Exhibit 99.1

Advancing bacteriophage therapeutics for patients with antibiotic - resistant infections January 7, 2019 NYSE American: APHB

Forward Looking Statement This presentation contains “forward - looking” statements that involve risks, uncertainties and assumptions . If the risks or uncertainties materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by such forward - looking statements . All statements other than statements of historical fact could be deemed forward - looking, including, but not limited to : statements related to the anticipated consummation of the merger transaction between AmpliPhi Biosciences Corporation (“ AmpliPhi ”) and C 3 J Therapeutics, Inc . , the financing to be completed immediately following the closing of the merger (the “Financing”), the expected benefits of the merger, milestones of the combined company following the closing of the merger, including the initiation and completion of clinical studies and the timing thereof ; the potential future of antibiotic resistance ; the planned development strategy ; bacteriophage technology being uniquely positioned to address the global threat of antibiotic resistance ; collaborations with third parties and the potential markets and market opportunities for product candidates ; potential market growth ; and any statements of assumptions underlying any of the items mentioned . These statements are based on estimates and information available to us at the time of this presentation and are not guarantees of future performance . Actual results could differ materially from our current expectations . You should not rely upon forward - looking statements as predictions of future events . Although we believe that the expectations reflected in the forward - looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward - looking statements will be achieved or occur . Moreover, we undertake no obligation to update publicly any forward - looking statements for any reason to conform these statements to actual results or to changes in our expectations except as required by law . We refer you to the documents that we file from time to time with the Securities and Exchange Commission (the “SEC”), including our Annual Report on Form 10 - K, Quarterly Reports on Form 10 - Q and Current Reports on Form 8 - K . These documents, including the sections therein entitled “Risk Factors,” identify important factors that could cause the actual results to differ materially from those contained in forward - looking statements . 2

Important Information for Investors and Shareholders This communication may be deemed to be solicitation material in respect of the proposed transaction between AmpliPhi and C 3 J Therapeutics, Inc . ("C 3 J") . In connection with the proposed transaction between AmpliPhi and C 3 J, AmpliPhi will file a proxy statement with the SEC . This communication is not a substitute for the proxy statement or any other documents that AmpliPhi may file with the SEC or send to AmpliPhi shareholders in connection with the proposed transaction . Before making any voting decision, investors and securityholders are urged to read the proxy statement and all other relevant documents filed or that will be filed with the SEC in connection with the proposed transaction as they become available because they will contain important information about the proposed transaction and related matters . You may obtain free copies of the proxy statement and all other documents filed or that will be filed with the SEC regarding the proposed transaction at the website maintained by the SEC www . sec . gov . Once filed, the proxy statement will be available free of charge on AmpliPhi’s website at www . ampliphibio . com or by contacting AmpliPhi’s Investor Relations by email at investor . ampliphibio . com or by phone at 858 - 829 - 0829 or by mail at Investor Relations, AmpliPhi Biosciences Corporation, 3579 Valley Centre Drive, Suite 100 , San Diego, CA 92130 . Participants in Solicitation : AmpliPhi , C 3 J and their respective directors and executive officers may be deemed to be participants in the solicitation of proxies from the holders of AmpliPhi’s common stock in connection with the proposed transaction . Information about AmpliPhi’s directors and executive officers is set forth in AmpliPhi’s Definitive Proxy Statement for its 2018 Annual meeting, which was filed with the SEC on November 9 , 2018 . Other information regarding the interests of such individuals, as well as information regarding C 3 J’s directors and executive officers and other persons who may be deemed participants in the proposed transaction, will be set forth in the proxy statement, which will be filed with the SEC . You may obtain free copies of these documents as described in the preceding paragraph . Non - Solicitation : This communication will not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities, nor will there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction . 3

SOLVING THE ANTIBIOTIC RESISTANCE CRISIS AmpliPhi Biosciences and C3J Therapeutics Merger AmpliPhi and C3J are combining businesses to build the World’s Leading Bacteriophage Company 4
Phage platforms led by seasoned drug development team • Robust natural phage discovery library • Synthetic biology to improve pharmacology and path to commercialization • Phage - specific GMP drug manufacturing capabilities (key differentiator) Robust pipeline with high impact clinical stage candidates • Existing Pharma partnership for large market indication • Phase 1/2 ready S. aureus candidate for bacteremia • Synthetic phage candidate for Pseudomonas lung infections (IND mid - 2019) Strong Board and executive leadership team with public market experience Strong balance sheet • Approximately $18 million in cash at closing 5 Combined Company A World - Leader in Phage Therapeutics
Overview of the Merger Transaction • Announced on January 4, 2019 • AmpliPhi will combine with C3J in a stock - for - stock transaction • Current C3J shareholders to own approximately 70% of combined company; current AmpliPhi securityholders to own approximately 30%; ownership split prior to new capital invested • Existing C3J shareholders have committed to invest an additional $10 million into the combined company • Combined company Board of Directors proportional to the ownership split • Merger expected to close in Q1 2019 6
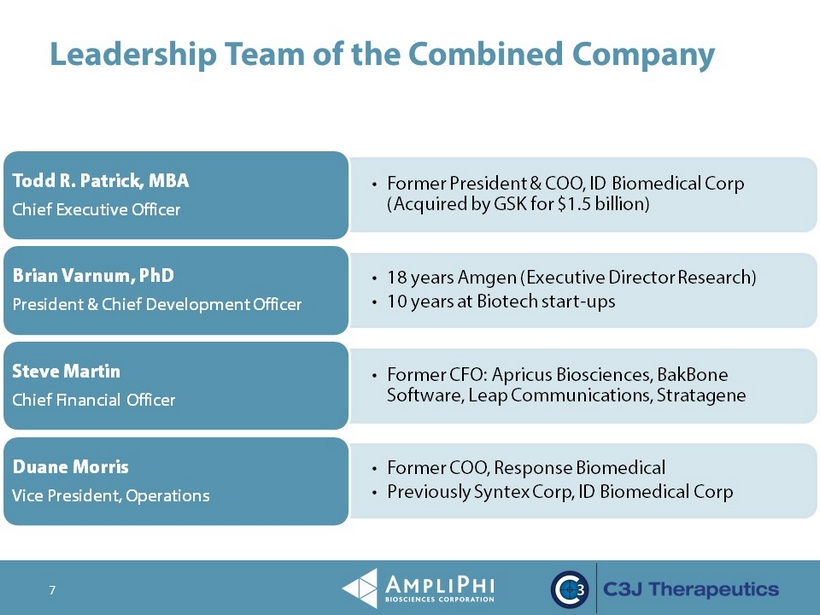
Leadership Team of the Combined Company 7 • Former President & COO, ID Biomedical Corp (Acquired by GSK for $1.5 billion) Todd R. Patrick, MBA Chief Executive Officer • 18 years Amgen (Executive Director Research) • 10 years at Biotech start - ups Brian Varnum, PhD President & Chief Development Officer • Former CFO : Apricus Biosciences, BakBone Software, Leap Communications, Stratagene Steve Martin Chief Financial Officer • Former COO, Response Biomedical • Previously Syntex Corp, ID Biomedical Corp Duane Morris Vice President, Operations

Introduction to Bacteriophage (Phage) • The most abundant life form on Earth • Natural predators of bacteria • Highly targeted, exists in harmony with prey • Specific phage engineering can enhance their killing ability, lowers resistance • Low cost of goods • Low cost to manufacture a dose • Replication at site of infection; requires fewer doses • Prior history as therapeutic agent • Antibiotics displaced phage use, drug - resistant threat revitalized phage use • Mechanism of action distinct from antibiotics 8
Unmet Need in Antibiotic Resistant Infections Phages Provide a Solution to an Urgent Public Health Threat Emergence of Drug - Resistant Bacterial Infections • The incidence of antibiotic - resistant continues to increase, representing a major challenge to human health • 2 million antibiotic - resistant infections; 23,000 deaths/year in US* • Deaths from bacterial infections expected to reach 10 million by 2050, surpassing cancer - related deaths* 9 More than 20 patients treated with phage therapy in 2017 - 18 • Serious or life - threatening infections not responding to antibiotics • Treatment under Emergency IND • Generally well tolerated * Centers for Disease Control and Prevention

10 FDA Backs Efforts to Advance New Innovations Supports Development of Phages as Non - Traditional Antimicrobials Source: Twitter Sept. 16, 2018; ”As more and more bacteria grow resistant to currently available antibiotics, we must tackle the issue on all fronts and seek new approaches to this persistent and potentially deadly problem” Scott Gottlieb, MD FDA Commissioner Strategic Directions: Facilitating Product Development Supporting Antimicrobial Stewardship Enhancing Antimicrobial Resistance Surveillance Enhancing Regulatory System “Push” incentives Greater research funding and shortening time to conduct trials GAIN Act, QIDP Designation, LPAD Pathway, Accelerators (CARB - X) “Pull” incentives Reimbursement reforms, i.e. a subscription - based model Encourage development of other types of antibacterials , incl. phage

Combined Pipeline Multiple Opportunities for Value Creation 11

12 • CDC estimates that 1.5 million Americans develop bacteremia each year and ~250,000 deaths occur as a direct result of infection 1 • 1 in 3 patients who die in hospital have bacteremia 1 • Bacteremia is the most expensive condition treated at U.S. hospitals, costing ~$24 billion annually 2 • S. aureus is the second most common pathogen associated with bacteremia, causing ~150,000 cases per year and ~30,000 deaths 3 4 • Infectious endocarditis – biofilm formation • MRSA – independent risk factor for death from bacteremia Lead Indication: S. aureus Bacteremia A Common and Deadly Condition 15.3% 12.4% 11.0% 8.3% 7.6% 6.6% 6.0% 5.0% 27.8% ESCHERICHIA … STAPHYLOCO … ENTEROCOCC … COAGULASE - … KLEBSIELLA SPP CANDIDA SPP STREPTOCOC … PSEUDOMON … OTHER … 1. CDC Data & Reports. https://www.cdc.gov/sepsis/datareports/index.html. Updated August 25, 2017. 2. Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project Statistical Brief No. 204. May 2016 3. Savage R. et al. 2016. CMAJ Open DOI:10.9778/cmajo.20160074 \ 4. Bassetti M et al. 2017. PLoS ONE; 12(2): e0170236

13 AB - SA01 Ready for Phase 1/2 trial in S. aureus bacteremia - 3 lytic phages - 3 ¨ 109 PFU per dose - Covers ~95% S. aureus strains, incl. MDR » Expanded Access: treated 15 patients with serious infections not responding to antibiotics • Bacteremia, endocarditis, PJI, LVAD infections, other • 300+ IV doses administered, well tolerated • 85% treatment success at end of therapy » FDA pre - IND feedback: ready for randomized controlled Phase 1/2 trial in S. aureus bacteremia
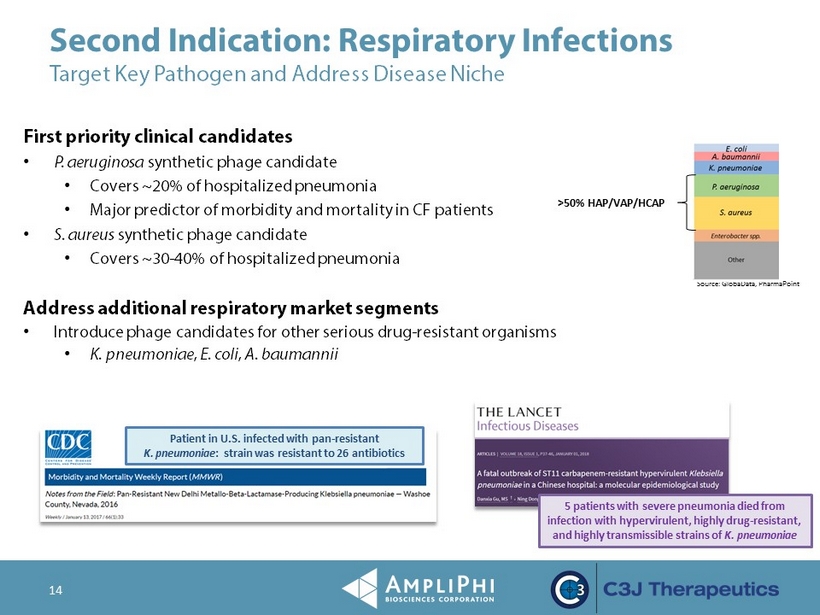
14 First priority clinical candidates • P. aeruginosa synthetic phage candidate • Covers ~20% of hospitalized pneumonia • Major predictor of morbidity and mortality in CF patients • S. aureus synthetic phage candidate • Covers ~30 - 40% of hospitalized pneumonia Address additional respiratory market segments • Introduce phage candidates for other serious drug - resistant organisms • K. pneumoniae , E. coli , A. baumannii Source: GlobaData , PharmaPoint Second Indication: Respiratory Infections Target Key Pathogen and Address Disease Niche >50% HAP/VAP/HCAP Patient in U.S. infected with pan - resistant K. pneumoniae : strain was resistant to 26 antibiotics 5 patients with severe pneumonia died from infection with hypervirulent, highly drug - resistant, and highly transmissible strains of K. pneumoniae
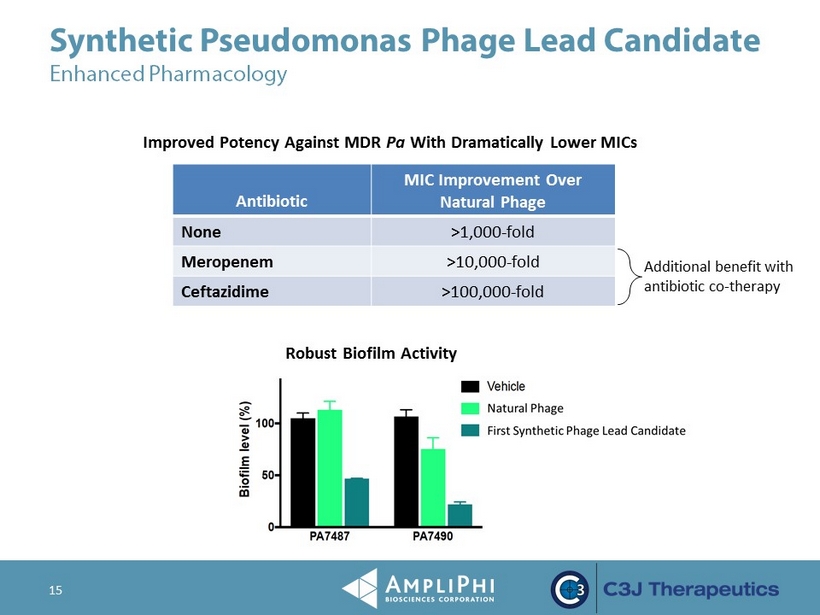
Synthetic Pseudomonas Phage Lead Candidate Enhanced Pharmacology 15 Antibiotic MIC Improvement Over Natural Phage None >1,000 - fold Meropenem >10,000 - fold Ceftazidime >100,000 - fold Additional benefit with antibiotic co - therapy Improved Potency Against MDR Pa With Dramatically Lower MICs Robust Biofilm Activity
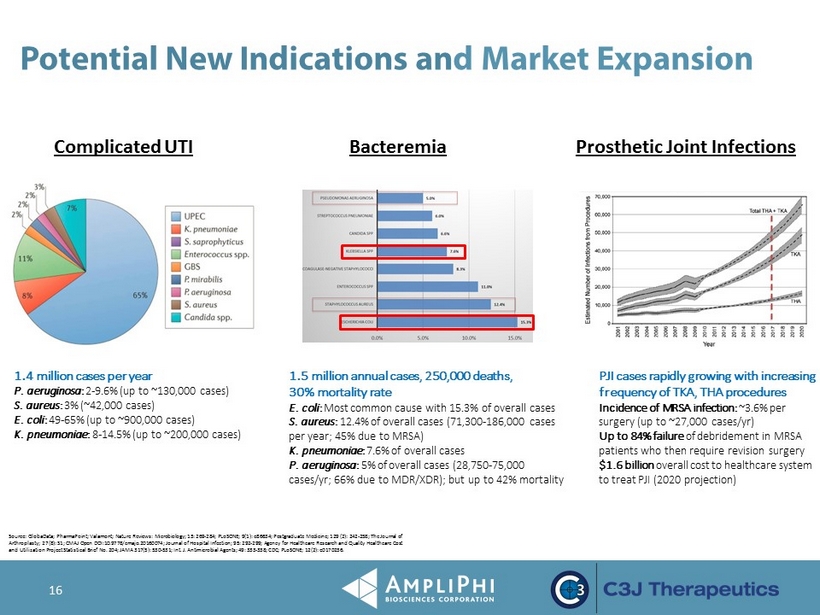
16 Potential New Indications an d Market Expansion Source: GlobaData ; PharmaPoint ; Valamont ; Nature Reviews: Microbiology; 13: 269 - 284; PLoSONE; 9(1): e86634; Postgraduate Medicine; 129 (2): 242 - 258; The Journal of Arthroplasty; 27(8): S1; CMAJ Open DOI:10.9778/cmajo.20160074; Journal of Hospital Infection; 95: 292 - 299; Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project Statistical Brief No. 204; JAMA 317(5): 530 - 531; Int. J. Antimicrobial Agents; 49: 333 - 338; CDC; PLoSONE ; 12(2): e0170236. Complicated UTI 1.4 million cases per year P. aeruginosa : 2 - 9.6% (up to ~130,000 cases) S. aureus : 3% (~42,000 cases) E. coli : 49 - 65% (up to ~900,000 cases) K. pneumoniae : 8 - 14.5% (up to ~200,000 cases) Bacteremia Prosthetic Joint Infections PJI cases rapidly growing with increasing frequency of TKA, THA procedures Incidence of MRSA infection: ~3.6% per surgery (up to ~27,000 cases/ yr ) Up to 84% failure of debridement in MRSA patients who then require revision surgery $1.6 billion overall cost to healthcare system to treat PJI (2020 projection) 1.5 million annual cases, 250,000 deaths, 30% mortality rate E. coli : Most common cause with 15.3% of overall cases S. aureus : 12.4% of overall cases (71,300 - 186,000 cases per year; 45% due to MRSA) K. pneumoniae : 7.6% of overall cases P. aeruginosa : 5% of overall cases (28,750 - 75,000 cases/ yr ; 66% due to MDR/XDR); but up to 42% mortality

17 Expected Milestones: 2019 - 2020 Multidrug - Resistant Infectious Disease • AB - SA01 • Initiate Phase 1/2 clinical study in bacteremia • Synthetic Pseudomonas phage candidate • Transition to GMP manufacturing • Conduct Pre - IND meeting and file IND • Initiate first - in - human clinical study in lung infections • Phase 2 pneumonia study • Advance both Staphylococcus and Pseudomonas phage candidates • ESKAPE pathogen phage • Advance new clinical candidate for market expansion and/or new indication • Partnering • Achieve R&D Milestone under existing C3J pharma partnership • New partnership potential (DX and/or Rx) • Grants and Alternative Investments Preventable Infectious Disease of the Microbiome • Synthetic S. mutans phage (dental caries product) • Initiate engineering • Initiate clinical trial partnership with leading academic institution

Investment Highlights • Platforms led by seasoned drug development team • Robust pipeline with high impact clinical stage candidates • Strong Board and Executive leadership team • Strong balance sheet with approximately $18 million in cash at closing • Multiple near - term regulatory milestones • Opportunity to build value with microbiome indications 18