Exhibit 99.1

Androxal ® A Mechanistically Consistent Approach to the Treatment of Secondary Hypogonadism

Repros Disclaimer Any statements made by the Company that are not historical facts contained in these slides (or in any oral accompanying discussion) are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and are subject to various risks, uncertainties and other factors that could cause the Company’s actual results, performance or achievements to differ materially from those expressed or implied by such forward - looking statements. These statements often include words such as “may,” “will,” “expect,” “anticipate,” “continue,” “estimate,” “project,” “potential,” “intend,” “belie ve, ” “plan,” “seek,” “could,” “can,” “should” or similar expressions. These statements are based on assumptions that the Company has made in light of the Company’s experience in the industry, as well as the Company’s perceptions of historical trends, current cond iti ons, expected future developments and other factors the Company believes are appropriate in these circumstances. Forward - looking statements include, but are not limited to, those relating to anticipated milestones for Androxal® and Proellex®, the conduct of planned clinical studies and the timing and nature of the results thereof, the markets for the Company’s products and the pot ent ial success of the Company in penetrating those markets and that the Company’s need for and use of financial resources. Such statements are based on current expectations that involve a number of known and unknown risks, uncertainties and other factor s that may cause actual events to be materially different from those expressed or implied by such forward - looking statements, including the ability to raise additional needed capital on a timely basis in order for it to continue to fund development of it s Androxal® and Proellex® programs, the ability to have success in the clinical development of its technologies, the reliabilit y o f interim results to predict final study outcomes, and such other risks as are identified in the Company's most recent Annual R epo rt on Form 10 - K and the subsequent quarterly report on Form 10 - Q and in the prospectus supplement and the accompanying prospectus included in the registration statement mentioned below. These documents are available on request from Repros or at www.sec.gov. Repros disclaims any intention or obligation to update or revise any forward - looking statements, whether as a resul t of new information, future events or otherwise. In this presentation, we rely on and refer to information and statistics regarding the pharmaceutical industry. We obtained t his information and these statistics from third - party sources, which we have supplemented where necessary with information from publicly available sources and our own internal estimates. Industry publications and surveys generally state that they have obtained information from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information. While we believe that each of these studies and publications is reliable, we have not independently verified suc h d ata, and we make no any representation as to the accuracy of such information. Similarly, we believe our internal research is reli abl e, but it has not been verified by any independent sources.
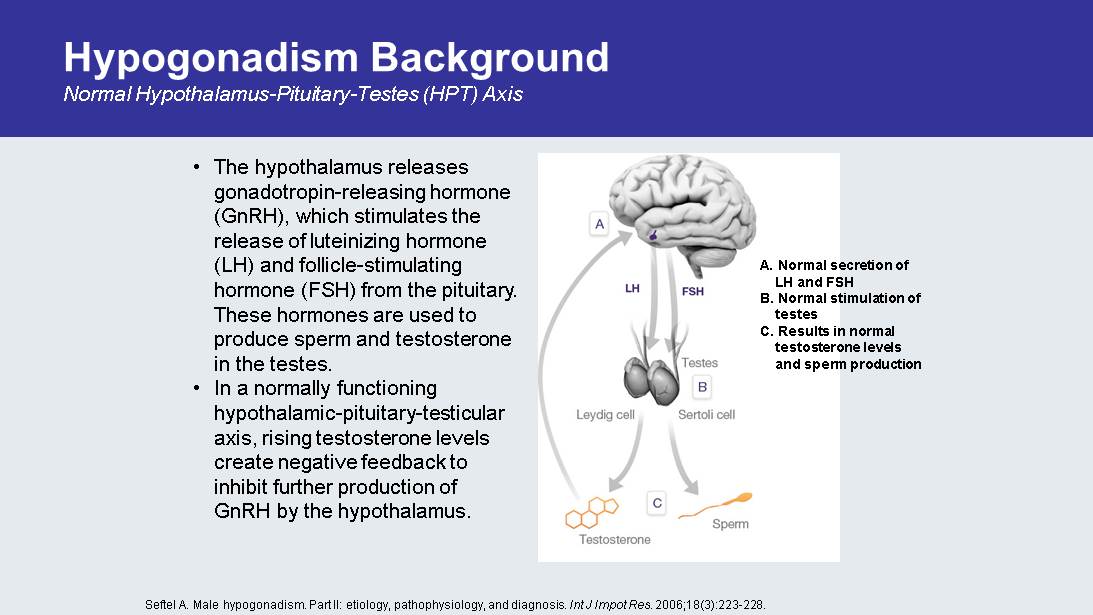
Hypogonadism Background Normal Hypothalamus - Pituitary - Testes (HPT) Axis A. Normal secretion of LH and FSH B. Normal stimulation of testes C. Results in normal testosterone levels and sperm production Seftel A. Male hypogonadism . Part II: etiology, pathophysiology , and diagnosis. Int J Impot Res . 2006;18(3):223 - 228. • The hypothalamus releases gonadotropin - releasing hormone ( GnRH ), which stimulates the release of luteinizing hormone (LH) and follicle - stimulating hormone (FSH) from the pituitary. These hormones are used to produce sperm and testosterone in the testes. • In a normally functioning hypothalamic - pituitary - testicular axis, rising testosterone levels create negative feedback to inhibit further production of GnRH by the hypothalamus.

Hypogonadism Background • Hypogonadism is a condition in which a male is unable to produce sufficient testosterone (T) . • A man is considered to be clinically hypogonadal if his morning testosterone is < 300 ng / dL (normal range 300 - 1040 ng / dL ). • In a recent study, hypogonadism was estimated to affect up to 13.8 million men in the United States . Another 17 million may be compromised due to obesity • There are two types of hypogonadism , primary and secondary . Overview

Hypogonadism Background • The less frequent type is primary , which is typically a result of a genetic defect, trauma or viral infections such as mumps. • This type of disorder is easily diagnosed by assessing both testosterone and the pituitary hormone that drives testicular synthesis of testosterone, luteinizing hormone (LH) . – LH pulses during the day, and in men with primary testicular failure, these pulses yield much higher levels of the hormone (>20 mIU /mL) compared to the normal range of 4 - 15 mIU / mL. Primary Hypogonadism

Hypogonadism Background • The more common dysfunction is secondary hypogonadism (85 - 95% of hypogonadal men). • This dysfunction is characterized by low testosterone and low/low normal LH and is a result of the pituitary not responding to the low T state by secreting more LH to drive T synthesis. • Historically, many believed this disorder was primarily age - related but growing evidence points to obesity playing a major role in this condition . – Obese men are estrogenized and estrogen provides negative feedback on the hypothalamic - pituitary axis suppressing the ability of the pituitary to sense the low T state and respond appropriately. Secondary Hypogonadism

Primary vs Secondary Hypogonadism Guay A et al. Int J Impot Res 22:9 - 19, 2011 . 0 10 20 30 40 50 <40 40-60 >60 Percentage Primary Secondary age groups (years) “The prevalence of secondary hypogonadism is far greater than that of primary hypogonadism .”
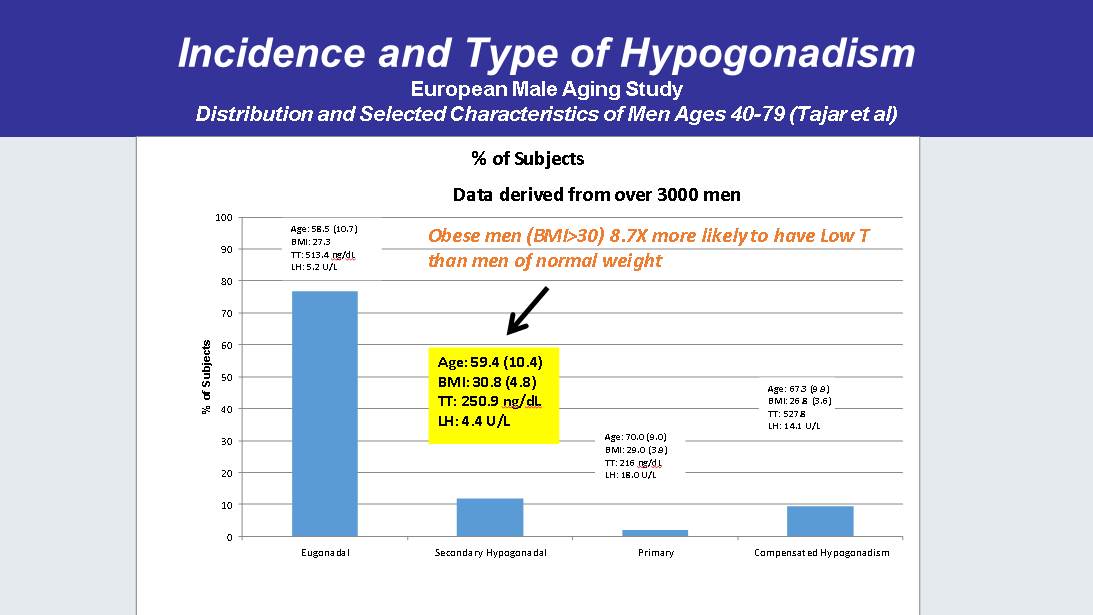
Incidence and Type of Hypogonadism European Male Aging Study Distribution and Selected Characteristics of Men Ages 40 - 79 ( Tajar et al) 0 10 20 30 40 50 60 70 80 90 100 Eugonadal Secondary Hypogonadal Primary Compensated Hypogonadism % of Subjects % of Subjects Age: 58.5 (10.7) BMI: 27.3 TT: 513.4 ng / dL LH: 5.2 U/L Age: 59.4 (10.4) BMI: 30.8 (4.8) TT: 250.9 ng / dL LH: 4.4 U/L Age: 70.0 (9.0) BMI: 29.0 (3.9) TT: 216 ng / dL LH: 18.0 U/L Age: 67.3 (9.9) BMI: 26.8 (3.6) TT: 527.8 LH: 14.1 U/L Data derived from over 3000 men Obese men (BMI>30) 8.7X more likely to have Low T than men of normal weight

• A man’s weight affects his T level more than his age. • Obese men, at even a young age, experience obesity - related Low T. • Weight loss significantly improves T levels in the secondary hypogonadal male. • The secondary hypogonadal male has functional but under - stimulated testes due to obesity caused by the negative feedback of estrogen. 40 - 44 45 - 49 50 - 54 55 - 59 60 - 64 65 - 69 70 - 74 75 - 79 Age Hypogonadism Background Weight Effect on T Production in the Secondary Hypogonadal Male Late onset hypogonadism : current concepts and controversies of pathogenesis, diagnosis and treatment, Ilpo Huhtaniemi , AJA (2014) 16, 192 - 202

BMI Distribution in Study of Secondary Hypogonadism , Repros : ZA - 003 (% of Subjects) 0 10 20 30 40 50 60 70 80 90 100 Underweight <18.5 Normal 18.5-24.9 Overweight 25-29.9 Obese>30 Weight Classification of Subjects Enrolled in ZA - 003 (n=204) Screening Criteria: T<300 ng/dL

The Average American Male at All Ages Was Overweight in 2000 Overweight BMI > 25 (6’ 190# male BMI=25.8) Obese BMI > 30 (6’ 230# male BMI =31.2) In 2010 there were ~90 million men in the US between the ages of 20 and 65 32% are obese

Hypogonadism Background Secondary Hypogonadism • Decreased secretion of LH (and FSH), caused by an abnormality in the anterior pituitary, leads to under stimulation of potentially normal testes. • When secondary hypogonadism is caused by hypothalamic dysfunction, secretion of gonadotropin - releasing hormone ( GnRH ) is reduced, causing reduced LH (and FSH) secretion and, ultimately, low testosterone. • Applying exogenous T creates negative feedback, thus worsening the condition. A. Decreased secretion of LH (and FSH) caused by an abnormality in the anterior pituitary B. Under stimulation of potentially normal testes C. Results in low testosterone D. Remarkably secondary hypogonadal men are generally fertile E. Exogenous T suppresses LH and FSH severely Seftel A. Male hypogonadism . Part II: etiology, pathophysiology , and diagnosis. Int J Impot Res . 2006;18(3):223 - 228. 2. Bhasin S, Cunningham GR, Hayes FJ, et al; and Endocrine Society Task Force. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice gu ide line. J Clin Endocrinol Metab . 2010;95:2536 - 2559 . Exogenous T creates negative feedback
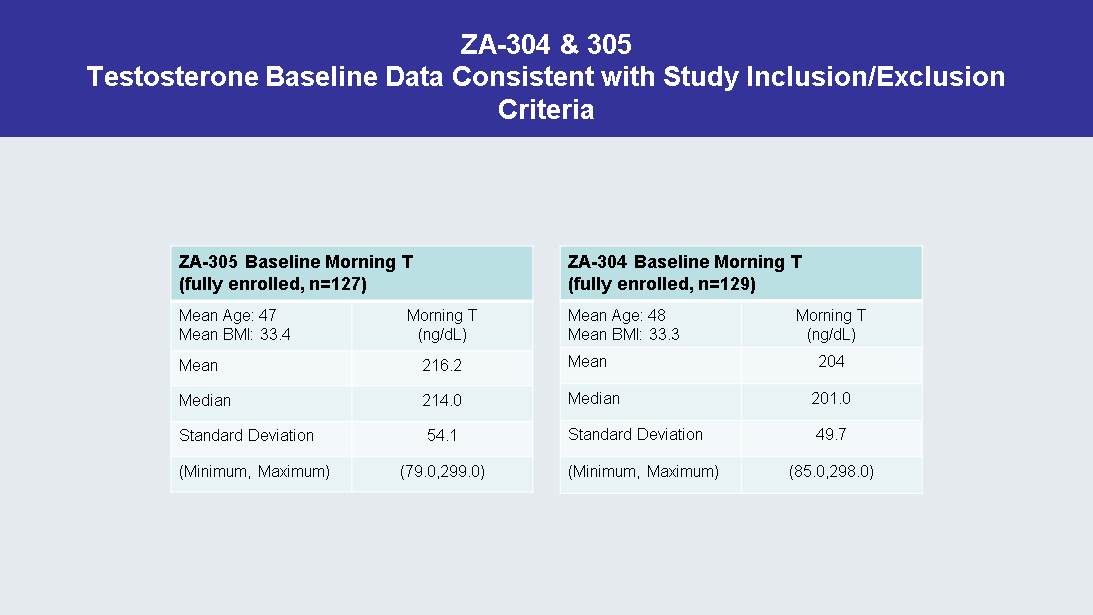
ZA - 304 & 305 Testosterone Baseline Data Consistent with Study Inclusion/Exclusion Criteria ZA - 305 Baseline Morning T (fully enrolled, n=127) Mean Age: 47 Mean BMI: 33.4 Morning T (ng/ dL ) Mean 216.2 Median 214.0 Standard Deviation 54.1 (Minimum, Maximum) (79.0,299.0) ZA - 304 Baseline Morning T (fully enrolled, n=129) Mean Age: 48 Mean BMI: 33.3 Morning T (ng/ dL ) Mean 204 Median 201.0 Standard Deviation 49.7 (Minimum, Maximum) (85.0,298.0)
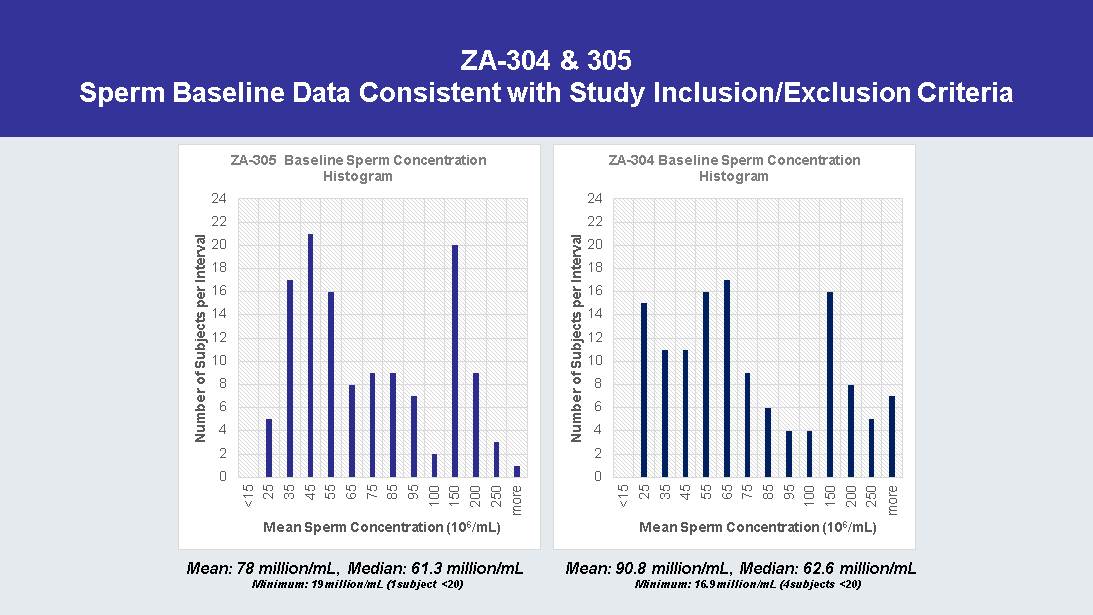
ZA - 304 & 305 Sperm Baseline Data Consistent with Study Inclusion/Exclusion Criteria 0 2 4 6 8 10 12 14 16 18 20 22 24 <15 25 35 45 55 65 75 85 95 100 150 200 250 more Number of Subjects per Interval Mean Sperm Concentration (10 6 /mL) ZA - 305 Baseline Sperm Concentration Histogram 0 2 4 6 8 10 12 14 16 18 20 22 24 <15 25 35 45 55 65 75 85 95 100 150 200 250 more Number of Subjects per Interval Mean Sperm Concentration (10 6 /mL) ZA - 304 Baseline Sperm Concentration Histogram Mean: 78 million/mL, Median: 61.3 million/mL Minimum: 19 million/mL (1 subject <20) Mean: 90.8 million/mL, Median: 62.6 million/mL Minimum: 16.9 million/mL (4 subjects <20)
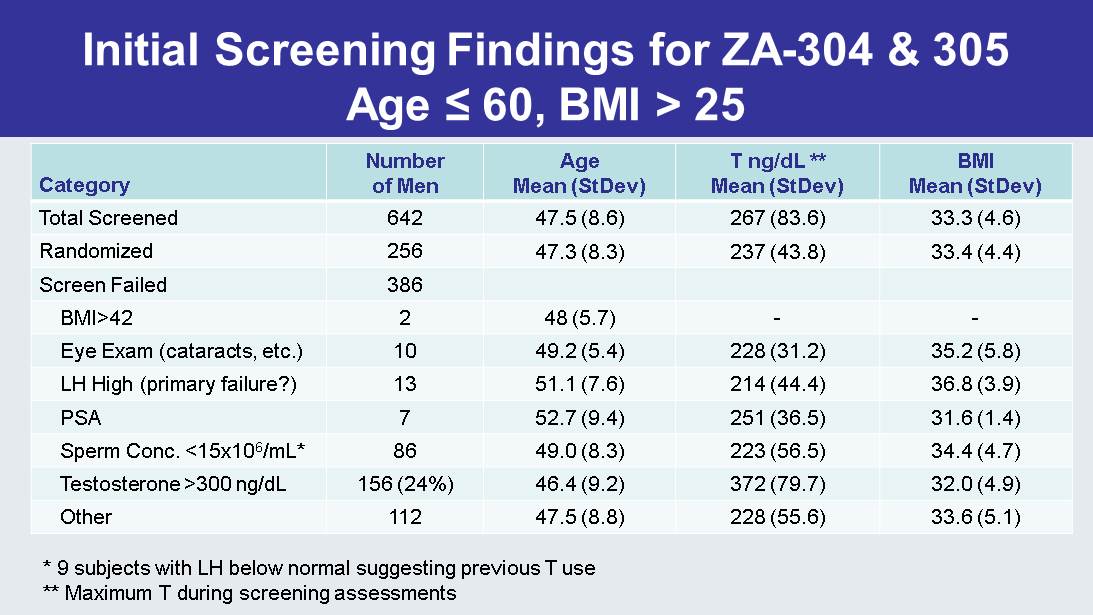
Initial Screening Findings for ZA - 304 & 305 Age ≤ 60, BMI > 25 Category Number of Men Age Mean ( StDev ) T ng/ dL ** Mean ( StDev ) BMI Mean ( StDev ) Total Screened 642 47.5 (8.6) 267 (83.6) 33.3 (4.6) Randomized 256 47.3 (8.3) 237 (43.8) 33.4 (4.4) Screen Failed 386 BMI>42 2 48 (5.7) - - Eye Exam (cataracts, etc.) 10 49.2 (5.4) 228 (31.2) 35.2 (5.8) LH High (primary failure?) 13 51.1 (7.6) 214 (44.4) 36.8 (3.9) PSA 7 52.7 (9.4) 251 (36.5) 31.6 (1.4) Sperm Conc. <15x10 6 /mL* 86 49.0 (8.3) 223 (56.5) 34.4 (4.7) Testosterone >300 ng/ dL 156 (24%) 46.4 (9.2) 372 (79.7) 32.0 (4.9) Other 112 47.5 (8.8) 228 (55.6) 33.6 (5.1) * 9 subjects with LH below normal suggesting previous T use ** Maximum T during screening assessments
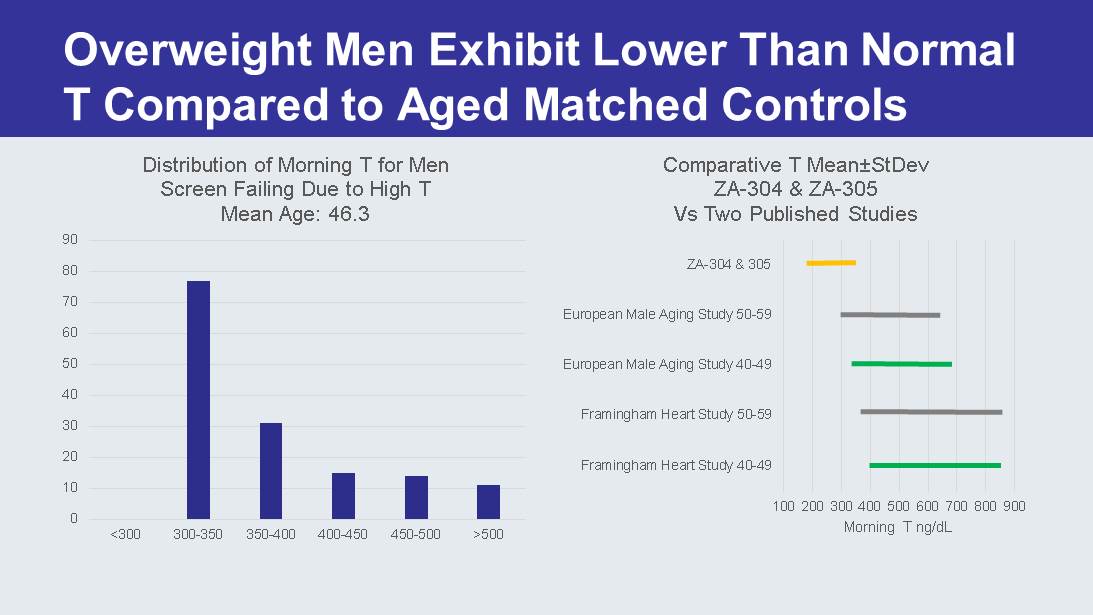
Overweight Men Exhibit Lower Than Normal T Compared to Aged Matched Controls 0 10 20 30 40 50 60 70 80 90 <300 300-350 350-400 400-450 450-500 >500 Distribution of Morning T for Men Screen Failing Due to High T Mean Age: 46.3 100 200 300 400 500 600 700 800 900 Framingham Heart Study 40-49 Framingham Heart Study 50-59 European Male Aging Study 40-49 European Male Aging Study 50-59 ZA-304 & 305 Morning T ng/ dL Comparative T Mean ± StDev ZA - 304 & ZA - 305 Vs Two Published Studies
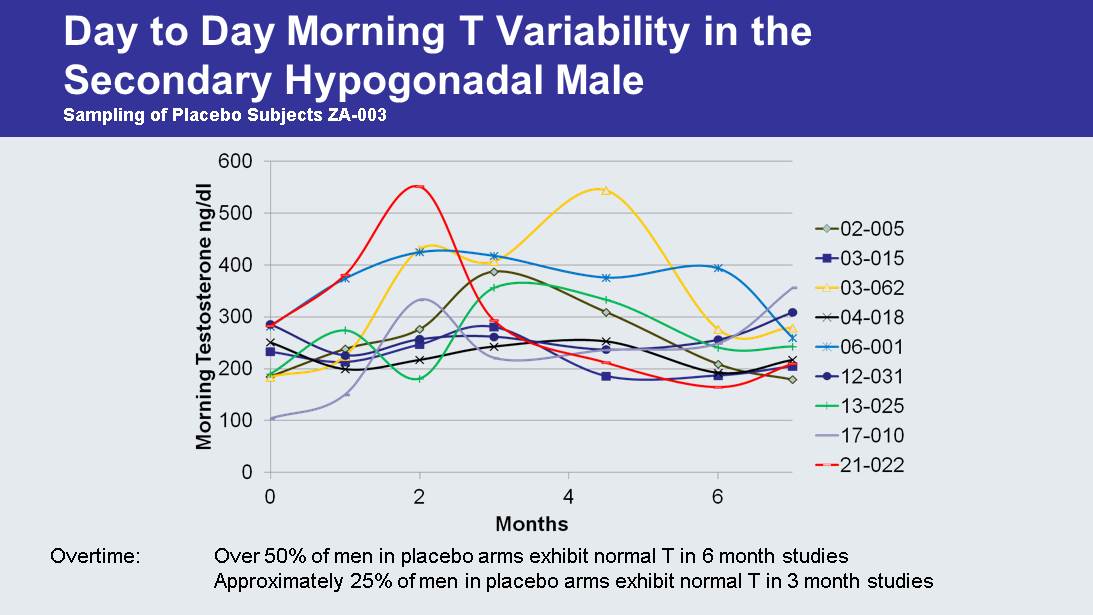
Day to Day Morning T Variability in the Secondary Hypogonadal Male Sampling of Placebo Subjects ZA - 003 Overtime: Over 50% of men in placebo arms exhibit normal T in 6 month studies Approximately 25% of men in placebo arms exhibit normal T in 3 month studies

So Why Not Just Give Secondary Hypogonadal Men Testosterone? • Unlike primary testicular failure, secondary hypogonadal men still have functional testes but their pituitary does not secrete appropriate levels of hormones to stimulate testicular function • Over time these men exhibit “ normal ” T unlike primary failures • The secondary hypogonadal male is generally still fertile • Hormone replacement worsens the underlying disorder by suppressing pituitary function to below normal levels and in over 30% of men completely shuts down the HPT axis • Secondary hypogonadism is reversible • Primary causes – Obesity – Stress or illness – Medication • There are no long - term studies regarding the effects of suppression of the Hypothalamic - Pituitary - Testes axis via hormone replacement in the secondary hypogonadal male • Does indiscriminate use of testosterone in the treatment of the largest cause of low T result in hormone dependence?
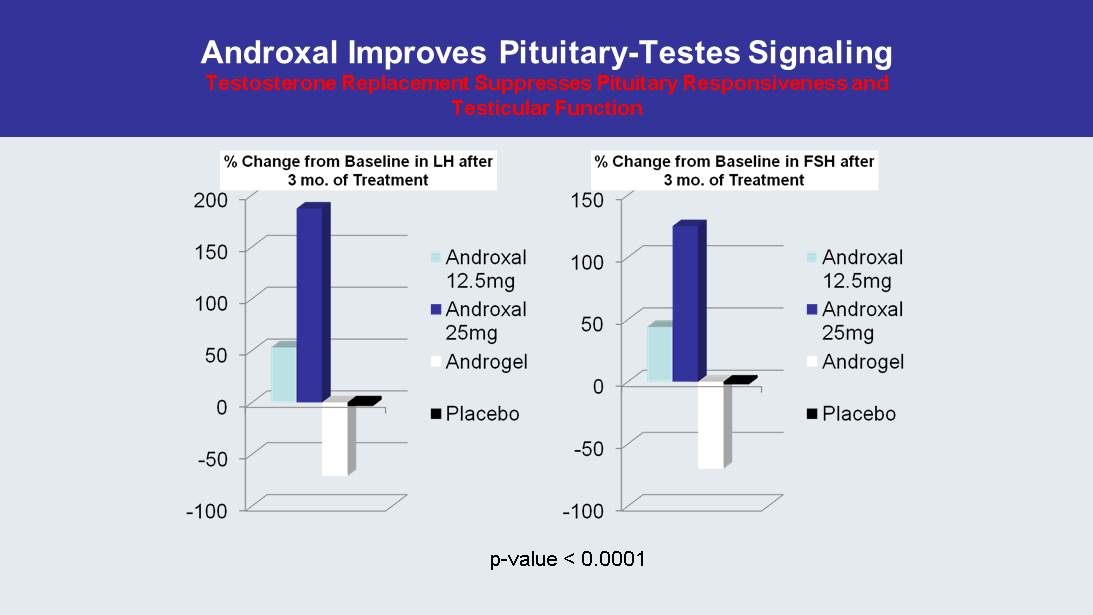
Androxal Improves Pituitary - Testes Signaling Testosterone Replacement Suppresses Pituitary Responsiveness and Testicular Function p - value < 0.0001

% of Subjects with FSH Below the Lower Limit of Normal and Below the Lower Limit of Detection (0.3 mIU/ml) after 3 months ZA - 003 “Completer” Analysis

Head to Head Comparison of Two Doses of Androxal to Testim and Placebo, Study ZA - 203 An approved T gel significantly suppresses LH and FSH in a clinically relevant manner and suppresses sperm concentration to near severe oligospermia (median sperm concentration 6.2 million/mL) after only three months of treatment 0 2 4 6 8 10 12 14 12.5 mg 25 mg PL Testim FSH Treatment Effect of Treatment on Median FSH p versus Testim Before After p<0.00001 p=0.0004 0 10 20 30 40 50 60 70 80 90 100 12.5 mg 25 mg PL Testim [Sperm] in Millions/ml Treatment Effect of Treatment on Median Sperm Concentration p versus Testim Baseline EOS p=0.012 p=0.0021 p=0.0049 0 2 4 6 8 10 12 12.5 mg 25 mg PL Testim LH Treatment Effect of Treatment on Median LH p versus Testim Before After p=0.0004 0 50 100 150 200 250 300 350 400 450 500 12.5 mg 25 mg PL Testim TT in ng/dL Treatment Effect of Treatment on Median Serum TT p versus placebo Before After p<0.00001 p=0.0002 p<0.00001

ZA - 204 Testosterone Replacement Induces Hormone Dependence in the Secondary Hypogonadal Male in 6 Week Exposure Study 0 100 200 300 400 500 600 Baseline End of Study End of Study +1 Week Mean Morning Testosterone (ng/ dL ) Androxal Androgel N = 31 Mean =263 StDev =88.7 N = 14 Mean =248 StDev =74.2 N = 24 Mean = 532 StDev =177.8 N = 13 Mean =242 StDev =89.8 P - value Androxal vs Androgel 1% <0.0001 N = 27 Mean =538 StDev =155 N = 14 Mean =452 StDev =243

Influence of Prior Testosterone ZA - 300, 6 month study
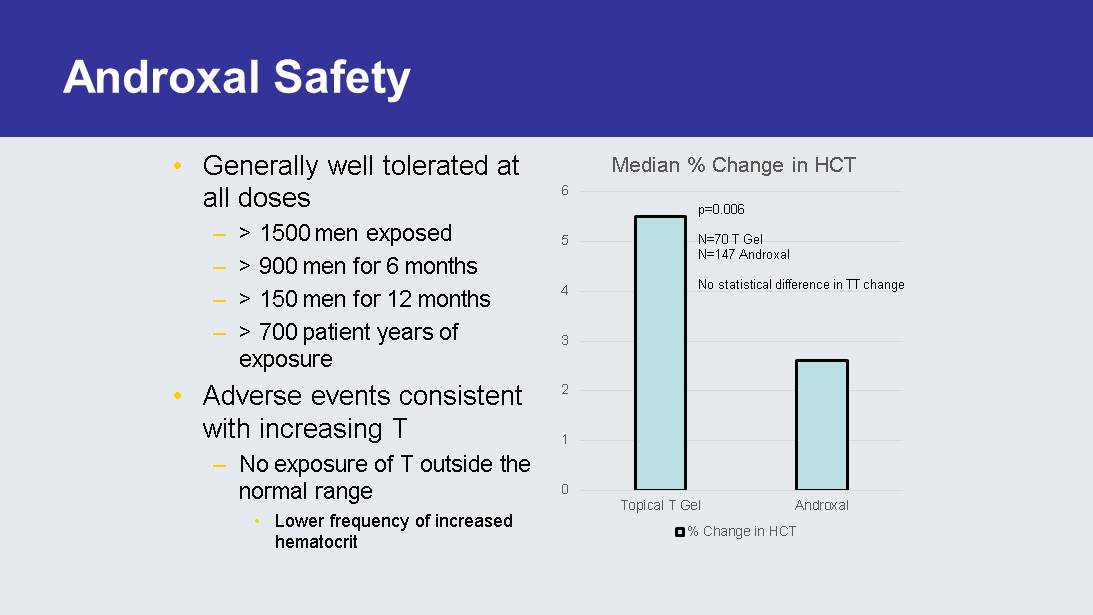
Androxal Safety • Generally well tolerated at all doses – > 1500 men exposed – > 900 men for 6 months – > 150 men for 12 months – > 700 patient years of exposure • Adverse events consistent with increasing T – No exposure of T outside the normal range • Lower frequency of increased hematocrit 0 1 2 3 4 5 6 Topical T Gel Androxal Median % Change in HCT % Change in HCT p=0.006 N=70 T Gel N=147 Androxal No statistical difference in TT change
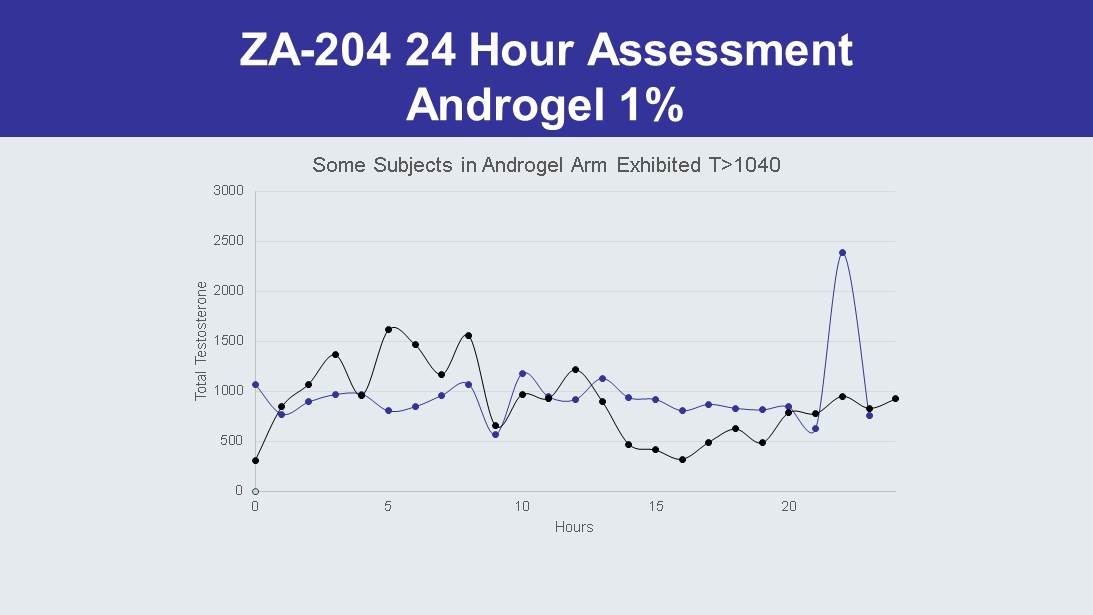
ZA - 204 24 Hour Assessment Androgel 1% 0 500 1000 1500 2000 2500 3000 0 5 10 15 20 Total Testosterone Hours Some Subjects in Androgel Arm Exhibited T>1040

Androxal Exhibits Unique Profile with Numerous Advantages vs Approved Hormone Replacement • The Androxal Advantages – Oral – Not controlled substance, cannot be abused – Less CV risk than T’s??? • No supernormal levels of T achieved – No transference risk – Restores normal function (no loss of testicular function) – Does not develop dependency – Avoids withdrawal symptoms – With lifestyle change can reverse disorder and result in no need for therapy

Upcoming Androxal Related Events • ZA - 305 Last Patient Last Visit 8/22/14 • Anticipated Release of 305 Data Mid 9 - 14 • FDA AdCom Meeting 9/17/14 • Anticipated Release of 304 Data Mid 10 - 14 • NYC Analyst Day Morning of Halloween • Submit Androxal NDA Around Year - end ‘14

Financial Summary • Cash and equivalents (as of 7/31/14, unaudited) $57.5 M • Cash runway: Through 2016* – Anticipate Androxal approved and launched • Current shares outstanding: 23.1 M shares – Warrants Outstanding – Series A – 877,137 (purchased in unit deal @ $2.45); Series B – 809,704 @ $2.49 exercise price. *Excludes marketing and commercialization expenses for Androxal