Table of Contents
Filed Pursuant to Rule 424(b)(3)
Registration No. 333-178102
PROSPECTUS

IMMUCOR, INC.
OFFER TO EXCHANGE
$400,000,000 aggregate principal amount of its 11.125% Senior Notes due 2019, the issuance of which has been registered under the Securities Act of 1933, as amended,
for
all of its outstanding 11.125% Senior Notes due 2019
We are offering to exchange, upon the terms and subject to the conditions set forth in this prospectus and the accompanying letter of transmittal, all of our new 11.125% Senior Notes due 2019 (the “exchange notes”) for all of our outstanding 11.125% Senior Notes due 2019 (the “outstanding notes” and collectively with the exchange notes, the “notes”). We are also offering the subsidiary guarantees of the exchange notes, which are described in this prospectus. The terms of the exchange notes are substantially identical to the terms of the outstanding notes except that the issuance of the exchange notes has been registered pursuant to an effective registration statement under the Securities Act of 1933, as amended (the “Securities Act”). We will pay interest on the notes on February 15 and August 15 of each year. The notes will mature on August 15, 2019.
The principal features of the exchange offer are as follows:
| • | We will exchange all outstanding notes that are validly tendered and not validly withdrawn prior to the expiration of the exchange offer for an equal principal amount of exchange notes. |
| • | You may withdraw tendered outstanding notes at any time prior to the expiration of the exchange offer. |
| • | The exchange offer expires at 5:00 p.m., New York City time, on January 13, 2012, unless extended. We do not currently intend to extend the expiration date. |
| • | The exchange of outstanding notes for exchange notes pursuant to the exchange offer will not be a taxable event for U.S. federal income tax purposes. |
| • | We will not receive any proceeds from the exchange offer. |
| • | We do not intend to apply for listing of the exchange notes on any securities exchange or automated quotation system. |
All untendered outstanding notes will continue to be subject to the restrictions on transfer set forth in the outstanding notes and in the indenture relating to the notes. In general, the outstanding notes may not be offered or sold except in a transaction registered under the Securities Act or pursuant to an exemption from, or in a transaction not subject to, the Securities Act and applicable state securities laws. Other than in connection with the exchange offer, we do not currently anticipate that we will register the outstanding notes under the Securities Act.
You should consider carefully the risk factors beginning on page 17 of this prospectus before participating in the exchange offer.
Each broker-dealer that receives exchange notes for its own account pursuant to the exchange offer must acknowledge that it will deliver a prospectus in connection with any resale of such exchange notes. The letter of transmittal states that by so acknowledging and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act. This prospectus, as it may be amended or supplemented from time to time, may be used by a broker-dealer in connection with resales of exchange notes received in exchange for outstanding notes where such outstanding notes were acquired by such broker-dealer as a result of market-making activities or other trading activities. We have agreed that, for a period of 180 days after the expiration date (as defined herein), we will make this prospectus available to any broker-dealer for use in connection with any such resale. See “Plan of distribution.”
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is December 14, 2011.
You should rely only on the information contained in this prospectus. We have not authorized any person to provide you with any information or represent anything about us or this offering that is not contained in this prospectus. If given or made, any such other information or representation should not be relied upon as having been authorized by us. We are offering to exchange the outstanding notes for the exchange notes only in places where the exchange offer is permitted. You should not assume that the information contained in this prospectus is accurate as of any date other than the date on the front cover of this prospectus.
Table of Contents
| Page | ||||
| ii | ||||
| ii | ||||
| iii | ||||
| iv | ||||
| 1 | ||||
| 17 | ||||
| 31 | ||||
| 39 | ||||
| 40 | ||||
| 41 | ||||
| 44 | ||||
| 49 | ||||
| Management’s discussion and analysis of financial condition and results of operations |
50 | |||
| 72 | ||||
| 83 | ||||
| 85 | ||||
| Security ownership of certain beneficial owners and management |
99 | |||
| 100 | ||||
| 102 | ||||
| 105 | ||||
| 168 | ||||
| 170 | ||||
| 172 | ||||
| 172 | ||||
| 172 | ||||
| F-1 | ||||
| II-1 | ||||
This prospectus incorporates important business and financial information about us that is not included or delivered with this prospectus. This information is available without charge to security holders upon written or oral request. Written or oral requests should be directed to Philip H. Moïse, Secretary of Immucor, Inc., at 3130 Gateway Drive, Norcross, Georgia 30071. Our telephone number is (770) 441-2051. You should request this information at least five business days in advance of the date on which you expect to make your decision with respect to the exchange offer. In any event, you must request this information prior to January 6, 2012, in order to receive the information prior to the expiration of the exchange offer.
i
Table of Contents
Where you can find additional information
We and BioArray Solutions Ltd. have filed with the Securities and Exchange Commission, or the SEC, a registration statement on Form S-4 under the Securities Act with respect to the exchange notes being offered hereby. This prospectus, which forms a part of the registration statement, does not contain all of the information set forth in the registration statement. For further information with respect to us, BioArray Solutions Ltd. or the exchange notes, we refer you to the registration statement. We are not currently subject to the informational requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act. As a result of the offering of the exchange notes, we will become subject to the informational requirements of the Exchange Act, and, in accordance therewith, will file reports and other information with the SEC. The registration statement, such reports and other information can be inspected and copied at the SEC located at 100 F Street, N.E., Washington D.C. 20549. Copies of such materials, including copies of all or any portion of the registration statement, can be obtained from the SEC at prescribed rates. You can call the SEC at 1-800-SEC-0330 to obtain information. Such materials may also be accessed electronically by means of the SEC’s home page on the Internet (http://www.sec.gov).
Under the terms of the indenture relating to the notes, we have agreed that, whether or not we are required to do so by the rules and regulations of the SEC, for so long as any of the notes remain outstanding, we will furnish to the trustee and holders of the notes the information specified therein in the manner specified therein. See “Description of exchange notes.”
Cautionary statement regarding forward-looking disclosure
This prospectus contains “forward-looking statements,” which include information concerning our plans, objectives, goals, strategies, future events, future revenues or performance, capital expenditures, financing needs and other information that is not historical information. Many of these statements appear, in particular, under the headings “Business,” “Selected historical consolidated financial data,” “Unaudited pro forma consolidated financial data” and “Management’s discussion and analysis of financial condition and results of operations.” When used in this prospectus, the words “estimate,” “expect,” “anticipate,” “project,” “plan,” “intend,” “believe” and variations of such words or similar expressions are intended to identify forward-looking statements. All forward-looking statements, including, without limitation, our examination of operating trends, are based upon our current expectations and various assumptions. We believe there is a reasonable basis for our expectations and beliefs, but there can be no assurance that we will realize our expectations or that our beliefs will prove correct.
There are a number of risks and uncertainties that could cause our actual results to differ materially from the forward-looking statements contained in this prospectus. Important factors that could cause our actual results to differ materially from those expressed as forward-looking statements are set forth in this prospectus, including but not limited to:
| • | our substantial indebtedness and potential additional future indebtedness; |
| • | restrictions in our debt agreements that limit our flexibility in operating our business; |
| • | our ability to generate cash to service our indebtedness; |
| • | lower industry blood demand and the subsequent impact on the business; |
| • | lower than expected demand for our instruments; |
| • | the decision of customers to defer capital spending; |
| • | the outcome of the administrative action (“notice of intent to revoke our biological license”) received from the Food and Drug Administration; |
ii
Table of Contents
| • | customer reaction to the Food and Drug Administration action and the subsequent impact on the business; |
| • | the strengthening of the U.S. dollar versus any of the functional currencies in which we operate and its adverse impact on reported results; |
| • | the unexpected change in the mix of instruments being purchased instead of acquired through other means, which could significantly change costs recognized in the period; |
| • | the consequences of the recent natural disasters in Japan; |
| • | the failure of customers to efficiently integrate our instruments into their blood banking operations; |
| • | increased competition in the sale of instruments and reagents, particularly in the United States; |
| • | unanticipated operational problems that result in non-compliance with Food and Drug Administration regulations; |
| • | product development obstacles; |
| • | regulatory obstacles; |
| • | the inability to hire and retain, and the unexpected loss of, key managers; |
| • | changes in interest rates; |
| • | the inability of our molecular immunohematology operations to attain expected revenue, gross margin and net income levels; |
| • | the outcome of any legal claims or regulatory investigations known or unknown; |
| • | customer and shareholder class action lawsuits; |
| • | our inability to protect our intellectual property, particularly as to the molecular immunohematology products, or our infringement of the intellectual property of others; |
| • | lower than expected market acceptance of the molecular immunohematology products; |
| • | the unexpected application of different accounting rules; |
| • | general economic conditions; |
| • | adverse developments with respect to our operation or performance, our products and our affiliates; and |
| • | other factors discussed in this prospectus, particularly in “Risk factors”, “Management’s discussion and analysis of financial condition and results of operations” and “Business.” |
All forward-looking statements attributable to us or persons acting on our behalf apply only as of the date they are made and are expressly made subject to the cautionary statements included in this prospectus. Except as may be required by law, we undertake no obligation to publicly update or revise any forward-looking statement to reflect events or circumstances occurring after the date they were made or to reflect the occurrence of unanticipated events.
Market, ranking, industry data and forecasts
This prospectus includes market share, ranking, industry data and forecasts that we obtained from industry publications and surveys, public filings and internal company sources. Industry publications, surveys and forecasts generally state that the information contained therein has been obtained from sources believed to be reliable, but there can be no assurance as to the accuracy or completeness of included information. We have not independently verified any of the data from third-party sources, nor have we ascertained the underlying economic
iii
Table of Contents
assumptions relied upon therein. Statements as to our market position and ranking are based on market data currently available to us, management’s estimates and assumptions we have made regarding the size of our markets within our industry. While we are not aware of any misstatements regarding our industry data presented herein, our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk factors” in this prospectus. We cannot guarantee the accuracy or completeness of such information contained in this prospectus.
We own or have rights to trademarks, service marks or trade names that we use in connection with the operation of our business. In addition, our names, logos and website names and addresses are our service marks or trademarks. Other trademarks, service marks and trade names appearing in this prospectus are the property of their respective owners. Some of the trademarks we own or have the right to use include “Immucor,” “Gamma,” “Capture,” “Galileo,” “Echo,” “NEO” and “BioArray.” Solely for convenience, the trademarks, service marks and tradenames referred to in this prospectus are listed without the ® and TM symbols, but we will assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors to these trademarks, service marks and trade names.
iv
Table of Contents
This summary contains basic information about us and the exchange offer. Because it is a summary, it does not contain all of the information that may be important to you. You should read this entire prospectus carefully, including the section entitled “Risk Factors” and our consolidated financial statements and the related notes included elsewhere in this prospectus, before participating in the exchange offer.
As part of the transactions described under “The transactions,” on August 19, 2011, IVD Acquisition Corporation (“Merger Sub”) merged with and into Immucor, Inc., with Immucor, Inc. being the surviving corporation, which we refer to as the “Acquisition.” In this prospectus, where we refer to or describe our business, the terms “we,” “us,” “our,” “the Company,” “Immucor,” “Issuer” and other similar terms refer to Immucor, Inc. and its subsidiaries, after giving effect to the consummation of the Transactions (as defined below), unless expressly stated otherwise or the context otherwise requires, and in particular, with respect to the historical financial information of Immucor. Unless we indicate otherwise or the context otherwise requires, information identified in this prospectus as “pro forma” gives effect to the consummation of the Transactions as if they had occurred on June 1, 2010. References in this prospectus to fiscal years are to our fiscal years, which end on May 31.
Please note that, unless otherwise noted, our presentation of any financial information for the quarter ended August 31, 2011 will include data from the “Predecessor” period, which covers the period preceding the Transactions (June 1, 2011 to August 19, 2011) and data from the “Successor” period which covers the period following the Transactions (August 20, 2011 to August 31, 2011).
Our company
We develop, manufacture and sell a complete line of reagents and automated systems that detect and identify certain properties of the cell and serum components of human blood for the purpose of blood transfusion. We target our offerings at hospitals, donor centers and reference laboratories for blood typing and antibody screening prior to transfusion. Our strategy is to drive automation in blood banks and transfusion centers around the world. Our strategy enables us to grow market share and develop long-term contractual relationships with our customers. Over the past five years, we have grown our net revenues at a compound annual growth rate (“CAGR”) of 10.5% from net revenues of $223.7 million in 2007 to $333.1 million in 2011.
Our industry
We are part of the in-vitro diagnostic blood typing and screening market, which generally seeks to prevent transfusion reactions through the testing of blood and blood components prior to transfusion. In the U.S., the Food and Drug Administration (the “FDA”) regulates the transfusion of human blood as a drug and as a biological product. The FDA regulates all phases of the blood banking industry, including donor selection and the collection, classification, storage, handling and transfusion of blood and blood components. The FDA requires that all facilities that manufacture products used for any of these purposes, and the products themselves, be registered or licensed by the FDA. See “Business—Regulation” for further discussion.
We estimate the current worldwide blood banking reagent and instrument market at approximately $1.2 billion. We conduct our business globally with manufacturing facilities in the U.S. and Canada. We have a direct distribution presence in the U.S., Canada, Western Europe and Japan, which represent most of the addressable market today. We sell through distributors in other international markets. Over the longer term, we believe emerging markets such as China, India and Brazil represent a growth opportunity as these markets develop and require automation. The long-term potential of the emerging markets is not included in the current estimated $1.2 billion market opportunity for instruments and reagents worldwide.
1
Table of Contents
The principal components of blood are red cells (the cellular portion) and plasma (the fluid portion). Attached to the exterior of red cells are antigens, which determine the blood group (A, B, AB or O) and type (Rh positive or Rh negative) of an individual’s blood. Plasma contains many different kinds of proteins, including antibodies that are produced by the body in response to foreign substances, such as foreign red cell antigens introduced in a transfusion. If foreign antigens are introduced into the patient’s blood in a transfusion, the patient’s body will produce antibodies to combat those foreign antigens, creating a potentially fatal reaction within the body.
Because of the potential reaction of antigens and antibodies, it is critical that healthcare providers correctly identify the antigens and antibodies present in patient blood and donor blood before a transfusion. If a donor’s red cells contain antigens that are recognized by antibodies in the patient’s plasma, the transfused red cells could be destroyed in a potentially life-threatening reaction. Also, if foreign antigens from donor red cells are introduced into a patient’s blood through a transfusion, the patient’s body can produce new antibodies in response to the foreign antigens. The production of these new antibodies, known as alloimmunization, can complicate future transfusions.
Because of the critical importance of matching patient and donor blood, highly skilled and educated technologists in hospitals, donor centers and reference laboratories generally perform procedures for testing compatibility. At present, many customers in the U.S. perform these tests manually, making blood testing laboratories much more labor intensive than other types of testing laboratories. In our direct markets outside of the U.S., a significant portion of the customers are performing these tests with automation.
Reagents
A reagent is a substance that is added during a test in order to bring about a reaction. The resulting reaction is used to confirm the presence of another substance. Our reagents are used to identify different properties of blood for the purpose of transfusion.
Most of our current reagent products are used in tests to (i) identify the blood group (A, B, AB, O) and type (Rh positive or negative), (ii) to detect and identify red cell antibodies or red cell antigens, (iii) to detect and identify platelet antibodies and (iv) to determine blood compatibility (crossmatch). The FDA requires the accurate testing of blood and blood components for the purpose of transfusion, using only reagents that have been licensed or cleared by the FDA.
We offer both traditional and proprietary reagents. Our serology instruments use both our traditional reagents, as well as our proprietary solid phase technology, marketed under the name Capture, to perform tests.
Traditional serology reagents
Under traditional agglutination blood testing techniques (the manual method), the technologist manually mixes serum with red blood cells in a test tube, performs several additional procedures, and then examines the mixture to determine whether there has been an agglutination reaction. A positive reaction occurs if the cells are drawn together in clumps by the presence of corresponding antibodies and antigens. Due to the critical importance of matching patient and donor blood, testing procedures using agglutination techniques are usually performed manually by highly educated and skilled technologists.
We estimate that approximately 60% of customers in the U.S. perform testing on a manual basis without the use of an automated instrument. The customers that perform testing on a manual basis are primarily in the small- to medium-sized hospital segment of the market. In the high volume segment of the U.S. market (large hospitals, donor centers and reference laboratories) and in our international direct markets, a significant portion of the customers are automated.
2
Table of Contents
Traditional reagents are generally used in a manual setting, but certain traditional reagent products are also used on our automated instruments. Traditional reagents accounted for approximately 60% of our revenue in fiscal 2011. We believe there is a slight amount of seasonality in our reagent business as fewer donations and elective surgical procedures are performed in our first fiscal quarter (June-August).
Capture reagents (solid phase technology)
In our proprietary solid phase blood test system, known as Capture, red cell or platelet antigens are bound to a microtitration plate as a solid support (the solid phase), and the bound reactant “captures” other reactants in a fluid state and binds those fluid reactants to the solid phase. In this testing system, patient (or donor) serum or plasma is placed in the well of a plastic microtitration plate on which antigen reactants have been bound. Our special proprietary indicator cells are then added. In a positive reaction, antibodies in the test sample are captured by the indicator cells and adhere to the bottom of the test well as a thin layer. In a negative reaction, there is no antibody attached to the indicator cells, and they settle to the bottom of the test well as a small cell button.
Positive and negative reactions using our proprietary Capture technology
These reactions occur rapidly and result in clearly defined, machine-readable test results that are often easier to interpret than the subjective results sometimes obtained from traditional agglutination technology (the manual method). Also, in batch test mode, the solid phase test results can generally be obtained in substantially less time than by traditional agglutination techniques.
We have FDA approval or clearance for the Capture-P products for platelet testing, the Capture-R products for red cell testing, and one infectious disease test, Capture-CMV, for acute cytomegalovirus antibody testing.
Serology instruments and instrument systems
Our automated instrument-reagent systems operate on a “razor/razor blade” model. We offer customers a selection of automated instruments (the “razors”). Our instruments are “closed systems,” meaning only our reagents can be used on our instruments. The “razor/ razor blade” business model generates a recurring revenue stream for us.
We designed our systems to be scalable, enabling laboratories to better match the instrument to their various needs based on the test volume in their laboratories and the complexity of the testing required. We design and own the rights to our instruments, even though they are produced by third-party manufacturers.
NEO—Targeted at donor centers, large volume hospitals and reference laboratories, NEO provides a fully automated solution to perform all routine blood bank tests, including blood grouping, antibody screening, crossmatch, direct antiglobulin test (DAT) and antibody identification. NEO replaced our previous high volume instrument, Galileo. With NEO, we believe there is also an opportunity to expand our automated footprint in the hospital market because of NEO’s added features, including STAT (immediate priority) functionality, a faster turnaround time and improved reliability. NEO uses our proprietary Capture reagent products as well as certain of our traditional reagents.
Echo—Targeted at small- to medium-sized hospitals as well as at integrated delivery networks (both hospital and lab systems) in combination with NEO, Echo has a broad test menu and uses both our proprietary Capture reagents as well as certain of our traditional reagents to perform its testing.
Capture Workstation (Semi-automated processor)—The Capture Workstation has semi-automated components for performing our proprietary Capture assays manually. It is marketed as a back-up system for our fully automated NEO and Echo instruments, or as a standalone test system for small laboratories looking to standardize testing.
3
Table of Contents
Molecular immunohematology technology
In many countries, blood pre-transfusion testing is limited to the prevention of transfusion reactions and not for the prevention of alloimmunization, which occurs when antigens foreign to the patient are inadvertently introduced into the patient’s blood system through transfusions. If alloimmunization occurs, the patient develops new antibodies in response to the foreign antigens, thereby complicating future transfusions. By using multiplex, cost-effective molecular testing, our molecular technology allows testing to prevent alloimmunization for better patient care.
In August 2008, we added molecular immunohematology to our product portfolio with our acquisition of BioArray Solutions Ltd. (“BioArray”). BioArray developed a flexible technology platform that combines semiconductor processing, microparticle chemistry and molecular biology for the DNA testing of blood for transfusion. It allows for a variety of multiplex DNA-based testing, combining DNA amplification (PCR) with detection and data analysis software. With the goal of improving transfusion medicine, we believe that molecular immunohematology, which we market as the BeadChip system, will bring a high degree of flexibility and performance to DNA and protein analysis.
Our Human Erythrocyte Antigen (“HEA”) molecular immunohematology product, our Human Platelet Antigen (“HPA”) molecular immunohematology product as well as our current semi-automated molecular immunohematology instrument, the Array Imaging System and BASIS database, are CE (“Conformité Européenne”) Marked denoting regulatory clearance in the European Union (“EU”). Our molecular offering is currently available for Research Use Only in the U.S.
Our strengths
Market leader in critical patient care segment. We are a leader in the worldwide in-vitro diagnostic blood typing and screening market. We are the market leader in the U.S. and one of the top three industry players worldwide, according to information compiled by a third-party consultant. Our leadership position is supported by the depth and breadth of our product offerings, including our extensive line of traditional reagents and our innovative instrumentation. Our instrumentation is a key differentiator for us. We are currently the only company to offer two fully automated instruments worldwide for serology testing to meet the different needs of our customers depending upon the test volume in their laboratory and the complexity of the testing required. Additionally, we are the only company to have brought four generations of automation to the market, more than any of our competitors. The breadth of our product offerings allows us to service the full range of customers, including donor centers, reference laboratories, small- and medium-sized hospitals, and large hospitals, both in the automated and manual market.
Attractive industry dynamics. Historically, demand for blood in the U.S. market has been stable with a low single-digit organic growth rate. Blood demand, which has a direct correlation to the number of blood transfusions performed, is a strong indicator of industry type and screen testing volumes. Between 2001 and 2010, the compounded annual organic growth rate for blood demand was approximately 1.5% in the U.S., despite challenges in the last two years due to the economic downturn. Over time, we believe testing volumes will benefit from an aging population and cyclical uplift in healthcare utilization, which is currently depressed because of the economy.
Additionally, there is a secular trend towards automation in the U.S. market, where the majority of customers still perform blood typing and screening on a manual basis. Automation represents an attractive value proposition for hospitals as it relieves lab technician shortage issues, improves operational efficiencies and enhances patient safety.
Another attractive industry dynamic is that our business faces no material direct government reimbursement issues. Blood bank instruments and reagents typically represent a nominal (0.05%) percent of hospital expense budgets.
4
Table of Contents
“Razor/razor blade” business model results in highly attractive credit profile. We believe that instrument placements are the most effective way to drive revenue growth. The tangible benefits for customers of automation include gains in operational efficiencies and improvements in patient safety. We intend to continue to pursue a “razor/razor blade” business model, in which our goal in placing instruments is to secure a long-term, contractual relationship with the customer for reagents. Our serology instruments are closed systems, so each instrument placed typically provides us with a recurring revenue stream through the sale of reagents. We continually innovate to ensure our automation offerings are competitive.
Defensible end-markets and stable customer base. In the U.S., the FDA regulates the transfusion of human blood as a drug and as a biological product. Due to the regulated nature of our industry, we believe there are significant barriers to entry for new competitors to enter the U.S. market. Our customer relationships tend to be “sticky” due to the long-term nature of customer contracts and high switching costs. In the U.S. market, for example, switching costs typically include retraining of staff, rewriting of standard operating procedures, change of laboratory setup and work flow, and an FDA-mandated validation process.
Meaningful cash flow generation capabilities. Our business supports a high cash flow conversion profile, with operating cash flows of approximately 105% of net income and 63% percent of EBITDA over the last five years. Our operating cash flow generation can be attributed to attractive gross margins and minimal working capital requirements. Annual purchases of property and equipment were approximately 2.7% of net sales, and movement from inventory to property and equipment for instruments placed on rental agreements was approximately 3.9% of net sales in fiscal 2011. The movement from inventory to property and equipment, which is reflected in working capital in our statement of cash flows, is similar to a capital expenditure in that it represents the amount that we record as property and equipment when we rent our instruments rather than sell them. In fiscal 2011, we produced approximately $102.1 million of operating cash flow. We believe that our business has the capability to support further growth while also taking advantage of operating leverage and continuing to generate strong cash flow. For a reconciliation of EBITDA to net income for the periods presented, see “Selected historical consolidated financial data.”
Sponsor with significant healthcare experience and ability to add value. Funds managed by TPG Capital, L.P. (“TPG” or “TPG Capital”) have been among the most active healthcare investors, investing in approximately 20 companies over the last four years across the healthcare industry. Benefiting from its distinguished history with similar transactions, TPG can leverage its deep healthcare expertise and relationships to assist us in driving future performance. TPG also has a large group of operations professionals who will support our efforts to drive ongoing growth and operating efficiencies.
Experienced management team with a strong track record. We have a highly experienced leadership team that has extensive experience in the industry. Our senior managers have effectively managed the business through the economic downturn and have successfully positioned us for domestic and international growth. In October 2011, we named William A. Hawkins as CEO, who brings years of experience building some of the most dynamic, innovative companies in the health care industry and we believe further strengthens our management team.
Our strategy
Our long-term growth drivers revolve around our automation strategy. We believe innovative instrumentation is the key to improving blood bank operations and patient safety, as well as increasing our market share around the world. In implementing our strategy, we are focused on the following:
Capitalize on the trend towards automation. We estimate that the majority of customers in the U.S. still perform blood typing and screening on a manual basis, particularly in the small- to medium-sized hospital segment. Given the labor shortages in blood banks and the strain on hospital budgets, we believe there are significant economic drivers behind the automation trend. Automation can allow customers to reduce headcount and/or overtime in the
5
Table of Contents
blood bank, which can be a benefit given the current shortage of qualified blood bank technologists. We also believe that automation can improve patient safety, increase operational efficiency and, for customers such as integrated delivery networks with multiple blood banks, permit the standardization of best practices. Given the reduction in both human and economic capital, we estimate that our instruments have an average payback period of one year or less, depending on the size of the lab. Hence, we believe our automated products represent an attractive value proposition for manual customers to switch to automation.
Continue to gain share in our direct markets. Outside of the U.S., we have direct distribution operations in Canada, Western Europe and Japan as well as a network of third-party distributors in other parts of the world. As of May 31, 2011, approximately 30% of our sales were from outside the U.S. We have been successful in growing our business in our international direct markets by placing instruments through competitive wins. These markets are highly automated, and the strength of our innovative automation offering has enabled us to gain new business, especially in Europe, where our market share is significantly lower than in the U.S. We have grown our net sales to third-party customers in Europe at a CAGR of 12.3% over the past four years, demonstrating the success of our automation strategy. We believe that our innovative instruments, our proprietary and traditional serology reagents and our new molecular immunohematology offering will enable us to continue to grow our market share outside the U.S.
Penetrate underserved emerging markets. Infrastructure in emerging markets is far less developed than that in the U.S. and Europe, representing a significant opportunity for growth through automation. We believe the markets of China, India and Brazil represent the largest opportunities based on the population size and the estimated low level of automation present in these markets today. The long-term potential of the emerging markets is not included in the current estimated $1.2 billion market opportunity for instruments and reagents worldwide. Given our innovative product offerings, we believe the emerging markets represent a significant opportunity for future growth.
Further develop our industry-leading molecular platform. We have invested substantial effort and research and development expense in developing our molecular diagnostics platform and believe it will become an increasingly strategic asset over time. Molecular solutions have the potential to significantly enhance patient safety and outcomes. Molecular immunohematology is a developing field worldwide, and we believe we are on the forefront of this exciting market. Our most significant molecular assays have received regulatory approval in the European Union, and we are working to achieve regulatory approval in the U.S.
The Sponsor
TPG Capital is a leading global private investment firm founded in 1992 with $48 billion of assets under management and offices in San Francisco, Beijing, Chongqing, Fort Worth, Hong Kong, Houston, London, Luxembourg, Melbourne, Moscow, Mumbai, New York, Paris, Sao Paulo, Shanghai, Singapore and Tokyo. TPG Capital has extensive experience with global public and private investments executed through leveraged buyouts, recapitalizations, spinouts, growth investments, joint ventures and restructurings.
6
Table of Contents
The transactions
On August 19, 2011, Immucor, Inc. was acquired by investment funds sponsored by TPG (collectively, the “Sponsor”) and certain co-investors in the Acquisition, valued at approximately $1.9 billion, including the assumption of approximately $1.1 billion of acquisition-related debt and the incurrence of approximately $55.3 million of collective transaction costs which were incurred by Immucor and the Sponsor. As a result of the Acquisition, our stock is no longer publicly traded. Currently, the issued and outstanding shares of Immucor, Inc. are indirectly owned by the Sponsor and certain co-investors.
To consummate the Acquisition, we entered into new debt financing consisting of (i) $715.0 million of senior secured credit facilities (the “Senior Credit Facilities”) comprised of: (a) a $100.0 million, 5-year revolving credit facility (the “Revolving Facility”), which was undrawn at closing and (b) a $615.0 million, 7-year term loan credit facility (the “Term Loan Facility”), and (ii) $400.0 million of outstanding notes, which we are offering to exchange, upon the terms and subject to the conditions set forth in this prospectus and the accompanying letter of transmittal, for all of our exchange notes.
We refer to the equity investments described above, the Acquisition and the related transactions, including the issuance and sale of the outstanding notes and the borrowings under our Senior Credit Facilities, as the “Transactions.”
For additional information regarding the Transactions, see “Description of other indebtedness” and “Description of exchange notes.”
7
Table of Contents
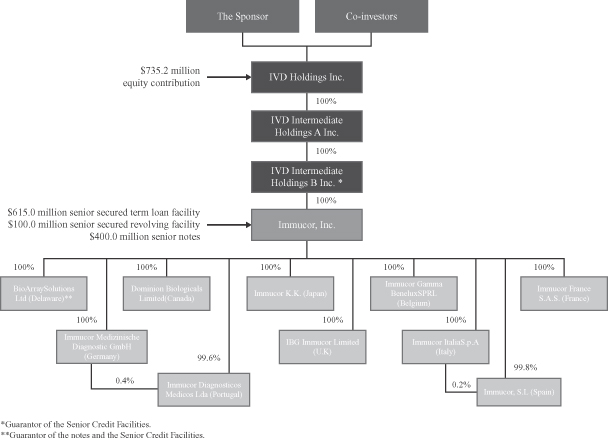
Corporate structure
Our corporate organizational structure is as follows:
Corporate information
Immucor’s corporate headquarters is located at 3130 Gateway Drive, Norcross, Georgia 30071. Our telephone number is (770) 441-2051. Our website is www.immucor.com. The information on our website is not deemed to be part of this prospectus, and you should not rely on it in connection with your decision whether or not to participate in the exchange offer.
8
Table of Contents
Ratio of earnings to fixed charges
The following table sets forth our ratio of earnings to fixed charges for each of the periods shown.
| (Predecessor) | (Combined) | (Pro forma) | ||||||||||||||||||||||||||||||
| Fiscal year ended May 31, | Quarter ended Aug. 31, 2011 |
Fiscal year
ended May 31, 2011 |
Quarter
ended August 31, 2011 |
|||||||||||||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | ||||||||||||||||||||||||||||
| Ratio of earnings to fixed charges |
110x | 106x | 96x | 100x | 92x | (a | ) | (b | )(c) | (b | )(c) | |||||||||||||||||||||
| (a) | The quarter ended August 31, 2011 reflects the addition of data from the Predecessor period and the Successor period to calculate the ratio on a combined basis. Our earnings were insufficient to cover fixed charges by $7.7 million for the quarter ended August 31, 2011. |
| (b) | The pro forma ratios of earnings to fixed charges have been developed by applying certain pro forma adjustments to our historical audited consolidated financial statements for the fiscal year ended May 31, 2011 and our unaudited interim consolidated financial statements for the period from June 1, 2011 through August 19, 2011 and for the period from August 20, 2011 to August 31, 2011, appearing elsewhere in this prospectus. For further information on pro forma adjustments and calculations, see “Unaudited pro forma consolidated financial information.” |
| (c) | Our pro forma earnings were insufficient to cover fixed charges by $17.9 million for the year ended May 31, 2011 and by $6.2 million for the quarter ended August 31, 2011. |
These ratios are computed by dividing the total earnings by the total fixed charges. For purposes of calculating the ratio of earnings to fixed charges, earnings represents pre-tax income from continuing operations plus fixed charges. Fixed charges consist of interest expense on all indebtedness plus amortization of deferred financing costs and the portion of rental expense that we believe is representative of the interest component of rental expense.
9
Table of Contents
The exchange offer
On August 19, 2011, we completed a private offering of the outstanding notes. Concurrently with the private offering, Merger Sub entered into a registration rights agreement (the “Registration Rights Agreement”) with J.P. Morgan Securities LLC , as representative of the several purchasers named in Schedule I to the Purchase Agreement (as defined in the Registration Rights Agreement). Concurrently with the consummation of the Acquisition, the Company and BioArray entered into a registration rights agreement joinder (the “Registration Rights Agreement Joinder”) pursuant to which the Company and BioArray assumed all of the rights and obligations of Merger Sub under the Registration Rights Agreement. Pursuant to the Registration Rights Agreement, we agreed, among other things, to file the registration statement of which this prospectus is a part. The following is a summary of the exchange offer. For more information please see “The exchange offer.” The “Description of exchange notes” section of this prospectus contains a more detailed description of the terms and conditions of the exchange notes.
| General |
The form and terms of the exchange notes are the same as the form and terms of the outstanding notes except that: |
| • | the issuance and sale of the exchange notes have been registered pursuant to an effective registration statement under the Securities Act; and |
| • | the holders of the exchange notes will not be entitled to the liquidation damages provision of the Registration Rights Agreement, which permits an increase in the interest rate on the outstanding notes in some circumstances relating to the timing of the exchange offer. See “The exchange offer.” |
| The exchange offer |
We are offering to exchange $400,000,000 aggregate principal amount of 11.125% Senior Notes due 2019 that have been registered under the Securities Act for all of our outstanding 11.125% Senior Notes due 2019. |
| The exchange offer will remain in effect for a limited time. We will accept any and all outstanding notes validly tendered and not withdrawn prior to 5:00 p.m., New York City time, on January 13, 2012. Holders may tender some or all of their outstanding notes pursuant to the exchange offer. However, outstanding notes may be tendered only in a denomination equal to $2,000 and any integral multiples of $1,000 in excess of $2,000. |
| Resale |
Based upon interpretations by the Staff of the SEC set forth in no-action letters issued to unrelated third-parties, we believe that the exchange notes may be offered for resale, resold or otherwise transferred by you without compliance with the registration and prospectus delivery requirements of the Securities Act, unless you: |
| • | are an “affiliate” of ours within the meaning of Rule 405 under the Securities Act; |
| • | are a broker-dealer that purchased the notes directly from us for resale under Rule 144A, Regulation S or any other available exemption under the Securities Act; |
10
Table of Contents
| • | acquired the exchange notes other than in the ordinary course of your business; |
| • | have an arrangement with any person to engage in the distribution of the exchange notes; or |
| • | are prohibited by law or policy of the SEC from participating in the exchange offer. |
| However, we have not obtained a no-action letter, and there can be no assurance that the SEC will make a similar determination with respect to the exchange offer. Furthermore, in order to participate in the exchange offer, you must make the representations set forth in the letter of transmittal that we are sending you with this prospectus. |
| Expiration date |
The exchange offer will expire at 5:00 p.m., New York City time, on January 13, 2012, unless we decide to extend it. We do not currently intend to extend the expiration date. |
| Conditions to the exchange offer |
The exchange offer is subject to certain customary conditions, some of which may be waived by us. See “The exchange offer—Conditions to the exchange offer.” |
| Procedures for tendering outstanding notes |
To participate in the exchange offer, you must properly complete and duly execute a letter of transmittal, which accompanies this prospectus, and transmit it, along with all other documents required by such letter of transmittal, to the exchange agent on or before the expiration date at the address provided on the cover page of the letter of transmittal. |
| In the alternative, you can tender your outstanding notes by following the automatic tender offer program, or ATOP, procedures established by The Depository Trust Company, or DTC, for tendering notes held in book-entry form, as described in this prospectus, whereby you will agree to be bound by the letter of transmittal and we may enforce the letter of transmittal against you. |
| If a holder of outstanding notes desires to tender such notes and the holder’s outstanding notes are not immediately available, or time will not permit the holder’s outstanding notes or other required documents to reach the exchange agent before the expiration date, or the procedure for book-entry transfer cannot be completed on a timely basis, a tender may be effected pursuant to the guaranteed delivery procedures described in this prospectus. |
| For more details, please read “The exchange offer—Procedures for tendering,” “The exchange offer—Book-entry transfer” and “The exchange offer—Guaranteed delivery procedures.” |
11
Table of Contents
| Special procedures for beneficial owners |
If you are a beneficial owner of outstanding notes that are registered in the name of a broker, dealer, commercial bank, trust company or other nominee, and you wish to tender those outstanding notes in the exchange offer, you should contact the registered holder promptly and instruct the registered holder to tender those outstanding notes on your behalf. If you wish to tender on your own behalf, you must, prior to completing and executing the letter of transmittal and delivering your outstanding notes, either make appropriate arrangements to register ownership of the outstanding notes in your name or obtain a properly completed bond power from the registered holder. The transfer of registered ownership may take considerable time and may not be able to be completed prior to the expiration date. |
| Withdrawal rights |
You may withdraw your tender of outstanding notes at any time prior to 5:00 p.m., New York City time, on the expiration date of the exchange offer. Please read “The exchange offer—Withdrawal of tenders.” |
| Acceptance of outstanding notes and delivery of exchange notes |
Subject to customary conditions, we will accept outstanding notes that are properly tendered in the exchange offer and not withdrawn prior to the expiration date. The exchange notes will be delivered promptly following the expiration date. |
| Consequences of failure to exchange outstanding notes |
If you do not exchange your outstanding notes in the exchange offer, you will no longer be able to require us to register the outstanding notes under the Securities Act, except in the limited circumstances provided under the Registration Rights Agreement. In addition, you will not be able to resell, offer to resell or otherwise transfer the outstanding notes unless we have registered the outstanding notes under the Securities Act, or unless you resell, offer to resell or otherwise transfer them under an exemption from the registration requirements of, or in a transaction not subject to, the Securities Act. |
| Dissenters’ rights |
Holders of outstanding notes do not have any appraisal or dissenters’ rights in connection with the exchange offer. We intend to conduct the exchange offer in accordance with the applicable requirements of the Exchange Act and the rules and regulations of the SEC. |
| Interest on the exchange notes and the outstanding notes |
The exchange notes will bear interest from the most recent interest payment date on which interest has been paid on the outstanding notes, or if no interest has been paid, the date the outstanding notes were issued. Holders whose outstanding notes are accepted for exchange will be deemed to have waived the right to receive interest accrued on the outstanding notes. |
| Broker-dealers |
Each broker-dealer that receives exchange notes for its own account in exchange for outstanding notes, where such outstanding notes were acquired by such broker-dealer as a result of market-making activities |
12
Table of Contents
| or other trading activities, must acknowledge that it will deliver a prospectus in connection with any resale of such exchange notes. See “Plan of distribution.” |
| Material U.S. federal income tax considerations |
The exchange of outstanding notes for exchange notes pursuant to the exchange offer will not constitute a taxable event for U.S. federal income tax purposes. Please read “Material United States federal income tax considerations.” |
| Exchange agent |
Deutsche Bank Trust Company Americas, the trustee under the indenture governing the notes, or the indenture, is serving as exchange agent in connection with the exchange offer. |
| Use of proceeds |
The issuance of the exchange notes will not provide us with any new proceeds. We are making the exchange offer solely to satisfy certain of our obligations under the Registration Rights Agreement. |
| Fees and expenses |
We will bear all expenses related to the exchange offer. Please read “The exchange offer—Fees and expenses.” |
13
Table of Contents
The exchange notes
The following summary contains basic information about the exchange notes and is not intended to be complete. For a more complete understanding of the exchange notes and the guarantees, please refer to the section entitled “Description of exchange notes” in this prospectus.
| Issuer |
Immucor, Inc. (successor by merger to IVD Acquisition Corporation). |
| Securities offered |
Up to $400.0 million in aggregate principal amount of 11.125% Senior Notes due 2019. The exchange notes and the outstanding notes will be considered to be a single class for all purposes under the indenture, including waivers, amendments, redemptions and offers to purchase. |
| Maturity date |
August 15, 2019. |
| Interest rate |
11.125% per year. |
| Interest payment dates |
February 15 and August 15, commencing February 15, 2012. |
| Optional redemption |
The exchange notes will be redeemable at our option, in whole or in part, at any time on or after August 15, 2015, at the redemption prices set forth in this prospectus, together with accrued and unpaid interest, if any, to the date of redemption. See “Description of exchange notes—Optional redemption.” |
| At any time prior to August 15, 2014, we may redeem up to 35% of the aggregate principal amount of the exchange notes and outstanding notes with the net proceeds of certain equity offerings at the redemption price set forth in this prospectus, plus accrued and unpaid interest, if any, to the redemption date. See “Description of exchange notes—Optional redemption.” |
| At any time prior to August 15, 2015, we may also redeem some or all of the exchange notes at a price equal to 100% of the principal amount of the exchange notes, plus accrued and unpaid interest, plus a “make-whole premium.” See “Description of exchange notes—Optional redemption.” |
| Change of control offer |
Upon the occurrence of specific kinds of changes of control, you will have the right, as holders of the exchange notes, to cause us to repurchase some or all of your exchange notes at 101% of their principal amount, plus accrued and unpaid interest to, but not including, the repurchase date. See “Description of exchange notes—Repurchase at the option of holders—Change of control.” |
| Asset disposition offer |
If the Issuer or its restricted subsidiaries sell assets, under certain circumstances, the Issuer will be required to use the net proceeds to make an offer to purchase the exchange notes at an offer price in cash in an amount equal to 100% of the principal amount of the exchange |
14
Table of Contents
| notes, plus accrued and unpaid interest to, but not including, the repurchase date. See “Description of exchange notes—Repurchase at the option of holders—Asset sales.” |
| Guarantees |
The exchange notes are guaranteed on a senior unsecured basis by our existing domestic subsidiary, BioArray Solutions Ltd., and all of our future direct and indirect domestic subsidiaries that guarantee the Senior Credit Facilities or our other indebtedness or indebtedness of any guarantor. Under certain circumstances, subsidiary guarantors may be released from their guarantees without the consent of the holders of the exchange notes. See “Description of exchange notes—Guarantees.” |
| Ranking |
The exchange notes and the subsidiary guarantees will be our and the guarantors’ senior unsecured obligations and will: |
| • | rank senior in right of payment to all of our and the subsidiary guarantors’ existing and future subordinated indebtedness; |
| • | rank equally in right of payment with all of our and the subsidiary guarantors’ existing and future senior indebtedness; |
| • | be effectively subordinated to any of our and the subsidiary guarantors’ existing and future secured debt, to the extent of the value of the assets securing such debt; and |
| • | be structurally subordinated to all of the existing and future liabilities (including trade payables) of each of our subsidiaries that do not guarantee the exchange notes. |
| As of August 31, 2011: |
| • | we had approximately $1,015 million of total indebtedness, all of which would rank equally with the exchange notes; |
| • | of our total indebtedness, we had approximately $615 million of secured indebtedness under our Senior Credit Facilities to which the exchange notes would be effectively subordinated; |
| • | we had commitments available to be borrowed under the Senior Credit Facilities of $100 million, which amount could increase by $150 million (or a greater amount if we meet specified financial ratios), the availability of which is subject to certain conditions; and |
| • | our non-guarantor subsidiaries’ total liabilities (including trade payables) would have been structurally senior to the exchange notes. |
| Covenants |
The indenture governing the exchange notes contains covenants that, among other things, limit our ability and the ability of our restricted subsidiaries to: |
| • | incur additional indebtedness; |
| • | pay dividends or make other distributions or repurchase or redeem our capital stock; |
15
Table of Contents
| • | prepay, redeem or repurchase certain debt; |
| • | make loans and investments; |
| • | sell assets; |
| • | incur liens; |
| • | enter into transactions with affiliates; |
| • | enter into agreements restricting our subsidiaries’ ability to pay dividends; and |
| • | consolidate, merge or sell all or substantially all of our assets. |
| These covenants are subject to a number of important exceptions and qualifications. In addition, if and for so long as the exchange notes have an investment grade rating from both Standard & Poor’s Ratings Group and Moody’s Investors Service, Inc. and no default under the indenture has occurred and is continuing, certain of the covenants listed above will be suspended. For more details, see “Description of exchange notes.” |
| No Public Market |
The exchange notes will be freely transferable but will be new securities for which there will not initially be a market. Accordingly, we cannot assure you whether a market for the exchange notes will develop or as to the liquidity of any market. The initial purchasers in the private offering of the outstanding notes have advised us that they currently intend to make a market in the exchange notes. The initial purchasers are not obligated, however, to make a market in the exchange notes, and any such market-making may be discontinued by the initial purchasers in their discretion at any time without notice. Accordingly, we cannot assure you that a liquid market for the exchange notes will develop or be maintained. |
| Risk Factors |
See “Risk factors” and the other information in this prospectus for a discussion of some of the factors you should carefully consider before participating in the exchange offer. |
16
Table of Contents
You should carefully consider the risks described below and all of the information contained in this prospectus before deciding whether to participate in the exchange offer. The risks and uncertainties described below are not the only risks and uncertainties that we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations. If any of those risks actually occurs, our business, financial condition and results of operations would suffer. In such case, you may lose all or part of your original investment in the notes. The risks discussed below also include forward-looking statements, and our actual results may differ substantially from those discussed in these forward-looking statements. See “Cautionary statement regarding forward-looking statements” in this prospectus.
Risks related to the company
Lower blood demand could negatively impact our financial results.
Our products are used to test blood prior to transfusion. Lower demand for blood in the markets in which we operate could result in lower testing volumes. For example, we believe the U.S. market has been experiencing lower demand for blood in fiscal 2010 and 2011. Lower blood demand could result from a variety of factors, such as fewer elective surgeries and more efficient blood utilization by hospitals. Blood is a large expense for hospital laboratories and pressure on hospital budgets due to macroeconomic factors and healthcare reform could force changes in the ways in which blood is used. Lower blood demand could negatively impact our revenue and profitability.
FDA administrative action could have a material and adverse effect on our business.
In June 2009, we announced that the FDA, in an administrative action based on a January 2009 inspection, issued a notice of intent to revoke our biologics license (“NOIR”) with respect to our Reagent Red Blood Cells and Anti-E (Monoclonal) Blood Grouping Reagent products. During June 2010, the FDA conducted an inspection of our facilities. During September 2010, we were notified by the FDA that while the June 2010 inspection “disclosed that substantive corrections have been made, some deviations continue.” Therefore, the FDA stated that the conditions outlined in the June 2009 NOIR administrative action remain in effect. The FDA stated that it will evaluate our overall compliance status at its next inspection. There is no set schedule for such FDA inspections, so we do not know when that next inspection will take place.
As part of its overview responsibility, the FDA makes plant and facility inspections on an unannounced basis. The FDA could seek to escalate the NOIR by revoking our biologics license for the products impacted if we fail to implement a corrective action plan that adequately addresses the deficiencies noted by the NOIR. The FDA could also take further regulatory actions, including seeking a consent decree, recalling or seizing of our products, ordering a total or partial shutdown of production, delaying future marketing clearances or approvals, and withdrawing or suspending of our current products from the market.
On-going governmental investigations and litigation could have a material and adverse effect on our business.
We are the subject of a number of investigations and private actions.
| • | The Federal Trade Commission (“FTC”) is investigating whether Immucor violated federal antitrust laws or engaged in unfair methods of competition, through three acquisitions made in the period from 1996 through 1999 or by restricting price competition. The FTC could decide to commence administrative and possibly federal court proceedings for purposes of determining whether there has been a violation and might seek to impose a variety of remedies for any violation including injunctive relief, divestiture of assets and/or disgorgement of profits. |
| • | We are the subject of a number of private civil actions by customers seeking class certification and alleging price fixing. Briefing relating to potential certification of a class began in September 2011, with plaintiffs’ motion to certify a class. Defendants must reply by February 2012, and the Court will determine whether to |
17
Table of Contents
| hear argument on plaintiffs’ motion. A decision on class certification could occur shortly after these filings and a hearing, if one is held. Were plaintiffs to prevail in one or more of the pending civil actions, we could have to pay significant amounts, including treble damages and attorneys’ fees, and these amounts would not be covered by our insurance policies. |
| • | We are the subject of a private civil action brought in the United States District Court for the Northern District of Georgia by a shareholder seeking class certification and alleging that the Company failed to disclose material information with respect to alleged violations of the antitrust laws and that the Company made inadequate disclosures about the results of FDA inspections and the Company’s quality control efforts. In June 2011, the Court granted our motion to dismiss the action and closed the case. In September 2011 the plaintiff appealed that dismissal to the United States Court of Appeal for the Eleventh Circuit, and that appeal is pending. Were this action to be reinstated and the plaintiff to prevail on behalf of a class, we could have to pay significant amounts, including damages and attorneys’ fees. |
The imposition of any of the above remedies could have a materially adverse impact on our business, financial condition and results of operations. In addition, regardless of the ultimate outcome in the above matters, we expect to incur significant expenses, including attorneys’ fees, in responding to and defending against issues raised in the above matters.
A catastrophic event at our Norcross, Georgia facility would prevent us from producing many of our reagent products.
Substantially all our reagent products are produced in our Norcross facility. While we have reliable supplies of most raw materials, our reagent production is highly dependent on the uninterrupted and efficient operation of the Norcross facility, and we currently have no plans to develop a third-party reagent manufacturing capability as an alternative source of supply. Therefore, if a catastrophic event occurred at the Norcross facility, such as a fire or tornado, many of those products could not be produced until the manufacturing portion of the facility was restored and cleared by the FDA. We maintain a disaster plan to minimize the effects of such a catastrophe, and we have obtained insurance to protect against certain business interruption losses. However, there can be no assurance that such coverage will be adequate or that such coverage will continue to remain available on acceptable terms, if at all.
Unforeseen product performance problems could prevent us from selling or result in a recall of the affected products.
In the event that we experience a product performance problem with either our instruments or our reagents, we may be required to, or may voluntarily, recall or suspend selling the products until the problem is resolved. We have from time to time initiated voluntary recalls of our products. Depending on the product as well as the availability of acceptable substitutes, such a product recall or suspension could significantly impact our operating results.
Poor product performance could increase operating costs and result in the loss of current or future customers.
Instrument performance and reliability is a key factor in satisfying current customers and attracting new customers. Poor performance or unreliability of instruments would not only increase maintenance costs but also could result in losing important current customers and an inability to gain new customers. Therefore, if we are unable to provide effective instrument support and service and minimize instrument down time, our revenues and financial results would be adversely affected.
Because we sell our products internationally, we could be adversely affected by fluctuations in foreign currency exchange rates.
In the fiscal year ended May 31, 2011, revenue outside the U.S. was approximately 30% of total revenue. As a result, fluctuations in foreign currency exchange rates, particularly the Euro, the Canadian Dollar, the British
18
Table of Contents
Pound and the Japanese Yen against the U.S. Dollar, could make our products less competitive and affect our sales and earnings levels. An increase in our revenue outside the U.S. would increase this exposure. We have not historically hedged against currency exchange rate fluctuations.
Gross margin volatility may negatively impact our profitability.
Our gross margin may be volatile from period to period due to various factors, including instrument sales, reagent product mix and manufacturing costs. With the introduction of the NEO serology instrument and as we continue to drive automation in the blood bank marketplace, we may experience increased instrument sales. The probable sales mix (in terms of instrument/reagent sales) could make it difficult for us to sustain the overall gross margins we have generated in the past. The higher margins on the Capture reagents used on our instruments may not be enough to offset the lower margins on the instruments themselves. For our reagent products, margins vary depending upon the product with rarer products generating higher margins. Depending upon the sales mix of these products, margins could vary significantly from period to period. Our reagent products are manufactured in-house. Margins for these products could be impacted based upon costs of raw materials and labor as well as overhead and the efficiency of our manufacturing operations from period to period. Margins may also be negatively impacted by increased competition. New market entrants or existing market participants seeking to gain market share may foster a competitive environment of pricing pressures and/or increased marketing and other expenditures that could negatively impact profitability.
If customers delay integrating our instruments into their operations, the growth of our business could be negatively impacted.
From time to time in the past, some of our customers have experienced significant delays between the purchase of an instrument and the time at which it has been successfully integrated into the customer’s existing operations and is generating reagent revenue at its expected annualized run rate. These delays may be due to a number of factors, including staffing and training issues and difficulties interfacing our instruments with the customer’s computer systems. Because our business operates on a “razor/razor blade” model, such integration delays result in delayed purchases of the reagents used with the instrument. A number of steps have mitigated these integration delays: improved performance of our field service staff, better instrument instructions, increased use of internet-based remote diagnostic tools, and more efficient scheduling of instrument installations. In addition, we have taken steps in the design of our next generation instruments intended to make it easier for our customers to integrate the instruments into existing operations. However, delays of customers successfully integrating instruments into their operations could adversely impact our future revenues and earnings growth.
We may not be successful in capitalizing on acquisitions of former distributors or newly established distribution networks outside the U.S.
An integral part of our strategy is to place our instruments in additional markets outside North America. To further this strategy, in the past few years we have acquired our former distribution businesses in Japan and the U.K. and established our own direct distribution organization in France. Our ability to grow successfully in overseas markets depends in part on our ability to achieve product acceptance and customer loyalty in these markets. Additionally, our operations in foreign countries present certain challenges and are subject to certain risks not necessarily present in our domestic operations, such as fluctuations in currency exchange rates, shipping delays, changes in applicable laws and regulations and various restrictions on trade. These factors could impact our ability to compete successfully in these markets, which could in turn negatively affect our international expansion goals, and could have a material adverse effect on our operating results.
Our financial performance is dependent on the timely and successful introduction of new products and services.
Our financial performance depends in part upon our ability to successfully develop and market next generation automated instruments and other products in a rapidly changing technological and economic environment. Our
19
Table of Contents
market share and operating results would be adversely affected if we fail to successfully identify new product opportunities and timely develop and introduce new instruments that achieve market acceptance, or if new products or technology are introduced in the market by competitors that could render Immucor’s instruments or reagents uncompetitive or obsolete. In addition, delays in the introduction of new products due to regulatory, developmental, or other obstacles could negatively impact our revenue and market share, as well as our earnings, including the potential impairment of goodwill related to our molecular immunohematology offering.
Global economic conditions may have a material adverse impact on our results.
Immucor is a global company with direct operations in the U.S., Canada, Western Europe and Japan and customers around the world. General economic conditions impact our customers, particularly hospitals. For our instruments, reduced capital budgets that result from negative economic conditions, such as a global recession, could result in lower instrument sales, which would negatively impact our future revenue, profitability and cash flow. A shift from capital purchases to rentals, which require no upfront cash outlay, could negatively impact our cash flow in the near term. Additionally, global economic conditions may adversely affect the ability of our customers to access funds to enable them to fund their operating and capital budgets. Budget constraints could slow our progress in driving automation in both our customer base and the blood banking industry as a whole, which could negatively impact our future revenues, profitability and cash flow.
We are highly dependent on our senior management team and other key employees, and the loss of one or more of these employees could adversely affect our operations.
Our success is dependent upon the efforts of our senior management and staff, including sales, technical and management personnel, many of whom have very specialized industry and technical expertise that is not easily replaced. For example, in April 2011 our Chief Operating Officer resigned, and in June 2011 our President and Chief Executive Officer retired. In October 2011, our replacement President and Chief Executive Officer resigned. If key individuals leave us, we could be adversely affected if suitable replacement personnel are not quickly recruited. Our future success depends on our ability to continue to attract, retain and motivate qualified personnel. There is intense competition for medical technologists, and in some markets there is a shortage of qualified personnel in our industry. If we are unable to continue to attract or retain highly qualified personnel, the development, growth and future success of our business could be adversely affected.
Supply chain interruptions could negatively impact our operations and financial performance.
Supply chain interruptions could negatively impact our operations and financial performance. The supply of any of our manufacturing materials may be interrupted because of poor vendor performance or other events outside our control, which may require us, among other things, to identify alternate vendors and result in lost sales and increased expenses. While such interruption could impact any of our third-party sourced materials, two particular areas of note are our instrument suppliers and our supply sources for rare antibodies or antigen combinations, which are described below.
We purchase our instruments from single-source suppliers. If the supply of any of our instruments were interrupted, due to the supplier’s financial problems or otherwise, we believe an alternative supplier could be found but that it would take in the range of 18 months to 24 months to transfer the technology and begin production with a new instrument supplier. The disruption of one of these supply relationships could cause us to incur costs associated with the development of an alternative source. Also, we may be required to obtain FDA clearance of the instrument if it is not built to the same specifications as with the previous supplier. The process of changing an instrument supplier could have an adverse impact on future growth opportunities during the transition period if supplies of finished goods on hand were insufficient to satisfy demand.
Some of our reagent products are derived from blood having particular or rare combinations of antibodies or antigens, which are found in a limited number of individuals. If we had difficulty in obtaining sufficient quantities of such blood and the supply was interrupted, we would need to establish a viable alternative, which
20
Table of Contents
may take both time and expense to either identify and/or develop and could have an adverse impact on our operations and financial position.
Distribution chain interruptions could negatively impact our operations and financial performance.
Distribution chain interruptions could negatively impact our operations and financial performance. Our international affiliates are supplied with a significant amount of product from our U.S. manufacturing facilities. If circumstances arose that disrupted our distribution of U.S.-sourced product internationally, we would need to establish an alternate distribution channel, which may take both time and expense to establish and could have an adverse impact on our operations and financial position.
We may be unable to adequately protect our proprietary technology.
We have a substantial patent portfolio of issued patents or pending patent applications supporting our molecular immunohematology offering. Also, we have one of the original six patents on our proprietary Capture technology still in force. Our competitiveness depends in part on our ability to maintain the proprietary nature of our owned and licensed intellectual property. Because the law is constantly changing, and unforeseen facts may arise, there is always a risk that patents may be found to be invalid or unenforceable. Therefore, there is no absolute certainty as to the exact scope of protection associated with any intellectual property. We believe our patents, together with our trade secrets and know-how, will prevent any current or future competitors from successfully copying and distributing our BeadChip and Capture products. However, there can be no assurance that competitors will not develop around the patented aspects of any of our current or proposed products or independently develop technology or know-how that is equivalent to or competitive with our technology and know-how. Any damage to our intellectual property portfolio could result in an adverse effect on our current or proposed products, our revenues and our operations.
Protecting our intellectual property rights is costly and time consuming. We may need to initiate lawsuits to protect or enforce our patents, or litigate against third-party claims, which would be expensive and, if we lose, may cause us to lose some of our intellectual property rights and reduce our ability to compete in the marketplace. Furthermore, these lawsuits may divert the attention of our management and technical personnel.
We may be subject to intellectual property rights infringement claims in the future, which are costly to defend, could require us to pay substantial damages and could limit our ability to use certain technologies in the future.
Our commercial success depends, in part, not only on protecting our own intellectual property but on not infringing the patents or proprietary rights of third parties. Were third parties to claim that we infringe on their intellectual property rights, responding to such claims, regardless of their merit, could be time consuming, result in costly litigation, divert management’s attention and resources and cause us to incur significant expenses. Our practices, products and technologies, particularly with respect to the field of molecular immunohematology, may not be able to withstand third-party claims, regardless of the merits of such claims.
As a result of such potential intellectual property infringement claims, we could be required or otherwise decide it is appropriate to discontinue manufacturing, using, or selling particular products subject to infringement claims or develop other technology not subject to infringement claims, which could be time-consuming and costly or may not be possible. In addition, to the extent potential claims against us are successful, we may have to pay substantial monetary damages or discontinue certain of our practices, products or technologies that are found to be in violation of another party’s rights. We also may have to seek third-party licenses to continue certain of our existing or planned product lines, thereby incurring substantial costs related to royalty payments for such licenses, which could negatively affect our gross margins. Also, license agreements can be terminated under appropriate circumstances. No assurance can be given that efforts to remediate any infringement would be successful or that licenses could be obtained on acceptable terms or that litigation will not occur.
21
Table of Contents
In the event there is a temporary or permanent injunction entered prohibiting us from marketing or selling certain of our products, or a successful claim of infringement against us requiring us to pay royalties to a third party and we fail to license such technology on acceptable terms and conditions or to develop or license a substitute technology, our business, results of operations or financial condition could be materially adversely affected.
We are controlled by the Sponsor, whose interests as an equity holder may conflict with yours as creditor.
We are controlled by the Sponsor. The Sponsor controls the election of our directors and thereby has the power to control our affairs and policies, including the appointment of management, the issuance of additional stock and the declaration and payment of dividends. The Sponsor will not have any liability for any obligations under the notes, and their interests may be in conflict with yours. For example, if we encounter financial difficulties or are unable to pay our debts as they mature, the Sponsor may pursue strategies that favor equity investors over debt investors. In addition, our equity holders may have an interest in pursuing acquisitions, divestitures, financing or other transactions that, in their judgment, could enhance their equity investments, even though such transactions may involve risk to you as a holder of the notes. Additionally, the Sponsor may make investments in businesses that directly or indirectly compete with us, or may pursue acquisition opportunities that may be complementary to our business and, as a result, those acquisition opportunities may not be available to us. For information concerning our arrangements with the Sponsor, see “Certain relationships and related party transactions.”
Risks relating to our industry
Government regulation may delay or prevent new product introduction and affect our ability to continue manufacturing and marketing existing products.
Our instruments, reagents and other products are subject to regulation by governmental and private agencies in the U.S. and abroad, which regulate the testing, manufacturing, packaging, labeling, distribution and marketing of medical supplies and devices. Certain international regulatory bodies also impose import and tax restrictions, tariff regulations, and duties on imported products. Delays in agency review can significantly delay new product introduction and may result in a product becoming “outdated” or losing its market opportunity before it can be introduced. Also, the FDA and international agencies have the authority to require a recall or modification of products in the event of a defect.
FDA clearance or approval generally is required before we can market new instruments or reagents in the U.S. or make significant changes to existing products. The process of obtaining marketing clearances and approvals from regulatory agencies for new products can be time consuming and expensive. There is no assurance that clearances or approvals will be granted or that agency review will not involve delays that would adversely affect our ability to commercialize our products.
If any of our products failed to perform in the manner represented during this clearance or approval process, particularly concerning safety issues, one or more of these agencies could require us to cease manufacturing and selling that product, or even recall previously-placed products, and to resubmit the product for clearance or approval before we could sell it again. Depending on the product, and the availability of acceptable substitutes, such an agency action could result in significantly reduced revenues and earnings for an indefinite period. See “—Risks related to the company—FDA administrative action could have a material and adverse effect on our business.”
Federal, state and foreign regulations regarding the manufacture and sale of our products are subject to change. We cannot predict what impact, if any, such changes might have on our business. In addition, there can be no assurance that regulation of our products will not become more restrictive in the future and that any such development would not have a material adverse effect on our business.
22
Table of Contents
The industry and market segment in which we operate are highly competitive, and we may not be able to compete effectively with larger companies with greater financial resources than we have.
Our industry and the markets we operate in are highly competitive. Some of our competitors have greater financial resources than we do, making them better equipped to fund research and development, manufacturing and marketing efforts, or license technologies and intellectual property from third parties. Moreover, competitive and regulatory conditions in many markets in which we operate restrict our ability to fully recoup our costs in those markets. Our competitors can be expected to continue to improve the design and performance of their products and to introduce new products with competitive price and performance characteristics. Although we believe that we have certain technological and other advantages over our competitors, maintaining these advantages will require us to continue to invest in research and development, sales and marketing and customer service and support. We cannot assure you that we will have sufficient resources to continue to make such investments at levels that our larger competitors could make or that we will be successful in maintaining such advantages.
Increased competition in the United States could negatively impact our revenues and profitability.
We could face increased competition in the U.S. market, which historically has had a limited number of market participants. For fiscal 2011, approximately 70% of our revenues were generated in the U.S., and our U.S. operations have higher gross margins than our operations outside the U.S. Additional competition in the U.S. could negatively impact our revenues and/or our profitability.
Changes in government policy may have a material adverse effect on our business.
Changes in government policy could have a significant impact on our business by increasing the cost of doing business, affecting our ability to sell our products and negatively impacting our profitability. Such changes could include modifications to existing legislation, such as U.S. tax policy, or entirely new legislation, such as the healthcare reform bill adopted in 2010. The Patient Protection and Affordable Care Act that became law in March 2010 imposes a new 2.3% excise tax on medical device makers beginning in 2013, which could have a material negative impact on our results of operations and our cash flows. Other elements of this legislation could meaningfully change the way healthcare is developed and delivered, and may materially impact numerous aspects of our business.
We may be exposed to product liability claims resulting from the use of products we sell and distribute.
Although product liability claims in our industry are infrequent, the expansion of our business in an increasingly litigious business environment may expose us to product liability claims related to the products we sell. We maintain insurance that includes product liability coverage, and we believe our insurance coverage is adequate for our business. However, there can be no assurance that insurance coverage for these risks will continue to be available or, if available, that it will be sufficient to cover potential claims or that the present level of coverage will continue to be available at a reasonable cost. A partially or completely uninsured successful claim against us could have a material adverse effect on us.
Risks related to our indebtedness and the exchange notes
Our substantial indebtedness could adversely affect our financial condition and prevent us from fulfilling our obligations under the exchange notes.
We have a significant amount of indebtedness. As of August 31, 2011, our total debt was $1,015 million and we had unused commitments of $100.0 million under our Senior Credit Facilities, which amount could increase by $150.0 million (or a greater amount if we meet specified financial ratios), the availability of which is subject to certain conditions.
23
Table of Contents
Subject to the limits contained in the credit agreement governing our Senior Credit Facilities and the indenture that governs the notes, we may be able to incur substantial additional debt from time to time to finance working capital, capital expenditures, investments or acquisitions, or for other purposes. If we do so, the risks related to our high level of debt could intensify. Specifically, our high level of debt could have important consequences to the holders of the exchange notes, including:
| • | making it more difficult for us to satisfy our obligations with respect to the exchange notes and our other debt; |
| • | requiring a substantial portion of our cash flows to be dedicated to debt service payments instead of other purposes, thereby reducing the amount of cash flows available for working capital, capital expenditures, acquisitions and other general corporate purposes; |
| • | limiting our ability to obtain additional financing to fund future working capital, capital expenditures, acquisitions or other general corporate requirements; |
| • | increasing our vulnerability to general adverse economic and industry conditions; |
| • | exposing us to the risk of increased interest rates as certain of our borrowings, including borrowings under our Senior Credit Facilities, are at variable rates of interest; |
| • | limiting our flexibility in planning for and reacting to changes in the industry in which we compete; |
| • | placing us at a disadvantage compared to other, less leveraged competitors; |
| • | increasing our cost of borrowing; and |
| • | preventing us from raising the funds necessary to repurchase all the notes tendered to us upon the occurrence of certain changes of control, which would constitute a default under the indenture governing the notes. |
In addition, the indenture that governs the notes and the credit agreement governing our Senior Credit Facilities contain restrictive covenants that limit our ability to engage in activities that may be in our long-term best interest. Our failure to comply with those covenants could result in an event of default which, if not cured or waived, could result in the acceleration of all our debt.
We may not be able to generate sufficient cash to service all of our indebtedness, including the exchange notes, and may be forced to take other actions to satisfy our obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments on or refinance our debt obligations, including the exchange notes, depends on our financial condition and operating performance, which are subject to prevailing economic and competitive conditions and to certain financial, business, legislative, regulatory and other factors beyond our control. We may be unable to maintain a level of cash flows from operating activities sufficient to permit us to pay the principal, premium, if any, and interest on our indebtedness, including the exchange notes.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we could face substantial liquidity problems and could be forced to reduce or delay investments and capital expenditures or to dispose of material assets or operations, seek additional debt or equity capital or restructure or refinance our indebtedness, including the exchange notes. We may not be able to effect any such alternative measures on commercially reasonable terms or at all and, even if successful, those alternative actions may not allow us to meet our scheduled debt service obligations. The credit agreement governing our Senior Credit Facilities and the indenture governing the notes restrict our ability to dispose of assets and use the proceeds from those dispositions and may also restrict our ability to raise debt or equity capital to be used to repay other indebtedness when it becomes due. We may not be able to consummate those dispositions or to obtain proceeds in an amount sufficient to meet any debt service obligations then due.
24
Table of Contents
In addition, we conduct a substantial portion of our operations through our subsidiaries, all of which except our single domestic subsidiary, BioArray Solutions Ltd., currently are foreign subsidiaries and will not be guarantors of the exchange notes or our other indebtedness. Accordingly, repayment of our indebtedness, including the exchange notes, is dependent on the generation of cash flow by our subsidiaries and their ability to make such cash available to us, by dividend, debt repayment or otherwise. Our non-guarantor subsidiaries do not have any obligation to pay amounts due on the exchange notes or our other indebtedness or to make funds available for that purpose. Our subsidiaries may not be able to, or may not be permitted to, make distributions to enable us to make payments in respect of our indebtedness, including the exchange notes. Each subsidiary is a distinct legal entity, and, under certain circumstances, legal and contractual restrictions may limit our ability to obtain cash from our subsidiaries. While the indenture governing the notes and the credit agreement governing our Senior Credit Facilities limit the ability of our subsidiaries to incur contractual restrictions on their ability to pay dividends or make other intercompany payments to us, these limitations are subject to qualifications and exceptions. In the event that we do not receive distributions from our subsidiaries, we may be unable to make required principal and interest payments on our indebtedness, including the exchange notes.
Our inability to generate sufficient cash flows to satisfy our debt obligations, or to refinance our indebtedness on commercially reasonable terms or at all, would materially and adversely affect our financial position and results of operations and our ability to satisfy our obligations under the exchange notes. If we cannot make scheduled payments on our debt, we will be in default and holders of the notes could declare all outstanding principal and interest to be due and payable, the lenders under our Senior Credit Facilities could terminate their commitments to loan money, the lenders could foreclose against the assets securing their borrowings and we could be forced into bankruptcy or liquidation. All of these events could result in you losing your original investment in the notes.
Despite our current level of indebtedness, we and our subsidiaries may still be able to incur substantially more debt. This could further exacerbate the risks to our financial condition described above.
We and our subsidiaries may be able to incur significant additional indebtedness in the future. Although the indenture governing the notes and the credit agreement governing our Senior Credit Facilities contain restrictions on the incurrence of additional indebtedness, these restrictions are subject to a number of qualifications and exceptions, and the additional indebtedness incurred in compliance with these restrictions could be substantial. If we incur any additional indebtedness that ranks equally with the exchange notes, subject to collateral arrangements, the holders of that debt will be entitled to share ratably with you in any proceeds distributed in connection with any insolvency, liquidation, reorganization, dissolution or other winding up of our Company. This may have the effect of reducing the amount of proceeds paid to you. These restrictions also will not prevent us from incurring obligations that do not constitute indebtedness. In addition, as of August 31, 2011, our Senior Credit Facilities had unused commitments of $100.0 million, which amount could increase by $150.0 million (or a greater amount if we meet specified financial ratios), the availability of which is subject to certain conditions. All of those borrowings would be secured indebtedness. If new debt is added to our current debt levels, the related risks that we and the guarantors now face could intensify. See “Description of other indebtedness” and “Description of exchange notes.”
The terms of the credit agreement governing our Senior Credit Facilities and the indenture governing the notes restrict our current and future operations, particularly our ability to respond to changes or to take certain actions.
The indenture governing the notes and the credit agreement governing our Senior Credit Facilities contain a number of restrictive covenants that impose significant operating and financial restrictions on us and may limit our ability to engage in acts that may be in our long-term best interest, including restrictions on our ability to:
| • | incur additional indebtedness; |
| • | pay dividends or make other distributions or repurchase or redeem our capital stock; |
25
Table of Contents
| • | prepay, redeem or repurchase certain debt; |
| • | make loans and investments; |
| • | sell assets; |
| • | incur liens; |
| • | enter into transactions with affiliates; |
| • | alter the businesses we conduct; |
| • | enter into agreements restricting our subsidiaries’ ability to pay dividends; and |
| • | consolidate, merge or sell all or substantially all of our assets. |
In addition, the restrictive covenants in the credit agreement governing our Senior Credit Facilities require us to maintain a maximum senior secured net leverage ratio to be tested on the last day of each fiscal quarter. Our ability to meet this financial covenant can be affected by events beyond our control.
A breach of the covenants under the indenture governing the notes or under the credit agreement governing our Senior Credit Facilities could result in an event of default under the applicable indebtedness. Such a default may allow the creditors to accelerate the related debt and may result in the acceleration of any other debt to which a cross-acceleration or cross-default provision applies. In addition, an event of default under the credit agreement governing our Senior Credit Facilities would permit the lenders under our Senior Credit Facilities to terminate all commitments to extend further credit under that facility. Furthermore, if we were unable to repay the amounts due and payable under our Senior Credit Facilities, those lenders could proceed against the collateral granted to them to secure that indebtedness. In the event our lenders or noteholders accelerate the repayment of our borrowings, we and our subsidiaries may not have sufficient assets to repay that indebtedness. As a result of these restrictions, we may be:
| • | limited in how we conduct our business; |
| • | unable to raise additional debt or equity financing to operate during general economic or business downturns; or |
| • | unable to compete effectively or to take advantage of new business opportunities. |
These restrictions may affect our ability to grow in accordance with our strategy.
Our variable rate indebtedness subjects us to interest rate risk, which could cause our debt service obligations to increase significantly.
Borrowings under our Senior Credit Facilities are at variable rates of interest and expose us to interest rate risk. If interest rates increase, our debt service obligations on the variable rate indebtedness will increase even though the amount borrowed remained the same, and our net income and cash flows, including cash available for servicing our indebtedness, will correspondingly decrease. Assuming all revolving loans are fully drawn, each one-eighth point change in the LIBOR interest rate above 1.5% would result in a $0.9 million change in annual interest expense on our indebtedness under our Senior Credit Facilities. We have entered into, and may continue to enter into, interest rate swaps that involve the exchange of floating for fixed rate interest payments in order to reduce interest rate volatility. However, we may not maintain interest rate swaps with respect to all of our variable rate indebtedness, and any swaps we enter into may not fully mitigate our interest rate risk.
The exchange notes will be effectively subordinated to our and our subsidiary guarantors’ indebtedness under the Senior Credit Facilities and any other secured indebtedness of ours to the extent of the value of the property securing that indebtedness.
The exchange notes will not be secured by our assets, the assets of our existing domestic subsidiary, BioArray Solutions Ltd., or any of our future subsidiary guarantors’ assets. As a result, the exchange notes and the
26
Table of Contents
guarantees will be effectively subordinated to our and our subsidiary guarantors’ indebtedness under the Senior Credit Facilities with respect to the assets that secure that indebtedness. As of August 31, 2011, we had a total unused availability under our Senior Credit Facilities of $100.0 million, which amount could increase by $150.0 million (or a greater amount if we meet specified financial ratios), the availability of which is subject to certain conditions. In addition, we may incur additional secured debt in the future. The effect of this subordination is that upon a default in payment on, or the acceleration of, any of our secured indebtedness, or in the event of bankruptcy, insolvency, liquidation, dissolution or reorganization of our Company or the subsidiary guarantors, the proceeds from the sale of assets securing our secured indebtedness will be available to pay obligations on the exchange notes only after all indebtedness under the Senior Credit Facilities and that other secured debt has been paid in full. As a result, the holders of the exchange notes may receive less, ratably, than the holders of secured debt in the event of our or our subsidiary guarantors’ bankruptcy, insolvency, liquidation, dissolution or reorganization.
The exchange notes will be structurally subordinated to all obligations of our existing and future subsidiaries that are not and do not become guarantors of the exchange notes.
The exchange notes will be guaranteed by our existing domestic subsidiary and by each of our existing and subsequently acquired or organized subsidiaries that guarantee the Senior Credit Facilities or that, in the future, guarantee our other indebtedness or indebtedness of another guarantor. Our existing domestic subsidiary, BioArray Solutions Ltd. will initially be the only guarantor of the exchange notes. Our subsidiaries that do not guarantee the exchange notes, including all of our non-domestic subsidiaries, will have no obligation, contingent or otherwise, to pay amounts due under the exchange notes or to make any funds available to pay those amounts, whether by dividend, distribution, loan or other payment. The exchange notes will be structurally subordinated to all indebtedness and other obligations of any non-guarantor subsidiary such that in the event of insolvency, liquidation, reorganization, dissolution or other winding up of any subsidiary that is not a guarantor, all of that subsidiary’s creditors (including trade creditors) would be entitled to payment in full out of that subsidiary’s assets before we would be entitled to any payment.
In addition, the indenture governing the exchange notes permits, subject to some limitations, the non-guarantor subsidiaries to incur additional indebtedness and does not contain any limitation on the amount of other liabilities, such as trade payables, that may be incurred by these non-guarantor subsidiaries.
In addition, our subsidiaries that provide, or will provide, guarantees of the exchange notes will be automatically released from those guarantees upon the occurrence of certain events, including the following:
| • | the designation of that subsidiary guarantor as an unrestricted subsidiary; |
| • | the release or discharge of any guarantee or indebtedness that resulted in the creation of the guarantee of the exchange notes by such subsidiary guarantor; or |
| • | the sale or other disposition, including the sale of substantially all the assets, of that guarantor. |
If any guarantee is released, no holder of the exchange notes will have a claim as a creditor against that subsidiary, and the indebtedness and other liabilities, including trade payables and preferred stock, if any, whether secured or unsecured, of that subsidiary will be effectively senior to the claim of any holders of the exchange notes. See “Description of exchange notes—Guarantees.”
We may not be able to repurchase the exchange notes upon a change of control.
Upon the occurrence of specific kinds of change of control events, we will be required to offer to repurchase all exchange notes at 101% of their principal amount, plus accrued and unpaid interest to the purchase date. Additionally, under the Senior Credit Facilities, a change of control (as defined therein) constitutes an event of default that permits the lenders to accelerate the maturity of borrowings under the credit agreement and terminate their commitments to lend. The source of funds for any purchase of the exchange notes and repayment of
27
Table of Contents
borrowings under our Senior Credit Facilities would be our available cash or cash generated from our subsidiaries’ operations or other sources, including borrowings, sales of assets or sales of equity. We may not be able to repurchase the exchange notes upon a change of control because we may not have sufficient financial resources to purchase all of the exchange notes that are tendered upon a change of control and repay our other indebtedness that will become due. We may require additional financing from third parties to fund any such purchases, and we may be unable to obtain financing on satisfactory terms or at all. Further, our ability to repurchase the exchange notes may be limited by law. In order to avoid the obligations to repurchase the exchange notes and events of default and potential breaches of the credit agreement governing our Senior Credit Facilities, we may have to avoid certain change of control transactions that would otherwise be beneficial to us.
In addition, some important corporate events, such as leveraged recapitalizations, may not, under the indenture that governs the notes, constitute a “change of control” that would require us to repurchase the exchange notes, even though those corporate events could increase the level of our indebtedness or otherwise adversely affect our capital structure, credit ratings or the value of the exchange notes. See “Description of exchange notes—Repurchase at the option of holders—Change of control.”
Holders of the exchange notes may not be able to determine when a change of control giving rise to their right to have the exchange notes repurchased has occurred following a sale of “substantially all” of our assets.
The definition of change of control in the indenture that governs the notes includes a phrase relating to the sale of “all or substantially all” of our assets. There is no precise established definition of the phrase “substantially all” under applicable law. Accordingly, the ability of a holder of exchange notes to require us to repurchase its notes as a result of a sale of less than all our assets to another person may be uncertain.
Because each guarantor’s liability under its guarantee may be reduced to zero, avoided or released under certain circumstances, you may not receive any payments from some or all of the guarantors.
You have the benefit of the guarantees of the subsidiary guarantors. However, the guarantees by the subsidiary guarantors are limited to the maximum amount that the subsidiary guarantors are permitted to guarantee under applicable law. As a result, a subsidiary guarantor’s liability under its guarantee could be reduced to zero, depending upon the amount of other obligations of such subsidiary guarantor. Further, under the circumstances discussed more fully below, a court under federal and state fraudulent conveyance and transfer statutes could void the obligations under a guarantee or further subordinate it to all other obligations of the subsidiary guarantor. See “—Federal and state fraudulent transfer laws may permit a court to void the exchange notes and/or the guarantees, and if that occurs, you may not receive any payments on the exchange notes.” In addition, you will lose the benefit of a particular guarantee if it is released under certain circumstances described under “Description of exchange notes—Guarantees.”
Federal and state fraudulent transfer laws may permit a court to void the exchange notes and/or the guarantees, and if that occurs, you may not receive any payments on the exchange notes.
Federal and state fraudulent transfer and conveyance statutes may apply to the issuance of the exchange notes and the incurrence of the guarantee of the subsidiary guarantor or any future guarantees of the exchange notes. Under federal bankruptcy law and comparable provisions of state fraudulent transfer or conveyance laws, which may vary from state to state, the exchange notes or the guarantees thereof could be voided as a fraudulent transfer or conveyance if we or any of the guarantors, as applicable, (a) issued the exchange notes or incurred the guarantees with the intent of hindering, delaying or defrauding creditors or (b) received less than reasonably equivalent value or fair consideration in return for either issuing the exchange notes or incurring the guarantees and, in the case of (b) only, one of the following is also true at the time thereof:
| • | we or any of the guarantors, as applicable, were insolvent or rendered insolvent by reason of the issuance of the exchange notes or the incurrence of the guarantees; |
28
Table of Contents
| • | the issuance of the exchange notes or the incurrence of the guarantees left us or any of the guarantors, as applicable, with an unreasonably small amount of capital or assets to carry on the business; |
| • | we or any of the guarantors intended to, or believed that we or such guarantor would, incur debts beyond our or the guarantor’s ability to pay as they mature; or |
| • | we or any of the guarantors were a defendant in an action for money damages, or had a judgment for money damages docketed against us or the guarantor if, in either case, the judgment is unsatisfied after final judgment. |
As a general matter, value is given for a transfer or an obligation if, in exchange for the transfer or obligation, property is transferred or a valid antecedent debt is secured or satisfied. A court would likely find that a subsidiary guarantor did not receive reasonably equivalent value or fair consideration for its guarantee to the extent the guarantor did not obtain a reasonably equivalent benefit directly or indirectly from the issuance of the exchange notes.
We cannot be certain as to the standards a court would use to determine whether or not we or the subsidiary guarantors were insolvent at the relevant time or, regardless of the standard that a court uses, whether the exchange notes or the guarantees would be subordinated to our or any of our subsidiary guarantors’ other debt. In general, however, a court would deem an entity insolvent if:
| • | the sum of its debts, including contingent and unliquidated liabilities, was greater than the fair saleable value of all of its assets; |
| • | the present fair saleable value of its assets was less than the amount that would be required to pay its probable liability on its existing debts, including contingent liabilities, as they become absolute and mature; or |
| • | it could not pay its debts as they became due. |
If a court were to find that the issuance of the exchange notes or the incurrence of a guarantee was a fraudulent transfer or conveyance, the court could void the payment obligations under the exchange notes or that guarantee, could subordinate the exchange notes or that guarantee to presently existing and future indebtedness of ours or of the related subsidiary guarantor or could require the holders of the exchange notes to repay any amounts received with respect to that guarantee. In the event of a finding that a fraudulent transfer or conveyance occurred, you may not receive any repayment on the exchange notes. Further, the avoidance of the exchange notes could result in an event of default with respect to our and our subsidiaries’ other debt that could result in acceleration of that debt.
Finally, as a court of equity, the bankruptcy court may subordinate the claims in respect of the exchange notes to other claims against us under the principle of equitable subordination if the court determines that (1) the holder of the exchange notes engaged in some type of inequitable conduct, (2) the inequitable conduct resulted in injury to our other creditors or conferred an unfair advantage upon the holders of the exchange notes and (3) equitable subordination is not inconsistent with the provisions of the bankruptcy code.
You may have difficulty selling the outstanding notes that you do not exchange.
If you do not exchange your outstanding notes for exchange notes in the exchange offer, you will continue to be subject to the restrictions on transfer of your outstanding notes described in the legend on your outstanding notes. The restrictions on transfer of your outstanding notes arise because we issued the outstanding notes under exemptions from, or in transactions not subject to, the registration requirements of the Securities Act and applicable state securities laws. In general, you may only offer or sell the outstanding notes if they are registered under the Securities Act and applicable state securities laws, or offered and sold under an exemption from these requirements. Except as required by the Registration Rights Agreement, we do not intend to register the outstanding notes under the Securities Act. The tender of outstanding notes under the exchange offer will reduce the principal amount of the currently outstanding notes. Due to the corresponding reduction in liquidity, this may have an adverse effect upon, and increase the volatility of, the market price of any currently outstanding notes that you continue to hold following completion of the exchange offer.
29
Table of Contents
Your ability to transfer the exchange notes may be limited by the absence of an active trading market and an active trading market may not develop for the exchange notes.
The exchange notes are new issues of securities for which there is no established trading market. We expect the exchange notes to be eligible for trading by “qualified institutional buyers,” as defined under Rule 144A, but we do not intend to list the exchange notes on any national securities exchange or include the exchange notes in any automated quotation system. The initial purchasers have advised us that they intend to make a market in the exchange notes as permitted by applicable laws and regulations. However, the initial purchasers are not obligated to make a market in the exchange notes, and, if commenced, they may discontinue their market-making activities at any time without notice. In addition, market making activities may be limited during the exchange offer or while the effectiveness of a shelf registration statement is pending.
Therefore, an active market for the exchange notes may not develop or be maintained, which would adversely affect the market price and liquidity of the exchange notes. In that case, the holders of the exchange notes may not be able to sell their notes at a particular time or at a favorable price.
Even if an active trading market for the exchange notes does develop, there is no guarantee that it will continue. Historically, the market for non-investment grade debt has been subject to severe disruptions that have caused substantial volatility in the prices of securities similar to the exchange notes. The market, if any, for the exchange notes may experience similar disruptions, and any such disruptions may adversely affect the liquidity in that market or the prices at which you may sell your exchange notes. In addition, subsequent to their initial issuance, the exchange notes may trade at a discount from their initial offering price, depending upon prevailing interest rates, the market for similar notes, our performance and other factors.
A lowering or withdrawal of the ratings assigned to our debt securities by rating agencies may increase our future borrowing costs and reduce our access to capital.
Our debt currently has a non-investment grade rating, and any rating assigned could be lowered or withdrawn entirely by a rating agency if, in that rating agency’s judgment, future circumstances relating to the basis of the rating, such as adverse changes, so warrant. Consequently, real or anticipated changes in our credit ratings will generally affect the market value of the exchange notes. Credit ratings are not recommendations to purchase, hold or sell the exchange notes. Additionally, credit ratings may not reflect the potential effect of risks relating to the structure or marketing of the exchange notes. Any downgrade by either Standard & Poor’s or Moody’s may result in higher borrowing costs.
Any future lowering of our ratings likely would make it more difficult or more expensive for us to obtain additional debt financing. If any credit rating initially assigned to the exchange notes is subsequently lowered or withdrawn for any reason, you may not be able to resell your exchange notes without a substantial discount.
If a bankruptcy petition were filed by or against us, holders of exchange notes may receive a lesser amount for their claim than they would have been entitled to receive under the indenture governing the notes.
If a bankruptcy petition were filed by or against us under the U.S. Bankruptcy Code after the issuance of the exchange notes, the claim by any holder of the exchange notes for the principal amount of the exchange notes may be limited to an amount equal to the sum of:
| • | the original issue price for the exchange notes; and |
| • | that unpaid portion of any discount on the exchange notes that does not constitute “unmatured interest” for purposes of the U.S. Bankruptcy Code. |
Any discount on the exchange notes that was not amortized as of the date of the bankruptcy filing would constitute unmatured interest. Accordingly, holders of the exchange notes under these circumstances may receive a lesser amount than they would be entitled to receive under the terms of the indenture governing the notes, even if sufficient funds are available.
30
Table of Contents
Purpose and effect of the exchange offer
Under the Registration Rights Agreement, the Company and the guarantors agreed to use reasonable efforts to (1) file a registration statement on an appropriate registration form with respect to a registered offer to exchange the outstanding notes for the exchange notes guaranteed by the guarantors, with terms substantially identical in all material respects to the outstanding notes, as applicable (except that the exchange notes will not contain terms with respect to transfer restrictions or any increase in annual interest rate) and (2) cause the registration statement to be declared effective under the Securities Act.
The Company and the guarantors have also agreed to use reasonable best efforts to file and to have become effective under the Securities Act a shelf registration statement for the resale of the outstanding notes and guarantees if the exchange offer is not available or cannot be effected prior to August 13, 2012. If the exchange offer is not completed or the shelf registration statement is not effective prior to August 13, 2012, additional interest on the outstanding notes will accrue at a rate of 0.25% per annum for the first 90-day period (which rate will be increased by an additional 0.25% per annum for each subsequent 90-day period that such additional interest continues to accrue, provided that the rate at which such additional interest accrues will in no event exceed 1.00% per annum) until the registration obligations are fulfilled.
Following the completion of the exchange offer, holders of outstanding notes not tendered will not have any further registration rights other than as set forth in the paragraphs below, and, subject to certain exceptions, the outstanding notes will continue to be subject to certain restrictions on transfer.
Subject to certain conditions, including the representations set forth below, the exchange notes will be issued without a restrictive legend and generally may be reoffered and resold without registration under the Securities Act. In order to participate in the exchange offer, a holder must represent to us in writing, or be deemed to represent to us in writing, among other things, that:
| • | the holder is not an “affiliate” of the Company or of any guarantor of the notes within the meaning of Rule 405 under the Securities Act; |
| • | the holder is not engaged and does not intend to engage in, and has no arrangement or understanding with any person to participate in, a distribution of the exchange notes in violation of the provisions of the Securities Act; |
| • | the holder is acquiring the exchange notes in its ordinary course of business; and |
| • | if the holder is a broker-dealer that will receive the exchange notes for its own account in exchange for the outstanding notes that were acquired as a result of market-making activities or other trading activities, that such holder will deliver a prospectus (or, to the extent permitted by law, make available a prospectus) meeting the requirements of the Securities Act in connection with any resales of the exchange notes. |
Based on an interpretation by the SEC’s staff set forth in no-action letters issued to third parties unrelated to us, we believe that, with the exceptions set forth below, the exchange notes issued in the exchange offer may be offered for resale, resold and otherwise transferred by the holder of exchange notes without compliance with the registration and prospectus delivery requirements of the Securities Act, unless the holder:
| • | is an “affiliate,” within the meaning of Rule 405 under the Securities Act, of ours; |
| • | is a broker-dealer that purchased outstanding notes directly from us for resale under Rule 144A or Regulation S or any other available exemption under the Securities Act; |
| • | acquired the exchange notes other than in the ordinary course of the holder’s business; |
| • | has an arrangement with any person to engage in the distribution of the exchange notes; or |
| • | is prohibited by any law or policy of the SEC from participating in the exchange offer. |
31
Table of Contents
Any holder who tenders in the exchange offer for the purpose of participating in a distribution of the exchange notes cannot rely on this interpretation by the SEC’s staff and must comply with the registration and prospectus delivery requirements of the Securities Act in connection with a secondary resale transaction. Each broker-dealer that receives exchange notes for its own account in exchange for outstanding notes, where such outstanding notes were acquired by such broker-dealer as a result of market-making activities or other trading activities, must acknowledge that it will deliver a prospectus in connection with any resale of such exchange notes. See “Plan of distribution.” Broker-dealers who acquired outstanding notes directly from us and not as a result of market-making activities or other trading activities may not rely on the staff’s interpretations discussed above, and must comply with the prospectus delivery requirements of the Securities Act in order to sell the exchange notes.
Terms of the exchange offer
Upon the terms and subject to the conditions set forth in this prospectus and in the letter of transmittal, we will accept any and all outstanding notes validly tendered and not withdrawn prior to 5:00 p.m., New York City time, on January 13, 2012, or such date and time to which we extend the exchange offer. We will issue $1,000 in principal amount of exchange notes in exchange for each $1,000 principal amount of outstanding notes accepted in the exchange offer. Holders may tender some or all of their outstanding notes pursuant to the exchange offer. Outstanding notes may be tendered only in a denomination equal to $2,000 and any integral multiples of $1,000 in excess of $2,000.
The exchange notes will evidence the same debt as the outstanding notes and will be issued under the terms of, and entitled to the benefits of, the indenture relating to the outstanding notes.
As of the date of this prospectus, $400 million in aggregate principal amount of outstanding notes are outstanding. This prospectus, together with the letter of transmittal, is being sent to the registered holders of the outstanding notes. We intend to conduct the exchange offer in accordance with the applicable requirements of the Exchange Act and the rules and regulations of the SEC promulgated under the Exchange Act.
We will be deemed to have accepted validly tendered outstanding notes when, as and if we have given oral (to be promptly confirmed in writing) or written notice thereof to Deutsche Bank Trust Company Americas, which is acting as the exchange agent. The exchange agent will act as agent for the tendering holders for the purpose of receiving the exchange notes from us. If any tendered outstanding notes are not accepted for exchange because of an invalid tender or the occurrence of certain other events set forth under the heading “—Conditions to the exchange offer,” any such unaccepted outstanding notes will be returned, without expense, to the tendering holder of those outstanding notes promptly after the expiration date unless the exchange offer is extended.
Holders who tender outstanding notes in the exchange offer will be required to pay brokerage commissions or fees, or, subject to the instructions in the letter of transmittal, transfer taxes, with respect to the exchange of outstanding notes in the exchange offer. We will pay certain charges and expenses applicable to the exchange offer. See “—Fees and expenses.”
Expiration date; extensions; amendments
The expiration date shall be 5:00 p.m., New York City time, on January 13, 2012, unless we, in our sole discretion, extend the exchange offer, in which case the expiration date shall be the latest date and time to which the exchange offer is extended. In order to extend the exchange offer, we will notify the exchange agent by oral (to be promptly confirmed in writing) or written notice prior to 9:00 a.m., New York City time, on the next business day after the previously scheduled expiration date and will also disseminate notice of any extension by press release or other public announcement prior to 9:00 a.m., New York City time on such date. Any such announcement will include the approximate number of securities deposited as of the date of the extension. We reserve the right, in our sole discretion:
| • | to delay accepting any outstanding notes, to extend the exchange offer or, if any of the conditions set forth under “—Conditions to the exchange offer” shall not have been satisfied, to terminate the exchange offer, by giving oral or written notice of that delay, extension or termination to the exchange agent, or |
32
Table of Contents
| • | to amend the terms of the exchange offer in any manner. |
Any delay in acceptance, extension, termination or amendment will be followed as promptly as practicable by oral (to be promptly confirmed in writing) or written notice to the registered holders of the outstanding notes. If we amend the exchange offer in a manner that we determine to constitute a material change, we will promptly disclose the amendment in a manner reasonably calculated to inform the holders of outstanding notes of that amendment, and we will extend the offer period, if necessary, so that at least five business days remain in the offer following notice of the material change.
Procedures for tendering
When a holder of outstanding notes tenders, and we accept such notes for exchange pursuant to that tender, a binding agreement between us and the tendering holder is created, subject to the terms and conditions set forth in this prospectus and the accompanying letter of transmittal. Except as set forth below, a holder of outstanding notes who wishes to tender such notes for exchange must, on or prior to the expiration date:
| • | transmit a properly completed and duly executed letter of transmittal, including all other documents required by such letter of transmittal, to Deutsche Bank Trust Company Americas, which will act as the exchange agent, at the address set forth below under the heading “—Exchange agent”; or |
| • | comply with DTC’s Automated Tender Offer Program, or ATOP, procedures described below. |
In addition, either:
| • | the exchange agent must receive the certificates for the outstanding notes and the letter of transmittal; |
| • | the exchange agent must receive, prior to the expiration date, a timely confirmation of the book-entry transfer of the outstanding notes being tendered, along with the letter of transmittal or an agent’s message; or |
| • | the holder must comply with the guaranteed delivery procedures described below. |
The term “agent’s message” means a message, transmitted to DTC and received by the exchange agent and forming a part of a book-entry transfer, or “book-entry confirmation,” which states that DTC has received an express acknowledgement that the tendering holder agrees to be bound by the letter of transmittal and that we may enforce the letter of transmittal against such holder.
The method of delivery of the outstanding notes, the letters of transmittal and all other required documents is at the election and risk of the holders. If such delivery is by mail, we recommend registered mail, properly insured, with return receipt requested. In all cases, you should allow sufficient time to assure timely delivery. No letters of transmittal or outstanding notes should be sent directly to us.
Signatures on a letter of transmittal or a notice of withdrawal must be guaranteed by an eligible institution unless the outstanding notes surrendered for exchange are tendered:
| • | by a registered holder of the outstanding notes; or |
| • | for the account of an eligible institution. |
An “eligible institution” is a firm which is a member of a registered national securities exchange or a member of the Financial Industry Regulatory Authority, Inc., or a commercial bank or trust company having an office or correspondent in the United States.
If outstanding notes are registered in the name of a person other than the signer of the letter of transmittal, the outstanding notes surrendered for exchange must be endorsed by, or accompanied by a written instrument or instruments of transfer or exchange, in satisfactory form to the exchange agent and as determined by us in our sole discretion, duly executed by the registered holder with the holder’s signature guaranteed by an eligible institution.
33
Table of Contents
We will determine all questions as to the validity, form, eligibility (including time of receipt) and acceptance of outstanding notes tendered for exchange in our sole discretion. Our determination will be final and binding. We reserve the absolute right to:
| • | reject any and all tenders of any outstanding note improperly tendered; |
| • | refuse to accept any outstanding note if, in our judgment or the judgment of our counsel, acceptance of the outstanding note may be deemed unlawful; and |
| • | waive any defects or irregularities or conditions of the exchange offer as to any particular outstanding note based on the specific facts or circumstances presented either before or after the expiration date, including the right to waive the ineligibility of any holder who seeks to tender outstanding notes in the exchange offer. |
Notwithstanding the foregoing, we do not expect to treat any holder of outstanding notes differently from other holders to the extent they present the same facts or circumstances.
Our interpretation of the terms and conditions of the exchange offer as to any particular outstanding notes either before or after the expiration date, including the letter of transmittal and the instructions to it, will be final and binding on all parties. Holders must cure any defects and irregularities in connection with tenders of outstanding notes for exchange within such reasonable period of time as we will determine, unless we waive such defects or irregularities.
Neither we, the exchange agent nor any other person shall be under any duty to give notification of any defect or irregularity with respect to any tender of outstanding notes for exchange, nor shall any of us incur any liability for failure to give such notification.
If trustees, executors, administrators, guardians, attorneys-in-fact, officers of corporations or others acting in a fiduciary or representative capacity sign the letter of transmittal or any outstanding notes or any power of attorney, these persons should so indicate when signing, and you must submit proper evidence satisfactory to us of those persons’ authority to so act unless we waive this requirement.
By tendering, each holder will represent to us that the person acquiring exchange notes in the exchange offer, whether or not that person is the holder, is obtaining them in the ordinary course of its business, and at the time of the commencement of the exchange offer neither the holder nor, to the knowledge of such holder, that other person receiving exchange notes from such holder has any arrangement or understanding with any person to participate in the distribution (within the meaning of the Securities Act) of the exchange notes issued in the exchange offer in violation of the provisions of the Securities Act. If any holder or any other person receiving exchange notes from such holder is an “affiliate,” as defined under Rule 405 of the Securities Act, of us, or is engaged in or intends to engage in or has an arrangement or understanding with any person to participate in a distribution (within the meaning of the Securities Act) of the notes in violation of the provisions of the Securities Act to be acquired in the exchange offer, the holder or any other person:
| • | may not rely on applicable interpretations of the staff of the SEC; and |
| • | must comply with the registration and prospectus delivery requirements of the Securities Act in connection with any resale transaction. |
Each broker-dealer that acquired its outstanding notes as a result of market-making activities or other trading activities, and thereafter receives exchange notes issued for its own account in the exchange offer, must acknowledge that it will comply with the applicable provisions of the Securities Act (including, but not limited to, delivering this prospectus in connection with any resale of such exchange notes issued in the exchange offer). The letter of transmittal states that by so acknowledging and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act. See “Plan of distribution” for a discussion of the exchange and resale obligations of broker-dealers.
34
Table of Contents
Acceptance of outstanding notes for exchange; delivery of exchange notes issued in the exchange offer
Upon satisfaction or waiver of all the conditions to the exchange offer, we will accept, promptly after the expiration date, all outstanding notes properly tendered and will issue exchange notes registered under the Securities Act in exchange for the tendered outstanding notes. For purposes of the exchange offer, we shall be deemed to have accepted properly tendered outstanding notes for exchange when, as and if we have given oral or written notice to the exchange agent, with written confirmation of any oral notice to be given promptly thereafter, and complied with the applicable provisions of the Registration Rights Agreement. See “—Conditions to the exchange offer” for a discussion of the conditions that must be satisfied before we accept any outstanding notes for exchange.
For each outstanding note accepted for exchange, the holder will receive an exchange note registered under the Securities Act having a principal amount equal to that of the surrendered outstanding note. Registered holders of exchange notes issued in the exchange offer on the relevant record date for the first interest payment date following the consummation of the exchange offer will receive interest accruing from the most recent date on which interest has been paid or, if no interest has been paid, from the issue date of the outstanding notes. Holders of exchange notes will not receive any payment in respect of accrued interest on outstanding notes otherwise payable on any interest payment date, the record date for which occurs on or after the consummation of the exchange offer. Under the Registration Rights Agreement, we may be required to make payments of additional interest to the holders of the outstanding notes under circumstances relating to the timing of the exchange offer.
In all cases, we will issue exchange notes for outstanding notes that are accepted for exchange only after the exchange agent timely receives:
| • | certificates for such outstanding notes or a timely book-entry confirmation of such outstanding notes into the exchange agent’s account at DTC; |
| • | a properly completed and duly executed letter of transmittal or an agent’s message; and all other required documents. |
If for any reason set forth in the terms and conditions of the exchange offer we do not accept any tendered outstanding notes, or if a holder submits outstanding notes for a greater principal amount than the holder desires to exchange, we will return such unaccepted or nonexchanged notes without cost to the tendering holder. In the case of outstanding notes tendered by book-entry transfer into the exchange agent’s account at DTC, the nonexchanged notes will be credited to an account maintained with DTC.
We will return the outstanding notes or have them credited to DTC, promptly after the expiration or termination of the exchange offer.
Book-entry transfer
The participant should transmit its acceptance to DTC on or prior to the expiration date or comply with the guaranteed delivery procedures described below. DTC will verify the acceptance and then send to the exchange agent confirmation of the book-entry transfer. The confirmation of the book-entry transfer will be deemed to include an agent’s message confirming that DTC has received an express acknowledgment from the participant that the participant has received and agrees to be bound by the letter of transmittal and that we may enforce the letter of transmittal against such participant. Delivery of exchange notes issued in the exchange offer may be effected through book-entry transfer at DTC. However, the letter of transmittal or facsimile thereof or an agent’s message, with any required signature guarantees and any other required documents, must:
| • | be transmitted to and received by the exchange agent at the address set forth below under “—The exchange agent” on or prior to the expiration date; or |
| • | comply with the guaranteed delivery procedures described below. |
35
Table of Contents
DTC’s ATOP program is the only method of processing the exchange offer through DTC. To tender outstanding notes through ATOP, participants in DTC must send electronic instructions to DTC through DTC’s communication system. In addition, such tendering participants should deliver a copy of the letter of transmittal to the exchange agent unless an agent’s message is transmitted in lieu thereof. DTC is obligated to communicate those electronic instructions to the exchange agent through an agent’s message. Any instruction through ATOP, such as an agent’s message, is at your risk and such instruction will be deemed made only when actually received by the exchange agent.
In order for your tender through ATOP to be valid, an agent’s message must be transmitted to and received by the exchange agent prior to the expiration date, or the guaranteed delivery procedures described below must be complied with. Delivery of instructions to DTC does not constitute delivery to the exchange agent.
Guaranteed delivery procedures
If a holder of outstanding notes desires to tender such notes and the holder’s outstanding notes are not immediately available, or time will not permit the holder’s outstanding notes or other required documents to reach the exchange agent before the expiration date, or the procedure for book-entry transfer cannot be completed on a timely basis, a tender may be effected if:
| • | the holder tenders the outstanding notes through an eligible institution; |
| • | prior to the expiration date, the exchange agent receives from such eligible institution a properly completed and duly executed notice of guaranteed delivery, acceptable to us, by facsimile transmission (receipt confirmed by telephone and an original delivered by guaranteed overnight courier), mail or hand delivery, setting forth the name and address of the holder of the outstanding notes tendered, the names in which such outstanding notes are registered, if applicable, the certificate number or numbers of such outstanding notes and the amount of the outstanding notes being tendered. The notice of guaranteed delivery shall state that the tender is being made and guarantee that within three New York Stock Exchange trading days after the expiration date, the certificates for all physically tendered outstanding notes, in proper form for transfer, or a book- entry confirmation, as the case may be, together with a properly completed and duly executed letter of transmittal or agent’s message with any required signature guarantees and any other documents required by the letter of transmittal will be deposited by the eligible institution with the exchange agent; and |
| • | the exchange agent receives the certificates for all physically tendered outstanding notes, in proper form for transfer, or a book-entry confirmation, as the case may be, together with a properly completed and duly executed letter of transmittal or agent’s message with any required signature guarantees and any other documents required by the letter of transmittal, within three New York Stock Exchange trading days after the expiration date. |
Withdrawal of tenders
You may withdraw tenders of your outstanding notes at any time prior to the expiration of the exchange offer.
For a withdrawal to be effective, you must send a written notice of withdrawal to the exchange agent at the address set forth below under “—Exchange agent.” Any such notice of withdrawal must:
| • | specify the name of the person that has tendered the outstanding notes to be withdrawn; identify the outstanding notes to be withdrawn, including the principal amount of such outstanding notes; and |
| • | where certificates for outstanding notes are transmitted, specify the name in which outstanding notes are registered, if different from that of the withdrawing holder. |
If certificates for outstanding notes have been delivered or otherwise identified to the exchange agent, then, prior to the release of such certificates, the withdrawing holder must also submit the serial numbers of the particular
36
Table of Contents
certificates to be withdrawn and signed notice of withdrawal with signatures guaranteed by an eligible institution unless such holder is an eligible institution. If outstanding notes have been tendered pursuant to the procedure for book-entry transfer described above, any notice of withdrawal must specify the name and number of the account at DTC, as applicable, to be credited with the withdrawn notes and otherwise comply with the procedures of such facility.
We will determine all questions as to the validity, form and eligibility (including time of receipt) of notices of withdrawal and our determination will be final and binding on all parties. Any tendered notes so withdrawn will be deemed not to have been validly tendered for exchange for purposes of the exchange offer. Any outstanding notes which have been tendered for exchange but which are not exchanged for any reason will be returned to the holder thereof without cost to such holder. In the case of outstanding notes tendered by book-entry transfer into the exchange agent’s account at DTC, the outstanding notes withdrawn will be credited to an account at the book-entry transfer facility. The outstanding notes will be returned promptly after withdrawal, rejection of tender or termination of the exchange offer. Properly withdrawn outstanding notes may be re-tendered by following one of the procedures described under “—Procedures for tendering” above at any time on or prior to 5:00 p.m., New York City time, on the expiration date.
Conditions to the exchange offer
Notwithstanding any other provision of the exchange offer, we may (a) refuse to accept any outstanding notes and return all tendered outstanding notes to the tendering holders, (b) extend the exchange offer and retain all outstanding notes tendered before the expiration of the exchange offer, subject, however, to the rights of holders to withdraw those outstanding notes, or (c) waive the unsatisfied conditions with respect to the exchange offer and accept all properly tendered outstanding notes that have not been withdrawn, if we determine, in our reasonable judgment, that (i) the exchange offer violates applicable laws or, any applicable interpretation of the staff of the SEC; (ii) an action or proceeding shall have been instituted or threatened in any court or by any governmental agency which might materially impair our ability to proceed with the exchange offer or a material adverse development shall have occurred in any existing action or proceeding with respect to us; or (iii) all governmental approvals that we deem necessary for the consummation of the exchange offer have not been obtained.
The foregoing conditions are for our sole benefit and may be asserted by us regardless of the circumstances giving rise to any such condition or may be waived by us in whole or in part at any time and from time to time. The failure by us at any time to exercise any of the foregoing rights shall not be deemed a waiver of any of those rights and each of those rights shall be deemed an ongoing right which may be asserted at any time and from time to time.
In addition, we will not accept for exchange any outstanding notes tendered, and no exchange notes will be issued in exchange for those outstanding notes, if at such time any stop order shall be threatened or in effect with respect to the registration statement of which this prospectus constitutes a part or the qualification of the indenture under the Trust Indenture Act of 1939. In any of those events we are required to use every reasonable effort to obtain the withdrawal of any stop order at the earliest possible time.
Effect of not tendering
Holders who desire to tender their outstanding notes in exchange for exchange notes registered under the Securities Act should allow sufficient time to ensure timely delivery. Neither the exchange agent nor we are under any duty to give notification of defects or irregularities with respect to the tenders of outstanding notes for exchange.
Outstanding notes that are not tendered or are tendered but not accepted will, following the consummation of the exchange offer, continue to accrue interest and to be subject to the provisions in the indenture regarding the transfer and exchange of the outstanding notes and the existing restrictions on transfer set forth in the legend on the outstanding notes and in the prospectus relating to the outstanding notes. After completion of the exchange
37
Table of Contents
offer, we will have no further obligation to provide for the registration under the Securities Act of those outstanding notes except in limited circumstances with respect to specific types of holders of outstanding notes and we do not intend to register the outstanding notes under the Securities Act. In general, outstanding notes, unless registered under the Securities Act, may not be offered or sold except pursuant to an exemption from, or in a transaction not subject to, the Securities Act and applicable state securities laws.
Exchange agent
All executed letters of transmittal should be directed to the exchange agent. Deutsche Bank Trust Company Americas has been appointed as exchange agent for the exchange offer. Requests for additional copies of this prospectus or of the letter of transmittal should be directed to the exchange agent addressed as follows:
| By mail, hand or overnight delivery: | By facsimile: | |||
| DB Services Americas, Inc. | 1-615-866-3889 | |||
| MS JCK01-D218 | For information or confirmation by telephone: | |||
| 5022 Gate Parkway, Suite 200 | 1-800-735-7777, Option 1 | |||
| Jacksonville, FL 32256 Attn: Reorganization Unit |
Fees and expenses
We will not make any payments to brokers, dealers or others soliciting acceptances of the exchange offer. The estimated cash expenses to be incurred in connection with the exchange offer will be paid by us and will include fees and expenses of the exchange agent, legal, printing and related fees and expenses. Notwithstanding the foregoing, holders of the outstanding notes shall pay all agency fees and commissions and underwriting discounts and commissions, if any, attributable to the sale of such outstanding notes or exchange notes.
Accounting treatment
We will record the exchange notes at the same carrying value as the outstanding notes, as reflected in our accounting records on the date of the exchange. Accordingly, we will not recognize any gain or loss for accounting purposes as the terms of the exchange notes are substantially identical to those of the outstanding notes. The expenses of the exchange offer will be amortized over the terms of the exchange notes.
Transfer taxes
Holders who tender their outstanding notes for exchange notes as part of the exchange offer will not be obligated to pay any transfer taxes in connection with that tender or exchange, except that holders who instruct us to register or issue exchange notes in the name of, or request that outstanding notes not tendered or not accepted in the exchange offer be returned to, a person other than the registered tendering holder will be responsible for the payment of any applicable transfer tax on those notes. If satisfactory evidence of payment of such taxes or exception therefrom is not submitted with the letter of transmittal, the amount of such transfer taxes will be billed directly to such tendering holder. If a transfer tax is imposed for any reason other than the transfer and exchange of outstanding notes to the Company or its order pursuant to the exchange offer, the amount of any such transfer taxes (whether imposed on the registered holder or any other person) will be payable by the applicable holder.
38
Table of Contents
On July 2, 2011, Immucor entered into a merger agreement with IVD Holdings Inc. (“Holding”) and Merger Sub, an indirect wholly owned subsidiary of Holding (the “Merger Agreement”). Holding and Merger Sub are corporations formed by the Sponsor solely for the purposes of completing the Acquisition and the other transactions described in this prospectus.
Pursuant to the Merger Agreement, Merger Sub accepted for payment all outstanding shares of the Company’s common stock at a cash purchase price of $27.00 per share (the “Offer Price”). Merger Sub was merged with and into the Company on August 19, 2011 in accordance with the “short-form” merger provisions available under Georgia law, upon which the Company became an indirect wholly-owned subsidiary of Holding. In connection with the Acquisition, each issued and outstanding share of the Company was converted into the right to receive the Offer Price.
In connection with the Acquisition, on August 19, 2011, (i) we entered into a credit agreement and related security and other agreements for a $615.0 million senior secured term loan facility and a $100.0 million senior secured revolving loan facility with certain lenders, Citibank, N.A., as administrative agent and collateral agent, Citigroup Global Markets Inc. and J.P. Morgan Securities LLC, as joint lead arrangers and joint lead bookrunners, UBS Securities LLC, as joint bookrunner, J.P. Morgan Securities LLC and UBS Securities LLC, as co-syndication agents and Deutsche Bank Securities Inc. and Royal Bank of Canada, as co-documentation agents and (ii) Merger Sub, as the initial issuer, and Deutsche Bank Trust Company Americas, as trustee (the “Trustee”), executed an indenture pursuant to which $400.0 million in aggregate principal amount of the outstanding notes were issued (the “initial indenture”) and Immucor, BioArray and the Trustee executed a supplemental indenture (the “supplemental indenture” and together with the initial indenture, the “indenture”) pursuant to which Immucor assumed the obligations of Merger Sub under the outstanding notes and BioArray guaranteed the outstanding notes on a senior basis. See “Description of other indebtedness” and “Description of exchange notes.”
39
Table of Contents
The exchange offer is intended to satisfy certain of our obligations under the Registration Rights Agreement. We will not receive any proceeds from the issuance of the exchange notes in the exchange offer. In exchange for the exchange notes, we will receive outstanding notes in like principal amount. We will retire or cancel all of the outstanding notes tendered in the exchange offer. Accordingly, issuance of the exchange notes will not result in any change in our capitalization.
40
Table of Contents
Selected historical consolidated financial data
The following table sets forth selected historical consolidated financial data for the periods ended and at the dates indicated below. Our selected historical consolidated financial data as of May 31, 2010 and May 31, 2011 and for the fiscal years ended May 31, 2009, May 31, 2010 and May 31, 2011 presented in this table has been derived from our historical audited consolidated financial statements included elsewhere in this prospectus. Our selected historical consolidated financial data as of May 31, 2007, May 31, 2008 and May 31, 2009 and for the fiscal years ended May 31, 2007 and May 31, 2008 presented in the table has been derived from our historical audited consolidated financial statements not included in this prospectus. The unaudited summary historical consolidated financial information as of and for the quarter ended August 31, 2010, as of August 31, 2011, and for the periods from June 1, 2011 to August 19, 2011 and August 20, 2011 to August 31, 2011 are derived from, and should be read in conjunction with, our unaudited condensed consolidated financial statements included elsewhere in this prospectus.
Our financial information in the table below for the quarter ended August 31, 2011 is presented separately for the Predecessor period and the Successor period.
The historical results presented below are not necessarily indicative of the results to be expected for any future period. The following information should be read in conjunction with the sections entitled “Management’s discussion and analysis of financial condition and results of operations” and our financial statements and the notes thereto contained elsewhere in this prospectus.
| Predecessor | Successor | |||||||||||||||||||||||||||||||||
| Fiscal year ended May 31, | For the period June 1, 2010 to August 31, 2010 (unaudited) |
For the period June 1, 2011 to August 19, 2011 (unaudited) |
For the period August 20, 2011 to August 31, 2011 (unaudited) |
|||||||||||||||||||||||||||||||
| (dollars in thousands) |
2007 | 2008 | 2009 | 2010 | 2011 | |||||||||||||||||||||||||||||
| Income statement data: |
||||||||||||||||||||||||||||||||||
| Net sales |
$ | 223,678 | $ | 261,199 | $ | 300,547 | $ | 329,073 | $ | 333,091 | $ | 83,641 | $ | 74,910 | $ | 11,390 | ||||||||||||||||||
| Cost of sales (exclusive of amortization shown separately below) |
65,923 | 75,710 | 84,536 | 95,349 | 96,175 | 23,974 | 22,955 | 7,156 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||||||
| Gross margin |
157,755 | 185,489 | 216,011 | 233,724 | 236,916 | 59,667 | 51,955 | 4,234 | ||||||||||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||||||||
| Research and development |
6,354 | 6,454 | 10,698 | 15,437 | 15,900 | 4,425 | 4,895 | 623 | ||||||||||||||||||||||||||
| Selling, general, administrative & distribution |
58,392 | 70,289 | 84,616 | 88,667 | 90,686 | 21,960 | 52,637 | 2,515 | ||||||||||||||||||||||||||
| Restructuring expenses |
1,051 | 646 | — | — | — | — | — | — | ||||||||||||||||||||||||||
| Amortization expenses |
346 | 357 | 3,739 | 4,278 | 4,333 | 1,080 | 931 | 1,648 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total operating expenses |
66,143 | 77,746 | 99,053 | 108,382 | 110,919 | 27,465 | 58,463 | 4,786 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Income (loss) from operations |
91,612 | 107,743 | 116,958 | 125,342 | 125,997 | 32,202 | (6,508 | ) | (552 | ) | ||||||||||||||||||||||||
| Non-operating income (expense) |
||||||||||||||||||||||||||||||||||
| Interest income |
2,841 | 4,263 | 1,957 | 454 | 706 | 213 | 142 | — | ||||||||||||||||||||||||||
| Interest expense |
(432 | ) | (371 | ) | (250 | ) | (33 | ) | (70 | ) | (14 | ) | — | (3,393 | ) | |||||||||||||||||||
| Other income (expense), net |
133 | 33 | (1,684 | ) | (551 | ) | 3,997 | 97 | 2,673 | (11 | ) | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total non-operating income (expense) |
2,542 | 3,925 | 23 | (130 | ) | 4,633 | 296 | 2,815 | (3,404 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Income (loss) before income taxes |
94,154 | 111,668 | 116,981 | 125,212 | 130,630 | 32,498 | (3,693 | ) | (3,956 | ) | ||||||||||||||||||||||||
| Income taxes |
34,086 | 40,214 | 40,798 | 42,629 | 41,303 | 11,079 | 2,681 | (1,514 | ) | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Net income (loss) |
$ | 60,068 | $ | 71,454 | $ | 76,183 | $ | 82,583 | $ | 89,327 | $ | 21,419 | $ | (6,374 | ) | $ | (2,442 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Cash flow data: |
||||||||||||||||||||||||||||||||||
| Net cash provided by (used in) operating activities |
$ | 58,279 | $ | 75,105 | $ | 79,822 | $ | 84,751 | $ | 102,111 | $ | 28,896 | $ | 25,588 | $ | (13,113 | ) | |||||||||||||||||
| Net cash used in investing activities |
(9,245 | ) | (15,693 | ) | (116,556 | ) | (6,304 | ) | (9,061 | ) | (1,219 | ) | (2,265 | ) | (1,940,294 | ) | ||||||||||||||||||
| Cash provided by (used in) financing activities |
10,175 | 72 | (1,396 | ) | (11,757 | ) | 2,578 | (310 | ) | 66 | 1,655,564 | |||||||||||||||||||||||
41
Table of Contents
| Predecessor | Successor | |||||||||||||||||||||||||||||||||
| Fiscal year ended May 31, | For the period June 1, 2010 to August 31, 2010 (unaudited) |
For the period June 1, 2011 to August 19, 2011 (unaudited) |
For the period August 20, 2011 to August 31, 2011 (unaudited) |
|||||||||||||||||||||||||||||||
| (dollars in thousands) |
2007 | 2008 | 2009 | 2010 | 2011 | |||||||||||||||||||||||||||||
| Balance sheet data: |
||||||||||||||||||||||||||||||||||
| Working capital |
$ | 162,865 | $ | 230,556 | $ | 192,562 | $ | 270,939 | $ | 378,979 | $ | 294,891 | $ | 381,259 | $ | 106,182 | ||||||||||||||||||
| Total assets |
275,478 | 364,950 | 451,340 | 519,834 | 633,127 | 549,351 | 652,395 | 2,013,869 | ||||||||||||||||||||||||||
| Long-term obligations, less current portion |
3,488 | — | — | — | — | — | — | 988,699 | ||||||||||||||||||||||||||
| Retained earnings (accumulated deficit) |
179,768 | 251,059 | 327,242 | 409,825 | 499,152 | 431,244 | 492,778 | (2,442 | ) | |||||||||||||||||||||||||
| Shareholders’ equity |
219,448 | 307,696 | 384,578 | 456,123 | 568,872 | 481,997 | 576,646 | 696,149 | ||||||||||||||||||||||||||
| Other financial data: |
||||||||||||||||||||||||||||||||||
| EBITDA(a) |
$ | 98,616 | $ | 116,152 | $ | 128,580 | $ | 141,360 | $ | 148,207 | $ | 36,654 | $ | 429 | $ | 1,580 | ||||||||||||||||||
| Adjusted EBITDA(a) |
101,606 | 119,797 | 136,168 | 147,857 | 151,151 | 39,474 | 37,969 | 1,391 | ||||||||||||||||||||||||||
| (a) | We have presented EBITDA and adjusted EBITDA (which is defined as net income before interest, taxes, depreciation and amortization (EBITDA) adjusted for the other items described in the notes below), which are non-GAAP financial measures. We present EBITDA and adjusted EBITDA in this prospectus because we consider them important supplemental measures of our performance and believe they are frequently used by securities analysts, investors and other interested parties in the evaluation of companies in our industry. EBITDA and adjusted EBITDA are not presentations made in accordance with GAAP, and our computation of EBITDA and adjusted EBITDA may vary from others in our industry. EBITDA and adjusted EBITDA should not be considered alternatives to operating income or net income, as a measure of operating performance or cash flow, as a measure of liquidity. EBITDA and adjusted EBITDA have important limitations as analytical tools, and you should not consider them in isolation, or as a substitute for analysis of our results as reported under GAAP. For example, EBITDA and adjusted EBITDA: |
| • | do not reflect our cash expenditures, or future requirements, for capital expenditures or contractual commitments; |
| • | do not reflect changes in, or cash requirements for, our working capital needs; |
| • | do not reflect the significant interest expense, or the cash requirements necessary to service interest or principal payments, on our debt; |
| • | exclude income tax payments that represent a reduction in cash available to us; |
| • | do not reflect any cash requirements for assets being depreciated and amortized that may have to be replaced in the future; and |
| • | do not reflect the impact of earnings or charges resulting from matters we consider not to be indicative of our ongoing operations. |
EBITDA and adjusted EBITDA are calculated by adjusting net income for the items summarized in the table below.
The following table is a reconciliation of net income to EBITDA and adjusted EBITDA for the periods indicated:
| Predecessor | Successor | |||||||||||||||||||||||||||||||||
| Fiscal year ended May 31, | For the period June 1, 2010 to August 31, 2010 |
For the period June 1, 2011 to August 19, 2011 |
For the period August 20, 2011 to August 31, 2011 |
|||||||||||||||||||||||||||||||
| (dollars in thousands) |
2007 | 2008 | 2009 | 2010 | 2011 | |||||||||||||||||||||||||||||
| Net income |
$ | 60,068 | $ | 71,454 | $ | 76,183 | $ | 82,583 | $ | 89,327 | $ | 21,419 | $ | (6,374 | ) | $ | (2,442 | ) | ||||||||||||||||
| Interest expense (income), net |
(2,409 | ) | (3,892 | ) | (1,707 | ) | (421 | ) | (636 | ) | (199 | ) | (142 | ) | 3,393 | |||||||||||||||||||
| Income tax expense |
34,086 | 40,214 | 40,798 | 42,629 | 41,303 | 11,079 | 2,681 | (1,514 | ) | |||||||||||||||||||||||||
| Depreciation and amortization |
6,871 | 8,376 | 13,306 | 16,569 | 18,213 | 4,383 | 4,264 | 2,143 | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| EBITDA |
$ | 98,616 | $ | 116,152 | $ | 128,580 | $ | 141,360 | $ | 148,207 | $ | 36,682 | $ | 429 | $ | 1,580 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Adjustments to EBITDA: |
||||||||||||||||||||||||||||||||||
| Stock-based compensation(i) |
$ | 3,123 | 3,678 | $ | 5,904 | $ | 5,946 | $ | 6,941 | $ | 1,739 | $ | 16,233 | — | ||||||||||||||||||||
| Transaction costs(ii) |
— | — | — | — | — | — | 18,863 | — | ||||||||||||||||||||||||||
| Other expense / (income)(iii) |
(133 | ) | (33 | ) | 1,684 | 551 | (3,997 | ) | 1,053 | 2,444 | (189 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Adjusted EBITDA |
$ | 101,606 | $ | 119,797 | $ | 136,168 | $ | 147,857 | $ | 151,151 | $ | 39,474 | $ | 37,969 | $ | 1,391 | ||||||||||||||||||
42
Table of Contents
| (i) | Reflects non-cash stock-based compensation expense related to the vesting of stock options. |
| (ii) | Transaction costs include legal, accounting and other costs related to the Transactions. |
| (iii) | Includes the following: |
| Predecessor | Successor | |||||||||||||||||||||||||||||||||
| Fiscal year ended May 31, | For the period June 1, 2010 to August 31, 2010 |
For the period June 1, 2011 to August 19, 2011 |
For the period August 20, 2011 to August 31, 2011 |
|||||||||||||||||||||||||||||||
| (dollars in thousands) |
2007 | 2008 | 2009 | 2010 | 2011 | |||||||||||||||||||||||||||||
| Exchange losses / (gains), net |
$ | (414 | ) | $ | (70 | ) | $ | 1,889 | $ | 362 | $ | 320 | $ | 71 | $ | (2,723 | ) | $ | 5 | |||||||||||||||
| Return of escrowed funds related to the BioArray acquisition |
— | — | — | — | (4,300 | ) | — | — | — | |||||||||||||||||||||||||
| Losses / (gains) on sale of fixed assets, net |
35 | 82 | 25 | 284 | 188 | 219 | 135 | — | ||||||||||||||||||||||||||
| Miscellaneous, net |
246 | (45 | ) | (230 | ) | (95 | ) | (205 | ) | 763 | 5,032 | (194 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total |
$ | (133 | ) | $ | (33 | ) | $ | 1,684 | $ | 551 | $ | (3,997 | ) | $ | 1,053 | $ | 2,444 | $ | (189 | ) | ||||||||||||||
43
Table of Contents
Unaudited pro forma consolidated financial information
The following unaudited pro forma consolidated statements of operations have been developed by applying pro forma adjustments to our historical audited consolidated financial statements for the fiscal year ended May 31, 2011 and our unaudited interim consolidated financial statements for the period from June 1, 2011 through August 19, 2011, the Predecessor period, and for the period from August 20, 2011 to August 31, 2011, the Successor period, appearing elsewhere in this prospectus. The unaudited pro forma consolidated statements of operations for the fiscal year ended May 31, 2011 and the quarter ended August 31, 2011 give effect to the Transactions as if they had occurred on June 1, 2010.
The unaudited pro forma adjustments are based upon available information and certain assumptions that we believe are reasonable under the circumstances. The unaudited pro forma consolidated financial data is presented for informational purposes only. The unaudited pro forma consolidated financial data does not purport to represent what our results of operations or financial condition would have been had the Transactions actually occurred on the dates indicated, nor does it purport to project our results of operations or financial condition for any future period or as of any future date. The unaudited pro forma consolidated financial data should be read in conjunction with the information included under the headings “The transactions,” “Selected historical consolidated financial data,” “Management’s discussion and analysis of financial condition and results of operations” and our historical audited and unaudited consolidated financial statements and related notes included elsewhere in this prospectus. All pro forma adjustments and their underlying assumptions are described more fully in the notes to our unaudited pro forma consolidated statements of operations.
The unaudited pro forma consolidated statements of operations give effect to adjustments that are (i) directly attributable to the Transactions, (ii) factually supportable and (iii) expected to have a continuing impact. The Acquisition has been accounted for using the acquisition method of accounting.
The unaudited pro forma consolidated statements of operations have not been adjusted to reflect certain non-recurring effects that we recorded in connection with the Transactions, such as (i) the effect on revenue from the write-off in deferred revenue and (ii) the impact on costs of sales from the increase of our inventory to fair value.
44
Table of Contents
Unaudited pro forma consolidated statement of operations
for the year ended May 31, 2011
| (dollars in thousands) |
Historical | Pro forma adjustments |
Unaudited pro forma |
|||||||||||
| Net sales |
$ | 333,091 | $ | — | $ | 333,091 | ||||||||
| Cost of sales (exclusive of amortization) |
96,175 | — | 96,175 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| Gross margin |
236,916 | — | 236,916 | |||||||||||
| Operating expenses: |
||||||||||||||
| Research and development |
15,900 | — | 15,900 | |||||||||||
| Selling and marketing |
36,431 | — | 36,431 | |||||||||||
| Distribution |
16,508 | — | 16,508 | |||||||||||
| General and administrative |
37,747 | 4,753 | (A)(B) | 42,500 | ||||||||||
| Amortization |
4,333 | 45,445 | (A) | 49,778 | ||||||||||
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
110,919 | 50,198 | 161,117 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| Income from operations |
125,997 | (50,198 | ) | 75,799 | ||||||||||
|
|
|
|
|
|
|
|||||||||
| Non-operating income (expense): |
||||||||||||||
| Interest income |
706 | (706 | ) | (C) | — | |||||||||
| Interest expense |
(70 | ) | (97,577 | ) | (D) | (97,647 | ) | |||||||
| Other, net |
3,997 | — | 3,997 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| Total non-operating income (expense) |
4,633 | (98,283 | ) | (93,650 | ) | |||||||||
|
|
|
|
|
|
|
|||||||||
| Income before income taxes |
130,630 | (148,482 | ) | (17,851 | ) | |||||||||
| Provision (benefit) for income taxes |
41,303 | (57,759 | ) | (F) | (16,456 | ) | ||||||||
|
|
|
|
|
|
|
|||||||||
| Net income (loss) |
$ | 89,327 | $ | (90,722 | ) | $ | (1,395 | ) | ||||||
|
|
|
|
|
|
|
|||||||||
See accompanying notes to unaudited pro forma consolidated statement of operations
45
Table of Contents
Unaudited pro forma consolidated statement of operations for the quarter ended August 31, 2011
| (dollars in thousands) |
Historical August 20, 2011 to August 31, 2011 |
Historical June 1, 2011 to August 19, 2011 |
Pro forma adjustments |
Pro forma June 1, 2011 to August 31, 2011 |
||||||||||||||||
| (Successor) | (Predecessor) | |||||||||||||||||||
| Net sales |
$ | 11,390 | $ | 74,910 | $ | — | $ | 86,300 | ||||||||||||
| Cost of sales (exclusive of amortization) |
7,156 | 22,955 | (2,414 | ) | (E) | 27,697 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Gross margin |
4,234 | 51,955 | 2,414 | 58,603 | ||||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Research and development |
623 | 4,895 | (885 | ) | (E) | 4.633 | ||||||||||||||
| Selling and marketing |
1,112 | 10,510 | (1,341 | ) | (E) | 10,281 | ||||||||||||||
| Distribution |
649 | 3,952 | (168 | ) | (E) | 4,433 | ||||||||||||||
| General and administrative |
754 | 38,175 | (28,454 | ) | (A),(B),(E) | 10,475 | ||||||||||||||
| Amortization |
1,648 | 931 | 9,866 | (A) | 12,445 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
4,786 | 58,463 | (20,982 | ) | 42,267 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Income from operations |
(552 | ) | (6,508 | ) | 23,396 | 16,336 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Non-operating income (expense): |
||||||||||||||||||||
| Interest income |
— | 142 | (142 | ) | (C) | — | ||||||||||||||
| Interest expense |
(3,393 | ) | — | (21,813 | ) | (D) | (25,206 | ) | ||||||||||||
| Other, net |
(11 | ) | 2,673 | — | 2,662 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total non-operating income (expense) |
(3,404 | ) | 2,815 | (21,955 | ) | (22,544 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Income before income taxes |
(3,956 | ) | (3,693 | ) | 1,441 | (6,208 | ) | |||||||||||||
| Provision (benefit) for income taxes |
(1,514 | ) | 2,681 | 560 | (F) | 1,727 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net income (loss) |
$ | (2,442 | ) | $ | (6,374 | ) | $ | 881 | $ | (7,935 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||||
See accompanying notes to unaudited pro forma consolidated statement of operations
46
Table of Contents
Notes to unaudited pro forma statements of operations for the fiscal year ended May 31, 2011 and for the quarter ended August 31, 2011
The unaudited pro forma consolidated statements of operations reflect our results for the fiscal year ended May 31, 2011 and for the fiscal quarter ended August 31, 2011, respectively. The unaudited pro forma consolidated statements of operations do not include, among other non-recurring adjustments, a charge for the non-cash inventory step-up that will have an impact on cost of sales during the approximate four-month period after the closing of the Acquisition during which our inventory on hand at such time is sold, and a charge for the write-off of deferred revenue, because these are not expected to have a continuing impact on the statements of income.
| (A) | The pro forma adjustments to depreciation resulting from recording our property and equipment at fair value pursuant to the acquisition method of accounting is calculated as follows: |
| For the year ended May 31, 2011 |
For the quarter
ended August 31, 2011 |
|||||||
| Pro forma |
$ | 15,615 | $ | 3,772 | ||||
| Historical |
13,862 | 3,333 | ||||||
|
|
|
|
|
|||||
| Adjustment |
$ | 1,753 | $ | 439 | ||||
|
|
|
|
|
|||||
The pro forma adjustments to amortization associated with intangible assets recorded at fair value pursuant to acquisition accounting is calculated as follows:
| For the year ended May 31, 2011 |
For the quarter ended August 31, 2011 |
|||||||
| Pro forma |
$ | 49,778 | $ | 12,445 | ||||
| Historical |
4,333 | 2,579 | ||||||
|
|
|
|
|
|||||
| Adjustment |
$ | 45,445 | $ | 9,866 | ||||
|
|
|
|
|
|||||
The unaudited pro forma consolidated statements of operations reflect adjustments to depreciation and amortization of property and equipment and identifiable intangible assets, including customer lists, existing technology and trade names, and below market leasehold interests based on our allocation of the purchase price over a weighted average useful life of 3 years for property and equipment and 16.6 years for definite-lived intangibles. Identifiable intangible assets have been amortized on a straight-line basis in the unaudited pro forma consolidated statements of operations.
| (B) | Adjustments relate to the following: |
| For the year ended May 31, 2011 |
For the quarter ended August 31, 2011 |
|||||||
| Adjustment to management fee(i) |
$ | 3,000 | $ | 644 | ||||
| Adjustment to transaction costs(ii) |
— | (18,863 | ) | |||||
|
|
|
|
|
|||||
| Total adjustment to general and administrative expenses (other than depreciation expenses described in Note A) |
$ | 3,000 | $ | (18,219 | ) | |||
|
|
|
|
|
|||||
| (i) | Represents the recurring estimated fees of $3.0 million associated with the management services agreement that we entered into with TPG Capital upon completion of the Transactions. |
| (ii) | Represents the elimination of historically recognized non-recurring costs directly related to the Transactions. |
47
Table of Contents
| (C) | Represents the elimination of historical interest income, as substantially all interest-bearing cash and cash equivalents have been used to fund the purchase consideration. |
| (D) | Represents estimated interest expense resulting from our new capital structure upon consummation of the Transactions: |
| For the year ended May 31, 2011 |
For the quarter ended August 31, 2011 |
|||||||
| Interest on term loan facility(i) |
$ | 44,587 | $ | 11,416 | ||||
| Interest on notes(i) |
44,500 | 11,367 | ||||||
| Amortization of capitalized debt issuance costs(ii) |
8,560 | 2,423 | ||||||
|
|
|
|
|
|||||
| Total interest expense |
$ | 97,647 | $ | 25,206 | ||||
|
|
|
|
|
|||||
| (i) | Represents interest on borrowings of $615.0 million under the senior secured term loan and $400.0 million aggregate principal of the notes that accrue interest at an interest rate of 7.25% and 11.125% respectively. A 0.125% increase in the floating rates above a floor of 1.5% on the senior secured term loan would increase our annual pro forma interest expense by approximately $0.8 million. |
| (ii) | Represents amortization of capitalized debt issuance costs incurred in connection with the establishment of the senior secured term loan facility and the senior secured revolving facility included in the Senior Credit Facilities and the notes over 5, 7 and 8 years, respectively. |
| (E) | Represents the effect of compensation expense and the related payroll tax that was recorded upon the accelerated vesting of share-based awards upon the change in control: |
| For the year ended May 31, 2011 |
For the quarter ended August 31, 2011 |
|||||||
| Adjustment for share-based compensation expense recorded in: |
||||||||
| Cost of sales |
$ | — | $ | (2,414 | ) | |||
| Research and development |
— | (885 | ) | |||||
| Selling and marketing |
— | (1,341 | ) | |||||
| Distribution |
— | (168 | ) | |||||
| General and administrative |
— | (10,674 | ) | |||||
|
|
|
|
|
|||||
| Total |
$ | — | $ | (15,482 | ) | |||
|
|
|
|
|
|||||
| (F) | Represents the effects of the above adjustments to income taxes based on the applicable tax rate of approximately 39%. |
48
Table of Contents
Ratio of earnings to fixed charges
The following table sets forth our ratio of earnings to fixed charges for each of the periods shown.
| (Predecessor) | (Combined) | (Pro forma) | ||||||||||||||||||||||||||||||
| Fiscal year ended May 31, | Quarter ended |
Fiscal year ended |
Quarter ended |
|||||||||||||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | Aug. 31, 2011 |
May 31, 2011 |
August 31, 2011 |
|||||||||||||||||||||||||
| Ratio of earnings to fixed charges |
110x | 106x | 96x | 100x | 92x | (a | ) | (b | )(c) | (b | )(c) | |||||||||||||||||||||
| (a) | The quarter ended August 31, 2011 reflects the addition of data from the Predecessor period and the Successor period to calculate the ratio on a combined basis. Our earnings were insufficient to cover fixed charges by $7.7 million for the quarter ended August 31, 2011. |
| (b) | The pro forma ratios of earnings to fixed charges have been developed by applying certain pro forma adjustments to our historical audited consolidated financial statements for the fiscal year ended May 31, 2011 and our unaudited interim consolidated financial statements for the period from June 1, 2011 through August 19, 2011 and for the period from August 20, 2011 to August 31, 2011, appearing elsewhere in this prospectus. For further information on pro forma adjustments and calculations, see “Unaudited pro forma consolidated financial information.” |
| (c) | Our pro forma earnings were insufficient to cover fixed charges by $17.9 million for the year ended May 31, 2011 and by $6.2 million for the quarter ended August 31, 2011. |
These ratios are computed by dividing the total earnings by the total fixed charges. For purposes of calculating the ratio of earnings to fixed charges, earnings represents pre-tax income from continuing operations plus fixed charges. Fixed charges consist of interest expense on all indebtedness plus amortization of deferred financing costs and the portion of rental expense that we believe is representative of the interest component of rental expense.
49
Table of Contents
Management’s discussion and analysis of financial condition
and results of operations
This discussion summarizes our consolidated operating results, financial condition and liquidity during each of the years in the three-year period ended May 31, 2011 and the fiscal quarter ended August 31, 2011. Our fiscal year ends on May 31.
You should read the following discussion of our results of operations and financial condition with the “Unaudited pro forma consolidated financial information,” “Selected historical consolidated financial data” and the historical audited and unaudited consolidated financial statements and related notes included elsewhere in this prospectus. This discussion contains forward-looking statements and involves numerous risks and uncertainties, including, but not limited to, those described in the “Risk factors” section of this prospectus. Actual results may differ materially from those contained in any forward-looking statements.
In management’s discussion and analysis, we have presented the results of operations and cash flows separately for the period from June 1, 2011 to August 19, 2011 (Predecessor), the period from August 20, 2011 to August 31, 2011 (Successor), and the three months ended August 31, 2010. We have prepared our discussion and analysis of the results of operations and cash flows by comparing the combined results of the Predecessor and Successor fiscal 2012 periods (three months ended August 31, 2011) with those of the Predecessor three months ended August 31, 2010. We believe this approach provides the most meaningful basis for the analysis and discussion of our results. Combined changes in operating results (i) have not been prepared on a pro forma basis as if the Acquisition occurred on the first day of the period, (ii) may not reflect the actual results we would have achieved absent the Acquisition, and (iii) may not be predictive of future results of operations.
Overview
The transactions
On July 2, 2011, we entered into a merger agreement pursuant to which, on August 19, 2011, Merger Sub merged with and into the Company, with the Company continuing as the surviving corporation and a wholly-owned indirect subsidiary of Holding. As a result of the merger, each outstanding share of Company common stock, other than shares of Company common stock held by Holding or Merger Sub, by the Company in treasury, by a wholly-owned subsidiary of the Company or by stockholders who validly exercised their appraisal rights under Georgia law, were converted into the right to receive cash in an amount equal to the offer price, subject to any required withholding of taxes. After that time, we continued our current operations, except that we ceased to be a public company, and our common stock ceased to be traded on the NASDAQ Stock Market. See “The transactions” in this prospectus for a more detailed description.
Effect of the acquisition
The application of purchase accounting as a result of the Acquisition required that our assets and liabilities be adjusted to their fair value as of the closing of the Acquisition. This resulted in an increase in our depreciation and amortization expense following the closing of the Acquisition because we wrote up our property, plant and equipment and our intangible assets to their fair value and then depreciated and amortized these higher amounts. In addition, we wrote up the value of our existing inventory by approximately $24.8 million to fair value, which will decrease our margins on that inventory when we sell it to our customers. Also, as a result of the Transactions, our borrowings and interest expense significantly increased. Any excess of purchase price over the fair value of our net assets, including identified intangible assets, was allocated to goodwill. Our indefinite-lived intangible assets and goodwill will be subject to periodic tests for impairment. We also recorded certain non-recurring charges during the Predecessor period in connection with the Transactions, including (i) the share-based compensation expense of approximately $15.5 million relating to the accelerated vesting of restricted stock, restricted stock units and stock options awarded to management and employees that occurred upon a change in control and (ii) certain non-recurring expenses related to the Transactions of approximately $18.9 million required to be expensed by accounting standards.
50
Table of Contents
Our business
We develop, manufacture and sell a complete line of reagents and automated systems used primarily by donor centers, hospitals and reference laboratories for testing to detect and identify certain properties of human blood for the purpose of blood transfusion. We have manufacturing facilities in the U.S. and Canada and sell our products through our direct sales network in the U.S., Canada, Western Europe and Japan as well as through third-party distributors in other markets.
We operate in a highly regulated industry and are subject to continuing compliance with multiple country-specific statutes, regulations and standards. For example, in the U.S., the FDA regulates all aspects of the blood banking industry, including the marketing of reagents and instruments used to detect and identify blood properties. Additionally, we are subject to government legislation that governs the delivery of healthcare. For example, in the U.S., the Patient Protection and Affordable Care Act was signed into law in March 2010 and contains elements that could meaningfully change the way healthcare is developed and delivered in the U.S. Included in the legislation is a 2.3% excise tax on medical device makers beginning in 2013.
In the markets of Western Europe, Canada and Japan, the testing of donor and patient blood for the purpose of transfusion is primarily automated. However, in the U.S., we estimate approximately 60% of laboratories perform this testing manually today. These laboratories are primarily in the small- to medium-sized hospital segment.
Our strategy is to drive automation in the blood bank with the goal of improving operations as well as patient safety. We continually innovate to ensure our automation offerings are competitive.
We offer two fully automated instruments for serology testing – NEO® and Echo® – to meet the different needs of our customers depending upon the volume in their laboratory and the complexity of the testing required. All of our serology instrumentation uses Capture® technology, our proprietary reagents, as well as traditional reagents to perform automated testing.
NEO is targeted at large hospitals, donor centers and reference laboratories and replaced our previous high volume serology instrument, Galileo.
Echo is a compact bench top, fully-automated walk-away serology instrument that meets the needs of the small- to medium-sized hospital market as well as integrated delivery networks, in conjunction with NEO, that want to standardize the operations of their laboratories.
In August 2008, we added molecular immunohematology to our product portfolio with our acquisition of privately-held BioArray. BioArray manufactures products which use DNA to identify certain properties of blood prior to transfusion. In many countries, blood pre-transfusion testing is limited to the prevention of transfusion reactions and not for the prevention of alloimmunization, which occurs when antigens foreign to the patient are inadvertently introduced into the patient’s blood system through transfusions. If alloimmunization occurs, the patient develops new antibodies in response to the foreign antigens, thereby complicating future transfusions. By using multiplex, cost-effective molecular testing, we believe that our molecular technology allows testing to prevent alloimmunization for better patient care.
Our Human Erythrocyte Antigen (“HEA”) molecular immunohematology product, our Human Platelet Antigen (“HPA”) molecular immunohematology product as well as our current semi-automated molecular immunohematology instrument, the Array Imaging System and BASIS™ database are CE (“Conformité Européenne”) Marked denoting regulatory clearance in the European Union (“EU”). Our molecular offering is currently available for Research Use Only in the U.S.
51
Table of Contents
Recent Developments
The following discusses recent material developments in our business.
Acquisition of the Company—On August 19, 2011, the Company was acquired through a merger transaction with Merger Sub, a wholly owned subsidiary of IVD Intermediate Holdings B, Inc. (the “Parent”). The Parent is a wholly owned indirect subsidiary of Holding, which was formed by investment funds affiliated with TPG Capital. The Acquisition was accomplished through a merger of Merger Sub with and into the Company, with the Company being the surviving company. As a result of the Acquisition, the Company became an indirect wholly owned subsidiary of Holding. Prior to August 19, 2011, the Company operated as a public company with common stock traded on the NASDAQ Stock Market.
Atypical items positively impacted fiscal 2011 net income—We had three atypical items in the fourth quarter of fiscal 2011 that, together on a net basis, positively impacted fully diluted earnings per share by approximately $0.07 per fully diluted share, net of tax as appropriate.
| • | In May 2011, $7.2 million of funds escrowed in connection with the BioArray acquisition were released and returned to the Company. In accordance with ASC 805, Business Combinations, $2.9 million of the $7.2 million returned to the Company was recorded to goodwill and the remaining $4.3 million, or approximately $0.06 per fully diluted share, was recorded to other income. Since the return of the escrowed funds was considered a return of purchase price, no tax was provided on these amounts. |
| • | In April 2011, a change in the state of New Jersey tax apportionment factors resulted in a positive adjustment of approximately $2.3 million, or $0.03 per fully diluted share, to our deferred tax liability that was established on the acquisition of BioArray. |
| • | In April 2011, we announced that our Chief Operating Officer resigned. The Company incurred approximately $2.4 million, or $0.02 per fully diluted share, net of tax, of expenses in association with his resignation, which partially offset the positive impact of the two items described above. |
Lower industry demand in the U.S. market—Beginning early in our fiscal year 2010, we believe the U.S. market began experiencing lower demand for blood because of the macroeconomic environment. Lower blood demand negatively impacts our reagent revenue as fewer blood transfusions result in lower testing volume. In our fiscal years 2010 and 2011, we believe industry demand in the U.S. market declined by approximately 3.5% in both years.
Critical accounting policies and estimates
General
We have identified the policies below as critical to our business operations and the understanding of our results of operations. The impact and any associated risks related to these policies on our business operations are discussed throughout “Management’s discussion and analysis of financial condition and results of operations” where such policies affect our reported and expected financial results. For a detailed discussion on the application of these and other accounting policies, see Note 1 to the audited consolidated financial statements included in this prospectus. Note that our preparation of this prospectus requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of our financial statements, and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates, and certain assumptions could prove to be incorrect. We believe that our most critical accounting policies and estimates relate to the following:
| • | Revenue recognition |
| • | Trade accounts receivable and allowance for doubtful accounts |
| • | Inventories |
| • | Goodwill |
| • | Taxation |
| • | Share-based compensation |
52
Table of Contents
Revenue recognition
In accordance with ASC 605, “Revenue Recognition,” we recognize revenue when the following four basic criteria have been met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services are rendered; (3) the fee is fixed and determinable; and (4) collectability is reasonably assured. Should changes in conditions cause management to determine these criteria are not met for certain future transactions, revenue recognized for any reporting period could be adversely affected.
Effective June 1, 2011, the Company prospectively adopted Accounting Standards Update (“ASU”) 2009-13, “Multiple-Deliverable Revenue Arrangements.” Under historical standards, the Company used the residual method to allocate arrangement consideration when vendor specific objective evidence existed for all undelivered elements, but not for the delivered elements. Under the new standards, the Company allocates revenue to all elements based on their relative selling prices. The selling prices of the various elements of our contractual arrangements are determined as follows:
Reagents (without price guarantees)—the selling price of reagents (without price guarantees) is based on vendor specific objective evidence (“VSOE”) of fair value by reference to the price our customers are required to pay for the reagents when sold separately.
Reagents (with price guarantees)—the selling price of reagents (with price guarantees) is based on management’s best estimate of selling price (“MBESP”). In determining MBESP, we consider the following: (1) our pricing practices as they relate to future price increases, (2) the overall economic conditions, and (3) competitor pricing. Revenue from the sale of the Company’s reagents (both with and without price guarantees) to end users is primarily recognized upon shipment when both title and risk of loss transfer to the customer, unless there are specific contractual terms to the contrary. Revenue from the sale of the Company’s reagents to distributors is recognized FOB customs clearance when both title and risk of loss transfer to the customer.
Instrument sales—the selling price of our instruments is based on MBESP. In determining MBESP, we consider the following: (1) the overall economic conditions, (2) the expected profit margin to be realized on the instruments, and (3) competitor pricing. Revenue from instrument sales is recognized when the instrument has been installed and accepted by the customer.
Consumables (part kits)—the selling price of our consumables is based on MBESP. In determining MBESP, we consider the following: (1) the overall economic conditions, (2) the expected profit margin to be realized on the agreement, and (3) competitor pricing. Revenue from consumables is recognized when the consumables have been delivered.
Training—the selling price of training is based on VSOE of fair value by reference to the price our customers are required to pay for training when training services are sold separately (through additional/incremental training sessions). Revenue from training services is recognized as the training services are provided.
General Support Agreements—the selling price of general support agreements is based on VSOE by reference to the price our customers are required to pay for the general support agreements when sold separately via renewals. Revenue from general support agreements is recognized over the term of the agreement.
Instrument leases—the selling price of instrument leases is based on MBESP. In determining MBESP, we consider the following: (1) the overall economic conditions, (2) the expected profit margin to be realized on the agreement, and (3) competitor pricing. Revenue from instrument leases is recognized over the term of the operating lease, which is generally 60 months.
Trade accounts receivable and allowance for doubtful accounts
Trade receivables at August 31, 2011, May 31, 2011 and May 31, 2010, totaling $67.6 million, $63.3 million and $59.6 million, respectively, are net of allowances for doubtful accounts of $10 thousand, $2.2 million and $2.1
53
Table of Contents
million, respectively. The allowance for doubtful accounts represents a reserve for estimated losses resulting from the inability of our customers to pay their debts. The collectability of trade receivable balances is regularly evaluated based on a combination of factors such as customer credit-worthiness, past transaction history with the customer, current economic industry trends and changes in customer payment patterns. If it is determined that a customer will be unable to fully meet its financial obligation, such as in the case of a bankruptcy filing or other material events impacting its business, a specific allowance for doubtful accounts is recorded to reduce the related receivable to the amount expected to be recovered. On August 19, 2011, in connection with the Acquisition, trade receivables were written down to the amount expected to be recovered and the allowance for doubtful accounts was set to zero.
Inventories
Typically, inventories are stated at the lower of cost (first-in, first-out basis) or market (net realizable value). Cost includes material, labor and manufacturing overhead. We also allocate certain production-related general and administrative costs to inventory and incurred approximately $2.8 million, $3.3 million and $3.0 million of such costs in fiscal 2011, 2010 and 2009, respectively. We had approximately $0.9 million and $1.2 million of general and administrative costs remaining in inventory as of May 31, 2011 and May 31, 2010, respectively.
We use a standard cost system as a tool to monitor production efficiency. The standard cost system applies estimated labor and manufacturing overhead factors to inventory based on budgeted production and efficiency levels, staffing levels and costs of operation, based on the experience and judgment of management. Actual costs and production levels may vary from the standard established and such variances are charged to the consolidated statement of income as a component of cost of sales. Since U.S. generally accepted accounting principles require that the standard cost approximate actual cost, periodic adjustments are made to the standard rates to approximate actual costs. In connection with the Acquisition of the Company on August 19, 2011, a fair value adjustment of $25.4 million increased inventory to net realizable value, which is currently greater than replacement cost. No material changes have been made to the inventory policy during the first quarter of fiscal 2012 or during fiscal 2011, 2010 or 2009.
Goodwill
Consistent with ASC 350, Intangibles—Goodwill and Other (“ASC 350”), goodwill and intangible assets with indefinite lives are not amortized but are tested for impairment annually or more frequently if impairment indicators arise. Intangible assets that have finite lives are amortized over their useful lives.
We evaluate the carrying value of goodwill at the beginning of the fourth fiscal quarter of each year and between annual evaluations if events occur or circumstances change that would more likely than not reduce the fair value of the reporting unit below its carrying amount. Such circumstances could include, but are not limited to: (1) a significant adverse change in legal factors or in business climate, (2) unanticipated competition, or (3) an adverse action or assessment by a regulator. When evaluating whether goodwill is impaired, we first assess qualitative factors to determine if it is more likely than not (defined as 50% or more) that the fair value of the reporting unit is less than its carrying amount. If it is determined that it is not more likely than not that the fair value of the reporting unit is less than its carrying amount, no additional steps are taken. If it is determined that it is more likely than not that the fair value of the reporting unit is less than its carrying amount, we then compare the fair value of the reporting unit to which the goodwill is assigned to the reporting unit’s carrying amount, including goodwill. The fair value of the reporting unit is estimated using primarily the income, or discounted cash flows, approach. If the carrying amount of a reporting unit exceeds its fair value, then the amount of the impairment loss must be measured. The impairment loss would be calculated by comparing the implied fair value of the reporting unit’s goodwill to its carrying amount. In calculating the implied fair value of the reporting unit’s goodwill, the fair value of the reporting unit is allocated to all of the other assets and liabilities of that unit based on their fair values. The excess of the fair value of a reporting unit over the amount assigned to its other assets and liabilities is the implied fair value of goodwill. An impairment loss would be recognized when the carrying amount of goodwill exceeds its implied fair value. Our evaluation of goodwill completed during fiscal 2011 resulted in no impairment charges.
54
Table of Contents
Income taxes
Our income tax policy records the estimated future tax effects of temporary differences between the tax bases of assets and liabilities and amounts reported in the accompanying consolidated balance sheets, as well as operating loss and tax credit carry-forwards. The value of our deferred tax assets assumes that we will be able to generate sufficient future taxable income in certain tax jurisdictions, based on estimates and assumptions. If these estimates and related assumptions change in the future, we may be required to record additional valuation allowances against our deferred tax assets resulting in additional income tax expense in our consolidated statements of income. In assessing the realizability of deferred tax assets, we consider whether it is more likely than not that some portion or all of the deferred tax assets will not be realized, and we consider the scheduled reversal of deferred tax liabilities, projected future taxable income, carry-back opportunities, and tax-planning strategies in making this assessment. We assess the need for additional valuation allowances quarterly. See Note 13 to the audited consolidated financial statements for the year ended May 31, 2011, which are included in this prospectus.
The calculation of income tax liabilities involves significant judgment by management in estimating the impact of uncertainties in the application of complex tax laws. Resolution of these uncertainties in a manner inconsistent with our expectations could have a material impact on our results of operations.
Share-based employee compensation
Consistent with the provisions of ASC 718, Compensation—Stock Compensation, compensation costs for grants of all share-based payments is based on the estimated grant date fair value. We attribute the value of share-based compensation to expense using the straight-line method.
We estimate the fair value of our share-based payment awards using the Black-Scholes option-pricing model (the “Black-Scholes model”). The Black-Scholes model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable. The Black-Scholes model requires the input of certain assumptions. Our stock options have characteristics significantly different from those of traded options, and changes in the assumptions can materially affect the fair value estimates.
We have calculated our additional paid in capital pool (“APIC pool”) based on the actual income tax benefits received from exercises of stock options granted after the effective date of ASC 718 using the long method. The APIC pool is available to absorb any tax deficiencies subsequent to the adoption of ASC 718. As of August 19, 2011, the APIC pool has been reset to zero.
55
Table of Contents
Results of operations
Comparison of the Successor Period from August 20, 2011 to August 31, 2011 and the Predecessor Period from June 1, 2011 to August 19, 2011 with the Predecessor three month period ended August 31, 2010
| Successor | Predecessor | |||||||||||||||||||||
| August 20, 2011 through August 31, 2011 |
June 1,
2011 through August 19, 2011 |
Three
Months Ended August 31, 2010 |
Change | |||||||||||||||||||
| Amount | % | |||||||||||||||||||||
| ($ in thousands) | ($ in thousands) | |||||||||||||||||||||
| Net sales |
$ | 11,390 | $ | 74,910 | $ | 83,641 | $ | 2,659 | 3 | % | ||||||||||||
| Gross margin |
4,234 | 51,955 | 59,667 | (3,478 | ) | -6 | % | |||||||||||||||
| Gross margin percentage |
37.2 | % | 69.4 | % | 71.3 | % | ||||||||||||||||
| Operating expenses |
4,786 | 58,463 | 27,465 | 35,784 | 130 | % | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Income (loss) from operations |
(552 | ) | (6,508 | ) | 32,202 | (39,262 | ) | -122 | % | |||||||||||||
| Non-operating income (expense) |
(3,404 | ) | 2,815 | 296 | (885 | ) | -299 | % | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Income (loss) before income tax |
(3,956 | ) | (3,693 | ) | 32,498 | (40,147 | ) | -124 | % | |||||||||||||
| Provision (benefit) for income tax |
(1,514 | ) | 2,681 | 11,079 | (9,912 | ) | -89 | % | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net income (loss) |
$ | (2,442 | ) | $ | (6,374 | ) | $ | 21,419 | $ | (30,235 | ) | -141 | % | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||
Revenue was $11.4 million in the Successor period and $74.9 million in Predecessor fiscal 2012 period compared to $83.6 million in Predecessor fiscal 2011 period. The overall increase of $2.7 million was due to favorable fluctuations in foreign currency exchange rates of $2.7 million. Weaker industry demand in the U.S. market continued to be the primary factor negatively impacting our year-over-year revenue growth rate.
Consolidated gross margin was 37.2% in the Successor period and 69.4% in Predecessor fiscal 2012 period yielding a combined consolidated gross margin of 65.1%. This was a decrease from the 71.3% achieved in the Predecessor fiscal 2011 period. Gross margins in the Successor period were negatively impacted by the amortization of the fair value adjustment to inventory and in the Predecessor fiscal 2012 period by the acceleration of share-based compensation expense both resulting from the Acquisition. The acceleration of share-based compensation expense and transaction costs relating to the Acquisition drove the 130% increase in operating expenses and the 141% decrease in net income.
Net sales
| Successor | Predecessor | |||||||||||||||||||||
| August 20, 2011 through August 31, 2011 |
June 1, 2011 through August 19, 2011 |
Three Months Ended August 31, 2010 |
Change | |||||||||||||||||||
| Amount | % | |||||||||||||||||||||
| ($ in thousands) | ($ in thousands) | |||||||||||||||||||||
| Traditional reagents |
$ | 6,296 | $ | 42,936 | $ | 49,621 | $ | (389 | ) | -1 | % | |||||||||||
| Capture reagents |
3,254 | 21,239 | 21,644 | 2,849 | 13 | % | ||||||||||||||||
| Instruments |
1,619 | 9,457 | 10,946 | 130 | 1 | % | ||||||||||||||||
| Molecular immunohematology |
221 | 1,278 | 1,430 | 69 | 5 | % | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||
| $ | 11,390 | $ | 74,910 | $ | 83,641 | $ | 2,659 | 3 | % | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||
Traditional reagent revenue was $6.3 million in the Successor period and $42.9 million in the Predecessor fiscal 2012 period compared to $49.6 million in the Predecessor fiscal 2011 period. The overall decrease was $0.4 million, or 1%. While traditional reagent revenue benefited by $1.4 million from fluctuations in foreign currency exchange rates, lower sales revenue from weaker industry demand in the U.S. market resulted in an overall decrease in traditional reagent revenue. Additionally, traditional reagent revenue is negatively impacted as we
56
Table of Contents
convert current manual customers to automation by placing an instrument. Instruments use approximately 70% Capture reagents and 30% traditional reagents so placing an instrument results in an increase in Capture reagent revenue and a decrease in traditional reagent revenue when the instrument is placed with a current customer. With our automation strategy, we expect this trend to continue.
Capture reagent revenue was $3.3 million in the Successor period and $21.2 million in the Predecessor fiscal 2012 period compared to $21.7 million in the Predecessor fiscal 2011 period. The overall increase of $2.8 million, or 13%, was primarily driven by incremental revenue from instrument placements. Sales of Capture reagents are largely dependent on the number of installed instruments requiring the use of our proprietary Capture technology. As we continue to place more instruments in the market, we expect revenue from Capture reagents to continue to increase as a percent of our total revenue.
Instrument revenue was $1.6 million in the Successor period and $9.4 million in the Predecessor fiscal 2012 period compared to $10.9 million in the Predecessor fiscal 2011 period. Overall, instrument revenue was essentially flat. During the Predecessor fiscal 2012 period, approximately $3.3 million of deferred revenue was recognized from previously placed instruments and the related service compared with $3.9 million recognized in the prior year quarter. As of August 31, 2011 and May 31, 2011, deferred instrument and service revenues totaled approximately $3.9 million and $13.6 million, respectively. The decrease in the deferred revenue balance is primarily due to an $8.5 million fair value adjustment arising from the Acquisition.
Molecular immunohematology revenue was $0.2 million in the Successor period and $1.3 million in the Predecessor fiscal 2012 period compared to $1.4 million in the Predecessor fiscal 2011 period. Overall, molecular immunohematology was essentially flat.
Gross margins(1)
| Successor | Predecessor | |||||||||||||||||||||||||||||
| August 20,
2011 through August 31, 2011 |
June 1, 2011 through August 19, 2011 |
Three Months Ended August 31, 2010 |
Change | |||||||||||||||||||||||||||
| Amount | Margin % | Amount | Margin % | Amount | Margin % | Amount | ||||||||||||||||||||||||
| (in ‘000) | (in ‘000) | (in ‘000) | (in ‘000) | |||||||||||||||||||||||||||
| Traditional reagents |
$ | 2,043 | 32.4 | % | $ | 32,481 | 75.6 | % | $ | 39,824 | 80.3 | % | $ | (5,300 | ) | |||||||||||||||
| Capture reagents |
2,455 | 75.4 | % | 16,887 | 79.5 | % | 17,389 | 80.3 | % | 1,953 | ||||||||||||||||||||
| Instruments |
(184 | ) | -11.4 | % | 2,053 | 21.7 | % | 1,816 | 16.6 | % | 53 | |||||||||||||||||||
| Molecular immunohematology |
(80 | ) | -36.2 | % | 534 | 41.8 | % | 638 | 44.6 | % | (184 | ) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| $ | 4,234 | 37.2 | % | $ | 51,955 | 69.4 | % | $ | 59,667 | 71.3 | % | $ | (3,478 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| (1) | The determination of gross margin is exclusive of amortization expense which is presented separately as an operating expense in the income statement. |
Gross margin on traditional reagents was 32.4% in the Successor period and 75.6% in the Predecessor fiscal 2012 period. The combined fiscal 2012 period margin of 70.1% was lower than the 80.3% in the Predecessor fiscal 2011 period. Gross margin in the Successor period included $2.4 million of expenses related to the amortization of the fair value of inventory and gross margin in the Predecessor fiscal 2012 period included $2.4 million of accelerated share-based compensation costs both arising from the Acquisition.
Capture reagents gross margin was 75.4% in the Successor period and 79.5% in the Predecessor fiscal 2012 period. The combined fiscal 2012 period margin of 79.0% was lower than the 80.3% in the Predecessor fiscal 2011 period. Gross margin decreased primarily due to an increase in reagent rentals and the related increase in the allocation of revenue from Capture reagents to instruments related to reagent rentals. In a reagent rental, the
57
Table of Contents
reagent revenue stream is used to fund all components of the customer’s acquisition, including the reagents themselves as well as the instrument and instrument-related items, such as training. Reagent gross margin is negatively impacted as a portion of reagent revenue is allocated to instruments over the life of the contract but none of the costs associated with the reagents are allocated.
Gross margin on instruments was negative 11.4% in the Successor period and 21.7% in the Predecessor fiscal 2012 period. The combined fiscal 2012 period margin of 16.9% was generally in line with the 16.6% in the Predecessor fiscal 2011 period. Gross margin in the Successor period was negative due to the reduction in the amount of deferred revenue recognized and the elimination of intercompany profit in ending inventory as a result of the Acquisition.
Operating expenses
| Successor | Predecessor | |||||||||||||||||||||
| August 20, 2011 through August 31, 2011 |
June 1, 2011 through August 19, 2011 |
Three Months Ended August 31, 2010 |
Change | |||||||||||||||||||
| Amount | % | |||||||||||||||||||||
| ($ in thousands) | ($ in thousands) | |||||||||||||||||||||
| Research and development |
$ | 623 | $ | 4,895 | $ | 4,425 | $ | 1,093 | 25 | % | ||||||||||||
| Selling and marketing |
1,112 | 10,510 | 9,142 | 2,480 | 27 | % | ||||||||||||||||
| Distribution |
649 | 3,952 | 4,032 | 569 | 14 | % | ||||||||||||||||
| General and administrative |
754 | 38,175 | 8,786 | 30,143 | 343 | % | ||||||||||||||||
| Amortization expense |
1,648 | 931 | 1,080 | 1,499 | 139 | % | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total operating expenses |
$ | 4,786 | $ | 58,463 | $ | 27,465 | $ | 35,784 | 130 | % | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||
Research and development expenses were $0.6 million in the Successor period and $4.9 million in the Predecessor fiscal 2012 period compared to $4.4 million in the Predecessor fiscal 2011 period. The overall increase of $1.1 million, or 25%, was primarily due to $0.9 million of compensation expense in the Predecessor fiscal 2012 period related to the vesting of all share-based awards in conjunction with the Acquisition.
Selling and marketing expenses were $1.1 million in the Successor period and $10.5 million in the Predecessor fiscal 2012 period compared to $9.1 million in the Predecessor fiscal 2011 period. The overall increase of $2.5 million, or 27%, was due to $1.3 million of compensation expense in the Predecessor fiscal 2012 period related to the vesting of all share-based awards in conjunction with the Acquisition. Additionally, there was an increase in other compensation related expenses in selling and marketing.
Distribution expenses were $0.6 million in the Successor period and $4.0 million in the Predecessor fiscal 2012 period compared to $4.0 million in the Predecessor fiscal 2011 period. The overall increase of $0.6 million, or 14%, was due to $0.2 million of compensation expense in the Predecessor fiscal 2012 period related to the vesting of all share-based awards in conjunction with the Acquisition. Increases in warehouse expenses and freight costs accounted for the majority of the remaining increase.
General and administrative expenses were $0.8 million in the Successor period and $38.2 million in the Predecessor fiscal 2012 period compared to $8.8 million in the Predecessor fiscal 2011 period. The overall increase of $30.1 million was primarily due to $18.9 million of transaction costs in the Predecessor fiscal 2012 period related to the Acquisition. Additionally, $10.7 million of compensation expense was recognized in the Predecessor fiscal 2012 period related to the vesting of all share-based awards in conjunction with the Acquisition.
Amortization expense was $1.6 million in the Successor period and $0.9 million in the Predecessor fiscal 2012 period compared to $1.1 million in the Predecessor fiscal 2011 period. The overall increase of $1.5 million was related to amortizing intangible assets which were recorded in the Successor period relating to the Acquisition.
58
Table of Contents
Non-operating income
| Successor | Predecessor | |||||||||||||||||||||
| August 20, 2011 through August 31, 2011 |
June 1, 2011 through August 19, 2011 |
Three Months Ended August 31, 2010 |
Change | |||||||||||||||||||
| Amount | % | |||||||||||||||||||||
| ($ in thousands) | ($ in thousands) | |||||||||||||||||||||
| Non-operating income (expense) |
$ | (3,404 | ) | $ | 2,815 | $ | 296 | $ | (885 | ) | nm | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||
Non-operating income (expense) was expense of $3.4 million in the Successor period and income of $2.8 million in the Predecessor fiscal 2012 period compared to $0.3 million in the Predecessor fiscal 2011 period. Realized foreign exchange gains relating to the settlement of intercompany balances accounts for $2.9 million of the income in the Predecessor fiscal 2012 period. Interest expense relating to the Company’s long-term debt accounts for $3.4 million of expense in the Successor period.
Income taxes
The provision for income taxes was a benefit of $1.5 million in the Successor period and expense of $2.7 million in the Predecessor fiscal 2012 period compared to $11.1 million in the Predecessor fiscal 2011 period. The overall reduction in income tax expense is primarily due to lower operating income. Utilization of foreign tax credits in the Predecessor fiscal 2012 period lowered the Company’s effective tax rate during the period. In the Successor period, the effective tax rate was higher due to the loss of the production activity deduction due to a pre-tax loss.
Comparison of years ended May 31, 2011 and May 31, 2010
| For the year ended May 31, | Change | |||||||||||||||
| (dollars in thousands) |
2011 | 2010 | Amount | % | ||||||||||||
| Net sales |
$ | 333,091 | $ | 329,073 | $ | 4,018 | 1 | % | ||||||||
| Gross margin(1) |
236,916 | 233,724 | 3,192 | 1 | % | |||||||||||
| Gross margin percentage |
71.1 | % | 71.0 | % | 0 | % | ||||||||||
| Operating expenses |
110,919 | 108,382 | 2,537 | 2 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income from operations |
125,997 | 125,342 | 655 | 1 | % | |||||||||||
| Non-operating income (expense) |
4,633 | (130 | ) | 4,763 | NM | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Income before income tax |
130,630 | 125,212 | 5,418 | 4 | % | |||||||||||
| Provision for income tax |
41,303 | 42,629 | (1,326 | ) | (3 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income |
$ | 89,327 | $ | 82,583 | $ | 6,744 | 8 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | The determination of gross margin is exclusive of amortization expense, which is presented separately as an operating expense in the income statement. |
Revenue increased by approximately $4.0 million, or approximately 1%, during the year ended May 31, 2011 compared with the prior year. While revenue increased year-over-year, the Company was negatively impacted in the current year by lower sales volume of reagents due to weaker industry demand in the U.S. market as well as by a decreased number of ship cycles when compared with the prior year. While ship cycles are typically consistent year-to-year, the timing of ship cycles is determined by the calendar, which can result in a different number of ship cycles between years. Revenue was negatively impacted by approximately $0.5 million in fiscal 2011 from foreign currency fluctuations. Approximately 70% of our fiscal 2011 consolidated revenue is from the U.S., and approximately 30% is from international sales largely denominated in local currency. Our significant
currencies besides the U.S. dollar are the Euro, the Canadian dollar, the British pound and the Japanese yen. As a result, our consolidated revenue expressed in dollars benefits when the U.S. dollar weakens and decreases when the U.S. dollar strengthens in relation to other currencies.
59
Table of Contents
For fiscal 2011, our consolidated gross margin was generally in line with the prior year. Gross margins in the prior year included costs related to the Quality Process Improvement Project of approximately $5.9 million, primarily for external consultants. There were no material external costs related to the Quality Process Improvement Project in the current year, which benefited margins. Gross margins in the current year were negatively impacted by the mix of instrument-related revenue, more instruments being expensed in fiscal 2011 compared with the prior year as well as by manufacturing variances related primarily to fewer ship cycles. Operating expenses increased by 2% when compared by the prior year, primarily due to increased distribution expenses and increased general and administrative expenses. Non-operating income increased by $4.8 million when compared with the prior year, primarily due to a return of funds escrowed in connection with the BioArray acquisition. Net income increased by approximately 8% in fiscal 2011 when compared with the prior year.
Net sales
| For the year ended May 31, | Change | |||||||||||||||
| (dollars in thousands) |
2011 | 2010 | Amount | % | ||||||||||||
| Traditional reagents |
$ | 199,826 | $ | 207,710 | $ | (7,884 | ) | (4 | )% | |||||||
| Capture products |
82,366 | 77,003 | 5,363 | 7 | % | |||||||||||
| Instruments |
45,112 | 39,680 | 5,432 | 14 | % | |||||||||||
| Molecular immunohematology |
5,787 | 4,680 | 1,107 | 24 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| $ | 333,091 | $ | 329,073 | $ | 4,018 | 1 | % | |||||||||
|
|
|
|
|
|
|
|||||||||||
Traditional reagent revenue decreased by approximately $7.9 million, or approximately 4%, in fiscal 2011 compared with fiscal 2010 primarily due to lower sales volume because of weaker industry demand in the U.S. market and fewer ship cycles in the current year compared with the prior year. Traditional reagent revenue is negatively impacted as we place more instruments in the market. Instruments use approximately 70% Capture reagents and 30% traditional reagents, so placing an instrument results in an increase in Capture reagent revenue and a decrease in traditional reagent revenue when the instrument is placed with a current customer. With our automation strategy, we expect this trend to continue. Additionally, we revised our go-to-market strategy at the beginning of the third quarter of fiscal 2011 to better address the economic downturn as well as the competitive pressures that we have historically experienced in our traditional reagent business.
Capture revenue increased by approximately $5.4 million, or approximately 7%, in fiscal 2011 when compared with the prior year primarily from incremental revenue from instrument placements. Capture’s year-over-year revenue growth rate was negatively impacted by weaker industry demand in the U.S. market. Sales of Capture reagents are largely dependent on the number of installed instruments requiring the use of our proprietary Capture technology. As we continue to place more instruments in the market, we expect revenue from Capture reagents to continue to increase as a percent of our total revenue.
Revenue from instruments increased by approximately $5.4 million, or approximately 14%, in fiscal 2011 compared with the prior year due to increased instrument placements. Instrument revenue is typically recognized over the life of either the instrument rental period or the underlying reagent contract period, dependent upon how the instrument was acquired. Historically, when instruments are sold (versus rented) revenue is deferred and recognized over the life of the underlying reagent contract period when the contract includes a price guarantee (which our contracts typically do). The proportion of instruments rented (versus sold) has increased over the past three years. In fiscal 2011, approximately $15.2 million of deferred revenue was recognized from previous instrument sales compared with $16.8 million recognized in fiscal 2010. In fiscal 2011, we deferred approximately $11.6 million of instrument and associated service revenues related to instrument sales compared with $11.6 million in the prior year. As of May 31, 2011 and 2010, deferred instrument and service revenues on the balance sheet totaled approximately $13.6 million and $16.7 million, respectively. The decrease in the deferred revenue balance is due to the increase in rentals as an acquisition option.
60
Table of Contents
Molecular immunohematology revenue increased by approximately $1.1 million in fiscal 2011 compared with fiscal 2010 due to new customers.
Gross margin
| For the year ended May 31, | Change | |||||||||||||||||||
| 2011 | 2010 | |||||||||||||||||||
| (dollars in thousands) |
Amount | Margin % | Amount | Margin % | Amount | |||||||||||||||
| Traditional reagents(1) |
$ | 160,630 | 80.4 | % | $ | 161,557 | 77.8 | % | $ | (927 | ) | |||||||||
| Capture products(1) |
65,305 | 79.3 | % | 62,732 | 81.5 | % | 2,573 | |||||||||||||
| Instruments(1) |
8,541 | 18.9 | % | 8,813 | 22.2 | % | (272 | ) | ||||||||||||
| Molecular immunonematology(1) |
2,440 | 42.2 | % | 622 | 13.3 | % | 1,818 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| $ | 236,916 | 71.1 | % | $ | 233,724 | 71.0 | % | $ | 3,192 | |||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| (1) | The determination of gross margin is exclusive of amortization expense, which is presented separately as an operating expense in the statements of income. |
Gross margins on traditional reagents increased to 80.4% in fiscal 2011 from 77.8% in the prior year. Gross margins in the prior year included expenses related to the remediation portion of our Quality Process Improvement Project, which was completed in the third quarter of fiscal 2010, of approximately $5.9 million. These Quality Process Improvement Project costs were primarily for external consultants. There were no material external costs related to the Quality Process Improvement Project in the current year periods, which benefited gross margins.
For fiscal 2011, Capture product gross margins decreased to 79.3% from 81.5% in the prior year primarily due to the allocation of revenue from Capture reagents to instruments related to reagent rentals. In a reagent rental, the reagent revenue stream is used to fund all components of the customer’s acquisition, including the reagents themselves as well as the instrument and instrument-related items, such as training. Reagent gross margin is negatively impacted as a portion of reagent revenue is allocated to instruments over the life of the contract but none of the costs associated with the reagents are allocated.
Gross margins on instruments decreased to 18.9% in fiscal 2011 from 22.2% in the prior year, primarily due to the mix of instrument revenue and more instruments being expensed in the current year as compared with the prior year. Where sales contracts have reagent price guarantee clauses (which our automation contracts typically do), instrument costs are expensed when the sale is made, but the related instrument revenue is deferred and recorded as income over the term of the contract. When an instrument is rented, revenue and expenses for the transaction are recognized evenly over the life of the contract. In fiscal 2011 and fiscal 2010, we recognized $15.2 million and $16.8 million, respectively, of deferred revenue related to instrument sales and service.
We expect molecular immunohematology gross margin to be volatile until production volumes are higher.
Operating expenses
| For the year ended May 31, |
Change | |||||||||||||||
| (dollars in thousands) |
2011 | 2010 | Amount | % | ||||||||||||
| Research and development |
$ | 15,900 | $ | 15,437 | $ | 463 | 3 | % | ||||||||
| Selling and marketing |
36,431 | 36,995 | (564 | ) | (2 | )% | ||||||||||
| Distribution |
16,508 | 14,831 | 1,677 | 11 | % | |||||||||||
| General and administrative |
37,747 | 36,841 | 906 | 2 | % | |||||||||||
| Amortization expense and other |
4,333 | 4,278 | 55 | 1 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
$ | 110,919 | $ | 108,382 | $ | 2,537 | 2 | % | ||||||||
|
|
|
|
|
|
|
|||||||||||
61
Table of Contents
Research and development expenses increased by approximately $0.5 million in fiscal 2011 compared with the prior year, primarily due to project-related expenses.
Selling and marketing expenses decreased by approximately $0.6 million in fiscal 2011 compared with fiscal 2010, primarily due to lower compensation expense.
Distribution expenses rose by approximately $1.7 million in fiscal 2011 over the prior year, primarily due to increased shipping supplies and freight costs.
General and administrative expenses increased by approximately $0.9 million in fiscal 2011 over the prior year, primarily due to expenses of approximately $2.3 million related to the resignation of the Company’s Chief Operating Officer, offset by lower legal expenses. Legal expenses related to the Department of Justice investigation and the related lawsuits totaled approximately $1.6 million in fiscal 2011, down from $2.9 million in fiscal 2010.
Amortization expense was generally in line with the prior year period.
Non-operating income (expense)
| For the year ended May 31, |
Change | |||||||||||
| (dollars in thousands) |
2011 | 2010 | Amount | |||||||||
| Non-operating income (expense) |
$ | 4,633 | $ | (130 | ) | $ | 4,763 | |||||
The year-over-year change in non-operating income (expense) was primarily attributable to the recognition of $4.3 million of other income related to the return of funds escrowed in association with the BioArray acquisition.
Income taxes
The provision for income taxes decreased $1.3 million in fiscal 2011 compared with fiscal 2010 primarily due to a New Jersey state tax law change relating to apportionment. The effective income tax rate was 31.6% in the current year compared with 34.0% in the prior year. The tax rate in the current year period was favorably impacted primarily by the New Jersey law change and a settlement of the escrow account related to the acquisition of BioArray. Since the return of the escrowed funds was considered a return of purchase price, no tax was provided on these amounts.
As a result of using compensation cost deductions arising from the exercise of nonqualified employee stock options and vesting of restricted shares for federal and state income tax purposes, we had an income tax benefit of approximately $1.8 million in fiscal 2011 and we had an income tax shortfall of approximately $0.2 million in fiscal 2010. As required by GAAP, the income tax benefits and income tax shortfall are recognized in our financial statements as a reduction of or an addition to additional paid-in capital rather than as an increase or reduction of the respective income tax provisions in the consolidated financial statements.
62
Table of Contents
Comparison of years ended May 31, 2010 and May 31, 2009
| For the year ended May 31, | Change | |||||||||||||||
| (dollars in thousands) |
2010 | 2009 | Amount | % | ||||||||||||
| Net sales |
$ | 329,073 | $ | 300,547 | $ | 28,526 | 9 | % | ||||||||
| Gross margin(1) |
233,724 | 216,011 | 17,713 | 8 | % | |||||||||||
| Gross margin percentage |
71.0 | % | 71.9 | % | (1 | )% | ||||||||||
| Operating expenses |
108,382 | 99,053 | 9,329 | 9 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Income from operations |
125,342 | 116,958 | 8,384 | 7 | % | |||||||||||
| Non-operating income (expense) |
(130 | ) | 23 | (153 | ) | 665 | % | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Income before income tax |
125,212 | 116,981 | 8,231 | 7 | % | |||||||||||
| Provision for income tax |
42,629 | 40,798 | 1,831 | 4 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net income |
$ | 82,583 | $ | 76,183 | $ | 6,400 | 8 | % | ||||||||
|
|
|
|
|
|
|
|||||||||||
| (1) | The determination of gross margin is exclusive of amortization expense, which is presented separately as an operating expense in the statements of income. |
Revenue increased by approximately $28.5 million, or approximately 9%, during the year ended May 31, 2010 compared with the prior year, primarily due to higher revenue in the U.S. market. Revenue also benefited by approximately $2.8 million in fiscal 2010 from foreign currency fluctuations. Approximately 72% of our fiscal 2010 consolidated revenue is from the U.S. and approximately 28% is from international sales largely denominated in local currency. Our significant currencies besides the U.S. dollar are the Euro, the Canadian Dollar, the British Pound and the Japanese Yen. As a result, our consolidated revenue expressed in dollars benefits when the U.S. dollar weakens and decreases when the U.S. dollar strengthens in relation to other currencies.
For fiscal 2010, our consolidated gross margin decreased to 71.0% from 71.9% achieved in fiscal 2009, primarily due to expenses related to our Quality Process Improvement Project, which were reflected in cost of sales. During fiscal 2010, we spent approximately $5.9 million on our Quality Process Improvement Project compared with approximately $2.4 million spent in fiscal 2009. Operating expenses increased approximately 9%, primarily due to our acquisition of BioArray (which closed on August 4, 2008), increased legal expenses and increased research and development expenses related to various projects, including our next generation instrument for molecular immunohematology. Net income increased approximately 8% in fiscal 2010 over the prior year.
Net sales
| For the year ended May 31, |
Change | |||||||||||||||
| (dollars in thousands) |
2010 | 2009 | Amount | % | ||||||||||||
| Traditional reagents |
$ | 207,710 | $ | 199,277 | $ | 8,433 | 4 | % | ||||||||
| Capture reagents |
77,003 | 64,145 | 12,858 | 20 | % | |||||||||||
| Instruments |
39,680 | 34,672 | 5,008 | 14 | % | |||||||||||
| Molecular immunohematology |
4,680 | 2,453 | 2,227 | 91 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| $ | 329,073 | $ | 300,547 | $ | 28,526 | 9 | % | |||||||||
|
|
|
|
|
|
|
|||||||||||
Traditional reagent revenue increased by approximately $8.4 million, or approximately 4%, in fiscal 2010 compared with fiscal 2009 primarily due to higher revenue in the U.S. market. Traditional reagent revenue was also negatively impacted by the economic downturn as well as the competitive pressures that we have historically experienced in our traditional reagent business. Traditional reagent sales, which accounted for approximately 63% of total revenue in fiscal 2010, have historically been a significant portion of our revenue. Our revenue mix is changing over time as we place more instruments in the market, which results in increased sales of our Capture reagents.
63
Table of Contents
Capture revenue increased by approximately $12.9 million, or approximately 20%, in fiscal 2010 over the prior year, primarily due to incremental revenue from instrument placements. Sales of Capture reagents are largely dependent on the number of installed instruments requiring the use of our proprietary Capture technology. As we continue to place more instruments in the market, we expect revenue from Capture reagents to continue to increase.
Revenue from instruments increased by approximately $5.0 million, or approximately 14%, in fiscal 2010 compared with the prior year due to increased instrument placements. Instrument revenue is typically recognized over the life of either the instrument rental period or the underlying reagent contract period. Historically, when instruments are sold (versus rented) revenue is deferred and recognized over the life of the underlying reagent contract period when the contract includes a price guarantee (which our contracts typically do). In fiscal 2010, approximately $16.8 million of deferred revenue was recognized from previous instrument sales compared with $16.9 million recognized in fiscal 2009. In fiscal 2010, we deferred approximately $11.6 million of instrument and associated service revenues related to instrument sales compared with $14.7 million in the prior year. As of May 31, 2010 and 2009, deferred instrument and service revenues on the balance sheet totaled approximately $16.7 million and $22.1 million, respectively. Over the past three years, the proportion of instruments rented (versus sold) has increased. Therefore, our deferred revenue balance has declined.
Molecular immunohematology revenue increased by approximately $2.2 million in fiscal 2010 compared with fiscal 2009, primarily due to the introduction of our molecular offering outside the U.S. Our molecular immunohematology products are a result of our August 4, 2008 BioArray acquisition.
Gross margin
| For the year ended May 31, | ||||||||||||||||||||
| 2010 | 2009 | Change | ||||||||||||||||||
| (dollars in thousands) |
Amount | Margin% | Amount | Margin% | Amount | |||||||||||||||
| Traditional reagents(1) |
$ | 161,557 | 77.8 | % | $ | 155,744 | 78.2 | % | $ | 5,813 | ||||||||||
| Capture reagents(1) |
62,732 | 81.5 | % | 54,411 | 84.8 | % | 8,321 | |||||||||||||
| Instruments(1) |
8,813 | 22.2 | % | 5,259 | 15.2 | % | 3,554 | |||||||||||||
| Molecular immunohematology(1) |
622 | 13.3 | % | 597 | 24.3 | % | 25 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| $ | 233,724 | 71.0 | % | $ | 216,011 | 71.9 | % | $ | 17,713 | |||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| (1) | The determination of gross margin is exclusive of amortization expense, which is presented separately as an operating expense in the statements of income. |
Gross margins on traditional reagents decreased to 77.8% in fiscal 2010 from 78.2% in the prior year, primarily due to expenses related to our Quality Process Improvement Project. During the current fiscal year, we spent approximately $5.9 million on our Quality Process Improvement Project compared with approximately $2.4 million spent in fiscal 2009. These costs were primarily reflected in traditional reagent cost of sales.
For fiscal 2010, Capture product gross margins decreased to 81.5% from 84.8% in the prior year primarily due to the allocation of revenue from Capture reagents to instruments related to reagent rentals as well as due to lower demand for reagents during the period. In a reagent rental, the reagent revenue stream is used to fund all components of the customer’s acquisition, including the reagents themselves as well as the instrument and instrument-related items, such as training. Reagent gross margin is negatively impacted as a portion of reagent revenue is allocated to instruments over the life of the contract but none of the costs associated with the reagents are allocated.
Gross margins on instruments increased to 22.2% in fiscal 2010 from 15.2% in the prior year, primarily due to sales mix. In the current year, more instruments were rented (versus sold) compared to the prior year. An instrument rental results in revenue and expenses related to the instrument being recognized evenly over the
64
Table of Contents
contract period. When we sell an instrument and the sales contract has reagent price guarantees, which our contracts typically do, the instrument costs are expensed upfront and the related instrument revenue is deferred and recognized over the contract period. Current year and prior year margins benefited from the recognition of revenue deferred from prior periods. In fiscal 2010 and fiscal 2009, we recognized $16.8 million and $16.9 million, respectively, of deferred revenue related to instrument sales and service.
Operating expenses
| For the year ended May 31, |
Change | |||||||||||||||
| (dollars in thousands) |
2010 | 2009(1) | Amount | % | ||||||||||||
| Research and development |
$ | 15,437 | $ | 10,698 | $ | 4,739 | 44 | % | ||||||||
| Selling and marketing |
36,995 | 38,315 | (1,320 | ) | (3 | )% | ||||||||||
| Distribution |
14,831 | 13,708 | 1,123 | 8 | % | |||||||||||
| General and administrative |
36,841 | 32,593 | 4,248 | 13 | % | |||||||||||
| Amortization expense and other |
4,278 | 3,739 | 539 | 14 | % | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
$ | 108,382 | $ | 99,053 | $ | 9,329 | 9 | % | ||||||||
|
|
|
|
|
|
|
|||||||||||
| (1) | Certain prior year operating expenses have been reclassified to conform with current year presentation. |
Research and development expenses increased by approximately $4.7 million in fiscal 2010 over the prior year, primarily due to recognizing a full year of BioArray expenses, which was acquired on August 4, 2008, and the development activities for the next generation molecular immunohematology instrument.
Selling and marketing expenses decreased by approximately $1.3 million in fiscal 2010 compared with fiscal 2009, primarily due to lower compensation expense.
Distribution expenses rose by approximately $1.1 million in fiscal 2010 over the prior year, primarily due to increased shipping and packaging costs.
General and administrative expenses increased by approximately $4.2 million in fiscal 2010 over the prior year, primarily due to legal expenses of $2.9 million related to the Department of Justice investigation and the related lawsuits.
Amortization expense increased by approximately $0.5 million in fiscal 2010 compared with fiscal 2009, primarily due to recognizing a full year of amortization of finite-lived intangibles that were recorded upon the acquisition of BioArray.
Non-operating income (expense)
| For the year
ended May 31, |
Change | |||||||||||
| (dollars in thousands) |
2010 | 2009 | amount | |||||||||
| Non-operating income (expense) |
$ | (130 | ) | $ | 23 | $ | (153 | ) | ||||
The year-over-year change in non-operating income (expense) was primarily attributable to lower interest income due to a decrease in interest rates.
Income taxes
The provision for income taxes increased $1.8 million in fiscal 2010 compared with fiscal 2009, primarily due to increased pre-tax income. The effective income tax rate was 34.0% in the current year compared with 34.9% in the prior year. The tax rate in the current year period was favorably impacted primarily by a settlement with a state taxing authority.
65
Table of Contents
As a result of using compensation cost deductions arising from the exercise of nonqualified employee stock options and vesting of restricted shares for federal and state income tax purposes, we had an income tax shortfall of approximately $0.2 million in fiscal 2010, and we realized income tax benefits of $3.3 million in fiscal 2009. As required by U.S. generally accepted accounting principles, the income tax shortfall and income tax benefits are recognized in our financial statements as a reduction of or an addition to additional paid-in capital rather than as an increase or reduction of the respective income tax provisions in the consolidated financial statements.
Liquidity and capital resources
Cash flow
Our principal source of liquidity is our operating cash flow. This cash-generating capability is one of our fundamental strengths and provides us with substantial financial flexibility in meeting our operating, investing and financing needs. We have adequate working capital and sources of capital to operate our current business and to meet our existing capital requirements.
The following table shows the cash flows provided by or used in operating, investing and financing activities for the Successor period, the Predecessor fiscal 2012 period, and the Predecessor fiscal 2011 period.
| (Successor) | (Predecessor) | |||||||||||||
| (dollars in thousands) |
August 20, 2011 to August 31, 2011 |
June 1, 2011 to August 19, 2011 |
Three Months Ended August 31, 2010 |
|||||||||||
| Net cash provided by (used in) operating activities |
$ | (13,113 | ) | $ | 25,588 | $ | 28,896 | |||||||
| Net cash used in investing activities |
(1,940,294 | ) | (2,265 | ) | (1,219 | ) | ||||||||
| Cash provided by (used in) financing activities |
1,655,564 | 66 | (310 | ) | ||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
14 | (3,029 | ) | 497 | ||||||||||
|
|
|
|
|
|
|
|||||||||
| Increase (decrease) in cash and cash equivalents |
$ | (297,829 | ) | $ | 20,360 | $ | 27,864 | |||||||
At August 31, 2011, we had working capital of $106.2 million compared with $294.9 million at August 31, 2010.
Our cash and cash equivalents were $25.1 million at August 31, 2011, as compared with $302.6 million at May 31, 2011. The reduction in our cash position resulted primarily from $301.1 million of net cash used in the Acquisition.
At May 31, 2011, we had working capital of $379.0 million, compared to $270.9 million of working capital at May 31, 2010. The following table shows the cash flows provided by or used in operating, investing and financing activities for fiscal years 2011, 2010 and 2009, as well as the effect of exchange rates on cash and cash equivalents for those same years:
| For the year ended May 31, | ||||||||||||
| (dollars in thousands) |
2011 | 2010 | 2009 | |||||||||
| Net cash provided by operating activities |
$ | 102,111 | $ | 84,751 | $ | 79,822 | ||||||
| Net cash used in investing activities |
(9,061 | ) | (6,304 | ) | (116,556 | ) | ||||||
| Cash provided by (used in) financing activities |
2,578 | (11,757 | ) | (1,396 | ) | |||||||
| Effect of exchange rate changes on cash and cash equivalents |
4,326 | (502 | ) | (465 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Increase (decrease) in cash and cash equivalents |
$ | 99,954 | $ | 66,188 | $ | (38,595 | ) | |||||
Our cash and cash equivalents were $302.6 million as of May 31, 2011, as compared to $202.6 million as of May 31, 2010. The increase in our cash position resulted from operating cash flow in the current year.
Operating activities. Cash flow from operations generally increases with net income.
66
Table of Contents
Net cash used in operating activities was $13.1 million for the Successor period and net cash generated by operating activities was $25.6 million for the Predecessor fiscal 2012 period compared with $28.9 million for the Predecessor fiscal 2011 period. Transaction costs of $16.9 million relating to the Acquisition in combination with the short duration of the Successor period of only 11 days drove the use of operating cash in the Successor period.
In fiscal 2011, net cash generated by operating activities was $102.1 million compared with $84.8 million in fiscal 2010. The year-over-year increase in cash flow from operating activities was primarily attributable to the $6.7 million year-over-year increase in net income and changes in working capital, including a decrease in inventories and an increase in accounts payable and accrued expenses and other liabilities.
In fiscal 2011, $7.2 million of escrowed funds related to the acquisition of BioArray were released and returned to us. In accordance with the contingent consideration guidance in ASC 805, Business Combinations, $2.9 million was recorded to goodwill, and the remaining $4.3 million was recorded to other income. The accrual of the $4.3 million amount reduced net cash generated by operating activities in fiscal 2011.
In fiscal 2010, net cash generated by operating activities was $84.8 million compared with $79.8 million in fiscal 2009. The year-over-year increase in cash flow from operating activities was primarily attributable to the $6.4 million year-over-year increase in net income.
Investing activities. Generally, the primary use of cash for investing activities is related to the purchase of property and equipment. However, in the Successor period we used a significant amount of cash in the Acquisition and in fiscal 2009 we used a significant amount of cash for acquisitions.
Net cash used in investing activities was $1.9 billion for the Successor period and $2.3 million for the Predecessor fiscal 2012 period, compared with $1.2 million for the Predecessor fiscal 2011 period. The purchase of property and equipment was the primary use of cash in both of the Predecessor periods of fiscal 2012 and fiscal 2011. In the Successor period, $0.9 million was used for the purchase of property and equipment and $1.9 billion was used in the Acquisition.
In fiscal 2011, $9.1 million of net cash was used in investing activities compared with $6.3 million of cash used in the fiscal 2010. In the current year, $9.1 million was used to purchase property and equipment. In the prior year, $6.3 million was used to purchase property and equipment.
In fiscal 2010, $6.3 million of net cash was used in investing activities compared with $116.6 million of cash used in the fiscal 2009. Cash used in investing activities in fiscal 2010 related to the purchase of property and equipment. In fiscal 2009, we paid $108.0 million for the acquisition of BioArray and our U.K. distributor as well as $8.6 million for the purchase of property and equipment.
Financing activities. For financing activities, the typical use of cash in the Predecessor period was for the repurchase of our common stock and the typical cash proceeds in the Predecessor period was in connection with the exercise of stock options. In the first quarter of fiscal 2012, we received significant proceeds from the incurrence of long-term debt, and used those proceeds in connection with the Acquisition.
Net cash provided by financing activities was $1.7 billion during the Successor period and $0.1 million during the Predecessor fiscal 2012 period, compared with $0.3 million of cash used by financing activities in the Predecessor fiscal 2011 period. During the Successor period, we received $991 million in proceeds from long-term debt. During the Predecessor fiscal 2012 period, we had a cash outflow of $0.5 million for payment of withholding taxes in compliance with the statutory tax withholding requirements on exercise of options and vesting of restricted shares in exchange for surrender of the Company’s shares of equal value, compared with a cash outflow of $0.4 million in the Predecessor fiscal 2011 period. The value of these reacquired shares is disclosed as ‘Repurchase of common stock’ under financing activities in the cash flow statement.
67
Table of Contents
Net cash provided by financing activities was $2.6 million during fiscal 2011, compared with $11.8 million used in financing activities in the prior year. Reflected in “repurchase of common stock” in the cash flow statement is approximately $0.5 million in withholding taxes we paid in fiscal 2011 compared with $0.3 million in fiscal 2010. This payment was in compliance with statutory tax withholding requirements for the exercise of options and vesting of restricted shares in exchange for surrender of our shares of equal value. During fiscal 2011, we received $1.3 million cash from the exercise of employee stock options compared with $0.3 million in the same period of the prior year. For fiscal 2011 we had a tax benefit of $1.8 million and in fiscal 2010 we had a tax shortfall of $0.2 million from the exercise of nonqualified employee stock options. During fiscal 2010, we used $11.6 million to repurchase shares of our common stock in the open market.
Net cash used in financing activities was $11.8 million during fiscal 2010, compared with $1.4 million in the prior year. During fiscal 2010, we used $11.6 million to repurchase shares of our common stock in the open market. Also reflected in “repurchase of common stock” in the cash flow statement is approximately $0.3 million in withholding taxes we paid. This payment was in compliance with statutory tax withholding requirements for the exercise of options and vesting of restricted shares in exchange for surrender of our shares of equal value. During fiscal 2010, we received $0.3 million cash from the exercise of employee stock options compared with $3.2 million in the same period of the prior year. For fiscal 2010 we had a tax shortfall of $0.2 million, and in fiscal 2009 we had a tax benefit of $3.3 million from the exercise of nonqualified employee stock options.
Stock repurchase program
The Company instituted a stock repurchase program in June 1998. In August 2009, the Board of Directors authorized the Company to repurchase an additional 2,000,000 shares of the Company’s common stock under this repurchase program, bringing the total authorized shares to 11,375,000.
We made no share repurchases during fiscal 2011. During fiscal 2010, approximately 650,000 shares were repurchased in the open market under the 1998 repurchase plan for approximately $11.6 million. During fiscal 2009, we repurchased approximately 295,000 shares in the open market for approximately $6.7 million. Shares that were repurchased by the Company were returned to the status of authorized but unissued.
As of May 31, 2011, 9,178,356 shares had been repurchased under the program. We terminated the program in connection with the Acquisition.
Contingencies
We record contingent liabilities resulting from asserted and unasserted claims against us when it is probable that a liability has been incurred and the amount of the loss is reasonably estimable. We disclose contingent liabilities when there is a reasonable possibility that the ultimate loss will exceed the recorded liability. Estimating probable losses requires analysis of multiple factors, in some cases including judgments about the potential actions of third-party claimants and courts. Therefore, actual losses in any future period are inherently uncertain. We are currently involved in certain legal proceedings as described under “Business—Legal proceedings.” We believe we have meritorious defenses to the claims and other issues asserted in such matters; however, there can be no assurance that such matters or any future legal matters will not have an adverse effect on the Company, our financial position or future results of operations. Contingent liabilities are described in Note 17 to the audited consolidated financial statements included in this prospectus.
Credit facilities
The Senior Credit Facilities are comprised of a $615.0 million senior secured term loan and a $100.0 million senior secured revolving loan facility. As of August 31, 2011, we had no outstanding loans under our senior secured revolving loan facility. The senior secured term loan was fully funded at the closing of the Acquisition. Amounts available under the senior secured revolving loan facility will be reduced by letters of credit utilization.
68
Table of Contents
The Senior Credit Facilities also allow an aggregate of $150.0 million (or a greater amount if we meet specified financial ratios) in uncommitted incremental facilities, the availability of which are subject to our meeting certain conditions. See “Description of other indebtedness.”
11.125% senior notes due 2019
On August 19, 2011, we issued $400.0 million in principal amount of outstanding notes. The outstanding notes bear interest at a rate of 11.125% per annum, and interest is payable semi-annually on February 15 and August 15 of each year, commencing on February 15, 2011. The outstanding notes mature on August 15, 2019.
For further information on the Senior Credit Facilities, the notes and related agreements, see “Description of other indebtedness” and “Description of exchange notes” and refer to the credit agreement, indenture and related agreements filed as exhibits to the registration statement of which this prospectus forms a part.
Outlook
We anticipate that cash generated by operations, the remaining funds available under our Senior Credit Facilities and existing cash and equivalents will be sufficient to meet working capital requirements, service our debt and finance capital expenditures over the next twelve months. We cannot assure you, however, that our business will generate sufficient cash flow from operations or that future borrowings will be available to us under our Senior Credit Facilities in amounts sufficient to enable us to repay our indebtedness, including the exchange notes, or to fund other liquidity needs. See “Risk factors—Risks related to our indebtedness and the exchange notes.”
Summary disclosures about contractual obligations
Contractual obligations
The following table sets forth our contractual obligations and other commitments as of August 31, 2011:
| Payments due by period | ||||||||||||||||||||
| (dollars in thousands) |
Less than 1 year |
1-3 years | 3-5 years | After 5 years | Total | |||||||||||||||
| Operating leases |
$ | 2,834 | $ | 4,754 | $ | 3,899 | $ | — | $ | 11,487 | ||||||||||
| Purchase obligations |
16,510 | — | — | — | 16,510 | |||||||||||||||
| Senior Credit Facilities(1)(2) |
4,613 | 12,300 | 12,300 | 585,788 | 615,000 | |||||||||||||||
| Notes(2) |
— | — | — | 400,000 | 400,000 | |||||||||||||||
| Interest |
83,553 | 177,945 | 176,256 | 224,142 | 661,897 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual cash obligations |
$ | 107,510 | $ | 194,999 | $ | 192,455 | $ | 1,209,930 | $ | 1,704,894 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | The Senior Credit Facilities are comprised of a $615.0 million senior secured term loan and a $100.0 million senior secured revolving loan facility. |
| (2) | Amounts shown do not include interest. |
In addition to the obligations in the table above, approximately $10.0 million of unrecognized tax benefits have been recorded as liabilities in accordance with ASC 740, “Income Taxes” (“ASC 740”), and we are uncertain as to if or when such amounts may be settled. Related to the unrecognized tax benefits not included in the table above, we have also recorded a liability for interest of $0.9 million.
The expected timing of payment of the obligations discussed above is estimated based on current information. The timing of payments and actual amounts paid may differ depending on the timing of receipt of services, or, for some obligations, changes to agreed-upon amounts.
69
Table of Contents
Off-balance sheet arrangements
As of August 31, 2011, we had no off-balance sheet arrangements.
Quantitative and qualitative disclosures about market risk
We are exposed to market risks for foreign currency exchange rates principally with the U.S. dollar versus the Euro, Canadian dollar, British pound and Japanese yen. Our financial instruments that can be affected by foreign currency fluctuations and exchange risks consist primarily of cash and cash equivalents and trade receivables denominated in currencies other than the U.S. dollar. We attempt to manage our exposure primarily by balancing assets and liabilities and maintaining cash positions in foreign currencies only at levels necessary for operating purposes. It has not been our practice to actively hedge our foreign subsidiaries’ assets or liabilities denominated in foreign currencies. To manage these risks, we regularly evaluate our exposure and, if warranted, may enter into various derivative transactions when appropriate. We do not hold or issue derivative instruments for trading or other speculative purposes. As part of accumulated other comprehensive income in shareholders’ equity, we recorded foreign currency translation gains of $13.9 million in fiscal 2011 and foreign currency translation losses of $5.2 million and $4.0 million in fiscal 2010 and 2009, respectively.
Additionally, we are exposed to interest rate risks related to cash and cash equivalents. It has been our practice to hold cash and cash equivalents in deposits that can be redeemed on demand and in investments with an original maturity of three months or less. The interest income earned from these deposits and investments is impacted by interest rate fluctuations.
Market risk in connection with our long-term debt
We are subject to interest rate risk in connection with our long-term debt. Our principal interest rate risk will relate to the term loan outstanding under our Senior Credit Facilities. We have approximately $615.0 million outstanding under our Senior Credit Facilities, bearing interest at variable rates. A 0.125% increase in these floating rates applicable to the indebtedness outstanding under our Senior Credit Facilities would increase our pro forma annual interest expense by approximately $0.8 million, assuming the senior secured term loan under the Senior Credit Facilities is fully funded and there are no borrowings under the revolving facility. The Senior Credit Facilities also allow an aggregate of $150.0 million (or a greater amount if we meet specified financial ratios) in uncommitted incremental facilities, the availability of which are subject to our meeting certain conditions, bearing interest at variable rates.
Recently issued accounting standards
Adopted by us in fiscal 2011 and 2012
In June 2009, the FASB issued an update to ASC 810, Consolidation (“ASC 810”). This update changes how a reporting entity determines when an entity that is insufficiently capitalized or is not controlled through voting (or similar rights) should be consolidated. The determination of whether a reporting entity is required to consolidate another entity is based on, among other things, the other entity’s purpose and design and the reporting entity’s ability to direct the activities of the other entity that most significantly impact the other entity’s economic performance. This update requires a reporting entity to provide additional disclosures about its involvement with variable interest entities and any significant changes in risk exposure due to that involvement. A reporting entity is now required to disclose how its involvement with a variable interest entity affects the reporting entity’s financial statements. The update is effective at the start of a reporting entity’s first fiscal year beginning after November 15, 2009. The adoption of this update to ASC 810 during the first quarter of fiscal 2011 did not have an impact on our consolidated financial statements.
In December 2009, the FASB issued Accounting Standards Update (“ASU”) 2009-16, Accounting for Transfers of Financial Assets (“ASU 2009-16”), which is an amendment of ASC 860, Transfers and Servicing. This update requires more information about the transfer of financial assets. More specifically, ASU 2009-16 eliminates the
70
Table of Contents
concept of a “qualified special purpose entity”, changes the requirements for derecognizing financial assets, and enhances the information reported to users of financial statements. This update is effective for fiscal years beginning on or after November 15, 2009. The adoption of ASU 2009-16 during the first quarter of fiscal 2011 did not have an impact on our consolidated financial statements.
In April 2010, the FASB issued ASU 2010-13, Effect of Denominating the Exercise Price of a Share-Based Payment Award in the Currency of the Market in Which the Underlying Equity Security Trades (“ASU 2010-13”), which is an amendment of ASC 718, Compensation—Stock Compensation. This update clarifies that an employee share-based payment award with an exercise price denominated in the currency of a market in which a substantial portion of the entity’s equity securities trades should not be considered to contain a condition that is not a market, performance, or service condition. Therefore, an entity would not classify such an award as a liability if it otherwise qualifies as equity. This update is effective for fiscal years and interim periods beginning on or after December 15, 2010; however, early application was permitted. The adoption of ASU 2010-13 during the first quarter of fiscal 2011 did not have an impact on our consolidated financial statements.
In October 2009, the FASB issued ASU 2009-13, Multiple Deliverables Revenue Arrangements (“ASU 2009-13”), which is an amendment of ASC 605-25, Revenue Recognition: Multiple Element Arrangements. This update addresses the accounting for multiple-deliverable arrangements to allow the vendor to account for deliverables separately instead of as one combined unit by amending the criteria for separating consideration in multiple-deliverable arrangements. This guidance establishes a selling price hierarchy for determining the selling price of a deliverable, which is based on (a) vendor-specific objective evidence, (b) third-party evidence or (c) best estimate of selling price. The residual method of allocation has been eliminated and arrangement consideration is now required to be allocated to all deliverables at the inception of the arrangement using the selling price method. Additionally, expanded disclosures will be required relating to multiple deliverable revenue arrangements. This update is effective for fiscal years beginning on or after June 15, 2010. The adoption of ASU 2009-13 during the first quarter of fiscal 2012 did not have a material impact on our consolidated financial statements.
Not yet adopted by us
In June 2011, the FASB issued ASU 2011-05, Presentation of Comprehensive Income (“ASU 2011-05”), which was issued to enhance comparability between entities that report under U.S. GAAP and IFRS, and to provide a more consistent method of presenting non-owner transactions that affect an entity’s equity. ASU 2011-05 eliminates the option to report other comprehensive income and its components in the statement of changes in stockholders’ equity and requires an entity to present the total of comprehensive income, the components of net income and the components of other comprehensive income either in a single continuous statement or in two separate but consecutive statements. This pronouncement is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011, which corresponds to our first quarter of fiscal 2013. Early adoption of the new guidance is permitted and full retrospective application is required. We are currently evaluating the effect that the provisions of this pronouncement will have on our financial statements.
In September 2011, the FASB issued ASU 2011-08, Testing Goodwill for Impairment (“ASU 2011-08”), which simplifies testing for impairment by allowing an entity to first assess qualitative factors and determine if it is more likely than not (defined as 50% or more) that the fair value of the reporting unit is less than its carrying amount. That determination can then be used to decide if it is necessary to perform the two-step goodwill impairment test. ASU 2011-08 is effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011, which corresponds to our first quarter of fiscal 2013. Early adoption is permitted in certain circumstances. We are currently evaluating the effect that the provisions of this pronouncement will have on our financial statements.
71
Table of Contents
Our company
We develop, manufacture and sell a complete line of reagents and automated systems that detect and identify certain properties of the cell and serum components of human blood for the purpose of blood transfusion. We target our offerings at hospitals, donor centers and reference laboratories for blood typing and antibody screening prior to transfusion.
Our strategy is to drive automation in blood banks and transfusion centers around the world. Our strategy enables us to grow market share and develop long-term contractual relationships with our customers. Over the past five years, we have grown our net revenues at a CAGR of 10.5% from net revenues of $223.7 million in 2007 to $333.1 million in 2011.
Our industry
We are part of the in-vitro diagnostic blood typing and screening market, which generally seeks to prevent transfusion reactions through the testing of blood and blood components prior to transfusion. In the U.S., the FDA regulates the transfusion of human blood as a drug and as a biological product. The FDA regulates all phases of the blood banking industry, including donor selection and the collection, classification, storage, handling and transfusion of blood and blood components. The FDA requires that all facilities that manufacture products used for any of these purposes, and the products themselves, be registered or licensed by the FDA. See “—Regulation” for further discussion.
We estimate the current worldwide blood banking reagent and instrument market at approximately $1.2 billion. We conduct our business globally with manufacturing facilities in the U.S. and Canada. We have a direct distribution presence in the U.S., Canada, Western Europe and Japan, which represent most of the addressable market today. We sell through distributors in other international markets. Over the longer term, we believe emerging markets such as China, India and Brazil represent a growth opportunity as these markets develop and require automation. The long-term potential of the emerging markets is not included in the current estimated $1.2 billion market opportunity for instruments and reagents worldwide.
The principal components of blood are red cells (the cellular portion) and plasma (the fluid portion). Attached to the exterior of red cells are antigens, which determine the blood group (A, B, AB or O) and type (Rh positive or Rh negative) of an individual’s blood. Plasma contains many different kinds of proteins, including antibodies that are produced by the body in response to foreign substances, such as foreign red cell antigens introduced in a transfusion. If foreign antigens are introduced into the patient’s blood in a transfusion, the patient’s body will produce antibodies to combat those foreign antigens, creating a potentially fatal reaction within the body.
Because of the potential reaction of antigens and antibodies, it is critical that healthcare providers correctly identify the antigens and antibodies present in patient blood and donor blood before a transfusion. If a donor’s red cells contain antigens that are recognized by antibodies in the patient’s plasma, the transfused red cells could be destroyed in a potentially life-threatening reaction. Also, if foreign antigens from donor red cells are introduced into a patient’s blood through a transfusion, the patient’s body can produce new antibodies in response to the foreign antigens. The production of these new antibodies, known as alloimmunization, can complicate future transfusions.
Because of the critical importance of matching patient and donor blood, highly skilled and educated technologists in hospitals, donor centers and reference laboratories generally perform procedures for testing compatibility. At present, many customers in the U.S. perform these tests manually, making blood testing laboratories much more labor intensive than other types of testing laboratories. In our direct markets outside of the U.S., a significant portion of the customers are performing these tests with automation.
72
Table of Contents
Our strengths
Market leader in critical patient care segment. We are a leader in the worldwide in-vitro diagnostic blood typing and screening market. We are the market leader in the U.S. and one of the top three industry players worldwide, according to information compiled by a third-party consultant. Our leadership position is supported by the depth and breadth of our product offerings, including our extensive line of traditional reagents and our innovative instrumentation. Our instrumentation is a key differentiator for us. We are currently the only company to offer two fully automated instruments worldwide for serology testing to meet the different needs of our customers depending upon the test volume in their laboratory and the complexity of the testing required. Additionally, we are the only company to have brought four generations of automation to the market, more than any of our competitors. The breadth of our product offerings allows us to service the full range of customers, including donor centers, reference laboratories, small- and medium-sized hospitals, and large hospitals, both in the automated and manual market.
Attractive industry dynamics. Historically, demand for blood in the U.S. market has been stable with a low single-digit organic growth rate. Blood demand, which has a direct correlation to the number of blood transfusions performed, is a strong indicator of industry type and screen testing volumes. Between 2001 and 2010, the compounded annual organic growth rate for blood demand was approximately 1.5% in the U.S., despite challenges in the last two years due to the economic downturn. Over time, we believe testing volumes will benefit from an aging population and cyclical uplift in healthcare utilization, which is currently depressed because of the economy.
Additionally, there is a secular trend towards automation in the U.S. market, where the majority of customers still perform blood typing and screening on a manual basis. Automation represents an attractive value proposition for hospitals as it relieves lab technician shortage issues, improves operational efficiencies and enhances patient safety.
Another attractive industry dynamic is that our business faces no material direct government reimbursement issues. Blood bank instruments and reagents typically represent a nominal (0.05%) percent of hospital expense budgets.
“Razor/razor blade” business model results in highly attractive credit profile. We believe that instrument placements are the most effective way to drive revenue growth. The tangible benefits for customers of automation include gains in operational efficiencies and improvements in patient safety. We intend to continue to pursue a “razor/razor blade” business model, in which our goal in placing instruments is to secure a long-term, contractual relationship with the customer for reagents. Our serology instruments are “closed systems,” so each instrument placed typically provides us with a recurring revenue stream through the sale of reagents. We continually innovate to ensure our automation offerings are competitive.
Defensible end-markets and stable customer base. In the U.S., the FDA regulates the transfusion of human blood as a drug and as a biological product. Due to the regulated nature of our industry, we believe there are significant barriers to entry for new competitors to enter the U.S. market. Our customer relationships tend to be “sticky” due to the long-term nature of customer contracts and high switching costs. In the U.S. market, for example, switching costs typically include retraining of staff, rewriting of standard operating procedures, change of laboratory setup and work flow, and an FDA-mandated validation process.
Meaningful cash flow generation capabilities. Our business supports a high cash flow conversion profile, with operating cash flows of approximately 105% of net income and 63% percent of EBITDA over the last five years. Our cash flow generation can be attributed to attractive gross margins and minimal working capital requirements. Annual purchases of property and equipment were approximately 2.7% of net sales, and movement from inventory to property and equipment for instruments placed on rental agreements was approximately 3.9% of net sales in fiscal 2011. The movement from inventory to property and equipment, which is reflected in working
73
Table of Contents
capital in our statement of cash flows, is similar to a capital expenditure in that it represents the amount that we record as property and equipment when we rent our instruments rather than sell them. In fiscal 2011, we produced approximately $102.1 million of operating cash flow. We believe that our business has the capability to support further growth while also taking advantage of operating leverage and continuing to generate strong cash flow. For a reconciliation of EBITDA to net income for the periods presented, see “Selected historical consolidated financial data.”
Sponsor with significant healthcare experience and ability to add value. Funds managed by TPG have been among the most active healthcare investors, investing in approximately 20 companies over the last four years across the healthcare industry. Benefiting from its distinguished history with similar transactions, TPG can leverage its deep healthcare expertise and relationships to assist us in driving future performance. TPG also has a large group of operations professionals who will support our efforts to drive ongoing growth and operating efficiencies.
Experienced management team with a strong track record. We have a highly experienced leadership team that has extensive experience in the industry. Our senior managers have effectively managed the business through the economic downturn and have successfully positioned us for domestic and international growth. In October 2011, we named William A. Hawkins as CEO, who brings years of experience building some of the most dynamic, innovative companies in the health care industry and we believe further strengthens our management team.
Our strategy
Our long-term growth drivers revolve around our automation strategy. We believe innovative instrumentation is the key to improving blood bank operations and patient safety, as well as increasing our market share around the world. In implementing our strategy, we are focused on the following:
Capitalize on the trend towards automation. We estimate that the majority of customers in the U.S. still perform blood typing and screening on a manual basis, particularly in the small- to medium-sized hospital segment. Given the labor shortages in blood banks and the strain on hospital budgets, we believe there are significant economic drivers behind the automation trend. Automation can allow customers to reduce headcount and/or overtime in the blood bank, which can be a benefit given the current shortage of qualified blood bank technologists. We also believe that automation can improve patient safety, increase operational efficiency and, for customers such as integrated delivery networks with multiple blood banks, permit the standardization of best practices. Given the reduction in both human and economic capital, we estimate that our instruments have an average payback period of one year or less, depending on the size of the lab. Hence, we believe our automated products represent an attractive value proposition for manual customers to switch to automation.
Continue to gain share in our direct markets. Outside of the U.S., we have direct distribution operations in Canada, Western Europe and Japan as well as a network of third-party distributors in other parts of the world. As of May 31, 2011, approximately 30% of our sales were from outside the U.S. We have been successful in growing our business in our international direct markets by placing instruments through competitive wins. These markets are highly automated, and the strength of our innovative automation offering has enabled us to gain new business, especially in Europe, where our market share is significantly lower than in the U.S. We have grown our net sales to third-party customers in Europe at a CAGR of 12.3% over the past four years, demonstrating the success of our automation strategy. We believe that our innovative instruments, our proprietary and traditional serology reagents and our new molecular immunohematology offering will enable us to continue to grow our market share outside the U.S.
Penetrate underserved emerging markets. Infrastructure in emerging markets is far less developed than that in the U.S. and Europe, representing a significant opportunity for growth through automation. We believe the markets of China, India and Brazil represent the largest opportunities based on the population size and the estimated low level of automation present in these markets today. The long-term potential of the emerging
74
Table of Contents
markets is not included in the current estimated $1.2 billion market opportunity for instruments and reagents worldwide. Given our innovative product offerings, we believe the emerging markets represent a significant opportunity for future growth.
Further develop our industry-leading molecular platform. We have invested substantial effort and research and development expense in developing our molecular diagnostics platform and believe it will become an increasingly strategic asset over time. Molecular solutions have the potential to significantly enhance patient safety and outcomes. Molecular immunohematology is a developing field worldwide, and we believe we are on the forefront of this exciting market. Our most significant molecular assays have received regulatory approval in the European Union, and we are working to achieve regulatory approval in the U.S.
Executing our automation strategy
We offer two fully automated instruments for serology testing – NEO® and Echo® – to meet the different needs of our customers depending upon the volume in their laboratory and the complexity of the testing required. All of our serology instrumentation uses Capture® technology, our proprietary reagents, as well as traditional reagents to perform automated testing.
NEO is targeted at large hospitals, donor centers and reference laboratories, NEO replaces our previous high volume instrument, Galileo.
Echo is a compact bench top, fully-automated walk-away serology instrument that meets the needs of the small- to medium-sized hospital market as well as integrated delivery networks that want to standardize the operations of their laboratories.
In August 2008, we added molecular immunohematology to our product portfolio with our acquisition of BioArray. BioArray developed a flexible technology platform that combines semiconductor processing, microparticle chemistry and molecular biology for the DNA testing of blood for transfusion. It allows for a variety of multiplex DNA-based testing, combining DNA amplification (PCR) with detection and data analysis software. With the goal of improving transfusion medicine, we believe that molecular immunohematology, which we market as the BeadChip system, will bring a high degree of flexibility and performance to DNA and protein analysis.
Our Human Erythrocyte Antigen (“HEA”) molecular immunohematology product, our Human Platelet Antigen (“HPA”) molecular immunohematology product as well as our current semi-automated molecular immunohematology instrument, the Array Imaging System and BASIS™ database are CE (“Conformité Européenne”) Marked denoting regulatory clearance in the European Union (“EU”). Our molecular offering is currently available for Research Use Only in the U.S.
Traditional serology reagents
A reagent is a substance that is added during a test in order to bring about a reaction. The resulting reaction is used to confirm the presence of another substance. Our reagents are used to identify different properties of blood for the purpose of transfusion.
Most of our current reagent products are used in tests to (i) identify the blood group (A, B, AB, O) and type (Rh positive or negative), (ii) to detect and identify red cell antibodies or red cell antigens, (iii) to detect and identify platelet antibodies and (iv) to determine blood compatibility (crossmatch). The FDA requires the accurate testing of blood and blood components for the purpose of transfusion, using only reagents that have been licensed or cleared by the FDA.
75
Table of Contents
Under traditional agglutination blood testing techniques (the manual method), the technologist manually mixes serum with red blood cells in a test tube, performs several additional procedures and then examines the mixture to determine whether there has been an agglutination reaction. A positive reaction occurs if the cells are drawn together in clumps by the presence of corresponding antibodies and antigens. Due to the critical importance of matching patient and donor blood, testing procedures using agglutination techniques are usually performed manually by highly educated and skilled technologists.
We estimate that approximately 60% of customers in the U.S. perform testing on a manual basis without the use of an automated instrument. The customers that perform testing on a manual basis are primarily in the small- to medium-sized hospital segment of the market. In the high volume segment of the U.S. market (large hospitals, donor centers and reference laboratories) and in our international direct markets, a significant portion of the customers are automated.
Traditional reagents are generally used in a manual setting, but certain traditional reagent products are also used on our automated instruments. Traditional reagents accounted for approximately 60% of our revenue in fiscal 2011. We believe there is a slight amount of seasonality in our reagent business as fewer donations and elective surgical procedures are performed in our first fiscal quarter (June-August).
Proprietary technology platforms
Capture reagents (solid phase technology)
In our proprietary solid phase blood test system, known as Capture, red cell or platelet antigens are bound to a microtitration plate as a solid support (the solid phase), and the bound reactant captures other reactants in a fluid state and binds those fluid reactants to the solid phase. In this testing system, patient or donor serum or plasma is placed in the well of a plastic microtitration plate on which antigen reactants have been bound. Our special proprietary indicator cells are then added. In a positive reaction, antibodies in the test sample are captured by the indicator cells and adhere to the bottom of the test well as a thin layer. In a negative reaction, there is no antibody attached to the indicator cells and they settle to the bottom of the test well as a small cell button. These reactions occur rapidly and result in clearly defined, machine-readable test results that are often easier to interpret than the subjective results sometimes obtained from traditional agglutination technology (manual method). Also, in batch test mode the solid phase test results can generally be obtained in substantially less time than by traditional agglutination techniques.
Molecular immunohematology technology
Our molecular immunohematology technology, which was obtained in August 2008 through our acquisition of BioArray, is marketed as the BeadChip system. BioArray developed a novel and flexible technology platform that allows for a variety of multiplex DNA-based testing, combining DNA amplification (PCR) with BeadChip detection and data analysis software. The platform combines semiconductor processing, microparticle chemistry and molecular biology to bring a high degree of flexibility and performance to DNA and protein analyses. Current BeadChip kits include Multiplex Human Erythrocyte Antigen and Human Platelet Antigen, both of which have received CE Mark approval. The BeadChip system is currently available for Research Use Only in the U.S.
Instruments and instrument systems
We offer customers a selection of automated analyzers. We designed our systems to be scalable, which enables laboratories to better match the instrument to their various needs based on the size of their testing facility, whether it is low, medium or high volume. While we design and own the rights to our instruments, our instruments are produced by third-party manufacturers.
76
Table of Contents
Serology
NEO—Targeted at donor centers, large-volume hospitals and reference laboratories, NEO provides a fully-automated solution to perform all routine blood bank tests, including blood grouping, antibody screening, crossmatch, direct antiglobulin test (DAT) and antibody identification. We believe there is opportunity to expand our automated footprint in the U.S. hospital market because of NEO’s added functionality, including STAT functionality, a faster turnaround time and improved reliability. NEO uses our proprietary Capture reagent products as well as certain traditional reagents.
ECHO—Echo is targeted at small- to medium-sized hospitals as well as at integrated delivery networks (both hospital and lab systems) in combination with NEO. Like NEO, Echo has a broad test menu and uses both our proprietary Capture reagents as well as certain traditional reagents to perform its testing.
CAPTURE WORKSTATION (Semi-automated Processor)—The Capture Workstation has semi-automated components for performing our proprietary Capture assays manually. It is marketed as a back-up system for our fully automated NEO and Echo instruments, or as a standalone test system for small laboratories looking to standardize testing.
Molecular
ARRAY IMAGING SYSTEM AND BASIS (SEMI-AUTOMATED)—Today, molecular testing using our BeadChip technology is a semi-automated process. The testing itself is primarily manual while the reading and interpretation of test results is automated with our Array Imaging System and BASIS database. This semi-automated system has received CE Mark approval in the EU and is available for Research Use Only in the U.S.
Research and development
We continually seek to improve our existing products and to develop new ones in order to increase our market share. Prior to sale, any new product requires regulatory approvals, including licensing or pre-market clearance from the FDA in the U.S. and CE marking in Western Europe. For the fiscal years ended May 31, 2011, 2010 and 2009, we spent approximately $15.9 million, $15.4 million and $10.7 million, respectively, for research and development. Research and development expenses have increased over the past three years due to the acquisition of BioArray in August 2008 and the subsequent development work on our molecular immunohematology offering.
Marketing and distribution
Our potential customers are donor centers, hospitals and reference laboratories. More than three-quarters of our current customers are hospitals, and the remainder are primarily donor centers and reference laboratories. No single customer represents 10% or more of our annual consolidated revenue.
We have a direct sales presence in the U.S., Canada, Western Europe and Japan. We sell through distributors in other regions of the world. We offer several instrument procurement options, including direct sales and rentals.
We typically seek to enter into both an instrument purchase or rental agreement with a customer and a reagent purchase agreement. Some of our agreements with our larger customers allow the customer to terminate the agreement without cause, usually with 60 days notice.
Backlog
As of August 31, 2011, there were no material backlogs for instruments or reagent products. At August 31, 2011, in accordance with U.S. generally accepted accounting principles, we had not recognized approximately $3.9 million in deferred revenue from extended warranty sales.
77
Table of Contents
Suppliers
We obtain raw materials from numerous outside suppliers and believe our business relationships with them are good. Some of our products are derived from blood having particular or rare combinations of antibodies or antigens, which are found in a limited number of individuals. To date, we have been able to obtain sufficient quantities of such blood for use in manufacturing our products, but there can be no assurance that a sufficient supply of such blood will always be available to us.
We source our serology instruments from single-source suppliers. Although we currently do not have a written contract with the supplier of our Echo instrument, we generally operate under the terms of past contractual arrangements with that supplier. We believe that our business relationship with our instrument suppliers is good. While these relationships are significant, we believe that other manufacturers could supply the instruments to us after a reasonable transition period. We have elected not to dual source our instruments because we consider our primary exposure to be the sudden bankruptcy of the suppliers. In the event a current instrument supplier experiences financial problems that prevent it from continuing to produce our instrument, we believe it would take in the range of 18 months to 24 months to transfer the technology and begin production with a new instrument supplier. While a change in an instrument supplier would disrupt our growth opportunities during the transition period, we do not believe it would have a long-term material financial impact on our existing business as 84.7% of our revenue for fiscal 2011 was generated from our reagents. During the transition period to a new supplier, there could be a material impact on our ability to execute our strategy of driving automation in the blood bank using a “razor/razor blade” model in which our sale of instruments is intended to establish a relationship in which we continue to sell reagents to a customer over time.
Regulation
The manufacture and sale of blood banking products is a highly regulated business and is subject to continuing compliance with multiple U.S., Canadian, Western European, Japanese and other country-specific statutes, regulations and standards that generally include licensing, product testing, facilities compliance, product labeling, post-market vigilance and consumer disclosure.
In the U.S., an FDA biologics license is issued for an indefinite period of time, subject to the FDA’s right to revoke the license. As part of its overview responsibility, the FDA makes facility inspections on an unannounced basis. In addition, each product manufactured by us is subject to formal product submissions and review processes by the FDA and other regulatory bodies, such as Health Canada, a European Union-recognized Notified Body and the Japanese Ministry of Health prior to authorization to market. Significant changes to our products or facilities can require additional submission and review prior to implementation. For example, we hold several FDA biologic licenses to manufacture blood grouping reagents, anti-human globulin reagents and reagent red blood cells. We must submit biologic license applications or 510(k) pre-market notifications to the FDA to obtain product licenses or market clearance for new products or instruments. To accomplish this, we must submit detailed product information to the FDA, perform a clinical trial of the product and demonstrate to the satisfaction of the FDA that the product meets certain efficacy and safety standards. There can be no assurance that any future product licenses, product clearances or instrument clearances will be obtained by us.
Our manufacturing and distribution facilities in the U.S., Germany and Canada are certified to ISO 13485:2003. This is an internationally recognized standard, and certification is required in order to continue product distribution in key markets such as the European Union and Canada. In addition, to allow continued marketing of our products in the European Union, we are required to maintain certification under the EC Full Quality Assurance System Assessment in accordance with the requirements of Annex IV of the IVD Medical Devices Directive 98/79/EC. This certification authorizes the use of the CE Mark on our products that allows products free access to all countries within the European Union. We have successfully completed certifications for CE marking of all products manufactured for the European market.
78
Table of Contents
In addition to the U.S., Western Europe, Canada and Japan, there are multiple countries worldwide that also impose regulatory barriers to market entry. We continue to maintain the product registrations and approvals necessary to access foreign markets.
In June 2009, we announced that the FDA, in an administrative action based on a January 2009 inspection, issued a notice of intent to revoke our biologics license with respect to our Reagent Red Blood Cells and Anti-E (Monoclonal) Blood Grouping Reagent products. Under this administrative action, we have the opportunity to demonstrate or achieve compliance before the FDA initiates revocation proceedings or takes other action. The FDA did not order the recall of any of our products or restrict us from selling these products. This administrative action was a follow on to a warning letter that we received in May 2008. We had been working on an FDA-approved remediation plan, submitted after the warning letter, but had failed to make adequate progress at the time of the FDA’s follow-up inspection in January 2009. In early calendar 2009 (during our fiscal third quarter of 2009), we formalized efforts to improve our quality systems through the Quality Process Improvement Project. In August 2009 in response to the June 2009 administrative action, we submitted a detailed remediation plan that outlined our actions and timelines to correct the FDA’s noted deficiencies from the January 2009 inspection. The Quality Process Improvement Project served as the basis for the detailed remediation plan. During our third fiscal quarter of 2010, we completed the remediation portion of the Quality Process Improvement Project. During June 2010 the FDA conducted an inspection of our facilities. During September 2010, we were notified by the FDA that while the June 2010 inspection “disclosed that substantive corrections have been made, some deviations continue.” Therefore, the FDA stated that the conditions outlined in the June 2009 NOIR administrative action remain in effect. The FDA stated that it will evaluate our overall compliance status at its next inspection. There is no set schedule for such FDA inspections so we do not know when that next inspection will take place. Since the June 2010 inspection, the FDA has resumed the normal approval process in considering our regulatory applications. Although we are prepared for such an inspection, we cannot predict whether the FDA will find further deviations or take further action with respect to the NOIR. See “Risk factors—Risks related to the company—FDA administrative action could have a material and adverse effect on our business.”
Properties
In the U.S., we lease our corporate office and main manufacturing facilities along with laboratories and warehouses, which are located in Norcross, Georgia. We also lease the manufacturing facility for our molecular immunohematology products, which is located in Warren, New Jersey. Outside the U.S., we lease our distribution facility in Germany as well as our sales offices in Western Europe and in Japan, with the exception of our Belgium sales office, which we own. We also own our Canadian manufacturing facility.
Our owned properties are not encumbered as security for any loan. We believe that our current facilities are adequate for our current and anticipated needs and do not foresee any difficulty in renewing leases that expire in the near term.
Environmental
Some of our processes generate hazardous waste and we have a U.S. Environmental Protection Agency identification number. We believe we are in compliance with applicable portions of the federal and state hazardous waste regulations.
Patents and trademarks
Since 1986, the U.S. Patent Office has issued six patents to us pertaining to our solid phase technology for serology testing, five of which have expired. The remaining patent expires in 2012. We believe that our trade secrets and know-how will help prevent any current or future competitors from successfully copying our solid phase products.
79
Table of Contents
When we acquired BioArray in August 2008, we acquired a substantial portfolio of issued patents or pending patent applications. Through the development of technologies supported by the patents, BioArray developed a novel and flexible technology platform that allows for a variety of DNA-based testing applications. The platform combines semiconductor processing, microparticle chemistry and molecular biology to bring a high degree of flexibility and performance to DNA and protein analysis. Using this technology platform, BioArray developed complete assay solutions called the BeadChip system. These patents expire between 2018 and 2025.
We use trademarks on several of the products we sell. These trademarks are protected by registration in the U. S. and other countries where such products are marketed using the trademarks. We consider these trademarks in the aggregate to be of material importance in the operation of our business.
Competition
Competition in the blood banking industry is based on quality of instrumentation and reagents, pricing, talent of the sales forces, ability to furnish a range of quality existing and new products, reliable technology, skilled and trained technicians, customer service and continuity of product supply. We believe we are well positioned to compete favorably in this industry principally because of the completeness, reliability and quality of our product line, our competitive pricing structure and our introduction of innovative products, such as our Capture technology and full line of automated instruments for serology testing and our molecular immunohematology offering. We also believe that continuing research efforts in the area of blood bank automation, the experience and expertise of our sales personnel and the expertise of our technical and customer support staff will enable us to remain competitive in the market.
Our principal competitors worldwide are Ortho-Clinical Diagnostics (“Ortho”), a Johnson & Johnson company, and Bio-Rad Laboratories, Inc. (“Bio-Rad”). Both Ortho and Bio-Rad sell instrumentation as well as reagents.
Financial information about geographic areas
We conduct our business globally with manufacturing facilities in the U.S. and Canada. We have sales operations in the U.S., Canada, several Western European countries and Japan. Roughly 30% of our revenues are generated by our international affiliates. In addition to the potentially adverse impact of foreign regulations (see “—Regulation”), we may be affected by more difficult market conditions outside the U.S., which could impact our revenues and profit margins. Also, there may be adverse consequences from fluctuations in foreign currency exchange rates, which may affect the competitiveness of our products and our profit margins because our affiliates sell our products predominantly in local currencies, but our cost structure is weighted towards the U.S. dollar.
For financial information about geographic areas, see Note 15 “Domestic and Foreign Operations” of the notes to the audited consolidated financial statements.
Employees
At May 31, 2011, we had a total of 757 full-time employees worldwide. We have a low staff turnover rate and consider our employee relations to be good. In addition to our full-time work force, we employ temporary and contract employees. None of our employees are represented by a labor union.
Legal proceedings
Government investigations
In October 2007, we reported that the FTC was investigating whether we violated federal antitrust laws or engaged in unfair methods of competition through three acquisitions made in the period from 1996 through 1999,
80
Table of Contents
and whether we or others engaged in unfair methods of competition by restricting price competition. The FTC letter requested that we provide certain documents and information to the FTC concerning those acquisitions and concerning our product pricing activities since then. In July 2008, the FTC formalized its document and information requests into a Civil Investigative Demand (“CID”) and also required us to provide certain additional information within the same general scope of its previous requests. The FTC has also required that we provide to them the documents we provided to the Department of Justice (see below). We have been cooperating with the FTC and we intend to continue cooperating, and we are assured that the issuance of a formal CID does not indicate any dissatisfaction with our cooperation. At this time, we cannot reasonably assess the timing or outcome of the investigation or its effect, if any, on our business.
In April 2009, we received a subpoena from the DOJ, requiring us to produce documents for the period beginning September 1, 2000 through April 23, 2009, pertaining to an investigation of possible violations of the federal criminal antitrust laws in the blood reagents industry. On November 8, 2010, we announced that the DOJ had informed us that it had closed its investigation. The investigation was closed with no further action taken on the part of the DOJ. In fiscal 2011, we incurred approximately $1.6 million in legal expenses related to the DOJ investigation and the related lawsuits, which are described in the following paragraph.
Class action litigation
Beginning in May 2009, a series of class action lawsuits has been filed against us, Ortho-Clinical Diagnostics, Inc. and Johnson & Johnson Health Care Systems, Inc. alleging that the defendants conspired to fix prices at which blood reagents are sold, asserting claims under Section 1 of the Sherman Act, and seeking declaratory and injunctive relief, treble damages, costs, and attorneys’ fees. All of these complaints make substantially the same allegations. The cases have been consolidated in the United States District Court for the Eastern District of Pennsylvania. In August 2010, the United States District Court for the Eastern District of Pennsylvania denied, in part, motions to dismiss for failure to state a cause of action and a motion to stay discovery filed by the Company and co-defendant Ortho-Clinical Diagnostics, Inc. The defendants filed a motion for reconsideration or for certification for interlocutory appeal with respect to the Court’s order on defendants’ motion to dismiss, and that motion was denied in December 2010. Discovery has now commenced in this litigation. No determination has been made as to whether any of the plaintiffs’ claims have merit or should be allowed to proceed as a class action. Briefing relating to potential certification of a class began in September 2011, with plaintiffs’ motion to certify a class. Defendants must reply by February 2012, and the Court will determine whether to hear argument on plaintiffs’ motion. A decision on class certification would occur after these filings and a hearing, if one is held. We intend to vigorously defend against these cases. However, were the plaintiffs to prevail in one or more of these cases, we could have to pay significant amounts, including treble damages and attorneys’ fees, and these amounts would not be covered by our insurance policies. At this time, we cannot reasonably assess the timing or outcome of this litigation or its effect, if any, on our business.
Private securities litigation in the United States District Court for the Northern District of Georgia against us and certain of our current and former directors and officers asserts federal securities fraud claims on behalf of a putative class of purchasers of the our common stock between October 19, 2005 and June 25, 2009. The case alleges that the defendants violated Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended, by failing to disclose that we had violated the antitrust laws, and challenges the sufficiency of our disclosures about the results of FDA inspections and our quality control efforts. In June 2011, the Court granted the defendants’ motion to dismiss the complaint and closed the case. In July 2011, the plaintiff moved that the Court reconsider its dismissal of the complaint, and the Court denied this motion to reconsider. In September 2011 plaintiffs appealed to the United States Circuit Court of Appeals for the Eleventh Circuit to reinstate the case. We will defend the case vigorously if it is reinstated. At this time, we cannot reasonably assess the timing or outcome of this litigation or its effect, if any, on our business.
81
Table of Contents
Litigation relating to the acquisition
In July 2011, in connection with the Acquisition, a series of six putative class action lawsuits were filed in the Superior Courts of Fulton County and Gwinnett County, Georgia, captioned as Hillary Kramer v. Immucor, Inc., et al., Civil Action No. 2011CV203124 (Fulton County), Babette C. Schorsch v. Immucor, Inc., et al., Civil Action No. 11A0776-1 (Gwinnett County), Allan Pillay v. Immucor, Inc., et al., Civil Action No. 2011CV203339 (Fulton County), Larry Macintyre v. Immucor, Inc., et al., Civil Action No. 2011CV203397 (Fulton County), Irene Dixon v. Immucor, Inc., et al., Civil Action No. 2011CV203567 (Fulton County) and Gilbert Rosenthal v. Immucor, Inc., et al., Civil Action No. 11A079463 (Gwinnett County). All of these actions were brought on behalf of our public shareholders against, in various combinations, ourselves, certain of our former individual directors, certain of our current and former executive officers, the Sponsor and certain of the Sponsor’s affiliates. The actions asserted claims for breaches of fiduciary duties against our board of directors in connection with the Acquisition, and for aiding and abetting the purported breaches of fiduciary duties by the Sponsor defendants. Some of the actions also included allegations that our Schedule 14D-9 filed with respect to the Acquisition failed to provide certain allegedly material information. The plaintiffs sought, among other things, preliminary and permanent relief, including injunctive relief enjoining the consummation of the Acquisition, rescission of the Acquisition to the extent it was consummated prior to the entry of a final judgment, and costs, expenses and disbursements of the action.
The Pillay case was dismissed without prejudice in August 2011; the Kramer, Macintyre and Dixon cases were dismissed without prejudice in September 2011; and the parties have agreed to stay the Rosenthal case in favor of the Schorsch case which remains pending in Gwinnett County. In the Schorsch case, which is the only case currently active, in August 2011 the Court denied plaintiff’s motion for preliminary injunction; and in October 2011 the plaintiff filed a Second Amended Complaint, which remains pending and which the defendants moved to dismiss on November 14, 2011. The Court has not yet ruled on defendants’ motions to dismiss the Schorsch case. We will defend this case vigorously if it is not dismissed. At this time, we cannot reasonably assess the timing or outcome of this litigation or its effect, if any, on our business.
Legal proceedings involving our former executive officers
Dr. Gioacchino De Chirico served as our President from July 2003 through June 2011 and as our Chief Executive Officer from September 2006 through June 2011. His current title is Past President and CEO; however, Dr. De Chirico does not serve as an executive officer or director of Immucor or any of our subsidiaries. He also served as our Chief Executive Officer from April to October 2004 but relinquished that position pending the board of directors’ review of payments made by our Italian subsidiary during his tenure as President of that subsidiary. In October 2004, a prosecutor in Milan, Italy alleged that our Italian subsidiary had made an improper €13,500 payment to an Italian physician for the purpose of securing business from the physician’s hospital. In 2005, based on its independent investigation, the Audit Committee of our board of directors concluded that the €13,500 payment was for services rendered by the physician at a medical conference.
The case in Milan was brought against the subsidiary and Dr. De Chirico. In 2007, the Company settled all charges against the subsidiary, and the subsidiary has not been subject to any continuing proceedings, fines or penalties related to that investigation. However, Dr. De Chirico, as an individual, chose not to settle, and his case proceeded to trial. In April 2008, the tribunal in Milan rendered a guilty verdict at the first level. The verdict was confirmed at the second level in March 2010, and in June 2011, Dr. De Chirico’s attorney was told that his appeal had been denied at the third level. However, under Italian law all penalties imposed under the verdict were immediately negated at the first level and cannot be reinstated.
In 2005, the SEC opened an investigation related to the Italian case. In 2007, Dr. De Chirico and we settled the SEC investigation, requiring Dr. De Chirico to pay a $30,000 civil penalty, without admitting any wrongdoing. We have not been subject to any continuing proceedings, fines or penalties related to the SEC investigation.
Other than as set forth above, we are not currently subject to any material legal proceedings, nor, to our knowledge, is any material legal proceeding threatened against us. However, from time to time, we are party to certain legal proceedings in the ordinary course of business.
82
Table of Contents
Directors and executive officers
Our current Board of Directors consists of three members. All of our directors, except Mr. Hawkins, who is an employee of the Company, are employees of TPG and are therefore deemed to be affiliates. Below is a list of the names, ages and positions, and a brief account of the business experience, of the individuals who serve as our directors and executive officers.
| Name |
Age | Position | ||||
| William A. Hawkins |
57 | President, Chief Executive Officer and Director | ||||
| Richard A. Flynt |
52 | Executive Vice President and Chief Financial Officer | ||||
| Philip H. Moïse |
62 | Executive Vice President, General Counsel and Secretary | ||||
| Jeffrey K. Rhodes |
37 | Director | ||||
| Todd B. Sisitsky |
40 | Director | ||||
William A. Hawkins serves as President and Chief Executive Officer and a director since he joined the Company in October 2011. From 2002 to June 2011, Mr. Hawkins served in a variety of executive capacities, including Chairman and CEO of Medtronic, one of the world’s largest and most innovative medical technology companies. Before joining Medtronic, Mr. Hawkins was the CEO of Novoste Corporation from 1998 to 2002. Mr. Hawkins serves on the board of visitors for the Duke University School of Engineering and the board of directors for the Guthrie Theater and the University of Minnesota Foundation. Within the past five years, Mr. Hawkins also served as a director of Deluxe Corporation. We believe Mr. Hawkins is qualified to sit on our board of directors because of his extensive experience, executive leadership and management experience in other companies in the healthcare industry.
Richard A. Flynt serves as Executive Vice President and Chief Financial Officer and joined the Company in December 2007. Mr. Flynt is a Certified Public Accountant with more than 25 years of financial management experience. He has most recently served as Vice President—Finance with McKesson Corporation in Alpharetta, Georgia. From July 2004 through January 2007, he was Senior Vice President—Corporate Controller & Chief Accounting Officer with Per-Se Technologies, Inc., which was acquired by McKesson Corporation in January 2007. From 1997 through 2004, he served in a variety of senior financial management positions with GNB Technologies, Inc., Exide Technologies, Inc. and GTS Energy. Prior to that, he had 15 years of public accounting experience with Ernst & Young LLP. Mr. Flynt earned a BBA and Masters of Accounting from the University of Georgia.
Philip H. Moïse serves as Executive Vice President, General Counsel and Secretary and joined the Company in April 2007. Before joining the Company, Mr. Moïse was in the private practice of law, most recently as a partner and Co-Chair of Life Sciences at Sutherland Asbill & Brennan LLP in Atlanta. During a 30-year career in private practice, he represented over 35 public and private companies in the life sciences and medical products industries, and he had represented the Company for more than 20 years. Mr. Moïse graduated with honors from The Citadel and Duke University School of Law.
Jeffrey K. Rhodes. Mr. Rhodes helps lead TPG’s investment activities in the healthcare services and pharmaceutical / medical device sectors. He is involved with TPG’s investments in Biomet, IMS Health, Immucor and Surgical Care Affiliates. Prior to joining TPG’s San Francisco office in 2005, Mr. Rhodes worked at McKinsey & Company and Article27 LTD, a start-up software company. He received his M.B.A. from the Harvard Business School, where he was a Baker Scholar. He earned his B.A., summa cum laude, in Economics from Williams College. Mr. Rhodes was appointed as a director in 2011. We believe Mr. Rhodes is qualified to sit on our board of directors because of his financial expertise as well as his experience as a director of other privately held companies in the healthcare industry.
83
Table of Contents
Todd B. Sisitsky. Mr. Sisitsky is a partner of TPG Capital, where he leads the firm’s investment activities in the healthcare services and pharmaceutical/medical device sectors. He played leadership roles in connection with TPG’s investments in Aptalis Pharma (GI-focused specialty pharmaceutical company, formerly Axcan Pharma), Biomet (leading orthopedic implant manufacturer), Fenwal Transfusion Therapies (blood product technologies business), IASIS Healthcare (Tennessee-based acute care hospital company), Surgical Care Affiliates (ambulatory surgery center business carved out from HealthSouth Corporation), HealthScope (hospital and pathology company based in Australia) and IMS Health (leading global data services and consulting business to several segments of the healthcare industry). Mr. Sisitsky also serves on the board of directors of the global not-for-profit organization, the Campaign for Tobacco Free Kids. Prior to joining TPG in 2003, Mr. Sisitsky worked at Forstmann Little & Company and Oak Hill Capital Partners. He received a M.B.A. from the Stanford Graduate School of Business, where he was an Arjay Miller Scholar, and earned his undergraduate degree from Dartmouth College, where he graduated summa cum laude. Mr. Sisitsky was appointed as a director in 2011. We believe Mr. Sisitsky is qualified to sit on our board of directors because of his financial expertise as well as his experience as a director of other privately held companies in the healthcare industry.
84
Table of Contents
Compensation discussion and analysis
This compensation discussion and analysis describes the material elements of compensation awarded to Named Executive Officers for our 2011 fiscal year. It should be read in conjunction with the Summary Compensation Table, related tables and the narrative disclosure. Unless otherwise specifically noted, the information contained in this section is stated as of May 31, 2011, the end of our 2011 fiscal year.
The Named Executive Officers for fiscal 2011 are:
| • | Dr. Gioacchino De Chirico, President and Chief Executive Officer for fiscal 2011 and until his retirement from an executive officer position in June 2011; |
| • | Richard A. Flynt, Executive Vice President and Chief Financial Officer; |
| • | Philip H. Moïse Executive Vice President, General Counsel and Secretary; and |
| • | Geoffrey S. Crouse, Executive Vice President and Chief Operating Officer until his resignation in April 2011. |
In connection with Dr. De Chirico’s retirement from an executive officer position in June 2011, Joshua H. Levine was appointed as our President and Chief Executive Officer effective June 10, 2011. Mr. Levine resigned from the Company effective October 17, 2011. William A. Hawkins was appointed as our President and Chief Executive Officer effective October 17, 2011. References to the Chief Executive Officer in this compensation discussion and analysis are to Dr. De Chirico, who served in that capacity for all of fiscal 2011. Neither Mr. Levine nor Mr. Hawkins was a Named Executive Officer for purposes of this compensation discussion and analysis because neither served during fiscal year 2011.
All references to the Committee in this Compensation Discussion and Analysis section are references to the Compensation Committee of our Board, as constituted immediately prior to the Acquisition. Though we currently expect the Board to continue to focus on the same basic elements, philosophies and objectives in respect of the compensation program for our executive officers, given that we are no longer a publicly-traded company, these elements, philosophies and objectives, as well as the executive compensation process described below, are subject to change as we continue our transition to being a privately-held company.
Overview
Our Compensation Committee (the “Committee”), which was comprised solely of independent directors, had responsibility over matters relating to compensation of executives, including cash incentive and other benefit plans, and non-employee directors of the Company, including reviewing the performance of management in achieving the Company’s goals and objectives and for setting the annual compensation of the Company’s executive officers. The Committee also had responsibility over matters relating to the Company’s equity incentive plans, including the sole authority to grant stock options and other equity incentive awards to employees, directors and any other eligible individuals from time to time and to administer the Company’s various equity incentive plans.
Cash bonuses that were paid to executive officers at the end of each fiscal year were conditioned on the achievement of certain net income targets and on company objectives. Under the terms of the bonus plans approved at the beginning of each fiscal year, the Compensation Committee had authority to adjust the net income target to account for non-recurring expenses not anticipated at the time the plan was approved. Long-term equity incentives (stock options and restricted stock) were awarded at the beginning of each fiscal year based on the salary and position of the recipient, and were influenced by the recipient’s performance.
Outside consultants assisted the Committee in establishing the overall compensation philosophy. Our consultants also provided market compensation information which the Committee used, together with other available
85
Table of Contents
information, to set compensation it determined to be in line with the current market rates and compensation paid by our peers. For fiscal year 2011, the compensation consultants retained independently by the Compensation Committee were Towers Watson. The consultant did not perform any other services for the Company.
The Committee also sought input from management, and the Chief Executive Officer had periodically met with Committee members to evaluate performance of other executive officers and recommend their compensation. However, no executive officer, including the Chief Executive Officer, had participated directly in any Committee meetings relating to his or her compensation, other than to provide information requested by the Committee, and the Chief Executive Officer was not present during any deliberations concerning his compensation. The significant aspects of management’s role were recommending performance targets and objectives, evaluating employee performance and recommending base salary increases. The Committee was not authorized under its Charter to delegate its authority to others in determining or recommending compensation for executive officers or directors.
Our compensation philosophy for fiscal year 2011
Primary objectives
The primary objectives of our executive compensation program were to support the achievement of our financial and strategic goals by attracting and retaining executives performing at the highest levels of our industry, and to reward the achievement of goals we believe drive shareholder value. To achieve these objectives, we intended that our executive compensation programs:
| • | Included performance measures that recognized overall company performance as well as individual performance; |
| • | Included plans that were competitive externally but flexible enough to deliver rewards based on an executive’s ability to influence our financial and organizational performance and to influence individual performances; |
| • | Reflected that outstanding company performance should be accompanied by outstanding rewards, and performance below expectations should result in lesser rewards or no rewards; |
| • | Reflected that outstanding individual performance should be accompanied by outstanding rewards within limits of company performance; |
| • | Provided flexibility and discretion in applying the compensation principles to appropriately reflect individual circumstances as well as changing business conditions and priorities; and |
| • | Included a mix of compensation that balances rewards for achievement of our short- and long-term financial goals and rewards linked to the performance of our stock. |
External benchmarks
In setting compensation for executive officers, we considered how medical diagnostics and other related companies in our industry compensate their executives. We refer to these companies as “peer companies” and considered how they compensate executives in positions comparable to our executive positions, that is, positions with similar duties, responsibilities, complexity and scope. The peer companies the Compensation Committee used for fiscal 2011 to compare compensation levels were:
| Affymetrix Inc. |
Myriad Genetics Inc. | |
| Align Technology, Inc. |
Orthofix, International N.V. | |
| American Medical Systems Holdings, Inc. |
Quidel Corp. | |
| Gen-Probe Inc. |
Steris Corp. | |
| Haemonetics Corp. |
Teleflex Incorporated | |
| Hill-Rom Holdings, Inc. |
Thoratec Corp. | |
| ICU Medical Inc. |
Zoll Medical Corp. |
86
Table of Contents
Certain positions are not specific to our industry. For those positions, our Compensation Committee also used compensation surveys conducted by national human resources consulting firms containing information about a broader group of companies in other industries, including public companies of comparable size to us. These compensation surveys, together with the peer group information, were used to consider compensation for all executives, including our Named Executive Officers.
Overall compensation opportunities were based on typical competitive market practices. However, while we considered levels of compensation at our peer companies to be an important measure, we expected that the application of other important factors would result in compensation being above peer-company medians for some executive officers and below peer-company medians for other executive officers, and this could vary from year to year. Actual executive compensation should have reflected our overall performance so that in years of strong company performance executives earn higher levels of compensation, and in years of weaker company performance executives should have earned lower levels of compensation.
Total compensation
Our primary compensation program included the following elements:
| • | Base salary, |
| • | Annual cash incentives (bonuses), and |
| • | Stock-based long-term incentives. |
Total compensation for our executive officers was weighted more heavily towards incentive-based remuneration, including equity-based compensation, because we believed such compensation had the greatest ability to influence our overall results. The mix of compensation elements was adjusted from time to time to best support our immediate and longer-term objectives.
Base salary
The purpose of base salary was to provide a competitive level of fixed compensation to help attract and retain the executives we needed to achieve our financial and strategic goals. Base salaries were generally established at a level designed to be competitive with base salaries for comparable positions among our peer companies, and were adjusted where appropriate to reflect the qualifications and contribution level of the individual executive. Outstanding individual performance or significant experience may have warranted a higher base salary for a particular executive as compared to those in comparable positions among our peer companies. Each executive officer’s base salary was reviewed on an annual basis and adjusted as appropriate to reflect individual performance and/or changes in the marketplace.
Annual cash incentives (bonuses)
The purpose of our annual cash bonus plan was to motivate and reward executives for achieving our shorter-term financial goals and strategic goals, and to provide competitive total compensation opportunities. Each executive’s annual bonus opportunity was designed with reference to the bonus opportunities of comparable positions at our peer companies, while also balancing the cost implications to us.
In April 2010, the Compensation Committee approved the FY 2011 Bonus Plan and Long-Term Incentive Plan for executive officers (the bonus portion of the Plan is referred to as the “2011 Bonus Plan” and the long-term incentive portion of the Plan is referred to as the “2011 Stock Incentive Plan”).
Under the terms of the 2011 Bonus Plan, the Company’s executive officers were eligible for bonuses based on three components, weighted equally:
| • | Net Income Component: actual net income compared to fiscal 2011 target net income; |
| • | Corporate Goals Component: the achievement of corporate goals established by the Compensation Committee for fiscal 2011; and |
87
Table of Contents
| • | Individual Goals Component: the achievement of individual performance objectives for fiscal 2011. |
Our Compensation Committee retained the ability to provide for bonuses for outstanding individual performance even when targeted metrics for overall company performance were not achieved. Annual bonuses could have increased if we exceed targeted net income.
Stock-based long-term incentives
The purposes of our long-term incentive program were to motivate executives to drive shareholder value by better aligning their interests with those of shareholders, and to provide competitive total compensation opportunities. We delivered long-term incentives through equity compensation plans because we believed equity ownership most clearly aligned the interests of our executives with those of our shareholders. Long-term incentives represented a comparably larger portion of the total compensation package for executives we believed were in a position to most directly influence our longer-term success. Long-term incentive grants were designed to be competitive with typical market practices among our peer companies and other comparable companies, taking into consideration individual performance, internal fairness and the overall cost of the program to the Company. Our Compensation Committee had generally favored an emphasis on the use of long-term equity incentive awards to motivate members of the Company’s senior management team, including the Chief Executive Officer and other Named Executive Officers. The ultimate value of the equity awarded depended on the long-term performance of our stock.
Long-term incentive awards were made under our 2005 Long-Term Incentive Plan. Under the plan, the Compensation Committee had the discretion to award many kinds of long-term incentives including stock options, restricted stock, SARs, deferred stock, and stock-based and cash-based long-term performance awards. As of the end of fiscal 2011, only stock options and restricted stock had been awarded under that plan. Stock options were granted at the closing market price on the date of grant and increased in value only if the Company’s stock price increased. In addition, all stock option grants required various minimum periods of employment beyond the date of the grant in order to exercise the option, and restricted stock awards required various minimum periods of employment in order for the recipient to gain unrestricted ownership of the shares awarded.
Benefits
Our executives received benefits consistent with our general employee population. These benefits, as for all employees, helped executives and their families protect themselves against financial risks associated with illness, disability, death and retirement through a combination of personal savings, company contributions and company-sponsored benefit plans.
During fiscal 2011, Named Executive Officers also received car allowances as disclosed in the Compensation Summary Table under the Executive Compensation section, and all officers were entitled to be reimbursed for a portion of the premiums on personal life insurance policies. The Compensation Committee eliminated executive car allowances for fiscal 2012.
Elements of compensation for fiscal 2011
The following section outlines the components of compensation and how we established pay levels for each of these components in fiscal 2011.
Base salary
The base salaries paid to our Named Executive Officers for fiscal 2011 were governed by the terms of their employment agreements with us, each of which had been approved by our Compensation Committee. These employment agreements contain the general terms of each Named Executive Officer’s employment and establish the minimum compensation that such Named Executive Officers are entitled to receive, but do not prohibit, limit
88
Table of Contents
or restrict our ability to pay additional compensation, whether in the form of base salary, bonus, stock options or otherwise. Our Compensation Committee reviewed base salaries of our Named Executive Officers annually and employment agreements were amended for any increases granted.
In August 2010, our Compensation Committee approved merit increases in the base salaries of Dr. De Chirico and Messrs. Flynt and Moïse. Mr. Crouse’s base salary for fiscal 2011 was determined pursuant to the terms of his employment agreement with us dated August 1, 2010. The table below shows the base salary for each Named Executive Officer for fiscal 2011.
| Gioacchino De Chirico |
President and Chief Executive Officer | $ | 595,000 | |||
| Richard A. Flynt |
Executive VP, Chief Financial Officer | $ | 365,000 | |||
| Philip H. Moïse |
Executive VP, General Counsel | $ | 503,000 | |||
| Geoffrey S. Crouse |
Executive VP, Chief Operating Officer | $ | 475,000 |
For fiscal 2011, the base salary of Mr. Moïse exceeded the median base salary of our peer companies and Mr. Crouse’s base salary exceeded the median base salary disclosed in salary surveys, while each of Dr. De Chirico’s and Mr. Flynt’s base salary fell below median levels for our peer companies.
Annual cash incentives—2011 Bonus Plan
Bonuses under the 2011 Bonus Plan, in which our Named Executive Officers participated, were based on the Company achieving specified goals for net income, product quality and new product introduction established by the Compensation Committee, and on achievement of individual performance objectives established for each participant at the beginning of the Company’s fiscal year. The 2011 Bonus Plan components were designed to support the Company’s long-term strategic goals and achievement of all bonus plan elements required successful execution throughout the organization.
The Named Executive Officers were eligible for bonuses based on three components, weighted equally:
| • | Net Income Component: actual net income compared to fiscal 2011 target net income (“Target Net Income”); |
| • | Corporate Goals Component: the achievement of corporate goals established by the Compensation Committee for fiscal 2011; and |
| • | Individual Goals Component: the achievement of individual performance objectives for fiscal 2011. |
The table below shows the maximum bonus awards that could have been earned by Named Executive Officers if actual net income was 100% or more of Target Net Income. Named Executive Officers could have earned bonus awards on a prorated basis if actual net income was between 90% and 100% of Target Net Income. No awards were paid if actual net income was less than 90% of Target Net Income. The amount an individual actually earned depended on the extent to which the goals for each bonus component were met.
| Maximum Bonus Award (% of Base Compensation)* |
100% of Target Net Income |
105% of Target Net Income |
110% of Target Net Income or Above |
|||||||||
| CEO |
45 | % | 67.5 | % | 90 | % | ||||||
| Other Named Executive Officers |
30 | % | 45 | % | 60 | % | ||||||
| * | Maximum bonus awards will be prorated for in-between percentage of net income. |
For fiscal 2011, Target Net Income was $91.4 million. Our actual net income for fiscal 2011 was $89.3 million. The Compensation Committee approved an adjustment to exclude expenses related to severance (net of tax) from net income. Net income as adjusted was $91.2 million, and we therefore achieved approximately 100% of Target Net Income. The Compensation Committee determined that the corporate component was 92% achieved for
89
Table of Contents
fiscal 2011. For the individual goals component, the Compensation Committee determined that each of the Named Executive Officers achieved 100% of their respective individual goals for the year. In August 2011, Dr. De Chirico and Messrs. Flynt and Moïse received the bonuses listed below under the Company’s 2011 Bonus Plan. Mr. Crouse did not receive a bonus because he was not employed by the Company at the time of payment.
| Gioacchino De Chirico |
$ | 260,461 | ||
| Richard A. Flynt |
$ | 106,519 | ||
| Philip H. Moïse |
$ | 146,792 |
Stock-based long-term incentives—2011 stock incentive plan
In April 2010, the Compensation Committee approved criteria for the issuance of equity-based awards for fiscal year 2011 and awarded stock options and restricted stock to our Named Executive Officers under our 2005 Long-Term Incentive Plan. The target value of awards for fiscal 2011 to the Named Executive Officers was as follows:
| Chief Executive Officer |
150% of base compensation | |
| Other Named Executive Officers |
100% of base compensation |
For the Chief Executive Officer, 35% of the target value was granted in stock options (based on their grant date value estimated using the Black-Scholes option pricing model) and 65% of the target value was granted in restricted stock (based on the market price of the Company’s stock on the date of grant). For the other Named Executive Officers, 25% of the target value was granted in stock options (based on their grant date value estimated using the Black-Scholes option pricing model) and 75% of the target value was granted in restricted stock (based on the market price of the Company’s stock on the date of grant).
In deciding on the number and kind of long-term incentive awards, the Compensation Committee considered the financial performance of the Company, such as increases in net income and operating income, as well as competitive market data on the amount and kind of long-term incentive grants made to executives at peer companies and other comparable companies. The Committee also sought input from management and from independent consultants retained by the Committee. The Compensation Committee then determined the number and kind of awards to be granted to Named Executive Officers occupying certain positions. A larger award, on a percentage basis, was made to the Chief Executive Officer because the Compensation Committee determined that he would have the most significant impact on the financial success of the Company for the time periods during which the awards would vest.
Special equity grant to Mr. Crouse
On July 16, 2010, Mr. Crouse was granted 100,000 stock options upon his promotion to Executive Vice President and Chief Operating Officer. The options were granted under our 2005 Long-Term Incentive Plan.
Specific details about the equity grants to the Named Executive Officers of the Company for fiscal 2011 are included in the “—Grants of plan-based awards” section below.
Section 162(m) of the Internal Revenue Code
Section 162(m) of the Internal Revenue Code prohibits us from deducting, for federal income tax purposes, certain compensation in excess of $1 million per year paid to certain persons named in the Summary Compensation Table unless the compensation meets particular criteria. One criterion is that the compensation be paid only upon the achievement of objective, pre-established performance goals and in accordance with certain procedural requirements. For fiscal year 2011, the Committee and management believed that Section 162(m) of the Internal Revenue Code did not impact the deductibility of executive compensation.
90
Table of Contents
Compensation Committee interlocks and insider participation
The members of the Compensation Committee during the fiscal year ended May 31, 2011 were James Clouser, Dr. Paul Holland, Ronny Lancaster, Dr. Paul Mintz, Mason Morfit and Joseph Rosen.
During the fiscal year ended May 31, 2011, no Named Executive Officer of the Company served either as: (1) a member of the compensation committee (or other board committee performing equivalent functions or, in the absence of any such committee, the entire board of directors) of another entity, one of whose executive officers served on the Compensation Committee (or other Board Committee performing equivalent functions or, in the absence of any such committee, the entire Board of Directors) of the Company; (2) a director of another entity, one of whose executive officers served on the Compensation Committee (or other Board Committee performing equivalent functions or, in the absence of any such committee, the entire Board of Directors) of the Company; or (3) a member of the compensation committee (or other board committee performing equivalent functions or, in the absence of any such committee, the entire board of directors) of another entity, one of whose executive officers served as a director of the Company.
Effective with the consummation of the Acquisition on August 19, 2011, a new Board of Directors was appointed. The new Board of Directors has not appointed a new Compensation Committee and will assume the responsibilities previously delegated to the Compensation Committee. None of our executive officers serve on the compensation committee (or its equivalent) or board of directors of another entity whose executive officer has served on our Board of Directors.
Executive compensation in fiscal year 2011
The following table sets forth the compensation earned by the Named Executive Officers for services rendered in all capacities to the Company for the 2011 fiscal year.
Fiscal 2011 Summary Compensation Table
| Name and Principal Position |
Year | Salary | Stock Awards(1) |
Option Awards(1) |
Non-Equity Incentive Plan Compensation(2) |
All Other Compensation(3) |
Total | |||||||||||||||||||||
| Dr. Gioacchino De Chirico |
2011 | $ | 595,776 | $ | 562,877 | $ | 302,813 | $ | 260,461 | $ | 65,629 | $ | 1,787,556 | |||||||||||||||
| 2010 | 571,304 | 565,841 | 294,823 | 50,624 | 66,194 | 1,548,786 | ||||||||||||||||||||||
| 2009 | 571,828 | 394,220 | 730,666 | 294,687 | (4) | 44,419 | 2,035,820 | |||||||||||||||||||||
| Richard A. Flynt |
2011 | 363,008 | 256,016 | 85,265 | 106,519 | 33,221 | 844,029 | |||||||||||||||||||||
| 2010 | 339,546 | 200,298 | 64,605 | 34,283 | 21,608 | 660,340 | ||||||||||||||||||||||
| 2009 | 292,662 | 121,292 | 224,815 | 106,575 | 23,167 | 768,511 | ||||||||||||||||||||||
| Philip H. Moïse |
2011 | 501,134 | 367,465 | 122,381 | 146,792 | 32,913 | 1,170,685 | |||||||||||||||||||||
| 2010 | 488,973 | 322,238 | 103,935 | 49,207 | 21,962 | 986,314 | ||||||||||||||||||||||
| 2009 | 472,115 | 194,944 | 361,321 | 171,463 | 19,441 | 1,219,284 | ||||||||||||||||||||||
| Geoffrey S. Crouse(5) |
2011 | 476,442 | 338,853 | 819,358 | — | 1,116,784 | 2,751,438 | |||||||||||||||||||||
| 2010 | 372,034 | 168,500 | 334,022 | 37,812 | 229,868 | 1,142,236 | ||||||||||||||||||||||
| 2009 | — | — | — | — | — | — | ||||||||||||||||||||||
| (1) | The amounts in these columns reflect the aggregate grant date fair value, as computed in accordance with Accounting Standards Codification 718, of awards pursuant to the Company’s equity incentive plans. Assumptions used in the calculation of these amounts for awards granted in fiscal year 2011 are included in Note 12, “Share-Based Compensation”, to the Company’s audited financial statements for the fiscal year ended May 31, 2011, included in the Company’s Annual Report on Form 10-K filed with the SEC on July 20, 2011. These referenced sections are deemed part of the disclosure required under Item 402 of SEC Regulation S-K. All the awards listed above were equity awards and consequently aggregate grant date fair value is fixed. |
| (2) | We paid bonuses for fiscal 2011 at the beginning of fiscal 2012 based on the achievement of approximately 100% of the fiscal 2011 Target Net Income, and corporate and individual goals established under the |
91
Table of Contents
| Company’s 2011 Bonus Plan at the end of fiscal 2010. The 2011 Bonus Plan is described in greater detail in the compensation discussion and analysis section of this report. |
| (3) | All Other Compensation consisted of the following: |
| Name |
Auto Allowance |
Financial Planning/ Legal Fees |
Insurance Premiums |
Company Contributions to Retirement and 401(k) Plans |
Severance Payments/ Accruals |
Total | ||||||||||||||||||
| Dr. Gioacchino De Chirico |
$ | 12,012 | $ | — | $ | 43,786 | $ | 9,831 | $ | — | $ | 65,629 | ||||||||||||
| Richard A. Flynt |
12,015 | — | 11,022 | 10,184 | — | 33,221 | ||||||||||||||||||
| Philip H. Moïse |
11,922 | — | 13,191 | 7,800 | — | 32,913 | ||||||||||||||||||
| Geoffrey S. Crouse |
10,582 | 6,823 | 5,447 | 8,723 | 1,085,209 | 1,116,784 | ||||||||||||||||||
| (4) | Dr. De Chirico was entitled to receive $337,687 under the 2009 Bonus Plan but voluntarily declined $43,000 of that amount, which was the portion related to the additional expenses incurred during fiscal 2009 related to the Company’s Quality Process Improvement Project. |
| (5) | Mr. Crouse joined the Company in August 2009. During fiscal 2011 he served as Executive Vice President, Chief Operating Officer until his resignation in April 2011. |
Grants of plan-based awards
Grants of stock-based awards for fiscal 2011 were made under the Company’s 2011 Stock Incentive Plan to Named Executive Officers, vice presidents, managers and supervisors generally based on the base compensation of the individual award recipient. All awards were made under the Company’s 2005 Long-Term Incentive Plan, which was adopted by our Board of Directors and approved by our shareholders in 2005.
The 2005 Long-Term Incentive Plan replaced our preexisting stock option plans which had been frozen and no longer were in effect except to the extent of awards that had been outstanding under these plans. Awards for up to 3,600,000 shares of our common stock may have been granted under the 2005 Long-Term Incentive Plan. There was a restriction on the number of shares that may have been used for awards other than stock options (1,800,000), but there was no restriction on the number of shares that may have been used for grants of non-incentive stock options. Options were granted at the closing market price on the date of the grant. Option awards generally vested equally over a four-year period and had a six-year contractual term. Restricted stock awards generally vested equally over a five-year period.
The 2005 Long-Term Incentive Plan provided for accelerated vesting of option and restricted stock awards if there was a change in control of the Company as defined in the 2005 Long-Term Incentive Plan, which would include, among other things, an acquisition, merger, liquidation and dissolution, or certain changes in majority ownership or of a majority of the Company’s Board of Directors.
92
Table of Contents
The following table includes information with respect to grants of plan-based awards to our Named Executive Officers during the fiscal year ended May 31, 2011.
Fiscal 2011 Grants Of Plan-Based Awards Table
| Name |
Grant Date |
Estimated Future Payouts Under Non-Equity Incentive Plan Awards(1) |
All Other Stock Awards: Number of Shares of Stock or Units(2) |
All Other Option Awards: Number of Securities Underlying Options(2)(3) |
Exercise or Base Price of Option Awards |
Grant Date Fair Value of Stock and Option Awards |
||||||||||||||||||||||||||
| Threshold | Target | Maximum | ||||||||||||||||||||||||||||||
| ($ /Sh) | ||||||||||||||||||||||||||||||||
| Dr. Gioacchino De Chirico |
||||||||||||||||||||||||||||||||
| Past President and |
6/7/10 | $ | $ | $ | 42,106 | 19.10 | $ | 302,813 | ||||||||||||||||||||||||
| 6/7/10 | 29,470 | 562,877 | ||||||||||||||||||||||||||||||
| — | 267,750 | 535,500 | ||||||||||||||||||||||||||||||
| Richard A. Flynt |
||||||||||||||||||||||||||||||||
| Executive Vice President |
6/7/10 | 11,856 | 19.10 | 85,265 | ||||||||||||||||||||||||||||
| 6/7/10 | 13,404 | 256,016 | ||||||||||||||||||||||||||||||
| — | 109,500 | 219,000 | ||||||||||||||||||||||||||||||
| Philip H. Moïse |
||||||||||||||||||||||||||||||||
| Executive Vice President |
6/7/10 | 17,017 | 19.10 | 122,381 | ||||||||||||||||||||||||||||
| 6/7/10 | 19,239 | 367,465 | ||||||||||||||||||||||||||||||
| — | 150,900 | 301,800 | ||||||||||||||||||||||||||||||
| Geoffrey S. Crouse |
||||||||||||||||||||||||||||||||
| Former Executive Vice President |
6/7/10 | 15,691 | 19.10 | 112,845 | ||||||||||||||||||||||||||||
| 7/16/10 | 100,000 | 18.53 | 706,513 | |||||||||||||||||||||||||||||
| 6/7/10 | 17,741 | 338,853 | ||||||||||||||||||||||||||||||
| — | 142,500 | 285,000 | ||||||||||||||||||||||||||||||
| (1) | Actual payouts in August 2011 to the Named Executive Officers under the 2011 Bonus Plan are reported under the Non-Equity Incentive Plan Compensation column in the Summary Compensation Table. The compensation discussion and analysis section of this report explains in greater detail the methodology used for calculating bonuses. |
| (2) | On June 7, 2010 as part of the annual group award under the 2011 Stock Incentive Plan, the Compensation Committee granted options to purchase shares of common stock and restricted share awards to employees of the Company, including Named Executive Officers, pursuant to the 2005 Long-Term Incentive Plan. The options vest 25% at each anniversary of the grant date and have a term of six years, and restricted shares vest 20% at each anniversary of the grant date. |
| (3) | On July 16, 2010, Mr. Crouse was granted stock options upon his promotion to Executive Vice President and Chief Operating Officer. |
93
Table of Contents
Outstanding equity awards at fiscal year-end
The following table shows outstanding equity awards to the Named Executive Officers at May 31, 2011:
Fiscal 2011 Outstanding Equity Awards At Fiscal Year-End Table
| Name |
Option Awards (1) | Stock Awards (2) | ||||||||||||||||||||||
| Number of Securities Underlying Unexercised Options |
Option Exercise Price |
Option Expiration |
Number of Shares or Units of Stock That Have Not Vested |
Market Value of Shares or Units of Stock That Have Not Vested |
||||||||||||||||||||
| Exercisable | Unexercisable | |||||||||||||||||||||||
| Dr. Gioacchino De Chirico |
28,368 | — | $ | 17.51 | 6/6/2012 | $ | ||||||||||||||||||
| 42,249 | 14,084 | 29.23 | 6/8/2013 | |||||||||||||||||||||
| 35,193 | 35,194 | 26.90 | 6/5/2014 | |||||||||||||||||||||
| 11,810 | 35,431 | 16.25 | 6/4/2015 | |||||||||||||||||||||
| — | 42,106 | 19.10 | 6/7/2016 | |||||||||||||||||||||
| 72,866 | 1,525,085 | |||||||||||||||||||||||
| Richard A. Flynt |
11,250 | 3,750 | 33.24 | 12/10/2013 | ||||||||||||||||||||
| 10,828 | 10,829 | 26.90 | 6/5/2014 | |||||||||||||||||||||
| 2,588 | 7,764 | 16.25 | 6/4/2015 | |||||||||||||||||||||
| — | 11,856 | 19.10 | 6/7/2016 | |||||||||||||||||||||
| 25,971 | 543,573 | |||||||||||||||||||||||
| Philip H. Moïse |
15,000 | — | 30.00 | 4/1/2013 | ||||||||||||||||||||
| 21,937 | 7,313 | 29.23 | 6/8/2013 | |||||||||||||||||||||
| 17,403 | 17,404 | 26.90 | 6/5/2014 | |||||||||||||||||||||
| 4,163 | 12,491 | 16.25 | 6/4/2015 | |||||||||||||||||||||
| — | 17,017 | 19.10 | 6/7/2016 | |||||||||||||||||||||
| 41,972 | 878,474 | |||||||||||||||||||||||
| Geoffrey S. Crouse (3) |
50,000 | — | 16.85 | 8/3/2015 | ||||||||||||||||||||
| 15,691 | — | 19.10 | 6/7/2016 | |||||||||||||||||||||
| 100,000 | — | 18.53 | 7/16/2016 | |||||||||||||||||||||
| (1) | All option awards are non-qualified stock options. Options granted prior to June 1, 2006 expire ten years from the date of grant. Options issued on or after June 1, 2006 expire six years from the date of grant. The vesting schedule for the options granted prior to June 1, 2006 is 50% on the second anniversary of the grant date and 25% on each of the third and fourth anniversaries of the grant date; the vesting schedule for the options granted on or after June 1, 2006 is 25% on each anniversary of the grant date. Restricted shares vest 20% at each anniversary of the date of grant. |
| (2) | The market value of outstanding stock awards is based on a per share value of $20.93, the closing market price of our stock on May 31, 2011. |
| (3) | Pursuant to the terms of Mr. Crouse’s employment agreement, all unvested stock options became exercisable on his resignation, and remain exercisable for their remaining terms. |
94
Table of Contents
Option exercises and stock vested
The table below presents information concerning options exercised and restricted shares vested during the 2011 fiscal year for each of the Named Executive Officers.
Fiscal 2011 Option Exercises And Stock Vested Table
| Option Awards | Stock Awards | |||||||||||||||
| Name |
Number of Shares Acquired on Exercise |
Value Realized on Exercise(1) |
Number of Shares Acquired on Vesting |
Value Realized on Vesting(2) |
||||||||||||
| Dr. Gioacchino De Chirico |
— | — | 14,214 | $ | 273,061 | |||||||||||
| Richard A. Flynt |
— | — | 3,367 | 65,050 | ||||||||||||
| Philip H. Moïse |
— | — | 6,675 | 128,155 | ||||||||||||
| Geoffrey S. Crouse(3) |
— | — | 27,741 | 581,163 | ||||||||||||
| (1) | The value realized equals the difference between the closing market price of our common stock on the date of exercise and the exercise price multiplied by the number of shares acquired on exercise. |
| (2) | The value realized equals the closing market price of our common stock on the vesting date multiplied by the number of shares acquired on vesting. |
| (3) | Pursuant to the terms of Mr. Crouse’s employment agreement, all restricted shares vested and all restrictions imposed on the restricted shares lapsed on his resignation. |
Employment agreements
We have written employment agreements with each of Messrs. Flynt and Moïse and Dr. De Chirico that provide for severance benefits in the event of termination without cause or by the employee for good reason in connection with a change in control. Each of these agreements is described below. We intend to enter into an employment agreement with Mr. Hawkins in the near future.
We had a written employment agreement with Mr. Crouse that provided for certain compensation in connection with his resignation. A description of the compensation he received in connection with his resignation is described below in the section “Quantification of potential amounts payable on termination”.
Messrs. Flynt and Moïse
The employment agreements with each of Messrs. Flynt and Moïse provide that if the Company terminates the employee’s employment without cause or the employee terminates his employment for good reason (each, a “qualifying termination”), then the Company must pay the employee two times his “average annual compensation”, as defined in the agreement. Average annual compensation is calculated as the employee’s current base salary plus the average of the bonuses paid to him over the last two fiscal years over which he was eligible to receive a bonus (or such lesser number of years as he was eligible to receive a bonus). Upon a qualifying termination, severance will be paid in approximately equal monthly installments over the two years following a qualifying termination, except that if the qualifying termination occurs within two years after certain change in control events of the Company, the severance will be paid in a lump sum on the fifth day following a qualifying termination.
Each of the agreements provides that, if there is a qualifying termination, the employee will be entitled to (1) a lump sum payment of health insurance costs for 18 months payable on the 38th day following such qualifying termination, and (2) three additional months of vesting on equity-based awards and up to nine additional months to exercise vested stock options (if any). No release of claims is required to receive the payments, except in respect to qualifying terminations that occur prior to a change in control. Each of the agreements provides that the Company will pay $30,000 to the employee for outplacement assistance on the fifth day following a qualifying termination after a change in control.
95
Table of Contents
None of the employment agreements include a change in control excise tax gross-up. Each of the agreements includes a “best net” provision, pursuant to which benefits will be reduced or “cut back” to the extent necessary to avoid an excise tax, if that would result in a better net after-tax benefit for the employee (taking into account the excise taxes the employee would pay on an unreduced benefit).
In consideration for the severance and benefits described above, Messrs. Flynt and Moïse are each subject to a two-year post-employment non-solicitation restriction and an 18-month post-employment non-competition restriction.
Dr. De Chirico
Dr. De Chirico retired from an executive officer position in April 2011. The following is a description of his employment agreement while he was an executive officer of the Company. Dr. De Chirico’s current employment agreement also provides for significant severance payments under certain circumstances.
Dr. De Chirico’s employment agreement provides that in the event of a qualifying termination (other than in connection with a change in control), he is entitled to severance equal to his average annual compensation payable through June 10, 2012, payable in approximately equal bi-weekly installments. In the event of a change in control and a qualifying termination prior to June 10, 2012, Dr. De Chirico is entitled to severance equal to two times his average annual compensation, which, in the case of certain change in control events, is payable in a single lump sum at the time of such termination.
In the event of a qualifying termination (whether or not following a change in control), Dr. De Chirico is also entitled to (i) a lump sum payment of health insurance costs for 18 months, payable on the date of such qualifying termination (rather than on June 10, 2012, the expiration date of his current agreement), and (ii) full vesting of outstanding equity-based awards, if any, on the date of such qualifying termination, and the right to exercise his stock options, if any, for their full remaining term.
The employment agreement does not include a change in control excise tax gross-up. The agreement includes a “best net” provision, pursuant to which benefits will be reduced or “cut back” to the extent necessary to avoid an excise tax, if that would result in a better net after-tax benefit for Dr. De Chirico (taking into account the excise taxes the employee would pay on an unreduced benefit).
In consideration for the severance payable under his agreement, Dr. De Chirico is subject to two-year post-employment non-solicitation and non-competition restrictions; however, Dr. De Chirico has also entered a consulting agreement with the Company that begins on June 10, 2012, under which he is also subject to non-solicitation and non-competition provisions. The consulting agreement with Dr. De Chirico commences on July 1, 2012, and has a four year term under which he will be paid $200,000 per year. The consulting agreement contains non-solicitation and non-competition provisions during the contract term.
Definitions of change of control, cause and good reason
The agreements with Messrs. Flynt and Moïse and Dr. De Chirico define change of control as the occurrence of one of the following events:
| • | Sale of the Company’s assets. The sale of all or substantially all of the Company’s assets to a single purchaser or group of associated purchasers, whether in a single transaction or a series of related transactions within a 12-month period. |
| • | Sale of the Company’s shares. The sale, exchange, or other disposition to a single purchaser or group of associated purchasers, in one transaction, or in a series of related transactions within a 12-month period, of 30% or more of the Company’s outstanding shares of capital stock. |
| • | Merger or consolidation. The merger or consolidation of the Company in a transaction or series of related transactions in which the Company’s shareholders receive or retain less than 50% of the outstanding voting shares of the new or surviving corporation. |
96
Table of Contents
Cause is defined in the agreements for Messrs. Flynt and Moïse as:
| • | Employee’s material dishonesty in connection with his employment with the Company that causes harm to the Company; |
| • | Employee’s continuing refusal to perform reasonable duties assigned to him that are consistent with employee’s position (subject to certain notice and cure periods); or |
| • | Employee’s breach of any of the material terms of the agreement (subject to certain notice and cure periods). |
In Dr. De Chirico’s agreement, cause is defined as employee’s dishonesty, employee’s continuing inability or refusal to perform reasonable duties assigned to him (unless such refusal occurs following the occurrence of a change of control), employee’s moral turpitude or employee’s breach of any material obligation to the Company under the agreement or any other agreement with the Company.
Good reason is defined in the agreements for Messrs. Flynt and Moïse as:
| • | the assignment to employee of any duties or responsibilities materially inconsistent with the scope of the duties or responsibilities associated with his title or position, or any material adverse change of his title, position or status or the circumstances of his employment; |
| • | any action or inaction by the Company which would materially and adversely affect employee’s base compensation or participation in, or materially reduce his benefits under, the Company’s benefit plans (including, without limitation, equity benefits) as of the effective date of the agreement or as may be increased thereafter, other than actions or inactions that apply to all executive officers of the Company generally; |
| • | a breach by the Company of any of the material terms of the agreement; |
| • | the Company’s relocation of its headquarters to, or requiring the employee to move his primary work location to, a place more than 30 miles from the Company’s current headquarters location in Norcross, Georgia. |
In Dr. De Chirico’s agreement, good reason is defined as the failure to make any material payments due to him under the agreement or a relocation of the Company’s headquarters to, or a requirement that he move his primary work location to, a place more than 30 miles from his principal residence. Under each of the agreements, the definition of good reason is subject to specified notice and cure periods.
Quantification of potential amounts payable on termination
The table below sets forth the estimated payments that would be made to each of the Named Executive Officers (other than Mr. Crouse) upon involuntary termination, a change of control, and death or permanent disability, based on each person’s base salary as of May 31, 2011 and cash bonus paid with respect to fiscal 2011. The actual amounts to be paid out can only be determined at the time of such Named Executive Officer’s separation from the Company. The information set forth in the table assumes, as necessary:
| • | The termination and/or the qualified change in control event occurred on May 31, 2011 (the last business day of our last completed fiscal year); |
| • | The price per share of our common stock on the date of termination is $20.93, the closing market price of our common stock on the NASDAQ Global Select Market on May 31, 2011. |
97
Table of Contents
The payments made to Mr. Crouse pursuant to his April 2011 resignation are described below the table.
Fiscal 2011 potential payments upon termination or change in control
| Name |
Benefit |
Before Change in Control Termination w/o Cause |
After Change in Control Termination w/o Cause |
Death or Disability |
||||||||||
| Dr. Gioacchino De Chirico |
Severance | $ | 753,377 | $ | 1,502,637 | $ | — | |||||||
| Past President and |
Stock Options(1) | — | 2,654,238 | 2,654,238 | ||||||||||
| Chief Executive Officer |
Restricted Stock(2) | — | 1,525,085 | 1,525,085 | ||||||||||
| Outplacement |
— | 30,000 | — | |||||||||||
| TOTAL(3) |
$ | 753,377 | $ | 5,711,960 | $ | 4,179,323 | ||||||||
|
|
|
|
|
|
|
|||||||||
| Richard A. Flynt |
Severance | $ | 229,172 | $ | 866,818 | $ | — | |||||||
| Executive Vice President |
Stock Options(1) | — | 715,785 | 715,785 | ||||||||||
| Chief Financial Officer |
Restricted Stock(2) | — | 543,573 | 543,573 | ||||||||||
| Outplacement |
— | 30,000 | — | |||||||||||
| TOTAL(3) |
$ | 229,172 | $ | 2,156,176 | $ | 1,259,358 | ||||||||
|
|
|
|
|
|
|
|||||||||
| Philip H. Moïse |
Severance | $ | — | $ | 1,198,267 | $ | — | |||||||
| Executive Vice President |
Stock Options(1) | — | 1,134,929 | 1,134,929 | ||||||||||
| General Counsel |
Restricted Stock(2) | — | 878,474 | 878,474 | ||||||||||
| Outplacement |
— | 30,000 | — | |||||||||||
| TOTAL(3) |
$ | $ | 3,241,670 | $ | 2,013,402 | |||||||||
|
|
|
|
|
|
|
|||||||||
| (1) | This value equals the difference between the closing market price of our common stock on May 31, 2011 and the exercise price, multiplied by the number of option shares subject to accelerated vesting. |
| (2) | This value is calculated based on the closing market price of our common stock on May 31, 2011. |
| (3) | The total excludes the annual cash incentive for fiscal 2011 paid in August 2011, which is already included as compensation earned in fiscal 2011 in the Summary Compensation Table. |
Mr. Crouse’s Resignation
Mr. Crouse resigned effective April 14, 2011. He was party to an amended and restated employment agreement with the Company dated as of August 1, 2010. Pursuant to the agreement, in connection with his resignation, he will be compensated at a rate equal to his average annual compensation (that is, his base salary plus average bonus over the last two years) for the remainder of the agreement term (through May 31, 2013), receive the equivalent of health insurance costs for 18 months and receive outplacement assistance. The aggregate amount of cash payments to be made to Mr. Crouse is $1,085,209. Also pursuant to his employment agreement, all of Mr. Crouse’s unvested stock options vested upon his resignation and will remain exercisable for their remaining term, and all restricted shares vested and all restrictions imposed on the restricted shares lapsed.
98
Table of Contents
Security ownership of certain beneficial owners
All of the outstanding shares of common stock of Immucor, Inc. are held by IVD Intermediate Holdings B Inc. IVD Intermediate Holdings A Inc. holds all of the outstanding stock of IVD Intermediate Holdings B Inc. IVD Holdings Inc. holds all of the outstanding stock of IVD Intermediate Holdings A Inc.
The following table describes the beneficial ownership of IVD Holdings Inc. common stock, as of November 14, 2011 (except as noted) by each person known to the Company to beneficially own more than five percent of IVD Holdings Inc.’s (“IVD Holdings”) common stock, each director of the Company, each executive officer of the Company named in the “Summary Compensation Table” and all directors and executive officers of the Company as a group. The beneficial ownership percentages reflected in the table below are based on 7,351,866 shares of IVD Holdings’ common stock outstanding as of November 14, 2011.
Except as described in the agreements mentioned above or as otherwise indicated in a footnote, each of the beneficial owners listed has, to our knowledge, sole voting, dispositive and investment power with respect to the indicated shares of common stock beneficially owned by them. Unless otherwise indicated in a footnote, the address for each individual listed below is c/o Immucor, Inc., 3130 Gateway Drive, Norcross, Georgia 30071.
| Name |
Shares of stock beneficially owned |
Percentage of shares of stock beneficially owned on a fully- diluted basis |
||||||
| 5% shareholders |
||||||||
| TPG Funds |
7,351,866 | (1) | 100.0 | % | ||||
| Directors and executive officers |
||||||||
| Jeffrey K. Rhodes |
0 | (2) | 0 | % | ||||
| Todd B. Sisitsky |
0 | (2) | 0 | % | ||||
| William A. Hawkins |
0 | 0 | % | |||||
| Richard A. Flynt |
0 | 0 | % | |||||
| Philip H. Moïse |
0 | 0 | % | |||||
| Dr. Gioacchino De Chirico (3) |
0 | 0 | % | |||||
| Geoffrey S. Crouse (4) |
0 | 0 | % | |||||
| All executive officers and directors as a group |
0 | 0 | % | |||||
| (1) | Includes 5,904,035 shares of common stock of IVD Holdings held by TPG Partners VI, L.P., a Delaware limited partnership (“Partners VI”), 1,224,497 shares held by TPG IVD Co-Invest, L.P. (“Co-Invest”), a Delaware limited partnership, 200,000 shares held by TPG Biotechnology Partners III, L.P., a Delaware limited partnership (“Biotech III”), and 23,334 shares held by TPG FOF VI SPV, L.P., a Delaware limited partnership (“FOF VI” and together with “Partners VI,” “Co-Invest,” and “Biotech III,” the “TPG Funds”). The general partner of Partners VI is TPG GenPar VI, L.P., a Delaware limited partnership, whose general partner is TPG GenPar VI Advisors LLC, a Delaware limited liability company, whose sole member is TPG Holdings I, L.P., a Delaware limited partnership (“Holdings I”), whose general partner is TPG Holdings I-A, LLC, a Delaware limited liability company, whose sole member is TPG Group Holdings (SBS), L.P., a Delaware limited partnership, whose sole general partner is TPG Group Holdings (SBS) Advisors, Inc., a Delaware corporation (“Group Advisors”). The general partner of Biotech III is TPG Biotechnology GenPar III, L.P., a Delaware limited partnership, whose general partner is TPG Biotechnology GenPar III Advisors, LLC, a Delaware limited liability company, whose sole member is Holdings I. The general partner of each of Co-Invest and FOF VI is TPG Advisors VI, Inc., a Delaware corporation (“Advisors VI”). Messrs. Bonderman and Coulter are directors, officers and sole shareholders of Group Advisors and Advisors VI and may therefore be deemed to beneficially own the shares held by the TPG Funds. Messrs. Bonderman and Coulter disclaim beneficial ownership of the shares held by the TPG Funds except to the extent of their pecuniary interest therein. The business address of each of Group Advisors and Messrs. Bonderman and Coulter is c/o TPG Capital, L.P., 301 Commerce Street, Suite 3300, Fort Worth, TX 76102. |
| (2) | Jeffrey K. Rhodes is a Principal and Todd Sisitsky is a Partner of TPG Capital, L.P., an affiliate of the TPG Funds. Neither Mr. Rhodes nor Mr. Sisitsky has voting or investment power over and disclaims beneficial ownership of the shares held by the TPG Funds. The business address of each of Messrs. Rhodes and Sisitsky is c/o TPG Capital, L.P., 301 Commerce Street, Suite 3300, Fort Worth, TX 76102. |
| (3) | Dr. Gioacchino De Chirico served as our President from July 2003 through June 2011 and as our Chief Executive Officer from September 2006 through June 2011. His current title is Past President and CEO; however, Dr. De Chirico does not serve as an executive officer or director of Immucor or any of our subsidiaries. |
| (4) | Geoffrey S. Crouse served as Executive Vice President and Chief Operating Officer until his resignation in April 2011. |
99
Table of Contents
Certain relationships and related party transactions
Management agreement
On August 19, 2011, we entered into a management services agreement with TPG and certain of its affiliates, as applicable (the “Manager”), pursuant to which the Manager (or its designees) will provide us with certain management services until December 31, 2021, with evergreen one-year extensions thereafter. Pursuant to such agreement, upon the closing of the Transactions, the Manager (or its designees) received a one-time transaction fee of $18.0 million and was reimbursed for all out-of-pocket expenses incurred by or on behalf of the Manager or its affiliates related to the Acquisition.
In addition, the management services agreement provides that the Manager (or its designees) will receive an aggregate annual retainer fee equal to $3.0 million. The management services agreement further provides that the Manager (or its designees) is entitled to receive fees in connection with certain subsequent financing, acquisition, disposition and change of control transactions equal to customary fees charged by internationally-recognized investment banks for serving as financial advisor in similar transactions. The management agreement also provides for reimbursement of out-of-pocket expenses incurred by the Manager (or its designees) after the consummation of the Transactions.
The management services agreement includes customary exculpation and indemnification provisions in favor of the Manager, its designees and each of their respective affiliates. The management services agreement may be terminated by TPG, or upon an initial public offering or change of control unless TPG agrees otherwise. In the event the management services agreement is terminated, we expect to pay the Manager (or its designees) all unpaid fees and expenses due with respect to periods prior to such termination plus the sum of the net present values of the aggregate annual retainer fees that would have been payable with respect to the period from the date of termination until the expiration date in effect immediately prior to such termination.
Indemnification of directors and officers; directors’ and officers’ insurance
The directors and officers of Immucor and its subsidiaries in office prior to August 19, 2011 are entitled under the Merger Agreement to continued indemnification and insurance coverage.
Monitoring related party transactions
The Board monitors the occurrence of related party transactions in connection with its general responsibility to oversee the adequacy and effectiveness of the Company’s accounting, financial controls and internal controls over financial reporting, including the Company’s systems to monitor and manage business risk and legal and ethical compliance programs. The Board relies principally on the Company’s management to identify transactions that are related party transactions. Any such transactions identified are considered not later than the Board’s next regularly-scheduled meeting, at which meeting the Board will determine what, if any, action may be warranted.
The Company’s code of conduct requires that employees avoid conflicts of interest, which are defined broadly to include transactions between the Company and the employee, a family member or an affiliated company. Any conflict of interest is required to be brought to the immediate attention of the Company’s General Counsel. A violation of the code of conduct may result in disciplinary action up to and including dismissal. A copy of the code of conduct is available on the Company’s website at www.immucor.com.
Director independence
Prior to the closing of the Acquisition, as required under NASDAQ Stock Market listing standards, a majority of the members of a listed company’s Board of Directors must have qualified as “independent,” as affirmatively
100
Table of Contents
determined by the Board of Directors. In addition, prior to the closing of the Acquisition, our corporate governance guidelines required that a majority of the members of our Board of Directors be independent in accordance with the applicable provisions of the Exchange Act, the rules adopted by the SEC and the applicable rules of the NASDAQ Stock Market. Our corporate governance guidelines also required that directors not serve on more than three other public company boards.
Consistent with the applicable NASDAQ Stock Market listing standards and the requirements of our corporate governance guidelines, prior to the closing of the Acquisition, the governance committee reviewed and analyzed the independence of each director. Our Board affirmatively determined that, prior to the closing of the Acquisition, all of the directors other than Mr. Levine were independent directors within the meaning of the applicable NASDAQ Stock Market listing standards.
After the closing of the Acquisition, all of our directors are employees of TPG or the Company. Accordingly, none of our current directors can be considered independent under the applicable NASDAQ Stock Market listing standards.
101
Table of Contents
Description of other indebtedness
Senior credit facilities
Overview
In connection with the Acquisition, we entered into a credit agreement and related security and other agreements for (1) a $615.0 million senior secured term loan facility and (2) a $100.0 million senior secured revolving loan facility with certain lenders, Citibank, N.A., as administrative agent and collateral agent, Citigroup Global Markets Inc. and J.P. Morgan Securities LLC, as joint lead arrangers and joint lead bookrunners, UBS Securities LLC, as joint bookrunner, J.P. Morgan Securities LLC and UBS Securities LLC, as co-syndication agents and Deutsche Bank Securities Inc. and Royal Bank of Canada, as co-documentation agents.
We drew the full amount of the Term Loan Facility on the closing date. Proceeds of the term loans on the closing date were, together with other sources of funds, used to finance a portion of the Transactions and related fees and expenses. We did not draw on the Revolving Facility at the closing of the Acquisition. As of August 31, 2011, we had no outstanding loans under our Revolving Facility. In addition to borrowings upon prior notice, our Revolving Facility includes borrowing capacity in the form of letters of credit and borrowings on same-day notice, referred to as swingline loans, in each case, up to $25.0 million, and is be available in U.S. dollars, GBP, Euros, Yen, Canadian dollars and in such other currencies as we and the administrative agent under the Revolving Facility may agree (subject to sublimits for such non-U.S. currencies).
The credit agreement governing our Senior Credit Facilities provides that, subject to certain conditions, we may request additional tranches of term loans and/or increase commitments under the Revolving Facility and/or the Term Loan Facility and/or add one or more incremental revolving credit facility tranches (provided there are no more than three such tranches with different maturity dates outstanding at any time) in an aggregate amount not to exceed (a) $150.0 million plus (b) an unlimited amount at any time, subject to compliance on a pro forma basis with a senior secured first lien net leverage ratio of no greater than 3.75:1.00 (with amounts alternatively consisting of debt securities and debt that is secured on a junior lien basis to the liens securing the Senior Credit Facilities or is unsecured subject to compliance with a senior secured net leverage ratio of no greater than 4.00:1.00). Availability of such additional tranches of term loans or revolving credit facilities and/or increased commitments is subject to, among other conditions, the absence of any default under the credit agreement governing our Senior Credit Facilities and the receipt of commitments by existing or additional financial institutions.
Interest rate and fees
Borrowings under our Senior Credit Facilities bear interest at a rate per annum equal to an applicable margin plus, at our option, either (a) in the case of borrowings in U.S. dollars, a base rate determined by reference to the highest of (1) the prime rate of Citibank, N.A., (2) the federal funds effective rate plus 0.50% and (3) a LIBOR rate determined by reference to the costs of funds for U.S. dollar deposits for an interest period of one month adjusted for certain additional costs, plus 1.00% or (b) in the case of borrowings in U.S. dollars or any other currency, a LIBOR rate determined by reference to the costs of funds for U.S. dollar deposits for the interest period relevant to such borrowing adjusted for certain additional costs, which, in the case of the Term Loan Facility only, shall be no less than 1.50%. The applicable margin for borrowings under our Senior Credit Facilities is 4.75% with respect to base rate borrowings and 5.75% with respect to LIBOR borrowings. The applicable margin for borrowings under the Revolving Facility is subject to a 0.25% step-down, when our senior secured net leverage ratio at the end of a fiscal quarter is less than or equal to 3.00:1.00.
In addition to paying interest on outstanding principal under our Senior Credit Facilities, we must pay customary agency fees and a commitment fee in respect of the unutilized commitments under the Revolving Facility, which commitment fee is currently 0.50% per annum. The commitment fee is subject to a 0.25% step-down, when our senior secured net leverage ratio at the end of a fiscal quarter is less than or equal to 3.00:1.00.
102
Table of Contents
Mandatory prepayments
The credit agreement governing our Senior Credit Facilities requires us to prepay, subject to certain exceptions, outstanding term loans with:
| • | 50% (subject to step-downs to 25% and 0% based upon our senior secured net leverage ratio) of our annual excess cash flow; |
| • | 100% of the net cash proceeds of certain asset sales and casualty and condemnation events, subject to reinvestment rights and certain other exceptions; and |
| • | 100% of the net cash proceeds of any incurrence or issuance of certain debt, other than the net cash proceeds of the notes offered hereby and other debt permitted under our Senior Credit Facilities. |
Voluntary prepayments
Voluntary prepayments of the Term Loan Facility in connection with repricing transactions on or prior to the first anniversary of the closing date are subject to a call premium of 1.0%. Otherwise, we are able to voluntarily prepay outstanding loans under our Senior Credit Facilities at any time without premium or penalty other than customary “breakage” costs with respect to LIBOR loans.
Amortization and final maturity
Beginning December 31, 2011, we are required to make scheduled quarterly payments each equal to 0.25% of the original principal amount of the term loans made on the closing date, with the balance due on the seventh anniversary of the closing date. There is not a scheduled amortization under our Revolving Facility. Any principal amount outstanding under our Revolving Facility is due and payable in full on the fifth anniversary of the closing date.
Guarantees and security
All obligations under our Senior Credit Facilities are unconditionally guaranteed by our immediate parent and certain of our existing direct or indirect wholly owned domestic subsidiaries, and will be required to be guaranteed by certain of our future direct or indirect wholly owned domestic subsidiaries. All obligations under our Senior Credit Facilities, and the guarantees of those obligations, are secured, subject to certain exceptions, by substantially all of our assets and the assets of our immediate parent and our subsidiary guarantors, including:
| • | a first-priority pledge of all of our capital stock directly held by our parent and a first-priority pledge of all of the capital stock directly held by us and our subsidiary guarantors (which pledge, in the case of (a) each domestic subsidiary that is directly owned by us or our subsidiary guarantors and that is a disregarded entity for United States federal income tax purposes and that has no material assets other than equity interests in one or more foreign subsidiaries that are controlled foreign corporations for United States federal income tax purposes or (b) foreign subsidiary is limited to 65% of the stock of such subsidiary); and |
| • | a first-priority security interest in substantially all of our parent’s, our and the subsidiary guarantor’s tangible and intangible assets. |
Certain covenants and events of default
Our Senior Credit Facilities contain a number of covenants that, among other things and subject to certain exceptions, restrict our ability and the ability of our subsidiaries to:
| • | incur additional indebtedness; |
| • | pay dividends on our capital stock or redeem, repurchase or retire our capital stock or our other indebtedness; |
103
Table of Contents
| • | make investments, loans and acquisitions; |
| • | create restrictions on the payment of dividends or other amounts to us from our restricted subsidiaries; |
| • | engage in transactions with our affiliates; |
| • | sell assets, including capital stock of our subsidiaries; |
| • | materially alter the business we conduct; |
| • | consolidate or merge; |
| • | incur liens; and |
| • | prepay or amend subordinated or unsecured debt. |
Although our immediate parent is not generally subject to the negative covenants under the Senior Credit Facilities, such parent is subject to a passive holding company covenant that limits its ability to engage in certain activities.
In addition, the credit agreement governing our Senior Credit Facilities requires us to comply with a maximum senior secured net leverage ratio financial maintenance covenant, to be tested on the last day of each fiscal quarter. The breach of this covenant is subject to certain equity cure rights.
The credit agreement governing our Senior Credit Facilities also contains certain customary representations and warranties, affirmative covenants and provisions relating to events of default (including upon a change of control).
104
Table of Contents
General
Certain terms used in this description are defined under the subheading “—Certain definitions.” In this description, (1) the term “Issuer” refers only Immucor and not to any of its subsidiaries, and (2) the terms “we,” “our” and “us” each refer to the Issuer and its consolidated Subsidiaries.
The Issuer issued $400.0 million in aggregate principal amount of 11.125% senior notes due 2019 (the “outstanding notes”) under an indenture dated as of August 19, 2011 (the “Indenture”) among the Issuer, the Guarantor and Deutsche Bank Trust Company Americas, as trustee (the “Trustee”). The outstanding notes were issued in a private transaction that was not subject to the registration requirements of the Securities Act. In this section, we refer to the exchange notes together with the outstanding notes as the “Notes”. Except as set forth herein, the terms of the Notes are substantially identical and include those stated in the Indenture and those made part of the Indenture by reference to the Trust Indenture Act.
The following description is only a summary of the material provisions of the Indenture. It does not purport to be complete and is qualified in its entirety by reference to the provisions of the Indenture, including the definitions therein of certain terms used below. We urge you to read the Indenture because it, and not this description, will define your rights as Holders of the Notes. You may request copies of the Indenture at our address set forth under the subheading “Available information.”
Exchange notes versus outstanding notes
The terms of the exchange notes are substantially identical to the outstanding notes except that the exchange notes will be registered under the Securities Act and will be free of any covenants regarding exchange registration rights.
Brief description of the notes
The Notes:
| • | are general, unsecured, senior obligations of the Issuer; |
| • | rank equally in right of payment with all existing and future Senior Indebtedness (including the Senior Credit Facilities) of the Issuer; |
| • | are effectively subordinated to all Secured Indebtedness of the Issuer (including the Senior Credit Facilities), to the extent of the value of the collateral securing such Secured Indebtedness; |
| • | are structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of Subsidiaries of the Issuer that do not guarantee the Notes; |
| • | are senior in right of payment to all future Subordinated Indebtedness of the Issuer; |
| • | are initially guaranteed on a senior unsecured basis by a Guarantor and will also be guaranteed in the future by each wholly-owned domestic Restricted Subsidiary of the Issuer, if any, subject to certain exceptions, that guarantees Indebtedness of the Issuer or any other Guarantor, including Indebtedness under the Senior Credit Facilities; and |
Guarantees
Our sole domestic subsidiary, BioArray Solutions, Ltd., as primary obligor and not merely as a surety, has, irrevocably and unconditionally, guaranteed, on an unsecured senior basis, the full and punctual payment when due, whether at maturity, by acceleration or otherwise, of all obligations of the Issuer under the Indenture and the Notes, whether for payment of principal of, premium, if any, or interest in respect of the Notes, expenses, indemnification or otherwise, on the terms set forth in the Indenture by executing the Indenture.
105
Table of Contents
In the future, subject to exceptions set forth under the caption “Certain covenants—Limitation on guarantees of indebtedness by restricted subsidiaries,” each direct and indirect domestic Wholly-Owned Subsidiary that is a Restricted Subsidiary of the Issuer that guarantees any Indebtedness of the Issuer or any other Guarantor will guarantee the Notes, subject to release as provided below or elsewhere in this “Description of exchange notes.” Each of the Guarantees of the Notes is a general, unsecured, senior obligation of each Guarantor, ranks equally in right of payment with all existing and future Senior Indebtedness of such Guarantor (including such Guarantor’s guarantee of the Senior Credit Facilities), is effectively subordinated to all Secured Indebtedness of such Guarantor (including such Guarantor’s guarantee of the Senior Credit Facilities) to the extent of the value of the collateral of such Guarantor securing such Secured Indebtedness, and ranks senior in right of payment to all future Subordinated Indebtedness of such Guarantor. Each of the Guarantees of the Notes is structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of Subsidiaries of each Guarantor that do not Guarantee the Notes.
Not all of the Issuer’s Subsidiaries have guaranteed the Notes. In the event of a bankruptcy, liquidation, reorganization or similar proceeding of any of these non-guarantor Subsidiaries, the non-guarantor Subsidiaries will pay the holders of their debt and their trade creditors before they will be able to distribute any of their assets to the Issuer. As a result, all of the existing and future liabilities of our non-guarantor Subsidiaries, including any claims of trade creditors, will be effectively senior to the Notes. The Indenture does not limit the amount of liabilities that are not considered Indebtedness that may be incurred by the Issuer or its Restricted Subsidiaries, including the non-guarantor Subsidiaries.
The obligations of each Guarantor under its Guarantee are limited as necessary to prevent the Guarantee from constituting a fraudulent conveyance under applicable law. This provision may not, however, be effective to protect a Guarantee from being voided under fraudulent transfer law, or may reduce the applicable Guarantor’s obligation to an amount that effectively makes its Guarantee worthless. If a Guarantee was rendered voidable, it could be subordinated by a court to all other indebtedness (including guarantees and other contingent liabilities) of the Guarantor, and, depending on the amount of such indebtedness, a Guarantor’s liability on its Guarantee could be reduced to zero. See “Risk factors—Risks related to our indebtedness and the exchange notes—Federal and state fraudulent transfer laws may permit a court to void the exchange notes and/or the guarantees, and if that occurs, you may not receive any payments on the exchange notes.”
Any Guarantor that makes a payment under its Guarantee will be entitled upon payment in full of all guaranteed obligations under the Indenture to a contribution from each other Guarantor in an amount equal to such other Guarantor’s pro rata portion of such payment based on the respective net assets of all the Guarantors at the time of such payment determined in accordance with GAAP.
Each Guarantor may consolidate with or merge with or into or sell all or substantially all its assets to the Issuer or another Guarantor without limitation or any other Person upon the terms and conditions set forth in the Indenture. See “—Certain covenants—Merger, consolidation or sale of all or substantially all assets.”
Each Guarantee by a Guarantor provides by its terms that it will be automatically and unconditionally released and discharged upon:
(1) (a) any sale, exchange or transfer (by merger or otherwise) of (i) the Capital Stock of such Guarantor, after which the applicable Guarantor is no longer a Restricted Subsidiary or (ii) all or substantially all the assets of such Guarantor, in each case if such sale, exchange or transfer is made in compliance with clauses (1) and (2) of the first paragraph under “—Repurchase at the option of holders—Asset sales”;
(b) the release or discharge of the guarantee by such Guarantor of Indebtedness under the Senior Credit Facilities, or the release or discharge of such other guarantee that resulted in the creation of such Guarantee, except a discharge or release by or as a result of payment under such guarantee (it being understood that a release subject to a contingent reinstatement is still a release, and that if any such
106
Table of Contents
guarantee is so reinstated, such Guarantee shall also be reinstated to the extent that such Guarantor would then be required to provide a Guarantee pursuant to the covenant described under “—Certain covenants—Limitation on guarantees of indebtedness by restricted subsidiaries”);
(c) the designation of any Restricted Subsidiary that is a Guarantor as an Unrestricted Subsidiary in compliance with the applicable provisions of the Indenture; or
(d) the exercise by the Issuer of its legal defeasance option or covenant defeasance option as described under “Legal defeasance and covenant defeasance” or the discharge of the Issuer’s obligations under the Indenture in accordance with the terms of the Indenture; and
(2) such Guarantor delivering to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for in the Indenture relating to such transaction have been complied with.
Ranking
The payment of the principal of, premium, if any, and interest on the Notes and the payment of any Guarantee ranks equally in right of payment to all existing and future Senior Indebtedness of the Issuer or the relevant Guarantor, as the case may be, including the obligations of the Issuer and such Guarantor under the Senior Credit Facilities.
The Notes and the Guarantees are effectively subordinated in right of payment to all of the Issuer’s and the Guarantors’ existing and future Secured Indebtedness to the extent of the value of the collateral securing such Secured Indebtedness. As of August 31, 2011, the Issuer and the Guarantors had $615 million of Secured Indebtedness outstanding, consisting of borrowings and the related guarantees under the Senior Credit Facilities. As of August 31, 2011, the Issuer also had (1) $100.0 million available for borrowing under the revolving credit facility included in the Senior Credit Facilities, which, if borrowed, would be Secured Indebtedness and (2) the option to raise additional tranches of term loans and/or increase commitments under the revolving credit facility and/or the term loan facility and/or add one or more incremental revolving credit facility tranches under the Senior Credit Facilities up to the sum of (a) $150.0 million and (b) if our Senior Secured First Lien Net Leverage Ratio (as defined in the credit agreement governing our Senior Credit Facilities) would be equal to or less than 3.75 to 1.00 on a pro forma basis (with amounts alternatively consisting of debt secured on a junior lien basis to the Liens securing the Senior Credit Facilities, subject to compliance with a Senior Secured Net Leverage Ratio (as defined in the credit agreement governing our Senior Credit Facilities) of no greater than 4.00:1.00), an unlimited amount, which, if borrowed, would be Secured Indebtedness.
Although the Indenture contains limitations on the principal amount of additional Indebtedness that the Issuer and the Issuer’s Restricted Subsidiaries (including the Guarantors) may incur, under certain circumstances the amount of such Indebtedness could be substantial and, in any case, such Indebtedness may be Secured Indebtedness or Senior Indebtedness. The Indenture does not limit the amount of additional Indebtedness that any direct or indirect parent company of the Issuer may incur. See “—Certain covenants—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock.”
Paying agent and registrar for the notes
The Issuer maintains one or more paying agents for the Notes. The initial paying agent for the Notes is the Trustee.
The Issuer also maintains one or more registrars and a transfer agent. The initial registrar and transfer agent with respect to the Notes is the Trustee. The registrar maintains a register reflecting ownership of the Notes outstanding from time to time. The registered Holder of a Note is treated as the owner of the Note for all purposes. Only registered Holders of Notes will have rights under the Indenture. The paying agent makes payments on the Notes and the transfer agent facilitates the transfer of Notes on behalf of the Issuer.
107
Table of Contents
The Issuer may change the paying agent, the registrar or the transfer agent without prior notice to the Holders. The Issuer or any of its Subsidiaries may act as a paying agent, registrar or transfer agent.
If any Notes are listed on an exchange and the rules of such exchange so require, the Issuer satisfies any requirement of such exchange as to paying agents, registrars and transfer agents and will comply with any notice requirements required under such exchange in connection with any change of paying agent, registrar or transfer agent.
Transfer and exchange
A Holder may transfer or exchange Notes in accordance with the Indenture. The registrar and the Trustee may require a Holder to furnish appropriate endorsements and transfer documents in connection with a transfer of Notes. Holders are required to pay all taxes due on transfer. The Issuer is not required to transfer or exchange any Note selected for redemption or tendered (and not withdrawn) for repurchase in connection with a Change of Control Offer or an Asset Sale Offer. Also, the Issuer is not required to transfer or exchange any Note for a period of 15 days before the mailing of a notice of redemption of Notes to be redeemed.
Principal, maturity and interest
The Issuer issued $400.0 million in aggregate principal amount of Notes in connection with the Acquisition. The Notes will mature on August 15, 2019. Subject to compliance with the covenant described below under “Certain covenants—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock,” the Issuer may issue additional Notes from time to time under the Indenture (“Additional Notes”). The Notes offered by the Issuer and any Additional Notes subsequently issued under the Indenture will be treated as a single class for all purposes under the Indenture, including waivers, amendments, redemptions and offers to purchase, except for certain waivers and amendments. Unless the context requires otherwise, references to “Notes” for all purposes of the Indenture and this “Description of exchange notes” include any Additional Notes that are actually issued. The Notes are issued in denominations of $2,000 and any integral multiples of $1,000 in excess of $2,000.
Interest on the Notes accrues at the rate of 11.125% per annum and is payable semi-annually in arrears on each February 15 and August 15, commencing February 15, 2012, to the Holders of Notes of record on the immediately preceding February 1 and August 1. Interest on the exchange notes accrues from the most recent date to which interest has been paid with respect to the outstanding notes or, if no interest has been paid with respect to such notes, from and including the Issue Date. Holders whose outstanding notes are accepted for exchange in the exchange offer will be deemed to have waived the right to receive interest accrued on the outstanding notes. Interest on the Notes is computed on the basis of a 360-day year comprised of twelve 30-day months.
Payment of principal, premium and interest
Cash payments of principal of, premium, if any, and interest on the Notes are payable at the office or agency of the Issuer maintained for such purpose or, at the option of the Issuer, cash payment of interest may be made by check mailed to the Holders of the Notes at their respective addresses set forth in the register of Holders; provided that (1) all cash payments of principal, premium, if any, and interest with respect to the Notes represented by one or more global notes registered in the name of or held by The Depository Trust Company (“DTC”) or its nominee will be made by wire transfer of immediately available funds to the accounts specified by the registered Holder or Holders thereof and (2) all cash payments of principal, premium, if any, and interest with respect to certificated Notes will be made by wire transfer to a U.S. dollar account maintained by the payee with a bank in the United States if such Holder elects payment by wire transfer by giving written notice to the Trustee or the paying agent to such effect designating such account no later than 30 days immediately preceding the relevant due date for payment (or such other date as the Trustee may accept in its discretion). Until otherwise designated by the Issuer, the Issuer’s office or agency is the office of the Trustee maintained for such purpose.
108
Table of Contents
Mandatory redemption; offers to purchase; open market purchases
Except as set forth below in the next succeeding paragraph, the Issuer is not required to make any mandatory redemption or sinking fund payments with respect to the Notes. However, under certain circumstances, the Issuer may be required to offer to purchase Notes as described under “—Repurchase at the option of holders.”
If the Notes would otherwise constitute “applicable high yield discount obligations” (“AHYDOs”) within the meaning of Section 163(i)(1) of the Internal Revenue Code of 1986, as amended (the “Code”), on or before the end of the first “accrual period” ending after the fifth anniversary of the “date of issue” (each within the meaning of Section 163(i)(2) of the Code) and prior to the end of each subsequent accrual period (the date of each such payment, an “AHYDO Payment Date”), the Issuer shall redeem a portion of each Note in an amount equal to the AHYDO Amount at a redemption price equal to 100% of the principal amount of the portion of each such Note being redeemed, plus accrued and unpaid interest, if any, through the date of redemption. For purposes of the foregoing, “AHYDO Amount” means, as of each AHYDO Payment Date, the amount (which may be zero) sufficient to ensure that the Notes will not be treated as an AHYDO. The AHYDO Amount shall be calculated by the Issuer. Each payment of the AHYDO Amount shall be treated for tax purposes as a payment of original issue discount on such Notes to the extent of the original issue discount that has accrued as of the date such payment is due and then as a payment of principal. It is the intention of the foregoing that the Notes will not be an AHYDO within the meaning of Section 163(i)(1) of the Code.
The Issuer or its Affiliates may at any time and from time to time purchase Notes in the open market or otherwise.
Optional redemption
At any time prior to August 15, 2014, the Issuer may, at its option and on one or more occasions, redeem up to 35.0% of the aggregate principal amount of Notes and Additional Notes issued under the Indenture at a redemption price equal to 100.0% of the aggregate principal amount thereof, plus a premium equal to the stated interest rate per annum on the Notes, plus accrued and unpaid interest, if any, to the Redemption Date, subject to the right of Holders of Notes of record on the relevant record date to receive interest due on the relevant interest payment date, with the net cash proceeds received by it from one or more Equity Offerings or a contribution to the Issuer’s common equity capital made with the net cash proceeds of a concurrent Equity Offering; provided that (a) at least 65.0% of the aggregate principal amount of Notes originally issued under the Indenture on the Issue Date and any Additional Notes issued under the Indenture after the Issue Date (excluding Notes held by the Issuer and its Subsidiaries) remains outstanding immediately after the occurrence of each such redemption; and (b) each such redemption occurs within 90 days of the date of closing of each such Equity Offering.
Notice of any redemption upon any Equity Offering may be given prior to the completion thereof, and any such redemption or notice may, at the Issuer’s discretion, be subject to one or more conditions precedent, including, but not limited to, completion of the related Equity Offering. If any Notes are listed on an exchange, and the rules of such exchange so require, the Issuer will notify the exchange of any such notice of redemption. In addition, the Issuer will notify the exchange of the principal amount of any Notes outstanding following any partial redemption of Notes.
In addition, at any time prior to August 15, 2015, the Issuer may at its option on one or more occasions redeem all or a part of the Notes, upon notice as described under “Selection and notice,” at a redemption price equal to the sum of (i) 100.0% of the principal amount of the Notes redeemed, plus (ii) the Applicable Premium as of the date of redemption plus (iii) accrued and unpaid interest and Additional Interest, if any, to the date of redemption (the “Redemption Date”), subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date.
Except pursuant to the preceding paragraphs, the Notes will not be redeemable at the Issuer’s option prior to August 15, 2015.
109
Table of Contents
On and after August 15, 2015, the Issuer may at its option redeem the Notes, in whole or in part, on one or more occasions, upon notice as described under “Selection and notice,” at the redemption prices (expressed as percentages of principal amount of the Notes to be redeemed) set forth below, plus accrued and unpaid interest, if any, to the Redemption Date, subject to the right of Holders of record on the relevant record date to receive interest due on the relevant interest payment date, if redeemed during the twelve-month period beginning on August 15 of each of the years indicated below:
| Year |
Percentage | |||
| 2015 |
105.563 | % | ||
| 2016 |
102.781 | % | ||
| 2017 and thereafter |
100.000 | % | ||
Selection and notice
If the Issuer is redeeming less than all of the Notes issued under the Indenture at any time, the Trustee will select the Notes to be redeemed (1) if the Notes are listed on an exchange, in compliance with the requirements of such exchange or (2) on a pro rata basis to the extent practicable, or, if the pro rata basis is not practicable for any reason, by lot or by such other method as the Trustee shall deem fair and appropriate in accordance with DTC procedures. Other than with respect to the payment of an AHYDO Amount (as described above under the subheading “—Mandatory redemption; offers to purchase; open market purchases”), no Notes of $2,000 or less can be redeemed in part.
Notices of redemption shall be delivered electronically or mailed by first-class mail, postage prepaid, at least 30 but not more than 60 days before the redemption date to each Holder of Notes at such Holder’s registered address or otherwise in accordance with the procedures of DTC, except that redemption notices may be delivered more than 60 days prior to a redemption date if the notice is issued in connection with a defeasance of the Notes or a satisfaction and discharge of the Indenture. If any Note is to be redeemed in part only, any notice of redemption that relates to such Note shall state the portion of the principal amount thereof that has been or is to be redeemed.
With respect to Notes represented by certificated notes, the Issuer will issue a new Note in a principal amount equal to the unredeemed portion of the original Note in the name of the Holder upon cancellation of the original Note; provided that new Notes will only be issued in denominations of $2,000 and integral multiples of $1,000 in excess of $2,000. Notes called for redemption become due on the date fixed for redemption. On and after the Redemption Date, interest ceases to accrue on the Notes or portions of them called for redemption.
Repurchase at the option of holders
Change of control
The Indenture provides that if a Change of Control occurs, unless the Issuer has previously or concurrently delivered a redemption notice with respect to all the outstanding Notes as described under “Optional redemption,” the Issuer will make an offer to purchase all of the Notes pursuant to the offer described below (the “Change of Control Offer”) at a price in cash (the “Change of Control Payment”) equal to 101.0% of the aggregate principal amount thereof plus accrued and unpaid interest, if any, to the date of repurchase, subject to the right of Holders of the Notes of record on the relevant record date to receive interest due on the relevant interest payment date. Within 30 days following any Change of Control, the Issuer will deliver notice of such Change of Control Offer electronically or by first-class mail, with a copy to the Trustee, to each Holder of Notes to the address of such Holder appearing in the security register or otherwise in accordance with the procedures of DTC with the following information:
(1) that a Change of Control Offer is being made pursuant to the covenant entitled “Change of control” and that all Notes properly tendered pursuant to such Change of Control Offer will be accepted for payment by the Issuer;
110
Table of Contents
(2) the purchase price and the purchase date, which will be no earlier than 30 days nor later than 60 days from the date such notice is delivered (the “Change of Control Payment Date”);
(3) that any Note not properly tendered will remain outstanding and continue to accrue interest;
(4) that unless the Issuer defaults in the payment of the Change of Control Payment, all Notes accepted for payment pursuant to the Change of Control Offer will cease to accrue interest on the Change of Control Payment Date;
(5) that Holders electing to have any Notes purchased pursuant to a Change of Control Offer will be required to surrender such Notes, with the form entitled “Option of Holder to Elect Purchase” on the reverse of such Notes completed, to the paying agent specified in the notice at the address specified in the notice prior to the close of business on the third Business Day preceding the Change of Control Payment Date;
(6) that Holders will be entitled to withdraw their tendered Notes and their election to require the Issuer to purchase such Notes; provided that the paying agent receives, not later than the close of business on the second Business Day prior to the expiration date of the Change of Control Offer, a facsimile transmission or letter setting forth the name of the Holder of the Notes, the principal amount of Notes tendered for purchase, and a statement that such Holder is withdrawing its tendered Notes and its election to have such Notes purchased;
(7) that Holders whose Notes are being purchased only in part will be issued new Notes and such new Notes will be equal in principal amount to the unpurchased portion of the Notes surrendered. The unpurchased portion of the Notes must be equal to at least $2,000 or any integral multiple of $1,000 in excess of $2,000;
(8) if such notice is delivered prior to the occurrence of a Change of Control, stating that the Change of Control Offer is conditional on the occurrence of such Change of Control; and
(9) the other instructions, as determined by the Issuer, consistent with the covenant described hereunder, that a Holder must follow in order to have its Notes repurchased.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of Notes pursuant to a Change of Control Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Indenture, the Issuer will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations described in the Indenture by virtue thereof.
On the Change of Control Payment Date, the Issuer will, to the extent permitted by law:
(1) accept for payment all Notes issued by it or portions thereof properly tendered pursuant to the Change of Control Offer;
(2) deposit with the paying agent an amount equal to the aggregate Change of Control Payment in respect of all Notes or portions thereof so tendered; and
(3) deliver, or cause to be delivered, to the Trustee for cancellation the Notes so accepted together with an Officer’s Certificate to the Trustee stating that such Notes or portions thereof have been tendered to and purchased by the Issuer.
The Senior Credit Facilities do, and future credit agreements or other agreements relating to Senior Indebtedness to which the Issuer becomes a party may, provide that certain change of control events with respect to the Issuer would constitute a default thereunder (including a Change of Control under the Indenture). If we experience a change of control that triggers a default thereunder, we could seek a waiver of such default or seek to refinance such Indebtedness. However, in the event we do not obtain such a waiver or do not refinance such Indebtedness, such default could result in amounts outstanding under such Indebtedness being declared due and payable.
111
Table of Contents
Our ability to pay cash to the Holders of Notes following the occurrence of a Change of Control may be limited by our then-existing financial resources. Therefore, sufficient funds may not be available when necessary to make any required repurchases.
The Change of Control purchase feature of the Notes may in certain circumstances make more difficult or discourage a sale or takeover of us and, thus, the removal of incumbent management. The Change of Control purchase feature is a result of negotiations between the Initial Purchasers and us. We have no present intention to engage in a transaction involving a Change of Control, although it is possible that we could decide to do so in the future. Subject to the limitations discussed below, we could, in the future, enter into certain transactions, including acquisitions, refinancings or other recapitalizations, that would not constitute a Change of Control under the Indenture, but that could increase the amount of Indebtedness outstanding at such time or otherwise affect our capital structure or credit ratings. Restrictions on our ability to incur additional Indebtedness are contained in the covenants described under “—Certain covenants—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock” and “—Certain covenants—Liens.” Such restrictions in the Indenture can be waived only with the consent of the Holders of a majority in principal amount of the Notes then outstanding. Except for the limitations contained in such covenants, however, the Indenture does not contain any covenants or provisions that may afford Holders of the Notes protection in the event of a highly leveraged transaction.
The Issuer is not required to make a Change of Control Offer following a Change of Control if a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the Indenture applicable to a Change of Control Offer made by the Issuer and purchases all Notes validly tendered and not withdrawn under such Change of Control Offer.
Notwithstanding anything to the contrary herein, a Change of Control Offer may be made in advance of a Change of Control, conditional upon such Change of Control, if a definitive agreement is in place for the Change of Control at the time of making of the Change of Control Offer.
The definition of “Change of Control” includes a disposition of all or substantially all of the assets of the Issuer and its Subsidiaries, taken as a whole, to any Person. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise, established definition of the phrase under applicable law. Accordingly, in certain circumstances there may be a degree of uncertainty as to whether a particular transaction would involve a disposition of “all or substantially all” of the assets of the Issuer and its Subsidiaries, taken as a whole. As a result, it may be unclear as to whether a Change of Control has occurred and whether a Holder of Notes may require the Issuer to make an offer to repurchase the Notes as described above.
The provisions under the Indenture relative to the Issuer’s obligation to make an offer to repurchase the Notes as a result of a Change of Control may be waived or modified with the written consent of the Holders of a majority in principal amount of the Notes then outstanding.
Asset sales
The Indenture provides that the Issuer will not, and will not permit any of its Restricted Subsidiaries to, consummate directly or indirectly an Asset Sale, unless:
(1) the Issuer or such Restricted Subsidiary, as the case may be, receives consideration at the time of such Asset Sale at least equal to the fair market value of the assets sold or otherwise disposed of; and
(2) except in the case of a Permitted Asset Swap, at least 75.0% of the consideration therefor received by the Issuer or such Restricted Subsidiary, as the case may be, is in the form of Cash Equivalents; provided that the amount of:
(a) any liabilities (as shown on the Issuer’s or such Restricted Subsidiary’s most recent balance sheet or in the footnotes thereto) of the Issuer or such Restricted Subsidiary, other than liabilities that are by their terms subordinated to the Notes or any Guarantor’s Guarantee of the Notes, that are assumed by
112
Table of Contents
the transferee of any such assets and for which the Issuer and all of its Restricted Subsidiaries have been validly released by all creditors in writing;
(b) any securities, notes or other obligations or assets received by the Issuer or such Restricted Subsidiary from such transferee that are converted by the Issuer or such Restricted Subsidiary into Cash Equivalents (to the extent of the Cash Equivalents received) within 180 days following the closing of such Asset Sale; and
(c) any Designated Non-cash Consideration received by the Issuer or such Restricted Subsidiary in such Asset Sale having an aggregate fair market value, taken together with all other Designated Non-cash Consideration received pursuant to this clause (c) that is at that time outstanding, not to exceed the greater of (x) $35.0 million and (y) 2.5% of Total Assets at the time of the receipt of such Designated Non-cash Consideration, with the fair market value of each item of Designated Non-cash Consideration being measured at the time received and without giving effect to subsequent changes in value,
shall be deemed to be Cash Equivalents for purposes of this provision and for no other purpose.
Within 365 days after the receipt of any Net Proceeds of any Asset Sale, the Issuer or such Restricted Subsidiary, at its option, may apply the Net Proceeds from such Asset Sale:
(1) to permanently reduce:
(a) Obligations under the Senior Credit Facilities, and to correspondingly reduce commitments with respect thereto;
(b) Obligations under Secured Indebtedness of the Issuer or a Restricted Subsidiary, other than Indebtedness owed to the Issuer or a Restricted Subsidiary, which is secured by a Lien that is permitted by the Indenture, and to correspondingly reduce commitments with respect thereto;
(c) Obligations under other Senior Indebtedness of the Issuer or a Restricted Subsidiary, other than Indebtedness owed to the Issuer or a Restricted Subsidiary (and to correspondingly reduce commitments with respect thereto); provided that the Issuer shall equally and ratably reduce Obligations under the Notes as provided under “Optional Redemption,” or through open-market purchases (to the extent such purchases are at or above 100.0% of the principal amount thereof) or by making an offer (in accordance with the procedures set forth below for an Asset Sale Offer) to all Holders to purchase their Notes at 100.0% of the principal amount thereof, plus the amount of accrued but unpaid interest, if any, on the amount of Notes to be repurchased, to the date of repurchase; or
(d) Indebtedness of a Restricted Subsidiary that is not a Guarantor, other than Indebtedness owed to the Issuer or another Restricted Subsidiary;
(2) to make (a) an Investment in any one or more businesses; provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Issuer or any of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) capital expenditures, (c) properties or (d) acquisitions of other assets, in each of (a), (b), (c) and (d), used or useful in a Similar Business; or
(3) to make an Investment in (a) any one or more businesses; provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Issuer or any of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) properties or (c) acquisitions of other assets that, in each of (a), (b) and (c), replace the businesses, properties and/or assets that are the subject of such Asset Sale;
provided that, in the case of clauses (2) and (3) above, a binding commitment shall be treated as a permitted application of the Net Proceeds from the date of such commitment so long as the Issuer or such other Restricted Subsidiary enters into such commitment with the good faith expectation that such Net Proceeds will be applied to
113
Table of Contents
satisfy such commitment within 180 days of such commitment (an “Acceptable Commitment”) and, in the event that any Acceptable Commitment is later cancelled or terminated for any reason before the Net Proceeds are applied in connection therewith, the Issuer or such Restricted Subsidiary enters into another Acceptable Commitment (a “Second Commitment”) within 180 days of such cancellation or termination; provided, further, that if any Second Commitment is later cancelled or terminated for any reason before such Net Proceeds are applied, then such Net Proceeds shall constitute Excess Proceeds.
Any Net Proceeds from the Asset Sale that are not invested or applied as provided and within the time period set forth in the preceding paragraph will be deemed to constitute “Excess Proceeds.” When the aggregate amount of Excess Proceeds exceeds $25.0 million, the Issuer shall make an offer to all Holders of the Notes and, if required by the terms of any Indebtedness that is pari passu with the Notes (“Pari Passu Indebtedness”), to the holders of such Pari Passu Indebtedness (an “Asset Sale Offer”), to purchase the maximum aggregate principal amount of the Notes and such Pari Passu Indebtedness that is in an amount equal to at least $2,000, that may be purchased out of the Excess Proceeds at an offer price in cash in an amount equal to 100.0% of the principal amount thereof (or accreted value thereof, if less), plus accrued and unpaid interest, if any, to the date fixed for the closing of such offer, in accordance with the procedures set forth in the Indenture. The Issuer will commence an Asset Sale Offer with respect to Excess Proceeds within ten Business Days after the date that Excess Proceeds exceed $25.0 million by delivering the notice required pursuant to the terms of the Indenture, with a copy to the Trustee or otherwise in accordance with the procedures of DTC. The Issuer may satisfy the foregoing obligations with respect to any Net Proceeds from an Asset Sale by making an Asset Sale Offer with respect to such Net Proceeds prior to the expiration of the relevant 365 days (or such longer period provided above) or with respect to Excess Proceeds of $25.0 million or less.
To the extent that the aggregate amount of Notes and such Pari Passu Indebtedness tendered pursuant to an Asset Sale Offer is less than the Excess Proceeds, the Issuer may use any remaining Excess Proceeds for general corporate purposes, subject to other covenants contained in the Indenture. If the aggregate principal amount of Notes or the Pari Passu Indebtedness surrendered by such holders thereof exceeds the amount of Excess Proceeds, the Trustee shall select the Notes, and the Issuer shall select such Pari Passu Indebtedness to be purchased on a pro rata basis based on the accreted value or principal amount of the Notes or such Pari Passu Indebtedness tendered with adjustments as necessary so that no Notes or Pari Passu Indebtedness will be repurchased in part in an unauthorized denomination. Upon completion of any such Asset Sale Offer, the amount of Excess Proceeds that resulted in the Asset Sale Offer shall be reset to zero (regardless of whether there are any remaining Excess Proceeds upon such completion).
Pending the final application of any Net Proceeds pursuant to this covenant, the holder of such Net Proceeds may apply such Net Proceeds temporarily to reduce Indebtedness outstanding under a revolving credit facility, including under the Senior Credit Facilities, or otherwise invest such Net Proceeds in any manner not prohibited by the Indenture.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of the Notes pursuant to an Asset Sale Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Indenture, the Issuer will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations described in the Indenture by virtue thereof.
The provisions under the Indenture relative to the Issuer’s obligation to make an offer to repurchase the Notes as a result of an Asset Sale may be waived or modified with the written consent of the Holders of a majority in principal amount of the then outstanding Notes.
Future credit agreements or other similar agreements to which the Issuer becomes a party may contain restrictions on the Issuer’s ability to repurchase Notes. In the event an Asset Sale occurs at a time when the Issuer
114
Table of Contents
is prohibited from purchasing Notes, the Issuer could seek the consent of its lenders to the repurchase of Notes or could attempt to refinance the borrowings that contain such prohibition. If the Issuer does not obtain such consent or repay such borrowings, the Issuer will remain prohibited from repurchasing Notes. In such a case, the Issuer’s failure to repurchase tendered Notes would constitute an Event of Default under the Indenture which would, in turn, likely constitute a default under such other agreements.
Certain covenants
Set forth below are summaries of certain covenants that are contained in the Indenture.
Changes in covenants when notes rated investment grade
During any period of time that (i) the Notes have Investment Grade Ratings from both Rating Agencies and (ii) no Default has occurred and is continuing under the Indenture (the occurrence of the events described in the foregoing clauses (i) and (ii) being collectively referred to as a “Covenant Suspension Event” and the date thereof being referred to as the “Suspension Date”) then, the covenants specifically listed under the following captions in this “Description of exchange notes” section of this prospectus will not be applicable to the Notes (collectively, the “Suspended Covenants”):
| (1) | “—Repurchase at the option of holders—Asset sales”; |
| (2) | “—Limitation on restricted payments”; |
| (3) | “—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”; |
| (4) | clause (4) of the first paragraph of “—Merger, consolidation or sale of all or substantially all assets”; |
| (5) | “—Transactions with affiliates”; |
| (6) | “—Dividend and other payment restrictions affecting restricted subsidiaries”; and |
| (7) | “—Limitation on guarantees of indebtedness by restricted subsidiaries.” |
During any period that the foregoing covenants have been suspended, the Issuer may not designate any of its Subsidiaries as Unrestricted Subsidiaries pursuant to the second sentence of the definition of “Unrestricted Subsidiary.”
If and while the Issuer and its Restricted Subsidiaries are not subject to the Suspended Covenants, the Notes will be entitled to substantially less covenant protection. In the event that the Issuer and its Restricted Subsidiaries are not subject to the Suspended Covenants under the Indenture for any period of time as a result of the foregoing, and on any subsequent date (the “Reversion Date”) one or both of the Rating Agencies withdraw their Investment Grade Rating or downgrade the rating assigned to the Notes below an Investment Grade Rating, then the Issuer and its Restricted Subsidiaries will thereafter again be subject to the Suspended Covenants under the Indenture with respect to future events. The period of time between the Suspension Date and the Reversion Date is referred to in this “Description of exchange notes” as the “Suspension Period.” The Guarantees of the Guarantors will be suspended during the Suspension Period. Additionally, upon the occurrence of a Covenant Suspension Event, the amount of Excess Proceeds from Net Proceeds shall be reset to zero.
Notwithstanding the foregoing, in the event of any such reinstatement, no action taken or omitted to be taken by the Issuer or any of its Restricted Subsidiaries prior to such reinstatement will give rise to a Default or Event of Default under the Indenture with respect to the Notes; provided that (i) with respect to Restricted Payments made after such reinstatement, the amount available to be made as Restricted Payments will be calculated as though the covenant described below under “—Limitation on restricted payments” had been in effect prior to, but not during, the Suspension Period; (ii) all Indebtedness incurred, or Disqualified Stock issued, during the Suspension Period will be classified to have been incurred or issued pursuant to clause (3) of the second paragraph of
115
Table of Contents
“—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”; provided that all Indebtedness outstanding on the Reversion Date under the Senior Credit Facilities shall be deemed incurred or issued pursuant to clause (1) of the second paragraph of “—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock” (up to the maximum amount of such Indebtedness that would be permitted to be incurred thereunder as of the Reversion Date and after giving effect to Indebtedness incurred prior to the Suspension Period and outstanding on the Reversion Date); (iii) any Affiliate Transaction entered into after such reinstatement pursuant to an agreement entered into during any Suspension Period shall be deemed to be permitted pursuant to clause (6) of the second paragraph of the covenant described under “—Transactions with affiliates”; (iv) any encumbrance or restriction on the ability of any Restricted Subsidiary to take any action described in clauses (1) through (3) of the first paragraph of the covenant described under “—Dividend and other payment restrictions affecting restricted subsidiaries” that becomes effective during any Suspension Period shall be deemed to be permitted pursuant to clause (a) of the second paragraph of the covenant described under “—Dividend and other payment restrictions affecting restricted subsidiaries”; and (v) no Subsidiary of the Issuer shall be required to comply with the covenant described under “—Limitation on guarantees of indebtedness by restricted subsidiaries” after such reinstatement with respect to any guarantee entered into by such Subsidiary during any Suspension Period.
There can be no assurance that the Notes will ever achieve or maintain Investment Grade Ratings.
Limitation on restricted payments
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly:
(I) declare or pay any dividend or make any payment or distribution on account of the Issuer’s or any of its Restricted Subsidiaries’ Equity Interests (in each case, solely in such Person’s capacity as holder of such Equity Interests), including any dividend or distribution payable in connection with any merger or consolidation other than:
(a) dividends or distributions by the Issuer payable solely in Equity Interests (other than Disqualified Stock) of the Issuer; or
(b) dividends or distributions by a Restricted Subsidiary so long as, in the case of any dividend or distribution payable on or in respect of any class or series of securities issued by a Restricted Subsidiary other than a Wholly-Owned Subsidiary, the Issuer or a Restricted Subsidiary receives at least its pro rata share of such dividend or distribution in accordance with its Equity Interests in such class or series of securities;
(II) purchase, redeem, defease or otherwise acquire or retire for value any Equity Interests of the Issuer or any direct or indirect parent company of the Issuer, including in connection with any merger or consolidation;
(III) make any principal payment on, or redeem, repurchase, defease or otherwise acquire or retire for value, in each case, prior to any scheduled repayment, sinking fund payment or maturity, any Subordinated Indebtedness, other than:
(a) Indebtedness permitted under clauses (7), (8) and (9) of the second paragraph of the covenant described under “—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”; or
(b) the purchase, repurchase or other acquisition of Subordinated Indebtedness purchased in anticipation of satisfying a sinking fund obligation, principal installment or final maturity, in each case due within one year of the date of purchase, repurchase or acquisition; or
(IV) make any Restricted Investment
(all such payments and other actions set forth in clauses (I) through (IV) above being collectively referred to as “Restricted Payments”),
116
Table of Contents
unless, at the time of and immediately after giving effect to such Restricted Payment:
(1) no Default shall have occurred and be continuing or would occur as a consequence thereof;
(2) immediately after giving effect to such transaction on a pro forma basis, the Issuer could incur $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first paragraph of the covenant described under “—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock” (the “Fixed Charge Coverage Test”); and
(3) such Restricted Payment, together with the aggregate amount of all other Restricted Payments made by the Issuer and its Restricted Subsidiaries after the Effective Date (including Restricted Payments permitted by clauses (1), (6)(c), (8) and (13) of the next succeeding paragraph, but excluding all other Restricted Payments permitted by the next succeeding paragraph), is less than the sum of (without duplication):
(a) 50.0% of the Consolidated Net Income of the Issuer for the period (taken as one accounting period and including the predecessor) beginning the first day of the fiscal quarter containing the Effective Date to the end of the most recently ended Test Period preceding such Restricted Payment, or, in the case such Consolidated Net Income for such period is a deficit, minus 100.0% of such deficit; plus
(b) 100.0% of the aggregate net cash proceeds and the fair market value of marketable securities or other property received by the Issuer after the Effective Date (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness or issue Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”) from the issue or sale of:
(i) (A) Equity Interests of the Issuer, including Treasury Capital Stock (as defined below), but excluding cash proceeds and the fair market value of marketable securities or other property received from the sale of:
(x) Equity Interests to any future, present or former employees, directors, officers, managers or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any direct or indirect parent company of the Issuer or any of the Issuer’s Subsidiaries after the Effective Date to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph; and
(y) Designated Preferred Stock;
and (B) to the extent such net proceeds are actually contributed to the Issuer, Equity Interests of any direct or indirect parent company of the Issuer (excluding contributions of the proceeds from the sale of Designated Preferred Stock of such company or contributions to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph); or
(ii) debt securities of the Issuer, in each case, that have been converted into or exchanged for such Equity Interests of the Issuer;
provided that this clause (b) shall not include the proceeds from (W) Refunding Capital Stock applied in accordance with clause (2) of the next succeeding paragraph, (X) Equity Interests or convertible debt securities of the Issuer sold to a Restricted Subsidiary, (Y) Disqualified Stock or debt securities that have been converted into Disqualified Stock or (Z) Excluded Contributions; plus
(c) 100.0% of the aggregate amount of cash and the fair market value of marketable securities or other property contributed to the capital of the Issuer following the Effective Date (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness or issue Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”) (other than by a Restricted Subsidiary and any Excluded Contributions); plus
117
Table of Contents
(d) 100.0% of the aggregate amount received in cash and the fair market value of marketable securities or other property received by means of:
(i) the sale or other disposition (other than to the Issuer or a Restricted Subsidiary) of Restricted Investments made by the Issuer or its Restricted Subsidiaries and repurchases and redemptions of such Restricted Investments from the Issuer or its Restricted Subsidiaries (other than by the Issuer or a Restricted Subsidiary) and repayments of loans or advances, and releases of guarantees, which constitute Restricted Investments made by the Issuer or its Restricted Subsidiaries, in each case after the Effective Date; or
(ii) the sale (other than to the Issuer or a Restricted Subsidiary) of the stock of an Unrestricted Subsidiary or a distribution from an Unrestricted Subsidiary (other than in each case to the extent the Investment in such Unrestricted Subsidiary constituted a Permitted Investment) or a dividend from an Unrestricted Subsidiary after the Effective Date; plus
(e) in the case of the redesignation of an Unrestricted Subsidiary as a Restricted Subsidiary or the merger, amalgamation or consolidation of an Unrestricted Subsidiary into the Issuer or a Restricted Subsidiary or the transfer of all or substantially all of the assets of an Unrestricted Subsidiary to the Issuer or a Restricted Subsidiary after the Effective Date, the fair market value of the Investment in such Unrestricted Subsidiary (or the assets transferred) at the time of the redesignation of such Unrestricted Subsidiary as a Restricted Subsidiary or at the time of such merger, amalgamation, consolidation or transfer of assets, other than to the extent the Investment in such Unrestricted Subsidiary constituted a Permitted Investment; provided that, in the case of this clause (e), if the fair market value of any marketable securities or other property (other than cash) contributed or received, or such Investment, as applicable, shall exceed $25.0 million, such fair market value shall be determined by the board of directors of the Issuer whose resolution with respect thereto will be delivered to the Trustee.
The foregoing provisions do not prohibit:
(1) the payment of any dividend or other distribution or the consummation of any irrevocable redemption within 60 days after the date of declaration of the dividend or other distribution or giving of the redemption notice, as the case may be, if at the date of declaration or notice, the dividend or other distribution or redemption payment would have complied with the provisions of the Indenture;
(2) (a) the redemption, repurchase, retirement or other acquisition of any Equity Interests (“Treasury Capital Stock”), including any accrued and unpaid dividends thereon, or Subordinated Indebtedness of the Issuer or any Restricted Subsidiary or any Equity Interests of any direct or indirect parent company of the Issuer, in exchange for, or out of the proceeds of the substantially concurrent sale or issuance (other than to a Restricted Subsidiary) of, Equity Interests of the Issuer or any direct or indirect parent company of the Issuer to the extent contributed to the Issuer (in each case, other than any Disqualified Stock) (“Refunding Capital Stock”), (b) the declaration and payment of dividends on Treasury Capital Stock out of the proceeds of the substantially concurrent sale or issuance (other than to a Subsidiary of the Issuer or to an employee stock ownership plan or any trust established by the Issuer or any of its Subsidiaries) of Refunding Capital Stock, and (c) if, immediately prior to the retirement of Treasury Capital Stock, the declaration and payment of dividends thereon was permitted under clauses (6) (a) or (b) of this paragraph, the declaration and payment of dividends on the Refunding Capital Stock (other than Refunding Capital Stock the proceeds of which were used to redeem, repurchase, retire or otherwise acquire any Equity Interests of any direct or indirect parent company of the Issuer) in an aggregate amount per year no greater than the aggregate amount of dividends per annum that were declarable and payable on such Treasury Capital Stock immediately prior to such retirement;
(3) the defeasance, redemption, repurchase, exchange or other acquisition or retirement of (i) Subordinated Indebtedness of the Issuer or a Guarantor made by exchange for, or out of the proceeds of the substantially concurrent sale of, new Indebtedness of the Issuer or a Guarantor or Disqualified Stock of the Issuer or a
118
Table of Contents
Guarantor or (ii) Disqualified Stock of the Issuer or a Guarantor made by exchange for, or out of the proceeds of the substantially concurrent sale of, Disqualified Stock of the Issuer or a Guarantor, that, in each case, is Refinancing Indebtedness incurred or issued, as applicable, in compliance with “—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”;
(4) a Restricted Payment to pay for the repurchase, retirement or other acquisition or retirement for value of Equity Interests (other than Disqualified Stock) of the Issuer or any direct or indirect parent company of the Issuer held by any future, present or former employee, director, officer, manager or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement (including, for the avoidance of doubt, any principal and interest payable on any Notes issued by the Issuer or any direct or indirect parent company of the Issuer in connection with any such repurchase, retirement or other acquisition), or any stock subscription or shareholder agreement, including any Equity Interest rolled over by management of the Issuer or any direct or indirect parent company of the Issuer in connection with the Transactions; provided that the aggregate amount of Restricted Payments made under this clause (4) does not exceed $15.0 million in any calendar year following the Effective Date (with unused amounts in any calendar year being carried over to the next two succeeding calendar years); provided, further, that each of the amounts in any calendar year under this clause (4) may be increased by an amount not to exceed:
(a) the cash proceeds from the sale of Equity Interests (other than Disqualified Stock) of the Issuer and, to the extent contributed to the Issuer, the cash proceeds from the sale of Equity Interests of any direct or indirect parent company of the Issuer, in each case to any future, present or former employees, directors, officers, managers or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies that occurs after the Effective Date, to the extent the cash proceeds from the sale of such Equity Interests have not otherwise been applied to the payment of Restricted Payments by virtue of clause (3) of the preceding paragraph; plus
(b) the cash proceeds of key man life insurance policies received by the Issuer or its Restricted Subsidiaries (or by any direct or indirect parent company to the extent contributed to the Issuer) after the Effective Date; less
(c) the amount of any Restricted Payments previously made with the cash proceeds described in clauses (a) and (b) of this clause (4);
and provided, further, that cancellation of Indebtedness owing to the Issuer from any future, present or former employees, directors, officers, managers or consultants of the Issuer (or their respective Controlled Investment Affiliates or Immediate Family Members), any direct or indirect parent company of the Issuer or any of the Issuer’s Restricted Subsidiaries in connection with a repurchase of Equity Interests of the Issuer or any of its direct or indirect parent companies will not be deemed to constitute a Restricted Payment for purposes of this covenant or any other provision of the Indenture;
(5) the declaration and payment of dividends to holders of any class or series of Disqualified Stock of the Issuer or any of its Restricted Subsidiaries or any class or series of Preferred Stock of any Restricted Subsidiary issued in accordance with the covenant described under “—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock” to the extent such dividends are included in the definition of “Fixed Charges”;
(6) (a) the declaration and payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by the Issuer or any of its Restricted Subsidiaries after the Effective Date;
(b) the declaration and payment of dividends to any direct or indirect parent company of the Issuer, the proceeds of which will be used to fund the payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by such parent company after the
119
Table of Contents
Effective Date; provided that the amount of dividends paid pursuant to this clause (b) shall not exceed the aggregate amount of cash actually contributed to the Issuer from the sale of such Designated Preferred Stock; or
(c) the declaration and payment of dividends on Refunding Capital Stock that is Preferred Stock in excess of the dividends declarable and payable thereon pursuant to clause (2) of this paragraph;
provided, in the case of each of (a), (b) and (c) of this clause (6), that for the most recently ended Test Period preceding the date of issuance of such Designated Preferred Stock or the declaration of such dividends on Refunding Capital Stock that is Preferred Stock, after giving effect to such issuance or declaration on a pro forma basis, the Issuer would have had a Fixed Charge Coverage Ratio of at least 2.00 to 1.00;
(7) payments made or expected to be made by the Issuer or any Restricted Subsidiary in respect of withholding or similar taxes payable by any future, present or former employee, director, officer, manager or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer or any of its Restricted Subsidiaries or any direct or indirect parent company of the Issuer and any repurchases of Equity Interests deemed to occur upon exercise of stock options or warrants or similar rights if such Equity Interests represent a portion of the exercise price of such options or warrants or similar rights;
(8) the declaration and payment of dividends on the Issuer’s common stock (or the payment of dividends to any direct or indirect parent company of the Issuer to fund a payment of dividends on such company’s common stock), following the first public offering of the Issuer’s common stock or the common stock of any direct or indirect parent company of the Issuer after the Effective Date, of up to 6.0% per annum of the net cash proceeds received by or contributed to the Issuer in or from any such public offering, other than public offerings with respect to the Issuer’s common stock registered on Form S-4 or Form S-8 and other than any public sale constituting an Excluded Contribution;
(9) Restricted Payments that are made with Excluded Contributions;
(10) other Restricted Payments in an aggregate amount taken together with all other Restricted Payments made pursuant to this clause (10) (in the case of Restricted Investments, at any one time outstanding (without giving effect to the sale of an Investment to the extent the proceeds of such sale do not consist of, or have not been subsequently sold or transferred for, Cash Equivalents)) not to exceed the greater of (a) $50.0 million and (b) 3.0% of Total Assets;
(11) distributions or payments of Securitization Fees;
(12) any Restricted Payment made in connection with the Transactions and the fees and expenses related thereto or owed to Affiliates, in each case with respect to any Restricted Payment made or owed to an Affiliate, to the extent permitted by the covenant described under “—Transactions with affiliates”;
(13) the repurchase, redemption or other acquisition or retirement for value of any Subordinated Indebtedness pursuant to the provisions similar to those described under “—Repurchase at the option of holders—Change of control” and “—Repurchase at the option of holders—Asset sales”; provided that the Issuer shall have made a Change of Control Offer or Asset Sale Offer, as applicable, to purchase the Notes on the terms provided in the Indenture applicable to Change of Control Offers or Asset Sale Offers, respectively, and all Notes validly tendered by Holders in such Change of Control Offer or Asset Sale Offer, as applicable, have been repurchased, redeemed, acquired or retired for value;
(14) the declaration and payment of dividends or distributions by the Issuer to, or the making of loans to, any direct or indirect parent company of the Issuer in amounts required for any direct or indirect parent company of the Issuer to pay, in each case without duplication,
(a) franchise and excise taxes and other fees, taxes and expenses required to maintain their corporate or other legal existence;
(b) so long as the Issuer is a member of a consolidated, combined or unitary group of which such direct or indirect parent company is a parent for foreign, federal, state or local income or other tax purposes,
120
Table of Contents
the relevant foreign, federal, state and local income or other taxes, to the extent such income or other taxes are attributable to the income of the Issuer and its Restricted Subsidiaries that are members of such group and, to the extent of the amount actually received from its Unrestricted Subsidiaries, in amounts required to pay such taxes to the extent attributable to the income of such Unrestricted Subsidiaries; provided that in each case the amount of such payments in or with respect to any taxable year does not exceed the amount that the Issuer and such Restricted Subsidiaries would be required to pay in respect of the relevant foreign, federal, state and local taxes for such taxable year were the Issuer, such Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes as a separate consolidated, combined or unitary group separately from any such parent company (or, if there are no such Restricted Subsidiaries or Unrestricted Subsidiaries, the Issuer on a separate company basis);
(c) customary salary, bonus and other benefits payable to employees, directors, officers and managers of any direct or indirect parent company of the Issuer to the extent such salaries, bonuses and other benefits are attributable to the ownership or operation of the Issuer and its Restricted Subsidiaries;
(d) general corporate operating and overhead costs and expenses of any direct or indirect parent company of the Issuer to the extent such costs and expenses are attributable to the ownership or operation of the Issuer and its Subsidiaries;
(e) fees and expenses other than to Affiliates of the Issuer related to any unsuccessful equity or debt offering of such parent company;
(f)(i) amounts payable pursuant to the Management Fee Agreement or any amendment thereto or replacement thereof so long as any such amendment or replacement is not disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole, as compared to the Management Fee Agreement as in effect on the Effective Date, solely to the extent such amounts are not paid directly by the Issuer or its Subsidiaries; or (ii) payments permitted under clauses (3), (4), (7), (12) or (19) of the covenant described under “—Transactions with affiliates”;
(g) cash payments in lieu of issuing fractional shares in connection with the exercise of warrants, options or other securities convertible into or exchangeable for Equity Interests of the Issuer or any direct or indirect parent company of the Issuer; and
(h) to finance Investments otherwise permitted to be made pursuant to this covenant; provided that (A) such Restricted Payment shall be made substantially concurrently with the closing of such Investment, (B) such direct or indirect parent company shall, immediately following the closing thereof, cause (1) all property acquired (whether assets or Equity Interests) to be contributed to the capital of the Issuer or one of its Restricted Subsidiaries or (2) the merger of the Person formed or acquired into the Issuer or one of its Restricted Subsidiaries (to the extent not prohibited by the covenant “—Merger, consolidation or sale of all or substantially all assets” below) in order to consummate such Investment, (C) such direct or indirect parent company and its Affiliates (other than the Issuer or a Restricted Subsidiary) receives no consideration or other payment in connection with such transaction except to the extent the Issuer or a Restricted Subsidiary could have given such consideration or made such payment in compliance with the Indenture, (D) any property received by the Issuer shall not increase amounts available for Restricted Payments pursuant to clause (3) of the preceding paragraph and (E) such Investment shall be deemed to be made by the Issuer or such Restricted Subsidiary pursuant to another provision of this covenant (other than pursuant to clause (9) hereof) or pursuant to the definition of “Permitted Investments” (other than clause (9) thereof); and
(15) the distribution, by dividend or otherwise, of shares of Capital Stock of, or Indebtedness owed to the Issuer or a Restricted Subsidiary by, Unrestricted Subsidiaries (other than Unrestricted Subsidiaries, the primary assets of which are Cash Equivalents);
provided that at the time of, and after giving effect to, any Restricted Payment permitted under clauses (10) and (15), no Default shall have occurred and be continuing or would occur as a consequence thereof.
121
Table of Contents
As of August 31, 2011, all of the Issuer’s Subsidiaries were Restricted Subsidiaries. The Issuer will not permit any Unrestricted Subsidiary to become a Restricted Subsidiary except pursuant to the next to the last sentence of the definition of “Unrestricted Subsidiary.” For purposes of designating any Restricted Subsidiary as an Unrestricted Subsidiary, all outstanding Investments by the Issuer and its Restricted Subsidiaries (except to the extent repaid) in the Subsidiary so designated will be deemed to be Restricted Payments in an amount determined as set forth in the penultimate sentence of the definition of “Investments.” Such designation will be permitted only if a Restricted Payment in such amount would be permitted at such time, whether pursuant to the first paragraph of this covenant or under clause (9) or (10) of the second paragraph of this covenant, or pursuant to the definition of “Permitted Investments,” and if such Subsidiary otherwise meets the definition of an Unrestricted Subsidiary. Unrestricted Subsidiaries will not be subject to any of the restrictive covenants set forth in the Indenture.
For the avoidance of doubt, this covenant shall not restrict the making of any “AHYDO catch up payment” with respect to, and required by the terms of, any Indebtedness of the Issuer or any of its Restricted Subsidiaries permitted to be incurred under the terms of the Indenture.
Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable, contingently or otherwise (collectively, “incur and collectively, an “incurrence”) with respect to any Indebtedness (including Acquired Indebtedness), and the Issuer will not issue any shares of Disqualified Stock and will not permit any Restricted Subsidiary to issue any shares of Disqualified Stock or Preferred Stock; provided that the Issuer may incur Indebtedness (including Acquired Indebtedness) or issue shares of Disqualified Stock, and, subject to the third paragraph of this covenant, any Restricted Subsidiary may incur Indebtedness (including Acquired Indebtedness), issue shares of Disqualified Stock and issue shares of Preferred Stock, if the Fixed Charge Coverage Ratio for the Issuer’s most recently ended Test Period preceding the date on which such additional Indebtedness is incurred or such Disqualified Stock or Preferred Stock is issued would have been at least 2.00 to 1.00, determined on a pro forma basis (including a pro forma application of the net proceeds therefrom), as if the additional Indebtedness had been incurred, or the Disqualified Stock or Preferred Stock had been issued, as the case may be, and the application of proceeds therefrom had occurred at the beginning of such Test Period.
The foregoing limitations do not apply to:
(1) the incurrence of Indebtedness pursuant to the Credit Facilities by the Issuer or any Restricted Subsidiary and the issuance and creation of letters of credit and bankers’ acceptances thereunder (with letters of credit and bankers’ acceptances being deemed to have a principal amount equal to the face amount thereof) in an aggregate principal amount not to exceed $865.0 million; provided that such amount will be reduced to the extent of any reduction or elimination by the lenders of any commitment under any Credit Facility in connection with any Qualified Securitization Facility for so long as such reduction or elimination of such commitment remains in effect but only to the extent Indebtedness under such Qualified Securitization Facility is outstanding;
(2) the incurrence by the Issuer and any Guarantor of Indebtedness represented by the Initial Notes and the related Guarantees and the exchange notes and related exchange guarantees to be issued in exchange for the Initial Notes and the related Guarantees pursuant to this exchange offer (but excluding any Additional Notes issued after the Effective Date and any exchange notes and related exchange guarantees to be issued in exchange therefor);
(3) Indebtedness of the Issuer and its Restricted Subsidiaries in existence on the Effective Date (other than Indebtedness described in clauses (1) and (2));
(4) Indebtedness (including Capitalized Lease Obligations) and Disqualified Stock incurred or issued by the Issuer or any Restricted Subsidiary and Preferred Stock issued by any Restricted Subsidiary, to finance the
122
Table of Contents
purchase, lease or improvement of property (real or personal) or equipment that is used or useful in a Similar Business, whether through the direct purchase of assets or the Capital Stock of any Person owning such assets in an aggregate principal amount, together with any Refinancing Indebtedness in respect thereof and all other Indebtedness, Disqualified Stock and/or Preferred Stock incurred or issued and outstanding under this clause (4) at such time, not to exceed the greater of (x) $40.0 million and (y) 2.5% of Total Assets (in each case, determined at the date of incurrence), so long as such Indebtedness, Disqualified Stock or Preferred Stock is incurred or issued at the date of such purchase, lease or improvement or within 270 days thereafter;
(5) Indebtedness incurred by the Issuer or any of its Restricted Subsidiaries constituting reimbursement obligations with respect to letters of credit issued in the ordinary course of business, including letters of credit in respect of workers’ compensation claims, health, disability or other employee benefits or property, casualty or liability insurance or self-insurance or other Indebtedness with respect to reimbursement-type obligations regarding workers’ compensation claims; provided that upon the drawing of such letters of credit or the incurrence of such Indebtedness, such obligations are reimbursed within 30 Business Days following such drawing or incurrence;
(6) Indebtedness arising from agreements of the Issuer or its Restricted Subsidiaries providing for indemnification, adjustment of purchase price, earnouts or similar obligations, in each case, incurred or assumed in connection with the disposition of any business, assets or a Subsidiary, other than guarantees of Indebtedness incurred by any Person acquiring all or any portion of such business, assets or a Subsidiary for the purpose of financing such acquisition; provided that such Indebtedness is not reflected on the balance sheet of the Issuer or any of its Restricted Subsidiaries (contingent obligations referred to in a footnote to financial statements and not otherwise reflected on the balance sheet will not be deemed to be reflected on such balance sheet for purposes of this clause (6));
(7) Indebtedness of the Issuer to a Restricted Subsidiary; provided that any such Indebtedness owing to a Restricted Subsidiary that is not a Guarantor is expressly subordinated in right of payment to the Notes; provided, further, that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such Indebtedness (except to the Issuer or another Restricted Subsidiary or any pledge of such Indebtedness constituting a Permitted Lien) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause (7) (to the extent such Indebtedness is then outstanding);
(8) Indebtedness of a Restricted Subsidiary to the Issuer or another Restricted Subsidiary; provided that if a Guarantor incurs such Indebtedness to a Restricted Subsidiary that is not a Guarantor, such Indebtedness is expressly subordinated in right of payment to the Guarantee of the Notes of such Guarantor; provided, further, that any subsequent issuance or transfer of any Capital Stock or any other event which results in any Restricted Subsidiary that is the obligee under such Indebtedness ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such Indebtedness (except to the Issuer or another Restricted Subsidiary or any pledge of such Indebtedness constituting a Permitted Lien) shall be deemed, in each case, to be an incurrence of such Indebtedness (to the extent such Indebtedness is then outstanding) not permitted by this clause (8);
(9) shares of Preferred Stock of a Restricted Subsidiary issued to the Issuer or another Restricted Subsidiary; provided that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such shares of Preferred Stock (except to the Issuer or another of its Restricted Subsidiaries or any pledge of such Capital Stock constituting a Permitted Lien) shall be deemed, in each case, to be an issuance of such shares of Preferred Stock (to the extent such Preferred Stock is then outstanding) not permitted by this clause (9);
(10) Hedging Obligations (excluding Hedging Obligations entered into for speculative purposes) for the purpose of limiting interest rate risk with respect to any Indebtedness permitted to be incurred under the Indenture, exchange rate risk or commodity pricing risk;
123
Table of Contents
(11) obligations in respect of self-insurance and obligations in respect of performance, bid, appeal and surety bonds and performance and completion guarantees and similar obligations provided by the Issuer or any of its Restricted Subsidiaries in the ordinary course of business;
(12) (a) Indebtedness or Disqualified Stock of the Issuer and Indebtedness, Disqualified Stock or Preferred Stock of the Issuer or any Restricted Subsidiary in an aggregate principal amount or liquidation preference up to 100.0% of the net cash proceeds received by the Issuer since immediately after the Effective Date from the issue or sale of Equity Interests of the Issuer or cash contributed to the capital of the Issuer (in each case, other than proceeds of Disqualified Stock or sales of Equity Interests to the Issuer or any of its Subsidiaries) as determined in accordance with clauses (3)(b) and (3)(c) of the first paragraph of “—Limitation on restricted payments” to the extent such net cash proceeds or cash have not been applied pursuant to such clauses to make Restricted Payments or to make other Investments, payments or exchanges pursuant to the second paragraph of “—Limitation on restricted payments” or to make Permitted Investments (other than Permitted Investments specified in clause (1) or (3) of the definition thereof) and (b) Indebtedness or Disqualified Stock of the Issuer and Indebtedness, Disqualified Stock or Preferred Stock of the Issuer or, subject to the third paragraph of this covenant, any Restricted Subsidiary not otherwise permitted hereunder in an aggregate principal amount or liquidation preference which, when aggregated with the principal amount and liquidation preference of all other Indebtedness, Disqualified Stock and Preferred Stock then outstanding and incurred pursuant to this clause (12)(b), does not exceed the greater of (x) $55.0 million and (y) 3.25% of Total Assets (in each case, determined at the date of incurrence) (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred or issued pursuant to this clause (12)(b) shall cease to be deemed incurred or outstanding for purposes of this clause (12)(b) but shall be deemed incurred or issued for the purposes of the first paragraph of this covenant from and after the first date on which the Issuer or such Restricted Subsidiary could have incurred or issued such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (12)(b));
(13) the incurrence or issuance by the Issuer of Indebtedness or Disqualified Stock or the incurrence or issuance by a Restricted Subsidiary of Indebtedness, Disqualified Stock or Preferred Stock which serves to refund, refinance, extend, replace, renew or defease any Indebtedness incurred or Disqualified Stock or Preferred Stock issued as permitted under the first paragraph of this covenant and clauses (2), (3), (4) and (12)(a) above, this clause (13) and clause (14) below or any Indebtedness incurred or Disqualified Stock or Preferred Stock issued to so refund, refinance, extend, replace, renew or defease such Indebtedness, Disqualified Stock or Preferred Stock; provided that any such Indebtedness, Disqualified Stock or Preferred Stock constitutes Refinancing Indebtedness;
(14) (a) Indebtedness or Disqualified Stock of the Issuer or, subject to the third paragraph of this covenant, Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary, incurred or issued to finance an acquisition or (b) Indebtedness, Disqualified Stock or Preferred Stock of Persons that are acquired by the Issuer or any Restricted Subsidiary or merged into the Issuer or a Restricted Subsidiary in accordance with the terms of the Indenture; provided that after giving effect to such acquisition or merger, either:
(i) the Issuer would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test, or
(ii) the Fixed Charge Coverage Ratio for the Issuer is equal to or greater than immediately prior to such acquisition or merger;
(15) Indebtedness arising from the honoring by a bank or other financial institution of a check, draft or similar instrument drawn against insufficient funds in the ordinary course of business; provided that such Indebtedness is extinguished within five Business Days of its incurrence;
(16) Indebtedness of the Issuer or any of its Restricted Subsidiaries supported by a letter of credit issued pursuant to the Credit Facilities, in a principal amount not in excess of the stated amount of such letter of credit;
(17)(a) any guarantee by the Issuer or a Restricted Subsidiary of Indebtedness or other obligations of any Restricted Subsidiary so long as the incurrence of such Indebtedness incurred by such Restricted Subsidiary
124
Table of Contents
is permitted under the terms of the Indenture, or (b) any guarantee by a Restricted Subsidiary of Indebtedness of the Issuer so long as the incurrence of such Indebtedness by the Issuer is permitted under the terms of the Indenture; provided that such guarantee under either clause (a) or clause (b) is incurred in accordance with the covenant described below under “—Limitation on guarantees of indebtedness by restricted subsidiaries”;
(18) Indebtedness consisting of Indebtedness issued by the Issuer or any of its Restricted Subsidiaries to future, present or former employees, directors, officers, managers and consultants thereof, their respective Controlled Investment Affiliates or Immediate Family Members, in each case to finance the purchase or redemption of Equity Interests of the Issuer or any direct or indirect parent company of the Issuer to the extent described in clause (4) of the second paragraph under “—Limitation on restricted payments”;
(19) customer deposits and advance payments received in the ordinary course of business from customers for goods purchased in the ordinary course of business;
(20) (a) Indebtedness owed on a short-term basis of no longer than 30 days to banks and other financial institutions incurred in the ordinary course of business of the Issuer and its Restricted Subsidiaries with such banks or financial institutions that arises in connection with ordinary banking arrangements to manage cash balances of the Issuer and its Restricted Subsidiaries and (b) Indebtedness in respect of Cash Management Services;
(21) Indebtedness incurred by a Restricted Subsidiary in connection with bankers’ acceptances, discounted bills of exchange or the discounting or factoring of receivables for credit management purposes, in each case incurred or undertaken in the ordinary course of business on arm’s length commercial terms;
(22) Indebtedness of the Issuer or any of its Restricted Subsidiaries consisting of (a) the financing of insurance premiums or (b) take-or-pay obligations contained in supply arrangements in each case, incurred in the ordinary course of business;
(23) the incurrence of Indebtedness of Foreign Subsidiaries of the Issuer in an amount not to exceed and together with any other Indebtedness incurred and outstanding under this clause (23) the greater of (i) $17.5 million and (ii) 1.0% of Total Assets (in each case, determined at the time of incurrence) (it being understood that any Indebtedness incurred pursuant to this clause (23) shall cease to be deemed incurred or outstanding for the purpose of this clause (23) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Issuer or such Restricted Subsidiaries could have incurred such Indebtedness under the first paragraph of this covenant without reliance on this clause (23)); and
(24) Indebtedness of the Issuer or any of its Restricted Subsidiaries undertaken in connection with cash management and related activities with respect to any Subsidiary or joint venture in the ordinary course of business.
Restricted Subsidiaries of the Issuer that are not Guarantors may not incur Indebtedness or Disqualified Stock or Preferred Stock under the first paragraph of this covenant or clause 12(b) or 14(a) of the second paragraph of this covenant if, after giving pro forma effect to such incurrence or issuance (including a pro forma application of the net proceeds therefrom), the aggregate amount of Indebtedness and Disqualified Stock and Preferred Stock of Restricted Subsidiaries that are not Guarantors incurred or issued pursuant to the first paragraph of this covenant and clauses 12(b) and 14(a) of the second paragraph of this covenant, collectively, would exceed the greater of (x) $40.0 million and (y) 2.5% of Total Assets.
For purposes of determining compliance with this covenant:
(1) in the event that an item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) meets the criteria of more than one of the categories of Permitted Indebtedness, Disqualified Stock or Preferred Stock described in clauses (1) through (24) above or is entitled to be incurred pursuant to the first paragraph of this covenant, the Issuer, in its sole discretion, will classify or reclassify such item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) and will only be required to
125
Table of Contents
include the amount and type of such Indebtedness, Disqualified Stock or Preferred Stock in one of the above clauses or under the first paragraph of this covenant; provided that all Indebtedness outstanding under the Credit Facilities on the Effective Date or any refinancing thereof that is secured by a Lien will, at all times, be treated as incurred on the Effective Date under clause (1) of the second paragraph above; and
(2) the Issuer is entitled to divide and classify an item of Indebtedness in more than one of the types of Indebtedness described in the first and second paragraphs above, subject to the proviso to clause (1) of this paragraph.
Accrual of interest or dividends, the accretion of accreted value, the accretion or amortization of original issue discount and the payment of interest or dividends in the form of additional Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, of the same class will not be deemed to be an incurrence of Indebtedness, Disqualified Stock or Preferred Stock for purposes of this covenant.
For purposes of determining compliance with any U.S. dollar-denominated restriction on the incurrence of Indebtedness, the U.S. dollar-equivalent principal amount of Indebtedness denominated in a foreign currency shall be calculated based on the relevant currency exchange rate in effect on the date such Indebtedness was incurred, in the case of term debt, or first committed, in the case of revolving credit debt; provided that if such Indebtedness is incurred to refinance other Indebtedness denominated in a foreign currency, and such refinancing would cause the applicable U.S. dollar-denominated restriction to be exceeded if calculated at the relevant currency exchange rate in effect on the date of such refinancing, such U.S. dollar-denominated restriction shall be deemed not to have been exceeded so long as the principal amount of such refinancing Indebtedness does not exceed (i) the principal amount of such Indebtedness being refinanced plus (ii) the aggregate amount of fees, underwriting discounts, premiums (including tender premiums) and other costs and expenses (including original issue discount, upfront fees or similar fees) incurred in connection with such refinancing.
The principal amount of any Indebtedness incurred to refinance other Indebtedness, if incurred in a different currency from the Indebtedness being refinanced, shall be calculated based on the currency exchange rate applicable to the currencies in which such respective Indebtedness is denominated that is in effect on the date of such refinancing. The principal amount of any non-interest bearing Indebtedness or other discount security constituting Indebtedness at any date shall be the principal amount thereof that would be shown on a balance sheet of the Issuer dated such date prepared in accordance with GAAP.
The Indenture provides that the Issuer will not, and will not permit any Guarantor to, directly or indirectly, incur any Indebtedness (including Acquired Indebtedness) that is contractually subordinated or junior in right of payment to any Indebtedness of the Issuer or such Guarantor, as the case may be, unless such Indebtedness is expressly subordinated in right of payment to the Notes or such Guarantor’s Guarantee to the extent and in the same manner as such Indebtedness is subordinated to other Indebtedness of the Issuer or such Guarantor, as the case may be.
The Indenture does not treat (1) unsecured Indebtedness as subordinated or junior to Secured Indebtedness merely because it is unsecured or (2) secured Senior Indebtedness as subordinated or junior to any other secured Senior Indebtedness merely because it has a junior priority lien with respect to the same collateral.
Liens
The Issuer will not, and will not permit any Guarantor to, directly or indirectly, create, incur or assume any Lien (except Permitted Liens) that secures Obligations under any Indebtedness or any related guarantee of Indebtedness, on any asset or property of the Issuer or any Guarantor, or any income or profits therefrom, or assign or convey any right to receive income therefrom, unless:
(1) in the case of Liens securing Subordinated Indebtedness, the Notes and related Guarantees are secured by a Lien on such property, assets or proceeds that is senior in priority to such Liens; and
(2) in all other cases, the Notes or the Guarantees are equally and ratably secured,
126
Table of Contents
except that the foregoing shall not apply to or restrict (a) Liens securing obligations in respect of the Notes and the related Guarantees, (b) Liens securing obligations in respect of Indebtedness permitted to be incurred under any Credit Facility, including any letter of credit facility relating thereto, that was permitted by the terms of the Indenture to be incurred pursuant to clause (1) of the second paragraph under “—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock” and (c) Liens securing obligations in respect of Indebtedness permitted to be incurred under the covenant described above under “—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”; provided that, with respect to Liens securing obligations in respect of Indebtedness permitted under this subclause (c), at the time of incurrence and after giving pro forma effect thereto and the application of the net proceeds therefrom, the Senior Secured Net Leverage Ratio for the Test Period immediately preceding the incurrence of such Lien would be no greater than 4.00 to 1.00.
Any Lien created for the benefit of the Holders of the Notes pursuant to this covenant shall be deemed automatically and unconditionally released and discharged upon the release and discharge of each of the Liens described in clauses (1) and (2) above.
Merger, consolidation or sale of all or substantially all assets
The Issuer may not consolidate or merge with or into or wind up into (whether or not the Issuer is the surviving Person), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) the Issuer is the surviving Person or the Person formed by or surviving any such consolidation or merger (if other than the Issuer) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made, is a Person organized or existing under the laws of the United States, any state thereof, the District of Columbia, or any territory thereof (such Person, as the case may be, being herein called the “Successor Company”); provided that in the case where the surviving Person is not a corporation, a co-obligor of the Notes is a corporation;
(2) the Successor Company, if other than the Issuer, expressly assumes all the obligations of the Issuer under the Notes pursuant to supplemental indentures or other documents or instruments;
(3) immediately after such transaction, no Default exists;
(4) immediately after giving pro forma effect to such transaction and any related financing transactions, as if such transactions had occurred at the beginning of the applicable Test Period,
(a) the Successor Company or the Issuer would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test, or
(b) the Fixed Charge Coverage Ratio for the Issuer would be equal to or greater than the Fixed Charge Coverage Ratio for the Issuer immediately prior to such transaction;
(5) each Guarantor, unless it is the other party to the transactions described above, in which case clause (1)(b) of the second succeeding paragraph shall apply, shall have by supplemental indenture confirmed that its Guarantee shall apply to such Person’s obligations under the Indenture, the Notes and the Registration Rights Agreement; and
(6) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Indenture.
The Successor Company will succeed to, and be substituted for the Issuer under the Indenture, the Guarantees, the Notes and the Registration Rights Agreement, as applicable. The foregoing clauses (3), (4), (5) and (6) shall not apply to the merger contemplated by the Transaction Agreement. Notwithstanding the immediately preceding clauses (3) and (4),
(1) any Restricted Subsidiary may consolidate with or merge into or transfer all or part of its properties and assets to the Issuer, and
127
Table of Contents
(2) the Issuer may merge with an Affiliate of the Issuer solely for the purpose of reincorporating the Issuer in the United States, any state thereof, the District of Columbia or any territory thereof so long as the amount of Indebtedness of the Issuer and its Restricted Subsidiaries is not increased thereby.
Subject to certain limitations described in the Indenture governing release of a Guarantee upon the sale, disposition or transfer of a Guarantor, no Guarantor will, and the Issuer will not permit any Guarantor to, consolidate or merge with or into or wind up into (whether or not such Guarantor is the surviving Person), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) (a) such Guarantor is the surviving Person or the Person formed by or surviving any such consolidation or merger (if other than such Guarantor) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made is a Person organized or existing under the laws of the jurisdiction of organization of such Guarantor, as applicable, or the laws of the United States, any state thereof, the District of Columbia, or any territory thereof (such Person being herein called the “Successor Person”);
(b) the Successor Person, if other than such Guarantor, expressly assumes all the obligations of such Guarantor under the Indenture and such Guarantor’s related Guarantee pursuant to supplemental indentures or other documents or instruments;
(c) immediately after such transaction, no Default exists; and
(d) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Indenture; and
(2) the transaction is made in compliance with clauses (1) and (2) under the first paragraph of the covenant described under “Repurchase at the option of holders—Asset sales.”
Subject to certain limitations described in the Indenture, the Successor Person will succeed to, and be substituted for, such Guarantor under the Indenture and such Guarantor’s Guarantee. Notwithstanding the foregoing, any Guarantor may (1) merge into or transfer all or part of its properties and assets to another Guarantor or the Issuer, (2) merge with an Affiliate of the Issuer solely for the purpose of reincorporating the Guarantor in the United States, any state thereof, the District of Columbia or any territory thereof, (3) convert into a corporation, partnership, limited partnership, limited liability company or trust organized or existing under the laws of the jurisdiction of organization of such Guarantor or (4) liquidate or dissolve if the Issuer determines in good faith that such action is in the best interests of the Issuer and is not materially disadvantageous to the Holders of the Notes.
Transactions with affiliates
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, make any payment to, or sell, lease, transfer or otherwise dispose of any of its properties or assets to, or purchase any property or assets from, or enter into or make or amend any transaction, contract, agreement, understanding, loan, advance or guarantee with, or for the benefit of, any Affiliate of the Issuer (each of the foregoing, an “Affiliate Transaction”) involving aggregate payments or consideration in excess of $5.0 million, unless:
(1) such Affiliate Transaction is on terms that are not materially less favorable to the Issuer or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Issuer or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis; and
(2) the Issuer delivers to the Trustee with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate payments or consideration in excess of $15.0 million, a resolution adopted by the majority of the board of directors of the Issuer approving such Affiliate Transaction and set forth in an Officer’s Certificate certifying that such Affiliate Transaction complies with clause (1) above.
128
Table of Contents
The foregoing provisions do not apply to the following:
(1) transactions between or among the Issuer and/or any of its Restricted Subsidiaries;
(2) Restricted Payments permitted by the provisions of the Indenture described above under the covenant “—Limitation on restricted payments” and Investments permitted by the definition of “Permitted Investments”;
(3) the payment of management, consulting, monitoring, advisory and other fees, indemnities and expenses pursuant to the Management Fee Agreement (plus any unpaid management, consulting, monitoring, advisory and other fees, indemnities and expenses accrued in any prior year) and the termination fees pursuant to the Management Fee Agreement, or any amendment thereto or replacement thereof so long as any such amendment or replacement is not disadvantageous in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole, as compared to the Management Fee Agreement as in effect on the Effective Date;
(4) the payment of reasonable and customary fees and compensation paid to, and indemnities and reimbursements and employment and severance arrangements provided on behalf of or for the benefit of, current or former employees, directors, officers, managers or consultants of the Issuer, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
(5) transactions in which the Issuer or any of its Restricted Subsidiaries, as the case may be, delivers to the Trustee a letter from an Independent Financial Advisor stating that such transaction is fair to the Issuer or such Restricted Subsidiary from a financial point of view or stating that the terms are not materially less favorable to the Issuer or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Issuer or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis;
(6) any agreement as in effect as of the Effective Date, or any amendment thereto (so long as any such amendment is not disadvantageous in any material respect in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole as compared to the applicable agreement as in effect on the Effective Date);
(7) the existence of, or the performance by the Issuer or any of its Restricted Subsidiaries of its obligations under the terms of, any stockholders agreement (including any registration rights agreement or purchase agreement related thereto) to which it was a party as of the Effective Date and any similar agreements which it may enter into thereafter; provided that the existence of, or the performance by the Issuer or any of its Restricted Subsidiaries of obligations under any future amendment to any such existing agreement or under any similar agreement entered into after the Effective Date shall only be permitted by this clause (7) to the extent that the terms of any such amendment or new agreement are not otherwise disadvantageous in any material respect in the good faith judgment of the board of directors of the Issuer to the Holders when taken as a whole as compared to the original agreement in effect on the Effective Date;
(8) the Transactions and the payment of all fees and expenses related to the Transactions, in each case as contemplated by the Rule 144A offering memorandum;
(9) transactions with customers, clients, suppliers, contractors, joint venture partners or purchasers or sellers of goods or services that are Affiliates, in each case in the ordinary course of business and otherwise in compliance with the terms of the Indenture which are fair to the Issuer and its Restricted Subsidiaries, in the reasonable determination of the board of directors of the Issuer or the senior management thereof, or are on terms at least as favorable as might reasonably have been obtained at such time from an unaffiliated party;
(10) the issuance of Equity Interests (other than Disqualified Stock) of the Issuer to any direct or indirect parent company of the Issuer or to any Permitted Holder or to any employee, director, officer, manager or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
(11) sales of accounts receivable, or participations therein, or Securitization Assets or related assets in connection with any Qualified Securitization Facility;
129
Table of Contents
(12) payments by the Issuer or any of its Restricted Subsidiaries to any of the Investors made for any financial advisory, financing, underwriting or placement services or in respect of other investment banking activities, including, without limitation, in connection with acquisitions or divestitures which payments are approved by a majority of the board of directors of the Issuer in good faith;
(13) payments and Indebtedness and Disqualified Stock (and cancellation of any thereof) of the Issuer and its Restricted Subsidiaries and Preferred Stock (and cancellation of any thereof) of any Restricted Subsidiary to any future, current or former employee, director, officer, manager or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement or any stock subscription or shareholder agreement that are, in each case, approved by the Issuer in good faith; and any employment agreements, stock option plans and other compensatory arrangements (and any successor plans thereto) and any supplemental executive retirement benefit plans or arrangements with any such employees, directors, officers, managers or consultants (or their respective Controlled Investment Affiliates or Immediate Family Members) that are, in each case, approved by the Issuer in good faith;
(14) investments by any of the Investors in securities of the Issuer or any of its Restricted Subsidiaries (and payment of reasonable out-of-pocket expenses incurred by such Investors in connection therewith) so long as (a) the investment is being offered generally to other investors on the same or more favorable terms and (b) the investment constitutes less than 5.0% of the proposed or outstanding issue amount of such class of securities;
(15) payments to or from, and transactions with, any joint venture in the ordinary course of business (including, without limitation, any cash management activities related thereto);
(16) payments by the Issuer (and any direct or indirect parent company thereof) and its Subsidiaries pursuant to tax sharing agreements among the Issuer (and any such parent company) and its Subsidiaries; provided that in each case the amount of such payments in any taxable year does not exceed the amount that the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent of amount received from Unrestricted Subsidiaries) would be required to pay in respect of foreign, federal, state and local taxes for such taxable year were the Issuer, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes separately from any such parent entity;
(17) any lease entered into between the Issuer or any Restricted Subsidiary, as lessee, and any Affiliate of the Issuer, as lessor, which is approved by a majority of the disinterested members of the board of directors of the Issuer in good faith;
(18) intellectual property licenses in the ordinary course of business; and
(19) the payment of reasonable out-of-pocket costs and expenses relating to registration rights and indemnities provided to stockholders of Holdings or any direct or indirect parent thereof pursuant to the stockholders agreement or the registration rights agreement entered into on or after the Effective Date in connection therewith.
Dividend and other payment restrictions affecting restricted subsidiaries
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create or otherwise cause or suffer to exist or become effective any consensual encumbrance or consensual restriction on the ability of any such Restricted Subsidiary to:
(1) (a) pay dividends or make any other distributions to the Issuer or any Restricted Subsidiary that is a Guarantor on its Capital Stock or with respect to any other interest or participation in, or measured by, its profits, or
(b) pay any Indebtedness owed to the Issuer or any Restricted Subsidiary that is a Guarantor;
(2) make loans or advances to the Issuer or any Restricted Subsidiary that is a Guarantor; or
130
Table of Contents
(3) sell, lease or transfer any of its properties or assets to the Issuer or any Restricted Subsidiary that is a Guarantor.
However, the preceding restrictions will not apply to encumbrances or restrictions existing under or by reason of:
(a) contractual encumbrances or restrictions in effect on the Effective Date, including pursuant to the Senior Credit Facilities and the related documentation and Hedging Obligations and the related documentation;
(b) the Indenture, the Notes and the guarantees thereof;
(c) purchase money obligations for property acquired in the ordinary course of business and Capital Lease Obligations that impose restrictions of the nature discussed in clause (3) above on the property so acquired;
(d) applicable law or any applicable rule, regulation or order;
(e) any agreement or other instrument of a Person acquired by or merged with and into the Issuer or any of its Restricted Subsidiaries in existence at the time of such acquisition or at the time it merges with or into the Issuer or any of its Restricted Subsidiaries or assumed in connection with the acquisition of assets from such Person (but, in any such case, not created in contemplation thereof), which encumbrance or restriction is not applicable to any Person, or the properties or assets of any Person, other than the Person so acquired and its Subsidiaries, or the property or assets of the Person so acquired and its Subsidiaries;
(f) contracts for the sale of assets, including customary restrictions with respect to a Subsidiary of the Issuer pursuant to an agreement that has been entered into for the sale or disposition of all or substantially all of the Capital Stock or assets of such Subsidiary;
(g) Secured Indebtedness otherwise permitted to be incurred pursuant to the covenants described under “—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock” and “—Liens” that limit the right of the debtor to dispose of the assets securing such Indebtedness;
(h) restrictions on cash or other deposits or net worth imposed by customers under contracts entered into in the ordinary course of business or arising in connection with any Permitted Liens;
(i) other Indebtedness, Disqualified Stock or Preferred Stock of Foreign Subsidiaries permitted to be incurred subsequent to the Effective Date pursuant to the provisions of the covenant described under “—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”;
(j) customary provisions in joint venture agreements and other similar agreements relating solely to such joint venture;
(k) customary provisions contained in leases, licenses or similar agreements, including with respect to intellectual property and other agreements, in each case, entered into in the ordinary course of business;
(l) restrictions created in connection with any Qualified Securitization Facility that, in the good faith determination of the Issuer, are necessary or advisable to effect such Qualified Securitization Facility;
(m) restrictions or conditions contained in any trading, netting, operating, construction, service, supply, purchase, sale or other agreement to which the Issuer or any of its Restricted Subsidiaries is a party entered into in the ordinary course of business; provided that such agreement prohibits the encumbrance of solely the property or assets of the Issuer or such Restricted Subsidiary that are the subject to such agreement, the payment rights arising thereunder or the proceeds thereof and does not extend to any other asset or property of the Issuer or such Restricted Subsidiary or the assets or property of another Restricted Subsidiary;
(n) customary provisions restricting subletting or assignment of any lease governing a leasehold interest of any Restricted Subsidiary;
(o) customary provisions restricting assignment of any agreement entered into in the ordinary course of business;
(p) restrictions arising in connection with cash or other deposits permitted under the covenant “—Liens”;
131
Table of Contents
(q) any other agreement governing Indebtedness entered into after the Effective Date that contains encumbrances and other restrictions that either (x) are, in the good faith judgment of the Issuer, no more restrictive in any material respect taken as a whole with respect to any Restricted Subsidiary than those encumbrances and other restrictions that are in effect on the Effective Date with respect to that Restricted Subsidiary pursuant to agreements in effect on the Effective Date or (y) do not prohibit (except upon a default or an event of default thereunder) the payment of dividends in an amount sufficient to make scheduled payments of cash interest on the Notes when due and will not otherwise materially impair the Issuer’s ability to make payments on the Notes when due, in each case in the good faith judgment of the Issuer; and
(r) any encumbrances or restrictions of the type referred to in clauses (1), (2) and (3) above imposed by any amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings of the contracts, instruments or obligations referred to in clauses (a) through (q) above; provided that such amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings are, in the good faith judgment of the Issuer, no more restrictive in any material respect with respect to such encumbrance and other restrictions taken as a whole than those prior to such amendment, modification, restatement, renewal, increase, supplement, refunding, replacement or refinancing.
Limitation on guarantees of indebtedness by restricted subsidiaries
The Issuer will not permit any of its Wholly-Owned Subsidiaries that are Restricted Subsidiaries (and non-Wholly-Owned Subsidiaries if such non-Wholly-Owned Subsidiaries guarantee other capital markets debt securities of the Issuer or any Guarantor), other than a Guarantor, a Foreign Subsidiary or a Securitization Subsidiary, to guarantee the payment of any Indebtedness of the Issuer or any other Guarantor unless:
(1) such Restricted Subsidiary within 30 days executes and delivers a supplemental indenture to the Indenture providing for a Guarantee by such Restricted Subsidiary, except that with respect to a guarantee of Indebtedness of the Issuer or any Guarantor, if such Indebtedness is by its express terms subordinated in right of payment to the Notes or such Guarantor’s Guarantee, any such guarantee by such Restricted Subsidiary with respect to such Indebtedness shall be subordinated in right of payment to such Guarantee substantially to the same extent as such Indebtedness is subordinated to the Notes; and
(2) such Restricted Subsidiary waives and will not in any manner whatsoever claim or take the benefit or advantage of, any rights of reimbursement, indemnity or subrogation or any other rights against the Issuer or any other Restricted Subsidiary as a result of any payment by such Restricted Subsidiary under its Guarantee;
provided that this covenant shall not be applicable to (i) any guarantee of any Restricted Subsidiary that existed at the time such Person became a Restricted Subsidiary and was not incurred in connection with, or in contemplation of, such Person becoming a Restricted Subsidiary and (ii) guarantees of any Qualified Securitization Facility by any Securitization Subsidiary. The Issuer may elect, in its sole discretion, to cause any Subsidiary that is not otherwise required to be a Guarantor to become a Guarantor, in which case such Subsidiary shall not be required to comply with the 30-day period described in clause (1) above.
Reports and other information
Notwithstanding that the Issuer may not be subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act or otherwise report on an annual and quarterly basis on forms provided for such annual and quarterly reporting pursuant to rules and regulations promulgated by the SEC, the Indenture requires the Issuer to file with the SEC:
(1) within 90 days after the end of each fiscal year (beginning with the fiscal year ending after the Issue Date), annual reports on Form 10-K, or any successor or comparable form, containing the information required to be contained therein, or required in such successor or comparable form;
132
Table of Contents
(2) within 45 days after the end of each of the first three fiscal quarters of each fiscal year (or, in the case of the fiscal quarter containing the Issue Date, 60 days after the end of such fiscal quarter), reports on Form 10-Q containing all quarterly information that would be required to be contained in Form 10-Q, or any successor or comparable form;
(3) within five (5) Business Days of the date on which an event would have been required to be reported on a Form 8-K or any successor or comparable form if the Issuer had been a reporting company under the Exchange Act, a current report relating to such event on Form 8-K or any successor or comparable form; provided, however, that no such report or information will be required to be so delivered if the Issuer determines in good faith that such event is not material to the holders of Notes or the business, assets, operations or financial condition of the Issuer and its Restricted Subsidiaries, taken as a whole;
(4) simultaneously with the delivery of each report referred to in clauses (1) and (2) above, the related consolidating financial statements reflecting the adjustments necessary to eliminate the accounts of Unrestricted Subsidiaries (if any) from such consolidated financial statements;
in each case, in a manner that complies in all material respects with the requirements specified in such form (subject, in the case of required financial information, to exceptions consistent with the presentation of financial information in the Rule 144A offering memorandum, to the extent filed within the times specified above); provided that the Issuer shall not be so obligated to file such reports with the SEC (i) if the SEC does not permit such filing or (ii) prior to the consummation of an exchange offer or the effectiveness of a shelf registration statement as required by the Registration Rights Agreement, in which event the Issuer will make available such information to the Trustee, the Holders of the Notes and prospective purchasers of Notes, in each case within 15 days after the time the Issuer would be required to file such information with the SEC, if it were subject to Sections 13 or 15(d) of the Exchange Act. In addition, to the extent not satisfied by the foregoing, the Issuer has agreed that, for so long as any Notes are outstanding, it will furnish to Holders and to securities analysts and prospective investors, upon their request, the information required to be delivered pursuant to Rule 144A(d)(4) under the Securities Act; provided, further, that any report required to be delivered under clause (1) or (2) above prior to the completion of the first fiscal year following the Effective Date shall not be required to contain all purchase accounting adjustments relating to the Transactions to the extent it is not practicable to include any such adjustments in such report.
In the event that any direct or indirect parent company of the Issuer of which the Issuer is a Wholly-Owned Subsidiary becomes a guarantor of the Notes, the Indenture permits the Issuer to satisfy its obligations in this covenant with respect to financial information relating to the Issuer by furnishing financial information relating to such parent; provided that, if and so long as such parent company shall have Independent Assets or Operations (as defined below), the same is accompanied by consolidating information that explains in reasonable detail the differences between the information relating to such parent, on the one hand, and the information relating to the Issuer and its Restricted Subsidiaries on a stand-alone basis, on the other hand. “Independent Assets or Operations” means, with respect to any such parent company, that such parent company’s total assets, revenues, income from continuing operations before income taxes and cash flows from operating activities (excluding in each case amounts related to its investment in the Issuer and the Restricted Subsidiaries), determined in accordance with GAAP and as shown on the most recent balance sheet of such parent company, is more than 3.0% of such parent company’s corresponding consolidated amount.
Notwithstanding the foregoing, such requirements shall be deemed satisfied prior to the commencement of the exchange offer or the effectiveness of the shelf registration statement by (1) the filing with the SEC of the exchange offer registration statement or shelf registration statement (or any other similar registration statement), and any amendments thereto, with such financial information that satisfies Regulation S-X of the Securities Act, subject to exceptions consistent with the presentation of financial information the Rule 144A offering memorandum, to the extent filed within the time periods specified above, or (2) by posting on its website and providing to the Trustee within 15 days of the time periods after the Issuer would have been required to file annual and interim reports with the SEC, the financial information (including the “Management’s discussion and
133
Table of Contents
analysis of financial condition and results of operations” section) that would be required to be included in such reports, subject to exceptions consistent with the presentation of financial information in the Rule 144A offering memorandum, to the extent filed within the times specified above.
Notwithstanding anything herein to the contrary, the Issuer will not be deemed to have failed to comply with any of its obligations hereunder for purposes of clause (3) under “—Events of default and remedies” until 60 days after the date any report hereunder is due.
To the extent any information is not provided within the time periods specified in this section “—Reports and other information” and such information is subsequently provided, the Issuer will be deemed to have satisfied its obligations with respect thereto at such time and any Default with respect thereto shall be deemed to have been cured.
The Issuer shall use its commercially reasonable efforts, consistent with its judgment as to what is prudent at the time, to participate in quarterly conference calls to discuss operating results and related matters. The Issuer shall issue a press release which will provide the date and time of any such call and will direct Holders, prospective investors and securities analysts to contact the investor relations office of the Issuer to obtain access to the conference call.
It is understood that the Trustee shall have no obligation whatsoever to determine whether or not such information, documents or reports have been posted on the Issuer’s website.
Payments for consent
The Issuer will not, and will not permit any of its Subsidiaries to, directly or indirectly, pay or cause to be paid any consideration to or for the benefit of any Holder for or as an inducement to any consent, waiver or amendment of any of the terms or provisions of the Indenture or the Notes unless such consideration is offered to be paid and is paid to all Holders that consent, waive or agree to amend in the time frame set forth in the solicitation documents relating to such consent, waiver or amendment.
Events of default and remedies
The Indenture provides that each of the following is an Event of Default:
(1) default in payment when due and payable, upon redemption, acceleration or otherwise, of principal of, or premium, if any, on the Notes;
(2) default for 30 days or more in the payment when due of interest or Additional Interest on or with respect to the Notes;
(3) failure by the Issuer or any Guarantor for 60 days after receipt of written notice given by the Trustee or the Holders of not less than 25.0% in principal amount of the then outstanding Notes to comply with any of its obligations, covenants or agreements (other than a default referred to in clause (1) or (2) above) contained in the Indenture or the Notes;
(4) default under any mortgage, indenture or instrument under which there is issued or by which there is secured or evidenced any Indebtedness for money borrowed by the Issuer or any of its Restricted Subsidiaries or the payment of which is guaranteed by the Issuer or any of its Restricted Subsidiaries, other than Indebtedness owed to the Issuer or a Restricted Subsidiary, whether such Indebtedness or guarantee now exists or is created after the issuance of the Notes, if both:
(a) such default either results from the failure to pay any principal of such Indebtedness at its stated final maturity (after giving effect to any applicable grace periods) or relates to an obligation other than the obligation to pay principal of any such Indebtedness at its stated final maturity and results in the holder or holders of such Indebtedness causing such Indebtedness to become due prior to its stated maturity; and
134
Table of Contents
(b) the principal amount of such Indebtedness, together with the principal amount of any other such Indebtedness in default for failure to pay principal at stated final maturity (after giving effect to any applicable grace periods), or the maturity of which has been so accelerated, aggregate $25.0 million or more at any one time outstanding;
(5) failure by the Issuer or any Significant Subsidiary (or any group of Restricted Subsidiaries that together (as of the latest audited consolidated financial statements of the Issuer) would constitute a Significant Subsidiary) to pay final judgments aggregating in excess of $25.0 million (net of amounts covered by insurance policies issued by reputable and creditworthy insurance companies), which final judgments remain unpaid, undischarged and unstayed for a period of more than 60 days after such judgment becomes final, and in the event such judgment is covered by insurance, an enforcement proceeding has been commenced by any creditor upon such judgment or decree which is not promptly stayed;
(6) certain events of bankruptcy or insolvency with respect to the Issuer or any Significant Subsidiary (or any group of Restricted Subsidiaries that together (as of the latest audited consolidated financial statements of the Issuer) would constitute a Significant Subsidiary); or
(7) the Guarantee of any Significant Subsidiary (or any group of Restricted Subsidiaries that together (as of the latest audited consolidated financial statements of the Issuer) would constitute a Significant Subsidiary) shall for any reason cease to be in full force and effect or be declared null and void in a final non-appealable judgment of a court of competent jurisdiction or any responsible officer of any Guarantor that is a Significant Subsidiary (or the responsible officers of any group of Restricted Subsidiaries that together (as of the latest audited consolidated financial statements of the Issuer) would constitute a Significant Subsidiary), as the case may be, denies that it has any further liability under its Guarantee or gives notice to such effect, other than by reason of the termination of the Indenture or the release of any such Guarantee in accordance with the Indenture.
If any Event of Default (other than of a type specified in clause (6) above) occurs and is continuing under the Indenture, the Trustee or the Holders of at least 25.0% in principal amount of the then total outstanding Notes may declare the principal, premium, if any, interest and any other monetary obligations on all the then outstanding Notes to be due and payable immediately.
Upon the effectiveness of such declaration, such principal of and premium, if any, and interest will be due and payable immediately. Notwithstanding the foregoing, in the case of an Event of Default arising under clause (6) of the first paragraph of this section, all outstanding Notes will become due and payable without further action or notice. The Indenture provides that the Trustee may withhold from the Holders notice of any continuing Default, except a Default relating to the payment of principal, premium, if any, or interest, if it determines that withholding notice is in their interest. In addition, the Trustee will have no obligation to accelerate the Notes if in the best judgment of the Trustee acceleration is not in the best interests of the Holders of the Notes.
The Indenture provides that the Holders of a majority in aggregate principal amount of the then outstanding Notes by notice to the Trustee may on behalf of the Holders of all of the Notes waive any existing Default and its consequences under the Indenture (except a continuing Default in the payment of interest on, premium, if any, or the principal of any Note held by a non-consenting Holder) and rescind any acceleration with respect to the Notes and its consequences (except if such rescission would conflict with any judgment of a court of competent jurisdiction). In the event of any Event of Default specified in clause (4) above, such Event of Default and all consequences thereof (excluding any resulting payment default, other than as a result of acceleration of the Notes) shall be annulled, waived and rescinded, automatically and without any action by the Trustee or the Holders, if within 20 days after such Event of Default arose:
(1) the Indebtedness or guarantee that is the basis for such Event of Default has been discharged;
(2) holders thereof have rescinded or waived the acceleration, notice or action (as the case may be) giving rise to such Event of Default; or
(3) the default that is the basis for such Event of Default has been cured.
135
Table of Contents
Subject to the provisions of the Indenture relating to the duties of the Trustee thereunder, in case an Event of Default occurs and is continuing, the Trustee will be under no obligation to exercise any of the rights or powers under the Indenture at the request or direction of any of the Holders of the Notes unless the Holders have offered to the Trustee indemnity or security reasonably satisfactory to the Trustee against any loss, liability or expense. Except to enforce the right to receive payment of principal, premium (if any) or interest when due, no Holder of a Note may pursue any remedy with respect to the Indenture or the Notes unless:
(1) such Holder has previously given the Trustee written notice that an Event of Default is continuing;
(2) Holders of at least 25.0% in principal amount of the total outstanding Notes have requested the Trustee to pursue the remedy;
(3) Holders of the Notes have offered the Trustee security or indemnity reasonably satisfactory to it against any loss, liability or expense;
(4) the Trustee has not complied with such request within 60 days after the receipt thereof and the offer of security or indemnity; and
(5) Holders of a majority in principal amount of the total outstanding Notes have not given the Trustee a direction inconsistent with such request within such 60-day period.
Subject to certain restrictions, under the Indenture the Holders of a majority in principal amount of the total outstanding Notes are given the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustee or of exercising any trust or power conferred on the Trustee. The Trustee, however, may refuse to follow any direction that conflicts with law or the Indenture or that the Trustee determines is unduly prejudicial to the rights of any other Holder of a Note or that would involve the Trustee in personal liability.
The Indenture provides that the Issuer is required to deliver to the Trustee annually a statement regarding compliance with the Indenture, and the Issuer is required, within five Business Days, upon becoming aware of any Default, to deliver to the Trustee a statement specifying such Default.
No personal liability of directors, officers, employees and stockholders
No past, present or future director, officer, employee, incorporator, member, partner or stockholder of the Issuer or any Guarantor or any of their parent companies (other than the Issuer and the Guarantors) shall have any liability, for any obligations of the Issuer or the Guarantors under the Notes, the Guarantees or the Indenture or for any claim based on, in respect of, or by reason of such obligations or their creation. Each Holder by accepting Notes waives and releases all such liability. The waiver and release are part of the consideration for issuance of the Notes. Such waiver may not be effective to waive liabilities under the federal securities laws, and it is the view of the SEC that such a waiver is against public policy.
Legal defeasance and covenant defeasance
The obligations of the Issuer and the Guarantors under the Indenture will terminate (other than certain obligations) and will be released upon payment in full of all of the Notes. The Issuer may, at its option and at any time, elect to have all of its obligations discharged with respect to the Notes and have each Guarantor’s obligation discharged with respect to its Guarantee (“Legal Defeasance”) and cure all then existing Events of Default except for:
(1) the rights of Holders of Notes to receive payments in respect of the principal of, premium, if any, and interest on the Notes when such payments are due solely out of the trust created pursuant to the Indenture;
(2) the Issuer’s obligations with respect to Notes concerning issuing temporary Notes, registration of such Notes, mutilated, destroyed, lost or stolen Notes and the maintenance of an office or agency for payment and money for security payments held in trust;
136
Table of Contents
(3) the rights, powers, trusts, duties and immunities of the Trustee, and the Issuer’s obligations in connection therewith; and
(4) the Legal Defeasance provisions of the Indenture.
In addition, the Issuer may, at its option and at any time, elect to have its obligations and those of each Guarantor released with respect to certain covenants that are described in the Indenture (“Covenant Defeasance”), and thereafter any omission to comply with such obligations shall not constitute a Default with respect to the Notes. In the event Covenant Defeasance occurs, certain events (not including bankruptcy, receivership, rehabilitation and insolvency events pertaining to the Issuer) described under “—Events of default and remedies” will no longer constitute an Event of Default with respect to the Notes.
In order to exercise either Legal Defeasance or Covenant Defeasance with respect to the Notes:
(1) the Issuer must irrevocably deposit with the Trustee, in trust, for the benefit of the Holders of the Notes, cash in U.S. dollars, U.S. dollar-denominated Government Securities, or a combination thereof, in such amounts as will be sufficient, in the opinion of a nationally recognized firm of independent public accountants, to pay the principal of, premium, if any, and interest due on the Notes on the stated maturity date or on the redemption date, as the case may be, of such principal, premium, if any, or interest on such Notes, and the Issuer must specify whether such Notes are being defeased to maturity or to a particular redemption date; provided, that upon any redemption that requires the payment of the Applicable Premium, the amount deposited shall be sufficient for purposes of the Indenture to the extent that an amount is deposited with the Trustee equal to the Applicable Premium calculated as of the date of the notice of redemption, with any deficit as of the date of redemption (any such amount, the “Applicable Premium Deficit”) only required to be deposited with the Trustee on or prior to the date of redemption. Any Applicable Premium Deficit shall be set forth in an Officer’s Certificate delivered to the Trustee simultaneously with the deposit of such Applicable Premium Deficit that confirms that such Applicable Premium Deficit shall be applied toward such redemption;
(2) in the case of Legal Defeasance, the Issuer shall have delivered to the Trustee an Opinion of Counsel confirming that, subject to customary assumptions and exclusions (a) the Issuer has received from, or there has been published by, the United States Internal Revenue Service a ruling or (b) since the issuance of the Notes, there has been a change in the applicable U.S. federal income tax law, in either case to the effect that, and based thereon such Opinion of Counsel shall confirm that, subject to customary assumptions and exclusions, the Holders of the Notes will not recognize income, gain or loss for U.S. federal income tax purposes, as applicable, as a result of such Legal Defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Legal Defeasance had not occurred;
(3) in the case of Covenant Defeasance, the Issuer shall have delivered to the Trustee an Opinion of Counsel confirming that, subject to customary assumptions and exclusions, the Holders of the Notes will not recognize income, gain or loss for U.S. federal income tax purposes as a result of such Covenant Defeasance and will be subject to such tax on the same amounts, in the same manner and at the same times as would have been the case if such Covenant Defeasance had not occurred;
(4) no Default (other than that resulting from borrowing funds to be applied to make such deposit and any similar and simultaneous deposit relating to other Indebtedness and, in each case, the granting of Liens in connection therewith) shall have occurred and be continuing on the date of such deposit;
(5) such Legal Defeasance or Covenant Defeasance shall not result in a breach or violation of, or constitute a default under the Senior Credit Facilities or any other material agreement or instrument (other than the Indenture) to which, the Issuer or any Guarantor is a party or by which the Issuer or any Guarantor is bound (other than that resulting from any borrowing of funds to be applied to make the deposit required to effect such Legal Defeasance or Covenant Defeasance and any similar and simultaneous deposit relating to other Indebtedness, and, in each case, the granting of Liens in connection therewith);
137
Table of Contents
(6) the Issuer shall have delivered to the Trustee an Opinion of Counsel to the effect that, as of the date of such opinion and subject to customary assumptions and exclusions following the deposit, the trust funds will not be subject to the effect of Section 547 of Title 11 of the United States Code;
(7) the Issuer shall have delivered to the Trustee an Officer’s Certificate stating that the deposit was not made by the Issuer with the intent of defeating, hindering, delaying or defrauding any creditors of the Issuer or any Guarantor or others; and
(8) the Issuer shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel (which Opinion of Counsel may be subject to customary assumptions and exclusions) each stating that all conditions precedent provided for or relating to the Legal Defeasance or the Covenant Defeasance, as the case may be, have been complied with.
Satisfaction and discharge
The Indenture will be discharged and will cease to be of further effect as to all Notes, when either:
(1) all Notes theretofore authenticated and delivered, except lost, stolen or destroyed Notes which have been replaced or paid and Notes for whose payment money has theretofore been deposited in trust, have been delivered to the Trustee for cancellation; or
(2) (a) all Notes not theretofore delivered to the Trustee for cancellation have become due and payable by reason of the making of a notice of redemption or otherwise, will become due and payable within one year or are to be called for redemption within one year under arrangements satisfactory to the Trustee for the giving of notice of redemption by the Trustee in the name, and at the expense, of the Issuer, and the Issuer or any Guarantor has irrevocably deposited or caused to be deposited with the Trustee as trust funds in trust solely for the benefit of the Holders of the Notes, cash in U.S. dollars, U.S. dollar-denominated Government Securities, or a combination thereof, in such amounts as will be sufficient without consideration of any reinvestment of interest to pay and discharge the entire indebtedness on the Notes not theretofore delivered to the Trustee for cancellation for principal, premium, if any, and accrued interest to the date of maturity or redemption; provided, that upon any redemption that requires the payment of the Applicable Premium, the amount deposited shall be sufficient for purposes of the Indenture to the extent that an amount is deposited with the Trustee equal to the Applicable Premium calculated as of the date of the notice of redemption, with any Applicable Premium Deficit only required to be deposited with the Trustee on or prior to the date of redemption. Any Applicable Premium Deficit shall be set forth in an Officer’s Certificate delivered to the Trustee simultaneously with the deposit of such Applicable Premium Deficit that confirms that such Applicable Premium Deficit shall be applied toward such redemption;
(b) no Default (other than that resulting from borrowing funds to be applied to make such deposit or any similar and simultaneous deposit relating to other Indebtedness and, in each case, the granting of Liens in connection therewith) with respect to the Indenture or the Notes shall have occurred and be continuing on the date of such deposit or shall occur as a result of such deposit, and such deposit will not result in a breach or violation of, or constitute a default under the Senior Credit Facilities or any other material agreement or instrument (other than the Indenture) to which the Issuer or any Guarantor is a party or by which the Issuer or any Guarantor is bound (other than resulting from any borrowing of funds to be applied to make such deposit and any similar and simultaneous deposit relating to other Indebtedness and, in each case, the granting of Liens in connection therewith);
(c) the Issuer has paid or caused to be paid all sums payable by it under the Indenture; and
(d) the Issuer has delivered irrevocable instructions to the Trustee to apply the deposited money toward the payment of the Notes at maturity or the redemption date, as the case may be.
In addition, the Issuer must deliver an Officer’s Certificate and an Opinion of Counsel to the Trustee stating that all conditions precedent to satisfaction and discharge have been satisfied.
138
Table of Contents
Amendment, supplement and waiver
Except as provided in the next two succeeding paragraphs, the Indenture, any Guarantee and the Notes may be amended or supplemented with the consent of the Holders of at least a majority in principal amount of the Notes then outstanding, including consents obtained in connection with a purchase of, or tender offer or exchange offer for, Notes, and any existing Default or compliance with any provision of the Indenture or the Notes issued thereunder may be waived with the consent of the Holders of a majority in principal amount of the then outstanding Notes, other than Notes beneficially owned by the Issuer or its Affiliates (including consents obtained in connection with a purchase of or tender offer or exchange offer for the Notes).
The Indenture provides that, without the consent of each affected Holder of Notes, an amendment or waiver may not, with respect to any Notes held by a non-consenting Holder:
(1) reduce the principal amount of such Notes whose Holders must consent to an amendment, supplement or waiver;
(2) reduce the principal of or change the fixed final maturity of any such Note or alter or waive the provisions with respect to the redemption of such Notes (other than provisions relating to the covenants described above under “—Repurchase at the option of holders”);
(3) reduce the rate of or change the time for payment of interest on any Note;
(4) waive a Default in the payment of principal of or premium, if any, or interest on the Notes, except a rescission of acceleration of the Notes by the Holders of at least a majority in aggregate principal amount of the Notes and a waiver of the payment default that resulted from such acceleration, or in respect of a covenant or provision contained in the Indenture or any Guarantee which cannot be amended or modified without the consent of all affected Holders;
(5) make any Note payable in money other than that stated therein;
(6) make any change in the provisions of the Indenture relating to waivers of past Defaults or the rights of Holders to receive payments of principal of or premium, if any, or interest on the Notes;
(7) make any change in these amendment and waiver provisions;
(8) impair the right of any Holder to receive payment of principal of, or premium, if any, or interest on such Holder’s Notes on or after the due dates therefor or to institute suit for the enforcement of any payment on or with respect to such Holder’s Notes;
(9) make any change to or modify the ranking of the Notes that would adversely affect the Holders; or
(10) except as expressly permitted by the Indenture, modify the Guarantees of any Significant Subsidiary in any manner adverse to the Holders of the Notes.
Notwithstanding the foregoing, the Issuer, any Guarantor (with respect to a Guarantee or the Indenture to which it is a party) and the Trustee may amend or supplement the Indenture and any Guarantee or Notes without the consent of any Holder:
(1) to cure any ambiguity, omission, mistake, defect or inconsistency;
(2) to provide for uncertificated Notes in addition to or in place of certificated Notes;
(3) to comply with the covenant relating to mergers, consolidations and sales of assets;
(4) to provide the assumption of the Issuer’s or any Guarantor’s obligations to the Holders;
(5) to make any change that would provide any additional rights or benefits to the Holders or that does not materially adversely affect the legal rights under the Indenture of any such Holder;
(6) to add covenants for the benefit of the Holders or to surrender any right or power conferred upon the Issuer or any Guarantor;
139
Table of Contents
(7) to comply with requirements of the SEC in order to effect or maintain the qualification of the Indenture under the Trust Indenture Act;
(8) to evidence and provide for the acceptance and appointment under the Indenture of a successor Trustee thereunder pursuant to the requirements thereof;
(9) to provide for the issuance of exchange notes or private exchange notes, which are identical to exchange notes except that they are not freely transferable;
(10) to add a Guarantor under the Indenture or to release a Guarantor in accordance with the terms of the Indenture;
(11) to conform the text of the Indenture, Guarantees or the Notes to any provision of this “Description of exchange notes” to the extent that such provision in this “Description of exchange notes” was intended to be a verbatim recitation of a provision of the Indenture, Guarantee or Notes, as provided in an Officer’s Certificate;
(12) to make any amendment to the provisions of the Indenture relating to the transfer and legending of Notes as permitted by the Indenture, including, without limitation to facilitate the issuance and administration of the Notes; provided that (a) compliance with the Indenture as so amended would not result in Notes being transferred in violation of the Securities Act or any applicable securities law and (b) such amendment does not materially and adversely affect the rights of Holders to transfer Notes; or
(13) to provide for the issuance of Additional Notes in accordance with the terms of the Indenture.
The consent of the Holders is not necessary under the Indenture to approve the particular form of any proposed amendment. It is sufficient if such consent approves the substance of the proposed amendment.
Notices
Notices given by publication or electronic delivery will be deemed given on the first date on which publication is made, and notices given by first-class mail, postage prepaid, will be deemed given five calendar days after mailing.
Concerning the trustee
The Indenture contains certain limitations on the rights of the Trustee thereunder, should it become a creditor of the Issuer, to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim as security or otherwise. The Trustee is permitted to engage in other transactions; however, if it acquires any conflicting interest, it must eliminate such conflict within 90 days, apply to the SEC for permission to continue as Trustee (if the Indenture has been qualified under the Trust Indenture Act) or resign.
The Indenture provides that the Holders of a majority in principal amount of the outstanding Notes will have the right to direct the time, method and place of conducting any proceeding for exercising any remedy available to the Trustee, subject to certain exceptions. The Indenture provides that in case an Event of Default shall occur (which shall not be cured), the Trustee is required, in the exercise of its power, to use the degree of care of a prudent person in the conduct of his own affairs. Subject to such provisions, the Trustee is under no obligation to exercise any of its rights or powers under the Indenture at the request of any Holder of the Notes, unless such Holder shall have offered to the Trustee security and indemnity reasonably satisfactory to it against any loss, liability or expense.
Additional information
Anyone who receives this prospectus may obtain a copy of the Indenture and the Registration Rights Agreement without charge by writing to Immucor, Inc., 3130 Gateway Drive, Norcross, Georgia, 30071, Attention: Investor Relations.
140
Table of Contents
Governing law
The Indenture, the Notes and any Guarantee are governed by and construed in accordance with the laws of the State of New York.
Certain definitions
Set forth below are certain defined terms used in the Indenture. For purposes of the Indenture, unless otherwise specifically indicated, the term “consolidated” with respect to any Person refers to such Person consolidated with its Restricted Subsidiaries and excludes from such consolidation any Unrestricted Subsidiary as if such Unrestricted Subsidiary were not an Affiliate of such Person.
“Acquired Indebtedness” means, with respect to any specified Person,
(1) Indebtedness of any other Person existing at the time such other Person is merged with or into or became a Restricted Subsidiary of such specified Person, including Indebtedness incurred in connection with, or in contemplation of, such other Person merging with or into or becoming a Restricted Subsidiary of such specified Person, and
(2) Indebtedness secured by a Lien encumbering any asset acquired by such specified Person.
“Acquisition” means the transactions contemplated by the Transaction Agreement.
“Additional Interest” means all additional interest then owing pursuant to the Registration Rights Agreement.
“Affiliate” of any specified Person means any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such specified Person. For purposes of this definition, “control” (including, with correlative meanings, the terms “controlling,” “controlled by” and “under common control with”), as used with respect to any Person, shall mean the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such Person, whether through the ownership of voting securities, by agreement or otherwise.
“Applicable Premium” means, with respect to any Note on any Redemption Date, the greater of:
(1) 1.0% of the principal amount of such Note; and
(2) the excess, if any, of (a) the present value at such Redemption Date of (i) the redemption price of such Note at August 15, 2015 (each such redemption price being set forth in the table appearing above under “—Optional redemption”), plus (ii) all required remaining scheduled interest payments due on such Note through August 15, 2015 (excluding accrued but unpaid interest to the Redemption Date), computed using a discount rate equal to the Treasury Rate as of such Redemption Date plus 50 basis points; over (b) the then outstanding principal amount of such Note.
“Asset Sale” means:
(1) the sale, conveyance, transfer or other disposition, whether in a single transaction or a series of related transactions (including by way of a Sale and Lease-Back Transaction) of property or assets of the Issuer or any of its Restricted Subsidiaries (each referred to in this definition as a “disposition”); or
(2) the issuance or sale of Equity Interests by any of the Issuer’s Restricted Subsidiaries or the sale by the Issuer or any of the Issuer’s Restricted Subsidiaries of Equity Interests in any of the Issuer’s Subsidiaries (other than Preferred Stock or Disqualified Stock of Restricted Subsidiaries issued in compliance with the covenant described under “—Certain covenants—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”), whether in a single transaction or a series of related transactions;
141
Table of Contents
in each case, other than:
(a) any disposition of Cash Equivalents or Investment Grade Securities or obsolete or worn out property or equipment in the ordinary course of business or any disposition of inventory or goods (or other assets) held for sale or no longer used in the ordinary course of business;
(b) the disposition of all or substantially all of the assets of the Issuer in a manner permitted pursuant to the provisions described above under “—Certain covenants—Merger, consolidation or sale of all or substantially all assets” or any disposition that constitutes a Change of Control pursuant to the Indenture;
(c) the making of any Restricted Payment that is permitted to be made, and is made, under the covenant described above under “—Certain covenants—Limitation on restricted payments” or any Permitted Investment;
(d) any disposition of assets or issuance or sale of Equity Interests of any Restricted Subsidiary in any transaction or series of related transactions with an aggregate fair market value of less than $10.0 million;
(e) any disposition of property or assets or the issuance of securities by a Restricted Subsidiary to the Issuer or by the Issuer or a Restricted Subsidiary to a Restricted Subsidiary;
(f) to the extent allowable under Section 1031 of the Internal Revenue Code of 1986, any exchange of like property (excluding any boot thereon) for use in a Similar Business;
(g) the lease, assignment or sub-lease of any real or personal property in the ordinary course of business;
(h) any issuance or sale of Equity Interests in, or Indebtedness or other securities of, an Unrestricted Subsidiary;
(i) foreclosures, condemnation or any similar action on assets or the granting of Liens not prohibited by the Indenture;
(j) sales of accounts receivable, or participations therein, or Securitization Assets or related assets in connection with any Qualified Securitization Facility or the disposition of an account receivable in connection with the collection or compromise thereof in the ordinary course of business;
(k) any financing transaction with respect to property built or acquired by the Issuer or any Restricted Subsidiary after the Effective Date, including Sale and Lease-Back Transactions and asset securitizations permitted by the Indenture;
(l) the sale or discount of inventory, accounts receivable or notes receivable in the ordinary course of business or the conversion of accounts receivable to notes receivable;
(m) the licensing or sub-licensing of intellectual property or other general intangibles in the ordinary course of business;
(n) any surrender or waiver of contract rights or the settlement, release or surrender of contract rights or other litigation claims in the ordinary course of business;
(o) the unwinding of any Hedging Obligations;
(p) sales, transfers and other dispositions of Investments in joint ventures to the extent required by, or made pursuant to, customary buy/sell arrangements between the joint venture parties set forth in joint venture arrangements and similar binding arrangements;
(q) the abandonment of intellectual property rights in the ordinary course of business, which in the reasonable good faith determination of the Issuer are not material to the conduct of the business of the Issuer and its Restricted Subsidiaries taken as a whole;
142
Table of Contents
(r) the granting of a Lien that is permitted under the covenant described above under “—Certain covenants—Liens”; and
(s) the issuance of directors’ qualifying shares and shares of Capital Stock of Foreign Subsidiaries issued to foreign nationals as required by applicable law.
“Business Day” means each day which is not a Legal Holiday.
“Capital Stock” means:
(1) in the case of a corporation, corporate stock or shares in the capital of such corporation;
(2) in the case of an association or business entity, any and all shares, interests, participations, rights or other equivalents (however designated) of corporate stock;
(3) in the case of a partnership or limited liability company, partnership or membership interests (whether general or limited); and
(4) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distributions of assets of, the issuing Person but excluding from all of the foregoing any debt securities convertible into Capital Stock, whether or not such debt securities include any right of participation with Capital Stock.
“Capitalized Lease Obligation” means, at the time any determination thereof is to be made, the amount of the liability in respect of a capital lease that would at such time be required to be capitalized and reflected as a liability on a balance sheet (excluding the footnotes thereto) prepared in accordance with GAAP.
“Cash Equivalents” means:
(1) United States dollars, Euros, Yen, Canadian Dollars, Sterling or any national currency of any Participating Member State of the EMU;
(2) in the case of any Foreign Subsidiary, such local currencies held by it from time to time in the ordinary course of business and not for speculation;
(3) readily marketable direct obligations issued or directly and fully and unconditionally guaranteed or insured by the United States government or any agency or instrumentality thereof the securities of which are unconditionally guaranteed as a full faith and credit obligation of such government with maturities of 12 months or less from the date of acquisition;
(4) certificates of deposit, time deposits and eurodollar time deposits with maturities of one year or less from the date of acquisition, demand deposits, bankers’ acceptances with maturities not exceeding one year and overnight bank deposits, in each case with any domestic or foreign commercial bank having capital and surplus of not less than $500.0 million in the case of U.S. banks and $100.0 million (or the United States dollar equivalent as of the date of determination) in the case of non-U.S. banks;
(5) repurchase obligations for underlying securities of the types described in clauses (3) and (4) above or clause (7) below entered into with any financial institution meeting the qualifications specified in clause (4) above;
(6) commercial paper rated at least P-2 by Moody’s or at least A-2 by S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another nationally recognized statistical rating agency) and in each case maturing within 12 months after the date of creation thereof;
(7) marketable short-term money market and similar highly liquid funds having a rating of at least P-2 or A-2 from either Moody’s or S&P, respectively (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another nationally recognized statistical rating agency);
143
Table of Contents
(8) readily marketable direct obligations issued by any state, commonwealth or territory of the United States or any political subdivision or taxing authority thereof having an Investment Grade Rating from either Moody’s or S&P (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another nationally recognized statistical rating agency) with maturities of 12 months or less from the date of acquisition;
(9) Investments with average maturities of 12 months or less from the date of acquisition in money market funds rated AAA- (or the equivalent thereof) or better by S&P or Aaa3 (or the equivalent thereof) or better by Moody’s (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another nationally recognized statistical rating agency); and
(10) investment funds investing substantially all of their assets in securities of the types described in clauses (1) through (9) above.
In the case of Investments by any Foreign Subsidiary or Investments made in a country outside the United States of America, Cash Equivalents shall also include (i) investments of the type and maturity described in clauses (1) through (10) above of foreign obligors, which Investments or obligors (or the parents of such obligors) have ratings described in such clauses or equivalent ratings from comparable foreign rating agencies and (ii) other short-term investments utilized by Foreign Subsidiaries in accordance with normal investment practices for cash management in investments analogous to the foregoing investments in clauses (1) through (10) and in this paragraph.
Notwithstanding the foregoing, Cash Equivalents shall include amounts denominated in currencies other than those set forth in clause (1) above; provided that such amounts are converted into any currency listed in clause (1) above as promptly as practicable and in any event within ten (10) Business Days following the receipt of such amounts.
“Cash Management Services” means any agreement or arrangement to provide cash management services, including treasury, depository, overdraft, credit card processing or credit or debit card, purchase card, electronic funds transfer and other cash management arrangements.
“Change of Control” means the occurrence of any of the following after the Effective Date:
(1) the sale, lease, transfer, conveyance or other disposition in one or a series of related transactions (other than by merger or consolidation) of all or substantially all of the assets of the Issuer and its Subsidiaries, taken as a whole, to any Person other than any Permitted Holder; or
(2) the Issuer becomes aware of (by way of a report or any other filing pursuant to Section 13(d) of the Exchange Act, proxy, vote, written notice or otherwise) the acquisition by (A) any Person (other than any Permitted Holder) or (B) Persons (other than any Permitted Holder) that are together a group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act, or any successor provision), including any such group acting for the purpose of acquiring, holding or disposing of securities (within the meaning of Rule 13d-5(b)(1) under the Exchange Act), in a single transaction or in a related series of transactions, by way of merger, consolidation or other business combination or purchase of beneficial ownership (within the meaning of Rule 13d-3 under the Exchange Act, or any successor provision) of 50.0% or more of the total voting power of the Voting Stock of the Issuer directly or indirectly through any of its direct or indirect parent holding companies, other than any transaction in which the Issuer shall become the Wholly-Owned Subsidiary of a Parent Company.
“Consolidated Depreciation and Amortization Expense” means with respect to any Person for any period, the total amount of depreciation and amortization expense of such Person and its Restricted Subsidiaries, including the amortization of deferred financing fees, debt issuance costs, and commissions, fees and expenses of such Person and its Restricted Subsidiaries for such period on a consolidated basis and otherwise determined in accordance with GAAP.
144
Table of Contents
“Consolidated Interest Expense” means, with respect to any Person for any period, without duplication, the sum of:
(1) consolidated interest expense of such Person and its Restricted Subsidiaries for such period, to the extent such expense was deducted (and not added back) in computing Consolidated Net Income (including (a) amortization of original issue discount resulting from the issuance of Indebtedness at less than par, (b) all commissions, discounts and other fees and charges owed with respect to letters of credit or bankers acceptances, (c) non-cash interest payments (but excluding any non-cash interest expense attributable to the movement in the mark to market valuation of Hedging Obligations or other derivative instruments pursuant to GAAP), (d) the interest component of Capitalized Lease Obligations, and (e) net payments, if any, made (less net payments, if any, received), pursuant to interest rate Hedging Obligations with respect to Indebtedness, and excluding (t) any expense resulting from the discounting of any Indebtedness in connection with the application of recapitalization accounting or, if applicable, purchase accounting in connection with the Transactions or any acquisition, (u) penalties and interest relating to taxes, (v) any Additional Interest and any “additional interest” with respect to other securities, (w) amortization of deferred financing fees, debt issuance costs, commissions, fees and expenses, (x) any expensing of bridge, commitment and other financing fees and any other fees related to the Transactions, (y) commissions, discounts, yield and other fees and charges (including any interest expense) related to any Qualified Securitization Facility and (z) any accretion of accrued interest on discounted liabilities and any prepayment premium or penalty); plus
(2) consolidated capitalized interest of such Person and its Restricted Subsidiaries for such period, whether paid or accrued; less
(3) interest income of such Person and its Restricted Subsidiaries for such period.
For purposes of this definition, interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by such Person to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP.
“Consolidated Net Income” means, with respect to any Person for any period, the aggregate of the Net Income of such Person and its Restricted Subsidiaries for such period, on a consolidated basis, and otherwise determined in accordance with GAAP; provided, however, that, without duplication,
(1) any net after-tax effect of extraordinary, non-recurring or unusual gains or losses (less all fees and expenses relating thereto) or expenses, and Transaction Expenses, relocation costs, integration costs, facility consolidation and closing costs, severance costs and expenses, one-time compensation charges, costs relating to pre-opening and opening costs for facilities, signing, retention and completion bonuses, costs incurred in connection with any strategic initiatives, transition costs, costs incurred in connection with non-recurring product and intellectual property development after the Effective Date, other business optimization expenses (including costs and expenses relating to business optimization programs), and new systems design and implementation costs and project start-up costs, operating expenses attributable to the implementation of cost-savings initiatives, and curtailments and modifications to pension and post-retirement employee benefit plans shall be excluded;
(2) the cumulative effect of a change in accounting principles and changes as a result of the adoption or modification of accounting policies during such period whether effected through a cumulative effect adjustment or a retroactive application, in each case in accordance with GAAP, shall be excluded;
(3) any net after-tax effect of gains or losses on disposal, abandonment or discontinuance of disposed, abandoned or discontinued operations, as applicable, shall be excluded;
(4) any net after-tax effect of gains or losses (less all fees, expenses and charges relating thereto) attributable to asset dispositions or abandonments or the sale or other disposition of any Capital Stock of any Person other than in the ordinary course of business shall be excluded;
145
Table of Contents
(5) the Net Income for such period of any Person that is an Unrestricted Subsidiary shall be excluded, and, solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of “—Certain covenants—Limitation on restricted payments,” the Net Income for such period of any Person that is not a Subsidiary or that is accounted for by the equity method of accounting shall be excluded; provided that Consolidated Net Income of such Person shall be increased by the amount of dividends or distributions or other payments that are actually paid in cash (or to the extent converted into cash) to such Person or a Restricted Subsidiary thereof in respect of such period;
(6) solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of “—Certain covenants—Limitation on restricted payments,” the Net Income for such period of any Restricted Subsidiary (other than any Guarantor) shall be excluded to the extent that the declaration or payment of dividends or similar distributions by that Restricted Subsidiary of its Net Income is not at the date of determination permitted without any prior governmental approval (which has not been obtained) or, directly or indirectly, by the operation of the terms of its charter or any agreement, instrument, judgment, decree, order, statute, rule, or governmental regulation applicable to that Restricted Subsidiary or its stockholders, unless such restriction with respect to the payment of dividends or similar distributions has been legally waived; provided that Consolidated Net Income of such Person will be increased by the amount of dividends or other distributions or other payments actually paid in cash (or to the extent converted into cash) to such Person or a Restricted Subsidiary thereof in respect of such period, to the extent not already included therein;
(7) effects of adjustments (including the effects of such adjustments pushed down to such Person and its Restricted Subsidiaries) in such Person’s consolidated financial statements pursuant to GAAP (including in the inventory, property and equipment, software, goodwill, intangible assets, in-process research and development, deferred revenue and debt line items thereof) resulting from the application of recapitalization accounting or purchase accounting, as the case may be, in relation to the Transactions or any consummated acquisition or the amortization or write-off of any amounts thereof, net of taxes, shall be excluded;
(8) any net after-tax effect of income (loss) from the early extinguishment or conversion of (a) Indebtedness, (b) Hedging Obligations or (c) other derivative instruments shall be excluded;
(9) any impairment charge or asset write-off or write-down, including impairment charges or asset write-offs or write-downs related to inventory, intangible assets, long-lived assets, investments in debt and equity securities or as a result of a change in law or regulation, in each case, pursuant to GAAP, and the amortization of intangibles arising pursuant to GAAP shall be excluded;
(10) any non-cash compensation charge or expense, including any such charge or expense arising from grants of stock appreciation or similar rights, stock options, restricted stock or other rights to, and any cash charges associated with the rollover, acceleration, or payout of, Equity Interests by management of such Person or of a Restricted Subsidiary or any of its direct or indirect parent companies in connection with the Transactions, shall be excluded;
(11) any fees, expenses or charges incurred during such period, or any amortization thereof for such period, in connection with any acquisition, Investment, Asset Sale, disposition, incurrence or repayment of Indebtedness (including such fees, expenses or charges related to the offering and issuance of the Notes and the syndication and incurrence of the Senior Credit Facilities), issuance of Equity Interests, refinancing transaction or amendment or modification of any debt instrument (including any amendment or other modification of the Notes and the Senior Credit Facilities) and including, in each case, any such transaction consummated prior to the Effective Date and any such transaction undertaken but not completed, and any charges or non-recurring merger costs incurred during such period as a result of any such transaction, in each case whether or not successful or consummated, shall be excluded;
(12) accruals and reserves that are established within twelve months after the Effective Date that are so required to be established as a result of the Transactions (or within twelve months after the closing of any acquisition that are so required to be established as a result of such acquisition) in accordance with GAAP shall be excluded;
146
Table of Contents
(13) to the extent covered by insurance and actually reimbursed, or, so long as such Person has made a determination that there exists reasonable evidence that such amount will in fact be reimbursed by the insurer and only to the extent that such amount is (a) not denied by the applicable carrier in writing within 180 days and (b) in fact reimbursed within 365 days of the date of the insurable event (with a deduction for any amount so added back to the extent not so reimbursed within such 365-day period), expenses, charges or losses with respect to liability or casualty events or business interruption shall be excluded;
(14) any noncash compensation expense resulting from the application of Accounting Standards Codification Topic No. 718, Compensation—Stock Compensation, shall be excluded; and
(15) the following items shall be excluded:
(a) any net unrealized gain or loss (after any offset) resulting in such period from Hedging Obligations and the application of Accounting Standards Codification Topic No. 815, Derivatives and Hedging;
(b) any net unrealized gain or loss (after any offset) resulting in such period from currency translation gains or losses including those related to currency remeasurements of Indebtedness (including any net loss or gain resulting from Hedging Obligations for currency exchange risk) and any other foreign currency translation gains and losses, to the extent such gain or losses are non-cash items; and
(c) any adjustments resulting for the application of Accounting Standards Codification Topic No. 460, Guarantees, or any comparable regulation.
In addition, to the extent not already included in the Consolidated Net Income of such Person and its Restricted Subsidiaries, notwithstanding anything to the contrary in the foregoing, Consolidated Net Income shall include the amount of proceeds received from business interruption insurance and reimbursements of any expenses and charges that are covered by indemnification or other reimbursement provisions in connection with any Permitted Investment or any sale, conveyance, transfer or other disposition of assets permitted under the Indenture.
Notwithstanding the foregoing, for the purpose of the covenant described under “—Certain covenants—Limitation on restricted payments” only (other than clause (3)(d) of the first paragraph thereof), there shall be excluded from Consolidated Net Income any income arising from any sale or other disposition of Restricted Investments made by such Person and its Restricted Subsidiaries, any repurchases and redemptions of Restricted Investments from such Person and its Restricted Subsidiaries, any repayments of loans and advances which constitute Restricted Investments by such Person or any of its Restricted Subsidiaries, any sale of the stock of an Unrestricted Subsidiary or any distribution or dividend from an Unrestricted Subsidiary, in each case only to the extent such amounts increase the amount of Restricted Payments permitted under such covenant pursuant to clause (3)(d) thereof.
“Consolidated Total Debt” means, as of the any date of determination, the aggregate principal amount of Indebtedness of the Issuer and its Restricted Subsidiaries outstanding on such date, determined on a consolidated basis in accordance with GAAP (but excluding the effects of any discounting of Indebtedness resulting from the application of purchase accounting in connection with the Transactions or any acquisition permitted under the Indenture), consisting only of Indebtedness for borrowed money, Capitalized Lease Obligations, debt obligations evidenced by bonds, notes, debentures, promissory notes or similar instruments and guarantees of Indebtedness of such types of a third Person; provided that Consolidated Total Debt shall not include Indebtedness in respect of (i) any letter of credit, except to the extent of unreimbursed obligations in respect of drawn letters of credit (provided that any unreimbursed amount under commercial letters of credit shall not be counted as Consolidated Total Debt until three (3) Business Days after such amount is drawn) and (ii) Hedging Obligations, except any unpaid termination payments thereunder.
“Contingent Obligations” means, with respect to any Person, any obligation of such Person guaranteeing any leases, dividends or other obligations that do not constitute Indebtedness (“primary obligations”) of any other Person (the “primary obligor”) in any manner, whether directly or indirectly, including, without limitation, any obligation of such Person, whether or not contingent:
(1) to purchase any such primary obligation or any property constituting direct or indirect security therefor;
147
Table of Contents
(2) to advance or supply funds
(a) for the purchase or payment of any such primary obligation, or
(b) to maintain working capital or equity capital of the primary obligor or otherwise to maintain the net worth or solvency of the primary obligor; or
(3) to purchase property, securities or services primarily for the purpose of assuring the owner of any such primary obligation of the ability of the primary obligor to make payment of such primary obligation against loss in respect thereof.
“Controlled Investment Affiliate” means, as to any Person, any other Person, other than any Investor, which directly or indirectly is in control of, is controlled by, or is under common control with such Person and is organized by such Person (or any Person controlling such Person) primarily for making direct or indirect equity or debt investments in the Issuer and/or other companies.
“Credit Facilities” means, with respect to the Issuer or any of its Restricted Subsidiaries, one or more debt facilities, including the Senior Credit Facilities or other financing arrangements (including, without limitation, commercial paper facilities or indentures) providing for revolving credit loans, term loans, letters of credit or other long-term Indebtedness, including any notes, mortgages, guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements or refundings thereof and any indentures or credit facilities or commercial paper facilities that replace, refund, supplement or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount permitted to be borrowed thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “—Certain covenants—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”) or adds Restricted Subsidiaries as additional borrowers or guarantors thereunder and whether by the same or any other agent, trustee, lender or group of lenders or holders.
“Default” means any event that is, or with the passage of time or the giving of notice or both would be, an Event of Default.
“Designated Non-cash Consideration” means the fair market value of non-cash consideration received by the Issuer or a Restricted Subsidiary in connection with an Asset Sale that is so designated as Designated Non-cash Consideration pursuant to an Officer’s Certificate, setting forth the basis of such valuation, executed by the principal financial officer of the Issuer, less the amount of Cash Equivalents received in connection with a subsequent sale, redemption or repurchase of, or collection or payment on, such Designated Non-cash Consideration.
“Designated Preferred Stock” means Preferred Stock of the Issuer or any direct or indirect parent company thereof (in each case other than Disqualified Stock) that is issued for cash (other than to a Restricted Subsidiary or an employee stock ownership plan or trust established by the Issuer or any of its Subsidiaries) and is so designated as Designated Preferred Stock, pursuant to an Officer’s Certificate executed by the principal financial officer of the Issuer or the applicable parent company thereof, as the case may be, on the issuance date thereof, the cash proceeds of which are excluded from the calculation set forth in clause (3) of the first paragraph of “—Certain covenants—Limitation on restricted payments.”
“Disqualified Stock” means, with respect to any Person, any Capital Stock of such Person which, by its terms, or by the terms of any security into which it is convertible or for which it is putable or exchangeable, or upon the happening of any event, matures or is mandatorily redeemable (other than solely as a result of a change of control or asset sale) pursuant to a sinking fund obligation or otherwise, or is redeemable at the option of the holder thereof (other than solely as a result of a change of control or asset sale), in whole or in part, in each case prior to the date 91 days after the earlier of the maturity date of the Notes or the date the Notes are no longer outstanding;
148
Table of Contents
provided that if such Capital Stock is issued to any plan for the benefit of employees of the Issuer or its Subsidiaries or by any such plan to such employees, such Capital Stock shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Issuer or its Subsidiaries in order to satisfy applicable statutory or regulatory obligations; provided, further, that any Capital Stock held by any future, current or former employee, director, officer, manager or consultant (or their respective Controlled Investment Affiliates or Immediate Family Members) of the Issuer, any of its Subsidiaries, any of its direct or indirect parent companies or any other entity in which the Issuer or a Restricted Subsidiary has an Investment and is designated in good faith as an “affiliate” by the board of directors of the Issuer (or the compensation committee thereof), in each case pursuant to any stock subscription or shareholders’ agreement, management equity plan or stock option plan or any other management or employee benefit plan or agreement shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Issuer or its Subsidiaries.
“EBITDA” means, with respect to any Person for any period, the Consolidated Net Income of such Person for such period (1) increased (without duplication) by the following, in each case (other than in the case of clause (h)) to the extent deducted (and not added back) in determining Consolidated Net Income for such period:
(a) provision for taxes based on income or profits or capital, including, without limitation, state, franchise and similar taxes, foreign withholding taxes (including any future taxes or other levies which replace or are intended to be in lieu of such taxes and any penalties and interest related to such taxes or arising from tax examinations) and the net tax expense associated with any adjustments made pursuant to clauses (1) through (15) of the definition of “Consolidated Net Income”; plus
(b) Fixed Charges of such Person for such period (including (x) net losses or Hedging Obligations or other derivative instruments entered into for the purpose of hedging interest rate risk, (y) bank fees and other deferred financing fees, and (z) costs of surety bonds in connection with financing activities, plus amounts excluded from Consolidated Interest Expense as set forth in clauses (1)(t) through (z) in the definition thereof); plus
(c) Consolidated Depreciation and Amortization Expense of such Person for such period; plus
(d) the amount of any restructuring charges or reserves, integration and facilities opening costs or other business optimization expenses (including cost and expenses relating to business optimization programs and new systems design and implementation costs) or accruals or reserves, and any one-time costs incurred in connection with Investments after the Effective Date, project start-up costs and costs related to the closure and/or consolidation of facilities; plus
(e) any other non-cash charges, including any write-offs or write-downs reducing Consolidated Net Income for such period (provided that if any such non-cash charges represent an accrual or reserve for potential cash items in any future period, (A) the Issuer may determine not to add back such non-cash charge in the current period and (B) to the extent the Issuer does decide to add back such non-cash charge, the cash payment in respect thereof in such future period shall be subtracted from EBITDA to such extent, and excluding amortization of a prepaid cash item that was paid in a prior period); plus
(f) the amount of any expense resulting from the application of Accounting Standards Codification Topic No. 810, Consolidation; plus
(g) the amount of management, monitoring, consulting, advisory and other fees (including termination fees) and indemnities and expenses paid or accrued in such period under the Management Fee Agreement or otherwise to the Investors to the extent otherwise permitted under “—Certain covenants—Transactions with affiliates”; plus
(h) the amount of “run rate” net cost savings and synergies (other than any of the foregoing related to Specified Transactions) projected by the Issuer in good faith to result from specified actions taken, committed to be taken or expected in good faith to be taken no later than twelve (12) months after the end of such period (calculated on a pro forma basis as though such cost savings and synergies had been realized on the first day of the period for which EBITDA is being determined), net of the amount of actual benefits
149
Table of Contents
realized during such period from such actions; provided that (A) such cost savings and synergies are reasonably identifiable and factually supportable (it is understood and agreed that “run-rate” means the full recurring benefit for a period that is associated with any action taken or expected to be taken, net of the amount of actual benefits realized during such period from such actions; provided that such benefit is expected to be realized within 12 months of taking such action), and (B) the aggregate amount of cost savings and synergies added pursuant to this clause (h) for any Test Period shall not exceed, after the Effective Date, the greater of (x) $15.0 million and (y) 10.0% of EBITDA for such Test Period (calculated prior to giving effect to any adjustment pursuant to this clause (h)); plus
(i) the amount of loss on sale of receivables, Securitization Assets and related assets to any Securitization Subsidiary in connection with a Qualified Securitization Facility; plus
(j) any costs or expense incurred by such Person or a Restricted Subsidiary pursuant to any management equity plan, stock option plan or any other management or employee benefit plan, agreement or any stock subscription or shareholder agreement, to the extent that such costs or expenses are funded with cash proceeds contributed to the capital of such Person or net cash proceeds of an issuance of Equity Interest of such Person (other than Disqualified Stock) solely to the extent that such cash proceeds are excluded from the calculation set forth in clause (3) of the first paragraph under “—Certain covenants—Limitation on restricted payments”; plus
(k) cash receipts (or any netting arrangements resulting in reduced cash expenditures) not representing EBITDA or Consolidated Net Income in any period to the extent non-cash gains relating to such income were deducted in the calculation of EBITDA pursuant to clause (2) below for any previous period and not added back; plus
(l) any net loss from disposed, abandoned or discontinued operations or from operations expected to be disposed of, abandoned or discontinued within twelve months after the end of such period; plus
(m) losses on asset sales (other than asset sales made in the ordinary course of business), disposals and abandonments; plus
(n) Specified Legal Expenses; and
(2) decreased (without duplication) by the following, in each case to the extent included in determining Consolidated Net Income for such period:
(a) non-cash gains increasing Consolidated Net Income of such Person for such period, excluding any non-cash gains that represent the reversal of an accrual or reserve for any anticipated cash charges in any prior period (other than any such accrual or reserve that has been added back to Consolidated Net Income in calculating EBITDA in accordance with this definition); plus
(b) any non-cash gains with respect to cash actually received in a prior period unless such cash did not increase EBITDA in such prior period; plus
(c) any net income from disposed, abandoned or discontinued operations or from operations expected to be disposed of, abandoned or discontinued within twelve months after the end of such period; plus
(d) extraordinary gains and unusual or non-recurring gains; plus
(e) gains on asset sales (other than asset sales made in the ordinary course of business), disposals and abandonments.
“Effective Date” means the Issue Date.
“EMU Legislation” means the legislative measures of the European Council for the introduction of, changeover to or operation of a single or unified European currency.
“Equity Interests” means Capital Stock and all warrants, options or other rights to acquire Capital Stock, but excluding any debt security that is convertible into, or exchangeable for, Capital Stock.
150
Table of Contents
“Equity Offering” means any public or private sale of common stock or Preferred Stock of the Issuer or any of its direct or indirect parent companies (excluding Disqualified Stock), other than:
(1) public offerings with respect to the Issuer’s or any direct or indirect parent company’s common stock registered on Form S-4 or Form S-8;
(2) issuances to any Subsidiary of the Issuer; and
(3) any such public or private sale that constitutes an Excluded Contribution.
“Euros” means the lawful currency of the Participating Member States introduced in accordance with the EMU Legislation.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Excluded Contribution” means net cash proceeds, marketable securities or Qualified Proceeds received by the Issuer from
(1) contributions to its common equity capital; and
(2) the sale (other than to a Subsidiary of the Issuer or to any management equity plan or stock option plan or any other management or employee benefit plan or agreement of the Issuer) of Capital Stock (other than Disqualified Stock and Designated Preferred Stock) of the Issuer;
in each case designated as Excluded Contributions pursuant to an Officer’s Certificate executed by the principal financial officer of the Issuer on the date such capital contributions are made or the date such Equity Interests are sold, as the case may be, and which are excluded from the calculation set forth in clause (3) of the first paragraph under “—Certain covenants—Limitation on restricted payments.”
“fair market value” means, with respect to any asset or liability, the fair market value of such asset or liability as determined by the Issuer in good faith.
“Financial Officer” means the Chief Financial Officer, the Treasurer or other financial officer of the Issuer, as appropriate.
“Fixed Charge Coverage Ratio” means, with respect to any Person for any period, the ratio of EBITDA of such Person for such period to the Fixed Charges of such Person for such period. In the event that the Issuer or any Restricted Subsidiary incurs, assumes, guarantees, redeems, repays, retires or extinguishes any Indebtedness (other than Indebtedness incurred or repaid under any revolving credit facility in the ordinary course of business for working capital purposes) or issues or redeems Disqualified Stock or Preferred Stock subsequent to the commencement of the period for which the Fixed Charge Coverage Ratio is being calculated but prior to or simultaneously with the event for which the calculation of the Fixed Charge Coverage Ratio is made (the “Fixed Charge Coverage Ratio Calculation Date”), then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect to such incurrence, assumption, guarantee, redemption, repayment, retirement or extinguishment of Indebtedness, or such issuance or redemption of Disqualified Stock or Preferred Stock, as if the same had occurred at the beginning of the applicable Test Period (and for the purposes of the numerator of Senior Secured Net Leverage Ratio, as if the same had occurred on the last day of the applicable Test Period).
For purposes of making the computation referred to above, any Specified Transaction that has been made by the Issuer or any of its Restricted Subsidiaries during any Test Period or subsequent to such Test Period and on or prior to or simultaneously with the Fixed Charge Coverage Ratio Calculation Date shall be calculated on a pro forma basis assuming that all such Specified Transactions (and the change in any associated fixed charge obligations and the change in EBITDA resulting therefrom) had occurred on the first day of the Test Period. If since the beginning of such Test Period any Person that subsequently became a Restricted Subsidiary or was merged with or into the Issuer or any of its Restricted Subsidiaries since the beginning of such Test Period shall
151
Table of Contents
have made any Specified Transaction that would have required adjustment pursuant to this definition, then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect thereto for such Test Period as if such Specified Transaction had occurred at the beginning of the applicable Test Period.
For purposes of this definition, whenever pro forma effect is to be given to any Specified Transaction (including the Transactions), the pro forma calculations shall be made in good faith by a Financial Officer of the Issuer and may include, for the avoidance of doubt, the amount of “run-rate” cost savings and synergies resulting from or related to any such Specified Transaction (including the Transactions) which is being given pro forma effect that have been or are expected to be realized and for which the actions necessary to realize such cost savings and synergies are taken or expected to be taken no later than 18 months after the date of any such Specified Transaction (in each case as though such cost savings and synergies had been realized on the first day of the applicable period and as if such cost savings and synergies were realized for the entirety of such period). For the purposes of the Indenture, “run-rate” means the full recurring benefit for a period that is associated with any action taken, committed to be taken or expected to be taken (including any savings expected to result from the elimination of a public target’s compliance costs with public company requirements), net of the amount of actual benefits realized during such period from such actions. If any Indebtedness bears a floating rate of interest and is being given pro forma effect, the interest on such Indebtedness shall be calculated as if the rate in effect on the Fixed Charge Coverage Ratio Calculation Date had been the applicable rate for the entire period (taking into account any Hedging Obligations applicable to such Indebtedness). Interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by a Financial Officer of the Issuer to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP. Interest on Indebtedness that may optionally be determined at an interest rate based upon a factor of a prime or similar rate, a eurocurrency interbank offered rate, or other rate, shall be deemed to have been based upon the rate actually chosen, or, if none, then based upon such optional rate chosen as the Issuer may designate.
“Fixed Charges” means, with respect to any Person for any period, the sum of, without duplication:
(1) Consolidated Interest Expense of such Person for such period;
(2) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Preferred Stock during such period; and
(3) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Disqualified Stock during such period.
“Foreign Subsidiary” means, with respect to any Person, any Restricted Subsidiary of such Person that is not organized or existing under the laws of the United States, any state thereof, the District of Columbia, or any territory thereof and any Restricted Subsidiary of such Foreign Subsidiary.
“GAAP” means generally accepted accounting principles in the United States of America which are in effect on the Issue Date. For purposes of this “Description of exchange notes,” the term “consolidated” with respect to any Person means such Person consolidated with its Restricted Subsidiaries and does not include any Unrestricted Subsidiary.
“Government Securities” means securities that are:
(1) direct obligations of the United States of America for the timely payment of which its full faith and credit is pledged; or
(2) obligations of a Person controlled or supervised by and acting as an agency or instrumentality of the United States of America the timely payment of which is unconditionally guaranteed as a full faith and credit obligation by the United States of America,
which, in either case, are not callable or redeemable at the option of the issuers thereof, and shall also include a depository receipt issued by a bank (as defined in Section 3(a)(2) of the Securities Act), as custodian with respect to any such Government Securities or a specific payment of principal of or interest on any such Government
152
Table of Contents
Securities held by such custodian for the account of the holder of such depository receipt; provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the Government Securities or the specific payment of principal of or interest on the Government Securities evidenced by such depository receipt.
“guarantee” means a guarantee (other than by endorsement of negotiable instruments for collection in the ordinary course of business), direct or indirect, in any manner (including letters of credit and reimbursement agreements in respect thereof), of all or any part of any Indebtedness or other obligations.
“Guarantee” means the guarantee by any Guarantor of the Issuer’s Obligations under the Indenture and the Notes.
“Guarantor” means each Restricted Subsidiary of the Issuer, if any, that Guarantees the Notes in accordance with the terms of the Indenture.
“Hedging Obligations” means, with respect to any Person, the obligations of such Person under any interest rate swap agreement, interest rate cap agreement, interest rate collar agreement, commodity swap agreement, commodity cap agreement, commodity collar agreement, foreign exchange contract, currency swap agreement or similar agreement providing for the transfer or mitigation of interest rate, currency or commodity risks either generally or under specific contingencies.
“Holder” means the Person in whose name a Note is registered on the registrar’s books.
“Holdings” means IVD Intermediate Holdings B Inc., a Delaware corporation and the direct parent of the Issuer.
“Immediate Family Members” means with respect to any individual, such individual’s child, stepchild, grandchild or more remote descendant, parent, stepparent, grandparent, spouse, former spouse, qualified domestic partner, sibling, mother-in-law, father-in-law, son-in-law and daughter-in-law (including adoptive relationships) and any trust, partnership or other bona fide estate-planning vehicle the only beneficiaries of which are any of the foregoing individuals or any private foundation or fund that is controlled by any of the foregoing individuals or any donor-advised fund of which any such individual is the donor.
“Immucor” means Immucor, Inc., a Georgia corporation.
“Indebtedness” means, with respect to any Person, without duplication:
(1) any indebtedness (including principal and premium) of such Person, whether or not contingent:
(a) in respect of borrowed money;
(b) evidenced by bonds, notes, debentures or similar instruments or letters of credit or bankers’ acceptances (or, without duplication, reimbursement agreements in respect thereof);
(c) representing the balance deferred and unpaid of the purchase price of any property (including Capitalized Lease Obligations) or services due more than twelve months after such property is acquired or such services are completed, except (i) any such balance that constitutes an obligation in respect of a commercial letter of credit, a trade payable or similar obligation to a trade creditor, in each case accrued in the ordinary course of business and (ii) any earn-out obligations until such obligation becomes a liability on the balance sheet of such Person in accordance with GAAP and if not paid after becoming due and payable;
(d) representing the net obligations under any Hedging Obligations; or
(e) during a Suspension Period only, obligations of the lessee for rental payments in respect of Sale and Lease-back Transactions in an amount equal to the present value of such obligations during the remaining term of the lease using a discount rate equal to the rate of interest implicit in such transaction determined in accordance with GAAP;
153
Table of Contents
if and to the extent that any of the foregoing Indebtedness (other than letters of credit (excluding commercial letters of credit) and Hedging Obligations) would appear as a liability upon a balance sheet (excluding the footnotes thereto) of such Person prepared in accordance with GAAP; provided that Indebtedness of any direct or indirect parent of the Issuer appearing upon the balance sheet of the Issuer solely by reason of push-down accounting under GAAP shall be excluded;
(2) to the extent not otherwise included, any obligation by such Person to be liable for, or to pay, as obligor, guarantor or otherwise, on the obligations of the type referred to in clause (1) of a third Person (whether or not such items would appear upon the balance sheet of the such obligor or guarantor), other than by endorsement of negotiable instruments for collection in the ordinary course of business; and
(3) to the extent not otherwise included, the obligations of the type referred to in clause (1) of a third Person secured by a Lien on any asset owned by such first Person, whether or not such Indebtedness is assumed by such first Person;
provided that notwithstanding the foregoing, Indebtedness shall be deemed not to include (a) Contingent Obligations incurred in the ordinary course of business, (b) reimbursement obligations under commercial letters of credit (provided that unreimbursed amounts under commercial letters of credit shall be counted as Indebtedness three (3) Business Days after such amount is drawn) or (c) obligations under or in respect of Qualified Securitization Facilities; provided, further, that Indebtedness shall be calculated without giving effect to the effects of Accounting Standards Codification Topic No. 815, Derivatives and Hedging, and related interpretations to the extent such effects would otherwise increase or decrease an amount of Indebtedness for any purpose under the Indenture as a result of accounting for any embedded derivatives created by the terms of such Indebtedness.
“Independent Financial Advisor” means an accounting, appraisal, investment banking firm or consultant to Persons engaged in Similar Businesses of nationally recognized standing that is, in the good faith judgment of the Issuer, qualified to perform the task for which it has been engaged.
“Initial Notes” means the $400.0 million aggregate principal amount of Notes issued on August 19, 2011.
“Initial Purchasers” means J.P. Morgan Securities LLC, Citigroup Global Markets Inc., UBS Securities LLC, Deutsche Bank Securities Inc. and RBC Capital Markets, LLC.
“Investment Grade Rating” means a rating equal to or higher than Baa3 (or the equivalent) by Moody’s and BBB- (or the equivalent) by S&P, or an equivalent rating by any other Rating Agency.
“Investment Grade Securities” means:
(1) securities issued or directly and fully guaranteed or insured by the United States government or any agency or instrumentality thereof (other than Cash Equivalents);
(2) debt securities or debt instruments with an Investment Grade Rating, but excluding any debt securities or instruments constituting loans or advances among the Issuer and its Subsidiaries;
(3) investments in any fund that invests exclusively in investments of the type described in clauses (1) and (2) which fund may also hold immaterial amounts of cash pending investment or distribution; and
(4) corresponding instruments in countries other than the United States customarily utilized for high quality investments.
“Investments” means, with respect to any Person, all investments by such Person in other Persons (including Affiliates) in the form of loans (including guarantees), advances or capital contributions (excluding accounts receivable, credit card and debit card receivables, trade credit, advances to customers, commission, travel and similar advances to employees, directors, officers, managers and consultants, in each case made in the ordinary course of business), purchases or other acquisitions for consideration of Indebtedness, Equity Interests or other
154
Table of Contents
securities issued by any other Person and investments that are required by GAAP to be classified on the balance sheet (excluding the footnotes) of the Issuer in the same manner as the other investments included in this definition to the extent such transactions involve the transfer of cash or other property. For purposes of the definition of “Unrestricted Subsidiary” and the covenant described under “—Certain covenants—Limitation on restricted payments”:
(1) “Investments” shall include the portion (proportionate to the Issuer’s equity interest in such Subsidiary) of the fair market value of the net assets of a Subsidiary of the Issuer at the time that such Subsidiary is designated an Unrestricted Subsidiary; provided that upon a redesignation of such Subsidiary as a Restricted Subsidiary, the Issuer shall be deemed to continue to have a permanent “Investment” in an Unrestricted Subsidiary in an amount (if positive) equal to:
(a) the Issuer’s “Investment” in such Subsidiary at the time of such redesignation; less
(b) the portion (proportionate to the Issuer’s equity interest in such Subsidiary) of the fair market value of the net assets of such Subsidiary at the time of such redesignation; and
(2) any property transferred to or from an Unrestricted Subsidiary shall be valued at its fair market value at the time of such transfer.
The amount of any Investment outstanding at any time shall be the original cost of such Investment, reduced by any dividend, distribution, interest payment, return of capital, repayment or other amount received in cash by the Issuer or a Restricted Subsidiary in respect of such Investment.
“Investor” means any of TPG Capital, L.P., TPG IVD Co-Invest, L.P. (for so long as TPG Capital, L.P. or any of its Affiliates retains control of the voting power thereof), and any of their respective Affiliates and funds or partnerships managed or advised by any of them or any of their respective Affiliates but not including, however, any portfolio company of any of the foregoing.
“Issue Date” means August 19, 2011.
“Issuer” means Immucor, as successor by merger to IVD Acquisition.
“IVD Acquisition” means IVD Acquisition Corporation, a Georgia corporation.
“Legal Holiday” means a Saturday, a Sunday or a day on which commercial banking institutions are not required to be open in the State of New York or place of payment.
“Lien” means, with respect to any asset, any mortgage, lien (statutory or otherwise), pledge, hypothecation, charge, security interest, preference, priority or encumbrance of any kind in respect of such asset, whether or not filed, recorded or otherwise perfected under applicable law, including any conditional sale or other title retention agreement, any lease in the nature thereof, any option or other agreement to sell or give a security interest in and any filing of or agreement to give any financing statement under the Uniform Commercial Code (or equivalent statutes) of any jurisdiction; provided that in no event shall an operating lease be deemed to constitute a Lien.
“Management Fee Agreement” means the management services agreement among TPG Capital, L.P. or certain of the management companies associated with it or their advisors, if applicable, IVD Holdings Inc. and the Issuer (and/or any of its direct or indirect parent companies).
“Management Stockholders” means the members of management (and their Controlled Investment Affiliates and Immediate Family Members) of the Issuer (or its direct parent) who are holders of Equity Interests of any direct or indirect parent companies of the Issuer on the Effective Date or will become holders of such Equity Interests in connection with the Acquisition.
“Moody’s” means Moody’s Investors Service, Inc. and any successor to its rating agency business.
155
Table of Contents
“Net Income” means, with respect to any Person, the net income (loss) of such Person, determined in accordance with GAAP and before any reduction in respect of Preferred Stock dividends.
“Net Proceeds” means the aggregate cash proceeds received by the Issuer or any of its Restricted Subsidiaries in respect of any Asset Sale, including any cash received upon the sale or other disposition of any Designated Non-cash Consideration received in any Asset Sale, net of the costs relating to such Asset Sale and the sale or disposition of such Designated Non-cash Consideration, including legal, accounting and investment banking fees, payments made in order to obtain a necessary consent or required by applicable law, and brokerage and sales commissions, any relocation expenses incurred as a result thereof, other fees and expenses, including title and recordation expenses, taxes paid or payable as a result thereof or any transactions occurring or deemed to occur to effectuate a payment under the Indenture (after taking into account any available tax credits or deductions and any tax sharing arrangements), amounts required to be applied to the repayment of principal, premium, if any, and interest on Senior Indebtedness required (other than required by clause (1) of the second paragraph of “—Repurchase at the option of holders—Asset sales”) to be paid as a result of such transaction and any deduction of appropriate amounts to be provided by the Issuer or any of its Restricted Subsidiaries as a reserve in accordance with GAAP against any liabilities associated with the asset disposed of in such transaction and retained by the Issuer or any of its Restricted Subsidiaries after such sale or other disposition thereof, including pension and other post-employment benefit liabilities and liabilities related to environmental matters or against any indemnification obligations associated with such transaction.
“Obligations” means any principal, interest (including any interest accruing on or subsequent to the filing of a petition in bankruptcy, reorganization or similar proceeding at the rate provided for in the documentation with respect thereto, whether or not such interest is an allowed claim under applicable state, federal or foreign law), premium, penalties, fees, indemnifications, reimbursements (including reimbursement obligations with respect to letters of credit and banker’s acceptances), damages and other liabilities, and guarantees of payment of such principal, interest, penalties, fees, indemnifications, reimbursements, damages and other liabilities, payable under the documentation governing any Indebtedness.
“Officer” means the Chairman of the board of directors, the Chief Executive Officer, the Chief Financial Officer, the President, any Executive Vice President, Senior Vice President or Vice President, the Treasurer or the Secretary of the Issuer.
“Officer’s Certificate” means a certificate signed on behalf of a Person by an Officer of such Person, who must be the principal executive officer, the principal financial officer, the treasurer or the principal accounting officer of such Person, that meets the requirements set forth in the Indenture.
“Opinion of Counsel” means a written opinion from legal counsel who is acceptable to the Trustee. The counsel may be an employee of or counsel to the Issuer or the Trustee.
“Parent Company” means any Person so long as such Person directly or indirectly holds 100.0% of the total voting power of the Capital Stock of the Issuer, and at the time such Person acquired such voting power, no Person and no group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision), including any such group acting for the purpose of acquiring, holding or disposing of securities (within the meaning of Rule 13d-5(b)(1) under the Exchange Act ) (other than any Permitted Holder), shall have beneficial ownership (within the meaning of Rule 13d-3 under the Exchange Act, or any successor provision), directly or indirectly, of 50.0% or more of the total voting power of the Voting Stock of such Person.
“Participating Member State” means each state so described in any EMU Legislation.
“Permitted Asset Swap” means the substantially concurrent purchase and sale or exchange of Related Business Assets or a combination of Related Business Assets and Cash Equivalents between the Issuer or any of its Restricted Subsidiaries and another Person; provided that any Cash Equivalents received must be applied in accordance with the covenant described under “—Repurchase at the option of holders—Asset sales.”
156
Table of Contents
“Permitted Holder” means any of the Investors and Management Stockholders and any group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members; provided that, in the case of such group and without giving effect to the existence of such group or any other group, such Investors and Management Stockholders, collectively, have beneficial ownership of more than 50.0% of the total voting power of the Voting Stock of the Issuer or any of its direct or indirect parent companies. Any Person or group whose acquisition of beneficial ownership constitutes a Change of Control in respect of which a Change of Control Offer is made in accordance with the requirements of the Indenture will thereafter, together with its Affiliates, constitute an additional Permitted Holder.
“Permitted Investments” means:
(1) any Investment in the Issuer or any of its Restricted Subsidiaries;
(2) any Investment in Cash Equivalents or Investment Grade Securities;
(3) any Investment by the Issuer or any of its Restricted Subsidiaries in a Person (including, to the extent constituting an Investment, in assets of a Person that represent substantially all of its assets or a division, business unit or product line, including research and development and related assets in respect of any product) that is engaged in a Similar Business if as a result of such Investment:
(a) such Person becomes a Restricted Subsidiary; or
(b) such Person, in one transaction or a series of related transactions, is merged or consolidated with or into, or transfers or conveys substantially all of its assets (or a division, business unit or product line, including research and development and related assets in respect of any product) to, or is liquidated into, the Issuer or a Restricted Subsidiary,
and, in each case, any Investment held by such Person; provided that such Investment was not acquired by such Person in contemplation of such acquisition, merger, consolidation or transfer;
(4) any Investment in securities or other assets not constituting Cash Equivalents or Investment Grade Securities and received in connection with an Asset Sale made pursuant to the provisions described under “—Repurchase at the option of holders—Asset sales” or any other disposition of assets not constituting an Asset Sale;
(5) any Investment existing on the Effective Date or made pursuant to binding commitments in effect on the Effective Date or an Investment consisting of any extension, modification or renewal of any Investment or binding commitment existing on the Effective Date; provided that the amount of any such Investment or binding commitment may be increased (a) as required by the terms of such Investment or binding commitment as in existence on the Effective Date (including as a result of the accrual or accretion of interest or original issue discount or the issuance of pay-in-kind securities) or (b) as otherwise permitted under the Indenture;
(6) any Investment acquired by the Issuer or any of its Restricted Subsidiaries:
(a) in exchange for any other Investment or accounts receivable held by the Issuer or any such Restricted Subsidiary in connection with or as a result of a bankruptcy, workout, reorganization or recapitalization of the issuer of such other Investment or accounts receivable (including any trade creditor or customer); or
(b) in satisfaction of judgments against other Persons; or
(c) as a result of a foreclosure by the Issuer or any of its Restricted Subsidiaries with respect to any secured Investment or other transfer of title with respect to any secured Investment in default;
(7) Hedging Obligations permitted under clause (10) of the covenant described in “—Certain covenants—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”;
(8) any Investment in a Similar Business taken together with all other Investments made pursuant to this clause (8) that are at that time outstanding, not to exceed the greater of (a) $40.0 million and (b) 2.5% of
157
Table of Contents
Total Assets (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
(9) Investments the payment for which consists of Equity Interests (other than Disqualified Stock) of the Issuer or any of its direct or indirect parent companies; provided that such Equity Interests will not increase the amount available for Restricted Payments under clause (3) of the first paragraph under the covenant described in “Certain covenants—Limitations on restricted payments”;
(10) guarantees of Indebtedness permitted under the covenant described in “—Certain covenants—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock,” performance guarantees and Contingent Obligations incurred in the ordinary course of business and the creation of liens on the assets of the Issuer or any Restricted Subsidiary in compliance with the covenant described under “—Certain covenants—Liens”;
(11) any transaction to the extent it constitutes an Investment that is permitted by and made in accordance with the provisions of the second paragraph of the covenant described under “—Certain covenants—Transactions with affiliates” (except transactions described in clauses (2), (5), (9) and (15) of such paragraph);
(12) Investments consisting of purchases and acquisitions of inventory, supplies, material or equipment or the licensing or contribution of intellectual property pursuant to joint marketing arrangements with other Persons;
(13) additional Investments, taken together with all other Investments made pursuant to this clause (13) that are at that time outstanding (without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities), not to exceed the greater of (a) $40.0 million and (b) 2.5% of Total Assets (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
(14) Investments in or relating to a Securitization Subsidiary that, in the good faith determination of the Issuer are necessary or advisable to effect any Qualified Securitization Facility or any repurchase obligation in connection therewith;
(15) advances to, or guarantees of Indebtedness of, employees not in excess of $5.0 million outstanding at any one time, in the aggregate;
(16) loans and advances to employees, directors, officers, managers and consultants for business-related travel expenses, moving expenses and other similar expenses or payroll advances, in each case incurred in the ordinary course of business or consistent with past practices or to fund such Person’s purchase of Equity Interests of the Issuer or any direct or indirect parent company thereof;
(17) advances, loans or extensions of trade credit in the ordinary course of business by the Issuer or any of its Restricted Subsidiaries;
(18) any Investment in any Subsidiary or any joint venture in connection with intercompany cash management arrangements or related activities arising in the ordinary course of business;
(19) Investments consisting of purchases and acquisitions of assets or services in the ordinary course of business;
(20) Investments made in the ordinary course of business in connection with obtaining, maintaining or renewing client contacts and loans or advances made to distributors in the ordinary course of business;
(21) Investments in prepaid expenses, negotiable instruments held for collection and lease, utility and workers compensation, performance and similar deposits entered into as a result of the operations of the business in the ordinary course of business;
(22) repurchases of the Notes;
158
Table of Contents
(23) Investments in Unrestricted Subsidiaries, taken together with all other Permitted Investments made pursuant to this clause (23) that are at the time outstanding, without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities, not to exceed the greater of (a) $35.0 million and (b) 2.0% of Total Assets; and
(24) Investments in the ordinary course of business consisting of Uniform Commercial Code Article 3 endorsements for collection or deposit and Article 4 customary trade arrangements with customers consistent with past practices.
“Permitted Liens” means, with respect to any Person:
(1) pledges or deposits by such Person under workmen’s compensation laws, unemployment insurance, other social security legislation or other insurance-related obligations (including, but not limited to, in respect of deductibles, self-insured retention amounts and premiums and adjustments thereto) or good faith deposits in connection with bids, tenders, contracts (other than for the payment of Indebtedness) or leases to which such Person is a party, or deposits to secure public or statutory obligations of such Person or deposits of cash or U.S. government bonds to secure surety or appeal bonds to which such Person is a party, or deposits as security for contested taxes or import duties or for the payment of rent, in each case incurred in the ordinary course of business;
(2) Liens imposed by law, such as carriers’, warehousemen’s and mechanics’ Liens, in each case for sums not yet overdue for a period of more than 30 days or being contested in good faith by appropriate actions or other Liens arising out of judgments or awards against such Person with respect to which such Person shall then be proceeding with an appeal or other proceedings for review if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(3) Liens for taxes, assessments or other governmental charges not yet overdue for a period of more than 30 days or not yet payable or subject to penalties for nonpayment or which are being contested in good faith by appropriate proceedings diligently conducted, if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(4) Liens in favor of issuers of performance and surety bonds or bid bonds or with respect to other regulatory requirements or letters of credit issued pursuant to the request of and for the account of such Person in the ordinary course of its business;
(5) survey exceptions, encumbrances, easements or reservations of, or rights of others for, licenses, rights-of-way, sewers, electric lines, telegraph and telephone lines and other similar purposes, or zoning or other restrictions as to the use of real properties or Liens incidental to the conduct of the business of such Person or to the ownership of its properties which were not incurred in connection with Indebtedness and which do not in the aggregate materially adversely affect the value of said properties or materially impair their use in the operation of the business of such Person and exceptions on title policies insuring liens granted on Mortgaged Properties (as defined in the Senior Credit Facilities);
(6) Liens securing obligations in respect of Indebtedness permitted to be incurred pursuant to clause (4), (12)(b), (13) or (23) of the second paragraph under “Certain covenants—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”; provided that (a) Liens securing Indebtedness, Disqualified Stock or Preferred Stock permitted to be incurred pursuant to clause (13) relate only to Refinancing Indebtedness that serves to refund or refinance Indebtedness, Disqualified Stock or Preferred Stock incurred under clause (4), (12)(b) or (13) of the second paragraph of “Certain covenants—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock,” (b) Liens securing Indebtedness permitted to be incurred pursuant to clause (23) extend only to the assets of Foreign Subsidiaries, and (c) Liens securing Indebtedness, Disqualified Stock or Preferred Stock to be incurred pursuant to clause (4) of the second paragraph under “Certain covenants—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock” extend only to the assets so purchased, leased or improved;
(7) Liens existing on the Effective Date;
159
Table of Contents
(8) Liens on property or shares of stock or other assets of a Person at the time such Person becomes a Subsidiary; provided that such Liens are not created or incurred in connection with, or in contemplation of, such other Person becoming such a Subsidiary; provided, further, that such Liens may not extend to any other property or other assets owned by the Issuer or any of its Restricted Subsidiaries;
(9) Liens on property or other assets at the time the Issuer or a Restricted Subsidiary acquired the property or such other assets, including any acquisition by means of a merger or consolidation with or into the Issuer or any of its Restricted Subsidiaries; provided that such Liens are not created or incurred in connection with, or in contemplation of, such acquisition; provided, further, that the Liens may not extend to any other property owned by the Issuer or any of its Restricted Subsidiaries;
(10) Liens securing obligations in respect of Indebtedness or other obligations of a Restricted Subsidiary owing to the Issuer or another Restricted Subsidiary permitted to be incurred in accordance with the covenant described under “Certain covenants—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”;
(11) Liens securing (x) Hedging Obligations and (y) obligations in respect of Cash Management Services;
(12) Liens on specific items of inventory or other goods and proceeds of any Person securing such Person’s obligations in respect of bankers’ acceptances or letters of credit issued or created for the account of such Person to facilitate the purchase, shipment or storage of such inventory or other goods;
(13) leases, subleases, licenses or sublicenses granted to others in the ordinary course of business which do not materially interfere with the ordinary conduct of the business of the Issuer or any of its Restricted Subsidiaries and do not secure any Indebtedness;
(14) Liens arising from Uniform Commercial Code financing statement filings regarding operating leases entered into by the Issuer and its Restricted Subsidiaries in the ordinary course of business or purported Liens evidenced by the filing of precautionary Uniform Commercial Code financing statements or similar public filings;
(15) Liens in favor of the Issuer or any Guarantor;
(16) Liens on equipment of the Issuer or any of its Restricted Subsidiaries granted in the ordinary course of business to the Issuer’s clients;
(17) Liens on accounts receivable, Securitization Assets and related assets incurred in connection with a Qualified Securitization Facility;
(18) Liens to secure any modification, refinancing, refunding, extension, renewal or replacement (or successive modification, refinancing, refunding, extensions, renewals or replacements) as a whole, or in part, of any Indebtedness secured by any Lien referred to in the foregoing clauses (6), (7), (8) and (9); provided that (a) such new Lien shall be limited to all or part of the same property that secured the original Lien (plus improvements on such property) and proceeds and products thereof, and (b) the Indebtedness secured by such Lien at such time is not increased to any amount greater than the sum of (i) the outstanding principal amount or, if greater, committed amount of the Indebtedness described under clauses (6), (7), (8) and (9) at the time the original Lien became a Permitted Lien under the Indenture, and (ii) an amount necessary to pay any fees and expenses (including original issue discount, upfront fees or similar fees) and premiums (including tender premiums), related to such refinancing, refunding, extension, renewal or replacement;
(19) deposits made or other security provided in the ordinary course of business to secure liability to insurance carriers;
(20) other Liens securing obligations in an aggregate amount at any one time outstanding not to exceed the greater of (a) $35.0 million and (b) 2.0% of Total Assets determined as of the date of incurrence;
(21) Liens securing judgments for the payment of money not constituting an Event of Default under clause (5) under “—Events of default and remedies”;
160
Table of Contents
(22) Liens in favor of customs and revenue authorities arising as a matter of law to secure payment of customs duties in connection with the importation of goods in the ordinary course of business;
(23) Liens (a) of a collection bank arising under Section 4-208 of the Uniform Commercial Code on items in the course of collection, (b) attaching to commodity trading accounts or other commodity brokerage accounts incurred in the ordinary course of business, and (c) in favor of banking institutions arising as a matter of law or under general terms and conditions encumbering deposits (including the right of set-off) and which are within the general parameters customary in the banking industry;
(24) Liens deemed to exist in connection with Investments in repurchase agreements permitted under “Certain covenants—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock”; provided that such Liens do not extend to any assets other than those that are the subject of such repurchase agreement;
(25) Liens encumbering reasonable customary deposits and margin deposits and similar Liens attaching to commodity trading accounts or other brokerage accounts incurred in the ordinary course of business and not for speculative purposes;
(26) Liens that are contractual rights of set-off (a) relating to the establishment of depository relations with banks or other deposit-taking financial institutions and not given in connection with the issuance of Indebtedness, (b) relating to pooled deposit or sweep accounts of the Issuer or any of its Restricted Subsidiaries to permit satisfaction of overdraft or similar obligations incurred in the ordinary course of business of the Issuer and its Restricted Subsidiaries or (c) relating to purchase orders and other agreements entered into with customers of the Issuer or any of its Restricted Subsidiaries in the ordinary course of business;
(27) during a Suspension Period only, Liens securing Indebtedness (other than Indebtedness that is secured equally and ratably with (or on a basis subordinated to) the Notes), and Indebtedness represented by Sale and Lease-Back Transactions in an amount not to exceed 12.5% of Total Assets at any one time outstanding;
(28) any encumbrance or restriction (including put and call arrangements) with respect to capital stock of any joint venture or similar arrangement pursuant to any joint venture or similar agreement;
(29) Liens arising out of conditional sale, title retention, consignment or similar arrangements for the sale or purchase of goods entered into by the Issuer or any Restricted Subsidiary in the ordinary course of business;
(30) Liens solely on any cash earnest money deposits made by the Issuer or any of its Restricted Subsidiaries in connection with any letter of intent or purchase agreement permitted under the Indenture;
(31) ground leases in respect of real property on which facilities owned or leased by the Issuer or any of its Subsidiaries are located;
(32) Liens on insurance policies and the proceeds thereof securing the financing of the premiums with respect thereto;
(33) Liens on Capital Stock of an Unrestricted Subsidiary that secure Indebtedness or other obligations of such Unrestricted Subsidiary;
(34) Liens on cash advances in favor of the seller of any property to be acquired in an Investment permitted under the Indenture to be applied against the purchase price for such Investment;
(35) any interest or title of a lessor, sublessor, licensor or sublicensor or secured by a lessor’s, sublessor’s, licensor’s or sublicensor’s interest under leases or licenses entered into by the Issuer or any of the Restricted Subsidiaries in the ordinary course of business; and
(36) deposits of cash with the owner or lessor of premises leased and operated by the Issuer or any of its Subsidiaries in the ordinary course of business of the Issuer and such Subsidiary to secure the performance of the Issuer’s or such Subsidiary’s obligations under the terms of the lease for such premises.
161
Table of Contents
For purposes of this definition, the term “Indebtedness” shall be deemed to include interest on such Indebtedness.
“Person” means any individual, corporation, limited liability company, partnership, joint venture, association, joint stock company, trust, unincorporated organization, government or any agency or political subdivision thereof or any other entity.
“Preferred Stock” means any Equity Interest with preferential rights of payment of dividends or upon liquidation, dissolution, or winding up.
“Qualified Proceeds” means the fair market value of assets that are used or useful in, or Capital Stock of any Person engaged in, a Similar Business.
“Qualified Securitization Facility” means any Securitization Facility (1) constituting a securitization financing facility that meets the following conditions: (a) the board of directors of the Issuer shall have determined in good faith that such Securitization Facility (including financing terms, covenants, termination events and other provisions) is in the aggregate economically fair and reasonable to the Issuer and the applicable Securitization Subsidiary, (b) all sales and/or contributions of Securitization Assets and related assets to the applicable Securitization Subsidiary are made at fair market value (as determined in good faith by the Issuer) and (c) the financing terms, covenants, termination events and other provisions thereof shall be market terms (as determined in good faith by the Issuer) or (2) constituting a receivables financing facility.
“Rating Agencies” means Moody’s and S&P or if Moody’s or S&P or both shall not make a rating on the Notes publicly available, a nationally recognized statistical rating agency or agencies, as the case may be, selected by the Issuer which shall be substituted for Moody’s or S&P or both, as the case may be.
“Refinancing Indebtedness” means (x) Indebtedness incurred by the Issuer or any Restricted Subsidiary, (y) Disqualified Stock issued by the Issuer or any Restricted Subsidiary or (z) Preferred Stock issued by any Restricted Subsidiary which, in each case, serves to extend, replace, refund, refinance, renew or defease any Indebtedness, Disqualified Stock or Preferred Stock, so long as
(1) the principal amount (or accreted value, if applicable) of such new Indebtedness, the amount of such new Preferred Stock or the liquidation preference of such new Disqualified Stock does not exceed the principal amount of (or accreted value, if applicable), plus any accrued and unpaid interest on, the Indebtedness, the amount of, plus any accrued and unpaid dividends on, the Preferred Stock or the liquidation preference of, plus any accrued and unpaid dividends on, the Disqualified Stock being so extended, replaced, refunded, refinanced, renewed or defeased (such Indebtedness, Disqualified Stock or Preferred Stock, the “Refinanced Debt”), plus the amount of any tender premium or premium required to be paid under the terms of the instrument governing such Refinanced Debt and any reasonable fees and expenses (including original issue discount, upfront fees or similar fees) incurred in connection with the issuance of such new Indebtedness, Preferred Stock or Disqualified Stock or the extension, replacement, refunding, refinancing, renewal or defeasance of such Refinanced Debt;
(2) such Refinancing Indebtedness has a Weighted Average Life to Maturity at the time such Refinancing Indebtedness is incurred which is not less than the remaining Weighted Average Life to Maturity of the Indebtedness, Disqualified Stock or Preferred Stock being extended, replaced, refunded, refinanced, renewed or defeased;
(3) such Refinancing Indebtedness has a final scheduled maturity date equal to or later than the final scheduled maturity date of the Indebtedness, Preferred Stock or Disqualified Stock being so extended, replaced, refunded, refinanced, renewed or defeased;
(4) to the extent such Refinancing Indebtedness extends, replaces, refunds, refinances, renews or defeases (i) Indebtedness subordinated to the Notes or any Guarantee thereof, such Refinancing Indebtedness is subordinated to the Notes or the Guarantee thereof at least to the same extent as the Indebtedness being
162
Table of Contents
extended, replaced, refunded, refinanced, renewed or defeased or (ii) Disqualified Stock or Preferred Stock, such Refinancing Indebtedness must be Disqualified Stock or Preferred Stock, respectively; and
(5) Refinancing Indebtedness shall not include:
(a) Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Issuer that is not a Guarantor that refinances Indebtedness or Disqualified Stock of the Issuer;
(b) Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Issuer that is not a Guarantor that refinances Indebtedness, Disqualified Stock or Preferred Stock of a Guarantor; or
(c) Indebtedness or Disqualified Stock of the Issuer or Indebtedness, Disqualified Stock or Preferred Stock of a Restricted Subsidiary that refinances Indebtedness, Disqualified Stock or Preferred Stock of an Unrestricted Subsidiary;
and, provided, further, that clauses (2) and (3) of this definition will not apply to any extension, replacement, refunding, refinancing, renewal or defeasance of any Indebtedness other than Indebtedness incurred under clauses (2) and (3) of the second paragraph of the covenant described under “—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock,” any Subordinated Indebtedness, Disqualified Stock and Preferred Stock.
“Registration Rights Agreement” means a registration rights agreement with respect to the Notes dated as of the Issue Date, among IVD Acquisition and the Initial Purchasers, as supplemented on the Effective Date by the joinder of Immucor and the Guarantor.
“Related Business Assets” means assets (other than Cash Equivalents) used or useful in a Similar Business; provided that any assets received by the Issuer or a Restricted Subsidiary in exchange for assets transferred by the Issuer or a Restricted Subsidiary shall not be deemed to be Related Business Assets if they consist of securities of a Person, unless upon receipt of the securities of such Person, such Person would become a Restricted Subsidiary.
“Restricted Investment” means an Investment other than a Permitted Investment.
“Restricted Subsidiary” means, at any time, any direct or indirect Subsidiary of the Issuer (including any Foreign Subsidiary) that is not then an Unrestricted Subsidiary; provided that upon an Unrestricted Subsidiary ceasing to be an Unrestricted Subsidiary, such Subsidiary shall be included in the definition of “Restricted Subsidiary.”
“Rule 144A offering memorandum” means the final offering memorandum of the outstanding notes, dated as of August 16, 2011.
“S&P” means Standard & Poor’s, a division of The McGraw-Hill Companies, Inc., and any successor to its rating agency business.
“Sale and Lease-Back Transaction” means any arrangement providing for the leasing by the Issuer or any of its Restricted Subsidiaries of any real or tangible personal property, which property has been or is to be sold or transferred by the Issuer or such Restricted Subsidiary to a third Person in contemplation of such leasing.
“SEC” means the U.S. Securities and Exchange Commission.
“Secured Indebtedness” means any Indebtedness of the Issuer or any of its Restricted Subsidiaries secured by a Lien.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Securitization Assets” means the accounts receivable, royalty or other revenue streams and other rights to payment and other assets related thereto subject to a Qualified Securitization Facility and the proceeds thereof.
163
Table of Contents
“Securitization Facility” means any of one or more receivables or securitization financing facilities, as amended, supplemented, modified, extended, renewed, restated or refunded from time to time, the Obligations of which are non-recourse (except for customary representations, warranties, covenants and indemnities made in connection with such facilities) to the Issuer or any of its Restricted Subsidiaries (other than a Securitization Subsidiary) pursuant to which the Issuer or any of its Restricted Subsidiaries sells or grants a security interest in its accounts receivable or Securitization Assets or assets related thereto to either (a) a Person that is not a Restricted Subsidiary or (b) a Securitization Subsidiary that in turn sells its accounts receivable to a Person that is not a Restricted Subsidiary.
“Securitization Fees” means distributions or payments made directly or by means of discounts with respect to any participation interest issued or sold in connection with, and other fees paid to a Person that is not a Securitization Subsidiary in connection with, any Qualified Securitization Facility.
“Securitization Subsidiary” means any Subsidiary formed for the purpose of, and that solely engages only in one or more Qualified Securitization Facilities and other activities reasonably related thereto.
“Senior Credit Facilities” means, collectively, the senior secured term loan facility and the senior secured revolving facility under that certain credit agreement, to be dated on or about the Effective Date, by and among the Issuer, Holdings, Citibank, N.A., as the administrative agent, and the lenders party thereto, including any guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements, refundings, refinancings or replacements thereof and any one or more indentures or credit facilities or commercial paper facilities with banks or other institutional lenders or investors that replace, refund, supplement or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof (provided that, such increase in borrowings is permitted under “—Certain covenants—Limitation on incurrence of indebtedness and issuance of disqualified stock and preferred stock” above) or adds Restricted Subsidiaries as additional borrowers or guarantors thereunder and whether by the same or any other agent, trustee, lender or group of lenders or holders.
“Senior Indebtedness” means:
(1) all Indebtedness of the Issuer or any Guarantor outstanding under the Senior Credit Facilities and the Notes and related Guarantees (including interest accruing on or after the filing of any petition in bankruptcy or similar proceeding or for reorganization of the Issuer or any Guarantor (at the rate provided for in the documentation with respect thereto, regardless of whether or not a claim for post-filing interest is allowed in such proceedings)), and any and all other fees, expense reimbursement obligations, indemnification amounts, penalties, and other amounts (whether existing on the Effective Date or thereafter created or incurred) and all obligations of the Issuer or any Guarantor to reimburse any bank or other Person in respect of amounts paid under letters of credit, acceptances or other similar instruments;
(2) all (x) Hedging Obligations (and guarantees thereof) and (y) obligations in respect of Cash Management Services (and guarantees thereof) owing to a lender under the Senior Credit Facilities or any Affiliate of such lender (or any Person that was a lender or an Affiliate of such lender at the time the applicable agreement giving rise to such Hedging Obligation was entered into); provided that such Hedging Obligations and obligations in respect of Cash Management Services, as the case may be, are permitted to be incurred under the terms of the Indenture;
(3) any other Indebtedness of the Issuer or any Guarantor permitted to be incurred under the terms of the Indenture, unless the instrument under which such Indebtedness is incurred expressly provides that it is subordinated in right of payment to the Notes or any related Guarantee; and
(4) all Obligations with respect to the items listed in the preceding clauses (1), (2) and (3);
164
Table of Contents
provided that Senior Indebtedness shall not include:
(a) any obligation of such Person to the Issuer or any of its Subsidiaries;
(b) any liability for federal, state, local or other taxes owed or owing by such Person;
(c) any accounts payable or other liability to trade creditors arising in the ordinary course of business;
(d) any Indebtedness or other Obligation of such Person which is subordinate or junior in any respect to any other Indebtedness or other Obligation of such Person; or
(e) that portion of any Indebtedness which at the time of incurrence is incurred in violation of the Indenture.
“Senior Secured Net Leverage Ratio” means, with respect to any Test Period, the ratio of (a) Consolidated Total Debt outstanding on the last day of such Test Period that is secured by a Lien on any property of the Issuer or any Restricted Subsidiary minus an aggregate amount of cash and Cash Equivalents not exceeding $75.0 million in the aggregate, included in the consolidated balance sheet of the Issuer as of such date, excluding cash and Cash Equivalents which are listed as “Restricted” on such balance sheet, to (b) EBITDA of the Issuer for such Test Period, in each case with such pro forma adjustments as are appropriate and consistent with the pro forma adjustment provisions set forth in the definition of Fixed Charge Coverage Ratio.
“Significant Subsidiary” means any Restricted Subsidiary that would be a “significant subsidiary” as defined in Article 1, Rule 1-02 of Regulation S-X, promulgated pursuant to the Securities Act, as such regulation is in effect on the Effective Date.
“Similar Business” means (1) any business engaged in by the Issuer or any of its Restricted Subsidiaries on the Effective Date, and (2) any business or other activities that are reasonably similar, ancillary, complementary or related to, or a reasonable extension, development or expansion of, the businesses in which the Issuer and its Restricted Subsidiaries are engaged on the Effective Date.
“Specified Legal Expenses” means all attorneys’ and experts’ fees and expenses and all other costs, liabilities and expenses paid or payable in connection with settling, investigating or defending or preparing to investigate or defend any threatened, pending, completed or future claim, demand, action, suit, proceeding, inquiry or investigation (whether civil, criminal, administrative or investigative) arising out of or related to antitrust, Federal Trade Commission or Department of Justice proceedings or securities law (other than in connection with the Transactions) not to exceed in aggregate $2.0 million in any Test Period.
“Specified Transaction” means (u) any designation of operations or assets of the Issuer or a Restricted Subsidiary as discontinued operations (as defined under GAAP), (v) any Investment that results in a Person becoming a Restricted Subsidiary, (w) any designation of a Subsidiary as a Restricted Subsidiary or an Unrestricted Subsidiary in compliance with the Indenture, (x) any purchase or other acquisition of a business of any Person, of assets constituting a business unit, line of business or division of any Person, (y) any Asset Sale (i) that results in a Restricted Subsidiary ceasing to be a Subsidiary of the Issuer or (ii) of a business, business unit, line of business or division of the Issuer or a Restricted Subsidiary, in each case whether by merger, consolidation or otherwise or (z) other Restricted Payment that by the terms of the Indenture requires a financial ratio to be calculated on a pro forma basis.
“Subordinated Indebtedness” means, with respect to the Notes,
(1) any Indebtedness of the Issuer which is by its terms subordinated in right of payment to the Notes, and
(2) any Indebtedness of any Guarantor which is by its terms subordinated in right of payment to the Guarantee of such entity of the Notes.
165
Table of Contents
“Subsidiary” means, with respect to any Person:
(1) any corporation, association, or other business entity (other than a partnership, joint venture, limited liability company or similar entity) of which more than 50.0% of the total voting power of shares of Capital Stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time of determination owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof or is consolidated under GAAP with such Person at such time; and
(2) any partnership, joint venture, limited liability company or similar entity of which
(a) more than 50.0% of the capital accounts, distribution rights, total equity and voting interests or general or limited partnership interests, as applicable, are owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof whether in the form of membership, general, special or limited partnership or otherwise, and
(b) such Person or any Restricted Subsidiary of such Person is a controlling general partner or otherwise controls such entity.
“Test Period” in effect at any time means the Issuer’s most recently ended four fiscal quarters for which internal financial statements are available (as determined in good faith by the Issuer).
“Total Assets” means the total assets of the Issuer and its Restricted Subsidiaries, determined on a consolidated basis in accordance with GAAP, as shown on the most recent balance sheet of the Issuer or such other Person as may be expressly stated.
“Transaction Agreement” means the Agreement and Plan of Merger, dated as of July 2, 2011, among IVD Holdings Inc., a Delaware corporation, IVD Acquisition and Immucor, as the same may be amended prior to the Issue Date.
“Transaction Expenses” means any fees or expenses incurred or paid by the Issuer or any Restricted Subsidiary in connection with the Transactions, including payments to officers, employees and directors as change of control payments, severance payments, special or retention bonuses and charges for repurchase or rollover of, or modifications to, stock options.
“Transactions” means the transactions contemplated by the Transaction Agreement (as amended through the Effective Date), the issuance of the Notes and borrowings under the Senior Credit Facilities as in effect on the Effective Date.
“Treasury Rate” means, as of any Redemption Date, the yield to maturity as of such Redemption Date of United States Treasury securities with a constant maturity (as compiled and published in the most recent Federal Reserve Statistical Release H.15 (519) that has become publicly available at least two Business Days prior to the Redemption Date (or, if such Statistical Release is no longer published, any publicly available source of similar market data)) most nearly equal to the period from the Redemption Date to August 15, 2015; provided that if the period from the Redemption Date to such date is less than one year, the weekly average yield on actively traded United States Treasury securities adjusted to a constant maturity of one year will be used.
“Trust Indenture Act” means the Trust Indenture Act of 1939, as amended (15 U.S.C. §§ 77aaa-777bbbb).
“Uniform Commercial Code” means the Uniform Commercial Code or any successor provision thereof as the same may from time to time be in effect in the State of New York.
“Unrestricted Subsidiary” means:
(1) any Subsidiary of the Issuer which at the time of determination is an Unrestricted Subsidiary (as designated by the Issuer, as provided below); and
166
Table of Contents
(2) any Subsidiary of an Unrestricted Subsidiary.
The Issuer may designate any Subsidiary of the Issuer (including any existing Subsidiary and any newly acquired or newly formed Subsidiary) to be an Unrestricted Subsidiary unless such Subsidiary or any of its Subsidiaries owns any Equity Interests or Indebtedness of, or owns or holds any Lien on, any property of, the Issuer or any Subsidiary of the Issuer (other than solely any Subsidiary of the Subsidiary to be so designated); provided that:
(1) any Unrestricted Subsidiary must be an entity of which the Equity Interests entitled to cast at least a majority of the votes that may be cast by all Equity Interests having ordinary voting power for the election of directors or Persons performing a similar function are owned, directly or indirectly, by the Issuer;
(2) such designation complies with the covenants described under “—Certain covenants—Limitation on restricted payments”; and
(3) each of (a) the Subsidiary to be so designated and (b) its Subsidiaries has not at the time of designation, and does not thereafter, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable with respect to any Indebtedness pursuant to which the lender has recourse to any of the assets of the Issuer or any Restricted Subsidiary.
The Issuer may designate any Unrestricted Subsidiary to be a Restricted Subsidiary; provided that, immediately after giving effect to such designation, no Default shall have occurred and be continuing and either: (1) the Issuer could incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Test or (2) the Fixed Charge Coverage Ratio for the Issuer would be equal to or greater than such ratio for the Issuer immediately prior to such designation, in each case, on a pro forma basis taking into account such designation.
Any such designation by the Issuer shall be notified by the Issuer to the Trustee by promptly filing with the Trustee a copy of the resolution of the board of directors of the Issuer or any committee thereof giving effect to such designation and an Officer’s Certificate certifying that such designation complied with the foregoing provisions.
“Voting Stock” of any Person as of any date means the Capital Stock of such Person that is at the time entitled to vote in the election of the board of directors of such Person.
“Weighted Average Life to Maturity” means, when applied to any Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, at any date, the quotient obtained by dividing:
(1) the sum of the products of the number of years from the date of determination to the date of each successive scheduled principal payment of such Indebtedness or redemption or similar payment with respect to such Disqualified Stock or Preferred Stock multiplied by the amount of such payment; by
(2) the sum of all such payments;
provided that for purposes of determining the Weighted Average Life to Maturity of any Indebtedness that is being extended, replaced, refunded, refinanced, renewed or defeased (the “Applicable Indebtedness”), the effects of any amortization or prepayments made on such Applicable Indebtedness prior to the date of the applicable extension, replacement, refunding, refinancing, renewal or defeasance shall be disregarded.
“Wholly-Owned Subsidiary” of any Person means a Subsidiary of such Person, 100.0% of the outstanding Equity Interests of which (other than directors’ qualifying shares and shares of Capital Stock of Foreign Subsidiaries issued to foreign nationals as required under applicable law) shall at the time be owned by such Person or by one or more Wholly-Owned Subsidiaries of such Person or by such Person and one or more Wholly-Owned Subsidiaries of such Person.
167
Table of Contents
The certificates representing the exchange notes will be issued in fully registered form without interest coupons (the “global notes”). The global notes will be deposited with the trustee as a custodian for DTC, as depositary, and registered in the name of a nominee of such depositary.
The global notes
We expect that pursuant to procedures established by DTC (a) upon the issuance of the global notes, DTC or its custodian will credit, on its internal system, the principal amount at maturity of the individual beneficial interests represented by such global notes to the respective accounts of persons who have accounts with such depositary and (b) ownership of beneficial interests in the global notes will be shown on, and the transfer of such ownership will be effected only through, records maintained by DTC or its nominee (with respect to interests of participants) and the records of participants (with respect to interests of persons other than participants). Ownership of beneficial interests in the global notes will be limited to persons who have accounts with DTC (“participants”) or persons who hold interests through participants. Holders may hold their interests in the global notes directly through DTC if they are participants in such system, or indirectly through organizations which are participants in such system.
Except as described below, owners of interests in the global notes will not have notes registered in their names, will not receive physical delivery of notes in certificated form and will not be considered the registered owners or “holders” thereof under the indenture for any purpose.
So long as DTC, or its nominee, is the registered owner or holder of the notes, DTC or such nominee, as the case may be, will be considered the sole owner or holder of the notes represented by such global notes for all purposes under the indenture. No beneficial owner of an interest in the global notes will be able to transfer that interest except in accordance with DTC’s procedures, in addition to those provided for under the indenture with respect to the global notes.
Payments of the principal of, premium (if any), interest (including additional interest) on, the global notes will be made to DTC or its nominee, as the case may be, as the registered owner thereof. None of us, the trustee or any paying agent will have any responsibility or liability for any aspect of the records relating to or payments made on account of beneficial ownership interests in the global notes or for maintaining, supervising or reviewing any records relating to such beneficial ownership interest.
We expect that DTC or its nominee, upon receipt of any payment of principal, premium, if any, interest (including additional interest) on the global notes, will credit participants’ accounts with payments in amounts proportionate to their respective beneficial interests in the principal amount of the global notes as shown on the records of DTC or its nominee. We also expect that payments by participants to owners of beneficial interests in the global notes held through such participants will be governed by standing instructions and customary practice, as is now the case with securities held for the accounts of customers registered in the names of nominees for such customers. Such payments will be the responsibility of such participants.
Transfers between participants in DTC will be effected in the ordinary way through DTC’s same-day funds system in accordance with DTC rules and will be settled in same day funds.
DTC has advised us that it will take any action permitted to be taken by a holder of notes (including the presentation of outstanding notes for exchange as described below) only at the direction of one or more participants to whose account the DTC interests in the global notes are credited and only in respect of such portion of the aggregate principal amount of notes as to which such participant or participants has or have given such direction. However, if there is an event of default under the indenture, DTC will exchange the global notes
168
Table of Contents
for certificated securities, which it will distribute to its participants and which will be legended as set forth in the final offering memoranda relating to the outstanding notes.
DTC has advised us as follows: DTC is a limited purpose trust company organized under the laws of the State of New York, a member of the Federal Reserve System, a “clearing corporation” within the meaning of the Uniform Commercial Code and a “Clearing Agency” registered pursuant to the provisions of Section 17A of the Exchange Act. DTC was created to hold securities for its participants and facilitate the clearance and settlement of securities transactions between participants through electronic book-entry changes in accounts of its participants, thereby eliminating the need for physical movement of certificates. Participants include securities brokers and dealers, banks, trust companies and clearing corporations and certain other organizations. Indirect access to the DTC system is available to others such as banks, brokers, dealers and trust companies that clear through or maintain a custodial relationship with a participant, either directly or indirectly (“indirect participants”).
Although DTC has agreed to the foregoing procedures in order to facilitate transfers of interests in the global notes among participants of DTC, it is under no obligation to perform such procedures, and such procedures may be discontinued at any time. Neither we nor the trustee will have any responsibility for the performance by DTC or its participants or indirect participants of their respective obligations under the rules and procedures governing their operations.
Certificated securities
Certificated securities shall only be issued in exchange for beneficial interests in the global notes (i) after there has occurred and is continuing an event of default with respect to the notes, (ii) if DTC is at any time unwilling or unable to continue as a depositary for the global notes or has ceased to be a clearing agency registered under the Exchange Act and a successor depositary is not appointed by us within 90 days or (iii) we, at our option, notify the trustee that we elect to cause the issuance of certificated notes and any participant requests a certificated note in accordance with DTC procedures.
Secondary market trading, global clearance and settlement under the book-entry system
Any permitted secondary market trading activity in the notes will be required by DTC to be settled in immediately available funds. We expect that secondary trading in any certificated notes will also be settled in immediately available funds.
169
Table of Contents
Material United States federal income tax considerations
The following discussion is a summary of certain U.S. federal income tax considerations applicable to the exchange of outstanding notes for exchange notes pursuant to the exchange offer. This summary is based on the Code, U.S. Treasury regulations promulgated thereunder, Internal Revenue Service (“IRS”) rulings and other published administrative positions, judicial decisions, and other applicable authorities, each as now in effect or in existence as of the date of this prospectus. All of these are subject to change, possibly with retroactive effect, or different interpretations, which may result in U.S. federal tax considerations different from those summarized below. No ruling has been requested from the IRS regarding the considerations discussed in this summary, and no assurance can be given that the IRS would not assert, or that a court would not sustain, a position contrary to any of the tax considerations discussed below.
This discussion is limited to holders who exchange their outstanding notes for exchange notes pursuant to the exchange offer. Moreover, the discussion is limited to holders who hold the notes as “capital assets” within the meaning of Section 1221 of the Code (generally, property held for investment). This summary does not address all aspects of U.S. federal income taxation that may be relevant to particular holders of notes in light of their specific circumstances (for example, holders subject to the alternative minimum tax provisions of the Code or to the Medicare tax on unearned income) or to holders that may be subject to special rules under U.S. federal income tax law, including:
| • | broker-dealers in stocks, securities or currencies; |
| • | persons that use a mark-to-market method of accounting; |
| • | banks and other financial institutions; |
| • | insurance companies; |
| • | regulated investment companies; |
| • | real estate investment trusts; |
| • | tax-exempt organizations; |
| • | persons holding notes as part of a synthetic security, a straddle or a hedging, integrated, conversion or constructive sale transaction; |
| • | persons who or that are, or may become, subject to the expatriation provisions of the Code; |
| • | individual retirement accounts or other tax deferred accounts; |
| • | U.S. persons whose functional currency is not the U.S. dollar; or |
| • | pass-through entities, including S corporations, partnerships and entities and arrangements classified as partnerships for U.S. federal tax purposes, or investors in such entities. |
The summary also does not discuss any aspect of state, local or foreign law, or U.S. federal estate and gift tax law, as applicable to holders of the notes.
If any entity or arrangement treated as a partnership for U.S. federal income tax purposes holds the notes and participates in the exchange offer, the U.S. federal income tax treatment of a partner generally will depend on the status of the partner and the activities of the partnership. If you are a partnership or a partner in a partnership, you should consult your own tax advisor as to the tax consequences of the exchange of outstanding notes for exchange notes pursuant to the exchange offer.
YOU SHOULD CONSULT YOUR OWN TAX ADVISORS WITH REGARD TO THE APPLICATION OF THE TAX CONSIDERATIONS DISCUSSED BELOW TO YOUR PARTICULAR SITUATION AND THE APPLICATION OF ANY OTHER U.S. FEDERAL AS WELL AS STATE OR LOCAL OR FOREIGN TAX
170
Table of Contents
LAWS AND TAX TREATIES, INCLUDING GIFT AND ESTATE TAX LAWS, IN CONNECTION WITH THE EXCHANGE OF OUTSTANDING NOTES FOR EXCHANGE NOTES PURSUANT TO THE EXCHANGE OFFER.
Exchange of outstanding notes for exchange notes pursuant to the exchange offer
The exchange of outstanding notes for exchange notes pursuant to the exchange offers will not constitute a taxable exchange for U.S. federal income tax purposes. Accordingly, a holder will not recognize any gain or loss upon the receipt of an exchange note for an outstanding note, a holder’s holding period for an exchange note will include the holding period of the outstanding note exchanged therefor, and such holder’s tax basis in the exchange note will be the same as its tax basis of the outstanding note immediately before the exchange.
171
Table of Contents
Each broker-dealer that receives exchange notes for its own account pursuant to the exchange offer must acknowledge that it will deliver a prospectus in connection with any resale of the exchange notes. This prospectus, as it may be amended or supplemented from time to time, may be used by a broker-dealer in connection with resales of exchange notes received in exchange for outstanding notes where the outstanding notes were acquired as a result of market-making activities or other trading activities. To the extent any such broker-dealer participates in the exchange offer, we have agreed that for a period of up to 180 days, we will use our commercially reasonable efforts to make this prospectus, as amended or supplemented, available to such broker-dealer for use in connection with any such resale.
We will not receive any proceeds from any sale of exchange notes by broker-dealers. Exchange notes received by broker-dealers for their own accounts pursuant to the exchange offer may be sold from time to time in one or more transactions in the over-the-counter market, in negotiated transactions, through the writing of options on the exchange notes or a combination of these methods of resale, at market prices prevailing at the time of resale, at prices related to the prevailing market prices or negotiated prices. Any resale may be made directly to purchasers or to through brokers or dealers who may receive compensation in the form of commissions or concessions from any broker-dealer or the purchasers of any exchange notes. Any broker-dealer that resells exchange notes that were received by it for its own account pursuant to the exchange offer and any broker or dealer that participates in a distribution of the exchange notes may be deemed to be an “underwriter” within the meaning of the Securities Act and any profit on any resale of exchange notes and any commissions or concessions received by these persons may be deemed to be underwriting compensation under the Securities Act. The letter of transmittal states that by acknowledging that it will deliver and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act.
We will not make any payments to brokers, dealers or others soliciting acceptances of the exchange offer. The estimated cash expenses to be incurred in connection with the exchange offer will be paid by us and will include fees and expenses of the exchange agent, legal, printing and related fees and expenses. Notwithstanding the foregoing, holders of the outstanding notes shall pay all agency fees and commissions and underwriting discounts and commissions, if any, attributable to the sale of such outstanding notes or exchange notes.
The validity and enforceability of the exchange notes and the related guarantees offered hereby will be passed upon for us by Ropes & Gray LLP, Boston, Massachusetts. In passing on the validity of the exchange notes and the guarantees, Ropes & Gray LLP relied upon the opinion of Bryan Cave LLP as to certain matters of the laws of the State of Georgia.
The audited financial statements and schedule included in this prospectus and elsewhere in the registration statement have been included in reliance upon the report of Grant Thornton LLP, independent registered public accountants, upon the authority of said firm as experts in accounting and auditing in giving said report.
172
Table of Contents
Index to consolidated financial statements
| Page | ||||
| Audited Financial Statements for the years ended May 31, 2011, 2010, and 2009 |
||||
| Report of Grant Thornton, LLP, independent registered public accounting firm |
F-2 | |||
| F-3 | ||||
| Audited consolidated statements of income for the years ended May 31, 2011, 2010 and 2009 |
F-4 | |||
| F-5 | ||||
| Audited consolidated statements of cash flows for the years ended May 31, 2011, 2010 and 2009 |
F-6 | |||
| F-7 | ||||
| F-38 | ||||
| Unaudited Financial Statements for the periods ended August 31, 2011 and 2010 |
||||
| F-39 | ||||
| F-40 | ||||
| F-41 | ||||
| F-42 | ||||
| F-43 | ||||
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm on Consolidated Financial Statements
Board of Directors and Shareholders
Immucor, Inc.
We have audited the accompanying consolidated balance sheets of Immucor, Inc. (a Georgia corporation) and subsidiaries (the “Company”) as of May 31, 2011 and 2010, and the related consolidated statements of income, shareholders’ equity and comprehensive income, and cash flows for each of the three years in the period ended May 31, 2011. Our audits of the basic consolidated financial statements included the financial statement schedule listed in the index on page F-1. These financial statements and the financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of May 31, 2011 and 2010, and the consolidated results of its operations and its cash flows for each of the three years in the period ended May 31, 2011 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the related financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of May 31, 2011, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated July 20, 2011 (not separately included herein) expressed an unqualified opinion.
/s/ GRANT THORNTON LLP
Atlanta, Georgia
July 20, 2011 (except for Notes 15 and 19 as to which the date is November 21, 2011)
F-2
Table of Contents
Immucor, Inc. and subsidiaries
Consolidated balance sheets
(Amounts in thousands, except share data)
| May 31, 2011 |
May 31, 2010 |
|||||||
| ASSETS |
||||||||
| CURRENT ASSETS: |
||||||||
| Cash and cash equivalents |
$ | 302,603 | $ | 202,649 | ||||
| Trade accounts receivable, net of allowance for doubtful accounts of $2,157 and $2,122 at May 31, 2011 and May 31, 2010, respectively |
63,324 | 59,578 | ||||||
| Inventories |
32,914 | 35,730 | ||||||
| Deferred income tax assets, current portion |
15,884 | 14,807 | ||||||
| Prepaid expenses and other current assets |
11,164 | 4,832 | ||||||
|
|
|
|
|
|||||
| Total current assets |
425,889 | 317,596 | ||||||
| PROPERTY AND EQUIPMENT, Net |
58,216 | 49,169 | ||||||
| GOODWILL |
93,767 | 94,336 | ||||||
| INTANGIBLE ASSETS, Net |
54,133 | 57,628 | ||||||
| DEFERRED INCOME TAX ASSETS |
376 | 540 | ||||||
| OTHER ASSETS |
746 | 565 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 633,127 | $ | 519,834 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY |
||||||||
| CURRENT LIABILITIES: |
||||||||
| Accounts payable |
$ | 10,790 | $ | 7,973 | ||||
| Accrued expenses and other current liabilities |
20,331 | 17,378 | ||||||
| Income taxes payable |
8,294 | 12,312 | ||||||
| Deferred revenue, current portion |
7,495 | 8,994 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
46,910 | 46,657 | ||||||
| DEFERRED REVENUE |
6,080 | 7,687 | ||||||
| DEFERRED INCOME TAX LIABILITIES |
9,264 | 7,368 | ||||||
| OTHER LONG-TERM LIABILITIES |
2,001 | 1,999 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
64,255 | 63,711 | ||||||
| COMMITMENTS AND CONTINGENCIES (Note 17) |
— | — | ||||||
| SHAREHOLDERS’ EQUITY: |
||||||||
| Common stock, $0.10 par value; authorized 120,000,000 shares, issued and outstanding 70,367,219 and 69,912,449 shares at May 31, 2011 and May 31, 2010, respectively |
7,037 | 6,991 | ||||||
| Additional paid-in capital |
45,729 | 36,256 | ||||||
| Retained earnings |
499,152 | 409,825 | ||||||
| Accumulated other comprehensive income |
16,954 | 3,051 | ||||||
|
|
|
|
|
|||||
| Total shareholders’ equity |
568,872 | 456,123 | ||||||
|
|
|
|
|
|||||
| Total liabilities and shareholders’ equity |
$ | 633,127 | $ | 519,834 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-3
Table of Contents
Immucor, Inc. and subsidiaries
Consolidated statements of income
(Amounts in thousands, except per share data)
| For the year ended May 31, | ||||||||||||
| 2011 | 2010 | 2009(1) | ||||||||||
| NET SALES |
$ | 333,091 | $ | 329,073 | $ | 300,547 | ||||||
| COST OF SALES |
||||||||||||
| (exclusive of amortization shown separately below) |
96,175 | 95,349 | 84,536 | |||||||||
|
|
|
|
|
|
|
|||||||
| GROSS MARGIN |
236,916 | 233,724 | 216,011 | |||||||||
| OPERATING EXPENSES |
||||||||||||
| Research and development |
15,900 | 15,437 | 10,698 | |||||||||
| Selling and marketing |
36,431 | 36,995 | 38,315 | |||||||||
| Distribution |
16,508 | 14,831 | 13,708 | |||||||||
| General and administrative |
37,747 | 36,841 | 32,593 | |||||||||
| Amortization expense |
4,333 | 4,278 | 3,739 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
110,919 | 108,382 | 99,053 | |||||||||
|
|
|
|
|
|
|
|||||||
| INCOME FROM OPERATIONS |
125,997 | 125,342 | 116,958 | |||||||||
| NON-OPERATING INCOME (EXPENSE) |
||||||||||||
| Interest income |
706 | 454 | 1,957 | |||||||||
| Interest expense |
(70 | ) | (33 | ) | (250 | ) | ||||||
| Other, net |
3,997 | (551 | ) | (1,684 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total non-operating income (expense) |
4,633 | (130 | ) | 23 | ||||||||
|
|
|
|
|
|
|
|||||||
| INCOME BEFORE INCOME TAXES |
130,630 | 125,212 | 116,981 | |||||||||
| PROVISION FOR INCOME TAXES |
41,303 | 42,629 | 40,798 | |||||||||
|
|
|
|
|
|
|
|||||||
| NET INCOME |
$ | 89,327 | $ | 82,583 | $ | 76,183 | ||||||
|
|
|
|
|
|
|
|||||||
| Earnings per share: |
||||||||||||
| Per common share—basic |
$ | 1.27 | $ | 1.18 | $ | 1.08 | ||||||
|
|
|
|
|
|
|
|||||||
| Per common share—diluted |
$ | 1.26 | $ | 1.17 | $ | 1.07 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Certain numbers have been reclassed to conform to current year presentation. |
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-4
Table of Contents
Immucor, Inc. and subsidiaries
Consolidated statement of shareholders’ equity and comprehensive income
(Amounts in thousands)
| Common stock | Additional paid-in capital |
Retained earnings |
Accumulated other comprehensive income* |
Total share- holders’ equity |
||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||
| BALANCE, MAY 31, 2008 |
70,136 | $ | 7,014 | $ | 37,325 | $ | 251,059 | $ | 12,298 | $ | 307,696 | |||||||||||||
| Shares issued under employee stock plan |
669 | 67 | 3,632 | — | — | 3,699 | ||||||||||||||||||
| Share-based compensation expense |
— | — | 5,904 | — | — | 5,904 | ||||||||||||||||||
| Stock repurchases and retirements |
(349 | ) | (35 | ) | (8,102 | ) | — | — | (8,137 | ) | ||||||||||||||
| Tax benefits related to stock-based compensation |
— | — | 3,253 | — | — | 3,253 | ||||||||||||||||||
| Comprehensive income (net of taxes): |
||||||||||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | — | (4,020 | ) | (4,020 | ) | ||||||||||||||||
| Net income |
— | — | — | 76,183 | — | 76,183 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total comprehensive income |
72,163 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| BALANCE, MAY 31, 2009 |
70,456 | $ | 7,046 | $ | 42,012 | $ | 327,242 | $ | 8,278 | $ | 384,578 | |||||||||||||
| Shares issued under employee stock plan |
125 | 12 | 324 | — | — | 336 | ||||||||||||||||||
| Share-based compensation expense |
— | — | 5,946 | — | — | 5,946 | ||||||||||||||||||
| Stock repurchases and retirements |
(669 | ) | (67 | ) | (11,843 | ) | — | — | (11,910 | ) | ||||||||||||||
| Tax shortfall related to stock-based compensation |
— | — | (183 | ) | — | — | (183 | ) | ||||||||||||||||
| Comprehensive income (net of taxes): |
||||||||||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | — | (5,227 | ) | (5,227 | ) | ||||||||||||||||
| Net income |
— | — | — | 82,583 | — | 82,583 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total comprehensive income |
77,356 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| BALANCE, MAY 31, 2010 |
69,912 | $ | 6,991 | $ | 36,256 | $ | 409,825 | $ | 3,051 | $ | 456,123 | |||||||||||||
| Shares issued under employee stock plan |
482 | 48 | 1,264 | — | — | 1,312 | ||||||||||||||||||
| Share-based compensation expense |
— | — | 6,941 | — | — | 6,941 | ||||||||||||||||||
| Stock repurchases and retirements |
(27 | ) | (2 | ) | (524 | ) | — | — | (526 | ) | ||||||||||||||
| Tax benefits related to stock-based compensation |
— | — | 1,792 | — | — | 1,792 | ||||||||||||||||||
| Comprehensive income (net of taxes): |
— | — | — | — | — | |||||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | — | 13,903 | 13,903 | ||||||||||||||||||
| Net income |
— | — | — | 89,327 | — | 89,327 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total comprehensive income |
103,230 | |||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| BALANCE, MAY 31, 2011 |
70,367 | $ | 7,037 | $ | 45,729 | $ | 499,152 | $ | 16,954 | $ | 568,872 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| * | Accumulated Other Comprehensive Income balance primarily consists of foreign currency translation adjustments and has no tax effect. |
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-5
Table of Contents
Immucor, Inc. and subsidiaries
Consolidated statements of cash flows
(Amounts in thousands)
| For the year ended May 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| OPERATING ACTIVITIES: |
||||||||||||
| Net income |
$ | 89,327 | $ | 82,583 | $ | 76,183 | ||||||
| Adjustments to reconcile net income to net cash provided by operating activities: |
||||||||||||
| Depreciation and amortization |
18,213 | 16,569 | 13,306 | |||||||||
| Loss on retirement of fixed assets |
1,072 | 538 | 352 | |||||||||
| Provision for doubtful accounts |
371 | 361 | 848 | |||||||||
| Share-based compensation expense |
6,941 | 5,946 | 5,904 | |||||||||
| Deferred income taxes |
941 | 4,994 | 5 | |||||||||
| Excess tax (benefits) shortfall from share-based compensation |
(1,792 | ) | 183 | (3,253 | ) | |||||||
| Accrued refund of BioArray escrowed funds(1) |
(4,256 | ) | — | — | ||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable, trade |
757 | (6,386 | ) | (6,310 | ) | |||||||
| Income taxes |
(2,790 | ) | 1,037 | 7,796 | ||||||||
| Inventories |
(8,248 | ) | (12,551 | ) | (4,647 | ) | ||||||
| Other assets |
875 | (102 | ) | (7,450 | ) | |||||||
| Accounts payable |
2,412 | (1,060 | ) | 796 | ||||||||
| Deferred revenue |
(3,595 | ) | (5,258 | ) | (2,247 | ) | ||||||
| Accrued expenses and other liabilities |
1,883 | (2,103 | ) | (1,461 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Cash provided by operating activities |
102,111 | 84,751 | 79,822 | |||||||||
| INVESTING ACTIVITIES: |
||||||||||||
| Purchases of property and equipment |
(9,061 | ) | (6,304 | ) | (8,602 | ) | ||||||
| Acquisition of businesses, net of cash acquired |
— | — | (107,954 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Cash used in investing activities |
(9,061 | ) | (6,304 | ) | (116,556 | ) | ||||||
| FINANCING ACTIVITIES: |
||||||||||||
| Repayments of long-term debt and capital leases |
— | — | (206 | ) | ||||||||
| Repurchase of common stock |
(526 | ) | (11,910 | ) | (7,621 | ) | ||||||
| Proceeds from exercise of stock options |
1,312 | 336 | 3,178 | |||||||||
| Excess tax benefits (shortfall) from share-based compensation |
1,792 | (183 | ) | 3,253 | ||||||||
|
|
|
|
|
|
|
|||||||
| Cash provided by (used in) financing activities |
2,578 | (11,757 | ) | (1,396 | ) | |||||||
| EFFECT OF EXCHANGE RATES ON CASH AND CASH EQUIVALENTS |
4,326 | (502 | ) | (465 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS |
99,954 | 66,188 | (38,595 | ) | ||||||||
| CASH AND CASH EQUIVALENTS AT BEGINNING OF YEAR |
202,649 | 136,461 | 175,056 | |||||||||
|
|
|
|
|
|
|
|||||||
| CASH AND CASH EQUIVALENTS AT END OF YEAR |
$ | 302,603 | $ | 202,649 | $ | 136,461 | ||||||
|
|
|
|
|
|
|
|||||||
| SUPPLEMENTAL INFORMATION: |
||||||||||||
| Tax paid |
$ | 43,090 | $ | 36,295 | $ | 32,528 | ||||||
| Interest paid |
$ | — | $ | — | $ | 36 | ||||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: |
||||||||||||
| Accrued refund of BioArray escrowed funds(1) |
$ | 2,935 | $ | — | $ | — | ||||||
| Movement from inventory to property and equipment for instruments placed on rental agreements |
$ | 12,857 | $ | 13,733 | $ | 6,707 | ||||||
| Shares surrendered for amounts due on stock options exercised |
$ | — | $ | — | $ | 516 | ||||||
| (1) | In fiscal 2011, the Company accrued the release of escrowed funds related to the acquisition of BioArray. In accordance with the contingent consideration guidance in ASC 805 “Business Combinations,” $2.9 million of the $7.2 million returned to the Company was recorded to Goodwill as it relates to items that were predefined as contingent items in the Merger Agreement and are considered measurement period adjustments. The $2.9 million appears in the Non-cash Investing and Financing Activities on the Consolidated Statement of Cash Flows. The remaining $4.3 million was recorded to Other Income as it is not considered a measurement period adjustment and appears as an adjustment to net income in the operating activities of the Consolidated Statement of Cash Flows. |
The accompanying notes are an integral part of these Consolidated Financial Statements.
F-6
Table of Contents
Immucor, Inc. and subsidiaries
Notes to consolidated financial statements
1. Nature of business and summary of significant accounting policies
Nature of business—Founded in 1982, Immucor, Inc., a Georgia corporation (“Immucor” or the “Company”), develops, manufactures and sells a complete line of reagents and automated systems used primarily by hospitals, reference laboratories and donor centers in a number of tests performed to detect and identify certain properties of the cell and serum components of human blood used for blood transfusion. The Company operates facilities in the U.S., Canada, Western Europe and Japan.
Consolidation policy—The consolidated financial statements include the accounts of the Company and all its subsidiaries. All significant inter-company balances and transactions have been eliminated in consolidation.
Use of estimates—The preparation of financial statements in conformity with generally accepted accounting principles in the U.S. requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Share-based compensation—The Company records share-based compensation expense in accordance with FASB Accounting Standard Codification™ (“ASC”) 718, “Compensation—Stock Compensation” (“ASC 718”). The Company values its share-based payment awards using the Black-Scholes option-pricing model based on grant date fair value estimates. The expense for each share-based payment award is expensed over the vesting period of the award using the straight-line method. See Note 12 of the notes to the consolidated financial statements for a detailed discussion of share-based compensation.
The Company has calculated its additional paid in capital pool (“APIC pool”) based on the actual income tax benefits received from exercises of stock options granted after the effective date of ASC 718 using the long method. The APIC pool is available to absorb future tax deficiencies subsequent to the adoption of ASC 718.
Concentration of credit risk—Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivable. In order to mitigate the concentration of credit risk, the Company places its cash and cash equivalents with multiple financial institutions. Cash and cash equivalents were $302.6 million and $202.6 million at May 31, 2011 and 2010, respectively, with approximately 83% and 80% of it located in the U.S.
Concentrations of credit risk with respect to accounts receivable are limited because a large number of geographically diverse customers make up the Company’s customer base, thus spreading the trade credit risk. At May 31, 2011, 2010 and 2009, no single customer or group of customers represented more than 10% of total consolidated net sales or trade accounts receivable. The Company controls credit risk through credit limits and monitoring procedures. At May 31, 2011 and 2010, the Company’s accounts receivable balances were $63.3 million and $59.6 million, respectively, with about 58% and 50%, respectively, of these accounts being of foreign origin, predominantly European. Companies and government agencies in some European countries require longer payment terms as a part of doing business. This may subject the Company to a higher risk of uncollectiblity. This risk is considered when the allowance for doubtful accounts is evaluated. The Company generally does not require collateral from its customers.
Concentration of production facilities and supplies—Substantially all of the Company’s reagent products are produced in its Norcross, Georgia facility in the U.S., and its reagent production is highly dependent on the uninterrupted and efficient operation of this facility. Therefore, if a catastrophic event occurred at the Norcross facility, such as a fire or tornado or if there is a production disruption for an extended period for any other reason, many of those products could not be produced until the manufacturing portion of the facility is restored and cleared by the FDA. The Company maintains a disaster plan to minimize the effects of such a catastrophe, and the Company has obtained insurance to protect against certain business interruption losses. While the Company purchases certain raw materials from a single supplier, the Company has reliable supplies of most raw materials.
F-7
Table of Contents
Cash and cash equivalents—The Company considers deposits that can be redeemed on demand and investments with an original maturity of three months or less when purchased to be cash and cash equivalents. Generally, cash and cash equivalents held at financial institutions are in excess of the Federal Deposit Insurance Corporation (FDIC) insurance limit. The Company limits its exposure to credit loss by placing its cash and cash equivalents in liquid investments with high quality financial institutions.
Inventories—Inventories are stated at the lower of cost (first-in, first-out basis) or market (net realizable value). Cost includes material, labor and manufacturing overhead. The Company also allocates certain production related general and administrative costs to inventory and incurred approximately $2.8 million, $3.3 million, and $3.0 million of such costs in fiscal 2011, 2010 and 2009, respectively. The Company had approximately $0.9 million and $1.2 million of general and administrative costs remaining in inventory as of May 31, 2011 and May 31, 2010, respectively.
The Company uses a standard cost system as a tool to monitor production efficiency. The standard cost system applies estimated labor and manufacturing overhead factors to inventory based on budgeted production and efficiency levels, staffing levels and costs of operation, based on the experience and judgment of management. Actual costs and production levels may vary from the standard established, and variances are charged to the consolidated statement of income as a component of cost of sales. Since U.S. generally accepted accounting principles require that the standard cost approximate actual cost, periodic adjustments are made to the standard rates to approximate actual costs. No material changes have been made to the inventory policy during fiscal 2011, 2010 or 2009.
Fair value of financial instruments—The carrying amounts reported in the consolidated balance sheets for cash and cash equivalents, accounts receivable and accounts payable approximate their fair values.
Property, plant and equipment—Property, plant and equipment is stated at cost less accumulated depreciation. Expenditures for replacements are capitalized, and the replaced items are retired. Normal maintenance and repairs are charged to operations. Major maintenance and repair activities that significantly enhance the useful life of the asset are capitalized. When property and equipment are retired, sold, or otherwise disposed of, the asset’s carrying amount and related accumulated depreciation are removed from the accounts and any gain or loss is included in operations. Depreciation is computed using the straight-line method over the estimated lives of the related assets ranging from three to thirty years. Carrying values of these assets are evaluated if impairment indicators arise.
Goodwill—In accordance with ASC 350, “Intangibles—Goodwill and Other” (“ASC 350”), goodwill and intangible assets with indefinite lives are tested for impairment annually or more frequently if impairment indicators arise. Intangible assets that have finite lives continue to be amortized over their useful lives.
The Company evaluates the carrying value of goodwill and intangible assets with indefinite lives at the end of the third quarter of each fiscal year and between annual evaluations if events occur or circumstances change that would more likely than not reduce the fair value of the reporting unit below its carrying amount. Such circumstances could include, but are not limited to: (1) a significant adverse change in legal factors or in business climate, (2) unanticipated competition, or (3) an adverse action or assessment by a regulator. When evaluating whether goodwill is impaired, the Company compares the fair value of the reporting unit to which the goodwill is assigned to the reporting unit’s carrying amount, including goodwill. The fair value of the reporting unit is estimated using primarily the income, or discounted cash flows, approach. If the carrying amount of a reporting unit exceeds its fair value, then the amount of the impairment loss must be measured. The impairment loss would be calculated by comparing the implied fair value of the reporting unit’s goodwill to its carrying amount. In calculating the implied fair value of the reporting unit’s goodwill, the fair value of the reporting unit is allocated to all of the other assets and liabilities of that unit based on their fair values. The excess of the fair value of a reporting unit over the amount assigned to its other assets and liabilities is the implied fair value of goodwill. An impairment loss would be recognized when the carrying amount of goodwill exceeds its implied fair value. The Company’s evaluation of goodwill and intangible assets with indefinite lives completed during fiscal years 2011, 2010 and 2009 resulted in no impairment charges.
F-8
Table of Contents
Deferred licensing costs—Deferred licensing costs with finite lives are amortized over their useful lives. In certain situations the deferred licensing costs are considered to have indefinite lives such as in the countries where the law and regulations are such that the barriers to obtaining a new license are very severe and upfront costs are high but, once the licenses are acquired, effort and costs required to maintain such licenses are minimal. The carrying values of assets with indefinite lives are not amortized but they are evaluated annually for impairment or more frequently if any triggering event which may impair the value of the asset occurs. There was no impairment charge related to deferred licensing costs in fiscal years 2011, 2010 or 2009.
Other intangible assets—Other intangible assets includes customer lists, developed product technology, trademarks and trade names. These intangible assets are amortized over their useful lives. Carrying values of these assets are evaluated when impairment indicators arise. There was no impairment charge related to other intangible assets in fiscal years 2011, 2010 or 2009.
Net sales relating to foreign operations—Sales to customers outside the United States were 32%, 28% and 27% of net sales in fiscal 2011, 2010 and 2009, respectively.
Foreign currency translation—The financial statements of foreign subsidiaries have been translated into U.S. Dollars in accordance with ASC 830-30, “Translation of Financial Statements” (“ASC 830-30”). The financial position and results of operations of the Company’s foreign subsidiaries are measured using the foreign subsidiary’s local currency as the functional currency. Revenues and expenses of such subsidiaries have been translated into U.S. Dollars at average exchange rates prevailing during the period. Assets and liabilities have been translated at the rates of exchange on the balance sheet date. The resulting translation gain and loss adjustments are recorded directly as a separate component of shareholders’ equity, unless there is a sale or complete liquidation of the underlying foreign investments. Foreign currency translation adjustments resulted in gains of $13.9 million in fiscal 2011 and losses of $5.2 million and $4.0 million in fiscal 2010 and 2009, respectively.
Gains and losses that result from foreign currency transactions are included in “other non-operating income (expense)” in the consolidated statements of income. In fiscal 2011, 2010 and 2009, we incurred net foreign currency transaction losses of $0.3 million, $0.4 million and $1.9 million, respectively.
Revenue recognition—In accordance with ASC 605, “Revenue Recognition” (“ASC 605”), the Company recognizes revenue when the following four basic criteria have been met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed and determinable; and (4) collectibility is reasonably assured.
| • | Reagent sales |
Revenue from the sale of the Company’s reagents to end users is primarily recognized upon shipment when both title and risk of loss transfer to the customer, unless there are specific contractual terms to the contrary. Revenue from the sale of the Company’s reagents to distributors is recognized FOB customs clearance when both title and risk of loss transfer to the customer.
| • | Instrument sales and rental |
Contracts for the sale or rental of our instruments typically have multiple deliverables. In such cases, we recognize revenue on the sale of instruments in accordance with ASC 605-25 “Revenue Recognition: Multiple-Element Arrangements” (“ASC 605-25”). Our instrument sales contracts with multiple deliverables include the sale or rental of an instrument (including delivery, installation and training), the servicing of the instrument during the first year, and, in many cases, price guarantees for reagents and consumables purchased during the contract period. We have determined the fair value of certain of these elements, such as training and first year service. We do not believe it is possible to determine the fair value of reagent contracts with price guarantees at the time of sale.
F-9
Table of Contents
If the contract contains reagent price guarantees, which our contracts typically do, the entire arrangement consideration is deferred and recognized over the related guarantee period. For contracts without reagent price guarantees, the sales price in excess of the fair values of training and service is allocated to the instrument itself and recognized upon shipment and completion of contractual obligations relating to training and/or installation. The fair value of a training session is recognized as revenue when services are provided. The fair value of first year service is recognized over the first year of the contract. The allocation of the total consideration, which is based on the estimated fair value of the units of accounting, requires judgment by management.
In revenue deferral situations, the costs related to the instruments are recognized when the instrument is installed and accepted by the customer.
Revenue from the sale of our instruments without multiple deliverables is generally recognized upon shipment and completion of contractual obligations. Revenue from rentals of our instruments is recognized over the term of the rental agreement. Instrument service contract revenue is recognized over the term of the service contract.
Instrument rentals are typically operating leases with 60 month terms. The assets subject to these leases are depreciated over the term of the lease using the straight line method.
Shipping and handling charges and sales tax—The amounts billed to customers for shipping and handling of orders are classified as revenue and reported in the statements of income as net sales. The costs of handling and shipping customer orders are reported in the operating expense section of the consolidated statements of income as distribution expense. For the years ended May 31, 2011, 2010 and 2009, these costs were $16.5 million, $14.8 million and $13.7 million, respectively. Sales taxes invoiced to customers and payable to government agencies are recorded on a net basis with the sales tax portion of a sales invoice directly credited to a liability account and the balance of the invoice credited to a revenue account.
Trade accounts receivable and allowance for doubtful accounts—Trade receivables at May 31, 2011 and May 31, 2010 were $63.3 million and $59.6 million, respectively, and were net of allowances for doubtful accounts of $2.2 million and $2.1 million, respectively. The allowance for doubtful accounts represents a reserve for estimated losses resulting from the inability of the Company’s customers to pay their debts. The collectibility of trade receivable balances is regularly evaluated based on a combination of factors such as customer credit-worthiness, past transaction history with the customer, current economic industry trends and changes in customer payment patterns. If it is determined that a customer will be unable to fully meet its financial obligation, such as in the case of a bankruptcy filing or other material events impacting its business, a specific allowance for doubtful accounts is recorded to reduce the related receivable to the amount expected to be recovered.
Advertising costs—Advertising costs are expensed as incurred and are classified as selling and marketing operating expenses in the consolidated statements of income. Advertising expenses were $0.5 million, $0.6 million and $0.7 million for the years ended May 31, 2011, 2010 and 2009, respectively.
Research and development costs—Research and development costs are expensed as incurred and are disclosed as a separate line item in the consolidated statements of income.
Loss contingencies—Certain conditions may exist as of the date the financial statements are issued that may result in a loss to the Company, but which will only be determined and resolved when one or more future events occur or fail to occur. The Company’s management and its legal counsel assess such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company’s legal counsel evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought therein.
F-10
Table of Contents
If the assessment of a contingency indicates that it is probable that a material loss is likely to occur and the amount of the liability can be estimated, then the estimated liability is accrued in the Company’s financial statements. If the assessment indicates that a potentially material loss contingency is not probable, but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material, would be disclosed.
Loss contingencies considered remote are generally not accrued or disclosed unless they involve guarantees, in which case the nature of the guarantee would be disclosed. Legal costs relating to loss contingencies are expensed as incurred.
Income taxes—The Company’s income tax policy is to record the estimated future tax effects of temporary differences between the tax bases of assets and liabilities and amounts reported in the accompanying consolidated balance sheets, as well as operating loss and tax credit carry-forwards. The value of the Company’s deferred tax assets assumes that the Company will be able to generate sufficient future taxable income in applicable tax jurisdictions, based on estimates and assumptions. If these estimates and related assumptions change in the future, the Company may be required to record additional valuation allowances against its deferred tax assets resulting in additional income tax expense in the Company’s consolidated statements of income. In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized, and the Company considers the scheduled reversal of deferred tax liabilities, projected future taxable income, carry-back opportunities, and tax-planning strategies in making this assessment. Management assesses the need for additional valuation allowances quarterly.
The calculation of income tax liabilities involves significant judgment in estimating the impact of uncertainties in the application of complex tax laws. The impact of an uncertain income tax position on an income tax return must be recognized at the largest amount that has a greater than 50% cumulative probability to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. This threshold and measurement attribute prescribed by the FASB will continue to require significant judgment by management.
Warranty—Each instrument sale includes a standard one-year service warranty. An extended warranty is sold separately. The start of the one-year standard service period generally coincides with when the instrument revenue itself is recognized (upon customer acceptance/training, etc.). Standard warranty revenues are thus deferred until the instrument revenues are recognized and are then amortized over a 12-month period. If the instrument revenue is deferred due to a price protection clause, the revenue from warranty is also deferred and recognized over the same period as the revenue from the instrument sale. The price charged for the extended warranty is considered to be the fair market value of a first year warranty built into the consideration arrangement and is in accordance with the guidance provided in ASC 605-25 “Revenue Recognition: Multiple-Element Arrangements.”
Impact of recently issued accounting standards—
Adopted by the company in fiscal 2011
In June 2009, the Financial Accounting Standards Board (“FASB”) issued an update to ASC 810, “Consolidation” (“ASC 810”). This update changes how a reporting entity determines when an entity that is insufficiently capitalized or is not controlled through voting (or similar rights) should be consolidated. The determination of whether a reporting entity is required to consolidate another entity is based on, among other things, the other entity’s purpose and design and the reporting entity’s ability to direct the activities of the other entity that most significantly impact the other entity’s economic performance. This update requires a reporting entity to provide additional disclosures about its involvement with variable interest entities and any significant changes in risk exposure due to that involvement. A reporting entity is now required to disclose how its
F-11
Table of Contents
involvement with a variable interest entity affects the reporting entity’s financial statements. The update is effective at the start of a reporting entity’s first fiscal year beginning after November 15, 2009. The adoption of this update to ASC 810 during the first quarter of fiscal 2011 did not have an impact on the Company’s consolidated financial statements.
In December 2009, the FASB issued ASU 2009-16, “Accounting for Transfers of Financial Assets” (“ASU 2009-16”), which is an amendment of ASC 860, “Transfers and Servicing.” This update requires more information about the transfer of financial assets. More specifically, ASU 2009-16 eliminates the concept of a “qualified special purpose entity”, changes the requirements for derecognizing financial assets, and enhances the information reported to users of financial statements. This update is effective for fiscal years beginning on or after November 15, 2009. The adoption of ASU 2009-16 during the first quarter of fiscal 2011 did not have an impact on the Company’s consolidated financial statements.
In April 2010, the FASB issued ASU 2010-13, “Effect of Denominating the Exercise Price of a Share-Based Payment Award in the Currency of the Market in Which the Underlying Equity Security Trades” (“ASU 2010-13”), which is an amendment of ASC 718, “Compensation—Stock Compensation. This update clarifies that an employee share-based payment award with an exercise price denominated in the currency of a market in which a substantial portion of the entity’s equity securities trades should not be considered to contain a condition that is not a market, performance, or service condition. Therefore, an entity would not classify such an award as a liability if it otherwise qualifies as equity. This update is effective for fiscal years and interim periods beginning on or after December 15, 2010, however, early application was permitted. The adoption of ASU 2010-13 during the first quarter of fiscal 2011 did not have an impact on the Company’s consolidated financial statements.
Not yet adopted by the company
In October 2009, the FASB issued ASU 2009-13, “Multiple Deliverables Revenue Arrangements” (“ASU 2009-13”), which is an amendment of ASC 605-25, “Revenue Recognition: Multiple Element Arrangements.” This update addresses the accounting for multiple-deliverable arrangements to allow the vendor to account for deliverables separately instead of as one combined unit by amending the criteria for separating consideration in multiple-deliverable arrangements. This guidance establishes a selling price hierarchy for determining the selling price of a deliverable, which is based on (a) vendor-specific objective evidence, (b) third-party evidence or (c) best estimate of selling price. The residual method of allocation has been eliminated and arrangement consideration is now required to be allocated to all deliverables at the inception of the arrangement using the selling price method. Additionally, expanded disclosures will be required relating to multiple deliverable revenue arrangements. This update will be effective for fiscal years beginning on or after June 15, 2010, which corresponds to the Company’s first quarter of fiscal 2012. Early adoption is permitted, however, the Company did not adopt early. Based on the current mix of instrument acquisition methods, the Company does not expect the adoption of this update to ASC 605-25 to have a material impact on its financial statements.
In June 2011, the FASB issued ASU 2011-05, “Presentation of Comprehensive Income” (“ASU 2011-05”), which was issued to enhance comparability between entities that report under U.S. GAAP and IFRS, and to provide a more consistent method of presenting non-owner transactions that affect an entity’s equity. ASU 2011-05 eliminates the option to report other comprehensive income and its components in the statement of changes in stockholders’ equity and requires an entity to present the total of comprehensive income, the components of net income and the components of other comprehensive income either in a single continuous statement or in two separate but consecutive statements. This pronouncement is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011, which corresponds to the Company’s first quarter of fiscal 2013. Early adoption of the new guidance is permitted and full retrospective application is required. The Company is currently evaluating the effect that the provisions of this pronouncement will have on its financial statements.
F-12
Table of Contents
2. Acquisitions
BioArray
On March 11, 2008, the Company signed an Agreement and Plan of Merger with BioArray Solutions Ltd. (“BioArray”), a privately-held company based in Warren, NJ. The Company completed the acquisition of BioArray on August 4, 2008. Pursuant to the agreement, the Company acquired 100% of the outstanding common stock of BioArray for an aggregate purchase price of $115.2 million, including approximately $2.4 million of acquisition-related transaction costs. The purchase consideration was paid in cash. The Company has included the financial results of BioArray in its consolidated financial statements beginning August 4, 2008.
BioArray is a pioneer in the field of molecular immunohematology or the DNA analysis of blood for the purpose of transfusion. Through the development of technologies supported by a substantial patent portfolio of issued or pending patents, BioArray developed a novel and flexible technology platform that allows for a variety of DNA-based testing. The platform combines semiconductor processing, microparticle chemistry and molecular biology to bring a high degree of flexibility and performance to DNA and protein analysis.
Using this technology platform, BioArray developed complete assay solutions called the BeadChipTM system. The BeadChip system includes BeadChips featuring proprietary array designs as well as the Array Imaging System and BASISTM database, which reads and interprets the results of the manual testing. The ensuing integrated assay delivery system enables users to simultaneously perform dozens of customized tests on each patient sample in a semi-automated fashion. The Company believes this versatility makes the BeadChip format ideally suited to a wide scope of applications.
As of the acquisition date, the BeadChip system was being sold to and used by a number of customers in the U.S. for Research Use Only purposes, which does not require FDA approval. After the acquisition was finalized, the Company began expanding the footprint of the BeadChip system outside of the U.S., particularly in Western Europe. The Company is in the process of developing an automated molecular immunohematology instrument. Additionally, we are working on achieving U.S. regulatory approval for the molecular offering. We believe these steps will facilitate the further commercialization of the BioArray solution. The current plan is to have a Research Use Only instrument available in calendar 2012.
Immucor believes that molecular immunohematology will revolutionize transfusion medicine. Immucor also believes that its acquisition of BioArray provides new, strategic growth markets for the Company in both its current market of transfusion, through an offering complementary to its current product offerings, as well as the potential new market of transplantation. The purchase price for BioArray, and the resulting goodwill, reflects the significant growth opportunities that both companies believe BioArray’s technology and the combination of the companies’ complementary strengths represents for future growth.
Immucor’s leadership in blood banking industry automation and BioArray’s leadership in molecular diagnostic systems for specialty transfusion applications should allow the combined company to develop and deliver more precise molecular immunohematology solutions to enhance patient outcomes. Immucor also believes its proven strength in developing automated instruments combined with its FDA licensing and CE Mark process experience, its established distribution network, its sales and marketing capabilities as well as its financial resources will generate enhanced long-term growth opportunities for the commercialization of BioArray’s BeadChip system.
The acquisition has been accounted for as a purchase business combination. Assets acquired and liabilities assumed were recorded at their fair values as of August 4, 2008. Acquisition-related transaction costs include investment banking, legal and accounting fees, and other external costs directly related to the acquisition.
In connection with the transaction, BioArray formed a new company intended to commercialize BioArray’s technology in fields outside of blood transfusion and transplantation. In April 2009, Immucor bought back the rights to the BioArray technology from the new company for approximately $1.0 million. As part of the liquidation of the new company, Immucor received approximately $140,000 in cash from the net assets, proportional to Immucor’s ownership interest.
F-13
Table of Contents
The transaction costs included a payment of $1.4 million to TM Capital Corp., an investment bank which represented the Company in a number of financial transactions, including the acquisition of BioArray Solutions in August 2008. Michael S. Goldman, one the Company’s former directors, is a Managing Director and founding principal of TM Capital Corp. With the receipt of these fees, as of August 4, 2008, Mr. Goldman no longer qualified as an “independent” director of the Company under Nasdaq Stock Market listing standards. As a result, Mr. Goldman resigned his positions as a member of the Compensation Committee and Governance Committee of the Board of Directors as of August 4, 2008 and did not stand for re-election at the 2008 annual meeting of shareholders.
Purchase price allocation
Under purchase accounting, the total purchase price was allocated to BioArray’s net tangible and identifiable intangible assets based on their estimated fair values as of August 4, 2008. The excess of the purchase price over the net tangible and identifiable intangible assets was recorded as goodwill. The allocation of the purchase price was based upon a valuation.
The following table details the purchase price allocation (in thousands):
| Current assets |
$ | 8,097 | ||
| Plant and equipment |
1,534 | |||
| Goodwill |
58,783 | |||
| Developed product technology |
51,000 | |||
| Deferred tax asset |
21,580 | |||
| Trademarks |
1,600 | |||
| Non-compete agreements |
1,650 | |||
|
|
|
|||
| Total assets acquired |
144,244 | |||
| Deferred tax liability |
(22,752 | ) | ||
| Liabilities assumed |
(6,328 | ) | ||
|
|
|
|||
| Total purchase price |
$ | 115,164 | ||
|
|
|
As of August 4, 2008, the Company made a preliminary determination of the amount of BioArray’s operating loss carry-forwards that will be available to reduce the Company’s future taxable income, based on the rules governing allowable carry-forward of losses when changes in ownership occur under Internal Revenue Code Section 382. This amount, which approximates $21.6 million, is recorded as a deferred tax asset. The net operating losses and credit carry-forwards will expire at various times between 2021 and 2028. The Company has acquired intangible assets, not including goodwill, totaling approximately $54.3 million in this transaction. The amortization of these intangibles is not deductible for tax purposes and hence the Company has recorded a deferred tax liability of approximately $22.8 million to offset the future book amortization related to these intangibles. None of the goodwill of approximately $58.8 million resulting from the acquisition of BioArray is deductible for tax purposes.
In fiscal 2011, escrowed funds of $7.2 million were released and returned to the Company. In accordance with the contingent consideration guidance in ASC 805 “Business Combinations,” $2.9 million of the $7.2 million returned to the Company was recorded to Goodwill as it relates to items that were predefined as contingent items in the Merger Agreement and are considered measurement period adjustments. The remaining $4.3 million was recorded to Other Income as it is not considered a measurement period adjustment.
F-14
Table of Contents
Identifiable intangible assets
In performing the purchase price allocation, the Company considered, among other factors, the intended future use of acquired assets, analyses of historical financial performance and estimates of future performance of BioArray’s products. The fair value of intangible assets was based, in part, on a valuation completed using an income approach. The rates used to discount net cash flows to their present values were based on the Company’s weighted average cost of capital and ranged from 13.5% to 15%. These discount rates were determined after consideration of the Company’s rate of return on equity and the weighted average return on invested capital. The following table sets forth the components of intangible assets associated with the acquisition:
| Intangible asset |
Fair value | Useful life | ||||||
| Developed product technology |
$ | 51,000 | 17 yrs. | |||||
| Trademarks |
1,600 | 17 yrs. | ||||||
| Non-compete agreements |
1,650 | 5 yrs. | ||||||
|
|
|
|||||||
| $ | 54,250 | |||||||
|
|
|
|||||||
Developed product technology represents the value assigned to BioArray’s technology platform, the BeadChip system. The BeadChip system is sold as a product platform that has a number of applications. It was not possible to identify income streams associated with the various technologies within the portfolio. As such, the asset was valued as a single portfolio of technologies. The valuation was developed based upon the projected future cash flows and other valuation factors attributable to anticipated future sales of the BeadChip system. Immucor is in the process of developing an automated instrument to further commercialize the BeadChip system. This project was started post-acquisition. While there is some risk that the FDA licensing or CE Mark process for the future automated instrument would not be successful, Immucor did not consider this regulatory risk to be significant enough to classify the technology portfolio that comprises the BeadChip system as in-process research and development.
Trademarks represent the estimated fair value of the BioArray trademark. Non-compete agreements represent the estimated fair value of agreements with BioArray’s former management team members. Useful lives of the developed product technology and trademarks were based on estimated economic useful lives and the non-compete agreements useful lives were based on the terms in the agreements. Intangible assets are being amortized using the straight-line method.
Pro forma financial information
The financial information in the table below summarizes the combined results of operations of Immucor and BioArray, on a pro forma basis, as though the companies had been combined as of the beginning of the period presented. The pro forma financial information is presented for informational purposes only and is not indicative of the results of operations that would have been achieved if the acquisition had taken place at the beginning of the period presented. Such pro forma financial information is based on the historical financial statements of Immucor and BioArray. This pro forma financial information is based on estimates and assumptions, which have been made solely for purposes of developing such pro forma information, including, without limitation, purchase accounting adjustments. The pro forma financial information presented below also includes depreciation and amortization based on the valuation of BioArray’s tangible assets and identifiable intangible assets resulting from the acquisition. The pro forma financial information presented below (in thousands, except per share data) does not reflect any synergies or operating cost reductions that may be achieved from the combined operations.
F-15
Table of Contents
| May 31, 2009 | ||||
| (in thousands) | ||||
| Revenue |
$ | 300,863 | ||
| Net Income |
$ | 73,633 | ||
| Earnings per share—basic |
$ | 1.05 | ||
|
|
|
|||
| Earnings per share—diluted |
$ | 1.03 | ||
|
|
|
|||
IBG Immucor limited
In June 2008, with the intent to sell its products directly in the United Kingdom, the Company acquired all of the outstanding shares of IBG Immucor Limited, a private company registered in, and the Company’s distributor for, the United Kingdom, for an aggregate cash purchase price of $4.4 million.
3. Inventory
Inventories are stated at the lower of cost (first-in, first-out basis) or market (net realizable value):
| May 31, | ||||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
| Raw materials and supplies |
$ | 9,506 | $ | 8,211 | ||||
| Work in process |
4,012 | 4,145 | ||||||
| Finished goods |
19,396 | 23,374 | ||||||
|
|
|
|
|
|||||
| $ | 32,914 | $ | 35,730 | |||||
|
|
|
|
|
|||||
4. Prepaid expenses and other current assets
| May 31, | ||||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
| Accrued refund of BioArray escrowed funds |
7,000 | — | ||||||
| Prepaid expenses |
2,919 | 3,944 | ||||||
| Other accruals |
1,245 | 888 | ||||||
|
|
|
|
|
|||||
| Prepaid expenses and other current assets |
$ | 11,164 | $ | 4,832 | ||||
|
|
|
|
|
|||||
5. Property and equipment
| May 31, | ||||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
| Land |
$ | 67 | $ | 62 | ||||
| Buildings and improvements |
3,073 | 1,702 | ||||||
| Leasehold improvements |
17,757 | 15,167 | ||||||
| Capital work-in-progress |
6,754 | 3,377 | ||||||
| Furniture and fixtures |
3,992 | 3,521 | ||||||
| Instruments at customer sites |
54,403 | 40,832 | ||||||
| Machinery and equipment |
34,826 | 34,164 | ||||||
|
|
|
|
|
|||||
| 120,872 | 98,825 | |||||||
| Less accumulated depreciation |
(62,656 | ) | (49,656 | ) | ||||
|
|
|
|
|
|||||
| Property, plant and equipment—net |
$ | 58,216 | $ | 49,169 | ||||
|
|
|
|
|
|||||
F-16
Table of Contents
Depreciation expense was $13.9 million in fiscal year 2011, $12.3 million in fiscal year 2010, and $9.6 million in fiscal year 2009. Depreciation expense is primarily included in cost of sales in the consolidated statements of income.
6. Goodwill
Changes in the carrying amount of goodwill for the years ended May 31, 2011 and 2010 were as follows:
| May 31, | ||||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
| Balance at beginning of year |
$ | 94,336 | $ | 97,255 | ||||
| Contingent consideration adjustment on the acquisition of BioArray |
(2,935 | ) | — | |||||
| Foreign currency translation adjustment and other |
2,366 | (2,919 | ) | |||||
|
|
|
|
|
|||||
| Balance at end of year |
$ | 93,767 | $ | 94,336 | ||||
|
|
|
|
|
|||||
In fiscal 2011, $7.2 million of escrowed funds related to the acquisition of BioArray were released and returned to the Company. In accordance with the contingent consideration guidance in ASC 805 “Business Combinations,” $2.9 million of the $7.2 million returned to the Company was recorded to Goodwill within the U.S. reporting segment as it relates to items that were predefined as contingent items in the Merger Agreement and are considered measurement period adjustments. The remaining $4.3 million was recorded to Other Income as it is not considered a measurement period adjustment.
Goodwill is tested for impairment at the end of the third quarter of each fiscal year or earlier if a triggering event occurs. Testing for impairment of goodwill confirmed that the carrying value of goodwill was not impaired, and consequently no impairment charges were recorded in the years ended May 31, 2011, 2010 and 2009.
7. Other intangible assets
| May 31, 2011 | May 31, 2010 | |||||||||||||||||||||||||||
| Weighted average life |
Cost | Accumulated amortization |
Net | Cost | Accumulated amortization |
Net | ||||||||||||||||||||||
| (in thousands) | (in thousands) | |||||||||||||||||||||||||||
| Intangible assets subject to amortization: |
||||||||||||||||||||||||||||
| Deferred licensing costs |
5 yrs | $ | 1,013 | $ | (579 | ) | $ | 434 | $ | 1,013 | $ | (502 | ) | $ | 511 | |||||||||||||
| Distribution rights |
10 yrs | 4,492 | (3,359 | ) | 1,133 | 4,492 | (2,863 | ) | 1,629 | |||||||||||||||||||
| Customer lists |
16 yrs | 5,215 | (2,117 | ) | 3,098 | 4,819 | (1,684 | ) | 3,135 | |||||||||||||||||||
| Non-compete agreements |
5 yrs | 1,650 | (935 | ) | 715 | 1,650 | (605 | ) | 1,045 | |||||||||||||||||||
| Developed product technology |
17 yrs | 51,097 | (8,597 | ) | 42,500 | 51,086 | (5,586 | ) | 45,500 | |||||||||||||||||||
| Trademarks / tradenames |
17 yrs | 1,615 | (281 | ) | 1,334 | 1,613 | (186 | ) | 1,427 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total amortizable assets |
65,082 | (15,868 | ) | 49,214 | 64,673 | (11,426 | ) | 53,247 | ||||||||||||||||||||
| Intangible assets not subject to amortization: |
||||||||||||||||||||||||||||
| Deferred licensing costs |
4,919 | — | 4,919 | 4,381 | — | 4,381 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total non-amortizable assets |
4,919 | — | 4,919 | 4,381 | — | 4,381 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total other intangible assets |
$ | 70,001 | $ | (15,868 | ) | $ | 54,133 | $ | 69,054 | $ | (11,426 | ) | $ | 57,628 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
F-17
Table of Contents
Amortization of intangible assets amounted to $4.4 million, $4.3 million and $3.7 million for the years ended May 31, 2011, 2010 and 2009, respectively. The following table presents our estimate of amortization expense for each of the next five fiscal years and thereafter (in thousands):
| Year ending May 31: |
||||
| 2012 |
4,301 | |||
| 2013 |
3,886 | |||
| 2014 |
3,609 | |||
| 2015 |
3,554 | |||
| 2016 |
3,554 | |||
| Thereafter |
30,310 | |||
|
|
|
|||
| $ | 49,214 | |||
|
|
|
|||
8. Accrued expenses and other current liabilities
| May 31, | ||||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
| Sales and other taxes payable |
$ | 4,620 | $ | 4,205 | ||||
| Salaries and wages |
11,912 | 9,524 | ||||||
| Professional fees and dealer commission |
1,095 | 1,080 | ||||||
| Royalties |
253 | 497 | ||||||
| Accruals for pricing discounts to dealers |
189 | 220 | ||||||
| Current portion of deferred leasehold improvement incentive |
1,096 | 914 | ||||||
| Other accruals |
1,166 | 938 | ||||||
|
|
|
|
|
|||||
| Accrued expenses and other current liabilities |
$ | 20,331 | $ | 17,378 | ||||
|
|
|
|
|
|||||
9. Deferred revenue
As described in Note 1, many of the Company’s instrument sales contracts involve multiple deliverables with price guarantees, and revenues from these contracts are deferred and recognized over the term of the agreements which are generally five years. The Company also defers revenue from service contracts over the term of the service agreements.
The additions to and recognition of deferred revenue for the year ended May 31, 2011 and May 31, 2010 were as follows:
| May 31, | ||||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
| Balance at beginning of year |
$ | 16,681 | $ | 22,093 | ||||
| Additions to deferred revenue from new contracts |
11,562 | 11,555 | ||||||
| Revenue recognized during the year |
(15,169 | ) | (16,814 | ) | ||||
| Foreign currency translation adjustment |
501 | (153 | ) | |||||
|
|
|
|
|
|||||
| Balance at end of year |
13,575 | 16,681 | ||||||
| Less: Deferred Revenue—current portion |
(7,495 | ) | (8,994 | ) | ||||
|
|
|
|
|
|||||
| Deferred Revenue—non-current portion |
$ | 6,080 | $ | 7,687 | ||||
|
|
|
|
|
|||||
The Company’s instruments have been acquired more often through rental agreements in the past three years than through sales. Revenue related to rental agreements is recognized over the rental period. These arrangements do not generate deferred revenue.
F-18
Table of Contents
10. Other long term liabilities
| May 31, | ||||||||
| 2011 | 2010 | |||||||
| (in thousands) | ||||||||
| Severance indemnity for employees |
$ | 1,224 | $ | 965 | ||||
| Employment agreement at acquisition |
— | 135 | ||||||
| Deferred leasehold improvement incentive |
1,873 | 1,813 | ||||||
|
|
|
|
|
|||||
| 3,097 | 2,913 | |||||||
| Less current portion |
(1,096 | ) | (914 | ) | ||||
|
|
|
|
|
|||||
| Other long term liabilities |
$ | 2,001 | $ | 1,999 | ||||
|
|
|
|
|
|||||
The Company credits leasehold improvement incentives received from landlords to rent expense over the remaining term of the lease agreement.
11. Common stock
Reserved shares
At May 31, 2011, 2,814,334 shares of common stock were reserved for future issuance upon the vesting of restricted stock or the exercise of stock options that have previously been issued and are still outstanding under the Immucor, Inc. 2005 Long-Term Incentive Plan and previous plans.
Stock repurchased
During fiscal 2011, 2010 and 2009, the Company either withheld from certain option exercises or reacquired from certain restricted shareholders an aggregate of 26,618, 18,921 and 35,088 shares valued at $0.5 million, $0.3 million and $1.0 million, respectively, in compliance with the statutory tax withholding requirements. The Company retired these shares and disclosed their value as ‘Stock repurchases and retirements’ in the consolidated statement of shareholders’ equity and comprehensive income and as ‘Repurchase of common stock’ under financing activities in the consolidated statements of cash flows.
During fiscal 2009, the Company also withheld 18,600 shares valued at $0.5 million from certain option exercises where the option exercise price was paid by having shares withheld. No shares were withheld for this purpose in fiscal 2011 or 2010. These shares were also retired and their values were disclosed as ‘Stock repurchases and retirements’ in the consolidated statement of shareholders’ equity and comprehensive income and as ‘Non-cash investing and financing activities’ in the consolidated statements of cash flows.
The shares acquired were returned to the status of authorized, but unissued shares.
Stock repurchase program
The Company currently has a stock repurchase program approved by the Board of Directors. In August 2009, the Board of Directors authorized the Company to repurchase an additional 2,000,000 shares of the Company’s common stock under this repurchase program, bringing the total authorized shares to 11,375,000.
During the year ended May 31, 2011, the Company did not repurchase any shares of common stock under the repurchase program. During the year ended May 31, 2010 and 2009, the Company spent $11.6 million and $6.7 million to repurchase 650,003 and 295,409 shares of its common stock at an average per share price of $17.78 and $22.52, respectively. Since 1998, the Company has repurchased 9,178,356 shares of its common stock having an aggregate value of approximately $69.2 million. An aggregate of 2,196,644 shares were available for repurchase under the program as of May 31, 2011.
F-19
Table of Contents
12. Share–based compensation
Impact of share-based compensation on income statement
The following table shows total share-based compensation expense before and after the tax effect for the years ended May 31, 2011, 2010 and 2009, included in the consolidated statements of income:
| For the year ended May 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| (in thousands) | ||||||||||||
| Pre-tax compensation expense |
$ | 6,941 | $ | 5,946 | $ | 5,904 | ||||||
| After tax compensation expense |
$ | 4,794 | $ | 4,143 | $ | 4,374 | ||||||
Valuation method used and assumptions
The Company estimates the fair value of stock options using a Black-Scholes valuation model. Key input assumptions used to estimate the fair value of stock options include the expected term until the exercise of the option, the expected volatility of the Company’s stock, risk-free rates of return and dividend yields, if any. The Company estimated the fair value of options at the grant date using the following weighted average assumptions:
| 2011 | 2010 | 2009 | ||||||||||
| Risk-free interest rate(1) |
1.65 | % | 2.15 | % | 3.13 | % | ||||||
| Expected volatility(2) |
43.41 | % | 45.47 | % | 42.41 | % | ||||||
| Expected life (years)(3) |
4.25 | 4.25 | 4.25 | |||||||||
| Expected dividend yield(4) |
— | — | — | |||||||||
| 1. | Based on the U.S. Treasury yield curve in effect at the time of grant. |
| 2. | Expected stock price volatility is based on the average historical volatility of the Company’s shares during the period corresponding to the expected life of the options. |
| 3. | Represents the period of time options are expected to remain outstanding. As the Company has so far only awarded “plain vanilla options” as described by ASC 718-10-S99, “Compensation—Stock Compensation: Overall: SEC Materials,” the Company used the “simplified method” for determining the expected life of the options granted. The simplified method calculates expected term as the sum of the vesting term and the original contractual term divided by two. The Company will continue to use the simplified method until such time that it has sufficient historical data for options with six-year contractual terms to estimate the expected term of share-based awards. |
| 4. | The Company has not paid dividends on its common stock and does not expect to pay dividends on its common stock in the near future. |
Plan summary
At an annual meeting of the Company’s shareholders held on December 13, 2005, the shareholders approved establishment of the Immucor, Inc. 2005 Long-Term Incentive Plan (the “2005 Plan”). The 2005 Plan replaced the Company’s preexisting stock option plans that were frozen and remain in effect only to the extent of awards outstanding under these plans. Under the 2005 Plan, the Company is able to award stock options, stock appreciation rights, restricted stock, deferred stock, and other performance-based awards as incentive and compensation to employees and directors. The Company’s shareholders approved 3,600,000 shares of the Company’s common stock to be used for grants under the 2005 Plan. There is a restriction on the number of shares that may be used for awards other than stock options (1,800,000), and a separate restriction on the number of shares that may be used for grants of incentive stock options (also 1,800,000), but there is no restriction on the number of shares that may be used for grants of non-incentive stock options. Options are granted at the closing market price on the date of the grant. Option awards generally vest equally over a four-year period and have a 6-year contractual term. Restricted stock awards generally vest equally over a five-year period. The 2005 Plan provides for accelerated vesting of option and restricted stock awards if there is a change in control, as defined in the plan.
F-20
Table of Contents
Stock option activity
Compensation costs for stock options with tiered vesting terms are recognized evenly over the vesting periods. Activity for the Company’s option plans was as follows for the years ended May 31, 2011, 2010 and 2009:
| Number of shares |
Weighted average exercise price |
Weighted average remaining contractual life (years) |
Aggregate intrinsic value(1) |
|||||||||||||
| (in thousands) | ||||||||||||||||
| Outstanding at May 31, 2008 |
2,449,941 | $ | 12.03 | 4.9 | $ | 37,551 | ||||||||||
| Granted |
866,995 | $ | 27.08 | |||||||||||||
| Exercised |
(639,274 | ) | $ | 5.79 | ||||||||||||
| Forfeited |
(78,566 | ) | $ | 26.53 | ||||||||||||
| Expired |
(43,000 | ) | $ | 11.51 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at May 31, 2009 |
2,556,096 | $ | 18.26 | 4.4 | $ | 9,948 | ||||||||||
| Granted |
362,531 | $ | 17.00 | |||||||||||||
| Exercised |
(52,350 | ) | $ | 6.41 | ||||||||||||
| Forfeited |
(141,255 | ) | $ | 25.57 | ||||||||||||
| Expired |
(64,925 | ) | $ | 25.10 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at May 31, 2010 |
2,660,097 | $ | 17.77 | 3.7 | $ | 14,611 | ||||||||||
| Granted |
376,842 | $ | 19.10 | |||||||||||||
| Exercised |
(374,687 | ) | $ | 3.50 | ||||||||||||
| Forfeited |
(78,940 | ) | $ | 24.31 | ||||||||||||
| Expired |
(124,146 | ) | $ | 25.24 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at May 31, 2011 |
2,459,166 | $ | 19.55 | 2.9 | $ | 10,076 | ||||||||||
| Exercisable at May 31, 2011 |
1,699,397 | $ | 18.33 | 2.4 | $ | 9,066 | ||||||||||
| 1) | The aggregate intrinsic value in the above table represents the total pre-tax intrinsic value (the difference between the Company’s closing stock price on the last trading day of the respective fiscal year and the exercise price, multiplied by the number of options) of in-the-money options. |
The weighted-average grant-date fair value of share options granted during fiscal years 2011, 2010 and 2009 was $7.14, $6.55 and $10.35, respectively. The total intrinsic value of share options exercised during fiscal years 2011, 2010 and 2009 was $6.0 million, $0.7 million and $14.7 million, respectively.
As of May 31, 2011, there was $3.9 million of total unrecognized compensation cost related to nonvested stock option awards. This compensation cost is expected to be recognized through March 2015, based on existing vesting terms with the weighted average remaining expense recognition period being approximately 2.0 years.
On April 21, 2009, the Compensation Committee approved the acceleration of the vesting of the stock options issued on June 8, 2007 to all employees other than officers of the Company. This action added approximately $0.9 million to the total compensation expense for fiscal 2009.
At May 31, 2011, 2010 and 2009, options for 1,699,397, 1,711,401 and 1,443,647 shares of common stock were exercisable, at weighted average exercise prices of $18.33, $14.47 and $11.65, respectively. In fiscal 2011, 2010 and 2009, all options were granted with an exercise price that agreed to the fair value of the shares at the date of grant.
F-21
Table of Contents
The following table as of May 31, 2011 sets forth by group of exercise price ranges, the number of options outstanding, weighted average exercise prices and weighted average remaining contractual lives of options outstanding, and the number and weighted average exercise prices of options currently exercisable.
| Options outstanding | Options exercisable | |||||||||||||||||||
| Range of exercise |
Number of shares |
Weighted average exercise price |
Weighted average contractual life |
Number of Shares |
Weighted average exercise price |
|||||||||||||||
| $ 0.01-$ 5.00 |
153,686 | $ | 1.90 | 0.9 | 153,686 | $ | 1.90 | |||||||||||||
| 5.01- 10.00 |
330,898 | 6.13 | 2.7 | 330,898 | 6.13 | |||||||||||||||
| 10.01- 20.00 |
792,654 | 17.77 | 3.2 | 402,349 | 17.47 | |||||||||||||||
| 20.01- 25.00 |
228,835 | 20.57 | 4.2 | 169,091 | 20.38 | |||||||||||||||
| 25.01- 30.00 |
841,279 | 27.85 | 2.6 | 574,779 | 28.13 | |||||||||||||||
| 30.01- 35.00 |
111,814 | 31.70 | 2.8 | 68,594 | 31.83 | |||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 2,459,166 | $ | 19.55 | 2.9 | 1,699,397 | $ | 18.33 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||
Restricted stock activity
The following is a summary of the changes in unvested restricted stock for the fiscal years ended May 31, 2011, 2010 and 2009:
| Number of shares |
Weighted- average grant- date fair value |
|||||||
| Nonvested stock outstanding at May 31, 2008 |
124,291 | $ | 21.65 | |||||
| Granted |
57,055 | 26.90 | ||||||
| Vested |
(30,120 | ) | 20.93 | |||||
| Forfeited |
(10,036 | ) | 24.55 | |||||
|
|
|
|||||||
| Nonvested stock outstanding at May 31, 2009 |
141,190 | $ | 23.72 | |||||
| Granted |
251,512 | 16.27 | ||||||
| Vested |
(72,501 | ) | 21.23 | |||||
| Forfeited |
(32,033 | ) | 17.45 | |||||
|
|
|
|||||||
| Nonvested stock outstanding at May 31, 2010 |
288,168 | $ | 18.54 | |||||
| Granted |
211,865 | 19.10 | ||||||
| Vested |
(106,701 | ) | 18.61 | |||||
| Forfeited |
(38,164 | ) | 18.49 | |||||
|
|
|
|||||||
| Nonvested stock outstanding at May 31, 2011 |
355,168 | $ | 18.86 | |||||
The total fair value of restricted shares vested was $2.1 million, $1.5 million and $0.8 million during fiscal years ended May 31, 2011, 2010 and 2009, respectively.
As of May 31, 2011, there was $4.7 million of total unrecognized compensation cost related to nonvested restricted stock awards. This compensation cost is expected to be recognized through June 2015, based on existing vesting terms with the weighted average remaining expense recognition period being approximately 3.4 years.
Shares available for future grants
As of May 31, 2011, a total of 1,095,803 shares were available for future grants.
F-22
Table of Contents
13. Income taxes
Sources of income before income taxes are summarized below (in thousands):
| Year ended May 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Domestic Operations |
$ | 105,060 | $ | 105,181 | $ | 107,843 | ||||||
| Foreign Operations |
25,570 | 20,031 | 9,138 | |||||||||
|
|
|
|
|
|
|
|||||||
| Income before income taxes |
$ | 130,630 | $ | 125,212 | $ | 116,981 | ||||||
|
|
|
|
|
|
|
|||||||
The provision for income taxes is summarized as follows (in thousands):
| Year ended May 31, | ||||||||||||
| 2011 | 2010(1) | 2009 | ||||||||||
| Current: |
||||||||||||
| Federal |
$ | 26,542 | $ | 28,815 | $ | 33,347 | ||||||
| Foreign |
7,718 | 6,080 | 4,606 | |||||||||
| State |
6,102 | 2,796 | 2,851 | |||||||||
|
|
|
|
|
|
|
|||||||
| 40,362 | 37,691 | 40,804 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Deferred: |
||||||||||||
| Federal |
3,551 | 4,134 | 91 | |||||||||
| Foreign |
263 | 279 | (626 | ) | ||||||||
| State |
(2,873 | ) | 525 | 529 | ||||||||
|
|
|
|
|
|
|
|||||||
| 941 | 4,938 | (6 | ) | |||||||||
|
|
|
|
|
|
|
|||||||
| Provision for income taxes |
$ | 41,303 | $ | 42,629 | $ | 40,798 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Certain prior year expenses have been reclassified to conform to current year presentation. |
Deferred income taxes reflect the net tax effects of: (a) temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and income tax purposes; and (b) operating loss and credit carry-forwards. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. Based on assessments of all available evidence including, but not limited to, the operating history and lack of profitability of certain subsidiaries, the Company is uncertain as to the ability to realize the subsidiaries’ net operating loss carry-forwards and tax benefits and, as a result, deferred tax valuation allowances have been recorded against these deferred tax assets in the following jurisdictions: Belgium, $1.4 million and France, $1.1 million. The net operating loss carry-forwards for France and Belgium do not expire; the federal operating loss carry-forwards for BioArray of $24.3 begin to expire in 2026; certain state operating loss carry-forwards for BioArray expire beginning in 2013; and credit carry-forwards of $2.2 million for BioArray expire beginning in 2021.
Due to a recent law change in New Jersey income tax apportionment factors, the Company experienced a positive adjustment of approximately $2.3 million to our deferred tax liability that was established on the acquisition of BioArray. Additionally, in fiscal 2011, the Company wrote off approximately $3.9 of net operating loss carry-forwards which were fully reserved in previous years. For the year ended May 31, 2011, the Company has no valuation allowances recorded against state deferred tax assets since all states with net operating losses carried forward are in a net deferred tax liability position, and the Company has scheduled the reversal of deferred tax liabilities to occur before the potential expiration of state net operating loss carry-forwards that exist as of May 31, 2011.
F-23
Table of Contents
The tax effects of significant items comprising the Company’s net deferred tax assets at May 31, 2011 and 2010 are as follows (in thousands):
| Year ended May 31, | ||||||||
| 2011 | 2010 | |||||||
| Deferred tax liabilities: |
||||||||
| Intangibles |
$ | (19,282 | ) | $ | (23,007 | ) | ||
| Property, Plant & Equipment |
(4,931 | ) | (2,075 | ) | ||||
| Prepaids and other |
(346 | ) | (457 | ) | ||||
| Deferred tax assets: |
||||||||
| Reserves not currently deductible |
4,376 | 4,191 | ||||||
| Deferred revenue |
2,923 | 4,027 | ||||||
| Compensation expense |
5,617 | 4,190 | ||||||
| Operating loss carry-forwards Credit carry-forwards |
|
11,580 2,736 |
|
|
19,291 2,770 |
| ||
| Other |
6,187 | 5,081 | ||||||
| Uniform capitalization |
603 | 684 | ||||||
|
|
|
|
|
|||||
| 9,463 | 14,695 | |||||||
| Valuation allowance |
(2,467 | ) | (6,716 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | 6,996 | $ | 7,979 | ||||
|
|
|
|
|
|||||
Deferred taxes are not provided for temporary differences of approximately $59.9 million, $43.3 million and $31.3 million as of May 31, 2011, 2010 and 2009, respectively, representing cumulative earnings of non-U.S. subsidiaries that are intended to be permanently reinvested. Computation of the potential deferred tax liability associated with these undistributed earnings is not practicable.
The Company’s effective tax rate differs from the federal statutory rate as follows:
| Year ended May 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Federal statutory tax rate |
35 | % | 35 | % | 35 | % | ||||||
| State income taxes, net of federal tax benefit |
— | 3 | 3 | |||||||||
| Production activity deduction |
(2 | ) | (2 | ) | (2 | ) | ||||||
| Change in analysis of uncertain income tax positions |
— | (1 | ) | — | ||||||||
| Other |
(1 | ) | (1 | ) | (1 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Effective tax rate |
32 | % | 34 | % | 35 | % | ||||||
|
|
|
|
|
|
|
|||||||
The following is a tabular reconciliation of the total amounts of unrecognized tax benefits, which is included in income taxes payable on the balance sheet, for the years ended May 31, 2011 and 2010 in (thousands):
| Unrecognized tax benefit—June 1, 2010 |
$ | 8,093 | ||
| Gross increases in unrecognized tax benefits as a result of tax positions taken during a prior period |
— | |||
| Gross decreases in unrecognized tax benefits as a result of tax positions taken during a prior period |
— | |||
| Gross increases in unrecognized tax benefits as a result of tax positions taken during current period |
2,107 | |||
| Gross decreases in unrecognized tax benefits as a result of tax positions taken during current period |
— | |||
| Decrease in unrecognized tax benefits relating to settlements with taxing authorities |
— | |||
| Reductions to unrecognized tax benefits as a result of the applicable statute of limitations |
(434 | ) | ||
| Unrecognized tax benefit—May 31, 2011 |
$ | 9,766 |
F-24
Table of Contents
The total balance of unrecognized tax benefits that would affect the effective tax rate, if recognized, is $3.6 million as of May 31, 2011. The unrecognized tax benefit is reflected as a current liability as it is likely to require payment during the twelve-month period ending May 31, 2012.
The Company recognizes interest and penalties accrued related to unrecognized tax benefits in income tax expense. The Company as of May 31, 2011, has recognized a liability for interest of $0.9 million. The Company has not recognized any accrued penalties since adoption of the update to ASC 740, “Income Taxes.”
The Company is subject to taxation in the U.S. and various states and foreign jurisdictions. The Company’s tax years for the fiscal years ended May 31, 2010, May 31, 2009 and May 31, 2008 remain subject to examination by U.S. tax authorities.
14. Earnings per share
The following table sets forth the computation of basic and diluted earnings per share in accordance with ASC 260, “Earnings per Share” (“ASC 260”). Basic earnings per common share are calculated by dividing net income by weighted-average common shares outstanding during the period. Diluted earnings per common share are calculated by dividing net income by weighted-average common shares plus dilutive potential common shares outstanding during the period. The following table details the calculation of basic and diluted earnings per share:
| Year ended May 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| (Shares and $ in thousands, except per share data) |
||||||||||||
| Numerator for basic and diluted earnings per share: |
||||||||||||
| Net Income |
$ | 89,327 | $ | 82,583 | $ | 76,183 | ||||||
|
|
|
|
|
|
|
|||||||
| Denominator: |
||||||||||||
| For basic earnings per share—weighted average shares basis |
70,073 | 70,062 | 70,382 | |||||||||
| Effect of dilutive stock options and restricted stock |
545 | 584 | 786 | |||||||||
|
|
|
|
|
|
|
|||||||
| Denominator for diluted earnings per share -adjusted weighted average shares basis |
70,618 | 70,646 | 71,168 | |||||||||
|
|
|
|
|
|
|
|||||||
| Earnings per common share—basic |
$ | 1.27 | $ | 1.18 | $ | 1.08 | ||||||
|
|
|
|
|
|
|
|||||||
| Earnings per common share—diluted |
$ | 1.26 | $ | 1.17 | $ | 1.07 | ||||||
|
|
|
|
|
|
|
|||||||
The effect of 1,672,617, 1,652,275 and 1,135,711 out-of-the-money options was excluded from the above calculation as inclusion of these securities would be anti-dilutive for the years ended May 31, 2011, 2010 and 2009, respectively.
On June 9, 2011, the Company, under an annual group award, issued 228,890 restricted stock units and 162,535 performance based restricted stock units. These grants are excluded in calculating the above diluted earnings per share as they were issued subsequent to year end but may have a dilutive effect on future earnings per share calculations.
15. Segment and geographic information
The Company’s operations and segments are organized around geographic areas. The foreign locations principally function as distributors of products primarily developed and manufactured by the Company in the United States and Canada. Effective August 4, 2008, U.S. operations also includes the operations of BioArray, which was acquired during the first quarter of fiscal 2009. The accounting policies applied in the preparation of the Company’s consolidated financial statements are applied consistently across all segments. Intersegment sales are recorded at market price and are eliminated in consolidation. The Other segment aggregates Canada and Japan, which were previously reported separately.
F-25
Table of Contents
Net sales by product group and segment information for the fiscal years ended May 31, 2011, 2010 and 2009 are summarized below (in thousands).
| Year Ended May 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Sales by product group: |
||||||||||||
| Traditional reagents |
$ | 199,826 | $ | 207,710 | $ | 199,277 | ||||||
| Capture reagents |
82,366 | 77,003 | 64,145 | |||||||||
| Instruments |
45,112 | 39,680 | 34,672 | |||||||||
| Molecular immunohematology |
5,787 | 4,680 | 2,453 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net sales |
$ | 333,091 | $ | 329,073 | $ | 300,547 | ||||||
|
|
|
|
|
|
|
|||||||
| For the Year Ended May 31, 2011 | ||||||||||||||||||||
| U.S. | Europe | Other | Elims | Consolidated | ||||||||||||||||
| Sales: |
||||||||||||||||||||
| Unaffiliated customers |
$ | 232,965 | $ | 68,569 | $ | 31,557 | $ | — | $ | 333,091 | ||||||||||
| Affiliates |
15,950 | 16,300 | 323 | (32,573 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net Sales |
$ | 248,915 | $ | 84,869 | $ | 31,880 | $ | (32,573 | ) | $ | 333,091 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income from operations |
100,774 | 13,397 | 11,819 | 7 | 125,997 | |||||||||||||||
| Depreciation |
9,093 | 4,258 | 529 | — | 13,880 | |||||||||||||||
| Amortization |
4,084 | 160 | 89 | — | 4,333 | |||||||||||||||
| Income tax expense |
33,322 | 4,171 | 3,802 | 8 | 41,303 | |||||||||||||||
| Interest income |
279 | 234 | 193 | — | 706 | |||||||||||||||
| Capital expenditures |
6,717 | 407 | 1,937 | — | 9,061 | |||||||||||||||
| Property & equipment, net |
39,957 | 14,318 | 3,941 | — | 58,216 | |||||||||||||||
| Goodwill |
70,946 | 7,214 | 15,607 | — | 93,767 | |||||||||||||||
| Total assets at period end |
677,861 | 92,866 | 64,992 | (202,592 | ) | 633,127 | ||||||||||||||
| For the Year Ended May 31, 2010 | ||||||||||||||||||||
| U.S. | Europe | Other | Elims | Consolidated | ||||||||||||||||
| Sales: |
||||||||||||||||||||
| Unaffiliated customers |
$ | 236,255 | $ | 65,096 | $ | 27,722 | $ | — | $ | 329,073 | ||||||||||
| Affiliates |
17,291 | 14,508 | 280 | (32,079 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net Sales |
$ | 253,546 | $ | 79,604 | $ | 28,002 | $ | (32,079 | ) | $ | 329,073 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income from operations |
105,447 | 9,698 | 9,782 | 415 | 125,342 | |||||||||||||||
| Depreciation |
7,879 | 3,956 | 456 | — | 12,291 | |||||||||||||||
| Amortization |
4,034 | 162 | 82 | — | 4,278 | |||||||||||||||
| Income tax expense |
36,271 | 3,214 | 2,879 | 265 | 42,629 | |||||||||||||||
| Interest income |
221 | 177 | 56 | — | 454 | |||||||||||||||
| Capital expenditures |
5,753 | 361 | 190 | — | 6,304 | |||||||||||||||
| Property & equipment, net |
36,334 | 10,945 | 1,890 | — | 49,169 | |||||||||||||||
| Goodwill |
73,881 | 6,235 | 14,220 | — | 94,336 | |||||||||||||||
| Total assets at period end |
594,005 | 69,151 | 51,337 | (194,659 | ) | 519,834 | ||||||||||||||
F-26
Table of Contents
| For the Year Ended May 31, 2009 | ||||||||||||||||||||
| U.S. | Europe | Other | Elims | Consolidated | ||||||||||||||||
| Sales: |
||||||||||||||||||||
| Unaffiliated customers |
$ | 220,076 | $ | 56,802 | $ | 23,669 | $ | — | $ | 300,547 | ||||||||||
| Affiliates |
17,707 | 14,023 | 371 | (32,101 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net Sales |
$ | 237,783 | $ | 70,825 | $ | 24,040 | $ | (32,101 | ) | $ | 300,547 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) from operations |
108,691 | 3,292 | 5,434 | (459 | ) | 116,958 | ||||||||||||||
| Depreciation |
5,623 | 3,537 | 407 | — | 9,567 | |||||||||||||||
| Amortization |
3,386 | 277 | 76 | — | 3,739 | |||||||||||||||
| Income tax (benefit) expense |
36,818 | 2,130 | 1,997 | (147 | ) | 40,798 | ||||||||||||||
| Interest income |
921 | 833 | 203 | — | 1,957 | |||||||||||||||
| Capital expenditures |
3,253 | 4,701 | 648 | — | 8,602 | |||||||||||||||
| Property & equipment, net |
30,625 | 10,855 | 1,981 | — | 43,461 | |||||||||||||||
| Goodwill |
76,586 | 7,089 | 13,580 | — | 97,255 | |||||||||||||||
| Total assets at period end |
524,397 | 70,979 | 42,607 | (186,643 | ) | 451,340 | ||||||||||||||
The company had net export sales to unaffiliated customers (in thousands):
| For the Year Ended May 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| (in thousands) | ||||||||||||
| United States |
$ | 6,100 | $ | 5,378 | $ | 5,035 | ||||||
| Europe |
6,609 | 5,947 | 5,657 | |||||||||
| Other |
2,269 | 2,073 | 1,794 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total net export sales |
$ | 14,978 | $ | 13,398 | $ | 12,486 | ||||||
|
|
|
|
|
|
|
|||||||
16. Retirement plan
The Company maintains a 401(k) retirement plan covering its U.S. employees who meet the plan requirements. The Company matches a portion of employee contributions, which vest immediately. During the years ended May 31, 2011, 2010 and 2009, the Company’s matching contributions to the plan were approximately $1.0 million, $0.9 million and $0.8 million, respectively.
The Company’s Canadian affiliate maintains a defined contribution pension plan covering all Canadian employees, except temporary employees. The Company matches a portion of employee contributions to the plan, and each employee vests in the Company’s matching contributions once they have been a participant continuously for two years. During each of the years ended May 31, 2011, 2010 and 2009, the Company’s matching contributions to the plan remained constant at approximately $0.1 million.
F-27
Table of Contents
17. Commitments and contingencies
Lease Commitments
In the United States, the Company leases office, warehousing and manufacturing facilities under several operating lease agreements expiring at various dates through fiscal 2017 with a right to renew for an additional term in the case of most of the leases. Certain of these leases contain escalation clauses. Outside the United States, the Company leases foreign office and warehouse facilities under operating lease agreements expiring at various dates through fiscal 2019. The total leasing expense for the Company was $4.3 million in fiscal 2011, $3.8 million in fiscal 2010 and $3.7 million in fiscal 2009.
The following is a schedule of approximate future annual lease payments under all operating leases that have initial or remaining non-cancelable lease terms as of May 31, 2011 (in thousands):
| Year ending May 31: |
||||
| 2012 |
$ | 2,905 | ||
| 2013 |
$ | 2,623 | ||
| 2014 |
$ | 2,263 | ||
| 2015 |
$ | 2,095 | ||
| 2016 |
$ | 2,010 | ||
| Thereafter |
$ | 318 | ||
|
|
|
|||
| $ | 12,214 | |||
|
|
|
|||
Purchase commitments
Purchase commitments made in the normal course of business was $15.1 million as of May 31, 2011.
Contingencies
In October 2007, we reported that the Federal Trade Commission (“FTC”) was investigating whether Immucor violated federal antitrust laws or engaged in unfair methods of competition through three acquisitions made in the period from 1996 through 1999, and whether Immucor or others engaged in unfair methods of competition by restricting price competition. The FTC letter requested that we provide certain documents and information to the FTC concerning those acquisitions and concerning our product pricing activities since then. In July 2008, the FTC formalized its document and information requests into a Civil Investigative Demand (“CID”) and also required us to provide certain additional information within the same general scope of its previous requests. The FTC has also required that we provide to them the documents we provided to the Department of Justice (see below). We have been cooperating with the FTC and we intend to continue cooperating, and we are assured that the issuance of a formal CID does not indicate any dissatisfaction with our cooperation. As was previously the case, at this time we cannot reasonably assess the timing or outcome of the investigation or its effect, if any, on our business.
In April 2009, we received a subpoena from the United States Department of Justice, Antitrust Division (“DOJ”), requiring us to produce documents for the period beginning September 1, 2000 through April 23, 2009, pertaining to an investigation of possible violations of the federal criminal antitrust laws in the blood reagents industry. On November 8, 2010, we announced that the DOJ had informed us that it had closed its investigation. The investigation was closed with no further action taken on the part of the DOJ.
Beginning in May 2009, a series of class action lawsuits has been filed against the Company, Ortho-Clinical Diagnostics, Inc. and Johnson & Johnson Health Care Systems, Inc. alleging that the defendants conspired to fix prices at which blood reagents are sold, asserting claims under Section 1 of the Sherman Act, and seeking declaratory and injunctive relief, treble damages, costs, and attorneys’ fees. All of these complaints make
F-28
Table of Contents
substantially the same allegations. The cases have been consolidated in the United States District Court for the Eastern District of Pennsylvania. In August 2010 the United States District Court for the Eastern District of Pennsylvania denied, in part, Motions to Dismiss for failure to state a cause of action and a Motion to Stay Discovery filed by the Company and co-defendant Ortho-Clinical Diagnostics, Inc. The defendants filed a Motion for Reconsideration or for Certification for Interlocutory Appeal with respect to the Court’s order on defendants’ motion to dismiss, and that motion was denied in December 2010. Discovery has now commenced in this litigation. No determination has been made whether any of the plaintiffs’ claims have merit or should be allowed to proceed as a class action. We intend to vigorously defend against these cases. At this time we cannot reasonably assess the timing or outcome of this litigation or its effect, if any, on our business.
Private securities litigation in the United States District Court of North Georgia against the Company and certain of its current and former directors and officers asserts federal securities fraud claims on behalf of a putative class of purchasers of the Company’s Common Stock between October 19, 2005 and June 25, 2009. The case alleges that the defendants violated Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended, by failing to disclose that Immucor had violated the antitrust laws, and challenges the sufficiency of the Company’s disclosures about the results of FDA inspections and the Company’s quality control efforts. On June 30, 2011, the District Court granted the defendants’ motion to dismiss the complaint and closed the case. Plaintiff could challenge that result, but has not done so as of July 20, 2011. The Company will defend the case vigorously if it is reinstated. At this time, the Company cannot reasonably assess the timing or outcome of this litigation or its effect, if any, on its business.
On July 12, 2011, a purported shareholder of the Company filed a putative class action lawsuit in the Superior Court of Fulton County, Georgia, captioned as Hillary Kramer v. Immucor, Inc., et al., Civil Action No. 2011CV203124. The action was brought on behalf of public shareholders of the Company and names as defendants Immucor, the individual directors of the Company, and TPG Capital and certain of its affiliates. The action asserts claims for breaches of fiduciary duties against the Company’s board of directors in connection with the proposed previously-announced transaction with TPG, and for aiding and abetting the purported breaches of fiduciary duties by the TPG defendants. The plaintiff seeks, among other things, preliminary and permanent relief, including injunctive relief enjoining the consummation of the proposed transaction, rescission of the proposed transaction to the extent it is consummated prior to the entry of a final judgment, and costs, expenses and disbursements of the action. At this time, the Company cannot reasonably assess the timing or outcome of this litigation or its effect, if any, on its business.
On July 15, 2011, a second putative class action challenging the proposed transaction was filed by a purported shareholder of Immucor. This action was filed in the Superior Court of Gwinnett County for the State of Georgia, and is captioned as Babette C. Schorsch v. Immucor, Inc., et al., Civil Action No. 11A0776-1. The action is brought on behalf of public shareholders of Immucor and names as defendants Immucor, the individual directors of Immucor, two Immucor executive officers, and TPG Capital and its affiliates. The action asserts claims for breaches of fiduciary duties against the Immucor board of directors in connection with the proposed transaction, and for aiding and abetting the purported breaches of fiduciary duties by the TPG defendants. The plaintiff seeks, among other things, injunctive relief enjoining the consummation of the proposed transaction, rescission of the proposed transaction to the extent it is consummated prior to the entry of a final judgment, and costs, expenses and disbursements of the action. At this time, Immucor cannot reasonably assess the timing or outcome of this litigation or its effect, if any, on its business.
On July 18, 2011, a third putative class action challenging the proposed transaction was filed by a purported shareholder of Immucor. This is the second case filed in the Superior Court of Fulton County for the State of Georgia, and is captioned as Allan Pillay v. Immucor, Inc., et al., Civil Action No. 2011CV203339. The action is brought on behalf of public shareholders of Immucor and names as defendants Immucor, the individual directors of Immucor, and TPG Capital and its affiliates. The action asserts claims for breaches of fiduciary duties against the Immucor board of directors in connection with the proposed transaction, including that the Immucor’s Schedule 14D-9 is misleading or incomplete, and a claim for aiding and abetting the purported breaches of
F-29
Table of Contents
fiduciary duties by the TPG defendants. The plaintiff seeks, among other things, a declaration that the action is maintainable as a class action, preliminary and permanent relief, including injunctive relief enjoining the consummation of the proposed transaction and rescission of the proposed transaction to the extent it is consummated prior to the entry of a final judgment, and costs, expenses and disbursements of the action. At this time Immucor cannot reasonably assess the timing or outcome of this litigation or its effect, if any, on its business.
Other than as set forth above, we are not currently subject to any material legal proceedings, nor, to our knowledge, is any material legal proceeding threatened against us. However, from time to time, we may become a party to certain legal proceedings in the ordinary course of business.
18. Selected quarterly financial data (unaudited)
| (In thousands, except per share amounts) | ||||||||||||||||||||||||
| Fiscal year ended |
Net sales | Gross profit |
Income from operations |
Net income |
Earnings per common share |
Earnings per common share— assuming dilution |
||||||||||||||||||
| May 31, 2011 |
||||||||||||||||||||||||
| First Quarter |
$ | 83,641 | $ | 59,667 | $ | 32,202 | $ | 21,419 | $ | 0.31 | $ | 0.30 | ||||||||||||
| Second Quarter |
81,546 | 58,385 | 31,957 | 21,063 | $ | 0.30 | $ | 0.30 | ||||||||||||||||
| Third Quarter |
83,349 | 59,019 | 33,867 | 22,686 | $ | 0.32 | $ | 0.32 | ||||||||||||||||
| Fourth Quarter |
84,555 | 59,845 | 27,971 | 24,159 | $ | 0.34 | $ | 0.34 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| $ | 333,091 | $ | 236,916 | $ | 125,997 | $ | 89,327 | $ | 1.27 | $ | 1.26 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| May 31, 2010 |
||||||||||||||||||||||||
| First Quarter |
$ | 83,071 | $ | 59,689 | $ | 33,342 | $ | 21,333 | $ | 0.30 | $ | 0.30 | ||||||||||||
| Second Quarter |
82,570 | 58,139 | 30,662 | 19,702 | $ | 0.28 | $ | 0.28 | ||||||||||||||||
| Third Quarter |
80,499 | 55,714 | 30,874 | 20,061 | $ | 0.29 | $ | 0.28 | ||||||||||||||||
| Fourth Quarter |
82,933 | 60,182 | 30,464 | 21,487 | $ | 0.31 | $ | 0.30 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| $ | 329,073 | $ | 233,724 | $ | 125,342 | $ | 82,583 | $ | 1.18 | $ | 1.17 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
The sum of the quarterly amounts of diluted earnings per common share for the fiscal year ended May 31, 2010 is different than the total due to rounding.
F-30
Table of Contents
19. Condensed consolidating financial information of guarantor subsidiaries
As part of the acquisition that occurred subsequent to year-end, as more fully described in Note 20, the Company incurred approximately $1 billion of debt that consists of the following: i) $615 million senior secured term loan facility, ii) $100 million senior secured revolving loan facility and iii) $400 million of high yield bonds.
As a result, the Company has outstanding certain indebtedness that is guaranteed by all of its U.S. subsidiaries and is now required to present the following information for all historical periods. The indebtedness is not guaranteed by the Company’s foreign subsidiaries. The guarantor subsidiaries are all wholly owned and the guarantees are made on a joint and several basis and are full and unconditional. Separate consolidated financial statements of the guarantor subsidiaries have not been presented because management believes that such information would not be material to investors. However, condensed consolidating financial information is presented. The condensed consolidating financial information of the Company is as follows:
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING BALANCE SHEETS
May 31, 2011
(Amounts in thousands)
| Immucor, Inc. |
Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| ASSETS |
||||||||||||||||||||
| CURRENT ASSETS: |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 250,386 | $ | — | $ | 52,297 | $ | (80 | ) | $ | 302,603 | |||||||||
| Accounts receivable, net |
25,914 | 377 | 37,033 | — | 63,324 | |||||||||||||||
| Intercompany receivable |
310 | — | 5,925 | (6,235 | ) | — | ||||||||||||||
| Inventories |
20,756 | 1,253 | 10,905 | — | 32,914 | |||||||||||||||
| Deferred income tax assets, current portion |
11,118 | 4,466 | 300 | — | 15,884 | |||||||||||||||
| Prepaid expenses and other current assets |
8,723 | 24,895 | 2,100 | (24,554 | ) | 11,164 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current assets |
317,207 | 30,991 | 108,560 | (30,869 | ) | 425,889 | ||||||||||||||
| PROPERTY AND EQUIPMENT, Net |
39,101 | 856 | 18,259 | — | 58,216 | |||||||||||||||
| INVESTMENT IN SUBSIDIARIES |
247,571 | — | 5 | (247,576 | ) | — | ||||||||||||||
| GOODWILL |
17,803 | 53,143 | 22,821 | — | 93,767 | |||||||||||||||
| INTANGIBLE ASSETS, Net |
1,050 | 45,695 | 7,388 | — | 54,133 | |||||||||||||||
| DEFERRED INCOME TAX ASSETS |
1,691 | — | 178 | (1,869 | ) | — | ||||||||||||||
| DEFERRED FINANCING COSTS |
— | — | — | — | — | |||||||||||||||
| OTHER ASSETS |
— | 99 | 647 | 376 | 1,122 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total assets |
$ | 624,423 | $ | 130,784 | $ | 157,858 | $ | (279,938 | ) | $ | 633,127 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY |
||||||||||||||||||||
| CURRENT LIABILITIES: |
||||||||||||||||||||
| Accounts payable |
6,324 | 1,463 | 3,083 | (80 | ) | 10,790 | ||||||||||||||
| Intercompany payable |
630 | 25,227 | 11,829 | (37,686 | ) | — | ||||||||||||||
| Accrued expenses and other current liabilities |
9,824 | 1,568 | 8,939 | — | 20,331 | |||||||||||||||
| Income taxes payable |
29,035 | — | 3,813 | (24,554 | ) | 8,294 | ||||||||||||||
| Deferred revenue, current portion |
4,778 | 31 | 2,686 | — | 7,495 | |||||||||||||||
| Current portion of long term debt |
— | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current liabilities |
50,591 | 28,289 | 30,350 | (62,320 | ) | 46,910 | ||||||||||||||
| LONG TERM DEBT |
— | — | — | — | — | |||||||||||||||
| DEFERRED REVENUE |
4,183 | 22 | 1,875 | — | 6,080 | |||||||||||||||
| DEFERRED INCOME TAX LIABILITIES |
— | 9,973 | 784 | (1,493 | ) | 9,264 | ||||||||||||||
| OTHER LONG-TERM LIABILITIES |
777 | — | 1,224 | — | 2,001 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities |
55,551 | 38,284 | 34,233 | (63,813 | ) | 64,255 | ||||||||||||||
| SHAREHOLDERS’ EQUITY: |
||||||||||||||||||||
| Total shareholders’ equity |
568,872 | 92,500 | 123,625 | (216,125 | ) | 568,872 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities and shareholders’ equity |
$ | 624,423 | $ | 130,784 | $ | 157,858 | $ | (279,938 | ) | $ | 633,127 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-31
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING BALANCE SHEETS
May 31, 2010
(Amounts in thousands)
| Immucor, Inc. |
Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| ASSETS |
||||||||||||||||||||
| CURRENT ASSETS: |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 171,144 | $ | — | $ | 31,783 | $ | (278 | ) | $ | 202,649 | |||||||||
| Accounts receivable, net |
29,684 | 376 | 29,518 | — | 59,578 | |||||||||||||||
| Intercompany receivable |
170 | 198 | 4,363 | (4,731 | ) | — | ||||||||||||||
| Inventories |
23,009 | 974 | 11,747 | — | 35,730 | |||||||||||||||
| Deferred income tax assets, current portion |
9,973 | 4,416 | 418 | — | 14,807 | |||||||||||||||
| Prepaid expenses and other current assets |
2,880 | 16,039 | 1,767 | (15,854 | ) | 4,832 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current assets |
236,860 | 22,003 | 79,596 | (20,863 | ) | 317,596 | ||||||||||||||
| PROPERTY AND EQUIPMENT, Net |
35,506 | 828 | 12,835 | — | 49,169 | |||||||||||||||
| INVESTMENT IN SUBSIDIARIES |
212,585 | — | 4 | (212,589 | ) | — | ||||||||||||||
| GOODWILL |
17,803 | 56,078 | 20,455 | — | 94,336 | |||||||||||||||
| INTANGIBLE ASSETS, Net |
1,589 | 49,239 | 6,800 | — | 57,628 | |||||||||||||||
| DEFERRED INCOME TAX ASSETS |
3,642 | — | 333 | (3,435 | ) | 540 | ||||||||||||||
| DEFERRED FINANCING COSTS |
— | — | — | — | — | |||||||||||||||
| OTHER ASSETS |
— | 99 | 466 | — | 565 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total assets |
$ | 507,985 | $ | 128,247 | $ | 120,489 | $ | (236,887 | ) | $ | 519,834 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY |
||||||||||||||||||||
| CURRENT LIABILITIES: |
||||||||||||||||||||
| Accounts payable |
4,357 | 1,327 | 2,567 | (278 | ) | 7,973 | ||||||||||||||
| Intercompany payable |
31 | 18,472 | 10,684 | (29,187 | ) | — | ||||||||||||||
| Accrued expenses and other current liabilities |
7,695 | 2,395 | 7,288 | — | 17,378 | |||||||||||||||
| Income taxes payable |
26,096 | — | 2,070 | (15,854 | ) | 12,312 | ||||||||||||||
| Deferred revenue, current portion |
6,465 | 21 | 2,508 | — | 8,994 | |||||||||||||||
| Current portion of long term debt |
— | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current liabilities |
44,644 | 22,215 | 25,117 | (45,319 | ) | 46,657 | ||||||||||||||
| LONG TERM DEBT |
— | — | — | — | — | |||||||||||||||
| DEFERRED REVENUE |
6,319 | 40 | 1,328 | — | 7,687 | |||||||||||||||
| DEFERRED INCOME TAX LIABILITIES |
— | 10,000 | 803 | (3,435 | ) | 7,368 | ||||||||||||||
| OTHER LONG-TERM LIABILITIES |
899 | — | 1,100 | — | 1,999 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities |
51,862 | 32,255 | 28,348 | (48,754 | ) | 63,711 | ||||||||||||||
| SHAREHOLDERS’ EQUITY: |
||||||||||||||||||||
| Total shareholders’ equity |
456,123 | 95,992 | 92,141 | (188,133 | ) | 456,123 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities and shareholders’ equity |
$ | 507,985 | $ | 128,247 | $ | 120,489 | $ | (236,887 | ) | $ | 519,834 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-32
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING STATEMENTS OF OPERATIONS
For the Year Ended May 31, 2011
(Amounts in thousands)
| Immucor, Inc. |
Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| NET SALES |
$ | 244,339 | $ | 4,577 | $ | 116,748 | $ | (32,573 | ) | $ | 333,091 | |||||||||
| COST OF SALES (exclusive of amortization shown separately below) |
67,726 | 3,549 | 57,473 | (32,573 | ) | 96,175 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| GROSS MARGIN |
176,613 | 1,028 | 59,275 | — | 236,916 | |||||||||||||||
| OPERATING EXPENSES: |
||||||||||||||||||||
| Research and development |
6,815 | 8,925 | 160 | — | 15,900 | |||||||||||||||
| Selling and marketing |
17,265 | 1,919 | 17,247 | — | 36,431 | |||||||||||||||
| Distribution |
9,932 | 130 | 6,446 | — | 16,508 | |||||||||||||||
| General and administrative |
24,899 | 2,892 | 9,956 | — | 37,747 | |||||||||||||||
| Amortization of intangibles |
539 | 3,544 | 250 | — | 4,333 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
59,450 | 17,410 | 34,059 | — | 110,919 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| INCOME (LOSS) FROM OPERATIONS |
117,163 | (16,382 | ) | 25,216 | — | 125,997 | ||||||||||||||
| NON-OPERATING INCOME (EXPENSE): |
||||||||||||||||||||
| Interest income |
279 | — | 527 | (100 | ) | 706 | ||||||||||||||
| Interest expense |
(56 | ) | — | (114 | ) | 100 | (70 | ) | ||||||||||||
| Other, net |
(103 | ) | 4,168 | (68 | ) | — | 3,997 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total non-operating income |
120 | 4,168 | 345 | — | 4,633 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| INCOME (LOSS) BEFORE INCOME TAXES |
117,283 | (12,214 | ) | 25,561 | — | 130,630 | ||||||||||||||
| PROVISION (BENEFIT) FOR INCOME TAXES |
42,051 | (8,721 | ) | 7,973 | — | 41,303 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| NET INCOME (LOSS) BEFORE EARNINGS OF CONSOLIDATED SUBSIDIARIES |
75,232 | (3,493 | ) | 17,588 | — | 89,327 | ||||||||||||||
| Net Income (Loss) of consolidated subsidiaries |
14,095 | — | — | (14,095 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| NET INCOME (LOSS) |
$ | 89,327 | $ | (3,493 | ) | $ | 17,588 | $ | (14,095 | ) | $ | 89,327 | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-33
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING STATEMENTS OF OPERATIONS
For the Year Ended May 31, 2010
(Amounts in thousands)
| Immucor, Inc. |
Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| NET SALES |
$ | 249,162 | $ | 4,385 | $ | 107,605 | $ | (32,079 | ) | $ | 329,073 | |||||||||
| COST OF SALES (exclusive of amortization shown separately below) |
70,433 | 4,115 | 52,880 | (32,079 | ) | 95,349 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| GROSS MARGIN |
178,729 | 270 | 54,725 | — | 233,724 | |||||||||||||||
| OPERATING EXPENSES: |
||||||||||||||||||||
| Research and development |
6,160 | 9,114 | 163 | — | 15,437 | |||||||||||||||
| Selling and marketing |
17,716 | 1,674 | 17,605 | — | 36,995 | |||||||||||||||
| Distribution |
8,839 | 91 | 5,901 | — | 14,831 | |||||||||||||||
| General and administrative |
21,643 | 3,866 | 11,332 | — | 36,841 | |||||||||||||||
| Amortization of intangibles |
539 | 3,495 | 244 | — | 4,278 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
54,897 | 18,240 | 35,245 | - | 108,382 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| INCOME (LOSS) FROM OPERATIONS |
123,832 | (17,970 | ) | 19,480 | — | 125,342 | ||||||||||||||
| NON-OPERATING INCOME (EXPENSE): |
||||||||||||||||||||
| Interest income |
220 | — | 422 | (188 | ) | 454 | ||||||||||||||
| Interest expense |
(15 | ) | (3 | ) | (203 | ) | 188 | (33 | ) | |||||||||||
| Other, net |
(469 | ) | — | (82 | ) | — | (551 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total non-operating income |
(264 | ) | (3 | ) | 137 | — | (130 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| INCOME (LOSS) BEFORE INCOME TAXES |
123,568 | (17,973 | ) | 19,617 | — | 125,212 | ||||||||||||||
| PROVISION (BENEFIT) FOR INCOME TAXES |
42,969 | (6,434 | ) | 6,094 | — | 42,629 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| NET INCOME (LOSS) BEFORE EARNINGS OF CONSOLIDATED SUBSIDIARIES |
80,599 | (11,539 | ) | 13,523 | — | 82,583 | ||||||||||||||
| Net Income (Loss) of consolidated subsidiaries |
1,984 | — | — | (1,984 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| NET INCOME (LOSS) |
$ | 82,583 | $ | (11,539 | ) | $ | 13,523 | $ | (1,984 | ) | $ | 82,583 | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-34
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING STATEMENTS OF OPERATIONS
For the Year Ended May 31, 2009
(Amounts in thousands)
| Immucor, Inc. |
Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| NET SALES |
$ | 234,908 | $ | 2,875 | $ | 94,865 | $ | (32,101 | ) | $ | 300,547 | |||||||||
| COST OF SALES (exclusive of amortization shown separately below) |
62,555 | 2,201 | 51,881 | (32,101 | ) | 84,536 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| GROSS MARGIN |
172,353 | 674 | 42,984 | — | 216,011 | |||||||||||||||
| OPERATING EXPENSES: |
||||||||||||||||||||
| Research and development |
6,190 | 4,335 | 173 | — | 10,698 | |||||||||||||||
| Selling and marketing |
19,562 | 1,446 | 17,307 | — | 38,315 | |||||||||||||||
| Distribution |
8,411 | — | 5,297 | — | 13,708 | |||||||||||||||
| General and administrative |
17,819 | 3,646 | 11,128 | — | 32,593 | |||||||||||||||
| Amortization of intangibles |
533 | 2,853 | 353 | — | 3,739 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
52,515 | 12,280 | 34,258 | — | 99,053 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| INCOME (LOSS) FROM OPERATIONS |
119,838 | (11,606 | ) | 8,726 | — | 116,958 | ||||||||||||||
| NON-OPERATING INCOME (EXPENSE): |
||||||||||||||||||||
| Interest income |
922 | — | 1,035 | — | 1,957 | |||||||||||||||
| Interest expense |
(85 | ) | 4 | (169 | ) | — | (250 | ) | ||||||||||||
| Other, net |
(1,689 | ) | — | 5 | — | (1,684 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total non-operating income |
(852 | ) | 4 | 871 | — | 23 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| INCOME (LOSS) BEFORE INCOME TAXES |
118,986 | (11,602 | ) | 9,597 | — | 116,981 | ||||||||||||||
| PROVISION (BENEFIT) FOR INCOME TAXES |
40,640 | (3,969 | ) | 4,127 | — | 40,798 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| NET INCOME (LOSS) BEFORE EARNINGS OF CONSOLIDATED SUBSIDIARIES |
78,346 | (7,633 | ) | 5,470 | — | 76,183 | ||||||||||||||
| Net Income (Loss) of consolidated subsidiaries |
(2,163 | ) | — | — | 2,163 | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| NET INCOME (LOSS) |
$ | 76,183 | $ | (7,633 | ) | $ | 5,470 | $ | 2,163 | $ | 76,183 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-35
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING CASH FLOW INFORMATION
For the year ended May 31, 2011
(Amounts in thousands)
| Immucor, Inc. |
Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| Net cash provided by (used in) operating activities |
$ | 82,767 | $ | 803 | $ | 17,731 | $ | 810 | $ | 102,111 | ||||||||||
| Net cash used in investing activities |
(6,112 | ) | (605 | ) | (2,344 | ) | — | (9,061 | ) | |||||||||||
| Net cash provided by financing activities |
2,578 | — | — | — | 2,578 | |||||||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
9 | — | 5,127 | (810 | ) | 4,326 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Increase (decrease) in cash and cash equivalents |
79,242 | 198 | 20,514 | — | 99,954 | |||||||||||||||
| Cash and cash equivalents at beginning of period |
171,144 | (278 | ) | 31,783 | — | 202,649 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents at end of period |
$ | 250,386 | $ | (80 | ) | $ | 52,297 | $ | — | $ | 302,603 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING CASH FLOW INFORMATION
For the year ended May 31, 2010
(Amounts in thousands)
| Immucor, Inc. |
Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| Net cash provided by (used in) operating activities |
$ | 78,454 | $ | 87 | $ | 6,006 | $ | 204 | $ | 84,751 | ||||||||||
| Net cash used in investing activities |
(8,250 | ) | (227 | ) | 2,173 | — | (6,304 | ) | ||||||||||||
| Net cash provided by financing activities |
(11,757 | ) | — | — | — | (11,757 | ) | |||||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
— | — | (298 | ) | (204 | ) | (502 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Increase (decrease) in cash and cash equivalents |
58,447 | (140 | ) | 7,881 | — | 66,188 | ||||||||||||||
| Cash and cash equivalents at beginning of period |
112,697 | (138 | ) | 23,902 | — | 136,461 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents at end of period |
$ | 171,144 | $ | (278 | ) | $ | 31,783 | $ | — | $ | 202,649 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-36
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING CASH FLOW INFORMATION
For the year ended May 31, 2009
(Amounts in thousands)
| Immucor, Inc. |
Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| Net cash provided by (used in) operating activities |
$ | 77,134 | $ | (84 | ) | $ | 3,003 | $ | (231 | ) | $ | 79,822 | ||||||||
| Net cash used in investing activities |
(118,096 | ) | (54 | ) | 1,594 | — | (116,556 | ) | ||||||||||||
| Net cash provided by financing activities |
(1,190 | ) | — | (206 | ) | — | (1,396 | ) | ||||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
— | — | (696 | ) | 231 | (465 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Increase (decrease) in cash and cash equivalents |
(42,152 | ) | (138 | ) | 3,695 | — | (38,595 | ) | ||||||||||||
| Cash and cash equivalents at beginning of period |
154,849 | — | 20,207 | — | 175,056 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents at end of period |
$ | 112,697 | $ | (138 | ) | $ | 23,902 | $ | — | $ | 136,461 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
20. Subsequent events
On July 2, 2011 the Company entered into a definitive agreement to be acquired by investment funds managed by TPG Capital. Under the terms of the agreement, Immucor shareholders will receive $27.00 in cash for each share of Immucor common stock they own. The transaction is expected to close in the second half of calendar 2011. The agreement was unanimously approved by the Immucor Board of Directors.
F-37
Table of Contents
Schedule II – Valuation and Qualifying Accounts
Years ended May 31, 2011, 2010 and 2009
| Beginning Balance |
Additions to Expense |
Deductions | Ending Balance |
|||||||||||||
| 2011 |
||||||||||||||||
| Allowance for doubtful accounts |
$ | 2,122 | $ | 371 | $ | (336 | ) | $ | 2,157 | |||||||
| Deferred income tax valuation allowance |
$ | 6,716 | $ | — | $ | (4,249 | ) | $ | 2,467 | |||||||
| 2010 |
||||||||||||||||
| Allowance for doubtful accounts |
$ | 2,179 | $ | 361 | $ | (418 | ) | $ | 2,122 | |||||||
| Deferred income tax valuation allowance |
$ | 6,134 | $ | 582 | $ | — | $ | 6,716 | ||||||||
| 2009 |
||||||||||||||||
| Allowance for doubtful accounts |
$ | 2,024 | $ | 848 | $ | (693 | ) | $ | 2,179 | |||||||
| Deferred income tax valuation allowance |
$ | 3,042 | $ | 3,092 | $ | — | $ | 6,134 | ||||||||
| Note 1: | “Deductions” for the “Allowance for doubtful accounts” represent accounts written off during the period less recoveries of accounts previously written off and exchange differences generated. |
F-38
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS
(Amounts in thousands, except share data)
| Successor | Predecessor | |||||||||
| August 31, 2011 | May 31, 2011 | |||||||||
| (unaudited) | ||||||||||
| ASSETS |
||||||||||
| CURRENT ASSETS: |
||||||||||
| Cash and cash equivalents |
$ | 25,134 | $ | 302,603 | ||||||
| Trade accounts receivable, net of allowance for doubtful accounts of $12 and $2,157 at August 31 and May 31, 2011, respectively |
67,562 | 63,324 | ||||||||
| Inventories |
57,026 | 32,914 | ||||||||
| Deferred income tax assets, current portion |
4,222 | 15,884 | ||||||||
| Prepaid expenses and other current assets |
5,072 | 11,164 | ||||||||
|
|
|
|
|
|||||||
| Total current assets |
159,016 | 425,889 | ||||||||
| PROPERTY AND EQUIPMENT, Net |
65,405 | 58,216 | ||||||||
| GOODWILL |
975,372 | 93,767 | ||||||||
| INTANGIBLE ASSETS, Net |
778,356 | 54,133 | ||||||||
| DEFERRED FINANCING COSTS |
34,481 | — | ||||||||
| OTHER ASSETS |
1,239 | 1,122 | ||||||||
|
|
|
|
|
|||||||
| Total assets |
$ | 2,013,869 | $ | 633,127 | ||||||
|
|
|
|
|
|||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY |
||||||||||
| CURRENT LIABILITIES: |
||||||||||
| Accounts payable |
$ | 10,246 | $ | 10,790 | ||||||
| Accrued expenses and other current liabilities |
24,891 | 20,331 | ||||||||
| Income taxes payable |
11,632 | 8,294 | ||||||||
| Deferred revenue, current portion |
3,270 | 7,495 | ||||||||
| Current portion of long term debt, net of debt discounts |
2,795 | — | ||||||||
|
|
|
|
|
|||||||
| Total current liabilities |
52,834 | 46,910 | ||||||||
| LONG TERM DEBT, NET OF DEBT DISCOUNTS |
988,699 | — | ||||||||
| DEFERRED REVENUE |
659 | 6,080 | ||||||||
| DEFERRED INCOME TAX LIABILITIES |
274,284 | 9,264 | ||||||||
| OTHER LONG-TERM LIABILITIES |
1,244 | 2,001 | ||||||||
|
|
|
|
|
|||||||
| Total liabilities |
1,317,720 | 64,255 | ||||||||
| COMMITMENTS AND CONTINGENCIES (Note 16) |
— | — | ||||||||
| SHAREHOLDERS’ EQUITY: |
||||||||||
| Successor: Common stock, $0.00 par value, 100 shares authorized, issued and outstanding as of August 31, 2011 |
— | — | ||||||||
| Predecessor: Common stock, $0.10 par value, 120,000,000 shares authorized, 70,367,219 issued and outstanding as of May 31, 2011 |
— | 7,037 | ||||||||
| Additional paid-in capital |
698,776 | 45,729 | ||||||||
| (Accumulated deficit) Retained earnings |
(2,442 | ) | 499,152 | |||||||
| Accumulated other comprehensive (loss) income |
(185 | ) | 16,954 | |||||||
|
|
|
|
|
|||||||
| Total shareholders’ equity |
696,149 | 568,872 | ||||||||
|
|
|
|
|
|||||||
| Total liabilities and shareholders’ equity |
$ | 2,013,869 | $ | 633,127 | ||||||
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these Condensed Consolidated Financial Statements.
F-39
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Amounts in thousands)
(Unaudited)
| Successor | Predecessor | |||||||||||||
| August 20, 2011 through August 31, 2011 |
June 1, 2011 through August 19, 2011 |
Three Months Ended August 31, 2010 |
||||||||||||
| NET SALES |
$ | 11,390 | $ | 74,910 | $ | 83,641 | ||||||||
| COST OF SALES (exclusive of amortization shown separately below) |
7,156 | 22,955 | 23,974 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| GROSS MARGIN |
4,234 | 51,955 | 59,667 | |||||||||||
| OPERATING EXPENSES: |
||||||||||||||
| Research and development |
623 | 4,895 | 4,425 | |||||||||||
| Selling and marketing |
1,112 | 10,510 | 9,142 | |||||||||||
| Distribution |
649 | 3,952 | 4,032 | |||||||||||
| General and administrative |
754 | 38,175 | 8,786 | |||||||||||
| Amortization of intangibles |
1,648 | 931 | 1,080 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
4,786 | 58,463 | 27,465 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| (LOSS) INCOME FROM OPERATIONS |
(552 | ) | (6,508 | ) | 32,202 | |||||||||
| NON-OPERATING INCOME (EXPENSE): |
||||||||||||||
| Interest income |
— | 142 | 213 | |||||||||||
| Interest expense |
(3,393 | ) | — | (14 | ) | |||||||||
| Other, net |
(11 | ) | 2,673 | 97 | ||||||||||
|
|
|
|
|
|
|
|||||||||
| Total non-operating income (expense) |
(3,404 | ) | 2,815 | 296 | ||||||||||
|
|
|
|
|
|
|
|||||||||
| (LOSS) INCOME BEFORE INCOME TAXES |
(3,956 | ) | (3,693 | ) | 32,498 | |||||||||
| PROVISION (BENEFIT) FOR INCOME TAXES |
(1,514 | ) | 2,681 | 11,079 | ||||||||||
|
|
|
|
|
|
|
|||||||||
| NET (LOSS) INCOME |
$ | (2,442 | ) | $ | (6,374 | ) | $ | 21,419 | ||||||
|
|
|
|
|
|
|
|||||||||
The accompanying notes are an integral part of these Condensed Consolidated Financial Statements.
F-40
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENT OF SHAREHOLDERS’ EQUITY AND
COMPREHENSIVE INCOME (LOSS)
(Unaudited, amounts in thousands)
| Retained Earnings |
Accumulated Other Comprehensive Income* |
Total Shareholders’ Equity |
||||||||||||||||||||||
| Additional Paid-In Capital |
||||||||||||||||||||||||
| Common Stock | ||||||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||
| Predecessor: |
||||||||||||||||||||||||
| BALANCE, May 31, 2011 |
70,367 | $ | 7,037 | $ | 45,729 | $ | 499,152 | $ | 16,954 | $ | 568,872 | |||||||||||||
| Shares issued under employee stock plan |
415 | 41 | 485 | — | — | 526 | ||||||||||||||||||
| Share-based compensation expense |
— | — | 16,233 | — | — | 16,233 | ||||||||||||||||||
| Stock repurchases and retirements |
(103 | ) | (10 | ) | (448 | ) | — | — | (458 | ) | ||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | — | (2,153 | ) | (2,153 | ) | ||||||||||||||||
| Net loss |
— | — | — | (6,374 | ) | — | (6,374 | ) | ||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total comprehensive loss |
(8,527 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| BALANCE, August 19, 2011 |
70,679 | $ | 7,068 | $ | 61,999 | $ | 492,778 | $ | 14,801 | $ | 576,646 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| * | Accumulated Other Comprehensive Income balance primarily consists of foreign currency translation adjustments and has no tax effect. |
| Additional Paid-In Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Income (Loss) |
Total Shareholders’ Equity |
|||||||||||||||||||||
| Common Stock | ||||||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||
| Successor: |
||||||||||||||||||||||||
| BALANCE, August 20, 2011 |
— | $ | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||
| Capital contribution from parent, net of costs |
100 | — | 698,776 | — | — | 698,776 | ||||||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||
| Net loss |
— | — | — | (2,442 | ) | — | (2,442 | ) | ||||||||||||||||
| Foreign currency translation adjustments |
— | — | — | — | 167 | 167 | ||||||||||||||||||
| Cash flow hedges, net of tax |
— | — | — | — | (352 | ) | (352 | ) | ||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total comprehensive loss |
(2,627 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| BALANCE, August 31, 2011 |
100 | $ | — | $ | 698,776 | $ | (2,442 | ) | $ | (185 | ) | $ | 696,149 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
The accompanying notes are an integral part of these Condensed Consolidated Financial Statements.
F-41
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited, amounts in thousands)
| Successor | Predecessor | |||||||||||||
| August 20, 2011 through August 31, 2011 |
June 1, 2011 through August 19, 2011 |
Three Months Ended August 31, 2010 |
||||||||||||
| OPERATING ACTIVITIES: |
||||||||||||||
| Net income (loss) |
$ | (2,442 | ) | $ | (6,374 | ) | $ | 21,419 | ||||||
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: |
||||||||||||||
| Depreciation and amortization |
2,038 | 4,321 | 4,383 | |||||||||||
| Non-cash interest expense |
224 | — | — | |||||||||||
| Loss on retirement of fixed assets |
— | 135 | 219 | |||||||||||
| Provision for doubtful accounts |
12 | 185 | 402 | |||||||||||
| Share-based compensation expense |
— | 16,233 | 1,739 | |||||||||||
| Deferred income taxes |
(1,234 | ) | (3,974 | ) | (13 | ) | ||||||||
| Excess tax benefit from share-based compensation |
— | — | (34 | ) | ||||||||||
| Changes in operating assets and liabilities: |
||||||||||||||
| Accounts receivable, trade |
(817 | ) | (3,938 | ) | (936 | ) | ||||||||
| Income taxes |
(382 | ) | 3,317 | 7,931 | ||||||||||
| Inventories |
2,663 | (3,242 | ) | (1,819 | ) | |||||||||
| Other assets |
(645 | ) | 6,459 | 458 | ||||||||||
| Accounts payable |
2,935 | (4,023 | ) | (1,709 | ) | |||||||||
| Deferred revenue |
(173 | ) | (920 | ) | (827 | ) | ||||||||
| Accrued expenses and other liabilities |
(15,292 | ) | 17,409 | (2,317 | ) | |||||||||
|
|
|
|
|
|
|
|||||||||
| Cash provided by (used in) operating activities |
(13,113 | ) | 25,588 | 28,896 | ||||||||||
| INVESTING ACTIVITIES: |
||||||||||||||
| Purchases of property and equipment |
(907 | ) | (2,265 | ) | (1,219 | ) | ||||||||
| Acquisition of Immucor, Inc. |
(1,939,387 | ) | — | — | ||||||||||
|
|
|
|
|
|
|
|||||||||
| Cash used in investing activities |
(1,940,294 | ) | (2,265 | ) | (1,219 | ) | ||||||||
| FINANCING ACTIVITIES: |
||||||||||||||
| Proceeds from long-term debt |
991,406 | — | — | |||||||||||
| Proceeds from capital contributions, net of costs |
698,776 | — | — | |||||||||||
| Payment of debt issuance costs |
(34,618 | ) | — | — | ||||||||||
| Repurchase of common stock |
— | (458 | ) | (351 | ) | |||||||||
| Proceeds from exercise of stock options |
— | 524 | 7 | |||||||||||
| Excess tax benefit from share-based compensation |
— | — | 34 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| Cash provided by (used in) financing activities |
1,655,564 | 66 | (310 | ) | ||||||||||
| EFFECT OF EXCHANGE RATES ON CASH AND CASH EQUIVALENTS |
14 | (3,029 | ) | 497 | ||||||||||
|
|
|
|
|
|
|
|||||||||
| INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS |
(297,829 | ) | 20,360 | 27,864 | ||||||||||
| CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD |
322,963 | 302,603 | 202,649 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| CASH AND CASH EQUIVALENTS AT END OF PERIOD |
$ | 25,134 | $ | 322,963 | $ | 230,513 | ||||||||
|
|
|
|
|
|
|
|||||||||
| SUPPLEMENTAL INFORMATION: |
||||||||||||||
| Tax paid |
$ | — | $ | 3,414 | $ | 3,154 | ||||||||
| Interest paid |
— | — | — | |||||||||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: |
||||||||||||||
| Movement from inventory to property and equipment of instruments placed on rental agreements |
315 | 1,618 | 3,324 | |||||||||||
The accompanying notes are an integral part of these Condensed Consolidated Financial Statements.
F-42
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
Notes to Condensed Consolidated Financial Statements
(Unaudited)
1. NATURE OF BUSINESS AND BASIS OF PRESENTATION
Nature of Business
Immucor, Inc. (“Immucor” and, together with its wholly owned subsidiaries, the “Company”) develops, manufactures and sells a complete line of reagents and automated systems used primarily by hospitals, donor centers and reference laboratories in a number of tests performed to detect and identify certain properties of human blood for the purpose of blood transfusion. The Company operates facilities in the United States, Canada, Western Europe and Japan. The unaudited condensed consolidated financial statements include the accounts of the Company and all of its subsidiaries.
Basis of Presentation
The Company was acquired on August 19, 2011 through a merger transaction with IVD Acquisition Corporation (“Merger Sub”), a wholly owned subsidiary of IVD Intermediate Holdings B, Inc. (the “Parent”). The Parent is a wholly owned indirect subsidiary of IVD Holdings, Inc. which was formed by investment funds affiliated with TPG Capital, L.P. (“TPG Capital”). The acquisition was accomplished through a merger of the Merger Sub with and into the Company, with the Company being the surviving company (the “Acquisition”). As a result of the merger, the Company became a wholly owned subsidiary of Parent. Prior to August 19, 2011, the Company operated as a public company with common stock traded on the NASDAQ Stock Market.
The Company continued as the same legal entity after the Acquisition, however, a new accounting basis was established upon accounting for the merger as a business combination. The accompanying unaudited condensed consolidated statements of operations, cash flows and equity are presented for two periods: Predecessor (June 1, 2011 to August 19, 2011) and Successor (August 20, 2011 to August 31, 2011), which relate to the period preceding the Acquisition and the period succeeding the Acquisition. Although the accounting policies followed by the Company are consistent for the Predecessor and Successor period, financial information for such periods has been prepared under two different historical-cost bases of accounting and is therefore not comparable. These results are not necessarily indicative of the results that may be achieved for the year ending May 31, 2012, or any other period.
The accompanying condensed consolidated financial statements are unaudited and have been prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”) for interim financial information, and the Securities and Exchange Commission’s (“SEC”) instructions for Form 10-Q and Article 10 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by U.S. GAAP for complete financial statements. In the opinion of management, all adjustments, consisting of normal recurring adjustments, considered necessary for a fair presentation of the unaudited condensed consolidated financial statements have been recorded in the interim periods presented. These unaudited, condensed consolidated financial statements should be read in conjunction with the Company’s audited, consolidated financial statements and related notes for the year ended May 31, 2011, included in the Company’s Annual Report on Form 10-K.
Basis of Consolidation
The condensed consolidated financial statements include the accounts of Immucor and all its subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
2. ACQUISITION
The Company was acquired on August 19, 2011 (the “Acquisition Date”) through the Acquisition described in Note 1.
F-43
Table of Contents
The Acquisition has been accounted for as a purchase business combination. Acquisition-related transaction costs include investment banking, legal and accounting fees, and other external costs directly related to the Acquisition. Transaction costs paid at closing totaled $88.3 million and include $35.0 million that was capitalized as debt issuance costs and $16.9 million which was incurred by the Company and included in general and administrative expense in the Predecessor fiscal 2012 period. The remaining $36.4 million was incurred by the Parent but paid by the Company out of equity proceeds. These costs have been reflected on the balance sheet as a reduction of the capital contribution from the parent. In addition, the company paid $2.0 million of transaction costs prior to closing that is also included in general and administrative expense in the Predecessor fiscal 2012 period.
Sources and Uses of Funds
The sources and uses of funds in connection with the Acquisition are summarized below (in thousands):
| Sources: |
||||
| Proceeds from Term Loan |
$ | 596,550 | ||
| Proceeds from Notes |
394,856 | |||
| Proceeds from equity contributions |
735,187 | |||
| Company cash used in transaction |
301,053 | |||
|
|
|
|||
| $ | 2,027,646 | |||
|
|
|
|||
| Uses: |
||||
| Equity purchase price |
$ | 1,939,387 | ||
| Transaction costs |
88,259 | |||
|
|
|
|||
| $ | 2,027,646 | |||
|
|
|
Preliminary Purchase Price Allocation
The Acquisition was recorded under the acquisition method of accounting by the Parent and pushed-down to the Company by allocating the purchase consideration of $1.9 billion to the cost of the assets acquired, including intangible assets, based on their estimated fair values at the Acquisition Date. The allocation of purchase price is based on management’s judgment after evaluating several factors, including, but not limited to, valuation assessments of tangible and intangible assets. The excess of the total purchase price of $975.3 million over the fair value of assets acquired and the liabilities assumed is recorded as goodwill. The goodwill arising from the Acquisition consists largely of the commercial potential of the Company and the value of the assembled workforce.
The following sets forth the Company’s preliminary purchase price allocation (in thousands):
| Cash on hand |
$ | 322,963 | ||
| Accounts receivable |
66,781 | |||
| Inventories |
60,000 | |||
| Property and equipment |
64,683 | |||
| Intangible assets |
779,860 | |||
| Goodwill |
975,299 | |||
| Current liabilities |
(55,863 | ) | ||
| Deferred revenue |
(4,107 | ) | ||
| Deferred tax assets and liabilities—net |
(274,071 | ) | ||
| Other assets and liabilities—net |
3,842 | |||
|
|
|
|||
| Total purchase price allocation: |
$ | 1,939,387 | ||
|
|
|
F-44
Table of Contents
The Company has not yet finalized its evaluation of the fair value of the assets acquired and liabilities assumed and will finalize the purchase price allocation relating to the Acquisition in the near future. Thus, the provisional measurements of fair value reflected are subject to change once this analysis is finalized. The final valuation could change the allocation of the purchase price, primarily as it relates to deferred income taxes, with a corresponding offset to goodwill.
The Company has acquired intangible assets, not including goodwill, totaling approximately $779.9 million in the Acquisition. The amortization of these intangibles is not deductible for tax purposes and hence the Company has recorded a deferred tax liability of approximately $291.7 million to offset the future book amortization related to these intangibles. None of the goodwill of approximately $975.3 million resulting from the Acquisition is deductible for tax purposes.
Identifiable Intangible Assets
In performing the purchase price allocation, the Company considered, among other factors, the intended future use of acquired assets, analyses of historical financial performance and estimates of future performance. The following table sets forth the components of intangible assets as of the Acquisition Date (in thousands):
| Intangible Asset |
Fair Value | Useful Life | ||||||
| Customer relationships |
$ | 455,000 | 20 | |||||
| Existing technology and trade names |
266,000 | 11 | ||||||
| Corporate trade name |
40,000 | 15 | ||||||
| Below market leasehold interests |
860 | 5 | ||||||
| In-process research and development |
18,000 | n/a | ||||||
|
|
|
|||||||
| $ | 779,860 | |||||||
|
|
|
|||||||
Customer relationships represent the fair value of the existing customer base.
Existing technologies relate to the serology instrument platforms (Galileo, NEO, and Echo); the Company’s proprietary Capture reagent technology; and the molecular immunohematology testing technology.
Corporate trade name represents the Immucor® company brand. Immucor is well recognized by customers as a company that provides an extensive selection of quality products including products that are not available elsewhere in the marketplace.
Below market leasehold interests represents the Company’s interest in the current leases, which provide for payments below comparable leases obtainable contemporaneously with the Acquisition.
Useful lives of the amortizable intangible assets were based on estimated economic useful lives and are being amortized using the straight-line method.
In-process research and development relates primarily to the molecular immunohematology business. The other projects valued relate to technological improvements for the serology instrument platforms, and generally are applicable to the current NEO and Echo instruments, and will thus be able to yield a cash flow impact relatively quickly upon approval and launch. In-process research and development is not amortized, but will be evaluated on a periodic basis to determine which projects remain in process.
F-45
Table of Contents
Pro forma Financial Information
The financial information in the table below summarizes the results of operations of the Company on a pro forma basis, as though the acquisition had occurred at the beginning of the period presented. The pro forma financial information is presented for informational purposes only and is not indicative of the results of operations that would have been achieved if the acquisition had taken place at the beginning of the period presented. Such pro forma financial information is based on the historical financial statements of the Company. This pro forma financial information is based on estimates and assumptions, which have been made solely for purposes of developing such pro forma information, including, without limitation, purchase accounting adjustments. The pro forma financial information presented below also includes depreciation and amortization based on the valuation of the Company’s tangible assets and identifiable intangible assets, interest expense and management fee resulting from the acquisition. The pro forma financial information presented below (in thousands) does not reflect any synergies or operating cost reductions that may be achieved.
| Three Months Ended | ||||||||
| August 31, 2011 | August 31, 2010 | |||||||
| Revenue |
$ | 86,300 | $ | 83,641 | ||||
| Net income (loss) |
$ | (7,935 | ) | $ | (1,735 | ) | ||
3. RELATED PARTY TRANSACTION
In connection with the Transactions, the Company entered into a management services agreement with the Sponsors pursuant to which they received on the closing date an aggregate transaction fee of $18 million in cash in connection with the Transactions. In addition, pursuant to such agreement, and in exchange for on-going consulting and management advisory services that will be provided to the Company, the Sponsors will receive an aggregate annual monitoring fee prepaid quarterly equal to $3 million. In the Successor period, $0.2 million was recorded for monitoring fees and is included in selling, general and administrative expenses in the statement of operations.
4. INVENTORIES
Typically inventories are stated at the lower of cost (first-in, first-out basis) or market (net realizable value). However, in relation to the Acquisition of the Company on August 19, 2011, a fair value adjustment of $25.4 million increased inventory to net realizable value, which is currently greater than replacement cost.
| Successor | Predecessor | |||||||||
| August 31, 2011 | May 31, 2011 | |||||||||
| (in thousands) | (in thousands) | |||||||||
| Raw materials and supplies |
$ | 9,896 | $ | 9,506 | ||||||
| Work in process |
10,774 | 4,012 | ||||||||
| Finished goods |
36,356 | 19,396 | ||||||||
|
|
|
|
|
|||||||
| $ | 57,026 | $ | 32,914 | |||||||
|
|
|
|
|
|||||||
F-46
Table of Contents
5. GOODWILL
| Successor | ||||
| August 31, 2011 | ||||
| (in thousands) | ||||
| Beginning balance, August 20, 2011 |
$ | — | ||
| Additions: |
||||
| Acquisition of Immucor, Inc. |
975,299 | |||
| Foreign currency translation adjustment |
73 | |||
|
|
|
|||
| Ending balance, August 31, 2011 |
$ | 975,372 | ||
|
|
|
|||
| Predecessor | ||||
| August 19, 2011 | ||||
| (in thousands) | ||||
| Beginning balance, May 31, 2011 |
$ | 93,767 | ||
| Foreign currency translation adjustment |
298 | |||
|
|
|
|||
| Ending balance, August 19, 2011 |
$ | 94,065 | ||
|
|
|
|||
6. OTHER INTANGIBLE ASSETS
| Successor | Predecessor | |||||||||||||||||||||||||||||
| August 31, 2011 | May 31, 2011 | |||||||||||||||||||||||||||||
| Weighted Average Life |
Cost | Accumulated Amortization |
Net | Cost | Accumulated Amortization |
Net | ||||||||||||||||||||||||
| (in thousands) | (in thousands) | |||||||||||||||||||||||||||||
| Intangible assets subject to amortization: |
||||||||||||||||||||||||||||||
| Customer lists |
20 yrs | $ | 455,144 | $ | (754 | ) | $ | 454,390 | $ | 5,215 | $ | (2,117 | ) | $ | 3,098 | |||||||||||||||
| Existing technology / trade names |
11 yrs | 266,000 | (800 | ) | 265,200 | — | — | — | ||||||||||||||||||||||
| Corporate trade name |
15 yrs | 40,000 | (88 | ) | 39,912 | — | — | — | ||||||||||||||||||||||
| Below market leasehold interests |
5 yrs | 860 | (6 | ) | 854 | — | — | — | ||||||||||||||||||||||
| Deferred licensing costs |
5 yrs | — | — | — | 1,013 | (579 | ) | 434 | ||||||||||||||||||||||
| Distribution rights |
10 yrs | — | — | — | 4,492 | (3,359 | ) | 1,133 | ||||||||||||||||||||||
| Non-compete agreements |
5 yrs | — | — | — | 1,650 | (935 | ) | 715 | ||||||||||||||||||||||
| Developed product technology |
17 yrs | — | — | — | 51,097 | (8,597 | ) | 42,500 | ||||||||||||||||||||||
| Trademarks / tradenames |
17 yrs | — | — | — | 1,615 | (281 | ) | 1,334 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total amortizable assets |
762,004 | (1,648 | ) | 760,356 | 65,082 | (15,868 | ) | 49,214 | ||||||||||||||||||||||
| Intangible assets not subject to amortization: |
||||||||||||||||||||||||||||||
| In-process research and development |
18,000 | — | 18,000 | — | — | — | ||||||||||||||||||||||||
| Deferred licensing costs |
— | — | — | 4,919 | — | 4,919 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total non-amortizable assets |
18,000 | — | 18,000 | 4,919 | — | 4,919 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total other intangible assets |
$ | 780,004 | $ | (1,648 | ) | $ | 778,356 | $ | 70,001 | $ | (15,868 | ) | $ | 54,133 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
F-47
Table of Contents
Amortization of intangible assets amounted to $1.6 million in the Successor fiscal 2012 period and $0.9 million in the Predecessor fiscal 2012 period compared with $1.1 million in the Predecessor fiscal 2011 period. The following table presents our estimate of amortization expense for each of the next five fiscal years and thereafter (in thousands):
| Year Ending May 31: |
||||
| 2012 |
39,021 | |||
| 2013 |
49,847 | |||
| 2014 |
49,847 | |||
| 2015 |
49,847 | |||
| 2016 |
49,847 | |||
| Thereafter |
521,947 | |||
|
|
|
|||
| $ | 760,356 | |||
|
|
|
7. LONG TERM DEBT
Long term debt consists of the following:
| Successor | Predecessor | |||||||||
| August 31, 2011 | May 31, 2011 | |||||||||
| (in thousands) | (in thousands) | |||||||||
| Term loan facility, net of $18,377 debt discounts |
$ | 596,624 | $ | — | ||||||
| Revolving facility |
— | — | ||||||||
| Notes, net of $5,130 debt discounts |
394,870 | — | ||||||||
|
|
|
|
|
|||||||
| 991,494 | — | |||||||||
| Less current portion |
(2,795 | ) | — | |||||||
| Long-term debt, net of current portion |
$ | 988,699 | $ | — | ||||||
|
|
|
|
|
|||||||
Senior Secured Credit Facilities, Security Agreement and Guaranty
In connection with the Acquisition of the Company on August 19, 2011, the Company entered into a credit agreement and related security and other agreements for (1) a $615 million senior secured term loan facility (the “Term Loan Facility”) and (2) a $100 million senior secured revolving loan facility (the “Revolving Facility,” and together with the Term Loan Facility, the “Senior Credit Facilities”) with certain lenders, Citibank, N.A., as administrative agent and collateral agent and the other agents party thereto. In addition to borrowings upon prior notice, the Revolving Facility includes borrowing capacity in the form of letters of credit and borrowings on same-day notice, referred to as swingline loans, in each case, up to $25.0 million, and will be available in U.S. dollars, GBP, Euros, Yen, Canadian dollars and in such other currencies as the Company and the administrative agent under the Revolving Facility may agree (subject to a sublimit for such non-U.S. currencies). The credit agreement governing the Senior Credit Facilities provides that, subject to certain conditions, the Company may request additional tranches of term loans and/or increase commitments under the Revolving Facility and/or the Term Loan Facility and/or add one or more incremental revolving credit facility tranches (provided there are no more than three such tranches with different maturity dates outstanding at any time) in an aggregate amount not to exceed (a) $150.0 million plus (b) an unlimited amount at any time, subject to compliance on a pro forma basis with a senior secured first lien net leverage ratio of no greater than 3.75:1.00 (with amounts alternatively consisting of debt securities and debt that is secured on a junior lien basis to the liens securing the Senior Credit Facilities or is unsecured subject to compliance with a senior secured net leverage ratio of no greater than 4.00:1.00). Availability of such additional tranches of term loans or revolving credit facilities and/or increased commitments is subject to, among other conditions, the absence of any default under the credit agreement governing the Senior Credit Facilities and the receipt of commitments by existing or additional financial institutions.
F-48
Table of Contents
Beginning on the last business day of December 2011, the Company is required to make scheduled quarterly payments each equal to 0.25% of the original principal amount of the loans under the Term Loan Facility made on the closing date, with the balance due and payable on the seventh anniversary of the closing date. The Company is also required to repay loans under the Term Loan Facility based on annual excess cash flows as defined in the agreement and upon the occurrence of certain other events set forth in the Term Loan Facility. The initial maturity date for the Revolving Credit Facility is five years from the closing date.
Borrowings under the Senior Credit Facilities bear interest at a rate per annum equal to an applicable margin plus, at the Company’s option, either (a) in the case of borrowings in U.S. dollars, a base rate determined by reference to the highest of (1) the prime rate of Citibank, N.A., (2) the federal funds effective rate plus 0.50% and (3) a LIBOR rate determined by reference to the costs of funds for U.S. dollar deposits for an interest period of one month adjusted for certain additional costs, plus 1.00% or (b) in the case of borrowings in U.S. dollars or another currency, a LIBOR rate determined by reference to the costs of funds for U.S. dollar deposits for the interest period relevant to such borrowing adjusted for certain additional costs, which, in the case of the Term Loan Facility only, shall be no less than 1.50%. The applicable margin for borrowings under the Senior Credit Facilities is currently 4.75% with respect to base rate borrowings and 5.75% with respect to LIBOR borrowings. The applicable margin for borrowings under the Revolving Facility is subject to a 0.25% step-down, when the Company’s senior secured net leverage ratio at the end of a fiscal quarter is less than or equal to 3:00 to 1:00. The effective interest rate was 7.25% as of August 31, 2011.
All obligations under the Senior Credit Facilities are unconditionally guaranteed by the Parent and certain of the Company’s existing and future wholly owned domestic subsidiaries (such subsidiaries collectively, the “Subsidiary Guarantors”), and are secured, subject to certain exceptions, by substantially all of the Company’s assets and the assets of the Parent and Subsidiary Guarantors, including, in each case subject to customary exceptions and exclusions:
| • | a first-priority pledge of all of the Company’s capital stock directly held by Parent and a first-priority pledge of all of the capital stock directly held by the Company and Subsidiary Guarantors (which pledge, in the case of the capital stock of each (a) domestic subsidiary that is directly owned by the Company or by any Subsidiary Guarantor and that is a disregarded entity for United States federal income tax purposes and that has no material assets other than equity interests in one or more foreign subsidiaries that are controlled foreign corporations for United States federal income tax purposes or (b) foreign subsidiary, is limited to 65% of the stock of such subsidiary); and |
| • | a first-priority security interest in substantially all of the Parent’s, the Company’s and the Subsidiary Guarantor’s other tangible and intangible assets. Parent has no material operations or assets other than the capital stock of the Company. |
The Senior Credit Facilities include restrictions on the Company’s ability and the ability of certain of its subsidiaries to, among other things, incur or guarantee additional indebtedness, pay dividends (including to Parent) on, or redeem or repurchase capital stock, make certain acquisitions or investments, materially change our business, incur or permit to exist certain liens, enter into transactions with affiliates or sell our assets to, or merge or consolidate with or into, another company.
Although the Parent is not generally subject to the negative covenants under the Senior Credit Facilities, the Parent is subject to a passive holding company covenant that limits its ability to engage in certain activities other than (i) owning equity interests in the Company and holding cash or property received by the Company, (ii) maintaining its legal existence and engaging in administrative matters related to being a holding company, (iii) performing its obligations under the Senior Credit Facilities, the Senior Notes due 2019 (“Notes”) and other financings not prohibited by the Senior Credit Facilities, (iv) engaging in public offerings of its securities and other equity issuances permitted under the Senior Credit Facilities, (v) providing indemnifications to officer and directors and (vi) engaging in activities incidental to the activities described above.
F-49
Table of Contents
In addition, the credit agreement governing the Senior Credit Facilities requires the Company to comply with a maximum senior secured net leverage ratio financial maintenance covenant, to be tested on the last day of each fiscal quarter beginning November 30, 2011. A breach of this covenant is subject to certain equity cure rights. The credit agreement governing the Senior Credit Facilities also contains certain customary representations and warranties, affirmative covenants and provisions relating to events of default, including upon change of control and a cross-default according to the terms of any indebtedness with an aggregate principal amount of $20 million or more. If an event of default occurs under the Senior Credit Facilities, the lenders may declare all amounts outstanding under the Senior Credit Facilities immediately due and payable. In such event, the lenders may exercise any rights and remedies they may have by law or agreement, including the ability to cause all or any part of the collateral security the Senior Credit Facilities to be sold.
There were no borrowings under the Revolving Facility and no outstanding stand-by letters of credit at August 31, 2011.
Indenture and the Senior Notes Due 2019
On August 19, 2011, the Company (as successor by merger to IVD Acquisition Corporation, the Merger Sub), issued $400 million in principal amount of Notes. The Notes bear interest at a rate of 11.125% per annum, and interest is payable semi-annually on February 15 and August 15 of each year, commencing on February 15, 2012. The Notes mature on August 15, 2019.
Subject to certain exceptions, the Notes are guaranteed on a senior unsecured basis by each of the Company’s current and future wholly owned domestic restricted subsidiaries (and non-wholly owned subsidiaries if such non-wholly owned subsidiaries guarantee the Company’s or another guarantor’s other capital market debt securities) that is a guarantor of certain debt of the Company or another guarantor, including the Senior Credit Facilities. The Notes are the Company’s senior unsecured obligations and rank equally in right of payment with all of the Company’s existing and future indebtedness that is not expressly subordinated in right of payment thereto. The Notes will be senior in right of payment to any future indebtedness that is expressly subordinated in right of payment thereto and effectively junior to (a) the Company’s existing and future secured indebtedness, including the Senior Credit Facilities described above, to the extent of the value of the collateral securing such indebtedness and (b) all existing and future liabilities of the Company’s non-guarantor subsidiaries.
The Indenture governing the Notes contains certain customary provisions relating to events of default and covenants, including without limitation, a cross-payment default provision and cross-acceleration provision in the case of a payment default or acceleration according to the terms of any indebtedness with an aggregate principal amount of $25 million or more, restrictions on the Company’s and certain of its subsidiaries’ ability to, among other things, incur or guarantee indebtedness; pay dividends on, redeem or repurchase capital stock; prepay, redeem or repurchase certain debt; sell or otherwise dispose of assets; make investments; issue certain disqualified or preferred equity; create liens; enter into transactions with the Company’s affiliates; designate the Company’s subsidiaries as unrestricted subsidiaries; enter into agreements restricting the Company’s restricted subsidiaries’ ability to (1) pay dividends, (2) make loans to the Company or any restricted subsidiary that is a guarantor or (3) sell, lease or transfer assets to the Company or any restricted subsidiary that is a guarantor; and consolidate, merge, or transfer all or substantially all of the Company’s assets. The covenants are subject to a number of exceptions and qualifications. Certain of these covenants, excluding without limitation those relating to transactions with the Company’s affiliates and consolidation, merger, or transfer of all or substantially all of the Company’s assets, will be suspended during any period of time that (1) the Notes have investment grade ratings and (2) no default has occurred and is continuing under the Indenture. In the event that the Notes are downgraded to below an investment grade rating, the Company and certain subsidiaries will again be subject to the suspended covenants with respect to future events.
The Company has been in compliance with its covenants during the terms of these agreements.
F-50
Table of Contents
Future Commitments
Debt repayment requirements over the next five fiscal years are as follows (in thousands):
| Year Ending May 31: |
||||
| 2012 |
3,076 | |||
| 2013 |
6,152 | |||
| 2014 |
6,152 | |||
| 2015 |
6,152 | |||
| 2016 |
6,152 | |||
| Thereafter |
987,316 | |||
|
|
|
|||
| $ | 1,015,000 | |||
|
|
|
Interest Expense
The significant components of interest expense are as follows (in thousands):
| Successor | Predecessor | |||||||||||||
| August 20, 2011 through August 31, 2011 |
June 1, 2011 through August 19, 2011 |
Three Months Ended August 31, 2010 |
||||||||||||
| Term loan |
$ | 1,643 | $ | — | $ | — | ||||||||
| Notes |
1,613 | — | — | |||||||||||
| Amortization of deferred financing costs |
137 | — | — | |||||||||||
| Other |
— | — | 14 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| Interest expense |
$ | 3,393 | $ | — | $ | 14 | ||||||||
|
|
|
|
|
|
|
|||||||||
8. DERIVATIVE FINANCIAL INSTRUMENTS
Interest Rate Swaps
In August 2011 during the Successor Period, the Company entered into floating-to-fixed interest rate swap agreements for an aggregate notional amount of $320 million related to a portion of the Company’s floating rate indebtedness. The purpose of entering into this swap is to eliminate all but small movements (due to possible differences in reset timing between the swap and the debt) in future debt interest payments. The objective of this swap is to protect the Company from variability in cash flows attributable to changes in LIBOR interest rates. The Company’s strategy is to use a pay fixed receive floating swap to convert the current or any replacement floating rate credit facility where LIBOR is consistently applied into a USD fixed rate obligation with the only variable piece remaining the difference in actual reset date when the swap and debt are not lined up. Consistent with the terms of the Company’s credit facility, these swaps include a LIBOR floor of 1.5%. These swap agreements, effective in August 2011, hedge a portion of contractual floating rate interest commitments through the expiration of the agreements in September of each year 2013 through 2016. As a result of entering into the swap agreements, the LIBOR rate associated with the Company’s indebtedness has been fixed at a weighted average rate of 1.8%.
Fair Value
As of the effective date, the Company designated the interest rate swap agreements as cash flow hedges. As cash flow hedges, unrealized gains are recognized as assets while unrealized losses are recognized as liabilities. The interest rate swap agreements are highly correlated to the changes in interest rates to which the Company is exposed. Unrealized gains and losses on these swaps are designated as effective or ineffective. The effective portion of such gains or losses is recorded as a component of accumulated other comprehensive income or loss, while the ineffective portion of such gains or losses will be recorded as a component of interest expense. Future realized gains and losses in connection with each required interest payment will be reclassified from accumulated other comprehensive income or loss to interest expense.
F-51
Table of Contents
The fair values of the interest rate swap agreements are estimated using industry standard valuation models using market-based observable inputs, including interest rate curves (level 2). A summary of the recorded assets (liabilities) included in the condensed consolidated balance sheet is as follows:
| Successor | Predecessor | |||||||||
| August 31, 2011 | May 31, 2011 | |||||||||
| (in thousands) | (in thousands) | |||||||||
| Interest rate swaps (included in other assets) |
$ | 269 | $ | — | ||||||
| Interest rate swaps (included in other liabilities) |
(839 | ) | — | |||||||
| Accumulated other comprehensive loss, net of tax |
(352 | ) | — | |||||||
9. FAIR VALUE
The Company uses a three-level fair value hierarchy that prioritizes the inputs used to measure fair value. This hierarchy requires entities to maximize the use of observable inputs and minimize the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
| • | Level 1—Quoted prices in active markets for identical assets or liabilities. |
| • | Level 2—Observable inputs, other than quoted prices included in Level 1, such as quoted prices for markets that are not active; or other inputs that are observable or can be corroborated by observable market data. |
| • | Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. This includes certain pricing models, discounted cash flow methodologies and similar techniques that use significant unobservable inputs. |
|
Fair Value at Reporting Date Using |
||||||||||||||||
| Description |
August 31, 2011 | Level 1 | Level 2 | Level 3 | ||||||||||||
| Derivatives |
||||||||||||||||
| Interest rate swaps (included in other assets) |
$ | 269 | $ | — | $ | 269 | $ | — | ||||||||
| Interest rate swaps (included in other liabilities) |
(839 | ) | — | (839 | ) | — | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total derivatives |
$ | (570 | ) | $ | — | $ | (570 | ) | $ | — | ||||||
|
|
|
|
|
|
|
|
|
|||||||||
The level 2 inputs used to calculate fair value were interest rates, volatility and credit derivative markets.
Financial assets and liabilities
The fair value of the Company’s debt is estimated to be $991 million at August 31, 2011.
The carrying amounts reported in the consolidated balance sheets for cash and cash equivalents, accounts payable and other current liabilities approximate fair value because of their short-term nature.
F-52
Table of Contents
10. COMPREHENSIVE INCOME (LOSS)
The components of comprehensive income (loss) for the three months ended August 31, 2011, separated into Predecessor and Successor periods, and the three months ended August 31, 2010 are as follows:
| Successor | Predecessor | |||||||||||||
| August 20, 2011 through August 31, 2011 |
June 1, 2011 through August 19, 2011 |
Three Months Ended August 31, 2010 |
||||||||||||
| (In thousands) | (In thousands) | (In thousands) | ||||||||||||
| Net (loss) income |
$ | (2,442 | ) | $ | (6,374 | ) | $ | 21,419 | ||||||
| Foreign currency translation adjustment |
167 | (2,153 | ) | 3,026 | ||||||||||
| Cash flow hedge, net of tax |
(352 | ) | — | — | ||||||||||
|
|
|
|
|
|
|
|||||||||
| Comprehensive (loss) income |
$ | (2,627 | ) | $ | (8,527 | ) | $ | 24,445 | ||||||
|
|
|
|
|
|
|
|||||||||
No tax effect is recorded for foreign currency translation adjustment for the Predecessor fiscal periods of 2012 and 2011 as the foreign net assets translated were deemed permanently invested.
11. REVENUE RECOGNITION
The Company recognizes revenue in accordance with accounting principles generally accepted in the United States of America, principally those required by the “Revenue Recognition” topic of the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) and those prescribed by the SEC. Revenue is recognized when the following four basic criteria have been met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed and determinable; and (4) collectibility is reasonably assured.
The Company’s revenue is generated from the following sources.
| • | Reagent sales |
| • | Instrument sales |
| • | Consumables (part kits) |
| • | Training |
| • | General Support Agreements |
| • | Instrument leases |
Effective June 1, 2011, the Company prospectively adopted Accounting Standards Update (“ASU”) 2009-13, “Multiple-Deliverable Revenue Arrangements.” Under historical standards, the Company used the residual method to allocate arrangement consideration when vendor specific objective evidence existed for all undelivered elements, but not for the delivered elements. Under the new standards, the Company allocates revenue to all elements based on their relative selling prices. The selling prices of the various elements of our contractual arrangements are determined as follows:
| • | Reagents (without price guarantees)—the selling price of reagents (without price guarantees) is based on vendor specific objective evidence (“VSOE”) of fair value by reference to the price our customers are required to pay for the reagents when sold separately. |
| • | Reagents (with price guarantees)—the selling price of reagents (with price guarantees) is based on management’s best estimate of selling price (“MBESP”). In determining MBESP, we consider the following: (1) our pricing practices as they relate to future price increases, (2) the overall economic conditions, and (3) competitor pricing. Revenue from the sale of the Company’s reagents (both with and without price guarantees) to end users is primarily recognized upon shipment when both title and risk of loss transfer to the customer, unless there are specific contractual terms to the contrary. Revenue from the sale of the Company’s reagents to distributors is recognized FOB customs clearance when both title and risk of loss transfer to the customer. |
F-53
Table of Contents
| • | Instruments—the selling price of our instruments is based on MBESP. In determining MBESP, we consider the following: (1) the overall economic conditions, (2) the expected profit margin to be realized on the instruments, and (3) competitor pricing. Revenue from instrument sales is recognized when the instrument has been installed and accepted by the customer. |
| • | Consumables (part kits)—the selling price of our consumables is based on MBESP. In determining MBESP, we consider the following: (1) the overall economic conditions, (2) the expected profit margin to be realized on the agreement, and (3) competitor pricing. Revenue from consumables is recognized when the consumables have been delivered. |
| • | Training—the selling price of training is based on VSOE of fair value by reference to the price our customers are required to pay for training when training services are sold separately (through additional/incremental training sessions). Revenue from training services is recognized as the training services are provided. |
| • | General Support Agreements—the selling price of general support agreements is based on VSOE by reference to the price our customers are required to pay for the general support agreements when sold separately via renewals. Revenue from general support agreements is recognized over the term of the agreement. |
| • | Instrument leases—the selling price of instrument leases is based on MBESP. In determining MBESP, we consider the following: (1) the overall economic conditions, (2) the expected profit margin to be realized on the agreement, and (3) competitor pricing. Revenue from instrument leases is recognized over the term of the operating lease, which is generally 60 months. |
The adoption of ASU 2009-13 did not have a material impact on the Company’s consolidated financial statements.
12. SHARE-BASED COMPENSATION
Predecessor share-based compensation
Plan summary
The Company has a Long-Term Incentive Plan that was approved by the shareholders in 2005 (the “2005 Plan”). Under the 2005 Plan, the Company is able to award stock options, stock appreciation rights, restricted stock, deferred stock, and other performance-based awards as incentive and compensation to employees and directors. The 2005 Plan provides for accelerated vesting of option and restricted stock awards if there is a change in control, as defined in the plan.
Plan activity
In an annual group grant in June 2011, the Company issued 162,535 performance based units and 228,890 restricted stock units with a grant date fair value of $19.85. These units had an original vesting period of 3 years.
Compensation expense
Share-based compensation of the predecessor reflects the fair value of employee share-based awards, including options, restricted stock, restricted stock units and performance units, which is typically recognized as expense on a straight line basis over the requisite service period of the award.
Immediately prior to the Acquisition, all outstanding awards became fully vested and the unrecognized compensation expense was recognized.
F-54
Table of Contents
A summary of share-based compensation recorded in the statements of income is as follows:
| Successor | Predecessor | |||||||||||||
| August 20, 2011 through August 31, 2011 |
June 1, 2011 through August 19, 2011 |
Three Months Ended August 31, 2010 |
||||||||||||
| (in thousands) | (in thousands) | |||||||||||||
| Share-based compensation |
$ | — | $ | 16,233 | $ | 1,739 | ||||||||
|
|
|
|
|
|
|
|||||||||
13. INCOME TAXES
As a result of the Acquisition, the Company will have a short tax year that coincides with the Predecessor period ending August 19, 2011. As such, the income tax provision for the Predecessor period reflects the income tax results that are expected to be reported on the short period return ending August 19, 2011. For the Successor 2012 period, the Company estimated its annual effective rate based on projected taxable income for the remainder of the year, adjusting as necessary for discrete events occurring in a particular period. The effective tax rate is applied to pre-tax book income to arrive at a tax provision for the period.
The effective tax rate for the Successor 2012 period, the Predecessor 2012 period, and the Predecessor 2011 period was 38.3%, (72.6)% and 34.1%, respectively. The difference between the federal statutory rate of 35% and the effective tax rate for the 11 day period ending August 31, 2011 primarily relates to state income taxes, valuation allowance on certain state net operating losses, and federal R&D credits earned. The difference between the federal statutory rate and the effective tax rate for the 79 day period ending August 19, 2011 primarily relates to the income taxes associated with the repatriation of foreign earnings in excess of foreign tax credits earned, the non-deductibility of certain transaction costs, and state income taxes.
Deferred income taxes reflect the net tax effects of: (a) temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and income tax purposes; and (b) operating loss and credit carry-forwards. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. In accounting for the Acquisition, the Company recorded a deferred tax liability of approximately $291.7 million associated with acquired intangible assets that have no income tax basis. This accounts for a significant portion of our gross deferred tax liabilities of approximately $309.3 million at August 31, 2011. These liabilities are offset by a gross deferred asset of approximately $36.9 million, which consists primarily of deferred tax assets associated with net operating loss carry-forwards.
In the Predecessor periods, the Company considered its investment in foreign subsidiaries to be permanently invested. Accordingly, no deferred tax liabilities were provided for on its investments in foreign subsidiaries. Subsequent to the Acquisition, the Company no longer considers itself to be permanently reinvested with respect to its accumulated and unrepatriated earnings as well as the future earnings of each foreign subsidiary. Accordingly, the Company has recorded a deferred tax liability associated its accumulated and unrepatriated earnings through the Acquisition date and will provide for deferred taxes on future earnings of its foreign subsidiaries. The Company continues to consider its investment in each foreign subsidiary in excess of its accumulated and unrepatriated earnings to be permanently reinvested and thus has not recorded a deferred tax liability on that amount.
14. SEGMENT AND GEOGRAPHIC INFORMATION
The Company’s operations and segments are organized around geographic areas. The foreign locations principally function as distributors of products primarily developed and manufactured by the Company in the United States and Canada. The accounting policies applied in the preparation of the Company’s consolidated financial statements are applied consistently across all segments. Intersegment sales are recorded at market price and are eliminated in consolidation. The Other segment consists of Canada and Japan.
F-55
Table of Contents
Net sales by product group and segment information for the three months ended August 31, 2011, separated into Predecessor and Successor periods, and the three months ended August 31, 2010 is summarized below (in thousands).
| Successor | Predecessor | |||||||||||
| August 20, 2011 to August 31, 2011 |
June 1, 2011 to August 19, 2011 |
June 1, 2010 to August 31, 2010 |
||||||||||
| Sales by product group: |
||||||||||||
| Traditional reagents |
$ | 6,296 | $ | 42,936 | $ | 49,621 | ||||||
| Capture reagents |
3,254 | 21,239 | 21,644 | |||||||||
| Instruments |
1,619 | 9,457 | 10,946 | |||||||||
| Molecular immunohematology |
221 | 1,278 | 1,430 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net sales |
$ | 11,390 | $ | 74,910 | $ | 83,641 | ||||||
|
|
|
|
|
|
|
|||||||
| Successor | ||||||||||||||||||||
| August 20, 2011 through August 31, 2011 | ||||||||||||||||||||
| U.S. | Europe | Other | Elims | Consolidated | ||||||||||||||||
| Sales: |
||||||||||||||||||||
| Unaffiliated customers |
$ | 7,530 | $ | 2,693 | $ | 1,167 | $ | — | $ | 11,390 | ||||||||||
| Affiliates |
375 | 510 | — | (885 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net Sales |
$ | 7,905 | $ | 3,203 | $ | 1,167 | $ | (885 | ) | $ | 11,390 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) from operations |
(161 | ) | 231 | 35 | (657 | ) | (552 | ) | ||||||||||||
| Goodwill |
696,600 | 163,588 | 115,184 | — | 975,372 | |||||||||||||||
| Intangible assets |
683,374 | 49,830 | 45,152 | — | 778,356 | |||||||||||||||
| Total assets at period end |
2,024,731 | 304,368 | 178,619 | (493,849 | ) | 2,013,869 | ||||||||||||||
| Predecessor | ||||||||||||||||||||
| June 1, 2011 through August 19, 2011 | ||||||||||||||||||||
| U.S. | Europe | Other | Elims | Consolidated | ||||||||||||||||
| Sales: |
||||||||||||||||||||
| Unaffiliated customers |
$ | 52,364 | $ | 15,100 | $ | 7,446 | $ | — | $ | 74,910 | ||||||||||
| Affiliates |
3,679 | 3,992 | 110 | (7,781 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net Sales |
$ | 56,043 | $ | 19,092 | $ | 7,556 | $ | (7,781 | ) | $ | 74,910 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) from operations |
(10,262 | ) | 1,619 | 2,171 | (36 | ) | (6,508 | ) | ||||||||||||
| Goodwill |
70,946 | 7,239 | 15,880 | — | 94,065 | |||||||||||||||
| Intangible assets |
45,871 | 1,139 | 6,592 | — | 53,602 | |||||||||||||||
| Total assets at period end |
732,603 | 92,440 | 39,199 | (211,847 | ) | 652,395 | ||||||||||||||
| Predecessor | ||||||||||||||||||||
| For the Three Months Ended August 31, 2010 | ||||||||||||||||||||
| U.S. | Europe | Other | Elims | Consolidated | ||||||||||||||||
| Sales: |
||||||||||||||||||||
| Unaffiliated customers |
$ | 59,801 | $ | 16,294 | $ | 7,546 | $ | — | $ | 83,641 | ||||||||||
| Affiliates |
3,643 | 4,338 | 59 | (8,040 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net Sales |
$ | 63,444 | $ | 20,632 | $ | 7,605 | $ | (8,040 | ) | $ | 83,641 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income (loss) from operations |
26,670 | 2,654 | 2,894 | (16 | ) | 32,202 | ||||||||||||||
| Goodwill |
73,881 | 6,500 | 14,582 | — | 94,963 | |||||||||||||||
| Intangible assets |
49,807 | 1,209 | 6,078 | — | 57,094 | |||||||||||||||
| Total assets at period end |
620,342 | 77,056 | 53,724 | (201,771 | ) | 549,351 | ||||||||||||||
F-56
Table of Contents
Net export sales to unaffiliated customers (in thousands):
| Successor | Predecessor | |||||||||||||
| August 20, 2011 through August 31, 2011 |
June 1, 2011 through August 19, 2011 |
Three Months Ended August 31, 2010 |
||||||||||||
| United States |
$ | 158 | $ | 1,417 | $ | 1,508 | ||||||||
| Europe |
219 | 964 | 1,669 | |||||||||||
| Other |
59 | 526 | 599 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| Total net export sales |
$ | 436 | $ | 2,907 | $ | 3,776 | ||||||||
|
|
|
|
|
|
|
|||||||||
15. CONDENSED CONSOLIDATING FINANCIAL INFORMATION OF GUARANTOR SUBSIDIARIES
The Company has outstanding certain indebtedness that is guaranteed by all if its U.S. subsidiaries. However, the indebtedness is not guaranteed by the Company’s foreign subsidiaries. The guarantor subsidiaries are all wholly owned and the guarantees are made on a joint and several basis and are full and unconditional. Separate consolidated financial statements of the guarantor subsidiaries have not been presented because management believes that such information would not be material to investors. However, condensed consolidating financial information is presented. The condensed consolidating financial information of the Company is as follows:
F-57
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING BALANCE SHEETS
August 31, 2011
(Amounts in thousands)
| Successor | ||||||||||||||||||||
| Immucor, Inc. | Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| ASSETS |
||||||||||||||||||||
| CURRENT ASSETS: |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 17,827 | $ | — | $ | 7,418 | $ | (111 | ) | $ | 25,134 | |||||||||
| Accounts receivable, net |
27,533 | 348 | 39,681 | — | 67,562 | |||||||||||||||
| Intercompany receivable |
211 | 20 | 7,806 | (8,037 | ) | — | ||||||||||||||
| Inventories |
34,706 | 2,015 | 20,305 | 57,026 | ||||||||||||||||
| Deferred income tax assets, current portion |
3,825 | 76 | 321 | — | 4,222 | |||||||||||||||
| Prepaid expenses and other current assets |
2,542 | 27,010 | 2,089 | (26,569 | ) | 5,072 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current assets |
86,644 | 29,469 | 77,620 | (34,717 | ) | 159,016 | ||||||||||||||
| PROPERTY AND EQUIPMENT, Net |
44,286 | 952 | 20,167 | — | 65,405 | |||||||||||||||
| INVESTMENT IN SUBSIDIARIES |
474,708 | — | 10,805 | (485,513 | ) | — | ||||||||||||||
| GOODWILL |
689,986 | 6,614 | 278,772 | — | 975,372 | |||||||||||||||
| INTANGIBLE ASSETS, Net |
672,880 | 10,494 | 94,982 | — | 778,356 | |||||||||||||||
| DEFERRED INCOME TAX ASSETS |
230 | 6,889 | — | (6,889 | ) | 230 | ||||||||||||||
| DEFERRED FINANCING COSTS |
34,481 | — | — | — | 34,481 | |||||||||||||||
| OTHER ASSETS |
269 | 98 | 642 | — | 1,009 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total assets |
$ | 2,003,484 | $ | 54,516 | $ | 482,988 | $ | (527,119 | ) | $ | 2,013,869 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY |
||||||||||||||||||||
| CURRENT LIABILITIES: |
||||||||||||||||||||
| Accounts payable |
7,259 | 1,098 | 2,000 | (111 | ) | 10,246 | ||||||||||||||
| Intercompany payable |
11,191 | 30,505 | 6,086 | (47,782 | ) | — | ||||||||||||||
| Accrued expenses and other current liabilities |
10,829 | 1,000 | 13,062 | — | 24,891 | |||||||||||||||
| Income taxes payable |
33,163 | — | 5,039 | (26,570 | ) | 11,632 | ||||||||||||||
| Deferred revenue, current portion |
1,881 | 5 | 1,384 | — | 3,270 | |||||||||||||||
| Current portion of long term debt |
2,795 | — | — | — | 2,795 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current liabilities |
67,118 | 32,608 | 27,571 | (74,463 | ) | 52,834 | ||||||||||||||
| LONG TERM DEBT |
988,699 | — | — | — | 988,699 | |||||||||||||||
| DEFERRED REVENUE |
570 | — | 89 | — | 659 | |||||||||||||||
| DEFERRED INCOME TAX LIABILITIES |
250,930 | — | 30,242 | (6,888 | ) | 274,284 | ||||||||||||||
| OTHER LONG-TERM LIABILITIES |
18 | — | 1,226 | — | 1,244 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities |
1,307,335 | 32,608 | 59,128 | (81,351 | ) | 1,317,720 | ||||||||||||||
| SHAREHOLDERS’ EQUITY: |
||||||||||||||||||||
| Total shareholders’ equity |
696,149 | 21,908 | 423,860 | (445,768 | ) | 696,149 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities and shareholders’ equity |
$ | 2,003,484 | $ | 54,516 | $ | 482,988 | $ | (527,119 | ) | $ | 2,013,869 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-58
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING BALANCE SHEETS
May 31, 2011
(Amounts in thousands)
| Predecessor | ||||||||||||||||||||
| Immucor, Inc. | Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| ASSETS |
||||||||||||||||||||
| CURRENT ASSETS: |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 250,386 | $ | — | $ | 52,297 | $ | (80 | ) | $ | 302,603 | |||||||||
| Accounts receivable, net |
25,914 | 377 | 37,033 | — | 63,324 | |||||||||||||||
| Intercompany receivable |
310 | — | 5,925 | (6,235 | ) | — | ||||||||||||||
| Inventories |
20,756 | 1,253 | 10,905 | — | 32,914 | |||||||||||||||
| Deferred income tax assets, current portion |
11,118 | 4,466 | 300 | — | 15,884 | |||||||||||||||
| Prepaid expenses and other current assets |
8,723 | 24,895 | 2,100 | (24,554 | ) | 11,164 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current assets |
317,207 | 30,991 | 108,560 | (30,869 | ) | 425,889 | ||||||||||||||
| PROPERTY AND EQUIPMENT, Net |
39,101 | 856 | 18,259 | — | 58,216 | |||||||||||||||
| INVESTMENT IN SUBSIDIARIES |
247,571 | — | 5 | (247,576 | ) | — | ||||||||||||||
| GOODWILL |
17,803 | 53,143 | 22,821 | — | 93,767 | |||||||||||||||
| INTANGIBLE ASSETS, Net |
1,050 | 45,695 | 7,388 | — | 54,133 | |||||||||||||||
| DEFERRED INCOME TAX ASSETS |
1,691 | — | 178 | (1,869 | ) | — | ||||||||||||||
| DEFERRED FINANCING COSTS |
— | — | — | — | — | |||||||||||||||
| OTHER ASSETS |
— | 99 | 647 | 376 | 1,122 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total assets |
$ | 624,423 | $ | 130,784 | $ | 157,858 | $ | (279,938 | ) | $ | 633,127 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY |
||||||||||||||||||||
| CURRENT LIABILITIES: |
||||||||||||||||||||
| Accounts payable |
6,324 | 1,463 | 3,083 | (80 | ) | 10,790 | ||||||||||||||
| Intercompany payable |
630 | 25,227 | 11,829 | (37,686 | ) | — | ||||||||||||||
| Accrued expenses and other current liabilities |
9,824 | 1,568 | 8,939 | — | 20,331 | |||||||||||||||
| Income taxes payable |
29,035 | — | 3,813 | (24,554 | ) | 8,294 | ||||||||||||||
| Deferred revenue, current portion |
4,778 | 31 | 2,686 | — | 7,495 | |||||||||||||||
| Current portion of long term debt |
— | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current liabilities |
50,591 | 28,289 | 30,350 | (62,320 | ) | 46,910 | ||||||||||||||
| LONG TERM DEBT |
— | — | — | — | — | |||||||||||||||
| DEFERRED REVENUE |
4,183 | 22 | 1,875 | — | 6,080 | |||||||||||||||
| DEFERRED INCOME TAX LIABILITIES |
— | 9,973 | 784 | (1,493 | ) | 9,264 | ||||||||||||||
| OTHER LONG-TERM LIABILITIES |
777 | — | 1,224 | — | 2,001 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities |
55,551 | 38,284 | 34,233 | (63,813 | ) | 64,255 | ||||||||||||||
| SHAREHOLDERS’ EQUITY: |
||||||||||||||||||||
| Total shareholders’ equity |
568,872 | 92,500 | 123,625 | (216,125 | ) | 568,872 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities and shareholders’ equity |
$ | 624,423 | $ | 130,784 | $ | 157,858 | $ | (279,938 | ) | $ | 633,127 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-59
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING STATEMENTS OF OPERATIONS
August 20, 2011 through August 31, 2011
(Amounts in thousands)
| Successor | ||||||||||||||||||||
| Immucor, Inc. | Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| NET SALES |
$ | 7,720 | $ | 185 | $ | 4,370 | $ | (885 | ) | $ | 11,390 | |||||||||
| COST OF SALES |
||||||||||||||||||||
| (exclusive of amortization shown separately below) |
4,835 | 226 | 2,980 | (885 | ) | 7,156 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| GROSS MARGIN |
2,885 | (41 | ) | 1,390 | — | 4,234 | ||||||||||||||
| OPERATING EXPENSES: |
||||||||||||||||||||
| Research and development |
287 | 331 | 5 | — | 623 | |||||||||||||||
| Selling and marketing |
577 | 67 | 468 | — | 1,112 | |||||||||||||||
| Distribution |
399 | 3 | 247 | — | 649 | |||||||||||||||
| General and administrative |
455 | 57 | 242 | — | 754 | |||||||||||||||
| Amortization of intangibles |
1,480 | 6 | 162 | — | 1,648 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
3,198 | 464 | 1,124 | — | 4,786 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| INCOME (LOSS) FROM OPERATIONS |
(313 | ) | (505 | ) | 266 | — | (552 | ) | ||||||||||||
| NON-OPERATING INCOME (EXPENSE): |
||||||||||||||||||||
| Interest income |
— | — | 11 | (11 | ) | — | ||||||||||||||
| Interest expense |
(3,401 | ) | — | (3 | ) | 11 | (3,393 | ) | ||||||||||||
| Other, net |
— | — | (11 | ) | — | (11 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total non-operating income (expense) |
(3,401 | ) | — | (3 | ) | — | (3,404 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| INCOME (LOSS) BEFORE INCOME TAXES |
(3,714 | ) | (505 | ) | 263 | — | (3,956 | ) | ||||||||||||
| PROVISION (BENEFIT) FOR INCOME TAXES |
(1,313 | ) | (177 | ) | (24 | ) | — | (1,514 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| NET INCOME (LOSS) BEFORE EARNINGS OF CONSOLIDATED SUBSIDIARIES |
(2,401 | ) | (328 | ) | 287 | — | (2,442 | ) | ||||||||||||
| Net Income (Loss) of consolidated subsidiaries |
(41 | ) | — | — | 41 | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| NET INCOME (LOSS) |
$ | (2,442 | ) | $ | (328 | ) | $ | 287 | $ | 41 | $ | (2,442 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-60
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING STATEMENTS OF OPERATIONS
June 1, 2011 through August 19, 2011
(Amounts in thousands)
| Predecessor | ||||||||||||||||||||
| Immucor, Inc. | Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| NET SALES |
$ | 55,063 | $ | 980 | $ | 26,648 | $ | (7,781 | ) | $ | 74,910 | |||||||||
| COST OF SALES |
||||||||||||||||||||
| (exclusive of amortization shown separately below) |
17,070 | 722 | 12,944 | (7,781 | ) | 22,955 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| GROSS MARGIN |
37,993 | 258 | 13,704 | — | 51,955 | |||||||||||||||
| OPERATING EXPENSES: |
||||||||||||||||||||
| Research and development |
2,390 | 2,471 | 34 | — | 4,895 | |||||||||||||||
| Selling and marketing |
5,321 | 568 | 4,621 | — | 10,510 | |||||||||||||||
| Distribution |
2,331 | 34 | 1,587 | — | 3,952 | |||||||||||||||
| General and administrative |
33,903 | 657 | 3,615 | — | 38,175 | |||||||||||||||
| Amortization of intangibles |
117 | 757 | 57 | — | 931 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
44,062 | 4,487 | 9,914 | — | 58,463 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| INCOME (LOSS) FROM OPERATIONS |
(6,069 | ) | (4,229 | ) | 3,790 | — | (6,508 | ) | ||||||||||||
| NON-OPERATING INCOME (EXPENSE): |
||||||||||||||||||||
| Interest income |
46 | — | 117 | (21 | ) | 142 | ||||||||||||||
| Interest expense |
— | — | (21 | ) | 21 | — | ||||||||||||||
| Other, net |
(246 | ) | 14 | 2,905 | — | 2,673 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total non-operating income (expense) |
(200 | ) | 14 | 3,001 | — | 2,815 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| INCOME (LOSS) BEFORE INCOME TAXES |
(6,269 | ) | (4,215 | ) | 6,791 | — | (3,693 | ) | ||||||||||||
| PROVISION (BENEFIT) FOR INCOME TAXES |
1,497 | (1,598 | ) | 2,782 | — | 2,681 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| NET INCOME (LOSS) BEFORE EARNINGS OF CONSOLIDATED SUBSIDIARIES |
(7,766 | ) | (2,617 | ) | 4,009 | — | (6,374 | ) | ||||||||||||
| Net Income (Loss) of consolidated subsidiaries |
1,392 | — | — | (1,392 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| NET INCOME (LOSS) |
$ | (6,374 | ) | $ | (2,617 | ) | $ | 4,009 | $ | (1,392 | ) | $ | (6,374 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-61
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING STATEMENTS OF OPERATIONS
Three Months Ended August 31, 2010
(Amounts in thousands)
| Predecessor | ||||||||||||||||||||
| Immucor, Inc. | Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| NET SALES |
$ | 62,303 | $ | 1,141 | $ | 28,237 | $ | (8,040 | ) | $ | 83,641 | |||||||||
| COST OF SALES |
||||||||||||||||||||
| (exclusive of amortization shown separately below) |
16,885 | 768 | 14,361 | (8,040 | ) | 23,974 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| GROSS MARGIN |
45,418 | 373 | 13,876 | — | 59,667 | |||||||||||||||
| OPERATING EXPENSES: |
||||||||||||||||||||
| Research and development |
2,039 | 2,348 | 38 | — | 4,425 | |||||||||||||||
| Selling and marketing |
4,472 | 447 | 4,223 | — | 9,142 | |||||||||||||||
| Distribution |
2,544 | 18 | 1,470 | — | 4,032 | |||||||||||||||
| General and administrative |
5,392 | 857 | 2,537 | — | 8,786 | |||||||||||||||
| Amortization of intangibles |
135 | 885 | 60 | — | 1,080 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
14,582 | 4,555 | 8,328 | — | 27,465 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| INCOME (LOSS) FROM OPERATIONS |
30,836 | (4,182 | ) | 5,548 | — | 32,202 | ||||||||||||||
| NON-OPERATING INCOME (EXPENSE): |
||||||||||||||||||||
| Interest income |
72 | — | 141 | — | 213 | |||||||||||||||
| Interest expense |
(10 | ) | — | (4 | ) | — | (14 | ) | ||||||||||||
| Other, net |
42 | (1 | ) | 56 | — | 97 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total non-operating income |
104 | (1 | ) | 193 | — | 296 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| INCOME (LOSS) BEFORE INCOME TAXES |
30,940 | (4,183 | ) | 5,741 | — | 32,498 | ||||||||||||||
| PROVISION (BENEFIT) FOR INCOME TAXES |
10,836 | (1,464 | ) | 1,707 | — | 11,079 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| NET INCOME (LOSS) BEFORE EARNINGS OF CONSOLIDATED SUBSIDIARIES |
20,104 | (2,719 | ) | 4,034 | — | 21,419 | ||||||||||||||
| Net Income (Loss) of consolidated subsidiaries |
1,315 | — | — | (1,315 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| NET INCOME (LOSS) |
$ | 21,419 | $ | (2,719 | ) | $ | 4,034 | $ | (1,315 | ) | $ | 21,419 | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-62
Table of Contents
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING CASH FLOW INFORMATION
August 20, 2011 through August 31, 2011
(Amounts in thousands)
| Successor | ||||||||||||||||||||
| Immucor, Inc. | Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| Net cash provided by (used in) operating activities |
$ | (11,908 | ) | $ | (7 | ) | $ | (1,198 | ) | $ | — | $ | (13,113 | ) | ||||||
| Net cash used in investing activities |
(1,940,133 | ) | (15 | ) | (146 | ) | — | (1,940,294 | ) | |||||||||||
| Net cash provided by financing activities |
1,655,564 | — | — | — | 1,655,564 | |||||||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
— | — | 14 | — | 14 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Increase (decrease) in cash and cash equivalents |
(296,477 | ) | (22 | ) | (1,330 | ) | — | (297,829 | ) | |||||||||||
| Cash and cash equivalents at beginning of period |
314,304 | (89 | ) | 8,748 | — | 322,963 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents at end of period |
$ | 17,827 | $ | (111 | ) | $ | 7,418 | $ | — | $ | 25,134 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING CASH FLOW INFORMATION
June 1, 2011 through August 19, 2011
(Amounts in thousands)
| Predecessor | ||||||||||||||||||||
| Immucor, Inc. | Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| Net cash provided by (used in) operating activities |
$ | 64,243 | $ | 144 | $ | (13,821 | ) | $ | (24,978 | ) | $ | 25,588 | ||||||||
| Net cash used in investing activities |
(393 | ) | (153 | ) | (1,719 | ) | — | (2,265 | ) | |||||||||||
| Net cash provided by (used in) financing activities |
68 | — | (25,085 | ) | 25,083 | 66 | ||||||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
— | — | (2,924 | ) | (105 | ) | (3,029 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Increase (decrease) in cash and cash equivalents |
63,918 | (9 | ) | (43,549 | ) | — | 20,360 | |||||||||||||
| Cash and cash equivalents at beginning of period |
250,386 | (80 | ) | 52,297 | — | 302,603 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents at end of period |
$ | 314,304 | $ | (89 | ) | $ | 8,748 | $ | — | $ | 322,963 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
IMMUCOR, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATING CASH FLOW INFORMATION
Three Months Ended August 31, 2010
(Amounts in thousands)
| Predecessor | ||||||||||||||||||||
| Immucor, Inc. | Guarantor | Non-Guarantors | Eliminations | Total | ||||||||||||||||
| Net cash provided by operating activities |
$ | 24,903 | $ | 254 | $ | 3,207 | $ | 532 | $ | 28,896 | ||||||||||
| Net cash used in investing activities |
(952 | ) | (104 | ) | (163 | ) | — | (1,219 | ) | |||||||||||
| Net cash used in financing activities |
(310 | ) | — | — | — | (310 | ) | |||||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
— | — | 1,029 | (532 | ) | 497 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Increase in cash and cash equivalents |
23,641 | 150 | 4,073 | — | 27,864 | |||||||||||||||
| Cash and cash equivalents at beginning of period |
171,144 | (278 | ) | 31,783 | — | 202,649 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents at end of period |
$ | 194,785 | $ | (128 | ) | $ | 35,856 | $ | — | $ | 230,513 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-63
Table of Contents
16. COMMITMENTS AND CONTINGENCIES
In October 2007, we reported that the Federal Trade Commission (“FTC”) was investigating whether Immucor violated federal antitrust laws or engaged in unfair methods of competition through three acquisitions made in the period from 1996 through 1999, and whether Immucor or others engaged in unfair methods of competition by restricting price competition. The FTC letter requested that we provide certain documents and information to the FTC concerning those acquisitions and concerning our product pricing activities since then. In July 2008, the FTC formalized its document and information requests into a Civil Investigative Demand (“CID”) and also required us to provide certain additional information within the same general scope of its previous requests. The FTC has also required that we provide to them the documents we provided to the Department of Justice (“DOJ”) as part of a DOJ investigation that was closed without further action in November 2010. We have been cooperating with the FTC and we intend to continue cooperating, and we are assured that the issuance of a formal CID does not indicate any dissatisfaction with our cooperation. As was previously the case, at this time we cannot reasonably assess the timing or outcome of the investigation or its effect, if any, on our business.
Beginning in May 2009, a series of class action lawsuits was filed against the Company, Ortho-Clinical Diagnostics, Inc. and Johnson & Johnson Health Care Systems, Inc. alleging that the defendants conspired to fix prices at which blood reagents are sold, asserting claims under Section 1 of the Sherman Act, and seeking declaratory and injunctive relief, treble damages, costs, and attorneys’ fees. All of these complaints make substantially the same allegations. The cases have been consolidated in the United States District Court for the Eastern District of Pennsylvania. In August 2010 the United States District Court for the Eastern District of Pennsylvania denied, in part, Motions to Dismiss for failure to state a cause of action and a Motion to Stay Discovery filed by the Company and co-defendant Ortho-Clinical Diagnostics, Inc. The defendants filed a Motion for Reconsideration or for Certification for Interlocutory Appeal with respect to the Court’s order on defendants’ motion to dismiss, and that motion was denied in December 2010. Discovery has now commenced in this litigation. No determination has been made whether any of the plaintiffs’ claims have merit or should be allowed to proceed as a class action. Briefing relating to potential certification of a class began in September 2011, with plaintiffs’ motion to certify a class. Defendants must reply by February 2012, and the Court will determine whether to hear argument on plaintiffs’ motion. A decision on class certification would occur after these filings and a hearing, if one is held. We intend to vigorously defend against these cases. At this time we cannot reasonably assess the timing or outcome of this litigation or its effect, if any, on our business.
Private securities litigation in the United States District Court of North Georgia against the Company and certain of its current and former directors and officers asserts federal securities fraud claims on behalf of a putative class of purchasers of the Company’s Common Stock between October 19, 2005 and June 25, 2009. The case alleges that the defendants violated Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended, by failing to disclose that Immucor had violated the antitrust laws, and challenges the sufficiency of the Company’s disclosures about the results of FDA inspections and the Company’s quality control efforts. On June 30, 2011, the District Court granted the defendants’ motion to dismiss the complaint and closed the case. Plaintiffs filed a motion to reconsider, which was denied. On September 28, 2011 plaintiffs filed a notice of appeal to the United States Circuit Court of Appeals for the Eleventh Circuit. The Company will defend the case vigorously if it is reinstated. At this time, the Company cannot reasonably assess the timing or outcome of this litigation or its effect, if any, on its business.
In July 2011, in connection with the Acquisition, a series of six putative class action lawsuits were filed in the Superior Courts of Fulton County and Gwinnett County, Georgia, captioned as Hillary Kramer v. Immucor, Inc., et al., Civil Action No. 2011CV203124 (Fulton County), Babette C. Schorsch v. Immucor, Inc., et al., Civil Action No. 11A0776-1 (Gwinnett County), Allan Pillay v. Immucor, Inc., et al., Civil Action No. 2011CV203339 (Fulton County), Larry Macintyre v. Immucor, Inc., et al., Civil Action No. 2011CV203397 (Fulton County), Irene Dixon v. Immucor, Inc., et al., Civil Action No. 2011CV203567 (Fulton County), and Gilbert Rosenthal v. Immucor, Inc., et al., Civil Action No. 11A079463 (Gwinnett County). All of these actions were brought on behalf of our public shareholders against, in various combinations, ourselves, our individual directors, certain of our executive officers, the Sponsor and certain of the Sponsor’s affiliates. The actions asserted claims for
F-64
Table of Contents
breaches of fiduciary duties against our board of directors in connection with the Acquisition, and for aiding and abetting the purported breaches of fiduciary duties by the Sponsor defendants. Some of the actions also included allegations that our Schedule 14D-9 filed with respect to the Acquisition failed to provide certain allegedly material information. The plaintiffs sought, among other things, preliminary and permanent relief, including injunctive relief enjoining the consummation of the Acquisition, rescission of the Acquisition to the extent it is consummated prior to the entry of a final judgment, and costs, expenses and disbursements of the action.
The Pillay case was dismissed without prejudice in August 2011; the Kramer, Macintyre and Dixon cases were dismissed without prejudice in September 2011; and the parties have agreed to stay the Rosenthal case in favor of the Schorsch case which remains pending in Gwinnett County. In the Schorsch case, which is the only case currently active, in August 2011 the Court denied plaintiff’s motion for preliminary injunction; and in October 2011 the plaintiff filed a Second Amended Complaint, which remains pending. The Court has not yet ruled on defendants’ motions to dismiss the Schorsch case. We will defend this case vigorously if it is not dismissed. At this time, we cannot reasonably assess the timing or outcome of this litigation or its effect, if any, on our business.
Other than as set forth above, we are not currently subject to any material legal proceedings, nor, to our knowledge, is any material legal proceeding threatened against us. However, from time to time, we may become a party to certain legal proceedings in the ordinary course of business.
17. RECENT ACCOUNTING PRONOUNCEMENTS
Adopted by the Company in fiscal 2012
In October 2009, the FASB issued ASU 2009-13, “Multiple Deliverables Revenue Arrangements” (“ASU 2009-13”), which is an amendment of ASC 605-25, “Revenue Recognition: Multiple Element Arrangements.” This update addresses the accounting for multiple-deliverable arrangements to allow the vendor to account for deliverables separately instead of as one combined unit by amending the criteria for separating consideration in multiple-deliverable arrangements. This guidance establishes a selling price hierarchy for determining the selling price of a deliverable, which is based on (a) vendor-specific objective evidence, (b) third-party evidence or (c) best estimate of selling price. The residual method of allocation has been eliminated and arrangement consideration is now required to be allocated to all deliverables at the inception of the arrangement using the selling price method. Additionally, expanded disclosures will be required relating to multiple deliverable revenue arrangements. This update is effective for fiscal years beginning on or after June 15, 2010. The adoption of ASU 2009-13 during the first quarter of fiscal 2012 did not have a material impact on the Company’s consolidated financial statements.
Not yet adopted by the Company
In June 2011, the FASB issued ASU 2011-05, “Presentation of Comprehensive Income” (“ASU 2011-05”), which was issued to enhance comparability between entities that report under U.S. GAAP and IFRS, and to provide a more consistent method of presenting non-owner transactions that affect an entity’s equity. ASU 2011-05 eliminates the option to report other comprehensive income and its components in the statement of changes in stockholders’ equity and requires an entity to present the total of comprehensive income, the components of net income and the components of other comprehensive income either in a single continuous statement or in two separate but consecutive statements. This pronouncement is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011, which corresponds to the Company’s first quarter of fiscal 2013. Early adoption of the new guidance is permitted and full retrospective application is required. The Company is currently evaluating the effect that the provisions of this pronouncement will have on its financial statements.
In September 2011, the FASB issued ASU 2011-08, “Testing Goodwill for Impairment” (“ASU 2011-08”), which simplifies testing for impairment by allowing an entity to first assess qualitative factors and determine if it
F-65
Table of Contents
is more likely than not (defined as 50% or more) that the fair value of the reporting unit is less than its carrying amount. That determination can then be used to decide if it is necessary to perform the two-step goodwill impairment test. ASU 2011-08 is effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011, which corresponds to the Company’s first quarter of fiscal 2013. Early adoption is permitted in certain circumstances. The Company is currently evaluating the effect that the provisions of this pronouncement will have on its financial statements.
18. SUBSEQUENT EVENTS
The Company has evaluated subsequent events through November 21, 2011, the date these financial statements were available to be issued. There have been no subsequent events after August 31, 2011 for which disclosure is required.
F-66
Table of Contents

IMMUCOR, INC.
Offer to Exchange
$400,000,000 aggregate principal amount of its 11.125% Senior Notes due 2019, the issuance of which has been registered under the Securities Act of 1933,
for
any and all of its outstanding 11.125% Senior Notes due 2019.
PROSPECTUS
Until the date that is 90 days from the date of this prospectus, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters with respect to their unsold allotments or subscriptions.