the allowance is increased to reflect the full lifetime ECL. If there is no realistic prospect of recovery, the asset is written off.
Expected credit losses are recognised in the income statement on financial assets measured at amortised cost and at fair value through other comprehensive income apart from equity investments.
Current equity investments
Current equity investments comprise equity investments which the Group holds with the intention to sell and which it may sell in the short term. Where acquired with this intention, they are measured at FVTPL. They are initially recorded at fair value and then remeasured at subsequent reporting dates to fair value. Unrealised gains and losses are recognised in the income statement. Dividend income is recognised in the income statement when the Group’s right to receive payment is established. Purchases and sales of current equity investments are accounted for on the trade date.
Other investments
Other investments comprise equity investments and investments in limited life funds. The Group has elected to designate the majority of its equity investments as measured at FVTOCI. They are initially recorded at fair value plus transaction costs and then remeasured at subsequent reporting dates to fair value. Unrealised gains and losses are recognised in other comprehensive income. On disposal of the equity investment, gains and losses that have been deferred in other comprehensive income are transferred directly to retained earnings.
Investments in limited life funds are measured at FVTPL. They are initially recorded at fair value and then remeasured at subsequent reporting dates to fair value. Unrealised gains and losses are recognised in the income statement.
Dividends on equity investments and distributions from funds are recognised in the income statement when the Group’s right to receive payment is established.
Purchases and sales of Other investments are accounted for on the trade date.
Trade receivables
Trade receivables are measured in accordance with the business model under which each portfolio of trade receivables is held. The Group has portfolios in each of the three business models under IFRS 9: to collect the contractual cash flows where there is no factoring agreement in place (measured at amortised cost); to sell the contractual cash flows where the trade receivables will be sold under a factoring agreement (measured at FVTPL); and both to collect and to sell the contractual cash flows where the trade receivables may be sold under a factoring arrangement (measured at FVTOCI). Trade receivables measured at amortised cost are carried at the original invoice amount less allowances for expected credit losses.
Expected credit losses are calculated in accordance with the simplified approach permitted by IFRS 9, using a provision matrix applying lifetime historical credit loss experience to the trade receivables. The expected credit loss rate varies depending on whether, and the extent to which, settlement of the trade receivables is overdue and it is also adjusted as appropriate to reflect current economic conditions and estimates of future conditions. For the purpose of determining credit loss rates, customers are classified into groupings that have similar loss patterns. The key drivers of the loss rate are the nature of the business unit and the location and type of customer.
When a trade receivable is determined to have no reasonable expectation of recovery it is written off, firstly against any expected credit loss allowance available and then to the income statement.
Subsequent recoveries of amounts previously provided for or written off are credited to the income statement. Long-term receivables are discounted where the effect is material.
Cash and cash equivalents
Cash held in deposit accounts is measured at amortised cost. Investments in money market funds are held at fair value through profit or loss because the funds fail the solely payments of principal and interest (SPPI) test.
Borrowings
All borrowings are initially recorded at the amount of proceeds received, net of transaction costs. Borrowings are subsequently carried at amortised cost, with the difference between the proceeds, net of transaction costs, and the amount due on redemption being recognised as a charge to the income statement over the period of the relevant borrowing.
Derivative financial instruments
Derivative financial instruments are used to manage exposure to market risks. The principal derivative instruments used by GSK are foreign currency swaps, interest rate swaps, foreign exchange forward contracts and options. The Group does not hold or issue derivative financial instruments for trading or speculative purposes.
Derivative financial assets and liabilities, including derivatives embedded in host contracts which have been separated from the host contract, are measured at fair value. Changes in the fair value of any derivative instruments that do not qualify for hedge accounting are recognised immediately in the income statement.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 2. Accounting principles and policies continued | | | |
Hedge accounting
Derivatives designated as the hedging instruments are classified at inception of hedge relationship as cash flow hedges, net investment hedges or fair value hedges.
Changes in the fair value of derivatives designated as cash flow hedges are recognised in other comprehensive income to the extent that the hedges are effective and accumulated in the cash flow hedge reserve. Ineffective portions are recognised in profit or loss immediately. Amounts deferred in the cash flow hedge reserve are reclassified to the income statement when the hedged item affects profit or loss, or if the hedged forecast transaction is to purchase a non-financial asset, the amount deferred in the cash flow hedge reserve is transferred directly from equity and included in the carrying value of the recognised non-financial asset.
Net investment hedges are accounted for in a similar way to cash flow hedges which are reclassified to the income statement when the hedged item affects profit or loss.
Changes in the fair value of derivatives designated as fair value hedges are recorded in the income statement, together with the changes in the fair value of the hedged asset or liability.
Taxation
Current tax is provided at the amounts expected to be paid, applying tax rates that have been enacted or substantively enacted by the balance sheet date. The tax charge for the period is recognised in the income statement, the statement of comprehensive income or directly in equity, according to the accounting treatment of the related transaction.
Deferred tax is provided in full on temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the financial statements. Deferred tax assets are recognised to the extent that it is probable that future taxable profits will be available against which the temporary differences can be utilised. Deferred tax is provided on temporary differences arising on investments in subsidiaries, associates and joint ventures, except where the timing of the reversal of the temporary difference can be controlled and it is probable that the temporary difference will not reverse in the foreseeable future. Deferred tax is provided using rates of tax that have been enacted or substantively enacted by the balance sheet date. Deferred tax assets and liabilities are offset when there is a legally enforceable right to offset current tax assets against current tax liabilities and when they relate to income taxes levied by the same tax authority and the Company and its subsidiaries intend to settle their current tax assets and liabilities on a net basis.
Deferred tax assets and liabilities are not recognised if the temporary differences arise from the initial recognition of goodwill or from the initial recognition of other assets and liabilities in a transaction (other than a business combination) that affects neither the accounting nor the taxable profit or loss. Unrecognised deferred tax assets are reassessed at each reporting date and are recognised to the extent that it has become probable that future taxable profits will allow the deferred tax asset to be recovered.
Where an uncertain tax position is identified, management will make a judgement as to what the probable outcome will be, assuming the relevant tax authority has full knowledge of the situation. Where it is assessed that an economic outflow is probable to arise, a provision is made for the best estimate of the liability. In estimating any such liability GSK applies a risk-based approach which takes into account, as appropriate, the probability that the Group would be able to obtain compensatory adjustments under international tax treaties. These estimates take into account the specific circumstances of each dispute and relevant external advice.
Discounting
Where the time value of money is material, balances are discounted to current values using appropriate discount rates. The unwinding of the discounts is recorded in finance income and finance expense.
Assets and liabilities held for sale or distribution and discontinued operations
Disposal groups are classified as held for sale or distribution if their carrying amount will be recovered principally through sale or a distribution to shareholders rather than through continuing use, they are available for sale or distribution in their present condition and the sale or distribution is considered highly probable. Assets held in Assets held for sale or distribution are measured at the lower of their carrying amount and fair value less costs to sell or distribute. Non-current assets included in Assets held for sale or distribution are not depreciated or amortised. Assets and liabilities classified as held for sale or distribution are presented in current assets and current liabilities separately from the other assets and liabilities in the balance sheet.
A discontinued operation is a component of the Group that has been disposed of, distributed or is classified as held for sale or distribution and that represents a separate major line of business. The results of discontinued operations are presented separately in the consolidated income statement, the consolidated statement of other comprehensive income and the consolidated statement of cash flows and comparatives are restated on a consistent basis.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| | |
| 3. Critical accounting judgements and key sources of estimation uncertainty |
In preparing the financial statements, management is required to make judgements about when or how items should be recognised in the financial statements and estimates and assumptions that affect the amounts of assets, liabilities, revenue and expenses reported in the financial statements. Actual amounts and results could differ from those estimates. The following are considered to be the critical accounting judgements and key sources of estimation uncertainty.
Turnover
Reported Group turnover for 2023 was £30,328 million (2022: £29,324 million).
Estimates
Gross turnover is reduced by rebates, discounts, allowances and product returns given or expected to be given, which vary by product arrangements and buying groups. These arrangements with purchasing organisations are dependent upon the submission of claims some time after the initial recognition of the sale. Accruals are made at the time of sale for the estimated rebates, discounts or allowances payable or returns to be made, based on available market information and historical experience.
Sales of pharmaceutical and vaccine products in the US have complex arrangements for rebates, discounts and allowances. Turnover of Commercial Operations products in the US for 2023 of £15,820 million (2022: £14,542 million) was after recording deductions of £16,539 million (2022: £15,272 million) for rebates, allowances, returns and other discounts. At 31 December 2023, the total accrual amounted to £5,951 million (2022: £5,855 million). Due to the nature of these accruals it is not practicable to give meaningful sensitivity estimates due to the large volume of variables that contribute to the overall rebates, chargebacks, returns and other revenue accruals.
As there can be significant variability in final outcomes, the Group applies a constraint when measuring the variable element within revenue, so that revenue is recognised at a suitably cautious amount. The objective of the constraint is to ensure that it is highly probable that a significant reversal of revenue will not occur when the uncertainties are resolved. The constraint is applied by making suitably cautious estimates of the inputs and assumptions used in estimating the variable consideration. Because the amounts are estimated they may not fully reflect the final outcome, and the amounts are subject to change dependent upon, amongst other things, the types of buying group and product sales mix. The constraints applied in recognising revenue mean that the risk of a material downward adjustment to revenue in the next financial year is low.
The level of accrual for rebates and returns is reviewed and adjusted regularly in the light of contractual and legal obligations, historical trends, past experience and projected market conditions. Market conditions are evaluated using wholesaler and other third-party analyses, market research data and internally generated information. It is reasonably possible that there could be a significant adjustment within the next 12 months to recognise additional revenue, if actual outcomes are better than the cautious constrained estimates.
Revenue is not recognised in full until it is highly probable that a significant reversal in the amount of cumulative revenue recognised will not occur. The amount of turnover recognised in the year from performance obligations satisfied in previous periods is set out in Note 6, ‘Turnover and segment information’, and is an indication of the level of sensitivity in the estimate.
Future events could cause the assumptions on which the accruals are based to change, which could materially affect the future results of the Group.
Taxation
The tax charge for the year was £756 million (2022: £707 million). At 31 December 2023, current tax payable was £500 million (2022: £471 million), non-current corporation tax payable was £75 million (2022: £127 million) and current tax recoverable was £373 million (2022: £405 million).
Judgement and estimates
The Group has open tax issues with a number of revenue authorities. Management makes a judgement of whether there is sufficient information to be able to make a reliable estimate of the outcome of the dispute. If insufficient information is available, no provision is made.
If sufficient information is available, in estimating a potential tax liability GSK applies a risk-based approach which takes into account, as appropriate, the probability that the Group would be able to obtain compensatory adjustments under international tax treaties. These estimates take into account the specific circumstances of each dispute and relevant external advice, are inherently judgemental and could change substantially over time as each dispute progresses and new facts emerge.
At 31 December 2023, the Group had recognised provisions of £584 million in respect of uncertain tax positions (2022: £551 million). Due to the number of uncertain tax positions held and the number of jurisdictions to which these relate, it is not practicable to give meaningful sensitivity estimates. No uncertain tax position is individually material to the Group.
Factors affecting the tax charge in future years are set out in Note 14, ‘Taxation’. GSK continues to believe that it has made adequate provision for the liabilities likely to arise from open assessments. Where open issues exist, the ultimate liability for such matters may vary from the amounts provided and is dependent upon the outcome of negotiations with the relevant tax authorities or, if necessary, litigation proceedings.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 3. Critical accounting judgements and key sources of estimation uncertainty continued |
Legal and other disputes
Legal costs for the year were £271 million (2022: £144 million). At 31 December 2023 provisions for legal and other disputes amounted to £267 million (2022: £218 million).
Judgement
Management makes a judgement of whether there is sufficient information to be able to make a reliable estimate of the likely outcome of the dispute and the legal and other expenses arising from claims against the Group. If insufficient information is available, no provision is made and disclosure of the claim is given.
The estimated provisions take into account the specific circumstances of each dispute and relevant external advice, are inherently judgemental and could change substantially over time as each dispute progresses and new facts emerge. Details of the status and various uncertainties involved in the significant unresolved disputes are set out in Note 47, ‘Legal proceedings’.
The company’s Directors, having taken legal advice, have established provisions after taking into account the relevant facts and circumstances of each matter and in accordance with accounting requirements. In respect of product liability claims related to certain products, there is sufficient history of claims made and settlements to enable management to make a reliable estimate of the provision required to cover unasserted claims.
The Group may become involved in legal proceedings, in respect of which it is not possible to meaningfully assess whether the outcome will result in a probable outflow, or to quantify or reliably estimate the liability. In these cases, appropriate disclosure about such cases would be provided, but no provision would be made and no contingent liability can be quantified.
The ultimate liability for legal claims may vary from the amounts provided and is dependent upon the outcome of litigation proceedings, investigations and possible settlement negotiations. The position could change over time and, therefore, there can be no assurance that any losses that result from the outcome of any legal proceedings will not exceed the amount of the provisions reported in the Group’s financial statements by a material amount.
Contingent consideration
The 2023 income statement charge for contingent consideration was £768 million (2022: £1,645 million).
At 31 December 2023, the liability for contingent consideration amounted to £6,662 million (2022: £7,068 million). Of this amount, £5,718 million (2022: £5,890 million) related to the acquisition of the former Shionogi-ViiV Healthcare joint venture in 2012.
Estimates
Any contingent consideration included in the consideration payable for a business combination is recorded at fair value at the date of acquisition. These fair values are generally based on risk-adjusted future cash flows discounted using appropriate post-tax discount rates. The fair values are reviewed on a regular basis, and any changes are reflected in the income statement. See Note 33, ‘Contingent consideration liabilities’.
Pensions and other post-employment benefits
Judgement
Where a surplus on a defined benefit scheme arises, or there is potential for a surplus to arise from committed future contributions, the rights of the Trustees to prevent the Group obtaining a refund of that surplus in the future are considered in determining whether it is necessary to restrict the amount of the surplus that is recognised. Three UK schemes are in surplus (2022: two UK schemes), with a combined surplus of £457 million at 31 December 2023 (2022: £109 million). There are further recognised pension surpluses totalling £177 million spread across five countries (2022: £120 million across five countries). GSK has made the judgement that these amounts meet the requirements of recoverability.
Estimates
The costs of providing pensions and other post-employment benefits are assessed on the basis of assumptions selected by management. These assumptions include future earnings and pension increases, discount rates, expected long-term rates of return on assets and mortality rates, and are disclosed in Note 31, ‘Pensions and other post-employment benefits’.
Discount rates are derived from AA rated corporate bond yields except in countries where there is no deep market in corporate bonds where government bond yields are used. A sensitivity analysis is provided in Note 31, ‘Pensions and other post-employment benefits’, a 0.25% reduction in the discount rate would lead to an increase in the net pension deficit of approximately £391 million and an increase in the annual pension cost of approximately £18 million. Similarly, a 0.25% increase in the discount rate would lead to a decrease in the net pension deficit of approximately £373 million and a decrease in the annual pension cost of approximately £18 million.
A 0.75% reduction in the discount rate would lead to an increase in the net pension deficit of approximately £1,231 million and an increase in the annual pension cost of approximately £51 million. Similarly, a 0.75% increase in the discount rate would lead to a decrease in the net pension deficit of approximately £1,071 million and a decrease in the annual pension cost of approximately £58 million. The selection of different assumptions could affect the future results of the Group.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| | |
| 4. New accounting requirements |
International Tax Reform - Pillar Two Model Rules - Amendments to IAS 12
The Group has adopted the amendments to IAS 12 which have been introduced in response to the OECD’s BEPS Pillar Two rules and include:
–A mandatory temporary exception to the recognition and disclosure of deferred taxes arising from the jurisdictional implementation of the Pillar Two model rules; and
–Disclosure requirements for affected entities to help users of the financial statements better understand an entity’s exposure to Pillar Two income taxes arising from that legislation.
GSK has applied the mandatory exception and is not recognising any deferred tax impact. Further information about the impact of the Pillar Two model framework, including the impact on the effective tax rate for 2024, is set out in Note 14, 'Taxation'.
Other amendments
The adoption of IFRS 17 Insurance Contracts and amendments to certain other IFRS accounting standards in the year ended 31 December 2023, did not have a material impact on the results or financial position of the Group.
Certain amendments to IFRS accounting standards and interpretations have been published that are not mandatory for 31 December 2023 reporting periods and have not been adopted early by the Group. These amendments and interpretations are not expected to have a material impact on the results or financial position of the Group in future reporting periods.
Amendments to IAS 7 Statement of Cash Flows and IFRS 7 Financial Instruments: Disclosures - Supplier Finance Arrangements, require additional disclosure of information about Group supplier finance arrangements. The disclosure requirements will apply for annual reporting periods beginning on or after 1 January 2024, but not for any interim periods ending on or before 31 December 2024.
The Group uses the average of exchange rates prevailing during the period to translate the results and cash flows of overseas subsidiaries, joint ventures and associates into sterling and period end rates to translate the net assets of those entities. The currencies which most influence these translations and the relevant exchange rates were:
| | | | | | | | | | | |
| 2023 | 2022 | 2021 |
| Average rates: | | | |
| US$/£ | 1.24 | 1.24 | 1.38 |
| Euro/£ | 1.15 | 1.17 | 1.16 |
| Yen/£ | 175 | 161 | 151 |
| | | | | | | | | | | |
| 2023 | 2022 | 2021 |
| Period end rates: | | | |
| US$/£ | 1.27 | 1.20 | 1.35 |
| Euro/£ | 1.15 | 1.13 | 1.19 |
| Yen/£ | 180 | 159 | 155 |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 6. Turnover and segment information |
Operating segments are reported based on the financial information provided to the Chief Executive Officer and the responsibilities of the GSK Leadership Team (GLT). GSK reports under two segments; Commercial Operations and Total R&D. Members of the GLT are responsible for each segment.
Originally GSK reported 2021 results under four segments: Pharmaceuticals, Pharmaceuticals R&D, Vaccines and Consumer Healthcare. However, the reporting of operating segments was changed in 2022 and with the demerger of Consumer Healthcare only two operating segments are reportable. Comparative information was retrospectively revised on a consistent basis in 2022. There is no change to the reportable segments in 2023.
R&D investment is essential for the sustainability of the business. However for segment reporting the Commercial Operating profits exclude allocations of globally funded R&D.
The Total R&D segment is the responsibility of the Chief Scientific Officer and is reported as a separate segment. The operating costs of this segment includes R&D activities across Specialty Medicines, including HIV and Vaccines. It includes R&D and some Selling, General and Administrative (SG&A) costs relating to regulatory and other functions.
The Group’s management reporting process allocates intra-Group profit on a product sale to the segment in which that sale is recorded, and the profit analyses below have been presented on that basis.
| | | | | | | | | | | |
| Turnover by segment | 2023
£m | 2022
£m | 2021
£m |
| Commercial operations | 30,328 | | 29,324 | | 24,696 | |
| 30,328 | | 29,324 | | 24,696 | |
For 2023, product sales are reported within three product groups: Vaccines, Specialty Medicines and General Medicines.
| | | | | | | | | | | |
| Commercial Operations: | 2023
£m | 2022
£m | 2021
£m |
| Shingles | 3,446 | | 2,958 | | 1,721 | |
| Meningitis | 1,260 | | 1,116 | | 961 | |
| RSV | 1,238 | | – | | – | |
| Influenza | 504 | | 714 | | 679 | |
| Established Vaccines | 3,266 | | 3,085 | | 2,970 | |
| 9,714 | | 7,873 | | 6,331 | |
| Pandemic Vaccines | 150 | | 64 | | 447 | |
| Vaccines | 9,864 | | 7,937 | | 6,778 | |
| | | |
| HIV | 6,444 | | 5,749 | | 4,777 | |
| Respiratory/Immunology and Other | 3,025 | | 2,609 | | 2,027 | |
| Oncology | 731 | | 602 | | 489 | |
| 10,200 | | 8,960 | | 7,293 | |
| Pandemic | 44 | | 2,309 | | 958 | |
| Specialty Medicines | 10,244 | | 11,269 | | 8,251 | |
| | | |
| Respiratory | 6,825 | | 6,548 | | 6,048 | |
| Other General Medicines | 3,395 | | 3,570 | | 3,619 | |
| General Medicines | 10,220 | | 10,118 | | 9,667 | |
| | | |
| Total Commercial Operations | 30,328 | | 29,324 | | 24,696 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 6. Turnover and segment information continued | |
During 2023, sales were made to three US wholesalers of £4,494 million (2022:£4,045 million; 2021: £3,159 million), £4,498 million (2022: £4,161 million; 2021: £3,081 million) and £3,531 million (2022: £3,227 million; 2021: £2,670 million) respectively, after allocating final-customer discounts to the wholesalers.
Revenue recognised in the year from performance obligations satisfied in previous periods totalled £1,751 million (2022: £1,601 million) including £728 million (2022: £898 million) impacting turnover arising from changes to prior year estimates of RAR (returns and rebates) accruals, £37 million (2022: £115 million) of milestone income and £986 million (2022: £588 million) of royalty income recognised in the current year.
| | | | | | | | | | | |
| Segment profit | 2023
£m | 2022 £m | 2021
£m |
| Commercial Operations | 14,656 | | 13,590 | | 11,467 | |
| Research and development | (5,607) | | (5,060) | | (4,567) | |
| Segment profit | 9,049 | | 8,530 | | 6,900 | |
| Corporate and other unallocated costs | (263) | | (379) | | (407) | |
| Other reconciling items between segment profit and operating profit | (2,041) | | (1,718) | | (2,136) | |
| Total Operating profit | 6,745 | | 6,433 | | 4,357 | |
| | | |
| Finance income | 115 | | 76 | | 14 | |
| Finance costs | (792) | | (879) | | (769) | |
| Gain/(loss) on disposal of interest in associates | 1 | | – | | (36) | |
| Share of after-tax profits/(losses) of associates and joint ventures | (5) | | (2) | | 33 | |
| Profit before taxation from continuing operations | 6,064 | | 5,628 | | 3,599 | |
| Taxation | (756) | | (707) | | (83) | |
| Profit after taxation for the year from continuing operations | 5,308 | | 4,921 | | 3,516 | |
Other reconciling items between segment profit and operating profit comprise items not specifically allocated to segment profit. These include impairment and amortisation of intangible assets; major restructuring costs, which include impairments of tangible assets and computer software; transaction-related adjustments related to significant acquisitions; proceeds and costs of disposals of products and businesses, significant legal charges and expenses on the settlement of litigation and government investigations, other operating income other than royalty income and other items. Please refer to the detail of Other reconciling items between segment profit and operating profit in the analysis of adjusting items (Group financial review).
| | | | | | | | | | | |
| Depreciation and amortisation by segment | 2023
£m | 2022 £m | 2021
£m |
| Commercial Operations | 893 | | 829 | | 915 | |
| Research and development | 572 | | 467 | | 378 | |
| Segment depreciation and amortisation | 1,465 | | 1,296 | | 1,293 | |
| Corporate and other unallocated depreciation and amortisation | 110 | | 112 | | 68 | |
Other reconciling items between segment depreciation and amortisation and total depreciation and
amortisation | 719 | | 739 | | 761 | |
| Total depreciation and amortisation | 2,294 | | 2,147 | | 2,122 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 6. Turnover and segment information continued | |
| | | | | | | | | | | |
| PP&E, intangible asset and goodwill impairment by segment | 2023
£m | 2022 £m | 2021
£m |
| Commercial Operations | 27 | | 29 | | 30 | |
| Research and development | 13 | | 32 | | 55 | |
| Segment impairment | 40 | | 61 | | 85 | |
| Corporate and other unallocated impairment | 35 | | 20 | | 63 | |
| Other reconciling items between segment impairment and total impairment | 432 | | 420 | | 392 | |
| Total impairment | 507 | | 501 | | 540 | |
| | | | | | | | | | | |
| PP&E and intangible asset impairment reversals by segment | | | |
| Commercial Operations | (16) | | (6) | | (8) | |
| Research and development | (9) | | (19) | | (2) | |
| Segment impairment reversals | (25) | | (25) | | (10) | |
| Corporate and other unallocated impairment reversals | (14) | | – | | – | |
| Other reconciling items between segment impairment reversals and total impairment reversals | – | | (1) | | (2) | |
| Total impairment reversals | (39) | | (26) | | (12) | |
| | | | | | | | | | | |
| Net operating assets by segment | | 2023
£m | 2022 £m |
| Commercial Operations | | 12,302 | | 10,288 | |
| Research and development | | 7,021 | | 7,299 | |
| Segment net operating assets | | 19,323 | | 17,587 | |
| Corporate and other unallocated net operating assets | | 625 | | 264 | |
| Net operating assets | | 19,948 | | 17,851 | |
| | | |
| Net debt | | (15,040) | | (17,197) | |
| Investments in associates and joint ventures | | 55 | | 74 | |
| Current equity investment | | 2,204 | | 4,087 | |
| Derivative financial instruments | | 16 | | 7 | |
| Current and deferred taxation | | 5,536 | | 5,176 | |
| Assets held for sale (excluding cash and cash equivalents) | | 76 | | 98 | |
| Net assets | | 12,795 | | 10,096 | |
The Commercial Operations segment includes the Shionogi-ViiV Healthcare contingent consideration liability of £5,718 million (2022: £5,890 million) and the Pfizer put option of £848 million (2022: £1,093 million).
Geographical information
The UK is regarded as being the Group’s country of domicile.
| | | | | | | | | | | |
| Turnover by location of customer | 2023
£m | 2022
£m | 2021
£m |
| UK | 693 | | 695 | | 656 | |
| US | 15,820 | | 14,542 | | 11,914 | |
| Rest of World | 13,815 | | 14,087 | | 12,126 | |
| External turnover | 30,328 | | 29,324 | | 24,696 | |
| | | | | | | | | | | |
| Non-current assets by location of subsidiary | | 2023
£m | 2022
£m |
| UK | | 6,464 | | 5,134 | |
| US | | 13,280 | | 14,024 | |
| Belgium | | 5,337 | | 5,415 | |
| Rest of World | | 6,606 | | 6,593 | |
| Non-current assets | | 31,687 | | 31,166 | |
Non-current assets by location excludes amounts relating to other investments, deferred tax assets, derivative financial instruments, pension assets, amounts receivable under insurance contracts and certain other non-current receivables. There are no other countries with individually material external revenue or non-current assets.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 7. Other operating income/(expense) |
| | | | | | | | | | | |
| 2023
£m | 2022 £m | 2021 £m |
Upfront settlement income(1) | – | | 922 | | – | |
| Fair value remeasurements of equity investments | (122) | | 256 | | 37 | |
| Disposal of businesses and assets | 61 | | 215 | | 552 | |
| Fair value remeasurements on contingent consideration recognised in business combinations | (791) | | (1,607) | | (1,058) | |
| Remeasurement of ViiV Healthcare put option liabilities and preferential dividends | 245 | | (85) | | (48) | |
| Fair value adjustments on derivative financial instruments | 7 | | 3 | | (4) | |
| Other income | 237 | | 61 | | 17 | |
| (363) | | (235) | | (504) | |
(1)On 1 February 2022, ViiV Healthcare reached agreement with Gilead Sciences, Inc (Gilead) to settle the global patent infringement litigation relating to the commercialisation of Gilead’s Biktarvy concerning ViiV Healthcare’s patents relating to dolutegravir, an anti-retroviral medication used, together with other medicines, to treat human immunodeficiency virus (HIV). Under the terms of the global settlement and licensing agreement, Gilead made an upfront payment of $1.25 billion (£922 million) to ViiV Healthcare on 15 February 2022. In addition, Gilead will also pay a 3% royalty on all future US sales of Biktarvy and in respect of the bictegravir component of any other future bictegravir-containing products sold in the US. These royalties will be payable by Gilead to ViiV Healthcare from 1 February 2022 until the expiry of ViiV Healthcare’s US Patent No. 8,129,385 on 5 October 2027 and will be recorded as royalty income in the income statement.
Fair value remeasurement on equity investments in 2023 included a loss of £17 million from the remeasurement of the Group’s retained investment in Haleon plc. See details in Note 22 'Current equity investments'.
Disposal of businesses and assets in 2023 primarily includes milestone income.
Disposal of businesses and assets in 2022 includes milestone income and the reversal of provisions no longer required.
Disposal of businesses and assets in 2021 included a net gain on disposal of the rights to the royalty stream for cabozantinib and a net gain on disposal of the cephalosporin antibiotic brands to Sandoz.
Fair value re-measurements on contingent consideration recognised as business combinations included a net charge of £934 million related to the acquisition of the former Shionogi-ViiV Healthcare joint venture, £187 million net credit payable to Novartis related to the Vaccines acquisition, together with fair value movements on related hedging contracts and a charge of £44 million relating to the contingent consideration on the acquisition of Affinivax primarily relating to the unwind of the discount.
Other income in 2023 primarily includes net income from dividends related to investments, including £49 million dividends received from the retained investment in Haleon plc.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | | | | | | | | | | |
| The following items have been included in operating profit: | 2023
£m | 2022 £m | 2021 £m |
| Employee costs (Note 9) | 8,473 | | 7,693 | | 7,680 | |
| Advertising | 835 | | 735 | | 433 | |
| Distribution costs | 199 | | 192 | | 169 | |
| Depreciation of property, plant and equipment | 892 | | 885 | | 855 | |
| Impairment of property, plant and equipment, net of reversals | 17 | | 70 | | 87 | |
| Depreciation of right of use assets | 190 | | 176 | | 179 | |
| Impairment of right of use assets | 10 | | 40 | | 5 | |
| Amortisation of intangible assets | 1,212 | | 1,086 | | 1,088 | |
| Impairment of intangible assets, net of reversals | 418 | | 365 | | 435 | |
| Impairment of tangible and intangible assets held for sale, net of reversals | 23 | | – | | 1 | |
| | | |
| Net foreign exchange (gains)/losses | 11 | | 11 | | (4) | |
| Inventories: | | | |
| Cost of inventories included in cost of sales | 6,576 | | 6,137 | | 5,885 | |
| Write-down of inventories | 979 | | 687 | | 800 | |
| Reversal of prior year write-down of inventories | (598) | | (483) | | (325) | |
| Short-term lease charge | 8 | | 6 | | 7 | |
| Low-value lease charge | 2 | | 2 | | 3 | |
| Variable lease payments | 17 | | 9 | | 10 | |
| Fees payable to the company’s auditor and its associates in relation to the Group (see below) | 22.0 | | 26.9 | | 31.7 | |
The reversals of prior year write-downs of inventories principally arise from the reassessment of usage or demand expectations prior to inventory expiration.
Net foreign exchange (gains)/losses include a net gain of £34 million (2022: £2 million loss; 2021: £35 million gain) arising from the recycling of exchange on liquidation or disposal of overseas subsidiaries. The recycling of exchange on disposal of overseas associates is £nil (2022: £nil). The recycling of exchange on disposal of overseas subsidiaries does not include recycling of exchange on disposal of Consumer Healthcare subsidiaries as this is reported as Profit after taxation on demerger of discontinued operations.
Included within operating profit are Major restructuring charges of £382 million (2022: £321 million; 2021: £424 million), see Note 10, ‘Major restructuring costs’.
| | | | | | | | | | | |
| Fees payable to the company’s auditor and its associates: | 2023
£m | 2022 £m | 2021 £m |
Audit of parent company and consolidated financial statements including attestation under
s.404 of Sarbanes-Oxley Act 2002 | 10.2 | | 10.9 | | 13.2 | |
| Audit of the company’s subsidiaries | 10.2 | | 9.7 | | 14.5 | |
| Total audit services | 20.4 | | 20.6 | | 27.7 | |
| Audit-related and other assurance services | 1.6 | | 6.3 | | 4.0 | |
| Total audit services, audit-related and other assurance services | 22.0 | | 26.9 | | 31.7 | |
The other assurance services provided by the auditor related to agreed upon procedures and other assurance services outside of statutory audit requirements. Audit related and other assurance services include £nil (2022: £4.4 million; 2021: £2.4 million) due to reporting accountant work performed in preparation for the Consumer Healthcare demerger.
In addition to the above, fees paid to the auditor in respect of the GSK pension schemes were:
| | | | | | | | | | | |
| 2023
£m | 2022 £m | 2021 £m |
| Audit | 0.2 | | 0.2 | | 0.2 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | | | | | | | | | | |
| 2023
£m | 2022 £m | 2021 £m |
| Wages and salaries | 6,706 | | 6,110 | | 5,858 | |
| Social security costs | 818 | | 763 | | 793 | |
| Pension and other post-employment costs, including augmentations (Note 31) | 356 | | 369 | | 415 | |
| Cost of share-based incentive plans | 321 | | 314 | | 345 | |
| Severance and other costs from integration and restructuring activities | 272 | | 137 | | 269 | |
| 8,473 | | 7,693 | | 7,680 | |
The Group provides benefits to employees, commensurate with local practice in individual countries, including in some markets, healthcare insurance, subsidised car schemes and personal life assurance.The cost of share-based incentive plans is analysed as follows:
| | | | | | | | | | | |
| 2023
£m | 2022 £m | 2021 £m |
| Share value plan | 244 | | 243 | | 258 | |
| Performance share plan | 58 | | 55 | | 51 | |
| Share option plans | 5 | | 4 | | 5 | |
| Cash settled and other plans | 14 | | 12 | | 31 | |
| 321 | | 314 | | 345 | |
The average number of persons employed by the Group (including Directors) during the year: | | | | | | | | | | | |
| 2023
Number | 2022 Number | 2021 Number |
| Manufacturing | 23,209 | 22,946 | 23,562 | |
| Selling, general and administration | 34,446 | 34,642 | 36,909 | |
| Research and development | 12,589 | 11,542 | 10,874 | |
| Total Continuing Operations | 70,244 | 69,130 | 71,345 | |
| | | |
| Discontinued Operations | – | 21,292 | 20,616 | |
| | | |
| Total | 70,244 | 90,422 | 91,961 |
Note: Consumer Healthcare was divested on 18 July 2022 and is shown as Discontinued Operations in the above table.The average monthly number of Group employees excludes temporary and contract staff.
The compensation of the Directors and senior management (members of the GLT) in aggregate, was as follows:
| | | | | | | | | | | |
| 2023
£m | 2022
£m | 2021
£m |
| Wages and salaries | 37 | | 31 | | 27 | |
| Social security costs | 4 | | 5 | | 3 | |
| Pension and other post-employment costs | 1 | | 2 | | 3 | |
| Cost of share-based incentive plans | 32 | | 28 | | 27 | |
| 74 | | 66 | | 60 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 10. Major restructuring costs |
Within the Pharmaceuticals sector, the highly regulated manufacturing operations and supply chains and long lifecycle of the business mean that restructuring programmes, particularly those that involve the rationalisation or closure of manufacturing or R&D sites, are likely to take several years to complete.
Major restructuring costs are those related to specific Board-approved Major restructuring programmes, including integration costs following material acquisitions, which are structural and are of a significant scale where the costs of individual or related projects exceed £25 million.
In January 2020, the Board approved a Separation Preparation programme to prepare for the separation of GSK into two companies. This programme is largely complete. After the acquisition of Sierra Oncology (July 2022) and Affinivax (August 2022), the Board approved a Major restructuring programme for the integration of significant acquisitions designed to integrate and achieve synergies. In June 2023 GSK acquired Bellus Health Inc.
The total restructuring costs of £382 million in 2023 (2022: £321 million; 2021: £424 million) were incurred in the following areas:
–Restructuring costs for separation of GSK into two companies
–Continued transformation of central functions, including GSK technology platforms and interfaces, to deliver greater digital synergies, simplification of applications and staff reductions
–The integration of acquisitions.
The analysis of the costs charged to operating profit under these programmes was as follows:
| | | | | | | | | | | |
| 2023
£m | 2022 £m | 2021 £m |
| Increase in provision for Major restructuring programmes (see Note 32) | 172 | | 138 | | 321 | |
| Amount of provision reversed unused (see Note 32) | (55) | | (111) | | (140) | |
| Impairment losses recognised | 33 | | 122 | | 14 | |
| Other non-cash charges/(credit) | 86 | | (7) | | 25 | |
| Other cash costs | 146 | | 179 | | 204 | |
| 382 | | 321 | | 424 | |
Provision reversals of £55 million reflected provision releases mainly related to the Separation Preparation programme. Asset impairments of £33 million and other non-cash charges of £86 million principally comprised fixed asset write-downs of manufacturing and administrative facilities and accelerated depreciation where asset lives have been shortened in the supply chain manufacturing network as a result of the Major restructuring programmes. All other charges have been or will be settled in cash and include site closure costs, consultancy and project management costs.
The analysis of Major restructuring charges by programme was as follows:
| | | | | | | | | | | |
| 2023 |
| Cash
£m | Non-cash
£m | Total
£m |
| Separation Preparation programme | 199 | | 117 | | 316 | |
| Significant acquisitions | 65 | | 1 | | 66 | |
| Legacy programmes | (1) | | 1 | | – | |
| 263 | | 119 | | 382 | |
| | | |
| | | | | | | | | | | |
| 2022 |
| Cash
£m | Non-cash
£m | Total £m |
| Separation Preparation programme | 177 | | 110 | | 287 | |
| Significant acquisitions | 20 | | – | | 20 | |
| Legacy programmes | 9 | | 5 | | 14 | |
| 206 | | 115 | | 321 | |
The analysis of Major restructuring charges by income statement line was as follows:
| | | | | | | | | | | |
| 2023
£m | 2022 £m | 2021 £m |
| Cost of sales | 164 | | 102 | | 102 | |
| Selling, general and administration | 216 | | 180 | | 277 | |
| Research and development | 2 | | 39 | | 45 | |
| 382 | | 321 | | 424 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | | | | | | | | | | |
| 2023
£m | 2022 £m | 2021 £m |
| Finance income arising from: | | | |
| Financial assets measured at amortised cost | 48 | | 31 | | 11 | |
| Financial assets measured at fair value through profit or loss | 60 | | 31 | | 2 | |
| Net gains arising from the forward element of forward contracts in net investment hedge relationships | – | | 12 | | – | |
| Other finance income | 7 | | 2 | | 1 | |
| 115 | | 76 | | 14 | |
| | | | | | | | | | | |
| 2023
£m | 2022 £m | 2021 £m |
| Finance expense arising on: | | | |
| Financial liabilities at amortised cost | (672) | | (789) | | (735) | |
| Net losses arising from: | | | |
| Financial instruments mandatorily measured at fair value through profit or loss | (23) | | 743 | | (565) | |
| Retranslation of loans | 25 | | (761) | | 565 | |
| Reclassification of hedges from other comprehensive income | (4) | | (2) | | (2) | |
| Unwinding of discounts on provisions | (15) | | (7) | | (2) | |
| Finance expense arising on lease liabilities | (38) | | (30) | | (27) | |
| Other finance expense | (65) | | (33) | | (3) | |
| (792) | | (879) | | (769) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 13. Associates and joint ventures |
The Group’s share of after-tax profits and losses of associates and joint ventures is set out below:
| | | | | | | | | | | |
| 2023
£m | 2022 £m | 2021 £m |
| Share of after-tax (losses)/profits of associates | (2) | | 1 | | 36 | |
| Share of after-tax losses of joint ventures | (3) | | (3) | | (3) | |
| (5) | | (2) | | 33 | |
During the year, the Group disposed of an investment in a joint venture for £nil consideration, with the release of related commitments for future capital contributions resulting in a net £1 million profit on disposal.
In May 2021, the Group agreed with Innoviva Inc. to sell all of its shares in Innoviva back to Innoviva for £277 million. Following the disposal, at 31 December 2023, 31 December 2022 and 31 December 2021, the Group held no significant individual associates.
Summarised income statement information in respect of Innoviva until May 2021 is set out below.
The results of Innoviva included in the summarised income statement information below represent the estimated earnings of Innoviva in the relevant periods, based on publicly available information. Figures for 2021 include share of Innoviva’s turnover, profit and total comprehensive income until the date of the disposal.
| | | | | | | | | | | |
| | | 2021
£m |
| Turnover | | | 108 | |
| Profit after taxation | | | 106 | |
| Total comprehensive income | | | 106 | |
Aggregated financial information in respect of GSK’s share of other associated undertakings and joint ventures is set out below:
| | | | | | | | | | | |
| 2023
£m | 2022
£m | 2021
£m |
| Share of after-tax losses | (5) | | (2) | | – | |
| Share of other comprehensive income/(expense) | 7 | | (9) | | 28 | |
| Share of total comprehensive income/(expense) | 2 | | (11) | | 28 | |
The Group’s sales to associates and joint ventures were £nil in 2023 (2022: £nil; 2021: £nil).
Please refer to the balance sheet information on Note 21, 'Investments in associates and joint ventures'.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
The Group’s tax charge is the sum of the total current and deferred tax expense.
| | | | | | | | | | | |
| Taxation charge based on profits for the year | 2023
£m | 2022 £m | 2021 £m |
| UK current year charge | 207 | | 200 | | 119 | |
| Rest of World current year charge | 1,371 | | 1,351 | | 593 | |
| Charge/(credit) in respect of prior periods | 43 | | (60) | | 219 | |
| Current taxation | 1,621 | | 1,491 | | 931 | |
| Deferred taxation | (865) | | (784) | | (848) | |
| 756 | | 707 | | 83 | |
In 2023, GSK made corporate income tax payments globally of £1.3 billion (2022: £1.5 billion), of which £205 million (2022: £48 million) was UK corporation tax paid to HMRC. These amounts are for corporate income tax only, and do not include the various other business taxes borne by GSK each year.
The deferred tax credits in each period reflect current year losses where offset against taxable profits in future periods is probable and the release of deferred tax liabilities. The latter relates primarily to the unwind of deferred tax liabilities on intangible assets.
The following table reconciles the tax charge calculated at the UK statutory rate on the Group profit before tax with the actual tax charge for the year.
| | | | | | | | | | | | | | | | | | | | |
| Reconciliation of taxation on Group profits | 2023 £m | 2023
% | 2022 £m | 2022
% | 2021 £m | 2021
% |
| Profit before tax | 6,064 | | | 5,628 | | | | 3,599 | | | |
| UK statutory rate of taxation | 1,425 | | 23.5 | | 1,069 | | 19.0 | | 685 | | 19.0 | |
| Differences in overseas taxation rates | 159 | | 2.6 | | 318 | | 5.6 | | 302 | | 8.4 | |
| Benefit of intellectual property incentives | (696) | | (11.5) | | (600) | | (10.7) | | (382) | | (10.6) | |
| R&D credits | (121) | | (2.0) | | (119) | | (2.1) | | (100) | | (2.8) | |
| Permanent differences on disposals, acquisitions and transfers | 10 | | 0.2 | | 275 | | 4.9 | | (3) | | (0.1) | |
| Other permanent differences | 102 | | 1.7 | | 82 | | 1.5 | | (4) | | (0.1) | |
| Re-assessments of prior year current tax estimates | 43 | | 0.7 | | (60) | | (1.1) | | 219 | | 6.1 | |
| Re-assessments of prior year deferred tax estimates | (147) | | (2.4) | | (233) | | (4.1) | | (281) | | (7.8) | |
| Changes in tax rates | (19) | | (0.3) | | (25) | | (0.4) | | (353) | | (9.8) | |
| Tax charge/tax rate | 756 | | 12.5 | | 707 | | 12.6 | | 83 | | 2.3 | |
As a global biopharmaceutical company, we have a substantial business and employment presence in many countries around the world. The impact of differences in overseas taxation rates arose from profits being earned in countries with tax rates higher than the UK statutory rate, the most significant of which in 2023 were Belgium and Japan. This adverse impact was offset by the benefit of intellectual property incentives such as the UK Patent Box and Belgian Innovation Income Deduction regimes, which provide a reduced rate of corporation tax on profits earned from qualifying patents. We claim these incentives in the manner intended by the relevant statutory or regulatory framework.
Permanent differences on disposals, acquisitions and transfers in 2022 includes tax on internal restructuring to simplify the group structure.
The Group’s tax rate is also influenced by updates to estimates of prior period tax liabilities following closure of open issues with tax authorities in various jurisdictions and changes in tax rates. The cumulative impact of these items in 2023 is a 2% reduction in the tax rate.
In 2021, ‘Changes in tax rates’ included credits in relation to the enactment of the increase in the headline rate of UK corporate income tax from 19% to 25% (effective 2023).
Future tax charges, and therefore our effective tax rate, may be affected by factors such as acquisitions, disposals, restructurings, the location of research and development activity, tax regime reforms and resolution of open matters as we continue to bring our tax affairs up to date around the world.
During 2023 the UK Government substantively enacted legislation introducing a global minimum corporate income tax rate, to have effect from 2024 in line with the Organisation for Economic Co-operation and Development’s (OECD) Pillar Two model framework. We anticipate that the rules will restrict our ability to benefit from innovation incentives, such as the UK and Belgium Patent Box regimes, and consequently our underlying effective tax rate is forecast to increase by around 2% from 2024.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 14. Taxation continued | |
| | | | | | | | | | | |
| Tax on items charged to equity and statement of comprehensive income | 2023
£m | 2022 £m | 2021 £m |
| Current taxation | | | |
| Share-based payments | (1) | | (3) | | – | |
| Defined benefit plans | (143) | | – | | – | |
| Fair value movements on cash flow hedges | – | | – | | 5 | |
| Fair value movements on equity investments | (6) | | 12 | | 36 | |
| (150) | | 9 | | 41 | |
| Deferred taxation | | | |
| Share-based payments | (6) | | 11 | | (11) | |
| Defined benefit plans | 184 | | (211) | | 223 | |
| Fair value movements on cash flow hedges | (1) | | (9) | | 3 | |
| Fair value movements on equity investments | (8) | | (68) | | (167) | |
| 169 | | (277) | | 48 | |
| Total charge/(credit) to equity and statement of comprehensive income | 19 | | (268) | | 89 | |
All of the above items have been charged to the statement of comprehensive income except for tax on share based payments.
Issues relating to taxation
The integrated nature of the Group’s worldwide operations involves significant investment in research and strategic manufacture at a limited number of locations, with consequential cross-border supply routes into numerous end-markets. In line with current OECD guidelines, we base our transfer pricing policy on the arm’s length principle and support our transfer prices with economic analysis and reports. However, different tax authorities may seek to attribute further profit to activities being undertaken in their jurisdiction potentially resulting in double taxation. The Group also has open items in several jurisdictions concerning such matters as the deductibility of particular expenses and the tax treatment of certain business transactions. GSK applies a risk based approach to determine the transactions most likely to be subject to challenge and the probability that the Group would be able to obtain compensatory adjustments under international tax treaties.
The calculation of the Group’s total tax charge therefore necessarily involves a degree of estimation and judgement in respect of certain items whose tax treatment cannot be finally determined until resolution has been reached with the relevant tax authority or, as appropriate, through a formal legal process. At 31 December 2023 the Group had recognised provisions of £584 million in respect of such uncertain tax positions (2022: £551 million). The net increase in recognised provisions during 2023 was driven by the reassessment of estimates, the agreement of a number of open issues with tax authorities in various jurisdictions and amounts related to discontinued operations. Whilst the ultimate liability for such matters may vary from the amounts provided and is dependent upon the outcome of agreements with the relevant tax authorities, or litigation where appropriate, the Group continues to consider that it has made appropriate provision for periods which are open and not yet agreed by the tax authorities.
A provision for deferred tax liabilities of £165 million as at 31 December 2023 (2022: £157 million) has been made in respect of taxation that would be payable on the remittance of profits by certain overseas subsidiaries. Whilst the aggregate amount of unremitted profits at the balance sheet date was approximately £18 billion (2022: £16 billion), the majority of these unremitted profits would not be subject to tax (including withholding tax) on repatriation, as UK legislation relating to company distributions provides for exemption from tax for most overseas profits, subject to certain exceptions. Deferred tax is not provided on temporary differences of £869 million (2022: £660 million) arising on unremitted profits as management has the ability to control any future reversal and does not consider such a reversal to be probable.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 14. Taxation continued | |
Movement in deferred tax assets and liabilities
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Accelerated
capital
allowances
£m | Intangible
assets
£m | Contingent consideration
£m | Intra-Group
profit
£m | Pensions & other post employment benefits
£m | Tax
losses
£m | Share
option
and award
schemes
£m | Other
net
temporary
differences
£m | Total |
At 1 January 2022 | (211) | | (3,711) | | 850 | | 999 | | 640 | | 1,450 | | 91 | | 1,554 | | 1,662 | |
| Exchange adjustments | (29) | | (264) | | – | | (40) | | 64 | | 6 | | 1 | | 160 | | (102) | |
| Credit/(charge) to income statement | 122 | | 126 | | 142 | | 258 | | (32) | | 104 | | (22) | | 190 | | 888 | |
| Credit/(charge) to statement of comprehensive income | – | | – | | – | | – | | 182 | | 42 | | (11) | | (12) | | 201 | |
| Acquisitions/Disposals | (1) | | (637) | | – | | – | | – | | 67 | | – | | 76 | | (495) | |
| R&D credits utilisation | – | | – | | – | | – | | – | | – | | – | | (76) | | (76) | |
| Transfer of assets for sale/distribution | 62 | | 3,667 | | – | | (118) | | (60) | | (8) | | (2) | | (250) | | 3,291 | |
| At 31 December 2022 | (57) | | (819) | | 992 | | 1,099 | | 794 | | 1,661 | | 57 | | 1,642 | | 5,369 | |
| Exchange adjustments | 11 | | 58 | | – | | (70) | | (24) | | (2) | | – | | (100) | | (127) | |
| Credit/(charge) to income statement | 72 | | 229 | | (71) | | 223 | | (15) | | 335 | | 12 | | 80 | | 865 | |
| Credit/(charge) to statement of comprehensive income | – | | – | | – | | – | | (184) | | – | | 5 | | 10 | | (169) | |
| Acquisitions/Disposals | – | | (144) | | – | | – | | – | | – | | – | | – | | (144) | |
| R&D credits utilisation | – | | – | | – | | – | | – | | – | | – | | (56) | | (56) | |
At 31 December 2023 | 26 | | (676) | | 921 | | 1,252 | | 571 | | 1,994 | | 74 | | 1,576 | | 5,738 | |
Deferred tax liabilities in relation to intangible assets predominately relate to temporary differences arising as a result of historic business combinations. Acquisitions within the year predominantly relate to Bellus Health (see Note 41, 'Acquisitions and disposals').
The Group continues to recognise deferred tax assets on future obligations in respect of contingent consideration amounts payable to minority shareholders. These payments are tax deductible at the point in time at which payment is made.
A deferred tax asset is recognised on intra-Group profits arising on inter-company inventory which are eliminated within the consolidated accounts. As intra-Group profits are not eliminated from the individual entities’ tax returns a temporary difference arises that will reverse at the point in time inventory is sold externally.
The deferred tax asset of £1,994 million (2022: £1,661 million) recognised on tax losses relates to trading losses. Such deferred tax assets are only recognised to the extent Group long-range forecasts indicate sufficient future taxable profits will be available to utilise such assets by around 2030. Other net temporary differences included accrued expenses for which a tax deduction is only available on a paid basis.
Deferred tax asset and liabilities are recognised on the balance sheet as follows:
| | | | | | | | |
| 2023
£m | 2022
£m |
| Deferred tax assets | 6,049 | | 5,658 | |
| Deferred tax liabilities | (311) | | (289) | |
| 5,738 | | 5,369 | |
| | | | | | | | | | | | | | |
| | 2023 | | 2022 |
| Unrecognised tax losses | Tax losses
£m | Unrecognised deferred tax asset
£m | Tax losses
£m | Unrecognised deferred tax asset
£m |
| Trading losses expiring: | | | | |
| Within 10 years | 939 | | 149 | | 967 | | 175 | |
| More than 10 years | 1,238 | | 66 | | 44 | | 13 | |
| Available indefinitely | 228 | | 47 | | 192 | | 41 | |
| At 31 December | 2,405 | | 262 | | 1,203 | | 229 | |
| | | | |
| Capital losses expiring: | | | | |
| Available indefinitely | 2,261 | | 567 | | 2,326 | | 548 | |
| At 31 December | 2,261 | | 567 | | 2,326 | | 548 | |
Deferred tax assets are only recognised where it is probable that future taxable profit will be available to utilise losses.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | | | | | | | | | | |
| 2023
pence | 2022
pence | 2021 pence |
| Basic earnings per share from continuing operations | 121.6 | | 110.8 | | 82.9 | |
| Basic earnings per share from discontinued operations | – | | 260.6 | | 26.7 | |
| Total basic earnings per share | 121.6 | | 371.4 | | 109.6 | |
| | | |
| Diluted earnings per share from continuing operations | 119.9 | | 109.2 | | 81.8 | |
| Diluted earnings per share from discontinued operations | – | | 257.0 | | 26.4 | |
| Total diluted earnings per share | 119.9 | | 366.2 | | 108.2 | |
Basic earnings per share has been calculated by dividing the profit attributable to shareholders by the weighted average number of shares in issue during the period after deducting shares held by the ESOP Trusts for the future exercise of share options and share awards and Treasury shares. The trustees have waived their rights to cash dividends on the GSK shares held by the ESOP Trusts.
Diluted earnings per share has been calculated after adjusting the weighted average number of shares used in the basic calculation to assume the conversion of all potentially dilutive shares. A potentially dilutive share forms part of the employee share schemes where its exercise price is below the average market price of GSK shares during the period and any performance conditions attaching to the scheme have been met at the balance sheet date.
The numbers of shares used in calculating basic and diluted earnings per share are reconciled below.
| | | | | | | | | | | |
| Weighted average number of shares in issue | 2023
millions | 2022
millions | 2021
millions |
| Basic | 4,052 | | 4,026 | | 4,003 | |
| Dilution for share options and awards | 59 | | 58 | | 49 | |
| Diluted | 4,111 | | 4,084 | | 4,052 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2023 | | | 2022 | | | 2021 |
| Paid/payable | Dividend
per share
(pence) | Total
dividend
£m | Paid | Dividend
per share
(pence) | Total
dividend
£m | Paid | Dividend
per share
(pence) | Total
dividend
£m |
| First interim | 13 July 2023 | 14.00 | | 567 | | 1 July 2022 | 17.50 | | 704 | | 8 July 2021 | 23.75 | | 951 | |
| Second interim | 12 October 2023 | 14.00 | | 568 | | 6 October 2022 | 16.25 | | 654 | | 7 October 2021 | 23.75 | | 951 | |
| Third interim | 11 January 2024 | 14.00 | | 568 | | 12 January 2023 | 13.75 | | 555 | | 13 January 2022 | 23.75 | | 952 | |
| Fourth interim | 11 April 2024 | 16.00 | | 649 | | 13 April 2023 | 13.75 | | 557 | * | 7 April 2022 | 28.75 | | 1,157 | |
| Total | | 58.00 | | 2,352 | | | 61.25 | | 2,470 | | | 100 | | 4,011 | |
* The estimate for the fourth interim dividend for 2022 disclosed in the 2022 annual report was £555 million, £2 million less than the dividend that was ultimately paid.
Under IFRS, interim dividends are only recognised in the financial statements when paid and not when declared. GSK normally pays a dividend two quarters after the quarter to which it relates and one quarter after it is declared. The 2023 financial statements recognise those dividends paid in 2023, namely the third and fourth interim dividends for 2022, and the first and second interim dividends for 2023.
The demerger of Consumer Healthcare in 2022 was effected by GSK declaring an interim dividend in specie of Haleon plc shares. The fair value of the distribution was £15,526 million.
The amounts recognised in each year were as follows:
| | | | | | | | | | | |
| 2023
£m | 2022
£m | 2021
£m |
| Cash dividends to shareholders | 2,247 | | 3,467 | | 3,999 | |
| Dividends in specie to shareholders in Haleon plc shares (Note 41) | – | | 15,526 | | – | |
| 2,247 | | 18,993 | | 3,999 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 17. Property, plant and equipment |
| | | | | | | | | | | | | | |
| Land and
buildings
£m | Plant,
equipment
and vehicles
£m | Assets in
construction
£m | Total
£m |
Cost at 1 January 2022 | 7,212 | | 11,816 | | 1,750 | | 20,778 | |
| Exchange adjustments | 403 | | 542 | | 105 | | 1,050 | |
| Additions through business combinations | 5 | | 8 | | 17 | | 30 | |
| Other additions | 13 | | 79 | | 1,153 | | 1,245 | |
| Capitalised borrowing costs | – | | – | | 21 | | 21 | |
| Disposals and write-offs | (64) | | (222) | | (5) | | (291) | |
| Reclassifications | 146 | | 689 | | (874) | | (39) | |
| Transfer to assets held for sale/distribution | (1,067) | | (1,959) | | (317) | | (3,343) | |
Cost at 31 December 2022 | 6,648 | | 10,953 | | 1,850 | | 19,451 | |
| Exchange adjustments | (189) | | (265) | | (44) | | (498) | |
| Additions through business combinations | – | | – | | – | | – | |
| Other additions | 11 | | 99 | | 1,185 | | 1,295 | |
| Capitalised borrowing costs | – | | – | | 36 | | 36 | |
| Disposals and write-offs | (136) | | (732) | | (16) | | (884) | |
| Reclassifications | 134 | | 701 | | (869) | | (34) | |
| Transfer to assets held for sale/distribution | (13) | | (52) | | (22) | | (87) | |
Cost at 31 December 2023 | 6,455 | | 10,704 | | 2,120 | | 19,279 | |
| | | | |
Depreciation at 1 January 2022 | (3,281) | | (6,744) | | – | | (10,025) | |
| Exchange adjustments | (191) | | (310) | | – | | (501) | |
| Charge for the year | (226) | | (726) | | – | | (952) | |
| Disposals and write-offs | 47 | | 181 | | – | | 228 | |
| Transfer to assets held for sale/distribution | 376 | | 1,130 | | – | | 1,506 | |
Depreciation at 31 December 2022 | (3,275) | | (6,469) | | – | | (9,744) | |
| Exchange adjustments | 90 | | 153 | | – | | 243 | |
| Charge for the year | (210) | | (682) | | – | | (892) | |
| Disposals and write-offs | 66 | | 662 | | – | | 728 | |
| Transfer to assets held for sale/distribution | 6 | | 29 | | – | | 35 | |
| Reclassifications | – | | (4) | | – | | (4) | |
Depreciation at 31 December 2023 | (3,323) | | (6,311) | | – | | (9,634) | |
| | | | |
Impairment at 1 January 2022 | (264) | | (514) | | (43) | | (821) | |
| Exchange adjustments | (9) | | (14) | | (1) | | (24) | |
| Disposals and write-offs | 9 | | 47 | | 5 | | 61 | |
| Impairment losses | (33) | | (45) | | (5) | | (83) | |
| Reversal of impairments | – | | 9 | | – | | 9 | |
| Transfer to assets held for sale/distribution | 37 | | 45 | | 2 | | 84 | |
Impairment at 31 December 2022 | (260) | | (472) | | (42) | | (774) | |
| Exchange adjustments | 4 | | 7 | | 1 | | 12 | |
| Disposals and write-offs | 27 | | 114 | | 13 | | 154 | |
| Impairment losses | (11) | | (32) | | – | | (43) | |
| Reversal of impairments | 3 | | 23 | | – | | 26 | |
| Transfer to assets held for sale/distribution | – | | – | | – | | – | |
| Reclassifications | – | | – | | – | | – | |
Impairment at 31 December 2023 | (237) | | (360) | | (28) | | (625) | |
Total depreciation and impairment at 31 December 2022 | (3,535) | | (6,941) | | (42) | | (10,518) | |
Total depreciation and impairment at 31 December 2023 | (3,560) | | (6,671) | | (28) | | (10,259) | |
Net book value at 1 January 2022 | 3,667 | | 4,558 | | 1,707 | | 9,932 | |
Net book value at 31 December 2022 | 3,113 | | 4,012 | | 1,808 | | 8,933 | |
Net book value at 31 December 2023 | 2,895 | | 4,033 | | 2,092 | | 9,020 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 17. Property, plant and equipment continued | |
The weighted average interest rate for capitalised borrowing costs in the year was 4% (2022: 4%). Disposals and write-offs in the year included a number of assets with nil net book value that are no longer in use in the business.
The impairment losses principally arose from decisions to rationalise facilities and were calculated based on fair value less costs of disposal. The fair value less costs of disposal valuation methodology uses significant inputs which are not based on observable market data, and therefore this valuation technique is classified as level 3 of the fair value hierarchy. These calculations determine the net present value of the projected risk-adjusted, post-tax cash flows of the relevant asset or cash generating unit, applying a discount rate of the Group post-tax weighted average cost of capital (WACC) of 7%, adjusted where appropriate for specific segment, country and currency risk.
Assets that continue to be used by the Group are generally assessed as part of their associated cash generating unit on a value in use basis. For value in use calculations, the post-tax cash flows do not include the impact of future uncommitted restructuring plans or improvements. Where an impairment is indicated and a pre-tax cash flow calculation is expected to give a materially different result, the test would be reperformed using pre-tax cash flows and a pre-tax discount rate. The Group WACC is equivalent to a pre-tax discount rate of approximately 9%.
Net impairment reversals have been credited to cost of sales: £1 million (2022: net impairment losses £11 million) and R&D: £5 million (2022: net impairment losses £7 million). Net impairment losses have been charged to SG&A: £23 million (2022: £55 million), after charging impairment losses of £27 million (2022: £34 million) arising from the Major restructuring programmes.
Reversals of impairment arose from subsequent reviews of the impaired assets where the conditions which gave rise to the original impairments were deemed no longer to apply. £17 million of the impairment reversal has been credited to cost of sales, £5 million of the impairment reversal has been credited to R&D expenses and £4 million of the impairment reversal has been credited to SG&A. During 2022, the full impairment reversal of £9 million was credited to cost of sales.
During 2023, £34 million (2022: £39 million) of computer software was reclassified from assets in construction to intangible assets on becoming ready for use.
The Group has assessed the qualitative and quantitative impact of climate related risks on asset recoverable amounts and concluded that there are no material impairments. As of 31 December 2023, £53 million has been capitalised in property, plant and equipment regarding the transition to a lower-carbon propellant.
| | | | | | | | | | | | | | |
| Land and buildings £m | Plant and equipment £m | Vehicles
£m | Total
£m |
| Net book value at 1 January 2022 | 633 | | 9 | | 98 | | 740 | |
| Exchange adjustments | 47 | | – | | 8 | | 55 | |
| Additions through business combinations | 53 | | – | | – | | 53 | |
| Other additions | 140 | | 2 | | 91 | | 233 | |
| Depreciation | (131) | | (3) | | (58) | | (192) | |
| Transfer to assets held for sale/distribution | (115) | | (1) | | (11) | | (127) | |
| Disposals | (27) | | (1) | | (8) | | (36) | |
| Impairments | (39) | | – | | – | | (39) | |
Net book value at 31 December 2022 | 561 | | 6 | | 120 | | 687 | |
| Exchange adjustments | (30) | | – | | (6) | | (36) | |
| Additions through business combinations | 1 | | – | | – | | 1 | |
| Other additions | 355 | | – | | 144 | | 499 | |
| Depreciation | (121) | | (2) | | (67) | | (190) | |
| Transfer to assets held for sale/distribution | – | | – | | – | | – | |
| Disposals | (11) | | – | | (9) | | (20) | |
| Impairments | (10) | | – | | – | | (10) | |
| Reclassifications | 6 | | – | | – | | 6 | |
Net book value at 31 December 2023 | 751 | | 4 | | 182 | | 937 | |
The Group has entered into some commitments for lease contracts that have not yet commenced. See Note 36, 'Commitments'.
An analysis of lease liabilities is set out in Note 30, ‘Net debt’.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | | | | | | | |
| 2023
£m | 2022
£m |
| Cost at 1 January | 7,046 | | 10,552 | |
| Exchange adjustments | (313) | | 550 | |
| Additions through business combinations (Note 41) | 109 | | 1,127 | |
| Other movements (Note 41) | (31) | | – | |
| Transfer to assets held for sale/distribution | – | | (5,183) | |
| Cost at 31 December | 6,811 | | 7,046 | |
| Net book value at 1 January | 7,046 | | 10,552 | |
| Net book value at 31 December | 6,811 | | 7,046 | |
All goodwill is allocated to the Group’s segments as follows:
| | | | | | | | |
| 2023
£m | 2022 £m |
| Commercial operations | 5,951 | | 6,148 | |
| Total R&D | 860 | | 898 | |
| Net book value at 31 December | 6,811 | | 7,046 | |
The recoverable amounts of the cash generating units are assessed using a fair value less costs of disposal model. Fair value less costs of disposal is calculated using a discounted cash flow approach, with a post-tax discount rate applied to the projected risk-adjusted post-tax cash flows and terminal value.
The discount rate used is based on the Group WACC of 7% (2022: 7%), as most cash generating units have integrated operations across large parts of the Group. The discount rate is adjusted where appropriate for specific segment, country and currency risks. The valuation methodology uses significant inputs which are not based on observable market data, therefore this valuation technique is classified as level 3 in the fair value hierarchy.
The R&D segment is evaluated on an arm's length pricing model, see assumptions below.
Details relating to the discounted cash flow models used in the impairment tests are as follows:
| | | | | | | | | | | |
| Valuation basis | Fair value less costs of disposal | | |
| Key assumptions | Sales growth rates Profit margins
Terminal growth rate
Discount rate Taxation rate | | |
| Determination of assumptions | Growth rates are internal forecasts based on both internal and external market information. Margins reflect past experience, adjusted for expected changes. Terminal growth rates based on management’s estimate of future long-term average growth rates. Discount rates based on Group WACC, adjusted where appropriate. Taxation rates based on appropriate rates for each jurisdiction. |
| Period of specific projected cash flows | Five years | | |
| Terminal growth rate and discount rate | | Terminal growth rate | Discount rate |
| 2023 | | |
| Commercial operations | 0% p.a | 7% p.a |
| R&D | 0% p.a | 7% p.a |
| 2022 | | |
| Commercial operations | 0% p.a | 7% p.a |
| R&D | 0% p.a | 7% p.a |
The terminal growth rate does not exceed the long-term projected growth rates for relevant markets, reflects the impact of future generic competition and take account of new product launches. Goodwill is monitored for impairment at the segmental level and the valuations indicated sufficient headroom such that a reasonably possible change to key assumptions is unlikely to result in an impairment of the related goodwill.
The Group has assessed the qualitative and quantitative impact of climate related risks on asset recoverable amounts and concluded that there are no material impairments.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 20. Other intangible assets |
| | | | | | | | | | | | | | |
| Computer
software
£m | Licences, patents, amortised brands
£m | Indefinite life
brands
£m | Total
£m |
Cost at 1 January 2022 | 2,424 | | 21,439 | | 18,626 | | 42,489 | |
| Exchange adjustments | 63 | | 934 | | 1,112 | | 2,109 | |
| Capitalised development costs | – | | 317 | | – | | 317 | |
| Additions through business combinations | – | | 2,964 | | – | | 2,964 | |
| Other additions | 149 | | 626 | | – | | 775 | |
| Disposals and asset write-offs | (203) | | (33) | | – | | (236) | |
| Transfer to assets held for sale/distribution | (513) | | (496) | | (19,772) | | (20,781) | |
| Reclassifications | 39 | | (34) | | 34 | | 39 | |
Cost at 31 December 2022 | 1,959 | | 25,717 | | – | | 27,676 | |
| Exchange adjustments | (30) | | (664) | | – | | (694) | |
| Capitalised development costs | – | | 363 | | – | | 363 | |
| Additions through business combinations | – | | 1,438 | | – | | 1,438 | |
| Other additions | 144 | | 525 | | – | | 669 | |
| Disposals and asset write-offs | (125) | | (13) | | – | | (138) | |
| Transfer to assets held for sale/distribution | 2 | | – | | – | | 2 | |
| Reclassifications | 34 | | (3) | | – | | 31 | |
Cost at 31 December 2023 | 1,984 | | 27,363 | | – | | 29,347 | |
| | | | |
Amortisation at 1 January 2022 | (1,369) | | (8,262) | | – | | (9,631) | |
| Exchange adjustments | (33) | | (307) | | – | | (340) | |
| Charge for the year | (204) | | (931) | | – | | (1,135) | |
| Disposals and asset write-offs | 129 | | 19 | | – | | 148 | |
| Transfer to assets held for sale | 254 | | 300 | | – | | 554 | |
Amortisation at 31 December 2022 | (1,223) | | (9,181) | | – | | (10,404) | |
| Exchange adjustments | 18 | | 174 | | – | | 192 | |
| Charge for the year | (203) | | (1,009) | | – | | (1,212) | |
| Disposals and asset write-offs | 100 | | 8 | | – | | 108 | |
| Transfer to assets held for sale/distribution | (3) | | – | | – | | (3) | |
| Reclassifications | 4 | | 1 | | – | | 5 | |
Amortisation at 31 December 2023 | (1,307) | | (10,007) | | – | | (11,314) | |
| | | | |
Impairment at 1 January 2022 | (91) | | (2,480) | | (208) | | (2,779) | |
| Exchange adjustments | (2) | | (138) | | (1) | | (141) | |
| Impairment losses | (72) | | (313) | | (17) | | (402) | |
| Transfer to assets held for sale/distribution | 10 | | 34 | | 226 | | 270 | |
| Reversal of impairments | 1 | | 17 | | – | | 18 | |
| Disposals and asset write-offs | 73 | | 7 | | – | | 80 | |
Impairment at 31 December 2022 | (81) | | (2,873) | | – | | (2,954) | |
| Exchange adjustments | 1 | | 70 | | – | | 71 | |
| Impairment losses | (23) | | (398) | | – | | (421) | |
| Transfer to assets held for sale/distribution | – | | – | | – | | – | |
| Reversal of impairments | 3 | | – | | – | | 3 | |
| Disposals and asset write-offs | 25 | | 11 | | – | | 36 | |
Impairment at 31 December 2023 | (75) | | (3,190) | | – | | (3,265) | |
| | | | |
Total amortisation and impairment at 31 December 2022 | (1,304) | | (12,054) | | – | | (13,358) | |
Total amortisation and impairment at 31 December 2023 | (1,382) | | (13,197) | | – | | (14,579) | |
Net book value at 1 January 2022 | 964 | | 10,697 | | 18,418 | | 30,079 | |
Net book value at 31 December 2022 | 655 | | 13,663 | | – | | 14,318 | |
Net book value at 31 December 2023 | 602 | | 14,166 | | – | | 14,768 | |
The weighted average interest rate for capitalised borrowing costs in the year was 4% (2022: 4%).
The net book value of computer software included £270 million (2022: £479 million) of internally generated costs.
The carrying value at 31 December 2023 of intangible assets, for which impairments have been charged in the year following those impairments, was £533 million (2022: £83 million), resulting from the appraisal of GSK’s assumptions related to in-licences and collaboration agreements. The carrying value at 31 December 2023 of intangible assets, for which impairment reversals have been charged in the year following those impairment reversals, was £nil million (2022: £776 million). No individual intangible asset accounted for a material impairment.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 20. Other intangible assets continued | |
Please refer to Note 2, 'Accounting principles and policies' to the Group’s accounting policy and estimate of the useful life for intangible assets over the exclusivity and non-exclusivity periods.
Amortisation and impairment losses, net of reversals, have been charged in the income statement as follows:
| | | | | | | | | | | | | | |
| Amortisation | Net impairment losses |
| 2023 £m | 2022 £m | 2023 £m | 2022 £m |
| Cost of sales | 668 | | 663 | | 1 | | 2 | |
| Selling, general and administration | 103 | | 116 | | 18 | | 66 | |
| Research and development | 441 | | 307 | | 399 | | 299 | |
| 1,212 | | 1,086 | | 418 | | 367 | |
Licences, patents, amortised brands etc. includes a large number of acquired licences, patents, know-how agreements and marketing rights, which are either marketed or in use, or still in development. Note 41, ‘Acquisitions and disposals’ gives details of additions through business combinations in the year. The book values of the largest individual items are as follows:
| | | | | | | | |
| 2023 £m | 2022 £m |
| Tesaro Assets | 2,656 | | 2,858 | |
| Meningitis Portfolio | 1,717 | | 1,855 | |
| Momelotinib | 1,470 | | 1,499 | |
| Camlipixant | 1,438 | | – | |
| Affinivax Assets | 1,429 | | 1,473 | |
| Dolutegravir (including Cabotegravir) | 1,059 | | 1,150 | |
| Iteos Assets | 443 | | 443 | |
| Alector Assets | 425 | | 509 | |
| Benlysta | 424 | | 541 | |
| Shingrix | 289 | | 288 | |
| Okairos | 198 | | 202 | |
| BMS Assets | 191 | | 196 | |
| CureVac Assets | 191 | | 178 | |
| Spero | 163 | | 163 | |
| RSV | 139 | | 40 | |
| Relvar/Breo/Anoro | 125 | | 181 | |
| Stiefel Trade Name | 116 | | 142 | |
| Wave Life Sciences | 116 | | – | |
| UCB | 115 | | 137 | |
| Arrowhead | 114 | | 90 | |
| DT | 104 | | 115 | |
| Fluarix/FluLaval | 100 | | 147 | |
| Vir Assets | 1 | | 159 | |
| Others | 1,143 | | 1,297 | |
| 14,166 | | 13,663 | |
After announcement on 13 December 2022, GSK and Wave Life Sciences Ltd. entered into a strategic collaboration in January 2023, to advance oligonucleotide therapeutics focusing on novel genetic targets.
On 28 June 2023, GSK has completed the acquisition of Bellus Health Inc, a late-stage biopharmaceutical company. The acquisition provides GSK access to camlipixant. (Refer to Note 41, 'Acquisitions and disposals').
The Group does not consider that any reasonably possible changes in the key assumptions would cause the recoverable amount of the intangible assets disclosed above to fall below their carrying values.
The Group has assessed the qualitative and quantitative impact of climate related risks on asset recoverable amounts and concluded that there are no material impairments.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 21. Investments in associates and joint ventures |
| | | | | | | | | | | | | | | | | | | | |
| Joint
ventures
£m |
Associates
£m
| 2023
Total
£m | Joint
ventures
£m |
Associates
£m
| 2022
Total
£m |
| At 1 January | 10 | | 64 | | 74 | | 12 | | 76 | | 88 | |
| Exchange adjustments | – | | (3) | | (3) | | 1 | | 1 | | 2 | |
| Additions | – | | – | | – | | – | | 1 | | 1 | |
| Disposals | (7) | | – | | (7) | | – | | – | | – | |
| Distributions received | – | | (11) | | (11) | | – | | (6) | | (6) | |
| Net fair value movements through other comprehensive income | – | | 7 | | 7 | | – | | (9) | | (9) | |
| Impairment of interest in associates | – | | – | | – | | – | | – | | – | |
Profit/(loss) after tax recognised in the consolidated income
statement | (3) | | (2) | | (5) | | (3) | | 1 | | (2) | |
| At 31 December | – | | 55 | | 55 | | 10 | | 64 | | 74 | |
During the year, the Group disposed of an investment in a joint venture for £nil consideration.
Please refer to the income statement information in Note 13, 'Associates and joint ventures'.
| | |
| 22. Current equity investments |
| | | | | | | | | | | | | | | | | | | | |
| Current | | | | | Investments measured at FVTPL
2023
£m | Investments measured at FVTPL
2022
£m |
| At 1 January | | | | | 4,087 | | – | |
| Additions | | | | | – | | 3,852 | |
| Net fair value movements through profit or loss | | | | | (17) | | 233 | |
| Disposals and Settlements | | | | | (1,863) | | – |
| Exchange adjustments | | | | | (3) | | 2 | |
| At 31 December | | | | | 2,204 | | 4,087 | |
Current equity investments represent Haleon plc shares held after the demerger of Consumer Healthcare. Shares are held for trading and measured at fair value through profit or loss (FVTPL) based on the Haleon plc share price. Changes in fair value are presented as Other operating income/(expense) in continuing operations. The Group’s investment in Haleon plc at the end of December 2023 is held by Glaxo Group Limited, 2.8% (2022: 5.4%), GSK Scottish Limited Partnership (No.1), 4.6% (2022: 4.7%), GSK Scottish Limited Partnership (No.2), nil (2022: 1.8%), GSK Scottish Limited Partnership (No.3), nil (2022: 1.0%) and the ESOP Trusts, nil (2022: 0.6%).
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | | | | | | | | | | | | | | | | | | | |
| Non-current | Investments designated as measured at FVTOCI
£m | Investments measured at FVTPL
£m | 2023 £m | Investments designated as measured at FVTOCI
£m | Investments measured at FVTPL
£m | 2022 £m |
| 1 January | 1,153 | | 314 | | 1,467 | | 1,927 | | 199 | | 2,126 | |
| Exchange adjustments | (26) | | (15) | | (41) | | 75 | | 25 | | 100 | |
| Additions | 93 | | 29 | | 122 | | 87 | | 63 | | 150 | |
| Net fair value movements through other comprehensive income | (253) | | – | | (253) | | (716) | | – | | (716) | |
| Net fair value movements through profit or loss | – | | (122) | | (122) | | – | | 27 | | 27 | |
| Held for sale | (16) | | – | | (16) | | – | | – | | – | |
| Disposals | (20) | | – | | (20) | | (220) | | – | | (220) | |
| 31 December | 931 | | 206 | | 1,137 | | 1,153 | | 314 | | 1,467 | |
Non-current other investments comprise non-current equity investments which are recorded at fair value at each balance sheet date. For investments traded in an active market, the fair value is determined by reference to the relevant stock exchange quoted bid price. For other investments, the fair value is estimated by management with reference to relevant available information, including the current market value of similar instruments, recent financing rounds and discounted cash flows of the underlying net assets. Net fair value movements include the impact of exchange losses of £37 million through Other comprehensive income and £nil through profit or loss (2022: gains of £134 million through Other comprehensive income and £nil through profit or loss). Other investments include listed investments of £741 million (2022: £823 million).
GSK has elected to designate the majority of its equity investments as measured at fair value through Other comprehensive income (FVTOCI). The most significant of these investments held at 31 December 2023 were in Crispr Therapeutics AG, which had a fair value at 31 December 2023 of £158 million (2022: £109 million) and Vir Biotechnology.Inc. which had a fair value at 31 December 2023 of £67 million (2022: £180 million). The fair value of the investment in Nimbus Therapeutics, LLC, disclosed as a significant investment at 31 December 2022, was £5 million at 31 December 2023 (2022: £139 million). The other investments include equity stakes in companies with which GSK has research collaborations and in companies which provide access to biotechnology developments of potential interest.
On disposal of equity investments measured at FVTOCI, the accumulated fair value movements are reclassified from the fair value reserve to retained earnings. Investments measured at FVTOCI with a fair value of £20 million (2022: £220 million) were disposed of during the year. The cumulative loss on these investments after tax was £26 million (2022: gain of £14 million).
Certain other investments, such as investments in funds with limited lives and investments acquired with an intention to sell, are measured at fair value through profit or loss (FVTPL). The most significant of these investments held at 31 December 2023 was SR One Capital Fund I-B, LP which had a fair value at 31 December 2023 of £102 million (2022: £211 million).
| | |
| 24. Other non-current assets |
| | | | | | | | |
| 2023
£m | 2022
£m |
| Amounts receivable under insurance contracts | 854 | | 857 | |
| Pension schemes in surplus | 634 | | 229 | |
| Other receivables | 96 | | 108 | |
| 1,584 | | 1,194 | |
Amounts receivable under insurance contracts are held at cash surrender value with movements through profit or loss.
Within the other receivables of £96 million (2022: £108 million), £27 million (2022: £34 million) is classified as financial assets of which £18 million (2022: £13 million) is classified as fair value through profit or loss. On the remaining balance of £9 million
(2022: £21 million), the expected credit loss allowance was immaterial at 31 December 2023 and 2022.
Other receivables include £7 million relating to carbon-based nature removal projects (2022: £2 million).
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| | | | | | | | |
| 2023
£m | 2022
£m |
| Raw materials and consumables | 1,594 | | 1,576 | |
| Work in progress | 2,449 | | 2,286 | |
| Finished goods | 1,455 | | 1,284 | |
| 5,498 | | 5,146 | |
As part of the TCFD one of the climate-related risks identified affects the metered dose inhalers (MDI). There is no impact on the recoverable value of the associated inventories held at year end.
| | |
| 26. Trade and other receivables |
| | | | | | | | |
| 2023
£m | 2022
£m |
| Trade receivables, net of loss allowance | 5,905 | | 5,452 | |
| Accrued income | 69 | | 19 | |
| Prepayments | 355 | | 343 | |
| Interest receivable | 2 | | 2 | |
| Employee loans and advances | 9 | | 11 | |
| Other receivables | 1,045 | | 1,226 | |
| 7,385 | | 7,053 | |
There were no trade or other receivable balances (2022: £nil) due from associates and joint ventures. The most significant component of other receivables comprises receivables for indirect and other taxes of £565 million (2022: £492 million). Other significant balances within other receivables are royalties receivable of £226 million (2022: £188 million) and an amount receivable from collaboration partners of £nil (2022: £263 million).
| | | | | | | | |
| Loss allowance - trade receivables | 2023
£m | 2022
£m |
| At 1 January | 91 | | 150 | |
| Exchange adjustments | (6) | | 9 | |
| Charge for the year | 11 | | 35 | |
| Transfer to assets held for sale | – | | (60) | |
| Subsequent recoveries of amounts provided for | (9) | | (19) | |
| Utilised | (2) | | (24) | |
| At 31 December | 85 | | 91 | |
Of the total trade receivables balance, £10 million (2022: £58 million) is considered credit impaired, against which a £8 million (2022: £26 million) expected credit loss allowance has been applied. No amount was purchased or originated credit impaired.
Within the other receivables of £1,045 million (2022: £1,226 million), £408 million (2022: £683 million) is classified as financial assets of which £nil (2022: £nil) is classified as held at fair value through profit or loss. At 31 December 2023, an expected credit loss allowance of £3 million (2022: £6 million) was recognised in respect of financial assets, with a release in expected credit loss allowance of £3 million (2022: £nil) reported in profit or loss during the year.
For more discussion on credit risk practices, please refer to Note 44, 'Financial instruments and related disclosures'.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 27. Cash and cash equivalents |
| | | | | | | | |
| 2023
£m | 2022
£m |
| Cash at bank and in hand | 748 | | 879 | |
| Cash equivalents | 2,188 | | 2,844 | |
| 2,936 | | 3,723 | |
Cash and cash equivalents included £190 million (2022: £200 million) not available for general use due to restrictions applying in the subsidiaries where it is held. Restrictions include exchange controls and taxes on repatriation. During 2022, £1,421 million was transferred to assets held for sale relating to Consumer Healthcare which was demerged during that year (see Note 41, 'Acquisitions and disposals').
| | | | | | | | |
| 2023
£m | 2022
£m |
| Property, plant and equipment | 60 | | 83 | |
| Other | 16 | | 15 | |
| 76 | | 98 | |
Non-current assets and disposal groups are transferred to assets held for sale when it is expected that their carrying amounts will be recovered principally through disposal and a sale is considered highly probable. They are held at the lower of carrying amount and fair value less costs to sell.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 29. Trade and other payables |
| | | | | | | | |
| 2023
£m | 2022
£m |
| Trade payables | 3,717 | | 3,866 | |
| Wages and salaries | 1,683 | | 1,488 | |
| Social security | 126 | | 126 | |
| ViiV Healthcare put option | 848 | | 1,093 | |
| Other payables | 346 | | 418 | |
| Deferred income | 222 | | 299 | |
| Customer return and rebate accruals | 6,799 | | 6,627 | |
| Other accruals | 2,103 | | 2,346 | |
| 15,844 | | 16,263 | |
Trade and other payables included £nil (2022: £nil) due to associates and joint ventures. The Group provides limited supplier financing arrangements to certain suppliers. The amounts involved at 31 December 2023 were not material.
Revenue recognised in the year that was included in deferred income at 1 January 2023 was £192 million (2022: £85 million).
Customer return and rebate accruals are provided for by the Group at the point of sale in respect of estimated rebates, discounts or allowances payable to customers. At 31 December 2023, customer return and rebate accruals included £5,781 million (2022: £5,717 million) in respect of US Commercial Operations. Accruals are made at the time of sale but the actual amounts paid are based on claims made some time after the initial recognition of the sale. As the amounts are estimated, they may not fully reflect the final outcome and are subject to change dependent upon, amongst other things, the types of buying group and product sales mix. The level of accrual is reviewed and adjusted quarterly in light of historical experience of actual amounts paid and any changes in arrangements. Future events could cause the assumptions on which the accruals are based to change, which could affect the future results of the Group.
Pfizer’s put option over its shareholding in ViiV Healthcare is currently exercisable. Pfizer may request an IPO of ViiV Healthcare at any time and if either GSK does not consent to such IPO or an offering is not completed within nine months, Pfizer could require GSK to acquire its shareholding. The amount of the liability for this put option, which is held on the gross redemption basis, is derived from an internal valuation of the ViiV Healthcare business, utilising both discounted forecast future cash flow and multiples-based methodologies.
The table below shows on an indicative basis the income statement and balance sheet sensitivity of the Pfizer put option to reasonably possible changes in key assumptions.
| | | | | | | | |
| Increase/(decrease) in financial liability and loss/(gain) in Income statement | 2023
£m | 2022
£m |
| 10% increase in sales forecasts* | 84 | | 100 | |
| 15% increase in sales forecasts* | 126 | | 149 | |
| 10% decrease in sales forecasts* | (84) | | (99) | |
| 15% decrease in sales forecast* | (126) | | (149) | |
| 1% (100 basis points) increase in discount rate | (18) | | (32) | |
| 1.50% (150 basis points) increase in discount rate | (26) | | (48) | |
| 1% (100 basis points) decrease in discount rate | 19 | | 35 | |
| 1.50% (150 basis points) decrease in discount rate | 28 | | 53 | |
| 10 cent appreciation of US Dollar | 54 | | 66 | |
| 15 cent appreciation of US Dollar | 85 | | 103 | |
| 10 cent depreciation of US Dollar | (46) | | (56) | |
| 15 cent depreciation of US Dollar | (67) | | (80) | |
| 10 cent appreciation of Euro | 22 | | 29 | |
| 15 cent appreciation of Euro | 34 | | 46 | |
| 10 cent depreciation of Euro | (18) | | (24) | |
| 15 cent depreciation of Euro | (26) | | (35) | |
* The sales forecast is for ViiV Healthcare sales only in respect of the ViiV Healthcare put option.
Other accruals includes interest accrued on financial liabilities at amortised cost of £162 million (2022: £207 million).
.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | | | | | | | | | | | | | |
| Listing exchange | | 2023
£m | 2022
£m |
| Current assets: | | | | |
| Liquid investments | | | 42 | | 67 | |
| Cash and cash equivalents | | | 2,936 | | 3,723 | |
| | | 2,978 | | 3,790 | |
| Short-term borrowings: | | | | |
| Commercial paper | | | (815) | | (1,191) | |
| Bank loans, overdrafts and other | | | (191) | | (448) | |
0.125% € Euro Medium Term Note 2023 | London Stock Exchange | | – | | (665) | |
0.000% € Euro Medium Term Note 2023 | London Stock Exchange | | – | | (443) | |
0.534% US$ Medium Term Note 2023 | New York Stock Exchange | | – | | (1,038) | |
3.000% US$ US Medium Term Note 2024 | New York Stock Exchange | | (784) | | – | |
1.375% € Euro Medium Term Note 2024 | London Stock Exchange | | (867) | | – | |
| Lease liabilities | | | (156) | | (167) | |
| | | (2,813) | | (3,952) | |
| Long-term borrowings: | | | | |
3.000% US$ US Medium Term Note 2024 | New York Stock Exchange | | – | | (829) | |
1.375% € Euro Medium Term Note 2024 | London Stock Exchange | | – | | (884) | |
4.000% € Euro Medium Term Note 2025 | London Stock Exchange | | (650) | | (663) | |
3.625% US$ US Medium Term Note 2025 | New York Stock Exchange | | (783) | | (827) | |
1.000% € Euro Medium Term Note 2026 | London Stock Exchange | | (608) | | (620) | |
1.250% € Euro Medium Term Note 2026 | London Stock Exchange | | (867) | | (885) | |
3.000% € Euro Medium Term Note 2027 | London Stock Exchange | | (434) | | (442) | |
3.375% £ Euro Medium Term Note 2027 | London Stock Exchange | | (306) | | (306) | |
3.875% US$ US Medium Term Note 2028 | New York Stock Exchange | | (1,370) | | (1,450) | |
0.883% ¥ Euro Medium Term Note 2028 | London Stock Exchange | | (235) | | – | |
1.250% £ Euro Medium Term Note 2028 | London Stock Exchange | | (745) | | (744) | |
3.375% US$ US Medium Term Note 2029 | New York Stock Exchange | | (778) | | (822) | |
1.375% € Euro Medium Term Note 2029 | London Stock Exchange | | (433) | | (441) | |
1.750% € Euro Medium Term Note 2030 | London Stock Exchange | | (650) | | (663) | |
3.125% € Euro Medium Term Note 2032 | London Stock Exchange | | (604) | | (616) | |
5.250% £ Euro Medium Term Note 2033 | London Stock Exchange | | (566) | | (640) | |
5.375% US$ US Medium Term Note 2034 | London Stock Exchange | | (390) | | (412) | |
1.625% £ Euro Medium Term Note 2035 | London Stock Exchange | | (745) | | (744) | |
6.375% US$ US Medium Note 2038 | New York Stock Exchange | | (2,139) | | (2,264) | |
6.375% £ Euro Medium Term Note 2039 | London Stock Exchange | | (627) | | (695) | |
5.250% £ Euro Medium Term Note 2042 | London Stock Exchange | | (472) | | (472) | |
4.200%US$ US Medium Term Note 2043 | New York Stock Exchange | | (385) | | (408) | |
4.250% £ Euro Medium Term Note 2045 | London Stock Exchange | | (366) | | (366) | |
| Other long-term borrowings | | | (1) | | (1) | |
| Lease liabilities | | | (1,051) | | (841) | |
| | | (15,205) | | (17,035) | |
| Net debt | | | (15,040) | | (17,197) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 30. Net debt continued | |
Current assets
Liquid investments are classified as financial assets at amortised cost. At 31 December 2023, they included US Treasury Notes and other government bonds. The effective interest rate on liquid investments at 31 December 2023 was approximately 0.9% (2022: approximately 0.1%). Liquid investment balances at 31 December 2023 earning interest at floating rates amount to £31 million (2022: £67 million). Liquid investment balances at 31 December 2023 earning interest at fixed rates amount to £11 million (2022: £nil).
Balances reported within cash and cash equivalents have an original maturity of three months or less. The effective interest rate on cash and cash equivalents at 31 December 2023 was approximately 4.7% (2022: approximately 3.1%). Cash and cash equivalents at 31 December 2023 earning interest at floating and fixed rates amounted to £2,720 million and £38 million respectively (2022: £3,441 and £10 million) and non-interest bearing holdings amounted to £178 million (2022: £272 million).
GSK’s policy regarding the credit quality of cash and cash equivalents is set out in Note 44, ‘Financial instruments and related disclosures’.
Short-term borrowings
GSK has a $10 billion (£7.8 billion) US commercial paper programme, of which $850 million (£667 million) was in issue at 31 December 2023 (2022: $900 million (£748 million)). GSK has a £5 billion Euro commercial paper programme, of which €170 million (£148 million) was in issue at 31 December 2023 (2022: €500 million (£443 million)). GSK has a £1.6 billion three-year committed facility and $2.2 billion (£1.7 billion) 364 day committed facility. The three-year committed facility was agreed in February 2022 and extended by one year in August 2023 to September 2026. The 364-day committed facility was agreed in September 2023. These facilities were undrawn at 31 December 2023.
The weighted average interest rate on commercial paper borrowings at 31 December 2023 was 5.1% (2022: 3.5%).
The weighted average interest rate on current bank loans and overdrafts at 31 December 2023 was 4.6% (2022: 7.8%).
The average effective pre-swap interest rate of notes classified as short-term at 31 December 2023 was 2.4% (2022: 0.4%).
Long-term borrowings
At the year-end, GSK had long-term borrowings of £15.2 billion (2022: £17.0 billion), of which £8.7 billion (2022: £11.1 billion) fell due in more than five years.
During 2023 through a bilateral buyback for outstanding Sterling Notes, GSK repurchased £76m of the 5.250% £ Euro Medium Term Note 2033 and £69m of the 6.375% £ Euro Medium Term Note 2039.
The average effective pre-swap interest rate of all notes in issue at 31 December 2023 was approximately 3.7% (2022: approximately 3.5%).
Long-term borrowings repayable after five years carry interest at effective rates between 1.5% and 6.6%, with repayment dates ranging from 2029 to 2045.
Both effective rates exclude the impact of one-off premiums associated with the early repayment of the Sterling Notes.
Pledged assets
The Group held pledged investments in US Treasury Notes with a par value of $54 million (£42 million), (2022: $56 million
(£47 million)) as security against irrevocable letters of credit issued on the Group’s behalf in respect of the Group’s self-insurance activity. Provisions in respect of self-insurance are included within the provisions for legal and other disputes discussed in Note 32, 'Other provisions’.
Lease liabilities
The maturity analysis of discounted lease liabilities recognised on the Group balance sheet is as follows:
| | | | | | | | |
| 2023
£m | 2022
£m |
| Rental payments due within one year | 156 | | 167 | |
| Rental payments due between one and two years | 214 | | 201 | |
| Rental payments due between two and three years | 134 | | 127 | |
| Rental payments due between three and four years | 114 | | 97 | |
| Rental payments due between four and five years | 88 | | 80 | |
| Rental payments due after five years | 501 | | 336 | |
| Total lease liabilities | 1,207 | | 1,008 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 31. Pensions and other post-employment benefits |
| | | | | | | | | | | |
| Pension and other post-employment costs | 2023
£m | 2022
£m | 2021
£m |
| UK pension schemes | 96 | | 114 | | 185 | |
| US pension schemes | 56 | | 48 | | 40 | |
| Other overseas pension schemes | 146 | | 154 | | 153 | |
| Unfunded post-retirement healthcare schemes | 58 | | 53 | | 37 | |
| 356 | | 369 | | 415 | |
| Analysed as: | | | |
| Funded defined benefit/hybrid pension schemes | 134 | | 152 | | 231 | |
| Unfunded defined benefit pension schemes | 35 | | 31 | | 23 | |
| Unfunded post-retirement healthcare schemes | 58 | | 53 | | 37 | |
| Defined benefit schemes | 227 | | 236 | | 291 | |
| Defined contribution pension schemes | 129 | | 133 | | 124 | |
| 356 | | 369 | | 415 | |
| | | |
| The costs of the defined benefit pension and post-retirement healthcare schemes are charged in the income statement as follows: |
| 2023
£m | 2022
£m | 2021
£m |
| Cost of sales | 94 | | 104 | | 106 | |
| Selling, general and administration | 91 | | 90 | | 136 | |
| Research and development | 42 | | 42 | | 49 | |
| 227 | | 236 | | 291 | |
GSK entities operate pension arrangements which cover the Group’s material obligations to provide pensions to retired employees. These arrangements have been developed in accordance with local practices in the countries concerned. Pension benefits can be provided by state schemes; by defined contribution schemes, whereby retirement benefits are determined by the value of funds arising from contributions paid in respect of each employee; or by defined benefit schemes, whereby retirement benefits are based on employee pensionable remuneration and length of service.
Pension costs of defined benefit schemes for accounting purposes have been calculated using the projected unit credit method. In certain countries pension benefits are provided on an unfunded basis, some administered by trustee companies. Formal, independent, actuarial valuations of the Group’s main plans are undertaken regularly, normally at least every three years.
Remeasurement movements in the year are recognised through the statement of comprehensive income. Discount rates are derived from AA rated corporate bond yields except in countries where there is no deep market in corporate bonds where government bond yields are used. Discount rates are selected to reflect the term of the expected benefit payments. Projected inflation rates and pension increases are long-term predictions based on the yield gap between long-term index-linked and fixed interest government bonds. In the UK, mortality rates are determined by adjusting the SAPS S3 standard mortality tables to reflect recent scheme experience. These rates are then projected to reflect improvements in life expectancy in line with the CMI 2022 projections with a long-term rate of improvement of 1.0% per year for both males and females. In the US, mortality rates are calculated using the PRI-2012 white collar table adjusted to reflect recent experience. These rates are projected using MP-2020 to allow for future improvements in life expectancy.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 31. Pensions and other post-employment benefits continued | |
The average life expectancy assumed now for an individual at the age of 60 and projected to apply in 2043 for an individual then at the age of 60 is as follows:
| | | | | | | | | | | | | | |
| UK | US |
| Male
Years | Female
Years | Male
Years | Female
Years |
| Current | 27.1 | 28.0 | 27.3 | 28.7 |
| Projected for 2043 | 28.2 | 29.2 | 28.9 | 30.2 |
The assets of funded schemes are generally held in separately administered trusts, either as specific assets or as a proportion of a general fund, or are insurance contracts. Assets are invested in different classes in order to maintain a balance between risk and return. Investments are diversified to limit the financial effect of the failure of any individual investment. The physical asset allocation strategy for three of the four UK plans is 36% in return-seeking assets and 64% in liability-matching assets. During 2019, a buy-in insurance contract was purchased to cover substantially all of the obligations of the other UK plan. At 31 December 2023, the value of the insurance contract was £387 million (2022: £402 million). The asset allocation of the US plans is currently set at 25% return-seeking assets and 75% liability-matching assets.
The pension plans are exposed to risk that arises because the estimated market value of the plans’ assets might decline, the investment returns might reduce, or the estimated value of the plans’ liabilities might increase.
In line with the agreed mix of return-seeking assets to generate future returns and liability-matching assets to better match future pension obligations, the Group has defined an overall long-term investment strategy for the plans, with investments across a broad range of assets. The main market risks within the asset and hedging portfolio are against credit risk, interest rates, long-term inflation, equities, property, currency and bank counterparty risk.
The plan liabilities are a series of future cash flows with relatively long duration. On an IAS 19 basis, these cash flows are sensitive to changes in the expected long-term inflation rate and the discount rate (AA corporate bond yield curve) where an increase in long-term inflation corresponds with an increase in the liabilities, and an increase in the discount rate corresponds with a decrease in the liabilities.
The interest rate risk and credit rate risk in the US are partially hedged. The targets are based on an accounting measure of the plan liabilities.
For the UK plans, there is an interest rate and inflation hedging strategy in place. The targets are based on an economic measure of the plan liabilities.
Climate-related impacts, along with other environmental, social and governance (ESG) considerations, can be financially material with regard both to expected returns and to risk implications. The incorporation of such considerations into investment policy is subject to local regulations and fiduciary obligations.
In the UK, the defined benefit pension schemes operated for the benefit of former Glaxo Wellcome employees and former SmithKline Beecham employees remain separate. These schemes were closed to new entrants in 2001 and subsequent UK employees are entitled to join a defined contribution scheme. In addition, the Group operates a number of post-retirement healthcare schemes, the principal one of which is in the US.
The UK defined benefit plans closed to future accrual effective from 31 March 2022. As a result, post closure the accrued benefits of active participants are revalued in line with inflation (RPI for the legacy Glaxo Wellcome plans and CPI for the legacy SmithKline Beecham plans subject to the relevant caps for each arrangement) rather than capped pay increases. From 1 April 2022, former defined benefits plans employees were transferred to the defined contribution plans. All defined benefit plan participants who were still active at 1 April 2022 received a defined pension contribution of £10,000 each in 2022.
The US cash balance pension plan closed to future accrual from 1 January 2021.
The Group has applied the following financial assumptions in assessing the defined benefit liabilities:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | UK | | | US | Rest of World |
| 2023
% pa | 2022
% pa | 2021
% pa | 2023
% pa | 2022
% pa | 2021
% pa | 2023
% pa | 2022
% pa | 2021
% pa |
| Rate of increase of future earnings | n/a | n/a | 2.00 | n/a | n/a | n/a | 3.20 | 3.40 | 2.90 |
| Discount rate | 4.60 | 4.80 | 2.00 | 5.00 | 5.30 | 2.70 | 3.10 | 3.40 | 1.10 |
| Expected pension increases | 2.90 | 3.10 | 3.20 | n/a | n/a | n/a | 2.50 | 2.40 | 2.30 |
| Cash balance credit/conversion rate | n/a | n/a | n/a | 4.00 | 3.90 | 2.00 | 0.60 | 0.80 | 0.20 |
| Inflation rate | 2.90 | 3.10 | 3.20 | 2.50 | 2.50 | 2.25 | 2.00 | 2.30 | 1.90 |
Sensitivity analysis detailing the effect of changes in assumptions is provided on page 205. The analysis provided reflects the assumption changes which have the most material impact on the results of the Group.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 31. Pensions and other post-employment benefits continued | |
The amounts recorded in the income statement and statement of comprehensive income for the three years ended 31 December 2023 in relation to the defined benefit pension and post-retirement healthcare schemes were as follows:
| | | | | | | | | | | | | | | | | |
| | | |
Pensions
| Post-retirement
benefits |
2023 | UK
£m | US
£m | Rest of World
£m | Group
£m | Group
£m |
| Amounts charged to operating profit | | | | | |
| Current service cost | – | | 5 | | 91 | | 96 | | 12 | |
| Past service cost | 3 | | – | | – | | 3 | | – | |
| Net interest cost | (5) | | 35 | | 16 | | 46 | | 47 | |
| Gains from settlements | – | | – | | (6) | | (6) | | – | |
| Expenses | 14 | | 16 | | – | | 30 | | (1) | |
| 12 | | 56 | | 101 | | 169 | | 58 | |
| | | | | |
Remeasurement gains/(losses) recorded in the statement of comprehensive income | 28 | | 45 | | 38 | | 111 | | (40) | |
| | | | | | | | | | | | | | | | | |
| | | |
Pensions
| Post-retirement
benefits |
2022 | UK
£m | US
£m | Rest of World
£m | Group
£m | Group
£m |
| Amounts charged to operating profit | | | | | |
| Current service cost | 13 | | 7 | | 126 | | 146 | | 22 | |
| Past service cost/(credit) | 6 | | – | | – | | 6 | | – | |
| Net interest (income)/cost | (11) | | 20 | | 9 | | 18 | | 32 | |
| Gains from settlements | – | | – | | (22) | | (22) | | – | |
| Expenses | 14 | | 21 | | – | | 35 | | (1) | |
| 22 | | 48 | | 113 | | 183 | | 53 | |
| | | | | |
Remeasurement gains/(losses) recorded in the statement of comprehensive income | (1,169) | | 36 | | 261 | | (872) | | 228 | |
| | | | | | | | | | | | | | | | | |
| | | |
Pensions
| Post-retirement
benefits |
2021 | UK
£m | US
£m | Rest of World
£m | Group
£m | Group
£m |
| Amounts charged to operating profit | | | | | |
| Current service cost | 53 | | 9 | | 119 | | 181 | | 17 | |
| Past service cost/(credit) | 27 | | 2 | | (10) | | 19 | | (3) | |
| Net interest (income)/cost | 3 | | 18 | | 7 | | 28 | | 22 | |
| Gains from settlements | – | | – | | (2) | | (2) | | – | |
| Expenses | 15 | | 12 | | 2 | | 29 | | – | |
| 98 | | 41 | | 116 | | 255 | | 36 | |
| | | | | |
Remeasurement gains/(losses) recorded in the statement of comprehensive income1 | 572 | | 98 | | 186 | | 856 | | 68 | |
The amounts included within past service costs in the UK included £3 million (2022: £6 million; 2021: £26 million) of augmentation costs which arose from Major restructuring programmes.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 31. Pensions and other post-employment benefits continued | |
A summarised balance sheet presentation of the Group defined benefit pension schemes and other post-retirement benefits is set out in the table below:
| | | | | | | | | | | |
| 2023
£m | 2022
£m | 2021
£m |
| Recognised in other non-current assets: | | | |
| Pension schemes in surplus | 634 | | 229 | | 741 | |
| Recognised in pensions and other post-employment benefits: | | | |
| Pension schemes in deficit | (1,397) | | (1,585) | | (1,870) | |
| Post-retirement benefits | (943) | | (994) | | (1,243) | |
| (2,340) | | (2,579) | | (3,113) | |
In the event of a plan wind-up, GSK believes the UK pension scheme rules provide the company with the right to a refund of surplus assets following the full settlement of plan liabilities. As a result, the net surplus in the UK defined benefit pension schemes is recognised in full.
The fair values of the assets and liabilities of the UK and US defined benefit pension schemes, together with aggregated data for other defined benefit pension schemes in the Group are as follows:
| | | | | | | | | | | | | | | | | |
At 31 December 2023 | | UK
£m | US
£m | Rest of World
£m | Group
£m |
| Equities: | – listed | 1,647 | | 447 | | 349 | | 2,443 | |
| – unlisted | – | | – | | 2 | | 2 | |
| Multi-asset funds | | 852 | | – | | – | | 852 | |
| Property: | – listed | – | | – | | – | | – | |
| – unlisted | 467 | | 119 | | 24 | | 610 | |
Corporate bonds: | – listed | 2,019 | | 698 | | 205 | | 2,922 | |
| – unlisted | – | | – | | 15 | | 15 | |
| Government bonds: | – listed | 4,897 | | 774 | | 527 | | 6,198 | |
| Insurance contracts | | 990 | | – | | 771 | | 1,761 | |
| Other (liabilities)/assets | | (1,374) | | 104 | | 89 | | (1,181) | |
| Fair value of assets | | 9,498 | | 2,142 | | 1,982 | | 13,622 | |
| Present value of scheme obligations | (9,222) | | (2,757) | | (2,406) | | (14,385) | |
| Net surplus/(obligation) | 276 | | (615) | | (424) | | (763) | |
| Included in other non-current assets | 457 | | – | | 177 | | 634 | |
| Included in pensions and other post-employment benefits | (181) | | (615) | | (601) | | (1,397) | |
| | 276 | | (615) | | (424) | | (763) | |
| Actual return on plan assets | | 647 | | 196 | | 138 | | 981 | |
The multi-asset funds comprise investments in pooled investment vehicles that are invested across a range of asset classes, increasing diversification within the growth portfolio. The value of funds in this asset class with a quoted market price is £209 million (2022: £211 million).
The ‘Other (liabilities)/assets’ category comprises cash and mark to market values of derivative positions.
Index-linked gilts held as part of a UK repo programme are included in government bonds. The related loan of £1,853 million at 31 December 2023 (2022: £2,376 million; 2021: £513 million) is deducted within ‘Other assets’.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 31. Pensions and other post-employment benefits continued | |
| | | | | | | | | | | | | | | | | |
At 31 December 2022 | | UK
£m | US
£m | Rest of World
£m | Group
£m |
| Equities: | – listed | 1,351 | | 437 | | 371 | | 2,159 | |
| – unlisted | – | | – | | 2 | | 2 | |
| Multi-asset funds | | 1,101 | | – | | – | | 1,101 | |
| Property: | – listed | – | | – | | 19 | | 19 | |
| – unlisted | 464 | | 140 | | 1 | | 605 | |
Corporate bonds: | – listed | 1,692 | | 779 | | 124 | | 2,595 | |
| – unlisted | – | | – | | 15 | | 15 | |
| Government bonds: | – listed | 4,048 | | 723 | | 558 | | 5,329 | |
| Insurance contracts | | 1,003 | | – | | 691 | | 1,694 | |
| Other (liabilities)/assets | | (645) | | 181 | | 89 | | (375) | |
| Fair value of assets | 9,014 | | 2,260 | | 1,870 | | 13,144 | |
| Present value of scheme obligations | (9,117) | | (3,030) | | (2,353) | | (14,500) | |
| Net surplus/(obligation) | (103) | | (770) | | (483) | | (1,356) | |
| | — | | — | | — | | — | |
| Included in other non-current assets | 109 | | – | | 120 | | 229 | |
| Included in pensions and other post-employment benefits | (212) | | (770) | | (603) | | (1,585) | |
| | (103) | | (770) | | (483) | | (1,356) | |
| | | | | |
| Actual return on plan assets | (4,710) | | (253) | | (550) | | (5,513) | |
| | | | | | | | | | | | | | | | | |
At 31 December 2021 | | UK
£m | US
£m | Rest of World
£m | Group
£m |
| Equities: | – listed | 3,954 | | 522 | | 731 | | 5,207 | |
| – unlisted | – | | – | | 4 | | 4 | |
| Multi-asset funds | | 1,415 | | – | | – | | 1,415 | |
| Property: | – listed | – | | – | | 68 | | 68 | |
| – unlisted | 502 | | 154 | | 1 | | 657 | |
Corporate bonds: | – listed | 1,503 | | 975 | | 140 | | 2,618 | |
| – unlisted | – | | – | | 15 | | 15 | |
| Government bonds: | – listed | 5,054 | | 724 | | 984 | | 6,762 | |
| Insurance contracts | | 1,334 | | – | | 917 | | 2,251 | |
| Other (liabilities)/assets | | (130) | | 149 | | 72 | | 91 | |
| Fair value of assets | 13,632 | | 2,524 | | 2,932 | | 19,088 | |
| Asset ceiling restrictions | | – | | – | | (26) | | (26) | |
| Present value of scheme obligations | (13,299) | | (3,248) | | (3,644) | | (20,191) | |
| Net surplus/(obligation) | 333 | | (724) | | (738) | | (1,129) | |
| | | | | |
| Included in Other non-current assets | 606 | | – | | 135 | | 741 | |
| Included in Pensions and other post-employment benefits | (273) | | (724) | | (873) | | (1,870) | |
| | 333 | | (724) | | (738) | | (1,129) | |
| | | | | |
| Actual return on plan assets | 541 | | 97 | | 48 | | 686 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 31. Pensions and other post-employment benefits continued | |
| | | | | | | | | | | | | | | | | |
| | | |
Pensions
| Post-retirement
benefits |
| Movements in fair values of assets | UK
£m | US
£m | Rest of World
£m | Group
£m | Group £m |
Assets at 1 January 2021 | 13,582 | | 2,635 | | 2,989 | | 19,206 | | – | |
| Exchange adjustments | – | | 31 | | (184) | | (153) | | – | |
| Interest income | 187 | | 57 | | 18 | | 262 | | – | |
| Expenses | (15) | | (12) | | – | | (27) | | – | |
| Settlements and curtailments | – | | – | | (7) | | (7) | | – | |
| Remeasurement | 354 | | 40 | | 30 | | 424 | | – | |
| Employer contributions | 139 | | 40 | | 133 | | 312 | | 105 | |
| Scheme participants’ contributions | 3 | | – | | 24 | | 27 | | 15 | |
| Benefits paid | (618) | | (267) | | (97) | | (982) | | (120) | |
Assets at 31 December 2021 | 13,632 | | 2,524 | | 2,906 | | 19,062 | | – | |
| Exchange adjustments | – | | 286 | | 122 | | 408 | | – | |
| Interest income | 271 | | 71 | | 28 | | 370 | | – | |
| Expenses | (14) | | (21) | | – | | (35) | | – | |
| Settlements and curtailments | – | | – | | (8) | | (8) | | – | |
| Remeasurement | (4,981) | | (324) | | (578) | | (5,883) | | – | |
| Employer contributions | 755 | | 50 | | 114 | | 919 | | 117 | |
| Scheme participants’ contributions | – | | – | | 15 | | 15 | | 18 | |
| Transfer to assets held for sale/distribution | – | | – | | (624) | | (624) | | – | |
| Benefits paid | (649) | | (326) | | (105) | | (1,080) | | (135) | |
Assets at 31 December 2022 | 9,014 | | 2,260 | | 1,870 | | 13,144 | | – | |
| Exchange adjustments | — | | (125) | | (84) | | (209) | | – | |
| Interest income | 430 | | 111 | | 60 | | 601 | | – | |
| Expenses | (14) | | (16) | | — | | (30) | | – | |
| Settlements and curtailments | – | | – | | 2 | | 2 | | – | |
| Remeasurement | 217 | | 85 | | 78 | | 380 | | – | |
| Employer contributions | 363 | | 125 | | 118 | | 606 | | 98 | |
| Scheme participants’ contributions | – | | – | | 11 | | 11 | | 18 | |
| Transfer to assets held for sale/distribution | – | | – | | – | | – | | – | |
| Benefits paid | (512) | | (298) | | (73) | | (883) | | (116) | |
Assets at 31 December 2023 | 9,498 | | 2,142 | | 1,982 | | 13,622 | | – | |
The final instalment of the cash funding or technical provision deficits of £1,080 million identified in the 31 December 2020 pension scheme valuations in three GSK UK Pension Schemes was paid in 2023.
During March 2022, GSK transferred 7,004 GSK Consumer Healthcare Holdings Limited (GSKCHH) C Ordinary Shares (representing 11.03%. (in aggregate) of GSK’s interest in GSKCHH to three Scottish Limited Partnerships (“SLPs”), each providing a funding mechanism for a separate GSK UK defined benefit pension scheme. As part of the steps relating to the demerger and separation, the SLPs transferred their applicable portion of GSKCHH C Ordinary Shares to Haleon plc (“Haleon”) in consideration for shares in Haleon. At the time of demerger the SLPs together held shares representing 7.5% of the total issued share capital of Haleon. The contributions were collateralised by the creation of three Scottish Limited Partnerships (SLPs). Each of the three principal UK defined benefit pension schemes (two benefiting current and former Glaxo Welcome employees, with the third benefiting current and former SmithKline Beecham employees) had an interest in one of the SLPs at the time of demerger.
| | | | | | | | | | | |
| Scottish Limited Partnership | General Partner | Limited Partners | |
| GSK (No. 1) Scottish Limited Partnership | GSK GP1 Ltd | GSK LP Ltd | Berkeley Square Pension Trustee Company Ltd acting on behalf of the GSK Pension Scheme (ceased to be a Limited Partner effective from 28 June 2023) |
| GSK (No. 2) Scottish Limited Partnership | GSK GP1 Ltd | GSK LP Ltd | Berkeley Square Pension Trustee Company Ltd acting on behalf of the GSK Pension Fund (ceased to be a Limited Partner effective from 28 June 2023) |
| GSK (No. 3) Scottish Limited Partnership | GSK GP2 Ltd | GSK LP Ltd | SmithKline Beecham Pension Plan Trustee Ltd acting on behalf of the SmithKline Beecham Pension Plan (ceased to be a Limited Partner effective from 28 June 2023) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 31. Pensions and other post-employment benefits continued | |
Each pension scheme, through its SLP interest received a distribution from that SLP with regards to the net proceeds of sales of Haleon shares and dividend income on the Haleon shares up to the amount equal to the agreed threshold (“Proceeds Threshold”). of £1,080 million increased by £7 million notional interest. Payment of this amount fully funded the cash funding or “technical provisions” deficits in the three pension schemes shown by the 31 December 2020 valuations. As at 31 December 2023, total cash contributions totalling £353 million (2022: £691 million; 2021: £44 million) were made towards the Proceeds Threshold leaving no further outstanding amount due to the UK pension schemes. The cash contributions included £17 million of distributions of dividends on Haleon shares from the SLPs to the Schemes.
The GSK UK Pension Schemes exited the SLP partnership on 28 June 2023 after receipt of the Proceeds Threshold and notional interest. The remaining economic interest in the SLPs is held by GSK LP Ltd, a 100% owned subsidiary of GSK plc and the GSK-controlled General Partner of each SLP.
Employer contributions for 2024, are estimated to be approximately £350 million in respect of defined benefit pension schemes and £80 million in respect of other post-retirement benefits.
| | | | | | | | | | | | | | | | | |
| | | |
Pensions
| Post-retirement
benefits |
| Movements in defined benefit obligations | UK £m | US
£m | Rest of World
£m | Group £m | Group £m |
Obligations at 1 January 2021 | (13,858) | | (3,445) | | (4,007) | | (21,310) | | (1,363) | |
| Exchange adjustments | – | | (40) | | 258 | | 218 | | 4 | |
| Service cost | (56) | | (9) | | (151) | | (216) | | (29) | |
| Past service cost | (28) | | (2) | | 25 | | (5) | | (12) | |
| Interest cost | (190) | | (76) | | (23) | | (289) | | (26) | |
| Settlements and curtailments | – | | – | | 17 | | 17 | | – | |
| Remeasurement | 218 | | 57 | | 164 | | 439 | | 78 | |
| Scheme participants’ contributions | (3) | | – | | (24) | | (27) | | (15) | |
| Benefits paid | 618 | | 267 | | 97 | | 982 | | 120 | |
Obligations at31 December 2021 | (13,299) | | (3,248) | | (3,644) | | (20,191) | | (1,243) | |
| Exchange adjustments | – | | (371) | | (124) | | (495) | | (125) | |
| Service cost | (13) | | (7) | | (126) | | (146) | | (22) | |
| Past service cost | (6) | | – | | – | | (6) | | – | |
| Interest cost | (260) | | (91) | | (37) | | (388) | | (32) | |
| Settlements and curtailments | – | | – | | 29 | | 29 | | – | |
| Remeasurement | 3,812 | | 360 | | 839 | | 5,011 | | 228 | |
| Scheme participants’ contributions | – | | – | | (15) | | (15) | | (18) | |
| Transfer to assets held for sale/distribution | – | | – | | 621 | | 621 | | 83 | |
| Benefits paid | 649 | | 326 | | 105 | | 1,080 | | 135 | |
Obligations at 31 December 2022 | (9,117) | | (3,031) | | (2,352) | | (14,500) | | (994) | |
| Exchange adjustments | – | | 166 | | 87 | | 253 | | 53 | |
| Service cost | – | | (5) | | (91) | | (96) | | (13) | |
| Past service cost | (3) | | – | | – | | (3) | | – | |
| Interest cost | (425) | | (145) | | (76) | | (646) | | (47) | |
| Settlements and curtailments | – | | – | | 4 | | 4 | | – | |
| Remeasurement | (189) | | (40) | | (40) | | (269) | | (40) | |
| Scheme participants’ contributions | – | | – | | (11) | | (11) | | (18) | |
| Transfer to assets held for sale/distribution | – | | – | | – | | – | | – | |
| Benefits paid | 512 | | 298 | | 73 | | 883 | | 116 | |
Obligations at 31 December 2023 | (9,222) | | (2,757) | | (2,406) | | (14,385) | | (943) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 31. Pensions and other post-employment benefits continued | |
The defined benefit pension obligation is analysed as follows:
| | | | | | | | | | | |
| 2023
£m | 2022 £m | 2021
£m |
| Funded | (13,782) | | (13,887) | | (19,419) | |
| Unfunded | (603) | | (613) | | (772) | |
| (14,385) | | (14,500) | | (20,191) | |
The liability for the US post-retirement healthcare scheme has been assessed using the same assumptions as for the US pension scheme, together with the assumption for future medical inflation of 6.75% (2022: 7%) in 2023, grading down to 5% in 2031 and thereafter. At 31 December 2023, the US post-retirement healthcare scheme obligation was £785 million (2022: £870 million; 2021: £1,059 million). Post-retirement benefits are unfunded.
The movement in the net defined benefit liability is as follows:
| | | | | | | | | | | |
| 2023
£m | 2022
£m | 2021
£m |
| At 1 January | (1,356) | | (1,129) | | (2,104) | |
| Exchange adjustments | 44 | | (87) | | 65 | |
| Service cost | (96) | | (146) | | (216) | |
| Past service cost | (3) | | (6) | | (5) | |
| Interest cost | (45) | | (18) | | (27) | |
| Settlements and curtailments | 6 | | 21 | | 10 | |
| Remeasurements: | | | |
| Return on plan assets, excluding amounts included in interest | 380 | | (5,883) | | 424 | |
| (Loss)/gain from change in demographic assumptions | 135 | | 92 | | (62) | |
| Gain/(loss) from change in financial assumptions | (137) | | 5,868 | | 716 | |
| Experience (loss)/gain | (267) | | (949) | | (215) | |
| Employer contributions | 606 | | 919 | | 312 | |
| Transfer to assets held for sale/distribution | – | | (3) | | – | |
| Expenses | (30) | | (35) | | (27) | |
| At 31 December | (763) | | (1,356) | | (1,129) | |
The remeasurements included within post-retirement benefits are detailed below:
| | | | | | | | | | | |
| 2023
£m | 2022 £m | 2021
£m |
| Gain from change in demographic assumptions | 7 | | 21 | | 19 | |
| Gain/(loss) from change in financial assumptions | (43) | | 219 | | 35 | |
| Experience gains | (4) | | (12) | | 24 | |
| (40) | | 228 | | 78 | |
The defined benefit pension obligation analysed by membership category is as follows:
| | | | | | | | | | | |
| 2023
£m | 2022
£m | 2021 £m |
| Active | 1,508 | | 1,390 | | 4,196 | |
| Retired | 8,730 | | 8,540 | | 11,115 | |
| Deferred | 4,147 | | 4,570 | | 4,880 | |
| 14,385 | | 14,500 | | 20,191 | |
The post-retirement benefit obligation analysed by membership category is as follows:
| | | | | | | | | | | |
| 2023
£m | 2022 £m | 2021 £m |
| Active | 277 | | 306 | | 494 | |
| Retired | 666 | | 688 | | 748 | |
| Deferred | – | | – | | 1 | |
| 943 | | 994 | | 1,243 | |
The weighted average duration of the defined benefit obligation is as follows:
| | | | | | | | | | | |
| 2023
years | 2022 years | 2021
years |
| Pension benefits | 11 | 12 | 15 |
| Post-retirement benefits | 10 | 10 | 12 |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 31. Pensions and other post-employment benefits continued | |
Sensitivity analysis
The effect of changes in assumptions used on the benefit obligations and on the 2024 annual defined benefit pension and post-retirement costs are detailed below. This information has been determined by taking into account the duration of the liabilities and the overall profile of the plan memberships.
| | | | | | | | | | | |
| 0.25%
increase
£m | | 0.25%
decrease
£m |
| Discount rate | | | |
| (Decrease)/increase in annual pension cost | (18) | | | 18 | |
| Increase/(decrease) in annual post-retirement benefits cost | 1 | | | (1) | |
| (Decrease)/increase in pension obligation | (373) | | | 391 | |
| (Decrease)/increase in post-retirement benefits obligation | (20) | | | 22 | |
| 0.75%
increase
£m | | 0.75%
decrease
£m |
| (Decrease)/increase in annual pension cost | (58) | | | 51 | |
| Increase/(decrease) in annual post-retirement benefits cost | 3 | | | (3) | |
| (Decrease)/increase in pension obligation | (1,071) | | | 1,231 | |
| (Decrease)/increase in post-retirement benefits obligation | (57) | | | 66 | |
| | | |
| 0.25%
increase
£m | | 0.25%
decrease
£m |
| Inflation rate | | | |
| Increase/(decrease) in annual pension cost | 16 | | | (14) | |
| Increase/(decrease) in pension obligation | 289 | | | (280) | |
| 0.75%
increase
£m | | 0.75%
decrease
£m |
| Increase/(decrease) in annual pension cost | 46 | | | (40) | |
| Increase/(decrease) in pension obligation | 897 | | | (803) | |
| | | |
| 1 year
increase
£m | | |
| Life expectancy | | | |
| Increase in annual pension cost | 20 | | | |
| Increase in annual post-retirement benefits cost | 2 | | | |
| Increase in pension obligation | 432 | | | |
| Increase in post-retirement benefits obligation | 32 | | | |
| | | |
| 1%
increase
£m | | |
| Rate of future healthcare inflation | | | |
| Increase in annual post-retirement benefits cost | 2 | | | |
| Increase in post-retirement benefits obligation | 34 | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | | | | | | | | | | | | | | | | |
| Legal
and other
disputes
£m | Major
restructuring
programmes
£m | Employee
related
provisions
£m | Other
provisions
£m | Total
£m |
At 1 January 2023 | 218 | | 351 | | 309 | | 306 | | 1,184 | |
| Exchange adjustments | (21) | | (13) | | (9) | | (5) | | (48) | |
| Charge for the year | 266 | | 172 | | 177 | | 124 | | 739 | |
| Reversed unused | (4) | | (55) | | (33) | | (143) | | (235) | |
| Unwinding of discount | 10 | | – | | – | | – | | 10 | |
| Utilised | (202) | | (169) | | (62) | | (88) | | (521) | |
| Transfer to assets held for sale/distribution | – | | – | | – | | – | | – | |
| Additions through business combinations | – | | – | | – | | – | | – | |
| Reclassifications and other movements | – | | (1) | | 1 | | 113 | | 113 | |
| Transfer to Pension obligations | – | | (3) | | – | | – | | (3) | |
At 31 December 2023 | 267 | | 282 | | 383 | | 307 | | 1,239 | |
| | | | | |
| To be settled within one year | 248 | | 215 | | 172 | | 109 | | 744 | |
| To be settled after one year | 19 | | 67 | | 211 | | 198 | | 495 | |
At 31 December 2023 | 267 | | 282 | | 383 | | 307 | | 1,239 | |
Legal and other disputes
The Group is involved in a substantial number of legal and other disputes, including notification of possible claims, as set out in Note 47, ‘Legal proceedings’. Provisions for legal and other disputes include amounts relating to product liability, anti-trust, government investigations, contract terminations and self insurance.
The Group may become involved in significant legal proceedings in respect of which it is not possible to meaningfully assess whether the outcome will result in a probable outflow, or to quantify or reliably estimate the liability, if any, that could result from ultimate resolution of the proceedings. In these cases, the Group would provide appropriate disclosures about such cases, but no provision would be made.
The net charge for the year of £262 million (including reversals and estimated insurance recoveries) primarily related to provisions for product liability cases, commercial disputes and various other government investigations.
The discount on the provision is £10 million in 2023 (2022:£3 million). The discount was calculated using risk-adjusted projected cash flows and risk-free rates of return.
In respect of product liability claims related to certain products, provision is made when there is sufficient history of claims made and settlements to enable management to make a reliable estimate of the provision required to cover unasserted claims, and to determine the probability of the outflow of cash. The ultimate liability for such matters may vary from the amounts provided and is dependent upon the outcome of litigation proceedings, investigations and possible settlement negotiations.
The Group’s position could change over time, and, therefore, there can be no assurance that any losses that result from the outcome of any legal proceedings will not exceed by a material amount the amount of the provisions reported in the Group’s financial accounts.
It is in the nature of the Group’s business that a number of these matters may be the subject of negotiation and litigation over many years. Litigation proceedings, including the various appeal procedures, often take many years to reach resolution, and out-of-court settlement discussions can also often be protracted. Indemnified disputes will result in a provision charge and a corresponding receivable.
The Group is in potential settlement discussions in a number of the disputes for which amounts have been provided and, based on its current assessment of the progress of these disputes, estimates that £248 million of the amount provided at 31 December 2023 will be settled within one year. For a discussion of legal issues, see Note 47, ‘Legal proceedings’.
Major restructuring programmes
During 2023, the Group had two major restructuring programmes: the Separation Preparation programme which focused on preparing for the separation of GSK into two companies and is now largely complete, plus the Significant Acquisitions programme which is focused on the integration of recent acquisitions.
Restructuring provisions primarily include severance costs when management has made a formal decision to eliminate certain positions and this has been communicated to the groups of employees affected and appropriate consultation procedures completed, where appropriate. No provision is made for staff severance payments that are paid immediately.
The discount on the provisions increased by £0.4 million in 2023 (2022: increased by £1 million).
Pension augmentation includes £3 million relating to the defined benefit plan arising from staff redundancies, as shown in Note 31, ‘Pensions and other post-employment benefits’.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 32. Other provisions continued | |
Employee related provisions
Employee related provisions include obligations for certain medical benefits to disabled employees and their spouses in
the US.
At 31 December 2023, the provision for these benefits amounted to £48 million (2022: £66 million). Other employee benefits reflect a variety of provisions for severance costs, jubilee awards and other long-service benefits.
Given the nature of these provisions, the amounts are likely to be settled over many years.
Other provisions
Included in other provisions are provisions for onerous contracts, insurance provisions and a number of other provisions including vehicle insurance and regulatory matters.
| | |
| 33. Contingent consideration liabilities |
The consideration for certain acquisitions includes amounts contingent on future events such as development milestones or sales performance. The Group has provided for the fair value of this contingent consideration as follows:
| | | | | | | | | | | | | | | | | |
| Shionogi-ViiV
Healthcare
£m | Affinivax
£m | Novartis
Vaccines
£m | Other
£m | Total
£m |
At 1 January 2021 | 5,359 | | – | | 477 | | 33 | | 5,869 | |
| Remeasurement through income statement | 1,026 | | – | | 32 | | 5 | | 1,063 | |
| Cash payments: operating cash flows | (721) | | – | | (21) | | – | | (742) | |
| Cash payments: investing activities | (105) | | – | | (9) | | – | | (114) | |
At 31 December 2021 | 5,559 | | – | | 479 | | 38 | | 6,076 | |
| Remeasurement through income statement | 1,431 | | 17 | | 231 | | (34) | | 1,645 | |
| Exchange movement through reserves | – | | 2 | | – | | – | | 2 | |
| Initial recognition from business combinations | – | | 482 | | – | | – | | 482 | |
| Cash payments: operating cash flows | (1,031) | | – | | (27) | | – | | (1,058) | |
| Cash payments: investing activities | (69) | | – | | (10) | | – | | (79) | |
At 31 December 2022 | 5,890 | | 501 | | 673 | | 4 | | 7,068 | |
| Remeasurement through income statement | 934 | | 44 | | (210) | | – | | 768 | |
| Exchange movement through reserves | – | | (29) | | – | | – | | (29) | |
| Cash payments: operating cash flows | (1,106) | | – | | (28) | | – | | (1,134) | |
| Cash payments: investing activities | – | | – | | (11) | | – | | (11) | |
At 31 December 2023 | 5,718 | | 516 | | 424 | | 4 | | 6,662 | |
Of the contingent consideration payable at 31 December 2023, £1,053 million (2022: £1,289 million) is expected to be paid within one year.
The consideration payable for the acquisition of the Shionogi-ViiV Healthcare joint venture, Affinivax and the Novartis Vaccines business are expected to be paid over a number of years. As a result, the total estimated liabilities are discounted to their present values, shown above. The Shionogi-ViiV Healthcare contingent consideration liability is discounted at 8% (2022: 8%), the Affinivax contingent consideration liability is discounted at 8.5% (2022: 9.9%) and the Novartis Vaccines contingent consideration liability is discounted at 7.5% (2022: 7.5%) for commercialised products and at 8.5% (2022: 8.5%) for pipeline assets.
The Shionogi-ViiV Healthcare and Novartis Vaccines contingent consideration liabilities are calculated principally based on the forecast sales performance of specified products over the lives of those products.
The Affinivax contingent consideration is based upon two potential milestone payments, each of $0.6 billion (£0.5 billion) which will be paid if certain paediatric clinical development milestones are achieved.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 33. Contingent consideration liabilities continued | |
The table below shows on an indicative basis the income statement and balance sheet sensitivity to reasonably possible changes
in key inputs to the valuations of the contingent consideration liabilities.
| | | | | | | | | | | | | | | | | | | | |
| | 2023 | 2022 |
| Increase/(decrease) in financial liability and loss/(gain) in income statement | Shionogi-ViiV Healthcare
£m | Affinivax
£m | Novartis
Vaccines
£m | Shionogi-ViiV
Healthcare
£m | Affinivax
£m | Novartis
Vaccines
£m |
| 10% increase in sales forecasts* | 539 | | N/A | 63 | | 556 | | N/A | 103 | |
| 15% increase in sales forecasts* | 807 | | N/A | 94 | | 834 | | N/A | 154 | |
| 10% decrease in sales forecasts* | (539) | | N/A | (62) | | (555) | | N/A | (103) | |
| 15% decrease in sales forecasts* | (808) | | N/A | (92) | | (833) | | N/A | (153) | |
| 1% increase in discount rate | (174) | | (12) | | (26) | | (199) | | (7) | | (55) | |
| 1.5% increase in discount rate | (256) | | (18) | | (38) | | (292) | | (10) | | (80) | |
| 1% decrease in discount rate | 184 | | 13 | | 30 | | 214 | | 7 | | 65 | |
| 1.5% decrease in discount rate | 281 | | 19 | | 47 | | 328 | | 11 | | 101 | |
| 10 cent appreciation of US Dollar | 386 | | 44 | | 11 | | 411 | | 45 | | 22 | |
| 15 cent appreciation of US Dollar | 604 | | 69 | | 17 | | 645 | | 71 | | 36 | |
| 10 cent depreciation of US Dollar | (330) | | (38) | | (8) | | (347) | | (38) | | (19) | |
| 15 cent depreciation of US Dollar | (478) | | (54) | | (12) | | (501) | | (56) | | (27) | |
| 10 cent appreciation of Euro | 91 | | N/A | 19 | | 109 | | N/A | 23 | |
| 15 cent appreciation of Euro | 144 | | N/A | 30 | | 171 | | N/A | 36 | |
| 10 cent depreciation of Euro | (79) | | N/A | (16) | | (91) | | N/A | (19) | |
| 15 cent depreciation of Euro | (113) | | N/A | (22) | | (130) | | N/A | (28) | |
| 10% increase in probability of milestone success | n/a | 75 | | 21 | | N/A | 82 | | 20 | |
| 10% decrease in probability of milestone success | n/a | (75) | | (10) | | N/A | (82) | | (10) | |
* The sales forecast is for ViiV Healthcare sales only in respect of the Shionogi-ViiV Healthcare contingent consideration.
| | |
| 34. Other non-current liabilities |
| | | | | | | | |
| 2023 £m | 2022
£m |
| Accruals | 4 | | 11 | |
| Deferred income | 254 | | 83 | |
| Other payables | 849 | | 805 | |
| 1,107 | | 899 | |
Other payables includes a number of employee-related liabilities including employee savings plans.
| | |
| 35. Contingent liabilities |
At 31 December 2023, contingent liabilities where GSK has a present obligation as a result of a past event, comprising guarantees and other items arising in the normal course of business, amounted to £32 million (2022: £58 million). At 31 December 2023, £0.2 million (2022: £0.5 million) of financial assets were pledged as collateral for contingent liabilities. Provision is made for the outcome of tax, legal and other disputes where it is both probable that the Group will suffer an outflow of funds and it is possible to make a reliable estimate of that outflow. If it is not possible to meaningfully assess whether the outcomes will result in a probable outflow, or to quantify or reliably estimate the liability, if any, no provision is recorded. Descriptions of the significant legal and other disputes to which the Group is a party are set out in Note 47, ‘Legal proceedings’.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | | | | | | | |
| Contractual obligations and commitments | 2023
£m | 2022
£m |
| Contracted for but not provided in the financial statements: | | |
| Intangible assets | 16,329 | | 10,659 | |
| Property, plant and equipment | 762 | | 743 | |
| Investments | 153 | | 138 | |
| Purchase commitments | 31 | | 161 | |
| Pensions and post-retirement benefits | – | | 345 | |
| Interest on loans | 5,446 | | 6,322 | |
| Future finance charges on leases | 254 | | 146 | |
| Lease contracts that have not yet commenced | 5 | | 395 | |
| 22,980 | | 18,909 | |
The commitments related to intangible assets include milestone payments, which are dependent on successful clinical development or on meeting specified sales targets, and which represent the maximum that would be paid if all milestones, however unlikely, are achieved. The amounts disclosed are not risk-adjusted or discounted. The increase in intangible asset commitments in 2023 is mainly attributable to new R&D collaborations including collaborations with Wave Life Sciences USA, Inc. and Shanghai Hansoh Biomedical Co. Ltd.
In 2022, GSK reached an agreement with the trustees of the UK pension schemes to make additional contributions of £1,080 million, to eliminate the pension deficit identified at the 31 December 2020 actuarial funding valuation. Prior to the Consumer Healthcare demerger, GSK agreed to collateralise this commitment and accelerate funding with additional contributions (Refer to Note 31 'Pensions and other post-employment benefits'). At 31 December 2023, £nil (2022: £345 million) additional contributions were unpaid.
Included within the total commitments above is £30 million related to nature based carbon removal projects that support GSK’s net-zero and nature positive goals and £46 million related to the transition to a lower-carbon propellant.
The table excludes any amounts already capitalised in the Financial Statements for the year end 2023.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 37. Share capital and share premium account |
Share Consolidation
Following completion of the Consumer Healthcare business demerger on 18 July 2022, GSK plc Ordinary shares were consolidated to maintain share price comparability before and after demerger. The consolidation was approved by GSK shareholders at a General Meeting held on 6 July 2022. Shareholders received 4 new Ordinary shares with a nominal value of 31¼ pence each for every 5 existing Ordinary shares which had a nominal value of 25 pence each. Earnings per share, diluted earnings per share, adjusted earnings per share and dividends per share were retrospectively adjusted to reflect the Share Consolidation in all the periods presented in years 2021 and 2022.
| | | | | | | | | | | |
| Ordinary shares of 25p each pre-share consolidation
Ordinary shares of 31¼p each post-share consolidation | Share
premium |
| Number | £m | £m |
| Share capital issued and fully paid: | | | |
At 1 January 2021 | 5,385,189,617 | | 1,346 | | 3,281 | |
| Issued under employee share schemes | 1,825,442 | | 1 | | 20 | |
| Ordinary shares acquired by ESOP Trusts | – | | – | | – | |
At 31 December 2021 | 5,387,015,059 | | 1,347 | | 3,301 | |
| Impact of share consolidation | (1,077,403,011) | | – | | – | |
| Issued under employee share schemes | 1,731,293 | | – | | 25 | |
| Ordinary shares acquired by ESOP Trusts | – | | – | | 114 | |
At 31 December 2022 | 4,311,343,341 | | 1,347 | | 3,440 | |
| Issued under employee share schemes | 802,642 | | 1 | | 9 | |
| Ordinary shares acquired by ESOP Trusts | – | | – | | 2 | |
At 31 December 2023 | 4,312,145,983 | | 1,348 | | 3,451 | |
At 31 December 2023, of the issued share capital, 58,817,197 shares were held in the ESOP Trusts, out of which 58,493,518 shares were held for the future exercise of share options and share awards and 323,679 shares were held for the Executive Supplemental Savings plan. 197,068,169 shares were held as Treasury shares and 4,056,260,617 shares were in free issue. All issued shares are fully paid and there are no shares authorised but not in issue. The nominal, carrying and market values of the shares held in the ESOP Trusts are disclosed in Note 45, ‘Employee share schemes’.
Retained earnings and other reserves amounted to £8,548 million at 31 December 2023 (2022: £5,811 million; 2021: £10,407 million) of which £451 million (2022: £463 million; 2021: £476 million) related to associates and joint ventures.
The cumulative translation exchange in equity is as follows:
| | | | | | | | | | | | | | |
| Net translation exchange included in: | |
| Retained
earnings
£m | Fair value
reserve
£m | Non-
controlling
interests
£m | Total
translation
exchange
£m |
| At 1 January 2021 | (539) | | (9) | | (161) | | (709) | |
| Exchange movements on overseas net assets and net investment hedges | (239) | | – | | (20) | | (259) | |
| Reclassification of exchange movements on liquidation or disposal of overseas subsidiaries and associates | (25) | | – | | – | | (25) | |
At 31 December 2021 | (803) | | (9) | | (181) | | (993) | |
| Exchange movements on overseas net assets and net investment hedges | 109 | | 4 | | (28) | | 85 | |
Reclassification of exchange movements on liquidation or disposal of overseas subsidiaries
and associates | 2 | | – | | – | | 2 | |
| Movement attributable to continuing operations | (692) | | (5) | | (209) | | (906) | |
Movement attributable to discontinued operations1 | 263 | | – | | 112 | | 375 | |
At 31 December 2022 | (429) | | (5) | | (97) | | (531) | |
| Exchange movements on overseas net assets and net investment hedges | (41) | | 19 | | (25) | | (47) | |
Reclassification of exchange movements on liquidation or disposal of overseas subsidiaries
and associates | (34) | | – | | – | | (34) | |
At 31 December 2023 | (504) | | 14 | | (122) | | (612) | |
(1)Includes £554 million reclassification to the consolidated income statement of net exchange gains related to the demerger of the Consumer Healthcare business.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 38. Movements in equity continued | |
The analysis of other comprehensive income by equity category is as follows:
| | | | | | | | | | | | | | |
2023 | Retained
earnings
£m | Other
reserves
£m | Non-
controlling
interests
£m | Total
£m |
| Items that may be subsequently reclassified to income statement: | | | | |
| Exchange movements on overseas net assets and net investment hedges | (41) | | 19 | | – | | (22) | |
Reclassification of exchange movements on liquidation or disposal of subsidiaries
and associates | (34) | | – | | – | | (34) | |
| Fair value movements on cash flow hedges | – | | (1) | | – | | (1) | |
| Tax on fair value movements on cash flow hedges | – | | 1 | | – | | 1 | |
| Reclassification of cash flow hedges to income | – | | 4 | | – | | 4 | |
| Items that will not be reclassified to income statement: | | | | |
| Exchange movements on overseas net assets of non-controlling interests | – | | – | | (25) | | (25) | |
| Fair value movements on equity investments | – | | (244) | | – | | (244) | |
| Tax on fair value movements on equity investments | – | | 14 | | – | | 14 | |
| Remeasurement on defined benefit plans | 71 | | – | | – | | 71 | |
| Tax on remeasurement defined benefit plans | (41) | | – | | – | | (41) | |
| Fair value movements on cash flow hedges | – | | (40) | | – | | (40) | |
| Total other comprehensive (expense)/income for the year | (45) | | (247) | | (25) | | (317) | |
| | | | | | | | | | | | | | |
2022 | Retained
earnings
£m | Other
reserves
£m | Non-
controlling
interests
£m | Total
£m |
| Items that may be subsequently reclassified to income statement: | | | | |
| Exchange movements on overseas net assets and net investment hedges | 109 | | 4 | | – | | 113 | |
Reclassification of exchange movements on liquidation or disposal of subsidiaries
and associates | 2 | | – | | – | | 2 | |
| Fair value movements on cash flow hedges | – | | (18) | | – | | (18) | |
| Tax on fair value movements on cash flow hedges | – | | 9 | | – | | 9 | |
| Reclassification of cash flow hedges to income | – | | 14 | | – | | 14 | |
| Items that will not be reclassified to income statement: | | | | |
| Exchange movements on overseas net assets of non-controlling interests | – | | – | | (28) | | (28) | |
| Fair value movements on equity investments | – | | (754) | | – | | (754) | |
| Tax on fair value movements on equity investments | – | | 56 | | – | | 56 | |
| Remeasurement on defined benefit plans | (786) | | – | | – | | (786) | |
| Tax on remeasurement defined benefit plans | 211 | | – | | – | | 211 | |
| Fair value movements on cash flow hedges | – | | (6) | | – | | (6) | |
| Other comprehensive (expense)/income for the year from continuing operations | (464) | | (695) | | (28) | | (1,187) | |
| Other comprehensive (expense)/income for the year from discontinued operations | 375 | | (19) | | – | | 356 | |
| Total other comprehensive (expense)/income for the year | (89) | | (714) | | (28) | | (831) | |
| | | | | | | | | | | | | | |
2021 | Retained
earnings
£m | Other
reserves
£m | Non-
controlling
interests
£m | Total
£m |
| Items that may be subsequently reclassified to income statement: | | | | |
| Exchange movements on overseas net assets and net investment hedges | (239) | | – | | – | | (239) | |
Reclassification of exchange movements on liquidation or disposal of subsidiaries
and associates | (25) | | – | | – | | (25) | |
| Fair value movements on cash flow hedges | – | | 5 | | – | | 5 | |
| Tax on fair value movements on cash flow hedges | – | | (8) | | – | | (8) | |
| Reclassification of cash flow hedges to income statement | – | | 12 | | – | | 12 | |
| Items that will not be reclassified to income statement: | | | | |
| Exchange movements on overseas net assets of non-controlling interests | – | | – | | (20) | | (20) | |
| Fair value movements on equity investments | – | | (911) | | – | | (911) | |
| Tax on fair value movements on equity investments | – | | 131 | | – | | 131 | |
| Remeasurement losses on defined benefit plans | 941 | | – | | – | | 941 | |
| Tax on remeasurement defined benefit plans | (223) | | – | | – | | (223) | |
| Other comprehensive (expense)/income for the year | 454 | | (771) | | (20) | | (337) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 38. Movements in equity continued | |
Information on net investment hedges is provided in part (d) of Note 44 ‘Financial instruments and related disclosures'.
The analysis of other reserves is as follows:
| | | | | | | | | | | | | | | | | |
| ESOP Trust
shares
£m | Fair value
reserve
£m | Cash flow
hedge reserve
£m | Other
reserves
£m |
Total
£m
|
At 1 January 2021 | (195) | | 1,302 | | (31) | | 2,129 | | 3,205 | |
| Exchange adjustments | (1) | | – | | – | | – | | (1) | |
Transferred to income and expenses in the year on impairments of equity
investments | 168 | | – | | – | | – | | 168 | |
| Transferred to retained earnings in the year on disposal of equity investments | – | | (139) | | – | | – | | (139) | |
| Net fair value movement in the year | – | | (780) | | 10 | | – | | (770) | |
At 31 December 2021 | (28) | | 383 | | (21) | | 2,129 | | 2,463 | |
| Exchange adjustments | (36) | | 28 | | 12 | | – | | 4 | |
| Transferred to retained earnings in the year on disposal of equity investments | – | | (21) | | 17 | | – | | (4) | |
| Balances derecognised on demerger | – | | – | | (169) | | – | | (169) | |
| Net fair value movement in the year | – | | (698) | | 141 | | – | | (557) | |
| Ordinary shares acquired by ESOP Trusts | (1,200) | | – | | – | | – | | (1,200) | |
| Write-down of shares held by ESOP Trusts | 911 | | – | | – | | – | | 911 | |
At 31 December 2022 | (353) | | (308) | | (20) | | 2,129 | | 1,448 | |
| Exchange adjustments | 26 | | (5) | | (2) | | – | | 19 | |
| Transferred to retained earnings in the year on disposal of equity investments | – | | 33 | | – | | – | | 33 | |
| Reclassification of cash flow hedges to income statement | – | | – | | 4 | | – | | 4 | |
| Hedging gain/(loss) transferred to non-financial assets | – | | – | | 36 | | – | | 36 | |
| Net fair value movement in the year (including tax) | – | | (230) | | (40) | | – | | (270) | |
| Ordinary shares acquired by ESOP Trusts | (285) | | – | | – | | – | | (285) | |
| Write-down of shares held by ESOP Trusts | 324 | | – | | – | | – | | 324 | |
At 31 December 2023 | (288) | | (510) | | (22) | | 2,129 | | 1,309 | |
Other reserves include various non-distributable merger and pre-merger reserves amounting to £1,849 million at 31 December 2023 (2022: £1,849 million; 2021: £1,849 million). Other reserves also include the capital redemption reserve created as a result of the share buy-back programme amounting to £280 million at 31 December 2023 (2022: £280 million; 2021: £280 million).
| | |
| 39. Non-controlling interests |
Total non-controlling interests includes the following individually material non-controlling interests. Other non-controlling interests are individually not material.
ViiV Healthcare
GSK holds 78.3% of the ViiV Healthcare sub-group, giving rise to a material non-controlling interest. Summarised financial information available at the latest practicable date in respect of the ViiV Healthcare sub-group is as follows:
| | | | | | | | | | | |
| 2023 £m | 2022 £m | 2021 £m |
| Turnover | 6,308 | | 5,619 | | 4,637 | |
| Profit after taxation | 2,034 | | 1,528 | | 1,087 | |
| Other comprehensive income/(expense) | (19) | | 94 | | (17) | |
| Total comprehensive income | 2,015 | | 1,622 | | 1,070 | |
| | | | | | | | |
| 2023 £m | 2022 £m |
| Non-current assets | 2,528 | | 2,716 | |
| Current assets | 3,330 | | 3,354 | |
| Total assets | 5,858 | | 6,070 | |
| | |
| Current liabilities | (3,881) | | (3,762) | |
| Non-current liabilities | (8,453) | | (8,983) | |
| Total liabilities | (12,334) | | (12,745) | |
| Net liabilities | (6,476) | | (6,675) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 39. Non-controlling interests continued | |
| | | | | | | | | | | |
| 2023 £m | 2022 £m | 2021 £m |
| Net cash inflow from operating activities | 2,192 | | 3,442 | | 2,128 | |
| Net cash outflow from investing activities | (2) | | (174) | | (287) | |
| Net cash outflow from financing activities | (2,463) | | (2,718) | | (1,608) | |
| Increase/(decrease) in cash and bank overdrafts in the year | (273) | | 550 | | 233 | |
The above financial information relates to the ViiV Healthcare group on a stand-alone basis, before the impact of Group-related adjustments, primarily related to the recognition of preferential dividends. The profit after taxation of £2,034 million (2022: £1,528 million; 2021: £1,087 million) is stated after charging preferential dividends payable to GSK and Pfizer and after a charge of £858 million (2022: £1,483 million; 2021: £1,218 million) for remeasurement of contingent consideration payable. This consideration is expected to be paid over a number of years.
The following amounts attributable to the ViiV Healthcare group are included in GSK’s financial statements:
| | | | | | | | | | | |
| 2023 £m | 2022 £m | 2021 £m |
| Share of profit for the year attributable to non-controlling interest | 373 | | 415 | | 196 | |
| Dividends paid to non-controlling interest | 398 | | 480 | | 224 | |
| Non-controlling interest in the consolidated balance sheet | (648) | | (611) | | (570) | |
| | |
| 40. Related party transactions |
At 31 December 2023, a loan of £0.8 million (2022: £nil) to Index Ventures and a loan of £0.6 million (2022: £nil ) to Medicxi Ventures I LP remained due to GSK. Cash distributions were received from investment in Medicxi Ventures I LP of £10.7 million (2022: Medicxi Ventures I LP of £6 million).
In December 2023, Qura Therapeutics LLC was liquidated, the investment and the associated commitment for future contributions were de-recognised from the balance sheet. An immaterial gain (less than £1 million) was recognised.
The Group had no other significant related party transactions which might reasonably be expected to influence decisions made by the users of these Financial Statements.
The aggregate compensation of the Directors and GLT is given in Note 9, ‘Employee costs’.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 41. Acquisitions and disposals |
Details of the acquisition and disposal of significant subsidiaries, associates, joint ventures and other businesses are given below:
2023
Business acquisitions
On 28 June 2023, GSK completed the acquisition of BELLUS Health Inc. ("Bellus") which was effected through a Plan of Arrangement (the “Arrangement”) pursuant to the Canada Business Corporations Act. The Arrangement was approved by Bellus’ shareholders on 16 June 2023. Upon completion, GSK acquired all outstanding common shares of Bellus for US$14.75 per common share in cash, representing a total equity value of US$2 billion (£1.6 billion). The acquisition provides GSK access to camlipixant, a potential best-in-class and highly selective P2X3 antagonist currently in phase III development for the first-line treatment of adult patients with refractory chronic cough (RCC).
| | | | | |
| Total
£m |
| Net assets acquired: | |
| Intangible assets | 1,438 | |
| Non-current equity investments | 2 | |
| Right of use assets | 1 | |
| Trade and other receivables | 96 | |
| Investments held as current assets | 51 | |
| Cash and cash equivalents | 148 | |
| Lease liabilities | (1) | |
| Trade and other payables | (103) | |
| Deferred tax liabilities | (136) | |
| 1,496 | |
| Non-controlling interest | – | |
| Goodwill | 109 | |
| Total consideration | 1,605 | |
In 2023, the provisional values of the identifiable assets and liabilities acquired in the Affinivax, Inc. business combination were updated for the finalisation of the fair value of intangible assets, resulting in an increase in intellectual property of £39 million, a decrease to goodwill of £31 million and a decrease to deferred tax of £8 million. The amounts recognised at 31 December 2022 have not been restated on the basis of materiality.
Business disposals
GSK completed no material business disposals in 2023.
Associates and joint ventures
GSK completed no material investments or disposals of associates or joint ventures during the year.
Cash flows
| | | | | | | | |
| Business Acquisitions
£m | Business Disposals
£m |
| Cash consideration (paid)/ received | (1,605) | | 68 | |
| Net deferred consideration paid | – | | (19) | |
| Transaction costs | (17) | | – | |
| Cash and cash equivalents acquired/(divested) | 148 | | – | |
| Cash (outflow)/inflow | (1,474) | | 49 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 41. Acquisitions and disposals continued | |
2022
Business acquisitions
On 1 July 2022, GSK completed the acquisition of 100% of Sierra Oncology, Inc., a California-based, late-stage biopharmaceutical company focused on targeted therapies for the treatment of rare forms of cancer, for $1.9 billion (£1.6 billion). The main asset is momelotinib which targets the medical needs of myelofibrosis patients with anaemia. Total transaction costs were £52 million.
On 15 August 2022, GSK completed the acquisition of 100% of Affinivax, Inc. a clinical-stage biopharmaceutical company based in Cambridge, Boston, Massachusetts focused on pneumococcal vaccine candidates. The consideration for the acquisition comprised an upfront payment of $2.2 billion (£1.8 billion) as adjusted for working capital acquired paid upon closing and two potential milestone payments each of $0.6 billion (£0.5 billion) to be paid upon the achievement of certain paediatric clinical development milestones. The estimated fair value of the contingent consideration payable was £482 million. The values were provisional and were subject to change. The total transaction costs were £71 million.
During 2022, no sales arising from the Sierra Oncology or Affinivax businesses were included in Group turnover and no revenue is expected until regulatory approval is received on the acquired assets.
GSK continues to support the ongoing development of the acquired assets and consequently these assets will be loss making until regulatory approval on these assets is received. The development of these assets has been integrated into the Group’s existing R&D activities, so it was impracticable to quantify these development costs or the impact on Total profit after taxation for the period ended 31 December 2022.
Goodwill of £1,127 million (£162 million for Sierra Oncology and £965 million for Affinivax), which is not expected to be deductible for tax purposes, has been recognised. The goodwill represents workforce in place, and specific synergies available to GSK from the business combinations. The goodwill has been allocated to the Group’s Commercial Operations and R&D segments (refer to Note 19 ‘Goodwill’ for allocation methodology).
| | | | | | | | | | | |
| Sierra Oncology
£m | Affinivax
£m | Total
£m |
| Net assets acquired | | | |
| Intangible assets | 1,497 | | 1,467 | | 2,964 | |
| Property, plant and equipment | – | | 30 | | 30 | |
| Right of use assets | 1 | | 52 | | 53 | |
| Inventory | 60 | | – | | 60 | |
| Trade and other receivables | 2 | | 17 | | 19 | |
| Cash and cash equivalents | 175 | | 109 | | 284 | |
| Lease liabilities | (1) | | (55) | | (56) | |
| Trade and other payables | (40) | | (77) | | (117) | |
| Taxation | (259) | | (236) | | (495) | |
| 1,435 | | 1,307 | | 2,742 | |
| Goodwill | 162 | | 965 | | 1,127 | |
| Total | 1,597 | | 2,272 | | 3,869 | |
| Total cash | 1,597 | | 1,790 | | 3,387 | |
| Fair value of contingent consideration | – | | 482 | | 482 | |
On 24 November 2022 GSK signed an agreement to buy out the 25% non-controlling interest in Glaxo Saudi Arabia Ltd for SAR94 million (£21 million), paid in 2023.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 41. Acquisitions and disposals continued | |
Demerger of Consumer Healthcare business
On 18 July 2022, GSK plc separated its Consumer Healthcare business from the GSK Group to form Haleon, an independent listed company. The separation was effected by way of a demerger of 80.1% of GSK’s 68% holding in the Consumer Healthcare business to GSK shareholders. Following the demerger, 54.5% of Haleon was held in aggregate by GSK shareholders, 6.0% was held by GSK (including shares received by GSK’s consolidated ESOP trusts) and 7.5% was held by certain Scottish Limited Partnerships (SLPs) set up to provide collateral for a funding mechanism pursuant to which GSK will provide additional funding for GSK’s UK defined benefit pension schemes (Note 31, 'Pensions and other post-employment benefits'). The aggregate ownership by GSK (including ownership by the ESOP trusts and SLPs) after the demerger of 13.5% was measured at fair value with changes through profit or loss. In 2022, Pfizer held 32% of Haleon after the demerger.
Under IFRIC 17 ‘Distributions of Non-cash Assets to Owners’ a liability and an equity distribution are measured at the fair value of the assets to be distributed when the dividend is appropriately authorised and it is no longer at the entity’s discretion. The liability and equity movement, and associated gain on distribution were recognised in Q3 2022 when the demerger distribution was authorised and occurred.
The asset distributed was the 54.5% ownership of the Consumer Healthcare business. The net carrying value of the Consumer Healthcare business in the consolidated financial statements, including the retained 13.5% and net of the amount attributable to the non-controlling interest, was approximately £11 billion at the end of June. GSK’s £6.3 billion share of the shareholder loans made in Q1 2022 in advance of the pre-separation dividends was eliminated in the consolidated financial statements. The assets distributed were reduced by Consumer Healthcare transactions up to 18 July that principally included pre-separation dividends declared and settled after the end of Q2 2022 and before 18 July 2022. Those dividends included: £10.4 billion (£7.1 billion attributable to GSK) of dividends funded by Consumer Healthcare debt that was partially on-lent during Q1 2022 and dividends of £0.6 billion (£0.4 billion attributable to GSK) from available cash balances.
The fair value of the 54.5% ownership of the Consumer Healthcare business distributed was £15.5 billion. This was measured by reference to the quoted average Haleon share price over the first five days of trading, this being a fair value measured with observable inputs which was considered to be representative of the fair value at the distribution date. A gain on distribution of this fair value less book value of the attributable net assets of the Consumer Healthcare business of £7.7 billion was recorded in the income statement in 2022. There was an additional gain of £2.4 billion to remeasure the retained 13.5% from its book value to fair value of £3.9 billion using the same fair value methodology as used for the distributed shares. The gain on distribution and on remeasurement of the retained stake upon demerger was presented as part of discontinued operations. Any future gains or losses on the retained stake in Haleon will be recognised in continuing operations. In addition, there was a reclassification of the Group’s share of cumulative exchange differences arising on translation of the foreign currency net assets of the divested subsidiaries and offsetting net investment hedges from reserves into the income statement of £0.6 billion. The total gain on demerger of Consumer Healthcare was £10.1 billion. These transactions were presented in profit from discontinued operations in 2022.
| | | | | |
| 2022
£m |
Fair value of the Consumer Healthcare business distributed (54.5%) | 15,526 | |
Fair value of the retained ownership in Haleon plc (13.5%) | 3,853 | |
| Total fair value | 19,379 | |
| Carrying amount of the net assets and liabilities distributed/de-recognised | (12,887) | |
| Carrying amount of the non-controlling interest de-recognised | 3,038 | |
| Gain on demerger before exchange movements and transaction costs | 9,530 | |
| Reclassification of exchange movements and net investment hedge movements on disposal of overseas subsidiaries | 554 | |
| Total gain on the demerger of Consumer Healthcare | 10,084 | |
Consumer Healthcare was presented as a discontinued operation as at 30 June 2022 and disclosed as such in the interim financial statements. The Consolidated Income Statement and Consolidated Cash Flow Statement distinguish discontinued operations from continuing operations. Comparative figures have been restated on a consistent basis. Financial information relating to the operations of Consumer Healthcare for the period is set out on the following page and includes financial information until 18 July 2022.
This financial information differs both in purpose and basis of preparation from the Historical Financial Information and the Interim Financial Information included in the Haleon prospectus and from that which was published by Haleon on 2 March 2023. As a result, whilst the two sets of financial information are similar, they are not the same because of certain differences in accounting and disclosure under IFRS.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 41. Acquisitions and disposals continued | |
| | | | | | | | |
| Total results | 2022
£m | 2021
£m |
| Turnover | 5,581 | 9,418 |
| Expense | (4,730) | | (7,575) | |
| Profit before tax | 851 | 1,843 |
| Taxation | (235) | | (263) | |
| Tax rate % | 27.6% | 14.3% |
| (Loss)/profit after taxation from discontinued operations: Consumer Healthcare | 616 | 1,580 |
| Other gains/(losses) on demerger | 2,433 | – |
| Remeasurement of discontinued operations distributed to shareholders on demerger | 7,651 | – |
| Profit after taxation on demerger of discontinued operations | 10,700 | 1,580 |
| Non-controlling interest in discontinued operations | 205 | 511 |
| Earnings attributable to shareholders from discontinued operations | 10,495 | 1,069 |
| Earnings per share from discontinued operations | 260.6p | 26.7p |
Other business disposals
There were no other material business disposals in 2022.
| | | | | | | | | | | |
| Cash flows | Business
acquisitions
£m | Business disposals - demerger
£m | Business disposals - other
£m |
| Cash consideration | (3,392) | | – | | – | |
| Net deferred consideration paid | – | | – | | (34) | |
| Cash and cash equivalents (divested)/acquired | 284 | | (933) | | (9) | |
| (3,108) | | (933) | | (43) | |
| Transaction costs paid | (79) | | (141) | | – | |
| Cash (outflow)/inflow | (3,187) | | (1,074) | | (43) | |
Cash consideration for business acquisitions included £5 million related to other business acquisition activity.
2021
Business acquisitions
GSK completed no material business acquisitions in 2021.
Business disposals
GSK made a number of business disposals for net cash consideration received in the year of £10 million. The profit on the disposal of the businesses in the year of £24 million was calculated as follows:
| | | | | |
| Total £m |
| Consideration: | |
| Cash consideration including currency forwards, purchase adjustments and deferred consideration | 10 | |
| Total | 10 | |
| |
| Net assets sold: | |
| Property, plant and equipment | 3 | |
| Cash and cash equivalents | 1 | |
| Other net assets | 1 | |
| Total | 5 | |
| |
| Costs: | |
| Deal costs | (16) | |
| Reclassification of exchange from other comprehensive income | 35 | |
| |
Gain on disposals in 2021 | 24 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 41. Acquisitions and disposals continued | |
Associates and joint ventures
On 20 May 2021 GSK agreed with Innoviva, Inc. (“Innoviva”) to sell all of its approximately 32 million shares of common stock of Innoviva back to Innoviva at a price of $12.25 per share, raising gross proceeds of approximately $392 million. Following settlement of the transaction, GSK will no longer hold any Innoviva stock. See details in Note 21 ‘Investment in associates and joint ventures’.
Cash flows
| | | | | | | | |
| Business disposals
£m | Associates
and joint ventures disposals
£m |
| Cash consideration received | 43 | | 277 | |
| Net deferred consideration paid | (51) | | – | |
| Transaction costs | (8) | | – | |
| Cash and cash equivalents (divested)/acquired | (1) | | – | |
| Cash (outflow)/inflow | (17) | | 277 | |
.
| | |
| 42. Adjustments reconciling Total profit after tax to operating cash flows |
| | | | | | | | | | | |
| 2023 £m | 2022 £m | 2021 £m |
| Total profit after tax from continuing operations | 5,308 | | 4,921 | | 3,516 | |
| | | |
| Tax on profits | 756 | | 707 | | 83 | |
| Share of after-tax (profits)/losses of associates and joint ventures | 5 | | 2 | | (33) | |
| Finance expense net of finance income | 677 | | 803 | | 755 | |
| Depreciation | 1,082 | | 1,061 | | 1,034 | |
| Amortisation of intangible assets | 1,212 | | 1,086 | | 1,088 | |
| Impairment and assets written off | 467 | | 481 | | 529 | |
| Profit on sale of businesses | – | | (36) | | (47) | |
| Profit on sale of intangible assets | (12) | | (185) | | (539) | |
| (Profit)/loss on sale of investments in associates | (1) | | – | | 36 | |
| Profit on sale of equity investments | – | | (1) | | (8) | |
| Changes in working capital: | | | |
| Decrease/(increase) in inventories | (424) | | (269) | | 51 | |
| (Increase) in trade receivables | (794) | | (158) | | (780) | |
| Increase/(decrease) in trade payables | (15) | | 494 | | 229 | |
| (Increase)/decrease in other receivables | 145 | | (458) | | (382) | |
| Contingent consideration paid (see Note 33) | (1,134) | | (1,058) | | (742) | |
| Other non-cash increase in contingent consideration liabilities | 492 | | 1,628 | | 1,063 | |
| Increase/(decrease) in other payables | 689 | | (5) | | 1,505 | |
| Decrease in pension and other provisions | (457) | | (962) | | (299) | |
| Share-based incentive plans | 307 | | 346 | | 343 | |
| Fair value adjustments | (107) | | (283) | | (31) | |
| Other | (100) | | (170) | | (122) | |
| Operating cash flow from continuing operations | 8,096 | | 7,944 | | 7,249 | |
| Operating cash flow from discontinued operations | – | | 932 | | 1,994 | |
| | | |
| Total cash generated from operations | 8,096 | | 8,876 | | 9,243 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 43. Reconciliation of net cash flow to movement in net debt |
| | | | | | | | | | | |
| 2023 £m | 2022 £m | 2021 £m |
| Net debt, at beginning of year, as adjusted | (17,197) | | (19,838) | | (20,780) | |
| Decrease in cash and bank overdrafts | (468) | | (7,597) | | (2,504) | |
| Decrease in liquid investments | (72) | | (1) | | (18) | |
| Issue of long-term loans | (223) | | (1,025) | | – | |
| Repayment of short-term notes | 2,116 | | 5,074 | | 2,304 | |
| Repayment of/(increase in) other short-term loans | 333 | | (1,021) | | (301) | |
| Repayment of long-term loans | 144 | | 1,594 | | – | |
| Repayment of lease liabilities | 197 | | 202 | | 181 | |
| Investments/(debt) of subsidiary undertakings acquired | 50 | | (24) | | – | |
| Exchange adjustments | 554 | | (1,531) | | 314 | |
| Other non-cash movements | (474) | | (207) | | (134) | |
| Decrease/(increase) in net debt from continuing operations | 2,157 | | (4,536) | | (158) | |
| Decrease/(increase) in net debt from discontinued operations | – | | 7,177 | | 1,100 | |
| | | |
| Total net debt at end of year | (15,040) | | (17,197) | | (19,838) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Analysis of changes in net debt | | At 1 January 2023
£m | Exchange
£m | Other
£m | Interest expense
£m | Change
in fair value
£m | Reclass-
ifications
£m | Cash flow
£m | At
31 December 2023
£m |
| Liquid investments | | 67 | | (4) | | 51 | | – | | – | | – | | (72) | | 42 | |
| | – | | – | | – | | – | | – | | – | | – | | – | |
| Cash and cash equivalents | | 3,723 | | (105) | | – | | – | | – | | – | | (682) | | 2,936 | |
| Overdrafts | | (298) | | 6 | | – | | – | | – | | – | | 214 | | (78) | |
| | 3,425 | | (99) | | – | | – | | – | | – | | (468) | | 2,858 | |
| Debt due within one year: | | | | | | | | | |
| Commercial paper | | (1,191) | | 56 | | – | | – | | – | | – | | 320 | | (815) | |
| European/US MTN & Bank facilities | | (2,146) | | 48 | | – | | – | | – | | (1,669) | | 2,116 | | (1,651) | |
| Lease liabilities | | (167) | | 12 | | (3) | | – | | – | | (195) | | 197 | | (156) | |
| Other | | (150) | | 21 | | 3 | | – | | – | | – | | 13 | | (113) | |
| | (3,654) | | 137 | | – | | – | | – | | (1,864) | | 2,646 | | (2,735) | |
| Debt due after one year: | | | | | | | | | |
| European/US MTN & Bank facilities | | (16,194) | | 469 | | – | | (19) | | – | | 1,669 | | (79) | | (14,154) | |
| Lease liabilities | | (841) | | 42 | | (447) | | – | | – | | 195 | | – | | (1,051) | |
| | (17,035) | | 511 | | (447) | | (19) | | – | | 1,864 | | (79) | | (15,205) | |
| | | | | | | | | |
| Net debt | | (17,197) | | 545 | | (396) | | (19) | | – | | – | | 2,027 | | (15,040) | |
| | | | | | | |
| Interest payable | | (207) | | 1 | | (29) | | (693) | | – | | – | | 766 | | (162) | |
| Derivative financial instruments | | 8 | | – | | – | | – | | 343 | | – | | (335) | | 16 | |
Total liabilities from financing
activities* | | (20,888) | | 649 | | (476) | | (712) | | 343 | | – | | 2,998 | | (18,086) | |
* Excluding cash and cash equivalents, overdrafts and liquid investments.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 43. Reconciliation of net cash flow to movement in net debt continued | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Analysis of changes in net debt | At 1 January 2022
£m | Exchange
£m | Other
£m | Interest expense
£m | Change
in fair value
£m | Reclass-
ifications
£m | Demerger
£m | Cash flow
£m | At
31 December 2022
£m |
| Liquid investments | 61 | | 7 | | – | | | | | | (1) | | 67 | |
| | | | | | | | | |
| Cash and cash equivalents | 3,861 | | 99 | | 1 | | – | | – | | – | | 7,496 | | (7,734) | | 3,723 | |
| Overdrafts | (450) | | 15 | | – | | – | | – | | – | | – | | 137 | | (298) | |
Liquid investments attributed to
continuing operations | 3,411 | | 114 | | 1 | | – | | – | | – | | 7,496 | | (7,597) | | 3,425 | |
Liquid investments attributed to
discontinued operations | 407 | | 37 | | – | | – | | – | | – | | (7,496) | | 7,052 | | – | |
| 3,818 | | 151 | | 1 | | – | | – | | – | | – | | (545) | | 3,425 | |
| | | | | | | | | |
| Debt due within one year: | | | | | | | | | |
| Commercial paper | (252) | | (30) | | – | | – | | – | | – | | – | | (909) | | (1,191) | |
| European/US MTN & Bank facilities | (2,596) | | (174) | | – | | – | | – | | (4,426) | | – | | 5,050 | | (2,146) | |
| Lease liabilities | (173) | | (14) | | 5 | | – | | – | | (186) | | – | | 201 | | (167) | |
| Other | (52) | | (2) | | (9) | | – | | – | | – | | – | | (87) | | (150) | |
Debt due within one year attributed
to continuing operations | (3,073) | | (220) | | (4) | | – | | – | | (4,612) | | – | | 4,255 | | (3,654) | |
Debt due within one year attributed
to discontinued operations | (72) | | (3) | | (15) | | – | | – | | (3) | | 1,559 | | (1,466) | | – | |
| (3,145) | | (223) | | (19) | | – | | – | | (4,615) | | 1,559 | | 2,789 | | (3,654) | |
| | | | | | | | | |
| Debt due after one year: | | | | | | | | | |
| European/US MTN & Bank facilities | (19,760) | | (1,386) | | – | | (43) | | – | | 4,426 | | – | 569 | | (16,194) | |
| Lease liabilities | (725) | | (59) | | (243) | | – | | – | | 186 | | – | | – | | (841) | |
Debt due after one year attributed to
continuing operations | (20,485) | | (1,445) | | (243) | | (43) | | – | | 4,612 | | – | | 569 | | (17,035) | |
Debt due after one year attributed to
discontinued operations | (87) | | (777) | | (6) | | (4) | | 48 | | 3 | | 10,059 | | (9,236) | | – | |
| (20,572) | | (2,222) | | (249) | | (47) | | 48 | | 4,615 | | 10,059 | | (8,667) | | (17,035) | |
| | | | | | | | | |
| Net debt | (19,838) | | (2,287) | | (267) | | (47) | | 48 | | – | | 11,618 | | (6,424) | | (17,197) | |
| | | | | | | | |
| Interest payable | (244) | | (5) | | (33) | | (865) | | – | | – | | 92 | | 848 | | (207) | |
| Derivative financial instruments | (22) | | – | | – | | – | | 670 | | – | | – | | (640) | | 8 | |
Total liabilities from financing
activities* | (23,983) | | (2,450) | | (301) | | (912) | | 718 | | – | | 11,710 | | (5,670) | | (20,888) | |
* Excluding cash and cash equivalents, overdrafts and liquid investments.
For further information on significant changes in net debt see Note 30, ‘Net debt’.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 44. Financial instruments and related disclosures |
The objective of GSK’s Treasury activities is to minimise the net cost of financial operations and reduce its volatility to benefit earnings and cash flows. GSK uses a variety of financial instruments to finance its operations and derivative financial instruments to manage market risks from these operations. Derivatives principally comprise foreign exchange forward contracts and swaps which are used to swap borrowings and liquid assets into currencies required for Group purposes as well as interest rate swaps which are used to manage exposure to financial risks from changes in interest rates. These financial instruments reduce the uncertainty of foreign currency transactions and interest payments.
Derivatives are used exclusively for hedging purposes in relation to underlying business activities and not as trading or speculative instruments.
Capital management
GSK’s financial strategy supports the Group’s strategic priorities and is regularly reviewed by the Board. GSK manages the capital structure of the Group through an appropriate mix of debt and equity.
The capital structure of the Group consists of net debt of £15 billion (see Note 30, ‘Net debt’) and total equity, including items related to non-controlling interests, of £13 billion (see ‘Consolidated statement of changes in equity’ on page 160). Total capital, including that provided by non-controlling interests, is £28 billion.
The Group continues to manage its financial policies to a credit profile that particularly targets ratings of at least A2/A (Moody's/S&P), through the cycle. The Group’s long-term credit rating with Standard & Poor’s is A (stable outlook) and with Moody’s Investor Services (‘Moody’s’) is A2 (stable outlook). The Group’s short-term credit ratings are A-1 and P-1 with Standard & Poor’s and Moody’s respectively.
Liquidity risk management
GSK’s policy is to borrow centrally in order to meet anticipated funding requirements. The strategy is to diversify liquidity sources using a range of facilities and to maintain broad access to financial markets. Each day, GSK sweeps cash to or from a number of global subsidiaries and central Treasury accounts for liquidity management purposes. GSK utilises both physical and notional cash pool arrangements as appropriate by location and currency. For notional cash pools, liquidity is drawn against foreign currency balances to provide both local funding and central liquidity as required and with balances actively managed and maintained to appropriate levels. As balances in notional pooling arrangements are not settled across currencies, gross cash and overdraft balances are reported.
At 31 December 2023, GSK had £2.8 billion of borrowings repayable within one year and held £3.0 billion of cash and cash equivalents and liquid investments of which £2.2 billion was held centrally.
GSK has access to short-term finance under a $10 billion (£7.8 billion) US commercial paper programme; $850 million (£667 million) was in issue at 31 December 2023 (2022: $900 million (£748 million)). GSK has access to short-term finance under a £5 billion Euro commercial paper programme; €170 million (£148 million) was in issue at 31 December 2023 (2022: €500 million (£443 million)). GSK has a £1.6 billion three-year and a $2.2 billion (£1.7 billion) 364 day committed facility. These committed facilities were undrawn at 31 December 2023. GSK considers this level of committed facilities to be adequate, given current liquidity requirements.
GSK has a £20 billion Euro Medium Term Note programme and at 31 December 2023, £9.2 billion of notes were in issue under this programme. The Group also had $8.4 billion (£6.6 billion) of notes in issue at 31 December 2023 under a US shelf registration. GSK’s borrowings mature at dates between 2024 and 2045.
The put option owned by Pfizer in ViiV Healthcare is exercisable. In reviewing liquidity requirements GSK considers that sufficient financing options are available should the put option be exercised.
Market risk
Interest rate risk management
GSK’s objective is to minimise the effective net interest cost and to balance the mix of debt at fixed and floating rates over time.
The Group’s main interest rate risk arises from borrowings and investments with floating rates and refinancing of maturing fixed rate debt where any changes in interest rates will affect future cash flows or the fair values of financial instruments. The policy on interest rate risk management limits the net amount of floating rate debt to a specific cap, reviewed and agreed no less than annually by the Board.
The majority of debt is issued at fixed interest rates and changes in the floating rates of interest do not significantly affect the Group’s net interest charge. Short-term borrowings including bank facilities are exposed to the risk of future changes in market interest rates as are the majority of cash and liquid investments.
Foreign exchange risk management
The Group’s objective is to minimise the exposure of overseas operating subsidiaries to transaction risk by matching local currency income with local currency costs where possible. Foreign currency transaction exposures arising on external and internal trade flows are selectively hedged. GSK’s internal trading transactions are matched centrally and inter-company payment terms are managed to reduce foreign currency risk. Where possible, GSK manages the cash surpluses or borrowing requirements of subsidiary companies centrally using forward contracts to hedge future repayments back into the originating currency.
In order to reduce foreign currency translation exposure, the Group seeks to denominate borrowings in the currencies of our principal assets and cash flows. These are primarily denominated in US Dollars, Euros and Sterling. Borrowings can be swapped into other currencies as required.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
Borrowings denominated in, or swapped into, foreign currencies that match investments in overseas Group assets may be treated as a hedge against the relevant assets. Forward contracts in major currencies are also used to reduce exposure to the Group’s investment in overseas assets (see ‘Net investment hedges’ section of this note for further details).
Credit risk
Credit risk is the risk that a counterparty will default on its contractual obligations resulting in financial loss to the Group and arises on cash and cash equivalents and favourable derivative financial instruments held with banks and financial institutions as well as credit exposures to wholesale and retail customers, including outstanding receivables.
The Group considers its maximum credit risk at 31 December 2023 to be £9,528 million (31 December 2022: £10,180 million) which is the total of the Group’s financial assets with the exception of ’Other investments’ (comprising equity investments) which bear equity risk rather than credit risk. See page 225 for details on the Group’s total financial assets. At 31 December 2023, GSK’s greatest concentration of credit risk was £1.2 billion with a wholesaler in the US (2022: £1.1 billion with a wholesaler in the US). See page 223 for further information on the Group’s credit risk exposure in respect of the three largest US wholesaler customers.
There has been no change in the estimation techniques or significant assumptions made during the current reporting period in assessing the loss allowance for financial assets at amortised cost or at FVTOCI since the adoption of IFRS 9 at the start of the 2018 reporting period.
Treasury-related credit risk
GSK sets global counterparty limits for each of GSK’s banking and investment counterparties based on long-term credit ratings from Moody’s and Standard & Poor’s. Usage of these limits is actively monitored.
GSK actively manages its exposure to credit risk, reducing surplus cash balances wherever possible. This is part of GSK’s strategy to regionalise cash management and to concentrate cash centrally as much as possible. The table below sets out the credit exposure to counterparties by rating for liquid investments, cash and cash equivalents and derivatives.
The gross asset position on each derivative contract is considered for the purpose of this table, although, under ISDA agreements, the amount at risk is the net position with each counterparty. Table (e) on page 233 sets out the Group’s financial assets and liabilities on an offset basis.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
At 31 December 2023, £44 million (2022: £60 million) of cash is categorised as held with unrated or sub-investment grade rated counterparties (lower than BBB-/Baa3). This exposure is concentrated in overseas banks used for local cash management or investment purposes, including: £18 million in Saudi Arabia with Saudi British Bank; £15 million with Halk Bank in the UK; £7 million in Nigeria held with United Bank for Africa, Zenith Bank, Access Bank and Stanbic IBTC Bank; £2 million in Brazil held with Banco Bradesco, Itau UniBanco, Banco Do Brasil and Caixa Economica Federal; and £1 million with Banco De La Produccion in Ecuador. Of the £55 million of bank balances and deposits held with BBB/Baa rated counterparties, £3.4 million was held with BBB-/Baa3 rated counterparties, including balances or deposits of £2.6 million with State Bank of India in India. These banks are used for local investment purposes.
GSK measures expected credit losses over cash and cash equivalents as a function of individual counterparty credit ratings and associated 12 month default rates. Expected credit losses over cash and cash equivalents and third-party financial derivatives are deemed to be immaterial and no such loss has been experienced during 2023.
Credit ratings are assigned by Standard & Poor’s and Moody’s respectively. Where the opinions of the two rating agencies differ, GSK assigns the lower rating of the two to the counterparty. Where local rating agency or Fitch data is the only source available, the ratings are converted to global ratings equivalent to those of Standard & Poor’s or Moody’s using published conversion tables. These credit ratings form the basis of the assessment of the expected credit loss on Treasury-related balances held at amortised cost being bank balances and deposits and Government securities.
| | | | | | | | | | | | | | | | | | | | |
2023 | AAA/Aaa
£m | AA/Aa
£m | A/A £m | BBB/Baa £m | BB+/Ba1
and below /unrated
£m | Total £m |
| Bank balances and deposits | – | | 28 | | 1,815 | | 55 | | 44 | | 1,942 | |
| US Treasury and Treasury repo only money market funds | 155 | | – | | – | | – | | – | | 155 | |
| Liquidity funds | 839 | | – | | – | | – | | – | | 839 | |
| Government securities | – | | 42 | | – | | – | | – | | 42 | |
| Third party financial derivatives | – | | – | | 130 | | – | | – | | 130 | |
| Total | 994 | | 70 | | 1,945 | | 55 | | 44 | | 3,108 | |
| | | | | | | | | | | | | | | | | | | | |
2022 | AAA/Aaa
£m | AA/Aa
£m | A/A £m | BBB/Baa £m | BB+/Ba1
and below /unrated
£m | Total £m |
| Bank balances and deposits | – | | – | | 1,215 | | 49 | | 60 | | 1,324 | |
| US Treasury and Treasury repo only money market funds | 146 | | – | | – | | – | | – | | 146 | |
| Liquidity funds | 2,253 | | – | | – | | – | | – | | 2,253 | |
| Government securities | – | | 67 | | – | | – | | – | | 67 | |
| Third party financial derivatives | – | | – | | 188 | | – | | – | | 188 | |
| Total | 2,399 | | 67 | | 1,403 | | 49 | | 60 | | 3,978 | |
GSK’s centrally managed cash reserves amounted to £2.2 billion at 31 December 2023, all available within three months. This includes £2.0 billion of cash managed by the Group for ViiV Healthcare, a 78.3% owned subsidiary. The Group has invested centrally managed liquid assets in bank deposits, Aaa/AAA rated US Treasury and Treasury repo only money market funds and Aaa/AAA rated liquidity funds.
Wholesale and retail credit risk
Outside the US, no customer accounts for more than 5% of the Group’s trade receivables balance.
In the US, in line with other pharmaceutical companies, the Group sells its products through a small number of wholesalers in addition to hospitals, pharmacies, physicians and other groups. Sales to the three largest wholesalers amounted to approximately 79% (2022:79%) of the sales of the US Commercial Operations business in 2023.
At 31 December 2023, the Group had trade receivables due from these three wholesalers totalling £3,319 million or 56% of total trade receivables (2022: £3,001 million or 55%). The Group is exposed to a concentration of credit risk in respect of these wholesalers such that, if one or more of them encounters financial difficulty, it could materially and adversely affect the Group’s financial results.
This concentration of trade receivables is reflective of standard market practice in the US pharmaceuticals sector where a significant portion of sales are made to these three wholesalers, as disclosed in Note 6 'Turnover and segment information'. GSK’s assessment is that there is limited credit risk associated with these customers.
The Group’s credit risk monitoring activities relating to these wholesalers include a review of their quarterly financial information and Standard & Poor’s credit ratings, development of GSK internal risk ratings, and establishment and periodic review of credit limits.
All new customers are subject to a credit vetting process and existing customers will be subject to a review at least annually. The vetting process and subsequent reviews involve obtaining information including the customer’s status as a government or private sector entity, audited financial statements, credit bureau reports, debt rating agency (e.g. Moody’s, Standard & Poor’s) reports, payment performance history (from trade references, industry credit groups) and bank references.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
Trade receivables consist of amounts due from a large number of customers, spread across diverse industries and geographical areas. Ongoing credit evaluation is performed on the financial condition of accounts receivable and, where appropriate, credit insurance is purchased or factoring arrangements put in place.
The amount of information obtained is proportional to the level of exposure being considered. The information is evaluated quantitatively (i.e. credit score) and qualitatively (i.e. judgement) in conjunction with the customer’s credit requirements to determine a credit limit.
Trade receivables are grouped into customer segments that have similar loss patterns to assess credit risk while other receivables and other financial assets are assessed individually. Historical and forward-looking information is considered to determine the appropriate expected credit loss allowance.
The Group believes there is no further credit risk provision required in excess of the allowance for expected credit losses (see Note 26, ‘Trade and other receivables’).
Credit enhancements
The Group uses credit enhancements including factoring and credit insurance to minimise the credit risk of the trade receivables in the Group. At 31 December 2023, £421 million (2022: £332 million) of trade receivables were insured in order to protect the receivables from loss due to credit risks such as default, insolvency and bankruptcy.
Each Group entity assesses the credit risk of its private customers to determine if credit insurance is required.
Factoring arrangements are managed locally by entities and are used to mitigate risk arising from large credit risk concentrations. All factoring arrangements are non-recourse.
Fair value of financial assets and liabilities excluding lease liabilities
The table on page 225 presents the carrying amounts and the fair values of the Group’s financial assets and liabilities excluding lease liabilities at 31 December 2023 and 31 December 2022.
The fair values of the financial assets and liabilities are included at the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
The following methods and assumptions are used to measure the fair values of significant financial instruments carried at fair value on the balance sheet:
–Other investments – equity investments traded in an active market determined by reference to the relevant stock exchange quoted bid price; other equity investments determined by reference to the current market value of similar instruments, recent financing rounds or the discounted cash flows of the underlying net assets
–Trade receivables carried at fair value – based on invoiced amount
–Interest rate swaps, foreign exchange forward contracts, swaps and options – based on the present value of contractual cash flows or option valuation models using market sourced data (exchange rates or interest rates) at the balance sheet date
–Cash equivalents carried at fair value – based on net asset value of the funds
–Contingent consideration for business acquisitions and divestments – based on present values of expected future cash flows.
The following methods and assumptions are used to estimate the fair values of significant financial instruments which are not measured at fair value on the balance sheet:
–Receivables and payables, including put options, carried at amortised cost – approximates to the carrying amount
–Liquid investments – approximates to the carrying amount
–Cash and cash equivalents carried at amortised cost – approximates to the carrying amount
–Long-term loans – based on quoted market prices (a level 1 fair value measurement) in the case of European and US Medium Term Notes; approximates to the carrying amount in the case of other fixed rate borrowings and floating rate bank loans
–Short-term loans, overdrafts and commercial paper – approximates to the carrying amount because of the short maturity of these instruments.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
| | | | | | | | | | | | | | | | | | | | |
| | | 2023 | 2022 |
| Notes | | Carrying
value
£m | Fair
value
£m | Carrying
value
£m | Fair
value
£m |
| | | | | | |
| Financial assets measured at amortised cost: | | | | | | |
| Other non-current assets | b | | 9 | | 9 | | 21 | | 21 | |
| Trade and other receivables | b | | 3,829 | | 3,829 | | 3,789 | | 3,789 | |
| Liquid investments | | | 42 | | 42 | | 67 | | 67 | |
| Cash and cash equivalents | | | 1,942 | | 1,942 | | 1,324 | | 1,324 | |
| | | | | | |
Financial assets measured at fair value through other comprehensive
income (FVTOCI): | | | | | | |
| Other investments designated at FVTOCI | a | | 931 | | 931 | | 1,153 | | 1,153 | |
| Trade and other receivables | a,b | | 2,541 | | 2,541 | | 2,327 | | 2,327 | |
| | | | | | |
| | | | | | |
Financial assets mandatorily measured at fair value through profit or loss
(FVTPL): | | | | | | |
| Current equity investments and other investments | a | | 2,410 | | 2,410 | | 4,401 | | 4,401 | |
| Other non-current assets | a,b | | 18 | | 18 | | 13 | | 13 | |
| Trade and other receivables | a,b | | 23 | | 23 | | 50 | | 50 | |
Held for trading derivatives that are not in a designated and
effective hedging relationship | a,d,e | | 98 | | 98 | | 165 | | 165 | |
| Cash and cash equivalents | a | | 994 | | 994 | | 2,399 | | 2,399 | |
| | | | | | |
Derivatives designated and effective as hedging instruments (fair value
movements through other comprehensive income) | a,d,e | | 32 | | 32 | | 25 | | 25 | |
| Total financial assets | | | 12,869 | | 12,869 | | 15,734 | | 15,734 | |
| | | | | | |
| Financial liabilities measured at amortised cost: | | | | | | |
| Borrowings excluding obligations under lease liabilities: | | | | | | |
| – bonds in a designated hedging relationship | d | | (5,348) | | (5,233) | | (6,322) | | (6,035) | |
| – other bonds | | | (10,456) | | (10,762) | | (12,017) | | (11,930) | |
| – bank loans and overdrafts | | | (191) | | (191) | | (447) | | (447) | |
| – commercial paper in a designated hedging relationship | | | (148) | | (148) | | (443) | | (443) | |
| – other commercial paper | | | (667) | | (667) | | (748) | | (748) | |
| – other borrowings | | | (1) | | (1) | | (2) | | (2) | |
| Total borrowings excluding lease liabilities | f | | (16,811) | | (17,002) | | (19,979) | | (19,605) | |
| Trade and other payables | c | | (13,383) | | (13,383) | | (14,065) | | (14,065) | |
| Other provisions | c | | (199) | | (199) | | (63) | | (63) | |
| Other non-current liabilities | c | | (54) | | (54) | | (84) | | (84) | |
| | | | | | |
| | | | | | |
| Financial liabilities mandatorily measured at fair value through profit or loss (FVTPL): | | | | | | |
| Contingent consideration liabilities | a,c | | (6,662) | | (6,662) | | (7,068) | | (7,068) | |
Held for trading derivatives that are not in a designated and
effective hedging relationship | a,d,e | | (78) | | (78) | | (77) | | (77) | |
| | | | | | |
Derivatives designated and effective as hedging instruments (fair value
movements through other comprehensive income) | a,d,e | | (36) | | (36) | | (106) | | (106) | |
| Total financial liabilities excluding lease liabilities | | | (37,223) | | (37,414) | | (41,442) | | (41,068) | |
| | | | | | |
| Net financial assets and financial liabilities excluding lease liabilities | | | (24,354) | | (24,545) | | (25,708) | | (25,334) | |
The valuation methodology used to measure fair value in the above table is described and categorised on page 224.
Trade and other receivables, Other non-current assets, Trade and other payables, Other provisions, Contingent consideration liabilities and Other non-current liabilities are reconciled to the relevant Notes on pages 227 to 228.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
Fair value of investments in GSK shares
At 31 December 2023, the Employee Share Ownership Plan (ESOP) Trusts held GSK shares with a carrying value of £288 million (2022: £354 million) and a market value of £853 million (2022: £861 million) based on quoted market price. The shares are held by the ESOP Trusts to satisfy future exercises of options and awards under employee incentive schemes. In 2023, the carrying value, which is the lower of cost or expected proceeds, of these shares has been recognised as a deduction from other reserves. At 31 December 2023, GSK held Treasury shares at a cost of £3,447 million (2022: £3,797 million) which has been deducted from retained earnings.
(a) Financial instruments held at fair value
The following tables categorise the Group’s financial assets and liabilities held at fair value by the valuation methodology applied in determining their fair value. Where possible, quoted prices in active markets are used (Level 1). Where such prices are not available, the asset or liability is classified as Level 2, provided all significant inputs to the valuation model used are based on observable market data. If one or more of the significant inputs to the valuation model is not based on observable market data, the instrument is classified as Level 3. Other investments classified as Level 3 in the tables below comprise equity investments in unlisted entities with which the Group has entered into research collaborations and investments which provide access to biotechnology developments of potential interest.
| | | | | | | | | | | | | | |
At 31 December 2023 | Level 1
£m | Level 2
£m | Level 3 £m | Total
£m |
| Financial assets at fair value | | | | |
| Financial assets measured at fair value through other comprehensive income (FVTOCI): | | | | |
| Other investments designated at FVTOCI | 741 | | – | | 190 | | 931 | |
| Trade and other receivables | – | | 2,541 | | – | | 2,541 | |
| | | | |
| Financial assets mandatorily measured at fair value through profit or loss (FVTPL): | | | | |
| Current equity investments and other investments | 2,204 | | – | | 206 | | 2,410 | |
| Other non-current assets | – | | – | | 18 | | 18 | |
| Trade and other receivables | – | | 23 | | – | | 23 | |
| Held for trading derivatives that are not in a designated and effective hedging relationship | – | | 98 | | – | | 98 | |
| Cash and cash equivalents | 994 | | – | | – | | 994 | |
| Derivatives designated and effective as hedging instruments (fair value movements through OCI) | – | | 32 | | – | | 32 | |
| 3,939 | | 2,694 | | 414 | | 7,047 | |
| Financial liabilities at fair value | | | | |
| Financial liabilities mandatorily measured at fair value through profit or loss (FVTPL): | | | | |
| Contingent consideration liabilities | – | | – | (6,662) | (6,662) |
| Held for trading derivatives that are not in a designated and effective hedging relationship | – | | (78) | – | (78) |
| Derivatives designated and effective as hedging instruments (fair value movements through OCI) | – | | (36) | – | (36) |
| – | | (114) | (6,662) | (6,776) |
| | | | | | | | | | | | | | |
At 31 December 2022 | Level 1
£m | Level 2
£m | Level 3 £m | Total
£m |
| Financial assets at fair value | | | | |
| Financial assets measured at fair value through other comprehensive income (FVTOCI): | | | | |
| Other investments designated at FVTOCI | 823 | | – | | 330 | | 1,153 |
| Trade and other receivables | – | | 2,327 | | – | | 2,327 |
| | | | |
| Financial assets mandatorily measured at fair value through profit or loss (FVTPL): | | | | |
| Current equity investments and other investments | 4,087 | | – | | 314 | | 4,401 |
| Other non-current assets | – | | – | | 13 | | 13 |
| Trade and other receivables | – | | 50 | | – | | 50 |
| Held for trading derivatives that are not in a designated and effective hedging relationship | – | | 165 | | – | | 165 |
| Cash and cash equivalents | 2,399 | | – | | – | | 2,399 |
| Derivatives designated and effective as hedging instruments (fair value movements through OCI) | – | | 25 | | – | | 25 |
| 7,309 | | 2,567 | | 657 | | 10,533 |
| Financial liabilities at fair value | | | | |
| Financial liabilities mandatorily measured at fair value through profit or loss (FVTPL): | | | | |
| Contingent consideration liabilities | – | | – | | (7,068) | | (7,068) |
| Held for trading derivatives that are not in a designated and effective hedging relationship | – | | (77) | | – | | (77) |
| Derivatives designated and effective as hedging instruments (fair value movements through OCI) | – | | (106) | | – | | (106) |
| – | | (183) | | (7,068) | | (7,251) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
Movements in the year for financial instruments measured using Level 3 valuation methods are presented below:
| | | | | | | | |
| 2023
£m | 2022
£m |
| At 1 January | (6,411) | | (5,657) | |
| Exchange adjustments | – | | 46 | |
| Net losses recognised in the income statement | (863) | | (1,627) | |
| Net (losses)/ gains recognised in other comprehensive income | (142) | | 91 | |
| Contingent consideration related to business acquisitions in the period | – | | (482) | |
| Settlement of contingent consideration liabilities | 1,145 | | 1,137 | |
| Additions | 57 | | 97 | |
| Disposals and settlements | (25) | | (16) | |
| Transfers from Level 3 | (9) | | – | |
| At 31 December | (6,248) | | (6,411) | |
Of the total net losses of £863 million (2022: £1,627 million) attributable to Level 3 financial instruments which were recognised in the income statement, £857 million (2022: £1,623 million) were in respect of financial instruments which were held at the end of the year and were reported in Other operating income/expense. Charges of £934 million (2022: £1,431 million) arose from remeasurement of the contingent consideration payable for the acquisition of the former Shionogi-ViiV Healthcare joint venture. A remeasurement gain of £210 million (2022: £231 million loss) arose from remeasurement of the contingent consideration payable for the acquisition of the Novartis Vaccines business. The acquisition of Affinivax in 2022 resulted in the additon of £482 million of contingent consideration to Level 3 financial liabilities, with charges of £44 million (2022: £17 million) arising on the remeasurement of the contingent consideration liability for the year. There were transfers of £9 million out of Level 3 financial instruments in the year (2022: no transfers into or out of Level 3 financial instruments). Movements arising on the translation of overseas net assets for consolidation into the Group accounts are recorded as exchange adjustments. Net gains and losses include the impact of other exchange movements.
Financial liabilities measured using Level 3 valuation methods at 31 December included £5,718 million (2022: £5,890 million) in respect of contingent consideration payable for the acquisition in 2012 of the former Shionogi-ViiV Healthcare joint venture. This consideration is expected to be paid over a number of years and will vary in line with the future performance of specified products and movements in certain foreign currencies. A further £424 million (2022: £673 million) is in respect of contingent consideration for the acquisition in 2015 of the Novartis Vaccines business. This consideration is expected to be paid over a number of years and will vary in line with the future performance of specified products, the achievement of certain milestone targets and movements in certain foreign currencies. As a result of the acquisition of Affinivax in 2022, contingent consideration payable of £516 million (2022: £501 million) is recognised at 31 December. This consideration is expected to be paid over a number of years and will vary in line with the achievement of certain development milestones and movements in the USD/GBP exchange rate. Sensitivity analysis on these balances is provided in Note 33, ‘Contingent consideration liabilities’.
(b) Trade and other receivables and Other non-current assets in scope of IFRS 9
The following table reconciles financial instruments within Trade and other receivables and Other non-current assets which fall within the scope of IFRS 9 to the relevant balance sheet amounts. The financial assets are predominantly non-interest earning. Non-financial instruments include tax receivables, pension surplus balances and prepayments, which are outside the scope of IFRS 9.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
| | | 2023 | | | | 2022 |
| At
FVTPL
£m | At
FVTOCI
£m | Amortised
cost
£m | Financial instruments
£m | Non-financial instruments
£m | Total
£m | At
FVTPL
£m | At
FVTOCI
£m | Amortised
cost
£m | Financial instruments
£m | Non-
financial instruments
£m | Total
£m |
Trade and other
receivables (Note 26) | 23 | 2,541 | 3,829 | 6,393 | 992 | 7,385 | 50 | 2,327 | 3,789 | 6,166 | 887 | 7,053 |
Other non-current assets
(Note 24) | 18 | – | 9 | 27 | 1,557 | 1,584 | 13 | – | 21 | 34 | 1,160 | 1,194 |
| | | | | | | | | | | | |
| 41 | 2,541 | 3,838 | 6,420 | 2,549 | 8,969 | 63 | 2,327 | 3,810 | 6,200 | 2,047 | 8,247 |
Trade and other receivables include trade receivables of £5,905 million (2022: £5,452 million). The Group has portfolios in each of the three business models under IFRS 9: £23 million (2022: £50 million), measured at FVTPL, is held to sell the contractual cash flows as the receivables will be sold under a factoring arrangement, £2,541 million (2022: £2,327 million), measured at FVTOCI, is held to either collect or sell the contractual cash flows as the receivables may be sold under a factoring agreement, and £3,341 million (2022: £3,075 million), measured at amortised cost, is held to collect the contractual cash flows and there is no factoring agreement in place.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
(c) Trade and other payables, Other provisions, Contingent consideration liabilities and Other non-current liabilities in scope of IFRS 9
The following table reconciles financial instruments within Trade and other payables, Other provisions, Contingent consideration liabilities and Other non-current liabilities which fall within the scope of IFRS 9 to the relevant balance sheet amounts. The financial liabilities are predominantly non-interest bearing. Non-financial instruments include payments on account, tax and social security payables and provisions which do not arise from contractual obligations to deliver cash or another financial asset, which are outside the scope of IFRS 9.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2023 | | | 2022 |
| At FVTPL
£m | Amortised cost
£m | Financial instruments
£m | Non-
financial instruments
£m |
Total
£m
| At FVTPL
£m |
Amortised cost
£m
| Financial instruments
£m | Non-
financial instruments
£m |
Total
£m
|
Trade and other payables
(Note 29) | – | | (13,383) | | (13,383) | | (2,461) | | (15,844) | | – | | (14,065) | | (14,065) | | (2,198) | | (16,263) | |
Other provisions
(Note 32) | – | | (199) | | (199) | | (1,040) | | (1,239) | | – | | (63) | | (63) | | (1,121) | | (1,184) | |
Contingent consideration
liabilities (Note 33) | (6,662) | | – | | (6,662) | | – | | (6,662) | | (7,068) | | – | | (7,068) | | – | | (7,068) | |
| Other non-current liabilities (Note 34) | – | | (54) | | (54) | | (1,053) | | (1,107) | | – | | (84) | | (84) | | (815) | | (899) | |
| (6,662) | | (13,636) | | (20,298) | | (4,554) | | (24,852) | | (7,068) | | (14,212) | | (21,280) | | (4,134) | | (25,414) | |
(d) Derivative financial instruments and hedging programmes
Derivatives are only used for economic hedging purposes and not as speculative investments and are classified as ‘held for trading’, other than designated and effective hedging instruments, and are presented as current assets or liabilities if they are expected to be settled within 12 months after the end of the reporting period, otherwise they are classified as non-current. The Group has the following derivative financial instruments:
| | | | | | | | | | | | | | | | | |
| 2023
Fair value | | 2022
Fair value |
| Assets
£m | Liabilities
£m | | Assets
£m | Liabilities
£m |
| Current | | | | | |
Cash flow hedges – Foreign exchange contracts
(net principal amount – £175 million (2022: £167 million)) | – | | (2) | | | 5 | | – | |
Net investment hedges – Foreign exchange contracts
(net principal amount – £12,339 million (2022: £7,197 million)) | 32 | | (34) | | | 20 | | (106) | |
| Derivatives designated and effective as hedging instruments | 32 | | (36) | | | 25 | | (106) | |
| Current | | | | | |
Foreign exchange contracts
(net principal amount – £10,375 million (2022: £5,908 million)) | 98 | | (78) | | | 163 | | (76) | |
| Embedded and other derivatives | – | | – | | | 2 | | (1) | |
| Derivatives classified as held for trading | 98 | | (78) | | | 165 | | (77) | |
| Total derivative instruments | 130 | | (114) | | | 190 | | (183) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
Fair value hedges
At 31 December 2023 and 31 December 2022, the Group had no designated fair value hedges.
Net investment hedges
At 31 December 2023, certain foreign exchange contracts were designated as net investment hedges in respect of the foreign currency translation risk arising on consolidation of the Group’s net investment in its European (Euro), American (USD), Singaporean (SGD), Canadian (CAD) and Japanese (JPY) foreign operations as shown in the table below.
The carrying value of bonds on page 225 included £5,348 million (2022: £6,322 million) that were designated as hedging instruments in net investment hedges.
Cash flow hedges
During 2022 and 2023, the Group entered into forward foreign exchange contracts which have been designated as cash flow hedges. These were entered into to hedge the foreign exchange exposure arising on cash flows from Euro denominated coupon payments relating to notes issued under the Group’s European Medium Term Note programme, and to hedge foreign currency payments due on acquisitions, and collaboration or licensing arrangements.
The Group manages its cash flow interest rate risk by using floating-to-fixed interest rate swaps. In addition, the Group carries a balance in reserves that arose from pre-hedging fluctuations in long-term interest rates when pricing bonds issued in prior years and in the current year. The balance is reclassified to finance costs over the life of these bonds.
Foreign exchange risk
In the current year, the Group has designated certain foreign exchange forward contracts and swaps as cash flow and net investment hedges. Foreign exchange derivative financial assets and liabilities are presented in the line ‘Derivative financial instruments’ (either as assets or liabilities) on the Consolidated balance sheet. The following tables detail the foreign exchange forward contracts and swaps outstanding at the end of the reporting period, as well as information on the related hedged items.
Hedge effectiveness is determined at the inception of the hedge relationship, and through periodic prospective effectiveness assessments to ensure that an economic relationship exists between the hedged item and hedging instrument. The Group enters into hedge relationships where the critical terms of the hedging instrument match exactly with the terms of the hedged item, and so a qualitative assessment of effectiveness is performed. If changes in circumstances affect the terms of the hedged item such that the critical terms no longer match exactly with the critical terms of the hedging instrument, the Group uses the hypothetical derivative method to assess effectiveness.
The main source of hedge ineffectiveness in these hedging relationships is the effect of the counterparty and the Group’s own credit risk on the fair value of the foreign exchange forward contracts and swaps, which is not reflected in the fair value of the hedged item attributable to changes in foreign exchange rates. No other sources of ineffectiveness emerged from these hedging relationships. No ineffectiveness was recorded from cash flow hedges in 2023 (2022: £nil). No ineffectiveness was recorded from net investment hedges (2022: £nil).
| | | | | | | | | | | | | | | | | |
| | 2023 |
| Hedging instruments |
Average exchange rate
|
Foreign
currency
| Net Notional
value
£m | Carrying
value
£m | Periodic change in value for calculating hedge
ineffectiveness
£m |
| Cash flow hedges | | | | | |
| Foreign exchange contracts | | | | | |
| Buy foreign currency: | | | | | |
| Less than 3 months | 1.27 | | USD | 145 | | (1) | | (1) | |
| 3 to 6 months | – | | – | | – | | – | | – | |
| Over 6 months | 1.25 | | USD | 35 | | (1) | | (1) | |
| Sell foreign currency: | | | | | |
| Less than 3 months | 1.16 | | EUR | (5) | | – | | – | |
| | | 175 | | (2) | | (2) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
| | | | | | | | | | | | | | | | | |
|
| 2023 |
| Hedging instruments |
Average exchange rate
|
Foreign
currency
| Net notional
value
£m | Carrying
value
£m | Periodic
change in
value for
calculating
hedge
ineffectiveness
£m |
| Net investment hedges | | | | | |
| Foreign exchange contracts | | | | | |
| Sell foreign currency: | | | | | |
| Less than 3 months | 1.15 | | EUR | 9,146 | | (12) | | 126 | |
| 181.42 | | JPY | 133 | | (1) | | 28 | |
| 1.27 | | USD | 2,633 | | 8 | | 97 | |
| Over 6 months | 1.67 | | CAD | 260 | | 2 | | 10 | |
| 1.66 | | SGD | 167 | | 1 | | 7 | |
| Borrowings: | | | | | |
| Less than 3 months | | EUR | 148 | | (148) | | 12 | |
| 3 to 6 months | | – | | – | | – | | – | |
| Over 6 months | | JPY | 236 | | (235) | | (3) | |
| | EUR | 5,127 | | (5,113) | | 125 | |
| | | 17,850 | | (5,498) | | 402 | |
| | | | | | | | | | | |
| 2023 |
| Hedged items | Periodic change in value
for calculating hedge ineffectiveness
£m | Cumulative balance in cash flow hedge reserve/foreign currency translation reserve
for continuing hedges
£m | Balance in cash flow hedge reserve arising from hedging relationships for which hedge accounting is no longer applied
£m |
| Cash flow hedges | | | |
Variability in cash flows from a highly probable forecast
transaction | 2 | | (2) | | – | |
| Net investment hedges | | | |
| Net investment in foreign operations | (402) | | (725) | | – | |
| | | | | | | | | | | | | | | | | |
| 2022 |
| Hedging instruments |
Average exchange rate
|
Foreign
currency
| Net notional
value
£m | Carrying
value
£m | Periodic change in value for calculating hedge
ineffectiveness
£m |
| Cash flow hedges | | | | | |
| Foreign exchange contracts | | | | | |
| Buy foreign currency: | | | | | |
| Less than 3 months | 1.23 | | USD | 100 | | 2 | | 2 | |
| 3 to 6 months | 1.16 | | EUR | 50 | | 2 | | 2 | |
| Over 6 months | 1.15 | | EUR | 24 | | 1 | | 1 | |
| Sell foreign currency: | | | | | |
| Less than 3 months | 1.14 | | EUR | (7) | | – | | – | |
| | | 167 | | 5 | | 5 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
| | | | | | | | | | | | | | | | | |
| | 2022 |
| Hedging instruments |
Average exchange rate
|
Foreign
currency
| Net notional
value
£m | Carrying
value
£m | Periodic change in value for calculating hedge
ineffectiveness
£m |
| Net investment hedges | | | | | |
| Foreign exchange contracts | | | | | |
| Sell foreign currency: | | | | | |
| Less than 3 months | 1.14 | | EUR | 6,559 | | (103) | | (317) | |
| 160.90 | | JPY | 194 | | (3) | | (9) | |
| 3 to 6 months | – | | – | | – | | – | | – | |
| Over 6 months | 1.57 | | CAD | 270 | | 18 | | 15 | |
| 1.59 | | SGD | 174 | | 2 | | 1 | |
| Borrowings: | | | | | |
| Less than 3 months | | EUR | 293 | | (293) | | (4) | |
| 3 to 6 months | | EUR | 150 | | (150) | | (3) | |
| Over 6 months | | EUR | 6,341 | | (6,322) | | (300) | |
| | | 13,981 | | (6,851) | | (617) | |
| | | | | | | | | | | |
| 2022 |
| Hedged items | Periodic change in value
for calculating hedge ineffectiveness
£m | Cumulative balance in cash flow hedge reserve/foreign currency translation reserve
for continuing hedges
£m | Balance in cash flow hedge reserve arising from hedging relationships for which hedge accounting is no longer applied
£m |
| Cash flow hedges | | | |
Variability in cash flows from a highly probable forecast
transaction | (2) | | 2 | | – | |
Variability in cash flows from foreign exchange exposure
arising on Euro denominated coupon payments relating to
debt issued | (3) | | 2 | | – | |
| Net investment hedges | | | | | – | |
| Net investment in foreign operations | 617 | | (1,120) | | – | |
£nil (2022: £3 million) of balances in the cash flow hedge reserve arise from hedging relationships for which hedge accounting is no longer applied.
The following table details the effectiveness of the hedging relationships and the amounts reclassified from the hedging reserve to profit or loss:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | 2023 |
| | | Amount reclassified to profit or loss | | Amount reclassified to balance sheet |
| Hedging
gains/(losses) recognised in reserves
£m | Amount
of hedge ineffectiveness
recognised in profit or loss
£m | Line item
in profit or
loss in
which hedge ineffectiveness is included | Hedged
future cash flows
no longer expected to occur
£m | Due to
hedged item affecting
profit or loss
£m |
Line item in profit or loss
in which reclassification adjustment
is included
| | Due to hedged item affecting balance sheet
£m | Line item
in balance
sheet in which reclassification adjustment is included |
| Cash flow hedges | | | | | | | | | |
| Variability in cash flows from a highly probable forecast transaction | (41) | | – | | Finance income or expense | – | | – | | Finance income or expense | | 37 | | Intangible assets |
| | | | | | | | | |
| Variability in cash flows from foreign exchange exposure arising on Euro denominated coupon payments relating to debt issued | (1) | | – | | Finance income or expense | – | | – | | Finance income or expense | | – | | – | |
| Net investment hedges | | | | | | | | | |
| Net investment in foreign operations | 402 | | – | | Finance income or expense | – | | 7 | | Other income or expense | | – | | – | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | 2022 |
| | | Amount reclassified to profit or loss | | Amount reclassified to balance sheet |
| Hedging
gains/(losses) recognised in reserves
£m | Amount
of hedge ineffectiveness
recognised in profit or loss
£m | Line item
in profit or
loss in
which hedge ineffectiveness is included | Hedged
future cash flows
no longer expected to occur
£m | Due to
hedged item affecting
profit or loss
£m | Line item in
profit or loss
in which
reclassification
adjustment
is included | | Due to hedged item affecting
balance sheet
£m | Line item
in balance
sheet in which reclassification adjustment is included |
| Cash flow hedges | | | | | | | | | |
Variability in cash flows from
a highly probable forecast transaction | (5) | | – | | Finance income or expense | – | | – | | – | | | 8 | | Intangible assets |
| | | | | | | | | |
| Variability in cash flows from foreign exchange exposure arising on Euro denominated coupon payments relating to debt issued | 4 | | – | | Finance income or expense | – | | (2) | | Finance income or expense | | – | | – | |
| Net investment hedges | | | | | | | | | |
| Net investment in foreign operations | (617) | | – | | Finance income or expense | – | | 194 | | Discontinued Operations (1) | | – | | – | |
(1)Reclassified to the Consolidated income statement on the demerger of the Consumer Healthcare business.
Interest rate risk
The Group manages its cash flow interest rate risk by using floating-to-fixed interest rate swaps, where at quarterly intervals the difference between fixed contract rates and floating rate interest amounts calculated by reference to the agreed notional principal amounts are exchanged.
There are none of these swaps outstanding at 31 December 2023 or at 31 December 2022.
The only impact on these financial statements of interest rate swaps is where the interest rate risk on an element of future debt issuance has been managed by entering into forward starting interest rate swaps, effectively to lock in the interest rates on the debt in advance. These were closed out at the time of issuing the debt, and the resulting gain or loss held in the Cash flow hedge reserve and reclassified to income statement as the interest payments on the debt impacted the income statement.
Forward starting interest rate swaps
Forward starting interest rate contracts, exchanging floating interest for fixed interest, were designated as cash flow hedges to hedge the interest variability of the interest cash flows associated with future fixed rate debt.
Interest rate swaps
Interest rate swap contract assets and liabilities are presented (when applicable) in the line ‘Derivative financial instruments’ (either as assets or liabilities) on the Consolidated balance sheet.
£21 million (2022: £24 million) of balances in the cash flow hedge reserve arise from hedge relationships for which hedge accounting is no longer applied.
The following table details the effectiveness of the hedging relationships and the amounts reclassified from the hedging reserve to profit or loss:
| | | | | | | | | | | | | | | | | | | | |
| | | 2023 |
| | | | Amount reclassified to profit or loss |
| Hedging
gains/(losses) recognised in reserves
£m | Amount
of hedge ineffectiveness
recognised
in profit or loss
£m | Line item
in profit or
loss in
which hedge ineffectiveness is included
| Due to
hedged future cash flows no longer expected to occur
£m | Due to
hedged item affecting
profit or loss
£m | Line item
in profit or loss in which reclassification adjustment is included
|
| Cash flow hedges | | | | | | |
Pre-hedging of long-term interest rates: Matured in the past | – | | – | | Finance income or expense | – | | 4 | | Finance income or expense |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
| | | | | | | | | | | | | | | | | | | | |
| | | 2022 |
| | | | Amount reclassified to profit or loss |
| Hedging
gains/(losses) recognised in reserves
£m | Amount
of hedge ineffectiveness recognised in profit or loss
£m | Line item
in profit or
loss in
which hedge ineffectiveness is included
| Due to
hedged future cash flows no longer expected to occur
£m | Due to
hedged item affecting
profit or loss
£m | Line item
in profit or loss in which reclassification adjustment is included
|
| Cash flow hedges | | | | | | |
| Pre-hedging of long-term interest rates: | | | | | | |
| Matured in the past | (23) | | – | | Finance income or expense | – | | 3 | | Finance income or expense |
(e) Offsetting of financial assets and liabilities
Financial assets and liabilities are offset and the net amount reported in the balance sheet where there is a legally enforceable right to offset the recognised amounts, and there is an intention to settle on a net basis or realise the asset and settle the liability simultaneously. There are also arrangements that do not meet the criteria for offsetting but still allow for the related amounts to be offset in certain circumstances, such as bankruptcy or the termination of a contract.
The following tables set out the financial assets and liabilities that are offset, or subject to enforceable master netting arrangements and other similar agreements but not offset, as at 31 December 2023 and 31 December 2022. The column ‘Net amount’ shows the impact on the Group’s balance sheet if all offset rights were exercised.
| | | | | | | | | | | | | | | | | |
31 December 2023 | Gross
financial
assets/
(liabilities)
£m | Gross
financial
(liabilities)/
assets set off
£m | Net financial assets/
(liabilities) per balance sheet
£m | Related amounts not
set off in the balance sheet
£m | Net
£m |
| Financial assets | | | | | |
| Trade and other receivables | 6,394 | | (1) | | 6,393 | | – | | 6,393 | |
| Derivative financial instruments | 130 | | – | | 130 | | (108) | | 22 | |
| | | | | |
| Financial liabilities | | | | | |
| Trade and other payables | (13,384) | | 1 | | (13,383) | | – | | (13,383) | |
| Derivative financial instruments | (114) | | – | | (114) | | 108 | | (6) | |
| | | | | | | | | | | | | | | | | |
31 December 2022 | Gross
financial
assets/
(liabilities)
£m | Gross
Financial
(liabilities)/
assets offset
£m | Net financial assets/
(liabilities)
£m | Related amounts not
offset
£m | Net
balance
£m |
| Financial assets | | | | | |
| Trade and other receivables | 6,166 | | – | | 6,166 | | – | | 6,166 | |
| Derivative financial instruments | 190 | | – | | 190 | | (163) | | 27 | |
| | | | | |
| Financial liabilities | | | | | |
| Trade and other payables | (14,065) | | – | | (14,065) | | – | | (14,065) | |
| Derivative financial instruments | (183) | | – | | (183) | | 163 | | (20) | |
Amounts which do not meet the criteria for offsetting on the balance sheet but could be settled net in certain circumstances principally relate to derivative transactions under ISDA (International Swaps and Derivatives Association) agreements where each party has the option to settle amounts on a net basis in the event of default of the other party. As there is presently not a legally enforceable right of offset, these amounts have not been offset in the balance sheet, but have been presented separately in the table above.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
(f) Debt interest rate repricing table
The following table sets out the exposure of the Group to interest rates on debt, including commercial paper. The maturity analysis of fixed rate debt is stated by contractual maturity and of floating rate debt by interest rate repricing dates. For the purpose of this table, debt is defined as all classes of borrowings other than lease liabilities.
| | | | | | | | |
| 2023 | 2022 |
| Total
debt
£m | Total
£m |
| Floating and fixed rate debt less than one year | (2,657) | | (3,785) | |
| Between one and two years | (1,434) | | (1,714) | |
| Between two and three years | (1,475) | | (1,490) | |
| Between three and four years | (740) | | (1,505) | |
| Between four and five years | (2,350) | | (748) | |
| Between five and ten years | (3,031) | | (4,736) | |
| Greater than ten years | (5,124) | | (6,001) | |
| Total | (16,811) | | (19,979) | |
| Original issuance profile: | | |
| Fixed rate interest | (15,847) | | (18,355) | |
| Floating rate interest | (964) | | (1,624) | |
| (16,811) | | (19,979) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
(g) Sensitivity analysis
The tables below illustrate the estimated impact on the income statement and equity as a result of hypothetical market movements in foreign exchange and interest rates in relation to the Group’s financial instruments. The range of variables chosen for the sensitivity analysis reflects management’s view of changes which are reasonably possible over a one-year period.
Foreign exchange sensitivity
The Group operates internationally and is primarily exposed to foreign exchange risk in relation to Sterling against movements in US Dollar, Euro and Japanese Yen. Foreign exchange risk arises from the translation of financial assets and liabilities which are not in the functional currency of the entity that holds them. Based on the Group’s net financial assets and liabilities as at 31 December, a weakening and strengthening of Sterling against these currencies, with all other variables held constant, is illustrated in the tables below. The tables exclude financial instruments that expose the Group to foreign exchange risk where this risk is fully hedged with another financial instrument.
| | | | | | | | |
| 2023 | 2022 |
| Income statement impact of non-functional currency foreign exchange exposures | Increase/(decrease) in
income
£m | Increase/(decrease) in
income
£m |
| 10 cent appreciation of the US Dollar | 61 | | 99 | |
| 15 cent appreciation of the US Dollar | 97 | | 155 | |
| 10 cent appreciation of the Euro | (4) | | (7) | |
| 15 cent appreciation of the Euro | (7) | | (12) | |
| 10 yen appreciation of the Yen | – | | – | |
| 15 yen appreciation of the Yen | – | | (1) | |
| | | | | | | | |
| 2023 | 2022 |
| Income statement impact of non-functional currency foreign exchange exposures | Increase/(decrease) in
income
£m | Increase/(decrease) in
income
£m |
| 10 cent depreciation of the US Dollar | (52) | | (84) | |
| 15 cent depreciation of the US Dollar | (76) | | (121) | |
| 10 cent depreciation of the Euro | 4 | | 6 | |
| 15 cent depreciation of the Euro | 5 | | 9 | |
| 10 yen depreciation of the Yen | – | | – | |
| 15 yen depreciation of the Yen | – | | – | |
The equity impact, shown below, for foreign exchange sensitivity relates to derivative and non-derivative financial instruments hedging the Group’s net investments in its European (Euro) foreign operations and cash flow hedges of its foreign exchange exposure arising on Euro denominated coupon payments relating to notes issued under the Group’s European Medium Term
Note programme.
| | | | | | | | |
| 2023 | 2022 |
| Equity impact of non-functional currency foreign exchange exposures | Increase/(decrease)
in equity
£m | Increase/(decrease)
in equity
£m |
| 10 cent appreciation of the US Dollar | (209) | | – | |
| 15 cent appreciation of the US Dollar | (327) | | – | |
| 10 cent appreciation of the Euro | (1,372) | | (1,290) | |
| 15 cent appreciation in Euro | (2,160) | | (2,034) | |
| | | | | | | | |
| 2023 | 2022 |
| Equity impact of non-functional currency foreign exchange exposures | Increase/(decrease)
in equity
£m | Increase/(decrease)
in equity
£m |
| 10 cent depreciation of the US Dollar | 178 | | – | |
| 15 cent depreciation of the US Dollar | 258 | | – | |
| 10 cent depreciation of the Euro | 1,152 | | 1,080 | |
| 15 cent depreciation of the Euro | 1,662 | | 1,557 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
The tables below present the Group’s sensitivity to a weakening and strengthening of Sterling against the relevant currency based on the composition of net debt as shown in Note 30, 'Net debt', adjusted for the effects of foreign exchange derivatives that are not part of net debt but affect future foreign currency cash flows.
| | | | | | | | |
| 2023 | 2022 |
| Impact of foreign exchange movements on net debt | (Increase)/decrease
in net debt
£m | (Increase)/decrease
in net debt
£m |
| 10 cent appreciation of the US Dollar | (622) | | (999) | |
| 15 cent appreciation of the US Dollar | (974) | | (1,570) | |
| 10 cent appreciation of the Euro | 386 | | 11 | |
| 15 cent appreciation of the Euro | 609 | | 17 | |
| 10 yen appreciation of the Yen | (5) | | 13 | |
| 15 yen appreciation of the Yen | (7) | | 20 | |
| | | | | | | | |
| 2023 | 2022 |
| Impact of foreign exchange movements on net debt | (Increase)/decrease
in net debt
£m | (Increase)/decrease
in net debt
£m |
| 10 cent depreciation of the US Dollar | 531 | | 846 | |
| 15 cent depreciation of the US Dollar | 769 | | 1,222 | |
| 10 cent depreciation of the Euro | (325) | | (9) | |
| 15 cent depreciation of the Euro | (468) | | (13) | |
| 10 yen depreciation of the Yen | 4 | | (12) | |
| 15 yen depreciation of the Yen | 6 | | (17) | |
Interest rate sensitivity
The Group is exposed to interest rate risk on its outstanding borrowings and investments where any changes in interest rates will affect future cash flows or the fair values of financial instruments.
The majority of debt is issued at fixed interest rates and changes in the floating rates of interest do not significantly affect the Group’s net interest charge, although the majority of cash and liquid investments earn floating rates of interest.
The table below hypothetically shows the Group’s sensitivity to changes in interest rates in relation to Sterling, US Dollar and Euro floating rate financial assets and liabilities. A 1% (100 basis points) or 1.5% (150 basis points) movement in EUR, USD or Sterling interest rates is not deemed to have a material effect on equity. A 1% (100 basis points) or 1.5% (150 basis points) decrease in EUR, USD or Sterling interest rates would have an equal and opposite impact to that shown below.
| | | | | | | | |
| 2023 | 2022 |
| Income statement impact of interest rate movements | Increase/(decrease)
in income
£m | Increase/(decrease)
in income
£m |
| 1% (100 basis points) increase in Sterling interest rates | 41 | | 36 | |
| 1.5% (150 basis points) increase in Sterling interest rates | 62 | | 55 | |
| 1% (100 basis points) increase in US Dollar interest rates | (34) | | (34) | |
| 1.5% (150 basis points) increase in US Dollar interest rates | (51) | | (51) | |
| 1% (100 basis points) increase in Euro interest rates | (9) | | (13) | |
| 1.5% (150 basis points) increase in Euro interest rates | (13) | | (19) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 44. Financial instruments and related disclosures continued | |
(h) Contractual cash flows for non-derivative financial liabilities and derivative instruments
The following tables provide an analysis of the anticipated contractual cash flows including interest payable for the Group’s non-derivative financial liabilities on an undiscounted basis. For the purpose of this table, debt is defined as all classes of borrowings except for lease liabilities. Interest is calculated based on debt held at 31 December without taking account of future issuance. Floating rate interest is estimated using the prevailing interest rate at the balance sheet date. Cash flows in foreign currencies are translated using spot rates at 31 December.
| | | | | | | | | | | | | | | | | | | | | | | |
At 31 December 2023 | Debt
£m | Interest
on debt
£m | Lease
liabilities
£m | Finance
charge
on lease
liabilities
£m | Trade payables
and other
liabilities not
in net debt
£m | | Total
£m |
| Due in less than one year | (2,660) | | (547) | | (156) | | (41) | | (14,526) | | – | | (17,930) | |
| Between one and two years | (1,436) | | (507) | | (214) | | (36) | | (1,469) | | – | | (3,662) | |
| Between two and three years | (1,477) | | (466) | | (134) | | (31) | | (1,150) | | – | | (3,258) | |
| Between three and four years | (742) | | (449) | | (114) | | (27) | | (1,406) | | – | | (2,738) | |
| Between four and five years | (2,359) | | (399) | | (88) | | (23) | | (940) | | – | | (3,809) | |
| Between five and ten years | (3,054) | | (1,611) | | (325) | | (75) | | (2,037) | | – | | (7,102) | |
| Greater than ten years | (5,172) | | (1,467) | | (176) | | (21) | | (1,043) | | – | | (7,879) | |
| Gross contractual cash flows | (16,900) | | (5,446) | | (1,207) | | (254) | | (22,571) | | – | | (46,378) | |
| | | | | | | | | | | | | | | | | | | | | | | |
At 31 December 2022 | Debt
£m | Interest
on debt
£m | Lease
liabilities
£m | Finance
charge
on lease
liabilities
£m | Trade payables
and other
liabilities not
in net debt
£m | | Total
£m |
| Due in less than one year | (3,786) | | (594) | | (167) | | (25) | | (15,362) | | | (19,934) | |
| Between one and two years | (1,717) | | (570) | | (201) | | (22) | | (1,097) | | | (3,607) | |
| Between two and three years | (1,496) | | (531) | | (127) | | (19) | | (1,034) | | | (3,207) | |
| Between three and four years | (1,508) | | (489) | | (97) | | (15) | | (1,277) | | | (3,386) | |
| Between four and five years | (751) | | (472) | | (80) | | (13) | | (1,008) | | | (2,324) | |
| Between five and ten years | (4,765) | | (1,810) | | (201) | | (41) | | (2,641) | | | (9,458) | |
| Greater than ten years | (6,063) | | (1,856) | | (135) | | (11) | | (1,134) | | | (9,199) | |
| Gross contractual cash flows | (20,086) | | (6,322) | | (1,008) | | (146) | | (23,553) | | | (51,115) | |
The table below provides an analysis of the anticipated contractual cash flows for the Group’s derivative instruments excluding equity options which do not give rise to cash flows, and other embedded derivatives, which are not material, using undiscounted cash flows. Cash flows in foreign currencies are translated using spot rates at 31 December. The gross cash flows of foreign exchange contracts are presented for the purpose of this table although, in practice, the Group uses standard settlement arrangements to reduce its liquidity requirements on these instruments.
| | | | | | | | | | | | | | | | | | | | | | | |
| 2023 | | 2022 |
| Gross cash inflows | | Gross cash outflows | | Gross cash inflows | | Gross cash outflows |
| Foreign exchange forward contracts and swaps
£m | | Foreign exchange forward contracts and swaps
£m | | Foreign exchange forward contracts and swaps
£m | | Foreign exchange forward contracts and swaps
£m |
| Less than one year | 31,961 | | | (31,944) | | | 24,418 | | | (24,410) | |
| Gross contractual cash flows | 31,961 | | | (31,944) | | | 24,418 | | | (24,410) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 45. Employee share schemes |
GSK operates several employee share schemes, including the Share Value Plan, whereby awards are granted to employees to acquire shares or ADS in GSK plc at no cost after a three-year vesting period and the Performance Share Plan, whereby awards are granted to employees to acquire shares or ADS in GSK plc at no cost, subject to the achievement by the Group of specified performance targets. The granting of these restricted share awards has replaced the granting of options to employees as the cost of the schemes more readily equates to the potential gain to be made by the employee. The Group also operates savings related share option schemes, whereby options are granted to employees to acquire shares in GSK plc at a discounted price.
Grants of restricted share awards are normally exercisable at the end of the three-year vesting or performance period. Awards are normally granted to employees to acquire shares or ADS in GSK plc but in some circumstances may be settled in cash. Grants under savings-related share option schemes are normally exercisable after three years’ saving. In accordance with UK practice, the majority of options under the savings-related share option schemes are granted at a price 20% below the market price ruling at the date of grant. Options under historical share option schemes were granted at the market price ruling at the date of grant.
The total charge for share-based incentive plans in 2023 was £321 million (2022: £314 million; 2021: £345 million). Of this amount, £244 million (2022: £243 million; 2021: £258 million) arose from the Share Value Plan. See Note 9, ‘Employee costs’ for further details.
GSK share award schemes
Share Value Plan
Under the Share Value Plan, share awards are granted to certain employees at no cost. The awards vest after two and a half to three years and there are no performance criteria attached. The fair value of these awards is determined based on the closing share price on the day of grant, after deducting the expected future dividend yield of 3.8% (2022: 3.2%; 2021: 3.8%) over the duration of the award.
| | | | | | | | | | | | | | |
| Number of shares and ADS issuable | Shares
Number (000) | Weighted
fair value | ADS
Number (000) | Weighted
fair value |
At 1 January 2021 | 28,874 | | | 16,116 | | |
| Awards granted | 11,220 | | £13.28 | | 6,358 | | $36.68 | |
| Awards exercised | (10,074) | | | (5,240) | | |
| Awards cancelled | (1,776) | | | (1,705) | | |
At 31 December 2021 | 28,244 | | | 15,529 | | |
| Awards granted | 10,987 | | £13.00 | | 6,133 | | $30.64 | |
| Awards exercised | (9,538) | | | (4,919) | | |
| Awards cancelled | (1,718) | | | (1,314) | | |
At 31 December 2022 | 27,975 | | | 15,429 | | |
| Awards granted | 11,548 | | £12.79 | | 6,449 | | $31.65 | |
| Awards exercised | (8,599) | | | (4,856) | | |
| Awards cancelled | (1,144) | | | (797) | | |
At 31 December 2023 | 29,780 | | | 16,225 | | |
Performance Share Plan
Under the Performance Share Plan, share awards are granted to Directors and senior executives at no cost. The percentage of each award that vests is based upon the performance of the Group over a defined measurement period with dividends reinvested during the same period. For awards granted from 2020, the performance conditions are based on four measures over a three-year performance period. These are adjusted free cash flow (30%), TSR (30%), R&D new product performance (20%) and pipeline progress (20%). For awards granted from 2022, the performance conditions are based on five measures over a three-year performance period. These are TSR (30%), pipeline progress (20%), profit measure (20%), sale measure (20%) and ESG environment (10%).
The fair value of the awards is determined based on the closing share price on the day of grant. For TSR performance elements, this is adjusted by the likelihood of that condition being met, as assessed at the time of grant.
During 2023, awards were made of 4.3 million shares at a weighted fair value of £12.40 and 1.0 million ADS at a weighted fair value of $29.96. At 31 December 2023, there were outstanding awards over 13.3 million shares and 2.7 million ADS.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 45. Employee share schemes continued | |
Share options and savings-related options
For the purposes of valuing savings-related options to arrive at the share-based payment charge, a Black-Scholes option pricing model has been used. The assumptions used in the model are as follows:
| | | | | | | | | | | |
| 2023 Grant | 2022 Grant | 2021 Grant |
| Risk-free interest rate | 4.57% | 3.37% | 0.74% |
| Dividend yield | 4.0% | 3.3% | 3.8% |
| Volatility | 34% | 36% | 27% |
| Expected life | 3 years | 3 years | 3 years |
Savings-related options grant price (including 20% discount) | £11.20 | £11.39 | £12.07 |
| | | | | | | | |
| Options outstanding for the Share Save Plan | Savings-related
share option schemes |
|
Number
000
| Weighted
exercise
price |
At 31 December 2023 | 6,196 | £11.13 |
| Range of exercise prices on options outstanding at year end | £10.34 | — £14.15 |
| Weighted average market price on exercise during year | | £14.32 |
| Weighted average remaining contractual life | | 1.9 years |
Options over 1.9 million shares were granted during the year under the savings-related share option scheme at a weighted average fair value of £4.08. At 31 December 2023, 4.2 million of the savings-related share options were not exercisable.
There has been no change in the effective exercise price of any outstanding options during the year.
Employee Share Ownership Plan Trusts
The Group sponsors Employee Share Ownership Plan (ESOP) Trusts to acquire and hold shares in GSK plc to satisfy awards made under employee incentive plans and options granted under employee share option schemes. The trustees of the ESOP Trusts purchase shares with finance provided by the Group by way of loans or contributions. The costs of running the ESOP Trusts are charged to the income statement. Shares held by the ESOP Trusts are deducted from other reserves and amortised down to the value of proceeds, if any, receivable from employees on exercise by a transfer to retained earnings. The trustees have waived their rights to dividends on the shares held by the ESOP Trusts.
At 31 December 2023, 58,817,197 shares were held in the ESOP Trusts, out of which 58,493,518 were held for the future exercise of share options and share awards and 323,679 shares were held for the Executive Supplemental Savings Plan.
| | | | | | | | |
| Shares held for share award schemes | 2023 | 2022 |
| Number of shares (000) | 58,817 | | 59,814 | |
| | |
| £m | £m |
| Nominal value | 18 | | 19 | |
| Carrying value | 288 | | 353 | |
| Market value | 853 | | 861 | |
| | |
| Shares held for share option schemes | 2023 | 2022 |
| Number of shares (000) | – | | 65 | |
| | |
| £m | £m |
| Nominal value | – | | – | |
| Carrying value | – | | 1 | |
| Market value | – | | 1 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
| | |
| 46. Principal Group companies |
The following represent the principal subsidiaries and their countries of incorporation of the Group at 31 December 2023. The equity share capital of these entities is shown in the percentage columns. All companies are incorporated in their principal country of operation except where stated.
| | | | | |
| England | % |
| Glaxo Group Limited | 100 | |
| Glaxo Operations UK Limited | 100 | |
| Glaxo Wellcome UK Limited | 100 | |
| GlaxoSmithKline Capital plc | 100 | |
| GlaxoSmithKline Export Limited | 100 | |
| GlaxoSmithKline Finance plc | 100 | |
GlaxoSmithKline Holdings Limited(a) | 100 | |
| GlaxoSmithKline IHC Limited | 100 | |
| GlaxoSmithKline Intellectual Property (No.2) Limited | 100 | |
| GlaxoSmithKline Intellectual Property (No.3) Limited | 100 | |
| GlaxoSmithKline Intellectual Property (No.4) Limited | 100 | |
| GlaxoSmithKline Intellectual Property Development Limited | 100 | |
| GlaxoSmithKline Intellectual Property Limited | 100 | |
| GlaxoSmithKline Research & Development Limited | 100 | |
GlaxoSmithKline Services Unlimited(a) | 100 | |
| GlaxoSmithKline UK Limited | 100 | |
| GlaxoSmithKline US Trading Limited | 100 | |
| Setfirst Limited | 100 | |
| SmithKline Beecham Limited | 100 | |
| ViiV Healthcare Finance Limited | 78.3 | |
| ViiV Healthcare UK (No.3) Limited | 78.3 | |
| Viiv Healthcare UK Limited | 78.3 | |
| | | | | |
| Europe | % |
| GlaxoSmithKline AG (Switzerland) | 100 | |
| Glaxo Wellcome Production S.A.S (France) | 100 | |
| GlaxoSmithKline B.V. (Netherlands) | 100 | |
| GlaxoSmithKline Biologicals SA (Belgium) | 100 | |
| GlaxoSmithKline GmbH & Co. KG (Germany) | 100 | |
| GlaxoSmithKline Manufacturing SpA (Italy) | 100 | |
| GlaxoSmithKline Pharma GmbH (Austria) | 100 | |
| GlaxoSmithKline Pharmaceuticals SA (Belgium) | 100 | |
| GlaxoSmithKline S.A. (Spain) | 100 | |
| GlaxoSmithKline S.p.A. (Italy) | 100 | |
| GlaxoSmithKline Single Member A.E.B.E. (Greece) | 100 | |
GlaxoSmithKline Trading Services Limited (Republic of Ireland)(b) | 100 | |
GSK Capital B.V. (Netherlands)(b) | 100 | |
| GSK Services Sp z o.o. (Poland) | 100 | |
| GSK Vaccines GmbH (Germany) | 100 | |
| GSK Vaccines S.r.l. (Italy) | 100 | |
| JSC GlaxoSmithKline Trading (Russia) | 100 | |
| Laboratoire GlaxoSmithKline (France) | 100 | |
| Laboratorios ViiV Healthcare, S.L. (Spain) | 78.3 | |
| ViiV Healthcare GmbH (Germany) | 78.3 | |
| ViiV Healthcare S.r.l. (Italy) | 78.3 | |
| ViiV Healthcare SAS (France) | 78.3 | |
| | | | | |
| Scotland | % |
| GSK (No.1) Scottish Limited Partnership | 100 | |
| | | | | |
| US | % |
| Affinivax, Inc | 100 | |
| Corixa Corporation | 100 | |
| GlaxoSmithKline Capital Inc. | 100 | |
| GlaxoSmithKline Holdings (Americas) Inc. | 100 | |
| GlaxoSmithKline LLC | 100 | |
| GSK Equity Investments, Limited | 100 | |
| Human Genome Sciences, Inc | 100 | |
| Stiefel Laboratories, Inc | 100 | |
| Tesaro, Inc. | 100 | |
| ViiV Healthcare Company | 78.3 | |
| | | | | |
| Others | % |
| Glaxo Saudi Arabia Limited (Saudi Arabia) | 100 | |
| Glaxo Wellcome Manufacturing Pte Ltd (Singapore) | 100 | |
| GlaxoSmithKline (Thailand) Limited (Thailand) | 100 | |
| GlaxoSmithKline Australia Pty Ltd (Australia) | 100 | |
| GlaxoSmithKline Brasil Limitada (Brazil) | 100 | |
| GlaxoSmithKline Far East B.V. (Taiwan) | 100 | |
| GlaxoSmithKline Ilaclari Sanayi ve Ticaret A.S. (Turkey) | 100 | |
| GlaxoSmithKline Inc. (Canada) | 100 | |
| GlaxoSmithKline K.K. (Japan) | 100 | |
| GlaxoSmithKline Korea Limited (Republic of Korea) | 100 | |
| GlaxoSmithKline Limited (Hong Kong) | 100 | |
| GlaxoSmithKline Mexico S.A. de C.V. (Mexico) | 100 | |
| GlaxoSmithKline Pakistan Limited (Pakistan) | 82.6 | |
| GlaxoSmithKline Pharmaceuticals Limited (India) | 75 | |
| GSK Enterprise Management Co, Ltd (China) | 100 | |
| GSK Pharma Vietnam Company Limited (Vietnam) | 100 | |
| ID Biomedical Corporation of Quebec (Canada) | 100 | |
| ViiV Healthcare K.K (Japan) | 78.3 | |
| ViiV Healthcare ULC (Canada) | 78.3 | |
| |
(a)Directly held wholly-owned subsidiary of GSK plc.
(b) Tax resident in UK.
The subsidiaries and associates listed above principally affect the figures in the Group’s financial statements. Each of GlaxoSmithKline Capital Inc., GlaxoSmithKline Capital plc, GlaxoSmithKline Finance plc, GSK Capital BV and GlaxoSmithKline LLC, is a wholly-owned finance subsidiary of the company, and the Company has fully and unconditionally guaranteed the securities issued by each of GlaxoSmithKline Capital Inc., GlaxoSmithKline Capital plc, GlaxoSmithKline Finance plc, GSK Capital BV and GlaxoSmithKline LLC.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| |
The Group is involved in significant legal and administrative proceedings, principally product liability, intellectual property, tax, anti-trust, consumer fraud and governmental investigations. The most significant of these matters, other than tax matters, are described below. The Group makes provision for these proceedings on a regular basis as summarised in Note 2, ‘Accounting principles and policies’ and Note 32, ‘Other provisions’. Note 2 also describes when disclosure is made of proceedings for which there is no provision. Legal expenses incurred and provisions related to legal claims are charged to selling, general and administration costs. The Group does not believe that information about the amount sought by plaintiffs, if that is known, would be meaningful with respect to those legal proceedings. This is due to a number of factors, including, but not limited to, the stage of proceedings, the entitlement of parties to appeal a decision and clarity as to theories of liability, damages and governing law.
At 31 December 2023, the Group’s aggregate provision for legal and other disputes (not including tax matters described in Note 14, ‘Taxation’) was £267 million. There can be no assurance that any losses that result from the outcome of any legal proceedings will not materially exceed the amount of the provisions reported in the Group’s financial statements. If this were to happen, it could have a material adverse impact on the results of operations of the Group in the reporting period in which the judgements are incurred or the settlements entered into.
Intellectual property
Intellectual property claims include challenges to the validity and enforceability of the Group’s patents on various products or processes as well as assertions of non-infringement of those patents. A loss in such cases could result in loss of patent protection for the product at issue. The consequences of any such loss could be a significant decrease in sales of that product and could materially affect future results of operations for the Group.
Coreg
In 2014, GSK initiated suit against Teva for inducing infringement of its patent relating to the use of carvedilol (Coreg) in decreasing mortality caused by congestive heart failure. In June 2017, the case proceeded to a jury trial in the US District Court for the District of Delaware. The jury returned a verdict in GSK’s favour, awarding GSK lost profits and reasonable royalties for a total award of $235.51 million. On 29 March 2018, the trial judge ruled on post-trial motions filed by Teva and found that substantial evidence at trial did not support the jury’s finding of induced infringement, overturning the jury award. GSK appealed, and on 2 October 2020, a divided panel of the Court of Appeals for the Federal Circuit reversed the district court’s ruling and reinstated the jury award in GSK’s favour.
On 2 December 2020, Teva filed a petition for rehearing en banc. The court granted Teva’s petition, but only for a rehearing by the three-member panel that issued the original decision. On 5 August 2021, the original panel issued its rehearing opinion where the majority again reinstated the jury’s damages award of $235.51 million in GSK’s favour.
Teva again filed a petition for rehearing en banc which was rejected by the Court of Appeals for the Federal Circuit on 11 February 2022. On 11 July 2022, Teva filed a petition for writ of certiorari with the Supreme Court of the United States seeking to overturn the Federal Court decision. On 15 May 2023, the US Supreme Court denied Teva’s request. Certain issues remain to be resolved at the District Court and the parties await the scheduling of a status conference.
Dolutegravir Proceedings
–Tivicay/Triumeq
In September 2021, ViiV Healthcare received a paragraph IV letter from Lupin relating to the Tivicay 5mg dosage for oral suspension, challenging only the crystal form patent. On 2 November 2021, ViiV Healthcare filed suit against Lupin in the US District Court for the District of Delaware. In March 2023, the parties reached a settlement, thereby concluding the matter.
–Juluca
On 12 June 2020, Cipla sent ViiV Healthcare a paragraph IV letter related to Juluca, and on 22 July 2020, ViiV Healthcare filed suit against Cipla in federal court in Delaware. In March 2023, the parties reached a settlement, thereby concluding the matter.
RSV
On 7 June 2022, Pfizer, Inc. filed suit in the London High Court challenging the validity and requesting revocation of three GSK European patents relating to RSV vaccine technology. Corresponding invalidity suits against additional patents were filed in the District Court of the Hague in the Netherlands in January 2023 and in the French-speaking Enterprise Court of Brussels in Belgium in March 2023. In each of those matters GSK counterclaimed that Pfizer’s RSV vaccine infringes GSK’s patents. On 2 August 2023, GSK filed a patent infringement suit against Pfizer in the United States District Court for the District of Delaware alleging infringement of four US GSK patents by Pfizer’s RSV vaccine, Abrysvo. Another two GSK patents were added to the US litigation on 30 November 2023.
The trial in the UK action took place in June 2023. A decision is expected by the end of Q2 2024. In the Netherlands, two separate first-instance hearings have been scheduled. The first was held on 26 January 2024 and the second is scheduled for 1 March 2024. In Belgium, trial on the merits is expected in Q3 2024 with a first instance decision likely in Q1 2025. A trial date in the US has yet to be set. GSK is seeking monetary compensation from Pfizer for Pfizer’s infringing sales of Abrysvo. GSK’s sales of Arexvy are not at issue in these litigations.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 47. Legal proceedings continued | |
Product liability
The Group is currently a defendant in a number of product liability lawsuits.
Avandia
There are two pending US class actions (both filed in 2010) by third-party payers which assert claims under the Racketeer Influenced and Corrupt Organizations Act (RICO) and state consumer protection laws. In December 2019, the Third Circuit Court of Appeals reversed the summary judgements granted in favour of the Group and remanded the third-party payer cases back to district court. Discovery is complete, and class certification and summary judgment briefing has been completed. A hearing on certain Daubert motions relating to experts was held on 1 February 2024. GSK has requested oral argument on class certification, which could be scheduled thereafter.
Zantac
In 2019, the Group was contacted by several regulatory authorities regarding the detection of N-Nitroso-dimethylamine (NDMA) in Zantac (ranitidine) products. Based on information available at the time and correspondence with regulators, the Group made the decision to suspend the release, distribution and supply of all dose forms of Zantac to all markets pending the outcome of the ongoing tests and investigations. Also, as a precautionary action, the Group made the decision to initiate a voluntary pharmacy/retail level recall of Zantac products globally.
On 30 April 2020, the European Medicines Agency (EMA) recommended the suspension of ranitidine medicines. Following the publication of the EMA’s recommendation, the Company communicated a decision not to re-enter the market. In the US, FDA requested that all manufacturers withdraw ranitidine products from the market.
The Group was named as a defendant in approximately 2,200 personal injury cases filed in the federal Zantac Multidistrict Litigation (MDL) court proceeding in the Southern District of Florida. In the MDL, plaintiffs originally identified 10 different types of cancers they wished to pursue. Plaintiffs subsequently dropped 5 of the 10 cancers, and proceeded only as to bladder, esophageal, stomach, liver, and pancreatic cancers, although plaintiffs in state courts continue to pursue claims beyond the 5 designated cancers. On 6 December 2022, the court presiding over the federal MDL proceeding granted Defendants’ Daubert motions, finding that Plaintiffs’ experts’ causation opinions regarding whether Zantac can cause the five cancers at issue in the MDL (liver, bladder, pancreatic, esophageal, and stomach) are unreliable and thus inadmissible. Without expert causation opinions, the MDL Court granted summary judgment to GSK and the other brand defendants. The MDL Court found that “there is no scientist outside this litigation who concluded ranitidine causes cancer, and the plaintiffs’ scientists within this litigation systemically utilized unreliable methodologies,” and failed to use “consistent, objective, science-based standards for the even-handed evaluation of data.” This ruling effectively dismissed approximately 2,200 filed cases in the MDL and is binding on all of the claims in the Census Registry. Approximately 13,000 Plaintiffs (which includes plaintiffs with filed cases and registry claimants) have appealed the MDL decision to the Eleventh Circuit Court of Appeals. Plaintiffs’ briefs are due on 10 April 2024. Following the Court’s Daubert decision, it entered a final order dismissing the medical monitoring and consumer class actions based on the reasoning in its Daubert holding. Plaintiffs have filed a notice of appeal in the medical monitoring and consumer class action cases.
GSK has been named as a defendant by approximately 78,000 plaintiffs in several US state jurisdictions. Of these plaintiffs, approximately 72,000 plaintiffs filed in Delaware. Most of the Delaware plaintiffs allege a cancer other than the five cancers being pursued by Plaintiffs in the MDL proceeding. The Delaware court held a general causation hearing on the admissibility of expert testimony for the 10 cancers Plaintiffs have decided to pursue (breast, colorectal, kidney, prostate, pancreatic, lung, bladder, liver, esophageal, and stomach) on 22-24 January 2024.
In the California Zantac litigation Cases JCCP 5150 (JCCP), the court issued a Sargon ruling in the first case scheduled for trial (Goetz). The court found that the plaintiff’s experts’ causation opinions are admissible and can be presented to a jury. The ruling applied only to the Goetz case and does not affect any other state court cases. On 23 June 2023, GSK reached a confidential settlement in the Goetz case. On 11 October 2023, GSK announced it had reached confidential settlements in the Cantlay/Harper case as well as the three remaining breast cancer bellwether cases in California. On 1 February 2024, GSK announced it had reached a confidential settlement in the Browne case filed in California state court. The case, which was set to begin trial on 20 February 2024, will be dismissed. On 28 February 2024, GSK reached a confidential settlement in the Boyd case filed in California state court. The case, which had been scheduled to begin trial on 2 April 2023, will now be dismissed as to GSK. The settlements reflect GSK’s desire to avoid the distraction related to protracted litigation. GSK does not admit any liability in the settlements and will continue to vigorously defend itself based on the facts and the science in all other Zantac cases. Additional bellwether cases in the JCCP have been and will be set for trial in Q2 and Q3 2024.
Multiple trials in other state courts have been set with dates in 2024 and 2025, including in Illinois, Texas, and Florida. The first of these cases is Valadez (colorectal) which is scheduled for trial on 25 April 2024. There are 14 additional cases in Illinois with trial dates in 2024 and 2025. Cases in Texas and Florida do not yet have firm trial dates, although trials are expected to occur in 2024 and 2025.
Outside the US, there are two proposed class actions pending against GSK in Ontario and Quebec, Canada along with a class action in Israel. In Canada, a certification hearing was held in October 2022 in the British Columbia proposed class action. This was the first class action to proceed to a certification hearing and the class action sought to certify a national class. In May 2023, the Court dismissed the proposed class action against the manufacturer defendants. An appeal from that decision was abandoned. The Ontario action will also be discontinued. There are also approximately 120 individual actions that have been filed in Canada.
Given the complex ownership and marketing of Zantac prescription and over-the-counter (OTC) medicine over many years, numerous claims involve several defendants. As a result, some defendants have served one another, including the Group, with notice of potential indemnification claims about possible liabilities connected particularly with Zantac OTC. Given the current stage of the proceedings, the Group cannot meaningfully assess what liability, if any, it may have, nor can it meaningfully assess the liability of other parties under relevant indemnification provisions.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 47. Legal proceedings continued | |
In addition, on 20 March 2020, the Department of Justice (DOJ) sent the Group notice of a civil investigation it had opened into allegations of False Claims Act violations by the Group related to Zantac. On 18 June 2020, the DOJ served a Civil Investigative Demand (CID) on the Group, formalising its request for documents. The Group continues to cooperate with the DOJ on the CID. On the same day, the New Mexico Attorney General filed a lawsuit against multiple defendants, including the Group, alleging violations of state consumer protection and false advertising statutes, among other claims.
On 11 November 2020, the Mayor & City of Baltimore filed an action against the Group alleging that Zantac increased the risk of cancer and/or caused cancer in Baltimore patients, and that the Group failed to warn of or concealed those risks. Fact and expert discovery is ongoing. The court has set a trial date of 2 June 2025.
Zofran
The Group was a defendant in over 400 product liability cases involving Zofran pending in a Multidistrict Litigation (MDL) proceeding in the District of Massachusetts. The cases alleged that children suffered birth defects due to their mothers’ ingestion of Zofran and/or generic ondansetron for pregnancy- related nausea and vomiting. Plaintiffs asserted that the Group sold Zofran knowing it was unsafe for pregnant women, failed to warn of the risks and illegally marketed Zofran “off-label” for use by pregnant women.
On 1 June 2021, the MDL Court granted the Group’s motion for summary judgment on federal pre-emption grounds. The Court found that the FDA was fully informed of all relevant safety information regarding Zofran and had repeatedly rejected any attempt to add a birth defect warning to the label. At that time, the Court granted judgment for the Group in all cases pending in the MDL (approximately 431 cases) and closed the MDL proceeding. Plaintiffs appealed this decision and, on 9 January 2023, the United States Court of Appeals for the First Circuit affirmed the district court’s decision in favour of the Group.
There remains one state court case and four proposed class actions in Canada, which are not currently active.
Sales and marketing and regulation
The Group’s marketing and promotion of its Pharmaceutical and Vaccine products are the subject of certain governmental investigations and private lawsuits brought by litigants under various theories of law.
GSK Korea – Proceedings under Fair Trade Laws
In August 2020, GSK Korea was indicted under Korea’s Monopoly Regulation and Fair Trade laws in relation to government tenders of HPV (Cervarix) and PCV (Synflorix) vaccines in 2018 and 2019. The prosecutor alleged that GSK Korea, through the actions of at least one of its employees, interfered with the tender process under the National Immunisation Programme by using “straw bidders.”
A former GSK Korea employee was also charged in his individual capacity by the prosecutor in relation to the same matter. Further, a number of wholesalers are co-defendants in the proceedings. On 1 February 2023, the court rendered a guilty verdict in respect of all defendants. GSK Korea was fined KRW70 million which is approximately £45,000. Appeal proceedings are ongoing.
The Korea Fair Trade Commission (KFTC) also commenced proceedings regarding the same matter. KFTC hearings took place in July 2023 and GSK Korea was found in violation of applicable fair trade law. The KFTC imposed a fine of KRW351 million which is approximately £212,000.
US electronic health records subpoena
On 19 March 2023, the Group received a subpoena from the United States Attorney’s Office for the Western District of Virginia, which is working with the United States Department of Justice Civil Division, seeking documents relating to the Group’s electronic health record programmes. The Group is cooperating with this enquiry.
Senate HELP Enquiry
The Group received a letter dated 8 January 2024 from majority members of the US Senate Health, Education, Labor and Pensions (“HELP”) Committee initiating an investigation into the pricing of inhalers for the treatment of asthma and COPD. The letter is similar to letters received by a number of other pharmaceutical companies and requests information on pricing, research in the treatment of respiratory diseases, patenting and business practices. The Group is cooperating with the enquiry.
Orange Book Challenge
In November 2023, the US Federal Trade Commission (FTC) wrote to the Group and identified five patents that it is challenging through an FDA Orange Book listing dispute process, reserving the right to take further action. A number of other companies were also contacted, with the FTC citing a total of 62 patents. As to the five patent listings challenged by the FTC, the Group has asked the FDA to remove four patents from the Orange Book with respect to certain products. It is the Group's position that these patents were properly listed at the time of the listing decision. No generic competition was impacted by the previous listings and all de-listed patents remain valid and enforceable. Subsequent to the FTC’s challenge, the Group received letters from US Senator Elizabeth Warren and US Congresswoman Pramila Jayapal, US Senator Amy Klobuchar, and US Senator Tammy Baldwin, reiterating the FTC position and requesting further information about the Group’s Orange Book-listed patents and the Group’s response to the FDA challenge process. The Group is cooperating with these enquiries.
Anti-trust/competition
Certain governmental actions and private lawsuits have been brought against the Group alleging violation of competition or anti-trust laws.
Lamictal
Purported classes of direct purchasers filed suit in the US District Court for the District of New Jersey alleging that the Group and Teva Pharmaceuticals unlawfully conspired to delay generic competition for Lamictal, resulting in overcharges to the purchasers, by entering into an allegedly anti-competitive reverse payment settlement to resolve patent infringement litigation. A separate count accuses the Group of monopolising the market.
On 13 December 2018, the trial judge granted plaintiffs’ class certification motion, certifying a class of direct purchasers. The Group filed a Rule 23(f) motion in the Court of Appeals for the Third Circuit, challenging the class certification decision. On 22 April 2020, the Court of Appeals vacated the lower court’s grant of class certification and remanded the issue back to the lower court for further analysis.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
| Strategic report | | | Governance and remuneration | | | Financial statements | | | Investor information | | 2023 Annual Report on Form 20-F |
| Notes to the financial statements continued |
| 47. Legal proceedings continued | |
On 9 October 2020, the district court heard argument on plaintiffs’ renewed motion for class certification after remand. On 9 April 2021, the district court denied Plaintiffs’ motion for class certification of the putative direct purchaser class, leaving a potential class of brand-only purchasers. Plaintiffs moved to supplement their expert report and seek additional discovery to support the addition of certain generic purchasers. On 21 January 2022, the district court denied Plaintiffs’ motion to supplement their expert report and seek additional discovery and held that the issue of generic purchasers had already been decided and denied in the court’s ruling on decertification. The parties conducted briefing on class certification as to the remaining brand-only purchasers, with plaintiffs also seeking to add a smaller category of purchasers.
On 1 February 2023, the district court denied Plaintiffs’ renewed class certification motion. A series of follow-on complaints have been filed in the US District Court for the Eastern District of Pennsylvania by groups of alleged purchasers. The cases have been consolidated with the previously pending case in the District of New Jersey. Discovery is ongoing.
Commercial and corporate
The Group is involved in certain contractual and/or commercial disputes.
Zejula Royalty Dispute
In October 2012, Tesaro, Inc. (now a wholly owned subsidiary of GSK) entered into two worldwide patent license agreements with AstraZeneca UK Limited related to niraparib (later approved as Zejula).
In May 2021, AstraZeneca filed a lawsuit against Tesaro in the High Court, England and Wales alleging that Tesaro failed to pay some of the royalties due under the license agreements. Tesaro has counterclaimed based on a calculated overpayment. Trial was held the week of 6 March 2023 and judgment was entered against the Group on 5 April 2023, ruling that all current uses of Zejula generate royalty-bearing sales under the wording of the two license agreements. On 12 June 2023, the Court of Appeal of England and Wales granted the Group’s request for permission to appeal the 5 April 2023 judgment. The appeal was heard on 17 January 2024 and on 9 February 2024 the Court of Appeal ruled in the Group’s favour, overturning the trial court’s judgment and determining that only Zejula sales for uses falling within the licensed patents could be deemed royalty-bearing. The appropriate quantum of royalties in accord with the Court of Appeal’s judgment may be the subject of further proceedings.
| | |
| 48. Post balance sheet events |
Acquisition of Aiolos Bio, Inc
On 9 January 2024, GSK announced it had entered into an agreement to acquire Aiolos Bio, Inc, (Aiolos) a clinical stage biopharmaceutical company focused on addressing the unmet treatment needs of patients with certain respiratory and inflammatory conditions, for an upfront payment of US$1 billion and up to US$400 million in certain success-based regulatory milestone payments. In addition, GSK will also be responsible for success-based milestone payments as well as tiered royalties owed to Jiangsu Hengrui Pharmaceuticals Co., Ltd. (Hengrui). The transaction was subject to customary conditions, including applicable regulatory agency clearances under the Hart- Scott-Rodino Act in the US, and subsequently closed on 14 February 2024. Given the timing of the closure of the transaction, GSK expects to disclose the provisional accounting for the acquisition in the Q1 2024 Results Announcement.
Disposal of shares in Haleon plc
On 17 January 2024, GSK completed the sale of 300 million shares in Haleon plc equivalent to 3.2% of Haleon plc’s issued share capital at a price of 326 pence per share, raising gross proceeds of £978 million. Following the sale, GSK holds approximately 385 million ordinary shares in Haleon plc, representing over 4.0% of the issued share capital of Haleon plc.
Investor Information In this section Number of Employees 246 Product development pipeline 247 Products, competition and intellectual property 251 Risk factors 254 Share capital and control 261 Dividends 263 Financial calendar 2023 264 Annual General Meeting 2023 264 Tax information for shareholders 265 Additional Information 267 Articles of Association of GSK plc 267 Material contracts 270 American Depositary Shares 271 Cyber Security 272 Code of Ethics 273 Supplemental Guarantor Information 273 Remuneration Policy 273 Principal Accountant Fees and Services 275 Shareholder services and contacts 276 US law and regulation 277 Report of Independent Public Accounting Firm 279 Corporate governance comparison 280 Group companies 287 Glossary of terms 296 2023 Annual Report on Form 20-F 245
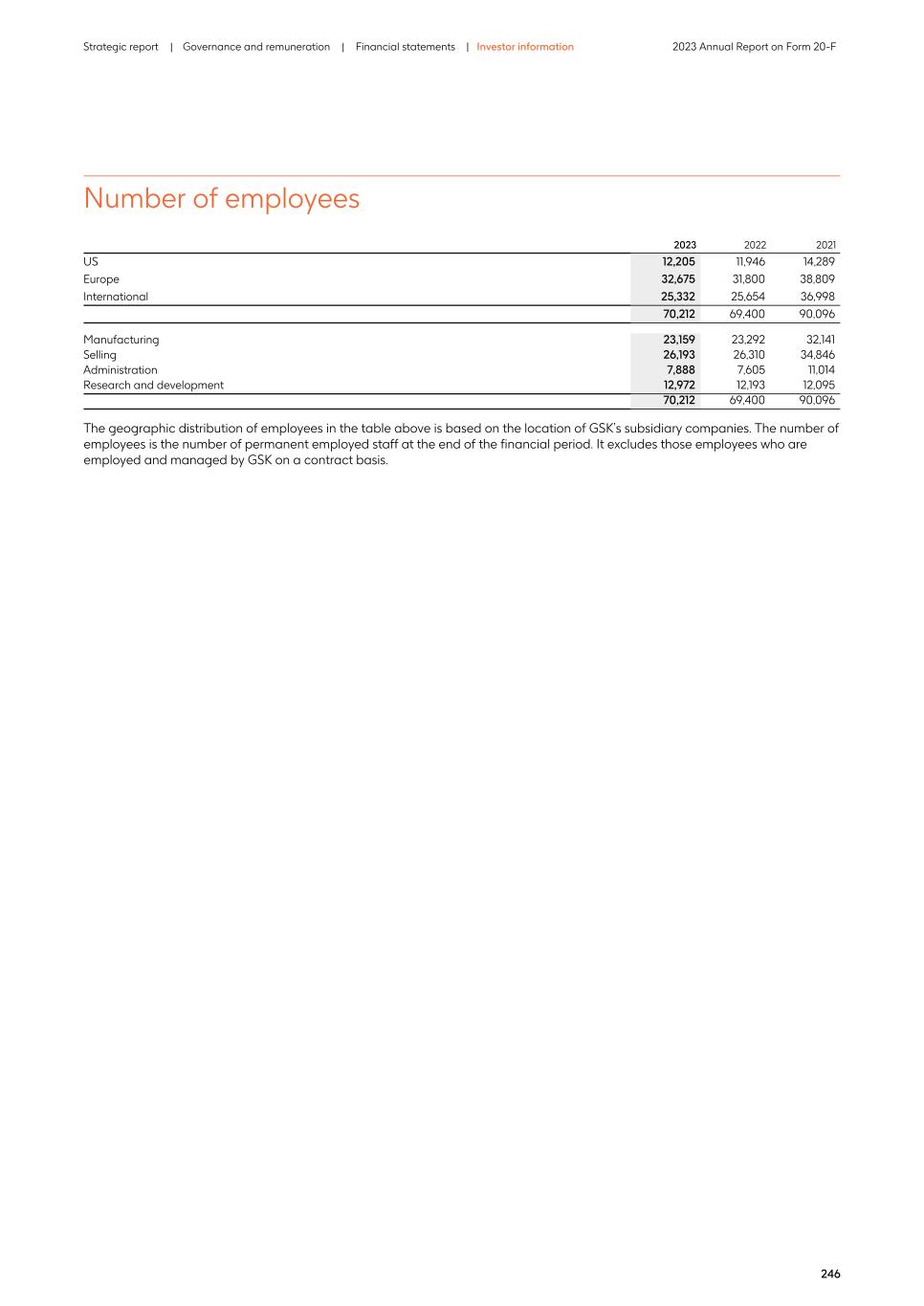
Number of employees 2023 2022 2021 US 12,205 11,946 14,289 Europe 32,675 31,800 38,809 International 25,332 25,654 36,998 70,212 69,400 90,096 Manufacturing 23,159 23,292 32,141 Selling 26,193 26,310 34,846 Administration 7,888 7,605 11,014 Research and development 12,972 12,193 12,095 70,212 69,400 90,096 The geographic distribution of employees in the table above is based on the location of GSK’s subsidiary companies. The number of employees is the number of permanent employed staff at the end of the financial period. It excludes those employees who are employed and managed by GSK on a contract basis. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F 246
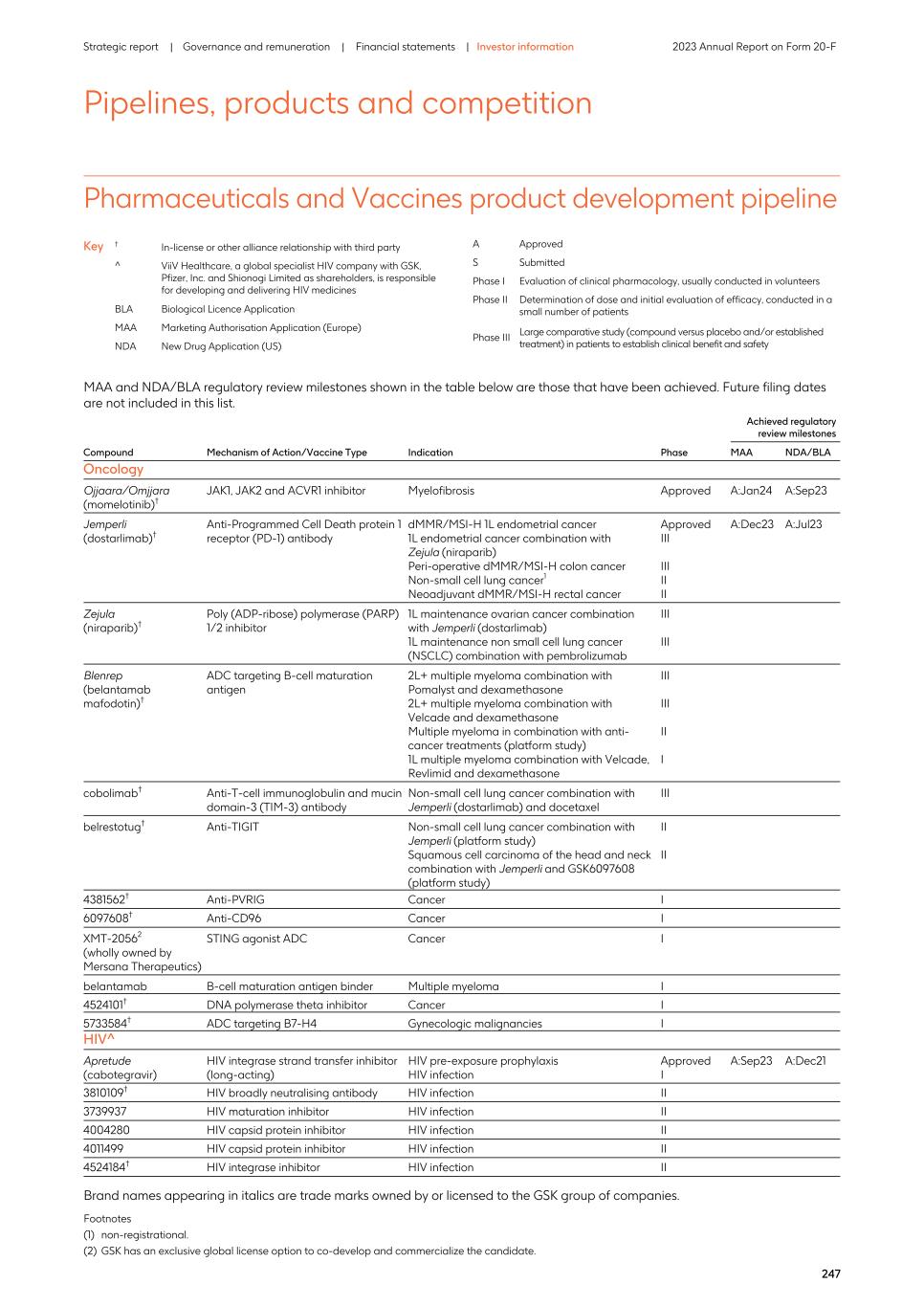
Pharmaceuticals and Vaccines product development pipeline Key † In-license or other alliance relationship with third party ^ ViiV Healthcare, a global specialist HIV company with GSK, Pfizer, Inc. and Shionogi Limited as shareholders, is responsible for developing and delivering HIV medicines BLA Biological Licence Application MAA Marketing Authorisation Application (Europe) NDA New Drug Application (US) A Approved S Submitted Phase I Evaluation of clinical pharmacology, usually conducted in volunteers Phase II Determination of dose and initial evaluation of efficacy, conducted in a small number of patients Phase III Large comparative study (compound versus placebo and/or established treatment) in patients to establish clinical benefit and safety MAA and NDA/BLA regulatory review milestones shown in the table below are those that have been achieved. Future filing dates are not included in this list. Achieved regulatory review milestones Compound Mechanism of Action/Vaccine Type Indication Phase MAA NDA/BLA Oncology Ojjaara/Omjjara (momelotinib)† JAK1, JAK2 and ACVR1 inhibitor Myelofibrosis Approved A:Jan24 A:Sep23 Jemperli (dostarlimab)† Anti-Programmed Cell Death protein 1 receptor (PD-1) antibody dMMR/MSI-H 1L endometrial cancer 1L endometrial cancer combination with Zejula (niraparib) Peri-operative dMMR/MSI-H colon cancer Non-small cell lung cancer1 Neoadjuvant dMMR/MSI-H rectal cancer Approved III III II II A:Dec23 A:Jul23 Zejula (niraparib)† Poly (ADP-ribose) polymerase (PARP) 1/2 inhibitor 1L maintenance ovarian cancer combination with Jemperli (dostarlimab) 1L maintenance non small cell lung cancer (NSCLC) combination with pembrolizumab III III IIIBlenrep (belantamab mafodotin)† ADC targeting B-cell maturation antigen 2L+ multiple myeloma combination with Pomalyst and dexamethasone 2L+ multiple myeloma combination with Velcade and dexamethasone Multiple myeloma in combination with anti- cancer treatments (platform study) 1L multiple myeloma combination with Velcade, Revlimid and dexamethasone III III II I cobolimab† Anti-T-cell immunoglobulin and mucin domain-3 (TIM-3) antibody Non-small cell lung cancer combination with Jemperli (dostarlimab) and docetaxel III belrestotug† Anti-TIGIT Non-small cell lung cancer combination with Jemperli (platform study) Squamous cell carcinoma of the head and neck combination with Jemperli and GSK6097608 (platform study) II II 4381562† Anti-PVRIG Cancer I 6097608† Anti-CD96 Cancer I XMT-20562 (wholly owned by Mersana Therapeutics) STING agonist ADC Cancer I belantamab B-cell maturation antigen binder Multiple myeloma I 4524101† DNA polymerase theta inhibitor Cancer I 5733584† ADC targeting B7-H4 Gynecologic malignancies I HIV^ Apretude (cabotegravir) HIV integrase strand transfer inhibitor (long-acting) HIV pre-exposure prophylaxis HIV infection Approved I A:Sep23 A:Dec21 3810109† HIV broadly neutralising antibody HIV infection II 3739937 HIV maturation inhibitor HIV infection II 4004280 HIV capsid protein inhibitor HIV infection II 4011499 HIV capsid protein inhibitor HIV infection II 4524184† HIV integrase inhibitor HIV infection II Brand names appearing in italics are trade marks owned by or licensed to the GSK group of companies. Footnotes (1) non-registrational. (2) GSK has an exclusive global license option to co-develop and commercialize the candidate. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Pipelines, products and competition 247
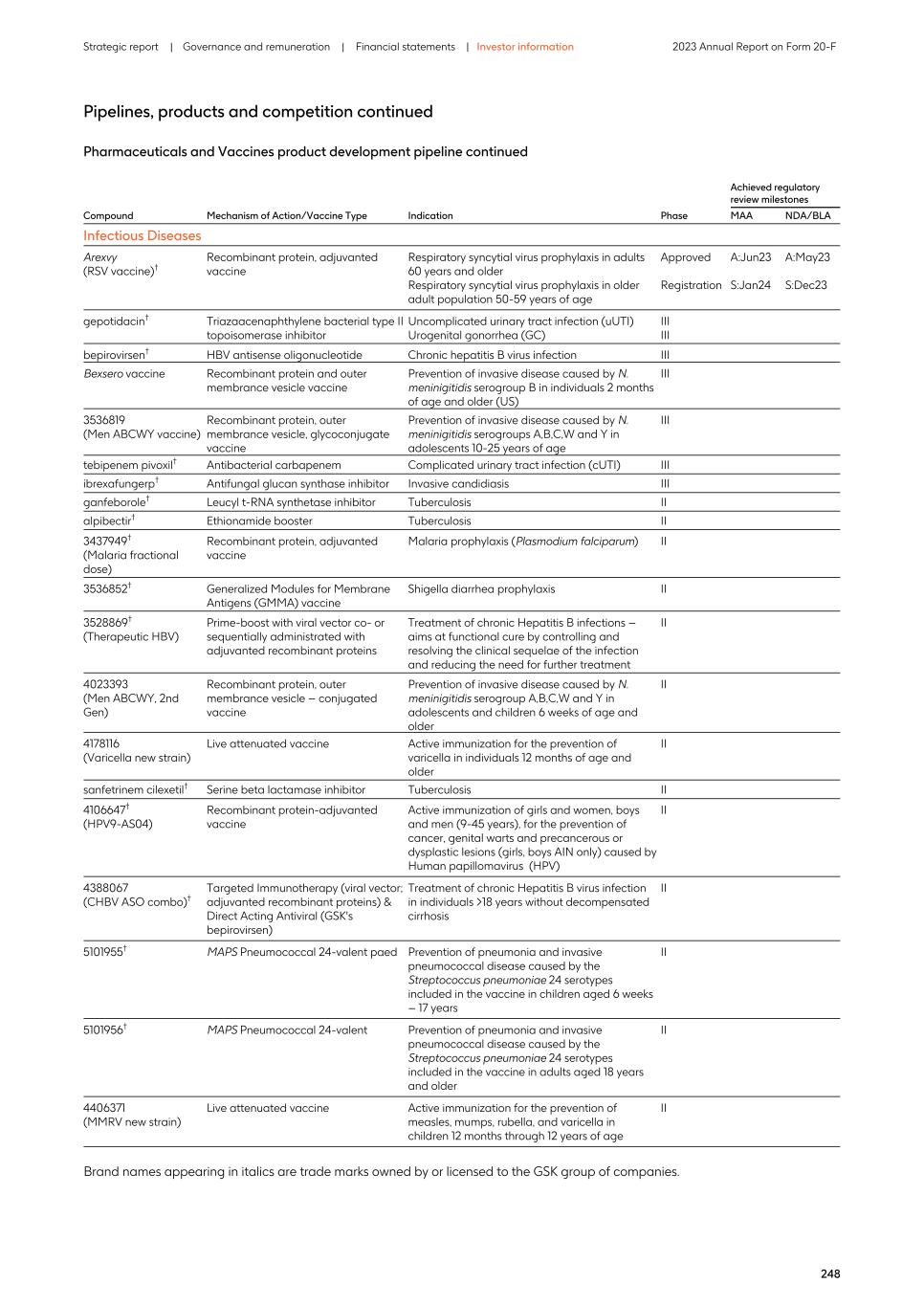
Achieved regulatory review milestones Compound Mechanism of Action/Vaccine Type Indication Phase MAA NDA/BLA Infectious Diseases Arexvy (RSV vaccine)† Recombinant protein, adjuvanted vaccine Respiratory syncytial virus prophylaxis in adults 60 years and older Respiratory syncytial virus prophylaxis in older adult population 50-59 years of age Approved Registration A:Jun23 S:Jan24 A:May23 S:Dec23 gepotidacin† Triazaacenaphthylene bacterial type II topoisomerase inhibitor Uncomplicated urinary tract infection (uUTI) Urogenital gonorrhea (GC) III III bepirovirsen† HBV antisense oligonucleotide Chronic hepatitis B virus infection III Bexsero vaccine Recombinant protein and outer membrance vesicle vaccine Prevention of invasive disease caused by N. meninigitidis serogroup B in individuals 2 months of age and older (US) III 3536819 (Men ABCWY vaccine) Recombinant protein, outer membrance vesicle, glycoconjugate vaccine Prevention of invasive disease caused by N. meninigitidis serogroups A,B,C,W and Y in adolescents 10-25 years of age III tebipenem pivoxil† Antibacterial carbapenem Complicated urinary tract infection (cUTI) III ibrexafungerp† Antifungal glucan synthase inhibitor Invasive candidiasis III ganfeborole† Leucyl t-RNA synthetase inhibitor Tuberculosis II alpibectir† Ethionamide booster Tuberculosis II 3437949† (Malaria fractional dose) Recombinant protein, adjuvanted vaccine Malaria prophylaxis (Plasmodium falciparum) II 3536852† Generalized Modules for Membrane Antigens (GMMA) vaccine Shigella diarrhea prophylaxis II 3528869† (Therapeutic HBV) Prime-boost with viral vector co- or sequentially administrated with adjuvanted recombinant proteins Treatment of chronic Hepatitis B infections – aims at functional cure by controlling and resolving the clinical sequelae of the infection and reducing the need for further treatment II 4023393 (Men ABCWY, 2nd Gen) Recombinant protein, outer membrance vesicle – conjugated vaccine Prevention of invasive disease caused by N. meninigitidis serogroup A,B,C,W and Y in adolescents and children 6 weeks of age and older II 4178116 (Varicella new strain) Live attenuated vaccine Active immunization for the prevention of varicella in individuals 12 months of age and older II sanfetrinem cilexetil† Serine beta lactamase inhibitor Tuberculosis II 4106647† (HPV9-AS04) Recombinant protein-adjuvanted vaccine Active immunization of girls and women, boys and men (9-45 years), for the prevention of cancer, genital warts and precancerous or dysplastic lesions (girls, boys AIN only) caused by Human papillomavirus (HPV) II 4388067 (CHBV ASO combo)† Targeted Immunotherapy (viral vector; adjuvanted recombinant proteins) & Direct Acting Antiviral (GSK's bepirovirsen) Treatment of chronic Hepatitis B virus infection in individuals >18 years without decompensated cirrhosis II 5101955† MAPS Pneumococcal 24-valent paed Prevention of pneumonia and invasive pneumococcal disease caused by the Streptococcus pneumoniae 24 serotypes included in the vaccine in children aged 6 weeks – 17 years II 5101956† MAPS Pneumococcal 24-valent Prevention of pneumonia and invasive pneumococcal disease caused by the Streptococcus pneumoniae 24 serotypes included in the vaccine in adults aged 18 years and older II 4406371 (MMRV new strain) Live attenuated vaccine Active immunization for the prevention of measles, mumps, rubella, and varicella in children 12 months through 12 years of age II Brand names appearing in italics are trade marks owned by or licensed to the GSK group of companies. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Pipelines, products and competition continued Pharmaceuticals and Vaccines product development pipeline continued 248
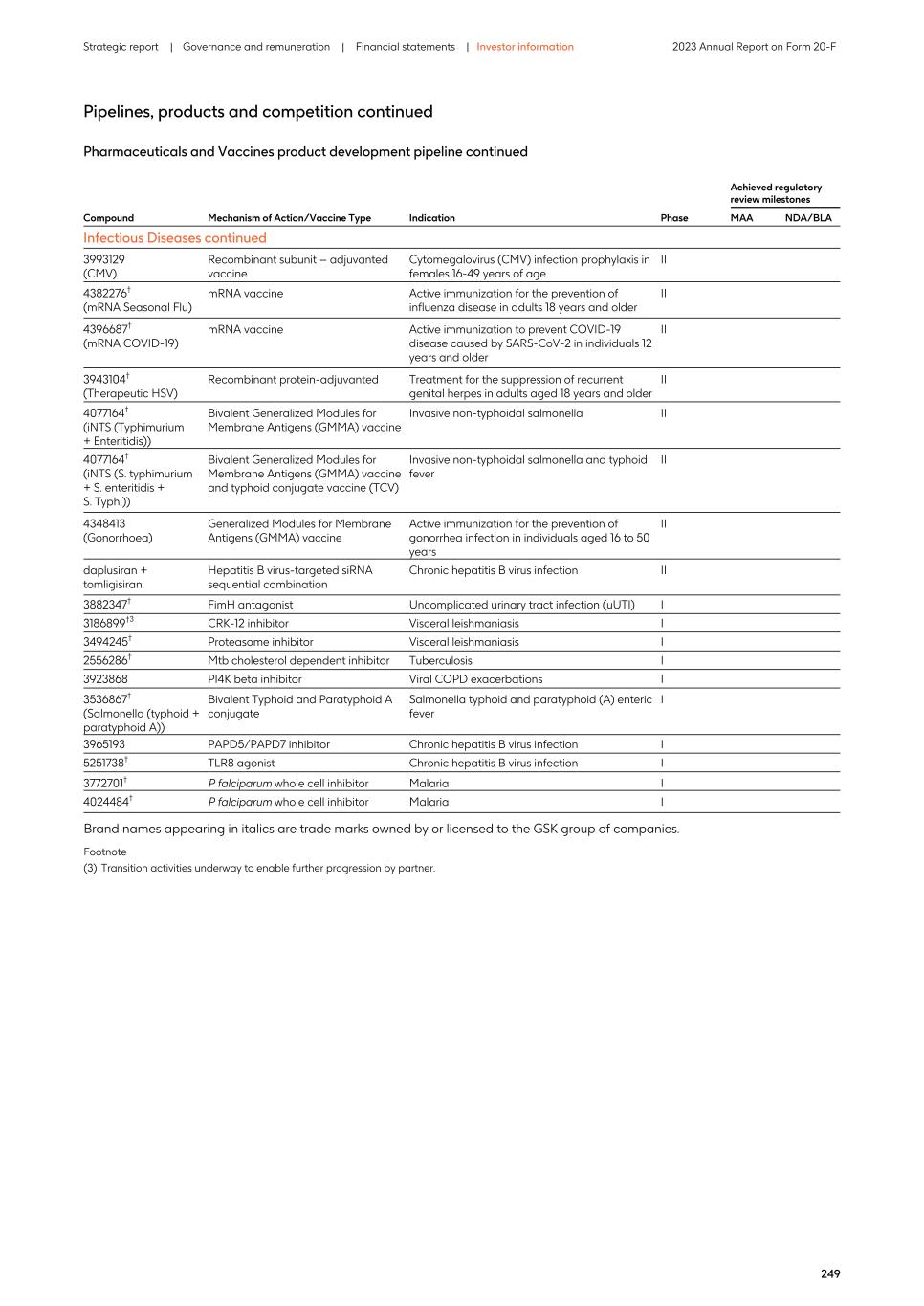
Achieved regulatory review milestones Compound Mechanism of Action/Vaccine Type Indication Phase MAA NDA/BLA Infectious Diseases continued 3993129 (CMV) Recombinant subunit – adjuvanted vaccine Cytomegalovirus (CMV) infection prophylaxis in females 16-49 years of age II 4382276† (mRNA Seasonal Flu) mRNA vaccine Active immunization for the prevention of influenza disease in adults 18 years and older II 4396687† (mRNA COVID-19) mRNA vaccine Active immunization to prevent COVID-19 disease caused by SARS-CoV-2 in individuals 12 years and older II 3943104† (Therapeutic HSV) Recombinant protein-adjuvanted Treatment for the suppression of recurrent genital herpes in adults aged 18 years and older II 4077164† (iNTS (Typhimurium + Enteritidis)) Bivalent Generalized Modules for Membrane Antigens (GMMA) vaccine Invasive non-typhoidal salmonella II 4077164† (iNTS (S. typhimurium + S. enteritidis + S. Typhi)) Bivalent Generalized Modules for Membrane Antigens (GMMA) vaccine and typhoid conjugate vaccine (TCV) Invasive non-typhoidal salmonella and typhoid fever II 4348413 (Gonorrhoea) Generalized Modules for Membrane Antigens (GMMA) vaccine Active immunization for the prevention of gonorrhea infection in individuals aged 16 to 50 years II daplusiran + tomligisiran Hepatitis B virus-targeted siRNA sequential combination Chronic hepatitis B virus infection II 3882347† FimH antagonist Uncomplicated urinary tract infection (uUTI) I 3186899†3 CRK-12 inhibitor Visceral leishmaniasis I 3494245† Proteasome inhibitor Visceral leishmaniasis I 2556286† Mtb cholesterol dependent inhibitor Tuberculosis I 3923868 PI4K beta inhibitor Viral COPD exacerbations I 3536867† (Salmonella (typhoid + paratyphoid A)) Bivalent Typhoid and Paratyphoid A conjugate Salmonella typhoid and paratyphoid (A) enteric fever I 3965193 PAPD5/PAPD7 inhibitor Chronic hepatitis B virus infection I 5251738† TLR8 agonist Chronic hepatitis B virus infection I 3772701† P falciparum whole cell inhibitor Malaria I 4024484† P falciparum whole cell inhibitor Malaria I Brand names appearing in italics are trade marks owned by or licensed to the GSK group of companies. Footnote (3) Transition activities underway to enable further progression by partner. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Pipelines, products and competition continued Pharmaceuticals and Vaccines product development pipeline continued 249
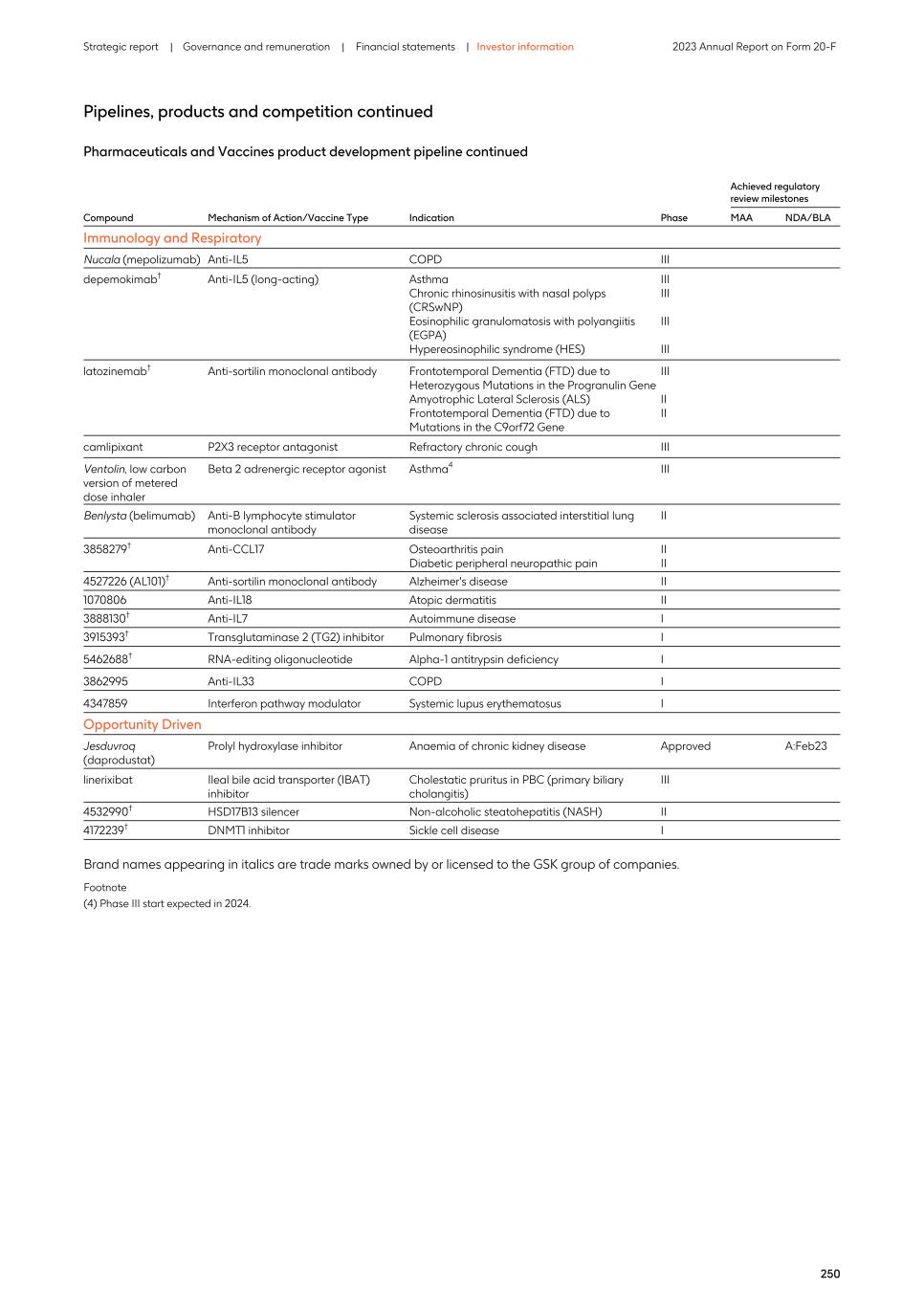
Achieved regulatory review milestones Compound Mechanism of Action/Vaccine Type Indication Phase MAA NDA/BLA Immunology and Respiratory Nucala (mepolizumab) Anti-IL5 COPD III depemokimab† Anti-IL5 (long-acting) Asthma Chronic rhinosinusitis with nasal polyps (CRSwNP) Eosinophilic granulomatosis with polyangiitis (EGPA) Hypereosinophilic syndrome (HES) III III III III latozinemab† Anti-sortilin monoclonal antibody Frontotemporal Dementia (FTD) due to Heterozygous Mutations in the Progranulin Gene Amyotrophic Lateral Sclerosis (ALS) Frontotemporal Dementia (FTD) due to Mutations in the C9orf72 Gene III II II camlipixant P2X3 receptor antagonist Refractory chronic cough III Ventolin, low carbon version of metered dose inhaler Beta 2 adrenergic receptor agonist Asthma4 III Benlysta (belimumab) Anti-B lymphocyte stimulator monoclonal antibody Systemic sclerosis associated interstitial lung disease II 3858279† Anti-CCL17 Osteoarthritis pain Diabetic peripheral neuropathic pain II II 4527226 (AL101)† Anti-sortilin monoclonal antibody Alzheimer's disease II 1070806 Anti-IL18 Atopic dermatitis II 3888130† Anti-IL7 Autoimmune disease I 3915393† Transglutaminase 2 (TG2) inhibitor Pulmonary fibrosis I 5462688† RNA-editing oligonucleotide Alpha-1 antitrypsin deficiency I 3862995 Anti-IL33 COPD I 4347859 Interferon pathway modulator Systemic lupus erythematosus I Opportunity Driven Jesduvroq (daprodustat) Prolyl hydroxylase inhibitor Anaemia of chronic kidney disease Approved A:Feb23 linerixibat Ileal bile acid transporter (IBAT) inhibitor Cholestatic pruritus in PBC (primary biliary cholangitis) III 4532990† HSD17B13 silencer Non-alcoholic steatohepatitis (NASH) II 4172239† DNMT1 inhibitor Sickle cell disease I Brand names appearing in italics are trade marks owned by or licensed to the GSK group of companies. Footnote (4) Phase III start expected in 2024. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Pipelines, products and competition continued Pharmaceuticals and Vaccines product development pipeline continued 250
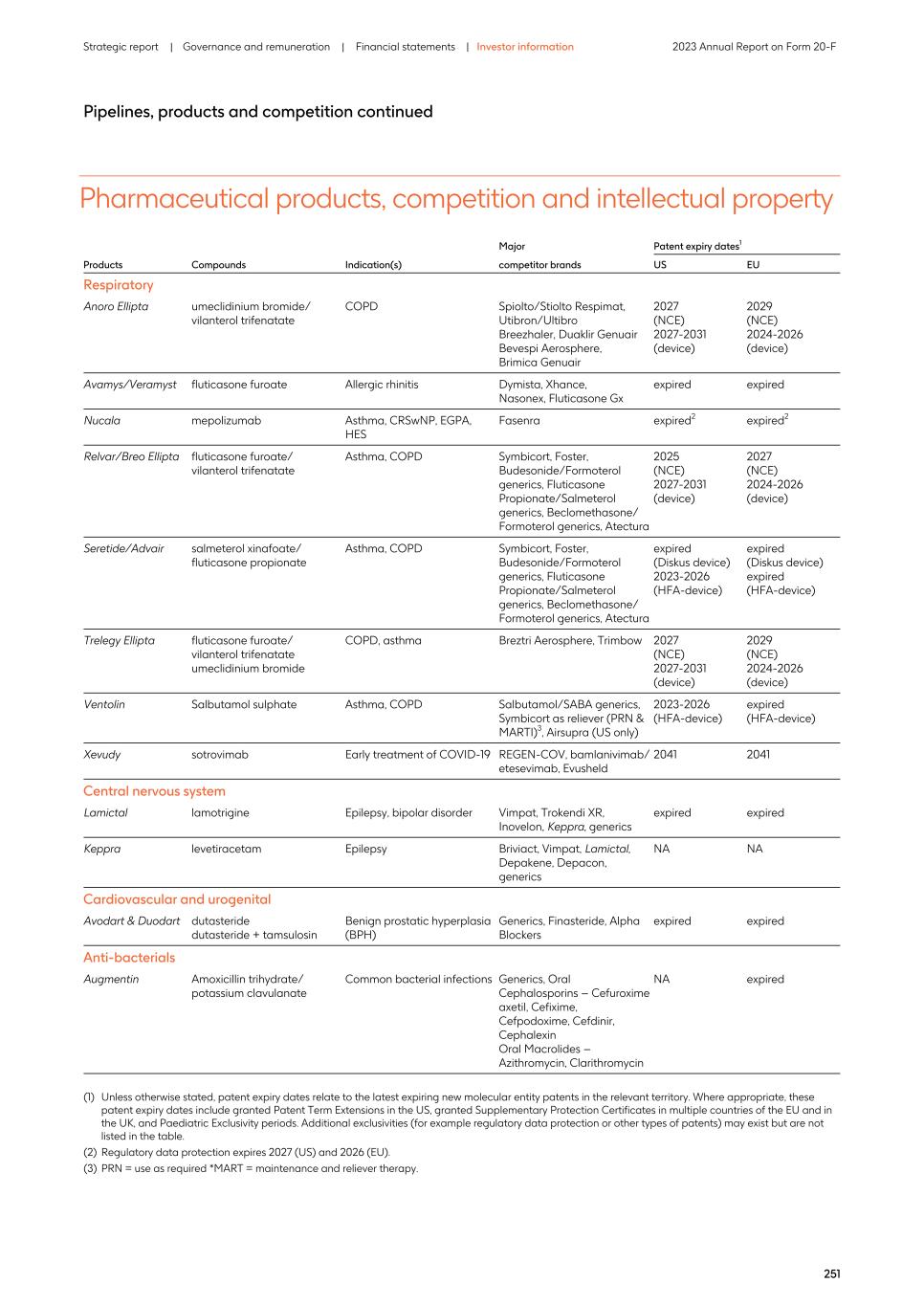
Pharmaceutical products, competition and intellectual property Major Patent expiry dates1 Products Compounds Indication(s) competitor brands US EU Respiratory Anoro Ellipta umeclidinium bromide/ vilanterol trifenatate COPD Spiolto/Stiolto Respimat, Utibron/Ultibro Breezhaler, Duaklir Genuair Bevespi Aerosphere, Brimica Genuair 2027 (NCE) 2027-2031 (device) 2029 (NCE) 2024-2026 (device) Avamys/Veramyst fluticasone furoate Allergic rhinitis Dymista, Xhance, Nasonex, Fluticasone Gx expired expired Nucala mepolizumab Asthma, CRSwNP, EGPA, HES Fasenra expired2 expired2 Relvar/Breo Ellipta fluticasone furoate/ vilanterol trifenatate Asthma, COPD Symbicort, Foster, Budesonide/Formoterol generics, Fluticasone Propionate/Salmeterol generics, Beclomethasone/ Formoterol generics, Atectura 2025 (NCE) 2027-2031 (device) 2027 (NCE) 2024-2026 (device) Seretide/Advair salmeterol xinafoate/ fluticasone propionate Asthma, COPD Symbicort, Foster, Budesonide/Formoterol generics, Fluticasone Propionate/Salmeterol generics, Beclomethasone/ Formoterol generics, Atectura expired (Diskus device) 2023-2026 (HFA-device) expired (Diskus device) expired (HFA-device) Trelegy Ellipta fluticasone furoate/ vilanterol trifenatate umeclidinium bromide COPD, asthma Breztri Aerosphere, Trimbow 2027 (NCE) 2027-2031 (device) 2029 (NCE) 2024-2026 (device) Ventolin Salbutamol sulphate Asthma, COPD Salbutamol/SABA generics, Symbicort as reliever (PRN & MARTI)3, Airsupra (US only) 2023-2026 (HFA-device) expired (HFA-device) Xevudy sotrovimab Early treatment of COVID-19 REGEN-COV, bamlanivimab/ etesevimab, Evusheld 2041 2041 Central nervous system Lamictal lamotrigine Epilepsy, bipolar disorder Vimpat, Trokendi XR, Inovelon, Keppra, generics expired expired Keppra levetiracetam Epilepsy Briviact, Vimpat, Lamictal, Depakene, Depacon, generics NA NA Cardiovascular and urogenital Avodart & Duodart dutasteride dutasteride + tamsulosin Benign prostatic hyperplasia (BPH) Generics, Finasteride, Alpha Blockers expired expired Anti-bacterials Augmentin Amoxicillin trihydrate/ potassium clavulanate Common bacterial infections Generics, Oral Cephalosporins – Cefuroxime axetil, Cefixime, Cefpodoxime, Cefdinir, Cephalexin Oral Macrolides – Azithromycin, Clarithromycin NA expired (1) Unless otherwise stated, patent expiry dates relate to the latest expiring new molecular entity patents in the relevant territory. Where appropriate, these patent expiry dates include granted Patent Term Extensions in the US, granted Supplementary Protection Certificates in multiple countries of the EU and in the UK, and Paediatric Exclusivity periods. Additional exclusivities (for example regulatory data protection or other types of patents) may exist but are not listed in the table. (2) Regulatory data protection expires 2027 (US) and 2026 (EU). (3) PRN = use as required *MART = maintenance and reliever therapy. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Pipelines, products and competition continued 251
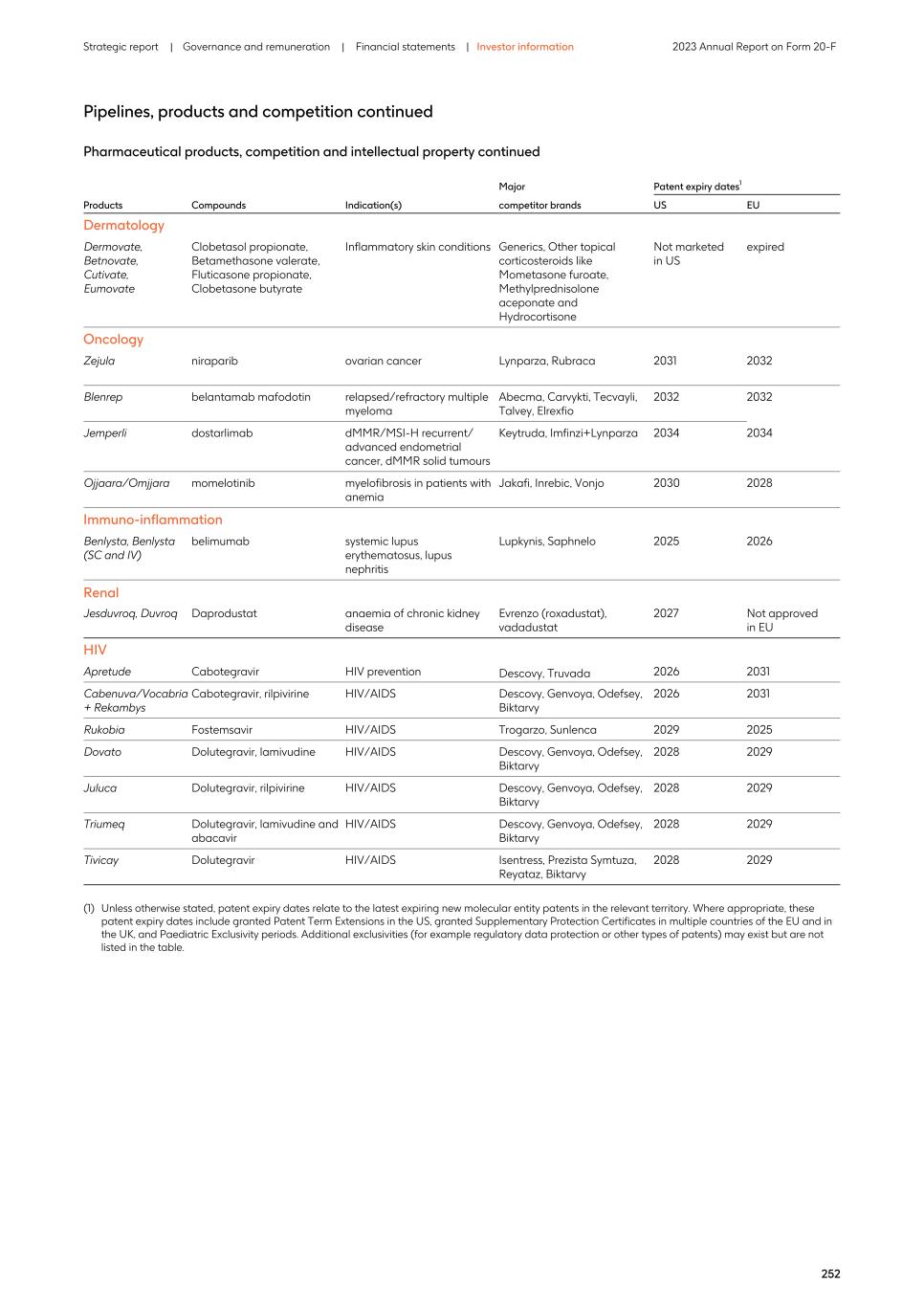
Major Patent expiry dates1 Products Compounds Indication(s) competitor brands US EU Dermatology Dermovate, Betnovate, Cutivate, Eumovate Clobetasol propionate, Betamethasone valerate, Fluticasone propionate, Clobetasone butyrate Inflammatory skin conditions Generics, Other topical corticosteroids like Mometasone furoate, Methylprednisolone aceponate and Hydrocortisone Not marketed in US expired Oncology Zejula niraparib ovarian cancer Lynparza, Rubraca 2031 2032 Blenrep belantamab mafodotin relapsed/refractory multiple myeloma Abecma, Carvykti, Tecvayli, Talvey, Elrexfio 2032 2032 Jemperli dostarlimab dMMR/MSI-H recurrent/ advanced endometrial cancer, dMMR solid tumours Keytruda, Imfinzi+Lynparza 2034 2034 Ojjaara/Omjjara momelotinib myelofibrosis in patients with anemia Jakafi, Inrebic, Vonjo 2030 2028 Immuno-inflammation Benlysta, Benlysta (SC and IV) belimumab systemic lupus erythematosus, lupus nephritis Lupkynis, Saphnelo 2025 2026 Renal Jesduvroq, Duvroq Daprodustat anaemia of chronic kidney disease Evrenzo (roxadustat), vadadustat 2027 Not approved in EU HIV Apretude Cabotegravir HIV prevention Descovy, Truvada 2026 2031 Cabenuva/Vocabria + Rekambys Cabotegravir, rilpivirine HIV/AIDS Descovy, Genvoya, Odefsey, Biktarvy 2026 2031 Rukobia Fostemsavir HIV/AIDS Trogarzo, Sunlenca 2029 2025 Dovato Dolutegravir, lamivudine HIV/AIDS Descovy, Genvoya, Odefsey, Biktarvy 2028 2029 Juluca Dolutegravir, rilpivirine HIV/AIDS Descovy, Genvoya, Odefsey, Biktarvy 2028 2029 Triumeq Dolutegravir, lamivudine and abacavir HIV/AIDS Descovy, Genvoya, Odefsey, Biktarvy 2028 2029 Tivicay Dolutegravir HIV/AIDS Isentress, Prezista Symtuza, Reyataz, Biktarvy 2028 2029 (1) Unless otherwise stated, patent expiry dates relate to the latest expiring new molecular entity patents in the relevant territory. Where appropriate, these patent expiry dates include granted Patent Term Extensions in the US, granted Supplementary Protection Certificates in multiple countries of the EU and in the UK, and Paediatric Exclusivity periods. Additional exclusivities (for example regulatory data protection or other types of patents) may exist but are not listed in the table. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Pipelines, products and competition continued Pharmaceutical products, competition and intellectual property continued 252
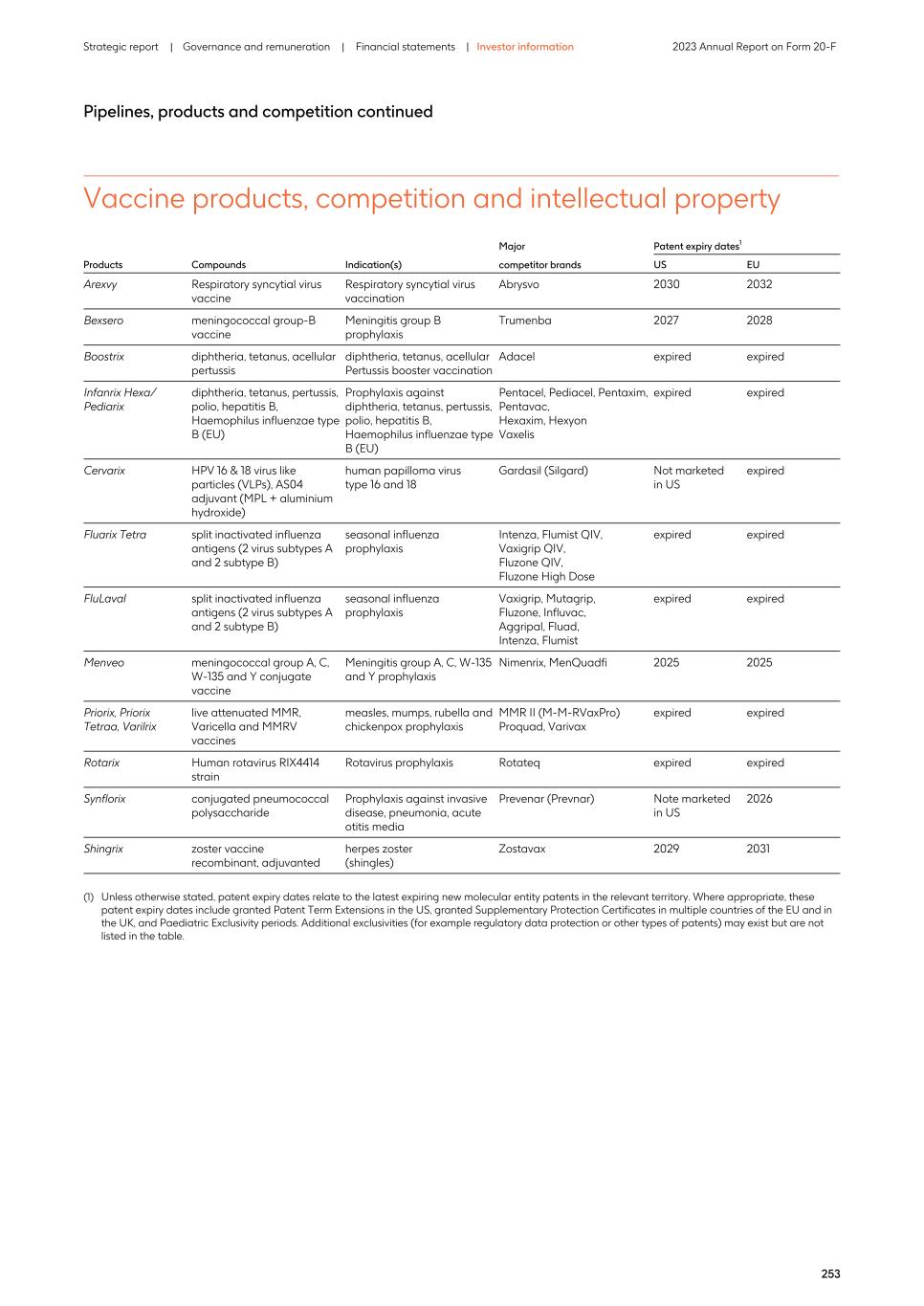
Vaccine products, competition and intellectual property Major Patent expiry dates1 Products Compounds Indication(s) competitor brands US EU Arexvy Respiratory syncytial virus vaccine Respiratory syncytial virus vaccination Abrysvo 2030 2032 Bexsero meningococcal group-B vaccine Meningitis group B prophylaxis Trumenba 2027 2028 Boostrix diphtheria, tetanus, acellular pertussis diphtheria, tetanus, acellular Pertussis booster vaccination Adacel expired expired Infanrix Hexa/ Pediarix diphtheria, tetanus, pertussis, polio, hepatitis B, Haemophilus influenzae type B (EU) Prophylaxis against diphtheria, tetanus, pertussis, polio, hepatitis B, Haemophilus influenzae type B (EU) Pentacel, Pediacel, Pentaxim, Pentavac, Hexaxim, Hexyon Vaxelis expired expired Cervarix HPV 16 & 18 virus like particles (VLPs), AS04 adjuvant (MPL + aluminium hydroxide) human papilloma virus type 16 and 18 Gardasil (Silgard) Not marketed in US expired Fluarix Tetra split inactivated influenza antigens (2 virus subtypes A and 2 subtype B) seasonal influenza prophylaxis Intenza, Flumist QIV, Vaxigrip QIV, Fluzone QIV, Fluzone High Dose expired expired FluLaval split inactivated influenza antigens (2 virus subtypes A and 2 subtype B) seasonal influenza prophylaxis Vaxigrip, Mutagrip, Fluzone, Influvac, Aggripal, Fluad, Intenza, Flumist expired expired Menveo meningococcal group A, C, W-135 and Y conjugate vaccine Meningitis group A, C, W-135 and Y prophylaxis Nimenrix, MenQuadfi 2025 2025 Priorix, Priorix Tetraa, Varilrix live attenuated MMR, Varicella and MMRV vaccines measles, mumps, rubella and chickenpox prophylaxis MMR II (M-M-RVaxPro) Proquad, Varivax expired expired Rotarix Human rotavirus RIX4414 strain Rotavirus prophylaxis Rotateq expired expired Synflorix conjugated pneumococcal polysaccharide Prophylaxis against invasive disease, pneumonia, acute otitis media Prevenar (Prevnar) Note marketed in US 2026 Shingrix zoster vaccine recombinant, adjuvanted herpes zoster (shingles) Zostavax 2029 2031 (1) Unless otherwise stated, patent expiry dates relate to the latest expiring new molecular entity patents in the relevant territory. Where appropriate, these patent expiry dates include granted Patent Term Extensions in the US, granted Supplementary Protection Certificates in multiple countries of the EU and in the UK, and Paediatric Exclusivity periods. Additional exclusivities (for example regulatory data protection or other types of patents) may exist but are not listed in the table. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Pipelines, products and competition continued 253

We outline below the principal risks and uncertainties relevant to GSK’s business, financial condition and operations that may affect our performance and ability to achieve our objectives. These are the risks that we believe could cause our actual results to differ materially from expected and historical results. Operating in the biopharmaceutical sector carries various inherent risks and uncertainties that may affect our business. We must comply with a broad range of laws and regulations which apply to the research and development, manufacturing, testing, approval, distribution, sales, and marketing of pharmaceutical and vaccine products. These affect the cost of product development, the time required to reach the market and the likelihood of doing so successfully on an uninterrupted basis. As rules and regulations change, government interpretation and policy evolves, and our business activities develop, the nature of a particular risk may also alter. Changes to regulatory regimes may be substantial. Any alteration in, and failure to comply with, applicable laws and regulations could materially and adversely affect our financial results. Similarly, our global business exposes us to litigation and government investigations, including product liability litigation, patent and antitrust litigation and sales and marketing litigation. Litigation and government investigations, and the related provisions we may make for unfavourable outcomes and increases in related costs, such as insurance premiums, could also materially and adversely affect our financial results. More detail on the status and various uncertainties in our significant unresolved disputes and potential litigation is set out in Note 47 ‘Legal proceedings’on page 241. Patient safety Risk definition The risk that GSK, including our third parties, fails to appropriately collect, assess, follow up, or report human safety information, including adverse events, from all potential sources or that GSK potentially fails to appropriately act on any relevant findings that may affect the benefit-risk profile of a medicine or vaccine in a timely manner. Risk impact GSK will not tolerate an unfavourable benefit-to-risk profile for patients who use our products. As the most important consequence of ineffective pharmacovigilance is the potential for harm to patients, we maintain robust processes for managing human safety information, conducting timely safety signal detection, and ensuring appropriate measures are in place to manage risks to patients. GSK also intends to fully comply with pharmacovigilance and other relevant regulations worldwide. Non-compliance could result in inspection findings, regulatory scrutiny, civil or criminal sanctions and either temporary or permanent loss of product marketing authorisation. We regularly review and respond to all patient safety risks to limit the potential for reputational damage, loss of trust by patients and healthcare providers, product-related litigation, and loss of shareholder confidence. Context We are accountable for safeguarding patients and clinical trial participants who receive our medicines and vaccines, whether in development or marketed, from harm. While an unforeseen event that unfavorably shifts the benefit/risk profile is not a probable occurrence, such an event cannot be fully discounted. Our Chief Medical Officer is the single point of accountability for benefit/risk decision-making. Cross-functional Safety Review Teams continually assess new safety and efficacy information for every GSK product throughout its life cycle. Our Global Safety Board, under the leadership of our Chief Medical Officer and Head of Global Safety, reviews product safety at established milestones and in every situation where there might be a potential impact on a benefit/risk profile. We must operate in a complex and restrictive pharmacovigilance regulatory environment, sometimes complicated by variable requirements between regulatory agencies. Such regulatory complexity is further illustrated by instances of regulatory agencies taking decisions on the safety of medicines and vaccines based on externally available data that may not be accessible to the marketing authorisation holder. This trend could inhibit our ability to make timely decisions and take appropriate action in relation to the safety of our products, or to confirm or refute conclusions asserted by external parties. This has the potential to extend beyond regulatory agencies to next-generation digital health data held by technology companies or other data custodians, and inaccessible by our industry and/or regulatory agencies. There are many sources of information that might trigger an increase in reporting related to products and/or adverse events (such as media coverage, social media, government health authorities, etc.). Ineffective management of patient safety risks could not only result in reputational damage, loss of trust by patients and healthcare providers, and decline in shareholder confidence, but could also increase the volume of product- related litigation, including class-action lawsuits, which is regularly faced by GSK and our industry in general. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Risk Factors 254

Product quality Risk definition The risk that GSK or our third parties potentially fail to ensure appropriate controls and governance of quality for development and commercial products are in place; compliance with industry practices and regulations in manufacturing and distribution activities; and terms of GSK product licenses and supporting regulatory activities are met. Risk impact A failure to ensure product quality could have far-reaching implications for patient safety, cause product launch delays, drug shortages or product recalls, and have regulatory, legal, and financial consequences. These could materially and adversely affect GSK’s reputation and financial results. Context The external environment for product quality remains challenging. An increase in supplier mergers in our supply network can create challenge in influencing their quality standards. The rapid advancement and use of digital technologies such as artificial intelligence and machine learning (AI/ML) within an evolving regulatory framework introduces both opportunity for modernisation and potential to impact product quality if not adequately controlled. There will be a need to adopt and adapt to new, updated guidance on this as it emerges. The threat of cyber-attacks and data breaches across the industry could risk the integrity of product quality data and its audit trail. Additionally, a gradual divergence in regulatory expectations during inspections, particularly from some health authorities, presents a challenge to our sites as they prepare for inspections. Retaining expertise in biopharma and the deep capability to support digital progression has the potential to be a challenge in a highly competitive environment. Financial controls and reporting Risk definition The risk that GSK fails to comply with current tax laws; fails to report accurate financial information in compliance with accounting standards and applicable legislation; or incurs significant losses due to treasury activities. Risk impact Non-compliance with existing or new financial or new ESG reporting and disclosure requirements, or changes to the recognition of income and expenses, could expose GSK to litigation and regulatory action and could materially and adversely affect our financial results. Failure to comply with changes in the substance or application of the laws governing transfer pricing, dividends, tax credits and intellectual property could also materially and adversely affect our financial results. Failure to comply with applicable laws and regulations could result in GSK being investigated by relevant government agencies and authorities and/or in legal proceedings against us. Government investigations and litigation, can be unpredictable and regardless of their outcome, may be costly, require significant management attention, and damage our reputation. Inconsistent application of treasury policies, transactional or settlement errors, or counterparty defaults could lead to significant losses. Context We are required by the laws of various jurisdictions to publicly disclose our financial results and any events that could materially affect the Group’s financial results. Regulators routinely review the financial statements of listed companies for compliance with new, revised, or existing accounting and regulatory requirements. We believe that we comply with the appropriate regulatory requirements concerning our financial statements and the disclosure of material information, including any transactions relating to business restructuring such as acquisitions and divestitures. However, should we be subject to an investigation into potential non-compliance with accounting and disclosure requirements, this could lead to restatements of previously reported results and significant penalties. Our Treasury group deals daily in high value transactions, mostly foreign exchange, and cash management transactions. These transactions involve market volatility and counterparty risk. The Group’s effective tax rate reflects the locations of our activities and the value they generate, which determine the jurisdictions in which profits arise and the applicable tax rates. These may be higher or lower than the UK statutory rate and may reflect regimes that encourage innovation and investment in R&D by providing tax incentives which, if changed, could affect GSK’s tax rate. In addition, the worldwide nature of our operations means that our cross-border supply routes, necessary to ensure supplies of medicines and vaccines, can result in conflicting claims from tax authorities as to the profits to be taxed in individual countries. This can lead to double taxation, with profits taxed in more than one country. The complexity of tax regulations also means that we may occasionally disagree with tax authorities on the technical interpretation of a particular area of tax law. The tax charge included in our financial statements is our best estimate of tax liability pending any audits by tax authorities. We expect there to be a continued focus on tax reform, driven by initiatives by the OECD and the EC to address the tax challenges arising from digitalisation of the economy. Together with domestic initiatives around the world, these may result in significant changes to established tax principles and an increase in tax authority disputes. Regardless of their merit or outcomes, these may be costly, divert management attention and adversely impact our reputation and relationship with key stakeholders. Laws, regulations, orders and other measures restrict dealings with certain countries, governments, government officials, entities, individuals, and the use of financial institutions and movement of funds. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Risk factors continued 255

Legal matters Risk definition The risk that GSK or our third parties potentially fail to comply with certain legal requirements for the development, supply and commercialisation of our products and operation of business, and specifically in relation to requirements for competition law, anti-bribery and corruption, and sanctions. Any failure to meet compliance and legal standards for these particular areas could lead to increasing scrutiny and enforcement from government agencies. Risk impact Failure to mitigate legal risk could expose GSK and associated persons to governmental investigation, regulatory action, and civil and criminal liability. It may compromise GSK’s ability to supply its products under certain government contracts. In addition, failure to manage legal risk could have substantial implications for GSK’s reputation and the credibility of senior leaders. It might erode investor confidence in our governance, risk management and future performance, and have a consequential negative impact on share performance. It could also lead to the imposition of significant financial penalties and the imposition of additional reporting obligations. Context The overall environment for anti-bribery and corruption, competition law and sanctions and export controls remains challenging. There continues to be a strong enforcement appetite for bribery investigations and prosecutions, with a particular focus on the conduct of multinational companies wherever they operate. The focus on sanctions, export controls and competition law enforcements has increased. From a sanctions perspective, we have seen penalties for violations levied on companies from a number of different industries. Merger control has seen increasing intervention with greater divergence in decisions and policy by enforcement agencies. Financial penalties handed down in these types of case are often very significant. Supportive aspects of the external environment include an increase in focus on corporate transparency. Advances in technology and the use of data analytics are also providing better platforms to streamline processes and detect potential issues. Commercial practices Risk definition The risk that GSK or our third parties potentially engage in commercial activities that fail to comply with laws, regulations, industry codes, and internal controls and requirements. Risk impact Failure to engage in activities that are consistent with the letter and spirit of the law, industry regulations, or the Group’s requirements relating to sales and promotion of medicines and vaccines; with appropriate interactions with healthcare professionals (HCPs), organisations and patients; with legitimate and transparent transfers of value; and with pricing and competition (or antitrust) regulations in commercial practices, including trade channel activities and business tendering, could materially and adversely affect our ability to deliver our strategy and long-term priorities. Additionally, it may result in incomplete awareness of the risk/benefit profile of our products and possibly suboptimal treatment of patients and consumers; governmental investigation, regulatory action and legal proceedings brought against the Group by governmental and private plaintiffs which could result in government sanctions, and criminal and/or financial penalties. Any practices that are found to be misaligned with our culture could also result in reputational harm and dilute the trust established with external stakeholders. Context We operate in a highly regulated and extremely competitive biopharma industry, amongst peers who make significant product innovations and technical advances and intensify price competition. Additional external factors include access limitations to our customers, macroeconomic inflationary dynamics, and pricing pressure across markets. To achieve our strategic objectives, we must continue to develop commercially viable new products, sustain reliable supply, and deliver additional uses for existing products that address the needs of patients, consumers, HCPs and payers. Financially, new products/indications carry with them an uncertainty of future success. Product development is costly, lengthy, and uncertain, and carries the potential for failure at any stage. Even after successful product development, we face challenges in how we launch, and our competitors’ products or pricing strategies could render our assets less competitive. We support product innovation through our continued focus on both in-person and virtual engagement, with a constant focus on our patient. Once we have an approved medicine or vaccine, it is our obligation to provide important information to the healthcare community in various ways, always in a responsible, legal, and ethical manner. Appropriate product promotion ensures HCPs have access to the information they need, that patients and consumers have the facts about the medicines and vaccines they require, and that products are prescribed, recommended, or used in a manner that provides healthcare benefit. We are committed to the ethical and responsible commercialisation of our products in support of our purpose to improve the quality of human life and get ahead of disease together. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Risk factors continued 256

Scientific and patient engagement Risk definition The risk that GSK or our third parties potentially fail to engage externally to gain insights, educate and communicate on the science of our medicines and associated disease areas, and provide grants and donations in a legitimate and transparent manner compliant with laws, regulations, industry codes and internal controls and requirements. Risk impact Without controls in place, the risk could result in real, perceived, or disguised promotion including off-label and prior authorisation promotion, and real or perceived provision of medical advice. This could lead to reputational damage, competitor complaints, regulatory inspections with subsequent corrective actions, or civil litigation. We must fully and appropriately engage externally to bring patient benefit, and to advance science and innovation, while delivering our strategy. Otherwise, we risk reducing the trust of the public, patients, healthcare professionals, payers, regulators, and governments. Context Scientific and patient engagements are diverse non- promotional activities directed at healthcare professionals, patients, payers, and external stakeholders. Such engagements aim to improve patient care through the exchange or provision of knowledge on the use of our products and related diseases. Scientific and patient engagement with external stakeholder groups is vital to GSK, as a research-based biopharma company that is ambitious for patients and to advance science and medicine. We expect our activities to be scientifically sound and accurate, conducted ethically and transparently, and compliant with applicable codes, laws, and regulations. There are many industry and local codes and laws and other regulations that apply (such as Privacy or Data integrity). That means measured risk-taking, rooted in sound ethical considerations, and principles-based decision-making, training, communication, and monitoring of such activities are key to managing the risk and enabling full and appropriate engagement. . Data ethics and privacy Risk definition The risk that GSK or our third parties potentially fail to ethically collect; use; re-use through artificial intelligence, data analytics or automation; secure; share and destroy personal information in accordance with laws, regulations, and internal controls and requirements. Risk impact Non-compliance with data privacy laws globally could lead to harm to individuals and GSK. It could also damage trust between GSK and individuals, communities, business partners and government authorities. Many countries have increased the enforcement powers of their data protection authorities by allowing them to impose significant fines, restrict cross-border data flows, or temporarily ban data processing. Many new national laws also enable individuals to bring collective legal actions against companies such as GSK for failing to follow data privacy laws. Context Data protection and privacy legislation is diverse, with limited global harmonisation or simplification, making it challenging for multinationals to standardise their approach to compliance. Governments are enforcing compliance with data protection and privacy laws more rigorously. The approach and focus of data protection and privacy regulators also differs between regions and countries, which creates further challenges for global organisations seeking to implement a single harmonised global privacy programme. Increases in the volume of data processed and advances in technology have resulted in a greater focus on data governance and the ethical use of personal information, over and above compliance with data privacy laws. Companies seeking to foster innovation in artificial intelligence and other new technologies are faced with evolving decisions from global policymakers on how best to promote trust in these systems and avoid unintended outcomes or harmful impacts. Additionally, there are a number of emerging laws concerning the localisation of data, restrictions on international transfers and data security, which are changing existing frameworks that GSK has previously relied upon. This increasing trend for data sovereignty affects our ability to drive medical innovation and to effectively operate internationally. Global regulators (such as the EU, UK, US and China) are also in the process of introducing legislation around the use of artificial intelligence and machine learning (AI/ML). There continues to be considerable uncertainty around the final version of these proposed laws. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Risk factors continued 257

Research practices Risk definition The risk that GSK or our third parties potentially fail to adequately conduct ethical and credible pre-clinical and clinical research, collaborate in research activities compliant with laws, regulations, and internal controls and requirements. Risk impact The potential impacts of the risk include harm to human subjects, reputational damage, failure to obtain the necessary regulatory approvals for our products, governmental investigation, legal proceedings brought against GSK by governmental and private plaintiffs (product liability suits and claims for damages), loss of revenue due to inadequate patent protection or inability to supply our products, and regulatory action such as fines, penalties, or loss of product authorisation. Poor data integrity and governance could compromise GSK’s R&D efforts and negatively impact our reputation. Any of these could materially and adversely affect our financial results and damage the trust of patients and customers. Context Research involving animals can raise ethical concerns. In many cases, however, research involving animals is the only way to investigate the effects of a potential new medicine in a living body other than in humans. Animal research provides critical information about the causes and mechanisms of diseases and therefore remains a vital part of our research. We continually seek ways in which we can minimise our use of animals in research, development, and testing, while complying with regulatory requirements and reducing the impact on the animals used. Human subject research is critical to assessing and demonstrating the safety and efficacy of our investigational products or further evaluating our products once they have been approved. This research includes clinical trials in healthy volunteers and patients and adheres to regulations and high ethical, medical, and scientific standards. We disclose the results of this research externally regardless of whether they reflect positively or negatively on our products, so that the scientific community can learn from the outcomes of our research. We also work with human biological samples which are fundamental to the discovery, development, and safety monitoring of our products. We are committed to managing human biological samples in accordance with relevant laws, regulations, and ethical principles, and in a manner that respects the interests of sample donors. Data is pivotal to our R&D strategy, and we are maximising the use of data to serve patients. Governing our data in accordance with relevant laws, regulations, contractual obligations, expectations, and our culture across data ethics, privacy, information and cyber security, and data integrity is essential. We use a wide variety of biological materials in the discovery, research, and development of our assets. We are committed to ensuring research is compliant with terms and conditions of licenses, agreements or authorisations under which we acquire, use, or transfer biological materials and technologies. Through the Convention on Biological Diversity (CBD) and the Nagoya Protocol, the international community has established a global framework regulating access to, and use of, genetic resources of non-human origin in research and development. We support the equitable access and fairness principles of access and benefit sharing (ABS) outlined in the CBD and the Nagoya Protocol. We also recognise the importance of appropriate, effective, and proportionate implementation measures at national and regional levels. Environment, health, and safety (EHS) Risk definition The risk that GSK or our third parties potentially fail to ensure appropriate controls and governance of the organisation's assets, facilities, infrastructure, and business activities, including execution of hazardous activities, handling of hazardous materials, or release of substances harmful to the environment that disrupts supply or harms employees, third parties or the environment. Risk impact Failure to manage EHS risks could lead to significant harm to people, the environment and the communities in which we operate, fines, inability to meet stakeholder expectations and regulatory requirements, litigation or regulatory action, and damage to the company’s reputation, which could materially and adversely affect our financial results. Context GSK is subject to the health, safety and environmental laws of various jurisdictions. These laws impose duties to protect people, the environment and the communities in which we operate. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Risk factors continued 258

Information and cyber security Risk definition The risk that GSK or our third parties potentially fail to ensure appropriate controls and governance to identify, protect, detect, respond, and recover from cyber incidents through unauthorised access, disclosure, theft, unavailability or corruption of GSK's information, key systems, or technology infrastructure in accordance with applicable laws, regulations, industry standards, internal controls and requirements. Risk impact Failure to adequately protect our information and systems against cyber security threats may cause harm to patients, workforce and customers, disruption to our business and/or loss of commercial or strategic advantage, regulatory sanction, or damage to our reputation. Context The external environment continues to be extremely challenging, making it hard to keep pace with increasingly sophisticated cyber security threats. Factors include increased geopolitical conflict and digital nationalism, rising frequency and severity of data breaches and the growing capability and sophistication of cyber threat actors with additional tools like generative AI to propagate their attacks. GSK’s business relies on operating a highly connected information network of internal and external systems which hold confidential research and development, manufacturing, commercial, workforce and financial data. This means that our systems and information have been and will continue to be targeted by cyber security threat actors. Acceleration in the use of digital, data and analytics, AI/ML and cloud computing capabilities to drive GSK’s pipeline, performance and productivity requires us to continuously adapt and strengthen our controls and defensive capabilities. GSK also relies on third-party contractors, partners and suppliers who face similar cyber security threats, which emphasises our focus on third party risk management. Additionally, hybrid working environments create a larger and more complex attack surface for cyber security threat actors to exploit. With employees accessing company resources from various locations and devices, new threats and vulnerabilities could arise. Supply continuity Risk definition The risk that GSK or our third parties potentially fail to deliver a continuous supply of compliant finished product or respond effectively to a crisis incident in a timely manner to recover and sustain critical supply operations. Risk impact We recognise how important the continuity of supply of our products is to the patients who rely on them. Supply disruption can lead to: – Product shortages and product recalls – Regulatory intervention – Reputational harm – Lost sales revenue Consequently, we need sophisticated end-to-end supply chain management with robust crisis management and business continuity plans in place to respond. Context We operate our supply chains in a continually evolving, highly regulated environment. There is no single set of global regulations which governs the manufacture and distribution of medicines, and we must adhere to the requirements in all those markets in which we licence, sell or manufacture our products. We rely upon our internal Quality Management System and our Internal Control Framework to ensure we maintain our licence to operate. Our complex end-to-end supply chains often involve third party suppliers, from Active Pharmaceutical Ingredient (API) manufacturers and raw material suppliers through to Third Party Logistics Providers and contract engineering firms. We have integrated risk management into our sourcing and day to day business processes, with emphasis on our Third- Party oversight. External factors continued to challenge supply continuity in 2023. The difficulties with sourcing bioscience materials has eased through the year. There is a new constraint with third party sterile manufacturing capacity which increases global competition for contract manufacturing operations. We continue to operate our global supply chains in a rapidly changing geopolitical environment. Increasing nationalism and friction between the US and China creates divergence from global supply strategy. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Risk factors continued 259

Climate change Risk definition Failure in the management of: – Physical climate and environmental risks; – Current and future regulatory requirements for environmental compliance, disclosure and taxes; – Delivery and performance of management environmental objectives; leading to: reduced supply chain resilience; product life cycle management issues, loss of trust/reputation with employees, investors, customers, regulators and other stakeholders; increased costs; loss of sales or market access; negative impacts on the environment. Risk impact We recognise that the way we respond to climate change and manage environmental risks affects our ability to supply products to patients and consumers and could lead to harm to the environment and our reputation. For example: – Changes to regulations governing the supply of high global warming potential (GWP) substances by the EU, UK and US governments could restrict our ability to manufacture metered dose inhalers; – Increasing levels of water stress could lead to interruptions to supply of water to our and third-party supply sites; – Increasing frequency and impact of extreme weather events that could disrupt GSK and third-party supplier sites; – Future regulatory policy responses to address climate change could lead to the imposition of carbon taxes by countries where we manufacture and source goods from third parties; – Failure to meet fast-evolving regulatory requirements on disclosures and environmental compliance could lead to regulatory actions or fines; – Failure to meet changing stakeholder expectations such as from health systems with increasing demands for low carbon medicines and vaccines, affecting demand for our products, which may have an adverse impact on our financial results and longer-term loss of trust, undermining the credibility of the company. Context It is increasingly understood that the interconnected effects of climate change, nature loss, and society’s impact on both are influencing human health. Internal and external expectations for companies to address their impact on the environment are increasing, as are the effects of climate change on operational resilience. Regulations on environmental compliance, disclosure and environmentally related taxation are rapidly evolving in jurisdictions around the world, such as the EU Corporate Sustainability Reporting Directive, and the proposed SEC Climate Ruling, which will require increasing levels of disclosure and data assurance. Our ability to meet our targets of reducing carbon emissions by 80% and 90% by 2030 and 2045, respectively, is based on successful regulatory outcomes from the programme to redevelop our Ventolin inhaler using a lower-carbon propellant. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Risk factors continued 260
Share capital and control Details of our issued share capital and the number of shares held in Treasury as at 31 December 2023 can be found in Note 37 to the financial statements, ‘Share capital and share premium account’. Our ordinary shares are listed on the London Stock Exchange (LSE) and are also quoted on the New York Stock Exchange (NYSE) in the form of American Depositary Shares (ADS). Each ADS represents two Ordinary Shares. For details of listed debt and where it is listed refer to Note 30 to the financial statements, ‘Net debt’. Holders of Ordinary Shares and ADS are entitled to receive dividends (when declared) and a copy of the company’s Annual Report (if elected). They are also entitled to attend, speak, appoint proxies and exercise voting rights at general meetings of the company There are no restrictions on the transfer, or limitations on the holding, of Ordinary Shares and ADS and no requirements to obtain approval prior to any transfers. No Ordinary Shares or ADS carry any special rights with regard to control of the company and there are no restrictions on voting rights. Major shareholders have the same voting rights per share as all other shareholders. There are no known arrangements under which financial rights are held by a person other than the holder of the shares and no known agreements on restrictions on share transfers or on voting rights. Shares acquired through the Group’s employee share plans rank equally with the other shares in issue and have no special rights. The trustees of our Employee Share Ownership Plan Trusts have waived their rights to dividends on shares held by those Trusts. Demerger of Haleon and Share Consolidation As reported previously, on 18 July 2022 the company completed the demerger of the Consumer Healthcare business from the Group. More details can be found on www.gsk.com/en-gb/ haleon-cmd-to-demerger-archive. On 19 July 2022, shareholders received four new GSK plc shares of nominal value of 31 1/4 pence each for every five GSK plc shares of nominal value of 25 pence each. The Group reduced its share holding in Haleon plc during the course of the financial year ended 31 December 2023 to 7.4%. More information can be found in Note 22 Current Equity Investments. On 17 January 2024, the Group reduced its shareholding by 3.2%, GSK now holds approximately 385 million ordinary shares in Haleon plc representing over 4.0% of the issued share capital of Haleon. More information can be found in Note 48 Post Balance Sheet Events. Exchange controls and other limitations affecting holders Other than certain economic sanctions, which may be in force from time to time, there are currently no applicable laws, decrees or regulations in force in the UK restricting the import or export of capital or restricting the remittance of dividends or other payments to holders of the company’s shares who are non-residents of the UK. Similarly, other than certain economic sanctions which may be in force from time to time, there are no limitations relating only to non-residents of the UK under English law or the company’s Articles of Association on the right to be a holder of, and to vote in respect of, the company’s shares. Interests in voting rights Other than as stated below, as far as as the company is aware, there are no persons with significant direct or indirect holdings in the company. Information provided to the company pursuant to the FCA's Disclosure Guidance and Transparency Rules (DTR 5) is published on a Regulatory Information Service and on the company’s website, gsk.com. The company has received notifications in accordance with DTR 5 of the following notifiable interests in the voting rights in the company’s issued share capital: 31 December 2023 23 February 2024 No. of voting rights Percentage of total voting rights(1) No. of voting rights Percentage of total voting rights(1) BlackRock, Inc. 231,975,400(2) 5.69 % 231,975,400(2) 5.69 % Dodge & Cox 253,464,108 (3) 5.04 % 253,464,108(3) 5.04 % (1) Percentage of total voting rights at the date of notification to the company. (2) Comprising an indirect interest in 229,134,683 Ordinary Shares and a holding of 2,840,717 Qualifying Financial Instruments (Contracts for Difference). (3) Comprising an indirect interest in 99,377,874 Ordinary Shares and 154,086,234 ADS. The company has not acquired or disposed of any interests in its own shares during the period under review. Share buy-back programme The Board has been authorised to issue and allot Ordinary Shares under Article 9 of the company’s Articles of Association. The power under Article 9 and the authority for the company to make purchases of its own shares are subject to shareholder authorities which are sought on an annual basis at our Annual General Meeting (AGM). Any shares purchased by the company may be cancelled, held as Treasury shares or used for satisfying share options and grants under the Group's employee share plans. Our programme covers purchases of shares for cancellation or to be held as Treasury shares, in accordance with the authority renewed by shareholders at the AGM in May 2023, when the company was authorised to purchase a maximum of just over 409 million shares. In determining specific share repurchase levels, the company considers the development of free cash flow during the year. No Treasury shares have been purchased since 2014. Details of shares purchased, cancelled, held as Treasury shares and subsequently transferred from Treasury to satisfy awards under the Group’s employee share plans are disclosed in Note 37 to the financial statements, ‘Share capital and share premium account’. The company confirms that it does not currently intend to make any market purchases in 2024. The company will continue to review the potential for future share buy-backs in line with its usual annual cycle and subject to return and ratings criteria. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Shareholder information 261
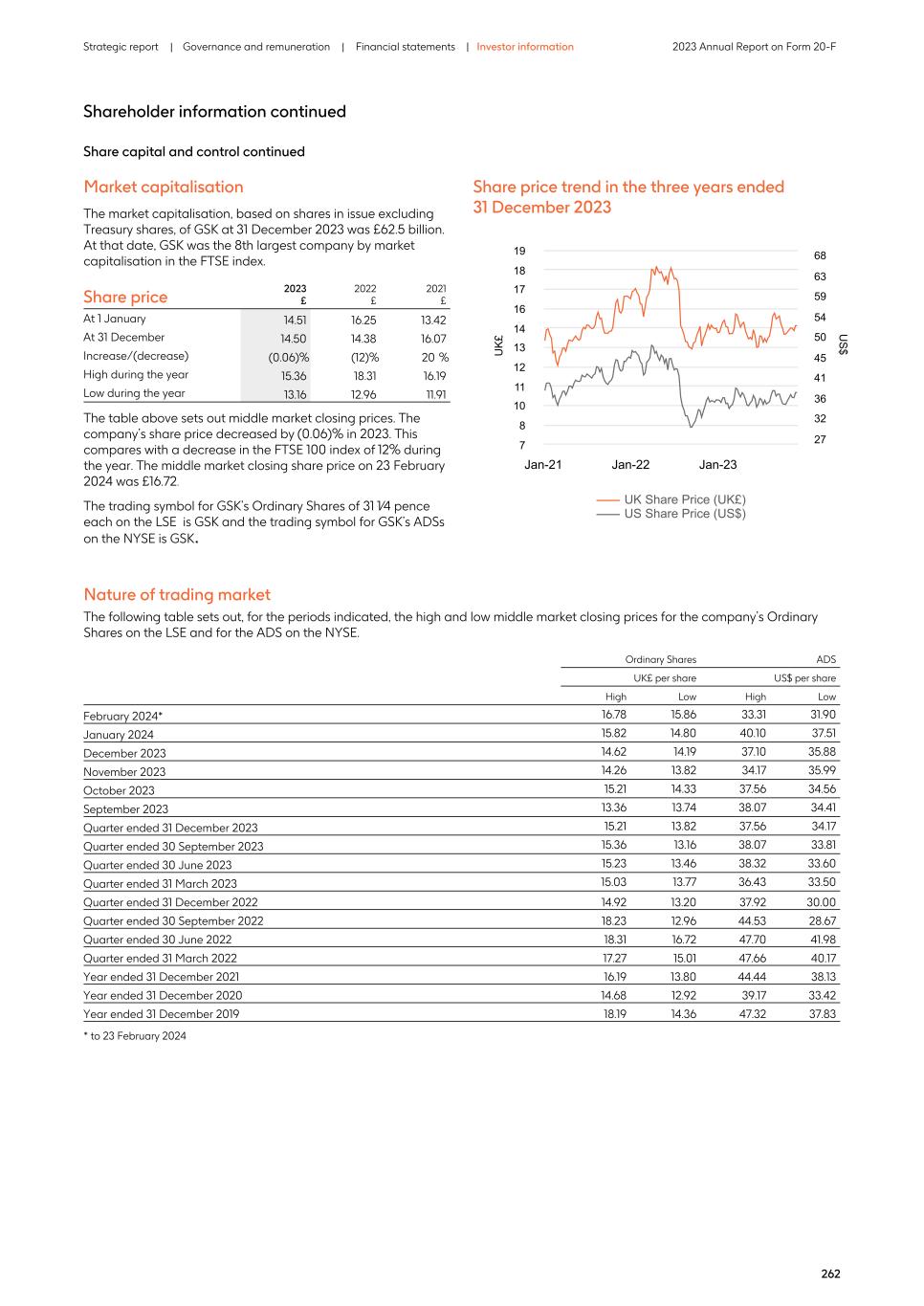
Market capitalisation The market capitalisation, based on shares in issue excluding Treasury shares, of GSK at 31 December 2023 was £62.5 billion. At that date, GSK was the 8th largest company by market capitalisation in the FTSE index. Share price 2023 £ 2022 £ 2021 £ At 1 January 14.51 16.25 13.42 At 31 December 14.50 14.38 16.07 Increase/(decrease) (0.06) % (12) % 20 % High during the year 15.36 18.31 16.19 Low during the year 13.16 12.96 11.91 The table above sets out middle market closing prices. The company’s share price decreased by (0.06)% in 2023. This compares with a decrease in the FTSE 100 index of 12% during the year. The middle market closing share price on 23 February 2024 was £16.72. The trading symbol for GSK’s Ordinary Shares of 31 1⁄4 pence each on the LSE is GSK and the trading symbol for GSK’s ADSs on the NYSE is GSK. Share price trend in the three years ended 31 December 2023 U K £ U S $ UK Share Price (UK£) US Share Price (US$) Jan-21 Jan-22 Jan-23 7 8 10 11 12 13 14 16 17 18 19 27 32 36 41 45 50 54 59 63 68 Nature of trading market The following table sets out, for the periods indicated, the high and low middle market closing prices for the company’s Ordinary Shares on the LSE and for the ADS on the NYSE. Ordinary Shares ADS UK£ per share US$ per share High Low High Low February 2024* 16.78 15.86 33.31 31.90 January 2024 15.82 14.80 40.10 37.51 December 2023 14.62 14.19 37.10 35.88 November 2023 14.26 13.82 34.17 35.99 October 2023 15.21 14.33 37.56 34.56 September 2023 13.36 13.74 38.07 34.41 Quarter ended 31 December 2023 15.21 13.82 37.56 34.17 Quarter ended 30 September 2023 15.36 13.16 38.07 33.81 Quarter ended 30 June 2023 15.23 13.46 38.32 33.60 Quarter ended 31 March 2023 15.03 13.77 36.43 33.50 Quarter ended 31 December 2022 14.92 13.20 37.92 30.00 Quarter ended 30 September 2022 18.23 12.96 44.53 28.67 Quarter ended 30 June 2022 18.31 16.72 47.70 41.98 Quarter ended 31 March 2022 17.27 15.01 47.66 40.17 Year ended 31 December 2021 16.19 13.80 44.44 38.13 Year ended 31 December 2020 14.68 12.92 39.17 33.42 Year ended 31 December 2019 18.19 14.36 47.32 37.83 * to 23 February 2024 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Shareholder information continued Share capital and control continued 262
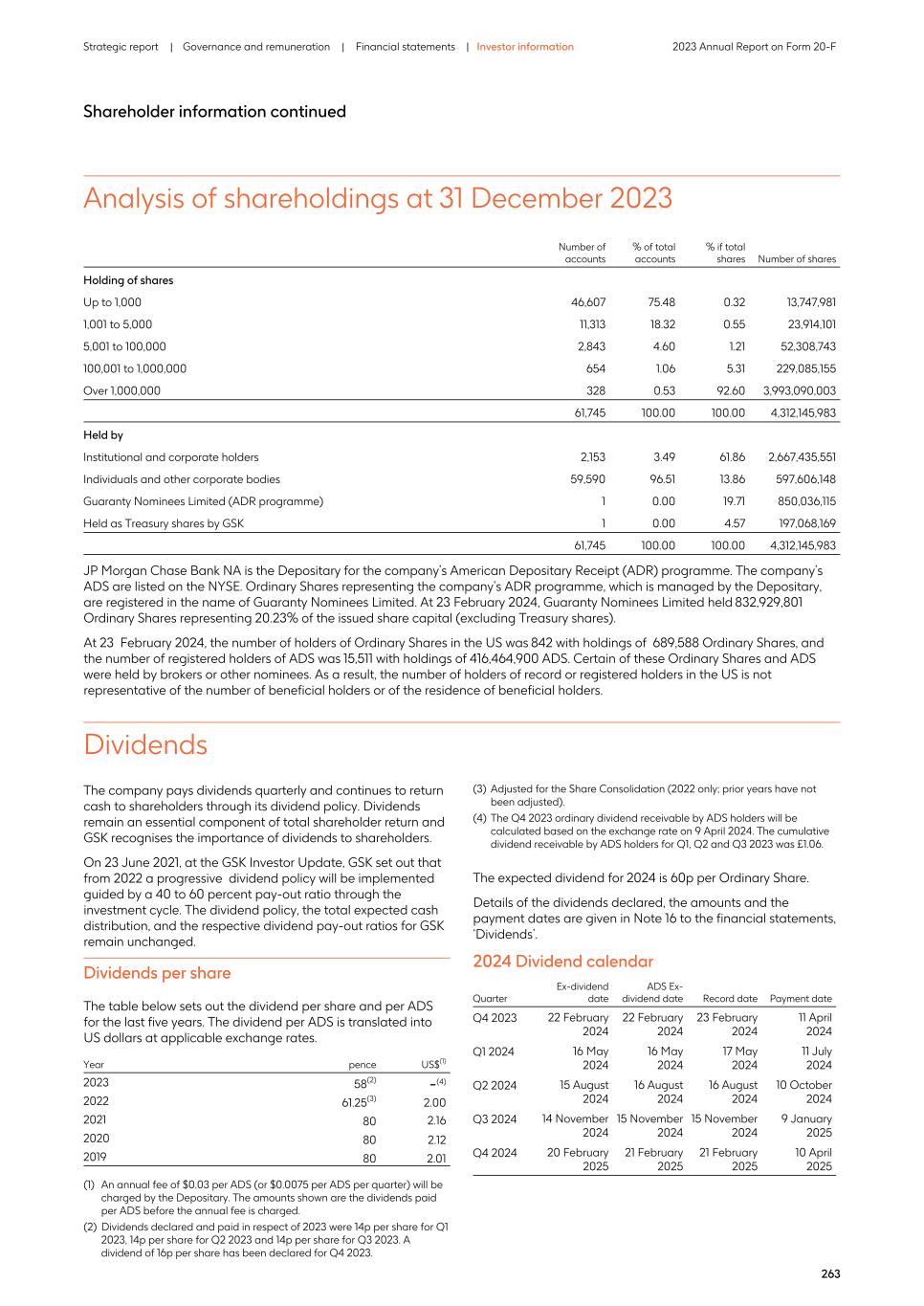
Analysis of shareholdings at 31 December 2023 Number of accounts % of total accounts % if total shares Number of shares Holding of shares Up to 1,000 46,607 75.48 0.32 13,747,981 1,001 to 5,000 11,313 18.32 0.55 23,914,101 5,001 to 100,000 2,843 4.60 1.21 52,308,743 100,001 to 1,000,000 654 1.06 5.31 229,085,155 Over 1,000,000 328 0.53 92.60 3,993,090,003 61,745 100.00 100.00 4,312,145,983 Held by Institutional and corporate holders 2,153 3.49 61.86 2,667,435,551 Individuals and other corporate bodies 59,590 96.51 13.86 597,606,148 Guaranty Nominees Limited (ADR programme) 1 0.00 19.71 850,036,115 Held as Treasury shares by GSK 1 0.00 4.57 197,068,169 61,745 100.00 100.00 4,312,145,983 JP Morgan Chase Bank NA is the Depositary for the company’s American Depositary Receipt (ADR) programme. The company’s ADS are listed on the NYSE. Ordinary Shares representing the company’s ADR programme, which is managed by the Depositary, are registered in the name of Guaranty Nominees Limited. At 23 February 2024, Guaranty Nominees Limited held 832,929,801 Ordinary Shares representing 20.23% of the issued share capital (excluding Treasury shares). At 23 February 2024, the number of holders of Ordinary Shares in the US was 842 with holdings of 689,588 Ordinary Shares, and the number of registered holders of ADS was 15,511 with holdings of 416,464,900 ADS. Certain of these Ordinary Shares and ADS were held by brokers or other nominees. As a result, the number of holders of record or registered holders in the US is not representative of the number of beneficial holders or of the residence of beneficial holders. Dividends The company pays dividends quarterly and continues to return cash to shareholders through its dividend policy. Dividends remain an essential component of total shareholder return and GSK recognises the importance of dividends to shareholders. On 23 June 2021, at the GSK Investor Update, GSK set out that from 2022 a progressive dividend policy will be implemented guided by a 40 to 60 percent pay-out ratio through the investment cycle. The dividend policy, the total expected cash distribution, and the respective dividend pay-out ratios for GSK remain unchanged. Dividends per share The table below sets out the dividend per share and per ADS for the last five years. The dividend per ADS is translated into US dollars at applicable exchange rates. Year pence US$(1) 2023 58(2) -(4) 2022 61.25(3) 2.00 2021 80 2.16 2020 80 2.12 2019 80 2.01 (1) An annual fee of $0.03 per ADS (or $0.0075 per ADS per quarter) will be charged by the Depositary. The amounts shown are the dividends paid per ADS before the annual fee is charged. (2) Dividends declared and paid in respect of 2023 were 14p per share for Q1 2023, 14p per share for Q2 2023 and 14p per share for Q3 2023. A dividend of 16p per share has been declared for Q4 2023. (3) Adjusted for the Share Consolidation (2022 only; prior years have not been adjusted). (4) The Q4 2023 ordinary dividend receivable by ADS holders will be calculated based on the exchange rate on 9 April 2024. The cumulative dividend receivable by ADS holders for Q1, Q2 and Q3 2023 was £1.06. The expected dividend for 2024 is 60p per Ordinary Share. Details of the dividends declared, the amounts and the payment dates are given in Note 16 to the financial statements, ‘Dividends’. 2024 Dividend calendar Quarter Ex-dividend date ADS Ex- dividend date Record date Payment date Q4 2023 22 February 2024 22 February 2024 23 February 2024 11 April 2024 Q1 2024 16 May 2024 16 May 2024 17 May 2024 11 July 2024 Q2 2024 15 August 2024 16 August 2024 16 August 2024 10 October 2024 Q3 2024 14 November 2024 15 November 2024 15 November 2024 9 January 2025 Q4 2024 20 February 2025 21 February 2025 21 February 2025 10 April 2025 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Shareholder information continued 263

Financial calendar 2024 Event Date Quarter 1 results announcement 1 May 2024 Annual General Meeting 8 May 2024 Quarter 2 results announcement 31 July 2024 Quarter 3 results announcement 30 October 2024 Preliminary/Quarter 4 Results announcement 5 February 2025 Annual Report publication February/March 2024 Annual Report distribution March 2024 Information about the company, including the share and ADS price, is available on our website at gsk.com. Information made available on the website does not constitute part of this Annual Report. Stock Exchange announcement notifications We provide shareholders with a service to receive automatic email notifications when we publish a stock exchange announcement. To receive email notifications, please sign up for announcements at gsk.com in the Investors section. Results announcements Results announcements are issued to the LSE and are available on its news service. They are also sent to the US Securities and Exchange Commission (SEC) and the NYSE, issued to the media and made available on our website. Financial reports The company publishes an Annual Report which is made available on our website from the date of publication. Shareholders may elect to receive notification by email of the publication of Annual Reports by registering on www.shareview.co.uk, and may also elect to receive a printed copy of the Annual Report by contacting our registrar, Equiniti Limited. Copies of previous Annual Reports are available on our website. Printed copies can also be obtained from our registrar (see page 276 for the contact details). Annual General Meeting 2024 Our Annual General Meeting (AGM) will be held at 2.30pm (UK time) on Wednesday, 8 May 2024 at the Royal Lancaster London, Lancaster Terrace, London W2 2TY and will also be broadcast live for you to join electronically. The AGM is the company’s principal forum for communication with private shareholders. In addition to the formal AGM business, there will be a presentation by the CEO on the performance of the Group and its future development. There will be an opportunity for questions to be asked of the Board and Chairs of the Board’s Committees will be available to take questions relating to their roles. Further details on how to access the AGM electronically or attend in person, ask questions and vote, can be found in the notice of Annual General Meeting 2024 (AGM Notice) which will be made available on our website at gsk.com on or around 25 March 2024. Investors holding shares through a nominee service should arrange with that service for them to be appointed as a proxy in respect of their shareholding to attend and vote at the meeting electronically. ADS holders wishing to attend the meeting electronically should refer to the AGM Notice for details on how to request a proxy appointment from the Depositary, JP Morgan Chase Bank NA. This will enable them to attend, ask questions and vote electronically on the business to be transacted at the meeting. ADS holders are reminded that if they do not instruct the Depositary as to the way in which the shares represented by their ADS should be voted by completing and returning the voting card provided by the Depositary, their shares will not be voted. Documents on display The Articles of Association of the company and Directors’ service contracts or, where applicable, letters of appointment between Directors and the company or any of its subsidiaries (and any side letters relating to severance terms and pension arrangements) are available for inspection at the company’s registered office and will be made available for inspection at the AGM. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Shareholder information continued 264

Tax information for shareholders A summary of certain UK tax and US federal income tax consequences for holders of shares and ADS who are citizens of the UK or the US is set out below. It is not a complete analysis of all the possible tax consequences of the purchase, ownership or sale of these securities. It is intended only as a general guide. Holders are advised to consult their advisers with respect to the tax consequences of the purchase, ownership or sale of their shares or ADS and the consequences under state and local tax laws in the US and the implications of the current UK/US tax conventions. US holders of ADS generally will be treated as the owners of the underlying shares for the purposes of the current UK/US double taxation conventions relating to income and gains (Income Tax Convention), estate and gift taxes (Estate and Gift Tax Convention), and for the purposes of the Internal Revenue Code of 1986, as amended. UK shareholders This summary only applies to a UK resident shareholder that holds shares as capital assets. Taxation of dividends For the 2023/24 UK tax year, UK resident individuals are entitled to a dividend tax allowance of up to £1,000, so that the first £1,000 of dividends received in a tax year will be free of tax. Dividends in excess of this allowance will be taxed at 8.75% for basic rate taxpayers, 33.75% for higher rate taxpayers and 39.35% for additional rate taxpayers. Note that from 6 April 2024 the dividend allowance will be reduced to £500. UK resident shareholders that are corporation taxpayers should note that dividends payable on ordinary shares are generally entitled to exemption from corporation tax. Taxation of capital gains UK resident shareholders may be liable for UK tax on gains on the disposal of shares or ADS. For disposals by individuals in the 2023/24 UK tax year, a taxable capital gain accruing on a disposal of shares or ADS will be taxed at 10% for basic rate taxpayers, or 20% if, after all allowable deductions, the individual’s taxable income for the year exceeds the basic rate income tax banding. Note this is following the use of any exemptions available to the individual taxpayer such as the annual exempt amount. Corporation taxpayers may be entitled to an indexation allowance which applies to reduce capital gains to the extent that such gains arise due to inflation. Indexation allowance may reduce a chargeable gain but will not create an allowable loss. For assets acquired on or before 1 January 2018, legislation in the Finance Act 2018 freezes the level of indexation allowance that is given in calculating a company’s chargeable gains at the value that would apply to the disposal of an asset in December 2017. For assets acquired from 1 January 2018 onwards, legislation in the Finance Act 2018 removes any indexation allowance on disposal. Inheritance tax Individual (UK-domiciled or otherwise) shareholders may be liable to UK inheritance tax on the transfer of shares or ADS. Exposure to a UK inheritance tax charge typically occurs on the death of the asset owner. However, transfers of shares (other than commercial sales) within seven years of death remain relevant to any inheritance tax exposure at death. Further, transfers to a trust arrangement during lifetime can give rise to an immediate inheritance tax charge. Tax may be charged on the amount by which the value of the shareholder’s estate is reduced as a result of any transfer by way of lifetime gift or other disposal at less than full market value. In the case of a bequest on death, tax may be charged on the value of the shares at the date of the shareholder’s death. Where an exposure to UK inheritance tax and US estate or gift tax exists, careful planning must be undertaken to understand the opportunity to utilise the US/UK Estate and Gift Double Tax Convention to manage tax credits and avoid double taxation. The overall exposure will be dependent on the specific circumstances of each situation and it is also important to note that tax charges may arise in other jurisdictions. Bespoke advice tailored to an individual’s personal circumstances should therefore be obtained from a tax professional. Stamp duty and stamp duty reserve tax UK stamp duty and/or stamp duty reserve tax (SDRT) will, subject to certain exemptions, be payable on the transfer of shares at a rate of 0.5% (rounded up to the nearest £5 in the case of stamp duty) of the consideration for the transfer. Notwithstanding this, provided that an instrument is executed in pursuance of the agreement that gave rise to the charge to SDRT and that instrument is stamped within six years of the agreement (including being stamped as exempt) any SDRT charge should be cancelled and any SDRT which has already been paid will be repaid. Where listed shares are transferred to a company connected to the transferor the chargeable consideration will be deemed to be not less than the market value of the shares transferred. This market value override also applies where non-listed shares are transferred to a company connected to the transferor where the consideration includes an issue of shares. US shareholders This summary only applies to a shareholder (who is a citizen or resident of the US or a domestic corporation or a person that is otherwise subject to US federal income tax on a net income basis in respect of the shares or ADS) that holds shares or ADS as capital assets, is not resident in the UK for UK tax purposes and does not hold shares for the purposes of a trade, profession or vocation that is carried on in the UK through a branch or agency. The summary also does not address the tax treatment of holders that are subject to special tax rules, such as banks, tax- exempt entities, insurance companies, dealers in securities or currencies, persons that hold shares or ADS as part of an integrated investment (including a ‘straddle’) comprised of a share or ADS and one or more other positions, and persons that own (directly, indirectly or constructively) 10% or more of the company’s stock (by vote or value), nor does it address tax treatment that may be applicable as a result of international income tax treaties. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Shareholder information continued 265

Taxation of dividends The gross amount of dividends received is treated as foreign source dividend income for US tax purposes. It is not eligible for the dividend received deduction allowed to US corporations. Dividends on ADS are payable in US dollars; dividends on Ordinary Shares are payable in sterling. Dividends paid in sterling will be included in income in the US dollar amount calculated by reference to the exchange rate on the day the dividends are received by the holder. Subject to certain exceptions for short-term or hedged positions, an individual eligible US holder will be subject to US taxation at a maximum federal rate of 23.8% plus applicable state and local tax in respect of qualified dividends. A qualified dividend as defined by the US Internal Revenue Service (IRS) is a dividend that meets the following criteria: 1. It must be issued by a US corporation, a corporation incorporated in a US possession, or a corporation that is eligible for the benefits of a comprehensive income tax treaty deemed satisfactory, as published by the IRS. 2. The dividends are not of a type listed by the IRS as dividends that do not qualify. 3. The required dividend holding period has been met. The shares must have been owned by you for more than 60 days of the ‘holding period’ – which is defined as the 121-day period that begins 60 days before the ex-dividend date, or the day in which the stock trades without the dividend priced in. For example, if a stock’s ex-dividend date is 1 October, the shares must be held for more than 60 days in the period between 2 August and 30 November of that year in order to count as a qualified dividend. Dividends that are not qualified are subject to taxation at the US federal graduated tax rates, at a maximum rate of 40.8%. Some types of dividends are automatically excluded from being qualified dividends, even if they meet the other requirements. These include (but are not limited to): – Capital gains distributions – Dividends on bank deposits – Dividends held by a corporation in an Employee Stock Ownership Plan (ESOP) – Dividends paid by tax-exempt corporations. US state and local tax rates on qualified and non-qualified dividends may vary and would be assessed in addition to the federal tax rates communicated above. Taxation of capital gains Generally, US holders will not be subject to UK capital gains tax, but will be subject to US tax on capital gains realised on the sale or other disposal of shares or ADS. Such gains will be long- term capital gains (subject to reduced rates of taxation for individual holders) if the shares or ADS were held for more than one year, from the date the shares were vested/released. Short- term capital gains can be subject to taxation of rates of up to 40.8%, whereas long-term capital gains may be subject to rates of up to 23.8%. State and local tax rates on capital gains may also apply. Information reporting and backup withholding Dividends and payments of the proceeds on a sale of shares or ADS, paid within the US or through certain US-related financial intermediaries, are subject to information reporting and may be subject to backup withholding unless the US holder is a corporation or other exempt recipient or provides a taxpayer identification number and certifies that no loss of exemption has occurred. Non-US holders generally are not subject to information reporting or backup withholding, but may be required to provide a certification of their non-US status in connection with payments received. Any amounts withheld will be allowed as a refund or credit against a holder’s US federal income tax liability provided the required information is furnished to the IRS. Estate and gift taxes Under the Estate and Gift Tax Convention, a US shareholder is not generally subject to UK inheritance tax. However, a US holder may be subject to US federal estate and gift tax. Stamp duty UK stamp duty and/or SDRT will, subject to certain exemptions, be payable on any transfer of shares to the ADS custodian or depositary at a rate of 1.5% of the amount of any consideration provided (if transferred on sale), or their value (if transferred for no consideration). However, no stamp duty or SDRT should be payable on the transfer of, or agreement to transfer an ADS or on transfers within the clearance service. Notwithstanding the above, where the clearance service operator has made an election under s97A Finance Act 1986, broadly the 1.5% stamp duty/SDRT charge should not arise on the transfer into the clearance service, but transfers to, and within, the system (where there is a change in beneficial ownership) would attract a 0.5% charge. Demerger and share consolidation A summary of certain UK and US tax consequences in respect of the demerger of Haleon plc and the consolidation of the company's share capital, relevant to the company’s shareholders who are resident (or, in the case of individuals, resident and domiciled) in the UK for UK tax purposes or who are citizens of or resident in the US for US tax purposes, is set out in Part 6 of the circular in relation to the Demerger and the Share Consolidation published on 1 June 2022 (Circular) (pages 83 to 89). The Circular, along with other information regarding the demerger and share consolidation can be found at gsk.com in the demerger section. Further information on the tax base cost allocation to assist UK shareholders apportion their base cost between their GSK plc shares and Haleon plc shares for UK capital gains tax purposes following the demerger, including a worked example, can be found in the Tax section at gsk.com in the demerger section. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Shareholder information continued Tax information for shareholders continued 266

Articles of Association of GSK plc The following is a summary of the principal provisions of the company’s Articles of Association (the “Articles”). Shareholders should not rely on this summary, but should instead refer to the current Articles which are filed with the Registrar of Companies in the UK and can be viewed on the company’s website. The Articles contain the fundamental provisions of the company’s constitution, and the rules for the internal management and control of the company. The company has no statement of objects in its Articles and accordingly its objects are unrestricted in accordance with the provisions of the Companies Act 2006. (a) Voting All resolutions put to the vote at general meetings, including electronic general meetings (see paragraph (h)), will be decided by poll. On a poll, every shareholder who is present in person or by proxy or, in the case of an electronic general meeting, who participates or is represented by proxy via an electronic platform shall have one vote for every Ordinary Share of which they are the holder. In the case of joint holders of a share, the vote of the senior who tenders a vote, whether in person or by proxy, shall be accepted to the exclusion of the votes of the other joint holders, and seniority shall be determined by the order in which the names stand on the register. Unless the Directors otherwise decide, the right to attend a general meeting and voting rights may not be exercised by a shareholder who has not paid to the company all calls and other sums then payable by them in respect of their Ordinary Shares. The right to attend a general meeting and voting rights may not be exercised by a shareholder who is subject to an order under Section 794 of the Companies Act 2006 because they have failed to provide the company with information concerning their interests in Ordinary Shares within the prescribed period, as required by Section 793 of the Companies Act 2006. (b) Transfer of Ordinary Shares Any shareholder may transfer their Ordinary Shares which are in certificated form by an instrument of transfer in any usual form or in any other form which the Directors may approve. Such instrument must be properly signed and stamped or certified (or otherwise shown to the satisfaction of the Directors as being exempt from stamp duty) and lodged with the company together with the relevant share certificate(s) and such other evidence as the Directors may reasonably require to show the right of the transferor to make the transfer. Any member may transfer title to their uncertificated Ordinary Shares by means of a relevant system, such as CREST. The transferor of a share is deemed to remain the holder until the transferee’s name is entered on the register. The Directors may decline to register any transfer of any Ordinary Share which is not fully paid. Registration of a transfer of uncertificated Ordinary Shares may be refused in the circumstances set out in the uncertificated securities rules, and where, in the case of a transfer to joint holders, the number of joint holders to whom the uncertificated Ordinary Share is to be transferred exceeds four. The Articles contain no other restrictions on the transfer of fully paid certificated Ordinary Shares provided: (i) the instrument of transfer is duly stamped or certified or otherwise shown to the satisfaction of the Directors to be exempt from stamp duty and is accompanied by the relevant share certificate and such other evidence of the right to transfer as the Directors may reasonably require; (ii) the transfer, if to joint transferees, is in favour of not more than four transferees; (iii) the instrument of transfer is in respect of only one class of shares; and (iv) the holder of the Ordinary Shares is not subject to an order under Section 794 of the Companies Act 2006. Notice of refusal to register a transfer must be sent to the transferee within two months of the instrument of transfer being lodged. The Directors may decline to register a transfer of Ordinary Shares by a person holding 0.25 per cent. or more of the existing Ordinary Shares if such person is subject to an order under Section 794 Companies Act 2006, after failure to provide the company with information concerning interests in those Ordinary Shares required to be provided under Section 793 of the Companies Act 2006, unless the transfer is carried out pursuant to an arm’s length sale. Provisions in the Articles will not apply to uncertificated Ordinary Shares to the extent that they are inconsistent with: (i) the holding of Ordinary Shares in uncertificated form; (ii) the transfer of title to Ordinary Shares by means of a system such as CREST; and (iii) any provisions of the relevant regulations. (c) Dividends and distribution of assets on liquidation The profits of the company which are available for distribution and permitted by law to be distributed and which the company may by ordinary resolution from time to time declare, upon the recommendation of the Directors to distribute by way of dividend, in respect of any accounting reference period shall be distributed by way of dividend among holders of Ordinary Shares. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Additional information 267

If in their opinion the company’s financial position justifies such payments, the Directors may, as far as any applicable legislation allows, pay interim dividends on shares of any class of such amounts and in respect of such periods as they think fit. Except in so far as the rights attaching to, or the terms of issue of, any share otherwise provide, all dividends will be declared, apportioned and paid pro rata according to the amounts paid up on the shares during any portion of the period in respect of which the dividend is paid. As the company has only one class of Ordinary Shares, the holders of such Ordinary Shares will be entitled to participate in any surplus assets on a winding-up in proportion to their shareholdings. (d) Variation of rights and changes in capital Subject to the provisions of any statute (including any orders, regulations or other subordinate legislation made under it) from time to time in force concerning companies in so far as it applies to the company (the “Companies Acts”), the rights attached to any class of shares may be varied with the written consent of the holders of three-quarters in nominal value of the issued shares of that class (excluding any shares of that class held as treasury shares) or with the sanction of a special resolution passed at a separate meeting of the holders of shares of that class. At every such separate meeting, the provisions of the Articles relating to general meetings shall apply, except the necessary quorum shall be at least two persons entitled to vote and holding or representing as proxy at least one-third in nominal value of the issued shares of the relevant class (excluding any shares of that class held as treasury shares) (but provided that at any adjourned meeting one holder of shares of the relevant class present in person or by proxy shall be a quorum). If new shares are created or issued which rank equally with any other existing shares, or if the company purchases or redeems any of its own shares, the rights of existing shares will not be regarded as changed or abrogated unless the terms of the existing shares expressly say otherwise. (e) Unclaimed dividends All dividends or other sums payable on or in respect of any Ordinary Shares which remain unclaimed may be invested or otherwise made use of by the Directors for the benefit of the company until claimed. Unless the Directors decide otherwise, any dividend or other sums payable on or in respect of any Ordinary Shares unclaimed after a period of six years from the date when declared or became due for payment will be forfeited and revert to the company. The company may stop sending dividend cheques or warrants by post, or employ such other means of payment in respect of any Ordinary Shares, if at least two consecutive payments have remained uncashed or are returned undelivered or if one payment has remained uncashed or is returned undelivered and the company cannot establish a new address for the holder after making reasonable enquiries; however, in either case, the company must resume sending cheques or warrants or employ such other means of payment if the holder or any person entitled to the Ordinary Shares by transmission requests the resumption in writing. (f) Untraced shareholders The company may sell any certificated Ordinary Shares in the company after using reasonable efforts to trace the holder of, or person entitled by transmission to, the Ordinary Shares and sending a notice to the registered address or last known address of the holder or other person entitled in accordance with the requirements of the Articles and waiting for three months if the Ordinary Shares have been in issue for at least ten years and during that period at least three dividends have become payable on them and have not been claimed or satisfied and, so far as any Director is aware, the company has not received any communication from the holder of the Ordinary Shares or any person entitled to them by transmission. Upon any such sale, the company will become indebted to the former holder of the Ordinary Shares or the person entitled to them by transmission for an amount equal to the net proceeds of sale unless and until forfeited. If no valid claim for the money has been received by the company during a period of two years from the date on which the relevant shares were sold by the company, the money will be forfeited and will belong to the company. (g) Limitations on rights of non-resident or foreign shareholders There are no limitations imposed by the Articles on the rights of non-resident or foreign shareholders except that there is no requirement for the company to serve notices on shareholders outside the United Kingdom and the United States, if no postal address in the United States or United Kingdom has been provided to the company. The company may choose not to serve, send or supply any notice to a particular shareholder where it considers this necessary or appropriate to deal with legal, regulatory or practical problems in, or under the laws of, any territory. (h) General meetings of shareholders The Articles rely on the Companies Act 2006 provisions dealing with the calling of general meeting. The company is required by the Companies Act 2006 to hold an annual general meeting each year. General meetings of shareholders may be called as necessary by the Directors and must be called promptly upon receipt of a requisition from shareholders. Under the Companies Act 2006, an annual general meeting must be called by notice of at least 21 clear days. A general meeting other than an annual general meeting may be called on not less than 14 clear days’ notice provided a special resolution reducing the notice period to 14 clear days has been passed at the immediately preceding annual general meeting or a general meeting held since that annual general meeting. The Directors may determine that a general meeting shall be held as a physical meeting or in combination with an electronic platform or platforms that enables members to participate in the meeting without physically attending (an electronic general meeting). Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Additional information continued 268

(i) Conflicts of interest The Directors may, subject to the provisions of the Articles, authorise any matter which would otherwise involve a Director breaching their duty under the Companies Acts to avoid conflicts of interest (each a “Conflict”). A Director seeking authorisation in respect of a Conflict shall declare to the other Directors the nature and extent of their Conflict as soon as is reasonably practicable and shall provide the other Directors with such details of the matter as are necessary to decide how to address the Conflict. The board may resolve to authorise the relevant Director in relation to any matter the subject of a Conflict, save that the relevant Director and any other Director with a similar interest shall not count towards the quorum nor vote on any resolution giving such authority, and, if the other Directors so decide, shall be excluded from any meeting of the Directors while the Conflict is under consideration. (j) Other Conflicts of Interest Subject to the provisions of the Companies Acts, and provided the nature and extent of a Director’s interest has been declared to the Directors, a Director may: (i) be party to, or otherwise interested in, any contract with the company, or in which the company has a direct or indirect interest; (ii) hold any other office or place of profit with the company (except that of auditor) in conjunction with their office of director for such period and upon such terms, including remuneration, as the Directors may decide; (iii) act by themselves or through a firm with which they are associated in a professional capacity for the company or any other company in which the company may be interested (otherwise than as auditor); (iv) be or become a director of, or employed by, or otherwise be interested in any holding company or subsidiary company of the company or any other company in which the company may be interested; and (v) be or become a director of any other company in which the company does not have an interest and which cannot reasonably be regarded as giving rise to a conflict of interest at the time of their appointment as director of that other company. No contract in which a Director is interested shall be liable to be avoided, and any Director who is so interested is not liable to account to the company or its shareholders for any benefit realised by the contract by reason of the Director holding that office or of the fiduciary relationship thereby established. However, no Director may vote on, or be counted in the quorum, in relation to any resolution of the board relating specifically to their own appointment (including remuneration) or the terms of their termination of appointment or relating to any contract in which they have an interest (subject to certain exceptions). Subject to the Companies Acts, the company may by ordinary resolution suspend or relax to any extent the provisions relating to directors’ interests or restrictions on voting or ratify any transaction not duly authorised by reason of a contravention of such provisions. (k) Directors’ remuneration Each of the Directors will be paid a fee at such rate as may from time to time be determined by the Directors, but the total fees paid to all of the directors for acting as directors (excluding any amounts paid under any other provision of the Articles) shall not exceed the higher of: (i) £3 million a year; and (ii) any higher amount as the company may by ordinary resolution decide. Such fees may be satisfied in cash or in shares or any other non-cash form. Any Director who devotes special attention to the business or performs any services which the Directors consider to extend beyond the ordinary services of a Director shall be entitled to receive such remuneration (whether by way of salary, commission or otherwise) as the Directors may decide. Each Director may be paid reasonable travelling, hotel and other incidental expenses they incur in attending and returning from meetings of the Directors or committees of the Directors, or general meetings of the company, or otherwise incurred in connection with the performance of their duties for the company. (l) Pensions and gratuities for Directors The Directors or any committee authorised by the Directors may provide benefits by the payment of gratuities, pensions or insurance or in any other manner for any Director or former Director or their relations, connected persons or dependants, but no benefits (except those provided for by the Articles) may be granted to or in respect of a Director or former Director who has not been employed by or held an executive office or place of profit under the company or any of its subsidiary undertakings or their respective predecessors in business without the approval of an ordinary resolution of the company. (m) Borrowing powers Subject to the provisions of the Companies Act 2006, the Directors may exercise all the company’s powers to borrow money; to mortgage or charge all or any of the company’s undertaking, property (present and future), and uncalled capital; to issue debentures and other securities; and to give security either outright or as collateral security for any debt, liability or obligation of the company or of any third party. (n) Retirement and removal of Directors A Director is subject to re-election at every annual general meeting of the company. In addition to any power of removal conferred by the Companies Acts the company may by special resolution remove any Director before the expiration of their period of office. No Director is required to retire by reason of their age, nor do any special formalities apply to the appointment or re-election of any Director who is over any age limit. No shareholding qualification for Directors shall be required. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Additional information continued 269

(o) Vacation of office The office of a director shall be vacated if: (i) they resign or offer to resign, and the board resolves to accept such offer; (ii) their resignation is requested by all of the other directors and all of the other directors are not less than three in number; (iii) they are or have been suffering from mental or physical ill health and the board resolves that their office be vacated; (iv) they are absent without permission of the board from meetings of the board (whether or not an alternate director appointed by them attends) for six consecutive months and the board resolves that their office is vacated; (v) they become bankrupt or compounds with their creditors generally; (vi) they are prohibited by law from being a director; or (vii)they are removed from office pursuant to the Articles or the Companies Acts. (p) Share rights Subject to any rights attached to existing shares, shares may be issued with such rights and restrictions as the company may by ordinary resolution decide, or (if there is no such resolution or so far as it does not make specific provision) as the board may decide. Such rights and restrictions shall apply as if they were set out in the Articles. Redeemable shares may be issued, subject to any rights attached to existing shares. The board may determine the terms, conditions and manner of redemption of any redeemable share so issued. Such terms and conditions shall apply to the relevant shares as if they were set out in the Articles. Subject to the articles, any resolution passed by the shareholders and other shareholders’ rights, the Board may decide how to offer, allot, grant options over or otherwise deal with any shares in the company. Material contracts Agreements with Novartis On April 22, 2014, GSK and Novartis AG (“Novartis”) entered into a three-part, inter-conditional transaction, which they executed, among other agreements, a share and business sale agreement relating to the vaccines business of Novartis. GSK’s shareholders approved the transaction on December 18, 2014. The transaction closed on March 2, 2015. Under the terms of the shareholders’ agreement, Novartis had the right to require GSK to purchase its shares in the consumer healthcare joint venture. On June 1, 2018, GSK acquired 100% of the shares in GlaxoSmithKline Consumer Healthcare Holdings Limited (“GSK Consumer Healthcare”) following cancellation of Novartis’s shares under the terms of a put option implementation agreement among GSK, Novartis and GSK Consumer Healthcare, among others. GSK continues to have obligations to pay further sales and milestone-based consideration to Novartis under the share and business sale agreement relating to the vaccines business of Novartis. Agreement with Pfizer On December 19, 2018, GSK, GSK Consumer Healthcare and Pfizer Inc. (“Pfizer”) entered into a Stock and Asset Purchase Agreement (the “SAPA”) pursuant to which the parties agreed to form a consumer healthcare joint venture (the “GSK/Pfizer JV”) through the acquisition by GSK Consumer Healthcare from Pfizer of Pfizer’s consumer healthcare business and the transfer by GSK to GSK Consumer Healthcare of those parts of the GSK consumer healthcare business not already part of GSK Consumer Healthcare as of the date of the SAPA (with certain limited exceptions). As consideration for the acquisition of its consumer healthcare business, Pfizer received shares in GSK Consumer Healthcare representing a 32% ownership interest in the GSK/Pfizer JV. GSK retained a controlling interest in GSK Consumer Healthcare of 68%. On July 31, 2019, the parties entered into an amendment to the SAPA, pursuant to which: (i) GSK Consumer Healthcare transferred by novation to GlaxoSmithKline Consumer Healthcare Holdings (No. 2) Limited (“GSK Consumer Healthcare (No. 2)”) all rights, title, interest, obligations duties and liabilities of GSK Consumer Healthcare under and in respect of the SAPA, (ii) the parties released GSK Consumer Healthcare from its obligations under the SAPA in exchange for GSK Consumer Healthcare (No. 2)’s assumption thereof and (iii) certain other amendments to the SAPA and other arrangements in connection with the closing of the transaction, including in relation to the delayed legal completion of the transaction in a number of jurisdictions due to regulatory constraints. The transaction closed on July 31, 2019. Each of GSK and Pfizer gave customary and broadly reciprocal representations and warranties to each other under the SAPA. GSK and Pfizer agreed to indemnify each other and GSK Consumer Healthcare (No. 2) (as applicable) in respect of losses (other than certain losses arising from tax matters, which are subject to a specific indemnity under the SAPA) relating to: (i) certain liabilities which the parties agreed will be retained by GSK or Pfizer; (ii) any breach of their respective covenants or agreements under the SAPA or the related ancillary agreements implementing the SAPA; or (iii) any breach of their respective representations and warranties given under the SAPA or the related ancillary agreements implementing the SAPA as of the date of completion of the transaction. GSK Consumer Healthcare (No. 2) agreed to indemnify GSK and Pfizer in respect of losses (other than certain losses arising from tax matters, which are subject to a specific indemnity under the SAPA) relating to: (i) liabilities which GSK Consumer Healthcare (No. 2) agreed to assume in connection with the transaction; (ii) liabilities resulting from the conduct of GSK Consumer Healthcare’s business other than those liabilities that GSK agreed to retain in connection with the transaction; and (iii) any breach of GSK Consumer Healthcare (No.2)’s post-completion covenants or agreements under the SAPA or the related ancillary agreements implementing the SAPA. On June 1, 2022, GSK, Pfizer, GSK Consumer Healthcare (No. 2) and Haleon plc (“Haleon”) entered into the second amendment agreement to the SAPA to implement certain amendments in connection with the demerger of the Consumer Healthcare business (the “Demerger”) and to include Haleon in the SAPA indemnity framework by way of a guarantee given by Haleon with respect to the indemnification obligations of GSK Consumer Healthcare (No. 2) under the SAPA. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Additional information continued 270

Demerger Agreements On June 1, 2022, GSK and Haleon entered into a demerger agreement (the “Demerger Agreement”) to effect the Demerger and to govern aspects of the relationship between GSK and Haleon following completion of the Demerger, including in respect of, among other things, confidentiality and certain indemnity obligations in connection with the issuance of shares by Haleon in connection with the Demerger. The Demerger Agreement contains certain customary indemnities under which GSK indemnifies Haleon in respect of liabilities, losses demands, claims, costs, taxes and damages arising, directly or indirectly, from or in consequence of certain claims. The Demerger Agreement also sets out how guarantees given by the GSK group for the benefit of companies in the Haleon group (or vice versa) will be dealt with following the Demerger. In addition, on June 1, 2022, GSK and Haleon entered into an exchange agreement with respect to the transfer by GSK of its B Ordinary Shares in GSK Consumer Healthcare to Haleon in exchange for the issuance of shares by Haleon. On June 1, 2022 GSK, GSK Consumer Healthcare and GSK Consumer Healthcare (No. 2) entered into an asset transfer framework agreement (the “Asset Transfer Framework Agreement”), setting out the framework for the transfer of certain businesses, assets, liabilities and employees that were excluded from the original perimeter of the GSK/Pfizer JV as contemplated in the SAPA and others that were included in the original perimeter of the GSK/Pfizer JV but had not yet legally transferred or to record the transfer of other assets under the SAPA, in each case from the GSK group to the Haleon group. The Asset Transfer Framework Agreement also sets out the framework for the transfer of certain businesses, assets, liabilities and employees from the Haleon group to the GSK group. On June 1, 2022, GSK, Pfizer, Anacor Pharmaceuticals, Inc., Haleon, GSK Consumer Healthcare, PF Consumer Healthcare Holdings LLC and GSK Consumer Healthcare (No. 2) entered into a Separation Co-operation and Implementation Agreement (the “SCIA”). The SCIA detailed certain actions to be taken and arrangements to be implemented to effect completion of, or which otherwise related to, the Demerger. The SCIA recorded the obligations of the parties relating to such matters and contained certain terms on which relations between the parties are governed following completion of the Demerger. The parties to the SCIA agreed to co-operate to achieve completion of the Demerger and undertook to take all steps required, and to enter into (or procure the entry into of) all documents required, to effect the Demerger. Further, on June 1, 2022, GlaxoSmithKline Services Unlimited, GlaxoSmithKline LLC, GlaxoSmithKline Consumer Healthcare (Overseas) Limited and GlaxoSmithKline Consumer Healthcare Holdings (US) LLC entered into a Transition Services Agreement, pursuant to which each group agreed to provide limited services to the other on commercial terms and on an arms’ length basis for a transitional period, effective from completion of the Demerger. Finally, on June 1, 2022, each of GlaxoSmithKline Trading Services Limited and GlaxoSmithKline Consumer Trading Services Limited entered into two Manufacturing and Supply Agreements with the other (each a “Manufacturing and Supply Agreement”). Pursuant to each Manufacturing and Supply Agreement, the parties agreed, to the extent required, to supply the other with pharmaceutical or Consumer Healthcare products (as the case may be) from the relevant manufacturing sites owned by each group after the Demerger on commercial terms and on an arms’ length basis. Following the completion of the Demerger on July 18, 2022, GSK does not have material obligations under these agreements to be performed on or after the date of this Annual Report on Form 20-F. American Depositary Shares Fees and charges payable by ADR holders JPMorgan Chase Bank, N.A. serves as the depositary (the “Depositary”) for GSK’s American Depositary Receipt (“ADR”) program. On July 29, 2019, GSK and the Depositary amended and restated the deposit agreement and further amended the deposit agreement on March 15, 2021 (the “Deposit Agreement”) between GSK, the Depositary and owners and holders of ADRs. Pursuant to the Deposit Agreement, ADR holders may be required to pay various fees to the Depositary, and the Depositary may refuse to provide any service for which a fee is assessed until the applicable fee has been paid. In particular, the Depositary, under the terms of the Deposit Agreement, shall charge (i) a fee of $5.00 per 100 American Depositary Shares (or portion thereof) for the issuance, delivery, reduction, cancellation or surrender (as the case may be) of American Depositary Shares (“ADSs”), (ii) a fee of U.S.$0.05 or less per ADS held (A) upon which any cash distribution is made pursuant to the Deposit Agreement or (B) in the case of an elective cash/stock dividend, upon which a cash distribution or an issuance of additional ADSs is made as a result of such elective dividend, (iii) a fee for the distribution or sale of securities, such fee being in an amount equal to the fee for the execution and delivery of ADSs referred to above which would have been charged as a result of the deposit of such securities but which securities or the net cash proceeds from the sale thereof are instead distributed by the Depositary to ADR holders entitled thereto, (iv) an aggregate fee of U.S.$0.05 or less per ADS per calendar year (or portion thereof) for services performed by the Depositary in administering the ADRs (which fee may be charged on a periodic basis during each calendar year and shall be assessed against ADR holders as of the record date or record dates set by the Depositary during each calendar year and shall be payable at the sole discretion of the Depositary by billing such Holders or by deducting such charge from one or more cash dividends or other cash distributions), and (v) a fee for the reimbursement of such fees, charges and expenses as are incurred by the Depositary and/or any of its agents (including, without limitation, the agent or agents of the Depositary (the “Custodian”) and expenses incurred on behalf of ADR holders in connection with compliance with foreign exchange control regulations or any law or regulation relating to foreign investment) in connection with the servicing of the ordinary shares or other Deposited Securities, the sale of securities (including, without limitation, Deposited Securities), the delivery of Deposited Securities or otherwise in connection with the Depositary’s or its Custodian’s compliance with applicable law, rule or regulation (which fees and charges shall be assessed on a proportionate basis against ADR holders as of the record date or dates set by the Depositary and shall be payable at the sole discretion of the Depositary by billing such ADR holders or by deducting such charge from one or more cash dividends or other cash distributions). Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Additional information continued 271

GSK will pay other charges and out of pocket expenses of the Depositary and any agent of the Depositary (except the Custodian) as specified in written agreements from time to time between GSK and the Depositary, except (i) stock transfer or other taxes and other governmental charges (which are payable by ADR holders or persons depositing ordinary shares), (ii) SWIFT, cable, telex and facsimile transmission and delivery charges incurred at the request of persons depositing, or ADR holders delivering ordinary shares, ADRs or Deposited Securities (which are payable by such persons or ADR holders), (iii) transfer or registration fees for the registration or transfer of Deposited Securities on any applicable register in connection with the deposit or withdrawal of Deposited Securities (which are payable by persons depositing ordinary shares or ADR holders withdrawing Deposited Securities) and (iv) in connection with the conversion of foreign currency into U.S. dollars, the Depositary shall deduct out of such foreign currency the fees, expenses and other charges charged by it and/or its agent (which may be a division, branch or affiliate) so appointed in connection with such conversion. The Depositary and/or its agent may act as principal for such conversion of foreign currency. Such charges may at any time and from time to time be changed by agreement between GSK and the Depositary. Direct and indirect payments by the Depositary The Depositary anticipates reimbursing GSK for certain expenses incurred by GSK that are related to the establishment and maintenance of the ADR program upon such terms and conditions as GSK and the Depositary may agree from time to time. The Depositary may make available to GSK a set amount or a portion of the Depositary fees charged in respect of the ADR program or otherwise upon such terms and conditions as GSK and the Depositary may agree from time to time. In 2023 the Depositary made payments of approximately $11.27 million. Under certain circumstances, including removal of the Depositary or termination of the ADR program by GSK, GSK is required to repay certain amounts paid to GSK and to compensate the Depositary for payments made or services provided on behalf of GSK. Cyber Security Risk management and strategy We use our corporate enterprise risk management and internal control framework to manage and oversee our Information and Cyber Security principal risk, and we follow our corporate governance hierarchy for risk reporting and escalation. Our Chief Information Security Officer (CISO) heads our Cyber Security Office and is responsible for identifying and putting in place controls and measures to help GSK mitigate and manage cyber security risks. This includes actively monitoring and initiating remediation or other actions to respond to cyber security intelligence and threats. It also includes ongoing investment in people, process and technology to improve our ability to prevent, detect, respond to and recover from any cyber security incidents. We monitor this risk using key risk indicators which include tolerance thresholds reported monthly to the business and quarterly through the governance channels. We also have a third-party security risk management programme to assess cyber security risk when selecting and on- boarding third parties like external partners and suppliers. We use widely accepted standards and frameworks to benchmark our internal environment and controls and help define our security objectives and desired security outcomes. While our standards and frameworks can evolve in response to our dynamic threat environment, we also rely on external frameworks including: – the National Institute of Standards and Technology (NIST) Cyber Security Framework for measuring the overall cyber readiness and maturity – the International Organisation for Standardisations (ISO) 27001/27002 for general information technology controls – Sarbanes-Oxley (SOX) for assessment of internal controls We also draw on third-party consultants' expertise in processes for assessing, identifying and managing cyber security risks. This year, our cyber security maturity programme, designed to reduce the risk of our data being compromised, has improved our security posture and our ability to detect, protect against, respond to and recover from malicious cyber activity. We also created an AI Governance Council, which includes the CISO, to assess and manage information security risks around adopting and scaling up AI at GSK. Information and Cyber Security Governance The Chief Digital and Technology Officer (CDTO) leads the Digital and Technology function, which includes the CISO and Cyber Security Office. The CDTO is the enterprise risk owner for our Information and Cyber Security principal risk, responsible for managing and reporting on the risk, and the enterprise risk plan. This plan includes a description of the risk, its context, our assessment and risk appetite, how we treat the risk and what actions we need to take to manage it in line with our corporate internal control framework. The CISO is responsible for risk coordination across the organisation, developing and overseeing the implementation of controls, and monitoring and reporting on the enterprise risk plan. Both the Board and the Audit & Risk Committee oversee our cyber security risk. The Risk Oversight and Compliance Council helps the Audit & Risk Committee to oversee the cyber security risks, and our strategies to address them. The CISO reports on cyber security risks throughout the year to the CDTO, Risk Oversight and Compliance Council and the Audit & Risk Committee. This reporting covers external insights, key risk indicators, management actions, updates on implementing the enterprise risk plan, progress on the cyber maturity programme, and escalations. The Cyber Security Office analyses potential cyber security incidents, supported by internal experts, and gives updates to the CISO. The CISO escalates any cyber incidents with potential for material impact to the Chief Compliance Officer and the CDTO, who in turn escalates to the GSK Leadership Team and Company Secretary, triggering review by the Disclosure Committee to determine materiality. Any material cyber security incidents are subsequently escalated to the Board and Audit & Risk Committee. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Additional information continued 272
Cyber Security Awareness, Training and Readiness Our cyber security awareness and training programmes include phishing simulations, monthly awareness campaigns and mandatory annual refreshers for all employees, new hires and high-risk roles. We run quarterly phishing simulation tests and related remedial trainings. We also offer optional training and an annual global event. These efforts aim to increase cyber security awareness and foster a culture that security is everyone’s responsibility. Also, we run periodic crisis simulation exercises for targeted functions to test our response to cyber security incidents. Compliance with various governmental cyber security regulations Our Cyber Security Office, guided by our General Counsel, works to stay abreast of emerging government regulations, trends, and compliance expectations regarding cyber security. As new regulatory guidance becomes available (including the U.S. Securities and Exchange Commission's rules on cyber security related disclosures), we respond with remedial compliance-related actions. Code of Ethics We have a number of well-established policies, including our Code of Conduct ("The Code") for all employees, including the CEO, CFO and other senior financial officers. The Code is available at https://www.gsk.com/en-gb/company/ governance/compliance/#the-code. During the year no waivers were granted from a provision of our code of ethics to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. Supplemental Guarantor Information As of 31 December 2023, GSK plc (the ‘Guarantor’) has fully and unconditionally guaranteed certain debt securities (‘Notes’) issued by GlaxoSmithKline Capital plc and GlaxoSmithKline Capital Inc. (the ‘Issuers’) in offerings under the Guarantor's and the Issuers' registration statement on Form F-3, including: GlaxoSmithKline Capital Inc.: • 3.625% Notes due 2025 • 3.875% Notes due 2028 • 5.375% Notes due 2034 • 6.375% Notes due 2038 • 4.200% Notes due 2043 GlaxoSmithKline Capital plc: • 3.000% Notes due 2024 • 3.375% Notes due 2029 The Issuers are 100% owned finance subsidiaries of GSK plc. The Issuers have no assets or operations other than those related to the issuance, administration and repayment of the Notes being registered and other non-registered securities guaranteed by GSK plc. GSK plc has fully and unconditionally guaranteed the Notes and no other subsidiary of GSK plc provides such guarantee. The Notes are listed on the New York Stock Exchange or the London Stock Exchange (in the case of 5.375% Notes due 2034). The guarantee is a full, irrevocable and unconditional guarantee of the principal, interest, premium, if any, and any other amounts payable in respect of the Notes. Remuneration Policy Loss of office payment policy for Executive Directors The company does not have a policy of fixed term contracts. Generally, contracts for new appointments will expire in line with the applicable policy on retirement age, which since 2009 has been 65. Contracts for existing Executive Directors will expire on the dates shown on page 147. Notice period on termination by the employing company or the Executive Director is 12 calendar months. The ability to impose a 12-month non-compete period (and a non-solicitation restriction) on an Executive Director is considered important by the company to have the ability to protect the Group’s intellectual property and staff. In light of this, the Committee believes that it would not be appropriate to provide for mitigation in the contracts. Termination of employment In the event that an Executive Director’s employment with the company terminates, the following policies and payments will apply. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Additional information continued 273
Element of Remuneration Loss of office payment policy Termination payment Termination by notice: 12 months’ annual salary payable on termination by the company (pro-rated where part of the notice period is worked). No termination payment is made in respect of any part of a notice period that extends beyond the contract expiry date. A bonus element is not normally included in the termination payment. However, the terms of the contracts seek to balance commercial imperatives and best practice. Redundancy: As above, for termination by notice. In the UK, only statutory redundancy pay will apply. In the US, general severance policy does not apply Retirement, death and ill-health, injury or disability: No termination payment. LTI awards PSP awards are governed by the plan rules as approved by shareholders. The following provisions will normally apply: Termination by notice: Unvested awards will lapse. Redundancy, retirement, death, ill-health, injury, disability or any other reason: Generally, awards will continue to vest over the original timescales subject to performance and pro-rated for time. In the event of a change of control, PSP awards will vest, taking into account performance to date and normally taking into account the proportion of the performance period that has elapsed. Alternatively, the awards may be exchanged for new awards. Annual bonus Termination by notice by individual: If an individual serves notice and the termination date falls before 31 December, th Termination by notice by the company, redundancy, retirement, death, ill-health, injury or disability: If the termination date falls during the financial year, eligible for pro-rated on-target bonus (if employed on 31 December, bonus payable based on actual results). Mandatorily deferred bonus under the DABP DABP deferred bonus awards in respect of mandatorily deferred bonus amounts are governed by the plan rules as approved by shareholders. The following provisions Termination for gross misconduct: Generally, unvested awards will lapse Any other reason: Generally, awards will vest in full on the original vesting date. In the event of a change of control, awards will vest or may be exchanged for new awards. Pensions Pension scheme contributions by the individual and the company, and any pension scheme benefit accruals, generally cease at the termination date in accordance with pension scheme rules. Access to pension scheme benefits is governed by the pension scheme rules and country legislation Benefits Generally, benefits will continue to apply until the termination date. The Committee may make payments in connection with an existing legal obligation or in respect of any claim related to the cessation of employment. This may include fees for outplacement assistance, legal and/or professional advice. Termination by notice by the company and retirement (US executives): In line with the policy applicable to US senior executives, they may become eligible, at a future date, to receive continuing medical and dental insurance after termination/retirement. Termination by mutual agreement In certain circumstances, it can be in the best interests of the company for the Board to manage proactively succession planning and the development of the senior talent pipeline. In such circumstances, the Board may therefore agree that an Executive’s departure will be by mutual agreement. In order for this to apply, the Committee will need to be satisfied that the Executive has demonstrated performance in line with expectations and where required they should have contributed to an orderly succession. In the case of an Executive Director, they would then be treated as a ‘good leaver’ for the purposes of GSK’s long-term incentive plans. If the termination date falls during the financial year, they would be eligible for a pro-rated on-target bonus and if they are employed on 31 December, the bonus payable would be based on actual results. The Committee does not anticipate the exercise of discretion provided by the PSP and DABP plan rules in respect of termination payments in a manner which would benefit an Executive Director. However, there may be unforeseen circumstances where this is in the best interests of the company and its shareholders. Where it is necessary to exercise discretion, explanations will be provided. Where an Executive Director leaves the company, the Committee will carry out an assessment of the individual’s performance and conduct over the time in role. If it is determined that the individual’s performance or conduct was contrary to the legitimate expectations of the company, the Committee reserves the right to apply appropriate mechanisms such as clawback or reduction or lapsing of outstanding incentive awards (malus), to ensure that any termination payments are in the best interests of the company and its shareholders (see page 135). Loss of office payment policy for Non-Executive Directors The Chair and other Non-Executive Directors are not entitled to receive any payments in respect of fees for loss of office when they retire or step down from the Board. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Additional information continued 274

Principal Accountant Fees and Services Audit Fees for 2023 and 2022 were paid to Deloitte LLP as follows: 2023 £m 2022 £m Audit Fees 20.4 20.6 Audit-Related Fees¹ 1.6 6.3 Tax Fees – – All Other Fees – – 1 The other assurance services provided by the auditor related to agreed upon procedures and other assurance services outside of statutory audit requirements. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Additional information continued 275

Shareholder services and contacts Registrar The company’s registrar is: Equiniti Limited Aspect House, Spencer Road, Lancing, BN99 6DA www.shareview.co.uk Tel: +44 (0)371 384 2991* Equiniti provides a range of services for shareholders: ADS Depositary The ADR programme is administered by JPMorgan Chase Bank, N.A.: Regular Correspondence: EQ Shareowner Services P.O. Box 64504 St. Paul, MN 55164-0504 Delivery of Stock Certificates and Overnight Mail: EQ Shareowner Services 1110 Centre Pointe Curve, Suite 101 Mendota Heights, MN 55120-4100 shareowneronline.com/informational/contact-us/ From the US: +1 877 353 1154 From outside the US: +1 651 453 2128 The Depositary also provides Global Invest Direct, a direct ADS purchase/sale and dividend reinvestment plan for ADS holders. For details on how to enrol, please visit www.adr.com or call the above helpline number to obtain an enrolment pack. Contacts Investor relations Investor relations may be contacted as follows: UK 980 Great West Road Brentford, Middlesex, TW8 9GS Tel: +44 (0)20 8047 5000 US 2929 Walnut Street Philadelphia PA 19104 Tel: +1 888 825 5249 (US toll free) Tel: +1 215 751 4000 (outside the US)­ GSK Response Center Tel: +1 888 825 5249 (US toll free) Tel: +1 215 751 4600 (outside the US) Share scam alert If you receive an unsolicited telephone call offering to sell or buy your shares, please take extra care. The caller may be part of a highly organised financial scam. If you are a UK shareholder, please contact the Financial Conduct Authority at www.fca.org.uk/consumers or on its consumer helpline: Tel: 0800 111 6768 (in the UK)* Tel: +44 207 066 1000 (outside the UK)* * Lines are open from 8.00am to 6.00pm, UK time, Monday to Friday, except UK public holidays, and 9.00am to 1.00pm on Saturdays. Donating shares to Save the Children In 2013, GSK embarked on an ambitious global partnership with Save the Children to share our expertise and resources with the aim of finding innovative ways to reduce the number of children dying from preventable diseases. Shareholders with a small number of shares, the value of which makes it uneconomical to sell, may wish to consider donating them to Save the Children. Donated shares will be aggregated and sold on behalf of Save the Children who will use the funds raised to help them reach the above goal.† To obtain a share donation form, please contact our registrar, Equiniti, which is managing the donation and sale of UK shares to Save the Children free of charge. † The provision of share dealing details is not intended to be an invitation or inducement to engage in an investment activity. Advice on share dealing should be obtained from a stockbroker or independent financial adviser. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures 276

US law and regulation A number of provisions of US law and regulation apply to the company because our shares are quoted on the NYSE in the form of ADS. NYSE rules In general, the NYSE rules permit the company to follow UK corporate governance practices instead of those applied in the US, provided that we explain any significant variations. This explanation is below in Corporate Governance. NYSE rules require us to file annual and interim written affirmations concerning the Audit & Risk Committee (ARC) and our statement on significant differences in corporate governance. Sarbanes-Oxley Act of 2002 Following a number of corporate and accounting scandals in the US, Congress passed the Sarbanes-Oxley Act of 2002. Sarbanes-Oxley is a wide-ranging piece of legislation concerned largely with financial reporting and corporate governance. As recommended by the Securities and Exchange Commission (SEC), the company has an established Disclosure Committee. The Committee reports to the CEO, the CFO and to the ARC. It is chaired by the Company Secretary and its members consist of senior managers from finance, legal, corporate communications and investor relations. Where appropriate, external legal counsel, the external auditors, our sponsor bank, and internal experts are invited to attend the Disclosure Committee’s meetings periodically. The Committee has responsibility for considering the materiality of information and, on a timely basis, determining the disclosure of that information. It has responsibility for the timely filing of reports with the SEC and the formal review of the Annual Report and the Annual Report on Form 20-F. In 2023, the Committee met 17 times, including for the purpose of receiving relevant and appropriate training. Sarbanes-Oxley requires that the Annual Report on Form 20-F contains a statement as to whether a member of the ARC is an audit committee financial expert, as defined in rules under Sarbanes-Oxley. Such a statement for the relevant members of the ARC (Charles Bancroft) is included in the Board Committee information area of the Corporate Governance report on page 110 and in his biography on page 100. Additional disclosure requirements arise under section 302 and section 404 of Sarbanes-Oxley in respect of disclosure controls and procedures and internal control over financial reporting. Section 302: Corporate responsibility for financial reports Sarbanes-Oxley requires the CEO and the CFO to complete formal certifications, confirming that: – they have each reviewed the Annual Report on Form 20-F – based on their knowledge, the Annual Report on Form 20-F contains no material misstatements or omissions; – based on their knowledge, the financial statements and other financial information fairly present, in all material respects, the financial condition, results of operations and cash flows as of the dates, and for the periods, presented in the Annual Report on Form 20-F; – they are responsible for establishing and maintaining disclosure controls and procedures that ensure that material information is made known to them, and have evaluated the effectiveness of these controls and procedures as at the year end, the results of such evaluation being contained in the Annual Report on Form 20-F; – they are responsible for establishing and maintaining internal control over financial reporting that provides reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; – they have disclosed in the Annual Report on Form 20-F any changes in internal controls over financial reporting during the period covered by the Annual Report on Form 20-F that have materially affected, or are reasonably likely to affect materially, the company’s internal control over financial reporting; and – they have disclosed, based on their most recent evaluation of internal control over financial reporting, to the external auditor and the ARC, all significant deficiencies and material weaknesses in the design or operation of internal controls over financial reporting which are reasonably likely to affect adversely the company’s ability to record, process, summarise and report financial information, and any fraud (regardless of materiality) involving persons that have a significant role in the company’s internal control over financial reporting. The Group has carried out an evaluation under the supervision and with the participation of its management, including the CEO and CFO, of the effectiveness of the design and operation of the Group’s disclosure controls and procedures as at 31 December 2023. There are inherent limitations to the effectiveness of any system of disclosure controls and procedures, including the possibility of human error and the circumvention or overriding of the controls and procedures. Accordingly, even effective disclosure controls and procedures can only provide reasonable assurance of achieving their control objectives. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued 277

Based on the Group’s evaluation, the CEO and CFO have concluded that, as at 31 December 2023, the disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed in the reports that the Group files and submits under the US Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported as and when required and that it is accumulated and communicated to management, including the CEO and CFO, as appropriate, to allow timely decisions regarding disclosure. Section 404: Management’s annual report on internal control over financial reporting In accordance with the requirements of section 404 of Sarbanes-Oxley, the following report is provided by management in respect of the company’s internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the US Securities Exchange Act of 1934, as amended (the Exchange Act)): – Management is responsible for establishing and maintaining adequate internal control over financial reporting for the Group. Internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS. – Management conducted an evaluation of the effectiveness of internal control over financial reporting based on the framework, Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organisations of the Treadway Commission (COSO). – Management has assessed the effectiveness of internal control over financial reporting, as at 31 December 2023 and has concluded that such internal control over financial reporting was effective. In addition, there have been no changes in the Group's internal control over financial reporting during 2023 that have materially affected, or are reasonably likely to affect materially, the Group's internal control over financial reporting. – Deloitte LLP, which has audited the consolidated financial statements of the Group for the year ended 31 December 2023, has also assessed the effectiveness of the Group's internal control over financial reporting under Auditing Standard No. 2201 of the Public Company Accounting Oversight Board (United States). Their audit report can be found below. Section 13(r) of the Exchange Act Section 13(r) of the Exchange Act requires issuers to make specific disclosure in their annual reports of certain types of dealings with Iran, including transactions or dealings with government-owned or-controlled entities, as well as dealings with entities sanctioned for activities related to terrorism or proliferation of weapons of mass destruction, even when those activities are not prohibited by US law and do not involve US persons. The Group exports certain medicines to Iran, via sales by non- US entities that are not subsidiaries of a US entity to a distributor in Iran pursuant to a specific licence issued by the Office of Foreign Assets Control. The Group does not regularly receive information regarding the identity of the distributor's downstream customers and intermediaries in Iran, and it is possible that these parties include entities, such as hospitals and pharmacies, that are owned directly or indirectly by the Iranian government or by persons or entities sanctioned in connection with terrorism or proliferation activities. As the Group does not regularly receive information regarding the identity of its distributor's downstream customers and intermediaries it cannot establish the proportion of gross revenue or sales potentially attributable to entities affiliated with the Iranian government or parties sanctioned for disclosable activities. As a result, the Group is reporting the entire gross revenues (£16.89 million) and net profits (£8.42 million) from the Group's sales to Iran in 2023. Some hospitals or other medical facilities in Lebanon may be affiliated with or controlled by Hezbollah or other groups that are designated by the United States pursuant to Executive Order 13224. Again, the Group does not deal directly with such hospitals or facilities and instead sells through distributors. The Group is unable to establish the proportion of gross revenue or sales potentially attributable to reportable activities. As a result, the Group is reporting the entire gross revenues (£6.02 million) and net losses (£4.2 million) from the Group's sales to Lebanon in 2023. In addition to Section 13(r) of the Exchange Act, US law generally restricts dealings by US persons and dealings that otherwise are subject to US jurisdiction with certain countries or territories that are subject to comprehensive sanctions, currently Crimea, Cuba, the so-called Donetsk People's Republic, Iran, the so-called Luhansk People's Republic, North Korea and Syria, as well as with the Government of Venezuela (though not with the country of Venezuela as a whole). The Group engages in some activity in certain such jurisdictions having assessed applicable licences and exemptions While we believe the Group complies with all applicable US sanctions in all material respects, such laws are complex and continue to evolve rapidly. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued US law and regulation continued 278

Report of Independent Registered Public Accounting Firm To the shareholders and the Board of Directors of GSK plc Opinion on Internal Control over Financial Reporting We have audited the internal control over financial reporting of GSK plc and subsidiaries (the “Group”) as at 31 December 2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, the Group maintained, in all material respects, effective internal control over financial reporting as at 31 December 2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by COSO. We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated financial statements as at and for the year ended 31 December 2023, of the Group and our report dated 5 March 2024, expressed an unqualified opinion on those financial statements. Basis for Opinion The Group’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying “Section 404: Management’s annual report on internal control over financial reporting” included on page 278 of the Form 20-F. Our responsibility is to express an opinion on the Group’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Group in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB. We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion. Definition and Limitations of Internal Control over Financial Reporting A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. /s/ Deloitte LLP London, United Kingdom 5 March 2024 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued 279

Corporate governance comparison Comparison of New York Stock Exchange Corporate Governance Standards and GSK plc’s corporate governance practice. The application of the New York Stock Exchange’s (“NYSE”) corporate governance standards is restricted for foreign companies, recognizing that they have to comply with domestic requirements. As a foreign private issuer, GSK plc (“GSK” or the “Company”) must comply with the following NYSE standards: 1. the Company must satisfy the audit committee requirements of Rule 10A-3 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”); 2. the Chief Executive Officer (the “CEO”) must promptly notify the NYSE in writing after any executive officer of the Company becomes aware of any non-compliance with any applicable provisions of the NYSE’s corporate governance standards; 3. the Company must submit an annual affirmation to the NYSE affirming GSK’s compliance with applicable NYSE corporate governance standards, and submit interim affirmations to the NYSE notifying it of specified changes to the audit committee or a change to the status of the Company as a foreign private issuer; and 4. the Company must provide a brief description of any significant differences between its corporate governance practices and those followed by US companies under the NYSE listing standards. As a Company listed on the London Stock Exchange, GSK is required to comply with the UK Listing Authority’s Listing Rules (the “Listing Rules”) and to report non-compliance with the UK Corporate Governance Code (the “UK Code”). The table below discloses differences between GSK’s current domestic corporate governance practices, which are based on the UK Code, and the NYSE corporate governance standards, applicable to US companies. Director Independence (303A.01 of the NYSE Manual) 1. Listed companies must have a majority of independent directors (as defined in Section 303A.02 of the NYSE Manual (see below). GSK complies with the equivalent domestic requirements contained in the UK Corporate Governance Code (the “UK Code”), the latest version of which was issued in July 2018. The UK Code provides that the board of directors of GSK (the “Board”) and its committees should have a combination of skills, experience and knowledge. Consideration should be given to the length of the service of the Board and membership should be regularly refreshed (Principle K). The Board should include an appropriate combination of Executive and Non- Executive Directors and, in particular, “independent” Non-Executive Directors (for the purpose of the UK Code) such that no one individual or small group of individuals can dominate the Board's decision-making. There should be a clear division of responsibilities between the leadership of the Board and the executive leadership of GSK’s business (Principle G). At least half the Board, excluding the Chair, should comprise Non-Executive Directors determined by the Board to be independent (Provision 11). The roles of Chair and Chief Executive should not be exercised by the same individual. If, exceptionally, this is proposed by the Board, major shareholders should be consulted ahead of appointment (Provision 9). The current Chair of the Board, Sir Jonathan Symonds, was considered independent on appointment (Provision 9). The Board considers that Elizabeth McKee Anderson, Charles Bancroft, Wendy Becker, Dr Anne Beal, Dr Hal Dietz, Dr Jesse Goodman, Urs Rohner and Dr Vishal Sikka are independent for the purpose of the UK Code. The independence and commitment of Dr Jesse Goodman and Urs Rohner, who have served on the Board for over six and nine years respectively, has been rigorously reviewed. Urs Rohner is scheduled to retire from the Board as planned in May 2024 at the conclusion of the AGM. A majority of the Board members are independent Non-Executive Directors and, in accordance with the requirements of the UK Code, the Board has appointed one of the independent Non-Executive Directors as Senior Independent Director to provide a sounding board for the Chair and act as an intermediary for other Directors and shareholders where necessary (Provision 12). In January 2012 the Board adopted a formal written role specification for the Senior Independent Director. NYSE Corporate Governance Standards Description of differences between GSK’s governance practice and the NYSE Corporate Governance Standards Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued 280

NYSE Independence Tests (303A.02 of the NYSE Manual) 2. In order to tighten the definition of “independent director” for purposes of these standards: (a) (i) No director qualifies as “independent” unless the board of directors affirmatively determines that the director has no material relationship with the listed company (either directly or as a partner, shareholder or officer of an organization that has a relationship with the company). (ii) In addition, in affirmatively determining the independence of any director who will serve on the compensation committee of the listed company's board of directors, the board of directors must consider all factors specifically relevant to determining whether a director has a relationship to the listed company which is material to that director's ability to be independent from management in connection with the duties of a compensation committee member, including, but not limited to: (b) In addition, a director is not independent if: (i) The director is, or has been within the last three years, an employee of the listed company, or an immediate family member is, or has been within the last three years, an executive officer, of the listed company. (ii) The director has received, or has an immediate family member who has received, during any twelve-month period within the last three years, more than $120,000 in direct compensation from the listed company, other than director and committee fees and pension or other forms of deferred compensation for prior service (provided such compensation is not contingent in any way on continued service). (iii) (A) The director is a current partner or employee of a firm that is the listed company’s internal or external auditor; (B) the director has an immediate family member who is a current partner of such a firm; (C) the director has an immediate family member who is a current employee of such a firm and personally works on the listed company’s audit; or (D) the director or an immediate family member was within the last three years a partner or employee of such a firm and personally worked on the listed company’s audit within that time. (iv) The director or an immediate family member is, or has been within the last three years, employed as an executive officer of another company where any of the listed company’s present executive officers at the same time serves or served on that company’s compensation committee. (v) The director is a current employee, or an immediate family member is a current executive officer, of a company that has made payments to, or received payments from, the listed company for property or services in an amount which, in any of the last three fiscal years, exceeds the greater of $1 million, or 2% of such other company’s consolidated gross revenues. (For the purposes of these standards “executive officer” is defined to have the meaning specified for the term “officer” in Rule 16a-1(f) under the Securities Exchange Act of 1934, as amended, the “Exchange Act”). GSK complies with the corresponding domestic requirements contained in the UK Code, which sets out the principles for GSK to determine whether a director is independent. The Board is required to identify each Non-Executive Director it considers to be independent. Circumstances which are likely to impair, or could appear to impair a non-executive director’s independence include, but are not limited to, whether a director: is or has been an employee of GSK within the last five years; (a) has, or has had within the last three years, a material business relationship with GSK either directly or as a partner, shareholder, director or senior employee of a body that has such a relationship with GSK; (b) has received or receives additional remuneration from GSK apart from a director's fee, participates in GSK's share option or a performance- related pay scheme, or is a member of GSK's pension scheme; (c) has close family ties with any of GSK's advisers, directors or senior employees; (d) holds cross-directorships or has significant links with other directors through involvement in other companies or bodies; (e) represents a significant shareholder; or (f) has served on the Board for more than nine years from the date of their first appointment. Where any of these or other relevant circumstances apply, and the Board nonetheless considers that the non-executive director is independent, a clear explanation should be provided (Provision 10). The Board considers all its Non-Executive Directors to be independent in character and judgment and has concluded that all its Non-Executive Directors are independent within the meaning of the UK Code, with the exception of Dr Hal Barron who transitioned from an Executive Director and Chief Scientific Officer role to a Non-Executive Director in August 2022. The Chair satisfied the independence criteria on appointment in accordance with the UK Code (Provision 9). The Chair should not remain in post beyond nine years from the date of their first appointment to the Board. To facilitate effective succession planning and the development of a diverse board, this period can be extended for a limited time (Provision 19). GSK complied with the UK Code requirement, and its Articles of Association, that all Directors should be subject to annual election or re-election by shareholders (Provision 18) at its Annual General Meeting in 2023 and intends to comply with this requirement at its 2024 Annual General Meeting. The UK Code also provides that the Board should undertake a formal and rigorous annual evaluation of its own performance and that of its committees, the Chair and individual Directors (Principle L and Provision 21). Annual evaluation of the Board should consider the Board’s composition, diversity and how effectively members work together to achieve objectives. Individual evaluation should demonstrate whether each director continues to contribute effectively (Principle L). GSK has complied with this requirement. In addition, the annual evaluation of the Board should be externally facilitated at least every three years and a statement should be made as to whether an external facilitator has any other connection with GSK or individual directors and the external facilitator should be identified in the Annual Report (Provision 21). Internally facilitated evaluations were conducted in 2015, 2016, 2018, 2021 and 2023. GSK conducted an externally facilitated evaluation in 2014, 2017, 2019, 2020 and 2022. The FRC’s Guidance on Board Effectiveness (“Guidance”) provides that all Directors should receive an induction on joining the Board and should regularly update and refresh their skills and knowledge. The Chair should ensure that new Directors receive a full, formal and tailored induction on joining the Board (Guidance, para 61, 75-76 & 81). The Chair should act on the results of the annual evaluation by recognising the strengths and addressing any weaknesses of the Board. Each Director should engage with this process and take appropriate action when development needs have been identified (Provision 22). NYSE Corporate Governance Standards Description of differences between GSK’s governance practice and the NYSE Corporate Governance Standards Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued Corporate governance comparison continued 281

Executive Sessions (303A.03 of the NYSE Manual) Meetings 3. To empower non-management directors to serve as a more effective check on management, the non-management directors of each listed company must meet at regularly scheduled executive sessions without management. GSK complies with the equivalent domestic requirements set out in the UK Code, which requires the Chair of GSK to hold meetings with the Non- Executive Directors without executives present (Provision 13). The Non- Executive Directors, led by the Senior Independent Director, also meet at least annually without the Chair present to appraise the Chair’s performance, and on other occasions as necessary (Provision 12). The UK Code provides that the Chair should demonstrate objective judgement and promote a culture of openness and debate. The Chair should facilitate the effective contribution of all Non-Executive Directors and constructive board relations, and ensure that directors receive accurate, timely and clear information (Principle F). In addition, the Chair should seek regular engagement with major shareholders in order to understand their views on governance and performance against the strategy. The Chair is responsible for ensuring that the Board as a whole has a clear understanding of the view of shareholders and stakeholders (Principle D and Provision 3). The Board should also understand the views of GSK’s other key stakeholders and keep engagement mechanisms under review so that they remain effective (Provision 5). Nominating / Corporate Governance Committee (303A.04 of the NYSE Manual) Nominations Committee 4. (a) Listed companies must have a nominating/corporate governance committee composed entirely of independent directors. (b) The nominating/corporate governance committee must have a written charter that addresses: (i) the committee’s purpose and responsibilities – which, at minimum, must be to: identify individuals qualified to become board members, consistent with criteria approved by the board, and to select, or to recommend that the board select, the director nominees for the next annual meeting of shareholders; develop and recommend to the board a set of corporate governance guidelines applicable to the corporation; and oversee the evaluation of the board and management; and (ii) an annual performance evaluation of the committee. GSK complies with the corresponding domestic requirements set out in the UK Code, which requires GSK to have a Nominations Committee that is comprised of a majority of independent Non-Executive Directors (Provision 17). In practice, GSK’s current Nominations & Corporate Governance Committee is comprised entirely of independent directors within the meaning of the UK Code. The Chair of the Board should not chair the committee when it is dealing with the appointment of their successor (Provision 17). GSK's Nominations & Corporate Governance Committee has written terms of reference in accordance with the UK Code. The terms of reference are available on GSK's website and explain the Nominations & Corporate Governance Committee's role and the authority delegated to it by the Board (Guidance, para 63). The Nominations & Corporate Governance Committee reviews the structure, size, diversity (including diversity of gender, social and ethnic backgrounds, and cognitive and personal strengths), and composition of the Board (evaluating the balance of skills, experience, independence and knowledge on the Board), leads the process for the appointment of members to the Board and the GSK Leadership Team (the “GLT”), and makes recommendations to the Board as appropriate. The Nominations & Corporate Governance Committee also monitors the planning of succession for the Board and senior management (Provision 17). The terms and conditions of appointment of the Chair and Non-Executive Directors are available for inspection (Guidance, para 96). The UK Code requires that GSK’s Annual Report describes the work of the Nominations Committee in discharging its duties, including the process it has used in relation to appointments, its approach to succession planning and how both support developing a diverse pipeline (Provision 23). Open advertising and/or an external search consultancy should generally be used for the appointment of a chair or a non-executive director. If an external search consultancy is engaged it should be identified in the Annual Report and a statement should be made as to whether it has any other connection with GSK or individual directors (Provision 20). This section should also include a description of how the Board evaluation has been conducted, the Board's policy on diversity and inclusion together with its objectives and linkage to GSK’s strategy, how it has been implemented and progress on achieving the objectives, and the gender balance of those in the senior management and their direct reports (Provision 23). GSK has complied with this requirement under the UK Code. As described above, there is an annual Board evaluation exercise, which also includes evaluation of the Board’s committees and individual Directors (Principle L and Provision 21). The Board is responsible for regularly reviewing its corporate governance standards and practices. The Company Secretary oversees corporate governance matters for the Group. The Company Secretary is responsible for advising the Board on all corporate governance matters (Provision 16). Domestic requirements do not mandate GSK to establish a distinct corporate governance committee. NYSE Corporate Governance Standards Description of differences between GSK’s governance practice and the NYSE Corporate Governance Standards Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued Corporate governance comparison continued 282

Compensation Committee (303A.05 of the NYSE Manual) Remuneration Committee 5 (a) Listed companies must have a compensation committee composed entirely of independent directors. Compensation committee members must satisfy the additional independence requirements specific to compensation committee membership set forth in Section 2(a)(ii) in the Section titled “NYSE Independence Tests” above. (b) The compensation committee must have a written charter that addresses: (i) the committee’s purpose and responsibilities – which, at a minimum, must be to have direct responsibility to: (A) review and approve corporate goals and objectives relevant to CEO compensation, evaluate the CEO’s performance in light of those goals and objectives, and, either as a committee or together with the other independent directors (as directed by the board), determine and approve the CEO’s compensation level based on this evaluation; (B) make recommendations to the board with respect to non-CEO executive officer compensation, and incentive-compensation and equity-based plans that are subject to board approval; and (C) prepare the disclosure required by Item 407(e)(5) of Regulation S-K under the Exchange Act; (ii) an annual performance evaluation of the compensation committee. (iii) The rights and responsibilities of the compensation committee set forth in Section 303A.05(c).: (c) The provision of other services to the listed company by the person that employs the compensation consultant, legal counsel or other adviser; (d) The amount of fees received from the listed company by the person that employs the compensation consultant, legal counsel or other adviser, as a percentage of the total revenue of the person that employs the compensation consultant, legal counsel or other adviser; (e) The policies and procedures of the person that employs the compensation consultant, legal counsel or other adviser that are designed to prevent conflicts of interest; (f) Any business or personal relationship of the compensation consultant, legal counsel or other adviser with a member of the compensation committee; (g) Any stock of the listed company owned by the compensation consultant, legal counsel or other adviser; and (h) Any business or personal relationship of the compensation consultant, legal counsel, other adviser or the person employing the adviser with an executive officer of the listed company. GSK complies with the equivalent domestic requirements set out in the UK Code, which requires GSK to have a Remuneration Committee comprising at least three independent Non-Executive Directors (Provision 32). In practice, GSK’s current Remuneration Committee is comprised entirely of independent directors within the meaning of the UK Code. GSK's Remuneration Committee has written terms of reference in accordance with the UK Code, which explain the Remuneration Committee’s role and the authority delegated to it by the Board and are available on GSK’s website (Guidance, para 63). The Remuneration Committee determines the terms of service and remuneration of the Executive Directors and members of the GLT and, with the assistance of external independent advisers, it evaluates and makes recommendations to the Board on overall executive remuneration policy (the Chair and the CEO are responsible for evaluating and making recommendations to the Board on the remuneration of Non-Executive Directors within the terms of the approved remuneration policy). It should review workforce remuneration and related policies and the alignment of incentives and rewards with culture, taking these into account when setting the policy for executive director remuneration (Provision 33). Where remuneration consultants are appointed, they should be identified in the Annual Report and a statement should be made as to whether they have any other connection with GSK or individual directors (Provision 35). The UK Code provides that the Remuneration Committee: (a) should take care to recognise and manage conflicts of interest when receiving views from Executive Directors or senior management, or when evaluating the advice of external third parties (Provision 35 & Guidance, para 129) and should have delegated responsibility for setting remuneration for all Executive Directors, the Chair and senior management (Provision 33); (b) should carefully consider the pension consequences and associated costs of basic salary increases and any other changes in pensionable remuneration, or contribution rates, particularly for Directors close to retirement (Provision 38); (c) should ensure that compensation commitments in Directors’ terms of appointment do not reward poor performance (Provision 39). Remuneration schemes should promote long-term shareholdings by Executive Directors that support alignment with long-term shareholder interests. A formal policy should be developed for post-employment shareholding requirements encompassing both unvested and vested shares (Provision 36). Remuneration schemes and policies should enable the use of discretion to override formulaic outcomes and include provisions that would enable GSK to recover and/or withhold sums or share awards specifying the circumstances in which it would be appropriate to do so (Provision 37); and (d) when determining Executive Director remuneration policy and practices, should address the following: (i) remuneration arrangements are transparent and promote effective engagement with shareholders and the workforce; (ii) the operation and rationale of remuneration structures are easy to understand; (iii) remuneration arrangements identify and mitigate reputational and other risks from excessive rewards and behavioural risks that can arise from target-based incentive plans; (iv) the range of possible values of rewards to individual Directors and any other limits or discretions are identified and explained at the time of approving the policy; (v) the link between individual awards, the delivery of strategy and the long-term performance of GSK should be clear; and (vi) incentive schemes should drive behaviours consistent with company purpose, values and strategy (Provision 40). The UK Code requires that remuneration of Non-Executive Directors should not include share options or other performance-related elements, but should reflect the time commitment and responsibilities of the role (Provision 34). The UK Code requires that notice or contract periods should be one year or less (Provision 39). As described above, there is an annual Board evaluation exercise, which also includes evaluation of the Board’s committees (Principle L and Provision 21). NYSE Corporate Governance Standards Description of differences between GSK’s governance practice and the NYSE Corporate Governance Standards Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued Corporate governance comparison continued 283

Audit Committee (303A.06 and 303A.07 of the NYSE Manual) Audit & Risk Committee 6. Listed companies must have an audit committee that satisfies the requirements of Rule 10A-3 under the Exchange Act. GSK complies with equivalent domestic requirements set out in the UK Code, which require that GSK has an Audit & Risk Committee that is comprised of at least three independent Non-Executive Directors (Provision 24). GSK considers all members of the Audit & Risk Committee to be independent. The Board has also satisfied itself, in line with the UK Code, that at least one member of the Audit & Risk Committee has recent and relevant financial experience and that the Audit & Risk Committee as a whole has competence relevant to the sector in which GSK operates (Provision 24). Under the UK Code, the main roles and responsibilities of the Audit & Risk Committee include: (a) monitoring the integrity of the financial statements of GSK and any formal announcements relating to GSK’s financial performance, reviewing significant financial reporting judgments contained in them (Provision 25); (b) providing advice (where requested by the Board) on whether the Annual Report and accounts, taken as a whole, is fair, balanced and understandable and provides the information necessary for shareholders to assess GSK’s position and performance, business model and strategy (Provision 25); (c) reviewing GSK’s internal financial controls and internal control and risk management systems (Provision 25); (d) monitoring and reviewing the effectiveness of GSK’s internal audit function (Provision 25); (e) conducting the tender process and making recommendations to the Board regarding the appointment, re-appointment and removal of the external auditor and approving the remuneration and terms of engagement of the external auditor (Provision 25); (f) reviewing and monitoring the external auditor’s independence and objectivity and the effectiveness of the audit process, taking into consideration relevant UK professional and regulatory requirements (Provision 25); (g) developing and implementing policy on the engagement of external auditors to supply non-audit services, ensuring there is prior approval of non-audit services, considering the impact this may have on independence, taking into account the relevant regulations and ethical guidance regarding the provision of non-audit services by the external audit firm, and to report to the Board on any improvement or action required (Provision 25); and (h) reporting to the Board on how it has discharged its responsibilities (Provision 25). The Audit & Risk Committee is also the means by which the Board reviews arrangements by which the staff of GSK may, in confidence, raise concerns about possible improprieties in matters of financial reporting or other matters (Provision 6). GSK's Audit & Risk Committee meets the requirements of Rule 10A-3 in that: – each member of the Audit & Risk Committee is deemed to be "independent" in accordance with the Exchange Act and applicable NYSE and UK requirements; – the Audit & Risk Committee, amongst other things, is responsible for recommending the appointment, compensation, maintenance of independence and oversight of the work of any registered public accounting firm engaged for the purpose of preparing or issuing an audit report or performing other audit, review or attest services for GSK, and each such accounting firm must report directly to the Audit & Risk Committee; – the Audit & Risk Committee has established a procedure for the receipt, retention and treatment of complaints regarding accounting, internal accounting controls or auditing matters, and for the confidential, anonymous submission by employees of concerns regarding questionable accounting or auditing matters; – the Audit & Risk Committee has the authority to engage independent counsel and other advisors as it determines necessary to carry out its duties; and – GSK must provide appropriate funding for the Audit & Risk Committee. The Board has determined that Charles Bancroft has the appropriate qualifications and background to be an “Audit Committee Financial Expert” as defined in rules promulgated by the SEC under the Exchange Act. NYSE Corporate Governance Standards Description of differences between GSK’s governance practice and the NYSE Corporate Governance Standards Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued Corporate governance comparison continued 284

7. (a) The audit committee must have a minimum of three members. All audit committee members must satisfy the requirements for independence set out in Section 303A.02 and, in the absence of an applicable exemption, Rule 10A-3(b)(1) under the Exchange Act. (b) The audit committee must have a written charter that addresses: (i) the committee’s purpose – which, at minimum, must be to: (A) assist board oversight of (1) the integrity of the listed company’s financial statements, (2) the listed company’s compliance with legal and regulatory requirements, (3) the independent auditor’s qualifications and independence, and (4) the performance of the listed company’s internal audit function and independent auditors (if the listed company does not yet have an internal audit function because it is availing itself of a transition period pursuant to Section 303A.00, the charter must provide that the committee will assist board oversight of the design and implementation of the internal audit function); and (B) prepare disclosure required by Item 407(d)(3)(i) of Regulation S-K (regarding the audit committee’s review and discussion of financial statements and certain other audit matters with management and auditors) (ii) an annual performance evaluation of the audit committee; and (iii) the duties and responsibilities of the audit committee – which, at a minimum, must include those set out in Rule 10A-3(b)(2), (3), (4) and (5) of the Exchange Act as well as to: (A) at least annually, obtain and review a report by the independent auditor describing: the firm’s internal quality-control procedures; any material issues raised by the most recent internal quality-control review, or peer review, of the firm, or by any inquiry or investigation by governmental or professional authorities, within the preceding five years, respecting one or more independent audits carried out by the firm, and any steps taken to deal with any such issues; and (to assess the auditor’s independence) all relationships between the independent auditor and the listed company; (B) meet to review and discuss the listed company’s annual audited financial statements and quarterly financial statements with management and the independent auditor, including reviewing the listed company’s specific disclosures under “Management’s Discussion and Analysis of Financial Condition and Results of Operations”; (C) discuss the listed company’s earnings press releases, as well as financial information and earnings guidance provided to analysts and rating agencies; (D) discuss policies with respect to risk assessment and risk management; (E) meet separately, periodically, with management, with internal auditors (or other personnel responsible for the internal audit function) and with independent auditors; (F) review with the independent auditor any audit problems or difficulties and management’s response; (G) set clear hiring policies for employees or former employees of the independent auditors; and (H) report regularly to the board of directors. (c) Each listed company must have an internal audit function. GSK complies with the equivalent domestic requirements set out in the UK Code, which requires that the Audit & Risk Committee should be comprised of a minimum of three independent Non-Executive Directors (Provision 24). GSK's Audit & Risk Committee has written terms of reference in accordance with the UK Code. The terms of reference are available on GSK's website and explain the Audit & Risk Committee’s role and the authority delegated to it by the Board (Guidance, para 63). The Audit & Risk Committee’s main responsibilities include monitoring and reviewing the financial reporting process, the system of internal control and risk management, overseeing the identification and management of risks, the external and internal process and for monitoring compliance with laws, regulations and ethical codes of practice, including review throughout the year of integrated assurance reports comprising business unit and associated consolidated internal audit reports. Where requested by the Board, the Audit & Risk Committee should provide advice on the following areas which the directors as a whole are required to explain in the Annual Report: + whether the annual report and accounts, taken as a whole, is fair, balanced and understandable and provides the information necessary for shareholders to assess GSK’s performance, business model and strategy (Principle M and Provision 27); and + when taking into account GSK’s position and principal risks, how the prospects of GSK have been assessed, over what period and why the period is regarded as appropriate. The Audit & Risk Committee should also advise whether there is a reasonable expectation that GSK will be able to continue in operation and meet its liabilities when falling due over the said period, drawing attention to any qualifications or assumptions as necessary (Provision 31). The UK Code requires that the Annual Report should describe the work of the Audit & Risk Committee in discharging its responsibilities (Provision 26). The Annual Report should include: – the significant issues that the committee considered in relation to the financial statements, and how these issues were addressed (Provision 26); – an explanation of how it has assessed the independence and effectiveness of the external audit process and the approach taken to the appointment or reappointment of the external auditor, information on the length of tenure of the current audit firm and when a tender was last conducted and advance notice of any retendering plans (Provision 26); – in the case of the Board not accepting the Audit & Risk Committee’s recommendation on the external auditor appointment, reappointment or removal, a statement from the Audit & Risk Committee explaining its recommendation and the reasons why the Board has taken a different position (Provision 26); and – if the external auditor provides non-audit services, an explanation of how auditor objectivity and independence are safeguarded (Provision 26). Please see section 6 above for a description of the main role and responsibilities of the Audit & Risk Committee. In accordance with the UK Code (Provision 25), the Audit & Risk Committee monitors and reviews the effectiveness of GSK’s internal audit function. NYSE Corporate Governance Standards Description of differences between GSK’s governance practice and the NYSE Corporate Governance Standards Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued Corporate governance comparison continued 285
Shareholder Approval of Equity Compensation Plans (303A.08 of the NYSE Manual) 8. Shareholders must be given the opportunity to vote on all equity- compensation plans and material revisions thereto, except for employment inducement awards, certain grants, plans and amendments in the context of mergers and acquisitions, and certain specific types of plans. However, these exempt grants, plans and amendments may be made only with the approval of the listed company's independent compensation committee or the approval of a majority of the listed company's independent directors. Companies must also notify the Exchange in writing when they use one of these exemptions. GSK complies with corresponding domestic requirements in the Listing Rules, which mandate that GSK must seek shareholder approval for employee share schemes and significant changes to existing schemes, save in circumstances permitted by the Listing Rules (Listing Rule 9.4). Please see section 5(c) above. Corporate Governance Guidelines (303A.09 of the NYSE Manual) 9. Listed companies must adopt and disclose corporate governance guidelines. GSK complies with corresponding domestic requirements in the Listing Rules and the UK Code, which require that GSK includes an explanation in its Annual Report of how it complies with the principles of the UK Code and a confirmation that it complies with the UK Code's provisions or, where it does not, provides an explanation of how and why it does not comply (Listing Rule 9.8.6). In addition, GSK is required to make certain mandatory corporate governance statements in the Directors’ Report in accordance with the FCA’s Disclosure Guidance and Transparency Rules, DGTR 7. GSK will comply with these requirements in its 2023 Annual Report. Code of Business Conduct and Ethics (303A.10 of the NYSE Manual) Code of Conduct 10. Listed companies must adopt and disclose a code of business conduct and ethics for directors, officers and employees, and promptly disclose any waivers of the code for directors or executive officers. GSK's Code of Conduct for all employees, including the CEO, CFO and other senior financial officers, is available on GSK's website. Foreign Private Issuer Disclosure (303A.11 of the NYSE Manual) 11. Listed foreign private issuers must disclose any significant ways in which their corporate governance practices differ from those followed by domestic companies under NYSE listing standards. GSK fulfils this requirement by publishing this disclosure in its Annual Report on Form 20-F. Listed foreign private issuers are required to provide this disclosure in the English language and in their annual reports filed on Form 20-F. GSK fulfils this requirement by including this disclosure in its Annual Report on Form 20-F. Certification Requirements (303A.12 of the NYSE Manual) 12. Each listed company and its CEO must file certain annual and interim certifications regarding compliance with the corporate governance requirements and certain other matters (although foreign private issuers are only required to comply with a subset of these requirements). GSK fulfils this requirement by filing the required certifications each year. Related Party Transactions (314.00 of the NYSE Manual) 13. A listed company's audit committee, or another independent body of the board of directors, shall conduct a reasonable prior review and oversight of all related party transactions for potential conflicts of interest and will prohibit such a transaction if it determines it to be inconsistent with the interests of the company and its shareholders. In the case of foreign private issuers, the term "related party transactions" refers to transactions required to be disclosed pursuant to Form 20-F, Item 7.B. GSK fulfils this requirement in respect of Directors and Officers via the Nominations & Corporate Governance Committee. In respect of any material transactions with other related parties (as set out in 314.00 of the NYSE Manual), the independent Directors of the Board (excluding the Executive Directors) fulfil this requirement. The Company’s Policy on Grant of Authority for Transactions reflects the requirement for the Board’s prior review and oversight in this regard. NYSE Corporate Governance Standards Description of differences between GSK’s governance practice and the NYSE Corporate Governance Standards Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued Corporate governance comparison continued 286
Group companies In accordance with Section 409 of the Companies Act 2006 a full list of subsidiaries, associates, joint ventures and joint arrangements, the address of the registered office and effective percentage of equity owned, as at 31 December 2023 are disclosed below. Unless otherwise stated the share capital disclosed comprises ordinary shares which are indirectly held by GSK plc. The percentage held by class of share is stated where this is less than 100%. Unless otherwise stated, all subsidiary companies have their registered office and are tax resident in their country of incorporation. Name Security Registered address Wholly owned subsidiaries 14245563 Canada Inc. Common 275 Armand-Frappier Boulevard, Laval ON H7V 4A7, Canada 14934792 Canada Inc. Common 100 Milverton Drive, Suite 800, Mississauga ON L5R 4H1, Canada 1506369 Alberta ULC Common 3500 855-2nd Street SW, Calgary AB T2P 4J8, Canada Action Potential Venture Capital Limited Ordinary GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom Adechsa GmbH (ii) Ordinary c/o PRV Provides Treuhandgesellschaft AG, Dorfstrasse 38, 6341, Baar, Switzerland Affinivax, Inc. Common Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States Allen & Hanburys Limited (ii) Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England Allen & Hanburys Pharmaceutical Nigeria Limited Ordinary 49, Town Planning Way, Ilupeju, Lagos, Nigeria Allen Pharmazeutika Gesellschaft m.b.H. Ordinary Wienerbergstraße 7, Wien, 1100, Austria, Austria Beecham Group p.l.c £0.05 Ordinary B; £0.20 Ordinary A 980 Great West Road, Brentford, Middlesex, TW8 9GS, England Beecham Pharmaceuticals (Pte) Limited Ordinary 38 Quality Road, Jurong Industrial Estate, Jurong, 618809, Singapore Beecham Portuguesa- Produtos Farmaceuticos e Quimicos, LDA Quota Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Alges, Portugal Beecham S.A. Ordinary Avenue Fleming 20, 1300 Wavre, Belgium Bellus Health Corp. Common The Corporation Trust Company, Corporation Trust Center, 1209 Orange Street, Wilmington DE 19801, United States Bellus Health Inc Common 275 Boulevard Armand Frappier, Laval QC H7V 4A7, Canada Biovesta Ilaçlari Ltd. Sti. (ii) Nominative Büyükdere Caddesi No. 173, 1.Levent Plaza B Blok, 1.Levent, Istanbul, 34394, Turkey Cascan GmbH & Co. KG Partnership Capital Prinzregentenplatz 9, 81675, Munich, Bavaria, Germany Cellzome GmbH Ordinary Meyerhofstrasse 1, 69117, Heidelberg, Germany Clarges Pharmaceutical Trustees Limited (ii) Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England Colleen Corporation Common Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States Corixa Corporation Common Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States Dealcyber Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England Desarrollo Energia Solar Alternativa S.L. Ordinary Severo Ochoa, 2, Parque Tecnologico de Madrid, Tres Cantos, 28760, Madrid, Spain Duncan Pharmaceuticals Philippines Inc. Common 23rd Floor, The Finance Centre, 26th Street Corner 9th Avenue, Bonifacio Global City, Taguig City, 1634, Philippines Etex Farmaceutica Ltda Social Capital Av. Andrés Bello 2457, Costanera Center, Torre 2, Piso 20, Providencia, Santiago, 7510689, Chile Glaxo Group Limited Ordinary GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom Glaxo Kabushiki Kaisha (ii) Ordinary 1-8-1 Akasaka Minato-ku, Tokyo, Japan Glaxo New Zealand Pension Plan Trustee Limited Ordinary Level 2 E.2, Generator at GridAKL, 12 Madden Street, Wynyard Quarter, Auckland, 1010, New Zealand Glaxo Operations UK Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England Glaxo Properties BV Ordinary Van Asch van Wijckstraat 55h, 3811 LP, Amersfoort, Netherlands Glaxo Saudi Arabia Limited Ordinary PO Box 22617, Area No 56 to 73, Warehouse City, First Stage Al Khomrah, Jeddah 21416, Saudi Arabia Glaxo Verwaltungs GmbH Ordinary Prinzregentenplatz 9, 81675, Munich, Bavaria, Germany Glaxo Wellcome Farmaceutica, Limitada Ordinary Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Alges, Portugal Glaxo Wellcome Manufacturing Pte Ltd Ordinary 1 Pioneer Sector 1, Jurong Industrial Estate, Jurong, 628413, Singapore Glaxo Wellcome Production Ordinary 23 rue François Jacob, 92500, Rueil-Malmaison, France Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued 287
Name Security Registered address Wholly owned subsidiaries continued Glaxo Wellcome Vidhyasom Limited (in liquidation) (ii) Ordinary 12th Floor Wave Place, 55 Wireless Road, Lumpini, Pathumwan, Bangkok, 10 330, Thailand Glaxo Wellcome, S.A. Ordinary Poligono Industrial Allendeduero, Avenida de Extremadura, 3, Aranda de Duero, 09400, Burgos, Spain Glaxo, S.A. Ordinary Severo Ochoa, 2, Parque Tecnologico de Madrid, Tres Cantos, 28760, Madrid, Spain Glaxochem Pte Ltd (iii) Ordinary 23 Rochester Park, 139234, Singapore GlaxoSmithKline - Produtos Farmaceuticos, Limitada Ordinary Quota Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Alges, Portugal GlaxoSmithKline (Cambodia) Co., Ltd. Ordinary 5th Floor DKSH Building, No.797 Preah Monivong Boulevard (Co, Sangkat Phsar Deum Thakov, Khan Chamkarmon, Phnom Penh, Cambodia GlaxoSmithKline (China) Investment Co Ltd Ordinary Room 901, 902, 903, 905, 908, 909 and 910, Unit 901, Floor 9, No. 56 Mid 4th East Ring Road, Chaoyang District, Beijing, China GlaxoSmithKline (China) R&D Company Limited Equity F1-3, No.18 Building, 999 Huanke Road, Pilot Free Trade Zone, Shanghai, 201 210, China GlaxoSmithKline (GSK) S.R.L. Ordinary Str. Dr. Nicolae D. Staicovici nr. 2, Opera Center II, etaj 4, sector 5, București, Romania, 050556 GlaxoSmithKline (Ireland) Limited Ordinary 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland GlaxoSmithKline (Israel) Ltd Ordinary 25 Basel Street, PO Box 10283, Petach-Tikva, 49002, Israel GlaxoSmithKline (Private) Limited (ii) Ordinary Unit 3, 20 Anthony Road, Msasa, Harare, Zimbabwe GlaxoSmithKline (Thailand) Limited Ordinary 12th Floor Wave Place, 55 Wireless Road, Lumpini, Pathumwan, Bangkok, 10 330, Thailand GlaxoSmithKline AB Ordinary Hemvarnsg. 9, 171 54, Solna, Sweden GlaxoSmithKline AG Ordinary Talstrasse 3 , 3053 Muenchenbuchsee, Switzerland GlaxoSmithKline Angola Unipessoal Limitada Quota Luanda, Bairro Petrangol, Estrada de Cacuaco n ° 288, Angola GlaxoSmithKline Argentina S.A. Ordinary Tucumán 1, piso 4, Buenos Aires, C1049AAA, Argentina GlaxoSmithKline AS Ordinary Drammensveien 288, Oslo, NO-0283, Norway GlaxoSmithKline Australia Pty Ltd Ordinary Level 4 , 436 Johnston Street , Abbotsford, Victoria, 3067, Australia GlaxoSmithKline B.V. Ordinary Van Asch van, Wijckstraat 55h, 3811 LP Amersfoort, The Netherlands, Netherlands GlaxoSmithKline Beteiligungs GmbH Ordinary Prinzregentenplatz 9, 81675, Munchen, Germany GlaxoSmithKline Biologicals Kft. Ordinary 2100 Gödöllõ, Homoki Nagy István utca 1, Hungary GlaxoSmithKline Biologicals S.A.S. Ordinary 637 Rue des Aulnois, Saint-Amand Les Eaux, 59230, France GlaxoSmithKline Biologicals SA Ordinary: Preference Rue de l'Institut 89 B-1330 Rixensart, Belgium GlaxoSmithKline Brasil Limitada Quotas Estrada dos Banderiantes, 8464, Rio de Janeiro, 22783-110, Brazil GlaxoSmithKline Capital Inc. Common Wilmington Trust SP Services, Inc., 1100 N. Market Street, 4th Floor, Wilmington DE 19890, United States GlaxoSmithKline (China) R&D Company Limited Equity F1-3, No.18 Building, 999 Huanke Road, Pilot Free Trade Zone, Shanghai, 201 210, China GlaxoSmithKline (GSK) S.R.L. Ordinary Str. Dr. Nicolae D. Staicovici nr. 2, Opera Center II, etaj 4, sector 5, București, Romania, 050556 GlaxoSmithKline (Ireland) Limited Ordinary 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland GlaxoSmithKline (Israel) Ltd Ordinary 25 Basel Street, PO Box 10283, Petach-Tikva, 49002, Israel GlaxoSmithKline (Private) Limited (ii) Ordinary Unit 3, 20 Anthony Road, Msasa, Harare, Zimbabwe GlaxoSmithKline (Thailand) Limited Ordinary 12th Floor Wave Place, 55 Wireless Road, Lumpini, Pathumwan, Bangkok, 10 330, Thailand GlaxoSmithKline AB Ordinary Hemvarnsg. 9, 171 54, Solna, Sweden GlaxoSmithKline AG Ordinary Talstrasse 3, 3053 Muenchenbuchsee, Switzerland GlaxoSmithKline Angola Unipessoal Limitada Quota Luanda, Bairro Petrangol, Estrada de Cacuaco n ° 288, Angola GlaxoSmithKline Argentina S.A. Ordinary Tucumán 1, piso 4, Buenos Aires, C1049AAA, Argentina GlaxoSmithKline AS Ordinary Drammensveien 288, Oslo, NO-0283, Norway GlaxoSmithKline Australia Pty Ltd Ordinary Level 4 , 436 Johnston Street , Abbotsford, Victoria, 3067, Australia GlaxoSmithKline B.V. Ordinary Van Asch van, Wijckstraat 55h, 3811 LP Amersfoort, The Netherlands, Netherlands GlaxoSmithKline Beteiligungs GmbH Ordinary Prinzregentenplatz 9, 81675, Munchen, Germany GlaxoSmithKline Biologicals Kft. Ordinary 2100 Gödöllõ, Homoki Nagy István utca 1, Hungary GlaxoSmithKline Biologicals S.A.S. Ordinary 637 Rue des Aulnois, Saint-Amand Les Eaux, 59230, France GlaxoSmithKline Biologicals SA Ordinary: Preference Rue de l'Institut 89 B-1330 Rixensart, Belgium Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued Group companies continued 288

Name Security Registered address Wholly owned subsidiaries continued GlaxoSmithKline Brasil Limitada Quotas Estrada dos Banderiantes, 8464, Rio de Janeiro, 22783-110, Brazil GlaxoSmithKline Capital Inc. Common Wilmington Trust SP Services, Inc., 1100 N. Market Street, 4th Floor, Wilmington DE 19890, United States GlaxoSmithKline Capital plc Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GlaxoSmithKline Caribbean Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GlaxoSmithKline Chile Farmaceutica Limitada Social Capital Av. Andrés Bello 2457, Torre 2, piso 20, Providencia, Santiago, Región Metropolitana, Chile GlaxoSmithKline Colombia S.A. Ordinary Avenida El Dorado, #69B-45/Piso 9, Bogota, Colombia GlaxoSmithKline Consumer Holding B.V. (ii) Ordinary Van Asch van Wijckstraat 55h, 3811 LP, Amersfoort, Netherlands GlaxoSmithKline doo Beograd-Novi Beograd (in liquidation) Ordinary Milutin Milankovic, 1J, Novi Beograd, Belgrade, 11070, Serbia GlaxoSmithKline Ecuador S.A. Ordinary Av 10 De Agosto N36-239, y Naciones Unidas, Edificio Electroectuatoriana, 2 do piso, Quito, Ecuador GlaxoSmithKline El Salvador S.A. de C.V. Ordinary Municipio de San Salvador, Departamento de San Salvador, El Salvador GlaxoSmithKline EOOD Ordinary 16 Nedelcho Bonchev str., Sofia, Sofiya, 1592, Bulgaria GlaxoSmithKline Export Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GlaxoSmithKline Export Panama S.A. Ordinary Panama City, Republic of Panama, Panama GlaxoSmithKline Far East B.V. Ordinary Van Asch van Wijckstraat 55h, 3811 LP, Amersfoort, Netherlands GlaxoSmithKline Finance plc Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GlaxoSmithKline GmbH & Co. KG Partnership Capital Prinzregentenplatz 9, 81675, Munchen, Germany GlaxoSmithKline Guatemala S.A. Ordinary 3ra. Av. 13-78 Zona 10, Torre Citibank, Nivel 8, Guatemala City, Guatemala GlaxoSmithKline Holding AS Ordinary Drammensveien 288, Oslo, NO-0283, Norway GlaxoSmithKline Holdings (Americas) Inc. Common Wilmington Trust SP Services Inc., 1100 North Market Street, 4th Floor, Wilmington, Delaware, 19890 GlaxoSmithKline Holdings (One) Limited (i) Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GlaxoSmithKline Holdings Limited (i) Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GlaxoSmithKline Holdings Pty Ltd Ordinary Level 4 , 436 Johnston Street , Abbotsford, Victoria, 3067, Australia GlaxoSmithKline Honduras S.A. Ordinary Tegucigalpa, MDC, Honduras GlaxoSmithKline IHC Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GlaxoSmithKline Ilaclari Sanayi ve Ticaret A.S. Nominative Büyükdere Caddesi No. 173, 1.Levent Plaza B Blok, 1.Levent, Istanbul, 34394, Turkey GlaxoSmithKline Inc. Class A Common; Class C Preference 100 Milverton Drive, Suite 800, Mississauga ON L5R 4H1, Canada GlaxoSmithKline Insurance Ltd. Ordinary c/o Trinity Corporate Services Ltd., Trinity Hall, 43 Cedar Avenue, Hamilton, Hamilton, HM12, Bermuda GlaxoSmithKline Intellectual Property (No.2) Limited Ordinary GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom GlaxoSmithKline Intellectual Property Development Limited Ordinary GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom GlaxoSmithKline Intellectual Property Holdings Limited A Ordinary; B Ordinary GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom GlaxoSmithKline Intellectual Property Limited Deferred; Ordinary GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom GlaxoSmithKline Intellectual Property Management Limited Ordinary GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom GlaxoSmithKline Investigación y Desarrollo, S.L. Ordinary Severo Ochoa 2 Parque Tecnológico de Madrid, Tres Cantos, 28760, Madrid Spain GlaxoSmithKline Investments Pty Ltd Ordinary Level 4, 436 Johnston Street, Abbotsford, Victoria, 3067, Australia GlaxoSmithKline K.K. Ordinary 1-8-1 Akasaka Minato-ku, Tokyo, Japan GlaxoSmithKline Korea Limited Ordinary 9F LS Yongsan Tower, 92 Hangang-daero, Yongsangu, Seoul, 04386, Korea, Republic of GlaxoSmithKline Latin America, S.A. Ordinary Panama City, Republic of Panama, Panama GlaxoSmithKline Limited Ordinary 23/F., Tower 6, The Gateway, 9 Canton Road, Tsimshatsui, Kowloon, Hong Kong GlaxoSmithKline Limited (ii) Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GlaxoSmithKline LLC LLC Interests Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States GlaxoSmithKline Manufacturing SpA Ordinary Viale dell’Agricoltura 7, 37135, Verona, Italy GlaxoSmithKline Maroc S.A. Ordinary 42-44 Angle Bd, Rachidi et Abou Hamed El Glaza, Casablanca, Morocco GlaxoSmithKline Medical and Healthcare Products Kft Ordinary 1062 Budapest, Andrassy ut 113, Hungary GlaxoSmithKline Mercury Limited (i) Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England Strategic report | Governance and remuneration | Financial statements | Investor information 2023
Annual Report on Form 20-F Other statutory disclosures continued Group companies continued 289
Name Security Registered address Wholly owned subsidiaries continued GlaxoSmithKline Mexico S.A. de C.V. Ordinary A; Ordinary B Av. Real Mayorazgo 130 Piso 20, Colonia Xoco, Alcaldia Benito Juárez, Ciudad de Mexico, 03330, Mexico GlaxoSmithKline NZ Limited Ordinary Level 2 E.2, Generator @GridAKL, 12 Madden Street, Wynyard Quarter, Auckland, 1010, New Zealand GlaxoSmithKline Oy Ordinary Porkkalankatu 20 A, Helsinki, 00180, Finland GlaxoSmithKline Peru S.A. Ordinary Av. Víctor Andrés Belaúnde N°147, Vía Principal N°133, Piso 7, Distrito de San Isidro, Lima, Peru GlaxoSmithKline Pharma A/S Ordinary Vallensbæk Company House III , Delta Park 37, DK-2665, Valle, Denmark GlaxoSmithKline Pharma GmbH Ordinary Wienerbergstraße 7, Wien, 1100, Austria, Austria GlaxoSmithKline Pharmaceutical Kenya Limited Ordinary P.O Box 78392-00507, Likoni Road, Nairobi, Kenya GlaxoSmithKline Pharmaceutical Nigeria Limited Ordinary 1 Industrial Avenue, Ilupeju, Ikeja, Lagos, PM B 21218, Nigeria GlaxoSmithKline Pharmaceutical Sdn Bhd Ordinary HZ.01, Horizon Penthouse, 1 Powerhouse, 1, Persiaran Bandar Utama, Bandar Utama, 47800 Petaling Jaya, Selangor, Malaysia GlaxoSmithKline Pharmaceuticals (Pvt) Ltd Ordinary 121 Galle Road, Kaldemulla, Moratuwa, Sri Lanka GlaxoSmithKline Pharmaceuticals Costa Rica S.A Ordinary Autopista Florencia del Castillo, kilómetro siete, Oficentro TerraCampus, edificio uno, cuarto piso, San Diego, Cartago, 30302, Costa Rica GlaxoSmithKline Pharmaceuticals SA Ordinary Avenue Fleming 20, 1300 Wavre, Belgium GlaxoSmithKline Pharmaceuticals Ukraine LLC Chartered Capital Pavla Tychyny avenue, 1-V, Kiev, 02152, Ukraine GlaxoSmithKline Philippines Inc Ordinary 23rd Floor, The Finance Centre, 26th Street Corner 9th Avenue, Bonifacio Global City, Taguig City, 1634, Philippines GlaxoSmithKline Pte Ltd Ordinary 23 Rochester Park, 139234, Singapore GlaxoSmithKline Puerto Rico, Inc. Common CORPORATION SERVICE COMPANY PUERTO RICO INC., c/o RVM Professional Services, LLC, A4 Reparto Mendoza, Humacao, 00791, Puerto Rico GlaxoSmithKline Republica Dominicana S.A. Ordinary Blue Mall Tower, Floor 23 Ave., Winston Churchill 95, Santo Domingo, Dominican Republic GlaxoSmithKline Research & Development Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GlaxoSmithKline S.A. Ordinary Severo Ochoa, 2, Parque Tecnologico de Madrid, Tres Cantos, 28760, Madrid, Spain GlaxoSmithKline S.p.A. Ordinary Viale dell’Agricoltura 7, 37135, Verona, Italy GlaxoSmithKline s.r.o. Ordinary Hvezdova 1734/2c, Prague, 4 140 00, Czech Republic GlaxoSmithKline Services GmbH & Co. KG Partnership Capital Prinzregentenplatz 9, 81675, Munchen, Germany GlaxoSmithKline Services Unlimited (i) Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GlaxoSmithKline Single Member A.E.B.E. Ordinary 266 Kifissias Avenue, Halandri, Athens, 152 32, Greece GlaxoSmithKline SL LLC LLC Interests Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States GlaxoSmithKline SL LP (ii)(viii) Partnership 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GlaxoSmithKline South Africa (Pty) Limited Ordinary Flushing Meadows Building, The Campus, 57 Sloane Street, Bryanston 2021, South Africa GlaxoSmithKline Trading Services Limited (iii) Ordinary 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland GlaxoSmithKline Tunisia S.A.R.L. Ordinary Immeuble REGUS, Lot B17, Centre Urbain Nord, Tunis, Tunisia GlaxoSmithKline UK Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GlaxoSmithKline Uruguay S.A. Registered Provisory Stock Victor Soliño 349, Montevideo, Montevideo, 11300, Uruguay GlaxoSmithKline US Trading Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GlaxoSmithKline Venezuela C.A. Ordinary calle Altagracia, edificio P&G, piso Mezzanina, torre Torre Sur, Urbanizacion Sorokaima, La Trinidad, Caracas, 1080, Venezuela, Bolivarian Republic of GlaxoSmithKline Vietnam Limited Liability Company (ii) Equity Capital The Metropolitan, 235 Dong Khoi Street, District 1, 7th Floor Unit 701, Ho Chi Minh City, Vietnam GlycoVaxyn AG (In liquidation) Common; Preferred A; Preferred B; Preferred C Grabenstrasse 3, 8952 Schlieren, Switzerland Groupe GlaxoSmithKline Ordinary 23 rue François Jacob, 92500, Rueil-Malmaison, France GSK Biopharma Argentina S.A. Nominative Non Endorseable Ordinary Tucumán 1, piso 4, Buenos Aires, C1049AAA, Argentina GSK (No.1) Scottish Limited Partnership (viii) Partnership 50 Lothian Road, Festival Square, Edinburgh, Scotland, EH3 9WJ, United Kingdom GSK (No.2) Scottish Limited Partnership (viii) Partnership 50 Lothian Road, Festival Square, Edinburgh, Scotland, EH3 9WJ, United Kingdom GSK (No.3) Scottish Limited Partnership (viii) Partnership 50 Lothian Road, Festival Square, Edinburgh, Scotland, EH3 9WJ, United Kingdom Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued Group companies continued 290
Name Security Registered address Wholly owned subsidiaries continued GSK Business Service Centre Sdn Bhd Ordinary Level 6, Quill 9, 112 Jalan Prof. Khoo Kay Kim, Petaling Jaya, 46300 Selangor, Malaysia GSK Capital B.V. (iii)(v) Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS GSK Capital K.K. Ordinary 1-8-1 Akasaka Minato-ku, Tokyo, Japan GSK Commercial Sp. z o.o. Ordinary ul. Rzymowskiego 53, 02-697, Warsaw, Poland GSK d.o.o., Ljubljana Ordinary Ameriška ulica 8, Ljubljana, 1000, Slovenia GSK Enterprise Management Co, Ltd Ordinary Floor 4, 18 Lane 999 Huanke Road, No. 1358 Zhongke Road, Shanghai, China GSK Equity Investments, Limited Units Corporation Service Company, 2595 Interstate Drive, Suite 103, Harrisburg PA 17110, United States GSK Finance (No.3) PLC Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GSK Finance (No 2) Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England GSK India Global Services Private Limited Equity Level 1, 2 & 3 Luxor North Tower, Bagmane Capital Business Park Outer Ring Road, Bangalore, Karnataka, 560037, India GSK International Holding and Finance BV Ordinary Van Asch van Wijckstraat 55h, 3811 LP, Amersfoort, Netherlands GSK Kazakhstan LLP Participation Interest Nursultan Nazarbayev Ave 273, Business center USKO, 3rd fl., Almaty, 050059, Kazakhstan GSK Life Sciences FZE Ordinary LB06015, Jebel Ali Freezone, Dubai, United Arab Emirates GSK Pharma India Private Limited Equity 1, Battery House, Bhulabhai Desai Raod, Mumbai, Maharashtra, 400026, India GSK Pharma Vietnam Company Limited Chartered Capital Unit 702/703 7th Floor, The Metropolitan Tower, 235 Dong Khoi Street, Ben Nghe Ward, District 1, Ho Chi Minh, Vietnam GSK Pharmaceutical Trading S.A. (ii) Ordinary Bucharest, 1-5 Costache Negri Street, Opera Center One, 5th floor, discussions room 01, District 5, Romania GSK PSC Poland sp. z o.o. Equal and indivisible shares ul. Grunwaldzka 189, Poznań, 60-322, Pol GSK Services Sp z o.o. Ordinary Ul. Grunwaldzka 189, 60-322, Poznan, Poland GSK Vaccines BV Ordinary Hullenbergweg 85, 1101 CL, Amsterdam, Netherlands GSK Vaccines GmbH Ordinary Emil-von-Behring-Str.76, 35041 Marburg, Germany GSK Vaccines Institute for Global Health S.r.l. Quota Via Fiorentina 1, 53100, Siena, Italy GSK Vaccines S.r.l. Quota Via Fiorentina 1, 53100, Siena, Italy GSK Vaccines Vertriebs GmbH Ordinary Rudolf-Diesel-Ring 27, 83607, Holzkirchen, Germany Human Genome Sciences, Inc. Common Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States ID Biomedical Corporation of Quebec Common 2323, boul. Du Parc Technologique, Québec Québec G1P 4R8, Canada Instituto Luso Farmaco, Limitada (ii) Quota Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Alges, Portugal InterPharma Dienstleistungen GmbH Quota Wienerbergstraße 7, Wien, 1100, Austria, Austria J&J Technologies, LC (ii) LLC Interests Corporation Service Company, 100 Shockoe Slip, 2nd Floor, Richmond VA 23219, United States JSC GlaxoSmithKline Trading Ordinary Leningradskiy Prospect 37A, Building 4, Floor 3, Premises XV, Room 1, 125167, Moscow, Russian Federation Laboratoire GlaxoSmithKline Ordinary 23 rue François Jacob, 92500, Rueil-Malmaison, France Laboratoire Pharmaceutique Algérien LPA Production SPA Ordinary Zone Industrielle Est, Boudouaou, Boumerdes, Algeria Laboratoire Pharmaceutique Algérien SPA Ordinary Zone Industrielle Est, Boudouaou, Boumerdes, Algeria Laboratoires Paucourt (ii) Ordinary 23 rue François Jacob, 92500, Rueil-Malmaison, France Laboratoires Saint-Germain (ii) Ordinary 23 rue François Jacob, 92500, Rueil-Malmaison, France Laboratorios Dermatologicos Darier, S.A de C.V. Ordinary A; Ordinary B Av. Real Mayorazgo 130 Piso 20, Colonia Xoco, Alcaldia Benito Juárez, Ciudad de Mexico, 03330, Mexico Laboratorios Farmaceuticos Stiefel (Portugal) LTDA (ii) Ordinary Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Alges, Portugal Laboratorios Stiefel de Venezuela SA Ordinary Calle Altagracia, edificio P&G, nivel Mezzanina,, piso Mezzanina, local Torre Sur, Urbanizacion Sorokaima, La Trinidad, Caracas, 1080, Venezuela, Bolivarian Republic of Laboratorios Stiefel Ltda. Ordinary Rua Professor Joao Cavalheiro Salem, no.1077, Bairro de Bonsucesso, Municipality of Guarulhos, Sao Paulo, CEP 07243-580, Brazil Laboratorios Wellcome De Portugal Limitada (ii) Quota Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Alges, Portugal Maxinutrition Limited (in liquidation) Ordinary C/O BDO LLP, 5 Temple Square, Temple Street, Liverpool, L2 5RH PT Glaxo Wellcome Indonesia Class A; Class B JL. Pulobuaran Raya Kav.III/ DD 2,3,4 KWS. Industri, Pulogadung, Jatinegara, Cakung, Jakarta Timur, Indonesia Setfirst Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued Group companies continued 291
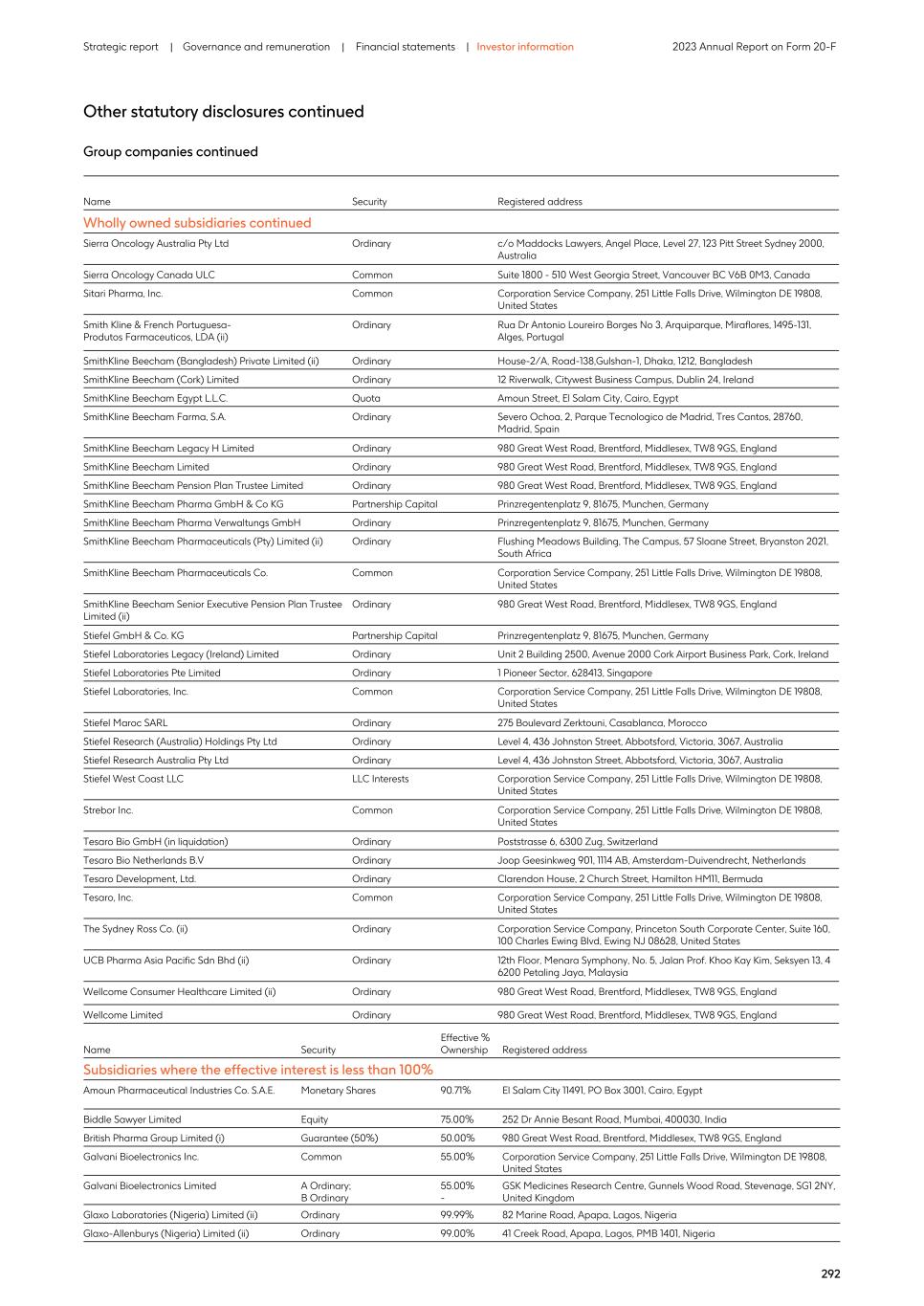
Name Security Registered address Wholly owned subsidiaries continued Sierra Oncology Australia Pty Ltd Ordinary c/o Maddocks Lawyers, Angel Place, Level 27, 123 Pitt Street Sydney 2000, Australia Sierra Oncology Canada ULC Common Suite 1800 - 510 West Georgia Street, Vancouver BC V6B 0M3, Canada Sitari Pharma, Inc. Common Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States Smith Kline & French Portuguesa- Produtos Farmaceuticos, LDA (ii) Ordinary Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Alges, Portugal SmithKline Beecham (Bangladesh) Private Limited (ii) Ordinary House-2/A, Road-138,Gulshan-1, Dhaka, 1212, Bangladesh SmithKline Beecham (Cork) Limited Ordinary 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland SmithKline Beecham Egypt L.L.C. Quota Amoun Street, El Salam City, Cairo, Egypt SmithKline Beecham Farma, S.A. Ordinary Severo Ochoa, 2, Parque Tecnologico de Madrid, Tres Cantos, 28760, Madrid, Spain SmithKline Beecham Legacy H Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England SmithKline Beecham Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England SmithKline Beecham Pension Plan Trustee Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England SmithKline Beecham Pharma GmbH & Co KG Partnership Capital Prinzregentenplatz 9, 81675, Munchen, Germany SmithKline Beecham Pharma Verwaltungs GmbH Ordinary Prinzregentenplatz 9, 81675, Munchen, Germany SmithKline Beecham Pharmaceuticals (Pty) Limited (ii) Ordinary Flushing Meadows Building, The Campus, 57 Sloane Street, Bryanston 2021, South Africa SmithKline Beecham Pharmaceuticals Co. Common Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States SmithKline Beecham Senior Executive Pension Plan Trustee Limited (ii) Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England Stiefel GmbH & Co. KG Partnership Capital Prinzregentenplatz 9, 81675, Munchen, Germany Stiefel Laboratories Legacy (Ireland) Limited Ordinary Unit 2 Building 2500, Avenue 2000 Cork Airport Business Park, Cork, Ireland Stiefel Laboratories Pte Limited Ordinary 1 Pioneer Sector, 628413, Singapore Stiefel Laboratories, Inc. Common Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States Stiefel Maroc SARL Ordinary 275 Boulevard Zerktouni, Casablanca, Morocco Stiefel Research (Australia) Holdings Pty Ltd Ordinary Level 4, 436 Johnston Street, Abbotsford, Victoria, 3067, Australia Stiefel Research Australia Pty Ltd Ordinary Level 4, 436 Johnston Street, Abbotsford, Victoria, 3067, Australia Stiefel West Coast LLC LLC Interests Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States Strebor Inc. Common Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States Tesaro Bio GmbH (in liquidation) Ordinary Poststrasse 6, 6300 Zug, Switzerland Tesaro Bio Netherlands B.V Ordinary Joop Geesinkweg 901, 1114 AB, Amsterdam-Duivendrecht, Netherlands Tesaro Development, Ltd. Ordinary Clarendon House, 2 Church Street, Hamilton HM11, Bermuda Tesaro, Inc. Common Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States The Sydney Ross Co. (ii) Ordinary Corporation Service Company, Princeton South Corporate Center, Suite 160, 100 Charles Ewing Blvd, Ewing NJ 08628, United States UCB Pharma Asia Pacific Sdn Bhd (ii) Ordinary 12th Floor, Menara Symphony, No. 5, Jalan Prof. Khoo Kay Kim, Seksyen 13, 4 6200 Petaling Jaya, Malaysia Wellcome Consumer Healthcare Limited (ii) Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England Wellcome Limited Ordinary 980 Great West Road, Brentford, Middlesex, TW8 9GS, England Name Security Effective % Ownership Registered address Subsidiaries where the effective interest is less than 100% Amoun Pharmaceutical Industries Co. S.A.E. Monetary Shares 90.71% El Salam City 11491, PO Box 3001, Cairo, Egypt Biddle Sawyer Limited Equity 75.00% 252 Dr Annie Besant Road, Mumbai, 400030, India British Pharma Group Limited (i) Guarantee (50%) 50.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England Galvani Bioelectronics Inc. Common 55.00% Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States Galvani Bioelectronics Limited A Ordinary; B Ordinary 55.00% - GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom Glaxo Laboratories (Nigeria) Limited (ii) Ordinary 99.99% 82 Marine Road, Apapa, Lagos, Nigeria Glaxo-Allenburys (Nigeria) Limited (ii) Ordinary 99.00% 41 Creek Road, Apapa, Lagos, PMB 1401, Nigeria Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued Group companies continued 292
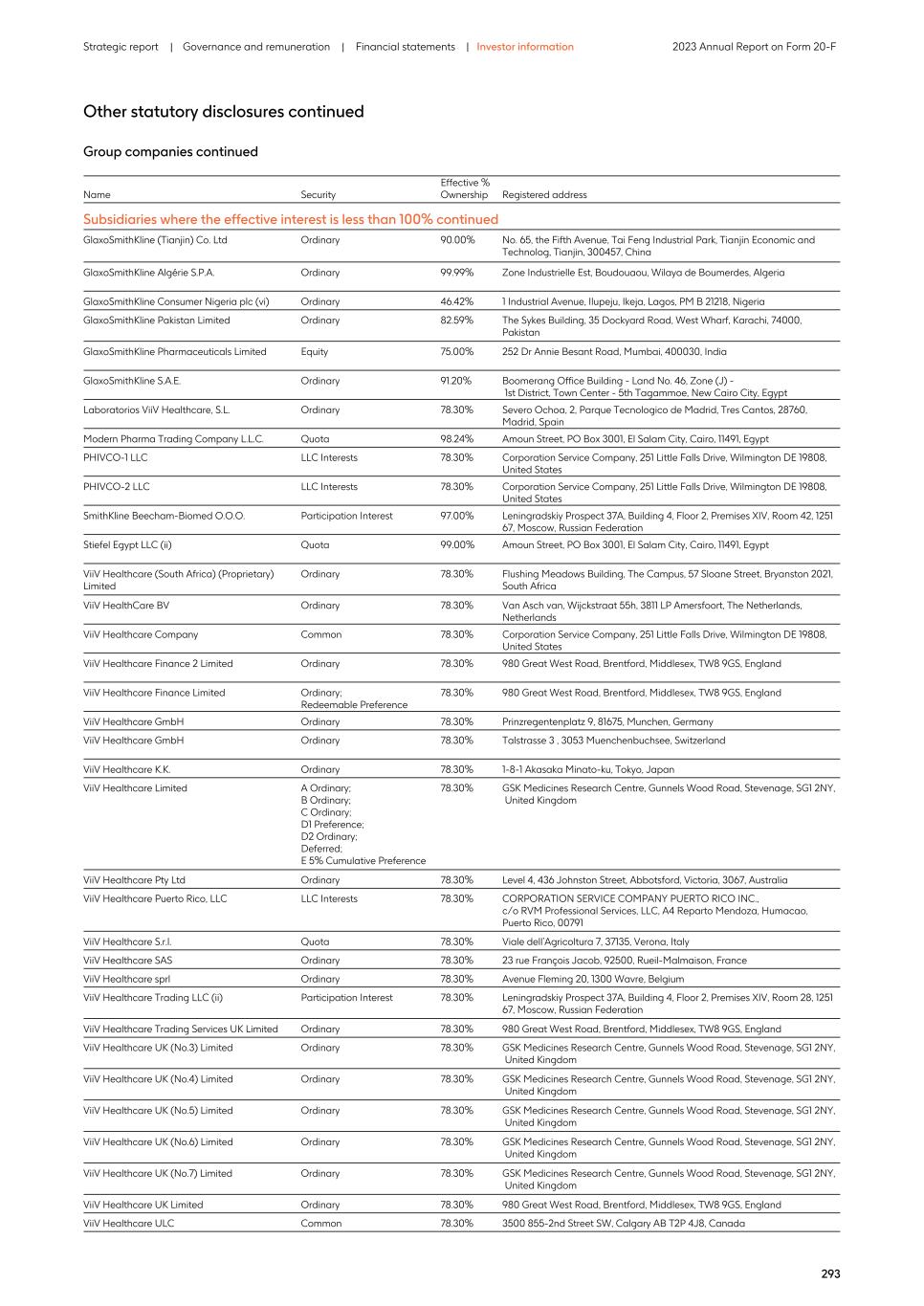
Name Security Effective % Ownership Registered address Subsidiaries where the effective interest is less than 100% continued GlaxoSmithKline (Tianjin) Co. Ltd Ordinary 90.00% No. 65, the Fifth Avenue, Tai Feng Industrial Park, Tianjin Economic and Technolog, Tianjin, 300457, China GlaxoSmithKline Algérie S.P.A. Ordinary 99.99% Zone Industrielle Est, Boudouaou, Wilaya de Boumerdes, Algeria GlaxoSmithKline Consumer Nigeria plc (vi) Ordinary 46.42% 1 Industrial Avenue, Ilupeju, Ikeja, Lagos, PM B 21218, Nigeria GlaxoSmithKline Pakistan Limited Ordinary 82.59% The Sykes Building, 35 Dockyard Road, West Wharf, Karachi, 74000, Pakistan GlaxoSmithKline Pharmaceuticals Limited Equity 75.00% 252 Dr Annie Besant Road, Mumbai, 400030, India GlaxoSmithKline S.A.E. Ordinary 91.20% Boomerang Office Building - Land No. 46, Zone (J) - 1st District, Town Center - 5th Tagammoe, New Cairo City, Egypt Laboratorios ViiV Healthcare, S.L. Ordinary 78.30% Severo Ochoa, 2, Parque Tecnologico de Madrid, Tres Cantos, 28760, Madrid, Spain Modern Pharma Trading Company L.L.C. Quota 98.24% Amoun Street, PO Box 3001, El Salam City, Cairo, 11491, Egypt PHIVCO-1 LLC LLC Interests 78.30% Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States PHIVCO-2 LLC LLC Interests 78.30% Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States SmithKline Beecham-Biomed O.O.O. Participation Interest 97.00% Leningradskiy Prospect 37A, Building 4, Floor 2, Premises XIV, Room 42, 1251 67, Moscow, Russian Federation Stiefel Egypt LLC (ii) Quota 99.00% Amoun Street, PO Box 3001, El Salam City, Cairo, 11491, Egypt ViiV Healthcare (South Africa) (Proprietary) Limited Ordinary 78.30% Flushing Meadows Building, The Campus, 57 Sloane Street, Bryanston 2021, South Africa ViiV HealthCare BV Ordinary 78.30% Van Asch van, Wijckstraat 55h, 3811 LP Amersfoort, The Netherlands, Netherlands ViiV Healthcare Company Common 78.30% Corporation Service Company, 251 Little Falls Drive, Wilmington DE 19808, United States ViiV Healthcare Finance 2 Limited Ordinary 78.30% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England ViiV Healthcare Finance Limited Ordinary; Redeemable Preference 78.30% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England ViiV Healthcare GmbH Ordinary 78.30% Prinzregentenplatz 9, 81675, Munchen, Germany ViiV Healthcare GmbH Ordinary 78.30% Talstrasse 3 , 3053 Muenchenbuchsee, Switzerland ViiV Healthcare K.K. Ordinary 78.30% 1-8-1 Akasaka Minato-ku, Tokyo, Japan ViiV Healthcare Limited A Ordinary; B Ordinary; C Ordinary; D1 Preference; D2 Ordinary; Deferred; E 5% Cumulative Preference 78.30% GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom ViiV Healthcare Pty Ltd Ordinary 78.30% Level 4, 436 Johnston Street, Abbotsford, Victoria, 3067, Australia ViiV Healthcare Puerto Rico, LLC LLC Interests 78.30% CORPORATION SERVICE COMPANY PUERTO RICO INC., c/o RVM Professional Services, LLC, A4 Reparto Mendoza, Humacao, Puerto Rico, 00791 ViiV Healthcare S.r.l. Quota 78.30% Viale dell’Agricoltura 7, 37135, Verona, Italy ViiV Healthcare SAS Ordinary 78.30% 23 rue François Jacob, 92500, Rueil-Malmaison, France ViiV Healthcare sprl Ordinary 78.30% Avenue Fleming 20, 1300 Wavre, Belgium ViiV Healthcare Trading LLC (ii) Participation Interest 78.30% Leningradskiy Prospect 37A, Building 4, Floor 2, Premises XIV, Room 28, 1251 67, Moscow, Russian Federation ViiV Healthcare Trading Services UK Limited Ordinary 78.30% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England ViiV Healthcare UK (No.3) Limited Ordinary 78.30% GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom ViiV Healthcare UK (No.4) Limited Ordinary 78.30% GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom ViiV Healthcare UK (No.5) Limited Ordinary 78.30% GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom ViiV Healthcare UK (No.6) Limited Ordinary 78.30% GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom ViiV Healthcare UK (No.7) Limited Ordinary 78.30% GSK Medicines Research Centre, Gunnels Wood Road, Stevenage, SG1 2NY, United Kingdom ViiV Healthcare UK Limited Ordinary 78.30% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England ViiV Healthcare ULC Common 78.30% 3500 855-2nd Street SW, Calgary AB T2P 4J8, Canada Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued Group companies continued 293
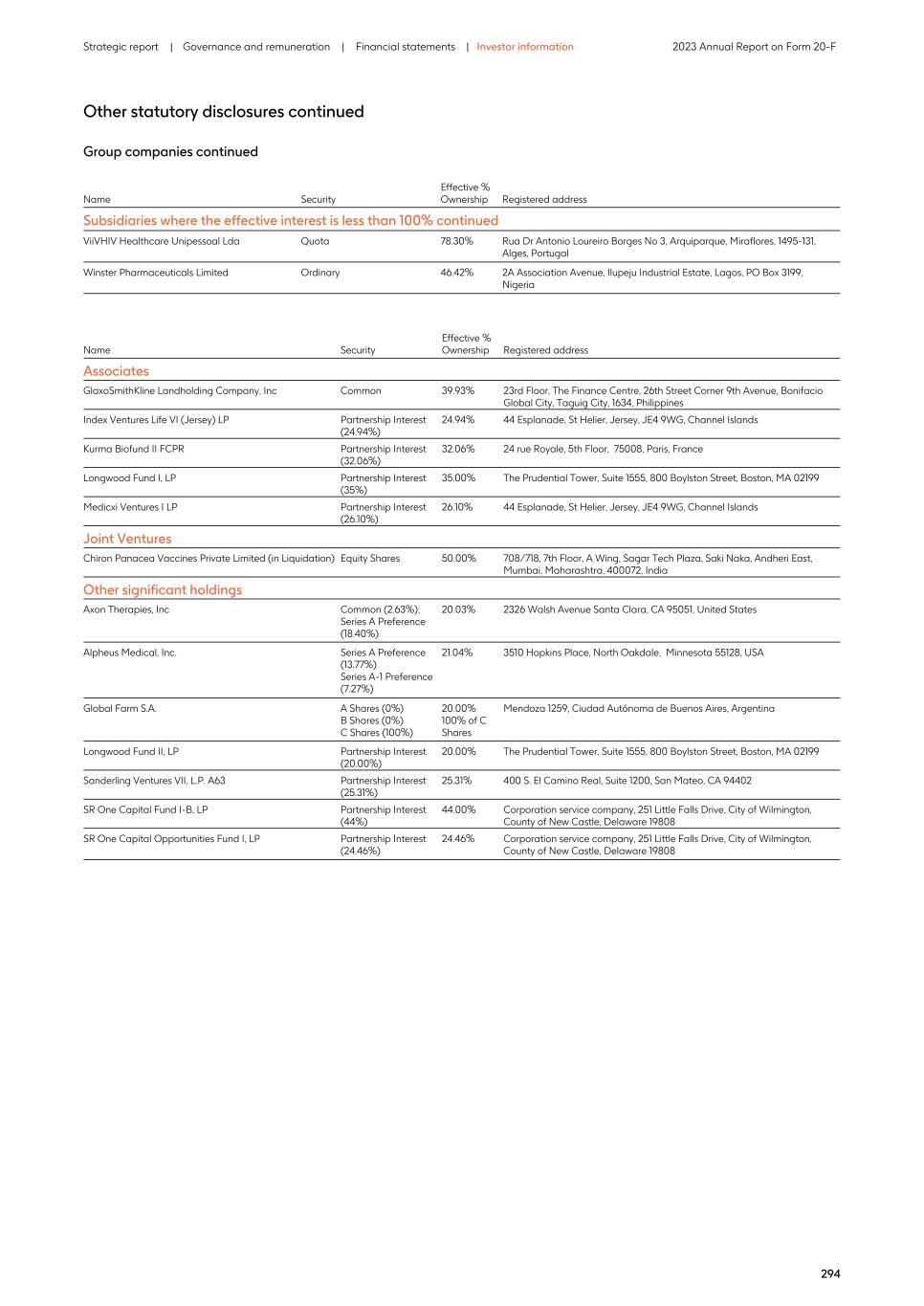
Name Security Effective % Ownership Registered address Subsidiaries where the effective interest is less than 100% continued ViiVHIV Healthcare Unipessoal Lda Quota 78.30% Rua Dr Antonio Loureiro Borges No 3, Arquiparque, Miraflores, 1495-131, Alges, Portugal Winster Pharmaceuticals Limited Ordinary 46.42% 2A Association Avenue, Ilupeju Industrial Estate, Lagos, PO Box 3199, Nigeria Name Security Effective % Ownership Registered address Associates GlaxoSmithKline Landholding Company, Inc Common 39.93% 23rd Floor, The Finance Centre, 26th Street Corner 9th Avenue, Bonifacio Global City, Taguig City, 1634, Philippines Index Ventures Life VI (Jersey) LP Partnership Interest (24.94%) 24.94% 44 Esplanade, St Helier, Jersey, JE4 9WG, Channel Islands Kurma Biofund II FCPR Partnership Interest (32.06%) 32.06% 24 rue Royale, 5th Floor, 75008, Paris, France Longwood Fund I, LP Partnership Interest (35%) 35.00% The Prudential Tower, Suite 1555, 800 Boylston Street, Boston, MA 02199 Medicxi Ventures I LP Partnership Interest (26.10%) 26.10% 44 Esplanade, St Helier, Jersey, JE4 9WG, Channel Islands Joint Ventures Chiron Panacea Vaccines Private Limited (in Liquidation) Equity Shares 50.00% 708/718, 7th Floor, A Wing, Sagar Tech Plaza, Saki Naka, Andheri East, Mumbai, Maharashtra, 400072, India Other significant holdings Axon Therapies, Inc Common (2.63%); Series A Preference (18.40%) 20.03% 2326 Walsh Avenue Santa Clara, CA 95051, United States Alpheus Medical, Inc. Series A Preference (13.77%) Series A-1 Preference (7.27%) 21.04% 3510 Hopkins Place, North Oakdale, Minnesota 55128, USA Global Farm S.A. A Shares (0%) B Shares (0%) C Shares (100%) 20.00% 100% of C Shares Mendoza 1259, Ciudad Autónoma de Buenos Aires, Argentina Longwood Fund II, LP Partnership Interest (20.00%) 20.00% The Prudential Tower, Suite 1555, 800 Boylston Street, Boston, MA 02199 Sanderling Ventures VII, L.P. A63 Partnership Interest (25.31%) 25.31% 400 S. El Camino Real, Suite 1200, San Mateo, CA 94402 SR One Capital Fund I-B, LP Partnership Interest (44%) 44.00% Corporation service company, 251 Little Falls Drive, City of Wilmington, County of New Castle, Delaware 19808 SR One Capital Opportunities Fund I, LP Partnership Interest (24.46%) 24.46% Corporation service company, 251 Little Falls Drive, City of Wilmington, County of New Castle, Delaware 19808 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued Group companies continued 294
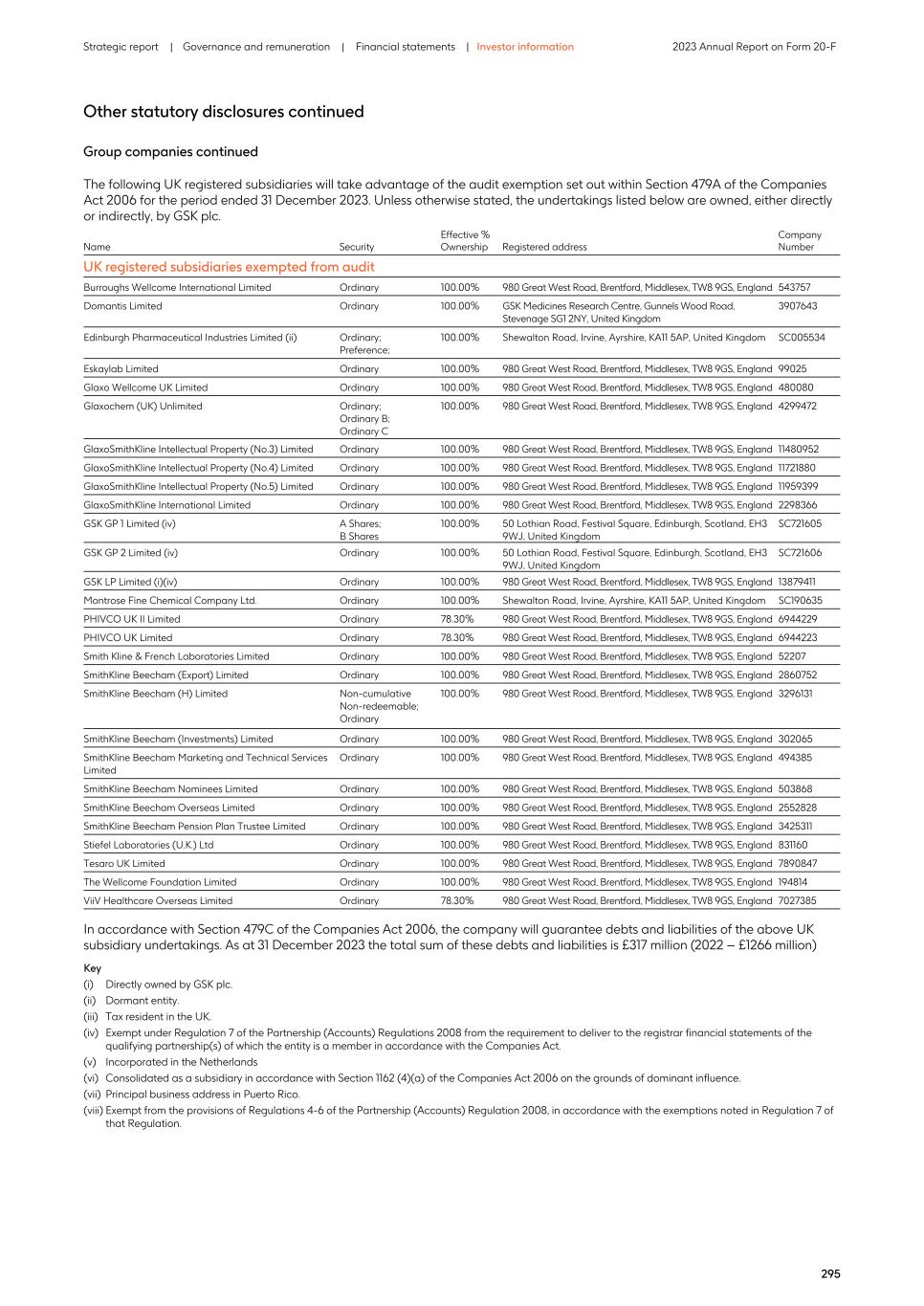
The following UK registered subsidiaries will take advantage of the audit exemption set out within Section 479A of the Companies Act 2006 for the period ended 31 December 2023. Unless otherwise stated, the undertakings listed below are owned, either directly or indirectly, by GSK plc. Name Security Effective % Ownership Registered address Company Number UK registered subsidiaries exempted from audit Burroughs Wellcome International Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 543757 Domantis Limited Ordinary 100.00% GSK Medicines Research Centre, Gunnels Wood Road, Stevenage SG1 2NY, United Kingdom 3907643 Edinburgh Pharmaceutical Industries Limited (ii) Ordinary; Preference; 100.00% Shewalton Road, Irvine, Ayrshire, KA11 5AP, United Kingdom SC005534 Eskaylab Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 99025 Glaxo Wellcome UK Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 480080 Glaxochem (UK) Unlimited Ordinary; Ordinary B; Ordinary C 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 4299472 GlaxoSmithKline Intellectual Property (No.3) Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 11480952 GlaxoSmithKline Intellectual Property (No.4) Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 11721880 GlaxoSmithKline Intellectual Property (No.5) Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 11959399 GlaxoSmithKline International Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 2298366 GSK GP 1 Limited (iv) A Shares; B Shares 100.00% 50 Lothian Road, Festival Square, Edinburgh, Scotland, EH3 9WJ, United Kingdom SC721605 GSK GP 2 Limited (iv) Ordinary 100.00% 50 Lothian Road, Festival Square, Edinburgh, Scotland, EH3 9WJ, United Kingdom SC721606 GSK LP Limited (i)(iv) Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 13879411 Montrose Fine Chemical Company Ltd. Ordinary 100.00% Shewalton Road, Irvine, Ayrshire, KA11 5AP, United Kingdom SC190635 PHIVCO UK II Limited Ordinary 78.30% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 6944229 PHIVCO UK Limited Ordinary 78.30% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 6944223 Smith Kline & French Laboratories Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 52207 SmithKline Beecham (Export) Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 2860752 SmithKline Beecham (H) Limited Non-cumulative Non-redeemable; Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 3296131 SmithKline Beecham (Investments) Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 302065 SmithKline Beecham Marketing and Technical Services Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 494385 SmithKline Beecham Nominees Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 503868 SmithKline Beecham Overseas Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 2552828 SmithKline Beecham Pension Plan Trustee Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 3425311 Stiefel Laboratories (U.K.) Ltd Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 831160 Tesaro UK Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 7890847 The Wellcome Foundation Limited Ordinary 100.00% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 194814 ViiV Healthcare Overseas Limited Ordinary 78.30% 980 Great West Road, Brentford, Middlesex, TW8 9GS, England 7027385 In accordance with Section 479C of the Companies Act 2006, the company will guarantee debts and liabilities of the above UK subsidiary undertakings. As at 31 December 2023 the total sum of these debts and liabilities is £317 million (2022 – £1266 million) Key (i) Directly owned by GSK plc. (ii) Dormant entity. (iii) Tax resident in the UK. (iv) Exempt under Regulation 7 of the Partnership (Accounts) Regulations 2008 from the requirement to deliver to the registrar financial statements of the qualifying partnership(s) of which the entity is a member in accordance with the Companies Act. (v) Incorporated in the Netherlands (vi) Consolidated as a subsidiary in accordance with Section 1162 (4)(a) of the Companies Act 2006 on the grounds of dominant influence. (vii) Principal business address in Puerto Rico. (viii) Exempt from the provisions of Regulations 4-6 of the Partnership (Accounts) Regulation 2008, in accordance with the exemptions noted in Regulation 7 of that Regulation. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued Group companies continued 295
Glossary of terms Terms used in the Annual Report US equivalent or brief description Accelerated capital allowances Tax allowance in excess of depreciation arising from the purchase of fixed assets that delay the charging and payment of tax. The equivalent of tax depreciation. American Depositary Receipt (ADR) Receipt evidencing title to an ADS. Each GSK ADR represents two Ordinary Shares American Depositary Shares (ADS) Listed on the New York Stock Exchange; represents two Ordinary Shares Basic earnings per share Basic income per share Called up share capital Ordinary Shares, issued and fully paid. CER growth Growth at constant exchange rates. The company GSK plc Currency swap An exchange of two currencies, coupled with a subsequent re-exchange of those currencies, at agreed exchange rates and dates Defined benefit plan Pension plan with specific employee benefits, often called ‘final salary scheme’. Defined contribution plan Pension plan with specific contributions and a level of pension dependent upon the growth of the pension fund. Derivative financial instrument A financial instrument that derives its value from the price or rate of some underlying item Diluted earnings per share Diluted income per share. Employee Share Ownership Plan Trusts Trusts established by the Group to satisfy share-based employee incentive plans Equity Shareholders’ funds Shareholders’ equity. Finance lease Capital lease. Freehold Ownership with absolute rights in perpetuity The Group GSK plc and its subsidiary undertakings. GSK GSK plc and its subsidiary undertakings. Hedging The reduction of risk, normally in relation to foreign currency or interest rate movements, by making off-setting commitments. Intangible fixed assets Assets without physical substance, such as computer software, brands, licences, patents, know-how and marketing rights purchased from outside parties. Ordinary share A fully paid up ordinary share in the capital of the company. Profit Income Profit attributable to shareholders Net income Share capital Ordinary Shares, capital stock or common stock issued and fully paid. Share option Stock option. Share premium account Additional paid-up capital or paid-in surplus (not distributable). Shares in issue The number of shares outstanding. Subsidiary An entity in which GSK exercises control. Treasury share Treasury stock. Turnover Revenue. UK Corporate Governance Code As required by the UK Listing Authority, the company has disclosed in the Annual Report how it has applied the best practice corporate governance provisions of the Financial Reporting Council’s UK Corporate Governance Code. Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued 296

Index Access 45 Accounting principles and policies 163 Acquisitions and disposals 214 Additional information 267 Adjustments reconciling Total profit after tax to operating cash flows 218 American depository shares 271 Annual General Meeting 2023 264 Approach to tax 92 Articles of Association of GSK plc 267 Assets held for sale 193 Associates and joint ventures 180 Audit & Risk Committee Report 121 Business model 06 Cash and cash equivalents 193 Cash generation and conversion 86 CEO’s statement 04 Chair’s statement 02 Chair’s Governance statement 106 Climate-related financial disclosure 57 Code of ethics 273 Commitments 209 Continuous engagement and key decisions 113 Consolidated balance sheet 159 Consolidated cash flow statement 161 Consolidated income statement 158 Consolidated statement of changes in equity 160 Consolidated statement of comprehensive income 158 Contingent consideration liabilities 207 Contingent liabilities 208 Corporate governance 98 Corporate governance comparison 280 Corporate Responsibility Committee Report 116 Critical accounting judgements and key sources of estimation uncertainty 169 Critical accounting policies 95 Cyber security 272 Demerger of Consumer Healthcare business 216 Directors and senior management 146 Directors’ interests in shares 144 Directors’ report 149 Directors’ statement of responsibilities 152 Dividends 184 Earnings per share 184 Employee costs 177 Employee share schemes 238 Environment 47 Ethical standards 51 Exchange rates 171 Finance expense 179 Finance income 179 Financial calendar 2024 264 Financial instruments and related disclosures 221 Financial performance summary 71 Financial position and resources 87 General Medicines 38,78 Glossary of terms 296 Goodwill 187 Group companies 287 Group financial review 70 GSK Leadership Team 104 Innovation 07 Inventories 192 Investments in associates and joint ventures 190 Investor relations 276 Legal proceedings 241 Major restructuring costs 178 Material contracts 270 Movements in equity 210 Net debt 195 New accounting requirements 171 Nominations & Corporate Governance Committee Report 119 Non-controlling interests 212 Non-controlling interests in ViiV Healthcare 74 Non-Executive Directors’ fees 143 Notes to the financial statements 162 Operating profit 176 Other intangible assets 188 Other investments 191 Other non-current assets 191 Other non-current liabilities 208 Other operating income/(expense) 175 Other provisions 206 Our culture and people 12 Our external environment 08 Our long-term priorities 07 Pensions and other post-employment benefits 197 Pharmaceutical products, competition and intellectual property 251 Pipeline 247 Post balance sheet events 244 Principal accountant fees and services 275 Principal group companies 240 Property, plant and equipment 185 Reconciliation of net cash flow to movement in net debt 219 Registrar 276 Related party transactions 213 Reliable supply 42 Remuneration Committee Chair's annual statement 127 Remuneration governance 141 Remuneration policy 273 Remuneration report 127 Reports of Independent Registered Public Accounting Firm 154, 279 Reporting framework 72 Research and development 15 Responsible business 44 Right of use assets 186 Risk Factors 254 Risk management 55 Science Committee report 117 Share capital and control 261 Share capital and share premium account 210 Share Consolidation 210 Shareholder information 261 Shareholder services and contacts 276 Specialty Medicines 35,77 Supplemental guarantor information 273 Task Force on Climate-related Financial Disclosures 57 Taxation 181 Tax information for shareholders 265 The Board 99 Three-year employee data 246 Trade and other payables 194 Trade and other receivables 192 Treasury policies 93 Trust 07 Turnover and segment information 172 US law and regulation 277 Using data responsibly 52 Vaccines 31,76 Vaccine products, competition and intellectual property 253 Strategic report | Governance and remuneration | Financial statements | Investor information 2023 Annual Report on Form 20-F Other statutory disclosures continued 297

GSK plc was incorporated as GlaxoSmithKline plc, an English public limited company on 6 December 1999. We were formed by a merger between Glaxo Wellcome plc and SmithKline Beecham plc. GSK acquired these two English companies on 27 December 2000 as part of the merger arrangements. Effective 15 May 2022 GlaxoSmithKline plc changed its name to GSK plc. On 18 July 2022, GSK plc separated its Consumer Healthcare business from the GSK Group to form Haleon, an independent listed company. Our shares are listed on the London Stock Exchange and the New York Stock Exchange. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is http://www.sec.gov. GSK’s internet address is gsk.com Brand names appearing in italics throughout this report are trade marks either owned by and/or licensed to GSK or associated companies. All other trade marks are the property of their respective owners. Cautionary statement regarding forward-looking statements This document and the Group’s other reports published or filed with or furnished to the US Securities and Exchange Commission (SEC), and any other written information released, or oral statements made, to the public in the future by or on behalf of the Group, may contain forward- looking statements. Forward-looking statements give the Group’s current expectations or forecasts of future events. An investor can identify these statements by the fact that they do not relate strictly to historical or current facts. They use words such as ‘anticipate’, ‘estimate’, ‘expect’, ‘intend’, ‘will’, ‘project’, ‘plan’, ‘believe’, ‘target’ and other words and terms of similar meaning in connection with any discussion of future operating or financial performance. In particular, these include statements relating to future actions, prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, dividend payments and financial results. Other than in accordance with its legal or regulatory obligations (including under the Market Abuse Regulation, the UK Listing Rules and the Disclosure and Transparency Rules of the Financial Conduct Authority), the Group undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. The reader should, however, consult any additional disclosures that the Group may make in any documents which it publishes and/or files with the SEC. All readers, wherever located, should take note of these disclosures. Accordingly, no assurance can be given that any particular expectation will be met and investors are cautioned not to place undue reliance on the forward- looking statements. Forward-looking statements are subject to assumptions, inherent risks and uncertainties, many of which relate to factors that are beyond the Group’s control or precise estimate. The Group cautions investors that a number of important factors, including those in this document, could cause actual results to differ materially from those expressed or implied in any forward-looking statement. Such factors include, but are not limited to, those discussed under ‘Risk Factors’ on pages 254 to 260 of this Annual Report on Form 20-F. Any forward-looking statements made by or on behalf of the Group speak only as of the date they are made and are based upon the knowledge and information available to the Directors on the date of this report. Notice regarding limitations on Director Liability under English Law Under the UK Companies Act 2006, a safe harbour limits the liability of Directors in respect of statements in and omissions from the Directors’ Report (for which see page 149 to 150), the Strategic report and the Annual report on remuneration. Under English law the Directors would be liable to the company, but not to any third party, if one or more of these reports contained errors as a result of recklessness or knowing misstatement or dishonest concealment of a material fact, but would otherwise not be liable. Pages 98 to 150, 152 to 153, and 245 to 295 inclusive comprise the Directors’ Report, pages 1 to 97 inclusive comprise the Strategic report and pages 127 to 148 inclusive comprise the Annual report on remuneration, each of which have been drawn up and presented in accordance with and in reliance upon English company law and the liabilities of the Directors in connection with these reports shall be subject to the limitations and restrictions provided by such law. About GSK 298

We unite science, technology and talent to get ahead of disease together. Head Office and Registered Office GSK plc 980 Great West Road Brentford, Middlesex TW8 9GS United Kingdom Tel: +44 (0)20 8047 5000 Registered number: 3888792
| | | | | |
Item 19 | Exhibits |
| |
| 1.1 | |
| |
2.1 | |
| |
2.2 | |
| |
2.3 | |
| |
| 2.4 | Long Term Debt Instruments: GSK plc is not party to any single instrument relating to long-term debt pursuant to which a total amount of securities exceeding 10% of its total assets (on a consolidated basis) is authorised to be issued. GSK plc hereby agrees to furnish to the Securities and Exchange Commission (the “Commission”), upon its request, a copy of any instrument defining the rights of holders of its long-term debt or the rights of holders of the long-term debt of any of its subsidiaries for which consolidated or unconsolidated financial statements are required to be filed with the Commission. |
| |
4.3 | |
| |
4.4 | |
| |
4.5 | |
| |
4.6 | |
| |
4.7 | |
| |
4.8 | |
| |
8.1 | |
| |
12.1 | |
| |
12.2 | |
| |
13.1 | |
| |
15.1 | |
| |
17 | |
| |
97.1 | |
| |
101.INS* | XBRL Instance Document |
| |
101.SCH* | XBRL Taxonomy Extension Schema Document |
| |
101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document 101.DEF**XBRL Taxonomy Extension Definition Linkbase Document 101.LAB**XBRL Taxonomy Extension Label Linkbase Document 101.PRE**XBRL Taxonomy Extension Presentation |
| |
| | | | | |
| Linkbase Document |
| |
| |
*In accordance with Rule 402 of Regulation S-T, the information in these exhibits shall not be deemed to be “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liability of that section, and shall not be incorporated by reference into any registration statement or other document filed under the Securities Act, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing. |
Signature
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
GSK plc
March 5, 2024
By: /s/ Julie Brown
Julie Brown
Chief Financial Officer


















































































































































































































