UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_____________________________________________________________________________________________
FORM 10-K
_____________________________________________________________________________________________
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the Fiscal Year Ended December 31 , 2022
OR
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the transition period from to .
Commission File No. 001-33093

(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) | (IRS Employer Identification No.) | |||||||
| (Address of Principal Executive Offices) | (Zip Code) | |||||||
Registrant’s telephone number, including area code: (858 ) 550-7500
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol | Name of Each Exchange on Which Registered | ||||||
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934. Yes ☐ No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definition of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
☒ | Accelerated Filer | ☐ | Non-accelerated Filer | ☐ | Smaller reporting company | Emerging growth company | ||||||||||||||||||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Exchange Act Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the Registrant’s voting and non-voting stock held by non-affiliates was approximately $1.0 billion based on the last sales price of the Registrant’s Common Stock on the Nasdaq Global Market of the Nasdaq Stock Market LLC on June 30, 2022. For purposes of this calculation, shares of Common Stock held by directors, officers and 10% stockholders known to the Registrant have been deemed to be owned by affiliates which should not be construed to indicate that any such person possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the Registrant or that such person is controlled by or under common control with the Registrant.
As of February 22, 2023, the Registrant had 17,076,658 shares of Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Table of Contents
| Part I | ||||||||
| Item 1. | ||||||||
| Item 1A. | ||||||||
| Item 1B. | ||||||||
| Item 2. | ||||||||
| Item 3. | ||||||||
| Item 4. | ||||||||
| Part II | ||||||||
| Item 5. | ||||||||
| Item 6. | ||||||||
| Item 7. | ||||||||
| Item 7A. | ||||||||
| Item 8. | ||||||||
| Item 9. | ||||||||
| Item 9A. | ||||||||
| Item 9B. | ||||||||
| Item 9C. | ||||||||
| Part III | ||||||||
| Item 10. | ||||||||
| Item 11. | ||||||||
| Item 12. | ||||||||
| Item 13. | ||||||||
| Item 14. | ||||||||
| Part IV | ||||||||
| Item 15. | ||||||||
| Item 16. | ||||||||
| GLOSSARY OF TERMS AND ABBREVIATIONS | |||||
| Abbreviation | Definition | ||||
| 2023 Notes | $750.0 million aggregate principal amount of convertible senior unsecured notes due 2023 | ||||
| Aldeyra | Aldeyra Therapeutics, Inc. | ||||
| Amgen | Amgen, Inc. | ||||
| ASC | Accounting Standards Codification | ||||
| ASU | Accounting Standards Update | ||||
| Aziyo | Aziyo Med, LLC | ||||
| Baxter | Baxter International, Inc. | ||||
| BeiGene | BeiGene, Ltd. | ||||
| BendaRx | BendaRx Corp. | ||||
| BLA | Biologics license application | ||||
| CASI | CASI Pharmaceuticals, Inc. | ||||
| cGMP | Current Good Manufacturing Practice | ||||
| Company | Ligand Pharmaceuticals Incorporated, including subsidiaries | ||||
| Convertible Note | Senior Convertible Promissory Note | ||||
| COPD | Chronic obstructive pulmonary disease | ||||
| Cormatrix | Cormatrix Cardiovascular, Inc. | ||||
| Corvus | Corvus Pharmaceuticals, Inc. | ||||
| CVR | Contingent value right | ||||
| CyDex | CyDex Pharmaceuticals, Inc. | ||||
| Daiichi Sankyo | Daiichi Sankyo Company, Ltd. | ||||
| Dianomi | Dianomi Therapeutics, Inc. | ||||
| DMF | Drug Master File | ||||
| ESG | Environmental, Social and Governance | ||||
| ECM | Extracellular matrix | ||||
| Eisai | Eisai Inc. | ||||
| EPA | Environmental Protection Agency | ||||
| ESPP | Employee Stock Purchase Plan, as amended and restated | ||||
| EU | European Union | ||||
| Exelixis | Exelixis, Inc. | ||||
| FASB | Financial Accounting Standards Board | ||||
| FDA | U.S. Food and Drug Administration | ||||
| FSGS | Focal segmental glomerulosclerosis | ||||
| FY 2022 | The Company's fiscal year ended December 31, 2022 | ||||
| FY 2021 | The Company's fiscal year ended December 31, 2021 | ||||
| FY 2020 | The Company's fiscal year ended December 31, 2020 | ||||
| GAAP | Generally accepted accounting principles in the United States | ||||
| GCSF | Granulocyte-colony stimulating factor | ||||
| Gilead | Gilead Sciences, Inc. | ||||
| HBV | Hepatitis B Virus | ||||
| Hikma | Hikma Pharmaceuticals PLC | ||||
| Hovione | Hovione FarmCiencia, S.A. | ||||
| Icagen | Icagen, Inc. | ||||
| IM | Intramuscular | ||||
| IND | Investigational New Drug | ||||
| IRS | Internal Revenue Service | ||||
| IV | Intravenous | ||||
| Jazz | Jazz Pharmaceuticals, Inc. | ||||
| Ligand | Ligand Pharmaceuticals Incorporated, including subsidiaries | ||||
| LTP | Liver targeting prodrug | ||||
| Marinus | Marinus Pharmaceuticals, Inc. | ||||
| Melinta | Melinta Therapeutics, Inc. | ||||
| Merck | Merck & Co., Inc. | ||||
| Metabasis | Metabasis Therapeutics, Inc. | ||||
| NDA | New Drug Application | ||||
| NOLs | Net Operating Losses | ||||
| Novan | Novan, Inc. | ||||
| Novartis | Novartis AG | ||||
| Nucorion | Nucorion Pharmaceuticals, Inc. | ||||
| OmniAb | OmniAb Operations, Inc. (f/k/a OmniAb, Inc.) | ||||
| OMT | Open Monoclonal Technology, Inc. | ||||
| Ono | Ono Pharmaceutical Co., Ltd. | ||||
| Opthea | Opthea Limited | ||||
| Orange Book | Publication identifying drug products approved by the FDA based on safety and effectiveness | ||||
| Palvella | Palvella Therapeutics, Inc. | ||||
| Par | Par Pharmaceutical, Inc. | ||||
| Pfenex | Pfenex Inc. | ||||
| Pfizer | Pfizer, Inc. | ||||
| Phoenix Tissue | Phoenix Tissue Repair | ||||
| PSU | Performance stock unit | ||||
| R&D | Research and Development | ||||
| RSU | Restricted stock unit | ||||
| Sage | Sage Therapeutics, Inc. | ||||
| SARM | Selective Androgen Receptor Modulator | ||||
| SEC | Securities and Exchange Commission | ||||
| Sedor | Sedor Pharmaceuticals, Inc., or RODES, Inc. | ||||
| Seelos | Seelos Therapeutics, Inc. | ||||
| Selexis | Selexis, SA | ||||
| Sermonix | Sermonix Pharmaceuticals, LLC | ||||
| SII | Serum Institute of India | ||||
| SQ Innovation | SQ Innovation, Inc. | ||||
| Sunshine Lake Pharma | Sunshine Lake Pharma Co., Ltd. | ||||
| Takeda | Takeda Pharmaceuticals Company Limited | ||||
| Taurus | Taurus Biosciences LLC | ||||
| Tax Act | The Tax Cuts and Jobs Act | ||||
| Teva | Teva Pharmaceuticals USA, Inc., Teva Pharmaceutical Industries Ltd. and Actavis, LLC | ||||
| Travere | Travere Inc. | ||||
| TR-Beta | Thyroid hormone receptor beta | ||||
| Vernalis | Vernalis plc | ||||
| Verona | Verona Pharma plc | ||||
| Viking | Viking Therapeutics | ||||
| xCella Biosciences | xCella Biosciences, Inc. | ||||
| Xi'an Xintong | Xi'an Xintong Medicine Research | ||||
| Zydus Cadila | Zydus Cadila Healthcare, Ltd | ||||
PART I
Cautionary Note Regarding Forward-Looking Statements:
You should read the following report together with the more detailed information regarding our company, our common stock and our financial statements and notes to those statements appearing elsewhere in this document.
This report contains forward-looking statements that involve a number of risks and uncertainties. Although our forward-looking statements reflect the good faith judgment of our management, these statements can only be based on facts and factors currently known by us. Consequently, these forward-looking statements are inherently subject to risks and uncertainties, and actual results and outcomes may differ materially from results and outcomes discussed in the forward-looking statements.
Forward-looking statements can be identified by the use of forward-looking words such as “believes,” “expects,” “may,” “will,” “plan,” “intends,” “estimates,” “would,” “continue,” “seeks,” “pro forma,” or “anticipates,” or other similar words (including their use in the negative), or by discussions of future matters such as those related to our future results of operations and financial position, royalties and milestones under license agreements, Captisol material sales, product development, and product regulatory filings and approvals, and the timing thereof, Ligand's status as a high-growth company, as well as other statements that are not historical. You should be aware that the occurrence of any of the events discussed under the caption “Risk Factors” could negatively affect our results of operations and financial condition and the trading price of our stock.
The cautionary statements made in this report are intended to be applicable to all related forward-looking statements wherever they may appear in this report. We urge you not to place undue reliance on these forward-looking statements, which speak only as of the date of this report. Except as required by law, we assume no obligation to update our forward-looking statements, even if new information becomes available in the future. This caution is made under the safe harbor provisions of Section 21E of the Securities Exchange Act of 1934, as amended.
References to “Ligand Pharmaceuticals Incorporated,” “Ligand,” the “Company,” “we,” “our” and “us” include Ligand Pharmaceuticals Incorporated and our wholly-owned subsidiaries.
Partner Information
Information regarding partnered products and programs comes from information publicly released by our partners and licensees.
Trademarks
This Annual Report on Form 10-K includes trademarks, trade names and service marks owned by us. Ligand®, Advasep®, BEPro™, Bonsity®, Captisol®, CyDex®, LTP®, LTP Technology™, Pelican Expression Technology™, PeliCRM™, Pfenex Expression Technology™ and XRPro® are protected under applicable intellectual property laws and are our property. All other trademarks, trade names and service marks including, but not limited to OmniAb® Kyprolis®, Evomela®, Veklury®, Livogiva®, Bonteo®, Zulresso®, Rylaze®, VAXNEUVANCE™, Pneumosil®, Minnebro®, Baxdela®, Conbriza®, Nexterone®, Noxafil®, Duavee®, OTORIN™, FILSPARI™ and LYTENAVA™ are the property of their respective owners. Solely for convenience, trademarks, trade names and service marks referred to in this report may appear without the ®, ™ or SM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the right of the applicable licensor to such trademarks, trade names and service marks. Use or display by us of other parties’ trademarks, trade dress or products is not intended to and does not imply a relationship with, or endorsement or sponsorship of, us by the trademark or trade dress owners.
1
| Item 1. | Business | ||||
Overview
Our business is focused on acquiring or funding programs and technologies that pharmaceutical companies use to discover and develop medicines. Our business model provides a diversified portfolio of biotech and pharmaceutical product revenue streams that are supported by an efficient and low corporate cost structure. The biotechnology industry is characterized by a binary clinical risk, in that, either a drug candidate is successfully developed and receives regulatory marketing approval, or the drug candidate fails in clinical trials. Our goal is to offer investors an opportunity to participate in the promise of the biotech industry in a profitable and diversified manner while mitigating the binary clinical risk associated with developing a single program.
Our business model is focused on funding mid to late-stage drug development in return for economic rights and out-licensing our technology platforms to help partners discover and develop medicines. We partner with other pharmaceutical companies to leverage what they do best (late-stage development, regulatory management and commercialization) ultimately to generate our revenue. Our Captisol platform technology is a chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs. Our Pelican Expression Technology is a robust, validated, cost-effective and scalable platform for recombinant protein production that is especially well-suited for complex, large-scale protein production where traditional systems are not. We have established multiple alliances, licenses and other business relationships with the world’s leading pharmaceutical companies including Amgen, Merck, Pfizer, Jazz, Takeda, Gilead Sciences and Baxter International.
Our revenue consists of three primary elements: royalties from commercialized products, sales of Captisol material, and contract revenue from license, milestone and other service payments. We selectively pursue acquisitions and drug development funding opportunities that address high unmet clinical needs to bring in new assets, pipelines, and technologies to aid in generating additional potential new revenue streams.
OmniAb Separation and Spin-Off
On March 23, 2022, we entered into an Agreement and Plan of Merger (the Merger Agreement), by and among our company, Avista Public Acquisition Corp. II (New OmniAb) and OmniAb, Inc., a Delaware corporation and then wholly-owned subsidiary of our company (OmniAb), and Orwell Merger Sub Inc. (Merger Sub), pursuant to which New OmniAb combined with OmniAb, our then-antibody discovery business (the OmniAb Business), in a Reverse Morris Trust transaction. Pursuant to a Separation and Distribution Agreement, dated as of March 23, 2022, among New OmniAb, our company and OmniAb (the Separation Agreement), we transferred the OmniAb Business, including certain of our related subsidiaries, to OmniAb and, in connection therewith, distributed (the Distribution) to Ligand stockholders 100% of the common stock of OmniAb. Immediately following the Distribution, in accordance with and subject to the terms and conditions of the Merger Agreement, Merger Sub merged with and into OmniAb (the Merger), with OmniAb continuing as the surviving company in the Merger and as a wholly-owned subsidiary of New OmniAb.
Technologies
Through a combination of research and acquisitions, we have created a partnered portfolio with a wide variety of underlying technologies. This diversification provides the added benefits of exposure to a wider variety of science, more licensing opportunities and lower impact of individual patent expiry.
Captisol Technology
Captisol is a patent-protected, chemically modified cyclodextrin with a structure designed to optimize the solubility and stability of drugs. This unique technology has enabled several FDA-approved products, including Gilead’s Veklury, Amgen’s Kyprolis, Baxter International’s Nexterone, Acrotech Biopharma’s and CASI Pharmaceuticals’ Evomela, Melinta Therapeutics’ Baxdela and Sage Therapeutics’ Zulresso. There are many Captisol-enabled products currently in various stages of development. We maintain a broad global patent portfolio for Captisol with the latest expiration date in 2035. Other patent applications covering methods of making Captisol, if issued, extend to 2041.
In addition to solid Captisol powder, we offer our partners access to cGMP manufactured aqueous Captisol concentrate. This product offering was established in 2017 to reduce cycle time and increase Captisol production capacity for large volume drug products. We maintain both Type IV and Type V DMFs with the FDA. These DMFs contain manufacturing and safety information relating to Captisol that our licensees can reference when developing Captisol-enabled drugs. We also have active DMFs in Japan, China and Canada. In 2022, commercial products using Captisol made up over half of our total royalty revenue.
2
Pelican Expression Technology™ Platform
The Pelican Expression Technology platform is a robust, validated, cost-effective and scalable platform for recombinant protein production, and is especially well-suited for complex, large-scale protein production. Global manufacturers have demonstrated consistent success with the platform and the technology is currently out-licensed for multiple commercial and development-stage programs. The versatility of the platform has been demonstrated in the production of enzymes, peptides, antibody derivatives and engineered non-natural proteins. Partners seek the platform as it contributes significant value to biopharmaceutical development programs by reducing timelines and costs associated with research and development through commercial manufacturing of therapeutics and vaccines. Given pharmaceutical industry trends toward large molecules with increased structural complexities, the Pelican Expression Technology platform is well positioned to meet these growing needs as one of the most comprehensive and broadly available, commercially validated protein production platform in the industry.
We acquired the Pelican Expression Technology through our acquisition of Pfenex in October 2020. Several of our partners have commercial products and late stage clinical product candidates utilizing Pelican Expression Technology. In 2022, commercial products acquired from the Pfenex acquisition made up over one-third of our total royalty revenue.
HepDirect, LTP, and BEPro Technology Platform
The HepDirect and LTP platforms are our proprietary liver-targeting prodrug technologies that can deliver many different chemical classes of drugs to the liver by using a chemical modification that renders an active pharmaceutical ingredient (API) biologically inactive until cleaved by a liver-specific enzyme. These technologies may improve the efficacy and/or safety of certain drugs and can be applied to marketed or new drug products to treat liver diseases or diseases caused by hemostasis imbalance of circulating molecules controlled by the liver.
The BEPro technology platform is a next generation prodrug technology distinct from HepDirect and LTP prodrug technologies, expanding use to non-liver related diseases. BEPro is specifically applicable to nucleotides and nucleotide analogs for the development of compounds with improved product profiles. Ligand has demonstrated improvements in cell penetration and oral, intravenous and inhaled pharmacokinetics with BEPro-enabled nucleotide analogs.
SUREtechnology Platform (owned by Selexis)
We acquired economic rights to various SUREtechnology Platform programs from Selexis. The SUREtechnology Platform, developed and owned by Selexis, is a novel technology that improves the way that cells are utilized in the development and manufacturing of recombinant proteins and drugs.
Recent Business Updates
Travere Therapeutics recently received FDA accelerated approval for FILSPARI (sparsentan) for the treatment of immunoglobulin A nephropathy (IgAN). FILSPARI is the first and only dual endothelin angiotensin receptor antagonist in development for rare kidney diseases and is the first non-immunosuppressive treatment indicated for IgAN. Travere anticipates a review decision by the EMA on the potential approval for sparsentan for the treatment of IgAN in Europe in the second half of 2023. Additionally, Travere announced that they expect to report top line results from the two-year confirmatory endpoints in the ongoing Phase 3 DUPLEX Study of sparsentan in focal segmental glomerulosclerosis (FSGS) in the second quarter of 2023, with anticipated submission for full approval in the second half of 2023 in both the U.S. and Europe. Travere reported that it ended 2022 with approximately $450 million in cash, cash equivalents and marketable securities, which would be available to support the commercial launch of sparsentan.
Novan announced it has submitted an NDA to the FDA seeking marketing approval for berdazimer gel, 10.3% (SB206) for the topical treatment of molluscum contagiosum (MC). MC is an infection that causes skin lesions and affects approximately six million people in the U.S. annually. Novan anticipates a potential first quarter 2024 approval assuming the filing is accepted by the FDA and standard review timelines.
Verona Pharma announced positive results of its Phase 3 ENHANCE-1 trial evaluating nebulized ensifentrine for the maintenance treatment of COPD. The ENHANCE-1 trial met its primary and key secondary endpoints demonstrating significant improvements in lung function, symptoms and quality of life measures. In addition, ensifentrine substantially reduced the rate and risk of COPD exacerbations. Ensifentrine was well tolerated over 24 and 48 weeks. In 2022, Verona announced that the Phase 3 ENHANCE-2 trial successfully met its primary endpoint and secondary endpoints evaluating lung function and symptoms, and also significantly reduced the rate and risk of COPD exacerbations. Verona plans to file an NDA for inhaled ensifentrine for the maintenance treatment of COPD with the FDA in the first half of 2023.
Viking Therapeutics announced the completion of patient enrollment in its Phase 2b clinical trial of VK2809, a novel liver-selective thyroid hormone receptor beta agonist, in patients with biopsy-confirmed non-alcoholic steatohepatitis (NASH). Viking expects to report data for the study's primary endpoint in the first half of 2023.
3
Palvella Therapeutics announced its initial closing of up to $37.7 million in financing with proceeds to be used to advance the development of QTORIN rapamycin for the treatment of pachyonychia congenita, microcystic lymphatic malformations (MLM), and for the prevention of basal cell carcinomas in Gorlin syndrome. Palvella expects top-line data in mid-2023 from the Phase 3 pivotal study evaluating QTORIN rapamycin in pachyonychia congenita. Palvella is currently enrolling patients in a multicenter Phase 2b clinical study in the U.S. and Europe for the prevention of basal cell carcinomas in patients with Gorlin syndrome, with data expected in the first half of 2023. Additionally, Palvella expects to report data in the first quarter of 2023 from a multicenter Phase 2 study in the U.S. investigating QTORIN rapamycin for the treatment of MLM.
In 2022, Jazz Pharmaceuticals announced FDA approval of Monday/Wednesday/Friday intramuscular dosing of Rylaze (asparaginase erwinia chrysanthemi (recombinant)-rywn) and submission of a supplemental BLA under the Real-time Oncology Review Program seeking approval for IV administration. Jazz also completed the Marketing Authorization Application submission to the EMA for both IV and IM administration, with a potential approval in 2023. Jazz is also advancing the program for potential submission, approval and launch in Japan.
Xi'an Xintong Pharmaceuticals announced pradefovir reached the primary and secondary endpoints in its Phase 3 clinical trial in China for the treatment of chronic hepatitis B. The 48-week statistical analysis showed that pradefovir was comparable to the first-line drug, tenofovir disoproxil fumarate, with a better safety profile. Xi'an Xintong has submitted a pre-NDA conference communication application with China’s National Medical Products Administration (NMPA) and expects to submit an NDA in the first quarter of 2023.
China Resources Double-Crane Pharmaceuticals announced the IND for CX2101A, a small molecule, RNA-dependent RNA polymerase inhibitor of SARS-CoV-2 that utilizes Ligand's proprietary BEPro prodrug technology, was approved by the NMPA for use in clinical trials for the treatment of novel coronavirus pneumonia in China.
Aldeyra announced the submission of an NDA to the FDA for topical ocular reproxalap for the treatment of signs and symptoms of dry eye disease. Reproxalap is a small-molecule modulator of RASP (reactive aldehyde species), which are elevated in ocular and systemic inflammatory disease.
Arcellx initiated a Phase 1 study of ARCL-002 in acute myeloid leukemia and myelodysplastic syndromes. ARCL-002 utilizes the Pelican Expression Technology.
Merck announced the European Medicines Agency has recommended approval of VAXNEUVANCE for active immunization for the prevention of invasive disease, pneumonia and acute otitis media caused by Streptococcus pneumoniae in individuals from 6 weeks to less than 18 years of age. VAXNEUVANCE is a 15-valent pneumococcal vaccine utilizing Ligand’s CRM197 vaccine carrier protein and is currently authorized for use in the European Union for individuals 18 years of age and older and is approved in the United States for individuals 6 weeks of age and older. In July 2022 Merck started a broad Phase 3 program for V116, their investigational 21-valent pneumococcal conjugate vaccine utilizing Ligand’s CRM197 vaccine carrier protein.
Sermonix Pharmaceuticals announced results of its ELAINE 1 Phase 2 study of lasofoxifene vs. fulvestrant in postmenopausal women with locally advanced or metastatic ER+/HER2- breast cancer and an ESR1 mutation. Median progression-free survival was 6.04 months for lasofoxifene vs. 4.04 months for fulvestrant (p=0.138). Objective response rate was 13.2% for lasofoxifene vs. 2.9% for fulvestrant, (p=0.12), with 1 complete response and 4 partial responses in the lasofoxifene arm vs. no complete responses and 1 partial response in the fulvestrant arm. While the study was not powered for statistical significance, all endpoints numerically favored lasofoxifene.
In February 2022, BeiGene, Ltd. announced the launch of KYPROLIS® (carfilzomib) for injection in China for patients with relapsed/refractory (R/R) multiple myeloma. KYPROLIS is licensed to BeiGene in China under a strategic collaboration with Amgen, and was approved in July 2021 by the China National Medical Products Administration (NMPA) in combination with dexamethasone for the treatment of adult patients with R/R multiple myeloma who have received at least two prior therapies, including a proteasome inhibitor and an immunomodulatory agent.
Outlook Therapeutics announced it submitted a BLA to the FDA for ONS-5010, an investigational ophthalmic formulation of bevacizumab for the treatment of wet age-related macular degeneration that, if approved, will be branded as LYTENAVA™ (bevacizumab-vikg).
Corporate and Governance Highlights
We are committed to policies and practices focused on environmental sustainability, positively impacting our social community and maintaining and cultivating good corporate governance. By focusing on such ESG policies and practices, we believe we can affect a meaningful and positive change in our community and maintain our open, collaborative corporate culture. We will continue our proactive shareholder and employee engagement in 2023. See www.ligand.com for information about our ESG policies and practices. The information contained on our website is not intended to be part of this filing.
4
Commercial and Clinical Stage Partnered Portfolio
We have a large portfolio of assets currently generating royalties and future potential revenue-generating programs, including over 100 fully-funded by our partners. Each white dot on our partnered pipeline chart below represents a fully-funded partnered program, with each section of the chart representing a major Ligand technology or platform.
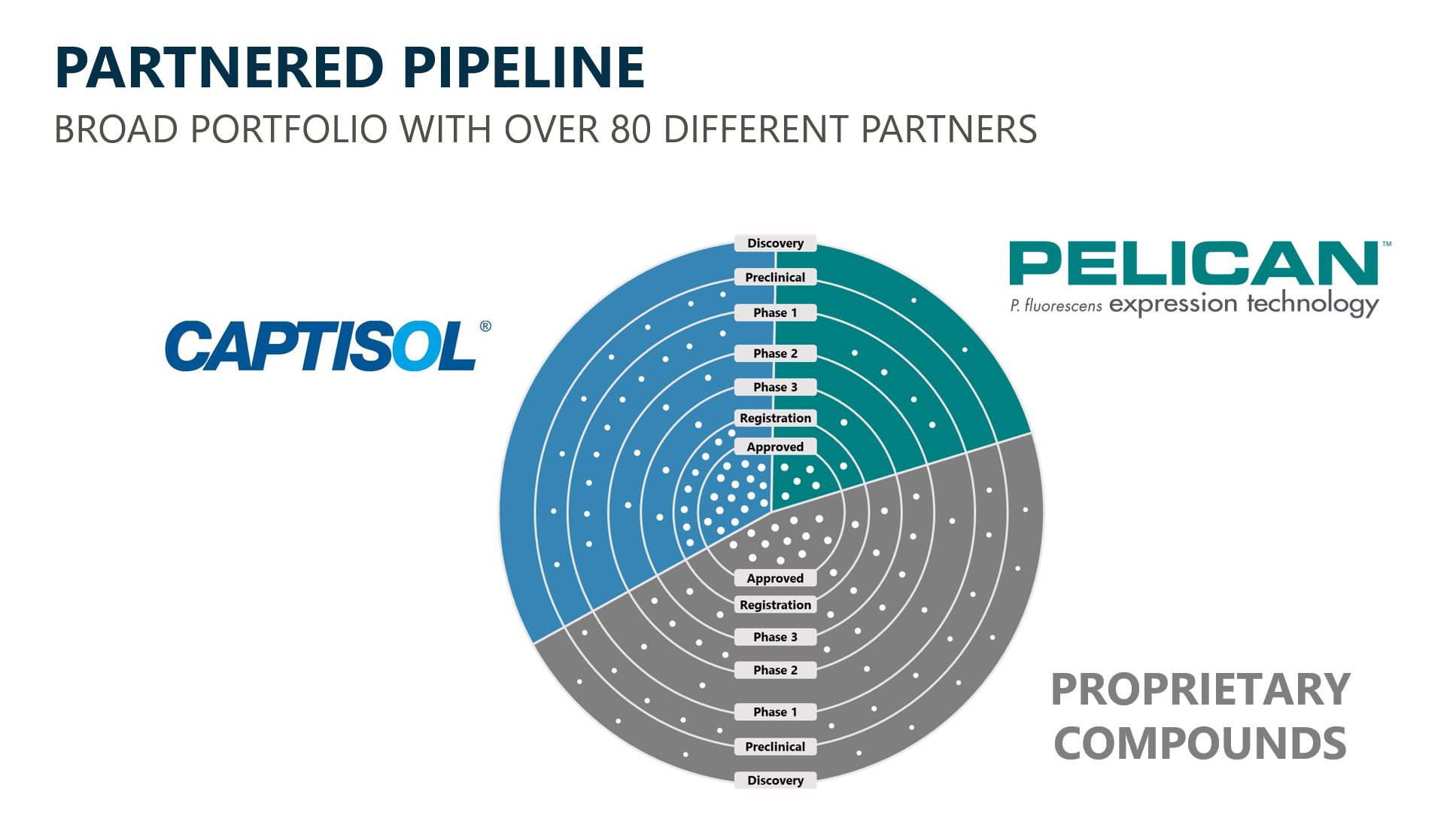
Royalties on Commercial Products
We currently receive royalties on more than ten commercial products. The following table provides an overview of our current portfolio of royalties:
| Product | Partner | Therapeutic Area | Royalty Rate | 2022 Royalty Revenue (in millions) | Estimated 2022 Product Revenue (in millions) | ||||||||||||
| Kyprolis | Amgen/Ono/Beigene | Cancer | 1.5% - 3.0% | $30.1 | $1,275.6 | ||||||||||||
| Teriparatide | Alvogen | Women's Health | 25%-40%¹ | $15.8 | N/A | ||||||||||||
| Evomela | Acrotech/CASI | Cancer | 20% | $10.2 | $51.0 | ||||||||||||
| Rylaze | Jazz | Cancer | Low single digit | $8.8 | $278.7 | ||||||||||||
| Nexterone | Baxter | Cardiovascular | Low single digit | $3.6 | $56.8 | ||||||||||||
| Pneumosil | Serum Institute | Infectious Disease | Low single digit | $2.6 | $114.7 | ||||||||||||
| Vaxneuvance | Merck | Infectious Disease | Low single digit | $1.1 | $159.0 | ||||||||||||
| Other | Various | Various | Various | $0.3 | $18.0 | ||||||||||||
(¹) We receive tiered profit sharing of 25% on quarterly profits less than $3.75 million, 35% on quarterly profits greater than $3.75 million but less than $7.5 million and 40% on quarterly profits greater than $7.5million. If therapeutic equivalence is achieved, quarterly profit changes to 50% of quarterly profits.
5
Portfolio Overview
We have assembled one of the largest portfolios of biopharmaceutical assets in the industry which provides investors the opportunity to participate in the biotech industry while mitigating the clinical binary risk typically associated with the industry. Our portfolio consists of assets which currently generate revenue through royalties on commercial products as well as Captisol sales on commercial products. In addition to these assets, we have a substantial pipeline of development stage assets that currently generate contractual payments through milestone and license fees with future potential for royalties and Captisol material sales for those programs under our Captisol technology.
| Approved | ||||||||
| Partner Name | Program | Therapeutic Area | ||||||
| Acrotech/CASI | Evomela | Cancer | ||||||
| Alvogen/Adalvo | Teriparatide | Women's Health | ||||||
| Alvogen/Hikma/Nanjing King-Friend | Voriconazole | Infectious Disease | ||||||
| Amgen/Beigene/Ono | Kyprolis | Cancer | ||||||
| Aziyo | ECM portfolio | Medical device/Cardiology | ||||||
| Baxter | Nexterone | Cardiovascular | ||||||
| Biocad | Teberif | Inflammatory/Metabolic | ||||||
| Exelixis/Daiichi-Sankyo | Minnebro | Cardiovascular | ||||||
| Gilead | Veklury | Infectious Disease | ||||||
| Jazz | Rylaze | Cancer | ||||||
| Melinta | Baxdela | Infectious Disease | ||||||
| Menarini | Frovatriptan | Central Nervous System | ||||||
| Merck | Noxafil-IV | Infectious Disease | ||||||
| Merck | Vaxneuvance | Infectious Disease | ||||||
| Par | Posaconazole | Infectious Disease | ||||||
| Pfizer | Duavee | Inflammatory/Metabolic | ||||||
| Pfizer | Vfend-IV | Infectious Disease | ||||||
| Sage | Zulresso | Central Nervous System | ||||||
| Sedor/Lupin | Sesquient | Central Nervous System | ||||||
| Serum Institute of India | Pneumosil | Infectious Disease | ||||||
| Zydus Cadila | Vivitra | Cancer | ||||||
| Zydus Cadila | Bryxta/ZyBev | Cancer | ||||||
| Zydus Cadila | Maropitant | Central Nervous System | ||||||
| Zydus Cadila | Exemptia | Inflammatory/Metabolic | ||||||
| Zydus Cadila | Vortuxi | Inflammatory/Metabolic | ||||||
| Phase 3/Pivotal or Regulatory Submission Stage | ||||||||
| Partner Name | Program | Therapeutic Area | ||||||
| Aldeyra | Reproxalap | Other/Undisclosed | ||||||
| BendaRx | Bendamustine | Oncology | ||||||
| Cantex | CX-01 | Oncology | ||||||
| Eisai | FYCOMPA | Central Nervous System | ||||||
| Escape Bio | S1P5 agonist | TBD | ||||||
| Marinus | Ganaxalone IV | Central Nervous System | ||||||
6
| Meridian | ML-141 | Oncology | ||||||
| Merck | V116 | Pneuococcal adult | ||||||
| Novan | SB206 | Infectious Disease | ||||||
| Novartis | Mekinist (CE-Trametinib) | Cancer | ||||||
| Opthea | OPT-302 | Ophthalmology | ||||||
| Outlook Therapeutics | ONS-5010 | Other/Undisclosed | ||||||
| Palvella | PTX-022 | Other/Undisclosed | ||||||
| Serum Institute | CRM197 | Infectious Disease | ||||||
| SQ Innovation | CE-Furosemide | Cardiovascular disease | ||||||
| Sunshine Lake | Vilazodone | Central Nervous System | ||||||
| Travere | Sparsentan | Severe and Rare | ||||||
| Verona | Ensifentrine (RPL554) | Respiratory Disease | ||||||
| Various | Teriparatide | Women's Health | ||||||
| Xi'an Xintong | Pradefovir | Infectious Disease | ||||||
| Phase 2 | ||||||||
| Partner Name | Program | Therapeutic Area | ||||||
| Acrivon | ACR-368 | Cancer | ||||||
| Corvus | Ciforadenant | Cancer | ||||||
| DeNovo | Lisfensine | Neurology | ||||||
| Merck | V116 | Infectious Disease | ||||||
| Oncternal | Cirmtuzumab | Cancer | ||||||
| Phoenix Tissue | PTR-01 | Genetic Disease | ||||||
| Seelos | Aplindore | Central Nervous System | ||||||
| Sermonix | Lasofoxifene | Cancer | ||||||
| Ohara Pharmaceuticals | JPH-203 | Cancer | ||||||
| Verona | Ensifentrine | Asthma | ||||||
| Verona | Ensifentrine | Cystic Fibrosis | ||||||
| Viking | VK5211 | Inflammatory/Metabolic | ||||||
| Viking | VK2809 | Inflammatory/Metabolic | ||||||
| Xi'an Xintong | MB07133 | Cancer | ||||||
| Phase 1 | ||||||||
| Partner Name | Program | Therapeutic Area | ||||||
| Apotex | Meloxicam | Migraine | ||||||
| China Resources Double Crane | CX2101A | COVID 19 | ||||||
| CSL | CSL-324 | Immunology | ||||||
| Foghorn | FHD-609/BRD9 | Cancer | ||||||
| Jazz | JZP-341 | Long Acting Erwinia Asparaginase | ||||||
| MEI Pharma | ME-344 | Cancer | ||||||
| Merck | V117 | Pneumococcal | ||||||
| Novartis | MIK-665 | Cancer | ||||||
| Novartis | BCL-201 | Cancer | ||||||
7
| Nucorion | NUC-1010 | Infectious disease | ||||||
| Revision Therapeutics | Rev0100 | Ophthalmology | ||||||
| Sage | SAGE-689 | Central Nervous System | ||||||
| Takeda | TAK-925 | Severe and Rare | ||||||
| Takeda | TAK-243 | Cancer | ||||||
| Vaxxas | Nanopatch | Infectious Disease | ||||||
| Viking | VK-0214 | Genetic Disease | ||||||
| Jupiter Biomed | Viright | Cancer | ||||||
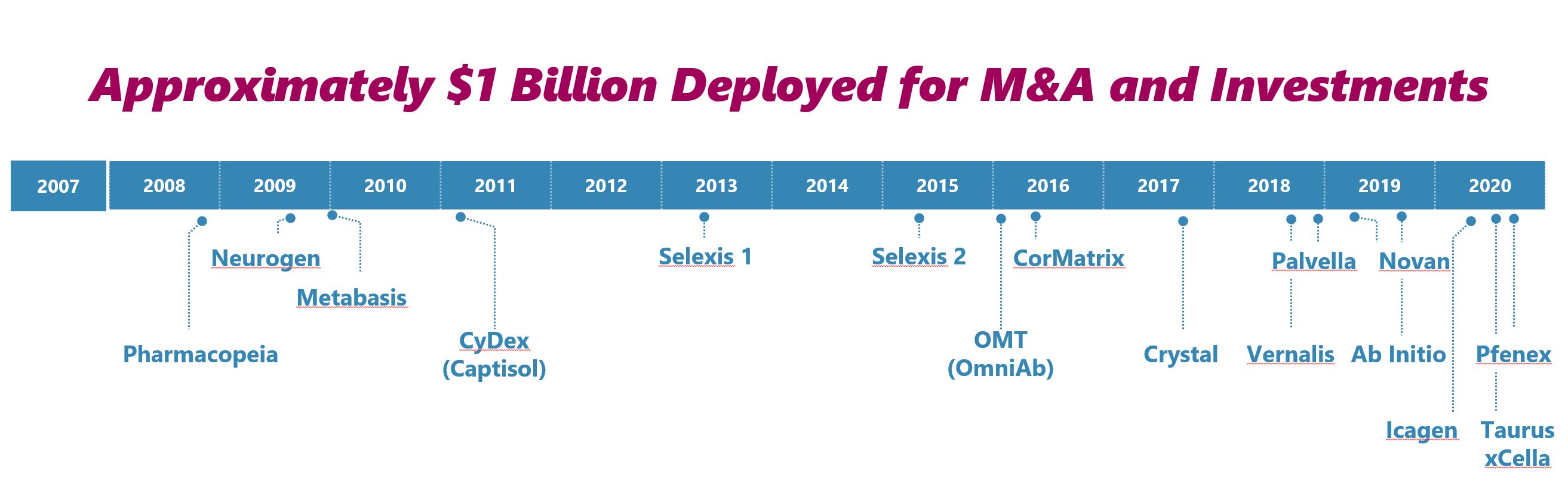
Acquisitions
We are a company devoted to identifying cutting-edge science and have exhibited a track record of capital deployment to create a high growth business model that operates with an efficient and low corporate cost structure. Over the last 15 years we have deployed approximately $1 billion of capital to build our portfolio. Following the spin-off of our OmniAb antibody discovery business, our strategy is to continue to expand our pipeline by acquiring additional revenue streams on both early and late stage drug product candidates from third parties, as well as acquiring commercial stage drugs for sale. Expanding our pipeline through these acquisitions can allow for further diversification across therapeutic areas and development stages. The following is a timeline of acquisitions we've completed over the last 15 years:
Selected Commercial Programs
The following programs represent important revenue-generating components of our current portfolio. For information about the royalties owed to us for these programs, see “Royalties” later in this business section.
Kyprolis (Amgen, Ono, BeiGene)
We supply Captisol to Amgen for use with Kyprolis (carfilzomib), and granted Amgen an exclusive product-specific license under our patent rights with respect to Captisol. Kyprolis is formulated with Ligand’s Captisol technology and is approved in the United States for the following:
•In combination with dexamethasone, lenalidomide plus dexamethasone, daratumumab plus dexamethasone, or daratumumab and hyaluronidase-fihj and dexamethasone, or isatuximab and dexamethasone for the treatment of patients with relapsed or refractory multiple myeloma who have received one to three lines of therapy.
•As a single agent for the treatment of patients with relapsed or refractory multiple myeloma who have received one or more lines of therapy.
Our agreement with Amgen may be terminated by either party in the event of material breach or bankruptcy, or unilaterally by Amgen with prior written notice, subject to certain surviving obligations. Absent early termination, the agreement will terminate upon expiration of the obligation to pay royalties. Under this agreement, we are entitled to receive revenue from clinical and commercial Captisol material sales and royalties on annual net sales of Kyprolis based on our patents and applications relating to the Captisol component of Kyprolis which are not expected to expire until 2033.
8
Teriparatide Injection Product (PF708) (Alvogen/Adalvo)
We acquired the Teriparatide Injection product with the acquisition of Pfenex in October 2020. Teriparatide Injection is a drug indicated for uses including the treatment of osteoporosis in certain patients at high risk for fracture. Teriparatide Injection was developed using our Pelican Expression Technology™ and was approved by the FDA in 2019 in accordance with the 505(b)(2) regulatory pathway, with FORTEO as the reference product. Our commercialization partner, Alvogen launched the product in June 2020 in the United States.
Our partner Alvogen has exclusively licensed the rights to commercialize and manufacture the Teriparatide Injection product in the United States, while Adalvo has the rights to commercialize in the EU, certain countries in the Middle East and North Africa (MENA), and the rest of world (ROW) territories (the latter defined as all countries outside of the EU, U.S. and MENA, excluding Mainland China, Hong Kong, Singapore, Malaysia and Thailand). In August 2020, marketing authorizationb throughout the EU was received under the trade name Livogiva and in December 2020 in Saudi Arabia under the name Bonteo. In December of 2022, we terminated a license agreement with Beijing Kangchen Biological Technology Co., Ltd. (Kangchen) thereby regaining the right to commercialize PF708 in Mainland China, Hong Kong, Singapore, Malaysia and Thailand along with a non-exclusive right to conduct development activities in such countries with respect to PF708.
In accordance with our agreements with Alvogen, we are eligible to receive tiered gross profit sharing of between 25% and 40% of quarterly profits prior to an “A” therapeutic equivalence designation, which increases to a flat 50% if an “A” rating is achieved.
In accordance with our EU, MENA and ROW agreements with Adalvo, we may be eligible to receive additional upfront and milestone payments of $1.5 million and may also be eligible to receive up to 60% of gross profit derived from product sales and regional license fees, if approved, depending on geography, cost of goods sold and sublicense fees.
Evomela (Acrotech and CASI)
We supply Captisol to, and receive royalties from, Acrotech Biopharma for sales of Evomela in the U.S., and CASI Pharmaceuticals for sales in China. Evomela received market approval by the NMPA in August of 2019. It is the only approved and commercially available melphalan product in China. Evomela is a Captisol-enabled melphalan IV formulation which is approved by the FDA for use in two indications:
•a high-dose conditioning treatment prior to autologous stem cell transplantation (ASCT) in patients with multiple myeloma; and
•for the palliative treatment of patients with multiple myeloma for whom oral therapy is not appropriate.
Evomela has been granted Orphan Designation by the FDA for use as a high-dose conditioning regimen for patients with multiple myeloma undergoing ASCT. The Evomela formulation avoids the use of propylene glycol, which has been reported to cause renal and cardiac side-effects that limit the ability to deliver higher quantities of therapeutic compounds. The use of the Captisol technology to reformulate melphalan is anticipated to allow for longer administration durations and slower infusion rates, potentially enabling clinicians to safely achieve a higher dose intensity of pre-transplant chemotherapy.
Under the terms of the license agreement, Acrotech Biopharma has marketing rights worldwide excluding China and CASI Pharmaceuticals has rights to market in China. We are eligible to receive over $50 million in potential milestone payments under this agreement, royalties on global net sales of the Captisol-enabled melphalan product and revenue from Captisol material sales. Acrotech and CASI’s obligation to pay royalties will expire at the end of the life of the relevant patents or when a competing product is launched, whichever is earlier, but in no event before ten years after the commercial launch. Our patents and applications relating to the Captisol component of melphalan are not expected to expire until 2033. As described herein, we have entered into a settlement agreement with Teva and Acrotech Biopharma (the holder of the NDA for Evomela) which will allow Teva to market a generic version of Evomela in the United States in 2026, or earlier under certain circumstances. Absent early termination, the agreement will terminate upon expiration of the obligation to pay royalties. The agreement may be terminated by either party for an uncured material breach or unilaterally by Acrotech and CASI by prior written notice.
Vaxneuvance (Merck)
Vaxneuvance, a 15-valent pneumococcal conjugate vaccine, also known as V114, was approved in the U.S. in July of 2021 for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F in adults 18 years of age and older, and subsequently in children 6 weeks through 17 years of age in June of 2022. Vaxneuvance was also approved in Europe in October 2022 for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years and older and in infants, children and adolescents from 6 weeks to less than 18 years of age. VAXNEUVANCE utilizes CRM197 vaccine carrier protein, which is produced using the patent-protected Pelican Expression Technology™ platform. We are entitled to low single digit royalties derived from net sales
9
of Vaxneuvance.
Pneumosil (Serum Institute of India, SII)
SII began commercialization of its 10-valent pneumococcal conjugate vaccine, Pneumosil, which is produced using CRM197 made in the Pelican Expression Technology platform, in the second quarter of 2020. Pneumosil is designed primarily to help fight against pneumococcal pneumonia among children, with an advantage of targeting the most prevalent serotypes of the bacterium causing serious illness in developing countries. Pneumosil achieved WHO Prequalification in December 2019, allowing the product to be procured by United Nations agencies and Gavi, the Vaccine Alliance, and subsequently achieved Indian Marketing Authorization in July 2020, and SII announced commercial launch of the product in India in December 2020.
Rylaze (Jazz Pharmaceuticals)
In July 2021, Jazz announced the US launch of Rylaze (asparaginase erwinia chrysanthemi (recombinant)-rywn), previously referred to as JZP458. Rylaze, which was approved by the FDA in June 2021, is a recombinant erwinia asparaginase used as a component of a multi-agent chemotherapeutic regimen for the treatment of acute lymphoblastic leukemia (ALL) or lymphoblastic lymphoma (LBL) in adult and pediatric patients one month or older who have developed hypersensitivity to E. coli-derived asparaginase. Additionally, Jazz is utilizing our technology for the development of PF745 (JZP341), a long-acting Erwinia asparaginase for the treatment of ALL and other hematological malignancies. Jazz has worldwide rights to develop and commercialize PF745.
Ligand is eligible to receive up to $155.5 million in milestone payments and tiered low to mid-single digit royalties based on worldwide net sales of any products resulting from this collaboration, including Rylaze.
Nexterone (Baxter)
We have a license agreement with Baxter, related to Baxter's Nexterone, a Captisol-enabled formulation of amiodarone, which is marketed in the United States and Canada. We supply Captisol to Baxter for use in accordance with the terms of the license agreement under a separate supply agreement. Under the terms of the license agreement, we will continue to earn milestone payments, royalties, and revenue from Captisol material sales. We earn royalties on net sales of Nexterone through early 2033.
Veklury (Gilead)
We supply Captisol to Gilead for sales of Veklury (remdesivir). Gilead received marketing approval from the FDA in October 2020. Veklury is an antiviral treatment of COVID-19 that is FDA approved. The product has regulatory approvals for the treatment of moderate or severe COVID-19 in over 70 countries. We are supplying Captisol to Gilead under a 10-year supply agreement. We are also supplying Captisol to Gilead’s voluntary licensing generic partners who are manufacturing remdesivir for 127 low- and middle-income countries. We receive our commercial compensation for this program through the sale of Captisol.
Zulresso (Sage)
We have a license agreement with Sage, related to Sage''s Zulresso, a Captisol-enabled formulation of brexanolone for the treatment of postpartum depression (PPD). Under the terms of the agreement, we receive royalties and revenue from Captisol material sales.
Noxafil-IV (Merck)
We have a supply agreement with Merck related to Merck’s NOXAFIL-IV, a Captisol-enabled formulation of posaconazole for IV use. NOXAFIL-IV is marketed in the United States, EU, Japan and Canada. We receive our commercial compensation for this program through the sale of Captisol.
Duavee or Duavive (Pfizer)
Pfizer is responsible for the marketing of bazedoxifene, a synthetic drug specifically designed to reduce the risk of osteoporotic fractures while also protecting uterine tissue. Pfizer has combined bazedoxifene with the active ingredient in Premarin to create a combination therapy for the treatment of post-menopausal symptoms in women. Pfizer is marketing the combination treatment under the brand names Duavee and Duavive in various territories. Net royalties on annual net sales of Duavee/Duavive are payable to us through the life of the relevant patent or ten years from the first commercial sale, whichever is longer, on a country by country basis.
Exemptia, Vivitra, Bryxta and Zybev (Zydus Cadila)
Zydus Cadila’s Exemptia (adalimumab biosimilar) is marketed in India for autoimmune diseases. Zydus Cadila uses the Selexis technology platform for Exemptia. We earn royalties on sales by Zydus Cadila for ten years following approval.
10
Zydus Cadila’s Vivitra (trastuzumab biosimilar) is marketed in India for breast cancer. Zydus Cadila uses the Selexis technology platform for Vivitra. We are entitled to earn royalties on sales by Zydus Cadila for ten years following approval.
Zydus Cadila’s Bryxta and Zybev (bevacizumab biosimilar) is marketed in India for various indications. Zydus Cadila uses the Selexis technology platform for Bryxta and Zybev. We earn royalties on sales by Zydus Cadila for ten years following approval.
Summary of Selected Development Stage Programs
We have multiple fully-funded partnered programs that are either in or nearing the regulatory approval process, or given the area of research or value of the license terms, we consider particularly noteworthy. We are eligible to receive milestone payments and royalties on these programs. This list does not include all of our partnered programs. In the case of Captisol-related programs, we are also eligible to receive revenue for the sale of Captisol material supply. The following table represents development stage assets with disclosed royalties:
| Development stage assets with disclosed royalties | ||||||||
| Program | Licensee | Royalty Rate | ||||||
| CE-Fosphenytoin | Sedor | 11% | ||||||
| CE-Meloxicam | Sedor | 8.0% - 10.0% | ||||||
| Ciforadenant | Corvus | Mid-single digit to low-teen royalty | ||||||
| DGAT-1 | Viking | 3.0% - 7.0% | ||||||
| Ensifentrine (RPL554) | Verona | Low to mid-single digit royalty | ||||||
| FBPase Inhibitor (VK0612) | Viking | 7.5% - 9.5% | ||||||
| Lasofoxifene | Sermonix | 6.0% - 10.0% | ||||||
| MB07133 | Xi'an Xintong | 6% | ||||||
| ME-344 | MEI Pharma | Low single digit royalty | ||||||
| Oral EPO | Viking | 4.5% - 8.5% | ||||||
| Pradefovir | Xi'an Xintong | 9% | ||||||
| PTX-022 | Palvella | 5.0% - 9.8% | ||||||
| SARM (VK5211) | Viking | 7.25% - 9.25% | ||||||
| SB206 | Novan | 7.0% - 10.0% | ||||||
| Sparsentan | Travere | 9% | ||||||
| TR Beta (VK2809 and VK0214) | Viking | 3.5% - 7.5% | ||||||
| Various | Nucorion | 4.0% - 9.0% | ||||||
| Various | Seelos | 4.0% - 10.0% | ||||||
Sparsentan (Travere)
In early 2012, Ligand licensed the world-wide rights to sparsentan to Travere Therapeutics. Travere recently received FDA accelerated approval for FILSPARI (sparsentan) for the treatment of immunoglobulin A nephropathy (IgAN). FILSPARI is the first and only dual endothelin angiotensin receptor antagonist in development for rare kidney diseases and is the first non-immunosuppressive treatment indicated for IgAN. Travere anticipates a review decision by the EMA on the potential approval for sparsentan for the treatment of IgAN in Europe in the second half of 2023. Additionally, Travere announced that they expect to report top line results from the two-year confirmatory endpoints in the ongoing Phase 3 DUPLEX Study of sparsentan in focal segmental glomerulosclerosis (FSGS) in the second quarter of 2023, with anticipated submission for full approval in the second half of 2023 in both the U.S. and Europe.
Under our license agreement with Travere, we are entitled to receive over $66 million in potential milestone payments, as well as 9% in royalties on any future worldwide sales.
TR-Beta - VK2809 and VK0214 (Viking)
Our partner, Viking, is developing VK2809, a novel selective thyroid hormone receptor beta (TR-beta) agonist with potential in multiple indications, including hypercholesterolemia, dyslipidemia and NASH. VK2809 is currently in a Phase 2b clinical trial (the VOYAGE study) in patients with biopsy-confirmed NASH. VK0214, another novel, orally available, TR-beta
11
agonist, is in development for the potential treatment of X-linked adrenoleukodystrophy (X-ALD). VK0214 is currently being evaluated in a Phase 1b clinical trial in patients with the adrenomyeloneuropathy (AMN) form of X-ALD. Under the terms of the agreement with Viking, we may be entitled to up to $375 million of development, regulatory and commercial milestones and tiered royalties on potential future sales. Our TR Beta programs partnered with Viking are subject to CVR sharing and a portion of the cash received will be paid out to CVR holders.
CRM197
CRM197 is a non-toxic mutant of diphtheria toxin. It is a well characterized protein and functions as a carrier for polysaccharides and haptens, making them immunogenic. CRM197 is used in prophylactic and therapeutic vaccine candidates. We have developed CRM197 production strains using our Protein Expression Technology platform and supply preclinical grade and cGMP CRM197 (PeliCRM™) to several vaccine development focused pharmaceutical customers.
Our partners Merck and SII have exclusively licensed unique production strains for use in their conjugate vaccine products and candidates for pneumococcal and meningitis bacterial infections. Pneumococcus bacterium (Streptococcus pneumoniae) is a leading cause of severe pneumonia and major cause of morbidity and mortality worldwide. In accordance with our CRM197 commercial license agreements with Merck, we are eligible to earn an additional $8 million in development and regulatory milestones and low single digit royalties derived from net sales, depending on territory. CRM-197 made in the Pelican Expression Technology platform is also used by Merck in its investigational vaccine candidates, including V116, a 21-valent pneumococcal conjugate vaccine currently in Phase 3 clinical trials.
Ensifentrine – RPL554 (Verona)
Ensifentrine is a first-in-class, selective dual inhibitor of phosphodiesterase 3 and 4 enzymes combining bronchodilator and non-steroidal anti-inflammatory activities in one compound. Ligand obtained the rights to ensifentrine in 2018 in the acquisition of Vernalis. Our partner, Verona Pharma, recently completed the Phase 3 ENHANCE-21 and ENHANCE-12 trials evaluating nebulized ensifentrine for the maintenance treatment of chronic obstructive pulmonary disease (COPD) and plans to file a NDA with the US FDA in the first half of 2023. Under the terms of our agreement with Verona, we are entitled to development and regulatory milestones, including a £5.0 million payment upon the first approval by any regulatory authority, and royalties on potential future sales.
SARM - VK5211 (Viking)
Viking is also developing VK5211, a novel SARM for patients recovering from hip-fracture. SARMs retain the beneficial properties of androgens without undesired side-effects of steroids or other less selective androgens. In a Phase 2 clinical trial, VK5211 demonstrated statistically significant, dose dependent increases in lean body mass. Under the terms of the agreement with Viking, we may be entitled to up to $270 million of development, regulatory and commercial milestones as well as tiered royalties on potential future sales.
Ganaxalone IV (Marinus)
Our partner, Marinus, is conducting Phase 3 clinical trials with Captisol-enabled ganaxolone IV in patients with refractory status epilepticus. Marinus has exclusive worldwide rights to Captisol-enabled ganaxolone, a GABAA receptor modulator, for use in humans. We are entitled to development and regulatory milestones, revenue from Captisol material sales, and royalties on potential future sales.
Ciforadenant – CPI-444 (Corvus)
Our partner, Corvus, is conducting a Phase 1b/2 clinical trial evaluating ciforadenant as a potential first line therapy for metastatic renal cell cancer (RCC) in combination with ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1). The Phase 1b/2 study is being conducted by the Kidney Cancer Research Consortium (KCRC) and is led by The University of Texas MD Anderson Cancer Center. Under the terms of our agreement with Corvus, we are entitled to development and regulatory milestones and tiered royalties on potential future sales. The aggregate potential milestone payments from Corvus are approximately $220 million for all indications.
FYCOMPA IV (Eisai)
Our partner, Eisai, is developing an intravenous Fycompa® (perampanel), formulated with Captisol, as a substitute in Japan for oral tablets as an adjunctive therapy in patients with partial onset seizures (including secondarily generalized seizures) or primary generalized tonic-clonic seizures. In August of 2022, Eisai announced it had filed a supplementary NDA in Japan for Fycompa IV seeking approval for the injection formulation as a new route of administration. We are entitled to revenue from Captisol material sales and tiered royalties on potential future sales.
12
SB206 (Novan)
We acquired certain economic rights to berdazimer gel, 10.3% (SB206) from Novan in May 2019. Berdazimer gel is a topical nitric-oxide antiviral gel for the treatment of viral skin infections, including molluscum contagiosum (MC). MC is an infection which causes skin lesions that affect approximately 6 million people in the United States annually, with the greatest incidence in children aged one to 14 years. Under a development funding and royalties agreement with Novan for berdazimer gel, Ligand is entitled to receive up to $20 million of milestone payments and tiered royalties of 7% to 10% on future worldwide sales of berdazimer gel.
PTX-022 (Palvella)
We acquired the economic rights to QTORIN™ 3.9% rapamycin anhydrous gel (QTORIN™ rapamycin, formerly PTX-022) from Palvella in December 2018. QTORIN™ rapamycin is a novel, topical formulation comprising high-strength rapamycin in development for the treatment of Pachyonychia Congenita (PC), treatment of Microcystic Lymphatic Malformations (Microcystic LM), and for the prevention of Basal Cell Carcinomas (BCCs) in Gorlin Syndrome (GS). Palvella expects to report top-line results of the Phase 3 VAPAUS study in PC in mid-2023.
Lasofoxifene (Sermonix)
Lasofoxifene is a selective estrogen receptor modulator for osteoporosis treatment and other diseases, discovered through the research collaboration between Pfizer and us. Our partner, Sermonix has a license for the development of oral lasofoxifene for the United States and additional territories and is currently developing lasofoxifene as a treatment for ESR1-mutated metastatic breast cancer. Under the terms of the agreement, we are entitled to receive over $45 million in potential regulatory and commercial milestone payments as well as royalties on potential future net sales.
Pradefovir (Xi'an Xintong)
Our Chinese licensee, Xi'an Xintong Medicine Research (following its acquisition of Chiva Pharmaceuticals), is developing pradefovir, an oral liver-targeting prodrug of the HBV DNA polymerase/reverse transcriptase inhibitor adefovir, for the potential treatment of HBV infection. Pradefovir was developed using Ligand’s HepDirect technology. Xi'an Xintong recently completed a Phase 3 HBV trial. We are entitled to an annual licensing maintenance fee and royalties on potential future sales.
MB07133 (Xi'an Xintong)
Chinese licensee Xi'an Xintong Medicine Research is also developing MB07133, a liver specific, HepDirect prodrug of cytarabine monophosphate, for the potential treatment of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. MB07133 is currently in Phase 1 in China. We are entitled to an annual licensing maintenance fee and royalties on potential future sales.
CX2101A (China Resources Double-Crane Pharmaceutical)
In October of 2021, Ligand signed a collaboration agreement granting China Resources Double-Crane Pharmaceutical Co., Ltd. (CRDC) exclusive Asia territorial rights to develop a novel investigational oral COVID-19 antiviral therapeutic compound using Ligand’s BEPro technology. Ligand received an upfront payment in respect of the collaboration, and clinical and regulatory milestone payments, and tiered royalties on net sales. CRDC will be responsible for all costs related to the program. BEPro is a proprietary prodrug technology that is specifically applicable to nucleotides and nucleotide analogs for the development of compounds with improved product profiles. In December of 2022, CRDC announced that the IND for CX2101A received a "Notice of Drug Clinical Trial Approval" issued by the State Drug Administration (NMPA), approving clinical trials of the drug for the treatment of novel coronavirus pneumonia in China. CX2101A is a small molecule compound that acts on RdRp (RNA-dependent RNA polymerase) of SARS-CoV-2, using the BEPro prodrug technology. CRDC is conducting a Phase 1 trial in China.
ONS-5010 (Outlook Therapeutics)
Outlook Therapeutics announced in October of 2022 that the US FDA has accepted for filing a BLA for ONS-5010 / LYTENAVA™ (bevacizumab-vikg), an investigational ophthalmic formulation of bevacizumab for the treatment of wet age-related macular degeneration (wet AMD). Outlook uses the Selexis technology platform for ONS-5010. The FDA set a PDUFA goal date of August 29, 2023 for the BLA. ONS-5010, if approved, is expected to receive 12 years of regulatory exclusivity in the United States. In December of 2022, Outlook announced the validation of its MAA by the EMA for ONS-5010. The decision for potential approval is expected from the European Commission in early 2024. We are entitled to earn royalties on sales of ONS-5010 by Outlook.
Milestone Payments
13
Our programs under license with our partners may generate milestone payments to us if our partners reach certain development, regulatory and commercial milestones. The following table represents the maximum value of our milestone payment pipeline by technology, development stage and partner (in thousands):
| Technology* | Stage* | Partner* | |||||||||||||||||||||
| Pelican | >$215,000 | Preclinical | > $1,000 | Viking | $1,500,000 | ||||||||||||||||||
| Captisol | > $170,000 | Clinical | > $120,000 | Jazz | $150,000 | ||||||||||||||||||
| LTP/Hep Direct/BEPro | > $310,000 | Regulatory | > $1,200,000 | Seelos | $100,000 | ||||||||||||||||||
| NCE/Other | > $1,850,000 | Commercial | > $1,250,000 | Travere | $70,000 | ||||||||||||||||||
| Total | >$2,500,000 | Total | >$2,500,000 | Other | >$750,000 | ||||||||||||||||||
| Total | >$2,500,000 | ||||||||||||||||||||||
*All tables exclude any annual access fees and collaboration revenue for development work.
Summary of selected programs available for license
We have a number of unpartnered programs focused on a wide-range of potential indications or disease eligible for further development or licensing:
| Program | Development Stage | Targeted Indication or Disease | ||||||||||||
| CE-Iohexol | Phase 2 | Diagnostics | ||||||||||||
| Luminespib/Hsp90 Inhibitor | Phase 2 | Oncology | ||||||||||||
| CE-Sertraline, Oral Concentrate | Phase 1 | Depression | ||||||||||||
| PF530 Interferon Beta | Phase 1 | Immunomodulatory | ||||||||||||
| PF582 Ranibizumab | Phase 1 | Ocular | ||||||||||||
| CCR1 Antagonist | Preclinical | Oncology | ||||||||||||
| CE-Busulfan | Preclinical | Oncology | ||||||||||||
| CE-Cetirizine Injection | Preclinical | Allergy | ||||||||||||
| CE-Silymarin for Topical formulation | Preclinical | Sun damage | ||||||||||||
| FLT3 Kinase Inhibitors | Preclinical | Oncology | ||||||||||||
| GCSF Receptor Agonist | Preclinical | Blood disorders | ||||||||||||
| PF529 Pegfilgrastim | Preclinical | Oncology | ||||||||||||
| PF810 Recombinant Peptide | Preclinical | Endocrine System | ||||||||||||
Manufacturing
We contract with a third party manufacturer, Hovione, for Captisol production. Hovione operates FDA-inspected sites in the United States, Macau, Ireland and Portugal. Manufacturing operations for Captisol are performed primarily at Hovione's Portugal and Ireland facilities. We believe we maintain adequate inventory of Captisol to meet our current partner needs and that our Captisol capacity will be sufficient to meet future partner needs.
In the event of a Captisol supply interruption, we are permitted to designate and, with Hovione’s assistance, qualify one or more alternate suppliers. If the supply interruption continues beyond a designated period, we may terminate the agreement. In addition, if Hovione cannot supply our requirements of Captisol due to an uncured force majeure event, we may also obtain Captisol from a third party and have previously identified such parties.
The current term of the agreement with Hovione is through December 2024. The agreement will automatically renew for successive two year renewal terms unless either party gives written notice of its intention to terminate the agreement no less than two years prior to the expiration of the initial term or renewal term. In addition, either party may terminate the agreement for the uncured material breach or bankruptcy of the other party or an extended force majeure event. We may terminate the agreement for extended supply interruption, regulatory action related to Captisol or other specified events. We have ongoing minimum purchase commitments under the agreement.
14
Competition
Some of the drugs we and our licensees and partners are developing may compete with existing therapies or other drugs in development by other companies. Furthermore, academic institutions, government agencies and other public and private organizations conducting research may seek patent protection with respect to potentially competing products or technologies and may establish collaborative arrangements with our competitors.
Our Captisol business may face competition from other suppliers of similar cyclodextrin excipients or other technologies that are aimed to increase solubility or stability of APIs.
Our competitive position also depends upon our ability to obtain patent protection or otherwise develop proprietary products or processes. For a discussion of the risks associated with competition, see below under “Item 1A. Risk Factors.”
Environmental, Health and Safety (EHS)
We are committed to providing a safe and healthy workplace, promoting environmental excellence in our communities, and complying with all relevant regulations and industry standards. We establish and monitor programs to reduce pollution, prevent injuries, and maintain compliance with applicable regulations. By focusing on such practices, we believe we can affect a meaningful, positive change in our community and maintain a healthy and safe environment. During 2022, we made good progress on our ESG efforts. We have initiated a $2.5 million solar investment at Kansas University Innovation Park; modified the Captisol manufacturing process resulting in water savings and packaging reduction; made ESG related charitable donations; and commenced numerous initiatives from our ESG-focused outreach committees. We expect to continue our effort and to refine our EHS policies and practices in 2023. More information on our EHS policies and initiatives is available on our website at www.ligand.com. The information contained on our website is not intended to be part of this filing.
Government Regulation
The research and development, manufacturing and marketing of pharmaceutical products are subject to regulation by numerous governmental authorities in the United States and other countries. We and our partners, depending on specific activities performed, are subject to these regulations. In the United States, pharmaceuticals are subject to regulation by both federal and various state authorities, including the FDA. The Federal Food, Drug and Cosmetic Act and the Public Health Service Act govern the testing, manufacture, safety, efficacy, labeling, storage, record keeping, approval, advertising and promotion of pharmaceutical products. These activities are subject to additional regulations that apply at the state level. There are similar regulations in other countries as well. For both currently marketed products and products in development, failure to comply with applicable regulatory requirements can, among other things, result in delays, the suspension of regulatory approvals, as well as possible civil and criminal sanctions. In addition, changes in existing regulations could have a material adverse effect on us or our partners. For a discussion of the risks associated with government regulations, see below under “Item 1A. Risk Factors.”
Patents and Proprietary Rights
We believe that patents and other proprietary rights are important to our business. Our policy is to file patent applications to protect technology, inventions and improvements to our inventions that are considered important to the development of our business. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position.
Patents are issued or pending for the following key products or product families. The scope and type of patent protection provided by each patent family is defined by the claims in the various patents. Patent term may vary by jurisdiction and depend on a number of factors including potential patent term adjustments, patent term extensions, and terminal disclaimers. For each product or product family, the patents and/or applications referred to are in force in at least the United States, and for most products and product families, the patents and/or applications are also in force in European jurisdictions, Japan and other jurisdictions.
Captisol
Patents and pending patent applications covering Captisol and methods of making Captisol are owned by us. The patents covering the Captisol product with the latest expiration date is set to be in 2033 (see, e.g., U.S. Patent No. 9,493,582 (expires Feb. 27, 2033)). Other patent applications covering methods of making Captisol, if issued, potentially have terms to 2041. We have asserted U.S. Patents 8,410,077, 9,200,088, and 9,493,582 against Teva in connection with their attempt to obtain FDA approval to manufacture and sell a generic version of Evomela®. We also own several patents and pending patent applications covering drug products containing Captisol as a component. Globally, we own approximately 390 issued patents covering all of the foregoing Captisol compositions, methods and related technology.
Ten Captisol patents in several families are listed in the Orange Book in connection with one or more prescription drugs currently on the market. These Captisol-enabled drugs include Nexterone (Baxter), Kyprolis (Amgen), Noxafil (Merck),
15
Evomela (Acrotech/CASI), Baxdela (Melinta) and Zulresso (Sage). These patents are listed in the table below, and each patent family containing these patents has pending and/or granted counterparts in Europe, China and Japan.
Orange Book-listed Captisol Patents | |||||||||||
Country | Patent No. | Title | Expiration (nominal) ‡ | ||||||||
United States | 7635773 | Sulfoalkyl Ether Cyclodextrin Compositions | 03/13/2029 | ||||||||
United States | 8410077 | Sulfoalkyl Ether Cyclodextrin Compositions | 03/13/2029 | ||||||||
United States | 9200088 | Sulfoalkyl Ether Cyclodextrin Compositions | 03/13/2029 | ||||||||
United States | 10117951 | Sulfoalkyl Ether Cyclodextrin Compositions | 03/13/2029 | ||||||||
United States | 9750822 | Sulfoalkyl Ether Cyclodextrin Compositions | 03/13/2029 | ||||||||
United States | 9493582 | Alkylated Cyclodextrin Compositions And Processes For Preparing And Using The Same | 2/27/2033 | ||||||||
United States | 10040872 | Alkylated Cyclodextrin Compositions And Processes For Preparing And Using The Same | 10/21/2033 | ||||||||
| United States | 10864183 | Injectable Nitrogen Mustard Compositions Comprising A Cyclodextrin Derivative And Methods Of Making And Using The Same | 5/28/2030 | ||||||||
| United States | 10940128 | Injectable Melphalan Compositions Comprising A Cyclodextrin Derivative And Methods Of Making And Using The Same | 5/28/2030 | ||||||||
| United States | 11020363 | Injectable Nitrogen Mustard Compositions Comprising A Cyclodextrin Derivative And Methods Of Making And Using The Same | 5/28/2030 | ||||||||
‡ Expiration dates are calculated as 20 years from the earliest nonprovisional filing date to which priority is claimed, and do not take into account disclaimers or extensions that are or may be available in these jurisdictions.
Subject to compliance with the terms of the respective agreements, our rights to receive royalty payments under our licenses with our exclusive licensors typically extend for the life of the patents covering such developments. For a discussion of the risks associated with patent and proprietary rights, see below under “Item 1A. Risk Factors.”
Kyprolis
Patents protecting Kyprolis include those owned by Amgen and those owned by us. The United States patent listed in the Orange Book relating to Kyprolis owned by Amgen with the latest expiration date is not expected to expire until 2029. Patents and applications owned by Ligand relating to the Captisol component of Kyprolis are not expected to expire until 2033. Amgen filed suit against several generic drug companies over their applications to make generic versions of Kyprolis. Several generics have settled with Amgen on confidential terms. However, it has been publicly reported that the U.S. launch date for at least Breckenridge Pharmaceuticals’ generic product will be on a date that is held as confidential in 2027 or sooner, depending on certain occurrences. One generic company, Cipla Limited/Cipla USA, Inc. chose not to settle the litigation with Amgen, and proceeded to trial. The District Court upheld the validity of patent claims from three of the patents and the judgment was upheld on appeal.
Ligand UK Development Limited
Under the terms of our sale of Vernalis (R&D) Limited to HitGen in December 2020, Ligand retained a portfolio of fully-funded shots on goal, which now include S65487, a Bcl-2 inhibitor, and S64315, an Mcl-1 inhibitor for treatment of cancers, both of which are partnered with Servier in collaboration with Novartis and VER250840 (an oral, selective Chk1 inhibitor for treatment of cancer). These programs and their IP are now owned by Ligand UK Development Limited, which has a worldwide patent portfolio of over 200 granted patents in over 70 countries. This patent portfolio is mature, with expected expiry dates between 2022 and 2033.
Pelican Expression Technology Platform
We acquired the Pelican Expression Technology platform through acquisition of Pfenex Inc. in October 2020. This acquisition brought a robust portfolio of patents and patent applications along with substantial know-how and trade secrets which protect various aspects of our core Pelican Expression Technology business. As of December 31, 2022, we were the sole owner of a patent portfolio that consisted of over 200 patents and 40 pending patent applications worldwide that provide material coverage for our platform technology, licensed products and product candidates. Our U.S. issued patents expire during the time period beginning in 2025 and ending in 2038. Our owned and exclusively licensed patent portfolio includes claims directed to methods for recombinant protein production and methods for rapid screening of an array of expression systems, tools for protein expression such as P. fluorescens promoters, secretion leaders, plasmid maintenance systems, improved methods for non-standard amino acid incorporation and fusion partners for peptide production. In addition, our IP covers
16
methods for producing certain classes of proteins such as cytokines, growth factors and antibody derivatives, as well as expression strains and methods for production, purification and formulation of certain vaccine antigens, peptides, therapeutic enzymes, human cytokines, etc.
Human Capital Management
We recognize and take care of our employees by offering a wide range of competitive pay, recognition, and benefit programs. We are proud to provide our employees the opportunity to grow and advance as we invest in their education and career development. As of December 31, 2022, we have 76 employees, of whom 49 are involved directly in scientific research and development activities.
We rely on skilled, experienced, and innovative employees to conduct the operations of our company. Our key human capital objectives include identifying, recruiting, retaining, incentivizing and integrating our existing and new employees. We frequently benchmark our compensation practices and benefits programs against those of comparable industries and in the geographic areas where our facilities are located. We believe that our compensation and employee benefits are competitive and allow us to attract and retain skilled labor throughout our organization. Our notable health, welfare and retirement benefits include:
•equity awards through our 2002 Stock Incentive Plan;
•subsidized health insurance;
•401(k) Plan with matching contributions;
•tuition assistance program; and
•paid time off.
We value diversity at all levels and continue to focus on extending our diversity and inclusion initiatives across our workforce. As of December 31, 2022, approximately 26% and 14% of our workforce are Asian and Hispanic, respectively. We believe that our business benefits from the different perspectives a diverse workforce brings.
We strive to maintain an inclusive environment free from discrimination of any kind, including sexual or other discriminatory harassment. Our employees have multiple avenues available through which inappropriate behavior can be reported, including a confidential hotline. All reports of inappropriate behavior are promptly investigated with appropriate action taken to stop such behavior.
Investor Information
Financial and other information about us is available on our website at www.ligand.com. We make available on our website, without charge, copies of our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. You may obtain copies of these documents by visiting the SEC’s website at www.sec.gov. In addition, we use Twitter (@Ligand_LGND) and our investor relations website as a means of disclosing material non-public information and for complying with our disclosure obligations under Regulation FD. Investors should monitor our Twitter account and our website, in addition to following our press releases, SEC filings, public conference calls and webcasts. These website addresses and the information accessible through our Twitter account are not intended to function as hyperlinks, and the information contained in our website and in the SEC’s website is not intended to be a part of this filing.
| ITEM 1A. | RISK FACTORS | ||||
The following is a summary description of some of the many risks we face in our business. You should carefully review these risks in evaluating our business, including the businesses of our subsidiaries. You should also consider the other information described in this report. Additional risks not presently known to us or that we currently deem immaterial also may impair our business.
Summary of Risks Related to our Business:
Our business is subject to numerous risks and uncertainties, including those described below. The principal risks and uncertainties affecting our business include, but are not limited to the following:
17
•Future revenue based on Kyprolis, Evomela, Teriparatide and Rylaze as well as royalties from our other partnered products, may be lower than expected;
•Future revenue from sales of Captisol material to our license partners may be lower than expected;
•We rely heavily on collaboration relationships to generate milestone and royalty payments and our collaboration partners have significant discretion when deciding whether to pursue any development program, and any failure by our partners to successfully develop a product candidate or a termination or breach of any of the related agreements, or a change in their strategy or the focus of their development and commercialization efforts with respect to our partnered programs, could reduce our milestone and license fee revenue, and potentially reduce future royalties;
•Our product candidates, and the product candidates of our partners, face significant development and regulatory hurdles prior to partnering and/or marketing which could delay or prevent licensing, sales-based royalties and/or milestone revenue;
•Third party intellectual property may prevent us or our partners from developing our potential products; our and our partners’ intellectual property may not prevent competition; and any intellectual property issues may be expensive and time consuming to resolve;
•Market acceptance and sales of any approved product will depend significantly on the availability and adequacy of coverage and reimbursement from third-party payors and may be affected by existing and future healthcare reform measures; and
•Our operating results may fluctuate significantly, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations or any guidance we may provide.
Risks Related to Our Business Operations and Reliance on Third Parties:
Future revenue based on Kyprolis, Evomela, Teriparatide and Rylaze as well as royalties from our other partnered products, may be lower than expected.
A significant portion of our royalty revenue is based on sales of Kyprolis by Amgen, sales of Evomela by Acrotech Biopharma, sales of Teriparatide by Alvogen/Adalvo and sales of Rylaze by Jazz. Royalties, including payments from the foregoing partners, are expected to be a substantial portion of our ongoing revenues for the foreseeable future. Any setback that may occur with respect to any of our partners' products, and in particular Kyprolis, could significantly impair our operating results and/or reduce our revenue and the market price of our stock. Setbacks for the products could include problems with shipping, distribution, manufacturing, product safety, marketing, government regulation or reimbursement, licenses and approvals, intellectual property rights, including failure by any of the foregoing partners to enforce their respective intellectual property rights, competition with existing or new products and physician or patient acceptance of the products, as well as higher than expected total rebates, returns, discounts, or unfavorable exchange rates. These products also are or may become subject to generic competition. For example, we entered into a settlement agreement with Teva and Acrotech Biopharma (the holder of the NDA for Evomela) which will allow Teva to market a generic version of Evomela in the United States on June 1, 2026, or earlier under certain circumstances. The entry of generic competition for Evomela may materially and adversely affect the revenue we derive from Evomela sales. Also, Amgen has settled patent litigation related to Kyprolis on confidential terms with several parties, but it has been publicly reported that the U.S. launch date for at least Breckenridge Pharmaceuticals’ applicable generic product will be “on a date that is held as confidential in 2027 or sooner, depending on certain occurrences.”
Future revenue from sales of Captisol material to our license partners may be lower than expected.
Revenues from sales of Captisol material to our collaborative partners, including Amgen and Gilead, represent a significant portion of our current revenues. Any setback that may occur with respect to Captisol could significantly impair our operating results and/or reduce the market price of our stock. Setbacks for Captisol could include problems with shipping, distribution, manufacturing, product safety, marketing, government regulation or reimbursement, licenses and approvals, intellectual property rights, competition with existing or new products and physician or patient acceptance of the products using Captisol. In addition, revenue from Captisol sales related to remdesivir may continue to decrease due to a number of factors, including alternative treatments for COVID-19 that have been or will be developed by other companies and the decrease in COVID-19 infections, in which case the commercial opportunity could be materially and adversely affected.
If products or product candidates incorporating Captisol material were to cause any unexpected adverse events, the perception of Captisol safety could be seriously harmed. If this were to occur, we may not be able to sell Captisol unless and until we are able to demonstrate that the adverse event was unrelated to Captisol, which we may not be able to do. Further, the FDA could require us to submit additional information for regulatory review or approval, including data from extensive safety
18
testing or clinical testing of products using Captisol. This would be expensive and it may delay the marketing of Captisol-enabled products and receipt of revenue related to those products, which could significantly impair our operating results and/or reduce the market price of our stock.
We obtain Captisol from Hovione, our third party manufacturer, primarily at their facilities in Ireland and Portugal. If Hovione were to cease to be able, for any reason, to supply Captisol to us in the amounts we require, or decline to supply Captisol to us, we would be required to seek an alternative source, which could potentially take a considerable length of time and impact our revenue and customer relationships. In the event of a Captisol supply interruption, we are permitted to designate and, with Hovione’s assistance, qualify one or more alternate suppliers, although there is no assurance that we could do so timely or at acceptable costs, if at all. In addition to manufacturing at Hovione’s facilities in Ireland and Portugal, we have processing capacity for Captisol in both the United States and England.
We maintain inventory of Captisol, which has a five-year shelf life, at three geographically dispersed storage locations in the United States and Europe. If we were to encounter problems maintaining our inventory, such as natural disasters, at one or more of these locations, it could lead to supply interruptions. In addition, we rely on Hovione to expand manufacturing capacity of Captisol and any failure by Hovione to timely implement such increased capacity could adversely affect our ability to supply Captisol to our partners. While we believe we maintain adequate inventory of Captisol to meet our current partner needs, and our Captisol capacity will be sufficient to meet future partner needs, our estimates and projections for Captisol demand may not be correct and any supply interruptions could materially adversely impact our operating results.
We currently depend on our arrangements with our partners and licensees to sell products using our Captisol technology. These agreements generally provide that our partners may terminate the agreements at will. If our partners discontinue sales of products using Captisol, fail to obtain regulatory approval for products using Captisol, fail to satisfy their obligations under their agreements with us, choose to utilize a competing product, or if we are unable to establish new licensing and marketing relationships, our financial results and growth prospects would be materially affected.
Further, under most of our Captisol outlicenses, the amount of royalties we receive will be reduced or will cease when the relevant patent expires. Our low-chloride patents and foreign equivalents are not expected to expire until 2033, our high purity patents and foreign equivalents, are not expected to expire until 2029 and our morphology patents and foreign equivalents are not expected to expire until 2026 in the United States, but the initially filed patents relating to Captisol expired starting in 2010 in the United States and in 2016 in most countries outside the United States. If our other intellectual property rights are not sufficient to prevent a generic form of Captisol from coming to market and if in such case our partners choose to terminate their agreements with us, our Captisol revenue may decrease significantly.
We rely heavily on collaboration relationships to generate milestone and royalty payments and our collaboration partners have significant discretion when deciding whether to pursue any development program, and any failure by our partners to successfully develop a product candidate or a termination or breach of any of the related agreements could reduce our milestone and license fee revenue, and potentially reduce future royalties.
Our strategy for developing and commercializing many of our product candidates includes entering into collaboration agreements, outlicenses, and development funding and royalty purchase agreements with corporate partners and others. These agreements give our collaboration partners significant discretion when deciding whether or not to pursue any development program. Our existing collaborations may not continue or be successful, and we may be unable to enter into future collaboration arrangements to develop and commercialize our unpartnered assets.
In addition, our collaborators may develop products, either alone or with others that compete with the types of products they are developing with us (or that we are developing on our own). This would result in increased competition for our or our partners' programs. If product candidates are approved for marketing under our collaboration programs, revenues we receive will depend on the manufacturing, marketing and sales efforts of our collaboration partners, who generally retain commercialization rights under the collaboration agreements. Generally, our current collaboration partners also have the right to terminate their collaborations at will or under specified circumstances. If any of our collaboration partners breach (for example, by not making required payments when due, or at all) or terminate their agreements with us or otherwise fail to conduct their collaboration activities successfully, including due to insolvency events, ongoing product development under these agreements will be delayed or terminated. Disputes or litigation may also arise with our collaborators (with us and/or with one or more third parties), including those over ownership rights to intellectual property, know-how or technologies developed with our collaborators. Such disputes or litigation could adversely affect our rights to one or more of our product candidates. Any such dispute or litigation could delay, interrupt or terminate the collaboration research, development and commercialization of certain potential products, create uncertainty as to ownership rights of intellectual property, or could result in litigation or arbitration. The occurrence of any of these problems could be time-consuming and expensive and could adversely affect our business.
19
Our collaboration partners may change their strategy or the focus of their development and commercialization efforts with respect to our partnered programs, and the success of our partnered programs could be adversely affected.
If our collaboration partners terminate their collaborations with us or do not commit sufficient resources to the development, manufacture, marketing or distribution of our partnered programs, we could be required to devote additional resources to our partnered programs, seek new collaboration partners or abandon such partnered programs, all of which could reduce our revenues and otherwise have an adverse effect on our business.
In addition, biopharmaceutical development is inherently uncertain and very few therapeutic candidates ultimately progress through clinical development and receive approval for commercialization. If our partners do not receive regulatory approval for a sufficient number of therapeutic candidates originating from our partnerships, we may not be able to sustain our business model.
Our product candidates, and the product candidates of our partners, face significant development and regulatory hurdles prior to partnering and/or marketing which could delay or prevent licensing, sales-based royalties and/or milestone revenue.
Before we or our partners obtain the approvals necessary to sell any of our unpartnered assets or partnered programs, we must show through preclinical studies and human testing that each potential product is safe and effective. We and/or our partners have a number of partnered programs and unpartnered assets moving toward or currently awaiting regulatory action. Failure to show any product's safety and effectiveness could delay or prevent regulatory approval of a product and could adversely affect our business. The product development and clinical trials process is complex and uncertain. For example, the results of preclinical studies and initial clinical trials may not necessarily predict the results from later large-scale clinical trials. In addition, clinical trials may not demonstrate a product's safety and effectiveness to the satisfaction of the regulatory authorities. A number of companies have suffered significant setbacks in advanced clinical trials or in seeking regulatory approvals, despite promising results in earlier trials. The FDA may also require additional clinical trials after regulatory approvals are received. Such additional trials may be expensive and time-consuming, and failure to successfully conduct those trials could jeopardize continued commercialization of a product.
The speed at which we and our partners complete our scientific studies and clinical trials depends on many factors, including, but not limited to, the ability to obtain adequate supplies of the products to be tested and patient enrollment. Patient enrollment is a function of many factors, including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial and other potential drug candidates being studied. Delays in patient enrollment for our or our partners’ trials may result in increased costs and longer development times. In addition, our partners have rights to control product development and clinical programs for products developed under our collaborations. As a result, these partners may conduct these programs more slowly or in a different manner than expected. Moreover, even if clinical trials are completed, we or our partners still may not apply for FDA or foreign regulatory approval in a timely manner or the FDA or foreign regulatory authority still may not grant approval.
Our product candidate discovery, early-stage development, and product reformulation programs may require substantial additional capital to complete successfully. Our partners’ development programs may require substantial additional capital to complete successfully, arising from costs to: conduct research, preclinical testing and human studies; establish pilot scale and commercial scale manufacturing processes and facilities; and establish and develop quality control, regulatory, marketing, sales and administrative capabilities to support these programs. While we expect to fund our research and development activities from cash generated from operations to the extent possible, if we are unable to do so, we may need to complete additional equity or debt financings or seek other external means of financing. These financings could depress our stock price. If additional funds are required to support our operations and we are unable to obtain them on terms favorable to us, we may be required to cease or reduce further development or commercialization of our products, to sell some or all of our technology or assets or to merge with another entity.
If the Distribution, together with certain related transactions, fails to qualify as a reorganization under Sections 355 and 368(a)(1)(D) of the Internal Revenue Code of 1986, as amended (the “Code”), or the Merger fails to qualify as a reorganization under Section 368(a) of the Code, we could incur significant tax liabilities.
The Distribution and the Merger were conditioned upon receipt of a tax opinion from outside counsel to the effect that the Distribution would qualify as a reorganization under Sections 355 and 368(a)(1)(D) of the Code, that the Merger would not cause Section 355(e) of the Code to apply to the Distribution and that the Merger would be treated as a reorganization under Section 368(a) of the Code. The opinion was delivered in connection with the closing of the Merger and was based on, among other things, certain facts, assumptions, representations and undertakings from us, OmniAb and New OmniAb, including those regarding the past and future conduct of the companies’ respective businesses and other matters. If any of these facts, assumptions, representations, or undertakings were incorrect or not satisfied, we may not be able to rely on the opinion, and we and our stockholders could be subject to significant U.S. federal income tax liabilities. In addition, the opinion is not binding on the IRS or the courts, and notwithstanding the opinion, the IRS could determine on audit that the Distribution or Merger does not qualify as a reorganization if it determines that any of the facts, assumptions, representations or undertakings on which the
20
opinion is based are not correct or have been violated or that the Distribution or Merger should be taxable for other reasons, including as a result of a significant change in stock or asset ownership after the Distribution. If the Distribution, together with certain related transactions, is ultimately determined not to qualify as a reorganization, or the Merger is ultimately determined not to qualify as a reorganization, we and our stockholders that are subject to U.S. federal income tax could incur significant U.S. federal income tax liabilities.
The anticipated benefits of the Separation and Merger may not be achieved.
We may not be able to achieve the full strategic and financial benefits expected to result from the Separation and Merger, including the potential that the Separation and Merger will:
•allow each business to pursue its own operational and strategic priorities and more quickly respond to trends, developments and opportunities in its respective markets;
•create two separate and distinct management teams focused on each business’s unique strategic priorities, target markets and corporate development opportunities;
•give each business opportunity and flexibility by pursuing its own investment, capital allocation and growth strategies consistent with its long-term objectives;
•allow investors to separately value each business based on the unique merits, performance and future prospects of each business, providing investors with two distinct investment opportunities;
•enhance the ability of each business to attract and retain qualified management and to better align incentive-based compensation with the performance of each separate business; and
•give each of New OmniAb and Ligand its own equity currency for use in connection with acquisitions.
We may not achieve the anticipated benefits of the Separation and Merger for a variety of reasons. Further, such benefits, if ultimately achieved, may be delayed. In addition, the Separation and Merger could materially and adversely affect our business, financial condition and results of operations.
The Separation and Distribution may expose Ligand to potential liabilities arising out of state and federal fraudulent conveyance laws and legal dividend requirements.
The Separation and Distribution are subject to review under various state and federal fraudulent conveyance laws. Fraudulent conveyance laws generally provide that an entity engages in a constructive fraudulent conveyance when (i) the entity transfers assets and does not receive fair consideration or reasonably equivalent value in return; and (ii) the entity: (a) is insolvent at the time of the transfer or is rendered insolvent by the transfer; (b) has unreasonably small capital with which to carry on its business; or (c) intends to incur or believes it will incur debts beyond its ability to repay its debts as they mature. An unpaid creditor or an entity acting on behalf of a creditor (including without limitation a trustee or debtor-in-possession in a bankruptcy by New OmniAb or Ligand or any of their respective subsidiaries) may bring an action alleging that the Separation or Distribution or any of the related transactions constituted a constructive fraudulent conveyance. If a court accepts these allegations, it could impose a number of remedies, including without limitation, voiding New OmniAb’s claims against Ligand, requiring New OmniAb stockholders to return to Ligand some or all of the shares of New OmniAb common stock issued via the Distribution and Merger, or providing Ligand with a claim for money damages against New OmniAb in an amount equal to the difference between the consideration received by Ligand and OmniAb’s fair market value at the time of the Distribution.
The measure of insolvency for purposes of the fraudulent conveyance laws will vary depending on which jurisdiction’s law is applied. Generally, an entity would be considered insolvent if (i) the present fair saleable value of its assets is less than the amount of its liabilities (including contingent liabilities); (ii) the present fair saleable value of its assets is less than its probable liabilities on its debts as such debts become absolute and matured; (iii) it cannot pay its debts and other liabilities (including contingent liabilities and other commitments) as they mature; or (iv) it has unreasonably small capital for the business in which it is engaged. We cannot assure you what standard a court would apply to determine insolvency or that a court would determine that New OmniAb or Ligand or any of their subsidiaries were solvent at the time of or after giving effect to the Distribution.
The Distribution of OmniAb common stock is also subject to review under state corporate distribution statutes. Under the DGCL, a corporation may only pay dividends to its stockholders either (i) out of its surplus (net assets minus capital) or (ii) if there is no such surplus, out of its net profits for the fiscal year in which the dividend is declared or the preceding fiscal year. Although Ligand intended to make the Distribution of OmniAb common stock entirely from surplus, we cannot assure you that a court will not later determine that some or all of the Distribution to Ligand stockholders was unlawful.
The Separation and the retirement of our CEO resulted in substantial changes in our Board of Directors and management.
21
The Separation resulted in substantial changes in our Board of Directors and management. In particular, Matthew Foehr, our former President and Chief Operating Officer, and Charles Berkman, our former Senior Vice President, General Counsel and Secretary, resigned from their positions with us upon the completion of the Separation to join management positions with New OmniAb. In connection with the Separation and the departure of the foregoing officers, Ligand appointed new officers. Matthew Korenberg, our former Executive Vice President, Finance and Chief Financial Officer, was appointed our President and Chief Operating Officer. Octavio Espinoza, our former Senior Vice President, Finance, was appointed our Chief Financial Officer. Andrew Reardon, our former Vice President, Special Counsel, was appointed Chief Legal Officer and Secretary. Furthermore, Sarah Boyce, Jennifer Cochran and Sunil Patel resigned as members of our Board of Directors in connection with the Separation to join the board of directors of New OmniAb. In addition, on December 5, 2022, John Higgins retired as our Chief Executive Officer and Todd Davis was appointed to that position. Mr. Higgins also resigned as a member of our Board of Directors effective December 31, 2022. These senior officer and board level changes could be disruptive to our operations, present significant management challenges and could harm our business.
Risks Related to Intellectual Property:
Third party intellectual property may prevent us or our partners from developing our potential products; our and our partners’ intellectual property may not prevent competition; and any intellectual property issues may be expensive and time consuming to resolve.
The manufacture, use or sale of our potential products or our licensees' products or potential products may infringe the patent rights of others. If others obtain patents with conflicting claims, we may be required to obtain licenses to those patents or to develop or obtain alternative technology. We may not be able to obtain any such licenses on acceptable terms, or at all. Any failure to obtain such licenses could delay or prevent us from pursuing the development or commercialization of our potential products, platform and technology.
Generally, our success will depend on our ability and the ability of our partners to obtain and maintain patents and other intellectual property rights for our and their potential products and technologies. Our patent position is uncertain and involves complex legal and technical questions for which legal principles are unresolved. Even if we or our partners do obtain patents, such patents may not adequately protect the technology we own or have licensed.
We permit our partners to list our patents that cover their branded products in the Orange Book. If a third party submits a new drug application (NDA) or abbreviated new drug application (ANDA) for a generic drug product that relies in whole or in part on studies contained in our partner’s NDA for their branded product, the third party will have the option to certify to the FDA that, in the opinion of that third party, the patents listed in the Orange Book for our partner’s branded product are invalid, unenforceable, or will not be infringed by the manufacture, use or sale of the third party’s generic drug product. A third party certification that a new product will not infringe Orange Book-listed patents, or that such patents are invalid, is called a paragraph IV patent certification. If the third party submits a paragraph IV patent certification to the FDA, a notice of the paragraph IV patent certification must be sent to the NDA owner and the owner of the patents that are subject to the paragraph IV patent certification notice once the third-party’s NDA or ANDA is accepted for filing by the FDA. A lawsuit may then be initiated to defend the patents identified in the notice. The filing of a patent infringement lawsuit within 45 days of the receipt of notice of a paragraph IV patent certification automatically prevents the FDA from approving the generic NDA or ANDA until the earlier of the expiration of a 30-month period, the expiration of the patents, the entry of a settlement order stating that the patents are invalid or not infringed, a decision in the infringement case that is favorable to the NDA or ANDA applicant, or such shorter or longer period as the court may order. If a patent infringement lawsuit is not initiated within the required 45-day period, the third-party’s NDA or ANDA will not be subject to the 30-month stay.
Several third-parties have challenged, and additional third parties may challenge, the patents covering our partner’s branded products, including Kyprolis and Evomela, which could result in the invalidation or unenforceability of some or all of the relevant patent claims. We may from time to time become party to litigation or other proceedings as a result of Paragraph IV certifications. For example, as a result of the settlement of one such matter, Teva will be permitted to market a generic version of Evomela® in the United States on June 1, 2026 or earlier under certain circumstances. The terms of the settlement agreement are otherwise confidential. Also, as noted above, Amgen has settled patent litigation related to Kyprolis on confidential terms with several parties, but it has been publicly reported that the U.S. launch date for at least Breckenridge Pharmaceuticals’ applicable generic product will be “on a date that is held as confidential in 2027 or sooner, depending on certain occurrences.”
In addition, we cannot assure you that all of the potentially relevant prior art information that was or is deemed available to a person of skill in the relevant art prior to the priority date of the claimed invention-relating to our and our partners’ patents and patent applications has been found. If such prior art exists, it can invalidate a patent or prevent a patent from issuing from a pending patent application, and we or our partners may be subject to a third party pre-issuance submission of prior art to the USPTO. Even if our patent applications do successfully issue and even if such patents cover our or our partner’s products or
22
potential products, third parties may initiate litigation or opposition, interference, re-examination, post-grant review, inter partes review, nullification or derivation action in court or before patent offices, or similar proceedings challenging the validity, enforceability or scope of such patents, which may result in the patent claims being narrowed or invalidated, may allow third parties to commercialize our or our partners’ products and compete directly with us and our partners, without payment to us or our partners, or limit the duration of the patent protection of our and our partners’ technology and products.
In addition, similar to what other companies in our industry have experienced, we expect our competitors and others may have patents or may in the future obtain patents and claim that making, having made, using, selling, offering to sell or importing our technologies infringes these patents. Defense of infringement and other claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of management and employee resources from our business. Parties making claims against us may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Parties making claims against us may be able to obtain injunctive or other relief, which could block our ability to develop, commercialize and sell products or services and could result in the award of substantial damages against us, including treble damages, attorney’s fees, costs and expenses if we are found to have willfully infringed. In the event of a successful claim of infringement against us, we may be required to pay damages and ongoing royalties and obtain one or more licenses from third parties, or be prohibited from selling certain products or services. As discussed above, we may not be able to obtain these licenses on acceptable or commercially reasonable terms, if at all, or these licenses may be non-exclusive, which could result in our competitors gaining access to the same intellectual property. In addition, we could encounter delays in product or service introductions while we attempt to develop alternative products or services to avoid infringing third-party patents or proprietary rights. Defense of any lawsuit or failure to obtain any of these licenses could prevent us from commercializing products or services, and the prohibition of sale of any of our technologies could materially affect our business and our ability to gain market acceptance for our technology.
Litigation or other proceedings to enforce or defend intellectual property rights are often very complex in nature, may be very expensive and time-consuming, may divert our management’s attention from our core business, and may result in unfavorable results that could adversely impact our ability to prevent third parties from competing with our partner’s products or technologies. Any adverse outcome of such litigation or other proceedings could result in one or more or our patents being held invalid or unenforceable, which could adversely affect our ability to successfully execute our business strategy and negatively impact our financial condition and results of operations. However, given the unpredictability inherent in litigation, we cannot predict or guarantee the outcome of these matters or any other litigation. Regardless of how these matters are ultimately resolved, these matters may be costly, time-consuming and distracting to our management, which could have a material adverse effect on our business. It may be necessary for us to pursue litigation or adversarial proceedings before the patent office in order to enforce our patent and proprietary rights or to determine the scope, coverage and validity of the proprietary rights of others. The outcome of any such litigation might not be favorable to us, and even if we were to prevail, such litigation could result in substantial costs and diversion of resources and could have a material adverse effect on our business, operating results or financial condition.
In addition, periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and or applications will be due to the U.S. and various foreign patent offices at various points over the lifetime of our and our licensees’ patents and/or applications. We have systems in place to remind us to pay these fees, and we rely on our outside patent annuity service to pay these fees when due. Additionally, the U.S. and various foreign patent offices require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with rules applicable to the particular jurisdiction. However, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If such an event were to occur, it could have a material adverse effect on our business.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during the course of this kind of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
Any conflicts with the patent rights of others could significantly reduce the coverage of our patents or limit our ability to obtain meaningful patent protection. For example, our European patent related to Agglomerated forms of Captisol was limited during an opposition proceeding, and the rejection of our European patent application related to High Purity Captisol was upheld on appeal. In addition, any determination that our patent rights are invalid may result in early termination of our agreements with our license partners and could adversely affect our ability to enter into new license agreements. We also rely on unpatented trade secrets and know-how to protect and maintain our competitive position. We require our employees, consultants, licensees and others to sign confidentiality agreements when they begin their relationship with us. These
23
agreements may be breached, and we may not have adequate remedies for any breach. In addition, our competitors may independently discover our trade secrets.
We may also need to initiate litigation, which could be time-consuming and expensive, to enforce our proprietary rights or to determine the scope and validity of others' rights. If this occurs, a court may find our patents or those of our licensors invalid or may find that we have infringed on a competitor's rights. In addition, if any of our competitors have filed patent applications in the United States which claim technology we also have invented, the United States Patent and Trademark Office may require us to participate in expensive interference proceedings to determine who has the right to a patent for the technology.
In addition, our agreements with some of our partners, suppliers or other entities with whom we do business require us to defend or indemnify these parties to the extent they become involved in infringement claims, including the types of claims described above. We could also voluntarily agree to defend or indemnify third parties in instances where we are not obligated to do so if we determine it would be important to our business relationships. If we are required or agree to defend or indemnify third parties in connection with any infringement claims, we could incur significant costs and expenses that could adversely affect our business, financial condition, results of operations and prospects. The occurrence of any of the foregoing problems could be time-consuming and expensive and could adversely affect our financial position, liquidity and results of operations.
If we are unable to obtain and maintain sufficient intellectual property protection for our products, platform and technology, or if the scope of the intellectual property protection obtained is not sufficiently broad, our competitors could develop and commercialize technologies or a platform similar or identical to ours, and our ability to successfully sell our platform and services may be impaired.
Our success depends in part on our ability to obtain and maintain adequate protection of the intellectual property we may own solely and jointly with others or otherwise have rights to, particularly patents, in the United States and in other countries with respect to our platform, our software and our technologies, without infringing the intellectual property rights of others.
We strive to protect and enhance the proprietary technologies that we believe are important to our business, including seeking patents intended to cover our platform and related technologies and uses thereof, as we deem appropriate. However, obtaining and enforcing patents in our industry is costly, time-consuming and complex, and we may fail to apply for patents on important products and technologies in a timely fashion or at all, or we may fail to apply for patents in potentially relevant jurisdictions. There can be no assurance that the claims of our patents (or any patent application that issues as a patent), will exclude others from making, using, importing, offering for sale, or selling products or services that are substantially similar to ours. We also rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. In countries where we have not sought and do not seek patent protection, third parties may be able to manufacture and sell our technology without our permission, and we may not be able to stop them from doing so. We may not be able to file and prosecute all necessary or desirable patent applications, or maintain, enforce and license any patents that may issue from such patent applications, at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. We may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the rights to patents licensed to third parties. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business.
It is possible that none of our pending patent applications will result in issued patents in a timely fashion or at all, and even if patents are granted, they may not provide a basis for intellectual property protection of commercially viable products or services, may not provide us with any competitive advantages, or may be challenged and invalidated by third parties or deemed unenforceable by a court. It is possible that others will design around our current or future patented technologies. As a result, our owned and licensed patents and patent applications comprising our patent portfolio may not provide us with sufficient rights to exclude others from commercializing technology and products similar to any of our products, platform and technology.
In addition, we may identify third party intellectual property and technology we may need to acquire or license in order to engage in our business, including to develop or commercialize new technologies. However, such licenses may not be available to us on acceptable terms or at all.
Issued patents directed to our platform and technology could be found invalid or unenforceable if challenged in court or before administrative bodies in the United States or abroad.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability. Some of our patents or patent applications (including licensed patents) may be challenged at a future point in time in opposition, derivation, reexamination, inter partes review, post-grant review or interference. Any successful third party challenge to our patents in this or any other proceeding could result in the unenforceability or invalidity of such patents or amendment to our patents in such a way that any resulting protection may lead to increased competition to our business, which could harm our business. In addition, in patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are
24
commonplace. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on certain aspects of our platform technologies. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us to license, develop or commercialize current or future products, platform and technology.
We may not be aware of all third party intellectual property rights potentially relating to our products, platform and technology. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until approximately 18 months after filing or, in some cases, not until such patent applications issue as patents. We or our licensors might not have been the first to make the inventions included in each of our pending patent applications and we or our licensors might not have been the first to file patent applications for these inventions. There is also no assurance that all of the potentially relevant prior art relating to our patents and patent applications or licensed patents and patent applications has been found, which could be used by a third party to challenge their validity, or prevent a patent from issuing from a pending patent application.
To determine the priority of these inventions, we may have to participate in interference proceedings, derivation proceedings or other post-grant proceedings declared by the USPTO that could result in substantial cost to us. The outcome of such proceedings is uncertain. No assurance can be given that other patent applications will not have priority over our patent applications. In addition, changes to the patent laws of the United States allow for various post-grant opposition proceedings that have not been extensively tested, and their outcome is therefore uncertain. Furthermore, if third parties bring these proceedings against our patents, we could experience significant costs and management distraction.
The validity, scope and enforceability of any patents that cover our partners’ biologic product candidate can be challenged by third parties.
For biologics, the Biologics Price Competition and Innovation Act of 2009, BPCIA, provides a mechanism for one or more third parties to seek FDA approval to manufacture or sell biosimilar or interchangeable versions of brand name biological products. Due to the large size and complexity of biological products, as compared to small molecules, a biosimilar must be “highly similar” to the reference product with “no clinically meaningful differences between the two.” The BPCIA does not require reference product sponsors to list patents in an Orange Book and does not include an automatic 30-month stay of FDA approval upon the timely filing of a lawsuit. The BPCIA, however, does require a formal pre-litigation process which includes the exchange of information between a biosimilar applicant and a reference biologic sponsor that includes the identification of relevant patents and each parties’ basis for infringement and invalidity. After the exchange of this information, sponsors may then initiate a lawsuit within 30 days to defend the patents identified in the exchange. If the biosimilar applicant successfully challenges the asserted patent claims it could result in the invalidation of, or render unenforceable, some or all of the relevant patent claims or result in a finding of non-infringement. Such litigation or other proceedings to enforce or defend intellectual property rights are often very complex in nature, may be very expensive and time-consuming, may divert our management’s attention from our core business, and may result in unfavorable results that could limit our partners’ ability to prevent third parties from competing with their products or product candidates.
We rely on in-licenses from third parties. If we lose these rights, our business may be materially and adversely affected, our ability to develop improvements to our technology platform and antibody discovery platform may be negatively and substantially impacted, and if disputes arise, we may be subjected to future litigation, as well as the potential loss of or limitations on our ability to incorporate the technology covered by these license agreements.
We are party to royalty-bearing license agreements that grant us rights to practice certain patent rights that are related to our products, platform and technology. In spite of our efforts to comply with our obligations under our in-license agreements, our licensors might conclude that we have materially breached our obligations under our license agreements and might therefore, including in connection with any aforementioned disputes, terminate the relevant license agreement, thereby removing or limiting our ability to develop and commercialize technology covered by these license agreements. If any such in-license is terminated, or if the licensed patents fail to provide the intended exclusivity, competitors or other third parties might have the freedom to market or develop technologies similar to ours. In addition, absent the rights granted to us under our license agreements, we may infringe the intellectual property rights that are the subject of those agreements, we may be subject to litigation by the licensor, and if such litigation by the licensor is successful we may be required to pay damages to our licensor, or we may be required to cease our development and commercialization activities that are deemed infringing, and in such event we may ultimately need to modify our activities or technologies to design around such infringement, which may be time- and resource-consuming, and which ultimately may not be successful. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
In addition, our rights to certain components of our technology platform, may be licensed to us on a non-exclusive basis. The owners of these non-exclusively licensed technologies are therefore free to license them to third parties, including our competitors, on terms that may be superior to those offered to us, which could place us at a competitive disadvantage.
25
Moreover, our licensors may own or control intellectual property that has not been licensed to us and, as a result, we may be subject to claims, regardless of their merit, that we are infringing or otherwise violating the licensor’s rights. In addition, certain of our agreements with third parties may provide that intellectual property arising under these agreements, such as data that could be valuable to our business, will be owned by the third party, in which case, we may not have adequate rights to use such data or have exclusivity with respect to the use of such data, which could result in third parties, including our competitors, being able to use such data to compete with us.
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
We or our licensors may be subject to claims that former employees, partners or other third parties have an interest in our or our in-licensed patents, trade secrets or other intellectual property as an inventor or co-inventor. Litigation may be necessary to defend against these and other claims challenging inventorship of our or our licensors’ ownership of our owned or in-licensed patents, trade secrets or other intellectual property. If we or our licensors fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property that is important to our systems, including our software, workflows, consumables, reagents, and transgenic animals. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees, and certain partners or partners may defer engaging with us until the particular dispute is resolved. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
If we are unable to protect the confidentiality of our information and our trade secrets, the value of our technology could be materially and adversely affected and our business could be harmed.
We rely on trade secrets and confidentiality agreements to protect our unpatented know-how, technology and other proprietary information, including parts of our technology platform, and to maintain our competitive position. However, trade secrets and know-how can be difficult to protect. In addition to pursuing patents on our technology, we take steps to protect our intellectual property and proprietary technology by entering into agreements, including confidentiality agreements, non-disclosure agreements and intellectual property assignment agreements, with our employees, consultants, academic institutions, corporate partners and, when needed, our advisers. However, we cannot be certain that such agreements have been entered into with all relevant parties, and we cannot be certain that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. For example, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements, and we may not be able to prevent such unauthorized disclosure, which could adversely impact our ability to establish or maintain a competitive advantage in the market. If we are required to assert our rights against such party, it could result in significant cost and distraction.
Monitoring unauthorized disclosure and detection of unauthorized disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, it would be expensive and time-consuming, and the outcome would be unpredictable. In addition, some courts both within and outside the United States may be less willing, or unwilling, to protect trade secrets. Further, we may need to share our trade secrets and confidential know-how with current or future partners, collaborators, contractors and others located in countries at heightened risk of theft of trade secrets, including through direct intrusion by private parties or foreign actors, and those affiliated with or controlled by state actors.
We also seek to preserve the integrity and confidentiality of our confidential proprietary information by maintaining physical security of our premises and physical and electronic security of our information technology systems, but it is possible that these security measures could be breached. If any of our confidential proprietary information were to be lawfully obtained or independently developed by a competitor or other third party, absent patent protection, we would have no right to prevent such competitor from using that technology or information to compete with us, which could harm our competitive position. If any of our trade secrets were to be disclosed to or independently discovered by a competitor or other third party, it could harm our business, financial condition, results of operations and prospects.
Risks Related to Government Regulation and Legal Proceedings:
Market acceptance and sales of any approved product will depend significantly on the availability and adequacy of coverage and reimbursement from third-party payors and may be affected by existing and future healthcare reform measures.
Sales of the products we license to our collaboration partners and the royalties we receive will depend in large part on the extent to which coverage and reimbursement is available from government and health administration authorities, private health
26
maintenance organizations and health insurers, and other healthcare payors. Significant uncertainty exists as to the reimbursement status of healthcare products. Healthcare payors, including Medicare, are challenging the prices charged for medical products and services. Government and other healthcare payors are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for medical products. Even if a product is approved by the FDA, insurance coverage may not be available, and reimbursement levels may be inadequate, to cover the costs associated with the research, development, marketing and sale of the product. If government and other healthcare payors do not provide adequate coverage and reimbursement levels for any product, market acceptance and any sales could be reduced.
From time to time, legislation is implemented to reign in rising healthcare expenditures. By way of example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the ACA, was enacted, which included a number of provisions affecting the pharmaceutical industry, including, among other things, annual, non-deductible fees on any entity that manufactures or imports some types of branded prescription drugs and increases in Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program. Since its enactment, there have been judicial, executive and Congressional challenges to certain aspects of the ACA. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA brought by several states without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden had issued an executive order to initiate a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA.
Other legislative changes have been proposed and adopted since the ACA was enacted, including aggregate reductions of Medicare payments to providers, which was temporarily suspended from March 1, 2020 through March 31, 2022, and reduced payments to several types of Medicare providers. Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. Most recently, in August 2022, the Inflation Reduction Act of 2022 (IRA), was signed into law. Among other things, the IRA requires manufacturers of certain drugs to engage in price negotiations with Medicare (beginning in 2026), with prices that can be negotiated subject to a cap; imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation (first due in 2023); and replaces the Part D coverage gap discount program with a new discounting program (beginning in 2025). The IRA permits the Secretary of the Department of Health and Human Services (HHS) to implement many of these provisions through guidance, as opposed to regulation, for the initial years. For that and other reasons, it is currently unclear how the IRA will be effectuated, and the impact of the IRA on our business and the pharmaceutical industry cannot yet be fully determined. Individual states in the United States have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. We cannot predict whether other legislative changes will be adopted, if any, or how such changes would affect our operations or financial condition.
If we or our commercialization partners market products in a manner that violates healthcare laws, we may be subject to civil or criminal penalties.
We and our collaboration partners are subject to federal and state healthcare laws, including fraud and abuse, anti-kickback, false claims, physician payment transparency and civil monetary penalties. These laws may impact, among other things, financial arrangements with physicians, sales, marketing and education programs and the manner in which any of those activities are implemented. If our operations or those of our collaboration partners are found to be in violation of any of those laws or any other applicable governmental regulations, we or our collaboration partners may be subject to penalties, including civil and criminal penalties, damages, fines, imprisonment, exclusion from government healthcare programs or the curtailment or restructuring of operations, any of which could adversely affect our ability to operate our business and our financial condition.
Changes in and actual or perceived failures to comply with applicable data privacy, security and protection laws, regulations, standards and contractual obligations may adversely affect our business, operations and financial performance.
We and our partners may be subject to federal, state, and foreign laws and regulations that govern data privacy and security. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing focus on privacy and data protection issues, which may affect our business and may increase our compliance costs and exposure to liability. In the United States, numerous federal and state laws and regulations govern the collection, use, disclosure, and protection of personal information, including state data breach notification laws, federal and state health information privacy laws, and federal and state consumer protection laws. Each of these laws is subject to varying
27
interpretations by courts and government agencies, creating complex compliance issues. If we fail to comply with applicable laws and regulations we could be subject to penalties or sanctions, including criminal penalties if we knowingly obtain or disclose individually identifiable health information from a covered entity in a manner that is not authorized or permitted by the Health Insurance Portability and Accountability Act, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, and regulations implemented thereunder (collectively, HIPAA) or applicable state laws.
Certain states have also adopted comparable privacy and security laws and regulations, which govern the privacy, processing and protection of health-related and other personal information. Such laws and regulations will be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us and our future customers and strategic partners. For example, the California Consumer Privacy Act of 2018 (CCPA) went into effect on January 1, 2020. The CCPA creates individual privacy rights for California consumers and increases the privacy and security obligations of entities handling certain personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. Further, the California Privacy Rights Act (CPRA) passed in California, and it significantly amends the CCPA. It will impose additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data. It will also create a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. The majority of the provisions went into effect on January 1, 2023, and additional compliance investment and potential business process changes may be required. Similar laws have passed in Virginia, Colorado, Connecticut and Utah, and have been proposed in other states and at the federal level, reflecting a trend toward more stringent privacy legislation in the United States. The enactment of such laws could have potentially conflicting requirements that would make compliance challenging. In the event that we are subject to or affected by HIPAA, the CCPA, the CPRA or other domestic privacy and data protection laws, any liability from failure to comply with the requirements of these laws could adversely affect our financial condition.
We are also or may become subject to rapidly evolving data protection laws, rules and regulations in foreign jurisdictions. For example, the European Union General Data Protection Regulation (GDPR) governs certain collection and other processing activities involving personal data about individuals in the European Economic Area (EEA). Among other requirements, the GDPR regulates transfers of personal data subject to the GDPR to third countries that have not been found to provide adequate protection to such personal data, including the United States; in July 2020, the Court of Justice of the European Union (CJEU) limited how organizations could lawfully transfer personal data from the European Union/EEA to the United States by invalidating the Privacy Shield for purposes of international transfers and imposing further restrictions on the use of standard contractual clauses (SCCs). In March 2022, the United States and European Union announced a new regulatory regime intended to replace the invalidated regulations; however, this new EU-US Data Privacy Framework has not been implemented beyond an executive order signed by President Biden on October 7, 2022 on Enhancing Safeguards for United States Signals Intelligence Activities. European court and regulatory decisions subsequent to the CJEU decision of July 2020 have taken a restrictive approach to international data transfers. As supervisory authorities issue further guidance on personal data export mechanisms, including circumstances where the SCCs cannot be used, and/or start taking enforcement action, we could suffer additional costs, complaints and/or regulatory investigations or fines, and/or if we are otherwise unable to transfer personal data between and among countries and regions in which we operate, it could affect the manner in which we provide our services, the geographical location or segregation of our relevant systems and operations, and could adversely affect our financial results.
Since the beginning of 2021, after the end of the transition period following the United Kingdom’s departure from the European Union, we are also subject to the United Kingdom data protection regime, which imposes separate but similar obligations to those under the GDPR and comparable penalties, including fines of up to £17.5 million or 4% of a noncompliant company’s global annual revenue for the preceding financial year, whichever is greater. As we continue to expand into other foreign countries and jurisdictions, we may be subject to additional laws and regulations that may affect how we conduct business.
Compliance with applicable data privacy and security laws, rules and regulations could require us to take on more onerous obligations in our contracts, require us to engage in costly compliance exercises, restrict our ability to collect, use and disclose data, or in some cases, impact our or our partners’ ability to operate in certain jurisdictions. Each of these constantly evolving laws can be subject to varying interpretations. If we fail to comply with any such laws, rules or regulations, we may face government investigations and/or enforcement actions, fines, civil or criminal penalties, private litigation or adverse publicity that could adversely affect our business, financial condition and results of operations.
28
Changes in funding for the FDA and other government agencies could hinder their ability to hire and retain key leadership and other personnel, or otherwise prevent new products and services from being developed or commercialized in a timely manner, which could negatively impact our business or the business of our partners.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the FDA have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs and biologics to be reviewed and/or approved by necessary government agencies, which would adversely affect our business or the business of our partners. For example, over the last several years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical FDA employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. If the timing of FDA’s review and approval of new products is delayed, the timing of our or our partners’ development process may be delayed which would result in delayed milestone revenues and materially harm our operations of business.
Separately, in response to the COVID-19 pandemic, the FDA postponed most inspections of domestic and foreign manufacturing facilities at various points. Even though the FDA has since resumed standard inspection operations of domestic facilities where feasible, the FDA has continued to monitor and implement changes to its inspectional activities to ensure the safety of its employees and those of the firms it regulates as it adapts to the evolving COVID-19 pandemic, and any resurgence of the virus or emergence of new variants may lead to further inspectional delays. Regulatory authorities outside the United States may adopt similar restrictions or other policy measures in response to the COVID-19 pandemic. If a prolonged government shutdown occurs, or if global health concerns continue to hinder or prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA or other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
If plaintiffs bring product liability lawsuits against us or our partners, we or our partners may incur substantial liabilities and may be required to limit commercialization of our approved products and product candidates.
As is common in our industry, our partners and we face an inherent risk of product liability as a result of the clinical testing of our product candidates in clinical trials and face an even greater risk for commercialized products. Although we are not currently a party to product liability litigation, if we are sued, we may be held liable if any product or product candidate we develop causes injury or is found otherwise unsuitable during product testing, manufacturing, marketing or sale. Regardless of merit or eventual outcome, liability claims may result in decreased demand for any product candidates, partnered products or products that we may develop, injury to our reputation, discontinuation of clinical trials, costs to defend litigation, substantial monetary awards to clinical trial participants or patients, loss of revenue and product recall or withdrawal from the market and the inability to commercialize any products that we develop. We have product liability insurance that covers our clinical trials up to a $10.0 million annual limit. Our insurance coverage may not be sufficient to cover all of our product liability related expenses or losses and may not cover us for any expenses or losses we may suffer. If we are sued for any injury caused by our product candidates, partnered products or any future products, our liability could exceed our total assets.
We face risks related to handling of hazardous materials and other regulations governing environmental safety.
Our operations are subject to complex and stringent environmental, health, safety and other governmental laws and regulations that both public officials and private individuals may seek to enforce. Our activities that are subject to these regulations include, among other things, our use of hazardous materials and the generation, transportation and storage of waste. Although we have secured clearance from the EPA historically, and currently are operating in material compliance with applicable EPA rules and regulations, our business could be adversely affected if we discover that we or an acquired business is not in material compliance with these rules and regulations. In the future, we may pursue the use of other surfactant substances that will require clearance from the EPA, and we may fail to obtain such clearance. Existing laws and regulations may also be revised or reinterpreted, or new laws and regulations may become applicable to us, whether retroactively or prospectively, that may have a negative effect on our business and results of operations. It is also impossible to eliminate completely the risk of accidental environmental contamination or injury to individuals. In such an event, we could be liable for any damages that result, which could adversely affect our business.
29
Risk Related to Our Strategic Transactions:
Any difficulties from strategic acquisitions could adversely affect our stock price, operating results and results of operations.
We may acquire companies, businesses and products that complement or augment our existing business. We may not be able to integrate any acquired business successfully or operate any acquired business profitably. Integrating any newly acquired business could be expensive and time-consuming. Integration efforts often take a significant amount of time, place a significant strain on managerial, operational and financial resources and could prove to be more difficult or expensive than we predict. The diversion of our management's attention and any delay or difficulties encountered in connection with any future acquisitions we may consummate could result in the disruption of our ongoing business or inconsistencies in standards and controls that could negatively affect our ability to maintain third-party relationships. Moreover, we may need to raise additional funds through public or private debt or equity financing, or issue additional shares, to acquire any businesses or products, which may result in dilution for stockholders or the incurrence of indebtedness.
As part of our efforts to acquire companies, business or product candidates or to enter into other significant transactions, we conduct business, legal and financial due diligence with the goal of identifying and evaluating material risks involved in the transaction. Despite our efforts, we ultimately may be unsuccessful in ascertaining or evaluating all such risks and, as a result, might not realize the intended advantages of the transaction. If we fail to realize the expected benefits from acquisitions we may consummate in the future or have consummated in the past, whether as a result of unidentified risks, integration difficulties, regulatory setbacks, litigation with current or former employees and other events, our business, results of operations and financial condition could be adversely affected. If we acquire product candidates, we will also need to make certain assumptions about, among other things, development costs, the likelihood of receiving regulatory approval and the market for such product candidates. Our assumptions may prove to be incorrect, which could cause us to fail to realize the anticipated benefits of these transactions.
In addition, we will likely experience significant charges to earnings in connection with our efforts, if any, to consummate acquisitions. For transactions that are ultimately not consummated, these charges may include fees and expenses for investment bankers, attorneys, accountants and other advisors in connection with our efforts. Even if our efforts are successful, we may incur, as part of a transaction, substantial charges for closure costs associated with elimination of duplicate operations and facilities and acquired in-process research and development charges. In either case, the incurrence of these charges could adversely affect our results of operations for particular quarterly or annual periods.
Other Risks:
Our business is subject to risks arising from epidemic diseases, such as the COVID-19 pandemic, which has impacted and could continue to impact our business.
The COVID-19 pandemic continues to impact worldwide public health and economic activity. A pandemic, including COVID-19 or other public health epidemic, poses the risk that we or our employees, contractors, including our CROs, suppliers, and other partners may be prevented from conducting business activities for an indefinite period of time, including due to spread of the disease within these groups or due to shutdowns that may be requested or mandated by governmental authorities. Although we have lifted most of the restrictions we previously imposed on in-person access to our facilities and currently do not believe the COVID-19 pandemic is having a material impact on our business, we cannot guarantee that the COVID-19 pandemic, including the emergence of variants, or a similar event, will not impact our operations in the future.
Several of our partners reported that their operations were impacted, including delays in research and development programs and deprioritizing clinical trials in favor of treating patients who have contracted the virus or to prevent the spread of the virus. This may lead to clinical trial protocol deviations or to discontinuation of treatment for patients who are currently enrolled in the clinical trials being conducted by us or our partners. In addition, certain of our partners reported negative impacts on product sales which will impact our royalty revenues. Although we believe that we and our partners have adjusted our business practices to the impacts of the COVID-19 pandemic, we may experience disruptions that could severely impact our business, drug manufacturing and supply chain, nonclinical activities and clinical trials and our partners’ business may be impacted in similar ways, including due to delays or difficulties in enrolling patients in clinical trials, diversion of healthcare resources away from the conduct of clinical trials, interruption of, or delays in receiving, supplies of Captisol or other product or product candidates from contract manufacturing organizations due to staffing shortages, production slowdowns or stoppages and disruptions in delivery systems, which may result in cancellations of Captisol orders or refunds if we fail to deliver Captisol timely, interruption or delays to discovery and development pipelines and difficulties launching or commercializing products, including due to reduced access to doctors as a result of social distancing protocols.
Further, the spread of COVID-19 has had and may continue to severely impact the trading price of shares of our common stock and could further severely impact our ability to raise additional capital on a timely basis or at all. The extent to which the
30
COVID-19 pandemic, or any other outbreak of an epidemic disease, impacts our results will depend on future developments that are highly uncertain and cannot be predicted, including new information that may emerge concerning the severity of the virus and the actions to contain its impact. Further, to the extent the COVID-19 pandemic or any other outbreak of an epidemic disease adversely affects our business and financial results, it may also have the effect of heightening many of the other risks described in this section.
Our operating results may fluctuate significantly, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations or any guidance we may provide.
Our quarterly and annual operating results may fluctuate significantly, which makes it difficult for us to predict our future operating results. These fluctuations may occur due to a variety of factors, many of which are outside of our control, including, but not limited to:
•the royalties from the sales of Kyprolis, Evomela and other products sold by our partners;
•the success of our collaboration partners’ preclinical and clinical programs;
•the timing of Captisol purchases for use in clinical trials and commercial products;
•the timing and cost of, and level of investment in, research, development, regulatory approval and commercialization activities relating to our internal development programs, which may change from time to time;
•expenditures that we may incur to acquire or develop additional product candidates and platform technologies; and
•future accounting pronouncements or changes in our accounting policies.
The cumulative effects of these factors could result in large fluctuations and unpredictability in our quarterly and annual operating results and revenues. This variability and unpredictability could result in our failing to meet the expectations of industry or financial analysts or investors for any period. If our revenue or operating results fall below the expectations of analysts or investors or below any forecasts we may provide to the market, or if the forecasts we provide to the market are below the expectations of analysts or investors, the price of our common stock could decline substantially. Such a stock price decline could occur even when we have met any previously publicly stated revenue or earnings guidance we may provide.
Changes or modifications in financial accounting standards, including those related to revenue recognition, may harm our results of operations.
From time to time, the FASB either alone or jointly with other organizations, promulgates new accounting principles that could have an adverse impact on our results of operations. For example, in May 2014, FASB issued an accounting standard for revenue recognition-Accounting Standards Codification Topic 606, Revenue from Contracts with Customers, or ASC 606-that supersedes most current revenue recognition guidance. The guidance requires a company to recognize revenue upon transfer of goods or services to a customer at an amount that reflects the expected consideration to be received in exchange for those goods or services. The guidance became effective in fiscal 2018.
Under ASC 606, Ligand estimates and books royalties in the same quarter that our partners report the sale of the underlying product. We rely on our partners’ earning releases and other information from our partners to determine the sales of our partners’ products and to estimate the related royalty revenues. If our partners report incorrect sales, or if our partners delay reporting of their earnings release, our royalty estimates may need to be revised and/or our financial reporting may be delayed.
Our ability to use our net operating loss carryforwards and certain other tax attributes to offset future taxable income may be subject to certain limitations.
As of December 31, 2022, we had U.S. federal and state net operating loss carryforwards (NOLs) of approximately $81.1 million and $168.3 million, respectively. Our federal NOLs expire through 2037 and our state NOLs begin to expire in 2029, if not utilized. Under the Tax Act, any federal NOLs arising in taxable years ending after December 31, 2017 will carry forward indefinitely. As of December 31, 2022, we had federal and California research and development tax credit carryforwards of approximately $8.5 million and $29.0 million, respectively. The federal research and development tax credit carryforwards expire in various years through 2040, if not utilized. The California research and development credit will carry forward indefinitely. Under Sections 382 and 383 of Internal Revenue Code of 1986, as amended (Code) if a corporation undergoes an “ownership change,” the corporation’s ability to use its pre-change NOLs and other pre-change tax attributes, such as research tax credits, to offset its future post-change income and taxes may be limited. In general, an “ownership change” occurs if there is a cumulative change in our ownership by “5% shareholders” that exceeds 50 percentage points over a rolling three-year period. Similar rules may apply under state tax laws. We believe we have experienced certain ownership changes in the past and have reduced our deferred tax assets related to NOLs and research and development tax credit carryforwards accordingly. In the event that it is determined that we have in the past experienced additional ownership changes, or if we experience one or more ownership changes as a result future transactions in our stock, then we may be further limited in our ability to use our NOLs and other tax assets to reduce taxes owed on the net taxable income that we earn in the event that we attain profitability. Furthermore, under the Tax Act, although the treatment of tax losses generated in tax years beginning before December 31,
31
2017 has generally not changed, tax losses generated in tax years beginning after December 31, 2017 may only offset 80% of our taxable income. This change may require us to pay federal income taxes in future years despite having potentially generated a loss for federal income tax purposes in prior years. Any such limitations on the ability to use our NOLs and other tax assets could adversely impact our business, financial condition and operating results.
The occurrence of a catastrophic disaster could disrupt our business, damage our facilities beyond insurance limits, increase our costs and expenses, or we could lose key data which could cause us to curtail or cease operations.
We are vulnerable to damage, business disruptions and/or loss of vital data from natural or man-made disasters, such as earthquakes, tornadoes, severe weather conditions, power loss, fire, floods and similar events, as well as from accidental loss or destruction. If any disaster were to occur, our ability to operate our business could be seriously impaired. We have property, liability, and business interruption insurance which may not be adequate to cover our losses resulting from disasters or other similar significant business interruptions, and we do not plan to purchase additional insurance to cover such losses due to the cost of obtaining such coverage. Any significant losses that are not recoverable under our insurance policies could seriously impair our business, financial condition and prospects. Our ability to obtain Captisol supply from our third-party manufactures could be disrupted if the operations of these manufacturers were affected by a natural or man-made disaster or other business interruption. In addition, we rely on our partners to generate most of our revenues through royalties, Captisol sales and development activities and any disruptions to their business as a result of such disasters could negatively impact our revenues.
We rely on information technology system and any failure, inadequacy, interruption or security lapse of our information technology systems, including any cyber security incidents, could harm our ability to operate our business effectively.
Our business is increasingly dependent on critical, complex and interdependent information technology systems, including internet-based systems, to support business processes as well as internal and external communications. We operate some of these systems and networks, but we also rely on third-party providers for various products and services across our operations. Despite the implementation of security measures, our information technology systems and those of our partners and third party service providers are vulnerable to attack, damage, and interruption from cyber-attacks, computer viruses and malware (e.g. ransomware), security breaches, unauthorized access, natural disasters, terrorism, war, telecommunication and electrical failures, hacking, phishing attacks and other social engineering schemes, employee theft or misuse, human error, fraud, denial or degradation of service attacks, sophisticated nation-state and nation-state-supported actors or unauthorized access or use by persons inside our organization, or persons with access to systems inside our organization.
Attacks upon information technology systems are increasing in their frequency, levels of persistence, sophistication and intensity, and are being conducted by sophisticated and organized groups and individuals with a wide range of motives and expertise. Furthermore, because the technologies used to obtain unauthorized access to, or to sabotage or disrupt, systems change frequently and often are not recognized until launched against a target, we may be unable to anticipate these techniques or implement adequate preventative measures. We may also experience security breaches that may remain undetected for an extended period. Even if identified, we may be unable to adequately investigate or remediate incidents or breaches due to attackers increasingly using tools and techniques that are designed to circumvent controls, to avoid detection, and to remove or obfuscate forensic evidence. As a result of the COVID-19 pandemic, or any future epidemic diseases, we may also face increased cybersecurity risks due to our reliance on internet technology and the number of our and our service providers’ employees who are (and may continue to be) working remotely, which may create additional opportunities for cybercriminals to exploit vulnerabilities. The White House, SEC and other regulators have also increased their focus on companies’ cybersecurity vulnerabilities and risks.
We and certain of our service providers are from time to time, subject to cyberattacks and security incidents. While we do not believe that we have experienced any significant system failures, accidents or security breaches, if such an event were to occur and cause interruptions in our or our critical third parties’ operations, it could lead to the loss of trade secrets or other intellectual property, as well as the public exposure of personal information of our employees and others, and could result in a material disruption of our clinical and commercialization activities and business operations, in addition to possibly requiring substantial expenditures to remedy. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and our business, reputation, and financial condition could be harmed. Any losses, costs or liabilities may not be covered by, or may exceed the coverage limits of, any or all applicable insurance policies.
Conversion of our outstanding convertible notes may result in losses, result in the dilution of existing stockholders, create downward pressure on the price of our common stock, and restrict our ability to take advantage of future opportunities.
In May 2018, we issued $750.0 million principal amount of the 2023 Notes. The sale of the 2023 Notes may affect our earnings per share figures, as accounting procedures require that we include in our calculation of earnings per share the number of shares of our common stock into which the 2023 Notes are convertible. The convertible notes may be converted into cash and shares of our common stock, if any (subject to our right or obligation to pay cash in lieu of all or a portion of such shares).
32
If shares of our common stock are issued to the holders of the convertible notes upon conversion, there will be dilution to our shareholders equity and the market price of our shares may decrease due to the additional selling pressure in the market. Any downward pressure on the price of our common stock caused by the sale or potential sale of shares issuable upon conversion of the convertible notes could also encourage short sales by third parties, creating additional selling pressure on our stock. Upon the occurrence of certain circumstances, holders of the convertible notes may require us to purchase all or a portion of their notes for cash, which may require the use of a substantial amount of cash. If such cash is not available, we may be required to sell other assets or enter into alternate financing arrangements at terms that may or may not be desirable. The existence of the convertible notes and the obligations that we incurred by issuing them may restrict our ability to take advantage of certain future opportunities, such as engaging in future debt or equity financing activities.
As of December 31, 2022, we had $76.9 million aggregate principal amount of 2023 Notes. The notes are convertible into cash, and if applicable, shares of our common stock under certain circumstances, including trading price conditions related to our common stock. Upon conversion, we are required to record a gain or loss for the difference between the fair value of the notes to be extinguished and their corresponding net carrying value. The fair value of the notes to be extinguished depends on our current incremental borrowing rate. If our incremental borrowing rate at the time of conversion is lower than the implied interest rate of the notes, we will record a loss in our consolidated statement of income during the period in which the notes are converted.
Impairment charges pertaining to goodwill, identifiable intangible assets or other long-lived assets from our mergers and acquisitions could have an adverse impact on our results of operations and the market value of our common stock.
The total purchase price pertaining to our acquisitions in recent years have been allocated to net tangible assets, identifiable intangible assets, in-process research and development and goodwill. To the extent the value of goodwill or identifiable intangible assets or other long-lived assets become impaired, we will be required to incur material charges relating to the impairment. Any impairment charges could have a material adverse impact on our results of operations and the market value of our common stock.
Our investments are subject to market and credit risks that could diminish their value and these risks could be greater during periods of extreme volatility or disruption in the financial and credit markets, which could adversely impact our business, financial condition, results of operations, liquidity and cash flows.
Our investments are subject to risks of credit defaults and changes in market values. Periods of macroeconomic weakness or recession, heightened volatility or disruption in the financial and credit markets could increase these risks, potentially resulting in other than temporary impairment of assets in our investment portfolio. Any event reducing the estimated fair value of these securities, other than on a temporary basis, could have a material and adverse effect on our business, results of operations, financial condition, liquidity and cash flows. If our investment manager, fails to react appropriately to difficult market, economic and geopolitical conditions, our investment portfolio could incur material losses.
We have a risk management framework in place to identify, assess and prioritize risks, including the market and credit risks to which our investments are subject. As part of that framework, we test our investment portfolio based on various market scenarios. Under certain stressed market scenarios, unrealized losses on our investment portfolio could lead to material reductions in its carrying value.
A decline in fair value below the amortized cost of a security requires management to assess whether an impairment has occurred. The decision on whether to record an impairment is determined in part by our assessment of the financial condition and prospects of a particular issuer, projections of future cash flows and recoverability of the particular security as well as management’s assertion of whether it is more likely than not that we will sell the particular security before recovery.
Our charter documents and concentration of ownership may hinder or prevent change of control transactions.
Provisions contained in our certificate of incorporation and bylaws may discourage transactions involving an actual or potential change in our ownership. In addition, our Board of Directors may issue shares of common or preferred stock without any further action by the stockholders. Our directors, officers and certain of our institutional investors collectively beneficially own a significant portion of our outstanding common stock. Such provisions and issuances may have the effect of delaying or preventing a change in our ownership. If changes in our ownership are discouraged, delayed or prevented, it would be more difficult for our current Board of Directors to be removed and replaced, even if you or our other stockholders believe that such actions are in the best interests of us and our stockholders.
Our amended and restated bylaws provide that the Court of Chancery of the State of Delaware will be the exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our amended and restated bylaws provide that the Court of Chancery of the State of Delaware is the exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty
33
owed by our directors, officers or other employees to us or our stockholders, (iii) any action asserting a claim arising pursuant to any provision of the General Corporation Law of Delaware or our amended and restated certificate of incorporation or amended and restated bylaws, or (iv) any action asserting a claim governed by the internal affairs doctrine. To the extent that any such claims may be based upon federal law claims, Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. Furthermore, Section 22 of the Securities Act provides for concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder, and as such, the exclusive jurisdiction clauses set forth above would not apply to such suits. The choice of forum provisions in our amended and restated bylaws may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees. By agreeing to these provisions, however, stockholders will not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder. Furthermore, the enforceability of similar choice of forum provisions in other companies’ certificates of incorporation and bylaws has been challenged in legal proceedings, and it is possible that a court could find these types of provisions to be inapplicable or unenforceable. If a court were to find the choice of forum provisions in our amended and restated bylaws to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could adversely affect our business and financial condition.
Our stock price has been volatile and could experience a sudden decline in value.
The market prices for securities of biotechnology and pharmaceutical companies have historically been highly volatile, and the market has experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. Continued volatility in the overall capital markets could reduce the market price of our common stock in spite of our operating performance. Further, high stock price volatility could result in higher share-based compensation expense.
Our common stock has experienced significant price and volume fluctuations and may continue to experience volatility in the future. Many factors may have a significant impact on the market price of our common stock, including, but not limited to, the following factors: results of or delays in our preclinical studies and clinical trials; the success of our collaboration agreements; publicity regarding actual or potential medical results relating to products under development by us or others; announcements of technological innovations or new commercial products by us or others; developments in patent or other proprietary rights by us or others; market perception of the OmniAb spin-off; comments or opinions by securities analysts or major stockholders or changed securities analysts' reports or recommendations; future sales or shorting of our common stock by existing stockholders; regulatory developments or changes in regulatory guidance; litigation or threats of litigation; economic and other external factors or other disaster or crises; the departure of any of our officers, directors or key employees; period-to-period fluctuations in financial results; and price and volume fluctuations in the overall stock market.
Our results of operations and liquidity needs could be materially negatively affected by market fluctuations and economic downturn.
Our results of operations could be materially negatively affected by economic conditions generally, both in the United States and elsewhere around the world. Concerns over inflation, energy costs, geopolitical issues, military conflicts, including the war between Russia and Ukraine, terrorism, public health emergencies or pandemics, the availability and cost of credit, and the U.S. financial markets have in the past contributed to, and may continue in the future to contribute to, increased volatility and diminished expectations for the economy and the markets. Sanctions imposed by the United States and other countries in response to military conflicts, including the war between Russia and Ukraine, may also adversely impact the financial markets and the global economy, and any economic countermeasures by the affected countries or others could exacerbate market and economic instability. In addition, the COVID-19 pandemic affected and may continue to affect, and any future epidemic diseases may affect, worldwide equity markets. Domestic and international equity markets periodically experience heightened volatility and turmoil. These events may have an adverse effect on us. In the event of a market downturn, our results of operations could be adversely affected by those factors in many ways, including making it more difficult for us to raise funds if necessary, and our stock price may further decline. We cannot provide assurance that our investments are not subject to adverse changes in market value. If our investments experience adverse changes in market value, we may have less capital to fund our operations.
| Item 1B. | Unresolved Staff Comments | ||||
None.
34
| Item 2. | Properties | ||||
The following table summarizes our principal facilities leased as of December 31, 2022, including the location and size of each facility, and their designated use. We believe our facilities are adequate for our current and near-term needs, and we will be able to locate additional facilities, as needed.
| Location | Approximate Square Feet | Operation | Lease Expiration Date | ||||||||
| San Diego, CA | 54,000 | Corporate headquarter office and laboratory | August 2032 | ||||||||
| Las Vegas, NV | 4,100 | Office | April 2028 | ||||||||
| Lawrence, KS | 3,700 | Office and laboratory | August 2032 | ||||||||
| Item 3. | Legal Proceedings | ||||
See “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (10), Commitments and Contingencies—Legal Proceedings.”
| Item 4. | Mine Safety Disclosures | ||||
Not applicable.
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities | ||||
Our common stock is traded on the Nasdaq Global Market under the symbol “LGND.” As of February 22, 2023, there were approximately 354 holders of record of the common stock.
Except for 2007, during which we declared a cash dividend on our common stock of $2.50 per share, we have not paid any dividends on our common stock in the past and currently do not expect to pay cash dividends or make any other distributions on common stock in the future. We expect to retain our future earnings, if any, for use in the operation and expansion of our business, to pay down debt and potentially for share repurchases. Any future determination to pay dividends on common stock will be at the discretion of our Board of Directors and will depend upon our financial condition, results of operations, capital requirements and such other factors as the board deems relevant.
During the fiscal year ended December 31, 2022, we did not repurchase any shares of our common stock under the stock repurchase program approved by our Board of Directors in September 2019, which allowed us to acquire up to $500 million of our common stock in open market and negotiated purchases for a period of up to three years. This stock repurchase program expired in September 2022.
The information required by Item 201(d) of Regulation S-K is incorporated by reference to the 2023 Annual Meeting Proxy Statement as defined in Item 10 below.
35
Performance Graph
The graph below shows the five-year cumulative total stockholder return assuming the investment of $100 and is based on the returns of the component companies weighted monthly according to their market capitalization. The graph compares total stockholder returns of our common stock, of all companies traded on the Nasdaq Stock market, as represented by the Nasdaq Composite® Index, and of the Nasdaq Biotechnology Stock Index, as prepared by The Nasdaq Stock Market Inc.
The stockholder return shown on the graph below is not necessarily indicative of future performance and we will not make or endorse any predictions as to future stockholder returns.
Value of $100 Invested Over Time
| 12/31/2017 | 12/31/2018 | 12/31/2019 | 12/31/2020 | 12/31/2021 | 12/31/2022 | |||||||||||||||||||||||||||||||||
| Ligand | $ | 100.00 | $ | 99.10 | $ | 76.16 | $ | 72.63 | $ | 112.80 | $ | 71.62 | ||||||||||||||||||||||||||
| NASDAQ Composite-Total Return | $ | 100.00 | $ | 97.12 | $ | 132.81 | $ | 192.47 | $ | 235.15 | $ | 158.65 | ||||||||||||||||||||||||||
| NASDAQ Biotechnology Index | $ | 100.00 | $ | 91.14 | $ | 114.02 | $ | 144.15 | $ | 144.18 | $ | 129.59 | ||||||||||||||||||||||||||
36
| Item 6. | [RESERVED] | ||||
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | ||||
Our Management's Discussion and Analysis of Financial Condition and Results of Operations (MD&A) will help readers understand our results of operations, financial condition, and cash flows. It is provided in addition to the accompanying consolidated financial statements and notes.
OmniAb Separation and Spin-Off
On March 23, 2022, we entered into the Merger Agreement, by and among our company, Avista Public Acquisition Corp. II (New OmniAb) and OmniAb, Inc., a Delaware corporation and then wholly-owned subsidiary of our company (OmniAb), and Orwell Merger Sub Inc. (Merger Sub), pursuant to which New OmniAb combined with OmniAb, our then-antibody discovery business (the OmniAb Business), in a Reverse Morris Trust transaction. Pursuant to the Separation Agreement, we transferred the OmniAb Business, including certain of our related subsidiaries, to OmniAb and, in connection therewith, distributed (the Distribution) to Ligand stockholders 100% of the common stock of OmniAb. Immediately following the Distribution on November 1, 2022, in accordance with and subject to the terms and conditions of the Merger Agreement, Merger Sub merged with and into OmniAb (the Merger), with OmniAb continuing as the surviving company in the Merger and as a wholly-owned subsidiary of New OmniAb. After the Distribution, we do not beneficially own any shares of common stock in OmniAb and no longer consolidate OmniAb into our financial results for periods ending after October 31, 2022. As a result, OmniAb's historical financial results through the Separation are reflected in our consolidated financial statements as discontinued operations.
Our MD&A is organized as follows:
•Results of Operations. Detailed discussion of our revenue and expenses from continuing operations for twelve months ended December 31, 2022, 2021 and 2020.
•Liquidity and Capital Resources. Discussion of key aspects of our consolidated statements of cash flows, changes in our financial position, and our financial commitments.
•Critical Accounting Policies and Estimates. Discussion of significant changes we believe are important to understand the assumptions and judgments underlying our consolidated financial statements.
•Recent Accounting Pronouncements. For summary of recent accounting pronouncements applicable to our consolidated financial statements, see “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (1), Basis of Presentation and Summary of Significant Accounting Policies.”
Results of Operations
Revenue
FY 2022 vs. FY 2021
| (Dollars in thousands) | 2022 | 2021(a) | Change | % Change | ||||||||||||||||||||||
| Royalties | $ | 72,527 | $ | 48,927 | $ | 23,600 | 48 | % | ||||||||||||||||||
| Captisol - Core | 16,429 | 23,423 | (6,994) | (30) | % | |||||||||||||||||||||
| Captisol - COVID | 88,066 | 140,827 | (52,761) | (37) | % | |||||||||||||||||||||
| Contract Revenue | 19,223 | 28,367 | (9,144) | (32) | % | |||||||||||||||||||||
| Total revenue | $ | 196,245 | $ | 241,544 | $ | (45,299) | (19) | % | ||||||||||||||||||
(a) Prior period amounts have been retrospectively adjusted to reflect the effects of the Separation.
Total revenue from continuing operations decreased by $45.3 million, or 19%, to $196.2 million in 2022 compared to $241.5 million in 2021 primarily due to the $52.8 million decrease in sales of COVID-related Captisol. The lower sales were due to reduced demand for remdesivir, a treatment for moderate or severe COVID-19. Core Captisol sales were $16.4 million
37
for the year ended December 31, 2022, compared with $23.4 million for the same period in 2021. The lower sales were due to the timing of customer orders. Royalty revenue increased by $23.6 million, or 48%, to $72.5 million in 2022 as compared to $48.9 million for the same period in 2021. The increase in royalty revenue is driven primarily by increases in sales of drugs using the Pelican platform - Rylaze, Pneumosil and Teriparatide, along with an increase in sales of Kyprolis. Contract revenue decreased year over year in 2022 by $9.1 million primarily due to the timing of partner milestone events.
FY 2021 vs. FY 2020
| (Dollars in thousands) | 2021(a) | 2020(a) | Change | % Change | ||||||||||||||||||||||
| Royalties | $ | 48,927 | $ | 33,796 | $ | 15,131 | 45 | % | ||||||||||||||||||
| Captisol - Core | 23,423 | 24,566 | (1,143) | (5) | % | |||||||||||||||||||||
| Captisol - COVID | 140,827 | 85,393 | 55,434 | 65 | % | |||||||||||||||||||||
| Contract Revenue | 28,367 | 19,807 | 8,560 | 43 | % | |||||||||||||||||||||
| Total revenue | $ | 241,544 | $ | 163,562 | $ | 77,982 | 48 | % | ||||||||||||||||||
(a) Prior period amounts have been retrospectively adjusted to reflect the effects of the Separation.
Total revenue from continuing operations increased by $78.0 million, or 48%, to $241.5 million in 2021 compared to $163.6 million in 2020 primarily due to the $55.4 million increase in sales of COVID-related Captisol. The higher sales were due to increased demand for remdesivir, a treatment for moderate or severe COVID-19. Core Captisol sales were $23.4 million for the year ended December 31, 2021, compared with $24.6 million for the same period in 2020. The lower sales were due to the timing of customer orders. Royalty revenue increased by $15.1 million, or 45%, to $48.9 million in 2021 as compared to $33.8 million for the same period in 2020. The increase in royalty revenue was driven primarily by the royalties from the sale of drugs using the Pelican platform - Rylaze, Pneumosil and Teriparatide. Contract revenue increased year over year in 2021 by $8.6 million primarily due to the timing of partner milestone events.
Royalty revenue is a function of our partners' product sales and the applicable royalty rate. Kyprolis royalty rate is under a tiered royalty rate structure with the highest being 3.0%. Evomela has a contractually fixed royalty rate of 20%. Teriparatide injection has a tiered gross profit share between 25% and 40% on sales that have been adjusted for certain deductible items as defined in the respective license agreement. The Rylaze royalty rate is in the low single digits. Contract revenue includes service revenue, license fees and development, regulatory and sales based milestone payments.
The following table represents royalty revenue by program:
| (in millions) | 2022 Estimated Partner Product Sales | Effective Royalty Rate | 2022 Royalty Revenue | 2021 Estimated Partner Product Sales | Effective Royalty Rate | 2021 Royalty Revenue | |||||||||||||||||
| Kyprolis | $ | 1,275.6 | 2.4% | $ | 30.1 | $ | 1,148.9 | 2.4% | $ | 27.5 | |||||||||||||
| Evomela | 51.0 | 20.0% | 10.2 | 50.5 | 20.0% | 10.1 | |||||||||||||||||
Teriparatide injection(1) | 47.2 | 33.5% | 15.8 | 12.9 | 41.1% | 5.3 | |||||||||||||||||
| Rylaze | 278.7 | 3.2% | 8.8 | 80.7 | 3.0% | 2.4 | |||||||||||||||||
| Other | 383.7 | 2.0% | 7.6 | 195.1 | 1.8% | 3.6 | |||||||||||||||||
| Total | $ | 2,036.2 | $ | 72.5 | $ | 1,488.1 | $ | 48.9 | |||||||||||||||
(1) - Teriparatide injection sales have been adjusted for certain deductible items as defined in the respective license agreement, and the royalty revenue is based on a tiered gross profit share.
Operating Costs and Expense
FY 2022 vs. FY 2021
38
| (Dollars in thousands) | 2022 | 2021(a) | Change | % Change | |||||||||||||||||||
| Cost of Captisol | $ | 52,827 | $ | 62,176 | $ | (9,349) | (15) | % | |||||||||||||||
| Amortization of intangibles | 34,237 | 34,222 | 15 | — | % | ||||||||||||||||||
| Research and development | 36,082 | 32,105 | 3,977 | 12 | % | ||||||||||||||||||
| General and administrative | 70,062 | 46,790 | 23,272 | 50 | % | ||||||||||||||||||
| Other operating income | — | (37,600) | 37,600 | (100) | % | ||||||||||||||||||
| Total operating costs and expenses | $ | 193,208 | $ | 137,693 | $ | 55,515 | 40 | % | |||||||||||||||
(a) Prior period amounts have been retrospectively adjusted to reflect the effects of the Separation.
Total operating costs and expenses from continuing operations for 2022 increased $55.5 million or 40% compared with 2021.
Cost of Captisol decreased year over year in 2022 primarily due to lower sales of Captisol during 2022, partially offset by the capacity ramp-up right of use asset impairment of $9.8 million recorded in the fourth quarter of 2022.
Amortization of intangibles remained steady in 2022 compared to 2021 as there have been no significant changes to the gross balance of intangible assets over these periods.
At any one time, we are working on multiple programs. As such, we generally do not track our R&D expenses on a specific program basis. Our R&D expenses increased by $4.0 million in 2022 compared to 2021 due to higher employee-related expenses and increased facility related expenses.
General and administrative expenses increased by $23.3 million in 2022 compared to 2021 primarily due to increases in stock compensation expense including a one-time charge associated with the retirement of our former CEO in the fourth quarter of 2022, headcount-related expenses and legal expenses.
Other operating income in 2021 was due to reducing the fair value of the CVR liability associated to the acquisition of Pfenex to zero, as the CVR payment expiration date passed on December 31, 2021 without achieving the triggering event. We did not have any other operating income in 2022.
FY 2021 vs. FY 2020
| (Dollars in thousands) | 2021(a) | 2020(a) | Change | % Change | |||||||||||||||||||
| Cost of Captisol | $ | 62,176 | $ | 30,419 | $ | 31,757 | 104 | % | |||||||||||||||
| Amortization of intangibles | 34,222 | 11,642 | 22,580 | 194 | % | ||||||||||||||||||
| Research and development | 32,105 | 40,503 | (8,398) | (21) | % | ||||||||||||||||||
| General and administrative | 46,790 | 60,012 | (13,222) | (22) | % | ||||||||||||||||||
| Other operating income | (37,600) | 600 | (38,200) | (6,367) | % | ||||||||||||||||||
| Total operating costs and expenses | $ | 137,693 | $ | 143,176 | $ | (5,483) | (4) | % | |||||||||||||||
(a) Prior period amounts have been retrospectively adjusted to reflect the effects of the Separation.
Total operating costs and expenses from continuing operations for 2021 decreased $5.5 million or 4% compared with 2020.
Cost of Captisol increased year over year in 2021 primarily due to higher sales of Captisol during 2021.
Amortization of intangibles increased year over year in 2021 primarily due to the acquisition of Pfenex in October 2020.
At any one time, we are working on multiple programs. As such, we generally do not track our R&D expenses on a specific program basis. Our R&D expenses decreased year over year in 2021 due to the sale of Vernalis R&D in October 2020.
General and administrative expenses decreased by $13.2 million in 2022 compared to 2021 primarily due to $20.7 million in acquisition and integration related costs in 2021 associated with the Pfenex acquisition in 2020. The decrease was partially offset by additional headcount related expenses in 2021.
Other operating income in 2021 was due to reducing the fair value of the CVR liability associated to the acquisition of Pfenex to zero, as the CVR payment expiration date passed on December 31, 2021 without achieving the triggering event.
We do not provide forward-looking estimates of costs and time to complete our ongoing research and development projects as such estimates would involve a high degree of uncertainty. Uncertainties include our inability to predict the outcome
39
of research and clinical studies, regulatory requirements placed upon us by regulatory authorities such as the FDA and EMA, our inability to predict the decisions of our partners, our ability to fund research and development programs, competition from other entities of which we may become aware in future periods, predictions of market potential for products that may be derived from our work, and our ability to recruit and retain personnel or third-party contractors with the necessary knowledge and skills to perform certain research. Refer to “Item 1A. Risk Factors” for additional discussion of the uncertainties surrounding our research and development initiatives.
Other income (expense)
FY 2022 vs. FY 2021
| (Dollars in thousands) | 2022 | 2021(a) | Change | % Change | |||||||||||||||||||
| Gain (loss) from short-term investments | $ | 28,540 | $ | (5,263) | $ | 33,803 | 642 | % | |||||||||||||||
| Interest income | 2,046 | 886 | 1,160 | 131 | % | ||||||||||||||||||
| Interest expense | (1,799) | (19,619) | 17,820 | 91 | % | ||||||||||||||||||
| Other expense, net | 4,187 | (7,650) | 11,837 | 155 | % | ||||||||||||||||||
| Total other income (expense), net | $ | 32,974 | $ | (31,646) | $ | 64,620 | 204 | % | |||||||||||||||
(a) Prior period amounts have been retrospectively adjusted to reflect the effects of the Separation.
The fluctuation in the gain (loss) from short-term investments is primarily driven by the changes in the fair value of our ownership in Viking common stock (an unrealized gain of $32.2 million in 2022 as compared to an unrealized loss of $9.6 million in 2021).
Interest income consists primarily of interest earned on our short-term investments. The year over year increase in 2022 is primarily due to the significant interest rate increases by the federal reserve during 2022.
Interest expense includes the 0.75% coupon cash interest expense in addition to the non-cash accretion of discount (including the amortization of debt issuance costs) on our 2023 Notes. The year over year decrease was primarily due to the adoption of ASU 2020-06 which significantly reduced the debt discount balance subject to amortization. See additional information in “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (1), Basis of Presentation and Summary of Significant Accounting Policies.” In addition, we carried a lower average debt outstanding balance during 2022 as compared 2021. During 2022, we repurchased $266.4 million in principal of the 2023 Notes for $261.4 million in cash, including accrued interest of $0.5 million. See additional information in “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (7), Convertible Senior Notes.”
Other expense, net, increased year over year in 2022 primarily due to a $4.2 million gain on our debt extinguishments in 2022 compared to $7.3 million loss on debt extinguishments in 2021. See additional information in “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (7), Convertible Senior Notes.”
FY 2021 vs. FY 2020
| (Dollars in thousands) | 2021(a) | 2020(a) | Change | % Change | |||||||||||||||||||
| Gain (loss) from short-term investments | $ | (5,263) | $ | (16,933) | $ | 11,670 | (69) | % | |||||||||||||||
| Interest income | 886 | 8,078 | (7,192) | (89) | % | ||||||||||||||||||
| Interest expense | (19,619) | (27,415) | 7,796 | 28 | % | ||||||||||||||||||
| Other expense, net | (7,650) | 62 | (7,712) | 12439 | % | ||||||||||||||||||
| Total other income (expense), net | $ | (31,646) | $ | (36,208) | $ | 4,562 | 13 | % | |||||||||||||||
(a) Prior period amounts have been retrospectively adjusted to reflect the effects of the Separation.
The fluctuation in the gain (loss) from short-term investments is primarily driven by the changes in the fair value of our ownership in Viking common stock (an unrealized loss of $9.6 million in 2021 as compared to an unrealized loss of $19.0 million in 2020).
Interest income consists primarily of interest earned on our short-term investments. The year over year decrease in 2021 resulted from the decrease in our short-term investment balances due to the usage of funds for the 2023 Notes repurchases.
40
Interest expense includes the 0.75% coupon cash interest expense in addition to the non-cash accretion of discount (including the amortization of debt issuance costs) on our 2023 Notes. The year over year decrease in 2021 was primarily due to lower average debt outstanding balance as compared to the prior year. During 2021, we repurchased $152.0 million in principal of the 2023 Notes for $156.0 million in cash, including accrued interest of $0.3 million. See “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (7), Convertible Senior Notes.”
Other expense, net, increased year over year in 2021 primarily due to a $7.3 million loss on our debt extinguishments compared to $2.5 million loss on debt extinguishments in 2020. See additional information in “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (7), Convertible Senior Notes.”
Income tax benefit (expense)
FY 2022 vs. FY 2021
| (Dollars in thousands) | 2022 | 2021 | Change | % Change | |||||||||||||||||||
| Income before income tax expense (benefit) from continuing operations | $ | 36,011 | $ | 72,205 | $ | (36,194) | (50) | % | |||||||||||||||
| Income tax benefit (expense) | (41,230) | 4,148 | (45,378) | (1,094) | % | ||||||||||||||||||
| Net income (loss) from continuing operations | $ | (5,219) | $ | 76,353 | $ | (81,572) | (107) | % | |||||||||||||||
| Effective Tax Rate | 114 | % | (6) | % | |||||||||||||||||||
Our effective tax rate for 2022 and 2021 was 114% and (6)%, respectively. Our tax rate is affected by recurring items, such as the U.S. federal and state statutory tax rates and the relative amounts of income we earn in those jurisdictions, which we expect to be fairly consistent in the near term. It is also affected by discrete items that may occur in any given year, but are not consistent from year to year. In 2022, the variance from the U.S. federal statutory rate of 21% was primarily due to limitations on the deductibility of stock-based compensation for certain officers and a discrete tax expense of $24.8 million related to the valuation allowance established during the fourth quarter of 2022 against deferred tax assets for California research and development credits and net operating losses. Beginning in 2022, the Tax Cuts and Jobs Act of 2017 requires taxpayers to capitalize and amortize R&D expenditures over five years for domestic research and fifteen years for foreign research pursuant to Section 174 of the Internal Revenue Code of 1986, as amended. We recorded an increase of $4.7 million to our current federal income tax expense and deferred tax assets for continuing operations during 2022 due to the capitalization of R&D under Section 174. In 2021, the variance from the U.S. federal statutory rate of 21% was attributable to the mix of earnings in jurisdictions with lower statutory rates than the U.S. federal statutory tax rate, and excess benefits from shared-based compensation. The items below also had an impact on the difference between our statutory U.S. rate.
2022
•$24.8 million (68.9%) increase from valuation allowance adjustments
•$5.9 million (16.3%) increase from Section 162(m) limitation
•$2.4 million (6.7%) decrease from the foreign-derived intangible income deduction
•$1.3 million (3.6%) increase due to excess tax benefits from share-based compensation which are recorded as a discrete item within the provision for income tax pursuant to ASU 2016-09
2021
•$12.1 million (16.7%) decrease due to excess tax benefits from share-based compensation which are recorded as a discrete item within the provision for income tax pursuant to ASU 2016-09
•$11.2 million (15.6%) increase from valuation allowance adjustments
•$8.1 million (11.1%) decrease from tax rate and law changes in the United Kingdom
•$8.0 million (11.1%) decrease due to the revaluation of contingent value rights
•$3.2 million (4.5%) increase from Section 162(m) limitation
•$3.1 million (4.3%) decrease from net operating loss carryforwards and credit adjustments
•$1.6 million (2.3%) decrease from research and development tax credits
Net Loss from Discontinued Operations
Net loss from discontinued operations for the years ended December 31, 2022, 2021 and 2020 was $28.1 million, $19.2 million and $9.6 million, respectively. See additional information in “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (2), Spin-off Of OmniAb.”
41
Liquidity and Capital Resources
At December 31, 2022, we had approximately $211.9 million in cash, cash equivalents, and short-term investments. Cash and cash equivalents and short-term investments decreased by $129.2 million from last year, due to factors described in the “Cash Flow Summary” below. Our primary source of liquidity, other than our holdings of cash, cash equivalents, and investments, which decreased during 2022 primarily from extinguishment of debt, has been cash flows from operations. Our ability to generate cash from operations provides us with the financial flexibility we need to meet operating, investing, and financing needs.
Historically, we have liquidated our short-term investments and/or issued debt and equity securities to finance our business needs as a supplement to cash provided by operating activities. Our short-term investments include U.S. government debt securities, investment-grade corporate debt securities, mutual funds and certificates of deposit. We have established guidelines relative to diversification and maturities of our investments in order to provide both safety and liquidity. These guidelines are periodically reviewed and modified to take advantage of trends in yields and interest rates. Additionally, we own certain securities which are classified as short-term investments that we received as a result of a milestone and an upfront license payment as well as 6.7 million shares of common stock in Viking.
On September 30, 2022, we entered into an At-The-Market Equity Offering Sales Agreement (Sales Agreement) with Stifel, Nicolaus & Company, Incorporated (Agent), under which we may, from time to time, sell shares of our common stock having an aggregate offering price of up to $100.0 million in “at the market” offerings through the Agent (ATM Offering). The shelf registration statement relating to such shares included a prospectus covering the offering, issuance and sale of up to $100.0 million of our common stock from time to time through the ATM Offering. The shares to be sold under the Sales Agreement may be issued and sold pursuant to the shelf registration statement. As of December 31, 2022 we have not issued any shares of common stock in the ATM Offering.
In May 2018, we issued the 2023 Notes with an aggregate principal amount of $750.0 million. A portion of the proceeds from such issuance totaling $49.7 million were used to repurchase 260,000 shares of our common stock. During 2021 and 2020, we repurchased $406.7 million in principal of the 2023 Notes for $378.8 million in cash, including accrued interest of $0.9 million. During 2022, we repurchased $266.4 million in principal of the 2023 Notes for $261.4 million in cash, including accrued interest of $0.5 million. After the repurchases, $76.9 million in principal amount of the 2023 Notes remain outstanding as of December 31, 2022.
We may continue to use cash on hand to repurchase additional 2023 Notes through open-market transactions, including through a Rule 10b5-1 trading plan to facilitate open-market repurchases, or otherwise, from time to time. The timing and amount of repurchase transactions will be determined by management based on the evaluation of market conditions, trading price of the 2023 Notes, legal requirements and other factors. The 2023 Notes were not convertible as of December 31, 2022. It is our intent and policy to settle conversions through combination settlement, which essentially involves payment in cash equal to the principal portion and delivery of shares of common stock for the excess of the conversion value over the principal portion. See detail in “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (7), Convertible Senior Notes.”
We are obligated to make payments to operating leases, including rental commitments on leases that have not yet commenced. For information on these obligations, see detail in “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (6), Leases.”
We also have commitments under our supply agreement with Hovione for Captisol purchases. The total purchase obligation as of December 31, 2022 was $28.7 million, of which $9.6 million is expected to be paid within a year and the remaining amount is expected to be paid between 1 to 3 years.
In September 2019, our Board of Directors approved a stock repurchase program authorizing the repurchase of up to $500.0 million of our common stock from time to time over a period of up to three years. Our prior $350.0 million stock repurchase program was terminated in connection with the approval of the new stock repurchase program. Our $500.0 million stock repurchase program expired in September 2022, and as of December 31, 2022 we do not have a repurchase program in place. See “Item 5. Market for Registrant's Common Equity, Related Stockholder Matters, and Issuer Purchase of Equity Securities.”
We believe that existing funds, cash generated from operations and existing sources of and access to financing are adequate to satisfy our needs for working capital; capital expenditure and debt service requirements; continued advancement of research and development efforts; potential stock repurchases; and other business initiatives we plan to strategically pursue, including acquisitions and strategic investments.
As of December 31, 2022, we had $3.5 million in fair value of contingent consideration liabilities associated with the acquisitions to be settled in future periods.
42
Cash Flow Summary
| (in thousands) | 2022 | 2021 | 2020 | |||||||||||||||||
| Net cash provided by (used in): | ||||||||||||||||||||
| Operating activities | $ | 137,850 | $ | 78,798 | $ | 54,586 | ||||||||||||||
| Investing activities | $ | 163,624 | $ | 30,523 | $ | 231,648 | ||||||||||||||
| Financing activities | $ | (275,990) | $ | (137,761) | $ | (310,545) | ||||||||||||||
In 2022, we generated cash from operations primarily from collections on our trade receivables. We generated cash from investing activities primarily from sale and maturity of short-term investments. During the year, we used cash for financing activities, including the payments related to the extinguishment of certain 2023 Notes.
In 2021, we generated cash from operations primarily due to the increase in net income. We generated cash from investing activities primarily from sale and maturity of short-term investments. During the year, we used cash for financing activities, including the payments related to the extinguishment of certain 2023 Notes, partially offset by cash received from issuance of common stock under employee stock plans and bond hedge settlement.
In 2020, we generated cash from operations primarily due to cash inflows from changes in deferred revenue. We generated cash from investing activities primarily from sale and maturity of short-term investments as well as the sale of the Vernalis R&D business; partially offset by cash used for the acquisition of Pfenex, and three additional acquisitions that are part of our discontinued operations, Icagen, xCella and Taurus. During the year, we used cash for financing activities, including the payments related to the extinguishment of certain 2023 Notes and stock repurchases.
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with GAAP requires estimates and assumptions that affect the reported amounts of assets and liabilities, revenues and expenses, and related disclosures of contingent liabilities in the consolidated financial statements and accompanying notes. The SEC has defined a company’s critical accounting policies as the ones that are most important to the portrayal of the company’s financial condition and results of operations, and which require the company to make its most difficult and subjective judgments, often as a result of the need to make estimates of matters that are inherently uncertain. Based on this definition, we have identified the critical accounting policies and judgments addressed below. We also have other key accounting policies, which involve the use of estimates, judgments, and assumptions that are significant to understanding our results. For additional information, see “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (1), Basis of Presentation and Summary of Significant Accounting Policies.” Although we believe that our estimates, assumptions, and judgments are reasonable, they are based upon information presently available. Actual results may differ significantly from these estimates under different assumptions, judgments, or conditions.
Revenue Recognition
We apply the following five-step model in accordance with ASC 606 in order to determine the revenue: (i) identification of the promised goods or services in the contract; (ii) determination of whether the promised goods or services are performance obligations, including whether they are distinct in the context of the contract; (iii) measurement of the transaction price, including the constraint on variable consideration; (iv) allocation of the transaction price to the performance obligations; and (v) recognition of revenue when (or as) the Company satisfies each performance obligation.
We receive royalty revenue on sales by our partners of products covered by patents that we or our partners own under contractual agreements. We do not have future performance obligations under these license arrangements. We generally satisfy our obligation to grant intellectual property rights on the effective date of the contract. However, we apply the royalty recognition constraint required under the guidance for sales-based royalties which requires a royalty to be recorded no sooner than the underlying sale occurs. Therefore, royalties on sales of products commercialized by our partners are recognized in the quarter the product is sold. Our partners generally report sales information to us on a one quarter lag. Thus, we estimate the expected royalty proceeds based on an analysis of historical experience and interim data provided by our partners including their publicly announced sales. Differences between actual and estimated royalty revenues are adjusted in the period in which they become known, typically the following quarter.
Our contracts with customers often will include variable consideration in the form of contingent milestone-based payments. We include contingent milestone based payments in the estimated transaction price when it is probable a significant reversal in the amount of cumulative revenue recognized will not occur. These estimates are based on historical experience,
43
anticipated results and our best judgment at the time. If the contingent milestone based payment is sales-based, we apply the royalty recognition constraint and record revenue when the underlying sale has taken place. Significant judgments must be made in determining the transaction price for our sales of intellectual property. Because of the risk that products in development with our partners will not reach development based milestones or receive regulatory approval, we generally recognize any contingent payments that would be due to us upon the occurrence of the development milestone or regulatory approval. Depending on the terms of the arrangement, we may also defer a portion of the consideration received if we have to satisfy a future obligation, which typically occurs with our contracts for R&D services.
For R&D services we recognize revenue over time and we measure our progress using an input method. The input methods we use are based on the effort we expend or costs we incur toward the satisfaction of our performance obligation. We estimate the amount of effort we expend, including the time it will take us to complete the activities, or the costs we may incur in a given period, relative to the estimated total effort or costs to satisfy the performance obligation. This results in a percentage that we multiply by the transaction price to determine the amount of revenue we recognize each period. This approach requires us to make numerous estimates and use significant judgement. If our estimates or judgements change over the course of the collaboration, they may affect the timing and amount of revenue that we recognize in the current and future periods.
Revenue from Captisol sales is recognized when control of Captisol material or intellectual property license rights is transferred to our customers in an amount that reflects the consideration we expect to receive from our customers in exchange for those products. A performance obligation is considered distinct from other obligations in a contract when it provides a benefit to the customer either on its own or together with other resources that are readily available to the customer and is separately identified in the contract. For Captisol material, we consider our performance obligation is satisfied at a point in time, once we have transferred control of the product, meaning the customer has the ability to use and obtain the benefit of the Captisol material or intellectual property license right. We recognize revenue for satisfied performance obligations only when we determine there are no uncertainties regarding payment terms or transfer of control. Sales tax and other taxes we collect concurrent with revenue-producing activities are excluded from revenue. We have elected to recognize the cost of freight and shipping when control over Captisol material has transferred to the customer as an expense in Cost of Captisol. We expense incremental costs of obtaining a contract when incurred if the expected amortization period of the asset that we would have recognized is one year or less or the amount is immaterial. We did not incur any incremental costs of obtaining a contract during the periods reported.
We occasionally have sub-license obligations related to arrangements for which we receive license fees, milestones and royalties. We evaluate the determination of gross as a principal versus net as an agent reporting based on each individual agreement.
Goodwill and Intangible Assets — Impairment Assessments
Goodwill
Goodwill is evaluated annually for impairment using either a quantitative or qualitative analysis. Goodwill is tested for impairment at the reporting unit level, and is based on the net assets for each reporting unit, including goodwill and intangible assets. Goodwill is assigned to each reporting unit, as this represents the lowest level that constitutes a business and is the level at which management regularly reviews the operating results. The Company performs a quantitative analysis using a discounted cash flow model and other valuation techniques, but may elect to perform a qualitative analysis. In addition, goodwill is evaluated for impairment whenever an event occurs or circumstances change that would indicate that it is more likely than not that the fair value of a reporting unit is less than its carrying amount. Events or circumstances that may result in an impairment review include changes in macroeconomic conditions, industry and market considerations, cost factors, overall financial performance, other relevant entity-specific events, specific events affecting the reporting unit or sustained decrease in share price.
The annual goodwill impairment test was performed using a qualitative analysis in 2022 and 2021. A qualitative analysis is performed by assessing certain trends and factors, including projected market outlook and growth rates, forecasted and actual sales and operating profit margins, discount rates, industry data, and other relevant qualitative factors. These trends and factors are compared to, and based on, the assumptions used in the most recent quantitative analysis performed for each reporting unit. The results of the qualitative analyses did not indicate a need to perform quantitative analysis.
Goodwill impairment testing was performed using quantitative analyses in 2022 for the OmniAb business and Ligand core business due to the reorganization of the Company’s business discussed in “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (2), Spin-off of OmniAb.” We used the relative fair value method to reallocate goodwill.
Intangible Assets
44
We regularly perform reviews to determine if an event occurred that may indicate the carrying values of our intangible assets are impaired. If indicators of impairment exist, we assess the recoverability of the affected long-lived assets by comparing its carrying amounts to its undiscounted cash flows. If the affected assets are not recoverable, we estimate the fair value of the assets and record an impairment loss if the carrying value exceeds the fair value. Factors that may indicate potential impairment include a significant decline in our stock price and market capitalization compared to net book value, significant changes in the ability of an asset to generate positive cash flows and the pattern of utilization of a particular asset.
In order to estimate the fair value of identifiable intangible assets, we estimate the present value of future cash flows from those assets. The key assumptions that we use in our discounted cash flow model are the amount and timing of estimated future cash flows to be generated by the asset over an extended period of time and a rate of return that considers the relative risk of achieving the cash flows, the time value of money, and other factors that a willing market participant would consider. Significant judgment is required to estimate the amount and timing of future cash flows and the relative risk of achieving those cash flows.
Assumptions and estimates about future values and remaining useful lives are complex and often subjective. They can be affected by a variety of factors, including external factors such as industry and economic trends, and internal factors such as changes in our business strategy and our internal forecasts. For example, if our future operating results do not meet current forecasts or if we experience a sustained decline in our market capitalization that is determined to be indicative of a reduction in fair value of our reporting unit, we may be required to record future impairment charges for purchased intangible assets. Impairment charges could materially decrease our future net income and result in lower asset values on our balance sheet.
Income Taxes
Our provision for income taxes, deferred tax assets and liabilities, and reserves for unrecognized tax benefits reflect our best assessment of estimated future taxes to be paid. Significant judgments and estimates based on interpretations of existing tax laws or regulations in the United States are required in determining our provision for income taxes. Changes in tax laws, statutory tax rates, and estimates of our future taxable income could impact the deferred tax assets and liabilities provided for in the consolidated financial statements and would require an adjustment to the provision for income taxes.
Deferred tax assets are regularly assessed to determine the likelihood they will be recovered from future taxable income. A valuation allowance is established when we believe it is more likely than not the future realization of all or some of a deferred tax asset will not be achieved. In evaluating our ability to recover deferred tax assets within the jurisdiction which they arise, we consider all available positive and negative evidence. Factors reviewed include the cumulative pre-tax book income for the past three years, scheduled reversals of deferred tax liabilities, our history of earnings and reliability of our forecasts, projections of pre-tax book income over the foreseeable future, and the impact of any feasible and prudent tax planning strategies.
We recognize the impact of a tax position in our financial statements only if that position is more likely than not of being sustained upon examination by taxing authorities, based on the technical merits of the position. Tax authorities regularly examine our returns in the jurisdictions in which we do business and we regularly assess the tax risk of our return filing positions. Due to the complexity of some of the uncertainties, the ultimate resolution may result in payments that are materially different from our current estimate of the tax liability. These differences, as well as any interest and penalties, will be reflected in the provision for income taxes in the period in which they are determined.
Share-Based Compensation
We measure and recognize compensation expense for all share-based payments, including restricted stock, ESPP and stock options, based on the estimated fair value. Restricted stock unit (RSU) and performance stock unit (PSU) are all considered restricted stock. The fair value of restricted stock is determined by the closing market price of our common stock on the date of grant. We recognize share-based compensation expense based on the fair value on a straight-line basis over the requisite service periods of the awards, taking into consideration of forfeitures as they occur. A PSU generally represents a right to receive a certain number of shares of common stock based on the achievement of corporate performance goals and continued employment during the vesting period. At each reporting period, we reassess the probability of the achievement of such corporate performance goals and any expense change resulting from an adjustment in the estimated shares to be released are treated as a cumulative catch-up in the period of adjustment. A limited amount of PSUs contain a market condition dependent upon the Company’s relative and absolute total stockholder return over a three-year period, with a range of 0% to 200% of the target amount granted to be issued under the award. Share-based compensation expense for these PSUs is measured using the Monte-Carlo simulation valuation model and is not adjusted for the achievement, or lack thereof, of the market conditions.
Conversion and Modification of Equity Awards Outstanding at Separation Date
In connection with the OmniAb Separation on November 1, 2022, under the provisions of the existing plans, we adjusted our outstanding equity awards in accordance with the Merger Agreement to preserve the intrinsic value of the awards immediately before and after the Distribution. Upon the Distribution, employees holding stock options, restricted stock units
45
and performance restricted stock units denominated in pre-Distribution Ligand stock received a number of otherwise-similar awards either in post-Distribution Ligand stock or in a combination of post-Distribution Ligand stock and OmniAb stock based on conversion ratios outlined for each group of employees in the Merger Agreement that we entered into in connection with the Distribution. The equity awards that were granted prior to March 2, 2022 were converted under the shareholder method, wherein employees holding outstanding equity awards received equity awards in both Ligand and OmniAb. For equity awards granted after March 2, 2022, for Ligand employees, the number of awards that were outstanding at the Separation were proportionately adjusted into post-Distribution Ligand stock to maintain the aggregate intrinsic value of the awards at the date of the Separation; for OmniAb employees, the number of awards that were outstanding at the Separation were proportionately adjusted into post-Distribution OmniAb stock to maintain the aggregate intrinsic value of the awards at the date of the Separation. The conversion ratio was determined based on the relative values of Ligand common stock in the “regular way” and “ex-distribution” markets during the five-trading day period prior to the closing of the business combination.
These modified awards otherwise retained substantially the same terms and conditions, including term and vesting provisions. Additionally, we will not incur any future compensation cost related to equity awards held by OmniAb employees and directors. We will incur future compensation cost related to OmniAb equity awards held by our employees.
Recent Accounting Pronouncements
For the summary of recent accounting pronouncements applicable to our consolidated financial statements, see “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (1), Basis of Presentation and Summary of Significant Accounting Policies.”
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | ||||
We are exposed to market risk from interest rates and equity prices which could affect our results of operations, financial condition and cash flows. We manage our exposure to these market risks through our regular operating and financing activities.
Investment Portfolio Risk
At December 31, 2022, our investment portfolio included investments in available-for-sale securities of $166.9 million, including the investment in Viking common stock of $63.1 million. These securities are subject to market risk and may decline in value based on market conditions.
Equity Price Risk
Our 2023 Notes include conversion and settlement provisions that are based on the price of our common stock at conversion or maturity of the notes, as applicable. As of December 31, 2022, the “if-converted value” did not exceed the principal amount of the 2023 Notes. See detail in “Item 8. Financial Statements and Supplementary Data—Notes to Consolidated Financial Statements—Note (7), Convertible Senior Notes.”
Foreign Currency Risk
Through our licensing and business operations, we are exposed to foreign currency risk. Foreign currency exposures arise from transactions denominated in a currency other than the functional currency and from foreign denominated revenues and profit translated into U.S. dollars. Our license partners sell our products worldwide in currencies other than the U.S. dollar. Because of this, our revenues from royalty payments are subject to risk from changes in exchange rates.
We purchase Captisol from Hovione, located in Lisbon, Portugal. Payments to Hovione are denominated and paid in U.S. dollars; however, the unit price of Captisol contains an adjustment factor which is based on the sharing of foreign currency risk between the two parties. The effect of an immediate 10% change in foreign exchange rates would not have a material impact on our financial condition, results of operations or cash flows. We do not currently hedge our exposures to foreign currency fluctuations.
Interest Rate Risk
We are exposed to changes in interest rates related primarily to our investment portfolio. Our investment policy and strategy are focused on the preservation of capital and supporting our liquidity requirements. We use a combination of internal and external management to execute our investment strategy. We typically invest in highly rated securities, with the primary objective of minimizing the risk of principal loss. Our investment policy generally requires securities to be investment grade and limits the amount of credit exposure to any one issuer. We have historically maintained a relatively short average maturity for our investment portfolio, and we believe a hypothetical 100 basis point adverse move in interest rates across all maturities would not materially impact the fair market value of the portfolio in either period.
46
| Item 8. | Consolidated Financial Statements and Supplementary Data | ||||
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | |||||
Report of Independent Registered Public Accounting Firm (PCAOB ID: | |||||
47
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Ligand Pharmaceuticals Incorporated
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Ligand Pharmaceuticals Incorporated (the Company) as of December 31, 2022 and 2021, and the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2022, and the related notes, (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2022, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 28, 2023 expressed an unqualified opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of the critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which they relate.
48
| Impairment assessment of finite-lived intangibles | ||||||||
| Description of the Matter | At December 31, 2022, the Company’s finite-lived intangible assets totaled $342.5 million. As discussed in Note 1 to the consolidated financial statements, the Company reviews finite-lived intangible assets for impairment whenever events or changes in circumstances indicate the carrying amount may not be recoverable. The Company did not identify indicators of impairment for its finite-lived intangibles at December 31, 2022. Auditing management’s assessment of impairment is challenging due to the degree of subjective auditor judgment necessary in evaluating management’s process to identify potential indicators of impairment and the related assessment of the severity of such indicators in determining whether a triggering event has occurred. A high degree of auditor judgment was required to evaluate potential triggering events which included market conditions, industry and economic trends, changes in regulations, clinical success and historical and forecasted financial results. The evaluation of triggering events could have a significant effect on the Company’s impairment assessment and the determination of whether further quantitative analysis of finite-lived intangible assets was required. | |||||||
| How We Addressed the Matter in Our Audit | We obtained an understanding of management’s process to identify indicators of impairment, including the qualitative analysis and related inputs and assumptions used in performing the analyses. We evaluated the design and tested the operating effectiveness of the controls that address the identification of indicators of impairment. For example, we tested controls over management’s assessment of indicators of impairment. To test the Company’s evaluation of indicators of impairment for finite-lived intangibles, our audit procedures included, among others, assessing the methodologies and testing the completeness and accuracy of the Company’s analysis of events or changes in circumstances. As part of our evaluation, we considered market conditions, industry and economic trends, changes in regulations, clinical success and historical and forecasted financial results, in assessing whether an indicator of impairments exists. | |||||||
| Assigning Goodwill in connection with the spin off of the OmniAb business | ||||||||
| Description of the Matter | As discussed in Note 2 to the consolidated financial statements, in March 2022, the Company entered an agreement to spin off the Company's OmniAb business. Coinciding with the signing of the agreement, the Company reorganized its reporting structure requiring the assignment of goodwill from the existing reporting unit to two reporting units. Management assigned goodwill using a relative fair value allocation which resulted in goodwill of $75.5 million being allocated to the OmniAb business. Auditing management’s assignment of goodwill was complex due to the significant judgement used by management when developing the fair value measurement of the reporting units, including the subjectivity involved in the selection of key assumptions related to estimating revenue and discount rates. | |||||||
| How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of controls over the Company’s assignment of goodwill based on the relative fair value of reporting units. For example, we tested controls over management’s determination of reporting units and management’s review of the significant assumptions described above along with the completeness and accuracy of the data used in these fair value estimates. To test the estimated fair value of the reporting units, our audit procedures included, among others, (i) assessing the suitability and application of the valuation methodologies selected, (ii) evaluating the significant assumptions, discussed above, and (iii) testing the underlying data used by the Company in its analysis. For example, we compared the significant assumptions used by management, to current industry and economic trends. We assessed the historical accuracy of management’s estimates and performed a sensitivity analysis of significant assumptions to evaluate the changes in the fair value of the reporting units that result from changes in assumptions. We also involved our valuation specialists to assist in the evaluation of the weighted-average cost of capital utilized in determining the fair value estimates. We tested the allocation of goodwill to the reporting units. We also evaluated the related disclosures. | |||||||
/s/ Ernst & Young LLP
We have served as the Company's auditor since 2016.
February 28, 2023
49
LIGAND PHARMACEUTICALS INCORPORATED
CONSOLIDATED BALANCE SHEETS
(in thousands, except par value)
| December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| ASSETS | |||||||||||
| Current assets: | |||||||||||
Cash and cash equivalents | $ | $ | |||||||||
| Short-term investments | |||||||||||
| Accounts receivable, net | |||||||||||
| Inventory | |||||||||||
| Income taxes receivable | |||||||||||
| Other current assets | |||||||||||
| Current assets of discontinued operations | |||||||||||
| Total current assets | |||||||||||
| Deferred income taxes, net | |||||||||||
| Intangible assets, net | |||||||||||
| Goodwill | |||||||||||
| Commercial license and other economic rights | |||||||||||
| Property and equipment, net | |||||||||||
| Operating lease assets | |||||||||||
| Finance lease assets | |||||||||||
| Other assets | |||||||||||
| Non-current assets of discontinued operations | |||||||||||
| Total assets | $ | $ | |||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable | $ | $ | |||||||||
| Accrued liabilities | |||||||||||
| Current contingent liabilities | |||||||||||
| Deferred revenue | |||||||||||
| Current operating lease liabilities | |||||||||||
| Current finance lease liabilities | |||||||||||
| 2023 convertible senior notes, net | |||||||||||
| Current liabilities of discontinued operations | |||||||||||
| Total current liabilities | |||||||||||
| 2023 convertible senior notes, net | |||||||||||
| Long-term contingent liabilities | |||||||||||
| Deferred income taxes, net | |||||||||||
| Long-term operating lease liabilities | |||||||||||
| Other long-term liabilities | |||||||||||
| Non-current liabilities of discontinued operations | |||||||||||
| Total liabilities | |||||||||||
| Commitments and contingencies | |||||||||||
| Stockholders’ equity: | |||||||||||
Preferred stock, $ | |||||||||||
Common stock, $ | |||||||||||
| Additional paid-in capital | |||||||||||
| Accumulated other comprehensive loss | ( | ( | |||||||||
| Retained earnings | |||||||||||
| Total stockholders’ equity | |||||||||||
| Total liabilities and stockholders’ equity | $ | $ | |||||||||
See accompanying notes to these consolidated financial statements.
LIGAND PHARMACEUTICALS INCORPORATED
50
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share amounts)
| Year Ended December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Revenues: | |||||||||||||||||
| Royalties | $ | $ | $ | ||||||||||||||
| Captisol | |||||||||||||||||
| Contract revenue | |||||||||||||||||
| Total revenues | |||||||||||||||||
| Operating costs and expenses: | |||||||||||||||||
Cost of Captisol | |||||||||||||||||
| Amortization of intangibles | |||||||||||||||||
| Research and development | |||||||||||||||||
| General and administrative | |||||||||||||||||
| Other operating (income) expense | ( | ||||||||||||||||
| Total operating costs and expenses | |||||||||||||||||
| Gain from sale of Vernalis R&D | |||||||||||||||||
| Income from continuing operations | |||||||||||||||||
| Other income (expense): | |||||||||||||||||
| Gain (loss) from short-term investments | ( | ( | |||||||||||||||
| Interest income | |||||||||||||||||
| Interest expense | ( | ( | ( | ||||||||||||||
| Other income (expense), net | ( | ||||||||||||||||
| Total other expense, net | ( | ( | |||||||||||||||
| Income before income tax from continuing operations | |||||||||||||||||
| Income tax benefit (expense) | ( | ||||||||||||||||
| Net income (loss) from continuing operations | ( | ||||||||||||||||
| Net loss from discontinued operations | ( | ( | ( | ||||||||||||||
| Net income (loss): | $ | ( | $ | $ | ( | ||||||||||||
| Basic net income (loss) from continuing operations per share | $ | ( | $ | $ | |||||||||||||
| Basic net loss from discontinued operations per share | $ | ( | $ | ( | $ | ( | |||||||||||
| Basic net income (loss) per share | $ | ( | $ | $ | ( | ||||||||||||
| Shares used in basic per share calculation | |||||||||||||||||
| Diluted net income (loss) from continuing operations per share | $ | ( | $ | $ | |||||||||||||
| Diluted net loss from discontinued operations per share | $ | ( | $ | ( | $ | ( | |||||||||||
| Diluted net income (loss) per share | $ | ( | $ | $ | ( | ||||||||||||
| Shares used in diluted per share calculation | |||||||||||||||||
See accompanying notes to these consolidated financial statements.
51
LIGAND PHARMACEUTICALS INCORPORATED
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(in thousands)
| Year Ended December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Net income (loss) | $ | ( | $ | $ | ( | ||||||||||||
| Unrealized net gain (loss) on available-for-sale securities, net of tax | ( | ( | ( | ||||||||||||||
| Foreign currency translation adjustment | ( | ||||||||||||||||
| Comprehensive income (loss) | $ | ( | $ | $ | ( | ||||||||||||
See accompanying notes to these consolidated financial statements.
52
LIGAND PHARMACEUTICALS INCORPORATED
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
(in thousands)
| Common Stock | Additional paid-in capital | Accumulated other comprehensive income (loss) | Retain earnings | Total stockholders’ equity | |||||||||||||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||||||||||||
| Balance at December 31, 2019 | $ | $ | $ | ( | $ | $ | |||||||||||||||||||||||||||||
| Issuance of common stock under employee stock compensation plans, net | — | — | — | ||||||||||||||||||||||||||||||||
| Share-based compensation | — | — | — | — | |||||||||||||||||||||||||||||||
| Repurchase of common stock | ( | ( | ( | — | — | ( | |||||||||||||||||||||||||||||
| Unrealized net loss on available-for-sale securities, net of deferred tax | — | — | — | ( | — | ( | |||||||||||||||||||||||||||||
| Foreign currency translation adjustment | — | — | — | ( | — | ( | |||||||||||||||||||||||||||||
| Reacquisition of equity due to 2023 debt extinguishment, net of tax | — | — | ( | — | — | ( | |||||||||||||||||||||||||||||
| — | — | — | — | ( | ( | ||||||||||||||||||||||||||||||
| Other tax adjustments | — | — | — | — | |||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( | ( | |||||||||||||||||||||||||||||
| Balance at December 31, 2020 | ( | ||||||||||||||||||||||||||||||||||
| Issuance of common stock under employee stock compensation plans, net | — | — | |||||||||||||||||||||||||||||||||
| Share-based compensation | — | — | — | — | |||||||||||||||||||||||||||||||
| Unrealized net loss on available-for-sale securities, net of deferred tax | — | — | — | ( | — | ( | |||||||||||||||||||||||||||||
| Reacquisition of equity due to 2023 debt extinguishment, net of tax | — | — | ( | — | — | ( | |||||||||||||||||||||||||||||
| Warrant and bond hedge unwind transactions | — | — | — | — | |||||||||||||||||||||||||||||||
| Net income | — | — | — | — | |||||||||||||||||||||||||||||||
| Balance at December 31, 2021 | ( | ||||||||||||||||||||||||||||||||||
| — | — | ( | — | ( | |||||||||||||||||||||||||||||||
| Issuance of common stock under employee stock compensation plans, net | — | ( | — | — | ( | ||||||||||||||||||||||||||||||
| Share-based compensation | — | — | — | — | |||||||||||||||||||||||||||||||
| Unrealized net loss on available-for-sale securities, net of deferred tax | — | — | — | ( | — | ( | |||||||||||||||||||||||||||||
| Bond hedge transaction | — | — | — | — | |||||||||||||||||||||||||||||||
| Distribution of OmniAb | — | — | ( | — | — | ( | |||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( | ( | |||||||||||||||||||||||||||||
| Balance at December 31, 2022 | $ | $ | $ | ( | $ | $ | |||||||||||||||||||||||||||||
See accompanying notes to these consolidated financial statements.
53
LIGAND PHARMACEUTICALS INCORPORATED
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| Year Ended December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Operating activities | |||||||||||||||||
| Net income (loss) | $ | ( | $ | $ | ( | ||||||||||||
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: | |||||||||||||||||
| Gain from sale of Vernalis R&D | ( | ||||||||||||||||
| Change in estimated fair value of contingent liabilities | ( | ( | |||||||||||||||
| Depreciation of fixed assets and amortization of intangible assets | |||||||||||||||||
| Loss (gain) short-term investments | ( | ||||||||||||||||
Amortization/accretion of premium (discount) on investments, net | |||||||||||||||||
| Amortization of debt discount and issuance fees | |||||||||||||||||
| Loss (gain) on debt extinguishment | ( | ||||||||||||||||
| Amortization of commercial license and other economic rights | ( | ( | |||||||||||||||
| Lease amortization expense | |||||||||||||||||
| Share-based compensation | |||||||||||||||||
| Deferred income taxes, net | ( | ( | |||||||||||||||
| Other | |||||||||||||||||
| Changes in operating assets and liabilities, net of acquisitions: | |||||||||||||||||
| Accounts receivable, net | ( | ( | |||||||||||||||
| Inventory | ( | ( | |||||||||||||||
| Accounts payable and accrued liabilities | ( | ( | |||||||||||||||
| Income taxes receivable | ( | ||||||||||||||||
| Deferred revenue | ( | ( | |||||||||||||||
| Other assets and liabilities | ( | ( | |||||||||||||||
| Net cash provided by operating activities | |||||||||||||||||
| Investing activities | |||||||||||||||||
Cash paid for acquisition, net of cash and restricted cash acquired | ( | ||||||||||||||||
| Purchases of property and equipment | ( | ( | ( | ||||||||||||||
| Purchases of short-term investments | ( | ( | ( | ||||||||||||||
| Proceeds from commercial license rights | |||||||||||||||||
| Proceeds from sale of short-term investments | |||||||||||||||||
| Proceeds from maturity of short-term investments | |||||||||||||||||
| Cash paid for equity method investment | ( | ( | |||||||||||||||
| Proceeds on sale of Vernalis R&D, net | |||||||||||||||||
| Other, net | ( | ( | |||||||||||||||
| Net cash provided by investing activities | |||||||||||||||||
| Financing activities | |||||||||||||||||
| Net cash transferred to OmniAb at separation | ( | ||||||||||||||||
| Repayment of debt | ( | ( | ( | ||||||||||||||
| Payments under finance lease obligations | ( | ( | ( | ||||||||||||||
| Cash paid for OmniAb transaction costs | ( | ||||||||||||||||
| Proceeds from bond hedge settlement | |||||||||||||||||
| Net proceeds from stock option exercises and ESPP | |||||||||||||||||
Taxes paid related to net share settlement of equity awards | ( | ( | ( | ||||||||||||||
| Share repurchases | ( | ||||||||||||||||
| Repurchase of warrants | ( | ||||||||||||||||
| Payments to CVR Holders | ( | ( | ( | ||||||||||||||
| Net cash used in financing activities | ( | ( | ( | ||||||||||||||
| Net increase (decrease) in cash, cash equivalents, and restricted cash | ( | ( | |||||||||||||||
54
| Cash, cash equivalents and restricted cash at beginning of year | |||||||||||||||||
| Cash, cash equivalents and restricted cash at end of year | $ | $ | $ | ||||||||||||||
| Supplemental disclosure of cash flow information | |||||||||||||||||
| Cash paid during the year: | |||||||||||||||||
| Interest paid | $ | $ | $ | ||||||||||||||
| Taxes paid | $ | $ | $ | ||||||||||||||
| Restricted cash in other current assets | $ | $ | $ | ||||||||||||||
| Supplemental schedule of non-cash investing and financing activities: | |||||||||||||||||
| Accrued inventory purchases | $ | $ | $ | ||||||||||||||
| Unrealized loss on available-for-sale investments | $ | ( | $ | ( | $ | ( | |||||||||||
| Purchase of fixed assets recorded in accounts payable | $ | $ | $ | ||||||||||||||
See accompanying notes to these consolidated financial statements.
55
Unless the context requires otherwise, references in this report to “Ligand,” “we,” “us,” the “Company,” and “our” refer to Ligand Pharmaceuticals Incorporated and its consolidated subsidiaries.
1. Basis of Presentation and Summary of Significant Accounting Policies
Business
On November 1, 2022, we completed the separation (the “Separation”) of our antibody discovery business and certain related assets and liabilities (the “OmniAb Business”) through a spin-off of OmniAb to Ligand’s shareholders of record as of October 26, 2022 on a pro rata basis (the “Distribution”) and merger (the “Merger”) of OmniAb with a wholly owned subsidiary of a separate public company, OmniAb, Inc. (formerly known as Avista Public Acquisition Corp. II (“New OmniAb”)), in a Reverse Morris Trust transaction pursuant to the Agreement and Plan of Merger, dated as of March 23, 2022 (the “Merger Agreement”), and the Separation and Distribution Agreement, dated as of March 23, 2022 (the “Separation Agreement”) (the Merger Agreement and Separation Agreement, collectively with the other related transaction documents, the “Transaction Agreements”). Pursuant to the Transaction Agreements, Ligand contributed to OmniAb cash and certain assets and liabilities constituting the OmniAb Business, including but not limited to the equity interests of Ab Initio Biotherapeutics, Inc., Crystal Bioscience, Inc., Icagen, LLC, Taurus Biosciences, LLC and xCella Biosciences, Inc.
After the spin-off of our OmniAb antibody discovery business, Ligand is a revenue-generating biopharmaceutical company focused on developing or acquiring technologies that help pharmaceutical companies discover and develop medicines. We operate in one business segment: development and licensing of biopharmaceutical assets.
Basis of Presentation and Principles of Consolidation
Our consolidated financial statements have been prepared in accordance with U.S. GAAP and include the accounts of our parent company and its wholly-owned subsidiaries. All intercompany transactions and balances have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires the use of estimates and assumptions that affect the amounts reported in the consolidated financial statements and the accompanying notes. Actual results may differ from those estimates.
Concentrations of Business Risk
Financial instruments that potentially subject us to significant concentrations of credit risk consist primarily of cash equivalents and investments. We invest excess cash principally in United States government debt securities, investment grade corporate debt securities, mutual funds and certificates of deposit. We maintain some cash and cash equivalents balances with financial institutions that are in excess of the Federal Deposit Insurance Corporation insurance limits. We have established guidelines relative to diversification and maturities that maintain safety and liquidity. These guidelines are periodically reviewed and modified to take advantage of trends in yields and interest rates.
56
Revenue from significant partners, which is defined as 10% or more of our total revenue, was as follows:
| Year ended December 31, | |||||||||||||||||
| 2022 | 2021(a) | 2020(a) | |||||||||||||||
| Partner A | |||||||||||||||||
| Partner B | |||||||||||||||||
| Partner C | < | < | < | ||||||||||||||
(a) Prior period amounts have been retrospectively adjusted to reflect the effects of the Separation.
We obtain Captisol primarily from two sites related to a single supplier, Hovione. If this supplier were not able to supply the requested amounts of Captisol from each site, and if our safety stocks of material were depleted, we would be unable to continue to derive revenues from the sale of Captisol until we obtained material from an alternative source, which could take a considerable length of time.
Cash Equivalents
Cash equivalents consist of highly liquid investments with maturities of three months or less from the date of acquisition.
Short-term Investments
Short-term investments primarily consist of investments in debt and equity securities. We classify our short-term investments as “available-for-sale”. Such investments are carried at fair value, with unrealized gains and losses on debt securities included in the statement of comprehensive income (loss), net of tax, and unrealized gains and losses on equity securities included the consolidated statement of operations. We determine the cost of investments based on the specific identification method. We determine the realized gains or losses on the sale of available-for-sale securities using the specific identification method and includes net realized gains and losses as a component of other income or expense within the consolidated statements of operations.
Debt securities consist of certificates of deposit, corporate debt securities, and securities of government-sponsored entities have effective maturities greater than three months and less than twenty-five months from the date of acquisition. Debt securities available-for-sale in an unrealized loss position are assessed for the current expected credit losses methodology. We start by assessing whether we intend to sell the security, or whether it is more likely than not that we will be required to sell the security before recovery of its amortized cost basis. If either of the criteria regarding intent or requirement to sell is met, the security’s amortized cost basis is written down to fair value through earnings. For debt securities available-for-sale that do not meet the aforementioned criteria, we evaluate whether the decline in fair value has resulted from credit losses or other factors. In making this assessment, we consider the extent to which fair value is less than amortized cost, any changes in interest rates, and any changes to the rating of the security by a rating agency, among other factors. If this assessment indicates that a credit loss exists, the present value of cash flows expected to be collected from the security is compared to the amortized cost basis of the security. If the present value of cash flows expected to be collected is less than the amortized cost basis, a credit loss exists and an allowance for credit losses is recorded, limited by the amount that the fair value is less than the amortized cost basis. Any impairment that has not been recorded through an allowance for credit losses is recognized in other comprehensive income or loss, as applicable.
57
Property and Equipment
Property and equipment are stated at cost, subject to review for impairment, and depreciated over the estimated useful lives of the assets, which generally range from to ten years , using the straight-line method. Amortization of leasehold improvements is recorded over the shorter of the lease term or estimated useful life of the related asset. Maintenance and repairs are charged to operations as incurred. When assets are sold, or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts and any gain or loss is included in operating income or expense.
Acquisitions
We first determine whether a set of assets acquired constitute a business and should be accounted for as a business combination. If the assets acquired are not a business, we account for the transaction as an asset acquisition. Business combinations are accounted for by using the acquisition method of accounting which requires us to use significant estimates and assumptions, especially with respect to intangible assets. We record the excess consideration over the aggregate fair value of tangible and intangible assets, net of liabilities assumed, as goodwill.
Under the acquisition method of accounting, we recognize separately from goodwill the identifiable assets acquired, the liabilities assumed, including contingent consideration and all contractual contingencies, generally at the acquisition date fair value. Contingent purchase consideration to be settled in cash are remeasured to estimated fair value at each reporting period with the change in fair value recorded in statement of operations. Costs that we incur to complete the business combination such as investment banking, legal and other professional fees are not considered part of consideration and we charge them to general and administrative expense as they are incurred.
Should the initial accounting for a business combination be incomplete by the end of a reporting period that falls within the measurement period, we report provisional amounts in our financial statements. During the measurement period, we adjust the provisional amounts recognized at the acquisition date to reflect new information obtained about facts and circumstances that existed as of the acquisition date that, if known, would have affected the measurement of the amounts recognized as of that date and we record those adjustments to our financial statements in the period of change, if any.
Contingent Liabilities
In connection with the acquisition of Pfenex in October 2020, we entered into a CVR agreement pursuant to which former equity holders of Pfenex received one nontransferable contractual right entitling such holder to receive $2.00 per share (or approximately $77.8 million total) in the event that Pfenex’s teriparatide injection product received notice from the FDA that such product is therapeutically equivalent with respect to FORTEO® (teriparatide injection) on or before December 31, 2021. The FDA did not provide notice of such event prior to the CVR expiration date and as a result, the Pfenex CVRs expired without payment.
In connection with the acquisition of CyDex in January 2011, we recorded a contingent liability for amounts potentially due to holders of the CyDex CVRs and former license holders. The liability is periodically assessed based on events and circumstances related to the underlying milestones, royalties and material sales.
In connection with the acquisition of Metabasis in January 2010, we issued Metabasis stockholders four tradable CVRs for each Metabasis share. The fair values of the CVRs are remeasured at each reporting date through the term of the related agreement.
Any change in fair value is recorded in our consolidated statement of operations. For additional information, see “Note (5), Fair Value Measurement and Note (8), Balance Sheet Account Details.”
Goodwill, Intangible Assets and Other Long-Lived Assets
Goodwill, which has an indefinite useful life, represents the excess of cost over fair value of net assets acquired. Goodwill is reviewed for impairment at least annually during the fourth quarter, or more frequently if an event occurs indicating the potential for impairment. During the goodwill impairment review, we assess qualitative factors to determine whether it is more
58
Commercial license and other economic rights
As of December 31, 2022 and 2021, commercial license and other economic rights consist of the following (in thousands):
| December 31, 2022 | December 31, 2021 | |||||||||||||||||||||||||||||||||||||
| Gross | Adjustments(1) | Net | Gross | Adjustments(2) | Net | |||||||||||||||||||||||||||||||||
| Aziyo and CorMatrix | $ | $ | ( | $ | $ | $ | ( | $ | ||||||||||||||||||||||||||||||
| Selexis and Dianomi | ( | ( | ||||||||||||||||||||||||||||||||||||
| Total | $ | $ | ( | $ | $ | $ | ( | $ | ||||||||||||||||||||||||||||||
(1) Amounts represent accumulated amortization to principal of $11.6 million and credit loss adjustments of $6.5 million as of December 31, 2022.
Commercial license and other economic rights as of December 31, 2022 represent a portfolio of future milestone and royalty payment rights acquired from Selexis in April 2013 and April 2015, CorMatrix in May 2016, and Dianomi in January 2019. Commercial license rights acquired are accounted for as financial assets, and other economic rights are accounted for as funded research and developments as further discussed below.
In May 2019, we entered into a development funding and royalties agreement with Novan, pursuant to which we would receive certain payments at specified milestones, as well as royalties on any future net sales of SB206, a product candidate being developed to treat molluscum contagiosum, and any other Novan products used for the treatment of molluscum (“Novan Molluscum Products”). We paid Novan an upfront payment of $12.0 million, which Novan is required to use to fund the development of SB206. We are not obligated to provide additional funding to Novan for the development or commercialization of SB206. Pursuant to the agreement, we would receive up to $20.0 million of milestone payments upon the achievement by Novan of certain regulatory milestones for SB206 or any other Novan Molluscum Product and commercial milestones. In addition to the milestone payments, Novan will pay us tiered royalties from 7.0 % to 10.0 % based on aggregate annual net sales of SB206 or any other Novan Molluscum Product in North America.
In December 2018, we entered into a development funding and royalties agreement with Palvella. Pursuant to the agreement, we will receive up to $8.0 million of milestone payments upon the achievement by Palvella of certain corporate, financing and regulatory milestones for PTX-022, a product candidate being developed to treat pachyonychia congenita. In addition to the milestone payments, Palvella will pay us tiered royalties from 5.0 % to 9.8 % based on aggregate annual worldwide net sales of any PTX-022 products, if approved, subject to Palvella’s right to reduce the royalty rates by making payments in certain circumstances. We made an upfront payment of $10.0 million, which Palvella is required to use to fund the development of PTX-022. We are not obligated to provide additional funding to Palvella for development or commercialization of PTX-022.
59
We determined the economic rights related to Novan and Palvella should be characterized as a funded research and development arrangement, thus we account for them in accordance with ASC 730-20, Research and Development Arrangement, and reduce our asset as the funds are expended by Novan and Palvella. As of December 31, 2019, Novan had used up the $12.0 million upfront payment provided by us. As such, our other economic rights related to Novan had been fully amortized as of December 31, 2019. As of December 31, 2020, the fund has been fully expended by Palvella and our cost basis for the asset has been reduced to zero , and therefore we will recognize milestones and royalties as revenue when earned. During 2020, we recorded a $3.0 million milestone from Palvella under contract revenue, which has been included in our consolidated statement of operations for the year ended December 31, 2020.
In May 2017, we entered into a royalty agreement with Aziyo pursuant to which we will receive royalties from certain marketed products that Aziyo acquired from CorMatrix. Pursuant to the agreement, we received $10.0 million in 2017 from Aziyo to buydown the royalty rates on the products CorMatrix sold to Aziyo. The agreement closed on May 31, 2017, in connection with the closing of the asset sale from CorMatrix to Aziyo (the “CorMatrix Asset Sale”). Per the agreement, we will receive a 5 % royalty on the products Aziyo acquired in the CorMatrix Asset Sale, reduced from the original 20 % royalty from CorMatrix pursuant to the previously disclosed interest purchase agreement, dated May 3, 2016 (the “Original Interest Purchase Agreement”) between CorMatrix and us. In addition, Aziyo has agreed to pay us up to $10.0 million of additional milestones tied to cumulative net sales of the products Aziyo acquired in the CorMatrix Asset Sale and to extend the term on these royalties by one year. The royalty agreement will terminate on May 31, 2027. In addition, in May 2017, we entered into an amended and restated interest purchase agreement (the “Amended Interest Purchase Agreement”) with CorMatrix, which supersedes in its entirety the Original Interest Purchase Agreement. Other than removing the commercial products sold to Aziyo in the CorMatrix Sale, the terms of the Amended Interest Purchase Agreement remain unchanged with respect to the CorMatrix developmental pipeline products, including the royalty rate of 5 % on such pipeline products. The Amended Interest Purchase Agreement will terminate 10 years from the date of the first commercial sale of such products.
We account for the Aziyo commercial license right as a financial asset in accordance with ASC 310, Receivables, and amortize the commercial license right using the effective interest method whereby we forecast expected cash flows over the term of the arrangement to arrive at an annualized effective interest. The annual effective interest associated with the forecasted cash flows from the royalty agreement with Aziyo as of December 31, 2022 is 19.0 %. Revenue is calculated by multiplying the carrying value of the commercial license right by the effective interest. The payments received in the year ended December 31, 2022 and 2021 were allocated accordingly between revenue and the amortization of the commercial license rights.
Prior to 2020, we accounted for commercial license rights related to developmental pipeline products such as Selexis and Dianomi on a non-accrual basis. These developmental pipeline products are non-commercialized, non-approved products that require FDA or other regulatory approval, and thus have uncertain cash flows. The developmental pipeline products are on a non-accrual basis as we are not yet able to forecast future cash flows given their pre-commercial stages of development. We will prospectively update the yield model under the effective interest method once the underlying products are commercialized and we can reliably forecast expected cash flows. Income will be calculated by multiplying the carrying value of the commercial license right by the effective interest rate. We regularly perform reviews to determine if any event has occurred that may indicate the carrying value of these commercial license rights are potentially impaired. If the affected commercial license rights are not recoverable, we estimate the fair value of the assets and record an impairment loss if the carrying value of the assets exceeds the fair value. During 2020, given the expected cash flow from the Selexis program, we started to account for the Selexis commercial license right as a financial asset in accordance with ASC 310, and amortize the commercial license right using the effective interest method whereby we forecast expected cash flows over the term of the arrangement to arrive at an annualized effective interest. The annual effective interest associated with the forecasted cash flows from the royalty agreement with Selexis as of December 31, 2022 is 24.6 %. Revenue is calculated by multiplying the carrying value of the commercial license right by the effective interest. The payments received in the year ended December 31, 2022 and 2021 were allocated accordingly between revenue and the amortization of the commercial license rights. We still accounted for commercial license rights related to Dianomi on a non-accrual basis as of December 31, 2022.
For commercial license rights, we have elected a prospective approach to account for changes in estimated cash flows and selected a method for determining when an impairment would be recognized and how to measure that impairment. In circumstances where our new estimate of expected cash flows is greater than previously expected, we will update our yield prospectively. In circumstances where our new estimate of expected cash flows is less than previously expected and below our original estimated yield we record an impairment. Impairment is recognized by reducing the financial asset to an amount that represents the present value of our most recent estimate of expected cash flows discounted by the original effective interest rate. In circumstances where our new estimate of expected cash flows is less than previously expected, but not below our original estimated yield, we update our yield prospectively.
As a result of adopting ASU 2016-13, Financial Instruments – Credit Losses: Measurement of Credit losses on Financial Instruments (Topic 326), we now recognize an allowance for current expected credit losses on the commercial license rights subject to credit risk. We recorded a $5.5 million pre-tax reserve for credit losses upon adoption of the standard on January 1,
60
Revenue Recognition
Our revenue is generated primarily from royalties on sales of products commercialized by our partners, Captisol material sales, and contract revenue for services, license fees and development, regulatory and sales based milestone payments.
We apply the following five-step model in accordance with ASC 606, Revenue from Contracts with Customers, in order to determine the revenue: (i) identification of the promised goods or services in the contract; (ii) determination of whether the promised goods or services are performance obligations, including whether they are distinct in the context of the contract; (iii) measurement of the transaction price, including the constraint on variable consideration; (iv) allocation of the transaction price to the performance obligations; and (v) recognition of revenue when (or as) the Company satisfies each performance obligation.
Royalties
We receive royalty revenue on sales by our partners of products covered by patents that we or our partners own under contractual agreements. We do not have future performance obligations under these license arrangements. We generally satisfy our obligation to grant intellectual property rights on the effective date of the contract. However, we apply the royalty recognition constraint required under the guidance for sales-based royalties which requires a royalty to be recorded no sooner than the underlying sale occurs. Therefore, royalties on sales of products commercialized by our partners are recognized in the quarter the product is sold. Our partners generally report sales information to us on a one quarter lag. Thus, we estimate the expected royalty proceeds based on an analysis of historical experience and interim data provided by our partners including their publicly announced sales. Differences between actual and estimated royalty revenues are adjusted in the period in which they become known, typically the following quarter.
Captisol Sales
Revenue from Captisol sales is recognized when control of Captisol material or intellectual property license rights is transferred to our customers in an amount that reflects the consideration we expect to receive from our customers in exchange for those products. A performance obligation is considered distinct from other obligations in a contract when it provides a benefit to the customer either on its own or together with other resources that are readily available to the customer and is separately identified in the contract. For Captisol material, we consider our performance obligation is satisfied at a point in time, once we have transferred control of the product, meaning the customer has the ability to use and obtain the benefit of the Captisol material or intellectual property license right. We recognize revenue for satisfied performance obligations only when we determine there are no uncertainties regarding payment terms or transfer of control. Sales tax and other taxes we collect concurrent with revenue-producing activities are excluded from revenue. We have elected to recognize the cost of freight and shipping when control over Captisol material has transferred to the customer as an expense in Cost of Captisol. We expense incremental costs of obtaining a contract when incurred if the expected amortization period of the asset that we would have recognized is one year or less or the amount is immaterial. We did not incur any incremental costs of obtaining a contract during the periods reported.
Contract Revenue
Our contracts with customers often will include variable consideration in the form of contingent milestone-based payments. We include contingent milestone based payments in the estimated transaction price when it is probable a significant reversal in the amount of cumulative revenue recognized will not occur. These estimates are based on historical experience, anticipated results and our best judgment at the time. If the contingent milestone based payment is sales-based, we apply the royalty recognition constraint and record revenue when the underlying sale has taken place. Significant judgments must be made in determining the transaction price for our sales of intellectual property. Because of the risk that products in development with our partners will not reach development based milestones or receive regulatory approval, we generally recognize any contingent payments that would be due to us upon the development milestone or regulatory approval. Depending on the terms of the arrangement, we may also defer a portion of the consideration received if we have to satisfy a future obligation, which typically occurs with our contracts for R&D services.
For R&D services we recognize revenue over time and we measure our progress using an input method. The input methods we use are based on the effort we expend or costs we incur toward the satisfaction of our performance obligation. We estimate the amount of effort we expend, including the time it will take us to complete the activities, or the costs we may incur in a given
61
period, relative to the estimated total effort or costs to satisfy the performance obligation. This results in a percentage that we multiply by the transaction price to determine the amount of revenue we recognize each period. This approach requires us to make numerous estimates and use significant judgement. If our estimates or judgements change over the course of the collaboration, they may affect the timing and amount of revenue that we recognize in the current and future periods.
We occasionally have sub-license obligations related to arrangements for which we receive license fees, milestones and royalties. We evaluate the determination of gross as a principal versus net as an agent reporting based on each individual agreement.
Deferred Revenue
Depending on the terms of the arrangement, we may also defer a portion of the consideration received if we have to satisfy a future obligation.
Disaggregation of Revenue
Royalty revenue for 2022, 2021 and 2020 for continuing operations are reported as below (in thousands):
| Year ended December 31, | ||||||||||||||||||||
| 2022 | 2021 | 2020 | ||||||||||||||||||
| Kyprolis | $ | $ | $ | |||||||||||||||||
| Evomela | ||||||||||||||||||||
| Teriparatide injection | ||||||||||||||||||||
| Rylaze | ||||||||||||||||||||
| Other | ||||||||||||||||||||
| $ | $ | $ | ||||||||||||||||||
The following table represents disaggregation of Captisol and contract revenue for continuing operations (in thousands):
| Year ended December 31, | ||||||||||||||||||||
| 2022 | 2021 | 2020 | ||||||||||||||||||
| Captisol | ||||||||||||||||||||
| Captisol - Core | $ | $ | $ | |||||||||||||||||
Captisol - COVID(a) | ||||||||||||||||||||
| $ | $ | $ | ||||||||||||||||||
| Contract | ||||||||||||||||||||
| Service Revenue | $ | $ | $ | |||||||||||||||||
| License Fees | ||||||||||||||||||||
| Milestone | ||||||||||||||||||||
| Other | ||||||||||||||||||||
| $ | $ | $ | ||||||||||||||||||
(a) Captisol - COVID represents revenue on Captisol supplied for use in formulation with remdesivir, an antiviral treatment for COVID-19.
62
Share-Based Compensation
We incur share-based compensation expense related to restricted stock, ESPP, and stock options.
Restricted stock unit (RSU) and performance stock unit (PSU) are all considered restricted stock. The fair value of restricted stock is determined by the closing market price of our common stock on the date of grant. We recognize share-based compensation expense based on the fair value on a straight-line basis over the requisite service periods of the awards, taking into consideration of forfeitures as they occur. PSU generally represents a right to receive a certain number of shares of common stock based on the achievement of corporate performance goals and continued employment during the vesting period. At each reporting period, we reassess the probability of the achievement of such corporate performance goals and any expense change resulting from an adjustment in the estimated shares to be released are treated as a cumulative catch-up in the period of adjustment. A limited amount of PSUs contain a market condition dependent upon the Company’s relative and absolute total stockholder return over a three-year period, with a range of 0 % to 200 % of the target amount granted to be issued under the award. Share-based compensation expense for these PSUs is measured using the Monte-Carlo simulation valuation model and is not adjusted for the achievement, or lack thereof, of the market conditions.
The Black-Scholes-Merton option-pricing model is used to estimate the fair value of stock purchases under our ESPP and stock options granted. The model assumptions include expected volatility, term, dividends, and the risk-free interest rate. We look to historical and implied volatility of our stock to determine the expected volatility. The expected term of an award is based on historical forfeiture experience, exercise activity, and on the terms and conditions of the stock awards. The expected dividend yield is determined to be 0 % given that except for 2007, during which we declared a cash dividend on our common stock of $2.50 per share, we have not paid any dividends on our common stock in the past and currently do not expect to pay cash dividends or make any other distributions on common stock in the future. The risk-free interest rate is based upon U.S. Treasury securities with remaining terms similar to the expected term of the share-based awards.
We grant options, RSUs and PSUs to employees and non-employee directors. Non-employee directors are accounted for as employees. Options and RSUs granted to certain non-employee directors typically vest one year from the date of grant. Options granted to employees typically vest 1/8 on the six month anniversary of the date of grant, and 1/48 each month thereafter for forty-two months . RSUs and PSUs granted to employees vest over three years . All option awards generally expire ten years from the date of grant.
Income Taxes
The provision for income taxes is computed using the asset and liability method, under which deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the financial reporting and tax bases of assets and liabilities, and for the expected future tax benefit to be derived from tax loss and credit carryforwards. Deferred tax assets and liabilities are determined using the enacted tax rates in effect for the years in which those tax assets are expected to be realized. The effect of a change in tax rates on deferred tax assets and liabilities is recognized in the provision for income taxes in the period that includes the enactment date.
Deferred tax assets are regularly assessed to determine the likelihood they will be recovered from future taxable income. A valuation allowance is established when we believe it is more likely than not the future realization of all or some of a deferred tax asset will not be achieved. In evaluating the ability to recover deferred tax assets within the jurisdiction which they arise we consider all available positive and negative evidence. Factors reviewed include the cumulative pre-tax book income for the past three years, scheduled reversals of deferred tax liabilities, history of earnings and reliable forecasting, projections of pre-tax book income over the foreseeable future, and the impact of any feasible and prudent tax planning strategies.
We recognize the impact of a tax position in our financial statements only if that position is more likely than not of being sustained upon examination by taxing authorities, based on the technical merits of the position. Tax authorities regularly examine our returns in the jurisdictions in which we do business and we regularly assess the tax risk of our return filing positions. Due to the complexity of some of the uncertainties, the ultimate resolution may result in payments that are materially
63
Income (Loss) Per Share
Basic income (loss) per share is calculated by dividing net income (loss) by the weighted-average number of common shares outstanding during the period. Diluted income per share is computed based on the sum of the weighted average number of common shares and potentially dilutive common shares outstanding during the period. Diluted loss per share is computed based on the sum of the weighted average number of common shares outstanding during the period.
Potentially dilutive common shares consist of shares issuable under the 2023 Notes, stock options and restricted stock. The 2023 Notes have a dilutive impact when the average market price of the Company’s common stock exceeds the applicable conversion price of the respective notes. It is our intent and policy to settle conversions through combination settlement, which essentially involves payment in cash equal to the principal portion and delivery of shares of common stock for the excess of the conversion value over the principal portion. Potentially dilutive common shares from stock options and restricted stock are determined using the average share price for each period under the treasury stock method. In addition, the following amounts are assumed to be used to repurchase shares: proceeds from exercise of stock options and the average amount of unrecognized compensation expense for stock options and restricted stock. In loss periods, basic net loss per share and diluted net loss per share are identical since the effect of otherwise dilutive potential common shares is anti-dilutive and therefore excluded.
| Year Ended December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Weighted average shares outstanding: | |||||||||||||||||
| Dilutive potential common shares: | |||||||||||||||||
| Restricted stock | |||||||||||||||||
| Stock options | |||||||||||||||||
| Shares used to compute diluted income per share | |||||||||||||||||
| Potentially dilutive shares excluded from calculation due to anti-dilutive effect | |||||||||||||||||
Comprehensive Income (Loss)
Comprehensive income (loss) represents net income (loss) adjusted for the change during the periods presented in unrealized gains and losses on available-for-sale debt securities, foreign currency translation adjustments, and reclassification adjustments for realized gains or losses included in net income (loss). The unrealized gains or losses are reported on the Consolidated Statements of Comprehensive Income (Loss).
Foreign Currency Translation
The British Pound Sterling was the functional currency of our subsidiary, Vernalis, which was sold in fourth quarter of the year ended December 31, 2020. For the year ended December 31, 2020, the corresponding financial statements have been translated into U.S. Dollars in accordance with ASC 830-30, Translation of Financial Statements. Assets and liabilities are translated at end-of-period rates while revenues and expenses are translated at average rates in effect during the period in which the activity took place. Equity is translated at historical rates and the resulting cumulative translation adjustments are included as a component of accumulated other comprehensive income (loss).
64
In August 2020, the FASB issued ASU 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”). The guidance simplifies the complexity associated with applying U.S. GAAP for certain financial instruments with characteristics of liabilities and equity. More specifically, the amendments focus on the guidance for convertible instruments and derivative scope exception for contracts in an entity’s own equity. Consequently, a convertible debt instrument, such as the Company’s 2023 Notes, will be accounted for as a single liability measured at its amortized cost, if no other features require bifurcation and recognition as derivatives. The new guidance also requires the if-converted method to be applied for all convertible instruments and requires additional disclosures. ASU 2020-06 is effective for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years.
We adopted this guidance effective January 1, 2022 under the modified retrospective approach and the comparative information has not been restated and continues to be presented according to accounting standards in effect for those periods. The cumulative effect of the change was recognized as an adjustment to the opening balance of retained earnings at the date of adoption and our 2023 Notes are no longer bifurcated into separate liability and equity components. The principal amount of the 2023 Notes is classified as a single liability measured at amortized cost in the consolidated balance sheet for the period ended December 31, 2022. Upon adoption of ASU 2020-06 on January 1, 2022, we recorded an adjustment to the 2023 Notes liability component, deferred tax liabilities, additional paid-in-capital and retained earnings. This adjustment was calculated based on the carrying amount of the 2023 Notes as if it had always been treated as a single liability measured at amortized cost. Furthermore, we recorded an adjustment to the debt issuance costs contra liability and equity (additional paid-in-capital) components under the same premise, as if debt issuance costs had always been treated as a contra liability only. Under this transition method, the cumulative effect of the accounting change increased the carrying amount of the 2023 Notes by $20.4 million, reduced deferred tax liabilities by $4.4 million, reduced additional paid-in capital by $51.1 million and increased retained earnings by $35.1 million. The net balance of the 2023 Notes at January 1, 2022 was $341.1 million which included an unamortized discount of $2.2 million.
Accounting Standards Not Yet Adopted
We do not believe that any other recently issued, but not yet effective accounting pronouncements, if adopted, would have a material impact on our consolidated financial statements or disclosures.
2. Spin-off of OmniAb
On March 23, 2022, we entered into the Separation Agreement to separate our OmniAb Business and the Merger Agreement, pursuant to which Avista Public Acquisition Corp. II (“APAC”) would combine with OmniAb, and acquire Ligand's OmniAb Business, in a Reverse Morris Trust transaction (collectively, the “Transactions”). In connection with the execution of the Merger Agreement, we made organizational changes to better align our organizational structure with our strategy and operations, and management reorganized the reportable segments to better reflect how the business is evaluated by the chief operating decision maker. Beginning in the first quarter of 2022, we operated the following two reportable segments: (1) OmniAb business and (2) Ligand core business. The OmniAb business segment is focused on enabling the discovery of therapeutic candidates for our partners by pairing antibody repertoires generated from our proprietary transgenic animals with our OmniAb business platform screening tools. The Ligand core business segment is a biopharmaceutical business focused on developing or acquiring technologies that help pharmaceutical companies deliver and develop medicines. We performed a fair value analysis utilizing a combination of income approach and market approach to determine the fair value of each segment in order to appropriately allocate the goodwill between the segments as of the announcement date. We reassigned goodwill using a relative fair value allocation method, which resulted in goodwill of a $105.7 million and $75.5 million being allocated to Ligand core business and OminAb business, respectively.
After the closing date of the Transactions on November 1, 2022, the historical financial results of OmniAb have been reflected in our consolidated financial statements as discontinued operations under GAAP for all periods presented through the date of the Distribution. Pursuant to the Transaction Agreements, Ligand contributed to OmniAb cash and certain specific assets and liabilities constituting the OmniAb Business. Pursuant to the Distribution, Ligand distributed on a pro rata basis to its shareholders as of October 26, 2022 shares of the common stock of OmniAb representing 100 % of Ligand’s interest in OmniAb. Immediately following the Distribution, Orwell Merger Sub Inc., a wholly-owned subsidiary of APAC, merged with and into OmniAb, with OmniAb continuing as the surviving company in the Merger and as a wholly owned subsidiary of New OmniAb. The entire transaction was completed on November 1, 2022, and following the Merger, New OmniAb is an independent, publicly traded company whose common stock trades on NASDAQ under the symbol “OABI.” After the Distribution, we do not beneficially own any shares of common stock in OmniAb and no longer consolidate OmniAb into our financial results for periods ending after November 1, 2022.
65
The transfer of assets and liabilities to OmniAb was effected through a contribution in accordance with the Merger Agreement, as summarized below (in thousands):
| As of November 1, 2022 | |||||
| ASSETS | |||||
| Current assets: | |||||
Cash and cash equivalents | $ | ||||
| Other current assets | |||||
| Total current assets | |||||
| Intangible assets, net | |||||
| Goodwill | |||||
| Property and equipment, net | |||||
| Operating lease assets | |||||
| Other assets | |||||
| Total assets | $ | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||
| Current liabilities: | |||||
| Current contingent liabilities | $ | ||||
| Deferred revenue | |||||
| Current operating lease liabilities | |||||
| Current finance lease liabilities | |||||
| Total current liabilities | |||||
| Long-term contingent liabilities | |||||
| Deferred income taxes, net | |||||
| Long-term operating lease liabilities | |||||
| Other long-term liabilities | |||||
| Total liabilities | |||||
| Net assets transferred to OmniAb | $ | ||||
Discontinued operations
In connection with the Merger, the Company determined its antibody discovery business qualified for discontinued operations accounting treatment in accordance with ASC 205-20. The following table summarizes revenue and expenses of the discontinued operations for the years ended December 31, 2022, 2021 and 2020 (in thousands):
| Year Ended December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Revenues: | |||||||||||||||||
| Royalties | $ | $ | $ | ||||||||||||||
| Contract revenue | |||||||||||||||||
| Total revenues | |||||||||||||||||
| Operating costs and expenses: | |||||||||||||||||
| Amortization of intangibles | |||||||||||||||||
| Research and development | |||||||||||||||||
| General and administrative | |||||||||||||||||
| Total operating costs and expenses | |||||||||||||||||
| Loss from operations | ( | ( | ( | ||||||||||||||
| Other income (expense): | |||||||||||||||||
| Gain from short-term investments | |||||||||||||||||
| Interest expense | ( | ( | |||||||||||||||
| Other income (expense), net | ( | ( | |||||||||||||||
| Total other expense, net | ( | ||||||||||||||||
| Loss before income tax | ( | ( | ( | ||||||||||||||
| Income tax benefit | |||||||||||||||||
| Net loss | $ | ( | $ | ( | $ | ( | |||||||||||
66
There were no assets and liabilities related to discontinued operations as of December 31, 2022, as all balances were transferred to OmniAb upon Separation. The following table summarizes the assets and liabilities of the discontinued operations as of December 31, 2021 (in thousands):
| December 31, 2021 | |||||
| ASSETS | |||||
| Current assets: | |||||
| Other current assets | $ | ||||
| Total current assets of discontinued operations | |||||
| Intangible assets, net | |||||
| Goodwill | |||||
| Property and equipment, net | |||||
| Operating lease assets | |||||
| Finance lease assets | |||||
| Other assets | |||||
| Total assets of discontinued operations | $ | ||||
| LIABILITIES | |||||
| Current liabilities: | |||||
| Current contingent liabilities | $ | ||||
| Deferred revenue | |||||
| Current operating lease liabilities | |||||
| Current finance lease liabilities | |||||
| Total current liabilities of discontinued operations | |||||
| Long-term contingent liabilities | |||||
| Deferred income taxes, net | |||||
| Long-term operating lease liabilities | |||||
| Other long-term liabilities | |||||
| Total liabilities of discontinued operations | $ | ||||
The following table summarizes the significant non-cash items, capital expenditures of the discontinued operations, and financing activities that are included in the consolidated statements of cash flows for the years ended December 31, 2022, 2021 and 2020 (in thousands):
| Year Ended December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Operating activities: | |||||||||||||||||
| Change in fair value of contingent consideration | $ | ( | $ | $ | |||||||||||||
| Depreciation and amortization | |||||||||||||||||
| Stock-based compensation expense | |||||||||||||||||
| Investing activities: | |||||||||||||||||
Cash paid for acquisition, net of cash acquired | $ | $ | $ | ( | |||||||||||||
| Purchase of property, plant and equipment | ( | ( | ( | ||||||||||||||
| Payments to CVR Holders | ( | ( | |||||||||||||||
| Financing activities: | |||||||||||||||||
| Payments to CVR Holders | $ | ( | $ | ( | $ | ( | |||||||||||
| Supplemental cash flow disclosures: | |||||||||||||||||
| Purchases of property, plant and equipment included in accounts payable and accrued expenses | $ | $ | $ | ||||||||||||||
3. Short-term Investments: Investment in Viking
67
is considered a related party as we maintain a seat on Viking's board of directors and we do not exert significant influence over Viking.
As of December 31, 2022 and December 31, 2021, we recorded our common stock in Viking in “short-term investments” at fair value of $63.1 million and $30.9 million, respectively. At December 31, 2020, we owned warrants to purchase up to 1.5 million shares of Viking's common stock at an exercise price of $1.50 per share, and during the year ended December 31, 2021 we exercised all outstanding Viking warrants. As of December 31, 2022 and December 31, 2021, we have zero 0.6 million Viking shares. See further discussion in “Note (5), Fair Value Measurement.”
4. Acquisitions
Excluding the impact of OmniAb, we completed one acquisition from January 1, 2020 through December 31, 2022, where we applied the acquisition method of accounting for business combinations. Accordingly, we recorded the tangible and intangible assets acquired and liabilities assumed at their estimated fair values as of the applicable date of acquisition.
Pfenex Acquisition
On October 1, 2020, we acquired Pfenex, which develops next-generation and novel protein therapeutics to improve existing therapies and create new therapies for biological targets linked to critical, unmet diseases using a protein expression technology platform.
The purchase price of $465.1 million included $429.6 million cash consideration paid upon acquisition, and a contingent CVR payment of up to $77.8 million in cash based on a certain specified milestone with an estimated initial fair value of $37.0 million. The CVR will only be paid in full if the milestone is achieved by December 31, 2021. The amount of the CVR included in purchase price was reduced by $1.5 million which was determined to be post-combination expense. The fair value of the CVR liability was determined using a probability adjusted income approach. These cash flows were then discounted to present value using a discount rate based on market participants' cost of debt reflective of the Company, which was 7.1 %. The liability is periodically assessed based on events and circumstances related to the underlying milestone, and any change in fair value is recorded in our consolidated statements of operations. During the year ended December 31, 2021, we wrote off the entire CVR liability of $37.6 million to , primarily due to not achieving the specific development and regulatory milestone by December 31, 2021 as defined by Pfenex CVR.
In connection with the acquisition, a portion of Pfenex's equity awards that were outstanding and unvested prior to the acquisition became fully vested per the terms of the merger agreement. The acceleration of vesting required us to allocate the fair value of the equity attributable to pre-combination service to the purchase price and the remaining amount was considered our post-combination expense. We paid $17.3 million in cash for equity compensation, which is attributable to pre-combination services and is reflected as a component of the total purchase price paid of $429.6 million. In addition, the fair value of equity compensation attributable to the post-combination service period was $8.7 million. These amounts were associated with the accelerated vesting of stock options previously granted to Pfenex employees and were fully paid in cash, which was recognized as general and administrative expenses during the fourth quarter of 2020.
We recorded $20.7 million of acquisition-related costs for legal, severance and other costs in connection with the acquisition within operating expenses in our consolidated statement of operations for 2020. The following table sets forth an allocation of the purchase price to the identifiable tangible and intangible assets acquired and liabilities assumed, with the excess recorded to goodwill (in thousands):
68
| Cash | $ | ||||
| Restricted cash | |||||
| Accounts and unbilled receivables | |||||
| Property and equipment, net | |||||
| Right-of-use asset | |||||
| Other assets | |||||
| Intangibles acquired | |||||
Goodwill(1) | |||||
| Accounts payable | ( | ||||
| Accrued liabilities | ( | ||||
| Deferred revenue | ( | ||||
| Lease liabilities | ( | ||||
| Other liabilities | ( | ||||
| Deferred tax liabilities, net | ( | ||||
| Total consideration | $ | ||||
The intangibles acquired and their weighted average useful life are as follows (in thousands, except useful lives):
| Approximate Fair Value | Estimated useful life (in years) | |||||||
| Contractual Relationships: | ||||||||
| Alvogen | $ | |||||||
| Merck | ||||||||
| Jazz | ||||||||
| SII | ||||||||
| Arcellx | ||||||||
| Acquired Technologies | ||||||||
| $ | ||||||||
The fair values of the contractual relationships were based on the discounted cash flow method that estimated the present value of the potential royalties, milestones and collaboration revenue streams derived from the licensing of the related technologies over the estimated contractual relationship period. The fair values of the acquired technologies were based on the discounted cash flow method that estimated the present value of the potential royalties, milestones, collaboration and product revenue streams derived from the licensing of the related technologies over the estimated useful lives. These projected cash flows were discounted to present value using discount rate, which varies from 12 % to 15 %. The intangible assets acquired are being amortized on a straight-line basis over the estimated useful life.
Approximately $2.0 million of revenue and $19.3 million of loss before income taxes of Pfenex were included in the consolidated statement of operations for the year ended December 31, 2020. The following summary presents our unaudited pro forma consolidated results of operations for the years ended December 31, 2020 and December 31, 2019 as if the Pfenex acquisition had occurred on January 1, 2019, which gives effect to certain transaction accounting adjustments, including amortization of acquired intangibles and stock based compensation expense for retained Pfenex employees. The transaction accounting adjustments do not include non-recurring adjustments related to Pfenex's executive salary, board of director compensation, and salary of Pfenex employees involved in the reduction of force as part of the acquisition, estimated to be $7.1 million in 2020 and $4.8 million in 2019. The pro forma financial information is not necessarily indicative of the operating results that would have occurred had the acquisition been consummated as if the date indicated, nor is it necessarily indicative of future operating results (in thousands, except per share amounts):
69
| Year Ended December 31, | ||||||||
| (Unaudited) | 2020 | 2019 | ||||||
| Revenue | $ | $ | ||||||
| Net Income (loss) | $ | ( | $ | |||||
| Net income (loss) per common share: | ||||||||
| Basic | $ | ( | $ | |||||
| Diluted | $ | ( | $ | |||||
5. Fair Value Measurement
We measure certain financial assets and liabilities at fair value on a recurring basis. Fair value is a market-based measurement that should be determined using assumptions that market participants would use in pricing an asset or liability. We establish a three-level hierarchy to prioritize the inputs used in measuring fair value. The levels are described in the below with level 1 having the highest priority and level 3 having the lowest:
Level 1 - Observable inputs such as quoted prices in active markets
Level 2 - Inputs other than the quoted prices in active markets that are observable either directly or indirectly
Level 3 - Unobservable inputs in which there is little or no market data, which require the Company to develop its own assumptions
70
The following table provides a summary of the assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2022 and 2021 (in thousands):
| Fair Value Measurements at Reporting Date Using | |||||||||||||||||||||||
| December 31, 2022 | Quoted Prices in Active Markets for Identical Assets | Significant Other Observable Inputs | Significant Unobservable Inputs | ||||||||||||||||||||
| Total | (Level 1) | (Level 2) | (Level 3) | ||||||||||||||||||||
| Assets: | |||||||||||||||||||||||
Short-term investments (1) | $ | $ | $ | $ | |||||||||||||||||||
Investment in Viking common stock (2) | |||||||||||||||||||||||
| Total assets | $ | $ | $ | $ | |||||||||||||||||||
| Liabilities: | |||||||||||||||||||||||
| Contingent liabilities - Cydex | $ | $ | $ | $ | |||||||||||||||||||
Contingent liabilities - Metabasis (3) | |||||||||||||||||||||||
| Liability for amounts owed to a former licensor | |||||||||||||||||||||||
| Total liabilities | $ | $ | $ | $ | |||||||||||||||||||
| Fair Value Measurements at Reporting Date Using | |||||||||||||||||||||||
December 31, 2021(a) | Quoted Prices in Active Markets for Identical Assets | Significant Other Observable Inputs | Significant Unobservable Inputs | ||||||||||||||||||||
| Total | (Level 1) | (Level 2) | (Level 3) | ||||||||||||||||||||
| Assets: | |||||||||||||||||||||||
Short-term investments (1) | $ | $ | $ | $ | |||||||||||||||||||
Investment in Viking common stock (2) | |||||||||||||||||||||||
| Total assets | $ | $ | $ | $ | |||||||||||||||||||
| Liabilities: | |||||||||||||||||||||||
| Contingent liabilities - Cydex | $ | $ | $ | $ | |||||||||||||||||||
Contingent liabilities - Metabasis (3) | |||||||||||||||||||||||
| Liability for amounts owed to a former licensor | |||||||||||||||||||||||
| Total liabilities | $ | $ | $ | $ | |||||||||||||||||||
(a) Prior period amounts have been retrospectively adjusted to reflect the effects of the Separation.
(1) Excluding our investment in Viking, our short-term investments in marketable debt and equity securities are classified as available-for-sale securities based on management's intentions and are at level 2 of the fair value hierarchy, as these investment securities are valued based upon quoted prices for identical or similar instruments in markets that are not active, and model-based valuation techniques for which all significant assumptions are observable in the market. Short-term investments in mutual funds are valued at their net asset value (NAV) on the last day of the period. We have classified marketable securities with original maturities of greater than one year as short-term investments based upon our ability and intent to use any and all of those marketable securities to satisfy the liquidity needs of our current operations. In addition, we have investment in warrants resulting from Seelos Therapeutics Inc. milestone payments that were settled in shares during the first quarter of 2019 and are at level 3 of the fair value hierarchy, based on Black Scholes value estimated by management on the last day of the period.
(2) Investment in Viking warrants, which we received as a result of Viking’s partial repayment of the Viking note receivable and our purchase of Viking common stock and warrants in April 2016, is classified as level 1 as the fair value is determined using quoted market prices in active markets for the same securities. The change of the fair value is recorded in “gain (loss) from short-term investments” in our consolidated statement of operations. See further discussion in “Note (3), Short-term Investments: Investment in Viking.”
(3) In connection with our acquisition of Metabasis in January 2010, we issued Metabasis stockholders four tradable CVRs, one CVR from each of four respective series of CVR, for each Metabasis share. The CVRs entitle Metabasis stockholders to cash payments as frequently as every six months as cash is received by us from proceeds from the sale or partnering of any of the Metabasis drug development programs, among other triggering events. The liability for the CVRs is determined using quoted prices in a market that is not active for the underlying CVR. The carrying amount of the liability may fluctuate significantly based upon quoted market prices and actual amounts paid under the agreements may be materially differ than the carrying amount of the liability. Several of the Metabasis drug development programs have been outlicensed to Viking, including VK2809. VK2809 is a novel selective TR-β agonist with potential in multiple indications, including hypercholesterolemia, dyslipidemia, NASH, and X-ALD. Under the terms of the agreement with Viking, we may be entitled to up to $375.0 million of development, regulatory and commercial milestones and tiered royalties on potential future sales including a $10.0 million payment upon initiation of a Phase 3 clinical trial.
71
A reconciliation of the level 3 financial instruments as of December 31, 2022 is as follows (in thousands):
| Liabilities | |||||
| Fair value of level 3 financial instruments as of December 31, 2021 | $ | ||||
| Fair value adjustments to contingent liabilities | ( | ||||
| Fair value of level 3 financial instruments as of December 31, 2022 | $ | ||||
A reconciliation of the level 3 financial instruments as of December 31, 2021 is as follows (in thousands):
| Liabilities | |||||
| Fair value of level 3 financial instruments as of December 31, 2020 | $ | ||||
| Payments to CVR holders and other contingency payments | ( | ||||
| Fair value adjustments to contingent liabilities | ( | ||||
| Fair value of level 3 financial instruments as of December 31, 2021 | $ | ||||
Assets Measured on a Non-Recurring Basis
We apply fair value techniques on a non-recurring basis associated with valuing potential impairment losses related to our goodwill, indefinite-lived intangible assets, and long-lived assets.
We evaluate goodwill and indefinite-lived intangible assets annually for impairment and whenever circumstances occur indicating that goodwill might be impaired. We determine the fair value of our reporting unit based on a combination of inputs, including the market capitalization of Ligand, as well as Level 3 inputs such as discounted cash flows, which are not observable from the market, directly or indirectly. We determine the fair value of our indefinite-lived intangible assets using the income approach based on Level 3 inputs.
Other than the finance lease equipment discussed in “Note (6), Leases”, there was no impairment of our goodwill, indefinite-lived assets, or long-lived assets recorded during the twelve months ended December 31, 2022.
Fair Value of Financial Instruments
In May 2018, we issued the 2023 Notes. We use quoted market rates in an inactive market, which are classified as a Level 2 input, to estimate the fair value of our 2023 Notes. The carrying value of the notes does not reflect the market rate. See “Note (7), Convertible Senior Notes” for additional information related to the fair value.
6. Leases
Finance lease
In May 2020 and January 2021, we entered into an agreement and the first amendment with Hovione, our third-party manufacturer, to increase our manufacturing of Captisol, respectively. The agreements are considered to include an embedded finance lease under ASC 842, Leases, as it provides the Company the right to use the underlying equipment to exclusively manufacture Captisol. As of December 31, 2021, we have fully paid consideration of $69.1 million for prepaid inventory and capacity ramp-up fee. We allocated consideration in the agreements between lease and non-lease components using relative standalone prices. Since the inception of the agreements, we have allocated $50.2 million of the consideration paid to the non-lease component which is accounted for as prepaid inventory and being amortized to cost of Captisol based on the usage. The remaining balance of $18.9 million was recognized as a right of use asset.
Given the current COVID status, our forecast for COVID-related Captisol has been significantly reduced, which triggered an indicator of impairment of the right of use asset as of December 31, 2022. We performed a recoverability test at the asset group level by comparing the sum of the estimated undiscounted future cash flows attributable to the asset group to its carrying value and identified the asset was impaired. We recorded a $9.8 million of impairment charge based on the fair value of the right of use asset which has been recognized in cost of Captisol in our consolidated statement of operations for the year ended December 31, 2022. As of December 31, 2022 the remaining right of use asset balance is $4.0 million which will be amortized straight-line over the remaining 6 years lease term.
72
Operating lease
We lease certain office facilities and equipment primarily under various operating leases. Our operating leases have remaining contractual terms up to ten years , some of which include options to extend the leases for up to five years . Our lease agreements do not contain any material residual value guarantees, material restrictive covenants, or material termination options. Our operating lease costs are primarily related to facility leases for administration offices and research and development facilities.
Lease assets and lease liabilities are recognized at the commencement of an arrangement where it is determined at inception that a lease exists. Lease assets represent the right to use an underlying asset for the lease term, and lease liabilities represent the obligation to make lease payments arising from the lease. These assets and liabilities are initially recognized based on the present value of lease payments over the lease term calculated using our incremental borrowing rate generally applicable to the location of the lease asset, unless the implicit rate is readily determinable. Lease assets also include any upfront lease payments made and lease incentives. Lease terms include options to extend or terminate the lease when it is reasonably certain that those options will be exercised.
In addition to base rent, certain of our operating leases require variable payments, such as insurance and common area maintenance. These variable lease costs, other than those dependent upon an index or rate, are expensed when the obligation for those payments is incurred. Leases with an initial term of 12 months or less are not recorded on the balance sheet, and the expense for these short-term leases and for operating leases is recognized on a straight-line basis over the lease term.
The depreciable life of lease assets and leasehold improvements is limited by the expected lease term, unless there is a transfer of title or purchase option reasonably certain of exercise.
Operating and Finance Lease Assets and Liabilities (in thousands):
| December 31, 2022 | December 31, 2021(a) | ||||||||||
| Assets | |||||||||||
| Operating lease assets | $ | $ | |||||||||
| Finance lease assets | |||||||||||
| Total lease assets | $ | $ | |||||||||
| Liabilities | |||||||||||
| Current operating lease liabilities | $ | $ | |||||||||
| Current finance lease liabilities | |||||||||||
| Long-term operating lease liabilities | |||||||||||
| Total lease liabilities | $ | $ | |||||||||
(a) Prior period amounts have been retrospectively adjusted to reflect the effects of the Separation.
Maturity of Operating and Finance Lease Liabilities as of December 31, 2022 (in thousands):
| Maturity Dates | Operating Leases | Finance Leases | ||||||||||||
| 2023 | $ | $ | ||||||||||||
| 2024 | ||||||||||||||
| 2025 | ||||||||||||||
| 2026 | ||||||||||||||
| 2027 | ||||||||||||||
| Thereafter | ||||||||||||||
| Total lease payments | ||||||||||||||
| Less imputed interest | ( | ( | ||||||||||||
| Present value of lease liabilities | $ | $ | ||||||||||||
As of December 31, 2022, our operating leases have a weighted-average remaining lease term of 9.3 years and a weighted-average discount rate of 7.1 %. As of December 31, 2021, our operating leases have a weighted-average remaining lease term of 2.9 years and a weighted-average discount rate of 5.1 %. Cash paid for amounts included in the measurement of operating lease liabilities was $1.7 million and $1.5 million for the twelve months ended December 31, 2022 and 2021, respectively. Operating lease expense was $0.7 million (net of sublease income of $0.7 million) and $1.0 million (net of sublease income of $0.4 million) for the twelve months ended December 31, 2022 and 2021, respectively.
73
7. Convertible Senior Notes
In May 2018, we issued $750 million aggregate principal amount of 2023 Notes, bearing cash interest at a rate of 0.75 % per year, payable semi-annually. The net proceeds from the offering, after deducting the initial purchasers' discount and offering expenses, were approximately $733.1 million. The 2023 Notes will be convertible into cash, shares of common stock, or a combination of cash and shares of common stock, at our election, based on a conversion rate as discussed below.
Holders of the 2023 Notes were entitled to convert the notes at any time prior to the close of business on the business day immediately preceding November 15, 2022, under any of the following circumstances:
(1) during any fiscal quarter (and only during such fiscal quarter) commencing after September 30, 2018, if, for at least 20 trading days (whether or not consecutive) during the 30 consecutive trading day period ending on the last trading day of the immediately preceding fiscal quarter, the last reported sale price of our common stock on such trading day was greater than 130 % of the conversion price on such trading day;
(2) during the five business day period immediately following any 10 consecutive trading day period, in which the trading price per $1,000 principal amount of notes was less than 98 % of the product of the last reported sale price of our common stock on such trading day and the conversion rate on each such trading day; or
(3) upon the occurrence of certain specified corporate events as specified in the indenture governing the notes.
In advance of the Distribution of the shares of common stock of OmniAb to Ligand’s shareholders on November 1, 2022, a notice of convertibility was delivered to the holders of the 2023 Notes. No holders exercised their right to convert their 2023 Notes during the applicable period for conversion. After we completed the Separation of the OmniAb Business, on November 15, 2022, the conversion rate was adjusted to 4.8390 shares of common stock per $1,000 principal amount of the 2023 Notes which represents a conversion price of approximately $206.65 per share. The maximum conversion rate of the 2023 Notes was adjusted to 6.2907 per $1,000 principal amount of the 2023 Notes which represents a conversion price of approximately $158.97 . The conversion rate for the 2023 Notes was adjusted in accordance with the requirements of the Indenture based on calculations determined with reference to a valuation period of the first 10 consecutive trading days after, and including, the ex-dividend date of the spin-off (as determined in the Indenture). The conversion rate and maximum conversion rate are subject to further adjustment under the circumstances and pursuant to the terms set forth in the Indenture.
The notes will have a dilutive effect to the extent the average market price per share of common stock for a given reporting period exceeds the current conversion price of $206.65 . In connection with the issuance of the 2023 Notes, we incurred $16.9 million of issuance costs, which primarily consisted of underwriting, legal and other professional fees. The portion of these costs allocated to the liability component totaling $13.7 million is amortized to interest expense using the effective interest method over the five years expected life of the 2023 Notes, and the effective interest rate as of December 31, 2022 is 0.5 %. During the year ended December 31, 2022 we recognized a total of $1.8 million in interest expense which includes $1.1 million in contractual interest expense and $0.7 million in amortized issuance costs.
It is our intent and policy to settle conversions through combination settlement, which essentially involves payment in cash equal to the principal portion and delivery of shares of common stock for the excess of the conversion value over the principal portion.
During 2021, we repurchased $152.0 million in principal of the 2023 Notes for $156.0 million in cash, including accrued interest of $0.3 million. We accounted for the repurchase as a debt extinguishment, which resulted in (1) a loss of $7.3 million reflected in other income (expense), net, in our consolidated statement of operations for the year ended December 31, 2021, (2) a $13.7 million reduction in debt discount, and (3) a $10.2 million reduction to additional paid in capital, related to the reacquisition of the equity component in our consolidated balance sheet as of December 31, 2021. After the repurchases, approximately $343.3 million in principal amount of the 2023 Notes remain outstanding.
74
During 2022, we repurchased $266.4 million in principal amount of the 2023 Notes for $261.4 million in cash, including accrued interest of $0.5 million We accounted for the repurchase as a debt extinguishment, which resulted in a gain of $4.2 million reflected in other income (expense), net, in our consolidated statement of operations for the year ended December 31, 2022, and a $1.3 million reduction in debt discount. After the repurchases, approximately $76.9 million in principal amount of the 2023 Notes remain outstanding.
Convertible Bond Hedge and Warrant Transactions
In conjunction with the 2023 Notes, in May 2018, we entered into convertible bond hedges and sold warrants covering 3,018,327 shares of our common stock to minimize the impact of potential dilution to our common stock and/or offset the cash payments we are required to make in excess of the principal amount upon conversion of the 2023 Notes. The convertible bond hedges have an exercise price of $206.65 per share and are exercisable when and if the 2023 Notes are converted. We paid $140.3 million for these convertible bond hedges. If upon conversion of the 2023 Notes, the price of our common stock is above the exercise price of the convertible bond hedges, the counterparties will deliver shares of common stock and/or cash with an aggregate value approximately equal to the difference between the price of common stock at the conversion date and the exercise price, multiplied by the number of shares of common stock related to the convertible bond hedge transaction being exercised. The convertible bond hedges and warrants described below are separate transactions entered into by us and are not part of the terms of the 2023 Notes. Holders of the 2023 Notes and warrants will not have any rights with respect to the convertible bond hedges.
Concurrently with the convertible bond hedge transactions, we entered into warrant transactions whereby we sold warrants covering 3,018,327 shares of common stock with an exercise price of $315.38 per share, subject to certain adjustments. We received $90.0 million for these warrants. The warrants have various expiration dates ranging from August 15, 2023 to February 6, 2024. The warrants will have a dilutive effect to the extent the market price per share of common stock exceeds the applicable exercise price of the warrants, as measured under the terms of the warrant transactions. The common stock issuable upon exercise of the warrants will be in unregistered shares, and we do not have the obligation and do not intend to file any registration statement with the SEC registering the issuance of the shares under the warrants.
In April 2020, in connection with the repurchases of $234.4 million in principal of the 2023 Notes for $203.8 million in cash, including accrued interest of $0.6 million, during the quarter ended March 31, 2020, we entered into amendments with Barclays Bank PLC, Deutsche Bank AG, London Branch, and Goldman Sachs & Co. LLC to the convertible note hedges transactions we initially entered into in connection with the issuance of the 2023 Notes. The amendments provide that the options under the convertible note hedges corresponding to such repurchased 2023 Notes will remain outstanding notwithstanding such repurchase.
In January 2021, in connection with the repurchases of approximately $20.3 million in principal of the 2023 Notes for approximately $19.1 million in cash, including accrued interest of $0.1 million, during the quarter ended December 31, 2020, we entered into amendments with Barclays Bank PLC, Deutsche Bank AG, London Branch, and Goldman Sachs & Co. LLC to the convertible note hedges transactions we initially entered into in connection with the issuance of the 2023 Notes. The amendments provide that the options under the convertible note hedges corresponding to such repurchased 2023 Notes will remain outstanding notwithstanding such repurchase.
During the year ended December 31, 2021, in connection with the repurchases of $152.0 million in principal of the 2023 Notes for $156.0 million in cash, including accrued interest of $0.3 million, we entered into Warrant Early Unwind Agreements and Bond Hedge Unwind Agreements with Barclays Bank PLC, Deutsche Bank AG, and Goldman Sachs & Co. LLC to unwind a portion of the convertible note hedges transactions we initially entered into in connection with the issuance of the 2023 Notes. We paid $18.4 million as part of the Warrant Early Unwind Agreements reducing the number of shares covered by the warrants from 3,018,327 to 2,559,254 . We received $18.9 million as part of the Bond Hedge Early Unwind Agreements reducing the number of options under the convertible bond hedges to 598,021 . These unwind transactions resulted in a $0.5 million net increase in additional paid-in-capital in our consolidated balance sheet as of December 31, 2021.
In August 2022, in connection with the repurchases of $227.8 million in principal of the 2023 Notes for $223.7 million in cash, including accrued interest of $0.4 million made during the six months ended June 30, 2022, we entered into Bond Hedge Unwind Agreements with Barclays Bank PLC, Deutsche Bank AG, and Goldman Sachs & Co. LLC to unwind a portion of the convertible note hedges transactions we initially entered into in connection with the issuance of the 2023 Notes. We received $0.2 million as part of these Bond Hedge Early Unwind Agreements reducing the number of options under the convertible bond hedges to 370,219 as of December 31, 2022. This transaction resulted in a $0.2 million net increase in additional paid-in-capital in our consolidated balance sheet as of December 31, 2022.
75
| December 31, 2022 | December 31, 2021 | ||||||||||
| Principal amount of 2023 Notes outstanding | $ | $ | |||||||||
| Unamortized discount (including unamortized debt issuance cost) | ( | ( | |||||||||
| Total long-term portion of notes payable | $ | $ | |||||||||
| Fair value of convertible senior notes outstanding (Level 2) | $ | $ | |||||||||
As of December 31, 2022, there were no events of default or violation of any covenants under our financing obligations.
8. Balance Sheet Account Details
Short-term Investments
| Cost | Gross unrealized gains | Gross unrealized losses | Estimated fair value | ||||||||||||||||||||
| December 31, 2022 | |||||||||||||||||||||||
| Short-term investments | |||||||||||||||||||||||
| Mutual Funds | $ | $ | $ | ( | $ | ||||||||||||||||||
| Bank deposits | ( | ||||||||||||||||||||||
| Commercial paper | |||||||||||||||||||||||
| Corporate bonds | ( | ||||||||||||||||||||||
| Corporate equity securities | ( | ||||||||||||||||||||||
| U.S. government securities | ( | ||||||||||||||||||||||
| Warrants | |||||||||||||||||||||||
| $ | $ | $ | ( | $ | |||||||||||||||||||
| December 31, 2021 | |||||||||||||||||||||||
| Short-term investments | |||||||||||||||||||||||
| Mutual fund | $ | $ | $ | ( | $ | ||||||||||||||||||
| Bank deposits | ( | ||||||||||||||||||||||
| Commercial paper | ( | ||||||||||||||||||||||
| Corporate bonds | ( | ||||||||||||||||||||||
| Corporate equity securities | ( | ||||||||||||||||||||||
| U.S. government securities | ( | ||||||||||||||||||||||
| Warrants | |||||||||||||||||||||||
| $ | $ | $ | ( | $ | |||||||||||||||||||
Gain (loss) from short-term investments on our consolidated statements of operations includes both realized and unrealized gain (loss) from our short-term investments in public equity and warrant securities, and realized gain (loss) from available-for-sale debt securities.
| December 31, 2022 | |||||||||||
| Amortized Cost | Fair Value | ||||||||||
| Within one year | $ | $ | |||||||||
| After one year through five years | |||||||||||
| Total | $ | $ | |||||||||
76
The following table summarizes our available-for-sale debt securities in an unrealized loss position (in thousands):
| Less than 12 months | 12 months or greater | Total | |||||||||||||||||||||||||||||||||
| Gross Unrealized Losses | Estimated Fair Value | Gross Unrealized Losses | Estimated Fair Value | Gross Unrealized Losses | Estimated Fair Value | ||||||||||||||||||||||||||||||
| December 31, 2022 | |||||||||||||||||||||||||||||||||||
| Bank deposits | $ | ( | $ | $ | $ | $ | ( | $ | |||||||||||||||||||||||||||
| Corporate bonds | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Commercial paper | |||||||||||||||||||||||||||||||||||
| U.S. Government Securities | ( | ( | |||||||||||||||||||||||||||||||||
| Total | $ | ( | $ | $ | ( | $ | $ | ( | $ | ||||||||||||||||||||||||||
| December 31, 2021 | |||||||||||||||||||||||||||||||||||
| Bank deposits | $ | ( | $ | $ | $ | $ | ( | $ | |||||||||||||||||||||||||||
| Corporate bonds | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Commercial paper | ( | ( | ( | ||||||||||||||||||||||||||||||||
| U.S. Government Securities | ( | ( | |||||||||||||||||||||||||||||||||
| Total | $ | ( | $ | $ | ( | $ | $ | ( | $ | ||||||||||||||||||||||||||
Our investment policy is capital preservation and we only invested in U.S.-dollar denominated investments. We held a total of 12 positions which were in an unrealized loss position as of December 31, 2022. We believe that we will collect the principal and interest due on our debt securities that have an amortized cost in excess of fair value. The unrealized losses are largely due to changes in interest rates and not to unfavorable changes in the credit quality associated with these securities that impacted our assessment on collectability of principal and interest. We do not intend to sell these securities nor do we believe that we will be required to sell these securities before the recovery of the amortized cost basis. Accordingly, no credit losses were recognized for the twelve months ended December 31, 2022.
| December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| Lab and office equipment | $ | $ | |||||||||
| Leasehold improvements | |||||||||||
| Computer equipment and software | |||||||||||
| Less accumulated depreciation and amortization | ( | ( | |||||||||
| $ | $ | ||||||||||
Depreciation of equipment is computed using the straight-line method over the estimated useful lives of the assets which ranges from to ten years . Leasehold improvements are amortized using the straight-line method over their estimated useful lives or their related lease term, whichever is shorter. Depreciation expense of $3.8 million, $2.4 million, and $1.5 million was recognized for the twelve months ended December 31, 2022, 2021, and 2020, respectively, and was included in operating expenses.
77
| December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| Indefinite-lived intangible assets | |||||||||||
| Goodwill | $ | $ | |||||||||
| Definite-lived intangible assets | |||||||||||
| Complete technology | |||||||||||
| Less: Accumulated amortization | ( | ( | |||||||||
| Trade name | |||||||||||
| Less: Accumulated amortization | ( | ( | |||||||||
| Customer relationships | |||||||||||
| Less: Accumulated amortization | ( | ( | |||||||||
| Contractual relationships | |||||||||||
| Less: Accumulated amortization | ( | ( | |||||||||
| Total goodwill and other identifiable intangible assets, net | $ | $ | |||||||||
Amortization of finite-lived intangible assets is computed using the straight-line method over the estimated useful life of the asset of up to 20 years. Amortization expense of $34.2 million, $34.2 million, and $11.6 million was recognized for the years ended December 31, 2022, 2021, and 2020, respectively. Estimated amortization expense for the years ending December 31, 2023 through 2027 is $34.1 no
| December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| Compensation | $ | $ | |||||||||
| Professional fees | |||||||||||
| Amounts owed to former licensees | |||||||||||
| Royalties owed to third parties | |||||||||||
| Return reserve and customer refunds | |||||||||||
| Acquisition related liabilities | |||||||||||
| Subcontractor | |||||||||||
| Supplier | |||||||||||
| Other | |||||||||||
| $ | $ | ||||||||||
Contingent liabilities:
In connection with the acquisition of CyDex in January 2011, we issued a series of CVRs and also assumed certain contingent liabilities. We may be required to make additional payments upon achievement of certain clinical and regulatory milestones to the CyDex shareholders and former license holders.
In connection with the acquisition of Metabasis in January 2010, we entered into four CVR agreements with Metabasis shareholders. The CVRs entitle the holders to cash payments as frequently as every six months as proceeds are received by us upon the sale or licensing of any of the Metabasis drug development programs and upon the achievement of specified milestones.
For CVRs associated with the Pfenex, see “Note (4), Acquisitions” for more information.
78
| December 31, 2020 | Payments | Fair Value Adjustment | December 31, 2021 | Payments | Fair Value Adjustment | Repurchases | December 31, 2022 | |||||||||||||||||||
| Cydex | $ | $ | ( | $ | ( | $ | $ | $ | ( | $ | $ | |||||||||||||||
| Metabasis | ( | |||||||||||||||||||||||||
| Pfenex | ( | |||||||||||||||||||||||||
| Total | $ | $ | ( | $ | ( | $ | $ | $ | ( | $ | $ | |||||||||||||||
9. Stockholders’ Equity
Share-based Compensation Expense
The following table summarizes share-based compensation expense from continuing operations (in thousands):
| December 31, | |||||||||||||||||
| 2022 | 2021(a) | 2020(a) | |||||||||||||||
| Share-based compensation expense as a component of: | |||||||||||||||||
| Research and development expenses | $ | $ | $ | ||||||||||||||
| General and administrative expenses | |||||||||||||||||
| $ | $ | $ | |||||||||||||||
(a) Prior period amounts have been retrospectively adjusted to reflect the effects of the Separation.
Conversion and Modification of Equity Awards Outstanding at Separation Date
In connection with the OmniAb Separation on November 1, 2022, under the provisions of the existing plans, we adjusted our outstanding equity awards in accordance with the Merger Agreement to preserve the intrinsic value of the awards immediately before and after the Distribution. Upon the Distribution, employees holding stock options, restricted stock units and performance restricted stock units denominated in pre-Distribution Ligand stock received a number of otherwise-similar awards either in post-Distribution Ligand stock or in a combination of post-Distribution Ligand stock and OmniAb stock based on conversion ratios outlined for each group of employees in the Merger Agreement that we entered into in connection with the Distribution. The equity awards that were granted prior to March 2, 2022 were converted under the shareholder method, wherein employees holding outstanding equity awards received equity awards in both Ligand and OmniAb. For equity awards granted after March 2, 2022, for Ligand employees, the number of awards that were outstanding at the Separation were proportionately adjusted into post-Distribution Ligand stock to maintain the aggregate intrinsic value of the awards at the date of the Separation; for OmniAb employees, the number of awards that were outstanding at the Separation were proportionately adjusted into post-Distribution OmniAb stock to maintain the aggregate intrinsic value of the awards at the date of the Separation. The conversion ratio was determined based on the relative values of Ligand common stock in the “regular way” and “ex-distribution” markets during the five-trading day period prior to the closing of the business combination.
These modified awards otherwise retained substantially the same terms and conditions, including term and vesting provisions. Additionally, we will not incur any future compensation cost related to equity awards held by OmniAb employees and directors. We will incur future compensation cost related to OmniAb equity awards held by our employees.
Stock Plans
In June 2022, our stockholders approved the amendment and restatement of the Ligand Pharmaceuticals Incorporated 2002 Stock Incentive Plan (the “2002 Plan”). The amended and restated 2002 Plan, which is referred to herein as the “Restated Plan” was amended to increase the shares available for issuance by 1.0 million.
On July 29, 2022, our board of directors (the “Board”) approved the Ligand Pharmaceuticals Incorporated 2022 Employment Inducement Plan (the “2022 Inducement Plan”). The terms of the 2022 Inducement Plan are substantially similar to the terms of the Restated Plan with the exception that incentive stock options may not be issued under the 2022 Inducement Plan and awards under the 2022 Inducement Plan may only be issued to eligible recipients under the applicable Nasdaq Listing Rules. The 2022 Inducement Plan was adopted by the Board without stockholder approval pursuant to Rule 5635(c)(4) of the Nasdaq Listing Rules. The Board has initially reserved 300,000 shares of the Company’s common stock for issuance pursuant to awards granted under the 2022 Inducement Plan.
As of December 31, 2022, there were 1.3 million shares available for future option grants or direct issuance under the Restated Plan and the 2022 Inducement Plan.
79
Following is a summary of our stock option plan activity and related information:
| Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term in Years | Aggregate Intrinsic Value (In thousands) | ||||||||||||||||||||
| Balance at January 1, 2020 | $ | $ | |||||||||||||||||||||
| Granted | $ | ||||||||||||||||||||||
| Exercised | ( | $ | |||||||||||||||||||||
| Forfeited | ( | $ | |||||||||||||||||||||
| Balance at December 31, 2020 | $ | $ | |||||||||||||||||||||
| Exercisable at December 31, 2020 | $ | $ | |||||||||||||||||||||
| Options vested and expected to vest as of December 31, 2020 | $ | $ | |||||||||||||||||||||
| Granted | $ | ||||||||||||||||||||||
| Exercised | ( | $ | |||||||||||||||||||||
| Forfeited | ( | $ | |||||||||||||||||||||
| Balance at December 31, 2021 | $ | $ | |||||||||||||||||||||
| Exercisable at December 31, 2021 | $ | $ | |||||||||||||||||||||
| Options vested and expected to vest as of December 31, 2021 | $ | $ | |||||||||||||||||||||
| Granted | $ | ||||||||||||||||||||||
| Exercised | ( | $ | |||||||||||||||||||||
| Forfeited | ( | $ | |||||||||||||||||||||
| Balance at October 31, 2022 | $ | $ | |||||||||||||||||||||
| Exercisable at October 31, 2022 | $ | $ | |||||||||||||||||||||
| Options vested and expected to vest as of October 31, 2022, before Separation and Regrant | $ | $ | |||||||||||||||||||||
| Cancellation due to Separation, Before Regrant | ( | ||||||||||||||||||||||
| Balance at November 1, 2022, Before Regrant | |||||||||||||||||||||||
Granted (1) | $ | ||||||||||||||||||||||
| Exercised | ( | $ | |||||||||||||||||||||
| Forfeited | ( | $ | |||||||||||||||||||||
| Balance at December 31, 2022 | $ | $ | |||||||||||||||||||||
| Exercisable at December 31, 2022 | $ | $ | |||||||||||||||||||||
| Options vested and expected to vest as of December 31, 2022 | $ | $ | |||||||||||||||||||||
The weighted-average grant-date fair value of all stock options granted during 2022, 2021 and 2020 was $28.90 , $80.08 , and $41.39 per share, respectively. The total intrinsic value of all options exercised during 2022, 2021 and 2020 was approximately $4.6 million, $77.3 million, and $11.9 million, respectively.
Cash received from options exercised, net of fees paid, in 2022, 2021 and 2020 was $2.6 million, $33.0 million and $2.5 million, respectively.
80
Following is a further breakdown of the options outstanding as of December 31, 2022:
| Range of exercise prices | Options outstanding | Weighted average remaining life in years | Weighted average exercise price | Options exercisable | Weighted average exercise price | ||||||||||||||||||||||||
$ | $ | $ | |||||||||||||||||||||||||||
$ | $ | $ | |||||||||||||||||||||||||||
$ | $ | $ | |||||||||||||||||||||||||||
$ | $ | $ | |||||||||||||||||||||||||||
$ | $ | $ | |||||||||||||||||||||||||||
$ | $ | $ | |||||||||||||||||||||||||||
$ | $ | $ | |||||||||||||||||||||||||||
$ | $ | $ | |||||||||||||||||||||||||||
$ | $ | $ | |||||||||||||||||||||||||||
$ | $ | $ | |||||||||||||||||||||||||||
| $ | $ | ||||||||||||||||||||||||||||
The assumptions used for the specified reporting periods and the resulting estimates of weighted-average grant date fair value per share of options granted:
| Year Ended December 31, | ||||||||||||||||||||
| 2022 | 2021 | 2020 | ||||||||||||||||||
| Risk-free interest rate | ||||||||||||||||||||
| Expected volatility | ||||||||||||||||||||
| Expected term | ||||||||||||||||||||
As of December 31, 2022, there was $32.7 million of total unrecognized compensation cost related to non-vested stock options under the 2002 Plan. That cost is expected to be recognized over a weighted average period of 2.7 years.
As of December 31, 2022, there was $4.8 million of total unrecognized compensation cost related to non-vested OmniAb stock options received upon aforementioned spin-off conversion. That cost is expected to be recognized over a weighted average period of 1.8 years.
81
Restricted Stock Activity
The following is a summary of our restricted stock activity and related information:
| Shares | Weighted-Average Grant Date Fair Value | ||||||||||
| Outstanding at January 1, 2020 | $ | ||||||||||
| Granted | $ | ||||||||||
| Vested | ( | $ | |||||||||
| Forfeited | $ | ||||||||||
| Outstanding at January 1, 2021 | $ | ||||||||||
| Granted | $ | ||||||||||
| Vested | ( | $ | |||||||||
| Forfeited | ( | $ | |||||||||
| Outstanding at December 31, 2021 | $ | ||||||||||
| Granted | $ | ||||||||||
| Vested | ( | $ | |||||||||
| Forfeited | ( | $ | |||||||||
| Outstanding at October 31, 2022, before Separation and Regrant | $ | ||||||||||
| Forfeited due to Separation, Before Regrant | ( | ||||||||||
| Balance at November 1, 2022, Before Regrant | |||||||||||
| Granted | $ | ||||||||||
| Vested | ( | $ | |||||||||
| Forfeited | ( | $ | |||||||||
| Balance at December 31, 2022 | $ | ||||||||||
As of December 31, 2022, unrecognized compensation cost related to non-vested stock awards under the 2002 Plan amounted to $12.4 million. That cost is expected to be recognized over a weighted average period of 1.7 years.
As of December 31, 2022, there was $1.2 million of total unrecognized compensation cost related to non-vested OmniAb stock awards received upon aforementioned spin-off conversion. That cost is expected to be recognized over a weighted average period of 1.0 years.
Employee Stock Purchase Plan
As of December 31, 2022, 35,881 shares of our common stock are available for future issuance under the Amended Employee Stock Purchase Plan, or ESPP. The ESPP permits eligible employees to purchase up to 1,250 shares of Ligand common stock per calendar year at a discount through payroll deductions. The price at which stock is purchased under the ESPP is equal to 85 % of the fair market value of the common stock on the first of a six month offering period or purchase date, whichever is lower. There were 8,479 , 8,448 and 6,455 shares issued under the ESPP in 2022, 2021 and 2020, respectively.
Share Repurchases
In September 2019, our Board of Directors approved a stock repurchase program authorizing the repurchase of up to $500.0 million of our common stock from time to time over a period of up to three years . This repurchase program expired in September 2022. During the year ended December 31, 2022 and 2021, we did no 934,079 shares for $78.0 million.
At-the Market Equity Offering Program
On September 30, 2022, we filed a registration statement on Form S-3 (the “Shelf Registration Statement”), which became automatically effective upon filing, covering the offering of common stock, preferred stock, debt securities, warrants and units.
On September 30, 2022, we also entered into an At-The-Market Equity Offering Sales Agreement (the “Sales Agreement”) with Stifel, Nicolaus & Company, Incorporated (the “Agent”), under which we may, from time to time, sell shares of our common stock having an aggregate offering price of up to $100.0 million in “at the market” offerings through the Agent (the
82
10. Commitment and Contingencies: Legal Proceedings
We record an estimate of a loss when the loss is considered probable and estimable. Where a liability is probable and there is a range of estimated loss and no amount in the range is more likely than any other number in the range, we record the minimum estimated liability related to the claim in accordance with ASC 450, Contingencies. As additional information becomes available, we assess the potential liability related to our pending litigation and revises our estimates. Revisions in our estimates of potential liability could materially impact our results of operations.
On October 31, 2019, we received three civil complaints filed in the U.S. District Court for the Northern District of Ohio on behalf of several Indian tribes. The Northern District of Ohio is the Court that the Judicial Panel on Multi-District Litigation (“JPML”) has assigned more than one thousand civil cases which have been designated as a Multi-District Litigation (“MDL”) and captioned In Re: National Prescription Opiate Litigation. The allegations in these complaints focus on the activities of defendants other than the Company and no individualized factual allegations have been advanced against us in any of the three complaints. We reject all claims raised in the complaints and intend to vigorously defend these matters.
CyDex, a wholly owned subsidiary of Ligand, and Baxter Healthcare Corp. (“Baxter”) are parties to a license agreement relating to Ligand’s Captisol® technology and, more specifically, relating to Captisol®-enabled Nexterone® (amiodarone HCl premixed injection). CyDex contended that Baxter has not paid all of the royalties due to CyDex under the terms of the license agreement and Baxter contends that it has overpaid royalties for several years. On April 6, 2021, Baxter initiated an arbitration with AAA pursuant to the arbitration provision of the license agreement. On April 21, 2021, CyDex filed an answering statement and counterdemand. On December 2, 2021, Baxter filed an Amended Notice of Arbitration Demand seeking a declaration limiting the “royalty term” of the license agreement to “the later of i) the expiration of the licensed [p]atent; or ii) when there are no longer any CyDex patents listed in the Orange Book for [Nexterone®].” Baxter later clarified its position, and asserted that royalties should have ceased being due upon the May 4, 2022 expiry of CyDex’s U.S. Patent No. 6,869,939. The parties conducted a three-day arbitration hearing May 24-26, 2022. In a September 9, 2022 Final Award, the Tribunal ruled in CyDex’s favor by (1) denying Baxter’s request for a partial refund of previously paid royalties, (2) granting CyDex’s request for underpaid royalties, and (3) concluding that “[g]oing forward Baxter shall pay CyDex” a royalty consistent with CyDex’s construction of the license agreement until, at least, the March 13, 2029 expiry of CyDex’s U.S. Patent No. 7,635,773.
On April 22, 2022, Pfenex Inc. (“Pfenex”), a wholly owned subsidiary of Ligand, received a notice of alleged breach from Beijing Kangchen Biological Technology Co., Ltd. (“Kangchen”) with respect to a Development and License Agreement , dated April 18, 2018, between Pfenex and Kangchen (“License Agreement”) pertaining to the development and commercialization of teriparatide in certain Southeast Asian countries. The allegations in the notice focused on the activities of Pfenex and other parties. On June 16, 2022, we rejected all claims raised by Kangchen in the notice. On June 24, 2022, Kangchen served Pfenex a notice of termination of the License Agreement and demanded initiation of the dispute resolution process in accordance with the License Agreement. On June 29, 2022, we again rejected all claims raised by Kangchen in the notice of termination and agreed to engage in the applicable dispute resolution process, including good faith negotiations between the parties. On October 20, 2022, we agreed to make a single lump sum payment to Kangchen in connection with a termination agreement that, among other things, terminates the License Agreement and releases all claims between the parties arising from the License Agreement. The payment was recorded as general and administrative expense during the year ended December 31, 2022. A termination agreement between Pfenex and Kangchen went into effect in December 2022.
From time to time, we may also become subject to other legal proceedings or claims arising in the ordinary course of our business. We currently believe that none of the claims or actions pending against us is likely to have, individually or in the aggregate, a material adverse effect on our business, financial condition or results of operations. Given the unpredictability inherent in litigation, however, we cannot predict the outcome of these matters.
11. Income Taxes
83
| Year Ended December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Current expense (benefit): | |||||||||||||||||
| Federal | $ | $ | $ | ||||||||||||||
| State | ( | ||||||||||||||||
| Foreign | |||||||||||||||||
| Deferred expense (benefit): | |||||||||||||||||
| Federal | ( | ( | ( | ||||||||||||||
| State | ( | ( | |||||||||||||||
| ( | ( | ||||||||||||||||
| Total income tax expense (benefit) | $ | $ | ( | $ | ( | ||||||||||||
A reconciliation of income tax expense (benefit) from continuing operations to the amount computed by applying the statutory federal income tax rate to the net income (loss) from continuing operations is summarized as follows (in thousands):
| Year Ended December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Tax at federal statutory rate | $ | $ | $ | ||||||||||||||
| State, net of federal benefit | ( | ( | |||||||||||||||
| FDII | ( | ( | ( | ||||||||||||||
| Rate change for changes in federal, foreign or state law | ( | ( | ( | ||||||||||||||
| Change in uncertain tax positions | ( | ( | |||||||||||||||
| Sale of Vernalis R&D | |||||||||||||||||
| Contingent liabilities | ( | ( | |||||||||||||||
| Foreign tax differential on income/loss of foreign subsidiaries | ( | ( | |||||||||||||||
| Research and development credits | ( | ( | |||||||||||||||
| Debt repurchases | |||||||||||||||||
| Subpart F income | |||||||||||||||||
| Share-based compensation | ( | ( | |||||||||||||||
| Provision to return adjustments | ( | ( | |||||||||||||||
| Officer compensation | |||||||||||||||||
| Change in valuation allowance | ( | ||||||||||||||||
| Other | ( | ||||||||||||||||
| $ | $ | ( | $ | ( | |||||||||||||
We remeasured certain deferred tax assets and liabilities based on the rates at which they are expected to reverse in the future, which is generally 21%. Significant components of our deferred tax assets and liabilities as of December 31, 2022 and 2021 are shown below. We assess the positive and negative evidence to determine if sufficient future taxable income will be generated to use the existing deferred tax assets. Our evaluation of evidence resulted in management concluding that the majority of our deferred tax assets will be realized. However, we maintain a valuation allowance to offset certain net deferred tax assets as management believes realization of such assets are uncertain as of December 31, 2022, 2021 and 2020. The valuation allowance increased $21.5 million in 2022, increased $11.4 million in 2021 and decreased $116.5 million in 2020.
We offset all deferred tax assets and liabilities by jurisdiction, as well as any related valuation allowance, and present them on our consolidated balance sheet as a non-current deferred income tax asset or liability (as applicable). Deferred tax assets
84
| December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| (in thousands) | |||||||||||
| Deferred tax assets: | |||||||||||
| Net operating loss carryforwards | $ | $ | |||||||||
| Research credit carryforwards | |||||||||||
| Stock compensation | |||||||||||
| Other | |||||||||||
| Valuation allowance for deferred tax assets | ( | ( | |||||||||
| Net deferred tax assets | $ | $ | |||||||||
| Deferred tax liabilities: | |||||||||||
| Identified intangibles | ( | ( | |||||||||
| Other | ( | ( | |||||||||
| Net deferred tax liabilities | $ | ( | $ | ( | |||||||
| Deferred income taxes, net | $ | ( | $ | ||||||||
Beginning in 2022, the Tax Cuts and Jobs Act of 2017 requires taxpayers to capitalize and amortize R&D expenditures over five years for domestic research and 15 years for foreign research pursuant to Section 174 of the Internal Revenue Code of 1986, as amended. We recorded an increase of $4.7 million to our current federal income tax expense and deferred tax assets for continuing operations during 2022 due to the capitalization of R&D under Section 174.
As of December 31, 2022, we had federal net operating loss carryforwards set to expire through 2037 of $81.1 million and $168.3 million of state net operating loss carryforwards that begin to expire in 2028. We also have $8.5 million of federal research and development credit carryforwards, which expire through 2040. We have $29.0 million of California research and development credit carryforwards that have no expiration date. In addition, we have approximately $96.1 million of non-U.S. net operating loss carryovers and approximately $15.6 million of non-U.S. capital loss carryovers that have no expiration date.
At December 31, 2021 we had federal net operating loss carryforwards set to expire through 2037 of $117.5 million and $170.1 million of state net operating loss carryforwards that begin to expire in 2028. We also had $9.3 million in federal research and development credit carryforwards, which expire through 2040, and $29.0 million of California research and development credit carryforwards that have no expiration date. In addition, we had approximately $101.6 million of non-U.S. capital loss carryovers and approximately $17.5 million of non-U.S. capital loss carryovers that have no expiration date.
Pursuant to Section 382 and 383 of the Internal Revenue Code of 1986, as amended, utilization of our net operating losses and credits may be subject to annual limitations in the event of any significant future changes in its ownership structure. These annual limitations may result in the expiration of net operating losses and credits prior to utilization. The deferred tax assets as of December 31, 2022 are net of any previous limitations due to Section 382 and 383.
We account for income taxes by evaluating a probability threshold that a tax position must meet before a financial statement benefit is recognized. The minimum threshold is a tax position that is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation processes, based on the technical merits of the position. Our remaining liabilities for uncertain tax positions are presented net of the deferred tax asset balances on the accompanying consolidated balance sheet.
| December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Balance at beginning of year | $ | $ | $ | ||||||||||||||
| Additions based on tax positions related to the current year | |||||||||||||||||
| Additions for tax positions of prior years | |||||||||||||||||
| Reductions for tax positions of prior years | ( | ( | ( | ||||||||||||||
| Balance at end of year | $ | $ | $ | ||||||||||||||
85
Included in the balance of unrecognized tax benefits at December 31, 2022 is $27.3 million of tax benefits that, if recognized would impact the effective rate. There are no positions for which it is reasonably possible that the uncertain tax benefit will significantly increase or decrease within twelve months.
We recognize interest and penalties related to uncertain tax positions in income tax expense. As of December 31, 2022 and December 31, 2021, we recognized an immaterial amount of interest and penalties. We file income tax returns in the United States, various state jurisdictions, United Kingdom, and Canada with varying statutes of limitations. The federal statute of limitation remains open for the 2019 tax year to the present. The state income tax returns generally remain open for the 2018 tax year through the present. Net operating loss and research credit carryforwards arising prior to these years are also open to examination if and when utilized. The Ligand California tax returns for 2019 and 2020 are currently under audit. We believe our reserve for unrecognized tax benefits and contingent tax issues is adequate with respect to all open years.
86
12. Selected Quarterly Financial Data (Unaudited)
The following table contains quarterly financial information for 2022 and 2021. The Company believes that the following information reflects all normal recurring adjustments necessary for a fair presentation of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
| Three months ended, | |||||||||||||||||
| March 31, 2022 | June 30, 2022 | September 30, 2022 | December 31, 2022 | Total | |||||||||||||
| (in thousands, except per share data) | |||||||||||||||||
| (unaudited) | |||||||||||||||||
| Total revenues | $ | $ | $ | $ | $ | ||||||||||||
| Research and development | $ | $ | $ | $ | $ | ||||||||||||
| General and administrative | $ | $ | $ | $ | $ | ||||||||||||
| Total operating costs and expenses | $ | $ | $ | $ | $ | ||||||||||||
| Net income (loss) from continuing operations | $ | ( | $ | $ | $ | ( | $ | ( | |||||||||
| Net loss from discontinued operations | $ | ( | $ | ( | $ | ( | $ | ( | $ | ( | |||||||
| Net income (loss) | $ | ( | $ | ( | $ | $ | ( | $ | ( | ||||||||
| Basic net income (loss) from continuing operations per share | $ | ( | $ | $ | $ | ( | $ | ( | |||||||||
| Basic net loss from discontinued operations per share | $ | ( | $ | ( | $ | ( | $ | ( | $ | ( | |||||||
| Diluted net income (loss) from continuing operations per share | $ | (0.77) | $ | 0.74 | $ | 0.56 | $ | (0.86) | $ | (0.31) | |||||||
| Diluted net loss from discontinued operations per share | $ | (0.15) | $ | (0.79) | $ | (0.54) | $ | (0.17) | $ | (1.67) | |||||||
| Shares used in the computation of basic net income (loss) per share | |||||||||||||||||
| Shares used in the computation of diluted net income (loss) per share | 16,824 | 17,058 | 17,132 | 16,890 | 16,868 | ||||||||||||
| Three months ended, | |||||||||||||||||
| March 31, 2021 | June 30, 2021 | September 30, 2021 | December 31, 2021 | Total | |||||||||||||
| (in thousands, except per share data) | |||||||||||||||||
| (unaudited) | |||||||||||||||||
| Total revenues | $ | $ | $ | $ | $ | ||||||||||||
| Research and development | $ | $ | $ | $ | $ | ||||||||||||
| General and administrative | $ | $ | $ | $ | $ | ||||||||||||
| Total operating costs and expenses | $ | $ | $ | $ | $ | ||||||||||||
| Net income (loss) from continuing operations | $ | $ | $ | $ | ( | $ | |||||||||||
| Net loss from discontinued operations | $ | ( | $ | ( | $ | ( | $ | ( | $ | ( | |||||||
| Net income (loss) | $ | $ | $ | $ | ( | $ | |||||||||||
| Basic net income (loss) from continuing operations per share | $ | $ | $ | $ | ( | $ | |||||||||||
| Basic net loss from discontinued operations per share | $ | ( | $ | ( | $ | ( | $ | ( | $ | ( | |||||||
| Diluted net income (loss) from continuing operations per share | $ | $ | $ | $ | ( | $ | |||||||||||
| Diluted net loss from discontinued operations per share | $ | ( | $ | ( | $ | ( | $ | ( | $ | ( | |||||||
| Shares used in the computation of basic net income (loss) per share | |||||||||||||||||
| Shares used in the computation of diluted net income (loss) per share | |||||||||||||||||
87
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | ||||
None.
| Item 9A. | Controls and Procedures | ||||
(a) Evaluation of Disclosure Controls and Procedures
We are responsible for maintaining disclosure controls and procedures designed to provide reasonable assurance that information required to be disclosed in reports we file under the Exchange Act is recorded, processed, summarized and reported within the specified time periods and accumulated and communicated to management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. The design of any system of controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected. As of the end of the period covered by this Annual Report on Form 10-K, we have carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, and have concluded our disclosure controls and procedures were effective at a reasonable assurance level as of December 31, 2022.
There have been no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2022 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
(b) Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting for the Company. Internal control over financial reporting is a process to provide reasonable assurance regarding the reliability of our financial reporting for external purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our financial statements in accordance with generally accepted accounting principles; providing reasonable assurance that receipts and expenditures are made in accordance with our management and directors; and providing reasonable assurance that
unauthorized acquisition, use or disposition of company assets that could have a material effect on our financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our financial statements would be prevented or detected.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework established by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) as set forth in the 2013 Internal Control-Integrated Framework. Based on our evaluation under the 2013 framework in Internal Control - Integrated Framework, management concluded that our internal controls over financial reporting were effective as of December 31, 2022.
Ernst & Young LLP, an independent registered public accounting firm, has audited the Company’s consolidated financial statements included in this Annual Report on Form 10-K and has issued an attestation report, included herein, on the effectiveness of our internal control over financial reporting as of December 31, 2022.
88
Report of Independent Registered Public Accounting Firm
The Stockholders and Board of Directors of Ligand Pharmaceuticals Incorporated
Opinion on Internal Control Over Financial Reporting
We have audited Ligand Pharmaceuticals Incorporated’s internal control over financial reporting as of December 31, 2022, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, Ligand Pharmaceuticals Incorporated (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2022, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2022 and 2021, the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2022, and the related notes and our report dated February 28, 2023 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
San Diego, California
February 28, 2023
89
| Item 9B. | Other Information | ||||
None
90
| Item 9C. | Disclosure Regarding Foreign Jurisdictions that Prevent Inspections | ||||
None
91
Part III
| Item 10. | Directors, Executive Officers and Corporate Governance | ||||
Code of Conduct
The Board of Directors has adopted a Code of Conduct and Ethics Policy (“Code of Conduct”) that applies to all officers, directors and employees. The Company will promptly disclose (1) the nature of any amendment to the Code of Conduct that applies to our principal executive officer, principal financial officer, principal accounting officer or controller or persons performing similar functions and (2) the nature of any waiver, including an implicit waiver, from a provision of our Code of Conduct that is granted to one of these specified officers, the name of such person who is granted the waiver and the date of the waiver on our website in the future. The Code of Conduct can be accessed via our website (http://www.ligand.com), Corporate Overview page. You may also request a free copy by writing to: Investor Relations, Ligand Pharmaceuticals Incorporated, 3911 Sorrento Valley Boulevard, Suite 110, San Diego, CA 92121.
The other information under Item 10 is hereby incorporated by reference to Ligand’s Definitive Proxy Statement to be filed with the SEC within 120 days of December 31, 2022.
| Item 11. | Executive Compensation | ||||
Item 11 is hereby incorporated by reference to Ligand’s Definitive Proxy Statement to be filed with the SEC within 120 days of December 31, 2022.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | ||||
Item 12 is hereby incorporated by reference to Ligand’s Definitive Proxy Statement to be filed with the SEC within 120 days of December 31, 2022.
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | ||||
Item 13 is hereby incorporated by reference to Ligand’s Definitive Proxy Statement to be filed with the SEC within 120 days of December 31, 2022.
| Item 14. | Principal Accountant Fees and Services | ||||
Item 14 is hereby incorporated by reference to Ligand’s Definitive Proxy Statement to be filed with the SEC within 120 days of December 31, 2022.
92
PART IV
| Item 15. | Exhibits and Financial Statement Schedule | ||||
(a) The following documents are included as part of this Annual Report on Form 10-K.
(1) Financial statements
(2) Schedules not included herein have been omitted because they are not applicable or the required information is in the consolidated financial statements or notes thereto.
(3) The following exhibits are filed as part of this Form 10-K and this list includes the Exhibit Index.
| Incorporated by Reference | ||||||||||||||||||||
Exhibit Number | Description of Exhibit | Form | File Number | Date of Filing | Exhibit Number | Filed Herewith | ||||||||||||||
| Asset Purchase Agreement, dated March 5, 2019, by and among Ligand Pharmaceuticals Incorporated and RPI Financial Trust | 8-K | 001-33093 | March 5, 2019 | 2.1 | ||||||||||||||||
Agreement and Plan of Merger, dated as of August 10, 2020, by and among Pfenex Inc., Ligand Pharmaceuticals Incorporated and Pelican Acquisition Sub, Inc. | 8-K | 001-33093 | August 11, 2020 | 2.1 | ||||||||||||||||
| Agreement for the Sale and Purchase of the Entire Issued Share Capital of Vernalis (R&D) Limited, dated as of October 11, 2020, by and among Ligand Pharmaceuticals Incorporated, Vernalis Limited, HitGen UK Ltd and HitGen Inc. | 8-K | 001-33093 | October 13, 2020 | 2.1 | ||||||||||||||||
| Agreement and Plan of Merger, dated as of March, 23, 2022, by and among Avista Public Acquisition Corp. II, Ligand Pharmaceuticals Incorporated, OmniAb, Inc. and Orwell Merger Sub Inc. | 8-K | 001-33093 | March 24, 2022 | 2.1 | ||||||||||||||||
| Separation and Distribution Agreement, dated as of March 23, 2022, by and among Avista Public Acquisition Corp. II, Ligand Pharmaceuticals Incorporated and OmniAb, Inc. | 8-K | 001-33093 | March 24, 2022 | 2.2 | ||||||||||||||||
| Amended and Restated Certificate of Incorporation of the Company. | S-4 | 333-58823 | July 9, 1998 | 3.1 | ||||||||||||||||
| Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Company, dated June 14, 2000 | 10-K | 0-20720 | March 29, 2001 | 3.5 | ||||||||||||||||
| Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Company, dated June 30, 2004 | 10-Q | 0-20720 | August 5, 2004 | 3.6 | ||||||||||||||||
| Certificate of Amendment of the Amended and Restated Certificate of Incorporation of the Company, dated November 17, 2010 | 8-K | 001-33093 | November 19, 2010 | 3.1 | ||||||||||||||||
| Certificate of Amendment of the Amended and Restated Certification of Incorporation of the Company, dated June 19, 2018 | S-8 | 333-233130 | August 8, 2019 | 3.6 | ||||||||||||||||
| Fourth Amended and Restated Bylaws of the Company | 8-K | 001-33093 | October 30, 2020 | 3.1 | ||||||||||||||||
93
| Specimen stock certificate for shares of the common stock of the Company | 10-K | 001-33093 | March 1, 2018 | 4.1 | ||||||||||||||||
| Indenture, dated as of May 22, 2018, between the Company and Wilmington Trust, National Association, as trustee, including the form of 0.75% Convertible Senior Notes due 2023 | 8-K | 001-33093 | May 22, 2018 | 4.1 | ||||||||||||||||
| Description of Registered Securities | 10-K | 001-33093 | February 24, 2021 | 4.3 | ||||||||||||||||
| 2002 Stock Incentive Plan (as amended and restated effective June 10, 2022) | DEF 14A | 001-33093 | April 22, 2022 | Appendix A | ||||||||||||||||
| 2002 Employee Stock Purchase Plan (as amended and restated effective June 6, 2019) | DEF | 001-33093 | April 24, 2019 | Appendix B | ||||||||||||||||
| Form of Stock Option Grant Notice and Stock Option Agreement under the Company’s 2002 Stock Incentive Plan | 10-K | 001-33093 | February 24, 2014 | 10.5 | ||||||||||||||||
| Form of Stock Issuance Agreement for non-employee directors under the Company’s 2002 Stock Incentive Plan | S-1 | 333-131029 | January 13, 2006 | 10.289 | ||||||||||||||||
| Form of Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement under the Company’s 2002 Stock Incentive Plan | 10-K | 001-33093 | March 1, 2018 | 10.6 | ||||||||||||||||
| Form of Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement under the Company’s 2002 Stock Incentive Plan - Performance-Based RSU Form | 10-K | 001-33093 | March 1, 2018 | 10.7 | ||||||||||||||||
| Form of Executive Officer Change in Control Severance Agreement | 8-K | 001-33093 | August 22, 2007 | 10.1 | ||||||||||||||||
| Amended and Restated Severance Plan, effective November 1, 2022 | X | |||||||||||||||||||
| Director Compensation and Stock Ownership Policy, as amended and restated, effective April 13, 2020 | X | |||||||||||||||||||
| 2022 Employment Inducement Plan | 10-Q | 001-33093 | August 9, 2022 | 10.2 | ||||||||||||||||
| Form of Stock Option Agreement under the Company’s 2022 Employment Inducement Plan | 10-Q | 001-33093 | August 9, 2022 | 10.3 | ||||||||||||||||
| Form of Restricted Stock Unit Award Agreement under the Company’s 2022 Employment Inducement Plan | 10-Q | 001-33093 | August 9, 2022 | 10.4 | ||||||||||||||||
| Form of Performance-Based Restricted Stock Unit Award Agreement under the Company’s 2022 Employment Inducement Plan | 10-Q | 001-33093 | August 9, 2022 | 10.5 | ||||||||||||||||
| Separation Agreement , effective December 12, 2022, by and between Ligand Pharmaceuticals Incorporated and John Higgins | X | |||||||||||||||||||
| Severance Agreement, effective December 5, 2022, by and between Ligand Pharmaceuticals Incorporated and Todd C. Davis | X | |||||||||||||||||||
| Tax Matters Agreement, dated as of November 1, 2022, by and among OmniAb, Inc.(f/k/a Avista Public Acquisition Corp. II) Ligand Pharmaceuticals Incorporated and OmniAb Operations, Inc. (f/k/a OmniAb, Inc.) | 8-K | 001-33093 | November 4, 2022 | 10.1 | ||||||||||||||||
| Amended and Restated Employee Matters Agreement, dated as of August 18, 2022, by and among Ligand Pharmaceuticals Incorporated, OmniAb Operations, Inc. (f/k/a OmniAb, Inc.), OmniAb, Inc. (f/k/a Avista Public Acquisition Corp. II) and Orwell Merger Sub Inc. | 10-Q | 001-33093 | November 8, 2022 | 10.1 | ||||||||||||||||
94
| TR Beta Contingent Value Rights Agreement, dated January 27, 2010, among the Company, Metabasis Therapeutics, Inc., David F. Hale and Mellon Investor Services LLC | 8-K | 001-33093 | January 28, 2010 | 10.2 | ||||||||||||||||
| General Contingent Value Rights Agreement, dated January 27, 2010, among the Company, Metabasis Therapeutics, Inc., David F. Hale and Mellon Investor Services LLC | 8-K | 001-33093 | January 28, 2010 | 10.4 | ||||||||||||||||
| Amendment of General Contingent Value Rights Agreement, dated January 26, 2011, among the Company, Metabasis Therapeutics, Inc., David F. Hale and Mellon Investor Services LLC | 8-K | 001-33093 | January 31, 2011 | 10.1 | ||||||||||||||||
| Amendment of General Contingent Value Rights Agreement dated May 20, 2014 among the Company, Metabasis Therapeutics, Inc., David F. Hale and Computershare Inc. | 8-K | 001-33093 | May 22, 2014 | 10.1 | ||||||||||||||||
| Amendment of TR Beta Contingent Value Rights Agreement dated May 20, 2014 among the Company, Metabasis Therapeutics, Inc., David F. Hale and Computershare, Inc. | 8-K | 001-33093 | May 22, 2014 | 10.2 | ||||||||||||||||
| Captisol® Supply Agreement, dated December 20, 2002, among CyDex, Inc., Hovione LLC, Hovione FarmaCiencia S.A., Hovione Pharmascience Limited and Hovione International Limited | 10-K | 001-33093 | March 3, 2011 | 10.1 | ||||||||||||||||
| 1st Amendment to Captisol® Supply Agreement, dated July 29, 2005, among CyDex, Inc., Hovione LLC, Hovione FarmaCiencia S.A., Hovione Pharmascience Limited and Hovione International Limited | 10-K | 001-33093 | March 3, 2011 | 10.101 | ||||||||||||||||
| 2nd Amendment to Captisol® Supply Agreement, dated March 1, 2007, among CyDex, Inc., Hovione LLC, Hovione FarmaCiencia S.A., Hovione Pharmascience Limited, and Hovione International Limited | 10-K | 001-33093 | March 3, 2011 | 10.102 | ||||||||||||||||
| 3rd Amendment to Captisol® Supply Agreement, dated January 25, 2008, among CyDex, Inc., Hovione LLC, Hovione FarmaCiencia S.A., Hovione Pharmascience Limited, and Hovione International Limited | 10-K | 001-33093 | March 3, 2011 | 10.103 | ||||||||||||||||
| 4th Amendment to Captisol® Supply Agreement, dated September 28, 2009, among CyDex Pharmaceuticals, Inc., Hovione LLC, Hovione FarmaCiencia S.A., Hovione Pharmascience Limited and Hovione International Limited | 10-K | 001-33093 | March 3, 2011 | 10.104 | ||||||||||||||||
| License Agreement, dated September 3, 1993, between CyDex L.C. and The University of Kansas | 10-K | 001-33093 | March 3, 2011 | 10.105 | ||||||||||||||||
| First Amendment to License Agreement, dated February 24, 1998, between CyDex, Inc. and The University of Kansas | 10-K | 001-33093 | March 3, 2011 | 10.106 | ||||||||||||||||
| Second Amendment to License Agreement, dated August 4, 2004, between CyDex, Inc. and The University of Kansas | 10-K | 001-33093 | March 3, 2011 | 10.107 | ||||||||||||||||
| Acknowledgement Agreement, dated February 22, 2008, between CyDex, Inc. and The University of Kansas | 10-K | 001-33093 | March 3, 2011 | 10.111 | ||||||||||||||||
| Exclusive License Agreement, dated June 4, 1996, between Pfizer, Inc. and The University of Kansas | 10-K | 001-33093 | March 3, 2011 | 10.108 | ||||||||||||||||
| Addendum to Nonexclusive License Agreement, dated December 11, 2001, between CyDex, Inc. and Pfizer, Inc. | 10-K | 001-33093 | March 3, 2011 | 10.11 | ||||||||||||||||
| License Agreement, by and between CyDex Pharmaceuticals, Inc. and Spectrum Pharmaceuticals, Inc., dated as of March 8, 2013 | 10-Q | 001-33093 | May 8, 2013 | 10.2 | ||||||||||||||||
| Supply Agreement, by and between CyDex Pharmaceuticals, Inc. and Spectrum Pharmaceuticals, Inc., dated as of March 8, 2013 | 10-Q | 001-33093 | May 8, 2013 | 10.3 | ||||||||||||||||
95
| Royalty Stream and Milestone Payments Purchase Agreement, dated April 29, 2013, between the Company and Selexis S.A. | 10-Q | 001-33093 | August 1, 2013 | 10.2 | ||||||||||||||||
| Master License Agreement dated May 21, 2014 among the Company, Metabasis Therapeutics, Inc. and Viking Therapeutics, Inc. | 10-Q | 001-33093 | August 5, 2014 | 10.2 | ||||||||||||||||
| First Amendment to Master License Agreement dated September 6, 2014 among the Company, Metabasis Therapeutics, Inc. and Viking Therapeutics, Inc. | 10-Q | 001-33093 | October 31, 2014 | 10.9 | ||||||||||||||||
| Second Amendment to Master License Agreement, dated April 8, 2015, among the Company, Metabasis Therapeutics, Inc. and Viking Therapeutics, Inc. | 10-Q | 001-33093 | August 5, 2015 | 10.1 | ||||||||||||||||
| Letter Agreement, dated as of August 12, 2014, between Bank of America, N.A. and the Company regarding the Base Issuer Warrant Transaction | 8-K | 001-33093 | August 18, 2014 | 10.2 | ||||||||||||||||
| Letter Agreement, dated as of August 12, 2014, between Deutsche Bank AG, London Branch and the Company regarding the Base Issuer Warrant Transaction | 8-K | 001-33093 | August 18, 2014 | 10.4 | ||||||||||||||||
| Letter Agreement, dated as of August 14, 2014, between Bank of America, N.A. and the Company regarding the Additional Issuer Warrant Transaction | 8-K | 001-33093 | August 18, 2014 | 10.6 | ||||||||||||||||
| Letter Agreement, dated as of August 14, 2014, between Deutsche Bank AG, London Branch and the Company regarding the Additional Issuer Warrant Transaction | 8-K | 001-33093 | August 18, 2014 | 10.8 | ||||||||||||||||
| Development Funding and Royalties Agreement, dated December 13, 2018, by and between Ligand Pharmaceuticals Incorporated and Palvella Therapeutics, Inc. | 10-K | 001-33093 | February 28, 2019 | 10.48 | ||||||||||||||||
| Sublicense Agreement between the Company, Pharmacopeia, Inc. and Retrophin LLC dated as of February 16, 2012, as amended through Amendment No. 5 to Sublicense Agreement, dated March 20, 2018. | 10-K | 001-33093 | February 28, 2022 | 10.37 | ||||||||||||||||
| Interest Purchase Agreement, dated May 3, 2016, between the Company and CorMatrix Cardiovascular, Inc. | 8-K/A | 001-33093 | May 9, 2016 | 10.1 | ||||||||||||||||
| Amended and Restated Interest Purchase Agreement, dated May 31, 2017, between the Company and CorMatrix Cardiovascular, Inc. | 10-Q | 001-033093 | August 9, 2017 | 10.2 | ||||||||||||||||
| Letter Agreement, dated as of May 17, 2018, between Barclays Capital Inc. and the Company regarding the Base Convertible Note Hedge Transaction | 8-K | 001-00393 | May 22, 2018 | 10.1 | ||||||||||||||||
| Letter Agreement, dated as of May 17, 2018, between Barclays Capital Inc. and the Company regarding the Base Issuer Warrant Transaction | 8-K | 001-00393 | May 22, 2018 | 10.2 | ||||||||||||||||
| Letter Agreement, dated as of May 17, 2018, between Deutsche Bank AG and the Company regarding the Base Convertible Note Hedge Transaction | 8-K | 001-00393 | May 22, 2018 | 10.3 | ||||||||||||||||
| Letter Agreement, dated as of May 17, 2018, between Deutsche Bank AG and the Company regarding the Base Issuer Warrant Transaction | 8-K | 001-00393 | May 22, 2018 | 10.4 | ||||||||||||||||
| Letter Agreement, dated as of May 17, 2018, between Goldman Sachs & Co. LLC and the Company regarding the Base Convertible Note Hedge Transaction | 8-K | 001-00393 | May 22, 2018 | 10.5 | ||||||||||||||||
| Letter Agreement, dated as of May 17, 2018, between Goldman Sachs & Co. LLC and the Company regarding the Base Issuer Warrant Transaction | 8-K | 001-00393 | May 22, 2018 | 10.6 | ||||||||||||||||
96
| Letter Agreement, dated as of May 18, 2018, between Barclays Capital Inc. and the Company regarding the Additional Convertible Note Hedge Transaction | 8-K | 001-00393 | May 22, 2018 | 10.7 | ||||||||||||||||
| Letter Agreement, dated as of May 18, 2018, between Barclays Capital Inc. and the Company regarding the Additional Warrant Transaction | 8-K | 001-00393 | May 22, 2018 | 10.8 | ||||||||||||||||
| Letter Agreement, dated as of May 18, 2018, between Deutsche Bank AG and the Company regarding the Additional Convertible Note Hedge Transaction | 8-K | 001-00393 | May 22, 2018 | 10.9 | ||||||||||||||||
| Letter Agreement, dated as of May 18, 2018, between Deutsche Bank AG and the Company regarding the Additional Warrant Transaction | 8-K | 001-00393 | May 22, 2018 | 10.10 | ||||||||||||||||
| Letter Agreement, dated as of May 18, 2018, between Goldman Sachs & Co. LLC and the Company regarding the Additional Convertible Note Hedge Transaction | 8-K | 001-00393 | May 22, 2018 | 10.11 | ||||||||||||||||
| Letter Agreement, dated as of May 18, 2018, between Goldman Sachs & Co. LLC and the Company regarding the Additional Warrant Transaction | 8-K | 001-00393 | May 22, 2018 | 10.12 | ||||||||||||||||
| Form of Indemnification Agreement between the Company and each of its directors | 10-K | 001-33093 | March 1, 2018 | 10.60 | ||||||||||||||||
| Form of Indemnification Agreement between the Company and each of its officers | 10-K | 001-33093 | March 1, 2018 | 10.61 | ||||||||||||||||
| Addendum, dated May 22, 2019, by and among Ligand Pharmaceuticals Incorporated, CyDex Pharmaceuticals, Inc., and Acrotech Biopharma LLC (as successor-in-interest to Spectrum Pharmaceuticals, Inc.), to that certain License Agreement between Ligand Pharmaceuticals Incorporated and Spectrum Pharmaceuticals, Inc., dated March 8, 2013 | 10-Q | 001-33093 | August 8, 2019 | 10.1 | ||||||||||||||||
| Call Option Amendment Agreement, dated April 6, 2020, between the Registrant and Barclays Bank PLC | 10-Q | 001-33093 | May 8, 2020 | 10.1 | ||||||||||||||||
| Call Option Amendment Agreement, dated April 6, 2020, between the Registrant and Deutsche Bank AG, London Branch | 10-Q | 001-33093 | May 8, 2020 | 10.2 | ||||||||||||||||
| Call Option Amendment Agreement, dated April 6, 2020, between the Registrant and Goldman Sachs & Co. LLC | 10-Q | 001-33093 | May 8, 2020 | 10.3 | ||||||||||||||||
| Call Option Amendment Agreement, dated January 28, 2021, between the Registrant and Barclays Bank PLC | 10-K | 001-33093 | February 24, 2021 | 10.67 | ||||||||||||||||
| Call Option Amendment Agreement, dated January 28, 2021, between the Registrant and Deutsche Bank AG, London Branch | 10-K | 001-33093 | February 24, 2021 | 10.68 | ||||||||||||||||
Call Option Amendment Agreement, dated January 28, 2021, between the Registrant and Goldman Sachs & Co. LLC | 10-K | 001-33093 | February 24, 2021 | 10.69 | ||||||||||||||||
| Supply agreement, dated December 22, 2015, by and between Cydex Pharmaceuticals, Inc. and Gilead Sciences, Inc. | 10-K | 001-33093 | February 24, 2021 | 10.72 | ||||||||||||||||
| Amendment to Supply Agreement, dated September 21, 2020, by and between Cydex Pharmaceuticals, Inc. and Gilead Sciences, Inc., which amends that certain Supply Agreement, dated December 2, 2015, by and between Cydex Pharmaceuticals, Inc. and Gilead Sciences, Inc. | 10-Q | 001-33093 | November 6, 2020 | 10.2 | ||||||||||||||||
| Subsidiaries of the Company | X | |||||||||||||||||||
| Consent of Independent Registered Public Accounting Firm | X | |||||||||||||||||||
97
| Certification by Principal Executive Officer, Pursuant to Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | X | |||||||||||||||||||
| Certification by Principal Financial Officer, Pursuant to Rules 13a-14(a) and 15d-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | X | |||||||||||||||||||
| Certifications by Principal Executive Officer and Principal Financial Officer, Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | X | |||||||||||||||||||
| 101 | The following financial information from our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, formatted in iXBRL (inline eXtensible Business Reporting Language): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Operations, (iii) Consolidated Statement of Comprehensive Income, (iv) Consolidated Statements of Stockholders' Equity, (v) Consolidated Statements of Cash Flows, and (vi) the Notes to Consolidated Financial Statements. | X | ||||||||||||||||||
| 104 | The cover page from the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2022, formatted in Inline XBRL and contained in Exhibit 101. | X | ||||||||||||||||||
† Confidential treatment has been requested for portions of this exhibit. These portions have been omitted and submitted separately to the Securities and Exchange Commission.
# Indicates management contract or compensatory plan.
* Certain schedules and annexes have been omitted in accordance with Item 601(a)(5) of Regulation S-K. A copy of any omitted schedule and/or annex will be furnished as a supplement to the U.S. Securities and Exchange Commission upon request.
** Pursuant to Item 601(b)(10) of Regulation S-K, certain confidential portions of this exhibit were omitted by means of marking such portions with an asterisk because the identified confidential portions (i) are not material and (ii) would be competitively harmful if publicly disclosed.
| Item 16. | Form 10-K Summary | ||||
None
98
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| LIGAND PHARMACEUTICALS INCORPORATED | |||||
| By: | /S/ TODD C. DAVIS | ||||
| Todd C. Davis, | |||||
| Chief Executive Officer | |||||
Date: February 28, 2023
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||||||||||||
| /s/ TODD C. DAVIS | Chief Executive Officer and Director (Principal Executive Officer) | February 28, 2023 | ||||||||||||
| Todd C. Davis | ||||||||||||||
| /s/ OCTAVIO ESPINOZA | Chief Financial Officer (Principal Financial and Accounting Officer) | February 28, 2023 | ||||||||||||
| Octavio Espinoza | ||||||||||||||
| /s/ JOHN W. KOZARICH | Director and Chairman of the Board | February 28, 2023 | ||||||||||||
| John W. Kozarich | ||||||||||||||
| /s/ JASON M. ARYEH | Director | February 28, 2023 | ||||||||||||
| Jason M. Aryeh | ||||||||||||||
| /s/ NANCY R. GRAY | Director | February 28, 2023 | ||||||||||||
| Nancy R. Gray | ||||||||||||||
| /s/ JASON HAAS | Director | February 28, 2023 | ||||||||||||
| Jason Haas | ||||||||||||||
| /s/ JOHN L. LAMATTINA | Director | February 28, 2023 | ||||||||||||
| John L. LaMattina | ||||||||||||||
| /s/ STEPHEN L. SABBA | Director | February 28, 2023 | ||||||||||||
| Stephen L. Sabba | ||||||||||||||
99