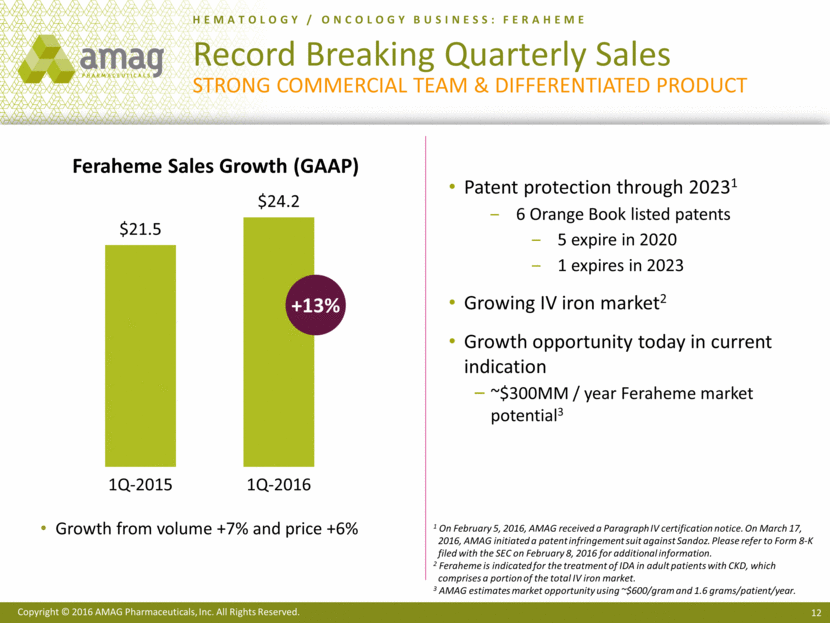Exhibit 99.1

FOR IMMEDIATE RELEASE
AMAG Pharmaceuticals Reports First Quarter 2016 Financial Results
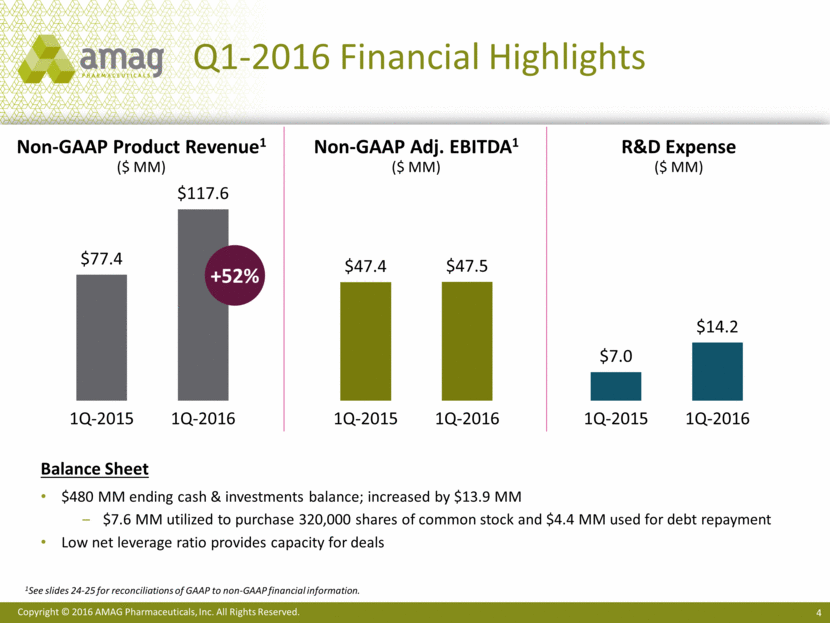
· First quarter 2016 non-GAAP product revenue increased 52% to $117.6 million1
· Entered into new Makena® partnership with a leading provider of home nursing services
Conference call scheduled for 8:00 a.m. ET today
WALTHAM, MA (May 3, 2016) – AMAG Pharmaceuticals, Inc. (NASDAQ: AMAG), a specialty pharmaceutical company with a diverse portfolio of products in the areas of maternal health, anemia management and cancer supportive care, today reported unaudited consolidated financial results for the first quarter ended March 31, 2016.
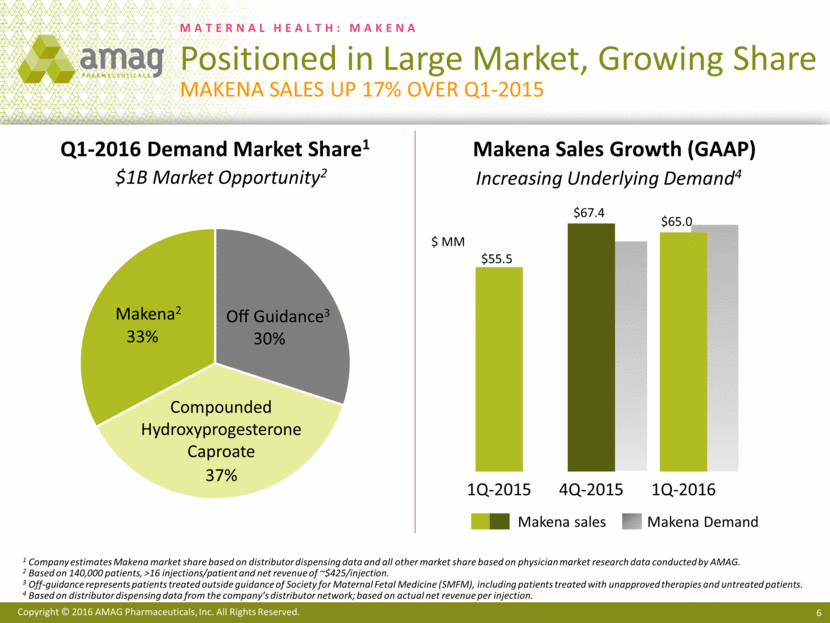
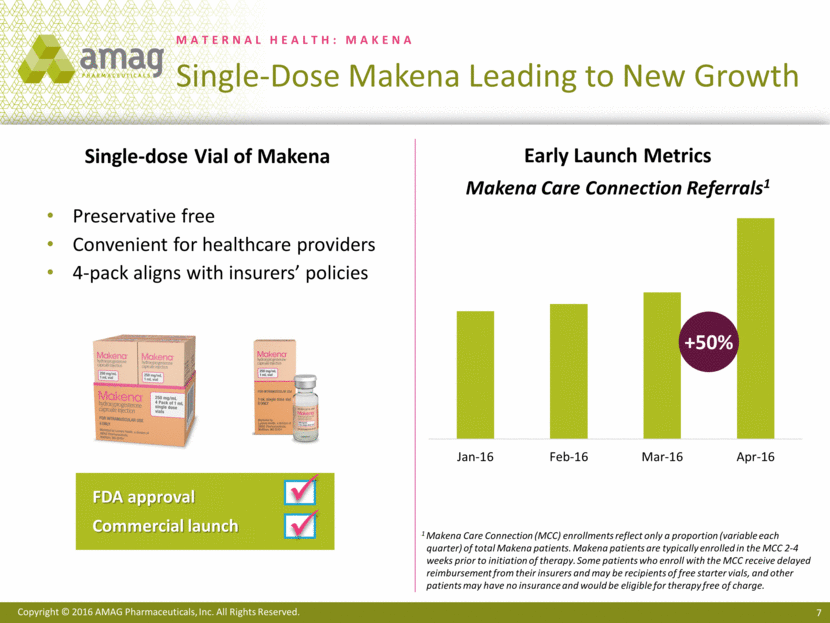
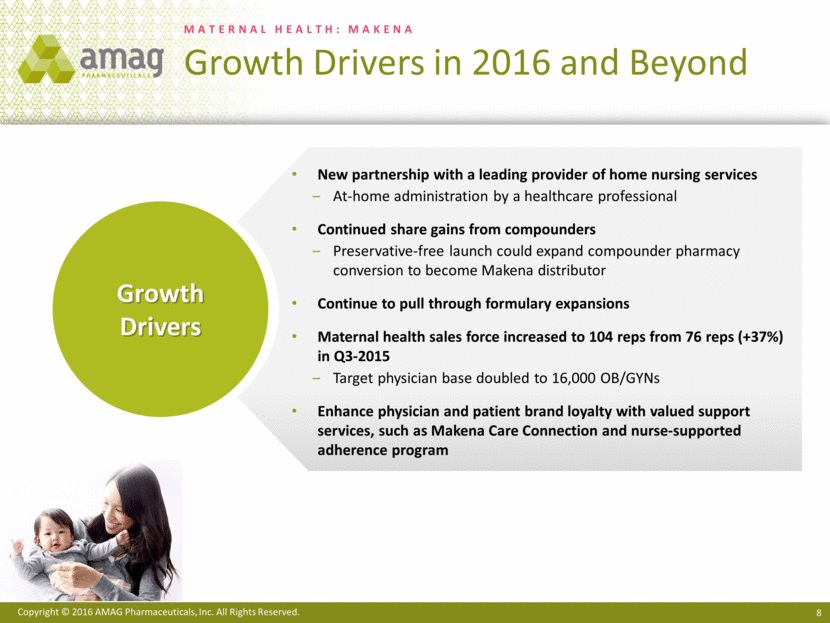
Most notably, the first quarter of 2016 included the approval of a single-dose, preservative-free formulation of Makena® (hydroxyprogesterone caproate injection). Prior to the commercial availability of this new Makena formulation, healthcare providers and patients who wanted a preservative-free formulation of hydroxyprogesterone caproate had to use non-FDA-approved, compounded versions. The company believes the commercial launch of the single-dose, preservative-free formulation of Makena will enable AMAG to capture significant additional share from the compounded segment of the market. The company estimates that compounding pharmacies currently hold approximately 37% of the Makena-eligible market. Additionally, AMAG entered into an agreement with a leading provider of home nursing services, which will exclusively administer Makena to all indicated patients who are eligible for their home health services.
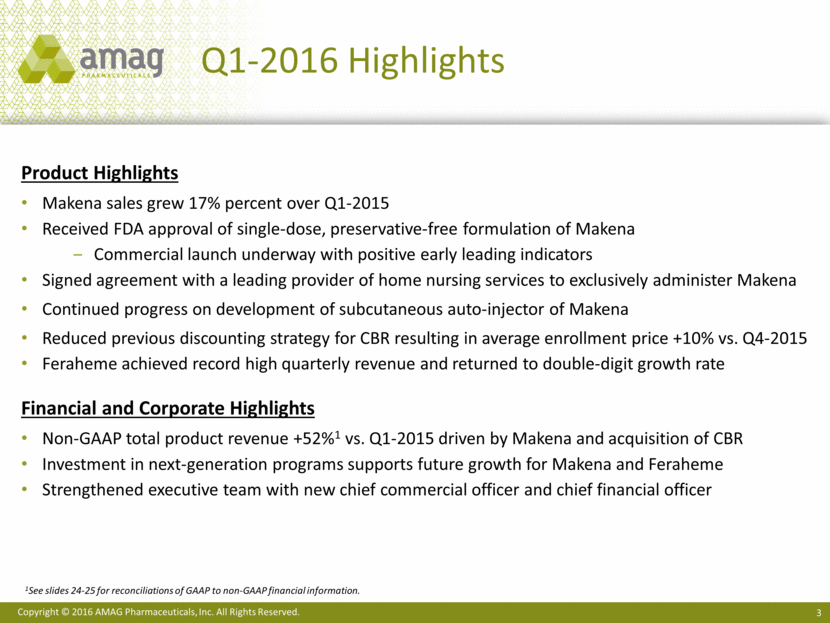
“We are off to a strong start in 2016, achieving goals on our next-generation program for Makena, including the commercial launch of our new single-dose, preservative-free formulation, as well as solidifying an important new strategic relationship to further expand access to FDA-approved Makena,” said William Heiden, AMAG’s chief executive officer. “First quarter 2016 sales of Makena increased 17% over the prior year, and early data from the recent launch of the new formulation of Makena, including April enrollment data at the Makena Care Connection that is up approximately 50% over March, suggests an acceleration in sales growth. We expect these trends to continue through the remainder of the year and are confirming our annual net product sales guidance for Makena.”
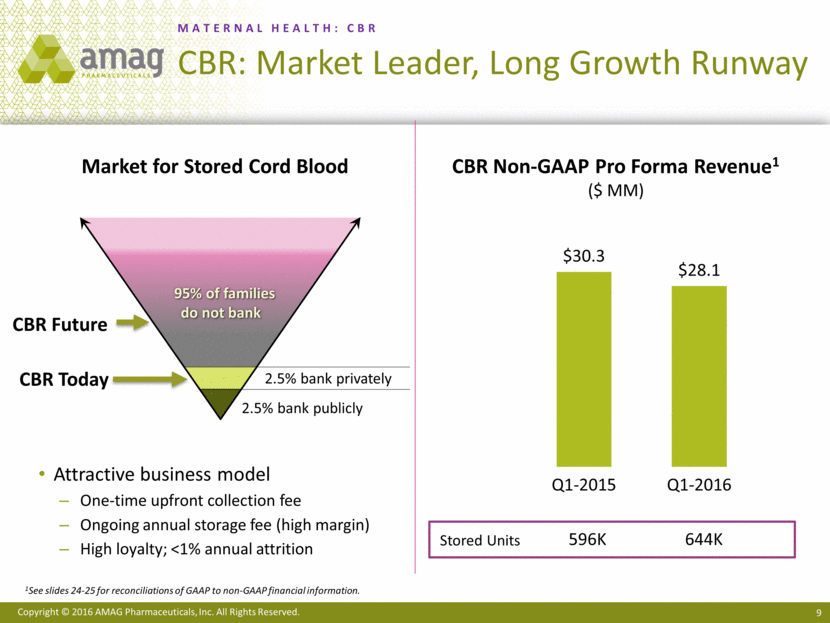
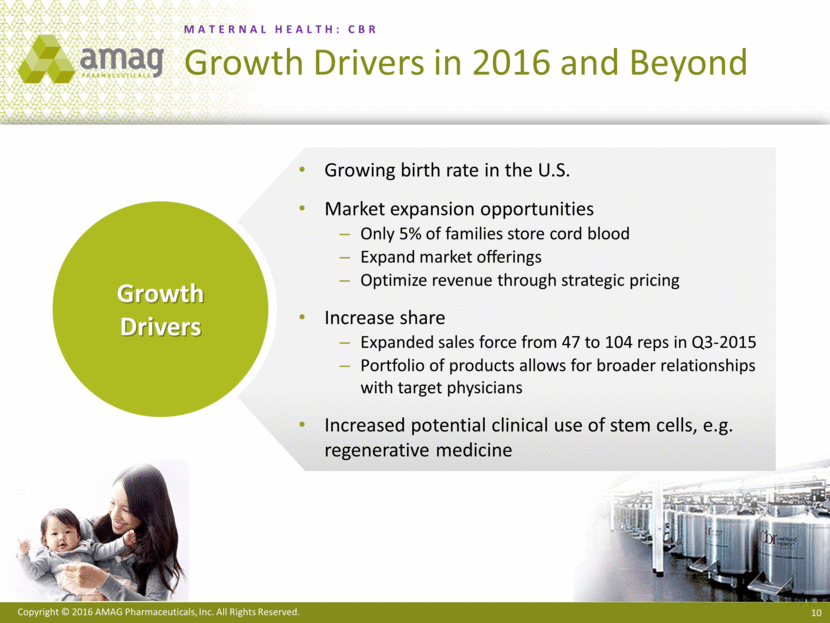
“CBR, our newborn stem cell preservation offering, as well as our anemia management and cancer supportive care products, performed in-line with our expectations and remain on track with our 2016 sales guidance,” added Mr. Heiden.
1 See summaries of non-GAAP adjustments for the three months ended March 31, 2016 and 2015 at the conclusion of this press release.
First Quarter 2016 and Recent Business Highlights:
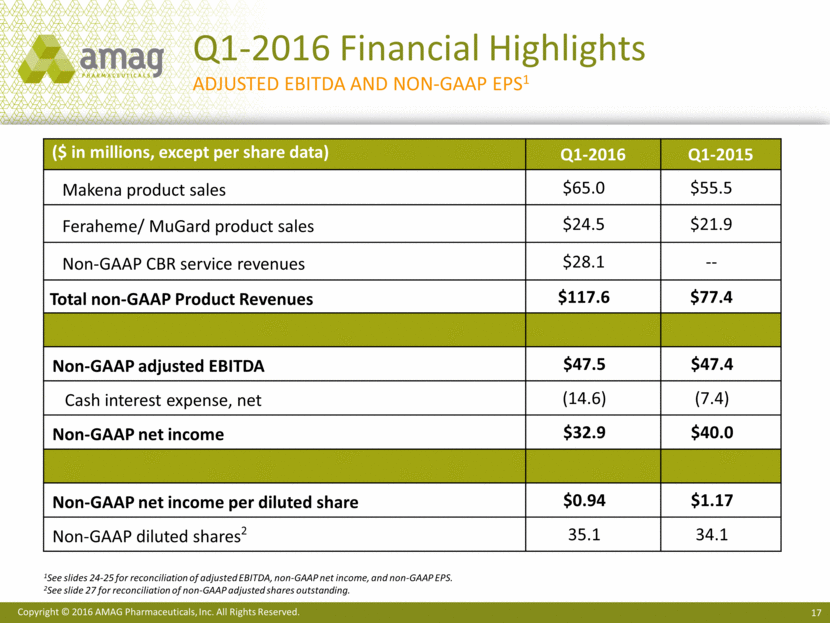
· Increased net product sales of Makena to $65 million, compared with $55.5 million in the first quarter of 2015. This growth in sales was driven by a 16% increase in volume as more at-risk pregnant women were treated with Makena. Net revenue per injection was up 1% versus the first quarter of 2015.
· Received approval from the Food and Drug Administration (FDA) in February 2016 for a single-dose, preservative-free formulation of Makena and began commercial promotion in April 2016.
· Entered into a new agreement with a leading provider of home nursing services, which had previously utilized compounded hydroxyprogesterone caproate and now will exclusively provide at-home administration of Makena.
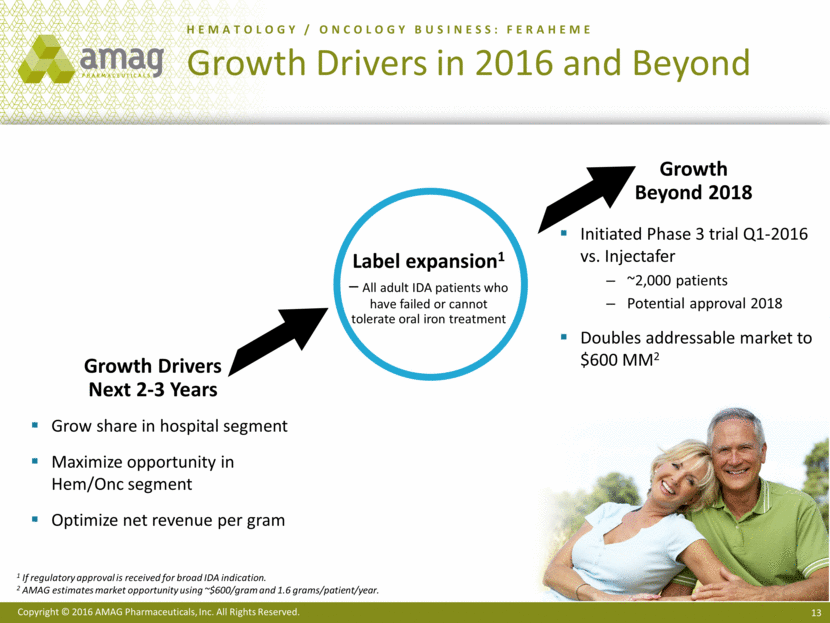
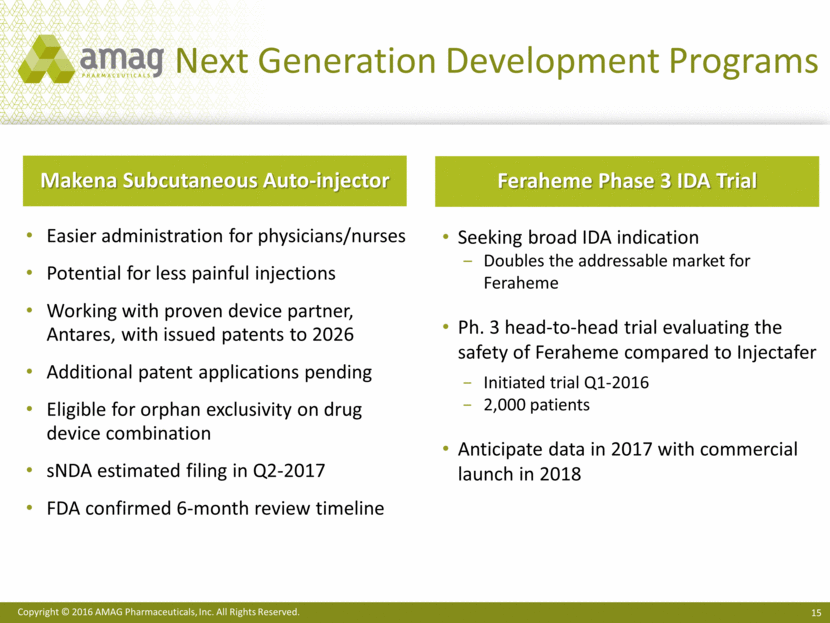
· Continued development of the next-generation program to deliver Makena subcutaneously via an auto-injector, with a range of activities underway, such as CMC work and pilot pharmacokinetic studies. The company currently anticipates filing the supplemental New Drug Application (sNDA) in the second quarter of 2017 and recently received confirmation from the FDA that the review time will be six months from submission.
· Generated a record $24.2 million of Feraheme® (ferumoxytol) sales in the first quarter of 2016, or 13% growth over the same period in the prior year. Strong execution of the Feraheme business strategies by the commercial team enabled the company to realize a 7% increase in volume.
· Began enrolling patients in a head-to-head, Phase 3 clinical trial evaluating the safety of Feraheme compared to Injectafer® (ferric carboxymaltose injection) in adults with iron deficiency anemia (IDA). This study is intended to support an sNDA filing to broaden the use of Feraheme beyond the current chronic kidney disease (CKD) indication to include all adult IDA patients who have failed or cannot tolerate oral iron treatment.
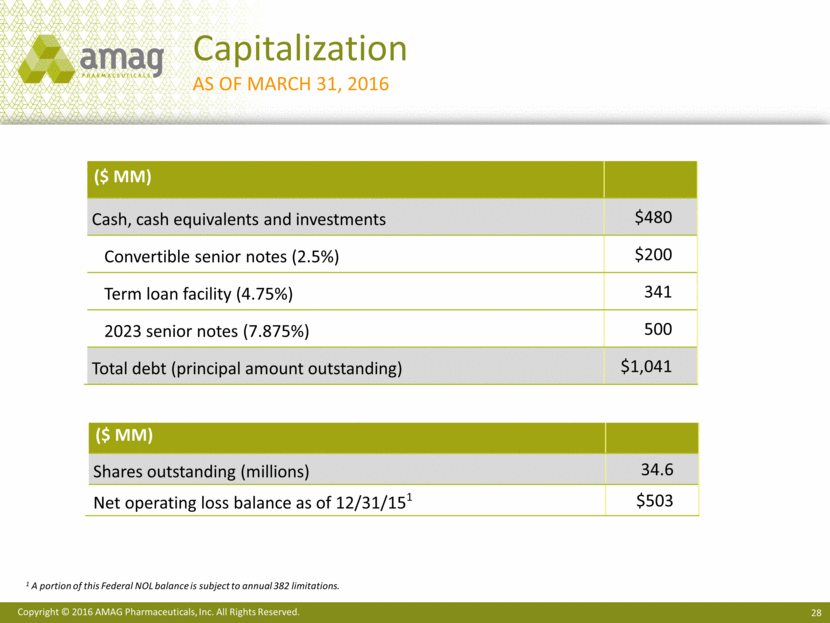
· Increased cash, cash equivalents and investments by $13.9 million to $480 million, net of $12 million utilized to purchase the company’s common stock and to repay debt.
· Strengthened the executive management team with the additions of Nik Grund as chief commercial officer and Ted Myles as chief financial officer.
First Quarter Ended March 31, 2016 (unaudited)
Financial Results (GAAP Basis)
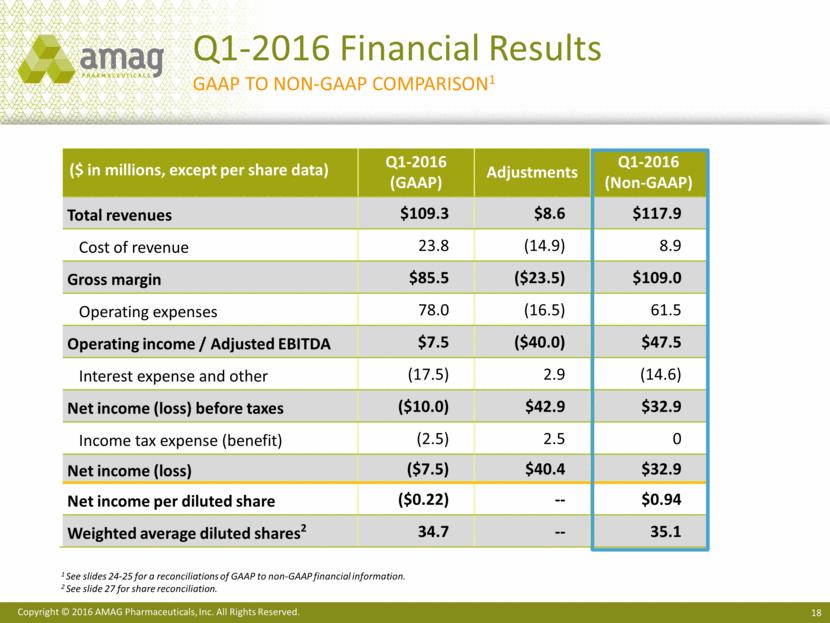
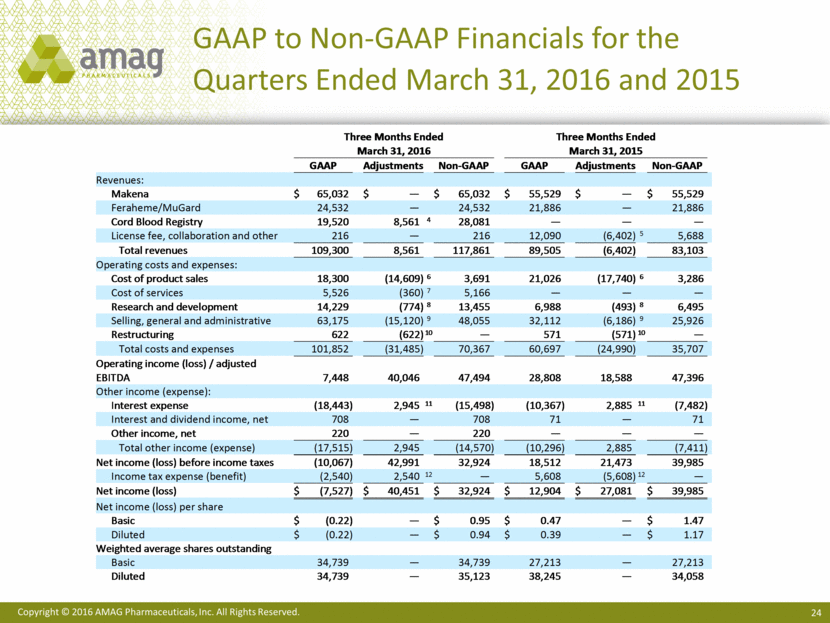
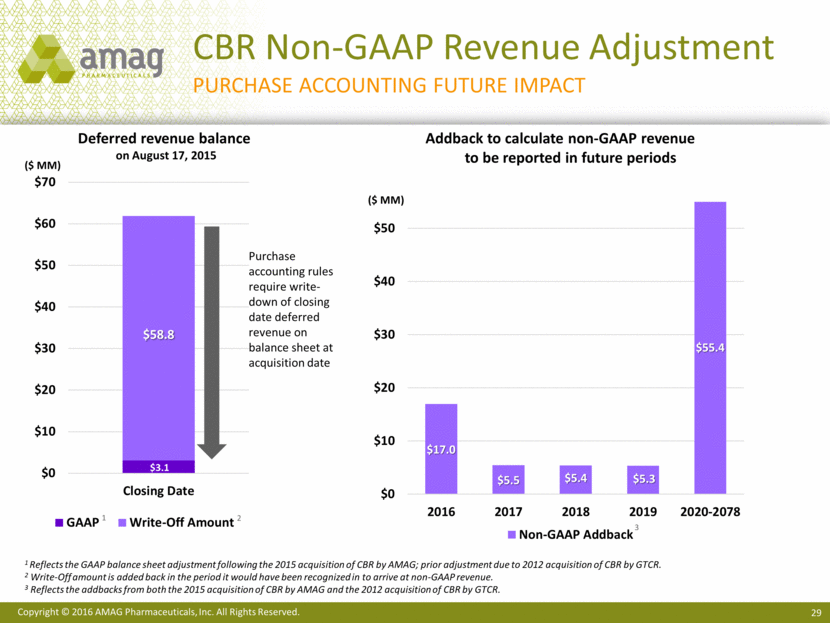
Total revenues for the first quarter of 2016 were $109.3 million, compared with $89.5 million in the first quarter of 2015. Net product sales of Makena were $65.0 million in the first quarter of 2016, compared with $55.5 million in the same period last year. Sales of Feraheme and MuGard® totaled $24.5 million in the first quarter of 2016, compared with $21.9 million in the first quarter of 2015. Service revenue from Cord Blood Registry® (CBR), which AMAG purchased in August 2015, totaled $19.5 million in the first quarter of 2016.
Costs of product sales and services totaled $23.8 million in the first quarter of 2016. In the first quarter of 2015, cost of product sales totaled $21.0 million and did not include CBR cost of services. Total operating expenses for the first quarter of 2016 were $78.0 million, compared with $39.7 million for the same period in 2015. The increase in operating expenses was primarily due to the acquisition of CBR in the third quarter of 2015, including the non-cash amortization of intangible assets, and higher research and development
costs. These R&D costs included the initiation of the company’s Phase 3 clinical trial to broaden the use of Feraheme to include all adult IDA patients, costs to support our Makena subcutaneous auto-injector and costs associated with preparing and filing with the FDA for a second source manufacturer of the single-dose, preservative-free formulation of Makena.
The company reported operating income of $7.4 million and a net loss of $7.5 million, or ($0.22) per basic and diluted share, for the first quarter of 2016, compared with operating income of $28.8 million and net income of $12.9 million, or $0.47 per basic share and $0.39 per diluted share, for the same period in 2015.
Financial Results (Non-GAAP Basis)1,2
Non-GAAP revenues totaled $117.9 million in the first quarter of 2016, up from $83.1 million in the first quarter of 2015. Non-GAAP CBR revenue totaled $28.1 million in the first quarter of 2016. The difference between GAAP and non-GAAP revenue for CBR represents purchase accounting adjustments related to deferred revenue. Non-GAAP revenue in 2015 excludes certain non-cash revenue related to the company’s ex-U.S. marketing agreement with its former partner, Takeda Pharmaceutical Company Limited.
Total costs and expenses on a non-GAAP basis totaled $70.4 million resulting in a gross margin of 92% and adjusted EBITDA margin of 40% for the first quarter of 2016. This compares to costs and expenses of $35.7 million in the same period of 2015, which resulted in a gross margin of 96% and adjusted EBITDA margin of 57%. The decline in gross margin resulted from the acquisition of CBR, which carries lower gross margins than the company’s pharmaceutical products. Investments in research and development to enhance the long-term revenue potential of Makena and Feraheme contributed to the lower adjusted EBITDA margin in the first quarter of 2016. Non-GAAP adjusted EBITDA for the first quarter of 2016 was $47.5 million, compared with $47.4 million for the same period in 2015.
After deducting cash interest expense, the company generated first quarter 2016 non-GAAP net income of $32.9 million, or $0.95 per non-GAAP basic share and $0.94 per non-GAAP diluted share. In the first quarter of 2015, non-GAAP net income totaled $40.0 million, or $1.47 per non-GAAP basic share and $1.17 per non-GAAP diluted share.
Balance Sheet Highlights
As of March 31, 2016, the company’s cash and investments totaled approximately $480 million and total debt (principal amount outstanding) was approximately $1.04 billion.
“We are reiterating our full year 2016 guidance for revenue, adjusted EBITDA and non-GAAP net income, including top-line revenue growth of approximately 40%, which underscores the strong recent trends for Makena and the overall underlying demand we are generating across our portfolio of products,” said Frank Thomas, president and chief operating officer. “During the quarter and throughout 2016, we are investing in our products through R&D to potentially expand our label for Feraheme and provide more patient- and provider-friendly versions of Makena. We believe these investments will enhance the long-term revenue potential of these products.”
2 See share count reconciliation at the conclusion of this press release.
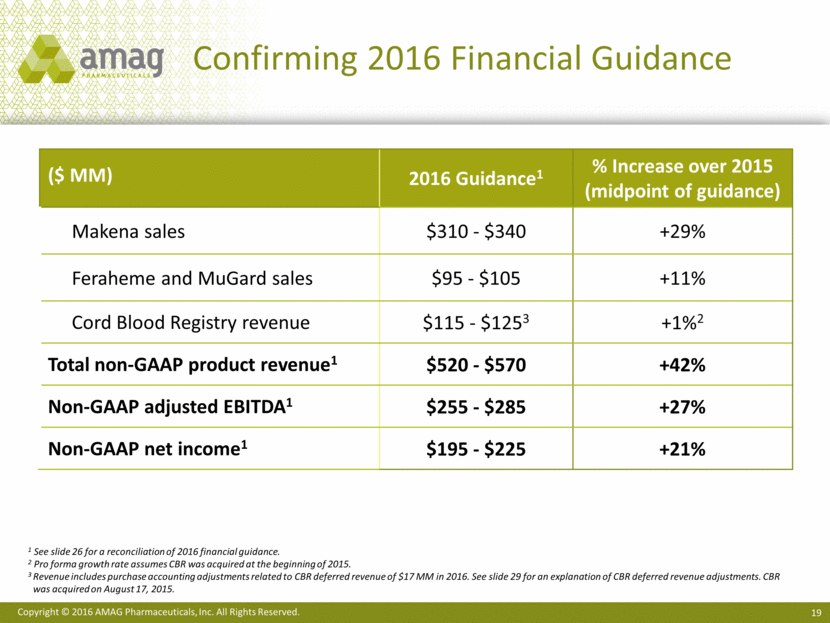
2016 Financial Guidance3
|
$ in millions |
|
2016 Guidance |
|
|
Makena sales |
|
$310 - $340 |
|
|
Feraheme and MuGard sales |
|
$95 - $105 |
|
|
Non-GAAP CBR revenue |
|
$115 -$125 |
|
|
Non-GAAP total product revenue |
|
$520 - $570 |
|
|
Non-GAAP adjusted EBITDA |
|
$255- $285 |
|
|
Non-GAAP net income |
|
$195- $225 |
|
Conference Call and Webcast Access
AMAG Pharmaceuticals, Inc. will host a conference call and webcast with slides today at 8:00 a.m. ET, during which management will discuss the company’s financial and operating results and recent developments. To access the conference call via telephone, please dial (877) 412-6083 from the United States or (702) 495-1202 for international access. A telephone replay will be available from approximately 11:00 a.m. ET on May 3, 2016 through midnight on May 10, 2016. To access a replay of the conference call, dial (855) 859-2056 from the United States or (404) 537-3406 for international access. The pass code for the live call and the replay is 90192659.
The call will be webcast with slides and accessible through the Investors section of the company’s website at www.amagpharma.com. The webcast replay will be available from approximately 11:00 a.m. ET on May 3, 2016 through midnight on June 3, 2016.
Use of Non-GAAP Financial Measures
AMAG has presented certain non-GAAP financial measures, including non-GAAP revenue, non-GAAP adjusted EBITDA (earnings before income taxes, depreciation and amortization), non-GAAP net income, non-GAAP diluted net income per share, and non-GAAP weighted average diluted shares. These non-GAAP financial measures exclude certain amounts, revenue, expenses or income, from the corresponding financial measures determined in accordance with accounting principles generally accepted in the U.S. (GAAP). Management believes this non-GAAP information is useful for investors, taken in conjunction with AMAG’s GAAP financial statements, because it provides greater transparency regarding AMAG’s operating performance. Management uses these measures, among other factors, to assess and analyze operational results and trends and to make financial and operational decisions. Non-GAAP information is not prepared under a comprehensive set of accounting rules and should only be used to supplement an understanding of AMAG’s operating results as reported under GAAP, not as a substitute for GAAP. In addition, these non-GAAP financial measures are unlikely to be comparable with non-GAAP information provided by other companies. The determination of the amounts that are excluded from non-GAAP financial measures is a matter of management judgment and depends upon, among other factors, the nature of the underlying expense or income amounts. Reconciliations between these non-GAAP financial measures and the most comparable GAAP financial measures are included in the tables accompanying this press release after the unaudited condensed consolidated financial statements.
3 See reconciliation of 2016 financial guidance of non-GAAP CBR revenue, non-GAAP adjusted EBITDA and non-GAAP net income at the conclusion of this press release.
About AMAG
AMAG is a biopharmaceutical company focused on bringing therapeutics to market that provide clear benefits and help improve people’s lives. Headquartered in Waltham, MA, AMAG possesses a diverse portfolio of products to support the health of patients in the areas of maternal health, anemia management and cancer supportive care. Through CBR®, the company also helps families to preserve newborn stem cells, which are used today in transplant medicine for certain cancers and blood, immune and metabolic disorders, and have the potential to play a valuable role in the ongoing development of regenerative medicine. For additional company information, please visit www.amagpharma.com.
About Makena® (hydroxyprogesterone caproate injection)
Makena® is a progestin indicated to reduce the risk of preterm birth in women pregnant with a single baby who have a history of singleton spontaneous preterm birth.
The effectiveness of Makena is based on improvement in the proportion of women who delivered <37 weeks of gestation. There are no controlled trials demonstrating a direct clinical benefit, such as improvement in neonatal mortality and morbidity.
Limitation of use: While there are many risk factors for preterm birth, safety and efficacy of Makena has been demonstrated only in women with a prior spontaneous singleton preterm birth. It is not intended for use in women with multiple gestations or other risk factors for preterm birth.
Makena should not be used in women with any of the following conditions: blood clots or other blood clotting problems, breast cancer or other hormone-sensitive cancers, or history of these conditions; unusual vaginal bleeding not related to the current pregnancy, yellowing of the skin due to liver problems during pregnancy, liver problems, including liver tumors, or uncontrolled high blood pressure.
Before patients receive Makena, they should tell their healthcare provider if they have an allergy to hydroxyprogesterone caproate, castor oil, or any of the other ingredients in Makena; diabetes or prediabetes, epilepsy, migraine headaches, asthma, heart problems, kidney problems, depression, or high blood pressure.
In one clinical study, certain complications or events associated with pregnancy occurred more often in women who received Makena. These included miscarriage (pregnancy loss before 20 weeks of pregnancy), stillbirth (fetal death occurring during or after the 20th week of pregnancy), hospital admission for preterm labor, preeclampsia (high blood pressure and too much protein in the urine), gestational hypertension (high blood pressure caused by pregnancy), gestational diabetes, and oligohydramnios (low amniotic fluid levels).
Makena may cause serious side effects including blood clots, allergic reactions, depression, and yellowing of the skin and the whites of the eyes. The most common side effects of Makena include injection site reactions (pain, swelling, itching, bruising, or a hard bump), hives, itching, nausea, and diarrhea. For additional product information, including full prescribing information, please visit www.makena.com.
About Feraheme® (ferumoxytol)
Feraheme received marketing approval from the FDA on June 30, 2009 for the treatment of IDA in adult CKD patients and was commercially launched by AMAG in the U.S. shortly thereafter. Ferumoxytol is protected in the U.S. by six issued patents covering the composition and dosage form of the product. Each issued patent is listed in the FDA’s Orange Book, the last of which expires in June 2023.
Fatal and serious hypersensitivity reactions including anaphylaxis have occurred in patients receiving Feraheme. Initial symptoms may include hypotension, syncope, unresponsiveness, cardiac/cardiorespiratory arrest. Feraheme is contraindicated in patients with a known hypersensitivity to Feraheme or any of its components, or a history of allergic reaction to any intravenous iron product.
For additional product information, please see full Prescribing Information, including Boxed Warning, available at www.feraheme.com.
About Cord Blood Registry® (CBR)
CBR is the world’s largest private newborn stem cell company. Founded in 1992, CBR is entrusted by parents with storing approximately 644,000 umbilical cord blood and cord tissue units. CBR is dedicated to advancing the clinical application of newborn stem cells by partnering with reputable research institutions on FDA-regulated clinical trials for conditions that have no cure today. For more information, visit www.cordblood.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA) and other federal securities laws. Any statements contained herein which do not describe historical facts, including, among others, AMAG’s belief that the single-dose, preservative-free formulation of Makena will allow AMAG to capture significant market share from the compounded segment of the market and the estimated market share of the compounded segment; AMAG’s expectations concerning the impact of strategic relationships on access to Makena; expectations that the recent acceleration in Makena sales growth will continue for the remainder of the year; AMAG’s belief that product revenues, including CBR and Feraheme, remain on track with 2016 sales guidance; expectations for AMAG’s next generation development programs for Makena, including the FDA review period for the auto-injector and the anticipated timing to file a sNDA for the subcutaneous auto-injector; expectations for AMAG’s Phase 3 clinical trial for the broader indication for Feraheme; AMAG’s expected 2016 first quarter financial results, including revenues and year-end cash and investment balances and total debt; AMAG’s 2016 financial guidance, including revenues, adjusted EBITDA and non-GAAP net income; and beliefs that AMAG’s investment in research and development of our products will enhance their long-term revenue potential, expand Feraheme’s label and provide more patient- and provider-friendly versions of Makena are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements.
Such risks and uncertainties include, among others, those risks identified in AMAG’s filings with the U.S. Securities and Exchange Commission (SEC), including its Annual Report on Form 10-K for the year ended December 31, 2015 and subsequent filings with the SEC. Any such risks and uncertainties could materially and adversely affect AMAG’s results of operations, its profitability and its cash flows, which would, in turn,
have a significant and adverse impact on AMAG’s stock price. AMAG cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made.
AMAG disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements.
AMAG Pharmaceuticals® and Feraheme® are registered trademark of AMAG Pharmaceuticals, Inc. MuGard® is a registered trademark of Abeona Therapeutics, Inc. Makena® is a registered trademark of AMAG Pharmaceuticals IP, Ltd. Cord Blood Registry® and CBR® are registered trademarks of CBR Systems, Inc.
– Tables Follow –
AMAG Pharmaceuticals, Inc.
Condensed Consolidated Statements of Operations
(unaudited, amounts in thousands, except for per share data)
|
|
|
Three Months Ended |
| ||||
|
|
|
March 31, |
| ||||
|
|
|
2016 |
|
2015 |
| ||
|
Revenues: |
|
|
|
|
| ||
|
Makena |
|
$ |
65,032 |
|
$ |
55,529 |
|
|
Feraheme/MuGard |
|
24,532 |
|
21,886 |
| ||
|
Cord Blood Registry |
|
19,520 |
|
— |
| ||
|
License fee, collaboration and other revenues |
|
216 |
|
12,090 |
| ||
|
Total revenues |
|
109,300 |
|
89,505 |
| ||
|
Operating costs and expenses: |
|
|
|
|
| ||
|
Cost of product sales |
|
18,300 |
|
21,026 |
| ||
|
Cost of services |
|
5,526 |
|
— |
| ||
|
Research and development expenses |
|
14,229 |
|
6,988 |
| ||
|
Selling, general and administrative expenses |
|
63,175 |
|
32,112 |
| ||
|
Restructuring expenses |
|
622 |
|
571 |
| ||
|
Total costs and expenses |
|
101,852 |
|
60,697 |
| ||
|
Operating income |
|
7,448 |
|
28,808 |
| ||
|
|
|
|
|
|
| ||
|
Other income (expense): |
|
|
|
|
| ||
|
Interest expense |
|
(18,443) |
|
(10,367) |
| ||
|
Interest and dividend income, net |
|
708 |
|
71 |
| ||
|
Other income (expense) |
|
220 |
|
— |
| ||
|
Total other income (expense) |
|
(17,515) |
|
(10,296) |
| ||
|
Net income (loss) before income taxes |
|
(10,067) |
|
18,512 |
| ||
|
Income tax expense (benefit) |
|
(2,540) |
|
5,608 |
| ||
|
Net income (loss) |
|
$ |
(7,527) |
|
$ |
12,904 |
|
|
|
|
|
|
|
| ||
|
Net income (loss) per share |
|
|
|
|
| ||
|
Basic |
|
$ |
(0.22) |
|
$ |
0.47 |
|
|
Diluted |
|
$ |
(0.22) |
|
$ |
0.39 |
|
|
|
|
|
|
|
| ||
|
Weighted average shares outstanding used to compute net income (loss) per share: |
|
|
|
|
| ||
|
Basic |
|
34,739 |
|
27,213 |
| ||
|
Diluted |
|
34,739 |
|
38,245 |
| ||
AMAG Pharmaceuticals, Inc.
Condensed Consolidated Balance Sheets
(unaudited, amounts in thousands)
|
|
|
March 31, 2016 |
|
December 31, 2015 |
| ||
|
Assets: |
|
|
|
|
| ||
|
Cash and cash equivalents |
|
$ |
203,389 |
|
$ |
228,705 |
|
|
Investments |
|
276,816 |
|
237,626 |
| ||
|
Accounts receivable, net |
|
82,754 |
|
85,678 |
| ||
|
Inventories |
|
42,002 |
|
40,645 |
| ||
|
Receivable from collaboration |
|
183 |
|
428 |
| ||
|
Prepaid and other current assets |
|
17,089 |
|
13,592 |
| ||
|
Total current assets |
|
622,233 |
|
606,674 |
| ||
|
Property, plant and equipment, net |
|
27,937 |
|
28,725 |
| ||
|
Goodwill |
|
639,484 |
|
639,188 |
| ||
|
Intangible assets, net |
|
1,180,124 |
|
1,196,771 |
| ||
|
Restricted cash |
|
2,593 |
|
2,593 |
| ||
|
Other long-term assets |
|
1,290 |
|
2,259 |
| ||
|
Total assets |
|
$ |
2,473,661 |
|
$ |
2,476,210 |
|
|
|
|
|
|
|
| ||
|
Liabilities and stockholders’ equity: |
|
|
|
|
| ||
|
Accounts payable |
|
$ |
3,427 |
|
$ |
4,906 |
|
|
Accrued expenses |
|
101,895 |
|
106,363 |
| ||
|
Current portion of long-term debt |
|
17,500 |
|
17,500 |
| ||
|
Current portion of acquisition-related contingent consideration |
|
98,436 |
|
96,967 |
| ||
|
Deferred revenues |
|
27,336 |
|
20,185 |
| ||
|
Total current liabilities |
|
248,594 |
|
245,921 |
| ||
|
Long-term liabilities: |
|
|
|
|
| ||
|
Long-term debt, net |
|
800,158 |
|
803,669 |
| ||
|
Convertible 2.5% notes, net |
|
172,822 |
|
170,749 |
| ||
|
Acquisition-related contingent consideration |
|
129,114 |
|
125,592 |
| ||
|
Deferred tax liabilities |
|
188,634 |
|
189,145 |
| ||
|
Deferred revenues |
|
7,659 |
|
5,093 |
| ||
|
Other long-term liabilities |
|
3,708 |
|
3,777 |
| ||
|
Total liabilities |
|
1,550,689 |
|
1,543,946 |
| ||
|
Total stockholders’ equity |
|
922,972 |
|
932,264 |
| ||
|
Total liabilities and stockholders’ equity |
|
$ |
2,473,661 |
|
$ |
2,476,210 |
|
AMAG Pharmaceuticals, Inc.
Reconciliation of Condensed Consolidated Statements of Operations to Non-GAAP Statements of Operations
(unaudited, amounts in thousands, except per share data)
|
|
|
Three Months Ended |
|
Three Months Ended |
| ||||||||||||||
|
|
|
March 31, 2016 |
|
March 31, 2015 |
| ||||||||||||||
|
|
|
GAAP |
|
Adjustments |
|
Non-GAAP |
|
GAAP |
|
Adjustments |
|
Non-GAAP |
| ||||||
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Makena |
|
$ |
65,032 |
|
$ |
— |
|
$ |
65,032 |
|
$ |
55,529 |
|
$ |
— |
|
$ |
55,529 |
|
|
Feraheme/MuGard |
|
|
24,532 |
|
|
— |
|
|
24,532 |
|
|
21,886 |
|
|
— |
|
|
21,886 |
|
|
Cord Blood Registry |
|
|
19,520 |
|
|
8,561 |
4 |
|
28,081 |
|
|
— |
|
|
— |
|
|
— |
|
|
License fee, collaboration and other |
|
|
216 |
|
|
— |
|
|
216 |
|
|
12,090 |
|
|
(6,402) |
5 |
|
5,688 |
|
|
Total revenues |
|
|
109,300 |
|
|
8,561 |
|
|
117,861 |
|
|
89,505 |
|
|
(6,402) |
|
|
83,103 |
|
|
Operating costs and expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of product sales |
|
|
18,300 |
|
|
(14,609) |
6 |
|
3,691 |
|
|
21,026 |
|
|
(17,740) |
6 |
|
3,286 |
|
|
Cost of services |
|
|
5,526 |
|
|
(360) |
7 |
|
5,166 |
|
|
— |
|
|
— |
|
|
— |
|
|
Research and development |
|
|
14,229 |
|
|
(774) |
8 |
|
13,455 |
|
|
6,988 |
|
|
(493) |
8 |
|
6,495 |
|
|
Selling, general and administrative |
|
|
63,175 |
|
|
(15,120) |
9 |
|
48,055 |
|
|
32,112 |
|
|
(6,186) |
9 |
|
25,926 |
|
|
Restructuring |
|
|
622 |
|
|
(622) |
10 |
|
— |
|
|
571 |
|
|
(571) |
10 |
|
— |
|
|
Total costs and expenses |
|
|
101,852 |
|
|
(31,485) |
|
|
70,367 |
|
|
60,697 |
|
|
(24,990) |
|
|
35,707 |
|
|
Operating income (loss) / adjusted EBITDA |
|
|
7,448 |
|
|
40,046 |
|
|
47,494 |
|
|
28,808 |
|
|
18,588 |
|
|
47,396 |
|
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense |
|
|
(18,443) |
|
|
2,945 |
11 |
|
(15,498) |
|
|
(10,367) |
|
|
2,885 |
11 |
|
(7,482) |
|
|
Interest and dividend income, net |
|
|
708 |
|
|
— |
|
|
708 |
|
|
71 |
|
|
— |
|
|
71 |
|
|
Other income, net |
|
|
220 |
|
|
— |
|
|
220 |
|
|
— |
|
|
— |
|
|
— |
|
|
Total other income (expense) |
|
|
(17,515) |
|
|
2,945 |
|
|
(14,570) |
|
|
(10,296) |
|
|
2,885 |
|
|
(7,411) |
|
|
Net income (loss) before income taxes |
|
|
(10,067) |
|
|
42,991 |
|
|
32,924 |
|
|
18,512 |
|
|
21,473 |
|
|
39,985 |
|
|
Income tax expense (benefit) |
|
|
(2,540) |
|
|
2,540 |
12 |
|
— |
|
|
5,608 |
|
|
(5,608) |
12 |
|
— |
|
|
Net income (loss) |
|
$ |
(7,527) |
|
$ |
40,451 |
|
$ |
32,924 |
|
$ |
12,904 |
|
$ |
27,081 |
|
$ |
39,985 |
|
|
Net income (loss) per share |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(0.22) |
|
|
— |
|
$ |
0.95 |
|
$ |
0.47 |
|
|
— |
|
$ |
1.47 |
|
|
Diluted |
|
$ |
(0.22) |
|
|
— |
|
$ |
0.94 |
|
$ |
0.39 |
|
|
— |
|
$ |
1.17 |
|
|
Weighted average shares outstanding |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
34,739 |
|
|
— |
|
|
34,739 |
|
|
27,213 |
|
|
— |
|
|
27,213 |
|
|
Diluted |
|
|
34,739 |
|
|
— |
|
|
35,123 |
|
|
38,245 |
|
|
— |
|
|
34,058 |
|
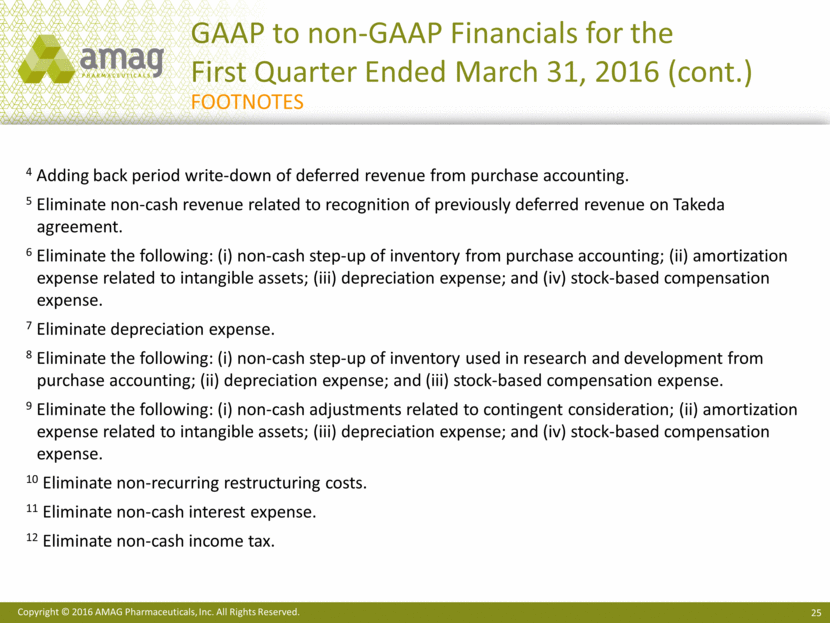
4 Adding back period write-down of deferred revenue from purchase accounting.
5 Eliminate non-cash revenue related to recognition of previously deferred revenue on Takeda agreement.
6 Eliminate the following: (i) non-cash step-up of inventory from purchase accounting; (ii) amortization expense related to intangible assets; (iii) depreciation expense; and (iv) stock-based compensation expense.
7 Eliminate depreciation expense.
8 Eliminate the following: (i) non-cash step-up of inventory used in research and development from purchase accounting; (ii) depreciation expense; and (iii) stock-based compensation expense.
9 Eliminate the following: (i) non-cash adjustments related to contingent consideration; (ii) amortization expense related to intangible assets; (iii) depreciation expense; and (iv) stock-based compensation expense.
10 Eliminate non-recurring restructuring costs.
11 Eliminate non-cash interest expense.
12 Eliminate non-cash income tax.
AMAG Pharmaceuticals, Inc.
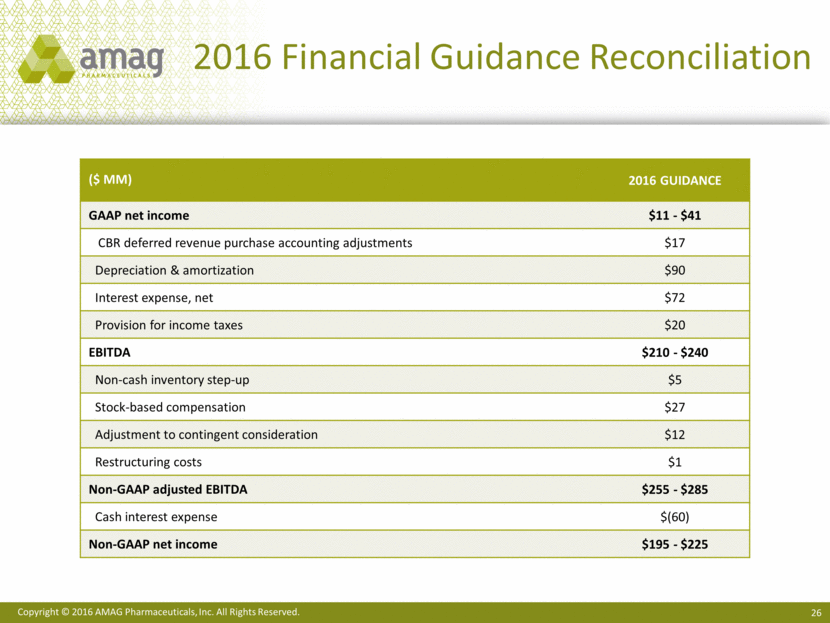
Reconciliation of 2016 Financial Guidance of Non-GAAP Adjusted EBITDA
and Non-GAAP Net Income
(unaudited, amounts in millions)
|
|
|
2016 |
|
|
|
Financial |
|
|
|
Guidance |
|
GAAP net income |
|
$11 - 41 |
|
Purchase accounting adjustments related to CBR deferred revenue |
|
17 |
|
Depreciation and amortization |
|
90 |
|
Interest expense, net |
|
72 |
|
Provision for income taxes |
|
20 |
|
EBITDA |
|
$210 - 240 |
|
Non-cash inventory step-up adjustments |
|
5 |
|
Stock-based compensation |
|
27 |
|
Adjustments to contingent consideration |
|
12 |
|
Restructuring costs |
|
1 |
|
Non-GAAP adjusted EBITDA |
|
$255 - 285 |
|
Cash interest expense |
|
(60) |
|
Non-GAAP net income |
|
$195 - 225 |
AMAG Pharmaceuticals, Inc.
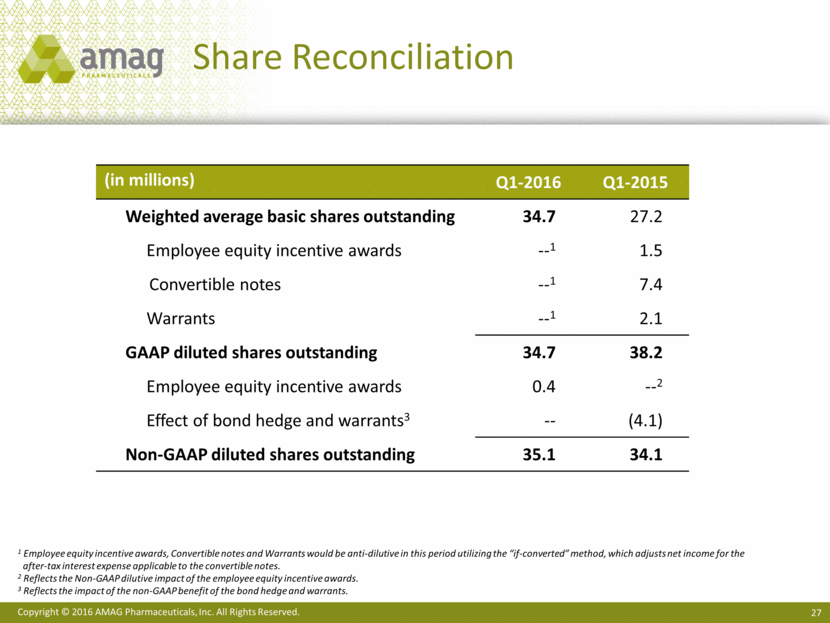
Share Count Reconciliation
(unaudited, amounts in millions)
|
|
|
Three Months Ended |
|
Three Months Ended |
|
|
|
|
March 31, 2016 |
|
March 31, 2015 |
|
|
Weighted average basic shares outstanding |
|
34.7 |
|
27.2 |
|
|
Employee equity incentive awards |
|
— |
13 |
1.5 |
|
|
Convertible notes |
|
— |
13 |
7.4 |
|
|
Warrants |
|
— |
13 |
2.1 |
|
|
GAAP diluted shares outstanding |
|
34.7 |
|
38.2 |
|
|
Employee equity incentive awards |
|
0.4 |
14 |
— |
|
|
Effect of bond hedge and warrants |
|
— |
|
(4.1) |
15 |
|
Non-GAAP diluted shares outstanding |
|
35.1 |
|
34.1 |
|
13 Employee equity incentive awards, Convertible notes and Warrants would be anti-dilutive in this period utilizing the “if-converted” method, which adjusts net income for the after-tax interest expense applicable to the convertible notes.
14 Reflects the Non-GAAP dilutive impact of the employee equity incentive awards.
15 Reflects the impact of the non-GAAP benefit of the bond hedge and warrants.
CONTACT:
AMAG Pharmaceuticals, Inc.
Linda Lennox
Vice President, Investor Relations
617-498-2846