
As filed with the Securities and Exchange Commission on February 9, 2024
Registration No. 333-268190
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
(
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
(Address, including zip code, and telephone number,
including area code, of registrant’s principal executive offices)
Corporation Service Company
1900 W. Littleton Boulevard
Littleton, Colorado 80120
Tel: (303) 832 7579
(Name, address, including zip code, and telephone number,
including area code, of agent for service of process)
Copies To:
Vincent J. McGill, Esq. Ellenoff Grossman & Schole LLP 1345 Avenue of the Americas New York, NY 10105 Telephone: (516) 220-6569 |
Louis A. Bevilacqua, Esq. Bevilacqua PLLC 1050 Connecticut Avenue, NW, Suite 500 Washington, DC 20036 (202) 869-0888 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Smaller
reporting company | |
| Emerging
growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the U.S. Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED FEBRUARY 9, 2024
PRELIMINARY PROSPECTUS
AiXin Life International, Inc.

[$9,500,000]
Shares of Common Stock
This is a public offering of common stock, par value $0.00001 per share (“common stock”), by AiXin Life International, Inc., a Colorado company. Throughout this prospectus, unless the context requires otherwise, all references to “AiXin Colorado” refer to AiXin Life International, Inc., a holding company, and references to “Aixin Life,” we,” “us,” “our,” the “Registrant,” the “Company” or “our Company” are to AiXin Colorado collectively with our PRC Subsidiaries and AiXin BVI. We are offering 2,375,000 shares of common stock on a firm commitment basis. We currently expect the public offering price to be $4.00 per share.
The offering is being made on a “firm commitment” basis by Boustead Securities, LLC. See “Underwriting.”
Our shares of common stock offered in this prospectus are shares of AiXin Colorado which has no material operations of its own. AiXin Colorado conducts substantially all of its operations through operating companies established in the People’s Republic of China, or the PRC, specifically, Chengdu AiXinZhonghong Biological Technology Co., Ltd, (“AiXinZhonghong”), AiXin Shangyan Hotel Management Co. Ltd. (“AiXin Hotel”), Chengdu Aixintang Haichuan Pharmacy (which together with certain affiliated entities is referred to as “Chengdu Aixintang Pharmacies”), and Yunnan Runcangsheng Technology Co., Ltd. (“Runcangsheng,” collectively with AiXinZhonghong, Aixin Hotel and Chengdu Aixintang Pharmacies, the “Chinese Operating Companies”). The Chinese Operating Companies are wholly owned through an intermediary company established in Hong Kong named HK AiXin International Group Co., Limited (“AiXin HK” and collectively with our Chinese Operating Companies our “PRC Subsidiaries”), which is owned by an intermediary company (AiXin (BVI) International Group, Ltd., “AiXin BVI”) established in the British Virgin Islands. We are not a Chinese operating company. We are a holding company and do not directly own any substantive business operations in China. Therefore, you will not directly hold any equity interests in our Chinese Operating Companies, AiXin BVI or AiXin HK. Our holding company structure involves unique risks to investors. Chinese regulatory authorities could disallow our operating structure, which would likely result in a material change in our operations and/or the value of our common stock, including causing the value of our common stock to significantly decline or become worthless. For a detailed description of risks related to our corporate structure, see “Risk Factors—Risks Relating to Our Holding Company Structure” for detailed discussions.
Additionally, as we conduct substantially all of our operations in China, we are subject to legal and operational risks associated with our business operations in China and our holding company in Hong Kong including risks related to the legal, political and economic policies of the PRC government, the relations between China and the United States, or Chinese or United States regulations, which risks could result in a material change in our operations or cause the value of our common stock to significantly decline or become worthless and affect our ability to offer or continue to offer securities to investors. PRC laws and regulations governing our current business operations are sometimes vague and uncertain, and we face the risk that changes in the policies of the PRC government could have a significant impact upon the business we may be able to conduct in the PRC and the profitability of such business. These risks associated with our being based in or conducting substantially all of our operations through operating companies established in China could cause the value of our securities to significantly decline or become worthless. Furthermore, these risks may result in a material change in our business operations or a complete hinderance of our ability to offer or continue to offer our securities to investors. The PRC government initiated a series of regulatory actions and statements to regulate business operations in China with little advance notice, including cracking down on illegal activities in securities markets, enhancing supervision over China-based companies listed overseas, adopting new measures to extend the scope of cybersecurity reviews, and expanding efforts to enforce anti-monopoly laws and regulations. As confirmed by our PRC counsel, Beijing Dentons Law Offices, LLP (Chengdu) (“Dentons” or “PRC Counsel”) the business conducted by our subsidiaries until now is not subject to cybersecurity review with the Cyberspace Administration of China, or CAC, given that we are not an operator of critical information infrastructure or an online platform operator as defined by the Measures for Cybersecurity Review (2021 version). In addition, as confirmed by our PRC Counsel, we are not subject to merger control review by China’s anti-monopoly enforcement agency due to the level of revenues reported by us and audited by our auditor KCCW Accountancy Corp, and the fact that we currently do not expect to propose or implement any acquisition of control of, or decisive influence over, any company with revenues within China of more than RMB400 million. To date, the statements and regulatory actions related to cybersecurity and anti-monopoly policies have had no impact on our daily business operations, the ability to accept foreign investments and list our securities on a U.S. or other foreign exchange. As of the date of this prospectus, no effective laws or regulations in the PRC explicitly require us to seek approval from the China Securities Regulatory Commission (the “CSRC”) or any other PRC governmental authorities for our overseas listing, nor has our Company or any of our subsidiaries received any inquiry, notice, warning or sanctions regarding our overseas listing from the CSRC or any other PRC governmental authorities. However, since these statements and regulatory actions are new, it is highly uncertain as to how soon legislative or administrative regulation making bodies will respond to ongoing developments and what existing or new laws or regulations or detailed implementation policies and interpretations will be promulgated or modified and the potential impact such new laws or modified laws and regulations will have on our daily business operations, the ability to accept foreign investments and list our securities on a U.S. or other foreign exchange. The Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies (the “Trial Measures”), came into effect March 31, 2023. The Trial Measures apply to overseas securities offerings and/or listings conducted by companies incorporated in the PRC, PRC domestic companies, and companies incorporated overseas with operations primarily in the PRC, indirect offerings. The Trial Measures require (1) the filing of the overseas offering and listing plan by the PRC domestic companies with the CSRC under certain conditions, and (2) the filing by the underwriter with the CSRC under certain conditions and (3) the submission of an annual report to the CSRC within the required timeline. Based on the advice of our PRC Counsel, this offering will be considered an indirect offering. However, our PRC Counsel has advised that because our common stock currently trades in the U.S., we are not required to submit filings to the CSRC before this offering is completed and this offering is not conditioned on CSRC approval. Rather, within three days of the closing of this offering we must submit filings to the CSRC in accordance with the Trial Measures. If we were to fail to comply with the post-offering filing obligations imposed by the Trial Measures or make a misrepresentation, misleading statement or material omission in the materials we submit to the CSRC, the CSRC would have the right to order rectification, issue a warning and impose a fine on us of between RMB1 million and RMB10 million and issuing a warning to the parties responsible for such failure, misrepresentation or material omission and impose a fine on each of such individuals ranging from RMB500,000 to RMB5 million. See “Risk Factors” for a discussion of these legal and operational risks and other information that should be considered before making a decision to purchase our common stock.
As a holding company, our ability to pay dividends to our shareholders and to service any debt we may incur at the holding company level may depend upon dividends or interest paid by our Chinese Operating Companies and AiXin HK (collectively, our “PRC Subsidiaries”). Current PRC regulations permit our PRC Subsidiaries to pay dividends to us only out of their accumulated profits, if any, determined in accordance with Chinese accounting standards and regulations. In addition, each of our PRC Subsidiaries is required to set aside at least 10% of its after-tax profits each year, if any, to fund a statutory reserve until such reserve reaches 50% of its registered capital. As of the date hereof, we have had no transactions that involved the transfer of cash or assets throughout our corporate structure. In order for us to pay dividends to our shareholders we will rely on the distribution of dividends through AiXin HK from our Chinese Operating Companies. As of the date hereof, none of our Chinese Operating Companies has distributed any cash or other assets to AiXin HK, including by way of dividends or interest payments, AiXin HK has not distributed any cash or other assets to AiXin BVI, and AiXin Colorado and Aixin BVI have not transferred any cash or other assets to any of our PRC Subsidiaries. AiXin Colorado does not currently plan or anticipate transferring cash or other assets from our operations in China to any non-Chinese entity. As of the date hereof, no transfers, dividends or distributions have been made to our investors. To the extent our cash or other assets is in one of our Chinese Operating Companies or AiXin HK, the funds or assets may not be available to fund operations or for use outside of mainland China or Hong Kong including for the payment of dividends to the shareholders of AiXin Colorado, due to interventions by the governments of PRC or Hong Kong, or the imposition of restrictions and limitations on the ability of the PRC Subsidiaries to use such cash or assets imposed by the government of mainland China or Hong Kong. See “Prospectus Summary – Summary of Risk Factors – Risks Relating to Our Holding Company Structure,” and “Risk Factors – Risks Relating to Our Holding Company Structure - We may rely on dividends and other distributions on equity paid by our PRC Subsidiaries to fund any cash and financing requirements we may have, and any limitation on the ability of our PRC Subsidiaries to make payments to us could have a material and adverse effect on our ability to conduct our business.” For a detailed description of limitations on the Company’s ability to transfer cash through our corporate structure, see “Prospectus Summary - Transfers of Cash to and from our Subsidiaries.”
Except for such limitations on our Company’s ability to transfer cash and other assets among our entities currently or hereafter imposed by the governments of the PRC and Hong Kong, there are no limitations on our Company’s ability to transfer cash and other assets through our corporate structure. Our Company does not have any cash management policies with respect to the transfer of cash among our entities other than requirements for the approval of management for transfers in excess of specified amounts and none of our entities is currently party to any debenture, loan or other agreement which imposes restrictions or otherwise limits our Company’s ability to transfer our cash and other assets. We have not adopted any policies that dictate how funds are transferred among our entities other than as necessary to comply with applicable laws. See “Prospectus Summary - Transfers of Cash to and from our Subsidiaries.”
Pursuant to the Holding Foreign Companies Accountable Act (“HFCAA”), the Public Company Accounting Oversight Board (United States) (the “PCAOB”) issued a Determination Report on December 16, 2021 which found that the PCAOB was then unable to inspect or investigate completely registered public accounting firms headquartered in mainland China of the PRC and Hong Kong, a Special Administrative Region and dependency of the PRC. In addition, the PCAOB’s report identified the specific registered public accounting firms which are subject to these determinations. Our registered public accounting firm, KCCW Accountancy Corp. is not headquartered in mainland China or Hong Kong and was not identified in this report as a firm subject to the PCAOB’s determinations. KCCW Accountancy Corp. is registered with the PCAOB and is subject to laws in the United States pursuant to which the PCAOB conducts regular inspections to assess KCCW Accountancy Corp.’s compliance with applicable professional standards. KCCW Accountancy Corp. has been inspected by the PCAOB on a regular basis, with the last inspection in 2022. On December 15, 2022, the PCAOB determined that the PCAOB was able to secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary. However, should PRC authorities obstruct or otherwise fail to facilitate the PCAOB’s access in the future, the PCAOB will consider the need to issue a new determination. On December 29, 2022, the Accelerating Holding Foreign Companies Accountable Act (“AHFCAA”) was enacted, which amended the HFCA Act by decreasing the number of non-inspection years from three years to two, thus reducing the time period before our common stock may be prohibited from trading or delisted if the PCAOB was unable to inspect our auditor. Notwithstanding the foregoing, if the PCAOB is not able to inspect and investigate completely our auditor’s work papers in China, you may be deprived of the benefits of such inspection which could result in a limitation on or a restriction to our access to the U.S. capital markets. Further, trading of our securities may be prohibited under the HFCAA and Nasdaq may determine to delist our securities if the PCAOB determines that it cannot inspect or investigate completely our auditor under the HFCAA. For more details, see “Risk Factors – Risk Associated with Our Company -A recent joint statement by the SEC and the Public Company Accounting Oversight Board (United States), or the “PCAOB,” proposed rule changes submitted by Nasdaq, and the newly enacted “Holding Foreign Companies Accountable Act” all call for additional and more stringent criteria to be applied to emerging market companies upon assessing the qualification of their auditors, especially the non-U.S. auditors who are not inspected by the PCAOB. These developments could add uncertainties to our offering.”
We are a reporting company under Section 12(g) of the Securities Exchange Act of 1934, as amended. Our common stock is currently quoted on the OTCQB (the “OTCQB”) under the symbol “AIXN.” The last reported closing price for our common stock on February 8, 2024, was $0.50 per share. There is a limited public trading market for our common stock. We have applied to list our common stock on the Nasdaq Capital Market under the symbol “AIXN.” There can be no assurance that our application will be approved. The closing of this offering is contingent upon the successful listing of our common stock on the Nasdaq Capital Market.
Investing in our securities involves a significant degree of risks. You should carefully consider the risk factors beginning on page 15 of this prospectus and set forth in the documents incorporated by reference herein before making any decision to invest in our securities.
Neither the U.S. Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this registration statement. Any representation to the contrary is a criminal offense.
| Per share | Total | |||||||
| Public offering price | $ | 4.00 | $ | 9,500,000 | ||||
| Underwriting discounts and commissions (1) | $ | 0.32 | $ | 760,000 | ||||
| Offering proceeds to us, before expenses | $ | 3.68 | $ | 8,740,000 | ||||
| (1) | Does not include additional items of compensation payable to Boustead Securities, LLC, the underwriter, which includes warrants to purchase 5% of the aggregate number of shares issued in this offering, with an exercise price equal to 125% of the price per share sold in this offering. We have also agreed to reimburse the underwriter for certain accountable expenses incurred by them. See “Underwriting.” |
We have also granted a 45-day option to the underwriter to purchase up to additional [356,250] shares of common stock solely to cover over-allotments, if any.
The underwriter expects to deliver our shares of common stock to purchasers in this offering on or about [●], 2024.

The date of this prospectus is [●], 2024
TABLE OF CONTENTS
| i |
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission (the “SEC” or the “Commission”). You should rely only on the information contained in this prospectus or any supplement or amendment hereto. Neither we, nor the underwriter have authorized any person to provide you with different information. Neither we, nor the underwriter are offering to sell, or seeking an offer to buy, our common stock in any jurisdiction where such offer or sale is not permitted. You should assume that the information contained in this prospectus and any supplement or amendment hereto is accurate only as of their respective dates, regardless of the time of delivery of this prospectus or of any sale of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
On October 27, 2020, we effected a 1-for-4 reverse split of our shares of common stock. Concurrently with the reverse split we reduced the number of our authorized shares of common stock from 900,000,000 shares to 500,000,000 shares. On February 17, 2023, we effected a 1-for-2 reverse split of our shares of common stock. At such time the number of our authorized shares of common stock remained 500,000,000 shares. On March 1, 2023, Quanzhong Lin entered into a Contribution Agreement wherein he agreed to contribute to the capital of AiXin Colorado seven million shares of common stock of AiXin Colorado effective upon effectiveness of the Registration Statement of which this prospectus is a part. Except as otherwise indicated, all share and per share numbers contained herein other than those set forth in the financial statements beginning on page F-1, have been adjusted to give effect to the 1-for-4 and 1-for-2 reverse stock splits and the reduction in the number of our shares of our common stock outstanding to occur as result of a contribution to our capital of 7 million shares of our common stock to be made by Mr. Quanzhong Lin, our Chairman, President, Chief Executive Officer and principal shareholder.
You should read this prospectus, together with additional information described under “Where You Can Find More Information”, beginning on page 81, before making an investment decision.
The market data and certain other statistical information used throughout this prospectus is based on independent industry publications, reports by market research firms or other independent sources that we believe to be reliable sources. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. We are responsible for all of the disclosure contained in this prospectus, and we believe these industry publications and third-party research, surveys and studies are reliable. While we are not aware of any misstatements regarding any third-party information presented in this prospectus, their estimates, in particular, as they relate to projections, involve numerous assumptions, are subject to risks and uncertainties, and are subject to change based on various factors. Some market and other data included herein, as well as the data of competitors as they relate to Aixin, is also based on our good faith estimates.
Unless the context otherwise requires, all references in this prospectus to:
| ● | “AiXin Colorado” refers to AiXin Life International, Inc., a Colorado corporation; | |
| ● | “AiXin BVI” refers to AiXin (BVI) International Group, Ltd., a British Virgin Islands corporation; | |
| ● | “AiXin HK” refers to HK AiXin International Group Co., Limited, a Hong Kong limited company; | |
| ● | “AiXinZhonghong” refers to Chengdu AiXinZhonghong Biological Technology Co., Ltd, a Chinese limited company and each of the affiliated Chinese limited companies owned by AiXinZhonghong which operates a pharmacy; | |
| ● | “AiXin Hotel” refers to Chengdu AiXin Shangyan Hotel Management Co., Ltd., a Chinese limited company; | |
| ● | “Aixintang Pharmacy” refers to Chengdu Aixintang Haichuan Pharmacy, a Chinese limited Company; | |
| ● | “Chengdu Aixintang Pharmacies” refers to Aixintang Pharmacy and each of the affiliated Chinese limited companies owned by Aixintang Pharmacy which operates a pharmacy; | |
| ● | “Runcangsheng” refers to Yunnan Runcangsheng Technology Co., Ltd. a Chinese limited company; | |
| ● | “Chinese Operating Companies” refers to, collectively, AiXinZhonghong, AiXin Hotel, Chengdu Aixintang Pharmacies and Runcangsheng; | |
| ● | “PRC Subsidiaries refers to, collectively, AiXin HK and the Chinese Operating Companies; | |
| ● | “Aixin Life,” “we,” “us,” “our,” the “Registrant”, the “Company,” or “our Company” refer to AiXin Colorado and our PRC Subsidiaries and Chinese Operating Companies, as appropriate; | |
| ● | “Exchange Act” refers to the Securities Exchange Act of 1934, as amended; | |
| ● | “SEC” or the “Commission” refers to the United States Securities and Exchange Commission; | |
| ● | “Securities Act” refers to the Securities Act of 1933, as amended; | |
| ● | “China,” “Chinese” or the “PRC” refers to the People’s Republic of China, including Hong Kong and Macau and excluding Taiwan; | |
| ● | all references to “RMB” or “Chinese Yuan” is to the legal currency of the People’s Republic of China; and | |
| ● | all references to “U.S. dollars,” “dollars,” “USD” or “$” are to the legal currency of the United States; |
AiXin Colorado’s reporting currency is the U.S. dollar. The functional currency of AiXin Colorado is the U.S. dollar and the functional currency of the Chinese Subsidiaries is the Chinese Renminbi (“RMB”). For the subsidiaries whose functional currencies are the RMB, all assets and liabilities are translated at exchange rates at the balance sheet date, which are 7.2960 and 6.8972 as of September 30, 2023 and December 31, 2022, respectively. Revenue and expenses are translated at the average yearly exchange rates, which are 7.0467, 6.7290 and 6.4508 for the three years ended December 31, 2023, 2022 and 2021, respectively, and 7.0343 for the nine months ended September 30, 2023. The equity is translated at historical exchange rates. Any translation adjustments resulting are not included in determining net income but are included in foreign exchange adjustments to other comprehensive loss, a component of equity.
| 1 |
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before investing in our securities, you should carefully read this entire prospectus, especially the risks of investing in our securities as discussed under “Risk Factors” and the financial statements and notes thereto herein. The following summary is qualified in its entirety by the detailed information appearing elsewhere in this prospectus.
Overview
Our Business
We, AiXin Life International, Inc. (“AiXin Colorado,” together with our Chinese Operating Companies and PRC Subsidiaries, as appropriate, referred to as “AiXin Life,” the “Company”, “we”, “us” and “our” unless specified otherwise) are a Colorado holding company with no material operations of our own. We conduct substantially all of our operations through our wholly-owned operating companies in the PRC. This is an offering of common stock of our Colorado holding company. Therefore, you will not directly own any equity interests in our operating companies.
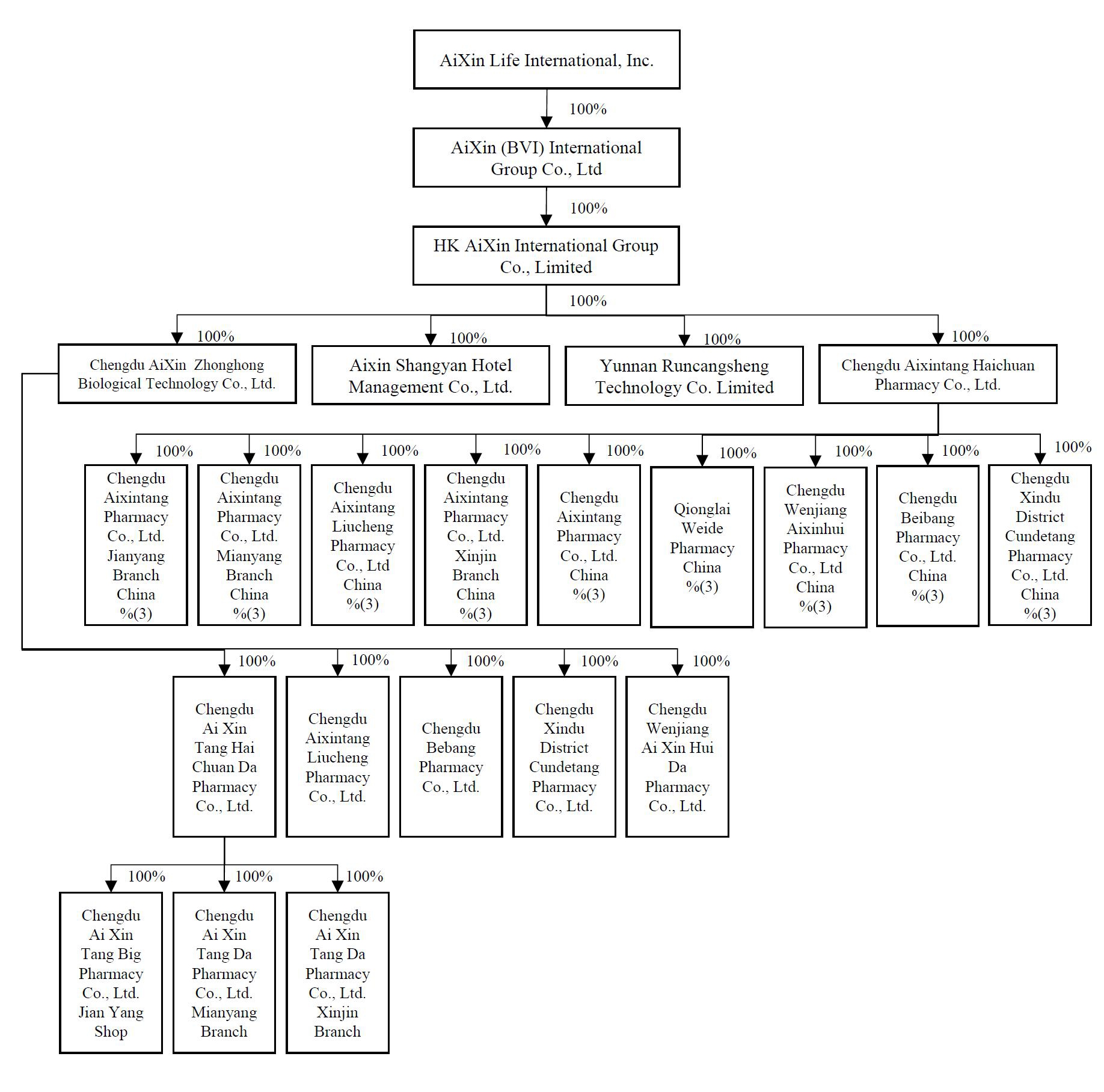
Our subsidiaries are (i) AiXin (BVI) International Group Co., Ltd. (“AiXin BVI”), 100% owned by AiXin Colorado, (ii) HK AiXin International Group Co., Limited, a Hong Kong limited company (“AiXin HK”), 100% owned by AiXin BVI, and the following entities, each of which was organized in the PRC, is wholly-owned by AiXin HK and which are collectively referred to as our “Chinese Operating Companies” (iii) Chengdu AiXinZhonghong Biological Technology Co., Ltd. and its wholly-owned subsidiaries, each of which operates a pharmacy (“AiXinZhonghong”), (iv) Yunnan Runcangsheng Technology Co., Ltd (“Yunnan Runcangsheng”), (v) Chengdu Aixintang Haichuan Pharmacy Co., Ltd. and its wholly-owned subsidiaries, each of which operates a pharmacy (“Chengdu Aixintang Pharmacies”) and (vi) AiXin Shangyan Hotel Management Co., Ltd. Our Chinese Operating Companies and AiXin HK are collectively referred to as our “PRC Subsidiaries.”
Our holding company structure involves unique risks to investors. Chinese regulatory authorities could disallow our operating structure, which would likely result in a material change in our operations and/or the value of our common stock, including causing the value of our common stock to significantly decline or become worthless. AiXin Colorado is listed on the OTCQB under the symbol “AIXN”.
| 2 |
We are focused on providing health and wellness products to the growing middle class in China. We currently develop, manufacture, market and sell premium-quality healthcare, nutritional products and wellness supplements, including herbs and greens, traditional Chinese remedies, functional products, such as weight management tools, probiotics, foods and drinks. We also offer products purchased from third parties. In addition, we have provided advertising and marketing services to clients which engaged us to market and distribute their products, though we have decreased this activity as we focus on distributing our products. We offer our products and those of clients for which we provide marketing services, through a diversified, omni-channel business model which generates revenues through retail and wholesale product sales, through company-owned pharmacies, direct marketing and on-line activities. Our marketing approach emphasizes proactively approaching customers such as by hosting marketing events for clients, which we believe is ideally suited to marketing the products we offer because sales of healthcare, nutritional products and supplements are strengthened by ongoing personal contact and support, coaching and education among the Company and our clients towards how to achieve a healthy and active lifestyle.
In December 2017, when we acquired AiXin BVI, it indirectly owned all of the capital stock of AiXinZhonghong, which began distributing nutritional products in 2013.
In September 2021, we completed the acquisition of nine retail pharmacies in Chengdu. Since that time we have added additional pharmacies and currently operate 25 pharmacies. We utilize these pharmacies to distribute health and wellness products and serve as learning centers for our clients. We intend to seek to continue to expand our chain of pharmacies and to use these outlets as part of our overall marketing strategy.
In September 2022, we acquired Yunnan Runcangsheng based in Yunnan Province which engages in the research, development, manufacture and wholesale distribution of health foods. Yunnan Runcangsheng operates a 13,000 square meter production facility, which houses R&D centers, extraction facilities, preparation workshops and a warehouse. Yunnan Runcangsheng has more than 30 sub brands and operates planting facilities where it grows some of the key ingredients used in its products. Many of the products it has developed are specifically targeted to disorders associated with changing eating habits and the presence of environmental toxins that are becoming more widespread through the use of western style pesticides and fertilizers.
In addition to our acquisitions in the health and nutritional sector, in July 2021, we acquired a hotel located in the Jinniu District, Chengdu City. The hotel covers more than 8,000 square meters and has a large restaurant that can accommodate 600 people, six luxury dining rooms, a 200 square meter music tea house, 13 private tea rooms, 108 guest rooms and other supporting facilities. We envision utilizing the hotel to conduct marketing events and seminars for our customers, and training sessions for our personnel at which we introduce new products and services intended to promote healthy living.
Business Objectives
Key elements of our business strategy are detailed below:
Leading brand of nutritional supplements. Our primary goal is to develop recognized brand names with a reputation as a provider of quality health and wellness products which deliver demonstrable benefits to our customers. Our objective is to provide potential customers with the information necessary to maintain a healthy lifestyle and to offer a variety of products whether the customer is looking to treat a specific health-related issue, maintain their overall wellness or improve their performance. Our premium, value-added offerings will include both proprietary branded products and other branded products provided by third parties to meet needs not met by our in-house products.
We believe that by offering a variety of branded exclusive products and a wide range of merchandise, and close customer support and services, we will be able to differentiate our Company from competitors and effectively compete against other food, drug and mass channel players, specialty stores, independent vitamin, supplement and natural food shops and online retailers.
| 3 |
Product development and innovation. With the acquisition of Yunnan Runcangsheng, we have the facilities and, more importantly, knowledgeable, experienced personnel to develop high-quality, innovative health foods and nutritional supplements that will be offered by our direct marketing team and available through our Company-owned pharmacies, our website and, as demand grows other select marketplaces and wholesale partners. To the extent desirable we will grow our own key ingredients. When we choose to use ingredients of third parties, they will be rigorously tested before they are added to our products, undergoing multiple quality checks to ensure that they meet our high standards for purity, composition and absence of contaminants.
We believe our ability to innovate and respond to customer needs by designing new products gives us a significant competitive advantage. At Yunnan Runcangsheng we directly employ scientists, nutritionists, formulators, and quality control experts who work at our research and manufacturing facilities.
A differentiated customer experience. We believe one of the key differentiators to our marketing strategy is the continued development of well-trained team members first used at AiXinZhonghong that work closely with customers. We will provide our team members with regular training and education focused on the benefits of a healthy lifestyle, the advantages of our products and solution-based selling. With their knowledge of the available products, our team members can engage customers in conversation, access the customer’s purchasing history through our data base, share product information and testimonials, and recommend solutions and help customers add complimentary products and build wellness regimens. We operate in a highly personalized, aspirational sector and believe that health food and nutritional supplement consumers often desire and seek out product expertise and knowledgeable customer service.
Our loyalty programs used early on by AiXinZhonghong will allow us to develop and maintain a large and loyal customer base, provide targeted offers and information, and connect with our customers on a regular basis. We routinely harness data generated by these programs to better understand customers’ buying behaviors and needs, so we can deliver a stronger experience, bring like-minded consumers into the channel and make well-informed decisions about our business.
As we grow and develop our customer base, we can use our hotel as a site for educational seminars, conferences and marketing events featuring well known experts in the wellness sector. These events can be used to increase customer loyalty and to educate our team members as to the benefits of a healthy lifestyle and our products so they can better serve our customers by guiding them to products that meet their needs.
Omni-channel development. We believe our diversified, omni-channel model, which includes company-owned pharmacies, direct marketing by our team members, individually and at large scale company sponsored marketing events, wholesale locations and on-line promotional activities, can differentiate us from many of our competitors, particularly those that rely exclusively on online marketing efforts. Our strategy is to give consumers a seamless, integrated experience across digital, mobile and in-store channels and in every interaction with us and our products. Through our website and in conversations with our well-trained team members, customers can research our products and purchase them online or at one of our local pharmacies or sales offices. We believe our physical store base provides a competitive advantage, allowing customers to experience our products and get expert advice from our team members.
Vertical Integration. We believe that our multi-channel distribution model, combined with our research, development and manufacturing capabilities offers us a unique advantage over our competitors. The daily feedback received from interactions between our team members and customers will be monitored to recognize what our customers want and rapidly develop products to meet their desires. Instead of simply responding to trends in the market, we will seek to bring new topical products to the market before they are generally available in the market.
| 4 |
Our Corporate Structure
We, AiXin Life International, Inc. (“AiXin Life” or “AiXin Colorado”), are a corporation organized under the laws of Colorado. We are a holding company with no material operations of our own. We conduct all of our operations through our wholly owned subsidiaries which are operating companies established in the PRC. Our operating companies in the PRC are wholly owned by HK AiXin International Group Co., Limited (“AiXin HK”), our wholly owned subsidiary organized in Hong Kong which, in turn is wholly owned by AiXin (BVI) International Group Co., Ltd. (“AiXin BVI”), an entity organized in the British Virgin Islands which is wholly owned by us.
Our subsidiaries are (i) AiXin BVI, (ii) AiXin HK, and the following entities, each of which was organized in the PRC and which are collectively referred to as our “Chinese Operating Companies” (iii) Chengdu AiXinZhonghong Biological Technology Co., Ltd., a Chinese limited company and its subsidiaries, each of which operates a pharmacy (“AiXinZhonghong”), (iv) Yunnan Runcangsheng Technology Co., Ltd (“Yunnan Runcangsheng”), (v) Chengdu Aixintang Haichuan Pharmacy Co., Ltd. and its subsidiaries, each of which operates a pharmacy and (“Chengdu Aixintang Pharmacies”) (vi) AiXin Shangyan Hotel Management Co., Ltd. Our Chinese Operating Companies and AiXin HK are collectively referred to as our “PRC Subsidiaries.” Unless the context otherwise requires, all references in this prospectus to “AiXin Colorado” refer to AiXin Life International, Inc., and references to “AiXin Life,” “we,” “us,” “our,” the “Registrant”, the “Company,” or “our Company” refer collectively to AiXin Colorado and its consolidated subsidiaries.
You will be purchasing shares of AiXin Colorado and will not hold a direct interest in any of its subsidiaries. For the avoidance of doubt, Chengdu Aixintang Haichuan Pharmacy Co., Ltd. owns 100% of the equity interests in each of the nine remaining pharmacy companies.
Summary of Risk Factors
Investing in our common stock involves a high degree of risk. Below is a summary of material factors that make an investment in our common stock speculative or risky. This summary does not address all of the risks that we face. Please refer to the information contained in and incorporated by reference under the heading “Risk Factors” on page 15 of this prospectus.
| 5 |
Risks Associated with Our Company
| ● | The health and wellness industry is highly competitive and we face fierce competition from numerous industry participants, including many larger, well capitalized nationally and internationally recognized companies, see “Risk Factors — Risks Associated with Our Company — We operate in a highly competitive industry and our failure to compete effectively could adversely affect our market share, revenues and growth prospects. |
| ● | We first entered the health and wellness business in 2017 when we acquired AiXin BVI. Consequently, we have a limited operating history on which to evaluate our prospects, see “Risk Factors — Risks Associated with Our Company — We have a limited operating history on which to judge our performance and assess our prospects for success.” |
| ● | We recently completed a significant acquisition and intend to seek to expand through future acquisitions. Our future expansion plans are subject to uncertainties and risks. For more details, see “Risk Factors – Risks Associated with Our Company – Our recently completed acquisition is subject to uncertainties and risks” and – “Our future expansion plans are subject to uncertainties and risks.” |
| ● | Our success depends upon our ability to anticipate and timely react to changes in consumers demands, see “Risk Factors – Risks Associated with Our Company - Failure to effectively anticipate consumer preferences could negatively impact the demand for our products and our ability to generate revenues and the market price of our common stock.” |
| ● | We may not succeed in our efforts to develop new products which could cause us to devote our resources to projects that do not succeed, see “Risk Factors – Risks Associated with Our Company—Resources devoted to product innovation may not yield new products that achieve commercial success.” |
| ● | Our success depends on our ability to attract and retain customers through a coordinated omni channel distribution network which includes pharmacies, direct marketing and online activities. If we do not succeed in these efforts we may fail to grow our customer base. For more details, see “Risk Factors – Risks Associated with Our Company – If we do not successfully develop and maintain a robust omni-channel experience for our customers, our business and results of operations could be materially and adversely affected.” |
| ● | If we fail to succeed in developing and protecting our brand names we could fail in our efforts to grow our customer base, see “Risk Factors – Risks Associated with Our Company —If we fail to develop and protect our brand names and reputation, we may not attract and retain new customers which could adversely affect our revenues and financial performance.” |
| ● | We are highly dependent upon consumer perception of our products and unfavorable publicity about us or our business may materially and adversely affect our reputation, business, results of operations and financial condition. For more details, see “Risk Factors – Risks Associated with Our Company –Unfavorable publicity about us or consumer perception of our products, the ingredients they contain and any similar products distributed by other companies could cause fluctuations in our operating results and could have a material adverse effect on our reputation, the demand for our products and our ability to generate revenues and the market price of our common stock.” |
● |
We face risks related to our ability to effectively source raw materials and any adverse change in the supply or cost of raw materials may adversely affect our business. See “Risk Factors – Risks Associated with Our Company -A significant disruption to our timely receipt of raw materials and inventory could adversely impact sales and operations or increase our transportation costs, which would decrease our profits. |
| ● | We are highly dependent upon the services of Quanzhong Lin, our President, Chief Executive Officer and principal shareholder. The loss of his services could have a Material adverse impact on our business. See, Risk Factors – Risks Associated with Our Company - Our business depends on the continued contributions made by Mr. Quanzhong Lin, our key executive officer. The loss of his services may result in a severe impediment to our business.” |
| 6 |
General Risks Associated with Business Operations in China
| ● | The Chinese government may intervene or influence our operations at any time or may exert more control over offerings conducted overseas and/or foreign investment in China-based issuers, which could result in material changes in our operations and/or the value of your common stock. For more details, see “Risk Factors – General Risks Associated with Business Operation in China - The PRC government has significant oversight and discretion over the conduct of a PRC company’s business operations or to exert control over any offering of securities conducted overseas and/or foreign investment in China-based issuers, and may intervene with or influence our operations, may limit or completely hinder our ability to offer or continue to offer securities to investors, and may cause the value of such securities to significantly decline or become worthless, as the government deems appropriate to further regulatory, political and societal goals.” |
| ● | On February 17, 2023, the CSRC released the Trial Measures, which came into effect March 31, 2023. The Trial Measures apply to overseas securities offerings and/or listings conducted by companies incorporated in the PRC, PRC domestic companies, directly and companies incorporated overseas with operations primarily in the PRC, indirect offerings. The Trial Measures require (1) the filings of the overseas offering and listing plan by the PRC domestic companies with the CSRC under certain conditions, (2) filings by the underwriter with the CSRC under certain conditions and (3) the submission of an annual report to the CSRC within the required timeline.
On the day it released the Trial Measures, the CSRC held a press conference and issued the Notice on Administration for the Filing of Overseas Offering and Listing by Domestic Companies (“Notice on Overseas Filing”), which, among others, clarified that: (i) PRC domestic companies that have submitted applications for an overseas offering and listing on or prior to the effective date of the Trial Measures but have not obtained approval from overseas regulatory authorities or stock exchanges may reasonably arrange the timing for submitting their filing applications with the CSRC, and should complete the filing before the completion of their overseas offering and listing; (ii) a six-month transition period will be granted to PRC companies in which they can complete an overseas offering and listing provided they obtained the approval for the offering from an overseas regulatory authority or stock exchange (such as the effectiveness of a registration statement in the United States) prior to March 31, 2023 and (iii) companies subject to the Trial Measures which have securities which trade in an overseas market prior to the effectiveness of the Trial Measures will need to file with the CSRC within three days of the completion of a follow-on offering.
Based on the advice of our PRC Counsel, this offering will be considered an indirect offering and we will need to fulfill filing obligations pursuant to the Trial Measures within three days of the completion of this offering. If we fail to comply with the post-offering filing obligations imposed by the Trial Measures or make a misrepresentation, misleading statement or material omission in the materials we submit to the CSRC, the CSRC would have the right to order rectification, issue a warning and impose a fine on us of between RMB1 million and RMB10 million and issuing a warning to the parties responsible for such failure, misrepresentation or material omission and impose a fine on each of such individuals ranging from RMB500,000 to RMB5 million.
The Trial Measures may subject us to additional compliance requirement in the future. Any failure to fully comply with new regulatory requirements may significantly limit or completely hinder our ability to offer or continue to offer our shares, cause significant disruption to our business operations, and severely damage our reputation, which would materially and adversely affect our financial condition and results of operations and cause our common stock to significantly decline in value or become worthless. See “Risk Factors – General Risks Associated with Business Operations in China — The CSRC has released the Trial Measures requiring Chinese domestic companies to complete filings with the CSRC if they complete an overseas offering and listing of their securities.” |
| ● | The Provisions on Strengthening the Confidentiality and Archives Administration Related to the Overseas Securities Offering and Listing by Domestic Enterprises (the “Confidentiality and Archives Provisions”), went into effect March 31, 2023. The Confidentiality and Archives Provisions require, among other items, that PRC domestic enterprises seeking to offer and list securities in overseas markets, establish an archival system which will maintain the confidentiality of information in accordance with applicable laws and regulations. Further, if a PRC domestic enterprise plans, directly or indirectly, to release documents or provide material that contain state secrets or government work secrets, or that will jeopardize national security or the public interest, it must strictly comply with all relevant governmental procedures and obtain approval of the appropriate regulatory before doing so. Although our PR Counsel has advised us that although we are subject to the Confidentiality and Archives Provisions, we believe that none of the documents or materials we intend to provide to parties outside of the PRC contains materials that would require us to make any filing or obtain any approval of a Chinese regulatory authority. Nevertheless, the determination of whether information contains state secrets, government work secrets or jeopardizes national security or the public interest is subjective and any failure or perceived failure by us or our subsidiaries to comply with the confidentiality and archive administration requirements under the Confidentiality and Archives Provisions could cause us to be referred for criminal investigation and held liable for such violations by the authorities in China. Further, PRC regulatory authorities could use these regulations to limit our disclosures or interfere with our operations. Any determination that we have violated the Confidentiality and Archives Provisions or use of these provisions to limit our disclosures could cause the value of our common stock to significantly decline in value or become worthless. See “Risk Factors – General Risks Associated with Business Operations in China — The CSRC has released rules for China-based companies seeking to conduct initial public offerings in foreign markets. The Chinese government may exert more oversight and control over offerings that are conducted overseas and foreign investment in China-based issuers, which could significantly limit or completely hinder our ability to offer or continue to offer our common stock to investors and could cause the value of our common stock to significantly decline or become worthless.” |
| ● | Article 26 of the Trial Measures came into effect on March 31, 2023. Article 26 provides, among other things, that any domestic entity or individual intending to provide documents and materials requested by an overseas securities regulatory agency in connection with an investigation may not provide such information without prior approval from the CSRC and competent authorities under the State Council. In addition, Article 11 of the Provisions on Strengthening Confidentiality and Archives Administration in Respect of Overseas Issuance and Listing of Securities by Domestic Enterprises, or Article 11 came into effect on March 31, 2023. Article 11 provides among other things, that any domestic company, securities firm and securities service agency shall obtain the consent of the CSRC or the relevant administrative authorities prior to cooperating in an inspection or investigation carried out by an overseas securities regulator or relevant administrative authorities or providing documents and materials for cooperating in the inspection or investigation.
If the Company were to provide information to any securities regulatory agency in violation of Article 26 or Article 11, it would be subject to fines and, if the information were deemed a state secret, government work secret or might jeopardize the national security of China or the public interest, as provided in the Confidentiality and Archives Provisions, it could cause the Company or the individuals responsible for the disclosure to be subject to criminal investigation. Likewise, if the Company refuses to provide information requested by any U.S. securities regulatory agency in circumstances that the Company believes would have caused it to violate Article 26 or Article 11, it will be subject in the United States to fines, penalties or delisting from Nasdaq. While detailed interpretation of or implementation rules of Article 26 and Article 11 have yet to be promulgated, the inability of an overseas securities regulator to directly conduct an investigation or evidence collection activities within China may further increase difficulties that you may face in protecting your interests, see “We could be subject to conflicting demands placed upon us by regulatory authorities in the United States and the PRC which could result in disciplinary actions if not resolved.” |
| ● | You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing actions in China against us or our management named in the prospectus based on foreign laws, including U. S. court judgements based on violations of the civil liability provisions of the U. S. federal securities laws. For more details, see “Risk Factors – General Risks Associated with Business Operations in China - You may have difficulty enforcing judgments against us.” |
| 7 |
| ● | Changes in the policies, regulations, rules and the enforcement of laws of the PRC government may be quick with little advance notice and could have a significant impact upon the business we may be able to conduct in the PRC and the profitability of such business. For more details, see “Risk Factors - General Risks Associated with Business Operations in China - Changes in the policies, regulations, rules and the enforcement of laws of the PRC government may be quick with little advance notice and could have a significant impact upon the business we may be able to conduct in the PRC and the profitability of such business.” |
| ● | Inflation could pose a risk to our business. For more details, see “Risk Factors - General Risks Associated with Business Operations in China - Inflation could pose a risk to our business.” |
| ● | There are uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations. For more details, see “Risk Factors – General Risks Associated with Business Operation in China - There are uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations.”. |
| ● | PRC regulations regarding acquisitions impose significant regulatory approval and review requirements, which could make it more difficult for us to pursue growth through acquisitions. For more details, see “Risk Factors – General Risks Associated with Business Operation in China - PRC regulations regarding acquisitions impose significant regulatory approval and review requirements, which could make it more difficult for us to pursue growth through acquisitions.” |
| ● | While the approval of the China Securities Regulatory Commission is not currently required for this offering, it may be required in the future in connection with this offering under the M&A Rules and, if required, we cannot predict whether we will be able to obtain such approval. For more details, see “Risk Factors – General Risks Associated with Business Operation in China - While the approval of the China Securities Regulatory Commission is not currently required for this offering, it may be required in the future in connection with this offering under the M&A Rules and, if required, we cannot predict whether we will be able to obtain such approval.” |
| ● | Our business may be subject to a variety of PRC laws and other obligations regarding cybersecurity and data protection. For more details, see “Risk Factors – General Risks Associated with Business Operation in China - Our business may be subject to a variety of PRC laws and other obligations regarding cybersecurity and data protection.” |
| ● | PRC regulations relating to investments in offshore companies by PRC residents may subject our PRC-resident beneficial owners or our PRC Subsidiaries to liability or penalties, limit our ability to inject capital into our PRC Subsidiaries or limit our PRC Subsidiaries’ ability to increase their registered capital or distribute profits. For more details, see “Risk Factors – General Risks Associated with Business Operation in China - PRC regulations relating to investments in offshore companies by PRC residents may subject our PRC-resident beneficial owners or our PRC Subsidiaries to liability or penalties, limit our ability to inject capital into our PRC Subsidiaries or limit our PRC Subsidiaries’ ability to increase their registered capital or distribute profits.” |
| 8 |
| ● | We may be treated as a resident enterprise for PRC tax purposes under the PRC Enterprise Income Tax Law, and we may therefore be subject to PRC income tax on our global income. For more details, see “Risk Factors – General Risks Associated with Business Operation in China - We may be treated as a resident enterprise for PRC tax purposes under the PRC Enterprise Income Tax Law, and we may therefore be subject to PRC income tax on our global income.” |
| ● | Restrictions on currency exchange may limit our ability to utilize our PRC revenue effectively. For more details, see “Risk Factors – General Risks Associated with Business Operation in China - Restrictions on currency exchange may limit our ability to utilize our PRC revenue effectively.” |
| ● | Introduction of new laws or regulations or changes to existing laws or regulations by the PRC government may adversely affect our business. For more details, see “Risk Factors – General Risks Associated with Business Operation in China - Introduction of new laws or changes to existing laws by the PRC government may adversely affect our business.” |
Risks Relating to Our Holding Company Structure
| ● | Substantial uncertainties exist with respect to the interpretation and implementation of the newly enacted Foreign Investment Law and how it may impact the viability of our current corporate structure, corporate governance and business operations. For more details, see “Risk Factors – Risks Relating to Our Holding Company Structure - Substantial uncertainties exist with respect to the interpretation and implementation of the newly enacted Foreign Investment Law and how it may impact the viability of our current corporate structure, corporate governance and business operations.” |
| ● |
We may rely on dividends and other distributions on equity paid by our PRC Subsidiaries and loans between us and our PRC Subsidiaries to fund any cash and financing requirements we or any of our PRC Subsidiaries may have, and any limitation on the ability of our PRC Subsidiaries to make payments to or transfer funds to us or our other PRC Subsidiaries could have a material and adverse effect on our ability to conduct our business and the ability of our PRC Subsidiaries to conduct their respective businesses. Current PRC regulations permit our PRC Subsidiaries to pay dividends to us only out of their accumulated profits, if any, determined in accordance with Chinese accounting standards and regulations. In addition, each of our PRC Subsidiaries is required to set aside at least 10% of its after-tax profits each year, if any, to fund a statutory reserve until such reserve reaches 50% of its registered capital. As of the date hereof, we have had no transactions that involved the transfer of cash or assets throughout our corporate structure. The PRC Subsidiaries have not transferred cash or other assets to AiXin BVI or AiXin Colorado, including by way of dividends or interest payments and AiXin BVI and AiXin Colorado have not transferred cash or other assets to any of our PRC Subsidiaries. AiXin Colorado does not currently plan or anticipate transferring cash or other assets from our operations in China to any non-Chinese entity. As of the date hereof, no transfers, dividends or distributions have been made to our investors. To the extent our cash or other assets is in one of our Chinese Operating Companies or AiXin HK, the funds or assets may not be available to fund operations or for use outside of mainland China or Hong Kong due to interventions by the governments of PRC or Hong Kong, or the imposition of restrictions and limitations on the ability of the PRC Subsidiaries to use such cash or assets imposed by the government of mainland China or Hong Kong. Except for such limitations on our Company’s ability to transfer cash and other assets among our entities currently or hereafter imposed by the governments of the PRC and Hong Kong, there are no limitations on our Company’s ability to transfer cash and other assets through our corporate structure. Our Company does not have any cash management policies with respect to the transfer of cash among our entities other than requirements for the approval of management for transfers in excess of specified amounts and none of our entities is currently party to any debenture, loan or other agreement which imposes restrictions or otherwise limits our Company’s ability to transfer our cash and other assets. We have not adopted any policies that dictate how funds are transferred among our entities other than as necessary to comply with applicable laws. For more details, see “Risk Factors – Risks Relating to Our Holding Company Structure - We may rely on dividends and other distributions on equity paid by our PRC Subsidiaries to fund any cash and financing requirements we may have, and any limitation on the ability of our PRC Subsidiaries to make payments to us could have a material and adverse effect on our ability to conduct our business.” and “Prospectus Summary - Transfers of Cash to and from our Subsidiaries.” |
| ● | PRC regulation of loans to and direct investment in PRC entities by offshore holding companies and governmental control of currency conversion may delay or prevent us from using the proceeds of this offering to make loans or additional capital contributions to our PRC Subsidiaries, which could materially and adversely affect our liquidity and our ability to fund and expand our business. For more details, see “Risk Factors – Risks Relating to Our Holding Company Structure - PRC regulation of loans to and direct investment in PRC entities by offshore holding companies and governmental control of currency conversion may delay or prevent us from using the proceeds of this offering to make loans or additional capital contributions to our PRC Subsidiaries, which could materially and adversely affect our liquidity and our ability to fund and expand our business.” |
Risks Relating to this Offering and Our Common Stock
| ● | Prior to this offering, we had a limited public market for our common stock and you may not be able to resell our shares at or above the price you paid, or at all. For more details, see “Risk Factors – Risks Relating to this Offering and Our Common Stock - Prior to this offering, we had a limited public market for our common stock and you may not be able to resell our shares at or above the price you paid, or at all.” |
| ● | Future sales of substantial amounts of our common stock by existing shareholders, including sales resulting from a foreclosure of shares pledged by holders of a substantial number of shares of our common stock, could adversely affect the price of our common stock. For more details, see “Risk Factors – Risks Relating to this Offering and Our Common Stock - The sale or availability for sale of substantial amounts of our common stock could adversely affect its market price.” |
| 9 |
| ● | The market price of our shares is likely to be highly volatile and subject to wide fluctuations in response to various factors. For more details, see “Risk Factors – Risks Relating to this Offering and Our Common Stock - The market price of our shares is likely to be highly volatile, which could result in substantial losses to investors.” |
| ● | We may never be able to pay dividends and are unlikely to do so. For more details, see “Risk Factors – Risks Relating to this Offering and Our Common Stock - Because we do not expect to pay dividends in the foreseeable future after this offering, you must rely on price appreciation of our common stock for return on your investment.” |
PRC Limitation on Overseas Listing and Share Issuances
As confirmed by our PRC Counsel, neither we nor our subsidiaries are currently required to obtain approval from Chinese authorities, including the China Securities Regulatory Commission, or CSRC, or Cybersecurity Administration Committee, or CAC, to list on U.S. exchanges or issue securities to foreign investors. We are, however, required by the Trial Measures to submit a filing with the CSRC within three days after the completion of this offering. If we were to fail to comply with the post-offering filing obligations imposed by the Trial Measures or make a misrepresentation, misleading statement or material omission in the materials we submit to the CSRC, the CSRC would have the right to order rectification, issue a warning and impose a fine on us of between RMB1 million and RMB10 million and issuing a warning to the parties responsible for such failure, misrepresentation or material omission and impose a fine on each of such individuals ranging from RMB500,000 to RMB5 million.
If our PRC Subsidiaries or AiXin Colorado were required to obtain approval in the future and were denied permission from Chinese authorities to list on U.S. exchanges, we would not be able to continue listing on any U.S. exchange, which would materially affect the interests of investors in our securities. It is uncertain when and whether we will be required to obtain permission from the PRC government to list on U.S. exchanges in the future, and even if such permission is obtained, whether it will be rescinded. Although we currently are not required to obtain permission from any of the PRC central or local government agencies and have not been denied the right to list on a U.S. exchange by any Chinese governmental authority, our operations could be adversely affected, directly or indirectly, by existing or future laws and regulations denying us the right to list on any U.S. exchange. If we inadvertently conclude that such approvals are not required when they are, or applicable laws, regulations, or interpretations change and we are required to obtain approval in the future, our operations and the interests of our shareholders could be adversely affected. For more detailed information, see “Risk Factors – General Risks Associated with Business Operations in China – While the approval of the China Securities Regulatory Commission is not currently required for this offering, it may be required in the future in connection with this offering under the M&A Rules and, if required, we cannot predict whether we will be able to obtain such approval.”
On February 17, 2023, the CSRC released regulations for the filing-based administration of direct and indirect overseas securities offerings and listings by domestic companies incorporated in Mainland China. The regulations became effective March 31, 2023. The regulations are comprised of the Trial Administrative Measures of Overseas Securities Offering and Listing by China-based Companies (the “Trial Measures”) and five supporting guidelines. The Trial Measures lay out the filing regulations for both direct and indirect overseas listings and clarify the criteria for determining whether a listing constitutes an indirect overseas listing in an overseas market.
An offering and listing by an overseas company will be deemed an indirect offering by a China-based company: if (i) more than 50% of the overseas company’s consolidated revenues, profit, total assets or net assets as indicated by its audited consolidated financial statements for the most recently completed fiscal year are attributable to PRC domestic companies, and (ii) one of (a) key components of its operations are carried out in Mainland China; (b) its principal places of business are located in Mainland China; or (c) the majority of the senior management members in charge of operations are Mainland China citizens or residents, is true. The determination is made on the basis of a “substance over form” review.
If a China-based company seeks to indirectly offer and list securities in an overseas market, it is required to designate a major Chinese operating entity to be responsible for processing all filings with the CSRC. With respect to an initial offering and listing overseas, the initial filing with the CSRC is to be made within three business days of the date on which the overseas application is submitted. In the case of a follow-on offering in the same overseas market where a Chinese based company has previously offered and listed securities, the issuer shall submit filings with the CSRC within three business days after the follow-on offering is completed.
In addition, an overseas offering and listing is prohibited if: (1) the intended securities offering and listing is specifically prohibited by PRC law, regulations or relevant national provisions; (2) the State Council determines that the intended securities offering and listing endanger the national security of the PRC; (3) in the past three years, the issuer or its controlling shareholders or the actual parties controlling the issuer have been convicted of committing corruption, bribery, embezzlement, misappropriation of property, or other criminal offenses disruptive to the order of the Chinese socialist market economy, or are under judicial investigation for suspicion of criminal offenses or major violations; (4) the issuer is suspected of committing any crimes or serious violations of laws and regulations and is under an investigation which has not been concluded; (5) there are material disputes with regard to the ownership of the securities of the relevant Chinese entity by its controlling shareholders or shareholders or by the parties actually in control of the issuer. The Trial Measures provide that in the event of an issuer’s breach of the Trial Measures, such as a failure to comply with the filing obligations, conducting an overseas securities offering and listing in violation of the Trial Measures or making a misrepresentation, misleading statement or material omission in the materials submitted to the CSRC by the issuer, the CSRC shall order rectification, issue warnings to the issuer, and, impose a fine between RMB1 million and RMB10 million on the issuer, and further, that the parties responsible for such breach, failure, misrepresentation or material omission shall be warned and each shall be subject to a fine ranging from RMB500,000 to RMB5 million.
On the date of the release of the Trial Measures, the CSRC held a press conference and issued the Notice on Administration for the Filing of Overseas Offering and Listing by China-based Companies, which clarified that: (i) China-based companies that have submitted applications for an overseas offering and listing on or prior to the effective date of the Trial Measures, but have not obtained approval from overseas regulatory authorities or stock exchanges may reasonably arrange the timing for submitting their filing applications with the CSRC and should complete the filing before the completion of their overseas offering and listing; (ii) a six-month transition period will be granted to China-based companies in which they can complete an overseas offering and listing provided that they have obtained approval from an overseas regulatory authority or stock exchange (such as effectiveness of a registration statement in the market of the United States) prior to March 31, 2023 and (iii) companies which have securities which trade in an overseas market prior to the effective date of the Trial Measures will need to file with the CSRC pursuant to the Trial Measures within three days of completion of a follow-on offering.
Because our PRC Subsidiaries have accounted for more than 50% of our consolidated revenues, profit, total assets and net assets from inception of our Company to date and key components of our operations are carried out in Mainland China, the offering by us pursuant to this registration statement is deemed by the CSRC to be an indirect offering and we will need to fulfill filing obligations pursuant to the Trial Measures after we have completed this offering. “Risk Factors – General Risks Associated with Business Operations in China — The CSRC has released the Trial Measures requiring Chinese domestic companies to complete filings with the CSRC if they complete an overseas offering and listing of their securities.”
The Provisions on Strengthening the Confidentiality and Archives Administration Related to the Overseas Securities Offering and Listing by Domestic Enterprises (the “Confidentiality and Archives Provisions”), came into effect March 31, 2023. The Confidentiality and Archives Provisions require, among other items, that PRC domestic enterprises seeking to offer and list securities in overseas markets, shall establish an archival system which will maintain the confidentiality of information in accordance with applicable laws and regulations. Further, if a PRC domestic enterprise plans, directly or indirectly, to release documents or provide material that contain state secrets or government work secrets, or that will jeopardize national security or the public interest, it must strictly comply with all relevant governmental procedures and obtain approval of the appropriate regulatory before doing so. Although our PRC Counsel has advised us that we are subject to the Confidentiality and Archives Provisions, we believe that none of the documents or materials we intend to provide to parties outside of the PRC contains materials that would require us to make any filing or obtain any approval of a Chinese regulatory authority. Nevertheless, the determination of whether information contains state secrets, government work secrets or jeopardizes national security or the public interest is subjective and any failure or perceived failure by us or our subsidiaries to comply with the confidentiality and archive administration requirements under the Confidentiality and Archives Provisions could cause us to be referred for criminal investigation and held liable for such violations by the authorities in China. Further, PRC regulatory authorities could use these regulations to limit our disclosures or interfere with our operations. Any determination that we have violated the Confidentiality and Archives Provisions or use of these provisions to limit our disclosures could cause the value of our common stock to significantly decline in value or become worthless. See “Risk Factors – General Risks Associated with Business Operations in China — The CSRC has released rules for China-based companies seeking to conduct initial public offerings in foreign markets. The Chinese government may exert more oversight and control over offerings that are conducted overseas and foreign investment in China-based issuers, which could significantly limit or completely hinder our ability to offer or continue to offer our common stock to investors and could cause the value of our common stock to significantly decline or become worthless.”
We also reviewed our operations with our PRC Counsel as to the applicability of the Measures for Cybersecurity review administered by the CAC. During this review we and our PRC Counsel determined that we are not a “critical information infrastructure operator” as we do not operate or maintain any infrastructure used by the public to communicate or transmit information. We further determined that we possess only limited personal information with respect to any individual, currently have personal information pertaining to fewer than 1 million individuals and the data we process in our business does not have a bearing on national security and thus may not be classified as core or important data by the authorities and, consequently, we and our PRC Counsel concluded that we are not subject to cybersecurity review by the CAC. See “Risk Factors - Our business may be subject to a variety of PRC laws and obligations regarding cybersecurity and date protection.”
As of the date of this prospectus, other than confirming with our PRC Counsel that our offering under this prospectus does not require the examination and approval of the CSRC in accordance with the existing PRC legislation and regulations, we have not received any inquiry, notice, warning, sanctions or regulatory objection to this offering from the CSRC, CAC or any other PRC governmental authorities, and we believe our PRC Subsidiaries have obtained all requisite permissions and approvals from PRC governmental authorities to operate our business as currently conducted under relevant PRC laws and regulations.
Currently, each of our Chinese Operating Companies holds and maintains a business license issued by the local market supervision and administration bureau and has received all requisite permissions and approvals in order to conduct and operate its business. As of the date of this prospectus, none of our Chinese Operating Companies has been denied a business license or punished by relevant governmental authorities due to its business operations. In addition, we and our non-PRC Subsidiaries have also received all requisite permissions and approvals in order to conduct and operate our business.
| 10 |
Transfers of Cash to and from our Subsidiaries
We (AiXin Colorado) are a Colorado holding company with no material operations of our own. We conduct all of our operations through operating companies established in the PRC. As a result, our ability to pay dividends to our shareholders and to service any debt we may incur may depend upon dividends paid by our PRC Subsidiaries. If any of our subsidiaries incur debt on its own, the instruments governing such debt may restrict its ability to pay dividends to us or otherwise transfer funds to us or any of our other subsidiaries. In addition, our PRC Subsidiaries are required to make appropriations to certain statutory reserve funds, which are not distributable as cash dividends except in the event of a liquidation of the Company at a time when it is solvent.
Current PRC regulations permit our PRC Subsidiaries to pay dividends to us through AiXin HK, our intermediate holding subsidiary in Hong Kong, only out of their accumulated profits, if any, determined in accordance with Chinese accounting standards and regulations. As of the date hereof, none of our PRC Subsidiaries has distributed any dividends to AiXin HK. Except for such limitations on our Company’s ability to transfer cash and other assets among our entities currently or hereafter imposed by the governments of the PRC and Hong Kong, there are no limitations on our Company’s ability to transfer cash and other assets through our corporate structure. Our Company does not have any cash management policies with respect to the transfer of cash among our entities other than requirements for the approval of management for transfers in excess of specified amounts and none of our entities is currently party to any debenture, loan or other agreement which imposes restrictions or otherwise limits our Company’s ability to transfer our cash and other assets. We have not adopted any policies that dictate how funds are transferred among our entities other than as necessary to comply with applicable laws.
The PRC government also imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies outside the PRC. Therefore, we may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency for the payment of dividends from our profits, if any, or for any other purpose. To the extent our cash or other assets is in one of our Chinese Operating Companies or AiXin HK, the funds or assets may not be available to fund operations or for use outside of mainland China or Hong Kong including for the payment of dividends to the shareholders of AiXin Colorado, due to interventions by the governments of PRC or Hong Kong, or the imposition of restrictions and limitations on the ability of the PRC Subsidiaries to use such cash or assets imposed by the government of mainland China or Hong Kong. See “Risk Factors – Risks Relating to Our Holding Company Structure - We may rely on dividends and other distributions on equity paid by our PRC Subsidiaries to fund any cash and financing requirements we may have, and any limitation on the ability of our PRC Subsidiaries to make payments to us could have a material and adverse effect on our ability to conduct our business.”
Cash dividends, if any, on our common stock will be paid in U.S. dollars. If we are considered a PRC tax resident enterprise for tax purposes, any dividends we pay to our overseas shareholders may be regarded as China-sourced income and as a result may be subject to PRC withholding tax at a rate of up to 10.0%.
Pursuant to the Arrangement between Mainland China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation and Tax Evasion on Income, or the Double Tax Avoidance Arrangement, the 10% withholding tax rate may be lowered to 5% if a Hong Kong resident enterprise owns no less than 25% of a PRC project. However, the 5% withholding tax rate does not automatically apply and certain requirements must be satisfied, including without limitation that (a) the Hong Kong enterprise must be the beneficial owner of the relevant dividends; and (b) the Hong Kong enterprise must directly hold no less than 25% share ownership in the PRC project during the 12 consecutive months preceding its receipt of the dividends. In current practice, a Hong Kong entity must obtain a tax resident certificate from the Hong Kong tax authority to apply for the 5% lower PRC withholding tax rate. As the Hong Kong tax authority will issue such a tax resident certificate on a case-by-case basis, we cannot assure you that we will be able to obtain the tax resident certificate from the relevant Hong Kong tax authority and enjoy the preferential withholding tax rate of 5% under the Double Taxation Arrangement with respect to dividends to be paid by any of our Chinese Operating Companies to AiXin HK. As of the date of this prospectus, we have not applied for the tax resident certificate from the relevant Hong Kong tax authority. AiXin HK intends to apply for the tax resident certificate at such time as one of our Chinese Operating Companies plans to declare and pay a dividend to AiXin HK. See “Risk Factors – Risks Relating to Our Holding Company Structure – We may rely on dividends and other distributions on equity paid by our PRC Subsidiaries to fund any cash and financing requirements we may have, and any limitation on the ability of our PRC Subsidiaries to make payments to us could have a material and adverse effect on our ability to conduct our business.”
As of the date hereof, we have had no transactions that involved the transfer of cash or assets throughout our corporate structure. The PRC Subsidiaries have not transferred cash or other assets to AiXin BVI or AiXin Colorado, including by way of dividends, and AiXin BVI and AiXin Colorado have not transferred cash or other assets to any of our PRC Subsidiaries. AiXin Colorado does not currently plan or anticipate causing any of our PRC Subsidiaries to transfer cash or other assets from our operations in China to any non-Chinese entity. As of the date hereof, no transfers, dividends, or distributions have been made to our investors. Our Company is not currently party to any debenture, loan or other agreement which imposes restrictions or otherwise limits our Company’s ability to transfer our cash and other assets and we have not adopted any policies that dictate how funds are transferred other than requirements for the approval of management for transfers in excess of specified amounts and as necessary to comply with applicable laws. Except for such limitations on our Company’s ability to transfer cash and other assets among our entities currently or hereafter imposed by the governments of the PRC and Hong Kong, there are no limitations on our Company’s ability to transfer cash and other assets through our corporate structure. See “Prospectus Summary - Transfers of Cash to and from our Subsidiaries.”
You may not have the Ability to Obtain an Adequate Remedy at Law Against us or our Management
AiXin Colorado, the entity in which you will acquire shares is a holding company. All of our operations are conducted by and practically all of our cash and other assets are owned by operating businesses in the PRC. In addition, nearly all of our officers and directors, specifically, Quanzhong Lin, Huiliang Jiao and Tianfeng Li are PRC nationals and residents of China and all of their assets are located outside the United States. In addition, Yao-Te Wang one of our directors is a resident of Taiwan, and all of his assets are located outside the United States. Christopher Lee, our remaining director, resides outside of the United States for a significant portion of time and portions of his assets are located outside the United States. In order to commence an action against any of our directors and officers, a United States Court may require that you effect personal service of the complaint on the intended defendant. Although there are international conventions for serving process on prospective defendants outside the United States, such service may take an extraordinary amount of time and in order to be completed, may require the cooperation of courts in the jurisdiction where the intended defendant resides. Further, should the individual seek to avoid service of process, it may be more difficult to effect service than it would be in the United States. As a result, it may be difficult for you to effect service of process upon us or our directors and officers outside of the United States and, in particular, inside China or to bring actions against us or our management in China.
Shareholder claims and other causes of action that are common in the United States, including securities law class actions and fraud claims, generally are difficult to pursue as a matter of law or practicality in China. For example, in China, there are significant legal and other obstacles to obtaining information needed for shareholder investigations or litigation outside China. Although the local authorities in China may establish a regulatory cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and administration, such regulatory cooperation with the securities regulatory authorities in the Unities States has not been effective. According to Article 177 of the PRC Securities Law which became effective in March 2020, no overseas securities regulator is allowed to directly conduct an investigation or evidence collection activities within the territory of the PRC. Accordingly, without the consent of competent PRC securities regulators and relevant authorities, no organization or individual may provide the documents and materials relating to securities business activities to overseas parties outside of the PRC.
China does not have treaties providing for the reciprocal recognition and enforcement of judgments of courts with the United States and many other countries and regions. Thus, there is no mechanism currently in place for enforcing a judgement in China. Even judgements from countries that have an enforcement treaty with China are often not enforceable in China. Therefore, recognition and enforcement in China of judgments of a court in any non-PRC jurisdiction in relation to any matter may be difficult or impossible. Further, although we are not s state owned enterprise, many businesses in China are owned in whole or in part by the Chinese government. If enforcement of a judgement against us would have an adverse impact on a state owned enterprise, local officials may be reluctant to cooperate in any enforcement action. Further, to the extent that a substantial portion of our outstanding shares of common stock are owned by Chinese residents that might be harmed by enforcement of a judgement against us, such residents may seek to prevent such enforcement.
We currently do not have Directors and Officers Insurance (“D&O Insurance”). Even if we obtain such insurance prior to completion of this offering, there is no guarantee that it will remain in place. The prevalence of D&O Insurance may be one of the reasons there is an active group of attorneys that seek to bring shareholder claims and other causes of action that are common in the United States, including securities law class actions and fraud claims, against public companies and their management. The absence of such insurance may make it more difficult for our shareholders to engage an attorney willing to commence an action against us or our management.
| 11 |
Holding Foreign Companies Accountable Act
Trading in our securities may be prohibited under the Holding Foreign Companies Accountable Act, or the HFCAA, if the PCAOB determines that it cannot inspect or investigate completely our auditor.
Pursuant to the HFCAA, the PCAOB issued a Determination Report on December 16, 2021 which found that the PCAOB was then unable to inspect or investigate completely registered public accounting firms headquartered in: (1) mainland China of the People’s Republic of China because of a position taken by one or more authorities in mainland China; and (2) Hong Kong, a Special Administrative Region and dependency of the PRC, because of a position taken by one or more authorities in Hong Kong. In addition, the PCAOB’s report identified the specific registered public accounting firms which are subject to these determinations. On August 26, 2022, the CSRC, the Ministry of Finance, and the PCAOB signed the Protocol to allow the PCAOB to inspect and investigate completely registered public accounting firms headquartered in mainland China and Hong Kong, consistent with the HFCAA. Pursuant to the fact sheet with respect to the Protocol disclosed by the SEC, the PCAOB shall have independent discretion to select any issuer audits for inspection or investigation and has the unfettered ability to transfer information to the SEC. On December 15, 2022, the PCAOB determined that the PCAOB was able to secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary. However, should PRC authorities obstruct or otherwise fail to facilitate the PCAOB’s access in the future, the PCAOB will consider the need to issue a new determination. On December 29, 2022, the AHCAA was enacted, which amended the HFCAA by decreasing the number of non-inspection years from three years to two, thus reducing the time period before our common stock may be prohibited from trading or delisted.
Our auditor, KCCW Accountancy Corp., is an independent public accounting firm registered with the PCAOB, and as an auditor of publicly traded companies in the U.S., is subject to laws in the U.S. pursuant to which the PCAOB conducts regular inspections to assess its compliance with the applicable professional standards. KCCW Accountancy Corp. is based in the United States and has been inspected by the PCAOB on a regular basis, with the last inspection in 2022. KCCW Accountancy Corp., is not headquartered in mainland China or Hong Kong and was not identified as a firm subject to the determinations announced by the PCAOB on December 16, 2021. Nevertheless, should the PCAOB be unable to fully conduct inspection of our auditor’s work papers in China, it will make it difficult to evaluate the effectiveness of our auditor’s audit procedures or equity control procedures. Investors may consequently lose confidence in our reported financial information and procedures or quality of the financial statements, which would adversely affect us and our securities. See “Risk Factors—Risks Associated with Our Company— A recent joint statement by the SEC and the Public Company Accounting Oversight Board (United States), or the “PCAOB,” proposed rule changes submitted by Nasdaq, and the newly enacted “Holding Foreign Companies Accountable Act” all call for additional and more stringent criteria to be applied to emerging market companies upon assessing the qualification of their auditors, especially the non-U.S. auditors who are not inspected by the PCAOB. These developments could add uncertainties to our offering.”
Corporate Information
AiXin Life International, Inc. was incorporated in the State of Colorado in 2001. We have a fiscal year-end of December 31. Our principal executive offices are located at Hongxing International Business Building 2, 14th FL, No. 69 Qingyun South Ave., Jinjiang District, Chengdu City, Sichuan Province, China and our telephone number is + (86) 313-6732526. We maintain a website at www.aixinjituan.com. The information contained on our website is not, and should not be interpreted to be, a part of this prospectus.
| 12 |
THE OFFERING
| Shares of common stock offered by us: | [2,375,000] shares of common stock, or [2,731,250] shares if the underwriter exercises the over-allotment option in full. | |
| Number of shares of common stock outstanding after this offering: (1) | [20,374,842] shares of common stock will be outstanding after this offering is completed, [20,731,092] shares if the underwriter exercises the over-allotment option in full. | |
| Over-allotment option: | We have granted the underwriter the right to purchase up to [356,250] additional shares of common stock from us at the public offering price less the underwriting discounts and commissions within 45 days from the date of this prospectus to cover over-allotments. | |
| Underwriter’s warrants: | We will issue to Boustead Securities, LLC, the underwriter, upon closing of this offering, compensation warrants, or the Underwriter’s Warrants, entitling the underwriter to purchase 5% of the aggregate number of shares of common stock issued in this offering, including shares issued pursuant to the exercise of the over-allotment option, at an exercise price of $5.00 per share (125% of the public offering price). The Underwriter’s Warrants will have a term of 5 years beginning on date of commencement of sales of the shares offered hereby and may be exercised upon commencement of sales of the shares offered hereby. The Underwriter’s Warrants may be exercised on a cashless basis. | |
| Use of proceeds: | Our proceeds from this offering are expected to be approximately $[9,500,000], before payment of the underwriter’s commissions and other expenses. We intend to use the proceeds from this offering for the purchase and sale of raw materials and developing our own brands, including working capital and general corporate purposes. See “Use of Proceeds” on page 38. | |
| Proposed Nasdaq Capital Market symbol: | We have applied to list our common stock on the Nasdaq Capital Market under the symbol “AIXN”. There can be no assurance that our application will be approved. The closing of this offering is contingent upon the successful listing of our common stock on the Nasdaq Capital Market. | |
| Lock-Up Agreements: | See “Underwriting” for more information. | |
| Risk factors: | Investing in our common stock is highly speculative and involves a significant degree of risk. As an investor you should be able to bear a complete loss of your investment. You should carefully consider the information set forth in the “Risk Factors” section beginning on page 15. | |
| OTCQB Market Symbol: | AIXN. | |
| Transfer Agent: | Securities Transfer Corporation. |
| (1) | The number of shares of our common stock to be outstanding after this offering is based on the number of shares outstanding as of February 8, 2024, reduced by seven million shares to be contributed to our capital by Quanzhong Lin upon effectiveness of the Registration Statement of which this prospectus is a part and gives no effect to shares which may be issued upon exercise of (i) the underwriter’s over-allotment option and (ii) the Underwriter’s Warrants. |
| 13 |
FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements within the meaning of Section 27A of the Securities Act and the Private Securities Litigation Reform Act of 1995, as amended. These forward-looking statements are based on our management’s belief and assumptions and on information currently available to our management. Although we believe that the expectations reflected in these forward-looking statements are reasonable, these statements relate to future events or our future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements.
In some cases, you can identify forward-looking statements by terminology such as “may,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under “Risk Factors” and elsewhere in this prospectus. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those implied or projected by the forward-looking statements. No forward-looking statement is a guarantee of future performance. You should read this prospectus and those documents which we have filed with the SEC as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from any future results expressed or implied by these forward-looking statements.
The forward-looking statements in this prospectus represent our views as of the date of this prospectus. We anticipate that subsequent events and developments may cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should therefore not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this prospectus.
You should also consider carefully the statements under “Risk Factors” and other sections of this prospectus, which address additional facts that could cause our actual results to differ from those set forth in the forward-looking statements. We caution investors not to place significant reliance on the forward-looking statements contained in this prospectus. We undertake no obligation to publicly update or review any forward-looking statements, whether as a result of new information, future developments or otherwise, except as otherwise required by law.
| 14 |
RISK FACTORS
You should carefully consider the risks described below and elsewhere in this prospectus, which could materially and adversely affect our business, results of operations or financial condition. Our business faces significant risks and the risks described below may not be the only risks we face. Additional risks not presently known to us or that we currently believe are immaterial may materially affect our business, results of operations, or financial condition. If any of these risks occur, the trading price of our common stock could decline, and you may lose all or part of your investment. You should consider our business and prospects in light of the challenges we face, including the ones discussed in this section. In the event that any of the events described in the risk factors below occur, it could have a material adverse effect on our operations and cash flow and cause the value of our securities to decline in value or become worthless.
Risks Associated with Our Company
We operate in a highly competitive industry and our failure to compete effectively could adversely affect our market share, revenues and growth prospects.
The market for health and wellness products is large, highly fragmented and intensely competitive. Current and prospective participants include specialty retailers, supermarkets, drugstores, mass merchants, multi-level marketing organizations, online merchants, mail-order companies and a variety of other smaller participants. The market is also highly sensitive to the introduction of new products, which may rapidly gain consumer acceptance. We compete for sales with heavily advertised brands manufactured by large pharmaceutical and food companies, as well as other brands, some of which have greater market presence, name recognition and financial, marketing and other resources, including some competitors that may spend more aggressively on advertising and promotional activities than we do. If we fail to compete effectively, we may lose business to other retailers.
Further, the ability of consumers to compare prices on a real-time basis through the use of smartphones and digital technology puts additional pressure on us to maintain competitive pricing. We compete in multiple product categories and sales channels, including pharmaceutical stores, wholesale to specialty store formats, direct marketing and increasingly internet-based and direct-sell retailers and vendors. Many factors affect the extent to which competition could affect our results, including as it relates to pricing, quality, assortment, marketing, promotions and advertising, service, locations, capital expenditures, category share and reputation, any of which could have a material effect on our results of operations.
We have a limited operating history on which to judge our performance and assess our prospects for future success.
We first entered the health and wellness business in December 2017 when we acquired AiXin BVI, which at that time owned all of the equity of AiXin Zhonghong, then engaged in the distribution of health and wellness foods. Consequently, we have a limited operating history on which to evaluate our prospects in the health and wellness industry. We may fail to continue our growth. You should not consider our historical growth and expansion of our business through acquisitions as indicative of our ability to grow in the future.
Our recently completed acquisition is subject to uncertainties and risks.
We recently completed a significant acquisition. There is no assurance that we will realize the benefits anticipated from our acquisition of Yunnan Runcangsheng. There is no assurance that the members of the management of Yunnan Runcangsheng that choose to join our Company can be successfully integrated into our management. The process of combining the operations of Yunnan Runcangsheng with our existing operations could have a material adverse effect on us and our financial condition.
Our future expansion plans are subject to uncertainties and risks.
We intend to seek to expand our operations through acquisitions. The implementation of such plan requires us to integrate any newly acquired business and its management teams. If we fail to effectively and efficiently implement our plan for acquisitions, we may not be successful in achieving profitable results. Even if we effectively and efficiently implement our future plans, there may be other unexpected events or factors that prevent us from achieving success. Our management has limited experience in effecting a rapid expansion and in managing larger operations. Our business, financial condition, results of operations and growth prospects may be materially and adversely affected if our future expansion plans fail to achieve positive results.
Failure to effectively anticipate consumer preferences could negatively impact the demand for our products and our ability to generate revenues and the market price of our common stock.
Our success also depends on our ability to anticipate and respond in a timely manner to changing consumer demand, consumer preferences, and shopping patterns regarding health and wellness products. Consumer preferences cannot be predicted with certainty and are subject to continual change and evolution. Additionally, our customers may have expectations about how they shop in stores or online, or more generally engage with businesses across different channels or media (through online and other digital or mobile channels or particular forms of social media), which may change over time which may make it more difficult for us to adapt to rapid changes in consumer preferences.
| 15 |
Our sales may decline significantly if we misjudge the market for our new products, which may result in significant inventory markdowns and lower margins, missed opportunities for other products, or inventory write-downs, and could have a negative impact on our reputation and profitability.
Resources devoted to product innovation may not yield new products that achieve commercial success.
Our ability to develop new and innovative products or identify and acquire new and innovative products from third-party vendors, depends on, among other factors, our ability to understand evolving market trends and translate our insights into identifying, and then designing and manufacturing or otherwise obtaining, commercially successful new products. If we are unable to do so, our customer relationships and product sales could be harmed significantly. The health and wellness industry is characterized by rapid and frequent changes in demand for products. Our failure to accurately predict these trends could harm our customer relationships and cause us to fail to grow our revenues. The development of new and innovative products requires significant investment in research and development and testing of new ingredients, formulas and possibly new production processes. The research and development process entails considerable uncertainty. Products may appear promising in development but fail to reach market within the expected time frame, or at all. Further, products also may fail to achieve commercial success. Finally, there is no guarantee that our development teams will be able to successfully respond to competitive products that could render some of our offerings obsolete.
If we do not successfully develop and maintain a robust omni-channel experience for our customers, our business and results of operations could be materially and adversely affected.
Omni-channel retailing, where customers are accessed through various coordinated channels, is rapidly evolving, and we must keep pace with changing customer expectations and new developments by our competitors. The growing middle class in China is increasingly seeking relevant information regarding healthy lifestyles and products that promote a healthy life. In addition, customers are increasingly shopping for products online instead of in traditional brick-and-mortar shopping centers and retail locations. As part of our omni-channel strategy, we anticipate the need to continue making investments in technology and to provide ready means for our customers to access the information and products they want in a comfortable environment. If we are unable to make, improve, or develop relevant channels to interact with customers in a timely manner, our ability to compete and our business and results of operations could be materially and adversely affected. In addition, if our online activities or our other customer-facing technology systems do not function as designed or we are unable to effectively blend our online and in-store platforms, we may experience a loss of customer confidence, lost sales, or data security breaches, any of which could materially and adversely affect our business and results of operations.
The lease with respect to our hotel expires at the end of 2025.
The term of the lease for our hotel in Chengdu expires December 31, 2025, and we do not have the unilateral right to extend the lease. There is no assurance that we will be able to renew the lease or that the rent we will be obligated to pay to secure such renewal will not be significantly above our current rent. Any failure to renew the lease will have a material adverse impact on our business. If the rent we are required to pay is substantially above the rent we are currently paying, unless we can increase our revenues, it will lead to a decrease in the net income derived from operating the hotel.
The operation of our hotel is subject to the business, financial and operating risks inherent to the hospitality industry, any of which could reduce the revenues derived at our hotel.
The operation of a hotel is subject to a number of business, financial and operating risks inherent to the hospitality industry. These include significant competition from multiple hospitality providers in which compete for the business of our guests; the financial condition of the owner of the property on which our hotel is situated; decreases in business and leisure travel; changes in operating costs, including employee compensation and benefits, energy, insurance, food and beverage and other supplies; increases in costs due to inflation or other factors that may not be fully offset by increases in revenues in our business, as well as increases in overall prices and the prices of our offerings due to inflation, which could weaken consumer demand for travel and the products and services we offer and adversely affect our revenues; changes in taxes and governmental regulations that influence or increase our operating costs; the costs and administrative burdens associated with complying with applicable laws and regulations; significant increases in cost for health care coverage for employees and potential government regulation with respect to health care coverage; shortages of labor or labor disruptions; changes in the desirability of the region in which our hotel is located; changes in the supply and demand for hotel services, including rooms, food and beverage and other products and services; and the costs required for climate change initiatives, including those resulting from regulatory changes or stakeholder or customer expectations.
Any of these factors could increase our costs or limit or reduce the prices we are able to charge hotel customers for hospitality products and services, or otherwise affect our ability to maintain our hotel. As a result, any of these factors can reduce our revenues and limit opportunities for growth.
Our efforts to utilize our hotel in Chengdu City to conduct marketing events for customers and training sessions for our employees may not anticipated benefits.
We envision using the hotel we acquired in Chengdu City to conduct marketing events for our customers and training sessions for our personnel. These efforts may not result in increased sales and may not improve our ability to train our personnel.
The failure to maintain permits and licenses could adversely impact our operations.
The manufacture and distribution of food products, and the sale of food and beverages at our hotel, as well as other aspects of our business, require that we maintain various permits and licenses and conduct our operations in accordance with applicable safety requirements and other regulations. Should we be unable to renew any of our licenses and permits, it could have a material adverse effect on our results of operations and the profitability of our business, even if the disruption is for only a limited period of time.
We may incur material product liability claims, or experience product recalls, which could increase our costs and adversely affect our sales and margin, reputation, revenues and operating income.
As a retailer, distributor and manufacturer of products for human consumption, we are subject to product liability claims if the use of our products is alleged to result in injury. The products that we sell consist of minerals, herbs, supplements and other ingredients that are classified as foods or dietary supplements. The products that we sell could contain contaminated substances, and some of the products we sell contain ingredients that do not have long histories of human consumption. Previously unknown adverse reactions resulting from human consumption of these ingredients could occur.
In addition, third-party manufacturers produce many of the products we sell. We rely on these manufacturers to ensure the integrity of their ingredients and formulations. As a distributor of products manufactured by third parties, we may also be liable for various product liability claims for products we do not manufacture. Our ultimate liability for these products that are manufactured by third parties depends on a number of factors, including our contractual relationship with the vendor, the creditworthiness of the vendor and any insurance that we have. Therefore, we may be unable to adequately protect ourselves against claims with respect to products manufactured by a third-party.
We may be subject to product liability claims, including, among others, that our products include inadequate instructions for use or inadequate warnings concerning possible side effects and interactions with other substances. Product liability claims could significantly damage our reputation and consumer confidence in our products, regardless of the merits or outcomes of such claims. Our litigation expenses could increase as well, which also could have a material adverse effect on our results of operations even if a product liability claim is unsuccessful or is not fully pursued.
We may be subject to product recalls, withdrawals or seizures if any of the products we manufacture or sell are believed to be adulterated, cause injury or illness or if we are alleged to have violated governmental regulations in the manufacturing, labeling, promotion, sale or distribution of such products. A significant recall, withdrawal or seizure of any of the products may require significant management attention, would likely result in substantial and unexpected costs and may materially and adversely affect our business, financial condition or results of operations. Furthermore, a recall, withdrawal or seizure of any of the products that we sell may adversely affect consumer confidence in our brands and thus decrease consumer demand for such products.
If we fail to develop and protect our brand names and reputation, we may not attract and retain new customers, which could adversely affect our revenues and financial performance.
We will invest significant resources to promote our brand names to obtain favorable public recognition for us and our products. If we do not achieve the reputation we envision, we may not be able to attract and retain a significant customer base, which could in turn adversely affect our revenues, profitability and the market price of our common stock.
Our ability to adequately protect our trade names, trademarks and patents could have an impact on our brand images and ability to penetrate new markets.
We believe that trade names and trademarks will be an important aspect of our business and an essential element of our strategy. We have applied for the registration of many of our trade names, trademarks and patents in China. Some of these applications have been granted and some of these registrations are currently pending approval from the corresponding departments. There can be no assurance that we will obtain such registrations or that the registrations we obtain will prevent the imitation of our products or infringement of our intellectual property rights by others. In particular, the laws of the PRC may not protect proprietary rights to the same extent as the laws of the U.S. Claims that others are infringing our intellectual property would be costly and would divert the attention of management and key personnel. Our failure to successfully protect our trademarks could diminish the value and effectiveness of our past and future marketing efforts and could cause customer confusion. This could in turn adversely affect our revenues, profitability and the market price of our common stock.
| 16 |
Unfavorable publicity about us or consumer perception of our products, including the nutritional supplements we distribute, the ingredients they contain and any similar products distributed by other companies could cause fluctuations in our operating results and could have a material adverse effect on our reputation, the demand for our products and our ability to generate revenues and the market price of our common stock.
We are highly dependent upon consumer perception of the safety and quality of our products, including our nutritional supplements, and the ingredients they contain, as well as that of similar products distributed by other companies. Consumer perception of products and the ingredients they contain can be significantly influenced by scientific research, national media attention and other publicity, including that generated via social media. A research report or publicity related to our products and the ingredients they contain that is perceived by our consumers as less favorable or that questions earlier favorable research or publicity could have a material adverse effect on our ability to generate revenues. Adverse publicity in the form of published scientific research or otherwise, whether or not accurate, that associates consumption of our products or the ingredients they contain or similar products distributed by other companies with illness or other adverse effects, that questions the benefits of our or similar products, or that claims that such products are ineffective could have a material adverse effect on our reputation, the demand for our products, our ability to generate revenues and the market price of our common stock.
We may be subject to health or advertising claims related to our customers.
Our products do are not medical treatments and we do not provide or medical advice, and we do not engage physicians or nurses to monitor the progress of our customers. A customer who uses our products and experiences health problems could allege or bring a lawsuit against us on the basis that those problems were caused or worsened by using our products. Further, customers who allege that they were deceived by any statements that we made in advertising or labeling could bring a lawsuit against us under consumer protection laws. We may ultimately be unsuccessful in defending ourselves against such claims. Also, defending ourselves against such claims, regardless of their merit and ultimate outcome, may be lengthy and costly, and could adversely affect our brand image, customer loyalty and results of operations.
We rely on third parties to provide us with nutritional supplements, fulfillment, customer service and Internet and networking services, the loss of any of which could cause our revenue, earnings or reputation to suffer.
In addition to the nutritional supplements we manufacture, we rely on third-parties to supply us with additional manufacturer the nutritional supplements we sell. We do not know if our vendors vendor directly sells the same product under a different brand or provides their supplements to other parties under private label brands. In each of these instances, sales of the same product under different brands could cause us to lose revenues and adversely impact our abilities to market our products. While we believe we could locate replacement suppliers for all supplements we acquire from third parties, it would likely take time to engage replacement suppliers which could adversely impact our revenues during such transition period and there are no assurances our costs would not increase to a level which makes the product unattractive to our customers.
We depend on the services of key executives and other skilled professionals and our ability to attract, train and retain highly qualified associates. Any failure to attract or retain such individuals could affect our business strategy and adversely impact our performance and results of operations.
Our senior executives are instrumental in setting our strategic direction, operating our business, identifying, recruiting and training personnel, identifying opportunities, designing new products and arranging necessary financing. In addition, other key employees below the executive level, with deep knowledge of our business, are critical to the execution and success of our strategy. We must attract, train and retain a growing number of qualified individuals.
Losing the services of any of these groups of individuals could adversely affect our business and we may be unable to identify candidates of sufficient experience and capabilities in a timely fashion or at all, which could negatively impact our business and operations. Further, our ability to control labor and benefit costs is subject to numerous external factors, including regulatory changes, prevailing wage rates, and healthcare and other insurance costs. We compete with other retail and non-retail businesses for these store and field associates and invest significant resources in training and motivating them. There is no assurance that we will be able to attract or retain qualified store and field associates in the future, which could have a material adverse effect on our business, financial condition and results of operations.
Compliance with new and existing laws and governmental regulations could increase our costs significantly and adversely affect our results of operations.
The processing, formulation, safety, manufacturing, packaging, labeling, advertising and distribution of our products are subject to numerous Chinese laws and regulations as well as laws and regulations imposed by provincial and local governments and agencies. Government regulations may prevent or delay the introduction, or require the reformulation, of our products, which could result in lost revenues and increased costs to us. Manufacturers and distributors of health and wellness products and dietary supplements and dietary ingredients are prohibited from marketing products that are adulterated or misbranded, and the governing agencies may take enforcement action against any adulterated or misbranded health and wellness product or dietary supplement on the market. If we violate applicable regulatory requirements, we may be subject to enforcement actions against us, which could have a material adverse effect on our business, prospects, financial condition, and results of operations. The government may not accept the evidence of safety for any new product or ingredient we may wish to market, may determine that a particular product or ingredient presents an unacceptable health risk based on reported serious adverse events or other information, and may determine that a particular claim or statement of efficacy or nutritional value that we use to support the marketing of a product is not substantiated, or is an unauthorized version of a “health claim.” Any of these actions could prevent us from marketing particular products or making certain claims or statements with respect to those products. We could also be required to remove a particular product from the market. Any future recall or removal would result in costs to us, including lost revenues from any products that we are required to remove from the market, any of which could be material. Any product recalls or removals could also lead to an increased risk of litigation and liability, substantial costs, and reduced growth prospects.
| 17 |
Additional or more stringent laws and regulations of health and wellness products have been considered from time to time. These developments could require reformulation of some products to meet new standards, recalls or discontinuance of some products not able to be reformulated, additional record-keeping requirements, increased documentation of the properties of some products, additional or different labeling, additional scientific substantiation, or other new requirements. Any of these developments could increase our costs significantly. In addition, regulators’ evolving interpretation of existing laws could have similar effects.
We depend upon a limited number of suppliers for certain raw materials and any significant disruption to our timely receipt of raw materials and inventory could adversely impact sales and operations or increase our transportation costs, which would decrease our profits.
We depend upon a limited number of suppliers for the raw materials necessary to produce certain of our products. Unexpected delays in the deliveries of raw materials from our vendors or inventories or increases in transportation costs (including through increased fuel costs) could significantly decrease our ability to make sales and earn profits. We must maintain sufficient levels of raw materials and inventories to operate our business successfully. However, we also must guard against accumulating excess inventory. If we fail to anticipate accurately either the market for the merchandise we offer or our customers’ purchasing habits, we may be forced to rely on markdowns or promotional sales to dispose of excess or slow-moving inventory, which could have a material adverse effect on our business, financial condition, and results of operations.
In addition, sales of adulterated products received from third parties could result in a product liability judgment or a widespread product recall that may negatively impact our sales and profitability for a period of time depending on product availability, competition reaction and consumer attitudes. Even if the product liability claim is unsuccessful or is not fully pursued, the negative publicity surrounding any assertions could adversely impact our reputation with existing and potential customers and our brand image.
Natural disasters (whether or not caused by climate change), unusually adverse weather conditions, pandemic outbreaks and terrorist acts in China and the response of the Chinese governments to such occurrences, could impair our ability to purchase, receive or replenish inventory or raw materials or cause customer traffic to decline, all of which could result in lost sales and otherwise adversely affect our financial performance.
The occurrence of one or more natural disasters, such as hurricanes, fires, floods and earthquakes (whether or not caused by climate change), unusually adverse weather conditions, pandemic outbreaks (including the recent outbreak of the coronavirus, or COVID-19) or terrorist acts in China and the response of the Chinese government to such occurrences, could adversely affect our operations and financial performance. To the extent these events result in the closure of one or more of our pharmacies, manufacturing facility or our corporate headquarters, or impact one or more of our key suppliers, our operations and financial performance could be materially adversely affected through lost sales. Such events could also cause a disruption in travel making it difficult for consumers to order our products, such as has happened as a result of lock-downs in Chengdu. In addition, these events could result in increases in fuel (or other energy) prices or a fuel shortage, the temporary lack of an adequate work force in a market, the temporary or long-term disruption in the supply of products from some suppliers, the temporary disruption in the transport of goods, the temporary reduction in the availability of products in our stores, expiration of inventory and disruption to our information systems.
| 18 |
We rely on third parties for the vast majority of our computing, storage, and other services related to our business. Any disruption of or interference with our use of the services of these third parties would negatively affect our operations and could seriously harm our business.
We rely upon third parties, including cloud based platforms and services, to process and store information and for online interactions with our customers. Any transition of the cloud services currently provided by the parties we rely upon to other platforms or to another cloud provider could be difficult to implement and would cause us to incur significant time and expense. Given this, any significant disruption of or interference with the cloud based services utilized by us, whether temporary, regular, or prolonged, would negatively impact our operations and our business would be seriously harmed.
As we increase the amount of personal and user data we store and process, we may become subject to complex and evolving laws, regulations, executive actions, rules, contractual obligations, policies, and other obligations regarding privacy, data protection, content, and other matters. Many of these obligations are subject to change and uncertain interpretations.
Currently, we collect, store, and use limited amounts of personal data and other sensitive information. As our business grows and we increase the amount of such data collect, store and use, we will become subject to a variety of laws, regulations, industry standards, policies, contractual requirements, executive actions, and other obligations relating to privacy, security, and data protection which are becoming increasingly stringent and subject to rapid change and uncertain interpretation. Preparing for and complying with these obligations could require us to devote significant resources. These obligations may necessitate changes to our services, information technologies, systems, and practices. In addition, these obligations may require us to change our business model.
Cyber-attacks could have a disruptive effect on our business.
From time to time we and our third-party service providers experience cyber-attacks, attempted and actual breaches of our or their information technology systems and networks or similar events, which could result in a loss of sensitive business or customer information, systems interruption or the disruption of our operations. The techniques that are used to obtain unauthorized access, disable or degrade service or sabotage systems change frequently and may be difficult to detect for long periods of time, we are accordingly unable to anticipate and prevent all data security incidents.
Even if we are fully compliant with legal standards and contractual or other requirements, we still may not be able to prevent security breaches involving sensitive data. The sophistication of efforts by hackers to gain unauthorized access to information systems has continued to increase in recent years and may continue to do so. Breaches, thefts, losses or fraudulent uses of customer, employee or company data could cause consumers to lose confidence in the security of our websites, mobile applications, point of sale systems and other information technology systems and, as a result of this loss in confidence, choose not to purchase from us. Such security breaches also could expose us to risks of data loss, business disruption, litigation, fines, regulatory charges and other costs or liabilities, any of which could adversely affect our business.
Our business may be materially adversely affected by global geopolitical conditions resulting from the ongoing Russia-Ukraine conflict and the Israel-Hamas conflict.
Global markets are experiencing volatility and disruption as a result of the geopolitical instability resulting from the ongoing Russia-Ukraine conflict and the Israel-Hamas conflict. The invasion of Ukraine by Russia and the Israel-Hamas conflict and the resulting measures that have been taken, and could be taken in the future, by NATO, the United States, the United Kingdom, the European Union, Israel and other countries have created global security concerns that could have a lasting impact on regional and global economies. Although the length and impact of the ongoing conflicts are highly unpredictable, they could lead to market disruptions, including significant volatility in commodity prices, credit and capital markets, as well as supply chain interruptions. Additionally, any resulting sanctions could adversely affect the global economy and financial markets and lead to instability and lack of liquidity in capital markets.
Any of the abovementioned factors, or any other negative impact on the global economy, capital markets or other geopolitical conditions resulting from the Russian invasion of Ukraine, the Israel-Hamas conflict and subsequent sanctions or related actions, could adversely affect our business. The extent and duration of the ongoing conflicts, resulting sanctions and any related market disruptions are impossible to predict, but could be substantial, particularly if current or new sanctions continue for an extended period of time or if geopolitical tensions result in expanded military operations on a global scale. Any such disruptions may also have the effect of heightening many of the other risks described in this section. If these disruptions or other matters of global concern continue for an extensive period of time, our business may be materially adversely affected.
We may be impacted by our ability to attract, develop and retain qualified associates and manage labor-related costs.
We believe one of our competitive advantages is providing a positive, engaging and satisfying experience for each customer, which requires us to have highly trained and engaged associates. Our success depends in part upon our ability to attract, develop and retain a sufficient number of qualified associates to enable us to grow our customer base. The turnover rate in the health and wellness industry is generally high, and qualified individuals of the requisite caliber and number needed may be in short supply. Competition for such qualified individuals or changes in labor laws could require us to incur higher labor costs. Our inability to recruit a sufficient number of qualified individuals may delay the expansion of our customer base and our growth. Significant increases in associate turnover rates or significant increases in labor-related costs could have a material adverse effect on our results of operations, financial condition and cash flows.
Large and similar sized competitors could steal our market share by offering lower prices.
We endeavor to provide high quality service to our clients at the best possible price, however, large and similar sized competitors might steal some of our market share by offering lower prices, causing us to lose some of our clients which could have a material adverse effect on our results of operations, financial condition and cash flows.
Our insurance may not be sufficient.
We carry insurance that we consider adequate in regard to the nature of the covered risks and the costs of coverage. We are not fully insured against all possible risks, nor are all such risks insurable.
Our business depends on the continued contributions made by Mr. Quanzhong Lin, our key executive officer. The loss of his services may result in a severe impediment to our business.
Our success is dependent upon the continued contributions made by our CEO and President, Mr. Quanzhong Lin. The Company has no “Key Man” insurance to cover the resulting losses in the event that any of our officer or directors should die or resign.
If Mr. Lin cannot serve the Company or is no longer willing to do so, the Company may not be able to find alternatives in a timely manner or at all. This would likely result in severe damage to our business operations and would have an adverse material impact on our financial position and operational results. To continue as a viable operation, the Company may have to recruit and train replacement personnel at a higher cost.
Our key executive does not devote his full business time to our operations.
Our President and Chief Executive Officer, Mr. Quanzhong Lin, is involved in a number of businesses and does not devote all of his working time to our business. Our positive reputation in Chengdu is derived from the standing of Mr. Lin in the Chengdu business community. If Mr. Lin does not devote sufficient time to our business, our operations could suffer, which would have an adverse material impact on our financial position and operational results.
Some of the other businesses engaged in by Mr. Lin could be deemed competitive with aspects of our business. Should such other businesses prove more successful than ours, Mr. Lin could choose to focus his attention on such businesses which could cause him to fail to devote sufficient attention to our business and our operations could suffer and our financial conditions and results of operations may be materially and adversely affected.
Our principal shareholder is not familiar with American business practices.
Mr. Quanzhong Lin, our founder and principal shareholder, is a citizen of the PRC and an active entrepreneur in Chengdu. Mr. Lin is not familiar with American business practices and is heavily influenced by the business culture in the PRC. There is a certain level of respect and prestige associated with being the Chinese principal of a company which is publicly traded in the U.S. Mr. Lin’s motivation for causing the business of AiXinZhonghong to become a part of a U.S. publicly-traded company may differ from those of American entrepreneurs and his values may cause him to operate the business differently than would an American entrepreneur which could have a material adverse effect on our results of operations, financial condition and cash flows
| 19 |
We may be adversely impacted by certain compliance or legal matters.
We, along with third parties with which we do business, are subject to complex compliance and litigation risks. The cost of defending against claims that might be brought against us or the ultimate resolution of such claims, whether by settlement or adverse court decision, may harm our business. Further, potential claimants may be encouraged to bring lawsuits based on a settlement from us or adverse court decisions against us. We cannot currently assess the likely outcome of such suits, but if the outcome were negative, it could have a material adverse effect on our reputation, results of operations, financial condition and cash flows.
In addition, we may be impacted by litigation trends, including class action lawsuits involving consumers and shareholders, that could have a material adverse effect on our reputation, the market price of our common stock, results of operations, financial condition and cash flows.
Failure to make adequate contributions to various employee benefits plans as required by PRC regulations may subject us to penalties.
Companies operating in China are required to participate in various government sponsored employee benefit plans, including certain social insurance, housing funds and other welfare-oriented payment obligations, and contribute to the plans in amounts equal to certain percentage of salaries, including bonuses and allowances, of employees up to a maximum amount specified by the local government from time to time at locations where they operate their businesses. The requirement of employee benefit plans has not been implemented consistently by the local governments in China given the different levels of economic development in different locations. Employers that fail to make adequate social insurance and housing provident fund contributions may be subject to late payment fees, fines and sanctions. If the relevant PRC authorities determine that we need to make supplemental contributions or that we are subject to late payment fees, fines or other legal sanctions, such as order of timely rectification, in relation to our failure to make social insurance and housing fund contributions in full for our employees, our business and financial condition may be adversely affected.
Our common stock may be delisted under the Holding Foreign Companies Accountable Act if the PCAOB is unable to inspect our auditors. The delisting of our common stock or the threat of their being delisted, may materially and adversely affect the value of your investment.
The HFCAA was enacted on December 18, 2020. The HFCAA states if the SEC determines that a company has filed audit reports issued by a registered public accounting firm that has not been subject to inspection by the PCAOB for three consecutive years beginning in 2021, the SEC shall prohibit the securities of such company from being traded on a national securities exchange or in the over the counter trading market in the U.S. On June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act which, if enacted, would amend the HFCAA and require the SEC to prohibit an issuer’s securities from trading on any U.S. stock exchange if its auditor is not subject to PCAOB inspections for two consecutive years instead of three.
On March 24, 2021, the SEC adopted interim final rules relating to the implementation of certain disclosure and documentation requirements of the HFCAA. A company will be required to comply with these rules if the SEC identifies it as having a “non-inspection” year under a process to be subsequently established by the SEC. The SEC is assessing how to implement other requirements of the HFCAA, including the listing and trading prohibition requirements described above. On September 22, 2021, the PCAOB adopted a final rule implementing the HFCAA, which provides a framework for the PCAOB to use when determining whether the PCAOB is unable to inspect or investigate completely registered public accounting firms located in a foreign jurisdiction because of a position taken by one or more authorities in that jurisdiction. On December 2, 2021, the SEC issued amendments to finalize rules implementing the submission and disclosure requirements in the HFCAA. The rules apply to registrants that the SEC identifies as having filed an annual report with an audit report issued by a registered public accounting firm that is located in a foreign jurisdiction and that PCAOB is unable to inspect or investigate completely because of a position taken by an authority in foreign jurisdictions. On December 16, 2021, the PCAOB issued a Determination Report which found that the PCAOB is unable to inspect or investigate completely registered public accounting firms headquartered in: (i) China, and (ii) Hong Kong. On August 26, 2022, the CSRC, the Ministry of Finance of the PRC, and the PCAOB signed a Statement of Protocol, or the Protocol, governing inspections and investigations of audit firms based in China and Hong Kong. Pursuant to the Protocol, the PCAOB shall have independent discretion to select any issuer audits for inspection or investigation and has the unfettered ability to transfer information to the SEC. Notwithstanding the foregoing, if the PCAOB is not able to inspect and investigate completely our auditor’s work papers in China, you may be deprived of the benefits of such inspection which could result in limitation or restriction to our access to the U.S. capital markets and trading of our securities may be prohibited under the HFCAA and Nasdaq may determine to delist our securities if the PCAOB determines that it cannot inspect or investigate completely our auditor under the HFCAA.
Our auditor is not headquartered in China or Hong Kong and was not identified in this report as a firm subject to the PCAOB’s determination.
| 20 |
The SEC may propose additional rules or guidance that could impact us if our auditor is not subject to PCAOB inspection. For example, on August 6, 2020, the President’s Working Group on Financial Markets, or the PWG, issued the Report on Protecting United States Investors from Significant Risks from Chinese Companies to the then President of the United States. This report recommended the SEC implement five recommendations to address companies from jurisdictions that do not provide the PCAOB with sufficient access to fulfil its statutory mandate. Some of the concepts of these recommendations were implemented with the enactment of the HFCAA. However, some of the recommendations were more stringent than the HFCAA. For example, if a company’s auditor was not subject to PCAOB inspection, the report recommended that the transition period before a company would be delisted would end on January 1, 2022.
The SEC has announced that the SEC staff is preparing a consolidated proposal for the rules regarding the implementation of the HFCAA and to address the recommendations in the PWG report. The implications of possible additional regulation in addition to the requirements of the HFCAA and what was recently adopted on December 2, 2021 are uncertain. While we understand that there has been dialogue among the CSRC, the SEC and the PCAOB regarding the inspection of PCAOB-registered accounting firms in China, there can be no assurance that we will be able to comply with requirements imposed by U.S. regulators. Such uncertainty could cause the market price of our common stock to be materially and adversely affected, and our securities could be delisted and prohibited from being traded on the national securities exchange earlier than would be required by the HFCAA. If our securities are unable to be listed on another securities exchange by then, such a delisting would substantially impair your ability to sell or purchase our common stock when you wish to do so, and the risk and uncertainty associated with a potential delisting would have a negative impact on the price of our common stock.
Further, new laws and regulations or changes in laws and regulations in both the United States and China could affect our ability to list our common stock on the Nasdaq Capital Market, which could materially impair the market for and market price of our common stock.
| 21 |
A joint statement by the SEC and the Public Company Accounting Oversight Board (United States), or the “PCAOB,” proposed rule changes submitted by Nasdaq, and the newly enacted “Holding Foreign Companies Accountable Act” and the “Accelerating Holding Foreign Companies Accountable Act” all call for additional and more stringent criteria to be applied to emerging market companies upon assessing the qualification of their auditors, especially the non-U.S. auditors who are not inspected by the PCAOB. These developments could add uncertainties to our offering.
On April 21, 2020, the SEC and the PCAOB released a joint statement highlighting the risks associated with investing in companies based in or having substantial operations in emerging markets including China. The joint statement emphasized the risks associated with lack of access for the PCAOB to inspect auditors and audit work papers in China and higher risks of fraud in emerging markets.
On May 18, 2020, Nasdaq filed three proposals with the SEC to (i) apply a minimum offering size requirement for companies primarily operating in a “Restrictive Market,” (ii) adopt a new requirement relating to the qualification of management or the board of directors for Restrictive Market companies, and (iii) apply additional and more stringent criteria to an applicant or listed company based on the qualifications of the company’s auditor. We are very likely to be deemed as a company primarily operating in a Restrictive Market under such proposed rules of Nasdaq. Therefore, Nasdaq might apply the additional and more stringent criteria for our initial and continued listing, which might cause delay or even denial of our listing application.
On December 18, 2020, the HFCAA was signed and became law. This legislation requires certain issuers of securities to establish that they are not owned or controlled by a foreign government. Specifically, an issuer must make this certification if the PCAOB is unable to audit specified reports because the issuer has retained a foreign public accounting firm not subject to inspection by the PCAOB. Furthermore, if the PCAOB is unable to inspect the issuer’s public accounting firm for three consecutive years, the issuer’s securities are banned from trade on a national exchange or through other methods. On December 29, 2022, the AHFCAA was enacted, which amended the HFCAA by decreasing the number of non-inspection years from three years to two, thus reducing the time period before our common stock may be prohibited from trading or delisted if the PCAOB were to determine that it could not inspect our auditor.
On March 24, 2021, the SEC announced that it had adopted interim final amendments to implement congressionally mandated submission and disclosure requirements of the HFCAA. The interim final amendments will apply to registrants that the SEC identifies as having filed an annual report on Forms 10-K, 20-F, 40-F or N-CSR with an audit report issued by a registered public accounting firm that is located in a foreign jurisdiction and that the PCAOB has determined it is unable to inspect or investigate completely because of a position taken by an authority in that jurisdiction. The SEC will implement a process for identifying such a registrant and any such identified registrant will be required to submit documentation to the SEC establishing that it is not owned or controlled by a governmental entity in that foreign jurisdiction, and will also require disclosure in the registrant’s annual report regarding the audit arrangements of, and governmental influence on, such a registrant.
On September 22, 2021, the PCAOB adopted rules to create a framework for the PCAOB to use when determining, as contemplated under the HFCAA, whether it is unable to inspect or investigate completely registered public accounting firms located in a foreign jurisdiction because of a position taken by one or more authorities in that jurisdiction.
On December 2, 2021, the SEC issued amendments to finalize the interim final rules previously adopted in March 2021 to implement the submission and disclosure requirements in the HFCAA. The rules apply to registrants that the SEC identifies as having filed an annual report with an audit report issued by a registered public accounting firm that is located in a foreign jurisdiction and that the PCAOB is unable to inspect or investigate completely because of a position taken by an authority in a foreign jurisdiction.
| 22 |
On December 16, 2021, the PCAOB issued a Determination Report which found that the PCAOB was then unable to inspect or investigate completely registered public accounting firms headquartered in: (1) mainland China of the People’s Republic of China, because of a position taken by one or more authorities in mainland China; and (2) Hong Kong, a Special Administrative Region and dependency of the PRC, because of a position taken by one or more authorities in Hong Kong. The PCAOB has made such designations as mandated under the HFCAA. Pursuant to each annual determination by the PCAOB, the SEC will, on an annual basis, identify issuers that have used non-inspected audit firms and thus are at risk of such suspensions in the future.
On August 26, 2022, the CSRC, the Ministry of Finance of the PRC, and the PCAOB signed a Statement of Protocol, or the Protocol, governing inspections and investigations of audit firms based in China and Hong Kong. Pursuant to the Protocol, the PCAOB shall have independent discretion to select any issuer audits for inspection or investigation and has the unfettered ability to transfer information to the SEC. On December 15, 2022, the PCAOB determined that the PCAOB was able to secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary. However, should PRC authorities obstruct or otherwise fail to facilitate the PCAOB’s access in the future, the PCAOB will consider the need to issue a new determination. Notwithstanding the foregoing, if the PCAOB is not able to inspect and investigate completely our auditor’s work papers in China, you may be deprived of the benefits of such inspection which could result in limitation or restriction to our access to the U.S. capital markets and trading of our securities may be prohibited under the HFCAA and Nasdaq may determine to delist our securities if the PCAOB determines that it cannot inspect or investigate completely our auditor under the HFCAA.
Our auditor, KCCW Accountancy Corp. (“KCCW CPA”), an independent public accounting firm registered with the PCAOB, and an auditor of publicly traded companies in the U.S., is subject to laws in the U.S. pursuant to which the PCAOB conducts regular inspections to assess its compliance with the applicable professional standards. Our auditor is based in the United States and has been inspected by the PCAOB on a regular basis, with the last inspection in 2022. Our auditor is not headquartered in mainland China or Hong Kong and was not identified as a firm subject to the determinations announced by the PCAOB on December 16, 2021. Nevertheless, should the PCAOB be unable to fully conduct inspection of our auditor’s work papers in China, it will make it difficult to evaluate the effectiveness of our auditor’s audit procedures or equity control procedures. Investors may consequently lose confidence in our reported financial information and procedures or quality of the financial statements, which would adversely affect us and our securities. Moreover, if trading in our securities is prohibited under the HFCAA in the future because the PCAOB determines that it cannot inspect or fully investigate our auditor at such future time, an exchange may determine to delist our securities.
There are uncertainties under the PRC Securities Law relating to the procedures and requisite timing for the U.S. securities regulatory agencies to conduct investigations and collect evidence within the territory of the PRC.
On December 28, 2019, the newly amended Securities Law of the PRC (the “PRC Securities Law”) was promulgated, which became effective on March 1, 2020. According to Article 177 of the PRC Securities Law (“Article 177”), the securities regulatory authority of the State Council may establish a regulatory cooperation mechanism with securities regulatory authorities of another country or region for the implementation of cross-border supervision and administration. Article 177 further provides that overseas securities regulatory authorities shall not engage in activities pertaining to investigations or evidence collection directly conducted within the territories of the PRC, and that no Chinese entities or individuals shall provide documents and information in connection with securities business activities to any organizations and/or persons aboard without the prior consent of the securities regulatory authority of the State Council and the competent departments of the State Council. As of the date of this prospectus, we are not aware of any implementing rules or regulations which have been published regarding application of Article 177.
As advised by our PRC Counsel, Article 177 is only applicable where the activities of overseas authorities constitute a direct investigation or evidence collection by such authorities within the territory of the PRC. Our principal business operation is conducted in the PRC. In the event that the U.S. securities regulatory agencies carry out an investigation on us such as an enforcement action by the Department of Justice, the SEC or other authorities, such agencies’ activities will constitute conducting an investigation or collecting evidence directly within the territory of the PRC and accordingly fall within the scope of Article 177. In that case, the U.S. securities regulatory agencies may have to consider establishing cross-border cooperation with the securities regulatory authority of the PRC by way of judicial assistance, diplomatic channels or establishing a regulatory cooperation mechanism with the securities regulatory authority of the PRC. However, there is no assurance that the U.S. securities regulatory agencies will succeed in establishing such cross-border cooperation in this particular case and/or establish such cooperation in a timely manner.
Furthermore, as the date of this prospectus, there have not been implementing rules or regulations regarding the application of Article 177, it remains unclear as to how it will be interpreted, implemented or applied by the Chinese Securities Regulatory Commission or other relevant government authorities. As such, there are uncertainties as to the procedures and requisite timing for the U.S. securities regulatory agencies to conduct investigations and collect evidence within the territory of the PRC. If the U.S. securities regulatory agencies are unable to conduct such investigations, there exists a risk that they may determine to suspend or de-register our registration with the SEC and may also delist our securities from the Nasdaq Capital Market or other applicable trading market within the US.
We could be subject to conflicting demands placed upon us by regulatory authorities in the United States and the PRC which could result in disciplinary actions if not resolved.
Article 26 of the Trial Measures issued by the CSRC on February 17, 2023 came into effect on March 31, 2023. Article 26 provides that if an overseas securities regulatory agency intends to conduct an investigation and collect evidence regarding an overseas offering or listing activities by a domestic company, and request assistance of the CSRC under relevant cross-border securities regulatory cooperation agreements, the CSRC may provide necessary assistance in accordance with the laws of the PRC. Any domestic entity or individual intending to provide documents and materials requested by an overseas securities regulatory agency in connection with an investigation may not provide such information without prior approval from the CSRC and competent authorities under the State Council. In addition, Article 11 of the Provisions on Strengthening Confidentiality and Archives Administration in Respect of Overseas Issuance and Listing of Securities by Domestic Enterprises which was jointly issued by the CSRC, the Ministry of Finance, the State Secrecy Administration and the State Archives Bureau on February 24, 2023 came into effect on March 31, 2023, and provides that, when an overseas securities regulator and the relevant competent authorities intends to conduct inspections or investigations to collect evidence from a domestic enterprise and any domestic securities firms and securities service agencies providing services regarding the overseas offering and listing activities of the domestic enterprise, the inspection or investigation shall be carried out under the appropriate cross-border regulatory cooperation agreement, and the CSRC or the relevant authorities shall provide the requisite assistance pursuant to the bilateral and multilateral cooperation mechanism. Further, any domestic company, securities firm and securities service agency shall obtain the consent of the CSRC or the relevant administrative authorities prior to cooperating in an inspection or investigation carried out by an overseas securities regulator or relevant administrative authorities or providing documents and materials for cooperating in the inspection or investigation.
As advised by our PRC Counsel, Article 26 and Article 11 are only applicable to information to be provided in response to a request made in connection with an investigation or other evidentiary collection proceeding instigated by a securities regulatory agency located outside of China. As this regulation has recently been adopted, clarifying regulations have not been issued and companies in China and their counsel generally have little experience in assessing when permission must be obtained before releasing information to a foreign regulatory agency. If the Company were to provide information to any securities regulatory agency in violation of Article 26 or Article 11, it would be subject to fines and, if the information were deemed a state secret, government work secret or might jeopardize the national security of China or the public interest, as provided in the Confidentiality and Archives Provisions, it could cause the Company or the individuals responsible for the disclosure to be subject to criminal investigation. Likewise, if the Company refuses to provide information requested by any U.S. securities regulatory agency in circumstances that the Company believes would have caused it to violate Article 26 or Article 11, it will be subject in the United States to fines, penalties or delisting from the Nasdaq Capital Market. While detailed interpretation of or implementation rules under Article 177, of Article 26 and Article 11 have yet to be promulgated, the inability of an overseas securities regulator to directly conduct an investigation or evidence collection activities within China may further increase difficulties that you may face in protecting your interests.
| 23 |
We are exposed to liabilities relating to environmental protection and safety laws and regulations.
Our operations are subject to comprehensive and frequently changing laws and regulations relating to environmental protection and health and safety. The discharge of waste and pollutants from our manufacturing operations into the environment may give rise to liabilities that may require us to incur costs to remedy such discharge. If we violate such laws or regulations, we may be required to implement corrective actions and could be subject to civil or criminal fines or penalties or other sanctions.
However, we cannot assure you that any environmental laws adopted in the future will not materially increase our operating costs and other expenses. We cannot assure you that we will not have to make significant capital or operating expenditures in the future in order to comply with existing or new laws and regulations or that we will comply with applicable environmental laws at all times. Such violations or liability could have a material adverse effect on our business, financial condition and results of operations.
We may require additional financing and our operations could be curtailed if we are unable to obtain required additional financing when needed.
We may need to obtain additional debt or equity financing to fund the immediate development of Yunnan Runcangsheng and future capital expenditures. While we do not anticipate seeking additional financing in the immediate future, any additional equity may result in dilution to the holders of our outstanding shares of capital stock. Additional debt financing may include conditions that would restrict our freedom to operate our business, such as conditions that:
| ● | limit our ability to pay dividends or require us to seek consent for the payment of dividends; | |
| ● | increase our vulnerability to general adverse economic and industry conditions; | |
| ● | require us to dedicate a portion of our cash flow from operations to payments on our debt, thereby reducing the availability of our cash flow to fund capital expenditures, working capital and other general corporate purposes; and | |
| ● | limit our flexibility in planning for, or reacting to, changes in our business and our industry. |
We cannot guarantee that we will be able to obtain any additional financing on terms that are acceptable to us, or at all.
General Risks Associated with Business Operations in China
| 24 |
The PRC government has significant oversight and discretion over the conduct of a PRC company’s business operations or to exert control over any offering of securities conducted overseas and/or foreign investment in China-based issuers, and may intervene with or influence our operations, may limit or completely hinder our ability to offer or continue to offer securities to investors, and may cause the value of such securities to significantly decline or become worthless, as the government deems appropriate to further regulatory, political and societal goals.
The PRC government may intervene or influence our operations at any time, which could result in a material change in our operations and/or the value of our common stock. For example, the PRC government has recently published new policies that significantly affected certain industries such as the education and internet industries, and we cannot rule out the possibility that it will release regulations or policies regarding any industry that could adversely affect the business, financial condition and results of operations of our Company. Furthermore, the PRC government has also recently indicated an intent to exert more oversight and control over securities offerings and other capital markets activities that are conducted overseas and foreign investment in China-based companies. Any such action, once taken by the PRC government, could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or in extreme cases, become worthless.
The PRC government initiated a series of regulatory actions and statements to regulate business operations in China with little advance notice, including cracking down on illegal activities in the securities market, enhancing supervision over China-based companies listed overseas using variable interest entity (“VIE”) structures, adopting new measures to extend the scope of cybersecurity reviews, and expanding anti-monopoly enforcement efforts. As confirmed by our PRC Counsel, we are not subject to cybersecurity review with the Cyberspace Administration of China, or CAC, given that we are not an operator of critical information infrastructure or an online platform operator as defined by the Measures for Cybersecurity Review (2021 version). See also “Risk Factors - General Risks Associated with Business Operations in China - Our business may be subject to a variety of PRC laws and other obligations regarding cybersecurity and data protection.” In addition, as confirmed by our PRC Counsel, we are not subject to merger control review by China’s anti-monopoly enforcement agency due to the level of our revenues and the fact that we currently do not expect to propose or implement any acquisition of control of, or decisive influence over, any company with revenues within China of more than RMB400 million. Currently, these statements and regulatory actions have had no impact on our daily business operations, the ability to accept foreign investments or list our securities on a U.S. or other foreign exchange. Since these statements and regulatory actions are new, it is highly uncertain how soon legislative or administrative regulation making bodies will respond and what existing or new laws or regulations or detailed implementations and interpretations will be modified or promulgated, if any, and the potential impact such modified or new laws and regulations will have on our daily business operation, the ability to accept foreign investments and list our securities on an U.S. or other foreign exchange.
| 25 |
The CSRC has released draft rules for China-based companies seeking to conduct initial public offerings in foreign markets. The Chinese government may exert more oversight and control over offerings that are conducted overseas and foreign investment in China-based issuers, which could significantly limit or completely hinder our ability to offer or continue to offer our common stock to investors and could cause the value of our common stock to significantly decline or become worthless.
On December 24, 2021, the CSRC released the Administrative Provisions of the State Council Regarding the Overseas Issuance and Listing of Securities by Domestic Enterprises (Draft for Comments) (the “Administrative Provisions”) and the Measures for the Overseas Issuance of Securities and Listing Record-Filings by Domestic Enterprises (Draft for Comments) (the “Measures”). The Administrative Provisions and Measures lay out the filing regulation arrangement for both direct and indirect overseas listing and clarify the determination criteria for indirect overseas listing in overseas markets. The Administrative Provisions and Measures, if enacted, may subject us to additional compliance requirement in the future. Any failure of us to fully comply with new regulatory requirements may significantly limit or completely hinder our ability to offer or continue to offer our common stock, cause significant disruption to our business operations, and severely damage our reputation, which would materially and adversely affect our financial condition and results of operations and cause our common stock shares to significantly decline in value or become worthless.
The CSRC has released the Trial Measures requiring Chinese domestic companies to complete filings with the CSRC if they complete an overseas offering and listing of their securities.
The Trial Measures, regulations for the filing-based administration of direct and indirect overseas securities offerings and listings by domestic companies incorporated in Mainland China went into effect March 31, 2023. The offering by us pursuant to this registration statement is deemed an indirect offering by the CSRC and we will need to fulfill filing obligations pursuant to the Trial Measures after we have completed this offering. If we were to fail to comply with the post-offering filing obligations imposed by the Trial Measures or make a misrepresentation, misleading statement or material omission in the materials we submit to the CSRC, the CSRC would have the right to order rectification, issue a warning and impose a fine on us of between RMB1 million and RMB10 million and issuing a warning to the parties responsible for such failure, misrepresentation or material omission and impose a fine on each of such individuals ranging from RMB500,000 to RMB5 million.
Any filings we make in accordance with the Trial Measures will provide the Chinese Government with additional information regarding our operations. Reviewing information we provide may cause the PRC government to exert oversight and control over our operations, securities offerings and other capital markets activities. Any such action could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or in extreme cases, become worthless.
The CSRC has released Provisions on Strengthening the Confidentiality and Archives Administration Related to the Overseas Securities Offering and Listing by Domestic Enterprises which may limit the activities of or disclosures made by companies in the PRC which, directly or indirectly, list their securities overseas.
The Confidentiality and Archives Provisions came into effect March 31, 2023. The Confidentiality and Archives Provisions require, among other items, that PRC domestic enterprises seeking to offer and list securities in overseas markets, establish an archival system which will maintain the confidentiality of information in accordance with applicable laws and regulations. Further, if a PRC domestic enterprise plans, directly or indirectly, to release documents or provide material that contain state secrets or government work secrets, or that will jeopardize national security or the public interest, it must strictly comply with all relevant governmental procedures and obtain approval of the appropriate regulatory before doing so. Although our PRC Counsel has advised us that we are subject to the Confidentiality and Archives Provisions, we believe that none of the documents or materials we intend to provide to parties outside of the PRC contains materials that would require us to make any filing or obtain any approval of a Chinese regulatory authority. Nevertheless, the determination of whether information contains state secrets, government work secrets or jeopardizes national security or the public interest is subjective and any failure or perceived failure by us or our subsidiaries to comply with the confidentiality and archive administration requirements under the Confidentiality and Archives Provisions could cause us to be referred for criminal investigation and held liable for such violations by the authorities in China. Further, PRC regulatory authorities could use these regulations to limit our disclosures or interfere with our operations. Any determination that we have violated the Confidentiality and Archives Provisions or use of these provisions to limit our disclosures could cause the value of our common stock to significantly decline in value or become worthless.
You may have difficulty in effecting service of legal process or bringing actions in China against us or our management named in the prospectus based on foreign laws.
We are a Colorado company conducting our operations in China through wholly owned subsidiaries with direct equity ownership and most of our assets are and will be located outside the United States. Almost all of our operations will be conducted in China. In addition, nearly all of our officers and directors, including Quanzhong Lin, Huiliang Jiao and Tianfeng Li are PRC nationals and residents of China and all of their assets are located outside the United States. In addition, Yao-Te Wang is a resident of Taiwan and resides in Taiwan. Christopher Lee, our remaining director, resides outside of the United States for a significant portion of time. As a result, it may be difficult for you to effect service of process upon us or our directors and officers inside China or to bring actions against us or our management in China.
Shareholder claims that are common in the United States, including securities law class actions and fraud claims, generally are difficult to pursue as a matter of law or practicality in China. For example, in China, there are significant legal and other obstacles to obtaining information needed for shareholder investigations or litigation outside China or otherwise with respect to foreign entities. Although the local authorities in China may establish a regulatory cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and administration, such regulatory cooperation with the securities regulatory authorities in the Unities States have not been efficient in the absence of mutual and practical cooperation mechanism. According to Article 177 of the PRC Securities Law which became effective in March 2020, no overseas securities regulator is allowed to directly conduct investigation or evidence collection activities within the territory of the PRC. Accordingly, without the consent of the competent PRC securities regulators and relevant authorities, no organization or individual may provide the documents and materials relating to securities business activities to overseas parties outside of the PRC.
You may have difficulty in enforcing foreign judgements in China against us or our management named in the prospectus.
China does not have treaties providing for the reciprocal recognition and enforcement of judgments of courts with the United States and many other countries and regions. Even judgements from countries that have an enforcement treaty with China are often not enforceable in China. Therefore, recognition and enforcement in China of judgments of a court in any non-PRC jurisdiction in relation to any matter not subject to a binding arbitration provision may be difficult or impossible.
It may also be difficult for you to enforce U.S. courts judgments based on violations of the civil liability provisions of the U.S. federal securities laws against us and our officers and directors, since most of them are residents of the PRC and those who are not residents of the PRC reside outside of the United States for all or a significant portion of time. In addition, there is uncertainty as to whether the courts of China would recognize or enforce judgments of U.S. courts.
Foreign exchange fluctuations may affect our business.
We accept payment for our products in RMB. Therefore, foreign exchange fluctuations may influence our business in unpredictable ways.
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions and the foreign exchange policy adopted by the PRC government. For instance, in August 2015, the People’s Bank of China, or PBOC, changed the way it calculates the mid-point price of Renminbi against the U.S. dollar, requiring the market-makers who submit for reference rates to consider the previous day’s closing spot rate, foreign-exchange demand and supply as well as changes in major currency rates. In fiscal year 2019 and 2020, the value of the Renminbi depreciated by approximately 6.9% and 5.5% against the U.S. dollar, respectively. In fiscal year 2021 and 2022 the value of the Renminbi appreciated by approximately 7.4% and 3.2% against the U.S. dollar. It is difficult to predict how market forces or PRC or U.S. government policy, including any interest rate increases by the Federal Reserve, may impact the exchange rate between the Renminbi and the U.S. dollar in the future. There remains significant international pressure on the PRC government to adopt a more flexible currency policy, including from the U.S. government, which has threatened to label China as a “currency manipulator,” which could result in greater fluctuation of the Renminbi against the U.S. dollar.
| 26 |
A substantial percentage of our revenues and costs are denominated in Renminbi, and a significant portion of our assets are also denominated in Renminbi. We are a holding company and we rely on dividends, loans and other distributions on equity paid by our operating subsidiaries in China. Any significant fluctuations in the value of the Renminbi may materially and adversely affect our liquidity and cash flows. Appreciation of the U.S. dollar against the Renminbi would have a negative effect on the U.S. dollar amount we would receive. Conversely, to the extent that we need to convert U.S. dollars into Renminbi for our operations, appreciation of the Renminbi against the U.S. dollar would have an adverse effect on the Renminbi amount we would receive.
Inflation could pose a risk to our business.
Inflation is an important factor that must be considered as we move forward. A change in the rate of inflation could influence the profits that we generate from our business. When the rate of inflation rises, the operational costs of running our Company would increase, such as labor costs, raw materials and public utilities, affecting our ability to provide our services at competitive prices. An increase in the rate of inflation would force our clients to search for other service providers, causing us to lose business and revenue.
Changes in the policies, regulations, rules and the enforcement of laws of the PRC government may be quick with little advance notice and could have a significant impact upon the business we may be able to conduct in the PRC and the profitability of such business.
The PRC’s economy is in a transition from a planned economy to a market oriented economy subject to five-year and annual plans adopted by the central government that set national economic development goals. Policies of the PRC government can have significant effects on the economic conditions of the PRC. The PRC government has confirmed that economic development will follow the model of a market economy. Under this direction, we believe that the PRC will continue to strengthen its economic and trading relationships with foreign countries and business development in the PRC will follow market forces. While we believe that this trend will continue, we cannot assure you that this will be the case. Changes in policies, regulations, rules and the enforcement of laws by the PRC government, which changes may be quick with little advance notice, could adversely affect our interests by. Although the PRC government has been pursuing economic reform policies for more than two decades, we cannot assure you that the government will continue to pursue such policies or that such policies may not be significantly altered, especially in the event of a change in leadership, social or political disruption, or other circumstances affecting the PRC’s political, economic and social environment.
There are uncertainties regarding the interpretation and enforcement of PRC laws, rules and regulations.
Most of our operations are conducted in the PRC, and are governed by PRC laws, rules and regulations. Our PRC Subsidiaries are subject to laws, rules and regulations applicable to foreign investment in China. The PRC legal system is a civil law system based on written statutes. Unlike the common law system, prior court decisions may be cited for reference but have limited precedential value.
In 1979, the PRC government began to promulgate a comprehensive system of laws, rules and regulations governing economic matters in general. However, China has not developed a fully integrated legal system, and recently enacted laws, rules and regulations may not sufficiently cover all aspects of economic activities in China or may be subject to significant degree of interpretation by PRC regulatory agencies and courts. In particular, because these laws, rules and regulations are relatively new, and because of the limited number of published decisions and the non-precedential nature of these decisions, and because the laws, rules and regulations often give the relevant regulator significant discretion in how to enforce them, the interpretation and enforcement of these laws, rules and regulations involve uncertainties and can be inconsistent and unpredictable. Therefore, it is possible that our existing operations may be found not to be in full compliance with relevant laws and regulations. In addition, the PRC legal system is based in part on government policies and internal rules, some of which are not published on a timely basis or at all, and which may have a retroactive effect. As a result, we may not be aware of our violation of these policies and rules until after the occurrence of the violation.
| 27 |
Any administrative and court proceedings in China may be protracted, resulting in substantial costs and diversion of resources and management attention. Since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy than in more developed legal systems. These uncertainties may impede our ability to enforce the contracts we have entered into and could materially and adversely affect our business, financial condition and results of operations.
PRC regulations regarding acquisitions impose significant regulatory approval and review requirements, which could make it more difficult for us to pursue growth through acquisitions.
Under the PRC Anti-Monopoly Law, companies undertaking acquisitions relating to businesses in China must notify the anti-monopoly enforcement agency in advance of any transaction where the parties’ revenues in the China market exceed certain thresholds and the buyer would obtain control of, or decisive influence over, the other party. In addition, on August 8, 2006, six PRC regulatory agencies, including the MOFCOM, the State-Owned Assets Supervision and Administration Commission, the State Administration of Taxation, the SAIC, the China Securities Regulatory Commission, or the CSRC, and the State Administration of Foreign Exchange, or SAFE, jointly adopted Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rules, which came into effect on September 8, 2006 and was amended on June 22, 2009. Under the M&A Rules, the approval of MOFCOM must be obtained in circumstances where overseas companies established or controlled by PRC enterprises or residents acquire domestic companies affiliated with such PRC enterprises or residents. Applicable PRC laws, rules and regulations also require certain merger and acquisition transactions to be subject to security review.
While the approval of the China Securities Regulatory Commission under the M & A Rules is not currently required for this offering, it may be required in the future in connection with this offering under the M&A Rules and, if required, we cannot predict whether we will be able to obtain such approval.
The application of the M&A Rules remains unclear. According to the results of the searches conducted by our PRC Counsel on the official website of the CSRC and its administrative license processing hall (https://neris.csrc.gov.cn/alappl/home/guideH), at present, only one administrative license related to overseas public offering and listing is enacted, that is, “examination and approval of overseas public offering shares and listing (including additional issuance) of joint-stock companies”. Such examination and approval license requirements are only applicable to issuers which are formed as PRC joint-stock companies in China under PRC law. None of our operating PRC Subsidiaries is formed as a PRC joint-stock company in China, and as such, we do not believe that we need CSRC approval. We do not believe that the current PRC regulations and rules including China Securities Law require explicitly and directly that the overseas listing of foreign issuers who indirectly hold the rights and interests of Chinese domestic enterprises be examined and approved by the CSRC. If CSRC approval is required, it is uncertain whether it would be possible for us to obtain the approval. Any failure to obtain or delay in obtaining CSRC approval for this offering would subject us to sanctions imposed by the CSRC and other PRC regulatory agencies.
| 28 |
While the application of the M&A Rules remains unclear, we believe, based on the advice of our PRC Counsel, based on its understanding of the current PRC laws, regulations and rules, that the CSRC’s approval is not required for this offering. We neither received nor were denied permission from CSRC or other PRC government agencies to list our securities on the Nasdaq Capital Market and issue our securities to foreign investors.
There remains some uncertainty as to how the M&A Rules will be interpreted or implemented in the context of an overseas offering. We cannot assure you that relevant PRC government agencies, including the CSRC, would reach the same conclusion as we do. If it is determined that CSRC approval is required for this offering, we may face sanctions by the CSRC or other PRC regulatory agencies for failure to obtain or delay in obtaining CSRC approval for this offering. These sanctions may include fines and penalties on our operations in China, limitations on our operating privileges in China, delays in or restrictions on the repatriation of the proceeds from this offering into the PRC, restrictions on or prohibition of the payments or remittance of dividends by our subsidiaries in China, or other actions that could have a material and adverse effect on our business, financial condition, results of operations, reputation and prospects, as well as the trading price of our securities. The CSRC or other PRC regulatory agencies may also take actions requiring us, or making it advisable for us, to halt this offering before the settlement and delivery of the securities that we are offering. Consequently, if you engage in market trading or other activities in anticipation of and prior to the settlement and delivery of the securities we are offering, you would be doing so at the risk that the settlement and delivery may not occur. In addition, if the CSRC or other regulatory agencies later promulgate new rules or explanations requiring that we obtain their approvals for this offering, we may be unable to obtain a waiver of such approval requirements.
As of the date of this prospectus, we have not received any inquiry, notice, warning, sanctions or regulatory objection to this offering from the CSRC or any other PRC governmental authorities, and our PRC Subsidiaries have obtained all requisite permissions and approvals from PRC governmental authorities to operate our business as currently conducted under relevant PRC laws and regulations.
As of the date of this prospectus, none of our PRC Subsidiaries has been denied or punished by relevant governmental authorities due to its business qualifications.
Our business may be subject to a variety of PRC laws and other obligations regarding cybersecurity and data protection.
Our business may be subject to PRC laws relating to the collection, use, sharing, retention, security, and transfer of confidential and private information, such as personal information and other data. These laws continue to develop, and the PRC government may adopt other rules and regulations in the future. Non-compliance could result in penalties or other significant legal liabilities.
Pursuant to the PRC Cybersecurity Law, which was promulgated by the Standing Committee of the National People’s Congress on November 7, 2016 and took effect on June 1, 2017, personal information and important data collected and generated by a critical information infrastructure operator in the course of its operations in China must be stored in China, and if a critical information infrastructure operator purchases internet products and services that affects or may affect national security, it should be subject to cybersecurity review by the Cyberspace Administration of China (“CAC”). Due to the lack of further interpretations, the exact scope of “critical information infrastructure operator” remains unclear.
| 29 |
On December 28, 2021, twelve Chinese government agencies jointly promulgated the Measures for Cybersecurity Review, which became effective on February 15, 2022, set forth the cybersecurity review mechanism for critical information infrastructure operators, and provided that critical information infrastructure operators who intend to purchase internet products and services that affect or may affect national security shall be subject to a cybersecurity review. On June 10, 2021, the Standing Committee of the National People’s Congress promulgated the PRC Data Security Law, which took effect in September 2021. The Data Security Law provides for a security review procedure for data activities that may affect national security. Moreover, the State Internet Information Office issued the Measures of Cybersecurity Review (Revised Draft for Comments, not yet effective) on July 10, 2021, which requires operators with personal information of more than 1 million users who want to list abroad to file a cybersecurity review with the CAC. Furthermore, the General Office of the Central Committee of the Communist Party of China and the General Office of the State Council jointly issued the Opinions on Severe and Lawful Crackdown on Illegal Securities Activities, which was available to the public on July 6, 2021. These opinions emphasized the need to strengthen the administration over illegal securities activities and the supervision of overseas listings by China-based companies. These opinions proposed to take effective measures, such as promoting the construction of relevant regulatory systems, to deal with the risks and incidents facing China-based overseas-listed companies and the demand for cybersecurity and data privacy protection. As these laws, opinions and the draft measures were recently issued, official guidance and interpretation of these remain unclear in several respects at this time, and the PRC government authorities have wide discretion in the interpretation and enforcement of these laws, opinions and draft measures. Therefore, it is uncertain whether the future regulatory changes would impose additional restrictions on our business.
The Data Security Law also sets forth the data security protection obligations for entities and individuals handling personal data, including that no entity or individual may acquire such data by stealing or other illegal means, and the collection and use of such data should not exceed the necessary limits The costs of compliance with, and other burdens imposed by, PRC Cybersecurity Law and any other cybersecurity and related laws may limit the utility of our internet sales channel of distribution and could have an adverse impact on our business. Further, if the enacted version of the Measures for Cybersecurity Review mandates clearance of cybersecurity review and other specific actions to be completed by companies like us, we face uncertainties as to whether such clearance can be timely obtained, or at all.
As confirmed by our PRC Counsel, we are not subject to the cybersecurity review by the CAC for this offering, given that we are not an operator of critical information infrastructure or an online platform operator as defined by the Measures for Cybersecurity Review (2021 version). However, there remains uncertainty as to how the Draft Measures will be interpreted or implemented and whether the PRC regulatory agencies, including the CAC, may adopt new laws, regulations, rules, or detailed implementation and interpretation related to the Draft Measures. If any such new laws, regulations, rules, or implementation and interpretation comes into effect, we will take all reasonable measures and actions to comply and to minimize the adverse effect of such laws on us.
We cannot assure you that PRC regulatory agencies, including the CAC, would take the same view as we do, and there is no assurance that we can fully or timely comply with such laws. In the event that we are subject to any mandatory cybersecurity review and other specific actions required by the CAC, we face uncertainty as to whether any clearance or other required actions can be timely completed, or at all. Given such uncertainty, we may be further required to shut down our website, or face other penalties, which could materially and adversely affect our business, financial condition, and results of operations.
| 30 |
PRC regulations relating to investments in offshore companies by PRC residents may subject our PRC-resident beneficial owners or our PRC Subsidiaries to liability or penalties, limit our ability to inject capital into our PRC Subsidiaries or limit our PRC Subsidiaries’ ability to increase their registered capital or distribute profits.
SAFE promulgated the Circular on Relevant Issues Concerning Foreign Exchange Control on Domestic Residents’ Offshore Investment and Financing and Roundtrip Investment through Special Purpose Vehicles, or SAFE Circular 37, on July 4, 2014, which replaced the former circular commonly known as “SAFE Circular 75.” SAFE Circular 37 requires PRC residents to register with local branches of SAFE in connection with their direct establishment or indirect control of an offshore entity, for the purpose of overseas investment and financing, with such PRC residents’ legally owned assets or equity interests in domestic enterprises or offshore assets or interests, referred to in SAFE Circular 37 as a “special purpose vehicle.” SAFE Circular 37 further requires amendment to the registration in the event of any significant changes with respect to the special purpose vehicle, such as changes in capital contributed by PRC individuals, share transfers or exchanges, mergers, divisions or other material events. In the event that a PRC shareholder holding interests in a special purpose vehicle fails to fulfill the required SAFE registration, the PRC Subsidiaries of that special purpose vehicle may be prohibited from making profit distributions to the offshore parent and from carrying out subsequent cross-border foreign exchange activities, and the special purpose vehicle may be restricted in its ability to contribute additional capital into its PRC Subsidiaries. Moreover, failure to comply with the various SAFE registration requirements could result in liability under PRC law for evasion of foreign exchange controls.
As we have little control over the registration procedures, we cannot assure you of the outcome of such registration, and we cannot assure you that any of our shareholders who are PRC residents will submit the required registration or amend or update their registration as required under Circular 37 and the Notice of the State Administration of Foreign Exchange on Further Simplifying and Improving the Foreign Exchange Administration Policies for Direct Investment [Hui Fa (2015) No.13] issued by SAFE with effect from June 1, 2015, or SAFE Notice 13, in a timely manner or at all. In addition, we may not be aware of the identities of all of our beneficial owners who are PRC residents. We do not have control over our beneficial owners and cannot assure you that all of our PRC-resident beneficial owners will comply with SAFE Circular 37 and subsequent implementation rules. The failure of our current and future beneficial owners who are PRC residents to register or amend their SAFE registrations in a timely manner pursuant to SAFE Circular 37 and subsequent implementation rules, may subject the beneficial owners or our PRC Subsidiaries to fines and legal sanctions. On February 13, 2015, SAFE promulgated a Notice on Further Simplifying and Improving Foreign Exchange Administration Policy on Direct Investment, or SAFE Notice 13, which became effective on June 1, 2015. Pursuant to SAFE Notice 13, entities and individuals are required to apply for foreign exchange registration of foreign direct investment and overseas direct investment, including those required under the SAFE Circular 37, with designated domestic banks, instead of SAFE. The designated domestic banks will directly review the applications and conduct the registration.
Furthermore, since it is unclear how the new SAFE regulations, and any future regulation concerning offshore or cross-border transactions, will be interpreted, amended and implemented by the relevant PRC government authorities, we cannot predict how these regulations will affect our business operations or future strategy. Failure to register or comply with relevant requirements may also limit our ability to contribute additional capital to our PRC Subsidiaries and limit our PRC Subsidiaries’ ability to distribute dividends to our Company. These risks may have a material adverse effect on our business, financial condition and results of operations.
We may be treated as a resident enterprise for PRC tax purposes under the PRC Enterprise Income Tax Law, and we may therefore be subject to PRC income tax on our global income.
Under the PRC Enterprise Income Tax Law and its implementing rules, both of which came into effect on January 1, 2008, enterprises established under the laws of jurisdictions outside of China with “de facto management bodies” located in China may be considered PRC tax resident enterprises for tax purposes and may be subject to the PRC enterprise income tax at the rate of 25% on their global income. “De facto management body” refers to a managing body that exercises substantive and overall management and control over the production and business, personnel, accounting books and assets of an enterprise. The State Administration of Taxation issued the Notice Regarding the Determination of Chinese-Controlled Offshore-Incorporated Enterprises as PRC Tax Resident Enterprises on the basis of de facto management bodies, or Circular 82, on April 22, 2009. Circular 82 provides certain specific criteria for determining whether the “de facto management body” of a Chinese-controlled offshore-incorporated enterprise is located in China. Although Circular 82 only applies to offshore enterprises controlled by PRC enterprises, not those controlled by foreign enterprises or individuals, the determining criteria set forth in Circular 82 may reflect the State Administration of Taxation’s general position on how the “de facto management body” test should be applied in determining the tax resident status of offshore enterprises, regardless of whether they are controlled by PRC enterprises. If we, AiXin Colorado and AiXin BVI, were to be considered a PRC resident enterprise, we would be subject to PRC enterprise income tax at the rate of 25% on our global income. In such case, our profitability and cash flow may be materially reduced as a result of our global income being taxed under the Enterprise Income Tax Law. We believe that none of our entities outside of China is a PRC resident enterprise for PRC tax purposes. However, the tax resident status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body.”
| 31 |
Restrictions on currency exchange may limit our ability to utilize our PRC revenue effectively.
We (AiXin Colorado) are a Colorado holding company with no material operations of our own. We conduct substantially all of our operations through the operating companies established in the PRC. We are a holding company and do not directly own any substantive business operations in China. Substantially all of our revenue is denominated in Renminbi. The Renminbi is currently convertible under the “current account,” which includes dividends, trade and service-related foreign exchange transactions, but requires approval from or registration with appropriate government authorities or designated banks under the “capital account,” which includes foreign direct investment and loans, including loans we may secure from our onshore subsidiaries.
The relevant PRC governmental authorities or the local bank may limit or eliminate our ability to purchase foreign currencies in the future for current account transactions.
Since 2016, PRC governmental authorities have imposed more stringent restrictions on outbound capital flows, including heightened scrutiny over “irrational” overseas investments for certain industries, as well as over four kinds of “abnormal” offshore investments, which are:
● investments through enterprises established for only a few months without substantive operation;
● investments with amounts far exceeding the registered capital of the onshore parent and not supported by its business performance shown on financial statements;
● investments in targets which are unrelated to an onshore parent’s main business; and
● investments with abnormal sources of Renminbi funding suspected to be involved in illegal transfer of assets or illegal operation of underground banking.
On January 26, 2017, SAFE promulgated the Circular on Further Improving Reform of Foreign Exchange Administration and Optimizing Genuineness and Compliance Verification, which tightened the authenticity and compliance verification of cross-border transactions and cross-border capital flow, including requiring banks to verify board resolutions, tax filing forms and audited financial statements before wiring foreign invested enterprises’ foreign exchange dividend distribution of over US$50,000. In addition, the Outbound Investment Sensitive Industry Catalogue (2018) lists certain sensitive industries that are subject to NDRC pre-approval requirements prior to remitting investment funds offshore, which subjects us to increased approval requirements and restrictions should we have overseas investments. Since a significant amount of our PRC revenue is denominated in Renminbi, any existing and future restrictions on currency exchange may limit our ability to utilize revenue generated in Renminbi to fund our business activities outside of the PRC, make investments, service any debt we may incur outside of China or pay dividends in foreign currencies to our shareholders.
Fluctuations in foreign exchange rates will impact our reported results.
The functional currency utilized by our PRC Subsidiaries is the RMB. Our financial results are reported in U.S. Dollars. Therefore, foreign exchange fluctuations will impact the reporting of our financial results and significant fluctuations in the value of the RMB relative to the U.S. Dollar may materially and adversely affect our financial results as reported in our filings with the SEC. Such variations in our perceived operating results may increase the volatility in the market for our common stock.
The disclosures in our reports and other filings with the SEC and our other public pronouncements are not subject to the scrutiny of any regulatory bodies in the PRC.
We are subject to the regulations of the SEC and our reports and other filings with the SEC are subject to SEC review in accordance with the rules and regulations promulgated by the SEC under the Securities Act and the Exchange Act. Our SEC reports and other disclosure and public pronouncements are not subject to the review or scrutiny of any PRC regulatory authority. For example, the disclosure in our SEC reports and other filings are not subject to the review by China Securities Regulatory Commission, a PRC regulator that is responsible for oversight of the capital markets in China. Accordingly, you should review our SEC reports, filings and our other public pronouncements with the understanding that no local regulator has done any review of us, our SEC reports, other filings or any of our other public pronouncements.
| 32 |
Introduction of new laws or changes to existing laws by the PRC government may adversely affect our business.
The PRC legal system is a codified legal system made up of written laws, regulations, circulars, administrative directives and internal guidelines. Unlike common law jurisdictions like the U.S., decided cases (which may be taken as reference) do not form part of the legal structure of the PRC and thus have no binding effect on subsequent cases with similar issues and fact patterns. Furthermore, in line with its transformation from a centrally-planned economy to a more free market-oriented economy, the PRC government is still in the process of developing a comprehensive set of laws and regulations. As the legal system in the PRC is still evolving, laws and regulations or the interpretation of the same may be subject to further changes. For example, the PRC government may impose more stringent environmental regulations which would affect our ability to comply with, or our costs to comply with, such regulations. Such changes, if implemented, may adversely affect our business operations and may reduce our profitability.
Risks Relating to Our Holding Company Structure
Substantial uncertainties exist with respect to the interpretation and implementation of the newly enacted Foreign Investment Law and how it may impact the viability of our current corporate structure, corporate governance and business operations.
On March 15, 2019, the PRC National People’s Congress approved the Foreign Investment Law, which came into effect on January 1, 2020 and replaced existing laws regulating foreign investment in the PRC and become the legal foundation for foreign investment in the PRC. Meanwhile, the Implementation Regulation of the Foreign Investment Law and the Measures for Reporting of Information on Foreign Investment came into effect as of January 1, 2020, which clarified and elaborated the relevant provisions of the Foreign Investment Law.
The Foreign Investment Law sets out the basic regulatory framework for foreign investments and proposes to implement a system of pre-entry national treatment with a restricted list for foreign investments, pursuant to which (i) foreign entities and individuals are prohibited from investing in the areas that are not open to foreign investments, (ii) foreign investments in the restricted industries must satisfy certain requirements under the law, and (iii) foreign investments in business sectors outside of the restricted list will be treated equally with domestic investments. The Foreign Investment Law also sets forth necessary mechanisms to facilitate, protect and manage foreign investments and proposes to establish a foreign investment information reporting system, through which foreign investors or foreign-invested enterprises are required to submit initial report, report of changes, report of deregistration and annual report relating to their investments to the Ministry of Commerce, or MOFCOM, or its local branches.
Although our operating structure is legal and permissible under the current Chinese law and regulations, including the Foreign Investment Law, Chinese regulatory authorities could disallow our operating structure, which would likely result in a material change in our operations and/or could cause the value of our common stock to significantly decline or become worthless.
We may rely on dividends and other distributions on equity paid by our PRC Subsidiaries to fund any cash and financing requirements we may have, and any limitation on the ability of our PRC Subsidiaries to make payments to us could have a material and adverse effect on our ability to conduct our business.
We are a Colorado holding company and we rely on dividends and other distributions on equity paid by our PRC Subsidiaries and loans between us and our PRC Subsidiaries to fund any cash and financing requirements we or any of our PRC Subsidiaries may have, including for the payment of dividends to our investors, the shareholders of AiXin Colorado. Any limitation on the ability of our PRC Subsidiaries to make payments to or transfer funds to us or our other PRC Subsidiaries could have a material and adverse effect on our ability to conduct our business and the ability of our PRC Subsidiaries to conduct their respective businesses. If our PRC Subsidiaries incur debt, the instruments governing the debt may restrict their ability to pay dividends or make other distributions to us. Under PRC laws and regulations, our PRC Subsidiaries may pay dividends only out of their respective accumulated profits as determined in accordance with PRC accounting standards and regulations. In addition, each of our PRC Subsidiaries is required to set aside at least 10% of its accumulated after-tax profits each year, if any, to fund a certain statutory reserve fund, until the aggregate amount of such fund reaches 50% of its registered capital. Such reserve funds cannot be distributed to us as dividends. At its discretion, each of our PRC Subsidiaries may allocate a portion of its after-tax profits based on PRC accounting standards to an enterprise expansion fund, or a staff welfare and bonus fund.
As of the date hereof, we have had no transactions that involved the transfer of cash or assets throughout our corporate structure. None of our Chinese Operating Companies has distributed any cash or other assets to AiXin HK, including by way of dividends or interest payments and AiXin Colorado has not transferred any cash or other assets to any of our PRC Subsidiaries. As of the date hereof, no transfers, dividends or distributions have been made to our investors. To the extent our cash or other assets is in one of our Chinese Operating Companies or AiXin HK, the funds or assets may not be available to fund operations or for use outside of mainland China or Hong Kong including for the payment of dividends to the shareholders of AiXin Life, due to interventions by the governments of PRC or Hong Kong, or the imposition of restrictions and limitations on the ability of the PRC Subsidiaries to use such cash or assets imposed by the government of mainland China or Hong Kong.
| 33 |
The PRC government may continue to strengthen its capital controls, and more restrictions and substantial vetting processes may be put forward by SAFE for cross-border transactions falling under both the current account and the capital account. Any limitation on the ability of our PRC Subsidiaries to pay dividends or make other kinds of payments to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our business, pay dividends, or otherwise fund and conduct our business.
Except for such limitations on our Company’s ability to transfer cash and other assets among our entities currently or hereafter imposed by the governments of the PRC and Hong Kong, there are no limitations on our Company’s ability to transfer cash and other assets through our corporate structure. Our Company does not have any cash management policies with respect to the transfer of cash among our entities other than requirements for the approval of management for transfers in excess of specified amounts and none of our entities is currently party to any debenture, loan or other agreement which imposes restrictions or otherwise limits our Company’s ability to transfer our cash and other assets and we have not adopted any policies that dictate how funds are transferred other than as necessary to comply with applicable laws. See “Prospectus Summary - Transfers of Cash to and from our Subsidiaries.”
In addition, the Enterprise Income Tax Law and its implementation rules provide that a withholding tax rate of up to 10% will be applicable to dividends payable by Chinese companies to non-PRC-resident enterprises unless otherwise exempted or reduced according to treaties or arrangements between the PRC central government and governments of other countries or regions where the non-PRC-resident enterprises are incorporated.
PRC regulation of loans to and direct investment in PRC entities by offshore holding companies and governmental control of currency conversion may delay or prevent us from using the proceeds of this offering to make loans or additional capital contributions to our PRC Subsidiaries, which could materially and adversely affect our liquidity and our ability to fund and expand our business.
We are an offshore holding company conducting our operations in China through our PRC Subsidiaries. We may make loans or provide guarantees for the benefit of our PRC Subsidiaries subject to the approval of or registration with governmental authorities and limitations of the amount, or we may make additional capital contributions to our PRC Subsidiaries. Any loans to our PRC Subsidiaries, which are treated as foreign-invested enterprises under PRC law, are subject to foreign exchange loan registrations. In addition, a foreign-invested enterprise, or FIE, shall use its capital pursuant to the principle of authenticity and self-use within its business scope. The capital of an FIE shall not be used for the following purposes: (i) directly or indirectly for payments beyond the business scope of the enterprise or payments prohibited by relevant laws and regulations; (ii) directly or indirectly for investments in securities or investments other than in banks’ principal-secured products unless otherwise provided by relevant laws and regulations; (iii) the granting of loans to non-affiliated enterprises, except where it is expressly permitted in the business license; and (iv) paying the expenses related to the purchase of real estate that is not for self-use (except for foreign-invested real estate enterprises).
In light of the requirements imposed by PRC regulations on loans to and direct investments in PRC entities by offshore holding companies, we cannot assure you that we will be able to complete the necessary government registrations or obtain the necessary government approvals on a timely basis, if at all, with respect to future loans by us to our PRC Subsidiaries or with respect to future capital contributions by us to our PRC Subsidiaries. If we fail to complete such registrations or obtain such approvals, our ability to use the proceeds from this offering and to capitalize or otherwise fund our PRC operations may be negatively affected, which could materially and adversely affect our liquidity and our ability to fund and expand our business.
Risks Related to this Offering and our Common Stock
Prior to this offering, we had a limited public market for our common stock and you may not be able to resell our shares at or above the price you paid or at all.
Prior to this offering, there was a limited public market for our common stock in the OTCQB. On many days during the years ended December 31, 2022, and 2023, and to date in 2024, no shares were traded. We cannot assure you that an active public market for our common stock will develop or that the market price of our shares will not decline below the public offering price. The public offering price of our shares may not be indicative of prices that will prevail in the trading market following the offering.
| 34 |
An active trading market for our common stock may not develop and the trading price for our common stock may fluctuate significantly.
We have applied to have our common stock approved for listing on the Nasdaq Capital Market. Prior to the completion of this offering, there has not been an active public market for our common stock, and we cannot assure you that a liquid public market for our common stock will develop even if it is listed on the Nasdaq Capital Market. The public offering price for our common stock offered hereby was determined by negotiation between us and the underwriter based upon several factors, and we can provide no assurance that the trading price of our common stock after this offering will not decline below the public offering price. As a result, investors in our securities may experience a significant decrease in the value of their common stock.
Nasdaq may apply additional and more stringent criteria for our initial and continued listing because we plan to have a small public offering and insiders will hold a large portion of our listed securities.
Nasdaq Listing Rule 5101 provides Nasdaq with broad discretionary authority over the initial and continued listing of securities in Nasdaq and Nasdaq may use such discretion to deny initial listing, apply additional or more stringent criteria for the initial or continued listing of particular securities, or suspend or delist particular securities based on any event, condition, or circumstance that exists or occurs that makes initial or continued listing of the securities on Nasdaq inadvisable or unwarranted in the opinion of Nasdaq, even though the securities meet all enumerated criteria for initial or continued listing on Nasdaq. In addition, Nasdaq has used its discretion to deny initial or continued listing or to apply additional and more stringent criteria in the instances, including but not limited to: (i) where the company engaged an auditor that has not been subject to an inspection by PCAOB, an auditor that PCAOB cannot inspect, or an auditor that has not demonstrated sufficient resources, geographic reach, or experience to adequately perform the company’s audit; (ii) where the company planned a small public offering, which would result in insiders holding a large portion of the company’s listed securities and Nasdaq was concerned that the offering size was insufficient to establish the company’s initial valuation, and there would not be sufficient liquidity to support a public market for the company; and (iii) where the company did not demonstrate sufficient nexus to the U.S. capital market, including having no U.S. shareholders, operations, or members of the board of directors or management. Nasdaq might apply the additional and more stringent criteria for our initial and continued listing, which might cause delay or even denial of our listing application.
The market price of securities of certain small cap companies, including companies whose operations are based in China, have been highly volatile and have raised the concerns of market regulators and if our common stock were to have such high volatility could cause Nasdaq to temporarily halt trading in or delist our securities.
The market prices of certain small cap companies, including companies whose operations are based in China, recently have been highly volatile. Such volatility in the market price of our common stock may cause Nasdaq, under the authority of Listing Rule 5101, to halt trading in our common stock or apply additional or more stringent criteria for the continued listing of our common stock, assuming initial listing is granted, even though our common stock then meets all enumerated criteria for initial or continued listing on the Nasdaq Capital Market.
If a limited number of participants in this offering purchase a significant percentage of the offering, the effective public float may be smaller than anticipated and the trading price of our common stock may be volatile which could subject us to securities litigation and make it more difficult for you to sell your shares and could cause Nasdaq to temporarily halt trading in or delist our common stock.
The market prices of certain small cap companies, including companies whose operations are based in China, recently have been highly volatile. As a company conducting a public offering, we are subject to the risk that a small number of investors will purchase a high percentage of the offering. We have not imposed any obligations on the underwriter as to the maximum number of shares it may place with individual investors. If, in the course of marketing the offering, the underwriter was to determine that demand for our shares was concentrated in a limited number of investors and such investors determined to hold their shares after the offering rather than trade them in the market, other shareholders could find the trading and price of our shares affected (positively or negatively) by the limited availability of our shares. If this were to happen, investors could find our shares to be more volatile than they might otherwise anticipate. Companies that experience such volatility in their share price may be more likely to be the subject of securities litigation. In addition, if a large portion of our public float were to be held by a few investors, smaller investors may find it more difficult to sell their shares. Further, such volatility in the market price of our common stock could cause Nasdaq, under the authority of Listing Rule 5101, to halt trading in our common stock or apply additional or more stringent criteria for the continued listing of our common stock, assuming initial listing is granted, even though our common stock then meets all enumerated criteria for initial or continued listing on the Nasdaq Capital Market.
| 35 |
The trading price of our common stock has been volatile.
The trading price for our common stock varied during the twelve-month period ended December 31, 2023, between a high of $11.00 on April 13, 2023 and a low of $1.00 on November 20, 2023. The last reported trade in our common stock on February 8, 2024, was $0.50. Our stock price is likely to continue to be volatile and subject to significant price and volume fluctuations in response to market and other factors. In addition, a substantial decline in the price of our securities that persists for a significant period of time could cause our securities to be delisted from the Nasdaq Capital Market, further reducing market liquidity. If an active market for our securities does not develop or cannot be sustained, the liquidity of an investor’s investment may be limited, and the price of our securities may decline. If an active market does not exist, investors may lose their entire investment. As a result of any of these factors, the market price of our securities at any given point in time may not accurately reflect our long-term value.
The market price of our shares is likely to be highly volatile, which could result in substantial losses to investors.
The trading price of our common stock may be volatile and could fluctuate widely due to factors beyond our control. This may happen because of the broad market and industry factors, like the performance and fluctuation of the market prices of other companies with business operations located mainly in China that have listed their securities in the United States. The securities of some companies with operations in China and securities listed in the United States have experienced significant volatility, including price declines in connection with their initial public offerings. The trading performance of these Chinese companies’ securities after their offerings may affect the attitudes of investors toward Chinese companies listed in the United States, including those of investors based in China, in general and consequently may impact the trading performance of our common stock, regardless of our actual operating performance. Such volatility in the price of our common stock including any stock-run up, may be unrelated to our actual or expected operating performance and financial condition or prospects, making it difficult for prospective investors to assess the rapidly changing value of our common stock.
In addition to market and industry factors, the trading price and volume of our common stock may be highly volatile due to factors specific to our operations, including the following:
| ● | variations in our actual and perceived operating results; |
| ● | news regarding gains or losses of customers or partners by us or our competitors; |
| ● | news regarding gains or losses of key personnel by us or our competitors; |
| ● | announcements of competitive developments, acquisitions or strategic alliances in our industry by us or our competitors; |
| ● | changes in earnings estimates or buy/sell recommendations by financial analysts; |
| ● | potential litigation; |
| ● | the imposition of fines or penalties related to our activities in the PRC and failure to comply with applicable rules and regulations; |
| ● | general market conditions or other developments affecting us or our industry; and |
| ● | the operating and stock price performance of other companies, other industries and other events or factors beyond our control. |
In addition, the stock market has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of particular companies. These fluctuations may be even more pronounced in the trading market for our shares shortly following this offering. If the market price of our common stock after this offering does not exceed the offering price, you may not realize any return on your investment in us and may lose some or all of your investment. Securities class action litigation has often been instituted against companies following periods of volatility in the overall market and in the market price of a company’s securities. This litigation, if instituted against us, could result in very substantial costs, divert our management’s attention and resources, and harm our business, operating results and financial condition. In addition, recent fluctuations in the financial and capital markets have resulted in volatility in securities prices.
| 36 |
Military or other conflicts in Ukraine, the Middle East or elsewhere may lead to increased volume and price volatility for publicly traded securities which could adversely impact the price of our common stock.
Military or other conflicts in Ukraine, the Middle East or elsewhere may lead to increased volume and price volatility for publicly traded securities, or affect the operations or financial condition of companies, and lead to national, regional or international economic disruptions and economic uncertainty, any of which could adversely impact the price of our common stock.
The sale or availability for sale of substantial amounts of our common stock could adversely affect its market price.
Sales of substantial amounts of our common stock in the public market after the completion of this offering, or the perception that these sales could occur, could adversely affect the market price of our common stock and could materially impair our ability to raise capital through equity offerings in the future. The common stock sold in this offering will be freely tradable without restriction or further registration under the Securities Act, and shares held by our existing shareholders may also be sold in the public market in the future subject to the restrictions in Rule 144 under the Securities Act and the applicable lock-up agreements. There will be [20,374,842] shares of common stock outstanding immediately after this offering or [20,731,092] shares assuming the full exercise of the underwriter’s over-allotment option. In connection with this offering, we and each of our directors and officers named in the section “Management,” have agreed not to sell any common stock for six months from the date of this prospectus without the prior written consent of the underwriter, subject to certain exceptions. However, the underwriter may release these securities from these restrictions at any time, subject to applicable regulations of the Financial Industry Regulatory Authority, Inc. (“FINRA”). We cannot predict what effect, if any, market sales of securities held by our significant shareholders or any other shareholder or the availability of these securities for future sale will have on the market price of our common stock. See “Underwriting” and “Shares Eligible for Future Sale” for a more detailed description of the restrictions on selling our securities after this offering.
If securities or industry analysts do not publish research or reports about our business, or if they adversely change their recommendations regarding our common stock, the market price for our common stock and trading volume could decline.
The trading market for our common stock will be influenced by research or reports that industry or securities analysts publish about our business. If one or more analysts who cover us downgrade our common stock, the market price for our common stock would likely decline. If one or more of these analysts cease to cover us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the market price or trading volume for our common stock to decline.
Because we do not expect to pay dividends in the foreseeable future after this offering, you must rely on price appreciation of our common stock for return on your investment.
To date, we have not paid dividends on our common stock. We currently intend to retain all of our available funds and any future earnings after this offering to fund the development and growth of our business. As a result, we do not expect to pay any cash dividends in the foreseeable future. Therefore, you should not rely on an investment in our common stock as a source for dividend income.
Our board of directors has complete discretion as to whether to distribute dividends. The timing, amount and form of future dividends, if any, will depend on, among other things, our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries, our financial condition, contractual restrictions and other factors deemed relevant by our board of directors. Accordingly, the return on your investment in our common stock will likely depend entirely upon any future price appreciation of our common stock. There is no guarantee that our common stock will appreciate in value after this offering or even maintain the price at which you purchased our common stock and you may even lose your entire investment.
You must rely on the judgment of our management as to the use of the net proceeds from this offering, and such use may not produce income or increase our share price.
We plan to use the net proceeds of this offering primarily for expanding our manufacturing facilities, pursuing business development opportunities and working capital and other general corporate purposes. See “Use of Proceeds.” However, our management will have considerable discretion in the application of the net proceeds received by us. You will not have the opportunity, as part of your investment decision, to assess whether proceeds are being used appropriately. The net proceeds may be used for corporate purposes that do not improve our efforts to achieve or maintain profitability or increase our share price. The net proceeds from this offering may be placed in investments that do not produce income or that lose value.
Our CEO has substantial influence over our Company. His interests may not be aligned with the interests of our other shareholders, and he could prevent or cause a change of control or other transactions.
As of the date of this prospectus, Quanzhong Lin, our Chairman of the Board of Directors and Chief Executive Officer, beneficially owns an aggregate of [58.14]% of our outstanding common stock. Upon completion of this offering, Mr. Lin will beneficially own approximately [36.98]% of our outstanding common stock.
Accordingly, Mr. Lin could have significant influence in determining the outcome of any corporate transaction or other matter submitted to the shareholders for approval, including mergers, consolidations, the appointment of directors and other significant corporate actions. Mr. Lin will also have the power to prevent or cause a change in control. Without the consent of Mr. Lin, we may be prevented from entering into transactions that could be beneficial to us or our minority shareholders. In addition, Mr. Lin could violate his fiduciary duties by diverting business opportunities from us to himself or others. The interests of Mr. Lin may differ from the interests of our other shareholders. The concentration in the ownership of our common stock shares may cause a material decline in the value of our common stock. For more information regarding Mr. Lin, see “Principal Shareholders.”
| 37 |
USE OF PROCEEDS
After deducting the underwriting commissions and estimated offering expenses payable by us, we expect to receive net proceeds of approximately $[7,695,000] from this offering. We anticipate that the proceeds will be applied as follows:
| Planned Actions | Amount | |||
| Working capital and general corporate purposes | $ | 2,355,000 | ||
| Satisfaction of a portion of accrued liabilities | 1,250,000 | |||
| Purchase of raw materials and supplies | 750,000 | |||
| Fund existing businesses operation (product design and manufacture) | 500,000 | |||
| Expansion of manufacturing facility | 1,250,000 | |||
| Expand online activities | 250,000 | |||
| Branding and marketing | 1,250,000 | |||
| Satisfaction of loans due third parties | 90,000 | |||
| Offering expenses | 950,000 | |||
| Underwriting commissions and expenses | 855,000 | |||
| TOTAL | $ | 9,500,000 | ||
The amount and timing of these expenditures will vary depending on a number of factors, including the amount of cash generated by our operations and the rate of growth, if any, of our business.
Although we may use a portion of the proceeds for the acquisition of, or investment in, companies, technologies, products or assets that complement our business, we have no present understandings, commitments or agreements to enter into any acquisitions or make any investments. We cannot assure you that we will make any acquisitions or investments in the future.
CAPITALIZATION
The following table sets forth our cash and cash equivalents, accounts payable, loans and advances from third parties and capitalization as of September 30, 2023:
| ● | on an actual basis; and | |
| ● | on a pro forma, as adjusted basis, to give effect to the sale of the shares of our common stock offered hereby at the public offering price of $4.00 and after deducting the underwriter’s commissions and estimated offering expenses payable by us and prior to the application of the net proceeds as described under “Use of Proceeds.” |
You should read this table in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements and related notes included elsewhere in this prospectus.
| September 30, 2023 | September 30, 2023 | September 30, 2023 | ||||||||||
| Actual | Pro Forma As Adjusted | Pro Forma As Adjusted, Assuming Exercise of the Over-Allotment Option in Full | ||||||||||
| (unaudited) | (unaudited) | (unaudited) | ||||||||||
| Cash and cash equivalents | $ | 412,659 | $ | 8,107,659 | $ | 9,418,659 | ||||||
| Inventory, net | 643,623 | 643,623 | 643,623 | |||||||||
| Accounts Payable | 599,373 | 599,373 | 599,373 | |||||||||
| Loans from third parties | 82,237 | 82,237 | 82,237 | |||||||||
| Accrued liabilities and other payables | $ | 2,136,132 | $ | 2,136,132 | $ | 2,136,132 | ||||||
| Common Stock, actual and pro forma as adjusted | 250 | 274 | 277 | |||||||||
| Additional Paid in Capital, actual and pro forma as adjusted | 14,882,538 | 22,577,514 | 23,888,511 | |||||||||
| Statutory Reserve | 151,988 | 151,988 | 151,988 | |||||||||
| Accumulated Deficit | (16,195,184 | ) | (16,195,184 | ) | (16,195,184 | ) | ||||||
| Accumulated other comprehensive income | 215,322 | 215,322 | 215,322 | |||||||||
| Total Stockholders’ equity (deficit) | $ | (945,086 | ) | $ | 6,749,914 | $ | 8,060,914 | |||||
| 38 |
DILUTION
If you invest in our common stock in this offering, you will experience immediate and substantial dilution to the extent of the difference between the public offering price per share you will pay in this offering and the pro forma as adjusted net tangible book value per share of our common stock after this offering.
Our net tangible book value as of September 30, 2023 was a deficit of $(947,926), or a deficit of approximately $(0.04) per share of common stock(1). Net tangible book value represents total tangible assets less total liabilities. Tangible assets represent total assets excluding goodwill and other intangible assets. Net tangible book value per share represents net tangible book value divided by the aggregate number of shares of common stock outstanding as of September 30, 2023.
If our common stock is sold in this offering at the public offering price of $4.00, assuming the underwriter does not exercise the option to purchase additional shares of our common stock after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, and the application of such net proceeds as described under “Use of Proceeds” our pro forma as adjusted net tangible book value as of September 30, 2023 would have been approximately $6,747,074, or approximately $0.33 per share of common stock(1). This represents an immediate increase in pro forma as adjusted net tangible book value of $0.37 per share of common stock to our existing stockholders and an immediate dilution (i.e., the difference between the offering price and the pro forma as adjusted net tangible book value after this offering) to investors participating in this offering of $3.67 per share of common stock. The following table illustrates this per share dilution to the new investors purchasing shares of common stock in this offering:
| Offering(2) | Full Over-allotment Post-offering(3) | |||||||
| Assumed offering price per share | $ | 4.00 | $ | 4.00 | ||||
| Net tangible book value per share as of September 30, 2023 | $ | (0.04 | ) | (0.04 | ) | |||
| Increase in net tangible book value per share attributable to this offering | $ | 0.37 | 0.43 | |||||
| Pro forma as adjusted net tangible book value per common stock after the offering | $ | 0.33 | 0.39 | |||||
| Dilution in pro forma as adjusted net tangible book value per share to investors in this offering | $ | 3.67 | 3.61 | |||||
| (1) | All per share amounts in this section give effect to the reduction in the number of our shares of common stock outstanding as a result of the contribution to our capital by Quanzhong Lin of seven million shares of our common stock to be effective upon effectiveness of the Registration Statement of which this prospectus is a part. |
| (2) |
Assumes gross proceeds from offering of 2,375,000 shares. |
| (3) | Assumes gross proceeds from offering of 2,731,250 shares, if over-allotment option is exercised in full. |
A $1.00 increase (decrease) in the public offering price of the shares offered hereby would increase (decrease) the pro forma as adjusted net tangible book value by $2,185,000, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriter’s commissions and estimated offering expenses payable by us.
| 39 |
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Our common stock is currently quoted on the OTCQB under the symbol “AIXN.” From January 22, 2021, until June 5, 2023, our common stock traded on the OTCQX
Trading in stocks quoted on the OTCQX and OTCQB is often thin and is characterized by wide fluctuations in trading prices due to many factors that may have little to do with a company’s operations or business prospects. We cannot assure you that there will be a market for our common stock in the future.
The following table sets forth the high and low trading prices of one share of our common stock for each fiscal quarter over the past two fiscal years, and from January 1, 2023 to the date of this prospectus. The quotations provided are for the over-the-counter market, which reflect interdealer prices without retail mark-up, mark-down or commissions, and may not represent actual transactions. Our common stock trades on a limited, sporadic and volatile basis. These high and low bid prices per share of common stock have been adjusted to give effect to the 1 for 4 reverse stock split of our common stock effected on October 27, 2020, and the 1 for 2 reverse stock split of our common stock effected on February 17, 2023. The prices set forth in the table below have not been adjusted to reflect any change as a result of the reduction in the number of our shares of common stock to be outstanding as a result of the seven million shares of our common stock to be contributed to our capital by Quanzhong Lin upon effectiveness of the Registration Statement of which this prospectus is a part.
| Year ended December 31, 2024 | High | Low | ||||||
| First Quarter (through February 8, 2024) | $ |
0.80 |
$ |
0.50 | ||||
| Year ended December 31, 2023 | High | Low | ||||||
| First Quarter | $ | 6.86 | $ | 1.60 | ||||
| Second Quarter | $ | 11.01 | $ | 1.17 | ||||
| Third Quarter | $ | 4.50 | $ | 2.25 | ||||
| Fourth Quarter | $ | 2.25 | $ | 1.00 | ||||
| Year ended December 31, 2022 | High | Low | ||||||
| First Quarter | $ | 6.98 | $ | 3.22 | ||||
| Second Quarter | $ | 5.49 | $ | 3.32 | ||||
| Third Quarter | $ | 4.00 | $ | 3.32 | ||||
| Fourth Quarter | $ | 6.80 | $ | 3.35 | ||||
Holders of Our Common Stock
As of February 8, 2024. there were [24,999,842] shares of common stock issued and outstanding. They were held by a total of approximately [650] shareholders of record. The holders of common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders. Holders of the common stock have no pre-emptive rights and no right to convert their common stock into any other securities. There are no redemption or sinking fund provisions applicable to the common stock.
Transfer Agent
The transfer agent for the common stock is Securities Transfer Corporation. The transfer agent’s address is 2901 N. Dallas Parkway, Suite 380, Plano Texas 75093, and its telephone number is +1 (469) 633-0101.
Dividend Policy
No cash dividends were paid on our shares of common stock since we acquired AiXin BVI. We do not foresee declaring any cash dividends on our common stock in the foreseeable future.
Securities Authorized for Issuance under Equity Compensation Plans
In 2019 we adopted the 2019 Equity Incentive Plan (the “2019 Plan”), which authorizes the issuance of shares of common stock for grants of stock options, stock appreciation rights, restricted stock, stock units, bonus stock, dividend equivalents, other stock related awards and performance awards that may be settled in cash, stock, or other property. The 2019 Plan authorizes the issuance of up to 625,000 shares.
We adopted the 2019 Plan to provide a means by which employees, directors, and consultants of our Company and those of our subsidiaries and other designated affiliates, which we refer to together as our affiliates, may be given an opportunity to purchase our common stock, to assist in retaining the services of such persons, to secure and retain the services of persons capable of filling such positions, and to provide incentives for such persons to exert maximum efforts for our success and the success of our affiliates. The material features of the 2019 Plan are outlined below. This summary is qualified in its entirety by reference to the complete text of the 2019 Plan which has been filed with the SEC.
| 40 |
SELECTED HISTORICAL FINANCIAL AND OPERATING DATA
The following tables set forth our summary historical financial data for the periods presented. The following summary financial data for the years ended December 31, 2022 and 2021 are derived from our audited financial statements appearing elsewhere in this prospectus; the summary financial data for the nine months ended September 30, 2023 and 2022 is unaudited.
This summary financial data should be read together with the historical financial statements and related notes to those statements, as well as “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” which are included elsewhere in this prospectus.
Balance Sheet Data
| December 31, | December 31, | September 30, | September 30, | |||||||||||||
| 2022 | 2021 | 2023 | 2022 | |||||||||||||
| Assets | ||||||||||||||||
| Current assets | ||||||||||||||||
| Cash and cash equivalents | $ | 510,128 | $ | 8,556,642 | $ | 412,659 | $ | 5,298,503 | ||||||||
| Restricted cash | 109,772 | 44,211 | 85,676 | 80,303 | ||||||||||||
| Accounts receivable, including related party, net | 562,581 | 45,923 | 250,561 | 196,376 | ||||||||||||
| Other receivables and prepaid expenses | 42,631 | 143,281 | 143,324 | 162,640 | ||||||||||||
| Advances to suppliers | 168,523 | 162,969 | 144,613 | 137,362 | ||||||||||||
| Inventory | 499,252 | 233,454 | 643,623 | 857,754 | ||||||||||||
| Advances to related parties | 83,102 | 19,055 | 384,588 | 92,642 | ||||||||||||
| Total current assets | 1,975,989 | 9,205,535 | 2,065,044 | 6,825,580 | ||||||||||||
| Property and equipment, net | 1,971,793 | 290,148 | 1,658,885 | 1,910,691 | ||||||||||||
| Intangible asset, net | 1,269 | 1,940 | 2,840 | 1,393 | ||||||||||||
| Deferred tax asset | 15,556 | 18,795 | 13,278 | 15,522 | ||||||||||||
| Security deposit | 86,992 | 94,153 | 82,237 | 84,347 | ||||||||||||
| Operating lease right-of-use assets | 999,285 | 2,049,775 | 457,793 | 1,173,616 | ||||||||||||
| Goodwill, net | - | - | - | - | ||||||||||||
| Total assets | $ | 5,050,884 | $ | 11,660,346 | $ | 4,280,077 | $ | 10,011,149 | ||||||||
| Liabilities and stockholders’ equity | ||||||||||||||||
| Current liabilities | ||||||||||||||||
| Accounts payable | $ | 398,469 | $ | 406,163 | $ | 599,373 | $ | 507,993 | ||||||||
| Accounts payable-related party | 165,958 | - | - | 160,911 | ||||||||||||
| Unearned revenue | 139,502 | 171,408 | 466,804 | 153,163 | ||||||||||||
| Taxes payable | 104,100 | 232,637 | 110,246 | 55,942 | ||||||||||||
| Accrued liabilities and other payables | 2,356,490 | 752,400 | 2,136,132 | 5,903,738 | ||||||||||||
| Government grant | 950,371 | - | 898,424 | 921,473 | ||||||||||||
| Loan from third parties | 86,992 | 94,153 | 82,237 | 84,347 | ||||||||||||
| Operating lease liabilities | 883,583 | 848,230 | 439,959 | 772,632 | ||||||||||||
| Advance from related parties | 236,882 | 1,947,154 | 333,919 | 726,650 | ||||||||||||
| Total current liabilities | 5,322,347 | 4,452,145 | 5,067,094 | 9,286,849 | ||||||||||||
| Operating lease liabilities - non-current | 194,725 | 1,138,710 | 158,069 | 382,152 | ||||||||||||
| Total liabilities | 5,517,072 | 5,590,855 | 5,225,163 | 9,669,001 | ||||||||||||
| Total stockholders’ equity (deficit) | (466,188 | ) | 6,069,491 | (945,086 | ) | 342,148 | ||||||||||
| Total liabilities and stockholders’ equity (deficit) | $ | 5,050,884 | $ | 11,660,346 | $ | 4,280,077 | $ | 10,011,149 | ||||||||
| 41 |
Statement of Operations Data:
| Years Ended December 31, | Nine Months Ended September 30, | |||||||||||||||
| 2022 | 2021 | 2023 | 2022 | |||||||||||||
| Sales revenue: | ||||||||||||||||
| Products | $ | 1,851,676 | $ | 742,624 | $ | 2,405,280 | $ | 888,690 | ||||||||
| Advertising | - | 1,944,811 | - | - | ||||||||||||
| Room revenues | 262,605 | 114,086 | 473,167 | 166,278 | ||||||||||||
| Food and beverage revenues | 475,746 | 201,755 | 356,009 | 356,840 | ||||||||||||
| Others | 118,533 | 62,957 | 63,233 | 100,543 | ||||||||||||
| Total revenue, net | 2,708,560 | 3,066,233 | 3,297,689 | 1,512,351 | ||||||||||||
| Operating costs and expenses | ||||||||||||||||
| Cost of goods sold | 1,103,160 | 535,485 | 1,136,411 | 493,905 | ||||||||||||
| Hotel operating costs | 1,739,948 | 744,594 | 1,387,993 | 1,334,041 | ||||||||||||
| Selling | 787,637 | 470,798 | 628,754 | 583,438 | ||||||||||||
| General and administrative | 1,395,008 | 1,033,830 | 1,268,119 | 752,113 | ||||||||||||
| (Reversal of) provision for bad debts | (119,274 | ) | (63,076 | ) | (10,250 | ) | 115,495 | |||||||||
| Stock-based compensation | 371,540 | 371,540 | 278,655 | 278,655 | ||||||||||||
| Total operating costs and expenses | 5,278,019 | 3,093,171 | 4,689,682 | 3,557,647 | ||||||||||||
| Loss from operations | (2,569,459 | ) | (26,938 | ) | (1,391,993 | ) | (2,045,296 | ) | ||||||||
| Non-operating income (expenses) | ||||||||||||||||
| Interest income | 4,876 | 4,113 | 692 | 3,636 | ||||||||||||
| Impairment loss | (3,823,770 | ) | - | - | (3,823,770 | ) | ||||||||||
| Other income | 51,856 | 63,064 | 52,395 | 41,789 | ||||||||||||
| Government grant income | - | - | 284,321 | - | ||||||||||||
| Other expenses | (31,651 | ) | (33,154 | ) | (5,022 | ) | (29,577 | ) | ||||||||
| Total non-operating income (expenses), net | (3,798,689 | ) | 34,023 | 332,386 | (3,807,922 | ) | ||||||||||
| (Loss) income before income tax | (6,368,148 | ) | 7,085 | (1,059,607 | ) | (5,853,218 | ) | |||||||||
| Income tax expense | 1,097 | 274,321 | 5,879 | 643 | ||||||||||||
| Net loss | (6,369,245 | ) | (267,236 | ) | (1,065,486 | ) | (5,853,861 | ) | ||||||||
| Other comprehensive items | ||||||||||||||||
| Foreign currency translation (loss) gain | (537,974 | ) | 122,283 | 42,473 | (152,137 | ) | ||||||||||
| Comprehensive loss | $ | (6,907,219 | ) | $ | (144,953 | ) | $ | (1,023,013 | ) | $ | (6,005,998 | ) | ||||
| 42 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Forward Looking Statements
The following discussion should be read in conjunction with our consolidated audited financial statements and related notes thereto and other financial information appearing elsewhere in this prospectus. In addition to historical information, the following discussion contains forward-looking statements that involve risks, uncertainties and assumptions. Where possible, we have tried to identify these forward-looking statements by using words such as “anticipate,” “believe,” “intends,” or similar expressions. As a result of many factors, including those factors set forth in the “Risk Factors” section of this prospectus, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in this prospectus.
This prospectus contains statements that we believe are, or may be considered to be, “forward-looking statements”. All statements other than statements of historical fact included in this prospectus regarding the prospects of our industry or our prospects, plans, financial position or business strategy, may constitute forward-looking statements. In addition, forward-looking statements generally can be identified by the use of forward-looking words such as “may,” “will,” “expect,” “intend,” “estimate,” “foresee,” “project,” “anticipate,” “believe,” “plans,” “forecasts,” “continue” or “could” or the negatives of these terms or variations of them or similar terms. Furthermore, such forward-looking statements may be included in various filings that we make with the Securities and Exchange Commission or press releases or oral statements made by or with the approval of one of our authorized executive officers. Although we believe that the expectations reflected in these forward-looking statements are reasonable, we cannot assure you that these expectations will prove to be correct. These forward-looking statements are subject to certain known and unknown risks and uncertainties, as well as assumptions that could cause actual results to differ materially from those reflected in these forward-looking statements. Readers are cautioned not to place undue reliance on any forward-looking statements contained herein, which reflect management’s opinions only as of the date hereof. Except as required by law, we undertake no obligation to revise or publicly release the results of any revision to any forward-looking statements. You are advised, however, to consult any additional disclosures we make in our reports to the SEC. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the cautionary statements contained in this prospectus.
You should read the matters described in “Risk Factors” and the other cautionary statements made in this prospectus, and incorporated by reference herein, as being applicable to all related forward-looking statements wherever they appear in this prospectus. We cannot assure you that the forward-looking statements in this prospectus will prove to be accurate and therefore prospective investors are encouraged not to place undue reliance on forward-looking statements.
Corporate History
AiXin Life International, Inc. (“AiXin Life” or “AiXin Colorado”) was incorporated under the laws of the State of Colorado on August 31, 2001. In February 2, 2017, Mr. Quanzhong Lin (Mr. Lin) purchased shares representing 65.0% of our then outstanding shares for $300,000. In December 2017, we issued additional shares of common stock to Mr. Lin increasing the percentage of our outstanding shares then owned by him to 80%, the sole stockholder of AiXin (BVI) International Group Co., Ltd. a British Virgin Islands corporation (“AiXin BVI”), for all of the outstanding shares of AiXin BVI. As a result, AiXin BVI became our wholly-owned subsidiary, and through AiXin BVI we now own all of the outstanding shares of HK AiXin International Group Co., Limited, a Hong Kong limited company (“AiXin HK”), which in turn owns all of the outstanding shares of Chengdu AiXinZhonghong Biological Technology Co., Ltd., a Chinese limited company (“AiXinZhonghong”), which markets and sells premium-quality nutritional products in China. AiXinZhonghong began distributing nutritional products in 2013.
For accounting purposes, the acquisition of AiXin BVI was accounted for as a reverse acquisition and treated as a recapitalization of the Company effected by a share exchange, with AiXin BVI as the accounting acquirer. Since neither AiXin BVI nor AiXin HK had operations prior to the date on which we acquired AiXin BVI, the historical consolidated financial statements of AiXinZhonghong are now the historical consolidated financial statements of the Company. The assets and liabilities of AiXinZhonghong were brought forward at their book value and no goodwill was recognized.
| 43 |
In September 2021 we completed the acquisition of nine pharmacies located in Chengdu. Since that time we have added additional pharmacies and currently operate 25 pharmacies. We utilize these pharmacies to distribute health and wellness products and serve as learning centers for our clients. The staff at our pharmacies is being trained to reach out to customers and make them aware of the benefits of our health and wellness products. In addition, the pharmacies serve as convenient locations where repeat customers can buy our products.
In September 2022, we acquired Yunnan Runcangsheng based in Yunnan Province which engages in the research, development, manufacture and wholesale distribution of health foods. Yunnan Runcangsheng operates a 13,000 square meter production facility, which houses R&D centers, extraction facilities, preparation workshops and a warehouse. Yunnan Runcangsheng has more than 30 sub brands and operates planting facilities where it grows some of the key ingredients used in its products. Many of the products it has developed are specifically targeted to alleviate symptoms associated with the increasingly competitive and pressured lifestyle of the Chinese middle class.
In addition to our acquisitions in the health and nutritional sector, in July 2021, we acquired a hotel located in the Jinniu District, Chengdu City. We envision utilizing the hotel to conduct marketing events and seminars for our customers, and training sessions for our personnel at which we introduce new products and services intended to promote healthy living.
We intend to look for additional opportunities to profit from the growing healthcare market in China. Though currently we are not party to any agreements, we will explore, among other opportunities, expanding our product line through internal research and acquiring complementary products from third parties, acquiring additional pharmacies and other retail outlets and operating nursing homes and possibly clinics which provide medical care to clients.
Our Business
We are focused on providing health and wellness products to the growing middle class in China. We currently develop, manufacture, market and sell premium-quality healthcare, nutritional products and wellness supplements, including herbs and greens, traditional Chinese remedies, functional products, such as weight management tools, probiotics, foods and drinks. We also offer products purchased from third parties. In addition, we have provided advertising and marketing services to clients which engage us to market and distribute their products, though we are likely to decrease this activity as we focus on distributing out products. We offer our products and those of clients for which we provide marketing services, through a diversified, omni-channel business model which generates revenues through retail and wholesale product sales, through company-owned pharmacies, direct marketing and online activities. Our marketing approach emphasizes proactively approaching customers such as by hosting marketing events for clients, which we believe is ideally suited to marketing the products we offer because sales of healthcare, nutritional products and supplements are strengthened by ongoing personal contact and support, coaching and education among the Company and our clients towards how to achieve a healthy and active lifestyle.
We believe the competitive strengths that will enable us to grow in the health and wellness market include our ability to design and manufacture products that are responsive to consumers’ needs as the lifestyle of China’s middle class evolves, our coordinated omni-channel distribution network where we enable consumers to obtain the information they need to improve their lifestyle on our website, at our pharmacies and through individual meetings with our team members.
Our ability to operate profitably and generate positive cash flow will be determined by our ability to attract a large and loyal customer base and provide the information and products they need cost effectively. Our revenue will largely be determined by our ability to achieve and maintain a strong brand name and company image, the volume of products we sell and the prices we can charge for such products, which will require that we compete effectively. Our costs will largely be determined by the cost of raw materials and acquired inventory, the labor used to design and manufacture products, and the costs incurred to deliver these products to the consumer.
Our Current Strategy
We intend to build a reputation as a provider of premium health and wellness products that seeks to improve our customers’ health and well-being. Our objective is to offer a variety of products for consumers interested in living well, whether they are looking to treat a health-related issue or simply maintain their overall wellness. Our premium, value-added offerings include both proprietary products developed and manufactured by us as well as products acquired from or sold on behalf of third parties. We believe our range of products and ability to develop new products, combined with the customer support and service we offer, differentiate us and allow us to effectively compete against food, drug and mass channel players, specialty stores, independent vitamin, supplement and natural food shops and online retailers.
| 44 |
We believe that the innovative capability we have as a result of our acquisition of Yunnan Runcangsheng is a significant competitive advantage. The vertical integration this gives us allows us to monitor the changing buying patterns of our customers to quickly and efficiently design new products they desire and drives increased efficiencies in manufacturing and supply chain.
Our retail strategy is to deliver a compelling experience at every customer touch point. We recognize that the growing Chinese middle class is searching for product expertise and knowledgeable customer service in choosing health and wellness products. We differentiate ourselves from competitors by educating our team members as to the benefits of our products with regular training that focuses on solution-based selling. With this training and information regarding a customer’s purchasing habits available in our data bases, members of our sales team can engage customers in conversation, share product information and testimonials, recommend solutions and help customers add complimentary products and build wellness regimens.
Our loyalty programs will allow us to develop and maintain a large and loyal customer base, provide targeted offers and information, and connect with our customers on a regular basis. We harness data generated by these programs to better understand customers’ buying behaviors and needs, so we can deliver a stronger experience, bring like-minded consumers into the channel and make well-informed decisions about the business. We currently collect, maintain and use customers’ data in accordance with all applicable laws and regulations and will strive to do so in the future.
We believe our diversified, omni-channel model, which includes company-owned pharmacies, direct marketing, large scale marketing events, and on-line activities, differentiates us from online-only competitors. Our strategy is to give consumers a seamless, integrated experience across all distribution channels. We believe our sales offices and pharmacies are a competitive advantage which enhances our ability to satisfy our customers, for example, by, allowing customers to experience our products and get expert advice from a coach. Our omni-channel model can enhance the customer experience and increase the lifetime value of our customers and we are implementing strategies to increase the use of on-line promotional activities to enhance our customers’ experiences.
Our business is dependent on consumer demand for our products and services. We believe that the significant growing middle class and changing lifestyles in China offer an opportunity to provide consumers with products that will enable them to maintain their health and well-being and deal with the ailments that accompany their changing lifestyles. Many of these customers are highly sensitive to the cost of our products and lack the knowledge to choose the optimal products. We believe that the combination of information and products we will provide will best serve to meet our customers’ needs.
| 45 |
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of our financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent liabilities. On an on-going basis, management evaluates past judgments and estimates, including those related to bad debts, accrued liabilities, and contingencies. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. We describe below those critical accounting policies that, as a result of judgments, uncertainties, uniqueness and complexities of the underlying accounting standards and operation involved could result in material changes to our financial position or results of operations under different conditions or using different assumptions.
Nine Months ended September 30, 2023 and 2022
The following table sets forth the results of our operations for the periods indicated as a percentage of net revenue. Because Runcangsheng was acquired in September 2022, it did not contribute to our financial results for the nine months ended September 30, 2022. Certain columns may not add due to rounding:
| Nine Months Ended September 30, | ||||||||||||||||
| 2023 | 2022 | |||||||||||||||
| $ | % of Revenue | $ | % of Revenue | |||||||||||||
| Revenue | $ | 3,297,689 | 100 | % | $ | 1,512,351 | 100 | % | ||||||||
| Operating costs and expenses | 4,689,682 | 142 | % | 3,557,647 | 235 | % | ||||||||||
| Income (loss) from operations | (1,391,993 | ) | (42 | )% | (2,045,296 | ) | (135 | )% | ||||||||
| Non-operating income (expense), net | 332,386 | 10 | % | (3,807,922 | ) | (252 | )% | |||||||||
| Loss before income tax | (1,059,607 | ) | (32 | )% | (5,853,218 | ) | (387 | )% | ||||||||
| Income tax expense | 5,879 | 0.2 | % | 643 | 0.04 | % | ||||||||||
| Net loss | $ | (1,065,486 | ) | (32 | )% | $ | (5,853,861 | ) | (387 | )% | ||||||
The following table shows our operations by business segment for the nine months ended September 30, 2023 and 2022.
| For the Nine Months Ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| Net revenue | ||||||||
| Products | $ | 1,064,668 | $ | 371,016 | ||||
| Pharmacies | 802,118 | 517,674 | ||||||
| Hotel | 892,409 | 623,661 | ||||||
| Manufacture and sale | 538,494 | - | ||||||
| Total revenues, net | $ | 3,297,689 | $ | 1,512,351 | ||||
| Operating costs and expenses | ||||||||
| Products | ||||||||
| Cost of goods sold | $ | 367,237 | $ | 98,809 | ||||
| Operating expenses | 1,249,461 | 961,885 | ||||||
| Pharmacies | ||||||||
| Cost of goods sold | 446,967 | 395,096 | ||||||
| Operating expenses | 361,200 | 467,315 | ||||||
| Hotel | ||||||||
| Hotel operating costs | 1,387,993 | 1,334,041 | ||||||
| Operating expenses | 106,219 | 300,501 | ||||||
| Manufacture and sale | ||||||||
| Cost of goods sold | 322,207 | - | ||||||
| Operating expenses | 448,398 | - | ||||||
| Total operating costs and expenses | $ | 4,689,682 | $ | 3,557,647 | ||||
| Loss from operations | ||||||||
| Products | $ | (552,030 | ) | $ | (689,678 | ) | ||
| Pharmacies | (6,049 | ) | (344,737 | ) | ||||
| Hotel | (601,803 | ) | (1,010,881 | ) | ||||
| Manufacture and sale | (232,111 | ) | - | |||||
| Loss from operations | $ | (1,391,993 | ) | $ | (2,045,296 | ) | ||
| 46 |
Revenue
Revenue was $3,297,689 in the nine months ending September 30, 2023, compared to $1,512,351 in the same period of 2022, an increase of $1,785,338 or 118%. The increase in revenue was mainly due to increases in direct sales of our nutritional products, increases in revenues from our hotel and pharmacies, and the generation of revenue by Runcangsheng which we did not own in the nine months of 2022. For the nine months ended September 30, 2023, we had $2,405,280 in product revenues (of which $1,064,668 were from direct sales, $802,118 were from sales at our pharmacies and $538,494 from sales by Runcangsheng) and hotel revenue of $892,409. For the nine months ended September 30, 2022, we had $888,690 product revenues (of which $371,016 were from direct sales and $517,674 were from the sales at our pharmacies), and hotel revenue of $623,661.
Operation Costs and Expenses
Cost of Goods Sold
Cost of goods sold was $1,136,411 for the nine months ended September 30, 2023, compared to $493,905 for the nine months ended September 30, 2022, an increase of $642,506 or 130%. The increase in our cost of goods sold is attributable to the increase in direct product sales, pharmacy sales and sales by Runcangsheng. The cost of goods sold for our direct product sales as a percentage of sales was 34% in 2023, compared to 27% for 2022. The cost of goods sold for products sold through our pharmacies as a percentage of pharmacy product sales was 56% in 2023, compared to 76% in 2022. The cost of goods sold as a percentage of sales by Runcangsheng was 60% in 2023, and no comparable costs were incurred in the nine months ended September 30, 2022. We were able to lower our cost of goods sold and increase our profit margin at out pharmacies significantly as a result of the manufacturing business we acquired when we purchased Runcangsheng, which enabled us to sell products we manufactured.
Hotel Operating Costs
Hotel operating costs were $1,387,993 and $1,334,041 for the nine months ended September 30, 2023 and 2022. The increase in hotel operating costs was mainly due to the increase in hotel sales but was partly offset by decreases in the cost of foods and fruits.
Operating Expenses
Operating expenses were $2,165,278 for the nine months ended September 30, 2023, compared to $1,729,701 for the same period of 2022, an increase of $435,577 or 25%. The increase in operating expenses was mainly due to the inclusion of the operating expenses of Runcangsheng.
Loss from Operations
Loss from operations was $1,391,993 in the nine months ended September 30, 2023, compared to $2,045,296 in the same period of 2022, a decrease of $653,303 or 32%. The decrease in our loss from operations for 2023 was due to the increases in our revenues which decreased the losses from our direct sales activities, pharmacies and hotel, partly offset by the loss incurred by our new subsidiary, Runcangsheng.
Non-operating Income
Non-operating income was $332,386 for the nine months ended September 30, 2023, compared to non-operating expenses of $3,807,922 for the nine months ended September 30, 2022. For the nine months ended September 30, 2023, we had interest income of $692 and other income $336,716, partly offset by other expenses of $5,022. For the nine months ended September 30, 2023, other income mainly consisted of a government grant of $284,321. For the nine months ended September 30, 2022, we had an impairment loss of $3,823,770 attributed to the acquisition of Runcangsheng and other expenses of $29,577, partly offset by interest income of $3,636 and other income of $41,789.
| 47 |
Income Tax Expense
Income tax expense was $5,879 and $643 for the nine months ended September 30, 2023 and 2022, respectively, an increase of $5,236 or 814% for the nine months ended September 30, 2023 compared with the same period of 2022.
Net Loss
Our net loss for the nine months ended September 30, 2023 was $1,065,486, compared to a net loss of $5,853,861 in the same period of 2022, a decrease of $4,788,375 or 82%. The decrease in the nine months ended September 30, 2023 was mainly due to increased sales and the absence of an impairment loss, partly offset by increased operating costs and expenses as explained above.
Results of Operations for the years ended December 31, 2022 and 2021
The following table sets forth the results of our operations for the periods indicated as a percentage of net revenue, certain columns may not add due to rounding:
| Years Ended December 31, | ||||||||||||||||
| 2022 | 2021 | |||||||||||||||
| $ | % of Revenue | $ | % of Revenue | |||||||||||||
| Revenue | $ | 2,708,560 | 100 | % | $ | 3,066,233 | 100 | % | ||||||||
| Operating costs and expenses | 5,278,019 | 195 | % | 3,093,171 | 101 | % | ||||||||||
| Loss from operations | (2,569,459 | ) | (95 | )% | (26,938 | ) | (1 | )% | ||||||||
| Non-operating income (expenses), net | (3,798,689 | ) | (140 | )% | 34,023 | 1 | % | |||||||||
| Income (loss) before income tax | (6,368,148 | ) | (235 | )% | 7,085 | (0.2 | )% | |||||||||
| Income tax expense | 1,097 | 0.04 | % | 274,321 | (9 | )% | ||||||||||
| Net loss | $ | (6,369,245 | ) | (235 | )% | $ | (267,236 | ) | (9 | )% | ||||||
The following table shows our operations by business segment for the years ended December 31, 2022 and 2021.
| For the Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Net revenue | ||||||||
| Advertising and products | $ | 823,930 | $ | 2,406,988 | ||||
| Pharmacies | 789,347 | 280,447 | ||||||
| Hotel | 856,884 | 378,798 | ||||||
| Manufacture and Sale | 238,399 | - | ||||||
| Total revenues, net | $ | 2,708,560 | $ | 3,066,233 | ||||
| Operating costs and expenses | ||||||||
| Advertising and products | ||||||||
| Cost of goods sold | $ | 171,345 | $ | 316,750 | ||||
| Operating expenses | 1,526,246 | 1,344,543 | ||||||
| Pharmacies | ||||||||
| Cost of goods sold | 578,092 | 218,735 | ||||||
| Operating expenses | 622,835 | 252,513 | ||||||
| Hotel | ||||||||
| Hotel operating costs | 1,739,948 | 744,594 | ||||||
| Operating expenses | 310,902 | 216,036 | ||||||
| Manufacture and Sale | ||||||||
| Cost of goods sold | 353,723 | - | ||||||
| Operating expenses | (25,072 | ) | - | |||||
| Total operating costs and expenses | $ | 5,278,019 | $ | 3,093,171 | ||||
| (Loss) income from operations | ||||||||
| Advertising and products | $ | (873,661 | ) | $ | 745,695 | |||
| Pharmacies | (411,580 | ) | (190,801 | ) | ||||
| Hotel | (1,193,966 | ) | (581,832 | ) | ||||
| Manufacture and Sale | (90,252 | ) | - | |||||
| Loss from operations | $ | (2,569,459 | ) | $ | (26,938 | ) | ||
| 48 |
Revenue
Revenue was $2,708,560 for the year ending December 31, 2022, compared to $3,066,233 in 2021, a decrease of $357,673 or 12%. The decrease in revenue was mainly due to the absence of advertising revenues. The imposition of COVID-19 restrictions prevented us from staging the types of events at which we market nutritional products. The decrease in advertising revenues was partly offset by increases in the revenues generated by our pharmacies and hotel and revenues resulting from the acquisition of Runcangsheng. Revenues and expenses as a result of the acquisition of our pharmacies, hotel and Runcangsheng began to be included in our financial results from their respective dates of acquisition. We completed the acquisition of our hotel in August 2021, our pharmacies in September 2021 and Runcangsheng in September 2022. For the year ended of December 31, 2022, we had no advertising revenues and $1,851,676 in product revenues (of which $823,930 were from direct sales, $789,347 represented sales at our pharmacies and $238,399 represented sales from Runcangsheng), and hotel revenue of $856,884. For 2021, we had advertising and product revenues of $2,406,988, pharmacies revenue of $280,447, and hotel revenue of $378,798.
Operating Costs and Expenses
Cost of Goods Sold
Cost of goods sold was $1,103,160 for the year ended December 31, 2022, compared to $535,485 for the year ended December 31, 2021, an increase of $567,675 or 106%. The increase in our cost of goods sold is attributable to an increase in pharmacy products sales, sales by Runcangsheng, and direct product sales as opposed to advertising revenues for which there is no cost of goods. The cost of goods sold for our direct product sales as a percentage of sales was 21% in 2022, compared to 69% for 2021. The cost of goods sold for products sold through our pharmacies as a percentage of pharmacy product sales was 73% in 2022, compared to 78% in 2021.
Hotel Operating Costs
Hotel operating costs were $1,739,948 for the year ended December 31, 2022. compared to $744,594 for 2021. The increase in hotel operating costs in 2022 was mainly due to the increased revenue as a result of increased activity at our hotel and the inclusion of the results of operations of the hotel for all of 2022 as opposed to the months beginning in August 2021.
Operating Expenses
Operating expenses were $2,434,911 for the year ended December 31, 2022, compared to $1,813,092 for the same period of 2021, an increase of $621,819 or 34%. The increase in operating expenses was mainly due to the inclusion of the operating expenses of our pharmacies and hotel for the entire year as opposed to only a portion of 2021 and the expenses of Runcangsheng.
Loss from Operations
Loss from operations was $2,569,459 in the year ended December 31, 2022, compared to $26,938 in 2021, an increase of $2,542,521 or 9,438%. The increase in our loss from operations for 2022 was due to the losses incurred by each of our segments. All of our operations and in particular our direct marketing activities and hotel business were materially adversely impacted by travel and work restrictions and limits on the number of people that might gather in one place imposed on a temporary basis in China and Chengdu to limit the spread of COVID-19.
Non-Operating Income (Expense)
Non-operating expense was $3,798,689 for the year ended December 31, 2022, compared to non-operating income of $34,023 for the year ended December 31, 2021. For the year ended December 31, 2022, we had an impairment loss of goodwill of $3,823,770 arising from the acquisition of Runcangsheng, and other expenses of $31,651,partly offset by interest income of $4,876 and other income of $51,856. For the year ended December 31, 2021, we had interest income of $4,113 and other income of $63,064 and other expenses of $33,154. The determination to write off all the goodwill resulting from the acquisition of Runcangsheng reflects the need to provide it with capital to achieve its business goals.
Income Tax Expense
Income tax expense was $1,097 and $274,321 for the years ended December 31, 2022 and 2021, respectively, a decrease of $273,224 or 100% for the year ended December 31, 2022 compared with 2021.
Net Loss
Our net loss for the year ended December 31, 2022 was $6,369,245, compared to $267,236 in 2021, an increase of $6,102,009 or 2,283%. The increased net loss in 2022 was mainly due to decreased sales revenue, increased operating costs and expense, and an impairment loss of $3,823,770 as explained above. The impairment loss was the result of our decision to write-off the entire amount of the goodwill resulting from the acquisition of Runcangsheng as the Company was in the development stage and will need additional capital to achieve its business goals.
| 49 |
Liquidity and Capital Resources
Summary of cash flows
Years ended December 31, 2022 and 2021
During the year ended of December 31, 2022, we used $1,624,565 in operations. As of December 31, 2022, cash and cash equivalents were $510,128 (excluding $109,772 of restricted cash), compared to $8,556,642 (excluding $44,211 of restricted cash) as of December 31, 2021. At December 31, 2022, we had a working capital deficit of $3,346,358 compared to working capital of $4,753,390 at December 31, 2021.
The following is a summary of cash provided by or used in each of the indicated types of activities during the years ended December 31, 2022 and 2021, respectively.
| December 31, 2022 | December 31, 2021 | |||||||
| Net cash used in operating activities | $ | (1,624,565 | ) | $ | (57,804 | ) | ||
| Net cash used in investing activities | $ | (3,522,369 | ) | $ | (4,431,513 | ) | ||
| Net cash (used in) provided by financing activities | $ | (2,389,593 | ) | $ | 5,221,864 | |||
Net cash used in operating activities
For the year ended December 31, 2022, net cash used in operating activities was $1,624,565. This reflects our net loss of $6,369,245, increased by a goodwill impairment of $3,823,770, non-cash related expenses including depreciation and amortization expense of $185,565, the change in deferred tax of $1,858, operating lease expenses of $837,425, stock-based compensation of $371,540, and an inventory impairment of $54,899, less changes in working capital of $576,330. The cash outflow from changes in working capital mainly resulted from an increase in outstanding accounts receivable of $425,961, payments of lease liabilities of $710,865, a change in accounts payable of $71,153, unearned revenue of $24,401 and taxes payable of $137,711, which was partly offset by cash inflows from accrued liabilities and other payables of $289,868, other receivable and prepaid expense of $109,439, inventory of $150,872, accounts payable to related party of $140,608, and advances to suppliers of $103,016.
For the year ended December 31, 2021, net cash used in operating activities was $57,804. This reflects our net loss of $267,236, adjusted by non-cash related expenses including depreciation and amortization expense of $96,106, operating lease expense of $411,607 and stock-based compensation of $371,540, and then decreased by change in deferred tax of $18,570, and changes in working capital of $651,251. The cash outflow from changes in working capital mainly resulted from unearned revenue of $122,897, payments of taxes payable of $57,467, payments of lease liabilities of $473,508 and payments of accrued liabilities of $142,027, partly offset by cash inflow from other receivables and prepaid expenses $94,992 and inventory of $69,738.
Net cash used in investing activities
For the year ended December 31, 2022, net cash used in investing activities was $3,522,369, mainly as a result of $3,812,027 paid for the acquisition of Runcangsheng, and purchases of property and equipment of $156,723, partly offset by cash acquired in connection with the acquisition of Runcangsheng of $446,381.
For the year ended December 31, 2021, net cash used in investing activities was $4,431,513, mainly for the acquisitions of our hotel and pharmacies of $4,517,620, partly offset by cash acquired in connection with the acquisitions of $87,448.
Net cash provided by (used in) financing activities
For the year ended December 31, 2022, net cash used in financing activities was $2,389,593 as a result of payments made against advances from related parties of $2,389,593.
For the year ended December 31, 2021, net cash provided by financing activities reflected a capital contribution of $4,386,070 and the proceeds from advances from related parties of $1,204,442, partially offset by the repayment of loans from third parties of $368,648.
| 50 |
Nine months ended September 30, 2023 and 2022
During the nine months ended September 30, 2023, we used $219,874 in operations. As of September 30, 2023, cash and cash equivalents were $412,659 (excluding $85,676 of restricted cash), compared to $510,128 (excluding $109,772 of restricted cash) as of December 31, 2022. At September 30, 2023, we had a working capital deficit of $3,002,050 compared to $3,346,358 at December 31, 2022.
The following is a summary of cash provided by or used in each of the indicated types of activities during the nine months ended September 30, 2023 and 2022, respectively.
| September 30, 2023 | September 30, 2022 | |||||||
| Net cash used in operating activities | $ | (219,874 | ) | $ | (1,412,550 | ) | ||
| Net cash (used in) provided by investing activities | $ | (218,556 | ) | $ | 393,896 | |||
| Net cash provided by (used in) financing activities | $ | 347,449 | $ | (1,525,860 | ) | |||
Net cash used in operating activities
For the nine months ended September 30, 2023, net cash used in operating activities was $219,874. This reflects our net loss of $1,065,486, adjusted by non-cash related expenses including depreciation and amortization expense of $308,114, a change in deferred tax of $1,481, a bad debt reversal of $10,250, an inventory impairment of $8,558, operating lease expenses of $618,085, government grant income $284,321 and stock-based compensation of $278,655, and then decreased by changes in working capital of $74,710. The cash outflow from changes in working capital mainly resulted from increases in other receivables and prepaid expense, including related parties of $116,468, in inventory of $186,658, in accounts payable from related party of $162,768, and in accrued liabilities and other payables of $136,638, and payments of lease liabilities of $500,056, partly offset by cash inflows from accounts receivable, including from related parties in the amount of $301,626, cash inflows from advances to suppliers of $133,520, cash inflows from accounts payable of $233,112, cash inflow from unearned revenue of $347,347, and taxes payable of $12,273.
For the nine months ended September 30, 2022, net cash used in operating activities was $1,412,550. This reflects our net loss of $5,853,861, increased by non-cash related expenses including depreciation and amortization expense of $82,921, the change in deferred tax of $1,419, bad debt expense of $115,495, operating lease expense of $632,496, stock-based compensation of $278,655, and impairment loss of 3,823,770, less changes in working capital of $493,445. The cash outflow from changes in working capital mainly resulted from an increase in accounts receivable of $126,720, payments of lease liabilities of $525,979, a change in inventory of $192,791, unearned revenue of $9,444 and taxes payable of $187,009, partly offset by cash inflows from accrued liabilities and other payables of $232,910, other receivable and prepaid expense of $100,801, accounts payable of $201,749, and advances to suppliers of $13,038.
Net cash used in investing activities
For the nine months ended September 30, 2023 and 2022, net cash used in investing activities was $218,556 included $212,607 for the purchase of fixed assets, $2,608 for the purchase of intangible assets, and $3,341 cash disposed of at the termination of a non-operating subsidiary. For the nine months ended September 30, 2022, net cash provided by investing activities was $393,896, mainly as a result of cash acquired in connection with the acquisition of subsidiaries of $446,381, partly offset by purchases of property and equipment of $52,485.
| 51 |
Net cash provided by financing activities
For the nine months ended September 30, 2023, net cash provided by financing activities were the result of capital contributions of $142,200 and proceeds from government grant of $284,321, partly offset by payments made against advances from related parties of $79,072. For the nine months ended September 30, 2022, net cash used in by financing activities was $1,525,860 as a result of payments made against advances from related parties of $1,525,860.
We substantially depleted our available cash and working capital during 2022 supporting our operations and completing the acquisition of Runcangsheng, further, in late 2023, we repaid to the Yizu Autonomous County People’s Government $978,647 received by Runcangsheng as a grant during the first half of 2022, and generated a $232,111 loss from operations in the nine months of 2023. It is likely that Runcangsheng will require additional capital to achieve its short-term operational goals and long range business plans. Further, we may need additional capital to maintain our other businesses. We may also have to raise additional financing as our working capital requirements are expected to increase in line with the growth of our business. In the past we have funded our operations through proceeds from private placements of equity and advances from our principal shareholder. Should we require capital to fund our business, we intend to finance our business by raising additional capital or, when available, borrowing additional funds. Additional issuances of equity or convertible debt securities will result in dilution to our current shareholders and could cause the price of our common stock to decrease. Further, such securities might have rights, preferences or privileges senior to our common stock. Additional financing may not be available upon acceptable terms, or at all. If adequate funds are not available or are not available on acceptable terms, we may not be able to take advantage of prospective new business endeavors or opportunities, which could significantly and materially restrict our business operations.
We are subject to all of the substantial risks inherent in the development of a new business enterprise within an extremely competitive industry. Due to the absence of a long-standing operating history and the emerging nature of the markets in which we compete, we anticipate operating losses until we can successfully implement our business strategy. Our revenue model is new and evolving, and we cannot be certain that it will be successful. The potential profitability of our business model is unproven. We may never ever achieve profitable operations. Our future operating results depend on many factors, including demand for our products, the level of competition, and the ability of our officers to manage our business and growth. As a result of the emerging nature of the market in which we compete, we may incur operating losses until such time as we can develop a substantial and stable revenue base. Additional development expenses may delay or negatively impact our ability to generate profits. Accordingly, we cannot assure you that our business model will be successful or that we can sustain revenue growth, achieve or sustain profitability, or continue as a going concern.
| 52 |
Our ability to obtain funds through the issuance of debt or equity is dependent upon the state of the financial markets at such time as we may seek to raise funds. The state of the capital market markets may be adversely impacted by various risks and uncertainties, including, but not limited to future and current impacts of global events such as COVID-19, the war in the Ukraine and the conflict in Palestine, increases in inflation and other risks detailed herein.
Impact of Inflation
Our results of operations may be affected by inflation, particularly rising prices for labor, raw materials, products and other operating costs, if we cannot pass such increases along to our customers in the form of higher prices for our products and services. Generally, our inventory turns multiple times per year and we anticipate that we will be able to increase prices on products to reflect increases in the cost of inventory.
Contingencies
We have no long-term fixed contractual obligations or commitments other than our operating lease obligations detailed in Note 11 to our financial statements.
Foreign Currency Translation Risk
Our operations are located in China, which may give rise to significant foreign currency risks from fluctuations and the degree of volatility in foreign exchange rates between the U.S. dollar and the Chinese Renminbi (“RMB”). All of our sales are in RMB. In the past years, RMB continued to appreciate against the U.S. dollar. As of December 31, 2022, the market foreign exchange rate had decreased to RMB6.8972 to one U.S. dollar. Our financial statements are translated into U.S. dollars using the closing rate method. The balance sheet items are translated into U.S. dollars using the exchange rates at the respective balance sheet dates. The capital and various reserves are translated at historical exchange rates prevailing at the time of the transactions while income and expenses items are translated at the average exchange rate for the period. All translation adjustments are included in accumulated other comprehensive income in the statement of equity. The foreign currency translation gain (loss) for the years ended December 31, 2022 and 2021 was a loss of $537,974 and a gain of $122,283, respectively.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements (as that term is defined in Item 303(a)(4)(ii) of Regulation S-K) as of September 30, 2023 that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources.
Contingencies
Our operations are conducted in the PRC and are subject to specific considerations and significant risks not typically associated with companies in North America and Western Europe. These include risks associated with, among others, the political, economic and legal environments in China and foreign currency exchange rates. Our results may be adversely affected by changes in PRC government policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad and rates and methods of taxation, among other things.
| 53 |
Our sales, purchases and expense transactions in China are denominated in RMB and all of our assets and liabilities in China are also denominated in RMB. The RMB is not freely convertible into foreign currencies under the current PRC law. In China, foreign exchange transactions are required by law to be transacted only by authorized financial institutions. Remittances in currencies other than RMB may require certain supporting documentation in order to affect the remittance.
Significant Accounting Policies
Our management’s discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which were prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as the reported net sales and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates and assumptions. We base our estimates on historical experience and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 to our consolidated financial statements, we believe the following accounting policies are the most critical to assist you in fully understanding and evaluating this management discussion and analysis.
Basis of Presentation
The accompanying financial statements are prepared in conformity with U.S. Generally Accepted Accounting Principles (“US GAAP”). The functional currency of AiXin is Chinese Renminbi (“RMB”). The accompanying financial statements are translated from RMB and presented in U.S. dollars (“USD”).
Accounts Receivable
We maintain an allowance for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of these reserves. As of September 30, 2023 and December 31, 2022, the bad debt allowance was $247,753 and $272,550, respectively.
Use of Estimates
In preparing financial statements in conformity with US GAAP, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements, as well as the reported amounts of revenues and expenses during the reporting period.
Significant estimates, required by management, include the recoverability of long-lived assets, allowance for doubtful accounts, and the reserve for obsolete and slow-moving inventories. Actual results could differ from those estimates.
Revenue Recognition
ASU No. 2014-09, Revenue from Contracts with Customers (“Topic 606”), became effective for us on January 1, 2018. Our revenue recognition disclosure reflects updated accounting policies that are affected by this new standard. We applied the “modified retrospective” transition method for open contracts for the implementation of Topic 606. As revenues are and have been primarily from the delivery of products and the performance of services, and we have no significant post-delivery obligations, this did not result in a material recognition of revenue on the accompanying consolidated financial statements for the cumulative impact of applying this new standard. We made no adjustments to previously-reported total revenues, as those periods continue to be presented in accordance with our historical accounting practices under Topic 605, Revenue Recognition.
| 54 |
Revenue from sale of goods under Topic 606 is recognized in a manner that reasonably reflects the delivery of our products and services to customers in return for expected consideration and includes the following elements:
| ● | executed contract(s) with customers that we believe are legally enforceable; | |
| ● | identification of performance obligation in the respective contract; | |
| ● | determination of the transaction price for each performance obligation in the respective contract; | |
| ● | allocation of the transaction price to each performance obligation; and | |
| ● | recognition of revenue only when we satisfy each performance obligation. |
Our revenue recognition policies for our operating segments are as follows:
Advertising and Products
Advertising Revenue
Commencing in the third quarter of 2019 we began to provide advertising services to our clients. Advertising contracts are signed to establish the price and advertising services to be provided. Pursuant to the advertising contracts, we provided advertising and marketing services to clients through exhibition events, conferences, and person-to-person marketing. We perform a credit assessment of each customer to assess the collectability of the contract price prior to entering into contracts.
Most of the advertisement contracts designated that we perform advertising services for the client through exhibition events, conferences, and person-to-person marketing during the contracted period, regardless of the number of such events. As such, we determined that the performance obligation is satisfied over time during the contracted period and revenue is recognized accordingly. Such advertising revenue amounted to $0 and $1,944,811 for the years ended December 31, 2022 and 2021, respectively.
All of the advertising revenue is subject to the PRC VAT of 6%. This VAT may be offset by VAT paid by us for raw materials and other materials purchased in China.
Product Revenues
Our revenue from sales of products is recognized when goods are delivered to the customer and no other obligation exists. We do not provide unconditional return or other concessions to customers. Our sales policy allows for the return of unopened products for cash after deducting certain service and transaction fees. As an alternative to returning a product, customers may request an exchange for products with the same value.
Product sales revenue represents the invoiced value of goods, net of value-added taxes (“VAT”). All of our products sold in China are subject to the PRC VAT of 13% since April 1, 2019. This VAT may be offset by VAT paid by for raw materials and other materials purchased in China. We record VAT payables and VAT receivables net of payments in the financial statements. The VAT tax return is filed offsetting the payables against the receivables. Sales and purchases are recorded net of VAT collected and paid as we act as an agent for the government.
| 55 |
Pharmacies
Our retail drugstores recognize revenue at the time the customer takes possession of the merchandise. For pharmacy sales, each prescription claim is its own arrangement with the customer and is a performance obligation. We generally receive payment from pharmacy customers we satisfy our performance obligations. We record a receivable when we have an unconditional right to receive payment and only the passage of time is required before payment is due. Sales revenue represents the invoiced value of goods, net of VAT. All of the products sold in our pharmacies are exempt from VAT as the pharmacies qualify for a small business exemption.
Hotel
Hotel revenues are primarily derived from the rental of rooms, food and beverage sales and other ancillary goods and services, including but not limited to souvenir, parking and conference reservations. Each of these products and services represents a distinct performance obligation and, in exchange for these services, we receive fixed amounts based on published rates or negotiated contracts. Payment is due in full at the time when the services are rendered or the goods are provided. Room rental revenue is recognized on a daily basis when rooms are occupied. Food and beverage revenue and other goods and services revenue are recognized when they have been delivered or rendered to the guests as the respective performance obligations are satisfied. All of the hotel’s goods sold in China are subject to the PRC VAT of 6%. This VAT may be offset by VAT paid by on raw materials and other materials purchased in China.
Manufacture and Sale
The Company’s new subsidiary Runcangsheng recognizes revenue at the time products are shipped as this satisfies its performance obligation. The Company records a receivable for the sales when it has an unconditional right to receive payment and only the passage of time is required before payment is due. Sales revenue represents the invoiced value of goods, net of VAT. All of the Company’s products sold in China are subject to the PRC VAT of 13% unless it is a qualified small subject to exemption.
Foreign Currency Translation and Comprehensive Income (Loss)
The functional currency of our business operations is RMB. For financial reporting purposes, RMB is translated into USD as the reporting currency. Assets and liabilities are translated at the exchange rate in effect at the balance sheet dates. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period.
Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity as “Accumulated other comprehensive income”. Gains and losses resulting from foreign currency transactions are included in income. There was no significant fluctuation in the exchange rate for the conversion of RMB to USD after the balance sheet date.
We use FASB ASC Topic 220, “Comprehensive Income”. Comprehensive income (loss) is comprised of net income (loss) and all changes to the statements of stockholders’ equity, except those due to investments by stockholders, changes in paid-in capital and distributions to stockholders. Comprehensive loss for years ended December 31, 2022 and 2021 consisted of net loss and foreign currency translation adjustments.
| 56 |
BUSINESS
Overview
We (AiXin Life International, Inc.) are a Colorado holding company with no material operations of our own. We conduct substantially all of our operations through our operating companies established in the PRC. From the time we acquired AiXin BVI in December 2017, our focus has been providing health and wellness products to the growing middle class in China. We currently develop, manufacture, market and sell premium-quality healthcare, nutritional products and wellness supplements, including herbs and greens, traditional Chinese remedies, functional products, such as weight management products, probiotics, foods and drinks. We also provide advertising and marketing services to clients which engage us to market and distribute their products. We offer our products and those of clients for which we provide marketing services, through a diversified, omni-channel business model which generates revenues through retail and wholesale product sales, through company-owned pharmacies, direct marketing and online activities.
In December 2017, when we acquired AiXin BVI, it indirectly owned all of the capital stock of AiXinZhonghong. AiXinZhonghong began distributing nutritional products in 2013. AiXinZhonghong offers nutritional products to its customers through a direct marketing team, at large scale entertainment style events which it organizes for the benefit of its clients and more recently, through online activities. In 2019, AiXinZhonghong began to provide marketing and distribution services to other manufacturers and suppliers of healthcare products. AiXinZhonghong has a proactive approach reaching out to customers to provide them with information as to how to live a healthy lifestyle. We believe this approach is ideally suited to marketing health and wellness products as sales of these products are strengthened by ongoing personal contact and support, coaching and education of clients, as to the benefits of a healthy and active lifestyle.
In September 2021 we completed the acquisition of nine pharmacies located in Chengdu. Since that time we have added additional pharmacies and currently operate 25 pharmacies. We utilize these pharmacies to distribute our health and wellness products and serve as learning centers for our clients along with their traditional business. We intend to seek to continue to expand our chain of pharmacies and to use these outlets as part of our overall marketing strategy. As part of our marketing efforts, we will educate the employees at our pharmacies as to the benefits of our health and wellness products so they can closely work with our clients.
In September 2022, we acquired Yunnan Runcangsheng which engages in the research, development, manufacture and wholesale distribution of health and wellness products. Yunnan Runcangsheng operates a 13,000 square meter production facility, including, R&D centers, extraction facilities, preparation workshops and a warehouse. Yunnan Runcangsheng has more than 30 brand names under which it distributes products and maintains and operates planting facilities where it grows some of the key ingredients used in its products. Many of the products it has developed are specifically targeted to alleviate ailments associated with the increasingly competitive and pressured lifestyle of Chinese people, such as hypertension and obesity which result from a more pressured yet sedentary lifestyle, symptoms associated with changing eating habits and the presence of environmental toxins that are becoming more widespread through the use of western style pesticides and fertilizers. When we entered into the agreement to acquire Yunnan Runcangsheng we began to distribute its products through our distribution channels and continue to do so.
In addition to our acquisitions in the health and nutritional sector, in July 2021, we acquired Chengdu AiXin Shangyan Hotel Management Co., Ltd (“AiXin Shangyan Hotel”) which operates the Shangyan Hotel located in the Jinniu District, Chengdu City. While we have continued the hotel’s pre-acquisition business, we intend to use the hotel to host events at which we educate our team members and customers as to the benefits of a healthy lifestyle, introduce new products and offer our complete product line for sale.
We intend to look for additional opportunities to profit from the growing healthcare market in China. Though currently we are not party to any agreements, we will explore, among other opportunities, expanding our product line through internal research and acquiring complementary products from third parties, acquiring additional pharmacies and other retail outlets and operating nursing homes and possibly clinics which provide medical care to clients.
Business Objectives
Key elements of our strategy are detailed below:
Leading brand of nutritional supplements. Our primary goal is to develop recognized brand names with a reputation as a provider of quality health and wellness products which deliver demonstrable benefits to our customers. Our objective is to provide members of the growing Chinese middle class with the information necessary to maintain a healthy lifestyle and to offer them a variety of products whether the customer is looking to treat a specific health-related issue, maintain their overall wellness or improve their performance. Our premium, value-added offerings will include both proprietary branded products and other branded products provided by third parties to meet needs not met by our in-house products.
We believe that by offering a variety of branded exclusive products and a wide range of merchandise, and close customer support and services, we will be able to differentiate our Company from competitors and effectively compete against other food, drug and mass channel players, specialty stores, independent vitamin, supplement and natural food shops and online retailers. We further believe that if we succeed in developing and maintaining a positive reputation, we will be able to offer additional products and services to our clients.
| 57 |
Product development and innovation. With the acquisition of Yunnan Runcangsheng we have the facilities and, more importantly, knowledgeable, experienced personnel, to develop high-quality, innovative health foods and nutritional supplement products that will be offered by our direct marketing team and available through our Company-owned pharmacies, our website and, as demand grows other select marketplaces and wholesale partners. To the extent desirable we will grow some of the key ingredients in our products. When we choose to use ingredients of third parties, they will be rigorously tested before they are added to our products, undergoing multiple quality checks to ensure that they meet our high standards for purity, composition and absence of contaminants.
We believe our capability to innovate and respond to customer needs by designing new products gives us a significant competitive advantage. At Yunnan Runcangsheng we directly employ scientists, nutritionists, formulators, and quality control experts who work at our research and manufacturing facilities.
A differentiated customer experience. We believe one of the key differentiators to our marketing strategy is the continued development of well-trained team members first used at AiXinZhonghong that work closely with customers. We will provide our team members with regular training and education focused on the benefits of a healthy lifestyle, the advantages of our products and solution-based selling. We will also maintain and make available to our team members data bases of our customers purchasing patterns. With their knowledge of the available products, our team members can engage customers in conversation, access the customer’s purchasing history through our data base, share product information and testimonials, and recommend solutions and help customers add complimentary products and build wellness regimens. We operate in a highly personalized, aspirational sector and believe that health food and nutritional supplement consumers often desire and seek out product expertise and knowledgeable customer service.
At AiXinZhonghong we developed loyalty programs to reward customers and incentivize them to introduce our products to their friends. A number of AiXinZhonghong’s customers in fact acted as independent marketers, buying products from us and distributing them to others at a profit. We intend to continue to use loyalty programs to develop and maintain a large and loyal customer base, provide targeted offers and information, and connect with our customers on a regular basis. We will harness data generated by these programs to better understand customers’ buying behaviors and needs, so we can deliver a more rewarding experience, encourage our customers to introduce our products to their friends and make well-informed decisions about the direction of our business.
As we grow and develop our customer base, we can use our hotel as a site for educational seminars, conferences and marketing events featuring well known experts in the wellness sector. These events can be used to increase customer loyalty and to educate our team members as to the benefits of a healthy lifestyle and our products so they can better serve our customers by guiding them to products that meet their needs.
Omni-channel development. We believe our diversified, omni-channel model, which includes company-owned offices open to clients, pharmacies, direct marketing by our team members, individually and at large scale company sponsored marketing events, wholesale locations and online activities, can differentiate us from many of our competitors, particularly those that rely exclusively on online marketing efforts. Our strategy is to give consumers a seamless, integrated experience across digital, mobile and in-store channels and in every interaction with us and our products.
Through our website and in conversations with our well-trained team members, customers can research our products and purchase them online or at one of our local pharmacies or sales offices. We believe our physical store base provides a competitive advantage, allowing customers to experience our products and get expert advice from our team members.
Our omni-channel model can enhance the customer experience and increase the value of a customer to us and we will implement strategies to blend our digital, online and in-store platforms, always stressing the availability and benefits of a conversation with one of our team members. These initiatives will include increased cross-channel marketing, online and in-store subscription services, giving customers the option of picking up online purchases at our stores, delivering products purchased online directly from stores, and providing educational content, information and advice online, at our pharmacies o through a personal visit by one of our team members.
| 58 |
Vertical Integration. We believe that our multi-channel distribution model, combined with our research, development and manufacturing capabilities offers us a unique advantage over our competitors. The daily feedback received from interactions between our team members and customers will be monitored to recognize what our customers want and rapidly develop products to meet their desires. Instead of simply responding to trends in the market, we will seek to bring new topical products to the market before they are generally available.
Products
Our pharmacies offer a wide variety of personal healthcare products and traditional Chinese remedies in addition to our health and wellness products. They began to carry the products developed by Yunnan Runcangsheng prior to completion of that acquisition. The primary products currently distributed by us, including those of Yunnan Runcangsheng, include rhino king, colostrum powder derived from goat’s milk, tong li ke, gammaoling granules, amoxicillin capsules, loratadine tablets, and fuyang granules.
Marketing
Our target customer is the growing population of middle- and upper-class Chinese who are willing to devote increasing amounts to healthcare products as their incomes grow. We believe that as this group grows, becomes more affluent and adopts a more western sedentary life-style and diet, it will experience obesity, hypertension, heart disease, back pain and some of the negative symptoms common in developed countries. Our marketing efforts include proactively approaching these customers through person-to-person marketing and by hosting educational and informative events, which we believe is ideally suited to marketing our products and those of our clients for which we perform advertising services because sales of health and wellness products and supplements are strengthened by ongoing personal contact and support, coaching and education towards how to achieve a healthy and active lifestyle.
We currently market our products through our pharmacies, at large scale marketing events which feature popular local entertainers, at our offices which customers are encouraged to use for social gatherings at wholesale and online. Key to our marketing efforts are the members of our marketing team who make themselves available to individual customers and work with such customers to develop a healthy well balanced dietary regimen. As part of our educational efforts, we distribute informational videos to our clients on-line and make multiple posts on social media daily. Off-line, in addition to distributing products through our pharmacies, we utilize person-to-person marketing to promote and sell products. These personal marketing efforts include hosting educational events and allowing customers to utilize our facilities for social gatherings.
In addition to our marketing efforts, through our loyalty program we encourage our customers to distribute our products to others. Our program for increasing the number of clients interested in reselling the products we offer and for developing new clients who demonstrate the ability to sell our products to others, is to provide them with compensation, in the form of quantity purchase discounts and other incentives, such as free meals, travel or vacations.
We offer our clients a customer satisfaction guarantee. Our sales policy allows for the return of unopened products for cash after deducting certain service and transaction fees. As an alternative to returning a product, customers can exchange a product for another of the same value.
Manufacturing
As a result of the acquisition of Yunnan Runcangsheng, we have a 13,000 square meter manufacturing center inclusive of office space, R&D centers, extraction workshops, preparation workshops, traditional Chinese medicine decoction pieces workshops and storage areas. The activities include research and development, extraction and intensive processing of traditional Chinese medicines and processing to incorporate active ingredients into tablets, capsules, granules and pills according to market demand and product attributes. The facility was designed and constructed and is operated in compliance with applicable national health food and pharmaceutical standards (GMP), and has achieved ISO quality system certification and HACCP food safety system certification.
| 59 |
Raw Materials
The raw materials used in products we manufacture are generally readily available, subject to fluctuations in price due to supply and demand issues. We do not look to enter into long-term contracts with any of our suppliers as the materials we need shifts as demand for our products changes.
Intellectual Property
We believe that we need to develop well recognized brand names and, when possible, obtain patents covering products we develop, their formulae or the processes by which they are manufactured. Runcangsheng has received patents on certain manufacturing processes and has used multiple brand names for its products. Given the early stage of Runcangsheng’s development and the limited historical operations of AiXinZhonghong, our products and brand names have yet to achieve the widespread recognition we envision. Further, we have yet to determine whether the patents Runcangsheng has received will prevent others from marketing competitive products or using manufacturing processes similar to those we have developed. See, “Risk Factors – Risks Associated with Our Company -, Our ability to adequately protect our trade names, trademarks and patents could have an impact on our brand images and ability to penetrate new markets.”
Competition
The category of nutritional products is very competitive and there are various channels through which such products are marketed to consumers, including direct selling, through the internet, through specialty retailers, pharmacies and discount channels of food, drug and mass merchandisers. We seek to differentiate ourselves by being familiar with our clients and providing a personalized sales experience and focusing on after-sale services where sales employees focus on the consultative sales process through product education and the frequent contact and support that many sales employees have with the clients. From a competitive standpoint, there are many providers and sales outlets of nutritional products in China. We believe that none have effectively combined the product, personal coaching, education and the product access provided by our sales employees and, further, that these efforts are compounded by the peer pressure our clients generate through our organized group sales presentations.
Employees
As of January 10, 2024, we had 207 employees allocated among the functions set forth in the table below
| Function | Number of employees | |||
| Administration | 37 | |||
| Finance | 20 | |||
| Manufacturing | 11 | |||
| Marketing (sales) | 118 | |||
| R&D | 6 | |||
| Procurement | 15 | |||
We believe that we maintain a good working relationship with our employees, and to date we have not experienced any significant labor disputes.
Government Regulations
We are subject to numerous regulations applicable to businesses generally, including regulations relating to working conditions, employee compensation and the maintenance of welfare plans, environmental regulation and truth in advertising. In addition, the manufacture and distribution of nutritional products is subject to many laws, governmental regulations, administrative determinations and guidance. Such laws, regulations and other constraints exist at the national, provincial and local levels, including regulations pertaining to: (1) the formulation, manufacturing, packaging, labeling, distribution, importation, sale and storage of products; (2) product claims and advertising, including direct claims and advertising by us, as well as claims and advertising by manufacturers and distributors of the products we offer, for which we may be held responsible; and (3) taxes. As a distributor, we are subject to only a portion of these laws and regulations. We believe that we are fully compliant with those applicable to our activities.
Prior to commencing manufacture or distribution of a product, the manufacturer or distributor may be required to obtain an approval, license or certification from the national, provincial or local government in China. Although we attempt to determine whether all regulatory requirements have been met, we cannot monitor the manufacture of products we acquire for distribution from third parties and cannot be certain that all applicable regulations are satisfied. Moreover, even if we were to determine that a manufacturer or distributor had the requisite license or certification at the beginning of a relationship, we might not become aware if it were to forfeit any regulatory approvals or fail to adhere to applicable requirements.
| 60 |
Dietary supplements are subject to regulation by the China Food and Drug Administration. China has highly restrictive nutritional supplement product regulations. Products marketed as “health foods” are subject to extensive laboratory and clinical analysis by government authorities, and the product registration process in China generally takes one to two years but may be substantially longer. We market both “health foods” and “general foods.” For products we distribute on behalf of or acquired from others, we are not in a position to obtain any required license, though we may be held liable if we were to distribute a product which had not been properly tested and registered with the authorities. There is some risk associated with the common practice in China of marketing a product as a “general food” while seeking “health food” classification. If government officials feel the categorization of a product distributed by us is inconsistent with product claims, ingredients or function, this could end or limit our ability to market such products.
As the middle class has grown, the number of manufacturers and distributors of nutritional supplements in China has dramatically increased. Many of these enterprises have often ignored applicable laws and distributed adulterated or inferior products. We believe this has created a marketing opportunity which we have tried to exploit as a trusted source of products on which our clients can rely. To the extent our reputation results from reviewing and testing products prior to distributing them, and then distributing only products determined to be safe, it is incumbent upon us to ensure that the manufacturers and distributors upon which we rely are trustworthy. A failure by any of these third parties could cause substantial damage to our reputation, business and financial results.
Direct selling and multi-level marketing are two forms of marketing regulated by various national, provincial and local government agencies in China. These laws and regulations are generally intended to prevent fraudulent or deceptive schemes, including “pyramid” schemes, which compensate participants primarily for recruiting additional participants without significant emphasis on product sales to consumers.
Under PRC regulations, “direct selling” refers to a type of business mode in which a company recruits door-to-door salesmen to sell products directly to ultimate consumers outside the companies’ fixed places of business. Businesses engaged in “direct selling” are required to obtain a license from the PRC government. A direct selling company is required to provide vocational training for, and conduct an examination of, any sales promoter it recruits, and obtain a certificate for each sales promoter after the sales promoter has passed the examination. A direct selling company is also required, when commencing operations, to deposit RMB20 million ($2.9 million) in a special account with a designated bank, which deposit is adjusted on a monthly basis to equal 15% of the operator’s sales from direct selling products up to RMB0.1 billion ($14.5 million).
We do not engage in direct selling activities subject to regulations prevalent in China since we do not employ sales personnel engaged in door-to-door sales outside our place of business.
Under PRC regulations, “multi-level marketing” refers to marketing, promotional and sales activities whereby organizers or operators take in new members and compensate each member based upon the number of new members introduced by such member, directly or indirectly, or based upon the level of sales generated by the members introduced by such member. The regulations also prohibit an organizer from requiring new members to deposit a sum of money as a condition to membership or requiring that members recruit additional members to establish a multi-level relationship. PRC regulations distinguish direct selling from multi-level marketing in that all direct sellers are normally trained by the direct selling company and any direct seller is not allowed to develop new followers or form multiple levels.
We are not in the direct selling category or multi-level marketing category since (i) we do not pay salaries or commissions to our member distributors, who decide as a matter of personal preference whether to introduce our products to relatives or friends based on their own personal experience of usage and/or trust of our Company’s products; (ii) we do not require individuals to deposit a sum of money to become a member; and (iii) we do not pay members to recruit individuals to join in or to form a multi-level relationship.
| 61 |
Nevertheless, the laws and regulations governing direct selling and multi-level marketing may be modified or reinterpreted from time to time, which may cause us to change our business model. Regulations are subject to discretionary interpretation by regulators and governmental authorities. There is often ambiguity and uncertainty with respect to the implication of direct selling and anti-pyramiding laws and regulations.
Properties
We currently operate 25 retail pharmacies in Chengdu. The pharmacies are operated subject to short term leases at market rate rents. In addition to the pharmacies, we maintain our administrative offices in Chengdu.
Yunnan Runcangsheng operates a 13,000 square meter production facility, including, R&D centers, extraction facilities, preparation workshops and a warehouse. Yunnan Runcangsheng has more than 30 brand names under which it distributes products and maintains and operates planting facilities where it grows some of the key ingredients used in its products. Runcangsheng acquired the right to use the production facility pursuant to a Development Agreement with the People’s Government of Luquan Li and Miao Autonomous County. The Development Agreement grants Runcangsheng the rights to use the facility for a period of five years ending November 25, 2025, provided Runcangsheng invests certain amounts in the development of the property and its business, including the construction of office space, manufacturing facilities and workshops. The governmental authorities appear to be satisfied with the progress Runcangsheng has made in the development of the property to date and have not expressed any intent to terminate the Agreement.
Shangyan Hotel Company owns and operates a hotel located in the Jinniu District, Chengdu City. The hotel covers more than 8,000 square meters and has a large restaurant that can accommodate 600 people, six luxury dining rooms, a 200 square meter music tea house, 13 private tea rooms, 108 guest rooms and other supporting facilities. The hotel is conveniently located, connected to the express ring line of Chengdu and the Chengdu bus system, within a 30-minute drive to Shuangliu International Airport. The hotel is equipped with all modern facilities, including central air conditioning and each guest room features luxury bedding, high-speed internet, a safe for valuables and minibar. To accommodate businesses, the banquet hall is equipped with advanced audio-visual equipment and dedicated high-speed wireless Internet to facilitate large group presentations. The staff includes a professional banquet team to ensure the success of any private function or business gathering. A full range of catering services, including Chinese-style boutique Sichuan cuisine are provided in a stylish environment. The hotel is leased pursuant to an agreement which expires at the end of December 2025 at an annual rent of approximately RMB5,856,000/.
Legal Proceedings
From time to time, we may become involved in legal proceedings or be subject to claims arising in the ordinary course of our business. We are not presently a party to any legal proceedings that in the opinion of our management, if determined adversely to us, would individually or taken together have a material adverse effect on our business, operating results, financial condition, or cash flows.
In December 2020, Jian Yiao (the “Plaintiff”) filed a complaint against Chengdu Aixintang Pharmacy Co., Ltd. (“Aixintang Pharmacy”) in Zhangjiagang People’s Court in Jiangsu Province. The complaint alleges that Mr. Yiao is entitled to $392,305 (RMB2,500,000) from Aixintang Pharmacy for breaching a purchase agreement entered in March 2020 (the “Purchase Agreement”). Aixintang Pharmacy claimed that the Purchase Agreement was the result of a forgery by an employee, and that Aixintang Pharmacy did not enter the Purchase Agreement. The Court determined that Aixintang Pharmacy breached the Purchase Agreement by not delivering the products ordered and ordered Aixintang Pharmacy to pay $392,305 (RMB2,500,000) to the Plaintiff. In December 2020, Aixintang Pharmacy filed a motion in the Jiangsu Suzhou Intermediate People’s Court against the verdict.
In February 2021, the judge in the Jiangsu Suzhou Intermediate People’s Court denied Aixintang Pharmacy’s motion and upheld the judgment from the first trial. In March 2021, Aixintang Pharmacy filed another motion to the Jiangsu High People’s Court on the basis that the Purchase Agreement was forged. In February 2022, Aixintang Pharmacy filed an appeal in Jiangsu High People’s Court against the judgment reached by Jiangsu Suzhou Intermediate People’s Court in February 2021. To date, this legal proceeding remains pending.
The incident which is the subject to the above lawsuit occurred before Aixintang Pharmacy was acquired by the Company. In November 2021, the Company and Mr. Lin agreed that Mr. Lin shall indemnify the Company against any losses arising from this legal proceeding.
The Company believes that current pending litigation will not have a material adverse effect on its consolidated financial position, results of operations or cash flows.
DIRECTORS AND EXECUTIVE OFFICERS
The name, address, age and titles of our executive officers and director are as follows:
The following table sets forth the names and ages of all directors and executive officers as of the end of the last fiscal year and on the date of this report:
| Name | Age | Position | ||
| Quanzhong Lin | 45 | Director, Chairman, President and Chief Executive Officer | ||
| Yao-Te Wang (1) (2) (3) | 46 | Director and Secretary | ||
| Christopher Lee (1) (2) (3) | 52 | Director | ||
| Huiliang Jiao (1) (2) (3) | 49 | Director | ||
| Tianfeng Li | 36 | Chief Financial Officer |
| (1) | Member of the Audit Committee |
| (2) | Member of the Compensation Committee |
| (3) | Member of the Nominating and Corporate Governance Committee |
| 62 |
Quanzhong Lin has served as a director, Chairman, President and Chief Executive Officer of our Company since February 2, 2017. Mr. Lin is a highly active entrepreneur in China, and currently serves as Chairman of AiXin Company Group, a diversified company which he founded in 2008. In addition to AiXin Company Group, Mr. Lin has founded a number of companies located in Chengdu City, Sichuan Province, China, engaged in various types of business, including pharmacies, retail outlets, hotel management services and global tourism.
In 2009, Mr. Lin founded QingBaiJiangJinWanXiang Daily Necessities store, predecessor to AiXinZhonghong Biotechnology Co., Ltd. From 2010 to 2013, Mr. Lin opened branches in Xindu and Xinjin district, officially entering the Chengdu market.
In September 2013, Mr. Lin founded Chengdu AiXin E-Commerce Company Ltd., which in the following twelve months opened branches in cities and counties including Huayuan and Wenjiang district, and Mianyang and Jianyang city. In April 2015, AiXin E-commerce Co., Ltd. changed its name to Chengdu AiXinZhonghong Biotechnology Co., Ltd., whose shares became listed on the Shanghai Stock Exchange (Ticker Symbol: 207448) in October 2015; and during 2015, AiXinZhonghong opened branches in Dujiangyan City, and Chongzhou City.
Yao-Te Wang has served as a director of our Company since December 12, 2017. He became our Secretary on June 20, 2023. Mr. Wang has been the Chief Executive Officer of Ivy Service Group (China), which is a transnational consultant company in China, since 2015. From January 2016 to June 2016, Mr. Wang participated in the overall operation planning for Chongqing Cultural Assets and Equity Exchange. From June 2015 to January 2016, Mr. Wang helped with the overall brand strategy development for Swire Group, who merged the biggest baking brand in Southwest China within more than 150 million RMB. From September 2014 to February 2015, Mr. Wang was the chairman special assistant for JECUI Health Science Company. From July 2012 to August 2014, Mr. Wang was the Chief Executive Officer of Ivy Service Group (Taipei). From August 2007 to June 2012, Mr. Wang was an instructor of National Defense University (Taipei), taught International Politics and Economic Analysis.
Christopher Lee was appointed as a Member of the Board of Directors of the Company on February 5, 2021. Mr. Lee has served as Chief Financial Officer of Semileds Corporation since September 2015. Mr. Lee joined Semileds Corporation in September 2014 and from November 2014 until his appointment as Chief Financial Officer, Mr. Lee was the interim Chief Financial Officer of Semileds Corporation. Semileds develops, manufactures and sells high performance light emitting diodes and is currently listed on The Nasdaq Stock Market. Mr. Lee has over 20 years of experience in accounting and finance, including US GAAP, PCAOB standards and SEC rules and regulations. Mr. Lee was a partner of KEDP CPA Group from August 2009 to June 2011 and a self-employed accountant from July 2011 to August 2014. Mr. Lee holds a BS degree in accounting from Ohio State University and a MS degree in business taxation from Golden Gate University and is licensed as a Certified Public Accountant (CPA) in the United States.
Huiliang Jiao was elected to the Board of Directors of our Company on December 1, 2022. Mr. Jiao received his degree from China Pharmaceutical University in 1997 where he majored in Pharmacy. Mr. Jiao joined Yunnan Runcangsheng Technology Co., Ltd. in April 2020, most recently serving as Chief Executive Officer. Mr. Jiao served as the general manager of Yunnan Shengshengyuan Technology Co., Ltd. from June 2016 until he joined Runcangsheng. From January 2007 until May 2016, Mr. Jiao was the general manager of Yunnan Shengcaofeng Biotechnology Co., Ltd. Throughout his career Mr. Jiao has been involved in the research and development of new products intended to improve individuals’ health and well-being, with an emphasis on functional products comprised of natural plants, foods and supplements intended to address obesity and other chronic conditions. He was named as an inventor on more than forty patents relating to the composition and manufacture of health foods. In addition to the development of health foods, Mr. Jiao has participated in the design and maintenance of production systems intended to meet the latest manufacturing standards.
Tianfeng Li, has been our Chief Financial Officer since December 1, 2022. Ms. Li also serves as Chief Financial Officer of the Company’s subsidiaries. Ms. Li has held various positions, most recently, Finance Manager, within the Finance Department of our subsidiary, Chengdu AiXin Pharmacy Co., Ltd., which she joined in May 2019. While serving at AiXin Pharmacy Ms. Li helped build the company’s finance team and established the development of its financial reporting and risk management systems. In addition, she presided over the preparation of the company’s financial statements, the completion of its annual audit, daily cash management, the preparation of the annual budget and the integration of newly acquired pharmacies. From May 2013 until joining AiXin Pharmacy in May 2019, Ms. Li served as the Finance Manager of Sichuan Jinxin Clean Energy Equipment Co., Ltd., where she was responsible for developing the company’s financial systems, preparing its budget and managing all aspects of cash management. At Sichuan Jinxin Clean Energy Equipment Co Ms. Li supervised a staff of five assistants. Ms. Li received a bachelor’s degree from The Open University of China in 2017, and has received a Certificate of Honor as an Intermediate Accountant.
| 63 |
There are no family relationships among any of our officers and directors.
Directors hold office until the next annual meeting of shareholders and until their successors have been duly elected and qualified. Officers are elected by the board of directors and hold office until the earliest of their death, resignation or removal from office.
Independence
Our board of directors has determined that each of Christopher Lee, Yao-Te Wang and Huiliang Jiao satisfies the definition of “independent director” in accordance with Rule 5605(a)(2) of the Marketplace Rules of The Nasdaq Stock Market, Inc. and Section 10(A)(m)(3) of the Securities Exchange Act of 1934, as amended.
Board Committees
Our board of directors has established standing committees in connection with the discharge of its responsibilities. These committees include an Audit Committee, a Compensation Committee and a Nominating and Corporate Governance Committee. Our board of directors has adopted written charters for each of these committees. Upon completion of this offering, copies of the charters will be available on our website. Our board of directors may establish other committees as it deems necessary or appropriate from time to time.
Audit Committee. Our Audit Committee consists of Messrs. Lee, Jiao and Wang, each of whom is independent. The Audit Committee assists the Board of Directors oversight of (i) the integrity of financial statements, (ii) our compliance with legal and regulatory requirements, (iii) the independent auditor’s qualifications and independence, and (iv) the performance of our internal audit function and independent auditor and prepares the report that the SEC requires to be included in our annual proxy statement. The Audit Committee operates under a written charter. Mr. Lee is the Chairman of our Audit Committee.
The Board of Directors determined that Mr. Lee possesses accounting or related financial management experience that qualifies him as financially sophisticated within the meaning of Rule 4350(d)(2)(A) of the Nasdaq Marketplace Rules and that he is an “audit committee financial expert” as defined by the rules and regulations of the SEC.
According to its charter, the Audit Committee shall consist of at least three members, each of whom shall be a non-employee director who has been determined by the Board to meet the independence requirements of Nasdaq, and also Rule 10A-3(b)(1) of the SEC, subject to the exemptions provided in Rule 10A-3(c). We do not have a website containing a copy of the Audit Committee Charter. The Audit Committee Charter describes the primary functions of the Audit Committee, including the following:
| ● | Oversee the Company’s accounting and financial reporting processes; | |
| ● | Oversee audits of the Company’s financial statements; | |
| ● | Discuss policies with respect to risk assessment and risk management, and discuss the Company’s major financial risk exposures and the steps management has taken to monitor and control such exposures; | |
| ● | Review and discuss with management the Company’s audited financial statements and review with management and the Company’s independent registered public accounting firm the Company’s financial statements prior to the filing with the SEC of any report containing such financial statements. | |
| ● | Recommend to the board that the Company’s audited financial statements be included in its annual report on Form 10-K for the last fiscal year; | |
| ● | Meet separately, periodically, with management, with the Company’s internal auditors (or other personnel responsible for the internal audit function) and with the Company’s independent registered public accounting firm; |
| 64 |
| ● | Be directly responsible for the appointment, compensation, retention and oversight of the work of any independent registered public accounting firm engaged to prepare or issue an audit report for the Company; | |
| ● | Take, or recommend that the board take, appropriate action to oversee and ensure the independence of the Company’s independent registered public accounting firm; and | |
| ● | Review major changes to the Company’s auditing and accounting principles and practices as suggested by the Company’s independent registered public accounting firm, internal auditors or management. |
Nominating and Corporate Governance Committee
The purpose of the Nominating and Corporate Governance Committee is to assist the Board of Directors in identifying qualified individuals to become members of our Board of Directors, in determining the composition of the Board of Directors and in monitoring the process to assess Board effectiveness. Each of Messrs. Lee, Jiao and Wang are members of the Nominating and Corporate Governance Committee. Mr. Wang serves as Chairman of the Nominating and Corporate Governance Committee. The Nominating and Corporate Governance Committee operates under a written charter.
| ● | Our Nominating and Corporate Governance Committee has, among the others, the following authority and responsibilities: | |
| ● | To determine and recommend to the Board, the criteria to be considered in selecting nominees for the director; | |
| ● | To identify and screen candidate consistent with such criteria and consider any candidates recommended by our stockholders pursuant to the procedures described in our proxy statement or in accordance with applicable laws, rules and regulations and provisions of our charter documents. | |
| ● | To select and approve the nominees for director to be submitted to a stockholder vote at the annual meeting of stockholders. |
Compensation Committee
The Compensation Committee is responsible for overseeing and, as appropriate, making recommendations to the Board of Directors regarding the annual salaries and other compensation of our executive officers and general employees and other policies, and for providing assistance and recommendations with respect to our compensation policies and practices. Each of Messrs. Lee, Jiao and Wang are members of the Compensation Committee. The Compensation Committee operates under a written charter. Mr. Jiao is the Chairman of Compensation Committee.
Our Compensation Committee has, among the others, the following responsibilities and authority.
| ● | The compensation committee may, in its sole discretion, retain or obtain the advice of a compensation consultant, legal counsel or other adviser. | |
| ● | The compensation committee shall be directly responsible for the appointment, compensation and oversight of the work of any compensation consultant, legal counsel and other adviser retained by the compensation committee or said group. | |
| ● | The Company must provide for appropriate funding, as determined by the compensation committee, for payment of reasonable compensation to a compensation consultant, legal counsel or any other adviser retained by the compensation committee or said group. |
| 65 |
| ● | The compensation committee select, or receive advice from, a compensation consultant, legal counsel or other adviser to the compensation committee or said group, other than in-house legal counsel, only after conducting an independence assessment with respect to the adviser as provided for in the Exchange Act. |
Board Leadership Structure and Role in Risk Oversight
Mr. Quanzhong Lin holds the positions of chief executive officer and chairman of the board of the Company. The board believes that Mr. Lin’s services as both chief executive officer and chairman of the board is in the best interest of the Company and its shareholders. Mr. Lin is a well-recognized successful entrepreneur in Chengdu. He possesses detailed and in-depth knowledge of the issues, opportunities and challenges facing the Company in its business and is thus best positioned to develop agendas that ensure that the Board’s time and attention are focused on the most critical matters relating to the business of the Company. His combined role enables decisive leadership, ensures clear accountability, and enhances the Company’s ability to communicate its message and strategy clearly and consistently to the Company’s shareholders, employees and customers.
The board has not designated a lead director. Given the limited number of directors comprising the Board, the independent directors call and plan their executive sessions collaboratively and, between meetings of the Board, communicate with management and one another directly. Under these circumstances, the directors believe designating a lead director to take on responsibility for functions in which they all currently participate might detract from rather than enhance performance of their responsibilities as directors.
Management is responsible for assessing and managing risk, subject to oversight by the board of directors. The board oversees our risk management policies and risk appetite, including operational risks and risks relating to our business strategy and transactions. Various committees of the board assist the board in this oversight responsibility in their respective areas of expertise.
Code of Ethics
We have adopted a Code of Ethical Business Conduct that applies to, among other persons, members of our board of directors, our Company’s officers including our Chief Executive Officer, employees, consultants and advisors. As adopted, our Code of Business Conduct and Ethics sets forth written standards that are designed to deter wrongdoing and to promote:
| 1. | honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; | |
| 2. | full, fair, accurate, timely, and understandable disclosure in reports and documents that we file with, or submit to, the SEC and in other public communications made by us; | |
| 3. | compliance with applicable governmental laws, rules and regulations; | |
| 4. | the prompt internal reporting of violations of the Code of Ethical Business Conduct to an appropriate person or persons identified in the Code of Ethical Business Conduct; and | |
| 5. | accountability for adherence to the Code of Ethical Business Conduct. |
Our Code of Code of Ethical Business Conduct requires, among other things, that all of our Company’s senior officers commit to timely, accurate and consistent disclosure of information; that they maintain confidential information; and that they act with honesty and integrity.
In addition, our Code of Ethical Business Conduct emphasizes that all employees, and particularly senior officers, have a responsibility for maintaining financial integrity within our Company, consistent with generally accepted accounting principles, and federal and state securities laws. Any senior officer who becomes aware of any incidents involving financial or accounting manipulation or other irregularities, whether by witnessing the incident or being told of it, must report it to our Company. Any failure to report such inappropriate or irregular conduct of others is to be treated as a severe disciplinary matter. It is against our Company policy to retaliate against any individual who reports in good faith the violation or potential violation of our Company’s Code of Ethical Business Conduct by another.
| 66 |
A copy of the Code of Ethics and Business Conduct was filed as an Exhibit to our report on Form 8-K filed on September 25, 2020, and is available on the SEC’s website, www.sec.gov.
EXECUTIVE COMPENSATION
The following table sets forth certain information about compensation paid, earned or accrued for services by each individual who served as our chief executive officer and chief financial officer for services rendered in all capacities during the year ended December 31, 2023. No other executive officer of our Company or other individual received total annual salary and bonus compensation in excess of $100,000 for the year ended December 31, 2023.
Summary Compensation Table
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Total ($) | |||||||||||
| Quanzhong Lin, President(1) | 2023 | $ | 20,100 | $ | $ | 20,100 | |||||||||
| 2022 | 20,822 | 20,822 | |||||||||||||
| Tiangfeng Li, Chief Financial Officer (2) | 2023 | $ | 26,500 | $ | $ | 26,500 | |||||||||
| 2022 | $ | 20,533 | $ | $ | 20,533 | ||||||||||
(1) Amounts attributed to Mr. Lin represent amounts paid as President and CEO of AiXinZhonghong.
(2) Amounts attributed to Ms. Li represent amounts paid as CFO of AiXinZhonghong.
Neither Mr. Lin nor Ms. Li has an employment agreement with the Company.
Narrative Disclosure to Summary Compensation Table
There are no annuity, pension or retirement benefits proposed to be paid to the officer or director or employees in the event of retirement at normal retirement date pursuant to any presently existing plan provided or contributed to by the Company or any of its subsidiaries, if any.
Stock Option Plan
In 2019 we adopted the 2019 Equity Incentive Plan (the “2019 Plan”), which authorizes the issuance of shares of common stock for grants of stock options, stock appreciation rights, restricted stock, stock units, bonus stock, dividend equivalents, other stock related awards and performance awards that may be settled in cash, stock, or other property. The 2019 Plan authorizes the issuance of up to 625,000 shares.
We adopted the 2019 Plan to provide a means by which employees, directors, and consultants of our Company and those of our subsidiaries and other designated affiliates, which we refer to together as our affiliates, may be given an opportunity to purchase our common stock, to assist in retaining the services of such persons, to secure and retain the services of persons capable of filling such positions, and to provide incentives for such persons to exert maximum efforts for our success and the success of our affiliates. The material features of the 2019 Plan are outlined below. This summary is qualified in its entirety by reference to the complete text of the 2019 Plan which has been filed with the SEC.
| 67 |
Grants of Plan-Based Awards
None of our executive officers was granted any options or equity awards during 2021 or 2020 or held any options or other equity awards as of the date hereof.
In October 2019, we issued an aggregate of 293,750 shares to employees and contractors under the 2019 Equity Incentive Plan.
Outstanding Equity Awards
There are no outstanding equity awards granted pursuant to the 2019 Equity Incentive Plan.
Compensation of Directors
The following table sets forth information regarding the compensation paid to, earned by or accrued for our directors during the fiscal year ended December 31, 2023.
| DIRECTOR COMPENSATION | ||||||||||||||||||||||||||||
| Name | Fees
Earned In
Cash | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Non-Qualified Deferred Compensation Earnings ($) | All
Other Compensation ($) | Total ($) | |||||||||||||||||||||
| Yao-Te Wang | $ | 37,000 | — | — | — | — | 0 | $ | 37,000 | |||||||||||||||||||
| Quanzhong Lin | $ | 0 | — | — | — | — | 0 | $ | 0 | |||||||||||||||||||
| Christopher Lee | $ | 9,600 | $ | 9,600 | ||||||||||||||||||||||||
| Huiliang Jiao | $ | 12,000 | — | — | — | — | — | $ | 12,000 | |||||||||||||||||||
Pension, Retirement or Similar Benefit Plans
There are no arrangements or plans in which we provide pension, retirement or similar benefits for directors or executive officers. We have no material bonus or profit sharing plans pursuant to which cash or non-cash compensation is or may be paid to our directors or executive officers, except that stock options may be granted at the discretion of the board of directors or a committee thereof.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Transactions with Related Persons
The following includes a summary of transactions since January 1, 2022 and certain ongoing transactions, or any currently proposed transaction, in which we were or are to be a participant and the amount involved exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets at year-end for the last two completed fiscal years, and in which any related person had or will have a direct or indirect material interest (other than compensation described under “Executive Compensation”). We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm’s-length transactions.
| 68 |
Advance to/from related parties
As of December 31, 2022 and 2021, the Company had accounts payable in the amount of $165,958 and $0, respectively, due to Luquan Shengcaofeng Biotechnology Co., Ltd., an affiliate of Runcangsheng, for the purchase of raw materials. This payable was satisfied during 2023.
From time to time, we have advanced certain amounts to pharmacies owned by Quanzhong Lin. The amount due from each of such entities as of the end of 2022 and 2021, and as of September 30, 2023, was as follows:
September 30, 2023 | December 31, 2022 | December 31, 2021 | ||||||||||
| Chengdu WenJiang Aixin Nanjiang Pharmacy Co., Ltd. | $ | 7,573 | $ | 9,708 | $ | 4,583 | ||||||
| Sichuan Aixin Investment Co., Ltd | 8,983 | 145 | 4,237 | |||||||||
| Huiliang Jiao | 135,328 | - | - | |||||||||
| Xiaoyan Zhou | 11,668 | - | - | |||||||||
| Chengdu Fuxiang Tang Pharmacy Co., Ltd. | 25,056 | 26,125 | - | |||||||||
| Chengdu WenJiang Aixin Huiwan Pharmacy Co., Ltd. | 3,888 | - | - | |||||||||
| Chengdu Heshengyuan Pharmacy Co., Ltd. | 3,379 | - | - | |||||||||
| Chengdu Zhiweibing Pharmacy Co., Ltd. | 7,859 | - | - | |||||||||
| Chengdu Tongtai Tang Pharmacy Co. Ltd. | 2,810 | - | - | |||||||||
| Chengdu city Wuhou District Xiaofei Pharmacy Co., Ltd | 8,115 | - | - | |||||||||
| Chongqing Aixin Hui Pharmacy Co., Ltd. | 402 | - | - | |||||||||
| Sichuan Xintang Xinfu Pharmacy Chain Co, Ltd | 344 | - | - | |||||||||
| Sichuan Aixintang Xinfu Chain Pharmacy Co., Ltd. | 302 | - | - | |||||||||
| Chengdu Wenjiang district Heneng hupu Pharmacy Co., Ltd | 26,822 | 34,622 | - | |||||||||
| Chengdu Cigu Foshou Pharmacy | 2,028 | - | - | |||||||||
| Mianyang Aixin Cunshan Pharmacy | 5,874 | - | - | |||||||||
| Chengdu Aixin International Travel Service Co., Ltd | 356 | - | - | |||||||||
| Chengdu Lisheng Huiren Tang Pharmacy Co., Ltd. | 133,801 | 12,502 | 10,235 | |||||||||
| Total | $ | 384,588 | $ | 83,102 | $ | 19,055 | ||||||
From time to time, we have received advances from certain affiliates. The amounts due to such affiliates as of the end of 2022 and 2021, and as of September 30, 2023 was as follows:
September 30, 2023 | December 31, 2022 | December 31, 2021 | ||||||||||
| Quanzhong Lin | $ | 243,323 | $ | 140,644 | $ | 1,822,705 | ||||||
| Yirong Shen | 84,978 | 89,892 | 97,292 | |||||||||
| Branch Manager | - | - | 1,667 | |||||||||
| Sichuan Haosen Haichuan business management Co. Ltd | 57 | - | - | |||||||||
| Sichuan Yunxi Pharmacy Co. Ltd | 449 | - | - | |||||||||
| Aixin Life Beauty | - | - | 7,724 | |||||||||
| Chengdu Yi Yan Tang Pharmacy Co. Ltd. | 162 | - | - | |||||||||
| Chengdu Aixin E-Commerce Company Ltd | - | - | 15,378 | |||||||||
| Chengdu Aixin International travel service Co, Ltd | 4,950 | 6,346 | 2,388 | |||||||||
| Total | $ | 333,919 | $ | 236,882 | $ | 1,947,154 | ||||||
All the entities in the above list are controlled by Quanzhong Lin. These advances to and from related parties were for working capital purposes, payable on demand, and bear no interest. Yirong Shen was a shareholder of Aixin Shangyan Hotel prior to the closing of Hotel Purchase Agreement and serves as the supervisor of Aixin Shangyan Hotel.
Acquisitions from a Major Shareholder
In September 2021, we completed the acquisition of nine pharmacies located in Chengdu by acquiring the entities which owned the pharmacies for an aggregate purchase price of RMB34,635,845, or approximately US$5.31 million (“Transfer Price”). Our Chairman, Quanzhong Lin, was the principal shareholder of the entity which owned the pharmacies we acquired.
In July we completed the acquisition of AiXin Shangyan Hotel. Shangyan Hotel Company, which owns and operates a hotel located in the Jinniu District, Chengdu City. The hotel covers more than 8,000 square meters and has a large restaurant that can accommodate 600 people, six luxury dining rooms, a 200 square meter music tea house, 13 private tea rooms, 108 guest rooms and other supporting facilities. We acquired the hotel through an acquisition of the outstanding equity of AiXin Shangyan Hotel of which Mr. Lin was the principal shareholder, for a purchase price of RMB7,598,887, or approximately $1.16 million (“Transfer Price”).
In connection with the acquisitions of the pharmacies and hotel we made payments to Mr. Lin in the aggregate amount of $4.50 million.
Forgiveness of Loan; Contribution of Shares
On December 31, 2021, Mr. Lin forgave a loan previously made to the Company in the amount of $6,912,513. This was treated as a contribution to capital. On March 1, 2023, for no consideration other than the expenses to be incurred by the Company in connection with this offering, Mr. Lin agreed to contribute to the capital of the Company seven million shares of the common stock of the Company.
In May 2014, we entered into a lease with Mr. Lin for use of an office. We renewed the lease until April 30, 2024 2023, with monthly rent of RMB5,000 ($[ ]), payable quarterly.
Other than the foregoing, none of the directors or executive officers of the Company, nor any person who owned of record or was known to own beneficially more than 5% of the Company’s outstanding shares of its common stock, nor any associate or affiliate of such persons or companies, had any material interest, direct or indirect, in any transaction that occurred since January 1, 2019, or in any proposed transaction, which has materially affected or will affect the Company.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth, as of February 8, 2024, certain information concerning the beneficial ownership of our common stock by (i) each stockholder known by us to own beneficially five percent or more of our outstanding common stock; (ii) each director; (iii) each named executive officer; and (iv) all of our executive officers and directors as a group, and their percentage ownership and voting power. The column entitled “Percentage of Shares Beneficially Owned—Before Offering” is based on [24,999,842] shares of our common stock outstanding and does not give effect to the reduction in the number of our shares of common stock to be outstanding as a result of the seven million shares of our common stock to be contributed to our capital by Quanzhong Lin upon effectiveness of the Registration Statement of which this prospectus is a part.
| 69 |
The information presented below regarding beneficial ownership of our voting securities has been presented in accordance with the rules of the Securities and Exchange Commission and is not necessarily indicative of ownership for any other purpose. Under these rules, a person is deemed to be a “beneficial owner” of a security if that person has or shares the power to vote or direct the voting of the security or the power to dispose or direct the disposition of the security. A person is deemed to own beneficially any security which such person has the right to acquire sole or shared voting or investment power within sixty (60) days through the conversion or exercise of any convertible security, warrant, option, or other right. More than one (1) person may be deemed to be a beneficial owner of the same securities. The percentage of beneficial ownership by any person as of a particular date is calculated by dividing the number of shares beneficially owned by such person, which includes the number of shares as to which such person has the right to acquire voting or investment power within sixty (60) days, by the sum of the number of shares outstanding as of such date including the number of such shares which such person has the right to acquire. Consequently, the denominator used for calculating such percentage may be different for each beneficial owner. Except as otherwise indicated below and under applicable community property laws, we believe that the beneficial owners of our common stock listed below have sole voting and investment power with respect to the shares shown.
| Name of Shareholder (1) | Amount and Nature of Beneficial Ownership | Percent of Common Stock Before the Offering | Percent of Common Stock After the Offering | |||||||||
| Directors and Executive Officers: | ||||||||||||
| Quanzhong Lin, Chairman and CEO | 14,534,676 | (2) | 58.14 | %(2) | 36.98 | %(3) | ||||||
| Yao-Te Wang, Director | 1,884,336 | 7.54 | %(2) | 9.25 | %(3) | |||||||
Christopher Lee, Director | 0 | - | ||||||||||
Huiliang Jiao, Director | 0 | - | ||||||||||
Tianfeng Li, CFO | 0 | - | ||||||||||
| All directors and executive officers as a group (five persons) | 16,419,012 | (2) | 65.68 | %(2) | 46.23 | %(3) | ||||||
| (1) | The address of each beneficial owner is c/o AiXin Life International, Inc., Hongxing International Business Building 2, 14th FL, No. 69 Qingyun South Ave., Jinjiang District, Chengdu City, Sichuan Province, China | |
| (2) | Does not give effect to the reduction in the number of our shares of common stock held by Mr. Lin as a result of the seven (7) million shares of our common stock to be contributed to our capital by him upon effectiveness of the Registration Statement of which this prospectus is a part. | |
| (3) | Gives effect to the reduction in the number of our shares of common stock held by Mr. Lin to 7,534,676 shares as a result of the contribution to our capital of the seven (7) million shares of our common stock referred to in footnote (2). |
DESCRIPTION OF CAPITAL STOCK
We have authorized capital stock consisting of 500,000,000 shares of common stock, $0.00001 par value per share and 20,000,000 shares of preferred stock, $0.001 par value per share.
As of the date of this prospectus, we have 24,999,842 shares of our common stock outstanding, without giving effect to the reduction in the number of our shares of common stock to be outstanding as a result of the seven million shares of our common stock to be contributed to our capital by Quanzhong Lin upon effectiveness of the Registration Statement of which this prospectus is a part.
The following description of our capital stock is a summary only and is subject to and qualified in its entirety by reference to the applicable provisions of the Colorado Business Corporations Act (“Colorado Act”), and our charter and Bylaws, copies of which have been filed as exhibits to the registration statement of which this prospectus is part. You should refer to, and read this summary together with, our Articles of Incorporation and Bylaws, each as amended and restated to date, to review all of the terms of our capital stock. Our Articles of Incorporation and amendments thereto are incorporated by reference as exhibits to the registration statement of which this prospectus is a part.
Common Stock
Holders of Shares of common stock have the right to cast one vote for each share of common stock in their name on the books of our Company, whether represented in person or by proxy, on all matters submitted to a vote of holders of common stock, including election of directors. There is no right to cumulative voting in election of directors. Except where a greater requirement is provided by statute, by our articles of incorporation, or by our bylaws, the presence, in person or by proxy duly authorized, of one or more holders of a majority of the outstanding Shares of our common stock constitutes a quorum for the transaction of business. The vote by the holders of a majority of outstanding Shares is required to effect certain fundamental corporate changes such as liquidation, merger, or amendment of our articles of incorporation.
| 70 |
There are no restrictions in our articles of incorporation or bylaws that prevent us from declaring dividends. The Colorado Act does, however, prohibit us from declaring dividends where, after giving effect to the distribution of the dividend (1) we would not be able to pay our debts as they become due in the usual course of business or (2) our total assets would be less than the sum of our total liabilities plus the amount that would be needed to satisfy the rights of stockholders who have preferential rights superior to those receiving the distribution.
Holders of Shares of our common stock are not entitled to preemptive or subscription or conversion rights, and no redemption or sinking fund provisions are applicable to our common stock. All outstanding Shares of common stock sold in the offering are, or when issued pursuant to the terms of any convertible securities or warrants will be, fully paid and non-assessable.
Each share of our common stock is entitled to equal dividends and distributions per share with respect to the common stock when, as and if declared by our Board of Directors. No holder of any shares of our common stock has a preemptive right to subscribe for any of our securities, nor are any shares of our common stock subject to redemption or convertible into other securities. Upon liquidation, dissolution or winding-up of the Company, and after payment to our creditors and preferred stockholders, if any, our assets will be divided pro rata on a share-for-share basis among the holders of our common stock. Each share of our common stock is entitled to one vote on all stockholder matters. Shares of our common stock do not possess any cumulative voting rights.
The presence of the persons entitled to vote a majority of the outstanding voting shares on a matter before the stockholders constitute the quorum necessary for the consideration of the matter at a stockholders’ meeting.
Except as otherwise required by law, the Articles of Incorporation, or any certificate of designations, (i) at all meetings of stockholders for the election of directors, a plurality of votes cast are sufficient to elect such directors; (ii) any other action taken by stockholders are be valid and binding upon the Company if the number of votes cast in favor of the action exceeds the number of votes cast in opposition to the action, at a meeting at which a quorum is present, except that adoption, amendment or repeal of the Bylaws by stockholders requires the vote of a majority of the shares entitled to vote; and (iii) broker non-votes and abstentions are considered for purposes of establishing a quorum but not considered as votes cast for or against a proposal or director nominee. Each stockholder has one vote for every share of stock having voting rights registered in his or her name, except as otherwise provided in any preferred stock designation setting forth the right of preferred stock stockholders.
The common stock does not have cumulative voting rights, which means that the holders of 51% of the common stock voting for election of directors can elect 100% of our directors if they choose to do so.
Preferred Stock
Our Articles of Incorporation gives our Board of Directors authority to issue shares of “blank check” preferred stock from time to time in one or more series, pursuant to resolutions adopted by the Board in accordance with the Colorado Act each having the voting powers, if any, designations, powers, preferences, and the relative, participating, optional, or other rights, if any, and the qualifications, limitations, or restrictions thereof, of any unissued series of preferred stock, to fix the number of shares constituting such series, and to increase or decrease the number of shares of any such series, but not below the number of shares thereof then outstanding, without the delay attendant to obtaining stockholder approval for such issuance.
We may issue preferred stock to effect a business combination, to raise capital or for other reasons. In addition, preferred stock could be utilized as a method of discouraging, delaying or preventing a change in control of our Company.
Our board of directors has no intention at the present time to create any additional series of preferred stock. The issuance of any new series of preferred stock could, depending on the terms of such series, impede the completion of a merger, tender offer or other takeover attempt.
| 71 |
Any future issuance of preferred stock may have the effect of delaying, deferring or preventing a change in control of us without further action by the shareholders and may adversely affect the voting and/or other rights of the holders of common stock or any other securities we may issue in the future. The issuance of shares of preferred stock, or the issuance of rights to purchase such shares, could be used to discourage an unsolicited acquisition proposal. For instance, the issuance of a series of preferred stock might impede a business combination by including class voting rights that would enable the holders to block such a transaction or facilitate a business combination by including voting rights that would provide a required percentage vote of the stockholders. In addition, under certain circumstances, the issuance of preferred stock could adversely affect the voting power of the holders of the common stock. Although our board of directors is required to make any determination to issue such stock based on its judgment as to the best interests of our stockholders, the board of directors could act in a manner that would discourage an acquisition attempt or other transaction that some, or a majority, of the stockholders might believe to be in their best interests or in which stockholders might receive a premium for their stock over the then market price of such stock. Our board of directors does not at present intend to seek stockholder approval prior to any issuance of currently authorized preferred stock, unless otherwise required by law.
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there existed only a limited public market for our common stock. Future sales of substantial amounts of our common stock in the public market, including shares issued upon exercise of outstanding warrants, or the anticipation of such sales, could adversely affect prevailing market prices of our common stock from time to time and could impair our ability to raise equity capital in the future.
Upon the closing of this offering, we will have [20,374,842] shares of our common stock issued and outstanding. In addition, we will have [118,750] shares of common stock issuable upon the exercise of the Underwriter’s Warrants.
Lock-Up
For further details on the lock-up agreements, see the section entitled “Underwriting – Lock-Up Agreements.”
Rule 144
In general, under Rule 144 of the Securities Act, as in effect on the date of this prospectus, any person who is not our affiliate at any time during the preceding three months, and who has beneficially owned their shares for at least six months, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares of our common stock provided current public information about us is available, and, after owning such shares for at least one year, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares of our common stock without restriction.
A person who is our affiliate or who was our affiliate at any time during the preceding three months, and who has beneficially owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell within any three-month period a number of shares that does not exceed the greater of:
| ● | 1% of the number of shares of our common stock then outstanding, which will equal approximately 242,499 shares immediately after this offering; or | |
| ● | the average weekly trading volume of our common stock during the four calendar weeks preceding the filing of a Notice of Proposed Sale of Securities pursuant to Rule 144 with respect to the sale. |
Sales under Rule 144 by our affiliates are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us.
| 72 |
UNDERWRITING
In connection with this offering, we will enter into an underwriting agreement with Boustead Securities, LLC, as the underwriter, with respect to the common stock in this offering. Under the terms and subject to the conditions contained in the underwriting agreement, the underwriter will agree to purchase from us on a firm commitment basis, and we have agreed to sell to the underwriter, the number of shares set opposite its name below, at the offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus:
| Name | Number of shares | |||
| Boustead Securities, LLC | ||||
| Total | ||||
The common stock sold by the underwriter to the public will initially be offered at the offering price set forth on the cover page of this prospectus. Any common stock sold by the underwriter to securities dealers may be sold at a discount from the public offering price not to exceed $[*] per share. If all of the shares of common stock are not sold at the public offering price, the underwriter may change the offering price and the other selling terms. The underwriter has advised us that that it does not intend to make sales to discretionary accounts.
The underwriting agreement will provide that the obligations of the underwriter to pay for and accept delivery of the common stock are subject to the passing upon certain legal matters by counsel and certain conditions such as confirmation of the accuracy of representations and warranties by us about our financial condition and operations and other matters. The obligation of the underwriter to purchase the common stock is conditioned upon our receiving approval to list the common stock on the Nasdaq Capital Market.
Over-Allotment Option
If the underwriter sells more shares of common stock than the total number set forth in the table above, we have granted to the underwriter an option, exercisable one or more times in whole or in part, not later than 45 days after the date of this prospectus, to purchase from us up to additional [356,250] shares of common stock (constituting up to 15% of the shares of common stock sold in this offering, excluding the shares subject to this option), at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus. The underwriter may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with this offering. Any shares of common stock issued or sold under the option will be issued and sold on the same terms and conditions as the other shares of common stock that are the subject of this offering.
Discounts and Commissions; Expenses
The underwriting discounts and commissions are a cash fee equal to eight percent (8%) of gross proceeds from the sale of securities in this offering. We have been advised by the underwriter that it proposes to offer the common stock to the public at the public offering price set forth on the cover of this prospectus and to dealers at a price that represents a concession not in excess of US$ per share under the public offering price. After the offering, the underwriter may change the public offering price and other selling terms.
The following table summarizes the public offering price and underwriting discounts and commissions payable to the underwriter by us in connection with this offering, assuming both the non-exercise and full exercise of the underwriter’s over-allotment option to purchase additional shares of common stock.
| Total | ||||||||||||
| Per Share | Total Without Exercise of Over- Allotment Option | Total With Full Exercise of Over- Allotment Option | ||||||||||
| Public offering price | $ | 4.00 | $ | 9,500,000 | $ | 10,925,000 | ||||||
| Underwriting discounts and commissions (8%)(1) | $ | 0.32 | $ | 760,000 | $ | 874,000 | ||||||
| Proceeds, before expenses, to us | $ | 3.68 | $ | 8,740,000 | $ | 10,051,000 | ||||||
| (1) | Does not include (i) the warrant to purchase a number of shares of common stock equal to 5% of the number of shares sold in the offering, (ii) a 1% non-accountable expense allowance or (iii) amounts representing reimbursement of certain out-of-pocket expenses, each as described below. |
We have agreed to pay a non-accountable expense allowance to the underwriter equal to 1% of the gross proceeds received at the closing of the offering.
We have agreed to reimburse the underwriter for reasonable out-of-pocket expenses incurred relating to the offering, regardless of whether the offering is consummated, not to exceed $125,000. Such accountable out-of-pocket expenses include no more than $75,000 in underwriter’s legal counsel fees, due diligence and other like expenses not to exceed $2,500; expenses relating to the preparation of bound volumes and Lucite cube mementos not to exceed $2,500; and background checks expenses not to exceed $7,500. Other than the aforementioned, any expense exceeding $2,500 shall be pre-approved in writing by us. Any advance that we pay to the underwriter to be applied against actual out-of-pocket accountable expenses will be refunded to us to the extent any portion of the advance is not actually incurred, in compliance with FINRA Rule 5110(g)(4)(A).
| 73 |
Underwriter’s Warrants
We have agreed to issue to the underwriter (or its permitted assignees) warrants to purchase up to a total of [*] shares of common stock, or [*] shares of common stock if the underwriter’s over-allotment option is exercised in full (constituting five percent (5%) of the shares of common stock sold in the offering) at an exercise price of $5.00 per share (125% of the public offering price). The Underwriter’s Warrants will be exercisable at any time, and from time to time, in whole or in part, commencing on the closing of the offering and expiring five (5) years from the date on which sales of the shares offered hereby commence, will have a cashless exercise provision and will terminate on the fifth (5th) anniversary of the effective date of the registration statement of which this prospectus is a part. The Underwriter’s Warrant will also provide for customary anti-dilution provisions and “piggyback” registration rights with respect to the registration of the common stock underlying the warrants for a period of seven (7) years from the commencement of sales of this offering. We have registered the Underwriter’s Warrant and the common stock underlying the Underwriter’s Warrant in this offering.
The Underwriter’s Warrants and the underlying shares are deemed compensation by FINRA and therefore will be subject to a 180-day lock-up pursuant to FINRA Rule 5110(e)(1). The underwriter (or permitted assignees) under FINRA Rule 5110(e)(1) will not sell, transfer, assign, pledge, or hypothecate these warrants or the securities underlying these warrants, nor will they engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the warrants or the underlying securities for a period of 180 days immediately following commencement of sale of this offering subject to certain exceptions permitted by FINRA Rule 5110(e)(2).
The registration statement of which this prospectus is a part also registers for sale the Underwriter’s Warrants and the common stock issuable upon exercise of such warrants, as a portion of the underwriting compensation payable in connection with this offering.
Indemnification
We have agreed to indemnify the underwriter and certain of its controlling persons against certain liabilities, including liabilities under the Securities Act. If we are unable to provide this indemnification, we will contribute to payments that the underwriter may be required to make for these liabilities.
Future Services
In the event we pursue a future financing, including a registered, underwritten public offering of securities (in addition to this offering), a public or private offering of securities (debt or equity), a merger, acquisition of another company or business, change of control, sale of substantially all assets, business combination, recapitalization or other similar transaction (regardless of whether we would be considered an acquiring party, a selling party or neither in such transaction), and such future financing is consummated (a) solely with investors with whom the Company has had a conference call or a meeting arranged by the underwriter within the twelve (12) month period ended December 30, 2024, and (b) within twelve (12) months from the completion of this offering, the underwriter shall be entitled to a cash fee equal to eight percent (8%) of the proceeds of such future financing and warrants to purchase five percent (5%) of the securities sold in such future financing, which warrants shall have an exercise price equal to 125% of the exercise price of the purchase price of the securities sold in such future financing and having a term of five years.
| 74 |
No Sales of Similar Securities
We have agreed not to offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any shares of our common stock or other securities convertible into or exercisable or exchangeable for shares of our common stock at a price per share that is less than the price per share in this offering, or modify the terms of any existing securities, whether in conjunction with another broker-dealer or on the Company’s own volition, for a period of twelve months following the date on which shares of our common stock are trading on the Nasdaq Capital Market, without the prior written consent of the underwriter.
Lock-Up Agreements
Our officers, directors, and holders of 5% or greater of our common stock have agreed to be locked up for a period of twelve months from the date on which the trading of our common stock commences on the Nasdaq Capital Market. Holders of 1%-4.99% of our common stock have agreed to be locked up for a period of six months from the date on which the trading of our common stock commences on the Nasdaq Capital Market, provided that if the aggregate of such holders shares were to equal or exceed 20% of our issued and outstanding shares on a fully diluted basis prior to the completing of this offering, then their lock up period shall be for twelve months from the date of trading of our common stock commences on the Nasdaq Capital Market. Holders of less than 1% of our common stock are not subject to any lock up provided that if the aggregate of such holders shares were to equal or exceed 5% of our issued and outstanding shares on a fully diluted basis prior to the completing of this offering, then their lock up period shall be for six months from the date of trading of our common stock commences on the Nasdaq Capital Market. During the lock-up period, without the prior written consent of the underwriter, they shall not, directly or indirectly, (i) offer, pledge, assign, encumber, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, any shares of common stock or any securities convertible into or exercisable or exchangeable for common stock, owned either of record or beneficially by any signatory of the lock-up agreement on the date of the prospectus or thereafter acquired; (ii) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the shares of common stock or any securities convertible into or exercisable or exchangeable for common stock, whether any such transaction described in clauses (i) or (ii) above is to be settled by delivery of common stock or such other securities, in cash or otherwise, or publicly announce an intention to do any of the foregoing; and (iii) make any demand for or exercise any right with respect to, the registration of any shares of common stock or any security convertible into or exercisable or exchangeable for common stock.
Trading; Nasdaq Listing
We have applied to list our common stock on the Nasdaq Capital Market under the symbol “AIXN”. There can be no assurance that our listing application will be approved by Nasdaq. The approval of our listing on the Nasdaq Capital Market is a condition of closing this offering.
Price Stabilization, Short Positions and Penalty Bids
In connection with the offering, the underwriter may purchase and sell shares in the open market. Purchases and sales in the open market may include short sales, purchases to cover short positions, which may include purchases pursuant to the over-allotment option, and stabilizing purchases. Short sales involve secondary market sales by an underwriter of a greater number of shares than they are required to purchase in the offering.
| ● | “Covered” short sales are sales of shares in an amount up to the number of shares represented by the over-allotment option. | |
| ● | “Naked” short sales are sales of shares in an amount in excess of the number of shares represented by the over-allotment option. | |
| ● | Covering transactions involve purchases of shares either pursuant to the over-allotment option or in the open market after the distribution has been completed in order to cover short positions. | |
| ● | To close a naked short position, an underwriter must purchase shares in the open market after the distribution has been completed. A naked short position is more likely to be created if an underwriter is concerned that there may be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. | |
| ● | To close a covered short position, an underwriter must purchase shares in the open market after the distribution has been completed or must exercise the over-allotment option. In determining the source of shares to close the covered short position, the underwriter will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. | |
| ● | Stabilizing transactions involve bids to purchase shares so long as the stabilizing bids do not exceed a specified maximum. |
Purchases to cover short positions and stabilizing purchases, as well as other purchases by an underwriter for its own account, may have the effect of preventing or retarding a decline in the market price of the common stock. They may also cause the price of the common stock to be higher than the price that would otherwise exist in the open market in the absence of these transactions. The underwriter may conduct these transactions in the over-the-counter market or otherwise. If the underwriter commence any of these transactions, they may discontinue them at any time.
| 75 |
Determination of Offering Price
In determining the public offering price, we and the underwriter have considered a number of factors, including:
| ● | the information set forth in this prospectus and otherwise available to the underwriter; | |
| ● | our prospects and the history and prospects for the industry in which we compete; | |
| ● | an assessment of our management; | |
| ● | our prospects for future revenues and earnings; | |
| ● | the general condition of the securities markets at the time of this offering; | |
| ● | the recent market prices of, and demand for, publicly traded securities of generally comparable companies; and | |
| ● | Other factors deemed relevant by the underwriter and us. |
The estimated public offering price set forth on the cover page of this preliminary prospectus is subject to change as a result of market conditions and other factors. Neither we nor the underwriter can assure investors that an active trading market will develop for our common stock, or that the common stock will trade in the public market at or above the initial public offering price.
Electronic Distribution
A prospectus in electronic format may be made available on the websites maintained by the underwriter. In addition, the common stock may be sold by the underwriter to securities dealers who resell the common stock to online brokerage account holders. Other than the prospectus in electronic format, the information on the underwriter’s website and any information contained in any other website maintained by the underwriter is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the underwriter in its capacity as underwriter and should not be relied upon by investors.
| 76 |
Other Relationships
The underwriter and its respective affiliates may in the future engage in investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. They may in the future receive customary fees and commissions for these transactions. Except for services provided in connection with this offering, the underwriter has not provided any financing, investment and/or advisory services to us during the 180 day period preceding the initial filing of the registration statement of which this prospectus forms a part, and as of the date of this prospectus, we do not have any agreement or arrangement with the underwriter to provide any of such services during the 60 day period following the effective date of such registration statement.
Offers Outside the United States
Other than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
The underwriter is expected to make offers and sales both in and outside the United States through its selling agents. Any offers and sales in the United States will be conducted by broker-dealers registered with the SEC.
Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriter is not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
European Economic Area — Belgium, Germany, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Regulation (EU) 2017/1129 (the “Prospectus Regulation”), as implemented in Member States of the European Economic Area (the “Relevant Member States”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Regulation:
| ● | to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; | |
| ● | to any legal entity that has two or more of (i) an average of at least 250 employees during its last financial year, (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements), and (iii) an annual net turnover of more than €50,000,000 (as shown in its last annual unconsolidated or consolidated financial statements); | |
| ● | to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive) subject to obtaining the prior consent of our Company or any underwriter for any such offer; or | |
| ● | in any other circumstances falling within Article 3(2) of the Prospectus Regulation, provided that no such offer of securities shall result in a requirement for the publication by our Company of a prospectus pursuant to Article 3 of the Prospectus Regulation; |
| 77 |
France
This document is not being distributed in the context of a public offering of financial securitestroffre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1, et seq. of the General Regulation of the French Autoritéestrairchés financiers (the “AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investissestraintifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (ceestraintreint d’investisseurs non-qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Societá e la Borsa (“CONSOB”)) pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
| ● | to Italian qualified investors, as defined in Article 100 of Decree No. 58 by reference to Article 34-3 of CONSOB Regulation no. 11971 of 14 May 1999, as amended (“Regulation no. 11971”); and |
| ● | in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a qualified investor solicits an offer from the issuer) under the paragraphs above must be:
| ● | made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and |
| ● | in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
| 78 |
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissão do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (the “SIX”), or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority.
This document is personal to the recipient only and not for general circulation in Switzerland.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (the “FSMA”)) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to our Company.
| 79 |
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (the “FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated. The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, such persons. Any person who is not such a person should not act or rely on this document or any of its contents.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”), pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the People’s Republic of China to legal or natural persons other than directly to “qualified domestic institutional investors.”
Hong Kong
The securities have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the securities has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to securities which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
| 80 |
All applicable provisions of the FSMA with respect to anything done by the underwriter in relation to the shares must be complied with in, from or otherwise involving the United Kingdom.]
LEGAL MATTERS
We are being represented by Ellenoff Grossman & Schole LLP with respect to certain legal matters as to United States federal securities and New York State law. The validity of the shares of our common stock offered hereby has been passed upon for us by Armstrong Teasdale LLP, Denver, Colorado. The underwriter has been represented in connection with this offering by Bevilacqua PLLC. Certain legal matters relating to the offering as to PRC law will be passed upon for us by Beijing Dentons Law Offices, LLP (Chengdu) and for the underwriter by [ ]. Ellenoff Grossman & Schole LLP may rely upon Beijing Dentons Law Offices, LLP (Chengdu) with respect to matters governed by PRC Law. Bevilacqua PLLC may rely upon [ ] with respect to matters governed by PRC law.
EXPERTS
KCCW Accountancy Corp., independent registered public accounting firm, has audited our financial statements as of and for the years ended December 31, 2022 and 2021 as set forth in their report.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act, with respect to the shares of common stock being offered by this prospectus. This prospectus does not contain all of the information in the registration statement and its exhibits. For further information with respect to us and the common stock offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street NE, Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street NE, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities. You may also request a copy of these filings, at no cost, by writing us at AiXin Life International Inc., Hongxing International Business Building 2, 14th FL, No. 69 Qingyun South Ave., Jinjiang District, Chengdu City, Sichuan Province, China
We are subject to the information reporting requirements of the Exchange Act, and file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information are available for inspection and copying at the public reference room and web site of the SEC referred to above. We also maintain a website at www.addentax.com, at which, following the closing of this offering, you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website incorporated by reference in, and is not part of, this prospectus.
| 81 |
FINANCIAL STATEMENTS
| F-1 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
AiXin Life International, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of AiXin Life International, Inc. (the “Company”) as of December 31, 2022 and 2021, the related consolidated statements of operations and comprehensive loss, stockholders’ equity (deficit), and cash flows for the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2022 and 2021, and the results of its operations and its cash flows for the years ended December 31, 2022 and 2021, in conformity with generally accepted accounting principles in the United States of America.
Consideration of the Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. As described in Note 2 to the financial statements, the Company incurred recurring losses from operations and has an accumulated deficit, which raises substantial doubt about its ability to continue as a going concern. Management’s plans with regard to these matters are described in Note 2. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current-period audit of the financial statements that was communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
| F-2 |
Critical Audit Matter Description
As described in Note 2 to the consolidated financial statements, the Company’s products revenue is derived from the delivery of its products. The sale of products by the Company is considered complete when the products are delivered at that time the ownership and risk of loss have been transferred to the customer.
The Company considers the contracts with its customer contain one performance obligation, and the Company is entitled to the consideration when performance obligation is satisfied at a point in time. The amount of revenue to be recognized is determined by the contracts between the Company and its customer. The Company recognizes revenue when the product is delivered.
The principal considerations for our determination that performing procedures relating to revenue recognition, specifically the identification and evaluation of the timing and amount of revenue recognition, is a critical audit matter, involved judgment exercised by management in identifying and evaluating the performance obligation. Auditor judgement is involved in performing our audit procedures to evaluate whether the timing and amount of revenue recognition was appropriately stated.
How the Critical Audit Matter Will Be Addressed in the Audit
Our audit procedures over determining the timing and amount of revenue recognition involved, among others, evaluation of management’s assessment in regard to the identification of performance obligation of revenue. We selected sales transactions and performed the following procedures:
- Evaluated the terms and conditions of each selected transaction and the appropriateness of the accounting treatment within the context of the five-step model prescribed by ASC 606, Revenue from Contracts with Customers, and evaluated whether management’s conclusions were appropriate.
- Tested the accuracy of management’s recognition of revenue for the performance obligation.
| /s/ KCCW Accountancy Corp. |
We have served as the Company’s auditor since 2019.
Diamond Bar, California
April 27, 2023

| F-3 |
AIXIN LIFE INTERNATIONAL, INC.
CONSOLIDATED BALANCE SHEETS
| December 31, | December 31, | |||||||
| 2022 | 2021 | |||||||
| Assets | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Restricted cash | ||||||||
| Accounts receivable, including related party, net | ||||||||
| Other receivables and prepaid expenses | ||||||||
| Advances to suppliers | ||||||||
| Inventory | ||||||||
| Advances to related parties | ||||||||
| Total current assets | ||||||||
| Property and equipment, net | ||||||||
| Intangible asset, net | ||||||||
| Deferred tax asset | ||||||||
| Security deposit | ||||||||
| Operating lease right-of-use assets | ||||||||
| Goodwill, net | ||||||||
| Total assets | $ | $ | ||||||
| Liabilities and stockholders’ equity | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | $ | ||||||
| Accounts payable-related party | ||||||||
| Unearned revenue | ||||||||
| Taxes payable | ||||||||
| Accrued liabilities and other payables | ||||||||
| Government grant | ||||||||
| Loan from third parties | ||||||||
| Operating lease liabilities | ||||||||
| Advance from related parties | ||||||||
| Total current liabilities | ||||||||
| Operating lease liabilities - non-current | ||||||||
| Total liabilities | ||||||||
| Stockholders’ equity (deficit) | ||||||||
| Undesignated preferred stock, $ par value, shares authorized, issued and outstanding | ||||||||
| Common stock, par value $ per share, shares authorized; shares issued and outstanding as of December 31, 2022 and 2021 | ||||||||
| Additional paid in capital | ||||||||
| Statutory reserve | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Accumulated other comprehensive income | ||||||||
| Total stockholders’ equity (deficit) | ( | ) | ||||||
| Total liabilities and stockholders’ equity (deficit) | $ | $ | ||||||
The accompanying notes are an integral part of these financial statements
| F-4 |
AIXIN LIFE INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Sales revenue: | ||||||||
| Products | $ | $ | ||||||
| Advertising | ||||||||
| Room revenues | ||||||||
| Food and beverage revenues | ||||||||
| Others | ||||||||
| Total revenue, net | ||||||||
| Operating costs and expenses | ||||||||
| Cost of goods sold | ||||||||
| Hotel operating costs | ||||||||
| Selling | ||||||||
| General and administrative | ||||||||
| (Reversal of) provision for bad debts | ( | ) | ( | ) | ||||
| Stock-based compensation | ||||||||
| Total operating costs and expenses | ||||||||
| Loss from operations | ( | ) | ( | ) | ||||
| Non-operating income (expenses) | ||||||||
| Interest income | ||||||||
| Impairment loss | ( | ) | ||||||
| Other income | ||||||||
| Other expenses | ( | ) | ( | ) | ||||
| Total non-operating income (expenses), net | ( | ) | ||||||
| (Loss) income before income tax | ( | ) | ||||||
| Income tax expense | ||||||||
| Net loss | ( | ) | ( | ) | ||||
| Other comprehensive items | ||||||||
| Foreign currency translation (loss) gain | ( | ) | ||||||
| Comprehensive loss | $ | ( | ) | $ | ( | ) | ||
| Loss per share - basic and diluted | $ | ) | $ | ) | ||||
| Weighted average shares outstanding - basic and diluted | ||||||||
The accompanying notes are an integral part of these financial statements
| F-5 |
AIXIN LIFE INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
| Common Stock | Additional | Accumulated other | ||||||||||||||||||||||||||
| Shares | Amount | paid in capital | Statutory reserves | Accumulated deficit | comprehensive income | Total | ||||||||||||||||||||||
| Balance at December 31, 2020 | $ | $ | $ | $ | ( | ) | $ | $ | ||||||||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||||||||||
| Acquisition of subsidiaries | - | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||
| Debt forgiven by major shareholder | - | |||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation | - | |||||||||||||||||||||||||||
| Balance at December 31, 2021 | ( | ) | ||||||||||||||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Balance at December 31, 2022 | $ | $ | $ | $ | ( | ) | $ | $ | ( | ) | ||||||||||||||||||
The accompanying notes are an integral part of these financial statements
| F-6 |
AIXIN LIFE INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments required to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | ||||||||
| Provision for bad debt | ||||||||
| Provision for inventory reserve | ||||||||
| Operating lease expense | ||||||||
| Stock-based compensation | ||||||||
| Deferred tax | ( | ) | ||||||
| Impairment loss | ||||||||
| Changes in assets and liabilities: | ||||||||
| Accounts receivable | ( | ) | ( | ) | ||||
| Accounts receivable - related parties | ( | ) | ||||||
| Other receivables and prepaid expenses | ||||||||
| Advances to suppliers | ||||||||
| Inventory | ||||||||
| Accounts payable | ( | ) | ( | ) | ||||
| Accounts payable - related party | ||||||||
| Unearned revenue | ( | ) | ( | ) | ||||
| Taxes payable | ( | ) | ( | ) | ||||
| Payment of operating lease liabilities | ( | ) | ( | ) | ||||
| Accrued liabilities and other payables | ( | ) | ||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Cash acquired from acquisition of subsidiaries | ||||||||
| Purchase of property and equipment | ( | ) | ( | ) | ||||
| Payment for acquisition of subsidiaries | ( | ) | ( | ) | ||||
| Net cash used in investing activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Change in advance from related parties | ( | ) | ||||||
| Repayment of loan from third parties | ( | ) | ||||||
| Capital contribution | ||||||||
| Net cash (used in) provided by financing activities | ( | ) | ||||||
| EFFECT OF EXCHANGE RATE CHANGE ON CASH AND CASH EQUIVALENTS AND RESTRICTED CASH | ( | ) | ||||||
| NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS AND RESTRICTED CASH | ( | ) | ||||||
| CASH AND CASH EQUIVALENTS AND RESTRICTED CASH, BEGINNING OF YEAR | ||||||||
| CASH AND CASH EQUIVALENTS AND RESTRICTED CASH, END OF YEAR | $ | $ | ||||||
| Supplemental Cash flow data: | ||||||||
| Income tax paid | $ | $ | ||||||
| Interest paid | $ | $ | ||||||
| Non-cash investing and financing activities | ||||||||
| Capital contribution from forgiveness of related party loan | $ | $ | ||||||
The accompanying notes are an integral part of these financial statements
| F-7 |
AIXIN LIFE INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. ORGANIZATION AND DESCRIPTION OF BUSINESS
Aixin
Life International, Inc. (the “Company” or “Aixin Life” or “we”) was incorporated under the laws
of the State of Colorado on August 31, 2001 under the name Mercari Communications Group, Ltd (“Mercari”). On February
2, 2017, Mr. Quanzhong Lin (Mr. Lin) purchased shares of the Company’s
common stock,
On December 12, 2017, the Company issued shares of common stock to Mr. Lin, the sole stockholder of AiXin (BVI) International Group Co., Ltd. a British Virgin Islands corporation (“AiXin BVI”), for his shares of AiXin BVI, pursuant to a Share Exchange Agreement.
As a result of the Share Exchange, AiXin BVI became the Company’s wholly-owned subsidiary, and the Company now owns all of the outstanding shares of HK AiXin International Group Co., Limited, a Hong Kong limited company (“AiXin HK”), which in turn owns all of the outstanding shares of Chengdu AiXinZhonghong Biological Technology Co., Ltd., a Chinese limited company (“AiXinZhonghong”), which markets and sells premium-quality nutritional products in China.
AiXin BVI was incorporated on September 21, 2017 as a holding company and AiXin HK was established in Hong Kong on February 25, 2016 as an intermediate holding company. AiXinZhonghong was established in the People’s Republic of China (“PRC”) on March 4, 2013, and on May 27, 2017, the local government of the PRC issued a certificate of approval regarding the foreign ownership of AiXinZhonghong by AiXin HK. Neither AiXin BVI nor AiXin HK had operations prior to December 12, 2017.
For accounting purposes, the acquisition of AiXin BVI was accounted for as a reverse acquisition and treated as a recapitalization of the Company effected by a share exchange, with AiXin BVI as the accounting acquirer. Since neither AiXin BVI nor AiXin HK had operations prior to December 12, 2017, the historical consolidated financial statements of AiXinZhonghong are now the historical consolidated financial statements of the Company. The assets and liabilities of AiXinZhonghong were brought forward at their book value and no goodwill was recognized.
Effective February 1, 2018, the Company changed its name to AiXin Life International, Inc. (“Aixin Life”).
The Company, through its indirectly owned AiXinZhonghong subsidiary, develops and distributes consumer products by offering a line of nutritional products. The Company sells the products through exhibition events, conferences, and person-to-person marketing. Beginning in 2019, the Company began to provide advertising services to clients who engaged the Company to help distribute their products. The Company’s business mainly focuses on a proactive approach to its customers such as hosting events for clients, which it believes is ideally suited to marketing its products because sales of nutrition products are strengthened by ongoing personal contact and support, coaching and education of its clients, as to the benefits of a healthy and active lifestyle.
On
May 25, 2021, AiXin HK entered into an Equity Transfer Agreement (the “Hotel Purchase Agreement”) with Chengdu Aixin Shangyan
Hotel Management Co., Ltd (“Aixin Shangyan Hotel”), and its two shareholders Quanzhong Lin and Yirong Shen (“Transferor”).
Pursuant to the Hotel Purchase Agreement, Aixin Life purchased
| F-8 |
On
June 2, 2021, AiXin HK entered into an Equity Transfer Agreement (the “Pharmacies Purchase Agreement”) with Chengdu Aixintang
Pharmacy Co., Ltd. and certain affiliated entities, each of which operates a pharmacy (together, “Aixintang Pharmacies”)
and its three shareholders, Quanzhong Lin, Ting Li and Xiao Ling Li (“Transferor”). Mr. Lin owned in excess of 95% of the
outstanding equity the Aixintang Pharmacies. The remaining equity interest was owned by Ting Li and Xiao Ling Li. Pursuant to the Pharmacies
Purchase Agreement, AiXin HK purchased all of the outstanding equity of Aixintang Pharmacies for an aggregate purchase price of RMB
On February 17, 2023, the Company effected a 1 for 2 reverse stock split. As a result of the reverse split, every two shares of the Company’s issued and outstanding common stock will be automatically combined and converted into one issued and outstanding share of common stock, par value $per share. The Company has approximately shares of outstanding common stock after the effect of reverse stock split. All share and earnings per share information has been retroactively adjusted to reflect the reverse stock split.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements are prepared in conformity with U.S. Generally Accepted Accounting Principles (“US GAAP”). The functional currency of AiXinZhonghong, Aixin Shangyan Hotel, Aixintang Pharmacies and Runcangsheng is the Chinese Renminbi (“RMB”). The accompanying consolidated financial statements are translated from RMB and presented in U.S. dollars (“USD”).
The consolidated financial statements include the accounts of the Company and its current wholly owned subsidiaries, AiXin HK, AiXinZhonghong, Aixin Shangyan Hotel, Aixintang Pharmacies and Runcangsheng. Intercompany transactions and accounts were eliminated in consolidation.
Going Concern
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The realization of assets and the satisfaction of liabilities in the normal course of business are dependent on, among other things, the Company’s ability to operate profitably, to generate cash flows from operations, and to pursue financing arrangements to support its working capital requirements.
The
Company has suffered losses from operations of $
| F-9 |
Management believes that it has developed a liquidity plan, as summarized below, that, if executed successfully, should provide sufficient liquidity to meet the Company’s obligations as they become due for a reasonable period of time, and allow the development of its core business. The plan includes:
● Gaining positive cash-inflow from operating activities through continuous cost reductions and the sales of higher margin products.
● Raising additional cash through loans from related parties and potential equity offerings
While the Company’s management believes that the measures described in the above liquidity plan will be adequate to satisfy its liquidity requirements for the twelve months after the date that the financial statements are issued, there is no assurance that the liquidity plan will be successfully implemented. Failure to successfully implement the liquidity plan may have a material adverse effect on its business, results of operations and financial position, and may adversely affect its ability to continue as a going concern. These consolidated financial statements do not include any adjustments related to the recoverability and classification of recorded assets or the amounts and classification of liabilities or any other adjustments that might be necessary should the Company be unable to continue as a going concern.
COVID – 19; The Invasion of Ukraine
On March 11, 2020, the World Health Organization announced that infections caused by the corona virus disease of 2019 (“COVID-19”) had become pandemic. In furtherance of its zero tolerance policy, the Government of China has adopted various regulations and orders, including mandatory quarantines, limits on the number of people that may gather in one location, closing non-essential businesses and travel bans to limit the spread of the disease. Many of these measures have been relaxed from time to time in various localities. However, beginning in the second half of 2021 to the end of 2022, the rate of COVID-19 cases has fluctuated in China and has increased in many provinces and cities including in Sichuan Province, where the Company is located. As a result of such increases there have been periodic short-term lockdowns and restrictions on travel in Sichuan Province. All of the Company’s operations, in particular its direct sales business and hotel, have been adversely impacted by the travel and work restrictions imposed on a temporary basis in China and Chengdu to limit the spread of COVID-19. Since January 2023, China has dropped all COVID restrictions.
In response to COVID-19, the Company has implemented procedures to promote employee and customer safety. These measures will not significantly increase its operating costs. However, the Company cannot predict with certainty what measures may be taken by its suppliers and customers and the impact these measures may have on its future financial position, results of operations or cash flows.
The invasion of Ukraine by the Russian Federation had an immediate impact on the global economy resulting in higher prices for oil and other commodities. The United States, United Kingdom, European Union and other countries responded to Russia’s invasion of Ukraine by imposing various economic sanctions and bans. Russia has responded with its own retaliatory measures. These measures have disrupted financial and economic markets. The global impact of these measures is continually evolving and cannot be predicted with certainty and there is no assurance that Russia’s invasion of Ukraine and responses thereto will not further disrupt the global economy and financial markets.
While the invasion of Ukraine and responses thereto have not interrupted the Company’s operations, these or future developments resulting from the invasion of Ukraine could make it difficult to access debt and equity capital on attractive terms, if at all, and impact the Company’s ability to fund business activities, including proposed acquisitions.
Use of Estimates
In preparing consolidated financial statements in conformity with US GAAP, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the consolidated financial statements, as well as the reported amounts of revenues and expenses during the reporting period.
| F-10 |
Significant estimates, required by management, include the recoverability of long-lived assets, allowance for doubtful accounts, and the reserve for obsolete and slow-moving inventories. Actual results could differ from those estimates.
Reclassification
Certain prior period amounts have been reclassified to conform to the current period presentation and had no effect on previously reported consolidated net income (loss) or accumulated deficit.
Cash and Cash Equivalents
For financial statement purposes, the Company considers all highly liquid investments with an original maturity of three months or less to be cash and cash equivalents.
Restricted Cash
The restricted cash reflects the temporary freeze of bank accounts of Aixintang Pharmacy Co., Ltd. (“Aixintang Pharmacy”) and its branches by the court during the appeal of a judgement against Aixintang Pharmacy (see Note 16 – litigation).
Accounts Receivable
The
Company’s policy is to maintain an allowance for potential credit losses on accounts receivable. Management reviews the composition
of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends
and changes in customer payment patterns to evaluate the adequacy of these reserves. As of December 31, 2022 and 2021, the bad debt allowance
was $
The following table summarizes the activity related to the Company’s Accounts Receivable allowance for doubtful accounts for the years ended December 31, 2022 and 2021:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Beginning balance | $ | $ | ||||||
| Additions* | ||||||||
| Provisions for bad debt | ||||||||
| Recoveries/Write offs | ( | ) | ( | ) | ||||
| Effect of translation | ( | ) | ||||||
| Ending balance | $ | $ | ||||||
| * |
Inventories
Inventories
mainly consists of health supplements, drugs, pharmaceutical and nutritional products, food and beverage, hotel supplies and consumables.
Inventories are valued at the lower of average cost or market, cost being determined on a moving weighted average method at the end of
the month. Management compares the cost of inventories with the net realizable value and an allowance is made for writing down inventories
to market value, if lower. Management periodically evaluates the composition of its inventories to identify slow-moving and obsolete
inventories to determine if a valuation allowance is required. The balance of reserve for inventory valuation as of December 31, 2022
and 2021 was $
| F-11 |
Property and Equipment
Property
and equipment are stated at cost, less accumulated depreciation, and impairment losses, if any. Major repairs and betterments that significantly
extend original useful lives or improve productivity are capitalized and depreciated over the period benefited. Maintenance and repairs
are expensed as incurred. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation
are removed from the respective accounts, and any gain or loss is included in operations. Depreciation of property and equipment is provided
using the straight-line method for substantially all assets with
| Office furniture | ||||
| Electronic equipment | ||||
| Machinery | ||||
| Leasehold improvements | ||||
| Vehicles |
Impairment of Long-Lived Assets
Long-lived assets, which include property and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable, but at least annually.
Recoverability of long-lived assets to be held and used is measured by comparing the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by it. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds its fair value. Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable. Based on its review, the Company believes that, as of December 31, 2022 and 2021, there were no significant impairments of its long-lived assets.
Goodwill
The Company evaluates goodwill for impairment annually or more frequently when an event occurs or circumstances change that indicate the carrying value may not be recoverable. In testing goodwill for impairment, the Company may elect to utilize a qualitative assessment to evaluate whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount. If the qualitative assessment indicates that goodwill impairment is more likely than not, the Company performs a two-step impairment test. The Company tests goodwill for impairment under the two-step impairment test by first comparing the book value of net assets to the fair value of the reporting units. If the fair value is determined to be less than the book value or qualitative factors indicate that it is more likely than not that goodwill is impaired, a second step is performed to compute the amount of impairment as the difference between the estimated fair value of goodwill and the carrying value. The Company estimates the fair value of the reporting units using discounted cash flows. Forecasts of future cash flows are based on our best estimate of future net sales and operating expenses, based primarily on expected category expansion, pricing, market segment share, and general economic conditions.
The Company completed the required testing of goodwill for impairment as of December 31, 2022, and determined that goodwill was impaired because of the current financial condition of the Company and the Company’s inability to generate future operating income without substantial sales volume increases, which are highly uncertain. Furthermore, the Company anticipates future cash flows indicate that the recoverability of goodwill is not reasonably assured.
The
goodwill write-down was reflected as an impairment loss, $
| F-12 |
Income Taxes
Income taxes are accounted for using an asset and liability method. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates, applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
The Company follows Accounting Standards Codification (“ASC”) Topic 740, which prescribes a more-likely-than-not threshold for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740 also provides guidance on recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods, and income tax disclosures.
Under ASC Topic 740, when tax returns are filed, it is likely that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the consolidated financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50% likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits in the accompanying balance sheets along with any associated interest and penalties that would be payable to the taxing authorities upon examination. Interest associated with unrecognized tax benefits is classified as interest expense and penalties are classified in selling, general and administrative expenses in the statement of income.
At December 31, 2022 and 2021, the Company did not take any uncertain positions that would necessitate recording a tax related liability.
Revenue Recognition
ASU No. 2014-09, Revenue from Contracts with Customers (“Topic 606”), became effective for the Company on January 1, 2018. The Company’s revenue recognition disclosure reflects its updated accounting policies that are affected by this new standard. The Company applied the “modified retrospective” transition method for open contracts for the implementation of Topic 606. As revenues are and have been primarily from the delivery of products and the performance of services, and the Company has no significant post-delivery obligations, this did not result in a material recognition of revenue on the Company’s accompanying consolidated financial statements for the cumulative impact of applying this new standard. The Company made no adjustments to its previously-reported total revenues, as those periods continue to be presented in accordance with its historical accounting practices under Topic 605, Revenue Recognition.
Revenue from sale of goods under Topic 606 is recognized in a manner that reasonably reflects the delivery of the Company’s products and services to customers in return for expected consideration and includes the following elements:
| ● | executed contract(s) with customers that the Company believes is legally enforceable; | |
| ● | identification of performance obligation in the respective contract; | |
| ● | determination of the transaction price for each performance obligation in the respective contract; | |
| ● | allocation of the transaction price to each performance obligation; and | |
| ● | recognition of revenue only when the Company satisfies each performance obligation. |
| F-13 |
The Company’s revenue recognition policies for its various operating segments are as follows:
Advertising and Products
Advertising Revenue
Commencing in the third quarter of 2019, AiXin Zhonghong began to provide advertising services to its clients. Advertising contracts are signed to establish the price and advertising services to be provided. Pursuant to the advertising contracts, the Company provides advertising and marketing services to its clients through exhibition events, conferences, and person-to-person marketing. The Company performs a credit assessment of the customer to assess the collectability of the contract price prior to entering into contracts.
Most
of the advertisement contracts designated that the Company perform such advertising services for its clients through exhibition events,
conferences, and person-to-person marketing during the contracted period, regardless of the number of such events. As such, the Company
determined that the performance obligation is satisfied over time during the contracted period and revenue is recognized accordingly.
Such advertising revenue amounted to $
All
of the advertising revenue is subject to the PRC VAT of
Product Revenue
The Company’s revenue from sale of products is recognized when goods are delivered to the customer and no other obligation exists. The Company does not provide unconditional return or other concessions to the customer. The Company’s sales policy allows for the return of unopened products for cash after deducting certain service and transaction fees. As an alternative to the product return option, the customers have options of asking for an exchange for products with the same value.
Sales
revenue of AiXin Zhonghong represents the invoiced value of goods, net of value-added taxes (“VAT”). All of the Company’s
products sold in China are subject to the PRC VAT of
Hotel
Hotel
revenues are primarily derived from the rental of rooms, food and beverage sales and other ancillary goods and services, including but
not limited to souvenir, parking and conference reservation. Each of these products and services represents a distinct performance obligation
and, in exchange for these services, the Company receives fixed amounts based on published rates or negotiated contracts. Payment is
due in full at the time when the services are rendered or the goods are provided. Room rental revenue is recognized on a daily basis
when rooms are occupied. Food and beverage revenue and other goods and services revenue are recognized when they have been delivered
or rendered to the guests as the respective performance obligations are satisfied. All of the hotel’s goods sold in China are subject
to the PRC VAT of
Pharmacies
The
Company’s retail drugstores (Aixintang Pharmacies) recognize revenue at the time the customer takes possession of the merchandise.
For pharmacy sales, each prescription claim is its own arrangement with the customer and is a performance obligation. Aixintang Pharmacies
generally receives payments from customers as it satisfies its performance obligation. The Company records a receivable when it has an
unconditional right to receive payment and only the passage of time is required before payment is due. Sales revenue represents the invoiced
value of goods, net of VAT. All of Aixintang Pharmacies’ products sold in China are eligible for the PRC VAT of
| F-14 |
Manufacture and Sale
The
Company’s new subsidiary Runcangsheng recognizes revenue at the time products are shipped as this satisfies its performance obligation.
The Company records a receivable for the sales when it has an unconditional right to receive payment and only the passage of time is
required before payment is due. Sales revenue represents the invoiced value of goods, net of value-added taxes (“VAT”). All
of the Company’s products sold in China are subject to the PRC VAT of
Unearned Revenue
The Company’s unearned revenue primarily consists of advances received from customers for the rental of hotel rooms prior to the delivery of service. The room rental services are delivered (normally within one year) based upon contract terms and customer demand.
Concentration of Credit Risk
The operations of the Company are in the PRC. Accordingly, the Company’s business, financial condition, and results of operations may be influenced by the political, economic, and legal environments in the PRC, and by the general state of the PRC economy.
The
Company has cash on hand and demand deposits in accounts maintained with state-owned banks within the PRC. Cash in state-owned banks
is covered by insurance up to RMB
During the year ended December 31, 2022, the Company had no customer that accounted for over 10% of its total revenue.
During the year ended December 31, 2021, the Company had two major customers that accounted for over 10% of its total revenue.
| Customer | Net
sales for the year ended December 31, 2021 | % of total revenue | ||||||
| A(1) | $ | % | ||||||
| B | % | |||||||
During the year ended December 31, 2022, the Company had three major suppliers that accounted for over 10% of its total purchases.
| Supplier | Net
purchases for the year ended December 31, 2022 | % of total purchase | ||||||
| C | $ | % | ||||||
| D (2) | % | |||||||
| F-15 |
During the year ended December 31, 2021, the Company had one major supplier accounted for over 10% of its total purchase.
| Supplier | Net
purchases for the year ended December 31, 2021 | % of total purchase | ||||||
| E | $ | % | ||||||
| (1) | Represented advertising revenues from this customer during the year ended December 31, 2021. The Company also purchased inventory from this customer in year ended December 31, 2021. |
| (2) | The Company purchased inventory from this supplier, Runcangsheng, in the year ended December 31, 2022. The Company acquired all of the outstanding equity of Runcangsheng on September 30, 2022 (see Note 17). |
Leases
The Company determines if an arrangement is a lease at inception under FASB ASC Topic 842, Right of Use Assets (“ROU”) and lease liabilities are recognized at commencement date based on the present value of remaining lease payments over the lease term. For this purpose, the Company considers only payments that are fixed and determinable at the time of commencement. As most of its leases do not provide an implicit rate, it uses its incremental borrowing rate based on the information available at commencement date in determining the present value of lease payments. The Company’s incremental borrowing rate is a hypothetical rate based on its understanding of what its credit rating would be. The ROU assets include adjustments for prepayments and accrued lease payments. The ROU asset also includes any lease payments made prior to commencement and is recorded net of any lease incentives received. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain that it will exercise such options.
ROU assets are reviewed for impairment when indicators of impairment are present. ROU assets from operating and finance leases are subject to the impairment guidance in ASC 360, Property, Plant, and Equipment, as ROU assets are long-lived nonfinancial assets.
ROU assets are tested for impairment individually or as part of an asset group if the cash flows related to the ROU asset are not independent from the cash flows of other assets and liabilities. An asset group is the unit of accounting for long-lived assets to be held and used, which represents the lowest level for which identifiable cash flows are largely independent of the cash flows of other groups of assets and liabilities. The Company recognized no impairment of ROU assets as of December 31, 2022 and 2021. Operating leases are included in operating lease ROU and operating lease liabilities (current and non-current), on the consolidated balance sheets.
Statement of Cash Flows
In accordance with ASC Topic 230, “Statement of Cash Flows,” cash flows from the Company’s operations are calculated based on the local currencies using the average translation rates. As a result, amounts related to assets and liabilities reported on the consolidated statements of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance sheets.
Fair Value of Financial Instruments
The carrying amounts of certain of the Company’s financial instruments, including cash and equivalents, accrued liabilities and accounts payable, approximate their fair value due to their short maturities. FASB ASC Topic 825, “Financial Instruments,” requires disclosure of the fair value of financial instruments held by the Company. The carrying amounts reported in the consolidated balance sheets for current liabilities each qualify as financial instruments and are a reasonable estimate of their fair value because of the short period of time between the origination of such instruments and their expected realization and the current market rate of interest.
Fair Value Measurements and Disclosures
ASC Topic 820, “Fair Value Measurements and Disclosures,” defines fair value, and establishes a three-level valuation hierarchy for disclosures of fair value measurement that enhances disclosure requirements for fair value measures. The three levels are defined as follow:
| ● | Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| F-16 |
| ● | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. | |
| ● | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
As of December 31, 2022 and 2021, the Company did not identify any assets and liabilities that are required to be presented on the balance sheet at fair value.
Foreign Currency Translation and Comprehensive Income (Loss)
The functional currency of the Company is RMB. For financial reporting purposes, RMB is translated into USD as the reporting currency. Assets and liabilities are translated at the exchange rate in effect at the balance sheet dates. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period.
Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity as “Accumulated other comprehensive income”. Gains and losses resulting from foreign currency transactions are included in income. There was no significant fluctuation in the exchange rate for the conversion of RMB to USD after the balance sheet date.
The Company uses FASB ASC Topic 220, “Comprehensive Income”. Comprehensive loss is comprised of net loss and all changes to the statements of stockholders’ equity, except those due to investments by stockholders, changes in paid-in capital and distributions to stockholders. Comprehensive income (loss) for the year ended December 31, 2022 and 2021 consisted of net income (loss) and foreign currency translation adjustments.
Basic income (loss) per share is computed on the basis of the weighted average number of common shares outstanding during the period.
Dilution is computed by applying the treasury stock method for options and warrants. Under this method, options and warrants are assumed to be exercised at the beginning of the period (or at the time of issuance, if later), and as if funds obtained thereby were used to purchase common stock at the average market price during the period.
As of December 31, 2022 and 2021, the Company did not have any potentially dilutive instruments.
Stock-Based Compensation
The Company periodically grants stock options, warrants and stock awards to employees and non-employees in non-capital raising transactions as compensation for services rendered. The Company accounts for stock option, stock warrant and stock award grants to employees based on the authoritative guidance provided by the FASB where the value of the award is measured on the date of grant and recognized over the vesting period. The Company accounts for stock option, stock warrant and stock award grants to non-employees in accordance with the authoritative guidance of the FASB where the value of the stock compensation is determined based upon the measurement date at either a) the date at which a performance commitment is reached, or b) at the date at which the necessary performance to earn the equity instruments is complete. Stock-based compensation charges generally are amortized over the vesting period on a straight-line basis. In certain circumstances where there are no future performance requirements by the employees and non-employees, option, warrant and award grants are immediately vested and the total stock-based compensation charge is recorded in the period of the measurement date.
Segment Reporting
ASC Topic 280, “Segment Reporting,” requires use of the “management approach” model for segment reporting. The management approach model is based on the way a company’s chief operating decision maker organizes segments within the Company for making operating decisions assessing performance and allocating resources. Reportable segments are based on products and services, geography, legal structure, management structure, or any other manner in which management disaggregates a company.
| F-17 |
The Company manages its business as four operating segments, advertising and products, pharmacies, hotels, and manufacture and sale, all of which are located in the PRC. All of its revenues are derived in the PRC. All long-lived assets are located in PRC.
The following table shows the Company’s operations by business segment for the year ended December 31, 2022 and 2021. Revenues and expenses for the Pharmacies, Hotel and Manufacture and sale segments commenced as of the respective dates of the completion of their acquisitions:
| For the Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Net revenue | ||||||||
| Advertising and products | $ | $ | ||||||
| Pharmacies | ||||||||
| Hotel | ||||||||
| Manufacture and sale | ||||||||
| Total revenues, net | $ | $ | ||||||
| Operating costs and expenses | ||||||||
| Advertising and products | ||||||||
| Cost of goods sold | $ | $ | ||||||
| Operating expenses | ||||||||
| Pharmacies | ||||||||
| Cost of goods sold | ||||||||
| Operating expenses | ||||||||
| Hotel | ||||||||
| Hotel operating costs | ||||||||
| Operating expenses | ||||||||
| Manufacture and sale | ||||||||
| Cost of goods sold | ||||||||
| Operating expense | ( | ) | ||||||
| Total operating costs and expenses | $ | $ | ||||||
| (Loss) income from operations | ||||||||
| Advertising and products | $ | ( | ) | $ | ||||
| Pharmacies | ( | ) | ( | ) | ||||
| Hotel | ( | ) | ( | ) | ||||
| Manufacture and Sale | ( | ) | ||||||
| (Loss) income from operations | $ | ( | ) | $ | ( | ) | ||
| Segment assets | As
of December 31, 2022 | As
of December 31, 2021 | ||||||
| Advertising and products | $ | $ | ||||||
| Pharmacies | ||||||||
| Hotel | ||||||||
| Manufacture and sale | ||||||||
| Total assets | $ | $ | ||||||
As the acquisition of Runcangsheng has been consummated as of December 31, 2022 (see Note 17), the revenues and operating results of the manufacture and sale segment will be included in the financial statements of the Company beginning on October 1, 2022.
| F-18 |
New Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments-Credit Losses (Topic 326), which requires entities to measure all expected credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts. This replaces the existing incurred loss model and is applicable to the measurement of credit losses on financial assets measured at amortized cost. This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2022. Early application will be permitted for all entities for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. The Company is currently evaluating the impact that the standard will have on its consolidated financial statements.
In January 2017, the FASB issued ASU No. 2017-04, Simplifying the Test for Goodwill Impairment. The guidance removes Step 2 of the goodwill impairment test, which requires a hypothetical purchase price allocation. A goodwill impairment will now be the amount by which a reporting unit’s carrying value exceeds its fair value, not to exceed the carrying amount of goodwill. The guidance should be adopted on a prospective basis. As a smaller reporting company, the standard will be effective for the Company for interim and annual reporting periods beginning after December 15, 2022, with early adoption permitted. The Company is currently evaluating the impact of adopting this standard on the Company’s consolidated financial statements presentation or disclosures.
In August 2020, the FASB issued ASU 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470- 20) and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity. This ASU (1) simplifies the accounting for convertible debt instruments and convertible preferred stock by removing the existing guidance in ASC 470-20, Debt: Debt with Conversion and Other Options, that requires entities to account for beneficial conversion features and cash conversion features in equity, separately from the host convertible debt or preferred stock; (2) revises the scope exception from derivative accounting in ASC 815-40 for freestanding financial instruments and embedded features that are both indexed to the issuer’s own stock and classified in stockholders’ equity, by removing certain criteria required for equity classification; and (3) revises the guidance in ASC 260, Earnings Per Share, to require entities to calculate diluted earnings per share (EPS) for convertible instruments by using the if-converted method. In addition, entities must presume share settlement for purposes of calculating diluted EPS when an instrument may be settled in cash or shares. For SEC filers, excluding smaller reporting companies, ASU 2020-06 is effective for fiscal years beginning after December 15, 2021 including interim periods within those fiscal years. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020. For all other entities, ASU 2020-06 is effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. Entities should adopt the guidance as of the beginning of the fiscal year of adoption and cannot adopt the guidance in an interim reporting period. The adoption of ASU 2020-06 is not expected to have any impact on the Company’s consolidated financial statements presentation or disclosures.
The Company’s management does not believe that any other recently issued, but not yet effective, authoritative guidance, if currently adopted, would have a material impact on the Company’s financial statement presentation or disclosures.
3. OTHER RECEIVABLES AND PREPAID EXPENSES
Other receivables and prepaid expenses consisted of the following at December 31, 2022 and 2021:
| December 31, 2022 | December 31, 2021 | |||||||
| Deposits | $ | $ | ||||||
| Prepaid expenses | ||||||||
| Employees’ social insurance | ||||||||
| Others | ||||||||
| Total | $ | $ | ||||||
| F-19 |
4. ADVANCES TO SUPPLIERS
The
Company had advances to suppliers of $
5. INVENTORIES
Inventories consisted of the following at December 31, 2022 and 2021:
| December 31, 2022 | December 31, 2021 | |||||||
| Raw material | $ | $ | ||||||
| Work in process | ||||||||
| Finished goods-health supplements | ||||||||
| Drugs, pharmaceutical and nutritional products | ||||||||
| Food and beverage, hotel supplies and consumables | ||||||||
| Total | $ | $ | ||||||
| Less: reserve for inventory | ||||||||
| Total inventories, net | $ | $ | ||||||
6. PROPERTY AND EQUIPMENT, NET
Property and equipment consisted of the following at December 31, 2022 and 2021:
| December 31, 2022 | December 31, 2021 | |||||||
| Vehicles | $ | $ | ||||||
| Office furniture | ||||||||
| Electronic equipment | ||||||||
| Machinery | ||||||||
| Leasehold improvements | ||||||||
| Other | ||||||||
| Total | ||||||||
| Less: Accumulated depreciation | ( | ) | ( | ) | ||||
| Property and equipment, net | $ | $ | ||||||
Depreciation
expense for the years ended December 31, 2022 and 2021 was $
7. INTANGIBLE ASSET, NET
Intangible asset consisted of the following at December 31, 2022 and 2021:
| December 31, 2022 | December 31, 2021 | |||||||
| Software | $ | $ | ||||||
| Less: Accumulated amortization | ( | ) | ( | ) | ||||
| Intangible asset, net | $ | $ | ||||||
Amortization
expense for the years ended December 31, 2022 and 2021 was $
| F-20 |
8. TAXES PAYABLE
Taxes payable consisted of the following at December 31, 2022 and 2021:
| December 31, 2022 | December 31, 2021 | |||||||
| Value-added | $ | $ | ||||||
| Income | ||||||||
| City construction | ||||||||
| Education | ||||||||
| Other | ||||||||
| Taxes payable | $ | $ | ||||||
9. ACCRUED LIABILITIES AND OTHER PAYABLES
Accrued liabilities and other payables consisted of the following at December 31, 2022 and 2021:
| December 31, 2022 | December 31, 2021 | |||||||
| Accrued employees’ social insurance | $ | $ | ||||||
| Accrued payroll and commission | ||||||||
| Accrued rent expense | ||||||||
| Construction payable | ||||||||
| Payable for equipment purchase | ||||||||
| Accrued professional fees | ||||||||
| Deposit | ||||||||
| Other payables | ||||||||
| Total | $ | $ | ||||||
10. LOAN FROM THIRD PARTIES
As
of December 31, 2022 and 2021, the Company had advances from former shareholders and unrelated third parties in an aggregate amount of
$
11. LEASE
Concurrent
with the completion of the sale of its rights to a portion of a building completed in 2019, the Company entered into an agreement to
lease a portion of the building back from the buyer over a lease term of
The
Company also has operating leases for other sales locations under various operating lease arrangements. The leases have remaining lease
terms of approximately
Aixin
Shangyan Hotel leases its hotel premises under an operating lease arrangement. The lease has a remaining lease term of approximately
Aixintang
Pharmacies lease retail pharmacy stores under operating lease arrangements, with remaining lease terms of
| F-21 |
Balance sheet information related to the Company’s leases is presented below:
| December 31, 2022 | December 31, 2021 | |||||||
| Operating Leases | ||||||||
| Operating lease right-of-use assets | $ | $ | ||||||
| Operating lease liabilities – current | $ | $ | ||||||
| Operating lease liability – non-current | ||||||||
| Total operating lease liabilities | $ | $ | ||||||
The following provides details of the Company’s lease expenses:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Operating lease expenses | $ | $ | ||||||
Other information related to leases is presented below:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Cash Paid For Amounts Included In Measurement of Liabilities: | ||||||||
| Operating cash flows from operating leases | $ | $ | ||||||
| Weighted Average Remaining Lease Term: | ||||||||
| Operating leases | ||||||||
| Weighted Average Discount Rate: | ||||||||
| Operating leases | % | % | ||||||
Maturities of lease liabilities were as follows:
| For the year ending December 31: | ||||
| 2023 | $ | |||
| 2024 | ||||
| 2025 | ||||
| 2026 | ||||
| Total lease payments | ||||
| Less: imputed interest | ( | ) | ||
| Total lease liabilities | ||||
| Less: current portion | ( | ) | ||
| Lease liabilities – non-current portion | $ | |||
12. RELATED PARTY TRANSACTIONS
Account payables to related parties
As
of December 31, 2022 and 2021, the Company had accounts payable to related party in the amount of $
Advance to related parties
Advance to related parties consisted of the following as of the periods indicated:
| December 31, 2022 | December 31, 2021 | |||||||
| Chengdu WenJiang Aixin Nanjiang Pharmacy Co., Ltd. | $ | $ | ||||||
| Sichuan Aixin Investment Co., Ltd. | ||||||||
| Chengdu Fuxiang Tang Pharmacy Co., Ltd. | ||||||||
| Chengdu Wenjiang district Heneng hupu Pharmacy Co., Ltd | ||||||||
| Chengdu Lisheng Huiren Tang Pharmacy Co., Ltd. | ||||||||
| Total | $ | $ | ||||||
| F-22 |
Advance from related parties
Advance from related parties consisted of the following as of the periods indicated:
| December 31, 2022 | December 31, 2021 | |||||||
| Quanzhong Lin | $ | $ | ||||||
| Yirong Shen | ||||||||
| Branch manager | ||||||||
| Chengdu Aixin E-Commerce Company Ltd. | ||||||||
| Chengdu Aixin International travel service Co, Ltd | ||||||||
| Aixin Life Beauty | ||||||||
| Total | $ | $ | ||||||
All the related party entities are controlled by Mr. Quanzhong Lin (the Chairman, President and major shareholder of Aixin Life). These advances to and from related parties were for working capital purpose, payable on demand, and bear no interest. Yirong Shen was a major shareholder of Aixin Shangyan Hotel prior to the closing of Hotel Purchase Agreement, and she serves as the supervisor of Aixin Shangyan Hotel.
Office lease from a Major Shareholder
In
May 2014, the Company entered a lease with its major shareholder for an office. The lease term was for three years expiring in May 2017
with an option to renew. The monthly rent was RMB
13. INCOME TAXES
The Company was incorporated in the United States of America (“USA”) and has operations in one tax jurisdiction, i.e. the PRC. The Company generated substantially all of its sales from its operations in the PRC for the years ended December 31, 2022 and 2021, and recorded income tax provision for the periods.
China
has a tax rate of
The components of the provision for income taxes for the years ended December 31, 2022 and 2021 consisted of the following:
| For the Year Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Current: | ||||||||
| China | $ | ( | ) | $ | ||||
| Total current | ( | ) | ||||||
| Deferred: | ||||||||
| China | ( | ) | ||||||
| Total deferred | ( | ) | ||||||
| Total income tax expense | $ | $ | ||||||
Deferred tax assets as of December 31, 2022 and 2021 consisted of the following:
| December 31, 2022 | December 31, 2021 | |||||||
| Deferred tax assets: | ||||||||
| Accumulated amortization | $ | $ | ||||||
| F-23 |
The following table reconciles the statutory rates to the Company’s effective tax rate for years ended December 31, 2022 and 2021:
| 2022 | 2021 | |||||||
| Statutory U.S. federal income tax rate | ( | )% | % | |||||
| Foreign tax rate differential | ( | )% | % | |||||
| Change in valuation allowances | % | % | ||||||
| Other | ( | )% | % | |||||
| Effective combined tax rate | % | % | ||||||
As
of December 31, 2022, the Company had approximately $
Uncertain Tax Positions
Interest
associated with unrecognized tax benefits are classified as income tax, and penalties are classified in selling, general and administrative
expenses in the statements of operations. For the years ended December 31, 2022 and 2021, the Company had
14. STOCKHOLDERS’ EQUITY
On August 17, 2020, by unanimous written consent in lieu of a meeting, the Board adopted resolutions authorizing a one (1)-for-four (4) reverse stock. The reverse stock split became effective on October 27, 2020. According to the Articles of Amendment, the Company is authorized to issue shares of blank check preferred stock at $ par value and shares of common stock at $ par value per share
Pursuant to resolutions adopted by the Board of Directors and the holders of a majority of the outstanding shares of common stock of AiXin Life International, Inc. on January 6, 2023, the Company filed an amendment to its Articles of Incorporation with respect to a proposed 1 for 2 “reverse” split of our common stock (the “Amendment”). Completion of the proposed reverse stock split was to be effected on a date determined by our Board of Directors only upon receipt of notice from the Financial Industry Regulatory Authority (“FINRA”) that it would process the proposed reverse stock split. The Company received notice from FINRA and its common stock began trading on a post-split basis on February 17, 2023.
As a result of the reverse split, every two shares of the Company’s issued and outstanding common stock will be automatically combined and converted into one issued and outstanding share of common stock. The Company has approximately shares of outstanding common stock after the effect of reverse stock split.
All share and earnings per share information has been retroactively adjusted to reflect the reverse stock split.
As of December 31, 2022 and 2021, the Company had common shares issued and outstanding.
Stock Awards Issued for Services
On
October 22, 2019, the Company granted and issued shares to its employees and contractors under its 2019 Equity Incentive Plan.
The stock awards were valued at $
| F-24 |
On
October 24, 2019, the Company granted and issued shares to its employees and contractors under its 2019 Equity Incentive Plan.
The stock awards were valued at $
The stock awards will vest over five () years from the grant date, and the grantee will forfeit a portion of the shares granted (“Shares Granted”) if the grantee is no longer employed by or contracted with the Company. Specifically, .
For the years ended December 31, 2022 and 2021, stock-based compensation expenses were $ each. As of December 31, 2022, unrecognized compensation expenses related to these stock awards are $. These expenses are expected to be recognized over years.
Forgiveness of shareholder’s loan
As
of December 31, 2021, the Company’s major shareholder Mr. Lin forgave his loan to the Company for $
Acquisition of Subsidiaries
As
of December 31, 2021, the Company completed the acquisitions of Aixin Shangyan Hotel and Aixintang Pharmacies (see Note 1). The acquisitions
were accounted for as acquisitions of entities under common control. In connection with the acquisitions, the Company made payments to
Mr. Lin in the aggregate amount of $
15. STATUTORY RESERVES
Pursuant to the PRC corporate law, the Company is now only required to maintain one statutory reserve by appropriating from its after-tax profit before declaration or payment of dividends. The statutory reserve represents restricted retained earnings.
Surplus reserve fund
The
Company is required to transfer
The
surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any,
and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion
to their shareholding or by increasing the par value of the shares currently held by them, provided that the remaining reserve balance
after such issue is not less than
Common welfare fund
Common
welfare fund is a voluntary fund to which the Company can elect to transfer
| F-25 |
This fund can only be utilized on capital items for the collective benefit of the Company’s employees, such as construction of dormitories, cafeteria facilities, and other staff welfare facilities. This fund is non-distributable other than upon liquidation.
16. OPERATING CONTINGENCIES
The Company’s operations in the PRC are subject to specific considerations and significant risks not typically associated with companies in North America and Western Europe. These include risks associated with, among others, the political, economic and legal environments and foreign currency exchange. The Company’s results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
The Company’s sales, purchases and expenses are denominated in RMB and all of the Company’s assets and liabilities are also denominated in RMB. The RMB is not freely convertible into foreign currencies under the current law. In China, foreign exchange transactions are required by law to be transacted only by authorized financial institutions. Remittances in currencies other than RMB may require certain supporting documentation to affect the remittance.
Litigation
The Company is, from time to time, involved in litigation incidental to the conduct of its business regarding merchandise sold, employment matters, and litigation regarding intellectual property rights.
In
December 2020, Jian Yiao (the “Plaintiff”) filed a complaint against Chengdu Aixintang Haichuan Pharmacy Co., Ltd.
(“Aixintang Pharmacy”, or the “Defendant”) in Zhangjiagang City People’s Court of Jiangsu
Province. The complaint alleges that Jian Yiao is entitled to RMB
In February 2021, the judge in the Jiangsu Suzhou Intermediate People’s Court denied the Defendant’s motion and upheld the judgment from the first trial. In March 2021, Aixintang Pharmacy filed another motion to the Jiangsu High People’s Court on the basis that the Purchase Agreement was forged. In February 2022, Aixintang Pharmacy filed an appeal in Jiangsu High People’s Court against the judgment reached by Jiangsu Suzhou Intermediate People’s Court in February 2021. As of the date of this report, this legal proceeding remains pending.
In November 2021, the Company and Mr. Quanzhong Lin agreed that Mr. Lin shall assume any losses arising from this legal proceeding. As such, the Company did not accrue contingent losses from this legal proceeding as of December 31, 2022 and 2021.
The Company believes that current pending litigation will not have a material adverse effect on its consolidated financial position, results of operations or cash flows.
17. ACQUISITIONS OF SUBSIDIARIES
Aixin Shangyan Hotel and Aixintang Pharmacies
In July and September, 2021, the Company completed the required governmental procedures and obtained the documents necessary to consider the acquisitions of Aixin Shangyan Hotel and Aixintang Pharmacies completed.
| F-26 |
Pursuant
to the Hotel Purchase Agreement, AiXin HK purchased all of the outstanding equity of Aixin Shangyan Hotel from Mr. Lin and the other
shareholder for a purchase price of RMB
Pursuant
to the Pharmacies Purchase Agreement, AiXin HK purchased
The acquisitions will be accounted for as acquisitions of entities under common control under ASC 805-50-15-6, and the assets and liabilities acquired will be measured and recorded at the carrying amount under ASC 805-50-30-5.
Runcangsheng
On
July 19, 2022, the Company entered into an Equity Transfer Agreement with Yunnan Shengshengyuan Technology Co., Ltd (“Shengshengyuan”)
and Yun Chen (collectively “the Sellers”), who own
Under
the terms of the Transfer Agreement, the Company agreed to purchase all of the outstanding equity interest of Yunnan Runcangsheng for
an aggregate purchase price of RMB
The following table summarizes the fair values of the assets acquired and liabilities assumed at the date of acquisition. Goodwill as a result of the acquisition of Runcangsheng is calculated as follows:
| Total purchase considerations | $ | |||
| Estimated fair value of assets acquired: | ||||
| Cash | $ | |||
| Accounts receivable | ||||
| Accounts receivable-related party | ||||
| Advance to suppliers | ||||
| Other receivables and prepaid expense | ||||
| Inventory | ||||
| Property and equipment | ||||
| Intangible assets | ||||
| Operating lease right-of-use assets | ||||
| Total assets acquired | ||||
| Estimated fair value of liabilities assumed: | ||||
| Accounts payable | ( | ) | ||
| Accounts payable-related party | ( | ) | ||
| Advance from customers | ( | ) | ||
| Government grant | ( | ) | ||
| Taxes payable | ( | ) | ||
| Operating lease liability | ( | ) | ||
| Accrued liabilities and other payables | ( | ) | ||
| Total liabilities assumed | ( | ) | ||
| Total net assets acquired | ||||
| Goodwill as a result of the acquisition | $ |
During the year ended December 31, 2022, the Company has recorded goodwill impairment in full amount.
The following condensed unaudited pro forma consolidated results of operations for the Company, Runcangsheng, Aixin Shangyan Hotel and Aixintang Pharmacies for the years ended December 31, 2022 and 2021 present the results of operations of the Company, Runcangsheng, Aixin Shangyan Hotel, and Aixintang Pharmacies as if the acquisitions occurred on January 1, 2022 and 2021, respectively.
| F-27 |
The pro forma results are not necessarily indicative of the actual results that would have occurred had the acquisitions been completed as of the beginning of the periods presented, nor are they necessarily indicative of future consolidated results.
For the Year Ended December 31, 2022 | ||||
| Revenue | $ | |||
| Operating costs and expenses | ||||
| Loss from operations | ( | ) | ||
| Other income | ||||
| Income tax expense | ||||
| Net loss | $ | ( | ) | |
For the Year Ended December 31, 2021 | ||||
| Revenue | $ | |||
| Operating costs and expenses | ||||
| Loss from operations | ( | ) | ||
| Other income | ||||
| Income tax expense | ||||
| Net loss | $ | ( | ) | |
18. SUBSEQUENT EVENT
The Company follows the guidance in FASB ASC 855-10 for the disclosure of subsequent events. The Company evaluated subsequent events through the date the financial statements were issued and determined the Company has no material subsequent events need to be disclosed.
| F-28 |
AIXIN LIFE INTERNATIONAL, INC.
CONSOLIDATED BALANCE SHEETS
| September 30, | December 31, | |||||||
| 2023 | 2022 | |||||||
| (Unaudited) | ||||||||
| Assets | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Restricted cash | ||||||||
| Accounts receivable, including related parties, net | ||||||||
| Other receivables and prepaid expenses, including related party | ||||||||
| Advances to suppliers | ||||||||
| Inventory, net | ||||||||
| Due from related parties | ||||||||
| Total current assets | ||||||||
| Property and equipment, net | ||||||||
| Intangible asset, net | ||||||||
| Deferred tax asset | ||||||||
| Security deposit | ||||||||
| Operating lease right-of-use assets | ||||||||
| Total assets | $ | $ | ||||||
| Liabilities and stockholders’ equity | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | $ | ||||||
| Accounts payable-related party | ||||||||
| Unearned revenue | ||||||||
| Taxes payable | ||||||||
| Accrued liabilities and other payables | ||||||||
| Government grant | ||||||||
| Loan from third parties | ||||||||
| Operating lease liabilities | ||||||||
| Due to related parties | ||||||||
| Total current liabilities | ||||||||
| Operating lease liabilities - non-current | ||||||||
| Total liabilities | ||||||||
| Stockholders’ deficit | ||||||||
| Undesignated preferred stock, $par value, shares authorized, issued and outstanding | ||||||||
| Common stock, par value $per share, shares authorized; shares issued and outstanding as of September 30, 2023 and December 31, 2022 | ||||||||
| Additional paid in capital | ||||||||
| Statutory reserve | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Accumulated other comprehensive income | ||||||||
| Total stockholders’ deficit | ( | ) | ( | ) | ||||
| Total liabilities and stockholders’ deficit | $ | $ | ||||||
| F-29 |
AIXIN LIFE INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(UNAUDITED)
Three Months Ended September 30, |
Nine Months Ended September 30, | |||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||
| Sales revenue: | ||||||||||||||||
| Products | $ | $ | $ | $ | ||||||||||||
| Room revenues | ||||||||||||||||
| Food and beverage revenues | ||||||||||||||||
| Others | ||||||||||||||||
| Total revenue, net | ||||||||||||||||
| Operating costs and expenses | ||||||||||||||||
| Cost of goods sold | ||||||||||||||||
| Hotel operating costs | ||||||||||||||||
| Selling | ||||||||||||||||
| General and administrative | ||||||||||||||||
| (Reversal of) provision for bad debts | ( | ) | ||||||||||||||
| Stock-based compensation | ||||||||||||||||
| Total operating costs and expenses | ||||||||||||||||
| Loss from operations | ( |
) | ( |
) | ( | ) | ( | ) | ||||||||
| Non-operating income (expenses) | ||||||||||||||||
| Interest income | ||||||||||||||||
| Impairment loss | ( |
) | ( | ) | ||||||||||||
| Other income | ||||||||||||||||
| Government grant income | ||||||||||||||||
| Other expenses | ( |
) | ( |
) | ( | ) | ( | ) | ||||||||
| Total non-operating income (loss), net | ( |
) | ( | ) | ||||||||||||
| Loss before income tax | ( |
) | ( |
) | ( | ) | ( | ) | ||||||||
| Income tax expense (benefit) | ( |
) | ||||||||||||||
| Net loss | ( |
) | ( |
) | ( | ) | ( | ) | ||||||||
| Other comprehensive items | ||||||||||||||||
| Foreign currency translation gain (loss) | ( |
) | ( | ) | ||||||||||||
| Comprehensive loss | $ | ( |
) | $ | ( |
) | $ | ( | ) | $ | ( | ) | ||||
| Loss per share - basic and diluted | $ | ) | $ | ) | $ | ) | $ | ) | ||||||||
| Weighted average shares outstanding | ||||||||||||||||
| F-30 |
AIXIN LIFE INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(UNAUDITED)
| Common Stock | Additional paid in | Statutory | Accumulated | Accumulated other comprehensive | ||||||||||||||||||||||||
| Shares | Amount |
capital | reserves | deficit | income | Total | ||||||||||||||||||||||
| Balance at December 31, 2022 | $ | $ | $ | $ | ( | ) | $ | $ | ( | ) | ||||||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||||||||||
| Disposal of subsidiary | - | |||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation | - | |||||||||||||||||||||||||||
| Balance at March 31, 2023 | ( | ) | ( | ) | ||||||||||||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||||||||||
| Shareholder contribution | - | |||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation | - | |||||||||||||||||||||||||||
| Balance at June 30, 2023 | ( | ) | ( | ) | ||||||||||||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation | - | |||||||||||||||||||||||||||
| Balance at September 30, 2023 | $ | $ | $ | $ | ( | ) | $ | $ | ( | ) | ||||||||||||||||||
| Balance at December 31, 2021 | $ | $ | $ | $ | ( | ) | $ | $ | ||||||||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation | - | |||||||||||||||||||||||||||
| Balance at March 31, 2022 | ( | ) | ||||||||||||||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Balance at June 30, 2022 | ( | ) | ||||||||||||||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Balance at September 30, 2022 | $ | $ | $ | $ | ( | ) | $ | $ | ||||||||||||||||||||
| F-31 |
AIXIN LIFE INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
| Nine Months Ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments required to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | ||||||||
| (Reversal of) provision for bad debts | ( | ) | ||||||
| Provision for inventory reserve | ||||||||
| Operating lease expense | ||||||||
| Stock based compensation | ||||||||
| Deferred tax | ||||||||
| Impairment loss | ||||||||
| Government grant income | ( | ) | ||||||
| Changes in assets and liabilities: | ||||||||
| Accounts receivable | ( | ) | ||||||
| Accounts receivable - related parties | ( | ) | ||||||
| Other receivables and prepaid expenses | ( | ) | ||||||
| Prepaid expense - related party | ( | ) | ||||||
| Advances to suppliers | ||||||||
| Inventory | ( | ) | ( | ) | ||||
| Accounts payable | ||||||||
| Accounts payable - related party | ( | ) | ||||||
| Unearned revenue | ( | ) | ||||||
| Taxes payable | ( | ) | ||||||
| Payment of lease liability | ( | ) | ( | ) | ||||
| Accrued liabilities and other payables | ( | ) | ||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Cash disposed at disposal of subsidiary | ( | ) | ||||||
| Cash acquired at acquisition of subsidiaries | ||||||||
| Purchase of property and equipment | ( | ) | ( | ) | ||||
| Purchase of intangible asset | ( | ) | ||||||
| Net cash used in investing activities | ( | ) | ||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Change in advance from related parties | ( | ) | ( | ) | ||||
| Capital contribution | ||||||||
| Proceeds from government grant | ||||||||
| Net cash provided by (used in) financing activities | ( | ) | ||||||
| EFFECT OF EXCHANGE RATE CHANGE ON CASH | ( | ) | ( | ) | ||||
| NET DECREASE IN CASH & RESTRICTED CASH | ( | ) | ( | ) | ||||
| CASH & RESTRICTED CASH, BEGINNING OF PERIOD | ||||||||
| CASH & RESTRICTED CASH, END OF PERIOD | $ | $ | ||||||
| Supplemental Cash flow data: | ||||||||
| Income tax paid | $ | $ | ||||||
| Interest paid | $ | $ | ||||||
| Non-cash investing and financing activities: | ||||||||
| Accrual of unpaid investment in subsidiary | $ | $ | ||||||
| Investment in subsidiary paid by shareholder on behalf of the Company | $ | $ | ||||||
| F-32 |
AIXIN LIFE INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
1. ORGANIZATION AND DESCRIPTION OF BUSINESS
Aixin
Life International, Inc. (the “Company” or “Aixin Life” or “we”) was incorporated under the laws
of the State of Colorado on August 31, 2001. On
February 2, 2017, Mr. Quanzhong Lin (Mr. Lin) purchased %
of the Company’s outstanding shares from China Concentric Capital Group for $
On
December 12, 2017, pursuant to a Share Exchange Agreement, in consideration for all of the outstanding shares of AiXin (BVI) International
Group Co., Ltd. a British Virgin Islands corporation (“AiXin BVI”), the Company issued to Mr. Lin, the sole stockholder of
AiXin BVI, shares of common stock then representing
As a result of the Share Exchange, AiXin BVI became the Company’s wholly-owned subsidiary, and the Company now owns all of the outstanding shares of HK AiXin International Group Co., Limited, a Hong Kong limited company (“AiXin HK”), which in turn owns all of the outstanding shares of Chengdu AiXinZhonghong Biological Technology Co., Ltd., a Chinese limited company (“AiXinZhonghong”), which markets and sells premium-quality nutritional products in China.
AiXin BVI was incorporated on September 21, 2017 as a holding company and AiXin HK was established in Hong Kong on February 25, 2016 as an intermediate holding company. AiXinZhonghong was established in the People’s Republic of China (“PRC”) on March 4, 2013, and on May 27, 2017, the local government of the PRC issued a certificate of approval regarding the foreign ownership of AiXinZhonghong by AiXin HK. Neither AiXin BVI nor AiXin HK had operations prior to December 12, 2017.
For accounting purposes, the acquisition of AiXin BVI was accounted for as a reverse acquisition and treated as a recapitalization of the Company effected by a share exchange, with AiXin BVI as the accounting acquirer. Since neither AiXin BVI nor AiXin HK had operations prior to December 12, 2017, the historical consolidated financial statements of AiXinZhonghong are now the historical consolidated financial statements of the Company. The assets and liabilities of AiXinZhonghong were brought forward at their book value and no goodwill was recognized.
Effective February 1, 2018, the Company changed its name to AiXin Life International, Inc. (“Aixin Life”).
The Company, through its indirectly owned AiXinZhonghong subsidiary, develops and distributes consumer products by offering a line of nutritional products. The Company sells the products through exhibition events, conferences, and person-to-person marketing. Beginning in 2019, the Company began to provide advertising services to clients who engaged the Company to help distribute their products. The Company’s business mainly focuses on a proactive approach to its customers such as hosting events for clients, which it believes is ideally suited to marketing its products because sales of nutrition products are strengthened by ongoing personal contact and support, coaching and education of its clients, as to the benefits of a healthy and active lifestyle.
On
May 25, 2021, AiXin HK entered into an Equity Transfer Agreement (the “Hotel Purchase Agreement”) with Chengdu Aixin Shangyan
Hotel Management Co., Ltd (“Aixin Shangyan Hotel”), and its two shareholders Quanzhong Lin and Yirong Shen (“Transferor”).
Pursuant to the Hotel Purchase Agreement, Aixin Life purchased
| F-33 |
On
February 17, 2023, the Company effected a
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements are prepared in conformity with U.S. Generally Accepted Accounting Principles (“US GAAP”). The functional currency of AiXinZhonghong, Aixin Shangyan Hotel, Aixintang Pharmacies, and Runcangsheng is the Chinese Renminbi (“RMB”). The accompanying consolidated financial statements are translated from RMB and presented in U.S. dollars (“USD”).
The consolidated financial statements include the accounts of the Company and its current wholly owned subsidiaries, AiXin HK, AiXinZhonghong, Aixin Shangyan Hotel, Aixintang Pharmacies, and Runcangsheng. Intercompany transactions and accounts were eliminated in consolidation. These unaudited interim financial statements should be read in conjunction with the annual consolidated financial statements and the accompanying notes contained in our Annual Report on Form 10-K for the year ended December 31, 2022.
Unaudited Interim Financial Information
These unaudited interim financial statements have been prepared in accordance with GAAP for interim financial reporting and the rules and regulations of the Securities and Exchange Commission that permit reduced disclosure for interim periods. Therefore, certain information and footnote disclosures normally included in financial statements prepared in accordance with GAAP have been condensed or omitted. In the opinion of management, all adjustments of a normal recurring nature necessary for a fair presentation of the financial position, results of operations and cash flows for the periods presented have been made. The results of operations for the interim periods presented are not necessarily indicative of the results to be expected for the year ending December 31, 2023.
Going Concern
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The realization of assets and the satisfaction of liabilities in the normal course of business are dependent on, among other things, the Company’s ability to operate profitably, to generate cash flows from operations, and to pursue financing arrangements to support its working capital requirements.
The Company has suffered net losses of $
| F-34 |
Management believes that it has developed a liquidity plan, summarized below, that, if executed successfully, should provide sufficient liquidity to meet the Company’s obligations as they become due for a reasonable period of time, and allow the development of its core business. The plan includes:
● Gaining positive cash-inflow from operating activities through continuous cost reductions and the sales of higher margin products.
● Raising cash through loans from related parties and potential equity offerings.
While the Company’s management believes that the measures in its liquidity plan including those described above will be adequate to satisfy its liquidity requirements for the twelve months after the date that these financial statements are issued, there is no assurance that the liquidity plan will be successfully implemented. Failure to successfully implement the liquidity plan may have a material adverse effect on its business, results of operations and financial position, and may adversely affect its ability to continue as a going concern. These consolidated financial statements do not include any adjustments related to the recoverability and classification of recorded assets or the amounts and classification of liabilities or any other adjustments that might be necessary should the Company be unable to continue as a going concern.
Global Uncertainties
The Company’s liquidity may be adversely impacted by various risks and uncertainties, including, but not limited to future and current impacts of global events such as a widespread health crisis, the continuation of the war in the Ukraine or the conflict in Palestine, the outbreak of another conflict or the expansion of the conflict in Palestine to other countries, the ongoing tensions between the United States and China, the Russian Federation and certain countries in the Middle East, increases in inflation, and other risks detailed in in the Company’s Annual Report on Form 10-K or other reports filed with the Securities and Exchange Commission.
While the invasion of Ukraine, the conflict in Palestine and responses thereto have not interrupted the Company’s operations, these or future developments which disrupt the international financial markets could make it difficult to access debt and equity capital on attractive terms, if at all, and impact the Company’s ability to fund business activities, including proposed acquisitions.
Use of Estimates
In preparing consolidated financial statements in conformity with US GAAP, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the consolidated financial statements, as well as the reported amounts of revenues and expenses during the reporting period.
Significant estimates required by management, include the recoverability of long-lived assets, allowance for doubtful accounts, and the reserve for obsolete and slow-moving inventories. Actual results could differ from those estimates.
Reclassification
Certain prior period amounts have been reclassified to conform to the current period presentation and had no effect on previously reported consolidated net income (loss) or accumulated deficit.
Cash and Cash Equivalents
For financial statement purposes, the Company considers all highly liquid investments with an original maturity of three months or less to be cash and cash equivalents.
Restricted Cash
The restricted cash was cash maintained in temporarily frozen bank accounts held by Aixintang Pharmacy and its branches by the court for a judgement against Aixintang Pharmacy which Aixintang Pharmacy is in the process of appealing (see Note 16 – litigation).
| F-35 |
Accounts Receivable
The
Company’s policy is to maintain an allowance for potential credit losses on accounts receivable. Management reviews the composition
of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends
and changes in customer payment patterns to evaluate the adequacy of these reserves. As of September 30, 2023 and December 31, 2022,
the bad debt allowance was $
The following table summarizes the activity related to the Company’s Accounts Receivable allowance for doubtful accounts for the nine months ended September 30, 2023 and 2022:
| For the nine Months ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| Beginning balance | $ | $ | ||||||
| Provision for bad debts | ||||||||
| Acquisition of subsidiary | ||||||||
| Recoveries/Write offs | ( | ) | ||||||
| Effect of translation | ( | ) | ( | ) | ||||
| Ending balance | $ | $ | ||||||
Inventories
Inventories
mainly consists of health supplements, drugs, pharmaceutical and nutritional products, food and beverage, hotel supplies and consumables.
Inventories are valued at the lower of average cost or market, cost being determined on a moving weighted average method at the end of
the month. Management compares the cost of inventories with the net realizable value and an allowance is made for writing down inventories
to market value, if lower. The Company recorded provision for inventory reserve of $
Property and Equipment
Property
and equipment are stated at cost, less accumulated depreciation, and impairment losses, if any. Major repairs and betterments that significantly
extend original useful lives or improve productivity are capitalized and depreciated over the period benefited. Maintenance and repairs
are expensed as incurred. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation
are removed from the respective accounts, and any gain or loss is included in operations. Depreciation of property and equipment is provided
using the straight-line method for substantially all assets with
| Office furniture | ||||
| Electronic equipment | ||||
| Machinery | ||||
| Leasehold improvements | ||||
| Vehicles |
Impairment of Long-Lived Assets
Long-lived assets, which include property and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable, but at least annually.
Recoverability of long-lived assets to be held and used is measured by comparing the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by it. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds its fair value. Fair value is generally determined using the asset’s expected future discounted cash flows or market value, if readily determinable. Based on its review, the Company believes that, as of September 30, 2023 and December 31, 2022, there were no significant impairments of its long-lived assets.
| F-36 |
Goodwill
The Company evaluates goodwill for impairment annually or more frequently when an event occurs or circumstances change that indicate the carrying value may not be recoverable. In testing goodwill for impairment, the Company may elect to utilize a qualitative assessment to evaluate whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount. If the qualitative assessment indicates that goodwill impairment is more likely than not, the Company performs a two-step impairment test. The Company tests goodwill for impairment under the two-step impairment test by first comparing the book value of net assets to the fair value of the reporting units. If the fair value is determined to be less than the book value or qualitative factors indicate that it is more likely than not that goodwill is impaired, a second step is performed to compute the amount of impairment as the difference between the estimated fair value of goodwill and the carrying value. The Company estimates the fair value of the reporting units using discounted cash flows. Forecasts of future cash flows are based on our best estimate of future net sales and operating expenses, based primarily on expected category expansion, pricing, market segment share, and general economic conditions.
The Company completed the required testing of goodwill for impairment as of December 31, 2022, and determined that goodwill was impaired because of the current financial condition of the Company and the Company’s inability to generate future operating income without substantial sales volume increases, which are highly uncertain. Furthermore, the uncertainty of the future cash flows indicates that the recoverability of goodwill is not reasonably assured.
The
goodwill write-down was reflected as an impairment loss, $
Income Taxes
Income taxes are accounted for using an asset and liability method. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates, applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
The Company follows Accounting Standards Codification (“ASC”) Topic 740, which prescribes a more-likely-than-not threshold for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740 also provides guidance on recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods, and income tax disclosures.
Under ASC Topic 740, when tax returns are filed, it is likely that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the consolidated financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50% likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits in the accompanying balance sheets along with any associated interest and penalties that would be payable to the taxing authorities upon examination. Interest associated with unrecognized tax benefits is classified as interest expense and penalties are classified in selling, general and administrative expenses in the statement of income.
At September 30, 2023 and December 31, 2022, the Company did not take any uncertain positions that would necessitate recording a tax related liability.
| F-37 |
Revenue Recognition
Revenue from sale of goods under Topic 606 is recognized in a manner that reasonably reflects the delivery of the Company’s products and services to customers in return for expected consideration and includes the following elements:
| ● | executed contract(s) with customers that the Company believes is legally enforceable; | |
| ● | identification of performance obligation in the respective contract; | |
| ● | determination of the transaction price for each performance obligation in the respective contract; | |
| ● | allocation of the transaction price to each performance obligation; and | |
| ● | recognition of revenue only when the Company satisfies each performance obligation. |
The Company’s revenue recognition policies for its various operating segments are as follows:
Products
The Company’s revenue from sales of products is recognized when goods are delivered to the customer and no other obligation exists. The Company does not provide unconditional return or other concessions to the customer. The Company’s sales policy allows for the return of unopened products for cash after deducting certain service and transaction fees. As an alternative to the product return option, the customers have the option of asking for an exchange for products with the same value.
Sales
revenue of AiXin Zhonghong represents the invoiced value of goods, net of value-added taxes (“VAT”). All of the Company’s
products sold in China are subject to the PRC VAT of
Hotel
Hotel
revenues are primarily derived from the rental of rooms, food and beverage sales and other ancillary goods and services, including but
not limited to souvenir, parking and conference reservation. Each of these products and services represents a distinct performance obligation
and, in exchange for these services, the Company receives fixed amounts based on published rates or negotiated contracts. Payment is
due in full at the time when the services are rendered or the goods are provided. Room rental revenue is recognized on a daily basis
when rooms are occupied. Food and beverage revenue and other goods and services revenue are recognized when they have been delivered
or rendered to the guests as the respective performance obligations are satisfied. All of the hotel’s goods sold in China are subject
to the PRC VAT of
Pharmacies
The
Company’s retail drugstores (Aixintang Pharmacies) recognize revenue at the time the customer takes possession of the merchandise.
For pharmacy sales, each prescription claim is its own arrangement with the customer and is a performance obligation. Aixintang Pharmacies
generally receives payments from customers as it satisfies its performance obligations. The Company records a receivable when it has
an unconditional right to receive payment and only the passage of time is required before payment is due. Sales revenue represents the
invoiced value of goods, net of VAT. All of Aixintang Pharmacies’ products sold in China are eligible for the PRC VAT of
Manufacture and Sale
The
Company’s new subsidiary Runcangsheng recognizes revenue at the time products are shipped as this satisfies its performance obligation.
The Company records a receivable for its sales when it has an unconditional right to receive payment and only the passage of time is
required before payment is due. Sales revenue represents the invoiced value of goods, net of value-added taxes (“VAT”). All
of the Company’s products sold in China are subject to the PRC VAT of
| F-38 |
Unearned Revenue
The Company’s unearned revenue primarily consists of advances received from customers for the purchase of products prior to the delivery of goods, and for the rental of hotel rooms prior to the delivery of service. The delivery of products and room rental services is (normally within one year) based upon contract terms and customer demand.
Concentration of Credit Risk
The operations of the Company are in the PRC. Accordingly, the Company’s business, financial condition, and results of operations may be influenced by the political, economic, and legal environments in the PRC, and by the general state of the PRC economy.
The
Company has cash on hand and demand deposits in accounts maintained with state-owned banks within the PRC. Cash in state-owned banks
is covered by insurance up to RMB
During
the three and nine months ended September 30, 2023 and 2022, the Company had no customer that accounted for over
During
the three months ended September 30, 2023, the Company had one supplier that accounted for
During
the nine months ended September 30, 2023, the Company had two suppliers that accounted for
During
the three months ended September 30, 2022, the Company had one supplier that accounted for
During
the nine months ended September 30, 2022, the Company had one supplier that accounted for
Leases
The Company determines if an arrangement is a lease at inception under FASB ASC Topic 842, Right of Use Assets (“ROU”) and lease liabilities are recognized at commencement date based on the present value of remaining lease payments over the lease term. For this purpose, the Company considers only payments that are fixed and determinable at the time of commencement. As most of its leases do not provide an implicit rate, it uses its incremental borrowing rate based on the information available at commencement date in determining the present value of lease payments. The Company’s incremental borrowing rate is a hypothetical rate based on its understanding of what its credit rating would be. The ROU assets include adjustments for prepayments and accrued lease payments. The ROU asset also includes any lease payments made prior to commencement and is recorded net of any lease incentives received. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain that it will exercise such options.
ROU assets are reviewed for impairment when indicators of impairment are present. ROU assets from operating and finance leases are subject to the impairment guidance in ASC 360, Property, Plant, and Equipment, as ROU assets are long-lived nonfinancial assets.
ROU assets are tested for impairment individually or as part of an asset group if the cash flows related to the ROU asset are not independent from the cash flows of other assets and liabilities. An asset group is the unit of accounting for long-lived assets to be held and used, which represents the lowest level for which identifiable cash flows are largely independent of the cash flows of other groups of assets and liabilities. The Company recognized no impairment of ROU assets as of September 30, 2023 and December 31, 2022. Operating leases are included in operating lease ROU and operating lease liabilities (current and non-current), on the consolidated balance sheets.
| F-39 |
Statement of Cash Flows
In accordance with ASC Topic 230, “Statement of Cash Flows,” cash flows from the Company’s operations are calculated based on the local currencies using the average translation rates. As a result, amounts related to assets and liabilities reported on the consolidated statements of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance sheets.
Fair Value of Financial Instruments
The carrying amounts of certain of the Company’s financial instruments, including cash and cash equivalents, accrued liabilities and accounts payable, approximate their fair value due to their short maturities. FASB ASC Topic 825, “Financial Instruments,” requires disclosure of the fair value of financial instruments held by the Company. The carrying amounts reported in the consolidated balance sheets for current liabilities each qualify as financial instruments and are a reasonable estimate of their fair value because of the short period of time between the origination of such instruments and their expected realization and the current market rate of interest.
Fair Value Measurements and Disclosures
ASC Topic 820, “Fair Value Measurements and Disclosures,” defines fair value, and establishes a three-level valuation hierarchy for disclosures of fair value measurement that enhances disclosure requirements for fair value measures. The three levels are defined as follow:
| ● | Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. | |
| ● | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. | |
| ● | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
As of September 30, 2023 and December 31, 2022, the Company did not identify any assets and liabilities that are required to be presented on the balance sheet at fair value.
Foreign Currency Translation and Comprehensive Income (Loss)
The functional currency of the Company is RMB. For financial reporting purposes, RMB is translated into USD as the reporting currency. Assets and liabilities are translated at the exchange rate in effect at the balance sheet dates. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period.
Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders’ equity as “Accumulated other comprehensive income”. Gains and losses resulting from foreign currency transactions are included in income. There was no significant fluctuation in the exchange rate for the conversion of RMB to USD after the balance sheet date.
The Company uses FASB ASC Topic 220, “Comprehensive Income”. Comprehensive loss is comprised of net loss and all changes to the statements of stockholders’ equity, except those due to investments by stockholders, changes in paid-in capital and distributions to stockholders. Comprehensive income (loss) for the three and nine months ended September 30, 2023 and 2022 consisted of net income (loss) and foreign currency translation adjustments.
Basic income (loss) per share is computed on the basis of the weighted average number of common shares outstanding during the period.
Dilution is computed by applying the treasury stock method for options and warrants. Under this method, options and warrants are assumed to be exercised at the beginning of the period (or at the time of issuance, if later), and as if funds obtained thereby were used to purchase common stock at the average market price during the period.
As of September 30, 2023 and December 31, 2022, the Company did not have any potentially dilutive instruments.
| F-40 |
Stock-Based Compensation
The Company periodically grants stock options, warrants and awards to employees and non-employees in non-capital raising transactions as compensation for services rendered. The Company accounts for stock option, stock warrant and stock award grants to employees based on the authoritative guidance provided by the FASB where the value of the award is measured on the date of grant and recognized over the vesting period. The Company accounts for stock option, stock warrant and stock award grants to non-employees in accordance with the authoritative guidance of the FASB where the value of the stock compensation is determined based upon the measurement date at either a) the date at which a performance commitment is reached, or b) at the date at which the necessary performance to earn the equity instruments is complete. Stock-based compensation charges generally are amortized over the vesting period on a straight-line basis. In certain circumstances where there are no future performance requirements by the employees and non-employees, option, warrant and award grants are immediately vested and the total stock-based compensation charge is recorded in the period of the measurement date.
Segment Reporting
ASC Topic 280, “Segment Reporting,” requires use of the “management approach” model for segment reporting. The management approach model is based on the way a company’s chief operating decision maker organizes segments within the Company for making operating decisions assessing performance and allocating resources. Reportable segments are based on products and services, geography, legal structure, management structure, or any other manner in which management disaggregates a company.
The Company manages its business as four operating segments, products, pharmacies, hotel, and manufacture and sales, all of which are located in the PRC. All of its revenues are derived in the PRC. All long-lived assets are located in PRC.
The following table shows the Company’s operations by business segment for the three months ended September 30, 2023 and 2022.
| For the Three Months Ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| Net revenue | ||||||||
| Products | $ | $ | ||||||
| Pharmacies | ||||||||
| Hotel | ||||||||
| Manufacture and sale | ||||||||
| Total revenues, net | $ | $ | ||||||
| Operating costs and expenses | ||||||||
| Products | ||||||||
| Cost of goods sold | $ | $ | ||||||
| Operating expenses | 421,417 | 301,624 | ||||||
| Pharmacies | ||||||||
| Cost of goods sold | ||||||||
| Operating expenses | ||||||||
| Hotel | ||||||||
| Hotel operating costs | ||||||||
| Operating expenses | ||||||||
| Manufacture and sale | ||||||||
| Cost of goods sold | ||||||||
| Operating expenses | ||||||||
| Total operating costs and expenses | $ | $ | ||||||
| Income (loss) from operations | ||||||||
| Products | $ | ( | ) | $ | ( | ) | ||
| Pharmacies | ( | ) | ||||||
| Hotel | ( | ) | ( | ) | ||||
| Manufacture and sale | ( | ) | ||||||
| Loss from operations | $ | ( | ) | $ | ( | ) | ||
| F-41 |
The following table shows the Company’s operations by business segment for the nine months ended September 30, 2023 and 2022.
| For the Nine Months Ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| Net revenue | ||||||||
| Products | $ | $ | ||||||
| Pharmacies | ||||||||
| Hotel | ||||||||
| Manufacture and sale | ||||||||
| Total revenues, net | $ | $ | ||||||
| Operating costs and expenses | ||||||||
| Products | ||||||||
| Cost of goods sold | $ | $ | ||||||
| Operating expenses | ||||||||
| Pharmacies | ||||||||
| Cost of goods sold | ||||||||
| Operating expenses | ||||||||
| Hotel | ||||||||
| Hotel operating costs | ||||||||
| Operating expenses | ||||||||
| Manufacture and sale | ||||||||
| Cost of goods sold | ||||||||
| Operating expenses | ||||||||
| Total operating costs and expenses | $ | $ | ||||||
| Loss from operations | ||||||||
| Products | $ | ( | ) | $ | ( | ) | ||
| Pharmacies | ( | ) | ( | ) | ||||
| Hotel | ( | ) | ( | ) | ||||
| Manufacture and sale | ( | ) | ||||||
| Loss from operations | $ | ( | ) | $ | ( | ) | ||
| Segment assets | As
of September 30, 2023 | As
of December 31, 2022 | ||||||
| Products | $ | $ | ||||||
| Pharmacies | ||||||||
| Hotel | ||||||||
| Manufacture and sale | ||||||||
| Total assets | $ | $ | ||||||
As the acquisition of Runcangsheng was consummated as of September 30, 2022 (see Note 17), the revenues and operating results of the manufacture and sale segment were included in the financial statements of the Company beginning on October 1, 2022.
| F-42 |
New Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments-Credit Losses (Topic 326), which requires entities to measure all expected credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts. This replaces the existing incurred loss model and is applicable to the measurement of credit losses on financial assets measured at amortized cost. This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2022. Early application will be permitted for all entities for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. The Company is currently evaluating the impact that the standard will have on its consolidated financial statements.
In January 2017, the FASB issued ASU No. 2017-04, Simplifying the Test for Goodwill Impairment. The guidance removes Step 2 of the goodwill impairment test, which requires a hypothetical purchase price allocation. A goodwill impairment will now be the amount by which a reporting unit’s carrying value exceeds its FV, not to exceed the carrying amount of goodwill. The guidance should be adopted on a prospective basis. As a smaller reporting company, the standard will be effective for the Company for interim and annual reporting periods beginning after December 15, 2022, with early adoption permitted. The adoption of ASU 2017-04 is not expected to have any impact on the Company’s consolidated financial statements presentation or disclosures.
In August 2020, the FASB issued ASU 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470- 20) and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity. This ASU (1) simplifies the accounting for convertible debt instruments and convertible preferred stock by removing the existing guidance in ASC 470-20, Debt: Debt with Conversion and Other Options, that requires entities to account for beneficial conversion features and cash conversion features in equity, separately from the host convertible debt or preferred stock; (2) revises the scope exception from derivative accounting in ASC 815-40 for freestanding financial instruments and embedded features that are both indexed to the issuer’s own stock and classified in stockholders’ equity, by removing certain criteria required for equity classification; and (3) revises the guidance in ASC 260, Earnings Per Share, to require entities to calculate diluted earnings per share (EPS) for convertible instruments by using the if-converted method. In addition, entities must presume share settlement for purposes of calculating diluted EPS when an instrument may be settled in cash or shares. For SEC filers, excluding smaller reporting companies, ASU 2020-06 is effective for fiscal years beginning after December 15, 2021 including interim periods within those fiscal years. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020. For all other entities, ASU 2020-06 is effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. Entities should adopt the guidance as of the beginning of the fiscal year of adoption and cannot adopt the guidance in an interim reporting period. The adoption of ASU 2020-06 is not expected to have any impact on the Company’s consolidated financial statements presentation or disclosures.
The Company’s management does not believe that any other recently issued, but not yet effective, authoritative guidance, if currently adopted, would have a material impact on the Company’s financial statement presentation or disclosures.
3. OTHER RECEIVABLES AND PREPAID EXPENSES
Other receivables and prepaid expenses consisted of the following at September 30, 2023 and December 31, 2022:
| September 30, 2023 | December 31, 2022 | |||||||
| Deposits | $ | $ | ||||||
| Prepaid expenses, including related party | ||||||||
| Employees’ social insurance | ||||||||
| Others | ||||||||
| Total | $ | $ | ||||||
4. ADVANCES TO SUPPLIERS
The
Company had advances to suppliers of $
| F-43 |
5. INVENTORIES
Inventories consisted of the following at September 30, 2023 and December 31, 2022:
| September 30, 2023 | December 31, 2022 | |||||||
| Raw material | $ | $ | ||||||
| Work in process | ||||||||
| Finished goods-health supplements | ||||||||
| Drugs, pharmaceutical and nutritional products | ||||||||
| Food and beverage, hotel supplies and consumables | ||||||||
| Total | $ | $ | ||||||
| Less: reserve for inventory | ||||||||
| Total inventories, net | $ | $ | ||||||
6. PROPERTY AND EQUIPMENT, NET
Property and equipment consisted of the following at September 30, 2023 and December 31, 2022:
| September 30, 2023 | December 31, 2022 | |||||||
| Vehicles | $ | $ | ||||||
| Office furniture | ||||||||
| Electronic equipment | ||||||||
| Machinery | ||||||||
| Leasehold improvements | ||||||||
| Other | ||||||||
| Total | ||||||||
| Less: Accumulated depreciation | ( | ) | ( | ) | ||||
| Property and equipment, net | $ | $ | ||||||
Depreciation
expense for the three months ended September 30, 2023 and 2022 was $
Depreciation
expense for the nine months ended September 30, 2023 and 2022 was $
7. INTANGIBLE ASSET, NET
Intangible asset consisted of the following at September 30, 2023 and December 31, 2022:
| September 30, 2023 | December 31, 2022 | |||||||
| Software | $ | $ | ||||||
| Less: Accumulated amortization | ( | ) | ( | ) | ||||
| Intangible asset, net | $ | $ | ||||||
Amortization
expense for the three months ended September 30, 2023 and 2022 was $
Amortization
expense for the nine months ended September 30, 2023 and 2022 was $
| F-44 |
8. TAXES PAYABLE
Taxes payable consisted of the following at September 30, 2023 and December 31, 2022:
| September 30, 2023 | December 31, 2022 | |||||||
| Value-added | $ | $ | ||||||
| Income | ||||||||
| City construction | ||||||||
| Education | ||||||||
| Other | ||||||||
| Taxes payable | $ | $ | ||||||
9. ACCRUED LIABILITIES AND OTHER PAYABLES
Accrued liabilities and other payables consisted of the following at September 30, 2023 and December 31, 2022:
| September 30, 2023 | December 31, 2022 | |||||||
| Accrued employees’ social insurance | $ | $ | ||||||
| Accrued payroll and commission | ||||||||
| Accrued rent expense | ||||||||
| Construction payable | ||||||||
| Payable for equipment purchase | ||||||||
| Accrued professional fees | ||||||||
| Deposit | ||||||||
| Other payables | ||||||||
| Total | $ | $ | ||||||
10. LOAN FROM THIRD PARTIES
As
of September 30, 2023 and December 31, 2022, the Company had advances from unrelated third parties of Aixin Shangyan Hotel in an aggregate
amount $
11. LEASE
AiXinZhonghong
leases its office on a monthly basis. AiXinZhonghong also has operating leases for other sales locations under various operating lease
arrangements. The leases have remaining lease terms of approximately
Aixin
Shangyan Hotel leases its hotel premises under an operating lease arrangement. The lease has a remaining lease term of approximately
Aixintang
Pharmacies lease retail pharmacy stores under operating lease arrangements, with remaining lease terms of
Runcangsheng
leases its office under an operating lease arrangement. The lease has a remaining lease term of approximately
Balance sheet information related to the Company’s leases is presented below:
| September 30, 2023 | December 31, 2022 | |||||||
| Operating Leases | ||||||||
| Operating lease right-of-use assets | $ | $ | ||||||
| Operating lease liabilities – current | $ | $ | ||||||
| Operating lease liability – non-current | ||||||||
| Total operating lease liabilities | $ | $ | ||||||
| F-45 |
The following provides details of the Company’s lease expenses:
| Three Months Ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| Operating lease expenses | $ | $ | ||||||
| Nine Months Ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| Operating lease expenses | $ | $ | ||||||
Other information related to leases is presented below:
| Nine Months Ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| Cash Paid for Amounts Included In Measurement of Liabilities: | ||||||||
| Operating cash flows from operating leases | $ | |||||||
| Weighted Average Remaining Lease Term: | ||||||||
| Operating leases | ||||||||
| Weighted Average Discount Rate: | ||||||||
| Operating leases | % | % | ||||||
Maturities of lease liabilities were as follows:
| For the year ending December 31: | ||||
| 2023 (excluding the nine months ended September 30, 2023) | $ | |||
| 2024 | ||||
| 2025 | ||||
| 2026 | ||||
| 2027 | ||||
| Thereafter | ||||
| Total lease payments | ||||
| Less: imputed interest | ( | ) | ||
| Total lease liabilities | ||||
| Less: current portion | ( | ) | ||
| Lease liabilities – non-current portion | $ |
12. RELATED PARTY TRANSACTIONS
Accounts receivable – related party
As
of September 30, 2023 and December 31, 2022, accounts receivable from related parties were $
Prepaid expense – related party
Prepaid expense – related party consisted of the following as of the periods indicated:
| September 30, 2023 | December 31, 2022 | |||||||
| Chengdu Aixin International Travel Service Co., Ltd | $ | $ | ||||||
Accounts payable – related party
Accounts payable – related party consisted of the following as of the periods indicated:
| September 30, 2023 | December 31, 2022 | |||||||
| Luquan Shengcaofeng Biotechnology Co., Ltd. | $ | $ | ||||||
Luquan Shengcaofeng Biotechnology Co., Ltd. is an entity controlled by Mr. Huiliang Jiao, a Director of the Company.
| F-46 |
Due from related parties
Due from related parties consisted of the following as of the periods indicated:
| September 30, 2023 | December 31, 2022 | |||||||
| Chengdu WenJiang Aixin Nanjiang Pharmacy Co., Ltd. | $ | $ | ||||||
| Sichuan Aixin Investment Co., Ltd | ||||||||
| Huiliang Jiao | ||||||||
| Xiaoyan Zhou | ||||||||
| Chengdu Fuxiang Tang Pharmacy Co., Ltd. | ||||||||
| Chengdu WenJiang Aixin Huiwan Pharmacy Co., Ltd. | ||||||||
| Chengdu Heshengyuan Pharmacy Co., Ltd. | ||||||||
| Chengdu Zhiweibing Pharmacy Co., Ltd. | ||||||||
| Chengdu Tongtai Tang Pharmacy Co. Ltd. | ||||||||
| Chengdu city Wuhou District Xiaofei Pharmacy Co., Ltd | ||||||||
| Chongqing Aixin Hui Pharmacy Co., Ltd. | ||||||||
| Sichuan Xintang Xinfu Pharmacy Chain Co, Ltd | ||||||||
| Sichuan Aixintang Xinfu Chain Pharmacy Co., Ltd. | ||||||||
| Chengdu Wenjiang district Heneng hupu Pharmacy Co., Ltd | ||||||||
| Chengdu Cigu Foshou Pharmacy | ||||||||
| Mianyang Aixin Cunshan Pharmacy | ||||||||
| Chengdu Aixin International Travel Service Co., Ltd | ||||||||
| Chengdu Lisheng Huiren Tang Pharmacy Co., Ltd. | ||||||||
| Total | $ | $ | ||||||
Due to related parties
Due to related parties consisted of the following as of the periods indicated:
| September 30, 2023 | December 31, 2022 | |||||||
| Quanzhong Lin | $ | $ | ||||||
| Yirong Shen | ||||||||
| Sichuan Haosen Haichuan business management Co. Ltd | ||||||||
| Sichuan Yunxi Pharmacy Co. Ltd | ||||||||
| Chengdu Yi Yan Tang Pharmacy Co. Ltd. | ||||||||
| Chengdu Aixin International travel service Co, Ltd | ||||||||
| Total | $ | $ | ||||||
The amounts due to and from related parties were for working capital purposes, payable on demand, and bear no interest. All the related party entities listed above are controlled by Mr. Quanzhong Lin (the Chairman, President and major shareholder of Aixin Life). Yirong Shen was a major shareholder of Aixin Shangyan Hotel prior to the closing of Hotel Purchase Agreement, and she serves as the supervisor of Aixin Shangyan Hotel. Tianming Long is a branch manager of Aixintang Pharmacies. Mr. Huiliang Jiao is the Director of the Company. Xiaoyan Zhou is the wife of Mr. Huiliang Jiao.
Office leases
In
May 2014, the Company entered a lease with its major shareholder for an office. The lease term was for three years expiring in May 2017
with an option to renew. The monthly rent was RMB
Runcangsheng
has an office lease with Xiaoyan Zhou, wife of Huiliang Jiao, the Company’s Director, from March 2020 to February 2023 with a monthly
rent of RMB
| F-47 |
13. INCOME TAXES
The Company was incorporated in the United States of America (“USA”) and has operations in one tax jurisdiction, i.e. the PRC. The Company generated substantially all of its sales from its operations in the PRC for the three and nine months ended September 30, 2023 and 2022, and recorded income tax provision for the periods.
China
has a tax rate of
Uncertain Tax Positions
Interest
associated with unrecognized tax benefits are classified as income tax, and penalties are classified in selling, general and administrative
expenses in the statements of operations. For the three and nine months ended September 30, 2023 and 2022, the Company had
14. STOCKHOLDERS’ EQUITY
On
August 17, 2020, by unanimous written consent in lieu of a meeting, the Board adopted resolutions authorizing a
Pursuant to resolutions adopted by the Board of Directors and the holders of a majority of the outstanding shares of common stock of AiXin Life International, Inc. on January 6, 2023, the Company filed an amendment to its Articles of Incorporation with respect to a proposed 1 for 2 “reverse” split of its common stock (the “Amendment”). Completion of the proposed reverse stock split was to be effected on a date determined by the Board of Directors only upon receipt of notice from the Financial Industry Regulatory Authority (“FINRA”) that it would process the proposed reverse stock split. The Company received notice from FINRA and its common stock began trading on a post-split basis on February 17, 2023.
As a result of the reverse split, every two shares of the Company’s issued and outstanding common stock were automatically combined and converted into one issued and outstanding share of common stock. The Company has approximately shares of outstanding common stock after giving effect to the reverse stock split and the elimination of fractional shares.
All share and earnings per share information has been retroactively adjusted to reflect the reverse stock split.
As of September 30, 2023, and December 31, 2022, the Company had common shares issued and outstanding.
Stock Awards Issued for Services
On
October 22, 2019, the Company granted and issued shares to its
employees and contractors under its 2019 Equity Incentive Plan. The stock awards were valued at $
On
October 24, 2019, the Company granted and issued shares to its
employees and contractors under its 2019 Equity Incentive Plan. The stock awards were valued at $
| F-48 |
The stock awards will vest over five () years from the grant date, and the grantee will forfeit a portion of the shares granted (“Shares Granted”) if the grantee is no longer employed by or contracted with the Company. Specifically,
For the three months ended September 30, 2023 and 2022, stock-based compensation expenses were $and $, respectively. For the nine months ended September 30, 2023 and 2022, stock-based compensation expenses were $and $, respectively. As of September 30, 2023, unrecognized compensation expenses related to these stock awards are $. These expenses are expected to be recognized over years.
Capital Contribution
During
the nine months ended September 30, 2023, the Company received capital contributions in the aggregate amount of $
15. STATUTORY RESERVES
Pursuant to the PRC corporate law, the Company is now only required to maintain one statutory reserve by appropriating from its after-tax profit before declaration or payment of dividends. The statutory reserve represents restricted retained earnings.
Surplus reserve fund
The
Company is required to transfer
The
surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any,
and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion
to their shareholding or by increasing the par value of the shares currently held by them, provided that the remaining reserve balance
after such issue is not less than
Common welfare fund
Common
welfare fund is a voluntary fund to which the Company can elect to transfer
This fund can only be utilized on capital items for the collective benefit of the Company’s employees, such as construction of dormitories, cafeteria facilities, and other staff welfare facilities. This fund is non-distributable other than upon liquidation.
16. OPERATING CONTINGENCIES
The Company’s operations in the PRC are subject to specific considerations and significant risks not typically associated with companies in North America and Western Europe. These include risks associated with, among others, the political, economic and legal environments and foreign currency exchange. The Company’s results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
The Company’s sales, purchases and expenses are denominated in RMB and all of the Company’s assets and liabilities are also denominated in RMB. The RMB is not freely convertible into foreign currencies under the current law. In China, foreign exchange transactions are required by law to be transacted only by authorized financial institutions. Remittances in currencies other than RMB may require certain supporting documentation to affect the remittance.
| F-49 |
Litigation
The Company is, from time to time, involved in litigation incidental to the conduct of its business regarding merchandise sold, employment matters, and litigation regarding intellectual property rights.
In
December 2020, Jian Yiao (the “Plaintiff”) filed a complaint against Chengdu Aixintang Pharmacy Co., Ltd. (“Aixintang
Pharmacy”, or the “Defendant”) in Zhangjiagang People’s Court in Jiangsu Province. The complaint alleges that
Jian Yiao is entitled to $
In February 2021, the judge in the Jiangsu Suzhou Intermediate People’s Court denied the Defendant’s motion and upheld the judgment from the first trial. In March 2021, Aixintang Pharmacy filed another motion to the Jiangsu High People’s Court on the basis that the Purchase Agreement was forged. In February 2022, Aixintang Pharmacy filed an appeal in Jiangsu High People’s Court against the judgment reached by Jiangsu Suzhou Intermediate People’s Court in February 2021. To date, this legal proceeding remains pending.
In November 2021, the Company and Mr. Quanzhong Lin agreed that Mr. Lin shall assume any losses arising from this legal proceeding. As such, the Company did not accrue contingent losses from this legal proceeding as of September 30, 2023.
The Company believes that current pending litigation will not have a material adverse effect on its consolidated financial position, results of operations or cash flows.
17. ACQUISITION OF SUBSIDIARIES
Runcangsheng
On
July 19, 2022, the Company entered into an Equity Transfer Agreement with Yunnan Shengshengyuan Technology Co., Ltd (“Shengshengyuan”)
and Yun Chen (collectively “the Sellers”), who own
Under
the terms of the Transfer Agreement, the Company purchased all of the outstanding equity interest of Yunnan Runcangsheng for an aggregate
purchase price of RMB
In addition to transferring their respective equity interest in Runcangsheng, both Sellers agreed to forgive any loans due to them from Runcangsheng. The acquisition was completed on September 30, 2022.
| F-50 |
The following table summarizes the fair values of the assets acquired and liabilities assumed at the date of acquisition. Goodwill as a result of the acquisition of Runcangsheng is calculated as follows:
| Total purchase considerations | $ | |||
| Estimated fair value of assets acquired: | ||||
| Cash | $ | |||
| Accounts receivable | ||||
| Accounts receivable-related party | ||||
| Advance to suppliers | ||||
| Other receivables and prepaid expense | ||||
| Inventory | ||||
| Property and equipment | ||||
| Intangible assets | ||||
| Operating lease right-of-use assets | ||||
| Total assets acquired | ||||
| Estimated fair value of liabilities assumed: | ||||
| Accounts payable | ( | ) | ||
| Accounts payable-related party | ( | ) | ||
| Advance from customers | ( | ) | ||
| Government grant | ( | ) | ||
| Taxes payable | ( | ) | ||
| Operating lease liability | ( | ) | ||
| Accrued liabilities and other payables | ( | ) | ||
| Total liabilities assumed | ( | ) | ||
| Total net assets acquired | ||||
| Goodwill as a result of the acquisition | $ |
During the year ended December 31, 2022, the Company recorded a goodwill impairment equal to the goodwill resulting from the acquisition of Runcangsheng.
The following condensed unaudited pro forma consolidated results of operations for the Company, Runcangsheng, Aixin Shangyan Hotel and Aixintang Pharmacies for the three and nine months ended September 30, 2022 present the results of operations of the Company, Runcangsheng, Aixin Shangyan Hotel, and Aixintang Pharmacies as if the acquisition of Runcangsheng occurred on January 1, 2022, respectively.
The pro forma results are not necessarily indicative of the actual results that would have occurred had the acquisition been completed as of the beginning of the periods presented, nor are they necessarily indicative of future consolidated results.
For the Three Months Ended September 30, 2022 | ||||
| Revenue | $ | |||
| Operating costs and expenses | ||||
| Loss from operations | ( | ) | ||
| Other expense | ( | ) | ||
| Income tax expense (benefit) | ( | ) | ||
| Net loss | $ | ( | ) | |
For the Nine Months Ended September 30, 2022 | ||||
| Revenue | $ | |||
| Operating costs and expenses | ||||
| Loss from operations | ( | ) | ||
| Other income | ||||
| Income tax expense | ||||
| Net loss | $ | ( | ) | |
18. SUBSEQUENT EVENT
The Company follows the guidance in FASB ASC 855-10 for the disclosure of subsequent events. The Company evaluated subsequent events through the date the financial statements were issued and determined the Company has no material subsequent events.
| F-51 |
AIXIN LIFE IINTERNATIONAL, INC.

[$9,500,000]
Shares of Common Stock
PRELIMINARY PROSPECTUS
Underwriter
BOUSTEAD SECURITIES, LLC
___________, 2024
You should rely only on the information contained in this prospectus. No dealer, salesperson or other person is authorized to give information that is not contained in this prospectus. This prospectus is not an offer to sell nor is it seeking an offer to buy these securities in any jurisdiction where the offer or sale is not permitted. The information contained in this prospectus is correct only as of the date of this prospectus, regardless of the time of the delivery of this prospectus or the sale of these securities.
Until , 2024, 25 days after the date of this prospectus, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriter with respect to their unsold allotments or subscriptions.
The date of this prospectus is [ ], 2024
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The following table sets forth the various expenses, all of which will be borne by us, in connection with the sale and distribution of the securities being registered, other than the underwriter commissions. All amounts shown are estimates except for the Securities and Exchange Commission registration fee and the FINRA filing fee.
| Description | Amount | |||
| Filing Fee - Securities and Exchange Commission | $ | 1,322.40 | ||
| FINRA Filing Fee | 9,815 | |||
| Nasdaq Application and Listing Fee | 75,000 | |||
| Attorney’s fees and expenses | 400,000 | * | ||
| Accountant’s fees and expenses | 175,000 | * | ||
| Transfer agent’s and registrar fees and expenses | 25,000 | * | ||
| Printing and engraving expenses | 125,000 | * | ||
| Miscellaneous expenses | 138,862.60 | * | ||
| Total | $ | 950,000.00 | * | |
* Estimated expenses.
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
The Colorado Business Corporations Act provides that a director or officer is not individually liable to the corporation or its stockholders or creditors for any damages as a result of any act or failure to act in his capacity as a director or officer unless it is proven that his act or failure to act constituted a breach of his fiduciary duties as a director or officer and his breach of those duties involved intentional misconduct, fraud or a knowing violation of law. The Articles of Incorporation or an amendment thereto may, however, provide for greater individual liability.
This provision is intended to afford directors and officers protection against and to limit their potential liability for monetary damages resulting from suits alleging a breach of the duty of care by a director or officer. As a consequence of this provision, stockholders of our Company will be unable to recover monetary damages against directors or officers for action taken by them that may constitute negligence or gross negligence in performance of their duties unless such conduct meets the requirements of Colorado law to impose such liability. The provision, however, does not alter the applicable standards governing a director’s or officer’s fiduciary duty and does not eliminate or limit the right of our Company or any stockholder to obtain an injunction or any other type of non-monetary relief in the event of a breach of fiduciary duty.
The Colorado Business Corporations Act also provides that under certain circumstances, a corporation may indemnify any person for amounts incurred in connection with a pending, threatened or completed action, suit or proceeding in which he is, or is threatened to be made, a party by reason of his being a director, officer, employee or agent of the corporation or serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, if such person (a) is not liable for a breach of fiduciary duty involving intentional misconduct, fraud or a knowing violation of law or such greater standard imposed by the corporation’s articles of incorporation; or (b) acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. Additionally, a corporation may indemnify a director, officer, employee or agent with respect to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor, if such person (a) is not liable for a breach of fiduciary duty involving intentional misconduct, fraud or a knowing violation of law or such greater standard imposed by the corporation’s articles of incorporation; or (b) acted in good faith and in a manner which he reasonably believed to be in or not opposed to the best interests of the corporation, however, indemnification may not be made for any claim, issue or matter as to which such a person has been adjudged by a court to be liable to the corporation or for amounts paid in settlement to the corporation, unless the court determines that the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper. To the extent that a director, officer, employee or agent of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding referred to above, or in defense of any claim, issue or matter therein, the corporation shall indemnify him against expenses, including attorneys’ fees, actually and reasonably incurred by him in connection with the defense.
| II-1 |
The Company’s By-laws provide in substance that every director and officer of the Company shall be entitled to indemnification against expense actually and necessarily incurred in any action suit or proceeding, in which he or she may be named as a party by reason of being or having been a director or officer of the Company, except to the extent that such officer is finally adjudicated to be liable for negligence or misconduct.
Neither our Bylaws nor our Articles of Incorporation include any specific indemnification provisions for our officers or directors against liability under the Securities Act. Additionally, insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Company pursuant to the foregoing provisions, or otherwise, the Company has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES.
Not Applicable
ITEM 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) Exhibits.
Pursuant to Item 601 of Regulation S-K:
A list of exhibits filed with this registration statement on Form S-1 is set forth on the Exhibit Index and is incorporated herein by reference.
ITEM 17. UNDERTAKINGS.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement to:
(i) Include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) Reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) Include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
| II-2 |
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date
(5) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
| II-3 |
EXHIBIT INDEX
| * | Filed herewith |
| ** | To be filed by amendment |
| *** | Previously filed |
| II-4 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Amendment to this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Chengdu, China, on February 9, 2024.
| AIXIN LIFE INTERNATIONAL, INC. | |
| /s/ Quanzhong Lin | |
| Quanzhong Lin | |
| CEO, President, Secretary and Director | |
| (Principal Executive Officer) |
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this amendment to this registration statement to be signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Quanzhong Lin | CEO, President, Secretary and Director | February 9, 2024 | ||
Quanzhong Lin
|
(Principal Executive Officer)
|
| ||
| /s/ Tianfeng Li | CFO and Treasurer | February 9, 2024 | ||
| Tianfeng Li | (Principal Financial and Accounting Officer) | |||
| * | Independent Director | February 9, 2024 | ||
| Yao-Te Wang | ||||
| * | Independent Director | February 9, 2024 | ||
| Christopher Lee | ||||
| * | Independent Director | February 9, 2024 | ||
| Huiliang Jiao |
| /s/ Quanzhong Lin | |||||
| ● | By Quanzhong Lin, Attorney-in-Fact |
| II-5 |