UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) | (Commission File Number) | (I.R.S. Employer Identification No.) |
(Address of principal executive offices) (Zip Code)
Registrant's telephone number, including area code:
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01. Other Events.
On November 1, 2022, Anika Therapeutics, Inc. (the “Company”) issued a press release announcing that Cingal® met its primary endpoint in a Phase III study (Cingal 19-01), demonstrating superiority over triamcinolone hexacetonide (TH) steroid alone at 26 weeks post-treatment. A copy of the press release is attached hereto as Exhibit 99.1 and incorporated herein by reference.
A top-line summary of the Company’s three Phase III clinical studies of Cingal is provided below.
Cingal 13-01 (Cingal vs placebo vs Monovisc alone)
The Cingal 13-01 Phase III study was a multicenter, randomized, double-blind, placebo-controlled active comparator study that evaluated subjects with osteoarthritis (OA) (Kellgren-Lawrence Grades K-L I/II/III) at 23 sites in Eastern Europe and Canada. In total, 368 patients were randomized into three treatment arms (Cingal (149 subjects), Monovisc® alone (150 subjects) and placebo (saline) (69 subjects)).
Cingal met the primary endpoint of this study and demonstrated superiority over placebo based on change from baseline in the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Pain Index at 12 weeks post-treatment (p=0.0013). Cingal also demonstrated superiority over saline at 26 weeks (p=0.0027). Cingal demonstrated superiority over Monovisc based on change from baseline in WOMAC Pain Index at week one (p=0.0367) and week three (p=0.0289). WOMAC Pain Index is a widely used set of standardized questionnaires for health professionals to evaluate the condition of patients with osteoarthritis of the knee.
As expected, there were no statistically significant differences between the Cingal and Monovisc treatment arms for change in WOMAC Pain Index at weeks 6, 12, 18 or 26, demonstrating that the HA component of Cingal provides the durable pain relief. The percent of Cingal subjects who met criteria for the OMERACT-OARSI Responder Index at 26 weeks was 92%. OMERACT-OARSI is a recognized method of evaluating responder rates based on meeting thresholds for improvement in pain and function.
Cingal was well-tolerated in this study, with only transient and non-serious adverse events (e.g., arthralgia, injection site bruising) related to the study injection.
Cingal 16-02 (Cingal vs Monovisc alone vs TH steroid alone)
The Cingal 16-02 Phase III study was a multicenter, randomized, double-blind, active comparator study that evaluated subjects with OA (Kellgren-Lawrence Grades K-L I/II/III) at 18 sites in Eastern Europe. In total, 557 patients were randomized into three treatment arms (Cingal (252 subjects), Monovisc alone (251 subjects) and TH steroid alone (74 subjects)).
In this study, while Cingal was numerically better than TH steroid alone after 26 weeks in change from baseline as measured by WOMAC Pain, the primary endpoint of superiority was not established. Likewise, while Cingal was numerically better than Monovisc alone at week 3 post-treatment, superiority was not established. The percent of Cingal subjects who met criteria for the OMERACT-OARSI Responder Index at 26 weeks was 91%.
Cingal was well-tolerated in this study, with only transient and non-serious adverse events (e.g., arthralgia, injection site pain, swelling, injection site reaction) related to the study injection.
Of note, the Cingal 16-02 study was an all-active study (it did not include a placebo arm), leading to the concern of expectation bias on the part of subjects, meaning that all patients received active treatment and therefore expected a pain improvement benefit. The design of the Cingal 19-01 study included a small placebo arm to address this potential bias, as well as modifying eligibility criteria to enroll patients with more severe OA (e.g., exclude K-L grade I patients and make baseline WOMAC Pain 50 mm instead of 40 mm).
Cingal 19-01 (Cingal vs TH steroid alone)
The Cingal 19-01 Phase III study was a multicenter, randomized, double-blind, placebo-controlled, active comparator study that evaluated subjects with more severe OA (Kellgren-Lawrence Grades (K-L) II/III) at 26 sites in the United States. In total, 231 subjects were randomized into three treatment arms (Cingal (99 subjects), TH steroid alone (99 subjects) and placebo (saline) (33 subjects)).
The pre-specified primary analysis of the primary endpoint of the study was to evaluate change from baseline in knee pain as measured by the WOMAC Pain Index at 26 weeks post-treatment, comparing the Cingal arm to the TH steroid arm.
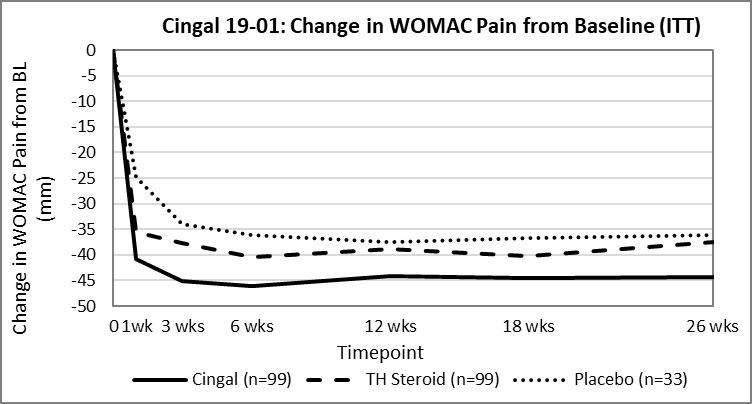
The 19-01 study met its primary endpoint and demonstrated superiority of Cingal over TH steroid at 26 weeks post-treatment based on change in WOMAC Pain Index from baseline (p=0.0406). The figure below shows the change in WOMAC Pain Index from baseline across all measured timepoints.

In addition, Cingal demonstrated strong performance for pain improvement with 66% improvement in WOMAC Pain Index (-44.3mm from baseline) and 90% of subjects met the criteria as OMERACT-OARSI Responders at 26 weeks post-treatment.
Cingal was well-tolerated in the study, with only transient and non-serious adverse events (e.g., arthralgia, injection site pain, swelling, stiffness, injection site reaction, injection site bruising, and transitory decreased range of motion) related to the study injection.
Cautionary Note Regarding Forward-Looking Statements
This Current Report on Form 8-K includes “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, statements about the results of the three completed Phase III clinical studies of Cingal, the clinical benefits and safety profile of Cingal, the timing and outcome of a planned meeting with the United States Food and Drug Administration (FDA) about the U.S. regulatory pathway for Cingal, the U.S. regulatory pathway generally, including the need for additional Phase III pivotal studies, and, if approved, the commercialization of this product candidate. The FDA may require that the Company conduct a single, full factorial pivotal study of Cingal confirming the clinical results observed in the Phase III clinical trials completed to date prior to any regulatory submission for approval. Forward-looking statements can be identified by such words as “will,” “likely,” “may,” “believe,” “expect,” “anticipate,” “intend,” “seek,” “designed,” “develop,” “would,” “future,” “can,” “could,” and other expressions that are predictions of or indicate future events and trends and that do not relate to historical matters. Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those set forth in, or implied by, such forward-looking statements. These risks and uncertainties include, but are not limited to, (i) the Company’s ability to successfully commence and/or complete clinical trials of its products on a timely basis or at all; (ii) the Company’s ability to obtain pre-clinical or clinical data to support domestic and international pre-market approval applications, 510(k) applications, or new drug applications, or to timely file and receive FDA or other regulatory approvals or clearances of its products; (iii) that such approvals will not be obtained in a timely manner or without the need for additional clinical trials, other testing or regulatory submissions, as applicable; (iv) the Company’s research and product development efforts and their relative success, including whether we have any meaningful sales of any new products resulting from such efforts; (v) the cost effectiveness and efficiency of the Company’s clinical studies, manufacturing operations, and production planning; (vi) the strength of the economies in which the Company operates or will be operating, as well as the political stability of any of those geographic areas; (vii) future determinations by the Company to allocate resources to products and in directions not presently contemplated; (viii) the Company’s ability to successfully commercialize its products, in the U.S. and abroad; (ix) the Company’s ability to provide an adequate and timely supply of its products to its customers; and (x) the Company’s ability to achieve its growth targets. For a discussion of other risks and uncertainties, and other important factors, any of which could cause the Company's actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in the Company’s Form 10-K for the year ended December 31, 2021 and subsequent filings with the Securities and Exchange Commission. All information in this Current Report on Form 8-K is as of the date hereof, and the Company undertakes no duty to update this information unless required by law.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
| Exhibit Number | Description |
| 99.1 | Press Release of Anika Therapeutics, Inc. dated November 1, 2022 |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Anika Therapeutics, Inc. | ||
| Date: November 1, 2022 | By: | /s/ Cheryl R. Blanchard |
| Cheryl R. Blanchard | ||
| President and Chief Executive Officer | ||