Exhibit 10.50
OFFICE LEASE AGREEMENT
BETWEEN
PACIFIC NORTH COURT HOLDINGS, L.P.,
AS LANDLORD
AND
ADAMIS PHARMACEUTICALS CORPORATION
AS TENANT
TABLE OF CONTENTS
|
1. Agreement to Let |
|
1 |
|
|
|
|
|
2. Principal Lease Provisions |
|
1 |
|
|
|
|
|
3. Term |
|
3 |
|
3.1. Description of Term |
|
3 |
|
3.2. Extension Rights |
|
3 |
|
3.2.1. Restrictions on Transferability of Option |
|
3 |
|
3.2.2. Conditions Terminating Tenant’s Rights to Exercise Option |
|
3 |
|
3.2.3. Conditions Terminating Tenant’s Option Rights |
|
3 |
|
3.2.4. Terms and Conditions of Extension of Term |
|
3 |
|
3.2.5. Determination of Then-Prevailing Rate |
|
4 |
|
|
|
|
|
4. Delivery of Possession |
|
4 |
|
4.1. Delivery Requirements |
|
4 |
|
4.2. Definition of Substantial Completion |
|
|
|
4.3. Final Completion |
|
|
|
|
|
|
|
5. Use of Premises and Common Areas |
|
4 |
|
5.1. Permitted Use of Premises |
|
4 |
|
5.2. Compliance with Laws |
|
4 |
|
5.3. Condition During Periods of Non-Use; Recapture |
|
5 |
|
5.4. Use of Common Areas |
|
5 |
|
5.5. General Covenants and Limitations on Use |
|
5 |
|
5.6. Access Rights |
|
6 |
|
5.7. Remedies for Breach |
|
6 |
|
|
|
|
|
6. Security Deposit |
|
6 |
|
|
|
|
|
7. Rent and Rent Adjustments |
|
7 |
|
7.1. Initial Monthly Rent |
|
7 |
|
7.2. Rental Adjustments |
|
7 |
|
7.3. Additional Rent |
|
7 |
|
7.4. General Rental Provisions |
|
7 |
|
|
|
|
|
8. Additional Rent |
|
7 |
|
8.1. Definitions |
|
7 |
|
8.1.1. Operating Expenses |
|
7 |
|
8.1.2. Excluded Costs |
|
8 |
|
8.1.3. Expense Year |
|
8 |
|
8.1.4. Tenant’s Share |
|
8 |
|
8.2. Adjustment of Operating Expenses |
|
9 |
|
8.2.1. Gross Up Adjustment When a Project is Less Than Fully Occupied |
|
9 |
|
8.2.2. Adjustment When Landlord Adds Additional Buildings to the Project |
|
9 |
|
8.2.3. Adjustment When Landlord Does Not Furnish a Service to All Tenants |
|
9 |
|
8.2.4. Additional Costs |
|
9 |
|
8.2.5. Common Areas |
|
9 |
|
8.3. Tax Expenses |
|
9 |
|
8.4. Calculation and Payment of Operating Expenses |
|
10 |
|
8.4.1. Calculation of Excess |
|
10 |
|
8.4.2. Statement/Payment of Operating Expenses |
|
10 |
|
8.5. Landlord’s Books and Records |
|
10 |
|
|
|
|
|
9. Utilities and Services |
|
11 |
|
9.1. Tenant’s Utility Costs |
|
11 |
|
9.2. Standard Tenant Services |
|
11 |
|
9.3. Over-Standard Tenant Use |
|
11 |
|
9.4. Conduit and Wiring |
|
12 |
|
9.5. Utilities Generally |
|
12 |
|
|
|
|
|
10. Maintenance |
|
12 |
|
10.1. Tenant’s Duties |
|
12 |
- i -
|
10.2. Landlord’s Duties |
|
12 |
|
|
|
|
|
11. Parking |
|
13 |
|
11.1. General Parking Rights |
|
13 |
|
11.2. Parking Ratios |
|
13 |
|
11.3. Parking Ratios |
|
|
|
|
|
|
|
12. Signs |
|
14 |
|
12.1. General Signage Conditions |
|
14 |
|
12.2. Tenant’s Individual Signage Rights |
|
14 |
|
12.2.1. Directory/Suite Signage |
|
14 |
|
12.2.2. Exterior Monument Signage |
|
|
|
|
|
|
|
13. Rules, Regulations, and Covenants |
|
14 |
|
|
|
|
|
14. Early Access/Insurance |
|
14 |
|
|
|
|
|
15. Tenant’s Liability Insurance |
|
14 |
|
|
|
|
|
16. Tenant’s Property Damage Insurance |
|
14 |
|
|
|
|
|
17. Tenant’s Additional Insurance |
|
15 |
|
|
|
|
|
18. Form of Tenant’s Insurance Policies |
|
15 |
|
|
|
|
|
19. Waiver of Subrogation |
|
15 |
|
|
|
|
|
20. Landlord’s Insurance |
|
16 |
|
|
|
|
|
21. Personal Property Taxes |
|
16 |
|
|
|
|
|
22. Alterations |
|
16 |
|
22.1. Request for Consent |
|
16 |
|
22.2. Minor Alterations |
|
16 |
|
22.3. Additional Requirements |
|
17 |
|
22.4. Ownership of Alterations |
|
17 |
|
|
|
|
|
23. Surrender of Premises and Holding Over |
|
18 |
|
|
|
|
|
24. Default |
|
18 |
|
|
|
|
|
25. Landlord’s Remedies |
|
19 |
|
25.1. Continuation of Lease |
|
19 |
|
25.2. Rent from Reletting |
|
19 |
|
25.3. Termination of Tenant’s Right to Possession |
|
19 |
|
25.4. Landlord’s Right to Cure Default |
|
20 |
|
25.5. Enforcement Costs |
|
20 |
|
|
|
|
|
26. Interest and Late Charges |
|
20 |
|
|
|
|
|
27. Landlord Default -Tenant’s Remedies |
|
20 |
|
|
|
|
|
28. Quarterly Payments |
|
|
|
|
|
|
|
29. Destruction |
|
20 |
|
|
|
|
|
30. Condemnation |
|
21 |
|
|
|
|
|
31. Assignment and Other Transfers |
|
22 |
|
31.1. Restriction on Transfer |
|
22 |
|
31.2. Transfer Provisions Generally |
|
22 |
|
31.3. Excess Rent and Recapture |
|
23 |
|
31.4. Permitted Transferee |
|
23 |
|
|
|
|
|
32. Landlord’s Reserved Rights |
|
24 |
|
32.1. General Rights Reserved |
|
24 |
|
32.2. Future Construction |
|
24 |
|
32.3. Relocation |
|
|
|
|
|
|
- ii -
|
33. Easements |
|
25 |
|
|
|
|
|
34. Access by Landlord |
|
25 |
|
|
|
|
|
35. Indemnity |
|
25 |
|
|
|
|
|
36. Exemption of Landlord from Liability |
|
26 |
|
|
|
|
|
37. Hazardous Substances |
|
26 |
|
37.1. Landlord’s Covenants |
|
26 |
|
37.2. Tenant’s Covenants |
|
26 |
|
37.3. Definition of Hazardous Materials |
|
27 |
|
|
|
|
|
38. Prohibition Against Mold, Lead-Based Paint, and Asbestos-Containing Materials |
|
27 |
|
|
|
|
|
39. Security Measures |
|
28 |
|
|
|
|
|
40. Subordination and Attornment |
|
28 |
|
|
|
|
|
41. Estoppel Certificate |
|
28 |
|
|
|
|
|
42. Waiver |
|
29 |
|
|
|
|
|
43. Brokers |
|
29 |
|
|
|
|
|
44. Limitations on Landlord’s Liability |
|
29 |
|
|
|
|
|
45. Sale or Transfer of Premises |
|
29 |
|
|
|
|
|
46. Quitclaim Deed |
|
29 |
|
|
|
|
|
47. No Merger |
|
30 |
|
|
|
|
|
48. Confidentiality |
|
30 |
|
|
|
|
|
49. Miscellaneous |
|
30 |
- iii -
STANDARD FORM
MODIFIED GROSS OFFICE LEASE
This Standard Form Modified Gross Office Lease (“Lease”) is entered into effective as of April 1, 2014, between PACIFIC NORTH COURT HOLDINGS, L.P., a California limited partnership (“Landlord”), and ADAMIS PHARMACEUTICALS CORPORATION, a Delaware corporation (“Tenant”), who agree as follows:
1. Agreement to Let. Landlord hereby leases to Tenant, and Tenant hereby leases from Landlord, upon all of the terms, provisions, and conditions contained in this Lease, (i) those certain premises described in the Principal Lease Provisions below (the “Premises”), consisting of a portion of that certain building described in the Principal Lease Provisions below (the “Building”), which is in turn a part of the Project (as described in the Principal Lease Provisions below), along with (ii) the non-exclusive right to use, in common with Landlord, Landlord’s invitees and licensees, and the other tenants and users of space within the Project, those portions of the Project intended for use by, or benefiting, tenants of the Project in common including, without limitation, the landscaped areas, passageways, walkways, hallways, elevators, parking areas, and driveways of the Building and the Project, but excluding all interior areas of the other buildings in the Project other than the Building (collectively, the “Common Areas”). This Lease confers no rights, however, to the roof, exterior walls, or utility raceways of the Building, nor rights to any other building in the Project, nor with regard to either the subsurface of the land below the ground level of the Project or with regard to the air space above the ceiling of the Premises; provided, however, that Tenant shall have the limited right to access systems and equipment exclusively serving the Premises (for which Tenant has maintenance and repair responsibilities pursuant to Paragraph 10.1, below) that may be located on the roof, in exterior or demising walls, in utility raceways, in the airspaces above the ceiling of the Premises, or in any other portion of the Building or the Common Areas for the sole purpose of maintaining, repairing, and replacing such systems and equipment.
2. Principal Lease Provisions. The following are the Principal Lease Provisions of this Lease. Other portions of this Lease explain and describe these Principal Lease Provisions in more detail and should be read in conjunction with this Paragraph. In the event of any conflict between the Principal Lease Provisions and the other portions of this Lease, the Principal Lease Provisions will control. (Terms shown in quotations are defined terms used elsewhere in this Lease)
2.1. “Project’’: That certain office project, commonly referred to as Torrey Reserve North Court, in San Diego, California, as more particularly depicted on the attached Exhibit ‘‘A.”
2.2. “Building”: That certain building within the Project as designated on the attached Exhibit “A”. sometimes referred to as Torrey Reserve - North Court Building I, whose mailing address is 11682 EI Camino Real, San Diego, California 92130.
2.3. “Premises”: Suite 300; consisting of a portion of the third (3rd) floor of the Building, as more particularly described on the attached Exhibit “B.”
2.4. Area of the Premises: Approximately 7,525 Rentable Square Feet of space in accordance with the Building Ownwers and Managers method of measuring Net Usuable Square Feet, (USF) and Net Rentable Square Feet (RSF). The term “Rentable Square Feet”, “Usable Square Footage,” and similar terms dealing with Rentable or Usable means of describing measurements of square footages, will have the meanings of such term adopted by the Building Owners and Managers Association International (relative to multi-tenant floors).
2.5. “Initial Lease Term”: Four (4) years plus any additional days required for the Initial Expiration Date to occur on the last day of a month as set forth in Paragraph 2.5.2, below, beginning as of the Lease Commencement Date and ending as of the Initial Expiration Date.
2.5.1. “Lease Commencement Date:” December 1, 2014.
2.5.2. “Initial Expiration Date”: That date which is four (4) years (plus, if such date is not the final day of a calendar month, however many days are left in the final calendar month of the Term) after the Lease Commencement Date.
2.5.3. Extension Rights: One (1) option to extend (Paragraph 3.2).
1
2.6. “Basic Monthly Rent”: $3.20 per Rentable Square Foot, net of electricity and other utilities, subject to adjustment pursuant to attached Addendum No.1. Basic Monthly Rent will always be due and payable on or before the first day of the applicable month, except that the first month’s Basic Monthly Rent will be due and payable upon the date of Landlord’s execution of this Lease.
2.7. “Rent Commencement Date”: The earlier of (i) the Lease Commencement Date, or (ii) the date that the Lease Commencement Date would have occurred, but for the occurrence of Tenant Delays (as defined in Exhibit ”C”).
2.8. “Security Deposit”: $170,000.00 Tenant’s Security Deposit-which is due and payable on or before November 1, 2014-does not constitute last month’s rent. Last month’s rent must be separately paid by Tenant on or before the first day of the last month of the Lease Term. If Tenant exercises any Option to Extend (as defined below) contained herein, then as a condition precedent to the effectiveness of Tenant’s exercise of such Option to Extend, Tenant shall pay to Landlord an amount equal to the difference between the Base Monthly Rent for the last year of such Extension Term (as defined below) and the amount of the Security Deposit then held by Landlord; which additional amount will be added to, and constitute a part of, the Security Deposit from that point forward.
2.9. “Base Year”: Calendar year 2014.
2.10. Guarantor: None.
2.11. Address for Landlord:
Pacific North Court Holdings, L.P.
c/o American Assets Trust Management, LLC
11455 EI Camino Real, Suite 200
San Diego, CA 92130
Attn: Property Management (Office)
2.12. Addresses for Tenant:
Legal Notices Addresses
(Prior to Occupancy)
Adamis Pharmaceuticals Corporation
11682 EI Camino Real, Suite 300
San Diego, CA 92130
Attn: Dennis J. Carlo
(Following Occupancy)
At the Premises
2.13. “Permitted Use”: The Premises shall be used for general office purposes, in accordance with all applicable laws, statutes, ordinances, and regulations and the provisions of this Lease, and for no other use.
2.14. Building Standard Operating Hours:
Monday through Friday: 7:00 a.m.-7:00 p.m.
Saturday: 9:00 a.m.-1:00 p.m.
(excluding Sundays and any local, state, and federal holidays)
2.15. Participating Brokers:
Landlord’s: CBRE, Inc. – Richard Balestri
Tenant’s: Ashcraft Investments, Inc. – William P. Driscoll
2
2.16. Initial Payment Amounts: $170,000.00, representing the Security Deposit, which amount is payable on or before November 1, 2014, and $24,080.00, representing the first month’s Basic Monthly Rent, which amount is payable on the date Tenant executes this Lease (to be adjusted on the Lease Commencement Date to reflect the actual first month’s Basic Monthly Rent based upon the actual Rentable Square Footage of the Premises if determined to be different than stated above pursuant to Paragraph 7.4, below).
3.1. Description of Term. The term of this Lease (‘‘Term’‘) shall commence on the “Lease Commencement Date”, and shall expire on the “Initial Expiration Date”, subject to (i) any extension rights described in Paragraph 3.2, below, and (ii) earlier termination by Landlord, as provided in this Lease. The term “Expiration Date”, as used in this Lease, shall mean the Initial Expiration Date, any earlier date upon which this Lease is terminated by Landlord, as provided below, or if the Term is extended pursuant to Paragraph 3.2, below, then the expiration date of any exercised Extension Term.
3.2. Extension Rights. Tenant shall, subject to all of the provisions of this Paragraph 3.2 (including all subparagraphs hereof), have the option to extend the Term (the “Option to Extend”) for one (1) additional term of three (3) years (the “Extension Term”), provided Tenant is in occupancy of not less than 50% of the Premises at the time of exercise of the Option to Extend and Tenant gives Landlord written notice via overnight nationally-recognized courier (such as FedEx or UPS), with signature acknowledgement by recipient required, of its election to exercise the Option to Extend no less than nine (9) months and no more than twelve (12) months prior to the then applicable Expiration Date of the Term. Such notice will constitute Tenant’s irrevocable election to extend the Term and may not subsequently be revoked by Tenant except as provided below. Time is of the essence with respect to the timing of such requirement to give notice to Landlord.
3.2.1. Restrictions on Transferability of Option. The Option to Extend is personal to the Tenant originally named in this Lease or any Permitted Transferee (as defined below) and may not be exercised by anyone other than such originally named Tenant or a Permitted Transferee.
3.2.2. Conditions Terminating Tenant’s Rights to Exercise Option. Tenant shall not have the right to exercise the Option to Extend, notwithstanding anything set forth above to the contrary: (a) during any period of time commencing from the date Landlord gives to Tenant a written notice that Tenant is in default under any provision of this Lease and continuing until the default alleged in said notice is cured; (b) during the period of time commencing on the day after a monetary obligation to Landlord is due from Tenant and unpaid (without any necessity for notice thereof to Tenant) and continuing until the obligation is paid; or (c) in the event that Landlord has given to Tenant two or more notices of default or two or more late charges have become payable under this Lease during the 12-month period prior to the time that Tenant attempts to exercise the Option to Extend. The period of time within which the Option to Extend may be exercised shall not be extended or enlarged by reason of Tenant’s inability to exercise the Option to Extend because of the foregoing provisions of this Paragraph 3.2.2, even if the effect thereof is to eliminate Tenant’s right to exercise the Option to Extend.
3.2.3. Conditions Terminating Tenant’s Option Rights. All rights with respect to the Option to Extend (including rights as to subsequent Extension Terms, if any) shall terminate and be of no further force or effect even after Tenant’s due and timely exercise of the Option to Extend, if, after such exercise, but prior to the commencement of the Extension Term, (a) Tenant fails to pay to Landlord a monetary obligation of Tenant for a period of ten days after such obligation became due (without imposing any obligation on the part of Landlord to give notice thereof to Tenant); (b) Tenant fails to cure a non-monetary default within 30 days after the date the Landlord gives notice to Tenant of such default or (c) Landlord gives to Tenant two or more notices of default or two or more late charges become payable for any monetary defaults, whether or not such defaults are cured.
3.2.4. Terms and Conditions of Extension of Term. If Tenant duly and timely exercises the Option to Extend, then this Lease shall remain in full force and effect for such additional three (3) year period, except that the Basic Monthly Rent will adjust as of the first day of the Extension Term such that for the first year of the Extension Term the Basic Monthly Rent shall be equal to the then prevailing base rental rate (ignoring tenant improvement and similar refurbishment or construction allowances, free rent, and other similar concessions–it being acknowledged that the Option to Extend reflects Tenant’s negotiated right to defer its decision whether to initially lease the Premises for such longer period of time, as opposed to Tenant’s right to enter into a new lease) for new leases of comparable Class A office space in the Solana Beach, as projected for the first day of the applicable Extension Term and determined pursuant to Paragraph 3.2.5, below (the “Then-Prevailing Rate”). The Basic Monthly Rent will thereafter increase in accordance with the provisions of attached Addendum No.1
3
3.2.5. Determination of Then-Prevailing Rate. If Tenant exercises the Option to Extend, then Landlord shall, within 15 business days of receipt of Tenant’s written notice of exercise, provide Tenant with written notice of the Then-Prevailing Rate and the calculation of the new Basic Monthly Rent to be effective during the first year of the Extension Term. Tenant shall have ten business days from the date of Landlord’s notice in which to (a) accept the Landlord’s determination of the Then-Prevailing Rate, (b) revoke Tenant’s election to exercise the Option to Extend, in which case Tenant’s Option to Extend shall be null and void, or (c) dispute Landlord’s determination of the Then-Prevailing Rate. If Tenant fails to notify Landlord, in writing, of its disagreement with Landlord’s determination of the Then-Prevailing Rate within such ten-business day period, then Tenant will be deemed to have accepted Landlord’s determination and Landlord’s determination shall be binding on both parties. If Tenant disputes such determination, then its notice to Landlord disputing such determination must set forth Tenant’s determination of the Then-Prevailing Rate. Upon receipt of Tenant’s notice, Landlord and Tenant shall promptly meet and, in good faith, attempt to agree upon the Then-Prevailing Rate. If Landlord and Tenant are unable to reach agreement upon the Then-Prevailing Rate within 30 days of the date of Landlord’s receipt of Tenant’s dispute notice, then the parties shall promptly submit such dispute to the San Diego office of the American Arbitration Association (the “AAA”), or its successor, for resolution before a single arbitrator (who must have at least ten years experience in the San Diego County commercial real estate market as a real estate broker or MAI appraiser) in accordance with Real Estate Industry Arbitration Rules of the AAA. Within ten days of the commencement of the arbitration, Landlord and Tenant shall each provide the arbitrator with their respective written determination of the Then-Prevailing Rate-which determination will not be disclosed by the arbitrator until both parties have submitted their respective written determinations. The arbitrator’s sole authority will be to select between the Landlord’s and the Tenant’s respective written determinations of the Then-Prevailing Rate, as set forth in the notices described above, as provided to the arbitrator in accordance with the preceding sentence; provided, however, if either party fails to timely submit such a written determination to the arbitrator, then the arbitrator shall use the written determination of such party as set forth in the notices described above as part of the initiation of the subject process. In no event may such arbitrator select any other amount as the Then-Prevailing Rate. The decision of the arbitrator shall be binding upon all parties and the cost of the arbitration shall be split equally between Landlord and Tenant.
4.1. Delivery Requirements. Tenant is currently in possession of the Premises pursuant to the terms of a sublease (“Sublease”) of the lease between Landlord and McDermott, Will & Emery (“MWE Lease”). On the Lease Commencement Date, the MWE Lease and Sublease shall terminate, and Landlord shall tender and Tenant shall accept possession of the Premises pursuant to the terms of this Lease.
5. Use of Premises and Common Areas.
5.1. Permitted Use of Premises. Tenant may use the Premises for the Permitted Use specified in the Principal Lease Provisions and for no other use without Landlord’s consent. Any change in the Permitted Use will require Landlord’s prior written consent, which consent may be granted or withheld in Landlord’s reasonable discretion.
5.2. Compliance with Laws. Landlord covenants that the Premises will comply with all applicable laws as of the Lease Commencement Date. Thereafter, Tenant shall comply with all laws concerning the Premises and/or Tenant’s use of the Premises, including without limitation the obligation at Tenant’s sole cost to alter, maintain, or restore the Premises in compliance with all applicable laws, even if such laws are enacted after the date of this Lease, and even if compliance entails costs to Tenant of a substantial nature. Such obligation to comply with laws shall include without limitation compliance with Title III of the Americans With Disabilities Act of 1990 (42 U.S.C. 12181 et seq.) (the “ADA”). In addition to the foregoing obligations of Tenant relative to the Premises, if Tenant’s particular use of the Premises (including the commencement of any Alterations, as defined below) results in the need for modifications or alterations to any other portion of the Project in order to comply with the ADA or other applicable laws, then Tenant shall additionally be responsible, upon demand, for the cost of such modifications and alterations plus a supervisory fee of ten percent of such cost payable to Landlord. Furthermore, pursuant to Section 1938 of the California Civil Code, Landlord confirms to the best of its knowledge that the entire Project has not undergone inspection by a Certified Access Specialist (CASp). Tenant shall indemnify, defend (with counsel satisfactory to Landlord), and hold Landlord (and its partners, members, shareholders, directors, officers, employees, agents, assigns, and any successors to Landlord’s interest in the Project) harmless from and against any and all losses, costs, demands, damages, expenses (including reasonable attorneys’ fees), claims, causes of action, judgments, penalties, fines, or liabilities, arising from Tenant’s failure to satisfy its obligations under this Paragraph including, without limitation, (i) any costs, expenses, and liabilities incurred by Landlord in connection with responding to any demand by any governmental authority that Landlord undertake any modifications or alterations which are Tenant’s responsibility pursuant to this Paragraph 5.2 or for which Tenant is obligated to reimburse Landlord hereunder, as well as (ii) any attorneys’ fees, costs, expenses, and liabilities incurred by Landlord in responding to, defending, pursuing, or otherwise being involved with any action, suit, or proceeding arising out of any claim relating to
4
the non-compliance of the Premises or the Project with the ADA or any similar law where such action, suit, or proceeding relates to, or arises from, Tenant’s use of the Premises or any Alterations, except to the extent caused by Landlord’s willful misconduct or negligent acts. Landlord shall comply with all applicable laws relating to the structural portions of the Building (including the foundation, structural roof, load bearing walls, structural columns and structural floor) and base building systems that serve the entire Building (including elevator, domestic water and condenser water distribution systems; and electrical distribution systems), provided that compliance with such applicable laws is not the responsibility of Tenant under this Lease. Landlord shall be permitted to include in Operating Expenses any costs or expenses incurred by Landlord under this Paragraph 5.2 to the extent consistent with the terms of Paragraph 8.1.1 below.
5.3. Condition During Periods of Non-Use; Recapture. During any period of time in which Tenant is not continuously using and occupying the Premises for the operation of its business, Tenant shall take such measures as may be necessary or desirable, in Landlord’s reasonable opinion, to secure the Premises from break-ins and use by unauthorized persons, to minimize the appearance of non-use, and to otherwise maintain the interior and exterior portions of Tenant’s Premises, including all windows and doors, in first class condition. Additionally, during any period of time in excess of 90 days in which Tenant is not continuously using and occupying the Premises during normal business hours. Landlord may, at its election, by giving written notice (the “Non-Use Recapture Notice”) to Tenant, recapture the Premises and terminate this Lease. If Landlord elects to exercise such right and delivers a Non-Use Recapture Notice to Tenant, and Tenant fails to cure such condition to Landlord’s reasonable satisfaction within five days of such Non-Use Recapture Notice, this Lease will automatically be deemed terminated as of the effective date stated in the Non-Use Recapture Notice, and Tenant shall surrender possession of the Premises and all improvements therein to Landlord as of such date (and any failure to do so shall constitute an immediate Event of Default hereunder).
5.4. Use of Common Areas. Tenant’s use of the Common Areas shall at all times comply with the provisions of all Rules (as defined below) regarding such use as Landlord may from time to time adopt. In no event shall the rights granted to Tenant to use the Common Areas include the right to store any property in the Common Areas, whether temporarily or permanently. Any property stored in the Common Areas may be removed by Landlord and disposed of, and the cost of such removal and disposal shall be payable by Tenant to Landlord upon demand. Additionally, in no event may Tenant use any portion of the Common Areas for loading, unloading, or parking, except in those areas specifically designated by Landlord for such purposes, nor for any group social event, sidewalk sale, employment fair or similar commercial or unauthorized purpose.
5.5. General Covenants and Limitations on Use. In addition to the Rules, Tenant and Tenant’s Invitees (as defined below) use of the Premises and the Project, will be subject to the following additional general covenants and limitations on use.
5.5.1. Tenant shall not do, bring, or keep anything in or about the Premises that will cause a cancellation of any insurance covering the Premises. If the rate of any insurance carried by Landlord is increased as a result of Tenant’s use other than for the Permitted Use or Tenant’s failure to continuously use and occupy the Premises, Tenant shall pay the amount of such increase to Landlord, within ten days after Landlord delivers to Tenant a written notice of such increase.
5.5.2. No noxious or unreasonably offensive activity shall be carried on, in or upon the Premises by Tenant or Tenant’s Invitees, nor shall anything be done or kept in the Premises which may be or become a public nuisance or which may cause unreasonable embarrassment, disturbance, or annoyance to others in the Project, or on adjacent or nearby property. To that end, Tenant additionally covenants and agrees that no light shall be emitted from the Premises which is unreasonably bright or causes unreasonable glare; no sounds shall be emitted from the Premises which are unreasonably loud or annoying; and no odor shall be emitted from the Premises which is or might be noxious or offensive to others in the Building, on the Project, or on adjacent or near-by property.
5.5.3. No unsightliness shall be permitted in the Premises which is visible from the Common Areas. Without limiting the generality of the foregoing, all equipment, objects, and materials shall be kept enclosed within the Premises and screened from view or in Common Areas trash enclosures; no refuse, scraps, debris, garbage, trash, bulk materials, or waste shall be kept, stored, or allowed to accumulate except as may be properly enclosed within appropriate containers in the Premises and promptly and properly disposed of.
5.5.4. The Premises shall not be used for sleeping or washing clothes, nor shall the Premises be used for cooking or the preparation, manufacture, or mixing of anything that might emit any offensive odor or objectionable noises or lights onto the Project or nearby properties. Additionally, Tenant shall be responsible for damage to the Project caused by any deliverymen who are at the Project to deliver goods to Tenant.
5
5.5.5. All pipes, wires, conduit, cabling, poles, antennas, and other equipment/facilities for or relating to utilities, telecommunications, computer equipment, or the transmission or reception of audio or visual signals must be kept and maintained enclosed within the Premises (except to the extent included as part of Landlord’s Work, Tenant’s Work, or otherwise approved by Landlord).
5.5.6. Tenant shall not keep or permit to be kept any bicycle, motorcycle, or other vehicle, nor any animal (excluding seeing-eye dogs), bird, reptile, or other exotic creature in the Premises.
5.5.7. Neither Tenant nor Tenant’s Invitees shall do anything that will cause damage or waste to the Project. Neither the floor nor any other portion of the Premises shall be overloaded. Tenant shall be responsible for all structural engineering required to determine structural load for items placed in the Premises by Tenant. Tenant shall fasten all files, bookcases, and like furnishings to walls in a manner to prevent tipping over in the event of earth movements. Landlord shall not be responsible for any damage or liability for such events. No machinery, equipment, apparatus, or other appliance shall be used or operated in or on the Premises that will in any manner injure, vibrate, or shake all or any part of the Project or be allowed to interfere with the equipment of any other tenant within the Project (or other property owned by Landlord or its affiliates), including, without limitation, interference with transmission and reception of telephone, telecommunications, television, radio, or similar signals.
5.6. Access Rights. Tenant will have 24 hour-a-day, seven day-a-week access to the Building and the Premises. Notwithstanding the foregoing, no failure of such access rights will constitute an eviction or a disturbance of Tenant’s use and possession of the Premises or relieve Tenant from paying Rent or performing any of its obligations under this Lease; except that Tenant shall be entitled to equitable abatement of its Rent (as defined below) obligations hereunder to the extent such lack of access is due to Landlord’s gross negligence or intentional misconduct and continues for a period in excess of three business days. Landlord will not be liable, under any circumstances, for a loss of or injury to property or for injury to or interference with Tenant’s business, including loss of profits through, in connection with, or incidental to a failure to furnish access under this Paragraph. Notwithstanding the foregoing, Landlord agrees to use reasonable efforts to promptly correct any such interruption of access.
5.7. Remedies for Breach. In the event of any breach of this Paragraph 5 by Tenant or Tenant’s Invitees, Landlord, at its election and in addition to its other rights and remedies under this Lease, may pay the cost of correcting such breach and Tenant shall immediately, upon demand, pay Landlord the cost thereof
6. Security Deposit. Upon Tenant’s execution of this Lease, Tenant shall deposit with Landlord good funds in the amount of the Security Deposit (if any) set forth in the Principal Lease Provisions, to secure the performance by Tenant of its obligations under this Lease, including Tenant’s obligations (i) to pay Basic Monthly Rent and Additional Rent (as defined below), (ii) to repair damages to the Premises and/or the Project caused by Tenant or Tenant’s agents, employees, contractors, licensees, and invitees (collectively, “Tenant’s Invitees”), (iii) to surrender the Premises in the condition required by Paragraph 24, below, and (iv) to remedy any other defaults by Tenant in the performance of any of its obligations under this Lease. If Tenant commits any default under this Lease, Landlord may, at its election, use the Security Deposit to cure such default, and to compensate Landlord for all damages actually suffered by Landlord which are directly attributable to such default, including, reasonable attorneys’ fees and costs incurred by Landlord. Upon demand by Landlord, Tenant shall promptly pay to Landlord a sum equal to any portion of the Security Deposit so used by Landlord, in order to maintain the Security Deposit in the amount set forth in the Principal Lease Provisions above (subject to increase as set forth below). Within 45 days following the Expiration Date or earlier termination of this Lease, Landlord shall deliver to Tenant, at Tenant’s last known address, any portion of the Security Deposit not used by Landlord, as provided in this Paragraph (less reductions of same pursuant to Section 6.1 below). Landlord may commingle the Security Deposit (and any advance Rent received by Landlord) with Landlord’s other funds and Landlord shall not pay interest on such Security Deposit to Tenant. Tenant waives the provisions of California Civil Code Section 1950.7 (or any successor statute), and any similar principals of law with respect to Landlord’s ability to apply the Security Deposit against future rent damages. Furthermore, upon lawful termination of the Lease as a result of Tenant’s default, Landlord shall be entitled to immediately apply the Security Deposit against damages computed under California Civil Code Section 1951.2, without the requirement that Tenant first be given notice and an opportunity to cure, and notwithstanding that the damages have not been finally adjudicated by a court.
6.1 Reduction of Security Deposit. Provided Tenant is not in default of any terms of this Lease at the time the Security Deposit is to be reduced, after the giving of any required notice and the expiration of any cure periods set forth in this Lease, such Security Deposit will be subject to annual reductions equal to Forty-Two Thousand Five Hundred and No/100 Dollars ($42,500.00), effective upon the first day of the thirteenth (13th), twenty-fifth (25th), and thirty-seventh (37th) months of the Term. Such reductions shall be issued as a credit which Tenant may use against future Rent. Upon the
6
thirty-seventh (37th) month of the Term, the remaining Forty-Two Thousand Five Hundred and No/100 Dollars ($42,500.00) of the Security Deposit shall remain in effect through the balance of the Term and any subsequent Term.
7.1. Initial Monthly Rent. Tenant shall pay to Landlord as minimum monthly rent, without deduction, setoff, prior notice, or demand, the Basic Monthly Rent described in the Principal Lease Provisions (subject to adjustment as provided in the attached Addendum), in advance, on or before the first day of each calendar month, beginning on the Rent Commencement Date and thereafter throughout the Term. If the Rent Commencement Date is other than the first day of a calendar month, then the Basic Monthly Rent payable by Tenant for the second month of the Term following the Rent Commencement Date (acknowledging that the first month’s rent is payable upon Lease execution) shall be prorated on the basis of the actual number of days during the Term occurring during the first partial calendar month thereof. Notwithstanding the foregoing, if Landlord is delayed in completion of Landlord’s Work due to any Tenant Delays, then in addition to the Basic Monthly Rent payable for the first month of the Term following the Rent Commencement Date, Tenant shall additionally pay to Landlord, upon the Rent Commencement Date, additional rent, at the rate of one-thirtieth of the Basic Monthly Rent per day, for the number of days of such delay.
7.2. Rental Adjustments. The Basic Monthly Rent shall be increased periodically in accordance with the provisions of attached Addendum No. 1 to this Lease.
7.3. Additional Rent. In addition to paying the Basic Monthly Rent pursuant to this Paragraph 7, Tenant shall pay to Landlord (in accordance with Paragraph 8 below), commencing on January 1, 2015, Tenant’s Share (as defined below) of the annual Operating Expenses (as defined below) that are in excess of the amount of Operating Expenses applicable to the Base Year. The amounts payable pursuant to this Paragraph, together with all other amounts of any kind (other than Basic Monthly Rent) payable by Tenant to Landlord under the terms of this Lease, constitute additional rent for the Premises and are collectively and individually referred to in this Lease as “Additional Rent.”
7.4. General Rental Provisions. All “Rent” (which includes Basic Monthly Rent and all “Additional Rent” hereunder) shall be paid to Landlord at the same address as notices are to be delivered to Landlord pursuant to the Principal Lease Provisions, as Landlord may change such address from time to time pursuant to the terms of this Lease.
8. Additional Rent.
8.1. Definitions. The following definitions apply in this Paragraph 8 (and elsewhere in this Lease):
8.1.1. Operating Expenses. Subject to the Excluded Costs (as defined below) relating to the Project, the term “Operating Expenses” means all expenses, costs, and amounts of every kind or nature that Landlord pays or incurs because of or in connection with the ownership, operation, management, maintenance, or repair of the Building, Common Areas and Project. Operating Expenses include, without limitation, the following amounts paid or incurred by Landlord relative to the Building, Common Areas and Project: (a) the cost of supplying utilities to all portions of the Project (other than tenant suites), including without limitation water, waste deposit, power, electricity, heating, ventilation, and air conditioning, (b) Tax Expenses and Insurance Expenses (as such terms are defined below), (c) the cost of providing janitorial services, window washing service and of operating, managing, maintaining, and repairing all building systems, including without limitation utility, mechanical, sanitary, storm drainage, and elevator systems, and the cost of supplies, tools, and equipment, as well as maintenance and service contracts in connection with those systems, (d) the cost of licenses, certificates, permits, and inspections relating to the operation of the Project, (e) the cost of consumable materials, contesting the validity or applicability of any government enactments that may affect the Operating Expenses, (f) the cost of maintenance, repair, and restoration of any parking areas or structures, including, without limitation, resurfacing, repainting, restriping, and cleaning costs, (g) fees, charges, and other costs, including administrative, management fees and accounting costs (or amounts in lieu of such fees), whether paid to Landlord, an affiliate of Landlord’s, or a third party, consulting fees, legal fees, and accounting fees of all persons engaged by Landlord or otherwise reasonably incurred by Landlord in connection with the operation, management, maintenance, and repair of the Project, (h) wages, salaries, and other compensation and benefits of all persons engaged in the operation, maintenance, repair, or security of the Project plus employer’s Social Security taxes, unemployment taxes, insurance, and any other taxes imposed on Landlord that may be levied on those wages, salaries, and other compensation and benefits. If any of Landlord’s employees provide services for more than one project of Landlord’s, only the prorated portion of those employees’ wages, salaries, other compensation and benefits, and taxes reflecting the percentage of their working time devoted to the Project will be included in the Operating Expenses, (i) payments under any easement, CC&R’s, license, operating agreement, declaration, restrictive covenant, or other instrument relating to the sharing of costs affecting the Project, (j) amortization (including interest on the unamortized cost at a rate equal to the floating commercial loan rate
7
announced from time to time by Bank of America as its “reference rate” (or a comparable rate selected by Landlord if such reference rate ceases to be published) plus three percentage points per annum) of the cost of acquiring or renting personal property used in the maintenance, repair, and operation of the Project, (k) reasonable reserves (it being acknowledged, that, among other amounts, any amount of reserves required by a Lender, as defined below, will be deemed reasonable), (I) fees and expenses for consultants retained, from time to time, by Landlord for the purposes of energy conservation, waste treatment, and water recycling and for the costs of any capital improvements, equipment or devices installed or paid for by Landlord or, at Landlord option, an annual amount sufficient, on the basis of Landlord’s experience or reasonable estimate, to establish in advance of the time for such installation a reserve to fund said costs, in order (i) to conform with any change in laws, rules, regulations or requirements of any governmental or quasi-governmental authority having jurisdiction or of the board of fire underwriters or similar insurance body or, (ii) to effect a labor saving, energy saving, or other economy (including, without limitation, as related to water recycling, waste treatment, and energy generation), amortized over the useful life of such capital improvement, equipment, or device (as reasonably determined by Landlord), and (m) the cost of maintenance of all heating, ventilating and air condition systems relating to individual premises and/or the Common Areas, other than HVAC systems exclusively serving other tenants’ premises that are directly paid for, or reimbursed, by such other tenants. All capital expenditures shall be amortized (including interest on the unamortized cost at the rate stated in subparagraph (j) of this Paragraph) over their useful life, as reasonably determined by Landlord’s certified public accountant.
8.1.2. Excluded Costs. “Excluded Costs” means the following expenses, as they relate to the Operating Expenses: (i) depreciation, principal, interest, and fees on mortgages or ground lease payments, except as otherwise provided herein, (ii) legal fees incurred in negotiating and enforcing tenant leases, disputes with other tenants, (iii) real estate brokers’ leasing commissions and advertising costs in connection with leasing space in the Project, (iv) initial improvements or alterations to tenant spaces in the Project, (v) the cost of providing any service directly to and paid directly by a single individual tenant, or costs incurred for the benefit of a single tenant, (vi) costs of any items to the extent Landlord actually receives reimbursement therefor from insurance proceeds, under warranties, or from a tenant or other third party (such costs shall be excluded or deducted – as appropriate – from Operating Expenses in the year in which the reimbursement is received), or which are paid out of reserves previously included in Operating Expenses, (vii) costs incurred due to Landlord’s breach of a law or ordinance, (viii) repairs necessitated by the gross negligence or willful misconduct of Landlord or Landlord’s employees, agents, or contractors, (ix) capital expenses other than those specifically included in the definition of Operating Expenses, (x) charitable or political contributions and membership fees or other payments to trade organizations, (xi) costs of Landlord’s Work which are to be borne by Landlord pursuant to attached Exhibit “C”, if any, (xii) rent and similar charges for Landlord’s on-site management office and/or leasing office or any other offices of Landlord or its affiliates, (xiii) Landlord’s general overhead expenses not related to the Project, (xiv) electric power and other utility costs for which any tenant directly contracts with and pays the local public service company, (xv) any bad debt loss, rent loss, or reserves for bad debts or rent loss, (xvi) any compensation paid to clerks, attendants or other persons in commercial concessions operated by Landlord, if any, provided that any compensation paid to any concierge at the Project shall be includable as an Operating Expense, (xvii) any costs expressly excluded from Operating Expenses elsewhere in this Lease, (xiix) costs (A) incurred to comply with laws relating to the removal of Hazardous Material (as defined in Paragraph 37.3 below) except for immaterial amounts completed in connection with routine maintenance and repairs; which was in existence in the Building or on the Project prior to the Lease Commencement Date, and was of such a nature that a federal, State or municipal governmental authority, if it then had knowledge of the presence of such Hazardous Material, in the state, and under the conditions that it then existed in the Building or on the Project, would have then required the removal of such Hazardous Material or other remedial or containment action with respect thereto; and (B) costs incurred to remove, remedy, contain, or treat Hazardous Material, which hazardous material is brought into the Building or onto the Project after the date hereof by Landlord or any other tenant of the Project and is of such a nature, at that time, that a federal, State or municipal governmental authority, if it had then had knowledge of the presence of such Hazardous Material, in the state, and under the conditions, that it then exists in the Building or on the Project, would have then required the removal of such Hazardous Material or other remedial or containment action with respect thereto except for immaterial amounts completed in connection with routine maintenance and repairs, (xix) any gifts provided to any entity whatsoever, including, but not limited to, Tenant, other tenants, employees, vendors, contractors, prospective tenants, and (xxi) any amount paid to Landlord or to subsidiaries or affiliates of Landlord for services in the Project to the extent the same exceeds the cost of such services rendered by qualified, first-class unaffiliated third parties on a competitive basis,
8.1.3. Expense Year. “Expense Year” means the Base Year, and each calendar year after the Base Year, in which any portion of the Lease Term falls, through and including the calendar year in which the Lease Term expires.
8.1.4. Tenant’s Share. “Tenant’s Share” means a fraction, the numerator of which is the total aggregate Rentable Square Feet in the Premises, and the denominator of 75,118 which is the total aggregate Rentable Square Feet in the Building. As of the Lease Commencement Date, the Tenant’s Share will be 10.02%. If either the Premises or the
8
Building are expanded or reduced, Tenant’s Share shall be appropriately adjusted. Tenant’s Share for the Expense Year in which that change occurs shall be determined on the basis of the number of days during the Expense Year in which each such Tenant’s Share was in effect.
8.2. Adjustment of Operating Expenses. Operating Expenses shall be adjusted as follows:
8.2.1. Gross Up Adjustment When a Project is Less Than Fully Occupied. If the occupancy of the total Rentable Square Footage of completed, partially occupied buildings within the Project (whose square footage is included in the calculation of the Project’s Rentable Square Footage pursuant to Paragraph 8.1.2. above) during any part of any Expense Year (including the Base Year) is less than 95%. Landlord shall make an appropriate adjustment to the variable components of the Operating Expenses for that Expense Year, as estimated by Landlord in its reasonable discretion using sound accounting and management principles, to determine the amount of Operating Expenses that would have been incurred had such buildings been 95% occupied. This amount shall be considered to have been the amount of Operating Expenses for that Expense Year. For purposes of this Paragraph 8.2. “variable components” include only those component expenses that are affected by variations in occupancy levels, such as nightly janitorial service to Tenants’ Premises or water usage.
8.2.2. Adjustment When Landlord Adds Additional Buildings to the Project. If Landlord adds additional buildings within the Project following the Base Year, Landlord shall make an appropriate adjustment to the Operating Expenses for the Base Year, as reasonably determined by Landlord using sound accounting and management principles, to determine the amount of Operating Expenses that would have been incurred for the Base Year if such additional building had been complete and 95% occupied during the Base Year.
8.2.3. Adjustment When Landlord Does Not Furnish a Service to All Tenants. If, during any part of any Expense Year (including the Base Year), Landlord is not furnishing a particular service or work (the cost of which, if furnished by Landlord, would be included in Operating Expenses) to a tenant (other than Tenant) that has undertaken to perform such service or work in lieu of receiving it from Landlord, Operating Expenses for that Expense Year shall be considered to be increased by an amount equal to the additional Operating Expenses that Landlord would reasonably have incurred during such period if Landlord had furnished such service or work to that tenant.
8.2.4. Additional Costs. If due to a change in the types of costs being incurred by Landlord as Operating Expenses (such as, for example, the commencement or cessation of security services—but not a mere change in how a particular cost is handled—such as going from an in-house to an outside landscaping service), the Base Year Operating Expenses need to be adjusted to eliminate the effect of such change, Landlord shall reasonably adjust the Base Year Operating Expenses and notify Tenant of such change in writing. Furthermore, Landlord shall have the right to reasonably decrease the amount of the Base Year Operating Expenses for purposes of calculating Increased Operating Expenses to eliminate the effect of abnormally high costs, or unusual costs, of a particular type or types (such as, by way of example, abnormally high energy costs associated with the “energy crisis” of 2001) occurring during the Base Year. There shall be no cap on Operating Expenses.
8.2.5. Common Areas. Landlord may elect to partition/separate portions of the Common Areas of the Project such that the Operating Expenses associated with such partitioned Common Areas are allocated to particular buildings or parcels within the Project.
8.3. Tax Expenses. “Taxes” means and refers to all federal, state, county, or local government or municipal taxes, school taxes, sewer rates, fees, charges, or other impositions of every kind or nature, whether general, special, ordinary, or extraordinary. Taxes include taxes, fees, and charges such as real property taxes, general and special assessments, transit taxes, leasehold taxes, and taxes based on the receipt of rent (including gross receipts or sales taxes applicable to the receipt of rent, unless required to be paid by Tenant), and personal property taxes imposed on Landlord’s fixtures, machinery, equipment, apparatus, systems, appurtenances, and other personal property used in connection with the Project or the Building, as the case may be, along with reasonable legal and other professional fees, costs and disbursements incurred in connection with proceedings to contest, determine or reduce real property taxes. Notwithstanding the foregoing, the following shall be excluded from Taxes: (a) all excess profits taxes, franchise taxes, gift taxes, capital stock taxes, inheritance and succession taxes, estate taxes, federal, state, and local income taxes, and other taxes applied or measured by Landlord’s general or net income (as opposed to rents, receipts, or income attributable to operations at the Building), and (b) personal property taxes attributable to property owned or installed by or for other tenants of the Project; ‘‘Tax Expenses” means the sum of all Taxes that are paid or incurred by Landlord because of or in connection with the ownership, leasing, and/or operation of the Project from time to time.
9
8.4. Calculation and Payment of Operating Expenses. Tenant’s Share of the increased Operating Expenses for any Expense Year shall be calculated and paid as follows:
8.4.1. Calculation of Excess. If Operating Expenses for any Expense Year (other than the Base Year) ending or beginning within the Lease Term exceeds the amount of Operating Expenses applicable to the Base Year, Tenant shall pay as Additional Rent to Landlord an amount equal to Tenant’s Share of that excess, in the manner stated below.
8.4.2. Statement/Payment of Operating Expenses. Tenant shall pay to Landlord, on the first day of each calendar month during the Lease Term, commencing January 1, 2015, as Additional Rent, without notice, demand, offset, or deduction (except as provided below), an amount (“Tenant’s Monthly Payment”) equal to one-twelfth of Tenant’s Share of the amount by which the Operating Expenses for each Expense Year following the Base Year exceed the Base Year Operating Expenses (such excess being referred to herein as the “Increased Operating Expenses”), as estimated (and subsequently reconciled) by Landlord in the most recently delivered Estimated Statement (as defined below). Landlord intends to deliver to Tenant, prior to the commencement of each Expense Year following the Base Year during the Lease Term, a written statement (“Estimated Statement”) setting forth Landlord’s estimate of the Operating Expenses and Increased Operating Expenses allocable to the ensuing Expense Year, and Tenant’s Share of such Increased Operating Expenses. Landlord may, at its option, during any Expense Year, deliver to Tenant a revised Estimated Statement, revising Landlord’s estimate of the Operating Expenses and Increased Operating Expenses, in accordance with Landlord’s most current estimate. Within approximately 90 days after the end of each Expense Year during the Lease Term, Landlord intends to deliver to Tenant a written statement (“Actual Statement”) setting forth the actual Operating Expenses allocable to the preceding Expense Year. Tenant’s failure to object to Landlord regarding the contents of an Actual Statement, in writing, within 90 days after delivery to Tenant of such Actual Statement, shall constitute Tenant’s absolute and final acceptance and approval of the Actual Statement. If the sum of Tenant’s Monthly Payments actually paid by Tenant during any Expense Year exceeds Tenant’s Share of the actual Increased Operating Expenses allocable to such Expense Year, then such excess will be credited against future Tenant’s Monthly Payments, unless such Expense Year was the Expense Year during which the Lease Expiration Date occurs (the “Last Calendar Year”), in which event either (i) such excess shall be credited against any monetary default of Tenant under this Lease, or (ii) if Tenant is not in default under this Lease, then Landlord shall (within the time frame for returning Tenant’s Security Deposit) pay to Tenant such excess. If the sum of Tenant’s Monthly Payments actually paid by Tenant during any Expense Year is less than Tenant’s Share of the actual Increased Operating Expenses allocable to such Expense Year, then Tenant shall, within thirty (30) days of delivery of the Actual Statement, pay to Landlord the amount of such deficiency. Landlord’s delay in delivering any Estimated Statement or Actual Statement will not release Tenant from its obligation to pay any Tenant’s Monthly Payment or any such excess upon receipt of the Estimated Statement or the Actual Statement, as the case may be. The references in this Paragraph to the actual Increased Operating Expenses allocable to an Expense Year, shall include, if such Expense Year is the Last Calendar Year, the actual Increased Operating Expenses allocable to the portion of such year prior to the Lease Expiration Date, calculated on a pro rata basis, without regard to the date of a particular expenditure. The provisions of this Paragraph 8.4 shall survive the termination of this Lease, and even though the Term has expired and Tenant has vacated the Premises, when the final determination is made of Tenant’s Share of Operating Expenses for the year in which this Lease terminates, Tenant shall immediately pay any increase due over the estimated expenses paid by Tenant pursuant hereto and conversely any overpayment made in Tenant’s estimated payments shall be immediately rebated by Landlord to Tenant.
8.5. Landlord’s Books and Records. If Tenant disputes the amount of Additional Rent stated in an Actual Statement within 90 days of Tenant’s receipt thereof, Tenant may, upon at least five business days’ notice to Landlord, request an opportunity to inspect and audit Landlord’s records and supporting documentation regarding such Actual Statement. Such inspection and audit must be conducted by an independent certified public accountant within 180 days of the date Tenant received the Actual Statement, shall be at Tenant’s sole cost and expense (except as provided below), and Landlord shall, at its election, either provide copies of such records and supporting documentation to Tenant or make such records and supporting documentation available to Tenant for its inspection at Landlord’s business office in San Diego, California during normal business hours. If Tenant fails to dispute the amount of Additional Rent stated in an Actual Statement within 90 days of Tenant’s receipt thereof, or Tenant’s audit fails to disclose a discrepancy in such Actual Statement within 180 days after Tenant’s receipt of the Actual Statement in question, then the Actual Statement will be deemed binding on Tenant. If it is determined as a result of Tenant’s timely audit of Landlord’s records (and Landlord’s certified public accountant’s concurrence therein) that Tenant was overcharged relative to the Operating Expenses, such overcharge shall entitle Tenant to a credit against its next payment of Operating Expenses in the amount of the overcharge plus, in the case of an overcharge exceeding three percent of the Operating Expenses, the reasonable third party costs of such audit (and if such credit occurs following the expiration of the Term, Landlord shall promptly pay the amount of such credit to Tenant). If it is determined as a result of Tenant’s timely audit of Landlord’s records (and Landlord’s certified public accountant’s concurrence therein), or otherwise, that Tenant was undercharged relative to the Operating Expenses, Tenant shall, within ten days of written demand, pay such undercharge to Landlord.
10
9. Utilities and Services.
9.1. Tenant’s Utility Costs. To the extent separately metered to the Premises, Tenant shall pay when due all bills for gas, electricity, and other utilities used at the Premises on and after the Rent Commencement Date and through and including the date of expiration of this Lease.
9.2. Standard Tenant Services. Subject to the terms and conditions contained herein, Landlord shall provide the following services during the Lease Term.
9.2.1.Subject to limitations imposed by all governmental rules, regulations and guidelines applicable thereto, Landlord shall provide heating and condenser water to facilitate the production of air conditioning (collectively, “HVAC”) when necessary for normal comfort for normal office use in the Premises during Building Standard Operating Hours.
9.2.2. Landlord shall provide adequate electrical wiring and facilities sufficient to provide electrical current to the Premises for Project-standard ordinary and customary office uses and excluding electrical power required for electric data processing equipment, computer rooms, special lighting in excess of Building standard lighting, or any other item of electrical equipment which (individually) consumes more than 1.8 kilowatts at rated capacity in which requires a voltage other than 120 volts single phase. In addition to the foregoing, Landlord shall replace lamps, starters, and ballasts for Project-standard lighting fixtures within the Premises upon Tenant’s request; the expense of which will be an Operating Expense. Tenant shall replace lamps, starters, and ballasts for non-Project-standard lighting fixtures within the Premises at Tenant’s sole expense. Landlord shall also provide electrical service in connection with Common Area needs, such as lighting.
9.2.3. Landlord shall provide city water from the regular Building outlets for drinking, lavatory and toilet purposes in the Building Common Areas.
9.2.4. Landlord shall provide five day per week ordinary and customary, basic janitorial services in and about the Premises in a manner consistent with other comparable buildings in the vicinity of the Building. Landlord shall not be required to provide janitorial services to above-Project-standard improvements installed in the Premises including but not limited to metallic trim, wood floor covering, glass panels, interior windows, kitchen/dining areas, executive washrooms, or shower facilities. Any janitorial services required by Tenant and provided by Landlord in excess of such ordinary and customary, basic janitorial services shall be separately paid for by Tenant, as Additional Rent, within ten days of written demand.
9.2.5. Landlord shall provide nonexclusive, non-attended automatic passenger elevator service during the Building Hours, shall have one elevator available at all other times, including on the Holidays, and shall provide nonexclusive, non-attended automatic passenger escalator service during Building Hours only.
9.2.6. Landlord shall provide nonexclusive freight elevator service subject to scheduling by Landlord.
Tenant shall cooperate fully with Landlord at all times and abide by all regulations and requirements that Landlord may reasonably prescribe for the proper functioning and protection of the HVAC, electrical, mechanical, and plumbing systems. Notwithstanding the foregoing, Tenant shall be responsible for all installation and recurring costs associated with utilities services at the Premises.
9.3. Over-Standard Tenant Use. Tenant shall not exceed the rated capacity of the Building’s electrical and other utility systems, which systems will be consistent in capacity with other first class office buildings built at or about the same time as the Building. In the event of any damage to any of the Project’s systems caused by Tenant’s use thereof in excess of ordinary and customary usage for a professional office. Tenant shall be responsible for all costs and expenses incurred by Landlord as a result of such over-use. In addition, if Tenant requires any utilities or services described in this Paragraph 9, which are to be provided by Landlord, in excess of the standard levels being provided by Landlord, or during hours other than Building Standard Operating Hours, Landlord shall have the right to impose reasonable restrictions on such usage and/or commercially reasonable charges therefor. The initial charge to Tenant for heating and air conditioning during hours other than Building Standard Operating Hours will be $45.00 per hour (or portion thereof), subject to increase over the Lease Term, including the Extension Term, if any. This charge will be based on Landlord’s actual cost and there will be no Landlord “add-on” or supervisory fee associated with this charge. Such charges are Additional Rent relative to the provision of such services and are not an offset to any Operating Expenses.
11
9.4. Conduit and Wiring. Installation of all types of conduit and wiring exclusively serving the Premises (other than as part of Landlord’s Work), including but not limited to Tenant’s Work, is subject to the requirements of Paragraph 22, below, Exhibit “C”, and the Landlord’s reasonable approval of the location, manner of installation, and qualifications of the installing contractor. All such conduit and wiring will, at Landlord’s option, become Landlord’s property upon the expiration of the Term. Upon expiration of the Term, Landlord may elect to require Tenant to remove such conduit and wiring at Tenant’s expense and return the Premises and the Common Areas to their pre-existing condition. If Landlord constructs new or additional utility facilities, including wiring, plumbing, conduits, and/or mains, resulting from Tenant’s changed or increased utility requirements, Tenant shall on demand promptly pay (or advance) to Landlord the cost of such items as Additional Rent.
9.5. Utilities Generally. Tenant agrees that, except as provided below, Landlord will not be liable for damages, by abatement of Rent or otherwise, for failure to furnish or delay in furnishing any service (including telephone and telecommunication services) or for diminution in the quality or quantity of any service. Such failure, delay, or diminution will not constitute an eviction or a disturbance of Tenant’s use and possession of the Premises or relieve Tenant from paying Rent or performing any of its obligations under this Lease, except that Tenant will be entitled to an equitable abatement of Rent for the period of such failure, delay, or diminution to the extent such failure, delay, or diminution is (i) is directly attributable to Landlord’s negligent acts or omissions, (ii) prevents Tenant from using, and Tenant does not use, the Premises or the affected portion thereof for the conduct of Tenant’s business operations therein, (iii) Tenant was using the Premises or such affected portion for the conduct of Tenant’s business operations immediately prior to the failure, and (iv) such failure, delay, or diminution continues for more than two consecutive business days (or ten business days in any twelve month period) after delivery of written notice of such failure, delay, or diminution from Tenant to Landlord. Landlord will not be liable, under any circumstances, for a loss of or injury to property or for injury to or interference with Tenant’s business, including loss of profits through, in connection with, or incidental to a failure to furnish any of the utilities or services under this Paragraph. Notwithstanding the foregoing, Landlord agrees to use reasonable efforts to promptly correct any such interruption of utilities or services. Tenant hereby waives the provisions of California Civil Code Section 1932(1) or any other applicable existing or future law, ordinance or governmental regulation permitting the termination of this Lease due to the interruption or failure of or inability to provide any services required to be provided by Landlord hereunder. If any governmental authority having jurisdiction over the Project imposes mandatory controls, or suggests voluntary guidelines applicable to the Project, relating to the use or conservation of water, gas, electricity, power, or the reduction of automobile emissions, Landlord, at its sole discretion, may comply with such mandatory controls or voluntary guidelines and, accordingly, require Tenant to so comply. Landlord shall not be liable for damages to persons or property for any such reduction, nor shall such reduction in any way be construed as a partial eviction of Tenant, cause an abatement of Rent, or operate to release Tenant from any of Tenant’s obligations under this Lease, except as specifically provided in this Paragraph 9.5.
10. Maintenance.
10.1. Tenant’s Duties. Tenant shall at its sole cost maintain, repair, replace, and repaint, all in first class condition, the interior of the Premises, all building systems exclusively serving the Premises and located within the Premises or the walls of the Premises, and any damage to the Premises or the Project resulting from the acts or omissions of Tenant or Tenant’s Invitees Tenant shall maintain all communications conduit, equipment, and wiring serving the Premises, whether in the Premises or not (and specifically including all of Tenant’s Work and all wiring, equipment, and conduit located on the roof of the Building), regardless of the ownership of said conduit or wiring, subject to Landlord’s reasonable approval of Tenant’s maintenance/repair contractor and manner of maintenance/repair. Notwithstanding anything to the contrary contained herein, Tenant shall pay any and all maintenance and recurring costs for supplemental HVAC units exclusively serving the Premises, or any portion thereof, upon presentation of invoice from Landlord. If Tenant fails to maintain, repair, replace, or repaint any portion of the Premises or the Project as provided above then following ten days’ written notice thereof to Tenant, Landlord may, at its election, maintain, repair, replace, or repaint any such portion of the Premises or the Project and Tenant shall promptly reimburse Landlord, as Additional Rent, for Landlord’s actual cost thereof, plus a supervisory fee in the amount of ten percent of Landlord’s actual cost. Notwithstanding the foregoing, if following Tenant’s payment (or performance) of its obligations under this Paragraph, Landlord receives payment from an insurer for such work, Tenant will be entitled to receive such proceeds (after Landlord has first been fully reimbursed for its costs and expenses relative thereto including Landlord’s costs and expenses in obtaining such proceeds) to the extent Tenant previously paid or incurred third party costs relative thereto..
10.2. Landlord’s Duties. Landlord shall, as part of the Operating Expenses, maintain, repair, replace, and repaint, all in good order and condition, consistent with other first-class office buildings in the vicinity of the Building, the Common Areas and all portions of the interior and exterior of the Building and any other buildings in the Project (including, without limitation, all electrical, mechanical, plumbing, fire/life safety, and other building systems), except to the extent of Tenant’s obligations as set forth in Paragraph 10.1, above. Landlord’s failure to perform its obligations set forth above will
12
not release Tenant of its obligations under this Lease, including without limitation Tenant’s obligation to pay Rent. Tenant waives the provisions of California Civil Code Section 1942 (or any successor statute), and any similar principles of law with respect to Landlord’s obligations for tenantability of the Premises and Tenant’s right to make repairs and deduct the expense of such repairs from rent. If Landlord fails to perform any of its repair and maintenance obligations under this Paragraph 10.2 and such failure materially and adversely impairs Tenant’s ability to use and occupy the Premises for the Permitted Use, Tenant will have the right, to perform such repairs and/or maintenance to the extent necessary to enable Tenant to resume its use and occupancy of the Premises. Notwithstanding the foregoing, prior to exercising such right, Tenant must, except as provided below in connection with an emergency, have given Landlord at least 30 days’ prior written notice of the nature of the problem and Tenant’s intention to exercise its rights under this Paragraph if such matter is not resolved within such 30-day period; provided, however, if the nature of thematter giving rise to such repair or maintenance obligation obligation will reasonably require more than 30 days to remedy and Landlord is proceeding with due diligence to remedy such matter, then such 30 day period will be extended for such additional time as may be necessary for Landlord to complete such repairs or maintenance. Notwithstanding the preceding sentence, in the case of an emergency which poses an imminent threat of death, injury, or severe damage to persons or property, the required notice from Tenant may be provided orally rather than in writing and for such shorter period of time (i.e., less than 30 days) as Tenant, in the exercise of its reasonable judgment deems appropriate under the exigent circumstances (however, at a minimum, Tenant shall at least contact Landlord telephonically prior to commencing such work so that Landlord may, make arrangements to handle such emergency itself). If Landlord fails to fulfill its repair and maintenance obligations under this Paragraph, and as a result thereof Tenant exercises the foregoing right to correct such matter, then Landlord shall reimburse Tenant for the reasonable third-party costs incurred by Tenant to complete such repairs and/or maintenance within 30 days after receipt of Tenant’s written demand therefor, together with copies of the paid invoices evidencing the costs so incurred. Any such repairs or maintenance performed by Tenant, as permitted herein, must be performed in a good and workmanlike manner by licensed contractors. Under no circumstances may Tenant offset any amount it is owed by Landlord pursuant to this Paragraph (or otherwise) against any Rent obligation under this Lease.
11. Parking.
11.1. General Parking Rights. Subject to the remaining provisions of this Paragraph 11, Landlord grants to Tenant (for the benefit of Tenant and Tenant’s Invitees) the right to the non-exclusive use of the unreserved parking area within the boundaries of and serving the Project (the “Parking Area”). Tenant’s use of the Parking Area shall be subject to such reasonable, non-discriminatory rules as Landlord may, in its sole discretion, adopt from time to time with respect to the Parking Area, (i) rules providing for the payment of charges or fees by users of the Parking Area , and in such event the charges or fees shall be deemed Additional Rent, (ii) rules limiting tenants of the Project ( Tenant) to the use of, or excluding the use of, certain parking spaces or certain portions of the Parking Area, in order to maintain the availability of accessible parking spaces for clients, guests, and invitees of tenants of the Project, and (iii) rules limiting tenants of the Project (including without limitation Tenant), and their employees, to the use of a restricted number of parking spaces or a restricted area. If Tenant, or any of Tenant’s employees, fails to comply with any such rules or requirement (such as, by way of example, parking in areas designated as visitor parking only), then Landlord will have the right to either have such vehicles towed from the Project at Tenant’s expense, or to charge Tenant $100.00 per day per car for any cars which are parked in violation of such requirements. Furthermore, Landlord shall have the right to immobilize such improperly parked vehicles by use of a “boot” or other device. Notwithstanding anything to the contrary in this Paragraph, Landlord may, at its election, construct improvements upon or otherwise alter in any manner the Parking Area, provided that Landlord makes parking available to Tenant elsewhere within the Project (or within a reasonable distance from the Premises that is equal to or greater than the applicable ratio described in Paragraph 11.2. below. Landlord reserves the right to grant certain tenants in the Project the exclusive right to park in specified areas of the Parking Area, to the exclusion of all other tenants. Tenant acknowledges that the exercise of the rights reserved to Landlord under this Paragraph may result in a decrease in the number of parking spaces available to Tenant and Tenant’s Invitees, and no such decrease shall affect Tenant’s obligations under this Paragraph or entitle Tenant to any abatement of Rent, provided the applicable parking ratio described in Paragraph 11.2. below, is maintained or exceeded.
11.2. Parking Ratios. As of the Rent Commencement Date (and subject to temporary interruptions in connection with Landlord’s continued development of the Project, as provided below), the parking ratio within the Project applicable to Tenant will be twenty-six (26) spaces based on four (4) spaces per 1,000 Usable Square Feet (“USF”) of space within the Premises. The foregoing (4:1,000 USF) parking ratio includes all spaces within the Project, including covered, uncovered, reserved, unreserved, handicap, and visitor parking spaces. Of the total spaces described above, a maximum of six (6) of said total spaces may be reserved in the parking garage by Tenant at the rate of $100.00 per space per month during the initial Lease Term. Tenant shall notify Landlord in writing upon execution of this Lease how many of the six (6) reserved spaces it chooses to reserved, and only those spaces shall be reserved for the entire initial Lease Term All unreserved parking shall be provided on a free and unassigned basis (i.e., first come, first served).
13
12. Signs.
12.1. General Signage Conditions. Landlord may at any time change the name of either or both of the Building and/or the Project and install, affix, and maintain all signs on the exterior and interior of the Building and other buildings within the Project as Landlord may, in Landlord’s sole discretion, desire. Tenant shall not have or acquire any property right or interest in the name of the Building or the Project. Subject to Tenant’s signage rights under Paragraph 12.2. below. Tenant may not place, construct, or maintain any sign, advertisement, awning, banner, or other exterior decoration (collectively, “sign”) inside or outside the Premises which is visible from the exterior of the Premises, or on the Building or any other portion of the Project, without Landlord’s prior written consent. Any sign that Tenant is permitted by Landlord to place, construct, or maintain in the Premises or on the Building or the Project (including pursuant to Paragraph 12.2. below) must comply with Landlord’s sign criteria applicable to the Project, including, criteria relating to size, color, shape, graphics, and location (collectively, the “Sign Criteria”), and shall comply with all applicable laws, ordinances, CC&R’s (or similar recorded instruments), rules, or regulations, and Tenant shall obtain any approvals required by such laws, ordinances, CC&R’s (or similar recorded instruments), rules, and regulations. Landlord makes no representation or warranty with respect to Tenant’s ability to obtain any such approval. Tenant shall, at Tenant’s sole cost, make any changes to any sign, whether in the Premises or on the Building, as required by any new or revised applicable laws, ordinances, rules, or regulations or any changes in the Project Sign Criteria. Tenant shall, additionally, maintain, repair, and replace all of Tenant’s signs (including, specifically, those installed pursuant to Paragraph 12.2. below) in first class condition. Nothing contained in this Paragraph 12 will limit the Landlord’s right to grant signage rights to other tenants of the Building, or to affect the signage rights of any tenant of the Building.
12.2. Tenant’s Individual Signage Rights. Subject to compliance with the requirements of Paragraph 12.1, above, Tenant is hereby granted the following signage rights in/on the Building and at the Project.
12.2.1. Directory/Suite Signage. The Building will be provided, at Landlord’s expense, with both a Project-standard lobby directory sign and suite signage. Tenant shall be entitled to be listed on such signs, subject to prior approval of the Tenant’s graphics by Landlord, if applicable.
13. Rules, Regulations, and Covenants. Tenant shall observe (and shall cause Tenant’s Invitees to observe) faithfully and comply strictly with any rules and regulations which Landlord may from time to time adopt for the Project (and provide Tenant with a copy of), as well as any recorded easement agreements, maintenance agreements, CC&R’s or like instruments affecting the Building and/or the Project, whether now existing or hereafter adopted or amended from time to time (all of the foregoing, collectively, “Rules”). The current Rules are attached as Exhibit “D”. Landlord has no duty or obligation to enforce any Rule against any other tenant, and Landlord will not be liable to Tenant for violation of any Rule by any other tenant, or any other tenant’s agents, employees, officers, independent contractors, customers, invitees, visitors, or licensees. Tenant acknowledges that Landlord reserves the right, from time to time, to enter into leases or other agreements by which Landlord agrees to restrict the use of all or any portion of the Project (including the Premises) from certain uses. All such leases and other agreements, whether now existing or entered into in the future, shall be binding upon Tenant and in no event shall Tenant utilize the Premises for any use so prohibited; provided, however, no such restriction may prevent Tenant from using the Premises for the Permitted Use.
14. Intentionally Deleted.
15. Tenant’s Liability Insurance. Tenant shall maintain, at Tenant’s sole cost and expense, Commercial General Liability Insurance covering the insured against (i) any and all Claims (as defined below) of bodily injury, personal injury and property damage (including loss of use thereof) arising out of or connection with Tenant’s use, occupancy and operations within the Premises and Building, and (ii) all contractual liabilities under this Lease, including, without limitation, indemnity provisions contained herein, for limits of liability not less than:
|
Bodily Injury and |
|
$2,000,000 each occurrence |
|
Property Damage Liability |
|
$4,000,000 annual aggregate |
|
|
|
|
|
Personal Injury Liability |
|
$2,000,000 each occurrence |
|
|
|
$4,000,000 annual aggregate |
|
|
|
0% Insured’s participation |
16. Tenant’s Property Damage Insurance. Tenant shall maintain, at Tenant’s sole cost and expense, Physical Damage Insurance covering (i) all office furniture, business and trade fixtures, office equipment, free-standing cabinet work, movable partitions, merchandise and all other items of Tenant’s property on the Premises installed by, for, or at the expense of Tenant, (ii) all Tenant improvements (installed and/or constructed per Exhibit “C” attached hereto), and any
14
other improvements which exist in the Premises as of the Commencement Date (excluding the base building structure and building systems), and (iii) all other improvements, Alterations, Personal Property and additions to the Premises. Such insurance shall be written on an “all risks” of physical loss or damage basis, for the full replacement cost value, new without deduction for depreciation of the covered items and in amounts that meet any co-insurance clauses of the policies of insurance and shall include coverage for damage or other loss caused by fire or other peril including, but not limited to, vandalism and malicious mischief, theft, water damage of any type, including sprinkler leakage, bursting or stoppage of pipes, coverage with respect to increased costs due to building ordinances, demolition coverage, boiler and machinery insurance and explosion. Such “full replacement value” shall be determined by the insurance company issuing such policy at the time the policy is initially obtained. Not more frequently than once every two years, either Landlord or Tenant may, at its election, notify the other that it elects to have the replacement value redetermined by an insurance company. Such redetermination shall be made promptly and in accordance with the rules and practices of the Board of Fire Underwriters, or a like board recognized and generally accepted by the insurance company, and Landlord and Tenant shall be promptly notified of the results by the company. Such policy shall be promptly adjusted according to such redetermination
17. Tenant’s Additional Insurance. In addition to the foregoing coverages, Tenant shall maintain, at Tenant’s sole cost and expense:
17.1. Worker’s Compensation and Employer’s Liability or other similar insurance pursuant to all applicable laws.
17.2. Business Interruption Insurance in amounts sufficient to reimburse Tenant for direct or indirect loss of earnings attributable to all perils commonly insured against by prudent tenants or attributable to prevention of access to the Premises or to the Project as a result of such perils, including, without limitation, reimbursement for payment of rental and all other monetary obligations required herein.
18. Form of Tenant’s Insurance Policies. The minimum limits of policies of insurance required of Tenant under this Lease shall in no event limit the liability of Tenant under this Lease. TheTenant’s liability insurance (i) shall name Landlord, American Assets Trust, Inc. and American Assets Trust, LP. and any other party with an insurable interest in the Project which the Landlord so specifies by written notice to Tenant, as an additional insured, including Landlord’s managing agent, American Assets Trust Management, LLC, as such agent may be changed from time to time; (ii) shall cover the liability assumed by Tenant under the indemnification provisions of this Lease; (iii) shall consist of “occurrence” based coverage, without provision for subsequent conversion to “claims” based coverage; (iv) shall be issued by an insurance company having a rating of not less than A XV in Best’s Insurance Guide or which is otherwise acceptable to Landlord and licensed or approved or permitted to do business in the State of California; (v) shall be primary insurance as to all Claims thereunder; (vi) be in form and content reasonably acceptable to Landlord; and (vii) shall provide that said insurance shall not be canceled unless thirty (30) days’ prior notice shall have been given to Landlord, and (viii) shall not provide for a deductible or co-insurance provision in excess of $10,000.00. Tenant shall deliver said policy or policies or certificates thereof or reasonable evidence that such insurance is in place to Landlord on or before the Commencement Date. In the event Tenant shall fail to procure such insurance, or to deliver such policies or certificate, Landlord may, at its option upon five (5) business days’ notice to Tenant, procure such policies for the account of Tenant unless Tenant provides same within such five (5) day period, and the cost thereof shall be paid to Landlord within five (5) days after delivery to Tenant of bills therefore. Tenant shall, at least 30 days prior to the expiration of each such policy, furnish Landlord with a renewal of or “binder” extending such policy. Not more frequently than once every two (2) years, if in the opinion of Landlord the amount or scope of such insurance at that time is not adequate, Tenant shall increase such insurance as reasonably required by Landlord.
19. Waiver of Subrogation. Landlord and Tenant release each other, Tenant’s Invitees, and Landlord’s guests, invitees, customers and licensees (collectively, “Landlord’s Invitees”) from all claims for damage, loss, or injury to the Project, to Tenant’s Personal Property, and to the fixtures and Alterations of either Landlord or Tenant in or on the Project to the extent such damage, loss or injury is covered by any insurance policies carried by Landlord and Tenant and in force at the time of such damage, or which would have been covered by insurance policies required by this Lease to be carried by Tenant, but which Tenant failed to carry. Subject to the remaining provisions of this Paragraph, Landlord and Tenant shall each cause all insurance policies obtained by it pursuant to this Lease to provide that the insurance company waives all right of recovery by way of subrogation against Landlord and Tenant in connection with any damage, loss, or injury covered by such policy. If any such policy cannot be obtained with a waiver of subrogation, or is obtainable only by the payment of an additional premium charge above that charged by insurance companies issuing policies without waiver of subrogation endorsements, the party undertaking to obtain such policy (the “Undertaking Party”) shall so notify the other party (the “Notified Party”). The Notified Party shall, within ten days after the giving of such notice, either obtain such policy from a company that is reasonably satisfactory to the Undertaking Party and that will issue such policy with a waiver of subrogation endorsement, or agree to pay the additional premium if such policy is obtainable at additional cost. If such
15
policy cannot be obtained with a waiver of subrogation endorsement or the Notified Party refuses to pay such additional premium, then the Undertaking Party shall not be required to obtain a waiver of subrogation endorsement with respect to such policy.
20. Landlord’s Insurance. Landlord shall at all times while this Lease is in effect maintain the following insurance, in such amounts and with such limits as Landlord may determine in its reasonable discretion: (i) Public liability and property damage insurance, and products liability insurance; (ii) all risk property insurance insuring the Building. In addition, Landlord may, at its election, maintain any of the following insurance, and any other insurance deemed appropriate or necessary, in Landlord’s sole discretion, in such amounts and with such limits as Landlord shall determine in its reasonable discretion: (i) coverage with respect to increased costs due to building ordinances, demolition coverage, and sprinkler leakage coverage; (ii) boiler and machinery insurance; (iii) fidelity insurance; (iv) Plate-glass insurance; (v) earthquake insurance; (vi) terrorism insurance, (vii) flood insurance; and (vii) rental interruption and/or business interruption insurance. The premiums, costs, expenses, and deductibles (or similar costs or charges) of and/or with respect to any such insurance (all of the preceding, collectively, “Insurance Expenses”) shall be included in Operating Expenses. Any such coverage may be part of an umbrella or blanket policy, whereupon the premiums, costs, and expenses hereof will be reasonably apportioned between the Building and the other properties so included under such policy(ies).
21. Personal Property Taxes. Tenant shall pay before delinquency all taxes, assessments, license fees, and other charges that are levied or assessed against, or based upon the value of, Tenant’s personal property installed or located in or on the Premises including without limitation trade fixtures, furnishings, equipment, Alterations, and inventory (collectively, “Tenant’s Personal Property”). On written demand by Landlord, Tenant shall furnish Landlord with satisfactory evidence of such payments. If any such taxes, assessments, license fees, and/or other charges are levied against Landlord or Landlord’s property, or if the assessed value of the Premises is increased by the inclusion of a value placed on Tenant’s Personal Property, and if Landlord pays such taxes, assessments, license fees, and/or other charges or any taxes based on the increased assessments caused by Tenant’s Personal Property, then Tenant, on demand, shall immediately reimburse Landlord, as Additional Rent, for the sum of such taxes, assessments, license fees, and/or other charges so levied against Landlord, or the proportion of taxes resulting from such increase in Landlord’s assessment. Landlord may, at its election, pay such taxes, assessments, license fees, and/or other charges or such proportion, and receive such reimbursement.
22. Alterations. Tenant shall not make any alterations, improvements, additions, installations, or changes of any nature in or to the Premises (any of the preceding, “Alterations”) unless Tenant first obtains Landlord’s written consent to such Alteration and otherwise complies with the provisions of this Paragraph 22; provided, however, no such consent will be required in connection with any Minor Alterations (as defined below).
22.1. Request for Consent. At least 15 days prior to making any Alterations, Tenant shall submit to Landlord, in written form, proposed detailed plans of such Alterations, which plans must (i) in the case of a Minor Alterations, be in sufficient detail to, among other things, provide Landlord with reasonable evidence that such Alterations are of a nature that Landlord’s consent is not required, and (ii) in the case of any other Alterations, in sufficient detail to allow Landlord and its consultants to fully evaluate the proposed Alterations and their affect upon the Premises and the Project. Landlord will not unreasonably withhold, condition, or delay its consent to any Alterations for which consent is required; except that, in the case of exterior Alterations or Alterations which will be visible from outside the Premises or which will affect any structural components of the Project, Landlord shall have the right to grant or withhold its consent in the exercise of its sole discretion. In addition to the foregoing requirements, if the proposed Alteration requires approval by or notice to the lessor of a ground or underlying lease or the holder of a deed of trust encumbering the Project, no Alteration shall be commenced until such approval has been received, or such notice has been given, as the case may be, and all applicable conditions and provisions of said superior lease or deed of trust with respect to the proposed Alteration or Alterations have been met or complied with at Tenant’s expense; and Landlord, if it approves the Alteration, will request such approval or give such notice expeditiously, as the case may be, and thereafter diligently pursue obtaining such approval.
22.2. Minor Alterations. Notwithstanding anything to the contrary contained herein, minor, interior cosmetic Alterations such as painting, wall papering, carpeting or hanging pictures or moving furniture and temporary partitions or cubicles (the aggregate cost of which will not exceed $10,000.00, and which Alterations will not be visible from outside the Premises or affect any structural components of the Project) will not require Landlord’s prior consent so long as (i) Tenant notifies Landlord in accordance with Paragraph 22.1 (i), and (ii) Tenant complies with all reasonable conditions which may be imposed by Landlord including, but not limited to, the requirements of Paragraph 22.3 below, Landlord’s selection of specific contractors or construction techniques and the requirements of the attached Exhibit “C.” Any Alterations meeting the foregoing requirements to avoid the necessity of obtaining Landlord’s consent are referred to herein as a “Minor Alterations.”
16
22.3. Additional Requirements. Tenant shall, prior to the commencement of any Alterations, and at Tenant’s sole cost, (i) acquire (and deliver to Landlord a copy of) any required permit from the appropriate governmental agencies to make such Alterations (any conditions of which permit Tenant shall comply with, at Tenant’s sole cost, in a prompt and expeditious manner), (ii) provide Landlord with ten business days’ prior written notice of the date the installation of the such Alterations is to commence, so that Landlord can post and record an appropriate notice of non-responsibility, (iii) pay Landlord the reasonable costs and expenses of Landlord for architectural, engineering, or other consultants which reasonably may be incurred by Landlord in determining whether to approve any such Alterations (excluding Minor Alterations), and (iv) if applicable, obtain (and deliver to Landlord proof of) reasonably adequate workers compensation insurance with respect to any of Tenant’s employees installing or involved with such Alterations (which insurance Tenant shall maintain on an occurrence basis in force until completion of the Alterations). In addition, Tenant shall comply with all reasonable conditions which may be imposed by Landlord relative to such Alterations including, but not limited to, (v) Landlord’s selection of specific contractors or construction techniques and (2) the requirements of the attached Exhibit “C” applicable to Tenant’s Work. Notwithstanding anything to the contrary contained in this Paragraph 22.3, in no event may Tenant remove any ceiling tiles or ceiling gridwork or lighting without Landlord’s prior consent, and any such consent may be conditioned upon requiring Tenant to post a deposit to cover the cost of restoring the Premises to their prior condition upon termination of the Term and to secure Tenant’s obligation to so restore the Premises.
22.4. Ownership of Alterations. All Alterations shall, upon the Expiration Date of this Lease, become the property of Landlord and shall remain on and be surrendered with the Premises on the Expiration Date; except that, Landlord may, at its election, require Tenant to remove any or all of the Alterations, provided that Landlord notifies Tenant in writing prior to commencement of the Alterations. If Landlord so elects to have the Alterations removed, Tenant shall, at its sole cost, on or before the Expiration Date, repair and restore the Premises to the condition of the Premises prior to the installation of the Alterations which are to be removed. Tenant shall pay all costs for Alterations and other construction done or caused to be done by Tenant and Tenant shall keep the Premises free and clear of all mechanics’ and materialmen’s liens resulting from or relating to any Alterations or other construction. Tenant may, at its election, contest the correctness or validity of any such lien provided that within 20 days after written demand by Landlord, Tenant procures and records a lien release bond, issued by a corporation satisfactory to Landlord and authorized to issue surety bonds in California, in an amount equal to 125% of the amount of the claim of lien, which bond meets the requirements of California Civil Code Section 8424 or any successor statute.
22.5. Control over Tenant’s Wi-Fi Use.
(a) Wi-Fi. Tenant shall have the right to install, at its sole cost and expense, a wireless intranet, Internet, and communications network (also known as “Wi-Fi”) utilizing IEEE 802.XX protocols within the Premises for the use of Tenant and its employees (the “Network”) subject to the provisions of this Paragraph 22.5 and the other provisions of Paragraph 22. All telecommunications service providers shall be subject to Landlord’s prior written approval with Landlord’s said approval not to be unreasonably withheld or delayed.
(b) No solicitation. Tenant shall not solicit, suffer, or permit other tenants or occupants of the Building to use the Network or any other communications service, including, without limitation, any wired or wireless Internet service that passes through, is transmitted through, or emanates from the Premises.
(c) Interference. Tenant agrees that the Network, Tenant’s communications equipment and the communications equipment of Tenant’s service providers located in or about the Premises or installed in the Building to service the Premises including, without limitation, any antennas, switches, or other equipment (collectively, ‘‘Tenant’s Communications Equipment”) shall be of a type and, if applicable, a frequency that will not cause radio frequency, electromagnetic, or other interference to any other party or any equipment of any other party including, without limitation, Landlord, other tenants, or occupants of the Building, Landlord reserves the right to cause Tenant to operate on a channel or frequency band that Landlord selects. In the event that Tenant’s Communications Equipment causes or is believed by Landlord to cause any such interference, upon written receipt of notice from Landlord of such interference, Tenant will promptly take all steps necessary to correct and eliminate the interference. If the interference is not eliminated within 24 hours (or a shorter period if Landlord believes a shorter period to be appropriate) then, upon notice from Landlord, Tenant shall use other channels or frequencies as determined solely by Landlord, or, at Landlord’s election, shut down the Tenant’s Communications Equipment pending resolution of the interference (with the exception of intermittent testing upon prior notice to, and with the prior approval of, Landlord). Landlord shall have no obligation or liability with respect to any interruption, curtailment or discontinuance of telecommunications services.
(d) Maintenance. Tenant shall maintain Tenant’s Telecommunications Equipment in good order and repair at its sole cost and expense.
17
(e) Acknowledgment. Tenant acknowledges that Landlord has granted and/or may grant lease rights, licenses, and other rights to other tenants and/or occupants of the Building and to telecommunications service providers.
23. Surrender of Premises and Holding Over.
23.1. Surrender. On the Expiration Date, Tenant shall surrender to Landlord the Premises and all Alterations (except for Alterations that Tenant is obligated to remove as expressly set forth above) in a first class and clean condition, less any normal wear and tear, free of trash and debris including cleaning of all flooring; all walls shall be patched and painted; all signage installed by Tenant on any portion of the Buildings or Project shall be removed and the surfaces repaired, including restoration of the signage mounting surfaces to their pre-existing condition; all sign circuits, electrical circuits, and lighting fixtures shall be in good operating condition; all roof penetrations arising from Tenant’s occupancy of the Premises shall be in a watertight condition; and all doors, windows, locks, and hardware shall be in operable condition upon the termination of this Lease. Tenant shall additionally, as of the Expiration Date, remove all of Tenant’s Personal Property and perform all repairs and restoration required by the removal of any Alterations or Tenant’s Personal Property, and Tenant shall surrender to Landlord all keys to the Premises (including without limitation any keys to any exterior or interior doors). Landlord may elect to retain or dispose of in any manner any Alterations or Tenant’s Personal Property that Tenant does not remove from the Premises on the Expiration Date as required by this Lease by giving written notice to Tenant. Any such Alterations or Tenant’s Personal Property that Landlord elects to retain or dispose of shall immediately upon notice to Tenant vest in Landlord. Tenant waives all claims against Landlord for any damage to Tenant resulting from Landlord’s retention or disposition of any such Alterations or Tenant’s Personal Property. Tenant will be liable to Landlord for Landlord’s costs for storing, removing (including related restoration work), or disposing of any such Alterations or Tenant’s Personal Property. If Tenant fails to surrender the Premises to Landlord on the Expiration Date in the condition required by this Paragraph, Tenant shall indemnify, defend, and hold Landlord harmless from and against all liabilities, damages, losses, costs, expenses, attorneys’ fees and claims resulting from such failure, including without limitation any claim for damages made by a succeeding tenant.
23.2. Holding Over. If Tenant, with Landlord’s consent, remains in possession of the Premises after the Expiration Date, such possession by Tenant shall be deemed to be a month-to-month tenancy terminable on 30-days’ written notice given at any time by Landlord or Tenant. During any such month-to-month tenancy, or any other holdover tenancy which is without Landlord’s consent, Tenant shall pay, as Basic Monthly Rent, 150% of the Basic Monthly Rent in effect immediately prior to the Expiration Date; which rental amount Tenant acknowledges is fair and reasonable under all of the facts and circumstances existing as of the date of this Lease. All provisions of this Lease except for those pertaining to Term shall apply to any such tenancy. Acceptance by Landlord of rent after such expiration or earlier termination shall not constitute consent to a holdover tenancy hereunder or result in a renewal. The foregoing provisions this Paragraph 23.2 are in addition to, and do not affect, Landlord’s right of re-entry or any rights of Landlord hereunder or as otherwise provided by law. Landlord expressly reserves the right to require Tenant to surrender possession of the Premises to Landlord as provided in this Lease upon expiration or other termination of this Lease. The provisions of this Paragraph 23.2 shall not be considered to limit or constitute a waiver of any other rights or remedies of Landlord provided in this Lease or at law.
24. Default. The occurrence of any of the following shall constitute a material default and breach of this Lease by Tenant (each an “Event of Default”):
24.1. The abandonment (as defined in the California Civil Code 1951.3) of the Premises by Tenant.
24.2. Tenant’s failure to make any payment of Rent (including late charges) as and when due. No grace period prior to the imposition of a late charge pursuant to Paragraph 26 below, shall extend the date when such Rent is due and payable, and Tenant shall be in default under this Lease if such payment is not timely made. In the case of Basic Monthly Rent, payments must be received on or before the first day of each calendar month, and Tenant shall be in default if such Rent is not paid by such date.
24.3. Tenant’s failure to timely deliver an estoppel certificate to Landlord in accordance with the provisions of Paragraph 41, below, or a subordination, non-disturbance, and attornment agreement in accordance with Paragraph 40, below.
24.4. Tenant’s failure to observe or perform any of the provisions of this Lease to be observed or performed by Tenant, other than described in the preceding three paragraphs, where such failure shall continue for a period of ten days after written notice of such failure from Landlord to Tenant; provided, however, that any such notice shall be in lieu of, and not in addition to, any notice required under applicable unlawful detainer statutes; and provided further, that if the nature of Tenant’s default is such that more than ten days are reasonably required for its cure, then Tenant shall not be deemed
18
to be in default if Tenant commenced such cure within such ten day period and thereafter diligently prosecutes such cure to completion within 60 days after Landlord’s written notice. Such written notice will be deemed to satisfy the statutory notice requirements of applicable unlawful detainer statutes and will be in lieu thereof (and not in addition thereto).
24.5. Intentionally Deleted.
24.6. Tenant’s failure to deliver to Landlord, within ten days after Landlord’s written request but not more than once per calendar year, any financial statement of Tenant (including without limitation a current annual balance sheet of Tenant) reasonably requested by Landlord, or if any financial statement given to Landlord by Tenant, or by any assignee, subtenant, or guarantor of Tenant, is materially false or evidences that Tenant’s net worth is negative, and Tenant fails to furnish to Landlord, within ten days after written notice from Landlord to Tenant, with cash as an additional security deposit in an amount equal to the aggregate Rental payable under this Lease for the six full calendar months immediately following such notice.
24.7. The making by Tenant of any general arrangement or assignment for the benefit of creditors; Tenant’s becoming bankrupt, insolvent or a “debtor” as defined in 11 U.S.C. Section 101, or any successor statute (unless, in the case of a petition filed against Tenant, such petition is dismissed within 60 days after its original filing); the institution of proceedings under the bankruptcy or similar laws in which Tenant is the debtor or bankrupt; the appointing of a trustee or receiver to take possession of substantially all of Tenant’s assets located at the Premises or of Tenants interest in this Lease (unless possession is restored to Tenant within 60 days after such taking); the attachment, execution, or judicial seizure of substantially all of Tenant’s assets located at the Premises or Tenant’s interest in this Lease (unless such attachment, execution, or judicial seizure is discharged within 60 days after such attachment, execution, or judicial seizure); or, if Tenant is a partnership or consists of more than one person or entity, any partners of the partnership or any such other person or entity becoming bankrupt or insolvent or making a general arrangement or assignment for the benefit of creditors.
24.8. Notwithstanding the foregoing, if (i) Tenant commits two similar defaults during any 12-month period or less, (ii) Tenant receives notices of default on each separate occasion, and (iii) each such default could have been cured within a 24-hour period from the date Tenant received notice of such default, then, as to any further, similar default that thereafter occurs during the same 12-month period. Landlord may treat such default as an Event of Default and exercise its remedies under Paragraph 26, below, without giving Tenant any further notice of default or opportunity to cure.
25. Landlord’s Remedies. Landlord shall have the following remedies if Tenant commits an Event of Default under this Lease. These remedies are not exclusive, but are cumulative and in addition to any remedies provided elsewhere in this Lease or now or later allowed by law.
25.1. Continuation of Lease. No act by Landlord shall terminate Tenant’s right to possession unless Landlord notifies Tenant in writing that Landlord elects to terminate Tenant’s right to possession. As long as Landlord does not terminate Tenant’s right to possession, Landlord may (i) continue this Lease in effect, (ii) continue to collect Rent when due and enforce all the other provisions of this Lease, and (iii) enter the Premises and relet them, or any part of them, to third parties for Tenant’s account, for a period shorter or longer than the remaining Term of this Lease. Tenant shall immediately pay to Landlord all costs Landlord incurs in such reletting, including, brokers’ commissions, attorneys’ fees, advertising costs, and expenses of remodeling the Premises for such reletting. The parties agree that Landlord is to have the remedy described in California Civil Code Section 1951.4 (which effectively provides that a lessor may continue a lease in effect after the lessee’s breach and recover rent as it becomes due), and the Tenant hereby acknowledges that this Lease meets the requirements of such statutory provision and that Tenant’s rights to sublet or assign hereunder are subject only to reasonable limitations.
25.2. Rent from Reletting. If Landlord elects to relet all or any portion of the Premises as permitted above, rent that Landlord receives from such reletting shall be applied to the payment of, in the following order and priority, (i) any indebtedness from Tenant to Landlord other than Rent due from Tenant, (ii) all costs incurred by Landlord in such reletting, and (iii) Rent due and unpaid under this Lease. After applying such payments as referred to above, any sum remaining from the rent Landlord receives from such reletting shall be held by Landlord and applied in payment of future Rent as it becomes due under this Lease. In no event shall Tenant be entitled to any excess rent received by Landlord unless and until all obligations of Tenant under this Lease, including all future obligations, are satisfied in full.
25.3. Termination of Tenant’s Right to Possession. Landlord may terminate Tenant’s right to possession of the Premises at any time, by notifying Tenant in writing that Landlord elects to terminate Tenant’s right to possession. Such written notice will result in the immediate termination of this Lease upon the date such right of possession is terminated. Upon termination of this Lease, Landlord has the right to recover from Tenant (i) the worth at the time of the award of the
19
unpaid Rent which had been earned at the time of such termination, (ii) the worth at the time of the award of the amount by which the unpaid Rent which would have been earned after such termination until the time of award exceeds the amount of such loss of Rent that Tenant proves could have been reasonably avoided, (iii) the worth at the time of the award of the amount by which the unpaid Rent for the balance of the Term after the time of award (had there been no such termination) exceeds the amount of such loss of Rent that Tenant proves could be reasonably avoided, and (iv) any other amount necessary to compensate Landlord for all detriment proximately caused by Tenant’s failure to perform Tenant’s obligations under this Lease or in the ordinary course of things would be likely to result therefrom. The “worth at the time of the award” of the amounts referred to in clauses (i) and (ii) above is to be computed by allowing interest at the Default Rate. The “worth at the time of the award” of the amount referred to in clause (iii) above is to be computed by discounting such amount at the discount rate of the Federal Reserve Bank of San Francisco at the time of award plus one percent.
25.4. Landlord’s Right to Cure Default. Landlord, at any time after Tenant commits an Event of Default, may cure such Event of Default at Tenant’s sole cost. If Landlord at any time, by reason of Tenant’s default or breach, pays any sum or does any act that requires the payment of any sum, such sum shall be due immediately from Tenant to Landlord at the time such sum is paid, along with a supervisory fee in the amount of ten percent of such amount so expended by Landlord, and shall be deemed Additional Rent under this Lease. If Tenant fails to timely pay any amount due under this Paragraph within ten business days of receipt of Landlord’s invoice for such costs, then (without curing such default) interest at the Default Rate shall accrue (and be immediately payable) on such overdue amount until it is paid.
25.5. Enforcement Costs. All costs and expenses incurred by Landlord in connection with collecting any amounts and damages owing by Tenant pursuant to the provisions of this Lease, or to enforce any provision of this Lease, including reasonable attorneys’ fees, whether or not any action is commenced by Landlord, shall be paid by Tenant to Landlord upon demand. If Tenant fails to timely pay any amount due under this Paragraph, then (without curing such default) interest at the Default Rate shall accrue (and be immediately payable) on such overdue amounts until it is paid.
26. Interest and Late Charges. Late payment by Tenant to Landlord of Rent will cause Landlord to incur costs not contemplated by this Lease, the exact amount of which would be impracticable or extremely difficult to fix. Such costs include, processing, collection and accounting charges, and late charges that may be imposed on Landlord by the terms of any deed of trust covering the Premises. Therefore, if any Rent (in the form of good funds) is not received by Landlord within ten days of its due date, then, without any requirement for notice to Tenant, Tenant shall owe and pay to Landlord an additional sum of ten percent of such overdue amount as a late charge. Such late charge represents a fair and reasonable estimate of the costs that Landlord will incur by reason of any late payment by Tenant, and therefore this Paragraph is reasonable under the circumstances existing at the time this Lease is made. Acceptance of such late charge by Landlord shall not constitute a waiver or cure of Tenant’s default with respect to such overdue amount, nor prevent Landlord from exercising any of the other rights and remedies available to Landlord under this Lease. In addition to the late charge payable by Tenant, as provided above, if any such Rent is not paid within 30 days of the date such Rent was due, then Tenant shall pay to Landlord interest on such overdue Rent (from such 30th day until all amounts, including interest, are paid in full) at the rate of seven percent above the “reference rate” announced from time to time by Bank of America, NT&SA (the “Default Rate”). If such reference rate ceases to be announced, then a comparable “prime rate” shall be utilized, as selected by Landlord.
27. Landlord Default – Tenant’s Remedies. If Landlord fails to cure a default by Landlord within any applicable cure period (or if no cure period is specified, then within 30 days of written notice from Tenant setting forth the nature of the claimed default; provided, however, if the nature of the cure of such default will reasonably require more than 30 days to complete and Landlord is proceeding with due diligence to remedy such matter, then such 30 day period will be extended for such additional time as may be necessary for Landlord to complete such cure), Tenant may, as Tenant’s sole remedy, remedy such default, whereupon Landlord shall reimburse Tenant for the reasonable third-party costs incurred by Tenant to remedy such default within 30 days after receipt of Tenant’s written demand therefor, together with copies of the paid invoices evidencing the costs so incurred. In no event will Tenant have any right to offset any amount owed by Landlord (regardless of whether Landlord is in default hereunder) against Tenant’s monetary obligations under this Lease).
28. Intentionally Deleted
29. Destruction. If the Building is totally or partially destroyed during the Term, rendering the Premises totally or partially inaccessible or unusable, then, subject to the remainder of this Paragraph, (i) Landlord shall promptly commence work necessary to restore the Building to substantially the same condition as it was in immediately before such destruction and shall diligently prosecute such restoration work until completed, (ii) Landlord shall not be required to
20
restore Tenant’s Alterations or Tenant’s Personal Property, unless they are an integral part of the Premises and they are specifically covered by insurance proceeds received by Landlord, such excluded items being the sole responsibility of Tenant to restore, (iii) such destruction shall not terminate this Lease (except as provided below), and (iv) all obligations of Tenant under this Lease shall remain in effect, except that the Basic Monthly Rent and Additional Rent shall be abated or reduced, between the date of such destruction and the date of Substantial Completion of restoration, by the ratio of (a) the Rentable Square Footage of the Premises rendered unusable or inaccessible by the destruction, to (b) the Rentable Square Footage of the Premises prior to such destruction. (The term “Substantial Completion” as used herein will mean completed to such an extent that the Premises may be used by Tenant for the Permitted Use, subject only to punchlist or correction items, and the restoration can be finally completed within 60 days and without material interference to Tenant’s occupancy and use of the Premises.) Notwithstanding anything to the contrary in this Paragraph, either party shall have ten business days from the date of Landlord’s determination that this sentence applies to the subject destruction/reconstruction, in which to terminate this Lease if Landlord determines that (1) it will likely take more than either (A) 330 days following the date of such casualty, or (B) 270 days from obtaining all required permits for such reconstruction, in which to complete such work, (2) such destruction (which is not de minimus in nature) occurs during the last year of the Term, or (3) then-existing laws do not permit such restoration. Additionally, Landlord may, at its election, terminate this Lease by so notifying Tenant in writing on or before the later of 60 days after such destruction or 30 days after Landlord’s receipt of the proceeds (or written notice of the amount of proceeds) from insurance maintained by Landlord, if (I) such destruction exceeds 20% of the then-replacement value of the Premises, the Building, or the Project, or (II) Landlord reasonably determines that the cost of such restoration will exceed the amount of insurance proceeds relating to such destruction actually received by Landlord from insurance maintained by Landlord, excluding deductibles, by more than five percent of such cost of restoration. If Landlord or Tenant so terminates this Lease, then (x) Landlord shall have no obligation to restore the Project , (y) Landlord shall retain all insurance proceeds under Landlord’s maintained policies relating to such destruction, and (z) this Lease shall terminate as of 30 days after such notice of termination from Landlord to Tenant. Tenant hereby waives the provisions of California Civil Code Sections 1932(2) and 1933(4) or any successor statute with respect to any destruction of the Premises. If Landlord restores the Premises following any such destruction, Tenant shall immediately refixturize, re-equip, and (if applicable) restock the Premises and shall re-open the Premises for business as soon thereafter as is reasonably practicable. If Tenant does not intend to so reopen the Premises for business, it must notify Landlord in writing within 20 business days of such damage or destruction, whereupon Landlord may cease its repair work and terminate this Lease. Additionally, if Landlord fails to Substantially Complete such restoration work within two hundred seventy (270) days, Tenant may, by 30 days’ written notice to Landlord delivered after such year (during which period of time such restoration is not Substantially Completed), terminate this Lease.
30. Condemnation. If during the Term, or during the period of time between the execution of this Lease and the Lease Commencement Date, there is any taking of all or any part of the Premises or any interest in this Lease by the exercise of any governmental power, whether by legal proceedings or otherwise, by any public or quasi-public authority, or private corporation or individual, having the power of condemnation (any of the preceding a “Condemnor”), or a voluntary sale or transfer by Landlord to any Condemnor, either under threat of condemnation or while legal proceedings for condemnation are pending (any of the preceding, a “Condemnation”), the rights and obligations of Landlord and Tenant shall be determined pursuant to this Paragraph. If such Condemnation is of the entire Premises, then this Lease shall terminate on the date the Condemnor takes possession of the Premises (the “Date of Condemnation”). If such Condemnation is of any portion, but not all, of the Premises, then this Lease shall remain in effect, except that, if the remaining portion of the Premises is rendered unsuitable for Tenant’s continued use of the Premises as conducted prior to Condemnation, then Tenant may elect to terminate this Lease, by so notifying Landlord in writing (the “Termination Notice”) within 30 days after the date that the nature and extent of the Condemnation have been determined. Such termination shall be effective on the earlier of (i) the date that is 30 days after the giving of the Termination Notice, or (ii) the Date of Condemnation. If Tenant does not give to Landlord the Termination Notice within such 30-day period, then all obligations of Tenant under this Lease shall remain in effect, except that (unless the Premises are restored as set forth below) Basic Monthly Rent and Tenant’s Share of Operating Expenses shall be reduced by the ratio of (a) the Rentable Square Footage of the Premises taken to (b) the Rentable Square Footage of the Premises immediately prior to the Date of Condemnation. Notwithstanding anything to the contrary in this Paragraph, if, within 30 days after Landlord’s receipt of the Termination Notice, Landlord notifies Tenant that Landlord at its cost will add to the remaining Premises (or substitute for the Premises other comparable space in the Project) so that the Rentable Square Footage of the Premises will be substantially the same after the Condemnation as they were before the Condemnation, and Landlord commences the restoration promptly and completes it within 150 days after Landlord so notifies Tenant, then all obligations of Tenant under this Lease shall remain in effect, except that Basic Monthly Rent and Additional Rent shall be abated or reduced during the period from the Date of Condemnation until the completion of such restoration by the ratio of (A) the Rentable Square Footage of the Premises taken to (B) the Rentable Square Footage of the Premises immediately prior to the Date of Condemnation. Unless Landlord restores the Premises pursuant to the preceding sentence, or unless Tenant gives to Landlord the Termination Notice within the relevant 30-day period, Tenant at its sole cost shall accomplish any restoration
21
required by Tenant to use the Premises. A temporary Condemnation of the Premises, or any part of the Premises, for less than 180 days, shall not constitute a Condemnation under this Paragraph; but the Basic Monthly Rent and Tenant’s Share of Operating Expenses shall abate as to the portion of the Premises affected during such temporary Condemnation, including any portion of the Premises rendered unsuitable for Tenant’s continued use as conducted prior to the Condemnation. All compensation, sums, or anything of value awarded, paid, or received on a total or partial Condemnation (the “Award”) shall belong to and be paid to Landlord. Tenant shall have no right to any part of the Award, and Tenant hereby assigns to Landlord all of Tenant’s right, title, and interest in and to any part of the Award, except that Tenant shall receive from the Award any sum paid expressly to Tenant from the Condemnor for Tenant’s Personal Property or for severance damages. Landlord and Tenant waive the provisions of any statute (including without limitation California Code of Civil Procedure Section 1265.130 or any successor statute) that allows Landlord or Tenant to petition the superior court (or any other court) to terminate this Lease in the event of a partial Condemnation of the Premises.
31. Assignment and Other Transfers.
31.1. Restriction on Transfer. Without Landlord’s prior written consent, which shall not be unreasonably withheld, conditioned or delayed and except as permitted by Paragraph 31.3, below, none of the following shall occur (nor be permitted by Tenant to occur), voluntarily, involuntarily, by operation of law, or otherwise (any of the following, a ‘‘Transfer’’): (i) any assignment, sublease, disposition, sale, concession, license, license agreement for the use of any portion of the Premises, mortgage, encumbrance, hypothecation, pledge, collateral assignment, or other transfer, by Tenant of this Lease, any interest in this Lease, or all or any portion of the Premises; or (ii) any assignment, disposition, sale, transfer, acquisition, or issuance of equitable interests (whether stock, partnership or otherwise) in Tenant, to or by any person, entity, or group of related persons or affiliated entities, whether in a Single transaction or in a series of related or unrelated transactions, which results in such person, entity, or group holding (or assigning, transferring, disposing of, or selling) 50% or more of the aggregate issued and outstanding equitable interests in Tenant.
31.2. Transfer Provisions Generally.
31.2.1. Landlord shall not be liable in damages to Tenant or to any proposed subtenant, assignee or other transferee (any of the preceding a “Proposed Transferee”) if such consent is adjudicated to have been unreasonably withheld, and, in such event, Tenant’s sole remedy shall be to have the proposed Transfer declared as valid as if Landlord’s consent had been given, although Tenant shall be entitled to reasonable attorney’s fees if Tenant is the prevailing party in such litigation. At least 30 days prior to entering into any proposed Transfer, Tenant shall submit to Landlord the sum of $500.00 (as payment toward Landlord’s and Landlord’s attorneys’ cost of reviewing, consenting to, rejecting and/or consummating any proposed Transfer), and a written notice (‘‘Tenant’s Notice”) which includes (i) a fully executed copy of the instrument of transfer (i.e., the sublease or assignment) relating to the proposed Transfer, along with all related agreements, documents, instruments, exhibits, and escrow instructions, (ii) the name and address of the Proposed Transferee, (iii) an abstract of the terms and conditions of the proposed Transfer, including without limitation the economics of such Proposed Transfer and the commencement or effective date of the proposed Transfer, which shall be at least 30 days after Tenant’s Notice is given, and (iv) the nature, character, and current banking, financial, and other credit information and references with respect to the Proposed Transferee and the business of the Proposed Transferee (including without limitation tax returns for the three most-recent years, a business plan with cash-flow projections and financial projections with assumptions and competitive market analysis), in reasonably sufficient detail to enable Landlord to determine the Proposed Transferee’s financial responsibility.
31.2.2. Within 30 days after Landlord’s receipt from Tenant of such sum and Tenant’s Notice, and all documentation requested of Tenant by Landlord, Landlord shall notify Tenant whether Landlord has consented to the proposed Transfer. Any consent by Landlord to any proposed Transfer shall not constitute a consent with respect to any other Transfer. If Landlord consents to any proposed Transfer, and Tenant fails to consummate such Transfer within 30 days of the commencement or effective date of the proposed Transfer (as set forth in Tenant’s Notice) or, if Tenant’s Notice fails to identify such a date, then within 150 days of the Tenant’s Notice, then such consent shall be deemed withdrawn and Tenant shall be required again to comply with this Paragraph before making a Transfer. Landlord shall not have unreasonably withheld its consent with respect to any Transfer if (among other things) Landlord shall not have received such sum or Tenant’s Notice, if the nature or character of the Proposed Transferee is not in keeping with the dignity and character of the Building and the surrounding area, if the Proposed Transferee’s proposed use is materially and adversely different than the Permitted Use or Tenant’s prior use, if the proposed Transfer will result in the diminution of the value or marketability of the Building or the Project, if Landlord is not reasonably satisfied that the Proposed Transferee is creditworthy, or if the proposed Transfer will conflict with or result in a breach of any of the provisions of, or constitute a default under, any agreement, instrument, or document to which Landlord is a party or by which the Project may be bound. No Transfer shall release or discharge Tenant from any liability, whether past, present, or future, under this Lease and Tenant shall continue to remain directly and primarily liable under this Lease (and not as a mere surety);
22
provided, however, as a condition to granting consent to any assignment (or like Transfer) Landlord may require the assigning Tenant to execute a guaranty on Landlord’s standard form—which guaranty shall serve to release such assigning Tenant from direct liability hereunder and such assigning Tenant will then only have liability for matters first accruing under this Lease thereafter pursuant to such guaranty. Tenant irrevocably assigns to Landlord, as security for Tenant’s obligations under this Lease, all rent and other amounts generated from any Transfer, and Landlord, as assignee and as special attorney-in-fact for Tenant, or a receiver for Tenant appointed on Landlord’s application, may collect such rent and other amounts and apply them toward Tenant’s obligations under this Lease; except that, unless a default occurs under this Lease, Tenant shall have the right to collect such rent and other amounts.
31.2.3 Unless otherwise agreed to by all parties, the Tenant’s Security Deposit (if any) shall be retained by Landlord and returned to the lawful tenant in possession of the Premises at the time of the Lease termination, subject to the terms and conditions of Paragraph 6 of this Lease. Any Transfer documentation shall contain the following provisions, which provisions whether contained in such Transfer documentation or not, shall apply to such Transfer: (a) Such Transfer shall be subject and subordinate to, and bound by, all provisions of this Lease; (b) No Proposed Transferee shall be permitted to enter into any Transfer without Landlord’s prior written consent; and (c) At Landlord’s option, in the event of cancellation or termination of this Lease for any reason or the surrender of this Lease, whether voluntarily, involuntarily, by operation of law or otherwise, prior to the expiration of such Transfer, the Proposed Transferee shall make full and complete attornment to Landlord for the balance of the term of such Transfer. Such attornment shall be evidenced by an agreement in form and substance reasonably satisfactory to Landlord that the Proposed Transferee shall execute and deliver to Landlord within five days after request by Landlord.
31.2.4. Tenant shall promptly reimburse Landlord for Landlord’s reasonable cost (less the $500.00 previously paid) of reviewing, consenting to, rejecting and/or consummating any proposed Transfer, including without limitation reasonable attorneys’ fees and costs/fees of Landlord’s Lender in connection therewith. If Tenant fails to pay such amount within twenty business days of written demand, Tenant shall be in default hereunder and Landlord shall have the right, in addition to its other rights and remedies under this Lease, to revoke its prior approval of the proposed Transfer if such Proposed Transferee has not yet taken over possession of the Premises.
31.3. Excess Rent and Recapture. Tenant shall promptly pay to Landlord, as and when received, 50% of all rents and other consideration after all of Tenant’s reasonable third-party expenses incurred in connection with such Transfer are deducted, of whatever nature, payable by the Proposed Transferee (or receivable by Tenant) pursuant to or as a result of any Transfer, which exceed (i) in the case of a sublease of a portion of the Premises, the portion of the Basic Monthly Rent that is allocable to the portion of the Premises subleased (such allocation based on the Rentable Square Footage of the portion subleased), or (ii) in the case of any other Transfer, the Basic Monthly Rent. Landlord additionally has the right, in the event Tenant indicates in the Tenant’s Notice that it desires to assign this Lease or sublet greater than 50% of the Premises, at its election, by giving written notice (the “Recapture Notice”) to Tenant within 15 days after receipt of Tenant’s Notice, to recapture the Premises and terminate this Lease. If Landlord elects to exercise such right and delivers a Recapture Notice to Tenant, this Lease shall automatically be deemed terminated as of the commencement or effective date stated in Tenant’s Notice for the proposed Transfer, and Tenant shall surrender possession of the Premises as of such date (and any failure to do so shall constitute a default hereunder); provided that, Tenant may in a written notice given within 15 days after receipt of the Recapture Notice withdraw the Tenant’s Notice, which shall thereafter be deemed of no force or effect.. Landlord’s giving of a Recapture Notice shall not constitute Landlord’s consent to Tenant’s proposed Transfer.
31.4. Permitted Transferee. Notwithstanding anything to the contrary contained in Paragraphs 31.1 or 31.3, above, no consent of Landlord will be required for, and no amounts will be payable to Landlord in connection with, any assignment or subletting to any of the following (any of which will constitute a “Permitted Transferee”):
31.4.1. Any parent, wholly-owned subsidiary, or other company of which Tenant owns all or substantially all of the voting and beneficial interests, or which company owns all or substantially all of the voting and beneficial interests in Tenant, and which parent, subsidiary, or other company has a net worth (determined in accordance with GAAP) equal to or greater than Tenant’s net worth as of the day before such transaction or as of the Lease Commencement Date, whichever is less;
31.4.2. Any surviving or successor entity resulting from a merger, consolidation, or sale of substantially all of the assets of Tenant, where the net worth of the resulting or acquiring company exceeds (as determined in accordance with GAAP), the net worth of the Tenant as of the day prior to such transaction or as of the Lease Commencement Date, whichever is less; or
23
31.4.3. Any sale of stock as part of a “public offering” on one of the nationally recognized securities exchanges (such as, without limitation, NYSE or NASDAQ).
Notwithstanding the foregoing, and as a condition precedent to the effectiveness of any such Transfer to a Permitted Transferee under Sections 31.4.1 or 31.4.2, at least 20 days prior to any proposed Transfer to a Permitted Transferee, Tenant shall notify Landlord in writing of its intention to undertake such a Transfer and provide Landlord with sufficient information to confirm that such entity will in fact be a Permitted Transferee and the assigning Tenant shall execute Landlord’s form guaranty—which guaranty shall serve to release such assigning Tenant from direct liability hereunder and such assigning Tenant will then only have liability for matters first accruing under this Lease thereafter pursuant to such guaranty (it being understood that if such assigning Tenant fails to execute such a Guaranty, then such assignment shall constitute an Event of Default, such Transfer will be void, and such assigning Tenant shall remain primarily liable hereunder). Landlord shall keep all such information confidential. Other than the right to engage in such a Transfer to a Permitted Transferee without Landlord’s consent, all other provisions of Paragraph 31.2 shall apply to such a Transfer under Sections 31.4.1 or 31.4.2.
32. Landlord’s Reserved Rights.
32.1. General Rights Reserved. In addition to the specific reserved rights identified in Paragraph 32.2, below, Landlord, as owner of the Project, in addition to Landlord’s other rights, reserves the right from time to time: (i) to temporarily utilize portions of the Common Areas for, among other things, entertainment, outdoor shows, displays, automobile and other product shows, the leasing of kiosks, or such other uses which, in Landlord’s reasonable judgment, are appropriate; (ii) to utilize the lighting standards and other areas or improvements in the Common Areas for advertising, notice purposes, or other reasonable purposes; (iii) to close any of the Common Areas to the extent required in the opinion of Landlord’s legal counsel to prevent a dedication of any of the Common Areas or the accrual of any rights to any person or to the public in and to any portion of the Common Areas; (iv) to close, temporarily, any of the Common Areas for maintenance purposes; (v) to designate other property outside the boundaries of the Project to become part of the Common Areas; (vi) to close off or otherwise utilize portions of the Common Areas while constructing improvements or making repairs or alterations to any portion of the Project; (vii) to utilize portions of the Common Areas, on a temporary basis, as a staging area for any construction work by Landlord or its affiliates, agents, tenants, or contractors; and (viii) to make any changes to the Common Areas, or any part of the Project, including without limitation changes to buildings or other improvements, the addition of new buildings or other improvements, and/or changes in (among other things) the location of driveways, entrances, exits, vehicular parking spaces, or the direction of the flow of traffic. In exercising such rights, Landlord agrees that (1) it will not materially adversely interfere with Tenant’s use of the Premises and further agrees to use commercially reasonable efforts to minimize any lesser interference with Tenant’s use of the Premises caused by the exercise of such rights; and (ii) in the event Landlord elects to close or restrict access to a material portion of the parking area pursuant to the rights reserved under this Paragraph 32.1, Landlord will provide substitute unreserved parking at no cost to Tenant.
32.2. Future Construction. Tenant acknowledges that, as more particularly provided below, the development of the Project is continuing and may, at Landlord’s election, include the construction of additional buildings and improvements within the Project, including in areas which currently constitute Common Areas. Tenant is entering into this Lease with a full understanding of the possible ramifications/effects of such future development work on its tenancy and the rental charged hereunder takes such factors into account. Tenant further acknowledges and agrees that Landlord may, from time to time, at its sole election, construct (including, without limitation, additional buildings), reconstruct (including without limitation the replacement of certain improvements with other improvements), improve (including tenant improvements), modify, expand, or otherwise alter the Project (collectively, “Construction Work”), or portions thereof (in no event however will Landlord have any obligation to do so). Tenant acknowledges that any such Construction Work will necessarily involve, among other things, the generation of noise, dust, and vibrations, barricading portions of the Project and the placement of scaffolding within the Project, demolition, structural alterations, storage of materials and equipment within the Project, and the presence of workmen within the Project, all of which may require the rearrangement of the Common Areas, including, without limitation, landscaping, parking areas (which may include the provision of temporary parking areas during periods of construction), roadways, lighting facilities, and the re-direction of vehicular and pedestrian traffic. Further, Landlord hereby reserves such licenses and easements in, on, above or below the Premises as may be reasonably required (i) for the installation, inspection, surveying, maintenance, or construction of mains, conduits, shafts, columns, footings, piers, pipes or other facilities to serve any building within the Project, or (ii) for any Construction Work; provided, however, Landlord will use its good faith efforts to minimize any unreasonable interference with Tenant’s use, occupancy, or enjoyment of the Premises as contemplated by this Lease. Except as provided below, Tenant waives any and all claims, defenses, rights of offset, or deductions based upon any inconvenience suffered by Tenant or any interruption of or interference with Tenant’s business including, without limitation, any loss of business, damage to property, loss of electronic information, or inconvenience to Tenant or Tenant’s Invitees as a result of or relating to such
24
Construction Work. Landlord hereby reserves for itself and its agents, employees, licensees and contractors, the right to enter the Premises to the extent reasonably necessary to pursue such Construction Work upon 24 hours’ prior notice to Tenant. The exercise of any of Landlord’s rights pursuant to this Paragraph will not entitle Tenant to any abatement of Rent or other claim, right of offset, or defense against Landlord, except that (a) Tenant shall have the right (subject to the provisions of Paragraph 19) to bring an action against Landlord (as Tenant’s sole remedy) in the event Tenant suffers any damages as a result of Landlord’s gross negligence or intentional misconduct in pursuing such Construction Work, and (b) if such Construction Work results in Tenant being unable to access the Premises, or portions thereof, for the Permitted Use for a period of greater than five business days, Tenant shall be entitled to equitable abatement of the Rent for such period of time during which it is unable to access the Premises. Tenant further acknowledges that expansion of the Project may affect the amount of the Lease Expenses and the portion thereof payable by Tenant. . In exercising such rights, Landlord agrees that (1) it will not materially adversely interfere with Tenant’s use of the Premises and further agrees to use commercially reasonable efforts to minimize any lesser interference with Tenant’s use of the Premises caused by the exercise of such rights; (ii) subject to Paragraph 11, Landlord will not materially adversely interfere with Tenant’s parking rights; and (iii) in the event Landlord elects to close or restrict access to a material portion of the parking area pursuant to the rights reserved under this Paragraph 32.1, Landlord will provide substitute unreserved parking at no cost to Tenant.
32.3. Intentionally Deleted.
33. Easements. Landlord may, at its election, from time to time, grant such easements, rights and dedications, and cause the recordation of parcel maps, easement and operating agreements, and restrictions affecting the Premises and the Project, provided that no such acts materially and adversely affect Tenant’s rights of ingress or egress to the Building and the Premises or Tenant’s right to use the Premises. Tenant shall promptly sign any documents or instruments to accomplish the foregoing upon request by Landlord. Tenant irrevocably appoints Landlord as Tenant’s special attorney-in-fact to execute and deliver such documents or instruments on behalf of Tenant if Tenant refuses or fails to do so within ten days of written request.
34. Access by Landlord. Landlord and any of Landlord’s Invitees shall have the right to enter the Premises at all reasonable times, during normal business hours if feasible under the circumstances, and upon 24 hours’ notice, if feasible under the circumstances, (i) to determine whether the Premises are in good condition and whether Tenant is complying with its obligations under this Lease, (ii) to do any necessary maintenance or make any restoration to the Premises that Landlord has the right or obligation to perform, (iii) to serve, post, or keep posted any notices required or allowed under this Lease, (v) to post “for sale” or “for rent” or “for lease” signs during the final nine months of the Term, (vi) to show the Premises to brokers, lenders, agents, prospective buyers, prospective tenants, or other persons interested in a listing of, financing, purchasing, or occupying the Project, the Premises or any portion of the Project or the Premises, and (vii) to shore the foundations, footings, and walls of the Project, and to erect scaffolding and protective barricades around and about the Premises, but not so as to prevent entry to the Premises, and to do any other act or thing necessary for the safety or preservation of the Premises if any excavation or other construction is undertaken or is about to be undertaken on any adjacent property or nearby street. In the event of an emergency Landlord shall have the right to enter the Premises at any time, without prior notice to Tenant. Landlord’s rights under this Paragraph extend, with Landlord’s consent, to the owner of adjacent property on which excavation or construction is to take place and the adjacent property owner’s agents, employees, officers, and contractors. Landlord shall not be liable for any inconvenience, disturbance, loss of business, nuisance, or other damage arising out of any entry on the Premises as provided in this Paragraph except damage resulting directly from the grossly negligent acts or willful misconduct of Landlord or Landlord’s Invitees. Tenant shall not be entitled to any abatement or reduction of Basic Monthly Rent or other Rent because of the exercise by Landlord of any rights under this Paragraph.
35. Indemnity. Tenant hereby agrees to indemnify, defend, protect, and hold harmless Landlord and its shareholders, officers, directors, agents, property managers, employees, contractors, and the partners comprising Landlord (if any) from and against all Claims (as defined below) and all costs, expenses, and attorneys’ fees incurred in the defense or handling of any such Claims or any action or proceeding brought on any of such Claims. For purposes of this Lease, the term “Claims” shall mean all liabilities, damages, losses, costs, expenses, attorneys’ fees, and claims (except to the extent they result from Landlord’s negligent acts or willful misconduct) arising from or which seek to impose liability under or because of (i) Tenant’s or Tenant’s Invitees’ use of the Premises, (ii) the conduct of Tenant’s business, (iii) any activity, work, or things done, permitted, or suffered by Tenant or any of Tenant’s Invitees in or about the Premises or elsewhere, (iv) any breach or default in the performance of any obligation to be performed by Tenant under this Lease, and/or (v) any negligence of Tenant or any of Tenant’s Invitees. If any action or proceeding is brought against Landlord or its shareholders, officers, directors, agents, property managers, employees, contractors, or the partners comprising Landlord (if any) by reason of any such Claims, Tenant upon notice from Landlord shall defend such action or proceeding at Tenant’s sole cost by legal counsel satisfactory to Landlord.
25
36. Exemption of Landlord from Liability. Except to the extent caused by Landlord’s grossly negligent acts or willful misconduct, Tenant assumes all risk of, Tenant waives all claims against Landlord in respect of, and Landlord shall not be liable for, any of the matters set forth in the preceding Paragraph or any of the following: injury to Tenant’s business, loss of income from such business, or damage or injury to the goods, wares, merchandise, or other property or the person of Tenant, Tenant’s Invitees, or any other persons in, upon, or about the Premises, whether such damage, loss, or injury is caused by or results from criminal acts, fire, steam, electricity, gas, water, rain, the breakage, leakage, obstruction or other defects of pipes, sewer lines, sprinklers, wires, appliances, plumbing, air-conditioning or lighting fixtures, or any other cause, conditions arising upon the Premises, or other sources or places, and regardless of whether the cause of such damage, loss, or injury or the means of repairing such damage, loss, or injury is inaccessible to Tenant. In connection with the foregoing, Tenant hereby waives any defense would otherwise be provided by Section 1542 of the California Civil Code (which states “A general release does not extend to claims which the creditor does not know or suspect to exist in his or her favor at the time of executing the release, which if known by him or her must have materially affected his or her settlement with the debtor”), or laws of a similar nature, which would limit any such release to matters known or suspected to exist by Tenant. This Lease shall not be affected or impaired by any change to any part of the Project or any sidewalks, streets or improvements nearby the Project.
37. Hazardous Substances.
37.1. Landlord’s Covenants. Landlord hereby notifies Tenant, and Tenant hereby acknowledges that, prior to the leasing of the Premises pursuant to this Lease, Tenant has been notified, pursuant to California Health and Safety Code Section 25359.7 (or any successor statute), that Landlord knows, or has reasonable cause to believe, that certain hazardous substances (as such term is used in such Section 25359.7), such as common cleaning supplies, office supplies, spillage of petroleum products from motor vehicles, and other consumer products, may have come (and may in the future come) to be located on or beneath the Premises and/or the Project. Notwithstanding the foregoing, Landlord shall not cause any unlawful accumulations of Hazardous Material (as defined below) to be generated, brought onto, used, stored, or disposed of in or about the Premises, the Building, or the Project by Landlord or its agents, employees, or contractors, except for limited quantities of standard office and janitorial supplies and petroleum and petroleum-related products commonly used on or at similar office projects. Furthermore, Landlord shall: (a) use, store, and dispose of all such permitted Hazardous Material in strict compliance with all applicable statutes, ordinances, and regulations in effect during the Lease Term that govern and/or relate to Hazardous Material, public health and safety and protection of the environment, and (b) comply at all times during the Lease Term with all environmental laws (as defined in Paragraph 37.2, below). Except as to those matters which are Tenant’s responsibility pursuant to Paragraph 37.2, below, Landlord shall be responsible, at its expense (or the expense of others; but not as an Operating Expense) to cause any unlawful accumulations of Hazardous Materials to be remediated in accordance with the requirements of all applicable environmental laws.
37.2. Tenant’s Covenants. Tenant covenants, represents, and warrants to the Landlord that its use of the Premises, the Building, and the Project will be in full compliance with all environmental laws. Tenant hereby agrees to indemnify Landlord against all actions, liabilities, damages, losses, costs, expenses, attorneys’ fees, and claims (except to the extent they arise as a result of Landlord’s grossly negligent acts or willful misconduct), arising from or relating to: (i) any discharges, releases, or threatened releases of any Hazardous Material into ambient air, water, or land by Tenant or Tenant’s Invitee’s from, on, under, or above the Premises, (ii) the manufacture, processing, distribution, use, treatment, storage, disposal, transport, or handling of pollutants, contaminants, or hazardous or toxic wastes, substances, or materials by Tenant or Tenant’s Invitees, from, on, or under, the Premises, or (iii) a violation of any environmental law on, under, or above the Premises by Tenant or Tenant’s Invitees (for purposes of this Lease, “environmental laws” shall mean any Federal, State, or local law, statute, regulation, ordinance, guideline, or common law principle relating to public health or safety or the use or control of the environment, including without limitation the Federal Comprehensive Environmental Response, Compensation and Liability Act of 1980, the Carpenter-Presley-Tanner Hazardous Substance Account Act, the California Hazardous Waste Control Law, the Federal Clean Air Act, the California Air Resources Act, the Federal Clean Water Act, the California Porter-Cologne Water Quality Control Act, the Federal Resource Conservation and Recovery Act, the California Nejedly-Z’berg-Dills Solid Waste Management and Recovery Act, and California Health and Safety Code Section 25359.7). Tenant agrees to promptly reimburse Landlord for all of Landlord’s costs arising from periodic monitoring of Tenant’s use, handling, or storage of Hazardous Substances at or surrounding the Premises. Tenant shall not cause or permit any Hazardous Material to be generated, brought onto, used, stored, or disposed of in or about the Premises, the Building, or the Project by Tenant or its agents, employees, contractors, subtenants, or invitees, except for limited quantities of standard office and janitorial supplies. Tenant shall: (a) use, store, and dispose of all such permitted Hazardous Material in strict compliance with all applicable statutes, ordinances, and regulations in effect during the Lease Term that govern and/or relate to Hazardous Material, public health and safety and protection of the environment, and (b) comply at all times during the Lease Term with all environmental laws. If the Premises are contaminated (or, due to the acts or omissions of Tenant or Tenant’s Invitees, the Project is contaminated) by any Hazardous Material during the Term,
26
then (1) Tenant shall promptly notify Landlord in writing of such contamination, and (2) Landlord may elect to either (A) demand that Tenant perform all remediation required by Landlord (to Landlord’s satisfaction and at Tenant’s sole cost, necessary to return the Premises (and/or the Project) to at least as good a condition as the Premises (or the Project) are in as of the date of this Lease, which Tenant shall immediately do upon receipt of notice from Landlord, or (B) proceed to cause such investigation, clean-up, and remediation work which Landlord deems necessary or desirable to be undertaken, whereupon the entire cost thereof (plus a supervisory fee equal to ten percent of such cost) will be payable by Tenant to Landlord upon demand as Additional Rent. If, after demand by Landlord, as provided in this Paragraph, Tenant does not promptly commence and diligently pursue such remediation, then Landlord may, at Landlord’s election, perform or cause to be performed such remediation and Tenant shall immediately, upon demand, pay the cost thereof to Landlord, plus a supervisory fee in the amount of ten percent of such cost. Tenant’s obligations and liability under this Paragraph shall survive the termination of Tenant’s tenancy and the Term of this Lease, except that nothing contained in this Paragraph shall be deemed to impose liability on Tenant for any problem arising after the Term of this Lease provided neither Tenant nor Tenant’s Invitees contributed to such problem during the Term of the Lease.
37.3. Definition of Hazardous Materials. As used in this Lease the term “Hazardous Material” shall mean any hazardous or toxic substance, material, or waste that is or becomes regulated by the United States, the State of California, or any local government authority having jurisdiction over the Building. Hazardous Material includes, without limitation: (a) any “hazardous substance”, as that term is defined in the Comprehensive Environmental Response, Compensation, and Liability Act of 1980 (CERCLA) (42 United States Code Sections 9601-9675); (b) “hazardous waste”, as that term is defined in the Resource Conservation and Recovery Act of 1976 (RCRA) (42 United States Code Sections 6901-6992k); (c) any pollutant, contaminant, or hazardous, dangerous, or toxic chemical, material, or substance, within the meaning of any other applicable federal, state, or local law, regulation, ordinance, or requirement (including consent decrees and administrative orders imposing liability or standards of conduct concerning any hazardous, dangerous, or toxic waste, substance, or material, now or hereafter in effect); (d) petroleum products; (e) radioactive material, including any source, special nuclear, or byproduct material as defined in 42 United States Code Sections 2011-2297; (f) asbestos in any form or condition; and (g) polychlorinated biphenyls (PCBs) and substances or compounds containing PCBs.
38. Prohibition Against Mold, Lead-Based Paint, and Asbestos-Containing Materials. Tenant shall not allow or permit any lead-based paint to be used in the Premises, nor shall Tenant allow or permit any condition to occur which could result in the growth of mold within the Premises. Additionally, Tenant shall not allow or permit any materials which contain asbestos in any form or concentration (“Asbestos-Containing Materials”) to be used or stored in the Premises or used in the construction of any improvements or alterations to the Premises, including, without limitation, building or construction materials and supplies. Such prohibition against Asbestos-Containing Materials shall apply regardless of whether the Asbestos-Containing Materials may be considered safe or approved for use by a manufacturer, supplier, or governmental authority, or by common use or practice. Landlord shall have the right, upon 24-hours’ written notice, to enter upon and conduct inspections of the Premises to determine Tenant’s compliance with this Paragraph. If Tenant violates the foregoing covenants relating to lead-based paint, mold, and Asbestos-Containing Materials (collectively “Prohibited Substances”), then (a) Tenant shall, upon notice from Landlord, immediately remove and remediate any damage from such Prohibited Substances at Tenant’s sole cost, (b) such removal and remediation shall comply with all applicable laws, regulations, and requirements, (c) Tenant shall reimburse Landlord for all expenses incurred in connection with any inspection and testing of the Premises conducted by Landlord, and (d) unless Tenant completes such removal within 30 days after notice from Landlord, Landlord may, at its election, do either or both of the following: (i) declare an Event of Default (without the requirement of any notice under Paragraph 24.4) and exercise Landlord’s remedies hereunder, including, without limitation, terminate this Lease upon ten days prior written notice to Tenant, and/or (ii) remove and remediate such Prohibited Substances and obtain reimbursement from Tenant for the cost of such removal and remediation, including a supervisory fee payable to Landlord in the amount of ten percent of the removal and disposal cost. Tenant shall indemnify Landlord and Landlord’s directors, officers, employees, and agents against all costs, liabilities, expenses, penalties, and claims for damages, including, without limitation, litigation costs and attorneys’ fees, arising from (A) the presence of Prohibited Substances upon the Premises, to the extent that such Prohibited Substances are used, stored, or otherwise permitted in the Premises or used in the construction of any Alterations by Tenant or Tenant’s agents, employees, representatives, or independent contractors, (B) any lawsuit, settlement, governmental order, or decree relating to the presence, handling, removal, or disposal of Prohibited Substances upon or from the Premises, to the extent that such Prohibited Substances are used, stored, or otherwise permitted in the Premises or used in the construction of any improvements or Alterations to the Premises by Tenant or Tenant’s agents, employees, representatives or independent contractors, or (C) Tenant’s failure to perform its obligations to remove such Prohibited Substances under this Paragraph. The provisions of this Paragraph shall not apply to any Prohibited Substances brought onto the Premises by Landlord or Landlord’s Invitees or resulting from the acts of Landlord or Landlord’s Invitees.
27
39. Security Measures. Tenant acknowledges that, although the Building may contain a restricted access entry system (if provided for as part of Landlord’s Work), (i) the Basic Monthly Rent does not include the cost of any security measures for any portion of the Project (ii) Landlord shall have no obligation to provide any such security measures, (iii) Landlord has made no representation to Tenant regarding the safety or security of the Project, and (iv) Tenant will be solely responsible for providing any security it deems necessary to protect itself, its property, and Tenant’s Invitees in, on, or about the Project. If Landlord provides any security measures at any time, then the cost thereof shall be included as part of the Operating Expenses, but Landlord will not be obligated to continue providing such security measures for any period of time, Landlord may discontinue such security measures without notice and without liability to Tenant, and Landlord will not be obligated to provide such security measures with any particular standard of care. Tenant assumes all responsibility for the security and safety of Tenant, Tenant’s property, and Tenant’s Invitees. Tenant releases Landlord from all claims (other than due to Landlord’s gross negligence or intentional misconduct) for damage, loss, or injury to Tenant, Tenant’s Invitees, and/or to the personal property of Tenant and/or of Tenant’s Invitees, even if such damage, loss, or injury is caused by or results from the criminal, reckless, or negligent acts of third parties. In connection with the foregoing, Tenant hereby waives any defense would otherwise be provided by Section 1542 of the California Civil Code (which states “A general release does not extend to claims which the creditor does not know or suspect to exist in his or her favor at the time of executing the release, which if known by him or her must have materially affected his or her settlement with the debtor”), or laws of a similar nature, which would limit any such release to matters known or suspected to exist by Tenant. Tenant is hereby instructed to conduct its own investigation through local police agencies regarding any criminal acts or dangerous conduct that has occurred in or near the Project. Landlord shall have no duty to warn Tenant of any criminal acts or dangerous conduct that has occurred in or near the Project, regardless of Landlord’s knowledge of such crimes or conduct, and Tenant hereby undertakes to remain informed regarding such issues.
40. Subordination and Attornment. This Lease and Tenant’s rights under this Lease are subject and subordinate to any mortgage, deed of trust, ground lease, or underlying lease (and to all renewals, modifications, consolidations, replacements, or extensions thereof), now or hereafter affecting the Premises. The provisions of this Paragraph shall be self-operative, and no further instrument of subordination shall be required. In confirmation of such subordination, however, Tenant shall promptly execute and deliver any commercially reasonable instruments that Landlord, any Lender, or the lessor under any ground or underlying lease, may request to evidence such subordination, provided such instrument contains customary non-disturbance language in favor of Tenant and is consistent with the provisions of the next sentence including, without limitation, a Subordination, Attornment, and Non-Disturbance Agreement in the form to be commercially reasonable and acceptable to Lender. If any Lender, or the lessor of any ground or underlying lease affecting the Premises, shall hereafter succeed to the rights of Landlord under this Lease, whether by foreclosure, deed in lieu of foreclosure, or otherwise, then (i) such successor landlord shall not be subject to any offsets or defenses which Tenant might have against Landlord, (ii) such successor landlord shall not be bound by any prepayment by Tenant of more than one month’s installment of Basic Monthly Rent or any other Rent, (iii) such successor landlord shall not be subject to any liability or obligation of Landlord except those arising after such succession, (iv) Tenant shall attorn to and recognize such successor landlord as Tenant’s landlord under this Lease, (v) Tenant shall promptly execute and deliver any commercially reasonable instruments that may be necessary to evidence such attornment, (vi) upon such attornment, this Lease shall continue in effect as a direct lease (whether separately documented or not) between such successor landlord and Tenant upon and subject to all of the provisions of this Lease, and (vii) Tenant shall be entitled to quiet enjoyment of the Premises for so long as Tenant is not in default under the terms of this Lease or any substitute lease referenced above. Notwithstanding the preceding provisions of this Paragraph, if any ground lessor or Lender elects to have this Lease prior to the lien of its ground lease, deed of trust, or mortgage, and gives written notice thereof to Tenant that this Lease shall be deemed prior to such ground lease, deed of trust, or mortgage, whether this Lease is dated prior or subsequent to the date of such ground lease, deed of trust, or mortgage, then this Lease shall be deemed to be prior to the lien of such ground lease or mortgage and such ground lease, deed of trust, or mortgage shall be deemed to be subordinate to this Lease.
41. Estoppel Certificate. Within ten days after written request from Landlord, Tenant shall execute and deliver to Landlord, in recordable form, a certificate (“Estoppel Certificate”) stating (i) that this Lease is unmodified and in full force and effect, or in full force and effect as modified, and stating all modifications, (ii) the then-current Basic Monthly Rent, (iii) the dates to which Basic Monthly Rent has been paid in advance, (iv) the amount of any security deposit, prepaid rent or other payment constituting Rent which has been paid, (v) whether or not Tenant or, to Tenant’s knowledge, Landlord is in default under this Lease and whether there currently exist any defenses or rights of offset under the Lease in favor of Tenant, (vi) that any Landlord’s Work required by this Lease is complete (or stating any exceptions) and (vii) such other matters as Landlord may reasonably request. Tenant’s failure to deliver such certificate within such ten day period shall be conclusive upon Tenant for the benefit of Landlord, and any successor in interest to Landlord, any lender or proposed lender, and any purchaser or proposed purchaser of the Project that, except as may be represented by Landlord, this Lease is unmodified and in full force and effect, no Rent has been paid more than 30 days in advance, neither Tenant nor Landlord is in default under this Lease, no defenses or rights of offset under the Lease exist in favor of Tenant, and that all
28
Landlord’s Work required by this Lease is complete. Landlord will similarly, in connection with any lending or Transfer transaction, upon ten days written request from Tenant, execute an estoppel certificate in favor of Tenant’s proposed lender or Transferee confirming (i) that this Lease is unmodified and in full force and effect, or in full force and effect as modified, and stating all modifications, (ii) the then-current Basic Monthly Rent, (iii) the dates to which Basic Monthly Rent has been paid in advance, (iv) the amount of any security deposit, prepaid rent, or other payment constituting Rent which has been paid, and (v) whether or not to the best of Landlord’s knowledge Tenant is in default under this Lease. The requirement for Tenant to execute and deliver to Landlord, the Estoppel Certificate, as required above, shall not be delayed, conditioned, or withheld for any reason; this requirement shall be an independent covenant of Tenant under this Lease.
42. Waiver. No delay or omission in the exercise of any right or remedy of Landlord in the event of any default or Event of Default by Tenant shall impair such right or remedy or be construed as a waiver. The receipt and acceptance by Landlord of delinquent Rent shall not constitute a waiver of any default other than the particular Rent payment accepted. Landlord’s receipt and acceptance from Tenant, on any date (the “Receipt Date”), of an amount less than the Rent actually due on such Receipt Date, or to become due at a later date but applicable to a period prior to such Receipt Date, shall not release Tenant of its obligation (i) to pay the full amount of such Rent due on such Receipt Date or (ii) to pay when due the full amount of such Rent to become due at a later date but applicable to a period prior to such Receipt Date. No act or conduct of Landlord, including without limitation, the acceptance of the keys to the Premises, shall constitute an acceptance by Landlord of the surrender of the Premises by Tenant before the Expiration Date. Only a written notice from Landlord to Tenant stating Landlord’s election to terminate Tenant’s right to possession of the Premises shall constitute acceptance of the surrender of the Premises and accomplish a termination of this Lease. Landlord’s consent to or approval of any act by Tenant requiring Landlord’s consent or approval shall not be deemed to waive or render unnecessary Landlord’s consent to or approval of any other or subsequent act by Tenant. Any waiver by Landlord of any default must be in writing and shall not be a waiver of any other default concerning the same or any other provision of this Lease. Tenant hereby waives any rights granted to Tenant under California Code of Civil Procedure Section 1179, California Civil Code Section 3275, and/or any successor statute(s). Tenant represents and warrants that if Tenant breaches this Lease and, as a result, this Lease is terminated, Tenant will not suffer any undue hardship as a result of such termination and, during the Term, will make such alternative or other contingency plans to provide for its vacation of the Premises and relocation in the event of such termination. Tenant acknowledges that Tenant’s waivers set forth in this Paragraph are a material part of the consideration for Landlord’s entering into this Lease and that Landlord would not have entered into this Lease in the absence of such waivers.
43. Brokers. Tenant represents that no real estate broker, agent, finder, or other person is responsible for bringing about or negotiating this Lease other than the Tenant’s broker, if any, listed in the Principal Lease Provisions, and Tenant has not dealt with any other real estate broker, agent, finder, or other person, relative to this Lease in any manner. Tenant shall indemnify, defend, and hold Landlord harmless from and against all liabilities, damages, losses, costs, expenses, attorneys’ fees and claims arising from any claims that may be made against Landlord by any real estate broker, agent, finder, or other person (other than as set forth above), alleging to have acted on behalf of or to have dealt with Tenant. Landlord shall be responsible, upon satisfaction of the requirements of a separate written listing agreement between Landlord and Landlord’s broker, for the payment of the commission due and owing to Landlord’s brokers identified in the Principal Lease Provisions (or any other brokers engaged by Landlord), pursuant to such separate written agreement between Landlord and Landlord’s broker. Landlord’s broker will in turn split such commission with Tenant’s broker as such parties may agree.
44. Limitations on Landlord’s Liability. If Landlord is in default of this Lease, and as a consequence Tenant recovers a money judgment against Landlord, such judgment shall be satisfied only out of the proceeds of sale received upon execution of such judgment and levy against the right, title, and interest of Landlord in the Project, and out of rent or other income from the Project receivable by Landlord or out of the consideration received by Landlord from the sale or other disposition of all or any part of Landlord’s right, title, and interest in the Project. Neither Landlord nor Landlord’s shareholders, members, officers, directors, agents, property managers, employees, contractors, or the partners comprising Landlord (if any) shall be personally liable for any deficiency.
45. Sale or Transfer of Premises. If Landlord sells or transfers the Project (whether voluntarily or involuntarily), Landlord, on consummation of the sale or transfer, shall be released from any liability thereafter accruing under this Lease. If any security deposit or prepaid rent has been paid by Tenant, Landlord may transfer the security deposit and/or prepaid rent to Landlord’s successor-in-interest and on such transfer Landlord shall be discharged from any further liability arising from the security deposit or prepaid rent.
46. Quitclaim Deed. Tenant shall execute and deliver to Landlord on the Expiration Date, promptly on Landlord’s request, a quitclaim deed to the Premises, in recordable form, designating Landlord as transferee.
29
47. No Merger. The voluntary or other surrender of this Lease by Tenant, or a mutual cancellation of this Lease, or a termination by Landlord, shall not work a merger, and shall, at the option of Landlord, terminate any existing subleases or may, at the option of Landlord, operate as an assignment to Landlord of any such subleases.
48. Confidentiality. Except as essential to the consummation of the transaction contemplated by this Lease (together with all amendments and addenda hereto):
48.1. Tenant shall keep and maintain the terms of this Lease and the transactions contemplated by this Lease or any aspect of this Lease in strict confidence; and
48.2. Tenant may not make or allow any notices, statements, disclosures, communication, or news releases concerning this Lease, the terms of this Lease and the transactions contemplated by this Lease or any aspect of this Lease.
48.3. Nothing provided herein, however, shall prevent Tenant from disclosing to its legal counsel and/or certified public accountants, prospective purchasers, or lenders the existence and terms of this Lease or any transaction under this Lease, or any aspect of this lease, or from complying with any governmental or court order or similar legal requirement which requires such party to disclose this Lease, the terms of this Lease, the transaction contemplated by this Lease and/or any aspect of this Lease; provided that such party uses reasonable and diligent good faith efforts to disclose no more than is absolutely required to be disclosed by such legal requirement. If Tenant violates this confidentiality provision, in addition to all other remedies to which Landlord may be entitled under law or in equity, Landlord shall be entitled to receive immediately the entire value of any rent relief, rent abatement, free rent, reimbursement, or other concession which Landlord has previously granted to Tenant.
48.4. Disclosure. Notwithstanding anything contained herein to the contrary, Landlord shall be entitled to disclose the terms of this Lease in connection with public filings and/or presentations of its parent and/or affiliates.
49. Miscellaneous.
49.1. This Lease may be executed in counterparts, each of which shall be deemed an original and all of which together shall constitute one document.
49.2. Within twenty days of written request, Tenant shall furnish to Landlord, not more than once every two (2) years financial statements certified by Tenant to be true and correct, reflecting Tenant’s then current financial condition. Such financial statements shall include a current balance sheet and a profit and loss statement covering the most recent 12-month period available.
49.3. Notwithstanding any other provision in this Lease to the contrary, Tenant shall refrain from selling or otherwise distributing any alcoholic beverages and such sales are expressly forbidden under this Lease notwithstanding the fact that Tenant may hold the appropriate license as issued and/or approved by the California Alcoholic Beverage Control Agency.
49.4. This Lease shall be governed by and construed in accordance with the laws of the state in which the Premises are located. If the Premises are located outside of California, then the references in this Lease to California statutes shall be deemed to include any relevant statute of the jurisdiction in which the Premises are located that is comparable to such California statutes.
49.5. For purposes of venue and jurisdiction, this Lease shall be deemed made and to be performed in the City of San Diego, California (whether or not the Premises are located in San Diego, California) and Landlord and Tenant hereby consent to the jurisdiction of the Courts of the County of San Diego.
49.6. Tenant covenants and agrees not to protest or in any way oppose any application for a license to serve or sell liquor filed by tenants or other users of space within the Project.
49.7. Whenever the context so requires, all words used in the singular shall be construed to have been used in the plural (and vice versa), each gender shall be construed to include any other genders, and the word “person” shall be construed to include a natural person, a corporation, a firm, a partnership, a joint venture, a limited liability company, a trust, an estate or any other entity.
30
49.8. Each provision of this Lease shall be valid and enforceable to the fullest extent permitted by law. If any provision of this Lease or the application of such provision to any person or circumstance shall, to any extent, be invalid or unenforceable, the remainder of this Lease, or the application of such provision to persons or circumstances other than those as to which it is held invalid or unenforceable, shall not be affected by such invalidity or unenforceability, unless such provision or such application of such provision is essential to this Lease.
49.9. In the event any litigation, arbitration, mediation, or other proceeding (“Proceeding”) is initiated by any party against any other party to enforce, interpret or otherwise obtain judicial or quasi-judicial relief in connection with this Lease the prevailing party in such Proceeding shall be entitled to recover from the unsuccessful party all costs, expenses, and reasonable attorney’s fees and expert witness fees relating to or arising out of such Proceeding (whether or not such Proceeding proceeds to judgment), and any post-judgment or post-award proceeding including without limitation one to enforce any judgment or award resulting from any such Proceeding. Any such judgment or award shall contain a specific provision for the recovery of all such subsequently incurred costs, expenses, and actual attorney’s fees and expert witness fees.
49.10. This Lease shall become effective and binding upon the parties when it has been executed by each of Landlord and Tenant; notwithstanding the fact that the Term of this Lease (i.e. Tenant’s rights of full occupancy hereunder) will not commence until the Lease Commencement Date.
49.11. Subject to any restriction on transferability contained in this Lease, this Lease shall be binding upon and shall inure to the benefit of the successors-in-interest and assigns of each party to this Lease. Nothing in this Paragraph shall create any rights enforceable by any person not a party to this Lease, except for the rights of the successors-in-interest and assigns of each party to this Lease, unless such rights are expressly granted in this Lease to other specifically identified persons.
49.12. The headings of the Paragraphs of this Lease have been included only for convenience, and shall not be deemed in any manner to modify or limit any of the provisions of this Lease, or be used in any manner in the interpretation of this Lease.
49.13. Time and strict and punctual performance are of the essence with respect to each provision of this Lease. All references to “days” in this Lease will refer to calendar days, unless such reference specifically indicates that “business days” are intended. Business days will mean and refer to all calendar days other than Saturdays, Sundays, and national or California state holidays.
49.14. Each party to this Lease and its legal counsel have had an opportunity to review and revise this Lease. The rule of construction that any ambiguities are to be resolved against the drafting party shall not be employed in the interpretation of this Lease or any Addendum or Exhibit to this Lease, and such rule of construction is hereby waived by Tenant.
49.15. All notices required or permitted to be given by Tenant to Landlord shall be in writing and shall be personally delivered, sent by certified mail, postage prepaid, return receipt requested, or sent by a nationally or locally recognized overnight express courier service that provides written confirmation of delivery to Landlord at the address set forth in the Principal Lease Provisions of this Lease. Each such notice or other communication shall be deemed given, delivered and received upon its actual receipt, except that if it is sent by mail in accordance with this Paragraph, then it shall be deemed given, delivered and received three days after the date such notice or other communication is deposited with the United States Postal Service in accordance with this Paragraph, and if it is sent by nationally recognized overnight express courier service, it shall be deemed given one business day after deposit with the courier. Landlord or Tenant must give a notice of a change of its address to the other, if such address changes. All notices required or permitted to be given to Tenant by Landlord shall, except as otherwise provided in this Lease, be in writing, and such notice shall be personally delivered, sent by certified mail, postage prepaid, return receipt requested, or sent by a nationally recognized overnight express courier service that provides written confirmation of delivery, to Tenant at the address set forth in the Principal Lease Provisions of this Lease. Each such notice or other communication shall be deemed given, delivered and received upon its actual receipt, except that if it is sent by mail in accordance with this Paragraph, then it shall be deemed given, delivered and received three days after the date such notice or other communication is deposited with the United States Postal Service in accordance with this Paragraph. Notwithstanding the foregoing, routine correspondence between Landlord and Tenant shall be deliverable by regular U.S. mail, by fax, or by other such means of delivery as may become customary.
31
49.16. lf more than one person is Tenant, then the obligations of Tenant under this Lease shall be the joint and several obligations of each of such persons; provided, however, that any act or signature of one or more of any of such persons and any notice or refund given to or served on anyone of such persons shall be fully binding on each of such persons.
49.17. AII provisions, whether covenants or conditions, to be performed or observed by Tenant shall be deemed to be both covenants and conditions. All indemnity, defense, and hold harmless obligations of Tenant hereunder shall survive the termination of this Lease.
49.18. Intentionally Deleted.
49.19. AII payments to be made by Tenant to Landlord under this Lease shall be in United States currency.
49.20. Any claim, demand, rights, or defense by Tenant that arises out of this Lease or the negotiations that preceded this Lease shall be barred unless Tenant commences an action thereon, or interposes a defense by reason thereof, within 12 months after the date of the inaction, omission, event, or action that gave rise to such claim, demand, right, or defense. Tenant acknowledges and understands, after having consulted with its legal counsel, that the purpose of this Paragraph is to shorten the period within which Tenant would otherwise have to raise such claims, demands, rights, or defenses under applicable laws.
49.21. This Lease, the Exhibits and Addenda, if any, attached hereto (which are incorporated herein by this reference), constitute all of the covenants, promises, assurances, representations, warranties, statements, agreements, conditions and understandings between Landlord and Tenant concerning the Premises and the Project, and there are no other covenants, promises, assurances, representations, warranties, statements, conditions, or understandings, either oral or written, between them. Except as herein otherwise provided, no subsequent alteration, change, modification, or addition to this Lease shall be binding upon Landlord or Tenant unless reduced to writing and signed by each of them. Notwithstanding the foregoing, the Landlord may, from time to time, establish and amend such Rules, regulations, and signage criteria, in a written form, for the benefit of the Project and Building, as it deems appropriate. Violations of such Rules, regulations, and signage criteria by Tenant or Tenant’s Invitees shall constitute a material default of this Lease.
49.22. This Lease, upon full execution, supersedes and revokes any and all previous leases governing the Premises, lease negotiations, arrangements, letters of intents, offers to lease, lease proposals or drafts, brochures, representations, and information conveyed, whether oral or written, between parties hereto or their respective representations or any other person purported to represent Landlord or Tenant. The Tenant acknowledges it has not been induced to enter into this Lease by any representations not set forth in the Leases, nor has it relied on any such representations. No such representations should be used in the interpretation or construction of this Lease and the Landlord shall have no liability for any consequences arising as a result of any such representations.
49.23. LANDLORD AND TENANT WAIVE THEIR RESPECTIVE RIGHTS TO TRIAL BY JURY OF ANY CONTRACT OR TORT CLAIM, COUNTERCLAIM, CROSS COMPLAINT, OR CAUSE OF ACTION IN ANY ACTION, PROCEEDING, OR HEARING BROUGHT BY EITHER PARTY AGAINST THE OTHER ON ANY MATTER ARISING OUT OF OR IN ANY WAY CONNECTED WITH THIS LEASE, THE RELATIONSHIP OF LANDLORD AND TENANT, OR TENANT’S USE OR OCCUPANCY OF THE PREMISES, INCLUDING ANY CLAIM OF INJURY OR DAMAGE OR THE ENFORCEMENT OF ANY REMEDY UNDER ANY CURRENT OR FUTURE LAW, STATUTE, REGULATION, CODE, OR ORDINANCE.
|
|
|
|
|
TENANT’S INITIALS |
|
LANDLORD’S INITIALS |
49.24. Consequential Damages. Notwithstanding anything to the contrary contained in this Lease, nothing in this Lease shall impose any obligations on Tenant or Landlord to be responsible or liable for, and each hereby releases the other from all liability for, consequential damages other than those consequential damages incurred by Landlord in connection with a holdover (without Landlord’s consent) of the Premises by Tenant after the expiration or earlier termination of this Lease or incurred by Landlord in connection with any repair, physical construction or improvement work performed by or on behalf of Tenant in the Project.
32
49.25. Anti-Money Laundering/OFAC Requirements. Tenant represents and warrants as follows, with the understanding that the Landlord will rely on the accuracy of these representations and warranties to establish the Landlord’s compliance with the laws enforced by the United States Department of Treasury’s Office of Foreign Assets Control (“OFAC”), and any other applicable laws, rules, regulations and other legal requirements relating to the combating of money laundering and/or terrorism (i.e., Patriot Act).
49.25.1. If Tenant is an entity (e.g., a corporation, partnership, limited liability company, trust), (i) Tenant has exercised due diligence to establish the identity of each person who possesses the power, directly or indirectly, to direct or cause the direction of Tenant’s management and policies; (ii) if ownership interests in Tenant are not publicly traded on an exchange or an organized over-the-counter market that is regulated by any foreign government, or any governmental body or regulatory organization empowered by a foreign government to administer or enforce its laws as they relate to securities matters, Tenant has exercised due diligence to establish the identity of each person who holds, directly or indirectly, a beneficial interest in Tenant; and (iii) if Tenant is a financial intermediary (e.g., a bank, brokerage firm, depository), Tenant has exercised due diligence to establish the identity of each of its account holders (each of the foregoing persons listed in this Paragraph being an “Affiliated Person”). Tenant (x) maintains records of all documents it uses to verify the identities of its Affiliated Persons; (y) will maintain all such records for a period of at least five (5) years after the expiration of the Lease; and (z) will make such documentation available to the Landlord at any time upon request.
49.25.2. Tenant is not a “Prohibited Person” (as defined below), none of its Affiliated Persons is a Prohibited Person, and Tenant is not acquiring, and does not intend to enter into this Lease for the direct or indirect benefit of any Prohibited Person. Tenant acknowledges and agrees that if, at any time, the Landlord determines that Tenant is or may be a Prohibited Person, or that any Prohibited Person holds or may hold a direct or indirect interest in Tenant, the Landlord may, in its sole discretion, terminate the Lease.
49.25.3. For purposes of the foregoing representations and warranties, “Prohibited Person” means any person or entity that acts or has acted (i) in contravention of any statute, rule, regulation or other legal requirement to which that person is subject relating to the combating of terrorism and/or money laundering, or (ii) on behalf of any person or organization (A) residing or having a place of business in a country or territory subject to embargo under laws enforced by OFAC, or (B) identified as a terrorist, terrorist organization, specially designated national or blocked person by OFAC, any other department, agency, division, board, bureau or other instrumentality of the United States Government, or any recognized international organization, multilateral expert group or governmental or industry publication. OFAC’s lists of specially designated nationals, blocked persons and embargoed countries and territories can be found at www.treas.gov/ofac.
49.25.4. Tenant acknowledges and agrees that, any provision of this Lease to the contrary notwithstanding, the Landlord may release confidential information regarding Tenant to law enforcement authorities and/or regulators if the Landlord determines, that it is in the best interests of the Landlord to do so in light of the Landlord’s obligations and/or potential liability under any applicable statute, rule, regulation or other legal requirement relating to the combating of terrorism and/or money laundering.
49.25.5. Tenant acknowledges and agrees that the foregoing representations and warranties are subject to Tenant’s indemnification obligations under this Lease.
49.25.6. If Tenant becomes aware of any fact or circumstance that may render any of the foregoing representations and warranties inaccurate in any respect, Tenant will immediately notify the Landlord.
33
|
LANDLORD: |
|
TENANT: |
||||
|
|
|
|
|
|
||
|
PACIFIC NORTH COURT HOLDINGS, L.P., |
|
ADAMIS PHARMACEUTICALS |
||||
|
a California limited partnership |
|
CORPORATION, a Delaware corporation |
||||
|
|
|
|
|
|
|
|
|
By: |
|
American Assets Trust Management, |
|
By: |
/s/ Dennis J. Carlo |
|
|
|
|
LLC, a Delaware limited liability |
|
|
Dennis J. Carlo |
|
|
|
|
company, as Agent |
|
|
President and CEO |
|
|
|
|
|
|
|
|
|
|
By: |
|
/s/ John W. Chamberlain |
|
Dated: April 1, 2014 |
|
|
|
|
|
John W. Chamberlain |
|
|
|
|
|
|
|
President and CEO |
|
|
|
|
|
|
|
|
|
|
|
|
|
By: |
|
/s/ James R. Durfey |
|
|
|
|
|
|
|
James R. Durfey |
|
|
|
|
|
|
|
V.P. of Office Leasing |
|
|
|
|
|
|
|
|
|
|
|
|
|
Dated: April 1, 2014 |
|
|
||||
34
ADDENDUM NO.1
TO STANDARD OFFICE LEASE
This Addendum to Lease (“Addendum”) constitutes part of the Office Lease Agreement (“Lease”) dated as of April 1, 2014 between PACIFIC NORTH COURT HOLDINGS, L.P., a California limited partnership (“Landlord”), and ADAMIS PHARMACEUTICALS CORPORATION, a Delaware corporation (‘‘Tenant’’). The terms of this Addendum are incorporated in the Lease for all purposes. All capitalized terms not otherwise defined in this Addendum are defined by the terms of the Lease.
|
1. |
BASIC MONTHLY RENT |
Basic Monthly Rent during the Lease Term shall be as follows:
|
Lease Period |
Approximate Basic Monthly Rent Per Rentable Square Foot
|
Actual Basic Monthly Rent for the Premises
|
|
12/1/2014 - 11/30/2015 |
$3.20 |
$24,080.00** |
|
12/1/2015 - 11/30/2016 |
$3.30 |
$24,802.40 |
|
12/1/2016 - 11/30/2017 |
$3.39 |
$25,546.47 |
|
12/1/2017 - 11/30/2018 |
$3.50 |
$26,312.87 |
In addition, Tenant shall pay for all individually and separately metered utilities.
**Tenant shall be granted a three (3) month full abatement of Basic Monthly Rent during months two (2) through four (4) of the Initial Lease Term and a partial abatement equal to seventy-five percent (75%) of Basic Monthly Rent during month five (5) of the Initial Lease Term. As such and provided Tenant is not in material default of this Lease, Tenant shall not be required to pay Basic Monthly Rent during the second (2nd) through fourth (4th) months of the Initial Lease Term, nor seventy-five (75%) of Basic Monthly Rent during the fifth (5th) month of the Initial Lease Term. If Tenant is deemed in material default of the Lease (after applicable notice and cure period), Tenant shall become fully liable for all funds abated and Landlord shall be entitled to exercise all of its rights and remedies with respect to collecting the monies so abated.
|
2. |
PROJECT EXPANSIONIMODIFICATION |
|
A. |
Further Construction. Tenant acknowledges that Landlord may, from time to time, at its sole election, construct, reconstruct, modify, expand, or otherwise alter the Project (collectively, “Construction Work”), or portions thereof (in no event however will Landlord have any obligation to do so). Tenant acknowledges that any such Construction Work will necessarily involve, among other things, the generation of noise, dust, and Vibrations, barricading portions of the Project and the placement of scaffolding within the Project, demolition, structural alterations, storage of materials and equipment within the Project, and the presence of workmen within the Project, all of which may require the rearrangement of parking areas, common areas, roadways, lighting facilities, and the re-direction of vehicular and pedestrian traffic. Except as provided below, Tenant waives any and all claims, defenses, rights of offset, or deductions based upon any inconvenience suffered by Tenant or any interruption of or interference with Tenant’s business including, without limitation, any loss of business, decreased sales, or inconvenience to Tenant or Tenant’s Invitees as a result of or relating to such Construction Work. Landlord hereby reserves for itself and its agents, employees, licensees and contractors, the right to enter the Premises to the extent reasonably necessary to pursue such Construction Work upon 48 hours’ prior written notice to Tenant. The exercise of any of Landlord’s rights pursuant to this paragraph will not entitle Tenant to any abatement of Rent or other claim, right of offset, or defense against Landlord, except that. Tenant shall have the right to bring an action against Landlord in the event Tenant suffers any damages as a result of Landlord’s negligence or intentional misconduct in pursuing such Construction Work. Tenant further acknowledges that expansion of the Project may affect the amount of the Operating Expenses and the portion thereof payable by Tenant. |
|
B. |
Reserved Rights. Landlord hereby reserves such licenses and easements in, on, above or below the Premises as may be reasonably required (i) for the installation, inspection, surveying, maintenance, or construction of mains, conduits, shafts, columns, footings, piers, pipes or other facilities to serve any building within the Project, or (ii) for any Construction Work; provided, however, Landlord will use its best efforts to minimize any unreasonable interference with Tenant’s use, occupancy, or enjoyment of the Premises as contemplated by this Lease. |
|
C. |
Right of Abatement. Notwithstanding anything to the contrary contained in Paragraph A, above, to the extent any Construction Work undertaken by Landlord materially adversely affects Tenant’s access to and reasonable use of the Premises then Tenant’s Base Monthly Rent will be equitably abated during the period such material adverse interference with Tenant’s business at the Premises continues as a result thereof. The foregoing right of abatement will constitute Tenant’s sole and absolute right against Landlord or otherwise in connection with any such Construction Work and Tenant releases and waives any other claims, defenses, or rights in connection therewith. |
|
D. |
Remodel. Landlord may in the future remodel, renovate or refurbish (“remodel”) all or any portion of the Project, which remodel may include the Premises. The remodeling will be done in accordance with design specifications prepared by the project architect and reviewed and approved by Landlord and, with respect to the interior of the Premises, also by Tenant. |
35
|
3. |
CONDITION OF THE PREMISES |
Tenant acknowledge that Tenant shall accept and occupy the Premises in its currently existing “as-is” condition pursuant to the terms of this Lease. Tenant acknowledges and agrees that Landlord has no obligation to improve the Premises, other than as may be set forth specifically in the Lease. In particular, Tenant acknowledges that any improvements or alterations needed to accommodate Tenant’s intended use shall be made solely at Tenant’s sole cost and expense, and strictly in accordance with the requirements of this Lease (including the requirement to obtain Landlord’s consent thereto), unless such improvements and alterations are specifically required of Landlord and expressly set forth in this Lease and in Exhibit “C”. Should tenant improvements be made to the Premises in the future, the Premises shall be constructed in accordance with the procedures outlined in Exhibit “C” of this Lease. Landlord shall have no responsibility to do any work required under any building codes or other governmental requirements not in effect or applicable on the Lease Commencement Date, including without limitation any requirements related to sprinkler retrofitting, seismic structural requirements, accommodation of disabled persons, or hazardous materials.
|
4. |
UTILITIES |
Notwithstanding the terms of Paragraph 9 of the Lease, the Premises are separately metered for electricity. Tenant shall make all arrangements for the establishment of an account or accounts with the appropriate utility provider(s), and Tenant shall make all payments with respect thereto for use of said utilities within the Premises during the term of the Lease, including any periods of early occupancy or holdover thereof. In the event the Premises are sub-metered and direct payment to the utility is impractical, Tenant shall reimburse Landlord or Landlord’s designee directly for all such costs, fees, and consumption charges arising from said sub-meter.
[Signature page to follow]
36
Unless modified by this Addendum, each term of the Lease remains unamended and in full force. The parties have executed this Addendum as of the date of the Lease.
|
LANDLORD: |
|
TENANT: |
|||
|
|
|
|
|
|
|
|
PACIFIC NORTH COURT HOLDINGS, L.P., |
|
ADAMIS PHARMACEUTICALS |
|||
|
a California limited partnership |
|
CORPORATION, a Delaware corporation |
|||
|
|
|
|
|
|
|
|
By: |
|
American Assets Trust Management, |
|
By: |
/s/ Dennis J. Carlo |
|
|
|
LLC, a Delaware limited liability |
|
|
Dennis J. Carlo |
|
|
|
company, as Agent |
|
|
President and CEO |
|
|
|
|
|
|
|
|
By: |
|
/s/ John W. Chamberlain |
|
Dated: April 1, 2014 |
|
|
|
|
John W. Chamberlain |
|
|
|
|
|
|
President and CEO |
|
|
|
|
|
|
|
|
|
|
|
By: |
|
/s/ James R. Durfey |
|
|
|
|
|
|
James R. Durfey |
|
|
|
|
|
|
V.P. of Office Leasing |
|
|
|
|
|
|
|
|
|
|
|
Dated: April 1, 2014 |
|
|
|||
37
EXHIBIT “A”
Project Site Plan
This Exhibit “A” is intended to show the approximate configuration of the Project and the Building as of the Commencement Date and is not a representation or warranty by Landlord as to the size, nature or exact configuration of the Project or Building.

38
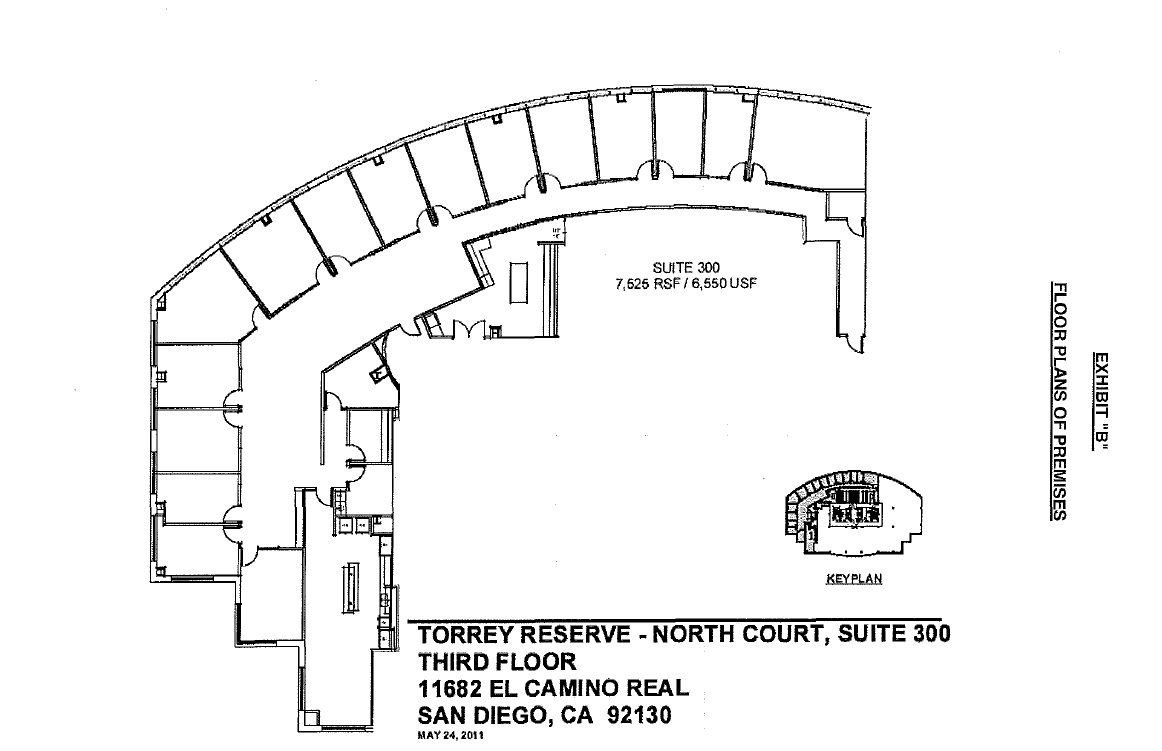
39
EXHIBIT“C”
WORK LETTER
General Recital: Tenant accepts the Premises in its “As-Is, Where-Is” condition.
40
EXHIBIT “D”
BUILDING RULES AND REGULATIONS
Tenant shall comply with the following Rules and Regulations. Landlord shall not be responsible to Tenant for the nonperformance of any of these rules and Regulations.
|
1. |
Locks; Keys. Tenant shall not alter any lock or install any new or additional locks or bolts on any doors or windows of the Premises without obtaining Landlord’s prior written consent. Tenant shall bear the cost of any lock changes or repairs required by Tenant. Landlord for the Premises shall furnish two keys, and any additional keys required by Tenant must be obtained from Landlord at a reasonable cost to be established by Landlord. |
|
2. |
Doors Opening to Public Corridors. All doors opening to public corridors must be kept closed at all times except for normal ingress to and egress from the Premises. |
|
3. |
Securing Doors; Admission to Building. Landlord reserves the right to close and keep locked all entrance and exit doors of the Building during the hours when Comparable Building are customarily closed and locked. When departing after the Building’s normal Business Hours, Tenant and Tenant’s employees and agents must be sure that the doors to the Building are securely closed and locked. Any person, including Tenant and Tenant’s employees and agents, who enters or leaves the Building at any time when it is locked or at any time considered to be after the Building’s normal Business Hours, may be required to sign the Building register. Access to the Building may be refused unless the person seeking access has proper identification or has previously arranged a pass for access to the Building. Landlord and its agents shall not be liable for damages for any error concerning the admission to, or exclusion from, the Building of any person. Landlord reserves the right, in the event of invasion, mob, riot, public excitement, or any other commotion, to prevent access to the Building or Project during the continuance of that event by any means it considers appropriate for the safety and protection of life and property. |
|
4. |
Furniture, Freight, and Equipment; Service Deliveries. No furniture, freight, or equipment of any kind may be brought into the Building without prior notice to Landlord. All moving activity into or out of the Building must be scheduled with Landlord and done only at a time and in the manner designated by Landlord. No service deliveries (other than Messenger services) shall be allowed between the hours of 4:00 PM and 6:00 PM, Monday through Friday. Landlord may at any time restrict the elevators and areas of the Building into which messengers may enter and may require that Tenant leave deliveries at the lobby security desk for pickup. Landlord may prescribe the weight, size, and position of all safes and other heavy property brought into the Building and the times and manner of moving those items within and out of the Building. Tenant shall not overload the floor of the Premises. If considered necessary by Landlord, safes and other heavy objects must stand on supports that are adequate to distribute the weight properly. Landlord shall not be responsible for loss of or damage to any safe or property. Any damage to any part of the Building or to its contents, occupants, or visitors caused by moving or maintaining any safe or other property referred to in this clause shall be the sole responsibility and expense of Tenant. |
|
5. |
Receipt of Deliveries; Use of Elevators. No furniture, packages, supplies, equipment, or merchandise may be received in the Building or carried up or down the elevators, except between those hours and in that specific elevator that Landlord shall designate. |
|
6. |
No Disturbance of Other Occupants. Tenant shall not disturb, solicit, or canvass any occupant of the Project and shall cooperate with Landlord and Landlord’s agents to prevent these actions. |
|
7. |
Use of Restrooms; Responsibility for Damage. The restrooms, urinals, wash bowls, and other apparatus shall not be used for any other purpose other than that for which they were constructed, and no foreign substance of any kind shall be thrown into them. The expense of any breakage, stoppage, or damage resulting from the violation of this rule shall be borne by the tenant who caused, or whose employees or agents caused, the breakage, stoppage, or damage. |
|
8. |
Heating and Air-Conditioning. Tenant shall not use any method of heating or air-conditioning, other than that supplied by Landlord, without Landlord’s prior written consent. |
41
|
9. |
Foul or Noxious Gases or Substances; Noninterference With Others. Tenant shall not use or keep, or allow to be used or kept, any foul or noxious gas or substance in or on the Premises. Tenant shall not allow the Premises to be occupied or used in a manner causing noise, odors, or vibrations that are offensive or objectionable to Landlord or other occupants of Project. |
|
10. |
Animals. Birds, and Vehicles. Tenant shall not bring into, or keep within, the Premise, Building or Project any animals, birds, or vehicles (e.g., bicycles). |
|
11. |
Cooking; No use of the Premises for Improper Purposes. No cooking shall be done or permitted on the Premises, except that Underwriter’s Laboratory (UL)-approved equipment and microwave ovens may be used in the Premises for heating food and brewing coffee, tea, hot chocolate, and similar beverages for employees and visitors. This must be in accordance with all applicable federal, state, and city laws, codes, ordinances, rules, and regulations. |
|
12. |
Telephone and Other Wires. Tenant may not introduce telephone wires or other wires into the Premises without first obtaining Landlord’s approval of the method and location of such introduction with Landlord’s said approval not to be unreasonably withheld or delayed. . No boring or cutting for telephone wires or other wires shall be allowed without Landlord’s consent. The location of telephones, call boxes, and other office equipment affixed to the Premises shall be subject to Landlord’s approval with Landlord’s said approval not to be unreasonably withheld or delayed. |
|
13. |
Exclusion or Expulsion. Landlord reserves the right to exclude or expel from the Project any person who, in Landlord’s judgment, is under the influence of alcohol or drugs or commits and act in violation of theses Rules and Regulations. |
|
14. |
Loitering Prohibited. Tenant and Tenant’s employees and agents shall not loiter in or on the entrances, corridors, sidewalks, lobbies, halls, stairways, elevators, or common areas for the purpose of smoking tobacco products or for any other purpose. Tenant and Tenant’s employees and agents shall not obstruct these areas but use them only as a means of ingress to and egress from the Premises. |
|
15. |
Operation of Electricity, Water, and Air Conditioning. Tenant shall not waste electricity, water, or air-conditioning and shall cooperate fully with Landlord to ensure the most effective operation of the Building’s heating and air-conditioning system. Tenant shall not adjust any controls of that heating and air-conditioning system. |
|
16. |
Disposal of Trash and Garbage. Tenant shall store all trash and garbage within the interior of the Premises. Tenant shall not place or have placed in the trash boxes or receptacles any material that may not or cannot be disposed of in the ordinary and customary manner of removing and disposing of trash in the vicinity of the Building. In disposing of trash and garbage, Tenant shall comply fully with any law or ordinance governing that disposal. All trash, garbage, and refuse disposal shall be made only through entry-ways and elevators provided for that purpose and shall be made only at times designated by Landlord. |
|
17. |
Compliance With Safety Regulations. Tenant shall comply with all safety, fire protection and evacuation procedures and regulations established by Landlord or by any government agency and participate in practice drills scheduled from time to time by Landlord. |
|
18. |
Protection of Premises. Tenant shall assume all responsibility, including keeping doors locked and other means of entry to the Premises closed, for protecting the Premises from theft, robbery, and pilferage. |
|
19. |
Awnings, Curtains, and Electrical Ceiling Fixtures. No awnings or other projection shall be attached to the outside walls of the Building without Landlord’s prior written consent. No curtains, blinds, shades, or screens, shall be attached to, hung in, or used in connection with any window or door of the Premises without Landlord’s prior written consent. All electrical ceiling fixtures hung in offices or spaces along the perimeter of the Building must be fluorescent or of a quality, type, design, and bulb color approved by Landlord. Tenant shall abide by Landlord’s regulations concerning the opening and closing of window coverings attached to those windows, if any, in the Premises that have a view of any interior portion of the Building or Building Common Area. |
42
|
20. |
Non-obstruction of Light. Tenant shall not cover or obstruct the sashes, sash doors, skylights, windows, and doors that reflect or admit light and air into halls, passageways, or other public places in the Building. Tenant shall not place any bottles, parcels, or other articles on the windowsills. |
|
21. |
Provision of Information to Tenant’s Employees. Tenant shall comply with requests by Landlord that Tenant informs Tenant’s employees of items of importance to Landlord. |
|
22. |
Hand Trucks and Similar Equipment. Without Landlord’s prior consent, Tenant shall not use, in any space or in the public halls of the Building, any hand trucks unless they are equipped with rubber tires and side guards or similar equipment. Tenant shall not bring any other vehicles of any kind into the Building. |
|
23. |
Use of Building’s Name or Likeness. Without Landlord’s prior written consent, Tenant shall not use the Building’s name or any photograph or other likeness of the Building in connection with, or in promoting or advertising, Tenant’s business, except that Tenant may include the Building’s name in the Tenant’s address. |
|
24. |
Parking Rules and Regulations. Without Landlord’s prior written consent, no automobile detailing or washing shall be permitted in the parking areas of the Building or Project. |
|
25. |
Rules Changes; Waivers. Landlord reserves the right at any time to change or rescind any one or more of these Rules and Regulations or to make any additional reasonable Rules and Regulations that, in Landlord’s judgment, may be necessary for: (a) The management, safety, care, and cleanliness of the Premises, Building, and Project; (b) The preservation of good order, and (c) The convenience of other occupants and tenants in the Premises, Building, and Project. |
|
26. |
Flammable. Tenant shall not have any open flames in the Premises, Building or Project at any time whatsoever, including, but not limited to, lit candles, lighters, matches or as it relates to cooking. No inflammable, explosive or dangerous fluids or substances shall be used or kept by Tenant in the Premises, Building or about the Property, except for those substances as are typically found in similar premises used for general office purposes and are being used by Tenant in a safe manner and in accordance with all applicable Laws. Tenant shall install and maintain, at Tenant’s sole cost and expense, an adequate, visibly marked (at all times properly operational) fire extinguisher next to any duplication or photocopying machine or similar heat producing equipment (which may or may not contain combustible material) in the Premises. Tenant shall install in the Premises as many other fire extinguishers in such locations as required by City code. Tenant shall not, without Landlord’s prior written consent, use, store, install, spill, remove, release or dispose of, within or about the Premises or any other portion of the Property, any asbestos-containing materials or any solid, liquid or gaseous material now or subsequently considered toxic or hazardous under the provisions of 42 U.S.C. Section 9601 et seq. or any other applicable environmental Law which may now or later be in effect. Tenant shall comply with all Laws pertaining to and governing the use of these materials by Tenant and shall remain solely liable for the costs of abatement and removal. |
Landlord may waive anyone or more of these Rules and Regulations for the benefit of any particular tenants. No waiver by Landlord shall be constructed as a waiver of those Rules and Regulations in favor of any other tenant, and no waiver shall prevent Landlord from enforcing those Rules and Regulations against any other tenant of the Project. Tenant shall be considered to have read these Rules and Regulations and to have agreed to abide by them as a condition of Tenant’s occupancy of the Premises.
43