Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended: December 31, 2012
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 000-52228
SORRENTO THERAPEUTICS, INC.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware | 33-0344842 | |
| (State or Other Jurisdiction of Incorporation or Organization) |
(I.R.S. Employer Identification No.) | |
| 6042 Cornerstone Ct. West, Suite B San Diego, California |
92121 | |
| (Address of Principal Executive Offices) | (Zip Code) |
(858) 210-3700
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, par value $0.0001 per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ¨ Yes x No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. ¨ Yes x No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to the filing requirements for at least the past 90 days. x Yes ¨ No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (Section 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). x Yes ¨ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). ¨ Yes x No
The aggregate market value of voting stock held by non-affiliates of the registrant is calculated based upon the closing sale price of the common stock on June 30, 2012 (the last trading day of the registrant’s second fiscal quarter of 2012), as reported on the Over-the-Counter Bulletin Board, and such aggregate market value was approximately $33,667,189.
At March 22, 2013, the registrant had 336,075,440 shares of common stock outstanding.
Table of Contents
SORRENTO THERAPEUTICS, INC.
ANNUAL REPORT ON FORM 10-K
FISCAL YEAR ENDED DECEMBER 31, 2012
| Page No. | ||||||
| 1 | ||||||
| Item 1. |
1 | |||||
| Item 1A. |
11 | |||||
| Item 1B. |
24 | |||||
| Item 2. |
24 | |||||
| Item 3. |
24 | |||||
| Item 4. |
24 | |||||
| 24 | ||||||
| Item 5. |
24 | |||||
| Item 6. |
25 | |||||
| Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
26 | ||||
| Item 7A. |
32 | |||||
| Item 8. |
32 | |||||
| Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
32 | ||||
| Item 9A. |
32 | |||||
| Item 9B. |
33 | |||||
| 33 | ||||||
| Item 10. |
33 | |||||
| Item 11. |
41 | |||||
| Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
46 | ||||
| Item 13. |
Certain Relationships, Related Transactions and Director Independence |
48 | ||||
| Item 14. |
50 | |||||
| Item 15. |
51 | |||||
i
Table of Contents
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, or Form 10-K, contains “forward-looking statements” that involve risks and uncertainties, as well as assumptions that, if they never materialize or prove incorrect, could cause our results to differ materially and adversely from those expressed or implied by such forward-looking statements. The forward-looking statements are contained principally in Item 1—“Business,” Item 1.A—“Risk Factors” and Item 7—“Management’s Discussion and Analysis of Financial Condition and Results of Operations” but appear throughout the Form 10-K. Examples of forward-looking statements include, but are not limited to our expectations, beliefs or intentions regarding our potential product offerings, business, financial condition, results of operations, strategies or prospects and other matters that do not relate strictly to historical facts or statements of assumptions underlying any of the foregoing. These statements are often identified by the use of words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “opportunity,” “plan,” “potential,” “predicts,” “seek,” “should,” “will,” or “would,” and similar expressions and variations or negatives of these words. These forward-looking statements are based on the expectations, estimates, projections, beliefs and assumptions of our management based on information currently available to management, all of which are subject to change. Such forward-looking statements are subject to risks, uncertainties and other factors that are difficult to predict and could cause our actual results and the timing of certain events to differ materially and adversely from future results expressed or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed under Item 1.A—“Risk Factors” in this Form 10-K. Furthermore, such forward-looking statements speak only as of the date of this Form 10-K. We undertake no obligation to update or revise publicly any forward-looking statements to reflect events or circumstances after the date of such statements for any reason, except as otherwise required by law.
| Item 1. | Business. |
Overview
We are a development stage biopharmaceutical company engaged in the discovery, development and commercialization of novel, proprietary drug candidates for the treatment of patients suffering from a variety of conditions, including cancer, inflammation, metabolic and infectious diseases. In 2012, we identified and advanced a number of potential drug candidates with the goal of selecting multiple lead products to progress into preclinical development activities in 2013. It is too early to assess which of these candidates, if any, will merit further evaluation in clinical trials. Our proprietary G-MAB® fully-human antibody library platform was designed to facilitate the rapid identification and isolation of highly specific antibody therapeutic product candidates that bind to disease targets appropriate for antibody therapy. Our initial investment in antibody expression and production capabilities enables us to make the product material necessary to conduct preclinical safety and efficacy testing in animal models.
Our therapeutic objective is to develop two classes of antibody drug products: (i) First in Class, or FIC, and/or (ii) Best in Class, or BIC, which may offer greater efficacy and/or fewer adverse events or side effects as compared to existing drugs. Although we intend to retain ownership and control of some product candidates by advancing them further into preclinical development, we will also consider partnerships with pharmaceutical or biopharmaceutical companies in order to balance the risks associated with drug discovery and development and maximize our stockholders’ returns. Our partnering objectives include generating revenue through license fees, milestone-related development fees and royalties by licensing rights to our product candidates.
Recent Development - IgDraSol Transactions
On March 7, 2013, we entered into an exclusive option agreement with IgDraSol, Inc., or IgDraSol, a private company focused on the development of oncologic agents for the treatment of metastatic breast cancer,
1
Table of Contents
which we refer to as MBC, non-small cell lung cancer, which we refer to as NSCLC, and other cancers. Pursuant to the option agreement, IgDraSol granted us an irrevocable option to acquire IgDraSol by means of an agreement and plan of merger. In consideration for entering into the option agreement, IgDraSol is to receive a non-refundable lump sum payment of $200,000. The close of the transaction and the lump sum payment are expected to occur within 51 days of the signing of the option agreement. If we exercise our option to acquire IgDraSol, we will, pursuant to the merger agreement, issue 76,199,198 shares of common stock to IgDraSol stockholders and, upon the achievement of a specified regulatory milestone, we will issue an additional 32,656,799 shares of common stock to former IgDraSol stockholders. If we do not exercise our option to acquire IgDraSol, we will be required to invest $500,000 in IgDraSol pari passu with other new investors of IgDraSol.
IgDraSol’s lead compound, Cynviloq™, is a micellar diblock copolymeric paclitaxel formulation drug product. Cynviloq™ is currently approved and marketed in several countries, including South Korea for MBC and NSCLC under the trade name Genexol-PM®, and has completed Phase 2 testing for potential advancement into registration trials in the U.S. IgDraSol has the exclusive U.S. distribution rights to Cynviloq™ from Samyang Biopharmaceuticals Corporation, a South Korean corporation.
Contemporaneously with the execution of the IgDraSol option agreement, on March 7, 2013, we and IgDraSol entered into an asset purchase agreement pursuant to which we agreed to purchase all documentation, equipment, information and other know-how related to the micellar nanoparticle technology encompassing Tocosol® and related technologies for a purchase price of $1,210,000. The transaction is expected to close within 45 days of the signing of the asset purchase agreement. Upon payment of such purchase price, we and IgDraSol intend to enter into a development services agreement pursuant to which approximately $3,000,000 in development services may be provided by IgDraSol for the development of Tocosol® and related technologies.
We and IgDraSol also entered into an initial services agreement dated March 7, 2013, pursuant to which IgDraSol is to provide certain product development and technology services related to our antibody platform in exchange for a payment of $1,000,000, which was paid to IgDraSol upon signing.
Collectively, the option agreement, asset purchase agreement, initial services agreement and development services agreement are referred to as the “IgDraSol Transactions”.
Corporate Information
On September 21, 2009, QuikByte Software, Inc., a Colorado corporation and shell company, or QuikByte, consummated its acquisition of Sorrento Therapeutics, Inc., a Delaware corporation and private concern, or STI, in a reverse merger, or the Merger. Pursuant to the Merger, all of the issued and outstanding shares of STI common stock were converted into an aggregate of 169,375,807 shares of QuikByte common stock and STI became a wholly owned subsidiary of QuikByte. The holders of QuikByte’s common stock immediately prior to the Merger held an aggregate of 55,708,320 shares of QuikByte’s common stock immediately following the Merger.
STI was originally incorporated as San Diego Antibody Company in California in 2006 and was renamed “Sorrento Therapeutics, Inc.” and reincorporated in Delaware in 2009, prior to the Merger. QuikByte was originally incorporated in Colorado in 1989. Following the Merger, on December 4, 2009, QuikByte reincorporated under the laws of the State of Delaware, or the Reincorporation. Immediately following the Reincorporation, on December 4, 2009, STI merged with and into QuikByte, the separate corporate existence of STI ceased and QuikByte continued as the surviving corporation, or the Roll-Up Merger. Pursuant to the certificate of merger filed in connection with the Roll-Up Merger, QuikByte’s name was changed from “QuikByte Software, Inc.” to “Sorrento Therapeutics, Inc.” We formed Sorrento Therapeutics, Inc. Hong Kong Limited effective December 4, 2012. Sorrento Hong Kong had no operations in 2012.
2
Table of Contents
Background to Antibodies
The Function of Antibodies
The human immune system protects the body against a variety of infections and other illnesses. Specialized cells work together with the other components of the immune system to recognize, neutralize and eliminate from the body numerous foreign substances, infectious organisms and malignant cells.
Antibodies are part of the body’s principal defense mechanism against disease-causing organisms, other foreign molecules and toxins. Antibodies are protein molecules capable of specifically recognizing substances potentially harmful to the human body, known as antigens, and binding to those antigens to neutralize or block them from interacting with and causing damage to the body. Antibodies are capable of recognizing and distinguishing between the subtlest of molecular differences in antigens. Antibodies that bind tightly to antigens are said to have “high affinity.”
Antibodies are naturally present in the blood and circulate for extended periods in order to perform their surveillance and defense functions. Antibodies are made in the immune system by human white blood cells, called leukocytes. Human leukocytes produce millions of different types of antibodies, all with varying shapes that allow them to specifically attach to and, as a result, neutralize different disease targets. For example, certain antibodies seek out and attach to viruses, bacteria and diseased cells, marking them for destruction by the human immune system. Others attach to specific disease targets and block their interaction with other molecules or can be used to deliver toxic agents to directly kill cancer cells.
As depicted below, the basic structure of an antibody comprises four polypeptides of two different sizes, two identical light chains and two identical heavy chains, named according to their relative size. The heavy and light chains are assembled within the white blood cell to form an antibody molecule. Each chain has a variable region, which contains the binding site for an antigen and gives the antibody its high specificity, and a constant region, which interacts with other parts of the immune system to facilitate the removal of the pathogen or foreign molecule. The genetic code determining the structure of a given variable region is referred to as immunoglobulin variable domain sequence.
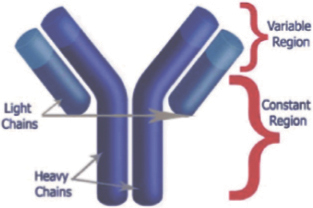
The repertoire of antibodies the body uses to defend itself is produced, in part, through random recombination of genes for the variable regions, as well as random pairing of the heavy and light chains. As a result, the immune system is able to adapt and produce antibodies against virtually any antigen. When an antibody encounters an antigen to which it binds, the white blood cell which produces this specific antibody proliferates to generate more antibodies against the target antigen. White blood cells that have differentiated to produce a specific antibody are called B lymphocytes.
3
Table of Contents
Antibodies as Products
Recent advances in the technologies for creating and producing antibody products, coupled with a better understanding of how antibodies and the immune system function, have further increased the already significant interest in the commercial development of antibodies as therapeutic products in key disease states.
We believe that, as products, antibodies have several potential clinical and commercial advantages over traditional therapies, including small molecule drugs and surgery. These advantages may include the following:
| • | fewer unwanted and uncomfortable side effects as a result of high specificity for the disease target; |
| • | greater patient compliance (use) as a result of more favorable pharmacokinetics over traditional therapies, including better absorption, distribution, metabolism and excretion; |
| • | enhanced ability to deliver various payloads, including drugs, radiation and toxins, to specific disease sites while avoiding surrounding (healthy) tissues; |
| • | reduced risk of failure while developing biobetter antibody therapeutics, since these antibodies target the same antigens as the existing, approved antibodies, yet are designed to deliver significant improvements, such as better dosing, fewer side effects and increased efficacy; and |
| • | overall lower cost of clinical development for the biobetter antibody therapeutic candidates. |
Monoclonal and Chimeric/Humanized Antibodies
The therapeutic antibodies marketed today generally belong to a class of molecules known as “monoclonal antibodies”, or mAbs. This term is used to refer to a homogeneous population of antibody molecules that are identical in their structure and functional characteristics. Historically, the approach to generating monoclonal antibodies has been to immortalize single antibody-producing white blood cells from mice, so that the cells are capable of reproducing over an indefinite period of time. Any of these immortalized, fused cells, known as hybridomas, can be made to produce one specific antibody with desired binding characteristics. The hybridomas can then be selected, cloned and expanded, allowing the large scale production of a murine mAb, or mouse antibody.
However, mouse antibodies are wholly composed of mouse protein sequences and tend to be recognized as “foreign” by the human immune system. When patients are repeatedly treated with mouse antibodies, they will begin to produce antibodies that effectively neutralize the mouse antibody, a reaction referred to as a Human Anti-Mouse Antibody, or HAMA, response. In many cases, the HAMA response prevents the mouse antibodies from having the desired therapeutic effect and may cause the patient to have an allergic reaction.
Recognizing the limitations of mouse mAbs, researchers have developed a number of approaches to make them appear more “human-like” to a patient’s immune system. For example, improved forms of mouse antibodies, referred to as “chimeric” and “humanized” antibodies, are genetically engineered and assembled from portions of mouse and human antibody gene fragments. While these chimeric and humanized antibodies are more human-like, they still retain a varying amount of the mouse antibody protein sequence, and accordingly may continue to trigger a HAMA response. Additionally, the chimeric/humanization process can be expensive and time-consuming, often requiring additional weeks or months of secondary manipulation after the initial generation of the mouse mAbs.
Human Antibodies
The probability of inducing a HAMA response can be reduced through the generation of antibody therapeutic products with fully-human protein sequences. Researchers have developed several antibody technologies to produce antibodies with 100% or fully-human protein sequences. One approach to generating human antibodies, known as “antibody display” technology, involves cloning and expressing human antibody
4
Table of Contents
genes in novel contexts, such as bacteriophages, which are viruses that infect bacteria, yeast or ribosome/mRNA complexes, in order to display libraries of antibody fragments for subsequent in vitro selection against antigens. Ribosomes are intracellular organelles that synthesize proteins. The information for the sequence of amino acids used to synthesize a given protein comes from the mRNA sequence, which is “read” by the ribosome. A ribosome/mRNA complex is mRNA attached to a ribosome for translation into a protein. Our proprietary technology, or the STI Technology, and the Winter II Technology discussed below are both antibody display technologies.
Another approach to develop human antibodies, called “human mouse technology”, is based on genetically-engineered strains of mice in which the attempt has been made to inactivate mouse antibody gene expression and to functionally replace it with human antibody gene expression. The so-called human mouse can be immunized with an antigen of interest, and if, after some time, which is often many months, a sufficient immune response has taken place, human antibody candidates may be obtained.
An additional approach involves the clonal isolation and expansion of human B-lymphocytes. This approach is generally limited to creating antibodies only to non-human antigens or antigens to which the lymphocyte donor had previously responded. Accordingly, it may not be suitable for targeting many key diseases, such as cancer and inflammatory and autoimmune disorders, for which appropriate therapy might require antibodies to human antigens.
Proprietary Human Antibody Library Technology
We believe the STI Technology is a significant improvement over traditional technologies used to construct large human antibody libraries. STI has the ability to produce fully-human antibody libraries of far greater diversity (that is, the number of different antibody species) and single-class libraries, such as Immunoglobulin G (IgG) for therapeutic antibodies or Immunoglobulin A (IgA) for anti-infective secretory antibodies. It is this dual advantage of high diversity and single class libraries that provides STI with what we believe is the premier antibody library asset in the industry. The fact that STI has generated a proprietary portfolio of fully-human antibody preclinical candidates, as both FIC and biobetters, provides evidence that the STI Technology is a formidable tool for discovering proprietary preclinical candidates.
The STI Technology was initially invented by Henry Ji, Ph.D., STI’s co-founder, Chief Executive Officer and President. A U.S. patent covering the STI Technology was issued in July 2008 and additional patent application families for the generation, display and screening of antibody libraries are pending. We also recently filed a group of patent applications covering significant improvements to the initial STI Technology, with the key improvements relating to what we believe is our ability to achieve greater library diversity and single-class libraries.
A traditional technology for the construction of human antibody libraries is the so-called “Winter II Technology”, developed by the Medical Research Council, at Cambridge, UK, The Scripps Research Institute in La Jolla, CA, or TSRI, and Stratagene, Inc. in La Jolla, CA. The Winter II Technology is a process to generate human antibody libraries via amplification of the variable regions of the heavy and light chains of human immunoglobulin genes obtained from human blood samples, followed by cloning and expression in a display system. The Winter II Technology process is covered by U.S. patents that begin to expire in 2018. We believe that the STI proprietary libraries do not follow the Winter II patents.
Our Technology Advantages
We believe the STI Technology may offer the following advantages over competing technologies:
| • | The STI Technology has been designed to provide the full spectrum of human immunoglobulin gene recombination in fully-human mAb libraries. Unlike chimeric and humanization technologies, the STI Technology has allowed the generation of antibodies with fully-human protein sequences without the challenges and limitations of animal-to-human gene transfer procedures. |
5
Table of Contents
| • | Because the STI Technology represents an in vitro human mAb library technology, it enables fast and cost-effective in vitro screening of a large number of antigens. The STI Technology is designed so that any antigen of interest can be investigated, without dependence on the successful induction of a host immune response against the antigen. As opposed to the human-mouse technology, the STI Technology does not require the establishment and maintenance of large animal facilities, which are quite costly to establish and maintain. In addition, a given human antigen may not induce an immune response in mice. In such cases, the “human-mouse” technology appears to be less suitable for delivering human antibody development candidates. |
In addition, we believe that our platform offers advantages over competing platforms, as we are an independent, development stage biotechnology company and, except for our limited license agreement with OPKO Health, Inc., or OPKO, as detailed below, we are not a party to agreements that restrict our right to enter into collaborative arrangements with third parties.
Competition
We compete in an industry characterized by intense competition and rapid technological change. We face, and will continue to face, competition in both the discovery and development of any of our product candidates. New discoveries and developments occur and are expected to continue to occur at a rapid pace. There are many companies, including major pharmaceutical and specialized biotechnology companies, engaged in activities similar to ours. Universities, governmental agencies and other public and private research organizations also conduct research and may market commercial products on their own or through joint ventures.
Many of these entities are significantly larger and have greater financial resources, technical staff, manufacturing, research and development resources, including personnel and technology, expertise in prosecution and enforcement of intellectual property rights and marketing capabilities than us, and many have significant experience in preclinical testing, human clinical trials, product manufacturing, marketing, sales and distribution and other regulatory approval and commercial procedures. They may also have a greater number of patents and greater legal resources to seek remedies for cases of alleged infringement of their patents, which may have the effect of blocking, delaying or compromising our own drug development process.
A number of biotechnology and pharmaceutical companies are developing new products for the treatment of the same diseases being targeted by us; in some instances, these products have already entered clinical trials or are already being marketed. Discoveries or commercial developments by our competitors may render some or all of our technologies or potential products obsolete or non-competitive.
Our Strategy
Our objective is to, either independently or through one or more partnerships with pharmaceutical or biopharmaceutical organizations, identify drug development candidates derived from our antibody libraries. In the event we are successful in identifying drug development candidates, we intend to actively seek partners with experience and expertise in antibody drug development for clinical development of these candidates. Our partnering objectives include generating revenue through license fees, milestone-related development fees and royalties by licensing rights to our development candidates. Key elements of our strategy are:
| • | Screening therapeutically/commercially attractive antigens. Utilizing the STI Technology, we have been and intend to continue screening clinically attractive antigens in the areas of infectious diseases, cancer, cardiovascular, or autoimmune and inflammatory diseases against our antibody libraries with the goal of identifying high quality, functional antibodies. We believe these antibodies represent potential novel and/or proprietary drug development candidates. The isolated human antibodies selected for respective antigens have been and will be subjected to further biochemical characterization and functional testing, such as binding affinity, specificity and kinetics, cellular functionality and |
6
Table of Contents
| animal model testing. The majority of the isolated human antibodies have and may undergo further optimization, applying for example in vitro maturation or molecular evolution to improve their affinity and specificity. |
| • | Establishing partnerships to enhance development efficiency. We intend to minimize technology risk and optimize development efficiency. For biobetter product candidates, the clinical development program established by the FIC provider is a significant advantage, as it represents a development strategy that has been shown to be successful. For FIC products, we expect to seek partnerships with biopharmaceutical companies with experience and expertise in the clinical indications under consideration for any drug candidates we develop. |
See the section entitled “Risk Factors” in this Form 10-K for a discussion of some of the risks relating to the execution of our business strategy.
Intellectual Property
The STI Technology is an antibody library generation technology that we believe represents a significant improvement over the traditional Winter II Technology. The STI Technology was initially invented by Henry Ji, Ph.D., STI’s co-founder and our Chief Executive Officer and President, and assigned to us by Dr. Ji.
A U.S. patent covering the STI Technology was issued in July 2008 and additional patent application families for the generation, display and screening of antibody libraries are pending. We also recently filed a group of patent applications covering significant improvements to the initial STI Technology, with the key improvements relating to what we believe is our ability to achieve greater library diversity and single-class libraries.
We rely on patents, trade secrets and proprietary know-how to protect our intellectual property rights. We plan to diligently prosecute and defend our patents and proprietary technology.
License Agreement with OPKO Health, Inc.
In June 2009, we entered into a limited license agreement, or the OPKO License, with OPKO pursuant to which we granted OPKO an exclusive, royalty-free, worldwide license under all U.S. and foreign patents and patent applications owned or controlled by us or any of our affiliates, or the STI Patents, to (i) develop, manufacture, use, market, sell, offer to sell, import and export certain products related to the development, manufacture, marketing and sale of drugs for ophthalmological indications, or the OPKO Field, and (ii) use and screen any population of distinct molecules covered by any claim of the STI Patents or which is derived by use of any process or method covered by any claim of the STI Patents to identify, select and commercialize certain products within the OPKO Field. Subject to certain limitations, OPKO will have the right to sublicense the foregoing rights granted under the OPKO License. Additionally, pursuant to the OPKO License, OPKO has granted us an exclusive, royalty-free, worldwide license to any patent or patent application owned or controlled by OPKO or any of its affiliates, or the OPKO Patents, to develop, use, make, market, sell and distribute certain products in any field of use, other than the OPKO Field, or the STI Field.
We have retained all rights in the STI Patents outside of the OPKO Field and we have agreed not to practice the OPKO Patents or the STI Patents outside the STI Field. Unless otherwise terminated in accordance with its terms, the License Agreement will expire upon the expiration of the last to expire patent within the STI Patents and OPKO Patents on a country-by-country basis.
License Agreement with The Scripps Research Institute
In January 2010, we entered into a license agreement, or the TSRI License, with TSRI. Under the TSRI License, TSRI granted us an exclusive, worldwide license to certain TSRI patent rights and materials based on
7
Table of Contents
quorum sensing for the prevention and treatment of Staphylococcus aureus, or Staph, infections, including Methicillin-resistant Staph, or MRSA. In consideration for the license, we issued TSRI a warrant for the purchase of common stock, and agreed to pay TSRI a nominal annual royalty, a running royalty based on any sales of licensed products by us or our affiliates and a royalty for any revenues generated by us through our sublicense of patent rights and materials licensed from TSRI under the TSRI License. The TSRI License requires us to indemnify TSRI for certain breaches of the agreement and other matters customary for license agreements. The parties may terminate the TSRI License at any time by mutual agreement. In addition, we may terminate the TSRI License by giving 60 days notice to TSRI and TSRI may terminate the TSRI License immediately in the event of certain breaches of the agreement by us or upon our failure to undertake certain activities in furtherance of commercial development goals. Unless terminated by us or TSRI, the term of the TSRI License will continue until the final expiration of all claims covered by the patent rights licensed by us under the agreement.
Collaboration Agreement
In July 2010, we entered into a feasibility study agreement, or the Collaboration Agreement, with a third party. Under the terms of the Collaboration Agreement, we provided certain antibody screening services for an upfront cash fee of $200,000 and were reimbursed $23,453 for certain costs and expenses associated with providing the services. We completed the screening services in March 2011.
Clinical Development
We currently focus our efforts primarily in the identification and isolation of human antibody drug candidates and further characterize these antibody candidates in in vitro and in vivo functional testing. Due to our limited financial resources, we intend to actively seek product development and commercialization partners from the biopharmaceuticals industry to help us advance in the clinical development of select product candidates.
Manufacturing, Marketing and Sales
We currently do not have any clinical or commercial manufacturing or sales capabilities. We may or may not manufacture the products we develop, if any. We intend to license to, or enter into strategic alliances with, larger companies in the biopharmaceutical businesses, which are equipped to manufacture, market and/or sell our products, if any, through their well-developed manufacturing capabilities and distribution networks. We intend to license some or all of our worldwide patent rights to more than one third party to achieve the fullest development, marketing and distribution of any products we develop.
Government Regulation
U.S. Regulations
We are further developing our internally discovered product candidates. The U.S. Food and Drug Administration, or FDA, regulates, among other things, the development, testing, manufacture, safety, efficacy, record-keeping, labeling, storage, approval, advertising, promotion, sale and distribution of biopharmaceutical products. Specifically, government authorities in the U.S., at the federal, state, and local level, and foreign countries extensively regulate, among other things, the following areas relating to products and product candidates labeled for use in humans:
| • | research and development; |
| • | testing, manufacture, labeling and distribution; |
| • | advertising, promotion, sampling and marketing; and |
| • | import and export. |
In particular, human therapeutic products are subject to rigorous preclinical and clinical trials to demonstrate safety and efficacy and other approval procedures of the FDA and similar regulatory authorities in foreign
8
Table of Contents
countries. Clinical trial programs in humans generally follow a three-phase process. Various federal, state, local, and foreign statutes and regulations also govern testing, manufacturing, labeling, distribution, storage and record-keeping related to such products and their promotion and marketing. The process of obtaining these approvals and the compliance with federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources. In addition, the current regulatory and political environment at FDA could lead to increased testing and data requirements which could impact regulatory timelines and costs.
Clinical trials involve the administration of the investigational product to healthy volunteers or to patients, under the supervision of qualified principal investigators. Each clinical study at each clinical site must be reviewed and approved by an independent institutional review board prior to the recruitment of subjects.
Clinical trials are typically conducted in three sequential phases, but the phases may overlap and different trials may be initiated with the same drug candidate within the same phase of development in similar or differing patient populations. Phase 1 studies may be conducted in a limited number of patients, but are usually conducted in healthy volunteer subjects. The drug is usually tested for safety and, as appropriate, for absorption, metabolism, distribution, excretion, pharmaco-dynamics and pharmaco-kinetics.
Phase 2 usually involves studies in a larger, but still limited patient population to evaluate preliminarily the efficacy of the drug candidate for specific, targeted indications, to determine dosage tolerance and optimal dosage and to identify possible short-term adverse effects and safety risks.
Phase 3 trials are undertaken to further evaluate clinical efficacy of a specific endpoint and to test further for safety within an expanded patient population at geographically dispersed clinical study sites.
The results generated from the preclinical studies and clinical trials, together with other detailed information, including information on the manufacture and composition of the product, are submitted to the FDA as part of a Biologics License Application, or BLA, requesting approval to market the product candidate. Under the Prescription Drug User Fee Act, as amended, the fees payable to the FDA for reviewing a BLA, as well as annual fees for commercial manufacturing establishments and for approved products, can be substantial. Each BLA submitted to the FDA for approval is typically reviewed for administrative completeness and reviewability within 45 to 60 days following submission of the application. If determined to be complete, the FDA will “file” the BLA, thus triggering a full review of the application. The FDA may refuse to file any BLA that it deems incomplete or not properly reviewable. The FDA’s established goals for the review of a BLA are six months for Priority applications and 10 months for Standard applications, whereupon a review decision is to be made. The FDA, however, may not approve a drug within these established goals and its review goals are subject to change from time to time. Further, the outcome of the review, even if generally favorable, may not be an actual approval but an “action letter” that describes additional work that must be done before the application can be approved.
Before approving a BLA, the FDA may inspect the facilities at which the product is manufactured and will not approve the product unless current Good Manufacturing Practices, or cGMP, compliance is satisfactory. The FDA may deny approval of a BLA if applicable statutory or regulatory criteria are not satisfied, or may require additional testing or information, which can delay the approval process. FDA approval of any application may include many delays or never be granted. If a product is approved, the approval will impose limitations on the indicated uses for which the product may be marketed, may require that warning statements be included in the product labeling, and may require that additional studies be conducted following approval as a condition of the approval, may impose restrictions and conditions on product distribution, prescribing or dispensing in the form of a risk management plan, or otherwise limit the scope of any approval. To market a product for other indicated uses, or to make certain manufacturing or other changes, requires FDA review and approval of a BLA Supplement or new BLA. Further post-marketing testing and surveillance to monitor the safety or efficacy of a product is required. Also, product approvals may be withdrawn if compliance with regulatory standards is not maintained or if safety or manufacturing problems occur following initial marketing. In addition, new government requirements may be established that could delay or prevent regulatory approval of our product candidates under development.
9
Table of Contents
Both before and after the FDA approves a product, the manufacturer and the holder or holders of the BLA for the product are subject to comprehensive regulatory oversight. For example, quality control and manufacturing procedures must conform to cGMP requirements, and the FDA periodically inspects manufacturing facilities to assess compliance with cGMP. Accordingly, manufacturers must continue to spend time, money and effort to maintain cGMP compliance.
Foreign Regulations
In addition to regulations in the U.S., we are subject to a variety of foreign regulatory requirements governing human clinical trials and marketing approval for drug products. The foreign regulatory approval process includes substantially all of the risks associated with FDA approval set forth above, as well as additional country-specific regulations. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
There can be no assurance that in the event we seek to develop any product candidate, we or any of our partners would be able to satisfy one or more of these requirements to conduct preclinical or clinical trials or receive any regulatory approvals.
Third-Party Reimbursement and Pricing Controls
In order to raise sufficient financial resources to continue to advance our product candidates, we will need to address pricing pressures and potential third-party reimbursement coverage for our product candidates. In the U.S. and elsewhere, sales of pharmaceutical products depend in significant part on the availability of reimbursement to the consumer from third-party payors, such as government and private insurance plans. Third-party payors are increasingly challenging the prices charged for medical products and services. It is and will continue to be time-consuming and expensive for us or our strategic collaborators to go through the process of seeking reimbursement from Medicare and private payors. Our products may not be considered cost effective, and coverage and reimbursement may not be available or sufficient to allow us to sell our products on a competitive and profitable basis.
In many foreign markets, including the countries in the European Union, pricing of pharmaceutical products is subject to governmental control. In the U.S., there have been, and we expect that there will continue to be, a number of federal and state proposals to implement similar governmental pricing control.
Employees
As of December 31, 2012, we had 18 employees and 13 consultants and advisors. A significant number of our management and our other employees and consultants have worked or consulted with pharmaceutical, biotechnology or medical product companies. While we have been successful in attracting skilled and experienced scientific personnel, there can be no assurance that we will be able to attract or retain the necessary qualified employees and/or consultants in the future. None of our employees are covered by collective bargaining agreements and we consider relations with our employees to be good.
Research and Development
Our research and development expenses totaled $3,830,404 and $2,570,406 in the years ended December 31, 2012 and 2011, respectively.
Address
Our principal executive offices are located at 6042 Cornerstone Ct. West, Suite B, San Diego, CA 92121, and our telephone number at that address is (858) 210-3700. Our website is www.sorrentotherapeutics.com. The contents of our website are not part of this Form 10-K.
10
Table of Contents
Available Information
We file electronically with the U.S. Securities and Exchange Commission, or SEC, our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to reports filed pursuant to Section 13(a) and 15(d) of the Securities Exchange Act of 1934, as amended. We make available on our website at www.sorrentotherapeutics.com, free of charge, copies of these reports, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Copies of our annual report will also be made available, free of charge, upon written request.
The public may read and copy any materials filed by us with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC at http://www.sec.gov. The contents of these websites are not incorporated into this filing. Further, our references to the URLs for these websites are intended to be inactive textual references only.
| Item 1A. | Risk Factors. |
Risks Related to Our Business
We are a development stage company subject to all of the risks and uncertainties of a new business, including the risk that we or our partners may never develop or market any products or generate product related revenues. We have incurred significant losses since our inception and anticipate that we will continue to incur losses for the foreseeable future. We are currently unprofitable and cannot assure you that we will ever become or remain profitable.
We are a development-stage biopharmaceutical company that began operating and commenced research and development activities in 2009. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. There is no assurance that our libraries of fully-human mAbs will be suitable for research, diagnostic or therapeutic use, or that we will be able to identify and isolate therapeutics product candidates, or develop, market and commercialize these candidates. We do not expect any of our product candidates to be commercially available for a number of years, if at all. Even if we are able to commercialize our product candidates, there is no assurance that these candidates would generate revenues or that any revenues generated would be sufficient for us to become profitable or thereafter maintain profitability.
We have not generated any product related revenues to date, and we do not expect to generate any such revenues for a number of years. Additionally, we have incurred operating losses since our inception and we expect to continue to incur significant operating losses for the foreseeable future. For the years ended December 31, 2012 and 2011, we had net losses of $4.8 million and $3.2 million, respectively. As of December 31, 2012, we had an accumulated deficit of $11.0 million. We also expect to continue to incur significant operating expenditures in the foreseeable future as we expand our research and development activities and seek to develop our technologies and product candidates. In the event that our operating losses are greater than anticipated or continue for longer than anticipated, we will need to raise significant additional capital sooner, or in greater amounts, than otherwise anticipated in order to be able to continue development of our technologies and maintain our operations. We cannot be certain that additional funding will be available on acceptable terms, or at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us we may have to significantly delay, scale back or discontinue the ongoing discovery and development of our product candidates. Any of these events could significantly harm our business, financial condition and prospects.
11
Table of Contents
We expect that we will require additional financing, and an inability to raise the necessary capital or to do so on acceptable terms would threaten the success of our business.
We believe that our current cash and cash equivalents balances will not be sufficient to meet our operating and capital requirements, as currently planned, for at least one year. Our future capital requirements will depend on many factors, including:
| • | the progress of the development of our core technology and product candidates; |
| • | the number of product candidates we pursue; |
| • | the time and costs involved in obtaining regulatory approvals; |
| • | the costs involved in filing and prosecuting patent applications and enforcing or defending patent claims; |
| • | our plans to establish sales, marketing and/or manufacturing capabilities; |
| • | our ability to establish, enforce and maintain selected strategic alliances and activities required for product commercialization; and |
| • | our revenues, if any, from successful development and commercialization of our product candidates. |
In order to carry out our business plan and implement our strategy, including the continued development of antibody libraries and the continued development of our pipeline of product candidates in both the FIC and biobetter market segments, we anticipate that we will need to obtain additional financing from time to time and may choose to raise additional funds through strategic collaborations, licensing arrangements, public or private equity or debt financing, a bank line of credit, asset sales, government grants, or other arrangements. We cannot be sure that any additional funding, if needed, will be available on terms favorable to us or at all. Furthermore, any additional equity or equity-related financing may be dilutive to our stockholders, and debt or equity financing, if available, may subject us to restrictive covenants and significant interest costs. If we obtain funding through a strategic collaboration or licensing arrangement, we may be required to relinquish our rights to certain of our product candidates or marketing territories.
In its report on our consolidated financial statements for the year ended December 31, 2012, our independent registered public accounting firm included an explanatory paragraph expressing substantial doubt regarding our ability to continue as a going concern. A “going concern” opinion means, in general, that our independent registered public accounting firm has substantial doubt about our ability to continue our operations without continuing infusions of capital from external sources and this opinion could impair our ability to finance our operations through the sale of debt or equity securities or commercial bank loans.
Further, the NIH has notified all grant recipients that due to the current Congressional budget sequestration, the NIH may not be able to issue continuation awards, or it may be required to negotiate a reduction in the scope of existing awards to meet the constraints imposed. Additionally, plans for new grants or cooperative agreements may be re-scoped, delayed, or canceled depending on the nature of the work and the availability of resources. As a result, we cannot assure you that we will receive the funding under our existing NIH grants, and we may not be successful in securing additional grants from the NIH in the future.
In addition, certain investors, including institutional investors, may be unwilling to invest in our securities since we are traded on the Over-the-Counter Bulletin Board, or OTCBB, and not on a national securities exchange. Our inability to raise capital when needed would harm our business, financial condition and results of operations, and could cause our stock price to decline or require that we wind down our operations altogether.
We have a limited operating history upon which to base an investment decision and we may be unable to successfully develop our technology or any product candidates.
We are a development stage company and have not demonstrated our ability to perform the functions necessary for the successful development or commercialization of the technology we are seeking to develop.
12
Table of Contents
Because we only recently commenced operations, we have a limited operating history upon which you can evaluate our business and prospects. In addition, as an early stage company, we have limited experience and have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the biopharmaceutical area.
The successful development, and any commercialization, of our technology and any product candidates would require us to successfully perform a variety of functions, including:
| • | developing our technology platform; |
| • | identifying, developing, manufacturing and commercializing product candidates; |
| • | entering into successful licensing and other arrangements with product development partners; |
| • | participating in regulatory approval processes; |
| • | formulating and manufacturing products; and |
| • | conducting sales and marketing activities. |
Our operations have been limited to organizing our company, acquiring, developing and securing our proprietary technology and identifying and obtaining early preclinical data for various product candidates. These operations provide a limited basis for you to assess our ability to continue to develop our technology, identify product candidates, develop and commercialize any product candidates we are able to identify and enter into successful collaborative arrangements with other companies, as well as for you to assess the advisability of investing in our securities. Each of these requirements will require substantial time, effort and financial resources.
Our potential product candidates are in early stages of development and any product candidates that we develop will require extensive preclinical and clinical testing before they are approved by the appropriate regulatory agency, if at all.
The FDA regulates, among other things, the development, testing, manufacture, safety, efficacy, record-keeping, labeling, storage, approval, advertising, promotion, sale and distribution of biopharmaceutical products. We are in the early stages of developing potential product candidates, and any candidates that we develop will require extensive preclinical and clinical testing before they will be approved by the FDA or another regulatory authority in a jurisdiction outside the U.S., if at all. We have not yet developed any product candidate; if we were to do so there are a number of requirements that we would be required to satisfy in order to begin conducting preclinical trials and there can be no assurance that we will develop product candidates or complete the steps necessary to allow us to commence these trials. We cannot predict with any certainty the results of preclinical testing or whether such trials would yield sufficient data to permit us, or those with whom we collaborate, to proceed with clinical development and ultimately submit an application for regulatory approval of our product candidates in the U.S. or abroad, or whether such applications would be approved by the appropriate regulatory agency. Further, our product candidates may not receive regulatory approval even if they are successful in clinical trials. If we do not receive regulatory approvals for our product candidates, we may not be able to continue our operations.
Our product development efforts may not be successful.
Our product development efforts for our FIC therapeutic antibodies are designed to focus on novel therapeutic approaches and technologies that have not been widely studied. We are applying these approaches and technologies in our attempt to discover new treatments for conditions that are also the subject of research and development efforts of many other companies. These approaches and technologies may never be successful.
13
Table of Contents
Our failure to find third party collaborators to assist or share in the costs of product development could materially harm our business, financial condition and results of operations.
Our strategy for the development and commercialization of our proprietary product candidates may include the formation of collaborative arrangements with third parties. Potential third parties include biopharmaceutical, pharmaceutical and biotechnology companies, academic institutions and other entities. Third-party collaborators may assist us in:
| • | funding research, preclinical development, clinical trials and manufacturing; |
| • | seeking and obtaining regulatory approvals; and |
| • | successfully commercializing any future product candidates. |
If we are not able to establish further collaboration agreements, we may be required to undertake product development and commercialization at our own expense. Such an undertaking may limit the number of product candidates that we will be able to develop, significantly increase our capital requirements and place additional strain on our internal resources. Our failure to enter into additional collaborations could materially harm our business, financial condition and results of operations.
In addition, our dependence on licensing, collaboration and other agreements with third parties may subject us to a number of risks. These agreements may not be on terms that prove favorable to us and may require us to relinquish certain rights in our product candidates. To the extent we agree to work exclusively with one collaborator in a given area, our opportunities to collaborate with other entities could be curtailed. Lengthy negotiations with potential new collaborators may lead to delays in the research, development or commercialization of product candidates. The decision by our collaborators to pursue alternative technologies or the failure of our collaborators to develop or commercialize successfully any product candidate to which they have obtained rights from us could materially harm our business, financial condition and results of operations.
We expect to rely on third parties to gain access to certain antigens.
We expect to gain access to antigens through contractual arrangements with leading academic researchers, through companies involved in supplying antigens, by isolating them ourselves, or from publicly available sources. In the event we are unable to access antigens in sufficient quantities, or at all, we may not be able to perform antibody discovery activities for certain antigens, which may have an adverse impact on our business and financial condition.
We expect to rely on third parties to conduct any clinical trials for any product candidates we develop, and if they do not properly and successfully perform their legal and regulatory obligations, as well as their contractual obligations to us, we may not be able to obtain regulatory approvals for any product candidates we develop.
In the event we develop product candidates, we expect to rely on contract research organizations and other third parties to assist us in managing, monitoring and otherwise carrying out these trials, including with respect to site selection, contract negotiation and data management. Because we would not control these third parties, they may not treat our clinical studies as their highest priority, or in the manner in which we would prefer, which could result in delays. Moreover, if third parties did not successfully carry out their duties under their agreements with us, if the quality or accuracy of the data they obtain is compromised due to failure to adhere to our clinical protocols or regulatory requirements, or if they otherwise failed to comply with clinical trial protocols or meet expected deadlines, the clinical trials conducted on our behalf may not meet regulatory requirements. If our clinical trials do not meet regulatory requirements or if these third parties need to be replaced, our clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval of some or all of the product candidates we may develop.
14
Table of Contents
If we cannot compete successfully against other biopharmaceutical companies, we may not be successful in developing and commercializing our technology and our business will suffer.
The biopharmaceutical space is characterized by intense competition and rapid technological advances. Even if we are able to develop our proprietary platform technology and additional antibody libraries, each will compete with a number of existing and future technologies and product candidates developed, manufactured and marketed by others. Specifically, we will compete against fully integrated pharmaceutical companies and smaller companies that are collaborating with larger pharmaceutical companies, academic institutions, government agencies and other public and private research organizations. Many of these competitors have validated technologies with products already FDA-approved or in various stages of development. In addition, many of these competitors, either alone or together with their collaborative partners, operate larger research and development programs and have substantially greater financial resources than we do, as well as significantly greater experience in:
| • | developing product candidates and technologies generally; |
| • | undertaking preclinical testing and clinical trials; |
| • | obtaining FDA and other regulatory approvals of product candidates; |
| • | formulating and manufacturing product candidates; and |
| • | launching, marketing and selling product candidates. |
If our technology fails to compete effectively against third party technologies, our business will be adversely impacted.
Because our development activities are expected to rely heavily on sensitive and personal information, an area which is highly regulated by privacy laws, we may not be able to generate, maintain or access essential patient samples or data to continue our research and development efforts in the future on reasonable terms and conditions, which may adversely affect our business.
We may have access to very sensitive data regarding patients whose tissue samples are used in our studies. This data will contain information that is personal in nature. The maintenance of this data is subject to certain privacy-related laws, which impose upon us administrative and financial burdens, and litigation risks. For instance, the rules promulgated by the Department of Health and Human Services under the Health Insurance Portability and Accountability Act, or HIPAA, create national standards to protect patients’ medical records and other personal information in the U.S. These rules require that healthcare providers and other covered entities obtain written authorizations from patients prior to disclosing protected health care information of the patient to companies. If the patient fails to execute an authorization or the authorization fails to contain all required provisions, then we will not be allowed access to the patient’s information and our research efforts can be substantially delayed. Furthermore, use of protected health information that is provided to us pursuant to a valid patient authorization is subject to the limits set forth in the authorization (i.e., for use in research and in submissions to regulatory authorities for product approvals). As such, we are required to implement policies, procedures and reasonable and appropriate security measures to protect individually identifiable health information we receive from covered entities, and to ensure such information is used only as authorized by the patient. Any violations of these rules by us could subject us to civil and criminal penalties and adverse publicity, and could harm our ability to initiate and complete clinical studies required to support regulatory applications for our proposed products. In addition, HIPAA does not replace federal, state, or other laws that may grant individuals even greater privacy protections. We can provide no assurance that future legislation will not prevent us from generating or maintaining personal data or that patients will consent to the use of their personal information, either of which may prevent us from undertaking or publishing essential research. These burdens or risks may prove too great for us to reasonably bear, and may adversely affect our ability to achieve profitability or maintain profitably in the future.
We may be exposed to liability claims associated with the use of hazardous materials and chemicals.
Our research and development activities may involve the controlled use of hazardous materials and chemicals. Although we believe that our safety procedures for using, storing, handling and disposing of these
15
Table of Contents
materials comply with federal, state and local laws and regulations, we cannot completely eliminate the risk of accidental injury or contamination from these materials. In the event of such an accident, we could be held liable for any resulting damages and any liability could materially adversely affect our business, financial condition and results of operations. We do not currently maintain hazardous materials insurance coverage. In addition, the federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous or radioactive materials and waste products may require us to incur substantial compliance costs that could materially harm our business.
If we are unable to retain and recruit qualified scientists and advisors, or if any of our key executives, key employees or key consultants discontinues his or her employment or consulting relationship with us, it may delay our development efforts or otherwise harm our business.
We may not be able to attract or retain qualified management and scientific and clinical personnel in the future due to the intense competition for qualified personnel among biotechnology, pharmaceutical and other businesses, particularly in the San Diego, California area. Our industry has experienced a high rate of turnover of management personnel in recent years. If we are not able to attract, retain and motivate necessary personnel to accomplish our business objectives, we may experience constraints that will significantly impede the successful development of any product candidates, our ability to raise additional capital and our ability to implement our overall business strategy.
We are highly dependent on the key members of our management and scientific staff, especially our Chief Executive Officer and President, Henry Ji, Ph.D. The loss of any of our key employees or key consultants could impede the achievement of our research and development objectives. Furthermore, recruiting and retaining qualified scientific personnel to perform research and development work in the future is critical to our success. We may be unable to attract and retain personnel on acceptable terms given the competition among biotechnology, biopharmaceutical and health care companies, universities and non-profit research institutions for experienced scientists. Certain of our current officers, directors, scientific advisors and/or consultants or certain of the officers, directors, scientific advisors and/or consultants hereafter appointed may from time to time serve as officers, directors, scientific advisors and/or consultants of other biopharmaceutical or biotechnology companies. We do not maintain “key man” insurance policies on any of our officers or employees. All of our employees are employed “at will” and, therefore, each employee may leave our employment at any time.
We plan to grant stock options or other forms of equity awards in the future as a method of attracting and retaining employees, motivating performance and aligning the interests of employees with those of our stockholders. If we are unable to implement and maintain equity compensation arrangements that provide sufficient incentives, we may be unable to retain our existing employees and attract additional qualified candidates. If we are unable to retain our existing employees, including qualified scientific personnel, and attract additional qualified candidates, our business and results of operations could be adversely affected.
We will need to increase the size of our company and may not effectively manage our growth.
Our success will depend upon growing our business and our employee base. Over the next 12 months, we plan to add additional employees to assist us with research and development. Our future growth, if any, may cause a significant strain on our management, and our operational, financial and other resources. Our ability to manage our growth effectively will require us to implement and improve our operational, financial and management systems and to expand, train, manage and motivate our employees. These demands may require the hiring of additional management personnel and the development of additional expertise by management. Any increase in resources devoted to research and product development without a corresponding increase in our operational, financial and management systems could have a material adverse effect on our business, financial condition, and results of operations.
Any disruption in our research and development facilities could adversely affect our business, financial condition and results of operations.
Our principal executive offices, which house our research and development programs, are located in San Diego, California. Our facilities may be affected by natural or man-made disasters. Earthquakes are of particular
16
Table of Contents
significance since our facilities are located in an earthquake-prone area. We are also vulnerable to damage from other types of disasters, including power loss, attacks from extremist organizations, fire, floods and similar events. In the event that our facilities were affected by a natural or man-made disaster, we may be forced to curtail our operations and/or rely on third-parties to perform some or all of our research and development activities. Although we believe we possess adequate insurance for damage to our property and the disruption of our business from casualties, such insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all. In the future, we may choose to expand our operations in either our existing facilities or in new facilities. If we expand our worldwide manufacturing locations, there can be no assurance that this expansion will occur without implementation difficulties, or at all.
International operations may expose us to foreign currency exchange rate fluctuations for all foreign currencies in which we do business and we may be materially adversely affected by these fluctuations.
We formed Sorrento Hong Kong effective December 4, 2012. Sorrento Hong Kong had no operations in 2012. In the event Sorrento Hong Kong becomes operational, we may have an international subsidiary that operates in a foreign currency which would expose us to foreign currency exchange rate fluctuations. We intend to hedge any foreign currency risks associated with potential transactions by entering into forward contracts. Although we may enter into such forward contracts, they may not be adequate to eliminate the risk of foreign currency exchange rate exposures. International operations may also expose us to currency fluctuations as we translate the financial statements of our international subsidiary to U.S. Dollars.
If we acquire companies or technologies in the future, they could prove difficult to integrate, disrupt our business, dilute stockholder value, and adversely affect our operating results and the value of our common stock.
As part of our business strategy, we may acquire, enter into joint ventures with, or make investments in complementary or synergistic companies, services, and technologies in the future. Acquisitions and investments involve numerous risks, including:
| • | difficulties in identifying and acquiring products, technologies, or businesses that will help our business; |
| • | difficulties in integrating operations, technologies, services, and personnel; |
| • | diversion of financial and managerial resources from existing operations; |
| • | the risk of entering new development activities and markets in which we have little to no experience; |
| • | risks related to the assumption of known and unknown liabilities; and |
| • | risks related to our ability to raise sufficient capital to fund additional operating activities. |
As a result, if we fail to properly evaluate acquisitions or investments, we may not achieve the anticipated benefits of any such acquisitions, we may incur costs in excess of what we anticipate, and management resources and attention may be diverted from other necessary or valuable activities.
Risks Related to the Proposed Acquisition of IgDraSol and the IgDraSol Transactions.
Completion of the proposed acquisition of Igdrasol and the related IgDraSol Transactions is subject to various closing conditions, involves significant costs, will require raising additional capital and will require considerable attention from our management. Failure to complete the acquisition could adversely affect our stock price and our future business and operations.
As more fully described above under Item 1, Recent Developments, on March 7, 2013, we entered into the IgDraSol Transactions. IgDraSol’s lead compound, Cynviloq™, is a micellar diblock copolymeric paclitaxel formulation drug product. Cynviloq™ has completed Phase 2 testing for potential advancement into registration
17
Table of Contents
trials in the U.S. IgDraSol is preparing for an “End of Phase 2” meeting with the FDA regarding Cynviloq™ targeted for the first half of 2013. As a formulation of paclitaxel, Cynviloq™ is potentially eligible for approval via FDA’s 505(b)(2) bioequivalence regulatory pathway versus albumin-bound paclitaxel (Abraxane®) in its currently approved MBC and NSCLC indications. However, there can be no assurance that Cynviloq™ is eligible for approval via FDA’s 505(b)(2) bioequivalence regulatory pathway or any other pathway acceptable to the Sorrento board of directors or shareholders.
The completion of the proposed acquisition of IgDraSol and activities under the IgDraSol Transactions are subject to the satisfaction of various closing conditions, and we cannot assure you that such conditions will be satisfied and that the acquisition will be successfully completed. In the event that the acquisition is not consummated, we will have spent considerable time and resources, and incurred substantial costs, including costs related to the acquisition, many of which must be paid even if the acquisition is not completed. If the acquisition is not consummated, our reputation in our industry and in the investment community could be damaged and, as a result, the market price of our common stock could decline.
We may fail to realize the anticipated benefits of the acquisition of IgDraSol and IgDraSol Transactions.
The success of the acquisition of IgDraSol and IgDraSol Transactions will depend on, among other things, our ability to combine our business with IgDraSol in a manner that does not materially disrupt existing relationships and that allows us to achieve development and operational synergies. If we are unable to achieve these objectives, the anticipated benefits of the acquisition may not be realized fully or at all or may take longer to realize than expected. In particular, the acquisition may not be accretive to our stock value or development pipeline in the near or long term.
We and IgDraSol have operated and will continue to operate independently until the potential close of the acquisition in the middle of 2013. It is possible that the integration process could result in the loss of key employees; the disruption of our ongoing business or the ongoing business of IgDraSol; or inconsistencies in standards, controls, procedures, or policies that could adversely affect our ability to maintain relationships with third parties and employees or to achieve the anticipated benefits of the acquisition. Integration efforts between the two companies will also divert management’s attention from our core business and other opportunities that could have been beneficial to our shareholders. An inability to realize the full extent of, or any of, the anticipated benefits of the acquisition, as well as any delays encountered in the integration process, could have an adverse effect on our business and results of operations, which may affect the value of the shares of our common stock after the completion of the acquisition. If we are unable to achieve these objectives, the anticipated benefits of the acquisition may not be realized fully or at all or may take longer to realize than expected. In particular, the acquisition may not be accretive to our stock value or development pipeline in the near or long term.
We expect to incur significant additional costs in connection with the acquisition of IgDraSol and the IgDraSol Transactions and in integrating the companies into a single business.
During the first half of 2013, we incurred significant legal and professional fees in connection with the IgDraSol acquisition. We expect to incur additional costs integrating the companies’ operations, higher development and regulatory costs, and personnel, which cannot be estimated accurately at this time. If the total costs of the integration of the two companies and advancement of the Cynviloq™ assets exceed the anticipated benefits of the acquisition, our financial results could be adversely affected.
Risks Related to Our Intellectual Property
Our ability to protect our intellectual property rights will be critically important to the success of our business, and we may not be able to protect these rights in the U.S. or abroad.
Our success, competitive position and future revenues will depend in part on our ability to obtain and maintain patent protection for our product candidates, methods, processes and other technologies, to prevent third parties from infringing on our proprietary rights and to operate without infringing upon the proprietary rights of
18
Table of Contents
third parties. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary rights are covered by valid and enforceable patents or are effectively maintained as trade secrets. We attempt to protect our proprietary position by filing U.S. and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. We have one issued U.S. patent and the examination of its European equivalent is currently in progress. In 2011, several improvement patent applications were filed for our proprietary antibody library technology. However, due to the difficulties of enforcing such antibody library technology, we filed a key patent application in the U.S. only and requested nonpublication. We have commenced generating a patent application portfolio of patents to protect each product candidate in our pipeline. However, the patent position of biopharmaceutical companies involves complex legal and factual questions, and therefore we cannot predict with certainty whether any patent applications that we have filed or that we may file in the future will be approved or any resulting patents will be enforced. In addition, third parties may challenge, seek to invalidate or circumvent any of our patents, once they are issued. Thus, any patents that we own or license from third parties may not provide any protection against competitors. Any patent applications that we have filed or that we may file in the future, or those we may license from third parties, may not result in patents being issued. Also, patent rights may not provide us with adequate proprietary protection or competitive advantages against competitors with similar technologies.
In addition, the laws of certain foreign countries do not protect our intellectual property rights to the same extent as do the laws of the U.S. If we fail to apply for intellectual property protection or if we cannot adequately protect our intellectual property rights in these foreign countries, our competitors may be able to compete more effectively against us, which could adversely affect our competitive position, as well as our business, financial condition and results of operations.
If any of our trade secrets, know-how or other proprietary information is disclosed, the value of our trade secrets, know-how and other proprietary rights would be significantly impaired and our business and competitive position would suffer.
Our success also depends upon the skills, knowledge and experience of our scientific and technical personnel and our consultants and advisors, as well as our licensors. To help protect our proprietary know-how and our inventions for which patents may be unobtainable or difficult to obtain, we rely on trade secret protection and confidentiality agreements. Unlike some of our competitors, we maintain our proprietary libraries for ourselves as we believe they have proven to be superior in obtaining strong binder product candidates. To this end, we require all of our employees, consultants, advisors and contractors to enter into agreements which prohibit the disclosure of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business. These agreements may not provide adequate protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure or the lawful development by others of such information. If any of our trade secrets, know-how or other proprietary information is disclosed, the value of our trade secrets, know-how and other proprietary rights would be significantly impaired and our business and competitive position would suffer.
Third party competitors may seek to challenge the validity of our patents, thereby rendering them unenforceable or we may seek to challenge third party competitor patents if such third parties seek to interpret or enforce a claim scope going well beyond the actual enabled invention.
Claims that we infringe upon the rights of third parties may give rise to costly and lengthy litigation, and we could be prevented from selling products, forced to pay damages, and defend against litigation.
Third parties may assert patent or other intellectual property infringement claims against us or our strategic partners or licensees with respect to our technologies and potential product candidates. If our products, methods, processes and other technologies infringe upon the proprietary rights of other parties, we could incur substantial costs and we may have to:
| • | obtain licenses, which may not be available on commercially reasonable terms, if at all, and may be non-exclusive, thereby giving our competitors access to the same intellectual property licensed to us; |
19
Table of Contents
| • | redesign our products or processes to avoid infringement; |
| • | stop using the subject matter validly claimed in the patents held by others; |
| • | pay damages; and |
| • | defend litigation or administrative proceedings which may be costly whether we win or lose, and which could result in a substantial diversion of our valuable management resources. |
Even if we were to prevail, any litigation could be costly and time-consuming and would divert the attention of our management and key personnel from our business operations. Furthermore, as a result of a patent infringement suit brought against us or our strategic partners or licensees, we or our strategic partners or licensees may be forced to stop or delay developing, manufacturing or selling technologies or potential products that are claimed to infringe a third party’s intellectual property unless that party grants us or our strategic partners’ or licensees’ rights to use its intellectual property. Ultimately, we may be unable to develop some of our technologies or potential products or may have to discontinue development of a product candidate or cease some of our business operations as a result of patent infringement claims, which could severely harm our business.
Our position as a relatively small company may cause us to be at a significant disadvantage in defending our intellectual property rights and in defending against infringement claims by third parties.
Litigation relating to the ownership and use of intellectual property is expensive, and our position as a relatively small company in an industry dominated by very large companies may cause us to be at a significant disadvantage in defending our intellectual property rights and in defending against claims that our technology infringes or misappropriates third party intellectual property rights. However, we may seek to use various post-grant administrative proceedings, including new procedures created under the America Invents Act, to invalidate potentially overly-broad third party rights. Even if we are able to defend our position, the cost of doing so may adversely affect our ability to grow, generate revenue or become profitable. Although we have not yet experienced patent litigation, we may in the future be subject to such litigation and may not be able to protect our intellectual property at a reasonable cost, or at all, if such litigation is initiated. The outcome of litigation is always uncertain, and in some cases could include judgments against us that require us to pay damages, enjoin us from certain activities or otherwise affect our legal or contractual rights, which could have a significant adverse effect on our business.
Risks Related to Ownership of Our Common Stock
The market price of our common stock may fluctuate significantly, and investors in our common stock may lose all or a part of their investment.
The market prices for securities of biotechnology and pharmaceutical companies have historically been highly volatile, and the market has from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. The market price of our common stock may fluctuate significantly in response to numerous factors, some of which are beyond our control, such as:
| • | announcements of the introduction of new products by our competitors; |
| • | market conditions in the pharmaceutical and biotechnology sectors; |
| • | announcements concerning product development results or intellectual property rights of others; |
| • | future issuances of common stock or other securities; |
| • | the addition or departure of key personnel; |
| • | legal disputes, government investigations and the results of any proceedings or lawsuits; |
| • | announcements by us or our competitors of acquisitions, investments or strategic alliances; and |
| • | general market conditions and other factors, including factors unrelated to our operating performance. |
Further, the equity markets in general have recently experienced extreme price and volume fluctuations. Continued market fluctuations could result in extreme volatility in the price of our common stock, which could cause a decline in the value of our common stock. Price volatility of our common stock might worsen if the trading volume of our common stock is low.
20
Table of Contents
Some or all of the “restricted” shares of our common stock issued to former stockholders of STI in connection with the Merger or held by other of our stockholders may be offered from time to time in the open market pursuant to an effective registration statement or Rule 144, and these sales may have a negative effect on the price of our common stock.
Trading of our common stock is limited, and trading restrictions imposed on us by applicable regulations may further reduce our trading, making it difficult for our stockholders to sell their shares.
Trading of our common stock is currently conducted on the OTCBB. The liquidity of our common stock is limited, not only in terms of the number of shares that can be bought and sold at a given price, but also as it may be adversely affected by delays in the timing of transactions and reduction in security analysts’ and the media’s coverage of us, if at all.
The foregoing factors may result in lower prices for our common stock than might otherwise be obtained and could also result in a larger spread between the bid and asked prices for our common stock. In addition, without a large public float, our common stock is less liquid than the stock of companies with broader public ownership, and, as a result, the trading price of our common stock may be more volatile. In the absence of an active public trading market, an investor may be unable to liquidate his investment in our common stock. Trading of a relatively small volume of our common stock may have a greater impact on the trading price of our stock than would be the case if our public float were larger. We cannot predict the price at which our common stock will trade at any given time.
We do not expect to pay dividends on our common stock, and investors will be able to receive cash in respect of their shares of our common stock only upon the sale of such shares.
We have no intention in the foreseeable future to pay any cash dividends on our common stock. Therefore, an investor in our common stock may obtain an economic benefit from the common stock only after an increase in its trading price and only then by selling the common stock.
Because our common stock is a “penny stock,” it may be more difficult for investors to sell shares of our common stock, and the market price of our common stock may be adversely affected.
According to the definition adopted by the SEC, our common stock is a “penny stock” because, among other things, its price is below $5.00 per share, it is not listed on a national securities exchange and we do not meet certain net tangible asset or average revenue requirements. Broker-dealers that sell penny stock must provide purchasers of such stock with a standardized risk-disclosure document prepared by the SEC. This document provides information about penny stock and the nature and level of risks involved in investing in penny stock. A broker must also give a purchaser, orally or in writing, bid and offer quotations and information regarding broker and salesperson compensation, make a written determination that the penny stock is a suitable investment for the purchaser and obtain the purchaser’s written agreement to the purchase. Broker-dealers must also provide customers that hold penny stock in their accounts with such broker-dealer a monthly statement containing price and market information relating to the penny stock. If a penny stock is sold to an investor in violation of the penny stock rules, the investor may be able to cancel its purchase and get its money back.
If applicable, the penny stock rules may make it difficult for investors to sell their shares of our common stock. Because of the rules and restrictions applicable to a penny stock, there is less trading in penny stock, and the market price of our common stock may be adversely affected. Also, many brokers choose not to participate in penny stock transactions. Accordingly, investors may not always be able to publicly resell their shares of our common stock at times and prices that they feel are appropriate.
Existing stockholders’ interest in us may be diluted by additional issuances of equity securities and raising funds through lending and licensing arrangements may restrict our operations or require us to relinquish proprietary rights.
We may issue additional equity securities to fund future expansion and pursuant to employee benefit plans. We may also issue additional equity for other purposes. These securities may have the same rights as our
21
Table of Contents
common stock or, alternatively, may have dividend, liquidation or other preferences to our common stock. The issuance of additional equity securities will dilute the holdings of existing stockholders and may reduce the share price of our common stock.
If we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish potentially valuable rights to our product candidates, potential products or proprietary technologies, or grant licenses on terms that are not favorable to us. If adequate funds are not available, our ability to achieve profitability or to respond to competitive pressures would be significantly limited and we may be required to delay, significantly curtail or eliminate the development of our product candidates.
Directors, executive officers, principal stockholders and affiliated entities own a significant percentage of our capital stock, and they may make decisions that you do not consider to be in your best interests or those of our other stockholders.
As of December 31, 2012, our directors, executive officers and principal stockholders beneficially owned, in the aggregate, over 68% of our outstanding voting securities. As a result, if some or all of them acted together, they would have the ability to exert substantial influence over the election of our board of directors and the outcome of issues requiring approval by our stockholders. This concentration of ownership may also have the effect of delaying or preventing a change in control of our company that may be favored by other stockholders. This could prevent transactions in which stockholders might otherwise recover a premium for their shares over current market prices.
Our certificate of incorporation, as amended, and bylaws provide for indemnification of officers and directors at our expense and limits their liability, which may result in a major cost to us and hurt the interests of our stockholders because corporate resources may be expended for the benefit of our officers and/or directors.
Our certificate of incorporation, as amended, bylaws and applicable Delaware law provide for the indemnification of our directors, officers, employees, and agents, under certain circumstances, against attorney’s fees and other expenses incurred by them in any litigation to which they become a party arising from their association with or activities on our behalf. We will also bear the expenses of such litigation for any of our directors, officers, employees, or agents, upon such person’s promise to repay us, therefore if it is ultimately determined that any such person shall not have been entitled to indemnification. This indemnification policy could result in substantial expenditures by us, which we will be unable to recover.
Our corporate documents and Delaware law contain provisions that could discourage, delay or prevent a change in control of our company, prevent attempts to replace or remove current management and reduce the market price of our common stock.
Provisions in our certificate of incorporation, as amended, and bylaws may discourage, delay or prevent a merger or acquisition involving us that our stockholders may consider favorable. For example, our certificate of incorporation, as amended, authorizes our board of directors to issue up to 100,000,000 shares of “blank check” preferred stock. As a result, without further stockholder approval, the board of directors has the authority to attach special rights, including voting and dividend rights, to this preferred stock. With these rights, preferred stockholders could make it more difficult for a third party to acquire us.
We are also subject to the anti-takeover provisions of the Delaware General Corporation Law. Under these provisions, if anyone becomes an “interested stockholder,” we may not enter into a “business combination” with that person for three years without special approval, which could discourage a third party from making a takeover offer and could delay or prevent a change in control of us. An “interested stockholder” means, generally, someone owning 15% or more of our outstanding voting stock or an affiliate of ours that owned 15% or more of our outstanding voting stock during the past three years, subject to certain exceptions as described in the Delaware General Corporation Law.
22
Table of Contents
Compliance with changing regulations concerning corporate governance and public disclosure may result in additional expenses.
There have been changing laws, regulations and standards relating to corporate governance and public disclosure, including the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, the Sarbanes-Oxley Act of 2002, or Sarbanes-Oxley, new regulations promulgated by the SEC and rules promulgated by the national securities exchanges. The Dodd-Frank Act, enacted in July 2010, expands federal regulation of corporate governance matters and imposes requirements on public companies to, among other things, provide stockholders with a periodic advisory vote on executive compensation and also adds compensation committee reforms and enhanced pay-for-performance disclosures. While some provisions of the Dodd-Frank Act are effective upon enactment, others will be implemented upon the SEC’s adoption of related rules and regulations. The scope and timing of the adoption of such rules and regulations is uncertain and, accordingly, the cost of compliance with the Dodd-Frank Act is also uncertain.
In addition, Sarbanes-Oxley specifically requires, among other things, that we maintain effective internal controls for financial reporting and disclosure of controls and procedures. In particular, we must perform system and process evaluation and testing of our internal controls over financial reporting to allow management to report on the effectiveness of our internal controls over financial reporting, as required by Section 404 of Sarbanes-Oxley. Our testing, or the subsequent testing by our independent registered public accounting firm, if and when required, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses. Our compliance with Section 404 will require that we incur substantial accounting expense and expend significant management efforts. We currently do not have an internal audit group, and we will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. Moreover, if we are not able to comply with the requirements of Section 404 in a timely manner, or if we or our independent registered public accounting firm identifies deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline, and we could be subject to sanctions or investigations by the SEC or other regulatory authorities, which would require additional financial and management resources.
These new or changed laws, regulations and standards are, or will be, subject to varying interpretations in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies, which could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. As a result, our efforts to comply with evolving laws, regulations and standards are likely to continue to result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities. Members of our board of directors and our principal executive officer and principal financial officer could face an increased risk of personal liability in connection with the performance of their duties. As a result, we may have difficulty attracting and retaining qualified directors and executive officers, which could harm our business. If the actions we take in our efforts to comply with new or changed laws, regulations and standards differ from the actions intended by regulatory or governing bodies, we could be subject to liability under applicable laws or our reputation may be harmed.
State securities laws may limit secondary trading, which may restrict the states in which and conditions under which you can sell shares.
Secondary trading in our common stock will not be possible in any state until our common stock is qualified for sale under the applicable securities laws of the state or there is confirmation that an exemption, such as listing in certain recognized securities manuals, is available for secondary trading in the state. If we fail to register or qualify, or to obtain or verify an exemption for the secondary trading of, our common stock in any particular state, the common stock could not be offered or sold to, or purchased by, a resident of that state. We currently do not intend and may not be able to qualify securities for resale in some or all of the states that do not offer manual exemptions and require shares to be qualified before they can be resold by our stockholders. In the event that a significant number of states refuse to permit secondary trading in our common stock, the liquidity for the common stock could be significantly impacted.
23
Table of Contents
| Item 1B. | Unresolved Staff Comments. |
None.
| Item 2. | Properties. |
We currently lease approximately 12,000 square feet of office and laboratory space in San Diego, California. Our initial lease expires in September 2014, but includes an option to extend the term of the lease for one additional four-year period. Including various supplemental amendments entered into through June 2012, the lease termination date expires in April 2017. These supplemental amendments contain an option to extend the term for the additional rental space by five years at the then prevailing rate. We believe that our current facilities are adequate to meet our needs for the foreseeable future and that, should it be needed, suitable additional space will be available to accommodate expansion of our operations on commercially reasonable terms.
| Item 3. | Legal Proceedings. |
We are not currently a party to any legal proceedings that, individually or in the aggregate, are deemed to be material to our financial condition or results of operations.
| Item 4. | Mine Safety Disclosures. |
None.
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
Market Information
Our common stock is traded on the OTCBB under the symbol “SRNE” and began quotation on the OTCBB on an unpriced basis in December 2006.
Our common stock trades only sporadically and has experienced in the past, and is expected to experience in the future, significant price and volume volatility.
The following table sets forth the range of high and low bid quotations for our common stock, as reported by the OTCBB, on a quarterly basis for the fiscal years ended December 31, 2012 and 2011. Quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
| 2012 | 2011 | |||||||||||||||
| First Quarter |
$ | 0.59 | $ | 0.20 | $ | 0.60 | $ | 0.20 | ||||||||
| Second Quarter |
0.77 | 0.12 | 0.30 | 0.20 | ||||||||||||
| Third Quarter |
0.45 | 0.15 | 0.28 | 0.15 | ||||||||||||
| Fourth Quarter |
0.25 | 0.06 | 0.24 | 0.10 | ||||||||||||
Holders of Record
As of March 8, 2013, there were 202 holders of record of our common stock.
24
Table of Contents
Dividend Policy
We paid no cash dividends in respect of our common stock during our two most recent fiscal years, and we have no plans to pay any dividends or make any other distributions in the foreseeable future. The payment by us of dividends, if any, in the future, rests within the discretion of our board of directors and will depend, among other things, upon our earnings, capital requirements and financial condition.
Stock Repurchases
In January 2011, we repurchased 1,104,135 unvested shares of restricted common stock for $43.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table sets forth additional information with respect to the shares of common stock that may be issued upon the exercise of options and other rights under our existing equity compensation plans and arrangements in effect as of December 31, 2012. The information includes the number of shares covered by, and the weighted average exercise price of, outstanding options and the number of shares remaining available for future grant, excluding the shares to be issued upon exercise of outstanding options.
| Plan Category |
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted-average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders(1) |
10,410,000 | $ | 0.14 | 3,610,000 | (2) | |||||||
| Equity compensation plans not approved by security holders(3) |
80,000 | $ | 0.04 | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Total |
10,490,000 | $ | 0.14 | 3,610,000 | ||||||||
|
|
|
|
|
|
|
|||||||
| (1) | Comprised of our 2009 Stock Incentive Plan, or the 2009 Plan. |
| (2) | Comprised solely of shares subject to awards available for future issuance under the 2009 Plan. Pursuant to the terms of the 2009 Plan, the share reserve of the 2009 Plan will automatically increase on the first day of each fiscal year, from fiscal year 2011 through 2019, by the lesser of (a) 1,200,000 shares, (b) one percent (1%) of outstanding shares of common stock as of the last day of the immediately preceding fiscal year (rounded down to the nearest whole share), and (c) such number of shares of common stock approved by the board of directors or applicable committee thereof. Effective January 1, 2013, 15,600,000 shares were authorized under the 2009 Plan, with 4,810,000 shares remaining available for future issuance under the plan. |
| (3) | Comprised solely of shares issued to non-employee directors prior to our adoption of the 2009 Plan. |
| Item 6. | Selected Financial Data. |
As a smaller reporting company, as defined by Item 10(f)(1) of Regulation S-K we are not required to provide the information set forth in this Item.
25
Table of Contents
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the financial statements and the related notes and other information that are included elsewhere in this Form 10-K. This discussion contains forward-looking statements based upon current expectations that involve risks and uncertainties, such as our plans, objectives, expectations and intentions. Actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of a number of factors, including those set forth under the cautionary note regarding “Forward-Looking Statements” contained elsewhere in this Form 10-K. Additionally, you should read the “Risk Factors” section of this Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
We are a development stage biopharmaceutical company focused on the discovery, development and commercialization of novel and/or proprietary drug candidates for the treatment of a variety of disease conditions, including cancer, inflammation, metabolic and infectious diseases. Through December 31, 2012, we identified and further developed a number of potential drug product candidates across various therapeutic areas, and intend to select several lead product candidates to progress into preclinical development activities in 2013. It is too early to assess which of these candidates, if any, will merit further evaluation in clinical trials. Our libraries were designed to facilitate the rapid identification and isolation of highly specific, antibody therapeutic product candidates that are fully-human and that bind to disease targets appropriate for antibody therapy. We built our initial antibody expression and production capabilities to enable us to make sufficient product material to conduct preclinical safety and efficacy testing in animal models.
Our therapeutic objective is to develop two classes of antibody drug products: (i) FIC and/or (ii) biobetters. Although we intend to retain ownership and control of some product candidates by advancing them further into preclinical development, we will also consider partnerships with pharmaceutical or biopharmaceutical organizations, with the appropriate experience and expertise, in order to balance the risks associated with drug discovery and development and maximize our stockholders’ returns. Our partnering objectives include generating revenue through license fees, milestone related development fees and royalties by licensing rights to our development candidates.
Recent Developments
Assignment Agreement. In January 2013, we entered into an Assignment Agreement, or the Assignment Agreement with Tien-Li Lee, M.D. and Jane Wu Lee, M.D. as individuals (collectively, the Lees) pursuant to which the Lees agreed to assign to us their right, title and interest throughout the world in and to certain inventions and patents that provide for the production of recombinant intravenous immuoglobulins. As consideration for the assignment, we: (i) issued the Lee’s 250,000 shares of our common stock upon execution of the Agreement, (ii) agreed to pay the Lees a total of $50,000 in five monthly installments of $10,000 beginning on February 1, 2013, and (iii) agreed to issue the Lees up to 2,000,000 shares of our common stock based upon the achievement of certain milestone events described in the Assignment Agreement.
Loan Agreement. In February 2013, we entered into a loan and security agreement with a bank pursuant to which the lender provided us loans to finance certain equipment, in an aggregate principal amount of up to $1,000,000. Under the loan agreement, the lender funded an initial equipment advance in the principal amount of $875,888 in February 2013 and agreed to fund, subject to customary conditions, an additional equipment advance in the principal amount of $124,112 on or prior to August 21, 2013. The loans under the loan agreement bear interest at a rate equal to the three-year U.S. Treasury note yield plus 4.65%, which is fixed on the date of each funding. Interest accrues on the initial outstanding advance at the fixed rate of 5.15%. We are obligated to pay interest-only on any loans funded under the loan agreement prior to April 30, 2013 until May 1, 2013, and
26
Table of Contents
thereafter to pay 36 consecutive equal monthly installments of principal and interest through April 1, 2016. We are obligated to pay equal monthly installments of principal and interest through April 1, 2016 on any loans funded under the loan agreement after April 30, 2013. All loans funded under the loan agreement mature on April 1, 2016.
Stock Purchase Agreement. In March 2013, we entered into a Stock Purchase Agreement and issued 33,658,305 shares of common stock, in a private placement transaction, at $0.18 per share for aggregate gross proceeds of $6,418,495.
IgDraSol Transactions. On March 7, 2013, we entered into the IgDraSol Transactions, as more fully described above under Item 1, Recent Developments. Pursuant to the option agreement, IgDraSol granted us an irrevocable option to acquire IgDraSol by means of the merger agreement. In consideration for entering into the option agreement, IgDraSol is to receive a non-refundable lump sum payment of $200,000. The close of the transaction and the lump sum payment are expected to occur within 51 days of the signing of the option agreement. If we exercise our option to acquire IgDraSol, we will, pursuant to the merger agreement, issue 76,199,198 shares of common stock to IgDraSol stockholders and, upon the achievement of a specified regulatory milestone, we will issue an additional 32,656,799 shares of common stock to former IgDraSol stockholders. If we do not exercise our option to acquire IgDraSol, we will be required to invest $500,000 in IgDraSol pari passu with other new investors of IgDraSol.
Contemporaneously with the execution of the option agreement, on March 7, 2013, we and IgDraSol entered into an asset purchase agreement for the purchase of all documentation, equipment, information and other know-how related to the micellar nanoparticle technology encompassing Tocosol® and related technologies for a purchase price of $1,210,000. The transaction is expected to close within 45 days of the signing of the asset purchase agreement. Upon payment of such purchase price, we and IgDraSol intend to enter into a development services agreement pursuant to which approximately $3,000,000 in development services may be provided by IgDraSol for the development of Tocosol® and related technologies.
We and IgDraSol also entered into an initial services agreement dated March 7, 2013, pursuant to which IgDraSol is to provide certain product development and technology services related to our antibody platform in exchange for a payment of $1,000,000, which was paid to IgDraSol upon signing.
Corporate Information
On September 21, 2009, QuikByte consummated its acquisition of STI in a reverse merger, as more fully described in Item 1 above. We formed Sorrento Hong Kong effective December 4, 2012. Sorrento Hong Kong had no operations in 2012.
Results of Operations
The following discussion of our operating results explains material changes in our results of operations for the years ended December 31, 2012 and 2011. The discussion should be read in conjunction with the consolidated financial statements and related notes included elsewhere in this Form 10-K.
Comparison of the Years Ended December 31, 2012 and 2011
Revenues. Revenues were $583,774 for the year ended December 31, 2012, as compared to $529,184 for the year ended December 31, 2011. The net increase of $54,590 is due to the following substantially offsetting factors: (i) grant revenues increased $254,590 due to increased activities under three active grants received from the National Institute of Allergy and Infectious Diseases, a division of the National Institutes of Health, or NIH, in the year ended December 31, 2012 as compared to only two active grants for the year ended December 31, 2011, and (ii) non-recurring collaboration revenue of $200,000 earned under a collaboration agreement upon delivering all screening services in 2011.
27
Table of Contents
In May 2010, we were awarded an Advanced Technology Small Business Technology Transfer Research grant to support our program to generate and develop novel antibody therapeutics and vaccines to combat Staph infections, including Methicillin-resistant Staph, or the Staph Grant award. The project period for this grant covered a two-year period, and as of December 31, 2011, the entire Phase 1 grant of $600,000 had been awarded. The Staph Grant award revenues for the years ended December 31, 2012 and 2011 and for the period from inception (January 25, 2006), or Inception, through December 31, 2012 were $119,379, $215,986 and $600,000, respectively.
In July 2011, we were awarded a second Advanced Technology Small Business Technology Transfer Research grant to support our program to generate and develop antibody therapeutics and vaccines to combat C. difficile infections, or the C. difficile Grant award. The project period for the C. difficile Grant award covers a two-year period which commenced in June 2011, and as of September 30, 2012, the entire Phase 1 grant of $600,000 had been awarded. The C. difficile Grant award revenues for the years ended December 31, 2012 and 2011 and for the period from Inception through December 31, 2012 were $335,579, $113,198 and $448,777, respectively.
In June 2012, we were awarded a third Advanced Technology Small Business Technology Transfer Research grant, with an initial award of $300,000, to support our program to generate and develop novel human antibody therapeutics to combat Staph infections, including Methicillin-resistant Staph, or the Staph Grant II award. The project period for the phase I grant covers a two-year period which commenced in June 2012, with a potential annual award of $300,000 per year. The Staph Grant II award revenues for the year ended December 31, 2012 and from Inception through December 31, 2012 was $128,816.
We had no other revenue during the years ended December 31, 2012 and 2011 as we have not yet developed any product candidates for commercialization or earned any licensing or royalty payments. We expect that any revenue we generate will fluctuate from quarter to quarter as a result of the unpredictability of the timing and amount of grant awards, research and development reimbursements and other payments received under our strategic collaborations.
Research and Development Expenses. Research and development expenses for the years ended December 31, 2012 and 2011 were $3,830,404 and $2,570,406, respectively. Research and development expenses include the costs to identify, isolate and advance human antibody drug candidates derived from our libraries, preclinical testing expenses and the expenses associated with fulfilling our development obligations related to the NIH grant awards, collectively the NIH Grants. Such expenses consist primarily of salaries and personnel related expenses, stock-based compensation expense, preclinical testing, laboratory supplies, consulting costs, depreciation and other expenses. The increase of $1,259,998 is primarily attributable to salaries, stock-based compensation expense, preclinical testing, depreciation, consulting and lab supply costs incurred in connection with our expanded research and development activities and receipt of the three NIH Grant awards in May 2010, July 2011 and June 2012, respectively. We expect research and development expenses to increase in absolute dollars as we incur incremental expenses associated with our efforts to identify, isolate and advance human antibody drug candidates derived from our libraries.
General and Administrative Expenses. General and administrative expenses for the years ended December 31, 2012 and 2011 were $1,605,978 and $1,201,220, respectively. General and administrative expenses consist primarily of salaries and personnel related expenses for executive, finance and administrative personnel, stock-based compensation expense, professional fees, infrastructure expenses, legal and accounting expenses and other general corporate expenses. The increase of $404,758 is primarily attributable to higher legal costs related to the annual shareholder meeting and general corporate matters. Other offsetting activities included a decrease in salaries expense related to the departure of our former Chief Executive Officer and increases in consulting expenses with the addition of our part time Chief Business Officer in the second half of 2012 and higher compliance costs associated with our public reporting obligations. We expect general and administrative expenses to increase in absolute dollars as we incur incremental expenses associated with ongoing operations and compliance with our public reporting obligations.
28
Table of Contents
Interest Income. Interest income for the years ended December 31, 2012 and 2011 was $7,300 and $5,951, respectively. The increase in interest income resulted from higher average cash balances in 2012 as compared to 2011. We expect that continued low interest rates will significantly limit our interest income in the near term.
Net Loss. Net loss for the years ended December 31, 2012 and 2011 was $4,845,308 and $3,236,491, respectively. The increase in net loss is mainly attributable to the expanded research and development and general and administrative activities.
Liquidity and Capital Resources
As of December 31, 2012 and 2011, we had $5,091,312 and $3,466,549 in cash and cash equivalents, respectively, primarily attributable to the closing of private placements aggregating gross proceeds of $6,000,000 and $2,000,000 in 2012 and 2011, respectively.
Cash Flows from Operating Activities. Net cash used for operating activities was $3,797,476 for 2012 and is primarily attributable to our net loss of $4,845,308, which was partially offset by $1,156,429 in non-cash activities relating to stock-based compensation and depreciation expense. Net cash used for operating activities was $2,743,375 for 2011 and primarily reflects a net loss of $3,236,491, which was partially offset by $457,253 in non-cash activities relating primarily to stock-based compensation expense.
We expect to continue to incur substantial and increasing losses and have negative net cash flows from operating activities as we seek to expand and support our technology portfolio and research and development activities.
Cash Flows from Investing Activities. Net cash used for investing activities was $547,884 for 2012 as compared to $1,051,737 for 2011. The net cash used related primarily to equipment acquired for research and development activities.
We expect to increase our investment in laboratory equipment as we seek to expand and progress our research and development capabilities.
Cash Flows from Financing Activities. Net cash provided by financing activities for 2012 and 2011 was $5,970,123 and $1,984,083, respectively, which were primarily derived from the sale of our common stock in private placement transactions.
Future Liquidity Needs. From inception through December 31, 2012, we have principally financed our operations through private equity financings with aggregate net proceeds of $15,451,451, as we have not generated any product related revenue from operations to date, and do not expect to generate significant revenue for several years, if ever. We will need to raise additional capital before we exhaust our current cash resources in order to continue to fund our research and development, including our long-term plans for preclinical trials and new product development, as well as to fund operations generally. As and if necessary, we will seek to raise additional funds through various potential sources, such as equity and debt financings, or through corporate collaboration and license agreements. We can give no assurances that we will be able to secure such additional sources of funds to support our operations, or, if such funds are available to us, that such additional financing will be sufficient to meet our needs.
In February 2013, we entered into a loan and security agreement with a bank which provided us with an equipment loan for up to $1,000,000 (see Note 10 of the Notes to the Consolidated Financial Statements). In March 2013, we entered into: (i) a stock purchase agreement and issued 33,658,305 shares of common stock for aggregate gross proceeds of $6,418,495, and (ii) the IgDraSol Transactions (see Note 10 of the Notes to the Consolidated Financial Statements).
We anticipate that we will continue to incur net losses into the foreseeable future as we: (i) continue to identify and advance a number of potential drug candidates into preclinical development activities, (ii) acquire
29
Table of Contents
IgDraSol and continue to fund its operations, and (iii) expand our corporate infrastructure, including the costs associated with being a public company. Without additional funding, we believe that we will not have sufficient funds to meet our obligations beyond October 2013. These conditions give rise to substantial doubt as to our ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
We plan to continue to fund our losses from operations and capital funding needs through public or private equity or debt financings, strategic collaborations, licensing arrangements, asset sales, government grants or other arrangements. However, we cannot be sure that such additional funds will be available on reasonable terms, or at all. If we are unable to secure adequate additional funding, we may be forced to make reductions in spending, extend payment terms with suppliers, liquidate assets where possible, and/or suspend or curtail planned programs. In addition, if we do not meet our payment obligations to third parties as they come due, we may be subject to litigation claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. Any of these actions could materially harm our business, results of operations, and future prospects.
Our actual cash requirements may vary materially from those now planned, however, because of a number of factors, including the actual costs incurred to effect and support the IgDraSol Transactions and related operating activities, the pursuit of development of product candidates, competitive and technical advances, costs of commercializing any potential product candidates, and costs of filing, prosecuting, defending and enforcing any patent claims and any other intellectual property rights. If we are unable to raise additional funds when needed, we may not be able to develop any product candidates, we could be required to delay, scale back or eliminate some or all of our research and development programs and we may need to wind down our operations altogether. Each of these alternatives would have a material adverse effect on our business.
If we raise additional funds by issuing equity securities, substantial dilution to existing stockholders would result. If we raise additional funds by incurring debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and specific financial ratios that may restrict our ability to operate our business.
In its report on our consolidated financial statements for the year ended December 31, 2012, our independent registered public accounting firm included an explanatory paragraph expressing substantial doubt regarding our ability to continue as a going concern. A “going concern” opinion means, in general, that our independent registered public accounting firm has substantial doubt about our ability to continue our operations without continuing infusions of capital from external sources and this opinion could impair our ability to finance our operations through the sale of debt or equity securities or commercial bank loans. Our ability to continue as a going concern depends, in large part, on our ability to obtain additional financing, which is uncertain. If we are unable to to do so, our business would be jeopardized and we may not be able to continue operations and have to liquidate our assets and may receive less than the value at which those assets were carried on our consolidated financial statements, and in this event it is likely that investors will lose all or part of their investment.
Additionally, recent global market and economic conditions have been unprecedented and challenging with tighter credit conditions and recession in most major economies. As a result of these market conditions, the cost and availability of credit has been and may continue to be adversely affected by illiquid credit markets and wider credit spreads. Concern about the stability of the markets generally and the strength of counterparties specifically has led many lenders and institutional investors to reduce, and in some cases, cease to provide credit to businesses and consumers. These factors have led to a decrease in spending by businesses and consumers alike, and a corresponding decrease in global infrastructure spending. Continued turbulence in the U.S. and international markets and economies and prolonged declines in business and consumer spending may adversely affect our liquidity and financial condition, including its ability to access the capital markets to meet liquidity needs.
30
Table of Contents
Further, the NIH has notified all grant recipients that due to the current Congressional budget sequestration, the NIH may not be able to issue continuation awards, or it may be required to negotiate a reduction in the scope of existing awards to meet the constraints imposed. Additionally, plans for new grants or cooperative agreements may be re-scoped, delayed, or canceled depending on the nature of the work and the availability of resources. As a result, we cannot assure you that we will receive the funding under our existing NIH grants, and we may not be successful in securing additional grants from the NIH in the future.
Related Party Transactions. There were no related party transactions in 2011. In December 2011, we entered into a Stock Purchase Agreement and issued 12,500,000 shares of common stock, in a private placement transaction, at $0.16 per share for aggregate gross proceeds of $2,000,000. In May 2012, pursuant to the December 2011 Stock Purchase Agreement, as amended and restated, we issued 37,500,000 shares of common stock, in a private placement transaction, at $0.16 per share for aggregate gross proceeds of $6,000,000. 6,250,000 of the shares were purchased by an investor, Hongye SD Group, LLC, of which Dr. Henry Ji, our Chief Executive Officer and President, is a managing director.
Critical Accounting Policies
Our consolidated financial statements are prepared in accordance with generally accepted accounting principles. The preparation of these consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and related disclosures. We evaluate our estimates and assumptions on an ongoing basis. Our estimates are based on historical experience and various other assumptions that we believe to be reasonable under the circumstances. Our actual results could differ from these estimates.
We believe the following accounting policies and estimates are most critical to aid in understanding and evaluating our reported financial results.
Cash and Cash Equivalents. We consider all highly liquid investments purchased with original maturities of three months or less to be cash equivalents. We minimize our credit risk associated with cash and cash equivalents by periodically evaluating the credit quality of our primary financial institution. The balance at times may exceed federally insured limits. As of December 31, 2012, we have not experienced any losses on such accounts.
Stock-Based Compensation. We account for stock-based compensation in accordance with authoritative guidance for stock-based compensation, which requires us to measure the cost of employee services received in exchange for equity incentive awards, including stock options, based on the grant date fair value of the award. The fair value is estimated using the Black-Scholes option pricing model. The resulting cost is recognized over the period during which the employee is required to provide services in exchange for the award, which is usually the vesting period. We recognize compensation expense over the vesting period using the straight-line method and classify these amounts in the consolidated statements of operations based on the department to which the related employee reports. To the extent that we issue future stock incentive awards to employees, our stock-based compensation expense will be increased by the additional unearned compensation resulting from such additional issuances.
We account for equity instruments, including restricted stock or stock options, issued to non-employees in accordance with authoritative guidance for equity based payments to non-employees. Stock options issued to non-employees are accounted for at their estimated fair value determined using the Black-Scholes option-pricing model. The fair value of options granted to non-employees is re-measured as they vest, and the resulting increase in value, if any, is recognized as expense during the period the related services are rendered. Restricted stock issued to non-employees is accounted for at its estimated fair value upon vesting. We evaluate the assumptions used to value stock awards to non-employees on a periodic basis. If factors change and we employ different assumptions, including any significant change in the estimated fair value of common stock, stock-based compensation expense may differ significantly from what we have recorded historically. In addition, to the extent
31
Table of Contents
that we issue future stock incentive awards to non-employees, our stock-based compensation expense will be increased by the additional unearned compensation resulting from such additional issuances.
Revenue Recognition. Our revenues are generated from grant awards and a Collaboration Agreement. The revenue from grant awards is based upon subcontractor costs and internal costs incurred that are specifically covered by each grant, and where applicable, plus a facilities and administrative rate that provides funding for overhead expenses. These revenues are recognized when expenses have been incurred by subcontractors or when we incur internal expenses that are related to the grant. The revenue from the Collaboration Agreement is derived from the completion of certain development services and the reimbursement of certain development costs incurred to provide such development services as provided for in the Collaboration Agreement. Revenue from upfront, nonrefundable service fees are recognized when earned, as evidenced by written acknowledgement from the collaborator, or other persuasive evidence that all service deliverables have been achieved, provided that the service deliverables are substantive and their achievability was not reasonably assured at the inception of the Collaboration Agreement. Any amounts received prior to satisfying our revenue recognition criteria are recorded as deferred revenue.
Off-Balance Sheet Arrangements
From our inception through December 31, 2012, we did not engage in any off-balance sheet arrangements, as defined in Item 303(a)(4) of Regulation S-K.
Recent Accounting Pronouncements
Refer to Note 2, “Nature of Operations and Summary of Significant Accounting Polices,” in the accompanying notes to the consolidated financial statements for a discussion of recent accounting pronouncements.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk. |
As a smaller reporting company, as defined by Item 10(f)(1) of Regulation S-K, we are not required to provide the information set forth in this Item.
| Item 8. | Financial Statements and Supplementary Data. |
Our consolidated financial statements and supplementary data required by this item are set forth at the pages indicated in Item 15(a)(1) and (a)(2), respectively, of this Form 10-K.
| Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure. |
None.
| Item 9A. | Controls and Procedures. |
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports filed under the Securities Exchange Act of 1934, as amended, or the Exchange Act, is recorded, processed, summarized and reported within the time periods specified in the SEC’s regulations, rules and forms and that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate, to allow for timely decisions regarding required disclosure.
In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of
32
Table of Contents
achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. As required by Rule 13a-15(b) promulgated by the SEC under the Exchange Act, we carried out an evaluation, under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered by this Form 10-K. Based on the foregoing, our chief executive officer and chief financial officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this Form 10-K.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) promulgated by the SEC under the Exchange Act. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control—Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2012.
This Form 10-K does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to rules of the SEC that permit us to provide only management’s report in this Form 10-K.
Changes in Internal Control Over Financial Reporting
There has been no change in our internal control over financial reporting during the quarter ended December 31, 2012 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | Other Information. |
None.
| Item 10. | Directors, Executive Officers and Corporate Governance. |
Directors and Executive Officers
The following table sets forth information concerning our executive officers and directors, including their ages, as of March 22, 2013:
| Name |
Age | Title(s) | ||||
| Dr. Henry Ji |
48 | Director, Chief Executive Officer and President | ||||
| Richard G. Vincent |
49 | Director, Chief Financial Officer and Secretary | ||||
| Dr. David Webb |
68 | Director | ||||
| Dr. Ernst-Guenter Afting |
70 | Director | ||||
| Cam Gallagher |
43 | Director | ||||
| Dr. Kim D. Janda |
55 | Director | ||||
| M. Scott Salka |
51 | Director | ||||
33
Table of Contents
Henry Ji, Ph.D. co-founded and has served as a director of Sorrento Therapeutics, Inc. since January 2006, served as its Chief Scientific Officer from November 2008 to September 2012, as its Interim Chief Executive Officer from April 2011 to September 2012, and as its Chief Executive Officer and President since September 2012. Dr. Ji also served as our Secretary from September 2009 to June 2011. In 2002, Dr. Ji founded BioVintage, Inc., a research and development company focusing on innovative life science technology and product development, and has served as its President since 2002. From 2001 to 2002, Dr. Ji served as Vice President of CombiMatrix Corporation, a publicly traded biotechnology company that develops proprietary technologies, including products and services in the areas of drug development, genetic analysis, molecular diagnostics and nanotechnology. During his tenure at CombiMatrix, Dr. Ji was responsible for strategic technology alliances with biopharmaceutical companies. From 1999 to 2001, Dr. Ji served as Director of Business Development, and in 2001 as Vice President, of Stratagene Corporation (later acquired by Agilent Technologies, Inc.) where he was responsible for novel technology and product licensing and development. In 1997, Dr. Ji co-founded Stratagene Genomics, Inc., a wholly owned subsidiary of Stratagene Corporation, and served as its President and Chief Executive Officer from its founding until 1999. Dr. Ji is the holder of several issued and pending patents in the life science research field and is the sole inventor of Sorrento Therapeutics Inc.’s intellectual property. Dr. Ji has a Ph.D. in Animal Physiology from the University of Minnesota and a B.S. in Biochemistry from Fudan University.
Dr. Ji has demonstrated significant leadership skills as President and Chief Executive Officer of Stratagene Genomics, Inc. and Vice President of CombiMatrix Corporation and Stratagene Corporation and brings more than 18 years of biotechnology and biopharmaceutical experience to his position on our board of directors. Dr. Ji’s extensive knowledge of the industry in which we operate, as well as his unique role in our day-to-day operations as our Chief Executive Officer and President, allows him to bring to our board of directors a broad understanding of the operational and strategic issues we face.
Richard G. Vincent joined us in January 2010, and served as our Chief Financial Officer, on a part-time basis, from February 2010 to September 2012 and has served as our Chief Financial Officer on a full time basis since September 2012. Since April 2008, Mr. Vincent has also served as a contract Chief Financial Officer for various other companies, including Elevation Pharmaceuticals, Inc., Meritage Pharma, Inc., Genoa Pharmaceuticals, Inc., Verus Pharmaceuticals, Inc., Chumby Industries, Inc. and Suneva Medical, Inc. From October 2004 to March 2009, Mr. Vincent served as Chief Financial Officer of Verus Pharmaceuticals, Inc., a specialty pharmaceutical company focused on developing and commercializing pediatric drug/device combination products for the treatment of asthma, allergies, and related diseases and conditions. From October 2003 to October 2004, Mr. Vincent served as Chief Financial Officer of Women First HealthCare, Inc., a public specialty pharmaceutical company focused on women’s healthcare and dermatology. Mr. Vincent also served as Senior Director of Finance for Elan Pharmaceuticals, Inc., a public biopharmaceutical company engaged in research, development and commercial activities primarily in neuroscience, autoimmune and severe chronic pain, from October 2001 to October 2003. From November 1995 to October 2001, Mr. Vincent served in various senior management capacities for several wireless and technology companies. From 1987 to 1995, Mr. Vincent held a number of positions with Deloitte & Touche LLP, the last of which was senior manager, where he specialized in emerging growth and publicly-reporting companies. Mr. Vincent became a Certified Public Accountant in California in 1989 and holds a B.S. degree in business with an emphasis in accounting from San Diego State University.
Mr. Vincent brings over 20 years of experience serving in key financial roles (including as chief financial officer for various companies) in a variety of privately-held and publicly-traded companies in the pharmaceutical and biopharmaceutical industries as well as serving in the public accounting industry for over nine years. He combines general business expertise and capital-raising experience, with a deep knowledge of financial matters and financial reporting obligations of a public company. In addition, Mr. Vincent has extensive corporate governance and operational management experience with both privately-held and publicly-traded companies, giving him a deep understanding of the challenges faced by public companies and allowing him to bring a variety of viewpoints and perspectives to our board of directors.
34
Table of Contents
David Webb, Ph.D. Since 2006 to the present, Dr. Webb has served on the Board of Directors of BIOCOM and Connect. Since August 2011, Dr. Webb has served as a member of the Board of Directors of the La Jolla Institute of Allergy and Immunology, and became Adjunct Professor, Department of Molecular Biology at The Scripps Research Institute in June 2011. Dr. Webb joined Celgene Corporation—San Diego in 2003 as Vice President, Research and in 2006 also became site head, Celgene, San Diego and retired in that role in June 2011. Celgene is a publicly-traded global biopharmaceutical company primarily engaged in the discovery, development and commercialization of innovative therapies designed to treat cancer and immune-inflammatory related diseases. Between 1995 and 2003, he held a series of senior management positions in several biotechnology companies where he developed and led drug discovery programs focused on inflammation, asthma, cancer and diabetes. These include Syrrx, OSI Pharmaceuticals, and Cadus Pharmaceutical Corporation where he was Vice President of Research and Chief Scientific Officer from 1995 to 1999 and adjunct professor of Microbiology and Immunology at New York Medical College. From 1987 to 1995, Dr. Webb was at Syntex, Inc. where he held the positions of Distinguished Scientist and later, Director, Institute of Immunology and Biological Sciences and was also a Consulting Professor of Cancer Biology at Stanford University. From 1973 to 1987, he was a member of the Department of Cell Biology, Roche Institute of Molecular Biology and Adjunct Associate Professor at Columbia University, College of Physicians and Surgeons. Dr. Webb earned his Ph.D. in Microbiology/Immunology at Rutgers, The State University of New Jersey-New Brunswick, and a B.A., M.A. in Biology from California State University-Fullerton.
Dr. Webb’s experience and qualifications for Board membership include his extensive background in the drug discovery, pre-clinical and clinical development, organizational development, including strategies and drug development programs, project management from target validation through lead optimization to full drug approval, business development expertise, development and managed research alliances for both domestic and international pharmaceutical and biotechnology companies, international project formation, profit and loss responsibilities, and development and management.
Ernst-Guenter Afting previously served as a director of the Company from September 2009 through April 1, 2010. From 1995 until his retirement in 2006, Dr. Afting served as President and Chief Executive Officer of the National Research Center for Environment and Health, GSF-National Research Center for Environment and Health GmbH, a governmental research center in Munich, Germany. From 1993 to 1995, he served as President and Chief Executive Officer of Roussel UCLAF, Paris, a pharmaceutical company. He was also a member of the board of directors of the Pharmaceutical Division of Hoechst Group from 1984 to 1993 and was chairman and Chief Executive Officer of the Divisional Pharmaceutical Board of Hoechst from 1992 to 1993. Dr. Afting was a member of the Advisory Committee on Science and Technology to German Chancellor Helmut Kohl from 1996 to 1997 and from 1996 to 2005 was a member of the German National Advisory Committee on Health Research to the State Secretaries of Science, Technology and Health. Dr. Afting has been a member of the medical faculty at the University of Goettingen since 1985. Dr. Afting currently serves on the boards of Intercell AG, Enanta Pharmaceuticals, Inc., and Olympus Europa GmbH. He received his Ph.D. in Chemistry and M.D. from the University of Freiburg/Breisgau, Germany.
With over 20 years of experience serving in key senior executive roles (including as president and chief executive officer) and as an active board member in a variety of privately-held and publicly-traded companies in the pharmaceutical and drug industries, Dr. Afting brings to our board of directors critical insights into the operational requirements of a public company. Dr. Afting’s experience and knowledge of the global biotechnology industry provides needed information to our board of directors and management and will be valuable to our overall business and strategies relating to our ongoing development efforts and transition to commercial activities.
Cam Gallagher has served as a director since September 2012. Mr. Gallagher currently serves as the sole Managing Director of Nerveda, LLC, a life science seed fund he founded in June 2009. Previously, from September 2007 until June 2009, Mr. Gallagher served as President and Chief Executive Officer of Nerveda, Inc., a privately held specialty pharmaceutical company in San Diego, California. Since October 2008,
35
Table of Contents
Mr. Gallagher has served as Chairman of the Board of Directors of DioGenix, Inc., a privately held molecular diagnostic company in Gaithersburg, Maryland. Mr. Gallagher is currently acting as the Senior Vice President of Business Development for Neurelis, Inc, a privately held specialty pharmaceutical company in San Diego, California. From December 2004 through June 2007, Mr. Gallagher served in various senior marketing positions of increasing responsibility at Verus Pharmaceuticals, Inc., a privately held specialty pharmaceutical company in San Diego, California. Mr. Gallagher holds an M.B.A. from the University of San Diego and a B.S. in Business Administration from the Ohio University.
Mr. Gallagher’s experiences in raising capital as a fund manager, President and Chief Executive Officer of a startup company, affiliations with multiple entrepeneurs across various therapeutic areas, and commercialization expertise with various biotechnology companies bring a great business and practical understanding of our industry. Mr. Gallagher brings a broad understanding of the operational, financial and strategic issues facing companies and his experience in all aspects of early commercial-stage companies provides valuable insight and perspective to our board of directors.
Kim D. Janda, Ph.D. has served as a director since April 2012. Dr. Janda has served as Ely R. Callaway, Jr. Chaired Professor in the Departments of Chemistry, Immunology and Microbial Science at The Scripps Research Institute since 1996 and as the Director of the Worm Institute of Research and Medicine (WIRM) at The Scripps Research Institute since 2005. Furthermore, Dr. Janda has served as a Skaggs Scholar within the Skaggs Institute of Chemical Biology, also at The Scripps Research Institute, since 1996. Dr. Janda holds a B.S. degree from the University of South Florida in Clinical Chemistry and a doctoral degree from the University of Arizona with Robert B. Bates in natural product total synthesis. A hallmark of his research is that Dr. Janda has been able to uniquely combine principles of medicinal chemistry together with modern molecular biology, immunology and neuropharmacology, allowing the creation of both synthetic/natural molecules and processes with biological, chemical and physical properties. Dr. Janda has published over 425 original publications in refereed journals and founded the biotechnological companies CombiChem, Drug Abuse Sciences and AIPartia. Dr. Janda is associate editor of Bioorg. & Med. Chem., PloS ONE and serves, or has served, on editorial boards of numerous journals including J. Comb. Chem., Chem. Reviews, J. Med. Chem., The Botulinum Journal, Bioorg. & Med. Chem. Lett., and Bioorg. & Med. Chem. Over a career of almost 25 years, Dr. Janda has provided numerous seminal contributions and is considered one of the first scientists to merge chemical and biological approaches into a cohesive research program. Dr. Janda serves on the Scientific Advisory Boards of Materia, Inc. and Singapore Ministry of Education (MOE), EP1 Physical Sciences.
Dr. Janda has almost 25 years of experience in life sciences and a very strong technical expertise relating to the discovery and development of antibody therapeutics, which gives him a unique understanding of the operational challenges and opportunities facing our company. As an experienced scientist and inventor on multiple patents in the life science industry, Dr. Janda brings critical insights into the operational requirements of a discovery and development antibody company as well as to our overall business and strategies relating to our ongoing development efforts, and serves as the chair of our Scientific Advisory Board.
M. Scott Salka has served as a director since February 2012. Mr. Salka currently serves as CEO of Aspyrian, CendR, and Vesper Biologics, all biotechnology companies. Previously, from 2001-2010, Mr. Salka served as CEO of Ambit Biosciences, a biotechnology company in San Diego, California. Since 2009, Mr. Salka has served on the Board of Directors of San Diego State University School of Business and has served on the Board of Directors of BIOCOM, the largest regional life science association in the world, since 2007. Mr. Salka holds an MBA from Carnegie Mellon University and a B.S. in Finance from San Diego State University.
Mr. Salka’s experience as a CEO of multiple biotechnology companies and knowledge of the biotechnology industry bring a great business and practical understanding of our industry. Mr. Salka brings a broad understanding of the operational, financial and strategic issues facing companies and his experience in all aspects of early-stage companies provides valuable insight and perspective to the Board.
36
Table of Contents
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, requires our directors and executive officers, and persons who beneficially own more than ten percent of a registered class of our equity securities, to file with the SEC initial reports of ownership and reports of changes of ownership of common stock and our other equity securities. Officers, directors and greater than ten percent stockholders are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file.
To our knowledge, based solely on a review of the copies of such reports furnished to us and written representations that no other reports were required, during the fiscal year ended December 31, 2012, no officer, director or greater than ten percent beneficial owner was delinquent with their Section 16(a) filing requirements.
Code of Ethics
We have adopted the Sorrento Therapeutics, Inc. Code of Business Conduct and Ethics that applies to all of our employees, executive officers and directors. The Code of Business Conduct and Ethics is available to stockholders on our Internet website at www.sorrentotherapeutics.com under “Corporate Governance.” If we make any substantive amendments to our Code of Business Conduct and Ethics or grant any waiver from a provision of the Code of Business Conduct and Ethics to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on our Internet website at www.sorrentotherapeutics.com under “Corporate Governance” and/or in our public filings with the SEC.
Consideration of Director Nominees
Director Qualifications
There are no specific minimum qualifications that our Board of Directors, or the Board, requires to be met by a director nominee recommended for a position on our Board, nor are there any specific qualities or skills that are necessary for one or more members of our Board to possess, other than as are necessary to meet the requirements of the rules and regulations applicable to us. The Board considers a potential director candidate’s experience, areas of expertise and other factors relative to the overall composition of our Board and its committees, including the following characteristics:
| • | the highest ethical standards and integrity and a strong personal reputation; |
| • | a background that provides experience and achievement in business, finance, biotechnology or other activities relevant to our business and activities; |
| • | a willingness to act on and be accountable for Board and, as applicable, committee decisions; |
| • | an ability to provide wise, informed and thoughtful counsel to management on a range of issues affecting us and our stockholders; |
| • | an ability to work effectively and collegially with other individuals; |
| • | loyalty and commitment to driving our success and increasing long-term value for our stockholders; |
| • | sufficient time to devote to Board and, as applicable, committee membership and matters; and |
| • | the independence requirements imposed by the SEC and the NYSE Amex. |
The Board retains the right to modify these qualifications from time to time.
Security Holder Nominations
The Board will consider director candidates recommended by our stockholders of record. The Board does not intend to alter the manner in which it evaluates candidates, including the minimum criteria set forth above, based on whether a candidate was recommended by a stockholder of record or not. Stockholders of record who wish to recommend individuals for consideration by the Board to become nominees for election to the Board at
37
Table of Contents
an annual meeting of stockholders must do so by delivering a written recommendation to the Board, c/o Sorrento Therapeutics, Inc., 6042 Cornerstone Ct. West, Suite B, San Diego, California 92121, Attn: Corporate Secretary, no later than the close of business on the 45th day nor earlier than the 75th day prior to the anniversary date of the initial mailing of our proxy statement for our preceding year’s annual meeting of stockholders. However, if the meeting date is more than 30 days before or after the 1 year anniversary of the preceding year’s annual meeting of stockholders, written recommendations must be received by the Secretary at the principal executive offices by not later than the close of business on the later of (i) the 90th day before such annual meeting or (ii) the 10th day following the day on which public announcement (as defined in our bylaws) of the date of such meeting is first made. Each written recommendation must set forth, among other information:
| • | the name and address of the stockholder of record and any beneficial owner on whose behalf the nomination is being made; |
| • | the class, series and number of our shares, and any of our convertible securities, that are beneficially owned by the stockholder of record and any beneficial owner on whose behalf the nomination is being made; |
| • | any proxy, contract, arrangement, understanding or relationship pursuant to which the stockholder of record and any beneficial owner on whose behalf the nomination is being made has the right to vote any of our voting securities; |
| • | any “short” interest in our securities held by the stockholder of record and any beneficial owner on whose behalf the nomination is being made; |
| • | the proposed director candidate’s name, age, business address and residential address; |
| • | complete biographical information for the proposed director candidate, including the proposed director candidate’s principal occupation or employment and business experience for at least the previous five years; |
| • | the class and number of our shares that are beneficially owned by the proposed director candidate as of the date of the written recommendation; and |
| • | any other information relating to the proposed director candidate that is required to be disclosed in solicitations for proxies for election of directors pursuant to Regulation 14A promulgated under the Exchange Act. |
Director candidate nominations from stockholders must be provided in writing and must include the written consent of each proposed nominee to serve as director if so elected.
If a proposed director candidate is recommended by a security holder in accordance with the procedural requirements discussed above, the Corporate Secretary will provide the foregoing information to the Board.
Board Leadership Structure
We have a separate Chairman of the Board, Dr. David Webb, and Chief Executive Officer, Dr. Henry Ji. We believe that having an independent director serve as our Chairman allows our Chief Executive Officer to focus on our business, while allowing the Chairman of the Board to fulfill his fundamental Board leadership role, which includes providing advice to and independent oversight of our Board.
The Chairman of the Board role requires significant additional commitment, particularly as the Board’s oversight responsibilities continue to grow due to our expanding business operations. Our Board is committed to good corporate governance and believes that it is appropriate for an independent, highly-qualified, director to serve as its Chairman.
Our Chairman of the Board is responsible for the orderly functioning of our Board and enhancing its effectiveness. Our Chairman guides Board processes, provides input on agenda items and presides at Board meetings. Our Chairman additionally acts as a liaison between our Board members and our executive
38
Table of Contents
management team, consulting regularly and providing guidance on Board-related matters. In the absence of the Chairman, another independent director typically presides at meetings of the Board.
Board’s Role in Risk Oversight
Our Board has an active role, as a whole and also at the committee level, in overseeing management of our risks. The Board regularly reviews information regarding our credit, liquidity and operations, as well as the risks associated with each. Our Compensation Committee is responsible for overseeing the management of risks relating to our executive compensation plans and arrangements. Our Audit Committee oversees management of financial risks and manages risks associated with the independence of the Board of Directors and potential conflicts of interest and oversees management of risks associated with health and safety concerns. While each committee is responsible for evaluating certain risks and overseeing the management of such risks, our entire Board is informed about such risks by the committees.
Information Regarding Board Committees
Our Board has established standing Audit and Compensation Committees to devote attention to specific subjects and to assist it in the discharge of its responsibilities. Both committees operate under a written charter adopted by our Board, each of which is available on our Internet website at www.sorrentotherapeutics.com under “Corporate Governance.” In February 2012, our Board formed a Science Committee which is currently comprised of Drs. Janda (Chairperson), Afting and Salka to provide assistance to the Board in fulfilling its oversight responsibilities relating to: (i) our research and development and technology strategies and initiatives; and (ii) significant trends in science and technology and the potential impact of such trends on our business and operations.
Audit Committee
We have a separately designated standing Audit Committee established in accordance with Section 3(a)(58)(A) of the Exchange Act. Our Audit Committee is currently comprised of Mr. Salka, Dr. Afting and Mr. Gallagher. Mr. Salka serves as the Chairperson of the Audit Committee. The functions of this Committee include, among others:
| • | evaluating our independent registered public accountant’s qualifications, independence and performance; |
| • | determining the engagement of our independent auditors; |
| • | approving the retention of our independent auditors to perform any proposed audit and permissible non-audit services; |
| • | monitoring the rotation of partners of our independent auditors on our engagement terms as required by law; |
| • | reviewing our financial statements; |
| • | reviewing our critical accounting policies and estimates; |
| • | discussing with our management and our independent auditors the results of the annual audit and the review of our quarterly financial statements; and |
| • | reviewing and evaluating, at least annually, the performance of the Audit Committee and its members, including compliance of the Audit Committee with its charter. |
Typically, the Audit Committee meets at least quarterly and with greater frequency if necessary. Our Board has adopted a written charter of the Audit Committee that is available to stockholders on our Internet website at www.sorrentotherapeutics.com under “Corporate Governance.”
Our common stock is quoted on the OTCBB. Since the OTCBB does not have rules regarding independence of audit committee members, the Board makes its determination as to the independence of our Audit Committee members based on the definition of “independence” as defined under the rules of the NYSE Amex. Under the applicable rules and regulations of the NYSE Amex, each member of a company’s audit committee must be considered independent in accordance with Section 803A of the NYSE Amex LLC Company Guide and Rule
39
Table of Contents
10A-3(b)(1) under the Exchange Act. Our Board reviews the NYSE Amex standards and Exchange Act definitions of independence for Audit Committee members on an annual basis and has determined that all members of our Audit Committee are independent (as independence is currently defined in Section 803A of the NYSE Amex Listed Company Manual). Our Board has determined that all members of our Audit Committee also meet the requirements for financial literacy under the NYSE Amex listing standards.
Our Board has determined that Mr. Salka is an audit committee financial expert, as defined under applicable SEC rules, and that both Dr. Afting and Mr. Gallagher meet the background and financial sophistication requirements under Section 803A of the NYSE Amex LLC Company Guide. In making these determinations, the Board made a qualitative assessment of Dr. Afting and Mr. Gallagher’s level of knowledge and experience based on a number of factors, including his formal education and experience. Both our independent registered public accounting firm and internal financial personnel regularly meet privately with our Audit Committee and have unrestricted access to this committee.
REPORT OF THE AUDIT COMMITTEE OF THE BOARD OF DIRECTORS
Our Audit Committee is composed of “independent” directors, as determined in accordance with Section 803A of the NYSE Amex LLC Company Guide and Rule 10A-3 of the Exchange Act. The Audit Committee operated pursuant to a written charter adopted by the Board, a copy of which may be viewed on our Internet website at www.sorrentotherapeutics.com under “Corporate Governance.”
As described more fully in its charter, the purpose of the Audit Committee is to assist our Board with its oversight responsibilities regarding the integrity of our financial statements, our compliance with legal and regulatory requirements, assessing the independent registered public accounting firm’s qualifications and independence and the performance of the persons performing internal audit duties for us and the independent registered public accounting firm. Management is responsible for preparation, presentation and integrity of our financial statements as well as our financial reporting process, accounting policies, internal audit function, internal accounting controls and disclosure controls and procedures. The independent registered public accounting firm is responsible for performing an independent audit of our financial statements in accordance with Standards of the Public Company Accounting Oversight Board and to issue a report thereon. The Audit Committee’s responsibility is to monitor and oversee these processes. The following is the Audit Committee’s report submitted to the Board for 2012.
The Audit Committee has:
| • | reviewed and discussed our audited consolidated financial statements with management and Mayer Hoffman McCann P.C., the independent registered public accounting firm; |
| • | discussed with Mayer Hoffman McCann P.C. the matters required to be discussed by AU Section 380, Communications with Audit Committees, as may be modified or supplemented; and |
| • | received from Mayer Hoffman McCann P.C. the written disclosures and the letter regarding their communications with the Audit Committee concerning independence as required by the Public Company Accounting Oversight Board and discussed the auditors’ independence with them. |
In addition, the Audit Committee has met separately with management and with Mayer Hoffman McCann P.C.
Based on the review and discussions referred to above, the Audit Committee recommended to the Board that the audited consolidated financial statements be included in our Annual Report on Form 10-K for the year ended December 31, 2012 for filing with the Securities and Exchange Commission.
Audit Committee
Mr. M. Scott Salka
Dr. Ernst Guenter Afting
Mr. Cam Gallagher
40
Table of Contents
This foregoing audit committee report is not “soliciting material,” is not deemed “filed” with the SEC, and shall not be deemed incorporated by reference by any general statement incorporating by reference this Annual Report on Form 10-K into any filing of ours under the Securities Act of 1933, as amended, or under the Securities Exchange Act of 1934, as amended, except to the extent we specifically incorporate this report by reference.
| Item 11. | Executive Compensation. |
Compensation Committee
Our Compensation Committee is comprised of Dr. Janda, Dr. Webb and Mr. Salka. Dr. Janda serves as the Chairperson of our Compensation Committee. The functions of this committee include, among others:
| • | determining the compensation and other terms of employment of our executive officers and reviewing and approving corporate performance goals and objectives relevant to such compensation; |
| • | evaluating and recommending the type and amount of compensation to be paid or awarded to our Board members; |
| • | evaluating and recommending to our Board the equity incentive plans, compensation plans and similar programs advisable for us, as well as modification or termination of existing plans and programs; |
| • | administering our equity incentive plans; |
| • | establishing policies with respect to equity compensation arrangements; |
| • | reviewing and approving the terms of any employment agreements, severance arrangements, change in control protections and any other compensatory arrangements for our executive officers; and |
| • | reviewing and evaluating, at least annually, the performance of the Compensation Committee and its members, including compliance of the Compensation Committee with its charter. |
Our Board has adopted a written charter of the Compensation Committee that is available to stockholders on our Internet website at www.sorrentotherapeutics.com under “Corporate Governance.” The Compensation Committee meets periodically throughout the year as necessary. The agenda for each meeting is usually developed by the Chairperson of the Compensation Committee, in consultation with our Chief Executive Officer and other representatives of senior management as necessary. The Compensation Committee meets regularly in executive session. However, from time to time, various members of management and other employees as well as outside advisors or consultants may be invited by the Compensation Committee to make presentations, provide financial or other background information or advice or otherwise participate in Compensation Committee meetings. The Chief Executive Officer may not participate in or be present during any deliberations or determinations of the Compensation Committee regarding his compensation. The charter of the Compensation Committee grants the Compensation Committee full access to all of our books, records, facilities and personnel, as well as authority to obtain, at our expense, advice and assistance from internal and external legal, accounting or other advisors and consultants and other external resources that the Compensation Committee considers necessary or appropriate in the performance of its duties. In particular, the Compensation Committee has the sole authority to retain or consult compensation consultants to assist in its evaluation of executive and director compensation, including the authority to approve the consultant’s reasonable fees and other retention terms.
The Compensation Committee meets outside the presence of all of our executive officers, including the named executive officers, in order to consider appropriate compensation for our Chief Executive Officer. For all other named executive officers, the Compensation Committee meets outside the presence of all executive officers except our Chief Executive Officer. The annual performance reviews of our executive officers are considered by the Compensation Committee when making decisions on setting base salary, targets for and payments under our bonus plan and grants of equity incentive awards. When making decisions on executive officers, the
41
Table of Contents
Compensation Committee considers the importance of the position to us, the past salary history of the executive officer and the contributions we expect the executive officer to make to the success of our business.
Compensation Philosophy
The primary goals of our Board with respect to executive compensation are to attract and retain talented and dedicated executives, to tie annual and long-term cash and stock incentives to achievement of specified performance objectives, and to create incentives resulting in increased stockholder value. To achieve these goals, our Compensation Committee recommends to our Board executive compensation packages, generally comprising a mix of salary, discretionary bonus and equity awards. Although we have not adopted any formal guidelines for allocating total compensation between equity compensation and cash compensation, we have implemented and maintain compensation plans that tie a substantial portion of our executives’ overall compensation to achievement of corporate goals.
Summary Compensation Table
The following table provides information regarding the compensation earned during the years ended December 31, 2012 and 2011 by our Chief Executive Officer and President and our Chief Financial Officer. We refer to these individuals as our “named executive officers.” There are no other executive officers who earned in excess of $100,000 for the year ended December 31, 2012.
| Name and Principal Position |
Year | Salary ($) |
Bonus ($) |
Option Awards ($) (1) |
Fees Earned Or Paid in Cash ($) |
All Other Compensation ($) (2) |
Total ($) |
|||||||||||||||||||||
| Henry Ji, Ph.D.(3) |
2012 | 255,237 | — | 77,500 | — | 19,255 | 351,992 | |||||||||||||||||||||
| 2011 | 240,000 | — | — | — | 22,371 | 262,371 | ||||||||||||||||||||||
| Richard Vincent(4) |
2012 | 183,506 | — | 936,250 | (5) | — | 4,739 | 1,124,495 | ||||||||||||||||||||
| 2011 | 117,525 | — | 63,500 | — | — | 181,025 | ||||||||||||||||||||||
| (1) | These amounts represent the aggregate grant date fair value of awards for grants of options to each named executive officer in the relevant fiscal year, computed in accordance with FASB ASC Topic 718. The dollar amounts listed do not necessarily reflect the dollar amounts of compensation actually realized or that may be realized by our named executive officers. The value as of the grant date for stock options is recognized over the number of days of service required for the stock option to vest in full. For a detailed description of the assumptions used for purposes of determining grant date fair value, see Note 6 to the financial statements included in this Annual Report on Form 10-K. |
| (2) | The amounts in this column consist of health and welfare benefits for Dr. Ji in 2012 and 2011 and for Mr. Vincent in 2012. |
| (3) | Dr. Ji served as our Chief Scientific Officer from November 2008 through September 2012, has served as a director since January 2006, as our Chief Executive Officer since April 2011 and as President since September 2012. |
| (4) | Mr. Vincent was appointed Chief Financial Officer in February 2010 and served us, on a part time basis, from January 2010 through September 2012. Mr. Vincent has served us on a full time basis as our Chief Financial Officer since September 2012. |
| (5) | Pursuant to a Stock Option Cancellation Agreement and Option Amendment dated as of September 30, 2012, Mr. Vincent forfeited an option to purchase 725,000 shares of common stock. This amount represents the aggregate grant date fair value of awards for grants of options Mr. Vincent received for the fiscal year ended December 31, 2012, including such forfeited option. The aggregate grant date fair value of the forfeited option was $217,500. |
42
Table of Contents
We paid no perquisites or other personal benefits to our executive officers during the years ended December 31, 2012 and 2011.
OUTSTANDING EQUITY AWARDS AT DECEMBER 31, 2012
The following table presents the outstanding option awards held by each of our named executive officers as of December 31, 2012.
| Option Award | Stock Awards | |||||||||||||||||||||||||||
| Name |
Option Grant Date |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Earned Options (#) Unexercisable |
Option Exercise Price ($) (3) |
Option Expiration Date |
Number of Shares or Units of Stock That Have Not Vested |
Market Value of Shares or Units of Stock That Have Not Vested |
|||||||||||||||||||||
| Henry Ji, Ph.D. |
02/16/10 | (1) | 75,000 | 75,000 | $ | 0.07 | 02/16/20 | — | — | |||||||||||||||||||
| 02/06/12 | (1) | — | 250,000 | $ | 0.16 | 02/06/22 | — | — | ||||||||||||||||||||
| Richard Vincent |
02/05/10 | (2) | 100,000 | — | $ | 0.07 | 02/05/20 | — | — | |||||||||||||||||||
| 03/07/11 | (1) | 250,000 | 250,000 | $ | 0.14 | 03/07/21 | — | — | ||||||||||||||||||||
| 02/06/12 | (1) | 31,250 | 93,750 | $ | 0.16 | 02/06/22 | — | — | ||||||||||||||||||||
| 09/30/12 | (4) | — | 2,275,000 | $ | 0.16 | 09/22/22 | — | — | ||||||||||||||||||||
| (1) | All of the options vest and become exercisable as to 25% of the shares subject to the option on each of the first four anniversaries of the date of grant. |
| (2) | All of the options vested and became exercisable on February 15, 2011. |
| (3) | Represents the fair market value of a share of our common stock, as determined by our Board, on the option’s grant date. |
| (4) | The options shall vest and become exercisable over a four-year period, with 1/4th of the shares subject to the option vesting on September 22, 2013 and 1/48th of the shares subject to the option vesting following each one-month period of service thereafter. |
Elements of Compensation
We evaluate individual executive performance with a goal of setting compensation at levels the Board or any applicable committee thereof believes are comparable with executives in other companies of similar size and stage of development while taking into account our relative performance and our own strategic goals. The compensation received by our named executive officers consists of the following elements:
Base Salary. Base salaries for our named executive officers are established based on the scope of their responsibilities and individual experience, taking into account competitive market compensation paid by other companies for similar positions within our industry.
Discretionary Annual Bonus. In addition to base salaries, our Board or the applicable committee thereof has the authority to award discretionary annual bonuses to our named executive officers. The annual incentive bonuses are intended to compensate officers for achieving corporate goals and value-creating milestones. No bonuses were awarded during 2012 or 2011.
Equity-Based Incentives. Salaries and bonuses are intended to compensate our executive officers for short-term performance. We also have adopted an equity incentive program intended to reward longer-term performance and to help align the interests of our named executive officers with those of our stockholders. We believe that long-term performance is achieved through an ownership culture that rewards performance by our named executive officers through the use of equity incentives. Our equity incentive plan has been established to
43
Table of Contents
provide our employees, including our named executive officers, with incentives to help align those employees’ interests with the interests of our stockholders.
When making equity-award decisions, the Compensation Committee considers market data, the grant size, the forms of long-term equity compensation available to it under our existing plans and the status of previously granted awards. The amount of equity incentive compensation granted reflects the executives’ expected contributions to our future success. Existing ownership levels are not a factor in award determination, as the Compensation Committee does not want to discourage executives from holding significant amounts of our stock.
Future equity awards that we make to our named executive officers will be driven by our sustained performance over time, our named executive officers’ ability to impact our results that drive stockholder value, their level of responsibility, their potential to fill roles of increasing responsibility, and competitive equity award levels for similar positions in comparable companies. Equity forms a key part of the overall compensation for each executive officer and is evaluated each year as part of the annual performance review process and incentive payout calculation.
The amounts awarded to the named executive officers are based on the Compensation Committee’s subjective determination of what is appropriate to incentivize the executives. Generally, the grants to named executive officers vest over a four-year period with 25% vesting on each anniversary of the grant date. All equity awards to our employees, including named executive officers, and to directors, have been granted and reflected in our financial statements, based upon the applicable accounting guidance, with the exercise price equal to the fair market value on the grant date based on the valuation determined by our Board.
Employment Agreements
Employment Agreement with Dr. Henry Ji. Dr. Ji is currently employed as our Chief Executive Officer and President, and is a party to an employment agreement entered into on September 21, 2012, and amended on October 18, 2012, or the Ji Employment Agreement. Pursuant to the Ji Employment Agreement, Dr. Ji will receive an initial annual base salary of $262,500, which amount will be subject to increase each year at the discretion of the Board or an authorized committee thereof. Dr. Ji will also be eligible to participate in an annual incentive program established by the Board. Dr. Ji’s target annual incentive compensation under such incentive program will be 60% of his then-applicable annual base salary. The annual bonus payable under the annual incentive program will be based on the achievement of individual and Company performance goals to be determined in good faith by the Board or an authorized committee of the Board. We have the right to terminate Dr. Ji’s employment at any time with or without “cause” or upon Dr. Ji’s death or disability, each as defined in the Ji Employment Agreement. Dr. Ji may resign with or without “good reason,” as defined in the Ji Employment Agreement, upon 30 days written notice. Under such circumstances, Dr. Ji will be entitled to receive any accrued but unpaid base salary as of the date of termination or resignation, any expenses owed to him and any amount accrued and arising from his participation in, or vested benefits accrued under, any employee benefit plans, programs or arrangements. The Ji Employment Agreement also includes provisions regarding severance. If Dr. Ji is terminated without cause or resigns for good reason, he will also be entitled to 12 months of his then-applicable base salary paid in a lump sum and 12 months of health care benefits continuation at our expense. If we terminate Dr. Ji for cause or he resigns without good reason, he shall not be entitled to further compensation. He shall have no obligation to seek other employment and any income so earned shall not reduce the foregoing amounts. The Ji Employment agreement also contains standard confidentiality, non-competition and non-solicitation covenants.
Employment Agreement with Richard Vincent. Mr. Vincent is currently employed as our Chief Financial Officer, and is a party to an employment agreement entered into on September 21, 2012 and amended on October 18, 2012, or the Vincent Employment Agreement. From January 2010 through September 2012, Mr. Vincent was a party to a consulting agreement whereby he was serving as our Chief Financial Officer on a part-time basis. Pursuant to the Vincent Employment Agreement, Mr. Vincent will receive an initial annual base
44
Table of Contents
salary of $280,000, which amount will be subject to increase each year at the discretion of the Board or an authorized committee thereof. Mr. Vincent will also be eligible to participate in an annual incentive program established by the Board. Mr. Vincent’s target annual incentive compensation under such incentive program will be 25% of his then-applicable annual base salary. The annual bonus payable under the annual incentive program will be based on the achievement of individual and Company performance goals to be determined in good faith by the Board or an authorized committee of the Board. We have the right to terminate Mr. Vincent’s employment at any time with or without “cause” or upon Mr. Vincent’s death or disability, each as defined in the Vincent Employment Agreement. Mr. Vincent may resign with or without “good reason,” as defined in the Vincent Employment Agreement, upon 30 days written notice. Under such circumstances, Mr. Vincent will be entitled to receive any accrued but unpaid base salary as of the date of termination or resignation, any expenses owed to him and any amount accrued and arising from his participation in, or vested benefits accrued under, any employee benefit plans, programs or arrangements. The Vincent Employment Agreement also includes provisions regarding severance. If Mr. Vincent is terminated without cause or resigns for good reason, he will also be entitled to 12 months of his then-applicable base salary paid in a lump sum and 12 months of health care benefits continuation at our expense. If we terminate Mr. Vincent for cause or he resigns without good reason, he shall not be entitled to further compensation. He shall have no obligation to seek other employment and any income so earned shall not reduce the foregoing amounts. The Vincent Employment agreement also contains standard confidentiality, non-competition and non-solicitation covenants.
Other Compensation. We intend to provide benefits and perquisites for our named executive officers at levels comparable to those provided to other executive officers in our industry. Our Board or any applicable committee thereof, in its discretion, may revise, amend or add to the benefits and perquisites of any named executive officer as it deems it advisable and in the best interest of the Company and our stockholders.
Non-Employee Director Compensation
The following table summarizes the total compensation paid to or earned by each of our directors who served during all or a portion of the year ended December 31, 2012.
| Name (1) |
Fees Earned or Paid in Cash ($) |
Option Awards ($) (2) |
Total ($) |
|||||||||
| Glenn Halpryn(3) |
5,273 | 42,000 | 47,273 | |||||||||
| Dr. Curtis Lockshin(3) |
5,273 | 24,900 | 30,173 | |||||||||
| Dr. Jane Hsiao(3) |
5,273 | 18,000 | 23,273 | |||||||||
| Dr. Kim Janda |
14,828 | 166,500 | 181,328 | |||||||||
| Diane D-S. Tang-Liu(3) |
3,773 | 22,200 | 25,973 | |||||||||
| Dr. David Webb(4) (5) |
9,583 | 157,500 | 167,083 | |||||||||
| Scott Salka(6) |
13,328 | 162,200 | 175,528 | |||||||||
| Cam Gallagher(4) |
7,986 | 140,000 | 147,986 | |||||||||
| Dr. Ernst-Guenter Afting(4) |
9,583 | 140,000 | 149,583 | |||||||||
| (1) | Dr. Ji, our Chief Executive Officer and President and Richard Vincent, our Chief Financial Officer, both named executive officers, are not included in this table as they are employees and thus receive no compensation for their service as directors. Dr. Ji’s and Mr. Vincent’s compensation for 2012 is included in the Summary Compensation Table above. |
| (2) | These amounts represent the aggregate grant date fair value of awards for grants of options to each listed director for the fiscal year ended December 31, 2012, computed in accordance with FASB ASC Topic 718. These amounts do not represent the actual amounts paid to or realized by the directors during the fiscal year ended December 31, 2012. The value as of the grant date for stock options is recognized over the number of months of service required for the stock option to vest in full. For a detailed description of the assumptions used for purposes of determining grant date fair value, see Note 6 to the Consolidated Financial Statements included in this Annual Report on Form 10-K. As of December 31, 2012, our non-employee directors held |
45
Table of Contents
| the following number of options: Dr. Janda—947,500; Dr. Webb—450,000; Mr. Salka—475,000; Mr. Gallagher—400,000; and Dr. Afting—440,000. |
| (3) | Mr. Halpryn, Dr. Lockshin, Dr. Hsiao and Ms. Tang-Liu’s service on the Board ceased effective as of September 6, 2012. |
| (4) | Dr. Webb, Mr. Gallagher, and Dr. Afting were appointed to the Board effective as of September 6, 2012. |
| (5) | Pursuant to a Stock Option Cancellation Agreement dated as of October 17, 2012, Dr. Webb forfeited an option to purchase 37,500 shares of common stock which was issued in September 2011. This amount represents the aggregate grant date fair value of awards for grants of options Dr. Webb received for the fiscal year ended December 31, 2012, excluding such forfeited option. The aggregate grant date fair value of the forfeited option was $4,987. |
| (6) | Mr. Salka was appointed to the Board effective as of February 23, 2012. |
Non-Employee Director Compensation Policy. We compensate non-employee members of our Board. Directors who are also employees do not receive cash or equity compensation for service on our Board in addition to compensation payable for their service as our employees.
From January 1, 2012 through September 5, 2012, each outside director received a quarterly fee of $1,500 for his or her service as a director.
Under our new non-employee director compensation policy, or Director Compensation Policy, which was adopted as of September 6, 2012, or the Effective Date, and amended as of December 12, 2012, we provide cash compensation in the form of an annual retainer of $25,000 for each non-employee director. We also pay an additional annual retainer of $5,000 to the chairman of our Board and $5,000 to the chairman of each of the audit, compensation and science committees. In addition, in the event our Board determines that a non-employee director’s service at any time requires extraordinary efforts, such non-employee director shall be eligible to receive additional compensation as may be approved by our Board in connection with such additional service. We have reimbursed and will continue to reimburse our non-employee directors for their reasonable travel expenses incurred in attending our Board and committee meetings.
Also under our Director Compensation Policy, any non-employee director who is (i) a non-employee director on the Effective Date or (ii) initially elected or appointed to our Board following the Effective Date, will be granted an option to purchase 400,000 shares of our common stock on the Effective Date or on the date of his or her initial election or appointment to our Board, as applicable (provided that such award shall be 450,000 shares of our common stock if the non-employee director is also serving as chairman of our Board). These options will be granted under our 2009 Stock Incentive Plan, or 2009 Plan. Such options will have an exercise price per share equal to the fair market value (as defined in the 2009 Plan) of our common stock on the date of grant.
The initial options granted to non-employee directors will vest in forty-eight equal monthly installments on the last day of each one-month period of service following the date of the initial option grant (or, with respect to the initial options granted to those individuals who were non-employee directors as of the Effective Date, following September 6, 2012), such that each initial option shall be 100% vested on the fourth anniversary of the date of grant (or, with respect to the initial options granted to those individuals who were non-employee directors as of the Effective Date, on September 6, 2016), subject to the non-employee director’s continuing service on our Board on those dates. The term of each option granted to a non-employee director will be ten years.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth information as of March 22, 2013, with respect to the beneficial ownership of shares of our common stock by:
| • | each person or group known to us to be the beneficial owner of more than five percent of our common stock; |
46
Table of Contents
| • | each of our directors; |
| • | each of our named executive officers; and |
| • | all of our current directors and executive officers as a group. |
This table is based upon information supplied by officers, directors and principal stockholders and a review of Schedules 13D and 13G, if any, filed with the SEC. Other than as set forth below, we are not aware of any other beneficial owner of more than five percent of our common stock as of March 22, 2013. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons and entities named in the table below have sole voting and investment power with respect to all shares of common stock that they beneficially own, subject to applicable community property laws.
Applicable percentage ownership is based on 336,075,440 shares of common stock outstanding as of March 22, 2013, adjusted as required by rules promulgated by the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include shares of common stock issuable pursuant to the exercise of stock options that are either immediately exercisable or exercisable on or before May 21, 2013, which is 60 days after March 22, 2013. These shares are deemed to be outstanding and beneficially owned by the person holding those options for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person.
Unless otherwise noted below, the address of each beneficial owner listed in the table is c/o Sorrento Therapeutics, Inc., 6042 Cornerstone Ct. West, Suite B, San Diego, California 92121.
| Beneficial Ownership of Common Stock | ||||||||
| Name of Beneficial Owner |
Number of Shares | Percentage of Class |
||||||
| Named Executive Officers and Directors: |
||||||||
| Dr. Henry Ji, Director, Chief Executive Officer and President |
58,541,532 | (1) | 17.4 | % | ||||
| Richard G. Vincent, Chief Financial Officer and Secretary |
381,250 | (2) | * | |||||
| Dr. Ernst-Guenter Afting, Director |
1,032,096 | (3) | * | |||||
| Dr. David Webb, Director |
75,000 | (4) | * | |||||
| Mr. M. Scott Salka, Director |
141,666 | (5) | * | |||||
| Mr. Cam Gallagher, Director |
66,666 | (6) | * | |||||
| Dr. Kim Janda, Director 10550 N. Torrey Pines Road, 8CC-582 La Jolla, CA 92037 |
414,166 | (7) | * | |||||
| All Officers and Directors as a Group (7 Persons) |
60,652,376 | (9) | 18.0 | % | ||||
| 5% Stockholders: |
||||||||
| Dr. Antonius Schuh, Former Chairman and Former Chief Executive Officer |
25,484,329 | (8) | 7.6 | % | ||||
| Dr. Henry Ji, Director, Chief Executive Officer and President |
58,541,532 | (1) | 17.4 | % | ||||
| Stephen Zaniboni |
25,484,329 | (10) | 7.6 | % | ||||
| OPKO Health, Inc. 4400 Biscayne Boulevard, Suite 900 Miami, Florida 33137 |
59,015,257 | 17.6 | % | |||||
| Donald Scifres One First Street, Suite 14 Los Altos, CA 94022 |
38,844,714 | (11) | 11.6 | % | ||||
| Halpryn Group VI, LLC 4400 Biscayne Boulevard, Suite 950 Miami, Florida 33137 |
20,168,115 | (12) | 6.0 | % | ||||
| Steven Jerry Glauser 1400 16th Street, Suite 510 Denver, Colorado 80202 |
21,292,847 | (13) | 6.3 | % | ||||
47
Table of Contents
| * | Less than 1%. |
| (1) | Comprised of 137,500 shares of common stock issuable pursuant to stock options exercisable within 60 days of March 22, 2013 and 52,154,032 shares of common stock held in family trusts, of which Dr. Ji is a co-trustee with his wife Vivian Q. Zhang. Each of Dr. Ji and Vivian Q. Zhang, while acting as co-trustees, have the power to act alone and have those actions binding on both trustees’ and the trusts’ assets, including voting and dispositive power over the shares of common stock held by the family trusts. |
| (2) | Comprised of 381,250 shares of common stock issuable pursuant to stock options exercisable within 60 days of March 22, 2013. |
| (3) | Comprised of 106,666 shares of common stock issuable pursuant to stock options exercisable within 60 days of March 22, 2013 and 925,430 shares of common stock held directly. |
| (4) | Comprised of 75,000 shares of common stock issuable pursuant to stock options exercisable within 60 days of March 22, 2013. Dr. Webb disclaims beneficial ownership of the shares of common stock gifted to and held by his two children. |
| (5) | Comprised of 141,666 shares of common stock issuable pursuant to stock options exercisable within 60 days of March 22, 2013. |
| (6) | Comprised of 66,666 shares of common stock issuable pursuant to stock options exercisable within 60 days of March 22, 2013. |
| (7) | Comprised of 414,166 shares of common stock issuable pursuant to stock options exercisable within 60 days of March 22, 2013. |
| (8) | Comprised of 25,484,329 shares of common stock held in a trust, of which Dr. Schuh is the trustee. |
| (9) | Comprised of shares included under “Named Executive Officers and Directors”. |
| (10) | These shares are held in a trust, of which Mr. Zaniboni is the trustee. |
| (11) | These shares are held in a trust account, of which Mr. Scifres is the trustee. |
| (12) | Comprised of 1,091,673 shares of common stock held directly, 17,184,228 shares of common stock held by Halpryn Group VI, LLC, of which Mr. Halpryn is a member, 555,556 shares of common stock held by Prine Intervest Limited, of which Mr. Halpryn is a member, and 1,336,658 shares of common stock held by IVC Investors, LLLP, in which Mr. Halpryn has an interest. Mr. Halpryn disclaims beneficial ownership of the shares of common stock held by each of Halpryn Group VI, LLC and IVC Investors, LLLP, except to the extent of any pecuniary interest therein. |
| (13) | Comprised of 4,108,619 shares of common stock held directly and 17,184,228 shares of common stock held by Halpryn Group VI, LLC, of which Mr. Glauser is a member. Mr. Glauser disclaims beneficial ownership of the shares of common stock held by Halpryn Group VI, LLC, except to the extent of any pecuniary interest therein. |
| Item 13. | Certain Relationships, Related Transactions and Director Independence. |
Certain Relationships and Related Transactions
The following is a description of transactions or series of transactions since January 1, 2012, or any currently proposed transaction, to which we have been a party, in which the amount involved in the transaction or series of transactions exceeds the lesser of $120,000 or one percent of the average of our total assets as of December 31, 2012 and December 31, 2011, and in which any of our directors, executive officers or persons who we know held more than five percent of any class of our capital stock, including their immediate family members, had or will have a direct or indirect material interest, other than compensation arrangements that are described under “Employment Agreements” above.
Common Stock Private Placement
In December 2011, we entered into a Stock Purchase Agreement with the Donald R. Scifres 2011 Annuity Trust Y, or the Scifres Trust, an accredited investor, and issued12,500,000 shares of common stock in consideration for an aggregate investment of $2,000,000. In May 2012, we entered into an Amended and
48
Table of Contents
Restated Stock Purchase Agreement, and issued 37,500,000 shares of common stock, in a private placement transaction, at $0.16 per share for aggregate gross proceeds of $6,000,000. 6,250,000 of the shares were purchased by an investor, Hongye SD Group, LLC, of which Dr. Henry Ji, our Chief Executive Officer and President, is a managing director.
The December 2011 Stock Purchase Agreement provides to SDL preemptive rights, except under certain conditions, for SDL to participate in any offer or sale by us of any capital stock or other securities of any type that are, or may become convertible, into capital stock, up to SDL’s pro rata portion, on the same terms, conditions and price provided in any subsequent placement of its securities. SDL’s preemptive rights do not apply to, among others things: (a) shares of common stock, options, warrants or other convertible securities issued to employees or officers or directors or outside consultants or contractors of the Company or any subsidiary pursuant to a plan, agreement or arrangement duly approved by our Board; (b) securities issued in connection with the acquisition of all or a substantial portion of the assets or the business of another entity by us; (c) securities issued in connection with a corporate partnering transaction, strategic alliance, technology transfer, license or similar transaction; or (d) any shares of common stock sold at a price equal to or greater than $0.16 per share (as adjusted for any stock splits, stock dividends, stock combinations, and similar events occurring after the date of the Stock Purchase Agreement, or the Purchase Price, or any other capital stock or other securities of any type whatsoever convertible into or exchangeable for our common stock at a price equal to or greater than the Purchase Price (as adjusted for any stock splits, stock dividends, stock combinations, and similar events occurring after the date of the Stock Purchase Agreement). SDL’s preemptive rights terminate on the earliest of (a) such time as SDL no longer is the owner of 40,000,000 shares of our common stock (as adjusted for any stock splits, stock combinations, and similar events occurring after the date of the Stock Purchase Agreement), (b) December 29, 2016, (c) the date our common stock is listed on a national exchange, or (d) the date of the closing of a sale or other disposition of all or substantially all of our assets or our merger into or consolidation with any corporation or other entity, in which the holders of our outstanding voting stock immediately prior to such transaction own, immediately after such transaction, securities representing less than 50% of our voting power or the surviving entity.
The Stock Purchase Agreement provides that we shall have one designee of SDL Ventures, LLC, an affiliate of the Scifres Trust, nominated for election to the board, subject to approval of the full board. The SDL-nominee must have relevant industry experience or academic experience and satisfy the independence requirements of the Nasdaq Global Market. We nominated M. Scott Salka for election to our board, and the full board approved the nomination of M. Scott Salka in February 2012.
The Stock Purchase Agreement provides piggy back registration rights to each investor in the December 2011 Financing, with such rights terminating upon the earlier of (a) seven years from the date of the Stock Purchase Agreement or (b) when such investor is entitled to sell all of the shares purchased pursuant to the Stock Purchase Agreement without volume restriction pursuant to Rule 144.
Stock Option Grants
We have granted stock options to our non-employee directors and executive officers.
Indemnification Agreements with Directors and Executive Officers
We have entered into indemnity agreements with certain directors, officers and other key employees of ours under which we agreed to indemnify those individuals under the circumstances and to the extent provided for in the agreements, for expenses, damages, judgments, fines, settlements and any other amounts they may be required to pay in actions, suits or proceedings which they are or may be made a party or threatened to be made a party by reason of their position as a director, officer or other agent of ours, and otherwise to the fullest extent permitted under Delaware law and our bylaws. We also have an insurance policy covering our directors and executive officers with respect to certain liabilities, including liabilities arising under the Securities Act of 1933,
49
Table of Contents
as amended, or otherwise. We believe that these provisions and insurance coverage are necessary to attract and retain qualified directors, officers and other key employees.
Review, Approval or Ratification of Transactions with Related Persons
The Board conducts an appropriate review of and oversees all related party transactions on a continuing basis and reviews potential conflict of interest situations where appropriate. The Board has not adopted formal standards to apply when it reviews, approves or ratifies any related party transaction. However, the Board has followed the following standards: (i) all related party transactions must be fair and reasonable and on terms comparable to those reasonably expected to be agreed to with independent third parties for the same goods and/or services at the time they are authorized by the Board and (ii) all related party transactions should be authorized, approved or ratified by the affirmative vote of a majority of the directors who have no interest, either directly or indirectly, in any such related party transaction.
Director Independence
The Board has the responsibility for establishing corporate policies and for our overall performance, although it is not involved in day-to-day operations. Our common stock is quoted on the OTCBB. Since the OTCBB does not have rules regarding director independence, the Board makes its determination as to director independence based on the definition of “independence” as defined under the rules of the NYSE Amex. Our Board consults with our counsel to ensure that the Board’s determinations are consistent with all relevant securities and other laws and regulations regarding the definition of “independent,” including those set forth in applicable NYSE Amex rules, as in effect from time to time. Consistent with these considerations, after review of all relevant transactions or relationships between each director, or any of his family members, us, our senior management and our independent auditors, our Board has determined that all of our directors, other than Dr. Ji and Mr. Vincent, are independent, as defined in Section 803A of the NYSE Amex LLC Company Guide.
| Item 14. | Principal Accountant Fees and Services. |
The following table represents aggregate fees billed to us for the years ended December 31, 2012 and 2011 by Mayer Hoffman McCann P.C., or Mayer Hoffman, our principal auditor for such periods. All fees described below were approved by the Audit Committee.
| Year Ended | ||||||||
| December 31, 2012 |
December 31, 2011 |
|||||||
| Audit Fees(1) |
$ | 57,000 | $ | 53,000 | ||||
| Audit-Related Fees |
— | — | ||||||
| Tax Fees |
— | — | ||||||
| All Other Fees |
— | — | ||||||
| Total Fees |
$ | 57,000 | $ | 53,000 | ||||
| (1) | Audit fees for the year ended December 31, 2012 related to our annual audit for 2012 and the review of our 2012 quarterly financial statements and other SEC filings. Audit fees for the fiscal year ended December 31, 2011 related to our annual audit for 2011 and the review of our 2011 quarterly financial statements and other SEC filings. |
Audit Committee’s Pre-Approval Policies and Procedures
The Audit Committee has adopted a policy for the pre-approval of audit and non-audit services rendered by our independent auditors, Mayer Hoffman. The policy generally pre-approves specified services in the defined categories of audit services, audit-related services and tax services up to specified amounts. Pre-approval may also be given as part of the Audit Committee’s approval of the scope of the engagement of the independent
50
Table of Contents
auditors or on an individual explicit case-by-case basis before the independent auditors are engaged to provide each service. The pre-approval of services may be delegated to one or more of the Audit Committee’s members, but the decision must be reported to the full Audit Committee at its next scheduled meeting. By the adoption of this policy, the Audit Committee has delegated the authority to pre-approve services to the Chairperson of the Audit Committee, subject to certain limitations.
The Audit Committee has determined that the rendering of the services other than audit services by Mayer Hoffman is compatible with maintaining the principal accountant’s independence.
| Item 15. | Exhibits and Financial Statement Schedules. |
(a)(1) Financial Statements
The Consolidated Financial Statements of Sorrento Therapeutics, Inc. and Report of Independent Registered Public Accounting Firm, are included in a separate section of this Form 10-K beginning on page F-1.
(a)(2) Financial Statement Schedules
The schedules required to be filed by this item have been omitted because of the absence of conditions under which they are required, or because the required information is included in the consolidated financial statements or the notes thereto.
(a)(3) Exhibits
| Exhibit |
Description | |
| 2.1* | Merger Agreement, dated July 14, 2009, by and among QuikByte Software, Inc., Sorrento Therapeutics, Inc., Sorrento Merger Corp., Inc., the Stockholders’ Agent and the Parent Representative (incorporated by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K filed with the SEC on July 14, 2009). | |
| 2.2 | First Amendment to Merger Agreement, dated August 26, 2009, by and among QuikByte Software, Inc., Sorrento Therapeutics, Inc., Sorrento Merger Corp., Inc., the Stockholders’ Agent and the Parent Representative (incorporated by reference to Exhibit 2.2 to the Registrant’s Current Report on Form 8-K filed with the SEC on August 26, 2009). | |
| 2.3 | Plan of Conversion (incorporated by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K filed with the SEC on October 23, 2009). | |
| 3.1 | Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed with the SEC on October 23, 2009). | |
| 3.2 | Certificate of Ownership and Merger (incorporated by reference to Exhibit 3.2 to the Registrant’s Current Report on Form 8-K filed with the SEC on December 7, 2009). | |
| 3.3 | Bylaws (incorporated by reference to Exhibit 3.2 to the Registrant’s Current Report on Form 8-K filed with the SEC on October 23, 2009). | |
| 4.1 | Specimen Common Stock Certificate (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K filed with the SEC on October 23, 2009). | |
| 9.1 | Form of Stockholder Voting Agreement by and among QuikByte Software, Inc. and the Stockholders of Sorrento Therapeutics, Inc. set forth on the signature page thereto, dated as of July 14, 2009 (incorporated by reference to Exhibit 9.1 to the Registrant’s Current Report on Form 8-K filed with the SEC on September 21, 2009). | |
51
Table of Contents
| Exhibit |
Description | |
| 10.1 | Form of Stock Purchase Agreement, dated September 18, 2009, by and among QuikByte Software, Inc. and the Investors listed on Exhibit A thereto (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed with the SEC on September 21, 2009). | |
| 10.2 | Escrow Agreement, dated September 21, 2009, by and among QuikByte Software, Inc., the Stockholders’ Agent, the Parent Representative and Bank of America, N.A. (incorporated by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K/A filed with the SEC on September 22, 2009). | |
| 10.3 | Stock Purchase Agreement dated December 21, 2010 by and among Sorrento Therapeutics, Inc. and the Investors whose names appear on the signature pages thereto (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed with the SEC on December 23, 2010). | |
| 10.4 | Stock Purchase Agreement dated December 29, 2011 by and between the Company and Donald R. Scifers 2011 Annuity Trust Y (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on form 8-K filed with the SEC on January 5, 2012). | |
| 10.5 | First Amendment to Stock Purchase Agreement, dated February 28, 2012 (the original agreement dated December 29, 2011), by and among Sorrento Therapeutics, Inc. and the Investors whose names appear on the signature pages thereto. (incorporated by reference to Exhibit 10.40 to the Registrant’s Quarterly Report on form 10-Q filed with the SEC on May 15, 2012). | |
| 10.6 | Second Amendment to Stock Purchase Agreement, dated March 30, 2012 (the original agreement dated December 29, 2011), by and among Sorrento Therapeutics, Inc. and the Investors whose names appear on the signature pages thereto. (incorporated by reference to Exhibit 10.41 to the Registrant’s Quarterly Report on form 10-Q filed with the SEC on May 15, 2012). | |
| 10.7 | Amended and Restated Stock Purchase Agreement dated May 14, 2012 by and between the Company and the investors whose names appear on the signature pages thereof. (incorporated by reference to Exhibit 10.1 to Registrant’s Current Report on form 8-K filed with the SEC on May 14, 2012). | |
| 10.8 | Standard Multi-Tenant Office Lease-Net, dated July 28, 2008, by and between Sorrento Therapeutics, Inc. and Suntree Garden, LLC (incorporated by reference to Exhibit 10.6 to the Registrant’s Current Report on Form 8-K filed with the SEC on September 21, 2009). | |
| 10.9 | First Amendment to Office Lease, dated August 18, 2009, by and between Sorrento Therapeutics, Inc. and Suntree Garden, LLC (incorporated by reference to Exhibit 10.7 to the Registrant’s Current Report on Form 8-K filed with the SEC on September 21, 2009). | |
| 10.10 | Second Amendment to Office Lease, dated October 1, 2009, by and between Sorrento Therapeutics, Inc. and Suntree Garden, LLC (incorporated by reference to Exhibit 10.10 to the Registrant’s Annual Report on Form 10-K filed with the SEC on March 25, 2010). | |
| 10.11 | Third Amendment to Office Lease, dated November 11, 2010, by and between Sorrento Therapeutics, Inc. and Suntree Garden, LLC (incorporated by reference to Exhibit 10.12 to the Registrant’s Annual Report on Form 10-K filed with the SEC on March 30, 2012). | |
| 10.12 | Fourth Amendment to Office Lease, dated January 17, 2011, by and between Sorrento Therapeutics, Inc. and Suntree Garden, LLC (incorporated by reference to Exhibit 10.13 to the Registrant’s Annual Report on Form 10-K filed with the SEC on March 30, 2012). | |
| 10.13 | Fifth Amendment to Office Lease, dated February 9, 2012, by and between Sorrento Therapeutics, Inc. and Suntree Garden, LLC (incorporated by reference to Exhibit 10.14 to the Registrant’s Annual Report on Form 10-K filed with the SEC on March 30, 2012). | |
52
Table of Contents
| Exhibit |
Description | |
| 10.14 | Sixth Amendment to Office Lease, dated June 20 2012, by and between Sorrento Therapeutics, Inc. and Suntree Garden, LLC. (incorporated by reference to Exhibit 10.15 to the Registrant’s Quarterly Report on Form 10-Q filed with the SEC on August 14, 2012). | |
| 10.15 | Share Purchase Agreement, dated June 10, 2009, by and between Sorrento Therapeutics, Inc. and OPKO Health, Inc. (incorporated by reference to Exhibit 10.8 to the Registrant’s Current Report on Form 8-K filed with the SEC on September 21, 2009). | |
| 10.16 | Limited License Agreement, dated June 10, 2009, by and between Sorrento Therapeutics, Inc. and OPKO Health, Inc. (incorporated by reference to Exhibit 10.9 to the Registrant’s Current Report on Form 8-K filed with the SEC on September 21, 2009). | |
| 10.17+ | Patent Assignment Agreement, dated June 10, 2009, by and between Henry H. Ji and Sorrento Therapeutics, Inc. (incorporated by reference to Exhibit 10.10 to the Registrant’s Current Report on Form 8-K filed with the SEC on September 21, 2009). | |
| 10.18+ | License Agreement, dated January 8, 2010, by and between The Scripps Research Institute and the Company (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed with the SEC on May 14, 2010). | |
| 10.19± | Form of Stock Option Agreement (incorporated by reference to Exhibit 10.11 to the Registrant’s Current Report on Form 8-K/A filed with the SEC on September 22, 2009). | |
| 10.20± | Form of Indemnity Agreement (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed with the SEC on October 23, 2009). | |
| 10.21± | Form of Indemnification Agreement (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed with the SEC on August 7, 2012). | |
| 10.22± | 2009 Stock Incentive Plan, and forms of agreements related thereto (incorporated by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed with the SEC on October 23, 2009). | |
| 10.23± | 2009 Equity Incentive Plan, and forms of agreement related thereto (incorporated by reference to Exhibit 10.17 to the Registrant’s Annual Report on Form 10-K filed with the SEC on March 25, 2010). | |
| 10.24± | Employment Agreement, dated September 21, 2012, by and between Sorrento Therapeutics, Inc. and Henry Ji, Ph.D. (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed with the SEC on November 8, 2012). | |
| 10.25± | First Amendment to Employment Agreement dated October 18, 2012, by and between Sorrento Therapeutics, Inc. and Henry Ji, Ph.D. (incorporated by reference to Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q filed with the SEC on November 8, 2012). | |
| 10.26± | Employment Agreement, dated September 21, 2012, by and between Sorrento Therapeutics, Inc. and Richard G. Vincent (incorporated by reference to Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q filed with the SEC on November 8, 2012). | |
| 10.27± | First Amendment to Employment Agreement, dated October 18, 2012, by and between Sorrento Therapeutics, Inc. and Richard G. Vincent (incorporated by reference to Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q filed with the SEC on November 8, 2012). | |
| 10.28± | Independent Director Compensation Policy | |
53
Table of Contents
| Exhibit |
Description | |
| 10.29± | Stock Option Cancellation Agreement and Option Amendment, dated September 30, 2012, by and between Sorrento Therapeutics, Inc. and Richard G. Vincent. | |
| 10.30± | Stock Option Cancellation Agreement, dated October 17, 2012, by and between Sorrento Therapeutics, Inc. and David Webb, Ph.D. | |
| 23.1 | Consent of Mayer Hoffman McCann P.C. | |
| 31.1 | Certification of Henry Ji, Ph.D., Principal Executive Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002, as amended. | |
| 31.2 | Certification of Richard Glenn Vincent, Principal Financial Officer, pursuant to Section 302 of the Sarbanes-Oxley Act of 2002, as amended. | |
| 32.1 | Certification of Henry Ji, Ph.D., Principal Executive Officer, and Richard Glenn Vincent, Principal Financial Officer, pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, as amended. | |
| 101.INS** | XBRL Instance Document | |
| 101.SCH** | XBRL Taxonomy Extension Schema Document | |
| 101.CAL** | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF** | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB** | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE** | XBRL Taxonomy Extension Presentation Linkbase Document | |
| * | Non-material schedules and exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K. The Registrant hereby undertakes to furnish supplementally copies of any of the omitted schedules and exhibits upon request by the SEC. |
| ** | Pursuant to Rule 406T of Regulation S-T, these interactive data files are deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933 or Section 18 of the Securities Act of 1934 and otherwise not subject to liability. |
| + | The SEC has granted confidential treatment with respect to certain portions of this exhibit. Omitted portions have been filed separately with the SEC. |
| ± | Management contract or compensatory plan. |
54
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: March 25, 2013 | SORRENTO THERAPEUTICS, INC. | |||||
| By: | /s/ HENRY JI | |||||
| Director, Chief Executive Officer & President | ||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature |
Title(s) |
Date | ||
| /S/ HENRY JI Henry Ji, Ph.D. |
Director, Chief Executive Officer & President (Principal Executive Officer) |
March 25, 2013 | ||
| /S/ RICHARD G. VINCENT Richard G. Vincent |
Director, Chief Financial Officer (Principal Financial and Accounting Officer) |
March 25, 2013 | ||
| /S/ DAVID WEBB David Webb, Ph.D. |
Director | March 25, 2013 | ||
| /S/ ERNST-GUNTER AFTING Ernst-Gunter Afting, Ph.D., M.D. |
Director | March 25, 2013 | ||
| /S/ CAM GALLAGHER Cam Gallagher |
Director | March 25, 2013 | ||
| /S/ KIM D. JANDA Kim D. Janda, Ph.D. |
Director | March 25, 2013 | ||
| /S/ M. SCOTT SALKA M. Scott Salka |
Director | March 25, 2013 | ||
55
Table of Contents
Sorrento Therapeutics, Inc.
(a Development Stage Company)
Index to Consolidated Financial Statements
| Page | ||||
| F-2 | ||||
| Consolidated Balance Sheets—As of December 31, 2012 and 2011 |
F-3 | |||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Sorrento Therapeutics, Inc. and Subsidiary
San Diego, California
We have audited the accompanying consolidated balance sheets of Sorrento Therapeutics, Inc. and Subsidiary (the “Company”) as of December 31, 2012 and 2011, and the related consolidated statements of operations, stockholders’ equity, and cash flows for the years then ended and for the period from January 25, 2006 (Inception) through December 31, 2012. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Sorrento Therapeutics, Inc. and Subsidiary as of December 31, 2012 and 2011, and the consolidated results of its operations and its cash flows for the years then ended and for the period from January 25, 2006 (Inception) through December 31, 2012, in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has experienced recurring losses, is dependent on future financing to fund its planned expenditures and had an accumulated deficit of approximately $10,950,000 at December 31, 2012. These conditions, among others, raise substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are also described in Note 2. The accompanying consolidated financial statements do not include any adjustments relating to the recoverability and classification of asset carrying amounts or the amount and classification of liabilities that might result should the Company be unable to continue as a going concern.
/s/ Mayer Hoffman McCann P.C.
San Diego, CA
March 25, 2013
F-2
Table of Contents
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Assets | ||||||||
| Current assets |
||||||||
| Cash and cash equivalents |
$ | 5,091,312 | $ | 3,466,549 | ||||
| Grants receivable |
79,760 | 61,238 | ||||||
| Prepaid expenses and other |
80,918 | 29,869 | ||||||
|
|
|
|
|
|||||
| Total current assets |
5,251,990 | 3,557,656 | ||||||
| Property and equipment, net |
1,480,989 | 988,445 | ||||||
| Other |
48,625 | 22,727 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 6,781,604 | $ | 4,568,828 | ||||
|
|
|
|
|
|||||
| Liabilities and stockholders’ equity | ||||||||
| Current liabilities |
||||||||
| Accounts payable |
$ | 439,533 | $ | 224,742 | ||||
| Accrued payroll and related |
77,744 | 88,510 | ||||||
| Accrued expenses |
66,896 | 46,087 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
584,173 | 359,339 | ||||||
| Commitments and contingencies (Note 7) |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.0001 par value; 100,000,000 shares authorized and no shares issued or outstanding |
— | — | ||||||
| Common stock, $0.0001 par value; 500,000,000 shares authorized and 300,117,135 and 262,347,135 shares issued and outstanding at December 31, 2012 and 2011, respectively |
30,012 | 26,235 | ||||||
| Additional paid-in capital |
17,117,718 | 10,288,245 | ||||||
| Deficit accumulated during the development stage |
(10,950,299 | ) | (6,104,991 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
6,197,431 | 4,209,489 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 6,781,604 | $ | 4,568,828 | ||||
|
|
|
|
|
|||||
See accompanying notes to consolidated financial statements.
F-3
Table of Contents
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF OPERATIONS
| Years Ended December 31, |
Period
from January 25, 2006 (Inception) through December 31, 2012 |
|||||||||||
| 2012 | 2011 | |||||||||||
| Revenues: |
||||||||||||
| Grant |
$ | 583,774 | $ | 329,184 | $ | 1,572,073 | ||||||
| Collaboration and reimbursable research and development costs |
— | 200,000 | 223,453 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
583,774 | 529,184 | 1,795,526 | |||||||||
| Expenses: |
||||||||||||
| Research and development |
3,830,404 | 2,570,406 | 8,203,326 | |||||||||
| General and administrative |
1,605,978 | 1,201,220 | 4,571,393 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total expenses |
5,436,382 | 3,771,626 | 12,774,719 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(4,852,608 | ) | (3,242,442 | ) | (10,979,193 | ) | ||||||
| Interest income |
7,300 | 5,951 | 28,894 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (4,845,308 | ) | $ | (3,236,491 | ) | $ | (10,950,299 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per share—basic and diluted |
$ | (0.02 | ) | $ | (0.01 | ) | ||||||
|
|
|
|
|
|||||||||
| Weighted average number of shares during the period—basic and diluted |
285,126,056 | 248,048,271 | ||||||||||
|
|
|
|
|
|||||||||
See accompanying notes to consolidated financial statements.
F-4
Table of Contents
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
| Common Stock | Additional Paid-in Capital |
Stockholder Note Receivable |
Deficit Accumulated During the Development Stage |
Total | ||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||
| Balance, January 25, 2006 (Inception) |
— | $ | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||
| Issuance of common stock for $400 cash to founders |
101,937,315 | 10,194 | (9,794 | ) | — | — | 400 | |||||||||||||||||
| Net loss |
— | — | — | — | (75,801 | ) | (75,801 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2006 |
101,937,315 | 10,194 | (9,794 | ) | — | (75,801 | ) | (75,401 | ) | |||||||||||||||
| Net loss |
— | — | — | — | (16,302 | ) | (16,302 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2007 |
101,937,315 | 10,194 | (9,794 | ) | — | (92,103 | ) | (91,703 | ) | |||||||||||||||
| Net loss |
— | — | — | — | (25,745 | ) | (25,745 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2008 |
101,937,315 | 10,194 | (9,794 | ) | — | (117,848 | ) | (117,448 | ) | |||||||||||||||
| Issuance of restricted common stock for $291 cash to consultants in March |
7,403,861 | 740 | (449 | ) | — | — | 291 | |||||||||||||||||
| Issuance of common stock for $10 cash and a $30 note to consultants in March |
1,019,374 | 102 | (62 | ) | (30 | ) | — | 10 | ||||||||||||||||
| Issuance of common stock for cash at $0.039 per share in June, net of issuance costs of $25,999 |
59,015,257 | 5,902 | 2,268,099 | — | — | 2,274,001 | ||||||||||||||||||
| Issuance of common stock for cash at $0.0448 per share in September |
44,634,374 | 4,463 | 1,995,537 | — | — | 2,000,000 | ||||||||||||||||||
| Issuance of common stock to former QuikByte stockholders in connection with the Merger |
11,073,946 | 1,107 | 99,279 | — | — | 100,386 | ||||||||||||||||||
| Costs associated with the Merger |
— | — | (168,767 | ) | — | — | (168,767 | ) | ||||||||||||||||
| Stock-based compensation |
— | — | 54,524 | — | — | 54,524 | ||||||||||||||||||
| Net loss |
— | — | — | — | (942,266 | ) | (942,266 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2009 |
225,084,127 | 22,508 | 4,238,367 | (30 | ) | (1,060,114 | ) | 3,200,731 | ||||||||||||||||
| Collection of note receivable |
— | — | — | 30 | — | 30 | ||||||||||||||||||
| Issuance of common stock for cash at $0.14 per share in December, net of issuance costs of $159,905 |
25,717,143 | 2,572 | 3,437,923 | — | — | 3,440,495 | ||||||||||||||||||
| Stock-based compensation |
— | — | 250,954 | — | — | 250,954 | ||||||||||||||||||
| Net loss |
— | — | — | — | (1,808,386 | ) | (1,808,386 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2010 |
250,801,270 | 25,080 | 7,927,244 | — | (2,868,500 | ) | 5,083,824 | |||||||||||||||||
| Repurchase of common stock |
(1,104,135 | ) | (110 | ) | 67 | — | (43 | ) | ||||||||||||||||
| Issuance of common stock in connection with the exercise of stock options |
150,000 | 15 | 13,110 | — | — | 13,125 | ||||||||||||||||||
| Issuance of common stock for cash at $0.16 per share in December, net of issuance costs of $28,999 |
12,500,000 | 1,250 | 1,969,751 | — | — | 1,971,001 | ||||||||||||||||||
| Reduction of stock issuance costs accrued in December 2010 |
— | — | 80,039 | — | — | 80,039 | ||||||||||||||||||
| Stock-based compensation |
— | — | 298,034 | — | — | 298,034 | ||||||||||||||||||
| Net loss |
— | — | — | — | (3,236,491 | ) | (3,236,491 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2011 |
262,347,135 | 26,235 | 10,288,245 | — | (6,104,991 | ) | 4,209,489 | |||||||||||||||||
| Issuance of common stock in connection with the exercise of stock options |
270,000 | 27 | 36,065 | — | — | 36,092 | ||||||||||||||||||
| Issuance of common stock for cash at $0.16 per share in May, net of issuance costs of $65,969 |
37,500,000 | 3,750 | 5,930,281 | — | — | 5,934,031 | ||||||||||||||||||
| Stock-based compensation |
— | — | 863,127 | — | — | 863,127 | ||||||||||||||||||
| Net loss |
— | — | — | — | (4,845,308 | ) | (4,845,308 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2012 |
300,117,135 | $ | 30,012 | $ | 17,117,718 | $ | — | $ | (10,950,299 | ) | $ | 6,197,431 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
F-5
Table of Contents
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended December 31, |
Period
from January 25, 2006 (Inception) through December 31, 2012 |
|||||||||||
| 2012 | 2011 | |||||||||||
| Operating activities |
||||||||||||
| Net loss |
$ | (4,845,308 | ) | $ | (3,236,491 | ) | $ | (10,950,299 | ) | |||
| Adjustments to reconcile net loss to net cash used for operating activities: |
||||||||||||
| Depreciation and amortization |
293,302 | 159,219 | 478,689 | |||||||||
| Stock-based compensation |
863,127 | 298,034 | 1,466,639 | |||||||||
| Increase (decrease) in cash resulting from changes in: |
||||||||||||
| Grants receivable |
(18,522 | ) | 184,807 | (79,760 | ) | |||||||
| Prepaid expenses and other |
(76,947 | ) | 15,635 | (109,393 | ) | |||||||
| Accounts payable |
(23,171 | ) | 42,943 | 176,946 | ||||||||
| Deferred revenue |
— | (200,000 | ) | — | ||||||||
| Accrued expenses and other liabilities |
10,043 | (7,522 | ) | 224,679 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used for operating activities |
(3,797,476 | ) | (2,743,375 | ) | (8,792,499 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Investing activities |
||||||||||||
| Purchases of property and equipment |
(547,884 | ) | (1,051,737 | ) | (1,721,717 | ) | ||||||
| Cash received in connection with Merger |
— | — | 104,860 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used for investing activities |
(547,884 | ) | (1,051,737 | ) | (1,616,857 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Financing activities |
||||||||||||
| Proceeds from issuance of common stock, net of issuance costs and repurchases |
5,934,031 | 1,970,958 | 15,451,451 | |||||||||
| Proceeds from exercise of stock options |
36,092 | 13,125 | 49,217 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
5,970,123 | 1,984,083 | 15,500,668 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net change in cash and cash equivalents |
1,624,763 | (1,811,029 | ) | 5,091,312 | ||||||||
| Cash and cash equivalents at beginning of period |
3,466,549 | 5,277,578 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of period |
$ | 5,091,312 | $ | 3,466,549 | $ | 5,091,312 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosures: |
||||||||||||
| Cash paid during the period for: |
||||||||||||
| Income taxes |
$ | 800 | $ | 800 | $ | 4,800 | ||||||
| Non-cash financing activities: |
||||||||||||
| In 2011, the Company reduced its stock issuance costs accrued in 2010 by $80,039.
In December 2012, the Company purchased equipment with an aggregate cost of $237,960, which has been included in accounts payable as of December 31, 2012. |
| |||||||||||
See accompanying notes to consolidated financial statements.
F-6
Table of Contents
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Reverse Merger Transaction and Accounting
Reverse Merger Transaction
On September 21, 2009, QuikByte Software, Inc., a Colorado corporation and shell company, or QuikByte, acquired Sorrento Therapeutics, Inc., a privately held Delaware corporation, or STI, in a reverse merger, or the Merger. Pursuant to the Merger, all of the issued and outstanding shares of STI common stock were converted, at an exchange ratio of 25.48433-for-1, into an aggregate of 169,375,807 shares of QuikByte common stock and STI became a wholly owned subsidiary of QuikByte. The holders of QuikByte’s common stock as of immediately prior to the Merger held an aggregate of 55,708,320 shares of QuikByte’s common stock, which consisted of: (i) 11,073,946 shares of common stock outstanding as of September 17, 2009, and (ii) 44,634,374 shares of common stock issued on September 18, 2009 in connection with a $2.0 million private placement. The accompanying financial statements share and per share information has been retroactively adjusted to reflect the exchange ratio in the Merger.
STI was originally incorporated as San Diego Antibody Company in California in 2006 and was renamed Sorrento Therapeutics, Inc. and reincorporated in Delaware in 2009, prior to the Merger. QuikByte was originally incorporated in Colorado in 1989. Following the Merger, on December 4, 2009, QuikByte reincorporated under the laws of the State of Delaware, or the Reincorporation. Immediately following the Reincorporation, on December 4, 2009, STI merged with and into QuikByte, the separate corporate existence of STI ceased and QuikByte continued as the surviving corporation, or the Roll-Up Merger. Pursuant to the certificate of merger filed in connection with the Roll-Up Merger, QuikByte’s name was changed from “QuikByte Software, Inc.” to “Sorrento Therapeutics, Inc.”, or the Company.
Reverse Merger Accounting
Immediately following the consummation of the Merger, the: (i) former security holders of STI common stock had an approximate 75% voting interest in QuikByte and the QuikByte stockholders retained an approximate 25% voting interest, (ii) former executive management team of STI remained as the only continuing executive management team for the Company, and (iii) Company’s ongoing operations consist solely of the ongoing operations of STI. Based primarily on these factors, the Merger was accounted for as a reverse merger and a recapitalization in accordance with generally accepted accounting principles in the U.S., or GAAP. As a result, these financial statements reflect the: (i) historical results of STI prior to the Merger, (ii) combined results of the Company following the Merger, and (iii) acquired assets and liabilities at their historical cost, which approximates their fair value at the Merger date. In connection with the Merger, the Company received cash of $104,860, other current assets of $20,150 and assumed accounts payable of $24,624.
2. Nature of Operations and Summary of Significant Accounting Policies
Nature of Operations and Basis of Presentation
The Company is a biopharmaceutical company focused on the discovery, development and commercialization of novel and proprietary biotherapeutics for the treatment of a variety of disease conditions, including cancer, inflammation, metabolic and infectious diseases. The Company’s objective is to either independently or through one or more partnerships with pharmaceutical or biopharmaceutical organizations identify drug development candidates derived from the libraries. See Note 10.
As of December 31, 2012, the Company has devoted substantially all of its efforts to product development, raising capital and building infrastructure, and has not realized revenues from its planned principal operations. Accordingly, the Company is considered to be in the development stage.
F-7
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The accompanying consolidated financial statements include the accounts of the Company’s wholly-owned subsidiary, Sorrento Therapeutics, Inc. Hong Kong Limited, or Sorrento Hong Kong, which was registered effective December 4, 2012. Sorento Hong Kong had no operations in 2012. All inter-company balances and transactions have been eliminated in consolidation.
Liquidity and Going Concern
The accompanying consolidated financial statements have been prepared on the going concern basis, which assumes that the Company will continue to operate as a going concern and which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. As reflected in the accompanying consolidated financial statements, the Company has a net loss of $4,845,308, net cash used for operations of $3,797,476 and net cash used for investing activities of $547,884, for the year ended December 31, 2012. As of December 31, 2012, the Company also has an accumulated deficit of $10,950,299 and working capital of $4,667,817.
In February 2013, the Company entered into a loan and security agreement with a bank which provided the Company with an equipment loan for up to $1,000,000. In March 2013, the Company entered into: (i) a stock purchase agreement and issued 33,658,305 shares of common stock for aggregate gross proceeds of $6,418,495, and (ii) the transactions with IgDraSol, Inc. (IgDraSol). The Company intends to acquire IgDraSol in 2013. See Note 10.
The Company anticipates that it will continue to incur net losses into the foreseeable future as it: (i) continues to identify and advance a number of potential drug candidates into preclinical development activities, (ii) acquires IgDraSol and continues to fund its operations, and (iii) expands its corporate infrastructure, including the costs associated with being a public company. Without additional funding, management believes that the Company will not have sufficient funds to meet its obligations beyond October 2013. These conditions give rise to substantial doubt as to the Company’s ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
The Company plans to continue to fund its losses from operations and capital funding needs through public or private equity or debt financings, strategic collaborations, licensing arrangements, asset sales, government grants or other arrangements. However, the Company cannot be sure that such additional funds will be available on reasonable terms, or at all. If the Company is unable to secure adequate additional funding, the Company may be forced to make reductions in spending, extend payment terms with suppliers, liquidate assets where possible, and/or suspend or curtail planned programs. In addition, if the Company does not meet its payment obligations to third parties as they come due, it may be subject to litigation claims. Even if the Company is successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. Any of these actions could materially harm the Company’s business, results of operations, and future prospects.
F-8
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
If the Company raises additional funds by issuing equity securities, substantial dilution to existing stockholders would result. If the Company raises additional funds by incurring debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and specific financial ratios that may restrict the Company’s ability to operate its business.
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Management believes that these estimates are reasonable; however, actual results may differ from these estimates.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with original maturities of three months or less to be cash equivalents. The Company minimizes its credit risk associated with cash and cash equivalents by periodically evaluating the credit quality of its primary financial institution. The balance at times may exceed federally insured limits. The Company has not experienced any losses on such accounts.
Fair Value of Financial Instruments
The Company’s financial instruments consist of cash and cash equivalents, grants receivable, prepaid expenses and other assets, accounts payable and accrued expenses. Fair value estimates of these instruments are made at a specific point in time, based on relevant market information. These estimates may be subjective in nature and involve uncertainties and matters of significant judgment and therefore cannot be determined with precision. As of December 31, 2012 and 2011, the carrying amount of cash and cash equivalents, grants receivable, prepaid expenses and other assets, accounts payable, accrued liabilities and deferred revenue are generally considered to be representative of their respective fair values because of the short-term nature of those instruments.
Grants Receivable
Grants receivable at December 31, 2012 and 2011 represent amounts due under several federal contracts with the National Institute of Allergy and Infectious Diseases, or NIAID, a division of the National Institutes of Health, or NIH, collectively, the NIH Grants. The Company considers the grants receivable to be fully collectible; accordingly, no allowance for doubtful amounts has been established. If amounts become uncollectible, they are charged to operations.
Property and Equipment
Property and equipment are carried at cost less accumulated depreciation. Depreciation of property and equipment is computed using the straight-line method over the estimated useful lives of the assets, which are generally three to five years. Leasehold improvements are amortized over the lesser of the life of the lease or the life of the asset.
F-9
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Impairment of Long-Lived Assets
The Company evaluates its long-lived assets with definite lives, such as property and equipment, for impairment. The Company records impairment losses on long-lived assets used for operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the carrying value of the assets. There have not been any impairment losses of long-lived assets through December 31, 2012.
Research and Development Costs
All research and development costs are charged to expense as incurred. Such costs primarily consist of lab supplies, contract services, stock-based compensation expense, salaries and related benefits.
Income Taxes
The provisions of the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 740-10, Uncertainty in Income Taxes, address the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under ASC 740-10, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by taxing authorities, based on the technical merits of the position. The Company has determined that it has no uncertain tax positions.
The Company accounts for income taxes using the asset and liability method to compute the differences between the tax basis of assets and liabilities and the related financial amounts, using currently enacted tax rates.
The Company has deferred tax assets, which are subject to periodic recoverability assessments. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount that more likely than not will be realized. The Company evaluates the recoverability of the deferred tax assets annually.
Revenue Recognition
The Company’s inception to date revenues are generated from three NIH and two U.S. Department of Treasury (or U.S. Treasury) grant awards and a feasibility study agreement, or the Collaboration Agreement, that the Company entered into with a third party in July 2010. The revenue from the NIH and U.S. Treasury grant awards are based upon subcontractor and internal costs incurred that are specifically covered by the grant, and where applicable, a facilities and administrative rate that provides funding for overhead expenses. These revenues are recognized when expenses have been incurred by subcontractors or when the Company incurs internal expenses that are related to the grant.
The revenue from the Collaboration Agreement is derived from the completion of certain development services and the reimbursement of certain development costs incurred to provide such development services. Revenue from upfront, nonrefundable service fees are recognized when earned, as evidenced by written acknowledgement from the collaborator, or other persuasive evidence that all service deliverables have been achieved, provided that the service deliverables are substantive and their achievability was not reasonably assured at the inception of the Collaboration Agreement. Any amounts received prior to satisfying the Company’s revenue recognition criteria are recorded as deferred revenue.
F-10
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Stock-Based Compensation
The Company accounts for stock-based compensation in accordance with FASB ASC Topic 718, which establishes accounting for equity instruments exchanged for employee services. Under such provisions, stock-based compensation cost is measured at the grant date, based on the calculated fair value of the award, and is recognized as an expense, under the straight-line method, over the employee’s requisite service period (generally the vesting period of the equity grant).
The Company accounts for equity instruments, including restricted stock or stock options, issued to non-employees in accordance with authoritative guidance for equity based payments to non-employees. Stock options issued to non-employees are accounted for at their estimated fair value determined using the Black-Scholes option-pricing model. The fair value of options granted to non-employees is re-measured as they vest, and the resulting increase in value, if any, is recognized as expense during the period the related services are rendered. Restricted stock issued to non-employees is accounted for at their estimated fair value as they vest.
Comprehensive Income (Loss)
Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. The Company is required to record all components of comprehensive income (loss) in the consolidated financial statements in the period in which they are recognized. Net income (loss) and other comprehensive income (loss), including foreign currency translation adjustments and unrealized gains and losses on investments, are reported, net of their related tax effect, to arrive at comprehensive income (loss). For the years ended December 31, 2012 and 2011, the comprehensive loss was equal to the net loss.
Net Loss Per Share
Net loss per share is presented as both basic and diluted net loss per share. Basic net loss per share excludes any dilutive effects of options, shares subject to repurchase and warrants. Diluted net loss per share includes the impact of potentially dilutive securities. During 2012 and 2011, the Company had securities outstanding which could potentially dilute basic earnings per share in the future, but were excluded from the computation of diluted net loss per share, as their effect would have been anti-dilutive.
These outstanding securities consist of the following:
| Years Ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| Unvested Restricted Common stock subject to repurchase |
336,454 | 1,677,430 | ||||||
| Outstanding options |
10,490,000 | 2,947,500 | ||||||
| Outstanding warrants |
200,000 | 200,000 | ||||||
| Weighted average exercise price of options |
$ | 0.14 | $ | 0.12 | ||||
F-11
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
3. Property and Equipment
Property and equipment consisted of the following as of December 31, 2012 and 2011:
| December 31, | ||||||||
| 2012 | 2011 | |||||||
| Furniture and fixtures |
$ | 15,739 | $ | 15,739 | ||||
| Office equipment |
23,039 | 10,691 | ||||||
| Lab equipment |
1,855,220 | 1,136,778 | ||||||
| Leasehold improvements |
65,679 | 10,623 | ||||||
|
|
|
|
|
|||||
| 1,959,677 | 1,173,831 | |||||||
| Less accumulated depreciation and amortization |
(478,688 | ) | (185,386 | ) | ||||
|
|
|
|
|
|||||
| $ | 1,480,989 | $ | 988,445 | |||||
|
|
|
|
|
|||||
Depreciation expense for the years ended December 31, 2012 and 2011 and for the period from inception (January 25, 2006) (“Inception”) through December 31, 2012 was $293,302, $159,219 and $478,689, respectively.
4. Significant Agreements and Contracts
License Agreement with OPKO Health, Inc.
In June 2009, the Company entered into a limited license agreement, or the OPKO License, with OPKO Health, Inc., or OPKO, pursuant to which the Company granted OPKO an exclusive, royalty-free, worldwide license under all U.S. and foreign patents and patent applications owned or controlled by the Company or any of its affiliates, or the STI Patents, to: (i) develop, manufacture, use, market, sell, offer to sell, import and export certain products related to the development, manufacture, marketing and sale of drugs for ophthalmological indications, or the OPKO Field, and (ii) use and screen any population of distinct molecules covered by any claim of the STI Patents or which is derived by use of any process or method covered by any claim of the STI Patents to identify, select and commercialize certain products within the OPKO Field. Subject to certain limitations, OPKO will have the right to sublicense the foregoing rights granted under the OPKO License. Additionally, pursuant to the OPKO License, OPKO has granted the Company an exclusive, royalty-free, worldwide license to any patent or patent application owned or controlled by OPKO or any of its affiliates to develop, use, make, market, sell and distribute certain products in any field of use, other than the OPKO Field, or the OPKO Patents.
The Company has retained all rights to the STI Patents outside of the OPKO Field and has agreed not to practice the OPKO Patents or the STI Patents outside the STI current field of use. Unless otherwise terminated in accordance with its terms, the License Agreement will expire upon the expiration of the last to expire patent within the STI Patents and OPKO Patents on a country-by-country basis.
License Agreement with The Scripps Research Institute
In January 2010, the Company entered into a license agreement, or the TSRI License, with The Scripps Research Institute, or TSRI. Under the TSRI License, TSRI granted the Company an exclusive, worldwide license to certain TSRI patent rights and materials based on quorum sensing for the prevention and treatment of Staphylococcus aureus (“Staph”) infections, including Methicillin-resistant Staph. In consideration for the license, the Company: (i) issued TSRI a warrant for the purchase of common stock, (ii) agreed to pay TSRI a
F-12
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
certain annual royalty commencing in the first year after certain patent filing milestones are achieved, (iii) agreed to pay a royalty on any sales of licensed products by the Company or its affiliates and a royalty for any revenues generated by the Company through its sublicense of patent rights and materials licensed from TSRI under the TSRI License. The TSRI License requires the Company to indemnify TSRI for certain breaches of the agreement and other matters customary for license agreements. The parties may terminate the TSRI License at any time by mutual agreement. In addition, the Company may terminate the TSRI License by giving 60 days notice to TSRI and TSRI may terminate the TSRI License immediately in the event of certain breaches of the agreement by the Company or upon the Company’s failure to undertake certain activities in furtherance of commercial development goals. Unless terminated earlier by either or both parties, the term of the TSRI License will continue until the final expiration of all claims covered by the patent rights licensed under the agreement. For the years ended December 31, 2012 and 2011 and for the period from Inception through December 31, 2012, the Company recorded $41,835, $4,991 and $127,345 in patent prosecution and maintenance costs associated with the TSRI License, respectively, which has been included in general and administrative expenses.
The fair value of the warrants to purchase Company common stock, issued in connection with the TSRI License in 2010, of $17,989 was determined using the Black-Scholes valuation model with the following weighted-average assumptions: risk-free interest rate of 2.48%, no dividend yield, expected term of 10 years, and volatility of 102%. Such fair value has been included in general and administrative expenses for the period from Inception through December 31, 2012.
NIH Grants
In May 2010, the NIAID awarded the Company an Advanced Technology Small Business Technology Transfer Research grant to support the Company’s program to generate and develop novel antibody therapeutics and vaccines to combat Staph infections, including Methicillin-resistant Staph, or the Staph Grant award. The project period for Phase 1 of the Staph Grant award covers a two-year period which commenced in June 2010 and ended in May 2012. The Company records revenue associated with the NIH Grants as the related costs and expenses are incurred. During the years ended December 31, 2012 and 2011 and for the period from Inception through December 31, 2012, the Company recorded $119,379, $215,986 and $600,000 of revenue associated with the Staph Grant award, respectively.
In July 2011, the NIAID awarded the Company a second Advanced Technology Small Business Technology Transfer Research grant to support the Company’s program to generate and develop antibody therapeutics and vaccines to combat C. difficile infections, or the C. difficile Grant award. The project period for the C. difficile Grant award covers a two-year period which commenced in June 2011, and as of September 30, 2012, the entire Phase 1 grant of $600,000 had been awarded. During the years ended December 31, 2012 and 2011 and for the period from Inception through December 31, 2012, the Company recorded $335,579, $113,198 and $448,777 of revenue associated with the C. difficile Grant award, respectively.
In June 2012, the NIAID awarded the Company a third Advanced Technology Small Business Technology Transfer Research grant, with an initial award of $300,000, to support the Company’s program to generate and develop novel human antibody therapeutics to combat Staph infections, including Methicillin-resistant Staph, or the Staph Grant II award. The project period for the phase I grant covers a two-year period which commenced in June 2012, with a potential annual award of $300,000 per year. During the year ended December 31, 2012 and for the period from Inception through December 31, 2012, the Company recorded $128,816 of revenue associated with the Staph Grant II award.
F-13
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Collaboration Agreement
In July 2010, the Company entered into the Collaboration Agreement, with a third party. Under the terms of the Collaboration Agreement, the Company provided certain antibody screening services for an upfront cash fee of $200,000 and was reimbursed for certain costs and expenses associated with providing the services, or the Development Costs. The upfront fee and reimbursable Development Costs were accounted for as separate units of accounting. The Company recorded the gross amount of the reimbursable Development Costs as revenue and the costs associated with these reimbursements are reflected as a component of research and development expense.
Any amounts received by the Company pursuant to the Collaboration Agreement prior to satisfying the Company’s revenue recognition criteria are recorded as deferred revenue. For the years ended December 31, 2012 and 2011 and for the period from Inception through December 31, 2012, the Company recognized $0, $200,000 and $223,453, respectively.
Assignment Agreement
Subsequent to year end, in January 2013, the Company entered into an Assignment Agreement, or the Assignment Agreement, with Tien-Li Lee, M.D. and Jane Wu Lee, M.D. as individuals (collectively, the Lees) pursuant to which the Lees agreed to assign to the Company their right, title and interest throughout the world in and to certain inventions and patents that provide for the production of recombinant intravenous immunoglobulin. As consideration for the assignment by the Lees under the Assignment Agreement, the Company: (i) issued the Lee’s 250,000 shares of the Company’s common stock upon execution of the Agreement, (ii) agreed to pay the Lees a total of $50,000 in five monthly installments of $10,000 beginning on February 1, 2013, and (iii) agreed to issue the Lees up to 2,000,000 shares of the Company’s common stock based upon the achievement of certain milestone events described in the Assignment Agreement. Unless otherwise terminated in accordance with its terms, the Assignment Agreement will expire upon the expiration of the last to expire patent within the assigned patent rights.
U.S. Treasury Grants
During 2010, the U.S. Treasury awarded the Company two one-time grants totaling $394,480 for investments in qualifying therapeutic discovery projects under section 48D of the Internal Revenue Code. The grants cover reimbursement for qualifying expenses incurred by the Company in 2010 and 2009. The proceeds from these grants are classified in “Revenues—Grant” in the Inception through December 31, 2012 consolidated statement of operations.
5. Loan and Security Agreement
Subsequent to year end, in February 2013, the Company entered into a loan and security agreement with a bank pursuant to which the lender provided the Company loans to finance certain equipment, in an aggregate principal amount of up to $1,000,000. Under the loan agreement, the lender funded the initial equipment advance in the principal amount of $875,888 in February 2013 and agreed to fund, subject to customary conditions, an additional equipment advance in the principal amount of $124,112 on or prior to August 21, 2013. The loans under the loan agreement bear interest at a rate equal to the three-year U.S. Treasury note yield plus 4.65%, which is fixed on the date of each funding. Interest accrues on the initial outstanding advance at the fixed rate of 5.15%.
The Company is obligated to pay interest-only on any loans funded under the loan agreement prior to April 30, 2013 until May 1, 2013, and thereafter to pay 36 consecutive equal monthly installments of principal
F-14
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
and interest through April 1, 2016. The Company is obligated to pay equal monthly installments of principal and interest through April 1, 2016 on any loans funded under the loan agreement after April 30, 2013. All loans funded under the loan agreement mature on April 1, 2016.
At the Company’s option, it may prepay all of the outstanding principal balance, subject to certain pre-payment fees ranging from 1% to 3% of the prepayment amount. In the event of a final payment of the loans under the loan agreement, either in the event of repayment of the loan at maturity or upon any prepayment, the Company is obligated to pay a final fee of $55,000.
The Company granted the lender a security interest in any equipment that is financed under the loan agreement. The Company is also subject to certain affirmative and negative covenants under the loan agreement, including limitations on its ability to: undergo certain change of control events; convey, sell, lease, license, transfer or otherwise dispose of any equipment financed by loans under the loan agreement; create, incur, assume, guarantee or be liable with respect to indebtedness, subject to certain exceptions; grant liens on any equipment financed under the loan agreement; and make or permit any payment on specified subordinated debt. In addition, under the loan agreement, subject to certain exceptions, the Company is required to maintain with the lender its primary operating, other deposit and securities accounts.
Future annual principal payments under the loan agreement are as follows:
| 2013 |
$ | 194,642 | ||
| 2014 |
291,963 | |||
| 2015 |
291,963 | |||
| 2016 |
97,320 | |||
|
|
|
|||
| Total payments |
$ | 875,888 | ||
|
|
|
6. Stockholders’ Equity (Deficit) and Related Party Transaction
Common Stock
In February 2006, in conjunction with the founding of the Company, 101,937,315 shares of common stock were issued to founders, at the pre-Merger par value, for total consideration of $400 in cash.
In March 2009, the Company issued 7,403,861 shares of restricted common stock to certain consultants, at the pre-Merger par value, for aggregate gross proceeds of $291.
In March 2009, the Company issued 1,019,374 shares of unrestricted common stock to certain consultants for aggregate cash gross proceeds of $10 and issued a note receivable for $30. The note was paid in full in 2010.
In June 2009, the Company issued 59,015,257 shares of common stock at $0.039 per share for aggregate gross proceeds of $2.3 million to OPKO in a private placement transaction. Related stock issuance costs totaled $25,999.
In September 2009, and in connection with the Merger, the Company: (i) issued 44,634,374 shares of common stock, in a private placement transaction, at $0.0448 per share for aggregate gross proceeds of $1,999,620, and (ii) issued 11,073,946 shares of common stock to the former stockholders of QuikByte in exchange for the net assets of QuikByte as well as all of their outstanding shares in QuikByte immediately prior to the Merger. Total stock issuance and Merger costs totaled $168,767.
F-15
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
In December 2010, the Company issued 25,717,143 shares of common stock, in a private placement transaction, at $0.14 per share for aggregate gross proceeds of $3.6 million. Related stock issuance costs were estimated at $159,905. In 2011, the Company reduced its stock issuance costs accrued in 2010 by $80,039.
In December 2011, the Company entered into a Stock Purchase Agreement and issued 12,500,000 shares of common stock, in a private placement transaction, at $0.16 per share for aggregate gross proceeds of $2,000,000. In May 2012, pursuant to the Stock Purchase Agreement, as amended and restated, the Company issued 37,500,000 shares of common stock, in a private placement transaction, at $0.16 per share for aggregate gross proceeds of $6,000,000. 6,250,000 of the shares were purchased by an investor, Hongye SD Group, LLC, of which Dr. Henry Ji, the Company’s Chief Executive Officer and President, is a managing director.
Subsequent to year end, in March 2013, the Company entered into a Stock Purchase Agreement and issued 33,658,305 shares of common stock, in a private placement transaction, at $0.18 per share for aggregate gross proceeds of $6,418,495. See Note 10.
Stock Incentive Plans
2009 Equity Incentive Plan
In February 2009, prior to the Merger, the Company’s Board of Directors approved the 2009 Equity Incentive Plan, or the EIP, under which 10,000,000 shares of common stock were reserved for issuance to employees, non-employee directors and consultants of the Company. The EIP provided for the grant of incentive stock options, non-incentive stock options, restricted stock awards and stock bonus awards to eligible recipients. In March 2009, the Company issued 7,403,861 restricted common stock awards to certain consultants for aggregate gross proceeds of $291. The restricted shares vest monthly over four years and the Company has the option to repurchase any unvested shares at the original purchase price upon any voluntary or involuntary termination. Any unvested shares immediately vest in the event of a merger, sale, or other transaction resulting in a change in control of the Company.
At December 31, 2012, 336,454 shares were unvested and subject to repurchase by the Company. The Company has the right of first refusal to purchase any proposed disposition of shares issued under the EIP. As a result of the Merger, no further shares are available for grant under the EIP. In January 2011, the Company repurchased 1,104,135 unvested shares of restricted common stock for $43.
2009 Non-Employee Director Grants
In September 2009, prior to the adoption of the 2009 Stock Incentive Plan, the Company’s Board of Directors approved the reservation and issuance of 200,000 nonstatutory stock options to the Company’s non-employee directors. The outstanding options vested on the one year anniversary of the vesting commencement date in October 2010. Such options are exercisable for up to 10 years from the grant date. No further shares are available for grant under this plan. The aggregate intrinsic value for such options as of December 31, 2012 was $10,816.
F-16
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The following table summarizes stock option activity as of December 31, 2011 and 2012, and the changes for the years then ended:
| Options Outstanding |
Weighted- Average Exercise Price |
|||||||
| Outstanding at December 31, 2010 |
120,000 | $ | 0.0448 | |||||
| Options Granted |
— | |||||||
| Options Canceled |
— | |||||||
| Options Exercised |
— | |||||||
|
|
|
|||||||
| Outstanding at December 31, 2011 |
120,000 | $ | 0.0448 | |||||
| Options Granted |
— | |||||||
| Options Canceled |
— | |||||||
| Options Exercised |
40,000 | $ | 0.0448 | |||||
|
|
|
|||||||
| Outstanding, Vested and Exercisable at December 31, 2012 |
80,000 | $ | 0.0448 | |||||
|
|
|
|||||||
2009 Stock Incentive Plan
In October 2009, the Company’s stockholders approved the 2009 Stock Incentive Plan, or the Stock Plan, which became effective in December 2009 and under which 12,000,000 shares of the Company’s common stock were initially reserved for issuance to employees, non-employee directors and consultants of the Company. Pursuant to the terms of the Stock Plan, such initial amount will be automatically increased annually on the first day of each fiscal year, beginning in 2011, by the lesser of: (i) 1% of the aggregate number of shares of the Company’s common stock outstanding on the last day of the immediately preceding fiscal year, (ii) 1,200,000 shares, or (iii) an amount approved by the administrator of the Stock Plan. As of December 31, 2012, 14,400,000 shares of the Company’s common stock were reserved for issuance. The Stock Plan provides for the grant of incentive stock options, non-incentive stock options, stock appreciation rights, restricted stock awards, unrestricted stock awards, restricted stock unit awards and performance awards to eligible recipients. Recipients of stock options shall be eligible to purchase shares of the Company’s common stock at an exercise price equal to no less than the estimated fair market value of such stock on the date of grant. The maximum term of options granted under the Stock Plan is ten years. Employee option grants will generally vest 25% on each anniversary of the original vesting date over four years. The vesting schedules for grants to non-employee directors and consultants will be determined by the Company’s Compensation Committee. Stock options are generally not exercisable prior to the applicable vesting date, unless otherwise accelerated under the terms of the applicable stock plan agreement. Unvested shares of the Company’s common stock issued in connection with an early exercise however, may be repurchased by the Company upon termination of the optionee’s service with the Company.
F-17
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The following table summarizes stock option activity as of December 31, 2011 and 2012, and the changes for the years then ended:
| Options Outstanding |
Weighted- Average Exercise Price |
Aggregate Intrinsic Value |
||||||||||
| Outstanding at December 31, 2010 |
1,960,000 | $ | 0.09 | |||||||||
| Options Granted |
1,780,000 | $ | 0.14 | |||||||||
| Options Canceled |
(762,500 | ) | $ | 0.11 | ||||||||
| Options Exercised |
(150,000 | ) | $ | 0.09 | ||||||||
|
|
|
|||||||||||
| Outstanding at December 31, 2011 |
2,827,500 | $ | 0.12 | $ | 115,700 | |||||||
| Options Granted |
8,535,000 | $ | 0.16 | |||||||||
| Options Canceled |
(722,500 | ) | $ | 0.15 | ||||||||
| Options Exercised |
(230,000 | ) | $ | 0.13 | ||||||||
|
|
|
|||||||||||
| Outstanding at December 31, 2012 |
10,410,000 | $ | 0.15 | $ | — | |||||||
|
|
|
|||||||||||
| Vested and Exercisable at December 31, 2012 |
2,320,000 | $ | 0.13 | $ | 54,750 | |||||||
|
|
|
|||||||||||
The Company uses the Black-Scholes valuation model to calculate the fair value of stock options. The fair value of employee stock options was estimated at the grant date using the following assumptions:
| Years Ended December 31, | ||||||||
| 2012 | 2011 | |||||||
| Weighted-average grant date fair value |
$ | 0.29 | $ | 0.11 | ||||
| Dividend yield |
— | — | ||||||
| Volatility |
103 | % | 102 | % | ||||
| Risk-free interest rate |
0.88 | % | 2.41 | % | ||||
| Expected life of options |
5.5 years | 5.7 years | ||||||
The assumed dividend yield was based on the Company’s expectation of not paying dividends in the foreseeable future. Due to the Company’s limited historical data, the estimated volatility incorporates the historical and implied volatility of comparable companies whose share prices are publicly available. The risk-free interest rate assumption was based on the U.S. Treasury’s rates for U.S. Treasury zero-coupon bonds with maturities similar to those of the expected term of the award being valued. The weighted average expected life of options was estimated using the average of the contractual term and the weighted average vesting term of the options.
The total employee stock-based compensation recorded as operating expenses was $324,958, $42,293, and $418,816 for the years ended December 31, 2012 and 2011 and for the period from Inception through December 31, 2012, respectively.
The total unrecognized compensation cost related to unvested stock option grants as of December 31, 2012 was $1,782,054 and the weighted average period over which these grants are expected to vest is 3.4 years.
The Company records equity instruments issued to non-employees as expense at their fair value over the related service period as determined in accordance with the authoritative guidance and periodically revalues the equity instruments as they vest. Stock-based compensation expense related to non-employee consultants recorded as operating expenses was $538,169, $255,741, and $1,047,823 for the years ended December 31, 2012 and 2011 and for the period from Inception through December 31, 2012, respectively.
F-18
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Common Stock Reserved for Future Issuance
Common stock reserved for future issuance consists of the following at December 31, 2012:
| Common stock warrants outstanding under the TSRI License |
200,000 | |||
| Common stock options outstanding under the EIP |
80,000 | |||
| Authorized for future grant or issuance under the Stock Plan |
14,020,000 | |||
|
|
|
|||
| 14,300,000 | ||||
|
|
|
7. Commitments and Contingencies
Litigation
In the normal course of business, the Company may be named as a defendant in one or more lawsuits. Management is currently not aware of any pending lawsuits.
Operating Lease
The Company leases its corporate office and laboratory space under a non-cancelable operating lease that, as amended, expires on September 30, 2014. The lease contains an option to extend the term by four years at the then prevailing rate. Effective April 1 2012, the Company entered into a supplemental amendment to add additional rental space, which expires in April 2017. This supplemental amendment contains an option to extend the term for the additional rental space by five years at the then prevailing rate. Through the end of the initial lease term, the lease provides for an average monthly base rent of $11,792 with scheduled annual base rent increases of 2.75%-3.00% over the initial lease term. For the additional rental space, the average monthly base rent beyond the initial term of the lease is $2,332. The Company has provided a security deposit of $22,757 to secure its obligations under the lease, which has been included in other assets in the accompanying financial statements.
Minimum future non-cancelable annual operating lease obligations are as follows for the years ending December 31:
| 2013 |
$ | 144,864 | ||
| 2014 |
117,101 | |||
| 2015 |
26,532 | |||
| 2016 |
28,944 | |||
| 2017 |
9,648 | |||
|
|
|
|||
| $ | 327,089 | |||
|
|
|
Rental expense paid for the years ended December 31, 2012 and 2011 and for the period from Inception through December 31, 2012 under the above lease totaled $128,300, $102,037 and $335,184, respectively.
F-19
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
8. Income Taxes
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s net deferred tax assets are as follows as of December 31, 2012 and 2011:
| 2012 | 2011 | |||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards and credits |
$ | 5,047,000 | $ | 2,404,000 | ||||
| Stock based compensation |
97,000 | 46,000 | ||||||
| Accrued expenses and other |
(99,000 | ) | (19,000 | ) | ||||
|
|
|
|
|
|||||
| Total deferred tax assets |
5,045,000 | 2,431,000 | ||||||
| Less valuation allowance |
(5,045,000 | ) | (2,431,000 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
The Company has evaluated the available evidence supporting the realization of its gross deferred tax assets, including the amount and timing of future taxable income, and has determined that it is more likely than not that the deferred tax assets will not be realized. Due to such uncertainties surrounding the realization of the domestic deferred tax assets, the Company maintains a valuation allowance of $5,045,000 against its deferred tax assets as of December 31, 2012. Realization of the deferred tax assets will be primarily dependent upon the Company’s ability to generate sufficient taxable income prior to the expiration of its net operating losses.
As of December 31 2012, the Company had net operating loss carryforwards of approximately $10,500,000 and $9,209,000 for federal and state income tax purposes, respectively. These may be used to offset future taxable income and will begin to expire in varying amounts in 2027 to 2032. The Company also has research and development credits of approximately $307,000 and $355,000 for federal and state income tax purposes, respectively. The federal credits may be used to offset future taxable income and will begin to expire in varying amounts in 2029 to 2032. The state credits may be used to offset future taxable income, such credits carryforward indefinitely.
The Company is subject to taxation in the U.S. and California jurisdictions. Currently, no historical years are under examination. The Company’s tax years ending December 31, 2012 and 2011 are subject to examination by the U.S. and state taxing authorities due to the carryforward of unutilized net operating losses and research and development credits.
Utilization of the Company’s net operating loss carryforwards and research and development credit carryforwards may be subject to a substantial annual limitation due to an “ownership change” that may have occurred, or that could occur in the future, as defined and required by Section 382 of the Internal Revenue Code of 1986, as amended (the “Code”), as well as similar state provisions. These ownership changes may limit the amount of net operating loss carryforwards and research and development credit carryforwards, and other tax attributes that can be utilized annually to offset future taxable income and tax, respectively. Any limitation may result in the expiration of a portion of the net operating loss carryforwards or research and development credit carryforwards before utilization.
In general, an “ownership change” results from a transaction or series of transactions over a three-year period resulting in an ownership change of more than 50% of the outstanding stock of a company by certain stockholders or public groups. The Company intends to complete a study in the future to assess whether an ownership change has occurred or whether there have been multiple ownership changes since the Company’s formation, and will complete such study before the use of any of the aforementioned attributes.
F-20
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
9. 401(k) Plan
The Company maintains a defined contribution 401(k) plan available to eligible employees. Employee contributions are voluntary and are determined on an individual basis, limited to the maximum amount allowable under federal tax regulations. The Company, at its discretion, may make certain contributions to the 401(k) plan. Through December 31, 2012, no such contributions were made.
10. Subsequent Events
Assignment Agreement
In January 2013, the Company entered into an Assignment Agreement, or the Assignment Agreement with Tien-Li Lee, M.D. and Jane Wu Lee, M.D. as individuals (collectively, the Lees) pursuant to which the Lees agreed to assign to the Company their right, title and interest throughout the world in and to certain inventions and patents that provide for the production of recombinant intravenous immunoglobulins. See Note 4.
Loan and Security Agreement
In February 2013, the Company entered into a loan and security agreement with a bank which provided the Company with an equipment loan for up to $1,000,000 (see Note 5).
Stock Purchase Agreement
In March 2013, the Company entered into a stock purchase agreement and issued 33,658,305 shares of common stock for aggregate gross proceeds of $6,418,495. See Note 6.
IgDraSol Transactions
On March 7, 2013, the Company entered into an exclusive option agreement with IgDraSol, a private company focused on the development of oncologic agents for the treatment of metastatic breast cancer, or MBC, non-small cell lung cancer, or NSCLC, and other cancers. Pursuant to the option agreement, IgDraSol granted the Company an irrevocable option to acquire IgDraSol by means of an agreement and plan of merger. In consideration for entering into the option agreement, IgDraSol is to receive a non-refundable lump sum payment of $200,000. The close of the transaction and the lump sum payment are expected to occur within 51 days of the signing of the option agreement. If the Company exercises its option to acquire IgDraSol, the Company will, pursuant to the merger agreement, issue 76,199,198 shares of common stock to IgDraSol stockholders and, upon the later achievement of a specified regulatory milestone, the Company will issue an additional 32,656,799 shares of common stock to former IgDraSol stockholders. If the Company does not exercise its option to acquire IgDraSol, the Company will be required to invest $500,000 in IgDraSol pari passu with other new investors of IgDraSol.
F-21
Table of Contents
SORRENTO THERAPEUTICS, INC.
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
IgDraSol’s lead compound, Cynviloq™, is a micellar diblock copolymeric paclitaxel formulation drug product. Cynviloq™ is currently approved and marketed in several countries, including South Korea for MBC and NSCLC under the trade name Genexol-PM®, and has completed Phase 2 testing for potential advancement into registration trials in the U.S. IgDraSol has the exclusive U.S. distribution rights to Cynviloq™ from Samyang Biopharmaceuticals Corporation, a South Korean corporation.
Contemporaneously with the execution of the option agreement, on March 7, 2013, the Company and IgDraSol entered into an asset purchase agreement pursuant to which the Company agreed to purchase all documentation, equipment, information and other know-how related to micellar nanoparticle technology encompassing Tocosol® and related technologies for a purchase price of $1,210,000. The transaction is expected to close within 45 days of the signing of the asset purchase agreement. Upon payment of such purchase price, IgDraSol and the Company intend to enter into a development services agreement pursuant to which approximately $3,000,000 in development services may be provided by IgDraSol for the development of Tocosol® and related technologies.
IgDraSol and the Company also entered into an initial services agreement dated March 7, 2013, or the initial services agreement, pursuant to which, IgDraSol is to provide certain product development and technology services related to the Company’s antibody platform in exchange for a payment of $1,000,000, which was paid to IgDraSol upon signing.
F-22