Exhibit 99.1

Androxal ® Analyst Day October 31, 2014 A Mechanistically Consistent Approach to the Treatment of Secondary Hypogonadism

Repros Disclaimer Any statements made by the Company that are not historical facts contained in these slides (or in any oral accompanying discussion) are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and are subject to various risks, uncertainties and other factors that could cause the Company’s actual results, performance or achievements to differ materially from those expressed or implied by such forward - looking statements . These statements often include words such as “may,” “will,” “expect,” “anticipate,” “continue,” “estimate,” “project,” “potential,” “intend,” “believe,” “plan,” “seek,” “could,” “can,” “should” or similar expressions . These statements are based on assumptions that the Company has made in light of the Company’s experience in the industry, as well as the Company’s perceptions of historical trends, current conditions, expected future developments and other factors the Company believes are appropriate in these circumstances .. Such statements are based on current expectations that involve a number of known and unknown risks, uncertainties and other factors that may cause actual events to be materially different from those expressed or implied by such forward - looking statements, including risks associated with the Company’s ability to continue to be able to raise additional capital on acceptable terms or at all in order to have available funding for the continued development of Androxal® and Proellex® ; the success of the clinical trials for Androxal® and Proellex® ; uncertainty related to the Company's ability to obtain approval of the Company’s products by the Food and Drug Administration, or FDA, and regulatory bodies in other jurisdictions ; uncertainty relating to the Company's patent portfolio, and such other risks as are identified in the Company's most recent Annual Report on Form 10 - K and the subsequent quarterly reports on Form 10 - Q . These documents are available on request from Repros or at www . sec . gov . Repros disclaims any intention or obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise . In this presentation, we rely on and refer to information and statistics regarding the pharmaceutical industry . We obtained this information and these statistics from third - party sources, which we have supplemented where necessary with information from publicly available sources and our own internal estimates . Industry publications and surveys generally state that they have obtained information from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information . While we believe that each of these studies and publications is reliable, we have not independently verified such data, and we make no any representation as to the accuracy of such information . Similarly, we believe our internal research is reliable, but it has not been verified by any independent sources .
Androxal Analyst Day Agenda • Quick overview of NDA status • KOL presentations – Key differences between TRT and restoration of the HPT axis in the treatment of the obese hypogonadal male – Review of 304 and 305 efficacy findings – Epidemiology of Hypogonadism • Key takeaways from the EMAS study – Pathogenesis of secondary hypogonadism in the obese male – Evidence for positive metabolic effects in normalizing T in the obese male – Analysis of Androxal safety (studies, 300, 301, 302, 303, 304, & 305) – Moderated round table and Q&A • Break • Quintiles presentation of “Payer and Physician” perspectives • Adjourn
Androxal NDA Status • FDA meeting to discuss NDA within the next two weeks • Anticipate NDA submission around year - end 2014 – Electronic submission ( Accenture Life Sciences contracted as consultant for content and service provider for assembly and cross linking) • Key NDA components – 23 preclinical studies – 8 Phase 1 studies – 4 Phase 2 studies – 7 Phase 3 plus 3 extension studies – Integrated summary of efficacy • Pivotal Studies 304, 305, 301 and 302 • Efficacy assessed in multiple trials totaling >1250 subjects with duration of exposure from 2 weeks up to > 1.5 years
Androxal NDA Status • Key NDA components (cont’d) – Integrated summary of safety • Exceeds ICH requirements for exposure • > 1500 men exposed (any dose, any duration) • > 900 men for 6 months • > 200 men for 12 months • > 700 patient years of exposure – CMC components • DMF submitted to FDA by supplier of API (enclomiphene citrate) • Module 3 of NDA – 13 clinical batches with stability demonstrated up to 30 months – 6 demonstration batches supporting 2 million capsule commercial batches
Edward D. Kim, M.D. Professor of Surgery/Urology The Changing Dynamic of the Treatment of Secondary Hypogonadism

• Testosterone therapy suppresses pituitary function and decreases testicular production of T • Exogenous T supplementation decreases sperm production. • Clomiphene, off - label, has become a standard therapy for men who desire to maintain future fertility. Rigourous studies are needed. Fertil Steril 2013; 99:718 - 24.
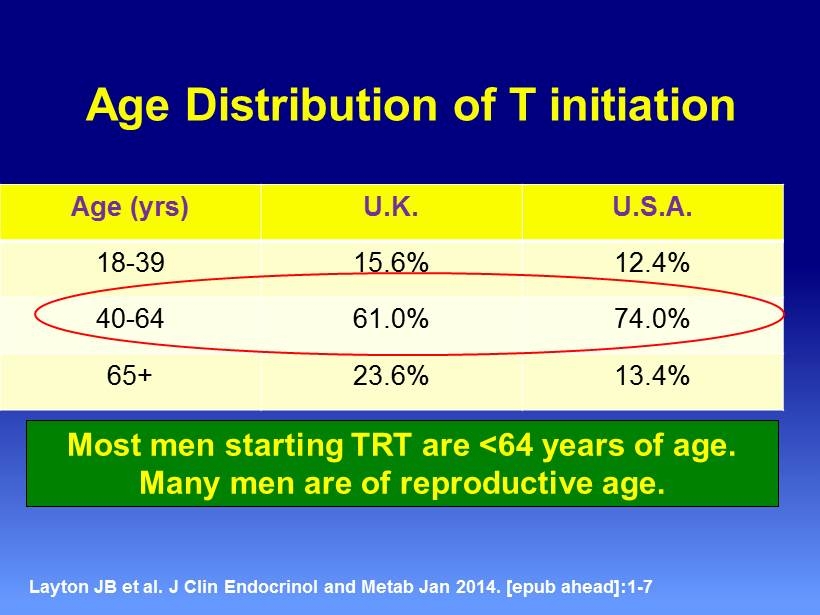
Age Distribution of T initiation Age ( yrs ) U.K. U.S.A. 18 - 39 15.6% 12.4% 40 - 64 61.0% 74.0% 65+ 23.6% 13.4% Layton JB et al. J Clin Endocrinol and Metab Jan 2014 . [ epub ahead ]:1 - 7 Most men starting TRT are <64 years of age. Many men are of reproductive age.

EAU Guidelines on Male Hypogonadism European Association of Urology 2012 “We recommend measurement of serum LH and FSH levels to distinguish between primary (testicular) and secondary (pituitary - hypothalamic) hypogonadism .”
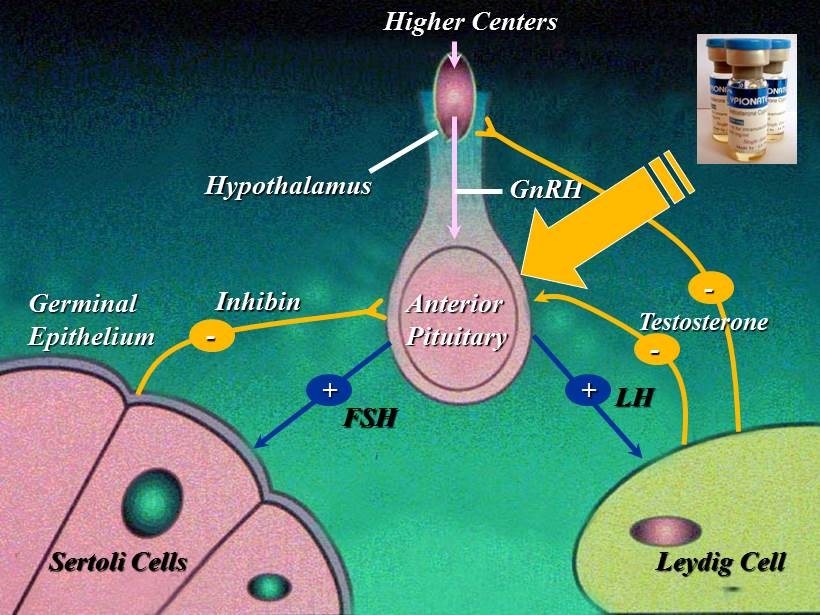
Higher Centers Hypothalamus Anterior Pituitary Sertoli Cells Leydig Cell Germinal Epithelium GnRH FSH LH + + Inhibin - - - Testosterone
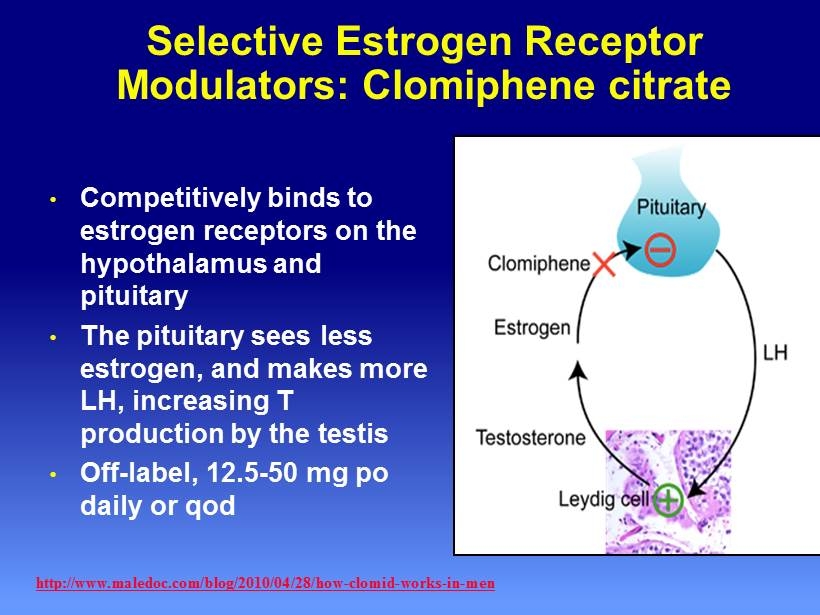
Selective Estrogen Receptor Modulators: Clomiphene citrate • Competitively binds to estrogen receptors on the hypothalamus and pituitary • The pituitary sees less estrogen, and makes more LH, increasing T production by the testis • Off - label, 12.5 - 50 mg po daily or qod http://www.maledoc.com/blog/2010/04/28/how - clomid - works - in - men

Key Clinical Findings from Androxal Studies
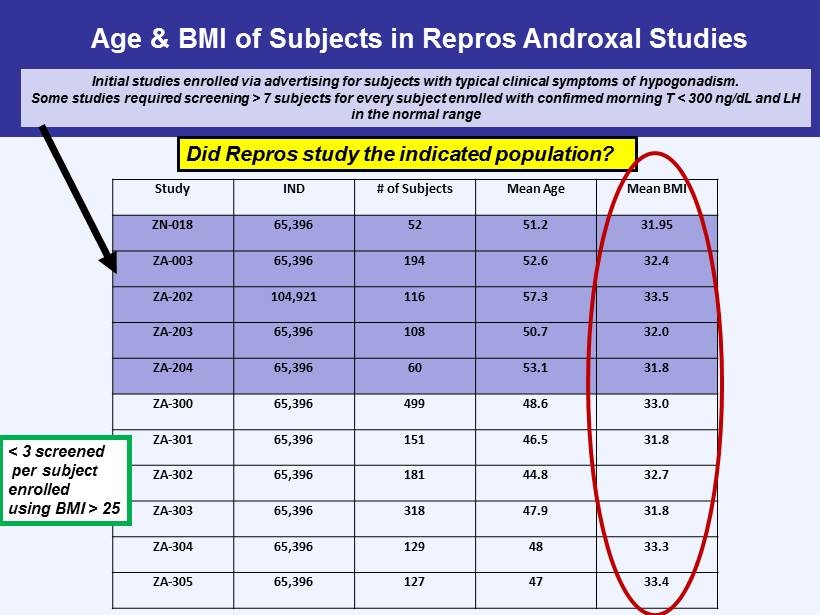
Age & BMI of Subjects in Repros Androxal Studies Study IND # of Subjects Mean Age Mean BMI ZN - 018 65,396 52 51.2 31.95 ZA - 003 65,396 194 52.6 32.4 ZA - 202 104,921 116 57.3 33.5 ZA - 203 65,396 108 50.7 32.0 ZA - 204 65,396 60 53.1 31.8 ZA - 300 65,396 499 48.6 33.0 ZA - 301 65,396 151 46.5 31.8 ZA - 302 65,396 181 44.8 32.7 ZA - 303 65,396 318 47.9 31.8 ZA - 304 65,396 129 48 33.3 ZA - 305 65,396 127 47 33.4 Initial studies enrolled via advertising for subjects with typical clinical symptoms of hypogonadism. Some studies required screening > 7 subjects for every subject enrolled with confirmed morning T < 300 ng/ dL and LH in the normal range < 3 screened per subject enrolled using BMI > 25 Did Repros study the indicated population?
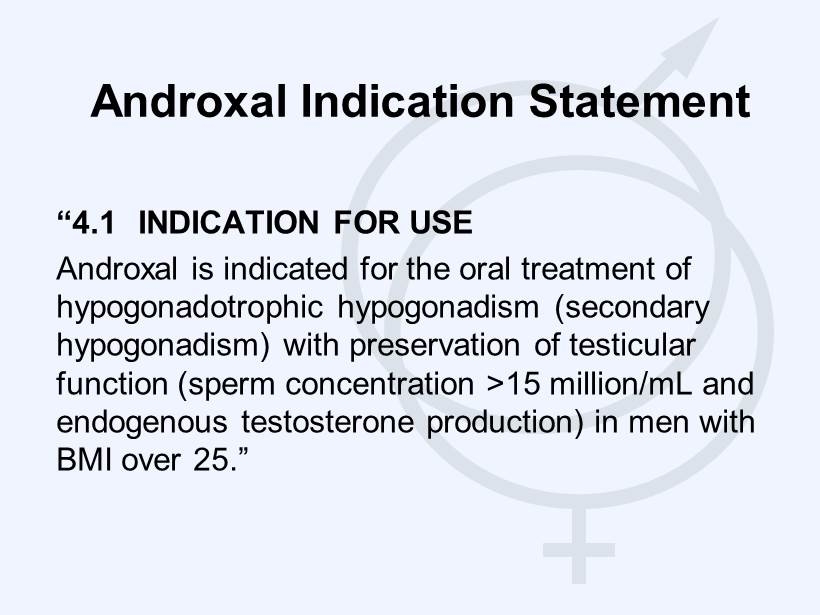
Androxal Indication Statement “4.1 INDICATION FOR USE Androxal is indicated for the oral treatment of hypogonadotrophic hypogonadism (secondary hypogonadism) with preservation of testicular function (sperm concentration >15 million/mL and endogenous testosterone production) in men with BMI over 25.”
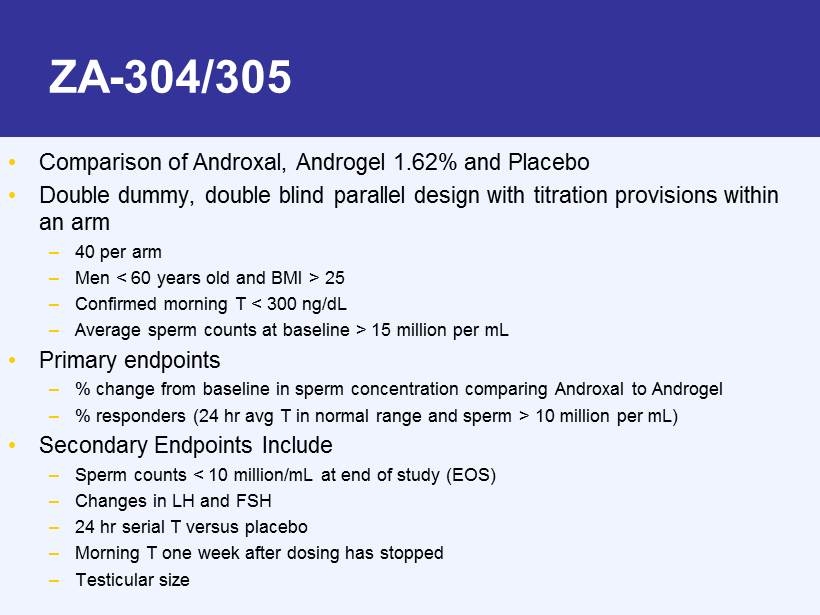
ZA - 304/305 • Comparison of Androxal, Androgel 1.62% and Placebo • Double dummy, double blind parallel design with titration provisions within an arm – 40 per arm – Men < 60 years old and BMI > 25 – Confirmed morning T < 300 ng/dL – Average sperm counts at baseline > 15 million per mL • Primary endpoints – % change from baseline in sperm concentration comparing Androxal to Androgel – % responders (24 hr avg T in normal range and sperm > 10 million per mL) • Secondary Endpoints Include – Sperm counts < 10 million/mL at end of study (EOS) – Changes in LH and FSH – 24 hr serial T versus placebo – Morning T one week after dosing has stopped – Testicular size
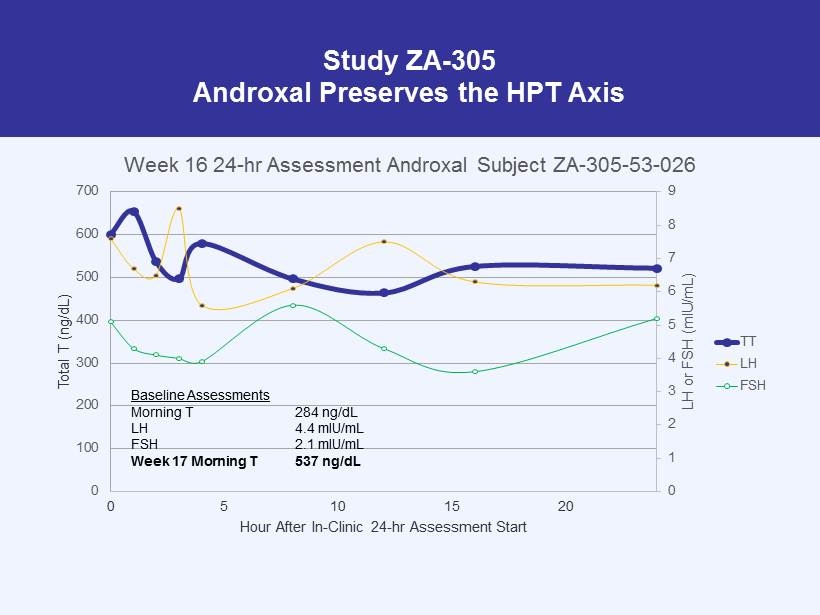
Study ZA - 305 Androxal Preserves the HPT Axis 0 1 2 3 4 5 6 7 8 9 0 100 200 300 400 500 600 700 0 5 10 15 20 LH or FSH ( mIU /mL) Total T (ng/dL) Hour After In - Clinic 24 - hr Assessment Start Week 16 24 - hr Assessment Androxal Subject ZA - 305 - 53 - 026 TT LH FSH Baseline Assessments Morning T 284 ng/dL LH 4.4 mIU/mL FSH 2.1 mIU/mL Week 17 Morning T 537 ng/dL
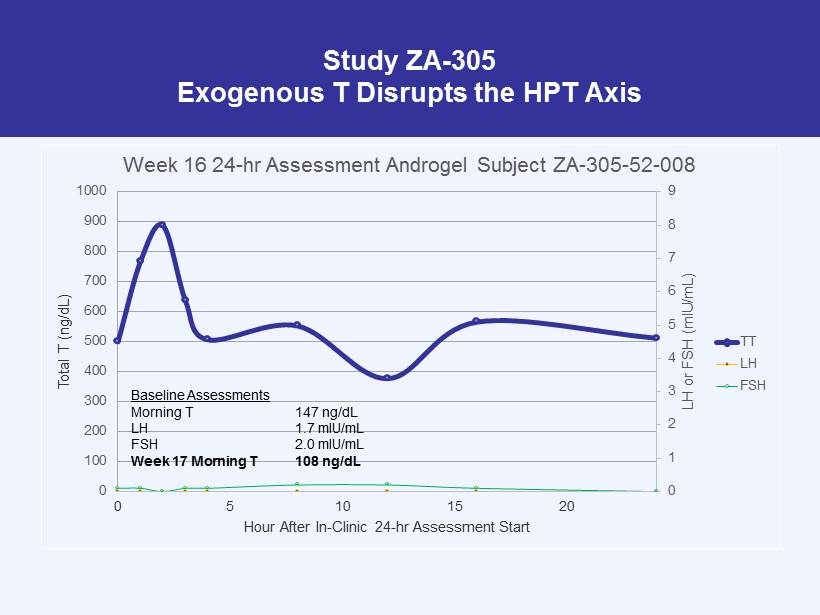
Study ZA - 305 Exogenous T Disrupts the HPT Axis 0 1 2 3 4 5 6 7 8 9 0 100 200 300 400 500 600 700 800 900 1000 0 5 10 15 20 LH or FSH ( mIU /mL) Total T (ng/dL) Hour After In - Clinic 24 - hr Assessment Start Week 16 24 - hr Assessment Androgel Subject ZA - 305 - 52 - 008 TT LH FSH Baseline Assessments Morning T 147 ng/dL LH 1.7 mIU/mL FSH 2.0 mIU/mL Week 17 Morning T 108 ng/dL
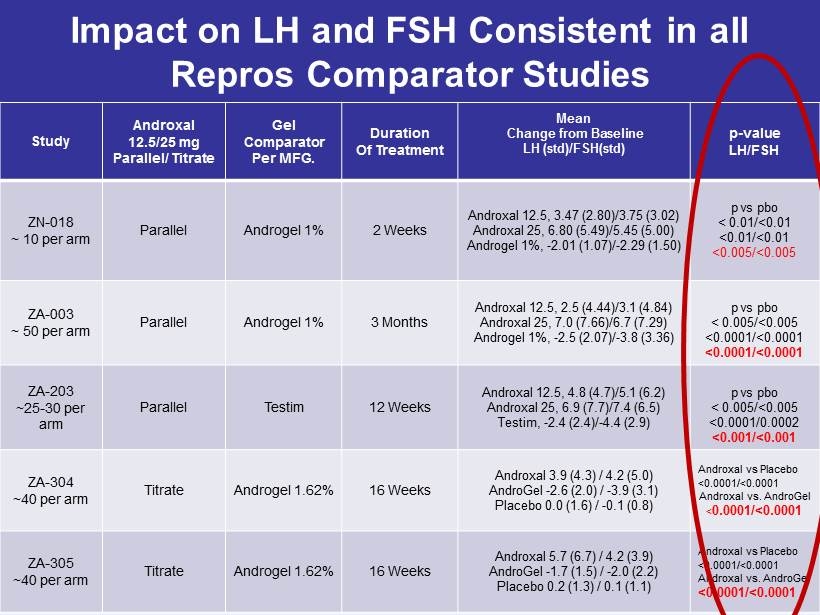
Impact on LH and FSH Consistent in all Repros Comparator Studies Study Androxal 12.5/25 mg Parallel/ Titrate Gel Comparator Per MFG. Duration Of Treatment Mean Change from Baseline LH ( std )/FSH( std ) p - value LH/FSH ZN - 018 ~ 10 per arm Parallel Androgel 1% 2 Weeks Androxal 12.5, 3.47 (2.80)/3.75 (3.02) Androxal 25, 6.80 (5.49)/5.45 (5.00) Androgel 1%, - 2.01 (1.07)/ - 2.29 (1.50) p vs pbo < 0.01/<0.01 <0.01/<0.01 <0.005/<0.005 ZA - 003 ~ 50 per arm Parallel Androgel 1% 3 Months Androxal 12.5, 2.5 (4.44)/3.1 (4.84) Androxal 25, 7.0 (7.66)/6.7 (7.29) Androgel 1%, - 2.5 (2.07)/ - 3.8 (3.36) p vs pbo < 0.005/<0.005 <0.0001/<0.0001 <0.0001/<0.0001 ZA - 203 ~25 - 30 per arm Parallel Testim 12 Weeks Androxal 12.5, 4.8 (4.7)/5.1 (6.2) Androxal 25, 6.9 (7.7)/7.4 (6.5) Testim , - 2.4 (2.4)/ - 4.4 (2.9) p vs pbo < 0.005/<0.005 <0.0001/0.0002 <0.001/<0.001 ZA - 304 ~40 per arm Titrate Androgel 1.62% 16 Weeks Androxal 3.9 (4.3) / 4.2 (5.0) AndroGel - 2.6 (2.0) / - 3.9 (3.1) Placebo 0.0 (1.6) / - 0.1 (0.8) Androxal vs Placebo <0.0001/<0.0001 Androxal vs. AndroGel < 0.0001/<0.0001 ZA - 305 ~40 per arm Titrate Androgel 1.62% 16 Weeks Androxal 5.7 (6.7) / 4.2 (3.9) AndroGel - 1.7 (1.5) / - 2.0 (2.2) Placebo 0.2 (1.3) / 0.1 (1.1) Androxal vs Placebo <0.0001/<0.0001 Androxal vs. AndroGel <0.0001/<0.0001
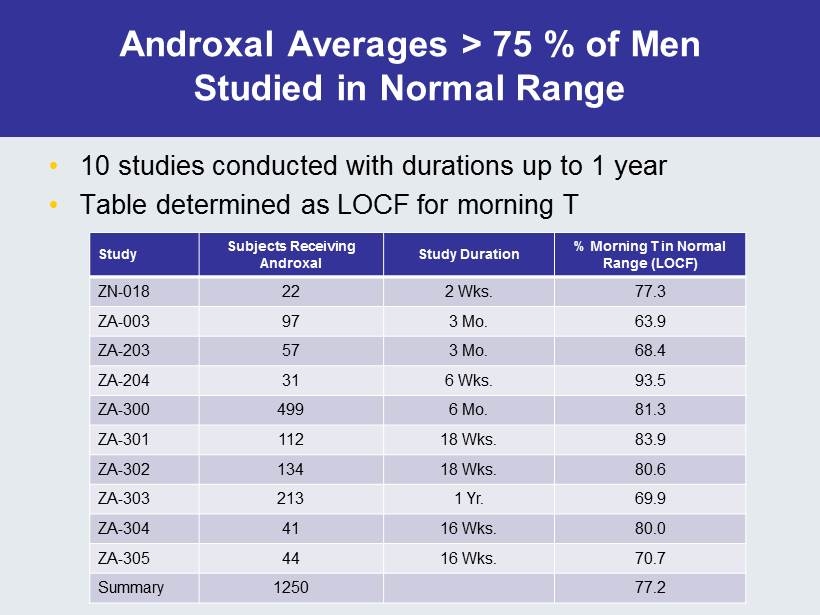
Androxal Averages > 75 % of Men Studied in Normal Range • 10 studies conducted with durations up to 1 year • Table determined as LOCF for morning T Study Subjects Receiving Androxal Study Duration % Morning T in Normal Range (LOCF) ZN - 018 22 2 Wks. 77.3 ZA - 003 97 3 Mo. 63.9 ZA - 203 57 3 Mo. 68.4 ZA - 204 31 6 Wks. 93.5 ZA - 300 499 6 Mo. 81.3 ZA - 301 112 18 Wks. 83.9 ZA - 302 134 18 Wks. 80.6 ZA - 303 213 1 Yr. 69.9 ZA - 304 41 16 Wks. 80.0 ZA - 305 44 16 Wks. 70.7 Summary 1250 77.2

What Predicts Response? • Higher baseline testosterone levels (>216 ng/dL) predict better response • Obesity is associated with lower Tlevels • Duration of hypogonadism and prior testosterone therapy may influence outcomes – Should met with hypogonadism be treated earlier?
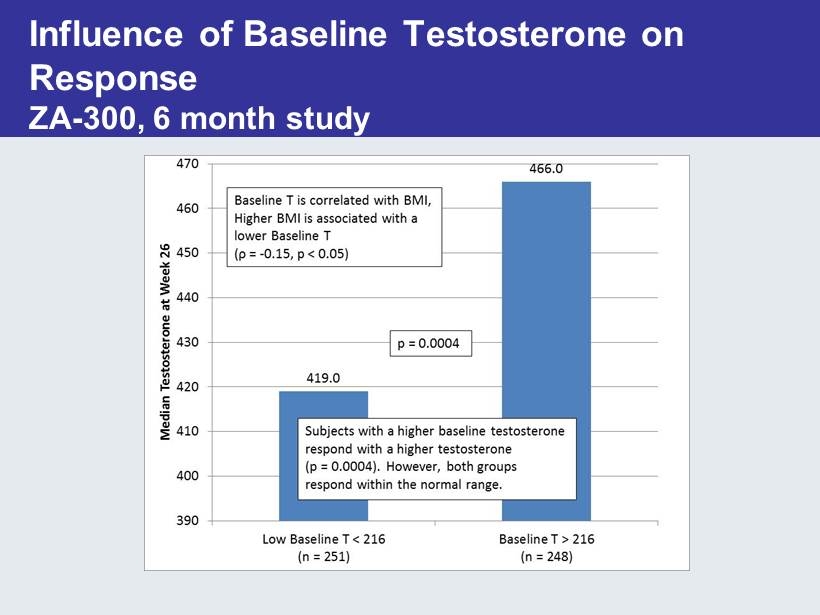
Influence of Baseline Testosterone on Response ZA - 300, 6 month study

Primary and Secondary Endpoints ZA - 304 & 305 Overall NDA Efficacy Findings & Clinical Implications Andrew McCullough, MD Professor of Surgery/Division of Urology Albany Medical College Director of Men’s Health/ Urological Institute of Northeastern NY Albany, NY
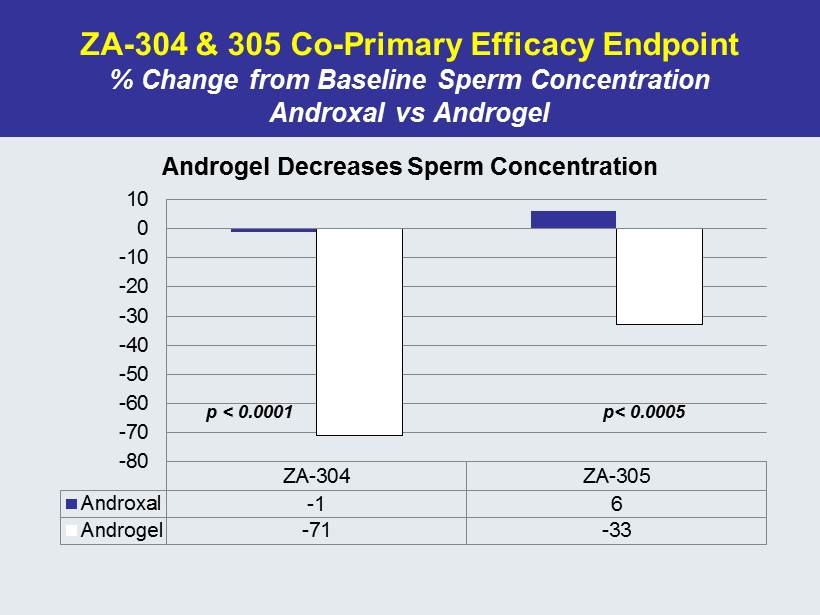
ZA - 304 & 305 Co - Primary Efficacy Endpoint % Change from Baseline Sperm Concentration Androxal vs Androgel ZA-304 ZA-305 Androxal -1 6 Androgel -71 -33 -80 -70 -60 -50 -40 -30 -20 -10 0 10 Androgel Decreases Sperm Concentration p < 0.0001 p< 0.0005
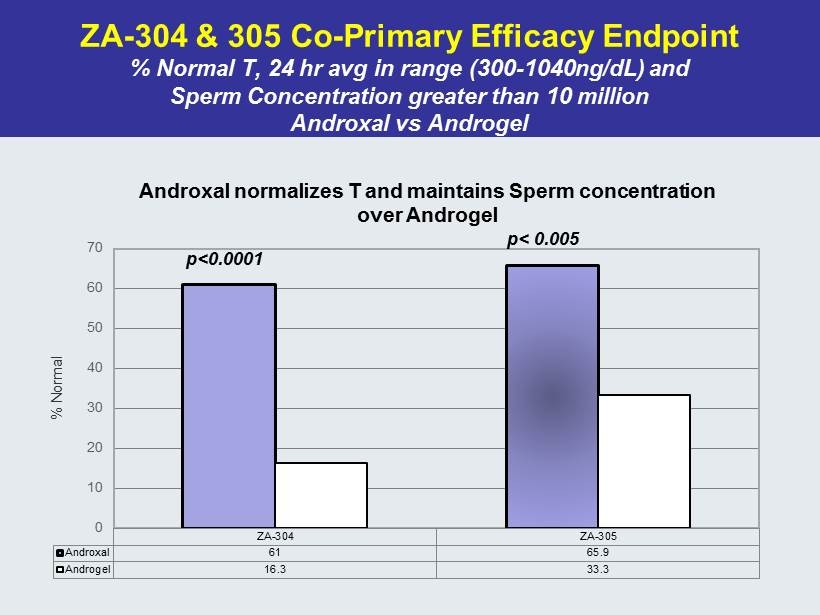
ZA - 304 & 305 Co - Primary Efficacy Endpoint % Normal T, 24 hr avg in range (300 - 1040ng/ dL ) and Sperm Concentration greater than 10 million Androxal vs Androgel ZA-304 ZA-305 Androxal 61 65.9 Androgel 16.3 33.3 0 10 20 30 40 50 60 70 % Normal Androxal normalizes T and maintains Sperm concentration over Androgel p<0.0001 p< 0.005
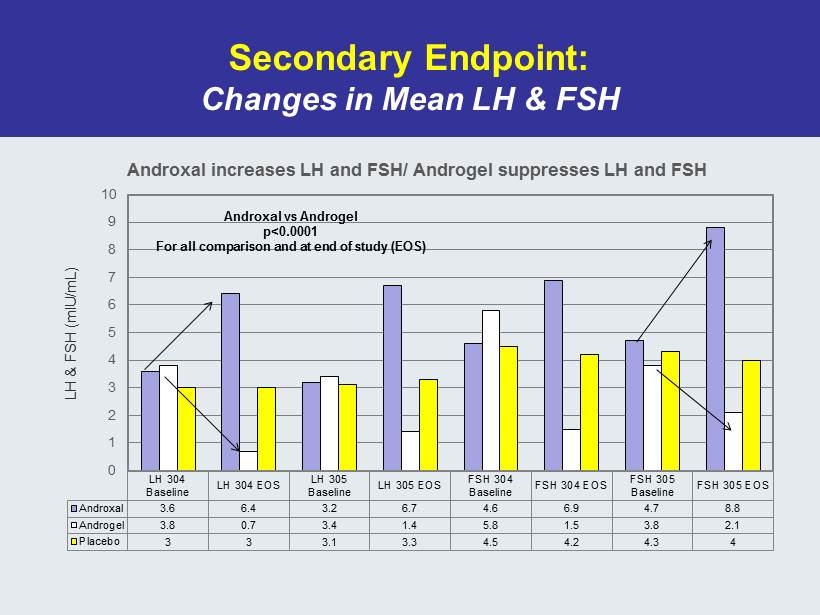
Secondary Endpoint: Changes in Mean LH & FSH LH 304 Baseline LH 304 EOS LH 305 Baseline LH 305 EOS FSH 304 Baseline FSH 304 EOS FSH 305 Baseline FSH 305 EOS Androxal 3.6 6.4 3.2 6.7 4.6 6.9 4.7 8.8 Androgel 3.8 0.7 3.4 1.4 5.8 1.5 3.8 2.1 Placebo 3 3 3.1 3.3 4.5 4.2 4.3 4 0 1 2 3 4 5 6 7 8 9 10 LH & FSH (mIU/mL) Androxal increases LH and FSH/ Androgel suppresses LH and FSH Androxal vs Androgel p<0.0001 For all comparison and at end of study (EOS)
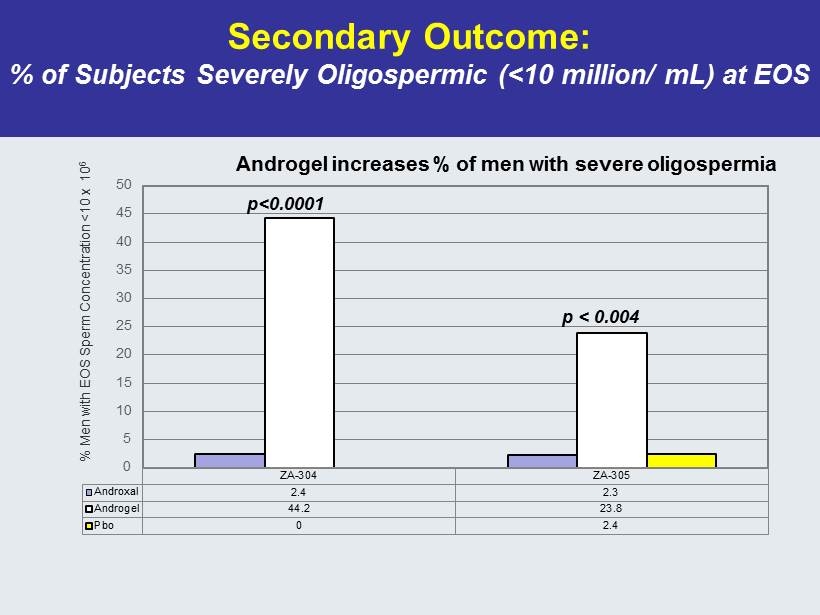
Secondary Outcome: % of Subjects Severely Oligospermic (<10 million/ mL) at EOS ZA-304 ZA-305 Androxal 2.4 2.3 Androgel 44.2 23.8 Pbo 0 2.4 0 5 10 15 20 25 30 35 40 45 50 % Men with EOS Sperm Concentration <10 x 10 6 Androgel increases % of men with severe oligospermia p<0.0001 p < 0.004
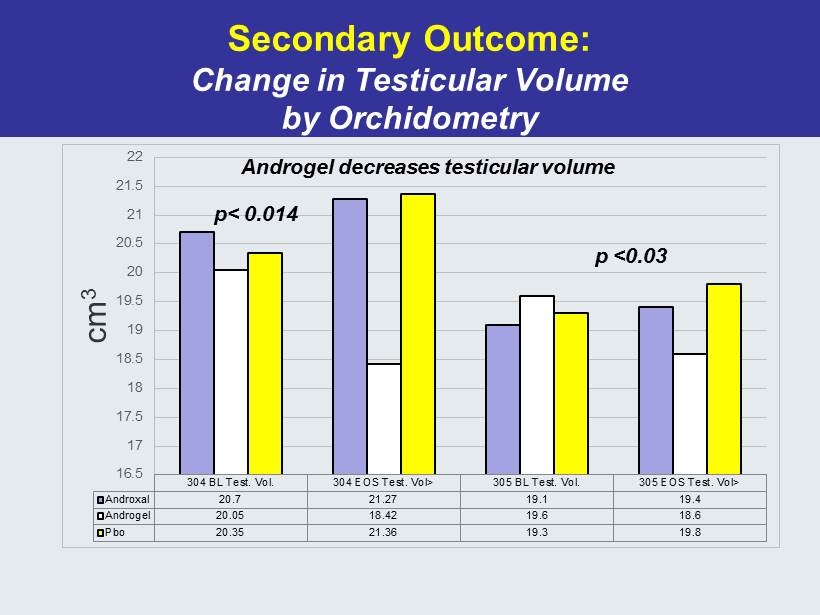
Secondary Outcome: Change in Testicular Volume by Orchidometry 304 BL Test. Vol. 304 EOS Test. Vol> 305 BL Test. Vol. 305 EOS Test. Vol> Androxal 20.7 21.27 19.1 19.4 Androgel 20.05 18.42 19.6 18.6 Pbo 20.35 21.36 19.3 19.8 16.5 17 17.5 18 18.5 19 19.5 20 20.5 21 21.5 22 cm 3 p< 0.014 p <0.03 Androgel decreases testicular volume
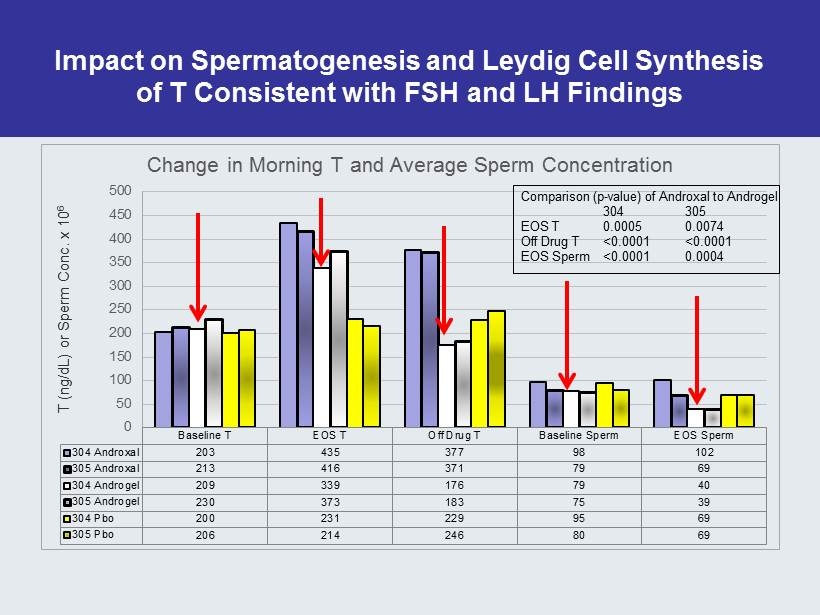
Impact on Spermatogenesis and Leydig Cell Synthesis of T Consistent with FSH and LH Findings Baseline T EOS T Off Drug T Baseline Sperm EOS Sperm 304 Androxal 203 435 377 98 102 305 Androxal 213 416 371 79 69 304 Androgel 209 339 176 79 40 305 Androgel 230 373 183 75 39 304 Pbo 200 231 229 95 69 305 Pbo 206 214 246 80 69 0 50 100 150 200 250 300 350 400 450 500 T (ng/dL) or Sperm Conc. x 10 6 Change in Morning T and Average Sperm Concentration Comparison (p - value) of Androxal to Androgel 304 305 EOS T 0.0005 0.0074 Off Drug T <0.0001 <0.0001 EOS Sperm <0.0001 0.0004
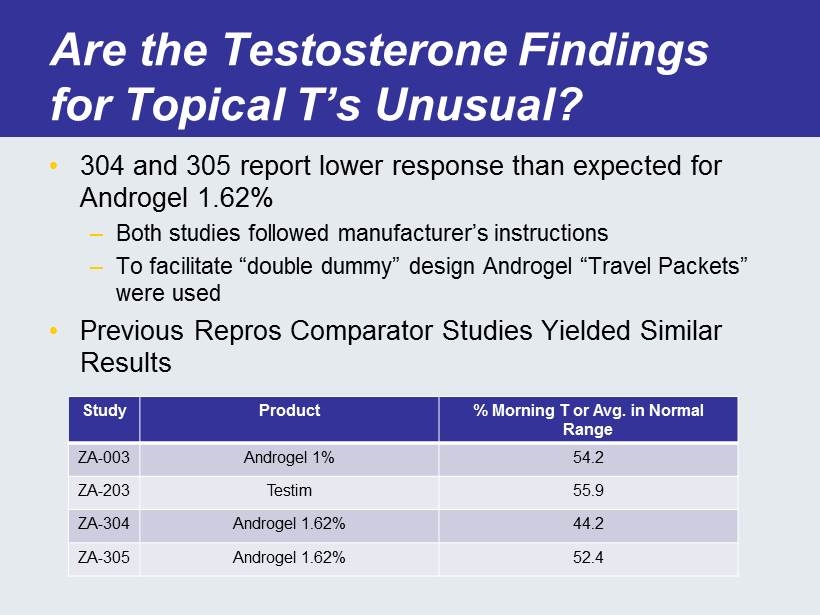
Are the Testosterone Findings for Topical T’s Unusual? • 304 and 305 report lower response than expected for Androgel 1.62% – Both studies followed manufacturer’s instructions – To facilitate “double dummy” design Androgel “Travel Packets” were used • Previous Repros Comparator Studies Yielded Similar Results Study Product % Morning T or Avg. in Normal Range ZA - 003 Androgel 1% 54.2 ZA - 203 Testim 55.9 ZA - 304 Androgel 1.62% 44.2 ZA - 305 Androgel 1.62% 52.4
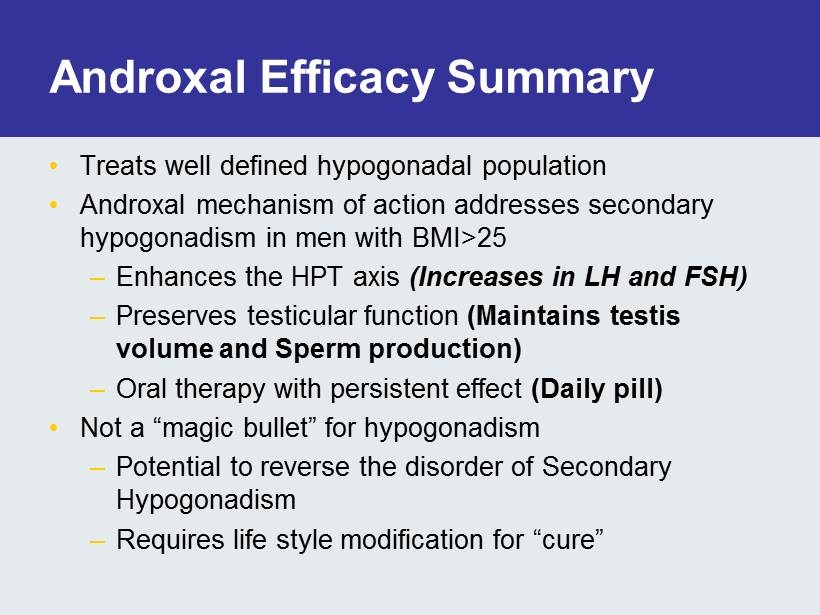
Androxal Efficacy Summary • Treats well defined hypogonadal population • Androxal mechanism of action addresses secondary hypogonadism in men with BMI>25 – Enhances the HPT axis (Increases in LH and FSH) – Preserves testicular function (Maintains testis volume and Sperm production) – Oral therapy with persistent effect (Daily pill) • Not a “magic bullet” for hypogonadism – Potential to reverse the disorder of Secondary Hypogonadism – Requires life style modification for “cure”

Glenn R. Cunningham, MD Professor of Medicine Molecular and Cellular Biology Baylor College of Medicine St. Luke’s - Baylor Diabetes Program Houston, Texas Prevalence of Hypogonadism in Middle - aged and Older Men & Implications for Clinical Management
Location of the EMAS centres. Lee D M et al. Int. J. Epidemiol. 2013;42:391-401 Published by Oxford University Press on behalf of the International Epidemiological Association ©The Author 2012; all rights reserved.
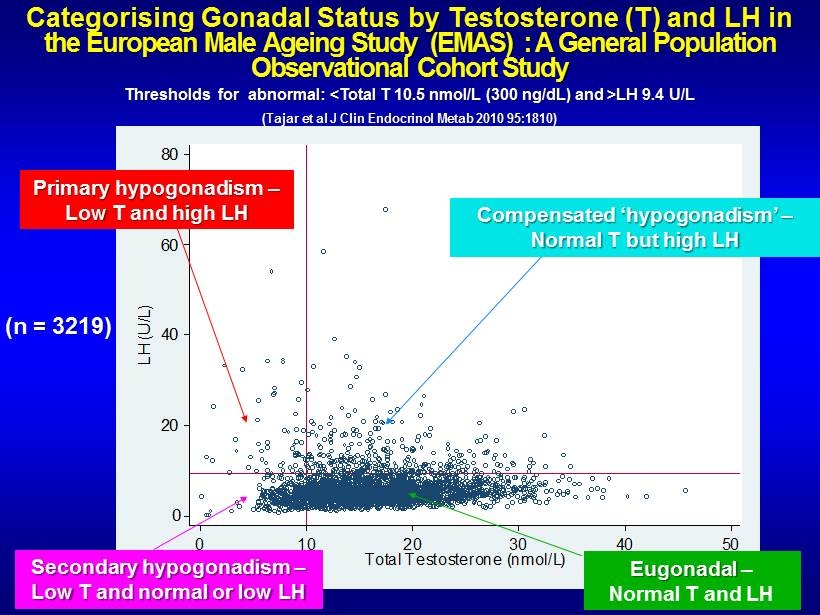
Categorising Gonadal Status by Testosterone (T) and LH in the European Male Ageing Study (EMAS) : A General Population Observational Cohort Study Thresholds for abnormal: <Total T 10.5 nmol /L (300 ng/ dL ) and >LH 9.4 U/L (Tajar et al J Clin Endocrinol Metab 2010 95:1810) 0 20 40 60 80 LH (U/L) 0 10 20 30 40 50 Total Testosterone (nmol/L) Secondary hypogonadism – Low T and normal or low LH Eugonadal – Normal T and LH Primary hypogonadism – Low T and high LH Compensated ‘ hypogonadism ’ – Normal T but high LH (n = 3219)
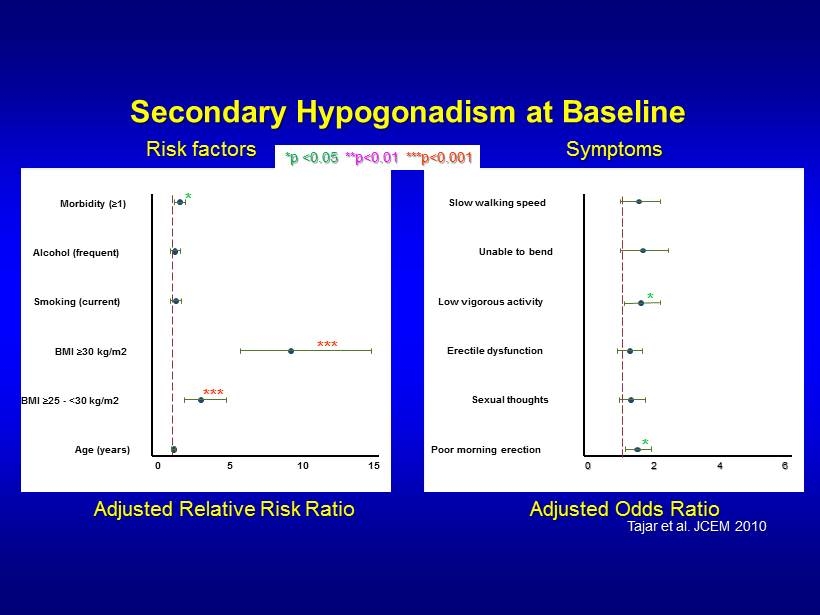
Secondary Hypogonadism at Baseline Age (years) BMI ≥ 25 - <30 kg/m2 BMI ≥ 30 kg/m2 Smoking (current) Alcohol (frequent) Morbidity ( ≥ 1) 0 5 10 15 *** *** * Poor morning erection Sexual thoughts Erectile dysfunction Low vigorous activity Unable to bend Slow walking speed 0 2 4 6 * * Adjusted Relative Risk Ratio *p <0.05 **p<0.01 ***p<0.001 Adjusted Odds Ratio Risk factors Symptoms Tajar et al. JCEM 2010
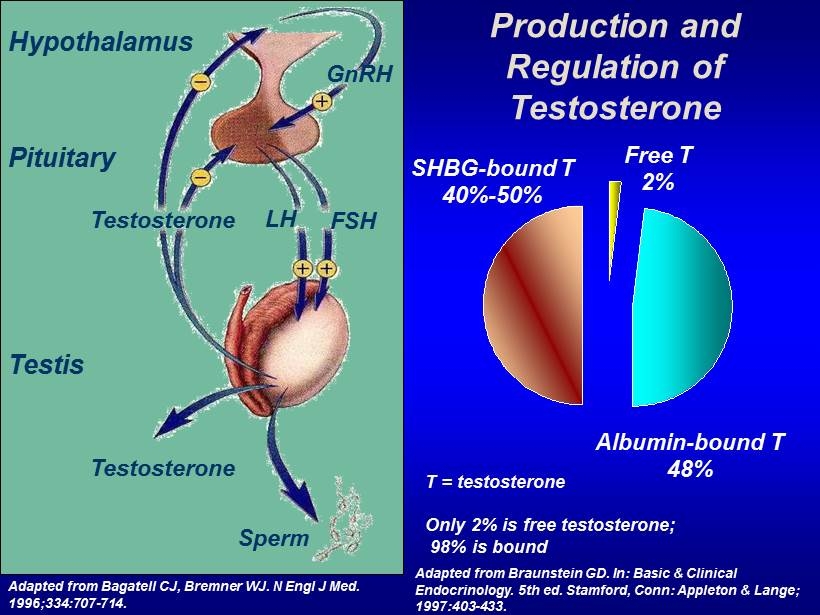
Production and Regulation of Testosterone T = testosterone Only 2% is free testosterone; 98% is bound Free T 2% SHBG - bound T 40% - 50% Albumin - bound T 48% Adapted from Bagatell CJ, Bremner WJ. N Engl J Med. 1996;334:707 - 714. GnRH LH FSH Testosterone Testosterone Sperm Hypothalamus Pituitary Testis Adapted from Braunstein GD. In: Basic & Clinical Endocrinology. 5th ed. Stamford, Conn: Appleton & Lange; 1997:403 - 433.
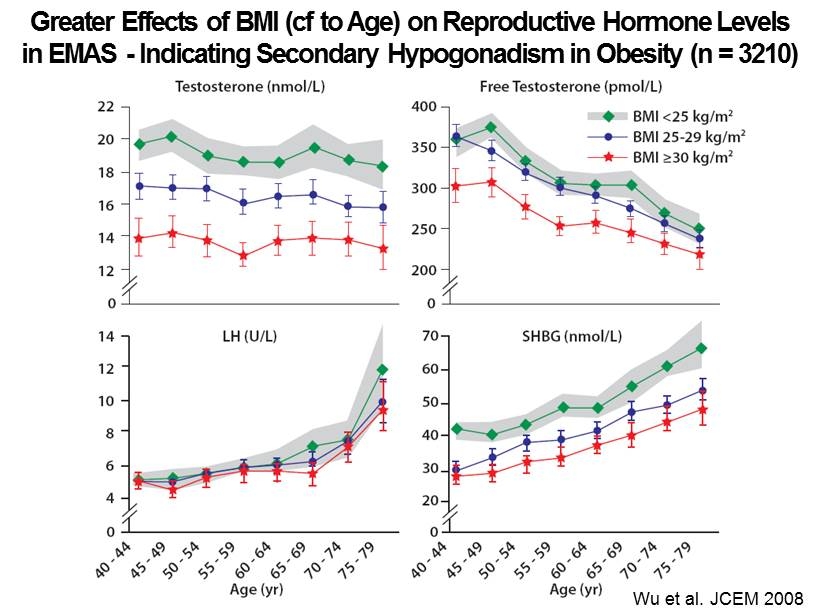
Wu et al. JCEM 2008 Greater Effects of BMI ( cf to Age) on Reproductive Hormone Levels in EMAS - Indicating Secondary Hypogonadism in Obesity (n = 3210)
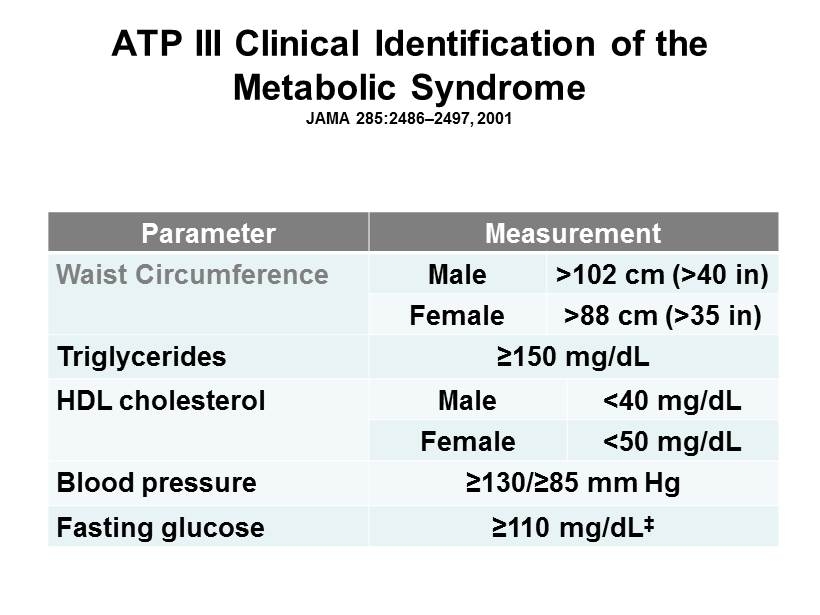
ATP III Clinical Identification of the Metabolic Syndrome JAMA 285:2486 – 2497, 2001 Parameter Measurement Waist Circumference Male >102 cm (>40 in) Female >88 cm (>35 in) Triglycerides ≥150 mg/dL HDL cholesterol Male <40 mg/dL Female <50 mg/dL Blood pressure ≥130/≥85 mm Hg Fasting glucose ≥110 mg/dL ‡

International Diabetes Federation Consensus Statement (2013) http://www.idf.org/metabolic - syndrome • Estimated that 20 - 25% of Worlds’ adult population have MetS • MetS increases risk of MI or CVA 3 - fold • MetS increases risk of death 2 - fold • MetS increases risk of T2DM 5 - fold

Institute of Human Development Centre for Endocrinology & Diabetes Manchester Academic Health Sciences Centre Institute of Human Development Centre for Endocrinology & Diabetes Manchester Academic Health Sciences Centre Two Separate Tracks of Hypogonadism 1. Secondary hypogonadism associated with obesity , independent of age and reversible 2. Primary hypogonadism - associated with ageing , independent of obesity, largely irreversible • Distinct clinical features and outcomes between tracks/categories • Require different management strategies
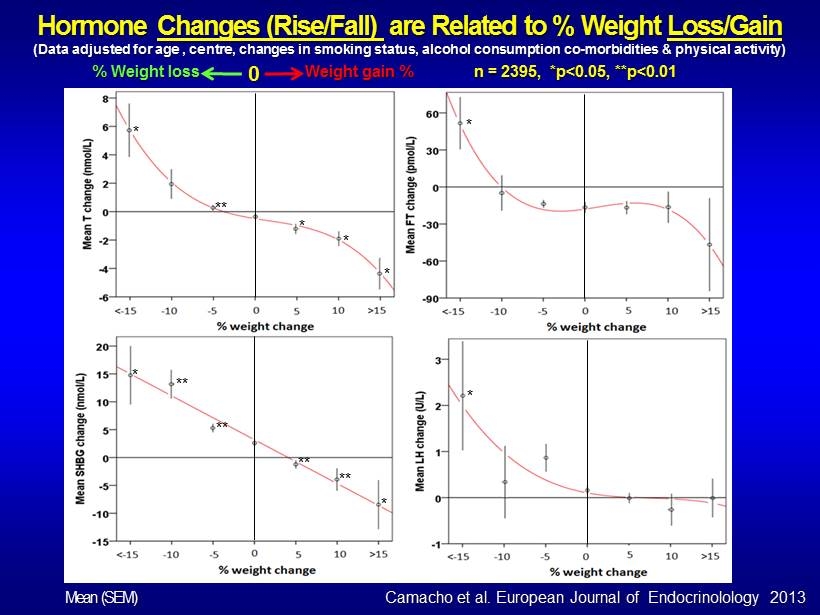
Hormone Changes (Rise/Fall) are Related to % Weight Loss/Gain (Data adjusted for age , centre, changes in smoking status, alcohol consumption co - morbidities & physical activity) n = 2395, *p<0.05, **p<0.01 % Weight loss Weight gain % 0 * ** * * * * * * ** ** ** ** * Camacho et al. European Journal of Endocrinolology 2013 Mean (SEM)

Institute of Human Development Centre for Endocrinology & Diabetes Manchester Academic Health Sciences Centre Institute of Human Development Centre for Endocrinology & Diabetes Manchester Academic Health Sciences Centre Clinical Implications • Secondary hypogonadism is closely associated with obesity and reversible suppression of hypothalamic/pituitary function • Diagnostic differentiation of secondary from primary hypogonadism is essential – measure T and LH • First line standard of care for obese men with low T should be weight loss • Strategies to mitigate gonadotrophin suppression thereby raising endogenous T and preserving fertility are preferable to exogenous T treatment in men with secondary hypogonadism

Male hypogonadism and its relationship with obesity and the metabolic syndrome Prof. Nelly Pitteloud Andrew Dwyer Lausanne, Switzerland
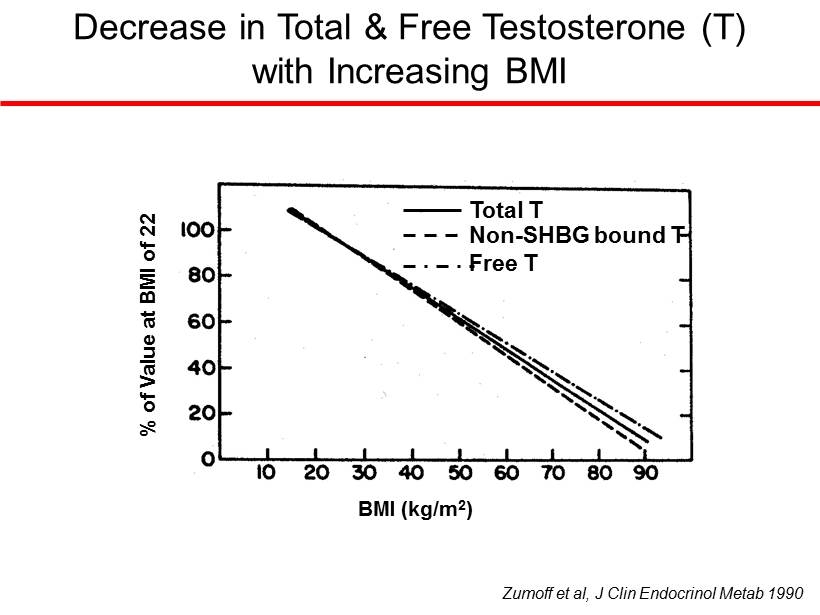
Total T Non - SHBG bound T Free T BMI (kg/m 2 ) % of Value at BMI of 22 Zumoff et al, J Clin Endocrinol Metab 1990 Decrease in Total & Free Testosterone (T) with Increasing BMI
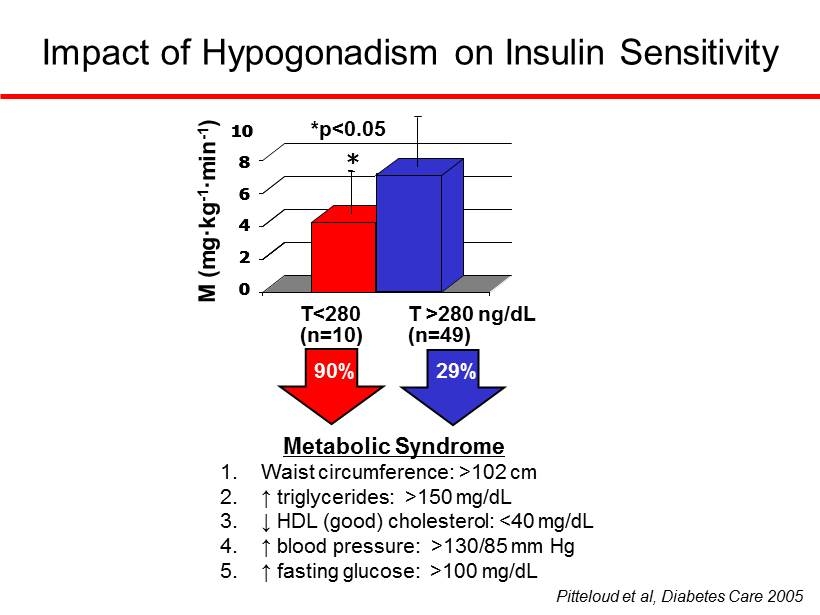
Impact of Hypogonadism on Insulin Sensitivity Metabolic Syndrome 1. Waist circumference: >102 cm 2. ↑ triglycerides: >150 mg/dL 3. ↓ HDL (good) cholesterol: <40 mg/dL 4. ↑ blood pressure: >130/85 mm Hg 5. ↑ fasting glucose: >100 mg/dL *p<0.05 0 2 4 6 8 10 * T<280 * 0 2 4 6 8 10 0 2 4 6 8 10 T >280 ng/dL (n=49) * (n=10) 90% 29% Pitteloud et al, Diabetes Care 2005
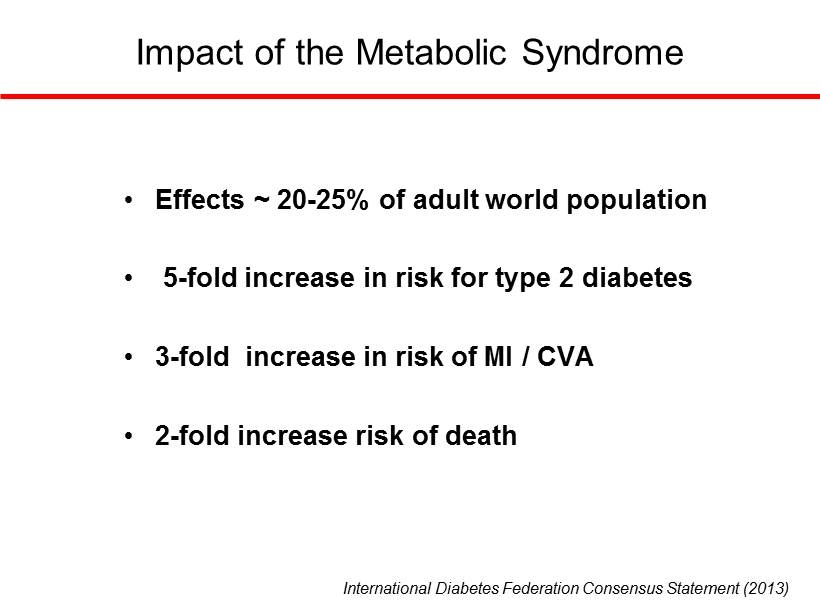
International Diabetes Federation Consensus Statement (2013) • Effects ~ 20 - 25% of adult world population • 5 - fold increase in risk for type 2 diabetes • 3 - fold increase in risk of MI / CVA • 2 - fold increase risk of death Impact of the Metabolic Syndrome
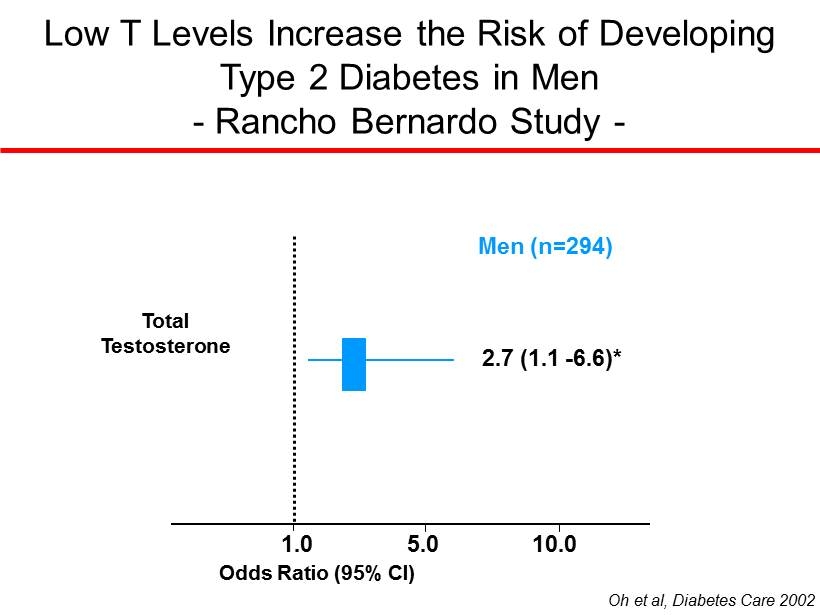
Low T Levels Increase the Risk of Developing Type 2 Diabetes in Men - Rancho Bernardo Study - 1.0 5.0 10.0 Oh et al, Diabetes Care 2002 Odds Ratio (95% CI) 2.7 (1.1 - 6.6)* Total Testosterone Men (n=294)
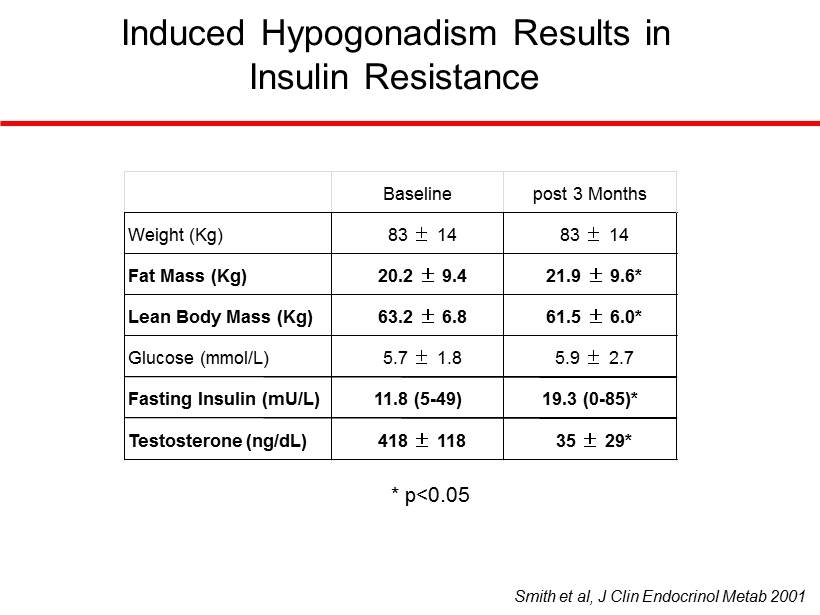
Induced Hypogonadism Results in Insulin Resistance Smith et al, J Clin Endocrinol Metab 2001 * p<0.05 Baseline post 3 Months Weight (Kg) 83 ± 14 83 ± 14 Fat Mass (Kg) 20.2 ± 9.4 21.9 ± 9.6* Lean Body Mass (Kg) 63.2 ± 6.8 61.5 ± 6.0* Glucose (mmol/L) 5.7 ± 1.8 5.9 ± 2.7 Fasting Insulin (mU/L) 11.8 (5 - 49) 19.3 (0 - 85)* Testosterone (ng/dL) 418 ± 118 35 ± 29*
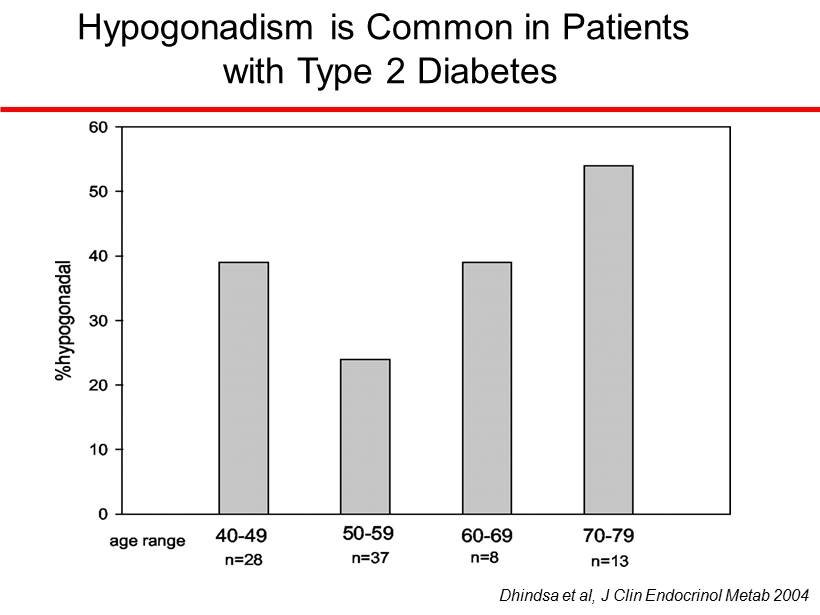
Hypogonadism is Common in Patients with Type 2 Diabetes Dhindsa et al, J Clin Endocrinol Metab 2004
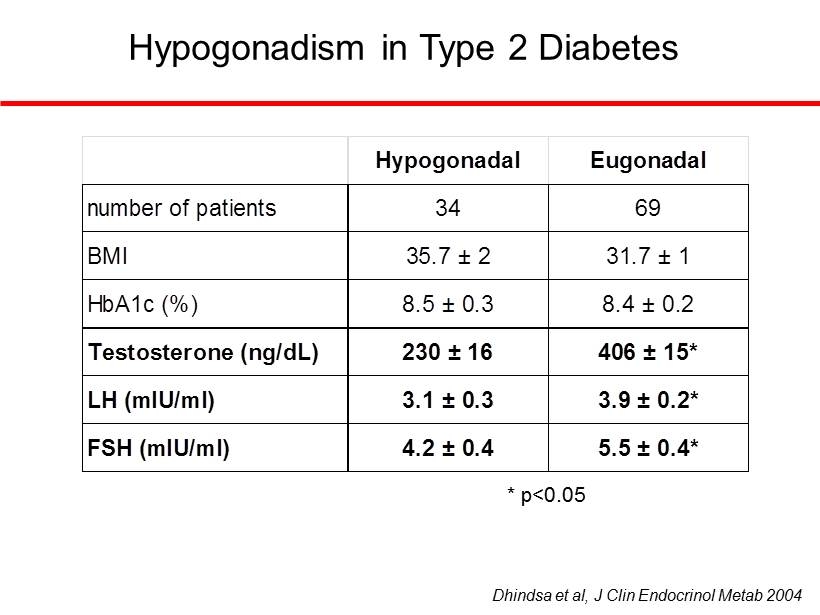
Hypogonadism in Type 2 Diabetes Hypogonadal Eugonadal number of patients 34 69 BMI 35.7 ± 2 31.7 ± 1 Testosterone (ng/dL) 230 ± 16 406 ± 15* LH (mIU/ml) 3.1 ± 0.3 3.9 ± 0.2* FSH (mIU/ml) 4.2 ± 0.4 5.5 ± 0.4* HbA1c (%) 8.5 ± 0.3 8.4 ± 0.2 Dhindsa et al, J Clin Endocrinol Metab 2004 * p<0.05 Hypogonadal Eugonadal number of patients 34 69 BMI 35.7 ± 2 31.7 ± 1 Testosterone (ng/dL) 230 ± 16 406 ± 15* LH (mIU/ml) 3.1 ± 0.3 3.9 ± 0.2* FSH (mIU/ml) 4.2 ± 0.4 5.5 ± 0.4* HbA1c (%) 8.5 ± 0.3 8.4 ± 0.2 Hypogonadal Eugonadal number of patients 34 69 BMI 35.7 ± 2 31.7 ± 1 Testosterone (ng/dL) 230 ± 16 406 ± 15* LH (mIU/ml) 3.1 ± 0.3 3.9 ± 0.2* FSH (mIU/ml) 4.2 ± 0.4 5.5 ± 0.4* HbA1c (%) 8.5 ± 0.3 8.4 ± 0.2
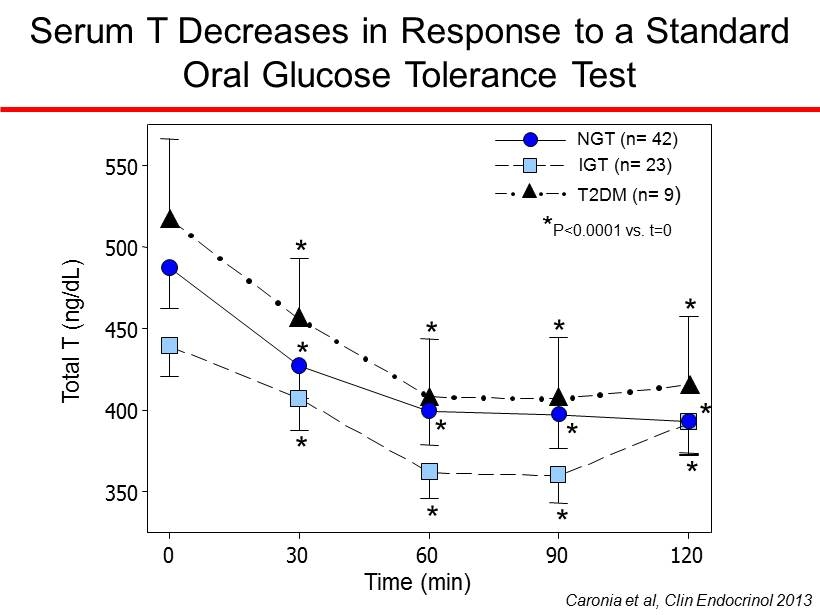
Serum T Decreases in Response to a Standard Oral Glucose Tolerance Test Total T (ng/dL) 350 400 450 500 550 * * * * * * * * * * * * Time (min) 0 30 60 90 120 NGT (n= 42) IGT (n= 23) T2DM (n= 9 ) * P<0.0001 vs. t=0 Caronia et al, Clin Endocrinol 2013
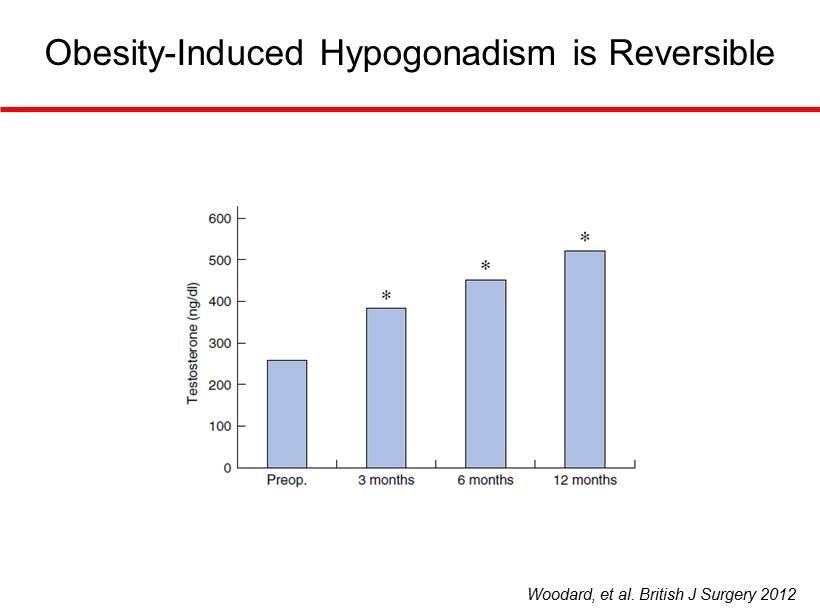
Woodard, et al. British J Surgery 2012 Obesity - Induced Hypogonadism is Reversible
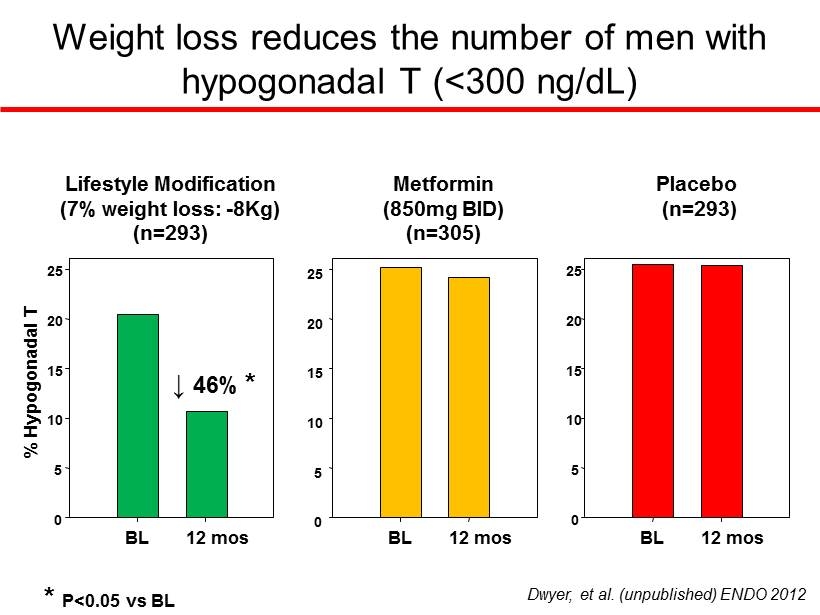
Weight loss reduces the number of men with hypogonadal T (<300 ng/dL) BL 12 mos % Hypogonadal T 0 5 10 15 20 25 BL 12 mos BL 12 mos 0 5 10 15 20 25 0 5 10 15 20 25 Lifestyle Modification (7% weight loss: - 8Kg) (n=293) Metformin (850mg BID) (n=305) Placebo (n=293) * P<0.05 vs BL ↓ 46% * Dwyer, et al. (unpublished) ENDO 2012
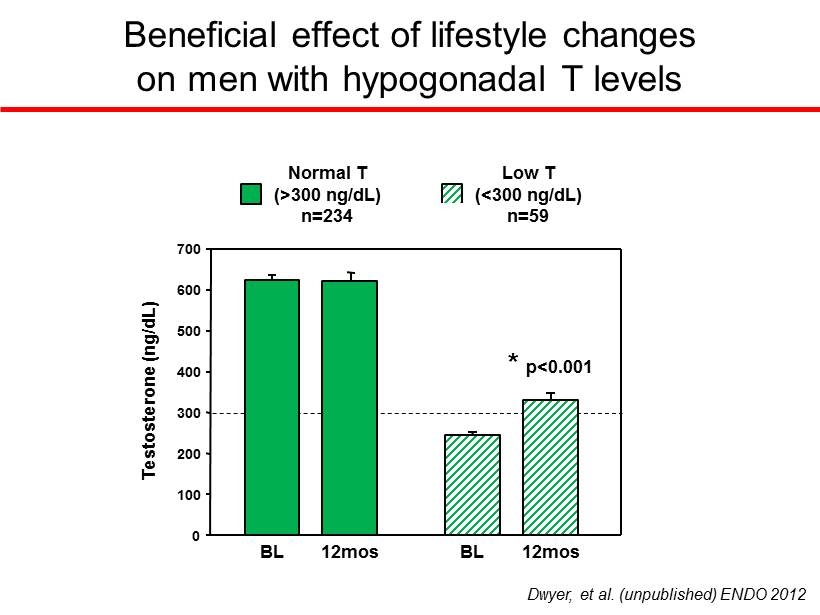
Beneficial effect of lifestyle changes on men with hypogonadal T levels 0 100 200 300 400 500 600 700 Normal T (>300 ng/dL) n=234 BL 12mos Low T (<300 ng/dL) n=59 BL 12mos Testosterone (ng/dL) Dwyer, et al. (unpublished) ENDO 2012 * p<0.001
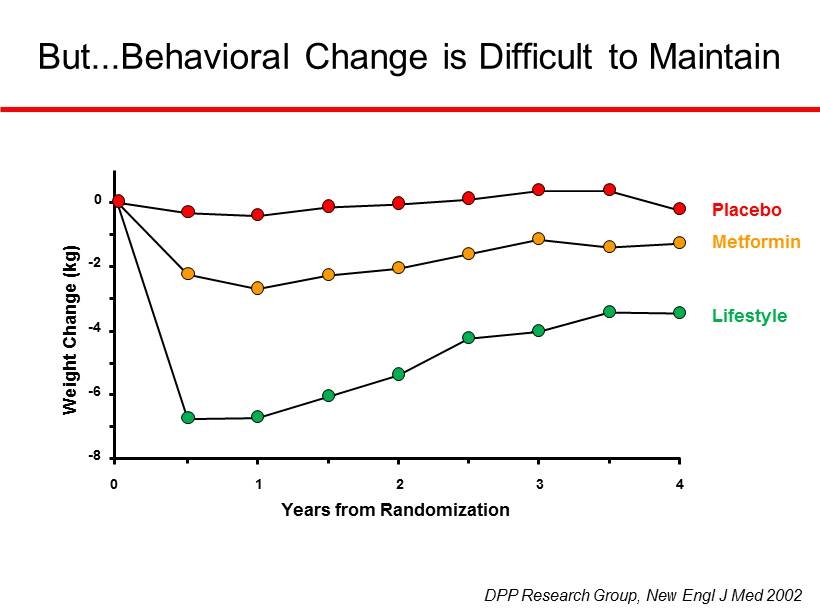
But...Behavioral Change is Difficult to Maintain Placebo Metformin Lifestyle DPP Research Group, New Engl J Med 2002 - 8 - 6 - 4 - 2 0 0 1 2 3 4 Years from Randomization Weight Change (kg)

There is sufficient data supporting a link between obesity, insulin resistance & 2˚ hypogonadism in men. The disruption of the reproductive axis caused by obesity is reversible. Obese men with type 2 diabetes are at particularly high risk for developing 2˚ hypogonadism. Summary & Take - Away Points

Thank you

Effects of Long - Term Testosterone Therapy in Obese Hypogonadal Men Analyst’s Day at The Waldorf Astoria, New York, New York, October 31, 2014 Prof . h.c . Dr. Farid Saad, Global Medical Affairs Andrology, Bayer Pharma AG , Berlin, Germany, Gulf Medical University, Research Department, Ajman, United Arab Emirates, and Hang Tuah University, Men’s Health Reproduction Study Center, Surabaya, Indonesia
The Economist, Dec 15, 2012
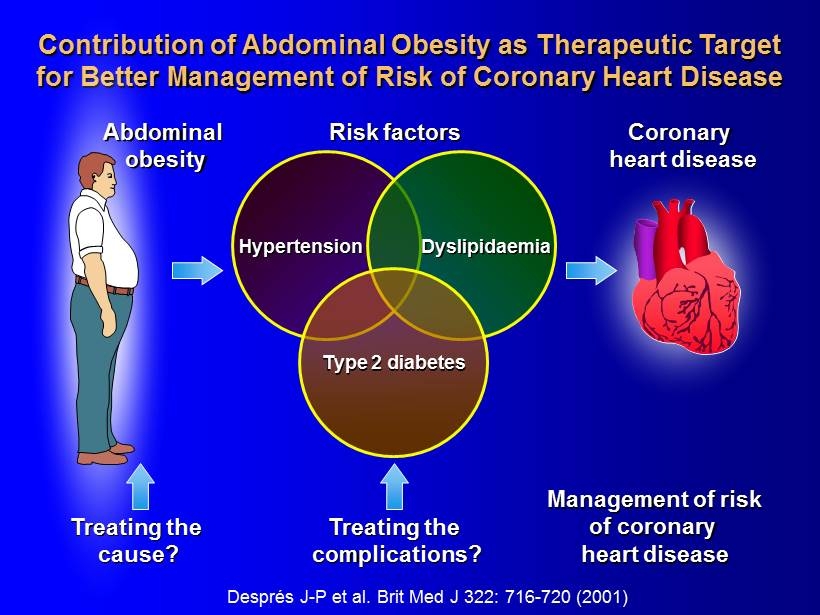
Després J - P et al. Brit Med J 322: 716 - 720 (2001) Contribution of Abdominal Obesity as Therapeutic Target for Better Management of Risk of Coronary Heart Disease Risk factors Coronary heart disease Abdominal obesity Treating the complications? Management of risk of coronary heart disease Treating the cause? Hypertension Dyslipidaemia Type 2 diabetes

Waist Circumference Is a Well - Established Cardio - Metabolic Risk Factor and a Component of All Current Definitions of the Metabolic Syndrome

What Are the Options for Treating Obesity ? 1. Lifestyle Interventions largely unsuccessful , typically resulting in U - shaped curve 2. Drugs largely unsuccessful , typically resulting in U - shaped curve ; targeting appetite control ; several drugs withdrawn from the market due to side effects 3. Bariatric ( Metabolic ) Surgery for BMI ≥ 40 kg/m 2 ; for BMI ≥ 35 kg/m 2 + type 2 diabetes ; expensive; not available to everyone ; successful but not risk - free ( loss of lean mass , osteoporosis , depression )

Approximately 50% of Obese Men Are Hypogonadal

Observational Registry Studies from Germany

362 Hypogonadal Men with Different Grades of Obesity from Two Ongoing Registry Studies
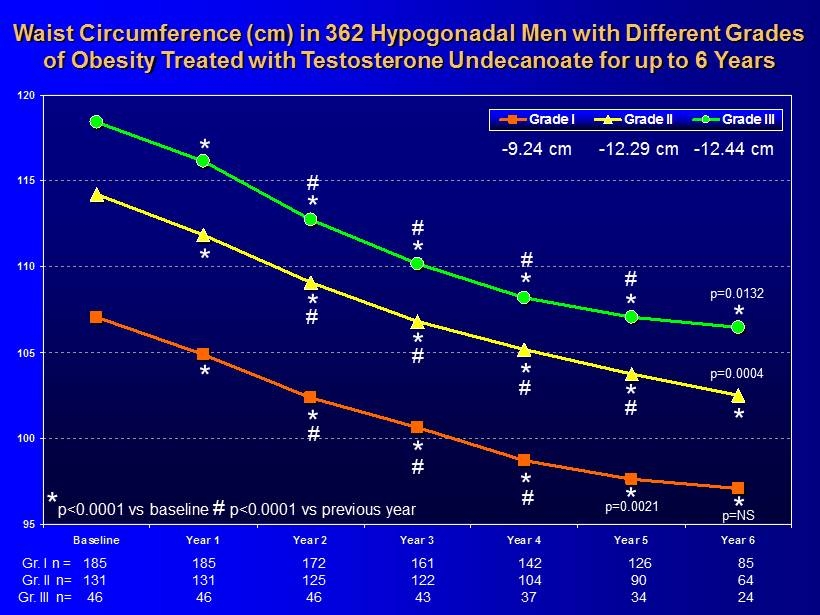
95 100 105 110 115 120 Baseline Year 1 Year 2 Year 3 Year 4 Year 5 Year 6 Grade I Grade II Grade III * * * * 185 161 142 126 85 185 172 * * 131 122 104 90 64 131 125 46 43 37 34 24 46 46 * * * * * * * * * * * * # # # # # # # # # # # p=0.0132 p=0.0004 p=0.0021 p=NS - 9.24 cm - 12.29 cm - 12.44 cm * p<0.0001 vs baseline # p<0.0001 vs previous year Waist Circumference (cm) in 362 Hypogonadal Men with Different Grades of Obesity Treated with Testosterone Undecanoate for up to 6 Years Gr. I n = Gr. II n= Gr. III n=
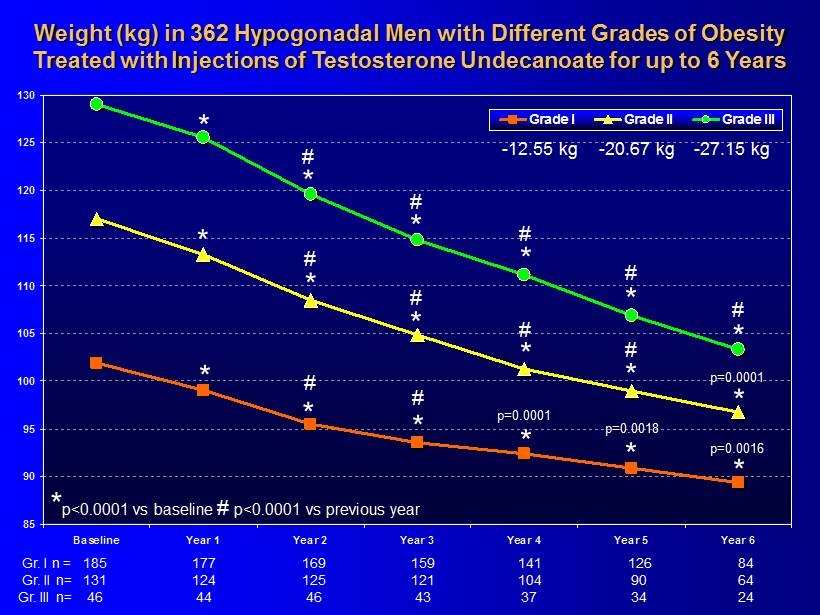
85 90 95 100 105 110 115 120 125 130 Baseline Year 1 Year 2 Year 3 Year 4 Year 5 Year 6 Grade I Grade II Grade III Weight (kg ) in 362 Hypogonadal Men with Different Grades of Obesity Treated with Injections of Testosterone Undecanoate for up to 6 Years * * * * 185 159 141 126 84 177 169 Gr. I n = * 131 121 104 90 64 124 125 Gr. II n= 46 43 37 34 24 44 46 Gr. III n= * * * * * * * * * * * * # # # # # # # # # # p=0.0001 p=0.0018 * # p=0.0001 p=0.0016 - 12.55 kg - 20.67 kg - 27.15 kg * p<0.0001 vs baseline # p<0.0001 vs previous year
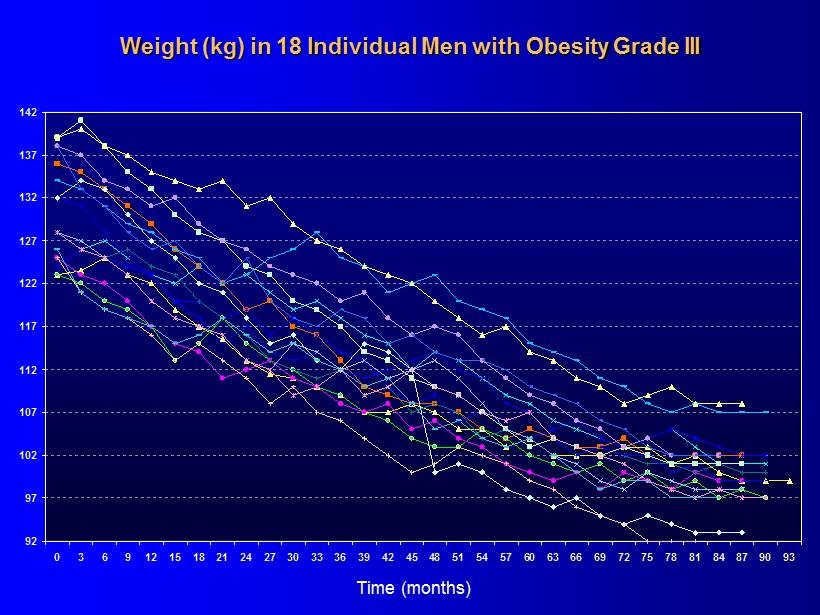
92 97 102 107 112 117 122 127 132 137 142 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 60 63 66 69 72 75 78 81 84 87 90 93 Weight (kg) in 18 Individual Men with Obesity Grade III Time ( months )
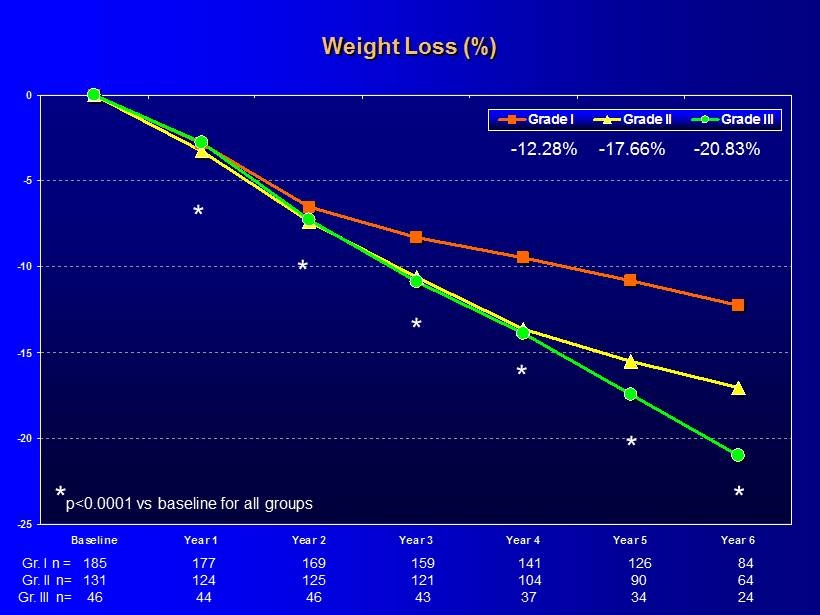
-25 -20 -15 -10 -5 0 Baseline Year 1 Year 2 Year 3 Year 4 Year 5 Year 6 Grade I Grade II Grade III * p<0.0001 vs baseline for all groups * * * * * * - 12.28% - 17.66% - 20.83% Weight Loss (%) 185 159 141 126 84 177 169 Gr. I n = 131 121 104 90 64 124 125 Gr. II n= 46 43 37 34 24 44 46 Gr. III n=
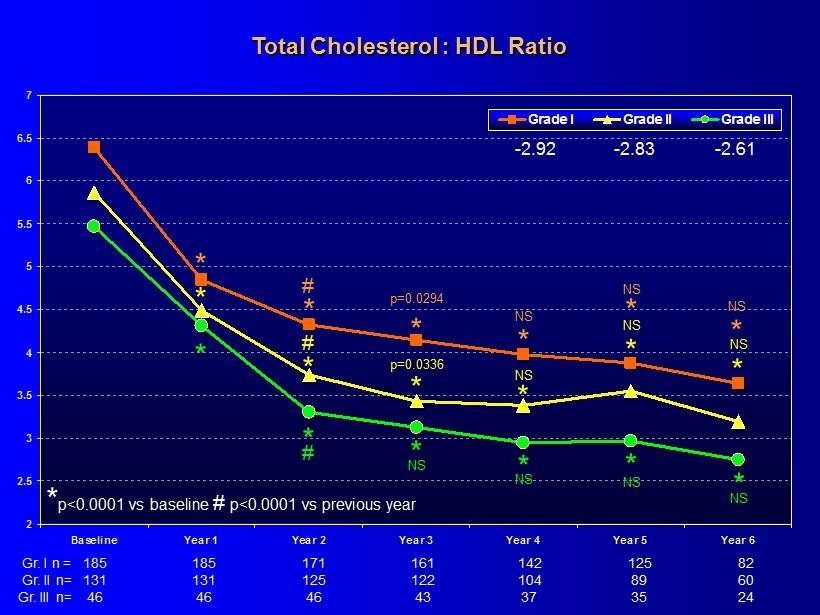
2 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 Baseline Year 1 Year 2 Year 3 Year 4 Year 5 Year 6 Grade I Grade II Grade III Total Cholesterol : HDL Ratio 185 161 142 125 82 185 171 131 122 104 89 60 131 125 46 43 37 35 24 46 46 - 2.92 - 2.83 - 2.61 * * * * * * * * p<0.0001 vs baseline # p<0.0001 vs previous year p=0.0294 * NS * NS * p=0.0336 * NS * NS * NS * NS * * NS * NS * NS # NS # # Gr. I n = Gr. II n= Gr. III n=
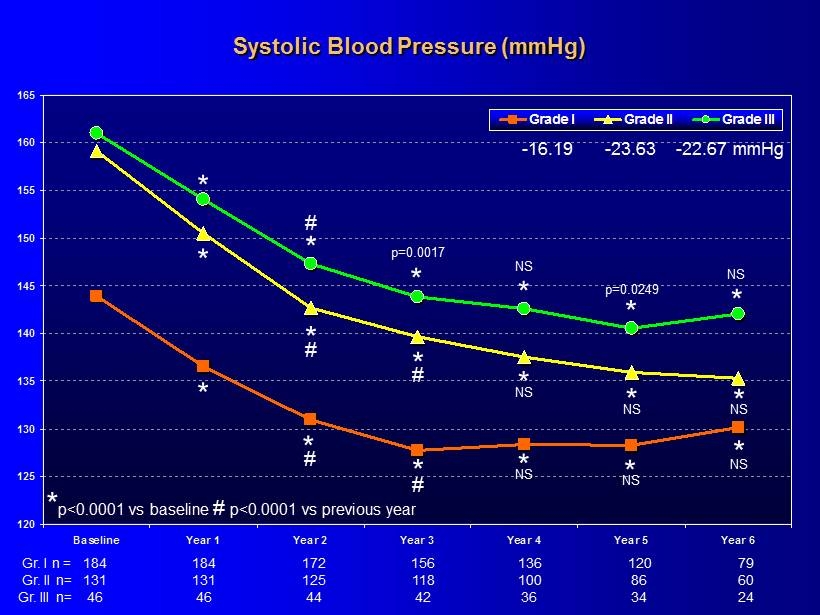
120 125 130 135 140 145 150 155 160 165 Baseline Year 1 Year 2 Year 3 Year 4 Year 5 Year 6 Grade I Grade II Grade III Systolic Blood Pressure (mmHg) * * * * 184 156 136 120 79 184 172 * * 131 118 100 86 60 131 125 46 42 36 34 24 46 44 * * * * * * * # # # # NS - 16.19 - 23.63 - 22.67 mmHg * p<0.0001 vs baseline # p<0.0001 vs previous year p=0.0017 NS p=0.0249 NS * # NS * NS * NS * NS * NS Gr. I n = Gr. II n= Gr. III n=
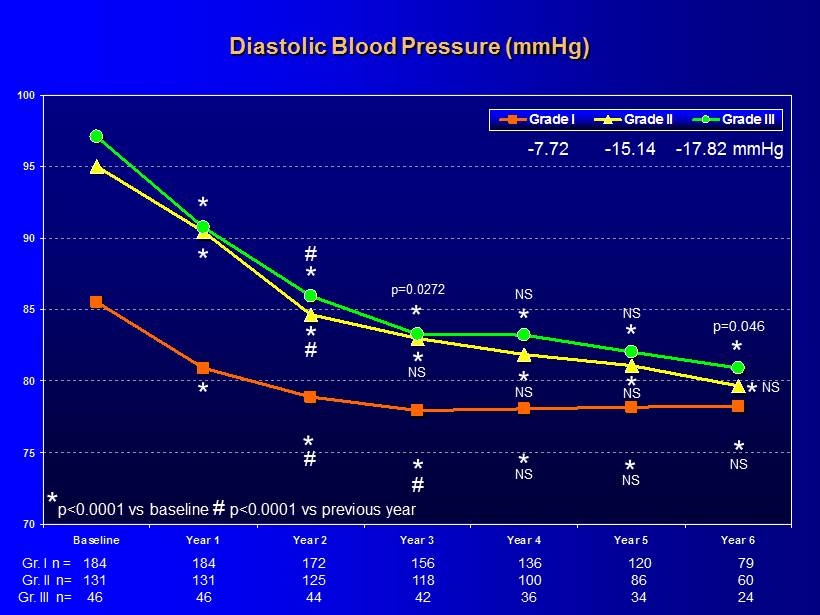
70 75 80 85 90 95 100 Baseline Year 1 Year 2 Year 3 Year 4 Year 5 Year 6 Grade I Grade II Grade III Diastolic Blood Pressure (mmHg) * * * * * * * * * * * * * # # # # NS - 7.72 - 15.14 - 17.82 mmHg * p<0.0001 vs baseline # p<0.0001 vs previous year p=0.0272 NS NS p =0.046 * NS * NS * NS * NS * NS NS 184 156 136 120 79 184 172 131 118 100 86 60 131 125 46 42 36 34 24 46 44 Gr. I n = Gr. II n= Gr. III n=

120 Hypogonadal Men with Type 2 Diabetes Mellitus (T2DM) from an Ongoing Registry Study
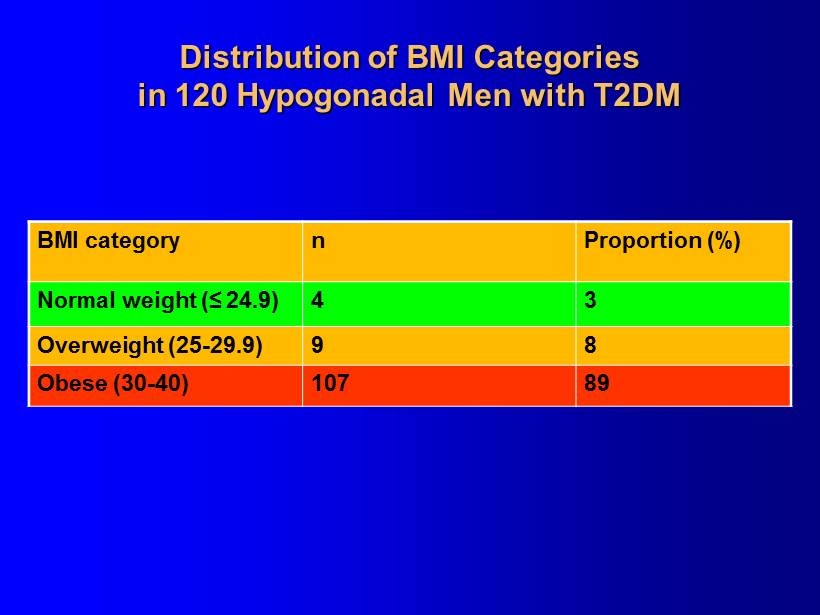
Distribution of BMI Categories in 120 Hypogonadal Men with T2DM BMI category n Proportion (%) Normal weight (≤ 24.9) 4 3 Overweight (25 - 29.9) 9 8 Obese (30 - 40) 107 89
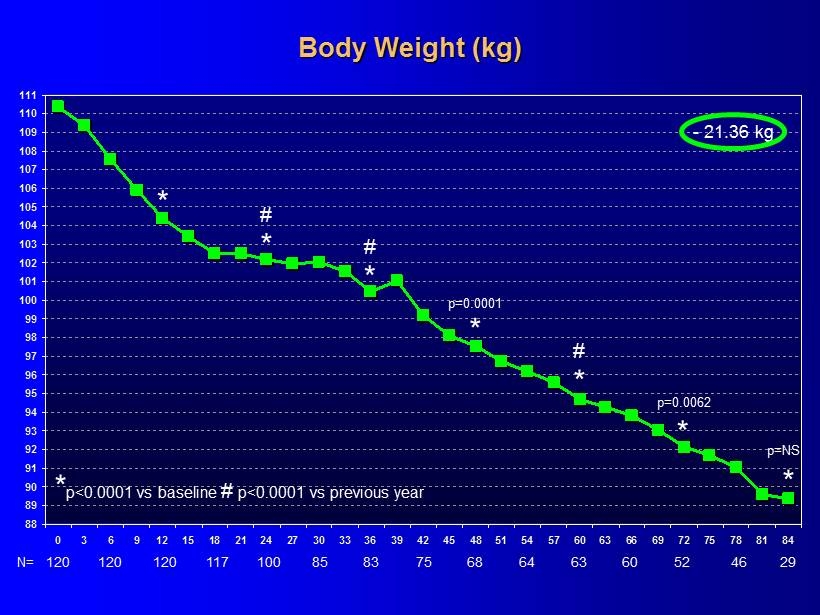
88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 60 63 66 69 72 75 78 81 84 * p<0.0001 vs baseline # p<0.0001 vs previous year * * # - 21.36 kg * p = NS 120 N= 120 120 117 100 85 83 75 68 64 63 60 29 52 46 * # * * # Body Weight (kg) * p =0.0001 p =0.0062
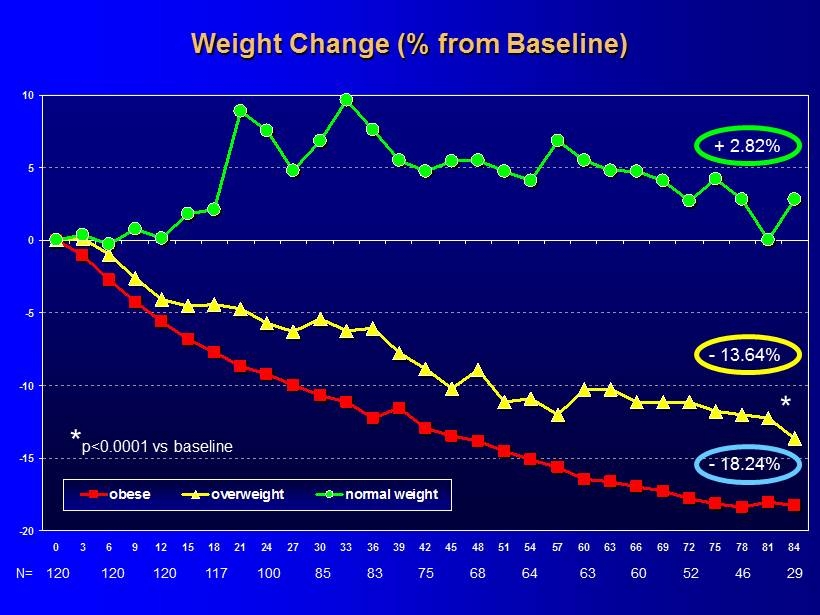
-20 -15 -10 -5 0 5 10 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 60 63 66 69 72 75 78 81 84 obese overweight normal weight Weight Change (% from Baseline) * p<0.0001 vs baseline + 2.82% * 120 N= 120 120 117 100 85 83 75 68 64 63 60 29 52 46 - 13.64% - 18.24%
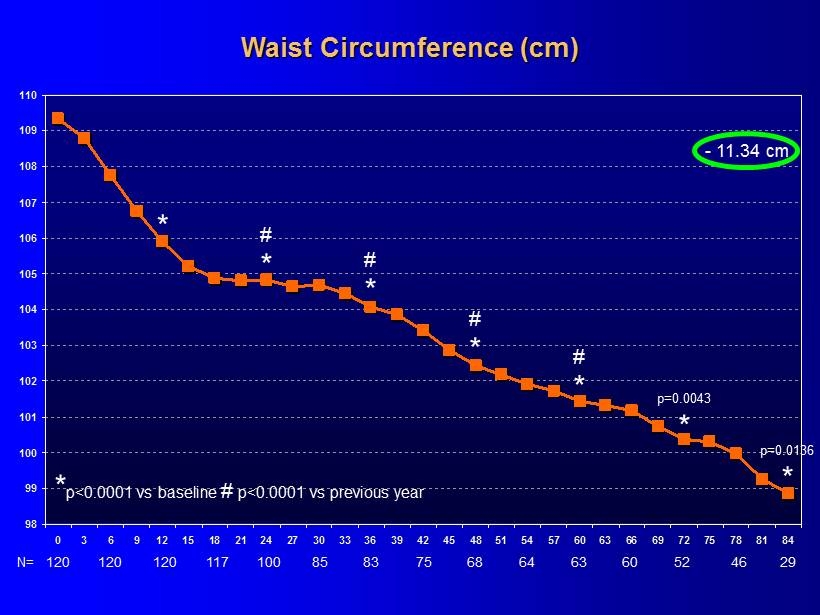
98 99 100 101 102 103 104 105 106 107 108 109 110 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 60 63 66 69 72 75 78 81 84 * p<0.0001 vs baseline # p<0.0001 vs previous year Waist Circumference (cm) * * # * - 11.34 cm p = 0.0043 120 N= 120 120 117 100 85 83 75 68 64 63 60 29 52 46 * # * # * # * p = 0.0136
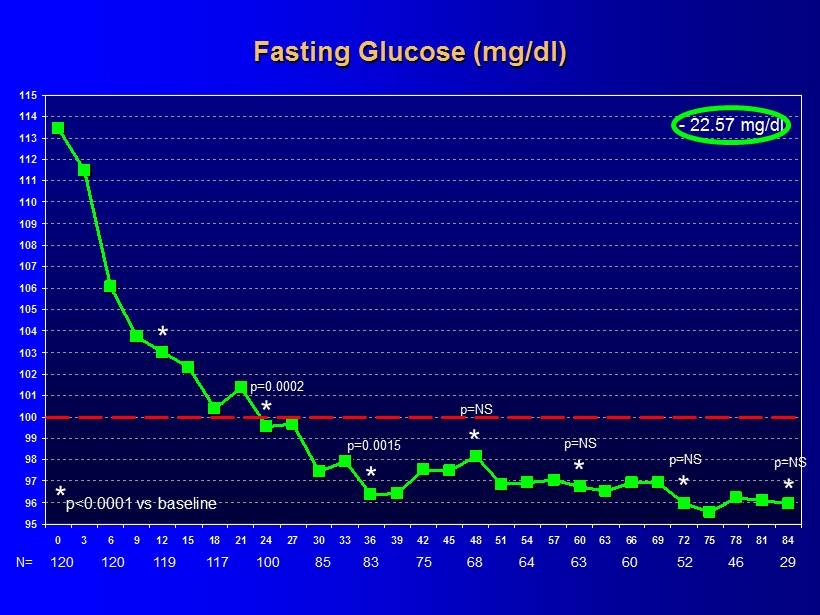
95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 60 63 66 69 72 75 78 81 84 * p<0.0001 vs baseline * * - 22.57 mg/dl Fasting Glucose (mg/dl) p = 0.0002 * * p = NS 120 N= 120 119 117 100 85 83 75 68 64 63 60 29 52 46 p = 0.0015 * p = NS * p = NS * p = NS
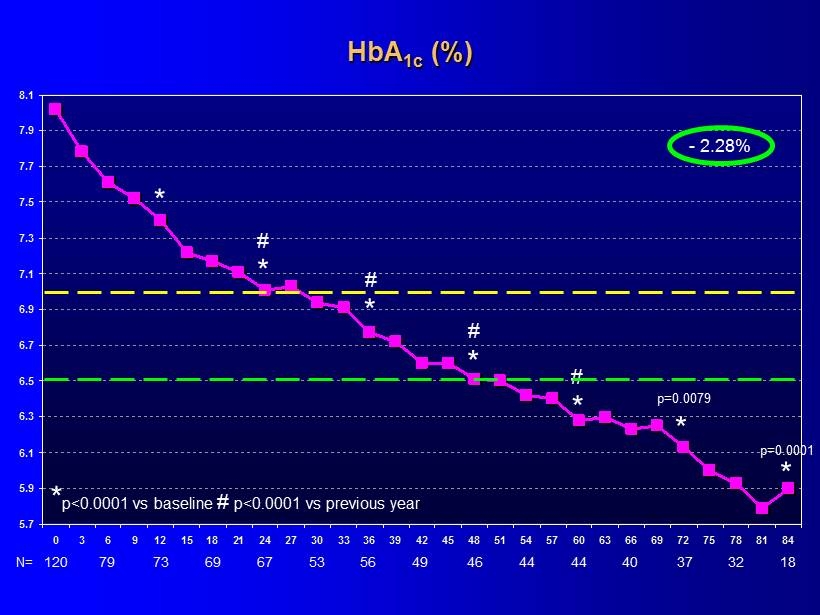
5.7 5.9 6.1 6.3 6.5 6.7 6.9 7.1 7.3 7.5 7.7 7.9 8.1 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 60 63 66 69 72 75 78 81 84 - 2.28% * p<0.0001 vs baseline # p<0.0001 vs previous year * * # * * * * * HbA 1c (%) 120 N= 79 73 69 67 53 56 49 46 44 44 40 18 37 32 # # # p = 0.0079 p = 0.0001
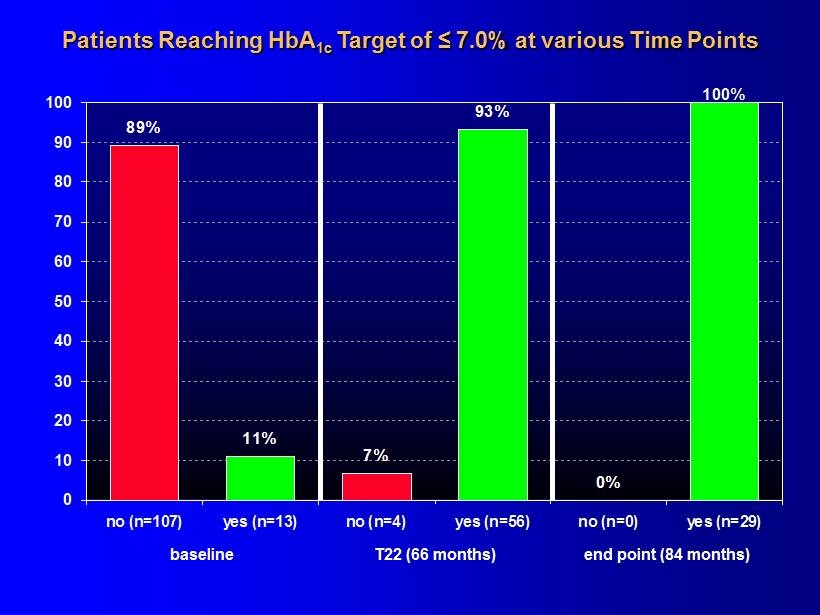
Patients Reaching HbA 1c Target of ≤ 7.0% at various Time Points 0% 100% 93% 7% 11% 89% 0 10 20 30 40 50 60 70 80 90 100 no (n=107) yes (n=13) no (n=4) yes (n=56) no (n=0) yes (n=29) baseline end point (84 months) T22 (66 months)
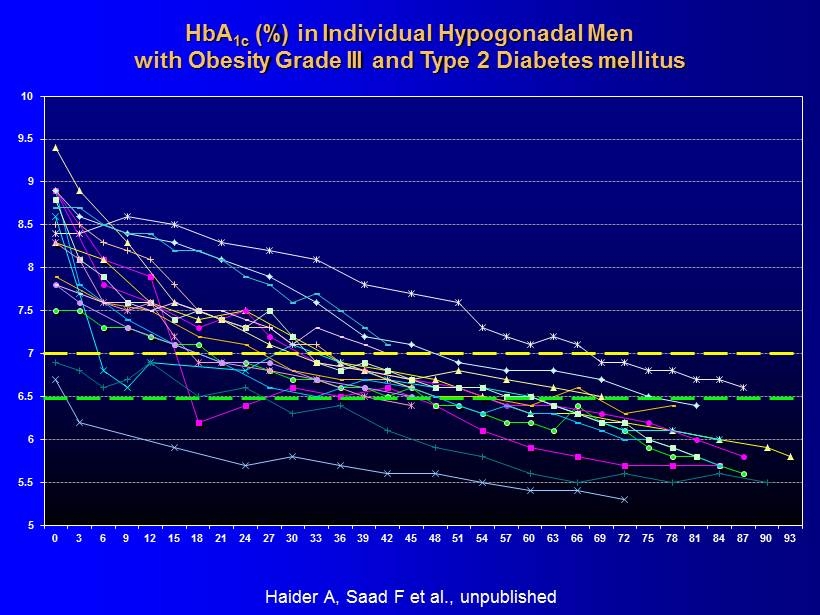
5 5.5 6 6.5 7 7.5 8 8.5 9 9.5 10 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 57 60 63 66 69 72 75 78 81 84 87 90 93 HbA 1c (%) in Individual Hypogonadal Men with Obesity Grade III and Type 2 Diabetes mellitus Haider A, Saad F et al ., unpublished

Observational Data Confirmed by A Controlled Study
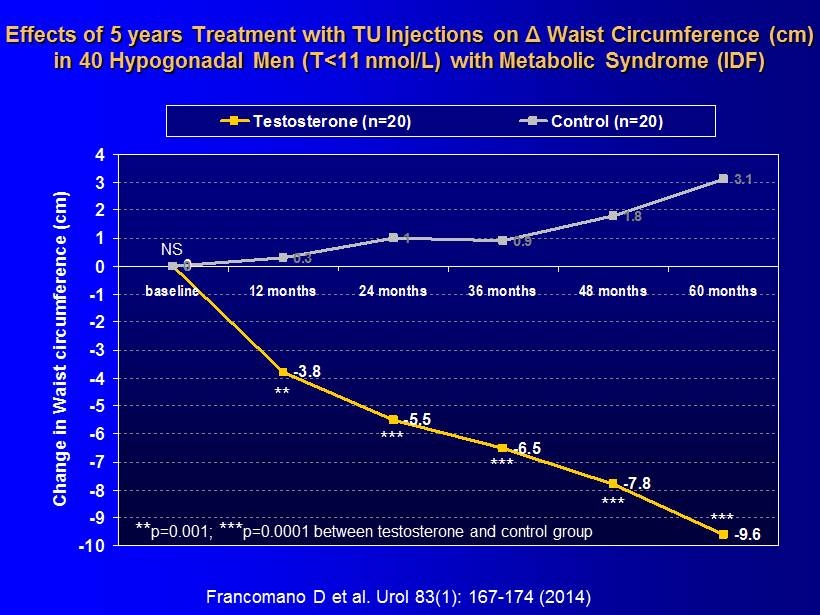
Effects of 5 years Treatment with TU Injections on Δ Waist Circumference (cm) in 40 Hypogonadal Men (T<11 nmol/L) with Metabolic Syndrome (IDF) 0 -3.8 -5.5 -6.5 -7.8 -9.6 0 0.3 1 0.9 1.8 3.1 -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 1 2 3 4 baseline 12 months 24 months 36 months 48 months 60 months Change in Waist circumference (cm) Testosterone (n=20) Control (n=20) ** *** *** *** *** ** p=0.001; *** p=0.0001 between testosterone and control group NS Francomano D et al. Urol 83(1): 167 - 174 (2014)
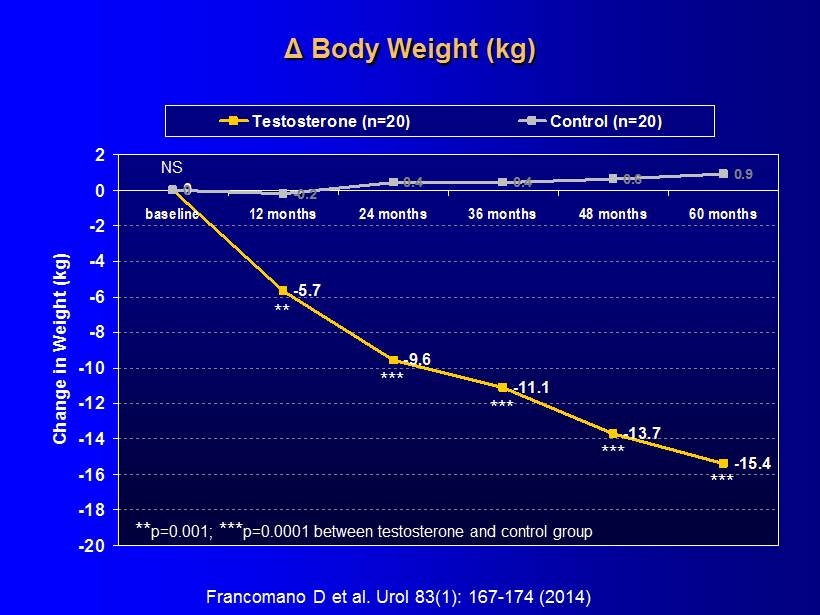
Δ Body Weight (kg) 0 -5.7 -9.6 -11.1 -13.7 -15.4 0 -0.2 0.4 0.4 0.6 0.9 -20 -18 -16 -14 -12 -10 -8 -6 -4 -2 0 2 baseline 12 months 24 months 36 months 48 months 60 months Change in Weight (kg) Testosterone (n=20) Control (n=20) ** *** *** *** *** ** p=0.001; *** p=0.0001 between testosterone and control group NS Francomano D et al. Urol 83(1): 167 - 174 (2014)
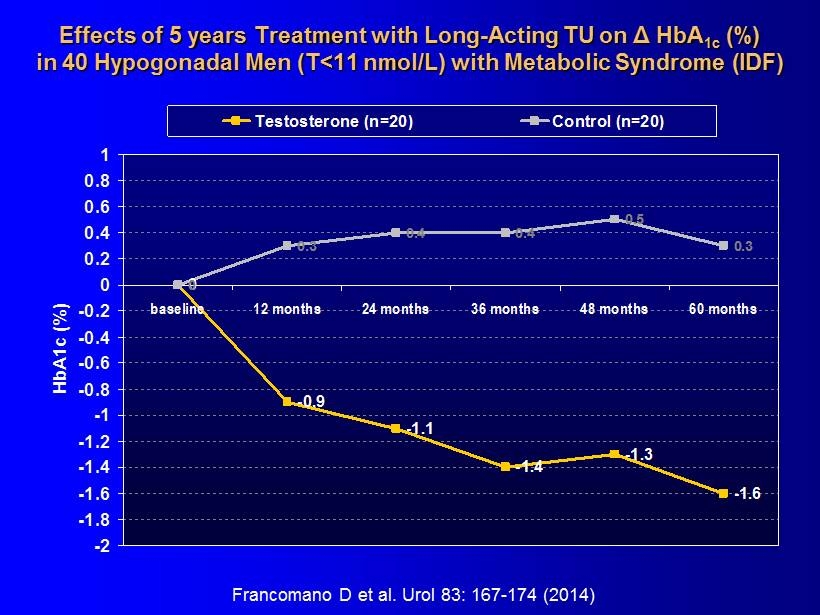
Effects of 5 years Treatment with Long - Acting TU on Δ HbA 1c (%) in 40 Hypogonadal Men (T<11 nmol/L) with Metabolic Syndrome (IDF) 0 -0.9 -1.1 -1.4 -1.3 -1.6 0 0.3 0.4 0.4 0.5 0.3 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0.2 0.4 0.6 0.8 1 baseline 12 months 24 months 36 months 48 months 60 months HbA1c (%) Testosterone (n=20) Control (n=20) Francomano D et al. Urol 83: 167 - 174 (2014)
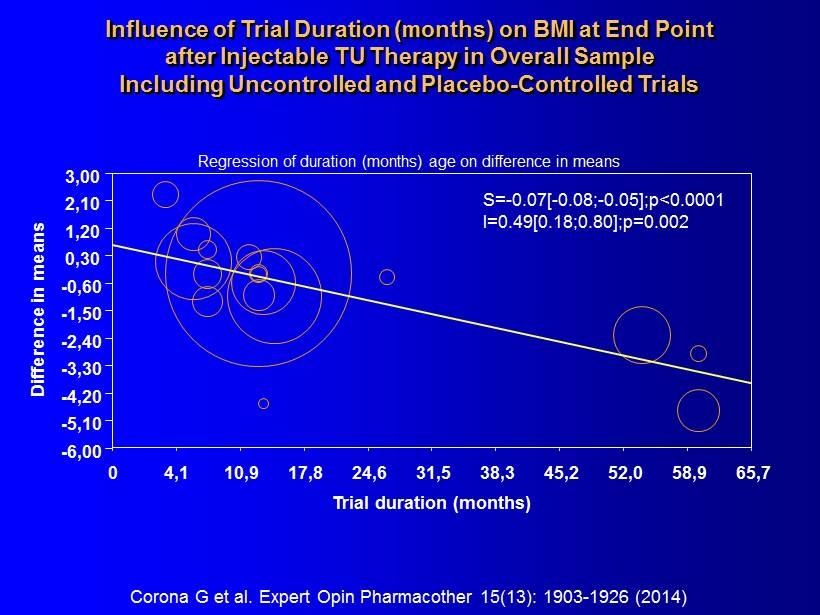
S= - 0.07[ - 0.08; - 0.05];p<0.0001 I=0.49[0.18;0.80];p=0.002 Trial duration (months) Difference in means 0 4,1 10,9 17,8 24,6 31,5 38,3 45,2 52,0 58,9 65,7 3,00 2,10 1,20 0,30 - 0,60 - 1,50 - 2,40 - 3,30 - 4,20 - 5,10 - 6,00 Corona G et al. Expert Opin Pharmacother 15(13): 1903 - 1926 (2014) Regression of duration ( months ) age on difference in means Influence of Trial Duration ( months ) on BMI at End Point after Injectable TU Therapy in Overall Sample Including Uncontrolled and Placebo - Controlled Trials

Summary Obesity is a risk factor for hypogonadism and vice versa. 50% of obese men are hypogonadal. In three independent registry studies of unselected hypogonadal men, 50 - 70% of men were obese at baseline. Testosterone therapy induces meaningful and sustained weight loss. In men with type 2 diabetes, testosterone therapy leads to meaningful and sustained improvement of diabetic control. These effects require long - term treatment.

Androxal Safety Review 31 October 2014 ▪ Discontinuation due to Adverse Events (DCAEs) ▪ Treatment Emergent Adverse Events ( TEAEs) ▪ Thrombotic events ▪ Prostate Specific Antigen (PSA) ▪ Other Safety Issues Related to Testosterone Therapy
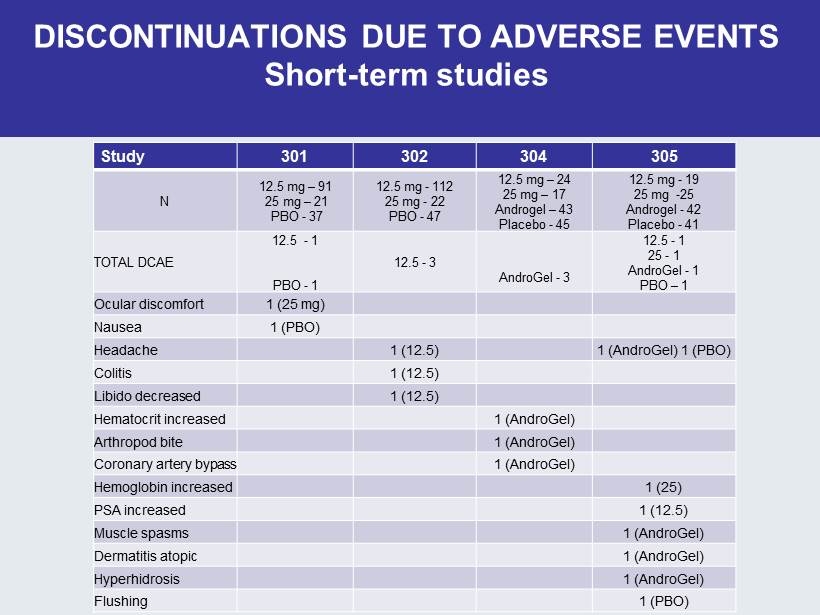
DISCONTINUATIONS DUE TO ADVERSE EVENTS Short - term studies Study 301 302 304 305 N 12.5 mg – 91 25 mg – 21 PBO - 37 12.5 mg - 112 25 mg - 22 PBO - 47 12.5 mg – 24 25 mg – 17 Androgel – 43 Placebo - 45 12.5 mg - 19 25 mg - 25 Androgel - 42 Placebo - 41 TOTAL DCAE 12.5 - 1 PBO - 1 12.5 - 3 AndroGel - 3 12.5 - 1 25 - 1 AndroGel - 1 PBO – 1 Ocular discomfort 1 (25 mg) Nausea 1 (PBO) Headache 1 (12.5) 1 (AndroGel) 1 (PBO) Colitis 1 (12.5) Libido decreased 1 (12.5) Hematocrit increased 1 (AndroGel) Arthropod bite 1 (AndroGel) Coronary artery bypass 1 (AndroGel) Hemoglobin increased 1 (25) PSA increased 1 (12.5) Muscle spasms 1 (AndroGel) Dermatitis atopic 1 (AndroGel) Hyperhidrosis 1 (AndroGel) Flushing 1 (PBO)
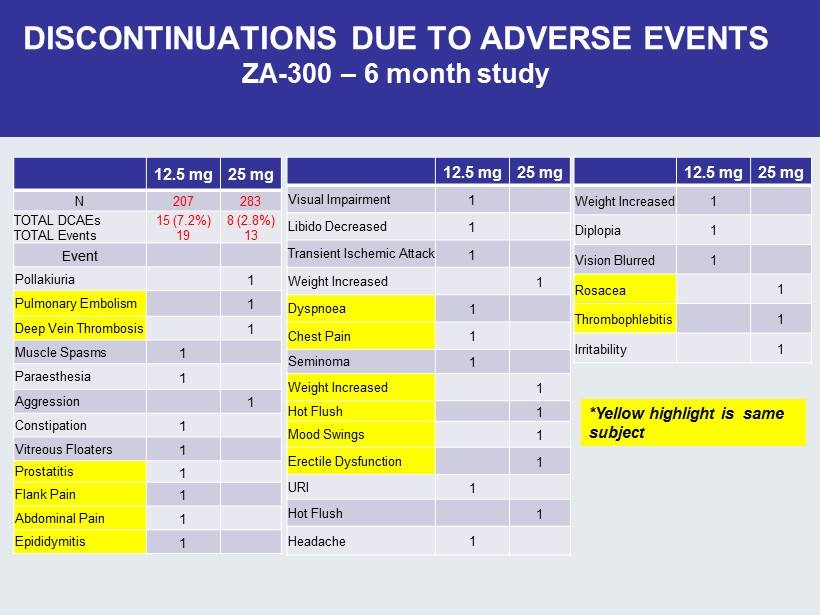
DISCONTINUATIONS DUE TO ADVERSE EVENTS ZA - 300 – 6 month study 12.5 mg 25 mg N 207 283 TOTAL DCAEs TOTAL Events 15 (7.2%) 19 8 (2.8%) 13 Event Pollakiuria 1 Pulmonary Embolism 1 Deep Vein Thrombosis 1 Muscle Spasms 1 Paraesthesia 1 Aggression 1 Constipation 1 Vitreous Floaters 1 Prostatitis 1 Flank Pain 1 Abdominal Pain 1 Epididymitis 1 12.5 mg 25 mg Visual Impairment 1 Libido Decreased 1 Transient Ischemic Attack 1 Weight Increased 1 Dyspnoea 1 Chest Pain 1 Seminoma 1 Weight Increased 1 Hot Flush 1 Mood Swings 1 Erectile Dysfunction 1 URI 1 Hot Flush 1 Headache 1 12.5 mg 25 mg Weight Increased 1 Diplopia 1 Vision Blurred 1 Rosacea 1 Thrombophlebitis 1 Irritability 1 *Yellow highlight is same subject
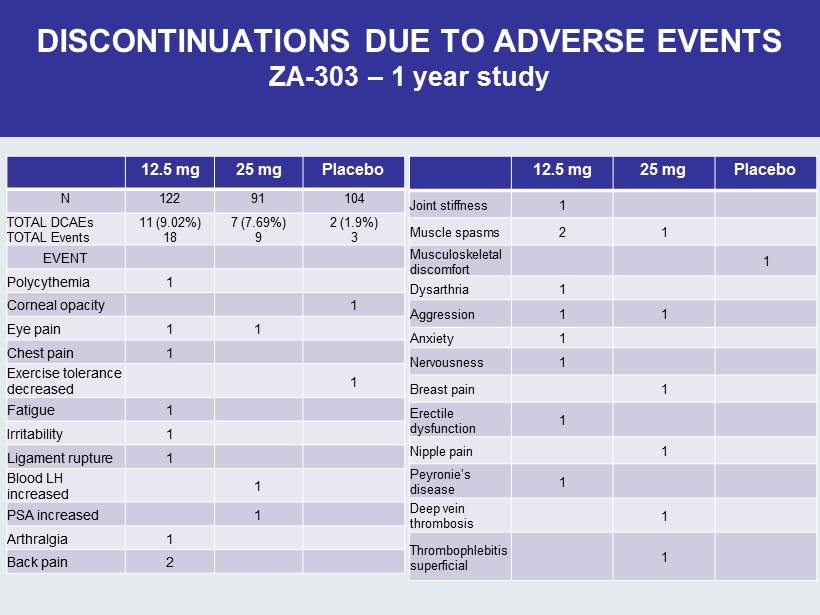
DISCONTINUATIONS DUE TO ADVERSE EVENTS ZA - 303 – 1 year study 12.5 mg 25 mg Placebo N 122 91 104 TOTAL DCAEs TOTAL Events 11 (9.02%) 18 7 (7.69%) 9 2 (1.9%) 3 EVENT Polycythemia 1 Corneal opacity 1 Eye pain 1 1 Chest pain 1 Exercise tolerance decreased 1 Fatigue 1 Irritability 1 Ligament rupture 1 Blood LH increased 1 PSA increased 1 Arthralgia 1 Back pain 2 12.5 mg 25 mg Placebo Joint stiffness 1 Muscle spasms 2 1 Musculoskeletal discomfort 1 Dysarthria 1 Aggression 1 1 Anxiety 1 Nervousness 1 Breast pain 1 Erectile dysfunction 1 Nipple pain 1 Peyronie’s disease 1 Deep vein thrombosis 1 Thrombophlebitis superficial 1
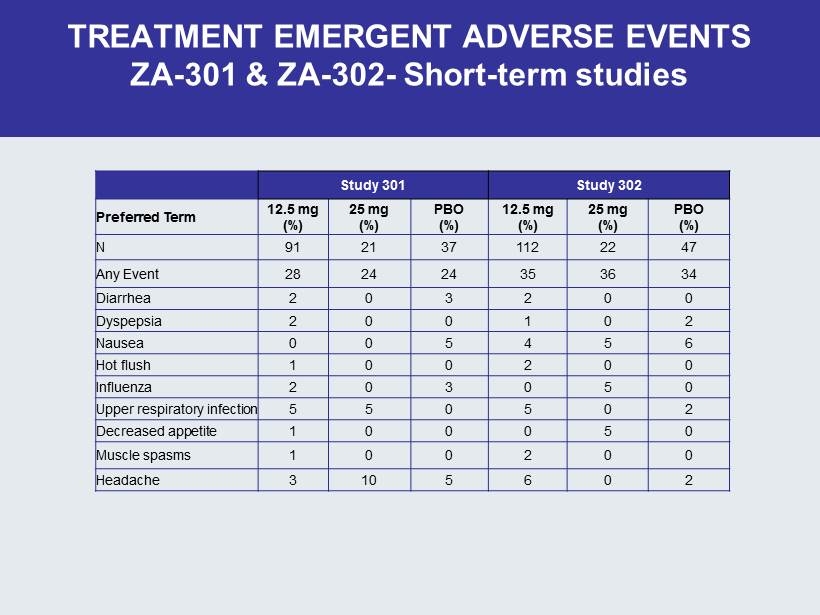
TREATMENT EMERGENT ADVERSE EVENTS ZA - 301 & ZA - 302 - Short - term studies Study 301 Study 302 Preferred Term 12.5 mg (%) 25 mg (%) PBO (%) 12.5 mg (%) 25 mg (%) PBO (%) N 91 21 37 112 22 47 Any Event 28 24 24 35 36 34 Diarrhea 2 0 3 2 0 0 Dyspepsia 2 0 0 1 0 2 Nausea 0 0 5 4 5 6 Hot flush 1 0 0 2 0 0 Influenza 2 0 3 0 5 0 Upper respiratory infection 5 5 0 5 0 2 Decreased appetite 1 0 0 0 5 0 Muscle spasms 1 0 0 2 0 0 Headache 3 10 5 6 0 2
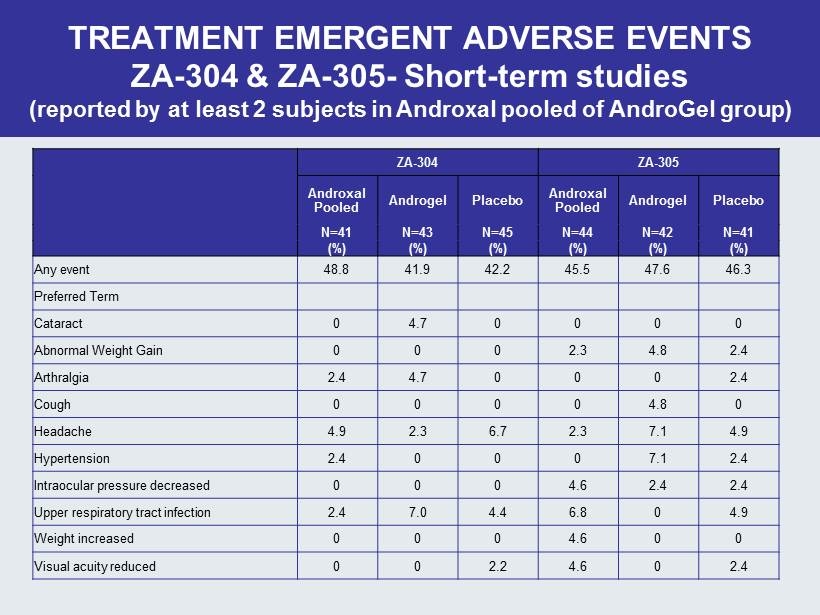
ZA - 304 ZA - 305 Androxal Pooled Androgel Placebo Androxal Pooled Androgel Placebo N=41 N=43 N=45 N=44 N=42 N=41 (%) (%) (%) (%) (%) (%) Any event 48.8 41.9 42.2 45.5 47.6 46.3 Preferred Term Cataract 0 4 .7 0 0 0 0 Abnormal Weight Gain 0 0 0 2.3 4.8 2.4 Arthralgia 2.4 4.7 0 0 0 2.4 Cough 0 0 0 0 4.8 0 Headache 4.9 2.3 6.7 2.3 7.1 4.9 Hypertension 2.4 0 0 0 7.1 2.4 Intraocular pressure decreased 0 0 0 4.6 2.4 2.4 Upper respiratory tract infection 2.4 7.0 4 .4 6.8 0 4.9 Weight increased 0 0 0 4.6 0 0 Visual acuity reduced 0 0 2.2 4.6 0 2.4 TREATMENT EMERGENT ADVERSE EVENTS ZA - 304 & ZA - 305 - Short - term studies (reported by at least 2 subjects in Androxal pooled of AndroGel group)
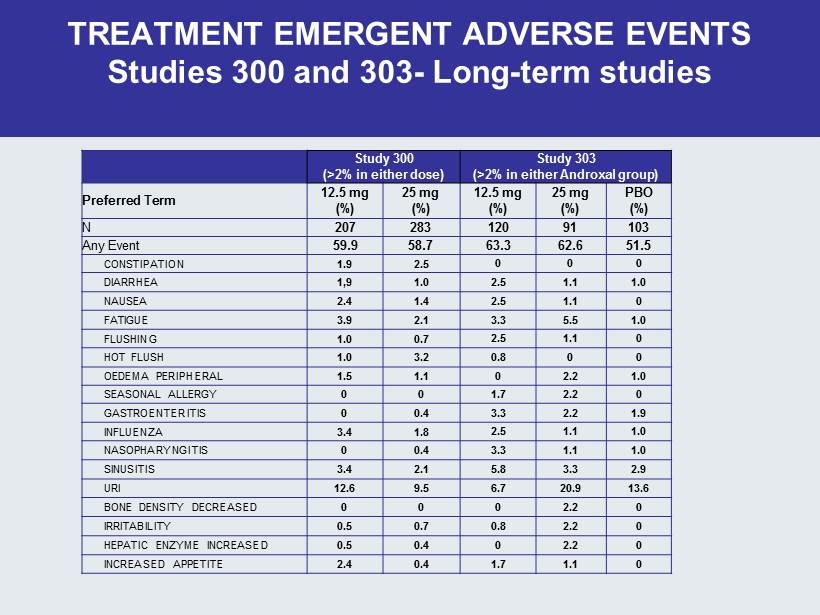
TREATMENT EMERGENT ADVERSE EVENTS Studies 300 and 303 - Long - term studies Study 300 (>2% in either dose) Study 303 (>2% in either Androxal group ) Preferred Term 12.5 mg (%) 25 mg (%) 12.5 mg (%) 25 mg (%) PBO (%) N 207 283 120 91 103 Any Event 59.9 58.7 63.3 62.6 51.5 CONSTIPATION 1.9 2.5 0 0 0 DIARRHEA 1,9 1.0 2.5 1.1 1.0 NAUSEA 2.4 1.4 2.5 1.1 0 FATIGUE 3.9 2.1 3.3 5.5 1.0 FLUSHING 1.0 0.7 2.5 1.1 0 HOT FLUSH 1.0 3.2 0.8 0 0 OEDEMA PERIPHERAL 1.5 1.1 0 2.2 1.0 SEASONAL ALLERGY 0 0 1.7 2.2 0 GASTROENTERITIS 0 0.4 3.3 2.2 1.9 INFLUENZA 3.4 1.8 2.5 1.1 1.0 NASOPHARYNGITIS 0 0.4 3.3 1.1 1.0 SINUSITIS 3.4 2.1 5.8 3.3 2.9 URI 12.6 9.5 6.7 20.9 13.6 BONE DENSITY DECREASED 0 0 0 2.2 0 IRRITABILITY 0.5 0.7 0.8 2.2 0 HEPATIC ENZYME INCREASED 0.5 0.4 0 2.2 0 INCREASED APPETITE 2.4 0.4 1.7 1.1 0
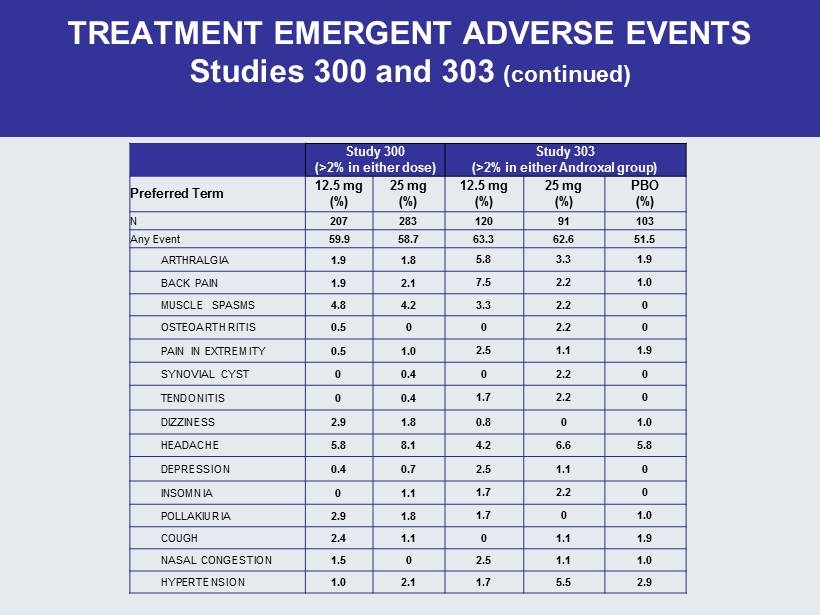
TREATMENT EMERGENT ADVERSE EVENTS Studies 300 and 303 (continued) Study 300 (>2% in either dose) Study 303 (>2% in either Androxal group ) Preferred Term 12.5 mg (%) 25 mg (%) 12.5 mg (%) 25 mg (%) PBO (%) N 207 283 120 91 103 Any Event 59.9 58.7 63.3 62.6 51.5 ARTHRALGIA 1.9 1.8 5.8 3.3 1.9 BACK PAIN 1.9 2.1 7.5 2.2 1.0 MUSCLE SPASMS 4.8 4.2 3.3 2.2 0 OSTEOARTHRITIS 0.5 0 0 2.2 0 PAIN IN EXTREMITY 0.5 1.0 2.5 1.1 1.9 SYNOVIAL CYST 0 0.4 0 2.2 0 TENDONITIS 0 0.4 1.7 2.2 0 DIZZINESS 2.9 1.8 0.8 0 1.0 HEADACHE 5.8 8.1 4.2 6.6 5.8 DEPRESSION 0.4 0.7 2.5 1.1 0 INSOMNIA 0 1.1 1.7 2.2 0 POLLAKIURIA 2.9 1.8 1.7 0 1.0 COUGH 2.4 1.1 0 1.1 1.9 NASAL CONGESTION 1.5 0 2.5 1.1 1.0 HYPERTENSION 1.0 2.1 1.7 5.5 2.9
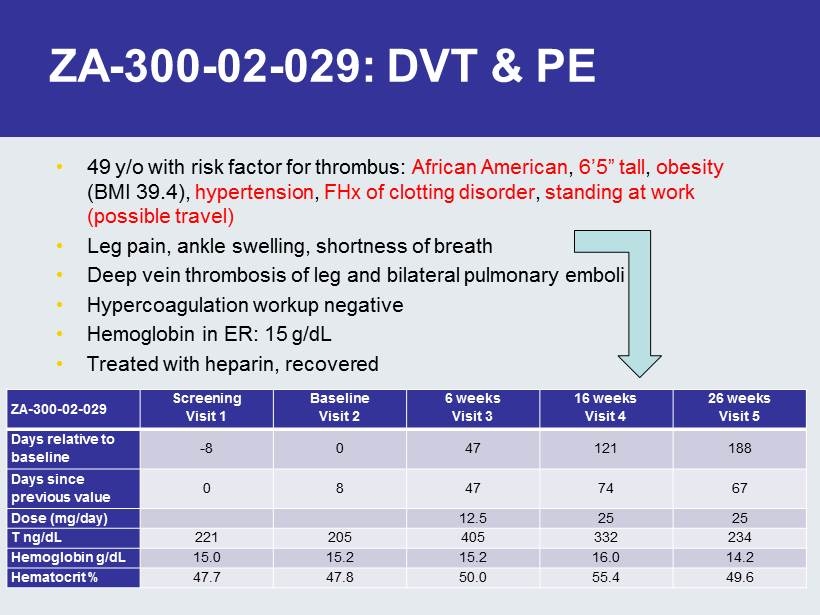
ZA - 300 - 02 - 029: DVT & PE • 49 y/o with risk factor for thrombus: African American , 6’5” tall , obesity (BMI 39.4), hypertension , FHx of clotting disorder , standing at work (possible travel) • Leg pain, ankle swelling, shortness of breath • Deep vein thrombosis of leg and bilateral pulmonary emboli • Hypercoagulation workup negative • Hemoglobin in ER: 15 g/ dL • Treated with heparin, recovered ZA - 300 - 02 - 029 Screening Visit 1 Baseline Visit 2 6 weeks Visit 3 16 weeks Visit 4 26 weeks Visit 5 Days relative to baseline - 8 0 47 121 188 Days since previous value 0 8 47 74 67 Dose (mg/day) 12.5 25 25 T ng/ dL 221 205 405 332 234 Hemoglobin g/dL 15.0 15.2 15.2 16.0 14.2 Hematocrit % 47.7 47.8 50.0 55.4 49.6
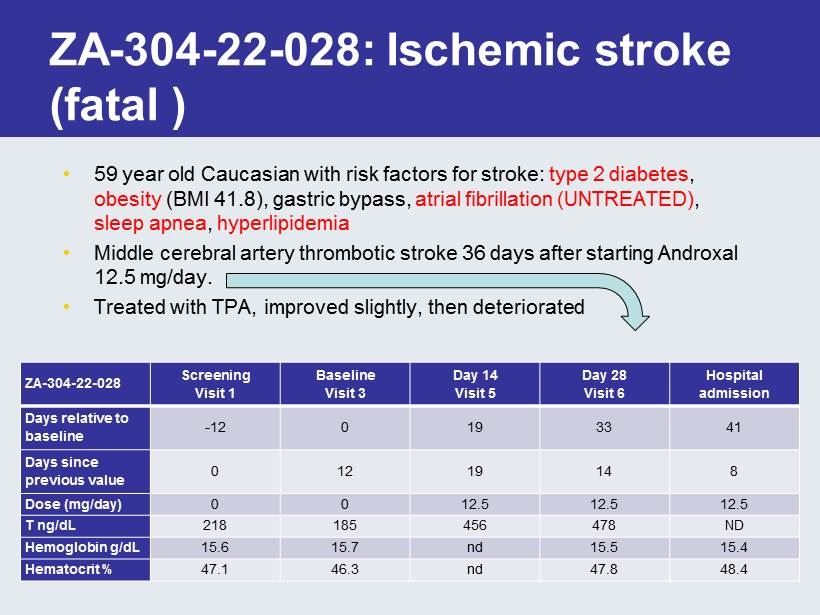
ZA - 304 - 22 - 028: Ischemic stroke (fatal ) • 59 year old Caucasian with risk factors for stroke: type 2 diabetes , obesity (BMI 41.8), gastric bypass, atrial fibrillation (UNTREATED) , sleep apnea , hyperlipidemia • Middle cerebral artery thrombotic stroke 36 days after starting Androxal 12.5 mg/day. • Treated with TPA, improved slightly, then deteriorated ZA - 304 - 22 - 028 Screening Visit 1 Baseline Visit 3 Day 14 Visit 5 Day 28 Visit 6 Hospital admission Days relative to baseline - 12 0 19 33 41 Days since previous value 0 12 19 14 8 Dose (mg/day) 0 0 12.5 12.5 12.5 T ng/ dL 218 185 456 478 ND Hemoglobin g/ dL 15.6 15.7 nd 15.5 15.4 Hematocrit % 47.1 46.3 nd 47.8 48.4

FDA Broadens Warning About Venous Blood Clots (19 June 2014) “The risk of venous blood clots is already included in the labeling of testosterone products as a possible consequence of polycythemia, an abnormal increase in the number of red blood cells that sometimes occurs with testosterone treatment . Because there have been postmarket reports of venous blood clots unrelated to polycythemia , FDA is requiring a change to drug labeling of all testosterone products to provide a more general warning regarding venous blood clots and to ensure this risk is described consistently in the labeling of all approved testosterone products . ”

PSA • PSA elevation is NOT an Adverse Drug Reaction • PSA elevation is an expected result of testosterone increase • PSA is not a cancer marker but a normal enzyme • Elevated PSA included in package insert for Axiron • Incidence and magnitude noted in Androxal studies is not alarming and has been reviewed by a panel of experts

OTHER SAFETY ISSUES RELATED TO TESTOSTERONE THERAPY • Cataracts – No evidence of cataract formation or progression in Androxal groups • Osteoporosis – Data suggests that Androxal subjects are no worse than placebo subjects – ZA - 303 found no deleterious bone effects in subjects with over 1 year of exposure
CONCLUSIONS • Androxal is well tolerated • Findings are consistent with known pharmacology • Thrombotic events (with or without Hct increase) are an established risk (similar to topical testosterone) • No unexpected safety issues

Preview of Commercial Findings Key Commercial Conclusions ▪ Payers and providers recognize room for improvement with current treatment options and are becoming more aware of the downsides of TRTs. ▪ Additional education will be important to inform physicians and payers as to the issues associated with testosterone replacement therapy. ▪ Both payers and providers reacted very positively to the Androxal profile and believe it would make a nice addition to the treatment of secondary hypogonadism and replace TRT use in many cases. ▪ Providers would expect to use it as a first line agent and would anticipate writing it for a majority of their new secondary hypogonadal patients. ▪ Payers would expect Androxal to be priced in line with other TRTs and would cover it at that price, with preferred positioning possible depending on contracting. 101

Q & A

Summary of Provider and Payer Perspectives Regarding Androxal October 31, 2014

Research Background Key Topics Quintiles conducted interviews with 30 physicians and 20 payers covering these key topics. • Role in managing patient treatment for primary and secondary hypogonadism • Understanding and perceptions of existing therapies and developments • Satisfaction with existing therapies for Secondary Hypogonadism • Unmet needs in current market Primary and Secondary Hypogonadism Treatment/Management • Impact of managed care in hypogonadism market • Awareness of new therapies for managing secondary hypogonadism • Relative importance of value drivers in prescribing behavior Value Drivers for Prescribing Treatment • Overall impressions of Androxal (Product X) • Potential to integrate Product X into hypogonadism treatment regimen • Ability for Product X to satisfy currently unmet needs in current market • Impact of managed care on Product X utilization Response to Androxal Product Profile • Credibility of value proposition to physicians and payers • Impact of value proposition on management and prescribing behavior • Overall attractiveness of Product X to physicians and payers • Critical Repros activities to maximize Product X uptake Response to Androxal Value Proposition 104

Providers 105
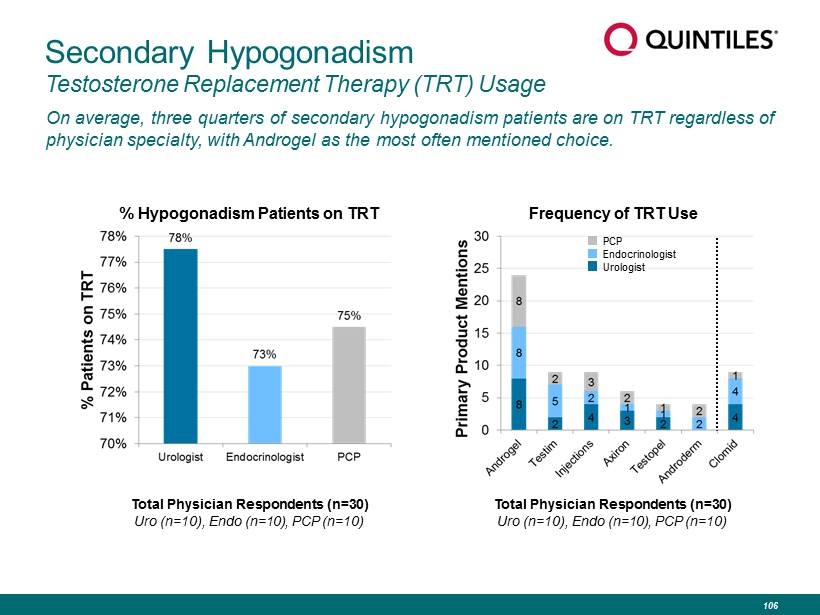
Secondary Hypogonadism Testosterone Replacement Therapy (TRT) Usage On average, three quarters of secondary hypogonadism patients are on TRT regardless of physician specialty, with Androgel as the most often mentioned choice. Total Physician Respondents (n=30) Uro (n=10), Endo (n=10), PCP (n=10) Frequency of TRT Use % Hypogonadism Patients on TRT PCP Endocrinologist Urologist Total Physician Respondents (n=30) Uro (n=10), Endo (n=10), PCP (n=10) 106
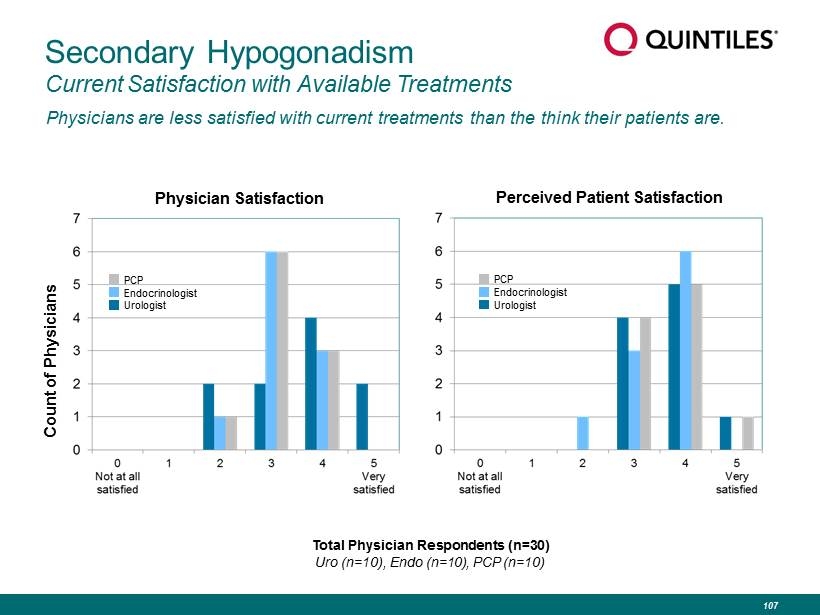
Secondary Hypogonadism Current Satisfaction with Available Treatments Physicians are less satisfied with current treatments than the think their patients are. Physician Satisfaction Perceived Patient Satisfaction Count of Physicians Total Physician Respondents (n=30) Uro (n=10), Endo (n=10), PCP (n=10) PCP Endocrinologist Urologist PCP Endocrinologist Urologist 107
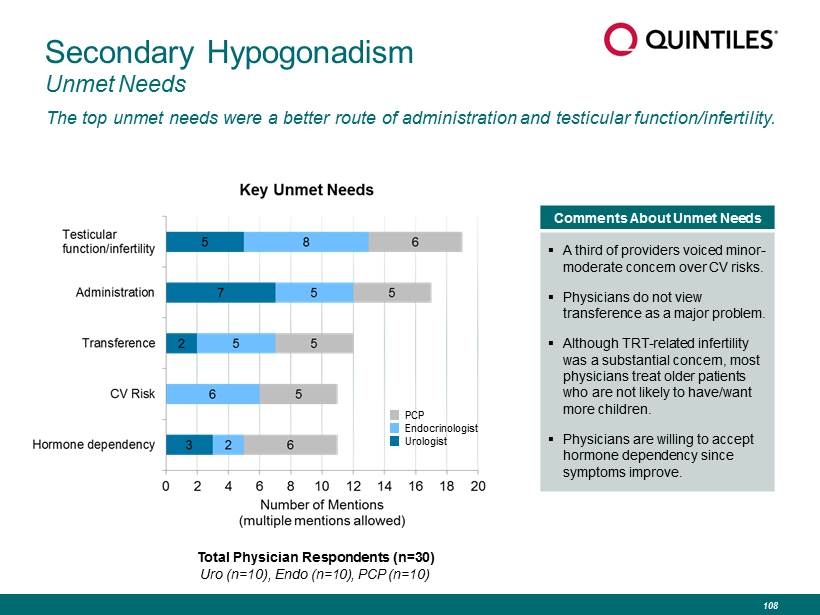
Secondary Hypogonadism Unmet Needs The top unmet needs were a better route of administration and testicular function/infertility. Comments About Unmet Needs ▪ A third of providers voiced minor - moderate concern over CV risks. ▪ Physicians do not view transference as a major problem. ▪ Although TRT - related infertility was a substantial concern, most physicians treat older patients who are not likely to have/want more children. ▪ Physicians are willing to accept hormone dependency since symptoms improve. PCP Endocrinologist Urologist Total Physician Respondents (n=30) Uro (n=10), Endo (n=10), PCP (n=10) 108
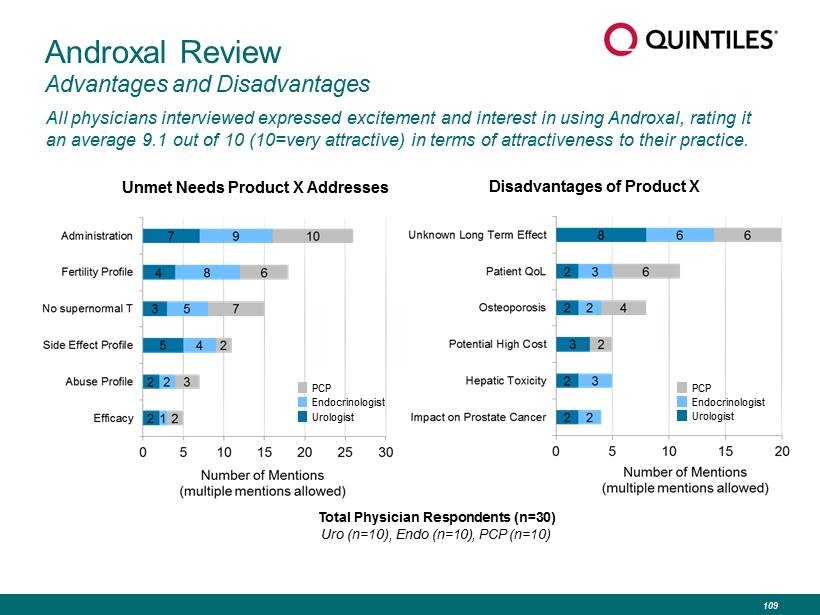
Androxal Review Advantages and Disadvantages All physicians interviewed expressed excitement and interest in using Androxal, rating it an average 9.1 out of 10 (10=very attractive) in terms of attractiveness to their practice. Unmet Needs Product X Addresses PCP Endocrinologist Urologist Total Physician Respondents (n=30) Uro (n=10), Endo (n=10), PCP (n=10) Disadvantages of Product X PCP Endocrinologist Urologist 109
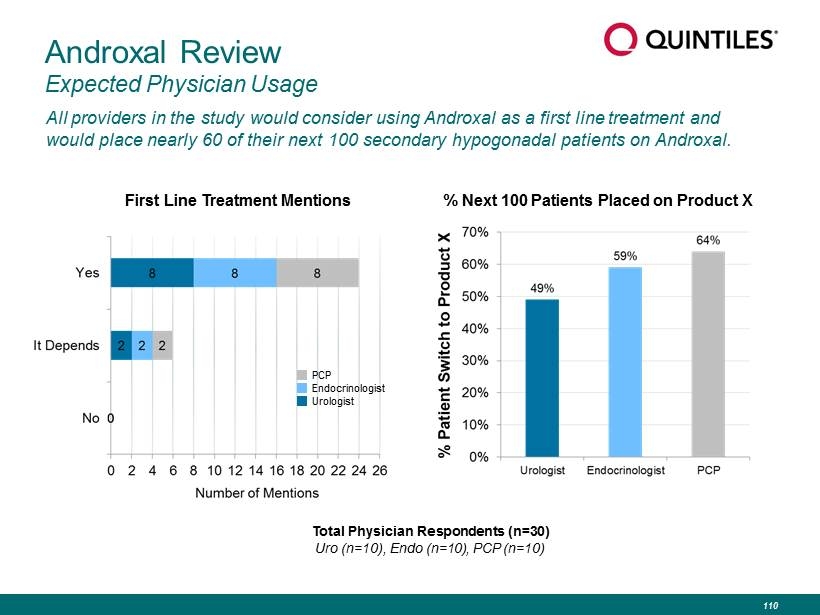
Androxal Review Expected Physician Usage All providers in the study would consider using Androxal as a first line treatment and would place nearly 60 of their next 100 secondary hypogonadal patients on Androxal. First Line Treatment Mentions Total Physician Respondents (n=30) Uro (n=10), Endo (n=10), PCP (n=10) PCP Endocrinologist Urologist % Next 100 Patients Placed on Product X 110

Payers 111
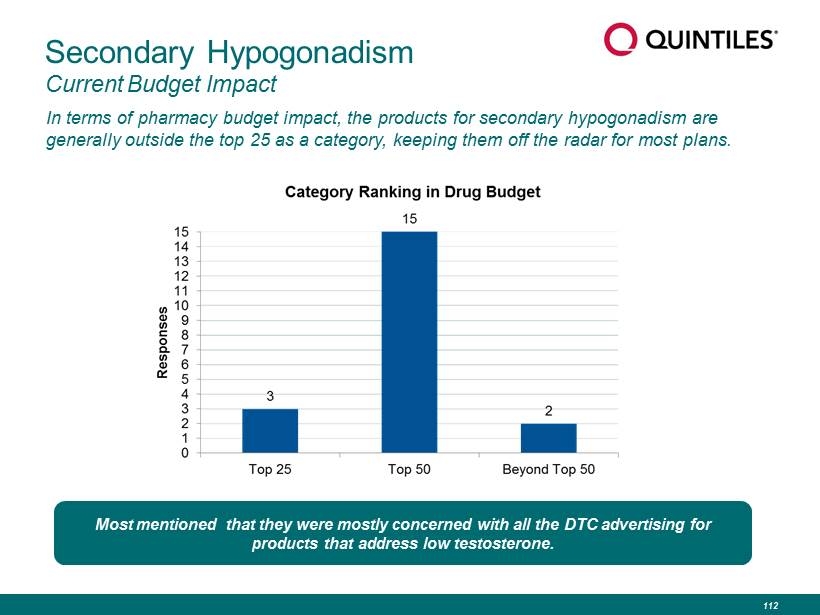
Secondary Hypogonadism Current Budget Impact In terms of pharmacy budget impact, the products for secondary hypogonadism are generally outside the top 25 as a category, keeping them off the radar for most plans. Most mentioned that they were mostly concerned with all the DTC advertising for products that address low testosterone. 112
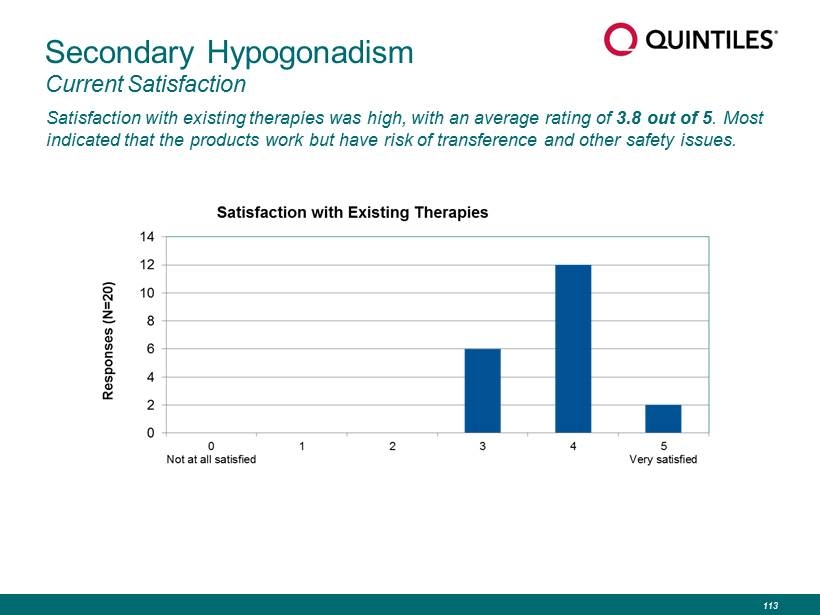
Secondary Hypogonadism Current Satisfaction Satisfaction with existing therapies was high, with an average rating of 3.8 out of 5 . Most indicated that the products work but have risk of transference and other safety issues. 113
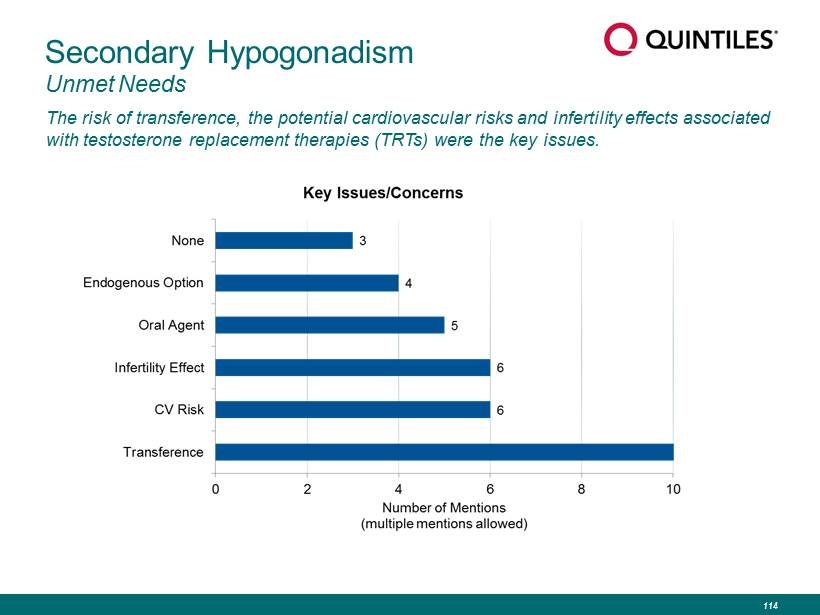
Secondary Hypogonadism Unmet Needs The risk of transference, the potential cardiovascular risks and infertility effects associated with testosterone replacement therapies (TRTs) were the key issues. 114
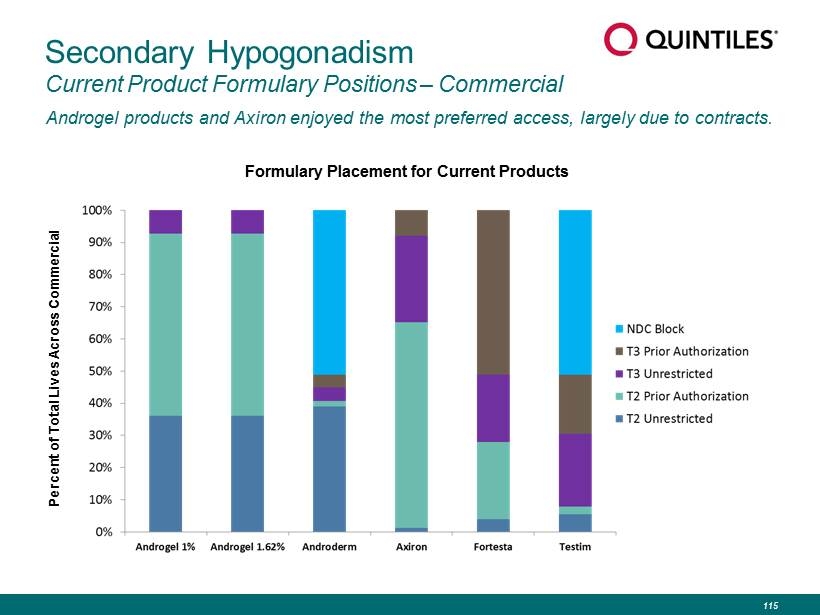
Secondary Hypogonadism Current Product Formulary Positions – Commercial Androgel products and Axiron enjoyed the most preferred access, largely due to contracts. Percent of Total Lives Across Commercial Formulary Placement for Current Products 115
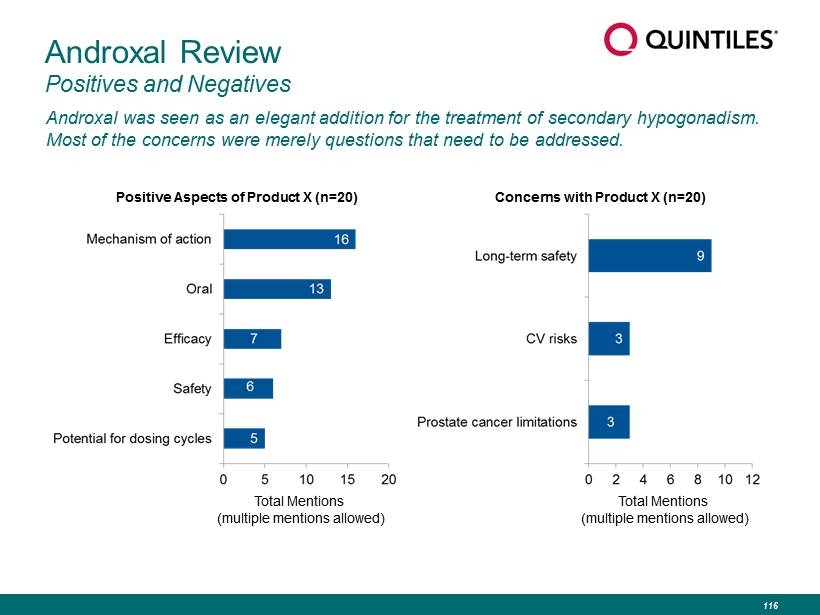
Androxal Review Positives and Negatives Androxal was seen as an elegant addition for the treatment of secondary hypogonadism. Most of the concerns were merely questions that need to be addressed. Total Mentions (multiple mentions allowed) Positive Aspects of Product X (n=20) Total Mentions (multiple mentions allowed) Concerns with Product X (n=20) 116
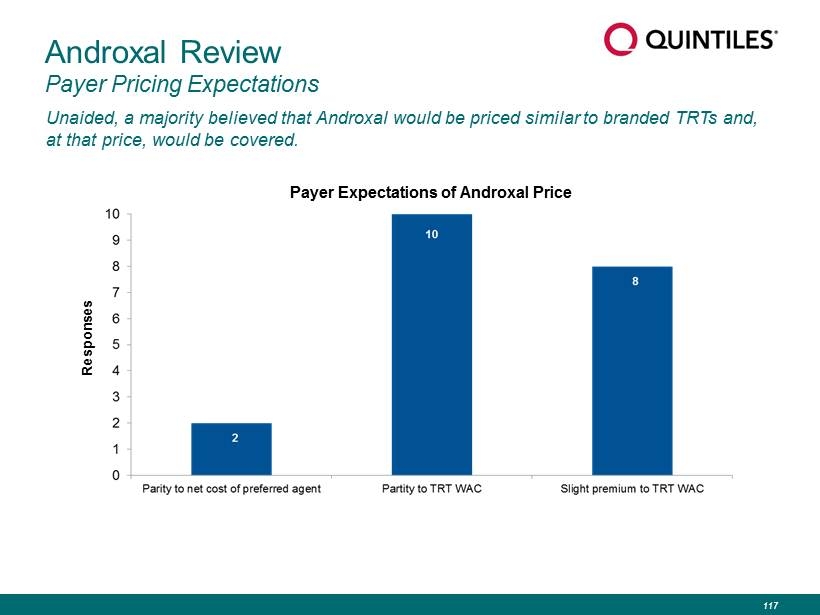
Unaided, a majority believed that Androxal would be priced similar to branded TRTs and, at that price, would be covered. Androxal Review Payer Pricing Expectations Responses Payer Expectations of Androxal Price 117

Conclusions Key Commercial Conclusions ▪ Payers and providers recognize room for improvement with current treatment options and are becoming more aware of the downsides of TRTs. ▪ Additional education will be important to inform physicians and payers as to the issues associated with testosterone replacement therapy. ▪ Both payers and providers reacted very positively to the Androxal profile and believe it would make a nice addition to the treatment of secondary hypogonadism and replace TRT use in many cases. ▪ Providers would expect to use it as a first line agent and would anticipate writing it for a majority of their new secondary hypogonadal patients. ▪ Payers would expect Androxal to be priced in line with other TRTs and would cover it at that price, with preferred positioning possible depending on contracting. 118

Thank you For further information and review of this presentation, please visit www.reprosrx.com