SCHEDULE 14A
(Rule 14a-101)
INFORMATION REQUIRED IN PROXY STATEMENT
SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934 (Amendment No. )
Filed by the Registrant x
Filed by a Party other than the Registrant o
Check the appropriate box:
| o | Preliminary Proxy Statement |
| o | Soliciting Material Under Rule 14a-12 |
| o |
Confidential, For Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| o | Definitive Proxy Statement |
| x | Definitive Additional Materials |
Regeneron Pharmaceuticals, Inc.
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if Other Than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| x | No fee required. | |
| o | Fee computed on table below per Exchange Act Rules 14a-6(i)(4) and 0-11. | |
| 1) | Title of each class of securities to which transaction applies:
| |
| 2) |
Aggregate number of securities to which transaction applies:
| |
| 3) |
Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined):
| |
| 4) |
Proposed maximum aggregate value of transaction:
| |
| 5) |
Total fee paid:
| |
| o | Fee paid previously with preliminary materials: | |
| o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the form or schedule and the date of its filing. | |
| 1) |
Amount previously paid:
| |
| 2) |
Form, Schedule or Registration Statement No.:
| |
| 3) | Filing Party:
| |
| 4) | Dated Filed:
| |

MORE SCIENCE, MORE IMPACT ANNUAL REPORT 2019

DEAR FELLOW SHAREHOLDERS,
As 2020 began, the world was presented with a major crisis as the SARS-CoV-2 (COVID-19) pandemic spread across the globe. Regeneron mobilized quickly, realizing that we were uniquely suited to bring forward potential solutions. We are extremely proud of how our talented team is responding to this public health challenge. Given our more than 30 years of investment in core technologies that improve the drug discovery and development process, and our track record of success with infectious diseases like Ebola, we are optimistic that we can make a meaningful and timely impact. We have two important COVID-19 research and development efforts ongoing: a global clinical trial of our IL-6 inhibitor Kevzara® (sarilumab) in hospitalized patients with severe or critical COVID-19; and the development of a novel antibody cocktail specifically designed to prevent or treat COVID-19 infection. We will be sharing updates on these programs as quickly as possible over the coming weeks and months.
Now, more than ever, we must remain focused on our mission to repeatedly bring important new medicines to patients with serious diseases. In 2019 we had a year of strong performance and delivery on this mission. We reached more and more patients through newly approved indications and record growth for our blockbuster treatments
02 Regeneron / Annual Report 2019

OUR BUSINESS IS SOLID, AND 2019 WAS A YEAR OF TREMENDOUS PROGRESS FOR REGENERON
EYLEA® (aflibercept) Injection and Dupixent® (dupilumab), made significant advancements throughout our preclinical and clinical pipelines, and delivered a public health breakthrough with an effective treatment for Ebola which is currently under review by the U.S. Food and Drug Administration (FDA).
Total revenues for 2019 were $7.9 billion, a 17 percent increase over 2018, which included U.S. EYLEA net product sales of $4.6 billion. Our collaborator Bayer recorded net product sales for EYLEA outside the U.S. of $2.9 billion, bringing EYLEA’s total global net product sales to $7.5 billion, a 12 percent increase compared to 2018. These figures show the continuing strength of this important treatment that has had double-digit sales growth for seven years without a single price increase. We remain confident in EYLEA’s ability to help even more patients as we expand our leadership position in wet age-related macular degeneration and diabetic eye diseases. Dupixent 2019 global net product sales, which are recorded by Sanofi, were $2.3 billion, an increase of 151 percent over the previous year. We have just begun to tap the potential of this first-in-class treatment option for several Type 2 inflammatory diseases, with many more studies underway.
In part due to Dupixent’s strong sales, our antibody collaboration with Sanofi became profitable for the first time in 2019, and we took important additional steps to strengthen Regeneron’s financial position. In the second quarter of 2020 we restructured our antibody collaboration with Sanofi to enhance profitability further and to simplify the commercial strategy for Praluent® (alirocumab). We will continue to collaborate with Sanofi on studying Kevzara for COVID-19, with Regeneron leading U.S.-based development and Sanofi leading development outside of the U.S.
Meanwhile, our research and development productivity continues, with notable progress in our growing immuno-oncology portfolio and diversification across the pipeline as a whole. We expanded our clinical program with Libtayo® (cemiplimab- rwlc), a PD-1 inhibitor, and moved
03 Regeneron / Annual Report 2019

multiple bispecific antibodies into the clinic, including the first in a whole new class of co-stimulatory bispecifics, which position us to become a leader in this emerging field. We continue to explore Dupixent in a variety of additional Type 2 inflammatory conditions, with late-stage trials underway in eosinophilic esophagitis, chronic obstructive pulmonary disease, prurigo nodularis and chronic spontaneous urticaria, as well as earlier studies in grass and food allergies.
From a public health perspective, we made a breakthrough in the fight against the devastating Ebola outbreak in the Democratic Republic of the Congo (DRC) with REGN-EB3’s impressive reduction in mortality compared to the prior standard- of-care in the PALM clinical trial. We are applying the same technologies against the novel coronavirus and hope for similar success.
All of this important research and groundbreaking medicine is built on three decades of investment in our VelociSuite® technologies. These proprietary end-to-end drug discovery and development tools allow us to quickly identify multiple antibody candidates against diseases—from Ebola to cancer to asthma to rare diseases, such as fibrodysplasia ossificans progressiva. Paired with our excellent clinical development and manufacturing capabilities, the possibilities are truly endless, and we feel that we are just at the beginning of what we can do using the power of science and technology.
Our commitment to continually advancing research through sophisticated and broadly applicable technology propelled our Regeneron Genetics Center® team to the major milestone of sequencing the exomes of over one million people as of
20+ approved and investigational medicines built using the power of VelociSuite
04 Regeneron / Annual Report 2019

February 2020. We are also applying our genetics and biology expertise to explore new modalities that are complementary to our world-class therapeutic antibodies. Key examples include our preclinical work in viral vector and gene therapy technologies, as well as ongoing collaborations with organizations who bring unique expertise in areas like gene silencing, gene editing and CAR-Ts.
In 2020 and beyond, we will continue to reinvest a significant portion of our growing revenue into our R&D efforts as we believe our scientific innovation and talent are our greatest differentiators. As we look toward the future of Regeneron we have begun to evaluate ex-U.S. commercialization opportunities, starting with exercising our co-commercialization rights for Dupixent in certain countries outside the U.S.
2019 was a busy and successful year for Regeneron, and we believe 2020 will be even more impactful. We will innovate against COVID-19 and the other serious diseases that continue to impact lives, even during a time of pandemic. We have our sights firmly set on the future as we expand the types of ailments we can treat and number of people we can help. The world needs us and the power of science, more than ever.
Sincerely,
Roy, Len, George
LEONARD S. SCHLEIFER M.D., Ph.D. Co-Founder, President and Chief Executive Officer
GEORGE D. YANCOPOULOS M.D., Ph.D. Co-Founder, President and Chief Scientific Officer
P. ROY VAGELOS M.D. Chairman of the Board
05 Regeneron / Annual Report 2019

2019 BY THE NUMBERS 13 U.S. and European regulatory approvals
13 U.S. and European regulatory approvals
80 patient advocacy and professional societies engaged across 20 disease states
30M doses of EYLEA administered from launch through early 2020
53 countries in which we conducted trials
20+ investigational medicines in clinical development
8,400+ patients enrolled in clinical trials
1M people sequenced by the Regeneron Genetics Center (as of February 2020)
27,800+ colleague volunteer hours
1 of 4 biotechs included on Dow Jones Sustainability World Index
06 Regeneron / Annual Report 2019

TABLE OF CONTENTS
25 CUTTING-EDGE RESEARCH AND TECHNOLOGY
30 SCIENCE-ENABLING BUSINESS STRATEGIES
08 SCIENTIFIC BREADTH AND DEPTH
33 RESPONSIBLE REGENERON

SCIENTIFIC BREADTH AND DEPTH
Our Approved Medicines 09
Robust Clinical Pipeline 10
Recent Publication Highlights 11
Excellence in Retinal Diseases 13
Broad Development in Inflammation and Allergic Diseases 15
Diverse and Flexible Immuno-oncology Approach 18
Rapid Response Approach in Infectious Diseases 21
Rare Disease and Pain Programs 24

7 REGENERON-DISCOVERED, FDA-APPROVED MEDICINES
1. Commercialized by Bayer ex-U.S.
2. Developed and commercialized under global collaboration with Sanofi
3. Commercialized by Sanofi ex-U.S., effective April 1, 2020
4. Commercialized exclusively by Sanofi
Please refer to Regeneron.com for more information on our marketed medicines, including full safety information.
09 Regeneron / Annual Report 2019
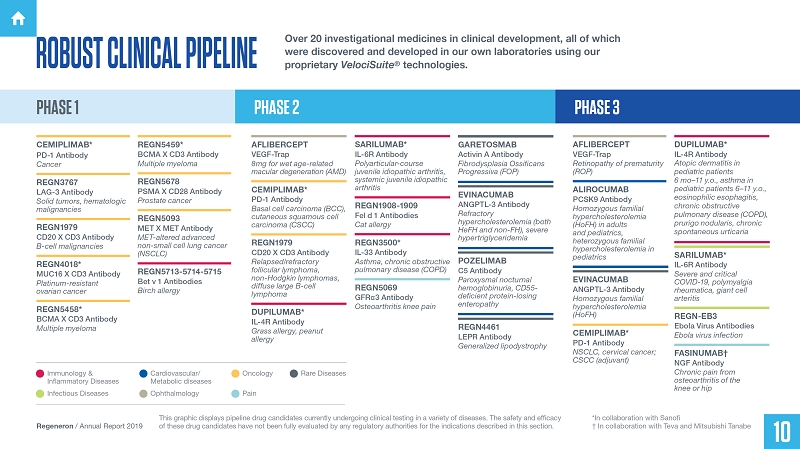
ROBUST CLINICAL PIPELINE
Over 20 investigational medicines in clinical development, all of which were discovered and developed in our own laboratories using our proprietary VelociSuite® technologies.
PHASE 1 PHASE 2 PHASE 3
CEMIPLIMAB* PD-1 Antibody Cancer
REGN3767 LAG-3 Antibody Solid tumors, hematologic malignancies
REGN3767 LAG-3 Antibody Solid tumors, hematologic malignancies
REGN1979 CD20 X CD3 Antibody B-cell malignancies
REGN4018* MUC16 X CD3 Antibody Platinum-resistant ovarian cancer
REGN5458* BCMA X CD3 Antibody Multiple myeloma
REGN5459* BCMA X CD3 Antibody Multiple myeloma
REGN5678 PSMA X CD28 Antibody Prostate cancer
REGN5093 MET X MET Antibody MET-altered advanced non-small cell lung cancer (NSCLC)
REGN5713-5714-5715 Bet v 1 Antibodies Birch allergy
AFLIBERCEPT VEGF-Trap 8mg for wet age-related macular degeneration (AMD)
CEMIPLIMAB* PD-1 Antibody Basal cell carcinoma (BCC), cutaneous squamous cell carcinoma (CSCC)
REGN1979 CD20 X CD3 Antibody Relapsed/refractory follicular lymphoma, non-Hodgkin lymphomas, diffuse large B-cell lymphoma
DUPILUMAB* IL-4R Antibody Grass allergy, peanut allergy
SARILUMAB* IL-6R Antibody Polyarticular-course juvenile idiopathic arthritis, systemic juvenile diopathic arthritis
REGN1908-1909 Fel d 1 Antibodies Cat allergy
REGN3500* IL-33 Antibody Asthma, chronic obstructive pulmonary disease (COPD)
REGN5069 GFRα3 Antibody Osteoarthritis knee pain
GARETOSMAB Activin A Antibody Fibrodysplasia Ossificans Progressiva (FOP)
EVINACUMAB ANGPTL-3 Antibody Refractory hypercholesterolemia (both HeFH and non-FH), severe hypertriglyceridemia
POZELIMAB C5 Antibody Paroxysmal nocturnal hemoglobinuria, CD55-deficient protein-losing enteropathy
REGN4461 LEPR Antibody Generalized lipodystrophy
Immunology & Inflammatory Diseases
Infectious Diseases Cardiovascular/Metabolic diseases Ophthalmology Rare Diseases Oncology Pain
This graphic displays pipeline drug candidates currently undergoing clinical testing in a variety of diseases. The safety and efficacy of these drug candidates have not been fully evaluated by any regulatory authorities for the indications described in this section.
*In collaboration with Sanofi
† In collaboration with Teva and Mitsubishi Tanabe
10 Regeneron / Annual Report 2019

A RANDOMIZED, CONTROLLED TRIAL OF EBOLA VIRUS DISEASE THERAPEUTICS
PUBLICATIONS HIGHLIGHTS
EFFICACY AND SAFETY OF DUPILUMAB IN PATIENTS WITH SEVERE CHRONIC RHINOSINUSITIS WITH NASAL POLYPS
200+ peer-reviewed scientific publications in 2019
~90 citations of data generated from the Regeneron Genetics Center
DUAL BLOCKADE OF IL-4 AND IL-13 WITH DUPILUMAB, AN IL-4Rα± ANTIBODY, IS REQUIRED TO ROADLY INHIBIT TYPE 2 INFLAMMATION
11 Regeneron / Annual Report 2019
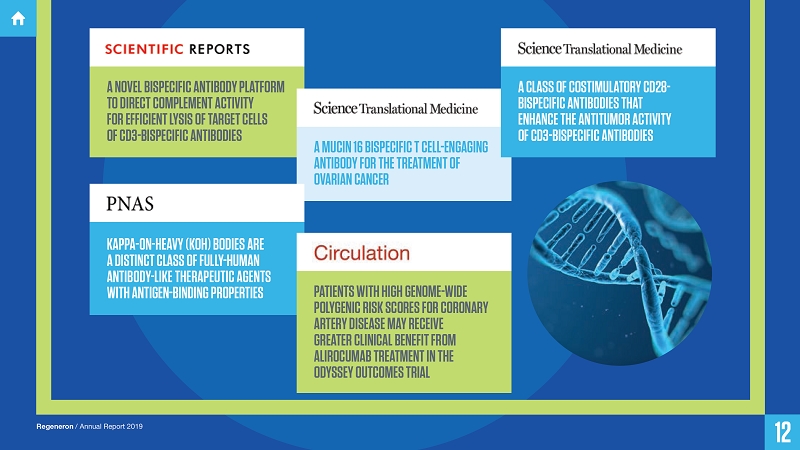
A NOVEL BISPECIFIC ANTIBODY PLATFORM TO DIRECT COMPLEMENT ACTIVITY FOR EFFICIENT LYSIS OF TARGET CELLS OF CD3-BISPECIFIC ANTIBODIES
A MUCIN 16 BISPECIFIC T CELL-ENGAGING ANTIBODY FOR THE TREATMENT OF OVARIAN CANCER
KAPPA-ON-HEAVY (KOH) BODIES ARE A DISTINCT CLASS OF FULLY-HUMAN ANTIBODY-LIKE THERAPEUTIC AGENTS WITH ANTIGEN-BINDING PROPERTIES
A CLASS OF COSTIMULATORY CD28-BISPECIFIC ANTIBODIES THAT ENHANCE THE ANTITUMOR ACTIVITY OF CD3-BISPECIFIC ANTIBODIES
PATIENTS WITH HIGH GENOME-WIDE POLYGENIC RISK SCORES FOR CORONARY ARTERY DISEASE MAY RECEIVE GREATER CLINICAL BENEFIT FROM ALIROCUMAB TREATMENT IN THE ODYSSEY OUTCOMES TRIAL
12 Regeneron / Annual Report 2019

EXCELLENCE IN RETINAL DISEASE EYLEA® (AFLIBERCEPT)
Help people protect and regain their vision. That was our goal when EYLEA was first approved, and it continues to drive our work nine years later. In 2019, EYLEA continued its run as the number one prescribed FDA-approved anti-VEGF treatment across its approved indications in the U.S. and finished the year with a total of $7.5 billion in annual global net product sales.1
Our focus on continually enhancing the value of EYLEA, for both physicians and patients, underpins its growth and is reflected in the multiple FDA approvals we received in 2019. 2 Among them were the approval and launch of the EYLEA pre-filled syringe, which gives physicians a more convenient and efficient way to administer EYLEA to patients, and the approval of EYLEA in diabetic retinopathy, a major milestone that extends the benefits of EYLEA to many additional patients.
The initial uptake of EYLEA for diabetic retinopathy has been strong, with positive feedback from ophthalmologists. Yet tremendous unmet need remains. Too often, diabetic retinopathy is undiagnosed and untreated due to a lack of visual symptoms in early stages, putting patients’ vision at risk. In the Phase 3 PANORAMA trial, EYLEA was shown to reduce the risk of vision threatening complications from Proliferative Diabetic Retinopathy or Anterior Segment Neovascularization, as well as center-involved Diabetic Macular Edema, compared to placebo, findings that underscore our efforts to advocate for proactive treatment and increased diabetic retinopathy screenings.
Through our clinical research, we are also preparing for our next chapter in ophthalmology innovation with an ongoing Phase 3 trial in retinopathy of prematurity. In addition, we expect to start two Phase 3 trials of high-dose EYLEA to evaluate 12-week or longer dosing intervals in wet AMD (PULSAR, sponsored by Bayer) and DME (PHOTON, sponsored by Regeneron).
EYLEA 2019 REGULATORY HIGHLIGHTS
• FDA approval for diabetic retinopathy
• FDA approval and launch of pre-filled syringe
1. Bayer records net product sales of EYLEA outside the U.S.
2. Sanofi records global net product sales of Dupixent
13 Regeneron / Annual Report 2019

THE PEOPLE WHO INSPIRE US
OUR HARD-WORKING IOPS TEAM
The pre-filled syringe for EYLEA was an eye-opening project for the Industrial Operations and Product Supply (IOPS) team.
“It was important for us to get EYLEA in a pre- filled syringe on the market so that doctors could dose patients more efficiently with fewer preparatory steps,” said Amy Walsh, Senior Director, Drug Product Manufacturing & Project Management in IOPS. “We also recognized that this pre-filled syringe could become the standard way EYLEA is administered to patients, so we challenged ourselves to examine closely each step of the manufacturing process to be able to deliver the safest and highest-quality device.”
For example, “after much research and discussion with the FDA around sterilization requirements, we opted to use vaporized hydrogen peroxide for our sterilization—even though we’d have to build our sterilization process from scratch. We believed the gentler approach may be a better fit than the more common ethylene oxide approach.”
Developing this new process meant finding new solutions and building equipment and methods that didn’t exist before. This included working with vendors to develop and source new machines, as well as coordinating across numerous internal and external teams to implement this process. After much hard work, the pre-filled syringe was approved by the FDA in August 2019. “It’s rewarding that all of our effort has made it simpler for doctors to treat their patients,” said Amy. “When we first learned it was approved, my reaction was happiness and pride in our team. We are proud that we developed this product form and of the impact it can have on clinical care. I’m thankful to work with such a great group of people!”
14 Regeneron / Annual Report 2019

BROAD DEVELOPMENT IN INFLAMMATION AND ALLERGIC DISEASES
DUPIXENT® (DUPILUMAB)
The unique ability of Dupixent to reduce excessive Type 2 inflammation continues to transform the treatment paradigms of several allergic and inflammatory diseases. With hundreds of thousands of patients now receiving treatment, Dupixent has generated impressive year- over-year growth, achieving total annual global net product sales of $2.3 billion in 2019. Yet even with approvals for several conditions and age groups, we are just beginning to realize the full potential of this innovative medicine.
In 2019, we added several new indications to Dupixent’s label, securing approvals in adolescents with moderate-to-severe atopic dermatitis (AD) and adults with chronic rhinosinusitis with nasal polyposis (CRSwNP) in the U.S., European Union (EU) and other countries around the world. We also worked diligently to extend the benefits of Dupixent to even more children suffering from asthma and atopic dermatitis. In August, we shared positive topline results from a Phase 3 trial in children aged six through 11 with severe AD. These data formed the basis for our regulatory applications in the U.S. and EU, with an FDA priority review expected by May 2020 and a European Commission (EC) decision by the end of 2020. In addition, our Phase 3 trial in children aged six through 11 with asthma is progressing forward, with a regulatory submission to the FDA targeted by 2021.
Finally, we continue to broaden the scope of our Dupixent development program. In late 2019, we initiated new late-stage trials in prurigo nodularis and chronic spontaneous urticaria. These add to our ongoing clinical trials in chronic obstructive pulmonary disease (COPD), peanut allergy and grass allergy, as well as in eosinophilic esophagitis.
DUPIXENT 2019 REGULATORY HIGHLIGHTS
• FDA and EC approvals for adolescents (ages 12-17) with moderate-to-severe AD
• Supplemental Biologics License Application (sBLA) and Marketing Authorization Application (MAA) submitted for 300 mg pre-filled pen; EC approval for 200 mg and 300 mg pre-filled pen (response to FDA complete response letter for 200 mg in progress)
• FDA and EC approval for CRSwNP (via Priority Review)
• EC approval for adults and adolescents with severe asthma
• Submitted sBLA for children ≥6 to <12 years with moderate-to-severe AD
15 Regeneron / Annual Report 2019

THE PEOPLE WHO INSPIRE US
PATIENTS AND THEIR FAMILIES
Jake, an 18-year-old from San Diego, CA, was diagnosed with severe atopic dermatitis (AD) when he was just six-months old.
During his childhood, symptoms of the disease covered up to 80 percent of his body, resulting in bloody arms and scars from constant scratching. Despite trying natural and prescription standard- of-care therapies, Jake’s AD persisted as he grew older, impacting his daily life. As he put it, “My AD kept me from feeling like a ‘normal’ kid my age.” Jake, who loves to play lacrosse, run and surf, gave up many of his hobbies and opted to wear long-sleeved shirts and long pants despite the California sun.
“There are many common misconceptions about AD,” said Jake. “People don’t think that my AD is a serious disease, but it is. Some people think that it’s related to poor hygiene or it’s contagious – and it’s neither of those things. It’s an inflammatory disease caused in part by a hypersensitive immune system. Even when I don’t have a visible rash, the inflammation is still active under my skin.”
Determined not to let AD take control of their son’s life, Jake’s parents, Jen and Tom, found a pediatric dermatologist who was enrolling patients in a clinical trial for an investigational biologic aimed at treating the underlying causes of AD. During the trial, Jake noticed his skin “became clear and the wounds turned to scars.” Today, Jake’s AD is under control, and he is feeling more confident. He is attending college in California and even chronicled his experience with AD in his college application essays.
16 Regeneron / Annual Report 2019

Building on the expertise established with Dupixent, we have made significant strides in investigating the potential of new approaches to treating allergic and inflammatory diseases:
REGN3500 REGN1908-1909 REGN5713-5714-5715
REGN3500 is an IL-33 antibody in Phase 2 trials for asthma and COPD. In June 2019, we announced, with our collaborator Sanofi, that our Phase 2 proof- of-concept trial in asthma had met its primary and secondary endpoints, improving loss of asthma control and lung function compared to placebo. Although the Phase 2 trial in COPD patients did not meet its primary endpoint, the company and Sanofi are reviewing the data further to determine who may yet benefit from this new potential drug.
Cat allergy is a major source of indoor allergies and a main risk factor for asthma. With limited treatments available, an estimated 500,000 Americans pursue laborious allergy desensitization, which can be difficult to tolerate and can take years to achieve significant effects. Our ongoing Phase 2 trial of REGN1908-1909, a Fel d 1 antibody, aims to provide relief, specifically to people who experience cat allergen-triggered asthma. In early studies, the investigational compound has been shown to markedly improve symptoms, with responses to treatment lasting at least one month.
Our Bet v 1 combination therapy, also known as REGN5713-5714-5715, is our latest investigational medicine to enter the clinic and is currently being studied in a Phase 1 trial for birch allergy.
17 Regeneron / Annual Report 2019

LIBTAYO 2019 REGULATORY HIGHLIGHTS
• Conditional EC approval for advanced CSCC
DIVERSE AND FLEXIBLE IMMUNOONCOLOGY APPROACH
LIBTAYO® (CEMIPLIMAB-RWLC)
In 2019, our PD-1 inhibitor Libtayo became the standard of care for advanced cutaneous squamous cell carcinoma (CSCC) generating global net product sales of $194 million.1 The rapid transformation of this treatment paradigm occurred in less than a year, underscoring the significant unmet need in advanced CSCC that Libtayo is addressing.
Our pivotal Phase 2 trial in advanced CSCC continues to show meaningful and durable clinical outcomes year after year. We are now exploring the ability of Libtayo to help patients with earlier stages of the disease, starting with a Phase 3 trial in adjuvant CSCC initiated in late 2019. In addition, our ongoing potentially registrational trials investigating Libtayo in cervical cancer, basal cell cancer (BCC) and non-small cell cancer (NSCLC) have made significant progress. Specifically, interim data from our Phase 3 trial in NSCLC showed positive early objective response rate results and our BCC trial is expected to report topline data in 2020.
Libtayo also continues to serve as the backbone for multiple investigational combination therapies both internally and with external collaborators. At the end of 2019, we had signed clinical collaborations and drug supply agreements with 10 companies, pairing Libtayo with a diverse range of novel cancer approaches that include oncolytic viruses, vaccines and gene therapies, among others.
1. Sanofi records net product sales of Libtayo outside the U.S.
18 Regeneron / Annual Report 2019

THE PEOPLE WHO INSPIRE US
PATIENTS AND THEIR FAMILIES
Unfortunately, Cathy was no stranger to skin cancer. Over the course of several decades, she had undergone multiple surgeries to remove growths of cutaneous squamous cell carcinoma (CSCC) on her head and upper body.
But when one of the growths of CSCC on her scalp penetrated her skull and the lining of her brain and aggressively returned despite major surgery, Cathy began to lose hope. Her doctor recommended a course of radiation over several weeks. However, being highly claustrophobic, Cathy did not think she could endure the confinement required for treatment, especially since the doctor did not think it would be curative. “I was ready to give up,” recalls Cathy. “I couldn’t bear the idea of radiation and there were just no other options. My husband and I decided to call our children to break the news. It was one of the hardest things I’ve ever done.”
One of her children, Lorah, happened to be a Regeneron Ph.D. scientist, and when she received the call, she told her mother there was actually one other option to consider: a FDA-approved immunotherapy medicine from Regeneron. “After reading the clinical trial results, I felt it was a good option for my mom given the situation,” Lorah said. Following a broader family conversation, Cathy discussed further with her doctor, and they decided to move forward.
“It was a new experience for everyone,” said Cathy. “I was the first person my doctor had ever treated with this medicine and also the first patient with metastatic CSCC to receive an immunotherapy at his hospital. So the day the doctor confirmed that my metastatic CSCC was no longer visible in my scan was momentous for everybody—it seemed like the whole hospital celebrated with me.” Since then, Cathy has also become a mentor to other patients with advanced CSCC through a program run by Regeneron and Sanofi.
19 Regeneron / Annual Report 2019

BISPECIFIC ANTIBODIES
Following the successful launch of Libtayo, we have continued to advance multiple investigational candidates from our bispecific antibody platform. Designed to closely resemble natural human antibodies, all of Regeneron bispecifics can bind to two different targets, opening up a diverse array of possibilities for targeting and killing cancer.
Regeneron currently has six bispecific antibodies in the clinic that fall into three categories. Our CD3-targeted bispecifics are designed to bridge cancer cells to the CD3 stimulatory receptors on T-cells, thus activating T-cell killing of cancer cells at the tumor site. Similarly, our CD28 costimulatory bispecifics also bridge cancer cells to T-cells. Designed to synergize with PD-1 inhibitors and/or CD-3 bispecifics, they costimulate T-cells via their CD28 receptors and thereby provide a signal needed to activate T cells more completely. In contrast, our third category of tumor-targeted bispecifics bind to proteins only on the cancer cell. In this way, they may affect various signaling pathways to hamper the cancer cells’ ability to survive and proliferate. BISPECIFIC ANTIBODIES
In 2019, we saw progress across all three categories of Regeneron bispecifics. Our first CD3 bispecific to enter the clinic, REGN1979 (CD20xCD3) continued to show positive results in patients with late-stage lymphomas, including in patients whose tumors did not respond to CAR-T therapy. In addition, we shared the first encouraging data from a second CD3 bispecific REGN5458 (BCMAxCD3) in multiple myeloma. We also initiated Phase 1/2 clinical trials for our first CD28 costimulatory bispecific, REGN5678 (PSMAxCD28), in prostate cancer and our first tumor-targeted bispecific, REGN5093 (METxMET), in MET-altered non-small cell lung cancer.
20 Regeneron / Annual Report 2019

RAPID RESPONSE IN INFECTIOUS DISEASES
REGN-EB3 FOR EBOLA
REGN-EB3 LED TO A NEARLY 90 PERCENT SURVIVAL RATE IN EBOLA PATIENTS WHO RECEIVED THE TREATMENT EARLY
One of our proudest moments of 2019 was learning that our investigational Ebola therapy, REGN-EB3, was so effective in preventing death compared to the prior antibody standard of care that the PALM clinical trial was halted early. The results, later published in The New England Journal of Medicine, found that REGN-EB3 demonstrated superior efficacy compared to the ZMapp control arm across multiple measures, including the primary endpoint of mortality at day 28 and secondary endpoint of reduction of number of days until the Ebola virus was no longer detected in the bloodstream. Given these groundbreaking results, we are working with the FDA to gain regulatory approval and with U.S. and global health authorities to determine appropriate stockpiling of REGN-EB3.
A cocktail of three antibodies, REGN-EB3 is a prime example of the power of Regeneron’s novel and proprietary VelocImmune® platform and associated VelociSuite technologies to generate and identify potential treatments rapidly, condensing a process that normally takes years into less than 12 months.
REGN-EB3 2019 REGULATORY HIGHLIGHTS
• Breakthrough Therapy designation granted by FDA
• BLA for Ebola under review with October 25, 2020 action deadline
21 Regeneron / Annual Report 2019

THE PEOPLE WHO INSPIRE US
HEALTHCARE PROVIDERS ON THE GROUND
While the city of Goma bustled around them, Drs. Patrice Kabongo and Ian Crozier sat quietly and intently focused on an infant in a small Ebola virus disease (EVD) treatment center in the Democratic Republic of Congo (DRC). Dressed head-to-toe in personal protective equipment, Crozier, an infectious diseases clinician deployed to North Kivu by the World Health Organization (WHO), held the infant, stabilizing an intravenous site tethered to an infusion bag containing Regeneron’s investigational antibody treatment. Kabongo, a physician with the DRC Ministry of Health, periodically monitored and recorded the infant’s vital signs, frequently reassuring the baby’s mother who watched anxiously nearby. She too was newly diagnosed with EVD and would soon be the second patient in Goma to receive similar treatment under an emergency use protocol enabled by the DRC and WHO. Her husband, the baby’s father, had arrived with severe illness too late to receive effective care and had died the night before; this hung heavy over the small team of healthcare workers determined the wife and infant would not meet the same fate.
“I have a particular experience of the suffering associated with this disease, much of it unfortunately at the bedsides of many patients in Western Africa who were not able to access effective supportive care and therapeutics,” said Crozier, who previously worked in Sierra Leone and has been on ground for much of the current outbreak in DRC. “While the suffering continues here, there is a new will and capacity to provide patients what they need: that bundle of care now includes specific therapeutics for EVD, and we need every tool we can get. These therapeutics were made available for compassionate use by companies like Regeneron, and global collaboration importantly enabled identification of the most effective treatments in the historic Pamoja Tulinde Maisha (PALM) randomized controlled trial.”
Among those benefiting under the team’s care were the baby and mother, who were both able to leave the Ebola Treatment Unit days later—a moment Crozier described as “truly gratifying.”
The entire Regeneron team is humbled by the dedication of healthcare workers on the frontline in fighting Ebola. Amidst risk of harm from violence and the virus, heath care workers in DRC have administered high-quality medical care that also supported a landmark clinical trial, setting an important model for future outbreaks and inspiring hope in the ongoing fight against COVID-19.
22 Regeneron / Annual Report 2019

NOVEL ANTIBODIES FOR COVID-19
With the emergence of the novel coronavirus SARS- CoV-2 in late 2019 and early 2020, the Regeneron team again responded with urgency, applying our core ‘rapid response’ technologies to identify hundreds of virus- neutralizing antibodies and selecting the most potent to move forward in therapeutic development. These novel antibodies are currently being scaled up for preclinical- and clinical-scale testing, with the goal of entering human clinical trials in June 2020. This therapeutic could be used to treat patients already infected with COVID-19 or to protect people not yet infected by the virus.
We are also investigating the potential of Kevzara, our FDA-approved medicine for moderate-to-severe rheumatoid arthritis, in hospitalized patients with severe or critical COVID-19. We are awaiting data from a Phase 2/3 clinical trial that is enrolling patients around the U.S. A separate global study is being led by our collaborator Sanofi. Kevzara is an IL-6 antibody and, based on promising data seen in China with another IL-6 inhibitor, we believe it may have efficacy in addressing the lung inflammation associated with COVID-19.
Based in large part on our experience with REGN-EB3, we are collaborating with the U.S. Department of Health and Human Services (HHS) and the Biomedical Advanced Research and Development Authority on both of these COVID-19 efforts under our existing collaboration agreement.
23 Regeneron / Annual Report 2019

RARE DISEASE AND PAIN PROGRAMS
EVINACUMAB POZELIMAB GARETOSMAB FASINUMAB
In mid-2019, we announced that our Phase 3 trial investigating evinacumab, an angiopoietin-like 3 (ANGPTL3) antibody, in patients with homozygous familial hypercholesterolemia (HoFH) met its primary endpoint. In the trial, adding evinacumab to other lipid-lowering therapies, including statins and PCSK9 inhibitors, decreased low-density lipoprotein (LDL) cholesterol by nearly 50 percent compared to lipid-lowering therapies alone. Currently, people living with HoFH, a severe, inherited form of high cholesterol, must often choose from treatment choices that can be time consuming or associated with potential side effects. With our positive Phase 3 data an FDA Breakthrough Therapy designation, we hope to offer patients a new option. We have begun a rolling BLA for evinacumab in the U.S. and plan to submit a regulatory filing in the EU in 2020.
In patients with the ultra-rare condition of paroxysmal nocturnal hemoglobinuria (PNH), genetic mutations cause the abnormal destruction of red blood cells in a process known as hemolysis. Left untreated or inadequately controlled, PNH can result in a range of chronic, sometimes life- threatening symptoms that include fatigue, shortness of breath and blood clots. Current treatments require intravenous infusion and may not adequately control the disease in approximately 50 percent of patients. With pozelimab, an antibody designed to block complement factor C5, our aim is to offer an effective and more convenient option. We saw positive initial early data from a Phase 2 trial presented in late 2019 and expect data from additional patients in 2020.
Fibrodysplasia ossificans progressive (FOP) is an extremely rare genetic disease that progressively replaces muscles, tendons and ligaments with bone, effectively locking patients into their own skeleton. After two decades of dedicated research into FOP, we reached a major milestone with the announcement of encouraging data from our first-in-human Phase 2 trial of garetosmab, an Activin A antibody. In the trial, treated patients experienced a reduction in new lesions compared to those given a placebo. These results validate our hypothesis that Activin A is responsible for the progression of FOP and informed discussions with regulatory authorities on potential submissions, the first of which is planned for 2020. Plans for a pediatric trial for this ultra-rare disease are also underway.
As the opioid crisis continues and the need for alternative chronic pain solutions remains, we are making progress with fasinumab, our nerve growth factor (NGF) antibody, for osteoarthritis pain. In 2019, we completed enrollment in our Phase 3 efficacy and safety studies. We expect to share a data update in 2020.
24 Regeneron / Annual Report 2019 24

CUTTING-EDGE RESEARCH AND TECHNOLOGY
Veloci-Bi® 26
Viral Vector and Gene Therapy Technologies 26
Gene Silencing and siRNA 26
Regeneron Genetics Center 28
Industrial Operations and Product Supply 29

VIRAL VECTOR AND GENE THERAPY TECHNOLOGIES
GENE SILENCING AND siRNA
Veloci-Bi allows us to create bispecific antibodies with no linkers or artificial sequences. As a result, our bispecifics are able to closely resemble natural human antibodies and have similar pharmacokinetics. As of early 2020, six investigational candidates developed with Veloci- Bi technology are in clinical trials for a diverse range of solid tumors and blood cancers.
Regeneron’s expertise in viral vector engineering, gene editing and DNA repair biology synergizes with our antibody technologies and gives us the ability to correct the abnormal function of genes at the heart of many genetic diseases. We have achieved proof of concept with adeno- associated virus (AAV) gene delivery of an antibody-guided enzyme-replacement therapy to potentially treat Pompe disease. We have also partnered with Decibel Therapeutics, Inc. to explore two other AAV-gene therapies, one designed to correct severe balance impairment and one intended to correct otoferlin deficiency, a cause of congenital deafness. Additionally, we are collaborating with Intellia Therapeutics, Inc. to tap the power of their high-efficiency CRISPR/Cas9 gene editing platform and have achieved proof of concept for several programs, including a Factor 9 gene insertion program in hemophilia B.
The underlying cause of many diseases can be traced back to the faulty functioning of one or more genes. Through our collaboration with Alnylam Pharmaceuticals, Inc., we are exploring innovative approaches to turn off, or silence, such genes using short interfering RNA (siRNA).
26 Regeneron / Annual Report 2019

THE PEOPLE WHO INSPIRE US
OUR SCIENCE-FOCUSED COLLABORATORS
What happens when two like-minded and innovation-focused companies bring their expertise together on a novel challenge? Beautiful science that we hope will lead to life-changing medicine.
In April 2019, we announced a broad new collaboration with Alnylam to explore RNA interference in ocular, central nervous system and liver diseases. Regeneron and Alnylam share complementary R&D strength and entrepreneurial spirits, making us well-suited to push beyond the core capabilities of either organization alone.
“We have a similar mindset when it comes to learning and understanding the science. Both teams love to ask lots of questions, challenge others to think differently, and dive deep into the details, which help us uncover ideas that might otherwise be obscured. Most importantly, we all try our best to do the right thing,” said Yi Zhang, Director of Clinical Pharmacology at Regeneron and the Clinical Pharmacology lead for the C5 complement-mediated diseases team with Alnylam.
Mangala Soundar, Senior Scientist, who works on the joint CNS programs and serves as the preclinical lead for Huntington disease research at Alnylam, agreed. “Working with Regeneron has opened doors to phenomenal capabilities and allowed us to expand our reach. We complement each other in a lot of ways and together make a stronger team,” she said.
Regeneron is bringing our industry-leading capabilities in human genetics and expertise in various other areas such as disease modeling technologies together with Alnylam’s pioneering work in gene silencing. Let’s see what we can do, together.
27 Regeneron / Annual Report 2019

REGENERON GENETICS CENTER
In 2019 and early 2020, our Regeneron Genetics Center celebrated two major achievements as they worked to further our understanding of the genetic underpinnings of disease and thus advance the future of medicine.
First, in mid-2019, the RGC team made the first tranche of genomic data from the UK Biobank cohort available to the global scientific research community. The batch of sequences from the first 50,000 UK Biobank participants has the potential to drive new discoveries and invites researchers from all over the world to join in this important effort. The team expects to complete sequencing for all of the 500,000 total participants by the end of 2020.
Second, as of February 2020, the RGC had sequenced the exomes of one million people, all with associated health record information. This makes the RGC the largest such genetics database in the world. From this unprecedented database, the team is generating important and actionable findings.
For example, using genetic analysis, our researchers identified a patient subgroup that may receive greater benefit from PSCK9 treatments in terms of cardiovascular events risk reduction. This finding was validated by real-world evidence in a long- running cardiovascular outcomes study. Similarly, the team used genetic evidence to help select COPD as a potential target for Regeneron’s IL-33 antibody. A variant in the HSD17B13 gene associated with reduced risk of or protection from certain chronic liver disease was first identified by RGC and Alnylam collaborators in 2018. The target is now a lead program for therapeutic intervention, with a potential RNAi therapeutic expected to enter the clinic this year.
The RGC continues to be an important and uniquely Regeneron asset, as our scientists discover new targets, inform our preclinical and clinical work and contribute to important genetic research efforts around the globe.
28 Regeneron / Annual Report 2019

INDUSTRIAL OPERATIONS AND PRODUCT SUPPLY
Our IOPS teams in Rensselaer, New York, and Raheen, Ireland, continue to maintain and advance industry- leading manufacturing, quality and supply chain practices for our clinical and commercial medicines. In 2019, the team completed seven successful global inspections and partner audits, including with the FDA and the U.S. Department of Agriculture. Our IOPS facility in Raheen became fully operational in 2019 and is now the largest biotech facility in Ireland. In recognition of their exceptional culture and commitment to continuous improvement, our IOPS Rensselaer team won the prestigious 2019 Shingo Prize for operational excellence.
In 2020, our IOPS team is demonstrating remarkable resiliency and flexibility as they enable rapidly shifting priorities and increased need for multiple products during the ongoing COVID-19 pandemic.
2019 SHINGO PRIZE
received by IOPS Rensselaer team
29 Regeneron / Annual Report 2019

SCIENCE-ENABLING BUSINESS STRATEGIES
Investing in Regeneron’s Future 31
Sanofi Collaboration Restructurings 32

INVESTING IN REGENERON’S FUTURE
As we strengthen Regeneron for the future, we are not only diversifying our portfolio through the science we pursue but also through the strategic investments we make.
Central to this approach is continuing to prioritize our investment in internal R&D capabilities. While the industry average R&D investment as a percentage of revenue hovers at approximately 20 percent, we routinely reinvest around 30 percent or more of our revenue into R&D. Our investment provides our researchers with the resources they need to gain critical insights into the underlying biology and mechanisms of diverse diseases, which fuel smarter, science-driven decisions throughout the preclinical and clinical research phases.
However, we recognize that not every area of scientific interest can be fully explored through our R&D capabilities alone. We continue to invest in collaborations with other innovative, science-minded companies in our industry. In April 2019, we announced that Alnylam and Regeneron will be working on a broad research initiative that covers the discovery, development and commercialization of RNAi therapies primarily in ocular and central nervous system disorders. A second collaboration, with Vyriad, Inc., was announced in November 2019, centered on jointly designing and validating novel Vesicular Stomatitis Virus (VSV)-based oncolytic virus treatments for diverse cancers using our VelociSuite technology. Although different in scope, these and other ongoing collaborations allow us to enhance our core capabilities, amplify our collective expertise and quickly branch into new technologies that may transform medicine.
In 2019, we also announced the initiation of a $1.0 billion share repurchase program as part of our strategic and diversified approach to capital allocation.
~30%
of annual revenue routinely reinvested into R&D
31 Regeneron / Annual Report 2019

SANOFI COLLABORATION RESTRUCTURINGS
We have collaborated with Sanofi for more than a decade, with our highly productive partnership evolving in step with our companies’ growth and development.
In January 2019, we announced the restructuring of our global Immuno-oncology Discovery and Development Agreement for new immuno-oncology cancer treatments. The revised agreement refocuses our ongoing collaboration to Libtayo and the clinical-stage BCMAxCD3 and MUC16xCD3 bispecific programs. The revised agreement allows Regeneron to retain all rights to its other immuno-oncology discovery and development programs and provides Sanofi increased flexibility to advance its early-stage immuno-oncology pipeline independently.
In addition, in the second quarter of 2020, we simplified our antibody collaboration for Praluent by restructuring into a royalty-based agreement. Sanofi gained sole ex-U.S. rights to Praluent while Regeneron gained sole U.S. rights to Praluent. Each company is now solely responsible for funding development and commercialization expenses in their respective territories. This restructuring does not impact Dupixent, and the companies will continue to collaborate on the potential use of Kevzara in COVID-19. Together with Sanofi, we agree these changes will help both companies increase efficiency and streamline operations.
32 Regeneron / Annual Report 2019

RESPONSIBLE REGENERON
Being a Responsible Corporate Citizen 34
2025 Global Responsibility Goals 37
New Environmental Targets to Help Protect and Restore the Planet 38

BEING A RESPONSIBLE CORPORATE CITIZEN
Regeneron’s mission is to use the power of science to bring new medicines to patients, over and over again. We are committed to operating responsibly, communicating transparently about our impacts and engaging all stakeholders in our mission. We strive to “do well by doing good” and have been publicly disclosing information about significant corporate responsibility matters since 2014.
For more details about our responsibility efforts and results, please refer to the 2019 Responsibility Report.
SELECT 2019 HONORS
• RobecoSAM and S&P Dow Jones Indices: Dow Jones Sustainability World Index
• Science: Top Employer
• Harvard Business Review: Best Performing CEOs
• Fast Company: Best Workplaces for Innovators
• IDEA Pharma: Pharmaceutical Invention Index
• Shingo Institute: The Shingo Prize
• Great Places to Work: Best Workplace in Ireland
• Civic 50: Most Community-Minded Companies in the U.S.
34 Regeneron / Annual Report 2019

Our responsibility strategy centers on three focus areas:
1. Improve the lives of people with serious diseases. As a science-focused company, we operate Regeneron with the long-term outlook required to turn rigorous scientific research into important new medicines. All seven of our approved medicines and all product candidates in our clinical pipeline are homegrown – discovered in Regeneron’s labs using our industry-leading, proprietary technologies. Our support for patients extends beyond the labs to disease education and awareness efforts, product support services and our commitment to drug access and responsible pricing.
2. Foster a culture of integrity and excellence. Regeneron’s culture is special and unique. Our culture includes our science-led mindset, our high ethical standards and our unbridled focus on solving big, complex problems. As we continue to grow, we remain committed to making significant investments to attract and retain top talent and facilitate the diverse and inclusive workforce we require to bring new medicines to people in need.
We are equally committed to conducting our business responsibly and ethically. This is demonstrated through the range of policies, practices and initiatives we have implemented, encompassing areas such as compliance, responsible sales and marketing, ethical clinical trials, responsible supply chain and product quality and safety.
3. Build sustainable communities. We believe that our role in creating a healthier world extends beyond creating life-transforming medicines to building a healthy living environment. In 2020, we announced a set of 2025 global responsibility goals and accompanying environmental targets, as shown on the subsequent two pages. We plan to begin reporting on our progress toward these goals in the 2020 Responsibility Report.
We also strengthen our communities through strategic philanthropic investments, product donations and the power of our employees’ talents and time. We are a long-standing supporter of science, technology, engineering and math (STEM) education, and make major philanthropic investments to inspire and celebrate future scientific innovators. These include our 10-year, $100-million commitment
35 Regeneron / Annual Report 2019

to the Regeneron Science Talent Search, the nation’s most prestigious pre-college science and mathematics competition, and our new five-year, $24-million commitment to the Regeneron International Science and Engineering Fair, the world’s largest pre-college science and engineering competition. In 2019, we also launched the Regeneron DNA Learning Center, a program of Cold Spring Harbor Laboratory. Our investments in STEM education represented approximately 86 percent of our 2019 corporate philanthropy grants, excluding medical grants and matched funds.
Also in 2019, 59 percent of Regeneron worldwide employees donated more than 27,800 hours to local non- profit organizations through our volunteer programs. This is in the top-quartile of corporate volunteer participation rates, and well above the 33 percent corporate average rate, according to a benchmarking study published in 2019 by Chief Executives for Corporate Purpose. Among our volunteer activities, we held our third annual Day for Doing Good, a company-wide day of service that had a record-high 57 percent employee participation.
IN 2019, 59 PERCENT OF REGENERON WORLDWIDE EMPLOYEES DONATED MORE THAN 27,800 HOURS OF THEIR TIME
36 Regeneron / Annual Report 2019

REGENERON’S 2025 RESPONSIBILITY GOALS
In 2019, we set global 2025 responsibility goals, which span across these three focus areas and the environmental and social issues that we believe are most significant to our business and stakeholders.
FOSTER A CULTURE OF INTEGRITY AND EXCELLENCE
Cultivate a leading workplace experience that is rooted in our unique science-driven culture.
Increase representation of qualified diverse individuals in leadership and foster inclusion across our organization.
Be vigilant in ensuring integrity remains at the core of how we operate.
Implement continuous improvements to uphold our high-quality, safe and reliable product supply.
Make Regeneron the safest part of people’s day by focusing on prevention in our drive towards zero incidents.
IMPROVE THE LIVES OF PEOPLE WITH SERIOUS DISEASES
Use the power of science to discover and advance important new medicines while continuing to make substantial investments into R&D.
Identify genetic insights that will support the discovery and advancement of tomorrow’s medicines through our Regeneron Genetics Center® .
Set fair, value-based prices for our medicines and break down barriers to patient access.
Support organizations that offer disease prevention, diagnosis and treatment for people touched by serious diseases.
BUILD SUSTAINABLE COMMUNITIES
Achieve our environmental targets to help protect and restore the planet.*
Foster the next generation of scientific innovators by providing STEM experiences to 2.5 million students.
Drive employee volunteer levels above national standards.
*See next page for specific environment targets.
37 Regeneron / Annual Report 2019

NEW ENVIRONMENTAL TARGETS TO HELP PROTECT AND RESTORE THE PLANET
WATER
WASTE
ENERGY & EMISSIONS
By 2021, achieve zero waste to landfill status at all Regeneron sites.**
By 2021, compost food waste at all sites with more than 2,000 employees.
By 2021, engage our top 30 suppliers, representing more than 50% of spend, to gather and report relevant Scope 3 greenhouse gas (GHG) emissions data.
By 2023, set global science-based targets for Scope 1 and 2 GHG emissions.
By 2025, improve water efficiencies by implementing global water mapping strategy and water stewardship program.
By 2025, develop and implement waste management plans to further increase our plastic recycling and reduce hazardous waste generation.
By 2025, match 50% of our electricity consumption with electricity from certified renewable energy sources.
By 2025, invest in the production of renewable power to meet our long-term electricity needs.
By 2025, reduce combined Scope 1 & 2 (market-based) GHG emissions per square meter by 30% based on 2016 peak baseline.
By 2035, match 100% of our electricity consumption with electricity from certified renewable energy sources.
**Excludes construction and demolition waste
2021 2025 2023 2035
38 Regeneron / Annual Report 2019
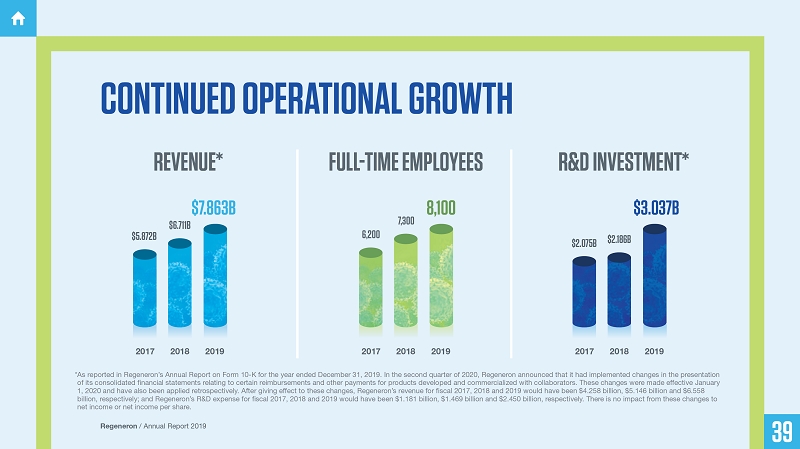
CONTINUED OPERATIONAL GROWTH
REVENUE* FULL-TIME EMPLOYEES R&D INVESTMENT*
$7.863B
$6.711B
$5.872B
2017 2018 2019
8,100
7,300
6,200
2017 2018 2019
$3.037B
$2.186B
$2.075B
2017 2018 2019
* As reported in Regeneron’s Annual Report on Form 10-K for the year ended December 31, 2019. In the second quarter of 2020, Regeneron announced that it had implemented changes in the presentation of its consolidated financial statements relating to certain reimbursements and other payments for products developed and commercialized with collaborators. These changes were made effective January 1, 2020 and have also been applied retrospectively. After giving effect to these changes, Regeneron’s revenue for fiscal 2017, 2018 and 2019 would have been $4.258 billion, $5.146 billion and $6.558 billion, respectively; and Regeneron’s R&D expense for fiscal 2017, 2018 and 2019 would have been $1.181 billion, $1.469 billion and $2.450 billion, respectively. There is no impact from these changes to net income or net income per share.
39 Regeneron / Annual Report 2019

FORWARD LOOKING STATEMENTS
This report includes forward-looking statements that involve risks and uncertainties relating to future events and the future performance of Regeneron Pharmaceuticals, Inc. (where applicable, together with its subsidiaries, “Regeneron” or the “Company”), and actual events or results may differ materially from these forward-looking statements. Words such as “anticipate,” “expect,” “intend,” “plan,” “believe,” “seek,” “estimate,” variations of such words, and similar expressions are intended to identify such forward-looking statements, although not all forward-looking statements contain these identifying words. These statements concern, and these risks and uncertainties include, among others, the impact of SARS-CoV-2 (the virus that has caused the COVID-19 pandemic) on Regeneron’s business and its employees, collaborators, suppliers, and other third parties on which Regeneron relies, Regeneron’s and its collaborators’ ability to continue to conduct research and clinical programs, Regeneron’s ability to manage its supply chain, net product sales of products marketed by Regeneron and/ or its collaborators (collectively, “Regeneron’s Products”), and the global economy; the nature, timing, and possible success and therapeutic applications of Regeneron’s Products and Regeneron’s product candidates and research and clinical programs now underway or planned, including without limitation EYLEA® (aflibercept) Injection, Dupixent® (dupilumab), Libtayo® (cemiplimab), Praluent® (alirocumab), Kevzara® (sarilumab), fasinumab, evinacumab, REGN-EB3, garetosmab, pozelimab, Regeneron’s immuno-oncology programs (including its costimulatory bispecific portfolio), Regeneron’s COVID-19 antibody program and other earlier-stage programs, and the use of human genetics in Regeneron’s research programs; the likelihood and timing of achieving any of Regeneron’s anticipated development and production milestones; unforeseen safety issues resulting from the administration of Regeneron’s Products and product candidates in patients, including serious complications or side effects in connection with the use of Regeneron’s Products and product candidates in clinical trials; the likelihood and timing of possible regulatory approval and commercial launch of Regeneron’s product candidates and new indications for Regeneron’s Products; the extent to which the results from the research and development programs conducted by Regeneron or its collaborators may be replicated in other studies and lead to therapeutic applications; ongoing regulatory obligations and oversight impacting Regeneron’s Products (such as EYLEA, Dupixent, Libtayo, Praluent, and Kevzara), research and clinical programs, and business, including those relating to patient privacy; determinations by regulatory and administrative governmental authorities which may delay or restrict Regeneron’s ability to continue to develop or commercialize Regeneron’s Products and product candidates; competing drugs and product candidates that may be superior to Regeneron’s Products and product candidates; uncertainty of market acceptance and commercial success of Regeneron’s Products and product candidates; the ability of Regeneron to manufacture and manage supply chains for multiple products and product candidates; the ability of Regeneron’s collaborators, suppliers, or other third parties (as applicable) to perform manufacturing, filling, finishing, packaging, labeling, distribution, and other steps related to Regeneron’s Products and product candidates; coverage and reimbursement determinations by third-party payers, including Medicare and Medicaid; unanticipated expenses; the costs of developing, producing, and selling products; the ability of Regeneron to meet any of
40 Regeneron / Annual Report 2019

FORWARD LOOKING STATEMENTS (Continued)
its financial projections or guidance, and changes to the assumptions underlying those projections or guidance; the potential for any license or collaboration agreement, including Regeneron’s agreements with Sanofi, Bayer, and Teva Pharmaceutical Industries Ltd. (or their respective affiliated companies, as applicable), to be cancelled or terminated without any further product success; and risks associated with intellectual property of others and pending or future litigation relating thereto (including without limitation the patent litigation and other related proceedings relating to Dupixent and Praluent), other litigation and other proceedings and government investigations relating to the Company and/ or its operations, the ultimate outcome of any such proceedings and investigations, and the impact any of the foregoing may have on Regeneron’s business, prospects, operating results, and financial condition. A more complete description of these and other material risks can be found in Regeneron’s filings with the U.S. Securities and Exchange Commission, including its Form 10-K for the fiscal year ended December 31, 2019, including in the section thereof captioned “Item 1A. Risk Factors.” Any forward-looking statements are made based on management’s current beliefs and judgment, and the reader is cautioned not to rely on any forward-looking statements made by Regeneron. Regeneron does not undertake any obligation to update publicly any forward-looking statement, whether as a result of new information, future events, or otherwise.
41 Regeneron / Annual Report 2019

CORPORATE INFORMATION
Common Stock and Related Matters
Our Common Stock is traded on The NASDAQ Global Select Market under the symbol “REGN.” Our Class A Stock is not publicly quoted or traded.
As of April 14, 2020, there were 170 shareholders of record of our Common Stock and 16 shareholders of record of our Class A Stock.
The closing sales price for the Common Stock on that date was $524.84. We have never paid cash dividends and do not anticipate paying any in the foreseeable future.
SEC Form 10-K
A copy of our 2019 Annual Report on Form 10-K filed with the Securities and Exchange Commission (which forms part of this 2019 Annual Report to Shareholders and is incorporated herein by reference) is available without charge from the Regeneron Investor Relations Department, reachable via invest@regeneron.com.
Shareholders’ Inquiries
Inquiries relating to stock transfer or lost certificates and notices of changes of address should be directed to our Transfer Agent, American Stock Transfer & Trust Co., 6201 15th Avenue, Brooklyn, New York 11219, (800) 937-5449, www. amstock.com/main. General information regarding the Company, recent press releases, and SEC filings are available on our website at www.regeneron.com, or can be obtained by contacting our Investor Relations Department at (914) 847-7741 or invest@regeneron.com.
Annual Meeting
The 2020 Annual Meeting of Shareholders will be held on June 12, 2020 at 10:30 a.m., Eastern Time, virtually via www.virtualshareholdermeeting.com/ REGN2020 and at the Westchester Marriott Hotel, 670 White Plains Road, Tarrytown, New York 10591. Due to the COVID-19 outbreak, we may change the venue for the in-person meeting or hold the Annual Meeting as a virtual-only event to the extent permitted under New York law. Please visit investor.regeneron.com for the most up-to-date information on the 2020 Annual Meeting, any procedures and limitations concerning in-person attendees, and information regarding any government-imposed limits on public gatherings applicable to the Annual Meeting that may be in effect at that time.
Corporate Office
777 Old Saw Mill River Road Tarrytown, New York 10591-6707 (914) 847-7400
Transfer Agent and Registrar
American Stock Transfer & Trust Co. 6201 15th Avenue Brooklyn, New York 11219
Independent Registered Public Accounting Firm
PricewaterhouseCoopers LLP
42 Regeneron / Annual Report 2019

REGENERON® , Science to Medicine® , Regeneron Genetics Center® and the following are registered trademarks of Regeneron Pharmaceuticals, Inc.: ARCALYST® , EYLEA® , Libtayo® (in the U.S.), VelociGene® , VelocImmune® , Veloci-Bi® , VelociMab® , VelociMouse® , VelociSuite® and ZALTRAP® . Praluent® , Dupixent® and Kevzara® are registered trademarks of Sanofi.

Design by addison.com