Exhibit 99.1
|
|
January 14, 2014
J.P. Morgan
2014 Healthcare Conference
Howard Robin
President & CEO
|
|
This presentation includes forward-looking statements regarding Nektar’s technology platform, drug candidates, clinical and regulatory objectives, market opportunity estimates, and royalty and milestone payment potential. Actual results could differ materially and these statements are subject to important risks detailed in Nektar’s filings with the SEC, including the Form 10-Q filed on November 7, 2013. Nektar undertakes no obligation to update forward-looking statements as a result of new information or otherwise.
|
|
| 3 |
|
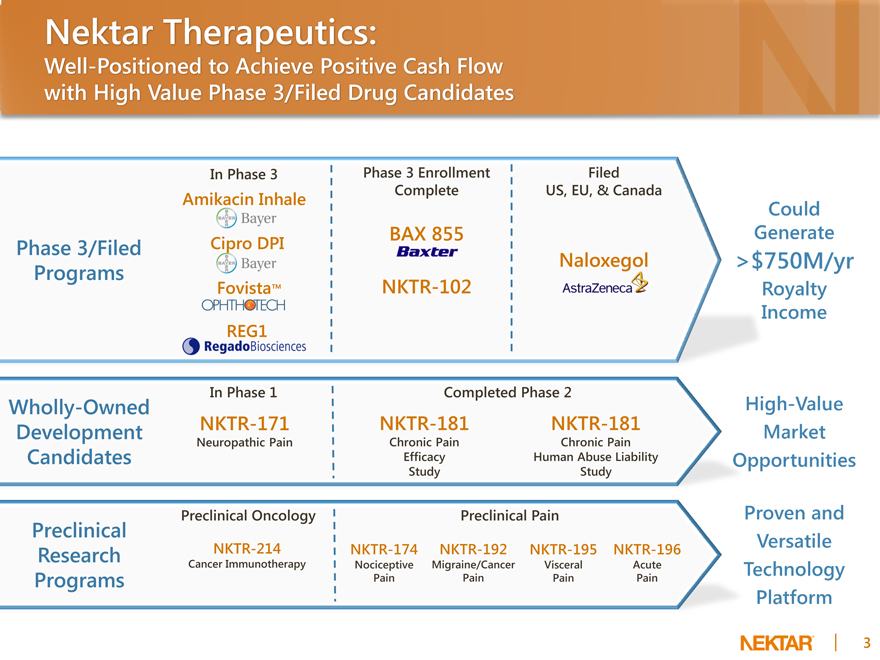
Nektar Therapeutics:
Well-Positioned to Achieve Positive Cash Flow with High Value Phase 3/Filed Drug Candidates
In Phase 3
Amikacin Inhale
Phase 3/Filed Cipro DPI Programs
Fovista™
REG1
Phase 3 Enrollment Complete
BAX 855
NKTR-102
Filed US, EU, & Canada
Naloxegol
In Phase 1
Wholly-Owned
Development NKTR-171
Neuropathic Pain
Candidates
Completed Phase 2
NKTR-181 NKTR-181
Chronic Pain Chronic Pain Efficacy Human Abuse Liability Study Study
Preclinical Oncology
Preclinical NKTR-214
Research Cancer Immunotherapy
Programs
Preclinical Pain
NKTR-174 NKTR-192 NKTR-195 NKTR-196
Nociceptive Migraine/Cancer Visceral Acute Pain Pain Pain Pain
Could Generate > $750M/yr Royalty Income High-Value Market Opportunities Proven and Versatile Technology Platform
Bayaer Fovista TM OPHIHOTECH RegadoBiosciences Baxter AstraZeneca
|
|
Naloxegol (NKTR-118):
Potential to be First Once-Daily Oral Tablet to Treat Opioid-Induced Constipation
NDA, MAA and NDS filings accepted in US, EU and Canada US PDUFA Date: September 16, 2014 AZ will participate along with other sponsors in an FDA Advisory Panel for OIC: March 10-11, 2014 (tentatively scheduled) AZ responsible for all development, regulatory and commercialization activities
Nektar eligible for up to: $175 million in regulatory/launch milestones in U.S. and Europe $375 million in sales milestones Significant, escalating double-digit royalties
Photo of an investigational product candidate
| 4 |
|
|
|
Opioid-Induced Constipation:
High Unmet Medical Need
$14.8 Billion
Of the $14.8 billion global opioid market, five markets account for ~80% of total unit volume: US (49%), UK (14%), Germany (5%), Canada (5%), France (5%).1 PCPs and Pain Management Specialists comprise the majority of prescribers in these markets.2
69 Million Patients
In these five markets, there are 69 million patients taking opioids for chronic pain (>30 days treatment).3 For these chronic pain opioid users, opioid induced constipation (OIC) is the most common side effect.4,5
28—35 Million Patients
Approximately 40-50% (28-35 million) patients taking opioids for chronic pain develop constipation.1,2,3* More than 50% of these patients do not get relief from laxatives.
| * |
|
Number of patients in major opioid markets |
SOURCE:1. IMS Health MIDAS MAT-2Q12, 2. IMS Health NPA MAT-2Q12, Cegedim MAT-2Q11) , 3IMS patient level data MAT-2Q09IMS patient level data MAT-2Q09, 4. Panchal, S et al. Opioid-Induced Bowel Dysfunction: Prevalence, Pathophysiology and Burden. Int J Clin Pract. 2007;61(7):1181-1187., 5. Reimer, K et al. Meeting the Challenges of Opioid-Induced Constipation in Chronic Pain Management – A Novel Approach. Pharmacology. 2009;83:10–17., 6 Pappagallo, M. (2001). Incidence, prevalence, and management of opioid bowel., dysfunction. Am J Surg 182(5A Suppl), 11S-18S.,7 Holzer Regulatory Peptides 155 (2009) 11-17.
| 5 |
|
|
|
| 6 |
|
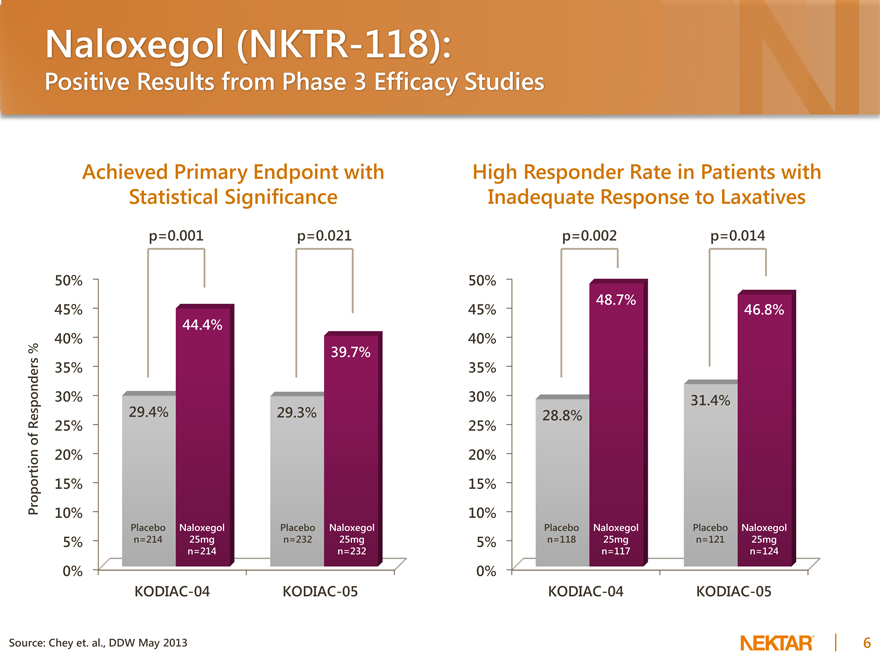
Naloxegol (NKTR-118):
Positive Results from Phase 3 Efficacy Studies
Achieved Primary Endpoint with Statistical Significance
p=0.001 p=0.021
Proportion of Responders %
50% 45% 40% 35% 30% 25% 20% 15% 10% 5% 0%
44.4%
39.7%
29.4% 29.3%
Placebo Naloxegol Placebo Naloxegol n=214 25mg n=232 25mg n=214 n=232
KODIAC-04 KODIAC-05
High Responder Rate in Patients with Inadequate Response to Laxatives
p=0.002 p=0.014
50% 45% 40% 35% 30% 25% 20% 15% 10% 5% 0%
48.7%
46.8%
31.4% 28.8%
Placebo Naloxegol Placebo Naloxegol n=118 25mg n=121 25mg n=117 n=124
KODIAC-04 KODIAC-05
Source: Chey et. al., DDW May 2013
|
|
| 7 |
|
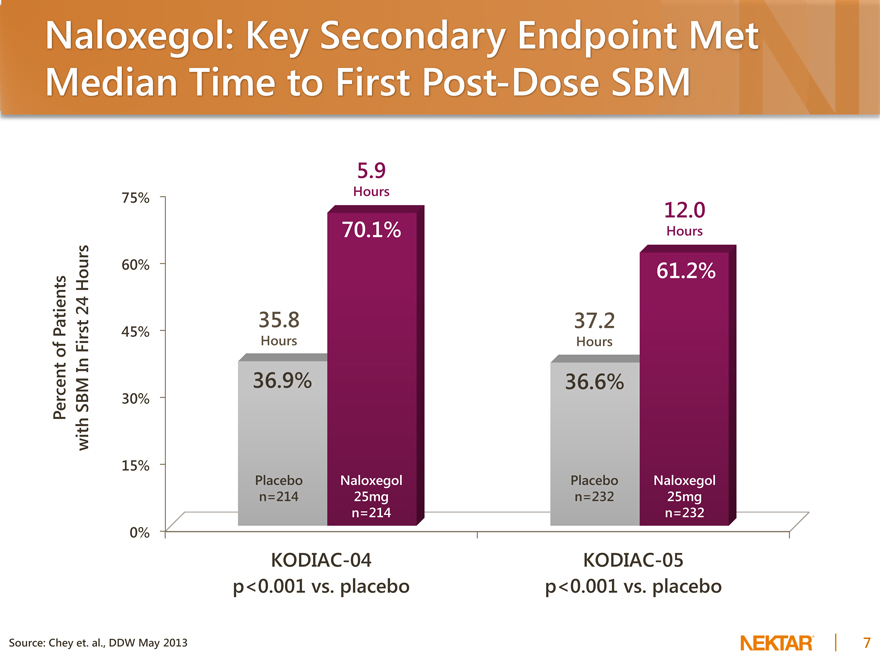
Naloxegol: Key Secondary Endpoint Met Median Time to First Post-Dose SBM
Percent of Patients with SBM In First 24 Hours
75% 60% 45% 30% 15% 0%
35.8
Hours
36.9%
Placebo n=214
5.9
Hours
70.1%
Naloxegol 25mg n=214
37.2
Hours
36.6%
Placebo n=232
12.0
Hours
61.2%
Naloxegol 25mg n=232
KODIAC-04 p<0.001 vs. placebo
KODIAC-05 p<0.001 vs. placebo
Source: Chey et. al., DDW May 2013
|
|
8
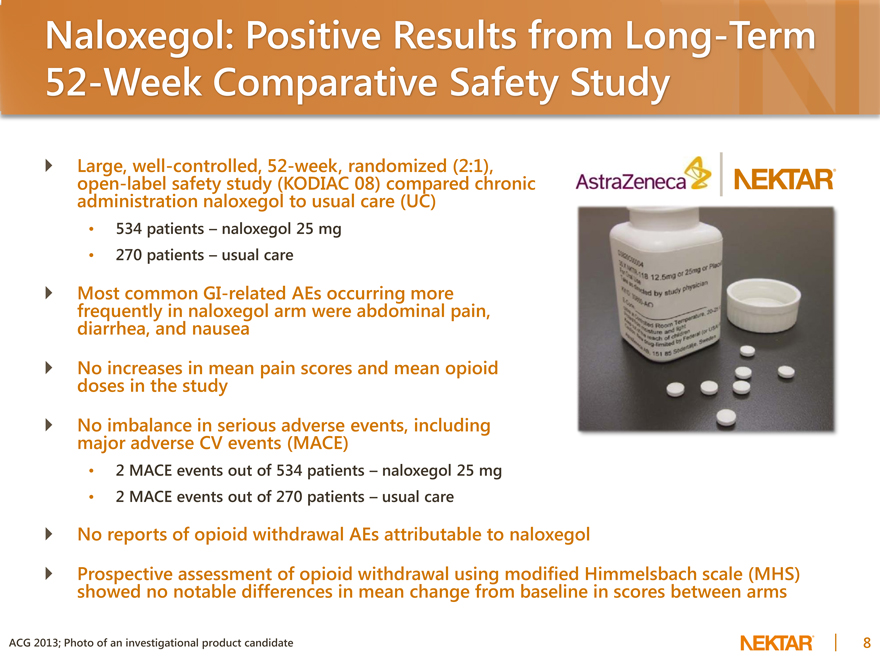
Naloxegol: Positive Results from Long-Term 52-Week Comparative Safety Study
Large, well-controlled, 52-week, randomized (2:1), open-label safety study (KODIAC 08) compared chronic administration naloxegol to usual care (UC)
534 patients – naloxegol 25 mg
270 patients – usual care
Most common GI-related AEs occurring more frequently in naloxegol arm were abdominal pain, diarrhea, and nausea No increases in mean pain scores and mean opioid doses in the study No imbalance in serious adverse events, including major adverse CV events (MACE)
2 MACE events out of 534 patients – naloxegol 25 mg
2 MACE events out of 270 patients – usual care
No reports of opioid withdrawal AEs attributable to naloxegol
Prospective assessment of opioid withdrawal using modified Himmelsbach scale (MHS) showed no notable differences in mean change from baseline in scores between arms
ACG 2013; Photo of an investigational product candidate
|
|
9
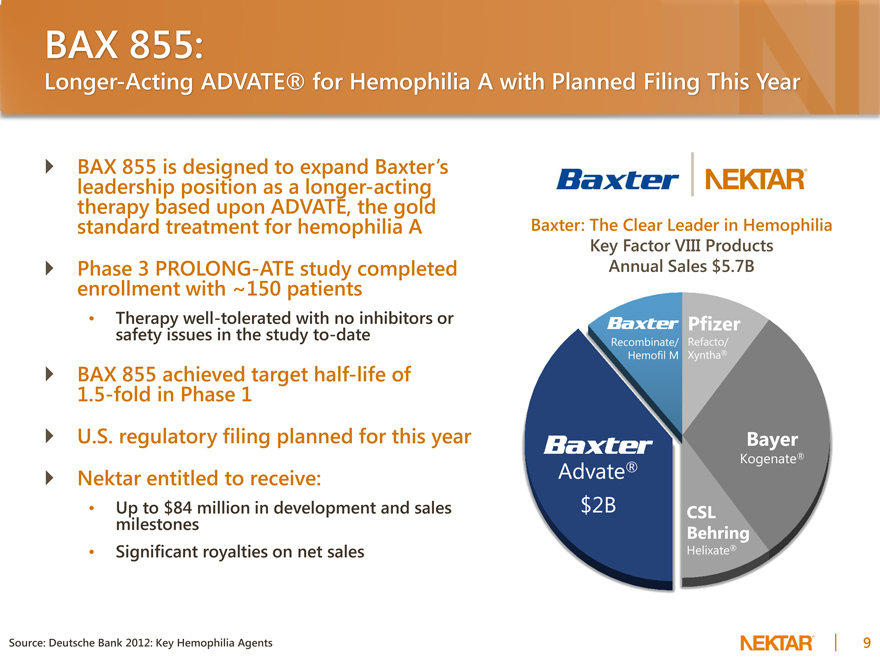
BAX 855:
Longer-Acting ADVATE® for Hemophilia A with Planned Filing This Year
BAX 855 is designed to expand Baxter’s leadership position as a longer-acting therapy based upon ADVATE, the gold standard treatment for hemophilia A Phase 3 PROLONG-ATE study completed enrollment with ~150 patients
Therapy well-tolerated with no inhibitors or safety issues in the study to-date
BAX 855 achieved target half-life of 1.5-fold in Phase 1
U.S. regulatory filing planned for this year
Nektar entitled to receive:
Up to $84 million in development and sales milestones
Significant royalties on net sales
Source: Deutsche Bank 2012: Key Hemophilia Agents
Baxter: The Clear Leader in Hemophilia
Key Factor VIII Products Annual Sales $5.7B
Pfizer
Recombinate/ Refacto/ Hemofil M Xyntha®
Bayer
Kogenate®
Advate®
$2B CSL Behring
Helixate®
|
|
Amikacin Inhale (BAY41-6551):
Bayer Phase 3 Program Ongoing with Data Expected in 1H 2015
Targeted delivery of amikacin directly to lungs to treat gram-negative ventilator pneumonia Amikacin inhale achieves greater lung exposure of antibiotic and lower systemic exposure1
65% of ICU pneumonias gram-negative with high mortality rate2
IV therapies can’t reach effective lung concentration at tolerable doses
SPA in place for Phase 3 Program
Primary endpoint: clinical response at test of cure visit (10-day treatment period)
Nektar royalties on net sales
30% U.S. flat royalty
22% average ex-U.S. royalty
Estimated global market: ~$700 million
1 Gram-negative Niederman et pneumonia”, al., “BAY41-6551 Intensive achieves Care bactericidal Med, Nov 2011; tracheal 2 aspirate Peleg et amikacin al - n engl concentrations j med 362;19 May in mechanically 2010 ventilated patients with
10
|
|
11
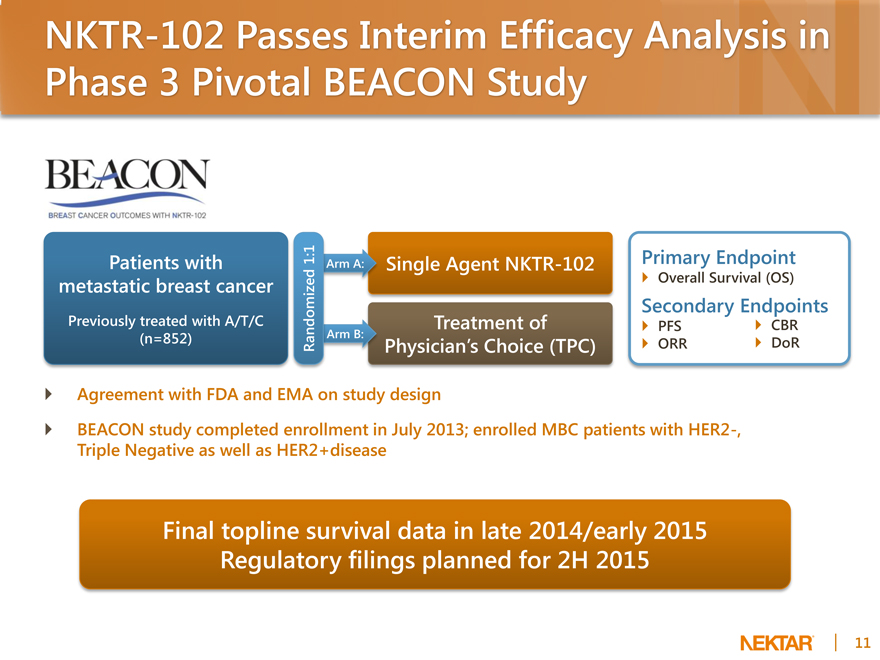
NKTR-102 Passes Interim Efficacy Analysis in Phase 3 Pivotal BEACON Study
Patients with metastatic breast cancer
Previously treated with A/T/C (n=852)
1:1
Arm A:
Randomized Arm B:
Single Agent NKTR-102
Treatment of
Physician’s Choice (TPC)
Primary Endpoint
Overall Survival (OS)
Secondary Endpoints
PFS CBR ORR DoR
Agreement with FDA and EMA on study design
BEACON study completed enrollment in July 2013; enrolled MBC patients with HER2-, Triple Negative as well as HER2+disease
Final topline survival data in late 2014/early 2015 Regulatory filings planned for 2H 2015
|
|
12
NKTR-102 Exceeded Primary Endpoint in Avastin-Resistant High-Grade Glioma Study
Glioma is one of the most deadly cancers
Limited data and limited survival in patients third-line or greater (after failing Avastin) NKTR-102 exceeded primary endpoint with 6-week PFS of 55%
6-Week PFS of 25% was needed to reach primary endpoint
50% of patients achieved stable disease (10/20 patients) with preliminary ORR at 6 weeks of 10%
As of January 2014, two patients still receiving study drug (one patient on study for over 12 months and one on study for seven months)
Mature PFS and OS data to be submitted for presentation at medical meeting in 2014
Preliminary data provided by Stanford Cancer Center
|
|
13
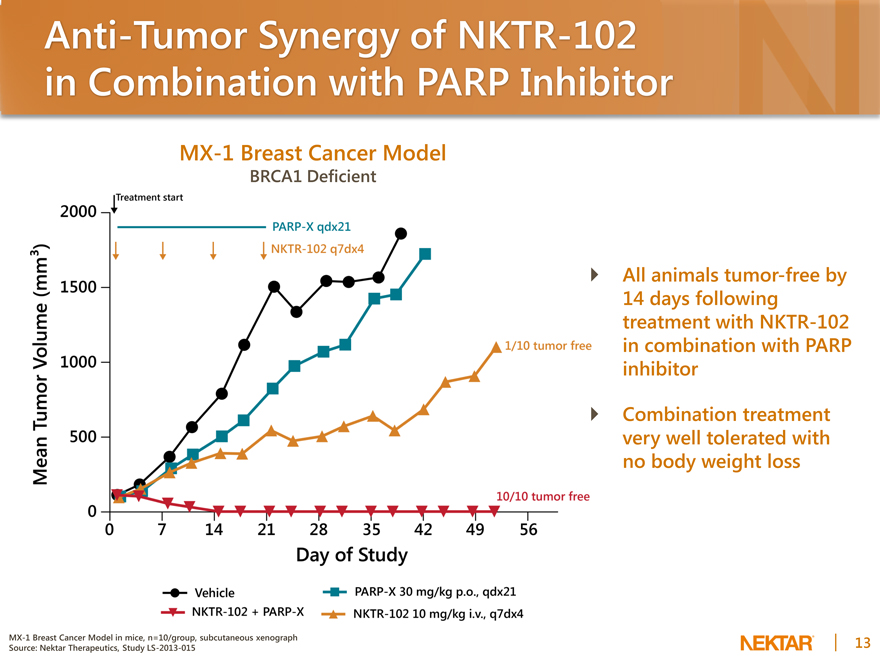
Anti-Tumor Synergy of NKTR-102 in Combination with PARP Inhibitor
MX-1 Breast Cancer Model
BRCA1 Deficient
All animals tumor-free by 14 days following treatment with NKTR-102 in combination with PARP inhibitor
Combination treatment very well tolerated with no body weight loss
MX-1 Breast Cancer Model in mice, n=10/group, subcutaneous xenograph Source: Nektar Therapeutics, Study LS-2013-015
2000 1500 1000 500 0 0 7 14 21 28 35 42 49 56 Day of Study Mean Tumor Volume (mm3)
Vehicle NKTR-102 + PARP-X PARP-X 30 mg/kg p.o., qdx21
nKTR-102 10 mg/kg i.v., q7dx4
Treatment start PARP-X qdx21 NKTR-102 q7dx4
1/10 tumor free 10/10 tumor free
|
|
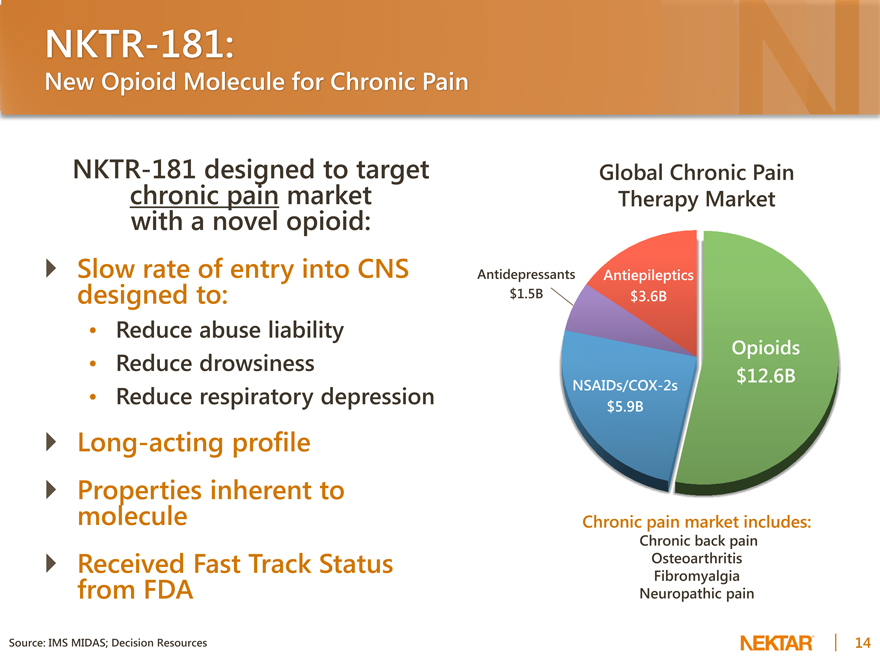
Global Chronic Pain Therapy Market
Antidepressants Antiepileptics $1.5B $3.6B
Opioids $12.6B
NSAIDs/COX-2s $5.9B
Chronic pain market includes:
Chronic back pain Osteoarthritis Fibromyalgia Neuropathic pain
NKTR-181:
New Opioid Molecule for Chronic Pain
NKTR-181 designed to target chronic pain market with a novel opioid: Slow rate of entry into CNS designed to:
Reduce abuse liability
Reduce drowsiness
Reduce respiratory depression
Long-acting profile
Properties inherent to molecule
Received Fast Track Status from FDA
Source: IMS MIDAS; Decision Resources
14
|
|
15
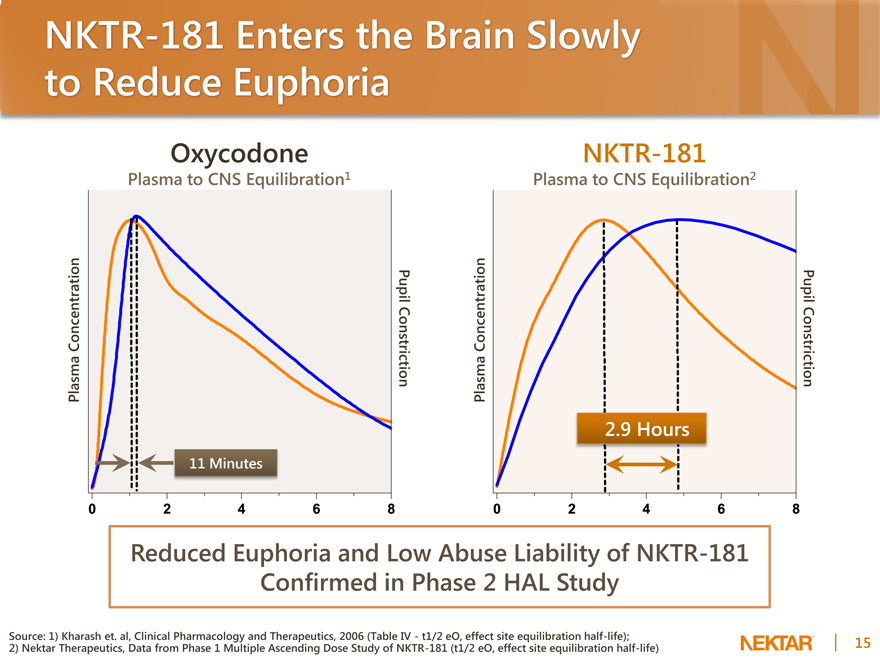
NKTR-181 Enters the Brain Slowly to Reduce Euphoria
Oxycodone
Plasma to CNS Equilibration1
Plasma Concentration
Pupil Constriction
11 Minutes
NKTR-181
Plasma to CNS Equilibration2
Plasma Concentration
2.9 Hours
Pupil Constriction
Reduced Euphoria and Low Abuse Liability of NKTR-181 Confirmed in Phase 2 HAL Study
Source: 2) Nektar 1) Therapeutics, Kharash et. al, Data Clinical from Pharmacology Phase 1 Multiple and Ascending Therapeutics, Dose 2006 Study (Table of NKTR-181 IV—t1/2 eO, (t1/2 effect eO, site effect equilibration site equilibration half-life); half-life)
|
|
Screening Titration 21-day Double-blind Period Period Placebo-controlled Period
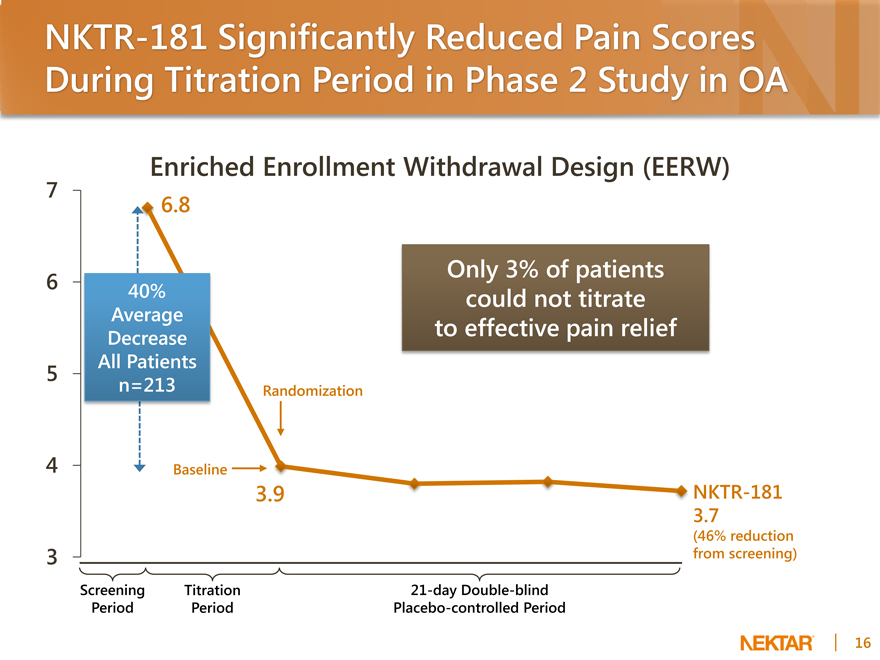
NKTR-181 Significantly Reduced Pain Scores During Titration Period in Phase 2 Study in OA
Enriched Enrollment Withdrawal Design (EERW)
| 7 |
|
6 5 4 3 |
6.8
40% Average Decrease All Patients n=213
Only 3% of patients could not titrate to effective pain relief
Randomization
Baseline
3.9
NKTR-181 3.7
(46% reduction from screening)
16
|
|
17
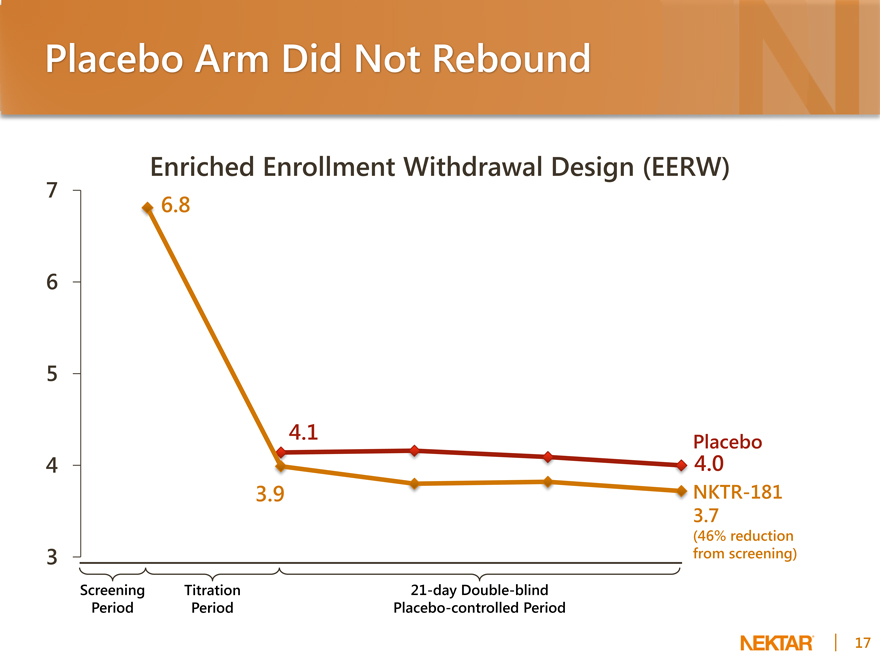
Placebo Arm Did Not Rebound
Enriched Enrollment Withdrawal Design (EERW)
7 6 5 4 3
6.8
4.1
3.9
Placebo
4.0
NKTR-181 3.7
(46% reduction from screening)
Screening Titration 21-day Double-blind Period Period Placebo-controlled Period
|
|
18
Reasons for Lack of Placebo Rebound
EERW design not optimal for a unique drug like NKTR-181 with low CNS side effects
EERW design adopted to study opioids with tolerability issues and high CNS side effects: typical drop-out rates >50%
NKTR-181 drop-out rate for AEs was only 18% because of low CNS side effects
With typical opioids, patients may be un-blinded when crossed to placebo in the EERW design because of removal of CNS side effects. The result is a significantly diminished placebo effect and a resulting rebound in pain scores.
Subjects were allowed to continue using their current non-opioid pain medications
NKTR-181 provided strong analgesia in titration, with 40% reduction of pain from baseline
This reduction at time of randomization allowed the background analgesics to maintain pain relief during the 3 week double-blind period, which prevented significant rebound.
WOMAC pain subscale is more reliable measurement than NRS
Pain scores collected in the clinic instead of self-reported patient diaries
Source: Leber and Davis, 1998
|
|
19
Next Step for NKTR-181:
Guidance from FDA and Key Pain Experts
Highly productive initial meeting with FDA under Fast
Track Status
First Phase 3 study can be started this year in patients with OA
Parallel design suitable for pivotal registration studies
No requirement to use EERW for registration
NKTR-181 is clearly an analgesic with a superior tolerability profile than other opioids
Recommend continued development in OA
|
|
20
Proposed Parallel Design Pivotal Study in Patients with Osteoarthritis
Blinded Titration to Analgesic Dose
12 Week Treatment Period
Patients with osteoarthritis (OA) of the knee (n~600)
Randomized 1:1
NKTR-181
Placebo
Primary Endpoint:
WOMAC Pain Subscale
Change from Baseline
Eliminate continued use of background medications Pain reduction measured by WOMAC Numeric Pain Subscale
Endpoint agreed to by FDA for approval in OA patients
Specific scale designed to measure OA pain
Pain scores collected in the clinic instead of self-reported patient diaries
Interim futility analysis at ~200 patients
|
|
21
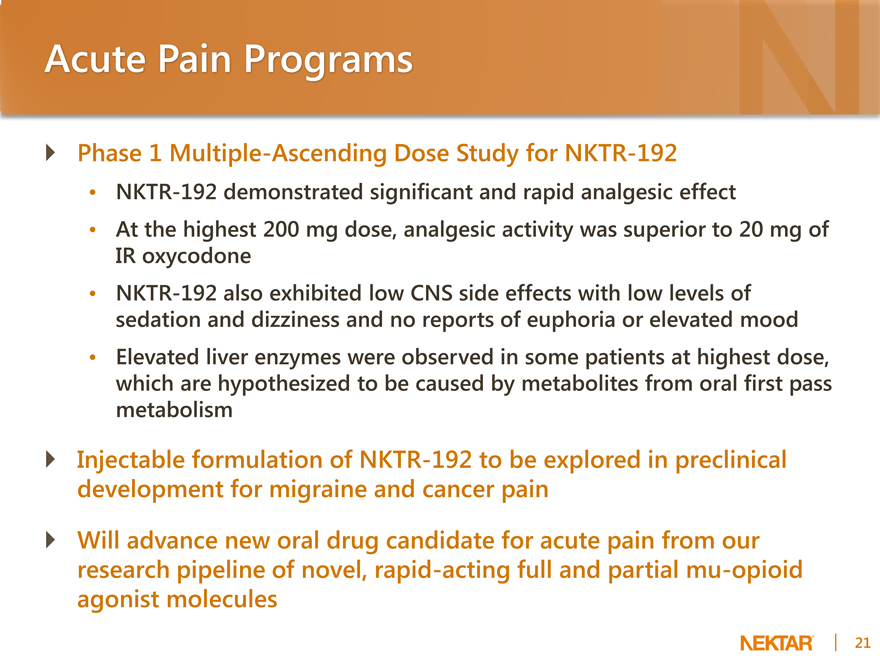
Acute Pain Programs
Phase 1 Multiple-Ascending Dose Study for NKTR-192
NKTR-192 demonstrated significant and rapid analgesic effect At the highest 200 mg dose, analgesic activity was superior to 20 mg of IR oxycodone NKTR-192 also exhibited low CNS side effects with low levels of sedation and dizziness and no reports of euphoria or elevated mood Elevated liver enzymes were observed in some patients at highest dose, which are hypothesized to be caused by metabolites from oral first pass metabolism
Injectable formulation of NKTR-192 to be explored in preclinical development for migraine and cancer pain Will advance new oral drug candidate for acute pain from our research pipeline of novel, rapid-acting full and partial mu-opioid agonist molecules
|
|
22
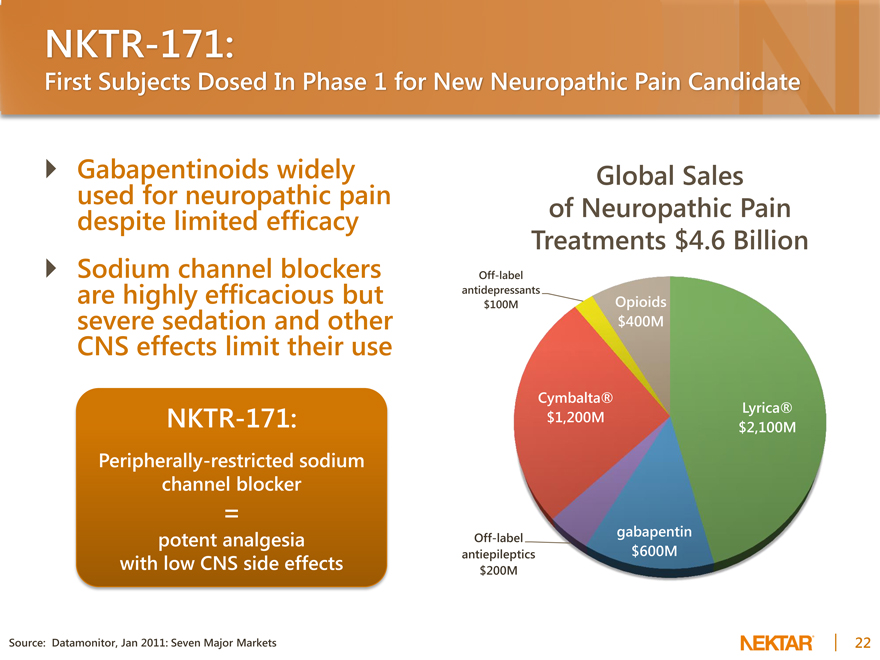
NKTR-171:
First Subjects Dosed In Phase 1 for New Neuropathic Pain Candidate
Gabapentinoids widely used for neuropathic pain despite limited efficacy Sodium channel blockers are highly efficacious but severe sedation and other CNS effects limit their use
NKTR-171:
Peripherally-restricted sodium channel blocker
=
potent analgesia with low CNS side effects
Source: Datamonitor, Jan 2011: Seven Major Markets
Global Sales of Neuropathic Pain Treatments $4.6 Billion
Off-label antidepressants
$100M Opioids $400M
Cymbalta®
Lyrica® $1,200M $2,100M
gabapentin $600M
Off-label antiepileptics $200M
|
|
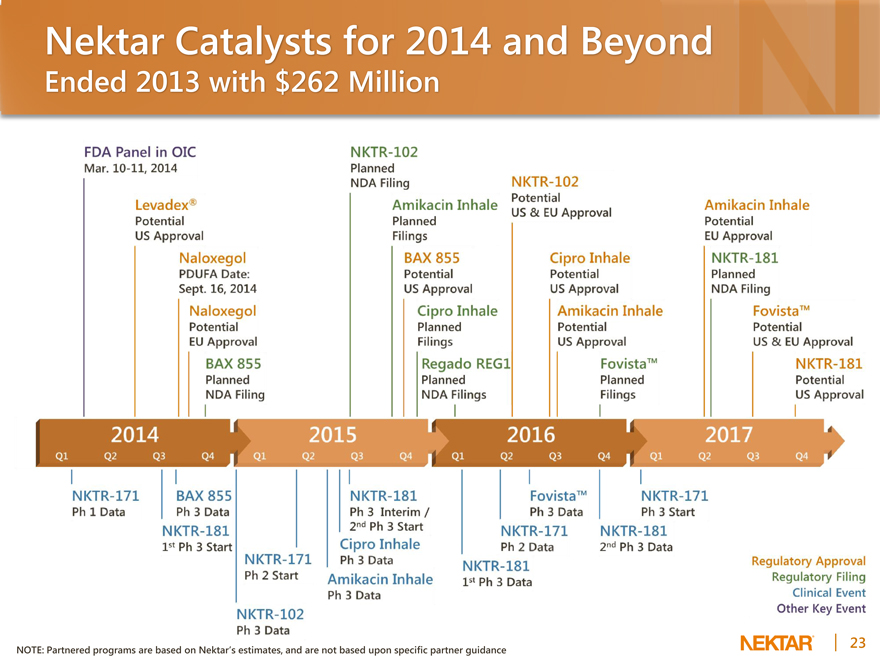
Nektar Catalysts for 2014 and Beyond
Ended 2013 with $262 Million
NOTE: Partnered programs are based on Nektar’s estimates, and are not based upon specific partner guidance
FDA Panel in OIC
Mar. 10-11, 2014 Levadex Potential US Approval Naloxegol PDUFA Date: Sept. 16, 2014 Naloxegol Potential EU Approval
BAX 855
Planned
NDA Filling NKTR Ph 1 Data BAX 855 Ph 3 Data 1st Ph 3 Start 2015 NKTR-102 Planned NDA Filling
Amikacin Inhale Planned Fillings
BAX 855 Potential US Approval
Cipro Inhale Planned Fillings
Regado REG1 NDA Fillings
NKTR-171 Ph2 Start NKTR-102
Ph 3 Data NKTR-181
Ph 3 Interim/
2nd Ph 3 Start Cipro Inhale Ph 3 Data
Amikacin Inhale
Ph 3 Data 2016
NKTR-102
Potential US & EU Approval Cipro Inhale Potential US Approval
Amikacin Inhale
Potential
US Approval
Fovista Planned Fillings
Fovista Ph 3 Data
NKTR-171 Ph 2 Data NKTR-181 1st Ph 3 Data
NKTR-181 2nd Ph 3 Data
2017 Amikacin Inhale Potential EU Approval NKTR-181
Planned
NDA Filling
Fovista
Potential
US & EU Approval
NKTR-181
Potential
US Approval