UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
(Mark One)
| For the fiscal year ended |
or
For the transition period from to to
| Commission file number |
(Exact name of registrant as specified in its charter)
|
|
|
|
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
|
|
|
|
|
(Address of principal executive offices) |
(Zip Code) |
| Registrant's telephone number, including area code | ( |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
Trading Symbol(s) |
Name of Each Exchange on which Registered |
||
|
|
|
|
||
|
|
N/A |
NYSE American |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ☐ Yes ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
|
|
☒ |
Smaller reporting company |
|
|
|
Emerging growth company |
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act.)
The aggregate market value of shares of common stock held by non-affiliates of the registrant on June 30, 2022 was $
The number of shares of common stock outstanding on March 17, 2023 was
DOCUMENTS INCORPORATED BY REFERENCE
None.
TABLE OF CONTENTS
|
PART I |
1 | ||
|
Item 1 |
Business |
1 | |
|
Item 1A |
Risk Factors |
12 | |
|
Item 1B |
Unresolved Staff Comments |
26 | |
|
Item 2 |
Properties |
26 | |
|
Item 3 |
Legal Proceedings |
26 | |
|
Item 4 |
Mine Safety Disclosure |
26 | |
|
PART II |
27 | ||
|
Item 5 |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
27 | |
|
Item 6 |
Selected Financial Data |
27 | |
|
Item 7 |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
28 | |
|
Item 7A |
Quantitative and Qualitative Disclosures About Market Risk |
36 | |
|
Item 8 |
Financial Statements and Supplementary Data |
36 | |
|
Item 9 |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
36 | |
|
Item 9A |
Controls and Procedures |
36 | |
|
Item 9B |
Other Information |
37 | |
|
Item 9C |
Disclosures Regarding Foreign Jurisdictions that Prevent Inspections |
37 | |
|
PART III |
38 | ||
|
Item 10 |
Directors, Executive Officers and Corporate Governance |
38 | |
|
Item 11 |
Executive Compensation |
41 | |
|
Item 12 |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
53 | |
|
Item 13 |
Certain Relationships and Related Transactions, and Director Independence |
55 | |
|
Item 14 |
Principal Accountant Fees and Services |
59 | |
|
PART IV |
60 | ||
|
Item 15 |
Exhibits, Financial Statement Schedules |
60 | |
The Private Securities Litigation Reform Act of 1995 (the “PSLRA”) provides a safe harbor for forward-looking statements made by or on behalf of the Company. Statements in this document which relate to other than strictly historical facts, such as statements about the Company’s plans and strategies, expectations for future financial performance, new and existing products and technologies, anticipated clinical and regulatory pathways, the ability to obtain, and timing of, regulatory approvals of the Company’s products, the timing and anticipated results of commercialization efforts, and anticipated markets for the Company’s products, are forward-looking statements within the meaning of the PSLRA. The words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” “project,” and similar expressions identify forward-looking statements that speak only as of the date hereof. Investors are cautioned that such statements involve risks and uncertainties that could cause actual results to differ materially from historical or anticipated results due to many factors including, but not limited to, our history of operating losses and uncertainty of future profitability, accumulated deficit, future capital needs, the outcome of any pending litigation, uncertainty of capital funding, dependence on royalties and grant revenue, limited product line and distribution channels, competition, risks of development of new products, our ability to maintain effective control over financial reporting, our ability to comply with NYSE American continued listing standards, the impact of the recent coronavirus pandemic, and other risks set forth below under Item 1A, “Risk Factors.” The Company undertakes no obligation to publicly update or revise any forward-looking statements.
PART I
Item 1. Business
Development of the Business
Navidea Biopharmaceuticals, Inc. (“Navidea,” the “Company,” “our” or “we”), a Delaware corporation (NYSE American: NAVB), is a biopharmaceutical company focused on the development and commercialization of precision immunodiagnostic agents and immunotherapeutics. Navidea is developing multiple precision-targeted products based on our Manocept platform to enhance patient care by identifying the sites and pathways of undetected disease and enable better diagnostic accuracy, clinical decision-making and targeted treatment.
Navidea’s Manocept platform is predicated on the ability to specifically target the CD206 mannose receptor expressed on activated macrophages. The Manocept platform serves as the molecular backbone of Tc99m tilmanocept, the first product developed and commercialized by Navidea based on the platform. Other than Tc99m tilmanocept, which the Company has a license to distribute outside of Canada, Mexico and the United States, none of the Company’s drug product candidates have been approved for sale in any market.
Our business is focused on two primary types of drug products: (i) diagnostic substances, including Tc99m tilmanocept and other diagnostic applications of our Manocept platform, and (ii) therapeutic development programs, including therapeutic applications of our Manocept platform. See Note 15 to the accompanying consolidated financial statements for more information about our business segments.
History
We were originally incorporated in Ohio in 1983 and reincorporated in Delaware in 1988. Since our inception, the majority of our efforts and resources have been devoted to the research and clinical development of radiopharmaceutical technologies primarily related to the intraoperative diagnosis and treatment of cancers. In recent years, we have expanded our focus to include the diagnosis of inflammatory diseases such as rheumatoid arthritis (“RA”) and the treatment of a range of other diseases.
Beginning in late 2011, the Company in-licensed two neuro-tracer product candidates, NAV4694 and NAV5001. The Company advanced the development of both product candidates over the course of 2012 through 2014, moving both into Phase 3 clinical trials. However, in May 2014, the Navidea Board of Directors announced that the Company would restructure its development efforts to focus on cost effective development of the Manocept platform and divest its neuro-tracer product candidates.
In December 2014, we announced the formation of a new business unit to further explore therapeutic applications for the Manocept platform, which was incorporated as Macrophage Therapeutics, Inc. (“MT”) in January 2015 as a majority-owned subsidiary of Navidea. Navidea also granted MT an exclusive sublicense for certain therapeutic applications of the Manocept technology. Effective March 1, 2019, Navidea terminated the sublicense to MT in accordance with its terms due to MT’s insolvency. Since then, Navidea has continued the development of therapeutic products based on the Manocept platform.
Technology and Product Candidates
Our primary development efforts over the last several years were focused on diagnostic products, including Tc99m tilmanocept, which the Company has a license to distribute outside of Canada, Mexico and the United States. Our more recent initiatives have been focused on diagnostic and therapeutic line extensions based on our Manocept platform.
During the ongoing COVID-19 global pandemic, the Company’s primary concern is the safety of its employees, the employees of its clinical trial sites, and the patients enrolled in its clinical trials. The Company is working hard to mitigate any safety risk along with any long-term impact on its clinical development programs. We do not believe there has been a significant impact to the Company’s clinical development and regulatory timelines resulting from the ongoing COVID-19 global pandemic. However, the COVID-19 outbreak delayed enrollment in our NAV3-32 clinical study in the United Kingdom due to national COVID-19-related shutdowns. In addition, the regulatory approval process in India was delayed by the impact of COVID-19 in that country.
As a brief overview of recent developments in the Company’s diagnostics area (additional details in following sections), Navidea has completed the Phase 2b clinical trial (NAV3-31) evaluating imaging repeatability, reproducibility, and stability, as well as the capacity of Tc99m tilmanocept imaging to serve as an early predictor of treatment efficacy of anti-tumor necrosis factor alpha (“TNFα”) therapy in patients with moderate to severe RA. In addition, the Company has completed enrollment into a Phase 2b clinical trial (NAV3-35) designed to accrue hand and wrist planar and single photon emission computed tomography/computed tomography (“SPECT/CT”) images from healthy subjects (with SPECT/CT imaging also done on a small group of RA patients) so that Navidea can complete a normative database in support of its RA imaging commercial product development. The Company’s currently enrolling pivotal Phase 3 trial for RA (NAV3-33) is the next step in the development plan for indications in RA. The additional Phase 2b trial (NAV3-32) correlating Tc99m tilmanocept uptake in RA-involved joints with CD206 immunohistochemistry findings from synovial biopsies is actively recruiting. In addition, the investigator-initiated Phase 2 cardiovascular (“CV”) study was completed at Massachusetts General Hospital and a manuscript has been published by the investigators. Results of this study provided to date have paralleled data in our earlier published article, and these data are supportive of Navidea’s hypothesis that tilmanocept can provide marked signal to background in a host of CV disease applications.
Manocept Platform - Diagnostics and Therapeutics Background
Navidea’s Manocept platform is predicated on the ability to specifically target the mannose receptor (CD206) expressed primarily on activated macrophages. This flexible and versatile platform serves as a molecular backbone for purpose-built targeted imaging molecules that may significantly impact patient care by providing enhanced diagnostic accuracy, clinical decision-making, and target-specific treatment. This CD206-targeted drug platform is applicable to a range of diagnostic modalities, including SPECT, positron emission tomography (“PET”), gamma-scanning and intra-operative and/or optical-fluorescence detection, as well as delivery of therapeutic compounds that target macrophages and their role in a variety of immune- and inflammation-involved diseases. The United States Food and Drug Administration (“FDA”)-approved sentinel node/lymphatic mapping agent, Tc99m tilmanocept, is representative of the ability to successfully exploit this mechanism to develop powerful new products and to expand this technology into additional diagnostic and therapeutic applications.
Activated macrophages play important roles in many disease states and are an emerging target in many diseases where diagnostic uncertainty exists. Impairment of the macrophage-driven disease mechanisms is an area of increasing and proven focus in medicine. The number of people affected by all the inflammatory diseases combined is estimated at more than 40 million in the United States and up to 700 million worldwide, making macrophage-mediated diseases an area of remarkable clinical importance. There are many recognized disorders having macrophage involvement, including RA, atherosclerosis/vulnerable plaque/cardiovascular disease, nonalcoholic steatohepatitis (“NASH”), inflammatory bowel disease (“IBD”), systemic lupus erythematosus, cancer generally including Kaposi’s sarcoma (“KS”), leishmaniasis, and others that span general clinical areas in cancer immunology, autoimmunity, infectious diseases, cardiology, central nervous system diseases, and inflammation. For the near term, we have selected target diseases that may, if successfully developed, benefit from this technology.
The Company has developed processes for producing the first four therapeutic Manocept immuno-construct series, the Manocept doxorubicin (“MAN-DOX”) series, which is designed to specifically target and kill or modify activated CD206+ macrophages by delivering doxorubicin, a Manocept paclitaxel series (“MAN-PAC”), and a Manocept Bisphosphonate series (“MAN-BIS”). The MAN-PAC and MAD-BIS series are designed to modify CD206+ macrophages to make them more proinflammatory. The Company has also created a Manocept dexamethasone (“MAN-DEX”) series, which is designed to inhibit the inflammatory activity of activated CD206+ macrophages by delivering a potent anti-inflammatory agent, dexamethasone. We have expended significant efforts in recent years to improve chemical syntheses and to produce sufficient quantities of Manocept constructs representing all 4 series agents, along with the concomitant analytical standards, to provide material for current and planned preclinical animal studies and future clinical trials. Evaluation of representative examples of constructs from all four series have been successfully performed in human macrophage cell culture assays with MAN-DOX and MAN-PAC advancing to evaluations in various syngeneic mouse models of cancer.
Manocept Platform – Immuno-Diagnostics Clinical Data
Rheumatoid Arthritis
Two Tc99m tilmanocept dose escalation studies in RA have been completed. The first study was completed and included 18 subjects (nine with active disease and nine healthy subjects) dosed subcutaneously (“SC”) with 50 and 200 µg/2mCi Tc99m tilmanocept (ClinicalTrials.gov NCT02683421). The results of this study were presented at five international meetings, including Biotechnology Innovation Organization, Society of Nuclear Medicine and Molecular Imaging (“SNMMI”), and The American College of Rheumatology (“ACR”). In addition, based on completion of extensive preclinical dosing studies pursuant to our dialog with the FDA, we have completed a Phase 1/2 study involving intravenous (“IV”) dosing of 39 subjects with IV-administered Tc99m tilmanocept (ClinicalTrials.gov NCT02865434). In conjunction with this study, we completed pharmacokinetic, pharmacodynamics and radiation dosimetry phases in human subjects as well. The majority of the costs of these studies were supported through a Small Business Innovation Research (“SBIR”) grant (NIH/NIAMSD Grant 1 R44 AR067583-01A1). Results of the Phase 1/2 study were presented at the June 2018 and June 2019 SNMMI meetings, the 2018 European League Against Rheumatism (“EULAR”) meeting and the 2018 ACR meeting. A manuscript intended for peer reviewed publication is in preparation.
The Phase 1/2 study enrolled subjects with active, moderate-to-severe RA, and healthy controls. Results from the completed trial demonstrated that Tc99m tilmanocept is well-tolerated with no serious adverse events, adverse drug reactions, or drug-related adverse events observed. Additionally, static planar images revealed joint-specific Tc99m tilmanocept localization in RA subjects to disease-involved joints of the shoulders, knees, hands, and feet, but no joint-specific localization in healthy control subjects, revealing potentially significant immunodiagnostic information about CD206-expressing synovial macrophage involvement in RA. An optimal imaging time window post-Tc99m tilmanocept IV administration, as well as optimal dosing, were also determined.
In April 2019, the Company received feedback from the FDA regarding the Company’s planned clinical studies to evaluate joint disease in patients with RA and monitor patient response to therapy. The Company’s proposed RA studies were discussed with the FDA during an in-person meeting and through follow-up collaborative efforts. The FDA communicated that the first study, a Phase 2b trial, was aligned with expectations for the studies and that they would continue to work with Navidea as the Company progressed into the second Phase 2b trial correlating Tc99m tilmanocept uptake in RA-involved joints with CD206 immunohistochemistry findings from synovial biopsies and into the planned Phase 3 clinical trial.
In May 2019, we began enrolling patients into the first Phase 2b study, (NAV3-31), entitled “Evaluation of the Precision and Sensitivity of Tilmanocept Uptake Value (“TUV”) on Tc99m Tilmanocept Planar Imaging” (ClinicalTrials.gov NCT03938636). This study, since completed, provided confirmatory support necessary to initiate Navidea’s Phase 3 study program. In October 2019, the Company performed its first interim analysis of this trial, covering subjects enrolling into Arms 1 and 2. The results of this interim analysis were in line with the Company’s hypotheses that Tc99m tilmanocept can provide robust, stable imaging in healthy subjects as well as in patients with active RA, and provide the fundamental information needed to keep moving forward into the Phase 3. A summary of these results was presented at the 2020 EULAR meeting. In May 2020, the Company announced the results of its second interim analysis, covering Arm 3 of the trial. This Arm mirrored the Phase 3 in design and provided information relevant for sample size calculation for the Phase 3 as well as support for the hypothesis that Tc99m tilmanocept imaging can provide an early indicator of treatment efficacy of anti-TNFα therapeutics. These interim results were presented at the 2020 ACR meeting. In June 2020, the Company announced full enrollment into this trial, with imaging events completed in each patient enrolled in Arm 3. A poster presentation based on the completed NAV3-31 study was presented at the 2022 ACR meeting. The Company also presented interim results of its NAV3-32 study at the 2022 ACR meeting.
In February 2021, the Company submitted its formal briefing book to the FDA, containing detailed analysis and discussion of the Company’s then-ongoing Phase 2b study (NAV3-31) and prior studies in RA as well as the design and statistical analysis plan for the proposed Phase 3 for FDA comment. Following the feedback received from the FDA at the end of March 2021, the Company continued to work toward completing the analysis of the full NAV3-31 trial dataset and submitted the resultant briefing book containing the results of this analysis in preparation for the standard End-of-Phase 2 Type B meeting, which took place on September 1, 2021. The Company had a constructive meeting with the FDA and, based on the discussion in this meeting and follow-up communication, made agreed-upon modifications to the trial design for the Phase 3 study (NAV3-33). The Company submitted the modified protocol back to the FDA and initiated the study in December 2021. Following additional feedback from the FDA, the Company made modifications to several of the objectives. Enrollment into the Phase 3 study is ongoing. The pivotal Phase 3 study program will determine Tc99m tilmanocept’s capability to serve as an early predictor of treatment response to anti-TNFα therapy in patients with RA. The current aim is for enrollment completion of the Phase 3 by end of 2023 with NDA submission targeted for late 2024 or early 2025.
Cardiovascular Disease
In collaboration with researchers at Massachusetts General Hospital, Navidea has completed two investigator-initiated clinical studies evaluating Tc99m tilmanocept’s ability to enable imaging of atherosclerotic plaques. Results of these studies provide strong preliminary evidence of the potential of Tc99m tilmanocept to accumulate specifically in and enable imaging of non-calcified atherosclerotic plaques. Non-calcified atherosclerotic plaques include plaques with morphologies indicating a high risk of rupture. Rupture of such plaques causes myocardial infarctions (heart attacks) and a significant portion of ischemic strokes. The studies compared aortic Tc99m tilmanocept uptake imaged by SPECT/CT in clinically asymptomatic subjects with intermediate Framingham Risk Scores (“FRS”) who were infected with Human Immunodeficiency Virus (“HIV”) as compared to healthy, uninfected, FRS and age-matched subjects. Tc99m tilmanocept SPECT/CT images were compared to aortic images of the same subjects obtained by contrast enhanced coronary computed tomography angiography and/or [18F]NaF PET/CT.
A nine-subject study to evaluate diagnostic imaging of emerging atherosclerosis plaque with the Tc99m tilmanocept product dosed SC was performed (ClinicalTrials.gov NCT02542371). The results of this study were presented at two major international meetings (Conference on Retroviruses and Opportunistic Infections and SNMMI, 2017) and published in early release in the Journal of Infectious Diseases in January 2017 (published in the circulated version, Journal of Infectious Diseases (2017) 215 (8): 1264-1269), confirming that the Tc99m tilmanocept product can both quantitatively and qualitatively target non-calcified plaque in the aortic arch of Acquired Immunodeficiency Syndrome (“AIDS”) patients (supported by NIH/NHLBI Grant 1 R43 HL127846-01). This study was later expanded to include up to 31 participants, achieved full enrollment, and a publication has resulted in the Journal of Infectious Diseases (2022) Nov 11; 226(10):1823-1833.
A second Phase 1/2 investigator-initiated study in cooperation with Massachusetts General Hospital in subjects with HIV was initiated that expanded the original study in both the scope of the drug administration as well as the diagnostic assessment of the subjects. This study enrolled both AIDS subjects and healthy controls in imaging non-calcified plaque using IV and SC-administered Tc99m tilmanocept and will expand the initial investigation to the assessment of aortic plaque as well as carotid and coronary arteries. Initial analysis suggested that the SC route of administration led to superior signal-to-background in areas of non-calcified plaque. These results are being further assessed.
Navidea was also awarded a $225,000 phase 1 Small Business Technology Transfer grant (1R41HL147640-01A1) entitled Gallium 68 Tilmanocept for PET Imaging of Atherosclerosis Plaques. This grant supported a research collaboration between Navidea and Dr. Suzanne Lapi of the University of Alabama Birmingham evaluating a mouse model of atherosclerosis. This work had as its aim the evaluation of [68]gallium tilmanocept and various next generation imaging agents for visualizing plaques. Activities began in the fourth quarter of 2019. All images have been acquired with efforts now focused on final data analysis.
Kaposi’s Sarcoma
We initiated and completed a study of KS in 2015 (ClinicalTrials.gov NCT022201420) and received additional funding from the National Institutes of Health (“NIH”) in 2016 to continue diagnostic studies in this disease. The new support not only continues the imaging of the cutaneous form of this disease but expands this to imaging of visceral disease via IV administration of Tc99m tilmanocept (NIH/NCI 1 R44 CA192859-01A1; ClinicalTrials.gov NCT03157167). This now-escalated study includes a pathology/biopsy component as well as an imaging component to determine pathology concordance with image assessment. We received Institutional Review Board approval of the clinical protocol and initiated a Phase 1/2 clinical study in KS in 2017. This trial has completed enrollment and imaging, with final data analysis and clinical study report preparation well underway.
Tuberculosis (“TB”)
In April 2019, the Company announced that Professor Mike Sathekge, MBChB, M. Med (Nuclear Medicine), PhD, Professor and Head of the Department of Nuclear Medicine in the Faculty of Health Sciences at the University of Pretoria/Steve Biko Academic Hospital, planned to initiate a comparative study evaluating the use of tilmanocept in patients with TB. The purpose of this ongoing study is to explore using 68Ga tilmanocept as an aid in TB patient management while contributing to the better understanding of the biology of TB granulomas. CD206+ macrophages constitute one of the most abundant cell types in TB granulomas. Therefore, a molecular probe such as 68Ga-labeled tilmanocept targeting mannose receptor CD206 expressed on macrophages holds great promise not only in understanding the biology of TB granulomas, but may also support future development of a tilmanocept-like drug delivery vehicle for delivering therapeutic interventions to TB granulomas. Navidea has provided tilmanocept for use in this study, and several subjects have been injected and imaged to date. Successful completion of this study could support an extended claim of 68Ga-tilmanocept.
Biomarker Application and Qualification
In November 2017, the Company commenced the qualification of the biomarker CD206 with the FDA Biomarker Section of The Center for Drug Evaluation and Research (“CDER”). As per FDA protocol, Navidea submitted a draft letter of intent (“LOI”) to CDER prior to the November 2017 meeting. According to the CDER directive, “the Biomarker Qualification Program was established to support the CDER’s work with external stakeholders to develop biomarkers that aid in the drug development process. Through the FDA’s Biomarker Qualification Program, an entity may request regulatory qualification of a biomarker for a particular context of use (“COU”) in drug development.” Following the meeting with the FDA, and because of Navidea’s data sets and the general external publication database, Navidea, in conjunction with FDA, is now reviewing the LOI with the FDA’s recommended consultants. Navidea has revised the LOI draft strategy in order to expedite the application process. In March 2018, Navidea had a follow-up meeting with the FDA’s assigned strategist, during which the potential to further narrow the LOI elements was reviewed. Navidea is continuing the process of finalizing the COU LOI and will be providing the background data sets for qualification review with the FDA/CDER. Additional meetings have taken place and the pursuit of this qualification is ongoing. A pivotal element of this will be the data obtained from the NAV3-32 clinical trial.
Manocept Platform – In-Vitro and Pre-Clinical Immunotherapeutics Data
The Company has been developing Manocept platform drug delivery constructs that carry various payloads. Chemical synthesis techniques have advanced considerably, resulting in more robust and reproducible synthesis protocols that provide products with chemical attributes indicative of enhanced in vivo activity. Particularly significant results included improved processes for adding mannose moieties to the amine-terminated linkers on the dextran backbone and significant advances in the design and functionality of the degradable linkers to which drug payloads are attached. These advances in chemical methods are the subject matter of several patent applications currently under review by the USPTO. Experiments utilizing human macrophage assays show that at treatment doses of MAN-DOX, MAN-PAC, and MAN-BIS below what is required to kill macrophages, all three construct series dramatically alter the immunological behavior of macrophages, making them more proinflammatory. Results indicate that MAN-BIS and MAN-PAC constructs are more active at altering macrophage behavior towards a proinflammatory status than MAN-DOX. In syngeneic mouse tumor experiments, the MAN-DOX and MAN-PAC constructs significantly synergized the activity of another anticancer therapy producing anti-tumor activity that was greater than either treatment alone. Consistent with the macrophage cell assay results, MAN-PAC was more active than MAN-DOX in synergizing the activity of the other anti-cancer therapy. Similar studies evaluating the MAD-BIS constructs in syngeneic mouse tumor models are pending. Results from the completed studies have been presented at the New York Academy of Sciences Frontiers in Cancer Immunotherapy 2021 (MAN-DOX, May 14, 2021), at the Tumor Myeloid-Directed Therapies Summit (MAN-BIS, June 2022), and at the Society for Immunotherapy of Cancer (SITC) (MAN-PAC, Nov. 10, 2022). Work involving a second generation Manocept dexamethasone-carrying construct (MAN-DEX) has progressed to evaluations in human macrophage culture assays, showing that the MAN-DEX suppressed the proinflammatory behavior of human macrophages as expected.
Kaposi’s Sarcoma
The novel MAN-DOX class constructs are designed to specifically deliver doxorubicin, a chemotoxin, which can kill KS tumor cells and their tumor-associated macrophages, potentially altering the course of cancer. We received additional funding to continue therapeutic studies in this disease with the goal of completing an investigational new drug (“IND”) submission for a Manocept construct (MAN-DOX class of compounds) consisting of tilmanocept linked to doxorubicin for the treatment of KS. Efforts supported by this grant (NIH/NCI 1 R44 CA206788-01) are now complete. The results greatly advanced our knowhow for robustly and reproducibly synthesizing MAN-DOX and related constructs carrying other payloads. The grant-supported efforts were presented at the New York Academy of Sciences Frontiers in Cancer Immunotherapy 2021.
Other Immunotherapeutic Applications
The Company continues to evaluate emerging data in other disease states to define areas of focus, development pathways and partnering options to capitalize on the Manocept platform, including ongoing studies in KS, RA and infectious diseases such as leishmaniasis. The immuno-inflammatory process is remarkably complex and tightly regulated with indicators that initiate, maintain and shut down the process. Macrophages are immune cells that play a critical role in the initiation, maintenance, and resolution of inflammation. They are activated and deactivated in the inflammatory process. Because macrophages may promote dysregulation that accelerates or enhances disease progression, diagnostic and therapeutic interventions that target macrophages may open new avenues for controlling inflammatory diseases. There can be no assurance that further evaluation or development will be successful, that any Manocept platform product candidate will ultimately achieve regulatory approval, or if approved, the extent to which it will achieve market acceptance.
Market Overview
Manocept Diagnostics and Macrophage Therapeutics Market Overview
Impairment of the macrophage-driven disease mechanism is an area of increasing focus in medicine. There are many recognized disorders having macrophage involvement, including RA, atherosclerosis/vulnerable plaque, Crohn’s disease, TB, systemic lupus erythematosus, KS, and others that span clinical areas in oncology, autoimmunity, infectious diseases, cardiology, and inflammation. The number of people affected by all the inflammatory diseases combined is estimated at more than 40 million in the United States, making these macrophage-mediated diseases an area of significant clinical importance. The Arthritis Foundation estimates that RA alone affects over 1.5 million people in the United States and as much as 1% of the worldwide population. Based on the most recent U.S. Medicare/Medicaid data, total annual societal costs of RA are estimated to be over $39 billion.
Tc99m Tilmanocept – Cancer Market Overview
Cancer is the second leading cause of death in the United States. The American Cancer Society (“ACS”) estimates that cancer will cause over 600,000 deaths in 2023 in the United States alone. Additionally, the ACS estimates that over 1.9 million new cancer cases will be diagnosed in the United States during 2023. The National Cancer Institute estimates that direct medical costs for cancer in the United States for 2020 were $208.9 billion. Cancer is also the second leading cause of death in Europe. The World Health Organization reports more than 3.7 million new cases and 1.9 million deaths in Europe each year.
Tc99m tilmanocept is approved by the FDA for use in solid tumor cancers where lymphatic mapping is a component of surgical management and for guiding sentinel lymph node biopsy in patients with clinically node negative breast cancer, head and neck cancer, melanoma or squamous cell carcinoma of the oral cavity. Tc99m tilmanocept has also received European approval in imaging and intraoperative detection of sentinel lymph nodes in patients with melanoma, breast cancer or localized squamous cell carcinoma of the oral cavity. If the potential of Tc99m tilmanocept as a radioactive tracing agent is ultimately realized, it may address not only the breast and melanoma markets on a procedural basis, but also assist in the clinical evaluation and staging of solid tumor cancers and expanding lymph node mapping to other solid tumor cancers such as prostate, gastric, colon, gynecologic, and non-small cell lung.
Marketing and Distribution
All of Navidea’s assets used in operating its business of developing, manufacturing and commercializing the Company’s radioactive diagnostic agent marketed under the Lymphoseek® trademark in Canada, Mexico and the United States were sold to Cardinal Health 414, LLC (“Cardinal Health 414”) in March 2017. Cardinal Health 414 has responsibility for marketing Lymphoseek in those territories.
Europe
Unlike in the United States, where institutions typically rely on radiopharmaceutical products that are compounded and delivered by specialized radiopharmacy distributors such as Cardinal Health 414, institutions in Europe predominantly purchase non-radiolabeled material and compound the radioactive product on-site. With respect to Tc99m tilmanocept commercialization in Europe, we initially chose a specialty pharmaceutical strategy that we believed would be supportive of premium product positioning and reinforce Tc99m tilmanocept's clinical value proposition, as opposed to a commodity or a generics positioning approach. On March 5, 2015, Navidea entered into an Exclusive License Agreement (as amended to date, the “License Agreement”) for the commercialization and distribution of a 50-microgram kit for radiopharmaceutical preparation (tilmanocept) in the European Union (“EU”) with SpePharm AG (“SpePharm,” an affiliate of Norgine BV), a European specialist pharmaceutical company with an extensive pan-European presence. Under the License Agreement, SpePharm had the exclusive right to develop, manufacture and commercialize the Company’s products approved for radiolabeling with Tc99m and containing Lymphoseek (collectively, the “Products”) in several jurisdictions abroad, including the United Kingdom, France, Germany, Australia and New Zealand (collectively, the “Licensed Territory”). In exchange for such rights, the Company was entitled to certain royalty payments. In accordance with the License Agreement, Navidea transferred responsibility for regulatory maintenance of the Tc99m tilmanocept Marketing Authorization to SpePharm. SpePharm was also responsible for production, distribution, pricing, reimbursement, sales, marketing, medical affairs, and regulatory activities.
On May 11, 2020 (the “Termination Date”), the Company entered into a Termination Agreement (the “Termination Agreement”) with SpePharm and Norgine BV (“Norgine”) which terminated the License Agreement. Pursuant to the Termination Agreement, the parties agreed that neither owed the other any payments due under the License Agreement as of the Termination Date and that, among other things, SpePharm no longer has any right in, nor claim to, any intellectual property owned by the Company or its affiliates anywhere in the world. SpePharm also agreed to perform certain wind-down activities (the “Wind-Down Activities”) during the six-month period following the Termination Date (the “Transition Period”), which Transition Period was extended by ninety days. The Wind-Down Activities included SpePharm transferring to the Company or its designee(s) the regulatory approvals controlled by SpePharm or its affiliates for the purpose of marketing, distributing and selling the Products in the Licensed Territory. SpePharm also transferred to the Company certain tenders and other customer and sales contracts related to the Products. Subject to the terms of the Termination Agreement, Norgine agreed to guarantee SpePharm’s performance of its obligations under the Termination Agreement. Although the Transition Period had elapsed, SpePharm continued to fulfill customer orders until the Company obtained the regulatory license required to distribute the product in Europe, which license was received during the fourth quarter of 2021.
On June 9, 2020, Navidea established a new European entity, Navidea Biopharmaceuticals Europe Limited (“Navidea Europe”), to address international development and commercialization needs for our technologies, including Tc99m tilmanocept. SpePharm has transferred the Tc99m tilmanocept Marketing Authorization to Navidea Europe, along with the responsibility for production and commercialization of Tc99m tilmanocept in the Licensed Territory. Navidea Europe has established relationships and executed agreements with third-party providers in order to fulfill such responsibilities. Navidea owns 100% of the outstanding shares of Navidea Europe.
China
In August 2014, Navidea entered into an exclusive agreement with Beijing Sinotau Medical Research Co., Ltd. (“Sinotau”), a pharmaceutical organization with a broad China focus in oncology and other therapeutic areas, who will develop and commercialize Tc99m tilmanocept in China. In exchange, Navidea will earn revenue based on unit sales to Sinotau, royalties based on Sinotau’s sales of Tc99m tilmanocept and milestone payments from Sinotau, including a $300,000 non-refundable upfront payment. As part of the agreement, Sinotau is responsible for costs and conduct of clinical studies and regulatory applications to obtain Tc99m tilmanocept approval by the China Food and Drug Administration (“CFDA”). Upon approval, Sinotau will be responsible for all Tc99m tilmanocept sales, marketing, market access and medical affairs activities in China and excluding Hong Kong, Macau and Taiwan. Navidea and Sinotau will jointly support certain pre-market planning activities with a joint commitment on clinical and market development programs pending CFDA approval.
India
In June 2017, Navidea entered into an exclusive license and distribution agreement with Sayre Therapeutics (“Sayre”) for the development and commercialization of Tc99m tilmanocept in India. Sayre specializes in innovative treatments and medical devices commercialization in South Asia. Under the terms of the agreement, Navidea received a $100,000 upfront payment and is eligible to receive milestone payments and double-digit royalties associated with the sale of Tc99m tilmanocept in India. The Company received regulatory approval for Tc99m tilmanocept in India in March 2022, however certain additional approvals, such as an import license and authorization to use an alternative manufacturer, must be obtained prior to commercial sales launch in India.
Summary
Tc99m tilmanocept is in various stages of approval in other global markets and sales to this point in these markets, to the extent there were any, have not been material. However, we believe that with international partnerships to complement our positions in the EU, China and India, we will help establish Tc99m tilmanocept as a global leader in lymphatic mapping, as we are not aware of any other company that has a global geographic range. However, it is possible that Tc99m tilmanocept will never achieve regulatory approval in any market outside the United States, the EU or India, or if approved, that it may not achieve market acceptance in any market. We may also experience difficulty in securing collaborative partners for other global markets or radiopharmaceutical products, or successfully negotiating acceptable terms for such arrangements. See Item 1A - “Risk Factors.”
Manufacturing
We currently use and expect to continue to be dependent upon contract manufacturers to manufacture each of our product candidates. We maintain a quality control and quality assurance program, including a set of standard operating procedures and specifications, with the goal that our products and product candidates are manufactured in accordance with current good manufacturing practices (“cGMP”) and other applicable domestic and international regulations. We are investing in additional manufacturing and supply chain resources, and have entered into development contracts with the established manufacturing companies Corden Pharma Switzerland, LLC for active pharmaceutical ingredient production and ROTOP Pharmaka GmbH for drug product manufacturing. It is likely that we will continue to rely on third-party manufacturers for our development and commercial products on a contract basis. We may not be successful in completing long-term agreements for the supply of Tc99m tilmanocept on terms acceptable to the Company, or at all. See Item 1A - “Risk Factors.”
Competition
Competition in the pharmaceutical and biotechnology industries is intense. We face competition from a variety of companies focused on developing inflammatory, oncology and CV disease diagnostic imaging agents and other diagnostic modalities. We compete with large pharmaceutical and other specialized biotechnology companies. We also face competition from universities and other non-profit research organizations. Many emerging medical product companies have corporate partnership arrangements with large, established companies to support the research, development, and commercialization of products that may be competitive with our products. In addition, a number of large established companies are developing proprietary technologies or have enhanced their capabilities by entering into arrangements with or acquiring companies with technologies applicable to the detection or treatment of cancer and other diseases targeted by our product candidates. Smaller companies may also prove to be significant competitors, particularly through collaborative arrangements with large pharmaceutical and established biotechnology companies. Many of these competitors have products that have been approved or are in development and operate large, well-funded research and development (“R&D”) programs. Many of our existing or potential competitors have substantially greater financial, R&D, regulatory, marketing, and production resources than we have. Other companies may develop and introduce products and processes competitive with or superior to ours.
We expect to encounter significant competition for our pharmaceutical products. Companies that complete clinical trials, obtain required regulatory approvals and commence commercial sales of their products before us may achieve a significant competitive advantage if their products work through a similar mechanism as our products and if the approved indications are similar. A number of biotechnology and pharmaceutical companies are developing new products for the diagnosis and/or treatment of the same diseases being targeted by us. In some instances, such products have already entered late-stage clinical trials or received FDA approval and may be marketed for some period prior to the approval of our products.
We believe that our ability to compete successfully will be based on our ability to create and maintain scientifically advanced “best-in-class” technology, develop proprietary products, attract and retain scientific personnel, obtain patent or other protection for our products, obtain required regulatory approvals and manufacture and successfully market our products, either alone or through third parties. We expect that competition among products cleared for marketing will be based on, among other things, product efficacy, safety, reliability, availability, price, and patent position. See Item 1A - “Risk Factors.”
Tc99m Tilmanocept Competition – Currently Approved Indications
Some surgeons who practice the lymphatic mapping procedure for which Tc99m tilmanocept is intended currently use other radiopharmaceuticals such as a sulfur colloid or other colloidal compounds. In addition, some surgeons still use vital blue dyes to assist in the visual identification of the draining lymphatic tissue around a primary tumor. In the EU and certain Pacific Rim markets, there are colloidal-based compounds with various levels of approved labeling for use in lymphatic mapping, although a number of countries still employ products used “off-label.”
Rheumatoid Arthritis Competition
Currently, no single test is available to diagnose and monitor RA. Rather, a rheumatologist will make a diagnosis based on several procedures that may include a physical exam, blood tests, and/or imaging tests, among others. The Arthritis Foundation states that the goals of RA treatment are to relieve symptoms, stop inflammation, prevent joint and organ damage, improve physical function and well-being, and reduce long-term complications. Medications for the treatment of RA currently fall into two categories: drugs that ease symptoms, such as nonsteroidal anti-inflammatory drugs, and drugs that slow disease activity. Drugs that slow disease activity include corticosteroids, biologic disease-modifying antirheumatic drugs (“bDMARDs”) and Janus kinase inhibitors. Many of these drugs are produced and sold by large pharmaceutical companies, including AbbVie, Amgen, Bristol Meyers Squibb, Johnson & Johnson, Merck, Pfizer and Roche, among others.
One of Navidea’s primary goals for its RA imaging product development program is to develop an imaging-based test that can predict patient-specific therapeutic responses to bDMARDs, especially anti-TNFα antibody therapy, which is the most commonly prescribed type of therapy for RA within this drug class. While no tests predicting therapeutic responses to bDMARDs have been approved by the FDA, Navidea is aware of other groups that are working to develop such tests, primarily blood-based biomarker assays. One or more of these tests could be approved by the FDA in the future, making it possible for them to compete directly with Navidea’s imaging-based RA test. Even without FDA approval, these tests could reduce the Company’s share of this market in the future, and at least one of these tests is currently available and used as a laboratory-based test that has not undergone the GDA approval process.
Patents and Proprietary Rights
The patent position of biotechnology companies, including Navidea, generally is highly uncertain and may involve complex legal and factual questions. Potential competitors may have filed applications, or may have been issued patents, or may obtain additional patents and proprietary rights relating to products or processes in the same area of technology as that used by the Company. The scope and validity of these patents and applications, the extent to which we may be required to obtain licenses thereunder or under other proprietary rights, and the cost and availability of licenses are uncertain. Our patent applications or those licensed to us may not result in additional patents being issued, and our patents or those licensed to us may not afford protection against competitors with similar technology; these patents may be designed around by others or others may obtain patents that we would need to license or design around.
We also rely upon unpatented trade secrets. Others may independently develop substantially equivalent proprietary information and techniques, or otherwise gain access to our trade secrets, or disclose such technology, or we may not be able to meaningfully protect our rights to our unpatented trade secrets.
We require our employees, consultants, advisers, and suppliers to execute a confidentiality agreement upon the commencement of an employment, consulting or manufacturing relationship with us. The agreement provides that all confidential information developed by or made known to the individual during the course of the relationship will be kept confidential and not disclosed to third parties except in specified circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual will be the exclusive property of our company. However, these agreements may not provide meaningful protection for our trade secrets in the event of an unauthorized use or disclosure of such information. We also employ a variety of security measures to preserve the confidentiality of our trade secrets and to limit access by unauthorized persons. However, these measures may not be adequate to protect our trade secrets from unauthorized access or disclosure. See Item 1A - “Risk Factors.”
Tilmanocept Intellectual Property
Tilmanocept is under license from the University of California, San Diego (“UCSD”) to Navidea for the exclusive world-wide rights in all diagnostic and therapeutic uses of tilmanocept, except for the use of Tc99m tilmanocept in lymphatic mapping in Canada, Mexico and the United States, which rights have been licensed directly to Cardinal Health 414 by UCSD. Navidea maintains license rights to Tc99m tilmanocept in the rest of the world, as well as a license to the intellectual property underlying the Manocept platform.
Tc99m tilmanocept and related compositions, including the Manocept backbone composition and methods of use, are the subject of multiple patent families including issued patents and patent applications in the United States and certain major foreign markets.
The first composition of matter patent covering tilmanocept was issued to UCSD in the United States in June 2002 and would have expired in May 2020. However, Navidea was granted a five-year patent term extension under the Hatch Waxman Act due to time lost in regulatory review. The claims of the composition of matter patent covering tilmanocept issued in the majority of major-market European countries in 2004 and would have expired in 2020. However, the Company has obtained supplemental protection certificates, extending the patent terms to 2025.
Patent applications have been filed by Navidea in the U.S. and certain major foreign markets related to manufacturing processes for tilmanocept, the first of which was issued in the U.S. in 2013. These patents and/or applications will expire between 2029 and 2034. Further patent applications have been filed by Navidea alone or with The Ohio State Innovation Foundation related to CD206 expressing cell-related disorders and diseases. These patents and/or applications are expected to expire between 2034 and 2043.
Government Regulation
The research, development, testing, manufacture, labeling, promotion, advertising, distribution and marketing, among other things, of our products are extensively regulated by governmental authorities in the United States and other countries. In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, Public Health Service Act, and their implementing regulations. Failure to comply with applicable U.S. requirements may subject us to administrative or judicial sanctions, such as FDA refusal to approve pending new drug applications or supplemental applications, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions and/or criminal prosecution. We also may be subject to regulation under the Occupational Safety and Health Act, the Atomic Energy Act, the Toxic Substances Control Act, the Export Control Act and other present and future laws of general application as well as those specifically related to radiopharmaceuticals.
Most aspects of our business are subject to some degree of government regulation in the countries in which we conduct our operations. As a developer, manufacturer and marketer of medical products, we are subject to extensive regulation by, among other governmental entities, the FDA and the corresponding state, local and foreign regulatory bodies in jurisdictions in which our products are intended to be sold. These regulations govern the introduction of new products, the observance of certain standards with respect to the manufacture, quality, safety, efficacy and labeling of such products, the maintenance of certain records, the tracking of such products, performance surveillance and other matters.
Failure to comply with applicable federal, state, local or foreign laws or regulations could subject us to enforcement action, including product seizures, recalls, withdrawal of marketing clearances, and civil and criminal penalties, any one or more of which could have a material adverse effect on our business. We believe that we are in substantial compliance with such governmental regulations. However, federal, state, local and foreign laws and regulations regarding the manufacture and sale of radiopharmaceuticals are subject to future changes. Such changes may have a material adverse effect on our company.
For some products, and in some countries, government regulation is significant and, in general, there is a trend toward more stringent regulation. In recent years, the FDA and certain foreign regulatory bodies have pursued a more rigorous enforcement program to ensure that regulated businesses like ours comply with applicable laws and regulations. We devote significant time, effort and expense addressing the extensive governmental regulatory requirements applicable to our business. To date, we have not received a warning letter from the FDA or any other regulatory bodies of alleged deficiencies in our compliance with the relevant requirements, nor have we recalled or issued safety alerts on any of our products. However, a warning letter, recall or safety alert, if it occurred, could have a material adverse effect on our company. See Item 1A - “Risk Factors.”
The FDA review processes could delay our Company's introduction of new products in the United States in the future. In addition, many foreign countries have adopted more stringent regulatory requirements that also have added to the delays and uncertainties associated with the development and release of new products, as well as the clinical and regulatory costs of supporting such releases. It is possible that delays in receipt of, or failure to receive, any necessary clearance for our new product offerings could have a material adverse effect on our business, financial condition or results of operations. See Item 1A - “Risk Factors.”
The U.S. Drug Approval Process
None of our drugs may be marketed in the United States until such drug has received FDA approval. The steps required before a drug may be marketed in the United States include:
|
● |
preclinical laboratory tests, animal studies and formulation studies; |
|
● |
submission to the FDA of an IND application for human clinical testing, which must become effective before human clinical trials may begin; |
|
● |
adequate and well-controlled human clinical trials to establish the safety and efficacy of the investigational product for each indication; |
|
● |
submission to the FDA of a New Drug Application (“NDA”); |
|
● |
satisfactory completion of FDA inspections of the manufacturing and clinical facilities at which the drug is produced, tested, and/or distributed to assess compliance with cGMPs and current good clinical practices (“cGCP”) standards; and |
|
● |
FDA review and approval of the NDA. |
Preclinical tests include laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies. The conduct of the preclinical tests and formulation of the compounds for testing must comply with federal regulations and requirements. The results of the preclinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND, which must become effective before human clinical trials may begin. An IND will automatically become effective 30 days after receipt by the FDA unless, before that time, the FDA raises concerns or questions about issues such as the conduct of the trials as outlined in the IND. In such a case, the IND sponsor and the FDA must resolve any outstanding FDA concerns or questions before clinical trials can proceed. We cannot be sure that submission of an IND will result in the FDA allowing clinical trials to begin.
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators. Clinical trials are conducted under protocols detailing the objectives of the study, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND.
Clinical trials typically are conducted in three sequential phases, but the phases may overlap or be combined. The study protocol and informed consent information for study subjects in clinical trials must also be approved by an institutional review board at each institution where the trials will be conducted. Study subjects must sign an informed consent form before participating in a clinical trial. Phase 1 usually involves the initial introduction of the investigational product into people to evaluate its short-term safety, dosage tolerance, metabolism, pharmacokinetics and pharmacologic actions, and, if possible, to gain an early indication of its effectiveness. Phase 2 usually involves trials in a limited subject population to (i) evaluate dosage tolerance and appropriate dosage, (ii) identify possible adverse effects and safety risks, and (iii) evaluate preliminarily the efficacy of the product candidate for specific indications. Phase 3 trials usually further evaluate clinical efficacy and further test its safety by using the product candidate in its final form in an expanded subject population. There can be no assurance that Phase 1, Phase 2 or Phase 3 testing will be completed successfully within any specified period of time, if at all. Furthermore, we or the FDA may suspend clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
The FDA and the IND sponsor may agree in writing on the design and size of clinical studies intended to form the primary basis of an effectiveness claim in an NDA application. This process is known as a Special Protocol Assessment (“SPA”). These agreements may not be changed after the clinical studies begin, except in limited circumstances. The existence of a SPA, however, does not assure approval of a product candidate.
Assuming successful completion of the required clinical testing, the results of the preclinical studies and of the clinical studies, together with other detailed information, including information on the manufacturing quality and composition of the investigational product, are submitted to the FDA in the form of an NDA requesting approval to market the product for one or more indications. The testing and approval process requires substantial time, effort and financial resources. Submission of an NDA requires payment of a substantial review user fee to the FDA. Before approving an NDA, the FDA usually will inspect the facility or the facilities where the product is manufactured, tested and distributed and will not approve the product unless cGMP compliance is satisfactory. If the FDA evaluates the NDA and the manufacturing facilities as acceptable, the FDA may issue an approval letter or a complete response letter. A complete response letter outlines conditions that must be met in order to secure final approval of the NDA. When and if those conditions have been met to the FDA’s satisfaction, the FDA will issue an approval letter. The approval letter authorizes commercial marketing of the drug for specific indications. As a condition of approval, the FDA may require post-marketing testing and surveillance to monitor the product’s safety or efficacy, or impose other post-approval commitment conditions.
The FDA has various programs, including fast track, priority review and accelerated approval, which are intended to expedite or simplify the process of reviewing drugs and/or provide for approval on the basis of surrogate endpoints. Generally, drugs that may be eligible for one or more of these programs are those for serious or life-threatening conditions, those with the potential to address unmet medical needs and those that provide meaningful benefit over existing treatments. Our drug candidates may not qualify for any of these programs, or, if a drug candidate does qualify, the review time may not be reduced or the product may not be approved.
After approval, certain changes to the approved product, such as adding new indications, making certain manufacturing changes or making certain additional labeling claims, are subject to further FDA review and approval. Obtaining approval for a new indication generally requires that additional clinical studies be conducted.
U.S. Post-Approval Requirements
Holders of an approved NDA are required to: (i) conduct pharmacovigilance and report certain adverse reactions to the FDA, (ii) comply with certain requirements concerning advertising and promotional labeling for their products, and (iii) continue to have quality control and manufacturing procedures conform to cGMP. The FDA periodically inspects the sponsor’s records related to safety reporting and/or manufacturing and distribution facilities; this latter effort includes assessment of compliance with cGMP. Accordingly, manufacturers must continue to expend time, money and effort in the area of production, quality control and distribution to maintain cGMP compliance. We use and will continue to use third-party manufacturers to produce our products in clinical and commercial quantities, and future FDA inspections may identify compliance issues at our facilities or at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct.
Marketing of prescription drugs is also subject to significant regulation through federal and state agencies tasked with consumer protection and prevention of medical fraud, waste and abuse. We must comply with restrictions on off-label use promotion, anti-kickback, ongoing clinical trial registration, and limitations on gifts and payments to physicians.
Non-U.S. Regulation
Before our products can be marketed outside of the United States, they are subject to regulatory approval similar to that required in the United States, although the requirements governing the conduct of clinical trials, including additional clinical trials that may be required, product licensing, pricing and reimbursement vary widely from country to country. No action can be taken to market any product in a country until an appropriate application has been approved by the regulatory authorities in that country. The current approval process varies from country to country, and the time spent in gaining approval varies from that required for FDA approval. In certain countries, the sales price of a product must also be approved. The pricing review period often begins after market approval is granted. Even if a product is approved by a regulatory authority, satisfactory prices may not be approved for such product.
In Europe, marketing authorizations may be submitted at a centralized, a decentralized or national level. The centralized procedure is mandatory for the approval of biotechnology products and provides for the grant of a single marketing authorization that is valid in all EU member states. A mutual recognition procedure is available at the request of the applicant for all medicinal products that are not subject to the centralized procedure.
The European Commission granted marketing authorization for Tc99m tilmanocept in the EU in November 2014, and a reduced-mass vial developed for the EU market was approved in September 2016.
While we are unable to predict the extent to which our business may be affected by future regulatory developments, we believe that our substantial experience dealing with governmental regulatory requirements and restrictions on our operations throughout the world, and our development of new and improved products, should enable us to compete effectively within this environment.
Regulation Specific to Radiopharmaceuticals
Our radiolabeled targeting agents and biologic products, if developed, would require a regulatory license to market from the FDA and from comparable agencies in foreign countries. The process of obtaining regulatory licenses and approvals is costly and time consuming, and we have encountered significant impediments and delays related to our previously proposed biologic products.
The process of completing pre-clinical and clinical testing, manufacturing validation and submission of a marketing application to the appropriate regulatory bodies usually takes a number of years and requires the expenditure of substantial resources, and any approval may not granted on a timely basis, if at all. Additionally, the length of time it takes for the various regulatory bodies to evaluate an application for marketing approval varies considerably, as does the amount of preclinical and clinical data required to demonstrate the safety and efficacy of a specific product. The regulatory bodies may require additional clinical studies that may take several years to perform. The length of the review period may vary widely depending upon the nature and indications of the proposed product and whether the regulatory body has any further questions or requests any additional data. Also, the regulatory bodies require post-marketing reporting and surveillance programs (pharmacovigilance) to monitor the side effects of the products. Our potential drug or biologic products may not be approved by the regulatory bodies or may not be approved on a timely or accelerated basis, or any approvals received may subsequently be revoked or modified.
The Nuclear Regulatory Commission (“NRC”) oversees medical uses of nuclear material through licensing, inspection, and enforcement programs. The NRC issues medical use licenses to medical facilities and authorized physician users, develops guidance and regulations for use by licensees, and maintains a committee of medical experts to obtain advice about the use of byproduct materials in medicine. The NRC (or the responsible Agreement State) also regulates the manufacture and distribution of these products. The FDA oversees the good practices in the manufacturing of radiopharmaceuticals, medical devices, and radiation-producing x-ray machines and accelerators. The states regulate the practices of medicine and pharmacy and administer programs associated with radiation-producing x-ray machines and accelerators. We may not be able to obtain all necessary licenses and permits and we may not be able to comply with all applicable laws. The failure to obtain such licenses and permits or to comply with applicable laws would have a materially adverse effect on our business, financial condition, and results of operations.
Corporate Information
Our executive offices are located at 4995 Bradenton Avenue, Suite 240, Dublin, OH 43017. Our telephone number is (614) 793-7500. “Navidea” and the Navidea logo are trademarks of Navidea Biopharmaceuticals, Inc. or its subsidiaries in the United States and/or other countries. Other trademarks or service marks appearing in this report may be trademarks or service marks of other owners.
Available Information
The address for our website is www.navidea.com. We make available free of charge on our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and amendments to such filings, as soon as reasonably practicable after each is electronically filed with, or furnished to, the Securities Exchange Commission (“SEC”). We do not charge for access to and viewing of these reports. Information in the investor section and on our website is not part of this Annual Report on Form 10-K or any of our other securities filings unless specifically incorporated herein by reference.
You may also review our electronically filed reports and other information that we file with the SEC on the SEC’s website at www.sec.gov. All statements made in any of our securities filings, including all forward-looking statements or information, are made as of the date of the document in which the statement is included, and we do not assume or undertake any obligation to update any of those statements or documents unless we are required to do so by law.
Financial Statements
Our consolidated financial statements and the related notes, including revenues, income (loss), total assets and other financial measures are set forth at pages F-1 through F-33 of this Annual Report on Form 10-K.
Human Capital Resources
As of March 17, 2023, we had 11 full-time and 4 part-time employees. None of our employees are represented by a collective bargaining agreement, we have not experienced any work stoppages, and we believe that our relationship with our employees is good.
We recognize that attracting, motivating and retaining talent is vital to our continued success. We aim to create an equitable, inclusive and empowering environment in which our employees can grow and advance their careers, with the overall goal of developing, expanding and retaining our workforce to support our current pipeline and future business goals. We value innovation, passion, data-driven decision making, persistence and honesty, and are building a diverse environment where our employees and consultants can thrive and be inspired to make exceptional contributions.
Our current management team, board of directors, and scientific advisors have significant experience in development and marketing of pharmaceutical product candidates from early stage discovery to clinical trials, regulatory approval and commercialization.
Our human capital resources objectives include identifying, recruiting, retaining, and incentivizing our existing and new employees. We maintain an equity incentive plan, the principal purposes of which are to attract, retain and reward personnel through the granting of stock-based compensation awards, in order to increase stockholder value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives. To facilitate talent attraction and retention, we strive to make our company a safe and rewarding workplace, with opportunities for our employees to grow and develop in their careers, supported by competitive compensation, benefits and health and wellness programs, and by programs that build connections between our employees.
In addition, as a result of the COVID-19 pandemic, we have taken steps to protect the health and safety of our employees in line with directives from state and the applicable local governments, as well as guidance from the Centers for Disease Control.
Item 1A. Risk Factors
An investment in our Common Stock, par value $0.001 per share (“Common Stock”) is highly speculative, involves a high degree of risk, and should be made only by investors who can afford a complete loss. You should carefully consider the following risk factors, together with the other information in this Annual Report on Form 10-K, including our financial statements and the related notes, before you decide to buy our Common Stock. If any of the following risks actually occur, our business, financial condition, or results of operations could be materially adversely affected, the trading of our Common Stock could decline, and you may lose all or part of your investment therein.
Summary of Risk Factors
Our business is subject to numerous risks and uncertainties, discussed in more detail in the following section. These risks include, among others, the following key risks:
Risks Related to Our Business, Financial Position and Capital Requirements
|
● |
If Cardinal Health 414, Sayre Therapeutics or Sinotau do not achieve commercial success with Tc99m tilmanocept, we may be unable to generate significant revenue from these relationships. |
|
● |
We may have difficulty raising additional capital, which could deprive us of necessary resources to pursue our business plans. |
|
● |
There may be future sales or other dilution of our equity, which may adversely affect the market price of shares of our Common Stock. |
|
● |
The Company has experienced recurring net losses and has used significant cash to fund its operations, and we expect to continue to incur substantial operating losses and may be unable to obtain additional financing, and we may not be able to continue as a going concern. |
Risks Related to Clinical Development, Regulatory Approval and Commercialization
|
● |
If we do not successfully develop any additional product candidates into marketable products, we may be unable to generate significant revenue or become profitable. |
|
● |
We may never obtain regulatory approval to manufacture or market our unapproved drug candidates and our approval to market our products or anticipated commercial launch may be delayed as a result of the regulatory review process. |
|
● |
Even if our drug candidates are successful in clinical trials, we may not be able to successfully commercialize them. |
|
● |
We may be unable to establish or contract for the pharmaceutical manufacturing capabilities necessary to develop and commercialize our potential products. |
Risks Related to Our Intellectual Property
|
● |
If any of our license agreements for intellectual property underlying our Manocept platform or any other products or potential products are terminated, we may lose the right to develop or market that product. |
|
● |
We may not have sufficient legal protection against infringement or loss of our intellectual property, and we may lose rights or protection related to our intellectual property if diligence requirements are not met, or at the expiry of underlying patents. |
|
● |
We and our collaborators may not be able to protect our intellectual property rights throughout the world. |
|
● |
We may become involved in disputes with licensors or potential future collaborators over intellectual property ownership, and publications by our research collaborators and scientific advisors could impair our ability to obtain patent protection or protect our proprietary information, which, in either case, could have a significant effect on our business. |
Risks Related to Our Business, Financial Position and Capital Requirements
We may have difficulty raising additional capital, which could deprive us of necessary resources to pursue our business plans.
We expect to devote significant capital resources to fund R&D and to maintain existing and secure new manufacturing resources. In order to support the initiatives envisioned in our business plan, we will likely need to raise additional funds through the sale of assets, public or private secured or unsecured debt or equity financing, collaborative relationships or other arrangements. Our ability to raise additional financing depends on many factors beyond our control, including the state of capital markets, the market price of our Common Stock and the development or prospects for development of competitive technology by others. Sufficient additional financing may not be available to us or may be available only on terms that would result in further dilution to the current owners of our Common Stock.
Our future expenditures on our programs are subject to many uncertainties, including whether our product candidates will be developed or commercialized with a partner or independently. Our future capital requirements will depend on, and could increase significantly as a result of, many factors, including:
|
● |
the final outcome of the Capital Royalty Partners II, L.P. (“CRG”) litigation and other litigation, including the outcome of any litigation involving Dr. Michael Goldberg; |
|
● |
the costs of seeking regulatory approval for our product candidates, including any nonclinical testing or bioequivalence or clinical studies, process development, scale-up and other manufacturing and stability activities, or other work required to achieve such approval, as well as the timing of such activities and approval; |
|
● |
the extent to which we invest in new technologies, product candidates, products or businesses; |
|
● |
the scope, prioritization and number of development and/or commercialization programs we pursue and the rate of progress and costs with respect to such programs; |
|
● |
the costs related to developing, acquiring and/or contracting for sales, marketing and distribution capabilities and regulatory compliance capabilities, if we commercialize any of our product candidates for which we obtain regulatory approval without a partner; |
|
● |
the timing and terms of any collaborative, licensing and other strategic arrangements that we may establish; |
|
● |
the extent to which we may need to expand our workforce to pursue our business plan, and the costs involved in recruiting, training, compensating and incentivizing new employees; |
|
● |
the effect of competing technological and market developments; and |
|
● |
the cost involved in establishing, enforcing or defending patent claims and other intellectual property rights. |
Our ability to raise additional capital may also be adversely impacted by potential worsening global economic conditions and the recent disruptions to, and volatility in, financial markets in the United States and worldwide resulting from the ongoing COVID-19 pandemic and Ukraine conflict. If we are unsuccessful in raising additional capital, or the terms of raising such capital are unacceptable, we may have to modify our business plan and/or significantly curtail our planned development activities, acquisition of new product candidates and other operations.
There may be future sales or other dilution of our equity, which may adversely affect the market price of shares of our Common Stock.
Our existing convertible preferred stock, warrants or other securities convertible into or exchangeable for our Common Stock, or securities we may issue in the future, may contain adjustment provisions that could increase the number of shares issuable upon exercise, conversion or exchange, as the case may be, and decrease the exercise, conversion or exchange price. The market price of our shares of Common Stock could decline as a result of sales of a large number of shares of our Common Stock or other securities in the market, the triggering of any such adjustment provisions or the perception that such sales could occur in the future.
Shares of Common Stock are subordinate to our existing and future indebtedness and preferred stock.
Shares of our Common Stock are equity interests that rank junior to our Series G Preferred Stock, to our indebtedness and to all creditor claims and other non-equity claims against us and our assets available to satisfy claims on us, including claims in a bankruptcy or similar proceeding. Our future indebtedness and preferred stock may restrict payments of dividends on our Common Stock.
Our amended and restated certificate of incorporation authorizes our board of directors to issue “blank check” preferred stock. This means our board of directors has the authority, without further action by our stockholders, to issue shares of preferred stock in one or more series and to set the terms and rights of the preferred stock. Any preferred stock that is issued may rank ahead of our Common Stock in terms of dividends and liquidation rights. If we issue additional shares of preferred stock in the future that has a preference over our Common Stock with respect to the payment of dividends or upon our liquidation, or if we issue additional shares of preferred stock with certain voting rights, the rights of holders of our Common Stock could be adversely affected.
Additionally, unlike indebtedness, where principal and interest customarily are payable on specified due dates, in the case of our Common Stock, (i) dividends are payable only when and if declared by our Board of Directors or a duly authorized committee of our Board of Directors, and (ii) as a corporation, we are restricted to making dividend payments and redemption payments out of legally available assets. We have never paid a dividend on our Common Stock and have no current intention to pay dividends in the future. Furthermore, our Common Stock places no restrictions on our business or operations or on our ability to incur indebtedness or engage in any transactions, subject only to the voting rights available to shareholders generally.
Raising additional capital may cause dilution to our stockholders or restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenue, we expect to obtain further funding through a combination of equity financings, debt financings, collaborations, licensing arrangements or other sources of financing, which may dilute our stockholders or restrict our operating activities. We do not have any committed external source of funds, and adequate additional financing may not be available to us on acceptable terms, or at all. To the extent that we raise additional capital through the sale of equity or convertible debt securities, stockholders’ ownership interests will be diluted, and the terms may include liquidation or other preferences that adversely affect our stockholders’ rights. Debt financing or preferred equity financings may result in imposition of covenants, increased fixed payment obligations or other restrictions that may affect our business. If we raise additional funds through upfront payments or milestone payments pursuant to strategic collaborations with third parties, we may have to relinquish valuable rights to product candidates or grant licenses on terms that are not favorable to us. If we are unable to raise additional funds when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves. In addition, securing financing could require a substantial amount of time and attention from our management and may divert a disproportionate amount of their attention away from day-to-day activities, which may adversely affect our management’s ability to oversee the development of product candidates.
The Company has experienced recurring net losses and has used significant cash to fund its operations, and we expect to continue to incur substantial operating losses and may be unable to obtain additional financing, and we may not be able to continue as a going concern.
Our ability to continue as a going concern is dependent on a combination of several factors, including our ability to raise capital by issuing debt or equity securities to investors, license or sell our product candidates to other pharmaceutical companies, and generate revenues from successfully developed products. If we are not able to continue our business as a going concern, we may be forced to liquidate our assets for an amount less than the value at which those assets are carried on our financial statements, and it is likely that investors will lose part or all of their investment.
The Company is currently engaged in litigation with Dr. Goldberg and CRG. In addition, the Company has experienced recurring net losses and has used significant cash to fund its operations. The Company has considerable discretion over the extent of development project expenditures and has the ability to curtail the related cash flows as needed. The Company also continues working to establish new sources of funding, including collaborations, potential equity investments, and additional grant funding that can augment the balance sheet. However, the extent to which COVID-19 impacts our business will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of COVID-19 and the actions to contain or treat its impact, among others. A significant outbreak of COVID-19 or other infectious diseases could result in a widespread health crisis that could adversely affect the economies and financial markets worldwide, resulting in an economic downturn that could impact our business, financial condition and results of operations, including our ability to obtain additional funding, if needed. Based on our current working capital and our projected cash burn, management believes that there is substantial doubt about the Company’s ability to continue as a going concern for a period of one year from the filing of this Annual Report on Form 10-K. No adjustments have been made to the accompanying consolidated financial statements of the Company as a result of this uncertainty. This disclosure with respect to our ability to continue as a going concern could materially limit our ability to raise additional funds through the issuance of equity or debt securities or otherwise.
If Cardinal Health 414, Sayre Therapeutics or Sinotau do not achieve commercial success with Tc99m tilmanocept, we may be unable to generate significant revenue from these relationships.
In March 2017, Navidea completed the sale to Cardinal Health 414 of all of its assets used in operating its business of developing, manufacturing and commercializing the Company’s radioactive diagnostic agent marketed under the Lymphoseek trademark in Canada, Mexico and the United States. Upon closing of the sale, the Supply and Distribution Agreement between Cardinal Health 414 and the Company was terminated and Cardinal Health 414 assumed responsibility for marketing Lymphoseek in those territories. Under the terms of the sale, Navidea is entitled to receive milestone payments (which, if paid, will be treated as additional purchase price) from Cardinal Health 414 based on net sales derived from Lymphoseek, subject, in each case, to Cardinal Health 414’s right to off-set.
Under the terms of our August 2014 agreement with Sinotau, as amended, Navidea is entitled to receive royalties and milestone payments based on Sinotau’s sales of Tc99m tilmanocept. Upon approval by the CFDA, Sinotau will be responsible for all Tc99m tilmanocept sales, marketing, market access and medical affairs activities in China, excluding Hong Kong, Macau and Taiwan. Tc99m tilmanocept has not yet received marketing approval in China, which may be delayed due to the coronavirus outbreak in China.
Under the terms of our June 2017 agreement with Sayre, Navidea is eligible to receive milestone payments and royalties associated with the sale of Tc99m tilmanocept in India. The Company received regulatory approval for Tc99m tilmanocept in India in March 2022, however certain additional approvals, such as an import license and authorization to use an alternative manufacturer, must be obtained prior to commercial sales launch in India.
Cardinal Health 414, Sayre or Sinotau may never achieve commercial success in North America, India, China, or any other global market, and they may never realize sales at levels necessary for us to achieve sales-based earnout, royalty or milestone payments.
Risks Related to Clinical Development, Regulatory Approval and Commercialization
If we do not successfully develop any additional product candidates into marketable products, we may be unable to generate significant revenue or become profitable.
Additional diagnostic and therapeutic applications of the Manocept platform, including diagnosis of RA, CV disease and solid tumor cancers, among others, are in various stages of pre-clinical and clinical development. Regulatory approval of additional Manocept-based product candidates may not be successful, or if successful, may not result in significant sales. Additional clinical testing for products based on our Manocept platform or other product candidates may not be successful and, even if they are, we may not be successful in developing any of them into a commercial product which will provide sufficient revenue to make us profitable.
Many companies in the pharmaceutical industry suffer significant setbacks in advanced clinical trials even after reporting promising results in earlier trials. Even if our Manocept trials are viewed as successful, we may not get regulatory approval for marketing of any Manocept product candidate. Our Manocept product candidates will be successful only if:
|
● |
they are developed to a stage that will enable us to commercialize them or sell related marketing rights; |
|
● |
we are able to commercialize them in clinical development or sell the marketing rights to third parties; and |
|
● |
upon being developed, they are approved by the regulatory authorities. |
We are dependent on the achievement of a number of these goals in order to generate future revenues. The failure to generate revenues from our Manocept-based product candidates may preclude us from continuing our R&D of these and other product candidates.
We may never obtain regulatory approval to manufacture or market our unapproved drug candidates and our approval to market our products or anticipated commercial launch may be delayed as a result of the regulatory review process.
Obtaining regulatory approval to market drugs to diagnose or treat diseases is expensive, difficult and risky. Preclinical and clinical data, as well as information related to the chemistry, manufacturing and control (“CMC”) processes of drug production, can be interpreted in different ways that could delay, limit or preclude regulatory approval. Negative or inconclusive results, adverse medical events during a clinical trial, or issues related to CMC processes could also delay, limit or prevent regulatory approval. Even if we receive regulatory clearance to market a particular product candidate, the approval could be conditioned on us conducting additional costly post-approval studies or could limit the indicated uses included in our labeling.
Clinical trials for our product candidates will be lengthy and expensive, and their outcome is uncertain.
Before obtaining regulatory approval for the commercial sale of any product candidates, we must demonstrate through preclinical testing and clinical trials that our product candidates are safe and effective for use in humans. Conducting clinical trials is a time consuming, expensive and uncertain process and may take years to complete.
We expect to sponsor efforts to explore the Manocept platform, whether in potential diagnostic or therapeutic uses. We continually assess our clinical trial plans and may, from time to time, initiate additional clinical trials to support our overall strategic development objectives. Historically, the results from preclinical testing and early clinical trials often do not predict the results obtained in later clinical trials. Frequently, drugs that have shown promising results in preclinical or early clinical trials subsequently fail to establish sufficient safety and efficacy data necessary to obtain regulatory approval. At any time during the clinical trials, we, the participating institutions, the FDA, the European Medicines Agency (“EMA”) or other regulatory authorities might delay or halt any clinical trials for our product candidates for various reasons, including:
|
● |
ineffectiveness of the product candidate; |
|
● |
discovery of unacceptable toxicities or side effects; |
|
● |
development of disease resistance or other physiological factors; |
|
● |
changes in local regulations as part of a response to the COVID-19 pandemic or other infectious disease outbreak, which may require us to change the ways in which our clinical trials are conducted, and which may result in unexpected costs, or to discontinue the clinical trials altogether; |
|
● |
delays or difficulties in enrolling patients in our clinical trials, including as a result of impacts associated with the COVID-19 pandemic; or |
|
● |
other reasons that are internal to the businesses of our potential collaborative partners, which reasons they may not share with us. |
While we have achieved some level of success in our clinical trials for Tc99m tilmanocept as indicated by the FDA and EMA approvals, the results of current and future trials for other product candidates that we may develop or acquire, are subject to review and interpretation by various regulatory bodies during the regulatory review process and may ultimately fail to demonstrate the safety or effectiveness of our product candidates to the extent necessary to obtain regulatory approval, or that commercialization of our product candidates is worthwhile. Any failure or substantial delay in successfully completing clinical trials and obtaining regulatory approval for our product candidates could materially harm our business.
We extensively outsource our clinical trial activities and usually perform only a small portion of the start-up activities in-house. We rely on independent third-party contract research organizations (“CROs”) to perform most of our clinical studies, including document preparation, site identification, screening and preparation, pre-study visits, training, post-study audits and statistical analysis. Many important aspects of the services performed for us by the CROs are out of our direct control. If there is any dispute or disruption in our relationship with our CROs, our clinical trials may be delayed. Moreover, in our regulatory submissions, we rely on the quality and validity of the clinical work performed by third-party CROs. If any of our CROs’ processes, methodologies or results were determined to be invalid or inadequate, our own clinical data and results and related regulatory approvals could be adversely impacted.
Even if our drug candidates are successful in clinical trials, we may not be able to successfully commercialize them.
We have dedicated and will continue to dedicate substantially all of our resources to the R&D of our Manocept technology and related compounds. There are many difficulties and uncertainties inherent in pharmaceutical R&D and the introduction of new products. A high rate of failure is inherent in new drug discovery and development. The process to bring a drug from the discovery phase to regulatory approval can take 12 to 15 years or longer and cost more than $1 billion. Failure can occur at any point in the process, including late in the process after substantial investment. As a result, most research programs will not generate financial returns. New product candidates that appear promising in development may fail to reach the market or may have only limited commercial success. Delays and uncertainties in the regulatory approval processes in the United States and in other countries can result in delays in product launches and lost market opportunities. Consequently, it is very difficult to predict which products will ultimately be approved. Due to the risks and uncertainties involved in the R&D process, we cannot reliably estimate the nature, timing, completion dates, and costs of the efforts necessary to complete the development of our R&D projects, nor can we reliably estimate the future potential revenue that will be generated from a successful R&D project.
Prior to commercialization, each product candidate requires significant research, development and preclinical testing and extensive clinical investigation before submission of any regulatory application for marketing approval. The development of radiopharmaceutical technologies and compounds, including those we are currently developing, is unpredictable and subject to numerous risks. Potential products that appear to be promising at early stages of development may not reach the market for a number of reasons including that they may:
|
● |
be found ineffective or cause harmful side effects during preclinical testing or clinical trials; |
|
● |
fail to receive necessary regulatory approvals; |
|
● |
be difficult to manufacture on a scale necessary for commercialization; |
|
● |
be uneconomical to produce; |
|
● |
fail to achieve market acceptance; or |
|
● |
be precluded from commercialization by proprietary rights of third parties. |
The occurrence of any of these events could adversely affect the commercialization of our product candidates. Products, if introduced, may not be successfully marketed and/or may not achieve customer acceptance. If we fail to commercialize products or if our future products do not achieve significant market acceptance, we will not likely generate significant revenues or become profitable.
If we fail to establish and maintain collaborations or if our partners do not perform, we may be unable to develop and commercialize our product candidates.
We have entered into collaborative arrangements with third parties to develop and/or commercialize product candidates and are currently seeking additional collaborations. Such collaborations might be necessary in order for us to fund our R&D activities and third-party manufacturing arrangements, seek and obtain regulatory approvals and successfully commercialize our existing and future product candidates. If we fail to enter into collaborative arrangements or fail to maintain our existing collaborative arrangements, the number of product candidates from which we could receive future revenues would decline.
Our dependence on collaborative arrangements with third parties will subject us to a number of risks that could harm our ability to develop and commercialize products including that:
|
● |
collaborative arrangements may not be on terms favorable to us; |
|
● |
disagreements with partners or regulatory compliance issues may result in delays in the development and marketing of products, termination of our collaboration agreements or time consuming and expensive legal action; |
|
● |
we cannot control the amount and timing of resources partners devote to product candidates or their prioritization of product candidates and partners may not allocate sufficient funds or resources to the development, promotion or marketing of our products, or may not perform their obligations as expected; |
|
● |
partners may choose to develop, independently or with other companies, alternative products or treatments, including products or treatments which compete with ours; |
|
● |
agreements with partners may expire or be terminated without renewal, or partners may breach collaboration agreements with us; |
|
● |
business combinations or significant changes in a partner’s business strategy might adversely affect that partner's willingness or ability to complete its obligations to us; and |
|
● |
the terms and conditions of the relevant agreements may no longer be suitable. |
The occurrence of any of these events could adversely affect the development or commercialization of our products.
Our pharmaceutical products will remain subject to ongoing regulatory review following the receipt of marketing approval. If we fail to comply with continuing regulations, we could lose these approvals and the sale of our products could be suspended.
Approved products may later cause adverse effects that limit or prevent their widespread use, force us to withdraw it from the market or impede or delay our ability to obtain regulatory approvals in additional countries. In addition, any contract manufacturer we use in the process of producing a product and its facilities will continue to be subject to FDA review and periodic inspections to ensure adherence to applicable regulations. After receiving marketing clearance, the manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion and record-keeping related to the product will remain subject to extensive regulatory requirements. We may be slow to adapt, or we may never adapt, to changes in existing regulatory requirements or adoption of new regulatory requirements.
If we fail to comply with the regulatory requirements of the FDA and other applicable U.S. and foreign regulatory authorities, or previously unknown problems with our products, manufacturers or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions, including:
|
● |
restrictions on the products, manufacturers or manufacturing processes; |
|
● |
warning letters; |
|
● |
civil or criminal penalties; |
|
● |
fines; |
|
● |
injunctions; |
|
● |
product seizures or detentions; |
|
● |
import bans; |
|
● |
voluntary or mandatory product recalls and publicity requirements; |
|
● |
suspension or withdrawal of regulatory approvals; |
|
● |
total or partial suspension of production; and |
|
● |
refusal to approve pending applications for marketing approval of new drugs or supplements to approved applications. |
If users of our products are unable to obtain adequate reimbursement from third-party payors, or if new restrictive legislation is adopted, market acceptance of our products may be limited and we may not achieve anticipated revenues.
Our ability to commercialize our products will depend in part on the extent to which appropriate reimbursement levels for the cost of our products and related treatment are obtained by governmental authorities, private health insurers and other organizations such as health maintenance organizations (“HMOs”). Generally, in Europe and other countries outside the United States, the government-sponsored healthcare system is the primary payor of patients’ healthcare costs. Third-party payors are increasingly challenging the prices charged for medical care. Also, the trend toward managed health care in the United States and the concurrent growth of organizations such as HMOs which could control or significantly influence the purchase of health care services and products, as well as legislative proposals to further reform health care or reduce government insurance programs, may all result in lower prices for our products if approved for commercialization. The cost containment measures that health care payors and providers are instituting and the effect of any health care reform could materially harm our ability to sell our products at a profit.
We may be unable to establish or contract for the pharmaceutical manufacturing capabilities necessary to develop and commercialize our potential products.
We are in the process of establishing third-party manufacturing capabilities for our compounds under development. We intend to rely on third-party contract manufacturers to produce sufficiently large quantities of drug materials that are and will be needed for clinical trials and commercialization of our potential products. Third-party manufacturers may not be able to meet our needs with respect to timing, quantity or quality of materials. If we are unable to contract for a sufficient supply of needed materials on acceptable terms, or if we should encounter delays or difficulties in our relationships with manufacturers, clinical trials for our product candidates may be delayed, thereby delaying the submission of product candidates for regulatory approval and the market introduction and subsequent commercialization of our potential products, and for approved products, any such delays, interruptions or other difficulties may render us unable to supply sufficient quantities to meet demand. Any such delays or interruptions may lower our revenues and potential profitability.
We and any third-party manufacturers that we may use must continually adhere to cGMPs and regulations enforced by the FDA through its facilities inspection program and/or foreign regulatory authorities where our products will be tested and/or marketed. If our facilities or the facilities of third-party manufacturers cannot pass a pre-approval plant inspection, the FDA and/or foreign regulatory authorities will not grant approval to market our product candidates. In complying with these regulations and foreign regulatory requirements, we and any of our third-party manufacturers will be obligated to expend time, money and effort on production, record-keeping and quality control to assure that our potential products meet applicable specifications and other requirements. The FDA and other regulatory authorities may take action against a contract manufacturer who violates cGMPs.
Our product supply and related patient access could be negatively impacted by, among other things: (i) product seizures or recalls or forced closings of manufacturing plants; (ii) disruption in supply chain continuity including from natural or man-made disasters at a critical supplier, as well as our failure or the failure of any of our suppliers to comply with cGMPs and other applicable regulations or quality assurance guidelines that could lead to manufacturing shutdowns, product shortages or delays in product manufacturing; (iii) manufacturing, quality assurance/quality control, supply problems or governmental approval delays; (iv) the failure of a sole source or single source supplier to provide us with the necessary raw materials, supplies or finished goods within a reasonable timeframe; (v) the failure of a third-party manufacturer to supply us with bulk active or finished product on time; and (vi) other manufacturing or distribution issues, including limits to manufacturing capacity due to regulatory requirements, and changes in the types of products produced, physical limitations or other business interruptions.
We may not be successful in securing and/or maintaining the necessary manufacturing, supply and/or radiolabeling capabilities for our product candidates in clinical development.
We may not be able to secure and/or maintain agreements or other purchasing arrangements with our subcontractors on terms acceptable to us, or that our subcontractors will be able to meet our production requirements on a timely basis, at the required levels of performance and quality, including compliance with FDA cGMP requirements. In the event that any of our subcontractors are unable or unwilling to meet our production requirements, we may not be able to establish an alternate source of supply without significant interruption in product supply or without significant adverse impact to product availability or cost. Any significant supply interruption or yield problems that we or our subcontractors experience would have a material adverse effect on our ability to manufacture our products and, therefore, a material adverse effect on our business, financial condition, and results of operations until a new source of supply is qualified. Any interruption in manufacturing across the supply chain, whether by natural disasters, global disease outbreaks such as COVID-19 or otherwise, could significantly and adversely affect our operations, and delay our R&D programs.
Risks Related to Our Intellectual Property
If any of our license agreements for intellectual property underlying our Manocept platform or any other products or potential products are terminated, we may lose the right to develop or market that product.
We have licensed intellectual property, including patents and patent applications relating to the underlying intellectual property for our Manocept platform, upon which all of our current product candidates are based. We may also enter into other license agreements or acquire other product candidates. The potential success of our product development programs depend on our ability to maintain rights under these licenses, including our ability to achieve development or commercialization milestones contained in the licenses. Under certain circumstances, the licensors have the power to terminate their agreements with us if we fail to meet our obligations under these licenses. We may not be able to meet our obligations under these licenses. If we default under any license agreement, we may lose our right to market and sell any products based on the licensed technology.
We may not have sufficient legal protection against infringement or loss of our intellectual property, and we may lose rights or protection related to our intellectual property if diligence requirements are not met, or at the expiry of underlying patents.
Our success depends, in part, on our ability to secure and maintain patent protection for our products and product candidates, to preserve our trade secrets, and to operate without infringing on the proprietary rights of third parties. While we seek to protect our proprietary positions by filing U.S. and foreign patent applications for our important inventions and improvements, domestic and foreign patent offices may not issue these patents. Third parties may challenge, invalidate, or circumvent our patents or patent applications in the future. Competitors, many of which have significantly more resources than we have and have made substantial investments in competing technologies, may apply for and obtain patents that will prevent, limit, or interfere with our ability to make, use, or sell our products either in the United States or abroad.
Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are or may be developing products. As the biotechnology and pharmaceutical industry expands and more patents are issued, the risk increases that we will be subject to claims that our products or product candidates, or their use, infringe the rights of others. In the United States, most patent applications are secret for a period of 18 months after filing, and in foreign countries, patent applications are secret for varying periods of time after filing. Publications of discoveries tend to significantly lag the actual discoveries and the filing of related patent applications. Third parties may have already filed applications for patents for products or processes that will make our products obsolete, limit our patents, invalidate our patent applications or create a risk of infringement claims.
Under U.S. patent law, we are currently subject to a “first to file” system of patent approval, as opposed to the former “first to invent” system. As a consequence, delays in filing patent applications for new product candidates or discoveries could result in the loss of patentability if there is an intervening patent application with similar claims filed by a third party, even if we or our collaborators were the first to invent.
We or our suppliers may be exposed to, or threatened with, future litigation by third parties having patent or other intellectual property rights alleging that our products, product candidates and/or technologies infringe their intellectual property rights or that the process of manufacturing our products or any of their respective component materials, or the component materials themselves, or the use of our products, product candidates or technologies, infringe their intellectual property rights. If one of these patents was found to cover our products, product candidates, technologies or their uses, or any of the underlying manufacturing processes or components, we could be required to pay damages and could be unable to commercialize our products or use our technologies or methods unless we are able to obtain a license to the patent or intellectual property right. A license may not be available to us in a timely manner or on acceptable terms, if at all. In addition, during litigation, a patent holder could obtain a preliminary injunction or other equitable remedy that could prohibit us from making, using or selling our products, technologies or methods.
Our currently held and licensed patents expire over the next two to seventeen years. Expiration of the patents underlying our technology, in the absence of extensions or other trade secret or intellectual property protection, may have a material and adverse effect on us.
In addition, it may be necessary for us to enforce patents under which we have rights, or to determine the scope, validity and unenforceability of other parties’ proprietary rights, which may affect our rights. There can be no assurance that our patents would be held valid by a court or administrative body or that an alleged infringer would be found to be infringing. The uncertainty resulting from the mere institution and continuation of any patent related litigation or interference proceeding could have a material and adverse effect on us.
We typically require our employees, consultants, advisers and suppliers to execute confidentiality and assignment of invention agreements in connection with their employment, consulting, advisory, or supply relationships with us. They may breach these agreements and we may not obtain an adequate remedy for breach. Further, third parties may gain unauthorized access to our trade secrets or independently develop or acquire the same or equivalent information.
We and our collaborators may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on all of our product candidates and products, when and if we have any, in every jurisdiction would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we or our licensors have not obtained patent protection to develop their own products. These products may compete with our products, when and if we have any, and may not be covered by any of our or our licensors' patent claims or other intellectual property rights.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology and/or pharmaceuticals, which could make it difficult for us to stop the infringement of our patents. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
The intellectual property protection for our product candidates depends on third parties.
With respect to Manocept and NAV4694, we have licensed certain issued patents and pending patent applications covering the respective technologies underlying these product candidates and their commercialization and use and we have licensed certain issued patents and pending patent applications directed to product compositions and chemical modifications used in product candidates for commercialization, and the use and the manufacturing thereof.
The patents and pending patent applications underlying our licenses do not cover all potential product candidates, modifications and uses. In the case of patents and patent applications licensed from UCSD, we did not have any control over the filing of the patents and patent applications before the effective date of the Manocept licenses and had limited control over the filing and prosecution of these patents and patent applications after the effective date of such licenses. In the case of patents and patent applications licensed from AstraZeneca, we have limited control over the filing, prosecution or enforcement of these patents or patent applications. We cannot be certain that such prosecution efforts have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents. We also cannot be assured that our licensors or their respective licensing partners will agree to enforce any such patent rights at our request or devote sufficient efforts to attain a desirable result. Any failure by our licensors or any of their respective licensing partners to properly protect the intellectual property rights relating to our product candidates could have a material adverse effect on our financial condition and results of operations.
We may become involved in disputes with licensors or potential future collaborators over intellectual property ownership, and publications by our research collaborators and scientific advisors could impair our ability to obtain patent protection or protect our proprietary information, which, in either case, could have a significant effect on our business.
Inventions discovered under research, material transfer or other such collaborative agreements may become jointly owned by us and the other party to such agreements in some cases and the exclusive property of either party in other cases. Under some circumstances, it may be difficult to determine who owns a particular invention, or whether it is jointly owned, and disputes could arise regarding ownership of those inventions. These disputes could be costly and time consuming and an unfavorable outcome could have a significant adverse effect on our business if we were not able to protect our license rights to these inventions. In addition, our research collaborators and scientific advisors generally have contractual rights to publish our data and other proprietary information, subject to our prior review. Publications by our research collaborators and scientific advisors containing such information, either with our permission or in contravention of the terms of their agreements with us, may impair our ability to obtain patent protection or protect our proprietary information, which could significantly harm our business.
General Risks
A pandemic, epidemic or outbreak of an infectious disease may adversely affect our business.
If a pandemic, epidemic or outbreak of an infectious disease occurs in the United States or worldwide, our development and commercialization efforts may be adversely affected. In December 2019, a novel strain of coronavirus, COVID-19, was identified in Wuhan, China. In January 2020, the World Health Organization declared this outbreak a “Public Health Emergency of International Concern,” and the U.S. Department of Health and Human Services declared a public health emergency to aid the U.S. healthcare community in responding to COVID-19. The spread of COVID-19 has impacted the global economy and our operations, including the interruption of our clinical trial activities in Europe and regulatory approval process in India. For example, the COVID-19 outbreak delayed enrollment in our NAV3-32 clinical study in the United Kingdom due to national COVID-19-related shutdowns in early 2021. Our clinical trial activities may be further delayed due to prioritization of hospital resources toward the outbreak, and some patients may be unwilling to enroll in our trials or be unable to comply with clinical trial protocols if quarantines impede patient movement or interrupt healthcare services, which would delay our ability to conduct clinical trials or release clinical trial results, and could delay our ability to obtain regulatory approval and commercialize our product candidates. The spread of an infectious disease, including COVID-19, may also result in the inability of our suppliers to deliver clinical drug supplies on a timely basis or at all. In addition, hospitals may reduce staffing and reduce or postpone certain treatments in response to the spread of an infectious disease. Such events may result in a period of business disruption, and in reduced operations, or doctors and medical providers may be unwilling to participate in our clinical trials, any of which could materially affect our business, financial condition and results of operations. The extent to which the global COVID-19 pandemic impacts our business will depend on future developments, which are highly uncertain and cannot be predicted, including new information that may emerge concerning the severity of COVID-19 and the actions to contain or treat its impact, among others. Any significant infectious disease outbreak, including the COVID-19 pandemic, could result in a widespread health crisis that could adversely affect the economies and financial markets worldwide, resulting in an economic downturn that could impact our business, financial condition and results of operations, including our ability to obtain additional funding, if needed. The Company has enhanced its business continuity plans to include measures to protect our employees in the event of infection in our corporate offices, or in response to potential mandatory quarantines.
We may lose out to larger or better-established competitors.
The biotech and pharmaceutical industries are intensely competitive. Many of our competitors have significantly greater financial, technical, manufacturing, marketing and distribution resources as well as greater experience in the industry than we have. The particular medical conditions our product lines address can also be addressed by other medical procedures or drugs. Many of these alternatives are widely accepted by physicians and have a long history of use.
To remain competitive, we must continue to launch new products and technologies. To accomplish this, we commit substantial efforts, funds, and other resources to R&D. A high rate of failure is inherent in the R&D of new products and technologies. We must make ongoing substantial expenditures without any assurance that our efforts will be commercially successful. Failure can occur at any point in the process, including after significant funds have been invested. Promising new product candidates may fail to reach the market or may only have limited commercial success because of efficacy or safety concerns, failure to achieve positive clinical outcomes, inability to obtain necessary regulatory approvals, limited scope of approved uses, excessive costs to manufacture, the failure to establish or maintain intellectual property rights, or infringement of the intellectual property rights of others. Even if we successfully develop new products or enhancements or new generations of our existing products, they may be quickly rendered obsolete by changing customer preferences, changing industry standards, or competitors' innovations. Innovations may not be accepted quickly in the marketplace because of, among other things, entrenched patterns of clinical practice or uncertainty over third-party reimbursement. We cannot state with certainty when or whether any of our products under development will be launched, whether we will be able to develop, license, or otherwise acquire compounds or products, or whether any products will be commercially successful. Failure to launch successful new products or new indications for existing products may cause our products to become obsolete, causing our revenues and operating results to suffer.
Physicians may use our competitors’ products and/or our products may not be competitive with other technologies. Tc99m tilmanocept is expected to continue to compete against sulfur colloid in the United States and other colloidal agents in the EU and other global markets. If our competitors are successful in establishing and maintaining market share for their products, our future earnout and royalty receipts may not occur at the rate we anticipate. In addition, our potential competitors may establish cooperative relationships with larger companies to gain access to greater R&D or marketing resources. Competition may result in price reductions, reduced gross margins and loss of market share.
We may be exposed to business risk, including product liability claims for any product candidates and products that we are able to commercialize.
The testing, manufacturing, marketing and use of any commercial products that we develop, as well as product candidates in development, involve substantial risk of product liability claims. These claims may be made directly by consumers, healthcare providers, pharmaceutical companies or others. In recent years, coverage and availability of cost-effective product liability insurance has decreased, so we may be unable to maintain sufficient coverage for product liabilities that may arise. In addition, the cost to defend lawsuits or pay damages for product liability claims may exceed our coverage. If we are unable to maintain adequate coverage or if claims exceed our coverage, our financial condition and our ability to clinically test our product candidates and market our products will be adversely impacted. In addition, negative publicity associated with any claims, regardless of their merit, may decrease the future demand for our products and impair our financial condition.
The administration of drugs in humans, whether in clinical studies or commercially, carries the inherent risk of product liability claims whether or not the drugs are actually the cause of an injury. Our products or product candidates may cause, or may appear to have caused, injury or dangerous drug interactions, and we may not learn about or understand those effects until the product or product candidate has been administered to patients for a prolonged period of time. We may be subject from time to time to lawsuits based on product liability and related claims, and we cannot predict the eventual outcome of any future litigation. We may not be successful in defending ourselves in the litigation and, as a result, our business could be materially harmed. These lawsuits may result in large judgments or settlements against us, any of which could have a negative effect on our financial condition and business if in excess of our insurance coverage. Additionally, lawsuits can be expensive to defend, whether or not they have merit, and the defense of these actions may divert the attention of our management and other resources that would otherwise be engaged in managing our business.
As a result of a number of factors, product liability insurance has become less available while the cost has increased significantly. We currently carry product liability insurance that our management believes is appropriate given the risks that we face. We will continually assess the cost and availability of insurance; however, there can be no guarantee that insurance coverage will be obtained or, if obtained, will be sufficient to fully cover product liabilities that may arise. If we are held liable for a claim against which we are not insured or for damages exceeding the limits of our insurance coverage, whether arising out of product liability matters, cybersecurity matters, or from some other matter, that claim could have a material adverse effect on our results of operations.
Security breaches and other disruptions could compromise our information and expose us to liability, which would cause our business and reputation to suffer.
In the ordinary course of our business, we collect and store sensitive data, including intellectual property, our proprietary business information and that of our suppliers and business partners, and personally identifiable information of employees and clinical trial subjects, in our data centers and on our networks. The secure maintenance and transmission of this information is critical to our operations and business strategy. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Any such breach could compromise our networks and the information stored there could be accessed, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, and regulatory penalties, disrupt our operations, and damage our reputation, which could adversely affect our business, revenues and competitive position.
Failure to comply with domestic and international privacy and security laws can result in the imposition of significant civil and criminal penalties. The costs of compliance with these laws, including protecting electronically stored information from cyber-attacks, and potential liability associated with failure to do so could adversely affect our business, financial condition and results of operations. We are subject to various domestic and international privacy and security regulations, including but not limited to The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”). HIPAA mandates, among other things, the adoption of uniform standards for the electronic exchange of information in common healthcare transactions, as well as standards relating to the privacy and security of individually identifiable health information, which require the adoption of administrative, physical and technical safeguards to protect such information. In addition, many states have enacted comparable laws addressing the privacy and security of health information, some of which are more stringent than HIPAA.
A security breach or privacy violation that leads to disclosure of consumer information (including personally identifiable information or protected health information) could harm our reputation, compel us to comply with disparate state and foreign breach notification laws and otherwise subject us to liability under laws that protect personal data, resulting in increased costs or loss of revenue.
Despite our efforts to protect against cyber-attacks and security breaches, hackers and other cyber criminals are using increasingly sophisticated and constantly evolving techniques, and we may need to expend substantial additional resources to continue to protect against potential security breaches or to address problems caused by such attacks or any breach of our safeguards. In addition, a data security breach could distract management or other key personnel from performing their primary operational duties.
The interpretation and application of consumer and data protection laws in the United States, Europe and elsewhere are often uncertain, contradictory and in flux. Among other things, foreign privacy laws impose significant obligations on U.S. companies to protect the personal information of foreign citizens. It is possible that these laws may be interpreted and applied in a manner that is inconsistent with our data practices, which could have a material adverse effect on our business. Complying with these various laws could cause us to incur substantial costs or require us to change our business practices in a manner adverse to our business.
We carry cyber risk insurance, which may limit our exposure to liability resulting from a security breach or other disruption in our information systems; however, we cannot assure you that such insurance policy will cover all liabilities that may result from security breaches or other disruption in our information systems.
We are subject to domestic and foreign anticorruption laws, the violation of which could expose us to liability, and cause our business and reputation to suffer.
We are subject to the U.S. Foreign Corrupt Practices Act and similar anti-corruption laws in other jurisdictions. These laws generally prohibit companies and their intermediaries from engaging in bribery or making other prohibited payments to government officials for the purpose of obtaining or retaining business, and some have record keeping requirements. The failure to comply with these laws could result in substantial criminal and/or monetary penalties. We operate in jurisdictions that have experienced corruption, bribery, pay-offs and other similar practices from time-to-time and, in certain circumstances, such practices may be local custom. We have implemented internal control policies and procedures that mandate compliance with these anti-corruption laws. However, we cannot be certain that these policies and procedures will protect us against liability. If our employees or other agents engage in such conduct, we might be held responsible and we could suffer severe criminal or civil penalties and other consequences that could have a material adverse effect on our business, financial position, results of operations and/or cash flow, and the market value of our Common Stock could decline.
Our international operations expose us to economic, legal, regulatory and currency risks.
Our operations extend to countries outside the United States and are subject to the risks inherent in conducting business globally and under the laws, regulations, and customs of various jurisdictions. These risks include: (i) failure to comply with a variety of national and local laws of countries in which we do business, including restrictions on the import and export of certain intermediates, drugs, and technologies, (ii) failure to comply with a variety of U.S. laws including the Iran Threat Reduction and Syria Human Rights Act of 2012; and rules relating to the use of certain “conflict minerals” under Section 1502 of the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) changes in laws, regulations, and practices affecting the pharmaceutical industry and the health care system, including but not limited to imports, exports, manufacturing, quality, cost, pricing, reimbursement, approval, inspection, and delivery of health care, (iv) fluctuations in exchange rates for transactions conducted in currencies other than the functional currency, (v) adverse changes in the economies in which we or our partners and suppliers operate as a result of a slowdown in overall growth, a change in government or economic policies, or financial, political, or social change or instability in such countries that affects the markets in which we operate, particularly emerging markets, (vi) differing local product preferences and product requirements, (vii) changes in employment laws, wage increases, or rising inflation in the countries in which we or our partners and suppliers operate, (viii) supply disruptions, and increases in energy and transportation costs, (ix) natural disasters, including droughts, floods, and earthquakes in the countries in which we operate, (x) local disturbances, terrorist attacks, riots, social disruption, or regional hostilities in the countries in which we or our partners and suppliers operate and (xi) government uncertainty, including as a result of new or changed laws and regulations. We also face the risk that some of our competitors have more experience with operations in such countries or with international operations generally and may be able to manage unexpected crises more easily. Furthermore, whether due to language, cultural or other differences, public and other statements that we make may be misinterpreted, misconstrued, or taken out of context in different jurisdictions. Moreover, the internal political stability of, or the relationship between, any country or countries where we conduct business operations may deteriorate. Changes in a country’s political stability or the state of relations between any such countries are difficult to predict and could adversely affect our operations, profitability and/or adversely impact our ability to do business there. The occurrence of any of the above risks could have a material adverse effect on our business, financial position, results of operations and/or cash flow, and could cause the market value of our Common Stock to decline.
Our failure to maintain continued compliance with the listing requirements of the NYSE American exchange could result in the delisting of our Common Stock.
Our Common Stock has been listed on the NYSE American exchange since February 2011. The rules of NYSE American provide that shares be delisted from trading in the event the financial condition and/or operating results of the Company appear to be unsatisfactory, the extent of public distribution or the aggregate market value of the Common Stock has become so reduced as to make further dealings on the NYSE American inadvisable, the Company has sold or otherwise disposed of its principal operating assets, or has ceased to be an operating company, or the Company has failed to comply with its listing agreements with the NYSE American. For example, the NYSE American may consider suspending trading in, or removing the listing of, securities of an issuer that has stockholders’ equity of less than (i) $2.0 million if such issuer has sustained losses from continuing operations and/or net losses in two of its three most recent fiscal years, (ii) $4.0 million if such issuer has sustained losses from continuing operations and/or net losses in three of its four most recent fiscal years, and (iii) $6.0 million if such issuer has sustained losses from continuing operations and/or net losses in its five most recent fiscal years. Navidea had stockholders’ (deficit) equity of approximately $(8.1) million and $625,000 as of December 31, 2022 and 2021, respectively, and has reported net losses from continuing operations in its five most recent fiscal years ended December 31, 2022.
On January 28, 2022, we received a deficiency letter from the NYSE American stating that the Company was not in compliance with the $6.0 million stockholders’ equity requirement of Section 1003(a)(iii) of the NYSE American Company Guide. As required by the NYSE American, the Company submitted a plan to the NYSE American by February 28, 2022 advising of actions it has taken or will take to regain compliance with the continued listing standards by July 28, 2023.
On April 8, 2022, the Company received a notification (the “Acceptance Letter”) from the NYSE American that the Company’s plan to regain compliance was accepted. The Acceptance Letter also stated that the Company is also not in compliance with the $2.0 million and $4.0 million stockholders’ equity requirements of Sections 1003(a)(i) and 1003(a)(ii), respectively, of the NYSE American Company Guide.
The NYSE American has granted the Company a plan period through July 28, 2023 to regain compliance with Sections 1003(a)(i), (ii) and (iii). If the Company is not in compliance with all continued listing standards by that date or if the Company does not make progress consistent with the plan during the plan period, the NYSE American may commence delisting procedures.
Our Common Stock will continue to be listed on the NYSE American while we attempt to regain compliance with the listing standard noted, subject to our compliance with other continued listing requirements. Our Common Stock will continue to trade under the symbol “NAVB,” but will have an added designation of “.BC” to indicate that we are not in compliance with the NYSE American’s listing standards. The NYSE American notification does not affect our business operations or our SEC reporting requirements and does not conflict with or cause an event of default under any of our material agreements.
The delisting of our Common Stock from the NYSE American likely would reduce the trading volume and liquidity in our Common Stock and may lead to decreases in the trading price of our Common Stock. The delisting of our Common Stock may also materially impair our stockholders’ ability to buy and sell shares of our Common Stock. In addition, the delisting of our Common Stock could significantly impair our ability to raise capital.
The price of our Common Stock has been, and may continue to be, highly volatile, and the value of your investment could decline significantly.
Our Common Stock traded as low as $0.16 per share and as high as $1.24 per share during the 12-month period ended February 28, 2023.
The following factors, some of which are beyond our control, may have a significant impact on the market price of our common stock:
|
● |
the impact of the global COVID-19 pandemic on our business, financial condition or prospects, including a decline in the volume of procedures using our product, potential delays and disruptions to global supply chains, manufacturing activities, logistics, operations, employees and contractors, the business activities of our suppliers, distributors, customers and other business partners, as well as the effects on worldwide economies, financial markets, social institutions, labor markets and healthcare systems; |
|
● |
our history of operating losses and uncertainty of future profitability; |
|
● |
our ability to successfully complete research and further development of our drug candidates; |
|
● |
the timing, cost and uncertainty of obtaining regulatory approvals of our drug candidates, including delays and additional costs related to the ongoing COVID-19 pandemic; |
|
● |
our ability to successfully commercialize our drug candidates, including delays or disruptions related to the ongoing COVID-19 pandemic; |
|
● |
our ability to raise capital sufficient to fund our development programs, including unavailability of funds or delays in receiving funds as a result of the ongoing COVID-19 pandemic; |
|
● |
delays in receipt of anticipated proceeds from our capital funding transactions and other receivables; |
|
● |
our dependence on royalties and grant revenue; |
|
● |
our limited product line and distribution channels; |
|
● |
advances in technologies and development of new competitive products; |
|
● |
our ability to maintain effective control over financial reporting; |
|
● |
the outcome of any pending litigation; and |
|
● |
our ability to comply with NYSE American continued listing standards. |
These factors may materially and adversely affect the market price of our Common Stock, which could result in substantial losses by our investors.
In addition, the stock market has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of companies like ours. Broad market and industry factors may negatively affect the market price of our Common Stock, regardless of our actual operating performance. Further, a systemic decline in the financial markets and related factors beyond our control may cause our share price to decline rapidly and unexpectedly. Price volatility of our Common Stock might be worse if the trading volume of our ordinary shares is low.
An investor’s ability to trade our Common Stock may be limited by trading volume.
During the 12-month period beginning on March 1, 2022 and ending on February 28, 2023, the average daily trading volume for our Common Stock on the NYSE American was approximately 182,000 shares. However, this trading volume may not be consistently maintained in the future. If the trading volume for our Common Stock decreases, there could be a relatively limited market for our Common Stock and the share price of our Common Stock would be more likely to be affected by broad market fluctuations, general market conditions, fluctuations in our operating results, changes in the market’s perception of our business and announcements made by us, our competitors or parties with whom we have business relationships. There may also be fewer institutional investors willing to hold or acquire our Common Stock. Such a lack of liquidity in our Common Stock may make it difficult for us to issue additional securities for financing or other purposes or to otherwise arrange for any financing that we may need in the future.
The market price of our Common Stock may be adversely affected by market conditions affecting the stock markets in general, including price and trading fluctuations on the NYSE American exchange.
The market price of our Common Stock may be adversely affected by market conditions affecting the stock markets in general, including price and trading fluctuations on the NYSE American. These conditions may result in (i) volatility in the level of, and fluctuations in, the market prices of stocks generally and, in turn, our shares of Common Stock, and (ii) sales of substantial amounts of our Common Stock in the market, in each case that could be unrelated or disproportionate to changes in our operating performance.
Because we do not expect to pay dividends on our Common Stock in the foreseeable future, stockholders will only benefit from owning Common Stock if it appreciates.
We have paid no cash dividends on any of our Common Stock to date, and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. As a result, with respect to our Common Stock, we do not expect to pay any cash dividends in the foreseeable future, and payment of cash dividends, if any, will also depend on our financial condition, results of operations, capital requirements and other factors and will be at the discretion of our Board of Directors. Furthermore, we are subject to various laws and regulations that may restrict our ability to pay dividends. We may also in the future become subject to contractual restrictions on, or prohibitions against, the payment of dividends. Due to our intent to retain any future earnings rather than pay cash dividends on our Common Stock and applicable laws, regulations and contractual obligations that may restrict our ability to pay dividends on our Common Stock, the success of your investment in our Common Stock will likely depend entirely upon any future appreciation and there is no guarantee that our Common Stock will appreciate in value.
Our future ability to utilize our net operating loss carryforwards and certain other tax attributes may be limited.
We have incurred substantial losses during our history, and we may never achieve profitability. To the extent that we continue to generate taxable losses, unused losses will carry forward to offset a portion of future taxable income, if any, subject to expiration of such carryforwards in the case of carryforwards generated prior to 2018. Additionally, we continue to generate business tax credits, including research and development tax credits, which generally may be carried forward to offset a portion of future taxable income, if any, subject to expiration of such credit carryforwards. Under Sections 382 and 383 of the Internal Revenue Code, if a corporation undergoes an “ownership change,” generally defined as a greater than 50 percentage point change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss carryforwards (“NOLs”), and other pre-change tax attributes (such as research and development tax credits) to offset its post-change income or taxes may be limited. The Company completed a Section 382 analysis through December 31, 2022 and believes that a Section 382 ownership change has not occurred. However, we may experience additional ownership changes in the future as a result of subsequent shifts in our stock ownership, some of which are outside of our control. As a result, if we earn net taxable income, our ability to use our pre-change NOLs or other pre-change tax attributes to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us. Additionally, for taxable years beginning after December 31, 2017, the deductibility of such U.S. federal net operating losses is limited to 80% of our taxable income in any future taxable year. There is a risk that due to changes under the Tax Cuts and Jobs Act, regulatory changes, or other unforeseen reasons, our existing NOLs or business tax credits could expire or otherwise be unavailable to offset future income tax liabilities. At the state level, there may also be periods during which the use of NOLs or business tax credits is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed. For these reasons, we may not be able to realize a tax benefit from the use of our NOLs or tax credits, even if we attain profitability.
We may have difficulty attracting and retaining qualified personnel and our business may suffer if we do not.
Our business has experienced a number of successes and faced several challenges in recent years that have resulted in several significant changes in our strategy and business plan, including the shifting of resources to support our current development initiatives. Our management will need to remain flexible to support our business model over the next few years. However, losing members of the Navidea team could have an adverse effect on our operations. Our success depends on our ability to attract and retain technical and management personnel with expertise and experience in the pharmaceutical industry, and the acquisition of additional product candidates may require us to acquire additional highly qualified personnel. The competition for qualified personnel in the biotechnology industry is intense and we may not be successful in hiring or retaining the requisite personnel. If we are unable to attract and retain qualified technical and management personnel, we will suffer diminished chances of future success.
Actual and anticipated changes to the regulations of the healthcare system and U.S. tax laws may have a negative impact on the cost of healthcare coverage and reimbursement of healthcare services and products.
The FDA and comparable agencies in other jurisdictions directly regulate many critical activities of life science, technology, and healthcare industries, including the conduct of preclinical and clinical studies, product manufacturing, advertising and promotion, product distribution, adverse event reporting, and product risk management. In both domestic and foreign markets, sales of products depend in part on the availability and amount of reimbursement by third-party payors, including governments and private health plans. Governments may regulate coverage, reimbursement, and pricing of products to control cost or affect utilization of products. Private health plans may also seek to manage cost and utilization by implementing coverage and reimbursement limitations. Substantial uncertainty exists regarding the reimbursement by third-party payors of newly approved healthcare products. The U.S. and foreign governments regularly consider reform measures that affect healthcare coverage and costs. Such reforms may include changes to the coverage and reimbursement of healthcare services and products. In particular, there have been recent judicial and Congressional challenges to the Patient Protection and Affordable Care Act (“PPACA”), which could have an impact on coverage and reimbursement for healthcare services covered by plans authorized by the PPACA, and we expect there will be additional challenges and amendments to the PPACA in the future.
In addition, various other healthcare reform proposals have emerged at the federal and state level. The recent changes to U.S. tax laws could also negatively impact the PPACA. We cannot predict what healthcare initiatives or tax law changes, if any, will be implemented at the federal or state level, however, government and other regulatory oversight and future regulatory and government interference with the healthcare systems could adversely impact our business.
We may not be able to maintain compliance with our internal controls and procedures.
We regularly review and update our internal controls, disclosure controls and procedures, and corporate governance policies. In addition, we are required under the Sarbanes Oxley Act of 2002 to report annually on our internal control over financial reporting. Any system of internal controls, however well designed and operated, is based in part on certain assumptions and can provide only reasonable, not absolute, assurances that the objectives of the system are met. Any failure or circumvention of the controls and procedures or failure to comply with regulation concerning control and procedures could have a material effect on our business, results of operation and financial condition. Any of these events could result in an adverse reaction in the financial marketplace due to a loss of investor confidence in the reliability of our financial statements, which ultimately could negatively affect the market price of our shares, increase the volatility of our stock price and adversely affect our ability to raise additional funding. The effect of these events could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors and our Board committees and as executive officers.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
We currently lease approximately 5,000 square feet of office space at 4995 Bradenton Avenue, Dublin, Ohio, as our principal offices, at a monthly base rent of approximately $3,000. The current lease term expires in June 2023. We believe this facility is in good condition and that suitable substitute space would be available if needed.
Item 3. Legal Proceedings
See Note 12 to the accompanying consolidated financial statements.
Item 4. Mine Safety Disclosure
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our Common Stock trades on the NYSE American exchange under the trading symbol “NAVB.” As of March 17, 2023, we had 373 holders of Common Stock of record. There were no repurchases of our Common Stock during the year ended December 31, 2022.
Stock Performance Graph
The following graph compares the cumulative total return on a $100 investment in each of the Common Stock of the Company, the Russell 3000, and the NASDAQ Biotechnology Index for the period from December 31, 2017 through December 31, 2022. This graph assumes an investment in the Company’s Common Stock and the indices of $100 on December 31, 2017 and that any dividends were reinvested.
COMPARISON OF 5-YEAR CUMULATIVE TOTAL RETURN*
Among Navidea Biopharmaceuticals, the Russell 3000 Index, and the NASDAQ Biotechnology Index
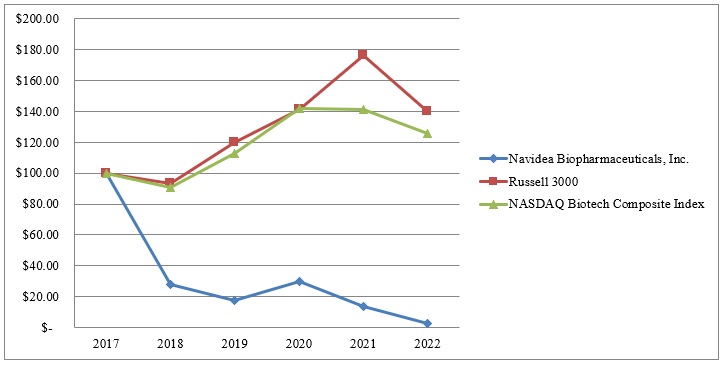
* $100 invested on 12/31/2017 in stock or index, including reinvestment of dividends.
|
|
|
Cumulative Total Return as of December 31, |
|
|||||||||||||||||||||
|
|
|
2017 |
|
|
2018 |
|
|
2019 |
|
|
2020 |
|
|
2021 |
|
|
2022 |
|
||||||
|
Navidea Biopharmaceuticals, Inc. |
|
$ |
100.00 |
|
|
$ |
27.78 |
$ |
17.50 |
$ |
29.86 |
$ |
13.89 |
$ |
2.92 |
|||||||||
|
Russell 3000 |
|
|
100.00 |
|
|
|
93.01 |
119.55 |
141.38 |
176.16 |
140.08 |
|||||||||||||
|
NASDAQ Biotechnology |
|
|
100.00 |
|
|
|
90.68 |
112.81 |
141.78 |
140.88 |
125.52 |
|||||||||||||
Dividend Policy
We did not declare or pay any dividends and we do not currently intend to pay dividends in the foreseeable future. We currently expect to retain future earnings, if any, for the foreseeable future, to finance the growth and development of our business.
Item 6. [Reserved]
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion should be read together with our Consolidated Financial Statements and the Notes related to those statements, as well as the other financial information included in this Form 10-K. Some of our discussion is forward-looking and involves risks and uncertainties. For information regarding risk factors that could have a material adverse effect on our business and future results, refer to Item 1A of this Form 10-K, “Risk Factors.”
The Company
Navidea Biopharmaceuticals, Inc. is a biopharmaceutical company focused on the development and commercialization of precision immunodiagnostic agents and immunotherapeutics. Navidea is developing multiple precision-targeted products based on our Manocept platform to enhance patient care by identifying the sites and pathways of undetected disease and enable better diagnostic accuracy, clinical decision-making and targeted treatment.
Navidea’s Manocept platform is predicated on the ability to specifically target the CD206 mannose receptor expressed on activated macrophages. The Manocept platform serves as the molecular backbone of Tc99m tilmanocept, the first product developed and commercialized by Navidea based on the platform. Other than Tc99m tilmanocept, which the Company has a license to distribute outside of Canada, Mexico and the United States, none of the Company’s drug product candidates have been approved for sale in any market.
We manage our business based on two primary types of drug products: (i) diagnostic substances, including Tc99m tilmanocept and other diagnostic applications of our Manocept platform, and (ii) therapeutic applications of our Manocept platform. See Note 15 to the consolidated financial statements for more information about our business segments.
In the near term, the Company intends to continue to develop our additional imaging product candidates into advanced clinical testing, as well as working to extend the regulatory approvals for use of the Tc99m tilmanocept product. We will also be evaluating potential funding and other resources required for continued development, regulatory approval and commercialization of any Manocept platform product candidates that we identify for further development, and potential options for advancing development.
Outlook
Our operating expenses in recent years have been focused primarily on support of both diagnostic and therapeutic applications of our Manocept platform, and Tc99m tilmanocept. We incurred approximately $6.0 million and $5.1 million in total on R&D activities during the years ended December 31, 2022 and 2021, respectively. Of the total amounts we spent on R&D during those periods, excluding costs related to our internal R&D headcount and our general and administrative staff which we do not currently allocate among the various development programs that we have underway, we incurred out-of-pocket charges by program as follows:
|
Development Program (a) |
2022 |
2021 |
||||||
|
Manocept Platform – Diagnostics |
$ | 3,558,378 | $ | 2,620,057 | ||||
|
Manocept Platform – Therapeutics |
468,640 | 653,733 | ||||||
|
Tc99m Tilmanocept (b) |
(97,162 |
) |
136,941 | |||||
|
(a) |
Certain development program expenditures were offset by grant reimbursement revenues totaling $51,007 and $87,898 during the years ended December 31, 2022 and 2021, respectively. |
|
(b) |
Changes in regulatory strategy resulted in the reversal of certain previously recorded expenses related to the Tc99m Tilmanocept development program during the year ended December 31, 2022. |
We plan to continue the advancement of our efforts with our Manocept platform during 2023. We currently expect our total research and development expenses, including both out-of-pocket charges as well as internal headcount and support costs, to be lower in 2023 than in 2022.
Tc99m tilmanocept is approved by the EMA for use in imaging and intraoperative detection of sentinel lymph nodes draining a primary tumor in adult patients with breast cancer, melanoma, or localized squamous cell carcinoma of the oral cavity in the EU. Similarly, Tc99m tilmanocept has been approved by the relevant regulatory agencies in the UK, India and Australia. We anticipate that we will incur costs to support our product, regulatory, manufacturing and commercial activities related to the sale of Tc99m tilmanocept in the EU, UK, India and Australia, as well as related to the potential marketing registration and sale of Tc99m tilmanocept in markets other than the EU, UK, India and Australia. There can be no assurance that Tc99m tilmanocept will achieve regulatory approval in any market other than the EU, UK, India and Australia, or if approved in those markets, that it will achieve market acceptance in those or any other markets. See Item 1A - “Risk Factors.”
We continue to evaluate existing and emerging data on the potential use of Manocept-related agents in the diagnosis, disease-staging and treatment of disorders in which macrophages are involved, such as RA, KS, NASH and other disease states, to define areas of focus, development pathways and partnering options to capitalize on the Manocept platform. We will also be evaluating potential funding and other resources required for continued development, regulatory approval and commercialization of any Manocept platform product candidates that we identify for further development, and potential options for advancing development. There can be no assurance of obtaining funding or other resources on terms acceptable to us, if at all, that further evaluation or development will be successful, that any Manocept platform product candidate will ultimately achieve regulatory approval, or if approved, the extent to which it will achieve market acceptance. See Item 1A - “Risk Factors.”
Results of Operations
Our pharmaceutical products and product candidates are not yet generating significant commercial revenue, therefore the discussion of our revenue focuses on the grant and other revenue and our operating variances focus on our product development programs and the supporting general and administrative expenses.
Years Ended December 31, 2022 and 2021
Sales Revenue. During 2022, we recognized revenue of $15,000 from sales of Lymphoseek in Europe and Australia. No sales revenue was recorded during 2021.
License Revenue. No license revenue was recorded during 2022. During 2021, we recognized license revenue of $46,000 related to net transitional sales from SpePharm in Europe.
Grant and Other Revenue. During 2022 and 2021, we recognized grant and other revenue of $51,000 and $486,000, respectively. Grant revenue of $51,000 and $88,000 during 2022 and 2021, respectively, was primarily related to an SBIR grant from the NIH supporting Manocept development. Other revenue during 2021 included $298,000 from LikeMinds, Inc. for the partial recovery of debts previously written off in 2015 and $100,000 from Cardinal Health 414 for reimbursement of certain research and development costs.
Research and Development Expenses. R&D expenses increased $828,000, or 16%, to $6.0 million during 2022 from $5.1 million during 2021. The increase was primarily due to net increases in drug project expenses related to (i) increased Manocept diagnostic development costs of $938,000 including increased manufacturing-related activities offset by decreased clinical trial and preclinical development costs; offset by (ii) decreased Tc99m tilmanocept development costs of $234,000 including decreased license fees, European regulatory consulting costs and manufacturing-related costs; and (iii) decreased Manocept therapeutic development costs of $185,000 including decreased clinical trial and preclinical development costs. The net increase in research and development expenses also included increased employee compensation including incentive-based awards of $440,000 and decreased regulatory consulting expenses of $122,000.
Selling, General and Administrative Expenses. Selling, general and administrative expenses increased $512,000, or 7%, to $8.0 million during 2022 from $7.5 million during 2021. Following the ruling by the Texas Court in August 2022, the Company recorded $2.6 million in legal fees pursuant to the CRG judgment during 2022. In addition, the increase was due to increases in insurance costs of $186,000 and depreciation and amortization of $19,000, partially offset by decreases in employee compensation including fringe benefits and incentive-based awards of $1.6 million, expenses related to European operations of $218,000, travel of $87,000, legal and professional services of $80,000, investor relations and shareholder services of $80,000, general office expenses of $58,000, facilities costs of $56,000, losses on the abandonment of certain intellectual property of $34,000 and franchise taxes of $17,000.
Other (Expense) Income. Other expense, net, was $1.1 million during 2022 compared to other income, net, of $346,000 during 2021. During 2022 and 2021, we recognized interest expense of $1.1 million and $9,000, respectively. Interest expense during 2022 included interest pursuant to the CRG judgment of $763,000, amortization of the discount on the Bridge Note of $208,000, and interest paid pursuant to the Bridge Note of $127,000. During 2021, we recognized a gain on extinguishment of debt of $366,000 resulting from forgiveness of our PPP loan. During 2022 and 2021, we recognized interest income of $9,000 and $3,000, respectively.
Liquidity and Capital Resources
Cash balances decreased to $2.0 million as of December 31, 2022 from $4.2 million as of December 31, 2021. The net decrease was primarily due to cash used to fund our operations of $9.0 million, principal payments on financed insurance premiums of $518,000, patent and trademark costs of $327,000 and purchases of equipment of $63,000, offset by net proceeds from issuance of preferred stock of $5.2 million and net proceeds from notes payable of $2.5 million.
Operating Activities. Cash used in operations was $9.0 million during 2022 compared to $10.2 million used in operations during 2021.
Receivables decreased to less than $1,000 as of December 31, 2022 from $93,000 as of December 31, 2021, primarily due to the receipt of receivables due from related parties.
Inventory, net increased to $427,000 as of December 31, 2022 from $151,000 as of December 31, 2021, primarily due to the manufacture of two batches of finished goods that were in process as of December 31, 2022 coupled with the purchase of materials to be used in manufacturing, offset by a $183,000 adjustment for expired or expiring materials and finished goods coupled with the allocation of finished goods for use in clinical trials.
Prepaid expenses and other current assets decreased to $780,000 as of December 31, 2022 from $908,000 as of December 31, 2021. The decrease was primarily due to application of an upfront contract payment related to a clinical study, reduction in the balance of prepaid credit cards due to reduced travel, normal amortization of prepaid insurance and the application of prepaid manufacturing-related costs to finished goods inventory, offset by an increase in prepaid costs related to efforts to raise capital.
Accounts payable increased to $2.1 million as of December 31, 2022 from $1.4 million as of December 31, 2021. The net increase in accounts payable was primarily due to increased amounts due for legal and professional services, deferred board of director fees, manufacturing-related activities and investor relations and shareholder services, offset by decreased amounts due for license fees, preclinical and clinical development activities, pharmacovigilance costs and regulatory consulting.
Accrued liabilities and other increased to $6.5 million as of December 31, 2022 from $3.1 million as of December 31, 2021. The net increase in accrued liabilities and other was primarily due to increased accruals for legal fees and associated interest pursuant to the CRG judgment, Manocept development costs and employee compensation, offset by decreased accruals related to the separation of our former Chief Executive Officer and employee benefits. Our payable and accrual balances will continue to fluctuate but will likely increase overall as we increase our clinical development activity related to the Manocept platform.
Deferred revenue, current increased to $800,000 as of December 31, 2022 from $41,000 as of December 31, 2021. The increase in deferred revenue, current was due to the receipt of $800,000 from a strategic partner pursuant to an API Development Agreement.
Investing Activities. Cash used in investing activities was $390,000 during 2022 compared to $329,000 during 2021. Patent and trademark costs used $327,000 and purchases of property and equipment used $63,000 during 2022. Patent and trademark costs used $304,000 and purchases of property and equipment used $25,000 during 2021.
Financing Activities. Cash provided by financing activities was $7.1 million during 2022 compared to $12.1 million during 2021. The $7.1 million provided by financing activities during 2022 consisted primarily of proceeds from the issuance of preferred stock and warrants of $6.2 million and proceeds from notes payable of $2.5 million, offset by payment of preferred stock and warrant issuance costs of $998,000, principal payments on financed insurance premiums of $518,000 and payment of debt issuance costs of $15,000. The $12.1 million provided by financing activities in 2021 consisted primarily of proceeds from issuance of preferred stock of $12.7 million, offset by principal payments on financed insurance premiums of $492,000, payment of preferred stock issuance costs of $70,000 and payment of tax withholdings related to stock-based compensation of $17,000.
Paycheck Protection Program Loan
The Coronavirus Aid, Relief, and Economic Security Act (the “CARES Act”) was enacted on March 27, 2020. Among the provisions contained in the CARES Act was the creation of the Payroll Protection Program (“PPP”) that provides for Small Business Administration (“SBA”) Section 7(a) loans for qualified small businesses. PPP loan proceeds are available to be used to pay for payroll costs, including salaries, commissions, and similar compensation, group health care benefits, and paid leaves; rent; utilities; and interest on certain other outstanding debt. On May 18, 2020, Fifth Third Bank (the “Lender”) funded a PPP loan to the Company in the amount of $366,000 (the “PPP Loan”). In accordance with the loan forgiveness requirements of the CARES Act, the Company used the proceeds from the PPP Loan primarily for payroll costs, rent and utilities. On February 23, 2021, the Lender notified the Company that the entire PPP Loan amount of $366,000 had been forgiven. See Note 10 to the accompanying consolidated financial statements.
Series D Preferred Stock
On August 31, 2020, the Company entered into a Stock Purchase Agreement and Letter of Investment Intent (the “Series D Preferred Stock Purchase Agreement”) with Keystone pursuant to which the Company agreed to issue to Keystone 150,000 shares of newly-designated Series D Redeemable Convertible Preferred Stock (the “Series D Preferred Stock”) for an aggregate purchase price of $15.0 million. Pursuant to the Series D Preferred Stock Purchase Agreement, Keystone agreed to purchase Series D Preferred Stock in amounts to be determined by Keystone in one or more closings before the end of the nine-month period following the date when the Company’s prospectus supplement to its existing registration statement on Form S-3 was filed with the SEC. Through July 7, 2021, Keystone purchased 72,500 shares of Series D Preferred Stock pursuant to the Series D Preferred Stock Purchase Agreement for an aggregate purchase price of $7.25 million, leaving a remaining balance of 77,500 shares of Series D Preferred Stock. On July 8, 2021, the Company entered into an Amendment to Stock Purchase Agreement and Letter of Investment Intent (the “Series D Amendment”) with Keystone pursuant to which Keystone purchased 22,077 shares of Series D Preferred Stock for an aggregate purchase price of approximately $2.2 million. After purchasing the 22,077 shares, Keystone has no further right or obligation to purchase shares of Series D Preferred Stock. Including the purchases pursuant to the Series D Amendment, Keystone’s purchases of Series D Preferred Stock pursuant to the Series D Purchase Agreement during the year ended December 31, 2021 totaled 76,827 shares of Series D Preferred Stock for an aggregate purchase price of approximately $7.7 million. All of the outstanding shares of Series D Preferred Stock were exchanged and cancelled pursuant to the Rights Offering completed in August 2022. See Note 13 to the accompanying consolidated financial statements.
Series E Preferred Stock
On March 2, 2021, the Company entered into a Stock Purchase Agreement and Letter of Investment Intent with an existing accredited investor, John K. Scott, Jr., pursuant to which the Company issued to Mr. Scott 50,000 shares of newly-designated Series E Redeemable Convertible Preferred Stock (the “Series E Preferred Stock”) for an aggregate purchase price of $5.0 million. On January 31, 2022, pursuant to the Certificate of Designations of the Series E Redeemable Convertible Preferred Stock dated March 2, 2021, the holder of the Series E Preferred Stock, John K. Scott, Jr., notified the Company that he was exercising his option to extend the Conversion Deadline (as defined therein) for an additional period of six months. All of the outstanding shares of Series E Preferred Stock were exchanged and cancelled in April 2022 pursuant to the Stock Exchange Agreement. See Note 13 to the accompanying consolidated financial statements.
Bridge Note and Preferred Stock Exchange
On April 10, 2022, the Company entered into a Stock Exchange and Loan Agreement (the “Stock Exchange Agreement”) with John K. Scott, Jr., the current Vice Chairman of our Board of Directors, pursuant to which Mr. Scott agreed to make a loan to the Company in the principal amount of up to $2.5 million, of which $1.5 million was funded on the closing date. The outstanding balance of the loan, which is evidenced by a Secured Term Note (“Bridge Note”), bears interest at a rate of 8% per annum, with payments of interest only to be made monthly over a period of two years. All outstanding principal and accrued and unpaid interest under the Bridge Note is due and payable on the second anniversary of the Stock Exchange Agreement. On July 1, 2022, Mr. Scott funded an additional $1.0 million under the Bridge Note. The Company’s obligations under the Bridge Note are secured by a first priority security interest in all of the Company’s assets and personal property pursuant to a Security Agreement. See Notes 2 and 8 to the accompanying consolidated financial statements.
As consideration and a partial inducement for Mr. Scott to make the Bridge Note, Mr. Scott agreed to deliver 50,000 shares of Series E Preferred Stock, representing 100% of the outstanding Series E Preferred Stock, to the Company in exchange for the Company’s issuance of 1,740 shares of Series F Redeemable Convertible Preferred Stock (“Series F Preferred Stock”) and 3,260 shares of Series G Redeemable Preferred Stock (“Series G Preferred Stock”). The number of shares of Common Stock that the Company may issue to Mr. Scott upon conversion of the Series F Preferred stock may not exceed that number of shares that would result in Mr. Scott owning more than 33.33% of the Company’s then outstanding shares of Common Stock unless the Company obtains stockholder approval to issue more than the 33.33% cap. See Notes 2 and 13 to the accompanying consolidated financial statements.
Rights Offering
On August 30, 2022, the Company closed on the Rights Offering to its stockholders and certain warrant holders as of August 3, 2022 of the right to purchase up to 35,000 Units at a subscription price of $1,000 per Unit. The Rights Offering resulted in the sale of 10,423 Units for aggregate gross proceeds of $6,173,000 to the Company. Each Unit consisted of one share of Series I Convertible Preferred Stock (“Series I Preferred Stock”) which is convertible into 2,222 shares of Common Stock and one Warrant to purchase an additional 2,222 shares of Common Stock at $0.45 per share. If exercised, additional gross proceeds of up to $11.6 million may be received through the exercise of Warrants issued in the Rights Offering.
Certain participants in the Rights Offering had the ability to pay the subscription price for their Units by cancelling or exchanging their shares of Series D Preferred Stock, Series F Preferred Stock and/or Series G Preferred Stock and the Company’s indebtedness evidenced by the Bridge Note, instead of paying by check or wire transfer of funds. The fair market value of the shares of each series of preferred stock and the Bridge Note to be cancelled or exchanged in the Rights Offering was determined by the Company’s Board of Directors with the assistance of an independent appraisal obtained by the Company. In order to help maximize Navidea’s ability to use its NOLs and other tax benefits in future years, Navidea’s Board of Directors exercised its discretion to limit the number of Units that John K. Scott, Jr. could purchase to 2,400 Units, which Mr. Scott elected to pay for by exchanging and surrendering all of his shares of Series F Preferred Stock. Of the total 10,423 Units sold in the Rights Offering, 4,250 Units were sold pursuant to the exchange and surrender of all outstanding shares of Navidea’s Series D Preferred Stock and Series F Preferred Stock. Neither the Series G Preferred Stock nor the Bridge Note was exchanged or cancelled pursuant to the Rights Offering.
Net proceeds after deducting fees and expenses of $998,401 related to the Rights Offering will be used to fund our pivotal Phase 3 clinical trial for RA, obtaining regulatory approvals, working capital, and for general corporate purposes. See Notes 2 and 13 to the accompanying consolidated financial statements.
Research and Development Expense Reimbursement
The Company has previously entered into an API Development Agreement with a strategic partner for assistance with the development and supply of the API used to manufacture Lymphoseek (technetium Tc 99m tilmanocept) that is sold by the Company in countries other than the United States, Canada and Mexico. Under the API Development Agreement, among other things, the strategic partner agreed to reimburse the Company for up to a total of $1.85 million of the Company’s out-of-pocket costs associated with such development, in two installments, subject to specified commercial and regulatory milestones. On August 11, 2022, the Company received the first installment in the amount of $800,000, which the strategic partner has the right to claw back if the Company does not satisfy certain commercial and regulatory milestones on or before March 31, 2023. The strategic partner is obligated, subject to certain conditions, to pay the remaining reimbursement amount upon the later of July 1, 2023 or satisfaction of specified commercial and regulatory milestones. See Note 2 to the accompanying consolidated financial statements.
Material Commitments
Bridge Note. On April 10, 2022, the Company entered into a Stock Exchange Agreement with John K. Scott, Jr., pursuant to which Mr. Scott loaned the Company $2.5 million. The Bridge Note bears interest at a rate of 8% per annum, with payments of interest only to be made monthly over a period of two years. All outstanding principal and accrued and unpaid interest under the Bridge Note is due and payable on the second anniversary of the Stock Exchange Agreement. As of December 31, 2022, there were approximately $2.8 million of payments remaining under the Bridge Note.
UCSD License Agreements. Under our license agreements with UCSD, we have exclusive world-wide rights to all diagnostic and therapeutic uses of tilmanocept, other than Tc99m tilmanocept used in lymphatic mapping in the United States, Canada and Mexico which rights are licensed directly to Cardinal Health 414 by UCSD. The UCSD license agreements include obligations for payments related to license fees, milestones, and royalties. As of December 31, 2022, the Company had accrued approximately $1.5 million of payments related to the UCSD license agreements for which we have not yet been invoiced. On February 16, 2023, the Company and UCSD executed an amendment to the license agreement for tilmanocept (other than Tc99m tilmanocept used in lymphatic mapping). The amendment released the Company from any and all obligations related to certain diligence requirements as defined in the license agreement. The amendment resulted in the reversal of approximately $1.2 million of accrued liabilities in the first quarter of 2023.
Financed Insurance Premiums. In November 2022, the Company prepaid $608,000 of insurance premiums through the issuance of a note payable to AFCO Premium Credit LLC with an interest rate of 7.85%. The note is payable in nine monthly installments of approximately $70,000, with the final payment due in August 2023. As of December 31, 2022, there were approximately $560,000 of payments remaining on the note.
Clinical Research Agreements. We have agreements in place with multiple clinical trial sites for conduct of our clinical studies, as well as with several contract research organizations for clinical trial-related services such as image and data management, monitoring, and statistical services. As of December 31, 2022, there were approximately $165,000 of payments currently due and an additional $365,000 accrued expenses related to clinical research agreements.
Manufacturing Development Agreements. We are investing in additional manufacturing and supply chain resources, and have entered into development contracts with the established manufacturing companies Corden Pharma Switzerland, LLC for active pharmaceutical ingredient production and ROTOP Pharmaka GmbH for drug product manufacturing. As of December 31, 2022, there were approximately $311,000 of payments currently due and an additional $212,000 of accrued expenses related to manufacturing development agreements.
Latkin Separation Agreement. On November 23, 2021, Jed A. Latkin signed a Separation Agreement and General Release (the “Separation Agreement”) in connection with his resignation from his positions as Chief Executive Officer, Chief Operating Officer and Chief Financial Officer, and as a director, on October 24, 2021 (the “Separation Date”). Pursuant to the Separation Agreement, among other things, the Company agreed to the continued payment of Mr. Latkin’s base salary of $490,000, less all relevant taxes and other withholdings, on the following basis: (i) for 12 months, 100% of his base salary, minus an aggregate $24,000 deducted monthly pro rata for reimbursement of Mr. Latkin’s attorney fees which were paid by the Company, and (ii) for 10 months following the expiration of the first 12-month period, 50% of his base salary. As of December 31, 2022, there were approximately $173,000 of payments remaining under the Separation Agreement.
CRG Litigation
See Notes 2 and 12 to the accompanying consolidated financial statements.
Platinum Litigation
See Notes 2 and 12 to the accompanying consolidated financial statements.
Goldberg Agreement and Litigation
See Notes 2 and 12 to the accompanying consolidated financial statements.
Summary
Our future liquidity and capital requirements will depend on a number of factors, including the ability to procure required financial resources, the ability of our distribution partners to achieve market acceptance of our products, our ability to complete the development and commercialization of new products, our ability to obtain milestone or development funds from potential development and distribution partners, regulatory actions by the FDA and international regulatory bodies, the outcome of any pending litigation, and intellectual property protection.
We plan to focus our resources during 2023 on development of products based on the Manocept platform. Although management believes that it will be able to achieve this objective, it is subject to a number of variables beyond our control, including the nature and timing of any partnering opportunities, the ability to modify contractual commitments made in connection with these programs, and the timing and expense associated with suspension or alteration of clinical trials, and consequently we expect to seek additional financing in order to support our planned development programs.
We will continue to evaluate our timelines, strategic needs, and balance sheet requirements. If we attempt to raise additional capital through debt, royalty, equity or otherwise, we may not be successful in doing so on terms acceptable to the Company, if at all. We may not be able to gain access and/or be able to secure new sources of funding, identify new development opportunities, successfully obtain regulatory approval for and commercialize new products, achieve significant product revenues from our products, or achieve or sustain profitability in the future.
The Company is currently engaged in litigation with Dr. Goldberg and CRG. As of December 31, 2022, the Company has accrued approximately $3.4 million of legal fees and interest pursuant to the CRG judgment. The amount of ultimate liability, if any, with respect to the Goldberg litigation is unknown.
The COVID-19 pandemic may negatively impact the Company’s operations, including possible effects on its financial condition, ability to access the capital markets on attractive terms or at all, liquidity, operations, suppliers, industry, and workforce. We do not believe there has been a significant impact to the Company’s clinical development and regulatory timelines resulting from the ongoing COVID-19 global pandemic. However, the COVID-19 outbreak delayed enrollment in our NAV3-32 clinical study in the United Kingdom due to national COVID-19-related shutdowns. In addition, the regulatory approval process in India has been delayed by the impact of COVID-19 in that country. The COVID-19 pandemic has adversely affected economies and financial markets worldwide, resulting in an economic downturn that could impact our business, financial condition and results of operations, including our ability to obtain additional funding. The Company will continue to evaluate the impact that the COVID-19 pandemic could have on the operations, financial position, and the results of operations and cash flows during fiscal year 2023 and beyond.
The current conflict between Ukraine and Russia has created volatility in the global capital markets and is continuing to have further global economic consequences, including disruptions of the global supply chain and energy markets. Any such volatility and disruptions may have adverse consequences on us or the third parties who operate in Europe on whom we rely. If the equity and credit markets deteriorate, including as a result of political unrest or war, it may make any debt or equity financing more difficult to obtain, more costly or more dilutive. The Company will continue to evaluate the impact that the Russia-Ukraine conflict could have on the operations, financial position, and the results of operations and cash flows during fiscal year 2023 and beyond.
The Company has experienced recurring net losses and has used significant cash to fund its operations. The Company has considerable discretion over the extent of development project expenditures and has the ability to curtail the related cash flows as needed. The Company also continues working to establish new sources of funding, including potential equity and/or debt financings, collaborations and additional grant funding that can augment the balance sheet. However, based on our current working capital and our projected cash burn, management believes that there is substantial doubt about the Company’s ability to continue as a going concern for a period of one year from the filing of this Annual Report on Form 10-K. No adjustments have been made to the accompanying consolidated financial statements as a result of this uncertainty. See Note 2 to the accompanying consolidated financial statements and Item 1A – “Risk Factors.”
As of December 31, 2022, we had no off-balance sheet arrangements.
Recent Accounting Standards
See Notes 1(q) and 1(r) to the accompanying consolidated financial statements.
Critical Accounting Policies
Revenue Recognition. We generate revenue from a grant to support a product development initiative. We generally recognize grant revenue when expenses reimbursable under the grant have been paid and payments under the grant become contractually due.
We also earn revenue from product sales to end customers, primarily in Europe. Revenue from product sales is generally recognized at the point where the customer obtains control of the goods and we satisfy our performance obligation, which occurs upon either shipment of the product or arrival at its destination, depending upon the shipping terms of the transaction. Our customers have no right to return products purchased in the ordinary course of business, however, we may allow returns in certain circumstances based on specific agreements.
In addition, we earn revenues related to our licensing and distribution agreements. The consideration we are eligible to receive under our licensing and distribution agreements typically includes upfront payments, reimbursement for research and development costs, milestone payments, and royalties. Each licensing and distribution agreement is unique and requires separate assessment in accordance with current accounting standards.
Research and Development. R&D expenses include both internal R&D activities and external contracted services. Internal R&D activity expenses include salaries, benefits, and stock-based compensation, as well as travel, supplies, and other costs to support our R&D staff. External contracted services include clinical trial activities, chemistry, manufacturing and control-related activities, and regulatory costs. R&D expenses are charged to operations as incurred. We review and accrue R&D expenses based on services performed and rely upon estimates of those costs applicable to the stage of completion of each project.
Debt. We evaluate newly-issued debt instruments in accordance with Accounting Standards Codification (“ASC”) 470, Debt. The Company evaluated the terms of the Bridge Note under these guidelines. Based on this evaluation, the Company recorded a debt discount related to the difference in the value of Mr. Scott’s Series E Preferred Stock and the Series F Preferred Stock and Series G Preferred Stock as well as debt issuance costs. The debt discount is being amortized as non-cash interest expense using the effective interest method over the term of the Bridge Note.
Preferred Stock. We evaluate newly-issued preferred stock in accordance with ASC 480, Distinguishing Liabilities from Equity, ASC 815, Derivatives and Hedging, ASC 470, Debt and Accounting Series Release (“ASR”) 268, Presentation in Financial Statements of “Redeemable Preferred Stocks.” The Company evaluated the provisions of the Series D, Series E, Series F and Series G Preferred Stock under the guidelines described above. Based on this evaluation, the Company determined that the Series D, Series E, Series F and Series G Preferred Stock are not mandatorily redeemable financial instruments and any obligation to issue a variable number of shares of Common Stock is not unconditional. Accordingly, the Series D, Series E, Series F and Series G Preferred Stock should be classified as equity. Neither the embedded conversion option in the Series D, Series E and Series F Preferred Stock, nor the embedded call option in the Series D, Series E, Series F and Series G Preferred Stock, meet the criteria to be separated from the Series D, Series E, Series F or Series G Preferred Stock and thus these features should not be bifurcated and accounted for as derivatives. Additionally, the Series D and Series E Preferred Stock each contain a beneficial conversion feature (“BCF”). Following the January 1, 2021 adoption of Accounting Standards Update (“ASU”) No. 2020-06, Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity, no BCF is recorded in the consolidated financial statements. Finally, the Company determined that the Series D, Series E and Series F Preferred Stock do not contain conversion features that could result in the Company being required to redeem a portion of the shares converted, thus the Series D, Series E and Series F Preferred Stock should not be classified in mezzanine equity. Similarly, the voluntary redemption feature of the Series G Preferred Stock cannot result in the Company being required to redeem the shares converted and the Series G Preferred Stock is not required to be classified in mezzanine equity.
Preferred Stock Issued with Warrants. We evaluate preferred stock issued with warrants in accordance with ASC 480, Distinguishing Liabilities from Equity, ASC 815, Derivatives and Hedging and ASR 268, Presentation in Financial Statements of “Redeemable Preferred Stocks.” The Company evaluated the provisions of the Series I Preferred Stock and the Warrants issued in the Rights Offering under the guidelines described above. Based on this evaluation, the Company determined that the Series I Preferred Stock and Warrants each meet the definition of a freestanding financial instrument and should be accounted for separately upon issuance.
|
● |
Series I Preferred Stock. The Series I Preferred Stock is not a mandatorily redeemable financial instrument and any obligation to issue a variable number of shares of Common Stock does not require liability classification. Accordingly, the Series I Preferred Stock should be classified as equity. The embedded conversion option in the Series I Preferred Stock does not meet the criteria to be separated from the Series I Preferred Stock and thus this feature should not be bifurcated and accounted for as a derivative. The subsequent rights offering privilege meets the criteria to be accounted for as a derivative, however, the scope exception is met and classification of the subsequent rights offering privilege in stockholders’ equity is appropriate. Because the Series I Preferred Stock is also classified in stockholders’ equity, no separate accounting is provided for the subsequent rights offering privilege. Finally, the Company determined that the Series I Preferred Stock does not contain conversion features that could result in the Company being required to redeem a portion of the shares converted, thus the Series I Preferred Stock should not be classified in mezzanine equity. |
|
● |
Warrants. The Warrants are not within the scope of ASC 480, therefore liability classification is not required. The Warrants have all of the characteristics to meet the definition of a derivative, however, they are considered to be indexed to the Company’s Common Stock and meet the other criteria to be classified in stockholders’ equity. Accordingly, the Warrants should be classified in stockholders’ equity upon issuance. The subsequent rights offering privilege meets the criteria to be accounted for as a derivative, however, the scope exception is met and classification of the subsequent rights offering privilege in stockholders’ equity is appropriate. Because the Warrants are also classified in stockholders’ equity, no separate accounting is provided for the subsequent rights offering privilege. |
Use of Estimates. The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. We base these estimates and assumptions upon historical experience and existing, known circumstances. Actual results could differ from those estimates.
Critical Estimates
Stock-Based Compensation. Stock-based payments to employees and directors, including grants of stock options and restricted stock, are recognized in the statements of operations based on their estimated fair values on the date of grant, subject to an estimated forfeiture rate. The fair value of each option award with time-based vesting provisions is estimated on the date of grant using the Black-Scholes option pricing model to value such stock-based payments and the portion that is ultimately expected to vest is recognized as compensation expense over either (1) the requisite service period or (2) the estimated performance period. The determination of fair value using the Black-Scholes option pricing model is affected by our stock price as well as assumptions regarding a number of complex and subjective variables, including expected stock price volatility, risk-free interest rate, expected dividends and projected employee stock option behaviors. The fair value of each option award with market-based vesting provisions is estimated on the date of grant using a Monte Carlo simulation to value such stock-based payments and the portion that is ultimately expected to vest is recognized as compensation expense over either (1) the requisite service period or (2) the estimated performance period. The determination of fair value using a Monte Carlo simulation is affected by our stock price as well as assumptions regarding a number of complex and subjective variables, including expected stock price volatility, risk-free interest rate, expected dividends and projected employee stock option behaviors.
We estimate the expected term based on the contractual term of the awards and employees' exercise and expected post-vesting termination behavior. Restricted stock awards are valued based on the closing stock price on the date of grant and amortized ratably over the estimated life of the award.
Since stock-based compensation is recognized only for those awards that are ultimately expected to vest, we have applied an estimated forfeiture rate to unvested awards for the purpose of calculating compensation cost. These estimates will be revised, if necessary, in future periods if actual forfeitures differ from estimates. Changes in forfeiture estimates impact compensation cost in the period in which the change in estimate occurs.
Contingent Liabilities. We are subject to legal proceedings and claims that arise in the normal course of business. In accordance with ASC Topic 450, Contingencies, we accrue for contingent liabilities when management determines it is probable that a liability has been incurred and the amount can be reasonably estimated. This determination requires significant judgment by management. As of the date of the filing of this Annual Report on Form 10-K, we are engaged in ongoing litigation with our former President and Chief Executive Officer, Dr. Michael Goldberg. In assessing whether we should accrue a liability in our financial statements as a result of this lawsuit, we considered various factors, including the legal and factual circumstances of the case, the trial records and post-trial rulings of the applicable courts, the current status of the proceedings, applicable law and the views of legal counsel.
We have concluded that a loss from the Goldberg case is not determinable or reasonably estimable and, therefore, a liability has not been recorded with respect to this case as of December 31, 2022. The amount of ultimate liability, if any, with respect to the Goldberg litigation is unknown.
Fair Value of Equity Transactions. Exchanges of preferred stock are recorded in the financial statements based on their estimated fair values at the date of exchange. The fair value of each series of preferred stock is estimated using the OPM Backsolve equity valuation method. The determination of fair value using the OPM Backsolve method is impacted by our stock price, the value of the most recent equity transaction and the features of each class of security, which can include rights such as liquidation preferences, required returns, conversion options, or other items. Differences in these features result in differences in value for each class of security. The OPM Backsolve method uses the economic rights, relationships and participation levels of the securities along with the Black-Scholes option pricing model to create an option-based equation for the equity capital structure of the company. When the value of one class of equity security is known, the OPM Backsolve method provides the ability to calculate a fair value for all other equity securities.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Not applicable to smaller reporting companies.
Item 8. Financial Statements and Supplementary Data
Our consolidated financial statements, and the related notes, together with the report of Marcum LLP dated March 27, 2023, are set forth at pages F-1 through F-33 attached hereto and incorporated herein by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
We maintain disclosure controls and procedures designed to ensure that information required to be disclosed in reports filed under the Exchange Act is recorded, processed, summarized, and reported within the specified time periods. As a part of these controls, our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Exchange Act.
Under the supervision and with the participation of our management, including our Chief Medical Officer and Vice President, Finance and Administration, we evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of December 31, 2022, and concluded that our disclosure controls and procedures were effective as of the end of the period covered by this report to ensure that information required to be disclosed by us in the reports that we file or submit is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure.
Our management understands that our disclosure controls and procedures do not guarantee that all errors and all improper conduct will be prevented. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, a design of a control system must reflect the fact that there are resource constraints, and the benefit of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of improper conduct, if any, have been detected. These inherent limitations include the realities that judgments and decision-making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more persons, or by management override of the control. Further, the design of any system of controls is also based in part upon assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations of a cost-effective control system, misstatements due to error or fraud may occur and may not be detected.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control system was designed to provide reasonable assurance to management and the Board of Directors regarding the preparation and fair presentation of published financial statements. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, and includes those policies and procedures that:
|
● |
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
|
● |
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP and that receipts and expenditures of the company are being made only in accordance with authorization of management and directors of the Company; and |
|
● |
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company's assets that could have a material effect on the financial statements. |
Under the supervision and with the participation of our management, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2022 based upon the criteria set forth in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based on our assessment we concluded that, as of December 31, 2022, our internal control over financial reporting was effective based on those criteria.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2022 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
None.
Item 9C. Disclosures Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Directors
Set forth below are the names and committee assignments of the persons who constitute our Board of Directors.
|
Name |
Age |
Committee(s) |
||
|
Amit Bhalla |
48 |
Audit |
||
|
Alexander L. Cappello |
67 |
Audit; Compensation, Nominating and Governance; Board Oversight |
||
|
John K. Scott, Jr. |
68 |
Compensation, Nominating and Governance; Board Oversight |
||
|
Joshua M. Wilson |
45 |
— |
||
|
Malcolm G. Witter |
69 |
Audit (Chair); Compensation, Nominating and Governance (Chair) |
Director Qualifications
The Board of Directors believes that individuals who serve on the Board should have demonstrated notable or significant achievements in their respective field; should possess the requisite intelligence, education and experience to make a significant contribution to the Board and bring a range of skills, diverse perspectives and backgrounds to its deliberations; and should have the highest ethical standards, a strong sense of professionalism and intense dedication to serving the interests of our stockholders. The following are qualifications, experience and skills for Board members which are important to our business and its future:
|
● |
General Management. Directors who have served in senior leadership positions bring experience and perspective in analyzing, shaping, and overseeing the execution of important operational and policy issues at a senior level. These directors’ insights and guidance, and their ability to assess and respond to situations encountered in serving on our Board of Directors, are enhanced by their leadership experience developed at businesses or organizations that operated on a global scale, faced significant competition, or involved other evolving business models. |
|
● |
Industry Knowledge. Because we are a pharmaceutical development company, education or experience in our industry, including medicine, pharmaceutical development, marketing, distribution, or the regulatory environment, is important because such experience assists our directors in understanding and advising our Company. |
|
● |
Business Development/Strategic Planning. Directors who have a background in strategic planning, business development, strategic alliances, mergers and acquisitions, and teamwork and process improvement provide insight into developing and implementing strategies for growing our business. |
|
● |
Finance/Accounting/Control. Knowledge of capital markets, capital structure, financial control, audit, reporting, financial planning, and forecasting are important qualities of our directors because such qualities assist in understanding, advising, and overseeing our Company’s capital structure, financing and investing activities, financial reporting, and internal control of such activities. |
|
● |
Board Experience/Governance. Directors who have served on other public company boards can offer advice and insights with regard to the dynamics and operation of a board of directors, the relations of a board to the chief executive officer and other management personnel, the importance of particular agenda and oversight matters, and oversight of a changing mix of strategic, operational, and compliance-related matters. |
Biographical Information
Set forth below is current biographical information about our directors, including the qualifications, experience and skills that make them suitable for service as a director. Each listed director’s respective experience and qualifications described below led the Compensation, Nominating and Governance (“CNG”) Committee of our Board of Directors to conclude that such director is qualified to serve as a member of our Board of Directors.
Director whose term continues until the 2023 Annual Meeting:
John K. Scott, Jr. has served as a director of Navidea since July 2021. Mr. Scott has served as the owner and manager of PCS, Inc. since 1997, where he is responsible for directing the acquisition, financing, sales and operations for land entitlement and development for privately owned condominium, apartment, hotel, single family and retail projects in California, Colorado and Texas. He has also served as the general partner of NJD, Ltd., a Texas limited partnership, since 1997 and as the managing member of Merging Interests, Inc. since 1980. Mr. Scott also has extensive experience in conducting due diligence, feasibility studies, financial analysis, cost estimates and transaction negotiations for the purchase, lease, development, marketing and sale of projects and properties. Mr. Scott earned a B.S. in agricultural economics with an emphasis on construction management and real estate from the University of Wisconsin.
Directors whose terms continue until the 2024 Annual Meeting:
Joshua M. Wilson has served as a director of Navidea since September 2022. Mr. Wilson is a seasoned banking and finance executive with more than 23 years of financial services and family office experience. During his career, Mr. Wilson has focused on raising capital and streamlining company operations for profit and non-profit entities, raising more than $500 million. Since June 2022 Mr. Wilson has served as the Chief Executive Officer for the UpSwing Foundation, focusing on raising capital for the vertical construction of UpSwing Foundation’s World Headquarters and overseeing all day-to-day operations of its business in support of the Foundation’s mission of “Connecting People and Catapulting Dreams-Together,” achieved through access to elite athletic facilities, multi-sport training, and scholarship opportunities. Mr. Wilson also currently serves as the Executive Director of G2G Ventures, a Colorado-based single-family office, focusing on the creation of its first four private equity partnership funds with assets in energy, industrial warehousing, biotechnology and biopharmaceuticals. From 2017 to June 2022, Mr. Wilson was State President-CO/WY-AZ-CA of First Western Trust Bank, and from 2011 to 2016, he served as Chief Financial Officer/Family Office Executive of Central Resources, Inc. Prior to that, Mr. Wilson held roles of increasing responsibility at multiple financial institutions, including Market President-Denver of First Western Trust Bank, Senior Vice President of Vectra Private Bank, and Vice President of Bank One/JP Morgan Chase. Mr. Wilson currently serves on the board of directors of Lynx Energy ULC and has prior board experience with First Western Trust. Mr. Wilson received his B.S. in Business Administration from Regis University.
Malcolm G. Witter has served as a director of Navidea since December 2020. Mr. Witter has over 40 years of operational and investment leadership experience, serving as investment banker, Chief Financial Officer, and advisor to many companies and private organizations. From 2016 to 2021, he served as the Corporate Development Regional Manager for USI Insurance Services (“USI”) where he was responsible for acquiring independent insurance agencies. From 2010 to 2016, Mr. Witter was Business Development Manager for Kibble & Prentice, Inc., a USI company. Prior to USI, Mr. Witter held roles at multiple financial institutions including Kibble & Prentice Financial, Compass Capital Fund Management, Bear, Stearns & Co., and Dean Witter Reynolds. Mr. Witter is a director of the Dean Witter Foundation and an Advisor to American Research Capital. Mr. Witter received his M.B.A. from the Stanford Graduate School of Business.
Directors whose terms continue until the 2025 Annual Meeting:
Amit Bhalla has served as a director of Navidea since May 2021. Mr. Bhalla has served as the Chief Financial Officer of Infinity BiologiX, LLC since November 2020. From 2015 to 2020, he served as Senior Healthcare Analyst for Lord, Abbett & Co as well as Investment Council Member for Lord, Abbett’s Healthcare Fund. Prior to that, Mr. Bhalla served in various roles including Vice President-Global Strategy & Development for Becton, Dickinson and Company, Director-Equity Research-Life Science Tools/Medical Technology for Citi, Vice President-Equity Research-Emerging Medical Technology and Analyst-Equity Research-Specialty Pharmaceuticals for Morgan Stanley, and Associate-Technical Operations/Research & Development for Johnson & Johnson’s Ortho-McNeil Pharmaceutical. Mr. Bhalla received his B.S. in biology from Cornell University and his M.B.A. from Tepper School of Business at Carnegie Mellon University.
Alexander L. Cappello has served as a director of Navidea since July 2021. Mr. Cappello has led several public and private companies over the past 48 years, including Cappello Global, LLC, a global investment bank, whose principals have transacted business in over 55 countries. He is also a director of The Cheesecake Factory Incorporated (Nasdaq), lead director of Virco Manufacturing Corporation (Nasdaq), lead director of The Agnew Companies and Caldera Medical Corp. Mr. Cappello is a director of RAND Corporation’s Center for Middle East Public Policy, the Center for Global Risk and Security, and the RAND-Russia Forum. Mr. Cappello is a former Chairman of Intelligent Energy, PLC (LSE), Inter-Tel (Nasdaq), and Geothermal Resources Intl. (AMEX), and a former director of Nano Financial Holdings and California Republic Bank. He is also a former advisor to the board of Gusmer Enterprises and former trustee of University of Southern California, and trustee and chairman of the investment committee of City of Hope. Mr. Cappello received a B.S. in management and finance from the Marshall School of Business at the University of Southern California.
Information About our Executive Officers
The following individuals are executive officers of Navidea and serve in the positions indicated below:
|
Name |
Age |
Position |
||
|
Michael S. Rosol, Ph.D. |
54 |
Chief Medical Officer |
||
|
Erika L. Eves |
53 |
Vice President, Finance and Administration |
Michael S. Rosol, Ph.D., has served as Chief Medical Officer of Navidea since December 2018. Prior to joining Navidea, Dr. Rosol served as Associate Director in the Clinical and Translational Imaging Group at Novartis Institutes for BioMedical Research from November 2016 to December 2018. Before that, he held positions as Senior Director of Business Development at Elucid Bioimaging, Inc. where he drove adoption of its Computer-Aided Phenotyping applications from May 2016 to November 2016, and as Chief Scientific Officer of MediLumine, Inc. from October 2015 to May 2016. Prior to those roles, he was the Head of the Translational Imaging Group at Novartis Pharmaceuticals Group from October 2012 to March 2015. His training and experience lie in the fields of biophysics, physiology, and biological/medical imaging, and his work has focused on cardiovascular imaging, preclinical and clinical imaging instrumentation and applications, animal models of human disease, pathophysiology, biomarkers, and imaging in toxicological and clinical trials. He has also served as faculty in Radiology and Director of two academic research imaging facilities. Dr. Rosol holds a Ph.D. from Boston University School of Medicine.
Erika L. Eves has served as Vice President, Finance and Administration of Navidea since November 2020. Ms. Eves has served the Company in several roles of increasing responsibility beginning in March 1992, including Accounting Clerk, Staff Accountant, Senior Accountant, Controller and Director of Finance and Administration. In addition to directing the financial operations of the Company, she is responsible for internal and external financial reporting including all SEC filings, maintaining a system of internal controls, and managing banking and vendor relationships. Ms. Eves earned a B.S.B.A. in Accounting from The Ohio State University and is a Certified Public Accountant.
Delinquent Section 16 Filings
Section 16(a) of the Exchange Act requires our officers and directors, and greater than 10% stockholders, to file reports of ownership and changes in ownership of our securities with the SEC. Copies of the reports are required by SEC regulation to be furnished to us. Based on our review of these reports and written representations from reporting persons, we believe that all reporting persons complied with all filing requirements during the fiscal year ended December 31, 2022, except for: (i) Mr. Scott, who had one late Form 4 filing related to his exchange of preferred stock, and (ii) Mr. Wilson, who had one late Form 3 filing due to delays in obtaining SEC filer codes.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to our directors, officers and all employees. The code of business conduct and ethics is posted on our website at www.navidea.com. The code of business conduct and ethics may also be obtained free of charge by writing to Navidea Biopharmaceuticals, Inc., Attn: Chief Financial Officer, 4995 Bradenton Avenue, Suite 240, Dublin, Ohio 43017.
Corporate Governance
Our Board of Directors is responsible for establishing broad corporate policies and reviewing our overall performance rather than day-to-day operations. The primary responsibility of our Board is to oversee the management of Navidea and, in doing so, serve the best interests of the Company and our stockholders. Our Board selects, evaluates and provides for the succession of executive officers and, subject to stockholder election, directors. It reviews and approves corporate objectives and strategies, and evaluates significant policies and proposed major commitments of corporate resources. Our Board also participates in decisions that have a potential major economic impact on the Company. Management keeps our directors informed of Company activity through regular communication, including written reports and presentations at Board and committee meetings.
Board of Directors Meetings
Our Board of Directors held a total of 18 meetings in the fiscal year ended December 31, 2022, and each of the directors attended at least 75 percent of the aggregate number of meetings of the Board of Directors and committees (if any) on which he served.
The Board of Directors has established the following committees to assist it in its oversight responsibilities: Audit Committee, Compensation, Nominating and Governance Committee, and Board Oversight Committee. The current membership and responsibilities of each committee are disclosed below.
Audit Committee
The Audit Committee of the Board of Directors selects our independent registered public accounting firm with whom the Audit Committee reviews the scope of audit and non-audit assignments and related fees, the accounting principles that we use in financial reporting, and the adequacy of our internal control procedures. The current members of our Audit Committee are Malcolm G. Witter (Chair), Amit Bhalla and Alexander L. Cappello, each of whom is “independent” under Section 803A of the NYSE American Company Guide, and each of whom meets the requirements of an “audit committee financial expert” as set forth in Section 407(d)(5) of Regulation S-K promulgated by the SEC. The Audit Committee held five meetings in the fiscal year ended December 31, 2022. The Board of Directors adopted a written Amended and Restated Audit Committee Charter on April 30, 2004. A copy of the Amended and Restated Audit Committee Charter is posted on the Company’s website at www.navidea.com.
Compensation, Nominating and Governance Committee
The CNG Committee of the Board of Directors discharges the Board’s responsibilities relating to the compensation of the Company's directors, executive officers and associates, identifies and recommends to the Board of Directors nominees for election to the Board, and assists the Board in the implementation of sound corporate governance principles and practices. With respect to its compensation functions, the CNG Committee evaluates and approves executive officer compensation and reviews and makes recommendations to the Board with respect to director compensation, including incentive or equity-based compensation plans; reviews and evaluates any discussion and analysis of executive officer and director compensation included in the Company’s annual report or proxy statement, and prepares and approves any report on executive officer and director compensation for inclusion in the Company’s annual report or proxy statement required by applicable rules and regulations; and monitors and evaluates, at the Committee’s discretion, matters relating to the compensation and benefits structure of the Company and such other domestic and foreign subsidiaries or affiliates, as it deems appropriate. The members of our CNG Committee are Malcolm G. Witter (Chair), Alexander L. Cappello and John K. Scott, Jr. The CNG Committee held 11 meetings in the fiscal year ended December 31, 2022. The Board of Directors adopted a written Compensation, Nominating and Governance Committee Charter on February 26, 2009. A copy of the Compensation, Nominating and Governance Committee Charter is posted on the Company’s website at www.navidea.com.
Board Oversight Committee
The Board Oversight Committee of the Board of Directors provides support and guidance to the Company’s executive leadership. In November 2021, following the resignation of the Company’s former Chief Executive Officer, Chief Operating Officer and Chief Financial Officer, Jed. A. Latkin, our Board of Directors established an Executive Leadership Committee to lead the Company on an interim basis until its next CEO was identified. The Executive Leadership Committee included Michael S. Rosol, Ph.D., our Chief Medical Officer, Erika L. Eves, our Vice President of Finance and Administration and Jeffrey G. Smith, our Vice President of Operations. Effective October 6, 2022, the Executive Leadership Committee was disbanded, and the Board Oversight Committee now works directly with Dr. Rosol. The current members of the Board Oversight Committee are Alexander L. Cappello and John K. Scott, Jr.
Item 11. Executive Compensation
Compensation Discussion and Analysis
Overview of Compensation Program. The CNG Committee of the Board of Directors is responsible for establishing and implementing our compensation policies applicable to senior executives and monitoring our compensation practices. The CNG Committee seeks to maintain compensation plans that are fair, reasonable and competitive. The CNG Committee is responsible for reviewing and approving senior executive compensation, awards under our cash bonus plan, and awards under our equity-based compensation plans.
Philosophy and Goals of Executive Compensation Plans. The CNG Committee’s philosophy for executive compensation is to:
|
● |
Pay for performance: The CNG Committee believes that our executives should be compensated based upon their ability to achieve specific operational and strategic results. Therefore, our compensation plans are designed to provide rewards for the individual’s contribution to our performance. |
|
● |
Pay commensurate with other companies categorized as value creators: The CNG Committee has set a goal that the Company should move toward compensation levels for senior executives that are, at a minimum, at the 25th to 75th percentile for similar executives in the workforce while taking into account current market conditions and Company performance. This allows us to attract, hire, reward and retain senior executives who formulate and execute our strategic plans and drive exceptional results. |
To assess whether our programs are competitive, the CNG Committee reviews compensation information of peer companies, national data and trends in executive compensation to help determine the appropriateness of our plans and compensation levels. These reviews, and the CNG Committee’s commitment to pay for performance, become the basis for the CNG Committee’s decisions on compensation plans and individual executive compensation payments.
The CNG Committee has approved a variety of programs that work together to provide a combination of basic compensation and strong incentives. While it is important for us to provide certain base level salaries and benefits to remain competitive, the CNG Committee’s objective is to provide compensation plans with incentive opportunities that motivate and reward executives for consistently achieving superior results. The CNG Committee designs our compensation plans to:
|
● |
Reward executives based upon overall company performance, their individual contributions and creation of stockholder value; |
|
● |
Encourage executives to make a long-term commitment to our Company; and |
|
● |
Align executive incentive plans with the long-term interests of stockholders. |
The CNG Committee reviews senior executive compensation levels at least annually. During the review process, the CNG Committee addresses the following questions:
|
● |
Do any existing compensation plans need to be adjusted to reflect changes in competitive practices, different market circumstances or changes to our strategic initiatives? |
|
● |
Should any existing compensation plans be eliminated or new plans be added to the executive compensation programs? |
|
● |
What are the compensation-related objectives for our compensation plans for the upcoming fiscal year? |
|
● |
Based upon individual performance, what compensation modifications should be made to provide incentives for senior executives to perform at superior levels? |
In addressing these questions, the CNG Committee considers input from management, outside compensation experts and published surveys of compensation levels and practices.
The CNG Committee does not believe that our compensation policies and practices for our employees give rise to risks that are reasonably likely to have a material adverse effect on the Company. Our incentive-based compensation is based on management’s evaluation of individual employee performance. The CNG Committee believes that such incentive-based compensation creates a strong motivation for Company employees to contribute towards the achievement of strong, sustainable performance, and believes that the Company has a strong set of internal controls that minimize the risk that financial performance can be misstated in order to achieve incentive compensation payouts.
In addition to the aforementioned considerations, the CNG Committee also takes into account the outcome of stockholder advisory (“say-on-pay”) votes on the compensation of our Chief Executive Officer and our next two highest-paid executive officers (the “Named Executive Officers”). At the Annual Meeting of Stockholders held on September 14, 2021, approximately 79% of our stockholders who cast a ballot voted in favor of the resolution relating to the compensation of our Named Executive Officers. The CNG Committee believes this vote affirmed our stockholders’ support of the Company’s executive compensation program. The CNG Committee will continue to consider the results of future say-on-pay votes when making future compensation decisions for the executive officers. The Company currently holds an advisory vote to approve the compensation of the Company’s Named Executive Officers every two years. The two-year frequency of advisory “say-on-pay” votes will continue until the next required vote on the frequency of advisory votes on executive compensation at the Company’s Annual Meeting of Stockholders to be held in 2023.
Scope of Authority of the CNG Committee. The Board of Directors has authorized the CNG Committee to establish the compensation programs for all executive officers and to provide oversight for compliance with our compensation philosophy. Annually, the CNG Committee recommends the compensation for our executive officers, including awards under incentive plans. The Chief Medical Officer provides input for the CNG Committee regarding the performance and appropriate compensation of the other officers. The CNG Committee gives considerable weight to the Chief Medical Officer’s evaluation of the other officers because of his or her direct knowledge of each officer’s performance and contributions. The CNG Committee also makes recommendations to the Board of Directors on appropriate compensation for the non-employee directors. In addition to overseeing the compensation of executive officers, the CNG Committee recommends or approves awards under short-term cash incentive and long-term equity-based compensation plans for all other employees. For more information on the CNG Committee’s role, see the CNG Committee’s charter, which can be found on our website at www.navidea.com.
Independent Compensation Expertise. The CNG Committee is authorized to periodically retain independent experts to assist in evaluating executive compensation plans and in setting executive compensation levels. These experts provide information on trends and best practices so the CNG Committee can formulate ongoing plans for executive compensation. The CNG Committee retained Frederic W. Cook & Co., Inc. (“F.W. Cook”) as its independent consultant to assist in the determination of the reasonableness and competitiveness of the compensation levels of its Named Executive Officers and Board of Directors for fiscal 2022. No conflict of interest exists that would prevent F.W. Cook from serving as independent consultant to the CNG Committee.
For fiscal 2022, F.W. Cook performed a benchmark compensation review of our key executive positions, including our Chief Medical Officer and our Board of Directors. F.W. Cook utilized published survey and proxy reported data from compensation peers, with market data aged to January 1, 2022, by an annualized rate of 3.0%, the expected pay increase in 2022 for executives in the life sciences industry.
In evaluating appropriate executive compensation, it is common practice to set targets at a point within the competitive marketplace. The CNG Committee sets its competitive compensation levels based upon its compensation philosophy. Following completion of the F.W. Cook study for 2022, the CNG Committee noted that the total cash compensation of our Chief Medical Officer was below the 25th percentile for an established peer group of companies.
Peer Group Companies. As part of their review, F.W. Cook surveyed the compensation levels at specific competitive benchmark companies. With input from the Board of Directors, F.W. Cook chose the peer companies because they are developmental life sciences companies and are similar to Navidea in revenue, employees and market capitalization. The selected peer group companies have market capitalization of less than $250 million and have comparable key executive positions. While the specific plans for these companies may or may not be used, it is helpful to review their compensation data to provide benchmarks for the overall compensation levels that will be used to attract, hire, retain and motivate our executives.
As competitors and similarly situated companies that compete for the same executive talent, F.W. Cook and the CNG Committee determined that the following peer group companies most closely matched the responsibilities and requirements of our executives:
|
Actinium Pharmaceuticals, Inc. |
ContraFect Corporation |
Marker Therapeutics, Inc. |
|
Advaxis |
Corvus Pharmaceuticals |
NanoViricides |
|
aTyr Pharma Inc. |
Genocea Biosciences, Inc. |
OncoSec Medical Incorporated |
|
Bellicum Pharmaceuticals, Inc. |
GeoVax Labs, Inc. |
Phio Pharmaceuticals Corp. |
|
Bio-Path Holdings, Inc. |
Idera Pharmaceuticals |
Regulus Therapeutics, Inc. |
|
Calithera Biosciences, Inc. |
Innovation Pharmaceuticals, Inc. |
T2 Biosystems, Inc. |
|
Cidara Therapeutics, Inc. |
Lumos Pharma, Inc. |
Vaccinex, Inc. |
F.W. Cook used the publicly available compensation information for these companies to analyze our competitive position in the industry. Base salaries and short-term and long-term incentive plans of the executives of these companies were reviewed to provide background and perspective in analyzing the compensation levels for our executives.
Specific Elements of Executive Compensation
Base Salary. Base salaries for senior executives are set using the CNG Committee’s philosophy that compensation should be competitive and based upon performance. Executives should expect that their base salaries, coupled with a cash bonus award, would provide them the opportunity to be compensated at or above the competitive market at the 25th to 75th percentile.
Based on competitive reviews of similar positions, industry salary trends, overall company results and individual performance, salary increases may be approved from time to time. The CNG Committee reviews and approves base salaries of all executive officers. In setting specific base salaries for fiscal 2022, the CNG Committee considered published proxy data for similar positions at peer group companies.
On September 9, 2022, the CNG Committee approved changes to base salaries for fiscal 2022, retroactive to January 1, 2022. The following table shows the changes in base salaries for the Named Executive Officers that were approved for fiscal 2022 compared to the approved salaries for fiscal 2021:
|
Named Executive Officer |
Fiscal 2022 Base Salary(a) |
Fiscal 2021 Base Salary(a) |
Change |
|||||||||
|
Michael S. Rosol, Ph.D. |
$ | 325,000 | $ | 240,000 | 35.4 |
% |
||||||
|
Erika L. Eves |
175,000 | 156,200 | 12.0 |
% |
||||||||
|
(a) |
The amount shown for fiscal 2022 and 2021 is the approved annual salary of the Named Executive Officer in effect at the end of each year. The actual amount paid to the Named Executive Officer during fiscal 2022 and 2021 is shown under “Salary” in the Summary Compensation table below. |
The following table shows the base salaries for the Named Executive Officers for fiscal 2023 compared to the approved salaries for fiscal 2022:
|
Named Executive Officer |
Fiscal 2023 Base Salary |
Fiscal 2022 Base Salary |
Change |
|||||||||
|
Michael S. Rosol, Ph.D. |
$ | 325,000 | $ | 325,000 | — |
% |
||||||
|
Erika L. Eves |
175,000 | 175,000 | — |
% |
||||||||
As of the date of filing this Annual Report on Form 10-K, 2023 salary changes have not yet been determined.
Short-Term Incentive Compensation. Our executive officers, along with our other employees, are eligible to participate in our annual cash bonus program, which has four primary objectives:
|
● |
Attract, retain and motivate top-quality executives who can add significant value to the Company; |
|
● |
Create an incentive compensation opportunity that is an integral part of the employee’s total compensation program; |
|
● |
Reward participants’ contributions to the achievement of our business results; and |
|
● |
Provide an incentive for individuals to achieve corporate objectives that are tied to our strategic goals. |
The cash bonus compensation plan provides each participant with an opportunity to receive an annual cash bonus based on each employee’s individual performance during the fiscal year. Cash bonus targets for senior executives are determined as a percentage of base salary, based in part on published proxy data for similar positions at peer group companies. The following are the key provisions of the cash bonus compensation plan for our Named Executive Officers:
|
● |
The plan is administered by the CNG Committee, which has the power and authority to establish, adjust, pay or decline to pay the cash bonus for each participant, including the power and authority to increase or decrease the cash bonus otherwise payable to a participant. However, the CNG Committee does not have the power to increase, or make adjustments that would have the effect of increasing, the cash bonus otherwise payable to any executive officer. |
|
● |
The CNG Committee is responsible for specifying the terms and conditions for earning cash bonuses, including establishing performance objectives for executive officers. |
|
● |
As soon as reasonably practicable after the end of each fiscal year, the CNG Committee determines whether and to what extent each performance objective has been achieved and the amount of the cash bonus to be paid to each executive officer. |
For fiscal 2022, the cash bonus for each executive officer was a function of the designated target bonus amount and individual performance objectives.
For fiscal 2022, the Board of Directors determined the cash bonus targets for Named Executive Officers as follows:
|
Named Executive Officer |
Target Cash Bonus |
Target Cash Bonus |
||||||
|
Michael S. Rosol, Ph.D. |
25.0 |
% |
$ | 81,250 | ||||
|
Erika L. Eves |
15.0 |
% |
26,250 | |||||
As of the date of filing this Annual Report on Form 10-K, 2022 bonus payouts have not yet been determined.
Long-Term Incentive Compensation. All Company employees are eligible to receive equity awards in the form of stock options, restricted stock or unrestricted stock. Equity instruments awarded under the Company’s equity-based compensation plan are based on the following criteria:
|
● |
Analysis of competitive information for comparable positions; |
|
● |
Evaluation of the value added to the Company by hiring or retaining specific employees; and |
|
● |
Each employee’s long-term potential contributions to our Company. |
Equity-based compensation is an effective method to align the interests of stockholders and management and focus management’s attention on long-term results. When awarding equity-based compensation the CNG Committee considers the impact the participant can have on our overall performance, strategic direction, financial results and stockholder value. Therefore, equity awards are primarily based upon the participant’s position in the organization, competitive necessity and individual performance. Stock option awards have vesting schedules over several years to promote long-term performance and retention of the recipient, and restricted stock awards may include specific performance criteria for vesting or vest over a specified period of time.
On September 9, 2022, the CNG Committee approved and adopted the terms and conditions of a long-term incentive plan (“LTIP”) that seeks to motivate and reward employees. The LTIP provides for the issuance of share-based awards to Named Executive Officers and other employees of the Company pursuant to the Navidea Biopharmaceuticals, Inc. 2014 Stock Incentive Plan. The target amount of the stock award under the LTIP for each employee was determined by the CNG Committee based on a variety of factors. Payout of the stock awards is based on the achievement of pre-established performance objectives and goals related to financing and FDA and EMA regulatory milestones for the Company’s Phase 3 clinical trial for rheumatoid arthritis (NAV3-33) over a 40-month performance period. The financing and EMA regulatory milestones will each comprise 5% of the total stock award payout for participants; the FDA regulatory milestones will comprise the remaining 90%. The payout amount is subject to downward adjustment based on the timing of the achievement of the particular milestone. In order to receive the payout, the participant generally will be required to continue to be employed through the date of the payout. Upon issuance of the stock award, the participant will be 100% vested in the stock award.
The CNG Committee established the target payout amount under the LTIP for its Named Executive Officers as follows:
|
Named Executive Officer |
Target Stock Award |
|||
|
Michael S. Rosol, Ph.D. |
260,000 | |||
|
Erika L. Eves |
120,000 | |||
Although equity awards may be made at any time as determined by the CNG Committee, they are generally made to all full-time employees pursuant to the LTIP. Based on completion of the public rights offering in August 2022, the CNG Committee decided to pay out 5% of the target stock award to all participants under the LTIP. On September 9, 2022, the following stock awards were made to the Named Executive Officers under the LTIP: 13,000 shares of common stock to Dr. Rosol and 6,000 shares of common stock to Ms. Eves. No FDA or EMA regulatory milestone was achieved during fiscal 2022.
On March 10, 2023, the CNG Committee amended the LTIP to award all remaining unearned LTIP stock awards as stock options. The LTIP stock options have an exercise price of $0.32 per share, and will expire on the tenth anniversary of the date of grant. The LTIP stock options will vest according to the performance objectives originally established for the LTIP as described above. On March 10, 2023, the following stock options were made to the Named Executive Officers under the LTIP: 247,000 stock options to Dr. Rosol and 114,000 stock options to Ms. Eves.
Other Benefits and Perquisites. The Named Executive Officers are generally eligible to participate in other benefit plans on the same terms as other employees. These plans include medical, dental, vision, disability and life insurance benefits, and our 401(k) retirement savings plan (the “401(k) Plan”).
Our paid time off (“PTO”) policy allows employees to carry up to 40 hours of unused PTO time forward to the next fiscal year. Any unused PTO time in excess of the amount eligible for rollover is generally forfeited.
We pay group life insurance premiums on behalf of all employees, including the Named Executive Officers. The benefit provides life insurance coverage at two times the employee’s annual salary plus $10,000, up to a maximum of $400,000.
We also pay group long-term disability insurance premiums on behalf of all employees, including the Named Executive Officers. The benefit provides long-term disability insurance coverage at 60% of the employee’s annual salary, up to a maximum of $10,000 per month, beginning 180 days after the date of disability and continuing through age 65.
401(k) Retirement Plan. All employees are given an opportunity to participate in our 401(k) Plan following a new-hire waiting period. Under the 401(k) Plan, participants may have pre-tax amounts, or post-tax amounts under a Roth option, withheld from their pay. The 401(k) Plan provides for a discretionary employer matching contribution (currently, a 100% match up to 6% of salary in the form of our Common Stock). Participants may invest their contributions in various fund options, but are prohibited from investing their contributions in our Common Stock. Participants are immediately vested in both their contributions and Company matching contributions. The 401(k) Plan qualifies under section 401 of the Internal Revenue Code, which provides that employee and company contributions and income earned on contributions are not taxable to the employee until withdrawn from the 401(k) Plan, and that the Company may deduct its contributions when made.
Report of Compensation, Nominating and Governance Committee
The CNG Committee is responsible for establishing, reviewing and approving the Company’s compensation philosophy and policies, reviewing and making recommendations to the Board regarding forms of compensation provided to the Company’s directors and officers, reviewing and determining cash and equity awards for the Company’s officers and other employees, and administering the Company’s equity incentive plans.
In this context, the CNG Committee has reviewed and discussed with management the Compensation Discussion and Analysis included in this annual report on Form 10-K. In reliance on the review and discussions referred to above, the CNG Committee recommended to the Board, and the Board has approved, that the Compensation Discussion and Analysis be included in this annual report on Form 10-K for filing with the SEC.
|
The Compensation, Nominating |
|
|
and Governance Committee |
|
|
Malcolm G. Witter (Chair) |
|
|
Alexander L. Cappello |
|
|
John K. Scott, Jr. |
Compensation, Nominating and Governance Committee Interlocks and Insider Participation
None of the members of our CNG Committee during the past year was an officer or employee of the Company. None of our executive officers currently serves, or in the past year served, as a member of a compensation committee (or other committee serving an equivalent function) or director of any entity that has one or more executive officers serving on our CNG Committee or our Board of Directors.
No director who served on the CNG Committee during 2022 had any relationships requiring disclosure by the Company under the SEC’s rules requiring disclosure of certain relationships and related-party transactions. None of the Company’s executive officers served as a director or a member of a compensation committee (or other committee serving an equivalent function) of any other entity, the executive officers of which served as a director of the Company or member of the CNG Committee during 2022.
Summary Compensation Table
The following table sets forth certain information concerning the annual and long-term compensation of our Named Executive Officers for the last two fiscal years.
Summary Compensation Table for Fiscal 2022
|
Named Executive Officer |
Year |
Salary |
(a) Stock Awards |
(a) Option Awards |
(b) Non-Equity Incentive Plan Compensation |
(c) All Other Compensation |
Total |
|||||||||||||||||||
|
Michael S. Rosol, Ph.D. (d) |
2022 |
$ |
336,667 |
$ |
3,640 |
$ |
— |
$ |
81,250 |
$ |
8,231 |
$ |
429,788 |
|||||||||||||
|
Chief Medical Officer |
2021 |
263,526 |
— |
133,037 |
63,057 |
9,731 |
469,351 |
|||||||||||||||||||
|
(Principal Executive Officer) |
||||||||||||||||||||||||||
|
Erika L. Eves (e) |
2022 |
$ |
181,667 |
$ |
1,680 |
$ |
— |
$ |
26,250 |
$ |
13,817 |
$ |
223,414 |
|||||||||||||
|
Vice President, |
2021 |
171,072 |
— |
23,481 |
29,614 |
12,563 |
236,730 |
|||||||||||||||||||
|
Finance & Administration |
||||||||||||||||||||||||||
|
(a) |
Amount represents the aggregate grant date fair value in the year granted in accordance with FASB ASC Topic 718. Assumptions made in the valuation of these awards are disclosed in Note 1(e) of the Notes to the Consolidated Financial Statements in this Form 10-K. |
|
(b) |
Amount represents the total non-equity incentive plan amounts which are disclosed for the year in which they were earned (i.e., the year to which the service relates). Non-equity incentive plan amounts for 2022 have not been approved by the Board of Directors as of the date of this filing and are therefore disclosed as 100% of the target amount. Once approved by the Board of Directors, the actual cash bonus amounts will be disclosed in a Current Report on Form 8-K. |
|
(c) |
Amount represents additional compensation as disclosed in the All Other Compensation Table below. |
|
(d) |
Dr. Rosol’s salary for the fiscal years ended December 31, 2022 and 2021 includes an additional $11,667 and $26,026, respectively, for his service on the Executive Leadership Committee. |
|
(e) |
Ms. Eves’s salary for the fiscal years ended December 31, 2022 and 2021 includes an additional $6,667 and $14,872, respectively, for her service on the Executive Leadership Committee. |
All Other Compensation
The following table describes each component of the amounts shown in the “All Other Compensation” column in the Summary Compensation Table above.
All Other Compensation Table for Fiscal 2022
|
Named Executive Officer |
Year |
(a) Employer Matching Contribution to 401(k) Plan |
(b) Employer Contribution to Health Savings Account |
Total All Other Compensation |
||||||||||
|
Michael S. Rosol, Ph.D. |
2022 |
$ |
7,231 |
$ |
1,000 |
$ |
8,231 |
|||||||
|
Chief Medical Officer |
2021 |
8,731 |
1,000 |
9,731 |
||||||||||
|
(Principal Executive Officer) |
||||||||||||||
|
Erika L. Eves |
2022 |
$ |
12,817 |
$ |
1,000 |
$ |
13,817 |
|||||||
|
Vice President, Finance & Administration |
2021 |
11,563 |
1,000 |
12,563 |
||||||||||
|
(a) |
Amount represents the value of the common stock accrued for contribution to the Named Executive Officer’s account in our 401(k) Plan as calculated on a quarterly basis. |
|
(b) |
Amount represents employer contributions to the Named Executive Officer’s Health Savings Account. |
Tax Consequences
The Tax Cuts and Jobs Act, which was enacted on December 22, 2017, included a number of significant changes to Section 162(m) of the Internal Revenue Code, such as the repeal of the qualified performance-based compensation exemption and the expansion of the definition of “covered employees” (for example, by including the chief financial officer and certain former Named Executive Officers as covered employees). As a result of these changes, except as otherwise provided in the transition relief provisions of the Tax Cuts and Jobs Act, compensation paid to any of our covered employees generally will not be deductible in 2022 or future years, to the extent that it exceeds $1 million.
Grants of Plan-Based Awards
The following table sets forth certain information about plan-based awards that we made to the Named Executive Officers during fiscal 2022. For information about the plans under which these awards were granted, see the discussion under “Short-Term Incentive Compensation” and “Long-Term Incentive Compensation” in the “Compensation Discussion and Analysis” section above.
Grants of Plan-Based Awards Table for Fiscal 2022
|
Estimated Future Payouts Under Non-Equity Incentive Plan Awards |
Estimated Future Payouts Under Equity Incentive Plan Awards |
All Other Stock Awards: Number of Shares |
All Other Option Awards: Number of Securities Underlying |
Exercise Price of Option |
Grant Date Fair Value of Stock and Option |
||||||||||||||||||||||||||||||
|
Named Executive Officer |
Grant Date |
Threshold |
Maximum |
Threshold |
Maximum |
of Stock |
Options |
Awards |
Awards |
||||||||||||||||||||||||||
|
Michael S. Rosol, Ph.D. |
N/A |
$ |
— |
$ |
81,250 |
— |
— |
— |
— |
$ |
— |
$ |
— |
(a) |
|||||||||||||||||||||
|
9/9/2022 |
— |
— |
— |
— |
13,000 |
— |
— |
3,640 |
(b) |
||||||||||||||||||||||||||
|
Erika L. Eves |
N/A |
$ |
— |
$ |
26,250 |
— |
— |
— |
— |
$ |
— |
$ |
— |
(a) |
|||||||||||||||||||||
|
9/9/2022 |
— |
— |
— |
— |
6,000 |
— |
— |
1,680 |
(b) |
||||||||||||||||||||||||||
|
(a) |
The Threshold column reflects the possibility that no minimum cash bonus awards will be payable. The Maximum column reflects the cash bonus awards payable if the Board of Directors, in its discretion, awards the maximum cash bonus. |
|
(b) |
These stock awards were made pursuant to the LTIP described above. The fair value on the date of grant is based on the closing price of our Common Stock of $0.28 per share. |
Outstanding Equity Awards
The following table presents certain information concerning outstanding equity awards held by the Named Executive Officers as of December 31, 2022.
Outstanding Equity Awards Table at Fiscal 2022 Year-End
|
Option Awards |
Stock Awards |
|||||||||||||||||||||||||||||
|
Number of Securities Underlying Unexercised Options (#) |
Market |
Equity Incentive Plan Awards |
||||||||||||||||||||||||||||
|
Named Executive |
Exercisable |
Unexercisable |
Option Exercise Price |
Option Expiration Date |
Note |
Number of Shares of Stock that Have Not Vested |
Stock Have Vested |
Number of Unearned Shares |
Market of Unearned Shares |
Note |
||||||||||||||||||||
|
Michael S. Rosol, |
6,250 | — | $ | 7.60 |
1/2/2029 |
(f) |
||||||||||||||||||||||||
|
Ph.D. |
16,667 | 8,333 | 1.06 |
2/6/2030 |
(h) |
|||||||||||||||||||||||||
| 8,333 | 16,667 | 2.56 |
2/15/2031 |
(i) |
||||||||||||||||||||||||||
| 18,750 | 81,250 | 1.08 |
12/27/2031 |
(j) |
||||||||||||||||||||||||||
|
Erika L. Eves |
625 | — | $ | 61.60 |
2/15/2023 |
(a) |
||||||||||||||||||||||||
| 625 | — | 35.40 |
1/28/2024 |
(b) |
||||||||||||||||||||||||||
| 625 | — | 33.00 |
3/26/2025 |
(c) |
||||||||||||||||||||||||||
| 1,000 | — | 10.20 |
4/25/2027 |
(d) |
||||||||||||||||||||||||||
| 1,200 | — | 7.20 |
2/20/2028 |
(e) |
||||||||||||||||||||||||||
| 2,400 | — | 3.00 |
2/7/2029 |
(g) |
||||||||||||||||||||||||||
| 4,000 | 2,000 | 1.06 |
2/6/2030 |
(h) |
||||||||||||||||||||||||||
| 4,167 | 8,333 | 2.56 |
2/15/2031 |
(i) |
||||||||||||||||||||||||||
|
(a) |
Options were granted February 15, 2013 and vested as to one-fourth on each of the first four anniversaries of the date of grant. |
|
(b) |
Options were granted January 28, 2014 and vested as to one-fourth on each of the first four anniversaries of the date of grant. |
|
(c) |
Options were granted March 26, 2015 and vested as to one-third on each of the first three anniversaries of the date of grant. |
|
(d) |
Options were granted April 25, 2017 and vested as to one-third on each of the first three anniversaries of the date of grant. |
|
(e) |
Options were granted February 20, 2018 and vested as to one-third on each of the first three anniversaries of the date of grant. |
|
(f) |
Options were granted January 2, 2019 and vested as to one-third on January 2, 2019, July 2, 2019 and January 2, 2020. |
|
(g) |
Options were granted February 7, 2019 and vest as to one-third on each of the first three anniversaries of the date of grant. |
|
(h) |
Options were granted February 6, 2020 and vest as to one-third on each of the first three anniversaries of the date of grant. |
|
(i) |
Options were granted February 15, 2021 and vest as to one-third on each of the first three anniversaries of the date of grant. |
|
(j) |
Options were granted December 27, 2021 and vest quarterly over four years beginning April 1, 2022. |
Options Exercised and Stock Vested
The following table presents, with respect to the Named Executive Officers, certain information about option exercises and restricted stock vested during fiscal 2022.
Options Exercised and Stock Vested Table for Fiscal 2022
|
Option Awards |
Stock Awards |
|||||||||||||||||
|
Named Executive Officer |
Number of Shares Acquired on Exercise |
Value Realized on Exercise |
Number of Shares Acquired on Vesting |
Value Realized on Vesting |
Note |
|||||||||||||
|
Michael S. Rosol, Ph.D. |
— |
$ |
— |
— |
$ |
— |
||||||||||||
|
Erika L. Eves |
— |
— |
— |
— |
||||||||||||||
Compensation of Non-Employee Directors
In October 2021, the Board of Directors retained the services of a compensation consultant, F.W. Cook, to evaluate the compensation of the non-employee directors. Based on the recommendation of F.W. Cook, our Board of Directors adopted a non-employee director compensation policy, beginning November 16, 2021. Under the policy, our non-employee directors are eligible to receive the following cash compensation for their services:
|
● |
an annual retainer of $42,500 for each Board member; |
|
● |
an additional annual retainer of $50,000 for the Chair of the Board; |
|
● |
an additional annual retainer of $35,000 for the Vice Chair of the Board; |
|
● |
an annual retainer of $10,000 for each Audit Committee member; |
|
● |
an additional annual retainer of $10,000 for the Chair of the Audit Committee; |
|
● |
an annual retainer $7,500 for each CNG Committee member; |
|
● |
an additional annual retainer of $7,500 for the Chair of the CNG Committee; and |
|
● |
an additional annual retainer of $100,000 for each member of the Board Oversight Committee. (a) |
|
(a) |
Mr. Cappello and Mr. Scott waived their Board Oversight Committee retainers effective July 1, 2022 and February 1, 2022, respectively. |
On November 16, 2021, each non-employee director received a grant of 30,000 shares of unrestricted common stock, which were payable in equal monthly issuances over 12 months, as well as 30,000 shares of restricted stock that vest as to one-third on each of the first three anniversaries of the date of grant. Following his September 2022 appointment to the Board of Directors, on November 3, 2022 Mr. Wilson was awarded 30,000 shares of restricted stock that vest as to one-third on each of the first three anniversaries of the date of grant.
The policy also provides for the reimbursement of our non-employee directors for reasonable and documented travel expenses to attend meetings of our Board of Directors and committees of our Board of Directors.
The aggregate number of equity awards outstanding as December 31, 2022 for each Director is set forth in the footnotes to the beneficial ownership table provided in the section entitled “Principal Stockholders.” Directors who are also officers or employees of Navidea do not receive any compensation for their services as directors.
The following table sets forth information concerning the compensation of our non-employee directors for the fiscal year ended December 31, 2022.
|
Name |
Fees Earned or Paid in Cash (a) |
Option Awards |
Stock Awards (b),(c),(d) |
All Other Compensation |
Total Compensation |
|||||||||||||||
|
Amit Bhalla |
$ | 52,500 | $ | — | $ | 16,813 | $ | — | $ | 69,313 | ||||||||||
|
Alexander L. Cappello (e) |
160,000 | — | 16,813 | — | 176,813 | |||||||||||||||
|
John K. Scott, Jr. (f) |
93,333 | — | 16,813 | — | 110,146 | |||||||||||||||
|
Joshua M. Wilson (g) |
10,625 | — | 7,092 | — | 17,717 | |||||||||||||||
|
Malcolm G. Witter |
77,500 | — | 16,813 | — | 94,313 | |||||||||||||||
|
(a) |
Amount represents fees earned during the fiscal year ended December 31, 2022 (i.e., the year to which the service relates). Monthly retainers are paid during the month in which they are earned. During 2022, Messrs. Bhalla, Scott, Wilson and Witter elected to defer receipt of fees payable in cash until the Company raises sufficient additional capital. Effective October 1, 2022, Mr. Cappello also elected to defer receipt of fees payable in cash until the Company raises sufficient additional capital. The value of the deferred cash payments is included in this amount. |
|
(b) |
Amounts shown do not reflect compensation actually received by the director but represent the aggregate grant date fair value in accordance with FASB ASC Topic 718. Amounts include the value of restricted stock awards as well as the value of unrestricted common stock issued or to be issued for fees earned during the fiscal year ended December 31, 2022 (i.e., the year to which the service relates). During 2022, Messrs. Scott and Witter elected to defer receipt of fees payable in unrestricted common stock until further notice. Effective October 1, 2022, Messrs. Bhalla and Cappello also elected to defer receipt of fees payable in unrestricted common stock until further notice. The value of the deferred stock grants is included in the Stock Awards column. On November 3, 2022, Mr. Wilson was issued 30,000 shares of restricted stock that vest as to one-third on each of the first three anniversaries of the date of grant. |
|
(c) |
As of December 31, 2022, the current non-employee directors held an aggregate of 90,000 shares of unvested restricted stock, with Messrs. Bhalla, Cappello and Witter each holding 20,000 shares, and Mr. Wilson holding 30,000 shares of unvested restricted stock. |
|
(d) |
During the year ended December 31, 2022, non-employee directors earned an aggregate of 105,000 shares of unrestricted common stock in partial payment of their fees. Of that amount, a total of 45,000 shares of unrestricted common stock were issued during the year ended December 31, 2022. As of December 31, 2022, a total of 72,869 shares of unrestricted common stock were deferred until further notice. |
|
(e) |
Mr. Cappello’s cash fees for the fiscal year ended December 31, 2022 include $50,000 for his service on the Board Oversight Committee. Mr. Cappello waived his Board Oversight Committee retainer effective July 1, 2022. |
|
(f) |
Mr. Scott’s cash fees for the fiscal year ended December 31, 2022 include $8,333 for his service on the Board Oversight Committee. Mr. Scott waived his Board Oversight Committee retainer effective February 1, 2022. |
|
(g) |
Mr. Wilson joined the Board of Directors effective September 30, 2022. The amounts shown reflect prorated cash fees and the value of restricted stock awarded to Mr. Wilson on November 3, 2022. |
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Equity Compensation Plan Information
The following table sets forth additional information as of December 31, 2022, concerning shares of our Common Stock that may be issued upon the exercise of options and other rights under our existing equity compensation plans and arrangements, divided between plans approved by our stockholders and plans or arrangements not submitted to our stockholders for approval. The information includes the number of shares covered by, and the weighted average exercise price of, outstanding options and other rights and the number of shares remaining available for future grants excluding the shares to be issued upon exercise of outstanding options, warrants, and other rights.
|
Plan Category |
(1) Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights |
(2) Weighted- Average Exercise Price of Outstanding Options, Warrants and Rights |
(3) Number of Securities Remaining Available for Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (1)) |
|||||||||
|
Equity compensation plans approved by security holders (a) |
702,805 | $ | 4.42 | 6,421,430 | ||||||||
|
Equity compensation plans not approved by security holders |
— | — | — | |||||||||
|
Total |
702,805 | $ | 4.42 | 6,421,430 | ||||||||
|
(a) |
Our stockholders ratified the 2014 Stock Incentive Plan (the “2014 Plan”) at the 2014 Annual Meeting of Stockholders held on July 17, 2014 and amended the 2014 Plan at the 2018, 2020 and 2022 Annual Meetings of Stockholders held on August 16, 2018, September 10, 2020 and December 8, 2022, respectively. The total number of shares available for awards under the 2014 Plan shall not exceed 7,750,000 shares, plus any shares subject to outstanding awards granted under prior plans and that expire or terminate for any reason. Although awards are still outstanding under the Fourth Amended and Restated 2002 Stock Incentive Plan (the “2002 Plan”), the 2002 Plan has expired and no new grants may be made from it. The total number of securities to be issued upon exercise of outstanding options includes 697,355 shares underlying options granted under the 2014 Plan and 5,450 shares underlying options granted under the 2002 Plan. |
Security Ownership of Principal Stockholders, Directors, Nominees and Executive Officers and Related Stockholder Matters
The following table sets forth, as of March 17, 2023, certain information with respect to the beneficial ownership of shares of our Common Stock by: (i) each person known to us to be the beneficial owner of more than 5% of our outstanding shares of Common Stock, (ii) each director or nominee for director of our Company, (iii) each of the Named Executive Officers, and (iv) our directors and executive officers as a group. Except as indicated in the footnotes to this table, the persons named in the table have sole voting and investment power with respect to all shares of our Common Stock shown as beneficially owned by them, subject to community property laws, where applicable. Percentage ownership is based on 32,851,252 shares of our Common Stock outstanding as of March 17, 2023. Shares underlying options or other rights to acquire our Common Stock that are exercisable within 60 days of March 17, 2023 are considered outstanding for the purpose of computing the percentage ownership of the person holding such options or other rights, but are not deemed outstanding for computing the percentage ownership of any other persons. The address of all directors and executive officers is c/o Navidea Biopharmaceuticals, Inc., 4995 Bradenton Avenue, Suite 240, Dublin, OH 43017.
|
Directors and Executive Officers |
Number of Shares Beneficially Owned |
Percent of Class |
|||||||
|
Amit Bhalla |
54,318 |
(a) |
* | ||||||
|
Alexander L. Cappello |
47,727 |
(b) |
* | ||||||
|
Erika L. Eves |
66,166 |
(c) |
* | ||||||
|
Michael S. Rosol, Ph.D. |
160,050 |
(d) |
* | ||||||
|
John K. Scott, Jr. |
18,785,384 |
(e) |
43.1 | % | |||||
|
Joshua M. Wilson |
— |
(f) |
* | ||||||
|
Malcolm G. Witter |
658,698 |
(g) |
2.0 | % | |||||
|
All directors and current executive officers as a group (7 persons) |
19,772,343 | 44.7 | % | ||||||
|
5% Beneficial Owners |
|||||||||
|
Irwin Bain |
1,946,617 |
(h) |
5.8 | % | |||||
|
(*) |
Less than one percent. |
|
|
(a) |
This amount includes 2,500 shares issuable upon exercise of options which are exercisable within 60 days and 3,750 shares that Mr. Bhalla has the right to receive within 60 days but has elected to defer, but does not include 20,000 shares of unvested restricted stock. |
|
|
(b) |
This amount includes 3,750 shares that Mr. Cappello has the right to receive within 60 days but has elected to defer, but does not include 20,000 shares of unvested restricted stock. |
|
|
(c) |
This amount includes 20,808 shares issuable upon exercise of options which are exercisable within 60 days and 35,729 shares in Ms. Eves’s account in the 401(k) Plan, but does not include 118,167 shares issuable upon exercise of options which are not exercisable within 60 days. |
|
|
(d) |
This amount includes 79,167 shares issuable upon exercise of options which are exercisable within 60 days and 25,158 shares in Dr. Rosol’s account in the 401(k) Plan, but does not include 324,083 shares issuable upon exercise of options which are not exercisable within 60 days. |
|
|
(e) |
This amount includes (i) 2,639 shares owned by Mr. Scott’s spouse, (ii) 7,500 shares owned by Mr. Scott’s children, (iii) 32,483 shares that Mr. Scott has the right to receive within 60 days but has elected to defer, (iv) 5,332,800 shares issuable upon conversion of Series I Convertible Preferred Stock and (v) 5,332,800 shares issuable upon exercise of warrants. Mr. Scott will not have the right to convert the Series I Preferred Stock or exercise the warrants to the extent that such conversion or exercise would cause Mr. Scott, together with his affiliates, to beneficially own in excess of 4.99% of the then outstanding common stock following such conversion or exercise. This amount excludes 30,000 shares of unvested restricted stock that Mr. Scott has the right to receive within 60 days but has elected to defer. |
|
|
(f) |
This amount does not include 30,000 shares of unvested restricted stock. |
|
|
(g) |
This amount includes (i) 2,500 shares issuable upon exercise of options which are exercisable within 60 days, (ii) 32,886 shares that Mr. Witter has the right to receive within 60 days but has elected to defer, (iii) 255,530 shares issuable upon conversion of Series I Convertible Preferred Stock and (iv) 255,530 shares issuable upon exercise of warrants. This amount excludes 20,000 shares of unvested restricted stock. |
|
|
(h) |
The number of shares beneficially owned is based on a Schedule 13G filed by Irwin Bain with the SEC on October 6, 2022. The address of Irwin Bain is 185 South Drexel Avenue, Bexley, OH 43209. |
All of our employees and directors, or any of their designees, are prohibited from (i) purchasing financial instruments (including prepaid variable forward contracts, equity swaps, collars, and exchange funds), or (ii) otherwise engaging in transactions (including “short sales” and arrangements involving a non-recourse pledge of securities), that hedge or offset, or are designed to hedge or offset, any decrease in the market value of shares of our common stock granted to such employee or director, or any of their designees, as part of their compensation, or held (directly or indirectly) by such employee or director, or any of their designees.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Certain Relationships and Related Transactions
We adhere to our Code of Business Conduct and Ethics, which states that no director, officer or employee of Navidea should have any personal interest that is incompatible with the loyalty and responsibility owed to our Company. We adopted a written policy regarding related party transactions in December 2015. When considering whether to enter into or ratify a related party transaction, the Audit Committee considers a variety of factors including, but not limited to, the nature and type of the proposed transaction, the potential value of the proposed transaction, the impact on the actual or perceived independence of the related party and the potential value to the Company of entering into such a transaction. All proposed transactions with a potential value of greater than $120,000 must be approved or ratified by the Audit Committee.
SEC disclosure rules regarding transactions with related persons require the Company to provide information about transactions with directors and executive officers as related persons, even though they may not have been related persons at the time the Company entered into the transactions described below.
Dr. Goldberg and Platinum
Dr. Michael Goldberg, our former President and Chief Executive Officer, previously managed a portfolio of funds for Platinum-Montaur Life Sciences LLC (“Platinum-Montaur”), an affiliate of Platinum Management (NY) LLC, Platinum Partners Value Arbitrage Fund L.P. (“PPVA”), Platinum Partners Capital Opportunity Fund (“PPCO”), Platinum Partners Liquid Opportunity Master Fund L.P., Platinum Liquid Opportunity Management (NY) LLC, and Montsant Partners LLC (collectively, “Platinum”), from May 2007 until December 2013.
In March 2017, the Company repaid to PPCO an aggregate of approximately $7.7 million in full satisfaction of the Company’s liabilities, obligations and indebtedness under the Platinum Loan Agreement between the Company and Platinum-Montaur, which were transferred by Platinum-Montaur to PPCO (the “Platinum Debt”). Subsequently, competing claims were made by Dr. Goldberg and by PPVA to the unpaid portion of the Platinum Debt. Platinum commenced litigation against the Company in November 2017. Platinum and the Company settled their dispute and Platinum’s lawsuit was dismissed in February 2022.
Goldberg Agreement and Litigation
In August 2018, Dr. Michael Goldberg resigned from his positions as an executive officer and a director of Navidea. In connection with Dr. Goldberg’s resignation, Navidea and Dr. Goldberg entered into an Agreement (the “Goldberg Agreement”) which set forth the terms of the separation from service. Among other things, the Goldberg Agreement provided that Dr. Goldberg would be entitled to 1,175,000 shares of our Common Stock, representing in part payment of accrued bonuses and payment of the balance of the Platinum debt. A portion of the 1,175,000 shares to be issued to Dr. Goldberg would be held in escrow for up to 18 months in order to reimburse Navidea in the event that Navidea is obligated to pay any portion of the Platinum debt to a party other than Dr. Goldberg. Further, the Goldberg Agreement provided that the Company’s subsidiary, Macrophage Therapeutics, Inc. (“MT”), would redeem all of Dr. Goldberg’s preferred stock and issue to Dr. Goldberg super voting common stock equal to 5% of the outstanding shares of MT. In November 2018, the Company issued 925,000 shares of our Common Stock to Dr. Goldberg, 250,000 of which were placed in escrow in accordance with the Goldberg Agreement.
On February 11, 2019, Dr. Goldberg represented to the MT Board that he had, without MT Board or shareholder approval, created a subsidiary of MT, transferred all of the assets of MT into the subsidiary, and then issued himself stock in the subsidiary. On February 19, 2019, Navidea notified MT that it was terminating the sublicense in accordance with its terms, effective March 1, 2019, due to MT’s insolvency. On February 20, 2019, the MT Board removed Dr. Goldberg as President and Chief Executive Officer of MT and from any other office of MT to which he may have been appointed or in which he was serving. Dr. Goldberg remains a member of the MT Board, together with John K. Scott, Jr. and Dr. Michael S. Rosol. Mr. Scott is also the Vice Chair of the Board of Directors of Navidea. On or about February 17, 2022, the Joint Official Liquidators and Foreign Representatives of PPVA executed the necessary paperwork to transfer its preferred stock in MT to Navidea.
New York Litigation Involving Dr. Goldberg
On February 20, 2019, Navidea filed a complaint against Dr. Goldberg in the United States District Court, Southern District of New York (the “District Court”), alleging breach of the Goldberg Agreement, as well as a breach of the covenant of good faith and fair dealing and to obtain a declaratory judgment that Navidea’s performance under the Goldberg Agreement is excused and that Navidea is entitled to terminate the Goldberg Agreement as a result of Dr. Goldberg’s actions. On April 26, 2019, Navidea filed an amended complaint against Dr. Goldberg which added a claim for breach of fiduciary duty seeking damages related to certain actions Dr. Goldberg took while CEO of Navidea. On June 13, 2019, Dr. Goldberg answered the amended complaint and asserted counterclaims against Navidea and third-party claims against MT for breach of the Goldberg Agreement, wrongful termination, injunctive relief, and quantum meruit.
On December 26, 2019, the District Court ruled on several motions related to Navidea and MT and Dr. Goldberg that substantially limited the claims that Dr. Goldberg can pursue against Navidea and MT. Specifically, the District Court found that certain portions of Dr. Goldberg’s counterclaims against Navidea and third-party claims against MT failed to state a claim upon which relief can be granted. Additionally, the District Court ruled that actions taken by Navidea and MT, including reconstituting the MT board of directors, replacing Dr. Goldberg with Mr. Latkin as Chief Executive Officer of MT, terminating the sublicense between Navidea and MT, terminating certain research projects, and allowing MT intellectual property to revert back to Navidea, were not breaches of the Goldberg Agreement.
The District Court also rejected Dr. Goldberg’s claim for wrongful termination as Chief Executive Officer of MT. In addition, the District Court found that Dr. Goldberg lacked standing to seek injunctive relief to force the removal of Dr. Claudine Bruck and Michael Rice from MT’s Board of Directors, to invalidate all actions taken by the MT Board on or after November 29, 2018 (the date upon which Dr. Bruck and Mr. Rice were appointed by Navidea to the Board of MT), or to reinstate the terminated sublicense between Navidea and MT.
In addition, the District Court found Navidea’s breach of fiduciary duty claim against Dr. Goldberg for conduct occurring more than three years prior to the filing of the complaint to be time-barred and that Dr. Goldberg is entitled to an advancement of attorneys’ fees solely with respect to that claim. To avoid further litigation expenses, the Company agreed to indemnify Dr. Goldberg solely with respect to the breach of fiduciary duty claim.
On January 31, 2020, Goldberg filed a motion for leave to amend his complaint to add back in claims for breach of contract, breach of the implied covenant of good faith and fair dealing, quantum meruit and injunctive relief. On April 1, 2020, the District Court denied Dr. Goldberg’s motion for leave to amend in its entirety.
On January 27, 2020, Dr. Goldberg filed a motion seeking additional advancement from Navidea for fees in connection with the New York Action and the Delaware Action. Navidea opposed the motion and the District Court referred the matters to a Magistrate Judge. On July 9, 2020, the Magistrate Judge issued her Report and Recommendation which recommended that: (1) the District Court decline to exercise jurisdiction over Dr. Goldberg’s motion as it pertained to expenses and fees incurred in defense of the Delaware Action; (2) the District Court decline to award any fees to Dr. Goldberg for the breach of fiduciary duty without additional motion practice on the issue; (3) the District Court find that Dr. Goldberg is entitled to advancement of his expenses and fees reasonably incurred in the defense of the remainder of the New York action subject to Dr. Goldberg’s posting of an undertaking; and (4) establish a protocol by which Dr. Goldberg could establish the amounts due for advancement.
On August 24, 2020, in connection with Dr. Goldberg’s motion for advancement, the District Court adopted the Magistrate Judge’s report and recommendation and found that while Dr. Goldberg was not being granted advancement of fees and expenses incurred in connection with either the Delaware Action or the assertion of third-party claims against MT, the Court ruled that Dr. Goldberg was entitled to advancement for the defense of the remaining claims asserted against him by Navidea in the New York action. The Court adopted a protocol by which additional motion practice will occur to determine the appropriate amount of fees to be advanced. Once that decision is made by the Magistrate Judge, subject to review by the District Court, Navidea will need to advance those fees to Dr. Goldberg conditioned upon Dr. Goldberg agreeing to pay those fees back to Navidea if it is determined that he is not entitled to indemnification.
On May 27, 2021, the District Court ordered that: (1) Dr. Goldberg be awarded $14,955 for indemnification for his attorneys’ fees for his defense of the breach of fiduciary duty claim; (2) Dr. Goldberg be advanced $1,237.50 for his attorneys’ fees subject to repayment; (3) Navidea should not be required to indemnify or advance any of the costs sought by Dr. Goldberg; (4) Dr. Goldberg is not entitled to advancement for the prosecution of his counterclaims and third-party claims; (5) Dr. Goldberg’s motion to hold Navidea in contempt be denied; and (6) Navidea should not be required to advance any additional fees or costs unless Dr. Goldberg presents his time records and costs in compliance with the District Court’s orders. The Company has made the payments ordered by the District Court.
On August 6, 2021, the Company moved for reconsideration of its obligations to advance fees in light of the Delaware Court’s decision dated June 23, 2021 (described below). On October 14, 2021, the Magistrate Judge recommended that Navidea’s motion for reconsideration be denied. On March 7, 2022, the District Court adopted the Report and Recommendation in part and permitted Dr. Goldberg to seek advancement for his fees incurred in defense of his claims since September 1, 2020. On April 8, 2022, Dr. Goldberg submitted a fee application seeking advancement of $143,172.55 for attorneys’ fees and disbursements for the time period September 1, 2020 through March 31, 2022. On March 15, 2023, the District Court adopted the Magistrate Judge’s report and recommendation that Dr. Goldberg’s application for fees allegedly incurred in connection with his defense of Navidea’s claims be denied as a sanction for failure to comply with prior court orders and that his application for fees incurred in connection with the successful prosecution of his prior fee applications be approved in the amount of $12,600. On March 17, 2023, the District Court confirmed that no further claims for advancement will be accepted by the Court in light of its March 15, 2023 Order.
Fact discovery and expert discovery in the New York Action have been completed. The Company has moved to disqualify Dr. Goldberg’s damages expert and briefing in the District Court was submitted on April 1, 2022. On November 9, 2022, the District Court issued an opinion granting the Company’s motion in part and precluding Dr. Goldberg’s damages expert from testifying on all but two issues. The Company anticipates that a briefing schedule for motions for summary judgment will be entered by the Court.
Delaware Litigation Involving Dr. Goldberg
On February 20, 2019, MT initiated a suit against Dr. Goldberg in the Court of Chancery of the State of Delaware (the “Delaware Court”), alleging, among other things, breach of fiduciary duty as a director and officer of MT and conversion, and to obtain a declaratory judgment that the transactions Dr. Goldberg caused MT to effect are void. On June 12, 2019, the Delaware Court found that Dr. Goldberg’s actions were not authorized in compliance with the Delaware General Corporate Law. Specifically, the Delaware Court found that Dr. Goldberg’s creation of a new subsidiary of MT and the purported assignment by Dr. Goldberg of MT’s intellectual property to that subsidiary were void. The Delaware Court’s ruling follows the order on May 23, 2019 in the case, in which it found Dr. Goldberg in contempt of its prior order holding Dr. Goldberg responsible for the payment of MT’s fees and costs to cure the damages caused by Dr. Goldberg’s contempt.
On June 23, 2021, the Delaware Court ruled in favor of MT and against Dr. Goldberg, finding that Dr. Goldberg breached his fiduciary duties to MT. Specifically, the Delaware Court ruled: “Dr. Goldberg attempted to take for himself that which belonged to [MT]. In doing so, he breached his duty of loyalty to [MT] stockholders. [MT] was absolutely justified in bringing this action to remedy (in this case undo) the harm caused by Dr. Goldberg’s misconduct.” The Delaware Court disagreed with MT’s arguments regarding damages and, other than awarding nominal damages, declined to award additional relief beyond that which it had previously granted. With respect to MT’s claim for conversion, the Delaware Court found that the claim was not supported because “Dr. Goldberg confirmed that he currently does not own or possess any intellectual property related to either Navidea or [MT]” and that “any IP Dr. Goldberg created while at Navidea or any of its subsidiaries was and remains the property of Navidea and its subsidiaries.” In addition, the Delaware Court denied Dr. Goldberg’s motion to hold MT’s directors and CEO in contempt, denied Dr. Goldberg’s motion to dismiss the lawsuit against him, and granted MT’s motion to dismiss Dr. Goldberg’s petition to remove MT’s board members. On December 9, 2021, Dr. Goldberg was ordered to reimburse MT in the amount of $66,796.33 and has paid that amount to MT. Neither party has appealed the Delaware Court’s decision and the Delaware Court’s decisions are now final.
See Note 12 to the accompanying consolidated financial statements.
Mr. Latkin and Platinum
Jed A. Latkin, our former Chief Executive Officer, Chief Operating Officer and Chief Financial Officer, was an independent consultant that served as a portfolio manager from 2011 through 2015 for two entities, namely Precious Capital and West Ventures, each of which were during that time owned and controlled, respectively, by PPVA and PPCO. Mr. Latkin was party to a consulting agreement with each of Precious Capital and West Ventures pursuant to which, as of April 2015, an aggregate of approximately $13 million was owed to him, which amount was never paid and Mr. Latkin has no information as to the current value. Mr. Latkin’s consulting agreements were terminated upon his ceasing to be an independent consultant in April 2015 with such entities. During his consultancy, Mr. Latkin was granted a .5% ownership interest in each of Precious Capital and West Ventures, however, to his knowledge he no longer owns such interests. In addition, PPVA owes Mr. Latkin $350,000 for unpaid consulting fees earned and expenses accrued in 2015 in respect of multiple consulting roles with them. Except as set forth above, Mr. Latkin has no other past or present affiliations with Platinum.
Macrophage Therapeutics, Inc. and Platinum
In March 2015, MT, our previously wholly-owned subsidiary, entered into a Securities Purchase Agreement to sell up to 50 shares of its Series A Convertible Preferred Stock (“MT Preferred Stock”) and warrants to purchase up to 1,500 common shares of MT (“MT Common Stock”) to Platinum and Dr. Goldberg (collectively, the “MT Investors”) for a purchase price of $50,000 per unit. A unit consisted of one share of MT Preferred Stock and 30 warrants to purchase MT Common Stock. Under the agreement, 40% of the MT Preferred Stock and warrants are committed to be purchased by Dr. Goldberg, and the balance by Platinum. The full 50 shares of MT Preferred Stock and warrants to be sold under the agreement are convertible into, and exercisable for, MT Common Stock representing an aggregate 1% interest on a fully converted and exercised basis. Navidea owns the remainder of the MT Common Stock. On March 11, 2015, definitive agreements with the MT Investors were signed for the sale of the first tranche of 10 shares of MT Preferred Stock and warrants to purchase 300 shares of MT Common Stock to the MT Investors, with gross proceeds to MT of $500,000. Platinum has since transferred its interests in MT to Navidea.
Director Independence
Our Board of Directors has adopted the definition of “independence” as described under the Sarbanes-Oxley Act of 2002 (Sarbanes-Oxley) Section 301, Rule 10A-3 under the Exchange Act and Section 803A of the NYSE American Company Guide. Our Board of Directors has determined that Messrs. Bhalla, Cappello, Scott and Witter meet the independence requirements.
Item 14. Principal Accountant Fees and Services
Our independent public accounting firm is Marcum LLP, PCAOB Auditor ID: 688. The Audit Committee has selected Marcum LLP as our independent registered public accounting firm for the years ended December 31, 2022 and 2021. In addition to retaining Marcum LLP to audit our consolidated financial statements for years ended December 31, 2022 and 2021, we may engage the firm from time to time during the year to perform other services.
Audit Fees. The aggregate fees billed and expected to be billed for professional services rendered by Marcum LLP, primarily related to the audit of the Company’s annual consolidated financial statements for the 2022 fiscal year, the reviews of the financial statements included in the Company’s Quarterly Reports on Form 10-Q for the 2022 fiscal year, and review of other SEC filings, were $439,546 (including direct engagement expenses).
The aggregate fees billed for professional services rendered by Marcum LLP, primarily related to the audit of the Company’s annual consolidated financial statements for the 2021 fiscal year, the reviews of the financial statements included in the Company’s Quarterly Reports on Form 10-Q for the 2021 fiscal year, and review of other SEC filings, were $347,055 (including direct engagement expenses).
Audit-Related Fees. No fees were billed by Marcum LLP for audit-related services for the 2022 or 2021 fiscal years.
Tax Fees. No fees were billed by Marcum LLP for tax-related services for the 2022 or 2021 fiscal years.
All Other Fees. No fees were billed by Marcum LLP for services other than those discussed above for the 2022 or 2021 fiscal years.
Pre-Approval Policy. The Audit Committee is required to pre-approve all auditing services and permitted non-audit services (including the fees and terms thereof) to be performed for the Company by its independent auditor or other registered public accounting firm, subject to the de minimis exceptions for permitted non-audit services described in Section 10A(i)(1)(B) of the Exchange Act that are approved by the Audit Committee prior to completion of the audit. The Audit Committee, through the function of the Chairman, has given general pre-approval for 100% of specified audit, audit-related, tax and other services.
PART IV
Item 15. Exhibits, Financial Statement Schedules
The following documents are filed as part of this report:
|
(1) |
The following Financial Statements are included in this Annual Report on Form 10-K on the pages indicated below: |
|
Report of Independent Registered Public Accounting Firm |
F-2 |
|
Consolidated Balance Sheets as of December 31, 2022 and 2021 |
F-4 |
|
Consolidated Statements of Operations for the years ended December 31, 2022 and 2021 |
F-6 |
|
Consolidated Statements of Stockholders’ (Deficit) Equity for the years ended December 31, 2022 and 2021 |
F-7 |
|
Consolidated Statements of Cash Flows for the years ended December 31, 2022 and 2021 |
F-8 |
|
Notes to the Consolidated Financial Statements |
F-9 |
|
(2) |
Financial statement schedules have been omitted because either they are not required or are not applicable or because the information required to be set forth therein is not material. |
|
(3) |
Exhibits: |
|
Exhibit Number |
Exhibit Description |
|
|
3.1 |
Amended and Restated Certificate of Incorporation of Navidea Biopharmaceuticals, Inc., as corrected February 18, 1994, and amended June 27, 1994, July 25, 1995, June 3, 1996, March 17, 1999, May 9, 2000, June 13, 2003, July 29, 2004, June 22, 2005, November 20, 2006, December 26, 2007, April 30, 2009, July 27, 2009, August 2, 2010, January 5, 2012, June 26, 2013 and August 18, 2016) (incorporated by reference to Exhibit 3.1 to the Company’s Annual Report on Form 10-K filed March 31, 2017). |
|
|
3.2 |
Certificate of Amendment to Amended and Restated Certificate of Incorporation of Navidea Biopharmaceuticals, Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed April 26, 2019). |
|
|
3.3 |
Second Amended and Restated By-Laws dated July 21, 1993, as amended July 18, 1995, May 30, 1996, July 26, 2007, November 7, 2013, April 2, 2021 and September 26,2022 (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed September 30, 2022). |
|
|
4.1 |
Certificate of Designations, Voting Powers, Preferences, Limitations, Restrictions, and Relative Rights of Series C Redeemable Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed May 12, 2020). |
|
|
4.2 |
Certificate of Elimination (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed September 2, 2020). |
|
|
4.3 |
Certificate of Designations, Voting Powers, Preferences, Limitations, Restrictions, and Relative Rights of Series D Redeemable Convertible Preferred Stock (incorporated by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K filed September 2, 2020). |
|
|
4.4 |
Certificate of Designations, Voting Powers, Preferences, Limitations, Restrictions, and Relative Rights of Series E Redeemable Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed March 4, 2021). |
|
|
4.5 |
Amended and Restated Certificate of Designations, Voting Powers, Preferences, Limitations, Restrictions, and Relative Rights of Series B Cumulative Convertible Preferred Stock (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed June 26, 2013). |
|
|
4.6 |
Form of Common Stock Certificate (incorporated by reference to Exhibit 4.3 to the Company’s Registration Statement on Form S-3 filed December 31, 2019). |
|
|
4.7 |
Description of Securities (incorporated by reference to Exhibit 4.3 to the Company’s Annual Report on Form 10-K filed March 18, 2020). |
|
|
4.8 |
Registration Rights Agreement, dated December 6, 2019, among Navidea Biopharmaceuticals, Inc. and the stockholders named therein (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed December 11, 2019). |
|
|
4.9 |
Form of Underwriter Warrants (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed June 17, 2019). |
|
|
4.10 |
Registration Rights Agreement, dated February 13, 2020, by and between Navidea Biopharmaceuticals, Inc. and John K. Scott, Jr. (incorporated by reference to Exhibit 4.5 to the Company’s Registration Statement on Form S-3 filed August 25, 2020). |
|
|
4.11 |
Form of Indenture (incorporated by reference to Exhibit 4.6 to the Company’s Registration Statement on Form S-3 filed February 8, 2021). |
|
|
4.12 |
Registration Rights Agreement, dated March 2, 2021, by and between Navidea Biopharmaceuticals, Inc. and John K. Scott, Jr. (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed March 4, 2021). |
|
|
4.13 |
Certificate of Designation of Series H Junior Participating Preferred Stock of Navidea Biopharmaceuticals, Inc. (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed April 12, 2022). |
|
|
4.14 |
Certificate of Designation of Voting Powers, Preferences, Limitations, Restrictions and Relative Rights of Series F Redeemable Convertible Preferred Stock of Navidea Biopharmaceuticals, Inc. (incorporated by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K filed April 12, 2022). |
|
Exhibit Number |
Exhibit Description |
|
4.15 |
Certificate of Designations of Voting Powers, Preferences, Limitations, Restrictions and Relative Rights of Series G Redeemable Preferred Stock of Navidea Biopharmaceuticals, Inc. (incorporated by reference to Exhibit 3.3 to the Company’s Current Report on Form 8-K filed April 12, 2022). |
|
|
4.16 |
Section 382 Rights Agreement, dated as of April 7, 2022, between Navidea Biopharmaceuticals, Inc. and Continental Stock Transfer & Trust Company, LLC, as Rights Agent (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed April 12, 2022). |
|
|
4.17 |
Registration Rights Agreement effective as of April 10, 2022 by and between Navidea Biopharmaceuticals, Inc. and John K. Scott, Jr. (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed April 12, 2022). |
|
|
4.18 |
Certificate of Designation of Preferences, Rights and Limitations of Series I Convertible Preferred Stock of Navidea Biopharmaceuticals, Inc. (incorporated by reference to Exhibit 4.18 to the Company’s Pre-Effective Amendment No. 3 to Form S-1 Registration Statement filed August 2, 2022). |
|
|
4.19 |
Form of Warrant to Purchase Common Stock (incorporated by reference to Exhibit 4.19 to the Company’s Pre-Effective Amendment No. 3 to Form S-1 Registration Statement filed August 2, 2022). |
|
|
4.20 |
Form of Warrant Agency Agreement between Navidea Biopharmaceuticals, Inc. and Continental Stock Transfer & Trust Company, LLC (incorporated by reference to Exhibit 4.20 to the Company’s Pre-Effective Amendment No. 3 to Form S-1 Registration Statement filed August 2, 2022). |
|
|
4.21 |
Form of Subscription Rights Certificate (incorporated by reference to Exhibit 4.21 to the Company’s Pre-Effective Amendment No. 3 to Form S-1 Registration Statement filed August 2, 2022). |
|
|
4.22 |
First Amendment to Section 382 Rights Agreement, dated as of January 10, 2023, between Navidea Biopharmaceuticals, Inc. and Continental Stock Transfer & Trust Company, LLC, as Rights Agent (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed January 10, 2023). |
|
|
10.1 |
License Agreement, dated December 9, 2011, between AstraZeneca AB and the Company (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the U.S. Securities and Exchange Commission) (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K/A filed April 11, 2012). |
|
|
10.2 |
Series HH Warrant to purchase Common Stock of Navidea Biopharmaceuticals, Inc. issued to GE Capital Equity Investments, Inc., dated June 25, 2013 (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K/A filed June 28, 2013). |
|
|
10.3 |
Series HH Warrant to purchase Common Stock of Navidea Biopharmaceuticals, Inc. issued to MidCap Financial SBIC, LP, dated June 25, 2013 (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K/A filed June 28, 2013). |
|
|
10.4 |
Office Lease, dated August 29, 2013, by and between Navidea Biopharmaceuticals, Inc. and BRE/COH OH LLC (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the U.S. Securities and Exchange Commission) (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed September 5, 2013). |
|
|
10.5 |
License Agreement, dated July 14, 2014, between the Company and the Regents of the University of California (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the U.S. Securities and Exchange Commission) (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed August 11, 2014). |
|
|
10.6 |
Form of Stock Option Agreement under the Navidea Biopharmaceuticals, Inc. 2014 Stock Incentive Plan (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed November 10, 2014). ^ |
|
|
10.7 |
Form of Restricted Stock Award and Agreement under the Navidea Biopharmaceuticals, Inc. 2014 Stock Incentive Plan (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed November 10, 2014). ^ |
|
|
10.8 |
Securities Exchange Agreement dated as of March 11, 2015 among Macrophage Therapeutics, Inc., Platinum-Montaur Life Sciences, LLC and Michael Goldberg, M.D. (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed May 11, 2015). |
|
Exhibit Number |
Exhibit Description |
|
10.9 |
Term Loan Agreement, dated as of May 8, 2015, by and among Navidea Biopharmaceuticals, Inc., as borrower, Macrophage Therapeutics, Inc. as guarantor, and Capital Royalty Partners II L.P., Capital Royalty Partners II – Parallel Fund “A” L.P. and Parallel Investment Opportunities Partners II L.P., as lenders (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K/A filed October 9, 2015). |
|
|
10.10 |
Security Agreement, dated as of May 15, 2015 among Navidea Biopharmaceuticals, Inc., as borrower, Macrophage Therapeutics, Inc. as guarantor, and Capital Royalty Partners II L.P., Capital Royalty Partners II – Parallel Fund “A” L.P. and Parallel Investment Opportunities Partners II L.P., as lenders, and Capital Royalty Partners II L.P., as control agent (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed May 15, 2015). |
|
|
10.11 |
Form of Series LL Warrant issued to Montsant Partners LLC and Platinum Partners Value Arbitrage Fund, L.P. (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed August 26, 2015). |
|
|
10.12 |
Amendment 1 to Term Loan Agreement by and among Navidea Biopharmaceuticals, Inc., as borrower, and Capital Royalty Partners II L.P., Capital Royalty Partners II – Parallel Fund “A” L.P. and Parallel Investment Opportunities Partners II L.P., as lenders, dated as of December 23, 2015 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed January 11, 2016). |
|
|
10.13 |
Form of Director Agreement (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed May 10, 2016). |
|
|
10.14 |
Asset Purchase Agreement, dated November 23, 2016, between Navidea Biopharmaceuticals, Inc. and Cardinal Health 414, LLC (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed November 30, 2016). |
|
|
10.15 |
Global Settlement Agreement dated March 3, 2017, by and among Navidea Biopharmaceuticals, Inc., Cardinal Health 414, LLC, Macrophage Therapeutics, Inc., Capital Royalty Partners II L.P., Capital Royalty Partners II (Cayman), L.P., Capital Royalty Partners II – Parallel Fund “A” L.P., Parallel Investment Opportunities Partners II L.P. and Capital Royalty Partners II – Parallel Fund “B” (Cayman) L.P. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed March 9, 2017). |
|
|
10.16 |
License-Back Agreement, dated March 3, 2017, between Navidea Biopharmaceuticals, Inc. and Cardinal Health 414, LLC (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed March 9, 2017). |
|
|
10.17 |
Series NN Warrant, dated March 3, 2017, issued to Cardinal Health 414, LLC (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed March 9, 2017). |
|
|
10.18 |
Series NN Warrant, dated March 3, 2017, issued to The Regents of the University of California (San Diego) (incorporated by reference to Exhibit 10.5 to the Company’s Current Report on Form 8-K filed March 9, 2017). |
|
|
10.19 |
Amended and Restated License Agreement, dated March 3, 2017, between Navidea Biopharmaceuticals, Inc. and The Regents of the University of California (San Diego) (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the Securities and Exchange Commission) (incorporated by reference to Exhibit 10.6 to the Company’s Current Report on Form 8-K filed March 9, 2017). |
|
|
10.20 |
Amendment to Asset Purchase Agreement dated April 2, 2018, between Navidea Biopharmaceuticals, Inc. and Cardinal Health 414, LLC (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed May 9, 2018). |
|
|
10.21 |
Agreement dated August 14, 2018, by and among Navidea Biopharmaceuticals, Inc., Macrophage Therapeutics, Inc. and Michael M. Goldberg, M.D. (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed November 9, 2018). |
|
|
10.22 |
Employment Agreement, effective October 1, 2018, by and between Navidea Biopharmaceuticals, Inc. and Jed A. Latkin (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed October 5, 2018).^ |
|
|
10.23 |
Form of Series OO Underwriter’s Common Stock Purchase Warrant issued to the underwriter’s designees on June 18, 2019 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed June 17, 2019). |
|
|
10.24 |
Termination Agreement, effective May 11, 2020, among Navidea Biopharmaceuticals, Inc., SpePharm AG, and Norgine BV (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed May 12, 2020). |
|
|
10.25 |
Employment Agreement, effective July 27, 2020, by and between Navidea Biopharmaceuticals, Inc. and Jed A. Latkin (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed July 31, 2020).^ |
|
Exhibit Number |
Exhibit Description |
|
10.26 |
Amended and Restated Equity Commitment Letter, dated August 14, 2020, by and between Navidea Biopharmaceuticals, Inc. and Mastiff Group, LLC (incorporated by reference to Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q filed August 14, 2020). |
|
|
10.27 |
Stock Purchase Agreement and Letter of Investment Intent, dated August 31, 2020, by and between Navidea Biopharmaceuticals, Inc. and Keystone Capital Partners, LLC (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed September 2, 2020). |
|
|
10.28 |
Stock Purchase Agreement, dated August 30, 2020, among Navidea Biopharmaceuticals, Inc., Mastiff Group, LLC and John K. Scott, Jr. (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed September 2, 2020). |
|
|
10.29 |
Stock Purchase Agreement and Letter of Investment Intent, dated March 2, 2021, by and between Navidea Biopharmaceuticals, Inc. and John K. Scott, Jr. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed March 4, 2021). |
|
|
10.30 |
Amendment to Stock Purchase Agreement and Letter of Investment Intent, dated July 8, 2021, by and between Navidea Biopharmaceuticals, Inc. and Keystone Capital Partners LLC (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed July 13, 2021). |
|
|
10.31 |
Separation Agreement and General Release, dated November 23, 2021, by and between Navidea Biopharmaceuticals, Inc. and Jed A. Latkin (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed November 26, 2021).^ |
|
|
10.32 |
Stock Exchange and Loan Agreement between Investor and Navidea Biopharmaceuticals, Inc., dated April 10, 2022 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed April 12, 2022). |
|
|
10.33 |
Secured Term Note, dated April 10, 2022 (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed April 12, 2022). |
|
|
10.34 |
Security Agreement dated as of April 10, 2022 by Navidea Biopharmaceuticals, Inc. in favor of John Kim Scott, Jr. (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed April 12, 2022). |
|
|
10.35 |
Navidea Biopharmaceuticals, Inc. 2014 Stock Incentive Plan (as amended and restated on August 16, 2018, September 10, 2020 and December 8, 2022) (incorporated by reference to Exhibit 10.1 to the Company’s Registration Statement on Form S-8 filed December 22, 2022). |
|
|
21.1 |
||
|
23.1 |
||
|
24.1 |
||
|
31.1 |
||
|
31.2 |
||
|
32.1 |
||
|
32.2 |
||
|
101.INS |
Inline XBRL Instance Document (the Instance Document does not appear in the Interactive Data File because it is XBRL) (1) |
|
|
101.SCH |
Inline XBRL Taxonomy Extension Schema Document (1) |
|
|
101.CAL |
Inline XBRL Taxonomy Extension Calculation Linkbase Document (1) |
|
|
101.DEF |
Inline XBRL Taxonomy Extension Definition Linkbase Document (1) |
|
|
101.LAB |
Inline XBRL Taxonomy Extension Label Linkbase Document (1) |
|
|
101.PRE |
Inline XBRL Taxonomy Extension Presentation Linkbase Document (1) |
|
|
104 |
Cover page Interactive Data File (formatted as Inline XBRL and combined in Exhibit 101.1) |
|
^ |
Management contract or compensatory plan or arrangement. |
|
* |
Filed herewith. |
|
** |
Furnished herewith. |
|
(1) |
These interactive data files shall not be deemed filed for purposes of Section 11 or 12 of the Securities Act of 1933, as amended, or Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to liability under those sections. |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Dated: March 27, 2023 | |||
|
NAVIDEA BIOPHARMACEUTICALS, INC. |
|||
|
(the Company) |
|||
|
By: |
/s/ Michael S. Rosol |
||
|
Michael S. Rosol, Ph.D. |
|||
|
Chief Medical Officer |
|||
|
(Principal Executive Officer) |
|||
|
By: |
/s/ Erika L. Eves |
||
|
Erika L. Eves |
|||
|
Vice President, Finance and Administration |
|||
|
(Principal Financial and Accounting Officer) |
|||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature |
Title |
Date |
|
/s/ Michael S. Rosol |
Chief Medical Officer |
March 27, 2023 |
|
Michael S. Rosol, Ph.D. |
(Principal Executive Officer) |
|
|
/s/ Erika L. Eves |
Vice President, Finance & Administration |
March 27, 2023 |
|
Erika L. Eves |
(Principal Financial Officer and Principal Accounting Officer) |
|
|
/s/ Alexander L. Cappello* |
Chairman of the Board of Directors |
March 27, 2023 |
|
Alexander L. Cappello |
||
|
/s/ John K. Scott, Jr.* |
Vice Chairman of the Board of Directors |
March 27, 2023 |
|
John K. Scott, Jr. |
||
|
/s/ Amit Bhalla* |
Director |
March 27, 2023 |
|
Amit Bhalla |
||
|
/s/ Joshua M. Wilson* |
Director |
March 27, 2023 |
|
Joshua M. Wilson |
||
|
/s/ Malcolm G. Witter* |
Director |
March 27, 2023 |
|
Malcolm G. Witter |
|
*By: |
/s/ Michael S. Rosol |
|
Michael S. Rosol, Ph.D., Attorney-in-fact |
NAVIDEA BIOPHARMACEUTICALS, INC.
FORM 10-K ANNUAL REPORT
As of December 31, 2022 and 2021
and for Each of the
Two Years in the Period Ended
December 31, 2022
FINANCIAL STATEMENTS
NAVIDEA BIOPHARMACEUTICALS, INC. and SUBSIDIARIES
Index to Consolidated Financial Statements
|
Consolidated Financial Statements of Navidea Biopharmaceuticals, Inc. |
|
|
Report of Independent Registered Public Accounting Firm (PCAOB ID |
F-2 |
|
Consolidated Balance Sheets as of December 31, 2022 and 2021 |
F-4 |
|
Consolidated Statements of Operations for the years ended December 31, 2022 and 2021 |
F-6 |
|
Consolidated Statements of Stockholders’ (Deficit) Equity for the years ended December 31, 2022 and 2021 |
F-7 |
|
Consolidated Statements of Cash Flows for the years ended December 31, 2022 and 2021 |
F-8 |
|
Notes to the Consolidated Financial Statements |
F-9 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
Navidea Biopharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Navidea Biopharmaceuticals, Inc. (the “Company”) as of December 31, 2022 and 2021, the related consolidated statements of operations, stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2022, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 2, the Company has experienced recurring net losses and has used significant cash to fund its operations. These conditions raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which they relate.
Valuation of Preferred Stock Exchanges
As described in Note 13 to the Company’s consolidated financial statements, on April 10, 2022, the Company entered into a Stock Exchange Agreement whereas the Company received a loan totaling $2.5 million. As consideration and a partial inducement to execute the transaction, the lender, who is also the holder of 100% of the Company's Series E Preferred Stock, delivered all 50,000 shares to the Company in exchange for the issuance of 1,740 shares of Series F Convertible Preferred Stock and 3,260 shares of Series G Preferred Stock.
Additionally, on August 30, 2022, the Company closed on the Rights Offering to its stockholders and certain warrant holders as of August 3, 2022 of the right to purchase up to 35,000 Units at a subscription price of $1,000 per Unit. Certain participants in the Rights Offering had the ability to pay the subscription price for their Units by cancelling or exchanging their shares of Series D Preferred Stock, Series F Preferred Stock and/or Series G Preferred Stock and the Company's indebtedness evidenced by a Bridge Note, instead of paying by check or wire transfer of funds.
Exchanges of preferred stock are recorded in the financial statements based on their estimated fair values at the date of exchange. The fair value of each series of preferred stock is estimated using the OPM Backsolve equity valuation method. The determination of fair value using the OPM Backsolve method is impacted by the Company stock price, the value of the most recent equity transaction and the features of each class of security, which can include rights such as liquidation preferences, required returns, conversion options, or other items. Differences in these features result in differences in value for each class of security. The OPM Backsolve method uses the economic rights, relationships and participation levels of the securities along with the Black-Scholes option pricing model to create an option-based equation for the equity capital structure of the Company. When the value of one class of equity security is known, the OPM Backsolve method provides the ability to calculate a fair value for all other equity securities.
We have identified the valuation of preferred stock exchanges as a critical audit matter. Changes in managements estimations and assumptions could have a material impact on the financial statements. Specifically, there was judgment applied by management in determining the estimated fair value at the date of the exchange. The valuation included making judgments about the methodologies and inputs to the valuation models. Auditing these matters involved especially challenging auditor effort due to the specialized skills and knowledge required to evaluate the valuation methodologies and the reasonableness of the inputs used to determine the estimated fair value at the date of the exchange.
How the Critical Audit Matter was Addressed in the Audit
Our audit procedures related to the valuation of preferred stock exchanges include the following, among others:
|
● |
To test the valuation of the instruments included in the exchanges, our audit procedures included, among others, evaluating the Company’s selection of valuation methodology, evaluating the methods and significant assumptions utilized by the Company’s valuation specialist, and testing the completeness and accuracy of the underlying data to support the significant assumptions, estimates, and inputs. |
|
● |
We involved our valuation specialist to assist with our evaluation of the methodology used by the Company and significant assumptions included in the fair value estimates. |
|
● |
Verified the mathematical accuracy of the valuation models used by the Company to determine the estimated fair values as of the date of the exchange. |
/s/
Marcum LLP
We have served as the Company’s auditor since 2016.
March 27, 2023
Navidea Biopharmaceuticals, Inc. and Subsidiaries
Consolidated Balance Sheets
|
December 31, |
||||||||
|
|
2022 |
2021 |
||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
|
Cash and cash equivalents |
$ | $ | ||||||
|
Receivables |
||||||||
|
Inventory, net |
||||||||
|
Prepaid expenses and other |
||||||||
|
Total current assets |
||||||||
|
Property and equipment |
||||||||
|
Less accumulated depreciation and amortization |
||||||||
|
Property and equipment, net |
||||||||
|
Right-of-use lease assets |
||||||||
|
Less accumulated amortization |
||||||||
|
Right-of-use lease assets, net |
||||||||
|
License agreements, patents and trademarks |
||||||||
|
Less accumulated amortization |
||||||||
|
License agreements, patents and trademarks, net |
||||||||
|
Other assets |
||||||||
|
Total assets |
$ | $ | ||||||
(continued)
Navidea Biopharmaceuticals, Inc. and Subsidiaries
Consolidated Balance Sheets (continued)
|
December 31, |
||||||||
|
|
2022 |
2021 |
||||||
| LIABILITIES AND STOCKHOLDERS’ (DEFICIT) EQUITY’ | ||||||||
| Current liabilities: | ||||||||
|
Accounts payable |
$ | $ | ||||||
|
Accrued liabilities and other |
||||||||
|
Notes payable, current |
||||||||
|
Lease liabilities, current |
||||||||
|
Deferred revenue, current |
||||||||
|
Total current liabilities |
||||||||
|
Lease liabilities, net of current portion |
||||||||
|
Note payable to related party, net of discount of $ |
||||||||
|
Deferred revenue |
||||||||
|
Total liabilities |
||||||||
| Commitments and contingencies (See Note 12) | ||||||||
| Stockholders’ (deficit) equity: | ||||||||
|
Preferred stock; $ par value; |
||||||||
|
Series D preferred stock; $ par value, |
||||||||
|
Series E preferred stock; $ par value, |
||||||||
|
Series G preferred stock; $ par value, |
||||||||
|
Series H preferred stock; $ par value, |
||||||||
|
Series I preferred stock; $ par value, |
||||||||
|
Common stock; $ par value; |
||||||||
|
Additional paid-in capital |
||||||||
|
Accumulated deficit |
( |
) |
( |
) |
||||
|
Total stockholders' deficit |
( |
) |
( |
) |
||||
|
Noncontrolling interest |
||||||||
|
Total Navidea stockholders’ (deficit) equity |
( |
) | ||||||
|
Total liabilities and stockholders’ (deficit) equity |
$ | $ | ||||||
See accompanying notes to consolidated financial statements.
Navidea Biopharmaceuticals, Inc. and Subsidiaries
Consolidated Statements of Operations
|
Years Ended December 31, |
||||||||
|
2022 |
2021 |
|||||||
| Revenue: | ||||||||
|
Sales revenue |
$ | $ | ||||||
|
License revenue |
||||||||
|
Grant and other revenue |
||||||||
|
Total revenue |
||||||||
|
Cost of revenue |
||||||||
|
Adjustments for expired and expiring inventory |
— | |||||||
|
Gross (loss) profit |
( |
) | ||||||
| Operating expenses: | ||||||||
|
Research and development |
||||||||
|
Selling, general and administrative |
||||||||
|
Total operating expenses |
||||||||
|
Loss from operations |
( |
) |
( |
) |
||||
| Other (expense) income: | ||||||||
|
Interest expense, net |
( |
) |
( |
) |
||||
|
Gain on extinguishment of debt |
||||||||
|
Other, net |
( |
) |
( |
) |
||||
|
Total other (expense) income, net |
( |
) | ||||||
|
Net loss before income taxes |
( |
) |
( |
) |
||||
|
Provision for income taxes |
( |
) | ||||||
|
Net loss |
( |
) |
( |
) |
||||
|
Net loss attributable to noncontrolling interest |
||||||||
|
Net loss attributable to Navidea and subsidiaries |
( |
) | ( |
) |
||||
|
Deemed dividend on preferred stock exchanged for Units |
( |
) |
||||||
|
Net loss attributable to common stockholders |
$ | ( |
) |
$ | ( |
) |
||
|
Loss attributable to common stockholders per common share (basic and diluted) |
$ | ( |
) |
$ | ( |
) |
||
|
Weighted average shares outstanding |
||||||||
See accompanying notes to consolidated financial statements.
Navidea Biopharmaceuticals, Inc. and Subsidiaries
Consolidated Statements of Stockholders’ (Deficit) Equity
|
Preferred Stock |
Preferred Stock Subscribed |
Preferred |
Common Stock Issued |
Common Stock Subscribed |
Common |
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
Shares |
Amount |
Shares |
Amount |
Stock Subscriptions Receivable |
Shares |
Amount |
Shares |
Amount |
Stock Subscriptions Receivable |
Additional Paid-In Capital | Accumulated Deficit | Non-controlling Interest | Total | |||||||||||||||||||||||||||||||||||||||||||
|
Balance, January 1, 2021 |
$ | $ | $ | ( |
) | $ | $ | $ | ( |
) | $ | $ | ( |
) | $ | $ | ||||||||||||||||||||||||||||||||||||||||
|
Issued restricted stock |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issued stock to 401(k) plan |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issued Series D Preferred Stock |
( |
) | ( |
) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issued stock upon conversion of Series D Preferred Stock |
( |
) | ( |
) | ( |
) | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Series D Preferred Stock subscribed |
( |
) | ( |
) | ( |
) | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issued Series E Preferred Stock, net of issuance costs |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issued stock upon stock option exercise |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issued stock in lieu of cash for payment of director fees |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Cancelled stock to pay employee tax obligations |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||||||||||||||||||||||||||||||||||||||||||
|
Common stock subscribed |
( |
) | ( |
) | ( |
) | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Stock compensation expense |
- | - | - | - | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Net loss |
- | - | - | - | ( |
) | ( |
) | ( |
) | ||||||||||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2021 |
( |
) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issued stock in lieu of cash bonuses |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issued stock to 401(k) Plan |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issued stock for payment of director fees |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
MT Preferred Stock reacquired due to Platinum settlement |
- | - | - | - | ( |
) | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Series E Preferred Stock exchanged for Series F and Series G Preferred Stock |
( |
) | ( |
) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issued Series I Preferred Stock, net of costs |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Series D and Series F Preferred Stock exchanged for Units in Rights Offering |
( |
) | ( |
) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Deemed dividend on Series D and Series F Preferred Stock exchanged for Units in Rights Offering |
- | - | - | - | ( |
) | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Series I Preferred Stock converted into Common Stock |
( |
) | ( |
) | ( |
) | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issued stock under long term incentive plan |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issued stock pursuant to warrant exercise |
( |
) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Issued restricted stock |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Stock compensation expense |
- | - | - | - | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Net loss |
- | - | - | - | ( |
) | ( |
) | ( |
) | ||||||||||||||||||||||||||||||||||||||||||||||
|
Balance, December 31, 2022 |
$ | - | $ | $ | - | $ | - | $ | $ | $ | $ | ( |
) | $ | $ | ( |
) | |||||||||||||||||||||||||||||||||||||||
See accompanying notes to consolidated financial statements.
Navidea Biopharmaceuticals, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
|
Years Ended December 31, |
||||||||
|
2022 |
2021 |
|||||||
| Cash flows from operating activities: | ||||||||
|
Net loss |
$ | ( |
) |
$ | ( |
) |
||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
|
Depreciation and amortization of property and equipment |
||||||||
|
Amortization of license agreements, patents and trademarks |
||||||||
|
Loss on disposal and abandonment of patents and equipment |
||||||||
|
Adjustments for expired and expiring inventory |
||||||||
|
Amortization of debt discount and issuance costs |
||||||||
|
Non-cash lease expense |
||||||||
|
Stock compensation expense |
||||||||
|
Gain on extinguishment of debt |
( |
) |
||||||
|
Value of stock issued to 401(k) plan for employer matching contributions |
||||||||
|
Value of stock issued in payment of employee bonuses |
||||||||
|
Value of stock issued in payment of director fees |
||||||||
|
Value of stock issued under long term incentive plan |
||||||||
| Changes in operating assets and liabilities: | ||||||||
|
Receivables |
( |
) |
||||||
|
Inventory |
( |
) | ||||||
|
Prepaid expenses and other assets |
||||||||
|
Accounts payable |
||||||||
|
Accrued and other liabilities |
||||||||
|
Lease liabilities |
( |
) |
( |
) |
||||
|
Deferred revenue |
||||||||
|
Net cash used in operating activities |
( |
) |
( |
) |
||||
| Cash flows from investing activities: | ||||||||
|
Payments for purchases of equipment |
( |
) |
( |
) |
||||
|
Patent and trademark costs |
( |
) |
( |
) |
||||
|
Net cash used in investing activities |
( |
) |
( |
) |
||||
| Cash flows from financing activities: | ||||||||
|
Proceeds from issuance of preferred stock and warrants, including collection of stock subscriptions |
||||||||
|
Payment of preferred stock issuance costs |
( |
) |
( |
) |
||||
|
Proceeds from issuance of common stock |
||||||||
|
Payment of tax withholdings related to stock-based compensation |
( |
) | ||||||
|
Proceeds from note payable |
||||||||
|
Payment of debt issuance costs |
( |
) |
|
|||||
|
Principal payments on notes payable |
( |
) |
( |
) |
||||
|
Net cash provided by financing activities |
||||||||
|
Net (decrease) increase in cash and cash equivalents |
( |
) | ||||||
|
Cash and cash equivalents, beginning of period |
||||||||
|
Cash and cash equivalents, end of period |
$ | $ | ||||||
See accompanying notes to consolidated financial statements.
Notes to the Consolidated Financial Statements
|
1. |
Organization and Summary of Significant Accounting Policies |
|
a. |
Organization and Nature of Operations: Navidea Biopharmaceuticals, Inc. (“Navidea,” the “Company,” or “we”), a Delaware Corporation (NYSE American: NAVB), is a biopharmaceutical company focused on the development and commercialization of precision immunodiagnostic agents and immunotherapeutics. Navidea is developing multiple precision-targeted products based on our Manocept platform to enhance patient care by identifying the sites and pathways of undetected disease and enable better diagnostic accuracy, clinical decision-making and targeted treatment. |
|
b. |
Principles of Consolidation: Our consolidated financial statements include the accounts of Navidea and our wholly-owned subsidiaries, Navidea Europe and Navidea UK, as well as those of our majority-owned subsidiary, MT. All significant inter-company accounts were eliminated in consolidation. |
|
c. |
Use of Estimates: The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. |
|
d. |
Revenue Recognition: We generate revenue from a grant to support one of our product development initiatives. We generally recognize grant revenue when expenses reimbursable under the grant have been paid and payments under the grant become contractually due. |
|
e. |
Stock-Based Compensation: As of December 31, 2022, we had instruments outstanding under two stock-based compensation plans; the Fourth Amended and Restated 2002 Stock Incentive Plan (“2002 Plan”) and the Amended and Restated 2014 Stock Incentive Plan (“2014 Plan”). Currently, under the 2014 Plan, we may grant incentive stock options, nonqualified stock options, and restricted stock awards to full-time employees and directors, and nonqualified stock options and restricted stock awards may be granted to our consultants and agents. Total shares authorized under the 2014 Plan is |
|
2022 |
2021 |
|||||||||||
|
Expected volatility |
- | - | ||||||||||
|
Weighted-average volatility |
||||||||||||
|
Expected forfeiture rate |
- | - | ||||||||||
|
Expected term (in years) |
- | - | ||||||||||
|
Risk-free rate |
- | - | ||||||||||
|
Expected dividends |
||||||||||||
|
f. |
Net Loss Per Share: Net loss per share is calculated in accordance with the two-class method. Under the two-class method, net loss is allocated between common stock and other participating securities based on their participation rights. We have determined that the outstanding nonvested restricted stock represents participating securities. Net losses are not allocated to the nonvested restricted stockholders for calculating net loss per share under the two-class method because nonvested restricted stockholders do not have contractual obligations to share in the losses of the Company. Basic net loss per share is calculated by dividing net loss attributable to common stockholders by the weighted average number of shares of common stock outstanding during the period, excluding the effects of any potentially dilutive instruments. Diluted net loss per share is calculated using the more dilutive of (a) the two-class method, or (b) treasury stock method, as applicable, to the potentially dilutive instruments. The weighted average number of shares of common stock outstanding during the period reflects additional common shares that would have been outstanding if dilutive potential shares of common stock had been issued. Potential shares of common stock that may be issued by the Company include convertible preferred stock, options and warrants. See Note 5. |
|
g. |
Research and Development Costs: R&D expenses include both internal R&D activities and external contracted services. Internal R&D activity expenses include salaries, benefits, and stock-based compensation, as well as travel, supplies, and other costs to support our R&D staff. External contracted services include clinical trial activities, manufacturing and control-related activities, and regulatory costs. R&D expenses are charged to operations as incurred. We review and accrue R&D expenses based on services performed and rely upon estimates of those costs applicable to the stage of completion of each project. |
|
h. |
Receivables: Receivables are recorded net of an allowance for doubtful accounts. We estimate an allowance for doubtful accounts based on a review and assessment of specific accounts and other receivables and write off accounts against the allowance account when deemed uncollectible. See Note 6. |
|
i. |
Inventory: All components of inventory are valued at the lower of cost (first-in, first-out) or net realizable value. We adjust inventory to net realizable value when the net realizable value is lower than the carrying cost of the inventory. Net realizable value is determined based on estimated sales activity and margins. We estimate a reserve for obsolete inventory based on management’s judgment of probable future commercial use, which is based on an analysis of current inventory levels, estimated future sales and production rates, and estimated shelf lives. See Note 7. |
|
j. |
Intangible Assets: Intangible assets consist primarily of license agreements, and patent and trademark costs. Intangible assets are stated at cost, less accumulated amortization. License agreements and patent costs are amortized using the straight-line method over the estimated useful lives of the license agreements and patents of approximately |
|
k. |
Leases: All of our leases are operating leases and are included in right-of-use lease assets, current lease liabilities and noncurrent lease liabilities on our consolidated balance sheets. These assets and liabilities are recognized at the commencement date based on the present value of remaining lease payments over the lease term using the Company’s incremental borrowing rates or implicit rates, when readily determinable. The discount rates used for each lease were based principally on the former Platinum debt. We used a “build-up” method where the approach was to estimate the risk/credit spread priced into the debt rate and then adjust that for the remaining term of each lease. Additionally, some market research was completed on the Company’s peer group. Short-term operating leases which have an initial term of 12 months or less are not recorded on the consolidated balance sheets. Lease expense for operating leases is recognized on a straight-line basis over the lease term. Lease expense is included in selling, general and administrative expenses on our consolidated statements of operations. See Note 11. |
|
l. |
Contingent Liabilities: We are subject to legal proceedings and claims that arise in the normal course of business. In accordance with ASC Topic 450, Contingencies, we accrue for contingent liabilities when management determines it is probable that a liability has been incurred and the amount can be reasonably estimated. This determination requires significant judgment by management. As of the date of the filing of this Annual Report on Form 10-K, we are engaged in separate matters of ongoing litigation with Capital Royalty Partners II, L.P. (“CRG”) and our former President and Chief Executive Officer, Dr. Michael Goldberg. In assessing whether we should accrue a liability in our financial statements as a result of these lawsuits, we considered various factors, including the legal and factual circumstances of the cases, the trial records and post-trial rulings of the applicable courts and appellate courts, the current status of the proceedings, applicable law and the views of legal counsel. |
|
m. |
Debt: We evaluate newly-issued debt instruments in accordance with Accounting Standards Codification (“ASC”) 470, Debt. The Company evaluated the terms of the Bridge Note under these guidelines. Based on this evaluation, the Company recorded a debt discount related to the difference in the value of Mr. Scott’s Series E Redeemable Convertible Preferred Stock (“Series E Preferred Stock”) and the Series F Redeemable Convertible Preferred Stock (“Series F Preferred Stock”) and Series G Redeemable Preferred Stock (“Series G Preferred Stock”) as well as debt issuance costs. The debt discount is being amortized as non-cash interest expense using the effective interest method over the term of the Bridge Note. See Notes 10 and 13. |
|
n. |
Preferred Stock: We evaluate newly-issued preferred stock in accordance with ASC 480, Distinguishing Liabilities from Equity, ASC 815, Derivatives and Hedging, ASC 470, Debt and Accounting Series Release (“ASR”) 268, Presentation in Financial Statements of “Redeemable Preferred Stocks.” The Company evaluated the provisions of the Series D, Series E, Series F and Series G Preferred Stock under the guidelines described above. Based on this evaluation, the Company determined that the Series D, Series E, Series F and Series G Preferred Stock are not mandatorily redeemable financial instruments and any obligation to issue a variable number of shares of Common Stock is not unconditional. Accordingly, the Series D, Series E, Series F and Series G Preferred Stock should be classified as equity. Neither the embedded conversion option in the Series D, Series E and Series F Preferred Stock, nor the embedded call option in the Series D, Series E, Series F and Series G Preferred Stock, meet the criteria to be separated from the Series D, Series E, Series F or Series G Preferred Stock and thus these features should not be bifurcated and accounted for as derivatives. Additionally, the Series D and Series E Preferred Stock each contain a beneficial conversion feature (“BCF”). Following the January 1, 2021 adoption of Accounting Standards Update (“ASU”) No. 2020-06, Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity, no BCF is recorded in the consolidated financial statements. Finally, the Company determined that the Series D, Series E and Series F Preferred Stock do not contain conversion features that could result in the Company being required to redeem a portion of the shares converted, thus the Series D, Series E and Series F Preferred Stock should not be classified in mezzanine equity. Similarly, the voluntary redemption feature of the Series G Preferred Stock cannot result in the Company being required to redeem the shares converted and the Series G Preferred Stock is not required to be classified in mezzanine equity. See Note 13. |
|
o. |
Preferred Stock Issued with Warrants: We evaluate preferred stock issued with warrants in accordance with ASC 480, Distinguishing Liabilities from Equity, ASC 815, Derivatives and Hedging and ASR 268, Presentation in Financial Statements of “Redeemable Preferred Stocks.” The Company evaluated the provisions of the Series I Preferred Stock and the Warrants issued in the Rights Offering under the guidelines described above. Based on this evaluation, the Company determined that the Series I Preferred Stock and Warrants each meet the definition of a freestanding financial instrument and should be accounted for separately upon issuance. |
|
i. |
Series I Preferred Stock: The Series I Convertible Preferred Stock (“Series I Preferred Stock”) is not a mandatorily redeemable financial instrument and any obligation to issue a variable number of shares of Common Stock does not require liability classification. Accordingly the Series I Preferred Stock should be classified as equity. The embedded conversion option in the Series I Preferred Stock does not meet the criteria to be separated from the Series I Preferred Stock and thus this feature should not be bifurcated and accounted for as a derivative. The subsequent rights offering privilege meets the criteria to be accounted for as a derivative, however, the scope exception is met and classification of the subsequent rights offering privilege in stockholders’ equity is appropriate. Because the Series I Preferred Stock is also classified in stockholders’ equity, no separate accounting is provided for the subsequent rights offering privilege. Finally, the Company determined that the Series I Preferred Stock does not contain conversion features that could result in the Company being required to redeem a portion of the shares converted, thus the Series I Preferred Stock should not be classified in mezzanine equity. See Note 13. |
|
ii. |
Warrants: The Warrants are not within the scope of ASC 480, therefore liability classification is not required. The Warrants have all of the characteristics to meet the definition of a derivative, however, they are considered to be indexed to the Company’s Common Stock and meet the other criteria to be classified in stockholders’ equity. Accordingly, the Warrants should be classified in stockholders’ equity upon issuance. The subsequent rights offering privilege meets the criteria to be accounted for as a derivative, however, the scope exception is met and classification of the subsequent rights offering privilege in stockholders’ equity is appropriate. Because the Warrants are also classified in stockholders’ equity, no separate accounting is provided for the subsequent rights offering privilege. See Note 13. |
|
p. |
Income Taxes: Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Due to the uncertainty surrounding the realization of the deferred tax assets in future tax returns, all of the deferred tax assets have been fully offset by a valuation allowance as of December 31, 2022 and 2021. |
|
q. |
Recently Adopted Accounting Standards: In May 2021, the Financial Accounting Standards Board (“FASB”) Issued ASU No. 2021-04, Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options. ASU 2021-04 was issued to clarify and reduce diversity in an issuer’s accounting for modifications or exchange of freestanding equity-classified written call options (for example, warrants) that remain equity-classified after modification or exchange. ASU 2021-04 requires that an entity treat a modification or exchange of a freestanding equity-classified written call option that remains equity-classified after modification or exchange be treated as an exchange of the original instrument for a new instrument. ASU 2021-04 also clarifies how an entity should measure and recognize the effect of a modification or exchange of a freestanding equity-classified written call option that remains equity-classified after modification or exchange. ASU 2021-04 is effective for all entities for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years, and should be implemented prospectively to modifications or exchanges occurring on or after the effective date of the amendments. Early adoption is permitted, including in an interim period. The adoption of ASU 2021-04 did not have a material impact on our consolidated financial statements. |
|
r. |
Accounting Standards to be Adopted: In June 2016, the FASB issued ASU No. 2016-13, Measurement of Credit Losses on Financial Instruments. ASU 2016-13 was issued to provide financial statement users with more useful information about the expected credit losses on financial instruments and other commitments to extend credit held by a reporting entity at each reporting date. ASU 2016-13 requires a financial asset (or group of financial assets) measured at amortized cost to be presented at the net amount expected to be collected. In addition, credit losses related to available-for-sale debt securities should be recorded through an allowance for credit losses. The amendments in ASU 2016-13 are effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years. Early application of the amendments is permitted. An entity should apply the amendments in ASU 2016-13 through a cumulative-effect adjustment to retained earnings as of the beginning of the first reporting period in which the guidance is effective (a modified-retrospective approach). The Company is currently evaluating the impact of adopting ASU 2016-13, however it is not expected to have a material impact on our consolidated financial statements. |
|
2. |
Liquidity |
The Company was engaged in litigation with Platinum-Montaur Life Sciences LLC (“Platinum-Montaur”), an affiliate of Platinum Management (NY) LLC, Platinum Partners Value Arbitrage Fund L.P., Platinum Partners Capital Opportunity Fund, Platinum Partners Liquid Opportunity Master Fund L.P., Platinum Liquid Opportunity Management (NY) LLC, and Montsant Partners LLC (collectively, “Platinum”). The Platinum lawsuit was settled and dismissed in January 2022. In addition, the Company is engaged in ongoing litigation with our former President and Chief Executive Officer, Dr. Michael Goldberg.
The Company is also engaged in ongoing litigation with CRG. On August 30, 2022, the District Court of Harris County, Texas (the “Texas Court”) made an oral ruling from the bench at the conclusion of the trial, awarding CRG approximately $
On April 10, 2022, the Company entered into a Stock Exchange and Loan Agreement (the “Stock Exchange Agreement”) with John K. Scott, Jr., the current Vice Chairman of our Board of Directors, pursuant to which Mr. Scott agreed to make a loan to the Company in the principal amount of up to $
On August 30, 2022, the Company closed on an offering to its stockholders and certain warrant holders as of August 3, 2022 of the right to purchase up to
The Company has previously entered into an API Development Funding and Access Agreement (“API Development Agreement”) with a strategic partner for assistance with the development and supply of the active pharmaceutical ingredient (“API”) used to manufacture Lymphoseek (technetium Tc 99m tilmanocept) that is sold by the Company in countries other than the United States, Canada and Mexico. Under the API Development Agreement, among other things, the strategic partner agreed to reimburse the Company for up to a total of $
We do not believe there has been a significant impact to the Company’s clinical development and regulatory timelines resulting from the ongoing COVID-19 global pandemic. However, the COVID-19 outbreak delayed enrollment in our NAV3-32 clinical study in the United Kingdom (“UK”) due to national COVID-19-related shutdowns. In addition, the regulatory approval process in India was delayed by the impact of COVID-19 in that country.
The current conflict between Ukraine and Russia has created volatility in the global capital markets and is continuing to have further global economic consequences, including disruptions of the global supply chain and energy markets. Any such volatility and disruptions may have adverse consequences on us or the third parties who operate in Europe on whom we rely. If the equity and credit markets deteriorate, including as a result of political unrest or war, it may make any debt or equity financing more difficult to obtain, more costly or more dilutive.
The Company has experienced recurring net losses and has used significant cash to fund its operations. The Company has considerable discretion over the extent of development project expenditures and has the ability to curtail the related cash flows as needed. The Company also continues working to establish new sources of funding, including potential equity and/or debt financings, collaborations and additional grant funding that can augment the balance sheet. However, based on our current working capital and our projected cash burn, management believes that there is substantial doubt about the Company’s ability to continue as a going concern for a period of one year from the filing of this Annual Report on Form 10-K.
|
3. |
Revenue from Contracts with Customers and Other Revenue |
Navidea is focused on the development and commercialization of precision immunodiagnostic agents and immunotherapeutics. We manage our business based on two primary types of drug products: (i) diagnostic substances, including Tc99m tilmanocept and other diagnostic applications of our Manocept platform, and (ii) therapeutic development programs, including all therapeutic applications of our Manocept platform. Tc99m tilmanocept, which the Company has a license to distribute outside of Canada, Mexico and the United States, is the only one of the Company’s drug product candidates that has been approved for sale in any market. Tc99 tilmanocept has only been approved for sale in the European Union (“EU”), the UK, India and Australia.
We earn revenue from product sales to end customers, primarily in Europe. Revenue from product sales is generally recognized at the point where the customer obtains control of the goods and we satisfy our performance obligation, which occurs upon either shipment of the product or arrival at its destination, depending upon the shipping terms of the transaction. Our customers have no right to return products purchased in the ordinary course of business, however, we may allow returns in certain circumstances based on specific agreements. Normal payment terms generally range from 30 to 90 days from invoice date, in accordance with each contract or purchase order.
The Company also recognizes revenue from up-front license fees and pre-market milestones after the cash has been received from its customers and the performance obligations have been met. Payments for sales-based royalties and milestones are generally received after the related revenue has been recognized and invoiced. Normal payment terms generally range from
Up-front and milestone payments received related to our license and distribution agreements in India and China are deferred until Tc99m tilmanocept has been approved by the regulatory authorities and product sales are authorized to commence in each of those countries. The Company received regulatory approval for Tc99m tilmanocept in India in late March 2022, however certain additional approvals, such as an import license and authorization to use an alternative manufacturer, must be obtained prior to commercial sales launch in India. It is not possible to determine with any degree of certainty whether or when regulatory approval for this product will be achieved in China, if at all. In addition, since sales of Tc99m tilmanocept have not yet begun in India or China, there is no basis for estimating whether, to what degree, or the rate at which the product will be accepted and utilized in these markets. Therefore, it is not possible to determine with any degree of certainty the expected sales in future periods in those countries. As such, the Company intends to recognize revenue from up-front and milestone payments on a straight-line basis beginning at the time of commercial sales launch in each country through the end of the initial term of each agreement. The initial term of each agreement is years in India and years in China.
The transaction price of a contract is the amount of consideration to which the Company expects to be entitled in exchange for transferring promised goods or services to a customer. Transaction prices do not include amounts collected on behalf of third parties (e.g., sales taxes). To determine the transaction price of a contract, the Company considers the terms of the contract. For the purpose of determining transaction prices, the Company assumes that the goods or services will be transferred to the customer as promised in accordance with existing contracts and that the contracts will not be cancelled, renewed, or modified.
When estimating a contract’s transaction price, the Company considers all the information (historical, current, and forecasted) that is reasonably available to it and identifies possible consideration amounts. Most of the Company’s contracts with customers include both fixed and variable components of the transaction price. Under those contracts, some or all of the consideration for satisfied performance obligations is contingent on events over which the Company has no direct influence. For example, regulatory approval or product sales volume milestones are contingent upon the achievement of those milestones by the distributor. Additionally, the prices charged to end users of Tc99m tilmanocept, upon which royalty payments are based in India and China, are set by the distributor in each of those countries.
The milestone payments have a binary outcome (that is, the Company will either receive all or none of each milestone payment) and can be estimated using the most-likely-amount method. Taking into account the constraint on variable consideration, the Company has assessed the likelihood of achieving the non-sales-based milestone payments in our current contracts and has determined that it is probable the milestones will be achieved and the Company will receive the consideration. Accordingly, it is probable that including those payments in the transaction price will not result in a significant revenue reversal when the contingency is resolved. Therefore, the amount of the non-sales-based milestone payments is included in the transaction price.
Royalties are estimated based on the expected value method because they are based on a variable amount of sales representing a range of possible outcomes. However, when taking into account the constraint on variable consideration, the estimate of future royalties included in the transaction price is generally $
Up-front fees, milestones and royalties are generally non-refundable. Therefore, the Company does not estimate expected refunds nor do we adjust revenue downward. The Company will evaluate and update the estimated transaction prices of its contracts with customers at the end of each reporting period.
During the years ended December 31, 2022 and 2021, the Company recognized revenue from contracts with customers of $
The following table disaggregates the Company’s revenue from contracts with customers for the years ended December 31, 2022 and 2021.
|
Years Ended December 31, |
||||||||
|
2022 |
2021 |
|||||||
| Sales revenue: | ||||||||
|
Tc99m tilmanocept – Europe |
$ | $ | ||||||
|
Tc99m tilmanocept – Australia |
||||||||
|
Total sales revenue |
||||||||
|
License revenue: |
||||||||
|
Tc99m tilmanocept - Europe |
$ | $ | ||||||
The following economic factors affect the nature, amount, timing and uncertainty of the Company’s revenue and cash flows as indicated:
Geographical Location of Customers. Drug pricing models vary among different markets, which in turn may affect the royalty rates and milestones we are able to negotiate with our distributors in those markets. Royalty rates and milestone payments vary by contract but may be based in part on the potential market size in each territory. In the case of Tc99m tilmanocept, royalty rates for Europe were lower than rates in India but higher than in China.
Status of Regulatory Approval. The majority of revenue from contracts with customers will generally be recognized after the product is approved for sale in each market. Each Tc99m tilmanocept customer operates in its own distinct regulatory environment, and the laws and pathways to drug product approval vary by market. Tc99m tilmanocept has been approved for sale in the EU and the UK, thus the Company recognized revenue from sales in Europe. Tc99m tilmanocept was approved for sale in India in March 2022, however product sales have not yet commenced. Tc99m tilmanocept has not yet been approved for sale in China and may never achieve approval in that market. The regulatory pathways and timelines in China will impact whether and when the Company recognizes the related royalties and milestones.
Through December 31, 2022, the Company has capitalized any contract-related costs as contract assets.
The following table summarizes the changes in contract liabilities, the current portion of which is included in accrued liabilities and other in the consolidated balance sheets during the years ended December 31, 2022 and 2021.
|
Years Ended December 31, |
||||||||
|
2022 |
2021 |
|||||||
|
Total deferred revenue related to contracts with customers, beginning of period |
$ | $ | ||||||
|
Deferred revenue related to milestones achieved |
||||||||
|
Deferred revenue related to milestones achieved, written off due to contract renegotiations |
( |
) | ||||||
|
Total deferred revenue related to contracts with customers, end of period |
$ | $ | ||||||
The Company had sales revenue receivable of $
In addition to revenue from contracts with customers, we also generate revenue from National Institutes of Health (“NIH”) grants to support various product development initiatives. The revenue recognition standard applies to revenue from contracts with customers. A customer is defined as a party that has contracted with an entity to obtain goods or services that are an output of the entity’s ongoing major or central operations in exchange for consideration. The Company’s ongoing major or central operations consist of the development and commercialization of precision immunodiagnostic agents and immunotherapeutics. The NIH and its various institutes are responsible for biomedical and public health research and provide major biomedical research funding to non-NIH research facilities and entities such as Navidea. While the Company will directly benefit from any knowledge gained from the project, there is also a public health benefit provided, which justifies the use of public funds in the form of the grants. Based on the nature of the Company’s operations and the terms of the grant awards, Navidea does not have a vendor-customer relationship with the NIH and the grant awards are outside the scope of the revenue recognition standard. Accordingly, the revenue recognition standard need not be applied to the NIH grants. During the years ended December 31, 2022 and 2021, the Company recognized grant revenue of $
Finally, we expect to recognize revenue from a strategic development partner up to a total of $1.85 million under the terms of the API Development Agreement. Based on the nature of the Company’s operations and the terms of the API Development Agreement, Navidea does not have a vendor-customer relationship with the strategic partner and amounts received under the API Development Agreement are outside the scope of the revenue recognition standard. Accordingly, the revenue recognition standard need not be applied to the API Development Agreement. On August 11, 2022, the Company received the first installment in the amount of $800,000, which the strategic partner has the right to claw back if the Company does not satisfy certain commercial and regulatory milestones on or before March 31, 2023. The Company recorded the first installment as deferred revenue, current on its consolidated balance sheets.
|
4. |
Stock-Based Compensation |
For the years ended December 31, 2022 and 2021, our total stock-based compensation expense, which includes reversals of expense and incremental expense for certain modified, forfeited or cancelled awards, was $
On November 23, 2021, our former Chief Executive Officer, Chief Operating Officer and Chief Financial Officer, Jed A. Latkin, signed a Separation Agreement and General Release (the “Separation Agreement”) in connection with his resignation from those positions and as a director on October 24, 2021 (the “Separation Date”). Pursuant to the Separation Agreement, among other things, the Company agreed to provide Mr. Latkin with certain separation benefits, commencing on the “Effective Date,” defined as the eighth day after Mr. Latkin signed, without revoking, the Separation Agreement. On the Effective Date, each of Mr. Latkin’s unvested stock options vested, and all of his vested stock options (covering
A summary of the status of our stock options as of December 31, 2022, and changes during the year then ended, is presented below.
|
Year Ended December 31, 2022 |
||||||||||||||||
|
Number of Options |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Life (in years) |
Aggregate Intrinsic Value |
|||||||||||||
|
Outstanding, January 1, 2022 |
$ | |||||||||||||||
|
Granted |
||||||||||||||||
|
Canceled and forfeited |
( |
) |
||||||||||||||
|
Expired |
( |
) |
||||||||||||||
|
Outstanding, December 31, 2022 |
$ |
|
$ | |||||||||||||
|
Exercisable, December 31, 2022 |
$ |
|
$ | |||||||||||||
The weighted average grant-date fair value of options granted in 2022 and 2021 was $
A summary of the status of our unvested restricted stock as of December 31, 2022, and changes during the year then ended, is presented below.
|
Year Ended December 31, 2022 |
||||||||
|
Number of Shares |
Weighted Average Grant-Date Fair Value |
|||||||
|
Unvested, January 1, 2022 |
$ | |||||||
|
Granted |
||||||||
|
Vested |
( |
) |
||||||
|
Unvested, December 31, 2022 |
$ | |||||||
During 2022 and 2021,
As of December 31, 2022, there was $
|
5. |
Loss Per Share |
Diluted loss per common share for the years ended December 31, 2022 and 2021 excludes the effects of
The Company’s unvested restricted stock awards contain nonforfeitable rights to dividends or dividend equivalents, whether paid or unpaid (referred to as “participating securities”). Therefore, the unvested stock awards are generally required to be included in the number of shares outstanding for both basic and diluted earnings per share calculations. However, due to our loss from continuing operations,
|
6. |
Receivables |
Receivables as of December 31, 2022 and 2021 consist of the following:
|
2022 |
2021 |
|||||||
|
Sales revenue |
$ | $ | ||||||
|
Related parties |
||||||||
|
License revenue |
||||||||
|
Grant revenue |
||||||||
|
Other |
||||||||
|
Total stock subscriptions and other receivables |
$ | $ | ||||||
As of December 31, 2022 and 2021, there was allowance for doubtful accounts. We believe that we have adequately addressed credit risks in estimating the allowance for doubtful accounts.
|
7. |
Inventory, Net |
The components of inventory, net as of December 31, 2022 and 2021 are presented in the following table:
|
December 31, 2022 |
December 31, 2021 |
|||||||
|
Materials |
$ | $ | ||||||
|
Work in process |
||||||||
|
Finished goods |
||||||||
|
Reserve for expiring finished goods |
( |
) |
||||||
|
Total inventory, net |
$ | $ | ||||||
During 2022, we reserved $
During 2022 and 2021, we utilized $
|
8. |
Property and Equipment |
The major classes of property and equipment are presented in the following table:
| Useful Life
(in years) |
2022 |
2021 |
||||||||||||
| Purchased software | $ | $ | ||||||||||||
| Production machinery and equipment | ||||||||||||||
|
Other machinery and equipment, primarily computers and research equipment |
– | |||||||||||||
| Leasehold improvements* | Term of Lease | |||||||||||||
| Furniture and fixtures | ||||||||||||||
| Total property and equipment | $ | $ | ||||||||||||
|
* |
|
During 2022 and 2021, we recorded $
|
9. |
Accounts Payable, Accrued Liabilities and Other |
Accounts payable as of December 31, 2022 and 2021 includes an aggregate of $
The Company pays director fees in both cash and stock. The cash portion of director fees due is included in accounts payable and the stock portion is included in accrued liabilities and other in the consolidated balance sheet as of December 31, 2022 and 2021. Certain directors have elected to defer receipt of cash and stock for director fees until the Company raises sufficient additional capital.
Under our license agreements with the University of California, San Diego (“UCSD”), we have exclusive world-wide rights to all diagnostic and therapeutic uses of tilmanocept, other than Tc99m tilmanocept used in lymphatic mapping in the United States, Canada and Mexico which rights are licensed to Cardinal Health 414. The UCSD license agreements include obligations for payments related to license fees, milestones, and royalties. As of December 31, 2022, the Company has accrued approximately $
Accrued liabilities and other as of December 31, 2022 and 2021 are presented in the following table:
|
2022 |
2021 |
|||||||
|
Contracted services |
$ | $ | ||||||
|
Compensation |
||||||||
|
Other |
||||||||
|
Total accrued liabilities and other |
$ | $ | ||||||
|
10. |
Notes Payable |
Bridge Note from John K. Scott, Jr.
On April 10, 2022, the Company entered into a Stock Exchange Agreement with John K. Scott, Jr., pursuant to which Mr. Scott agreed to make a loan to the Company in the principal amount of up to $
As consideration and partial inducement for Mr. Scott to enter into the Bridge Note, the Company exchanged all
Interest expense related to the Bridge Note totaled $
IPFS Corporation
In November 2020, we prepaid $
Interest expense related to the IPFS notes payable totaled $
AFCO Premium Credit LLC
In November 2022, we prepaid $
Interest expense related to the AFCO note payable totaled $
Paycheck Protection Program
The CARES Act was enacted on March 27, 2020. Among the provisions contained in the CARES Act was the creation of the PPP that provides for SBA Section 7(a) loans for qualified small businesses. PPP Loan proceeds are available to be used to pay for payroll costs, including salaries, commissions, and similar compensation, group health care benefits, and paid leaves; rent; utilities; and interest on certain other outstanding debt. On May 18, 2020, the Lender funded the PPP Loan in the amount of $
Summary
During the years ended December 31, 2022 and 2021, we recorded interest expense of $
|
11. |
Leases |
We currently lease approximately
In addition, we leased approximately
We currently lease office equipment at a monthly payment of $
Total operating lease expense was $
The following table presents information about the amount, timing and uncertainty of cash flows arising from the Company’s operating leases as of December 31, 2022.
|
Maturity of Lease Liabilities |
Operating Lease Payments |
|||
|
2023 |
$ | |||
|
2024 |
||||
|
Total undiscounted operating lease payments |
||||
|
Less imputed interest |
||||
|
Present value of operating lease liabilities |
$ | |||
|
Balance Sheet Classification |
||||
|
Current lease liabilities |
$ | |||
|
Noncurrent lease liabilities |
||||
|
Total operating lease liabilities |
$ |
|
Other Information |
||||
|
Weighted-average remaining lease term for operating leases (in years) |
||||
|
Weighted-average discount rate for operating leases |
% |
Cash paid for amounts included in the present value of operating lease liabilities was $
|
12. |
Commitments and Contingencies |
We are subject to legal proceedings and claims that arise in the ordinary course of business. In accordance with ASC Topic 450, Contingencies, we make a provision for a liability when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated. Although the outcome of any litigation is uncertain, in our opinion, the amount of ultimate liability, if any, with respect to these actions, will not materially affect our financial position.
CRG Litigation
The Company has been engaged in ongoing litigation with CRG, in its capacity as a lender and as control agent for other affiliated lenders party to the CRG Loan Agreement (collectively, the “CRG Lenders”), in the Texas Court relating to CRG’s claims of default under the terms the CRG Loan Agreement. Following a trial in December 2017, the Texas Court ruled that the Company’s total obligation to CRG was in excess of $
In April 2018, CRG asserted claims against Navidea and MT for alleged breaches of the GSA and Loan Agreement entered into by Navidea arising from Navidea’s challenge to CRG’s drawing down on letters of credit in the full amount of $
On November 21, 2021, the Texas Court entered an interlocutory judgment declaring that CRG did not breach the GSA, but that Navidea did breach the GSA and the indemnification provision of the CRG Loan Agreement. In the interlocutory order, the Texas Court sua sponte awarded as damages reasonable attorneys' fees in an amount, if any, to be determined at trial. CRG made a claim of approximately $
The Company had also been engaged in ongoing litigation with CRG in the Court of Common Pleas of Franklin County, Ohio (the “Ohio Court”) related to Navidea’s claims that the CRG Lenders fraudulently induced Navidea to enter into a settlement agreement and breached the terms of the same through certain actions taken by the CRG Lenders in connection with the GSA, pursuant to which Navidea agreed to pay up to $
Platinum Litigation
In November 2017, Platinum-Montaur commenced an action against the Company in the Supreme Court of the State of New York, County of New York (the “New York Supreme Court”), seeking damages of approximately $
On November 30, 2018, Platinum-Montaur filed a notice of appeal with the United States Court of Appeals for the Second Circuit (the “Second Circuit”) claiming that the District Court erred in dismissing Platinum-Montaur’s claims for breach of contract and unjust enrichment. On January 22, 2019, Platinum-Montaur filed its brief in the Second Circuit, asking the Second Circuit to reverse the District Court and remand the case to the District Court for further proceedings. The Second Circuit held oral argument in this matter on September 5, 2019. On November 25, 2019, the Second Circuit issued a decision which remanded the case to the District Court for further consideration of whether the District Court had jurisdiction over the case following removal from the New York Supreme Court. The Second Circuit did not address the merits of Platinum-Montaur’s allegations against Navidea. By agreement of the parties, the case was remanded from the District Court to the New York Supreme Court. Navidea filed a Motion to Dismiss on June 4, 2020, and on September 2, 2020, the New York Supreme Court granted the Motion to Dismiss. Platinum-Montaur filed a Notice of Appeal of the New York Supreme Court’s decision on September 23, 2020 and the appeal was docketed with the Appellate Department-First Division. Platinum-Montaur perfected an appeal of the judgment in favor of the Company on or about June 28, 2021. In February 2022, Platinum and the Company settled their dispute and Platinum’s lawsuit was dismissed.
Goldberg Agreement and Litigation
In August 2018, Dr. Goldberg resigned from his positions as an executive officer and a director of Navidea. In connection with Dr. Goldberg’s resignation, Navidea and Dr. Goldberg entered into an Agreement (the “Goldberg Agreement”) which set forth the terms of the separation from service. Among other things, the Goldberg Agreement provided that Dr. Goldberg would be entitled to
On February 11, 2019, Dr. Goldberg represented to the MT Board that he had, without MT Board or shareholder approval, created a subsidiary of MT, transferred all of the assets of MT into the subsidiary, and then issued himself stock in the subsidiary. On February 19, 2019, Navidea notified MT that it was terminating the sublicense in accordance with its terms, effective March 1, 2019, due to MT’s insolvency. On February 20, 2019, the MT Board removed Dr. Goldberg as President and Chief Executive Officer of MT and from any other office of MT to which he may have been appointed or in which he was serving. Dr. Goldberg remains a member of the MT Board, together with John K. Scott, Jr. and Dr. Michael S. Rosol. Mr. Scott is also the Vice Chair of the Board of Directors of Navidea. On or about February 17, 2022, the Joint Official Liquidators and Foreign Representatives of PPVA executed the necessary paperwork to transfer its preferred stock in MT to Navidea.
New York Litigation Involving Dr. Goldberg
On February 20, 2019, Navidea filed a complaint against Dr. Goldberg in the United States District Court, Southern District of New York (the “District Court”), alleging breach of the Goldberg Agreement, as well as a breach of the covenant of good faith and fair dealing and to obtain a declaratory judgment that Navidea’s performance under the Goldberg Agreement is excused and that Navidea is entitled to terminate the Goldberg Agreement as a result of Dr. Goldberg’s actions. On April 26, 2019, Navidea filed an amended complaint against Dr. Goldberg which added a claim for breach of fiduciary duty seeking damages related to certain actions Dr. Goldberg took while CEO of Navidea. On June 13, 2019, Dr. Goldberg answered the amended complaint and asserted counterclaims against Navidea and third-party claims against MT for breach of the Goldberg Agreement, wrongful termination, injunctive relief, and quantum meruit.
On December 26, 2019, the District Court ruled on several motions related to Navidea and MT and Dr. Goldberg that substantially limited the claims that Dr. Goldberg can pursue against Navidea and MT. Specifically, the District Court found that certain portions of Dr. Goldberg’s counterclaims against Navidea and third-party claims against MT failed to state a claim upon which relief can be granted. Additionally, the District Court ruled that actions taken by Navidea and MT, including reconstituting the MT board of directors, replacing Dr. Goldberg with Mr. Latkin as Chief Executive Officer of MT, terminating the sublicense between Navidea and MT, terminating certain research projects, and allowing MT intellectual property to revert back to Navidea, were not breaches of the Goldberg Agreement.
The District Court also rejected Dr. Goldberg’s claim for wrongful termination as Chief Executive Officer of MT. In addition, the District Court found that Dr. Goldberg lacked standing to seek injunctive relief to force the removal of Dr. Claudine Bruck and Michael Rice from MT’s Board of Directors, to invalidate all actions taken by the MT Board on or after November 29, 2018 (the date upon which Dr. Bruck and Mr. Rice were appointed by Navidea to the Board of MT), or to reinstate the terminated sublicense between Navidea and MT.
In addition, the District Court found Navidea’s breach of fiduciary duty claim against Dr. Goldberg for conduct occurring more than three years prior to the filing of the complaint to be time-barred and that Dr. Goldberg is entitled to an advancement of attorneys’ fees solely with respect to that claim. To avoid further litigation expenses, the Company agreed to indemnify Dr. Goldberg solely with respect to the breach of fiduciary duty claim.
On January 31, 2020, Goldberg filed a motion for leave to amend his complaint to add back in claims for breach of contract, breach of the implied covenant of good faith and fair dealing, quantum meruit and injunctive relief. On April 1, 2020, the District Court denied Dr. Goldberg’s motion for leave to amend in its entirety.
On January 27, 2020, Dr. Goldberg filed a motion seeking additional advancement from Navidea for fees in connection with the New York Action and the Delaware Action. Navidea opposed the motion and the District Court referred the matters to a Magistrate Judge. On July 9, 2020, the Magistrate Judge issued her Report and Recommendation which recommended that: (1) the District Court decline to exercise jurisdiction over Dr. Goldberg’s motion as it pertained to expenses and fees incurred in defense of the Delaware Action; (2) the District Court decline to award any fees to Dr. Goldberg for the breach of fiduciary duty without additional motion practice on the issue; (3) the District Court find that Dr. Goldberg is entitled to advancement of his expenses and fees reasonably incurred in the defense of the remainder of the New York action subject to Dr. Goldberg’s posting of an undertaking; and (4) establish a protocol by which Dr. Goldberg could establish the amounts due for advancement.
On August 24, 2020, in connection with Dr. Goldberg’s motion for advancement, the District Court adopted the Magistrate Judge’s report and recommendation and found that while Dr. Goldberg was not being granted advancement of fees and expenses incurred in connection with either the Delaware Action or the assertion of third-party claims against MT, the Court ruled that Dr. Goldberg was entitled to advancement for the defense of the remaining claims asserted against him by Navidea in the New York action. The Court adopted a protocol by which additional motion practice will occur to determine the appropriate amount of fees to be advanced. Once that decision is made by the Magistrate Judge, subject to review by the District Court, Navidea will need to advance those fees to Dr. Goldberg conditioned upon Dr. Goldberg agreeing to pay those fees back to Navidea if it is determined that he is not entitled to indemnification.
On May 27, 2021, the District Court ordered that: (1) Dr. Goldberg be awarded $
On August 6, 2021, the Company moved for reconsideration of its obligations to advance fees in light of the Delaware Court’s decision dated June 23, 2021 (described below). On October 14, 2021, the Magistrate Judge recommended that Navidea’s motion for reconsideration be denied. On March 7, 2022, the District Court adopted the Report and Recommendation in part and permitted Dr. Goldberg to seek advancement for his fees incurred in defense of his claims since September 1, 2020. On April 8, 2022, Dr. Goldberg submitted a fee application seeking advancement of $ for attorneys’ fees and disbursements for the time period September 1, 2020 through March 31, 2022. On March 15, 2023, the District Court adopted the Magistrate Judge’s report and recommendation that Dr. Goldberg’s application for fees allegedly incurred in connection with his defense of Navidea’s claims be denied as a sanction for failure to comply with prior court orders and that his application for fees incurred in connection with the successful prosecution of his prior fee applications be approved in the amount of $
Fact discovery and expert discovery in the New York Action have been completed. The Company has moved to disqualify Dr. Goldberg’s damages expert and briefing in the District Court was submitted on April 1, 2022. On November 9, 2022, the District Court issued an opinion granting the Company’s motion in part and precluding Dr. Goldberg’s damages expert from testifying on all but two issues. The Company anticipates that a briefing schedule for motions for summary judgment will be entered by the Court.
Delaware Litigation Involving Dr. Goldberg
On February 20, 2019, MT initiated a suit against Dr. Goldberg in the Court of Chancery of the State of Delaware (the “Delaware Court”), alleging, among other things, breach of fiduciary duty as a director and officer of MT and conversion, and to obtain a declaratory judgment that the transactions Dr. Goldberg caused MT to effect are void. On June 12, 2019, the Delaware Court found that Dr. Goldberg’s actions were not authorized in compliance with the Delaware General Corporate Law. Specifically, the Delaware Court found that Dr. Goldberg’s creation of a new subsidiary of MT and the purported assignment by Dr. Goldberg of MT’s intellectual property to that subsidiary were void. The Delaware Court’s ruling follows the order on May 23, 2019 in the case, in which it found Dr. Goldberg in contempt of its prior order holding Dr. Goldberg responsible for the payment of MT’s fees and costs to cure the damages caused by Dr. Goldberg’s contempt.
On June 23, 2021, the Delaware Court ruled in favor of MT and against Dr. Goldberg, finding that Dr. Goldberg breached his fiduciary duties to MT. Specifically, the Delaware Court ruled: “Dr. Goldberg attempted to take for himself that which belonged to [MT]. In doing so, he breached his duty of loyalty to [MT] stockholders. [MT] was absolutely justified in bringing this action to remedy (in this case undo) the harm caused by Dr. Goldberg’s misconduct.” The Delaware Court disagreed with MT’s arguments regarding damages and, other than awarding nominal damages, declined to award additional relief beyond that which it had previously granted. With respect to MT’s claim for conversion, the Delaware Court found that the claim was not supported because “Dr. Goldberg confirmed that he currently does not own or possess any intellectual property related to either Navidea or [MT]” and that “any IP Dr. Goldberg created while at Navidea or any of its subsidiaries was and remains the property of Navidea and its subsidiaries.” In addition, the Delaware Court denied Dr. Goldberg’s motion to hold MT’s directors and CEO in contempt, denied Dr. Goldberg’s motion to dismiss the lawsuit against him, and granted MT’s motion to dismiss Dr. Goldberg’s petition to remove MT’s board members. On December 9, 2021, Dr. Goldberg was ordered to reimburse MT in the amount of $ and has paid that amount to MT. Neither party has appealed the Delaware Court’s decision and the Delaware Court’s decisions are now final.
NYSE American Continued Listing Standards
On January 28, 2022, the Company received a notification from the NYSE American LLC (the “NYSE American”) stating that the Company was not in compliance the $6.0 million stockholders’ equity requirement of Section 1003(a)(iii) of the NYSE American Company Guide. As required by the NYSE American, the Company submitted a plan to the NYSE American by February 28, 2022 advising of actions it has taken or will take to regain compliance with the continued listing standards by July 28, 2023.
On April 8, 2022, the Company received a notification (the “Acceptance Letter”) from the NYSE American that the Company’s plan to regain compliance was accepted. The Acceptance Letter also stated that the Company is also not in compliance with Sections 1003(a)(i) and 1003(a)(ii) of the NYSE American Company Guide, which require an issuer to have stockholders’ equity of (i) $2.0 million or more if it has reported losses from continuing operations and/or net losses in two out of its three most recent fiscal years, and (ii) $4.0 million or more if it has reported losses from continuing operations in three out of its four most recent fiscal years. The Acceptance Letter noted that the Company had stockholders’ equity of $
The NYSE American has granted the Company a plan period through July 28, 2023 to regain compliance with Sections 1003(a)(i), (ii) and (iii). If the Company is not in compliance with all continued listing standards by that date or if the Company does not make progress consistent with the plan during the plan period, the NYSE American may commence delisting procedures.
|
13. |
Equity |
Platinum Settlement
As discussed in Note 12, Platinum and the Company settled their dispute and Platinum’s lawsuit was dismissed in January 2022. As part of the settlement, Platinum returned their six shares of MT Preferred Stock, representing
Series D Preferred Stock
On August 31, 2020, the Company entered into a Stock Purchase Agreement and Letter of Investment Intent (the “Series D Preferred Stock Purchase Agreement”) with Keystone pursuant to which the Company agreed to issue to Keystone
Through July 7, 2021, Keystone purchased
Series E Preferred Stock
On March 2, 2021, the Company entered into a Series E Preferred Stock Purchase Agreement with an existing accredited investor, John K. Scott, Jr. pursuant to which the Company issued to Mr. Scott
Under the Series E Preferred Stock Purchase Agreement, Mr. Scott was granted a right of first offer with respect to future issuances of Company securities (the “Right of First Offer”); provided, however, that in no event shall Mr. Scott have such right if the acquisition of any of such securities would result in Mr. Scott beneficially holding more than
In connection with the private placement, the Company entered into a registration rights agreement (the “Registration Rights Agreement”), pursuant to which, among other things, the Company prepared and filed with the Securities and Exchange Commission (the “SEC”) a registration statement on Form S-1 to register for resale the maximum number of Series E Conversion Shares (as defined below) issuable upon conversion of the Series E Preferred Stock. In the event that both (i) the number of shares of Common Stock beneficially held by Mr. Scott fell below 20% of the outstanding Common Stock on an as-converted basis, as determined in accordance with Section 13(d) of the Exchange Act and the rules thereunder, and (ii) Mr. Scott was an affiliate (as that term is defined under Rule 144) at the time of the Reload Request (as defined below), the Company, upon written request from Mr. Scott (the “Reload Request”), would have been required prepare and file with the SEC one, and only one, additional registration statement covering the resale of those shares of Common Stock owned by Mr. Scott as of the date of the Reload Request that, as of such time, were not registered for resale under the Securities Act of 1933, as amended (the “Securities Act”). The securities issued in the offering were not registered under the Securities Act, and could not have been offered or sold absent registration or availability of an applicable exemption from registration.
Except with respect to transactions which may adversely affect any right, preference, privilege or voting power of the Series E Preferred Stock, the Series E Preferred Stock had no voting rights. Whenever the Company’s Board of Directors declared a dividend on Common Stock, each record holder of a share of Series E Preferred Stock on the record date set by the Board of Directors would have been entitled to receive an amount equal to such dividend declared on one share of Common Stock multiplied by the number of shares of Common Stock (the “Series E Conversion Shares”) into which such share of Series E Preferred Stock could have been converted on the record date, without regard to any conversion limitations in the Series E Preferred Certificate of Designation of Preferences, Rights and Limitations (the “Series E Preferred Certificate”). Holders of the Series E Preferred Stock could have converted some or all of the Series E Preferred Stock into Series E Conversion Shares at a fixed price of $
On January 31, 2022, pursuant to the Series E Preferred Certificate dated March 2, 2021, Mr. Scott exercised his option to extend the Conversion Deadline (as defined therein) for an additional period of six months. All of the outstanding shares of Series E Preferred Stock were exchanged and cancelled pursuant to the Stock Exchange Agreement in April 2022. There were
NOL Rights Agreement
On April 7, 2022, the Company’s Board of Directors adopted an NOL rights plan in the form of a Section 382 Rights Agreement (“NOL Rights Agreement”) to preserve and protect the Company’s net operating loss carryforwards (“NOLs”) and other tax assets. As of December 31, 2022, the Company had approximately $
Under the NOL Rights Agreement, the Board declared a non-taxable dividend of one preferred share purchase right for each outstanding share of common stock of the Company, each right initially representing the right to purchase one of a share of our Series H Junior Participating Preferred Stock. The rights will be exercisable only if a person or group acquires
The rights issued under the NOL Rights Agreement will expire on the earliest of (i) April 6, 2025; (ii) the effective date of the repeal of Section 382 or any successor statute if the Board determines in its sole discretion that the NOL Rights Agreement is no longer necessary or desirable for the preservation of NOLs or other tax benefits; (iii) the first day of a taxable year of the Company to which the Board determines in its sole discretion that no NOLs or other Tax Benefits may be carried forward; or (iv) the day following the certification of the voting results of the Company’s 2022 annual meeting of stockholders if at or before such annual meeting a proposal to approve the NOL Rights Agreement has not been approved by stockholders, unless the Rights are earlier redeemed or exchanged by the Company, or upon the occurrence of certain transactions. See Note 19(a).
Stock Exchange and Loan Agreement
On April 10, 2022, the Company entered into a Stock Exchange Agreement with John K. Scott, Jr., pursuant to which Mr. Scott agreed to make a loan to the Company in the principal amount of up to $
As consideration and a partial inducement for Mr. Scott to make the loan, at the closing, Mr. Scott delivered
In connection with the Stock Exchange Agreement, the Company entered into a Registration Rights Agreement with Mr. Scott, pursuant to which the Company agreed to file a registration statement with the SEC to register the resale of the shares issuable to Mr. Scott upon conversion of the Series F Preferred Stock.
Rights Offering
On August 30, 2022, the Company closed on the Rights Offering to its stockholders and certain warrant holders as of August 3, 2022 of the right to purchase up to
Both the Series I Preferred Stock and the Warrants are standalone instruments and each is classified as stockholders’ equity. We measured the fair value of the Series I Preferred Stock based on the market price of the underlying Common Stock on the closing date of the Rights Offering. We measured the fair value of the Warrants using the Black-Scholes option pricing model as of the closing date of the Rights Offering. The assumptions used to calculate fair value of the Warrants included volatility of
Certain participants in the Rights Offering had the ability to pay the subscription price for their Units by cancelling or exchanging their shares of Series D Preferred Stock, Series F Preferred Stock and/or Series G Preferred Stock and the Company’s indebtedness evidenced by the Bridge Note, instead of paying by check or wire transfer of funds. The fair market value of the shares of each series of preferred stock and the Bridge Note to be cancelled or exchanged in the Rights Offering was determined by the Company’s Board of Directors with the assistance of an independent appraisal obtained by the Company. In order to help maximize Navidea’s ability to use its NOLs and other tax benefits in future years, Navidea’s Board of Directors exercised its discretion to limit the number of Units that John K. Scott, Jr. could purchase to
In accordance with U.S. GAAP, we determined that the fair value of the Series D Preferred Stock and Series F Preferred Stock that was surrendered pursuant to the Rights Offering was approximately $
Net proceeds after deducting fees and expenses of $
401(k) Employer Match
During the years ended December 31, 2022 and 2021, we issued
Bonuses Paid in Stock
During the year ended December 31, 2022, we issued
Long Term Incentive Plan
On September 9, 2022, the Company’s Board of Directors approved and adopted the terms and conditions of a long-term incentive plan (“LTIP”) that seeks to motivate and reward employees. The LTIP provides for the issuance of share-based awards to employees of the Company pursuant to the 2014 Plan. The target amount of the stock award under the LTIP for each employee was determined based on a variety of factors. Payout of the stock awards is based on the achievement of pre-established performance objectives and goals related to financing and U.S. Food and Drug Administration (“FDA”) and European Medicines Agency (“EMA”) regulatory milestones for the Company’s Phase 3 clinical trial for rheumatoid arthritis (NAV3-33). The financing and EMA regulatory milestones will each comprise
Although the Company did not fully satisfy the financing milestone, based on completion of the Rights Offering, the Board of Directors decided to pay out
Stock Warrants
On October 25, 2022, the Holders of Series LL warrants to purchase
As of December 31, 2022, there are warrants outstanding to purchase
The following table summarizes information about our outstanding warrants as of December 31, 2022:
|
Exercise Price |
Number of Warrants |
Expiration Date |
|||||||
|
Series HH |
$ | 6/25/2023 | |||||||
|
Series OO |
6/13/2024 | ||||||||
|
Series PP |
8/30/2027 | ||||||||
|
Total warrants |
$ |
* |
|||||||
|
* |
|
Common Stock Reserved
As of December 31, 2022, we have reserved
An additional
|
14. |
Income Taxes |
The components of our deferred tax assets (“DTAs”) as of December 31, 2022 and 2021 are summarized in the following table:
|
As of December 31, |
||||||||
|
2022 |
2021 |
|||||||
| Deferred tax assets: | ||||||||
|
Net operating loss carryforwards |
$ | $ | ||||||
|
R&D credit carryforwards* |
||||||||
|
Stock compensation |
||||||||
|
Intangibles |
||||||||
|
Accrued expenses |
||||||||
|
Capitalized §174 expenses |
||||||||
|
Disallowed interest expense |
||||||||
|
Temporary differences |
||||||||
|
Deferred tax assets before valuation allowance |
||||||||
|
Valuation allowance |
( |
) |
( |
) |
||||
|
Net deferred tax assets |
$ | $ | ||||||
| * |
footnote
Current accounting standards require a valuation allowance against DTAs if, based on the weight of available evidence, it is more likely than not that some or all of the DTAs may not be realized. Due to the uncertainty surrounding the realization of these DTAs in future tax returns, all of the DTAs have been fully offset by a valuation allowance as of December 31, 2022 and 2021.
In assessing the realizability of DTAs, management considers whether it is more likely than not that some portion or all of the DTAs will not be realized. The ultimate realization of DTAs is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities (including the impact of available carryback and carryforward periods) and projected future taxable income in making this assessment. Based upon the level of historical taxable income and projections for future taxable income over the periods in which the DTAs are deductible, management believes it is more likely than not that the Company will not realize the benefits of these deductible differences or tax carryforwards as of December 31, 2022.
As of December 31, 2022 and 2021, we had U.S. net operating loss carryforwards of $
There were no expirations of U.S. NOL carryforwards during 2022 or 2021. U.S. R&D credit carryforwards of $
The details of our U.S. net operating loss and federal R&D credit carryforward amounts and expiration dates as of December 31, 2022 are summarized in the following table:
|
As of December 31, 2022 |
||||||||||||||||||
|
U.S. Net Operating Loss Carryforwards |
U.S. R&D Credit Carryforwards |
|||||||||||||||||
|
Generated |
Expiration |
Amount |
Generated |
Expiration |
Amount |
|||||||||||||
|
2003 |
2023 | $ | — | 2003 | 2023 | $ | ||||||||||||
|
2004 |
2024 | — | 2004 | 2024 | ||||||||||||||
|
2005 |
2025 | — | 2005 | 2025 | ||||||||||||||
|
2006 |
2026 | — | 2006 | 2026 | ||||||||||||||
|
2007 |
2027 | — | 2007 | 2027 | ||||||||||||||
|
2008 |
2028 | — | 2008 | 2028 | ||||||||||||||
|
2009 |
2029 | — | 2009 | 2029 | ||||||||||||||
|
2010 |
2030 | — | 2010 | 2030 | ||||||||||||||
|
2011 |
2031 | — | 2011 | 2031 | ||||||||||||||
|
2012 |
2032 | 2012 | 2032 | |||||||||||||||
|
2013 |
2033 | 2013 | 2033 | |||||||||||||||
|
2014 |
2034 | 2014 | 2034 | |||||||||||||||
|
2015 |
2035 | 2015 | 2035 | |||||||||||||||
|
2016 |
2036 | 2016 | 2036 | |||||||||||||||
|
2017 |
2037 | — | 2017 | 2037 | ||||||||||||||
|
2018 |
N/A | — | 2018 | 2038 | ||||||||||||||
|
2019 |
N/A | 2019 | 2039 | |||||||||||||||
|
2020 |
N/A | 2020 | 2040 | |||||||||||||||
|
2021 |
N/A | 2021 | 2041 | — | ||||||||||||||
|
2022 |
N/A | 2022 | 2042 | — | ||||||||||||||
| Total carryforwards | $ | Total carryforwards | $ | |||||||||||||||
Under Sections 382 and 383 of the Internal Revenue Code (“IRC”) of 1986, as amended, the utilization of U.S. net operating loss and R&D tax credit carryforwards may be limited under the change in stock ownership rules of the IRC. The Company completed a Section 382 analysis through December 31, 2022 and believes that a Section 382 ownership change has not occurred.
Reconciliations between the statutory federal income tax rate and our effective tax rate for continuing operations are presented in the following table:
|
2022 |
2021 |
|||||||||||||||
|
Amount |
% |
Amount |
% |
|||||||||||||
|
Benefit at statutory rate |
$ | ( |
) |
( |
)% |
$ | ( |
) |
( |
)% |
||||||
|
Adjustments to valuation allowance |
% |
% |
||||||||||||||
|
Adjustments to R&D credit carryforwards |
( |
) |
( |
)% |
||||||||||||
|
Permanent items and other |
% | ( |
) | ( |
)% |
|||||||||||
|
Provision (benefit) per financial statements |
$ | $ | ||||||||||||||
|
15. |
Segments |
We report information about our operating segments using the “management approach” in accordance with current accounting standards. This information is based on the way management organizes and reports the segments within the enterprise for making operating decisions and assessing performance. Our reportable segments are identified based on differences in products, services and markets served. There were inter-segment sales. We manage our business based on primary types of drug products: (i) diagnostic substances, including Tc99m tilmanocept and other diagnostic applications of our Manocept platform, and (ii) therapeutic development programs, including therapeutic applications of our Manocept platform.
The information in the following tables is derived directly from each reportable segment’s financial reporting:
|
Year Ended December 31, 2022 |
Diagnostics |
Therapeutics |
Corporate |
Total |
||||||||||||
|
Sales revenue: |
||||||||||||||||
|
United States |
$ | $ | $ | $ | ||||||||||||
|
International |
||||||||||||||||
|
Grant and other revenue |
||||||||||||||||
|
Total revenue |
||||||||||||||||
|
Cost of revenue |
||||||||||||||||
|
Adjustments for expired and expiring inventory |
||||||||||||||||
|
Research and development expenses |
||||||||||||||||
|
Selling, general and administrative expenses, excluding depreciation and amortization (1) |
||||||||||||||||
|
Depreciation and amortization (2) |
||||||||||||||||
|
Loss from operations (3) |
( |
) |
( |
) |
( |
) |
( |
) |
||||||||
|
Other expense, net (4) |
( |
) | ( |
) |
||||||||||||
|
Net loss |
( |
) |
( |
) |
( |
) |
( |
) |
||||||||
| Total assets, net of depreciation and amortization: | ||||||||||||||||
|
United States |
$ | $ | $ | $ | ||||||||||||
|
International |
||||||||||||||||
|
Capital expenditures |
||||||||||||||||
|
Year Ended December 31, 2021 |
Diagnostics |
Therapeutics |
Corporate |
Total |
||||||||||||
|
Royalty revenue |
$ | $ | $ | $ | ||||||||||||
|
License revenue |
||||||||||||||||
|
Grant and other revenue |
||||||||||||||||
|
Total revenue |
||||||||||||||||
|
Research and development expenses, excluding depreciation and amortization |
||||||||||||||||
|
Selling, general and administrative expenses, excluding depreciation and amortization (1) |
||||||||||||||||
|
Depreciation and amortization (2) |
||||||||||||||||
|
Loss from operations (3) |
( |
) |
( |
) |
( |
) |
( |
) |
||||||||
|
Other income (4) |
|
|||||||||||||||
|
Provision for income taxes |
( |
) |
( |
) |
( |
) | ( |
) | ||||||||
|
Net loss |
( |
) |
( |
) |
( |
) |
( |
) |
||||||||
| Total assets, net of depreciation and amortization: | ||||||||||||||||
|
United States |
$ | $ | $ | $ | ||||||||||||
|
International |
||||||||||||||||
|
Capital expenditures |
||||||||||||||||
|
(1) |
|
|
(2) |
|
|
(3) |
|
|
(4) |
|
|
16. |
Material Agreements |
|
a. |
Research and Development Agreements: In January 2002, we completed a license agreement with UCSD for the exclusive world-wide rights to Tc99m tilmanocept for use in lymphatic mapping. The license agreement was effective until the later of the expiration date of the longest-lived underlying patent. In July 2014, we amended the license agreement to extend the agreement until the third anniversary of the expiration date of the longest-lived underlying patent. Under the terms of the license agreement, UCSD granted us the exclusive rights to make, use, sell, offer for sale and import licensed products as defined in the agreement and to practice the defined licensed methods during the term of the agreement. We could also sublicense the patent rights, subject to certain sublicense terms as defined in the agreement. In consideration for the license rights, we agreed to pay UCSD a license issue fee of $ |
In connection with the sale of the rights to sell Tc99m tilmanocept in the United States, Canada and Mexico to Cardinal Health 414, LLC (“Cardinal Health 414”), the Company amended and restated its Tc99m tilmanocept license agreement with UCSD pursuant to which UCSD granted a license to the Company to exploit certain intellectual property rights owned by UCSD and, separately, Cardinal Health 414 entered into a license agreement with UCSD pursuant to which UCSD granted a license to Cardinal Health 414 to exploit certain intellectual property rights owned by UCSD for Cardinal Health 414 to sell Tc99m tilmanocept in the United States, Canada and Mexico. Total costs related to the UCSD license agreement for net sales and royalties of Tc99m tilmanocept outside the United States, Canada and Mexico were $
In July 2014, the Company executed an expanded license agreement for the exclusive world-wide rights to all diagnostic and therapeutic uses of tilmanocept (other than Tc99m tilmanocept used in lymphatic mapping). The license agreement is effective until the third anniversary of the expiration date of the longest-lived underlying patent. Under the terms of the license agreement, UCSD has granted us the exclusive rights to make, use, sell, offer for sale and import licensed products as defined in the agreement and to practice the defined licensed methods during the term of the agreement. We may also sublicense the patent rights, subject to certain sublicense terms as defined in the agreement. As consideration for the license rights, we agreed to pay UCSD a license issue fee of $
|
b. |
Separation Agreement: Effective July 27, 2020 through October 24, 2021, Jed A. Latkin was employed under an employment agreement that provided for an annual base salary of $ |
|
17. |
Employee Benefit Plan |
We maintain an employee benefit plan under Section 401(k) of the IRC (the “401(k) Plan”). The 401(k) Plan allows employees to make contributions and we may, but are not obligated to, match a portion of the employee’s contribution with our Common Stock, up to a defined maximum. The Company’s current matching contribution rate is
|
18. |
Supplemental Disclosure for Statements of Cash Flows |
We paid interest aggregating $
|
19. |
Subsequent Events |
The Company has evaluated events and transactions subsequent to December 31, 2022 and through the date these consolidated financial statements were included in this Annual Report on Form 10-K and filed with the SEC.
|
a. |
Amendment to NOL Rights Agreement: On January 10, 2023, the Company entered into the First Amendment to Section 382 Rights Agreement (“First Amendment”), which amended the NOL Rights Agreement. The First Amendment reduced the “exchange ratio”" from five shares of Common Stock per right to three shares of Common Stock per right. |
|
b. |
UCSD Amendment: On February 16, 2023, the Company and UCSD executed an amendment to the license agreement for the exclusive world-wide rights to all diagnostic and therapeutic uses of tilmanocept (other than Tc99m tilmanocept used in lymphatic mapping). The amendment released the Company from any and all obligations related to certain diligence requirements as defined in the license agreement. The amendment resulted in the reversal of approximately $ |

