Filed Pursuant to Rule 433
Issuer Free Writing Prospectus dated May 1, 2017
Registration No. 333-217169

Leading the Phage Therapy Renaissance Announcing Personalized Precision Phage (P 3 ) Therapies for Antibiotic - Resistant Infections May 1, 2017 NYSE MKT: APHB This free writing prospectus should be read together with the issuer’s registration statement on Form S - 1 (File No. 333 - 217169) (including the prospectus therein), as amended. The following information supplements the information contained in the registration statement.

2 This presentation contains “forward - looking” statements that involve risks, uncertainties and assumptions. If the risks or uncer tainties materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by such forward - looking statements. All state ments other than statements of historical fact could be deemed forward - looking, including, but not limited to: the potential future of antibiotic resistance; t he expected timing of additional clinical trials, including Phase I or II clinical trials; the drug product candidates to be supplied by AmpliPhi for clinical trials; planned business strategies, inc luding our personalized precision phage (P 3 ) therapy strategy and the expected benefits thereof; bacteriophage technology being uniquely positioned to address the globa l threat of antibiotic resistance; our expectations with respect to the $1.8 million Australian tax rebate; the protection of i nte llectual property; the activities to be performed by specific parties in connection with clinical trials; the potential use of bacteriophages to treat bacterial infe cti ons; timing for manufacturing scale - up and the purification and formulation of bacteriophages; research and development plans; the development of bacteriophage - based thera pies; the ability to select combinations of phages to formulate product candidates; the ability to manufacture product candidates; the safety and efficac y o f product candidates; potential and expected financing arrangements; collaborations with third parties and the potential markets for product candidates; potentia l m arket growth; the development of personalized precision phage therapies and planned approach for administering such therapies; the potential benefits of perso nal ized precision phage therapies; and any statements of assumptions underlying any of the items mentioned. These statements are based on estimates and information ava ilable to us at the time of this presentation and are not guarantees of future performance. Actual results could differ materially from our current expectatio ns. You should not rely upon forward - looking statements as predictions of future events. Although we believe that the expectations reflected in the forward - looking s tatements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward - looking statements will be achieved or occur. Moreover, we undertake no obligation to update publicly any forward - looking statements for any reason to conform these statement s to actual results or to changes in our expectations except as required by law. We refer you to the documents that we file from time to time with the Securities and Exchange Commission (the “SEC”), specifi cal ly our Annual Report on Form 10 - K and our Current Report on Form 8 - K filed with the SEC on May 1, 2017. These documents, including the sections therein entitled “ Risk Factors,” identify important factors that could cause the actual results to differ materially from those contained in forward - looking statements. We have filed a registration statement (including a prospectus) with the SEC (File No 333 - 217169 ) for the offering to which this presentation relates. The registration statement can be accessed through the following link: https :// www.sec.gov/Archives/edgar/data/921114/000114420417023490/0001144204 - 17 - 023490 - index.htm . Before you invest, you should read the prospectus in that registration statement and other documents we have filed with the SEC for mor e complete information about our company and the offering. You may obtain these documents for free by visiting the SEC’s website at www.sec.gov , or alternatively from the offices of H.C. Wainwright & Co., LLC, H.C. Wainwright & Co., LLC, 430 Park Avenue, 4th Floor, New York, New York 10022. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale o f t hese securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the sec uri ties laws of any such state or other jurisdiction. Safe Harbor Statement

3 Antibiotic - resistance is a major global health threat Bacteriophage technology is uniquely positioned to address this threat • Precisely targeted, differentiated mechanism of action, synergistic with antibiotics AmpliPhi is pioneering bacteriophage technology to treat multidrug - resistant (MDR) infections • 10+ years of bacteriophage R&D, with $60M+ invested • Wholly owned cGMP manufacturing facility dedicated to phage production AB - SA01 S. aureus program is well - positioned for Phase 2 in chronic rhinosinusitis (CRS) • Two Phase 1 studies completed (CRS and topical) • Positive feedback from Type B meeting with FDA in Q1 2017 AB - PA01 P. aeruginosa program is estimated 9 months from Phase 1 in cystic fibrosis (CF) • Positive feedback received from the Medicines and Healthcare products Regulatory Agency (MHRA) regarding planning a Phase 1 in patients with CF • Phase 1 expected to be conducted with Dr. Jane Davies at Royal Brompton Hospital in London Expect to achieve clinical evidence for phage technology platform by treating serious MDR infections under compassionate - use regulatory guidelines Executive Summary
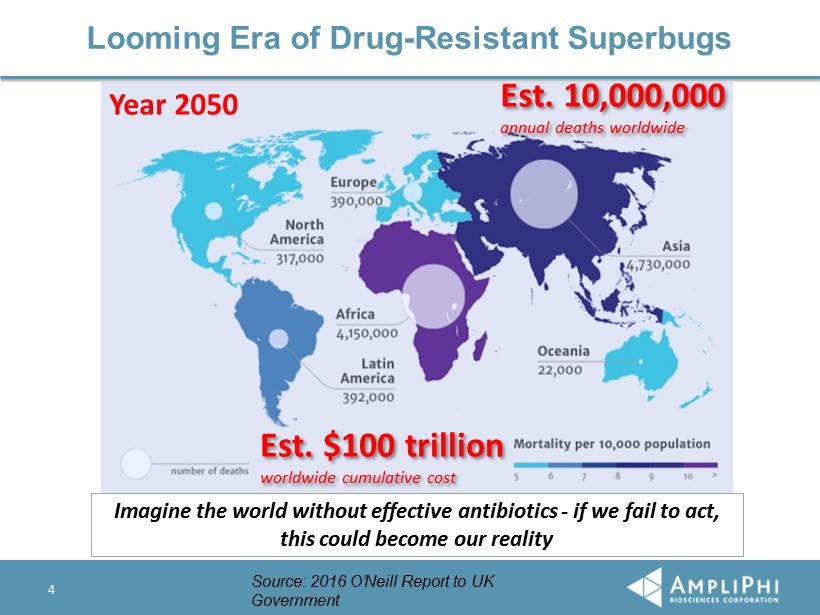
Year 2050 Looming Era of Drug - Resistant Superbugs 4 Est. $100 trillion worldwide cumulative cost Imagine the world without effective antibiotics - if we fail to act, this could become our reality Est. 10,000,000 annual deaths worldwide Source: 2016 O’Neill Report to UK Government

The World Awaits a Response 5
The Power of Bacteriophages as Targeted Antibacterials Harnessing ancient predator - prey relationship to kill bacteria • Naturally - occurring viruses • infect and kill only bacteria • prey on specific bacterial strains • penetrate biofilms Phages are the most abundant and diverse organisms on Earth • Humans evolved with phage in and on us • There are a variety of phage types capable of infecting and killing most, if not all, strains of bacteria Discovered in 1915 and used broadly in U.S. and Europe prior to development of antibiotics • Efficacy of approach has been broadly, yet anecdotally, demonstrated • 20 th century challenges: purification, characterization, narrow spectrum 6

Society is losing the race against pathogenic bacteria 7 The Time for Phage Therapies Is Now Characterization, sequencing, purification, endotoxin removal, synthetic biology • Selecting and optimizing therapeutic phages • proprietary combinations designed to maximize efficacy and minimize resistance • Hybridizing and synthesizing traits to create enhanced phages Urgent need Better technology Modern techniques
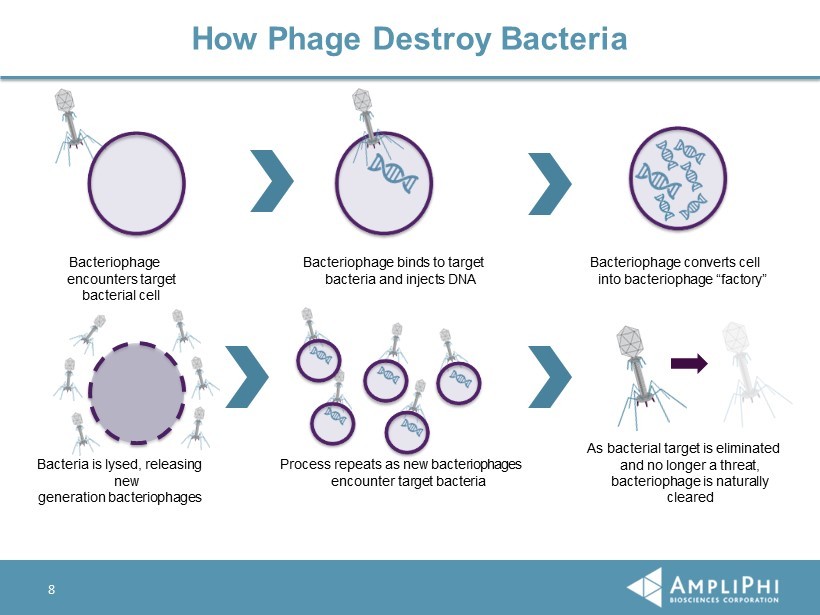
How Phage Destroy Bacteria Bacteriophage encounters target bacterial cell Bacteriophage binds to target bacteria and injects DNA Bacteriophage converts cell into bacteriophage “factory” Bacteria is lysed, releasing new generation bacteriophages Process repeats as new bacteriophages encounter target bacteria As bacterial target is eliminated and no longer a threat, bacteriophage is naturally cleared 8

9 AmpliPhi’s Phage Library Targets Bacteria on WHO Priority Pathogens List Source: World Health Organization, 2017 Priority Pathogens List published Feb. 25, 2017 Priority 1: CRITICAL Acinetobacter baumannii , carbapenem - resistant Pseudomonas aeruginosa , carbapenem - resistant Enterbacteriaceae , carbapenem - resistant, 3 rd generation cephalosporin - resistant Enterococcus faecium , vancomycin - resistant Staphylococcus aureus, methicillin - resistant, vancomycin intermediate and resistant Helicobacter pylori, clarithromycin - resistant Campylobacter, fluoroquinolone - resistant Salmonella spp. , fluoroquinolone - resistant Neisseria gonorrhoeae , 3 rd generation cephalosporin - resistant, fluoroquinolone - resistant Priority 2: HIGH Priority 3: MEDIUM Streptococcus pneumoniae , penicillin - non - susceptible Haemophilus influenzae , ampicillin - resistant Shigella spp. , fluoroquinolone - resistant

10 Patent protection ▪ Protection for sequential administration of phage and antibiotics ▪ Prosecuting both biofilm - dependent and independent induced sensitivity ▪ Broad species coverage in EU, Australia, Japan, Canada; U.S. protection for Pseudomonas Phages can exert selection pressure on MDR bacteria to induce sensitivity to antibiotics ▪ Bacteria have limited degrees of freedom to mutate around therapies ▪ Phage attack causes evolutionary trade - off in bacteria, restoring antibiotic sensitivity ▪ E.g., efflux pump* ▪ Recent publications demonstrate POC Phage Therapy Has the Potential to Reinvigorate Antibiotic Efficacy (*) Chan et al. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa . Scientific Reports 2016

Bacteriophage - based technology • Mechanism of action radically different from traditional antibiotics • Targeted, flexible and diverse R&D expertise • Staphylococcus aureus: 3 - phage cocktail completed two Phase 1 trials • Pseudomonas aeruginosa: 4 - phage cocktail in development • Inventory of phages against S. aureus , Salmonella , P. aeruginosa , Enterococcus , E. coli , Klebsiella , Enterobacter , Acinetobacter , Streptococcus Manufacturing expertise • Scale - up manufacturing, purification, formulation of bacteriophages • Wholly owned, dedicated cGMP phage manufacturing facility 11 Company Overview
Management M. Scott Salka Chief Executive Officer Igor Bilinsky , Ph.D. Chief Operating Officer Steve Martin Chief Financial Officer Alex Gaidamaka, Ph.D., D.V.M. VP CMC Sandra Morales, Ph.D. VP Research Carrie Langlais Furr , Ph.D. VP Regulatory & Project Management 12 Board of Directors Jeremy Curnock Cook, Chair Louis Drapeau Paul Grint , M.D. Wendy Johnson Mike Perry, D.V.M., Ph.D . Vijay Samant M. Scott Salka ~25 in R&D and Manufacturing Team

13 Historical Focus on Phage 1.0 Phage 1.0 Phage 2.0 Phage 3.0 AB - SA01, AB - PA01 Wild - type Bacterial infections with high unmet need Pipeline programs: CRS, CF, otitis, etc. Personalized Wild - type Antibiotic - resistant serious or life - threatening bacterial infections Precision medicine for infectious diseases Engineered phages with improved properties for treatment of serious bacterial infections

Development Pipeline Indication Collaborator Discovery Preclinical Phase 1 Phase 2 AB - SA01 (3 - Phage cocktail targeting Staphylococcus aureus ) Chronic Rhinosinusitis (CRS) Seek partner for Phase 2 Topical indications / wound care AB - PA01 (4 - Phage cocktail targeting Pseudomonas aeruginosa ) Cystic Fibrosis (CF) Seek partner or foundation funding CRS Otitis Seek partner 14

15 University of Adelaide Patient 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Days Completed Phase 1 Trial in Chronic Rhinosinusitis Low Dose BID (10 8 PFU/mL) High Dose BID (10 9 PFU/mL) Primary Endpoints ▪ Well tolerated, no drug - related SAEs Secondary Observations ▪ Reduction in S. aureus bacterial load at end of trial compared to baseline in all patients ▪ Reduction in mucosal edema, discharge and polyps by endoscopic images (Lund - Kennedy scale) ▪ Patient - reported symptom improvement ▪ No patient drop outs Nasal Wash Administration FDA Type B meeting Feb., 2017 : positive feedback on previously submitted detailed development proposal to commence a Phase 2

16 U.S. Army under CRADA Primary Endpoints (all subjects) • AB - SA01 well tolerated throughout the trial ▪ No treatment - emergent AEs considered definitely or probably related to AB - SA01 ▪ No severe or high - grade treatment - emergent AEs, SAEs or discontinuation of treatment due to treatment - emergent AEs ▪ All laboratory values and vital sign parameters within normal ranges Subject 1 2 3 Days Topically administered AB - SA01 and placebo to opposite forearms, covered with occlusive dressings Treatment for 3 consecutive days and monitored for 2 weeks following treatment ▪ Low Dose QD ( 10 8 PFU/mL) ▪ High Dose QD ( 10 9 PFU/mL) Completed Intact Skin Phase 1 Trial
Ljubljana, Slovenia: • ~6,000 square feet - Clean rooms - QC & process development - GMP storage Conducting: • Fermentation (40 - liter scale) • Purification • Aseptic fill • Product QC and release • Stability APHB cGMP Bioreactor 17 In - House cGMP Certified Manufacturing Facility
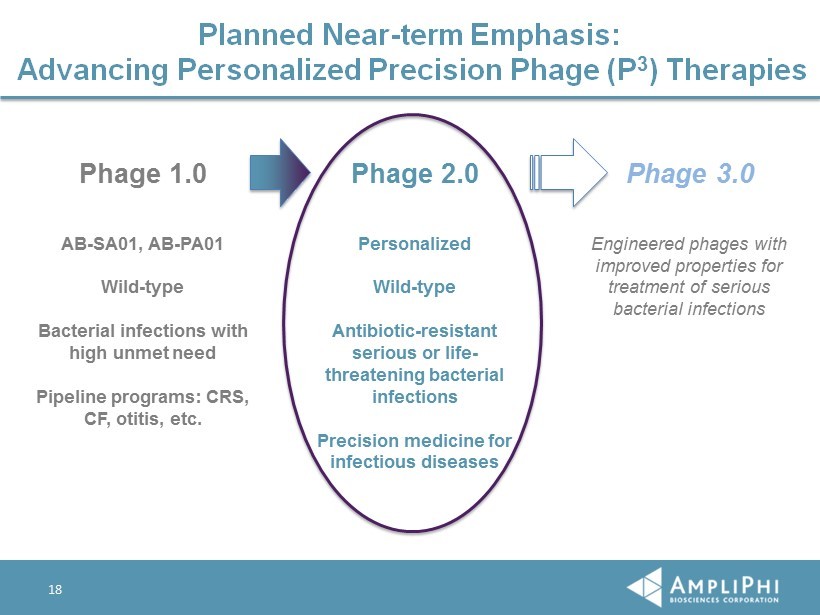
18 Planned Near - term Emphasis: Advancing Personalized Precision Phage (P 3 ) Therapies Phage 1.0 Phage 2.0 Phage 3.0 AB - SA01, AB - PA01 Wild - type Bacterial infections with high unmet need Pipeline programs: CRS, CF, otitis, etc. Personalized Wild - type Antibiotic - resistant serious or life - threatening bacterial infections Precision medicine for infectious diseases Engineered phages with improved properties for treatment of serious bacterial infections

19 Resistant Bacterial Infections Responsible for >20,000 Deaths per Year in the U.S. Source: Pharmacy & Therapeutics 2015; CDC 2014 (*) Community associated U.S. incidence of resistant hospital associated infections Annual deaths attributed to resistant infections Invasive methicillin - resistant Staphylococcus aureus (MRSA) 72,000 11,000 Drug - resistant Streptococcus pneumoniae ~1M * 7,000 Vancomycin - resistant Enterococci (VRE ) 20,000 1,300 Carbapenem - resistant Enterobacteriaceae (CRE) 9,000 600 MDR Acinetobacter 7,000 500 MDR Pseudomonas aeruginosa 6,000 400 >20,000
Positive FDA Feedback at Type B Meeting in February 2017 FDA expressed support for precision phage therapy for patients with serious or life - threatening infections • “CBER acknowledged that phage therapy is an exciting approach to treatment of multidrug - resistant organisms and expressed a commitment to addressing the unique regulatory challenges that might arise during product development.” Data from compassionate - use cases could inform approval pathway • “CBER stated that the clinical safety and effectiveness data collected during development, including from emergency case studies, could inform future discussions for clinical development and ultimately, the regulatory pathway to approval.” FDA is open for continued discussion • FDA suggested various potential next steps, e.g., Master File, Type C Meeting, etc. 20
Compassionate - use Case: Patient Suffering from Refractory P. aeruginosa Bladder Infection • 67 - year - old female underwent intra - abdominal resections and radiation for adenocarcinoma • Contracted symptomatic P. aeruginosa bladder infection • Multiple courses of antibiotics over 2 - year period: gentamicin, ceftazidime, ciprofloxacin, and meropenem • Only option: removal of bladder and urethra • Personalized 6 - phage therapy developed and administered via catheter for 10 days • 6 days after initial dose, bacteria became sensitive to meropenem • Patient experienced durable microbiological cure for >2 years until she succumbed to cancer • No phage - resistant bacteria were detected 21 Khawaldeh et al. (2011). Bacteriophage therapy for refractory Pseudomonas aeruginosa urinary tract infection. J Med Microbiol .

Compassionate - use Case: Critically Ill Patient Suffering from MDR A. baumannii Infection • 69 - year - old male suffering from MDR Acinetobacter baumannii abdominal infection • Multiple courses of antibiotics over 4 months: vancomycin, meropenem, colistin, tigecycline, azithromycin, and rifampin • Critically ill; in a coma for several weeks • Multi - disciplinary team with UCSD, Texas A&M, SDSU, US Navy • Personalized phage therapy developed within 10 days • Administered IP and IV, under Emergency IND at UCSD • Patient emerged from coma 4 days after initial phage administration • Patient experienced durable microbiological cure and continues doing well 22 “Intravenous applications of phage therapy to treat a terminally ill patient who was infected with a multidrug - resistant A. baumannii ,” Dr. Biswajit Biswas, U.S. Navy’s Medical Research Center, presented at Centennial Celebration of Bacteriophage Research, Institut Pasteur, Paris. April 26, 2017
• Establish center to provide P 3 Therapies for patients with antibiotic - resistant infections under compassionate use • Serve patients who have few or no other therapeutic options • Demonstrate real - world clinical evidence • Optimize treatment regimens and expand therapeutic phage libraries • Support commercial approval of P 3 therapies based on real - world evidence 23 Vision: Conquering MDR Infections
• We believe there is a strong interest in phage therapy for antibiotic - resistant infections from leading hospitals and infectious disease physicians in Australia • Favorable regulatory environment for treating patients under compassionate - use: physician has ability to administer treatment without prior regulatory approval in emergency situations • Established AmpliPhi research facility with experienced team in phage production and purification • Concentrated population centers, access to patients • Potential availability for government funding and approximately 40% R&D tax rebate 24 Australia Is an Attractive Location for Initial P 3 Therapy Clinical Trials

25 Planned P 3 Therapies Operating Model Nationwide network • Identify patients • Provide treatment Collect data Optimize regimen AmpliPhi Estimates • < 7 day turnaround for common pathogens • < 14 days for first encounter with new pathogen • On - going pathogen surveillance National Advisory Council Pathogen isolate Personalized bacteriophage therapy AmpliPhi Local lead hospital Phage library Screening Bioinformatics Real - time manufacturing

26 P 3 T Planned Regulatory Strategy: Leverage Compassionate - use Clinical Data to Inform Path to Approval Demonstrate clinical utility of P 3 T Define path to approval in consultation with FDA Obtain registration - enabling data and commercialize • Compassionate - use for patients with serious and life - threatening infections • Start in Australia • Utilize GMP S. aureus and P. aeruginosa phages • Optimize therapeutic regimens • Expand phage libraries for MDR pathogens • Publish clinical evidence • Present compassionate - use clinical data to regulatory authorities • Define scope of data required for approval • 21 st Century Cures Act • Real - world evidence • LPAD limited patient population framework • Generate additional data as agreed with FDA • Expand to other major markets • Precedents of personalized therapies (DC vaccine, CAR - T) and targeted therapies (Rx/ Dx , basket trial design) 2017 – 2018 * 2018+ * * AmpliPhi estimated timelines
27 • Partnerships have helped support development • $9.0M gross raised in 2016 through issuance of common stock • Received $0.9M in tax rebates from Australian Government in 2016 • Filed for $1.8M in tax rebates from Australian Government; expect receipt in mid - 2017 subject to Australian tax authorities review • $2.2M cash balance as of March 31, 2017 • 1.65M shares outstanding and 2.6M fully diluted as of April 30, 2017 * Funding and Capitalization *Share amounts outstanding include common stock only. Fully diluted includes outstanding warrants and stock options. Fully di lut ed amount does not include shares issuable under the 2016 Equity Incentive Plan or the ESPP Plan or the shares that are potentia lly issuable pursuant to the Common Stock Issuance Agreement. See the Preliminary Prospectus dated May 1, 2017 as filed with the SE C.

28 Antibiotic - resistance is a major global health threat We believe bacteriophage technology is uniquely positioned to address this threat • Precisely targeted, differentiated mechanism of action, synergistic with antibiotics AmpliPhi is pioneering bacteriophage technology to treat MDR infections • 10+ years of bacteriophage R&D, with $60M+ invested • Wholly owned cGMP manufacturing facility dedicated to phage • S. aureus program is well - positioned for Phase 2 • P. aeruginosa program is estimated to be 9 months from Phase 1 initiation Potential to achieve proof - of - concept of P 3 Therapy by end of 2017 Potential to address significant commercial opportunity In Summary