

UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a)
of the
Securities Exchange Act of 1934 (Amendment No. )
 |
Filed by the Registrant |  |
Filed by a Party other than the Registrant |
| Check the appropriate box: | |
 |
Preliminary Proxy Statement |
 |
Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
 |
Definitive Proxy Statement |
 |
Definitive Additional Materials |
 |
Soliciting Material under §240.14a-12 |

(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
| Payment of Filing Fee (Check all boxes that apply): | |
 |
No fee required |
 |
Fee paid previously with preliminary materials |
 |
Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11 |




| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
2022 was another productive year at Regeneron, marked by significant achievements that positioned us to reach more patients with our medicines while advancing our robust investigational pipeline and novel technologies. We are inspired by the incredible talent and dedication of our team who relentlessly pursue our mission of turning promising science into meaningful medicines. As we mark Regeneron’s 35th year, we reflect on the fundamentals that have brought us to this point and look ahead with confidence that the best is yet to come.
We believe the business strategy we pursued for decades will continue to drive our future success: we consistently prioritize investment in Regeneron’s research capabilities, homegrown technologies, and high-performing team – and we continue to complement our internal capabilities with highly productive external collaborations. Our strong performance in 2022 represents the fruits of this strategy and fuels the virtuous cycle of continuous reinvestment into the research and development (“R&D”) engine that produced EYLEA® (aflibercept) Injection, Dupixent® (dupilumab), Libtayo® (cemiplimab), and six other Regeneron medicines approved by the U.S. Food and Drug Administration (the “FDA”). This engine is also producing the medicines of the future, generating our clinical pipeline of over 30 distinct and largely homegrown assets, which we believe bodes well for our future prospects.
Looking ahead to 2023 and beyond, we expect to retain and grow our leadership positions in ophthalmology and allergic diseases while expanding our portfolio to include best-in-class oncology bispecific and costimulatory bispecific antibodies, as well as novel genetics medicine approaches such as CRISPR, gene silencing, and gene therapy.
In 2022, Regeneron, together with its collaborators, delivered record global net product sales of EYLEA, Dupixent, and Libtayo, contributing to full-year GAAP net income of $4.34 billion and total revenues of $12.17 billion. Total revenues grew 17% compared to 2021 when excluding contributions from our COVID-19 antibody cocktail REGEN-COV®/Ronapreve™ (casirivimab and imdevimab).1 In addition to investing nearly $3.6 billion in Regeneron’s R&D capabilities in 2022, we have continued to execute on our capital allocation strategy. In 2022, we invested approximately $1.3 billion in business development initiatives and returned significant value to our shareholders through share repurchases, with $2.1 billion of our common stock repurchased in 2022 and nearly $10 billion repurchased since 2019.
These strong financial results were driven by execution on the following three strategic imperatives.
Fortify our leadership in the treatment of retinal diseases:
EYLEA continued to be the No. 1 prescribed anti-VEGF treatment for retinal diseases in 2022, capturing approximately 75% of the branded market share and reaching more than 57 million injections globally since its launch 11 years ago. Global net product sales grew 4% year-over-year to $9.65 billion in 2022.2 In February 2023, EYLEA gained FDA approval as the first pharmacological treatment for infants with prematurity of retinopathy, expanding our opportunity to help some of the most vulnerable patients with unmet needs.
In 2022, we also shared positive clinical trial results for aflibercept 8 mg, which demonstrated that extended dosing regimens in neovascular age-related macular degeneration and diabetic macular edema achieved non-inferiority in vision gains and a consistent safety profile compared to EYLEA’s eight-week dosing regimen. Given prescribers’ decade-plus experience with EYLEA, we believe that, over time, there is an opportunity for aflibercept 8 mg to significantly reduce the treatment burden for patients and to become a new standard of care for these conditions. Our Biologics License Application was accepted by the FDA for priority review, and we anticipate a decision by late June of this year.
1 |
The casirivimab and imdevimab antibody cocktail is known as REGEN-COV in the United States and Ronapreve in other countries. Regeneron records net product sales of REGEN-COV in the United States and Roche records net product sales of Ronapreve outside the United States. REGEN-COV was authorized under an emergency use authorization from the FDA for COVID-19 from November 2020 until January 2022 when the emergency use authorization was revised to exclude its use in geographic regions where infection or exposure is likely due to a variant that is not susceptible to the treatment. Revenues excluding REGEN-COV and Ronapreve is a measure not calculated in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). See Appendix A for a definition of this measure and a reconciliation of this measure to the most directly comparable GAAP financial measure. |
2 |
Our collaborator Bayer records net product sales for EYLEA outside the United States. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
Maintain and grow Dupixent:
2022 was another banner year for Dupixent, which is now approved in five related type 2 inflammatory diseases. Global net product sales were approximately $8.7 billion in 2022, a year-over-year increase of 40%.3 Despite new competition in 2022, Dupixent strengthened its market-leading position in atopic dermatitis, asthma, and nasal polyps, and was also approved in new indications (eosinophilic esophagitis and prurigo nodularis), additional geographies, and patient populations as young as six months of age.
These approvals meaningfully expanded the Dupixent commercial opportunity, with potential to reach even more patients across established and new indications. Regeneron and Sanofi recently announced exciting results from a pivotal trial demonstrating the potential of Dupixent to become the first biologic to treat chronic obstructive pulmonary disease in patients with type 2 inflammation by showing significant reduction in exacerbations compared to placebo and improvement in lung function and quality of life. We look forward to discussing these results with regulatory authorities.
Advance our hematology/oncology portfolio:
We made significant progress advancing our oncology portfolio and pipeline in 2022. Full-year global net product sales for Libtayo, our homegrown PD-1 inhibitor, grew 26% year-over-year to $578 million,4 and the medicine is now FDA approved in five indications. In addition, Regeneron acquired Sanofi’s share of global rights to Libtayo, which we viewed as a necessary step toward realizing the clinical and commercial potential of our diverse oncology portfolio. We can now fully explore multiple combinations with Libtayo as our foundation – including with our LAG3 antibody, fianlimab, costimulatory CD28 bispecifics, and CD3 bispecifics, among others. In 2022, we also completed our first-ever acquisition of another biotechnology company, Checkmate Pharmaceuticals, Inc., adding a new modality to our portfolio of potential combination-ready approaches for difficult-to-treat cancers and deepening our commitment to oncology.
In 2023, we plan to report results from multiple studies and complete key regulatory submissions that will further establish our leadership in oncology.
Beyond delivering on these three strategic imperatives in 2022, we were also focused on building the next chapter of scientific leadership at Regeneron.
We made important progress in our human genetics research and genetics medicine technology efforts in 2022. The Regeneron Genetics Center® (“RGC”) marked its tenth year and has built one of the largest and most diverse genomic biobanks in the world, yielding many actionable insights – another example of strategic reinvestment that has produced tangible benefits for our preclinical and clinical pipelines. In 2022, we announced that RGC scientists uncovered rare genetic loss-of-function mutations in the CIDEB gene that are associated with substantial protection from liver disease, including nonalcoholic steatohepatitis (“NASH”) and cirrhosis. Discoveries such as this open the door to potential new medicines for notoriously hard-to-treat diseases, whether via antibody therapeutics or other modalities.
Our Regeneron Genetics Medicine group is exploring such approaches through strategic collaborations. For example, in collaboration with Intellia Therapeutics, Inc., we are working to advance potentially groundbreaking applications of CRISPR technology. In 2022, interim results from an ongoing Phase 1 study of NTLA-2001 showed our CRISPR/Cas9 therapy has the potential to become the first single-dose, in vivo treatment to halt and even reverse the underlying cause of transthyretin (ATTR) amyloidosis. We are continuing to advance this program in 2023, as well as other genetics medicine projects, including those that explore gene silencing in hard-to-treat diseases such as NASH and Alzheimer’s disease.
Finally, while we have entered a new phase of the COVID-19 pandemic, the virus is still present and disproportionately affecting people who are immunocompromised. Since need remains, we continue to identify and study novel antibodies that may provide treatment for, and protection against, new strains. We are progressing a unique candidate that appears to neutralize all known variants of the COVID-19 virus by binding to a highly conserved epitope of the spike protein, and we hope to begin clinical trials this year.
In order to advance all this exciting work across the full drug discovery and development spectrum, we need a strong and committed team. We now have more than 12,000 dedicated employees across seven countries and are honored to be ranked once again among the top employers by Science magazine. We are particularly proud of our industry-leading Industrial Operations and Product Supply team, who emphasize continual improvement and teamwork to ensure our medicines are safe and available for patients around the globe. To keep up with this talented and growing workforce,
3 |
Our collaborator Sanofi records global net product sales of Dupixent. |
4 |
Prior to July 1, 2022, our collaborator Sanofi recorded net product sales of Libtayo outside the United States. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
we are taking steps to ensure we retain our nimble and innovative culture and to invest in our facilities, with a $1.8 billion project underway at our headquarters in Tarrytown, N.Y. and a new 350,000 square foot manufacturing facility in Rensselaer, N.Y.
Our employees do not just focus on getting things done, but care about how we do this – with precision, ingenuity, and the highest ethical standards. These priorities are consistent with our company motto of “Doing Well by Doing Good” and our company values and behaviors known as The Regeneron Way. We invite you to read more about our efforts to improve the lives of people with serious diseases, sustain our culture of integrity and excellence, and build sustainable communities in our 2022 Responsibility Report and our inaugural Diversity, Equity, and Inclusion (“DEI”) Annual Impact Report. Notably, we believe our DEI strategy to build a “Better Workplace, Better Science, Better World” provides a framework to cultivate talent, fuel discovery, and advance our business.
On a personal note from Roy, this will be my last letter to shareholders. I have had the privilege of serving as Chair for nearly three decades and watched with pride as Regeneron has transformed from a small biotechnology company with big ideas into a successful research-based biopharmaceutical company that improves the lives of millions of patients. I am confident that the Company under the leadership of Len, George, and Christine Poon, who will assume the role of lead independent director, will continue to bring forward value to society and shareholders.
Collectively, we remain assured in our near- and long-term growth prospects, with an increasingly diverse commercial portfolio reaching new patients and geographies, clinical trial initiations expected for numerous new therapeutic candidates, and one of the most innovative R&D engines in the industry. In our 35th year, we are proud to say that Regeneron is extremely well positioned to continue executing on our mission and delivering breakthroughs for patients and healthcare professionals around the world.
Sincerely,

|

|

|
P. Roy Vagelos, M.D., |
Leonard S. Schleifer, M.D., Ph.D., |
George D. Yancopoulos, M.D., Ph.D., |
Chair of the Board |
President and Chief Executive Officer |
President and Chief Scientific Officer |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |

| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |

REGENERON PHARMACEUTICALS, INC.
777 Old Saw Mill River Road
Tarrytown, New York 10591-6707
The 2023 Annual Meeting of Shareholders of Regeneron Pharmaceuticals, Inc. (the “Company”) will be held on Friday, June 9, 2023, commencing at 10:30 a.m., Eastern Time, virtually via the Internet at www.virtualshareholdermeeting.com/REGN2023, for the following purposes:
to elect four Class II directors for a three-year term;
to ratify the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2023;
to cast an advisory vote to approve the compensation of the Company’s Named Executive Officers as disclosed in these proxy materials (say-on-pay);
to cast an advisory vote on whether future say-on-pay votes should be held every one, two, or three years (say-on-frequency);
if properly presented, to vote on a non-binding shareholder proposal; and
to act upon such other matters as may properly come before the meeting and any adjournment(s) or postponement(s) thereof.
The board of directors has fixed the close of business on April 11, 2023 as the record date for determining shareholders entitled to notice of, and to vote at, the Annual Meeting and at any adjournment(s) or postponement(s) thereof.
Pursuant to the rules of the Securities and Exchange Commission (the “SEC”), we have elected to use the “Notice and Access” method of providing our proxy materials over the Internet. Accordingly, we will mail, beginning on or about April 21, 2023, a Notice of Internet Availability of Proxy Materials to our shareholders of record and beneficial owners as of the record date (other than (i) those who previously elected to receive proxy materials by e-mail, (ii) those who have previously asked to receive paper copies of the proxy materials, and (iii) shareholders who participate and hold shares of common stock in the Regeneron Pharmaceuticals, Inc. 401(k) Savings Plan or the Regeneron Ireland Share Participation Plan). As of the date of mailing of the Notice of Internet Availability of Proxy Materials, all shareholders and beneficial owners will have the ability to access all of the proxy materials on a website referenced in the Notice of Internet Availability of Proxy Materials.
The Notice of Internet Availability of Proxy Materials also contains a toll-free telephone number, an e-mail address, and a website where shareholders can request a paper or electronic copy of the proxy statement, our 2022 annual report, and/or a form of proxy relating to the Annual Meeting. These materials are available free of charge. The Notice also contains information on how to access and vote the form of proxy.
We have opted to hold the Annual Meeting as a virtual-only meeting, which means that you will not be able to attend the Annual Meeting in person. All shareholders will be able to attend the Annual Meeting and participate electronically, which will allow them to vote their shares on the date of the Annual Meeting and ask questions during the meeting. Please visit our website at http://newsroom.regeneron.com for the most up-to-date information about the Annual Meeting. We have designed the format of the Annual Meeting to ensure that shareholders are afforded similar rights and opportunities to participate as they would at an in-person meeting.
As Authorized by the Board of Directors,

Joseph J. LaRosa
Executive Vice President, General Counsel and Secretary
April 21, 2023
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |

| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
TABLE OF CONTENTS
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ i |
TABLE OF CONTENTS (CONT.)
NOTE REGARDING FORWARD-LOOKING STATEMENTS AND NON-GAAP FINANCIAL MEASURES: See Appendix A for important information regarding forward-looking statements and financial measures not calculated in accordance with U.S. Generally Accepted Accounting Principles contained in this proxy statement.
NOTE REGARDING TRADEMARKS AND PRODUCT NAMES: “ARCALYST®,” “Evkeeza®,” “EYLEA®,” “Inmazeb®,” “Libtayo®”, “Praluent®” (in the United States), “REGEN-COV®,” Regeneron®,” “Regeneron Genetics Center®,” “VelociGene®,” “VelocImmune®,” and “ZALTRAP®” are trademarks of Regeneron Pharmaceuticals, Inc. (“Regeneron”). This proxy statement refers to products marketed or otherwise commercialized by Regeneron, its collaborators, and other parties. Consult the product label in each territory for specific information about such products.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ ii |
Meeting Date: |
Time: |
Location: |
Record Date: |
June 9, 2023 |
10:30 a.m., ET |
Online at |
APRIL 11, 2023 |
|
Matter |
Board Vote Recommendation |
1 |
Election of four Class II directors for a three-year term |
FOR each director nominee 
|
2 |
Ratification of the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2023 |
FOR 
|
3 |
Advisory vote to approve the compensation of the Company’s Named Executive Officers as disclosed in these proxy materials (say-on-pay) |
FOR 
|
4 |
Advisory vote on the frequency of future say-on-pay votes (say-on-frequency) |
Every ONE year
|
5 |
Non-binding shareholder proposal, if properly presented |
AGAINST 
|
See “General Information about the Meeting and Other Matters” starting on page 110 for questions and answers related to the annual meeting, how to vote, and other matters.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 1 |
As you review this year’s proposals, we encourage you to consider our compensation and governance practices in light of our track record of long-term shareholder value creation and delivering on our mission of repeatedly bringing important new medicines to patients living with serious diseases.
Shareholder value creation over the last decade driven by relentless innovation and product pipeline progress
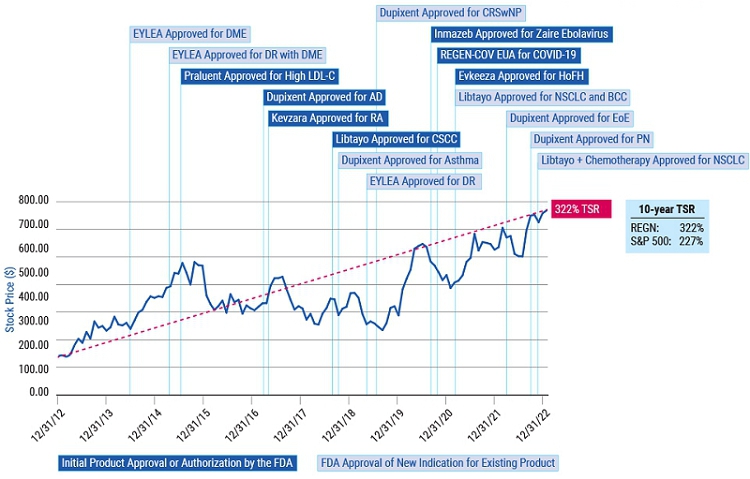
DME = diabetic macular edema; DR = diabetic retinopathy; LDL-C = low-density lipoprotein cholesterol; AD = atopic dermatitis; RA = rheumatoid arthritis; CSCC = cutaneous squamous cell carcinoma; CRSwNP = chronic rhinosinusitis with nasal polyposis; EUA = Emergency Use Authorization from FDA; FDA = U.S. Food and Drug Administration; HoFH = homozygous familial hypercholesterolemia; NSCLC = non-small cell lung cancer; BCC = basal cell carcinoma; EoE = eosinophilic esophagitis; and PN = prurigo nodularis. Consult the product label in each approved territory for specific information about such products and indications.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 2 |
In the last decade, we have actively and regularly engaged with our shareholders to receive feedback on many important areas including governance, compensation, and corporate responsibility matters. Shareholder feedback is discussed with management and, depending on the topic, relayed for consideration to the appropriate committee of the board of directors (typically the Compensation Committee or the Corporate Governance and Compliance Committee), the full board, or both. In recent years, shareholder input resulted in specific changes to our compensation and corporate governance practices and policies. For example, after careful consideration of shareholder feedback following the 2021 annual shareholder meeting, in 2022 our board of directors voluntarily changed the frequency of our say-on-pay votes from every three years to every year.
Since the 2022 annual shareholder meeting, we reached out to shareholders collectively representing over 60% of the public shares (i.e., shares of common stock outstanding as of December 31, 2022, excluding shares held by our directors and executive officers). This outreach resulted in one-on-one discussions with shareholders representing nearly 50% of our public shares.

Our 2022 outreach built on an active outreach program in prior years and focused on, among other matters:
the board’s director refreshment efforts, including how the recent election of Dr. Thompson is consistent with the board’s director refreshment philosophy (discussed further in the subsection “Board of Directors—Board Governance—Director Refreshment Philosophy”);
the Company’s classified board structure, including relevant considerations unique to the Company and its long-term orientation (discussed further in the subsection “Board of Directors—Board Governance—Board Structure”);
the Company’s dual-class capital structure, including relevant mitigating factors such as the steady decline in the number of shares of Class A stock outstanding since the Company’s initial public offering (“IPO”) and the relatively low percentage of overall votes controlled by the Class A shareholders, particularly when compared to other founder-led companies (discussed further in the subsection “The Company—Corporate Governance—Capital Structure”); and
investor views of the newly mandated “pay-versus-performance” disclosure (which we provide in the subsection “Compensation Dashboard—Additional Compensation Information—Pay Versus Performance”).
We had meaningful and candid discussions about these and other corporate governance topics in 2022, and the feedback received from shareholders was discussed in detail at the board level and has already shaped the recent meeting agenda and discussion in the boardroom. For example, as described further in the subsection “Board of Directors—Board Governance—Board Structure,” 2022 shareholder feedback led the Corporate Governance and Compliance Committee to request, review, and discuss a detailed analysis of the costs and benefits of the Company’s classified board structure in order to facilitate the Committee’s continued consideration of this feature of Regeneron’s governance. We remain committed to continued engagement with shareholders to understand your viewpoints and better convey the board’s approach to corporate governance with the goal of ensuring independent oversight of management’s execution of our business strategy and the continued creation of sustainable shareholder value.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 3 |

From Left to Right:
Bonnie L. Bassler, Ph.D., Michael S. Brown, M.D., N. Anthony Coles, M.D., Joseph L. Goldstein, M.D., Christine A. Poon, Arthur F. Ryan, Leonard S. Schleifer, M.D., Ph.D., George L. Sing, Marc Tessier-Lavigne, Ph.D., Craig B. Thompson, M.D., P. Roy Vagelos, M.D., George D. Yancopoulos, M.D., Ph.D., Huda Y. Zoghbi, M.D.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 4 |
As the first substantive order of business at the 2023 Annual Meeting, you have an opportunity to vote on four members of our board of directors. This is the correct starting point not only because the board oversees Regeneron, but also because understanding the Regeneron board leads to a better understanding of the Company and its business model.
At Regeneron, we lead with science as we pursue our mission of repeatedly bringing important new medicines to patients living with serious diseases. Our business is built on investment in our deep scientific and technological capabilities, which drives our research, preclinical development, clinical, and manufacturing efforts.
The composition of our board is shaped by this business model and the recognition that our board members must have predominantly science-based backgrounds to effectively provide robust, independent oversight of management. The current makeup of our board reflects this principle: Eight of our 13 directors are members of the National Academy of Sciences, and our board includes two Nobel laureates and holders of many scientific awards. In addition, the board includes individuals with experience building shareholder value through all stages of corporate development. Various members also bring substantial governance, financial, policy, and management expertise gained from their professional backgrounds and their service on other boards.
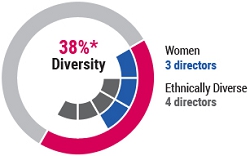
The board’s composition also reflects our commitment to ensure diversity of thought, experience, attributes, and background, as demonstrated by the fact that three of our board’s current 13 members are women and four members are racially or ethnically diverse. The following Board Diversity Matrix sets forth certain diversity information as self-reported by the current members of the board.
Board Diversity Matrix* |
||
Total Number of Directors |
13 |
|
|
Female |
Male |
Part I: Gender Identity |
|
|
Directors |
3 |
10 |
Part II: Demographic Background |
|
|
African American or Black |
- |
1 |
Asian |
1 |
1 |
White |
1 |
8 |
Directors who identify as Middle Eastern/Arab American: 1 Directors who are Military Veterans: 2 |
||
*
As of April 21, 2023. There have been no changes to this information since the publication of the Board Diversity Matrix dated as of November 14, 2022, which is posted on our website. |
||
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 5 |
|
*
As of April 21, 2023. On April 14, 2023, Dr. Vagelos notified the Company of his decision not to stand for re-election at the 2023 Annual Meeting. Following the 2023 Annual Meeting, the board is expected to be comprised of 12 members, of which 42% are diverse by gender and/or race/ethnicity. |
The board and the Corporate Governance and Compliance Committee seek to ensure that our directors as a group possesses the mix of skills and experiences to provide effective oversight and guidance to management to execute on the Company’s long-term strategy. The board and the Committee also consider succession planning for board and committee chairs for purposes of continuity and to maintain relevant expertise and depth of experience.
The table below summarizes key qualifications, skills, or attributes most relevant to the decision to nominate the director to serve on the board of directors. A mark indicates a specific area of focus or expertise on which the board of directors relies most. The lack of a mark does not mean the director does not possess that qualification or skill. Each director biography below describes these qualifications and relevant experience in more detail. We believe the table below demonstrates the breadth and diversity of the collective experience, expertise, and skills of our board of directors.
Experience, Expertise, or Attribute |
Bonnie L. Bassler, Ph.D. |
Michael S. Brown, M.D. |
N. Anthony Coles, M.D. |
Joseph L. Goldstein, M.D. |
Christine A. Poon |
Arthur F. Ryan |
Leonard S. Schleifer, M.D., Ph.D. |
George L. Sing |
Marc Tessier- Lavigne, Ph.D. |
Craig B. Thompson, M.D. |
P. Roy Vagelos, M.D. |
George D. Yancopoulos, M.D., Ph.D. |
Huda Y. Zoghbi, M.D. |
Industry Experience |
● |
● |
● |
● |
● |
● |
● |
● |
● |
● |
● |
● |
|
Executive/Leadership Experience |
● |
● |
● |
● |
● |
● |
● |
● |
● |
● |
● |
● |
● |
Science/Biotech Background |
● |
● |
● |
● |
● |
|
● |
● |
● |
● |
● |
● |
● |
Research/Academic Experience |
● |
● |
● |
● |
● |
|
● |
|
● |
● |
● |
● |
● |
Business Strategy/Operations Experience |
|
|
● |
|
● |
● |
● |
● |
● |
● |
● |
● |
|
Financial Expertise |
|
|
● |
|
● |
● |
● |
● |
|
● |
● |
|
|
Public Company CEO Experience |
|
|
● |
|
|
● |
● |
|
|
|
● |
|
|
National Academy of Sciences Membership |
● |
● |
|
● |
|
|
|
|
● |
● |
● |
● |
● |
Diverse by Gender |
● |
|
|
|
● |
|
|
|
|
|
|
|
● |
Diverse by Race/Ethnicity |
|
|
● |
|
● |
|
|
● |
|
|
|
|
● |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 6 |

Director since: 1991 Age: 82 Independent |
|
Board and Committee Membership—2022 Attendance |
|
Board of Directors: |
7/7 |
Corporate Governance and Compliance Committee: |
5/5 |
Technology Committee: |
2/2 |
Regeneron Securities Beneficially Owned as of April 11, 2023 |
|
Common Stock: |
5,000 |
Options: |
3,537 |
Restricted Stock Units (“RSUs”): |
1,153 |
Career Highlights |
•
Professor of Molecular Genetics and Internal Medicine and Chair of the Department of Molecular Genetics at The University of Texas Southwestern Medical Center at Dallas since 1977 •
Member of the boards of trustees of The Rockefeller University and the Howard Hughes Medical Institute •
Nobel Prize for Physiology or Medicine in 1985 (jointly with Dr. Brown) •
U.S. National Medal of Science in 1988 (jointly with Dr. Brown) |
Scientific Society Memberships |
•
The National Academy of Sciences •
The National Academy of Medicine •
The Royal Society of London |
Dr. Goldstein’s extensive research experience, his distinguished scientific and academic credentials, including his receipt of the Nobel Prize for Physiology or Medicine in 1985, and his substantial understanding of the Company gained through his service as a director, led to the board’s decision to nominate Dr. Goldstein for reelection to the board. |
1 |
Biographical information is given, as of April 11, 2023, for each nominee and for each of the other directors whose term of office will continue after the 2023 Annual Meeting. All nominees are presently directors and, with the exception of Dr. Thompson, were previously elected by the shareholders. None of the corporations or other organizations referred to below with which a director has been or is currently employed or otherwise associated is a parent, subsidiary, or affiliate of the Company. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 7 |

Director since: 2010 Age: 70 Independent |
|
Board and Committee Membership—2022 Attendance |
|
Board of Directors: |
7/7 |
Compensation Committee (Chair): Corporate Governance and Compliance Committee: |
12/12
5/5 |
Regeneron Securities Beneficially Owned as of April 11, 2023 |
|
Common Stock: |
790 |
Options: |
62,894 |
RSUs: |
1,153 |
Career Highlights |
•
Former Executive-in-Residence in the Department of Management and Human Resources and former Dean and John W. Berry, Sr. Chair in Business at The Max M. Fisher College of Business at The Ohio State University •
Former vice chair, worldwide chair of pharmaceuticals, member of the executive committee, and director at Johnson & Johnson •
Held senior leadership positions at Bristol-Myers Squibb Company, including president of international medicines and president of medical devices •
Former member of the Supervisory Board of Royal Philips Electronics and the board of directors of Decibel Therapeutics, Inc. |
Other Public Boards |
•
Prudential Financial, Inc. •
The Sherwin-Williams Company |
Ms. Poon’s extensive expertise in domestic and international business operations, including sales and marketing and commercial operations, and her deep strategic and operational knowledge of the pharmaceutical industry, led to the board’s decision to nominate Ms. Poon for reelection to the board. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 8 |

Director since: 2022 Age: 70 Independent |
|
Board and Committee Membership—2022 Attendance* |
|
Board of Directors: |
2/2 |
Technology Committee: |
1/1 |
* Dr. Thompson was elected as a member of the board and the Technology Committee effective October 3, 2022. |
|
Regeneron Securities Beneficially Owned as of April 11, 2023 |
|
Common Stock: |
0 |
Options: |
2,399 |
RSUs: |
207 |
Career Highlights |
•
Former President and Chief Executive Officer of Memorial Sloan Kettering Cancer Center •
Co-founder of Agios Pharmaceuticals, Inc. •
Former director of Merck & Co., Inc. |
Scientific Society Memberships |
•
The National Academy of Sciences •
The National Academy of Medicine •
The American Academy of Arts and Sciences •
The American Society for Clinical Investigation •
The Association of American Physicians |
Other Public Boards |
•
Charles River Laboratories International, Inc. |
Dr. Thompson’s extensive research and leadership experience in the pharmaceutical and healthcare industries, as well as his experience as a corporate director, led to the board’s decision to nominate Dr. Thompson for reelection to the board. |
|
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 9 |

Director since: 2016 Age: 68 Independent |
|
Board and Committee Membership—2022 Attendance |
|
Board of Directors: |
6/7 |
Compensation Committee: |
11/12 |
Technology Committee: |
2/2 |
Regeneron Securities Beneficially Owned as of April 11, 2023 |
|
Common Stock: |
0 |
Options: |
27,289 |
RSUs: |
1,153 |
Career Highlights |
•
Professor in the departments of Pediatrics, Molecular and Human Genetics, and Neurology and Neuroscience at Baylor College of Medicine since 1994 •
Director of the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital •
Howard Hughes Medical Institute Investigator •
Pearl Meister Greengard Prize •
March of Dimes Prize in Developmental Biology •
Vanderbilt Prize in Biomedical Science |
Scientific Society Memberships |
•
The National Academy of Sciences •
The Institute of Medicine •
The American Association for the Advancement of Science |
Dr. Zoghbi’s extensive research experience and her scientific and academic career and accomplishments led to the board’s decision to nominate Dr. Zoghbi for reelection to the board. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 10 |

Director since: 2017 Age: 62 Independent |
|
Board and Committee Membership—2022 Attendance |
|
Board of Directors: |
6/7 |
Audit Committee: |
8/9 |
Regeneron Common Stock Beneficially Owned as of April 11, 2023 |
|
Common Stock: |
11 |
Options: |
3,537 |
RSUs: |
1,153 |
Career Highlights |
•
President and Chief Executive Officer since 2019 and Chair since 2018 of Cerevel Therapeutics Holdings, Inc., the parent entity of Cerevel Therapeutics, Inc. •
Chair and CEO of TRATE Enterprises LLC, a privately-held company, since 2013 •
Former Chief Executive Officer and Chair of the Board of Yumanity Therapeutics, Inc. •
Former President, Chief Executive Officer and Chair of the Board of Onyx Pharmaceuticals, Inc. •
Former President, Chief Executive Officer, and member of the board of directors of NPS Pharmaceuticals, Inc. •
Previously held various leadership positions in the biopharmaceutical and pharmaceutical industries, including at Merck & Co., Inc., Bristol-Myers Squibb Company, and Vertex Pharmaceuticals Incorporated •
Former director of Laboratory Corporation of America Holdings, Campus Crest Communities, Inc., CRISPR Therapeutics AG, and McKesson Corporation |
Other Public Boards |
•
Cerevel Therapeutics Holdings, Inc. |
Dr. Coles’s experience as a seasoned executive and corporate director with extensive knowledge of highly regulated biopharmaceutical and pharmaceutical companies, as well as his in-depth knowledge and understanding of the regulatory environment in which Regeneron operates, led the board to conclude that Dr. Coles should serve as a director. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 11 |

Director since: 2003 Age: 80 Independent |
|
Board and Committee Membership—2022 Attendance |
|
Board of Directors (Presiding Director): |
7/7 |
Audit Committee: |
9/9 |
Corporate Governance and Compliance Committee (Chair): |
5/5 |
Regeneron Securities Beneficially Owned as of April 11, 2023 |
|
Common Stock: |
17,800 |
Options: |
3,537 |
RSUs: |
1,153 |
Career Highlights |
•
Former Chief Executive Officer and Chair of the Board of Prudential Financial, Inc. •
President and Chief Operating Officer of Chase Manhattan Bank from 1990 to 1994 •
Managed Chase’s worldwide retail bank between 1984 and 1990 •
Non-executive director of the Royal Bank of Scotland Group plc from 2008 to 2013 •
Director of Citizens Financial Group, Inc. from 2009 to 2019 |
Mr. Ryan’s substantial leadership experience as a chief executive officer of leading companies in the banking and insurance industries, and his extensive business experience and financial expertise, led the board to conclude that Mr. Ryan should serve as a director. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 12 |

Director since: 1988 Age: 73 Independent |
|
Board and Committee Membership—2022 Attendance |
|
Board of Directors: |
7/7 |
Audit Committee (Chair): |
9/9 |
Compensation Committee: |
12/12 |
Regeneron Securities Beneficially Owned as of April 11, 2023 |
|
Common Stock: |
27,117 |
Options: |
50,614 |
RSUs: |
1,153 |
Career Highlights |
•
Chief Executive Officer of GanD, Inc. since 2016 •
Chair of Grace Science, LLC since 2017 •
Extensive venture capital and leadership experience in the biotechnology sector and high technology |
Mr. Sing’s extensive healthcare and financial expertise as a healthcare venture capital investor and biomedical company chief executive officer, his executive leadership experience, and his substantial knowledge of the Company led the board to conclude that Mr. Sing should serve as a director. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 13 |

Director since: 2011 Age: 63 Independent |
|
Board and Committee Membership—2022 Attendance |
|
Board of Directors: |
7/7 |
Technology Committee: |
2/2 |
Regeneron Securities Beneficially Owned as of April 11, 2023 |
|
Common Stock: |
1,187 |
Options: |
16,362 |
RSUs: |
1,153 |
Career Highlights |
•
President of Stanford University since 2016 •
President of The Rockefeller University and Carson Family Professor and head of the Laboratory of Brain Development at The Rockefeller University from 2011 to 2016 •
Executive Vice President and Chief Scientific Officer at Genentech, Inc. from 2003 to 2011 •
Professor at Stanford University from 2001 to 2003 and at the University of California, San Francisco from 1991 to 2001 •
Former member of the board of directors of Pfizer Inc., Agios Pharmaceuticals, Inc., and Juno Therapeutics, Inc. |
Scientific Society Memberships |
•
The National Academy of Sciences •
The National Academy of Medicine •
The Royal Society of London •
The Royal Society of Canada |
Other Public Boards |
•
Denali Therapeutics Inc. |
Dr. Tessier-Lavigne’s distinguished scientific and academic background, and his significant industry experience, including experience in senior scientific leadership roles at a leading biopharmaceutical company, led the board to conclude that Dr. Tessier-Lavigne should serve as a director. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 14 |

Director since: 2016 Age: 60 Independent |
|
Board and Committee Membership—2022 Attendance |
|
Board of Directors: |
7/7 |
Corporate Governance and Compliance Committee: |
5/5 |
Technology Committee: |
2/2 |
Regeneron Securities Beneficially Owned as of April 11, 2023 |
|
Common Stock: |
0 |
Options: |
20,945 |
RSUs: |
1,153 |
Career Highlights |
•
Chair of the Department of Molecular Biology since 2013 and Squibb Professor in Molecular Biology since 2003 at Princeton University •
Howard Hughes Medical Institute Investigator •
Former President of the American Society for Microbiology •
Former member of the board of the American Association for the Advancement of Science, the National Science Foundation, and the American Academy of Microbiology •
MacArthur Foundation Fellowship •
Lounsbery Award •
Shaw Prize for Life Science and Medicine •
Gruber Prize in Genetics •
Wolf Prize in Chemistry •
Canada Gairdner International Award •
Former director of Kaleido Biosciences, Inc. |
Scientific Society Memberships |
•
The National Academy of Sciences •
The National Academy of Medicine •
The American Academy of Arts and Sciences •
The Royal Society of London •
The American Philosophical Society |
Other Public Boards |
•
Cidara Therapeutics, Inc. •
Royalty Pharma plc |
Dr. Bassler’s extensive research experience and her scientific and academic career and accomplishments, as well as her experience as a corporate director, led to the board to conclude that Dr. Bassler should serve as a director. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 15 |

Director since: 1991 Age: 81 Independent |
|
Board and Committee Membership—2022 Attendance |
|
Board of Directors: |
7/7 |
Corporate Governance and Compliance Committee: |
5/5 |
Technology Committee (Chair): |
2/2 |
Regeneron Securities Beneficially Owned as of April 11, 2023 |
|
Common Stock: |
11,662 |
Options: |
8,656 |
RSUs: |
1,153 |
Career Highlights |
•
Distinguished Chair in Biomedical Sciences since 1989 and Regental Professor of Molecular Genetics and Internal Medicine and Director of the Jonsson Center for Molecular Genetics since 1985 at The University of Texas Southwestern Medical Center at Dallas •
Nobel Prize for Physiology or Medicine in 1985 (jointly with Dr. Goldstein) •
U.S. National Medal of Science in 1988 (jointly with Dr. Goldstein) |
Scientific Society Memberships |
•
The National Academy of Sciences •
The National Academy of Medicine •
The Royal Society of London |
Dr. Brown’s distinguished scientific and academic background, including his receipt of the Nobel Prize for Physiology or Medicine in 1985, and his significant industry experience gained through his service on the board of directors of the Company and of a leading pharmaceutical company, led the board to conclude that Dr. Brown should serve as a director. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 16 |

Director since: 1988 Age: 70 |
|
Board and Committee Membership—2022 Attendance |
|
Board of Directors: |
7/7 |
Technology Committee: |
2/2 |
Regeneron Securities Beneficially Owned as of April 11, 2023 |
|
Class A Stock: |
1,726,565 |
Common Stock: |
683,096 |
Options: |
852,188 |
Career Highlights |
•
Founded the Company in 1988; built and managed the Company over the past 35 years together with Regeneron’s founding scientist, Dr. Yancopoulos •
Director, President, and Chief Executive Officer of the Company since its inception •
Former Chair of the Board of the Company from 1990 through 1994 •
Licensed physician certified in Neurology by the American Board of Psychiatry and Neurology |
Dr. Schleifer’s significant industry and leadership experience, as well as his incomparable knowledge of the Company and an in-depth understanding of the complex research, drug development, and business issues facing companies in the biopharmaceutical sector, led the board to conclude that Dr. Schleifer should serve as a director. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 17 |

Director since: 2001 Age: 63 |
|
Board and Committee Membership—2022 Attendance |
|
Board of Directors: |
7/7 |
Technology Committee: |
2/2 |
Regeneron Securities Beneficially Owned as of April 11, 2023 |
|
Class A Stock: |
42,750 |
Common Stock: |
1,222,370 |
Options: |
795,799 |
Career Highlights |
•
Founding scientist of the Company; built and managed the Company since 1989 together with Dr. Schleifer •
President and Chief Scientific Officer of the Company •
Director of the Company since 2001 •
11th most highly cited scientist in the world in the 1990s •
Principal inventor and/or developer, together with key members of his team, of the nine FDA-approved drugs the Company has developed, EYLEA® (aflibercept) Injection, Praluent® (alirocumab), Dupixent® (dupilumab), Kevzara® (sarilumab), Libtayo® (cemiplimab), Evkeeza® (evinacumab-dgnb), Inmazeb® (atoltivimab, maftivimab and odesivimab-ebgn), ZALTRAP® (ziv-aflibercept) Injection for Intravenous Infusion, and ARCALYST® (rilonacept) Injection for Subcutaneous Use, as well as of its foundation technologies, including the TRAP technology, VelociGene®, and VelocImmune® |
Scientific Society Memberships |
•
The National Academy of Sciences |
Dr. Yancopoulos’s significant industry and scientific experience and distinguished record of scientific expertise, as well as his extensive knowledge of the Company and an in-depth knowledge of the Company’s technologies and research and development programs, led the board to conclude that Dr. Yancopoulos should serve as a director. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 18 |
The board has a standing Audit Committee, Compensation Committee, and Corporate Governance and Compliance Committee, each of which is comprised entirely of independent directors. The Corporate Governance and Compliance Committee is responsible for reviewing and recommending for the board’s selection candidates to serve on our board of directors and for overseeing all aspects of the Company’s compliance program other than financial compliance. The board also has a standing Technology Committee, which provides direct oversight of our research and clinical development programs, plans, and policies. The board has adopted charters for the Audit Committee, Compensation Committee, Corporate Governance and Compliance Committee, and Technology Committee, current copies of which are available on our website at www.regeneron.com under the “Corporate Governance” heading on the “Investors & Media” page.
We show below information on the membership, key functions, recent focus areas, and number of meetings of each board committee during 2022.
AUDIT |
|
Members |
Key Functions of the Committee |
George L. Sing, Chair N. Anthony Coles, M.D. Arthur F. Ryan |
•
Select the independent registered public accounting firm, review and approve its engagement letter, and monitor its independence and performance •
Review the overall scope and plans for the annual audit by the independent registered public accounting firm •
Approve performance of non-audit services by the independent registered public accounting firm and evaluate the performance and independence of the independent registered public accounting firm •
Review and approve the Company’s periodic financial statements and the results of the year-end audit •
Review and discuss the adequacy and effectiveness of the Company’s accounting and internal control policies and procedures •
Evaluate the internal audit process for establishing the annual audit plan; review and approve the appointment and replacement of the Company’s Chief Audit Executive, if applicable, and any outside entities providing internal audit services and evaluate their performance on an annual basis •
Review the independent registered public accounting firm’s recommendations concerning the Company’s financial practices and procedures •
Oversee the Company’s risk management program •
Discuss with management the Company’s major financial risk exposures and the steps management has taken to monitor and control such exposures •
Establish procedures for the receipt, retention, and treatment of complaints received by the Company regarding accounting, internal accounting controls, or auditing matters and for the confidential, anonymous submission by employees of concerns regarding questionable accounting or auditing matters •
Review and approve any related person transaction •
Prepare an annual report of the Audit Committee for inclusion in the Company’s proxy statement |
Number of Meetings Held in 2022 |
|
9 |
|
Recent Focus Areas |
|
•
Regeneron’s international expansion and related audit matters •
Impact of the Inflation Reduction Act •
Cybersecurity risk assessment •
Succession planning for the Company’s finance, information technology, and real estate & facilities management organizations |
|
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 19 |
COMPENSATION |
|
Members |
Key Functions of the Committee |
Christine A. Poon, Chair George L. Sing Huda Y. Zoghbi, M.D. |
•
Evaluate the performance of the Chief Executive Officer and other executive officers of the Company •
Recommend compensation for the Chief Executive Officer for approval by the non-employee members of the board of directors •
Approve compensation for other executive officers •
Approve the total compensation budget for all Company employees •
Oversee the Company’s compensation and benefit philosophy and programs generally •
Oversee the Company’s strategies and policies related to human capital management, including with respect to workplace environment and culture; talent recruitment, development, and retention; and employee engagement* •
Review and approve annually the corporate goals and objectives applicable to the compensation of the Chief Executive Officer and the goals and objectives of the Company’s executive compensation programs •
Review and approve the Compensation Discussion and Analysis to be included in the Company’s proxy statement •
Prepare an annual report of the Compensation Committee for inclusion in the Company’s proxy statement |
Number of Meetings Held in 2022 |
|
12 |
|
Recent Focus Areas |
|
•
Retention of key leaders •
Potential broader use of performance restricted stock units and other pay mix considerations •
Assessment of the impact of inflation on employee groups and implementation of related measures •
New “pay-versus-performance” disclosure rules •
Design of annual cash incentive program |
|
CORPORATE GOVERNANCE AND COMPLIANCE |
|
Members |
Key Functions of the Committee |
Arthur F. Ryan, Chair Bonnie L. Bassler, Ph.D. Michael S. Brown, M.D. Joseph L. Goldstein, M.D. Christine A. Poon |
•
Identify qualified individuals to become members of the board and recommend such candidates to the board •
Assess the functioning of the board and its committees and make recommendations to the board concerning the appropriate size, function, and needs of the board •
Review, and make recommendations to the board regarding, non-employee director compensation •
Make recommendations to the board regarding corporate governance matters and practices •
Oversee all aspects of the Company’s comprehensive compliance program other than financial compliance •
Oversee the Company’s key corporate responsibility initiatives and conduct a periodic review of environmental, social, and governance (“ESG”) matters* |
Number of Meetings Held in 2022 |
|
5 |
|
Recent Focus Areas |
|
•
Director refreshment efforts, including the appointment of Dr. Craig B. Thompson •
Corporate governance expectations of shareholders and other stakeholders •
Compliance program and data privacy •
ESG matters |
|
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 20 |
TECHNOLOGY |
|
Members |
Key Functions of the Committee |
Michael S. Brown, M.D., Chair Bonnie L. Bassler, Ph.D. Joseph L. Goldstein, M.D. Marc Tessier-Lavigne, Ph.D. P. Roy Vagelos, M.D. Huda Y. Zoghbi, M.D. Leonard S. Schleifer, M.D., Ph.D.** George D. Yancopoulos, M.D., Ph.D.** Number of Meetings Held in 2022 2 |
•
Oversee, review, and evaluate the Company’s research and clinical development programs, plans, and policies •
Identify and discuss emerging scientific and technology issues and trends, including their impact on Regeneron’s research and development programs, plans, or policies •
Identify and assess new leaders within research & development and global development organizations |
Recent Focus Areas |
|
•
The Company’s immuno-oncology programs •
Recent advances and discoveries of the Regeneron Genetics Center® and the Company’s research and preclinical development pipeline
|
|
*
The full board retains oversight of the Company’s strategies and policies related to diversity, equity, and inclusion. See the subsection “The Company—Corporate Governance—Corporate Responsibility” for more information. **
Ex Officio Member. |
|
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 21 |
Pursuant to the Company’s Certificate of Incorporation, the board of directors is divided into three classes, denominated Class I, Class II, and Class III, with members of each class holding office for staggered three-year terms. There are currently four members in each of Class I, Class II, and Class III. In accordance with the requirements of New York law, Dr. Thompson was not assigned to any particular class upon his election by the board in October 2022 and, if he is elected by shareholders at the 2023 Annual Meeting, he will join Class II. The respective terms of the directors expire (in all cases, subject to the election and qualification of their successors and to their earlier death, resignation, or removal) as follows:
The terms of the Class I Directors expire at the 2025 Annual Meeting;
The terms of the Class II Directors expire at the 2023 Annual Meeting; and
The terms of the Class III Directors expire at the 2024 Annual Meeting.
On April 14, 2023, Dr. Vagelos, a Class II Director, notified the Company of his decision not to stand for re-election at the 2023 Annual Meeting. Following the 2023 Annual Meeting, subject to the election of the other Class II Directors nominated for reelection at the 2023 Annual Meeting, the board is expected to be comprised of 12 members equally apportioned among the classes. See the subsection “Board Leadership Structure” below for more information.
Regeneron continues to operate with the long-term outlook required to turn rigorous scientific research into important new medicines. To deliver on this mission, we manage our business for the long term and pursue our core strategy of creating and advancing a high-quality, internally developed product pipeline. In response to shareholder feedback, in January 2023 the Corporate Governance and Compliance Committee requested, reviewed, and discussed a detailed analysis of the costs and benefits of the Company’s classified board structure in order to facilitate the Committee’s continued consideration of this feature of Regeneron’s governance. Following this review, at present the board and the Committee continue to believe that the classified board structure aligns with the Company’s long-term orientation and enables the board to provide appropriate and expert oversight of management over the course of the development cycle of our products, which the board believes ultimately drives the creation of sustainable shareholder value. Furthermore, the board believes three-year terms enhance the independence of our non-employee directors by providing continuity of service and protecting them against potential pressures from special interest groups or others who might have an agenda contrary to the long-term interests of shareholders. The board and/or the Committee will periodically review and continue to consider whether the classified board structure aligns with the Company’s long-term strategic objectives.
The board held 7 meetings in 2022, of which five were regular and two were special meetings. The Regeneron Board of Directors Corporate Governance Guidelines provide that directors are expected to attend all or substantially all meetings of the board and the committees on which they serve. All directors in office at that time attended at least 75% of the total number of meetings of the board and committees of the board on which they served.
According to the Regeneron Board of Directors Corporate Governance Guidelines, board members are expected to attend the Company’s Annual Meeting of Shareholders. All of the directors attended our 2022 Annual Meeting of Shareholders.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 22 |
The Corporate Governance and Compliance Committee is responsible for ensuring that our board is comprised of highly qualified directors with diverse skillsets and backgrounds who will serve as stewards of investor capital and drive the Company’s scientific focus to ensure the continued creation of long-term shareholder value. The Committee seeks to ensure that our board as a whole possesses the mix of skills and experiences to provide effective oversight and guidance to management to execute on the Company’s long-term strategy. The Committee’s refreshment philosophy prioritizes skills that it considers important and desirable based on the Company’s current needs and business priorities, while recognizing that our board members must have predominantly science-based backgrounds to effectively provide robust, independent oversight of management. The Committee also works to ensure that various members of the board bring substantial governance, financial, policy, and management expertise gained from their professional backgrounds and their service on other boards.
The Committee believes it is desirable to maintain a mix of longer-tenured, experienced directors who have developed enhanced knowledge and understanding of, and valuable insight into, the Company and its operations and newer directors with fresh perspectives. As a result, we do not impose director tenure limits or a mandatory retirement age. The Committee has considered the perspectives of some shareholders regarding longer-tenured directors but believes that longer-serving directors with experience and institutional knowledge bring critical skills to the boardroom. In particular, the Committee believes that continuity on the board allows for longer-tenured directors to make meaningful contributions and provide effective oversight of management during the complete drug discovery and development cycle. Accordingly, while director tenure is taken into consideration when evaluating the board’s composition, the Committee believes that imposing arbitrary limits on director tenure would deprive the board of the valuable contributions of its most experienced members.
To ensure a robust approach to director suitability, evaluation, and refreshment, the Committee has adopted refreshment mechanisms that include the following:
A formal annual board and committee self-evaluation, as discussed further below;
A requirement to offer resignation upon material change in principal employment; and
A policy that limits director service to no more than four public boards (including Regeneron) and requires notification prior to appointment to another public or for-profit company board.
In 2022, Dr. Thompson was elected as a member of the board of directors. Dr. Thompson is a renowned healthcare business leader with exceptional senior executive experience and scientific expertise in multiple therapeutic areas, including oncology. His distinguished career, physician-scientist background, and expertise in oncology will be assets to the Company as we continue to expand our oncology footprint. Dr. Thompson was recommended for consideration by the Corporate Governance and Compliance Committee by a non-management director of the board.
The Corporate Governance and Compliance Committee will consider a nominee for election to the board of directors recommended by a shareholder of record if the shareholder submits the recommendation in compliance with the requirements of our Guidelines Regarding Director Nominations, which are available on our website at www.regeneron.com under the “Corporate Governance” heading on the “Investors & Media” page.
In considering potential candidates for the board of directors, the Corporate Governance and Compliance Committee considers factors such as whether or not a potential candidate: (1) possesses relevant expertise; (2) brings skills and experience complementary to those of the other members of the board; (3) has sufficient time to devote to the affairs of the Company; (4) has demonstrated excellence in his or her field; (5) has the ability to exercise sound business judgment; (6) has the commitment to rigorously represent the long-term interests of the Company’s shareholders; (7) possesses a diverse background and experience, including with respect to race, age, and gender; and (8) should be recommended in light of such other factors as the Corporate Governance and Compliance Committee may determine from time to time.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 23 |
Candidates for director are reviewed in the context of the current composition of the board of directors, the operating requirements of the Company, and the long-term interests of shareholders. In conducting the assessment, the Committee considers the individual’s independence, experience, skills, background, and diversity, including with respect to race, age, and gender, along with such other factors as it deems appropriate, given the current needs of the board and the Company to maintain a balance of knowledge, experience, and capabilities. When recommending a slate of director nominees each year, the Corporate Governance and Compliance Committee reviews the current composition of the board of directors in order to recommend a slate of directors who, with the continuing directors, will provide the board with the requisite diversity of skills, expertise, experience, and viewpoints necessary to effectively fulfill its duties and responsibilities.
In the case of an incumbent director whose term of office is set to expire, the Corporate Governance and Compliance Committee reviews such director’s overall service to the Company during the director’s term and also considers the director’s interest in continuing as a member of the board. In the case of a new director candidate, the Corporate Governance and Compliance Committee also reviews whether the nominee is “independent,” based on our Corporate Governance Guidelines, applicable listing standards of the Nasdaq Stock Market LLC, and applicable SEC and other relevant rules and regulations, if necessary.
The Corporate Governance and Compliance Committee may employ a variety of methods for identifying and evaluating nominees for the board of directors. In addition, the Corporate Governance and Compliance Committee may consider candidates recommended by other directors, management, search firms, shareholders, or other sources. When conducting searches for new directors, the Corporate Governance and Compliance Committee will take reasonable steps to include diverse candidates in the pool of nominees and any search firm will affirmatively be instructed to seek to include diverse candidates. Candidates recommended by shareholders will be evaluated on the same basis as candidates recommended by our directors or management or by third-party search firms or other sources. Candidates may be evaluated at regular or special meetings of the Corporate Governance and Compliance Committee. The Committee also considers succession planning for board and committee chairs for purposes of continuity and to maintain relevant expertise and depth of experience.

On an annual basis, the board of directors, the Audit Committee, the Compensation Committee, and the Corporate Governance and Compliance Committee conduct self-assessments to ensure effective performance and to identify opportunities for improvement. As the first step in the self-assessment process, directors complete a comprehensive questionnaire, which asks them to consider various topics related to board and committee composition, structure, effectiveness, and responsibilities, as well as satisfaction with the schedule, materials, and discussion topics. Each committee, as well as the board as a whole, then reviews and assesses the responses and presents its findings and recommendations to the board of directors. The results of the assessments are then discussed by the board of directors and the respective committees in executive session, with a view toward taking action to address any issues presented. Results requiring additional consideration are addressed at subsequent board and committee meetings, where appropriate.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 24 |
Annual Self-Assessment Process
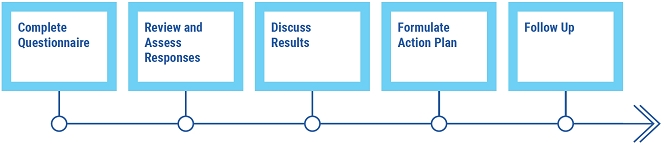
While this formal self-assessment is conducted on an annual basis, directors share perspectives, feedback, and suggestions year round, both inside and outside the boardroom.
Regeneron’s charter documents give shareholders the right to call a special shareholder meeting upon the written request of at least 25% of the total number of votes entitled to be cast by shareholders.
The board of directors has determined that each of the following currently serving directors is independent as defined in the listing standards of the Nasdaq Stock Market LLC and our Corporate Governance Guidelines: Bonnie L. Bassler, Ph.D., Michael S. Brown, M.D., N. Anthony Coles, M.D., Joseph L. Goldstein, M.D., Christine A. Poon, Arthur F. Ryan, George L. Sing, Marc Tessier-Lavigne, Ph.D., Craig B. Thompson, M.D., and Huda Y. Zoghbi, M.D. These individuals are affiliated with numerous educational institutions, hospitals, charities, and corporations, as well as civic organizations and professional associations. The board of directors considered each of these relationships and determined that none of these relationships conflicted with the interests of the Company or would impair their independence or judgment. In accordance with our Corporate Governance Guidelines, the board conducts executive sessions of independent directors presided by the Chair of the Corporate Governance and Compliance Committee following each regularly scheduled board meeting, as discussed further below.
The board of directors has determined that each of the current members of the Audit Committee, Messrs. Ryan and Sing and Dr. Coles, qualifies as an “audit committee financial expert” as that term is defined by SEC rules, and is independent as defined for audit committee members in the listing standards of the Nasdaq Stock Market LLC and SEC rules.
In addition, the board of directors has determined that each of the current members of the Compensation Committee, Ms. Poon, Mr. Sing, and Dr. Zoghbi, meets the additional independence criteria applicable to compensation committee members under the listing standards of the Nasdaq Stock Market LLC and qualifies as a “Non-Employee Director” pursuant to Rule 16b-3 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
The board of directors recognizes that one of its key responsibilities is to establish and evaluate an appropriate leadership structure for the board so as to provide effective oversight of management. The board of directors believes that a company’s board leadership structure should take into account relevant corporate governance and strategic considerations (such as board size and composition, director tenure, and experience of the management team) and that the board should maintain flexibility and adjust the leadership structure as appropriate.
Regeneron’s current board leadership structure (in effect since 1995) is based on the separation of the roles of the Chief Executive Officer and the Chair of the Board, with Dr. Vagelos serving as Chair and Dr. Schleifer serving as President and Chief Executive Officer. The board has determined that this leadership structure is currently appropriate for the Company. This determination was based in part on Dr. Vagelos’s extensive leadership experience, business acumen, and deep understanding of the healthcare industry, as well as the fact that in the board’s view Dr. Vagelos is
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 25 |
able to directly challenge senior management on both scientific and business matters. The perspectives gained from his extensive industry experience have made Dr. Vagelos an invaluable resource to both the board and the management team and have also allowed him to effectively serve as a sounding board for the other directors.
Based on the way in which he has executed his role as Chair, the board considers Dr. Vagelos to be an impartial director. However, given that Dr. Vagelos does not meet the independence requirements set forth in the listing standards of the Nasdaq Stock Market LLC, and to enable even more robust independent oversight, Mr. Ryan, an independent director and Chair of the Corporate Governance and Compliance Committee, currently serves as Presiding Director and chairs all executive sessions of independent directors (which generally follow each regularly scheduled board meeting). Mr. Ryan’s substantial leadership experience as a chief executive officer of leading companies in the banking and insurance industries, his extensive business experience and financial expertise, and his prior service as a director of several publicly traded companies have made him well suited to effectively serve as an additional layer of independent oversight.
The board believes that this governance arrangement has bolstered the board’s leadership structure and its goal of ensuring independent oversight of management’s execution of our business strategy and the continued creation of sustainable shareholder value.
On April 14, 2023, Dr. Vagelos notified the Company of his decision not to stand for re-election at the 2023 Annual Meeting. On the same day, the board of directors adopted a board leadership succession plan, based on which the board plans to elect Drs. Schleifer and Yancopoulos as Co-Chairs of the Board to succeed Dr. Vagelos as Chair and to establish the role of Lead Independent Director (with Ms. Poon expected to be designated by the independent directors as the Lead Independent Director), each effective following the conclusion of the 2023 Annual Meeting. Pursuant to this board leadership succession plan, Drs. Schleifer and Yancopoulos will retain their roles as President and Chief Executive Officer and President and Chief Scientific Officer, respectively. In planning to elect Drs. Schleifer and Yancopoulos as Co-Chairs, the board of directors considered their incomparable knowledge and demonstrated leadership of the Company for over three decades, as well as the fact that Dr. Schleifer previously served as Chair of the Board from 1990 through 1994. The board also believes that their ability to speak as Co-Chairs of the Board as well as Presidents of the Company will provide strong unified leadership for the Company. In the board’s view, electing Drs. Schleifer and Yancopoulos as Co-Chairs, combined with a strong Lead Independent Director appointed by the independent directors, will provide balanced leadership and effective oversight of management and be in the best interest of the Company and its shareholders.
The board will continue to periodically evaluate and determine, with input from the independent directors, an appropriate leadership structure for the board so as to provide effective oversight of management.
The board executes its oversight responsibility for risk management directly and through its committees, as follows:
The Audit Committee, which includes our Presiding Director as a member, oversees the Company’s risk management program. The risk management program focuses on the most significant risks the Company faces in the short-, intermediate-, and long-term timeframe. The Company’s Chief Audit Executive, who reports independently to the Committee, facilitates the risk management program. Audit Committee meetings include discussions of specific risk areas throughout the year, including, among others, those relating to cybersecurity, and reports from the Chief Audit Executive on the Company’s enterprise risk profile on an annual basis. Recently, the Audit Committee independently engaged a consultant to conduct a cybersecurity assessment and preparedness analysis.
The Compensation, Corporate Governance and Compliance, and Technology Committees oversee risks associated with their respective areas of responsibility.
As part of its overall review of the Company’s compensation policies and practices, the Compensation Committee generally considers the risks associated with these policies and practices while designing performance incentives that align executives’ interests with those of long-term shareholders. At least annually the Compensation Committee reviews and considers a compensation program risk assessment performed by its independent compensation consultant.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 26 |
The Corporate Governance and Compliance Committee oversees all aspects of the Company’s comprehensive compliance program other than financial compliance and considers legal and regulatory compliance risks, including corporate responsibility initiatives that are expected to have a significant impact on the Company’s ability to deliver sustained growth. The Company’s Chief Compliance Officer, who reports to our Executive Vice President, General Counsel and Secretary, provides regular updates directly to the Corporate Governance and Compliance Committee (generally given at every regularly scheduled meeting of the Committee), and relevant information is shared with the full board as part of the Corporate Governance and Compliance Committee’s report to the board.
The Technology Committee considers risks associated with our research and development programs.
The board is kept abreast of its committees’ risk oversight and other activities via reports of the committee chairs to the full board at regular board meetings. The board considers specific risk topics, including risks associated with our strategic plan, drug access and pricing (discussed further below), our finances, and our development activities. In addition, the board receives detailed regular reports from members of our senior management that include discussions of the risks and exposures involved in their respective areas of responsibility. Further, the board is routinely informed by the appropriate members of senior management of developments internal and external to the Company that could affect our risk profile. Specific risk topics may also be considered at executive sessions of independent directors, which are chaired by our Presiding Director.
The board is closely involved in and provides oversight of all key pricing determinations for our products, which we endeavor to make in a thoughtful and well-informed manner with fairness and affordability in mind. We believe we have the appropriate governance mechanisms and internal processes in place to ensure that pricing decisions are thoroughly and appropriately vetted prior to implementation and are made in line with our values and commitments. This includes routine presentations to our board of directors on drug pricing strategies, practices, and trends. See “The Company—Corporate Governance—Corporate Responsibility” for more information.
The Compensation Committee is responsible for overseeing the Company’s general compensation objectives and programs. We describe below under “Compensation-Related Matters—Compensation Discussion and Analysis—Compensation Processes” the role of the Compensation Committee, as well as the role of our executive officers, in decisions regarding executive compensation (particularly with respect to our Named Executive Officers (as defined below under “Compensation-Related Matters—Compensation Discussion and Analysis”)).
The Compensation Committee has the sole authority to retain its own third-party compensation consultants, and in 2022 utilized the services of Pay Governance LLC, an independent compensation consultant. Advice and recommendations provided by Pay Governance LLC in 2022 related to both executive compensation (discussed in the section “Compensation-Related Matters” below) and director compensation matters (discussed in the subsection “Compensation of Directors” below). As discussed further below, the Corporate Governance and Compliance Committee has adopted a policy requiring the annual review of non-employee director compensation by an independent compensation consultant and separately engaged Pay Governance LLC for that purpose.
Management also retains another compensation consultant for its own use. In 2022, management used the services of Radford (part of Aon Hewitt, a business unit of Aon plc), a compensation consultant focused on the technology and life sciences sectors. Radford provided various consulting services to us, including analyzing the competitiveness of specific compensation programs; preparing surveys of competitive pay practices; and assisting management in the development and analysis of executive compensation recommendations. Reports prepared by Radford that relate to executive compensation may also be shared with the Compensation Committee, the full board, or another committee of the board.
Our board of directors has always viewed shareholder feedback as critical for its decision-making and has taken several actions after careful consideration of the feedback received. In the past several years, the board and management have conducted regular and extensive outreach. Annually, we seek to engage in direct one-on-one discussions with shareholders collectively representing in excess of 50% of the shares of common stock outstanding (excluding shares held by our directors and executive officers), which we refer to as “public shares.” We encourage
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 27 |
director participation in our outreach, and our Presiding Director (who is also the Chair of our Corporate Governance and Compliance Committee) and the Chair of our Compensation Committee led many of these discussions as representatives of the board in recent years. We also routinely engage with our shareholders through regular investor-relations channels, industry and corporate governance conferences, and informal exchanges in other settings.

Our active outreach program solicits and gathers feedback on a broad range of matters, including board structure and composition, executive compensation, shareholder rights, and corporate responsibility and other ESG topics. The feedback received is a key input in board and relevant committee discussions, and has consistently been a significant driver of actions taken over the last several years. We have a track record of taking actions in response to shareholder input, as described in the section “Compensation Related Matters—Compensation Discussion and Analysis—Compensation Processes—Shareholder Input and Outreach and the 2022 Say-on-Pay Vote Result.”
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 28 |
The general philosophy we have applied to compensation of our non-employee directors and the current Chair of the Board is similar to the executive compensation philosophy outlined in the “Compensation-Related Matters” section of this proxy statement. This philosophy, including its emphasis on equity compensation, is consistent with the Company’s long-term business orientation and has helped ensure alignment of directors’ interests with those of Regeneron shareholders.
Non-employee director compensation matters are subject to annual review. The Corporate Governance and Compliance Committee makes recommendations to the board of directors regarding, and the board of directors determines, the compensation of non-employee directors. The Corporate Governance and Compliance Committee evaluates the appropriate level and form of compensation for non-employee directors and recommends changes to such compensation to the board of directors when appropriate. Directors who are Company employees receive no additional compensation for serving on our board of directors or its committees. In determining compensation recommendations for the non-employee directors, the Corporate Governance and Compliance Committee considers, among other things, the qualifications, expertise, and demands on our directors, practices of similar companies in the biotechnology industry (including the Peer Group2), and any comparative information provided by independent compensation consultants. In addition, since November 2018, the Corporate Governance and Compliance Committee has required the annual review of non-employee director compensation by an independent compensation consultant, and has engaged Pay Governance LLC for this purpose since 2021.
The process governing the compensation arrangements of the current Chair of the Board is described under “Compensation Arrangements of the Chair of the Board of Directors” below.
The current compensation program for our non-employee directors and the current Chair of the Board (which has been effective for our non-employee directors and the Chair of the Board since January 2019 and December 2018, respectively, and is further described below) is referred to in this section as the “Current Compensation Program.”3
Our philosophy for non-employee director compensation is simple: to attract the most highly qualified directors with a diverse skillset who will serve as stewards of the Company’s long-term prospects and scientific focus. There is significant competition within our industry for highly qualified directors, particularly those with distinguished scientific credentials (such as members of the National Academy of Sciences) and the caliber of science-based backgrounds that we seek in our board members from the industry. With this in mind, our non-employee director compensation program emphasizes equity compensation primarily in the form of stock options, which reward increases in stock price, over cash fees. The board of directors believes that this emphasis is consistent with the Company’s long-term business orientation and has helped ensure alignment of directors’ interests with those of Regeneron shareholders. Under the Current Compensation Program, we have utilized value-denominated equity compensation awards (granted in the form of stock options and a relatively small percentage of RSUs) for our non-employee directors. This feature of the Current Compensation Program is meant to, among other things, ensure greater stability in reported non-employee director compensation on a year-over-year basis. The board of directors believes that the Current Compensation Program is consistent with Regeneron’s philosophy for non-employee director compensation.
2 |
See “Compensation-Related Matters— Compensation Discussion and Analysis—Compensation Processes—Peer Data” below for a list of the companies included in our Peer Group. |
3 |
The Current Compensation Program was implemented following the previously disclosed settlement of two shareholder derivative actions (the “Settlement”) and complies with the terms of the Settlement. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 29 |
In 2022, each non-employee director received an annual retainer of $90,000 and an annual committee retainer of $10,000 for each standing committee of the Company’s board of directors on which the director served. In addition, each chair of each standing committee received an additional annual retainer of $10,000. Compared to cash compensation of non-employee directors in our Peer Group, the 2022 annual retainer for board service was below the median and the additional retainers provided to our committee chairs were at or below the median.4
Non-employee directors are reimbursed for their actual expenses incurred in connection with their activities as directors, which include travel, hotel, and food and entertainment expenses (as applicable). In addition, directors are eligible to participate in the Regeneron Matching Gift Program, which is also available to eligible employees. Under this program, the Company matches contributions made by directors and employees to eligible tax-exempt organizations up to an annual maximum amount of $5,000 per director or employee.
The January 2022 and January 2023 annual equity awards to our non-employee directors were made in accordance with the Current Compensation Program and complied with the respective limits imposed by the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan and the Settlement.
With respect to each of the January 2022 and January 2023 annual equity awards, the board of directors (upon the recommendation of the Corporate Governance and Compliance Committee) determined that the targeted aggregate grant date fair value of such equity awards would be set at $600,000 per non-employee director and consist of stock options with a grant date fair value of $480,000 (or 80% thereof) and RSUs with a grant date fair value of $120,000 (or 20% thereof). The January 2022 annual equity awards to our non-employee directors are shown in the table below. On January 3, 2023, each of the then-serving non-employee directors (other than Dr. Thompson) received an annual equity award comprised of stock options representing 1,929 shares of common stock (with a grant date fair value of $480,215) and 165 RSUs (with a grant date fair value of $119,772). The aggregate grant date fair value of the January 2023 equity awards to these directors was $599,987 per non-employee director. As a non-employee director newly elected effective October 3, 2022, Dr. Thompson received a prorated annual equity award on January 3, 2023 consisting of 477 options (with a grant date fair value of $118,750) and 40 RSUs (with a grant date fair value of $29,036). See “Equity Awards to New Directors” below for more information.
Similar to the process undertaken in respect of the January 2022 annual equity awards, the Corporate Governance and Compliance Committee recommended the approval of, and the board of directors approved, the terms of the January 2023 annual equity awards after consideration of the review, analysis, and recommendations of the Corporate Governance and Compliance Committee’s independent compensation consultant. Such analysis focused on, among other matters, the market practices of companies in our Peer Group, other relevant industry and market data points, Regeneron’s non-employee director compensation philosophy (including its emphasis on long-term incentives), and the terms of the Settlement.
The exercise price of a non-employee director stock option is equal to the fair market value of a share of the Company’s common stock on the date of grant (determined as the average of the high and low sales price per share of the Company’s common stock on the Nasdaq Global Select Market on the date of grant or, if such date is not a trading day, on the last preceding date on which there was a sale of the Company’s common stock on the Nasdaq Global Select Market).
4
Based on information reported by our Peer Group companies in 2022. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 30 |
Under the Current Compensation Program, a pro-rata portion of each equity award (i.e., each stock option and RSU award) equal to the portion of one year that has passed from its date of grant vests on the date of the Company’s first annual shareholder meeting following the date of grant, and the remaining portion vests on the first anniversary of the date of grant. The RSU awards contain mandatory deferral provisions, according to which the shares underlying the RSUs will generally not be delivered to the non-employee director until the earliest of (i) the termination of the non-employee director’s service as a member of the board, (ii) the seventh anniversary of the RSU grant date, and (iii) the date of a change in control (as defined in the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan (or its predecessor)). A non-employee director may, subject to compliance with applicable tax rules, elect in writing a maximum deferral period longer than the seventh anniversary of the grant date. Other than as discussed below, the vesting of equity awards is generally subject to continued service on the board, and stock option awards generally expire ten years following the date of grant.
To the extent they remain unvested and outstanding, equity awards granted to a non-employee director continue to vest following the retirement of that director provided applicable conditions relating to the length of the director’s service and the director’s age have been met. If a non-employee director’s service as a member of the board is terminated as a result of his or her death, all of the director’s equity awards will immediately vest in full.
To the extent they remain unvested and outstanding, equity awards granted to non-employee directors become fully vested automatically upon a change in control of the Company. Each non-employee director has the right to nullify this acceleration of vesting, in whole or in part, if it would cause the director to pay excise taxes under the requirements of the Internal Revenue Code.
Under the Current Compensation Program, any newly elected non-employee director will receive an initial equity award with an aggregate grant date fair value equal to 5/3rds of the aggregate grant date fair value of the most recent annual equity award to a non-employee director; and, with respect to the annual equity award to a non-employee director in respect of the first year of his or her service, the aggregate grant date fair value of such annual award will be prorated based on the date as of which the non-employee director first becomes a member of the board of directors. The initial equity award to Dr. Thompson (shown in the table below) and his prorated January 2023 annual equity award (discussed above) were consistent with these guidelines.
On December 31, 1998, we entered into an employment agreement with the current Chair of the board of directors, Dr. Vagelos. Pursuant to the terms of his employment agreement, Dr. Vagelos has received an annual salary of $100,000 as a non-officer employee.
Under the Current Compensation Program, Dr. Vagelos has received an annual equity award with an aggregate grant date fair value equal to 10 times the aggregate grant date fair value of the corresponding non-employee director annual award. In December 2022, the Compensation Committee determined that stock options and RSUs would comprise 80% and 20% of the 2022 annual equity award to Dr. Vagelos, respectively, and set the targeted aggregate grant date fair value of such award at (but no more than) ten times the aggregate grant date fair value of the corresponding annual equity award for our non-employee directors. As in prior years, Dr. Vagelos’s 2022 annual equity award reflected, among other things, Dr. Vagelos’s key contributions as the Company had gained momentum as a fully integrated biotech company with multiple class-leading products, as well as Dr. Vagelos’s crucial role as a trusted advisor to the CEO, other senior managers, and the non-employee directors (as described further in the subsection “Board Governance—Board Leadership Structure” above).
The 2022 stock option award granted to Dr. Vagelos vests ratably over four years and contains change-of-control provisions consistent with those described above for equity grants to non-employee directors. The 2022 RSU award granted to Dr. Vagelos vests 50% on the second anniversary of the date of grant and 50% of the fourth anniversary of the date of grant and contains change-of-control provisions consistent with those described above for equity grants to non-employee directors. In addition, the award agreements relating to Dr. Vagelos’s equity awards provide that each such award will continue to vest in accordance with the terms of the applicable award agreement following his qualified retirement (as defined in the applicable Company policy; Dr. Vagelos currently meets the policy requirement).
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 31 |
The following table and explanatory footnotes provide information with respect to compensation paid to Dr. Vagelos and each non-employee director for their service in 2022 in accordance with the policies, plans, and employment agreement described above:
A |
B |
C |
D |
E |
F |
G |
H |
||||
Name |
Fees earned or paid in cash ($) |
Stock awards1 ($) |
Option awards1,2 ($) |
Non-equity incentive plan compensation ($) |
Change in pension value and non-qualified deferred compensation earnings |
All other compensation3 ($) |
Total ($) |
||||
Bonnie L. Bassler, Ph.D. |
110,000 |
119,490 |
|
480,539 |
|
— |
— |
5,000 |
4 |
715,029 |
|
Michael S. Brown, M.D. |
120,000 |
119,490 |
|
480,539 |
|
— |
— |
5,000 |
4 |
725,029 |
|
N. Anthony Coles, M. D. |
100,000 |
119,490 |
|
480,539 |
|
— |
— |
5,000 |
4 |
705,029 |
|
Joseph L. Goldstein, M.D. |
110,000 |
119,490 |
|
480,539 |
|
— |
— |
— |
|
710,029 |
|
Christine A. Poon |
120,000 |
119,490 |
|
480,539 |
|
— |
— |
— |
|
720,029 |
|
Arthur F. Ryan |
120,000 |
119,490 |
|
480,539 |
|
— |
— |
5,000 |
4 |
725,029 |
|
George L. Sing |
120,000 |
119,490 |
|
480,539 |
|
— |
— |
— |
|
720,029 |
|
Marc Tessier-Lavigne, Ph.D. |
100,000 |
119,490 |
|
480,539 |
|
— |
— |
— |
|
700,029 |
|
Craig B. Thompson, M.D.5 |
24,457 |
199,641 |
|
800,169 |
|
— |
— |
— |
|
1,024,267 |
|
P. Roy Vagelos, M.D. |
— |
1,199,501 |
|
4,799,762 |
|
— |
— |
110,000 |
6 |
6,109,263 |
|
Huda Y. Zoghbi, M.D. |
110,000 |
119,490 |
|
480,539 |
|
— |
— |
5,000 |
4 |
715,029 |
|
1
The amounts in columns C and D reflect the respective aggregate grant date fair values of RSUs and options awarded during the year ended December 31, 2022 pursuant to the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. Valuation assumptions and methodologies used in the calculation of these amounts do not take into account expected forfeitures and are otherwise described in Note 13 to the Company’s audited financial statements for the fiscal year ended December 31, 2022 included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 filed with the U.S. Securities and Exchange Commission (“SEC”) on February 6, 2023 (the “2022 Annual Report”). 2
At December 31, 2022, the non-employee directors and Dr. Vagelos had the following aggregate number of stock option awards outstanding: Dr. Bassler: 22,376; Dr. Brown: 13,968; Dr. Coles: 2,707; Dr. Goldstein: 6,320; Ms. Poon: 62,064; Mr. Ryan: 2,707; Mr. Sing: 49,784; Dr. Tessier-Lavigne: 15,532; Dr. Thompson: 3,216; Dr. Vagelos: 611,277; and Dr. Zoghbi: 26,459. 3
See the subsection “Compensation-Related Matters–Compensation Dashboard—Additional Compensation Information–Perquisites and Personal Benefits” for information regarding director air transportation in accordance with guidelines approved by our board of directors. 4
Consists of a Company contribution paid or payable on or before April 11, 2023 under the Regeneron Matching Gift Program in respect of charitable gifts made in 2022. 5
Dr. Thompson was elected as a member of the board of directors on October 3, 2022; accordingly, his fees were prorated based on his election date. The table also reflects his initial equity award granted effective as of his October 3, 2022 election date. 6
Consists of (i) $100,000 for the salary paid pursuant to the terms of our employment agreement with Dr. Vagelos, (ii) $5,000 for a Company contribution paid or payable on or before April 11, 2023 under the Regeneron Matching Gift Program in respect of a charitable gift made in 2022, and (iii) $5,000 for 401(k) Savings Plan matching contributions in respect of 2022. |
|||||||||||
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 32 |

PROPOSAL 1
|
|
The board of directors, upon the recommendation of the Corporate Governance and Compliance Committee, has nominated for election at the 2023 Annual Meeting Joseph L. Goldstein, M.D., Christine A. Poon, Craig B. Thompson, M.D., and Huda Y. Zoghbi, M.D., as Class II Directors for a three-year term expiring at the 2026 Annual Meeting. |
The board of directors unanimously recommends a vote FOR the election of each of these nominees. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 33 |
Our leadership team possesses deep and diverse industry knowledge, a passion for science, and a shared commitment to help transform lives. All officers of the Company are appointed annually and serve at the pleasure of the board of directors. The names, positions, ages, and background of the Company’s executive officers as of April 11, 2023 are set forth below. There are no family relationships between any of our directors and executive officers. None of the corporations or other organizations referred to below with which an executive officer has previously been employed or otherwise associated is a parent, subsidiary, or affiliate of the Company.

|
Leonard S. Schleifer, M.D., Ph.D., 70, founded the Company in 1988, has been a director and its President and Chief Executive Officer since its inception, and served as Chair of the Board from 1990 through 1994. Dr. Schleifer, together with Regeneron’s founding scientist, Dr. Yancopoulos, built and has managed the Company over the past 35 years. Dr. Schleifer received his M.D. and Ph.D. in Pharmacology from the University of Virginia. Dr. Schleifer is a licensed physician and is certified in Neurology by the American Board of Psychiatry and Neurology. |

|
George D. Yancopoulos, M.D., Ph.D., 63, joined Dr. Schleifer in 1989 as founding scientist of the Company, and together they built and have managed the Company since then. Dr. Yancopoulos is currently President and Chief Scientific Officer, and has served on the board since 2001. He received his M.D. and Ph.D. from Columbia University. Dr. Yancopoulos was the 11th most highly cited scientist in the world in the 1990s, and in 2004 he was elected to be a member of the National Academy of Sciences. Dr. Yancopoulos, together with key members of his team, is a principal inventor and/or developer of the nine FDA-approved drugs the Company has developed, EYLEA, Praluent, Dupixent, Kevzara, Libtayo, Evkeeza, Inmazeb, ZALTRAP, and ARCALYST, as well as of its foundation technologies, including the TRAP technology, VelociGene®, and VelocImmune®. |

|
Christopher Fenimore, 52, has been Senior Vice President, Controller since January 2021. He previously served as Vice President, Controller from March 2017 to December 2020, as Vice President, Deputy Controller from January 2017 to March 2017, and as Vice President, Financial Planning from January 2012 to December 2016. Prior to joining the Company in 2003, he was Vice President, Finance at Mojave Therapeutics, Inc. Mr. Fenimore’s prior experience includes working as a supervising senior accountant at KPMG, as well as healthcare industry-focused venture capital and investment banking roles. Mr. Fenimore holds an M.A. in Biotechnology from Columbia University, an M.B.A. in Professional Accounting from Rutgers Business School, and a B.A. in Economics from Rutgers University. Mr. Fenimore is a Certified Public Accountant in the State of New York. |

|
Robert E. Landry, 59, has been Executive Vice President, Finance since January 2019 and Chief Financial Officer since October 2013. From September 2013 to December 2018, he served as Senior Vice President, Finance. Previously, Mr. Landry served as Senior Vice President, Treasurer, at Pfizer Inc. from October 2012 to August 2013 and Senior Vice President—Finance, Pfizer’s Diversified Business, from October 2009 to October 2012. Prior to those roles, Mr. Landry held a number of positions at Wyeth, which was acquired by Pfizer Inc. in October 2009, including Treasurer and Principal Corporate Officer from 2007 to 2009, Director of Pharmaceutical Marketing and Sales of Wyeth’s Australian affiliate from 2006 to 2007, and Chief Financial Officer of Wyeth’s Australian and New Zealand affiliates from 2004 to 2006. Mr. Landry holds a B.B.A. in Accounting from the University of Notre Dame. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 34 |

|
Joseph J. LaRosa, 64, has been Executive Vice President, General Counsel and Secretary since January 2019. From September 2011 to December 2018, he served as Senior Vice President, General Counsel and Secretary. Before joining Regeneron, Mr. LaRosa was Senior Vice President, General Counsel, and Secretary at Nycomed US Inc. Mr. LaRosa’s prior experience includes working in a number of senior legal positions at Schering-Plough Corporation from 1993 to 2009, where he was a corporate officer and served most recently as Vice President, Legal Affairs, and a member of the Operations Management Team. Mr. LaRosa received his J.D. from New York University School of Law. |

|
Marion McCourt, 63, has been Executive Vice President, Commercial since January 2021. She previously served as Senior Vice President, Commercial from February 2018 to December 2020. From April 2017 until joining the Company, Ms. McCourt served as the Principal Operating Officer and the Chief Operating Officer and President of Axovant Sciences, Inc. Ms. McCourt previously served as chief operating officer of Medivation, Inc. from February 2016 until its acquisition by Pfizer Inc. in September 2016. Previously, Ms. McCourt worked at Amgen Inc., where she most recently served as a Vice President in U.S. Commercial Operations from February 2014 to January 2016. From May 2013 to January 2014, Ms. McCourt served as Vice President and General Manager at Amgen where she was responsible for the bone health and primary care business unit. From 2012 to 2013, she was Chief Operating Officer for AstraZeneca U.S., a division of AstraZeneca plc. Her responsibilities included oversight and leadership of all U.S. commercial functions, including medical affairs, business development, finance, human resources, legal, operations, and corporate affairs. During her 12-year tenure at AstraZeneca, Ms. McCourt was President and Chief Executive Officer of AstraZeneca Canada Inc. from 2011 to 2012 and also held various other roles at AstraZeneca Pharmaceuticals LP, a subsidiary of AstraZeneca plc. Ms. McCourt received her B.S. in Biology from Lafayette College. |

|
Andrew J. Murphy, Ph.D., 65, has been Executive Vice President, Research since January 2019. He previously served as Senior Vice President, Research, Regeneron Laboratories from January 2013 to December 2018, as Vice President, Target Discovery from May 2005 to December 2012, as Vice President, Gene Discovery and Bioinformatics from January 2001 to May 2005, and Director of Genomics and Bioinformatics from May 1999 to December 2000. Dr. Murphy is a co-inventor of several of the Company’s key technologies, including VelociGene® and VelocImmune®, and continues to lead several technology centers and therapeutic focus areas. He received his B.S. in Molecular Biology at the University of Wisconsin, and his Ph.D. in Human Genetics from Columbia University, College of Physicians and Surgeons. |

|
Neil Stahl, Ph.D., 66, has been Executive Vice President, Research and Development since January 2015. He previously served as Senior Vice President, Research and Development Sciences from January 2007 to December 2014, as Senior Vice President, Preclinical Development and Biomolecular Sciences from December 2000 to December 2007, and as Vice President, Preclinical Development and Biomolecular Sciences from January 2000 to December 2000. He joined the Company in 1991. Before becoming Vice President, Biomolecular Sciences in 1997, Dr. Stahl was Director, Cytokines and Signal Transduction. Dr. Stahl received his Ph.D. in Biochemistry from Brandeis University. |

|
Daniel P. Van Plew, 50, has been Executive Vice President and General Manager, Industrial Operations and Product Supply since January 2016. From April 2008 to December 2015, Mr. Van Plew served as Senior Vice President and General Manager, Industrial Operations and Product Supply. Prior to that date, he served as Vice President and General Manager, Industrial Operations and Product Supply since joining the Company in 2007. From 2006 until 2007, Mr. Van Plew served as Executive Vice President, R&D and Technical Operations of Crucell Holland B.V., a global biopharmaceutical company. Between 2004 and 2006, Mr. Van Plew held positions of increasing responsibility at Chiron Biopharmaceuticals, part of Chiron Corporation, a biotechnology company, most recently as Senior Director, Vacaville Operations. From 1998 until 2004, Mr. Van Plew held various managerial positions in the health and life sciences practice at Accenture, Ltd., a management consulting business. Mr. Van Plew received his M.S. in Chemistry from The Pennsylvania State University and his M.B.A. from Michigan State University. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 35 |

Regeneron is committed to good corporate governance, which we believe promotes the long-term interests of shareholders, strengthens the accountability of the board of directors and management, and helps build trust in the Company. The following chart summarizes key information regarding our corporate governance.
Board and Other Governance Information |
2023* |
Size of Board |
13 |
Number of Independent Directors |
10 |
Number of Female Directors |
3 |
Number of Directors Diverse by Race or Ethnicity |
4 |
Separate Chair and Chief Executive Officer |

|
Majority Voting in the Election of Directors |

|
Director Resignation Policy |

|
Number of Meetings of the Board of Directors Held in 2022 |
7 |
Executive Sessions of Independent Directors without Management Present |

|
Independent Presiding Director |

|
Code of Business Conduct and Ethics Applicable to All Employees, Officers, and Directors |

|
Annual Board and Committee Self-Evaluations |

|
Stock Ownership Guidelines for Directors and Senior Executives |

|
Annual Say-On-Pay Vote |

|
Active Shareholder Engagement |

|
Shareholder Right to Call Special Shareholder Meeting |

|
*
As of April 11, 2023. On April 14, 2023, Dr. Vagelos notified the Company of his decision not to stand for re-election at the 2023 Annual Meeting. At the conclusion of the 2023 Annual Meeting, Dr. Vagelos’s term will expire and the size of the board is expected to be reduced to 12 members, 10 of which are independent. As described under “Board of Directors—Board Governance—Board Leadership Structure” above, on April 14, 2023, the board of directors adopted a board leadership succession plan based on which the board plans to elect Drs. Schleifer and Yancopoulos as Co-Chairs of the Board to succeed Dr. Vagelos as Chair and to establish the role of Lead Independent Director (with Ms. Poon expected to be designated by the independent directors as the Lead Independent Director), each effective following the conclusion of the 2023 Annual Meeting. Pursuant to this board leadership succession plan, Drs. Schleifer and Yancopoulos will retain their roles as President and Chief Executive Officer and President and Chief Scientific Officer, respectively. |
|
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 36 |
Our corporate governance has been enhanced based on shareholder feedback over the last ten years, including by adopting the corporate governance practices shown below. See “Compensation Discussion and Analysis—Compensation Processes—Shareholder Input and Outreach and the 2022 Say-on-Pay Vote Result” for more information.
While the Company has dual-class stock, no new shares of Class A stock, par value $0.001 per share (“Class A stock”), have been issued since our IPO in 1991, and the number of shares of Class A stock outstanding has been steadily decreasing since that time, from 10.9 million to 1.8 million as of the record date for the 2023 Annual Meeting. At the time of our IPO, outstanding shares of Class A stock represented approximately 95% of total shareholder votes. As of April 11, 2023, outstanding shares of Class A stock represented only approximately 14% of total shareholder votes. The voting rights granted holders of Class A stock cannot be unilaterally changed by the board of directors or a vote of the holders of common stock; such rights may be amended only with the affirmative vote of holders of the majority of the shares of Class A stock. We believe that the high percentage (over 85%) of total shareholder votes held by the holders of common stock, coupled with other rights afforded to Regeneron shareholders (such as majority voting in the election of directors and the shareholders’ right to call a special shareholder meeting upon the written request of at least 25% of the total number of votes entitled to be cast), ensures that holders of common stock are heard and their rights as shareholders are protected.
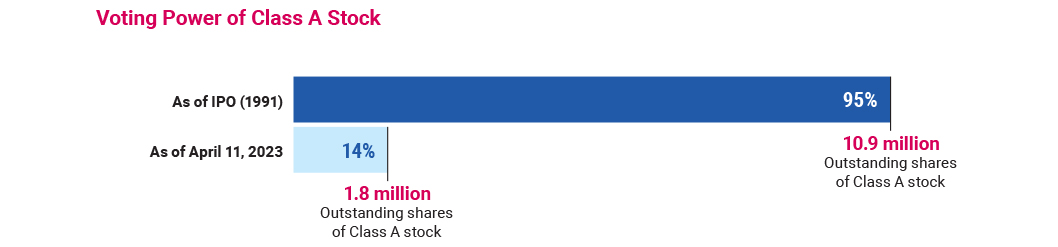
The board of directors has adopted a code of business conduct and ethics that applies to all of our employees, officers, and directors. You can find links to this code on our website at www.regeneron.com under the “Corporate Governance” heading on the “Investors & Media” page. We may satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding an amendment to, or a waiver from, a provision of our code of business conduct and ethics that applies to our principal executive officer, principal financial officer, principal accounting officer, or controller, or persons performing similar functions, by posting such information on our website where it is accessible through the same link noted above.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 37 |
Under our Corporate Governance Guidelines, the board of directors is required to periodically review with our CEO Regeneron’s plan for succession to the offices of the CEO and other senior executive positions. In recent years, the board and its committees conducted a multi-year formal succession planning and talent review, which included succession planning for the CEO and other senior management positions and incorporated diversity, equity, and inclusion (“DEI”) considerations as a strategic priority. The applicable committees of the board of directors advanced this formal review by focusing on certain assigned functions and roles within the Company. As part of this process, the Audit Committee reviewed functions and roles within the Company’s finance, information technology, and real estate & facilities management organizations, while the Compensation Committee and the Technology Committee reviewed functions and roles within the Company’s commercial and certain other general and administrative organizations and the Company’s research & development and global development organizations, respectively. Implementation of the succession planning and talent review plan continued in 2022 and 2023 to date.
In addition to formal succession planning, directors also have exposure to Regeneron leaders through board and committee presentations and discussions and informal events and interactions with key talent throughout the year, both in small-group and one-on-one settings.
Regeneron’s mission is to use the power of science to repeatedly bring new medicines to patients. We are committed to operating responsibly, communicating transparently about our impacts, and engaging key stakeholders in our mission. We strive to “Do Well by Doing Good,” which guides our approach to corporate responsibility. We disclose detailed information about significant corporate responsibility matters in our annual responsibility reports.
Our board of directors and management team recognize the importance of corporate responsibility matters. The Company’s policy is to take into consideration the long-term interests of the Company, its shareholders, and other stakeholders, including patients, employees, the healthcare community, regulators, partners, suppliers, and local communities. While the full board has retained oversight of the Company’s strategies and policies related to diversity, equity, and inclusion, the board’s Corporate Governance and Compliance Committee has been tasked with oversight of corporate responsibility as set forth in its charter. Under our Corporate Governance Guidelines, the Corporate Governance and Compliance Committee is responsible for conducting a periodic review of ESG matters and overseeing the Company’s key corporate responsibility initiatives, including those expected to have a significant impact on the Company’s ability to deliver sustained growth. Management, who has the responsibility for formulating and implementing such initiatives and matters, has established for these purposes a Responsibility Committee comprised of cross-functional business leaders.
Our responsibility strategy centers on three focus areas:
Improve the lives of people with serious diseases
Foster a culture of integrity and excellence
Build sustainable communities
As shown below, our 2025 responsibility goals span across these three areas, focusing on the environmental and social issues that we believe are most significant to our business and stakeholders.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 38 |
*
Our accompanying set of environmental targets are outlined in detail in our 2022 Responsibility Report. |
In 2022, we made significant progress toward achieving many of these responsibility goals. Select highlights within each focus area are summarized below, and a detailed discussion of our progress is included in our 2022 Responsibility Report.
Our responsibility efforts and progress across these focus areas have garnered recognitions. For example, in 2022, we were included in the Dow Jones Sustainability World Index for the fourth year in a row and the Dow Jones Sustainability North America Index for the third year in a row. These global and regional indices are comprised of corporate leaders in ESG practices.
We have also continued to enhance our responsibility reporting. Our 2022 Responsibility Report continues to align with the Sustainability Accounting Standards Board (“SASB”) framework and with the Global Reporting Initiative (“GRI”) Universal Standards. In 2023, we also published on our website our third annual Task Force for Climate-related Financial Disclosures (“TCFD”) report on Regeneron’s climate-related risks and opportunities and, as discussed below, our inaugural DEI Annual Impact Report.
Improve the lives of people with serious diseases. As a science-focused company, we operate Regeneron with the long-term outlook required to turn rigorous scientific research into important new medicines. All nine of our approved medicines and almost all of the product candidates in our clinical pipeline are homegrown—discovered in Regeneron’s labs using our industry-leading, proprietary technologies. Our support for patients extends beyond the labs to disease education and awareness efforts, patient assistance programs, and our commitment to access to medicine and responsible pricing. We strive to make thoughtful and well-informed pricing decisions with fairness and affordability in mind and are guided in this endeavor by our board of directors, which is closely involved in and provides oversight of all key pricing determinations. We believe we have the appropriate governance mechanisms and internal processes in place to ensure that pricing decisions are thoroughly and appropriately vetted prior to implementation and are made in line with our values and commitments. This includes routine presentations to our board of directors on drug pricing strategies, practices, and trends.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 39 |
In 2022, our commitment to improving lives was highlighted by securing regulatory approvals for several new indications for existing products and making significant progress in our product pipeline of approximately 35 product candidates as of the date of our 2022 Annual Report, including potential new treatments for ultra-rare and rare diseases. We are proud of these scientific accomplishments and the potentially life-changing benefits they can deliver to patients.
We are dedicated to making sure our medicines are available to everyone who needs them, including those in low- and middle-income countries (“LMICs”). For example, since 2018, we have worked with the World Health Organization, U.S. Food and Drug Administration (“FDA”), and other global organizations to offer Inmazeb, our treatment for the infection caused by Zaire ebolavirus, under a compassionate use protocol in response to Ebola outbreaks in affected African countries. We have an executive-sponsored Inmazeb access working group and we proactively engage public health agencies, non-governmental agencies, and others in our industry to ensure continued access to Inmazeb in LMICs. Similarly, to ensure rapid and broad access to REGEN-COV, our COVID-19 therapy that was previously authorized under an emergency use authorization from the FDA, our partner Roche utilized global national income tiered pricing to address affordability challenges in LMICs. We remain committed to addressing the ongoing threat of COVID-19, and, in early 2023, announced the discovery of a next-generation COVID-19 antibody that has demonstrated high neutralization potency against all known SARS-CoV-2 variants and lineages to date.
Foster a culture of integrity and excellence. Regeneron’s unique culture makes us who we are. Our culture includes our science-led mindset, our high ethical standards, and our unwavering focus on solving big, complex problems. We are equally committed to conducting our business responsibly and ethically. This is demonstrated through the range of policies, practices, and initiatives we have implemented, encompassing areas such as compliance, responsible sales and marketing, ethical clinical trials, responsible supply chain, and product quality and safety.
As we grow, we remain committed to making significant investments to attract and retain top talent and promote DEI within our workforce and our communities. These efforts are crucial to achieving our 2025 responsibility goal to increase representation of diverse individuals in leadership and foster inclusion. We believe our commitment to DEI allows us to better drive innovation and achieve our mission to repeatedly bring important new medicines to patients with serious diseases. In 2022, we advanced our DEI strategy focused on creating a better workplace, better science, and better world by, among other things, introducing inclusive leadership education for some of our most senior leaders, launching a pilot mentorship program focused on our diverse talent base, and continuing our efforts to ensure that our clinical trials represent the people who are most likely to benefit from the medicine under investigation if approved. In addition, to increase access to clinical trials, we utilize DEI principles in making clinical trial decisions, such as the design and selection of sites. Our board of directors receives periodic updates on our DEI efforts and continues to monitor our progress.
In 2023, we published on our website our inaugural DEI Annual Impact Report, which includes a detailed discussion of our DEI strategic framework, governance model, goals, and initiatives. We have also continued to share our DEI progress and metrics in our 2022 Responsibility Report and publish consolidated EEO-1 data (data from annual reports submitted to the U.S. Equal Employment Opportunity Commission) on our website.
Build sustainable communities. Our commitment to protect the health of the planet is closely tied to our mission to improve the health of patients. As part of our 2025 responsibility goals, we set ambitious medium- and longer-term environmental sustainability targets. We report on our continued progress toward these goals and targets in our Responsibility Reports.
We also strengthen our communities through strategic philanthropic investments, product donations, and the power of our employees’ talents and time. We are a long-standing supporter of science, technology, engineering and math (“STEM”) education, and make major philanthropic investments to inspire and celebrate future scientific innovators, including our ten-year, $100-million commitment to the Regeneron Science Talent Search, the nation’s most prestigious pre-college science and mathematics competition; and our five-year, $24-million commitment to the Regeneron International Science and Engineering Fair, the world’s largest pre-college science and engineering competition, which commenced in 2020. Over the past three years, we have provided STEM experiences to nearly 1.7 million students, putting us on track to achieve our goal of reaching 2.5 million STEM students by 2025.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 40 |
We also continue to foster employee volunteerism to reach our 2025 global responsibility goal of driving employee volunteer levels above national standards. In 2022, nearly 7,000 employees volunteered approximately 31,200 hours, including approximately 55% of our employees who volunteered nearly 20,000 hours to approximately 190 nonprofits during our sixth annual company-wide service event, Day for Doing Good.
We are proud to have been named to the Civic 50 for the sixth consecutive year. The Civic 50 recognizes the most community-minded companies in the United States.
For more information about our responsibility efforts and results, please refer to the 2022 Responsibility Report available on our website.
We are committed to adhering to the highest ethical standards when engaging in any political activities. Reflecting this commitment, the board of directors (upon the recommendation of the Corporate Governance and Compliance Committee) has adopted the Company’s Corporate Political Contributions Policy, a formal written policy that, together with our code of business conduct and ethics, sets forth our policies and procedures on political contributions and political activity. The policy is available on our website at www.regeneron.com under the “Transparency & Policies” heading on the “Responsibility” page.
We maintain robust stock ownership guidelines for our directors, executive officers, and other senior executives, as shown below.
Non-Employee Directors |
|
CEO, CSO, and Chair of the Board |
|
Other Executive Officers and Senior Executives |
Must own shares with a value at least 3x their annual retainers. |
|
Must own shares with a value at least 6x their respective base salaries. |
|
Must own shares with a value at least 2x their respective base salaries. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 41 |

The board of directors has adopted a written policy for the review, approval, or ratification of related person transactions. The Company considers transactions (or a series of related transactions) in which the Company is a participant, the amount involved exceeds $10,000 in any calendar year, and a director, officer, more than 5% holder of our voting securities, any immediate family member of any of the foregoing, or any related entity of any of the foregoing has a direct or indirect material interest to constitute related person transactions. The policy provides for a standing pre-approval of transactions with any passive institutional shareholder who holds more than 5% of our voting securities and transactions where all shareholders receive proportional benefits. With respect to any new transaction that is deemed pre-approved, the Audit Committee receives a summary of each such transaction and retains the ability to require that one or more of such transactions be subject to the standard approval procedures. The policy also requires that the arrangements relating to a permanent, full-time employment of an immediate family member of a director or executive officer hired by the Company be approved in accordance with the policy. In addition, in the event a person is or becomes a director or executive officer of the Company and an immediate family member of such person is a permanent, full-time employee of the Company, no material, outside-of-the-ordinary-course-of-business change in the terms of employment, including compensation, is permitted to be made without the prior approval of the Audit Committee (except, if the immediate family member is himself or herself an executive officer of the Company, any proposed change in the terms of employment is reviewed and approved in the same manner as compensatory arrangements of other executive officers).
The board of directors has determined that the members of the Audit Committee are best suited to review and approve related person transactions. Accordingly, each related person transaction (other than a transaction that is deemed pre-approved as described above) must be reviewed and approved or ratified by the members of the Audit Committee, other than any member of the Audit Committee that has an interest in the transaction. Under the policy, the Chair of the Audit Committee is delegated the authority to approve certain related person transactions that require urgent review and approval.
When reviewing, approving, or ratifying a related person transaction, the Audit Committee will consider several factors, including the benefits to the Company, the impact on a director’s independence in the event that a director or his/her immediate family is involved in the transaction, the terms of the transaction, and the terms available to unrelated third parties or to employees in general, if applicable. Related person transactions are approved only if the Audit Committee (or the Chair of the Audit Committee pursuant to delegated authority in the circumstances noted above) determines that they are in, or are not inconsistent with, the best interests of the Company and our shareholders.
Dr. Tessier-Lavigne has served as the President of Stanford University in 2022 and 2023 to date. In 2022, we made payments to Stanford University of approximately $145,500 in the aggregate consisting of a medical education fellowship grant and contractual royalty and software technology license payments. In 2023 through the end of the first quarter, we made payments to Stanford of approximately $118,500 in the aggregate consisting of a payment for services provided in connection with a clinical trial entered into in the ordinary course of business and a contractual royalty payment.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 42 |
Our Certificate of Incorporation provides that, to the fullest extent permitted under the New York Business Corporation Law, no director or officer of our Company shall be personally liable to the Company or its shareholders for monetary damages for any breach of fiduciary duty in such capacity. In addition, our Amended and Restated By-Laws provide that we shall indemnify our directors and certain of our other personnel against expenses (including attorneys’ fees) and certain other liabilities, including judgments, fines, and amounts paid in settlement, arising out of or incurred as a result of legal actions brought or threatened against them by reason of their position in our Company, subject to certain qualifications and provided that each such person acted in good faith, in a manner that they reasonably believed was in the Company’s best interest, and, where applicable, not unlawful. Subject to the provisions of our Certificate of Incorporation, our Amended and Restated By-Laws, and the New York Business Corporation Law, we may also advance expenses of the individuals entitled to indemnification.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 43 |

The Audit Committee has appointed PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2023. PricewaterhouseCoopers LLP (or its predecessor) has audited the Company’s financial statements for the past 34 years. The Audit Committee believes that the continued engagement of one independent registered public accounting firm contributes to higher quality audit work and greater operational efficiencies by leveraging PricewaterhouseCoopers LLP’s deep institutional knowledge of our operations and business, accounting policies and practices, and internal controls. SEC rules and PricewaterhouseCoopers LLP policies require the lead audit partner to be rotated at least every five years. The Audit Committee and its Chair are involved in the selection of PricewaterhouseCoopers LLP’s lead audit partner pursuant to this rotation. A new lead audit partner was selected beginning with the fiscal year 2022 audit. Additionally, in order to ensure continuing auditor independence, the Audit Committee periodically considers whether there should be a regular rotation of the Company’s independent registered public accounting firm.
The Audit Committee and the board of directors believe that the continued retention of PricewaterhouseCoopers LLP to serve as the Company’s independent registered public accounting firm is in the best interests of the Company and its shareholders.
The board of directors has directed that the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for fiscal year 2023 be submitted for ratification by the shareholders at the 2023 Annual Meeting. Shareholder ratification of the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for fiscal year 2023 is not required by the Company’s charter documents or otherwise, but is being pursued as a matter of good corporate practice. If shareholders do not ratify the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for fiscal year 2023, the board of directors will consider the matter at its next meeting. Even if the selection is ratified, the Audit Committee may in its discretion select a different independent registered public accounting firm at any time during the year if it determines that such a change would be in the best interests of the Company and its shareholders.
PricewaterhouseCoopers LLP has advised the Company that it will have in attendance at the 2023 Annual Meeting a representative who will be afforded an opportunity to make a statement, if such representative desires to do so, and will respond to appropriate questions presented at the 2023 Annual Meeting.
Aggregate fees incurred related to services provided to the Company by PricewaterhouseCoopers LLP for the years ended December 31, 2022 and 2021 were:
|
2022 ($) |
2021 ($) |
Audit Fees |
3,125,087 |
2,594,572 |
Audit-Related Fees |
— |
64,500 |
Tax Fees |
81,709 |
303,101 |
All Other Fees |
6,335 |
4,194 |
Total Fees |
3,213,131 |
2,966,367 |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 44 |
Audit Fees. Audit fees in 2022 and 2021 were primarily for professional services rendered for the audit of the Company’s financial statements for the fiscal year, including attestation services required under Section 404 of the Sarbanes-Oxley Act of 2002, technical accounting consultations related to the annual audit, and reviews of the Company’s quarterly financial statements included in its Form 10-Q filings.
Audit-Related Fees. Audit-related fees in 2021 were for professional services in connection with the planned liquidation of certain of the Company’s subsidiaries and the filing of a registration statement on Form S-3.
Tax Fees. Tax fees in 2022 and 2021 were for tax planning and advisory services.
All Other Fees. All other fees in 2022 and 2021 were for annual subscriptions to accounting resources.
The Audit Committee has adopted a policy regarding the pre-approval of audit and permitted non-audit services to be performed by the Company’s independent registered public accounting firm, PricewaterhouseCoopers LLP. The Audit Committee will, on an annual basis, consider and, if appropriate, approve the provision of audit and non-audit services by PricewaterhouseCoopers LLP. The Audit Committee has approved a general provision of $75,000 for accounting advisory and other permissible consulting engagements. Management is responsible for notifying the Audit Committee of the status of accounting advisory and other permissible consulting engagements at regularly scheduled Audit Committee meetings and, if the Audit Committee so determines, the general provision is replenished to $75,000. The Audit Committee did not utilize the de minimis exception to the pre-approval requirements to approve any services provided by PricewaterhouseCoopers LLP during fiscal 2022 or 2021.
We have reviewed the audited financial statements of the Company for the year ended December 31, 2022, which are included in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022, and met with both management and PricewaterhouseCoopers LLP, the Company’s independent registered public accounting firm, to discuss those financial statements. The Audit Committee has discussed with the Company’s independent registered public accounting firm the matters required to be discussed by the applicable requirements of the Public Company Accounting Oversight Board (the “PCAOB”) and the Securities and Exchange Commission. The Audit Committee also discussed with the independent registered public accounting firm their independence relative to the Company and received and reviewed the written disclosures and the letter from the independent registered public accounting firm required by the PCAOB.
Based on the foregoing discussions and review, the Audit Committee recommended to the board of directors that the audited financial statements of the Company for the year ended December 31, 2022 be included in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2022 for filing with the Securities and Exchange Commission.
We have appointed PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2023. This appointment was based on a variety of factors, including PricewaterhouseCoopers LLP’s competence in the fields of accounting and auditing.
The Audit Committee
George L. Sing, Chair
N. Anthony Coles, M.D.
Arthur F. Ryan
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 45 |

PROPOSAL 2
|
The board of directors unanimously recommends a vote FOR |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 46 |
The following table sets forth, as of April 11, 2023, the number of shares of the Company’s Class A stock and common stock beneficially owned by each of the Company’s directors, each of the NEOs referred to below under “Compensation-Related Matters—Compensation Discussion and Analysis,” all directors and executive officers as a group, and each other person or group of persons known by the Company to beneficially own more than 5% of the outstanding shares of Class A stock or common stock, based upon (unless indicated otherwise) information obtained from such persons, and the percentage that such shares represent of the number of outstanding shares of Class A stock and common stock, respectively.
The Class A stock is convertible on a share-for-share basis into common stock. The Class A stock is entitled to ten votes per share and the common stock is entitled to one vote per share. No new shares of Class A stock have been issued since our IPO in 1991. See “The Company—Corporate Governance—Capital Structure” for more information. We have determined beneficial ownership in accordance with the rules of the SEC. Except as otherwise indicated in the footnotes below, we believe, based on the information furnished or otherwise available to us, that the persons named in the table below have sole voting and investment power with respect to all shares of Class A stock and common stock shown as beneficially owned by them, subject to applicable community property laws. We have based our calculation of percentage of shares of a class beneficially owned on 1,818,146 shares of Class A stock and 107,892,108 shares of common stock outstanding as of April 11, 2023, except that for each person listed who beneficially owns Class A stock (and for directors and executive officers as a group), the number of shares of common stock beneficially owned by that person (and by directors and executive officers as a group) and the percentage ownership of common stock of such person assume the conversion on April 11, 2023 of all shares of Class A stock listed as beneficially owned by such person (or persons in the case of directors and executive officers as a group) into common stock and also that no other shares of Class A stock beneficially owned by others are so converted.
In computing the number of shares of common stock beneficially owned by a person (and by directors and executive officers as a group) and the percentage ownership of common stock of such person (and by directors and executive officers as a group), shares of common stock subject to options, PSUs, RSUs, or other convertible securities (if any) held by that person (and by directors and executive officers as a group) that are exercisable or releasable as of April 11, 2023 or are exercisable or releasable within sixty days after April 11, 2023 are deemed to be outstanding. Such shares are not deemed to be outstanding, however, for the purpose of computing the percentage ownership of common stock of any other person.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 47 |
Name and Address of Beneficial Owner |
Shares of Class A Stock Beneficially Owned1 |
|
Shares of Common Stock Beneficially Owned1 |
||||||
Number |
Percent of Class |
Number2 |
Percent of Class |
||||||
Beneficial Owners of More than 5% of Class A Stock or Common Stock (Other than Directors and Executive Officers): |
|
|
|
|
|
|
|
|
|
FMR LLC3 245 Summer Street |
— |
|
— |
|
|
11,096,984 |
|
10.3 |
% |
BlackRock, Inc.4 55 East 52nd Street |
— |
|
— |
|
|
9,166,817 |
|
8.5 |
% |
The Vanguard Group, Inc.5 100 Vanguard Blvd. |
— |
|
— |
|
|
8,722,448 |
|
8.1 |
% |
Capital World Investors6 333 South Hope Street |
— |
|
— |
|
|
6,315,819 |
|
5.9 |
% |
JPMorgan Chase & Co.7 383 Madison Avenue |
— |
|
— |
|
|
6,101,044 |
|
5.7 |
% |
Directors and Executive Officers:8 |
|
|
|
|
|
|
|
|
|
Leonard S. Schleifer, M.D., Ph.D. |
1,726,565 |
9 |
95.0% |
|
|
3,261,849 |
10 |
3.0 |
% |
P. Roy Vagelos, M.D. |
— |
|
— |
|
|
1,059,966 |
11 |
1.0 |
% |
George D. Yancopoulos, M.D., Ph.D. |
42,750 |
12 |
2.3% |
|
|
2,060,919 |
13 |
1.9 |
% |
Bonnie L. Bassler, Ph.D. |
— |
|
— |
|
|
22,098 |
14 |
|
29 |
Michael S. Brown, M.D. |
— |
|
— |
|
|
21,471 |
15 |
|
29 |
N. Anthony Coles, M.D. |
— |
|
— |
|
|
4,701 |
16 |
|
29 |
Joseph L. Goldstein, M.D. |
— |
|
— |
|
|
9,690 |
17 |
|
29 |
Robert E. Landry |
— |
|
— |
|
|
105,100 |
18 |
|
29 |
Marion McCourt |
— |
|
— |
|
|
48,043 |
19 |
|
29 |
Andrew J. Murphy, Ph.D. |
— |
|
— |
|
|
323,608 |
20 |
|
29 |
Christine A. Poon |
— |
|
— |
|
|
64,837 |
21 |
|
29 |
Arthur F. Ryan |
— |
|
— |
|
|
22,490 |
22 |
|
29 |
George L. Sing |
— |
|
— |
|
|
78,884 |
23 |
|
29 |
Marc Tessier-Lavigne, Ph.D. |
— |
|
— |
|
|
18,702 |
24 |
|
29 |
Craig B. Thompson, M.D. |
— |
|
— |
|
|
2,606 |
25 |
|
29 |
Daniel P. Van Plew |
— |
|
— |
|
|
114,187 |
26 |
|
29 |
Huda Y. Zoghbi, M.D. |
— |
|
— |
|
|
28,442 |
27 |
|
29 |
All Directors and Executive Officers as a Group (20 persons) |
1,769,315 |
|
97.3% |
|
|
7,918,673 |
28 |
7.0 |
% |
1
The inclusion in this table of any Class A stock or common stock, as the case may be, deemed beneficially owned does not constitute an admission of beneficial ownership of those shares. 2
For each person listed who beneficially owns Class A stock (and for directors and executive officers as a group), the number of shares of common stock listed includes the number of shares of Class A stock listed as beneficially owned by such person (or persons in the case of directors and executive officers as a group). |
|||||||||
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 48 |
3
Based solely on an amendment to a Schedule 13G jointly filed by FMR LLC and Abigail P. Johnson on February 9, 2023. According to this amendment, FMR LLC has sole voting power as to 10,677,607 of the shares reported as beneficially owned and sole dispositive power as to all of the shares reported as beneficially owned. Abigail P. Johnson is a Director, the Chairman, and the Chief Executive Officer of FMR LLC. Members of the Johnson family, including Abigail P. Johnson, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the voting power of FMR LLC. The Johnson family group and all other Series B shareholders have entered into a shareholders’ voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders’ voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, as amended, to form a controlling group with respect to FMR LLC. 4
Based solely on an amendment to a Schedule 13G filed by BlackRock, Inc. on February 3, 2023. According to this amendment, BlackRock, Inc. has sole voting power as to 8,336,590 of the shares reported as beneficially owned and sole dispositive power as to all of the shares reported as beneficially owned. 5
Based solely on an amendment to a Schedule 13G filed by The Vanguard Group, Inc. on February 9, 2023. According to this amendment, The Vanguard Group, Inc. has shared voting power as to 150,986, sole dispositive power as to 8,284,063, and shared dispositive power as to 438,385 of the shares reported as beneficially owned. 6
Based solely on an amendment to a Schedule 13G filed by Capital World Investors on February 13, 2023. According to this amendment, Capital World Investors, a division of Capital Research and Management Company, has sole voting power as to 6,297,182 of the shares reported as beneficially owned and dispositive power as to all of the shares reported as beneficially owned. 7
Based solely on an amendment to a Schedule 13G filed by JPMorgan Chase & Co. on January 11, 2023. According to this filing, JPMorgan Chase & Co. has sole voting power as to 5,535,989, shared voting power as to 28,639, sole dispositive power as to 6,063,413, and shared dispositive power as to 34,499 of the shares reported as beneficially owned. 8
The address for each director and executive officer is c/o Regeneron Pharmaceuticals, Inc., 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707. 9
Includes 15,775 shares of Class A stock held in trust for the benefit of Dr. Schleifer’s son, of which Dr. Schleifer is a trustee. 10
Includes (i) 852,188 shares of common stock purchasable upon the exercise of options granted pursuant to the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan, the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan, the Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan, or the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan (collectively, the “Long-Term Incentive Plans”) that are exercisable or become so within sixty days after April 11, 2023; (ii) 129,969 shares of common stock held in a grantor retained annuity trust of which Dr. Schleifer is the trustee; (iii) 11,178 shares of common stock held in a trust for the benefit of Dr. Schleifer’s family members, of which Dr. Schleifer’s spouse is a trustee; (iv) 100 shares of common stock held in a charitable foundation of which Dr. Schleifer is a director and officer; and (v) 5,896 shares of common stock held in an account under the Company’s 401(k) Savings Plan. 11
Includes (i) 515,491 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; (ii) 1,963 shares of common stock held in an account under the Company’s 401(k) Savings Plan; (iii) 125,493 shares of common stock held in a charitable lead annuity trust, of which Dr. Vagelos is the trustee; (iv) 23,461 shares of common stock held in a trust for his grandchildren, of which Dr. Vagelos’s wife is the trustee; (v) 3,609 shares of common stock held in trusts for his grandchildren, of which Dr. Vagelos and/or his wife are trustees; (vi) 39,860 shares of common stock and 42,242 shares of common stock held by the Marianthi Foundation and the Pindaros Foundation, respectively, both of which are charitable foundations of which Dr. Vagelos is a director and an officer; and (vii) 1,163 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 11, 2023 upon termination of service. Dr. Vagelos disclaims beneficial ownership of the shares held by the charitable foundations referenced in (vi). 12
Of these shares, 23,367 shares are held in trust for the benefit of Dr. Yancopoulos’s children and certain other family members; Dr. Yancopoulos is a trustee of the trust. The remaining 19,383 shares are held in trusts for the benefit of Dr. Yancopoulos’s children, of which Dr. Yancopoulos is the trustee. 13
Includes (i) 795,799 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; (ii) 5,869 shares of common stock held in an account under the Company’s 401(k) Savings Plan; (iv) 569,653 shares of common stock held in trust for the benefit of Dr. Yancopoulos’s children and certain other family members, of which Dr. Yancopoulos is a trustee; (v) 196,848 shares of common stock held in trusts for the benefit of Dr. Yancopoulos’s children; and (vi) 450,000 shares of common stock held in grantor retained annuity trusts of which Dr. Yancopoulos is the trustee. 14
Consists of (i) 20,945 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; and (ii) 1,153 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 11, 2023 upon termination of service. 15
Consists of (i) 8,656 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023, which are held in a trust of which Dr. Brown and his spouse are trustees for the benefit of Dr. Brown’s immediate family members; (ii) 6,162 shares of common stock held in a trust of which Dr. Brown and his spouse are trustees for the benefit of Dr. Brown’s immediate family members; (iii) 5,000 shares of common stock held in a trust of which Dr. Brown’s spouse is trustee for the benefit of Dr. Brown’s immediate family members; (iv) 500 shares of common stock held by a family charitable foundation, of which Dr. Brown is a director and an officer and his wife is a director; and (v) 1,153 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 11, 2023 upon termination of service. Dr. Brown disclaims beneficial ownership of the shares referenced in (iii) and (iv). 16
Includes (i) 3,537 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; and (ii) 1,153 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 11, 2023 upon termination of service. 17
Includes (i) 3,537 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; and (ii) 1,153 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 11, 2023 upon termination of service. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 49 |
18
Includes (i) 72,928 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; (ii) 27,601 shares of restricted stock (“RSAs”); and (iii) 250 shares of common stock held in an account under the Company’s 401(k) Savings Plan. 19
Includes (i) 27,102 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; (ii) 19,579 RSAs; and (iii) 138 shares of common stock held in an account under the Company’s 401(k) Savings Plan. 20
Includes (i) 258,571 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; (ii) 43,152 RSAs; and (iii) 4,290 shares of common stock held in an account under the Company’s 401(k) Savings Plan. 21
Includes (i) 62,894 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; and (ii) 1,153 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 11, 2023 upon termination of service. 22
Includes (i) 3,537 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; and (ii) 1,153 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 11, 2023 upon termination of service. 23
Includes (i) 50,614 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; (ii) 750 shares of common stock held by Mr. Sing’s spouse; (iii) 400 shares of common stock held by Mr. Sing’s spouse as custodian for the benefit of their son; (iv) 1,000 shares of common stock held in a trust for benefit of Mr. Sing’s son; and (v) 1,153 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 11, 2023 upon termination of service. 24
Includes (i) 16,362 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; and (ii) 1,153 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 11, 2023 upon termination of service. 25
Consists of (i) 2,399 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; and (ii) 207 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 11, 2023 upon termination of service. 26
Includes (i) 79,238 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; (ii) 20,419 RSAs; and (iii) 1,065 shares of common stock held in an account under the Company’s 401(k) Savings Plan. 27
Consists of (i) 27,289 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; and (ii) 1,153 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 11, 2023 upon termination of service. 28
Includes (i) 3,346,342 shares of common stock purchasable upon exercise of options granted pursuant to the Long-Term Incentive Plans that are exercisable or become so within sixty days after April 11, 2023; (ii) 139,330 RSAs; (iii) 11,747 shares of common stock issuable upon settlement of RSUs that are releasable within sixty days after April 11, 2023; and (iv) 27,100 shares of common stock held in an account under the Company’s 401(k) Savings Plan. 29
Represents less than 1%. |
The Company has established a process for shareholders to send communications to the members of the board of directors. Shareholders may send such communications by mail addressed to the full board, a specific member or members of the board, or a particular committee of the board, at 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707, Attention: Corporate Secretary. All such communications will be opened by our Corporate Secretary for the sole purpose of determining whether the contents represent a message to our directors. Any contents that are not in the nature of advertising, promotions of a product or service, or patently offensive material will be forwarded promptly to the addressee. In the case of communications to the board or any individual director or group or committee of directors, the Corporate Secretary will make sufficient copies of the contents to send to such director or each director who is a member of the group or committee to which the communication is addressed.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 50 |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 51 |
At the upcoming 2023 Annual Meeting, we seek your input on the compensation of our “Named Executive Officers”1 as discussed in this proxy statement through the advisory vote commonly referred to as “say-on-pay” (Proposal No. 3). We also seek your input on the frequency of future say-on-pay votes through the advisory vote commonly referred to as “say-on-frequency” (Proposal No. 4). In recognition of shareholder preference and feedback, we now hold say-on-pay votes every year and are recommending that shareholders vote at the 2023 Annual Meeting for continuing this practice. Say-on-pay votes help us to assess whether shareholders approve of our compensation program and philosophy and whether they view our compensation decisions in a particular year as responsive to any concerns they may have articulated. In “Compensation Discussion and Analysis–Compensation Processes–Shareholder Input and Outreach and the 2022 Say-on-Pay Vote Result,” we discuss in detail how we have responded to recent shareholder feedback on compensation and other matters.
As you read the following Compensation Discussion and Analysis and review this year’s compensation-related proposals, we encourage you to consider Regeneron’s compensation program in light of Regeneron’s long-term performance as well as the program’s business objectives. Among the compensation program’s key objectives are to pay for performance, drive the creation of long-term, sustainable shareholder value, deliver compensation that is competitively positioned among our peers, and align with the pursuit and achievement of both our short-term and long-term goals.
Shareholder value creation over the last decade driven by relentless innovation and product pipeline progress

DME = diabetic macular edema; DR = diabetic retinopathy; LDL-C = low-density lipoprotein cholesterol; AD = atopic dermatitis; RA = rheumatoid arthritis; CSCC = cutaneous squamous cell carcinoma; CRSwNP = chronic rhinosinusitis with nasal polyposis; EUA = Emergency Use Authorization from FDA; FDA = U.S. Food and Drug Administration; HoFH = homozygous familial hypercholesterolemia; NSCLC = non-small cell lung cancer; BCC = basal cell carcinoma; EoE = eosinophilic esophagitis; and PN = prurigo nodularis. Consult the product label in each approved territory for specific information about such products and indications.
1 |
Our “Named Executive Officers” or “NEOs” are identified below under “Compensation Discussion and Analysis.” |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 52 |
The following Compensation Discussion and Analysis (“CD&A”) describes the philosophy, objectives, and structure of our 2022 executive compensation program.2 This CD&A is intended to be read in conjunction with the subsequent tables presented under “Compensation Dashboard–2022 Executive Compensation Tables,” which provide further historical compensation information for our “Named Executive Officers” or “NEOs” identified below.3

|
LEONARD S. SCHLEIFER, President and Chief Executive Officer |
|

|
GEORGE D. YANCOPOULOS, President and Chief Scientific Officer |

|
ROBERT E. LANDRY Executive Vice President, Finance and Chief Financial Officer |
|

|
DANIEL P. VAN PLEW Executive Vice President and General Manager, Industrial Operations and Product Supply |

|
ANDREW J. MURPHY, Executive Vice President, Research |
|

|
MARION MCCOURT Executive Vice President, Commercial |
2 |
In this section, “we,” “us,” and “our” refer to the Company and, where applicable, to the Compensation Committee of the Company’s board of directors. |
3 |
This year, our NEOs as determined in accordance with SEC rules consist of the following: our President and Chief Executive Officer (“CEO”); our Executive Vice President, Finance and Chief Financial Officer (“CFO”); and our three other highest-paid executives for 2022, our Executive Vice President and General Manager, Industrial Operations and Product Supply, our Executive Vice President, Research, and our Executive Vice President, Commercial. In addition, as in prior years, we have included our President and Chief Scientific Officer (“CSO”) as an NEO because of the importance of his role to Regeneron, his historical inclusion as an NEO in our proxy statements, and the fact that the annualized grant date fair market value of the five-year, front-loaded equity award granted to him in 2020 would have resulted in his qualifying as an NEO in respect of 2022. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 53 |
Regeneron is a unique company at the forefront of medical science. We focus on long‐term growth through internal innovation. Our compensation model and broad-based equity program reinforce our company culture and are designed to reward long‐term, sustainable performance and to promote an environment where employees are empowered to focus on our mission and drug discovery and development. The use of equity awards for all employees encourages them to think and act like owners, aligns the interests of our workforce with the long-time horizons for which we manage the business, and creates a shared incentive to deliver the type of successful research and commercialization efforts that create sustainable value for shareholders.
Over the last 10 years, we have delivered significant, sustainable value to shareholders, as demonstrated by total shareholder return of 322% in that period, and brought to market several groundbreaking medicines that help patients with serious diseases. Over the same period, we have embraced a model of enhanced shareholder engagement. We have worked to foster long-term relationships and trust with our shareholders and to strengthen the alignment of our executive compensation program with their perspectives and interests without detracting from the unique and effective culture that this program has helped create. Shareholder feedback continues to inform our review of compensation and corporate governance practices.
Our capital allocation strategy begins with investment in best-in-class R&D capabilities, the most important of which are our people. Our compensation program includes some unique features, and we believe that the success of the program in meeting its objectives is in part attributable to these features. We view equity-based compensation as a powerful tool to secure the commitment of true experts for the time it takes to do what was thought impossible only a decade ago. Our business accomplishments, record of scientific excellence, and industry-leading retention rate demonstrate the effectiveness of our approach.
We believe our performance in 2022 was supported by our compensation program, as shaped by our corporate culture and shareholder feedback. In 2022, we made significant progress in all key areas of our business, as shown in the chart below. We also received positive feedback on our compensation program, as demonstrated by the overwhelming support of the say-on-pay vote in 2022 at 87.8% of the votes cast, and continued to engage with shareholders on executive compensation and other governance topics. The compensation decisions made in 2022 were generally consistent year-over-year and reflected our commitment to Regeneron’s long-standing compensation philosophy as well as shareholder input.
Business Accomplishments and Shareholder Value Creation Supported by
Unique Compensation Program Shaped by Corporate Culture and Shareholder Feedback
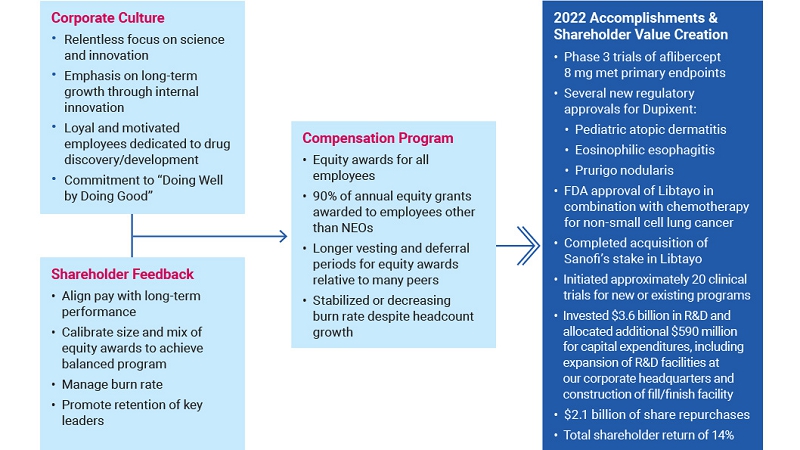
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 54 |

We focus on long-
term growth through internal innovation.
Our compensation model and broad-based equity program reinforce our company culture and are designed to reward long-term, sustainable performance.
An “overnight breakthrough” in the biotechnology industry takes years of research and drug development spearheaded by a collection of the world’s top scientists, staff, and businesspeople that are supported by substantial and patient capital investment. Our corporate culture and pay philosophy are the interlinked product of the unique challenges at the forefront of medical science, which require agility, persistence, and top-tier talent.
Regeneron’s culture is defined by loyal and motivated employees with an entrepreneurial spirit who are dedicated to the Company’s mission to use the power of science to invent medicines for people with serious diseases. Our employees’ engagement, commitment, and achievements are key drivers of pipeline success and our long-term performance. For Regeneron to succeed, our compensation model must reward long-term, sustainable performance and reinforce a culture where employees are empowered to pursue fulfilling careers and focus on our mission and drug discovery and development. Therefore, our compensation model is designed to encourage experimentation, innovation, and long-term thinking within an appropriate governance framework.
Like many companies, we use equity-based pay to align employees’ interests with our long-term success; but unlike many peers, a key part of our pay philosophy is to award equity-based pay to all employees. This unique approach ensures that when we deliver for patients and for shareholders, everyone shares in the upside growth, even over the multiyear timeframes required to bring new medicines to market. Emphasizing the overall success of the Company thus allows us to experiment, pursue innovative approaches, and follow the science. In recent years, approximately 90% of our annual employee equity grants were awarded to employees other than our NEOs, representing an exceptionally broad-based equity plan. We believe this approach encourages our employees to think and act like owners and supports our robust employee engagement and industry-leading retention,4 as shown below.

Our corporate culture also is shaped by our commitment to “Doing Well by Doing Good,” which has inspired the Company for the last 35 years. This commitment, paired with unrelenting focus on science and innovation, is reflected in our 2025 global responsibility goals. In 2022, we took important steps toward achieving these goals, as discussed in the subsection “The Company–Corporate Governance–Corporate Responsibility.”
4
Based on Regeneron’s turnover rate compared to the industry average. In 2022, our turnover rate was 9.0% compared to an industry average of 21.9%. Industry average is based on data of U.S. life sciences companies reported in Aon’s 2022 Salary Increase and Turnover Study. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 55 |
In 2022, we made significant progress in all key areas of our business. We also continued to demonstrate our commitment to driving sustainable, long-term shareholder value creation, including by investing $3.6 billion in research and development and $590 million in capital expenditures (primarily to expand R&D facilities at our corporate headquarters and construct a fill/finish manufacturing facility). We highlight select 2022 accomplishments below and provide a comprehensive overview of our 2022 achievements, as well as the Compensation Committee’s assessment of those achievements, in the subsections “Components of Executive Pay: What We Pay and Why We Pay It—Annual Cash Incentives” and “Compensation Dashboard—Additional Compensation Information—Annual Cash Incentives.”
Regulatory Actions |
Clinical and Significant Pipeline Advances |
|
|
•
EYLEA: Pediatric exclusivity granted by FDA based on completion of retinopathy of prematurity study, extending period of U.S. market exclusivity by an additional six months through May 17, 2024 •
Dupixent:
•
FDA approval for the treatment of adults and adolescents with eosinophilic esophagitis (“EoE”) (first and only treatment approved by the FDA for this indication) •
FDA and European Commission (“EC”) approvals for the treatment of prurigo nodularis (first and only targeted medicine specifically indicated to treat this disease) •
FDA approval for pediatric atopic dermatitis (first biologic approved for children 6 months-5 years of age)
•
Libtayo:
•
FDA approval in combination with chemotherapy for the treatment of non-small cell lung cancer (“NSCLC”)
•
EC approval and Ministry of Health, Labour and Welfare (“MHLW”) approval in Japan for the treatment of cervical cancer |
•
Aflibercept 8 mg: Reported that Phase 3 trials in diabetic macular edema (“DME”) and wet age-related macular degeneration (“wet AMD”) met their primary endpoints
•
Dupixent: Reported that Phase 3 trial in EoE in pediatrics met its primary endpoint
•
Oncology Programs:
•
Presented potentially pivotal Phase 2 data for odronextamab (bispecific antibody targeting CD20 and CD3) in B-cell non-Hodgkin lymphoma and linvoseltamab (bispecific antibody targeting BCMA and CD3) in multiple myeloma
•
Presented positive data from Phase 1 studies of fianlimab (in combination with Libtayo) in advanced melanoma and NSCLC
•
Presented positive initial data for two novel bispecific antibodies in solid tumors–REGN5093 (targeting two distinct MET epitopes) and ubamatamab (targeting MUC16 and CD3) •
Reported encouraging initial data from an ongoing Phase 1/2 trial investigating REGN5678 (bispecific antibody targeting PSMA and CD28) in combination with Libtayo in prostate cancer
•
Pipeline Growth: Initiated approximately 20 clinical trials for new or existing programs |
|
|
|
Commercial and Operational Execution |
Financial Execution and Talent Management |
|
•
EYLEA: Full-year 2022 U.S. net product sales increased 8% to $6.26 billion versus 2021
•
Dupixent: Full-year 2022 global net product sales increased 40% to $8.68 billion1 versus 2021
•
Libtayo: Full-year 2022 global net product sales increased 26% to $578.0 million1 versus 2021
•
Commercial Expansion:
•
Completed acquisition of Sanofi’s stake in Libtayo, providing Regeneron with exclusive worldwide development, commercialization, and manufacturing rights to the medicine
•
Established commercial capabilities in additional countries outside the United States
•
Industrial Operations and Product Supply: Expanded manufacturing capabilities while substantially rebuilding bulk and finished goods inventory (following resource reallocation to the production of COVID-19 monoclonal antibodies) |
•
Revenue: Full-year 2022 revenues decreased 24% to $12.17 billion versus 2021; excluding REGEN-COV and Ronapreve,2 revenues increased 17%3 versus 2021 •
EPS: GAAP and non-GAAP diluted net income per share, or EPS, for full-year 2022 of $38.22 and $44.98,3 respectively
•
Share Repurchase Programs: Repurchased $2.1 billion of common stock
•
Global Growth and Retention: Headcount reached nearly 12,000 employees, up 14% year-over-year, while turnover rate stayed at less than half the industry average |
|
|
|
1
Our collaborator Sanofi records global net product sales of Dupixent and, prior to July 1, 2022, recorded net product sales of Libtayo outside the United States. |
2
The casirivimab and imdevimab antibody cocktail is known as REGEN-COV® in the United States and RonaproveTM in other countries. The Company records net product sales of REGEN-COV in the United States and Roche records net product sales of Ronapreve outside the United States. REGEN-COV was authorized under an emergency use authorization from the FDA for COVID-19 from November 2020 until January 2022 when the EUA was revised to exclude its use in geographic regions where infection or exposure is likely due to a variant that is not susceptible to the treatment. |
3
Revenues excluding REGEN-COV and Ronapreve, non-GAAP net income, and non-GAAP net income per share, or EPS, are not measures calculated in accordance with GAAP. See Appendix A for a definition of these measures and a reconciliation of each of these measures to the most directly comparable GAAP financial measure. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 56 |
Our stock performed well in 2022, delivering a total shareholder return (“TSR”) of 14%. In addition, our stock outperformed the benchmarks shown below over the 3-year and 5-year periods ended December 31, 2022. While we cannot predict or control the performance of our stock price in any particular period, we believe that our Company’s performance is best assessed from a long-term perspective, consistent with the long-term nature of the drug discovery and development cycle. We also believe that if we continue to deliver pipeline, operational, and financial results, the creation of shareholder value and stock price appreciation will follow.
Regeneron TSR vs. Market*
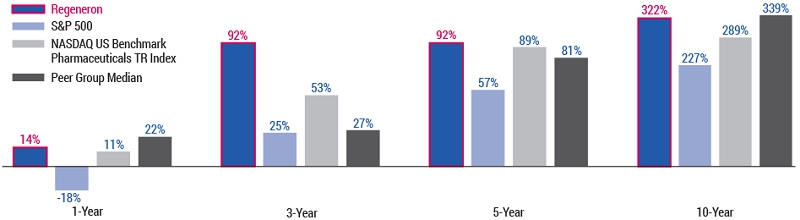
*
TSR data reflect total returns (stock price appreciation and, if applicable, reinvested dividends). |
We remain committed to our compensation philosophy and always strive to design our compensation program to support the long-term success of Regeneron. However, the specific features of our compensation program continue to evolve as the Company grows, our competition changes, and our business and operating environments transform. A decade ago, we had one-fifth of the employees we have today, a Company-wide equity compensation program comprised entirely of stock options, and equity award guidelines that sized all awards based on the number of underlying shares, resulting in an annual burn rate of almost 5% of common shares outstanding. In recognition of the impact of our growth on the use of equity and in response to shareholder feedback, commencing in 2013, we implemented eight straight years of equity award guideline reductions; and in 2019 and 2020, we introduced and then expanded the use of full-value awards as a component of our annual equity awards. Thanks in part to these changes and the continued calibration of the size and mix of annual equity awards Company-wide, we achieved a burn rate of 2.7% in 2022, the lowest level in more than a decade, despite keeping our broad-based equity program intact and growing to nearly 12,000 employees.
Key compensation decisions and compensation-related outcomes in 2022 are highlighted below.
No Additional Equity Awards for CEO and CSO. Consistent with the commitment of the board and the Compensation Committee in December 2020 when our CEO and CSO were each granted front-loaded performance restricted stock units (“PSUs”) in lieu of five years’ worth of annual equity awards (a pledge that was reiterated during our subsequent shareholder engagement), no equity awards were granted to these executives in 2022. See “Compensation Discussion and Analysis–Components of Executive Pay: What We Pay and Why We Pay It–Annual Equity Awards” for more information on these PSU awards.
Further Reduction of Burn Rate Through Continued Calibration of Size and Mix of Equity Awards to Other NEOs and Broad-Based Employees. In 2022, our burn rate was 2.7%, the lowest level in over a decade, despite keeping our broad-based equity program intact and increasing the number of our employees by 14% year-over-year, as shown in the chart below. As had been the case since 2019, the Compensation Committee used a mix of stock options and full-value equity awards for NEOs below the CEO/CSO level and for other employees in 2022, with the full-value awards consisting of restricted stock awards (“RSAs”) and, for employees outside the United States, restricted stock units (“RSUs”).
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 57 |
Regeneron Stock Utilization vs. Headcount*

*
Burn rate calculated by dividing (a) the sum of (i) the number of shares subject to equity awards (stock options, RSAs, and RSUs) granted during the year and (ii) PSUs earned during the year (if any), by (b) the basic weighted-average number of shares of common stock and Class A stock outstanding during the year. Headcount numbers based on the number of employees as of December 31 of the applicable year. |
Company Performance Multiplier for Annual Cash Incentive Awards Approved at 1.9 (same as in 2021). For 2022, the Compensation Committee determined and approved the Company performance multiplier for annual cash incentive awards to all eligible employees at 1.9 (out of a possible range of 0 to 2.0). In so doing, the Compensation Committee, following a thorough assessment within a pre-established framework that included consideration of the Company’s progress toward a targeted selection of potential milestones identified at the beginning of the year, recognized another year of outstanding Company performance. See the subsection “Components of Executive Pay: What We Pay and Why We Pay It—Annual Cash Incentives” for more information on the Committee’s process in setting the Company performance multiplier for 2022.
In addition to establishing an executive compensation program that aligns with our corporate culture and pay philosophy, we seek the views of our shareholders and annually engage with our investors to solicit ideas, input, and direct feedback. We do this not only formally through our say-on-pay vote (which we now hold annually in recognition of shareholder feedback and preference) but also through direct discussions with shareholders and informal exchanges in other settings. In the last decade, we have actively and regularly engaged with shareholders to receive feedback on many important areas including governance, compensation, and corporate responsibility matters. Feedback from these outreach efforts informs the Compensation Committee’s decision-making when evaluating our current compensation program and considering any potential modifications.
At our 2022 annual shareholder meeting, our say-on-pay proposal received the support of 87.8% of votes cast, the highest level of support in the last decade. The Compensation Committee believes that our compensation program has been appropriately structured to attract, retain, and motivate our executive team, aligning executive interests with those of shareholders. Nevertheless, we continued to engage with investors in 2022 to make sure we understand their perspectives and views of our compensation program and philosophy. Since the 2022 annual shareholder meeting, we reached out to shareholders collectively representing over 60% of the public shares (i.e., shares of common stock outstanding as of December 31, 2022, excluding shares held by our directors and executive officers). This outreach resulted in one-on-one discussions with shareholders representing nearly 50% of our public shares.
The feedback we received in 2022 was generally in support of our executive pay program and continued to be an important factor in board and committee discussions. See the subsection “Compensation Discussion and Analysis–Compensation Processes–Shareholder Input and Outreach and the 2022 Say-on-Pay Vote Result” for a more detailed discussion of our shareholder engagement program as well as a summary of recent actions taken in response to shareholder feedback.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 58 |
Our compensation program’s structure and design are shaped by our corporate culture, defined by employees dedicated to our mission; our business strategy and ongoing, company-wide pay philosophy; and our process for seeking and carefully considering valuable shareholder feedback each year. The compensation program for our NEOs follows the same principles we apply throughout the organization, with an even greater emphasis on equity compensation to ensure our leadership’s interests are aligned with our long-term shareholders.

To create an effective executive compensation structure, our Compensation Committee relied on the following compensation elements for our NEOs in 2022. These compensation elements are generally consistent with the compensation framework utilized in recent years (except that our CEO and CSO did not receive any PSUs or other equity awards in 2022).
|
Period |
Element |
Objective |
Performance Measured/Rewarded |

|
Annual |
Base Salary |
Recognizes an individual’s role and responsibilities and serves as an important retention vehicle |
Reviewed annually and set based on market competitiveness, individual performance, and other internal considerations |

|
Annual |
Annual Cash Incentive |
Motivates and rewards our executives for short-term achievements and milestones towards our long-term goals |
Corporate performance (CEO and CSO); corporate performance and individual contributions to that achievement (other NEOs) |
Long-Term |
Stock Options (NEOs other than CEO and CSO) |
Aligns the interests of other NEOs with shareholders; rewards shareholder value creation after the date of grant |
Vest in equal annual increments over four years; 10-year term |
|
Long-Term |
RSAs (NEOs other than CEO and CSO) |
Reinforces long-term focus and rewards high performance |
Vest 50% on the second anniversary of the date of grant and 50% on fourth anniversary of the date of grant |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 59 |
Our Compensation Committee independently oversees the executive compensation program with the support of an independent compensation consultant and management. Our compensation program demonstrates strong governance, minimizing inappropriate risk-taking behavior while protecting shareholder rights and interests. The following is a summary of Regeneron’s key executive compensation best practices and policies.

|
Align pay with performance |
All CEO and CSO direct pay is performance-based and “at-risk” (except for base salary)
94% of CEO direct pay “at-risk” in 2022
(see next page)

|
Align our compensation philosophy and program design with our culture and business strategy |
Long-term oriented
Focused on product pipeline
Employee/shareholder mindset
|
|
|

|
Align management and shareholder interests |
Equity compensation is a key component of our compensation program
Alignment over the long term: stock options vest over four years with a ten-year term; RSAs vest over four or five years; 2020 PSUs (granted to CEO and CSO) include five-year performance and vesting periods and a three-year post-vesting holding period

|
Maintain a strong recoupment (clawback) policy |
Applies to bonus and other incentive compensation
Applies regardless of whether paid or payable in cash, equity, or otherwise and regardless of whether earned or vested
|
|
|

|
Maintain robust stock ownership guidelines |
CEO and CSO: Shares with a value at least
6x base salary
Other NEOs: Shares with a value at least
2x base salary

|
Retain an independent compensation consultant |
Independent compensation consultant provides advice directly to the Compensation Committee on all key compensation decisions, as well as recommendations for compensation plans, budgets, and strategies
|
|
|

|
Annual say-on-pay votes |
Say-on-pay votes now held annually in recognition of shareholder feedback and preference

|
Actively and regularly engage with shareholders on executive compensation matters |
Robust engagement program in the last decade
In 2022, reached out to shareholders collectively representing over 60% of public shares and held one-on-one discussions with shareholders representing approximately 50% of public shares

|
Reprice or exchange stock options |

|
Provide excise tax gross-ups in any new compensation plans or arrangements |
|
|
|

|
Provide excessive perquisites |

|
Allow hedging or pledging of securities |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 60 |
Our executive compensation program is designed to pay for performance, drive the creation of long-term, sustainable shareholder value, deliver compensation that is competitively positioned amongst our peers, encourage a shareholder mindset, and align with the pursuit and achievement of both our short-term and long-term strategic goals. Our pay program must attract and retain talented leaders who can innovate and execute. Managing our business for the long term is core to our culture, as demonstrated by our history of growing through innovation and through a pipeline of internally developed medicines. Further, the compensation program must support the board’s and management’s broader objectives, such as those relating to research and product development; commercialization and access; manufacturing operations; and human capital management, including promotion of a diverse and inclusive workplace.
We strive to achieve these objectives by considering the following key compensation program tenets when designing and determining appropriate compensation structures:
Drive innovation through ownership culture Our objective is to create and reinforce a culture where employees think and act like owners, are empowered to pursue fulfilling careers, and focus on our mission and drug discovery and development. We believe a broad-based equity program that incentivizes long-term performance and promotes employee retention is a key ingredient in achieving this culture of ownership and innovation. |
Prioritize design simplicity and long-term orientation Tying compensation to long-term, Company-wide success and straightforward Company goals has enabled us to encourage decision-making that we believe is consistent with the long-term sustainability of our Company and our reputation. Our objective is to remain nimble, to encourage evolutionary ideas when strategies need to change, and to have the ability to pivot quickly if needed, without being hindered by overly complex compensation structures. |
|
|
|
|
Provide at-risk, performance-based equity to all employees A key part of our pay philosophy since our inception has been to award equity-based pay to all employees, not just senior executives, to ensure that when we deliver for patients and for shareholders, everyone shares in the potential upside growth. In line with this goal, approximately 90% of the employee equity grants in each of the last five years were awarded to our employees other than our NEOs. We believe this approach represents one of our competitive advantages. |
Align with shareholder interests Our objective has always been to ensure close alignment with shareholder interests. All of the direct pay of our CEO and CSO, except for base salary, depends on performance and is “at-risk.” For our CEO, at-risk pay comprised 94% of his direct pay in 2022, which is significantly higher than the percentage of at-risk compensation for CEOs in our Peer Group, as shown in the charts below.5 More broadly, the long-term nature of our equity program is consistent with the drug discovery and development cycle and, therefore, helps drive the creation of long-term shareholder value. Further information about pay-versus-performance alignment is provided in the subsection “Compensation Dashboard—Additional Compensation Information—Pay Versus Performance.” |
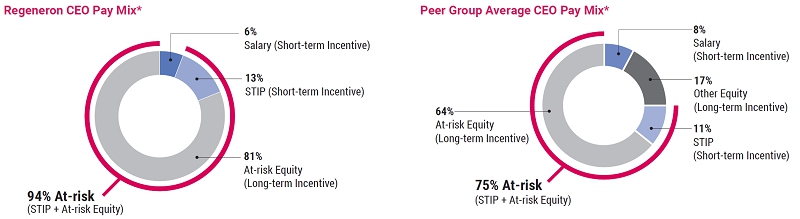
*
“At-risk Equity” consists of stock options and any equity awards with performance-based vesting conditions (such as PSUs). For purposes of this chart, we annualized the five-year, front-loaded PSU award that was granted to our CEO in 2020. “Other Equity” consists of all other equity awards, such as time-based RSAs and RSUs. “At-risk Equity” and “Other Equity” reflect the grant date fair value of such equity awards. “STIP” consists of bonus and/or other applicable compensation provided under short-term, non-equity incentive plans. “At-risk” compensation consists of STIP and At-risk Equity. Total compensation amounts reflect direct compensation (total reported compensation, other than amounts reported as “All other compensation” and (if applicable) amounts reported under “Change in pension value and nonqualified deferred compensation earnings”). |
5 |
We define “direct pay” or “direct compensation” as total compensation as reported in the Summary Compensation Table in the applicable proxy statement, other than the amounts reported as “All other compensation” and (if applicable) amounts reported under “Change in pension value and nonqualified deferred compensation earnings.” Peer Group CEO pay mix is based on the median for each compensation component using data reported for 2021. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 61 |
 |
BASE SALARIES |
|
 |
ANNUAL CASH INCENTIVES |
|
 |
ANNUAL EQUITY AWARDS |
|
 |
PERQUISITES AND PERSONAL BENEFITS |
|
 |
POTENTIAL SEVERANCE BENEFITS |
Objective |
COMPENSATION COMPONENTS |
||||
Base Salaries |
Annual Cash Incentives |
Annual Equity Awards |
Perquisites and Personal Benefits |
Potential Severance Payments |
|
Attract and retain top talent |
● |
● |
● |
● |
● |
Provide stability and manage risk |
● |
|
|
|
● |
Reward annual performance |
|
● |
|
|
|
Balance immediate focus with pursuit of sustainable long-term performance |
|
|
● |
|
● |
Align our employees’ interests with those of shareholders and reward exceptional performance |
|
● |
● |
|
|
Promote a culture of scientific innovation, teamwork, and ethical behavior |
● |
● |
● |
● |
● |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 62 |
The base salary component of NEO pay generally comprises a steadily smaller percentage of overall compensation as executives’ level of responsibility rises.
We consider factors including the executive’s scope of responsibilities, experience, and annual performance when setting base salaries. We also consider base salaries of comparable positions in the region, among our peers, and in the broader biopharmaceutical industry. See the subsections “Compensation Processes—Independent Compensation Consultant” and “Compensation Processes—Peer Data” for further information regarding the role of the Compensation Committee’s independent compensation consultant and our use of Peer Group data for purposes of setting compensation of our NEOs.
Named Executive Officer |
2022 Base Salary ($) |
2023 Base Salary ($) |
2023 vs. 2022 Change (%) |
||
Leonard S. Schleifer, M.D., Ph.D. |
1,811,995 |
|
1,875,415 |
|
3.51 |
George D. Yancopoulos, M.D., Ph.D. |
1,811,995 |
|
1,875,415 |
|
3.51 |
Robert E. Landry |
851,600 |
|
881,406 |
|
3.51 |
Daniel P. Van Plew |
900,000 |
|
931,500 |
|
3.51 |
Andrew J. Murphy, Ph.D. |
750,000 |
|
776,250 |
|
3.51 |
Marion McCourt |
706,300 |
|
731,021 |
|
3.51 |
1
Reflects a 3.5% merit increase consistent with market practice. |
|||||
Our NEOs are eligible for cash incentives based on annual performance. We use these annual incentive opportunities to reward short-term achievements and milestones towards our long-term goals.
We focus on our overall corporate performance to determine the cash incentives of our CEO and our CSO. Our other NEOs’ cash incentives are assessed on both our overall corporate performance and on their individual contributions. As shown in the table below, the annual cash incentive opportunity for each NEO in 2022 was a function of the NEO’s cash incentive target (as a percentage of base salary); the weight and multiplier for the corporate performance component; and, if applicable, the weight and multiplier for the individual performance component. Each NEO’s cash incentive target as a percentage of base salary has remained the same for the last three years.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 63 |
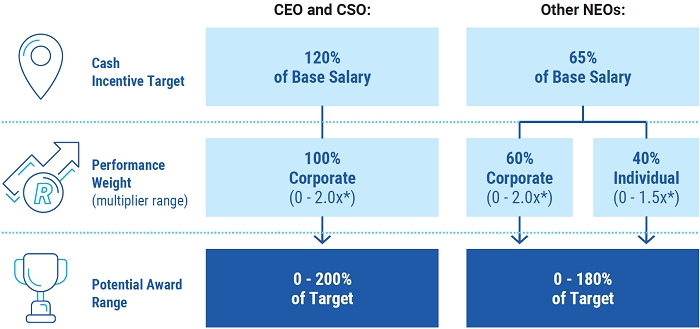
*
Reflects the historical range used by the Compensation Committee. In extraordinary cases, the Committee may exceed this range. The range for the corporate performance multiplier shown above has been exceeded only once in the last decade. |
Using this formula, cash incentives are typically capped at 200% of target for our CEO and CSO and 180% of target for our other NEOs. In addition, cash incentives for each NEO and certain other senior executives are capped at the amounts previously allocated to such executives by the Compensation Committee when setting up the cash incentive pool under the Cash Incentive Bonus Plan toward the beginning of every calendar year. See “Compensation Dashboard—Additional Compensation Information—Annual Cash Incentives.”
The Compensation Committee utilizes an in-depth process to review the Company’s performance every year, culminating in the Committee’s determination of the Company performance multiplier for cash incentives awarded at year-end.

1 Select Milestone Goals Presented at beginning of year, focused on product pipeline and development |
2 Progress Updates Throughout the Year at regularly scheduled meetings of the board of directors |
3 Final Presentation on Performance including a scorecard on select milestone goals and a more holistic assessment across organization |
4 Outcome: Committee Decision on Company performance multiplier for year |
At the regularly scheduled meeting of the board of directors in January of each year, our CEO presents a targeted selection of potential milestones for the upcoming year that, if achieved, would have a substantial impact on the short-, medium-, and/or long-term success of the Company. These milestones are generally focused on our product pipeline and development in recognition of the importance of innovation as a key component of the Company’s business strategy and valuation, as well as the critical role of the development pipeline in the Company’s long-term success. Throughout the year, the board of directors is apprised of key developments related to these milestones. At the end of the year, the Committee receives a final presentation on Company performance for the year, which includes a discussion of whether and to what extent the select milestones have been achieved in the given year as well as a more
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 64 |
detailed overview of achievements and developments across the organization. This presentation is accompanied by management’s assessment of Company performance for the year. The Committee then conducts its own, holistic assessment of the Company’s performance, with a primary focus on the following three categories:
factors related to our product pipeline and development for both the near- and long-term;
factors regarding our financial performance and operations; and
factors related to our talent, culture, and corporate responsibility.
Shown immediately below are the 2022 milestones identified at the beginning of the year and Regeneron’s progress toward achieving them as of the time the Committee made its final assessment of Company performance for purposes of the Company performance multiplier. See “Compensation Dashboard—Additional Compensation Information—Annual Cash Incentives” for a more detailed presentation of the 2022 accomplishments reviewed by the Committee when setting the Company performance multiplier for 2022.
Program |
Milestone |
Status* |
Aflibercept 8 mg |
Report positive results from Phase 3 studies in wet AMD and DME |
Achieved |
Dupixent (dupilumab) |
Complete rolling supplemental Biologics License Application (“sBLA”) submission for eosinophilic esophagitis in adults and adolescents with priority review and approval in late 2022 |
Achieved |
FDA approval of sBLA for children aged 6 months to 5 years with moderate-to-severe atopic dermatitis |
Achieved |
|
Antibodies to SARS-CoV-2 virus |
Agree with FDA on regulatory pathway for an accelerated emergency use authorization (“EUA”) for treatment of Omicron (B.1.1.529) variant with “next generation” antibodies; obtain such EUA assuming significant number of COVID-19 cases still ongoing |
Delayed progress due to speed of Omicron mutations showing resistance to “next-generation” antibodies |
Fully enroll a prevention study with “next-generation” antibody or antibodies active against Omicron variant |
||
Libtayo (cemiplimab) |
FDA approval of sBLA for the treatment of NSCLC in combination with chemotherapy |
Achieved |
Linvoseltamab |
Report positive results from potentially pivotal Phase 2 study in multiple myeloma |
Achieved |
Initiate Phase 1 and Phase 3 studies exploring combinations with standard of care and additional combination studies |
Achieved in Part Phase 1 initiation achieved; |
|
Odronextamab (CD20 and CD3 bispecific antibody) |
Report positive results from potentially pivotal Phase 2 study in B-cell non-Hodgkin lymphoma; submit BLA for approval |
Achieved in Part Positive Phase 2 results; BLA submission delayed |
Initiate OLYMPIA Phase 3 program and additional combination studies |
Phase 3 initiation in process |
|
Solid tumor bispecific antibodies |
Obtain proof of concept with a costimulatory bispecific combination therapy |
Achieved |
Obtain proof of concept for REGN5093 (bispecific antibody targeting two distinct MET epitopes) from Phase 1 study in MET-altered advanced NSCLC |
Achieved |
|
*
As of the time the Committee determined the Company performance multiplier for 2022. |
||
Based on a holistic assessment of these milestones as well as other relevant Company performance factors as discussed in this proxy statement, the Compensation Committee set the Company performance multiplier at 1.9 for 2022. In setting the multiplier at 1.9, the Compensation Committee recognized outstanding Company performance in 2022 while also identifying areas of possible improvement. See the subsection “Compensation Dashboard—Additional Compensation Information—Annual Cash Incentives” for additional information.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 65 |
The personal performance multiplier may range from 0 to 1.5. For the explanation of individual factors considered in the cash incentive decisions, see the subsection “Compensation Dashboard—Additional Compensation Information—Annual Cash Incentives.”
In determining the level of 2022 cash incentives earned, we calculated the NEOs’ respective target cash incentive amounts (which, for our CEO, was set approximately at the median of the Peer Group) and applied the Company performance multiplier based on the Company’s achievement of 2022 objectives; and, for the four NEOs who also have a personal performance component, we applied a personal performance multiplier. For the four NEOs with a personal performance component, the personal performance component had a 40% weighting and the Company performance component had a 60% weighting.
Based on the achievement of corporate goals and, where applicable, individual goals (discussed above and in the subsection “Compensation Dashboard—Additional Compensation Information—Annual Cash Incentives”) in the past year, our NEOs earned the followed cash incentives in 2022:
Named Executive Officer |
2022 Base Salary ($) |
Cash Incentive Target (as percentage of base salary) |
|
Personal Performance Multiplier |
Company Performance Multiplier |
2022 Total Cash Incentive Payout ($) |
||||
Leonard S. Schleifer, M.D., Ph.D. |
1,811,995 |
|
120% |
|
|
n/a |
|
1.9 |
|
4,131,349 |
George D. Yancopoulos, M.D., Ph.D. |
1,811,995 |
|
120% |
|
|
n/a |
|
1.9 |
|
4,131,349 |
Robert E. Landry |
851,600 |
|
65% |
|
|
1.5 |
|
1.9 |
|
963,160 |
Daniel P. Van Plew |
900,000 |
|
65% |
|
|
1.5 |
|
1.9 |
|
1,017,900 |
Andrew J. Murphy, Ph.D. |
750,000 |
|
65% |
|
|
1.5 |
|
1.9 |
|
848,250 |
Marion McCourt |
706,300 |
|
65% |
|
|
1.4 |
|
1.9 |
|
780,462 |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 66 |
Equity grants represent the largest portion of the value awarded annually to our NEOs for compensation purposes. These awards are designed to incentivize delivery of sustainable long-term value, which we believe is created by focusing on the discovery, development, and commercialization of new medicines. Our Compensation Committee utilizes a customized framework for determining the size and mix of the annual equity awards of our NEOs and other executives, which is periodically reassessed in light of our business objectives, feedback from our shareholders, and market practices. This framework and the relevant considerations underlying our equity program and the types of awards we have utilized in recent years are summarized below.

|
Performance-Based Sizing and Value Delivery |
•
In determining the size of equity awards to our NEOs, we take into account: •
Corporate performance (assessment of Regeneron’s corporate accomplishments, particularly as they relate to our product pipeline); and/or •
Individual performance (assessment of performance against the corporate goals established by the board of directors for the CEO and the goals established by the Committee and/or the CEO for the other NEOs, as well as an assessment of the individual’s importance to the Company’s future performance). •
Both stock options and PSUs are performance-based: •
Stock options only deliver value if we deliver stock price appreciation for shareholders after grant. No amount of time will make a stock option deliver any value unless the company’s stock price increases. •
PSUs (which were most recently granted in 2020 but not utilized for any NEOs in 2021 or 2022) only vest if the relevant performance criteria related to TSR are satisfied. They are designed to reward top performance; reward exceptional long-term shareholder value creation; and ensure stability and continuity of leadership to support the achievement of the next phase of Regeneron’s ambitious product, pipeline, and talent development plans. |
|

|
Long-Term Value Creation |
•
Stock option grants have ten-year terms and four-year vesting provisions (generally requiring our employees, including our NEOs, to remain employed for four years in order for all options to vest*) to align with long-term value creation and the development cycle of our products. •
PSUs granted in 2019 and 2020 incorporate a long-term, five-year performance period. In addition, the PSUs granted in 2020 require a three-year deferral and holding period, thus promoting and rewarding value creation over eight years. •
RSAs/RSUs promote long-term employment: •
RSAs/RSUs awarded as a component of annual equity awards vest 50% on the second anniversary of the date of grant and 50% on the fourth anniversary of the date of grant, which is a more backloaded vesting schedule than is typical in the industry. •
Special RSAs awarded to our NEOs in recent years vest 100% on the fifth anniversary of the date of grant. No special RSAs were awarded to our NEOs in 2021 or 2022. |
|
|
Meaningful Holding Requirements |
•
We require NEOs to retain a significant amount of equity within five years of their employment with Regeneron: •
Our CEO and CSO must own shares with a value at least 6x their respective base salaries. •
Our other NEOs must own shares with a value at least 2x their respective base salaries. •
In addition, PSUs awarded in 2020 require a three-year deferral and holding period following vesting, except in certain limited circumstances. |
|
|
Risk-Mitigating Policies and Practices |
•
We have a recoupment (clawback) policy that enables us to reduce or recoup equity and other incentive compensation for compliance violations by NEOs and other covered officers and employees; importantly, the policy covers both financial and non-financial violations.** •
We prohibit our NEOs from hedging or pledging Regeneron securities they hold. •
We periodically evaluate each NEO’s grant history and prior grant size amounts, as well as the amount of outstanding unvested and/or vested but unexercised stock options and other equity awards (if applicable) held, including whether such awards are “in-the-money.” These considerations inform future NEO grant sizes and the mix of equity awards utilized. |
*
In the case of our CEO, this is subject to the terms of his employment agreement. See the subsection “Compensation Dashboard—2022 Executive Compensation Tables—Post-Employment Compensation—Leonard S. Schleifer, M.D., Ph.D. Employment Agreement.” In the case of our CSO, unvested stock options held by him will continue to vest following his qualified retirement (as defined in the applicable Company policy). |
**
See the subsection “Compensation Processes—Risk Assessment” below for further information about the recoupment (clawback) policy. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 67 |
In applying this framework in 2022, the Compensation Committee determined to grant our NEOs (except our CEO and CSO, who did not receive any equity awards in 2022) the target grant date fair value of the equity awards shown in the table below, with 60% of the grant date fair value of each NEO award allocated to stock options and 40% to RSAs. The 2022 annual equity awards to the NEOs were expressed in dollar terms (i.e., based on their grant date fair value). This approach allows for value delivery to be normalized and predictable year-over-year, an important consideration for the top talent we seek to attract and retain, and aligns with industry peers of similar size, maturity, and growth trajectory. It has also resulted in lowering the Company’s burn rate in recent years and helped address feedback relating to the levels of burn rate viewed as acceptable by some of our shareholders. In 2022, our burn rate was 2.7%, the lowest level in over a decade, despite keeping our broad-based equity program intact and increasing the number of our employees by 14% year-over-year.
Named Executive Officer |
Annual Stock Options |
|
Annual RSAs |
Total ($)** |
|||||||
($)** |
(#) |
($)** |
(#) |
||||||||
Leonard S. Schleifer, M.D., Ph.D.* |
— |
|
— |
|
|
— |
|
— |
|
— |
|
George D. Yancopoulos, M.D., Ph.D.* |
— |
|
— |
|
|
— |
|
— |
|
— |
|
Robert E. Landry |
4,200,000 |
|
14,222 |
|
|
2,800,000 |
|
3,853 |
|
7,000,000 |
|
Daniel P. Van Plew |
4,140,000 |
|
14,019 |
|
|
2,760,000 |
|
3,798 |
|
6,900,000 |
|
Andrew J. Murphy, Ph.D. |
4,500,000 |
|
15,238 |
|
|
3,000,000 |
|
4,129 |
|
7,500,000 |
|
Marion McCourt |
3,240,000 |
|
10,971 |
|
|
2,160,000 |
|
2,973 |
|
5,400,000 |
|
*
Drs. Schleifer and Yancopoulos did not receive any equity awards in 2022. See “Update on 2020 PSU Awards” below for more information. **
Represents the target grant date fair value of the awards. See “Compensation Dashboard—2022 Executive Compensation Tables—2022 Summary Compensation Table” for the actual grant date fair value of such awards. |
|
||||||||||
All of the equity awards granted to the NEOs in 2022 are subject to the Company’s policy regarding recoupment or reduction (clawback) of incentive compensation for compliance violations, including after such awards have been earned/vested.
Stock options represented 60% of the grant date fair value of the annual equity awards granted to our NEOs in 2022 (other than our CEO and CSO, who did not receive any equity awards in 2022). The use of stock options for 2022 annual equity awards to these NEOs was based on our long-held view that stock options are a useful compensation tool when used thoughtfully as part of a well-designed compensation program because they are inherently performance-based, requiring stock price appreciation before there is any real value earned, and simple. No amount of time will make a stock option deliver any value unless the company’s stock price increases. In addition, stock options incentivize these NEOs to take actions that increase shareholder value over the entire 10-year option term, which we believe is consistent with the drug discovery and development cycle.
Stock options awarded to our NEOs in 2022 have an exercise price of $726.53 per share, the average of the high and low sales price per share of our common stock as quoted on the Nasdaq Global Select Market on the date of grant. These grants consist of non-qualified stock options and vest ratably over a period of four years. Except as set forth below in the subsection “Compensation Dashboard—2022 Executive Compensation Tables—Post-Employment Compensation,” stock option vesting ceases, and unvested stock options are forfeited, upon termination of employment.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 68 |
We granted time-based RSAs as a component of the annual equity awards for our NEOs in 2022 (other than our CEO and CSO, who did not receive any equity awards in 2022), representing 40% of the grant date fair value of the annual equity award for each of these executives. In continuing to use RSAs/RSUs as a component of the equity award mix for the 2022 annual equity awards to our NEOs and other employees, the Compensation Committee took into account, among other factors, shareholder feedback about the annual rate of equity compensation dilution, retention considerations, and employee input. The RSAs vest 50% on the second anniversary of the date of grant and 50% on the fourth anniversary of the date of grant, which is a more backloaded vesting schedule than is typical in the industry.
As previously disclosed, in 2020 our CEO and CSO received front-loaded PSU awards in lieu of five years’ worth of annual equity awards (2020 through 2024). Consistent with the board’s commitment at the time of these grants, as reaffirmed by the board in response to subsequent shareholder feedback, the Compensation Committee granted no equity awards to our CEO or CSO in 2021 or 2022. In the most recent shareholder engagement cycle, a number of investors expressed an interest in receiving an update on performance to date as it relates to the 2020 PSUs. We have included the disclosure below to address this request.
In designing the 2020 PSU awards, the Compensation Committee and the non-employee members of our board intentionally set challenging goals that would require extraordinary success in our scientific endeavors and business execution. The results to date suggest the awards have been working in the way they were intended. Over the first two years of the five-year performance period (2021 and 2022), our stock delivered a cumulative TSR of 51%, thanks in large part to the business achievements since the date of grant (including those summarized above in the subsection “Compensation Program Overview—2022 Business Highlights”).
Despite significant stock price appreciation since the time of grant (from $478.30 to a new high of $837.55 as of the date of this proxy statement), the design of these awards ensured that only 20% of the maximum number of the 2020 PSUs awarded have been earned to date and thus eligible to vest in 2025 based on the satisfaction of the threshold performance goal corresponding to a stock price of $628. Importantly, no additional PSUs (i.e., the remaining 80% of the maximum number of the 2020 PSUs awarded) may be earned until the third anniversary of the grant date in December 2023. To the extent any earned PSUs subsequently vest at the conclusion of the five-year performance period in December 2025, they will remain subject to an additional three-year deferral and holding period until December 2028. The eight-year life of these awards is of longer duration than is typical in our industry and the broader market and was consciously selected by the Compensation Committee to further our corporate culture and pay philosophy through alignment with the drug discovery and development cycle and long-term shareholder value creation.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 69 |
Similar to our other employees, our NEOs may participate in Company-wide health, disability, life insurance, and other benefit plans, as well as our 401(k) Savings Plan. See details concerning the 401(k) Savings Plan in the subsection “Compensation Dashboard—Additional Compensation Information—Perquisites and Personal Benefits.” Our NEOs are eligible to receive a limited number of additional perquisites. These include financial and tax planning assistance, which are taxable benefits.
In addition, our CEO is entitled to life insurance, long-term disability, medical malpractice insurance premiums, and additional tax and financial planning services pursuant to his employment agreement. These are described in footnote 5 to the Summary Compensation Table.
Our CEO and CSO are also eligible for various benefits under our board-approved security policy, the purpose of which is to ensure increased efficiencies and providing a more secure environment for these executives. Based on the recommendation of an independent, third-party security study, our security policy and related guidelines require our CEO and CSO (as well as their spouses and children when they accompany them) to use, as much as practicable, Company-provided aircraft for all business and personal air travel. The security policy also provides for 24/7 personal security services for each of Drs. Schleifer and Yancopoulos.
Additional information regarding perquisites and other personal benefits provided to our NEOs in, or with respect to, 2022 is given in the applicable footnotes to the Summary Compensation Table and in the subsection “Compensation Dashboard—Additional Compensation Information—Perquisites and Personal Benefits.”
The following points are key to understanding our change-in-control and other severance provisions:
Outstanding equity award agreements (with the exceptions and qualifications described in the subsection “Compensation Dashboard—Additional Compensation Information—Potential Severance Payments”) for all employees other than Dr. Vagelos include a governance best-practice “double trigger” provision for the acceleration of vesting of awards granted thereunder only upon a without-cause termination by the Company within two years of a change in control.
We have a policy against excise tax gross-up provisions for payments contingent on a change in control of Regeneron in contracts, compensatory plans, and other arrangement with the Company’s officers (including NEOs) with the exception of the CEO under his existing employment agreement or amendments to it.
Regeneron has no pension, deferred compensation, or retirement plans for U.S.-based employees other than our 401(k) Savings Plan described above.
For additional details, see the subsection “Compensation Dashboard—Additional Compensation Information—Potential Severance Payments.”
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 70 |
The Compensation Committee is responsible for overseeing the Company’s general compensation objectives and programs. The Compensation Committee evaluates the performance of our NEOs and approves their compensation—in the case of the CEO, subject to approval of the non-employee members of the board of directors. The Compensation Committee operates under a written charter adopted by the board of directors and regularly reviews and reassesses the adequacy of its charter. A copy of the current charter is available on our website at www.regeneron.com under the “Corporate Governance” heading on the “Investors & Media” page.
Annual salaries for the following year and year-end cash incentives and equity awards for all employees are determined in December of each year based on Company and individual performance, as well as other factors, which may include compensation trends among our Peer Group and in the biotechnology industry in general. With respect to our CEO, this process is formalized in the Compensation Committee’s charter, which specifies that the Compensation Committee is to annually present the proposed annual compensation of the CEO to the non-employee members of the board of directors for approval. The non-employee directors and the current Chair of the Board have also been involved in reviewing the Company’s performance for purposes of setting the annual cash incentive.
We make our annual equity awards on a regular, pre-set schedule. The meetings at which such grants are approved are generally scheduled well in advance of the grant date, without regard to the timing of earnings or other major announcements. We generally grant annual equity awards to eligible employees whose performance is determined to merit an annual grant, including the NEOs, at a meeting held during December.
Pursuant to the terms of the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan, stock option awards are granted with an exercise price equal to the average of the high and low sales price per share of our common stock as quoted on the Nasdaq Global Select Market on the date of the grant or, if such date is not a trading day, on the last preceding date on which there was a sale of our common stock on the Nasdaq Global Select Market.
We periodically evaluate the personal benefits and perquisites afforded to our NEOs. The Compensation Committee also regularly meets in executive session to discuss any of the matters that fall within its responsibilities.
Members of our senior management play a role in the overall executive compensation process and assess performance of other officers. They also recommend for the Compensation Committee’s approval the salary, cash incentive, and equity grant budgets for non-officers and make specific recommendations for salary increases, cash incentives, and equity grants for other officers. For our NEOs (other than our CEO), recommendations to the Compensation Committee regarding their compensation are made by our CEO, who also evaluates their performance. Our CEO’s performance is evaluated directly by the Compensation Committee based on the Company’s overall corporate performance against annual goals that are presented to the board of directors at the beginning of each year, as discussed above.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 71 |
Fostering long-term relationships and maintaining trust with our shareholders has been a key priority for us. We seek shareholder feedback through our now-annual say-on-pay votes as well as through discussions with our shareholders in connection with our annual shareholder meetings and in the “off-season,” where we discuss compensation, governance, and other issues of importance and interest to our shareholders. This outreach complements the many touchpoints our investor relations team has with shareholders throughout the year. In addition, on a more informal basis, we engage with our shareholders through industry and corporate governance conferences and informal exchanges in other settings.
Say-on-pay vote. Our shareholders are provided with an opportunity to cast a non-binding, advisory vote on our executive compensation program every year. We switched to the annual cadence of say-on-pay votes last year in recognition of shareholder feedback and preference. Our most recent advisory say-on-pay vote was held at our 2022 annual shareholder meeting, at which this advisory proposal was approved by 87.8% of the votes cast. This was the highest level of say-on-pay support in the last decade (up from 70.2% at the prior meeting at which this vote was on the ballot). Despite the greater approval rate of our compensation program by our shareholders evidenced by this result, we continued to engage with investors in 2022 to make sure we understand their perspectives and views of our compensation program and philosophy.
Shareholder outreach. In the last decade, we have actively and regularly engaged with our shareholders to receive feedback on many important areas including governance, compensation, and corporate responsibility matters and are committed to continued engagement with portfolio managers and investment stewardship teams. Shareholder feedback is discussed with management and, depending on the topic, relayed for consideration to the appropriate committee of the board of directors (typically the Compensation Committee or the Corporate Governance and Compliance Committee), the full board, or both. In recent years, shareholder input resulted in specific changes to our compensation and corporate governance practices and policies. For example, after careful consideration of shareholder feedback following the 2021 annual shareholder meeting, in 2022 our board of directors voluntarily changed the frequency of our say-on-pay votes from every three years to every year. See the table further below for other recent changes informed by shareholder feedback.
Since the 2022 annual shareholder meeting, we reached out to shareholders collectively representing over 60% of the public shares (i.e., shares of common stock outstanding as of December 31, 2022, excluding shares held by our directors and executive officers). This outreach resulted in one-on-one discussions with shareholders representing nearly 50% of our public shares.

| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 72 |
Our 2022 outreach built on an active outreach program in prior years and focused on, among other matters:
the board’s director refreshment efforts, including how the recent election of Dr. Thompson is consistent with the board’s director refreshment philosophy (discussed further in the subsection “Board of Directors—Board Governance–Director Refreshment Philosophy”);
the Company’s classified board structure, including relevant considerations unique to the Company and its long-term orientation (discussed further in the subsection “Board of Directors—Board Governance–Board Structure”);
the Company’s dual-class capital structure, including relevant mitigating factors such as the steady decline in the number of shares of Class A stock outstanding since the Company’s IPO and the relatively low percentage of overall votes controlled by the Class A shareholders, particularly when compared to other founder-led companies (discussed further in the subsection “The Company—Corporate Governance–Capital Structure”); and
investor views of the newly mandated “pay-versus-performance” disclosure (which we provide in the subsection “Compensation Dashboard—Additional Compensation Information—Pay Versus Performance”).
We had meaningful and candid discussions about these and other corporate governance topics in 2022, and the feedback received from shareholders was discussed in detail at the board level and has already shaped the recent meeting agenda and discussion in the boardroom. For example, as described further in the subsection “Board of Directors—Board Governance–Board Structure,” 2022 shareholder feedback led the Corporate Governance and Compliance Committee to request, review, and discuss a detailed analysis of the costs and benefits of the Company’s classified board structure in order to facilitate the Committee’s continued consideration of this feature of Regeneron’s governance.
With respect to executive compensation, feedback provided in 2022 was generally positive, with some variation among our shareholders. A number of shareholders indicated that they understood and supported Regeneron’s compensation program while others had questions about, among other matters, potential use of specific performance metrics in equity awards, potential broader use of performance-based share units, the process for determining the 2021 Company performance multiplier, and performance to date under the 2020 PSUs. We have included additional disclosure in this proxy statement to better communicate our views on key compensation points (including under “Components of Executive Pay: What We Pay and Why We Pay It—Annual Cash Incentives” and “Components of Executive Pay: What We Pay and Why We Pay It—Annual Equity Awards—Update on 2020 PSU Awards”). We will continue to consider shareholder feedback in executive compensation decisions.
Set forth below are certain select changes to executive compensation and governance practices and other actions taken by the board and/or the relevant board committee in the last five years that were informed by shareholder feedback.
Board Refreshment
Elected Dr. Craig B. Thompson, former President and Chief Executive Officer of Memorial Sloan Kettering Cancer Center, to the Board (2022).
Say-on-Pay
Voluntarily adopted an annual say-on-pay vote (2022).
Enhanced Disclosure
Enhanced proxy statement disclosure surrounding board composition and refreshment, board structure and leadership, board oversight of pricing decisions/access to medicine, annual cash incentive determinations, 2020 PSUs, and certain other corporate governance matters (2022-2023).
Pay-for-Performance Alignment
Introduced PSUs as a component of CEO and CSO equity awards (2019).
Granted 100% of CEO and CSO equity awards in the form of PSUs (2020).
Reaffirmed commitment to issue no additional equity awards for CEO and CSO before December 2025 (2021/2022).
Management Stability
Increased holding/vesting requirements for CEO and CSO by incorporating a five-year performance period and a subsequent three-year holding period in most recent equity awards (2020).
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 73 |
Dilution/Burn Rate Concerns
Recalibrated equity award mix (stock options and RSAs/RSUs) for NEOs below the CEO/CSO level and other employees (2019-2021).
Annual Cash Incentives
Enhanced the process by which the Compensation Committee determines the Company performance multiplier for annual cash incentives; provided more detailed disclosure regarding this process (2019-2022).
Compensation of Non-Employee Directors and Chair of the Board
Introduced new compensation program for our non-employee directors and the Chair of the Board, thereby reducing the annual grant date fair value of their equity awards by nearly 50% (2018).
Corporate Responsibility
Increased the breadth and depth of ESG data collection and reporting; aligned annual Responsibility Report with the SASB framework and, since 2021, published a separate annual report on climate-related risks and opportunities aligned with the recommendations developed by the TCFD (2017-2022).
Bolstered DEI initiatives and reporting, including by hiring Chief DEI Officer in 2021, making a commitment to increase diversity in leadership and strengthen a culture of inclusion as part of global ESG goals, and publishing consolidated EEO-1 data (data from annual reports submitted to the U.S. Equal Employment Opportunity Commission) on Regeneron’s website (2018-2022).
The Compensation Committee has the sole authority to retain, at the Company’s expense, one or more third-party compensation consultants to assist the Compensation Committee in performing its responsibilities and to terminate the services of the consultant if the Compensation Committee deems it appropriate. The Compensation Committee (and, as discussed above with respect to non-employee director compensation matters, the Corporate Governance and Compliance Committee) has utilized the services of Pay Governance LLC since September 2021. In order to maintain its independence, the Compensation Committee retained Pay Governance LLC directly, and Pay Governance LLC performed services for the Compensation Committee exclusively at the Compensation Committee’s direction. The Compensation Committee periodically evaluates the independence of its compensation consultant. In accordance with applicable listing standards of the Nasdaq Stock Market LLC and SEC rules, in 2022 the Compensation Committee evaluated the independence of Pay Governance LLC; and, on the basis of this evaluation, concluded that the engagement of Pay Governance LLC did not raise any conflicts of interest.
The Compensation Committee’s consultant reviews management recommendations for compensation plans, budgets, and strategies, and also advises the Compensation Committee on how regulations and trends in executive compensation nationally and specifically in the pharmaceutical and biopharmaceutical industries may be relevant to the Company. It also assists with developing the Peer Group; provides comparative compensation information for our CEO and CSO and other senior executives (using the Peer Group and other compensation data as described below); reviews senior management’s compensation recommendations for other officers, including the other NEOs; and provides general advice to the Compensation Committee on compensation matters, including facilitating the articulation and periodic review of the Company’s compensation philosophy and replenishment of our long-term equity incentive plan.
For purposes of setting our NEOs’ and other senior executives’ compensation, we use comparative compensation information from a relevant peer group of companies (referred to in this proxy statement as the “Peer Group”). In 2022, we selected the companies in the Peer Group with the assistance of Pay Governance LLC based on factors including, but not limited to, the following:
research and development orientation;
market capitalization;
number of employees;
stage of development; and
total revenues.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 74 |
The Peer Group is also meant to provide a representative sample of companies with which we compete for talent. We periodically reassess the composition of the Peer Group and make changes as appropriate, taking into account factors such as changes in the Company’s market capitalization and merger-and-acquisition activity impacting the existing Peer Group companies.
AbbVie Inc. |
BioMarin Pharmaceutical Inc. |
Incyte Corporation |
Alnylam Pharmaceuticals, Inc. |
Bristol-Myers Squibb Company |
Merck & Co., Inc. |
Amgen Inc. |
Eli Lilly and Company |
Seagen Inc. |
Biogen Inc. |
Gilead Sciences, Inc. |
Vertex Pharmaceuticals Incorporated |
|
||
The Compensation Committee reviewed the Peer Group in June 2022 and, based on the recommendation of Pay Governance LLC, did not make any changes with the exception of the removal of Alexion Pharmaceuticals, Inc. due to its acquisition by AstraZeneca PLC. As part of its assessment, the Compensation Committee took into account that Regeneron ranked at the 45th-50th percentile in the Peer Group based on market capitalization, revenues for the last four completed quarters, and the then-available reported number of employees, based on the data provided to the Committee, as shown in the table below.
Company |
Market Capitalization ($ Millions) |
Revenues Last Four Quarters ($ Millions) |
Employees (as of last SEC filing) |
|
90-Day Average (as of 5/15/22) |
30-Day Average (as of 5/15/22) |
|||
AbbVie Inc. |
$265,498 |
$278,791 |
$56,725 |
50,000 |
Eli Lilly and Company |
$243,568 |
$265,419 |
$29,323 |
35,000 |
Merck & Co., Inc. |
$206,200 |
$219,562 |
$53,978 |
67,500 |
Bristol-Myers Squibb Company |
$152,689 |
$162,055 |
$46,960 |
32,200 |
Amgen Inc. |
$129,600 |
$131,270 |
$26,316 |
24,200 |
Gilead Sciences, Inc. |
$78,599 |
$77,354 |
$27,472 |
14,400 |
Vertex Pharmaceuticals Incorporated |
$63,373 |
$68,387 |
$7,948 |
3,900 |
Biogen Inc. |
$31,262 |
$30,460 |
$10,820 |
9,610 |
Seagen Inc. |
$24,829 |
$25,217 |
$1,669 |
2,675 |
Alnylam Pharmaceuticals, Inc. |
$18,197 |
$18,088 |
$880 |
1,665 |
Incyte Corporation |
$16,498 |
$17,254 |
$3,115 |
2,094 |
BioMarin Pharmaceutical Inc. |
$15,198 |
$14,946 |
$1,880 |
3,045 |
Summary Statistics: |
|
|
|
|
75th Percentile |
$166,067 |
$176,432 |
$33,732 |
32,900 |
Median |
$70,986 |
$72,870 |
$18,568 |
12,005 |
25th Percentile |
$23,171 |
$23,435 |
$2,806 |
2,953 |
Regeneron Pharmaceuticals, Inc. |
$69,172 |
$72,922 |
$16,508 |
10,492 |
Percentile Rank |
P49 |
P50 |
P49 |
P47 |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 75 |
In making the compensation decisions in December 2022, we used data from publicly filed proxy statements of the companies in the Peer Group (as compiled by the Compensation Committee’s compensation consultant) to review each component of compensation of our NEOs against their peers in the Peer Group as well as their total annual compensation in relation to the Peer Group, while taking into account various factors such as the executives’ respective performance, past compensation history, experience, and role in the Company’s success. We use Peer Group data as a point of reference for measurement, but Peer Group data do not represent the only factor considered and there is no targeted pay level percentile. The Compensation Committee retains discretion in determining the nature and extent of the use of Peer Group data.
We believe that the Company’s programs balance risk and potential reward in a manner that is appropriate to the Company’s circumstances and in the best interests of the Company’s shareholders over the long term. We also believe that the Company’s compensation and benefits programs do not create risks that are reasonably likely to have a material adverse effect on the Company. We regularly review the Company’s compensation and benefits programs, including its executive compensation program and its incentive-based compensation programs (such as sales incentive plans). At least annually, the Compensation Committee reviews and considers a compensation program risk assessment performed by its independent compensation consultant.
The Company’s compensation and governance-related policies are further enhanced by our stock ownership guidelines applicable to our senior officers and our policy regarding recoupment or reduction (clawback) of incentive compensation of our officers and other specified employees for compliance violations. Our policy regarding recoupment or reduction (clawback) of incentive compensation for compliance violations applies to bonus and other incentive compensation, regardless of whether paid or payable in cash, equity, or otherwise and regardless of whether such compensation has been earned or vested. In addition, the policy covers both financial and non-financial violations resulting in a significant harm to the Company’s business, prospects, results of operations, or financial condition. Under the policy, the board and any designated committee of the board have full discretion to make recoupment and reduction decisions as they may deem appropriate subject to applicable law, the Company’s compensation plans in effect from time to time, and all relevant contractual obligations.
We also have adopted policies against hedging and pledging of our securities by our directors and employees, including the NEOs; and against including excise tax gross-up provisions with respect to payments contingent upon a change in control of Regeneron in contracts, compensatory plans, or other arrangements with the Company’s executive officers, including the NEOs (other than the existing employment agreement with our CEO or any amendments thereto, which we expressly exempted).
These policies demonstrate Regeneron’s continued commitment to robust corporate governance and are meant to reduce compensation-related risks and ensure greater alignment of the interests of our employees, including the NEOs, and those of the Company and our shareholders.
We take tax considerations into account in making our compensation-related assessments and decisions.
Prior to the enactment of the Tax Cuts and Jobs Act in December 2017, Section 162(m) of the Internal Revenue Code generally limited the deductibility for federal income tax purposes of compensation in any year paid to the CEO and the other NEOs (other than the Chief Financial Officer) (the “covered employees”) to the extent such compensation exceeded $1 million, subject to certain exceptions. “Performance-based” compensation, as defined under Section 162(m) of the Internal Revenue Code, was exempt from such deduction limitation if specified requirements set forth in the Internal Revenue Code and applicable Treasury regulations were met. The Regeneron Pharmaceuticals, Inc. Cash Incentive Bonus Plan, adopted prior to the enactment of the Tax Cuts and Jobs Act, allows (but does not require) awards thereunder to be subject to the attainment of performance goals in order to qualify for this performance-based compensation exception.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 76 |
Under the Tax Cuts and Jobs Act, which generally became effective for us commencing with our 2018 fiscal year, the exception under Section 162(m) for performance‐based compensation is no longer generally available, subject to transition relief for certain grandfathered arrangements in effect as of November 2, 2017. Further, the definition of covered employees has been expanded to include our CFO. In addition, once one of our NEOs is considered a covered employee subject to the deduction limitation of Section 162(m), the NEO will remain a covered employee so long as he or she receives compensation from us. Despite the elimination of the performance-based compensation exception, we have continued to use the Cash Incentive Bonus Plan for annual cash incentives of the NEOs because we believe it furthers our compensation philosophy and objectives regardless of tax treatment. The Compensation Committee will continue to review the full impact of Section 162(m) (as revised by the Tax Cuts and Jobs Act) on the Company, our compensation programs, and executive compensation trends generally as it makes compensation-related assessments and decisions in the future.
Due to the requirements set forth in Section 274(e)(2) of the Internal Revenue Code, Company-provided personal and guest air travel (which is provided by the Company only to the extent permitted under board-approved guidelines and a security policy adopted by the board based on an independent, third-party security study) results in a partial disallowance of the related corporate tax deductions. In 2022, this disallowance amounted to approximately $3.2 million.
We take into account the deductibility of compensation in determining NEOs’ compensation. However, we reserve the right to use our judgment to authorize compensation payments that are not deductible, such as when we believe that such payments are necessary to maintain the flexibility needed to attract talent, promote executive retention, reward performance, or attain other Company objectives, or as required to comply with the Company’s contractual commitments.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 77 |
We, the members of the Compensation Committee, have reviewed and discussed with management the Compensation Discussion and Analysis set forth above. Based on that review and discussion, we have recommended to the board of directors that the Compensation Discussion and Analysis be included in this proxy statement.
The Compensation Committee
Christine A. Poon, Chair
George L. Sing
Huda Y. Zoghbi, M.D.
None of the members of the Compensation Committee is currently, or has been at any time since our formation, one of our officers or employees. None of our executive officers serves as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our board of directors or Compensation Committee.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 78 |

The following table and accompanying footnotes provide information regarding compensation earned by, or paid to, our NEOs during the last three fiscal years (other than with respect to Ms. McCourt, who qualified as an NEO for 2022 but not for 2021 or 2020).
A |
B |
|
C |
|
E |
|
F |
|
G |
|
I |
|
J |
Name and principal position |
Year |
|
Salary ($)1 |
|
Stock awards ($)2 |
|
Option awards ($)2 |
Non-Equity Incentive Plan Compensation ($)3 |
All Other Compensation ($)4 |
Total ($) |
|||
Leonard S. Schleifer, M.D., Ph.D. President and Chief Executive Officer |
2022 |
|
1,811,995 |
|
— |
|
— |
|
4,131,349 |
|
1,060,725 |
5 |
7,004,069 |
2021 |
|
1,767,800 |
|
— |
|
— |
|
4,030,584 |
|
672,130 |
|
6,470,514 |
|
2020 |
|
1,480,119 |
|
130,000,032 |
|
— |
|
3,567,714 |
|
302,256 |
|
135,350,121 |
|
George D. Yancopoulos, M.D., Ph.D. |
2022 |
|
1,811,995 |
|
— |
|
— |
|
4,131,349 |
|
650,352 |
6 |
6,593,696 |
2021 |
|
1,767,800 |
|
— |
|
— |
|
4,030,584 |
|
599,980 |
|
6,398,364 |
|
2020 |
|
1,258,096 |
|
130,000,032 |
|
— |
|
3,004,391 |
|
120,800 |
|
134,383,319 |
|
Robert E. Landry Executive Vice President, Finance and Chief Financial Officer |
2022 |
|
851,600 |
|
2,799,320 |
|
4,199,713 |
|
963,160 |
|
24,700 |
7 |
8,838,493 |
2021 |
|
822,800 |
|
2,559,468 |
|
3,839,805 |
|
1,052,182 |
|
24,200 |
|
8,298,455 |
|
2020 |
|
825,577 |
|
949,560 |
|
3,459,499 |
|
938,872 |
|
24,195 |
|
6,197,703 |
|
Daniel P. Van Plew Executive Vice President and General Manager, Industrial Operations and Product Supply |
2022 |
|
900,000 |
|
2,759,361 |
|
4,139,777 |
|
1,017,900 |
|
36,499 |
8 |
8,853,537 |
2021 |
|
822,800 |
|
2,799,882 |
|
4,199,871 |
|
930,587 |
|
20,950 |
|
8,774,090 |
|
2020 |
|
825,577 |
|
3,409,560 |
|
3,459,499 |
|
938,872 |
|
20,945 |
|
8,654,453 |
|
Andrew J. Murphy, Ph.D. Executive Vice President, Research |
2022 |
|
750,000 |
|
2,999,842 |
|
4,499,737 |
|
848,250 |
|
24,700 |
9 |
9,122,529 |
2021 |
|
724,500 |
|
2,231,397 |
|
3,347,323 |
|
819,410 |
|
24,200 |
|
7,146,830 |
|
2020 |
|
726,923 |
|
5,025,288 |
|
4,335,258 |
|
887,250 |
|
24,195 |
|
10,998,914 |
|
Marion McCourt Executive Vice President, Commercial10 |
2022 |
|
706,300 |
|
2,159,974 |
|
3,239,703 |
|
780,462 |
|
13,500 |
11 |
6,899,939 |
1
The reported salary amounts for 2020 give effect to a 27th pay period in 2020 as a result of the Company’s payroll schedule, which was accelerated to avoid a delayed funds disbursement. This resulted in each NEO receiving one additional paycheck in 2020 based on the base salaries then in effect. |
2
The amounts in columns E and F reflect the respective aggregate grant date fair values (disregarding estimated forfeitures) of PSUs or RSAs (as applicable) and stock option awards granted in 2022, 2021, and 2020, respectively, pursuant to the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. Valuation assumptions and methodologies used in the calculation of these amounts for 2022 are included in Note 13 to the Company’s audited financial statements for the fiscal year ended December 31, 2022, included in the 2022 Annual Report. |
3
Non-equity incentive plan compensation amounts (consisting of cash incentives paid to the NEOs in respect of the relevant year under the Regeneron Pharmaceuticals, Inc. Cash Incentive Bonus Plan) are shown in the year in which they were accrued and earned. |
4
See the subsection “Additional Compensation Information—Perquisites and Personal Benefits” below for further information. Certain 2022 perquisites and other personal benefits are quantified for each of the NEOs in the footnotes to this table below based on the actual additional cost incurred by us in providing the perquisite or other personal benefit. |
5
Consists of (i) $20,724 for life insurance premiums, (ii) $7,887 for disability insurance premiums, (iii) $28,158 for medical malpractice insurance premiums, (iv) $13,500 for 401(k) Savings Plan matching contributions in respect of 2022, (v) $11,200 for tax and financial planning advisory services, (vi) $220,551 for personal use of Company-provided aircraft, and (vii) $758,705 for secure car transportation/personal and residential security services (in the case of clauses (vi)-(vii), in accordance with our security policy and calculated as described in the subsection “Additional Compensation Information—Perquisites and Personal Benefits” below). |
6
Consists of (i) $13,500 for 401(k) Savings Plan matching contributions in respect of 2022, (ii) $2,200 for tax and financial planning advisory services, (iii) $250,000 for personal use of Company-provided aircraft, and (iv) $384,652 for secure car transportation/personal and residential security services (in the case of clauses (iii)-(iv), in accordance with our security policy and calculated as described in the subsection “Additional Compensation Information—Perquisites and Personal Benefits” below). |
7
Consists of (i) $13,500 for 401(k) Savings Plan matching contributions in respect of 2022 and (ii) $11,200 for tax and financial planning advisory services. |
8
Consists of (i) $10,250 for 401(k) Savings Plan matching contributions in respect of 2022, (ii) $11,200 for tax and financial planning advisory services, and (iii) $15,049 for temporary home residence security monitoring services (in the case of clause (iii), calculated as described in the subsection “Additional Compensation Information—Perquisites and Personal Benefits” below). |
9
Consists of $13,500 for 401(k) Savings Plan matching contributions in respect of 2022 and (ii) $11,200 for tax and financial planning advisory services. |
10
Ms. McCourt qualified as an NEO for 2022 but not for 2021 or 2020. |
11
Consists of $13,500 for 401(k) Savings Plan matching contributions in respect of 2022. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 79 |
The following table and explanatory footnotes provide information regarding the annual cash incentive and equity awards granted to our NEOs during 2022.
A |
B |
|
C |
D |
E |
I |
J |
K |
|
L |
|
Estimated Possible Payouts Under Non-Equity Incentive Plan Awards1 |
All other stock awards: number of shares of stock or units (#) |
All other option awards: number of securities underlying options (#) |
Exercise or base price of option awards ($/Sh)2 |
Closing price of Company common stock on grant date ($/Sh)2 |
Grant date fair value of stock and option awards ($)3 |
||||
Name |
Grant date |
|
($) |
Target ($) |
Maximum ($) |
|||||
Leonard S. Schleifer, M.D., Ph.D. |
— |
|
— |
2,174,394 |
4,419,243 |
— |
— |
— |
— |
— |
George D. Yancopoulos, M.D., Ph.D. |
— |
|
— |
2,174,394 |
4,419,243 |
— |
— |
— |
— |
— |
Robert E. Landry |
— |
|
— |
553,540 |
1,019,825 |
— |
— |
— |
— |
— |
12/16/2022 |
4 |
— |
— |
— |
— |
14,222 |
726.53 |
723.17 |
4,199,713 |
|
12/16/2022 |
5 |
— |
— |
— |
3,853 |
— |
— |
— |
2,799,320 |
|
Daniel P. Van Plew |
— |
|
— |
585,000 |
1,189,796 |
— |
— |
— |
— |
— |
12/16/2022 |
4 |
— |
— |
— |
— |
14,019 |
726.53 |
723.17 |
4,139,777 |
|
12/16/2022 |
5 |
— |
— |
— |
3,798 |
— |
— |
— |
2,759,361 |
|
Andrew J. Murphy, Ph.D. |
— |
|
— |
487,500 |
1,019,825 |
— |
— |
— |
— |
— |
12/16/2022 |
4 |
— |
— |
— |
— |
15,238 |
726.53 |
723.17 |
4,499,737 |
|
12/16/2022 |
5 |
— |
— |
— |
4,129 |
— |
— |
— |
2,999,842 |
|
Marion McCourt |
— |
|
— |
459,095 |
849,854 |
— |
— |
— |
— |
— |
12/16/2022 |
4 |
— |
— |
— |
— |
10,971 |
726.53 |
723.17 |
3,239,703 |
|
12/16/2022 |
5 |
— |
— |
— |
2,973 |
— |
— |
— |
2,159,974 |
|
1
Cash incentive awards under the Regeneron Pharmaceuticals, Inc. Cash Incentive Bonus Plan. The actual cash incentive awards earned in respect of 2022 and paid out in January 2023 are reported as “Non-Equity Incentive Plan Compensation” in the Summary Compensation Table above. The maximum amount in this column represents the maximum cash incentive allocated to each executive by the Compensation Committee in March 2022 under the Cash Incentive Bonus Plan. 2
These options have an exercise price equal to the average of the high and low sales price per share of the Company’s common stock on the date of grant. Therefore, the closing price of our common stock on the grant date may be higher or lower than the exercise price of these options. 3
The amounts in this column represent the grant date fair value (disregarding estimated forfeitures) of the awards made pursuant to the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. The valuation assumptions and methodologies used in the calculation of these amounts are included in Note 13 to the Company’s audited financial statements for the fiscal year ended December 31, 2022, included in the 2022 Annual Report. 4
The NEO received a non-qualified stock option award that vests subject to continued employment at a rate of 25% per year over the first four years of the maximum ten-year option term. 5
The NEO received an annual RSA that vests 50% on the second anniversary of the date of grant and 50% on the fourth anniversary of the date of grant, subject to the NEO’s continued employment. |
||||||||||
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 80 |
The following table and explanatory footnotes provide information regarding unexercised stock options and PSUs or RSAs (as applicable) held by our NEOs as of December 31, 2022.
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
|||||
|
Option Awards |
Stock Awards |
||||||||||||
Name |
Number of securities underlying unexercised options exercisable (#) |
Number of securities underlying unexercised options unexercisable (#) |
Equity incentive plan awards: number of securities underlying unexercised unearned options (#) |
Option exercise price ($) |
Option expiration date |
Number of shares or units of stock that have not vested (#) |
Market value of shares or units of stock that have not vested ($) |
Equity incentive plan awards: number of unearned shares, units or other rights that have not vested (#) |
Equity incentive plan awards: market or payout value of unearned shares, units or other rights that have not vested ($) |
|||||
Leonard S. Schleifer, M.D., Ph.D. |
60,959 |
20,319 |
1 |
— |
372.46 |
12/11/2029 |
— |
|
— |
|
— |
|
— |
|
129,013 |
— |
|
— |
381.40 |
12/12/2028 |
— |
|
— |
|
— |
|
— |
|
|
139,474 |
— |
|
— |
378.98 |
12/12/2027 |
— |
|
— |
|
— |
|
— |
|
|
146,815 |
— |
|
— |
381.92 |
12/16/2026 |
— |
|
— |
|
— |
|
— |
|
|
172,723 |
— |
|
— |
555.67 |
12/16/2025 |
— |
|
— |
|
— |
|
— |
|
|
203,204 |
— |
|
— |
399.66 |
12/16/2024 |
— |
|
— |
|
— |
|
— |
|
|
— |
— |
|
— |
— |
— |
— |
|
— |
|
248,108 |
2 |
179,007,441 |
4 |
|
— |
— |
|
— |
— |
— |
— |
|
— |
|
25,155 |
3 |
18,149,081 |
4 |
|
TOTAL |
852,188 |
20,319 |
|
|
|
|
|
|
|
|
273,263 |
|
197,156,522 |
|
George D. Yancopoulos, M.D., Ph.D. |
60,959 |
20,319 |
1 |
— |
372.46 |
12/11/2029 |
— |
|
— |
|
— |
|
— |
|
129,013 |
— |
|
— |
381.40 |
12/12/2028 |
— |
|
— |
|
— |
|
— |
|
|
139,474 |
— |
|
— |
378.98 |
12/12/2027 |
— |
|
— |
|
— |
|
— |
|
|
146,815 |
— |
|
— |
381.92 |
12/16/2026 |
— |
|
— |
|
— |
|
— |
|
|
146,815 |
— |
|
— |
555.67 |
12/16/2025 |
— |
|
— |
|
— |
|
— |
|
|
172,723 |
— |
|
— |
399.66 |
12/16/2024 |
— |
|
— |
|
— |
|
— |
|
|
— |
— |
|
— |
— |
— |
— |
|
— |
|
248,108 |
2 |
179,007,441 |
4 |
|
— |
— |
|
— |
— |
— |
— |
|
— |
|
25,155 |
3 |
18,149,081 |
4 |
|
TOTAL |
795,799 |
20,319 |
|
|
|
|
|
|
|
|
273,263 |
|
197,156,522 |
|
Robert E. Landry |
— |
14,222 |
5 |
— |
726.53 |
12/16/2032 |
— |
|
— |
|
— |
|
— |
|
4,240 |
12,718 |
6 |
— |
644.54 |
12/08/2031 |
— |
|
— |
|
— |
|
— |
|
|
9,976 |
9,974 |
7 |
— |
492.00 |
12/09/2030 |
— |
|
— |
|
— |
|
— |
|
|
18,375 |
6,125 |
1 |
— |
372.46 |
12/11/2029 |
— |
|
— |
|
— |
|
— |
|
|
20,000 |
— |
|
— |
381.40 |
12/12/2028 |
— |
|
— |
|
— |
|
— |
|
|
23,337 |
— |
|
— |
378.98 |
12/12/2027 |
— |
|
— |
|
— |
|
— |
|
|
3,800 |
— |
|
— |
381.92 |
12/16/2026 |
— |
|
— |
|
— |
|
— |
|
|
— |
— |
|
— |
— |
— |
3,853 |
8 |
2,779,901 |
4 |
— |
|
— |
|
|
— |
— |
|
— |
— |
— |
3,971 |
9 |
2,865,037 |
4 |
— |
|
— |
|
|
— |
— |
|
— |
— |
— |
965 |
10 |
696,238 |
4 |
— |
|
— |
|
|
— |
— |
|
— |
— |
— |
1,312 |
12 |
946,595 |
4 |
— |
|
— |
|
|
— |
— |
|
— |
— |
— |
5,000 |
13 |
3,607,450 |
4 |
— |
|
— |
|
|
— |
— |
|
— |
— |
— |
12,500 |
14 |
9,018,625 |
4 |
— |
|
— |
|
|
TOTAL |
79,728 |
43,039 |
|
|
|
|
27,601 |
|
19,913,845 |
|
|
|
|
|
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 81 |
A |
B |
C |
|
D |
E |
F |
G |
|
H |
I |
J |
|||
|
Option Awards |
Stock Awards |
||||||||||||
Name |
Number of securities underlying unexercised options exercisable (#) |
Number of securities underlying unexercised options unexercisable (#) |
Equity incentive plan awards: number of securities underlying unexercised unearned options (#) |
Option exercise price ($) |
Option expiration date |
Number of shares or units of stock that have not vested (#) |
Market value of shares or units of stock that have not vested ($) |
Equity incentive plan awards: number of unearned shares, units or other rights that have not vested (#) |
Equity incentive plan awards: market or payout value of unearned shares, units or other rights that have not vested ($) |
|||||
Daniel P. Van Plew |
— |
14,019 |
5 |
— |
726.53 |
12/16/2032 |
|
|
|
|
|
|
|
|
4,637 |
13,911 |
6 |
— |
644.54 |
12/08/2031 |
— |
|
|
— |
|
— |
— |
||
9,976 |
9,974 |
7 |
— |
492.00 |
12/09/2030 |
— |
|
|
— |
|
— |
— |
||
18,375 |
6,125 |
1 |
— |
372.46 |
12/11/2029 |
— |
|
|
— |
|
— |
— |
||
46,250 |
— |
|
— |
381.40 |
12/12/2028 |
— |
|
|
— |
|
— |
— |
||
— |
— |
|
— |
— |
— |
3,798 |
8 |
2,740,219 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
4,344 |
9 |
3,134,153 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
965 |
10 |
696,238 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
5,000 |
11 |
3,607,450 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
1,312 |
12 |
946,595 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
5,000 |
13 |
3,607,450 |
4 |
— |
— |
|||
TOTAL |
79,238 |
44,029 |
|
|
|
|
20,419 |
|
|
14,732,104 |
|
|
|
|
Andrew J. Murphy, Ph.D. |
— |
15,238 |
5 |
— |
726.53 |
12/16/2032 |
— |
|
|
— |
|
— |
— |
|
3,696 |
11,087 |
6 |
— |
644.54 |
12/08/2031 |
— |
|
|
— |
|
— |
— |
||
12,500 |
12,500 |
7 |
— |
492.00 |
12/09/2030 |
— |
|
|
— |
|
— |
— |
||
18,375 |
6,125 |
1 |
— |
372.46 |
12/11/2029 |
— |
|
|
— |
|
— |
— |
||
25,000 |
— |
|
— |
381.40 |
12/12/2028 |
— |
|
|
— |
|
— |
— |
||
50,000 |
— |
|
— |
378.98 |
12/12/2027 |
— |
|
|
— |
|
— |
— |
||
34,000 |
— |
|
— |
381.92 |
12/16/2026 |
— |
|
|
— |
|
— |
— |
||
35,000 |
— |
|
— |
555.67 |
12/16/2025 |
— |
|
|
— |
|
— |
— |
||
40,000 |
— |
|
— |
399.66 |
12/16/2024 |
— |
|
|
— |
|
— |
— |
||
40,000 |
— |
|
— |
270.43 |
12/13/2023 |
— |
|
|
— |
|
— |
— |
||
|
|
|
|
|
|
|
4,129 |
8 |
2,979,032 |
4 |
|
|
||
— |
— |
|
— |
— |
— |
3,462 |
9 |
2,497,798 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
964 |
10 |
695,516 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
8,285 |
11 |
5,977,545 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
1,312 |
12 |
946,595 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
10,000 |
13 |
7,214,900 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
15,000 |
14 |
10,822,350 |
4 |
— |
— |
|||
TOTAL |
258,571 |
44,950 |
|
|
|
|
43,152 |
|
|
31,133,736 |
|
|
|
|
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 82 |
A |
B |
C |
|
D |
E |
F |
G |
|
H |
I |
J |
|||
|
Option Awards |
Stock Awards |
||||||||||||
Name |
Number of securities underlying unexercised options exercisable (#) |
Number of securities underlying unexercised options unexercisable (#) |
Equity incentive plan awards: number of securities underlying unexercised unearned options (#) |
Option exercise price ($) |
Option expiration date |
Number of shares or units of stock that have not vested (#) |
Market value of shares or units of stock that have not vested ($) |
Equity incentive plan awards: number of unearned shares, units or other rights that have not vested (#) |
Equity incentive plan awards: market or payout value of unearned shares, units or other rights that have not vested ($) |
|||||
Marion McCourt |
— |
10,971 |
5 |
— |
726.53 |
12/16/2032 |
— |
|
|
— |
|
— |
— |
|
3,564 |
10,689 |
6 |
— |
644.54 |
12/08/2031 |
— |
|
|
— |
|
— |
— |
||
12,500 |
12,500 |
7 |
— |
492.00 |
12/09/2030 |
— |
|
|
— |
|
— |
— |
||
6,438 |
3,412 |
1 |
— |
372.46 |
12/11/2029 |
— |
|
|
— |
|
— |
— |
||
10,000 |
— |
|
— |
381.40 |
12/12/2028 |
— |
|
|
— |
|
— |
— |
||
— |
— |
|
— |
— |
— |
2,973 |
8 |
2,144,990 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
3,338 |
9 |
2,408,334 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
537 |
10 |
387,440 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
2,000 |
11 |
1,442,980 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
731 |
12 |
527,409 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
10,000 |
14 |
7,214,900 |
4 |
— |
— |
|||
— |
— |
|
— |
— |
— |
2,500 |
15 |
1,803,725 |
4 |
— |
— |
|||
TOTAL |
32,502 |
37,572 |
|
|
|
|
22,079 |
|
|
15,929,778 |
|
|
|
|
1
This stock option award was granted to the NEO on December 11, 2019 and vests at a rate of 25% per year over the first four years of the option term. |
2
This PSU award was granted to the NEO on December 31, 2020 and has a five-year performance period from the date of grant. Based on performance as of December 31, 2021 in accordance with SEC rules, the number of PSUs shown in this table assumes a target level of payout. |
3
This PSU award was granted to the NEO on December 11, 2019 and has a five-year performance period from the date of grant. Based on performance as of December 31, 2021 in accordance with SEC rules, the number of PSUs shown in this table assumes a maximum level of payout. |
4
Reflects the closing price of $721.49 per share of the Company’s common stock on the Nasdaq Global Select Market on December 30, 2022. |
5
This stock option award was granted to the NEO on December 16, 2022 and vests at a rate of 25% per year over the first four years of the option term. |
6
This stock option award was granted to the NEO on December 8, 2021 and vests at a rate of 25% per year over the first four years of the option term. |
7
This stock option award was granted to the NEO on December 9, 2020 and vests at a rate of 25% per year over the first four years of the option term. |
8
This RSA was granted to the NEO on December 16, 2022 and vests 50% on the second anniversary of the date of grant and 50% on the fourth anniversary of the date of grant, subject to the NEO’s continued employment. |
9
This RSA was granted to the NEO on December 8, 2021 and vests 50% on the second anniversary of the date of grant and 50% on the fourth anniversary of the date of grant, subject to the NEO’s continued employment. |
10
This RSA was granted to the NEO on December 9, 2020 and vests 50% on the second anniversary of the date of grant and 50% on the fourth anniversary of the date of grant, subject to the NEO’s continued employment. |
11
This RSA was granted to the NEO on December 9, 2020 and vests 100% on the fifth anniversary of the date of grant, subject to the NEO’s continued employment. |
12
This RSA was granted to the NEO on December 11, 2019. The amount shown in this table consists of the remaining 50% of the RSA that will vest on the fourth anniversary of the date of grant, subject to the NEO’s continued employment. |
13
This RSA was granted to the NEO on December 11, 2019 and vests 100% on the fourth anniversary of the date of grant, subject to the NEO’s continued employment. |
14
This RSA was granted to the NEO on December 12, 2018 and vests 100% on the fifth anniversary of the date of grant, subject to the NEO’s continued employment. |
15
This RSA was granted to the NEO on February 12, 2018 and vests 100% on the fifth anniversary of the date of grant, subject to the NEO’s continued employment. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 83 |
The following table and explanatory footnotes provide information with regard to amounts realized by our NEOs during 2022 as a result of the exercise of stock options or the vesting of RSAs.
|
Option awards |
|
Stock awards |
||
Name (a) |
Number of shares acquired on exercise (#) (b) |
Value realized on exercise ($)1 ( c) |
|
Number of shares acquired on vesting (#) (d) |
Value realized on vesting ($)2 (e) |
Leonard S. Schleifer, M.D., Ph.D. |
— |
— |
|
— |
— |
George D. Yancopoulos, M.D., Ph.D. |
— |
— |
|
— |
— |
Robert E. Landry |
35,265 |
11,097,488 |
|
965 |
726,915 |
Daniel P. Van Plew |
164,000 |
45,282,380 |
|
965 |
726,915 |
Andrew J. Murphy, Ph.D. |
40,000 |
19,388,300 |
|
965 |
726,915 |
Marion McCourt |
13,800 |
4,246,309 |
|
538 |
405,265 |
1
Amounts reflect the difference between the exercise price of the option(s) and, as applicable (depending on the manner of exercise), the average of the high and low sales price per share of the Company’s common stock on the Nasdaq Global Select Market on the exercise date(s) or the market price at which the relevant options were exercised on the exercise date(s). 2
Amount reflects the average of the high and low sales price per share of the Company’s common stock on the Nasdaq Global Select Market on the vesting date. |
|||||
As discussed in “Compensation Dashboard—Additional Compensation Information—Potential Severance Payments,” our NEOs are entitled to certain severance benefits upon the voluntary or involuntary termination of their employment. We provide additional information regarding the severance benefits available to our NEOs in the tables set out below in this subsection. For our CEO, the table shows the amounts payable under his employment agreement upon his involuntary or not-for-cause termination, termination in connection with a corporate change of control, and in the event of his disability or death. For the other NEOs, the table shows their post-termination compensation arrangements under our change in control severance plan upon an involuntary or not-for-cause termination in connection with a corporate change of control.
We entered into an employment agreement with our CEO, Dr. Schleifer, effective as of December 20, 2002, providing for his employment with the Company through December 31, 2003 and continuing thereafter on a year-by-year basis. On November 14, 2008, this employment agreement was amended and restated to bring the employment agreement into compliance with Section 409A of the Internal Revenue Code (“Section 409A”). Pursuant to this agreement, we agreed that in the event that Dr. Schleifer’s employment is terminated by us other than for cause (as defined in the employment agreement) or is terminated by Dr. Schleifer for good reason (as defined in the employment agreement to include specified acts of constructive termination (which Dr. Schleifer has agreed will not include the election of Dr. Yancopoulos as Co-Chair of the Board), together called an “involuntary termination”), we will pay Dr. Schleifer an amount equal to 125% of the sum of his base salary plus his average cash incentive paid over the prior three years. This amount will be paid in a lump-sum severance payment. In addition, we will continue to provide Dr. Schleifer and his dependents medical, dental, and life insurance benefits for 18 months. Subject to the discussion in the following paragraph, in the event that Dr. Schleifer’s employment terminates for any reason other than for cause, (i) all of his unvested stock options will continue to vest in accordance with the terms of the applicable award grant and he will be entitled to exercise the stock options throughout their original term, which is generally ten years from the date of grant, and (ii) all of his unvested PSUs from the 2019 grant will remain outstanding, and vesting and forfeiture shall be determined in the manner set forth in the applicable award agreement, without regard to such termination of employment. The treatment of Dr. Schleifer’s unvested PSUs from the 2020 grant upon certain termination events is
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 84 |
governed by the terms of the 2020 PSU award agreement, notwithstanding any provision to the contrary in his employment agreement. The terms of the 2020 PSU award agreement provide that (i) in the case of a termination of employment due to death or disability, the PSUs remain outstanding and may be earned during their term (and, to the extent earned, are not subject to the three-year mandatory deferral and holding period (the “Holding Period”)); (ii) in the case of a termination by the Company without cause or a departure by Dr. Schleifer for good reason (each as defined in his employment agreement), earnout is measured as of termination and all PSUs earned as of that date vest and remain subject to the Holding Period; and (iii) in the case of a change in control, earnout is measured as of the date of the change in control (using the applicable transaction price as the ending stock price), and all PSUs earned as of that date vest with no Holding Period and any unearned PSUs are forfeited immediately.
Upon an involuntary termination (i.e., a termination by the Company without cause or by Dr. Schleifer for good reason, each as defined in the employment agreement) within three years after a change of control of the Company or within three months prior to such a change of control, we will pay Dr. Schleifer an amount equal to three times the sum of his annual base salary plus his average cash incentive over the prior three years. This amount will be paid in a lump-sum severance payment. In addition, we will continue to provide Dr. Schleifer and his dependents medical, dental, and life insurance benefits for 36 months. Upon such an involuntary termination in connection with a change of control, Dr. Schleifer’s outstanding stock options will vest immediately and remain exercisable throughout their original term, which is generally ten years from the date of grant. In addition, pursuant to the terms of his 2019 PSU award agreement, any such PSUs that vest upon a change of control (as a result of performance exceeding the relevant TSR goal for the period from the grant date to the date of the change of control) will become deliverable to Dr. Schleifer upon the earlier of (x) the five-year anniversary of the grant date or (y) a termination of Dr. Schleifer’s employment by the Company without cause or by Dr. Schleifer for good reason, in each case within two years after such a change of control. Pursuant to the terms of his 2020 PSU award agreement, any such PSUs that vest upon a change of control as discussed above will be immediately deliverable to Dr. Schleifer (with no Holding Period). If aggregate severance payments to Dr. Schleifer in connection with a change of control exceed certain thresholds set forth in the Internal Revenue Code, then we will pay him an additional amount to cover any resulting excise tax obligations, unless the excise taxes could be eliminated by reducing Dr. Schleifer’s cash severance payments and benefits under the agreement by less than 10%, in which case such benefits and payments will be reduced accordingly.
The following table reflects the potential payments to our CEO under his employment agreement assuming a termination effective December 31, 2022 under different scenarios (including following a change of control), as well as upon death or disability. The information in the table below is based on the assumptions set forth in the footnotes to the table; actual values and amounts may differ from those presented below.
|
Cash Severance ($) |
Benefits Continuation ($) |
Death Benefits4 ($) |
Disability Benefits ($) |
Value of Accelerated Stock Options/PSUs ($) |
Cutback/ Gross-up6 ($) |
Total Amount ($) |
|||||
Involuntary Termination Following a Change of Control1 |
16,091,445 |
2 |
299,767 |
3 |
— |
— |
|
167,557,088 |
5 |
— |
$183,948,300 |
|
Involuntary Termination |
6,704,769 |
7 |
144,872 |
8 |
— |
— |
|
150,956,631 |
9 |
— |
$157,806,272 |
|
Death |
— |
|
113,786 |
10 |
— |
— |
|
— |
11 |
— |
$113,786 |
11 |
Disability |
— |
|
144,872 |
8 |
— |
951,297 |
12 |
— |
13 |
— |
$1,096,169 |
|
1
For purposes of these calculations, (i) we used Dr. Schleifer’s 2022 base salary and the annual cash incentives paid to Dr. Schleifer for performance in 2019, 2020, and 2021, respectively; (ii) we assumed that Dr. Schleifer received his annual cash incentive that was earned in 2022 and paid in 2023 (described in the Summary Compensation Table above); (iii) we took into consideration, for purposes of determining whether Dr. Schleifer was entitled to receive a gross-up payment under the terms of his employment agreement, the fact that Dr. Schleifer’s stock options continue to vest according to their original vesting schedule following a voluntary or involuntary termination (other than in connection with a change of control); (iv) we assumed a 6.7% annual increase in medical premiums, 4% annual increase in dental premiums, and no increase in annual disability or life insurance premiums; (v) we assumed that the medical and dental insurance benefits received in 2023, 2024, and 2025 would be taxable and that Dr. Schleifer would be eligible for a tax gross-up for these benefits under the terms of his employment agreement; (vi) although Dr. Schleifer’s employment agreement provides for restrictive covenants, including a six-month non-compete obligation, no specific value has been ascribed to these covenants solely for purposes of assessing excise tax liabilities and potential cutbacks; and (vii) although certain payments to Dr. Schleifer would be subject to potential delays upon separation of service under Section 409A, we did not attempt to determine which, if any, payments would be delayed or revise the values to reflect any such delay. |
||||||||||||
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 85 |
2
Equal to three times the sum of (a) Dr. Schleifer’s 2022 base salary and (b) the average annual cash incentive paid to Dr. Schleifer for performance in the three completed years prior to the termination date. For purposes of this calculation, we used Dr. Schleifer’s annual cash incentives for performance in 2019, 2020, and 2021. |
3
Equal to the estimated cost of providing Dr. Schleifer and his dependents medical, dental, and life insurance benefits for 36 months. |
4
We maintain $1 million of term life insurance covering Dr. Schleifer payable to his designated beneficiary. |
5
Equal to the sum of (a) the aggregate amount of the differences between the exercise prices of Dr. Schleifer’s accelerated stock options and the closing sales price per share of the Company’s common stock on the Nasdaq Global Select Market on December 30, 2022, the last business day of 2022, of $721.49 and (b) the value of 25,155 PSUs from his 2019 grant and 197,253 PSUs from his 2020 grant that would have been earned had the applicable vesting determination been made on December 31, 2022 in respect of performance for the period from the date of grant to December 31, 2022. In the case of the 2020 PSUs, this calculation assumes a change of control transaction price equal to the closing sales price per share of the Company’s common stock on the Nasdaq Global Select Market on December 30, 2022. |
6
Under Dr. Schleifer’s employment agreement, if payments due in connection with a change of control are subject to excise taxes under Section 280G of the Internal Revenue Code, we will cut back the payments if the excise tax can be eliminated by reducing his cash severance payments and benefits by less than 10%. Otherwise, we will pay him an additional “gross up” amount so that his after-tax benefits are the same as though no excise tax had been applied. We have determined that Dr. Schleifer would not have been subject to excise taxes if he had been terminated on December 31, 2022 as a result of a change of control. |
7
Equal to 1.25 times the sum of (a) Dr. Schleifer’s 2022 base salary and (b) the average annual cash incentive paid to Dr. Schleifer for performance in the three completed years prior to the termination date. For purposes of this calculation, we used Dr. Schleifer’s year-end cash incentive awards for performance in 2019, 2020, and 2021. |
8
Equal to the estimated cost of providing Dr. Schleifer and his dependents medical, dental, and life insurance benefits for 18 months. |
9
Equal to the value of 209,229 PSUs from Dr. Schleifer’s 2020 grant that would have been earned had a vesting determination been made on December 31, 2022 in respect of performance for the period from the date of grant to December 31, 2022 (and disregarding that PSUs not earned as of such termination date would have 12 additional months to be earned under the terms of the applicable award agreement). In addition, under such a scenario (i) all of Dr. Schleifer’s unvested stock options would continue to vest in accordance with the terms of the applicable award grant and he would be entitled to exercise all of his stock options throughout the remainder of their original term, and (ii) all of his unvested PSUs from the 2019 grant would remain outstanding (with vesting and forfeiture determined in the manner set forth in the applicable award agreement). |
10
Equal to the estimated cost of providing Dr. Schleifer’s dependents medical and dental benefits for 18 months. |
11
As discussed in “Compensation Dashboard—Additional Compensation Information—Potential Severance Payments,” unvested stock options held by any employee (including Dr. Schleifer) become immediately exercisable upon his or her death. The value of such accelerated stock options would have been $7,091,941. In addition, any PSUs held by Dr. Schleifer will remain outstanding upon his death, and any vesting or forfeiture of such PSUs will be determined following the completion of the applicable performance period as it otherwise would have been determined if he remained employed by the Company for the duration of such performance period. |
12
Represents 35% of Dr. Schleifer’s 2022 salary over a period of 18 months. We have assumed long-term disability coverage exists pursuant to Dr. Schleifer’s employment agreement for the remaining 65% of Dr. Schleifer’s salary. |
13
In the case of a termination of Dr. Schleifer’s employment due to disability, (i) all of his unvested stock options would continue to vest in accordance with the terms of the applicable award grant and he would be entitled to exercise the stock options throughout their original term, and (ii) any PSUs held by Dr. Schleifer would remain outstanding (with vesting and forfeiture determined in the manner set forth in the applicable award agreement). |
Each of the NEOs, other than our CEO, participates in our change in control severance plan that was adopted by the board of directors on January 20, 2006. The purposes of the plan are (i) to help us retain key employees, (ii) to help maintain the focus of such employees on our business and to mitigate the distractions caused by the possibility that we may be the target of an acquisition, and (iii) to provide certain benefits to such employees in the event their employment is terminated (or constructively terminated) after, or in contemplation of, a change in control. On November 14, 2008, the change in control severance plan was amended and restated to bring it into compliance with Section 409A.
Under the plan, each participant is entitled to receive a cash severance payment in an amount equal to one, or, in designated cases, including with respect to the NEOs other than Dr. Schleifer, two times the sum of the participant’s annual base salary and his or her average annual cash incentive over the prior three years if, within two years after or 180 days before a change in control, either the participant resigns his or her employment for Good Reason (as defined in the plan) or the participant’s employment is terminated by the Company for any reason other than Cause (as defined in the plan). This amount will be paid in a lump-sum severance payment. A participant so terminated is also entitled to receive a pro-rata annual cash incentive for the year in which he or she is terminated based on the portion of the year the participant was employed by us. In addition, for either one or two years, as the case may be, plan participants will receive continuation of health care coverage and welfare benefits provided by us, to the extent permitted by our benefit plans, at a cost no greater than what the participant’s cost would have been if he or she had continued his or her employment with the Company.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 86 |
In the event that a plan participant resigns his or her employment for Good Reason (which generally conforms to the definition in Section 409A), or the participant’s employment is terminated by the Company for any reason other than Cause, in either case within two years after or 180 days before a change in control, then, unless otherwise provided in an award agreement, the participant’s stock options and other equity awards granted under our long-term incentive plans that would have vested prior to or upon the change in control will become vested on the change in control date, and the exercise period of such equity awards, and other equity awards held by the participant that otherwise would have expired, will be extended to the later of (i) 30 days following the first date after a change in control in which the shares underlying the equity award may be traded, and (ii) the permitted exercise date in the plan or grant assuming the change in control happened immediately prior to the participant’s termination. However, in no event will any stock option or other equity award be extended (i) beyond the expiration date of the grant, or (ii) such that the grant will be subject to the additional tax under Section 409A of the Internal Revenue Code.
In the event that a participant would become subject to a “golden parachute” excise tax under Section 4999 of the Internal Revenue Code as a result of severance benefits and payments, the severance benefits and payments owed to the participant shall be reduced to an amount one dollar less than the amount that would subject the participant to the excise tax, unless the total severance benefits/payments net of the excise taxes are greater than the amount that the participant would receive following any such reduction.
The following table shows the potential payments to our NEOs (other than our CEO), upon their hypothetical termination (other than for Cause) or resignation for Good Reason, in the two years following, or the 180 days prior to, a change in control. The information in the table below assumes an effective termination or resignation date of December 31, 2022 and is further based on the assumptions set forth in the footnotes to the table; actual values and amounts may differ from those presented below.
|
Cash Severance1 ($) |
Benefits Continuation2 ($) |
Value of Accelerated Stock Options/RSAs/PSUs3 ($) |
Cutback4 ($) |
Total Amount5 ($) |
George D. Yancopoulos, M.D., Ph.D. |
10,046,313 |
91,417 |
167,557,088 |
— |
177,694,818 |
Robert E. Landry |
3,571,499 |
157,431 |
25,319,238 |
— |
29,048,168 |
Daniel P. Van Plew |
3,552,483 |
151,638 |
20,229,298 |
— |
23,933,419 |
Andrew J. Murphy, Ph.D. |
3,082,373 |
104,698 |
36,993,315 |
— |
40,180,386 |
Marion McCourt |
2,801,941 |
57,325 |
20,811,812 |
— |
23,671,078 |
1
Equal to two times the sum of (a) the NEO’s 2022 base salary and (b) the average annual cash incentives paid to the NEO over the prior three years. 2
Equal to the estimated cost of providing each NEO and his dependents medical, dental, vision, disability, and life insurance coverage for 24 months, plus the estimated cost of providing each NEO tax and financial planning advisory services for 24 months. 3
For stock options, equal to the aggregate amount of the differences between the exercise prices of each NEO’s accelerated “in-the-money” stock options and the closing sales price per share of the Company’s common stock on the Nasdaq Global Select Market on December 30, 2022, of $721.49. In the case of Messrs. Landry and Van Plew, Dr. Murphy, and Ms. McCourt, the amounts also include the value as of December 30, 2022 of accelerated RSAs. In the case of Dr. Yancopoulos, the amount includes the value of 25,155 PSUs from his 2019 grant and 197,253 PSUs from his 2020 grant that would have been earned had the applicable vesting determination been made on December 31, 2022 in respect of performance for the period from the date of grant to December 31, 2022. In the case of the 2020 PSUs, this calculation assumes a change of control transaction price equal to the closing sales price per share of the Company’s common stock on the Nasdaq Global Select Market on December 30, 2022. 4
We have determined (using the assumptions outlined in footnote 5) that none of the NEOs listed in the table above would have been subject to any cutbacks or excise taxes if terminated on December 31, 2022. 5
For purposes of these calculations, (i) we used base salaries as of December 31, 2022, and annual cash incentives paid to the NEOs for performance in 2019, 2020, and 2021, respectively; (ii) we assumed that each NEO received his annual cash incentive that was earned in 2022 and paid in 2023 (described in the Summary Compensation Table above); (iii) we took into consideration, for purposes of determining whether each NEO was subject to a reduction under the terms of the change in control severance plan, the fact that each NEO’s equity awards may vest in full or in part following a change in control (parachute payments for time vesting stock options and restricted stock were valued using Internal Revenue Code Treas. Reg. Section 1.28G-1 Q&A 24(c)); (iv) we assumed a 6.7% annual increase in medical premiums, 4% annual increase in dental and vision premiums, and no increase in disability or life insurance premiums or employer cost of tax and financial planning advisory services for 2023 and 2024; (v) we assumed that the medical insurance benefits received in 2023 and 2024 would be taxable and that the NEOs would be eligible for a tax gross-up for these benefits under the terms of the change in control severance plan; (vi) although the change in control severance plan provides for restrictive covenants, including a one-year covenant prohibiting the solicitation of Company employees, no specific value has been ascribed to these covenants for purposes of assessing excise tax liabilities and potential cutbacks; and (vii) although certain payments to the NEOs would be subject to potential delays upon separation of service under Section 409A, we did not attempt to determine which, if any, payments would be delayed or revise the values to reflect any such delay. |
|||||
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 87 |
In 2016, we adopted our Cash Incentive Bonus Plan for purposes of allowing our annual cash incentives to qualify as performance-based compensation under Section 162(m) of the Internal Revenue Code and permitting us to deduct cash incentive compensation that might otherwise not be deductible by reason of Section 162(m) (as then in effect). Although the Tax Cuts and Jobs Act eliminated the performance-based compensation exception for compensation paid in 2018 and beyond (as discussed under “Compensation Processes—Tax Implications” above), we have continued to use the Cash Incentive Bonus Plan for annual cash incentives because we believe it furthers our compensation philosophy and objectives regardless of tax treatment. For 2022 annual cash incentives for the NEOs, in March 2022 the Compensation Committee set up a cash incentive pool under the Cash Incentive Bonus Plan; specified maximum allocations of such pool to the NEOs and certain other senior executives; and established a R&D-related performance goal consisting of (i) the submission of one or more Investigational New Drug Applications, Biologics License Applications, or supplemental Biologics License Applications with the FDA (or its equivalent outside the United States) or (ii) the approval of any regulatory filing of the type described in clause (i) by the FDA or the applicable regulatory authority outside the United States. In November 2022, the Compensation Committee determined that Regeneron’s performance in 2022 exceeded the established goal, thus enabling the funding of the cash incentive pool. The Compensation Committee then exercised “negative discretion” (as permitted under the Plan) to reduce the respective allocations of such pool for each NEO. In exercising negative discretion for the NEOs, the Compensation Committee determined that their annual cash incentives should be set consistent with the Company’s historical practice of using a formula that utilizes their respective cash incentive targets, a corporate performance component, and, if applicable, an individual performance component, as described below.
The targets for the 2022 annual cash incentives for the NEOs were set as percentages of their respective base salaries as follows: Dr. Schleifer—120%; Dr. Yancopoulos—120%; Mr. Landry—65%; Mr. Van Plew—65%; Dr. Murphy—65%; and Ms. McCourt—65%.
For 2022, Dr. Schleifer’s cash incentive target was set approximately at the median of the Peer Group. In determining the cash incentive target for Dr. Yancopoulos, the Compensation Committee took into consideration the importance of his scientific leadership as President & CSO and the significant contributions he has made to the success of the Company and, specifically, to the discovery and development of the Company’s commercial products, its pipeline of internally developed product candidates, and its platform technologies. The Compensation Committee determined that there were no meaningful comparative data for Dr. Yancopoulos relating to similarly situated executives and that his cash incentive target for 2022 would be set to equal Dr. Schleifer’s. In determining the cash incentive targets for 2022 for Messrs. Landry and Van Plew, Dr. Murphy, and Ms. McCourt, the Compensation Committee took into consideration the compensation of similarly situated executive officers at companies in the Peer Group.
The cash incentives were determined through the use of both an individual and a Company performance component with a range of 0 to 1.5 for the personal performance multiplier and a range of 0 to 2.0 for the Company performance multiplier, depending upon performance during the year. Both the personal performance multiplier and the Company performance multiplier were determined by the Compensation Committee for each NEO based on the Committee’s assessment of the Company’s performance relative to the general corporate goals described below and, in the case of each of Messrs. Landry and Van Plew, Dr. Murphy, and Ms. McCourt, the NEO’s personal performance during the year.
With respect to 2022, the Compensation Committee approved a personal performance multiplier of 1.5 for each of Messrs. Landry and Van Plew and Dr. Murphy and a personal performance multiplier of 1.4 for Ms. McCourt. The personal performance component accounted for 40% of these NEOs’ cash incentives. The Company component was based on a Company performance multiplier that was determined based on the Company’s overall corporate performance (as described below) in 2022, including against 2022 milestone goals that were presented to the board of directors in January 2022. This Company performance component accounted for 60% of the cash incentives awarded to Messrs. Landry and Van Plew, Dr. Murphy, and Ms. McCourt. In the case of Drs. Schleifer and Yancopoulos, the
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 88 |
Compensation Committee focused exclusively on overall Company performance in 2022 (as described above) when determining their cash incentives and did not utilize a personal performance multiplier.
In determining the annual cash incentive for Mr. Landry, the Compensation Committee gave special consideration to Mr. Landry’s leadership of and accomplishments in the Company’s accounting, finance, and tax functions and across his other responsibilities, including the successful execution of the Company’s capital allocation priorities in 2022 through investment in R&D capabilities, pursuit and funding of business development opportunities, and the return of cash to our shareholders through share buybacks. In the case of Mr. Van Plew, the Compensation Committee focused primarily on Mr. Van Plew’s leadership of and accomplishments in the Company’s Industrial Operations and Product Supply organization, including efforts to rebuild bulk and finished goods inventory (following the drawdown of safety stock levels for certain of our products resulting from resource reallocation to the production of our COVID-19 monoclonal antibodies); preparations for new product launches and expanded manufacturing capacity; and continued progress toward completing fill/finish facilities. In the case of Dr. Murphy, the Compensation Committee considered the progress and continued expansion of the Company’s research and preclinical development pipeline. In the case of Ms. McCourt, the Compensation Committee considered her leadership of and performance across the portfolio of the Company’s commercialized products, including continued growth in net product sales and market share of EYLEA, Dupixent, and Libtayo (including record net product sales of one or more of these products in the applicable quarterly periods of 2022); the successful launch of three new indications for Dupixent in the United States; and the acquisition of global rights to Libtayo.
With respect to 2022, the Compensation Committee set the Company performance multiplier at 1.9. For a discussion of the process by which the Compensation Committee determined the Company performance multiplier in 2022, see the subsection “Compensation Discussion and Analysis—Components of Executive Pay: What We Pay and Why We Pay It—Annual Cash Incentives.” As part of its holistic assessment of Company performance in 2022, the Committee considered the achievements and relevant developments described below in the following three categories: (1) our product pipeline and development for both the near- and long-term; (2) our financial performance and operations; and (3) our talent, culture, and corporate responsibility. The factors analyzed under category (1) continued to be a key measure for the Compensation Committee’s assessment and calculating the Company performance multiplier. This recognizes the importance of innovation as a key component of the Company’s business strategy and valuation, as well as the critical role of the development pipeline in the Company’s long-term success.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 89 |
(1) PRODUCT PIPELINE AND DEVELOPMENT (PRIMARY FACTORS) |
||
Regulatory & Clinical Milestones; Commercial Support •
Approval of new products or indications by the FDA or applicable regulatory authorities outside the United States •
Regulatory submissions for new products and new indications •
Breakthrough Therapy or orphan drug designations by the FDA (or its equivalent outside the United States) •
Data readouts and key publications from potentially pivotal/registrational studies •
Initiation of new Phase 3 or Phase 2 studies |
||
Achievements/Relevant Developments in 2022:* |
||
EYLEA •
Approved by Ministry of Health, Labour and Welfare (“MHLW”) for retinopathy or prematurity (“ROP”) in Japan •
Committee for Medicinal Products for Human Use (“CHMP”) of the European Medicines Agency (“EMA”) recommended approval of EYLEA for ROP •
Pediatric exclusivity granted by FDA •
Filed sBLA for every-16-weeks dosing regimen in patients with diabetic retinopathy •
Filed sBLA with FDA for ROP Aflibercept 8mg •
Reported that Phase 3 trials in diabetic macular edema (“DME”) and wet age-related macular degeneration (“wet AMD”) met their primary endpoints |
Dupixent •
Approved by FDA for atopic dermatitis in pediatrics (6 months–5 years of age) •
Approved by FDA for eosinophilic esophagitis (“EoE”) in adults and adolescents •
Approved by FDA for prurigo nodularis (“PN”) •
Approved by EC for severe asthma in pediatrics (6–11 years of age) •
Filed regulatory applications in the EU and Japan for atopic dermatitis in pediatric patients •
Filed regulatory applications in the EU for EoE and PN •
Filed regulatory application in Japan for PN |
Libtayo •
Approved by FDA in combination with chemotherapy for NSCLC •
Approved by EC for cervical cancer •
Withdrew sBLA for cervical cancer •
Filed regulatory application in Japan for cervical cancer Evkeeza •
Filed sBLA for HoFH in pediatrics Kevzara •
Filed sBLA for polymyalgia rheumatica |
Progress in Earlier-Stage Clinical Programs; New Candidates Advanced into Clinical Development •
Data readouts and key publications from existing Phase 1 studies •
Initiation of new Phase 1 studies •
Notable early research milestones and collaborations |
||
Achievements/Relevant Developments in 2022: |
||
Data Readouts •
Announced positive interim data from an ongoing Phase 1 clinical study of NTLA-2001 for transthyretin (ATTR) amyloidosis (in collaboration with Intellia Therapeutics, Inc.) •
Presented positive data from Phase 1 trials of fianlimab (in combination with Libtayo) in advanced melanoma and NSCLC •
Announced promising data from ongoing Phase 1 study of ALN-HSD, an investigational RNAi therapeutic targeting HSD17B13 in development for the treatment of nonalcoholic steatohepatitis (“NASH”) |
Advancement of New Product Candidates •
Submitted several new Investigational New Drug Applications with the FDA or their equivalent outside the United States |
Early Research Milestones and Publications •
The Regeneron Genetics Center® announced the discovery of rare genetic loss-of-function mutations in the CIDEB gene that are associated with substantial protection from liver disease, including serious diseases such as NASH and cirrhosis |
*
Descriptions in this table are based on information available to the Committee when setting the Company performance multiplier for 2022. |
||
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 90 |
(2) FINANCE AND OPERATIONS (SECONDARY FACTORS) |
|
Financial Metrics; Capital Structure •
Growth in total revenues •
Growth in net product sales for key marketed products •
Growth in profitability metrics •
Collaboration agreements •
Finance projects |
Operational & Manufacturing •
Marketing structure & strategy •
Pricing, policy & legal developments •
Successful completion of audits •
Expansion of facilities •
Increase in manufacturing capabilities |
Achievements/Relevant Developments in 2022: |
||
•
Total revenue for the first nine months of 2022 was $8.76 billion •
Full-year non-GAAP net income and diluted non-GAAP EPS forecast each projected to exceed the 2022 board-approved plan •
EYLEA global net product sales of $7.31 billion in the first nine months of 2022 (representing 7% growth vs. the first nine months of 2021) •
Dupixent global net product sales of $6.23 billion in the first nine months of 2022 (representing 41% growth vs. the first nine months of 2021) •
Libtayo global net product sales of $409.2 million in the first nine months of 2022 (representing 21% growth vs. the first nine months of 2021) •
Kevzara global net product sales of $276.8 million in the first nine months of 2022 (representing 18% growth vs. the first nine months of 2021) |
•
Executed over $2.8 billion in share repurchases and business development initiatives in the first nine months of 2022 •
Completed acquisition of Sanofi’s stake in Libtayo, providing Regeneron with exclusive worldwide development, commercialization, and manufacturing rights to the medicine •
Completed acquisition Checkmate Pharmaceuticals, Inc., the Company’s first acquisition of a public company •
Achieved favorable rulings with respect to, among other matters, inter partes review proceedings before the U.S. Patent and Trademark Office and opposition proceedings before the European Patent Office |
•
Established commercial capabilities in certain jurisdictions outside the United States |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 91 |
(3) TALENT, CULTURE, AND CORPORATE RESPONSIBILITY (SECONDARY FACTORS) |
||
Talent Management & Retention •
Growth of global workforce to support our long-term strategic objectives •
Employee retention and below-industry attrition rate •
Outside recognition and employee feedback |
||
Achievements/Relevant Developments in 2022: |
||
•
Global company growth continued, with headcount approach 12,000 employees, up 14% year-over-year •
Turnover rate well below the industry median •
Hired key positions for global expansion efforts •
Obtained favorable results from employee engagement survey, which exceeded certain pharmaceutical industry benchmarks |
•
Earned the following accolades and rankings from independent organizations: •
#3 ranking in Science Magazine’s ‘Top Biopharma Companies to Work For’ •
Fortune magazine’s list of ‘Best Workplaces in Biotechnology and Pharmaceuticals’ •
Great Places to Work: ‘Best Workplaces in New York’ •
Great Places to Work: ‘Best Workplaces in Ireland’ •
Forbes: America’s Best-in-State Employers, New York •
IDEA Pharma: Pharmaceutical Invention Index |
|
Corporate Responsibility •
ESG activities, reporting, ratings, and rankings •
Corporate giving •
Philanthropic and citizenship programs |
||
Achievements/Relevant Developments in 2022: |
||
•
Engaged public health agencies, government and non-governmental agencies, and others to facilitate continued access to Regeneron’s Ebola and COVID-19 treatments in low- and lower-middle-income countries •
Developed an enterprise-wide climate action plan toward meeting Regeneron’s 2025 energy and emissions targets •
Provided engaging employee volunteerism opportunities, with more than 50% of the global workforce registering to participate in Regeneron’s annual company-wide service event, Day for Doing Good |
•
Earned the following accolades and rankings from independent organizations: •
Civic 50: Most Community-Minded Companies in the Nation •
Prix Galien: Best Biotechnology Product of 2022 for Inmazeb •
Newsweek: America’s Most Responsible Companies •
Fast Company World Changing Ideas: Honorable Mention •
Scrip Awards: Community Partnership of the Year (together with Society for Science) •
FTSE4Good Index constituent •
Favorable rankings from certain ESG ratings firms |
|
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 92 |
All employees who participate in our 401(k) Savings Plan, including the NEOs, are eligible to receive certain matching contributions. In each plan year, we contribute to each participant’s account a matching contribution (in the form of shares of our common stock) equal to 50% of a specified percentage of the participant’s compensation that the participant has contributed to the plan (which was 10% with respect to each of the last three years), up to a maximum level established under the Internal Revenue Code. Each of our NEOs participated in our 401(k) Savings Plan during 2022 and received matching contributions in the aggregate amount of $13,500 ($10,250 in the case of Mr. Van Plew) in the form of shares of our common stock. The contributions were paid quarterly up and until the maximum level was reached and are included in the compensation amounts reported for each of our NEOs in the Summary Compensation Table included in this proxy statement. As with all employees, the number of shares of common stock that each NEO received on a quarterly basis was determined using the average market price per share of our common stock during the applicable quarter.
To achieve increased efficiencies and a more secure traveling environment, the Company provides air transportation for certain executive and director travel in accordance with guidelines approved by our board of directors. Based on the recommendation of an independent, third-party security study, the guidelines and our security policy require Drs. Schleifer and Yancopoulos (as well as their spouses and children when they accompany them) to use, as much as practicable, Company-provided aircraft for all business and personal air travel. Regeneron covers the cost of any such personal air travel for up to $250,000 in incremental cost (as described below) annually for each of Drs. Schleifer and Yancopoulos. Family members or other guests may accompany our NEOs and directors during Company-provided air business travel, space permitting, so long as they cover any incremental cost related to such guests (other than with respect to the family members of Drs. Schleifer and Yancopoulos as described above). In addition, in limited circumstances personal use of Company-provided air travel by our other NEOs or directors may be permitted if authorized by the Chair and any incremental cost is paid by the lead passenger. Any required reimbursement or other payment of the incremental cost is made to the extent permitted by applicable Federal Aviation Administration rules.
We determine the incremental cost of any Company-provided personal or guest air travel based on the direct variable operating cost. Items included in the calculation include (as applicable) fuel costs; landing, non-home-base hangar or aircraft parking, and ground handling fees; in-flight catering; travel, lodging, and other expenses for flight crew; and other trip-related variable cost, including the use of our fractional jet interests. Because Company-provided air transportation is used primarily for business travel, incremental costs exclude fixed costs that generally do not change based on usage, such as (as applicable) flight crew salaries; aircraft purchase or lease costs; depreciation; insurance costs; certain maintenance fees based on minimum usage; and home-base hangar costs. When the aircraft is already flying to a destination for business purposes, only the direct variable costs associated with the guest (for example, catering), if any, are included in determining the aggregate incremental cost to Regeneron. If any aircraft flies empty before picking up or after dropping off a passenger for personal reasons, this “deadhead” segment would be included in the aggregate incremental cost based on the methodology described above. The amount of disallowed corporate tax deductions attributable to Company-provided personal and guest air travel is not included in the NEO incremental cost calculation.
The security policy also covers secure car transportation, on-site residential security at the primary residence for each of Drs. Schleifer and Yancopoulos, and 24/7 personal security services for Drs. Schleifer and Yancopoulos. Such services are rendered by third-party providers and/or full-time employees of the Company depending on availability and time of day. The incremental costs of such services are calculated based on the methodology described below and exclude costs that the Company would have incurred in any event, such as the ordinary wages, taxes, and benefits of drivers that are employed full-time by the Company for business travel and the costs of security services provided at the Company’s offices during normal business hours. We generally calculate the incremental costs of such services based on (a) an assumed fuel cost per mile (based on then applicable standard mileage rates published by the Internal Revenue Service) times total miles traveled in connection with secure car transportation for personal travel or security services outside of the office; (b) the amount paid by the Company to the third-party providers of such services or any overtime wages of full-time employees of the Company attributed to such services based on the number of hours expended; and (c) the costs of leasing or renting vehicles dedicated to the provision of such services. In addition, in 2022, the Compensation Committee approved the temporary provision of home residence security services for Mr. Van Plew, as had been determined to be necessary based on an assessment by the Company’s Corporate Security team.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 93 |
The incremental cost associated with these services for Mr. Van Plew was comprised of the amount paid by the Company to the third-party provider of such services.
Amounts associated with personal or guest Company-provided air and secure car transportation/personal and residential security services are imputed as income to the NEOs to the extent required by applicable tax regulations. The NEOs do not receive a tax gross-up from us to cover their personal income tax obligations in respect of any such imputed income.
The amounts disclosed in the “All other compensation” column of the Summary Compensation Table relating to personal and guest use of Company-provided air transportation, secure car transportation, and personal and residential security services in accordance with our security policy attributable to Drs. Schleifer and Yancopoulos, as well as the amount disclosed for Mr. Van Plew for temporary residential security services, are based on the incremental cost resulting from such transportation/services as described above.
The Corporate Governance and Compliance Committee monitors business and any personal or guest Company-provided air travel on a periodic basis.
Additional information regarding perquisites and other personal benefits provided to our NEOs in, or with respect to, 2022 is given in the applicable footnotes to the Summary Compensation Table included in this proxy statement.
Except for equity award agreements for Dr. Vagelos and the 2020 PSU award agreements,6 outstanding equity award agreements for all employees include a “double trigger” provision for the acceleration of vesting of unvested equity awards upon a termination by the Company without cause or by the employee for good reason within two years following a change in control. Dr. Vagelos’s stock option, PSU, and RSU awards contain change-of-control provisions consistent with those applicable to the non-employee director equity awards, as described under “Board of Directors–Compensation of Directors” above.
Our CEO has an employment agreement that provides for certain severance benefits following termination, including following death or disability, resignation following defined “good reason” events, or termination in connection with a change in control. The other NEOs are covered by a change in control severance plan, which provides certain benefits to them and other designated officers if they are terminated in connection with a change in control. In addition, in the case of our CSO, stock option, RSA, and PSU award agreements applicable to his awards provide that he would have a “good reason” for terminating his employment with Regeneron upon or within two years after the occurrence of a change in control if the employment of our CEO has ended due to our CEO’s involuntary termination (as defined in the CEO’s employment agreement). Information regarding applicable payments under this employment agreement and change in control severance plan is provided in the subsection “2022 Executive Compensation Tables—Post-Employment Compensation.”
Our NEOs will forfeit any unvested stock options or RSAs upon the termination of their employment for any reason (including disability or retirement) other than death, except as provided in our employment agreement with our CEO, in our change in control severance plan, and in certain stock option agreements with our CSO. In the event of the death of an employee, any unvested stock options held by such employee become immediately exercisable, and any shares subject to RSAs/RSUs will become fully vested. In the case of PSU awards to our CEO and CSO (as well as the 2019 PSU award to Dr. Vagelos), upon a termination of employment due to death (or, in the case of 2020 PSU awards, also disability), the PSU award will remain outstanding, and any vesting of the PSUs will be determined following the completion of the applicable performance period. For information regarding the value of accelerated stock options, RSAs, and PSUs held by our CEO and other NEOs (as applicable) as of December 31, 2022, see the subsection “2022 Executive Compensation Tables—Post-Employment Compensation” under “Value of Accelerated Stock Options/PSUs” in the table entitled “Potential Severance Payments under Dr. Schleifer’s Employment Agreement” (for our CEO) and under “Value of Accelerated Stock Options/RSAs/PSUs” in the table entitled “Potential Payments under Change in Control Severance Plan” (for other NEOs).
6 |
In the case of the PSUs granted to our CEO and CSO in 2020, if there is a change in control, earnout is measured as of the date of the change in control (using the applicable transaction price as the ending stock price), and all PSUs earned as of that date vest with no Holding Period and any unearned PSUs are forfeited immediately. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 94 |
Except as provided below, when employees retire, they forfeit all unvested stock options, RSAs, and PSUs, including the PSUs granted to our CEO and CSO in 2020. An employee considered “retirement eligible” upon separation under our employee policies as in effect from time to time has the remaining life of the 10-year stock option term to exercise stock options that are vested as of the date of his or her retirement. In addition, in the case of (i) stock option award agreements with our CEO (as provided in his employment agreement), our CSO, and Dr. Vagelos and (ii) the 2019 PSU award agreements with our CEO and CSO and Dr. Vagelos, awards thereunder will continue to vest in accordance with the terms of the applicable award agreement so long as they are “retirement eligible” upon separation.
The award agreements for the 2020 PSU awards to our CEO and CSO provide that if the recipient’s employment with the Company is terminated without Cause or the recipient leaves his employment with the Company for a Good Reason (each as defined in or incorporated by reference into the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan or the award agreement), earnout is measured as of termination and all PSUs earned as of that date vest and remain subject to a mandatory deferral and holding period of three years after vesting. In this circumstance, PSUs not earned as of such termination date have 12 additional months to be earned and, to the extent earned, will vest on the fifth anniversary of the grant date or the first anniversary of the termination, whichever comes first (with the holding period beginning upon such vesting). Unearned PSUs are forfeited at the end of the 12 months. See the table entitled “Potential Severance Payments under Dr. Schleifer’s Employment Agreement” in the subsection “2022 Executive Compensation Tables—Post-Employment Compensation” for the value of accelerated 2020 PSU awards to our CEO under such a termination scenario as of December 31, 2022 (which value would be the same in respect of the 2020 PSU awards to our CSO under a corresponding termination scenario).
The change-in-control severance benefits provided to our NEOs are designed to promote stability and continuity of our senior management and are intended to preserve employee morale and productivity and encourage retention in the face of the disruptive impact of an actual, threatened, or rumored change in control of the Company. These severance benefits were established following a review of comparable practices at the Company’s peer companies and with the advice of the Compensation Committee’s consultant.
We have no pension, deferred compensation, or retirement plans applicable to our NEOs, other than our 401(k) Savings Plan described above.
As required by Section 953(b) of the Dodd-Frank Wall Street Reform and Consumer Protection Act and Item 402(u) of Regulation S-K, we are required to disclose the median of the annual total compensation of our employees (excluding our principal executive officer), the annual total compensation of our principal executive officer, Dr. Schleifer, and the ratio of these two amounts.
We have determined the total compensation of our median employee (based on the 2022 annual total compensation of our employees, excluding Dr. Schleifer) to be $162,115. The total 2022 compensation of Dr. Schleifer, as reported in the Summary Compensation Table above, was $7,004,069. Accordingly, the ratio of the 2022 annual total compensation of Dr. Schleifer to the median of the 2022 annual total compensation of our employees was approximately 43 to 1.7
For 2022, we identified the median employee as of December 31, 2022 by (i) aggregating for each applicable employee (a) annual base salary for salaried employees (or wages plus overtime, based on annual work schedule, for permanent hourly employees), (b) the target annual cash incentive, and (c) the grant date fair value of any equity awards granted during 2022, and (ii) ranking this compensation measure from lowest to highest. This calculation was performed for all employees, excluding Dr. Schleifer, whether employed on a full-time, part-time, or seasonal basis. For purposes of identifying the median employee, we converted amounts paid in foreign currencies to U.S. dollars based on the applicable 2022 average exchange rate.
7 |
This ratio would have been approximately 204 to 1 if the five-year, front-loaded PSU award granted to Dr. Schleifer in 2020 in lieu of five years of annual equity awards (i.e., until the Company’s regular year-end grant cycle in December 2025) were annualized over such five-year period. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 95 |
We believe that the pay ratio reported above is a reasonable estimate calculated in a manner consistent with SEC rules based on our internal records and the methodology described above. Because the SEC rules for identifying the median compensated employee and calculating the pay ratio based on that employee’s annual total compensation allow companies to adopt a variety of methodologies, to apply certain exclusions, and to make reasonable estimates and assumptions that reflect their employee populations and compensation practices, the pay ratio reported by other companies may not be comparable to the pay ratio reported above, as other companies have different employee populations and compensation practices and may utilize different methodologies, exclusions, estimates, and assumptions in calculating their own pay ratios.
As required by Item 402(v) of Regulation S-K, the following table and accompanying footnotes and discussion provide certain information regarding executive compensation and measures of Company performance in the last three fiscal years. Except where expressly stated, the information presented below was not considered by the Compensation Committee in structuring our executive compensation program for the years presented, and the reader should instead refer to the section “Compensation Discussion and Analysis” for a description of the philosophy, objectives, and structure of our pay program.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
A |
B |
|
C |
|
D |
|
E |
|
F |
G |
|
H |
|
I |
|
|
|
|
|
|
|
|
|
Value of Initial Fixed $100 Investment Based On: |
|
|
|
|
|
||
Year |
Summary Compensation Table Total for PEO ($)1 |
Compensation “Actually Paid” to PEO ($)1,2,3 |
Average Summary Compensation Table Total for Non-PEO NEOs ($)1 |
Average Compensation “Actually Paid” to Non-PEO NEOs ($)1,2,3 |
TSR ($) |
Peer |
Net Income ($) |
($)5 |
|||||||
2022 |
|
|
|
|
|
|
|
||||||||
2021 |
|
|
|
|
|
|
|
||||||||
2020 |
|
|
|
|
|
|
|
||||||||
1
2
The amounts shown for “Compensation “Actually Paid”” have been calculated in accordance with Item 402(v) of Regulation S K and do not reflect compensation actually earned, realized, or received by the Company’s NEOs. These amounts reflect total compensation as reported in the Summary Compensation Table with certain adjustments as described in footnote 3 below. 3
“Compensation “Actually Paid”” reflects the exclusions and inclusions of equity awards for the PEO and the Non-PEO NEOs as set forth below and calculated in accordance with FASB ASC Topic 718 using the valuation methodologies and assumptions set forth in the calculation of the grant date fair value of these awards as disclosed in the Company’s audited financial statements for the fiscal year in which the equity awards were granted. The “Exclusion of Stock Awards and Option Awards from Summary Compensation Table” columns in the tables below reflect the aggregate amounts reported in the Summary Compensation Table for the applicable year in the “Stock Awards” and “Option Awards” columns. |
|
||||||||||||||
|
Year |
|
Summary Compensation Table Total for PEO ($) |
Exclusion of Stock Awards and Option Awards from Summary Compensation Table for PEO ($) |
Inclusion of Item 402(v) Equity Award Values for PEO ($) |
Compensation “Actually Paid” to PEO ($) |
|
|||
|
2022 |
|
7,004,069 |
|
|
|
98,786,583 |
|
||
|
2021 |
|
6,470,514 |
|
|
|
123,744,652 |
|
||
|
2020 |
|
135,350,121 |
|
( |
|
|
153,035,522 |
|
|
|
Year |
|
Average Summary Compensation Table Total for Non-PEO NEOs ($) |
Exclusion of Stock Awards and Option Awards from Summary Compensation Table for Non-PEO NEOs ($) |
Inclusion of Item 402(v) Equity Award Values for Non-PEO NEOs($) |
Average Compensation “Actually Paid” to Non-PEO NEOs ($) |
|
|||
|
2022 |
|
8,061,639 |
|
( |
|
|
32,174,465 |
|
|
|
2021 |
|
7,548,928 |
|
( |
|
|
40,168,860 |
|
|
|
2020 |
|
40,058,597 |
|
( |
|
|
48,557,559 |
|
|
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 96 |
The “Inclusion of Item 402(v) Equity Award Values” columns in the tables above are derived from the dollar values set forth in the following tables: |
|
Year |
Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year for PEO ($) |
Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards for PEO ($) |
Vesting-Date Fair Value of Equity Awards Granted During Year that Vested During Year for PEO ($) |
Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year for PEO ($) |
Fair Value at Last Day of Prior Year of Equity Awards Forfeited During Year for PEO ($) |
Value of Dividends or Other Earnings Paid on Stock or Option Awards Not Otherwise Included for PEO ($) |
Total - Inclusion of Equity Values for PEO ($) |
|
|
2022 |
|
|
|
|
||||
|
2021 |
|
|
|
|
||||
|
2020 |
|
|
|
|
||||
|
Year |
Average Year-End Fair Value of Equity Awards Granted During Year That Remained Unvested as of Last Day of Year for Non-PEO NEOs ($) |
Average Change in Fair Value from Last Day of Prior Year to Last Day of Year of Unvested Equity Awards for Non-PEO NEOs ($) |
Average Vesting-Date Fair Value of Equity Awards Granted During Year that Vested During Year for Non-PEO NEOs ($) |
Average Change in Fair Value from Last Day of Prior Year to Vesting Date of Unvested Equity Awards that Vested During Year for Non-PEO NEOs ($) |
Average Fair Value at Last Day of Prior Year of Equity Awards Forfeited During Year for Non-PEO NEOs ($) |
Average Value of Dividends or Other Earnings Paid on Stock or Option Awards Not Otherwise Included for Non-PEO NEOs ($) |
Total—Average Inclusion of Equity Values for Non-PEO NEOs ($) |
|
2022 |
|
|
|||||
|
2021 |
|
|
|||||
|
2020 |
|
|
|||||
4
5
Pursuant to Item 402(v) of Regulation S-K, we determined our stock price to be the most important financial performance measure used to link Company performance to Compensation “Actually Paid” to our PEO and other NEOs in 2022. This performance measure may not have been the most important financial performance measure for years 2021 and 2020 and we may determine a different financial performance measure to be the most important such measure in future years. |
||||||||
The following chart sets forth the relationship between Compensation “Actually Paid” to our PEO, the average of Compensation “Actually Paid” to our Non-PEO NEOs, each as set forth in the Table above, and the Company’s cumulative TSR over the three-year period from 2020 through 2022.
PEO and Average NEO Compensation “Actually Paid” Versus Regeneron TSR, 2020-2022

| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 97 |
The following chart sets forth the relationship between Compensation “Actually Paid” to our PEO, the average of Compensation “Actually Paid” to our Non-PEO NEOs, and our net income during years 2020 through 2022, each as set forth in the table above.
PEO and Average NEO Compensation “Actually Paid” Versus Regeneron Net Income, 2020-2022

The following chart sets forth the relationship between Compensation “Actually Paid” to our PEO, the average of Compensation “Actually Paid” to our Non-PEO NEOs, and the closing price of our common stock on the last trading day of years 2020 through 2022, each as set forth in the table above.
PEO and Average NEO Compensation “Actually Paid” Versus Regeneron Stock Price, 2020-2022
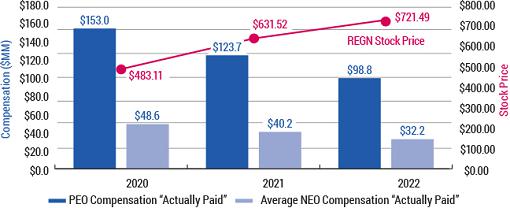
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 98 |
The following chart compares our cumulative TSR over the three-year period from 2020 through 2022 to that of the NQ US Pharma Index over the same time period.
Comparison of Regeneron Cumulative TSR to the NQ US Pharma Total Return Index, 2020-2022
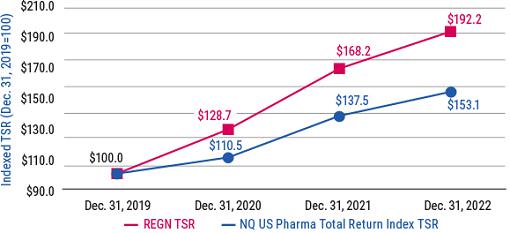
The following table presents the financial and non-financial performance measures that the Company considers to have been the most important in linking Compensation “Actually Paid” to our PEO and our Non-PEO NEOs in 2022 to Company performance. The measures in this table are not ranked.
|
|
|
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 99 |
The Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan (referred to in this subsection as the “Plan”) is the only plan currently used by the Company to grant equity awards. The Plan was approved by shareholders and is designed to promote best practices by reinforcing the alignment between equity compensation arrangements for employees and non-employee directors and the interests of shareholders. The provisions that promote such best practices include:
Provision |
Description |
No Discounted Stock Options or Stock Appreciation Rights |
Stock options and stock appreciation rights are not granted with an exercise or base price less than the fair market value of common stock (as defined in the Plan) on the date of grant. |
No Stock Option or Stock Appreciation Right Re-pricing or Exchange |
Except for equitable adjustments in connection with specific corporate transactions (such as stock splits, recapitalizations, reorganizations, mergers, consolidations, and similar transactions), the Plan does not permit a decrease in the exercise price or base price of a stock option or stock appreciation right granted under the Plan through settlement, cancellation, forfeiture, exchange, surrender, or otherwise below the fair market value of common stock (as defined in the Plan) on the date of grant. |
Recoupment (Clawback) Policy |
Awards granted to our officers and other employees under the Plan are subject to recoupment or reduction in accordance with the terms of our policy regarding recoupment or reduction of incentive compensation (sometimes referred to as our “clawback policy”). |
Independent Administration |
The Plan is administered by the Compensation Committee, which is intended to be comprised solely of non-employee directors each of whom meets the additional independence criteria applicable to compensation committee members under the listing standards of the Nasdaq Stock Market LLC and qualifies as a “Non-Employee Director” pursuant to Rule 16b-3 under the Exchange Act. |
No “Evergreen” Provision |
The Plan does not contain an “evergreen” feature pursuant to which the shares authorized for issuance thereunder can be automatically replenished. |
No Tax Gross-ups |
The Plan does not provide for any tax gross-ups. |
The following table summarizes some key metrics relating to the equity component of our compensation program. When evaluating the information below, it is important to keep in mind the 46% increase in the number of our employees over the 2020—2022 period and our all-employee equity award strategy encompassing initial equity grants to all new hires as well as a comprehensive annual equity program covering all levels of employees. See “Compensation Discussion and Analysis—Compensation Program Overview — 2022 Compensation Highlights” for more information on our burn rate, which in 2022 was at the lowest level in more than a decade, and headcount growth over the same time period.
|
|
2022 |
|
2021 |
|
2020 |
Unadjusted Burn Rate1 |
|
2.71% |
|
3.14% |
|
3.25% |
Adjusted Burn Rate1 |
|
4.00% |
|
4.53% |
|
4.15% |
Overhang2 |
|
25.01% |
|
26.92% |
|
29.27% |
Dilution3 |
|
15.63% |
|
16.71% |
|
18.98% |
1
Calculated by dividing (a) the sum of the number of shares subject to (i) stock options, RSAs, and RSUs granted during the year and (ii) PSUs earned during the year (if any), by (b) the basic weighted-average number of shares of common stock and Class A stock outstanding during the year. For “Adjusted Burn Rate,” a multiplier of 2.5 is applied to RSAs, RSUs, and PSUs. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 100 |
2
Calculated by dividing (a) the sum of (i) the number of shares subject to equity awards (stock options and unvested RSAs, RSUs, and PSUs (assuming, in the case of PSUs, maximum payouts earned)) outstanding at the end of the year and (ii) the number of shares available for future grants under the Plan at the end of the year, by (b) the sum of (i) the number of shares of common stock and Class A stock outstanding at the end of the year, (ii) the shares subject to equity awards (stock options and unvested RSAs, RSUs, and PSUs (assuming, in the case of PSUs, maximum payouts earned)) outstanding at the end of the year, and (iii) the number of shares available for future grants under the Plan at the end of the year. |
3
Calculated by dividing the number of shares subject to equity awards (stock options and unvested RSAs, RSUs, and PSUs (assuming, in the case of PSUs, maximum payouts earned)) outstanding at the end of the year by the sum of (i) the number of shares of common stock and Class A stock outstanding at the end of the year and (ii) the shares subject to equity awards (stock options and unvested RSAs, RSUs, and PSUs (assuming, in the case of PSUs, maximum payouts earned)) outstanding at the end of the year. |
|
The following table shows information with respect to securities authorized for issuance under the equity compensation plans maintained by the Company as of December 31, 2022.
A |
B |
C | |
Plan Category |
Number of securities to be issued upon exercise of outstanding options, warrants, and rights
|
Weighted-average exercise price of outstanding options, warrants, and rights ($) |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column A)
|
Equity compensation plans approved by security holders1 |
17,367,2713 |
481.624 |
15,872,560 |
Equity compensation plans not approved by security holders2 |
— |
— |
44,246 |
Total |
17,367,271 |
481.62 |
15,916,806 |
1
The equity compensation plans approved by the security holders are the Regeneron Pharmaceuticals, Inc. Second Amended and Restated 2000 Long-Term Incentive Plan; the Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan; the Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan; and the Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan. The Second Amended and Restated Regeneron Pharmaceuticals, Inc. 2014 Long-Term Incentive Plan is the only plan currently used by the Company to grant equity awards. 2
The equity compensation plan not approved by the security holders is the Executive Stock Purchase Plan. It was adopted in 1989 and provides for the Compensation Committee of the board of directors to award employees, directors, consultants, and other individuals who render service to the Company the right to purchase Class A stock at a price set by the Compensation Committee. The Plan provides for the vesting of shares as determined by the Compensation Committee; should the Company’s relationship with a Plan participant terminate before all shares are vested, unvested shares will be repurchased by the Company at a price per share equal to the original amount paid by the Plan participant. As of December 31, 2022, there were no unvested shares and 44,246 shares of Class A stock available for future grants under the Plan. 3
This amount includes (i) 15,636,891 shares to be issued upon exercise of outstanding options, (ii) 230,444 shares to be issued upon vesting of outstanding RSUs, and (iii) 1,499,936 shares to be issued upon vesting of outstanding PSUs (assuming, in the case of PSUs, maximum payouts earned). 4
The calculation of the weighted-average exercise price does not include the 230,444 shares to be issued upon vesting of RSUs or the 1,499,936 shares to be issued upon vesting of PSUs (assuming, in the case of PSUs, maximum payouts earned), as RSUs and PSUs do not have an exercise price. 5
This amount is net of 2,467,042 outstanding RSAs. As these shares are considered issued and outstanding upon grant, they are not included in the amounts reported in column A. |
|||
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 101 |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 102 |
As required by Section 14A of the Exchange Act, we are seeking, on an advisory basis, shareholder approval of the compensation of our NEOs as disclosed above (“say-on-pay” proposal). Specifically, shareholders are being asked to approve the following advisory resolution:
RESOLVED, that the shareholders of Regeneron Pharmaceuticals, Inc. hereby approve the compensation of the Company’s Named Executive Officers, as disclosed in the Company’s proxy statement relating to the Company’s 2023 Annual Meeting (the “Proxy Statement”) pursuant to the compensation disclosure rules of the Securities and Exchange Commission (which disclosure includes the Compensation Discussion and Analysis, the compensation tables, and the related compensation information contained in the Proxy Statement).
In determining to recommend that shareholders approve the say-on-pay proposal, the board of directors considered, among other factors discussed under “Compensation Discussion and Analysis” above, that the Company had achieved its corporate goals in 2022. Information regarding compensation of the relevant NEOs for 2022 is provided in the Summary Compensation Table included in this proxy statement.
The board of directors unanimously recommends a vote FOR approval of the compensation of our Named Executive Officers as disclosed in this proxy statement. As an advisory vote, this proposal is non-binding on the Company. However, the board of directors and the Compensation Committee value your opinion and will review and consider the voting results in connection with their ongoing evaluation of our compensation program.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 103 |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 104 |
As required by Section 14A of the Exchange Act, at least once every six years we submit to a vote of our shareholders a non-binding advisory proposal on the desired frequency of future say-on-pay votes. Shareholders may vote to hold say-on-pay votes every one, two, or three years, or may abstain.
Our shareholders last voted on this question at the 2017 annual shareholder meeting. At that meeting, a frequency of every three years received approximately 55% of the votes cast on the proposal, consistent with the board of directors’ recommendation at such time. Effective at the 2022 annual shareholder meeting, our board of directors voluntarily changed the frequency of our say-on-pay votes from every three years to every year in recognition of shareholder feedback and preference. In light of that feedback and the importance we place on shareholder engagement, including as it relates to executive compensation matters, the board of directors is recommending that shareholders vote for continuing to conduct say-on-pay votes every year.
Because this shareholder vote is advisory, it will not be binding on the Company or the board of directors. However, the Compensation Committee and the board of directors will take into account the outcome of the vote when determining the frequency of future say-on-pay votes.
The enclosed proxy allows you to vote for a frequency of every one, two, or three years, or to abstain. Shareholders are not voting to approve or disapprove the board of directors’ recommendation.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 105 |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 106 |
The following shareholder proposal has been submitted to the Company for action at the 2023 Annual Meeting by Boston Common Asset Management, on behalf of Boston Common ESG Impact US Equity Fund (“Boston Common”), a shareholder representing that Boston Common beneficially owned continuously for at least one year shares of the Company’s common stock worth at least $25,000, along with co-filers. The Company is not responsible for any inaccuracies this shareholder proposal may contain. As explained below, the board of directors unanimously recommends a vote AGAINST this proposal. The text of the proposal follows:
RESOLVED, that shareholders of Regeneron Pharmaceuticals Inc. (“Regeneron”) ask the Board of Directors to establish and report on a process by which the impact of extended patent exclusivities on product access would be considered in deciding whether to apply for secondary and tertiary patents. Secondary and tertiary patents are patents applied for after the main active ingredient/molecule patent(s) and which relate to the product. The report on the process should be prepared at reasonable cost, omitting confidential and proprietary information, and published on Regeneron’s website.
Supporting Statement
Access to medicines, especially costly specialty drugs, is the subject of consistent and widespread public debate in the U.S. A 2021 Rand Corporation analysis concluded that U.S. prices for branded drugs were nearly 3.5 times higher than prices in 32 OECD member countries.1 The Kaiser Family Foundation has “consistently found prescription drug costs to be an important health policy area of public interest and public concern.”2
This high level of concern has driven policy responses. The Inflation Reduction Act empowers the federal government to negotiate some drug prices.3 State measures, including drug price transparency legislation and Medicaid purchasing programs, have also been adopted.4 The House Committee on Oversight and Reform (the “Committee”) launched an investigation into drug pricing in January 2019.5
Intellectual property protections on branded drugs play an important role in maintaining high prices and impeding access. When a drug’s patent protection ends, generic manufacturers can enter the market, reducing prices. But branded drug manufacturers may try to delay competition by extending their exclusivity periods.
Among the abuses described by the Committee’s December 2021 report is construction of a “patent thicket,” which consists of many “secondary patents covering the formulations, dosing, or methods of using, administering, or manufacturing a drug” granted after the drug’s primary patent, covering its main active ingredient or molecule, has been granted.6 The U.S. Patent and Trademark Office, partly in response to a letter from six U.S. Senators requesting measures to address patent thickets,7 recently issued a request for public comment on initiatives to “adequately protect[ ] innovation while not unnecessarily delaying generic and biosimilar competition.”8
Regeneron markets Eylea, which treats eye disorders. According to I-MAK, of the 135 patent applications filed on Eylea, 65% were filed after the drug was approved by the Food and Drug Administration.9 According to I-MAK, such post-approval filings “indicat[e] an attempt to prolong existing exclusivity.”10
In our view, a process that considers the impact of extended exclusivity periods on patient access would ensure that Regeneron considers not only whether it can apply for secondary and tertiary patents but also whether it should do so. Regeneron’s current approach subjects the company to reputational risks and potential regulatory blowback resulting from high drug prices and perceptions regarding abusive patenting practices.
1
www.rand.org/news/press/2021/01/28.html |
2
www.kff.org/health-costs/poll-finding/public-opinion-on-prescription-drugs-and-their-prices/ |
3
www.kff.org/medicare/issue-brief/explaining-the-prescription-drug-provisions-in-the-inflation-reduction-act/ |
4
www.americanprogress.org/article/state-policies-to-address-prescription-drug-affordability-across-the-supply-chain/ |
5
oversight.house.gov/sites/democrats.oversight.house.gov/files/DRUG%20PRICING%20REPORT%20WITH%20APPENDIX%20v3.pdf, at i. |
6
oversight.house.gov/sites/democrats.oversight.house.gov/files/DRUG%20PRICING%20REPORT%20WITH%20APPENDIX%20v3.pdf, at 79. |
7
www.leahy.senate.gov/imo/media/doc/20220608%20Letter%20to%20PTO%20on%20repetitive%20patents.pdf |
8
www.govinfo.gov/content/pkg/FR-2022-10-04/pdf/2022-21481.pdf |
9
www.i-mak.org/wp-content/uploads/2022/09/Overpatented-Overpriced-2022-FINAL.pdf, at 6. |
10
www.i-mak.org/wp-content/uploads/2022/09/Overpatented-Overpriced-2022-FINAL.pdf, at 6. |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 107 |
Management’s Statement in Opposition
We believe that the report requested by the proposal is unnecessary and against the interest of our company and shareholders. After careful consideration, the board of directors unanimously recommends that shareholders vote AGAINST this shareholder proposal. The reasons for the board’s recommendation include the following:
Patent protection facilitates continued innovation and enables our mission of bringing important new medicines to patients.
For the last 35 years, Regeneron has generated a robust pipeline of diverse product candidates designed to address unmet patient needs. We protect intellectual property (“IP”) rights in our innovations and discoveries to facilitate continued innovation, protect patients, and promote collaboration.
As our currently marketed products and most of our product candidates are biologics, bringing them to market may cost more than bringing traditional, small-molecule drugs to market due to the complexity associated with the research, development, production, supply, and regulatory review of such products.
Bringing a single successful product to market, particularly a biological product, can cost billions of dollars and take many years to discover, test, validate, and get approved. By protecting our IP, we prevent others from inappropriately benefiting from our hard work and investment, thereby protecting shareholder interests.
Patent protection also helps ensure patients receive authentic, safe, and effective treatments by preventing others from making substandard copies or counterfeits of our medicines. Patent protection is also foundational to promoting global collaborations (including by allowing the free sharing of information without fear of misappropriation), allowing us to share our ideas and advancements with the objective of spurring additional innovations.
At Regeneron, innovation often does not stop at the initial approval of a product. For instance, we typically continue to make significant R&D investments relating to additional indications and applications for our products (such as conducting new clinical studies in potential additional indications). By way of example, since its initial approval by the U.S. Food and Drug Administration (“FDA”) in wet AMD in November 2011, EYLEA has been approved for the treatment of several additional indications in the United States and other jurisdictions. Similarly, Dupixent has been approved to treat a range of diseases following the initial FDA approval in atopic dermatitis, including asthma, chronic rhinosinusitis with nasal polyposis, and eosinophilic esophagitis; and in our oncology portfolio, we are combining our foundational product Libtayo (which has now received FDA approval in three distinct indications) with other product candidates in our development pipeline to investigate new therapeutic approaches in difficult-to-treat cancers. These and other efforts require us to continue to take innovative steps with respect to our commercialized products that, if successful, ultimately benefit patients.
Since our founding, we have been responsible when it comes to pricing our medicines. We have publicly recognized our responsibility to set fair, value-based prices for our medicines and break down barriers to patient access and already publicly disclose our pricing philosophy.
Our pricing philosophy outlines our approach to pricing, with fairness, affordability, and access at the forefront. You can access our pricing philosophy on our website at www.regeneron.com on the “Transparency & Policies” page under the “Responsibility” heading.
We have been responsible when it comes to pricing our medicines. For example, we have not increased the price of EYLEA since its initial approval in 2011, despite significant ongoing R&D investments as discussed above.
One of our previously announced 2025 responsibility goals is to set fair, value-based prices for our medicines and break down barriers to patient access. We report our progress towards this and other goals in our annual Responsibility Report. We recognize that the medicines we create are only useful if patients in need can access and afford them. To that end, we are committed to developing pricing approaches that facilitate patient access and foster medical innovation through engagement with our stakeholders. For example, in recent years, we have engaged the U.S. Biomedical Advanced Research and Development Authority (BARDA), public health organizations, non-governmental agencies, and others in our industry to help facilitate access to our COVID-19 treatment in low- and middle-income countries.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 108 |
We help make our products more accessible through our product support and other programs. These programs, for example, help healthcare providers and patients navigate health insurance plans and offer co-pay support to eligible commercial patients. We also provide patient assistance programs that are designed to increase access to medicine and provide education on using medicines safely and appropriately. Our programs help eligible patients throughout their treatment journey with insurance eligibility support, patient resources, financial assistance, and access to free medicine.
In 2022, we provided financial support to 900,000 patients, including $850 million in commercial co-payments paid by Regeneron to help eligible commercially insured patients with their out-of-pocket costs. We also continued to provide free medicine to eligible patients who do not have insurance or cannot afford the cost of the drug. In 2022, Regeneron’s Patient Assistance Programs provided free medicine to approximately 60,000 eligible patients, a value of more than $1.5 billion (based on 2022 year-end wholesale acquisition cost).
Our board provides robust oversight of risks associated with our strategic plan, drug access, and pricing.
Our board is closely involved in and provides oversight of all key pricing determinations for our products, which we endeavor to make in a thoughtful and well-informed manner with fairness and affordability in mind.
The board (directly and/or through one or more of its committees) also considers specific risk topics, including risks associated with our strategic plan and drug access and pricing, as it executes its oversight responsibility for risk management.
We believe we have the appropriate governance mechanisms and internal processes in place to ensure that pricing decisions are thoroughly and appropriately vetted prior to implementation and are made in line with our values and commitments. This includes management’s routine presentations to the board on drug pricing strategies, practices, and trends as well as developments internal and external to Regeneron that could affect our risk profile.
As Regeneron already publicly discloses the Company’s governance and strategy as it relates to drug pricing and IP, requiring Regeneron to prepare a separate report with this information would place an undue administrative burden on the Company without providing meaningful additional information to shareholders.
In addition to relevant disclosures in our proxy statements and Responsibility Reports, Regeneron provides comprehensive information in our annual reports on Form 10-K on our patents and other IP, including a detailed listing of patents that are of particular relevance to key products. This type of disclosure aims to enhance investors’ understanding of Regeneron’s patent portfolio and IP positioning of our products. We do not believe the separate report requested by the proposal would provide meaningful additional information to shareholders to merit the resources required to provide the report.
| The board of directors unanimously recommends a vote AGAINST this shareholder proposal. |  |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 109 |
June 9, 2023
10:30 a.m., ET
The Annual Meeting will be held virtually via the Internet at www.virtualshareholdermeeting.com/REGN2023. We have designed the format of the Annual Meeting to ensure that shareholders are afforded similar rights and opportunities to participate as they would at an in-person meeting.
Instructions on how to attend and participate via the Internet are posted at www.virtualshareholdermeeting.com/REGN2023. To vote or submit questions during the meeting, you will need the 16-digit control number included on the Notice of Internet Availability of Proxy Materials (the “Notice”) or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received.
Only shareholders of record at the close of business on the record date, April 11, 2023, are entitled to vote at the Annual Meeting. As of April 11, 2023, 107,892,108 shares of common stock and 1,818,146 shares of Class A stock were issued and outstanding. The common stock and the Class A stock vote together on all matters as a single class, with the common stock being entitled to one vote per share and the Class A stock being entitled to ten votes per share. To vote your shares, you will need the 16-digit control number included on the Notice or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received.
Election of four Class II directors for a three-year term
Ratification of the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2023
Advisory vote to approve the compensation of the Company’s Named Executive Officers as disclosed in these proxy materials (say-on-pay)
Advisory vote on the frequency of future say-on-pay votes (say-on-frequency)
Non-binding shareholder proposal, if properly presented
Shareholders who use the 16-digit control number that was furnished to them (either on the Notice or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received) to log on to the meeting will be able to submit questions during the meeting, as time permits. If you wish to submit a question during the Annual Meeting, log on to the virtual meeting website using the 16-digit control number, type your question into the “Ask a Question” field, and click “Submit.” Questions and answers may be arranged by topic and substantially similar questions may be answered once. If we run out of time to answer all of the questions submitted, we will provide responses to the questions not addressed on our website at www.regeneron.com on the “Investors & Media” page.
A replay of the Annual Meeting will be made publicly available approximately 24 hours after the Annual Meeting at www.virtualshareholdermeeting.com/REGN2023. The replay will be available for one year.
The “Notice and Access” rules of the SEC permit us to furnish proxy materials, including this proxy statement and our 2022 Annual Report, to our shareholders by providing access to such documents on the Internet instead of mailing printed copies. Most shareholders received the Notice and will not receive printed copies of the proxy materials unless they request them. This method reduces the environmental impact of the Annual Meeting. The Notice will be mailed beginning on or about April 21, 2023. The Notice includes instructions on how you may access and review all of our proxy materials and the 2022 Annual Report via the Internet. The Notice also includes instructions on how you may
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 110 |
vote your shares. If you would like to receive a paper or electronic copy of our proxy materials, you should follow the instructions in the Notice for requesting such materials. Any request to receive proxy materials by mail or e-mail will remain in effect until you revoke it.
No. The Notice identifies the items to be voted on at the Annual Meeting, but you cannot vote by marking the Notice and returning it. The Notice provides instructions on how to vote by Internet, by requesting and returning a paper proxy card, or by voting at the meeting.
We sent you the Notice regarding this proxy statement because Regeneron’s board of directors is asking (technically called soliciting) holders of common stock and Class A stock to provide proxies to be voted at our 2023 Annual Meeting of Shareholders or at any adjournment(s) or postponement(s) of the meeting.
If you vote by proxy in time for it to be voted at the Annual Meeting, one of the individuals named as your proxy will vote your shares as you have directed. If you submit a proxy, but no indication is given as to how to vote your shares as to a proposal, your shares will be voted in the manner recommended by the board of directors. The board of directors knows of no matter, other than those indicated above under “What is on the agenda for the meeting?”, to be presented at the Annual Meeting. If any other matter properly comes before the Annual Meeting, the persons named and designated as proxies will vote your shares in their discretion.
Shareholders who previously elected to receive proxy materials by e-mail will not receive the Notice in the mail. Instead, these shareholders should have received an e-mail with links to the proxy materials and the proxy voting website. Shareholders who have previously asked to receive paper copies of the proxy materials and shareholders who participate and hold shares of common stock in the Regeneron Pharmaceuticals, Inc. 401(k) Savings Plan or the Regeneron Ireland Share Participation Plan will receive paper copies of the proxy materials.
The presence at the Annual Meeting, in person or by proxy, of the holders as of the record date of shares of common stock and Class A stock having a majority of the voting power of all shares of common stock and Class A stock outstanding on the record date will constitute a quorum for the transaction of business at the Annual Meeting. Shares held as of the record date by holders who are present or represented by proxy at the Annual Meeting but who have abstained from voting or have not voted with respect to some or all of such shares on any proposal to be voted on at the Annual Meeting will be counted as present for purposes of establishing a quorum.
We recommend that shareholders vote by proxy even if they plan to attend the Annual Meeting via the Internet. If you are a shareholder of record, there are three ways to vote by proxy:
Via the Internet. You may vote by proxy via the Internet by visiting www.proxyvote.com. You will need the 16-digit control number included on the Notice or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received. You may vote via the Internet through 11:59 p.m., Eastern Time, on June 8, 2023.
Via telephone. You may vote by proxy via telephone by calling the toll-free number found on the proxy card or the voting instruction form. You will need the 16-digit control number included on the proxy card or voting instruction form. You may vote via telephone through 11:59 p.m., Eastern Time, on June 8, 2023.
By mail. If you received printed copies of the proxy materials, you may vote by proxy by completing the proxy card or voting instruction form and returning it in the envelope provided.
You may attend the Annual Meeting via the Internet at www.virtualshareholdermeeting.com/REGN2023. Shareholders who use the 16-digit control number that was furnished to them (either on the Notice or, if you received a paper copy of the proxy materials, the proxy card or voting instruction form you received) to log on to the meeting will be able to vote during the meeting.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 111 |
We will have technicians ready to assist you with any technical difficulties you may have accessing the virtual meeting website. If you encounter any difficulties accessing the virtual meeting website during the meeting time, please call the technical support number that will be posted at www.virtualshareholdermeeting.com/REGN2023.
If you participate and hold shares of common stock in the Regeneron Pharmaceuticals, Inc. 401(k) Savings Plan, you may provide voting instructions to Fidelity Management Trust Company, the plan’s trustee, (1) through the Internet at www.proxyvote.com by 11:59 p.m., Eastern Time, on June 6, 2023, (2) by calling 1-800-690-6903 by 11:59 p.m., Eastern Time, on June 6, 2023, or (3) by returning your completed proxy card by mail. The trustee will vote your shares in accordance with your instructions. If you do not provide timely voting instructions to the trustee, the trustee will vote your shares in the same proportion as the shares for which the trustee receives voting instructions from other participants in the plan.
If you participate and hold shares of common stock in the Regeneron Ireland Share Participation Plan, you may provide voting instructions to Mercer Ireland Limited, who administers the Plan on behalf of Irish Pensions Trust Limited, the trustees of the Plan. You will receive a voting instruction form by mail sent directly to your home address, which you should complete, sign, and return to Mercer by mail using the enclosed pre-paid envelope or as an e-mail attachment in accordance with the instructions provided by Mercer.
Yes. You may change your vote or revoke your proxy at any time before the proxy is exercised by voting again electronically through the Internet or by telephone, by mailing a new proxy card or voting instruction form, or by attending the Annual Meeting (via the Internet) and voting. If you are a record holder, you may also revoke your proxy by filing with the Secretary of the Company, at or before the taking of the vote at the Annual Meeting, a written notice of revocation bearing a later date than the proxy you previously submitted. Attendance at the Annual Meeting will not have the effect of revoking a proxy unless you vote at the Annual Meeting. If you hold your shares through a broker, bank, or other nominee in “street name,” you will need to contact them or follow the instructions in the voting instruction form used by the firm that holds your shares to revoke your proxy. Only your latest dated proxy we receive at or prior to the Annual Meeting will be counted.
Solicitation of proxies may be made by mail, in person, or by telephone by officers, directors, and other employees of the Company and by employees of the Company’s transfer agent, American Stock Transfer & Trust Company, LLC (“AST”), and employees of Broadridge Financial Solutions, Inc. (“Broadridge”). We will reimburse AST, Broadridge, and our banks, brokers, and other custodians, nominees, and fiduciaries for their respective reasonable costs in the preparation and mailing of proxy materials to shareholders. In addition, we have engaged Innisfree M&A Incorporated to assist in the solicitation of proxies and provide related advice and informational support for a service fee of $25,000 and the reimbursement of customary disbursements and expenses. We will bear all costs of the solicitation of proxies.
The board of directors recommends that you vote:
 |
FOR election of each of the four nominated Class II directors (Proposal No. 1); |
 |
FOR ratification of the appointment of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for 2023 (Proposal No. 2); and |
 |
FOR approval of the compensation of the Company’s Named Executive Officers as disclosed in these proxy materials (say-on-pay) (Proposal No. 3). |
 |
A frequency of every one year for the advisory vote regarding the frequency of future say-on-pay votes (Proposal No. 4) |
 |
AGAINST approval of a non-binding shareholder proposal (Proposal No. 5) |
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 112 |
The following table summarizes the voting requirements applicable to the proposals to be voted on at the Annual Meeting:
Proposal |
Vote Required |
Effect of Abstentions and Broker Non-Votes* |
Broker Discretionary Voting Allowed?+ |
|
1 |
Election of Directors |
Majority of the votes cast. In accordance with our director resignation policy, an incumbent director who fails to receive the required number of votes in an uncontested election will be required to tender his or her resignation to the Chair of the board of directors for consideration by the Corporate Governance and Compliance Committee. |
No effect—not considered votes cast on this proposal |
No—brokers without voting instructions will not be able to vote on this proposal |
2 |
Ratification of the Appointment of PricewaterhouseCoopers LLP |
Majority of the votes cast |
No effect—not considered votes cast on this proposal |
Yes—brokers without voting instructions will have discretionary authority to vote |
3 |
Say-on-Pay |
Non-binding, advisory proposal. We will consider the matter approved if it receives the affirmative vote of a majority of the votes cast |
No effect—not considered votes cast on this proposal |
No—brokers without voting instructions will not be able to vote on this proposal |
4 |
Say-on-Frequency |
Non-binding, advisory proposal. We will consider the option that receives a plurality of the votes cast to be the recommendation of shareholders |
No effect—not considered votes cast on this proposal |
No—brokers without voting instructions will not be able to vote on this proposal |
5 |
Non-Binding Shareholder Proposal |
Non-binding proposal. We will consider the matter approved if it receives the affirmative vote of a majority of the votes cast |
No effect—not considered votes cast on this proposal |
No—brokers without voting instructions will not be able to vote on this proposal |
*
As noted above, abstentions will be counted as present for purposes of establishing a quorum at the Annual Meeting. +
Only relevant if you are the beneficial owner of shares held in “street name.” If you are a shareholder of record and you do not cast your vote, no votes will be cast on your behalf on any of the items of business at the Annual Meeting. |
||||
If any other matter is properly brought before the Annual Meeting, such matter also will be determined by the affirmative vote of a majority of the votes cast at the Annual Meeting.
A shareholder wishing to present a proposal at the 2024 Annual Meeting of Shareholders and have the proposal included in our proxy statement must submit the proposal in writing and it must be received by the Company at its principal executive offices at 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707 by December 23, 2023, and must satisfy the other conditions established by the SEC.
Under our Amended and Restated By-Laws, proposals of shareholders (other than proposals to be included in our proxy statement) and nominations of directors for election at the 2024 Annual Meeting may be made only by a shareholder of record who has given notice of the proposal or nomination to the Secretary of the Company at our principal executive offices no earlier than 90 days and no later than 60 days prior to the meeting; provided that if less than 70 days’ notice or public disclosure of the date of the 2024 Annual Meeting is given or made to shareholders, notice by the shareholder in order to be timely must be received no later than the close of business on the tenth day following the day on which such notice of the annual meeting was first mailed or such public disclosure of the annual meeting was made, whichever first occurs. The notice must contain certain information as specified in our Amended and Restated By-Laws. Assuming our 2024 Annual Meeting is held on June 14, 2024 in accordance with the Company’s past practice, and at least 70 days’ notice or prior public disclosure of the date of the 2024 Annual Meeting is given or made to shareholders, notice of such proposals or nominations would need to be given no earlier than
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 113 |
March 16, 2024 and no later than April 15, 2024. In addition to satisfying the requirements under our Amended and Restated By-Laws relating to nominations of directors, including the deadline for written notice, to comply with the SEC’s “universal proxy rules,” shareholders who intend to solicit proxies in support of director nominees other than the Company’s nominees at the 2024 Annual Meeting in compliance with Rule 14a-19 under the Exchange Act must provide written notice containing the information required by Rule 14a-19 to our Corporate Secretary at 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707 no later than April 10, 2024.
Applicable rules permit brokerage firms and the Company to send one Notice (or one annual report, proxy statement, and Notice in the case of shareholders who have elected to receive paper copies of our proxy materials) to multiple shareholders who share the same address under certain circumstances. This practice is known as “householding.” We believe that householding will provide greater convenience for our shareholders, as well as cost savings for us, by reducing the number of duplicate documents that are sent to your home. Consequently, we have implemented the practice of householding for shares held in “street name” and intend to deliver only one copy of the applicable proxy materials to multiple shareholders sharing the same address. If you wish to receive separate copies of the proxy statement for the 2023 Annual Meeting, the 2022 Annual Report, or the Notice, you may find these materials at our internet website (www.regeneron.com) or you may stop householding for your account and receive separate printed copies of these materials by contacting our Investor Relations Department, at Regeneron Pharmaceuticals, Inc., 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707, or by calling us at 914-847-7000, and these materials will be promptly delivered to you. If you hold shares registered in your name (sometimes called a shareholder of record), you can elect householding for your account by contacting us in the same manner described above. Any shareholder may stop householding for your account by contacting our Investor Relations Department at the address and/or phone number included above. If you revoke your consent, you will be removed from the householding program within 30 days of receipt of your revocation and each shareholder at your address will receive individual copies of our disclosure documents.
We know of no other matters to be brought before the Annual Meeting, except as set forth in this proxy statement. If any other matter is properly presented at the Annual Meeting upon which a vote may properly be taken, shares represented by duly executed and timely submitted proxies will be voted on any such matter in accordance with the judgment of the persons named as proxies in the enclosed proxy card. Discretionary authority for them to do so is contained in the enclosed proxy card.
Interested shareholders may obtain without charge a copy of our 2022 Annual Report (without exhibits), which includes our audited financial statements for the fiscal year ended December 31, 2022, required to be filed with the SEC, by making a written request to Regeneron Pharmaceuticals, Inc., 777 Old Saw Mill River Road, Tarrytown, New York 10591-6707, Attention: Investor Relations, or by calling our Investor Relations Department at (914) 847-7000.
If you previously requested to receive proxy materials through the mail, or by means of an e-mail with links to the proxy materials and the proxy voting website, your election will remain in effect until you revoke it. Shareholders currently receiving paper copies of our proxy materials, and shareholders who received a paper copy of the Notice, may instead elect to receive all future proxy materials electronically through an e-mail with a link to these documents on the Internet. Receiving these documents online conserves resources, saves the Company the cost of producing and mailing documents to your home or business, and gives you an automatic link to the proxy voting site.
If your shares are registered in your name or you hold shares in the Company Stock Fund in the Company’s 401(k) Savings Plan, to enroll in the electronic delivery service, vote your shares through the Internet at www.proxyvote.com and, when prompted, indicate that you agree to receive or access shareholder communications electronically in future years. If your shares are not registered in your name, to enroll in the electronic delivery service, check the information provided to you by your bank or broker, or contact your bank or broker for instructions on how to elect to view future proxy statements and annual reports over the Internet.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 114 |
This proxy statement includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron Pharmaceuticals, Inc. (where applicable, together with its subsidiaries, “Regeneron” or the “Company”), and actual events or results may differ materially from these forward-looking statements. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate,” variations of such words, and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the impact of SARS-CoV-2 (the virus that has caused the COVID-19 pandemic) on Regeneron’s business and its employees, collaborators, and suppliers and other third parties on which Regeneron relies, Regeneron’s and its collaborators’ ability to continue to conduct research and clinical programs, Regeneron’s ability to manage its supply chain, net product sales of products marketed or otherwise commercialized by Regeneron and/or its collaborators or licensees (collectively, “Regeneron’s Products”), and the global economy; the nature, timing, and possible success and therapeutic applications of Regeneron’s Products and product candidates being developed by Regeneron and/or its collaborators or licensees (collectively, “Regeneron’s Product Candidates”) and research and clinical programs now underway or planned, including without limitation EYLEA® (aflibercept) Injection, Dupixent® (dupilumab), Libtayo® (cemiplimab), Praluent® (alirocumab), Kevzara® (sarilumab), Evkeeza® (evinacumab), Inmazeb® (atoltivimab, maftivimab, and odesivimab-ebgn), aflibercept 8 mg, pozelimab, odronextamab, itepekimab, fianlimab, garetosmab, linvoseltamab, REGN5713-5714-5715, Regeneron’s other oncology programs (including its costimulatory bispecific portfolio), Regeneron’s and its collaborators’ earlier-stage programs, and the use of human genetics in Regeneron’s research programs; the likelihood and timing of achieving any of Regeneron’s anticipated development and production milestones; safety issues resulting from the administration of Regeneron’s Products and Regeneron’s Product Candidates in patients, including serious complications or side effects in connection with the use of Regeneron’s Products and Regeneron’s Product Candidates in clinical trials; the likelihood, timing, and scope of possible regulatory approval and commercial launch of Regeneron’s Product Candidates and new indications for Regeneron’s Products, including without limitation those listed above; the extent to which the results from the research and development programs conducted by Regeneron and/or its collaborators may be replicated in other studies and/or lead to advancement of product candidates to clinical trials, therapeutic applications, or regulatory approval; ongoing regulatory obligations and oversight impacting Regeneron’s Products, research and clinical programs, and business, including those relating to patient privacy; determinations by regulatory and administrative governmental authorities which may delay or restrict Regeneron’s ability to continue to develop or commercialize Regeneron’s Products and Regeneron’s Product Candidates; competing drugs and product candidates that may be superior to, or more effective than, Regeneron’s Products and Regeneron’s Product Candidates; uncertainty of market acceptance and commercial success of Regeneron’s Products and Regeneron’s Product Candidates and the impact of studies (whether conducted by Regeneron or others and whether mandated or voluntary) or recommendations and guidelines from governmental authorities and other third parties on the commercial success of Regeneron’s Products and Regeneron’s Product Candidates; the ability of Regeneron to manufacture and manage supply chains for multiple products and product candidates; the ability of Regeneron’s collaborators, licensees, suppliers, or other third parties (as applicable) to perform manufacturing, filling, finishing, packaging, labeling, distribution, and other steps related to Regeneron’s Products and Regeneron’s Product Candidates; the availability and extent of reimbursement of Regeneron’s Products from third-party payors, including private payor healthcare and insurance programs, health maintenance organizations, pharmacy benefit management companies, and government programs such as Medicare and Medicaid; coverage and reimbursement determinations by such payors and new policies and procedures adopted by such payors; unanticipated expenses; the costs of developing, producing, and selling products; the ability of Regeneron to meet any of its financial projections or guidance, and changes to the assumptions underlying those projections or guidance; the potential for any license or collaboration agreement, including Regeneron’s agreements with Sanofi and Bayer (or their respective affiliated companies, as applicable) to be cancelled or terminated; and risks associated with intellectual property of other parties and pending or future litigation relating thereto (including without limitation the patent litigation and other related proceedings relating to EYLEA, Praluent, and REGEN-COV® (casirivimab and imdevimab)), other litigation and other proceedings and government investigations relating to the Company and/or its operations,
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 115 |
the ultimate outcome of any such proceedings and investigations, and the impact any of the foregoing may have on Regeneron’s business, prospects, operating results, and financial condition. A more complete description of these and other material risks can be found in Regeneron’s filings with the U.S. Securities and Exchange Commission, including its Form 10-K for the fiscal year ended December 31, 2022, including in the section thereof captioned “Item 1A. Risk Factors.” Any forward-looking statements are made based on management’s current beliefs and judgment, and the reader is cautioned not to rely on any forward-looking statements made by Regeneron. Regeneron does not undertake any obligation to update (publicly or otherwise) any forward-looking statement, whether as a result of new information, future events, or otherwise.
This proxy statement uses non-GAAP net income, non-GAAP net income per share, and total revenues excluding REGEN-COV and RonapreveTM, which are financial measures that are not calculated in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). These non-GAAP financial measures are computed by excluding certain non-cash and other items from the related GAAP financial measure. Non-GAAP adjustments also include the estimated income tax effect of reconciling items. The Company makes such adjustments for items the Company does not view as useful in evaluating its operating performance. For example, adjustments may be made for items that fluctuate from period to period based on factors that are not within the Company’s control (such as the Company’s stock price on the dates share-based grants are issued) or items that are not associated with normal, recurring operations (such as changes in applicable laws and regulations). Management uses these non-GAAP measures for planning, budgeting, forecasting, assessing historical performance, and making financial and operational decisions, and also provides forecasts to investors on this basis. Additionally, such non-GAAP measures provide investors with an enhanced understanding of the financial performance of the Company’s core business operations. However, there are limitations in the use of these and other non-GAAP financial measures as they exclude certain expenses that are recurring in nature. Furthermore, the Company’s non-GAAP financial measures may not be comparable with non-GAAP information provided by other companies. Any non-GAAP financial measure presented by Regeneron should be considered supplemental to, and not a substitute for, measures of financial performance prepared in accordance with GAAP. A reconciliation of the Company’s historical GAAP to non-GAAP results is included below.
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 116 |
Reconciliation of GAAP to Non-GAAP Financial Information (Unaudited) (In millions, except per share data)
|
Year Ended December 31, |
|
2022 |
||
GAAP R&D |
$ 3,592.5 |
|
R&D: Stock-based compensation expense |
406.8 |
|
R&D: Acquisition-related integration costs |
17.0 |
|
Non-GAAP R&D |
$ 3,168.7 |
|
GAAP SG&A |
$ 2,115.9 |
|
SG&A: Stock-based compensation expense |
256.4 |
|
SG&A: Acquisition-related integration costs and other |
6.6 |
|
Non-GAAP SG&A |
$ 1,852.9 |
|
GAAP COGS |
$ 800.0 |
|
COGS: Stock-based compensation expense |
61.8 |
|
COGS: Intangible asset amortization expense |
34.8 |
|
COGS: Charges related to REGEN-COV |
196.6 |
|
Non-GAAP COGS |
$ 506.8 |
|
GAAP other income (expense), net |
$ 119.9 |
|
Other income/expense: (Gains) losses on investments |
36.8 |
|
Non-GAAP other income (expense), net |
$ 156.7 |
|
GAAP net income |
$ 4,338.4 |
|
Total of GAAP to non-GAAP reconciling items above |
1,016.8 |
|
Income tax effect of GAAP to non-GAAP reconciling items |
(191.3) |
|
Non-GAAP net income |
$ 5,163.9 |
|
Non-GAAP net income per share - basic |
$ 48.22 |
|
Non-GAAP net income per share - diluted
|
$ 44.98 |
|
Shares used in calculating: |
|
|
GAAP net income per share - basic |
107.1 |
|
GAAP net income per share - diluted |
113.5 |
|
Non-GAAP net income per share - basic |
107.1 |
|
Non-GAAP net income per share - diluted |
114.8 |
|
|
Year Ended December 31, |
|||
2022 |
2021 |
|||
Revenue reconciliation: |
|
|
|
|
Total revenues |
$12,172.9 |
|
$16,071.7 |
|
REGEN-COV net product sales in the United States |
— |
|
5,828.0 |
|
Global gross profit payment from Roche in connection with sales of Ronapreve |
627.3 |
|
361.8 |
|
Total revenues excluding REGEN-COV and Ronapreve |
$ 11,545.6 |
|
$ 9,881.9 |
|
| 2023 PROXY STATEMENT AND NOTICE OF ANNUAL SHAREHOLDER MEETING |  |
/ 117 |


