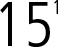0000078003DEF 14AFALSE00000780032023-01-012023-12-31iso4217:USD00000780032022-01-012022-12-3100000780032021-01-012021-12-3100000780032020-01-012020-12-310000078003ecd:NonPeoNeoMemberpfe:ProRataTargetBonusAwardMember2023-01-012023-12-31000007800312023-01-012023-12-310000078003ecd:PeoMemberpfe:StockAndOptionAwardsAdjustmentsMember2023-01-012023-12-310000078003ecd:PeoMemberpfe:ChangeInPensionValueMember2023-01-012023-12-310000078003ecd:PeoMember2023-01-012023-12-310000078003ecd:PeoMemberpfe:EquityAwardsGrantedDuringTheYearMember2023-01-012023-12-310000078003ecd:PeoMemberpfe:EquityAwardsGrantedInPriorYearsUnvestedMember2023-01-012023-12-310000078003ecd:PeoMemberpfe:EquityAwardsGrantedInPriorYearsVestedMember2023-01-012023-12-310000078003ecd:PeoMemberpfe:StockAndOptionAwardsAdjustmentsMember2022-01-012022-12-310000078003ecd:PeoMemberpfe:ChangeInPensionValueMember2022-01-012022-12-310000078003ecd:PeoMember2022-01-012022-12-310000078003ecd:PeoMemberpfe:EquityAwardsGrantedDuringTheYearMember2022-01-012022-12-310000078003ecd:PeoMemberpfe:EquityAwardsGrantedInPriorYearsUnvestedMember2022-01-012022-12-310000078003ecd:PeoMemberpfe:EquityAwardsGrantedInPriorYearsVestedMember2022-01-012022-12-310000078003ecd:PeoMemberpfe:StockAndOptionAwardsAdjustmentsMember2021-01-012021-12-310000078003ecd:PeoMemberpfe:ChangeInPensionValueMember2021-01-012021-12-310000078003ecd:PeoMember2021-01-012021-12-310000078003ecd:PeoMemberpfe:EquityAwardsGrantedDuringTheYearMember2021-01-012021-12-310000078003ecd:PeoMemberpfe:EquityAwardsGrantedInPriorYearsUnvestedMember2021-01-012021-12-310000078003ecd:PeoMemberpfe:EquityAwardsGrantedInPriorYearsVestedMember2021-01-012021-12-310000078003ecd:PeoMemberpfe:StockAndOptionAwardsAdjustmentsMember2020-01-012020-12-310000078003ecd:PeoMemberpfe:ChangeInPensionValueMember2020-01-012020-12-310000078003ecd:PeoMember2020-01-012020-12-310000078003ecd:PeoMemberpfe:EquityAwardsGrantedDuringTheYearMember2020-01-012020-12-310000078003ecd:PeoMemberpfe:EquityAwardsGrantedInPriorYearsUnvestedMember2020-01-012020-12-310000078003ecd:PeoMemberpfe:EquityAwardsGrantedInPriorYearsVestedMember2020-01-012020-12-310000078003pfe:StockAndOptionAwardsAdjustmentsMemberecd:NonPeoNeoMember2023-01-012023-12-310000078003ecd:NonPeoNeoMemberpfe:ChangeInPensionValueMember2023-01-012023-12-310000078003ecd:NonPeoNeoMember2023-01-012023-12-310000078003ecd:NonPeoNeoMemberpfe:EquityAwardsGrantedDuringTheYearMember2023-01-012023-12-310000078003pfe:EquityAwardsGrantedInPriorYearsUnvestedMemberecd:NonPeoNeoMember2023-01-012023-12-310000078003ecd:NonPeoNeoMemberpfe:EquityAwardsGrantedInPriorYearsVestedMember2023-01-012023-12-310000078003pfe:StockAndOptionAwardsAdjustmentsMemberecd:NonPeoNeoMember2022-01-012022-12-310000078003ecd:NonPeoNeoMemberpfe:ChangeInPensionValueMember2022-01-012022-12-310000078003ecd:NonPeoNeoMember2022-01-012022-12-310000078003ecd:NonPeoNeoMemberpfe:EquityAwardsGrantedDuringTheYearMember2022-01-012022-12-310000078003pfe:EquityAwardsGrantedInPriorYearsUnvestedMemberecd:NonPeoNeoMember2022-01-012022-12-310000078003ecd:NonPeoNeoMemberpfe:EquityAwardsGrantedInPriorYearsVestedMember2022-01-012022-12-310000078003pfe:StockAndOptionAwardsAdjustmentsMemberecd:NonPeoNeoMember2021-01-012021-12-310000078003ecd:NonPeoNeoMemberpfe:ChangeInPensionValueMember2021-01-012021-12-310000078003ecd:NonPeoNeoMember2021-01-012021-12-310000078003ecd:NonPeoNeoMemberpfe:EquityAwardsGrantedDuringTheYearMember2021-01-012021-12-310000078003pfe:EquityAwardsGrantedInPriorYearsUnvestedMemberecd:NonPeoNeoMember2021-01-012021-12-310000078003ecd:NonPeoNeoMemberpfe:EquityAwardsGrantedInPriorYearsVestedMember2021-01-012021-12-310000078003pfe:StockAndOptionAwardsAdjustmentsMemberecd:NonPeoNeoMember2020-01-012020-12-310000078003ecd:NonPeoNeoMemberpfe:ChangeInPensionValueMember2020-01-012020-12-310000078003ecd:NonPeoNeoMember2020-01-012020-12-310000078003ecd:NonPeoNeoMemberpfe:EquityAwardsGrantedDuringTheYearMember2020-01-012020-12-310000078003pfe:EquityAwardsGrantedInPriorYearsUnvestedMemberecd:NonPeoNeoMember2020-01-012020-12-310000078003ecd:NonPeoNeoMemberpfe:EquityAwardsGrantedInPriorYearsVestedMember2020-01-012020-12-31000007800322023-01-012023-12-31000007800332023-01-012023-12-31000007800342023-01-012023-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the
Securities Exchange Act of 1934
(Amendment No. )
Filed by the Registrant x
Filed by a Party Other Than the Registrant ¨
Check the Appropriate Box:
| | | | | | | | |
| ¨ | Preliminary Proxy Statement |
| |
| ¨ | Confidential, for Use of the Commission Only (as Permitted by Rule 14a-6(e)(2)) |
| |
| x | Definitive Proxy Statement |
| | |
| ¨ | Definitive Additional Materials |
| |
| ¨ | Soliciting Material Pursuant to §240.14a-12 |
|
| Pfizer Inc. |
| (Name of Registrant as Specified In Its Charter) |
|
| (Name of Person(s) Filing Proxy Statement, if other than the Registrant) |
|
| Payment of filing fee (Check all boxes that apply): |
| |
| x | No fee required |
| |
| ¨ | Fee paid previously with preliminary materials |
| |
| ¨ | Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11 |
| | |
| | | | | | | | |
| | |
| | |
| A Letter from Pfizer’s Chairman & Chief Executive Officer | |
| | |
| | |
| | | | | | | | | | | | | | |
| | | | |
| | Dear Shareholders, While 2023 was a year of several challenges, it was also a year of significant accomplishments. A year that I believe sets up our company for incredible progress in 2024 and beyond, as we celebrate 175 years of breakthrough innovations that serve patients around the world. | |
| | |
| | |
First, the hard truth. We missed our 2023 initial projections, largely due to the decline in sales of our COVID-19 products.
We took corrective steps. We eliminated many uncertainties related to COVID-19 products and reduced COVID-19 product inventory to reflect anticipated future demand; we improved our commercial structure; and we launched a cost-realignment program to right-size our cost base. Difficult, but necessary, decisions were made, enabling us to be more efficient and effective. I am proud that we met our challenges with courage, compassion, and renewed commitment to our purpose.
In terms of achievements, there were many in 2023, among them:
•Approximately 618 million patients around the world were treated with our medicines and vaccinesi;
•We received a record number of FDA approvals for new molecular entities;
•We completed our acquisition of Seagen, a critical step in our ambition to end cancer as we know it.
These accomplishments, combined with the resilience of our colleagues, are why I am optimistic about the future of Pfizer and what we will deliver to patients.
Patient Impact
In 2022, we set an ambitious goal of changing one billion lives per year by 2027. I am proud to share the progress we made toward this goal in 2023. Approximately 618 million patients around the world were treated with our medicines and vaccinesi. We streamlined our processes, enabling us to deliver breakthroughs more quickly without compromising quality. Additionally, we expanded our portfolio to help ensure that we are meeting the needs of different patient populations and addressing unmet patient needs.
Performance
For the full year 2023, we reported revenues of $58.5 billion, reflecting an operational decrease of 41% year over year, primarily due to a significant decline in revenues for our COVID-19 products. Excluding our COVID-19 products, we achieved 7% operational revenue growth, solidly in line with our expectations for non-COVID-19 product revenuesii.
Bright Future
2023 was a record-breaking year for FDA approvals for Pfizer, with nine new molecular entity approvals and many more approvals for new indications in already approved products. Our research and development success rates are notably higher than industry benchmarks, and we significantly reduced the time it takes to bring new products to market compared to five years ago. We are on an exciting trajectory that builds on a legacy of discovering, developing, and delivering breakthroughs that have changed patients’ lives since 1849.
i. The patients treated metric is calculated from Pfizer and third-party datasets. This estimate does not include Seagen patients treated. Figures may be limited given the coverage provided by external sources (e.g., calendar duration, geographic and product coverage) and are subject to change. Numbers are estimates and, in some cases, use global volume, daily dosage, and number of treatment days to facilitate calculations. Methodologies to calculate estimates may vary by product type given the nature of the product and available data. Patients taking multiple Pfizer products may be counted as multiple patients towards total. Numbers do not include comprehensive estimated patient counts from Ex-U.S. Access & Affordability programs. Historical estimates may periodically be subject to revision due to restatements in the underlying data source.
ii. Operational revenue growth/decline excludes the impact of foreign exchange. For additional information on the company’s operational revenue performance, see the “Our 2023 Performance – Total Revenues” and the “Analysis of the Consolidated Statements of Income” sections in Management’s Discussion and Analysis of Financial Condition and Results of Operations in our 2023 Annual Report on Form 10-K.
| | | | | | | | |
2024 Proxy Statement Pfizer | | i |
| | |
| A Letter from Pfizer’s Chairman & Chief Executive Officer |
Health Equity
As a company with equity as a core value, we have a deep passion and commitment to achieving global health equity. As part of this commitment, we have made substantive progress with our initiative, “An Accord for a Healthier World,” which aims to provide greater access to medicines and vaccines to 1.2 billion people living in 45 lower-income countries around the world. Seven countries have signed framework supply agreements with us, and we are actively working with their governments to help enable a seamless supply of selected products.
Our health equity work is just one element of Pfizer’s broader environmental, social, and governance (ESG) strategy. Learn more in our 2023 Impact Report.
Conquering Cancer
Our recent acquisition of Seagen is a turning point in our oncology story. With Seagen’s leading antibody-drug conjugate technology and Pfizer’s capabilities and scale, we are confident that we will accelerate breakthroughs in cancer medicines to patients around the world. The combined commercial infrastructure of Pfizer and Seagen is three times the size of Seagen alone in the U.S. Our oncology pipeline doubled in size and is now armed with technologies that can help transform outcomes for patients.
Digital Transformation
For years, Pfizer has been harnessing Digital and Artificial Intelligence (AI) across the entire company to drive innovation and productivity on a global scale. For example, supercomputing, AI, and virtual in silico screening accelerate our scientific research by reducing computational times by 80-90%, and our industry-first Digital Operations Center and AI-powered manufacturing processes are increasing throughput by 20%, enabling us to deliver more medicines to patients faster.
Building Trust in Science
Pfizer is a science-based company. We rely on facts. Providing accurate information – especially about vaccines and medicines – can avoid deadly consequences. We are committed to doing everything we can to build trust in science and promote accurate science-based information. These are key priorities for us, and we are advancing them through our digital channels and collaboration with healthcare organizations and key stakeholders.
Closing
I want to express my deepest gratitude to my incredible colleagues at Pfizer for another remarkable year of achievements and advancements. I am proud that Pfizer was once again recognized as one of the most innovative and ethical companies. We were named one of Fortune’s “World’s Most Admired Companies” and one of the “World’s Most Ethical Companies” by Ethisphere.
In 2024, Pfizer celebrates its 175th anniversary as one of the world’s most innovative and trusted companies in healthcare. Proud as we are of what we have delivered to the world, our focus, as always, is on the future and building on our legacy through flawless execution.
I believe Pfizer is well-positioned for consistent sustainable growth potential going forward. We have the right products, the right people, and the right plan for great success in 2024 and beyond.
Simply put, we will outdo yesterday.
Thank you for your continued support of our important work.
| | | | | |
| Dr. Albert Bourla Chairman & Chief Executive Officer |
We encourage you to read our 2023 Annual Report on Form 10-K, which includes our audited consolidated financial statements as of and for the year ended December 31, 2023, and the sections captioned “Risk Factors” and “Forward-Looking Information and Factors that May Affect Future Results,” for a description of the substantial risks and uncertainties related to the forward-looking statements included herein.
| | | | | | | | |
ii | | Pfizer 2024 Proxy Statement |
| | | | | | | | |
| | |
| | |
| A Message from Pfizer’s Lead Independent Director | |
| | |
| | |
| | | | | | | | | | | | | | |
| | | | |
| | Dear Shareholders, I would like to express my gratitude on behalf of the Board of Directors for your investment and interest in Pfizer. Serving as your Lead Independent Director is a great privilege, and I am honored to work closely with the Chairman and my fellow Directors as we fulfill our fiduciary responsibilities to you, our valued shareholders. | |
| | |
| | |
Ensuring that we are well-informed about Pfizer's business performance is critical to the Board. Throughout 2023, we had regular discussions with management, which included reviewing the company's performance, corporate strategy, significant business initiatives, capital allocation priorities, and business development opportunities.
In addition, we worked closely with Pfizer's leaders to oversee significant initiatives for the company over the course of the year, including:
•The successful acquisition of Seagen, which will help propel Pfizer towards its goal of achieving world-class oncology leadership.
•The company’s reorganization of its R&D platform operations to further enhance its focus, speed, and execution in the field of oncology.
•Changes to the executive leadership team, detailed in this Proxy Statement.
Our Board is comprised of Directors with diverse skills and experiences that are highly relevant to Pfizer's business needs and overall strategy. We are proud of the Board's diverse composition, with each Director bringing a unique set of perspectives, experiences, and abilities to Pfizer. This diversity ensures that our Board functions effectively and efficiently. This year’s Proxy Statement includes enhanced disclosures that further highlight the skillsets of our Directors and how they align with Pfizer’s strategy. This disclosure was created in direct response to feedback received from our shareholders during the year.
The Board is also committed to its continuous improvement. We conduct annual evaluations to assess our performance and to ensure and enhance our overall effectiveness. In 2023, we engaged an external third-party provider to help conduct the annual Board and Committee evaluations, a process that proved to be highly productive and beneficial in providing us with constructive feedback.
In closing, we would like to express our appreciation to our shareholders for their confidence in Pfizer. We are grateful for the valuable feedback they have shared with the company, including in direct conversations with members of the Board, which meaningfully informs our governance and oversight practices.
Thank you for your continued support. Your vote is important, so please take the time to read the Proxy Statement and cast your vote.
| | | | | |
| Mr. Shantanu Narayen Lead Independent Director |
| | | | | | | | |
2024 Proxy Statement Pfizer | | iii |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
| | | |
| | Notice of 2024 Annual Meeting and Proxy Statement | |
| | | | | | | |
| | | | |
| Voting Your Shares. For information regarding how to vote your shares by telephone, by internet, by mail or at the virtual Annual Meeting, see “Annual Meeting Information – Voting” later in this Proxy Statement. | | | MEETING TIME AND DATE |
| | |
| | 9:00 a.m., Eastern Daylight Time (EDT), on Thursday, April 25, 2024 |
| |
Items of Business 1.To elect 12 members of the Board of Directors, each until our next Annual Meeting and until his or her successor has been duly elected and qualified. 2.To ratify the selection of KPMG LLP as our independent registered public accounting firm for 2024. 3.To approve the Amended and Restated Pfizer Inc. 2019 Stock Plan. 4.To conduct an advisory vote to approve our executive compensation. 5.To consider 4 shareholder proposals, if properly presented at the Annual Meeting. 6.To transact any other business that properly comes before the Annual Meeting or any adjournment or postponement of the Meeting. Materials To Review This booklet contains our Notice of 2024 Annual Meeting and Proxy Statement. Our 2023 Annual Report on Form 10-K is included as Appendix A and is followed by certain Corporate and Shareholder Information. None of Appendix A or the Corporate and Shareholder Information on the back inside cover are a part of our proxy solicitation materials. This Notice of 2024 Annual Meeting and Proxy Statement and a proxy card or voting instruction form are being mailed or made available to shareholders starting on or about March 14, 2024. Margaret M. Madden Senior Vice President and Corporate Secretary, Chief Governance Counsel March 14, 2024 | | VIRTUAL MEETING ONLY |
| |
The 2024 Annual Meeting will be held in a virtual meeting format only. To access the virtual Annual Meeting, please visit https://meetnow.global/PFE2024. We designed the format of the virtual Annual Meeting to ensure that our shareholders who attend the virtual Annual Meeting will be afforded comparable rights and opportunities to participate as they would at an in-person meeting. |
|
|
| RECORD DATE |
|
| February 28, 2024 |
|
|
|
|
|
| | | | | |
| Important Notice Regarding the Availability of Proxy Materials for the Annual Meeting of Shareholders to Be Held on April 25, 2024. This Notice of 2024 Annual Meeting and Proxy Statement and the 2023 Annual Report on Form 10-K are available on our website at https://investors.pfizer.com/Investors/Financials/Annual-Reports/default.aspx. Except as stated otherwise, information on our website is not considered part of this Proxy Statement. |
| | | | | | | | |
iv | | Pfizer 2024 Proxy Statement |
| | | | | | | | |
| | |
| | |
| Our Business and Strategy | |
| | |
| | |
| Pfizer Inc. is a research-based, global biopharmaceutical company. We apply science and our global resources to bring therapies to people that extend and significantly improve their lives through the discovery, development, manufacture, marketing, sale and distribution of biopharmaceutical products worldwide. We work across developed and emerging markets to advance wellness, prevention, treatments and cures that challenge the most feared diseases of our time. We collaborate with healthcare providers, governments and local communities to support and expand access to reliable, affordable healthcare around the world. | |
2023 Milestones
| | | | | | | | | | | | | | | | | | | | |
| | | | | | |
Key Regulatory Approvals | | | Regulatory Submissions | | | Pivotal Study Starts |
| | | 7 | | | 6 |
| | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | |
| Research & Development | | | Manufacturing | | | Employees Globally | | | Global Reach | |
| 112 | | | 37 | | | ~88,000 | | | ~200 | |
| projects in our current product pipeline (as of January 30, 2024) | | | sites worldwide | | | | | | countries and territories where we supply our products | |
| | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | |
| Revenues | | | Patients Treated | | | Shareholder Returns |
~$58.5B | | | ~618M | | | $9.2B |
in 2023 | | | patients treated globally with our medicines and vaccines in 20232 | | | to shareholders through cash dividends in 2023 |
| | | | | | |
Unless indicated otherwise, the information contained in this summary is as of December 31, 2023.
(1) Includes, as two separate approvals: (i) U.S. Food and Drug Administration approval for the Omicron XBB.1.5-adapted monovalent COVID-19 vaccine for individuals 12 years and older; and (ii) Emergency Use Authorization for the Omicron XBB.1.5-adapted monovalent COVID-19 vaccine in individuals 6 months through 11 years of age.
(2) The patients treated metric is calculated from Pfizer and third-party datasets. This estimate does not include Seagen patients treated. Figures may be limited given the coverage provided by external sources (e.g., calendar duration, geographic and product coverage) and are subject to change. Numbers are estimates and in some cases use global volume, daily dosage and number of treatment days to facilitate calculations. Methodologies to calculate estimates may vary by product type given the nature of the product and available data. Patients taking multiple Pfizer products may be counted as multiple patients towards total. Numbers do not include comprehensive estimated patient counts from Ex-U.S. Access & Affordability programs. Historical estimates may periodically be subject to revision due to restatements in the underlying data source.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 1 |
| | |
| Our Business and Strategy |
Our 2023 Performance Overview
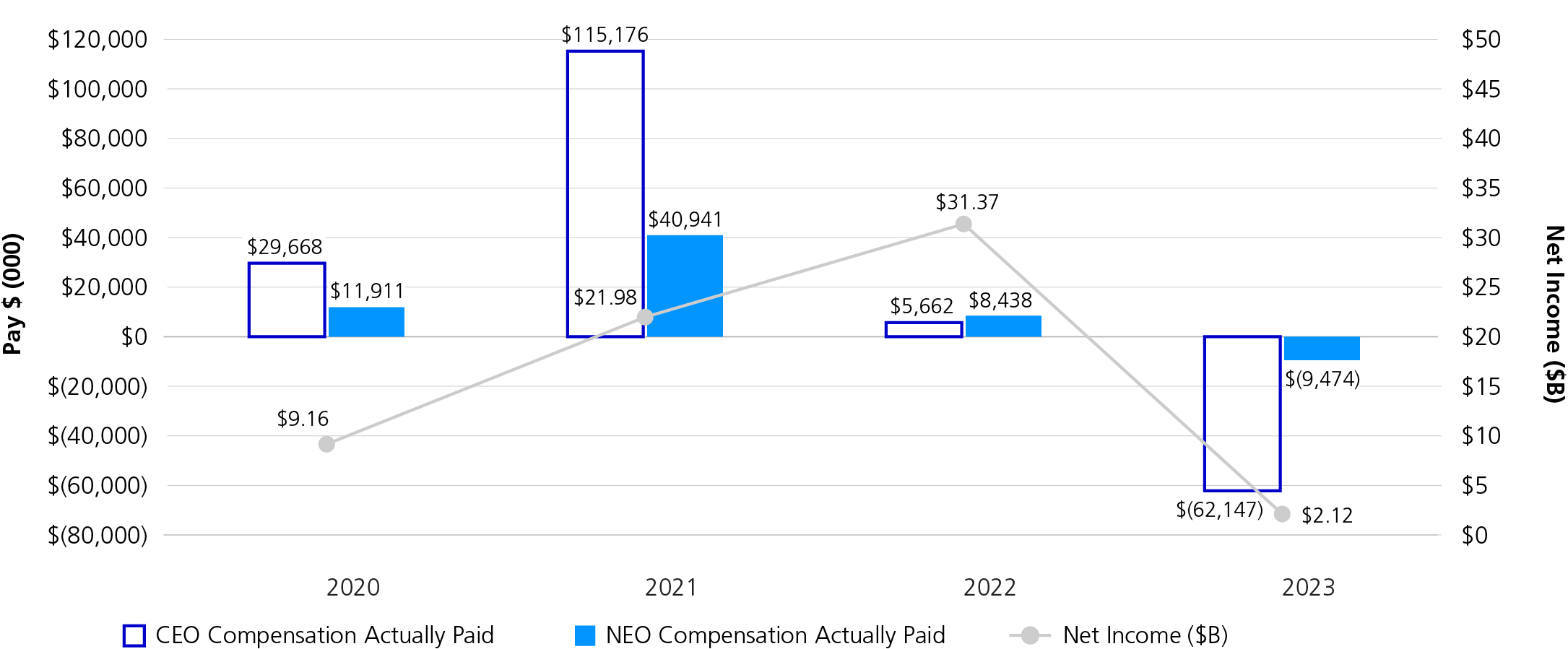
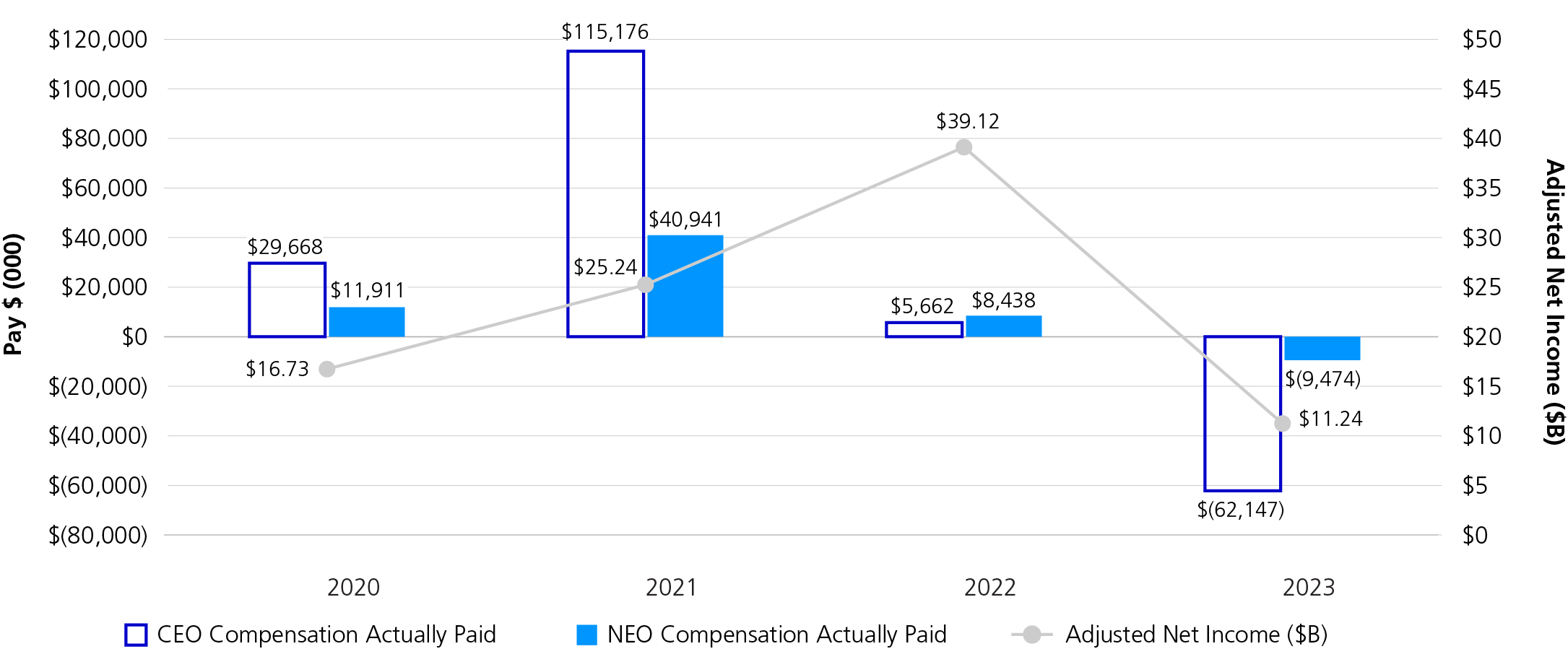
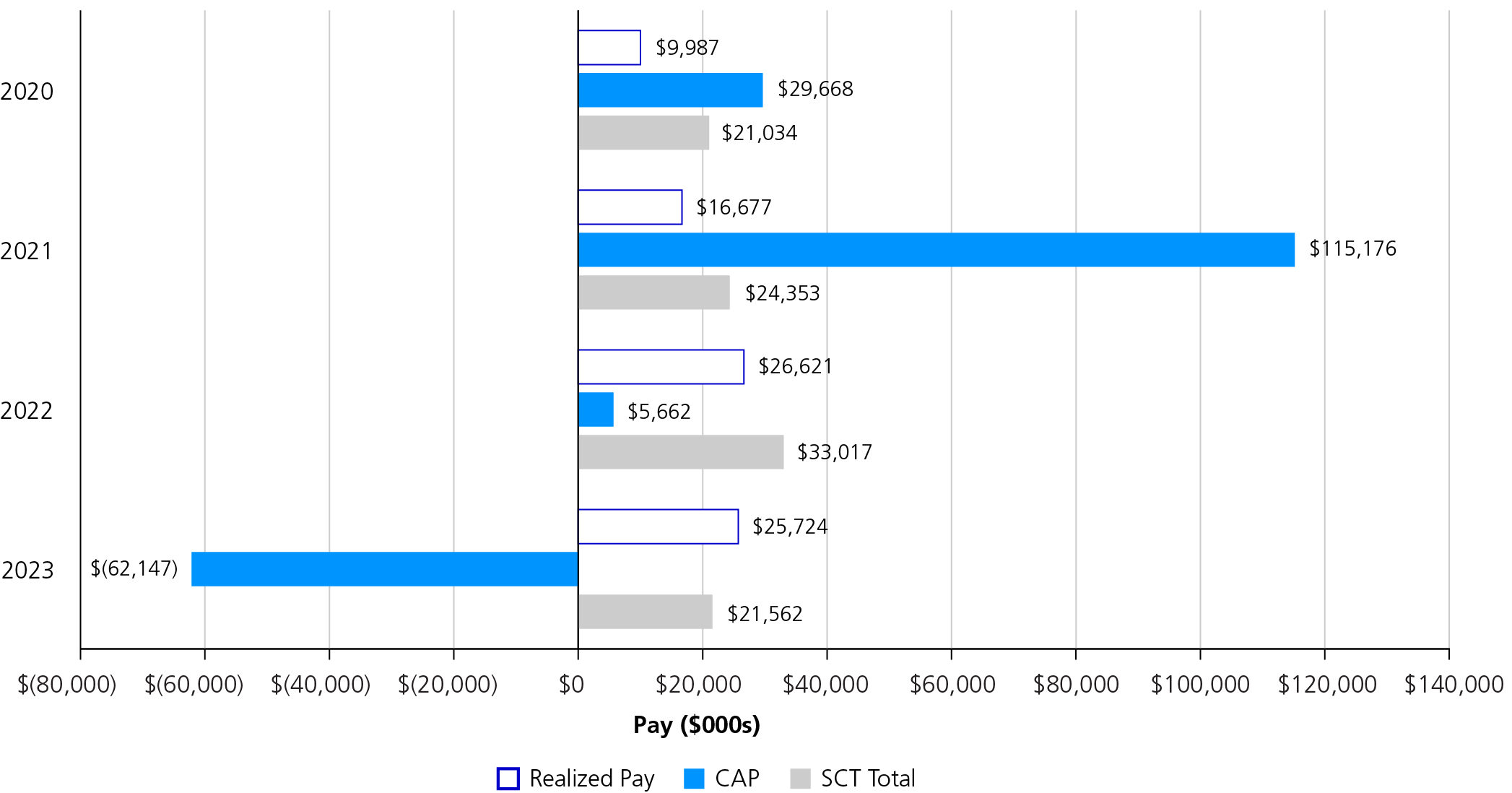
2023 was a challenging year for Pfizer after experiencing a peak year of financial performance in 2022. Full-year 2023 revenues were $58.5 billion, reflecting a 41% operational decline, primarily due to the expected decline in revenues related to COVID-19 products. Despite the challenges, a number of significant achievements created a strong foundation for the year ahead and fortified our potential for long-term growth.
We executed on our strategic priorities and introduced a number of new medicines and vaccines, achieving a record number of U.S. Food and Drug Administration (FDA) approvals for nine new molecular entities (NMEs) that are expected to favorably impact Pfizer’s performance in the coming years. Notably, we introduced a vaccine to help alleviate the significant burden of respiratory syncytial virus (RSV) in higher-risk populations. Pfizer currently is the only company with an RSV vaccine to help protect older adults, as well as infants through maternal immunization, and this development is reflective of the continued growth of our vaccine portfolio. We also launched several new indications for our existing in-line brands that further enhanced our leadership positions across oncology, vaccines and inflammation and immunology, while continuing to prioritize and advance our pipeline.
In December 2023, we acquired Seagen, one of the largest investments in Pfizer’s history, and a critical step toward our goal to achieve world-class oncology leadership. We are now poised to accelerate the next generation of potential breakthrough treatments and bring new hope to people living with cancer everywhere. Our industry-leading oncology portfolio now includes over 25 approved medicines and biosimilars across more than 40 indications, including nine that are either blockbuster or have the potential to be blockbuster.
To help ensure we are well positioned for growth moving forward, we launched a multi-year, enterprise-wide cost realignment program that aims to align our costs with our longer-term revenue expectations and made changes to our commercial organization.
As a science-driven global biopharmaceutical company, we remain focused on advancing our pipeline, executing on our commercial launches, and deploying capital responsibly to enhance shareholder value. Our commitment to being a force for good in the world continues, as we expanded our Accord for a Healthier World initiative which aims to provide the full portfolio of Pfizer’s medicines and vaccines for which we have global rights on a not-for-profit basis to 45 lower-income countries.
We are proud of the positive impact we are having on human lives around the world. Our ability to fulfill our purpose, Breakthroughs that change patients’ lives, remains a core focus and underscores our commitment to helping address the needs of society to help sustain long-term value creation for all stakeholders.
ADVANCING OUR R&D PIPELINE
As of January 30, 2024, we had the following number of projects in various stages of R&D:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Phase 1 | à | | | | Phase 2 | à | | | | Phase 3 | à | | | | Registration | à | | | | Total | | |
| 41 Experimental products tested for first time in human clinical trials | | | | 34 Trials focused on product’s effectiveness, ideal dosage and delivery method | | | | 31 Randomized trials to test results of earlier trials on larger populations to analyze risks and benefits | | | | 6 Applications filed with appropriate regulatory authorities when trial results warrant | | | | 112 | | |
| | | | | | | | |
2 | | Pfizer 2024 Proxy Statement |
| | |
| Our Business and Strategy |
By the end of 2023, Pfizer achieved an end-to-end success rate of 17 percent – from first in human (FIH) to approval at a NME level. We are sustaining higher success rates through focused efforts in therapeutic areas where we believe we are equipped to make the biggest impact on patients’ lives.
| | | | | | | | | | | | | | |
Clinical Trial Success Rates*
(NMEs only) | Phase 1
(3-year avg.) | Phase 2
(5-year avg.) | Phase 3/Registration
(5-year avg.) | End-to-End
Success Rate |
Pfizer(1) (through 2023) | 35 | % | 58 | % | 83 | % | 17 | % |
Industry(2) (through 2022) | 42 | % | 32 | % | 71 | % | 10 | % |
* The analysis includes only studies involving NMEs. The analysis does not include Seagen NMEs.
(1)Success rates for Phase 1 are based on a 3-year rolling average (2021-2023); rates for Phase 2 and Phase 3/Registration represent a 5-year rolling average (2019-2023).
(2)Success rates are based on a 5-year rolling average for Phase 2 and Phase 3 studies, and a 3-year rolling average for Phase 1 studies, with the cut-off for the analysis ending on fiscal year-end 2022, which is the most recent information available. The “industry” in this analysis was based on the Pharmaceutical Benchmarking Forum’s participant companies: AbbVie Inc.; Astellas Pharma, Inc; Bayer AG; Bristol-Myers Squibb Company; Eli Lilly and Company; Gilead Sciences, Inc.; Johnson & Johnson; Merck & Co., Inc.; Novartis AG; Pfizer; Roche Holding AG and Sanofi.
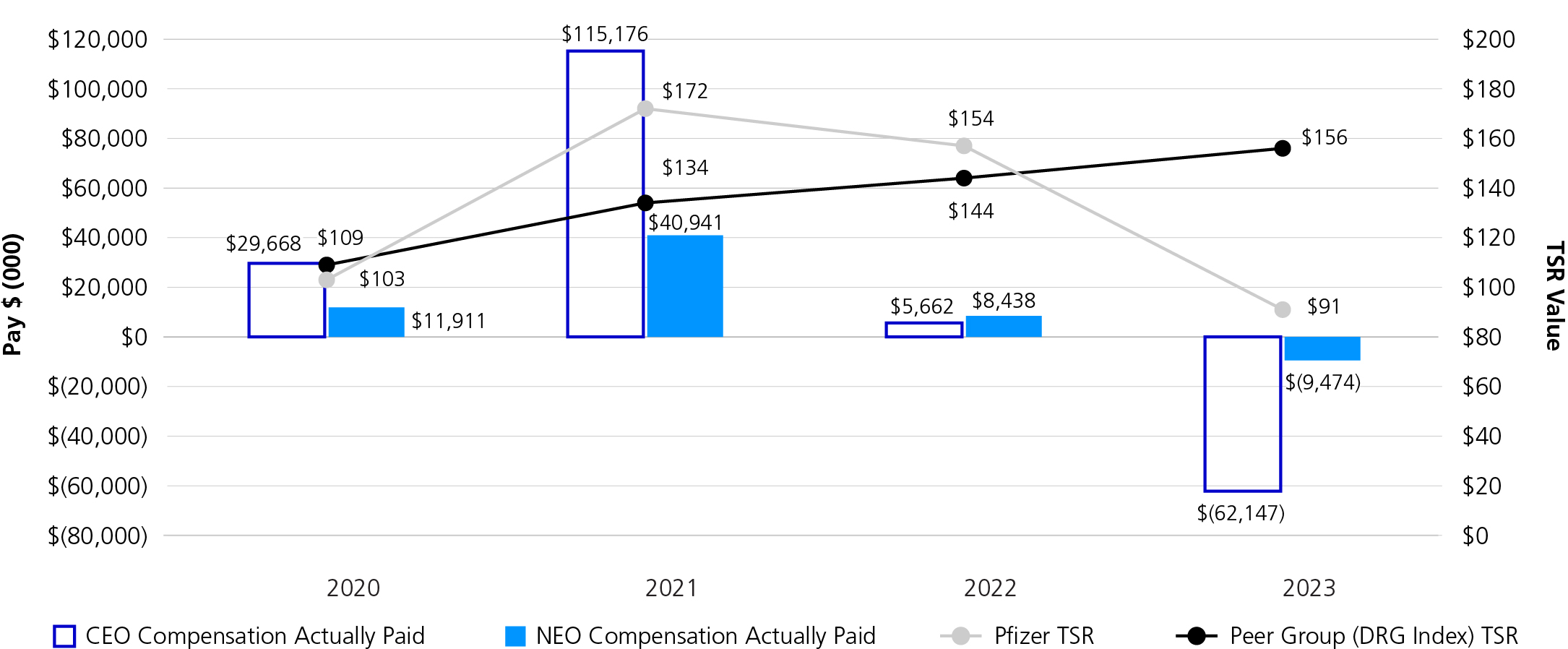
OUR TOTAL SHAREHOLDER RETURN (TSR)
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | |
Quarterly
Dividends | | One-year
TSR | | Three-year
TSR | | Five-year
TSR | | Capital Returned to Shareholders (cash dividends) |
3% á | | (41.2%) â | | (12.2%) â | | (15.7%) â | | $9.2B |
| Compared to 2022 | | Year-End 2023 | | Year-End 2023 | | Year-End 2023 | | in 2023 |
| | | | | | | | |
THE BOARD’S OVERSIGHT OF STRATEGY
The Board and its Committees oversee our corporate strategy, including significant business and organizational initiatives, capital allocation priorities and potential value-enhancing business development opportunities (including acquisitions, licenses, and collaborations) intended to support our strategy. Accordingly, the Board engages in robust discussions regarding our corporate strategy at every meeting and, at least annually, receives a formal update on the company’s short- and long-term objectives, including the company’s operating plan, long-term corporate strategic plan, and competitive landscape. In addition, the Board’s Committees oversee the aspects of our strategy associated with their respective areas of responsibility.
OUR APPROACH TO ESG: CONNECTED TO OUR PURPOSE AND STRATEGY
Our environmental, social and governance (ESG) priorities are aligned with our corporate strategy. Our commitment to ESG is rooted in our purpose, as we strive to serve patients and communities with innovation and sustainable approaches to discovering, developing, and bringing medicines and vaccines to market. With senior leader support and collaboration at all levels, we aim to improve health outcomes, build trust, create shared value, and make a positive impact on society for years to come.
Pfizer's 2023 Impact Report, which is informed by several globally recognized frameworks including the Global Reporting Initiative (GRI), Sustainability Accounting Standards Board (SASB), and Task Force on Climate-Related Financial Disclosure (TCFD), includes further details on our ESG goals and progress. In addition, our consolidated EEO-1 Reports are available on our website.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 3 |
| | |
| Our Business and Strategy |
The Board’s Oversight of ESG Matters
Pfizer’s Board is engaged on and supports ESG. The Governance & Sustainability Committee is primarily responsible for oversight of our ESG strategy, reporting, policies and practices. The Committee regularly receives updates from management on Pfizer’s progress as measured against the company’s ESG strategy, metrics, and goals to further enhance ESG performance. The Committee also receives updates on the regulatory environment that may impact our ESG strategy and reporting requirements.
The Board Committees oversee specific elements of our ESG program associated with their respective areas of responsibility:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Board Oversight |
| | | | | | | | | | | | | | |
| Governance & Sustainability | | | | Compensation | | | | Audit | | | | Regulatory and Compliance | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| •ESG strategy, reporting, policies and practices •Human capital management, including succession planning, culture, diversity, equity and inclusion, pay equity and talent management •Political and lobbying activities •Climate change program •Reputational risk factors •Board diversity | | | | •Executive compensation program (which includes a short-term incentive program that incorporates an ESG Scorecard), including approving compensation of our executive officers •Human capital management, which may include executive diversity, pay equity, inclusion, recruiting, retention, career development and succession planning (in collaboration with the Governance & Sustainability Committee) | | | | •Enterprise Risk Management (ERM) program (which includes reviewing and receiving briefings on priority issues that fall under ERM and ESG) •Company culture (compliance related concerns, workplace behavior, harassment and retaliation) | | | | •Compliance program •Ethics and integrity, including company culture •Product quality and safety •Quality and compliance governance framework and risk management •Healthcare-related regulatory and compliance risks in connection with the development, manufacturing, supply and marketing of products and risk mitigation efforts | |
| | | | | | | | | | | | | | |
Culture and Diversity, Equity and Inclusion (DEI)
The Board also recognizes the critical importance and value of Pfizer’s colleagues and the need to build and sustain a culture where colleagues of diverse backgrounds, abilities and experiences contribute their unique viewpoints and perspectives to all aspects of the business. Management establishes and reinforces the company’s culture, which the Board and its Committees oversee. Pfizer’s commitment to DEI is a choice we make, both mindfully and actively. We support our Equity value by listening, learning, and adapting to better support our colleagues and the communities where we live and serve. We believe that every person deserves to be seen, heard and cared for, which happens when we are inclusive and act with integrity. Our leaders set the tone for the company, embracing accountability and transparency, promoting an inclusive culture and supporting a speak-up culture in which colleagues are encouraged to share views and raise concerns without fear of retaliation. Please see https://www.pfizer.com/about/responsibility/diversity-and-inclusion.
| | | | | | | | |
4 | | Pfizer 2024 Proxy Statement |
| | | | | | | | |
| | |
| | |
| Item 1 Election of Directors | |
| | |
| | |
| All twelve members of our Board are standing for re-election. In an uncontested Director election, the number of votes cast “for” a Director nominee must exceed the number of votes cast “against” that nominee. Our Corporate Governance Principles (Principles) contain detailed procedures to be followed in the event that one or more Directors do not receive a majority of the votes cast “for” his or her election at the Annual Meeting. Each nominee elected as a Director will continue in office until our next Annual Meeting and until his or her successor has been duly elected and qualified, or until a Director’s earlier death, resignation, removal or retirement. While we expect each nominee to be able to serve if elected, if any nominee is not able to serve, the persons appointed by the Board and named as proxies in the proxy materials or, if applicable, their substitutes (the Proxy Committee), may vote their proxies for substitute nominees, unless the Board chooses to reduce the number of Directors. Criteria for Board Membership GENERAL CRITERIA •Proven integrity and independence, with a record of substantial achievement in an area of relevance to Pfizer •Ability and sufficient time, energy and attention to make a meaningful contribution to the Board’s advising, counseling and oversight roles •Prior or current leadership experience with major complex organizations, including within the scientific, government service, educational, finance, marketing, technology or not-for-profit sectors, with some members of the Board being widely recognized as leaders in the fields of medicine or biological sciences •Commitment to enhancing Pfizer’s long-term growth •Broad experience, diverse perspectives, and the ability to exercise sound judgment, and a judicious and critical temperament that will enable objective appraisal of management’s plans and programs •Diversity with respect to gender, age, race, ethnicity, background, professional experience and perspectives. The Board and each Committee conduct annual evaluations to help ensure that each of its members individually, and the Board as a whole, continue to meet the criteria for Board membership. Based on these activities and their review of the current composition of the Board, in December 2023, the Governance & Sustainability Committee and the Board determined that the criteria for Board membership have been satisfied, and the Board nominated the existing Directors as the slate of Director nominees for election at the 2024 Annual Meeting of Shareholders. Selection of Candidates DIRECTOR SKILLS CONSIDERATIONS AND COMMITMENT TO DIVERSITY In recruiting and selecting Director candidates, the Governance & Sustainability Committee considers the size of the Board and those skills outlined in our skills matrix. This matrix guides the Committee in determining whether a particular Board member or candidate possesses one or more of the requisite skills, as well as whether those skills and/or other attributes qualify him or her for service on a particular committee. The Committee also considers a range of additional factors, including other positions the Director or candidate holds; other boards on which he or she serves; the results of the Board and Committee evaluations; each Director’s and candidate’s projected retirement date; each Director’s and candidate's demonstrated ability and sufficient time, energy and attention to make a meaningful contribution; their independence; their attendance (if applicable); and the company’s current and future business needs, particularly in light of the company’s evolving strategic priorities. Pursuant to its charter, the Governance & Sustainability Committee of the Board is responsible for considering a diverse pool of candidates to fill positions on the Board; however, the company does not have a formal policy on Board diversity. Pfizer’s Principles provide that Directors should be selected so that the Board maintains its diverse composition, with diversity reflecting gender, age, race, ethnicity, background, professional experience and perspectives. | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 5 |
| | |
| Item 1 — Election of Directors |
PROCESS FOR SELECTING DIRECTOR NOMINEES
Consistent with the objective of maintaining the Board’s diverse composition, during 2023, the Governance & Sustainability Committee conducted a needs assessment, identified and reviewed Director candidates and followed the robust process below to review potential nominees. The Board did not elect any new Directors during 2023.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 1 | | | | 2 | | | | 3 | | | | 4 | |
| Needs
Assessment | | | | Candidate
Identification | | | | Screening | | | | Nomination
and Onboarding | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| Define skills and diversity criteria based on: •Gaps to fill due to Board turnover/succession planning •Current and future business needs •Results of Board evaluation •Management team priorities | | | | Identify candidates through: •Board member recommendations •Executive Leadership Team (ELT) recommendations •Search agencies and recruiters •Shareholders •Other sources | | | | Review of qualifications: •Skills matrix •Integrity and independence requirements •Past experience and perspectives •Other positions the candidate holds or has held •Diversity Committee members and, as appropriate, other Board members and management interview qualified candidates. | | | | Select Director nominees best suited to serve the interests of the company and its shareholders. Following election, all new independent Directors undergo a comprehensive onboarding process, which includes: •Meetings with members of the ELT and other senior leaders; and •An in-depth review of a broad set of materials that provide information on the company and Board-related matters. | |
| | | | | | | | | | | | | | |
Director Independence
Our Board of Directors has adopted Director Qualification Standards (Standards) to evaluate and determine Director independence. Our Standards meet, and in some respects exceed, the independence requirements of the New York Stock Exchange (NYSE). To qualify as independent under our Standards, a non-employee Director must have no material relationship with Pfizer other than as a Director. The Standards include additional strict guidelines for Directors and their immediate families and can be found on our website at https://investors.pfizer.com/Investors/Corporate-Governance/The-Pfizer-Board-Policies/default.aspx.
Under our Standards, certain relationships and transactions are not considered to be material transactions that would impair a Director’s independence, including the following:
•the Director is an employee, or an immediate family member of the Director is an executive officer, of another company that does business with Pfizer, and our annual sales to, or purchases from, the other company in each of the last three fiscal years amounted to less than 1% of the annual revenues of the other company;
•the Director, or an immediate family member of the Director, is an executive officer of another company, and our indebtedness to the other company or its indebtedness to Pfizer amounts to less than 1% of the total consolidated assets of the other company; and
•contributions to not-for-profit entities in which a Director, or a Director’s spouse, serves as an executive officer, which amount to less than 2% of that organization’s latest publicly available total revenues (or $1 million, whichever is greater).
Drs. Desmond-Hellmann, Hobbs, Hockfield and Littman, and Mr. Echevarria, are employed at medical, scientific or academic institutions with which Pfizer engages in ordinary-course business transactions. Mr. Narayen is the chief executive officer of Adobe Inc., a company with which Pfizer engages in ordinary-course business transactions. Dr. Gottlieb is a Resident Fellow of the American Enterprise Institute (AEI). In 2023, Pfizer made a payment to AEI related to a corporate sponsorship. We reviewed our transactions with and payments to each of these entities and found that these transactions/payments were made in the ordinary-course of business and were below the levels set forth in our Standards.
| | | | | | | | |
6 | | Pfizer 2024 Proxy Statement |
| | |
| Item 1 — Election of Directors |
Independence Assessment. Together with Pfizer’s legal counsel, the Governance & Sustainability Committee reviewed the applicable legal and NYSE standards for Board and Committee member independence, as well as our Standards. A summary of the answers to annual questionnaires completed by each of the Directors and a report of transactions with Director-affiliated entities were also made available to the Committee. On the basis of these reviews, the Committee delivered a report to the full Board of Directors, and the Board made its independence determinations based upon the Committee’s report and the supporting information.
The Board has determined that all of our current Directors (other than Dr. Albert Bourla) are independent of the company and its management and meet Pfizer’s criteria for independence. The independent Directors are Drs. Susan Desmond-Hellmann, Scott Gottlieb, Helen H. Hobbs, Susan Hockfield and Dan R. Littman; Ms. Suzanne Nora Johnson; and Messrs. Ronald E. Blaylock, Joseph J. Echevarria, Shantanu Narayen, James Quincey and James C. Smith.
Our 2024 Director Nominees
The Governance & Sustainability Committee and the Board believe that each nominee for Director brings a strong and unique set of perspectives, experiences and skills to Pfizer that creates an effective and well-functioning Board.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | |
| Name | Age | Independent | Director Since | Audit | Compensation | Governance & Sustainability | Regulatory and Compliance | Science and Technology | Other Public Boards |
| | | | | | | | | |
| Ronald E. Blaylock | 64 | ü | 2017 | l | l | | | | 2 |
Albert Bourla, DVM, Ph.D. Chairman and CEO | 62 | | 2018 | | | | | | – |
Susan Desmond-
Hellmann, M.D., M.P.H. | 66 | ü | 2020 | | | l | | l | – |
| Joseph J. Echevarria | 66 | ü | 2015 | l | | Chair | | | 2 |
| Scott Gottlieb, M.D. | 51 | ü | 2019 | | | | Chair | l | 1 |
Helen H. Hobbs, M.D. | 71 | ü | 2011 | | | l | l | Chair | – |
Susan Hockfield, Ph.D. | 73 | ü | 2020 | | | | l | l | – |
Dan R. Littman, M.D., Ph.D. | 71 | ü | 2018 | | | l | l | l | – |
Shantanu Narayen Lead Independent Director | 60 | ü | 2013 | | | | | | 1 |
Suzanne Nora Johnson | 66 | ü | 2007 | Chair | | | l | | 1 |
| James Quincey | 59 | ü | 2020 | | l | | | | 1 |
James C. Smith | 64 | ü | 2014 | l | Chair | | | | – |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 7 |
| | |
| Item 1 — Election of Directors |
DIRECTOR KEY SKILLS AND EXPERIENCE
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Director Key Skills and Experience |
| | | | | | | | | | | |
| | | | | | | | | | | | | |
Business Leadership & Operations Experience serving in a senior leadership role develops skills in core management areas and provides a valuable practical understanding of the operations of complex organizations | l | l | l | l | | | l | | l | l | l | l |
| | | | | | | | | | | | | |
| | | | | | | | | | | | |
International Business Leadership experience in organizations that operate across diverse and dynamic political systems, economic conditions, and regulatory environments provides valuable perspectives for oversight of the risks and opportunities within Pfizer’s extensive global business operations | | l | | l | | | | | l | l | l | l |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Medicine & Science Knowledge of relevant sciences and experience as a healthcare provider provides Directors with a deep understanding of Pfizer’s key therapeutic areas and an appreciation for our mission to deliver breakthroughs that change patients’ lives | | l | l | | l | l | l | l | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Healthcare & Pharma Experience as an executive and/or in an operational role at a pharmaceutical or biotechnology focused organization or company provides Directors with a deep understanding of Pfizer’s business and key strategic and operational considerations | | l | l | | l | l | | l | | l | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Finance & Accounting Expertise in finance, capital markets and financial reporting processes enables Directors to effectively monitor and assess Pfizer’s performance and capital allocation decisions, and oversee accurate financial reporting and robust controls | l | | | l | | | | | l | l | l | l |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Risk Management Experience identifying, managing and mitigating key strategic and operational risks promotes effective oversight of Pfizer’s risks and opportunities and contributes to effective oversight of strategy in a variety of operating environments | l | | | l | | | | | l | l | | l |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Academia Experience in a leadership or senior advisory position at a scientific, research or academic institution provides Directors with deep technical subject matter expertise related to the intricacies of Pfizer’s R&D pipeline | | | l | | | l | l | l | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Human Capital Management Experience with human capital management responsibilities assists the Board in overseeing succession planning, talent development and Pfizer’s executive compensation program | | l | | | | | | | l | | l | l |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Government & Public Policy Understanding of the complex regulatory and governmental environment in which Pfizer operates allows the Board to oversee the company’s long-term strategy by incorporating current and potential changes in public policy and regulation | | | | l | l | | l | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Technology & Cybersecurity Experience understanding and overseeing information technology and cybersecurity matters is critical to mitigating risks to our business, and to Board oversight of Pfizer’s actions to address innovation and competitiveness in a rapidly evolving technological age | | | l | | | | | | l | | l | |
| | | | | | | | | | | | |
| | | | | | | | |
8 | | Pfizer 2024 Proxy Statement |
| | |
| Item 1 — Election of Directors |
DIRECTOR DIVERSITY
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Diversity Attributes1 |
| | | | | | | | | | | |
| Gender Expression | Female | | | l | | | l | l | | | l | | |
| Male | l | l | | l | l | | | l | l | | l | l |
| Race/Ethnicity | Asian | | | | | | | | | l | | | |
| Black | l | | | | | | | | | | | |
| Latino | | | | l | | | | | | | | |
| White | | l | l | | l | l | l | l | | l | l | l |
(1)Diversity attributes are self-identified.
| | | | | |
| Your Board of Directors recommends a vote “FOR” the election of each of these nominees as Director. |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 9 |
| | |
| Item 1 — Election of Directors |
Director Nominees
| | | | | | | | | | | |
| | | |
| | Ronald E. Blaylock KEY SKILLS & EXPERIENCE Business Leadership & Operations/Risk Management: Mr. Blaylock’s extensive experience in private equity and investment banking brings business leadership, financial expertise and risk management skills to the Board. In addition, Mr. Blaylock’s service on the compensation committees of other public companies enables him to bring valuable insights to Pfizer’s Board and Compensation Committee. Finance & Accounting: Mr. Blaylock’s significant financial background, including as the founder and managing partner of GenNx360 Capital Partners and the founder of Blaylock & Company, brings substantial financial expertise and a unique perspective to the Board on issues of importance relating to finance. BACKGROUND Founder, Managing Partner of GenNx360 Capital Partners, a private equity firm focused on investing in industrial and business services companies in the U.S. middle market since 2006. Prior to launching GenNx360 Capital Partners, Mr. Blaylock founded and managed Blaylock & Company, an investment banking firm. He also held senior management positions at UBS, PaineWebber Group and Citicorp. Director of CarMax, Inc. and W.R. Berkley Corporation, an insurance holding company. Former Director of Advantage Solutions Inc. (from 2019 to 2022) and Urban One, Inc. (from 2002 until 2019). Member of the Board of Trustees of Carnegie Hall. Member of the Board of Overseers of New York University Stern School of Business. Board Member of the Mental Health Coalition. |
| | |
Age: 64 Director Since: 2017 Board Committees: Audit and Compensation Key Skills: Business Leadership & Operations Finance & Accounting Risk Management Other Current Public Boards:
CarMax, Inc. and W.R. Berkley Corporation | | |
| | | | | | | | | | | |
| | | |
| | Albert Bourla, DVM, Ph.D. KEY SKILLS & EXPERIENCE Business Leadership & Operations/Human Capital Management/International Business/Healthcare & Pharma: Dr. Bourla has over 25 years of leadership experience and a demonstrated track record for delivering strong business results. Dr. Bourla has deep knowledge of the global healthcare industry as he has held a number of senior global positions across a range of businesses in five different countries (including eight different cities) over the course of his career, which enables him to provide important insights and perspectives to our Board on the company’s commercial, strategic, manufacturing and global product development functions. As Chairman and CEO, Dr. Bourla provides an essential link between management and the Board regarding management’s business perspectives. In addition, his experience on the Board of Pharmaceutical Research and Manufacturers of America (PhRMA) enables him to bring a broad perspective on issues facing our industry. Medicine & Science: Dr. Bourla brings expertise in medicine and science to the Board through his distinguished career at Pfizer. Since joining Pfizer in 1993, Dr. Bourla has served in various leadership positions with increasing responsibility within Pfizer’s former Animal Health and global commercial organizations. In addition, he is a Doctor of Veterinary Medicine and holds a Ph.D. in the Biotechnology of Reproduction from the Veterinary School of Aristotle University. BACKGROUND Chairman of the Board of Pfizer since January 2020; Chief Executive Officer of Pfizer since January 2019; Chief Operating Officer of Pfizer from January 2018 until December 2018; Group President, Pfizer Innovative Health from June 2016 until December 2017; Group President, Global Innovative Pharma Business of Pfizer from February 2016 until June 2016 (responsible for Vaccines, Oncology and Consumer Healthcare from 2014). President and General Manager of Established Products Business Unit of Pfizer from 2010 until 2013. Board member of PhRMA and of The Pfizer Foundation, which promotes access to quality healthcare. Co-Chair of the Board of Directors of the Partnership for New York City and Member of the Board of Catalyst. |
| | |
Chairman and CEO Age: 62 Director Since: 2018 Key Skills: Business Leadership & Operations Healthcare & Pharma International Business Medicine & Science Human Capital Management Other Current Public Boards: None | | |
| | | | | | | | |
10 | | Pfizer 2024 Proxy Statement |
| | |
| Item 1 — Election of Directors |
| | | | | | | | | | | |
| | | |
| | Susan Desmond-Hellmann, M.D., M.P.H. KEY SKILLS & EXPERIENCE Business Leadership & Operations: Dr. Desmond-Hellmann brings strong leadership, expertise in business operations and global perspectives to the Board through her experiences as former Chief Executive Officer of the Bill & Melinda Gates Foundation, where she oversaw the creation of the Gates Medical Research Institute (GMRI), as former President of Product Development at Genentech and as Chancellor of the University of California, San Francisco (UCSF). Medicine & Science/Healthcare & Pharma/Academia: Dr. Desmond-Hellmann’s background reflects significant achievements in medicine, healthcare and academia. She brings expertise in medicine and science from her leadership roles in product development and clinical cancer research. Through her experiences at a biotechnology company and at a pharmaceutical institute, she brings healthcare and pharma industry expertise. In addition, she has significant achievements in academia through her service as a distinguished professor at UCSF. Pfizer and the Board benefit from her depth of experience and expertise in medicine, healthcare and academia. Technology & Cybersecurity: Dr. Desmond-Hellmann brings an expertise in technology and innovation from her previous experiences at Genentech and as a director on other public company boards, including Meta. BACKGROUND Board Member of National Resilience, Inc. and Stand Up To Cancer. Senior Advisor at Lazard, Inc. in the Healthcare Group. Senior Advisor at GMRI from 2020 to 2021. CEO of the Bill & Melinda Gates Foundation, a private foundation committed to enhancing global healthcare, reducing extreme poverty and expanding educational opportunities, from 2014 to 2020. She served as the first female, and ninth overall, Chancellor of UCSF from 2009 to 2014. Dr. Desmond-Hellmann remains an Adjunct Professor at UCSF. Member of the President’s Council of Advisors on Science and Technology. From 1995 through 2009, she was employed at Genentech where she served as President of Product Development from 2005 to 2009, overseeing pre-clinical and clinical development, business development and product portfolio management. Prior to Genentech, she was Associate Director, Clinical Cancer Research at Bristol-Myers Squibb Pharmaceutical Research Institute. Director of: (i) Meta from 2013 to 2019; and (ii) Procter & Gamble from 2010 to 2017. Received the Hockfield Cancer Research Prize (2023). |
| | |
Age: 66 Director Since: 2020 Board Committees: Governance & Sustainability and Science and Technology Key Skills: Business Leadership & Operations Healthcare & Pharma Medicine & Science Academia Technology & Cybersecurity Other Current Public Boards: None | | |
| | | | | | | | | | | |
| | | |
| | Joseph J. Echevarria KEY SKILLS & EXPERIENCE Business Leadership & Operations/International Business/Risk Management: Mr. Echevarria’s 36-year career at Deloitte brings financial expertise and international business, leadership and operational and risk management skills to the Board. Finance & Accounting: Mr. Echevarria’s financial acumen, including his significant audit experience, expertise in accounting issues and service on the audit committees of other public companies, is an asset to Pfizer’s Board and Audit Committee. Government & Public Policy: Pfizer also benefits from Mr. Echevarria’s breadth and diversity of experience, which includes his former public service on the President’s Export Council. BACKGROUND Served as the CEO of Deloitte LLP, a global provider of professional services, from 2011 until his retirement in 2014. During his 36-year tenure with Deloitte, served in various leadership roles, including Deputy Managing Partner, Southeast Region, Audit Managing Partner and U.S. Managing Partner and Chief Operating Officer. Since 2022, Mr. Echevarria has served as CEO of the University of Miami (UM), and served as Trustee of UM since 2011. Serves as Chair Emeritus of former President Obama’s My Brother’s Keeper Alliance and as an advisor to the Obama Foundation. Chairman of the Board of The Bank of New York Mellon Corporation. Director of Unum Group, a provider of financial protection benefits. Director of Xerox Holdings Corporation from 2017 until 2023. Former member of the Presidential Commission on Election Administration. |
| | |
Age: 66 Director Since: 2015 Board Committees: Audit and Governance & Sustainability (Chair) Key Skills: Business Leadership & Operations Finance & Accounting International Business Risk Management Government & Public Policy Other Current Public Boards: The Bank of New York Mellon Corporation and Unum Group | | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 11 |
| | |
| Item 1 — Election of Directors |
| | | | | | | | | | | |
| | | |
| | Scott Gottlieb, M.D. KEY SKILLS & EXPERIENCE Government & Public Policy/Medicine & Science/Healthcare & Pharma: Dr. Gottlieb brings significant expertise in health care, public policy and the biopharmaceutical industry to Pfizer's Board and the Regulatory and Compliance and Science and Technology Committees. Through his work as a physician and his tenure at the U.S. Food and Drug Administration (FDA), Dr. Gottlieb has demonstrated an understanding of patient needs, the public policy environment and the rapidly changing dynamics of biopharmaceutical research and development. BACKGROUND Partner, New Enterprise Associates, Inc.’s Healthcare Investment Team and Resident Fellow of the American Enterprise Institute since 2019. Served as the 23rd Commissioner of the FDA from 2017 to 2019. Prior to serving as Commissioner of the FDA, Dr. Gottlieb held several roles in the public and private sectors, including serving as a Venture Partner to New Enterprise Associates, Inc. from 2007 to 2017. Director of Illumina, Inc. Director of Aetion, Inc. a private healthcare data technology company, Tempus, a private technology company and Comanche Biopharma, a private maternal medicine biopharmaceutical company. Board Member of National Resilience, Inc. Scientific Advisory Board Member of CellCarta. Member of the National Academy of Medicine and a contributor to the financial news network CNBC. |
| | |
Age: 51 Director Since: 2019 Board Committees: Regulatory and Compliance (Chair) and Science and Technology Key Skills: Healthcare & Pharma Medicine & Science Government & Public Policy Other Current Public Boards: Illumina, Inc. | | |
| | | | | | | | | | | |
| | | |
| | Helen H. Hobbs, M.D. KEY SKILLS & EXPERIENCE Academia/Medicine & Science/Healthcare & Pharma: Dr. Hobbs’ background reflects significant achievements in academia and medicine. She has served as a faculty member at the University of Texas Southwestern Medical Center for more than 30 years and is a leading geneticist in liver and heart disease, areas in which Pfizer has significant investments and experience. Pfizer benefits from her experience, expertise, achievements and recognition in both medicine and science. BACKGROUND Investigator, Howard Hughes Medical Institute since 2002, Professor of Internal Medicine and Molecular Genetics and Director of the McDermott Center for Human Growth and Development at the University of Texas Southwestern Medical Center. Board Member of Atavistik Bio. Scientific Advisory Board Member of the Chan Zuckerberg Initiative, Scientific Advisor of the Column Group and Colossal Biosciences. Member of the American Society for Clinical Investigation and the Association of American Physicians. Elected to the National Academy of Medicine in 2004, the American Academy of Arts and Sciences in 2006, and the National Academy of Sciences in 2007. Received the Pearl Meister Greengard Award (2015); the Breakthrough Prize in Life Sciences (2015); the Passano Award (2016); the Harrington Prize for Innovation in Medicine (2018); the Lefoulon-Delalande Grand Prize in Science (2018); the Gerald D. Aurbach Award for Outstanding Translational Research (2019); the Anitschkow Prize (2019); and Ross Prize in Molecular Medicine (2023). |
| | |
Age: 71 Director Since: 2011 Board Committees: Governance & Sustainability, Regulatory and Compliance, and Science and Technology (Chair) Key Skills: Healthcare & Pharma Medicine & Science Academia Other Current Public Boards: None | | |
| | | | | | | | |
12 | | Pfizer 2024 Proxy Statement |
| | |
| Item 1 — Election of Directors |
| | | | | | | | | | | |
| | | |
| | Susan Hockfield, Ph.D. KEY SKILLS & EXPERIENCE Academia/Business Leadership & Operations/Medicine & Science: Dr. Hockfield has strong leadership skills, having served as the first woman and first life scientist President of the Massachusetts Institute of Technology (MIT) from 2004 to 2012 and as Dean of the Graduate School of Arts and Sciences from 1998 to 2002 and Provost from 2003 to 2004 at Yale University. Her background also reflects significant achievements in academia and science as she has served as a professor of Neuroscience at the Yale University School of Medicine (1985-2004) and MIT (2004-present). Pfizer benefits from her experience, expertise, achievements and recognition in both medicine and science. Government & Public Policy: Pfizer benefits from Dr. Hockfield’s breadth and depth of experience in the public policy space, including her public service as Science Envoy with the U.S. Department of State, co-chair of the Advanced Manufacturing Partnership, as a member of a Congressional Commission evaluating the Department of Energy laboratories, and as President and Chair of the American Association for the Advancement of Science. BACKGROUND Professor of Neuroscience and President Emerita at MIT. Served as MIT’s sixteenth president from 2004 to 2012. Member, Koch Institute for Integrative Cancer Research at MIT. Prior to joining MIT, she was the William Edward Gilbert Professor of Neurobiology, Dean of the Graduate School of Arts and Sciences from 1998 to 2002 and Provost from 2003 to 2004 at Yale University. Board Member of Repertoire Immune Medicines, Cajal Neuroscience and Break Through Cancer. Founding co-chair of the Advanced Manufacturing Partnership. Fellow of the American Association for the Advancement of Science. Member of the American Academy of Arts and Sciences and the Society for Neuroscience. Recipient of the Charles L. Branch BrainHealth Award, Charles Judson Herrick Award from the American Association of Anatomists, the Wilbur Lucius Cross Award from Yale University, the Meliora Citation from the University of Rochester, the Golden Plate Award from the Academy of Achievement, the Amelia Earhart Award from the Women’s Union, the Edison Achievement Award, the Pinnacle Award for Lifetime Achievement from the Greater Boston Chamber of Commerce and the Geoffrey Beene Builders of Science Award from Research!America. She previously served as a Director of General Electric Company from 2006 until 2018 and of Qualcomm Incorporated from 2012 until 2016. |
| | |
Age: 73 Director Since: 2020 Board Committees: Regulatory and Compliance and Science and Technology Key Skills: Business Leadership & Operations Medicine & Science Academia Government & Public Policy Other Current Public Boards: None | | |
| | | | | | | | | | | |
| | | |
| | Dan R. Littman, M.D., Ph.D. KEY SKILLS & EXPERIENCE Medicine & Science/Healthcare & Pharma/Academia: Dr. Littman’s background reflects significant achievements in medicine, healthcare and academia. He has served as a faculty member at the NYU Langone Medical Center for more than 25 years and is a renowned immunologist and molecular biologist. Pfizer benefits from his experience, expertise, achievements and recognition in both medicine and science. In addition, his experiences as a member of the National Academy of the Sciences and the National Academy of Medicine enable him to bring a broad perspective of the scientific and medical community to the Board. BACKGROUND Helen L. and Martin S. Kimmel Professor of Molecular Immunology, Department of Pathology at NYU Grossman School of Medicine (NYU Grossman). Professor, Department of Microbiology at NYU Grossman since 1995 and Investigator, Howard Hughes Medical Institute, since 1987. Professor of Microbiology and Immunology at the University of California, San Francisco from 1985 to 1995. Member of the National Academy of the Sciences and the National Academy of Medicine. Fellow of the American Academy of Arts and Sciences and the American Academy of Microbiology. Founding Scientific Advisory Board Member of Vedanta Biosciences and Scientific co-founder and Advisory Board Member of Immunai, Inc. Member of Scientific Advisory Boards at the Cancer Research Institute, the Broad Institute, IMIDomics, Scleroderma Research Foundation, Sonoma Biotherapeutics, Whitehead Institute of MIT Board of Advising Scientists and the Ragon Institute of MGH, MIT and Harvard. Member of the Scientific Steering Committee of Parker Institute of Cancer Immunotherapy. Awarded the New York City Mayor’s Award for Excellence in Science and Technology (2004), the Ross Prize in Molecular Medicine (2013) and the Vilcek Prize in Biomedical Science (2016). |
| | |
Age: 71 Director Since: 2018 Board Committees: Governance & Sustainability, Regulatory and Compliance and Science and Technology Key Skills: Healthcare & Pharma Medicine & Science Academia Other Current Public Boards: None | | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 13 |
| | |
| Item 1 — Election of Directors |
| | | | | | | | | | | |
| | | |
| | Shantanu Narayen KEY SKILLS & EXPERIENCE Business Leadership & Operations/International Business/Finance & Accounting/Human Capital Management: Mr. Narayen’s experience as Chair and CEO of Adobe Inc. (Adobe) brings strong leadership and human capital management skills to the Board, and his past roles in worldwide product development provide valuable global operations experience. He also serves as a member and Vice Chairman of US-India Strategic Partnership Forum. Through his experiences as a director on another public board, he provides a broad perspective on issues facing public companies and governance matters. Technology & Cybersecurity/Risk Management: Pfizer benefits from Mr. Narayen’s extensive knowledge in technology, product innovation and leadership in the digital marketing category through his experience in the technology industry. In addition, his deep knowledge and understanding of business risks through his leadership at a global technology company provide further insight and perspective to the Board. BACKGROUND Chair since 2017 and Chief Executive Officer since 2007 of Adobe, one of the largest and most diversified software companies in the world. President of Adobe until December 2021. Prior to his appointment as CEO, he held various leadership roles at Adobe, including President and Chief Operating Officer, Executive Vice President of Worldwide Products, and Senior Vice President of Worldwide Product Development. Vice Chairman of US-India Strategic Partnership Forum. Consistently named one of the world’s best CEOs by Barron’s magazine and, in 2020, ranked as a Fortune “Businessperson of the Year.” |
| | |
Lead Independent Director Age: 60 Director Since: 2013 Key Skills: Business Leadership & Operations Finance & Accounting International Business Human Capital Management Risk Management Technology & Cybersecurity Other Current Public Boards: Adobe Inc. | | |
| | | | | | | | | | | |
| | | |
| | Suzanne Nora Johnson KEY SKILLS & EXPERIENCE Business Leadership & Operations/Risk Management/International Business: Ms. Nora Johnson’s careers in law and investment banking, including serving in various leadership roles at Goldman Sachs Group, Inc. (Goldman Sachs), provide valuable business experience and critical insights into the roles of the law and finance when evaluating strategic transactions. Finance & Accounting: Ms. Nora Johnson also brings financial expertise to the Board, providing an understanding of financial statements, corporate finance, accounting, capital markets and risk management. Healthcare & Pharma: Ms. Nora Johnson’s extensive knowledge of healthcare through her role in healthcare investment banking and investing, as well as her involvement with not-for-profit organizations, such as in scientific research (The Carnegie Institution) and healthcare policy (The Brookings Institution) provide touchstones of public opinion and exposure to diverse, global points of view. BACKGROUND Retired Vice Chairman, Goldman Sachs, since 2007. During her 21-year tenure with Goldman Sachs, she served in various leadership roles, including Chair of the Global Markets Institute, Head of Global Research, and Head of Global Health Care. Board Chair of Intuit Inc.; Co-Chair, Board of Trustees of The Brookings Institution; Member of the Board of Trustees of the Carnegie Institution of Washington; and Chair of the Board of Trustees of the University of Southern California. Member of the American Academy of Arts and Sciences. Director of American International Group, Inc. from 2008 to 2020 and Visa, Inc. from 2007 to 2022. |
| | |
Age: 66 Director Since: 2007 Board Committees: Audit (Chair) and Regulatory and Compliance Key Skills: Business Leadership & Operations Finance & Accounting Healthcare & Pharma International Business Risk Management Other Current Public Boards: Intuit Inc. | | |
| | | | | | | | |
14 | | Pfizer 2024 Proxy Statement |
| | |
| Item 1 — Election of Directors |
| | | | | | | | | | | |
| | | |
| | James Quincey KEY SKILLS & EXPERIENCE Business Leadership & Operations/International Business/Finance & Accounting/Human Capital Management: Mr. Quincey’s experience as Chairman and CEO of The Coca-Cola Company brings strong business and leadership and human capital management skills, including extensive experience in leading business operations in international markets, such as Latin America and Europe, to the Board. He also brings a high level of financial experience acquired through his various leadership positions at The Coca-Cola Company, managing complex financial transactions, mergers and acquisitions, business strategy and international operations. Technology & Cybersecurity: Mr. Quincey also brings expertise in information technology to Pfizer’s Board. In his leadership position at The Coca-Cola Company, he is responsible for the company’s information technology function. BACKGROUND Chairman and Chief Executive Officer of The Coca-Cola Company, a total beverage company with products sold in more than 200 countries and territories. He was appointed Chairman of the Board in 2019 and CEO in 2017. Prior to his appointment as CEO in 2017, he held various leadership roles at The Coca-Cola Company, including President and Chief Operating Officer from 2015 to 2017, President of the Europe Group, President of the Northwest Europe and Nordics business unit and President of the Mexico division. Director of US-China Business Council and Catalyst. |
| | |
Age: 59 Director Since: 2020 Board Committees: Compensation Key Skills: Business Leadership & Operations Finance & Accounting International Business Human Capital Management Technology & Cybersecurity Other Current Public Boards: The Coca-Cola Company | | |
| | | | | | | | | | | |
| | | |
| | James C. Smith KEY SKILLS & EXPERIENCE Business Leadership & Operations/Finance & Accounting/Human Capital Management/International Business/Risk Management: Through Mr. Smith’s experience as former President and CEO of Thomson Reuters Corporation (Thomson Reuters) he brings valuable leadership, finance, international business, risk management and human capital management skills to our Board. Pfizer benefits from Mr. Smith’s organizational expertise and leadership experience, developed through numerous senior management roles and on notable merger and acquisition activities, including the acquisition and subsequent integration of two of the information industry’s preeminent firms, as well as his strong operational and international expertise. Mr. Smith’s previous experience running global Human Resources for the Thomson Corporation informs his strong advocacy for culture and talent development. BACKGROUND Chairman of the Thomson Reuters Foundation, a London-based charity supported by Thomson Reuters. President and Chief Executive Officer of Thomson Reuters, a provider of intelligent information for businesses and professionals from 2012 through March 2020, its Chief Operating Officer from September 2011 to December 2011, and Chief Executive Officer, Thomson Reuters Professional Division, from 2008 to 2011. Prior to the acquisition of Reuters Group PLC by The Thomson Corporation in 2008, served as Chief Operating Officer of Thomson Corporation and as President and Chief Executive Officer of Thomson Learning’s Academic and Reference Group. Director of Refinitiv, a privately held global provider of financial market data and infrastructure until its acquisition by the London Stock Exchange Group in January 2021. Member of the Board of Governors of Marshall University. Member of the Board of Trustees of the Brookings Institution. Director of Thomson Reuters from 2012 until 2020. |
| | |
Age: 64 Director Since: 2014 Board Committees: Audit and Compensation (Chair) Key Skills: Business Leadership & Operations Finance & Accounting International Business Human Capital Management Risk Management Other Current Public Boards: None | | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 15 |
| | | | | | | | | | | | | | | | | |
| | |
| | |
| Governance | |
| | |
| | |
| Overview We are committed to maintaining and enhancing our history of excellence in governance, which promotes our shareholders’ long-term interests, strengthens Board and management accountability, and helps support our standing as a trusted corporate citizen. GOVERNANCE MATERIALS AVAILABLE ON OUR WEBSITE Our corporate governance policies and procedures are reviewed at least annually by the Governance & Sustainability Committee and the full Board and are updated periodically in response to changing regulatory requirements, evolving practices and trends, issues raised by our shareholders and other stakeholders, and otherwise as circumstances warrant. You can view our Principles, policies and other corporate governance materials on our website at https://investors.pfizer.com/Investors/Corporate-Governance/The-Pfizer-Board-Policies/default.aspx and https://www.pfizer.com/about/responsibility/compliance/code-of-conduct. Website references and their hyperlinks have been provided throughout this Proxy Statement for convenience only. The content on any referenced websites is not a part of our proxy solicitation materials. BOARD LEADERSHIP STRUCTURE Given the dynamic and competitive environment in which we operate, the Board believes that its optimal leadership structure may vary as circumstances warrant. Our Principles provide the Board with flexibility to determine its optimal model for independent Board leadership at any given time. The Board does not favor any specific leadership structure. The independent Directors evaluate the Board’s leadership structure at least annually. During its review, the Governance & Sustainability Committee considers the company’s current operating environment, peers’ Board leadership structures, best practices and investor feedback. When and if the positions of Chairman and CEO are combined, or the Chairman is not an independent Board member, the independent Directors will elect a strong Lead Independent Director with a clearly defined role and responsibilities. Please see the Charter of the Lead Independent Director available at https://investors.pfizer.com/Investors/Corporate-Governance/The-Pfizer-Board-Policies/default.aspx. | |
| | | | | |
| | | 2023 ANNUAL REVIEW OF LEADERSHIP STRUCTURE In December 2023, following a thorough review by the Governance & Sustainability Committee, the independent Directors evaluated the Board’s leadership structure taking into consideration the company’s performance under the current operating and governance environment, executive leadership changes and investor feedback. The Committee, with input from the other independent Directors, determined that it would be in the best interest of the company and its shareholders for Dr. Bourla, Pfizer’s CEO, to continue serving as Chairman of the Board in 2024. The Board concluded that Dr. Bourla’s deep scientific, industry and regulatory expertise, along with his extensive company knowledge, helps enable him to effectively lead the Board and execute company strategies. Dr. Bourla’s leadership capabilities and business acumen, developed over 25-plus years of experience, proved to be especially beneficial during 2023 as the company faced numerous challenges, including uncertain patient demand for its COVID-19 products in a post-pandemic environment. Under Dr. Bourla’s leadership, the company acquired Seagen, reorganized its commercial operations and appointed new executive leadership. Additionally, we launched an enterprise-wide cost realignment program that aims to align our costs with our longer-term revenue expectations. We believe these strategic decisions, as well as the acquisition of Seagen and other organizational changes, will help position Pfizer for the future. The independent Directors also re-elected Mr. Shantanu Narayen as Lead Independent Director in 2024. Mr. Narayen has demonstrated strong leadership skills, risk oversight abilities, and expertise in technology and product innovation during his tenure as a Director and Lead Independent Director. His strong independent leadership, global leadership experience, and commitment to the Board make him well-suited for this independent leadership role. Accordingly, the independent Directors remain confident in Mr. Narayen’s ability to continue as Lead Independent Director for 2024. OUR BOARD LEADERSHIP STRUCTURE IS FURTHER STRENGTHENED BY: •the strong, independent oversight exercised by our Board, composed entirely of independent Directors other than Dr. Bourla, and its key Committees; •the independent leadership provided by Pfizer’s Lead Independent Director, who has robust, well-defined responsibilities under a Board-approved charter; and •Board and Committee processes and procedures that provide substantial independent oversight of our CEO’s performance, including regular executive sessions of the independent Directors (which take place at every Board meeting and are led by our Lead Independent Director), an annual evaluation of our CEO’s performance against predetermined goals, as well as an assessment of the CEO’s interactions with the Board in his role as Chairman. | | |
| | | | | |
| | | | | | | | |
16 | | Pfizer 2024 Proxy Statement |
THE BOARD’S ROLE IN RISK OVERSIGHT
The Board believes that its leadership structure and the Enterprise Risk Management (ERM) program support the effective risk oversight function of the Board. Management is responsible for assessing and managing risk, including through the ERM program, subject to oversight by the Board. The ERM program provides a framework for risk identification and management. Each risk is prioritized and assigned to a member or members, as appropriate, of our ELT, the company’s senior-most leadership and decision-making management body.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | THE BOARD The Board considers significant enterprise risk topics, including, among others: risks associated with our strategic plan, our capital structure, our R&D and business development activities, drug pricing, access and reimbursement, manufacturing and supply, cybersecurity, our ESG program, culture and human capital management and pipeline and portfolio strategy. In addition, it receives regular reports from members of our ELT that include discussions of the risks involved in their respective areas of responsibility. The Board is routinely informed of developments that could affect our risk profile or other aspects of our business. The Board is kept informed of its Committees’ risk oversight and other activities through reports by the Committee Chairs to the full Board. These reports are presented at every regular Board meeting. | |
| | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | AUDIT COMMITTEE The Audit Committee has primary responsibility for overseeing Pfizer’s ERM program. Pfizer’s Chief Internal Auditor, who reports to the Committee, facilitates the ERM program in coordination with the Legal Division and Compliance Division and helps ensure that ERM is integrated into our strategic and operating planning process. The Committee meets throughout the year, with agendas that include discussions of individual risk areas, including areas posing potential reputational risk to Pfizer, as well as an annual summary of the ERM program. As part of the ERM discussions, the Committee reviews and receives information and briefings concerning risks to Pfizer associated with drug pricing, access and reimbursement. The Committee also oversees the company’s information security (including cybersecurity) and technology risk management programs, which are fully integrated into the overall ERM program. The Committee receives regular briefings concerning Pfizer’s information security and technology risks and risk management practices, which are led by Pfizer’s Chief Information Security Officer. | | | | | REGULATORY AND COMPLIANCE COMMITTEE The Regulatory and Compliance Committee is responsible for reviewing and overseeing Pfizer’s ethics & compliance program, including evaluating its effectiveness. The Committee reviews and receives information and briefings about current and emerging compliance and quality risks and regulatory, enforcement and other external factors that may affect our business operations, risk management, performance, or strategy, as we innovate to deliver on our purpose and advance public health. The Committee’s primary responsibilities include overseeing Pfizer’s healthcare law compliance and quality risk management, its culture of integrity and the status of compliance with applicable laws, regulations and internal procedures. Periodically, the Regulatory and Compliance Committee and the Audit Committee hold joint sessions to discuss risks relevant to both Committees’ areas of risk oversight, including an annual discussion of the ERM program. | | | | | OTHER BOARD COMMITTEES The Board’s other Committees oversee risks associated with their respective areas of responsibility. For example: •The Compensation Committee considers the risks associated with our compensation policies and practices for both executive compensation and compensation generally. •The Governance & Sustainability Committee considers risks relating to the company’s: –ESG strategy and reporting; –human capital management; –lobbying priorities and activities; –political spending; and –potential reputational risk factors. •The Science and Technology Committee evaluates the soundness/risks associated with our technologies. | |
| | | | | | | | | | | | | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 17 |
THE BOARD’S ROLE IN SUCCESSION PLANNING
Management Succession Planning
Succession planning for Pfizer’s senior management positions helps ensure continuity of leadership and is critical to the company’s success. The Board is responsible for succession planning for the CEO and certain other senior management positions, and discusses succession planning regularly in executive sessions. To assist the Board, the CEO annually provides the Board with an assessment of potential successors to the CEO role, as well as to certain senior management positions. When appropriate, the Board will meet with these individuals.
In addition, the Governance & Sustainability Committee reviews the succession plans relating to positions held by elected corporate officers with the CEO and will make recommendations to the Board with respect to potential successors to fill these roles, as appropriate.
During 2023, in light of the company’s reorganization of its commercial business to further advance its emphasis on oncology and help improve focus, speed and execution, and based on management’s recommendation, the Governance & Sustainability Committee recommended, and the Board approved, certain changes to the ELT, including the elections of: (i) Dr. Chris Boshoff as Chief Oncology Officer, Executive Vice President; (ii) Mr. Aamir Malik as Chief U.S. Commercial Officer, Executive Vice President; and (iii) Mr. Alexandre de Germay as Chief International Commercial Officer, Executive Vice President.
Board Succession Planning
The Governance & Sustainability Committee focuses on Board succession planning on a continuous basis and considers the size of the Board, our skills matrix and upcoming retirements, all in light of the company’s strategic priorities. In performing this function, it recruits and recommends nominees for election as Directors. The goal is to achieve a Board that provides effective oversight of the company with appropriate diversity of gender, age, race, ethnicity, background, professional experience and perspectives. The Board does not believe in automatic annual re-nomination.
The Board’s self-evaluation process, retirement policy and term limit are important determinants for continuing service and ensuring ongoing refreshment. Non-employee Directors will not be nominated for election to the Board after their 73rd birthday, except in the case of a non-employee Director who has not yet attained 15 years of service. In such case, the Director will have a term limit of up to 15 years such that a Director will not be nominated for election to the Board if the 15-year anniversary of his or her service on the Board would occur prior to the end of the term for which nominees are being nominated. This policy helps ensure continuity of Directors who possess key skills and expertise that are critical to the Board’s oversight of the company. On the recommendation of the Governance & Sustainability Committee, the Board may waive either requirement as to any Director if it deems a waiver to be in the best interests of the company.
| | | | | | | | |
18 | | Pfizer 2024 Proxy Statement |
EVALUATION OF BOARD EFFECTIVENESS
The Board is committed to continuous improvement and uses annual evaluations to evaluate performance and improve effectiveness.
2023 Evaluation Process
| | | | | | | | | | | | | | | | | |
| | | | | |
| | FEBRUARY Board & Committee Evaluation | The Governance & Sustainability Committee initiates, conducts and oversees the process, which, in 2023, consisted of engaging a third-party provider to conduct Board and Committee evaluations through individual Director interviews, which focused on a variety of topics, including Board and Committee leadership. |
|
|
| | |
| â | |
| | |
| APRIL Presentation of Evaluation Results | The results of the full Board and each Committee evaluation were presented by the third-party provider to the Governance & Sustainability Committee and reported to the full Board by the Chair of the Governance & Sustainability Committee. The results were fully discussed in executive session at a subsequent Board meeting. |
| | |
| â | |
| | |
| JUNE Follow-up | The Board and Committees discussed topics requiring additional consideration to be addressed at future Board and Committee meetings. |
| | |
| â | |
| | |
| OCTOBER Evaluation of Existing Process | The Governance & Sustainability Committee reviewed the effectiveness of the overall evaluation process and considered whether, for 2024, to: •utilize a self-evaluation questionnaire; •incorporate individual Director evaluations into the process; or •conduct the evaluation through an external third-party provider. After reviewing and discussing the comprehensive feedback provided by the Board and Committees’ self-evaluations, the Governance & Sustainability Committee determined it would revert back to its original self-evaluation questionnaire process for its 2024 annual evaluation of the Board and its Committees. In addition, the Committee determined it would use a third-party provider every two to three years. |
| |
| |
| | | | | |
Board and Committee Information
During 2023, the Board met 7 times. Each of our Directors attended 75% or more of the total meetings of the Board and the Committees on which he or she served. In accordance with our Principles, all Directors attended our 2023 Annual Meeting.
COMMITTEE REFRESHMENT
The Board, upon recommendation from the Governance & Sustainability Committee, reviews and determines the composition of the Committees and appoints the Committee Chairs. Through periodic committee refreshment, we balance the benefits derived from continuity and depth of experience with those gained from fresh perspectives and enhancing our Directors’ understanding of different aspects of our business. There were no changes to Committee compositions in 2023.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 19 |
BOARD COMMITTEES
| | | | | | | | | | | |
| | | |
| | The Audit Committee Chair: Suzanne Nora Johnson The Committee’s primary responsibilities include: •the appointment, compensation, retention and oversight of our independent registered public accounting firm (the firm); •reviewing and discussing, with the firm, Internal Audit and management, the adequacy and effectiveness of internal control over financial reporting; •reviewing and consulting with management, Internal Audit and the firm on matters related to the annual audit, the published financial statements, earnings releases and the accounting principles applied; •reviewing reports from management relating to the status of compliance with laws, regulations and internal procedures and policies; •reviewing and approving, based on discussion with the Chief Financial Officer, the appointment, replacement or dismissal of the Chief Internal Auditor and reviewing, with the Chief Financial Officer, the performance of the Chief Internal Auditor; and •reviewing and discussing with management the company’s policies with respect to risk assessment and risk management, including with respect to information security and technology risks (including cybersecurity). The Committee has established policies and procedures for the pre-approval of all services provided by the firm. It also has established procedures for the receipt, retention and treatment, on a confidential basis, of complaints received by Pfizer regarding its accounting, internal controls and auditing matters. Further details of the role of the Audit Committee, as well as the Audit Committee Report, may be found in “Item 2 — Ratification of Selection of Independent Registered Public Accounting Firm” on page 35. The Audit Committee is governed by a Board-approved Charter which is available on our website at https://investors.pfizer.com/Investors/Corporate-Governance/Board-Committees--Charters/. |
| | |
Additional Committee Members: Ronald E. Blaylock Joseph J. Echevarria James C. Smith All Members are Independent and Financially Literate All Members qualify as “Audit Committee Financial Experts” as defined by the Securities and Exchange Commission Meetings Held in 2023: 11 | | |
| | | |
| | | | | | | | | | | |
| | | |
| | The Compensation Committee Chair: James C. Smith The Committee reviews and approves the company’s overall compensation philosophy and oversees the administration of our executive compensation and benefit programs, policies and practices. Its responsibilities also include: •establishing and monitoring performance against short-term and long-term incentive (LTI) plan goals, and approving the short-term incentive plan pool performance and LTI plan goals, and LTI awards; •establishing objectives for the CEO and reviewing the goals approved by the CEO for our executive officers, including the Named Executive Officers (NEOs), as well as evaluating the performance and setting compensation for the CEO and reviewing and approving the compensation of the ELT; •reviewing and assessing annually, potential risks to the company from its compensation program and related policies; •evaluating the efficacy of the company’s compensation policy and strategy in achieving gender and minority pay parity, positive social impact and attracting a diverse workforce; and •collaborating with the Governance & Sustainability Committee on responsibilities delegated by the Board relating to human capital management. The Committee has the authority to delegate any of its responsibilities to another committee, officer and/or subcommittee, as the Committee may deem appropriate in its sole discretion, subject to applicable law, rules, regulations and NYSE listing standards. The Compensation Committee is governed by a Board-approved Charter which is available on our website at https://investors.pfizer.com/Investors/Corporate-Governance/Board-Committees--Charters/. Compensation Committee Interlocks and Insider Participation. During 2023 and as of the date of this Proxy Statement, none of the members of the Committee was or is an officer or employee of Pfizer, and no executive officer of the company served or serves on the compensation committee or board of any company that employed or employs any member of Pfizer’s Compensation Committee or Board of Directors. |
| | |
Additional Committee Members: Ronald E. Blaylock James Quincey All Members are Independent All Members are “non-employee directors” as defined in Rule 16b-3 under the Securities Exchange Act of 1934 Meetings Held in 2023: 8 | | |
| | | |
| | | | | | | | |
20 | | Pfizer 2024 Proxy Statement |
| | | | | | | | | | | |
| | | |
| | The Governance & Sustainability Committee Chair: Joseph J. Echevarria The Committee oversees the practices, policies and procedures of the Board and its committees. Its responsibilities include: •recommending and recruiting Director candidates so that the Board maintains its diverse composition, with diversity reflecting gender, age, race, ethnicity, background, professional experience and perspectives; •overseeing the evaluations of the Board and its Committees; •reviewing our Principles and Director Qualification Standards; •overseeing the company’s ESG strategy and reporting and maintaining an informed status on political spending and lobbying priorities and activities; and •overseeing the company’s policies and practices related to human capital management, including succession planning, culture, diversity, equity and inclusion, pay equity and talent management. The Governance & Sustainability Committee is governed by a Board-approved Charter which is available on our website at https://investors.pfizer.com/Investors/Corporate-Governance/Board-Committees--Charters/. |
| | |
Additional Committee Members: Susan Desmond-Hellmann, M.D., M.P.H. Helen H. Hobbs, M.D. Dan R. Littman, M.D., Ph.D. All Members are Independent Meetings Held in 2023: 5 | | |
| | | |
| | | | | | | | | | | |
| | | |
| | The Regulatory and Compliance Committee Chair: Scott Gottlieb, M.D. The Committee’s primary responsibilities include: •assisting the Board with overseeing quality and compliance risk management in areas of healthcare compliance across the company’s core functions; and •reviewing and overseeing the company’s ethics & compliance program and related activities through review of reports and information from management, legal counsel and third parties covering: (i) the effectiveness of the compliance program; (ii) proactive quality and compliance risk management; and (iii) significant regulatory and compliance healthcare-related matters. The Committee makes recommendations to the Compensation Committee concerning the extent, if any, to which the incentive-based compensation of any executive, senior manager, compliance personnel and/or attorney involved in any significant misconduct resulting in certain government or regulatory action, or other person with direct supervision over such employee, should be reduced, extinguished or recouped. The Regulatory and Compliance Committee is governed by a Board-approved Charter which is available on our website at https://investors.pfizer.com/Investors/Corporate-Governance/Board-Committees--Charters/. |
| | |
Additional Committee Members: Helen H. Hobbs, M.D. Susan Hockfield, Ph.D. Dan R. Littman, M.D., Ph.D. Suzanne Nora Johnson All Members are Independent Meetings Held in 2023: 4 | | |
| | | |
| | | | | | | | | | | |
| | | |
| | The Science and Technology Committee Chair: Helen H. Hobbs, M.D. The Committee is responsible for periodically examining management’s strategic direction of and investment in the company’s biopharmaceutical R&D and technology initiatives. Its responsibilities include: •monitoring progress of Pfizer’s R&D pipeline; •evaluating the quality, direction and competitiveness of the company’s R&D programs; and •reviewing Pfizer’s approach to acquiring and maintaining key scientific technologies and capabilities. The Committee also identifies and evaluates emerging issues, assesses the performance of R&D leaders, and evaluates the sufficiency of review by external scientific experts. The Science and Technology Committee is governed by a Board-approved Charter which is available on our website at https://investors.pfizer.com/Investors/Corporate-Governance/Board-Committees--Charters/. |
| | |
Additional Committee Members: Susan Desmond-Hellmann, M.D., M.P.H. Scott Gottlieb, M.D. Susan Hockfield, Ph.D. Dan R. Littman, M.D., Ph.D. All Members are Independent Meetings Held in 2023: 5 | | |
| | | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 21 |
Governance & Sustainability Committee Report
The following are examples of how we worked to achieve the Board’s objectives to maintain and enhance Pfizer's record of excellence in corporate governance and Board oversight in 2023 and early 2024.
Board Leadership Structure: In late 2023, the Committee and the independent Directors conducted a thorough annual review of the Board’s leadership structure, and the independent Directors unanimously determined to maintain the current leadership structure, with Dr. Bourla as Chairman and CEO, and Mr. Narayen as Lead Independent Director.
Board and Committee Matters: We assessed the Directors’ qualifications for serving on various Committees and their independence. We also engaged a third-party provider to conduct Board and Committee evaluations through individual Director interviews and reviewed the results. In addition, we reviewed, among other factors, Director service on other boards and other commitments held by Directors. As a result, we determined that all Directors are in compliance with the company’s Principles and have sufficient time, energy, and attention to effectively serve on our Board. Additionally, the Committee reviewed and, where appropriate, recommended changes to our Principles and other governance documents. Further, in light of new U.S. Securities and Exchange Commission (SEC) regulations governing Rule 10b5-1 stock trading plans, we reviewed and recommended updates to our related processes. We also reviewed the non-employee Director compensation program, in consultation with Meridian Compensation Partners, LLC, and recommended no changes.
Board Succession Planning: We continued our Board succession planning to identify and assess potential Director candidates. We considered several factors, including a review of our skills matrix. We conducted a needs assessment and considered a diverse pool of candidates based on recommendations provided by our Chairman and CEO, the independent Directors, management, external advisors, the Board’s evaluation and other resources. The Board did not elect any new Directors during 2023.
Commercial Reorganization: Pursuant to our Charter, we make recommendations to the Board with respect to the selection of elected corporate officers. During 2023, in light of the company’s reorganization of its commercial business to further advance its emphasis on oncology and help improve focus, speed and execution, and based on management’s recommendation, the Committee recommended, and the Board approved, certain changes to the ELT, including the elections of: (i) Dr. Chris Boshoff as Chief Oncology Officer, Executive Vice President; (ii) Mr. Aamir Malik as Chief U.S. Commercial Officer, Executive Vice President; and (iii) Mr. Alexandre de Germay as Chief International Commercial Officer, Executive Vice President.
Environmental, Social and Governance Strategy: Throughout the year, we engaged with management to review and discuss the company’s ESG priorities and its progress, as well as changes and potential regulatory developments as a result of the evolving ESG external environment in the U.S. and internationally.
Public Policy/Corporate Political Spending/Lobbying Activities: Under our Charter, we are informed of company issues related to public policy, including political spending policies and practices. In addition, management provides regular updates to the Committee on the company’s work on legislative and regulatory policies, including the benefits and risks derived from our association with certain trade and other organizations.
Legislative and Regulatory Developments: We continued to monitor and evaluate corporate governance and executive compensation developments, including SEC rules and NYSE listing standards through reports provided by management.
Shareholder Engagement: We engaged in reviews of shareholder and stakeholder communications at each of our meetings and were informed of shareholder feedback received during Pfizer’s year-round investor outreach, which included the participation of the Chair of the Governance & Sustainability Committee, Mr. Echevarria, the Lead Independent Director, Mr. Narayen, and the Chair of the Compensation Committee, Mr. Smith, when appropriate. The Committee was also kept apprised of all shareholder proposals received and discussions with the proponents.
THE GOVERNANCE & SUSTAINABILITY COMMITTEE
Joseph J. Echevarria, Chair
Susan Desmond-Hellmann, M.D., M.P.H.
Helen H. Hobbs, M.D.
Dan R. Littman, M.D., Ph.D.
| | | | | | | | |
22 | | Pfizer 2024 Proxy Statement |
Regulatory and Compliance Committee Report
The Committee assists the Board with the oversight of healthcare-related regulatory and compliance risk management. Under the terms of its Charter, the Committee receives reports regarding the company’s ethics & compliance program, for which management has primary responsibility.
In 2023, we received reports and discussed with management, including the Chief Compliance, Quality and Risk Officer and the General Counsel, significant healthcare-related regulatory and compliance risks and related compliance program initiatives, functions and risk management.
Among the matters considered were:
•management of potential healthcare regulatory and compliance risks relating to the development, manufacture, supply and commercialization of Pfizer products, and efforts to mitigate those risks;
•certain compliance- and quality-related matters, government and internal investigations, regulatory actions and significant regulatory communications and other legal proceedings;
•results of internal audits conducted in areas within the Committee’s oversight;
•updates on the company’s quality and compliance governance framework and risk management;
•updates regarding compliance with the requirements of Pfizer’s Corporate Integrity Agreement;
•updates regarding the integration of acquired companies into Pfizer’s compliance program;
•anti-retaliation policies and procedures and any retaliation claims received by Pfizer; and
•Pfizer’s culture of integrity and our policies supporting speak-up, open-door and anti-retaliation, and the tone set by leaders throughout the organization.
In our activities, we considered potential risks and steps Pfizer has taken to mitigate risk in areas within our oversight.
THE REGULATORY AND COMPLIANCE COMMITTEE
Scott Gottlieb, M.D., Chair
Helen H. Hobbs, M.D.
Susan Hockfield, Ph.D.
Dan R. Littman, M.D., Ph.D.
Suzanne Nora Johnson
| | | | | | | | |
2024 Proxy Statement Pfizer | | 23 |
Shareholder Outreach
OUR ENGAGEMENT PROCESS
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | à | | | | à | | | | à | | | |
| | | | | | | | | | | |
| Spring | | | Summer | | | Fall | | | Winter | |
| | | | | | | | | | | |
| | | | | | | | | | | | | | |
| Discuss Proxy Statement voting items with institutional investors | | | | Discuss proxy voting season results and investor feedback with the Board to determine appropriate next steps, if any | | | | Solicit feedback from a variety of investors representing a significant number of shares outstanding | | | | Share investor feedback from fall outreach meetings with the Governance & Sustainability Committee and full Board | |
| | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | Review governance trends and best practices | | | | | | | | Adopt new or modify our existing governance practices in response, if needed | |
| | | | | Develop strategy for fall outreach meetings | | | | | | | | Develop strategy for proxy season outreach meetings | |
INVESTOR OUTREACH
Investor feedback is an important factor for Pfizer and the Board and essential to maintaining our strong governance practices. Throughout the year, we routinely seek opportunities to engage with our investors to hear their views concerning Pfizer’s corporate governance policies and practices, including ESG, and to discuss current and emerging trends.
During 2023, we contacted our 50 largest and some smaller, but engaged, institutional investors, representing approximately 44% of our shares outstanding, and invited them to engage in a dialogue on various topics. We met with over 30 investors, representing approximately 33% of our shares outstanding. In addition, we engaged with proxy advisory firms to hear their perspectives on governance and ESG topics and the prior and upcoming proxy seasons. While the engagements were primarily conducted by management, Board members also participated. During 2023, Mr. Echevarria, Chair of the Governance & Sustainability Committee, Mr. Narayen, Lead Independent Director, and Mr. James Smith, Chair of the Compensation Committee, participated in certain discussions with investors. Furthermore, during the proxy season, we engaged with every shareholder proposal proponent to hear their concerns, aiming to reach mutual agreements to address them.
In addition to connecting with our institutional investors, we also remain responsive to our retail investors’ and other stakeholders’ inquiries. This past year, we continued our outreach to proactively include retail investors through a retail investor program, led by Corporate Affairs. The program, “Investor Insights,” is designed to create a more cohesive relationship with Pfizer’s retail holders by sharing certain informative content regarding Pfizer’s corporate strategy through digital platforms.
In February 2024, we hosted an “Oncology Innovation Day,” during which Pfizer business executives and scientific leadership outlined our strategic priorities for the newly formed Pfizer Oncology organization.
| | | | | | | | |
24 | | Pfizer 2024 Proxy Statement |
Summary of Certain 2023 Shareholder Discussions
During our discussions, we strive for a collaborative approach and a variety of perspectives, which helps inform our understanding of stakeholder interests and motivations and fosters a mutual understanding of governance and related priorities. Items on the meeting agendas for 2023 covered a range of topics, including the Board, human capital, executive compensation, political expenditures, sustainable business risks and climate change. Please see below for highlights from our discussions.
| | | | | | | | | | | |
| | | |
| | Board of Directors: Investors generally responded positively to the composition and refreshment of the Board. Some investors asked about skills the Board may seek in future candidates and the timing of any upcoming retirements. We addressed questions regarding the rotation process for Committee Chairs and members, as well as the Board’s annual evaluation process, which involved the use of a third-party provider in 2023. Most investors were pleased with our current level of Board gender diversity, which stands at 33%; however, a few expressed a preference for a higher percentage. Some investors inquired whether the Board anticipated the need for any new skills given the company’s deepening focus on oncology. Finally, we sought investor feedback on our proxy disclosures, especially regarding the Board. Investors provided positive feedback; however, a few investors requested more details about Director skills and the importance of those skills to our business. Action taken: Feedback was shared with the Governance & Sustainability Committee and the full Board. See enhanced disclosures regarding Board composition and Director skills throughout this Proxy Statement. | |
| | | |
| | | |
| | | |
| | Human Capital: Investors expressed interest in Pfizer’s diversity, equity, and inclusion (DEI) goals, as well as our practices regarding pay equity. They inquired whether any changes were planned in response to the scrutiny impacting DEI programs. We were also asked about colleague morale in light of the challenges and opportunities facing our business during the year. We received questions regarding our plans to conduct a racial equity assessment, as well as requests for updates on our progress and the expected publication date for the results. Action taken: Feedback was shared with the Governance & Sustainability Committee. Pfizer intends to publish the results of a racial equity assessment in the spring of 2024, as per our agreement with the Service Employees International Union (SEIU). The SEIU submitted a shareholder proposal requesting the assessment in 2022. For additional information concerning our DEI initiatives and progress, please see Pfizer’s 2023 Impact Report and our website at https://www.pfizer.com/about/responsibility/diversity-and-inclusion. Please note that these documents are not a part of our proxy solicitation materials. | |
| | | |
| | | |
| | | |
| | Executive Compensation: We discussed the various elements of the executive compensation program. Investors asked if the Compensation Committee was considering any modifications to the executive compensation program in 2024. Investor feedback remained positive regarding the use of ESG metrics in the short-term incentive plan. Some investors expressed interest in seeing Pfizer include additional ESG metrics, such as “access to medicines,” in the future. A few investors inquired about the compensation packages for certain departing senior executives, and investors requested that we provide details in our 2024 Proxy Statement. Action taken: Feedback was shared with the Compensation Committee and the Governance & Sustainability Committee. The Compensation Committee determined in early 2024 that ESG metrics will remain unchanged and continue to be included in our short-term incentive program. Details concerning the departures of senior executives and related compensation decisions are included in this Proxy Statement. Please see the “Compensation Discussion and Analysis” section for additional information. | |
| | | |
| | | |
| | | |
| | Political Expenditures: A few investors asked about our political contributions and lobbying activities and disclosures. Some were interested in discussing the shareholder proposal requesting a congruency report, voted on at the 2023 Annual Meeting of Shareholders, as well as our discussions with the shareholder proponent. Overall feedback was positive about our existing disclosures, including the company’s “Industry Associations - Congruency Report”. Since the report was originally published in late 2021, investors asked about a timeline for expected updates. Investors were also interested in discussing the impact of the Inflation Reduction Act of 2022 on our business. Action taken: Feedback was shared with the Governance & Sustainability Committee. Pfizer published an updated “Industry Associations - Congruency Report” in early 2024. For additional information, please visit our website at https://www.pfizer.com/about/programs-policies/political-partnerships. | |
| | | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 25 |
| | | | | | | | | | | |
| | | |
| | Sustainable Business Risks: Investors reinforced their expectation that our ESG priorities remain balanced and aligned with our business priorities. We received some questions about Pfizer’s process to ensure product quality and safety, including Board and Committee oversight. We explained that the Regulatory and Compliance Committee has oversight over product quality, safety, compliance and risk management across R&D and Medical, Manufacturing and Supply, and Commercial. Some investors expressed interest in viewing further details about our product quality and safety outcomes. We explained that patient health and safety are foundational to all we do and shared information about how we maintain high standards in product quality and safety through effective quality management systems and processes, including how product quality and safety performance indicators are regularly monitored to proactively identify opportunities for continuous improvement. Action taken: Feedback was shared with the Governance & Sustainability Committee. Pfizer’s 2023 Impact Report provides information on our quality management system, including inspection outcomes data. We continue to evaluate and update this reporting annually. Please note that the 2023 Impact Report is not a part of our proxy solicitation materials. | |
| | | |
| | | |
| | | |
| | Climate Change: We received questions concerning Pfizer’s 2040 Net-Zero goal, which was announced in June 2022. Overall, investors were pleased with Pfizer’s response to climate change, and were interested in discussing our near-term Scope 1 and 2 initiatives to help achieve our goals, including partnering with our supply chains and increasing our reliance on renewable energy. Action taken: Feedback was shared with the Governance & Sustainability Committee. For additional information concerning progress in 2023 in achieving Pfizer’s ESG strategy and climate goals, please see Pfizer’s 2023 Impact Report. Please note that the 2023 Impact Report is not a part of our proxy solicitation materials. | |
| | | |
SHAREHOLDER INQUIRIES
We communicate with our shareholders through various platforms, including via our corporate website, digital and print media, webcasts and live events, including our annual meeting of shareholders, investor presentations and healthcare industry presentations. In 2023, in addition to meeting with institutional investors, we responded to more than 800 inquiries from individual shareholders sent to the Board or the Office of the Corporate Secretary.
At each Governance & Sustainability Committee meeting, we share investor and other stakeholder feedback directly with the Committee. We view communication between our shareholders and the Board as a dialogue and, when appropriate, members of our Board engage directly with our shareholders.
How to Communicate with Our Directors
Shareholders and other interested parties may communicate with any of our Directors, including the Lead Independent Director and the Audit Committee Chair, as follows:
By mail: Write to the Corporate Secretary, Pfizer Inc., 66 Hudson Boulevard East, New York, NY, 10001-2192; or
By e-mail: Go to Pfizer’s website at https://investors.pfizer.com/Investors/Corporate-Governance/Contact-Our-Directors/default.aspx.
Shareholder communications are distributed to the Board, or to any individual Director or Directors, as appropriate, depending on the facts and circumstances outlined in the communication. The Board has requested that certain items that are unrelated to the duties and responsibilities of the Board be redirected or excluded, as appropriate.
| | | | | | | | |
26 | | Pfizer 2024 Proxy Statement |
Public Policy Engagement and Political Participation
The Governance & Sustainability Committee maintains an informed status on the company’s issues related to public policy and corporate political spending practices. The Committee receives periodic updates and reviews Pfizer’s Political Action Committee (PAC) and Corporate Political Contributions Report (PAC Report) prior to its annual publication. The Committee also reviews the company’s “Industry Associations – Congruency Report.” In addition, management regularly informs the Committee of Pfizer’s public policy priorities and its efforts to educate lawmakers in support of those priorities.
PUBLIC POLICY ENGAGEMENT FOR GLOBAL PUBLIC HEALTH
It is fundamental to our business that we engage on public policy issues that may affect our ability to meet patients’ needs and enhance shareholder value. These issues include advancing biomedical research and healthcare innovation, advocating for intellectual property (IP) protections, and improving patient access to care. In addition, we regularly work with policymakers and industry and trade groups to help create and maintain an innovative environment where we can cultivate new medicines, bring them to market and ensure that patient health and safety remain a priority.
To advance our business objectives, we are also members of industry and trade groups, including the Pharmaceutical Research and Manufacturers of America, the National Association of Manufacturers, the U.S. Chamber of Commerce and the Business Roundtable. These organizations, along with the others to which we belong, represent both the industry and the business community at large in an effort to bring about consensus on broad policy issues. Our support of these organizations is evaluated annually by our U.S. Government Relations leaders based on their expertise in healthcare policy and advocacy.
In addition to healthcare policy, we realize these organizations may engage in a variety of other issues that extend beyond the scope of our priorities. Our participation as a member of these groups comes with the understanding that we may not always agree with every position held by the organization and/or its other members. Nevertheless, we monitor where and to what extent our trade associations are misaligned with the company on such issues and we will advocate for the trade association to come into alignment, as appropriate. If and when a trade association’s misalignment outweighs the benefits to Pfizer and its stakeholders, we consider whether to reduce our involvement with the organization or end our involvement altogether.
We believe value exists in making sure our positions on issues important to Pfizer and our industry are communicated and understood within those organizations. Please see Pfizer’s “Industry Associations – Congruency Report” at https://www.pfizer.com/about/programs-policies/political-partnerships. The “Industry Associations – Congruency Report” is not a part of our proxy solicitation materials.
Corporate Political Contributions
At Pfizer, we have adopted a formal policy for making corporate political and PAC contributions in the U.S., which applies to Pfizer and the Pfizer PAC. The policy is designed to ensure that our political expenditures are made in compliance with all federal, state, and local election laws, as applicable. In addition, in 2010, Pfizer adopted a policy that prohibits the company from making direct independent expenditures, including direct contributions to 527 independent expenditure committees, a decision supported by executives and affirmed by the Governance & Sustainability Committee of our Board. The PAC Report also explains that Pfizer typically does not make contributions to 527 Issue Organizations. However, if requested to make such a contribution, it would undergo review and approval by the Political Contributions Policy Committee (PCPC). The PCPC, co-chaired by Pfizer’s Chief Corporate Affairs Officer and the Chief Compliance, Quality & Risk Officer, consists of senior leaders from various divisions. Any contributions made to 527 Issue Organizations would be disclosed in the PAC Report. In addition, 501(c)(4) organizations may not use Pfizer’s funds to benefit directly or indirectly any specific federal, state, or local official, candidate, political party committee or political committee.
Our disclosures comply with all federal, state and local laws and reporting requirements governing corporate political contributions. All corporate political contributions are published annually in the PAC Report in compliance with Pfizer’s corporate policy.
We regularly discuss our political contributions disclosures with investors and other stakeholders to help ensure our disclosures meet their needs. Over the years, shareholder engagement has influenced our level of disclosure and helped to develop or modify related policies. See “Shareholder Outreach” above for more information. | | | | | | | | |
2024 Proxy Statement Pfizer | | 27 |
Policies and Procedures for Approval and Oversight of Corporate and PAC Political Expenditures
All corporate and PAC political spending decisions undergo a rigorous review process conducted monthly by the PAC Steering Committee. The Committee, composed of nine cross-divisional colleagues, reviews and approves all PAC and corporate political contributions. The PAC is a non-partisan, employee-run organization that enables employees to participate in the American political process. The Committee ensures that each contribution advances our business objectives and is not based on the political preferences or views of any individual colleague. In addition, the Committee considers contributions to lawmakers on a case-by-case basis using the following criteria:
•Prioritization of candidates who support policies that impact our purpose and uphold our core values, which include healthcare, tax and an intellectual property ecosystem that supports innovation and patient access to medicines;
•Representation where colleagues live and work; and
•Elected officials’ conduct and statements.
Our PAC support does not imply an endorsement of a candidate’s position on any social or religious issue, and we always consider a candidate’s ethical conduct in our evaluation to help ensure Pfizer’s values are upheld.
Further, all PAC and corporate contribution requests are shared with the PCPC.
FEDERAL AND STATE LOBBYING ACTIVITY
The company’s U.S. Government Relations leaders are responsible for the company’s lobbying activities. The Governance & Sustainability Committee is responsible for maintaining an informed status on the company’s lobbying priorities and activities through periodic reports from management. In addition, all colleague communications with government and regulatory officials are governed by Pfizer’s internal policies and procedures, which include guidelines available on our website at https://www.pfizer.com/about/responsibility/compliance/code-of-conduct.
Reporting and Compliance Features
Federal Lobbying
•Pfizer’s disclosures and lobbying activities comply with the Honest Leadership and Open Government Act of 2007. These reports may be viewed at https://lda.senate.gov/system/public/.
•In addition, we voluntarily report the portion of our dues used by trade associations for federal lobbying activity. See https://www.pfizer.com/about/programs-policies/political-partnerships.
State Lobbying
•We are fully compliant with state registration and reporting requirements.
•Links to states’ reporting entities, where state lobbying reports are filed, may be accessed at: https://www.pfizer.com/about/programs-policies/political-partnerships.
| | | | | | | | |
28 | | Pfizer 2024 Proxy Statement |
Pfizer Policies on Business Conduct
All of our colleagues, including our Chief Executive Officer, Chief Financial Officer and Controller, are required to abide by Pfizer’s policies on business conduct to help ensure that our business is conducted in a consistently legal and ethical manner. Pfizer’s policies form the foundation of a comprehensive process that includes compliance with corporate policies and procedures, an open relationship among colleagues to foster ethical business conduct, and an utmost commitment to integrity. Our policies and procedures cover all major areas of business conduct, including employment practices, conflicts of interest, anti-corruption, transparency, privacy, product communications, intellectual property, the protection of confidential information and the responsible use of artificial intelligence, and require strict adherence to laws and regulations applicable to the conduct of our business. In addition, we strive to ensure fair competition in all our business dealings, including, among other things, distribution agreements, rebates and discounts to customers, patent, copyright, and trademark licenses, territorial restrictions on resellers, and pricing policy generally. We are committed to competing fairly and following the applicable antitrust and competition laws of all countries in which we operate.
Code of Conduct training is assigned to all new colleagues upon hire and to existing colleagues regularly. The training includes a certification to confirm that colleagues agree to abide by the Code of Conduct and that they understand their responsibility to report and have reported any potential violations of law, regulations, ethical standards or Pfizer policy.
Colleagues are required to report any conduct that they believe to be an actual or apparent violation of Pfizer’s policies on business conduct. Retaliation in any form against any colleague who seeks advice, raises a concern, reports misconduct, or provides information in an investigation is prohibited. Our Audit Committee, working with Pfizer’s Compliance Division, has procedures to receive, retain and treat complaints received regarding accounting, internal accounting controls, or auditing matters and to allow for confidential and anonymous submissions by employees with concerns regarding questionable accounting or auditing matters.
The full text of our Code of Conduct, including information regarding how to report allegations of misconduct, is posted on our website at https://www.pfizer.com/about/responsibility/compliance/code-of-conduct. We will disclose any future amendments to, or waivers from, provisions of these ethics policies and standards affecting our Chief Executive Officer, Chief Financial Officer, Controller and executive officers on our website as promptly as practicable, as may be required under applicable SEC and NYSE rules.
CODE OF CONDUCT FOR DIRECTORS
Our Directors are required to comply with a Code of Business Conduct and Ethics for Members of the Board of Directors (the Director Code). It is intended to focus the Board and the Directors on areas of ethical risk, help them recognize and deal with ethical issues, provide mechanisms to report unethical conduct, and foster a culture of honesty and accountability. The Director Code covers all areas of professional conduct relating to service on our Board, including conflicts of interest, unfair or unethical use of corporate opportunities, strict protection of confidential information, compliance with applicable laws and regulations, and oversight of ethics and compliance by employees of the company.
The Director Code is available on our website at https://investors.pfizer.com/Investors/Corporate-Governance/The-Pfizer-Board-Policies/default.aspx.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 29 |
Other Governance Practices and Policies
DERIVATIVES TRADING/HEDGING POLICY
We have a policy that prohibits employees, including the NEOs, and Directors from purchasing or selling options on our common stock or engaging in short sales of our common stock. In addition, the policy prohibits trading in puts, calls, straddles, equity swaps or other derivative securities, including exchange funds, that are directly linked to our common stock (sometimes referred to as “hedging”).
RELATED PERSON TRANSACTIONS AND INDEMNIFICATION
Related Person Transaction Approval Policy
Pfizer has adopted a Related Person Transaction Approval Policy (the Policy) administered by the Governance & Sustainability Committee. The Policy applies to any transaction or series of transactions in which Pfizer or a subsidiary is a participant, the amount involved exceeds $120,000, and a related person under the Policy has a direct or indirect material interest. Under the Policy, management determines whether a transaction requires review by the Governance & Sustainability Committee.
Transactions requiring review are referred to the Governance & Sustainability Committee for approval, ratification or other action. Based on its consideration of all of the relevant facts and circumstances, the Governance & Sustainability Committee decides whether or not to approve such transactions and approves only those transactions that are deemed to be in the best interests of the company. If the company becomes aware of an existing transaction with a related person that has not been approved under this Policy, the matter is referred to the Governance & Sustainability Committee. The Governance & Sustainability Committee evaluates all options available, including ratification, revision or termination of such transaction. The Governance & Sustainability Committee then provides a summary of such transactions, including their terms, structure and business purpose, as well as the Governance & Sustainability Committee’s approval decision, to the Audit Committee for their information.
Transactions with Related Persons
Certain of our NEOs and other executive officers have family members who are also employed by Pfizer in non-executive positions. Specifically, Dr. Bourla’s sister-in-law is employed as Colleague Experience Designer, People Experience and Mr. Douglas Lankler’s daughter is employed as Pipeline Project Manager, Pfizer Digital. In addition, our Chief Compliance, Quality and Risk Officer, Executive Vice President, Mr. Rady Johnson’s daughter is employed as Global Study Manager, Pfizer Research and Development. The total annual compensation of each of Dr. Bourla’s sister-in-law, and Messrs. Lankler’s and Johnson’s daughters for fiscal 2023 (including any incentive compensation) did not, individually, exceed $250,000. Additionally, each of Dr. Bourla’s sister-in-law, and Messrs. Lankler’s and Johnson’s daughters, are employed on an “at will” basis and compensated on the same basis as Pfizer’s other employees of similar function, seniority and responsibility. The Governance & Sustainability Committee reviewed and approved or ratified each of these related party transactions in accordance with the Policy and provided a summary of such transactions, and its approval decision, to the Audit Committee.
Indemnification
We indemnify our Directors and our elected officers to the fullest extent permitted by law so that they will be free from undue concern about personal liability in connection with their service to Pfizer. Our By-laws require indemnification, and we have also entered into agreements with those individuals that contractually obligate us to provide this indemnification to them.
| | | | | | | | |
30 | | Pfizer 2024 Proxy Statement |
| | | | | | | | | | | |
| | | |
| | |
| Non-Employee Director Compensation | |
| | | |
| | | |
| Non-Employee Director Compensation Our non-employee Directors receive cash compensation, as well as equity compensation in the form of Pfizer stock units, for their service. In 2023, we provided the following compensation: | |
| Compensation Element | Direct Compensation Program | |
| Board Member Annual Cash and Equity Retainer | $155,000, payable quarterly in cash, and grant of $205,000 in stock units | |
| Chair of Each Board Committee (Additional Cash Fee) | $30,000 | |
| Lead Independent Director (Additional Cash Fee) | $50,000 | |
| Stock Ownership Guidelines | Required to own Pfizer common stock and/or deferred stock units with a value of at least five times their annual cash retainer ($775,000). New directors are subject to milestones toward this requirement.(1) | |
| Cash Compensation | Directors can defer all or a portion of their annual cash retainers until they cease to be members of the Board. At a Director’s election, the cash retainer fees can be invested in an account credited with Pfizer stock units or deemed invested in the same investments available to Pfizer employees under certain deferred compensation plans.(2) | |
| Equity Compensation | Directors who have met the stock ownership requirements as of December 31 of the prior year are permitted each year to elect to defer units granted in the following year or to receive the equivalent in shares.(3) | |
| The Pfizer Foundation Matching Gift Program* | The Pfizer Foundation matches eligible contributions up to a maximum of $20,000 per Director, per calendar year. | |
| | | |
| (1)Currently all Directors comply with our stock ownership guidelines. (2)The number of Pfizer stock units is based on the closing price of Pfizer’s common stock on the last business day of the fiscal quarter in which the retainer is earned. The number of stock units in a Director’s account is increased by additional stock units based on the value of any dividends on the common stock. Upon distribution, the amount attributable to stock units held in his or her account is paid in cash or in shares of Pfizer stock, at the Director’s election. The amount of any cash payments is determined by multiplying the number of Pfizer stock units in the account by the closing price of our common stock on the last business day before the payment date. (3)All of the eligible non-employee Directors will defer their Pfizer stock units granted in 2024. The number of units in a Director’s account is increased by additional stock units based on the value of any dividends on the common stock. Deferred stock units are not payable until the Director ceases to be a member of the Board, at or after which time they are paid in cash or in shares of Pfizer stock, at the Director’s election. The amount of any cash payment is determined by multiplying the number of Pfizer stock units in the account by the closing price of our common stock on the last business day before the payment date. * The Pfizer Foundation is a charitable organization established by Pfizer Inc. It is a separate legal entity from Pfizer Inc. with distinct legal restrictions. | |
| | | |
| Our Governance & Sustainability Committee is responsible for reviewing and advising on the compensation of our non-employee Directors. To assist with this duty, they engage an independent compensation consultant to perform regular periodic reviews of our non-employee Director compensation program, which includes an analysis of market trends and best practices and peer comparison with our Pharmaceutical Peer and General Industry Comparator Groups. The compensation program for our non-employee Directors was last reviewed in April 2023 by the Governance & Sustainability Committee, in consultation with Meridian Compensation Partners, LLC, and they determined that the program remains competitive amongst Pfizer’s peers and continues to attract and retain highly engaged and qualified independent Directors; accordingly, no changes were recommended. In addition to the above, under our Director compensation program, any newly elected Director receives a pro-rata grant of Pfizer stock units based upon the ratio of the Director’s period of service as a Director during the 12-month period beginning as of the most recent Annual Meeting prior to election multiplied by $205,000, as of the date of grant. In 2024, upon election at the 2024 Annual Meeting, each non-employee Director will receive Pfizer stock units in accordance with the Director compensation program (which currently provides for a grant value of $205,000 as of the date of grant), provided the Director continues to serve as a Director following the meeting. | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 31 |
| | |
| Non-Employee Director Compensation |
Under the Pfizer Inc. 2019 Stock Plan, the aggregate value of Pfizer stock units granted, plus cash retainer paid to a non-employee Director during a 12-month period, may not exceed $800,000. The limit on non-employee Director compensation will remain unchanged in the Amended and Restated Pfizer Inc. 2019 Stock Plan, which is the subject of the proposal in “Item 3 — Approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan,” later in this Proxy Statement. Dr. Bourla does not receive any compensation for his service as a Director. For additional information regarding Dr. Bourla’s compensation, see the “Compensation Discussion and Analysis” section later in this Proxy Statement. THE PFIZER FOUNDATION MATCHING GIFT PROGRAM
Our non-employee Directors may participate in the Pfizer Foundation Matching Gift Program. In 2023, under this program, the Pfizer Foundation matched contributions to eligible Internal Revenue Code 501(c)(3) tax-exempt organizations, up to a maximum of $20,000 per year, per Director. Contributions to religious organizations, private foundations and organizations that do not accept donations from the Pfizer Foundation, as well as to individuals, are not eligible for a match.
2023 Director Compensation Table
The following table sets forth the compensation provided for our non-employee Directors who served in 2023.
| | | | | | | | | | | | | | |
| Name | Fees Earned or Paid in Cash ($) | Stock Awards ($)(1) | All Other Compensation ($)(2) | Total ($) |
| Ronald E. Blaylock | 155,000 | | 205,000 | | 20,000 | | 380,000 | |
| Susan Desmond-Hellmann, M.D., M.P.H. | 155,000 | | 205,000 | | — | | 360,000 | |
| Joseph J. Echevarria | 185,000 | | 205,000 | | — | | 390,000 | |
Scott Gottlieb, M.D. | 185,000 | | 205,000 | | 27,201 | | 417,201 | |
| Helen H. Hobbs, M.D. | 185,000 | | 205,000 | | 20,000 | | 410,000 | |
| Susan Hockfield, Ph.D. | 155,000 | | 205,000 | | 3,000 | | 363,000 | |
| Dan R. Littman, M.D., Ph.D. | 155,000 | | 205,000 | | 31,400 | | 391,400 | |
| Shantanu Narayen | 205,000 | | 205,000 | | 20,000 | | 430,000 | |
| Suzanne Nora Johnson | 185,000 | | 205,000 | | 20,000 | | 410,000 | |
| James Quincey | 155,000 | | 205,000 | | 20,000 | | 380,000 | |
| James C. Smith | 185,000 | | 205,000 | | — | | 390,000 | |
(1)The number of units granted was determined by dividing the grant date value of the award, $205,000, by $38.74, the closing price of the company’s common stock on April 27, 2023. At the end of 2023, the aggregate number of stock units (including dividend equivalents) held by each current non-employee Director was as follows: Mr. Blaylock, 49,726, Dr. Desmond-Hellman, 20,241, Mr. Echevarria, 101,845, Dr. Gottlieb, 26,354, Dr. Hobbs, 103,313, Dr. Hockfield, 22,713, Dr. Littman, 41,524, Mr. Narayen, 130,538, Ms. Nora Johnson, 92,181, Mr. Quincey, 38,377, and Mr. Smith, 116,971.
(2)The amounts in this column for Drs. Hobbs, Hockfield and Littman, Messrs. Blaylock, Narayen and Quincey, and Ms. Nora Johnson represent charitable contributions made in 2023 under our Pfizer Foundation Matching Gift Program. Certain charitable contributions by our Directors are not eligible for matching contributions under the program and, therefore, the amounts in the above table may not reflect all such contributions made by our Directors. The amounts for Drs. Hobbs and Littman and Ms. Nora Johnson also include matching contributions for charitable contributions made in December 2022. The amount for Dr. Gottlieb represents $24,670 for security provided on the advice of internal and external third-party security experts due to heightened risks, which is the direct cost we incurred in providing this benefit, and $2,531 for personal expenses related to business travel to our Board meetings.
| | | | | | | | |
32 | | Pfizer 2024 Proxy Statement |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
| | |
| Securities Ownership | |
| | | | | | | |
| | | | | | | |
| The table below shows the number of shares of our common stock beneficially owned (as of the close of business on January 31, 2024) by each of our Directors and each NEO, as well as the number of shares beneficially owned by all of our current Directors and executive officers as a group. Together, these individuals beneficially own less than one percent (1%) of our common stock outstanding. The table and footnotes also include information about Total Shareholder Return Units (TSRUs), Profit Units (PTUs), stock units, Restricted Stock Units (RSUs) and deferred performance-related share awards credited to the accounts of our Directors and executive officers under various compensation and benefit plans. For additional information, see the “Non-Employee Director Compensation” section earlier, and the “Compensation Discussion and Analysis” section later in this Proxy Statement. | |
| | | | | | | |
| | Number of Shares or Units | |
| Beneficial Owners | Common Stock | (1) | Stock Units | | | |
| Ronald E. Blaylock | 13,000 | | (2) | 49,726 | | (4) | | |
| Albert Bourla, DVM, Ph.D. | 316,779 | | (3) | 847,554 | | (5) | | |
| David M. Denton | 16,445 | | | — | | | | |
| Susan Desmond-Hellmann, M.D., M.P.H. | 3,408 | | (2) | 20,241 | | (4) | | |
| Mikael Dolsten, M.D., Ph.D. | 174,395 | | (3) | 314,400 | | (5) | | |
| Joseph J. Echevarria | — | | | 101,845 | | (4) | | |
| Scott Gottlieb, M.D. | 9,000 | | | 26,354 | | (4) | | |
| Helen H. Hobbs, M.D. | — | | | 103,313 | | (4) | | |
| Susan Hockfield, Ph.D. | — | | | 22,713 | | (4) | | |
| Angela Hwang* | 68,764 | | (2)(3) | 76,723 | | (5) | | |
| Douglas M. Lankler | 136,997 | | (3) | 95,027 | | (5) | | |
| Dan R. Littman, M.D., Ph.D. | — | | | 41,524 | | (4) | | |
| Aamir Malik | 374 | | — | | | | |
| Shantanu Narayen | — | | | 130,538 | | (4) | | |
| Suzanne Nora Johnson | 10,000 | | | 92,181 | | (4) | | |
| William Pao, M.D., Ph.D.** | — | | | — | | | | |
| James Quincey | — | | | 38,377 | | (4) | | |
| James C. Smith | 3,542 | | (2) | 116,971 | | (4) | | |
| All Directors and Executive Officers as a Group (23) | 1,080,244 | | | 2,153,476 | | | | |
| | | | | | | |
| * Effective December 15, 2023, Ms. Hwang ceased serving as an executive officer. ** Effective July 27, 2023, Dr. Pao ceased serving as an executive officer. (1)Individuals beneficially own less than one percent (1%) of our common stock outstanding. (2)Includes the following shares held in the names of family members or trust: Mr. Blaylock, 4,750; Dr. Desmond-Hellmann, 3,408; Ms. Hwang, 8,532; and Mr. Smith, 1,542 shares. Mr. Blaylock, Ms. Hwang and Mr. Smith disclaim beneficial ownership of such shares. (3)Includes shares credited under the Pfizer Savings Plan and/or deferred shares relating to previously vested awards under Pfizer’s share award programs. (5)Includes stock units (each equivalent to a share of Pfizer common stock) to be settled in cash following the officer’s separation from service, held under the Pfizer Supplemental Savings Plan (PSSP) and/or the Pfizer Deferred Compensation Plan (DCP). The PSSP and the DCP are described later in this Proxy Statement. Also includes the following PTUs (each equivalent to a share of Pfizer common stock) as of January 31, 2024: Dr. Dolsten, 195,574 PTUs. This column does not include the following stock appreciation rights in the form of TSRUs as of January 31, 2024: Dr. Bourla, 4,612,352 of which 507,669 settled in February 2024; Mr. Denton, 344,256; Dr. Dolsten, 1,304,933; Ms. Hwang, 1,298,330 of which 144,397 settled in February 2024; Mr. Lankler, 1,166,405 of which 190,754 settled in February 2024; Mr. Malik, 352,113; and Dr. Pao, 23,717. See “Compensation Tables—2023 Outstanding Equity Awards at Fiscal Year-End Table” and “—Estimated Payments and Benefits upon Termination Table” for a discussion of the vesting of RSUs, TSRUs and PTUs. | | | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 33 |
Beneficial Owners
The following table shows persons or entities known by us to be a beneficial owner of more than 5% of our common stock:
| | | | | | | | |
| Name and Address of Beneficial Owner | Shares of Pfizer Common Stock | Percent of Class |
| | |
The Vanguard Group(1) 100 Vanguard Boulevard Malvern, PA 19355 | 506,479,807(1) | 8.97 | % |
| | |
| | |
BlackRock, Inc.(2) 50 Hudson Yards New York, NY 10001 | 434,748,255(2) | 7.7 | % |
| | |
| | |
State Street Corporation(3) State Street Financial Center One Congress Street, Suite 1 Boston, MA 02114 | 287,875,814(3) | 5.10 | % |
| | |
(1)The information is based solely on a Schedule 13G/A filed by Vanguard on February 13, 2024 (the Vanguard 13G/A). According to the Vanguard 13G/A, includes sole voting power with respect to 0 shares, shared voting power with respect to 7,098,817 shares, sole dispositive power with respect to 481,625,108 shares, and shared dispositive power with respect to 24,854,699 shares.
(2)The information is based solely on a Schedule 13G/A filed by BlackRock on January 26, 2024 (the BlackRock 13G/A). According to the BlackRock 13G/A, includes sole voting power with respect to 392,763,728 shares, shared voting power with respect to 0 shares, sole dispositive power with respect to 434,748,255 shares, and shared dispositive power with respect to 0 shares.
(3)The information is based solely on a Schedule 13G/A filed by State Street on January 29, 2024 (the State Street 13G/A). According to the State Street 13G/A, includes sole voting power with respect to 0 shares, shared voting power with respect to 145,103,185 shares, sole dispositive power with respect to 0 shares, and shared dispositive power with respect to 287,482,006 shares.
Delinquent Section 16(a) Reports
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our Directors and certain of our officers to file reports of holdings and transactions in Pfizer equity with the SEC and the NYSE. Based on our records and other information, we believe that in 2023 our Directors and our officers who were subject to Section 16(a) met all applicable filing requirements, except as follows: due to an inadvertent administrative error by the company in February 2023, a transaction for Jennifer Damico, Senior Vice President and Controller, for the withholding of 360 shares to satisfy tax obligations upon the vesting of restricted stock units was reported late.
| | | | | | | | |
34 | | Pfizer 2024 Proxy Statement |
| | | | | | | | | | | |
| | | |
| | |
| Item 2 Ratification of Selection of Independent Registered Public Accounting Firm | |
| | | |
| | | |
| The Audit Committee is directly responsible for the appointment, compensation, retention and oversight of our independent registered public accounting firm (the firm). The Committee conducts a comprehensive annual evaluation of the firm’s qualifications, performance and independence. It considers whether the firm should be rotated and considers the advisability and potential impact of selecting a different firm. In evaluating and selecting the company’s firm, the Committee considers, among other things, historical and recent performance of the current firm, an analysis of known significant legal or regulatory proceedings related to the firm, external data on audit quality and performance, including recent Public Company Accounting Oversight Board (PCAOB) reports, industry experience, audit fee revenues, firm capabilities and audit approach, and the independence and tenure of the firm. The Committee also annually evaluates the firm’s commitment to diversity and inclusion, as well as how its values align with Pfizer’s values — courage, excellence, equity, and joy. The Audit Committee selected, and the Board of Directors ratified the selection of, KPMG LLP (KPMG) as our firm for 2024. We have not been able to determine the specific year that KPMG or its predecessor firms began serving as our auditor; however, we are aware that KPMG or its predecessor firms have served as our auditor since at least 1942. In accordance with SEC rules and KPMG policies, audit partners are subject to rotation requirements to limit the number of consecutive years an individual partner may provide audit services to our company. For lead and concurring review partners, the maximum number of consecutive years of service in that capacity is five years. The process for selection of the lead audit partner under this rotation policy involves a meeting between the Chair of the Audit Committee and the candidate for the role, as well as discussion by the full Committee and with management. The Audit Committee and the Board of Directors determined that the continued retention of KPMG as our firm is in the best interest of Pfizer and our shareholders, and we are asking our shareholders to ratify the selection of KPMG as our firm for 2024. Although ratification is not required by our By-laws or otherwise, the Board is submitting the selection of KPMG to our shareholders for ratification because we value our shareholders’ views on our firm and as a matter of good corporate practice. In the event that our shareholders fail to ratify the selection, it will be considered a recommendation to the Board and the Audit Committee to consider the selection of a different firm. Even if the selection is ratified, the Audit Committee may in its discretion select a different firm at any time during the year if it determines that such a change would be in the best interests of Pfizer and our shareholders. Representatives of KPMG will attend the Annual Meeting to answer questions and will have the opportunity to make a statement if they desire to do so. | |
| | | |
| | Your Board of Directors recommends a vote “FOR” the ratification of KPMG LLP as independent registered public accounting firm for 2024. | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 35 |
| | |
| Item 2 — Ratification of Selection of Independent Registered Public Accounting Firm |
Audit and Non-Audit Fees
The following reflects KPMG fees for the audit of our financial statements for the years ended December 31, 2023 and 2022, and fees billed for other services rendered by KPMG during those periods.
| | | | | | | | |
| 2023
($) | 2022
($) |
Audit fees(1) | 30,582,000 | | 25,234,000 | |
Audit-related fees(2) | 1,234,000 | | 1,349,000 | |
Tax fees(3) | 3,745,000 | | 1,737,000 | |
All other fees(4) | — | | — | |
| Total | 35,561,000 | | 28,320,000 | |
(1)Principally for audit work performed on the consolidated financial statements and internal control over financial reporting, as well as statutory audits. The increase in audit fees in 2023 was primarily due to fees for strategic initiatives.
(2)Principally related to audits of employee benefit plans.
(3)Principally for services related to tax compliance and reporting and analysis services.
(4)KPMG did not provide any “other services” during the period.
POLICY ON AUDIT COMMITTEE PRE-APPROVAL OF AUDIT AND PERMISSIBLE NON-AUDIT SERVICES
Consistent with requirements of the SEC and the PCAOB regarding auditor independence, the Audit Committee has responsibility for appointing, setting the compensation of and overseeing the performance of the firm. In recognition of this responsibility, the Committee has established a policy to pre-approve all audit and permissible non-audit services provided by the firm.
Prior to engagement of the firm for the next year’s audit, management submits for Audit Committee approval a list of services and related fees expected to be rendered during that year within each of the following categories of services:
| | | | | |
| Services | Description |
| |
| Audit services | These services include audit work performed on the financial statements (including financial statements prepared in connection with strategic transactions) and internal control over financial reporting, as well as work that generally only the independent registered public accounting firm can reasonably be expected to provide, including comfort letters, statutory audits, and discussions surrounding the proper application of financial accounting and/or reporting standards. |
| |
| |
| Audit-related services | These services are for assurance and related services that are traditionally performed by the independent registered public accounting firm, including due diligence related to mergers and acquisitions, employee benefit plan audits, and special procedures required to meet certain regulatory requirements. |
| |
| |
| Tax services | These include all services, except those services specifically related to the audit of the financial statements that are included in the first category, performed by the independent registered public accounting firm’s tax personnel, including tax analysis; assisting with coordination of execution of tax-related activities, primarily in the area of corporate development; supporting other tax-related regulatory requirements; and tax compliance and reporting. |
| |
| |
| All other services | These are services not captured in the audit, audit-related or tax categories. Pfizer generally does not request such services from the firm. |
| |
Prior to engagement, the Audit Committee pre-approves services within each category, and the fees for each category are budgeted. The Committee requires the firm and management to report actual versus budgeted fees periodically by category of service. During the year, circumstances may arise when it may become necessary to engage the firm for additional services not contemplated in the original pre-approval. In those instances, the Committee requires specific pre-approval before engaging the firm.
The Committee may delegate pre-approval authority to one or more of its members who must report, for informational purposes only, any pre-approval decisions to the Committee at its next scheduled meeting.
| | | | | | | | |
36 | | Pfizer 2024 Proxy Statement |
| | | | | | | | | | | |
| | | |
| | |
| Audit Committee Report | |
| | | |
| | | |
| The Audit Committee reviews Pfizer’s financial reporting process on behalf of the Board of Directors. Management has the primary responsibility for the financial statements and the reporting process, including the system of internal controls. The Committee met and held discussions with management and the independent registered public accounting firm (the firm) regarding the fair and complete presentation of Pfizer’s results and the assessment of Pfizer’s internal control over financial reporting. We discussed significant accounting policies applied in Pfizer’s financial statements, as well as, when applicable, alternative accounting treatments, and critical audit matters addressed during the audit. Management represented to the Committee that the consolidated financial statements were prepared in accordance with accounting principles generally accepted in the United States of America, and the Committee reviewed and discussed the consolidated financial statements with management and the firm. The Committee discussed with the firm matters required to be discussed under applicable Public Company Accounting Oversight Board (PCAOB) and U.S. Securities and Exchange Commission standards. In addition, the Committee reviewed and discussed with the firm its independence from Pfizer and its management. As part of that review, we received the written disclosures and the letter required by applicable requirements of the PCAOB regarding the firm’s communications with the Audit Committee concerning independence, and the Committee discussed the firm’s independence from Pfizer. We also considered whether the firm’s provision of non-audit services to Pfizer is compatible with the auditor’s independence. The Committee concluded that the firm is independent from Pfizer and its management. As part of our responsibilities for oversight of Pfizer’s Enterprise Risk Management program, we reviewed and discussed company practices with respect to risk assessment and risk management, including discussions of individual risk areas, as well as an annual summary of the overall program. The Committee discussed with Pfizer’s Internal Audit Department and the firm the overall scope of and plans for their respective audits. The Committee meets with the Chief Internal Auditor, Chief Compliance, Quality and Risk Officer and representatives of the firm, in regular and executive sessions, to discuss the results of their examinations, the evaluations of Pfizer’s internal controls, and the overall quality of Pfizer’s financial reporting and compliance programs. In reliance on the reviews and discussions referred to above, the Committee has recommended to the Board of Directors, and the Board has approved, that the audited financial statements be included in Pfizer’s Annual Report on Form 10-K for the year ended December 31, 2023, for filing with the U.S. Securities and Exchange Commission. The Committee has selected, and the Board of Directors has ratified, the selection of the firm for 2024. THE AUDIT COMMITTEE Suzanne Nora Johnson, Chair Ronald E. Blaylock Joseph J. Echevarria James C. Smith The Audit Committee Report does not constitute soliciting material, and shall not be deemed to be filed or incorporated by reference into any Company filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that the Company specifically incorporates the Audit Committee Report by reference therein. | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 37 |
| | | | | | | | |
| | |
| | |
| Item 3 Approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan | |
| | |
| | |
The Pfizer Inc. 2019 Stock Plan (the 2019 Plan) was originally approved at the 2019 Annual Meeting of Shareholders. On February 22, 2024, on the recommendation of the Compensation Committee (the Committee), the Board of Directors (the Board) approved the Amended and Restated Pfizer Inc. 2019 Stock Plan (the Amended and Restated 2019 Plan), subject to shareholder approval at the 2024 Annual Meeting of Shareholders. If the Amended and Restated 2019 Plan is approved by our shareholders, it will become effective April 25, 2024 (the Effective Date).
The company is requesting that our shareholders approve the Amended and Restated 2019 Plan to increase the number of shares of common stock reserved for issuance thereunder by 320,000,000 shares. The number of shares originally authorized for issuance under the 2019 Plan was 400,000,000. As of January 31, 2024, 247,268,253 shares remained available for grant under the 2019 Plan, which is the only Pfizer plan under which equity-based compensation may currently be awarded to executives, other employees and non-employee Directors. After the Effective Date of the Amended and Restated 2019 Plan, awards will continue to be made pursuant to the Amended and Restated 2019 Plan and no awards will be granted under any other equity plans of the company. In addition to the change to increase the number of shares of common stock reserved for issuance, the Amended and Restated 2019 Plan includes certain minor amendments to the 2019 Plan as described below, which do not require shareholder approval, and reflect developments in equity and compensation practice:
•An expanded definition of “Cause” in the context of termination of employment, that includes (i) willful misconduct or gross negligence which materially and demonstrably results in financial harm to the company; (ii) willful breach of duty in the course of service or employment; (iii) misappropriation of funds or other property of the company or any Subsidiary; or (iv) conduct which is a violation of company policy or which materially interferes with the employee’s ability to perform his or her duties.
•Express reference to the Pfizer Inc. Recoupment Policy, which was adopted in compliance with the Dodd-Frank Wall Street Reform and Consumer Protection Act (Dodd-Frank Act) and listing requirements.
In the opinion of the Committee and the Board, an increase in the number of shares available for grant is necessary as a part of our continuing objective to attract, retain and motivate employees and to align the interests of our employees with those of our shareholders.
The 320,000,000 shares of common stock newly reserved for issuance under the Amended and Restated 2019 Plan, plus the shares remaining available for grant as of the Effective Date (and any shares that are added back to the share reserve in the event of expiration, forfeiture or cancellation in accordance with plan terms), are expected to provide us with sufficient shares to cover the awards to be granted over the next four (4) to five (5) years. However, the actual duration of the share reserve will depend on currently unknown factors such as changes in participation, future grant practices, competitive market practices, acquisitions and divestitures, forfeiture rates, and the company’s future stock price. As discussed in further detail below, in determining the share reserve, the Committee and the Board took into account, among other things, our stock price and volatility, share usage, burn rate and dilution, the existing terms of our outstanding awards, and our fungible share counting ratio of 3:1 for full-value share awards under the Amended and Restated 2019 Plan.
We are also seeking approval of the Amended and Restated 2019 Plan in order to: (i) comply with New York Stock Exchange (NYSE) rules requiring stockholder approval of equity compensation plans; and (ii) allow the Committee to grant incentive stock options to employee participants in the Amended and Restated 2019 Plan.
Overview
Equity-Based Compensation — Key Component of Compensation Program
Equity-based compensation is a key component of our total compensation package. As a worldwide biopharmaceutical company, attracting, retaining and motivating specialized talent is critical to achieving our strategic and operating goals, including our goal to increase shareholder value.
| | | | | | | | |
38 | | Pfizer 2024 Proxy Statement |
| | |
Item 3 — Approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan |
Highlights of the Amended and Restated 2019 Plan and Best Practices
The Plan and our other related governance practices and policies contain provisions that are consistent with the interests of our shareholders and with our corporate governance practices. In addition, the company maintains a no hedging or pledging policy.
| | | | | | | | | | | | | | |
The Amended and Restated 2019 Plan DOES... | | The Amended and Restated 2019 Plan DOES NOT... |
| Provide for a minimum one-year vesting period subject to certain limited exceptions | | | Provide for automatic single-trigger vesting on a change of control (except where an acquirer does not assume outstanding awards) |
| Subject the payment of dividends and dividend equivalents on an award to the vesting of the award | | | Permit repricing or the buyout of underwater stock options or Stock Appreciation Rights (SARs) without shareholder approval |
| Limit the number of shares and the cash amounts that may be granted or paid to any non-employee Director in a year | | | Permit the grant of stock options, Total Shareholder Return Units (TSRUs) or SARs with below-market grant prices |
| Provide for the recycling of shares back to the plan pool only in the event of expiration, forfeiture or cancellation of awards (i.e., no “liberal share recycling”) | | | Provide for excise tax gross-ups |
| Provide for the forfeiture/clawback of incentive awards under certain circumstances | | | Contain any “evergreen” provisions that automatically add shares to the plan reserve |
| | | | Provide for the grant of reload stock options |
Amended and Restated Share Reserve Information
The following table presents information regarding outstanding equity awards and the shares available for future awards under the company’s equity plans as of December 31, 2023. Additional share information presented as of January 31, 2024 is provided in footnotes to this table below.
| | | | | |
Number of Stock Options Outstanding | 28,452,100 |
| ‒‒ Weighted-Average Exercise Price of Outstanding Stock Options | $32.66 |
‒‒ Weighted-Average Remaining Contractual Term of Outstanding Stock Options | 1.7 years |
Number of TSRUs Outstanding | 163,572,245 |
| ‒‒ Weighted-Average Exercise Price of Outstanding TSRUs | $36.83 |
‒‒ Weighted-Average Remaining Contractual Term of Outstanding TSRUs | 2.0 years |
Number of Full Value Awards Outstanding (Counting Performance Awards at Maximum Payout) | 67,434,384 |
A. Number of Shares Available for Future Grant*/**/*** | 247,830,527 |
| B. Additional Share Request Under Proposal | 320,000,000 | |
Shares Remaining Available After Shareholder Approval (A+B) | 567,830,527 |
* The 2019 Plan is the only active equity plan under which shares may be granted. Awards of stock options, TSRUs, and SARs reduce the available pool by one share each; all other awards reduce the available pool by three shares each (calculated assuming maximum payout of performance awards).
** Includes 67,734,387 shares assumed from the remaining shares available from the stock plan of Seagen which may be issued solely to legacy employees of Seagen and newly hired employees after the date of acquisition.
*** As of January 31, 2024, the shares available for future grants are 247,268,253 (net of 562,274 from December 31, 2023 for the following transactions: (i) less December 2023 ad hoc grants recorded in January 2024 and first quarter 2024 dividend equivalent units of 1,871,937, plus (ii) cancelled/forfeited stock options, Restricted Stock Units (RSUs), TSRUs, performance share awards (PSAs) and portfolio performance shares (PPSs) of 1,309,663). Note: This amount will be reduced by approximately 140,720,000 shares that will be subject to annual long-term incentive awards granted in February 2024.
Usage of Shares Authorized for Grant
Overhang
As of December 31, 2023, we had approximately 507,300,000 shares of our common stock subject to outstanding equity awards and available for future grant under the 2019 Plan, which represented approximately 8.24% of diluted common shares outstanding (or “overhang percentage”). The 320,000,000 new shares proposed to be included in the Amended and Restated 2019 Plan share reserve would increase the overhang percentage by an additional 4.54 percentage points to approximately 12.78%.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 39 |
| | |
Item 3 — Approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan |
Share Usage and Burn Rate
| | | | | | | | | | | | | | | | | |
| | Fiscal Year
2023 | Fiscal Year
2022 | Fiscal Year
2021 | Average |
A | Stock Options Granted | 635,210 | | 429,271 | | 778,697 | | 614,393 | |
B | TSRUs/PTSRUs* Granted | 26,631,130 | | 22,478,807 | | 34,522,249 | | 27,877,395 | |
C | Restricted Stock Units (RSUs) Granted | 10,006,946 | | 9,616,699 | | 10,893,311 | | 10,172,319 | |
D | Performance Awards Granted (at Maximum) | 10,373,452 | | 8,594,761 | | 11,594,968 | | 10,187,727 | |
E | Total Share-Based Awards Granted (A+B+C+D) | 47,646,738 | | 41,119,538 | | 57,789,225 | | 48,851,834 | |
F | Basic Weighted-Average Common Shares Outstanding | 5,643,066,544 | | 5,608,003,154 | | 5,601,421,475 | | 5,617,497,058 | |
G | Annual Burn Rate (E / F) | 0.84% | 0.73% | 1.03% | 0.87% |
H | Burn Rate (A+B+(3x(C+D)))/ F | 1.57% | 1.38% | 1.83% | 1.59% |
* Performance Total Shareholder Return Units
Dilution
| | | | | | | | | | | | | | | | | |
| | Fiscal Year
2023 | Fiscal Year
2022 | Fiscal Year
2021 | Average |
I | Total Share-Based Awards Outstanding at Year-End | 259,458,729 | | 285,118,493 | | 322,423,224 | | 289,000,149 | |
J | Shares Available for Future Grant at Year-End | 247,830,527 | | 269,789,591 | | 315,077,752 | | 277,565,957 | |
K | Common Stock Outstanding at Year-End | 5,646,748,750 | | 5,616,102,732 | | 5,619,230,577 | | 5,627,360,686 | |
L | Dilution (I+J)/(I+J+K) | 8.24% | 8.99% | 10.19% | 9.15% |
Reasons for Seeking Shareholder Approval
We use equity compensation as a key tool for the attraction, retention and motivation of the best available talent. If shareholder approval is not obtained, we will continue to operate under the terms of the 2019 Plan. Based on similar usage and depending on stock price, we are seeking shareholder approval approximately one year before we expect the current share reserve under the 2019 Plan to be depleted.
Additional Information About the Plan
The following is a summary of the principal features of the Amended and Restated 2019 Plan. This summary is not a complete description of all of the provisions of the Amended and Restated 2019 Plan and is qualified in its entirety by reference to the Amended and Restated 2019 Plan, which is attached to this Proxy Statement as “Annex 1”. Purpose
The general purpose of the Amended and Restated 2019 Plan is to allow Pfizer to continue to utilize equity awards to attract, retain and motivate employees and to further align the interests of our employees with those of Pfizer’s shareholders. Non-qualified stock options, incentive stock options, TSRUs, SARs, RSUs, PSAs, PPSs, Breakthrough Performance Awards (BPAs) and other stock unit awards may be granted under the Amended and Restated 2019 Plan.
Administration and Plan Term
The selection of employee participants in the Amended and Restated 2019 Plan and the level of participation of each employee participant will be determined by the Committee. The Governance & Sustainability Committee will make such determinations as to any grants to non-employee Directors. The Committee may delegate any or all of its authority to administer the Amended and Restated 2019 Plan as it deems appropriate, except that no delegation may be made to an employee of Pfizer in the case of awards made to individuals who are subject to Section 16 of the Securities Exchange Act of 1934, as amended.
The Amended and Restated 2019 Plan will terminate on the tenth anniversary of the Effective Date, unless terminated earlier by the Board or the Committee.
| | | | | | | | |
40 | | Pfizer 2024 Proxy Statement |
| | |
Item 3 — Approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan |
Shares Subject to the Amended and Restated 2019 Plan; Share Counting
Subject to adjustments for changes in capitalization, a total of 320,000,000 shares will be authorized for grant pursuant to awards under the Amended and Restated 2019 Plan, plus the number of shares that remain available for grant as of the Effective Date under the 2019 Plan, and the number of shares that are subject to outstanding awards granted under the 2019 Plan as of the Effective Date that terminate, expire, or are forfeited, cancelled, or settled for cash. The Amended and Restated 2019 Plan provides that awards other than stock options and SARs/TSRUs will be counted against the Amended and Restated 2019 Plan maximum in a 3-to-1 ratio. For example, if we grant an award of 100 RSUs and 100 PSAs, we would reduce the shares available for grant under the Amended and Restated 2019 Plan by 300 shares and 600 shares, respectively.
Any shares that terminate, expire, or are forfeited, cancelled or settled in cash, may be used for the future grant of awards to the extent of such termination, expiration, forfeiture, cancellation or settlement. Any shares that again become available for future grants shall be added back as one (1) share for options or TSRUs or SARs, and as three (3) shares for awards other than options, TSRUs or SARs, including in each case with respect to awards granted under the 2019 Plan. Shares subject to awards under the Amended and Restated 2019 Plan or an award granted under the 2019 Plan that is outstanding on the Effective Date may not again be made available for issuance or delivery if such shares are (i) shares that were subject to a stock-settled TSRU/SAR and were not issued upon the net settlement or net exercise of such TSRU/SAR, (ii) shares delivered or withheld by the company to pay the exercise price of an option, (iii) shares delivered to or withheld by the company to pay the withholding taxes related to an award, (iv) shares withheld by the company in connection with the net settlement of an award, or (v) shares repurchased on the open market with the proceeds of an option exercise.
The shares to be delivered under the Amended and Restated 2019 Plan will be made available from authorized but unissued shares of Pfizer common stock, from treasury shares and/or from shares purchased in the open market or otherwise.
Limitations on Awards under the Amended and Restated 2019 Plan
No more than 320,000,000 shares may be granted as incentive stock options under the Amended and Restated 2019 Plan.
As noted above in the section entitled “Non-Employee Director Compensation,” pursuant to the Amended and Restated 2019 Plan, the dollar value of equity awards and/or the cash retainer that may be granted to any one non-employee Director under the Amended and Restated 2019 Plan or otherwise is limited to an aggregate value (at grant) of $800,000 in any calendar year. The limitations are not intended to reflect an intention to grant awards at such levels.
Eligibility
All employees of the company and its affiliates, as well as the company’s non-employee Directors, are eligible to participate in the Amended and Restated 2019 Plan. From time to time, the Committee will determine who will be granted awards, and the number of shares subject to such grants. As of January 31, 2024, approximately 37,642 employees (including 37,630 employees, 12 executive officers) and 11 non-employee Directors were eligible under the current criteria to receive awards under the Amended and Restated 2019 Plan.
Minimum Vesting Period
The Amended and Restated 2019 Plan prescribes a minimum vesting period of at least twelve months for an award, provided that the Committee may grant awards without regard to the foregoing minimum requirement with respect to a maximum of five (5) percent of the shares of Common Stock reserved for issuance under the Amended and Restated 2019 Plan. Any substitute awards, cash-denominated awards and awards to non-employee Directors that vest on the earlier of the first anniversary of the date of grant or the next annual meeting of shareholders which is at least 50 weeks after the immediately preceding annual meeting are excluded from such minimum vesting period.
No Dividends or Dividend Equivalents on Unvested Awards
Notwithstanding any provision of the Amended and Restated 2019 Plan to the contrary, dividends, dividend equivalents and dividend equivalent units will only be paid if, and to the extent, the underlying award vests, regardless of whether vesting is contingent upon the achievement of performance goals or time.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 41 |
| | |
Item 3 — Approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan |
Prohibition on Repricing
The Amended and Restated 2019 Plan does not permit the repricing of options, TSRUs or SARs, or the exchange of underwater options, TSRUs or SARs for cash or stock/units, and options, TSRUs and SARs may not be granted at a discount to the fair market value of our common stock on the grant date (i.e., with an exercise price lower than the fair market value) without shareholder approval. The limited circumstance of the assumption or substitution of awards in a transaction that involves the adjustment of awards in order to preserve aggregate value would not be considered a repricing for this purpose.
Transferability
Unless otherwise determined by the Committee, awards granted under the Amended and Restated 2019 Plan may not be transferred except by will or the laws of descent and distribution and, during his or her lifetime, any options or awards may be exercised only by the participant, unless otherwise permitted by the Amended and Restated 2019 Plan. The Amended and Restated 2019 Plan explicitly prohibits the transfer of awards to third parties for consideration.
Certain Adjustments
In the event of any change in the number or kind of outstanding shares of common stock of the company by reason of a recapitalization, merger, consolidation, reorganization, separation, liquidation, stock split, stock dividend, extraordinary cash dividend, combination of shares or any other change in the corporate structure or shares of stock of the company, an appropriate adjustment will be made consistent with applicable provisions of the Code and Treasury Department rulings and regulations, and as the Committee, in its sole and absolute discretion deems equitable or appropriate, including:
•In the number and kind of shares for which any options or awards may thereafter be granted, both in the aggregate and as to each optionee or award holder;
•In the number and kind of shares or other property, including cash, subject to outstanding options and awards;
•In the option or exercise price, if applicable; and
•Other adjustments as the Committee deems appropriate.
Change in Control
Unless the Committee or the Board determines otherwise at the time of grant, in the event a participant’s employment is involuntarily terminated without cause during the 24-month period following a change in control:
•Any unvested options and SARs will vest and remain exercisable for their full term in accordance with the terms of the grant, as applicable;
•Any unvested TSRUs will continue to vest and will be settled in accordance with the terms of the grant, as applicable;
•Any vested options, TSRUs, and SARs will remain exercisable for their full term or be settled in accordance with the terms of the grant, as applicable;
•In general, performance awards will continue to vest and become payable in accordance with the terms of the grant, as applicable;
•The restrictions on any restricted stock awards will lapse, and will become fully vested and transferable to the full extent of the original grant, as applicable; and
•RSUs, other stock unit awards, and any other awards will continue to vest and become payable in accordance with the terms of the grant, as applicable.
Additionally, the Committee or the Board may provide for awards to be cancelled in exchange for a cash payment in connection with a change in control. However, if the option price per share under any outstanding option is equal to or greater than the price per share in the change in control, or the value (change in stock price plus projected dividend equivalents) of any outstanding TSRU or SAR is negative, the Board may cancel such award without the payment of any consideration.
Recoupment Policy
Awards under the Amended and Restated 2019 Plan are subject to the company’s policies on recoupment of gains realized from any awards as may be in effect from time to time. All awards granted under the Amended and Restated 2019 Plan will be subject to recoupment in accordance with the Pfizer Inc. Recoupment Policy which was adopted in compliance with NYSE listing standards and the Dodd-Frank Act.
| | | | | | | | |
42 | | Pfizer 2024 Proxy Statement |
| | |
Item 3 — Approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan |
Amendment and Revocation
The Board may amend or revoke the Amended and Restated 2019 Plan, but may not, without prior approval of our shareholders:
•Increase the maximum number of shares of common stock that may be issued under the Amended and Restated 2019 Plan;
•Extend the term of the Amended and Restated 2019 Plan or of options granted under the 2019 Plan;
•Change the eligibility criteria;
•Reprice any option, TSRU or SAR except as provided for in the Amended and Restated 2019 Plan; or
•Take any other action that requires shareholder approval to comply with any tax or regulatory requirement.
Additionally, the Board may not take any action with respect to an affected participant without such participant’s consent if the action would materially impair the participant’s rights under any outstanding award.
Types of Awards
Stock Options
Options granted under the Amended and Restated 2019 Plan may be either non-qualified stock options or incentive stock options qualifying under Section 422 of the Code. The option price may not be less than the fair market value of the stock on the date the option is granted. On January 31, 2024, the closing price of our shares traded on the NYSE, as published in the Wall Street Journal, was $27.08 per share.
The option price is payable in cash or, if the grant provides, in common stock. Generally, all options terminate after a 10-year period from the date of the grant. The Amended and Restated 2019 Plan also provides for the automatic exercise of options that are due to expire in the event that the option price is less than the fair market value of the underlying shares.
The Committee determines the terms of each stock option grant at the time of the grant.
Total Shareholder Return Units / Stock Appreciation Rights
A TSRU or SAR represents a right to receive the excess of (i) the fair market value of one share of common stock on the date of the settlement pursuant to the terms of the grant plus dividends, if applicable, over (ii) the grant price of the right on the grant date, as specified by the Committee. TSRUs and SARs may, but need not, be granted in tandem with options. The Committee determines the terms of each TSRU/SAR at the time of the grant. Any freestanding TSRU/SAR may not be granted with a grant price that is less than the fair market value of the stock on the date the TSRU/SAR is granted and cannot have a term longer than 10 years. Distributions to the recipient may be made in common stock, in cash or in a combination of both as determined by the Committee.
Restricted Stock Awards
Restricted stock is stock issued with such contingencies or restrictions as the Committee may impose. Until the conditions or contingencies are satisfied or lapse, the stock is subject to forfeiture. Unless the Committee determines otherwise, a recipient of a restricted stock award has the same voting, dividend and other rights as holders of common stock, except that the Amended and Restated 2019 Plan prohibits the payment of dividends on unearned/unvested awards. If the participant ceases to be an employee before the end of the contingency or restricted period, the award is forfeited, subject to such exceptions as authorized by the Committee.
Restricted Stock Units
A restricted stock unit or an RSU is an award of a right to receive, in cash or shares, as the Committee may determine, the fair market value of one share of Pfizer common stock, on such terms and conditions as the Committee may determine.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 43 |
| | |
Item 3 — Approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan |
Performance-Based Awards
The Amended and Restated 2019 Plan has been designed to permit the Committee to grant performance-based awards that are earned subject to the achievement of set performance goals, including PSAs, PPSs, performance cash awards and other awards/units. A performance award may be in any form of award permitted under the Amended and Restated 2019 Plan. The Committee may select periods during which performance criteria chosen by the Committee are measured for the purpose of determining the extent to which a performance award has been earned. The Committee decides whether the performance levels have been achieved, what amount of the award will be paid and the form of payment, which may be cash, stock or other property or any combination. The Amended and Restated 2019 Plan has also been designed to permit the grant of performance-based awards that are denominated in cash.
Performance goals may be based on the achievement of specified levels of company performance (or performance of an applicable unit or division of the company) under one or more of the measures described in the Amended and Restated 2019 Plan, relative to the performance of other corporations or comparable businesses, or may provide for the inclusion or exclusion of specified extraordinary, nonrecurring charges.
U.S. Tax Treatment of Options and Awards
The following is a summary of certain United States federal income tax consequences with respect to certain awards that may be granted pursuant to the Amended and Restated 2019 Plan. The following discussion is a brief summary only, and reference is made to the Internal Revenue Code of 1986, as amended and the regulations and interpretations issued thereunder for a complete statement of all relevant federal tax consequences. This summary is not intended to be exhaustive and does not describe state, local or foreign tax consequences of participation in the Amended and Restated 2019 Plan.
Incentive Stock Options
An incentive stock option results in neither taxable income to the optionee, nor a deduction to the Company at the time it is granted or exercised. If the optionee holds the stock received as a result of an exercise of an incentive stock option for at least two years from the date of the grant and one year from the date of exercise, then the gain realized on disposition of the stock is treated as a long-term capital gain. If the shares are disposed of during this period, however (i.e., a “disqualifying disposition”), then the optionee will include the income, as ordinary compensation for the year of the disposition, in an amount equal to the excess, if any, of the fair market value of the shares, upon exercise of the option over the option price (or, if less, the excess of the amount realized upon disposition over the option price). The excess, if any, of the sale price over the fair market value on the date of exercise will be a short-term capital gain. In such case, the company will be entitled to a deduction, in the year of such a disposition, for the amount includible in the optionee’s income as compensation, subject to Section 162(m) of the Code. The optionee’s tax basis in the shares acquired upon exercise of an incentive stock option is equal to the option price paid, plus any amount includible in his or her income as a result of a disqualifying disposition.
Non-Qualified Stock Options
A non-qualified stock option results in no taxable income to the optionee or deduction to the company at the time it is granted. An optionee exercising a non-qualified stock option will, at that time, realize taxable compensation in the amount of the excess of the then market value of the shares over the option price. Subject to the applicable provisions of the Code, including Section 162(m), a deduction for federal income tax purposes will be allowable to the company in the year of exercise in an amount equal to the taxable compensation realized by the optionee. The optionee’s tax basis in shares received upon exercise is equal to the sum of the option price plus the amount includible in his or her income as compensation upon exercise.
Any gain (or loss) upon subsequent disposition of the shares will be a long- or short-term gain (or loss), depending upon the holding period of the shares.
If a non-qualified stock option is exercised by tendering previously owned shares of the company’s common stock in payment of the option price, then, instead of the treatment described above, the following will apply: a number of new shares equal to the number of previously owned shares tendered will be considered to have been received in a tax-free exchange; the optionee’s basis and holding period for such number of new shares will be equal to the basis and holding period of the previously owned shares exchanged. The optionee will have compensation income equal to the fair market value on the date of exercise of the number of new shares received in excess of such number of exchanged shares; the optionee’s basis in such excess shares will be equal to the amount of such compensation income; and the holding period in such shares will begin on the date of exercise.
| | | | | | | | |
44 | | Pfizer 2024 Proxy Statement |
| | |
Item 3 — Approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan |
Total Shareholder Return Units / Stock Appreciation Rights
Generally, the recipient of a stand-alone TSRU/SAR will not recognize taxable income at the time the stand-alone TSRU/SAR is granted. If an employee receives the appreciation inherent in the TSRU/SAR (change in stock price plus dividends from grant date to settlement date) in cash, the cash will be taxed as ordinary income to the employee at the time it is received. If an employee receives the appreciation inherent in the TSRU/SAR in stock, the value is converted into stock which is taxable as ordinary income at the fair market value of the stock.
In general, there will be no federal income tax deduction allowed to the company upon the grant or termination of TSRU/SARs. However, upon the settlement of a TSRU/SAR, the company will be entitled to a deduction equal to the amount of ordinary income the recipient is required to recognize as a result of the settlement, subject to Section 162(m) of the Code.
Restricted Stock Awards / Performance Stock Awards
No income will be recognized at the time of grant by the recipient of a restricted stock award or performance stock award while such award is subject to a substantial risk of forfeiture. Generally, at the time the substantial risk of forfeiture terminates with respect to a stock award, the then fair market value of the stock awarded will constitute ordinary income to the employee. Subject to the applicable provisions of Section 162(m), a deduction for federal income tax purposes will be allowable to the company in an amount equal to the compensation realized by the employee.
Other Awards
In the case of an award of RSUs, performance awards, dividend equivalents or dividend equivalent units or other stock or cash awards, the recipient will generally recognize ordinary income in an amount equal to any cash received and the fair market value of any shares received on the date of payment or delivery. In that taxable year, the company will receive a federal income tax deduction in an amount equal to the ordinary income which the recipient has recognized, subject to Section 162(m) of the Code.
Tax Treatment of Awards to Non-Employee Directors and to Employees Outside the U.S.
The grant and settlement of awards under the Amended and Restated 2019 Plan with respect to non-employee Directors and employees outside of the U.S., as well as the exercise of options or stock appreciation rights by employees outside of the U.S., may be taxed on a different basis.
New Plan Benefits
No awards made under the 2019 Plan prior to the date of the 2024 Annual Meeting were granted subject to shareholder approval of this Item 3. Future awards to be made under the Amended and Restated 2019 Plan are subject to the discretion of the Committee, or in the case of awards to non-employee Directors, the Governance & Sustainability Committee, and accordingly, are not currently determinable. Additionally, the 2024 awards granted to executive officers and all other employees would not have been increased if they had been made under the proposed Amended and Restated 2019 Plan, rather than under the 2019 Plan.
The “Summary Compensation Table” and the “2023 Grants of Plan-Based Awards Table” appearing elsewhere in this Proxy Statement show the awards that were made under the 2019 Plan in 2023 to our NEOs. As discussed herein under “Non-Employee Director Compensation,” each person who is serving as a non-employee Director of the company following the 2024 Annual Meeting will be granted an award of Pfizer stock units with a grant date dollar value equal to $205,000, which would result in the grant of stock units with an aggregate grant date dollar value of approximately $2,255,000 if all non-employee Director nominees receive an award following the 2024 Annual Meeting. | | | | | | | | |
2024 Proxy Statement Pfizer | | 45 |
| | |
Item 3 — Approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan |
Additional Plan Information
The outstanding aggregate number of shares subject to stock options and other equity awards under the 2019 Plan since its inception through January 31, 2024, is set forth in the table below. The closing price of a share of Pfizer common stock on January 31, 2024 was $27.08.
| | | | | | | | | | | | | | |
| Name | Number of Options/TSRUs Granted (#)(1) | Average Per Share Exercise Price ($) | Number of Shares Subject to Other Stock Awards or Unit Awards (#)(2) | Market Value of Shares Subject to Stock or Unit Awards ($)(3) |
| Albert Bourla, DVM, Ph.D. | 3,546,053 | | 37.55 | | 620,226 | | 16,795,720 | |
| David M. Denton | 344,256 | | 47.01 | | 128,199 | | 3,471,637 | |
| Mikael Dolsten, M.D., Ph.D. | 1,304,933 | | 36.91 | | 220,941 | | 5,983,082 | |
| Douglas M. Lankler | 809,769 | | 36.60 | | 128,883 | | 3,490,152 | |
| Aamir Malik | 352,113 | | 43.95 | | 142,757 | | 3,865,864 | |
| Angela Hwang | 999,509 | | 36.80 | | 165,706 | | 4,487,318 | |
William Pao, M.D., Ph.D. | 23,717 | | 42.30 | | 6,467 | | 175,134 | |
All current executive officers as a group (excluding those listed above) | 8,124,152 | | 37.99 | | 1,717,086 | | 46,498,695 | |
| All current directors who are not executive officers as a group | 0 | | 0 | | 271,622 | | 7,355,524 | |
| All nominees for election as directors as a group | 0 | | 0 | | 0 | | 0 | |
| Each associate of any such directors, executive officers, or nominees | 0 | | 0 | | 0 | | 0 | |
| Each other person who received or is to receive 5% of such options, warrants, or rights | 0 | | 0 | | 0 | | 0 | |
| All employees, including all current officers who are not executive officers, as a group | 120,230,902 | | 36.52 | | 41,827,125 | | 1,132,678,555 | |
(1)Represents 132,451,326 TSRUs and 3,284,078 stock options.
(2)Other stock awards were granted in the form of RSUs, PTUs, PPSs and PSAs. Performance-based awards are reflected assuming “target” performance. Please see the “Compensation Discussion and Analysis” section of this Proxy Statement for additional details on the performance-based awards. (3)Amounts calculated based on $27.08, the closing price of the company’s common stock on January 31, 2024.
| | | | | |
| Your Board of Directors recommends a vote “FOR” the approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan. |
| | | | | | | | |
46 | | Pfizer 2024 Proxy Statement |
| | | | | | | | | | | |
| | | |
| | |
| Item 4 2024 Advisory Approval of Executive Compensation | |
| | | |
| | | |
| Our executive compensation program aligns interests of participants, including key executives, with the long-term interests of our shareholders; attracts, retains and motivates participants, including key executives, to drive our business and financial performance; and links a significant portion of the individual’s executive compensation to the achievement of pre-established performance metrics directly tied to our business goals and strategies. The Compensation Committee believes that Pfizer’s pay-for-performance executive compensation program is consistent with the goals of its executive compensation philosophy to drive performance and increase shareholder value. This philosophy is intended to align each executive’s compensation with Pfizer’s short- and long-term performance and to provide the compensation and incentives needed to attract, motivate and retain key executives crucial to our long-term success. In accordance with this philosophy, our executive compensation program delivers a significant portion of the total compensation opportunity for each of our executives (including the Named Executive Officers, or NEOs) as long-term compensation directly tied to Pfizer’s total shareholder return and other performance factors that measure our progress against our strategic goals and operating plans, as well as individual performance. Additionally, in setting target levels of compensation and the value and level of award opportunities, the Compensation Committee considers the median compensation values of our Pharmaceutical Peer and General Industry Comparator Groups. 2023 Advisory Vote on Executive Compensation Our executive compensation program received significant shareholder support and was approved, on an advisory basis, by 92.8% of the votes cast at the 2023 Annual Meeting. Our Compensation Committee and the other members of our Board believe that this level of approval of our executive compensation program indicates our shareholders’ strong support of our compensation philosophy and goals. The consistent high level of support from our shareholders over the past several years is indicative of our Committee’s commitment to compensating our executives in a manner that effectively links pay and performance. We believe it is also reflective of market best practices, strong shareholder engagement and continuously striving to enhance our programs by ensuring they align with our evolving strategic priorities, market trends and reflect feedback received from our shareholders. 2023 Pay-for-Performance 2023 was a year of change for Pfizer following a peak year of financial performance in 2022. Despite these challenges, we continued to execute on our strategic priorities and took steps to help ensure Pfizer is well positioned for future growth. Under the leadership of Dr. Bourla and the executive team, we achieved a record number of U.S. FDA approvals for nine NMEs, including a vaccine for RSV, and launched several new indications for our existing in-line brands that further enhanced our leadership position in oncology, vaccines and inflammation and immunology. With our acquisition of Seagen, we took a critical step toward our goal to achieve world-class oncology leadership. In 2023, we also took steps to transform the company for potential future growth by launching a cost realignment program and making changes to our commercial organization. In addition, we continued to focus on our ESG goals. Consistent with the company’s pay for performance philosophy, based on the Compensation Committee’s review of the company’s financial performance against the pre-established targets and their view that the ELT members were most able to impact financial performance, the Committee determined that no bonuses would be paid to the CEO and executive officers, including the NEOs, for the 2023 performance year, despite positive performance against pipeline and ESG goals. Given the unique challenges Pfizer faced in 2023 and in consideration of individual contributions, the Committee believes that the compensation of our NEOs for 2023 is reasonable and appropriate, aligned with the performance of our company and designed to ensure that our executive’s interests align with shareholders’ interest. Please see the “Compensation Discussion and Analysis” section for additional information. In deciding how to cast your vote on this proposal, the Board requests that you consider the structure of our executive compensation program in connection with our 2023 performance, which is more fully discussed in the Compensation Discussion and Analysis section. The Compensation Discussion and Analysis section also contains more details about how we implement our philosophy and goals, and how we apply these principles to our compensation program. In particular, we discuss how we set compensation targets and other objectives and evaluate performance against those targets and objectives to ensure that performance is appropriately rewarded. Please see the “Compensation Discussion and Analysis” section for additional information. | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 47 |
| | |
Item 4 — 2024 Advisory Approval of Executive Compensation |
2024 Advisory Vote on Executive Compensation
The Board is presenting this proposal, which gives shareholders the opportunity to endorse or not endorse our executive pay program, on an advisory basis, by voting “FOR” or “AGAINST” (or abstaining from voting on) the following resolution:
“RESOLVED, that the shareholders of Pfizer Inc. approve, on an advisory basis, the compensation of the company’s Named Executive Officers, as disclosed pursuant to the compensation disclosure rules of the U.S. Securities and Exchange Commission, including the Compensation Discussion and Analysis, the compensation tables and any related material disclosed in this Proxy Statement.”
Although the advisory vote is non-binding, the Board values shareholders’ opinions and our Compensation Committee will review the results of the vote and will consider shareholders’ concerns and take into account the outcome of the vote when considering future decisions concerning our executive compensation program.
| | | | | |
| Your Board of Directors recommends a vote ”FOR” the approval, on an advisory basis, of the compensation of the Company’s named executive officers. |
| | | | | | | | |
48 | | Pfizer 2024 Proxy Statement |
| | | | | | | | | | | |
| | | |
| | |
| Compensation Committee Report | |
| | | |
| | | |
| The Compensation Committee has reviewed and discussed with management the following Compensation Discussion and Analysis section of Pfizer’s 2024 Proxy Statement. Based on our review and discussions, we have recommended to the Board of Directors that the Compensation Discussion and Analysis be included in Pfizer’s 2024 Proxy Statement. THE COMPENSATION COMMITTEE James C. Smith, Chair Ronald E. Blaylock James Quincey | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 49 |
| | | | | | | | | | | |
| | | |
| | |
| Executive Compensation | |
| | | |
| | | |
| Compensation Discussion and Analysis This Compensation Discussion and Analysis (CD&A) describes Pfizer’s executive compensation program for 2023 and certain elements of our 2024 program. It explains how the Compensation Committee of the Board (the Committee) made 2023 performance year compensation decisions for our executives, including the following Named Executive Officers (NEOs): Albert Bourla, DVM, Ph.D. — Chairman and Chief Executive Officer (CEO) David M. Denton — Chief Financial Officer (CFO), Executive Vice President (EVP) Mikael Dolsten, M.D., Ph.D. — Chief Scientific Officer, President, Pfizer Research & Development Douglas M. Lankler — General Counsel, Executive Vice President (EVP) Aamir Malik — Chief U.S. Commercial Officer, Executive Vice President (EVP)(1) Angela Hwang — Former Chief Commercial Officer (CCO) and President, Global Biopharmaceuticals Business (GBB)(2) William Pao, M.D., Ph.D. — Former Chief Development Officer (CDO), Executive Vice President (EVP)(3) (1)Chief Business Innovation Officer, Executive Vice President, prior to December 15, 2023. (2)Effective December 15, 2023, Ms. Hwang ceased serving as an executive officer and is currently serving as Advisor to the Chief Executive Officer. (3)Effective July 27, 2023, Dr. Pao ceased serving as CDO and as an executive officer and terminated employment on August 15, 2023. Table of Contents | |
|
| | |
| | | |
| | | |
|
| | |
| | | |
| | | |
|
| | |
| | | |
| | | |
|
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
|
| | |
| | | |
| | | |
|
| | |
| | | |
| | | | | | | | |
50 | | Pfizer 2024 Proxy Statement |
Executive Summary
PFIZER’S EXECUTIVE COMPENSATION: PAY-FOR-PERFORMANCE PHILOSOPHY
Our executive compensation program is designed to attract and retain highly qualified executives and incentivize them to create value and advance the interests of our shareholders. Pfizer’s executive compensation program is consistent with the goals of its executive compensation philosophy to align pay and performance and increase shareholder value. (See “Our Business and Strategy” earlier in this Proxy Statement.) This philosophy is evidenced by the Committee’s determination that, based on the company’s performance against the annual pre-set financial goals for the Global Performance Plan (GPP) Program, the Executive Leadership Team (ELT) members (including the NEOs) would not receive payouts from the short-term annual incentive plan for the 2023 performance year. (See “Annual Incentives Objectives and Results (For Annual Incentive Purposes)” later in this Proxy Statement.) | | | | | | | | | | | |
| | | |
| | OUR PHILOSOPHY •Aligns each executive’s compensation with Pfizer’s short- and long-term performance and provides the compensation and incentives needed to attract, motivate and retain key executives crucial to Pfizer’s long-term success; •Delivers a significant portion of the total compensation opportunity for each of our executives (including the NEOs) as long-term incentives that are directly aligned with shareholders’ interests and tied to Pfizer’s absolute and relative total shareholder return (TSR) and to other performance factors that measure our progress against the goals of our strategic and operating plans; and •Benchmarks compensation against that of our Pharmaceutical Peer and General Industry Comparator Groups with consideration of company market capitalization and complexity — as indicated by revenues, range of products, international operations and other factors — to set target levels of compensation and determine the value and level of award opportunities. | |
| | | |
2023 NEO PAY MIX
Pfizer’s executive compensation program is designed to strengthen the link between pay and performance by having a significant amount of the executives’ compensation tied to the achievement of pre-established performance metrics directly related to our business goals and strategies. Using year-end salary and target short- and long-term incentive awards, our pay mix is as follows:
| | | | | | | | | | | | | | |
CEO – 2023 Target Total Direct Compensation | Other Active NEOs – 2023 Target Total Direct Compensation (Average) |
| | | | |
| n | Year-End Salary |
| | |
| n | Annual Short-Term Incentive (Target) |
| | |
| n | Annual Long-Term Incentive (Target) |
| | |
Additionally, our stock ownership guidelines promote the alignment of interests with shareholders by requiring the CEO to own Pfizer stock with a value equal to at least eight times his base salary and for each other NEO to own Pfizer stock with a value equal to at least four times their respective base salary. These guidelines include progressive steps to reach these ownership levels within five years (see “Stock Ownership and Holding Requirements” later in this Proxy Statement). | | | | | | | | |
2024 Proxy Statement Pfizer | | 51 |
COMPENSATION PRACTICES
Compensation Risk Assessment
As part of our compensation program, we conduct an annual comprehensive assessment of the potential compensation-related risks to Pfizer of the following compensation programs:
Executive Compensation Program. The Committee’s independent advisor conducts a risk assessment of the executive compensation program at the direction of, and subject to review by, the Committee. It focuses on: (1) ensuring an appropriate balance in our program structure to mitigate compensation-related risk by using an appropriate mix of cash versus stock, short-term versus long-term measurements and financial versus non-financial goals; and (2) best-practice policies to mitigate compensation-related risk including recoupment provisions covering clawbacks and forfeitures, stock ownership guidelines, equity administration rules, and insider-trading and hedging/pledging prohibitions.
Global Compensation Program. An assessment of our global sales-incentive and commission plans is conducted annually by management and reviewed by the Committee and its independent advisor. The assessment takes into consideration the plan metrics, plan participation rates, recovery/clawback provisions and other risk-mitigation factors, as well as the maximum potential payouts.
Based on the results of these assessments, the Committee does not believe that the compensation programs create risks that are reasonably likely to have a material adverse effect on our company.
Leading Compensation Practices
| | | | | | | | | | | | | | |
| What We Do | | What We Do Not Do |
| Risk Mitigation | | | Permit Hedging or Pledging of Pfizer Stock |
| Compensation Recovery/Clawback | | | Provide Employment Agreements |
| Stock Ownership Requirements | | | Provide “Single Trigger” Change in Control Payments or Benefits or Change in Control Agreements |
| Minimum Vesting Period on Long-Term Incentives | | | Provide Repricing of Outstanding Long-Term Incentives |
| 100% Performance-Based Annual Long-Term Incentives | | | Provide “Gross-Ups” For Excise Taxes or Perquisites |
| Multiple Metrics across Short-Term and Long-Term Incentive Programs | | | Provide Cash Severance Exceeding 2.99 times (base salary and target bonus) |
| Short-Term Incentive Plan with ESG Scorecard metrics | | | |
| Robust Investor Outreach | | | |
| Independent Compensation Consultant | | | |
Advisory Vote on Executive Compensation and Shareholder Outreach Program
| | | | | |
We are committed to open and continued communications with our shareholders and have a robust outreach program. Our executive compensation program has received strong shareholder support of, on average, 93.9% of the votes cast over the past ten years. At the 2023 and 2022 Annual Meetings, it received support of 92.8% and 92.7% of the votes cast, respectively. See “Shareholder Outreach” for more information. Our Committee and the other members of our Board view this consistently high level of support as indicative of our commitment to effectively linking pay and performance. The feedback we received during our shareholder outreach, as well as our shareholders’ votes, reflects strong support for our executive compensation program, pay-for-performance compensation philosophy and goals, market best practices and focus on shareholders’ interests. | |
| | | | | | | | |
52 | | Pfizer 2024 Proxy Statement |
2023 Executive Compensation Program Summary
| | | | | | | | | | | | | | | | | | | | |
| Element | Type/Form | Performance Measures | Program Design | | Objectives | |
| | | | | | |
| Salary | Cash | Fixed cash compensation; reviewed annually and adjusted, as appropriate | A fixed amount of compensation for performing day-to-day responsibilities based on market data, job scope, responsibilities and experience. Generally reviewed annually for a potential increase based on a number of factors, including market levels, performance and compensation practices that are equitable within the organization. | | Provides competitive level of fixed compensation that helps attract and retain high-performing executive talent. | |
| | | | | | |
| | | | | | |
Annual Short-Term Incentive/Global Performance Plan (GPP) | Cash | Funded based on Pfizer’s performance and weighted as follows: | Aggregate pool is funded based on the performance against Pfizer’s annual financial goals, the achievement of pre-set pipeline goals and three ESG metrics. Individual awards are based on operating unit/function and individual performance measured over the performance year. | | Provides incentive to executives for achieving short-term results that create sustained future growth potential and long-term shareholder value. | |
| Metrics | | |
| | |
| | | | |
| Total Revenue (40%) | A leading indicator of performance and value creation; provides a clear focus on growth; an important measure in our industry; understandable with a clear line of sight and employee impact. | | |
| | | |
| | | |
Adjusted Diluted EPS (40%) | A measure of income that provides focus on profitable growth and expense control; viewed as a strong indicator of sustained performance over the long term; understandable with a clear line of sight and employee impact. | | |
| | | |
| | | |
Cash Flow from Operations (20%) | A measure that provides focus on generating cash in the short term to fund operations and research and to return funds to shareholders in the form of dividends and share repurchases; focuses managers on expense control and on improving working capital; a strong link to long-term shareholder value creation. | | |
| | | |
| | | |
Modifiers of up to +/- 30 percentage points (PP): Pipeline Achievement (25 PP) and ESG Scorecard (5 PP) | To recognize the progress and delivery of the R&D pipeline and our progress against three ESG metrics from our ESG Scorecard. | | |
| | | | | | |
Annual Long-Term Incentive Compensation (100% Performance-Based Equity) | 5- and 7-Year Total Shareholder Return Units (TSRUs) Represents 25% (each) of total annual grant value (50% in total) | Absolute TSR | 5- and 7-Year TSRUs generally vest three years from the grant date and are settled on the fifth or seventh anniversary of the grant date, respectively. The value earned is equal to the difference between the Settlement Price (the 20-day average of the closing prices of Pfizer common stock ending on the settlement date) and the Grant Price (the closing stock price on the date of grant), plus the value of dividend equivalents accumulated over the term. This value, if any, is converted into shares by dividing it by the settlement price; no value is received if the TSR is negative. | | Provides direct alignment with shareholders as awards are tied to absolute TSR. | |
| | | | | |
| | | | | |
Performance Share Awards (PSAs) Represents 50% of total annual grant value | Adjusted Net
Income (NI)*
and relative TSR | PSAs have a three-year performance period starting on January 1st of the year of grant and generally vest on the third anniversary of the grant with value delivered, if any, based on performance. Paid based on the company’s performance against a combination of three one-year adjusted net income* goals, set annually, and relative TSR, as compared to the NYSE Arca Pharmaceutical Index (DRG Index or DRG), over a three-year period. The maximum payout is 200% of target but is capped at target if the TSR for the performance period is negative. The payout range for the operating metric range is 0%-150%, and the relative TSR metric can drive the overall payout range as high as 200%. Dividend equivalents paid during the performance period are applied to the number of shares actually earned under the award. Earned PSAs, including the dividend equivalents, are paid in cash to active colleagues and in shares to former colleagues. | | Provides alignment with shareholders by aligning compensation to operational goals and relative TSR over a three-year performance period. | |
* Adjusted Net Income, as the PSA performance measure, is defined as U.S. GAAP net income attributable to Pfizer Inc. common shareholders before the impact of amortization of intangible assets, certain acquisition-related items, discontinued operations and certain significant items; and is adjusted to reflect budgeted foreign exchange (FX) rates for the year and further refined to exclude certain other unbudgeted or non-recurring items including acquired in-process research and development expenses.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 53 |
2023 Executive Compensation Program Summary (continued)
| | | | | | | | | | | | | | |
| Element | Plan/Program | Program Design | Objectives | |
| | | | |
| Retirement | Savings Plan | A qualified savings plan providing participants with the opportunity to defer a portion of their eligible pay up to the IRC limitations (on a pre-tax, after-tax or Roth basis) and receive a company matching contribution (i.e., defer 6.0% to receive a 4.5% matching contribution). In addition, since 2018, all participants receive an age- and service-weighted company-provided Retirement Savings Contribution (RSC) (5% to 9% of eligible pay). | Provides retirement benefits through elective deferrals, company matching contributions and RSC, up to Internal Revenue Code (IRC) limits. | |
| Supplemental Savings Plan | A non-qualified savings plan providing participants a pre-tax savings opportunity relating to amounts in excess of the IRC limitations under the same formulas/features (matching contributions and RSC) as the qualified savings plan noted above. | Allows for deferrals, company matching contributions and RSC in excess of IRC limits. | |
| Pension Plan** | Qualified pension plan provides retirement income for eligible participants based on years of service and final average earnings; frozen as of December 31, 2017. | Provides retirement income based on tenure and compensation, up to IRC limits. | |
| | | |
| Supplemental Pension Plan** | Non-qualified pension plan provides retirement income relating to compensation in excess of the IRC limitations under the same formula as the qualified pension plan noted above; frozen as of December 31, 2017. | Provides retirement income based on tenure and compensation in excess of IRC limits. | |
| | | |
| | | | |
| | | | |
| Other | Perquisites | Certain other benefits provided to executives by the company consisting of limited reimbursement for personal financial planning services, home security and additional security services, as deemed necessary, as well as certain personal travel benefits for the CEO and other NEOs (including other Executive Leadership Team (ELT) members). | Provides additional benefits consistent with competitive practices and safety concerns; increases efficiencies and allows more productive use of NEOs’ time, and therefore, greater focus on Pfizer-related activities. | |
** Plans were closed to new participants effective January 1, 2011 and benefits were frozen on December 31, 2017 for all participants.
| | | | | | | | |
54 | | Pfizer 2024 Proxy Statement |
SECTION 1 — Elements of Our Executive Compensation Program
2023 SALARIES
The table below shows the annual salaries for our NEOs set by the Committee, which are based on its review of competitive market practices and individual performance:
| | | | | | | | | | | |
| Salary* | |
| Name | 2022
($) | 2023 ($) | Increase (%) |
| A. Bourla | 1,750,000 | | 1,800,000 | | 2.9 | |
| D. Denton | 1,250,000 | | 1,312,500 | | 5.0 | |
| M. Dolsten | 1,550,000 | | 1,612,000 | | 4.0 | |
| D. Lankler | 1,155,000 | | 1,212,750 | | 5.0 | |
| A. Malik | 1,248,000 | | 1,310,400 | | 5.0 | |
| A. Hwang | 1,292,000 | | 1,356,600 | | 5.0 | |
| W. Pao | 1,200,000 | | 1,260,000 | | 5.0 | |
* Salary is typically approved at the Committee’s February meeting of the respective year and effective April 1 of that year.
2023 ANNUAL INCENTIVE AWARD/GLOBAL PERFORMANCE PLAN (GPP)
The Committee determined the funding of the annual incentive plan based on the company’s performance against three pre-set weighted financial goals tied to Pfizer’s annual operating plan, its achievement of pre-set pipeline goals related to sustained portfolio delivery, progress on the ESG Scorecard and consideration of other qualitative factors. Achievement versus our financial goals is measured using the same key operating assumptions as those in our annual budget.(1)
Determining Annual Incentive Pool
The Committee has determined that its evaluation process (illustrated below) provides the appropriate limited flexibility to determine the final GPP pool funding based upon a holistic review of Pfizer’s overall performance with a strong focus on financial performance against pre-established goals, considering the performance on the pipeline and ESG Scorecard, as well as other qualitative factors. Upon completion of its review, the Committee approved the GPP pool funding.
GPP Pool Funding Process
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| Financial Goals: Committee determined the funding level of the plan using a performance matrix with three financial goals | | | | | | Pipeline and ESG Scorecard Modifiers and Other Qualitative Factors: Committee considered these factors as modifiers to the funding level calculated using the financial goals | | | | | Leaders significantly differentiated pay to be more closely aligned with individual performance and contributions. | |
| | | | | | | | | | | | | | | | | | | |
| 40% Total Revenue
40% Adjusted Diluted EPS
20% Cash Flow from Operations
| | | | | | Up to +/- 30 Percentage Points (PP): | | | | | | | | | | |
| | | | | | | | | | Adjust +/- depending on the Committee’s evaluation of Other Qualitative Factors | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | +/- 25 PP Pipeline Achievements +/- 5 PP ESG Scorecard | | | | | | | | | |
| | | à | | | | | à | | | à | | | |
| | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
| Plan Funding Capped at 200% | | | | | |
| | | | | | |
(1)Includes budgeted FX rates, business development activity (e.g., acquisitions or divestitures), planned increases in the pricing of our medicines, planned capital allocation activities, such as share repurchases and dividend payments (share repurchases in excess of budgeted amounts are removed from the calculation of the financial results for GPP purposes), and/or other operational factors (e.g., losses of exclusivity), as well as certain other qualitative criteria. In general, legal settlements will remain in the adjusted numbers as part of ordinary course of business. However, anomalous settlements, including but not limited to, acquisition-related activities and discontinued operations, are reviewed by the Controller and CFO to determine if they should be treated as a “Certain Significant Item” (CSI). CSI designations are ultimately reviewed by the Compensation Committee and the Board of Directors as part of their review of the financial statements. See “Financial Measures” for a comparison of U.S. GAAP revenues and U.S. GAAP diluted EPS and non-GAAP total revenue and non-GAAP Adjusted Diluted EPS for annual incentive purposes, respectively. | | | | | | | | |
2024 Proxy Statement Pfizer | | 55 |
| | | | | | | | |
| | |
| In determining the funding level, the Committee evaluates the performance measured against the selected financial metrics and the modifiers (Pipeline Achievement and ESG Scorecard), as well as other qualitative factors annually considering the following: •Consistency with best practices in our industry; •Support of the annual operating plan; •Reinforcement of Pfizer’s portfolio strategy, promotion of decisions and behaviors aligned with maximizing near-term business results while supporting the achievement of the company’s long-term goals — while not encouraging unnecessary or excessive risk-taking; and •R&D modifier measuring achievement on key pipeline goals and a modifier for the ESG Scorecard progress, which both have the potential to drive long-term shareholder value. | |
| | |
ANNUAL INCENTIVES OBJECTIVES AND RESULTS (FOR ANNUAL INCENTIVE PURPOSES)
The selected performance measures are linked to a combination of the company’s annual financial goals and strategic goals that help drive long-term value creation. In the first quarter of 2023, the Committee set the target financial goals and, working in collaboration with the Board’s Science and Technology Committee and management’s Sustainability Steering Committee, set the goals for the pipeline achievement matrix and the ESG Scorecard, respectively, for annual incentive purposes. The Committee continued to take a rigorous, holistic approach designed to ensure that the financial goals and modifiers (Pipeline Achievement and ESG Scorecard) are set at levels to drive strong performance and create long-term value. As such, the Committee believes the achievement of the established financial, pipeline and ESG goals requires exceptional performance and execution without encouraging unnecessary or excessive risk-taking. The Committee then determined that a sufficient degree of stretch existed in the various goals (see “Determining Annual Incentive Pool” for additional information). This multi-factor approach for annual incentive bonus funding provides the Committee with the ability to utilize a holistic approach to the process and is the fundamental principle underlying our GPP program.
For 2023, the Committee assessed company performance against our annual targets used for our GPP program. Although the financial performance for our non-COVID products largely met their objectives, the lower-than-expected demand and resulting sales for our COVID-19 products caused the company to underperform against our annual financial targets. While performance against the pipeline and ESG modifiers generated a positive modifier, the Committee, in consultation with its independent compensation consultant, and using a holistic approach, decided that no bonuses would be paid to the ELT, including the NEOs employed at the end of the year.
| | | | | | | | |
56 | | Pfizer 2024 Proxy Statement |
The following tables outline the 2023 range details (as applicable) and for financial metrics, the applicable 2022 Results.
Financial
| | | | | | | | | | | | | | | | | | | | |
| | | | | | |
Financial Goals — Goals are set utilizing a budgeting approach that considers prior year’s performance, expected growth, the impact of business development activities, impact of losses of exclusivity and fluctuations in foreign exchange rates. Given that certain factors can change in any specific period, the Committee believes that in its determination of whether goals are challenging and rigorous, it should consider all relevant factors and not merely a year-over-year comparison. These financial results are different than our results under GAAP. The 2022 results included significant COVID-related reported revenue (over $56 billion), and the 2023 goals appropriately reflected materially lower expectations for COVID product revenue. |
| | | | | | |
Financial Objectives / (Weighting)
(For Annual Incentive Purposes) | 2022 Results
($) | | | 2023 Threshold ($)(1) | 2023 Target ($)(1) | 2023 Results ($)(1) |
Total Revenue(2) (40%) | 104.5 billion | | | 64.3 billion | 68.8 billion | 59.3 billion |
Adjusted Diluted EPS(3) (40%) | 6.80 | | | 3.04 | 3.29 | 1.95 |
Cash Flow from Operations(4) (20%) | 29.3 billion | | | 7.1 billion | 10.6 billion | 9.3 billion |
(1)2023 Threshold, Target, and Results for Annual Incentive Purposes.
(2)Total Revenue for annual incentive purposes is based on budgeted FX rates assumed in each respective year and excludes certain other unbudgeted or non-recurring items. Therefore, 2023 and 2022 results differ from U.S. GAAP revenues of $58.5 billion and $100.3 billion, respectively.
(3)Adjusted Diluted EPS for annual incentive purposes is based on budgeted FX rates assumed in each respective year and excludes certain other unbudgeted or non-recurring items including acquired in-process research and development expenses. See “Non-GAAP Financial Measure: Adjusted Income — Certain Significant Items” in the Management’s Discussion and Analysis of Financial Condition and Results of Operations (MD&A) section in Pfizer’s 2023 Annual Report on Form 10-K for information about significant substantive and/or unusual items that are evaluated on an individual basis.
(4)2023 and 2022 Results exclude certain discretionary timing items for compensation purposes (non-GAAP amounts).
NOTE: See “Financial Measures” for a comparison of 2023 and 2022 U.S. GAAP revenues and U.S. GAAP diluted EPS and non-GAAP total revenue and non-GAAP Adjusted Diluted EPS for annual incentive purposes, respectively. Adjusted Diluted EPS is defined as U.S. GAAP Diluted EPS before the impact of amortization of intangible assets, certain acquisition-related items, discontinued operations and certain significant items. Non-GAAP total revenue and non-GAAP Adjusted Diluted EPS for annual incentive purposes are not, and should not, be viewed as substitutes for U.S. GAAP revenues and U.S. GAAP diluted EPS, respectively. For more information on revenues, see “Our 2023 Performance — Total Revenues” in the MD&A in Pfizer’s 2023 Annual Report on Form 10-K. Non-Financial
| | | | | |
| |
Pipeline Achievement Goals — The pipeline goals consist of four metrics from signs of clinical activity to product approvals measured by projected peak year revenues. These goals align with the company’s end-to-end pipeline development and reinforce Pfizer’s portfolio strategy and culture. At the end of the year, the Science and Technology (S&T) Committee of the Board and Portfolio Management Team (PMT)* review, pressure test and validate the achievements and provide the Committee with a scoring recommendation based on the performance against each pre-set goal. Using the scoring recommendation as a guideline, the Committee then evaluates the pipeline performance holistically to determine the modifier to be applied. |
| |
Pipeline Objectives
(For Annual Incentive Purposes) | Performance Range |
Pipeline Growth (e.g., signs of clinical activity, proof of concept, pivotal study starts and product approvals measured by peak year revenues.) | Up to +25 PP (Above) |
| 0 (zero) PP (Target) |
| Up to -25 PP (Below) |
* Committee composed of members of senior management that governs major pipeline investment and strategic R&D priorities.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 57 |
| | | | | | | | |
| | |
ESG Scorecard — The three ESG metrics selected align with our strategy and are consistent with key guiding principles. Incorporating ESG into our bonus program amplifies our focus on long-term value creation for our shareholders and promotes our commitment to equity and long-term sustainability. These key performance indicators are holistic drivers of our future success as a company. The Committee assesses the individual ESG metrics without assigning a particular weight to each goal. Based on the Committee’s evaluation of each achievement within the ESG Scorecard, the Committee considers the overall performance to determine a combined modifier score. |
| | |
ESG Objectives
(For Annual Incentive Purposes) | 2023 Goals | 2023 Results* |
| Percentage of Vice President and higher roles held by women (globally) | 44.3 | % | 44.8 | % |
| Percentage of Vice President and higher roles held by minorities (U.S.) | 28.3 | % | 30.5 | % |
Greenhouse Gas Emissions (in metric tons CO2e) | ≤1.10M Mt | 1.09M Mt |
* The 2023 Results exclude Seagen data (employees and GHG emissions).
2023 Annual Incentive Awards
The Committee reviewed the company’s 2023 performance and noted that the company did not achieve the financial targets set for 2023 under the terms of the GPP. Although the Committee recognized Dr. Bourla’s and the ELT members’ (including the NEOs) continued leadership and contributions, the Committee determined that, consistent with the GPP terms, the ELT members (including the NEOs) employed at the end of the year would not receive annual incentive awards for the 2023 performance year.
Annual incentive award targets and payout ranges for 2023, as well as the actual annual incentive award for each of the NEOs, are:
| | | | | | | | | | | | | | | | | |
| 2023 Salary(1) ($) | Target Award as a % of Salary(2) | Target Award
($) | Maximum Award(3) ($) | Actual Award
($) |
Name | A | B | C = A x B | D = C x 250% |
| A. Bourla | 1,787,671 | | 200% | 3,575,342 | | 8,938,355 | | 0 | |
D. Denton | 1,297,089 | | 100% | 1,297,089 | | 3,242,723 | | 0 | |
| M. Dolsten | 1,596,712 | | 100% | 1,596,712 | | 3,991,780 | | 0 | |
| D. Lankler | 1,198,510 | | 90% | 1,078,659 | | 2,696,648 | | 0 | |
| A. Malik | 1,295,014 | | 100% | 1,295,014 | | 3,237,535 | | 0 | |
| A. Hwang | 1,340,671 | | 100% | 1,340,671 | | 3,351,678 | | 0 | |
W. Pao(4) | 768,821 | | 90% | 691,939 | | 1,729,848 | | 691,939 | |
(1)Represents 2023 daily salary earned during the performance period the participant is eligible to participate in the bonus program (GPP).
(3)Maximum award is 250% of the respective individual’s target award, subject to rounding.
(4)Dr. Pao was paid a pro-rata target bonus in accordance with the terms of the GPP in effect at the time of his involuntary termination. His target bonus for the full year was $1,120,685.
| | | | | | | | |
58 | | Pfizer 2024 Proxy Statement |
2023 ANNUAL LONG-TERM INCENTIVE AWARD PROGRAM
Pfizer’s annual long-term incentive compensation for our NEOs (and the other ELT members) is entirely in the form of performance-based equity-related awards using two vehicles that incentivize long-term value creation:
| | | | | | | | | | | | | | | | | | | | |
| Type/Weighting | | | 5- and 7-Year Total Shareholder Return Units (TSRUs)
(25% each of value at grant) | | | Performance Share Awards (PSAs)
(50% of value at grant) |
| Program Design (metrics, vesting and objectives) | | | Deliver value based on long-term alignment with shareholders by linking rewards to absolute TSR over a five- or seven-year period. Vests on the third anniversary of grant; settled on fifth or seventh anniversary of grant. | | | Aligns rewards to both a strategic financial performance metric, NI(1), over three one-year periods and relative TSR(2) performance as compared to the DRG Index over a three-year period. Vests on the third anniversary of grant. |
| Value Delivered | | | Difference between the Settlement Price(2) and the Grant Price (both as described in the “Executive Summary” section of this Proxy Statement), plus dividend equivalents accumulated during the term. TSRUs have no value if TSR is negative. | | | Amount earned based on performance (payout range is 0% to 200% of target award value) plus dividend equivalents for the three-year performance period on the shares earned. |
| Formula | | | (# of TSRUs granted × [Settlement Price(2) - Grant Price + Dividend Equivalents]) / Settlement Price(2) | | | Average of the three annual NI(1) Performance Factors percentage adjusted by modifier as follows: +/- 1.5 × the first 20 percentage point differential between Pfizer’s TSR % and DRG Index TSR %(3) and +/- 2.0 × the differential over 20 percentage points(3) |
| | | = Shares delivered(4) | | | = PSA percentage earned and delivered in cash(5) |
(1)Adjusted Net Income, as the PSA performance measure, is defined as U.S. GAAP net income attributable to Pfizer Inc. common shareholders before the impact of amortization of intangible assets, certain acquisition-related items, discontinued operations and certain significant items; and is adjusted to reflect budgeted FX rates for the year and further refined to exclude certain other unbudgeted or non-recurring items including acquired in-process research and development expenses.
(2)The settlement price is the 20-day average of Pfizer’s closing stock prices ending on the settlement date of the TSRUs. For PSAs, the TSR is calculated based on the average of the closing stock prices for the 30 trading days immediately prior to start and end of each three-year performance period.
(3)Positive or negative adjustment.
(4)TSRUs have no value if TSR is negative.
(5)PSA payout is delivered in cash to active colleagues and in shares to former colleagues; payout is capped at target if TSR is negative.
2023 Grant Value of Annual Long-Term Incentive Awards
The 2023 grant value of each NEO’s regular annual long-term incentive award opportunity was set by the Committee based on competitive market data (targeted to approximate the market median), relative duties and responsibilities, the individual’s future advancement potential, the individual’s impact on Pfizer’s results, and for retention purposes.
| | | | | | | | | | | | | | |
| Name | 5-Year TSRUs Value(1) ($) (25%) | 7-Year TSRUs Value(1) ($) (25%) | PSAs Value(1) ($) (50%) | Total Grant Value of Annual LTI Awards(2) ($) |
| A. Bourla | 4,500,000 | | 4,500,000 | | 9,000,000 | | 18,000,000 | |
| D. Denton | 1,125,000 | | 1,125,000 | | 2,250,000 | | 4,500,000 | |
| M. Dolsten | 1,500,000 | | 1,500,000 | | 3,000,000 | | 6,000,000 | |
| D. Lankler | 875,000 | | 875,000 | | 1,750,000 | | 3,500,000 | |
| A. Malik | 1,125,000 | | 1,125,000 | | 2,250,000 | | 4,500,000 | |
| A. Hwang | 1,125,000 | | 1,125,000 | | 2,250,000 | | 4,500,000 | |
W. Pao(3) | 875,000 | | 875,000 | | 1,750,000 | | 3,500,000 | |
(1)Consistent with historical practice, the grant value is converted into TSRUs and PSAs using the value/closing stock price on the first trading day of the week of grant. The actual value of the grant may differ due to the change in the value of the TSRUs/PSAs between the conversion date and the date of grant.
(2)The amounts shown represent the full value of the annual grant, which is different from the 2023 amount reported in the "Summary Compensation Table” which reports the value of TSRUs granted in 2023, and the value of one-third of each of the 2021, 2022 and 2023 PSA grants, in accordance with applicable accounting rules. The Committee considers the full value in its determination of annual compensation. (3)Under the terms of grant, upon termination without cause, Dr. Pao was entitled to retain a portion of the value of TSRUs and PSAs granted, based upon the ratio of days worked from grant until termination of employment over the full vesting period. As such, he retained and currently holds approximately 16% of the TSRUs and PSAs granted to him in February 2023.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 59 |
PERFORMANCE SHARE AWARDS
PSAs align rewards to both a strategic financial performance metric, NI(1), over three one-year periods and relative TSR performance as compared to the DRG Index over a three-year period. The number of PSAs earned at the end of the three-year performance period was determined as set forth below:
2021 PSAs (PERFORMANCE PERIOD 2021-2023)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | NI Goals ($B) | | | | | | | |
Fiscal
Year | Metric(1) | | Threshold
($) | Target
($) | Maximum
($) | Actual
Results | Performance Factor(2)/(3) | | Relative TSR Modifier(2)/(3) | Final 2021 PSA Payout(4) |
| 2021 | NI & TSR | | 17.11 | 18.11 | > | $25.24B | 150.00% | | Pfizer TSR | -15.62% | (A) | 100% |
| 2022 | NI & TSR | | 36.02 | 37.02 | > | $39.12B | 150.00% | | DRG TSR | 42.22% | (B) | -105.7% |
| 2023 | NI & TSR | | 18.06 | 19.06 | > | $11.24B | 0% | | | -57.85% | | |
| | | | | × (Factors) | | |
| 3-Yr. Avg. | 100% (A) | | | -105.7% (B) | Payout % | 0% (C) |
| | | | | |
PSA Formula: Average of the three annual (NI) % Performance Factors + ((1.5 x the first 20 percentage points of differential between Pfizer’s TSR % – DRG Index TSR %) + (2.0 x the differential over 20 percentage points)) |
(A) 100% + (B) (1.5 x -20%) + (2.0 x -37.85%) = (C) 0% Payout(4) |
(1)Adjusted Net Income is defined as U.S. GAAP net income attributable to Pfizer Inc. common shareholders before the impact of amortization of intangible assets, certain acquisition-related items, discontinued operations and certain significant items; and is adjusted to reflect budgeted FX rates for the year and further refined to exclude certain other unbudgeted or non-recurring items including acquired in-process research and development expenses.
(2)The payout range from the operating metric factor is 0% to 150%, with an overall maximum of 200% after the application of the relative TSR modifier. TSR is calculated based on the average of the closing stock prices for the 30 trading days immediately prior to start and end of each three-year performance period.
(3)The 2022 PSAs (performance period 2022-2024) and 2023 PSAs (performance period 2023-2025) have not completed the full three-year performance period. The table above shows the final performance factor for each applicable year which overlaps with portions of the three one-year goals for the 2022 PSAs and 2023 PSAs performance period (for 2022 PSAs – fiscal year 2022 and 2023; and for 2023 PSAs – fiscal year 2023).
(4)Payout range 0% to 200%, subject to rounding.
2021 PSA PAYOUT (PERFORMANCE PERIOD 2021-2023)(1)
| | | | | | | | | | | | | | |
| Name | Award Value At Grant ($)(2) | Target Award At Grant
(#) | Actual Award Earned (#) | Actual Award Value ($) |
A. Bourla | 7,000,000 | | 204,320 | | 0 | | 0 | |
| M. Dolsten | 3,000,000 | | 87,565 | | 0 | | 0 | |
| D. Lankler | 1,750,000 | | 51,080 | | 0 | | 0 | |
| A. Hwang | 2,250,000 | | 65,674 | | 0 | | 0 | |
(1)Messrs. Denton and Malik, and Dr. Pao did not receive a 2021 PSA grant.
(2)The amounts shown represent the PSA portion (50%) of the annual grant value, which is different from the 2021 amount reported in the Summary Compensation Table in this Proxy Statement.
| | | | | | | | |
60 | | Pfizer 2024 Proxy Statement |
Additional Benefits and Perquisites
Additional benefit and perquisite programs are provided to the CEO and the other NEOs (and other ELT members), as briefly described below. Further details are included elsewhere in this Proxy Statement.
•Retirement Programs — Provide retirement income through the: (1) Savings Plan and Supplemental Savings Plans, which are defined contribution plans providing an account consisting of employee elective deferrals, company matching contributions and the RSC plus investment returns and (2) for colleagues employed prior to 2011 and for service through 2017, a pension benefit based on tenure and compensation through the Pension Plan and Supplemental Pension Plan (frozen plans). The benefits provided to our executives and the determination of such benefits are generally the same as those for all other eligible colleagues. (See “Retirement Benefits” later in this Proxy Statement.) •Perquisites — Provide for limited reimbursement for personal financial planning services, certain home security, other security expenses, as deemed necessary and certain personal travel benefits for the CEO and the other NEOs (and other ELT members). The Committee believes that these benefits are appropriate and consistent with market practices. (See “Perquisites” later in this Proxy Statement.) | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
| 2024 COMPENSATION ACTIONS Salary, Target Annual Incentive and Annual Long-Term Incentive Awards At the February 2024 meeting, the Committee approved the following April 2024 salaries, 2024 annual incentive targets and February 2024 long-term incentive awards, for the NEOs continuing to serve as executive officers in 2024: | |
| | | | | | | |
| Name | April 1, 2024 Salary ($) | 2024 Target Annual Incentive(1)(2) (%) | 2024 Target Annual Incentive(2) ($) | 2024 LTI Award Value(3) ($) | Total Direct Compensation ($) | |
| A. Bourla | 1,800,000 | | 200% | 3,600,000 | | 18,000,000 | | 23,400,000 | | |
| D. Denton | 1,358,400 | | 100% | 1,346,988 | | 4,500,000 | | 7,205,388 | | |
| M. Dolsten | 1,668,400 | | 100% | 1,654,377 | | 6,000,000 | | 9,322,777 | | |
| D. Lankler | 1,255,200 | | 90% | 1,120,181 | | 3,500,000 | | 5,875,381 | | |
| A. Malik | 1,356,300 | | 100% | 1,344,888 | | 4,500,000 | | 7,201,188 | | |
| | | | | | | |
| Note: Dr. Pao and Ms. Hwang’s compensation data are not reported in the supplemental table above. Upon his involuntary termination, Dr. Pao ceased serving as an executive officer and CDO on July 27, 2023 and departed from the company following a transition period on August 15, 2023. Ms. Hwang ceased serving as an executive officer on December 15, 2023 and is currently serving as Advisor to the CEO for transition purposes, until a date to be determined. She will not receive a merit increase in 2024 and her salary is therefore unchanged from 2023. Additionally, Ms. Hwang did not receive a 2024 annual long-term incentive award. Upon termination of employment at the conclusion of the transition period, Ms. Hwang will be involuntarily terminated without cause due to her position being eliminated, and at such time, she will be eligible for a prorated annual incentive award for 2024 under the current terms of the GPP. (1)The Committee evaluated the target annual incentive, and in consultation with the Committee’s independent advisor affirmed the target incentive percentages. (2)Target annual incentive is calculated by multiplying the target incentive percentage by the salary earned during 2024 (estimated for purposes of this table). (3)These awards included 50% of the award value granted as 5- and 7-Year TSRUs and the remaining 50% granted as PSAs. The long-term incentive award values are converted into units, subject to rounding, on the day of grant, using the closing stock price/value on February 27, 2024 of $26.89. The 5-Year TSRU values were converted to TSRUs using $6.98 and the 7-Year TSRU values were converted to TSRUs using $7.90, representing the estimated value at grant using the Monte Carlo Simulation model as of February 27, 2024 (grant date). See Equity Award Grant Practices later in this Proxy Statement. | |
| | | | | | | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 61 |
SECTION 2 — How We Determine Executive Compensation
ROLE OF THE COMPENSATION COMMITTEE
The Committee, composed solely of independent Directors, determines the compensation for each of our executives (including the NEOs) and oversees the design and administration of our executive compensation program. Each year, it reviews and performs a comprehensive assessment and analysis of the executive compensation program, including the elements of each NEO’s compensation, with input from the Committee’s independent compensation consultant. As part of the compensation review, a “tally sheet” for each NEO (and the other ELT members) is provided with the details of the NEO’s target and actual total compensation elements, stock ownership, as well as benefits information, accumulated deferred compensation, and outstanding equity award values. The Committee believes that tally sheets are a useful tool in evaluating total compensation in relation to competitive market pay and internal pay equity. The independent Directors review and ratify all decisions by the Committee relating to the compensation of our executives (including the NEOs).
The Committee annually conducts an independence assessment of its advisors including the compensation consultant, consistent with NYSE listing standards and SEC rules governing our Proxy Statement disclosure.
ROLE OF THE INDEPENDENT COMPENSATION CONSULTANT
The Committee continued to utilize Meridian Compensation Partners, LLC (Meridian) as its independent compensation consultant. The independent compensation consultant advises the Committee on executive compensation matters including current trends and the impact to compensation. The Committee assessed the ongoing engagement of Meridian for 2024 in accordance with our policy on criteria and assessment of the firm’s independence and concluded that the engagement of Meridian should continue and does not raise any conflicts of interest or similar concerns.
Fees Paid. The total amount of fees paid to Meridian for services provided to the Compensation Committee in 2023 was $237,240 (including reasonable travel and business expenses, as applicable). In addition, Meridian serves as the independent advisor to the Governance & Sustainability Committee regarding non-employee Director compensation matters, as appropriate, and received $33,530 for services relating to Director compensation for the same time frame.
HOW WE ESTABLISH TARGETS
Target total direct compensation for each executive is intended to approximate the market median (50th percentile of the market), as defined by survey and public data relating to our Pharmaceutical Peers and General Industry Comparator Group. On an annual basis, the Committee reviews the total compensation opportunity of each NEO (as well as the other ELT members). This includes cash compensation (salary and target annual short-term incentive) and target annual long-term incentive compensation, as well as perquisites, retirement benefits and health and insurance benefits. The Committee, with the advice of its independent compensation consultant, then sets each NEO’s compensation target for the current year. This generally involves establishing an annual short-term incentive opportunity and a long-term incentive award.
We recognize that while some information is available on the performance of our non-U.S.-based peer companies, the compensation data can be limited in terms of comparable benchmarks and other information compared to peers with U.S. pay models. The Committee utilizes data on the pharmaceutical industry and complex multi-national companies as they provide useful comparative compensation data for our annual benchmarking analysis and are also a source of potential talent for Pfizer.
| | | | | | | | |
62 | | Pfizer 2024 Proxy Statement |
The following explains the Committee’s approach for determining our executive pay targets.
| | | | | | | | | | | | | | | | | |
Objective | | We target the median compensation values of our peer and comparator groups to help determine an appropriate total compensation level and pay mix for our executives. The groups are selected based on their having comparable scope, complexity, revenue and similar compensation models. We establish a competitive pay framework using our comparator groups’ median compensation values, to help determine the optimum pay mix of base pay, annual short- and long-term incentive targets. The framework is a general guide to determine the preliminary salary recommendation, target annual short-term incentive award opportunity, and target annual long-term incentive value for each executive position. In addition to using the peer data for our annual benchmark analysis and as a source for potential talent, the peer data is used to benchmark: •Plan design (both short-term and long-term) •Performance metrics •Perquisites •Share usage •Stock ownership guidelines Note: The actual total compensation and/or amount of each compensation element for an individual executive may be more or less than this median to reflect individual performance, responsibilities, and internal equity, among other factors. |
| | | | | |
| Peers | | 2023 Pharmaceutical Peers (broad mix of large companies from the pharmaceutical industry) | 2023 General Industry Comparators (broad mix of large, non-pharmaceutical, multi-national companies of similar size and complexity) |
| Pharma* (12 peers) | General Industry** (19 peers) |
| AbbVie Inc. Amgen Inc. AstraZeneca PLC Bristol-Myers Squibb Company Eli Lilly and Company Gilead Sciences, Inc. | GSK plc Johnson & Johnson Merck & Co., Inc. Novartis AG* Roche Holding AG* Sanofi* | 3M Company Abbott Laboratories The Boeing Company Caterpillar Inc. Chevron Corporation The Coca-Cola Company Comcast Corporation ConocoPhillips Exxon Mobil Corporation Honeywell International Inc. International Business Machines Corporation | Lockheed Martin Corporation Mondelez International, Inc. PepsiCo, Inc. The Procter & Gamble Company RTX Corporation (formerly Raytheon Technologies) UnitedHealth Group Incorporated United Parcel Service, Inc. Verizon Communications Inc. |
* The Committee recognizes that while data are available on the performance of certain of our non-U.S.-based peer companies, the compensation data in some cases are limited in terms of comparable benchmarks and may use different pay models as compared to Pfizer’s pay model.
** Effective in 2023, our General Industry Comparators were changed as General Electric Company was removed and Exxon Mobil Corporation and UnitedHealth Group Incorporated were added.
PFIZER COMPARISON TO PEER GROUP MEDIANS
The table below compares our 2023 revenue, net income and market capitalization to the median revenue, net income and market capitalization for our Pharmaceutical Peer and General Industry Comparator Groups.
| | | | | | | | | | | |
| In Billions | Pfizer ($) | Pharmaceutical Peer
Group Median** ($) | General Industry Comparator Group Median ($) |
| Revenue* | 58.5 | 45.0 | | 74.3 |
| Reported Net Income* | 2.1 | 10.0 | | 10.5 |
| Market Capitalization* | 155.3 | 194.2 | 165.9 |
* Revenue and Net Income based on published earnings releases. Market Capitalization as of February 15, 2024.
** Excludes Novartis AG, Roche Holding AG and Sanofi.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 63 |
SECTION 3 — How We Evaluate Performance:
2023 Compensation Decisions
LINKING PAY AND PERFORMANCE
This section highlights the Committee’s process for its key compensation decisions for 2023.
Setting Performance Objectives
The performance objectives for the NEOs reflect the goals that the CEO and the Committee believed our executives should focus on during the period in order to achieve Pfizer’s business goals, including financial, operating and/or strategic plans. The Committee monitors and reviews the progress on the individual performance objectives (measured on two six-month periods (semester basis)) for our NEOs.
Determining 2023 Awards — Paying for Performance
The Committee determined the bonus pool funding based on a holistic review of the company’s overall performance (see “2023 Annual Incentive Award/Global Performance Plan (GPP)”). Consistent with the company’s pay for performance philosophy, based on the Committee’s review of the company’s financial performance against the pre-established targets and their view that the ELT members were most able to impact financial performance, the Committee determined that no bonuses would be awarded to the CEO and executive officers, including the NEOs, for the 2023 performance year, despite positive performance against the pipeline and ESG goals. NEO Performance-Year Total Direct Compensation (Performance-Year TDC) and Summary Compensation Table Total Direct Compensation (SCT TDC)
The Committee uses a Performance-Year TDC approach to determine the competitiveness of our total direct compensation (TDC) versus peers, and to evaluate the alignment of pay and performance. The compensation decisions for the NEOs reflect their contributions to the company’s overall performance and that of their respective role, area and/or function. The table below provides the Performance-Year TDC versus the SCT TDC for the NEOs and is not intended as a substitute for the SCT. The difference between the Performance-Year TDC (Column D) and SCT Total (Column F), as illustrated below, is attributed to the SCT reporting requirements for PSAs, and other amounts included in Column F, as applicable, such as change in pension value, retirement contributions and the cost of security services. Accounting rules require that long-term incentive grants made during the year be included but only after the goals are set; therefore, due to the use of three one-year goals, the SCT TDC (Column E) and SCT Total (Column F) reflect the full value of the TSRU grants made in 2023 and one-third of each outstanding PSA grant related to that goal for the 2021, 2022 and 2023 PSAs. The Performance-Year TDC reflects the full value of both the TSRU and the PSA grants made in February 2024 (the year following the performance year).
Despite Dr. Bourla’s strong individual performance, Pfizer’s 2023 financial performance was below pre-established targets set at the beginning of the year; therefore, consistent with the terms of the GPP, the Compensation Committee with the support of the other independent members of the Board and in consultation with the Committee’s independent advisor, did not award him a bonus for 2023. However, it did award him a 2024 LTI award with a grant value of $18.0 million. The Committee and the Board believe that this grant provides an appropriate performance-based incentive opportunity and recognizes Dr. Bourla for his leadership and focus on Pfizer’s long-term strategy, including developing our product pipeline.
Performance-Year TDC (Column D) for Dr. Bourla is $19.8 million compared to Dr. Bourla’s 2023 SCT Total value (Column F) of $21.6 million, with the most significant differences being the All Other Compensation (AOC) value of $2.3 million, as these amounts are not included in the Performance-Year TDC, offset in part by the difference in the reporting of the LTI awards noted above. The AOC details are included in the SCT footnotes (include, among other things, $0.8 million for security services and $1.3 million in Savings Plan contributions).
Performance-Year LTI Value (Column C) for Dr. Bourla is the February 2024 grant of $18.0 million. The accounting value for the LTI awards reported in the SCT ($17.5 million) includes the February 2023 TSRU grants ($8.8M) and one-third of the 2021, 2022 and 2023 PSA grants. The TSRU grants made in February 2024 will be reflected in the 2024 SCT and the PSA portion of the LTI award made in February 2024 will be reflected in the SCT over three years (2024, 2025 and 2026).
| | | | | | | | |
64 | | Pfizer 2024 Proxy Statement |
| | | | | | | | | | | | | | | | | | | | | | | |
| Performance-Year Compensation(1) | | Summary Compensation Table(2) |
Name | Year-End
Salary (A)
($) | Annual Short-
Term Incentive
Award (paid in 2024) (B)
($) | Annual LTI Award(3) (granted in February 2024) (C) ($) | Total Direct
Compensation
(D = A + B + C)
($) | | Total Direct Compensation(4) (Salary + Bonus + Non-Equity Incentive + equity awards valued on accounting basis) (E) ($) | Total(4) (Total Direct Compensation (E) + Change in Pension Value + All Other Compensation) (F) ($) |
| A. Bourla | 1,800,000 | | 0 | | 18,000,000 | | 19,800,000 | | | 19,294,370 | | 21,562,064 | |
| D. Denton | 1,312,500 | | 0 | | 4,500,000 | | 5,812,500 | | | 4,828,372 | | 5,279,536 | |
| M. Dolsten | 1,612,000 | | 0 | | 6,000,000 | | 7,612,000 | | | 7,632,333 | | 8,858,700 | |
D. Lankler | 1,212,750 | | 0 | | 3,500,000 | | 4,712,750 | | | 4,719,182 | | 5,477,798 | |
| A. Malik | 1,310,400 | | 0 | | 4,500,000 | | 5,810,400 | | | 4,821,475 | | 5,420,734 | |
Note: Dr. Pao and Ms. Hwang’s compensation data are not reported in the supplemental table above. Upon his involuntary termination, Dr. Pao ceased serving as an executive officer and CDO on July 27, 2023 and departed from the company following a transition period on August 15, 2023. Ms. Hwang ceased serving as an executive officer on December 15, 2023 and is currently serving as Advisor to the CEO for transition purposes, until a date to be determined. She will not receive a merit increase in 2024 and her salary is therefore unchanged from 2023. Additionally, Ms. Hwang did not receive a 2024 annual long-term incentive award. Upon termination of employment at the conclusion of the transition period, Ms. Hwang will be involuntarily terminated without cause due to her position being eliminated, and at such time, she will be eligible for a prorated annual incentive award for 2024 under the current terms of the GPP.
(1)The performance-year TDC calculation includes the value of the annual long-term incentive grant, attributable to, and in consideration of the individual’s performance in the performance year (made in the following February), without regard to when the annual LTI performance goals are set for PSAs.
(2)Amounts reflected are the SCT Total amounts and SCT TDC, both of which reflect the grants made during the year for which the applicable performance goals for PSAs have been set under GAAP rules. The accounting rules provide that PSAs are included when the applicable goals are set; therefore, one-third of the PSAs is included in the SCT TDC in each of the three performance years as a result of the use of three, separately established annual goals.
(3)Annual LTI Award (Column C) amounts represent the 2024 annual LTI award value, which includes the value of the TSRUs and the full PSA grant value, and is reflective of LTI awards associated with the NEO’s respective roles. These grant values differ from the accounting values shown in the 2023 SCT.
(4)SCT TDC (Column E) includes salary, bonus, non-equity incentive compensation and equity awards made during the year with their value based on accounting rules (which reflects, as applicable, the TSRUs granted in 2023, and the value of one-third of each of the 2021, 2022 and 2023 PSAs). Ms. Hwang did not receive a 2024 annual long-term incentive award. The SCT “Total” (Column F) is composed of Total Direct Compensation (Column E), plus the change in pension value and All Other Compensation.
Compensation Actions Relating to the NEOs
The Committee assessed each active NEO’s 2023 performance based on the contributions made during the year. The 2023 performance information regarding our NEOs (excluding our former CCO and CDO) is included in the “2023 NEO Performance Summaries” section elsewhere in this Proxy Statement. The Committee did not assess Ms. Hwang and Dr. Pao’s performance as each ceased to serve as an executive officer on December 15, 2023 and July 27, 2023, respectively. Pursuant to the terms of the GPP in effect on the date of his involuntary termination without cause, Dr. Pao received a prorated target bonus for 2023. As discussed earlier in this Proxy Statement, Ms. Hwang did not receive a bonus for the 2023 performance year nor did she receive a February 2024 LTI award. | | | | | | | | |
2024 Proxy Statement Pfizer | | 65 |
LEADERSHIP TRANSITION
The company made several leadership changes as a result of the reorganization decisions made by the company during 2023, including eliminating the following ELT roles:
Angela Hwang (former Chief Commercial Officer (CCO) and President, GBB)
Effective December 15, 2023, Ms. Hwang ceased serving as the CCO and President, GBB and agreed to assist with the reorganization and transition of her duties. She is currently serving as Advisor to the Chief Executive Officer continuing until a date to be determined. As a result of the elimination of Ms. Hwang’s role, which the company determined to be an involuntary termination without cause (due to position elimination and reorganization) under the Executive Severance Plan, she will be entitled to severance payments and benefits under the terms of Pfizer’s applicable plans and policies upon her termination of employment. This will include severance under the Executive Severance Plan in accordance with its terms. Outstanding equity awards will be treated in accordance with the terms of the grants with no enhanced treatment.
William Pao (former Chief Development Officer (CDO), EVP)
Effective July 27, 2023, Dr. Pao ceased serving as Chief Development Officer, EVP. Dr. Pao’s position was eliminated leading to his involuntary termination without cause. He was paid his salary until his separation date (August 15, 2023), and he received the following benefits pursuant to the terms of the Executive Severance Plan, which provides for severance benefits to ELT members upon an involuntary termination without cause, and related severance provisions of other plans and programs:
•A cash severance payment of $2,394,000, equal to one year’s base salary and target bonus, was paid in the seventh month following his termination (March 2024), consistent with the plan terms (see further description under Executive Severance Plan later in this Proxy Statement). Severance benefits were subject to Dr. Pao’s execution and non-revocation of a release of claims in favor of the company and continued compliance with restrictive covenants and his confidentiality obligations.
•A cash payment of $691,939, which represents a prorated target annual incentive award pursuant to the terms of the GPP in effect upon his involuntary termination. This payment was made on September 29, 2023.
•Outstanding equity awards were treated in accordance with the terms of the applicable award agreements.
•Reimbursement for qualifying financial counseling expenses of up to $15,000 in 2023 and $8,750 for 2024 expenses through August 15, 2024.
•Continuation of certain health and insurance coverage for 24 months at active employee rates.
The benefits provided are consistent with the terms of the plans and programs in which Dr. Pao participated and no enhanced treatment was provided in connection with his termination.
Under the terms of Dr. Pao’s offer letter and the applicable Pfizer plans, the unvested portions of his sign-on awards (including a cash sign-on award, time-based restricted stock units, and credit to the Pfizer Supplemental Savings Plan (PSSP)) vested upon his involuntary termination without cause. The sign-on awards were “make-whole awards” to replace the value of his forfeited equity awards from his prior employer. All payments made to Dr. Pao are consistent with the existing contractual entitlements.
| | | | | | | | |
66 | | Pfizer 2024 Proxy Statement |
2023 NEO PERFORMANCE SUMMARIES
The following is a summary of each NEO’s individual accomplishments for 2023.
| | | | | | | | |
| | |
| Albert Bourla, DVM, Ph.D. Chairman and CEO | |
| | |
| | |
| Under Dr. Bourla’s leadership in 2023, Pfizer saw significant achievements that position the company for success in 2024 and beyond, as it continues to deliver on its purpose: Breakthroughs that change patients’ lives. •Treated more than 600 million patients (globally)1 with our medicines and vaccines in 2023. •Ranked the #1 pharmaceutical company in terms of pharmaceutical-only products revenues in 2023. •Achieved record number of approvals in 2023: 9 new molecular entity approvals from the FDA – a record number for Pfizer and 3 times more than the second in our industry. •Successfully renegotiated government contracts for Comirnaty and Paxlovid. •Successfully completed the acquisition of Seagen, potentially contributing more than $10 billion2 in risk-adjusted revenues in 2030, with potential significant growth beyond 2030. Seagen’s proprietary, world-leading antibody drug conjugate (ADC) technology together with the scale and strength of Pfizer’s capabilities and expertise, positions us to significantly advance the global fight against cancer. | |
| | |
| | | | | | | | |
| | |
| David M. Denton CFO, EVP | |
| | |
| | |
| Mr. Denton was responsible for our financial management and played a key role in driving our financial results. •Returned $9.2 billion to shareholders through cash dividends in 2023. •Successfully closed the acquisition of Seagen on December 14, 2023, including the raising of $39 billion in short- and long-term financing, furthering Pfizer’s priority to achieve world-class oncology leadership and to deliver cancer medicines that help patients live better and longer lives. •Continued to reinvest capital into initiatives intended to enhance the future growth prospects of the company, including $10.7 billion in internal research and development projects and approximately $43.8 billion invested in completed business development transactions, net of cash acquired. | |
| | |
| | | | | | | | |
| | |
| Mikael Dolsten, M.D., Ph.D. Chief Scientific Officer, President, Pfizer Research & Development | |
| | |
| | |
| Dr. Dolsten continued to drive the focus on executing on our robust pipeline and other R&D related initiatives. •Delivered 15 regulatory approvals covering internal medicine, rare disease, oncology, vaccines, and inflammation and immunology; achieved seven proof of concepts and eight signs of clinical activity for early-stage assets. •Advanced Pfizer’s innovative research pipeline achieving 67 advancements3 from Phase 1 to registration, with a focus on six key therapeutic areas: inflammation and immunology, internal medicine, oncology, rare diseases, vaccines, and anti-infectives. Initiated six pivotal studies. •Contributed to the acquisition of Seagen, furthering Pfizer’s priority to achieve world-class oncology leadership and to deliver cancer medicines that help patients live better and longer lives. •Ensured successful pharmacovigilance and medical support for all Pfizer products and clinical programs. | |
| | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 67 |
| | | | | | | | |
| | |
| Douglas M. Lankler General Counsel, EVP | |
| | |
| | |
| Mr. Lankler continued to provide legal advice and counsel on Pfizer's legal matters. •Provided comprehensive legal support for all issues related to our COVID-19 product portfolio (vaccine and oral therapy), including our contracting with governments. •Achieved favorable resolutions of several significant litigation matters. •Successfully closed the acquisition of Seagen on December 14, 2023, including supporting the raising of short- and long-term financing and obtaining the Federal Trade Commission’s clearance for the deal, furthering Pfizer’s priority to achieve world-class oncology leadership and to deliver cancer medicines that help patients live better and longer lives. •Supported the commercial business’s launch of 13 products. | |
| | |
| | | | | | | | |
| | |
| Aamir Malik Chief U.S. Commercial Officer, EVP4 | |
| | |
| | |
| Mr. Malik was responsible for developing and executing near and long term strategies to drive growth for Pfizer. •Successfully negotiated and closed the $43 billion acquisition of Seagen on December 14, 2023, furthering Pfizer’s priority to achieve world-class oncology leadership and to deliver cancer medicines that help patients live better and longer lives. •Led Pfizer’s Portfolio Management Team (the senior-most portfolio governance body), applying rigorous analysis to approve business development investments and prioritize the R&D portfolio, resulting in eight signs of clinical activity, seven proof of concepts, six pivotal study starts, 15 regulatory approvals, and 13 early-stage scientific collaborations executed in 2023. •Launched Pfizer Ignite (a new initiative offering strategic guidance and end-to-end R&D services to select innovative biotech companies aligned with Pfizer’s R&D focus areas), signing collaborations with a number of new innovative biotech companies in 2023. •Drove creation of refreshed Purpose Blueprint, Pfizer’s updated set of strategic goals and priorities that employees will strive to achieve over the next few years. | |
| | |
(1)The patients treated metric is calculated from Pfizer and third-party datasets. This estimate does not include Seagen patients treated. Figures may be limited given the coverage provided by external sources (e.g., calendar duration, geographic and product coverage) and are subject to change. Numbers are estimates and in some cases use global volume, daily dosage and number of treatment days to facilitate calculations. Methodologies to calculate estimates may vary by product type given the nature of the product and available data. Patients taking multiple Pfizer products may be counted as multiple patients towards total. Numbers do not include comprehensive estimated patient counts from Ex-U.S. Access & Affordability programs. Historical estimates may periodically be subject to revision due to restatements in the underlying data source.
(2)The potential revenue contribution from Seagen is a forward-looking statement subject to risks and uncertainties that could cause actual results to differ materially, including uncertainties related to clinical trial outcomes, regulatory decisions, and competitive developments, as well as the matters described under the "Risk Factors" section in Pfizer’s 2023 Annual Report on Form 10-K, which is incorporated by reference.
(3)Includes Seagen.
(4)Chief Business Innovation Officer, Executive Vice President, prior to December 15, 2023.
| | | | | | | | |
68 | | Pfizer 2024 Proxy Statement |
SECTION 4 — Benefit Programs
The following outlines some of our benefit programs available to eligible U.S.-based colleagues, including eligible NEOs (unless otherwise noted):
| | | | | |
| Plan/Eligibility | Description of Benefit |
| |
Pension and Savings Plans: •Savings Plan (qualified defined contribution savings plan) •Supplemental Savings Plan (non-qualified plan) •Pension Plan (qualified defined benefit pension plan (frozen*)) •Supplemental Pension Plan (non-qualified plan (frozen*))
* Benefits under the Pension Plan and Supplemental Pension Plan were frozen in 2017 for all participants, although participants may continue to grow into retirement plan milestones. | All eligible colleagues earn retirement benefits through age- and service-weighted annual company-provided Retirement Savings Contributions (RSC) (5%-9%) on salary and bonus to the Pfizer Savings Plan (PSP), and, as applicable, to the Pfizer Supplemental Savings Plan (PSSP), in addition to our matching contributions to these plans. The PSP permits eligible U.S. colleagues, including NEOs, to make pre- and after-tax and/or Roth contributions, from their eligible pay, up to certain limits and to receive company matching contributions. We also maintain the PSSP which permits participants, including NEOs, to make pre-tax contributions in excess of IRC limits on qualified plans and provides applicable matching contributions and the RSC for amounts not permitted under the PSP. |
| |
All eligible U.S. colleagues accumulate retirement benefits through the savings plans in the form of elective deferrals, matching contributions and/or the RSC. |
Insurance Plans Medical, dental, life and long-term disability insurance. | Programs are designed to provide certain basic quality of life benefits and protections to U.S. eligible colleagues, including the NEOs, and at the same time enhance our attractiveness as an employer of choice. The cost of these plans is shared between the colleague and the company. The company’s cost of coverage for the NEOs ranges up to approximately $30,000 annually based on the coverage selected. |
Supplemental Individual Disability Insurance Additional disability insurance coverage. | This is an optional individual disability benefit providing for coverage in excess of the limit provided under the company’s group long-term disability plan. Participants pay the full cost of this additional insurance coverage. |
Deferred Compensation Executives may elect to defer certain compensation into the Deferred Compensation Plan (DCP). | Annual incentive awards and performance share award settlements may be deferred under the DCP. Deferrals into the DCP may be notionally invested in a selection of investment options, Pfizer stock unit funds, and/or a cash equivalent fund. |
Retiree Healthcare Benefits Pfizer maintains post-retirement medical coverage. | Generally, access to post-retirement medical coverage at the colleague’s cost is available to active colleagues who are at least age 55 with at least 10 years of service. A retiree medical subsidy is provided to those colleagues with at least 15 years of service (after age of 40). For U.S. eligible colleagues, including the NEOs, the total company-provided subsidy can range from $61,500 to $275,000 (based on service after age 40, subject to a cap of 25 years, and coverage tier). The subsidy may only be used to cover Pfizer’s share of the cost. Coverage at the retiree’s cost may continue after the subsidy is depleted. |
Executive Severance Plan Provides severance benefits to NEOs (and the other ELT members) in the event of involuntary termination of employment without Cause (including position elimination or reorganization-related termination (other than for Cause)). | Cash severance is equal to the greater of: (a)one times pay (defined as base salary plus target annual incentive), or (b)13 weeks’ pay plus three weeks’ pay per full year of service, subject to a maximum of 104 weeks’ pay. The Committee adopted an executive severance policy, effective in 2023, which provides that without shareholder approval, cash severance paid to our executives (including the NEOs) cannot exceed 2.99 times of base salary and target bonus. Since 2009, the Executive Severance Plan has provided and continues to provide, cash severance with a cap of 2 year's pay (104 weeks), which aligns with the current executive severance policy. In addition to the cash severance, participants may continue participation in certain health and insurance benefits at active employee rates for a period of time and receive outplacement assistance. |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 69 |
SECTION 5 — Other Compensation Programs and Policies
PERQUISITES
We provide a limited number of perquisites to our NEOs, such as limited personal use of company aircraft, limited reimbursement for certain financial counseling and home security services (plus additional security services, as discussed below) and, solely for the CEO, use of a car and driver (as described below). The transportation benefits provide increased efficiencies and enable more productive use of our executives’ time and, in turn, greater focus on Pfizer-related activities. Since 2021, due to the significant ongoing and increased threats, the Committee approved providing personal security to the CEO and other NEOs, as deemed necessary. The Committee believes these perquisites contribute to executive security, recruitment and retention, and are consistent with market practice.
We do not provide tax “gross-ups” for perquisites provided to the NEOs, except in the case of certain relocation expenses (consistent with our relocation policy for U.S.-based colleagues generally). Therefore, executives (including the NEOs) pay applicable taxes due on these perquisites. The Committee reviews and carefully considers the reasonableness of and rationale for providing these perquisites.
| | |
| Perquisite/Description |
| Car and Driver |
For the CEO: For security reasons, a car and driver are available to the CEO for personal use (including commuting) and the cost does not need to be reimbursed to the company. Spouse/partner travel is generally considered personal use and the incremental cost of such travel must be reimbursed to the company. For tax purposes, the cost of the personal use of the car and fuel is imputed as income to the CEO. All taxes on this income are paid by the CEO and no gross-up payment for these taxes is made by the company. Tax regulations provide the cost of the driver is not reportable as income to the CEO as a result of the recommendations contained in an independent, third-party security study. The unreimbursed incremental cost to the company of personal use of a car and driver by Dr. Bourla in 2023 is reflected in the “All Other Compensation” column in the SCT and the related footnotes. |
For the other NEOs: Cars and drivers are available for business reasons; NEOs (other than the CEO) are required to reimburse the company for personal use of cars and drivers. |
| Aircraft Usage |
For the CEO: The Board has determined that the CEO must use company-provided aircraft for all air travel, including personal travel, to the maximum extent practicable, based on the recommendations contained in an independent, third-party security study. This study also recommends that the CEO’s spouse use company-provided aircraft when accompanying the CEO, to the maximum extent practicable. Personal travel by the CEO is subject to disclosure. Travel by the spouse is generally considered personal use and is subject to taxation and disclosure. |
For the other NEOs: Company aircraft are available for business travel and limited personal travel. Personal use is permitted only with the prior approval of the CEO or his designees and is subject to other limitations. Travel on company aircraft by Pfizer executives to attend boards of directors’ meetings at external companies is treated as personal travel. Personal travel is subject to taxation and all taxes are paid by the executives. |
| Financial Counseling and Security |
| We provide an allowance of up to $15,000 per year to the NEOs for financial counseling services, which may include tax preparation and estate planning services. Reimbursement for appropriate home security systems and monitoring charges is provided to the NEOs. Also, Pfizer may, based on the advice from its independent security consultant, and other security experts, provide additional security services for our executives, as deemed appropriate. All taxes applicable to these benefits are paid by the respective executive. |
The value of perquisites, based on the incremental cost to the company, is included in “All Other Compensation” in the SCT as described above and includes the personal use of a company-provided car and driver and aircraft. The incremental cost for personal use of aircraft consists of the variable costs we incurred to operate the aircraft for such use. As our aircraft are used primarily for business travel, this does not include fixed or non-variable costs that would be incurred regardless of such personal use, such as crew salaries and benefits, insurance, aircraft purchase or leasing costs, depreciation, and scheduled maintenance.
| | | | | | | | |
70 | | Pfizer 2024 Proxy Statement |
TAX POLICIES
Effective January 1, 2018, the exception from the IRC Section 162(m) deduction limit for performance-based compensation was repealed, such that compensation paid to our NEOs in excess of $1.0 million is generally not deductible unless it qualifies for transition relief. The company will utilize the transition relief provisions for eligible compensation to the extent possible. As in prior years, the Committee believes that it is important to continue to maintain flexibility and the ability to pay competitive compensation by not requiring compensation to be deductible.
EQUITY AWARD GRANT PRACTICES
The Committee’s long-standing practice has been to approve equity awards to eligible colleagues, including the NEOs, at its meeting held in late February (which are then ratified by the independent Directors at the full Board meeting typically, the fourth Thursday of the month). The Committee and full Board meetings are generally scheduled at least one-year in advance. The Committee neither grants equity awards in anticipation of the release of material nonpublic information, nor is the timing of filings of material nonpublic information based on equity award grant dates.
Effective for the 2024 equity awards, the Committee approved modifications to Pfizer’s equity award grant practices, such that the grant date of the annual equity awards will be the third business day following the expected Annual Report on Form 10-K filing, but no earlier than the second business day following the actual 10-K filing. The Committee approved the February 2024 annual equity awards at its February 21, 2024 meeting. These awards were then ratified by the independent Directors at the Board’s February 22, 2024 meeting with a grant date of February 27, 2024 (the close of the third business day following the 10-K filing).
Equity grants to certain newly hired colleagues, including executive officers, are effective on the last trading day of the month they begin work at Pfizer. Special equity grants to continuing colleagues are effective on the last trading day of the month in which the award is approved or such later date as determined at the time of approval. Where applicable, the exercise/grant price for an award will be equal to the closing market price of our common stock on the grant date. Our equity incentive plan prohibits the repricing or exchange/cash out of equity awards without shareholder approval.
DERIVATIVES TRADING/HEDGING POLICY
Our policy prohibits all employees, including the NEOs, and Directors from purchasing or selling options on our common stock or engaging in short sales of our shares. It also prohibits trading in puts, calls, straddles, equity swaps or other derivative securities including exchange funds, that are directly linked to our common stock (sometimes referred to as “hedging”).
COMPENSATION RECOVERY/CLAWBACK
The company adopted a clawback policy, effective October 2, 2023, that complies with the new SEC rules under the Dodd-Frank Wall Street Reform and Consumer Protection Act and NYSE rules. The newly adopted policy provides that Pfizer will seek recovery, in the event of a required accounting restatement, of erroneously awarded incentive-based compensation received by current and former executive officers. We have historically maintained a clawback policy, and these recovery/clawback policies are in addition to any policies or recovery requirements provided under the new SEC rules. Our equity and cash incentive awards contain compensation recovery/clawback provisions that authorize the cancellation and/or reduction of outstanding awards and the return of shares and/or cash paid and/or gain realized from an award, if the NEOs, other executives or employees: (i) engage in any activity in competition with the company; (ii) engage in any activity inimical, contrary or harmful to the interests of the company (or directly supervise any employee who engages in such activity) or that violates any company policies; or (iii) disclose or misuse any confidential information or material concerning the company. All equity awards are subject to cancellation or reduction in payout by the Committee during their performance/vesting periods and until they are paid out or settled and for a period of one-year after settlement. Under the terms of the grants, in general, participants will not receive their awards following termination of employment until the original settlement date. In addition, the Compensation Committee may, if permitted by law, make retroactive adjustments to any cash- or equity-based incentive compensation paid to NEOs and other executives upon an accounting restatement resulting from material noncompliance with financial reporting requirements under federal securities law. Where applicable, and as determined by the Committee, we will seek to recover any amounts deemed to have been inappropriately received by any executive officer.
Furthermore, in accordance with the terms of the Regulatory and Compliance Committee’s (RCC) Charter, if a government or regulatory action occurs that, in the judgment of the RCC, has caused significant financial or reputational damage to the company or otherwise indicates a significant compliance or regulatory issue within the company, then the RCC shall make a written recommendation to the Compensation Committee concerning the extent, if any, to which the incentive-based compensation of any executive, senior manager, compliance personnel and/or attorney involved in the conduct at issue or with direct supervision over an employee that engaged in the conduct at issue should be reduced, extinguished, or recovered.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 71 |
The company will disclose our decision to take action when required by, and in compliance with, SEC rules and regulations and other applicable laws. In addition, when legally permissible to do so, we will disclose a decision to take action when the facts and circumstances of the matter have been publicly disclosed in the company’s filings with the SEC and where disclosure can be made without prejudicing the company and its shareholders.
STOCK OWNERSHIP AND HOLDING REQUIREMENTS
Our NEOs are required to maintain certain levels of ownership of Pfizer stock. The CEO is required to own Pfizer common stock equal in value to at least eight times his annual salary. Each of the other NEOs is required to own Pfizer common stock with a value equal to at least four times their annual base salary. Ownership includes shares owned directly or in a family trust controlled by the executive, plus shares and certain units (e.g., unvested RSUs and deferred units, excluding TSRUs and performance shares) held through various Pfizer plans and programs.
Our guidelines allow meeting these targets with certain milestones over five years. Until the applicable milestone is reached, the executive must hold and may not sell shares (except to meet tax-withholding obligations). Once the level is met, in accordance with the rules set forth in the guidelines, NEOs must hold and may not sell shares if it would cause their ownership to fall below that level.
We believe that these requirements align the interests of our NEOs with those of our shareholders. Additionally, long-term incentive awards continue to vest and settle in accordance with their stated terms following an NEO’s retirement, rather than vesting upon retirement, thereby maintaining the alignment with shareholders into retirement.
Consistent with our policy prohibiting the pledging of Pfizer stock, none of our NEOs or other executive officers have pledged Pfizer stock as collateral for personal loans or other obligations.
All of the NEOs have met the requirements (full or applicable interim) under the guidelines (as noted below).
2023 Stock Ownership(1)
| | | | | | | | |
Name(2) | Full Guidelines | 12/31/2023 Multiple |
| A. Bourla | 8X | 18.41 |
D. Denton(3) | 4X | 1.08 |
| M. Dolsten | 4X | 5.20 |
| D. Lankler | 4X | 5.47 |
A. Malik(3) | 4X | 1.05 |
(1)Determined using Pfizer closing stock price, base salary and shares held as of December 29, 2023 (rounded).
(2)Excludes Ms. Hwang and Dr. Pao’s stock ownership as they were not executive officers as of December 31, 2023.
(3)Subject to interim milestone guidelines, which have been met. Messrs. Denton and Malik are on track to be in compliance with the full guidelines.
| | | | | | | | |
72 | | Pfizer 2024 Proxy Statement |
Compensation Tables
SUMMARY COMPENSATION TABLE
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name and
Principal Position | Year | Salary
($) | Bonus ($)(1) | Stock Awards ($)(2) | Option Awards ($)(3) | Non-Equity Incentive Plan Compensation ($)(4) | Change In Pension Value and Non- Qualified Deferred Compensation Earnings ($)(5) | All Other Compensation ($)(6) | Total
($) |
A. Bourla Chairman and Chief Executive Officer | 2023 | 1,787,500 | | — | | 8,745,187 | | 8,761,683 | | 0 | 8,440 | | 2,259,254 | | 21,562,064 | |
| 2022 | 1,737,500 | | — | | 9,296,191 | | 9,526,444 | | 7,650,000 | | 2,473,747 | | 2,333,571 | | 33,017,453 | |
| 2021 | 1,687,500 | | — | | 6,180,808 | | 7,050,649 | | 8,000,000 | | 49,901 | | 1,384,361 | | 24,353,219 | |
D. Denton Chief Financial Officer, EVP(7) | 2023 | 1,296,875 | | — | | 1,341,079 | | 2,190,418 | | 0 | — | 451,164 | | 5,279,536 | |
| 2022 | 833,333 | | 5,000,000 | | 4,000,012 | | 2,250,008 | | 1,838,355 | | — | 10,522,450 | | 24,444,158 | |
M. Dolsten Chief Scientific Officer, President, Pfizer Research & Development | 2023 | 1,596,500 | | — | | 3,115,268 | | 2,920,565 | | 0 | | 413,970 | 812,397 | | 8,858,700 | |
| 2022 | 1,535,000 | | — | | 3,471,267 | | 2,931,212 | | 3,530,972 | | — | | 749,782 | | 12,218,233 | |
| 2021 | 1,478,750 | | — | | 2,531,393 | | 3,021,712 | | 3,250,000 | | — | | 633,135 | | 10,914,990 | |
D. Lankler General Counsel, EVP(8) | 2023 | 1,198,313 | | — | | 1,817,208 | | 1,703,661 | | 0 | 196,724 | 561,892 | | 5,477,798 | |
2022 | 1,143,750 | | — | | 2,160,626 | | 1,709,885 | | 2,264,931 | | — | 547,163 | | 7,826,355 | |
| 2021 | 1,102,500 | | — | | 1,587,984 | | 1,762,662 | | 2,191,860 | | — | 484,366 | | 7,129,372 | |
A. Malik Chief U.S. Commercial Officer, EVP(9) | 2023 | 1,294,800 | | — | | 1,336,257 | | 2,190,418 | | 0 | — | | 599,259 | | 5,420,734 | |
A. Hwang Former Chief Commercial Officer and President, Global Biopharmaceuticals Business(10) | 2023 | 1,340,450 | | — | | 2,336,483 | | 2,190,418 | | 0 | 146,531 | 679,897 | | 6,693,779 |
| 2022 | 1,276,500 | | — | | 2,661,590 | | 2,198,403 | | 2,936,438 | | — | 673,437 | | 9,746,368 |
| 2021 | 1,220,000 | | — | | 1,975,798 | | 2,266,282 | | 2,984,700 | | — | 586,154 | | 9,032,934 |
W. Pao Former Chief Development Officer, EVP(11) | 2023 | 772,500 | | 691,939 | | 577,860 | | 1,703,661 | | — | | — | | 2,505,158 | 6,251,118 |
| 2022 | 937,500 | | 5,000,000 | | 5,999,988 | | — | | 1,565,557 | | — | | 3,760,971 | | 17,264,016 | |
(1)Bonus. Shown in this column for 2022 are Mr. Denton and Dr. Pao’s one-time sign-on cash payments. See the Leadership Transition section in the 2023 Proxy Statement. For 2023, the amount shown for Dr. Pao is the pro-rata target bonus payable in accordance with the terms of the GPP in effect at the time of his involuntary termination.
(2)Stock Awards. Represents for each year one-third of the grant date fair value of PSAs granted in that year and the prior two years (e.g., for 2023: 2023, 2022 and 2021) in accordance with ASC Topic 718.
Consistent with the applicable accounting rules, the amounts listed include one-third of each of these awards, as only the 2023 PSA’s first-year goal, 2022 PSA’s second-year goal and 2021 PSA’s third-year goal of the three-year performance period beginning in 2023 were set in 2023. The maximum potential values of the PSAs at the grant date, February 23, 2023 (using closing stock price of $42.30) based on the units included here (one-third of the grants in 2023, 2022 and 2021 (as applicable)) would be as follows (subject to rounding): Dr. Bourla—$17,490,374; Mr. Denton—$2,682,158; Dr. Dolsten—$6,230,536; Mr. Lankler—$3,634,416; Mr. Malik—$2,672,514; Ms. Hwang—$4,672,966; and Dr. Pao—$1,155,720. The PSA grant date fair values have been determined using Pfizer’s closing stock price on the respective grant date.
However, the Committee considered the full value of the annual LTI award (without regard to when performance goals are set) when making the grant, and the below amounts represent the full 2023 grant date fair values (per share/unit) using the closing stock price on February 23, 2023 of $42.30 for the PSAs, and for the 5-Year and 7-Year TSRUs, the Monte Carlo values of $10.60 and $12.18, respectively, as discussed below:
| | | | | | | | | | | | | | | | | | | | | | | |
| A. Bourla | D. Denton | M. Dolsten | D. Lankler | A. Malik | A. Hwang | W. Pao |
| A. PSAs at Target ($) | 8,915,698 | | 2,228,914 | | 2,971,913 | | 1,733,623 | | 2,228,914 | | 2,228,914 | | 1,733,623 | |
| B. TSRUs ($) | 8,761,683 | | 2,190,418 | | 2,920,565 | | 1,703,661 | | 2,190,418 | | 2,190,418 | | 1,703,661 | |
C. 2023 LTI Award (Full Grant Date Fair Value) ($) (A + B) (Subject to Rounding) | 17,677,381 | | 4,419,332 | | 5,892,478 | | 3,437,284 | | 4,419,332 | | 4,419,332 | | 3,437,284 | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 73 |
(3)Option Awards. Represents the aggregate grant date fair values of the TSRUs under GAAP. The grant date fair values have been determined in accordance with ASC Topic 718 using the Monte Carlo Simulation model, based on the assumptions and methodologies described in our 2023 Annual Report on Form 10-K (Note 13. Share-Based Payments).
(4)Non-Equity Incentive Plan Compensation. Represents annual incentive awards, earned under the GPP for the performance year noted and paid to the NEO early in the following year. As further described in the “Compensation Discussion and Analysis,” NEOs did not receive a payout under the GPP for fiscal year 2023. (5)Change in Pension Value and Non-Qualified Deferred Compensation Earnings. Represents the change in the actuarial present value of the NEO’s accumulated pension benefits in 2023, 2022 and 2021, as applicable, measured against the prior year’s value. The pension amounts reported in the SCT are zero ($0) as the change in value, as applicable, was negative, as follows unless otherwise noted for 2022 and 2021, respectively: Dr. Dolsten—($1,512,532) and ($71,761); Mr. Lankler—($1,320,685) and ($98,472); and Ms. Hwang—($862,403) and ($21,713). The 2022 amount for Dr. Bourla reflects his attainment of the “Rule of 90” (age plus service equal to or greater than 90) in 2022. This provides him with an unreduced pension benefit upon his retirement. Additionally, the value takes into account as a payment his one-time election of a notional transfer to the Pfizer Supplemental Savings Plan of $12,682,274, which was the present value of his post-2004 Supplemental Pension Plan benefit of July 1, 2022. Net of taxes ($182,270), the amount of the transfer was $12,500,004. Further information regarding pension plans is included in “The Pension Plan and Supplemental Pension Plan Summary” later in this Proxy Statement. The pension amount for 2023 represents the difference between the December 31, 2023 and December 31, 2022 present values of age 65 accrued pensions, or the current benefit if the NEO is eligible for an unreduced pension under the Pfizer Consolidated Pension Plan (the Pension Plan or the PCPP) and Pfizer Consolidated Supplemental Pension Plan for U.S. and Puerto Rico Employees (Supplemental Pension Plan), based on the assumptions reflected in the company’s financials as of December 31, 2023, as shown below: a.Discount Rate: 5.45% for qualified pension plans; 5.39% for non-qualified pension plans.
b.Lump Sum Interest Rates: For Pfizer Retirement Annuity Plan (the Pfizer Sub-Plan), rates based on implied forward rates developed from the November 2023 full yield curve published by the IRS in December 2023 for Pension Protection Act (PPA) funding calculation purposes, adjusted based on the movement in the Mercer Yield Curve spot rates during December 2023. For Wyeth Retirement Plan U.S. (the Wyeth Sub-Plan), based on Pension Benefit Guaranty Corporation (PBGC) rates derived from 12-year spot rates from the projected full yield curve described in the preceding sentence.
c.Percent Electing Lump Sum:
i.75% relating to the Pfizer Sub-Plan (only applies to the extent the executive is eligible to receive a lump sum).
ii.70% relating to the Pfizer benefit formula in the Supplemental Pension Plan.
iii.85% relating to the Wyeth Sub-Plan and the Wyeth benefit formula in the Supplemental Pension Plan.
d.Mortality Table for Lump Sum: Unisex mortality table specified by IRC Section 417(e), with projected mortality improvements.
e.Mortality Table For Annuities: Pri-2012 annuitant mortality table. No collar adjustments were made for the qualified plan, and a white-collar adjustment was reflected for the non-qualified plan.
f.Mortality Improvement Scale for Annuities: MMP-2021 projection scale, which assumes an ultimate rate of improvement of 1.2% for ages below 65 and grades linearly to zero at age 115. The grade-down period to the ultimate rate occurs over a 15-year period based on year of birth, with the grade-down period weighted 67% for birth cohorts and 33% for the ultimate rates of improvement.
(6)All Other Compensation. Column amounts represent the incremental cost to the company of perquisites, matching contributions, an RSC and other contributions, as applicable, under the Savings Plan and the Supplemental Savings Plan received by each NEO as detailed in the table below:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Perquisites and Other Compensation | | Employer Contributions | |
| Name | Aircraft Usage
($) | Financial Counseling
($) | Car Usage
($) | Security ($)(a) | Other ($)(b) | Savings Plan ($)(c) | Supplemental Savings Plan ($)(c) | Total ($) |
| A. Bourla | 154,520 | | 15,000 | | 23,733 | | 789,495 | | 2,443 | | 16,500 | | 1,257,563 | | 2,259,254 | |
| D. Denton | 70,194 | | 15,000 | | — | | — | | 2,871 | | 14,488 | | 348,611 | | 451,164 | |
| M. Dolsten | 88,856 | | 15,000 | | — | | 14,630 | | 1,702 | | 42,900 | | 649,309 | | 812,397 | |
| D. Lankler | 87,691 | | 4,446 | | — | | 1,110 | | 1,107 | | 42,900 | | 424,638 | | 561,892 | |
| A. Malik | 80,637 | | 12,947 | | — | | — | | 1,498 | | 37,828 | | 466,349 | | 599,259 | |
| A. Hwang | 89,581 | | 10,000 | | — | | — | | 2,936 | | 42,900 | | 534,480 | | 679,897 | |
| W. Pao | — | | — | | — | | — | | 2,424,431 | | 14,850 | | 65,877 | | 2,505,158 | |
a.Due to heightened security risks including threats made against our executives, additional security protection, pursuant to an independent security study and the advice of other security experts, has been provided to certain executives, where deemed appropriate, including some of our NEOs.
b.Represents the value of incidental items provided in connection with attendance at Pfizer business meetings. Also, includes the nominal values from our colleague-to-colleague recognition program linked to our Pfizer values for all NEOs excluding Messrs. Lankler and Malik. For Mr. Denton and Dr. Pao, includes relocation-related benefits pursuant to our relocation policy of $2,216 and $10,996, respectively. Consistent with our policy for all U.S. relocations, these amounts include certain gross-up payments of $1,081 and $2,972, respectively. Additionally, for Dr. Pao includes accrued unused vacation pay of $19,385 and severance payment of $2,394,000, as further described in the “Estimated Payments and Benefits upon Termination Table” below. For additional details on Dr. Pao’s severance, see the “Leadership Transition” section elsewhere in this Proxy Statement. c.Under the Savings Plan (up to IRC limits) and Supplemental Savings Plan, our NEOs, consistent with all other participants, are eligible for matching contributions of up to 4.5% of their eligible pay (base salary and bonus) and a retirement savings contribution of 5% to 9% (based on age and service) of eligible pay.
(7)Effective May 2, 2022, Mr. Denton joined Pfizer as Chief Financial Officer, Executive Vice President; as such he was not an NEO in 2021.
(8)Mr. Lankler was not an NEO in 2022. The 2022 data has been added to comply with SEC requirements due to his status as an NEO in 2021 and in 2023.
(9)Mr. Malik joined Pfizer in August 2021 and was not an NEO in 2021 and 2022.
(10)Effective December 15, 2023, Ms. Hwang ceased serving as an executive officer and will serve as Advisor to the Chief Executive Officer for a transition period.
(11)Dr. Pao ceased serving as an executive officer and CDO on July 27, 2023 and departed from the company following a transition period on August 15, 2023. He was not an NEO in 2021.
| | | | | | | | |
74 | | Pfizer 2024 Proxy Statement |
2023 GRANTS OF PLAN-BASED AWARDS TABLE
This table provides additional information about non-equity incentive awards and long-term equity incentive awards granted to our NEOs during 2023. The long-term incentive awards were made under the 2019 Stock Plan and are described in the CD&A section “Elements of Our Executive Compensation Program.” | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Estimated Future Payouts Under Non-Equity Incentive Plan Awards(1) | | Estimated Future Payouts Under Equity Incentive Plan Awards(2) | All Other Stock Awards: Number of Shares or Units (#) | All Other TSRU Awards: Number of Securities Underlying TSRUs(3)(4) (#) | Exercise or Base Price of TSRU Awards ($/Sh) | Grant Date Fair Value of Stock and TSRU Awards(4) ($) |
Name | Grant Date | Threshold ($) | Target ($) | Maximum ($) | | Threshold (#) | Target(3) (#) | Maximum (#) |
A. Bourla | | 0 | 3,575,342 | | 8,938,355 | | | | | | | | | |
| 2/23/2023 | | | | | | | | | 413,280 | | 42.30 | 4,380,768 | |
| 2/23/2023 | | | | | | | | | 359,681 | | 42.30 | 4,380,915 | |
| 2/23/2023 | | | | | 0 | 206,742 | | 413,484 | | | | | 8,745,187 | |
D. Denton | | 0 | 1,297,089 | | 3,242,723 | | | | | | | | | |
| 2/23/2023 | | | | | | | | | 103,320 | | 42.30 | 1,095,192 |
| 2/23/2023 | | | | | | | | | 89,920 | | 42.30 | 1,095,226 |
| 2/23/2023 | | | | | 0 | 31,704 | | 63,408 | | | | 1,341,079 | |
M. Dolsten | | 0 | 1,596,712 | | 3,991,780 | | | | | | | | | |
| 2/23/2023 | | | | | | | | | 137,760 | | 42.30 | 1,460,256 | |
| 2/23/2023 | | | | | | | | | 119,894 | | 42.30 | 1,460,309 | |
| 2/23/2023 | | | | | 0 | 73,647 | | 147,294 | | | | | 3,115,268 | |
D. Lankler | | 0 | 1,078,659 | | 2,696,648 | | | | | | | | | |
| 2/23/2023 | | | | | | | | | 80,360 | | 42.30 | 851,816 | |
| 2/23/2023 | | | | | | | | | 69,938 | | 42.30 | 851,845 | |
| 2/23/2023 | | | | | 0 | 42,960 | | 85,920 | | | | | 1,817,208 | |
A. Malik | | 0 | 1,295,014 | | 3,237,535 | | | | | | | | | |
| 2/23/2023 | | | | | | | | | 103,320 | | 42.30 | 1,095,192 | |
| 2/23/2023 | | | | | | | | | 89,920 | | 42.30 | 1,095,226 | |
| 2/23/2023 | | | | | 0 | 31,590 | | 63,180 | | | | 1,336,257 | |
A. Hwang | | 0 | 1,340,671 | | 3,351,678 | | | | | | | | | |
| 2/23/2023 | | | | | | | | | 103,320 | | 42.30 | 1,095,192 |
| 2/23/2023 | | | | | | | | | 89,920 | | 42.30 | 1,095,226 |
| 2/23/2023 | | | | | 0 | 55,236 | | 110,472 | | | | 2,336,483 | |
W. Pao | | 0 | 691,939 | | 1,729,848 | | | | | | | | | |
| 2/23/2023 | | | | | | | | | 80,360 | | 42.30 | 851,816 | |
| 2/23/2023 | | | | | | | | | 69,938 | | 42.30 | 851,845 | |
| 2/23/2023 | | | | | 0 | 13,661 | | 27,322 | | | | | 577,860 | |
(1)Amounts are the threshold, target and maximum annual incentive award payout for the January 1, 2023 - December 31, 2023 performance period. The actual 2023 payout is reported in the “Summary Compensation Table” in the “Non-Equity Incentive Plan Compensation” column. (2)Amounts are the threshold, target and maximum share payouts under our PSAs. The “target” represents one-third of each of the 2021, 2022 and 2023 PSA grants (as noted earlier in this Proxy Statement). There is no payment for below threshold performance so the amount is zero. See “SCT footnote 2” for further information on the full value of the 2023 PSA grant. (3)Long-term incentive grant values were converted into units using the closing stock price/value on the first trading day of the week of grant. The PSA values were converted using the closing stock price of $42.70 on February 21, 2023; the 5-Year and 7-Year TSRU values were converted into TSRUs using $10.89 and $12.51, respectively, the estimated values using the Monte Carlo Simulation model as of February 21, 2023. PSAs generally vest three years from the grant date. The 5-Year and 7-Year TSRUs also generally vest three years from the grant date and are settled on the fifth or seventh anniversary of the grant date, respectively.
(4)Represent the award values as of the grant date (GAAP and as required under Regulation S-K). The values for the PSAs, 5-Year and 7-Year TSRUs are shown at the respective grant date fair values as of: February 23, 2023 of $42.30, $10.60 and $12.18, respectively, in accordance with ASC Topic 718. The performance-based conditions that apply are discussed in the “Performance Share Awards” section detailed earlier in this Proxy Statement. | | | | | | | | |
2024 Proxy Statement Pfizer | | 75 |
2023 OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END TABLE
The following table details the outstanding equity awards held by our NEOs as of December 31, 2023.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | TSRU Awards(2) | | Stock Awards(2) |
Name | Grant Date/ Performance Share Period(1) | Number of Securities Underlying Unexercised TSRUs Vested (#) | Number of Securities Underlying Unexercised TSRUs Unvested (#) |
TSRU Exercise Price ($) |
TSRU Expiration Date
| | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) |
| A. Bourla | 2/23/2017 | 127,674 | | 27.34 | 2/23/2024 | | | | | |
| 2/22/2018 | 238,399 | | | 30.17 | 2/22/2025 | | | | | |
| 2/28/2019 | 379,995 | | | 38.71 | 2/28/2024 | | | | | |
| 2/28/2019 | 320,231 | | | 38.71 | 2/28/2026 | | | | | |
| 2/27/2020 | 582,823 | | 31.31 | 2/27/2025 | | | | | |
| 2/27/2020 | 499,353 | | 31.31 | 2/27/2027 | | | | | |
| 2/25/2021 | | 491,626 | 33.82 | 2/25/2026 | | | | | |
| 2/25/2021 | | 424,782 | 33.82 | 2/25/2028 | | | | | |
| 2/24/2022(3) | | 412,081 | | 45.96 | 2/24/2027 | | | | | |
| 2/24/2022(3) | | 362,427 | | 45.96 | 2/24/2029 | | | | | |
| 2/23/2023 | | 413,280 | | 42.30 | 2/23/2028 | | | | | |
| 2/23/2023 | | 359,681 | | 42.30 | 2/23/2030 | | | | | |
| 1/1/2021–12/31/2023 | | | | | | | | 204,320 | | 5,882,373 | |
| 1/1/2022–12/31/2024(3) | | | | | | | | 205,133 | | 5,905,779 | |
| 1/1/2023–12/31/2025 | | | | | | | | 210,773 | | 6,068,155 | |
| D. Denton | 5/31/2022 | | 80,703 | | 53.04 | 5/31/2027 | | | | | |
| 5/31/2022 | | 70,313 | | 53.04 | 5/31/2029 | | | | | |
| 2/23/2023 | | 103,320 | | 42.30 | 2/23/2028 | | | | | |
| 2/23/2023 | | 89,920 | | 42.30 | 2/23/2030 | | | | | |
| 5/31/2022(2) | | | | | | 32,587 | | 938,182 | | | |
| 1/1/2022–12/31/2024 | | | | | | | | 42,421 | | 1,221,301 | |
| 1/1/2023–
12/31/2025 | | | | | | | | 52,693 | | 1,517,031 | |
| | | | | | | | |
76 | | Pfizer 2024 Proxy Statement |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | TSRU Awards(2) | | Stock Awards(2) |
Name | Grant Date/ Performance Share Period(1) | Number of Securities Underlying Unexercised TSRUs Vested (#) | Number of Securities Underlying Unexercised TSRUs Unvested (#) | TSRU Exercise Price ($) | TSRU Expiration Date
| | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) |
| M. Dolsten | 2/27/2020 | 224,163 | | 31.31 | 2/27/2025 | | | | | |
| 2/27/2020 | 192,059 | | 31.31 | 2/27/2027 | | | | | |
| 2/25/2021(3) | | 210,697 | 33.82 | 2/25/2026 | | | | | |
| 2/25/2021(3) | | 182,050 | 33.82 | 2/25/2028 | | | | | |
| 2/24/2022(3) | | 126,794 | | 45.96 | 2/24/2027 | | | | | |
| 2/24/2022(3) | | 111,516 | | 45.96 | 2/24/2029 | | | | | |
| 2/23/2023 | | 137,760 | | 42.30 | 2/23/2028 | | | | | |
| 2/23/2023 | | 119,894 | | 42.30 | 2/23/2030 | | | | | |
| 3/3/2022(4) | | | | | | 66,496 | | 1,914,431 | | | |
| 3/3/2022(4) | | | | | | 43,355 | | 1,248,176 | | | |
| 11/7/2022(4) | | | | | | 53,302 | | 1,534,551 | | | |
| 11/7/2022(4) | | | | | | 29,477 | | 848,638 | | | |
| 1/1/2021–12/31/2023(3) | | 87,565 | 2,520,996 |
| 1/1/2022–12/31/2024(3) | | 63,118 | 1,817,167 |
| 1/1/2023–12/31/2025 | | 70,258 | 2,022,728 |
| D. Lankler | 2/23/2017 | 95,755 | | 27.34 | 2/23/2024 | | | | | |
| 2/22/2018 | 85,824 | | 30.17 | 2/22/2025 | | | | | |
| 2/28/2019 | 94,998 | | 38.71 | 2/28/2024 | | | | | |
| 2/28/2019 | 80,058 | | 38.71 | 2/28/2026 | | | | | |
| 2/27/2020 | 156,914 | | 31.31 | 2/27/2025 | | | | | |
| 2/27/2020 | 134,441 | | 31.31 | 2/27/2027 | | | | | |
| 2/25/2021 | | 122,907 | 33.82 | 2/25/2026 | | | | | |
| 2/25/2021 | | 106,195 | 33.82 | 2/25/2028 | | | | | |
| 2/24/2022 | | 63,397 | | 45.96 | 2/24/2027 | | | | | |
| 2/24/2022 | | 55,758 | | 45.96 | 2/24/2029 | | | | | |
| 2/28/2022 | | 10,566 | | 46.94 | 2/28/2027 | | | | | |
| 2/28/2022 | | 9,294 | | 46.94 | 2/28/2029 | | | | | |
| 2/23/2023 | | 80,360 | | 42.30 | 2/23/2028 | | | | | |
| 2/23/2023 | | 69,938 | | 42.30 | | 2/23/2030 | | | | | |
| 1/1/2021–12/31/2023 | | 51,080 | | 1,470,593 | |
| 1/1/2022–12/31/2024 | | 31,559 | | 908,584 | |
| 1/1/2022–12/31/2024 | | 5,260 | | 151,435 | |
| 1/1/2023–12/31/2025 | | 40,984 | | 1,179,929 | |
| | | | | | | | | | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 77 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | TSRU Awards(2) | | Stock Awards(2) |
Name | Grant Date/ Performance Share Period(1) | Number of Securities Underlying Unexercised TSRUs Vested (#) | Number of Securities Underlying Unexercised TSRUs Unvested (#) | TSRU Exercise Price ($) | TSRU Expiration Date
| | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) |
| A. Malik | 2/24/2022 | | 84,529 | | 45.96 | 2/24/2027 | | | | | |
| 2/24/2022 | | 74,344 | | 45.96 | 2/24/2029 | | | | | |
| 2/23/2023 | | 103,320 | | 42.30 | 2/23/2028 | | | | | |
| 2/23/2023 | | 89,920 | | 42.30 | | 2/23/2030 | | | | | |
| 8/31/2021(2) | | | | | | 47,263 | | 1,360,688 | | | |
| 1/1/2022–12/31/2024 | | | | | | | | 42,079 | | 1,211,454 | |
| 1/1/2023–12/31/2025 | | | | | | | | 52,693 | | 1,517,031 | |
| A. Hwang | 2/23/2017 | 17,732 | | 27.34 | 2/23/2024 | | | | | |
| 2/22/2018 | 47,680 | | 30.17 | 2/22/2025 | | | | | |
| 2/28/2019 | 126,665 | | 38.71 | 2/28/2024 | | | | | |
| 2/28/2019 | 106,743 | | 38.71 | 2/28/2026 | | | | | |
| 2/27/2020 | 179,330 | | 31.31 | 2/27/2025 | | | | | |
| 2/27/2020 | 153,647 | | 31.31 | 2/27/2027 | | | | | |
| 2/25/2021 | | 158,023 | | 33.82 | 2/25/2026 | | | | | |
| 2/25/2021 | | 136,537 | | 33.82 | 2/25/2028 | | | | | |
| 2/24/2022 | | 95,095 | | 45.96 | 2/24/2027 | | | | | |
| 2/24/2022 | | 83,637 | | 45.96 | 2/24/2029 | | | | | |
| 2/23/2023 | | 103,320 | | 42.30 | 2/23/2028 | | | | | |
| 2/23/2023 | | 89,920 | | 42.30 | | 2/23/2030 | | | | | |
| 1/1/2021–12/31/2023 | | | | | | | | 65,674 | | 1,890,754 | |
| 1/1/2022–12/31/2024 | | | | | | | | 47,339 | | 1,362,890 | |
| 1/1/2023–12/31/2025 | | | | | | | | 52,693 | | 1,517,031 | |
| W. Pao | 2/23/2023 | | 12,681 | | 42.30 | 2/23/2028 | | | | | |
| 2/23/2023 | | 11,036 | | 42.30 | 2/23/2030 | | | | | |
| 1/1/2023–12/31/2025 | | | | | | | | 6,467 | | 186,193 | |
(1)Subject to rounding. We have included a column showing the grant dates of TSRUs, RSUs, Profit Units (PTUs) and the associated performance periods for the PSAs. The PSAs shown represent the full grant for the applicable grant date (irrespective of when the goals are set). Under the terms of the vested TSRUs, retirement eligible colleagues may “exercise” their vested TSRUs and convert them to PTUs which are settled on the settlement date of the underlying TSRUs. The PTUs noted above include the dividend equivalent units accrued from the exercise date to year-end, as applicable; however the dividend equivalent units are not included in the tables below.
(2)All TSRUs vest on the third anniversary of grant date and are settled on the 5th or 7th anniversary of grant, as applicable. Mr. Denton’s RSUs vest 50% on each of the first and second anniversaries of the grant date. Mr. Malik’s RSUs vest on the third anniversary of the grant date.
(3)Dr. Bourla’s February 24, 2022 annual grant consists of the following awards (subject to rounding), that are not eligible for retirement treatment and vest on the third anniversary of the grant:
| | | | | | | | | | | |
| Grant Date | 5-Year TSRUs | 7-Year TSRUs | PSAs |
| 2/24/2022 | 105,662 | | 92,930 | | 52,598 | |
| | | | | | | | |
78 | | Pfizer 2024 Proxy Statement |
Dr. Dolsten’s annual grants (detailed below) include the following awards (subject to rounding), that are not eligible for retirement treatment and vest on the third anniversary of the grant:
| | | | | | | | | | | |
| Grant Date | 5-Year TSRUs | 7-Year TSRUs | PSAs |
| 2/25/2021 | 35,116 | | 30,342 | | 14,594 | |
| 2/24/2022 | 21,132 | | 18,586 | | 10,520 | |
(4)Dr. Dolsten exercised the following TSRUs and received the following resulting PTUs:
| | | | | | | | | | | | | | |
| Exercise Date | TSRUs Exercised | TSRUs | PTUs | Distribution Date |
| 3/3/2022 | TSRU 2017 7YR | 127,674 | | 62,022 | | 2/23/2024 |
| 3/3/2022 | TSRU 2019 5YR | 158,331 | | 40,437 | | 2/28/2024 |
| 11/7/2022 | TSRU 2018 7YR | 127,146 | | 50,938 | | 2/22/2025 |
| 11/7/2022 | TSRU 2019 7YR | 133,430 | | 28,170 | | 2/28/2026 |
| Total: | | 181,567 | | |
2023 OPTION/TSRU EXERCISES AND STOCK VESTED TABLE
The following table provides additional information about the value realized by the NEOs on TSRU award settlements and unit award vesting that occurred during 2023. Note: The “exercises” of the TSRUs listed above are not reported in the table below until the PTUs are distributed on the original TSRU settlement date and they are shown in the Profit Units column. The information from this table and tables from prior years was used in the realized pay table elsewhere in this Proxy Statement.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| TSRU
Awards | | Option
Awards | | Restricted Stock/Restricted Stock Units/Profit Units(1) | | Performance Shares 2020-2022 Paid February 2023(2) |
| Name | Number
of Shares
Acquired
on Exercise
(#) | Number
of Shares
Withheld
to Cover
Taxes (#) | Value Realized on Exercise ($)(3) | | Number
of Shares
Acquired
on Exercise
(#) | Value
Realized
on Exercise
($) | | Number
of Shares
Acquired
on Vesting
(#) | Number
of Shares
Withheld
to Cover
Taxes (#) | Value
Realized
on Vesting
($) | | Number
of Shares
Acquired
on Vesting
(#) | Number
of Shares
Withheld
to Cover
Taxes (#) | Value
Realized
on Vesting
($) |
A. Bourla | — | | — | | — | | | — | | — | | | 224,410 | | 105,413 | | 9,418,948 | | | 355,999 | | — | | 14,517,641 | |
D. Denton(4) | — | | — | | — | | | — | | — | | | 31,778 | | 16,223 | | 1,208,193 | | | — | | — | | — | |
M. Dolsten | — | | — | | — | | | — | | — | | | 153,481 | | 69,670 | | 6,321,653 | | | 136,922 | | — | | 5,583,672 | |
| D. Lankler | — | | — | | — | | | — | | — | | | 102,610 | | 46,267 | | 4,292,988 | | | 95,846 | | — | | 3,908,601 | |
A. Hwang(3) | 33,572 | | 13,789 | | 1,415,676 | | | — | | — | | | — | | — | | — | | | 109,539 | | — | | 4,466,984 | |
W. Pao(4) | — | | — | | — | | | — | | — | | | 121,656 | | 67,277 | | 4,521,535 | | | — | | — | | — | |
Note: This table excludes Mr. Malik as he did not have any LTI award settlements in 2023.
(1)The amounts reported in this column represent the payment of PTUs from the exercises on December 16, 2022 for Dr. Bourla, November 11, 2021 and December 17, 2021 for Dr. Dolsten, and December 14, 2022 for Mr. Lankler of TSRUs granted on February 25, 2016 and February 22, 2018 that settled on February 25, 2023 and February 22, 2023, respectively. The fair market values for the PTUs at settlement were $41.75 and $42.38 for the February 25, 2016 and February 22, 2018 grants, respectively. Additionally, Drs. Bourla and Dolsten and Mr. Lankler deferred the dividend equivalent units resulting from the PTUs award, in the amount of $34,851, $132,897 and $21,181, respectively. The amount also includes RSUs for Mr. Denton and Dr. Pao as disclosed in note 4 (below).
(2)Represents PSAs earned over the 2020-2022 performance period which were denominated in shares and then converted and paid in cash based on the fair market value of $40.78 per share on February 27, 2023.
(3)Represents TSRUs, which were granted on: (i) February 25, 2016, and settled on February 25, 2023, using the settlement price (20-day average) of $43.44 and a fair market value of $41.75; and (ii) February 22, 2018, and settled on February 22, 2023, using the settlement price (20-day average) of $43.70 and a fair market value of $42.38.
(4)Mr. Denton’s RSUs represent the first 50% of the RSU award granted on May 31, 2022, which vested and was distributed on May 31, 2023 at a fair market value of $38.02. The remaining 50% will vest on the second anniversary of the grant date. Dr. Pao’s RSUs represents the RSU award granted on March 31, 2022, one-third of which vested and was distributed on March 31, 2023 at a fair market value of $40.80. The remaining two-thirds vested due to his involuntary termination on August 15, 2023 at a fair market value of $35.39.
RETIREMENT BENEFITS
The following shows the present value of accumulated benefits payable to each of our NEOs (who is a participant) under the Pfizer Consolidated Pension Plan (the Pension Plan or the PCPP) and the Pfizer Consolidated Supplemental Pension Plan for United States and Puerto Rico Employees (the Supplemental Pension Plan). The Pension Plan and the Supplemental Pension Plan were closed to new participants effective January 1, 2011 and were frozen for future accruals and eligible salary on December 31, 2017.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 79 |
2023 PENSION BENEFITS TABLE(1)
| | | | | | | | | | | | | | | | | | | | | | | |
| Name | Plan Name | Number of Years of Credited Service (#) | Age 65 Single-Life Annuity Payment ($) | Present Value of Accumulated Benefit ($)(2) | Payments During Last Fiscal Year ($) | Immediate Annuity Payable on 12/31/2023 ($) | Lump Sum
Value
($) |
A. Bourla(3) | Pension Plan | 24 | 99,747 | | 1,296,531 | | — | | 99,747 | | 1,253,181 | |
| Supplemental Plan | | 8,697 | | 92,120 | | — | | 8,697 | | 97,206 | |
M. Dolsten(4) | Pension Plan | 9 | 41,404 | | 532,437 | | — | | 41,404 | | 516,899 | |
| Supplemental Plan | | 431,209 | | 5,479,434 | | — | | 431,209 | | 5,288,936 | |
D. Lankler(5) | Pension Plan | 18 | 64,207 | | 669,827 | | — | | 52,681 | | 733,231 | |
| Supplemental Plan | | 237,457 | | 2,388,311 | | — | | 189,357 | | 2,607,312 | |
A. Hwang | Pension Plan | 21 | 86,987 | | 736,126 | | — | | 62,921 | | 854,809 | |
| Supplemental Plan | | 142,914 | | 1,207,599 | | — | | 103,374 | | 1,404,395 | |
(1)Messrs. Denton and Malik and Dr. Pao did not participate in these plans.
(2)Based on the December 31, 2023 assumptions used in determining our financial statement disclosure. See the “Summary Compensation Table” footnote, for the assumptions used. (3)The benefits shown for Dr. Bourla reflect an offset attributable to the value of 12,797,337 Greek drachma (GRD) in cumulative employer contributions that Pfizer made to the Greek TSAY (Greece Fund for Health Professionals), a government sponsored plan on his behalf from 1993 to 1999. For this purpose, benefits were converted from GRD to United States dollars (USD) using exchange rates (.003119) and (.003252) as of December 31, 2022 and December 31, 2023, respectively.
(4)The retirement benefits for Dr. Dolsten attributable to service prior to 2012 are based on the provisions of the Wyeth Sub-Plan formula and the Wyeth Supplemental Executive Retirement Plan formula.
(5)The retirement benefits for Mr. Lankler attributable to service prior to 2012 are based on the provisions of the Warner-Lambert Sub-Plan formula and the Warner-Lambert Company Supplemental Pension Income Plan formula.
The PCPP retains both the Pfizer and legacy company pension formulas, including: the Wyeth Retirement Plan U.S. (the Wyeth Sub-Plan), the Warner-Lambert Retirement Plan (the Warner-Lambert Sub-Plan) and the Pfizer Retirement Annuity Plan (the Pfizer Sub-Plan) formulas. Included are benefits earned under the related Supplemental Pension Plan, which includes both the Pfizer and legacy company pension formulas including the Wyeth Supplemental Executive Retirement Plan and the Warner-Lambert Company Supplemental Pension Income Plan (collectively, the Supplemental Plans). Pension benefits for all eligible U.S.-based colleagues, including the eligible NEOs, were provided under the Pension Plan and Supplemental Plan formulas.
For the purpose of computing the “Lump Sum Value” shown in the table (above), interest rates as of January 1, 2024 are 5.58% for annuity payments expected to be made during the first 5 years, 5.66% for payments after 5 and up to 20 years, and 5.56% for payments made after 20 years. For the portion of the Wyeth formula benefit accrued prior to January 1, 2012, the lump sum assumption as of January 1, 2024 is based on the Unisex 1994 Group Annuity Mortality table blended 50% Male and 50% Female, and an interest rate of 3.30%, which is based on PBGC rates derived from the 12-year spot rates from the full yield curve developed for purposes of calculating lump sums under IRC Section 417(e).
We have presented additional information in the table in lieu of a sub-table and to enhance the narrative that follows the table. We have included an additional column titled “Age 65 Single-Life Annuity Payment” in the table above. This column represents the amount payable upon attaining age 65 (or current age, if later), assuming termination of employment on December 31, 2023, except for Mr. Lankler whose Warner- Lambert Sub-Plan benefits were determined as of age 62 (the earliest unreduced retirement age under the Warner-Lambert Sub-Plan).
The immediately payable pension benefit and the lump sum value of that benefit for those NEOs who meet the criteria for benefit commencement under the Pension Plan are also listed in the table above. For Mr. Lankler, the benefit earned prior to 2012 is not payable as a lump sum, and thus the lump sums shown for this portion of his benefit are illustrative. The lump sum values for Mr. Lankler’s benefits based on the Warner-Lambert Sub-Plan formula are the present values of the 50% joint and survivor annuity.
| | | | | | | | |
80 | | Pfizer 2024 Proxy Statement |
THE PENSION PLAN AND SUPPLEMENTAL PENSION PLAN SUMMARY
The Pension Plan is a frozen, funded, tax-qualified, defined benefit pension plan and the Supplemental Pension Plan is a frozen, unfunded, non-qualified excess benefit plan both of which provide benefits to certain colleagues, including the NEOs, other than Messrs. Denton and Malik and Dr. Pao. The following table summarizes the terms of both pension plans, including the legacy plan benefits for our NEOs.
Pfizer’s Pension Plan(1)
| | | | | | | | | | | |
| Pfizer Benefits | Legacy Plan Benefits(2) |
| Name | All NEOs (other than Messrs. Denton and Malik and Dr. Pao) | Dr. Dolsten | Mr. Lankler |
| | | |
| | | |
| Time Frame | Frozen on December 31, 2017 | Pension benefits earned prior to January 2012 | Pension benefits earned prior to January 2012 |
| | | |
| | | |
| Plans | Pfizer Sub-Plan/ Pfizer Supplemental Pension Plan | Wyeth Sub-Plan/ Supplemental Plans | Warner-Lambert Sub-Plan/ Supplemental Plans |
| | | |
| | | |
| Pension Earnings | Highest five-calendar years’ average of salary and annual bonus(1) earned for the year (as of December 31, 2017). Benefits on earnings up to the tax code limit are included under the Pension Plan; benefits on excess earnings are accrued under the Supplemental Pension Plan | Highest five-years’ average of the last 10 years of salary and annual bonus paid during the year (as of December 31, 2017). Benefits on earnings up to the tax code limit are included under the Pension Plan; benefits on excess earnings are accrued under the Supplemental Pension Plan | Annual salary as of January 1st of the year and bonus paid during the year (through December 31, 2011). Benefits on earnings up to the tax code limit are included under the Pension Plan; benefits on excess earnings are accrued under the Supplemental Pension Plan |
| | | |
| | | |
| Formula | Greater of (1.4% of Pension Earnings) x (years of service); or (1.75% of Pension Earnings – 1.5% primary social security benefit) x (years of service) (as of December 31, 2017; capped at 35 years) | (2% of Pension Earnings –1/60th of annual primary social security benefit as of December 31, 2017) x (years of service) (as of December 31, 2011, capped at 30) | (1.5% of Pension Earnings) + (Monthly flat dollar benefit per year of service) (through December 31, 2011) |
| | | |
| | | |
| Form of Payment | Annuity or Lump sum | Annuity or Lump sum | Annuity |
| | | |
(1)Bonuses other than the annual short-term incentive are not included in Pension Earnings under any of the plans.
(2)Dr. Bourla’s prior pension benefit from 1993 to 1999 accrued under the government sponsored Greek TSAY (Greece Fund for Health Professionals), which is not a Pfizer sponsored program and as such is not listed. Dr. Dolsten has both a Pfizer Sub-Plan and Wyeth Sub-Plan benefit. Mr. Lankler has both a Pfizer Sub-Plan and a Warner-Lambert Sub-Plan benefit.
General
Contributions to the Pension Plan are made entirely by Pfizer and are paid into a tax-exempt trust from which benefits are paid. Qualified pension plans, such as the frozen Pension Plan, limit the annual earnings that may be considered in calculating benefits and the maximum annual pension benefit. The frozen Supplemental Plan (non-qualified plan), provides, out of Pfizer’s general assets, benefits which are substantially equal to the difference between the benefits which would have been paid in the absence of these IRC limits and the amount that may be provided under the Pension Plan. The Supplemental Plans are unfunded; however, in certain circumstances Pfizer or a predecessor company established and funded trusts to provide for obligations under the Supplemental Plans.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 81 |
Early Retirement Provisions
Under the Pfizer Sub-Plan and Pfizer Supplemental Plan, the normal retirement age is 65. Colleagues may begin benefits earlier, subject to the following provisions:
•If a colleague terminates employment where the sum of their age and years of service equals or exceeds 90, the colleague is entitled to receive an unreduced early payment of either an annuity or an equivalent lump sum. Dr. Bourla attained this milestone during 2022.
•If a colleague retires on or after age 55 having 10 or more years of service, the colleague may elect to receive either an early retirement annuity or lump sum payment, reduced by 4% per year for each year (prorated for partial years) that the benefit commences between the benefit commencement date and age 65. Dr. Dolsten attained this milestone in 2018. Ms. Hwang and Mr. Lankler attained this milestone during 2020.
If a colleague with a vested benefit does not satisfy any of the above criteria, the colleague may elect to receive an annuity starting on or after age 55, reduced by 6% per year for each year (prorated for partial years) prior to age 65, certain exclusions apply.
Under the Warner-Lambert Sub-Plan and Warner-Lambert Supplemental Plan, the normal retirement age is 65. Colleagues may begin benefits earlier, subject to the following provision:
•If a colleague retires on or after age 55 having 5 or more years of service, the colleague may elect to receive an early retirement annuity (or lump sum, if elected within 6 months of termination) on an unreduced basis beginning at age 62 or on a reduced basis prior to age 62 reflecting a reduction of 3% per year for each year (prorated for partial years) between the later of age 60 or the benefit commencement date and age 62 and by 6% per year for each year (prorated for partial years) that the benefit commences between the benefit commencement date (if earlier than age 60) and age 60. Mr. Lankler attained this milestone during 2020.
Under the Wyeth Sub-Plan and Wyeth Supplemental Executive Retirement Plan, the normal retirement age is 65. Colleagues may begin benefits earlier, subject to the following provisions:
•If a colleague retires on or after age 55 having 10 or more years of service, the colleague may elect to receive either an early retirement annuity or lump sum payment, reduced by 3% per year for each year (prorated for partial years) that the benefit commences between the benefit commencement date and age 65. Dr. Dolsten attained this milestone in 2018.
If a colleague with a vested benefit does not satisfy the above criteria, the colleague may elect to receive an annuity or lump sum payment starting on or after age 55, reduced actuarially using a 7.50% interest rate and the GATT2003 mortality table, for the period from age 65 to the benefit commencement date.
| | | | | | | | |
82 | | Pfizer 2024 Proxy Statement |
2023 NON-QUALIFIED DEFERRED COMPENSATION TABLE(1)
This table summarizes activity during 2023 and account balances in our various non-qualified savings and deferred compensation plans for our NEOs. The PSSP and DCP plans permit the executives to defer eligible earnings on a pre-tax basis. Other than employer contributions to the PSSP, the account balances in these plans are generally attributable to employee deferrals of previously earned compensation and the earnings on amounts in the plans. In addition to employee deferrals, generally, the PSSP has two types of company contributions: company matching contributions and the RSC, described in more detail below. The PSSP is a non-qualified supplemental savings plan that provides for the deferral of compensation, related company matching contributions based on the executive’s contributions and the RSC that otherwise could have been made under the related tax-qualified PSP but for the application of certain IRC limitations. In addition, the PSSP accepts transfers of Post-2004 Supplemental Pension Plan benefits into the plan which are treated as re-deferrals as required by IRC Section 409A.
| | | | | | | | | | | | | | | | | | | | |
| Name | Plan(2) | Executive
Contributions in
2023 ($) | Pfizer Contributions in 2023 ($)(3) | Aggregate Earnings in
2023 ($) | Aggregate
Withdrawals/
Distributions ($) | Aggregate Balance at 12/31/2023 ($)(4) |
| A. Bourla | PSSP | 637,525 | | 1,257,563 | | 20,776 | | — | | 25,707,209 | |
| Deferred PSA | — | | — | | (5,495,465) | | — | | 7,920,119 | |
| Deferred RSU | 32,873 | | — | | (1,352,123) | | — | | 1,944,878 | |
| Total: | 670,398 | | 1,257,563 | | (6,826,812) | | — | | 35,572,206 | |
| D. Denton | PSSP | 130,390 | | 348,611 | | 1,944,237 | | — | | 11,595,002 | |
| Total: | 130,390 | | 348,611 | | 1,944,237 | | — | | 11,595,002 | |
| M. Dolsten | PSSP | 287,848 | | 649,309 | | 4,880 | | — | | 6,097,264 | |
| Deferred RSU | 125,413 | | — | | (951,322) | | — | | 18,264,829 | |
| Total: | 413,261 | | 649,309 | | (946,442) | | — | | 24,362,093 | |
| D. Lankler | PSSP | 187,995 | | 424,638 | | (11,790) | | — | | 6,053,423 | |
| Deferred RSU | 19,979 | | — | | (4,839) | | — | | 15,140 | |
| Total: | 207,974 | | 424,638 | | (16,629) | | — | | 6,068,563 | |
| A. Malik | PSSP | 955,731 | | 466,349 | | 639,188 | | — | | 4,041,703 | |
| Deferred GPP | 1,514,301 | | — | | 282,072 | | — | | 1,796,373 | |
| Total: | 2,470,032 | | 466,349 | | 921,260 | | — | | 5,838,076 | |
| A. Hwang | PSSP | 236,813 | | 534,480 | | (895,756) | | — | | 2,599,910 | |
| Deferred PSA | — | | — | | (76,817) | | — | | 110,710 | |
| Total: | 236,813 | | 534,480 | | (972,573) | | — | | 2,710,620 | |
| W. Pao | PSSP | 125,101 | | 65,877 | | 567,132 | | — | | 3,592,559 | |
| Total: | 125,101 | | 65,877 | | 567,132 | | — | | 3,592,559 | |
(1)Contribution amounts in this table have been reflected in the “Summary Compensation Table” and prior years’ summary compensation tables, as applicable (based on the year contributions were earned). Aggregate earnings are not reflected in the “Summary Compensation Table” and were not reflected in prior years’ summary compensation tables. For Dr. Bourla’s contributions, see Footnote 4 below. (2)The PSSP contributions were based on the executive’s deferral election and the salary shown in the “Summary Compensation Table,” as well as annual incentive awards paid in 2023, previously reported in 2022. For Dr. Dolsten, the reported PSSP values include legacy Wyeth Supplemental Employee Savings Plan (Wyeth SESP) earnings and balances that he participated in prior to 2012. The Wyeth SESP is an unfunded, non-qualified supplemental savings plan. A rabbi trust was established to meet all or a portion of our obligations under the Wyeth SESP. (3)Represents PSSP company matching contributions and RSC earned in 2023 and reported in the “Summary Compensation Table” under the “All Other Compensation” column. (4)Amounts reported in the Aggregate Balance column reflect the cumulative value of the NEOs’ account balances, including employee contributions, company matching contributions/RSC, other employer contributions, withdrawals and investment earnings thereon as of December 31, 2023. The amounts reported do not include the fourth quarter 2023 matching contributions or the RSC earned in 2023 because they were credited in early 2024; such amounts are included under the “Pfizer Contributions in 2023” column and in the “Summary Compensation Table” under the “All Other Compensation” column (based on the year contributions were earned). Additionally, the amount in the “Aggregate Balance” column reported for Dr. Bourla includes a one-time notional transfer of $12,500,004 from the post-2004 Supplemental Pension Plan benefit to the PSSP in August 2022. | | | | | | | | |
2024 Proxy Statement Pfizer | | 83 |
PFIZER SAVINGS PLANS
General
U.S.-based colleagues (including the NEOs) who meet the eligibility requirements may elect to participate in the PSP and the PSSP. Employer matching contributions and RSC amounts, if applicable, are reflected in the “All Other Compensation” column of the “Summary Compensation Table” or prior years’ summary compensation tables, as applicable. Note that investment earnings have not been included in the “Summary Compensation Table.” Savings Plan
The PSP is a tax-qualified retirement savings plan into which participating colleagues may contribute a percentage of their salary and bonus (regular earnings).
| | | | | | | | | | | | | | |
| Participants | Employee
Contributions | Company Matching Contributions | Timing | Tax Law Restrictions |
| | | | |
| All NEOs | Up to 30% of “regular earnings” on a pre-tax basis, Roth basis and/or after-tax basis subject to IRC earnings cap of $330,000 | Matching contributions are equal to 100% of the first 3% of “regular earnings” contributed and 50% of the next 3% of “regular earnings” contributed | Immediately vested; matching contributions are made shortly after the end of each quarter provided the employee is employed at the end of each quarter, unless the employee terminated employment due to retirement, death or disability. Distributable as a lump sum or in partial payments | “Annual Additions”* limited to $66,000 ($73,000 if over age 50) Elective annual deferrals (pre-tax/Roth basis) limited to $22,500 ($30,000 if over age 50) |
| | | | |
* Includes matching contributions, RSC, pre-tax contributions, Roth contributions and after-tax contributions.
Retirement Savings Contribution
| | | | | | | | |
| Participants | Company Contributions | Timing |
| | |
| All NEOs | Age- and service-weighted annual company contribution from 5% to 9% of “regular earnings” which is vested after three years of service. (9% contribution when age and service equals or exceeds 65) | Made early in the following year but only if the employee is employed on December 31st of the respective year, unless the employee terminated employment due to retirement, death or disability. Subject to three-year cliff vesting |
| | |
Supplemental Savings Plan
The PSSP provides employees the opportunity to make contributions and receive the crediting of company contributions equal to the difference between the amount that would have been allocated to an employee’s account, if the IRC limits described above under “Savings Plan — Employee Contributions” and “Savings Plan — Tax Law Restrictions” columns did not exist and the amount was actually allocated under the PSP.
| | | | | | | | | | | | | | |
| Participants | Employee
Contributions | Company Contributions | Timing | Form of Payment |
| | | | |
| All NEOs | May contribute up to 30% of “regular earnings” on a pre-tax basis | Matching contributions and RSC: Same as PSP above | Same as PSP above | Lump sum (default) or in 2 to 20 annual installments (as elected) following termination from service |
| | | | |
| | | | | | | | |
84 | | Pfizer 2024 Proxy Statement |
ESTIMATED PAYMENTS AND BENEFITS UPON TERMINATION TABLE
The following table shows the estimated payments and benefits payable upon a hypothetical termination of employment under the Executive Severance Plan and other plans and programs that provide for benefits upon certain terminations, under the various termination scenarios as of December 31, 2023, based upon the closing price of our common stock on December 29, 2023 (the last trading date in the year).
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Termination Without
Cause | | Termination on
Change in Control | | Death or
Disability |
| Name | Severance(1) (A)($) | Other(2) (B)($) | Long-Term Award Payouts(3) (C)($) | Total
(A+B+C)($) | | Long-Term Award Payouts(4) (D)($) | Total
(A+B+D)($) | | Long-Term Award Payouts(4) ($) |
| A. Bourla | 10,696,154 | | 54,548 | | 12,928,368 | | 23,679,070 | | | 17,856,307 | | 28,607,009 | | 17,856,307 | |
| D. Denton | 2,625,000 | | 36,557 | | 2,013,848 | | 4,675,405 | | | 3,676,514 | | 6,338,071 | | 3,676,514 | |
| M. Dolsten | 3,596,000 | | 51,994 | | 4,774,303 | | 8,422,297 | | | 6,360,891 | | 10,008,885 | | 6,360,891 | |
D. Lankler | 3,766,522 | | 54,667 | | 2,865,428 | | 6,686,617 | | | 3,710,542 | | 7,531,731 | | 3,710,542 | |
| A. Malik | 2,620,800 | | 54,548 | | 2,537,265 | | 5,212,613 | | | 4,089,174 | | 6,764,522 | | 4,089,174 | |
A. Hwang(5) | 4,748,100 | | 54,548 | | 3,684,116 | | 8,486,764 | | | 4,770,676 | | 9,573,324 | | 4,770,676 | |
Note: As Dr. Pao ceased serving as an executive officer on July 27, 2023 and terminated employment on August 15, 2023, he is not shown in the table above. In accordance with the terms of the Executive Severance Plan, Dr. Pao received or will receive (i) cash severance equal to $2,394,000 (representing one year’s base salary and target bonus, based on the Executive Severance Plan formula), (ii) 24 months of active employee medical, dental and life insurance coverage, (iii) reimbursement of qualifying financial counseling expenses up to $15,000 in 2023 and $8,750 for expenses through August 15, 2024, and (iv) treatment for outstanding long-term incentive awards held at termination in accordance with the terms of the grants. Based on the closing stock price of $35.39 on August 15, 2023, the equity awards had a value of approximately $3.0 million. The foregoing severance benefits were subject to Dr. Pao’s execution and non-revocation of a release of claims against the company and continued compliance with restricted covenants and confidentiality obligations. For additional details on Dr. Pao’s severance, see the “Leadership Transition” section elsewhere in this Proxy Statement. (1)Severance equal to the greater of: (a) one year’s pay (base salary and target bonus); or (b) 13 weeks’ pay plus 3 weeks’ pay per full year of service, subject to a maximum of 104 weeks. These amounts do not include a bonus payment for the year of termination, if any, under the GPP. In general, under the terms of the GPP, in effect as of December 31, 2023, the individual may receive a pro-rata bonus for the year of termination in the company’s discretion (including a lower than target or no bonus amounts).
(2)The company’s cost of 24 months of medical, vision, dental and life insurance coverage with employee paying active employee rates. The amounts vary based on the coverages selected.
(3)The value of the long-term incentive awards that the participants would have been entitled to as of December 31, 2023 upon a termination without Cause.
For retirement eligible participants (all NEOs other than Messrs. Denton and Malik), all unvested awards, granted in 2022 and earlier (excluding off-cycle and supplemental awards), will continue to vest and will be distributed according to the original terms of the award. Awards granted in 2023 would prorate and settle on the original settlement date. For Messrs. Denton and Malik, their RSUs (received as “make-whole awards” upon joining Pfizer) would vest and distribute and their TSRUs and PSAs would prorate and settle on the original settlement date.
(4)These amounts represent the value of the long-term incentive awards that the participants would have been entitled to as of December 31, 2023 upon a termination on the date of a change in control, death or disability. For awards granted in 2016 and later, in all cases other than death for which the awards vest and settle, the awards will continue to vest and will be settled on the original settlement date.
(5)As further described in the “Leadership Transition” section elsewhere in this Proxy Statement, Ms. Hwang ceased serving as executive officer on December 15, 2023 and is currently serving as Advisor to the CEO continuing until a date to be determined. Upon termination of her employment at the conclusion of the transition period, Ms. Hwang will be involuntarily terminated without cause due to her position being eliminated, and at such time, she will be eligible for a prorated annual incentive award for 2024 under the terms of the GPP, severance and other benefits pursuant to the Executive Severance Plan and other plans and programs that provide for benefits upon an involuntary termination. | | | | | | | | |
2024 Proxy Statement Pfizer | | 85 |
POTENTIAL PAYMENTS UPON DISABILITY, DEATH, RETIREMENT OR CHANGE IN CONTROL
The NEOs are eligible for the following potential payments upon terminations due to disability, death, retirement or a termination following a change in control (subject to the plan provisions):
| | | | | |
| Disability | |
| |
| Benefits Program | •Company-paid long-term disability benefit equal to 50% of pay (salary and bonus) with optional employee purchase of 60% or 70% of pay. Covered pay maximum $500,000. Individual supplemental policy, if employee has purchased it, may provide a higher coverage. •Health and life insurance benefits for 24 months for those who are approved to receive long-term disability benefits due to an injury or illness. •Savings Plan and Supplemental Savings Plan contributions will cease for those who are terminated due to disability (after short-term disability ends). |
| Long-Term Incentive Program | •Vested TSRUs/PTUs will settle on the original settlement date. •Unvested TSRUs will continue to vest and settle on the original settlement date. •PSAs will continue to vest and settle based on the actual performance at the end of the performance period. •RSUs will continue to vest and be paid according to the original vesting schedule. |
| |
| Death | |
| |
| Benefits Program | •Life insurance death benefits of one times pay (salary plus bonus) with a maximum death benefit of $2.0 million. •Additional death benefits of up to eight times pay (salary plus bonus), if the employee purchased additional coverage with a maximum supplemental death benefit of $4.0 million. •Upon the death of an employee, pension and savings plan benefits and deferred compensation are payable in accordance with the terms of the plans and the executive’s prior elections (if any). Additionally, health insurance coverage continues for family members at no cost for three months, and afterwards either COBRA or retiree medical coverage (if eligible) is available. |
| Long-Term Incentive Program | •Vested TSRUs/PTUs are immediately settled. •Unvested TSRUs are vested and settled. •PSAs immediately vest and are paid out at target. •RSUs immediately vest and are paid in full. |
| |
| Retirement | |
| |
| Benefits Program | |
| Long-Term Incentive Program | If a participant retires after attaining either age 62 with 5 years of continuous and uninterrupted service (for annual grants starting in 2022) or 55 with 10 years of continuous and uninterrupted service, both measured from the most recent hire date, or after attaining age and years of service totaling 90 or more after the first anniversary of the grant date: •Vested TSRUs/PTUs will be settled on the original settlement date. •Unvested TSRUs continue to vest and will be settled on the original settlement date. •PSAs will continue to vest and will be settled based on the actual performance at the end of the performance period. •RSUs (other than off-cycle grants) will continue to vest and be paid at the end of the original vesting schedule. ◦Off-cycle grants are typically forfeited. Generally, if retirement occurs prior to the first anniversary of the grant date, the unvested portion of these long-term incentive awards is forfeited. Based on age and years of service, all active NEOs (excluding Messrs. Denton and Malik) are currently eligible for retirement treatment and had long-term incentive awards with a value of $10,273,855 for Dr. Bourla, $3,615,132 for Dr. Dolsten, $2,530,612 for Mr. Lankler and $3,253,644 for Ms. Hwang, as of December 31, 2023 had they retired on that date. These amounts do not include $4,076,627 for Dr. Bourla, $6,493,576 for Dr. Dolsten, $1,555,028 for Mr. Lankler and $1,031,950 for Ms. Hwang, representing the current value of their vested but unsettled TSRUs (and PTUs, as applicable) as of December 31, 2023. The actual amount received by these NEOs for their long-term incentive awards will be determined on the settlement date (in respect of TSRUs, PTUs and PSAs) based on the values at the respective time and is not tied to retirement or other separation from service. |
| |
| Change in Control |
| |
| Long-Term Incentive Program | If a participant’s employment is terminated other than for Cause within 24 months following a change in control: •Vested TSRUs/PTUs will settle on the original settlement date. •Unvested TSRUs will continue to vest and settle on the original settlement date. •PSAs will continue to vest and are settled based on the actual performance at the end of the performance period. •RSUs will continue to vest and be paid according to the original vesting schedule. |
| |
| | | | | | | | |
86 | | Pfizer 2024 Proxy Statement |
CEO Pay Ratio
The 2023 annual total compensation for Dr. Bourla was 291 times the annual total compensation of the median-paid employee as follows:
| | | | | | | | |
| Annual Total Compensation | |
| Albert Bourla: | $21,562,064 | | (1) |
| Median-Paid Employee | $74,008 | | (2) |
| Ratio | 291:1 | |
(1)As reported in the “Total” column of the Summary Compensation Table.
(2)Cash compensation (including overtime pay) of $66,427; equity of $0; change in pension of $0 plus all other compensation of $7,581.
The annual total compensation for purposes of the pay ratio was determined using the requirements for the SCT. As permitted under the SEC disclosure rules, the same median-paid employee selected for the 2023 Proxy Statement has been identified as the median-paid employee for the second year in this 2024 Proxy Statement, as there were no significant changes in our employee population (as of November 1, 2023) or to the median-paid employee’s compensation arrangements in 2023 that would significantly affect the pay ratio disclosure. As the median-paid employee was located outside of the U.S., the annual total compensation was converted to U.S. dollars using the spot exchange rate as of the last business day of the year (December 29, 2023).
To identify the median-paid employee for the 2023 Proxy Statement (who is also the median-paid employee for the 2024 Proxy Statement), we took the following steps:
1.Calculated the annual total cash compensation (annual base salary rate + actual incentive bonus paid during the prior 12 months, if applicable) for all employees of the company as of November 1, 2022. We believe that annual total cash compensation is a consistently applied compensation measure at Pfizer and most appropriate for determining the median-paid employee, as annual LTI awards are not granted widely to employees. We used actual annual total cash compensation (converted to USD based on the foreign exchange rate in effect on the last day of the prior month (October 31, 2022)), and did not make any assumptions or adjustments to the amounts determined.
2.The median-paid employee is selected by ranking the annual total cash compensation from lowest to highest of all employees (excluding the CEO, but including the other NEOs, full-time and part-time employees and employees on leave). In the event that there are multiple employees with compensation that is substantially similar to the median total cash compensation, this subset data is resorted by employee identification numerical order. The median employee from this subset with the substantially similar median compensation ultimately will be deemed to be the median-paid employee.
Pay–Versus–Performance Table (2020-2023)
The following table reports the compensation of our Principal Executive Officer (PEO or referred to herein as CEO) and the average compensation of the other non-CEO NEOs as reported in the Summary Compensation Table for the past four fiscal years, as well as Compensation Actually Paid (CAP) as calculated under the SEC Pay-Versus-Performance (PVP) disclosure requirements and certain performance measures required by the rules. The disclosure covers our four most-recent fiscal years, which will expand next year to a rolling five years. Dollar amounts reported as CAP are computed in accordance with Item 402(v) of Regulation S-K, and our Board believes that it is important to recognize that these amounts do not reflect the actual amount of compensation earned by or paid to our CEO and NEOs during the applicable fiscal year.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Summary Compensation Table (SCT) Total for CEO*
($) | Compensation Actually Paid to CEO(1)/(2)/(3) ($) | Average SCT Total for (non-CEO) NEOs*
($) | Average Compensation Actually Paid to (non-CEO) NEOs(1)/(2)/(3) ($) | | Value of initial fixed $100 investment based on | | (GAAP) | | | Company Selected Measure
(Non-GAAP) | |
Year
| | TSR (Pfizer) ($) | TSR (Peer Group)** ($) | | Net Income ($B) | | | Adj. Net Income***
($B) | |
| 2023 | 21,562,064 | | (62,146,536) | | 6,330,278 | | (9,473,859) | | | 91 | 156 | | 2.12 | | | 11.24 | | |
| 2022 | 33,017,453 | | 5,662,152 | | 14,842,288 | | 8,437,687 | | | 154 | 144 | | 31.37 | | | 39.12 | | |
| 2021 | 24,353,219 | | 115,175,594 | | 9,289,461 | | 40,940,768 | | | 172 | 134 | | 21.98 | | | 25.24 | | |
| 2020 | 21,033,570 | | 29,667,753 | | 9,599,590 | | 11,911,035 | | | 103 | 109 | | 9.16 | | | 16.73 | | |
(Amounts are subject to rounding.)
| | | | | | | | |
2024 Proxy Statement Pfizer | | 87 |
* SCT Total. As noted earlier in this Proxy Statement, reflects the grants made during the year for which the applicable performance goals have been set under GAAP rules. The accounting rules provide that PSAs are deemed granted and therefore included in the SCT when the applicable goals are set; therefore, one-third of the PSAs is included in the SCT Total in each of the three performance years as a result of the use of three, separately established annual goals. Additionally, for 2022, equity awards reported in the SCT includes the “make-whole awards” for Mr. Denton and Dr. Pao in connection with their hiring, as detailed in the Leadership Transition section in the 2023 Proxy Statement. For 2023, the average amount shown includes Dr. Pao’s pro-rata target bonus award ($691,939) payable in accordance with the terms of the GPP in effect at the time of his involuntary termination.
** Peer Group TSR. Represents the DRG Index (NYSE ARCA Pharmaceutical Index) peer group.
*** Adjusted Net Income. Results used for PSA purposes. Adjusted Net Income is defined as U.S. GAAP net income attributable to Pfizer Inc. common shareholders before the impact of amortization of intangible assets, certain acquisition-related items, discontinued operations and certain significant items; and is adjusted to reflect budgeted FX rates for the year and further refined to exclude certain other unbudgeted or non-recurring items including acquired in-process research and development expenses.
(1)To calculate CAP, as defined by the SEC, the following deductions and additions were made to the SCT Total compensation:
CEO — Summary Compensation Table Total to CAP Reconciliation
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Year | CEO/Principal Executive Officer (PEO) | Reported Summary Compensation Table (SCT) Total ($) | Deductions: Reported Value of Stock and Option Awards ($) | Deductions: Reported Change in the Actuarial Present Value of Pension ($) | SCT Adjusted Total
($) | Fair Value of Grant During the Year at 12/31 ($) | Change in Fair Value of Prior Years’ Awards (Unvested at 12/31) ($) | Change in Fair Value of Prior Years’ Awards that Vested During Applicable Year ($) | CAP to CEO ($) |
| | A | B | C | D=A-B-C | E | F | G | H=D+E+F+G |
| 2023 | Bourla | 21,562,064 | 17,506,870 | 8,440 | 4,046,754 | 2,676,420 | | (54,869,053) | | (14,000,657) | | (62,146,536) | |
| 2022 | Bourla | 33,017,453 | | 18,822,635 | | 2,473,747 | 11,721,071 | | 23,195,458 | | (19,441,173) | | (9,813,204) | | 5,662,152 | |
| 2021 | Bourla | 24,353,219 | 13,231,457 | 49,901 | 11,071,861 | 47,742,308 | | 57,799,698 | | (1,438,273) | | 115,175,594 | |
| 2020 | Bourla | 21,033,570 | 11,680,768 | 1,367,780 | 7,985,022 | 20,171,525 | | 2,516,212 | | (1,005,006) | | 29,667,753 | |
(Amounts are subject to rounding.)
Average Non-CEO NEOs — Summary Compensation Table Total to CAP Reconciliation
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Year
| Non-CEO NEOs(i)
| Reported Summary Compen-sation Table (SCT) Total ($) | Deductions: Reported Value of Stock and Option Awards ($) | Deductions: Reported Change in the Actuarial Present Value of Pension ($) | SCT Adjusted Total
($) | Fair Value of Grant During the Year at 12/31 ($) | Change in Fair Value of Prior Years’ Awards (Unvested at 12/31) ($) | Change in Fair Value of Prior Years’ Awards that Vested During Applicable Year ($) |
Avg. CAP to (non-CEO) NEOs ($) |
| A | B | C | D=A-B-C | E | F | G | H=D+E+F+G |
| 2023 | Denton, Dolsten, Lankler, Malik, Hwang, and Pao(ii) | 6,330,278 | | 3,903,883 | | 126,204 | | 2,300,191 | | 583,665 | | (9,788,919) | | (2,568,796) | | (9,473,859) | |
| 2022 | Denton, Pao, Dolsten, Hwang, and D’Amelio(ii) | 14,842,288 | | 6,029,494 | | — | | 8,812,794 | | 6,608,542 | | (4,530,369) | | (2,453,280) | | 8,437,687 | |
| 2021 | D’Amelio, Dolsten, Hwang, Lankler and Young(ii) | 9,289,461 | | 4,583,167 | | 53,838 | | 4,652,456 | | 16,368,787 | | 20,441,286 | | (521,762) | | 40,940,768 | |
| 2020 | D’Amelio, Dolsten, Hwang, and Young | 9,599,590 | | 4,416,359 | | 1,050,895 | | 4,132,336 | | 7,370,367 | | 1,057,547 | | (649,214) | | 11,911,035 | |
(Amounts are subject to rounding.)
(i) Except for Messrs. Denton and Malik and Dr. Pao, the NEOs met the criteria for retirement treatment on their equity awards for the respective year.
(ii) For 2023, Ms. Hwang and Dr. Pao were no longer serving as executive officers at fiscal-year end (former CCO and CDO, respectively). For 2022, Mr. D’Amelio was no longer serving as an executive officer at fiscal year-end (former CFO). For 2021, Mr. Young was no longer serving as an executive officer at fiscal year-end (former Group President, Chief Business Officer).
(2)The Monte Carlo Simulation used to determine values for the TSRUs uses the valuation date (or the prior business day where the valuation date falls on the weekend or holiday) assumptions of: stock price, expected dividend yield, risk-free interest rate, and stock price volatility, as determined in accordance with ASC Topic 718. However, for outstanding awards granted prior to the Upjohn/Mylan transaction (closed November 2020) the valuations of these grants utilized an adjusted grant price which factored in the value of the dividends prior to the close, consistent with what shareholders would have already received. The PSAs valuation methodology utilized the equity intrinsic value accounting, with the applicable performance conditions applied and dividend equivalents accrued based on the applicable performance conditions. For Mr. Denton and Dr. Pao’s RSU “make-whole awards”, dividend equivalent units are accumulated during the vesting period, at the applicable dividend dates, and reinvested as additional RSUs which are settled in shares with the underlying RSUs on the vesting date.
(3)As the pension plan was frozen, no service cost was included in the calculation of CAP. Additionally, dividends are not paid on PSAs until they settle and then solely on the earned shares.
| | | | | | | | |
88 | | Pfizer 2024 Proxy Statement |
CEO AND AVERAGE NON-CEO NEO CAP PAY-VERSUS-PERFORMANCE
The following illustrates the relationship between the CAP of our CEO and average non-CEO NEOs (Avg. NEO) and company performance as well as peer performance.
•The 4-year compensation history of the CEO and Avg. NEO shows that the disclosed CAP aligns with Pfizer’s TSR which outperformed the DRG Index TSR for 2022 and 2021; tracked the DRG Index for 2020 and underperformed for 2023. The values are based on a $100 investment made on December 31, 2019.
•Considering the significant weighting of long-term stock-based incentives in our pay mix (75%-80% for the CEO and 60%-70% on average for the NEOs, of target total direct compensation) due to the intended alignment between the financial interests of our executives and shareholders, the CAP values are significantly influenced by Pfizer’s stock price. This is illustrated by the impact of year-over-year stock price declines since 2021. When comparing year-end stock prices in 2021 ($59.05) to 2022 ($51.24) to 2023 ($28.79), there was a reduction of approximately 13% for 2022 vs. 2021 ($51.24 vs. $59.05), and 44% for 2023 vs. 2022 ($28.79 vs. $51.24) respectively, which resulted in a significant decline in CAP values for 2022 and 2023. The significant weighting of long-term stock-based incentives in our mix is further demonstrated when the stock price increases, when comparing the 2020 year-end stock price of $36.81 to the 2021 year-end stock price of $59.05, which is an approximate 60% increase. This stock price increase resulted in a year-over-year change in value of outstanding long-term incentives which is reflected in the increase in the CAP value in 2021 compared to the CAP value in 2020.
The graphs below show the alignment between pay versus performance.
CEO and Average NEOs CAP versus TSR Performance*
* TSR value based on $100 investment of Pfizer versus DRG Index as of December 31, 2019.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 89 |
CEO and Average NEOs CAP versus Net Income (GAAP)
CEO and Average NEOs CAP versus Adjusted Net Income (Non-GAAP)
Overall, the Committee believes the executive compensation program strikes an appropriate balance between incentivizing our executives based on performance, as well as utilizing market competitive pay practices. This is also evidenced by the performance metrics the Committee selected to link pay with performance as described in the section below. See our “Compensation Discussion and Analysis” for additional information regarding Pfizer’s pay-for-performance executive compensation program. | | | | | | | | |
90 | | Pfizer 2024 Proxy Statement |
COMPANY PERFORMANCE METRICS
Pfizer’s executive compensation program appropriately aligns pay and performance as the Committee seeks to utilize metrics that incentivize and strengthen our alignment to the Compensation Philosophy, as well as our focus on long-term sustainable growth. The metrics (non-GAAP) listed below are the performance metrics the Committee deems as the most important financial performance measures used to link compensation actually paid to our NEOs to the company’s performance for the most recently completed fiscal year, as further described in our Compensation Discussion and Analysis within the sections titled “2023 Annual Incentive Award/Global Performance Plan (GPP)” and “2023 Annual Long-Term Incentive Award Program”. | | |
| Most Important Performance Measures |
| Adjusted Net Income |
| Total Revenue |
| Adjusted Diluted EPS |
| Cash Flow from Operations |
CEO REALIZED PAY TABLE (SUPPLEMENTAL)
The supplemental table and graphs below compare the realized pay for Dr. Bourla over four years with the disclosed SCT Total and CAP.
| | | | | | | | | | | | | | |
Year
| SCT Total
($) | CAP
($) | | Realized Pay(1) ($) |
| 2023 | 21,562,064 | | (62,146,536) | | | 25,724,089 | |
| 2022 | 33,017,453 | | 5,662,152 | | | 26,621,180 | |
| 2021 | 24,353,219 | | 115,175,594 | | 16,676,919 | |
| 2020 | 21,033,570 | | 29,667,753 | | 9,986,957 | |
(Amounts are subject to rounding.)
(1)Realized Pay is defined as base salary, short-term incentive bonus paid on account of the performance year, and payouts/settlements of long-term incentive awards during the applicable year.
| | | | | | | | | | | | | | | | | |
Year
| Salary
($) | Bonus
($) | LTI Settlements
($) | | Realized Pay
($) |
| 2023 | 1,787,500 | | 0 | 23,936,589 | | | 25,724,089 | |
| 2022 | 1,737,500 | | 7,650,000 | | 17,233,680 | | | 26,621,180 | |
| 2021 | 1,687,500 | | 8,000,000 | 6,989,419 | | | 16,676,919 | |
| 2020 | 1,650,000 | | 5,491,800 | 2,845,157 | | | 9,986,957 | |
(Amounts are subject to rounding.)
| | | | | | | | |
2024 Proxy Statement Pfizer | | 91 |
Supplemental Table: Realized Pay Comparison
The graph above illustrates our CEO’s realized pay compared to the CAP and SCT Total for 2020-2023. For 2023, the year-end stock price declined from 2022, therefore resulting in a negative CAP value attributable to the decline in the value of outstanding equity awards. The CAP value differs significantly from the realized pay and SCT Total.
| | | | | | | | |
92 | | Pfizer 2024 Proxy Statement |
This table provides certain information as of December 31, 2023 with respect to our equity compensation plans:
EQUITY COMPENSATION PLAN INFORMATION
| | | | | | | | | | | | | | | | | |
| Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (A) | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights
(B) | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (A)) (C) |
| Equity compensation plans approved by security holders | 259,458,729 | | (1) | $36.22 | 156,478,884 | |
|
| Equity compensation plans not approved by security holders | 0 | | N/A | 91,351,643 | | (2) |
| Total | 259,458,729 | | $36.22 | 247,830,527 | | (3) |
(1)This amount includes the following (subject to rounding):
•28,452,100 shares to be issued upon the exercise of outstanding stock options, of which 9,697,389 were granted from the Pfizer Inc. 2004 Stock Plan, as amended and restated (the 2004 Stock Plan) with a weighted-average exercise price of $30.62, 15,457,834 were granted from the Pfizer Inc. 2014 Stock Plan (the 2014 Stock Plan) with a weighted-average exercise price of $33.16, and 3,296,877 were granted from the Pfizer Inc. 2019 Stock Plan (2019 Stock Plan) with a weighted-average exercise price of $36.31.
•4,733,569 (maximum) Performance Share Awards (PSAs) to be issued, but not yet earned as of December 31, 2023, which were granted under the 2019 Stock Plan. The number of shares (to inactive participants) or cash (to active participants) at vesting, if any, to be issued pursuant to such outstanding awards will be determined upon the achievement of predetermined goals related to two measures: Adjusted Net Income over three one-year periods and the relative three-year TSR as compared to the DRG Index. Since these awards have no exercise price, they are not included in the weighted-average exercise price calculation in column (B).
•35,797,257 (maximum) Portfolio Performance Shares (PPSs) to be issued, but not yet earned as of December 31, 2023, of which 5,584,155 were granted from the 2014 Stock Plan and 30,213,102 were granted from the 2019 Stock Plan. The number of shares, if any, to be issued pursuant to such outstanding awards will be determined upon the achievement of predetermined goals related to Pfizer’s long-term product portfolio during a three or five-year performance period, as applicable, from the year of the grant date. Since these awards have no exercise price, they are not included in the weighted-average exercise price calculation in column (B).
•25,843,539 shares subject to RSUs of which 385 were granted under the 2014 Stock Plan and 25,843,154 were granted from the 2019 Stock Plan. Since these awards have no exercise price, they are not included in the weighted-average exercise price calculation in column (B).
•163,572,245 TSRUs not yet settled as of December 31, 2023. This includes the following shares granted under our stock plans:
| | | | | | | | | | | | | | |
| Pfizer Stock Plans | Vested TSRUs | Weighted–Average
Grant Price | Non–Vested TSRUs | Weighted–Average
Grant Price |
| 2014 Stock Plan | 37,050,702 | | $37.51 | 0 | | N/A |
| 2019 Stock Plan | 48,848,219 | | $31.42 | 77,673,324 | | $39.92 |
The number of shares, if any, to be issued pursuant to outstanding TSRUs will be determined by the difference between the settlement price and the grant price, plus the dividends equivalents accumulated, if applicable during a 5- or 7-year term. The settlement price is the 20-day average closing stock price ending on the fifth or seventh anniversary of the grant.
•1,060,019 Profit Units (PTUs), which are converted units from exercises of vested TSRU grants of which 877,699 were under the 2014 Stock Plan and 182,320 were under the 2019 Stock Plan.
(2)This amount 91,351,643 represents the aggregate number of shares that were assumed under the 2019 Stock Plan from the remaining shares available under the Seagen Inc. (Seagen), Arena Pharmaceuticals (Arena), Biohaven Pharmaceuticals (Biohaven), and Global Blood Therapeutics (GBT) stock plans, which are exempt from shareholder approval requirements pursuant to NYSE Listed Company Manual Rule 303A.08. The assumed shares may be issued under the terms of the 2019 Stock Plan to legacy employees of the acquired companies and newly hired employees after the dates of the respective acquisitions.
(3)This amount represents the number of shares available, 247,830,527 for issuance pursuant to stock options and awards that could be granted in the future under the 2019 Stock Plan inclusive of the shares we assumed from the remaining shares available under the Seagen, Arena, Biohaven, and GBT stock plans which can be issued to legacy employees of the acquired companies and newly hired employees after the dates of respective acquisitions. Under the 2019 Stock Plan, any option or TSRU granted reduces the available number of shares on a one-to-one basis and any whole share award granted (such as PSA, PPS or RSU) reduces the available number of shares on a three-to-one basis.
On October 15, 2009, Pfizer acquired Wyeth and assumed the Wyeth Management Incentive Plan (the MIP Plan), pursuant to which no subsequent awards have been or will be made. As of December 31, 2023, 56 Pfizer shares were issuable in settlement of the participants’ accounts, which are delivered in lump sums and installments upon separation from Pfizer, subject to meeting the requirements of the MIP Plan. Information regarding these shares is not included in the above table.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 93 |
Financial Measures
The following table contains a comparison of 2023 and 2022 U.S. GAAP to non-GAAP revenues and U.S. GAAP diluted EPS to non-GAAP adjusted diluted EPS for annual incentive purposes relating to “Annual Incentives Objectives and Results (For Annual Incentive Purposes)” within this Proxy Statement (Unaudited). These financial measures for annual incentive purposes utilize budgeted exchange rates for the year and exclude certain other unbudgeted or non-recurring items. Therefore, these financial measures are different from those utilized in our press releases and Management’s Discussion and Analysis of Financial Condition and Results of Operations in our 2023 Annual Report on Form 10-K. | | | | | | | | |
| (Billions, except per common share data) | 2023 | 2022 |
GAAP Revenues | $58.5 | | $100.3 | |
| Foreign exchange impact relative to rates in effect for budget purposes | 0.7 | 4.4 | |
| Exclusion of non-recurring items | 0.1 | (0.2) | |
| Non-GAAP Revenues for Annual Incentive Purposes | $59.3 | | $104.5 | |
GAAP Diluted EPS* | $0.37 | $5.47 | |
| Amortization of intangible assets—net of tax | 0.67 | 0.50 | |
| Acquisition-related items—net of tax | 0.24 | 0.12 | |
| Discontinued operations—net of tax | — | | — | |
| Certain significant items—net of tax | 0.55 | 0.49 | |
| Non-GAAP Adjusted Diluted EPS* | $1.84 | | $6.58 | |
| Foreign exchange impact relative to rates in effect for budget purposes | 0.09 | 0.39 | |
| Acquired in-process research and development expenses—net of tax | — | | 0.11 | |
| Exclusion of non-recurring items | 0.02 | (0.28) | |
| Non-GAAP Adjusted Diluted EPS for Annual Incentive Purposes | $1.95 | | $6.80 | |
* See the “Non-GAAP Financial Measure: Adjusted Income” section of the MD&A in Pfizer’s 2023 Annual Report on Form 10-K. Amounts may not add due to rounding.
| | | | | | | | |
94 | | Pfizer 2024 Proxy Statement |
| | | | | | | | | | | |
| | | |
| We expect the following proposal (Item 5 on the proxy card) to be presented by the shareholder at the Annual Meeting. The company is not responsible for any inaccuracies this shareholder proposal may contain. As explained below, the Board unanimously recommends that you vote “AGAINST” this shareholder proposal. Item 5 – Adopt an Independent Board Chair Policy Mr. Kenneth Steiner, 14 Stoner Ave., 2M, Great Neck, NY 11021-2100, who represents that he owns no less than 500 shares of Pfizer common stock, has notified Pfizer that he will present the following proposal at the 2024 Annual Meeting: The Shareholder’s Resolution Proposal 5 — Independent Board Chairman Shareholders request that the Board of Directors adopt an enduring policy, and amend the governing documents as necessary in order that 2 separate people hold the office of the Chairman and the office of the CEO as follows: Selection of the Chairman of the Board The Board requires the separation of the offices of the Chairman of the Board and the Chief Executive Officer. Whenever possible, the Chairman of the Board shall be an Independent Director. The Board has the discretion to select a Temporary Chairman of the Board who is not an Independent Director to serve while the Board is seeking an Independent Chairman of the Board. This policy could be phased in when there is a contract renewal for our current CEO or for the next CEO transition. This proposal topic won 52% support at Boeing and 54% support at Baxter International in 2020. Boeing then adopted this proposal topic. There should be a rule against a person who has been a CEO and a Chairman at the same time being named as Lead Director. Mr. Shantanu Narayen, Pfizer Lead Director had years in the dual jobs of CEO and Chairman. Past and present holders of both jobs at the same time would seem to have a special affinity with the Pfizer person who now has the 2 most important single jobs at Pfizer — Chairman and CEO. A special affinity is inconsistent with the oversight role of a Lead Director. A lead director is no substitute for an independent board chairman. A lead director cannot call a special shareholder meeting and cannot even call a special meeting of the board. A lead director can delegate most of his lead director duties to others and then simply rubber-stamp it. There is no way shareholders can be sure of what goes on. A lead director can be given a list of duties but there is no rule that prevents the Chairman from overriding the lead director in any of the so-called lead director duties. The Pfizer Board said it wanted the flexibility to implement the leadership structure best suited for Pfizer but failed to state how the best leadership structure would be determined. Thus there should be at least 250 words from the Pfizer Board of Directors on how the best leadership structure is determined if they are serious in opposing this proposal and not just platitudes. It is time for an Independent Board Chairman since Pfizer stock dropped from $47 to $30 in the year before submittal of this proposal. Please vote yes: Independent Board Chairman — Proposal 5 | |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 95 |
Board of Directors’ Statement in Opposition to the Proposal
The Board of Directors recommends a vote AGAINST this proposal.
•Imposing a strict policy that mandates the separation of the roles of Chairman and CEO is unnecessary and would bring no additional benefits to Pfizer or our shareholders.
•The Board is in the best position to determine the most effective leadership structure for Pfizer and our shareholders. It is of utmost importance to maintain the Board’s flexibility to implement a leadership structure optimal for the company and all stakeholders.
•If the Chairman and CEO positions are held by the same individual, the independent Directors elect a Lead Independent Director with significant authority, and well-defined responsibilities under a Board-approved charter.
•Pfizer’s shareholder rights and governance practices, including the election of a Lead Independent Director, establish a robust leadership structure that supports independent board oversight.
•Pfizer seeks investor feedback regularly on its Board leadership structure and composition among other governance topics.
•Proposals on this topic have been voted on previously at Annual Meetings and have consistently failed to receive majority shareholder support.
PFIZER’S SHAREHOLDER RIGHTS AND GOVERNANCE PRACTICES SUPPORT INDEPENDENT BOARD OVERSIGHT
Apart from the Chairman and CEO, Dr. Albert Bourla, the Board consists entirely of independent Directors who exercise strong, independent oversight. This independence is further enhanced by the composition of our Audit, Compensation, Governance & Sustainability, Regulatory and Compliance and Science and Technology Committees, which are comprised solely of independent Directors.
Pfizer’s existing shareholder rights and robust corporate governance policies, including annual director elections, a majority vote standard in uncontested Director elections, a shareholder special meeting right and proxy access, establish a solid framework for ensuring independent Board oversight of the company and for effectively challenging and overseeing management, including the CEO. Our corporate governance policies are supported by the Board’s practices, such as regular executive sessions of the independent Directors, led by our Lead Independent Director, at every Board meeting. These sessions cover various topics, including succession planning and the evaluation of senior leaders, including the Chairman and CEO.
Furthermore, the Board’s independent oversight is strengthened through ongoing refreshment. Since 2020, Pfizer has elected three new independent Directors who bring fresh and diverse perspectives to the Board. As a result, the average Director tenure on the Board is currently eight years.
A FLEXIBLE LEADERSHIP STRUCTURE IS MOST EFFECTIVE FOR PFIZER AND OUR SHAREHOLDERS
The Board values having the flexibility to select the optimal Board leadership structure for Pfizer and our shareholders. Our Corporate Governance Principles reflect this view by stating that independent Directors annually elect a Chairman upon the recommendation of the Governance & Sustainability Committee. The Chairman may or may not be the CEO, and if the CEO also serves as Chairman, a Lead Independent Director is elected. The independent Directors, with their diverse backgrounds, are best suited to assess the appropriate leadership structure based on the company’s circumstances and challenges.
The Board does not have a preferred structure and recognizes that the right structure may vary as circumstances warrant. In addition, the Board conducts an annual evaluation of its leadership structure and considers alternative leadership structures based on various criteria.
PFIZER CONDUCTS AN ANNUAL REVIEW OF THE BOARD’S LEADERSHIP STRUCTURE
In December 2023, following a thorough review by the Governance & Sustainability Committee, the independent Directors also evaluated the Board’s leadership structure considering the company’s recent performance and executive leadership changes, in addition to, investor feedback. The Committee, with input from the other independent Directors, determined that it would be in the best interest of the company and its shareholders for Dr. Bourla, Pfizer’s CEO, to continue serving as Chairman of the Board in 2024. The Board concluded Dr. Bourla’s leadership capabilities and business acumen, developed over 25-plus years of experience, proved to be especially beneficial during 2023 as the company faced numerous challenges, including uncertain patient demand for its COVID-19 products in a post-pandemic environment. Under Dr. Bourla’s leadership, the company acquired Seagen, reorganized its commercial operations and appointed new executive leadership. Additionally, we launched an enterprise-wide cost realignment program that aims to align our costs with our longer-term revenue expectations. We believe these strategic decisions and other organizational changes will help position Pfizer for the future.
| | | | | | | | |
96 | | Pfizer 2024 Proxy Statement |
The independent Directors also re-elected Mr. Shantanu Narayen as Lead Independent Director in 2024. Mr. Narayen has demonstrated strong leadership skills, risk oversight abilities, and expertise in technology and product innovation during his tenure as a Director and Lead Independent Director. His strong independent leadership, global leadership experience, and commitment to the Board make him well-suited for this independent leadership role. Accordingly, the independent Directors remain confident in Mr. Narayen’s ability to continue as Lead Independent Director for 2024.
OUR LEAD INDEPENDENT DIRECTOR ROLE PROVIDES STRONG, INDEPENDENT LEADERSHIP
The position of Lead Independent Director comes with a clear mandate, significant authority, and well-defined responsibilities under a Board-approved charter, and includes:
•Presiding at all meetings of the Board, including executive sessions of the independent Directors;
•Calling meetings of independent Directors;
•Leading the annual evaluation of the Chairman and CEO;
•Functioning as a liaison between the Chairman and CEO and the independent Directors;
•Approving information sent to the Board;
•Authorizing retention of outside advisors and consultants; and
•Acting as liaison to shareholders and keeping apprised of inquiries from shareholders and is involved in responding to these inquiries when appropriate.
SUMMARY
The Board is best positioned to determine its optimal leadership structure and should maintain the flexibility to do so. Requiring the separation of the roles of Chairman and CEO through a rigid and enduring policy is unnecessary and not beneficial for Pfizer and our shareholders. The Board remains committed to engaging with investors and taking their feedback into account among other considerations when evaluating its leadership structure. While shareholders may have differing views, the Board continues to receive strong shareholder support for its approach and flexibility in choosing its optimal leadership structure. At the 2023 Annual Meeting of Shareholders, this proposal received 34.7% support of the votes cast.
| | | | | |
| Accordingly, your Board of Directors recommends a vote “AGAINST” this proposal. |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 97 |
We expect the following proposal (Item 6 on the proxy card) to be presented by the shareholder(s) at the Annual Meeting. The company is not responsible for any inaccuracies this shareholder proposal may contain. As explained below, the Board unanimously recommends that you vote “AGAINST” this shareholder proposal.
Item 6 – Publish a Congruency Report on Political, Lobbying, Electioneering Expenditures
Tara Health Foundation, 47 Kearny Street, San Francisco, CA 94108, which represents that it owns at least 1,840 shares of Pfizer common stock, and a co-filer have notified Pfizer that they will present the following proposal at the 2024 Annual Meeting:
The Shareholder’s Resolution
WHEREAS: Public data collected by OpenSecrets.org show that Pfizer and its corporate PAC rank in the top 1% of political donors.1
Pfizer policy states that “political contributions are made to support the election of candidates, political parties and committees that support public policies important to the industry.”2 Pfizer notes these expenditures “are subject to robust internal procedures designed to align these efforts with [its] public policy priorities, applicable law, and patient-centric agenda.”3
However, Pfizer’s political expenditures appear to be misaligned with the company’s stated values and public policy priorities, threatening the company’s bottom line if it leads to investor action or consumers taking their business elsewhere.
Pfizer listed “Gender Equality” as a Sustainable Development Goal in its ESG report, stating: “We aim to end discrimination against women, ensure equal opportunities for leadership and access to reproductive health.” Yet in 2018, Pfizer was a top contributor to leading efforts by 527 groups seeking to strike down the Affordable Care Act – which has made prescription drugs more accessible for millions – and contributes to PhRMA, which donates to numerous organizations opposing congressional efforts to reform drug pricing. The proponents further estimate that since the beginning of the 2020 election cycle, Pfizer and its employee PACs have donated at least $5 million to politicians and political organizations working to weaken women’s access to reproductive healthcare.
This pattern spending has drawn scrutiny from STAT, Bloomberg News, Huffington Post, The Minnesota Daily, CQ ESG Briefing, Agenda (a Financial Times publication) and Forbes.
Proponents believe Pfizer should establish policies and reporting systems that minimize risk to reputation and brand by addressing possible missteps in corporate electioneering and political spending that contrast with its stated healthcare objectives.
RESOLVED: Pfizer publish an annual report, at reasonable expense, analyzing the congruency of political, lobbying, and electioneering expenditures during the preceding year against publicly stated company values and policies, including Pfizer’s stated goal to “end discrimination against women, ensure equal opportunities for leadership and access to reproductive health.” Such a report should identify and explain trends of incongruent expenditures, and state whether the identified incongruencies have or will lead to a change in future expenditures or contributions.
SUPPORTING STATEMENT
Proponents recommend, at management discretion, that Pfizer include in its analysis metrics that illuminate the degree to which political contributions align with stated values and policy priorities year over year, and present such metrics in the aggregate. Proponents further recommend that the report also contain management’s analysis of risks to our company’s brand, reputation, or shareholder value of political spending, including expenditures for electioneering communications, that conflict with publicly stated company values. “Expenditures for electioneering communications” means spending, from the corporate treasury and from the PACs, directly or through a third party, at any time during the year, on printed, internet or broadcast communications, which are reasonably susceptible to interpretation as in support of or opposition to a specific candidate.
1https://www.opensecrets.org/orgs/pfizer-inc/summary?id=D000000138
2https://cdn.pfizer.com/pfizercom/investors/corporate/802a-Political-Contributions-JAN2018.pdf
3https://www.pfizer.com/sites/default/files/investors/financial_reports/annual_reports/2022/files/Pfizer_ESG_Report.pdf
| | | | | | | | |
98 | | Pfizer 2024 Proxy Statement |
Board of Directors’ Statement in Opposition to the Proposal
The Board of Directors recommends a vote AGAINST this proposal.
•The report requested by the proposal is unnecessary. Pfizer already provides transparent disclosures concerning the company’s public policy priorities and political activities in its Political Action Committee (PAC) and Corporate Political Contributions Report (the PAC Report).
•In addition, we publish an “Industry Associations – Congruency Report” (Congruency Report) that evaluates the alignment between Pfizer and four significant trade associations across six areas of public policies significant to Pfizer.
•Given our existing reporting, adopting the request of the proposal is unnecessary and would divert the company’s public policy focus away from our core business priorities to social issues, particularly those outside our area of expertise, and not integral to accomplishing our strategic objectives.
•Furthermore, our commitment to remaining bipartisan in the political process is well-known, and we prioritize maintaining a strong reputation in this regard.
•Shareholder proposals on this topic have been previously voted on at Annual Meetings and have consistently failed to receive majority support.
PFIZER PAC AND CORPORATE POLITICAL CONTRIBUTIONS REPORT
The PAC Report is published annually and provides detailed information by recipient and amount of Pfizer PAC and Pfizer Inc. contributions to political committees, corporate contributions in state and local elections and certain contributions to trade associations. Our disclosures fully comply with all federal, state, and local laws, including reporting requirements for our PAC and corporate political contributions. The PAC Report clearly explains Pfizer’s rationale for these political expenditures, including the company’s support for bipartisan candidates who value Pfizer’s purpose “to discover, develop and deliver Breakthroughs that change patients’ lives.”
Additionally, the PAC Report outlines how Pfizer’s political expenditures align with our public policy priorities, such as protecting intellectual property, supporting a patient-centric healthcare system that promotes access and innovation, protecting patients from insurance barriers and counterfeit medicines, protecting Medicare Part D and ensuring affordable out-of-pocket costs for patients. To view the PAC Report, see https://www.pfizer.com/about/programs-policies/political-partnerships.
In 2010, Pfizer adopted a policy that prohibits the company from making direct independent expenditures, including direct contributions to 527 independent expenditure committees, a decision supported by executives and affirmed by the Governance & Sustainability Committee of our Board. The PAC Report also explains that Pfizer typically does not make contributions to 527 Issue Organizations. However, if requested to make such a contribution, it would undergo review and approval by the Political Contributions Policy Committee (PCPC). The PCPC, co-chaired by Pfizer’s Chief Corporate Affairs Officer and the Chief Compliance, Quality & Risk Officer, consists of senior leaders from various divisions. Any contributions made to 527 Issue Organizations would be disclosed in the PAC Report.
The Pfizer PAC Steering Committee, composed of Pfizer employees from different divisions of the company, reviews and approves all political contribution requests monthly. Contributions to candidate or elected officials are based on their voting record on policies that support and advance innovation incentives, manufacturing, a secure supply chain, patient affordability, and intellectual property rights. Our PAC support and corporate political contributions do not imply an endorsement of a candidate or elected official’s stance on any social or religious issue. Furthermore, before making any final funding decisions are made, we consider a candidate’s or elected official’s ethical conduct to ensure Pfizer’s values are upheld.
INDUSTRY ASSOCIATIONS – CONGRUENCY REPORT
In 2024, Pfizer published an updated Congruency Report that outlines the public policy positions of Pfizer and four major trade associations across six areas of public policies significant to Pfizer: Patient Access to Healthcare; Intellectual Property; Trade; Tax; Diversity, Equity, and Inclusion; and Climate Change. The initial Congruency Report was published in 2021 in response to shareholder feedback.
The Congruency Report compares Pfizer’s and the trade associations’ positions on policy issues and describes the degree of alignment and areas of misalignment. If areas of misalignment exist, if possible, we will advocate for the trade association to come into alignment. If a trade association’s misalignment outweighs the benefits to Pfizer and its stakeholders, we consider whether to reduce or end our involvement with the organization.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 99 |
Investor feedback about the Congruency Report continues to be overwhelmingly positive. The Congruency Report is available on Pfizer’s website. See https://www.pfizer.com/about/programs-policies/political-partnerships.
BOARD OVERSIGHT OF CORPORATE POLITICAL CONTRIBUTIONS
Pursuant to its Charter, the Governance & Sustainability Committee (the Committee) maintains an informed status on the company’s priorities and activities related to public policy, including political spending policies and practices. The Committee receives regular reports from management, which include an overview of the benefits derived from the company’s association with certain trade and other organizations and reviews of the company’s PAC and Congruency Reports. In addition, the Committee monitors public policy issues that may pose a reputational risk to the company.
SUMMARY
Pfizer regularly evaluates its political contribution reporting practices to ensure compliance with all applicable laws and regulations and to meet the needs of stakeholders. Our robust policies and practices regarding corporate political spending, along with our existing enhanced disclosures, address the concerns raised by this proposal. Therefore, the additional disclosures requested by the proposal are unnecessary and not in the best interests of the company and our shareholders. At Pfizer’s 2023 Annual Meeting of Shareholders, this proposal received support from 14.1% of the votes cast.
| | | | | |
| Accordingly, your Board of Directors recommends a vote “AGAINST” this proposal. |
| | | | | | | | |
100 | | Pfizer 2024 Proxy Statement |
We expect the following proposal (Item 7 on the proxy card) to be presented by the shareholder at the Annual Meeting. The company is not responsible for any inaccuracies this shareholder proposal may contain. As explained below, the Board unanimously recommends that you vote “AGAINST” this shareholder proposal.
Item 7 – Amend Director Resignation Processes
New York City Carpenters Pension Fund, 395 Hudson Street, 9th Floor, New York, NY 10014, which represents that it owns at least 79,400 shares of Pfizer common stock, has notified Pfizer that it will present the following proposal at the 2024 Annual Meeting:
The Shareholder’s Resolution
Resolved: That the shareholders of Pfizer, Inc. (“Company”) hereby request that the board of directors take the necessary action to amend its director election resignation bylaw that requires each director nominee to submit an irrevocable conditional resignation to the Company to be effective upon the director's failure to receive the required shareholder majority vote support in an uncontested election. The proposed amended resignation bylaw shall require the Board to accept a tendered resignation absent the finding of a compelling reason or reasons to not accept the resignation. Further, if the Board does not accept a tendered resignation and the director remains as a “holdover” director, the resignation bylaw shall stipulate that should a “holdover” director fail to be re-elected at the next annual election of directors, that director's new tendered resignation will be automatically effective 30 days after the certification of the election vote. The Board shall report the reasons for its actions to accept or reject a tendered resignation in a Form 8-K filing with the U.S. Securities and Exchange Commission.
SUPPORTING STATEMENT
The Proposal requests that the Board amend its director resignation bylaw to enhance director accountability. The Company has established in its bylaws a majority vote standard for use in an uncontested director election, an election in which the number of nominees equal the number of open board seats. Under applicable state corporate law, a director's term extends until his or her successor is elected and qualified, or until he or she resigns or is removed from office. Therefore, an incumbent director who fails to receive the required vote for election under a majority vote standard continues to serve as a “holdover” director until the next meeting of shareholders. A Company resignation bylaw currently addresses the continued status of an incumbent director who fails to be re-elected by requiring such director to tender his or her resignation for Board consideration.
The proposed new director resignation bylaw will set a more demanding standard of review for addressing director resignations then that contained in the Company 's current resignation bylaw. The resignation bylaw will require the reviewing directors to articulate a compelling reason or reasons for not accepting a tendered resignation and allowing an un-elected director to continue to serve as a “holdover” director. Importantly, if a director's resignation is not accepted and he or she continues as a “holdover” director but again fails to be elected at the next annual meeting of shareholders, that director's new tendered resignation will be automatically effective 30 days following the election vote certification. While providing the Board latitude to accept or not accept the initial resignation of an incumbent director that fails to receive majority vote support, the amended bylaw will establish the shareholder vote as the final word when a continuing ”holdover” director is not re-elected. The Proposal's enhancement of the director resignation process will establish shareholder voting in director elections as a more consequential governance right.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 101 |
Board of Directors’ Statement in Opposition to the Proposal
The Board of Directors recommends a vote AGAINST this proposal.
•Amending the company’s By-laws as requested by the proposal is unnecessary and will not provide any additional benefit to Pfizer or our shareholders. Pfizer’s existing majority voting standard and director resignation policy adequately address the concerns raised by the proponent, while also allowing the Board to exercise its judgment, consistent with its fiduciary duties, and consider the specific circumstances surrounding a Director’s resignation in the event of his or her failure to secure a majority vote in an uncontested election.
•The shareholder proponent has expressed no concerns with Pfizer’s Board of Directors. In addition, Pfizer’s Director nominees consistently receive high levels of shareholder support. For example, at the 2023 Annual Meeting, no Director received less than 94% support.
•Adopting the policy requested by the proposal could be competitively harmful, as it would place us as an outlier among our peers and the majority of public companies and could make it more difficult to recruit highly qualified director candidates.
PFIZER’S CURRENT MAJORITY VOTING STANDARD AND DIRECTOR RESIGNATION POLICY ARE ROBUST AND REFLECT BEST PRACTICES
Pfizer’s Corporate Governance Principles already contain a robust majority voting standard and director resignation policy consistent with best practices. In an uncontested election, any director nominee who does not receive a majority of votes cast “for” his or her election must tender his or her resignation promptly following the failure to receive the required vote. Within 90 days of the certification of the shareholder vote, Pfizer’s Governance & Sustainability Committee (the Committee) must make a recommendation to the Board as to whether the Board should accept the resignation. The Board is required to decide whether to accept the resignation and disclose its decision-making process in a Form 8-K filed with the SEC. Pfizer only nominates candidates for election or re-election to the Board who agree to tender an irrevocable resignation upon failure to receive the required vote, effective upon Board acceptance of such resignation.
In considering a Director’s resignation, the Committee and the Board may consider any factors they deem relevant, and their decision is subject to their fiduciary duties under Delaware law. Any Director who tenders his or her resignation pursuant to this provision may not participate in the Committee’s recommendation or Board action regarding whether to accept the resignation.
The shareholder proposal requests that Pfizer modify this policy to require that the Board accept a tendered resignation when he or she fails to achieve a majority of “for” votes absent the finding of a “compelling” reason or reasons to not accept the resignation. This action would impose a more rigid policy that would remove necessary discretion from the Board at critical times. The Board must be able to accept or reject any such resignation after thoughtful deliberation and consideration of any appropriate and relevant factors consistent with the Board’s fiduciary duties. In addition, the “compelling reason” standard in the proposal is ambiguous and could invite potential litigation over the Board’s business judgment as to whether a reason was compelling or not.
Our current director resignation policy is consistent with those of the vast majority of S&P 500 companies, which are not automatically effective, and do not impose a “compelling” reasons standard. Pfizer’s existing majority voting standard and director resignation policy adequately address the concerns raised by the proponent, while also allowing the Board to exercise its judgment, consistent with its fiduciary duties, and consider the specific circumstances surrounding a Director’s resignation in the event of his or her failure to secure a majority vote in an uncontested election. Adopting the policy requested by the proposal would place us as an outlier among our peers and the majority of public companies and could place us at a competitive disadvantage in recruiting high caliber directors best positioned to oversee Pfizer’s complex and global business.
SUMMARY
Pfizer’s current policies and practices concerning its majority voting standard coupled with its Director resignation policy are considered best practice and already satisfy the concerns raised by this proposal. The proponent cites no evidence of concerns with Pfizer’s Board of Directors. Pfizer’s Director nominees consistently receive high levels of shareholder support. Therefore, the Board views adoption of this proposal as unnecessary and not in the best interest of Pfizer or our shareholders.
| | | | | |
| Accordingly, your Board of Directors recommends a vote “AGAINST” this proposal. |
| | | | | | | | |
102 | | Pfizer 2024 Proxy Statement |
We expect the following proposal (Item 8 on the proxy card) to be presented by the shareholder at the Annual Meeting. The company is not responsible for any inaccuracies this shareholder proposal may contain. As explained below, the Board unanimously recommends that you vote “AGAINST” this shareholder proposal.
Item 8 – Publish a Report on Corporate Contributions
National Center for Public Policy Research, 2005 Massachusetts Avenue, NW, Washington, DC 20036, which represents that it owns at least $2,000 worth of shares of Pfizer common stock, has notified Pfizer that it will present the following proposal at the 2024 Annual Meeting:
The Shareholder’s Resolution
Whereas: Corporate contributions should enhance the image of Pfizer in the eyes of the public. Increased disclosure of these contributions would serve to create greater goodwill for Pfizer. It would also allow the public to better voice its opinions on our corporate giving strategy. Inevitably, some organizations might be viewed more favorably than others. This could be useful in guiding Pfizer's public policy and philanthropic decision making in the future. Corporate giving should ultimately enhance shareholder value in line with the Company 's fiduciary duty.
Resolved: Shareholders request Pfizer list the recipients of corporate contributions to third-party public policy or nonprofit organizations of $5,000 or more on Pfizer's website, along with the amount contributed and any material limitations or monitoring of the contributions.
SUPPORTING STATEMENT
While Pfizer currently reports on its political and lobbying contributions, current disclosure is insufficient for shareholders to evaluate the proper use of corporate assets given to third-party public policy organizations and how those assets should be used.
According to Pfizer, “Essential aspects of our business are being challenged by barriers to access, counterfeit medicines, illegal importation, and challenges to intellectual property protection. For this reason, we actively participate in public policy dialogues to explain our perspectives.”1 It goes on to note its “Third Party Funding Criteria” of “think tanks and legislative organizations.”2
Such statements suggest that Pfizer funds groups seeking to prevent the sale of dangerous counterfeit drugs or improve access to life-saving treatments. What it doesn't suggest is Pfizer donating untold sums of money to promote divisive agendas outside its fiduciary remit.
For instance, Pfizer is listed on the Human Rights Campaign's (HRC) website as a “Platinum Partner.”3 The HRC indoctrinates children as young as 5-years-old with radical gender ideology and instruction on sexual orientation by pushing books and lesson plans in schools.4
Pfizer also “generously” funds the HRC's “Healthcare Equality Index”(HEl).5 To earn a perfect HEI score, “hospitals must display LGBT symbols, solicit and use patients' preferred pronouns, and conduct trainings on LGBT issues.”6 They must also “provide the same treatments for gender dysphoria that they provide for other medical conditions-meaning a hospital that uses puberty blockers to treat precocious puberty cannot withhold the drugs from children who say they're transgender.”7
It has become clear that promoting extreme, partisan ideology creates reputational and legal risk. Company bottom-lines, and therefore value to shareholders, decrease when companies engage in overtly political and divisive partnerships. Following Bud Light's similar embrace of partisanship, its revenue fell $395 million in North America when compared to the same time a year ago.8 This is roughly 10 percent of its revenue in the months following its leap into contentious politics.9 Target's market cap fell over $15 billion amid backlash for similar actions.10 And Disney stock fell 44 percent in 2022 — its worst performance in nearly 50 years – amid its decision to pursue extreme partisan agendas.11
1https://www.pfizer.com/about/programs-policies/political-partnerships
2https://cdn.pfizer.com/pfizercom/Third-Party-Funding-Criteria-30AUG2022.pdf
3https://www.hrc.org/about/corporate-partners
| | | | | | | | |
2024 Proxy Statement Pfizer | | 103 |
4https://hrc-prod-requests.s3-us-west-2.amazonaws.com/welcoming-schools/documents/WS-Lesson-l-Am-Jazz-Book-Transgender.pdf?mtime20210509204030&focal=none; https://hrc-prod-requests.s3-us-west-2.amazonaws.com/welcoming-schools/documents/WS_LGBTQ_Definitions_for_Students.pdf?mtime=20200713131845&focal=none; https://hrc-prod-requests.s3-us-west-2.amazonaws.com/welcoming-schools/documents/WS-Lesson-They-She-He-Easy-as-ABC-Understand-Pronouns.pdf; https://hrc-prod-requests.s3-us-west-2.amazonaws.com/welcoming-schools/documents/WS_Simple_Ways_to_Include_LGBTQ_Material.pdf?mtime=20200713132045&focal=none;
5https://www.hrc.org/resources/healthcare-equality-index
6https://hrc-prod-requests.s3-us-west-2.amazonaws.com/HEI-2024-Scoring-Criteria-Tiers.pdf;
https://freebeacon.com/latest-news./how-left-wing-activist-group-teamed-up-with-big-pharma-to-push-radical-gender-ideology-on-american-hospitals/
7https://hrc-prod-requests.s3-us-west-2.amazonaws.com/HEI-2024-Scoring-Criteria-Tiers.pdf;
https://freebeacon.com/latest-news/how-left-wing-activist-group-teamed-up-with-big-pharma-to-push-radical-gender-ideology-on-american-hospitals/
8https://www.cnn.com/2023/08/03/business/anheuser-busch-revenue-bud-light-intl-hnk/index.html;
9https://www.theguardian.com/business/2023/aug/03/bud-light-revenue-sales-anheuser-busch
10https://www.foxbusiness.com/media/target-market-cap-losses-hit-15-7-billion-share-near-52-week-low-amid-woke-backlash; https://nypost.com/2023/05/23/target-to-remove-some-lgbtq-merchandise-after-facing-customer-backlash/?dicbo=v2-x4CMNWo
11https://www.washingtonexaminer.com/policy/economy/disney-has-Iost-50-billion-in-value-since-war-with-florida-began; https://www.hollywoodreporter.com/business/business-news/disney-stock-2022-1235289239/; https://markets.businessinsider.com/news/stocks/disney-stock-price-decline-bob-iger-pandemic-inflation-recession-streaming-2022-12; https://www.foxnews.com/media/disneys-decline-shows-woke-focus-alienating-fans-wsj-column
| | | | | | | | |
104 | | Pfizer 2024 Proxy Statement |
Board of Directors’ Statement in Opposition to the Proposal
The Board of Directors recommends a vote AGAINST this proposal.
•Adoption of this proposal is unnecessary. Pfizer is committed to corporate transparency, as evidenced by our existing robust public disclosures about our corporate contributions, including charitable contributions.
•The intention behind the shareholder proposal, as stated in the supporting statement, is not to further enhance transparency, but rather to emphasize Pfizer's affiliation with a specific organization.
•The company’s existing reports and additional information publicly available about our corporate contributions is more than ample for our shareholders to understand the nature of our activities. The additional report requested by the proposal is overly prescriptive, unnecessary, and will provide no additional benefit to the company and our shareholders.
PFIZER’S EXISTING DISCLOSURES AND PROCESSES ARE ROBUST
Pfizer partners with many medical, scientific, patient, and civic organizations to support their programs and activities, such as health education and scientific research. This support takes many forms, including grants, charitable contributions and partnerships for efforts that strengthen communities, and work toward a healthier world. Working with these organizations allows Pfizer to better understand the needs of the patients who take our medicines, while helping these medical, scientific, and patient organizations inform and address the needs of those they serve.
Pfizer U.S. medical, scientific and patient education grants, charitable contributions, and other healthcare-related funding are detailed in the company’s U.S. Medical, Scientific, Patient and Civic Organizations Funding Report1. Additionally, Pfizer has a strong tradition of funding external, independent, not-for-profit organizations to support shared goals and to demonstrate our commitment to programs and activities that provide broad public benefit, advance medical care, and improve patient outcomes. These external, independent, not-for-profit organizations include patient advocacy groups, professional medical associations, and other U.S.-based charitable organizations with 501(c)(3) tax status.
Pfizer strives to ensure that our support of these organizations is conducted in strict compliance with relevant laws and regulations, industry codes, external standards and internal Pfizer policies and procedures. Consistent with this, Pfizer provides limited support for certain healthcare related qualified 501(c)(3) organizations, explained in the “Healthcare Charitable Contributions” section of our website.2 Programs eligible for Healthcare Charitable Contributions3 are limited to the following: (i) patient education, including health screening; (ii) patient advocacy for disease awareness; and (iii) patient access to care (e.g., transportation costs). Pfizer does not support studies or research through its Healthcare Charitable Contributions program.
Furthermore, we also engage on public policy issues that may affect our ability to meet patients’ needs and enhance shareholder value. These issues include advancing biomedical research and healthcare innovation, advocating for intellectual property protections, and improving patient access to healthcare. We work with policymakers and industry and trade groups to help create and maintain an innovative environment where we can cultivate new medicines, bring them to market and ensure that patient health and safety remain a priority. Pfizer provides detailed disclosures regarding our corporate political contributions and lobbying activities on the company’s website at https://www.pfizer.com/about/programs-policies/political-partnerships.
Moreover, our disclosures regarding corporate political contributions are reviewed by the Governance & Sustainability Committee (Committee), who oversees the company’s public policy priorities and activities. The Committee also oversees the company’s environmental, social, and governance strategy, and corporate citizenship matters, and monitors public policy issues that may pose reputational risks.
SUMMARY
Pfizer has a strong and detailed system of internal governance that helps to ensure our contributions align with our business priorities, goals, internal policies, and legal requirements. The existing reports and additional publicly available information about our corporate contributions is more than ample for our shareholders to understand the nature of our activities. The additional reporting requested by the proponent is unnecessary, and would divert valuable time and resources away from important matters, and therefore, is not in the best interests of the company and our shareholders.
(1)https://cdn.pfizer.com/pfizercom/responsibility/grants_contributions/Yes_Report_FY-2022.pdf
(2)https://www.pfizer.com/about/responsibility/global-impact/charitable-contributions
(3)https://www.pfizer.com/independentgrants
| | | | | |
| Accordingly, your Board of Directors recommends a vote “AGAINST” this proposal. |
| | | | | | | | |
2024 Proxy Statement Pfizer | | 105 |
| | | | | | | | | | | |
| | | |
| | |
| Annual Meeting Information | |
| | | |
| | | | | | | | | | | | | | | | | |
| | |
| Annual Meeting WHEN AND WHERE? | |
| | | Date and Time | Location | |
| | | April 25, 2024
9:00 a.m., EDT | Virtual Meeting Only: Please visit https://meetnow.global/PFE2024 The 2024 Annual Meeting (the Annual Meeting or the Meeting) will be held in virtual format only through a live video webcast with the option to ask live questions. We designed the format to ensure that our shareholders who attend the meeting will be afforded comparable rights and opportunities to participate as they would at an in-person meeting, allowing for broader shareholder attendance at no cost to the shareholder. Closed captioning will be provided. | |
| | |
| HOW DO I ATTEND THE ANNUAL MEETING? Please visit https://meetnow.global/PFE2024 to attend the Annual Meeting, where additional information will be available, including the Rules of Conduct and Meeting Procedures. Shareholders may log in to the Meeting website beginning at 8:45 a.m. EDT on the day of the Meeting and can attend the Meeting, ask questions and vote their shares. You will be required to enter a control number, which can be found on your Notice of Internet Availability (Notice), proxy card, electronic notification or voting instructions included with your proxy materials. | |
| | | | | |
| | | | | |
| | | Registered Shareholders | Please visit https://meetnow.global/PFE2024 and enter your 15-digit control number found on the Notice, proxy card or electronic notification included with your proxy materials. | |
| | | Beneficial Owners (Shareholders who hold shares through an intermediary, such as a bank or broker) | Please visit https://meetnow.global/PFE2024 and enter your control number found on the voting instructions included with your proxy materials. Access to the Meeting website will be available on the day of the Meeting beginning at 8:45 a.m. EDT. Beneficial owners should check with their intermediary through which they hold their shares to confirm whether it is necessary to register in advance of the annual meeting. If registration is required, please see “How Do I Register in Advance of the Annual Meeting?” below. | |
| | | Guests | Please visit https://meetnow.global/PFE2024 and join the Annual Meeting as a “Guest.” You will not have the ability to ask questions or vote during the virtual meeting if you join the meeting as a Guest. | |
| | | Proponents of Shareholder Proposals | The proponent of a shareholder proposal included in this Proxy Statement should notify the company in writing of the individual authorized to present the proposal at the Meeting at least two weeks before the Annual Meeting. | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | |
| | HOW DO I REGISTER IN ADVANCE OF THE ANNUAL MEETING? While we expect the vast majority of beneficial owners will be able to attend the Annual Meeting, vote their shares and ask questions using the control number received with their proxy materials, as described above, we recommend that beneficial owners confirm this ability with the intermediary through which they hold their shares such as a bank or broker. If your intermediary does not provide for the ability to access the Annual Meeting using the control number found on the voting instructions included with your proxy materials, you will be required to request a legal proxy from your intermediary to register in advance of the Annual Meeting to participate in the Annual Meeting. To register, you must submit proof of your proxy power (legal proxy) reflecting your ownership of Pfizer common stock, which can be obtained from your intermediary, and your email address. Requests for registration should be directed to Computershare and be received no later than 5:00 p.m., EDT, on April 19, 2024 at the following: •By e-mail: Forward an image of your legal proxy to legalproxy@computershare.com along with your name and email address. Requests for registration must be labeled as “Legal Proxy”; or •By mail: Computershare, Pfizer Legal Proxy, P.O. Box 43001, Providence, RI, 02940-3001. You will receive a confirmation email from Computershare of your registration and a new control number, which will be 15-digits, which will allow you to attend the Annual Meeting, vote your shares, ask questions at the meeting and submit questions in advance of the Meeting. If you have already voted your shares and then request a legal proxy, your original vote will be invalidated and you will be required to vote your shares again. | | |
| | | | | | | |
| | | | | | | | |
106 | | Pfizer 2024 Proxy Statement |
| | |
Annual Meeting Information |
HOW DO I ASK A QUESTION AT THE ANNUAL MEETING?
•IN ADVANCE OF THE ANNUAL MEETING: During the two weeks prior the Meeting, shareholders may submit questions via the Meeting website. See instructions below. The deadline to submit questions in advance is April 23, 2024 at 5:00 p.m. EDT.
◦Registered Shareholders: Registered shareholders can submit questions electronically via the Meeting website. Please visit https://meetnow.global/PFE2024 and enter the 15-digit control number included on the Notice, proxy card or electronic notification included with your proxy materials when prompted and follow the instructions on-screen.
•DURING THE ANNUAL MEETING: Shareholders will have the opportunity to ask live questions during the meeting. Details on how to ask live questions will be provided on the Meeting website provided below.
◦Registered Shareholders and Beneficial Owners: Please visit https://meetnow.global/PFE2024 and enter the control number included on your Notice, proxy card, voting instruction form or electronic notification when prompted and follow the instructions on-screen.
◦Note: Beneficial owners should check with their intermediary through which they hold their shares to confirm whether it is necessary to register in advance of the Annual Meeting in order to attend the Meeting. If registration is required, please see “How Do I Register in Advance of the Annual Meeting?” above. We will hold a live Q&A session, during which we intend to answer as many questions as time permits. Questions must comply with the Rules of Conduct and Meeting Procedures, which will be available on the Meeting website, and be pertinent to Pfizer, our shareholders and the Meeting matters.
Questions and answers may be grouped by topic and substantially similar questions may be grouped and answered once.
Questions and answers to any pertinent questions not addressed during the Meeting, will be published following the meeting on our Investors website at https://investors.pfizer.com/Investors/Overview/default.aspx.
WHAT IF I HAVE TECHNICAL QUESTIONS?
Beginning at 8:30 a.m. EDT on the day of the Meeting and through the conclusion of the Meeting, our support team will be ready to assist shareholders with any technical difficulties accessing and participating in the Meeting. Should you require assistance, please call the support team listed on the virtual Annual Meeting website at https://meetnow.global/PFE2024 or by phone, within the U.S., U.S. territories & Canada: +1-888-724-2416 or outside of the U.S., U.S. territories & Canada:+1-781-575-2748.
WILL THE VIRTUAL ANNUAL MEETING BE AVAILABLE FOR REPLAY?
A replay of the Annual Meeting will be made publicly available at https://investors.pfizer.com/Investors/Events--Presentations approximately 24 hours after the Meeting. The replay will be available for approximately one year.
WHAT IS A QUORUM FOR THE MEETING?
The presence of holders representing a majority of the voting power of all shares of Pfizer stock issued and outstanding and entitled to vote at the Annual Meeting, logging into the Meeting using the control number or represented by proxy, is necessary to constitute a quorum. Abstentions and broker non-votes are counted as present and entitled to vote for purposes of determining a quorum.
Voting
WHO IS ENTITLED TO VOTE AT THE MEETING?
Holders of Pfizer common stock at the close of business on February 28, 2024 (the Record Date), are entitled to receive the Notice of 2024 Annual Meeting and Proxy Statement and to vote their shares at the Meeting. As of that date, there were 5,662,541,291 shares of the company’s common stock outstanding and entitled to vote. Each share of common stock is entitled to one vote on each matter properly brought before the Meeting.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 107 |
| | |
| Annual Meeting Information |
HOW DO I VOTE?
Whether or not you plan to attend the Meeting, we urge you to review your proxy materials and vote your shares in advance of the Meeting.
| | | | | |
| |
| By Mail | Complete, sign and date the accompanying proxy card or voting instruction form and return it in the prepaid envelope. If you are a registered shareholder and return your signed proxy card, but do not indicate your voting preferences, the persons named in the proxy card will vote the shares represented by your proxy card as recommended by the Board of Directors. If you are a registered shareholder and you do not have the prepaid envelope, please send your completed proxy card by regular mail to Pfizer Inc., Proxy Services, c/o Computershare Investor Services, P.O. Box 43119, Providence, RI, 02940-5110, or by overnight mail to Pfizer Inc., Proxy Services, c/o Computershare, 150 Royall St., Suite 101, Canton, MA 02021. |
| |
| |
| By Telephone or via the Internet | Registered Shareholders: Pfizer has established telephone and Internet voting procedures for registered shareholders. These procedures are designed to authenticate your identity, to allow you to give your voting instructions and to confirm that those instructions have been properly recorded. •By Telephone: You can vote by calling the toll-free telephone number on your proxy card. Please have your proxy card handy when you call. Easy-to-follow voice prompts will allow you to vote your shares and confirm that your instructions have been properly recorded. If you are located outside the United States, Puerto Rico and Canada, see your proxy card for additional instructions. •By Internet: The website for Internet voting is www.investorvote.com/PFE. Please have your notice, proxy card or electronic notification handy when you go to the website. As with telephone voting, you can confirm that your instructions have been properly recorded. If you vote on the Internet, you also can request electronic delivery of future proxy materials. Telephone and Internet voting facilities for registered shareholders will be available until the polls close on April 25, 2024. Beneficial owners: The availability of telephone and Internet voting for beneficial owners will depend on the voting processes of your broker, bank or other holder of record. We, therefore, recommend that you follow the voting instructions in the materials you receive. If you vote by telephone or on the Internet, you do not have to return your proxy card or voting instruction form. |
| |
| |
| At the Virtual Annual Meeting | If you were a registered shareholder at the close of business on February 28, 2024 and have your control number, you may vote your shares during the virtual Annual Meeting by following the instructions available on the virtual Annual Meeting website. Please visit https://meetnow.global/PFE2024 to access the virtual Annual Meeting. If you hold your shares through an intermediary, such as a bank or broker, and your intermediary does not require registration prior to the annual meeting, please visit https://meetnow.global/PFE2024 to vote your shares during the virtual Annual Meeting. If your intermediary requires registration prior to the annual meeting, see “How Do I Register in Advance of the Annual Meeting?” |
| |
IS THERE A LIST OF REGISTERED SHAREHOLDERS ENTITLED TO VOTE AT THE MEETING?
As required by Delaware law, the names of registered shareholders entitled to vote at the virtual Annual Meeting will be available to registered shareholders for ten days prior to the Meeting for any purpose germane to the Meeting, between the hours of 8:45 a.m. and 4:30 p.m. EDT, at our principal executive offices at 66 Hudson Boulevard East, New York, NY, 10001-2192, Attention: Corporate Secretary. Registered shareholders must make an appointment.
| | | | | | | | |
108 | | Pfizer 2024 Proxy Statement |
| | |
Annual Meeting Information |
WHAT SHARES ARE INCLUDED ON THE PROXY CARD?
If you are a registered shareholder, you will receive a proxy card for all the shares you hold of record:
•in certificate form;
•in book-entry form; and
•in book-entry form in the Computershare Investment Plan.
If you are a Pfizer employee, you will receive either a proxy card or an electronic notification and/or a voting instruction form for all the Pfizer shares you hold:
•in a Pfizer savings plan; and/or
•in Grantor Trusts for deferred stock received by certain Pfizer and legacy Wyeth employees.
Your proxy card will serve as a voting instruction form for the applicable savings plan and/or Grantor Trust.
If you are a Pfizer employee and you do not vote your shares or specify your voting instructions on your proxy card or voting instruction form, the administrator of the applicable savings plan and/or the trustee of a Grantor Trust, as the case may be, will vote your shares in accordance with the terms of your plan and/or Grantor Trust.
To allow sufficient time for voting by the administrator of the applicable savings plan and/or the trustee of a Grantor Trust, your voting instructions must be received by 10:00 a.m., Eastern Daylight Time, on April 22, 2024.
If you hold Pfizer shares through any other company plan, you will receive voting instructions from that plan’s administrator, as applicable.
If you are a beneficial owner, you will receive voting instructions from your broker, bank or other holder of record.
WHAT ARE THE VOTING REQUIREMENTS TO ELECT THE DIRECTORS AND TO APPROVE EACH OF THE PROPOSALS DISCUSSED IN THIS PROXY STATEMENT?
| | | | | | | | |
| Proposal | Vote Required | Broker Discretionary Voting Allowed |
| Election of Directors | Majority of Votes Cast* | No |
| Ratification of KPMG LLP | Majority of Votes Cast | Yes |
Approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan | Majority of Votes Cast | No |
Advisory Approval of Executive Compensation | Majority of Votes Cast | No |
Shareholder Proposals | Majority of Votes Cast | No |
* Any nominee who does not receive a majority of votes cast “for” his or her election would be required to tender his or her resignation promptly following the failure to receive the required vote. Within 90 days of the certification of the shareholder vote, the Governance & Sustainability Committee would then be required to make a recommendation to the Board as to whether the Board should accept the resignation, and the Board would be required to decide whether to accept the resignation and disclose its decision-making process. In a contested election, the required vote would be a plurality of votes cast. Full details of this policy are set forth in our Principles on our website.
Abstentions/Broker Non-Votes: If you abstain from voting or there is a broker non-vote on any matter, your abstention or broker non-vote will not affect the outcome of such vote, because abstentions and broker non-votes are not considered to be votes cast under our By-laws.
HOW WILL MY SHARES BE VOTED AT THE MEETING?
At the Meeting, the Proxy Committee appointed by the Board of Directors will vote your shares as you instruct. If you sign your proxy card and return it without indicating how you would like to vote your shares, your shares will be voted as the Board of Directors recommends, which is:
•FOR the election of each of the Director nominees named in this Proxy Statement;
•FOR the ratification of the selection of KPMG LLP as our independent registered public accounting firm for the 2024 fiscal year;
•FOR the approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan;
•FOR the approval, on an advisory basis, of the compensation of our Named Executive Officers; and
•AGAINST the shareholder proposals.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 109 |
| | |
| Annual Meeting Information |
WHO WILL COUNT THE VOTES?
Representatives of our transfer agent, Computershare, will tabulate the votes and act as inspectors of election.
WHAT CAN I DO IF I CHANGE MY MIND AFTER I VOTE?
If you are a registered shareholder, you can revoke your proxy before it is exercised by:
•giving written notice to our Corporate Secretary;
•delivering a valid, later-dated proxy, or a later-dated vote by telephone or on the Internet, in a timely manner; or
•voting at the virtual Annual Meeting by following the instructions available on the virtual Annual Meeting website.
If you are a beneficial owner of shares, you may submit new voting instructions by contacting your broker, bank or other holder of record and following its instructions for how to do so.
Proxy Materials
WHY DID I RECEIVE THESE PROXY MATERIALS?
We are providing these proxy materials in connection with the solicitation by the Board of Directors of Pfizer Inc., a Delaware corporation, of proxies to be voted at our 2024 Annual Meeting of Shareholders and at any adjournment or postponement of the Meeting.
The Notice of 2024 Annual Meeting and Proxy Statement and a proxy card or voting instruction form are being mailed or made available to shareholders starting on or about March 14, 2024.
CAN I ACCESS THE PROXY MATERIALS AND THE 2023 ANNUAL REPORT ON FORM 10-K ON THE INTERNET?
This Notice of 2024 Annual Meeting and Proxy Statement and the 2023 Annual Report on Form 10-K are available on our website at https://investors.pfizer.com/financials/annual-reports/default.aspx. Instead of receiving future proxy statements and accompanying materials by mail, most shareholders can elect to receive an e-mail that will provide electronic links to them. Opting to receive your proxy materials online will conserve natural resources and will save us the cost of producing documents and mailing them to you, and will also give you an electronic link to the proxy voting site.
Registered Shareholders: If you vote on the Internet at www.investorvote.com/PFE, simply follow the prompts to enroll in the electronic proxy delivery service. You also may enroll in the electronic proxy delivery service at any time in the future by going directly to http://www.computershare.com/investor and following the enrollment instructions.
Beneficial Owners: Please check the information provided in the proxy materials sent to you by your broker, bank or other holder of record regarding the availability of this service.
HOW CAN I ELIMINATE DUPLICATE MAILINGS?
A single Notice of 2024 Annual Meeting and Proxy Statement and 2023 Annual Report, along with individual proxy cards, or individual Notices of Internet Availability, may be delivered in one envelope to multiple shareholders having the same last name and address and to individuals with more than one account registered at Computershare with the same address unless contrary instructions have been received from an affected shareholder. If you would like to enroll in this service or receive individual copies of all documents, now or in the future, please contact Computershare by calling 1-800-733-9393 or writing to Computershare at P.O. Box 43119, Providence, RI, 02940-5110. We will deliver a separate copy of all documents to a shareholder at a shared address to which a single copy of the documents was delivered promptly upon request to the address or telephone number provided above.
| | | | | | | | |
110 | | Pfizer 2024 Proxy Statement |
| | |
Annual Meeting Information |
Other Questions
WHAT IS A BROKER NON-VOTE?
If you are a beneficial owner whose shares are held of record by a broker, you must instruct the broker how to vote your shares. If you do not provide voting instructions, your shares will not be voted on any proposal on which the broker does not have discretionary authority to vote. This is called a “broker non-vote.“ In these cases, the broker can register your shares as being present at the Annual Meeting for purposes of determining the presence of a quorum, but will not be able to vote on those matters for which specific authorization is required under NYSE rules.
If you are a beneficial owner whose shares are held of record by a broker, your broker has discretionary voting authority under NYSE rules to vote your shares on the ratification of KPMG LLP as our independent registered public accounting firm, even if the broker does not receive voting instructions from you. However, your broker does not have discretionary authority to vote on the election of Directors, the approval of the Amended and Restated Pfizer Inc. 2019 Stock Plan, the advisory approval of executive compensation or the shareholder proposals without instructions from you, in which case a broker non-vote will occur and your shares will not be voted on these matters.
WHAT IS THE DIFFERENCE BETWEEN HOLDING SHARES AS A REGISTERED SHAREHOLDER AND HOLDING SHARES AS A BENEFICIAL OWNER?
If your shares are registered in your name with Pfizer’s transfer agent, Computershare, you are the ”registered shareholder” of those shares. This Notice of 2024 Annual Meeting and Proxy Statement and any accompanying materials have been provided directly to you by Pfizer.
If your shares are held in a stock brokerage account or by a bank or other holder of record, you are considered the “beneficial owner” of those shares, and this Notice of 2024 Annual Meeting and Proxy Statement and any accompanying documents have been provided to you by your broker, bank or other holder of record. As the beneficial owner, you have the right to direct your broker, bank or other holder of record how to vote your shares by using the voting instruction form or by following their instructions for voting by telephone or on the Internet.
WHO WILL PAY FOR THE COST OF THIS PROXY SOLICITATION?
Pfizer will pay the cost of soliciting proxies. Proxies may be solicited on our behalf by our Directors, officers or employees in person or by telephone, mail, electronic transmission and/or facsimile transmission. We have hired Morrow Sodali LLC, 333 Ludlow Street, 5th Floor, South Tower, Stamford CT 06902, to distribute and solicit proxies. We will pay Morrow Sodali LLC a fee of $35,000, plus reasonable expenses, for these services.
Other Business
The Board is not aware of any matters that are expected to come before the 2024 Annual Meeting other than those referred to in this Proxy Statement. If any other matter should properly come before the Annual Meeting, the Proxy Committee intends to vote the proxies in accordance with its best judgment.
The Chairman of the Meeting may refuse to allow the transaction of any business, or to acknowledge the nomination of any person, not made in compliance with our By-laws and the procedures described below.
| | | | | | | | |
2024 Proxy Statement Pfizer | | 111 |
| | |
| Annual Meeting Information |
Submitting Proxy Proposals and Director Nominations for the 2025 Annual Meeting
| | | | | | | | |
| Type | Deadline | Submission Requirements* |
Proposals for Inclusion in Our 2025 Proxy Materials | November 14, 2024 | Must comply with Rule 14a-8 under the Securities Exchange Act of 1934, as amended |
Director Nominations Pursuant to Our Proxy Access By-law | Between October 15, 2024 and November 14, 2024 | Must include the information set forth in our By-laws |
Other Proposals or Nominations to be Brought before Our 2025 Annual Meeting | If the 2025 Annual Meeting is to be held within 25 days before or after the anniversary of the date of this year’s Annual Meeting (April 25, 2024), then Pfizer must receive your notice not less than 90 days nor more than 120 days in advance of the anniversary of the 2024 Annual Meeting, or no earlier than December 26, 2024 and no later than January 25, 2025. If the 2024 Annual Meeting is to be held on a date not within 25 days before or after such anniversary, then Pfizer must receive it no later than 10 days following the first to occur of: •the date on which notice of the date of the 2024 Annual Meeting is mailed; or •the date public disclosure of the date of the 2024 Annual Meeting is made. | Must include the information set forth in our By-laws |
* Proposals and/or nominations must be received at our principal executive offices at 66 Hudson Boulevard East, New York, NY, 10001-2192, Attention: Corporate Secretary.
For any other meeting, nominations (other than by proxy access) or items of business must be received by the 10th day following the date on which public disclosure of the date of the meeting is made.
Upon written request, we will provide, without charge, a copy of our By-laws. Requests should be directed to our principal executive offices at 66 Hudson Boulevard East, New York, NY, 10001-2192, Attention: Corporate Secretary.
CONSIDERATION OF POTENTIAL DIRECTOR CANDIDATES
On an ongoing basis, the Governance & Sustainability Committee considers potential candidates identified on its own initiative, as well as those identified by other Directors, members of management, search firms, shareholders and other sources (including individuals seeking to join the Board). Shareholders who wish to recommend candidates may contact the Governance & Sustainability Committee as described in “How to Communicate with Our Directors” earlier in this Proxy Statement. All candidates are required to meet the criteria outlined in this Proxy Statement, as well as those outlined in our Principles and other governing documents, as applicable, as determined by the Governance & Sustainability Committee. Further, each candidate must also meet the criteria in our Director Qualification Standards outlined above under “Director Independence.” Shareholder-recommended candidates will be evaluated by the Governance & Sustainability Committee in the same manner as other nominees. Shareholder nominations must be made according to the procedures required under our By-laws (including via our proxy access by-law) and described above.
| | | | | | | | |
112 | | Pfizer 2024 Proxy Statement |
Amended and Restated Pfizer Inc. 2019 Stock Plan
SECTION 1. PURPOSE.
The purpose of the Pfizer Inc. 2019 Stock Plan, as hereby amended and restated (the “Plan”) is to furnish a material incentive to employees and non-employee Directors of the Company and its Affiliates by making available to them the benefits of an increased common stock ownership in the Company through stock options and other incentive awards. It is believed that these incentives stimulate the efforts of employees and non-employee Directors towards the continued success of the Company and its Affiliates, as well as assist in the recruitment and retention of employees and non-employee Directors.
The Plan, in the form set forth herein, shall become effective as of the Effective Date (as defined below) and is an amendment and restatement of the Pfizer Inc. 2019 Stock Plan which first became effective as of April 25, 2019.
SECTION 2. DEFINITIONS.
As used in the Plan, the following terms shall have the meanings set forth below:
(a) “Affiliate” shall mean (i) any Person that directly, or through one or more intermediaries, controls, or is controlled by, or is under common control with, the Company or (ii) any entity in which the Company has a significant equity interest, as determined by the Committee; and except as limited by Section 5 of the Plan, the employees of such entity or Person described in (i) or (ii) above are eligible to participate in the Plan, as determined by the Committee.
(b) “Award” shall mean any Option, Stock Appreciation Right, Restricted Stock Award, Restricted Stock Unit, Performance Award, Performance Share Award, Performance Cash Award, Portfolio Performance Share Award, Total Shareholder Return Unit, Other Stock Unit Award, Dividend Equivalents, Dividend Equivalent Units with respect to any of the forgoing, if applicable, or any other right, interest or option relating to Shares issued and delivered pursuant to the provisions of the Plan.
(c) “Award Agreement” shall mean any written or electronic agreement, contract or other instrument or document evidencing any Award granted by the Committee hereunder, which in the sole and absolute discretion of the Committee may, but need not, be signed or acknowledged by the Company or the Participant.
(d) “Board” shall mean the Company’s Board of Directors.
(e) “Cause” shall mean, (i) the Participant’s willful misconduct or gross negligence which materially and demonstrably results in financial harm to the Company; (ii) willful breach of duty in the course of service or employment; (iii) the Participant’s misappropriation of funds or other property of the Company or any Subsidiary or the plea of guilty by the Participant to or conviction of the Participant for the commission of a felony; or (iv) the conduct by the Participant which is a violation of Company policy or which materially interferes with the Participant’s ability to perform his or her duties; provided, however, that to the extent a Participant is also eligible to receive benefits pursuant to any Company plan or individual agreement that provides for separation or severance benefits upon a termination without “cause,” the definition of “cause” in such other plan or agreement shall apply. No act or failure to act shall be deemed “willful” unless done, or omitted to be done, not in good faith and without reasonable belief that the action or omission was in the best interest of the Company and its Affiliates.
(f) “Change in Control” shall mean the consummation of any of the following events: (i) at any time during the initial twelve-month period following the Effective Date and each successive twelve-month period thereafter, at least a majority of the Board shall cease to consist of “Continuing Directors” (meaning directors of the Company who either were directors as of the Effective Date, or who subsequently became directors and whose election, or nomination for election by the Company’s stockholders, was approved by a majority of the then Continuing Directors, provided that any director whose initial assumption of office is in connection with an actual or threatened election contest, including but not limited to a consent solicitation, relating to the election of directors of the Company shall not qualify as a “Continuing Director”); or (ii) any “person” or “group” (as determined for purposes of Section 13(d)(3) of the Exchange Act, except any majority-owned subsidiary of the Company or any employee benefit plan of the Company or any trust thereunder), shall have acquired “beneficial ownership” (as determined for purposes of Securities and Exchange Commission (“SEC”) Regulation 13d-3) of
| | | | | | | | |
2024 Proxy Statement Pfizer | | I |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
Shares having 30% or more of the voting power of all outstanding Shares, unless such acquisition is approved by a majority of the directors of the Company in office immediately preceding such acquisition; or (iii) a merger or consolidation to which the Company is a party is consummated, in which outstanding Shares are converted into shares of another company (other than a conversion into shares of voting common stock of the successor corporation or a holding company thereof representing more than 50% of the voting power of all capital stock thereof outstanding immediately after the merger or consolidation) or other securities (of either the Company or another company) or cash or other property; or (iv) the sale of all, or substantially all, of the Company’s assets occurs; or (v) the stockholders of the Company approve a plan of complete liquidation of the Company.
(g) “Change in Control Price” means, with respect to a Share, the closing price of such Share reported on the New York Stock Exchange Composite Tape on the date of a Change in Control or Change in Control Event, or if no such price is reported for that date, the closing price on the next preceding date for which such price was reported. To the extent the consideration paid in any such transaction described above consists in full or in part of securities or other noncash consideration, the value of such securities or other noncash consideration shall be determined in the sole discretion of the Board.
(h) “Code” shall mean the Internal Revenue Code of 1986, as amended from time to time, and any successor thereto.
(i) “Committee” shall mean the Compensation Committee of the Board or such other persons or committee to whom it has delegated any authority, as may be appropriate. A person may serve on the Compensation Committee only if he or she is a “Non-Employee Director” for purposes of Rule 16b-3 under the Exchange Act.
(j) “Company” shall mean Pfizer Inc., a Delaware corporation.
(k) “Director” or “Non-Employee Director” shall mean a member of the Board.
(l) “Dividend Equivalents” are equal to the dividends a Share of Company stock would have earned over the settlement period for Total Shareholder Return Units (TSRUs) and from grant to payment for Performance Awards (Performance Share Awards (PSAs) and Portfolio Performance Shares (PPSs)). This value is included into the TSRU calculation for the shares settled and as additional Shares for the PSAs and PPSs that are paid. Dividends and Dividend Equivalents that can be earned with respect to an Award may be accumulated, but shall only become payable if and to the extent the underlying Award is vested, and shall be subject to the same restrictions and risk of forfeiture as the underlying Award.
(m) “Dividend Equivalent Units” (DEUs) are a credit to an individual’s Restricted Stock Units (RSUs) account equivalent to the amount of dividends that would be paid on the same number of actual Shares of Company stock. “DEUs” are “reinvested” and become additional RSUs. DEUs are also paid on any Profit Units (PTUs) resulting from a TSRU exercise. Dividends and DEUs that can be earned with respect to an Award may be accumulated, but shall only become payable if and to the extent the underlying Award is vested, and shall be subject to the same restrictions and risk of forfeiture as the underlying Award.
(n) “Effective Date” shall mean the date the Plan is or was last approved by the stockholders of the Company.
(o) “Employee” shall mean any employee of the Company or any Affiliate. For any and all purposes under this Plan, the term “Employee” shall not include a person hired as an independent contractor, leased employee, consultant or a person otherwise designated by the Committee, the Company or an Affiliate at the time of hire or such later time as not eligible to participate in or receive benefits under the Plan or not on the payroll, even if such ineligible person is subsequently determined to be a common law employee of the Company or an Affiliate or otherwise an employee by any governmental or judicial authority. Unless otherwise determined by the Committee in its sole and absolute discretion, for purposes of the Plan, an Employee shall be considered to have terminated employment or services and to have ceased to be an Employee if his or her employer ceases to be an Affiliate, even if he or she continues to be employed by such employer.
(p) “Exchange Act” shall mean the Securities Exchange Act of 1934, as amended.
(q) “Executive Leadership Team” shall mean the Chief Executive Officer of the Company and the group of corporate executive officers of the Company whose positions report directly to the Chief Executive Officer (or individuals designated as members of this group by the Chief Executive Officer), or any successor to such group.
(r) “Fair Market Value” shall mean, with respect to Shares, as of any date, the closing price for the Shares as reported on the New York Stock Exchange for that date or, if no such price is reported for that date, the closing price on the next preceding date for which such price was reported, unless otherwise determined by the Committee. For purposes of achieving an exemption from Section 409A in the case of affected Participants governed by Section 409A, Fair Market Value of the Shares shall be determined in a manner consistent with Section 409A and any applicable regulations.
(s) “Grant Date” shall mean the date on which an Award is granted.
| | | | | | | | |
II | | Pfizer 2024 Proxy Statement |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
(t) “Incentive Stock Option” shall mean an Option granted under Section 6 that is intended to meet the requirements of Section 422 of the Code or any successor provision thereto.
(u) “Key Employee” means an Employee treated as a “specified employee” as of his or her Separation from Service under Code Section 409A(a)(2)(B)(i), i.e., a key employee (as defined in Code Section 416(i) without regard to paragraph (5) thereof) of the Company or its Affiliates if the Company’s stock is publicly traded on an established securities market or otherwise. Key Employees shall be determined under rules adopted by the Company in accordance with Section 409A. Notwithstanding the foregoing, the Committee may, under the alternative permissible methods allowable under Section 409A, adopt an alternative identification and effective date for purposes of determining which employees are Key Employees.
(v) “Nonqualified Stock Option” shall mean either an Option granted under Section 6 that is not intended to be an Incentive Stock Option or an Incentive Stock Option that has been disqualified.
(w) “Option” shall mean any right granted to a Participant under the Plan allowing such Participant to purchase Shares at such price or prices and during such period or periods as the Committee shall determine.
(x) “Other Stock Unit Award” shall mean any right granted to a Participant by the Committee pursuant to Section 10.
(y) “Participant” shall mean an Employee or a Non-Employee Director who is selected by the Committee or the Board from time to time in their sole discretion to receive an Award under the Plan.
(z) “Performance Award” shall mean any Award (which shall include Performance Shares, Portfolio Performance Shares or Performance Cash) granted pursuant to Section 9, containing performance goals to be achieved as established by the Committee.
(aa) “Performance Cash” shall mean any grant pursuant to Section 9 of a cash-denominated award, which value may be paid to the Participant by delivery of such property as the Committee shall determine, including, without limitation, cash, Shares, other property, or any combination thereof, upon achievement of such performance goals during the Performance Period established by the Committee with respect to such grant.
(bb) “Performance Period” shall mean a period, as established by the Committee at the time any Performance Award is granted or at any time thereafter, during which any performance goals specified by the Committee with respect to such Award are to be measured.
(cc) “Performance Share” shall mean any grant pursuant to Section 9 of a unit valued by reference to a designated number of Shares, which value may be paid to the Participant by delivery of such property as the Committee shall determine, including, without limitation, cash, Shares, other property, or any combination thereof, upon achievement of such performance goals during the Performance Period established by the Committee with respect to such grant.
(dd) “Performance Share Award” or “PSA” shall mean an award of a Performance Share under Section 9.
(ee) “Person” shall mean any individual, corporation, partnership, association, limited liability company, joint-stock company, trust, unincorporated organization or government or political subdivision thereof.
(ff) “Portfolio Performance Share” or “PPS” shall mean any grant pursuant to Section 9 of a unit valued by reference to a designated number of Shares, which value may be paid to the Participant by delivery of such property as the Committee shall determine, including, without limitation, cash, Shares, other property, or any combination thereof, upon achievement of such performance goals during the Performance Period established by the Committee with respect to such grant.
(gg) “Portfolio Performance Share Awards” shall mean an award of Portfolio Performance Shares under Section 9.
(hh) “Prior Plan” shall mean the Company’s 2014 Stock Plan.
(ii) “Restricted Stock” shall mean any Share issued pursuant to Section 8 with the restriction that the holder may not sell, transfer, pledge or assign such Share and with such other restrictions as the Committee, in its sole and absolute discretion, may impose (including, without limitation, any restriction on the right to vote such Share, and the right to receive any cash dividends), which restrictions may lapse separately or in combination at such time or times, in installments or otherwise, as the Committee may deem appropriate.
(jj) “Restricted Stock Award” shall mean an award of Restricted Stock under Section 8.
(kk) “Restricted Stock Unit” or “RSU” shall mean any unit issued pursuant to Section 8 representing a Share with such restrictions as the Committee, in its sole and absolute discretion, may impose, which restrictions may lapse separately or in combination at such time or times, in installments or otherwise, as the Committee may deem appropriate, and which shall have the right to receive DEUs as determined by the Committee.
| | | | | | | | |
2024 Proxy Statement Pfizer | | III |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
(ll) “Restricted Stock Unit Award” shall mean an award of Restricted Stock Units under Section 8.
(mm) “Restriction Period” shall mean the period of time as specified by the Committee, before Restricted Shares, Restricted Stock Units or Other Stock Unit Awards become non-forfeitable and issuable to a Participant within the meaning of Sections 8 and 10.
(nn) “Retirement” shall mean having attained a minimum age of 55 and a minimum of 10 years of continuous, uninterrupted service measured from the most recent hire date at the time of a Participant’s separation from the Company, unless determined otherwise by the Committee, and which shall also constitute a Separation from Service.
(oo) “Section 409A” shall mean Section 409A of the Code and the regulations and other guidance issued thereunder by the U.S. Treasury or Internal Revenue Service.
(pp) “Separation from Service” means a “separation from service” within the meaning of Section 409A.
(qq) “Shares” shall mean the shares of common stock of the Company.
(rr) “Stock Appreciation Right” shall mean any right granted to a Participant pursuant to Section 7 to receive, upon exercise by the Participant, the excess of (i) the Fair Market Value of one Share on the date of exercise over (ii) the grant price of the right on the Grant Date, or if granted in connection with an outstanding Option on the Grant Date of the related Option, as specified by the Committee in its sole and absolute discretion, which, except in connection with an adjustment provided in Section 4(d), shall not be less than the Fair Market Value of one Share on such Grant Date of the right or the related Option, as the case may be. Any payment by the Company in respect of such right may be made in cash, Shares, other property, or any combination thereof, as the Committee, in its sole and absolute discretion, shall determine.
(ss) “Substitute Awards” shall mean Awards granted or Shares issued by the Company in assumption of, or in substitution or exchange for, awards previously granted, or the right or obligation to make future awards, by a company acquired by the Company or an Affiliate or with which the Company or an Affiliate combines.
(tt) “Total and Permanent Disability” shall mean total and permanent disability as determined in accordance with rules established by the Committee, and in compliance with Section 409A.
(uu) “Total Shareholder Return Unit” or “TSRU” shall mean any right granted to a Participant pursuant to Section 7 to receive the excess of (i) the Fair Market Value of one Share on the date of the settlement pursuant to the terms of the grant, over (ii) the grant price of the right on the Grant Date, as specified by the Committee in its sole and absolute discretion, which, except in connection with an adjustment provided in Section 4(d), shall not be less than the Fair Market Value of one Share on such Grant Date of the right. Such Total Shareholder Return Unit may or may not accumulate Dividend Equivalents, at the Committee’s discretion. Any payment by the Company in respect of such right may be made in cash or Shares as the Committee, in its sole and absolute discretion, shall determine. Except with respect to the right to exercise and the accumulation of Dividend Equivalents, for all purposes of this Plan, TSRUs shall be treated the same as Stock Appreciation Rights.
(vv) “Total Shareholder Return Unit Award” shall mean an award of Total Shareholder Return Units under Section 7.
SECTION 3. ADMINISTRATION.
(a) The Plan shall be administered by the Committee. The Committee shall have full power and authority, subject to such orders or resolutions not inconsistent with the provisions of the Plan as may from time to time be adopted by the Board, to (i) select the Employees of the Company and its Affiliates to whom Awards may from time to time be granted hereunder; (ii) determine the type or types of Award to be granted to each Participant hereunder; (iii) determine the number of Shares to be covered by or relating to each Award granted hereunder; (iv) determine the vesting, exercisability, transferability, and payment of Awards, including the authority to accelerate the vesting of Awards; (v) determine the terms and conditions, not inconsistent with the provisions of the Plan, of any Award granted hereunder; (vi) determine whether, to what extent and under what circumstances Awards may be settled in cash, Shares or other property or cancelled or suspended, consistent with the terms of the Plan; (vii) determine whether, to what extent, and under what circumstances shares or cash paid to or gain realized by the Participant based on an Award shall be returned to the Company, consistent with the terms of the Plan; (viii) determine whether, to what extent, and under what circumstances a Participant may be ineligible to retain an Award; (ix) determine whether, to what extent, and under what circumstances payment of cash, Shares, other property and other amounts payable with respect to an Award made under the Plan shall be deferred either automatically or at the election of the Participant, consistent with the terms of the Plan; (x) interpret and administer the Plan and any instrument or agreement entered into under the Plan; (xi) establish such rules, regulations and sub-plans and appoint such agents as it shall deem appropriate for the proper administration of the Plan; and (xii) make any other determination and take any other action that the Committee deems necessary or desirable for administration of the Plan. The Committee
| | | | | | | | |
IV | | Pfizer 2024 Proxy Statement |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
may, in its sole and absolute discretion, and subject to the provisions of the Plan, from time to time delegate any or all of its authority to administer the Plan to any other persons or committee as it deems necessary or appropriate for the proper administration of the Plan; provided, however, that in no event shall an employee of the Company be delegated the authority to grant Awards to, or amend Awards held by, individuals who are subject to Section 16 of the Exchange Act; provided further, that any delegation of administrative authority shall only be permitted to the extent it is permissible under applicable securities laws or the rules of any securities exchange or automated quotation system on which the Shares are listed, quoted or traded. Any delegation hereunder shall be subject to the restrictions and limits that the Board or Committee specifies at the time of such delegation. At all times, the delegate appointed under this Section 3 shall serve in such capacity as deemed necessary or desirable by the Committee, in its sole and absolute discretion. The decisions of the Committee shall be final, conclusive and binding with respect to the interpretation and administration of the Plan and any grants made hereunder. The Committee shall make, in its sole and absolute discretion, all determinations arising in the administration, construction or interpretation of the Plan and Awards under the Plan, including the right to construe disputed or doubtful Plan or Award terms and provisions, and any such determination shall be conclusive and binding on all Persons.
(b) The Committee shall be authorized to make adjustments in performance criteria or in the terms and conditions of other Awards in recognition of unusual or nonrecurring events affecting the Company or its financial statements or changes in applicable laws, regulations or accounting principles or as otherwise provided in Section 12. The Committee may correct any defect, supply any omission or reconcile any inconsistency in the Plan or any Award in the manner and to the extent it shall deem desirable to carry it into effect. In the event that the Company shall assume outstanding employee benefit awards or the right or obligation to grant future awards in connection with the acquisition of or combination with another corporation or business entity, the Committee may, in its sole and absolute discretion, make such adjustments in the terms of Awards under the Plan as it shall deem appropriate. The Committee, in its sole and absolute discretion, may, consistent with the Plan, design any Award to satisfy specific requirements of obtaining a tax, regulatory or accounting benefit or to avoid any adverse tax, regulatory or accounting result, provided, however, that the Company makes no representation that any Award will satisfy any such particular requirement or achieve any particular result, does not covenant to maintain any particular tax, regulatory or accounting status, and the failure of any Award to satisfy any such requirement or achieve a particular result shall not create any liability to any Participant or beneficiary. The Committee and each member thereof, shall be indemnified and held harmless to the fullest extent permitted by law for any and all actions taken pursuant to, and in accordance with the terms of the Plan.
SECTION 4. SHARES SUBJECT TO THE PLAN.
(a)Subject to adjustment as provided in Section 4(c) and Section 4(d), the total number of Shares authorized for grant on or after the Effective Date pursuant to Awards under the Plan is equal to three hundred twenty million (320,000,000) Shares, plus, the number of Shares that remain available for issuance under the Plan as of the Effective Date, provided that no more than four hundred million (400,000,000) Shares may be granted as Incentive Stock Options. Any Shares granted in connection with Options, TSRUs and Stock Appreciation Rights shall be counted against this limit as one (1) Share for every one (1) Option, TSRU or Stock Appreciation Right awarded. Any Shares granted in connection with Awards other than Options, TSRUs and Stock Appreciation Rights shall be counted against this limit as three (3) Shares for every one (1) Share granted in connection with such Award or by which the Award is valued by reference. After the Effective Date of the Plan, no awards may be granted under the Prior Plan. Any Shares issued hereunder may consist, in whole or in part, of authorized and unissued Shares, treasury Shares or Shares purchased in the open market or otherwise.
(b)Notwithstanding any other provision of the Plan to the contrary, any Awards granted under the Plan (excluding, for this purpose, any (i) Substitute Awards, (ii) Shares delivered in lieu of fully vested cash-denominated Awards and (iii) Awards to Non-Employee Directors that vest on the earlier of the one year anniversary of the date of grant or the next annual meeting of stockholders which is at least 50 weeks after the immediately preceding year’s annual meeting) shall be granted subject to a minimum vesting period of at least twelve (12) months, such that no such Awards shall vest prior to the first anniversary of the applicable grant date; provided, that, the Committee may grant any such Awards without regard to the foregoing minimum vesting requirement with respect to a maximum of five (5) percent of the shares of Common Stock reserved for issuance under the Plan pursuant to Section 4(a) hereof (subject to adjustment under Section 4(c)).
| | | | | | | | |
2024 Proxy Statement Pfizer | | V |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
(c)In the event of any merger, reorganization, consolidation, recapitalization, stock dividend, extraordinary cash dividend, stock split, reverse stock split, spin-off, split-off or similar transaction or other change in corporate structure affecting the Shares, such adjustments and other substitutions shall be made to the Plan and to Awards as the Committee, in its sole and absolute discretion, deems equitable or appropriate, including, without limitation, such adjustments in the aggregate number, class and kind of securities (or property, including cash) that may be delivered under the Plan, in the aggregate or to any one Participant, in the number, class, kind and option or exercise price of securities subject to outstanding Awards granted under the Plan (including, if the Committee deems appropriate, the substitution of similar options to purchase the shares of, or other awards denominated in the shares of, another company) as the Committee may determine to be appropriate, in its sole and absolute discretion; provided, however, that the number of Shares subject to any Award shall always be a whole number and further provided that in no event may any change be made to an Incentive Stock Option which would constitute a modification within the meaning of Section 424(h)(3) of the Code. Moreover, notwithstanding anything herein to the contrary, an adjustment to an Award under this Section 4(c) may not be made in a manner that would result in the grant of a new Option, TSRU or Stock Appreciation Right under Section 409A, unless the Committee specifically determines that such adjustment is desirable and will not cause the modified award to create adverse tax consequences under Section 409A.
(d)Any Shares subject to Awards, or awards under the Prior Plan that are outstanding on the Effective Date, that terminate, expire, or are forfeited, cancelled or settled in cash, either in whole or in part, shall be added to the Shares available for Awards under the Plan to the extent of such termination, forfeiture, cancellation or settlement. Any Shares that again become available for future grants pursuant to the preceding sentence shall be added back as one (1) Share if such Shares were subject to Options, TSRUs or Stock Appreciation Rights or options, TSRUs or stock appreciation rights under the Prior Plan and as three (3) Shares if such Shares were subject to Awards other than Options, TSRUs or Stock Appreciation Rights or awards other than options, TSRUs or stock appreciation rights under the Prior Plan. In addition, in the case of any Substitute Award, Shares delivered or deliverable in connection with such assumed or Substitute Award shall not reduce the number of Shares authorized for grant in Section 4(a) above, and Shares subject to a Substitute Award shall not be added to the Shares available for Awards under the Plan. Additionally, in the event that a company acquired by the Company or any Affiliate or with which the Company or any Affiliate combines has shares available under a pre-existing plan approved by stockholders and not adopted in contemplation of such acquisition or combination, the shares available for grant pursuant to the terms of such pre-existing plan (as adjusted, to the extent appropriate, using the exchange ratio or other adjustment or valuation ratio or formula used in such acquisition or combination to determine the consideration payable to the holders of common stock of the entities party to such acquisition or combination) may be used for Awards under the Plan and shall not reduce the Shares authorized for grant under the Plan (and Shares subject to such Awards shall not be added to the Shares available for Awards under the Plan); provided that Awards using such available shares shall not be made after the date awards or grants could have been made under the terms of the pre-existing plan, absent the acquisition or combination, and shall only be made to individuals who were not Employees or Directors prior to such acquisition or combination. Notwithstanding the foregoing, Shares subject to an Award under the Plan or an award under the Prior Plan, may not again be made available for issuance or delivery under the Plan if such Shares are (i) Shares that were subject to a stock-settled Stock Appreciation Right or TSRUs, or a stock-settled stock appreciation right or TSRU under the Plan or the Prior Plan, and were not issued upon the net settlement or net exercise thereof; (ii) Shares delivered to or withheld by the Company to pay the exercise price of an Option or an option under the Plan or the Prior Plan; (iii) Shares delivered to or withheld by the Company to pay the withholding taxes relating to an Award or an award under the Plan or the Prior Plan; (iv) Shares withheld by the Company in connection with the net settlement of an Award; or (v) Shares repurchased on the open market with the proceeds of an Option exercise or the exercise of an option under the Prior Plan.
SECTION 5. ELIGIBILITY.
Any Employee or Non-Employee Director shall be eligible to be selected as a Participant; provided, however, that Incentive Stock Options shall only be awarded to Employees of the Company, or a parent or Affiliate, within the meaning of Section 422 of the Code.
Notwithstanding any provision in this Plan to the contrary, the Non-Employee Directors, including a designated committee of the Board composed solely of Non-Employee Directors, shall have the authority, in their sole and absolute discretion, to select Non-Employee Directors as Participants who are eligible to receive Awards other than Incentive Stock Options under the Plan. The Non-Employee Directors shall set the terms of any such Awards in their sole and absolute discretion, and the Non-Employee Directors shall be responsible for administering and construing such Awards in substantially the same manner that the Committee administers and construes Awards to Employees; provided, however, that no Non-Employee Directors shall be granted Awards and / or paid a cash retainer in any calendar year under this Plan or any other arrangement with the Company having an aggregate value of more than eight hundred thousand dollars ($800,000). Any compensation that is deferred shall be counted toward this limit for the year in which it was first earned, and not when paid or settled if later.
| | | | | | | | |
VI | | Pfizer 2024 Proxy Statement |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
SECTION 6. STOCK OPTIONS.
Options may be granted hereunder to any Participant; either alone or in addition to other Awards granted under the Plan and shall be subject to the following terms and conditions:
(a)Option Price. Other than in connection with Substitute Awards, the exercise price per Share shall be not less than the Fair Market Value of the Shares on the date the Option is granted.
(b)Number of Shares. The Option shall state the number of Shares covered thereby.
(c)Exercise of Option. Unless otherwise determined by the Committee, an Option will be deemed exercised by the optionee, or in the event of death, an Option shall be deemed exercised by the estate of the optionee, or by a person who acquired the right to exercise such Option by bequest or inheritance or by reason of the death of the optionee, or in the event of the optionee’s Total and Permanent Disability, an Option shall be deemed exercised by a person having a legally binding power of attorney for the optionee, in each case upon delivery of (i) a notice of exercise to the Company or its representative, or by using other methods of notice as the Committee shall adopt, and (ii) accompanying payment of the exercise price or other methods of satisfying the exercise price as approved by the Committee and in accordance with any restrictions as the Committee shall adopt. The notice of exercise, once delivered, shall be irrevocable. Notwithstanding the above, and unless the Committee determines otherwise, in the event that the Option is not exercised by the last day on which it is exercisable, and the exercise price per Share is below the Fair Market Value of a Share on such date in an amount determined by the Committee or its delegate, the Option shall be deemed exercised on such date, with a spread equal to the Fair Market Value of the Shares on such date minus the exercise price, and the resulting proceeds net of the exercise price, any required tax withholding (subject to Section 16(j)) and any applicable costs shall be paid to the optionee or the optionee’s legal representative. In no event may any Option granted hereunder be exercised for a fraction of a Share.
(d)Term of Option. The Committee shall determine the term of each Option, except that the period for Incentive Stock Options shall not exceed ten (10) years from the Grant Date. A Nonqualified Stock Option may be exercisable for a period of up to ten (10) years so as to conform with or take advantage of governmental requirements, statutes or regulations, but in no event longer than the Option’s term.
(e)Termination of Option. All Options shall terminate upon their expiration, their surrender, upon breach by the optionee of any provisions of the Option, or in accordance with any other rules and procedures incorporated into the terms and conditions governing the Options as the Committee shall deem advisable or appropriate.
(f)Termination of Employment. Except as otherwise set forth in the Plan, the terms relating to the treatment of an outstanding Option in the event of the Participant’s termination of employment shall be determined by the Committee at the time of grant and shall be set forth in the applicable Award Agreement.
(g)Incorporation by Reference. The Option shall contain a provision that all the applicable terms and conditions of this Plan are incorporated by reference therein.
(h)Other Provisions. The Option shall also be subject to such other terms and conditions as the Committee shall deem advisable or appropriate, consistent with the provisions of the Plan as herein set forth. In addition, Incentive Stock Options shall contain such other provisions as may be necessary to meet the requirements of the Code and the Treasury Department rulings and regulations issued thereunder with respect to Incentive Stock Options.
(i)Exemption from Section 409A. It is intended that all Options granted under this Plan will be exempt from Section 409A. Nevertheless, the Company does not represent, covenant or guarantee that any particular Award made under the Plan will qualify for favorable tax treatment (e.g., as in Incentive Stock Options) or will avoid unfavorable tax consequences to the Participant (e.g., Section 409A penalties).
| | | | | | | | |
2024 Proxy Statement Pfizer | | VII |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
SECTION 7. STOCK APPRECIATION RIGHTS AND TOTAL SHAREHOLDER RETURN UNITS.
(a)Grant of a Stock Appreciation Right or Total Shareholder Return Unit (TSRU). Stock Appreciation Rights or TSRUs may be granted hereunder to any Participant, either alone (“freestanding”) or in addition to other Awards granted under the Plan and may, but need not, relate to a specific Option granted under Section 6. The provisions of Stock Appreciation Rights or TSRUs need not be the same with respect to each recipient. Any Stock Appreciation Right or TSRU related to a Nonqualified Stock Option may be granted at the same time such Option is granted or at any time thereafter before exercise or expiration of such Option. Any Stock Appreciation Right or TSRU related to an Incentive Stock Option must be granted at the same time such Option is granted. In the case of any Stock Appreciation Right or TSRU related to any Option, the Stock Appreciation Right or TSRU or applicable portion thereof shall terminate and no longer be exercisable upon the termination or exercise of the related Option, except that a Stock Appreciation Right or TSRU granted with respect to less than the full number of Shares covered by a related Option shall not be reduced until the exercise or termination of the related Option exceeds the number of Shares not covered by the Stock Appreciation Right or TSRU. Any Option related to any Stock Appreciation Right or TSRU shall no longer be exercisable to the extent the related Stock Appreciation Right or TSRU has been exercised or settled, as applicable.
(b)Terms. The Committee may impose such terms and conditions or restrictions on the exercise of any Stock Appreciation Right or TSRU, as it shall deem advisable or appropriate; provided that a Stock Appreciation Right or TSRU shall not: (i) have an exercise price less than Fair Market Value of a Share on the Grant Date other than in connection with Substitute Awards, or (ii) a term of greater than ten (10) years. Notwithstanding the above, and unless the Committee determines otherwise, in the event that the Stock Appreciation Right is not exercised or settled by the last day on which it is exercisable, and the exercise price per share of such Stock Appreciation Right is below the Fair Market Value of a Share on such date in an amount to be determined by the Committee or its delegate, the Stock Appreciation Right shall be deemed exercised on such date, with a spread equal to the Fair Market Value of the Shares on such date minus the exercise price, and the resulting proceeds net of any required tax withholding (subject to Section 16(j)) and any applicable costs shall be paid to the Participant or the Participant’s legal representative.
(c)Termination of Employment. Except as otherwise set forth in the Plan, the terms relating to the treatment of an outstanding Stock Appreciation Right or TSRU in the event of the Participant’s termination of employment shall be determined by the Committee at the time of grant and shall be set forth in the applicable Award Agreement.
(d)Section 409A. Stock Appreciation Rights or TSRUs may be granted hereunder by the Committee either (i) in a manner consistent with Section 409A such that the Stock Appreciation Right or TSRU will not provide for a deferral of compensation under Section 409A, or (ii) in a manner that is intended from grant to subject the Stock Appreciation Right or TSRU to Section 409A. In the event Stock Appreciation Rights or TSRUs are granted to be so subject to Section 409A, then the Stock Appreciation Right or TSRU shall be settled and paid in a single lump sum (i) as of a specified date, (ii) upon the Participant’s Separation from Service, or (iii) the earlier of (i) or (ii) hereof, as specified and set forth by the Committee in an Award Agreement at the time of grant, and shall otherwise be granted, administered, settled and paid in accordance with Section 409A. Notwithstanding the foregoing, to the extent necessary to avoid the imposition of taxes under Section 409A, any such settlement and payment may not be made to a Key Employee upon a Separation from Service before the date which is 6 months after the date of the Key Employee’s Separation from Service (or, if earlier, the date of death of the Key Employee).
| | | | | | | | |
VIII | | Pfizer 2024 Proxy Statement |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
SECTION 8. RESTRICTED STOCK AND RESTRICTED STOCK UNITS.
(a)Grant of Restricted Stock or Restricted Stock Unit. A Restricted Stock Award or Restricted Stock Unit Award shall be subject to restrictions imposed by the Committee at the time of grant for the Restriction Period. Restricted Stock Awards or Restricted Stock Unit Awards may be issued hereunder to Participants for no cash consideration or for such minimum consideration as may be required by applicable law, either alone or in addition to other Awards granted under the Plan. Any Award of Restricted Stock or a Restricted Stock Unit shall also be subject to such other terms and conditions as the Committee shall deem advisable or appropriate, consistent with the provisions of the Plan as herein set forth. Unless otherwise provided in the Award Agreement, beginning on the date of grant of the Restricted Stock Award, the Participant shall become a stockholder of the Company with respect to all Shares subject to the Award Agreement and shall have all of the rights of a stockholder, including the right to vote such Shares and the right to receive distributions made with respect to such Shares, except as otherwise provided in this Plan. A Participant who holds a Restricted Stock Unit Award shall only have those rights specifically provided for in the Award Agreement; provided, however, in no event shall the Participant have voting rights with respect to such Award, Dividend Equivalents or DEUs. Dividend Equivalents and DEUs can be earned with respect to the Restricted Stock Unit Awards at the discretion of the Committee. Dividends, Dividend Equivalents or DEUs that can be earned on Restricted Stock Awards or Restricted Stock Unit Awards, as applicable, may be accumulated, but shall only become payable if and to the extent the underlying Restricted Stock or Restricted Stock Unit Award is vested, and shall be subject to the same restrictions and risk of forfeiture as the underlying award.
(b)Termination of Employment. Except as otherwise set forth in the Plan, the terms relating to the treatment of an outstanding Restricted Stock Award or Restricted Stock Unit Award in the event of the Participant’s termination of employment, shall be determined by the Committee at the time of grant and shall be set forth in the applicable Award Agreement.
(c)Registration. Any Restricted Stock issued hereunder may be evidenced in such manner, as the Committee, in its sole and absolute discretion, shall deem appropriate, including, without limitation, book entry registration or issuance of a stock certificate or certificates. In the event any stock certificates are issued in respect of Shares of Restricted Stock awarded under the Plan, such certificates shall be registered in the name of the Participant and shall bear an appropriate legend referring to the terms, conditions and restrictions applicable to such Award.
(d)Section 409A. Restricted Stock Unit Awards may be granted hereunder by the Committee either (i) in a manner consistent with Section 409A such that the Restricted Stock Unit Awards will not provide for a deferral of compensation under Section 409A, or (ii) in a manner that is intended from grant to subject the Restricted Stock Unit Awards to Section 409A. In the event Restricted Stock Unit Awards are granted to be subject to Section 409A, then the Restricted Stock Unit Awards shall be settled and paid in a single lump sum (i) as of a specified date, (ii) upon the Participant’s Separation from Service, or (iii) the earlier of (i) or (ii) hereof, as specified and set forth by the Committee in an Award Agreement at the time of grant, and shall otherwise be granted, administered, settled and paid in accordance with Section 409A. Notwithstanding the foregoing, to the extent necessary to avoid the imposition of taxes under Section 409A, any such settlement and payment may not be made to a Key Employee upon a Separation from Service before the date which is 6 months after the date of the Key Employee’s Separation from Service (or, if earlier, the date of death of the Key Employee).
SECTION 9. PERFORMANCE AWARDS, PERFORMANCE SHARE AWARDS, AND PORTFOLIO PERFORMANCE SHARES.
(a)Grant of Performance Awards. Performance Awards (which can include Performance Share Awards, Portfolio Performance Share Awards and Performance Cash Awards) may be paid in cash, Shares, other property, or any combination thereof, and may be subject to such other terms and conditions as the Committee shall deem advisable or appropriate, consistent with the provisions of the Plan as set forth, in the sole and absolute discretion of the Committee at the time of payment. The performance levels to be achieved for each Performance Period and the amount of the Award to be distributed shall be conclusively determined by the Committee. Performance Awards will be paid in a lump sum prior to the 15th day of the third month of the year immediately following the year in which the close of the Performance Period occurs in accordance with the applicable short-term deferral exception provisions of Section 409A, or, in accordance with procedures established by the Committee and the applicable provisions of Section 409A, or on a deferred basis pursuant to Section 15 hereof, if applicable. Dividend Equivalents that can be earned with respect to Performance Awards may be accumulated, but shall only become payable if and to the extent the underlying Performance Awards are achieved, and shall be subject to the same restrictions and risk of forfeiture as the underlying award.
(b)Termination of Employment. Except as otherwise set forth in the Plan, the terms relating to the treatment of an outstanding Performance Award in the event of the Participant’s termination of employment shall be determined by the Committee at the time of grant and shall be set forth in the applicable Award Agreement.
| | | | | | | | |
2024 Proxy Statement Pfizer | | IX |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
(c)Section 409A. In the event Performance Awards are subject to Section 409A, then the Performance Award shall be settled and paid in a single lump sum (i) as of a specified date, (ii) upon the Participant’s Separation from Service, or (iii) the earlier of (i) or (ii) hereof, in accordance with rules established by the Committee at the time of grant, and shall otherwise be granted, administered, settled and paid in accordance with Section 409A. Notwithstanding the foregoing, to the extent necessary to avoid the imposition of taxes under Section 409A, any such settlement and payment may not be made to a Key Employee upon a Separation from Service before the date which is 6 months after the date of the Key Employee’s Separation from Service (or, if earlier, the date of death of the Key Employee).
SECTION 10. OTHER STOCK UNIT AWARDS.
(a)Stock and Administration. Awards that are valued by reference to, or are otherwise based on, Shares may be granted hereunder to Participants, either alone or in addition to other Awards granted under the Plan, and such Other Stock Unit Awards shall also be available as a form of payment in the settlement of other Awards granted under the Plan. Other Stock Unit Awards may be paid in Shares, cash or any other form of property, as the Committee shall determine. Subject to the provisions of the Plan, the Committee shall have sole and absolute discretion to determine the Employees to whom and the time or times at which such Awards shall be made, the number of Shares to be issued or delivered pursuant to such Awards, and all other conditions of the Awards. Any Other Stock Unit Awards shall be subject to such other terms and conditions as the Committee shall deem advisable or appropriate, consistent with the provisions of the Plan as herein set forth.
(b)Termination of Employment. Except as otherwise set forth in the Plan, the terms relating to the treatment of an outstanding Other Stock Unit Award in the event of the Participant’s termination of employment shall be determined by the Committee at the time of grant and shall be set forth in the applicable Award Agreement.
(c)Other Provisions. Shares (including securities convertible into Shares) subject to Awards granted under this Section 10 may be issued for no cash consideration or for such minimum consideration as may be required by applicable law.
(d)Section 409A. Other Stock Unit Awards may be granted hereunder by the Committee (i) in a manner consistent with Section 409A such that the Other Stock Unit Awards will not provide for a deferral of compensation under Section 409A, or (ii) in a manner that is intended from grant to subject the Other Stock Unit Award to Section 409A. In the event Other Stock Unit Awards are granted to be subject to Section 409A, then the Other Stock Unit Awards shall be settled and paid in a single lump sum (i) as of a specified date, (ii) upon the Participant’s Separation from Service, or (iii) the earlier of (i) or (ii) hereof, as specified by the Committee at the time of grant or otherwise in a fashion which is compliant with Section 409A, and shall otherwise be granted, administered, settled and paid in compliance with Section 409A. Notwithstanding the foregoing, to the extent necessary to avoid the imposition of taxes under Section 409A, any such settlement and payment may not be made to a Key Employee upon a Separation from Service before the date which is six (6) months after the date of the Key Employee’s Separation from Service (or, if earlier, the date of death of the Key Employee).
SECTION 11. CHANGE IN CONTROL PROVISIONS.
(a)Unless the Committee or Board shall determine otherwise at the time of grant with respect to a particular Award, and notwithstanding any other provision of the Plan to the contrary, in the event a Participant’s employment or service is involuntarily terminated by the Company without Cause (as determined by the Committee or Board in its sole and absolute discretion) during the 24-month period following a Change in Control, and provided that, with respect to any Awards that are considered deferred compensation under Section 409A, the Participant’s involuntary termination of employment or service also constitutes a Separation from Service:
(i)any Options and Stock Appreciation Rights outstanding and which are not then exercisable or vested shall upon such involuntary termination fully vest and become exercisable for their full term. TSRUs will continue to vest according to the original vesting schedule at grant and settle in accordance with the terms of grant. Options, TSRUs and Stock Appreciation Rights shall remain in effect for the respective terms of such Award as set forth in the applicable Award Agreement notwithstanding such involuntary termination;
(ii)any vested Options, TSRUs and Stock Appreciation Rights outstanding shall upon such involuntary termination remain in effect and be exercisable for the respective terms of such Award or settled as applicable, as set forth in the applicable Award Agreement notwithstanding such involuntary termination;
(iii)any Restricted Stock Unit shall upon such involuntary termination continue to vest according to the original vesting schedule at grant and shall be paid in accordance with the original schedule;
| | | | | | | | |
X | | Pfizer 2024 Proxy Statement |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
(iv)all Performance Awards, Performance Share Awards, Performance Cash Awards and Portfolio Performance Share Awards shall upon such involuntary termination continue to vest according to the original vesting and distribution schedule at grant and will be paid at the end of the performance period based on the applicable performance criteria;
(v)the restrictions applicable to any Restricted Stock shall upon such involuntary termination lapse, and such Restricted Stock shall immediately become free of all restrictions, limitations or conditions and become fully vested and transferable to the full extent of the original grant;
(vi)any Other Stock Unit Awards or any other Awards shall upon such involuntary termination continue to vest and be paid according to the original vesting and distribution schedule at grant; and
(vii)notwithstanding any other provision of this Section 11(a), the proceeds, from exercise, settlement or otherwise, of any Options, Stock Appreciation Rights, TSRUs, Restricted Stock, Restricted Stock Units, Performance Shares, Performance Share Awards, Portfolio Performance Share Awards or Other Stock Unit Awards that are considered deferred compensation under Section 409A shall be subject to the terms of Section 19 of the Plan.
(b)Change in Control Cash Out. Notwithstanding any other provision of the Plan, in the event of a Change in Control, or, with respect to Options, TSRUs, Stock Appreciation Rights, Restricted Stock, Restricted Stock Units, Performance Awards, Performance Share Awards, Performance Cash Awards, Portfolio Performance Awards or Other Stock Unit Awards that are considered deferred compensation under Section 409A, in the event of a Change in Control that is also a “Change in Control Event” described in Section 409A(a)(2)(A)(v) or otherwise under Section 409A, (i) the Committee or Board may, in its sole and absolute discretion, provide in the terms of the Award that is intended to be exempt from Section 409A, that such Awards shall, upon the occurrence of a Change in Control, be cancelled in exchange for a cash payment to be made within 60 days of the Change in Control (and the Participant shall have no discretion to choose the date of payment): (A) in an amount equal to the amount by which the Fair Market Value per Share on the date of the payment exceeds the option price per Share under the Option, if any, multiplied by the number of Shares to be issued and delivered under the Option; (B) in an amount equal to the value of the TSRUs (change in stock price plus projected dividend equivalents, multiplied by the number of the TSRUs granted); (C) in an amount equal to the value of the Stock Appreciation Rights (change in stock price multiplied by the number of the Stock Appreciation Rights granted); or (D) in an amount equal to the Fair Market Value per Share on the date of the payment for the Restricted Stock, Restricted Stock Units, Performance Awards, Performance Share Awards, Portfolio Performance Awards or Other Stock Unit Awards, or (ii) the Committee or Board may, in its sole and absolute discretion, provide in the terms of an Award that is deferred compensation under Section 409A, that such Awards shall, upon the occurrence of a Change in Control Event, be cancelled in exchange for a cash payment to be made within 60 days of the Change in Control Event (and the Participant shall have no discretion to choose the date of payment): (A) in an amount equal to the amount by which the Change in Control Price per Share exceeds the option price per Share under the Option if any, multiplied by the number of Shares to be issued and delivered under the Option; (B) in an amount equal to the value of the TSRUs (change in stock price plus projected dividend equivalents, multiplied by the number of the TSRUs granted); (C) in an amount equal to the value of the Stock Appreciation Rights (change in stock price multiplied by the number of the Stock Appreciation Rights granted); or (D) in an amount equal to the Change in Control Price per Share for the Restricted Stock, Restricted Stock Units, Performance Awards, Performance Share Awards, Portfolio Performance Awards or Other Stock Unit Awards. However, if the option price per Share under any outstanding Option is equal to or greater than the Change in Control Price per Share, or the value (change in stock price plus projected dividend equivalents) of any outstanding TSRU or the value (change in stock price) of any outstanding Stock Appreciation Right is negative, the Board may cancel such Award without the payment of any consideration. For the avoidance of doubt, the Committee or Board may, in its sole and absolute discretion, determine the appropriate treatment of Awards upon a Change in Control.
(c)Notwithstanding the above, if the Change in Control is the result of a transaction pursuant to Section 2(f)(iii) and the surviving entity does not assume, substitute or replace Awards, such Awards shall become fully vested and, (except with respect to TSRUs unless exercisable by their terms), immediately exercisable or transferable to the full extent of the original grant upon the Change in Control and shall be distributed, settled or paid in full within 60 days of the Change in Control as provided in Section 11(b) above with respect to each Award that is intended to be exempt from Section 409A, and each Award that is considered deferred compensation under Section 409A, respectively.
| | | | | | | | |
2024 Proxy Statement Pfizer | | XI |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
SECTION 12. PERFORMANCE GOALS.
(a)If an Award is designated by the Committee as subject to this Section 12, then the lapsing of restrictions thereon and the distribution of cash, Shares or other property pursuant thereto, as applicable, may be subject to the achievement of one or more objective performance goals established by the Committee, which, in the discretion of the Committee may be based on the attainment of specified levels of one or any combination of the following: (i) shareholder return; (ii) total shareholder return; (iii) cost targets or reductions, savings, productivity or efficiencies; (iv) operating income, income before or after taxes, net income, or adjusted net income; (v) earnings per share, adjusted earnings per share, earnings before or after taxes, earnings before or after interest, depreciation and/or amortization (“EBITDA”), adjusted EBITDA, economic earnings, or extraordinary or special items or book value per share (which may exclude nonrecurring items); (vi) operating profit or margins or operating expenses, (vii) working capital measures; (viii) return on assets (gross or net), return on equity or return on invested capital; (ix) cash flow measures; (x) market share; (xi) revenues; (xii) strategic business criteria, consisting of one or more objectives based on meeting specified market penetration, geographic business expansion, and goals relating to acquisitions, divestitures, joint ventures and similar transactions, and budget comparisons; (xiii) personal professional objectives, including any of the foregoing performance goals, the implementation of policies and plans, the negotiation of transactions, formation of joint ventures, research or development collaborations, and the completion of other corporate transactions and any combination of, or a specified increase in, any of the foregoing; (xiv) economic value added to the Company or the Affiliate or division of the Company for or within which the Participant is primarily employed; or (xv) such other criteria established by the Committee. Where applicable, the Performance Goals may be expressed in terms of attaining a specified level of the particular criteria or the attainment of any or a specific percentage increase or decrease in the particular criteria. Such performance goals also may be based on the achievement of specified levels of Company performance (or performance of an applicable Affiliate or division of the Company) under one or more of the measures described above relative to the performance of other corporations. The Committee shall have the authority to make equitable adjustments to the Performance Goals as may be determined by the Committee, in its sole and absolute discretion.
SECTION 13. AMENDMENTS AND TERMINATION.
(a)The Board may amend, alter, suspend, discontinue or terminate the Plan or any portion thereof at any time; provided, however, that no such amendment, alteration, suspension, discontinuation or termination shall be made without (a) stockholder approval if such approval is necessary to qualify for or comply with any tax or regulatory requirement or in order to satisfy any rules of the stock exchange on which the Shares are traded or other applicable law for which the Board deems it necessary or desirable to qualify, satisfy or comply, or (b) the consent of the affected Participant, if such action would materially impair the rights of such Participant under any outstanding Award. Notwithstanding anything in the Plan to the contrary, the Board may not (except pursuant to Section 4(c) or in connection with a Change in Control), without the approval of the Company’s stockholders, cancel an Option, TSRU or Stock Appreciation Right in exchange for cash and may not add the shares underlying a canceled Option, TSRU or Stock Appreciation Right to the Shares available for Awards under the Plan, when the exercise or grant price per share exceeds the Fair Market Value of one Share or take any action with respect to an Option, TSRU or Stock Appreciation Right that would be treated as a repricing under the rules and regulations of the principal securities exchange on which the Shares are traded, including a reduction of the exercise price of an Option or the grant price of a TSRU or Stock Appreciation Right or the exchange of an Option, TSRU or Stock Appreciation Right for another Award. In addition, notwithstanding the above, any termination of the Plan shall comply with Section 409A to the extent necessary in order to avoid adverse tax consequences to Participants under Section 409A.
(b)The Committee may delegate to another committee, as it may appoint, the authority to take any action consistent with the terms of the Plan, either before or after an Award has been granted, which such other committee deems necessary or advisable to comply with any government laws or regulatory requirements of a foreign country or for local tax reasons or reasons of local custom, including but not limited to, modifying or amending the terms and conditions governing any Awards, or establishing any local country plans as sub-plans to this Plan. In addition, under all circumstances, the Committee may make non-substantive administrative changes to the Plan as to conform with or take advantage of governmental requirements, statutes or regulations.
(c)The Committee may amend the terms of any Award theretofore granted, prospectively or retroactively, but no such amendment shall (a) unless otherwise required or advisable under applicable law (as determined by the Board), materially impair the rights of any Participant without his or her consent, or (b) cause any Award intended to be exempt from Section 409A to become subject to Section 409A. Notwithstanding the foregoing, the Committee may amend the terms of any award heretofore granted, prospectively or retroactively, in order to cure any potential defects under Section 409A, in a manner deemed appropriate by the Committee in its sole and absolute discretion, without the consent of the Participant. Any change or adjustment to an outstanding Incentive Stock Option shall not, without the consent of the Participant, be made in a manner so as to constitute a “modification” that would cause such Incentive Stock Option to fail to continue to qualify as an Incentive Stock Option. Notwithstanding the foregoing, any adjustments made pursuant to Section 4(c) shall not be subject to these restrictions.
| | | | | | | | |
XII | | Pfizer 2024 Proxy Statement |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
SECTION 14. DIVIDENDS.
Subject to the provisions of the Plan and any Award Agreement, the recipient of an Award (including, without limitation, any deferred Award) may, if so determined by the Committee, be entitled to receive, cash or stock dividends, or Dividend Equivalents with respect to the number of Shares covered by the Award, as determined by the Committee, in its sole and absolute discretion, and the Committee may provide that such amounts (if any) shall be deemed to have been reinvested in additional Shares or otherwise reinvested; provided, however, that dividends and or Dividend Equivalents that can be earned with respect to an Award, shall only become payable if and to the extent the underlying Award vests, regardless of whether or not vesting is contingent upon the achievement of performance goals or time and provided further, however, that if the receipt of any such Dividend Equivalents granted with respect to Options, TSRUs, Restricted Stock, Other Stock Unit Awards and Stock Appreciation Rights is contingent upon the exercise of the Options or TSRUs or Stock Appreciation Right, or the vesting of the Restricted Stock, Performance Shares, or Other Stock Unit Awards, then the Options, Restricted Stock, Performance Shares, Other Stock Unit Awards, or Stock Appreciation Rights shall be granted and administered in accordance with all applicable provisions of Section 409A.
SECTION 15. DEFERRAL OF AWARDS UNDER THE COMPANY’S DEFERRED COMPENSATION PLAN.
Except as otherwise provided in this Plan, the Committee may provide upon the granting of an Award hereunder, other than an Award that is intended to be a stock right which does not constitute a deferral of compensation within the meaning of Treasury Regulations Section 1.409A-1(a)(5) so that it is subject to the requirement that it not include any feature for the deferral of compensation until an event enumerated in such provision, that it is eligible to be deferred under, and pursuant to the terms and conditions of, the Pfizer Inc. Deferred Compensation Plan, as may be amended or restated from time to time (or under any other Company deferred compensation plan or deferral election form). Any such deferral shall be in accordance with the terms of such plan and in compliance with the applicable provisions of Section 409A.
SECTION 16. GENERAL PROVISIONS.
(a)An Award may not be sold, pledged, assigned, hypothecated, transferred, or disposed of in any manner other than by will or by the laws of descent or distribution and may be exercised, during the lifetime of the Participant, only by the Participant; provided that the Committee, in its sole and absolute discretion, may permit additional transferability, on a general or specific basis, other than to a third party for consideration, and may impose conditions and limitations on any permitted transferability.
(b)No Employee or Non-Employee Director shall have the right to be selected to receive an Award under this Plan or, having been so selected, to be selected to receive a future Award grant. Neither the Award nor any benefits arising out of this Plan shall constitute part of a Participant’s employment or service contract with the Company or any Affiliate and, accordingly, this Plan and the benefits hereunder may be terminated at any time in the sole and absolute discretion of the Company without giving rise to liability on the part of the Company or any Affiliate for severance payments. The Awards under this Plan are not intended to be treated as compensation for any purpose including under any other Company plan.
(c)No Employee or Non-Employee Director shall have any claim to be granted any Award under the Plan, and there is no obligation for uniformity of treatment of Employees or Participants under the Plan.
(d)The prospective recipient of any Award under the Plan shall not, with respect to such Award, be deemed to have become a Participant, or to have any rights with respect to such Award until and unless such recipient shall have accepted any Award Agreement or other instrument evidencing the Award.
(e)Nothing in the Plan or any Award granted under the Plan shall be deemed to constitute an employment or service contract or confer or be deemed to confer on any Employee or Participant any right to continue in the employ or service of, or to continue any other relationship with, the Company or any Affiliate or limit in any way the right of the Company or any Affiliate to terminate an Employee’s employment or Participant’s service at any time, with or without Cause.
(f)All Shares delivered under the Plan pursuant to any Award shall be subject to such stock transfer orders and other restrictions as the Committee may deem advisable under the rules, regulations and other requirements of the Securities and Exchange Commission, any stock exchange upon which the Shares are then listed, and any applicable federal, state or local securities law, and the Committee may cause a legend or legends to be put on any such certificates to make appropriate reference to such restrictions.
| | | | | | | | |
2024 Proxy Statement Pfizer | | XIII |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
(g)In appropriate circumstances, the Committee in its sole and absolute discretion may determine that an Award shall be cancelled, or the Shares or cash paid or gain realized from an Award shall be returned to the Company. Additionally, Awards are subject to the Company’s policies on recoupment of gains realized from any Awards as may be in effect from time to time. All Awards granted under the Plan will be subject to recoupment in accordance with the Pfizer Inc. Recoupment Policy and any other clawback policy that the Company is required to adopt pursuant to the listing standards of any national securities exchange or association on which the Company’s securities are listed or as is otherwise required by the Dodd-Frank Wall Street Reform and Consumer Protection Act or other applicable law.
(h)No Award granted hereunder shall be construed as an offer to sell securities of the Company, and no such offer shall be outstanding, unless and until the Committee in its sole and absolute discretion has determined that any such offer, if made, would comply with all applicable requirements of the U.S. federal securities laws and any other laws to which such offer, if made, would be subject.
(i)Except as otherwise required in any applicable Award Agreement or by the terms of the Plan, recipients of Awards under the Plan shall not be required to make any payment or provide consideration other than the rendering of services.
(j)The Company and its Affiliates shall be authorized to withhold from any Award granted or payment due under the Plan, and/or to withhold from wages or other cash compensation paid to the Participant, the amount of withholding taxes due in respect of an Award or payment hereunder and to take such other action as may be necessary in the opinion of the Company or Affiliate to satisfy all obligations for the payment of such taxes. Such other actions may include, without limitation, the requirement that the Participant execute a market sale of Shares or other consideration received pursuant to the Award. The Committee shall be authorized to establish procedures for elections by Participants to satisfy such obligation for the payment of such taxes by delivery of or transfer of Shares to the Company (in a manner limited so as to avoid adverse accounting treatment for the Company), or by directing the Company to retain Shares with a value not to exceed the maximum statutory rate of the Participant’s applicable jurisdiction(s) or otherwise deliverable in connection with the Award with a value at the Participant’s minimum statutory required tax withholding rate (up to the maximum statutory tax rate) of the Participant’s applicable jurisdiction(s) (in a manner limited so as to avoid adverse accounting treatment for the Company and permitted under applicable withholding rules promulgated by the Internal Revenue Service or other applicable governmental entity).
(k)Nothing contained in the Plan shall prevent the Board from adopting other or additional compensation arrangements, subject to stockholder approval if such approval is required; and such arrangements may be either generally applicable or applicable only in specific cases.
(l)Any Award shall contain a provision that it may not be exercised at a time when the exercise thereof or the issuance of Shares thereunder would constitute a violation of any federal or state law or listing requirements of the New York Stock Exchange for such Shares or a violation of any foreign jurisdiction where Awards are or will be granted under the Plan. Without limiting the foregoing, the Company shall have no obligation to issue or deliver Shares subject to Awards granted hereunder prior to: (i) obtaining any approvals from governmental agencies that the Company determines are necessary or advisable, and (ii) completion of any registration or other qualification with respect to the Shares under any applicable law in the United States or any jurisdiction outside of the United States or ruling of any governmental body that the Company determines to be necessary or advisable or at a time when such registration or qualification is not current, has been suspended or otherwise has ceased to be effective. The inability or impracticability of the Company to obtain or maintain authority from any regulatory body having jurisdiction, which authority is deemed by the Company’s counsel to be necessary to the lawful issuance and sale of any Shares hereunder, shall relieve the Company of any liability in respect of the failure to issue or sell such Shares as to which such requisite authority shall not have been obtained, and shall constitute circumstances in which the Committee may determine to amend or cancel awards pertaining to such Shares, with or without consideration to the affected Participants.
(m)The provisions of the Plan shall be construed, regulated and administered according to the laws of the State of New York without giving effect to principles of conflicts of law, except to the extent superseded by any controlling Federal statute.
(n)The Committee may amend the terms of any Award heretofore granted, prospectively or retroactively, in order to cure any potential defects under Section 409A, in a manner deemed appropriate by the Committee in its sole and absolute discretion, without the consent of the Participant. Nothing in this Section 16(n) shall be construed as an admission that any of the compensation and/or benefits payable under this Plan constitutes “deferred compensation” subject to Section 409A.
| | | | | | | | |
XIV | | Pfizer 2024 Proxy Statement |
| | |
Annex 1 — Amended and Restated Pfizer Inc. 2019 Stock Plan |
(o)If any provision of the Plan is or becomes or is deemed invalid, illegal or unenforceable in any jurisdiction, or would disqualify the Plan or any Award under any law deemed applicable by the Committee, such provision shall be construed or deemed amended to conform to applicable laws or if it cannot be construed or deemed amended without, in the determination of the Committee, materially altering the intent of the Plan, it shall be stricken and the remainder of the Plan shall remain in full force and effect.
(p)Awards may be granted to Participants who are foreign nationals or employed outside the United States, or both, on such terms and conditions different from those applicable to Awards to Employees employed in the United States as may, in the judgment of the Committee, be necessary or desirable in order to recognize differences in local law or tax policy. The Committee also may impose conditions on the exercise or vesting of Awards in order to minimize the Company’s obligation with respect to tax equalization for Employees on assignments outside their home country.
(q)If approved by the Committee in its sole and absolute discretion, an Employee’s absence or leave because of military or governmental service, Total and Permanent Disability or other reason shall not be considered an interruption of employment for any purpose under the Plan; provided, however, that to the extent an Award under this Plan is subject to Section 409A, such absence or leave shall be considered a Separation from Service to the extent provided by Section 409A.
SECTION 17. TERM OF PLAN.
The Plan shall terminate on the tenth anniversary of the Effective Date, unless sooner terminated by the Board pursuant to Section 13; provided, however, in no event may an Incentive Stock Option be granted more than ten (10) years after the earlier of (i) the date of the adoption of the Plan as hereby amended and restated by the Board or (ii) the Effective Date.
SECTION 18. COMPLIANCE WITH SECTION 16.
With respect to Participants subject to Section 16 of the Exchange Act (“Members”), transactions under the Plan are intended to comply with all applicable conditions of Rule 16b-3 or its successors under the Exchange Act. To the extent that compliance with any Plan provision applicable solely to such Members that is included solely for purposes of complying with Rule 16b-3 is not required in order to bring a transaction by such Member in compliance with Rule 16b-3, it shall be deemed null and void as to such transaction, to the extent permitted by law and deemed advisable by the Committee. To the extent any provision in the Plan or action by the Committee involving such Members is deemed not to comply with an applicable condition of Rule 16b-3, it shall be deemed null and void as to such Members, to the extent permitted by law and deemed advisable by the Committee.
SECTION 19. COMPLIANCE WITH SECTION 409A.
The intent of the parties is that payments and benefits under the Plan comply with Section 409A to the extent subject thereto or an exemption therefrom, and, accordingly, to the maximum extent permitted, the Plan shall be interpreted and be administered to be in compliance therewith. Any payments described in the Plan that are due within the “short-term deferral period” as defined in Section 409A shall not be treated as deferred compensation unless applicable law requires otherwise. Notwithstanding anything to the contrary in the Plan, no payment or distribution under this Plan that constitutes an item of deferred compensation under Section 409A and becomes payable by reason of a Participant’s termination of employment or service with the Company will be made to such Participant until such Participant’s termination of employment or service constitutes a Separation from Service. Notwithstanding anything to the contrary in the Plan, to the extent required in order to avoid accelerated taxation and/or tax penalties under Section 409A, amounts that would otherwise be payable and benefits that would otherwise be provided during the six (6) month period immediately following the Participant’s termination of employment shall instead be paid on the first business day after the date that is six (6) months following the Participant’s separation from service (or upon the Participant’s death, if earlier). In addition, for purposes of the Plan, each amount to be paid or benefit to be provided to the Participant pursuant to the Plan, which constitutes deferred compensation subject to Section 409A, shall be construed as a separate identified payment for purposes of Section 409A. For any payment under the Plan that constitutes deferred compensation under Section 409A, to the extent required in order to avoid accelerated taxation and/or tax penalties under Section 409A, a Change in Control shall be deemed to have occurred under the Plan with respect to such payment only if a change in the ownership or effective control of the Company or a change in ownership of a substantial portion of the assets of the Company shall also be deemed to have occurred under Section 409A. The Company makes no representation that any or all of the payments or benefits described in this Plan will be exempt from or comply with Section 409A and makes no undertaking to preclude Section 409A from applying to any such payment. The Participant shall be solely responsible for the payment of any taxes and penalties incurred under Section 409A.
| | | | | | | | |
2024 Proxy Statement Pfizer | | XV |