Exhibit 99.1

Palatin Technologies November 2012 Carl Spana, Ph.D. President & CEO Stephen T. Wills, CPA/MST CFO / COO

The statements in this presentation that relate to future plans, events or performance are forward - looking statements, which are made pursuant to the safe harbor provisions of Section 21 E of the Securities Exchange Act of 1934 . Such forward - looking statements involve significant risks and uncertainties, and actual results, events and performance may differ materially from those expressed or implied in this presentation . Factors that could cause such differences include, but are not limited to, risks pertaining to product development, clinical trial outcomes, regulatory requirements and actions, availability of required financing and other sources of funds, corporate partnering agreements, development of the markets for the Company’s products and other risks disclosed in the Company’s most recent Annual Report on Form 10 - K and other periodic reports filed with the Securities and Exchange Commission . The forward - looking statements in this presentation do not constitute guarantees of future performance . The Company undertakes no obligation to publicly update these forward - looking statements to reflect events or circumstances that occur after the date of this presentation . Forward - Looking Statement 2

3 Palatin Technologies, Inc. (NYSE MKT: PTN) is a biopharmaceutical company developing targeted, receptor - specific peptide therapeutics for the treatment of diseases with significant unmet medical need and commercial potential. Company Profile

Palatin Investment Opportunity 4 Well positioned to drive growth through internal pipeline/collaborations Lead product: bremelanotide (BMT) for the treatment of female sexual dysfunction (FSD) ◦ Unmet medical need with multi - billion dollar market opportunity ◦ Phase 2B clinical trial positive - Top - line data released November 8th ◦ Extensive clinical & CMC data, proof of principle established PL - 3994 targeting natriuretic receptors for pulmonary indications ◦ Novel mechanism of action – initial indication acute treatment of severe asthma ◦ Phase 1 subcutaneous studies complete, phase 2 program ready MCR - 4 obesity/diabetes collaboration with AstraZeneca ◦ Proof of principle established ◦ Multiple preclinical compounds under evaluation ◦ AstraZeneca responsible for clinical development and costs ◦ ~$145M in milestones plus royalties Targeting new collaborations for our natriuretic and melanocortin research programs

Pipeline 5 Program Indication Preclinical Phase I Phase II Phase III Melanocortin Receptor (MCR) Programs Bremelanotide Female Sexual Dysfunction MCR-4 Agonist (partnered with AstraZeneca) Obesity and Diabetes Next generation peptide Female Sexual Dysfunction and Erectile Dysfunction Natriuretic Receptor Programs PL-3994: Subcutaneous Acute Severe Asthma PL-3994: Subcutaneous Cardio Pulmonary Indications Ongoing active program Next step dependent on licensing or partnering


Female Sexual Dysfunction Overview Female Sexual Dysfunction (FSD) is defined as persistent or recurring problems during one or more of the stages of sexual response with associated distress FSD is prevalent in U.S. adult women ◦ ~43% experiencing some symptoms of FSD ◦ ~23% experiencing associated distress FSD has a significant impact on patient self - image, relationships, and general well - being 7

FSD: Significant Untapped Market Opportunity No FDA approved treatments available 50 million pre - menopausal women in US o 22% reported a sexual problem o 9% were distressed by their sexual problems • One - third sought formal care Market size – U.S. o Potential presenting pre - menopausal women ~1.5 million o Annual sales >$2 billion 8

First - in - class melanocortin receptor 4 agonist for FSD On - demand use with rapid onset of activity and well - tolerated Novel mechanism of action activating endogenous pathways involved in sexual arousal response Evaluated in 30 clinical studies showing efficacy in both FSD and ED Bremelanotide Profile 9
BMT Subcutaneous Development 10 Demonstrated optimal pharmacokinetics ◦ Subcutaneous on - demand formulation: less variation in patient exposure ◦ Good therapeutic dosing window to avoid potential AE’s (GI and BP effects) associated with over - exposure Completed and submitted preclinical toxicology to FDA ◦ Reproductive and carcinogenicity studies completed Proven manufacturing capabilities ◦ Drug API manufactured by Lonza at commercial scale
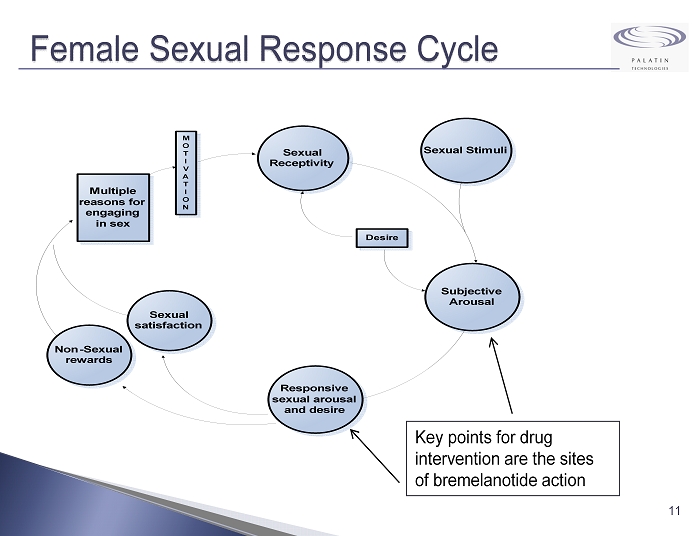
Female Sexual Response Cycle 11 Key points for drug intervention are the sites of bremelanotide action Sexual satisfaction Non-Sexual rewards Multiple reasons for engaging in sex M O T I V A T I O N Subjective Arousal Sexual Receptivity Desire Sexual Stimuli Responsive sexual arousal and desire

Bremelanotide FSD Clinical Data Studies of sexual function in female rodent and primate models demonstrated pronounced effects on perceptive and appetitive sexual behaviors Multiple Phase 2 efficacy studies ◦ Double - blind, placebo - controlled ◦ In - clinic dosing and “at - home” use ◦ SC or IN dosing in premenopausal and postmenopausal FSD patients ◦ Significant effects on improving vaginal engorgement, improving arousal and desire, as well as improving orgasm Phase 2 data supported movement into Phase 2B at - home studies 12

BMT Phase 2B FSD Clinical Trial 13 Dose ranging 16 week at - home placebo - controlled study ◦ Subcutaneous dosing: placebo; 0.75 mg BMT; 1.25 mg BMT and 1.75 mg BMT ◦ Target enrollment: 400 patients Used FDA accepted and validated endpoints ◦ Increase in Satisfying Sexual Events (SSE’s) ◦ A s measured by event log ◦ Female Sexual Function Index (FSFI) ◦ 19 - item questionnaire measuring improvement in arousal, desire and overall sexual function ◦ Female Sexual Distress Scale - DAO (FSDS - DAO) ◦ 15 - item questionnaire that measures personal distress associated with FSD Objectives ◦ Evaluating safety and efficacy in premenopausal patients with FSD, HSDD, FSAD and FSD/HSDD Positive top - line data reported 4Q12

Phase 2B Clinical Data 14 Analysis of the primary and key secondary endpoints of 327 pre - menopausal patients shows clinically meaningful and statistically significant effects for BMT vs. placebo Primary endpoint ◦ Improvement in the number of satisfying sexual events BMT vs. Placebo (p=0.018) ‒ BMT mean change from baseline 1.6 to 2.4 (50% increase) ‒ Placebo mean change from baseline 1.7 to 1.9 (12% increase) Key secondary endpoints ◦ FSFI - total score mean change 3.55 vs. 1.88 (p=0.0017) ◦ FSDS - DAO total score mean change - 11.1 vs. - 6.8 (p=0.036) Analysis was for 1.25 mg & 1.75 mg pooled vs. placebo 1.75 mg dose analysis also demonstrated clinically meaningful and statistical significance on all of the above endpoints

Phase 2B Clinical Data 15 395 premenopausal FSD patients were randomized to receive drug or placebo 26 patients met the predefined BP withdrawal criteria and were evenly distributed among placebo and active arms of the study 12 patients withdrew for AE’s (2 on placebo), predominately emesis, nausea and flushing No SAE’s attributed to BMT occurred during the study Drug treated subjects had ~2mmHg change in BP predominately during the first 4 hours of dosing Most common AE’s on drug were nausea, flushing, headache and emesis

BMT FSD Next Steps 16 Complete data analysis by 4Q12 End - of - Phase 2 meeting with FDA 1Q13 Full data presentation at scientific conferences 1Q13 Corporate partnership Start of Phase 3 program targeted for 4Q13
Summary of BMT FSD Program Patient Testimony “ I was focused on sex, I wasn’t thinking of anything else.” “Quality of orgasms was better - more intense, lasted longer.” 17 Multiple Phase 2 studies establish proof - of - concept in FSD Phase 2B study met all key objectives and supports transition to Phase 3 development Subcutaneous formulation has consistent pharmacokinetics and is well - tolerated BMT continues to demonstrate a positive patient experience

Melanocortin Receptor 4 Agonist Obesity & Diabetes

AstraZeneca Obesity Collaboration Exclusive global licensing and research collaboration ◦ $10M upfront and $12M milestones received ◦ $145M in additional milestones ◦ Royalties upon commercialization Clinical proof - of - concept studies for MCR - 4 mechanism have been completed ◦ Primary objectives met: significant decrease in food intake and weight loss Multiple lead d rug c andidates under development ◦ Clinical candidate development selection targeted 4Q12 ◦ AZD2820 (initial clinical candidate) development halted during Phase 1 program for compound specific safety concern 19

Melanocortins and Obesity Overview MCR - 4 neurons located in the hypothalamus play a key role in coordinating central, peripheral and behavioral signals of energy balance MCR - 4 neurons are activated by a variety of appetite suppressing signals resulting in decreased hunger and increased energy expenditure Many of the drugs with potent anorexigenic effects work by activating MCR - 4; this includes drugs recently approved by the FDA Loss of function mutations in MCR - 4 cause early onset obesity and diabetes 20


Medical need for new therapy with faster onset acting independently of the beta - agonist mechanism Acute Severe Asthma Asthma episode in which symptoms do not adequately respond to current therapies Patients often require emergency room (ER) treatment ER Standard of Care requires several hours and patients remain at risk Recent FDA guidance has questioned the safety of beta - agonist monotherapy 22

PL - 3994 Market Opportunity 23 *Individual treatment cost $ 1 , 000 **Crossover use of asthma therapeutics in COPD approx . 30 % of asthma market Administration Indication Stage Market Potential Subcutaneous Acute Severe Asthma ER Phase 2 >$ 500M* Nebulized Acute Severe Asthma ER Rescue M edication PRN Preclinical >$1B Inhalation/DPI Broader and Chronic COPD/Asthma ** Planned >$3B » >2 million patient ER visits per year » ~400K treatment naïve patients (35% of treatment naïve population) » >1 million 2 refractory patients

PL - 3994 Overview 24 Guanylate Cyclase - A (GC - A) activators have clinical precedent as effective bronchodilators in asthmatics ◦ PL - 3994: novel direct acting GC - A activator NPR - A agonism relaxes smooth muscle in airways ◦ Effect maintained under conditions of 2 overload Half - life of 3 hours in man Desirable properties for development as a subcutaneous or inhaled therapy Enhances human airway smooth muscle responsiveness to beta - agonists

-10 -9 -8 -7 -6 -5 0 20 40 60 80 100 PL3994 PL5731 Vehicle Agonist (M) % Inhibition of contraction 0 20 40 60 80 100 120 140 160 ISO ISO + PL-3994 10 -6 10 -5 10 -4 10 -7 10 -8 10 -9 [ISO] M Relaxation (%) 0 10 20 30 40 50 10 -6 10 -4 10 -8 10 -10 [hBNP] M [PL-3994] M [compound] M Relaxation (%) PL - 3994 in Asthma Disease Models 0 5 10 15 D 2 in pulmonary inflation pressure (cmH O) 1 m g/kg 10 m g/kg 100 m g/kg control PL - 3994 Dose Isolated guinea - pig trachea Intra - tracheal instillation of PL - 3994; effect on inflation pressure in guinea pigs Human lung responsiveness to beta - agonists Human lung slice [Compound] M [ISO] M [Agonist] M 25

PL - 3994 Program Subcutaneous formulation ◦ Phase 2 proof - of - principle study in stable asthmatics ◦ Placebo - controlled dose escalation crossover study design ◦ Objective is to determine if patients can have a clinically meaningful increase in pulmonary function (FEV 1 ) Nebulized formulation activities ◦ Physical chemical studies ◦ Animal toxicology program 26

Milestones Bremelanotide Phase 2B study in FSD patients ◦ Complete patient enrollment 1Q12 x ◦ Positive Top - line data released 4Q12 x ◦ EOP2 meeting targeted 1Q13 ◦ Corporate collaboration 2013 AstraZeneca MCR development obesity/diabetes program ◦ Clinical candidate selection 4Q12 ◦ Phase 1 clinical trial 4Q13 PL - 3994 in Acute Asthma ◦ Corporate collaboration target 1 H13 Melanocortin inflammation program ◦ Corporate collaboration 1H13 ◦ Clinical candidate development selection 1H13 27

Financial Snapshot Ticker Symbol NYSE MKT: PTN Shares Outstanding – September 30, 2012 ◦ Common 38.9M ◦ Fully Diluted 133.6M Market Capitalization – September 30, 2012 $27.0M Cash and Cash Equivalents Balance – September 30, 2012 $33.5M 28

Thank You