EXHIBIT 99
CEL-SCI CORPORATION
Common
Stock
THESE
SECURITIES INVOLVE A HIGH DEGREE OF RISK. SEE "RISK
FACTORS".
THESE
SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE SECURITIES
AND EXCHANGE COMMISSION OR ANY STATE SECURITIES COMMISSION, NOR HAS
THE COMMISSION OR ANY STATE SECURITIES COMMISSION PASSED UPON THE
ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE
CONTRARY IS A CRIMINAL OFFENSE.
This
Prospectus relates to shares (the "Shares") of common stock (the
"Common Stock") of CEL-SCI Corporation which may be issued pursuant
to certain employee compensation plans adopted by CEL-SCI. The
employee compensation plans provide for the grant, to selected
employees of CEL-SCI and other persons, of either shares of
CEL-SCI’s common stock or options to purchase shares of
CEL-SCI’s common stock. Persons who received Shares pursuant
to the Plans and who are offering such shares to the public by
means of this Prospectus are referred to as the "Selling
Shareholders".
CEL-SCI
has Incentive Stock Option Plans, Non-Qualified Stock Option Plans,
Stock Bonus Plans, Stock Compensation Plans and a 2014 Incentive
Stock Bonus Plan. In some cases these plans are collectively
referred to as the "Plans". The terms and conditions of any stock
grants and the terms and conditions of any options, including the
price of the shares of Common Stock issuable on the exercise of
options, are governed by the provisions of the respective Plans and
any particular agreements between CEL-SCI and the Plan
participants.
The
Selling Shareholders may offer the shares from time to time in
negotiated transactions in the over-the-counter market, at fixed
prices which may be changed from time to time, at market prices
prevailing at the time of sale, at prices related to such
prevailing market prices or at negotiated prices. The Selling
Shareholders may effect such transactions by selling the Shares to
or through securities broker/dealers, and such broker/dealers may
receive compensation in the form of discounts, concessions, or
commissions from the Selling Shareholders and/or the purchasers of
the Shares for whom such broker/dealers may act as agent or to whom
they sell as principal, or both (which compensation as to a
particular broker/dealer might be in excess of customary
commissions). See "Selling Shareholders" and "Plan of
Distribution".
None of
the proceeds from the sale of the Shares by the Selling
Shareholders will be received by CEL-SCI. CEL-SCI has agreed to
bear all expenses (other than underwriting discounts, selling
commissions and fees and expenses of counsel and other advisers to
the Selling Shareholders). CEL-SCI has agreed to indemnify the
Selling Shareholders against certain liabilities, including
liabilities under the Securities Act of 1933, as amended (the
"Securities Act").
1
The
purchase of the securities offered by this prospectus involves a
high degree of risk. Risk factors include the lack of revenues and
history of loss, need for additional capital and need for FDA
approval. See the “Risk Factors” section of this
prospectus, beginning on page 17, for additional Risk
Factors.
Neither
the Securities and Exchange Commission nor any state securities
commission has approved or disapproved of these securities or has
passed upon the accuracy or adequacy of this prospectus. Any
representation to the contrary is a criminal offense.
The
date of this Prospectus is February __, 2018.
2
AVAILABLE INFORMATION
CEL-SCI
is subject to the information requirements of the Securities
Exchange Act of 1934 (the "Exchange Act") and in accordance
therewith, files reports and other information with the Securities
and Exchange Commission (the "Commission"). Proxy statements,
reports and other information concerning CEL-SCI can be inspected
and copied at the Commission's office at 100 F Street, NE,
Washington, D.C. 20549. Certain information concerning CEL-SCI is
also available at the Internet Web Site maintained by the
Securities and Exchange Commission at www.sec.gov. This Prospectus
does not contain all information set forth in the Registration
Statement of which this Prospectus forms a part and exhibits
thereto which CEL-SCI has filed with the Commission under the
Securities Act and to which reference is hereby made.
DOCUMENTS INCORPORATED BY REFERENCE
The
following documents filed with the Commission by CEL-SCI
(Commission File No. 001-11889) are incorporated by reference into
this prospectus:
●
our Annual Report
on Form 10-K for the fiscal year ended September 30,
2017;
●
our Current Reports
on Form 8-K filed with the SEC on October 6, 2017, November 3,
2017, November 22, 2017, December 1, 2017, December 12, 2017,
December 20, 2017, December 21, 2017, January 4, 2018 and January
16, 2018;
●
the description of
our common stock contained in our Registration Statement on Form
8-A filed with the SEC on July 2, 1996 and all amendments and
reports updating that description; and the description of our
Series S warrants contained in our Registration Statement on Form
8-A filed with the SEC on January 3, 2014 and all amendments and
reports updating that description.
CEL-SCI
will provide, without charge, to each person to whom a copy of this
Prospectus is delivered, including any beneficial owner, upon the
written or oral request of such person, a copy of any or all of the
documents incorporated by reference herein (other than exhibits to
such documents, unless such exhibits are specifically incorporated
by reference into this Prospectus). Requests should be directed
to:
CEL-SCI
Corporation
8229
Boone Blvd., Suite 802
Vienna,
Virginia 223l4
(703)
506-9460
Attention:
Secretary
3
All
documents filed with the Commission by CEL-SCI pursuant to Sections
13(a), 13(c), 14 or 15(d) of the Exchange Act subsequent to the
date of this prospectus and prior to the termination of this
offering shall be deemed to be incorporated by reference into this
prospectus and to be a part of this prospectus from the date of the
filing of such documents. Any statement contained in a document
incorporated or deemed to be incorporated by reference shall be
deemed to be modified or superseded for the purposes of this
prospectus to the extent that a statement contained in this
prospectus or in any subsequently filed document which also is or
is deemed to be incorporated by reference in this prospectus
modifies or supersedes such statement. Such statement so modified
or superseded shall not be deemed, except as so modified or
superseded, to constitute a part of this prospectus.
Investors are
entitled to rely upon information in this prospectus or
incorporated by reference at the time it is used by CEL-SCI to
offer and sell securities, even though that information may be
superseded or modified by information subsequently incorporated by
reference into this prospectus.
CEL-SCI
has filed with the Securities and Exchange Commission a
Registration Statement under the Securities Act of l933, as
amended, with respect to the securities offered by this prospectus.
This prospectus does not contain all of the information set forth
in the Registration Statement. For further information with respect
to CEL-SCI and such securities, reference is made to the
Registration Statement and to the exhibits filed with the
Registration Statement. Statements contained in this prospectus as
to the contents of any contract or other documents are summaries
which are not necessarily complete, and in each instance reference
is made to the copy of such contract or other document filed as an
exhibit to the Registration Statement, each such statement being
qualified in all respects by such reference. The Registration
Statement and related exhibits may also be examined at the
Commission’s internet site (www.sec.gov).
4
TABLE OF CONTENTS
|
|
Page
|
|
|
|
|
THE COMPANY
|
6
|
|
|
|
|
FORWARD LOOKING STATEMENTS
|
16
|
|
|
|
|
RISK FACTORS
|
17
|
|
|
|
|
COMPARATIVE SHARE DATA
|
41
|
|
|
|
|
DILUTION
|
43
|
|
|
|
|
USE OF PROCEEDS
|
43
|
|
|
|
|
MARKET FOR CEL-SCI’S COMMON STOCK
|
44
|
|
|
|
|
SELLING SHAREHOLDERS
|
45
|
|
|
|
|
PLAN OF DISTRIBUTION
|
49
|
|
|
|
|
DESCRIPTION OF SECURITIES
|
50
|
5
THE COMPANY
CEL-SCI
Corporation was formed as a Colorado corporation in 1983.
CEL-SCI’s principal office is located at 8229 Boone
Boulevard, Suite 802, Vienna, VA 22182. CEL-SCI’s
telephone number is 703-506-9460 and its web site is
www.cel-sci.com. CEL-SCI does not incorporate the
information on its website into this prospectus, and you should not
consider it part of this prospectus.
CEL-SCI makes its
electronic filings with the Securities and Exchange Commission
(SEC), including its annual reports on Form 10-K, quarterly reports
on Form 10-Q, current reports on Form 8-K and amendments to these
reports available on its website free of charge as soon as
practicable after they are filed or furnished to the
SEC.
In this
prospectus, unless otherwise specified or the context requires
otherwise, the terms “CEL-SCI,” the
“Company,” “we,” “us” and
“our” to refer to CEL-SCI Corporation. Our fiscal year
ends on September 30.
CEL-SCI’S PRODUCTS
We are
dedicated to research and development directed at improving the
treatment of cancer and other diseases by using the immune system,
the body’s natural defense system. We are currently focused
on the development of the following product candidates and
technologies:
1)
Multikine®
(Leukocyte Interleukin, Injection), or Multikine, an
investigational immunotherapy under development for
the potential treatment of certain head and neck
cancers;
2)
L.E.A.P.S. (Ligand
Epitope Antigen Presentation System) technology, or LEAPS, with two
investigational therapies, LEAPS-H1N1-DC, a product candidate under
development for the potential treatment of pandemic influenza in
hospitalized patients, and CEL-2000 and CEL-4000, vaccine product
candidates under development for the potential treatment of
rheumatoid arthritis.
MULTIKINE
Our
lead investigational therapy, Multikine, is currently being
developed as a potential therapeutic agent directed at using the
immune system to produce an anti-tumor immune response. Data from
Phase 1 and Phase 2
clinical trials suggest that Multikine may help the immune system
“see” the tumor and then attack it, enabling the
body’s own anti-tumor immune response to fight the tumor.
Multikine is the trademark we have registered for this
investigational therapy, and this proprietary name is subject to
review by the U.S. Food and Drug Administration, or FDA, in
connection with our future anticipated regulatory submission for
approval. Multikine
has not been licensed or approved for sale, barter or exchange by
the FDA or any other regulatory agency, such as the European
Medicine Agency, or EMA. Neither has its safety or efficacy been
established for any use.
Multikine is an
immunotherapy product candidate comprised of a patented defined
mixture of 14 human natural cytokines and is manufactured in a
proprietary manner in our manufacturing facility. We spent over 10
years and more than $80 million developing and validating the
manufacturing process for Multikine. The pro-inflammatory cytokine
mixture includes interleukins, interferons, chemokines and
colony-stimulating factors, which contain elements of the
body’s natural mix of defenses against cancer.
6
Multikine is
designed to be used in a different way than immune therapy is
generally being used. Generally, immunotherapy is given to patients
who have already failed other treatments of such as surgery,
radiation and/or chemotherapy and most of the time it is
administered systemically. Multikine on the other hand is
administered locally to treat tumors and their microenvironments
before any other therapy has been administered because it is
believed that is the time when the immune system is thought to be
most amenable to activation against the tumor. For example, in the Phase 3 clinical trial,
Multikine is injected locally at the site of the tumor and near the
adjacent draining lymph nodes as a first line of treatment before
surgery, radiation and/or chemotherapy because that is when the
immune system is thought to be strongest. The goal is to help the
intact immune system recognize and kill the tumor micro metastases
that usually cause recurrence of the cancer. In short, we believe
that the local administration and administration of Multikine and
its administration before weakening of the immune system by
chemotherapy and radiation will result in better anti-tumor
response than if Multikine were administered as a second- or
later-line therapy. In clinical studies of Multikine,
administration of the investigational therapy to head and neck
cancer patients has demonstrated the potential for lesser or no
appreciable toxicity.
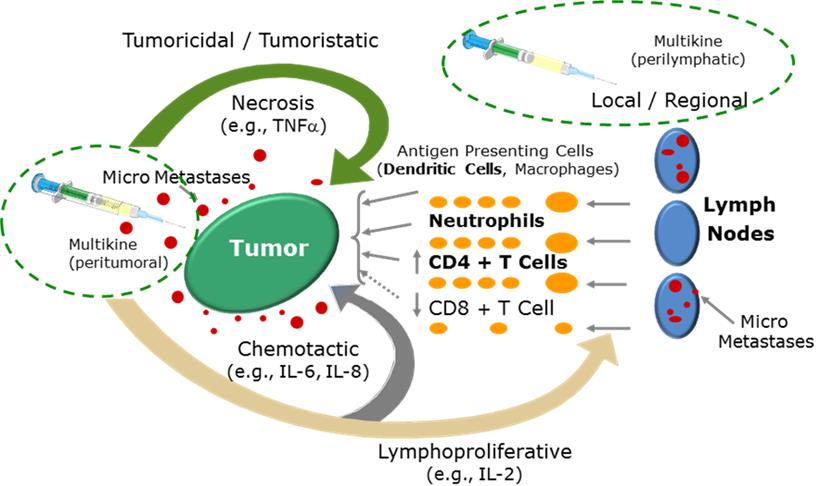
Source: Adapted from Timar et al., Journal of Clinical Oncology
23(15) May 20, 2005
7
The
first indication CEL-SCI is pursuing for its investigational drug
product candidate Multikine is an indication for the neoadjuvant
therapy in patients with squamous cell carcinoma of the head and
neck, or SCCHN (hereafter also referred to as advanced primary head
and neck cancer).
SCCHN
is a type of head and neck cancer, and CEL-SCI believes that, in
the aggregate, there is a large, unmet medical need among head and
neck cancer patients. CEL-SCI believes the last FDA approval of a
therapy indicated for the treatment of advanced primary head and
neck cancer was over 50 years ago. In the aggregate, head and neck
cancer represents about 6% of the world’s cancer cases, with
approximately over 650,000 patients diagnosed worldwide each year,
and nearly 60,000 patients diagnosed annually in the United States.
Multikine investigational immunotherapy was granted Orphan Drug
designation for neoadjuvant therapy in patients with SCCHN by the
FDA in the United States.
8
CEL-SCI’s
Phase 3 clinical trial is currently primarily under the management
of two clinical research organizations, or CROs: ICON Inc., or
ICON, and Ergomed Clinical Research Limited, or
Ergomed.
The
Phase 3 study was designed with the objective that if the study
endpoint, which is an improvement in overall survival of the
subjects treated with the Multikine treatment regimen plus the
current standard of care (SOC) as compared to subjects treated with
the current SOC only, is satisfied, the study results are expected
to be used to support applications that we plan to submit to
regulatory agencies in order to seek commercial marketing approvals
for Multikine in major markets around the world. This assessment
can only be made when a certain number of deaths have occurred in
the two main comparator groups of the study.
The
primary endpoint for the protocol for this Phase 3 head and neck
cancer study required that a 10% increase in overall survival be
obtained in the Multikine group which also is administered CIZ (CIZ
= low dose (non-chemotherapeutic) of cyclophosphamide, indomethacin
and Zinc-multivitamins) all of which are thought to enhance
Multikine activity), plus Standard of Care (Surgery + Radiotherapy
or Chemoradiotherapy) arm of the study over the Control comparator
(Standard of Care alone) arm. As the study was designed, the final
determination of whether this endpoint had been successfully
reached could only be determined when 298 events (deaths) had
occurred in the combined comparator arms of the study.
Nine
hundred twenty-eight (928) newly diagnosed head and neck cancer
patients have been enrolled in this Phase 3 cancer study and all
the patients who have completed treatment continue to be followed
for protocol-specific outcomes in accordance with the Study
Protocol. The last patient was enrolled in the study in September
2016. Approximately 135 patients were enrolled in the study from
2011 to 2013, about 195 were enrolled in 2014, about 340 in 2015,
and about 260 in 2016. The study protocol assumed an overall
survival rate of about 55% at 3 years for the SOC treatment group
alone. At this point in the study the 928 patients enrolled in the
study are being followed-up as required by the study
protocol.
Since
CEL-SCI launched its Phase 3 clinical trial for Multikine, CEL-SCI
has incurred expenses of approximately $45.9 million as of
September 30, 2017 on direct costs for the Phase 3 clinical trial.
CEL-SCI estimates it will incur additional expenses of
approximately $13.0 million for the remainder of the Phase 3
clinical trial. It should be noted that this estimate is based only
on the information currently available in CEL-SCI’s contracts
with the Clinical Research Organizations responsible for managing
the Phase 3 clinical trial and does not include other related
costs, e.g., the manufacturing of the drug. This number may be
affected by the rate of death accumulation in the study, foreign
currency exchange rates, and many other factors, some of which
cannot be foreseen today. It is therefore possible that the cost of
the Phase 3 clinical trial will be higher than currently
estimated.
Throughout the
course of the Phase 3 study, an Independent Data Monitoring
Committee, or IDMC, has met periodically to review safety data from
the Phase 3 study, and the IDMC is expected to continue doing so
throughout the remainder of the Phase 3 study. At various points in
the study at which the IDMC has completed review of the safety data
and has issued recommendations, it has recommended that the Phase 3
study may continue. However, on one occasion, in the spring of
2014, the IDMC made a recommendation that the study be closed. This
recommendation by the IDMC was reversed following review of
additional information submitted by us, and the IDMC recommended
that the study continue. In the spring of 2016, with close to 800
patients enrolled, the IDMC made a recommendation that enrollment
in the Phase 3 study should stop, but that patients already
enrolled in the study should continue treatment and follow-up. We
responded to this letter and indicated we would address the
remaining three requests, generally relating to study design
considerations that were not part of the IDMC recommendation in
follow-up correspondence. Subsequent to this correspondence, we
submitted a complete response to the IDMC addressing all of their
requests, as well as providing to them a copy of the suggested
protocol amendment which was sent to the FDA for review and
comment, as was requested by the IDMC. The IDMC did not provide any
additional response following the additional submission to
them.
9
On
September 26, 2016, we received notice from the FDA that the Phase
3 clinical trial in advanced primary head and neck cancer has been
placed on partial clinical hold. In August 2017, the FDA removed
the clinical hold on the Phase 3 study and informed us that all
clinical trial activities under this Investigational New Drug
application (IND) may resume.
In
September 2016, the last patient was accrued to the study. A total
of 928 randomized patients were enrolled in the study as of that
date. In December 2016, and again in February 2017 the IDMC
reviewed the data from the Study, but offered no recommendation as
they awaited the outcome of discussions with the FDA regarding the
clinical hold, which was subsequently removed by the FDA on August
10, 2017.
On
December 7, 2017 CEL-SCI announced that the IDMC had completed its
most recent review of the Phase 3 study data. The data from all 928
enrolled patients were provided to the IDMC by the clinical
research organization (CRO) responsible for data management of this
Phase 3 study. The IDMC made the following observation and
recommendation, a) the IDMC saw no evidence of any significant
safety questions and b) the IDMC recommends continuing the
study.
On
December 11, 2017 we announced that the Phase 3 clinical study was
fully enrolled and will not need to enroll more
patients.
Ultimately, the
decision as to whether our drug product candidate is safe and
effective can only be made by FDA and/or by other regulatory
authorities based upon an assessment of all of the data from an
entire drug development program submitted as part of an application
for marketing approval. As detailed elsewhere in this prospectus,
the current Phase 3 clinical study for our investigational drug may
or may not be able to be used as the pivotal study supporting a
marketing application in the United States, and, if not, at least
one entirely new Phase 3 pivotal study would need to be conducted
to support a marketing application in the United
States.
Prior
to starting the Phase 3 study, we had tested Multikine in over 200
patients. The following is a summary of results from our last Phase
2 study conducted with Multikine. This study employed the same
treatment protocol as is being followed in our Phase 3
study:
●
Reported potential for improved
survival: In a follow-up analysis of the Phase 2 clinical
study population, which used the same dosage and treatment regimen
as is being used in the Phase 3 study, head and neck cancer
patients with locally advanced primary disease who received the
investigational therapy Multikine as first-line investigational
therapy, followed by surgery and radiotherapy, were reported by the
clinical investigators to have had a 63.2% overall survival, or OS,
rate at a median of 3.33 years from surgery. This percentage of OS
was arrived at as follows: of the 21 subjects enrolled in the Phase
2 study, the consent for the survival follow-up portion of the
study was received from 19 subjects. OS was calculated using the
entire treatment population that consented to the follow-up portion
of the study (19 subjects), including two subjects who, as later
determined by three pathologists blinded to the study, did not have
oral squamous cell carcinoma, or OSCC. These two subjects were thus
not evaluable per the protocol and were not included in the
pathology portion of the study for purposes of calculating complete
response rate, as described below, but were included in the OS
calculation. The overall survival rate of subjects receiving the
investigational therapy in this study was compared to the overall
survival rate that was calculated based upon a review of 55
clinical trials conducted in the same cancer population (with a
total of 7,294 patients studied), and reported in the peer reviewed
scientific literature between 1987 and 2007. Review of this
literature showed an approximate survival rate of 47.5% at 3.5
years from treatment. Therefore, the results of our final Phase 2
study were considered to be potentially favorable in terms of
overall survival, recognizing the limitations of this early-phase
study. It should be noted that an earlier investigational therapy
Multikine study appears to lend support to the overall survival
findings described above - Feinmesser et al Arch Otolaryngol. Surg.
2003. However, no definitive conclusions can be drawn from these
data about the potential efficacy or safety profile of this
investigational therapy. Moreover, further research is required,
and these results must be confirmed in the Phase 3 clinical trial
of this investigational therapy that is currently in progress.
Subject to completion of that Phase 3 clinical trial and the
FDA’s review and acceptance of our entire data set on this
investigational therapy, we believe that these early-stage clinical
trial results indicate the potential for our Multikine product
candidate to become a treatment for advanced primary head and neck
cancer, if approved.
10
●
Reported average of 50% reduction in tumor
cells in Phase 2 trials (based on 19 patients evaluable by
pathology, having OSCC): The clinical investigators who
administered the three-week Multikine treatment regimen used in the
Phase 2 study reported that, as was determined in a controlled
pathology study, Multikine administration appeared to have caused,
on average, the disappearance of about half of the cancer cells
present at surgery (as determined by histopathology assessing the
area of Stroma/Tumor (Mean+/- Standard Error of the Mean of the
number of cells counted per filed)) even before the start of
standard therapy, which normally includes surgery, radiation and
chemotherapy (Timar et al JCO 2005).
●
Reported 10.5% complete response in the final
Phase 2 trial (based on 19 patients evaluable by pathology, having
OSCC): The
clinical investigators who administered the three-week Multikine
investigational treatment regimen used in the Phase 2 study
reported that, as was determined in a controlled pathology study,
the tumor apparently was no longer present (as determined by
histopathology) in approximately 10.5% of evaluable patients with
OSCC (Timar et al JCO 2005). In the original study, 21 subjects
received Multikine, two of which were later excluded, as subsequent
analysis by three pathologists blinded to the study revealed that
these two patients did not have OSCC. Two subjects in this study
had a complete response, leaving a reported complete response rate
of two out of 19 assessable subjects with OSCC (or 10.5%) (Timar et
al, JCO 2005).
●
Adverse events reported in clinical
trials: In clinical trials conducted to date with the
Multikine investigational therapy, adverse events which have been
reported by the clinical investigators as possibly or probably
related to Multikine administration included pain at the injection
site, local minor bleeding and edema at the injection site,
diarrhea, headache, nausea, and constipation.
Subsequently, an
analysis on the 21 subjects originally treated with Multikine in
the study to evaluate overall survival was conducted, as described
above. In connection with the follow-up portion of the study for
overall survival, we also conducted an unreported post-hoc analysis
of complete response rate in the study population, which included
subjects who provided consent for the follow-up and who also had
OSCC. Two of the 21 subjects did not re-consent for follow-up, and
two of the remaining 19 subjects were excluded from the post-hoc
complete response rate analysis as they had previously been
determined by pathology analysis to not have OSCC. The two complete
responders with OSCC both consented to the follow-up study.
Therefore, the post-hoc analysis of complete response was based on
a calculation of the two complete responders out of 17 evaluable
subjects who consented to the follow-up analysis and who also had
OSCC (or 11.8%).
Furthermore, we
reported an overall response rate of 42.1% based on the number of
evaluable patients who experienced a favorable response to the
treatment, including those who experienced minor, major and
complete responses. Out of the 19 evaluable patients, two
experienced a complete response, two experienced a major response,
and four experienced a minor response to treatment. Thus, we
calculated the number of patients experiencing a favorable response
as eight patients out of 19 (or 42.1%) (Timar et al, JCO
2005).
11
The
clinical significance of these and other data, to date, from the
multiple Multikine clinical trials, is not yet known. These
preliminary clinical data do suggest the potential to demonstrate a
possible improvement in the clinical outcome for patients treated
with Multikine.
Peri-Anal Warts and Cervical Dysplasia in HIV/HPV Co-Infected
Patients
HPV is
a very common sexually transmitted disease in the United States and
other parts of the world. It can lead to cancer of the cervix,
penis, anus, esophagus and head and neck. Our focus in HPV,
however, is not on developing an antiviral for the potential
treatment or prevention of HPV in the general population. Instead,
our focus is on developing an immunotherapy product candidate
designed to be administered to patients who are immune-suppressed
by other diseases, such as HIV, and who are therefore less able or
unable to control HPV and its resultant or co-morbid diseases. Such
patients have limited treatment options available to
them.
One
condition that is commonly associated with both HIV and HPV is the
occurrence of anal intraepithelial dysplasia, or AIN, and anal and
genital warts. The incidence of AIN in HIV-infected people is
estimated to be about 25%. The incidence of anal HPV infection in
HIV-infected men who have sex with men, or MSM, is estimated to be
as high as 95%. In the aggregate, the United States and Europe have
about 875,000 HIV-infected patients with AIN (assuming AIN
prevalence of approximately 25% of the aggregate HIV-infected
population). Persistent HPV infection in the anal region is thought
to be responsible for up to 80% of anal cancers, and men and women
who are HIV positive have a 30-fold increase in their risk of anal
cancer. Persistent HPV infection can
also be a precursor to cervical cancer, as well as certain head and
neck cancers.
In
October 2013, we signed a cooperative research and development
agreement, or CRADA, with the U.S. Naval Medical Center, San Diego,
or the USNMC. Pursuant to this agreement, the USNMC was to conduct
a Phase 1 study, approved by the Human Subjects Institutional
Review Board, of our investigational immunotherapy, Multikine, in
HIV/HPV co-infected men and women with peri-anal warts. The purpose
of this study was to evaluate the safety and clinical impact of
Multikine as a potential treatment of peri-anal warts and assess
its effect on AIN in HIV/HPV co-infected men and
women.
In July
2015, we added a clinical site at the University of California, San
Francisco, or UCSF, and Key Opinion Leader, or KOL, to the ongoing
Phase 1 study. In August 2016, the U.S. Navy discontinued this
Phase 1 study because of difficulties in enrolling patients. UCSF
is continuing with the study. We will not continue with this
indication because 1) the patient enrollment is extremely slow and
2) we need to focus our resources on the Phase 3study.
In
October 2013, we entered into a co-development and profit sharing
agreement with Ergomed for development of Multikine as a potential
treatment of HIV/HPV co-infected men and women with peri-anal
warts.
The treatment
regimen for this Phase 1 study of up to 15 HIV/HPV co-infected
patient volunteers with peri-anal warts is identical to the regimen
that was used in an earlier Institutional Review Board-approved
Multikine Phase 1 study in HIV/HPV co-infected patients, which was
conducted at the University of Maryland. In that study, the Multikine
investigational therapy was administered to HIV/HPV co-infected
women with cervical dysplasia, resulting in visual and histological
evidence of clearance of lesions in three out of the eight
subjects.
12
Furthermore, in
this cervical dysplasia Phase 1 study, the number of HPV viral
sub-types in three volunteer subjects tested were reduced
post-treatment with Multikine, as opposed to pre-treatment, as
determined by in situ polymerase chain reaction performed on tissue
biopsy collected before and after Multikine treatment. As reported
by the investigators in the earlier study, the study volunteers,
except one subject volunteer, all appeared to tolerate the
treatment with no reported serious adverse events.
MANUFACTURING FACILITY
Before
starting the Phase 3 clinical trial, for reasons related to
regulatory considerations, the Company needed to build a dedicated
manufacturing facility to produce Multikine. This facility has been
completed and validated, and has produced multiple clinical lots
for the Phase 3 clinical trial. The facility has also passed review
by a European Union Qualified Person on several
occasions.
The
Company’s lease on the manufacturing facility expires on
October 31, 2028.
CEL-SCI
completed validation of its new manufacturing facility in January
2010. The state-of-the-art facility is being used to manufacture
Multikine for CEL-SCI’s Phase 3 clinical trial. In addition
to using this facility to manufacture Multikine, CEL-SCI, only if
the facility is not being used for Multikine, may offer the use of
the facility as a service to pharmaceutical companies and others,
particularly those that need to “fill and finish” their
drugs in a cold environment (4 degrees Celsius, or approximately 39
degrees Fahrenheit). Fill and finish is the process of filling
injectable drugs in a sterile manner and is a key part of the
manufacturing process for many medicines. However, priority will
always be given to Multikine as management considers the
preparation for a final marketing approval to be more important
than offering fill and finish services.
LEAPS
Our
patented T-cell Modulation Process, referred to as LEAPS (Ligand
Epitope Antigen Presentation System), uses
“heteroconjugates” to direct the body to choose a
specific immune response. LEAPS is designed to stimulate the human
immune system to more effectively fight bacterial, viral and
parasitic infections as well as autoimmune, allergies,
transplantation rejection and cancer, when it cannot do so on its
own. Administered like a vaccine, LEAPS combines T-cell binding
ligands with small, disease-associated peptide antigens, and may
provide a new method to treat and prevent certain
diseases.
The
ability to generate a specific immune response is important because
many diseases are often not combated effectively due to the
body’s selection of the “inappropriate” immune
response. The capability to specifically reprogram an immune
response may offer a more effective approach than existing vaccines
and drugs in attacking an underlying disease.
On
September 19, 2017, we announced that it has been awarded a Phase 2
Small Business Innovation Research (SBIR) grant in the amount of
$1.5 million from the National Institute of Arthritis Muscoskeletal
and Skin Diseases, which is part of the National Institutes of
Health (NIH). This grant will provide funding to allow us to
advance our first LEAPS product candidate, CEL-4000, towards an
Investigational New Drug (IND) application, by funding GMP
manufacturing, IND enabling studies, and additional mechanism of
action studies. The work is being conducted at our research
laboratory and Rush University Medical Center in Chicago, Illinois
in the laboratories of Tibor Glant, MD, Ph.D., The Jorge O. Galante
Professor of Orthopedic Surgery and Katalin Mikecz, MD, Ph.D.
Professor of Orthopedic Surgery & Biochemistry. The grant was
awarded based on published data described below by Dr. Glant's team
in collaboration with our showing that the administration of a
proprietary peptide using our LEAPS technology prevented the
development, and lessened the severity, including inflammation, of
experimental proteoglycan induced arthritis (PGIA or GIA) when it
was administered after the disease was induced in the
animals.
13
In July
2014, the Company announced that it has been awarded a Phase 1
Small Business Innovation Research (SBIR) grant in the amount of
$225,000 from the National Institute of Arthritis Muscoskeletal and
Skin Diseases, which is part of the National Institutes of Health.
The grant funded the development of our LEAPS technology as a
potential treatment for rheumatoid arthritis, an autoimmune disease
of the joints. The work was conducted at Rush University Medical
Center in Chicago, Illinois in the laboratories of Tibor Glant, MD,
Ph.D., The Jorge O. Galante Professor of Orthopedic Surgery;
Katalin Mikecz, MD, Ph.D. Professor of Orthopedic Surgery &
Biochemistry; and Allison Finnegan, Ph.D. Professor of
Medicine.
With
the support of the SBIR grant, CEL-SCI is developing two new drug
candidates, CEL-2000 and CEL-4000, as potential rheumatoid
arthritis therapeutic vaccines. The data from animal studies using
the CEL-2000 treatment vaccine demonstrated that it could be used
as an effective treatment against rheumatoid arthritis with fewer
administrations than those required by other anti-rheumatoid
arthritis treatments currently on the market for arthritic
conditions associated with the Th17 signature cytokine
TNF- . The data for
CEL-4000 indicates it could be effective against rheumatoid
arthritis cases where a Th1 signature cytokine (IFN-c) is dominant.
CEL-2000 and CEL-4000 have the potential to be a more
disease-specific therapy, significantly less expensive, act at an
earlier step in the disease process than current therapies and may
be useful in patients not responding to existing rheumatoid
arthritis therapies. CEL-SCI believes this represents a large unmet
medical need in the rheumatoid arthritis market.
In
February 2017 and November 2016, CEL-SCI announced new preclinical
data that demonstrate its investigational new drug candidate
CEL-4000 has the potential for use as a therapeutic vaccine to
treat rheumatoid arthritis. This efficacy study was supported in
part by the SBIR Phase I Grant and was conducted in collaboration
with Drs. Katalin Mikecz and Tibor Glant, and their research team
at Rush University Medical Center in Chicago, IL.
In
March 2015, CEL-SCI and its collaborators published a review
article on vaccine therapies for rheumatoid arthritis based in part
on work supported by the SBIR grant. The article is entitled
“Rheumatoid arthritis vaccine therapies: perspectives and
lessons from therapeutic Ligand Epitope Antigen Presentation System
vaccines for models of rheumatoid arthritis” and was
published in Expert Rev. Vaccines 1 - 18 and can be found at
http://www.ncbi.nlm.nih.gov/ pubmed/25787143.
In
August 2012, Dr. Zimmerman, CEL-SCI’s Senior Vice President
of Research, Cellular Immunology, gave a Keynote presentation at
the OMICS 2nd International Conference on Vaccines and Vaccinations
in Chicago. This presentation showed how the LEAPS peptides
administered altered only select cytokines specific for each
disease model, thereby improving the status of the test animals and
even preventing death and morbidity. These results support the
growing body of evidence that provides for its mode of action by a
common format in these unrelated conditions by regulation of Th1
(e.g., IL12 and IFN-c) and their action on reducing
TNF- and other
inflammatory cytokines as well as regulation of antibodies to these
disease associated antigens. This was also illustrated by a
schematic model showing how these pathways interact and result in
the overall effect of protection and regulation of cytokines in a
beneficial manner.
In
February 2010, CEL-SCI announced that its CEL-2000 vaccine
demonstrated that it was able to block the progression of
rheumatoid arthritis in a mouse model, where a Th17 signature
cytokine (TNF- ) is
dominant. The results were published in the scientific
peer-reviewed Journal of International Immunopharmacology (online
edition) in an article titled “CEL-2000: A Therapeutic
Vaccine for Rheumatoid Arthritis Arrests Disease Development and
Alters Serum Cytokine / Chemokine Patterns in the Bovine Collagen
Type II Induced Arthritis in the DBA Mouse Model” Int
Immunopharmacol. 2010 Apr; 10(4):412-21 http://www.
ncbi.nlm.nih.gov/pubmed/20074669.
14
Using
the LEAPS technology, CEL-SCI has created a potential peptide
treatment for H1N1 (swine flu) hospitalized patients. This LEAPS
flu treatment is designed to focus on the conserved, non-changing
epitopes of the different strains of Type A Influenza viruses
(H1N1, H5N1, H3N1, etc.), including “swine”,
“avian or bird”, and “Spanish Influenza”,
in order to minimize the chance of viral “escape by
mutations” from immune recognition. Therefore, one should
think of this treatment not really as an H1N1 treatment, but as a
potential pandemic flu treatment. CEL-SCI’s LEAPS flu
treatment contains epitopes known to be associated with immune
protection against influenza in animal models.
In
September 2009, the U.S. FDA advised CEL-SCI that it could proceed
with its first clinical trial to evaluate the effect of LEAPS-H1N1
treatment on the white blood cells of hospitalized H1N1 patients.
This followed an expedited initial review of CEL-SCI's regulatory
submission for this study proposal.
In
November 2009, CEL-SCI announced that The Johns Hopkins University
School of Medicine had given clearance for CEL-SCI’s first
clinical study to proceed using LEAPS-H1N1. Soon after the start of
the study, the number of hospitalized H1N1 patients dramatically
declined and the study was unable to complete the enrollment of
patients.
Additional work on
this treatment for the pandemic flu is being pursued in
collaboration with the National Institute of Allergy and Infectious
Diseases (NIAID), part of the National Institutes of Health, USA.
In May 2011 NIAID scientists presented data at the Keystone
Conference on “Pathogenesis of Influenza: Virus-Host
Interactions” in Hong Kong, China, showing the positive
results of efficacy studies in mice of LEAPS H1N1 activated
dendritic cells (DCs) to treat the H1N1 virus. Scientists at the
NIAID found that H1N1-infected mice treated with LEAPS-H1N1 DCs
showed a survival advantage over mice treated with control DCs. The
work was performed in collaboration with scientists led by Kanta
Subbarao, M.D., Chief of the Emerging Respiratory Diseases Section
in NIAID’s Division of Intramural Research, part of the
National Institutes of Health, USA.
In July
2013, CEL-SCI announced the publication of the results of influenza
studies by researchers from the NIAID in the Journal of Clinical
Investigation (www.jci.org/articles/view/67550). The studies
described in the publication show that when CEL-SCI’s
investigational J-LEAPS Influenza Virus treatments were used
“in vitro” to activate DCs, which when injected into
influenza infected mice, arrested the progression of lethal
influenza virus infection in these mice. The work was performed in
the laboratory of Dr. Subbarao.
Even
though the various LEAPS drug candidates have not yet been given to
humans, they have been tested in vitro with human cells. They have
induced similar cytokine responses that were seen in these animal
models, which may indicate that the LEAPS technology might
translate to humans. The LEAPS candidates have demonstrated
protection against lethal herpes simplex virus (HSV1) and H1N1
influenza infection, as a prophylactic or therapeutic agent in
animals. They have also shown some level of efficacy in animals in
two autoimmune conditions, curtailing and sometimes preventing
disease progression in arthritis and myocarditis animal models.
CEL-SCI’s belief is that the LEAPS technology may be a
significant alternative to the vaccines currently available on the
market for these diseases.
None of
the LEAPS investigational products have been approved for sale,
barter or exchange by the FDA or any other regulatory agency for
any use to treat disease in animals or humans. The safety or
efficacy of these products has not been established for any use.
Lastly, no definitive conclusions can be drawn from the
early-phase, preclinical-trials data involving these
investigational products. Before obtaining marketing approval from
the FDA in the United States, and by comparable agencies in most
foreign countries, these product candidates must undergo rigorous
preclinical and clinical testing which is costly and time consuming
and subject to unanticipated delays. There can be no assurance that
these approvals will be granted.
15
FORWARD LOOKING STATEMENTS
This
prospectus and the documents that are incorporated or deemed to be
incorporated by reference into this prospectus, contain or
incorporate by reference “forward-looking statements”
within the meaning of the Private Securities Litigation Reform Act
of 1995, Section 27A of the Securities Act of 1933, as amended, and
Section 21E of the Securities Exchange Act of 1934, as amended. You
can generally identify these forward-looking statements by
forward-looking words such as “anticipates,”
“believes,” “expects,”
“intends,” “future,” “could,”
“estimates,” “plans,” “would,”
“should,” “potential,”
“continues” and similar words or expressions (as well
as other words or expressions referencing future events, conditions
or circumstances). These forward-looking statements involve risks,
uncertainties and other important factors that may cause our actual
results, performance or achievements to be materially different
from any future results, performance or achievements expressed or
implied by such forward-looking statements, including, but not
limited to:
●
the progress and
timing of, and the amount of expenses associated with, our
research, development and commercialization activities for our
product candidates, including Multikine;
●
our expectations
regarding the timing, costs and outcome of any pending or future
litigation matters, lawsuits or arbitration proceedings, including
but not limited to the pending arbitration proceeding we initiated
against our former clinical research organization, or
CRO;
●
the success of our
clinical studies for our product candidates;
●
our ability to
obtain U.S. and foreign regulatory approval for our product
candidates and the ability of our product candidates to meet
existing or future regulatory standards;
●
our expectations
regarding federal, state and foreign regulatory
requirements;
●
the therapeutic
benefits and effectiveness of our product candidates;
●
the safety profile
and related adverse events of our product candidates;
●
our ability to
manufacture sufficient amounts of Multikine or our other product
candidates for use in our clinical studies or, if approved, for
commercialization activities following such regulatory
approvals;
●
our plans with
respect to collaborations and licenses related to the development,
manufacture or sale of our product candidates;
●
our expectations as
to future financial performance, expense levels and liquidity
sources;
●
our ability to
compete with other companies that are or may be developing or
selling products that are competitive with our product
candidates;
●
anticipated trends
and challenges in our potential markets; and
●
our ability to
attract, retain and motivate key personnel.
16
All
forward-looking statements contained herein are expressly qualified
in their entirety by this cautionary statement, the risk factors
set forth under the heading “Risk Factors” and
elsewhere in this prospectus and in the documents incorporated or
deemed to be incorporated by reference into this prospectus. The
forward-looking statements contained in this prospectus and any
document incorporated or deemed to be incorporated by reference in
this prospectus, speak only as of their respective
dates. Except to the extent required by applicable laws
and regulations, we undertake no obligation to update these
forward-looking statements to reflect new information, events or
circumstances after the date of this prospectus or to reflect the occurrence of
unanticipated events. In light of these risks and uncertainties,
the forward-looking events and circumstances described in this
prospectus and the documents that are incorporated by reference
into this prospectus may not occur and actual results could differ
materially from those anticipated or implied in such
forward-looking statements. Accordingly, you are cautioned not to
place undue reliance on these forward-looking
statements.
RISK FACTORS
Investors should be
aware that this offering involves the risks described below, which
could adversely affect the price of our common stock. In
addition to the other information contained in this prospectus, the
following factors should be considered carefully in evaluating an
investment in the securities offered by this
prospectus. The risks and uncertainties we described are
not the only ones facing us. Additional risks not
presently known to us, or that we currently deem immaterial, may
also impair our business operations. If any of these
risks were to occur, our business, financial condition, results of
operations and liquidity would likely suffer. In that
event, the trading price of our common stock would decline, and you
could lose all or part of your investment. Some
statements in this Prospectus, including statements in the
following risk factors, constitute forward-looking statements. See
“Forward-Looking Statements.”
Risks Related to CEL-SCI
CEL-SCI has identified material weaknesses in its internal control
over financial reporting which could, if not remediated, result in
material misstatements in CEL-SCI’s financial
statements.
CEL-SCI’s
management is responsible for establishing and maintaining adequate
internal control over its financial reporting, as defined in Rule
13a-15(f) under the Exchange Act. CEL-SCI’s management
identified material weaknesses in the internal control over
financial reporting as of September 30, 2016. A material weakness
is a deficiency, or a combination of deficiencies, in internal
control over financial reporting, such that there is a reasonable
possibility that a material misstatement of CEL-SCI’s annual
or interim financial statements will not be prevented or detected
on a timely basis.
17
CEL-SCI
discovered an error in the way it accounted for the lease for its
manufacturing facility. The accounting error was determined to be a
material weakness in CEL-SCI’s internal control over
financial reporting as of September 30, 2016 relating to
CEL-SCI’s financial close process for non-routine
transactions including the accounting for leases and the assessment
of impairment of long-lived assets. The errors were identified
during the course of the preparation of its financial statements
and other financial data for its fiscal year ended September 30,
2017, as well as its assessment of its disclosure controls and
procedures and internal control over financial reporting as of that
date. This resulted in CEL-SCI filing an amended 10-K/A for the
year ended September 30, 2016, that disclosed these material
weaknesses and the impact of the restatement to the previously
issued financial statements. In addition, as part of the
Company’s audit of the financial statements for the year
ended September 30, 2017, there was an error noted in how the
Company accounted for certain warrants issued in a financing
transaction. These warrants were initially recorded as liability
instruments but met the criteria under ASC 815 to be treated as
equity instruments. These material weaknesses continue to exist at
September 30, 2017 and CEL-SCI is in the process of remediating
these material weaknesses.
If the
remedial measures CEL-SCI has begun implementing that are designed
to address these material weaknesses are insufficient to address
these material weaknesses, or if additional material weaknesses or
significant deficiencies in CEL-SCI’s internal control are
discovered or occur in the future, the financial statements may
contain material misstatements and CEL-SCI could be required to
restate its financial results.
We have incurred significant losses since inception, and we
anticipate that we will continue to incur significant losses for
the foreseeable future and may never achieve or maintain
profitability.
We have
a history of net losses, expect to incur substantial losses and
have negative operating cash flow for the foreseeable future, and
may never achieve or maintain profitability. Since the date of our
formation and through September 30, 2017, we incurred net losses of
approximately $300 million. We have relied principally upon the
proceeds from the public and private sales of our securities to
finance our activities to date. To date, we have not commercialized
any products or generated any revenue from the sale of products,
and we do not expect to generate any product revenue for the
foreseeable future. We do not know whether or when we will generate
product revenue or become profitable.
We are
heavily dependent on the success of Multikine which is under
clinical development. We cannot be certain that Multikine will
receive regulatory approval or be successfully commercialized even
if we receive regulatory approval. Multikine is our only product
candidate in late-stage clinical development, and our business
currently depends heavily on its successful development, regulatory
approval and commercialization. We have no drug products for sale
currently and may never be able to develop approved and marketable
drug products.
Even if
we succeed in developing and commercializing one or more of our
product candidates, we expect to incur significant operating and
capital expenditures as we:
●
continue to
undertake preclinical development and clinical trials for product
candidates;
●
seek regulatory
approvals for product candidates; and
●
implement
additional internal systems and infrastructure.
18
To
become and remain profitable, we must succeed in developing and
commercializing our product candidates, which must generate
significant revenue. This will require us to be successful in a
range of challenging activities, including completing preclinical
testing and clinical trials of our product candidates, discovering
or acquiring additional product candidates, obtaining regulatory
approval for these product candidates and manufacturing, marketing
and selling any products for which we may obtain regulatory
approval. We are only in the preliminary stages of most of these
activities. We may never succeed in these activities and, even if
we do, may never generate revenue that is significant enough to
achieve profitability.
Even if
we do achieve profitability, we may not be able to sustain or
increase profitability on a quarterly or annual basis. Our failure
to become and remain profitable could depress the value of our
company and could impair our ability to raise capital, expand our
business, maintain our research and development efforts, diversify
our product offerings or even continue our operations. A decline in
the value of our company could cause our stockholders to lose all
or part of their investment.
We will require substantial additional capital to remain in
operation. A failure to obtain this necessary capital when needed
could force us to delay, limit, reduce or terminate our product
candidates’ development or commercialization
efforts.
As of
September 30, 2017, we had cash and cash equivalents of
approximately $2.4 million. We believe that we will continue to
expend substantial resources for the foreseeable future developing
Multikine, LEAPS and any other product candidates or technologies
that we may develop or acquire. These expenditures will include
costs associated with research and development, potentially
obtaining regulatory approvals and having our products
manufactured, as well as marketing and selling products approved
for sale, if any. In addition, other unanticipated costs may arise.
Because the outcome of our current and anticipated clinical trials
is highly uncertain, we cannot reasonably estimate the actual
amounts necessary to successfully complete the development and
commercialization of our product candidates.
Our
future capital requirements depend on many factors,
including:
●
the rate of
progress of, results of and cost of completing Phase 3 clinical
development of Multikine for the treatment of certain head and neck
cancers;
●
the results of our
applications to and meetings with the FDA, the EMA and other
regulatory authorities and the consequential effect on our
operating costs;
●
assuming favorable
Phase 3 clinical results, the cost, timing and outcome of our
efforts to obtain marketing approval for Multikine in the United
States, Europe and in other jurisdictions, including the
preparation and filing of regulatory submissions for Multikine with
the FDA, the EMA and other regulatory authorities;
●
the scope,
progress, results and costs of additional preclinical, clinical, or
other studies for additional indications for Multikine, LEAPS and
other product candidates and technologies that we may develop or
acquire;
●
the timing of, and
the costs involved in, obtaining regulatory approvals for LEAPS if
clinical studies are successful;
19
●
the cost and timing
of future commercialization activities for our products, if any of
our product candidates are approved for marketing, including
product manufacturing, marketing, sales and distribution
costs;
●
the revenue, if
any, received from commercial sales of our product candidates for
which we receive marketing approval;
●
the cost of having
our product candidates manufactured for clinical trials and in
preparation for commercialization;
●
our ability to
establish and maintain strategic collaborations, licensing or other
arrangements and the financial terms of such
agreements;
●
the costs involved
in preparing, filing and prosecuting patent applications and
maintaining, defending and enforcing our intellectual property
rights, including litigation costs, and the outcome of such
litigation; and
●
the extent to which
we acquire or in-license other products or
technologies.
Based on the current operating plan, and absent
any future financings or strategic partnerships, CEL-SCI believes
that its existing cash and cash equivalents and investments will be
sufficient to fund its projected operating expenses and capital
expenditure requirements into March 2018. However, CEL-SCI’s
operating plan may change as a result of many factors currently
unknown to CEL-SCI, and CEL-SCI may need additional funds sooner
than planned. Additional funds may not be available when we
need them on terms that are acceptable to us, or at all. If
adequate funds are not available to us on a timely basis, we may be
required to delay, limit, reduce or terminate preclinical studies,
clinical trials or other development activities for Multikine,
LEAPS, or any other product candidates or technologies that we
develop or acquire, or delay, limit, reduce or terminate our sales
and marketing capabilities or other activities that may be
necessary to commercialize our product candidates. Due to recurring losses
from operations and future liquidity needs, there is substantial
doubt about our ability to continue as a going concern without
additional capital becoming available. The doubt about our
ability to continue as a going concern could have an adverse impact
on our ability to execute our business plan, result in the
reluctance on the part of certain suppliers to do business with us,
or adversely affect our ability to raise additional debt or equity
capital.
The costs of our product candidates development and clinical trials
are difficult to estimate and will be very high for many years,
preventing us from making a profit for the foreseeable future, if
ever.
Clinical and other
studies necessary to obtain approval of a new drug can be time
consuming and costly, especially in the United States, but also in
foreign countries. Our estimates of the costs associated with
future clinical trials and research may be substantially lower than
what we actually experience. It is impossible to predict what we
will face in the development of a product candidate, such as
Multikine. The purpose of clinical trials is to provide both us and
regulatory authorities with safety and efficacy data in humans. It
is relatively common to revise a trial or add subjects to a trial
in progress. The difficult and often complex steps necessary to
obtain regulatory approval, especially that of the FDA, and the
EMA, involve significant costs and may require several years to
complete. We expect that we will need substantial additional
financing over an extended period of time in order to fund the
costs of future clinical trials, related research, and general and
administrative expenses.
20
The
extent of our clinical trials and research programs are primarily
based upon the amount of capital available to us and the extent to
which we receive regulatory approvals for clinical trials. We have
established estimates of the future costs of the Phase 3 clinical
trial for Multikine, but, as explained above, our estimates may not
prove correct.
An adverse determination in any current or future lawsuits or
arbitration proceedings to which we are a party could have a
material adverse effect on us.
We are
currently involved in a pending arbitration proceeding, CEL-SCI
Corporation v. inVentiv Health Clinical, LLC (f/k/a PharmaNet LLC)
and PharmaNet GmbH (f/k/a PharmaNet AG). We initiated the
proceedings against inVentiv Health Clinical, LLC, or inVentiv, the
former third-party CRO, and are seeking payment for damages related
to inVentiv’s prior involvement in the Phase 3 clinical trial
of Multikine. The arbitration claim, initiated under the Commercial
Rules of the American Arbitration Association, alleges (i) breach
of contract, (ii) fraud in the inducement, and (iii) common law
fraud. Currently, we are seeking at least $50 million in damages in
our amended statement of claim.
In an
amended statement of claim, we asserted the claims set forth above
as well as an additional claim for professional malpractice. The
arbitrator subsequently granted inVentiv’s motion to dismiss
the professional malpractice claim based on the “economic
loss doctrine” which, under New Jersey law, is a legal
doctrine that, under certain circumstances, prohibits bringing a
negligence-based claim alongside a claim for breach of contract.
The arbitrator denied the remainder of inVentiv’s motion,
which had sought to dismiss certain other aspects of the amended
statement of claim. In particular, the arbitrator rejected
inVentiv’s argument that several aspects of the amended
statement of claim were beyond the arbitrator’s
jurisdiction.
In
connection with the pending arbitration proceedings, inVentiv has
asserted counterclaims against us for (i) breach of contract,
seeking at least $2 million in damages for services allegedly
performed by inVentiv; (ii) breach of contract, seeking at least $1
million in damages for the alleged use of inVentiv’s name in
connection with publications and promotions in violation of the
parties’ contract; (iii) opportunistic breach, restitution
and unjust enrichment, seeking at least $20 million in disgorgement
of alleged unjust profits allegedly made by us as a result of the
purported breaches referenced in subsection (ii); and (iv)
defamation, seeking at least $1 million in damages for allegedly
defamatory statements made about inVentiv. We believe
inVentiv’s counterclaims are meritless and intend to
vigorously defend against them. However, if such defense is
unsuccessful, and inVentiv successfully asserts any of its
counterclaims, such an adverse determination could have a material
adverse effect on our business, results, financial condition and
liquidity.
In
October 2015, we signed an arbitration funding agreement with a
company established by Lake Whillans Litigation Finance, LLC, a
firm specializing in funding litigation expenses. Pursuant to the
agreement, an affiliate of Lake Whillans provided us with $5
million in funding for litigation expenses to support our
arbitration claims against inVentiv. The funding will only be
repaid if we receive proceeds from the arbitration.
The hearing
(the “trial”) started on September 26, 2016 and was
originally scheduled to end in November/December of 2016.
On November 13, 2017, CEL-SCI
announced that the last witness in the arbitration hearing
testified on November 8, 2017, and no further witnesses or
testimony are expected. With that final witness, the testimony
phase of the arbitration concluded. All that remained after
November 8, 2017 at the trial level were closing statements and
post-trial submissions.
21
Additionally, we
may be the target of claims asserting violations of securities
fraud and derivative actions, or other litigation or arbitration
proceedings in the future. Any future litigation could result in
substantial costs and divert management’s attention and
resources. These lawsuits or arbitration proceedings may result in
large judgments or settlements against us, any of which could have
a material adverse effect on our business, operating results,
financial condition and liquidity.
Compliance with changing regulations concerning corporate
governance and public disclosure may result in additional
expenses.
Changing laws,
regulations and standards relating to corporate governance and
public disclosure may create uncertainty regarding compliance
matters. New or changed laws, regulations and standards are subject
to varying interpretations in many cases. As a result, their
application in practice may evolve over time. We are committed to
maintaining high standards of corporate governance and public
disclosure. Complying with evolving interpretations of new or
changing legal requirements may cause us to incur higher costs as
we revise current practices, policies and procedures, and may
divert management time and attention from potential
revenue-generating activities to compliance matters. If our efforts
to comply with new or changed laws, regulations and standards
differ from the activities intended by regulatory or governing
bodies due to ambiguities related to practice, our reputation may
also be harmed. Further, our board members, chief executive
officer, and other executive officers could face an increased risk
of personal liability in connection with the performance of their
duties. As a result, we may have difficulty attracting and
retaining qualified board members and executive officers, which
could harm our business.
We have not established a definite plan for the marketing of
Multikine, if approved.
We have
not established a definitive plan for marketing nor have we
established a price structure for any of our product candidates, if
approved. However, we intend, if we are in a position to do so, to
sell Multikine ourselves in certain markets where it is approved,
and or to enter into written marketing agreements with various
third parties with established sales forces in such markets. The
sales forces in turn would, we believe, focus on selling Multikine
to targeted cancer centers, physicians and clinics involved in the
treatment of head and neck cancer. We have already licensed future
sales of Multikine, if approved, to three companies: Teva
Pharmaceuticals in Israel, Turkey, Serbia and Croatia; Orient
Europharma in Taiwan, Singapore, Hong Kong, Malaysia, South Korea,
the Philippines, Australia and New Zealand; and Byron BioPharma,
LLC in South Africa. We believe that these companies will have the
resources to market Multikine appropriately in their respective
territories, if approved, but there is no guarantee that they will.
There is no assurance that we will be able to find qualified
third-party partners to market our product in other areas, on terms
that are favorable to us, or at all.
We may
encounter problems, delays and additional expenses in developing
marketing plans with third parties. In addition, even if Multikine,
if approved, is cost-effective and demonstrated to increase overall
patient survival, we may experience other limitations involving the
proposed sale of Multikine, such as uncertainty of third-party
coverage and reimbursement. There is no assurance that we can
successfully market Multikine, if approved, or any other product
candidates we may develop.
22
We
hope to expand our clinical development capabilities in the future,
and any difficulties hiring or retaining key personnel or managing
this growth could disrupt our operations.
We are
highly dependent on the principal members of our management and
development staff. If the Phase 3 Multikine clinical trial is
successful, we expect to expand our clinical development and
manufacturing capabilities, which will involve hiring additional
employees. Future growth will require us to continue to implement
and improve our managerial, operational and financial systems and
to continue to retain, recruit and train additional qualified
personnel, which may impose a strain on our administrative and
operational infrastructure. The competition for qualified personnel
in the biopharmaceutical field is intense. We are highly dependent
on our ability to attract, retain and motivate highly qualified
management and specialized personnel required for clinical
development. Due to our limited resources, we may not be able to
manage effectively the expansion of our operations or recruit and
train additional qualified personnel. If we are unable
to retain key personnel or manage our future growth effectively, we
may not be able to implement our business plan.
If
product liability or patient injury lawsuits are brought against
us, we may incur substantial liabilities and may be required to
limit clinical testing or future commercialization of Multikine or
our other product candidates.
We face
an inherent risk of product liability as a result of the clinical
testing of Multikine and other product candidates, and will face an
even greater risk if we commercialize any of our product
candidates. For example, we may be sued if our Multikine or LEAPS
product candidates, or any other future product candidates,
allegedly cause injury or are found to be otherwise unsuitable
during clinical testing, manufacturing or, if approved, marketing
or sale. Any such product liability claims may include allegations
of defects in manufacturing, defects in design, a failure to warn
of dangers inherent in the product candidate, negligence, strict
liability and a breach of warranties. Claims could also be asserted
under state consumer protection acts.
Furthermore,
Multikine is made, in part, from components of human blood. There
are inherent risks associated with products that involve human
blood such as possible contamination with viruses, including
hepatitis or HIV. Any possible contamination could cause injuries
to patients who receive contaminated Multikine, or could require us
to destroy batches of Multikine, thereby subjecting us to possible
financial losses, lawsuits and harm to our business.
If we
cannot successfully defend ourselves against product liability
claims, we may incur substantial liabilities or be required to
limit or cease the clinical testing or commercialization of our
product candidates, if approved. Even a successful defense would
require significant financial and management resources. Regardless
of the merits or eventual outcome, liability claims may result
in:
●
decreased demand
for Multikine or our other product candidates, if
approved;
●
injury to our
reputation;
●
withdrawal of
existing, or failure to enroll additional, clinical trial
participants;
●
costs to defend any
related litigation;
●
a diversion of
management’s time and our resources;
23
●
substantial
monetary awards to trial participants or patients;
●
product candidate
recalls, withdrawals or labeling, marketing or promotional
restrictions;
●
loss of
revenue;
●
inability to
commercialize Multikine or our other product candidates;
and
●
a decline in the
price of our common stock.
Although we have
product liability insurance for Multikine in the amount of $10.0
million, the successful prosecution of a product liability case
against us could have a materially adverse effect upon our business
if the amount of any judgment exceeds our insurance coverage. Any
claim that may be brought against us could result in a court
judgment or settlement in an amount that is not covered, in whole
or in part, by our insurance or that is in excess of the limits of
our insurance coverage. Our insurance policies also have various
exclusions, and we may be subject to a claim for which we have no
coverage. We may have to pay any amounts awarded by a court or
negotiated in a settlement that exceed our coverage limitations or
that are not covered by our insurance, and we may not have, or be
able to obtain, sufficient capital to pay such amounts. We
commenced the Phase 3 clinical trial for Multikine in December
2010. Although no claims have been brought to date, participants in
our clinical trials could bring civil actions against us for any
unanticipated harmful effects allegedly arising from the use of
Multikine or any other product candidate that we may attempt to
develop.
Our commercial success depends, in part, upon attaining significant
market acceptance of our product candidates, if approved, among
physicians, patients, healthcare payors and major operators of
cancer clinics.
Even if we obtain
regulatory approval for our product candidates, any resulting
product may not gain market acceptance among physicians, healthcare
payors, patients and the medical community, which are critical to
commercial success. Market acceptance of any product candidate for
which we receive approval depends on a number of factors,
including:
●
the efficacy and
safety as demonstrated in clinical trials;
●
the timing of
market introduction of such product candidate as well as
competitive products;
●
the clinical
indications for which the drug is approved;
●
the approval,
availability, market acceptance and reimbursement for the companion
diagnostic;
●
acceptance by
physicians, major operators of cancer clinics and patients of the
drug as a safe and effective treatment;
●
the potential and
perceived advantages of such product candidate over alternative
treatments, especially with respect to patient subsets that are
targeted with such product candidate;
●
the safety of such
product candidate seen in a broader patient group, including its
use outside the approved indications;
●
the cost of
treatment in relation to alternative treatments;
24
●
the availability of
adequate reimbursement and pricing by third-party payors and
government authorities;
●
relative
convenience and ease of administration;
●
the prevalence and
severity of adverse side effects; and
●
the effectiveness
of our sales and marketing efforts.
If our
product candidates are approved but fail to achieve an adequate
level of acceptance by physicians, healthcare payors and patients,
we will not be able to generate significant revenues, and we may
not become or remain profitable.
Our Independent Registered Public Accountants have included in
their report on our financial statements a paragraph stating that
we may be unable to continue as a going concern.
As a
result of our recurring losses from operations, our independent
registered public accounting firm, BDO USA, LLP, has issued a
report in connection with their audit of our financial statements
for the year ended September 30, 2017, that included an explanatory
paragraph referring to our recurring losses from operations and
expressing substantial doubt in our ability to continue as a going
concern without additional capital becoming available. The doubt
about our ability to continue as a going concern could have an
adverse impact on our ability to execute our business plan, result
in the reluctance on the part of certain suppliers to do business
with us, or adversely affect our ability to raise additional debt
or equity capital.
25
Risks Related to Government Approvals
Our product candidates must undergo rigorous preclinical and
clinical testing and regulatory approvals, which could be costly
and time-consuming and subject us to unanticipated delays or
prevent us from marketing any products.
Our
product candidates are subject to premarket approval from the FDA
in the United States, the EMA in the European Union, and by
comparable agencies in most foreign countries before they can be
sold. Before obtaining marketing approval, these product candidates
must undergo costly and time consuming preclinical and clinical
testing which could subject us to unanticipated delays and may
prevent us from marketing our product candidates. There can be no
assurance that such approvals will be granted on a timely basis, if
at all.
Clinical testing is expensive and can take many
years to complete, and its outcome is inherently uncertain. Failure
can occur at any time during the clinical trial process. The
results of preclinical studies and early clinical trials of
our product candidates may not be
predictive of the results of later-stage clinical trials. A number
of companies in the biopharmaceutical industry have suffered
significant setbacks in advanced clinical trials due to lack of
efficacy or adverse safety profiles, notwithstanding promising
results in earlier trials. Our current and future clinical trials
may not be successful.
Although
we are involved in a Phase 3 clinical trial for Multikine and the
trial is fully enrolled, we do not know if this one study will be
sufficient for approval, if successful.
Clinical
trials can be delayed for a variety of reasons, including delays
related to:
●
the availability of
financial resources needed to commence and complete our planned
trials;
●
obtaining
regulatory approval to commence a trial;
●
reaching agreement
on acceptable terms with prospective contract research
organizations, or CROs, and clinical trial sites, the terms of
which can be subject to extensive negotiation and may vary
significantly among different CROs and trial sites;
●
obtaining
Institutional Review Board, or IRB, approval at each clinical trial
site;
●
recruiting suitable
patients to participate in a trial;
●
having patients
complete a trial or return for post-treatment
follow-up;
●
clinical trial
sites deviating from trial protocol or dropping out of a
trial
Patient
enrollment, a significant factor in the timing of clinical trials,
is affected by many factors including the competence of the CRO
running the study, size and nature of the patient population, the
proximity of patients to clinical sites, the eligibility criteria
for the trial, the design of the clinical trial, competing clinical
trials and clinicians' and patients' perceptions as to the
potential advantages of the drug being studied in relation to other
available therapies, including any new drugs that may be approved
for the indications we are investigating. Furthermore, we rely on
CROs and clinical trial sites to ensure the proper and timely
conduct of our clinical trials and while we have agreements
governing their committed activities, we have limited influence
over their actual performance.
26
It
remains possible that the regulatory authorities could determine
that the Phase 3 study is not sufficient to support a marketing
application in the United States. Under this circumstance, at least
one entirely new Phase 3 clinical trial would need to be conducted
to support a marketing application in the United States. If there
is a need to conduct an additional Phase 3 clinical trial, any such
requirement would have significant and severe material consequences
for us and could impact our ability to continue as a going
concern.
We
could also encounter significant delays and/or need to terminate a
development program for a product candidate if physicians encounter
unresolved ethical issues associated with enrolling patients in
clinical trials of our product candidates in addition to existing
treatments that have established safety and efficacy profiles.
Further, a clinical trial may be suspended or terminated by us, one
or more of the IRBs for the institutions in which such trials are
being conducted, by us upon a final recommendation by the
Independent Data Monitoring Committee, or IDMC, with which we agree
for such trial, or by the FDA or other regulatory authorities, due
to a number of factors, including failure to conduct the clinical
trial in accordance with regulatory requirements or our clinical
protocols, as a result of inspection of the clinical trial
operations or trial site(s) by the FDA or other regulatory
authorities, the imposition of a clinical hold or partial clinical
hold, unforeseen safety issues or adverse side effects, failure to
demonstrate a benefit from using a product candidate, changes in
governmental regulations or administrative actions or lack of
adequate funding to continue the clinical trial. The occurrence of
any one or more of these events would have significant and severe
material consequences for us and could impact our ability to
continue as an ongoing concern.
If we
experience termination of, or delays in the completion of, any
clinical trial of our product candidates, the commercial prospects
for our product candidates will be harmed, and our ability to
generate product revenues will be delayed. In addition, any delays
in completing our clinical trials will increase our costs, slow our
product development and approval process and jeopardize our ability
to commence product sales and generate revenues. Any of these
occurrences may harm our business, prospects, financial condition
and results of operations significantly. Many of the factors that
cause, or lead to, a delay in the commencement or completion of
clinical trials may also ultimately lead to a delay or the denial
of regulatory approval for our product candidates.
We
cannot be certain when or under what conditions we will undertake
future clinical trials. A variety of issues may delay the Phase 3
clinical trial for Multikine. Early trials for our other product
candidates, or the plans for later trials, may not satisfy the
requirements of regulatory authorities, such as the FDA. We may
fail to find subjects willing to enroll in our trials. We
manufacture Multikine in our own manufacturing facility, but rely
on third-party vendors to manage the trial process and other
activities, and these vendors may fail to meet appropriate
standards. Accordingly, the clinical trials relating to our product
candidates may not be completed on schedule, the FDA or foreign
regulatory agencies may order us to stop or modify our research, or
these agencies may not ultimately approve any of our product
candidates for commercial sale. Varying interpretations of the data
obtained from pre-clinical and clinical testing could delay, limit
or prevent regulatory approval of our product candidates. The data
collected from our clinical trials may not be sufficient to support
regulatory approval of our various product candidates, including
Multikine. Our failure to adequately demonstrate the safety and
efficacy of any of our product candidates would delay or prevent
regulatory approval of our product candidates in the United States,
which could prevent us from achieving profitability. Although we
had positive results in our Phase 2 trials for Multikine, those
results were for a very small sample set, and we will not know how
Multikine will perform in a larger set of subjects until we are
well into, or complete, our Phase 3 clinical trial.
27
The
development and testing of product candidates and the process of
obtaining regulatory approvals and the subsequent compliance with
appropriate federal, state, local and foreign statutes and
regulations require the expenditure of substantial time and
financial resources. Failure to comply with the applicable U.S.
requirements at any time during the product development process,
approval process or after approval, may subject an applicant to
administrative or judicial sanctions. FDA sanctions could include,
among other actions, refusal to approve pending applications,
withdrawal of an approval, a clinical hold, termination of the
Phase 3 study, warning letters, product recalls or withdrawals from
the market, product seizures, total or partial suspension of
production or distribution, injunctions, fines, refusals of
government contracts, restitution, disgorgement or civil or
criminal penalties. Any agency or judicial enforcement action could
have a material adverse effect on us.
The
requirements governing the conduct of clinical trials,
manufacturing and marketing of our product candidates, including
Multikine, outside the United States vary from country to country.
Foreign approvals may take longer to obtain than FDA approvals and
can require, among other things, additional testing and different
trial designs. Foreign regulatory approval processes include all of
the risks associated with the FDA approval process. Some of those
agencies also must approve prices for products approved for
marketing. Approval of a product by the FDA or the EMA does not
ensure approval of the same product by the health authorities of
other countries. In addition, changes in regulatory requirements
for product approval in any country during the clinical trial
process and regulatory agency review of each submitted new
application may cause delays or rejections.
We have
only limited experience in filing and pursuing applications
necessary to gain regulatory approvals. Our lack of experience may
impede our ability to obtain timely approvals from regulatory
agencies, if at all. We will not be able to commercialize Multikine
and other product candidates until we have obtained regulatory
approval. In addition, regulatory authorities may also limit the
types of patients to which we or our third-party partners may
market Multikine or our other product candidates. Any failure to
obtain or any delay in obtaining required regulatory approvals may
adversely affect our or our third-party partners’ ability to
successfully market our product candidates.
Even if we obtain regulatory approval for our investigational
products, we will be subject to stringent, ongoing government
regulation.
If our
investigational products receive regulatory approval, either in the
United States or internationally, those products will be subject to
limitations on the approved indicated uses for which the product
may be marketed or to the conditions of approval, and may contain
requirements for potentially costly post-marketing testing,
including Phase 4 clinical trials, and surveillance of the
safety and efficacy of the investigational products. We will
continue to be subject to extensive regulatory requirements. These
regulations are wide-ranging and govern, among other
things:
●
product design,
development and manufacture;
●
product application
and use
●
adverse drug
experience;
●
product advertising
and promotion;
28
●
product
manufacturing, including good manufacturing practices
●
record keeping
requirements;
●
registration and
listing of our establishments and products with the FDA, EMA and
other state and national agencies;
●
product storage and
shipping;
●
drug sampling and
distribution requirements;
●
electronic record
and signature requirements; and
●
labeling changes or
modifications.
We and
any of our third-party manufacturers or suppliers must continually
adhere to federal regulations setting forth requirements, known as
current, Good Manufacturing Practices, or cGMPs, and their foreign
equivalents, which are enforced by the FDA, the EMA and other
national regulatory bodies through their facilities inspection
programs. If our facilities, or the facilities of our contract
manufacturers or suppliers, cannot pass a pre-approval plant
inspection or fail such inspections in the future, the FDA, EMA or
other national regulators will not approve our marketing
applications for our product candidates, or may withdraw any prior
approval. In complying with cGMP and foreign regulatory
requirements, we and any of our potential third-party manufacturers
or suppliers will be obligated to expend time, money and effort in
production, record-keeping and quality control to ensure that our
product candidates meet applicable specifications and other
requirements.
If we
do not comply with regulatory requirements at any stage, whether
before or after marketing approval is obtained, we may be subject
to, among other things, license suspension or revocation, criminal
prosecution, seizure, injunction, fines, be forced to remove a
product from the market or experience other adverse consequences,
including restrictions or delays in obtaining regulatory marketing
approval for such products or for other product candidates for
which we seek approval. This could materially harm our financial
results, reputation and stock price. Additionally, we may not be
able to obtain the labeling claims necessary or desirable for
product promotion. If we or other parties identify adverse effects
after any of our products are on the market, or if manufacturing
problems occur, regulatory approval may be suspended or withdrawn.
We may be required to reformulate our products, conduct additional
clinical trials, make changes in product labeling or indications of
use, or submit additional marketing applications to support any
changes. If we encounter any of the foregoing problems, our
business and results of operations will be harmed and the market
price of our common stock may decline.
The FDA and other governmental authorities’
policies may change and additional government regulations may be
enacted that could prevent, limit or delay regulatory approval
of our product candidates. If
we are slow or unable to adapt to changes in existing requirements
or the adoption of new requirements or policies, or if we are not
able to maintain regulatory compliance, we may lose any marketing
approval that we may have obtained, which would adversely affect
our business, prospects and ability to achieve or sustain
profitability. We cannot predict the extent of adverse
government regulations which might arise from future legislative or
administrative action. Without government approval, we will be
unable to sell any of our product candidates.
29
Our product candidates may cause undesirable side effects or have
other properties that could delay or prevent their regulatory
approval, limit the commercial profile of an approved label, or
result in significant negative consequences following marketing
approval, if any.
Undesirable side
effects caused by our product candidates could cause us or
regulatory authorities to interrupt, delay or halt clinical trials
and could result in a more restrictive label or the delay or denial
of regulatory approval by the FDA or other comparable foreign
authorities. Results of our clinical trials could reveal a high and
unacceptable severity and/or prevalence of these or other side
effects. In such an event, our trials could be suspended or
terminated and the FDA or comparable foreign regulatory authorities
could order us to cease further development of, or deny approval
of, our product candidates for any or all targeted indications. The
drug-related side effects could affect patient recruitment or the
ability of enrolled patients to complete the trial or result in
potential product liability claims. Any of these occurrences may
harm our business, financial condition and prospects
significantly.
Additionally if one
or more of our product candidates receives marketing approval, and
we or others later identify undesirable side effects caused by such
products, a number of potentially significant negative consequences
could result, including:
●
regulatory
authorities may withdraw approvals of such product;
●
regulatory
authorities may require additional warnings on the
label;
●
we may be required
to create a medication guide outlining the risks of such side
effects for distribution to patients;
●
we could be sued
and held liable for harm caused to patients; and
●
our reputation may
suffer.
Any of
these events could prevent us from achieving or maintaining market
acceptance of the particular product candidate, if approved, and
could significantly harm our business, results of operations and
prospects.
We rely on third parties to conduct our preclinical and clinical
trials. If these third parties do not successfully carry out their
contractual duties and meet regulatory requirements, or meet
expected deadlines, we may not be able to obtain regulatory
approval for or commercialize our product candidates and our
business could be substantially harmed.
We have
relied upon and plan to continue to rely upon third-party CROs to
prepare for, conduct, monitor and manage data for our preclinical
and clinical programs. We rely on these parties for all aspects of
the execution of our preclinical and clinical trials, and although
we diligently oversee and carefully manage our CROs, we directly
control only certain aspects of their activities and rely upon them
to provide timely, complete, and accurate reports on their conduct
of our studies. Although such third parties provide support and
represent us for regulatory purposes in the context of our clinical
trials, ultimately we are responsible for ensuring that each of our
studies is conducted in accordance with the applicable protocol,
legal, regulatory, and scientific standards, and our reliance on
the CROs does not relieve us of our regulatory responsibilities. We
and our CROs acting on our behalf as well as principal
investigators and trial sites are required to comply with Good
Clinical Practice, or GCP, and other applicable requirements, which
are implemented through regulations and guidelines enforced by the
FDA, the Competent Authorities of the Member States of the European
Economic Area, or EEA, and comparable foreign regulatory
authorities for all of our products in clinical development.
Regulatory authorities enforce these GCPs through periodic
inspections of trial sponsors, principal investigators, and trial
sites. If we or any of our CROs fail to comply with applicable GCPs
or other applicable regulations, the clinical data generated in our
clinical trials may be determined to be unreliable and we may
therefore need to enroll additional subjects in our clinical
trials, or the FDA, EMA or comparable foreign regulatory
authorities may require us to perform an additional clinical trial
or trials before approving our marketing applications. Moreover, if
we or any of our CROs, principal investigators, or trial sites,
fail to comply with applicable regulatory and GCP requirements,
then we, our CROs, principal investigators, or trial sites may be
subject to enforcement actions, such as fines, warning letters,
untitled letters, clinical holds, civil or criminal penalties,
and/or injunctions. We cannot assure you that upon inspection by a
given regulatory authority, such regulatory authority will
determine that any of our clinical trials comply with GCP
regulations. In addition, our clinical trials must be conducted
with product produced under GMP regulations. Our failure to comply
with these regulations may require us to delay or repeat clinical
trials, which would delay the regulatory approval
process.
30
For
example, we are currently involved in a dispute with our former CRO
relating to the conduct of our Phase 3 study where we allege (i)
breach of contract, (ii) fraud in the inducement and (iii) fraud.
In connection with this dispute, we have alleged that our CRO
failed to properly select, monitor and supervise the study sites
and principal investigators, ensure proper enrollment of subjects,
and ensure strict compliance with the Phase 3 trial protocol and
GCP and other applicable regulatory requirements.
If any
of our relationships with our third-party CROs terminate, we may
not be able to enter into arrangements with alternative CROs or to
do so on commercially reasonable terms. In addition, our CROs are
not our employees, and except for remedies available to us under
our agreements with such CROs, we cannot control whether or not
they devote sufficient time and resources to our on-going clinical,
nonclinical and preclinical programs. If CROs do not successfully
fulfill their regulatory obligations, carry out their contractual
duties or obligations or meet expected deadlines, if they need to
be replaced or if the quality or accuracy of the clinical data they
obtain is compromised due to the failure to adhere to our clinical
protocols, regulatory requirements or for other reasons, our
clinical trials may be extended, delayed or terminated, and we may
not be able to obtain regulatory approval for or successfully
commercialize our product candidates. As a result, our results of
operations and the commercial prospects for our product candidates
would be harmed, our costs could increase and our ability to
generate revenues could be delayed.
Switching or adding
additional CROs involves additional cost and requires management
time and focus. In addition, there is a natural transition period
when a new CRO commences work. As a result, delays may occur, which
can materially impact our ability to meet our desired clinical
development timelines. Though we diligently oversee and carefully
manage our relationships with our CROs, there can be no assurance
that we will not encounter similar challenges or delays in our
clinical development in the future or that these delays or
challenges will not have a material adverse impact on our business,
financial condition and prospects.
We have obtained orphan drug designation from the FDA for Multikine
for neoadjuvant, or primary, therapy in patients with squamous cell
carcinoma of the head and neck, but we may be unable to maintain
the benefits associated with orphan drug designation, including the
potential for market exclusivity.
Under
the Orphan Drug Act, the FDA may grant orphan drug designation to a
drug or biologic intended to treat a rare disease or condition,
which is defined as one occurring in a patient population of fewer
than 200,000 in the United States, or a patient population greater
than 200,000 in the United States where there is no reasonable
expectation that the cost of developing the drug or biologic will
be recovered from sales in the United States. In the United States,
orphan drug designation entitles a party to financial incentives
such as opportunities for grant funding towards clinical trial
costs, tax advantages and user-fee waivers. In addition, if a
product that has orphan drug designation subsequently receives the
first FDA approval for the disease for which it has such
designation, the product is entitled to orphan drug exclusivity,
which means that the FDA may not approve any other applications,
including a full Biologics License Application, or BLA, to market
the same biologic for the same indication for seven years, except
in limited circumstances, such as a showing of clinical superiority
to the product with orphan drug exclusivity or where the
manufacturer is unable to assure sufficient product
quantity.
31
Even
though we have received orphan drug designation for Multikine for
the treatment of squamous cell carcinoma of the head and neck, we
may not be the first to obtain marketing approval of a product for
the orphan-designated indication due to the uncertainties
associated with developing pharmaceutical products. In addition,
exclusive marketing rights in the United States may be limited if
we seek approval for an indication broader than the
orphan-designated indication, or may be lost if the FDA later
determines that the request for designation was materially
defective or if we are unable to assure sufficient quantities of
the product to meet the needs of patients with the rare disease or
condition. Further, even if we obtain orphan drug exclusivity for a
product candidate, that exclusivity may not effectively protect the
product candidate from competition because different drugs with
different active moieties can be approved for the same condition.
Even after an orphan product is approved, the FDA can subsequently
approve another drug with the same active moiety for the same
condition if the FDA concludes that the later drug is safer, more
effective, or makes a major contribution to patient care. Orphan
drug designation neither shortens the development time or
regulatory review time of a drug nor gives the drug any advantage
in the regulatory review or approval process.
Our current and future relationships with healthcare professionals,
principal investigators, consultants, potential customers and
third-party payors in the United States and elsewhere may be
subject, directly or indirectly, to applicable healthcare laws and
regulations.
Healthcare
providers, physicians and third-party payors in the United States
and elsewhere will play a primary role in the recommendation and
prescription of any drug candidates for which we obtain marketing
approval. Our current and future arrangements with healthcare
professionals, principal investigators, consultants, potential
customers and third-party payors may expose us to broadly
applicable healthcare laws, including, without
limitation:
●
the federal
Anti-Kickback Statute, which prohibits, among other things, persons
from knowingly and willfully soliciting, offering, receiving or
providing remuneration, directly or indirectly, in cash or in kind,
to induce or reward, or in return for, either the referral of an
individual for, or the purchase, lease, order or recommendation of,
any good, facility, item or service, for which payment may be made,
in whole or in part, under federal and state healthcare programs
such as Medicare and Medicaid. A person or entity does not need to
have actual knowledge of the statute or specific intent to violate
it to have committed a violation. In addition, the Affordable Care
Act provides that the government may assert that a claim including
items or services resulting from a violation of the federal
Anti-Kickback Statute constitutes a false or fraudulent claim for
purposes of the False Claims Act;
●
federal civil and
criminal false claims laws, including the federal False Claims Act,
which impose criminal and civil penalties, including civil
whistleblower actions, against individuals or entities for, among
other things, knowingly presenting, or causing to be presented, to
the federal government, including the Medicare and Medicaid
programs, claims for payment that are false or fraudulent or making
a false statement to avoid, decrease or conceal an obligation to
pay money to the federal government;
●
the civil monetary
penalties statute, which imposes penalties against any person or
entity who, among other things, is determined to have presented or
caused to be presented a claim to a federal health program that the
person knows or should know is for an item or service that was not
provided as claimed or is false or fraudulent;
32
●
the federal Health
Insurance Portability and Accountability Act of 1996, or HIPAA,
which created new federal criminal statutes that prohibit knowingly
and willfully executing, or attempting to execute, a scheme to
defraud any healthcare benefit program or obtain, by means of false
or fraudulent pretenses, representations or promises, any of the
money or property owned by, or under the custody or control of, any
healthcare benefit program, regardless of the payor
(e.g., public or private), knowingly and willfully embezzling
or stealing from a health care benefit program, willfully
obstructing a criminal investigation of a healthcare offense and
knowingly and willfully falsifying, concealing or covering up by
any trick or device a material fact or making any materially false
statements in connection with the delivery of, or payment for,
healthcare benefits, items or services relating to healthcare
matters. A person or entity does not need to have actual knowledge
of the statute or specific intent to violate it to have committed a
violation;
●
HIPAA, as amended
by the Health Information Technology for Economic and Clinical
Health Act of 2009, or HITECH, and their respective implementing
regulations, which impose obligations on covered entities,
including healthcare providers, health plans, and healthcare
clearinghouses, as well as their respective business associates
that create, receive, maintain or transmit individually
identifiable health information for or on behalf of a covered
entity, with respect to safeguarding the privacy, security and
transmission of individually identifiable health
information;
●
the federal
Physician Payments Sunshine Act and its implementing regulations,
which impose annual reporting requirements for certain
manufacturers of drugs, devices, biologicals and medical supplies
for payments and “transfers of value” provided to
physicians and teaching hospitals, as well as ownership and
investment interests held by physicians and their immediate family
members; and
●
analogous state and
foreign laws, such as state anti-kickback and false claims laws,
which may apply to sales or marketing arrangements and claims
involving healthcare items or services reimbursed by
non-governmental third-party payors, including private insurers;
state laws that require pharmaceutical companies to comply with the
pharmaceutical industry’s voluntary compliance guidelines and
the relevant compliance guidance promulgated by the federal
government or otherwise restrict payments that may be made to
healthcare providers; state and foreign laws that require drug
manufacturers to report information related to payments and other
transfers of value to physicians and other healthcare providers or
marketing expenditures; and state and foreign laws governing the
privacy and security of health information in certain
circumstances, many of which differ from each other in significant
ways and often are not preempted by HIPAA, thus complicating
compliance efforts.
Efforts
to ensure that our future business arrangements with third parties
will comply with applicable healthcare laws and regulations may
involve substantial costs. It is possible that governmental
authorities will conclude that our business practices may not
comply with current or future statutes, regulations or case law
involving applicable fraud and abuse or other healthcare laws. If
our operations are found to be in violation of any of these laws or
any other governmental regulations, we may be subject to
significant civil, criminal and administrative penalties,
including, without limitation, damages, fines, imprisonment,
exclusion from participation in government healthcare programs,
such as Medicare and Medicaid, and the curtailment or restructuring
of our operations, all of which could significantly harm our
business. If any of the physicians or other healthcare providers or
entities with whom we expect to do business, including our current
and future collaborators, are found not to be in compliance with
applicable laws, they may be subject to criminal, civil or
administrative sanctions, including exclusions from participation
in government healthcare programs, which could also adversely
affect our business.
33
Failure to obtain or maintain adequate coverage and reimbursement
for our product candidates, if approved, could limit our ability to
market those products and decrease our ability to generate
revenue.
Sales
of our product candidates will depend substantially, both
domestically and abroad, on the extent to which the costs of our
product candidates will be paid by health maintenance, managed
care, pharmacy benefit, and similar healthcare management
organizations, or reimbursed by government authorities, private
health insurers and other third-party payors. We anticipate that
government authorities and other third-party payors will continue
efforts to contain healthcare costs by limiting the coverage and
reimbursement levels for new drugs. If coverage and reimbursement
are not available, or are available only to limited levels, we may
not be able to successfully commercialize our product candidates.
Even if coverage is provided, the approved reimbursement amount may
not be high enough to allow us to establish or maintain pricing
sufficient to realize a return on our investment. It is difficult
to predict at this time what third-party payors will decide with
respect to the coverage and reimbursement for our product
candidates.
Healthcare legislative reform measures may have a material adverse
effect on our business and results of operations.
In the
United States, there have been and continue to be a number of
legislative initiatives to contain healthcare costs that may result
in more limited coverage or downward pressure on the price we may
otherwise receive for our product candidates. For example, in March
2010, the Patient Protection and Affordable Care Act, as amended by
the Health Care and Education Reconciliation Act, or collectively,
the Affordable Care Act, was passed, which substantially changes
the way health care is financed by both governmental and private
insurers, and significantly impacts the U.S. pharmaceutical
industry. The Affordable Care Act, among other things, addressed a
new methodology by which rebates owed by manufacturers under the
Medicaid Drug Rebate Program are calculated for drugs that are
inhaled, infused, instilled, implanted or injected, increased the
minimum Medicaid rebates owed by manufacturers under the Medicaid
Drug Rebate Program and extended the rebate program to individuals
enrolled in Medicaid managed care organizations, established annual
fees and taxes on manufacturers of certain branded prescription
drugs, and established the Center for Medicare and Medicaid
Innovation with broad authority to test and implement new payment
models under Medicare and Medicaid, which are designed to reduce
expenditures while preserving and enhancing quality of
care.
Foreign governments often impose strict price controls, which may
adversely affect our future profitability.
We
intend to seek approval to market Multikine in both the United
States and foreign jurisdictions. If we obtain approval in one or
more foreign jurisdictions, we will be subject to rules and
regulations in those jurisdictions relating to Multikine. In some
foreign countries, particularly in the European Union, prescription
drug pricing is subject to governmental control. In these
countries, pricing negotiations with governmental authorities can
take considerable time after the receipt of marketing approval for
a drug candidate. Coverage and reimbursement decisions in one
foreign jurisdiction may impact decisions in other countries. To
obtain reimbursement or pricing approval in some countries, we may
be required to conduct clinical trials that demonstrate our product
candidate is more effective than current treatments and that
compare the cost-effectiveness of Multikine to other available
therapies. If reimbursement of Multikine is unavailable or limited
in scope or amount, or if pricing is set at unsatisfactory levels,
we may be unable to achieve or sustain profitability.
34
Risks Related to Intellectual Property
We may not be able to achieve or maintain a competitive position,
and other technological developments may result in our proprietary
technologies becoming uneconomical or obsolete.
We are
involved in a biomedical field that is undergoing rapid and
significant technological change. The pace of change continues to
accelerate. The successful development of product candidates from
our compounds, compositions and processes, through research
financed by us, or as a result of possible third-party licensing
arrangements with pharmaceutical or other companies, is not
assured. We may fail to apply for patents on important technologies
or product candidates in a timely fashion, or at all.
Many
companies are working on drugs designed to cure or treat cancer or
cure and treat viruses, such as HPV or H1N1. Many of these
companies have financial, research and development, and marketing
resources which are much greater than ours and are capable of
providing significant long-term competition either by establishing
in-house research groups or by forming collaborative ventures with
other entities. In addition, smaller companies and non-profit
institutions are active in research relating to cancer and
infectious diseases. The future market share of Multikine or our
other product candidates, if approved, will be reduced or
eliminated if our competitors develop and obtain approval for
products that are safer or more effective than our product
candidates. Moreover, the patent positions of pharmaceutical
companies are highly uncertain and involve complex legal and
factual questions for which important legal principles are often
evolving and remain unresolved. As a result, the validity and
enforceability of patents cannot be predicted with certainty. In
addition, we do not know whether:
●
we were the first
to make the inventions covered by each of our issued patents and
pending patent applications;
●
we were the first
to file patent applications for these inventions;
●
others will
independently develop similar or alternative technologies or
duplicate any of our technologies;
●
any of our pending
patent applications will result in issued patents;
●
any of our patents
will be valid or enforceable;
●
any patents issued
to us or our collaboration partners will provide us with any
competitive advantages, or will be challenged by third
parties;
●
we will be able to
develop additional proprietary technologies that are
patentable;
●
the U.S. government
will exercise any of its statutory rights to our intellectual
property that was developed with government funding;
or
●
our business may
infringe the patents or other proprietary rights of
others.
35
Our patents might not protect our technology from competitors, in
which case we may not have any advantage over competitors in
selling any products that we may develop.
Our
commercial success will depend in part on our ability to obtain
additional patents and protect our existing patent position, as
well as our ability to maintain adequate intellectual property
protection for our technologies, product candidates, and any future
products in the United States and other countries. If we do not
adequately protect our technology, product candidates and future
products, competitors may be able to use or practice them and erode
or negate any competitive advantage we may have, which could harm
our business and ability to achieve profitability. The laws of some
foreign countries do not protect our proprietary rights to the same
extent or in the same manner as U.S. laws, and we may encounter
significant problems in protecting and defending our proprietary
rights in these countries. We will be able to protect our
proprietary rights from unauthorized use by third parties only to
the extent that our proprietary technologies, product candidates
and any future products are covered by valid and enforceable
patents or are effectively maintained as trade
secrets.
Certain
aspects of our technologies are covered by U.S. and foreign
patents. In addition, we have a number of new patent applications
pending. There is no assurance that the applications still pending
or which may be filed in the future will result in the issuance of
any patents. Furthermore, there is no assurance as to the breadth
and degree of protection any issued patents might afford us.
Disputes may arise between us and others as to the scope and
validity of these or other patents. Any defense of the patents
could prove costly and time consuming and there can be no assurance
that we will be in a position, or will deem it advisable, to carry
on such a defense. A suit for patent infringement could
result in increasing costs, delaying or halting development, or
even forcing us to abandon a product candidate. Other
private and public concerns, including universities, may have filed
applications for, may have been issued, or may obtain additional
patents and other proprietary rights to technology potentially
useful or necessary to us. We are not currently aware of any such
patents, but the scope and validity of such patents, if any, and
the cost and availability of such rights are impossible to
predict.
Much of our intellectual property is protected as trade secrets or
confidential know-how, not as a patent.
We
consider proprietary trade secrets and/or confidential know-how and
unpatented know-how to be important to our
business. Much of our intellectual property pertains to
our manufacturing system, certain aspects of which may not be
suitable for patent filings and must be protected as trade secrets
and/or confidential know-how. This type of information
must be protected diligently by us to protect its disclosure to
competitors, since legal protections after disclosure may be
minimal or non-existent. Accordingly, much of the value
of this intellectual property is dependent upon our ability to keep
our trade secrets and know-how confidential.
To
protect this type of information against disclosure or
appropriation by competitors, our policy is to require our
employees, consultants, contractors and advisors to enter into
confidentiality agreements with us. However, current or former
employees, consultants, contractors and advisers may
unintentionally or willfully disclose our confidential information
to competitors, and confidentiality agreements may not provide an
adequate remedy in the event of unauthorized disclosure of
confidential information. Enforcing a claim that a third party
obtained illegally, and is using, trade secrets and/or confidential
know-how is expensive, time consuming and unpredictable. The
enforceability of confidentiality agreements may vary from
jurisdiction to jurisdiction.
36
In
addition, in some cases a regulator considering our application for
product candidate approval may require the disclosure of some or
all of our proprietary information. In such a case, we
must decide whether to disclose the information or forego approval
in a particular country. If we are unable to market our
product candidates in key countries, our opportunities and value
may suffer.
Failure
to obtain or maintain trade secrets and/or confidential know-how
trade protection could adversely affect our competitive position.
Moreover, our competitors may independently develop substantially
equivalent proprietary information and may even apply for patent
protection in respect of the same. If successful in obtaining such
patent protection, our competitors could limit our use of such
trade secrets and/or confidential know-how.
We may be subject to claims challenging the inventorship or
ownership of our patents and other intellectual
property.
We may
also be subject to claims that former employees, collaborators or
other third parties have an ownership interest in our patents or
other intellectual property. We may be subject to ownership
disputes in the future arising, for example, from conflicting
obligations of consultants or others who are involved in developing
our product candidates. Litigation may be necessary to defend
against these and other claims challenging inventorship or
ownership. If we fail in defending any such claims, in addition to
paying monetary damages, we may lose valuable intellectual property
rights, such as exclusive ownership of, or right to use, valuable
intellectual property. Such an outcome could have a material
adverse effect on our business. Even if we are successful in
defending against such claims, litigation could result in
substantial costs and be a distraction to management and
employees.
Risks Related to our Common Stock
You may experience future dilution as a result of future equity
offerings or other equity issuances.
We
expect that significant additional capital will be needed in the
future to continue our planned operations. To raise additional
capital, we may in the future offer additional shares of our common
stock or other securities convertible into or exchangeable for our
common stock. To the extent we raise additional capital
by issuing equity securities, our stockholders may experience
substantial dilution. These sales may result in material dilution
to our existing stockholders, and new investors could gain rights
superior to our existing stockholders.
Our outstanding options, warrants and convertible notes may
adversely affect the trading price of our common
stock.
As of
December 31, 2017, there were outstanding warrants, convertible
notes and options which allow the holders to purchase 13,413,158
shares of common stock that may be issued upon the exercise of
outstanding warrants, with a weighted average exercise price of
$7.45 per share, 1,133,355 shares that
may be issued upon the conversion of outstanding notes, with a
weighted average conversion price of $1.96 per share and
1,231,812 shares that may be issued upon the exercise of
outstanding options, with a weighted average exercise price of
$15.56 per share . The outstanding options, notes and warrants
could adversely affect our ability to obtain future financing or
engage in certain mergers or other transactions, since the holders
of options and warrants can be expected to exercise them at a time
when we may be able to obtain additional capital through a new
offering of securities on terms more favorable to us than the terms
of the outstanding options and warrants. For the life of the
options, notes and warrants, the holders have the opportunity to
profit from a rise in the market price of our common stock without
assuming the risk of ownership. The issuance of shares upon the
exercise or conversion of outstanding options, notes and warrants
will also dilute the ownership interests of our existing
stockholders.
37
Our ability to utilize our net operating loss carryforwards and
certain other tax attributes may be limited.
Under
Section 382 of the Internal Revenue Code of 1986, as amended, if a
corporation undergoes an “ownership change” (generally
defined as a greater than 50% change (by value) in its equity
ownership over a three-year period), the corporation’s
ability to use its pre-change net operating loss carryforwards and
other pre-change tax attributes to offset its post-change income
may be limited. As a result of our public offerings and other
transactions, we may experience ownership changes in the future
based on subsequent shifts in our stock ownership, some of which
are outside our control. As a result, our ability to use our
pre-change net operating loss carryforwards and other pre-change
tax attributes to offset U.S. federal taxable income may be subject
to limitations, which could result in increased tax liability to
us.
Since we do not intend to pay dividends on our common stock, any
potential return to investors will result only from any increases
in the price of our common stock.
At the
present time, we intend to use available funds to finance our
operations. Accordingly, while payment of dividends rests within
the discretion of our board of directors, no common stock dividends
have been declared or paid by us and we have no intention of paying
any common stock dividends in the foreseeable future. Additionally,
any future debt financing arrangement may contain terms prohibiting
or limiting the amount of dividends that may be declared or paid on
our common stock. Any return to our shareholders will
therefore be limited to appreciation in the price of our common
stock, which may never occur. If our stock price does
not increase, our shareholders are unlikely to receive any return
on their investments in our common stock.
The price of our common stock has been volatile and is likely to
continue to be volatile, which could result in substantial losses
for our shareholders.
Our
stock price has been, and is likely to continue to be, volatile. As
a result of this volatility, our shareholders may not be able to
sell their shares at or above its current market price. The market
price for our common stock may be influenced by many factors,
including:
●
actual or
anticipated fluctuations in our financial condition and operating
results;
●
actual or
anticipated changes in our growth rate relative to our
competitors;
●
competition from
existing products or new products or product candidates that may
emerge;
●
development of new
technologies that may make our technology less
attractive;
●
changes in
physician, hospital or healthcare provider practices that may make
our product candidates less useful;
●
announcements by
us, our partners or our competitors of significant acquisitions,
strategic partnerships, joint ventures, collaborations or capital
commitments;
●
developments or
disputes concerning patent applications, issued patents or other
proprietary rights;
●
the recruitment or
departure of key personnel;
38
●
failure to meet or
exceed financial estimates and projections of the investment
community or that we provide to the public;
●
actual or
anticipated changes in estimates as to financial results,
development timelines or recommendations by securities
analysts;
●
variations in our
financial results or those of companies that are perceived to be
similar to us;
●
changes to coverage
and reimbursement levels by commercial third-party payors and
government payors, including Medicare, and any announcements
relating to reimbursement levels;
●
general economic,
industry and market conditions; and
●
the other factors
described in this “Risk Factors” section.
We have been advised that we are not in compliance with certain
continued listing standards of the NYSE American.
On
December 9, 2016, we received a letter from the NYSE American, our
current listing exchange, which advised us that, based upon our
quarterly report for the quarter ended June 30, 2016, we were
noncompliant with certain continued listing standards of the NYSE
American. We can maintain our listing by submitting a plan of
compliance by January 9, 2017. This plan must advise of actions we
have taken or will take to regain compliance with the continued
listing standards by June 11, 2018. We submitted such a plan on
January 9, 2017. On February 24, 2017, the NYSE American accepted
our plan of compliance and granted us until June 11, 2018 to regain
compliance with the continued listing standards. Although, the NYSE
American will not normally remove the securities if an issuer has a
market capitalization of at least $50 million if we do not make
sufficient progress under the plan to reestablish compliance by
June 11, 2018, the staff of the exchange may initiate proceedings
to delist our securities from the NYSE American. We may appeal a
delisting determination in accordance with the rules of the
exchange.
The
letter from the NYSE American has no immediate effect on the
listing of our securities on the exchange.
Under our amended bylaws, stockholders that initiate certain
proceedings may be obligated to reimburse us and our officers and
directors for all fees, costs and expenses incurred in connection
with such proceedings if the claim proves
unsuccessful.
On
February 18, 2015, we adopted new bylaws which include a
fee-shifting provision in Article X for stockholder claims. Article
X provides that in the event any stockholder initiates or asserts a
claim against us, or any of our officers or directors, including
any derivative claim or claim purportedly filed on our behalf, and
the stockholder does not obtain a judgment on the merits that
substantially achieves, in substance and amount, the full remedy
sought, then the stockholder will be obligated to reimburse us and
any of our officers or directors named in the action, for all fees,
costs and expenses of every kind and description that we or our
officers or directors may incur in connection with the
claim. In adopting Article X, it is our intent
that:
39
●
all actions,
including federal securities law claims, would be subject to
Article X;
●
the phrase “a
judgment on the merits” means the determination by a court of
competent jurisdiction on the matters submitted to the
court;
●
the phrase
“substantially achieves, in both substance and amount”
means the plaintiffs in the action would be awarded at least 90% of
the relief sought;
●
only persons who
were stockholders at the time an action was brought would be
subject to Article X; and
●
only the directors
or officers named in the action would be allowed to
recover.
The
fee-shifting provision contained in Article X of our bylaws is not
limited to specific types of actions, but is rather potentially
applicable to the fullest extent permitted by law. Fee-shifting
bylaws are relatively new and untested. The case law and potential
legislative action on fee-shifting bylaws are evolving and there
exists considerable uncertainty regarding the validity of, and
potential judicial and legislative responses to, such bylaws. For
example, it is unclear whether our ability to invoke our
fee-shifting bylaw in connection with claims under the federal
securities laws, would be pre-empted by federal law. Similarly, it
is unclear how courts might apply the standard that a claiming
stockholder must obtain a judgment that substantially achieves, in
substance and amount, the full remedy sought. The application of
our fee-shifting bylaw in connection with such claims, if any, will
depend in part on future developments of the law. We cannot assure
you that we will or will not invoke our fee-shifting bylaw in any
particular dispute. In addition, given the unsettled state of the
law related to fee-shifting bylaws, such as ours, we may incur
significant additional costs associated with resolving disputes
with respect to such bylaw, which could adversely affect our
business and financial condition.
If a
stockholder that brings any such claim, suit, action or proceeding
is unable to obtain the required judgment, the attorneys’
fees and other litigation expenses that might be shifted to a
claiming stockholder are potentially significant. This fee-shifting
bylaw, therefore, may dissuade or discourage stockholders (and
their attorneys) from initiating lawsuits or claims against us or
our directors and officers. In addition, it may impact the
fees, contingency or otherwise, required by potential
plaintiffs’ attorneys to represent our stockholders or
otherwise discourage plaintiffs’ attorneys from representing
our stockholders at all. As a result, this bylaw may limit the
ability of stockholders to affect our management and direction,
particularly through litigation or the threat of
litigation.
The provision of our amended bylaws requiring exclusive venue in
the U.S. District Court for Delaware for certain types of lawsuits
may have the effect of discouraging lawsuits against us and our
directors and officers.
Article
X of our amended bylaws provides that stockholder claims brought
against us, or our officers or directors, including any derivative
claim or claim purportedly filed on our behalf, must be brought in
the U.S. District Court for the district of Delaware and that with
respect to any such claim, the laws of Delaware will
apply.
40
The
exclusive forum provision may limit a stockholder’s ability
to bring a claim in a judicial forum the stockholder finds
favorable for disputes with us or our directors or officers, and
may have the effect of discouraging lawsuits with respect to claims
that may benefit us or our stockholders.
COMPARATIVE SHARE DATA
|
|
Number of
Shares
|
|
Shares
outstanding as of January 31, 2018
|
13,993,462
|
The
number of shares outstanding as of January 31, 2018 excludes shares
which may be issued upon the exercise of the options or warrants,
or the conversion of the note, described below.
|
|
|
Number of Shares
|
|
Reference
|
|
Shares issuable upon exercise of Series N warrants
|
|
85,339
|
|
A
|
|
Shares issuable upon exercise of options granted
|
|
|
|
|
|
to CEL-SCI's officers, directors,
employees,
|
|
|
|
|
|
consultants, and third parties
|
|
1,231,240
|
|
B
|
|
Shares issuable upon exercise of Series S warrants
|
|
1,037,120
|
|
C
|
|
Shares issuable upon exercise of Series V warrants
|
|
810,127
|
|
D
|
|
Shares issuable upon exercise of Series W warrants
|
|
688,930
|
|
E
|
|
Shares issuable upon exercise of Series X warrants
|
|
120,000
|
|
F
|
|
Shares issuable upon exercise of Series Y warrants
|
|
26,000
|
|
G
|
|
Shares issuable upon exercise of Series Z warrants
|
|
264,000
|
|
H
|
|
Shares issuable upon exercise of Series ZZ warrants
|
|
20,000
|
|
H
|
|
Shares issuable upon exercise of Series AA warrants
|
|
200,000
|
|
I
|
|
Shares issuable upon exercise of Series BB warrants
|
|
16,000
|
|
I
|
|
Shares issuable upon exercise of Series CC warrants
|
|
680,480
|
|
J
|
|
Shares issuable upon exercise of Series DD warrants
|
|
1,360,960
|
|
J
|
|
Shares issuable upon exercise of Series EE warrants
|
|
1,360,960
|
|
J
|
|
Shares issuable upon exercise of Series FF warrants
|
|
68,048
|
|
J
|
|
Shares issuable upon exercise of Series GG warrants
|
|
400,000
|
|
K
|
|
Shares issuable upon exercise of Series HH warrants
|
|
20,000
|
|
K
|
|
Shares issuable upon exercise of Series II warrants
|
|
600,000
|
|
L
|
|
Shares issuable upon exercise of Series JJ warrants
|
|
30,000
|
|
L
|
|
Shares issuable upon exercise of Series KK warrants
|
|
395,970
|
|
M
|
|
Shares issuable upon exercise of Series LL warrants
|
|
26,398
|
|
M
|
|
Shares issuable upon conversion of notes
|
|
597,633
|
|
N
|
|
Shares issuable upon exercise of Series MM warrants
|
|
893,491
|
|
N
|
|
Shares issuable upon conversion of notes
|
|
480,349
|
|
O
|
|
Shares issuable upon exercise of Series NN warrants
|
|
539,300
|
|
O
|
|
Shares issuable upon exercise of Series OO warrants
|
|
60,000
|
|
P
|
|
Shares issuable upon the exercise of Series PP
warrants
|
|
1,750,000
|
|
Q
|
|
Shares issuable upon the exercise of Series QQ
warrants
|
|
87,500
|
|
Q
|
|
Shares issuable upon the exercise of Series RR
warrants
|
|
583,057
|
|
R
|
|
Shares issuable upon the exercise of Series SS
warrants
|
|
1,289,478
|
|
S
|
41
A.
As of January 31,
2018, 85,339 Series N warrants entitle the holders to purchase one
share of the Company's common stock at a price of $3.00 per share
at any time prior to August 18, 2018.
B.
The options are
exercisable at prices ranging from $1.59 to $415 per share. CEL-SCI
may also grant options to purchase additional shares under its
Incentive Stock Option and Non-Qualified Stock Option
Plans.
C.
On January 12, 2018
the exercise price of the Series S warrants was changed to $3.00
per share for a three month period which will end on April 12,
2018. After this date, the exercise price will revert to $31.25 per
share. The Series S warrants expire on October 11,
2018.
D.
The Series V
warrants were immediately exercisable at a price of $19.75 and
expire on May 28, 2020. As of January 31, 2018, none of the Series
V warrants had been exercised.
E.
The Series W
warrants are exercisable at a price of $16.75 and expire on October
28, 2020. As of January 31, 2018, none of the Series W warrants had
been exercised.
F.
The Series X
warrant are exercisable at a price of $9.25 per share at any time
on or before January 13, 2021. As of January 31, 2018, none of the
Series X warrants had been exercised.
G.
The Series Y
warrant are exercisable at a price of $12.00 per share at any time
on or before February 15, 2021. As of January 31, 2018, none of the
Series Y warrants had been exercised.
H.
The Series Z
warrants may be exercised at any time on or before November 23,
2021 at a price of $13.75 per share. The Series ZZ warrants may be
exercised at any time on or before May 18, 2021 at a price of
$13.75 per share. As of January 31, 2018, none of the Series Z or
ZZ warrants had been exercised.
I.
The Series AA
warrants may be exercised at any time on or before February 22,
2022 at a price of $13.75 per share. The Series BB warrants may be
exercised at any time on or before August 22, 2021 at a price of
$13.75 per share. As of January 31, 2018, none of the Series AA or
BB warrants had been exercised.
J.
The Series CC
warrants may be exercised at any time on or before December 8, 2021
at a price of $5.00 per share. The Series DD warrants may be
exercised at any time on or before March 1, 2018 at a price of
$4.50 per share. The Series EE warrants may be exercised at any
time on or before March 1, 2018 at a price of $4.50 per share. The
Series FF warrants may be exercised at any time on or before
December 1, 2021 at a price of $3.90625 per share. As of January
31, 2018, none of the Series CC, DD, EE or FF warrants had been
exercised.
K.
The Series GG
warrants may be exercised at any time on or after August 23, 2017
and on or before August 23, 2022 at a price of $3.00 per share. The
Series HH warrants may be exercised at any time on or before
February 16, 2022 at a price of $3.125 per share. As of January 31,
2018, none of the Series GG or HH warrants had been
exercised.
L.
The Series II
warrants may be exercised at any time on or after September 14,
2017 and on or before September 14, 2022 at a price of $3.00 per
share. The Series JJ warrants may be exercised at any time on or
before March 8, 2022 at a price of $3.125 per share. As of January
31, 2018, none of the Series II or JJ warrants had been
exercised.
42
M.
The Series KK
warrants may be exercised at any time on or after November 3, 2017
and on or before November 3, 2022 at a price of $3.035 per share.
The Series LL warrants may be exercised at any time on or before
April 30, 2022 at a price of $3.59375 per share. As of January 31,
2018, none of the Series KK or LL warrants had been
exercised.
N.
The convertible
notes in the principal amount of $1.51 million are convertible into
shares of common stock at a fixed conversion price of $1.69. The
notes bear interest at 4% per year and are due and payable on
September 21, 2018. As of January 31, 2018, notes in the principal
amount of $500,000 had been converted into 295,858 shares of common
stock. The Series MM warrants are exercisable at a price of $1.86
per share at any time on or before June 22, 2022. As of January 31,
2018, none of the Series MM warrants had been
exercised.
O.
The convertible
notes in the principal amount of $1.235 million are convertible
into shares of common stock at a fixed conversion price of $2.29.
The notes bear interest at 4% per year and are due and payable on
September 21, 2018. As of January 31, 2018, notes in the principal
amount of $135,000 had been converted into 58,952 shares of common
stock. The Series NN warrants are exercisable at a price of $2.52
per share at any time on or before July 24, 2022. As of January 31,
2018, none of the Series NN warrants had been
exercised.
P.
The Series OO
warrants may be exercised at any time on or after January 31, 2018
and on or before July 31, 2022 at a price of $2.52 per share. As of
January 31, 2018, none of the Series OO warrants had been
exercised.
Q.
The Series PP
warrants may be exercised at any time on or after February 28, 2018
and on or before February 28, 2023 at a price of $2.30 per share.
The Series QQ warrants may be exercised at any time on or after
February 22, 2018 and on or before August 22, 2022 at a price of
$2.50 per share. As of January 31, 2018, none of the Series PP or
QQ warrants had been exercised.
R.
The Series RR
warrants may be exercised at any time on or before October 30, 2022
at a price of $1.65 per share. As of January 31, 2018, none of the
Series RR warrants had been exercised.
S.
The Series SS
warrants may be exercised at any time on or after June 20, 2018 and
on or before December 18, 2022 at a price of $2.09 per share. As of
January 31, 2018, none of the Series SS warrants had been
exercised.
DILUTION
As of
January 31, 2018, CEL-SCI’s net book value was less than
$0.01 per share. If the price paid for shares in this offering is
greater than $0.01 per share, an investor will suffer dilution
equal in amount to the difference between the price paid for the
shares and CEL-SCI’s net tangible book value at the time of
purchase.
USE OF PROCEEDS
All of
the shares offered by this prospectus are being offered by certain
owners of CEL-SCI’s common stock (the Selling Shareholders)
and were issued by CEL-SCI in connection with CEL-SCI's employee
stock option, bonus and compensation plans. None of the proceeds
from this offering will be received by CEL-SCI. Expenses expected
to be incurred by CEL-SCI in connection with this offering are
estimated to be approximately $10,000. The Selling Shareholders
have agreed to pay all commissions and other compensation to any
securities broker/dealers through whom they sell any of the
Shares.
43
MARKET FOR CEL-SCI’S COMMON STOCK
Our
common stock is publicly traded on the NYSE American under the
symbol “CVM”. The following table sets forth, for the
periods indicated, the high and low intraday sale prices of our
common stock as reported by the NYSE American. The high and low
prices have been adjusted to reflect a 25-for-1 reverse stock split
which became effective on the NYSE American on June 15,
2017.
|
|
HIGH
|
LOW
|
|
FY 2018
|
|
|
|
Second
Quarter (through January 31, 2018)
|
$2.50
|
$1.85
|
|
First
Quarter (through December 31, 2017)
|
$2.14
|
$1.60
|
|
|
|
|
|
FY 2017
|
|
|
|
Fourth
Quarter (through September 30, 2017)
|
$3.69
|
$1.57
|
|
Third
Quarter (through June 30, 2017)
|
$4.00
|
$1.46
|
|
Second
Quarter (through March 31, 2017)
|
$4.50
|
$1.75
|
|
First
Quarter (through December 31, 2016)
|
$7.75
|
$1.50
|
|
|
|
|
|
FY 2016
|
|
|
|
Fourth
Quarter (through September 30, 2016)
|
$13.50
|
$6.00
|
|
Third
Quarter (through June 30, 2016)
|
$15.00
|
$11.00
|
|
Second
Quarter (through March 31, 2016)
|
$16.50
|
$9.00
|
|
First
Quarter (through December 31, 2015)
|
$18.75
|
$9.00
|
44
As of
January 31, 2018, there were 13,993,462
outstanding shares of
CEL-SCI’s common stock outstanding held by approximately 800
holders of record.
Holders
of common stock are entitled to receive dividends as may be
declared by the Board of Directors out of legally available funds
and, in the event of liquidation, to share pro rata in any
distribution of CEL-SCI’s assets after payment of
liabilities. The Board of Directors is not obligated to declare a
dividend. CEL-SCI has not paid any dividends on its common stock
and CEL-SCI does not have any current plans to pay any common stock
dividends.
The
provisions in CEL-SCI’s Articles of Incorporation relating to
CEL-SCI’s preferred stock allow CEL-SCI’s directors to
issue preferred stock with rights to multiple votes per share and
dividend rights which would have priority over any dividends paid
with respect to CEL-SCI’s common stock. The
issuance of preferred stock with such rights may make more
difficult the removal of management, even if such removal would be
considered beneficial to shareholders generally, and will have the
effect of limiting shareholder participation in certain
transactions such as mergers or tender offers if such transactions
are not favored by incumbent management.
The
market price of CEL-SCI’s common stock, as well as the
securities of other biopharmaceutical and biotechnology companies,
have historically been highly volatile, and the market has from
time to time experienced significant price and volume fluctuations
that are unrelated to the operating performance of particular
companies. Factors such as fluctuations in CEL-SCI’s
operating results, announcements of technological innovations or
new therapeutic products by CEL-SCI or its competitors,
governmental regulation, developments in patent or other
proprietary rights, public concern as to the safety of products
developed by CEL-SCI or other biotechnology and pharmaceutical
companies, and general market conditions may have a significant
effect on the market price of CEL-SCI’s common
stock.
SELLING SHAREHOLDERS
CEL-SCI
has issued (or may in the future issue) shares of its common stock
to various persons pursuant to certain employee compensation plans
adopted by CEL-SCI. The employee compensation plans provide for the
grant or issuance to selected employees of CEL-SCI and other
persons of shares of CEL-SCI’s common stock or options to
purchase shares of CEL-SCI’s common stock. Persons who
received shares pursuant to the Plans and who are offering such
shares to the public by means of this Prospectus are referred to as
the “Selling Shareholders”.
CEL-SCI
has adopted a number of Stock Option, Stock Bonus and Stock
Compensation Plans. A summary description of these Plans follows.
In some cases these Plans are collectively referred to as the
“Plans”.
Incentive Stock
Option Plans. CEL-SCI has Incentive Stock Option Plans which
authorize the issuance of shares of CEL-SCI’s Common Stock to
persons that exercise options granted pursuant to the Plan. Only
Company employees may be granted options pursuant to the Incentive
Stock Option Plans.
Non-Qualified Stock
Option Plans. CEL-SCI has Non-Qualified Stock Option Plans
which authorize the issuance of shares of CEL-SCI’s Common
Stock to persons that exercise options granted pursuant to the
Plans. CEL-SCI’s employees, directors, officers, consultants
and advisors are eligible to be granted options pursuant to the
Plans, provided however that bona fide services must be rendered by
such consultants or advisors and such services must not be in
connection with the offer or sale of securities in a
capital-raising transaction or for directly or indirectly promoting
or maintaining a market for CEL-SCI’s securities. The option
exercise price is determined on the date the option is
granted.
45
Stock Bonus
Plans. CEL-SCI has Stock Bonus Plans which allow for the
issuance of shares of Common Stock to its employees, directors,
officers, consultants and advisors. However bona fide services must
be rendered by the consultants or advisors and such services must
not be in connection with the offer or sale of securities in a
capital-raising transaction or for directly or indirectly promoting
or maintaining a market for CEL-SCI’s
securities.
Stock Compensation
Plans. CEL-SCI’s Stock Compensation Plans provides for
the issuance of shares of its common stock to officers, directors
and employees of CEL-SCI, as well as consultants to CEL-SCI, that
agree to receive shares of CEL-SCI’s common stock in lieu of
all or part of the compensation owed to them by CEL-SCI. However,
bona fide services must be rendered by consultants and the services
must not be in connection with the offer or sale of securities in a
capital-raising transaction or for directly or indirectly promoting
or maintaining a market for CEL-SCI’s
securities.
2014 Incentive
Stock Bonus Plan.
CEL-SCI’s
2014 Incentive Stock Bonus Plan provides for the issuance of shares
of its common stock to officers, directors and employees of CEL-SCI
as well as consultants to CEL-SCI when CEL-SCI reaches certain
performance goals which are established from time to time by
CEL-SCI’s board of directors. The primary purpose of the plan
is to 1) align the interests of those CEL-SCI employees whose work
is essential to CEL-SCI’s ability to commercialize its
patented Multikine technology with those of CEL-SCI’s
shareholders through performance based compensation and 2) to tie
these key employees to CEL-SCI for the rest of the foreseeable drug
development phase of Multikine.
On
August 6, 2014 CEL-SCI’s board of directors granted stock
awards (“Awarded Shares”) pursuant to the 2014
Incentive Stock Bonus Plan to the persons (the
“Grantees”) and in the amounts shown
below.
|
Grantee
|
Awarded Shares
(1)
|
|
|
|
|
Geert
Kersten
|
232,000
|
|
Eyal
Talor
|
124,000
|
|
Patricia
Prichep
|
124,000
|
|
John
Cipriano
|
64,000
|
(1)
The Awarded Shares
(or a portion of the shares) will only be earned based upon the
achievement of certain significant milestones leading to the
commercialization of CEL-SCI’s Multikine technology or
significant increases in the market price of CEL-SCI’s common
shares.
Upon the
achievement of the following performance goals, a percentage of the
Awarded Shares will be earned by the Grantees and will no longer be
subject to being forfeited to CEL-SCI.
46
i.
Upon either (a) the
enrollment of 350 patients in the Phase 3 head and neck cancer
study or (b) the closing price of a share of CEL-SCI’s common
stock on the primary exchange on which such common stock is then
traded exceeds $87.50 for ten consecutive trading days, each
Grantee shall earn 25% of the Awarded Shares.
ii.
Upon either (a) the
full enrollment of patients in the Phase 3 head and neck cancer
study or (b) the start of a pivotal clinical trial for Multikine
(the "Proprietary Technology") in a disease indication other than
head and neck cancer or (c) the closing price of a share of
CEL-SCI’s common stock on the primary exchange on which the
common stock is then traded exceeds $150.00 for ten consecutive
trading days, each Grantee will earn 50% of the Awarded Shares,
less any of the Awarded Shares previously earned.
iii.
Upon either (a) the
end of the Phase 3 head and neck cancer study or any other pivotal
study involving CEL-SCI’s proprietary technology, or (b) the
closing price of a share of CEL-SCI’s common stock on the
primary exchange on which the common stock is then traded exceeds
$225.00 for ten consecutive trading days, each Grantee will earn
75% of the Awarded Shares, less any of the Awarded Shares
previously earned.
iv.
Upon either (a) the
filing of the first marketing application for any pharmaceutical
based upon CEL-SCI’s proprietary technology in the USA,
Canada, UK, Germany, France, Italy, Spain, Japan, or Australia, or
(b) the closing price of a share of CEL-SCI’s common stock on
the primary exchange on which the common stock is then traded
exceeds $300.00 for ten consecutive trading days, each Grantee will
earn 100% of the Awarded Shares, less any of the Awarded Shares
previously earned.
The stock price per
share will be proportionately adjusted in the event of any stock
splits, stock dividends; recapitalizations or similar
events.
The
Grantees may not sell, convey, transfer, pledge, encumber or
otherwise dispose of the Awarded Shares until the shares are
earned.
The Grantees
will forfeit and return to CEL-SCI all Awarded Shares that have not
been earned as of August 5, 2024.
Notwithstanding the
above, upon the occurrence of a Level One Change in Control, all
Awarded Shares which have not previously been earned will vest and
all restrictions pertaining to the Awarded Shares (other than as
may be provided by applicable securities laws) which have not
previously been earned will lapse. Upon the occurrence of a Level
Two Change in Control, if during the period commencing on the date
that is 12 months prior to the occurrence of the Level Two Change
in Control and ending on the date that is 48 months following the
Level Two Change in Control, the Grantee's employment with the
Company is terminated, other than for Cause, or the Grantee
terminates his employment on account of Good Reason, all Awarded
Shares will vest and all restrictions pertaining to the Awarded
Shares (other than as may be provided by applicable securities
laws) will lapse.
47
(i)
A Level One Change
in Control will occur upon (a) the acquisition by any individual,
entity or group of beneficial owners (within the meaning of Rule
13d-3 of the Securities and Exchange Commission) of 50% or more of
either (1) the then outstanding shares of the common stock of
CEL-SCI, or (2) the combined voting power of the then outstanding
voting securities of CEL-SCI entitled to vote in the election of
directors or (b) a majority of the Board consists of persons who
were not nominated or appointed in the first instance by the
Board.
(ii)
A Level Two Change
in Control will occur upon acquisition by any individual, entity or
group of beneficial ownership (within the meaning of Rule 13d-3 of
the Securities and Exchange Commission) of 20% or more of either
(1) the then outstanding shares of CEL-SCI’s common stock, or
(2) the combined voting power of CEL-SCI’s then outstanding
voting securities entitled to vote in the election of
directors.
(iii)
Cause means (a)
conviction of, or pleas of nolo contendere, by the Grantee for a
felony or dishonesty while performing his employment duties, (b) a
Grantee's violation of any non-competition, non-solicitation,
confidentiality or other restrictive covenant agreement applicable
to the Grantee or (c) the Grantee's continued failure to materially
carry out his duties as an employee which failure has not been
cured within 30 days after the Grantee receives written notice of
such failure.
(iv)
Good Reason means
(a) a reduction in compensation (including benefits) of the Grantee
or (b) the Grantee being assigned any duties which are materially
inconsistent with the duties of the Grantee immediately prior to
the occurrence of the Level Two Change in Control or (c) the office
at which the Grantee performs his duties is more than 10 miles from
the office at which the Grantee performed his duties immediately
prior to the occurrence of the Level Two Change in
Control.
Summary. The
following lists, as of January 31, 2018 the options and shares
granted pursuant to the Plans. Each option represents the right to
purchase one share of CEL-SCI's common stock.
|
Name of
Plan
|
Total Shares Reserved Under Plans
|
Shares Reserved for Outstanding
Options
|
Shares Issued as Stock
Bonus/Compensation
|
Remaining Options/Shares Under
Plans
|
|
|
|
|
|
|
|
Incentive
Stock Option Plans
|
138,400
|
124,758
|
N/A
|
454
|
|
Non-Qualified
Stock Option Plans
|
1,187,200
|
1,106,482
|
N/A
|
47,018
|
|
Stock
Bonus Plans
|
383,760
|
N/A
|
222,574
|
161,153
|
|
Stock
Compensation Plans
|
134,000
|
N/A
|
115,590
|
18,410
|
|
2014
Incentive Stock Bonus Plan
|
640,000
|
N/A
|
624,000
|
16,000
|
48
Shares
issuable upon the exercise of options granted to CEL-SCI’s
officers and directors pursuant to the Non-Qualified Stock Option
Plans, as well as shares issued pursuant to the Stock Bonus Plans,
are being offered by means of this Prospectus. The following table
lists the shareholdings of CEL-SCI’s officers and directors
and the shares offered by means of this prospectus as of January
31, 2018.
|
|
|
Number of Shares Being Offered
|
|
|
||
|
Name of Selling Shareholder
|
Number of Shares Owned
|
Option Shares (2)
|
Bonus Shares
|
Stock Compensation Shares
|
Number of shares which will be owned on completion
of the Offering
|
Percent of Class
|
|
|
|
|
|
|
|
|
|
Geert
R. Kersten
|
371,339(1)
|
234,953
|
116,000
|
--
|
487,339
|
4.07%
|
|
Patricia
B. Prichep
|
79,634
|
147,868
|
62,000
|
--
|
141,634
|
1.16%
|
|
Eyal
Talor, Ph.D.
|
49,257
|
145,963
|
62,000
|
--
|
111,257
|
1.01%
|
|
Daniel
H. Zimmerman, Ph.D.
|
14,558
|
47,100
|
--
|
--
|
14,558
|
*
|
|
John
Cipriano
|
16,000
|
95,500
|
32,000
|
--
|
48,000
|
*
|
|
Peter
R. Young, Ph.D.
|
1,191
|
50,187
|
--
|
--
|
1,191
|
*
|
|
Bruno
Baillavoine
|
933
|
37,500
|
--
|
--
|
933
|
*
|
|
Robert
Watson
|
--
|
10,000
|
--
|
--
|
--
|
*
|
* Less
than 1%.
(1)
Includes shares
held in trusts for the benefit of Mr. Kersten’s children and
shares held in the de Clara Trust, for which Mr. Kersten is a
beneficiary.
(2)
Represents shares
issued or issuable upon exercise of stock options. The options held
by CEL-SCI’s officers and directors are exercisable at prices
of between $1.84 and $172.50 per share.
Mr.
Kersten is an officer and director of CEL-SCI. Dr. Young, Mr.
Baillavoine and Mr. Watson are directors of CEL-SCI. The other
persons in the foregoing table are officers of
CEL-SCI.
CEL-SCI
has filed with the Commission under the Securities Act of 1933 a
Form S-8 registration statement of which this prospectus forms a
part with respect to the resale of the shares from time to time in
the public market or in privately negotiated
transactions.
PLAN OF DISTRIBUTION
The
Selling Shareholders may sell the Shares offered by this Prospectus
from time to time in negotiated transactions in the public market
at fixed prices which may be changed from time to time, at market
prices prevailing at the time of sale, at prices related to such
prevailing market prices or at negotiated prices. The Selling
Shareholders may effect such transactions by selling the Shares to
or through broker/dealers, and such broker/dealers may receive
compensation in the form of discounts, concessions, or commissions
from the Selling Shareholders and/or the purchasers of the Shares
for which such broker/dealers may act as agent or to whom they may
sell, as principal, or both (which compensation as to a particular
broker/dealer may be in excess of customary
compensation).
The
Selling Shareholders and any broker/dealers who act in connection
with the sale of the Shares hereunder may be deemed to be
“underwriters” within the meaning of Section 2(11) of
the Securities Acts of 1933, and any commissions received by them
and profit on any resale of the Shares as principal might be deemed
to be underwriting discounts and commissions under the Securities
Act. CEL-SCI has agreed to indemnify the Selling Shareholders and
any securities broker/dealers who may be deemed to be underwriters
against certain liabilities, including liabilities under the
Securities Act as underwriters or otherwise.
49
CEL-SCI
has advised the Selling Shareholders that they and any securities
broker/dealers or others who may be deemed to be statutory
underwriters will be subject to the prospectus delivery
requirements under the Securities Act of 1933. CEL-SCI has also
advised each Selling Shareholder that in the event of a
“distribution” of the shares owned by the Selling
Shareholder, such Selling Shareholder, any “affiliated
purchasers”, and any broker/ dealer or other person who
participates in such distribution may be subject to Rule 102 under
the Securities Exchange Act of 1934 (“1934 Act”) until
their participation in that distribution is completed. A
“distribution” is defined in Rule 102 as an offering of
securities “that is distinguished from ordinary trading
transactions by the magnitude of the offering and the presence of
special selling efforts and selling methods". CEL-SCI has also
advised the Selling Shareholders that Rule 101 under the 1934 Act
prohibits any “stabilizing bid” or “stabilizing
purchase” for the purpose of pegging, fixing or stabilizing
the price of the common stock in connection with this
offering.
DESCRIPTION OF SECURITIES
Common Stock
CEL-SCI
is authorized to issue 600,000,000 shares of common stock, (the
"common stock"). Holders of common stock are each entitled to cast
one vote for each share held of record on all matters presented to
shareholders. Cumulative voting is not allowed; hence, the holders
of a majority of the outstanding shares of common stock can elect
all directors.
Holders
of common stock are entitled to receive such dividends as may be
declared by the Board of Directors out of funds legally available
therefor and, in the event of liquidation, to share pro rata in any
distribution of CEL-SCI's assets after payment of liabilities. The
board is not obligated to declare a dividend. It is not anticipated
that dividends will be paid in the foreseeable future.
Holders
of common stock do not have preemptive rights to subscribe to
additional shares if issued by CEL-SCI. There are no conversion,
redemption, sinking fund or similar provisions regarding the common
stock. All of the outstanding shares of common stock are fully paid
and non-assessable.
Preferred Stock
CEL-SCI
is authorized to issue up to 200,000 shares of preferred stock.
CEL-SCI's Articles of Incorporation provide that the Board of
Directors has the authority to divide the preferred stock into
series and, within the limitations provided by Colorado statute, to
fix by resolution the voting power, designations, preferences, and
relative participation, special rights, and the qualifications,
limitations or restrictions of the shares of any series so
established. As the Board of Directors has authority to establish
the terms of, and to issue, the preferred stock without shareholder
approval, the preferred stock could be issued to defend against any
attempted takeover of CEL-SCI. As of the date of this prospectus no
shares of preferred stock were outstanding.
50
Rights Agreement
In
November 2007, CEL-SCI declared a dividend of one Series A Right
and one Series B Right, or collectively the Rights, for each share
of CEL-SCI’s common stock which was outstanding on November
9, 2007. When the Rights become exercisable, each Series
A Right will entitle the registered holder, subject to the terms of
a Rights Agreement, to purchase from CEL-SCI one share of
CEL-SCI’s common stock at a price equal to 20% of the market
price of CEL-SCI’s common stock on the distribution date,
although the price may be adjusted pursuant to the terms of the
Rights Agreement. If after a person or group of
affiliated persons has acquired 15% or more of CEL-SCI’s
common stock or following the commencement of a tender offer for
15% or more of CEL-SCI’s outstanding common stock (i) CEL-SCI
is acquired in a merger or other business combination and CEL-SCI
is not the surviving corporation, (ii) any person consolidates or
merges with CEL-SCI and all or part of CEL-SCI’s common
shares are converted or exchanged for securities, cash or property
of any other person, or (iii) 50% or more of CEL-SCI’s
consolidated assets or earning power are sold, proper provision
will be made so that each holder of a Series B Right will
thereafter have the right to receive, upon payment of the exercise
price of $100 (subject to adjustment), that number of shares of
common stock of the acquiring company which at the time of such
transaction has a market value that is twice the exercise price of
the Series B Right.
The
description and terms of the Rights are set forth in a Rights
Agreement between the Company and Computershare Trust Company,
N.A., as Rights Agent.
Distribution of Rights
Initially,
stockholders will not receive separate certificates for the Rights
as the Rights will be represented by outstanding common stock
certificates. Until the exercise date, the Rights cannot be bought,
sold or otherwise traded separately from the common stock.
Certificates for common stock will carry a notation that indicates
that Rights are attached to the common stock and incorporate the
terms of the Rights Agreement.
Separate
certificates representing the Rights will be distributed as soon as
practicable after the earliest to occur of:
●
15 business days
following a public announcement that a person or group of
affiliated or associated persons has acquired beneficial ownership
of 15% or more of CEL-SCI’s outstanding common stock,
or
●
15 business days
(or such later date as may be determined by action of
CEL-SCI’s board of directors prior to such time as any person
or group of affiliated persons has acquired 15% or more of
CEL-SCI’s common stock) following the commencement of, or
announcement of an intention to make, a tender offer or exchange
offer the consummation of which would result in the beneficial
ownership by a person or group of 15% or more of such outstanding
common stock.
The
earlier of such dates described above is called the
“distribution date.”
51
Until
the distribution date (or earlier redemption or expiration of the
Rights), the surrender for transfer of any certificates for common
stock outstanding as of the record date, even without such
notation, will also constitute the transfer of the Rights
associated with the common stock represented by such certificate.
As soon as practicable following the distribution date, separate
certificates evidencing the Rights will be mailed to holders of
record of the common stock as of the close of business on the
distribution date, and such separate right certificates alone will
evidence the Rights.
Exercise
and Expiration
The
holders of the Rights are not required to take any action until the
Rights become exercisable. The Rights are not exercisable until the
distribution date. Holders of the Rights will be
notified by CEL-SCI that the Rights have become
exercisable. The Rights will expire on October 30, 2020,
unless the expiration date is extended or unless the Rights are
earlier redeemed by CEL-SCI as described below.
Redemption
At any
time prior to the distribution date, CEL-SCI’s board of
directors may redeem the Rights in whole, but not in part, at a
price of $0.0001 per Right. Subject to the foregoing, the
redemption of the Rights may be made effective at such time, on
such basis and with such conditions as CEL-SCI’s board of
directors in its sole discretion may establish. Immediately upon
any redemption of the Rights, the right to exercise the Rights will
terminate and the only entitlement of the holders of Rights will be
to receive the redemption price.
Exchange Option
At any
time after a person or group of affiliated persons has acquired 15%
or more of CEL-SCI’s common stock or following the
commencement of a tender offer for 15% or more of CEL-SCI’s
outstanding common stock, and prior to the acquisition by such
person of 50% or more of the outstanding common stock,
CEL-SCI’s board of directors may exchange the Rights (other
than Rights owned by such person or group which have become void),
in whole or in part, at an exchange ratio of one share of common
stock per Right (subject to adjustment).
Warrants Held by Private Investors
See
“Comparative Share Data” for information concerning
CEL-SCI’s outstanding options, warrants and convertible
securities.
Transfer Agent
Computershare Trust
Company, Inc., of Denver, Colorado, is the transfer agent for
CEL-SCI's common stock.
52