Exhibit 99.1
|
|

|
NEWS RELEASE
CONTACTS:
Actavis
Investors:
Lisa DeFrancesco
(862) 261-7152
Media:
Charlie Mayr David Belian
(862) 261-8030 (862) 261-8141
Forest
Investors:
Frank J. Murdolo
(212) 224-6714
Media:
Amanda Kaufman
(646) 231-7316
Actavis to Acquire Forest Laboratories, Inc. for ~$25 Billion in an Equity and Cash Transaction
- Creates an Innovative New Model in Specialty Pharmaceuticals Leadership, with $15 Billion in Revenue and a Growing ~$7 Billion North American Specialty Brand Business -
- Blockbuster Franchises in CNS, Gastroenterology, Women’s Health, Urology and Cardiovascular -
- Broader Portfolios and New Therapeutic Categories including Infectious Disease, Respiratory, Cystic Fibrosis and Dermatology -
- Combination Expected to Generate in Excess of $4 Billion in Free Cash Flow in 2015 to Drive Rapid Deleveraging -
- Immediately Accretive to Non-GAAP Earnings; Double-Digit Accretion Expected in 2015 and 2016 -
- Opportunity for Substantial Synergies and Savings of ~$1 Billion -
- Anticipate Closing in Mid-Year 2014, Pending Approvals -
DUBLIN, IRELAND and NEW YORK, NY – February 18, 2014 – Actavis plc (NYSE: ACT) and Forest Laboratories, Inc. (NYSE: FRX) today announced that they have entered into a definitive agreement under which Actavis will acquire Forest for a combination of cash and equity valued at approximately $25 billion or $89.48 per Forest share ($26.04 in cash and 0.3306 Actavis shares for each share of Forest common stock). The per share consideration represents a premium of approximately 25 percent per share over Forest’s stock price, and a premium of approximately 31 percent over Forest’s 10-day volume weighted average stock price, as of the close of trading on February 14, 2014. If successfully completed, the transaction will combine two of the world’s fastest-growing specialty pharmaceutical companies, with combined annual revenues of over $15 billion anticipated for 2015.
“With this strategic combination, we create an innovative new model in specialty pharmaceuticals leadership, with size and scale, a balanced offering of strong brands and generics, a focus on strategic, lower-risk drug development, and—most important—the ability to drive sustainable organic growth,” said Paul Bisaro, Chairman and CEO of Actavis. “Bolstered by one of the deepest and most diversified product portfolios in the industry with an exceptionally strong pipeline, this transaction creates a powerful engine for generating long-term, double-digit revenue and earnings growth.
“The combination of Actavis and Forest is expected to yield double-digit accretion to non-GAAP earnings in 2015 and 2016, with significant annual free cash flow generation of greater than $4 billion in 2015, enabling us to rapidly de-lever. The combination has the potential to realize approximately $1 billion in operating and tax synergies, before any manufacturing synergies or revenue synergies, while we anticipate continuing to invest over $1 billion per year in R&D.”
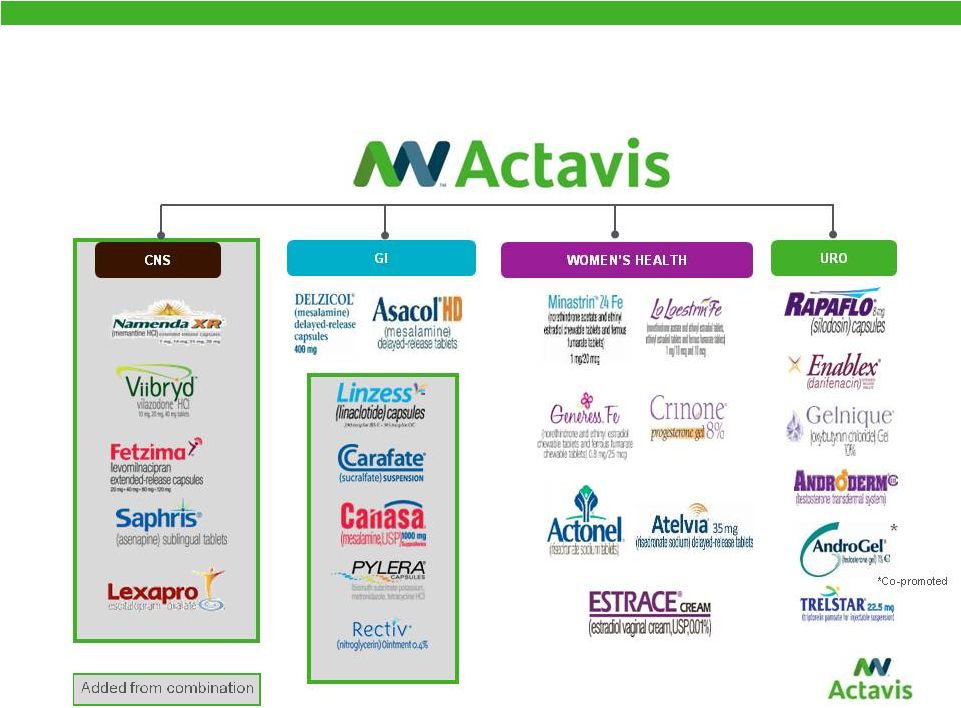
On a pro forma combined basis for full year 2014, the combined company will have an approximately $2 billion CNS franchise; Gastroenterology (GI) and Women’s Health franchises valued at approximately $1 billion each; a Cardiovascular franchise that generates approximately $500 million; and Urology and Dermatology/Established Brand franchises approaching $500 million a year in sales each.
“The combination of Forest with Actavis creates a specialty company with annual sales of approximately $15 billion, a diversified portfolio and a geographically balanced business,” said Brent Saunders, CEO and President of Forest. “This compelling combination gives us more optionality to drive future growth and sustainable shareholder value due to our expanded geographic and therapeutic presence, ability to drive new product flow through R&D, strong
balance sheet and consistent cash flow. The terms of the agreement provide Forest shareholders with cash and the opportunity to participate in the future growth of our new, stronger combined company.
“Forest is a great fit with Actavis due to our strong legacy in branded specialty and primary care pharmaceuticals with a best in class commercial team, a top-notch drug development organization and a long history of successful partnerships. The acquisition builds on our blockbuster line call strategy in CNS and GI and dramatically extends our reach beyond the U.S. market,” added Saunders. “By joining forces with Actavis, we become more relevant to key physicians and customers through blockbuster franchises in CNS, Women’s Health, GI and Urology, as well as Actavis’ global generics business.”
Management of the New Actavis Following Close
“In addition to being financially and commercially compelling, this transaction fundamentally transforms Actavis, positioning it for a new and even more exciting future,” explained Bisaro. “In five short years, my management team has transformed Watson, and now Actavis, from a U.S. generics company to a leader on the global specialty pharmaceutical stage. Brent and his team, in a short period, have made dramatic progress in rejuvenating Forest into a leader in North American brands.
“As Chairman of Actavis, I am in a unique and enviable position of having two exceptionally experienced and successful management teams committed to creating a new future for the combined company. I am especially pleased that Brent will be joining the Actavis Board of Directors and has agreed to work with me following the close to build a world class company focused on sustainable double digit growth. Over the next several months, as we prepare for the integration and closing, our teams will define the structure necessary to capitalize on Actavis’ global leadership in brand, generic, biosimilar and OTC pharmaceuticals.”
The combined company will be led by Paul Bisaro, Chairman and CEO of Actavis plc. The integration of the two companies will be led by the Actavis and Forest senior management teams, with integration planning expected to begin immediately in order to assure a rapid transition to a single company following close. Actavis has agreed that three members of the Forest Board of Directors will be named to the Actavis Board of Directors following the close.
The proposed transaction has been unanimously approved by the Boards of Directors of Actavis and Forest, and is enthusiastically supported by the management teams of both
companies. The transaction is subject to the approval of the shareholders of both companies, as well as customary regulatory approvals, including a Hart-Scott-Rodino review in the United States.
Financially Compelling Transaction
| • | The acquisition is expected to generate double-digit accretion in 2015 and 2016, including approximately $1 billion in operating and tax synergies to be realized within three years following the close. These synergies exclude any additional revenue or manufacturing synergies. These synergies are in addition to standalone synergies announced publicly by Forest as part of its Project Rejuvenate and acquisition of Aptalis. |
| • | The combination of Actavis and Forest will result in Specialty Brand revenues comprising approximately 50 percent of total combined company pro forma revenues, when compared to approximately 30 percent of North American specialty brand revenues for standalone Actavis. |
| • | The combination would generate strong free cash flow in excess of $4 billion in 2015. |
| • | Strong cash flow will enable the combined company to rapidly de-lever the balance sheet to under 3.5x debt to pro forma adjusted EBITDA by the end of 2014. |
Significantly Expanded North American Specialty Portfolio
| • | The combined company will create blockbuster product franchises in the CNS, Gastroenterology, Women’s Health, Urology and Cardiovascular therapeutic categories. |
| • | The combined company will have emerging and sustainable portfolios in Infectious Disease, Respiratory, Cystic Fibrosis and Dermatology therapeutic categories. |
Expanded U.S. Specialty Sales and Marketing
| • | The combination creates a world-class commercial organization competing across multiple market segments. |
| • | The combined company U.S. sales force has extraordinary marketing reach with primary care physicians, psychiatrists, neurologists, infectious disease specialists, cardiologists, pulmonologists, gastroenterologists, OB-Gyn’s, urologists and dermatologists. |
| • | The combined business will be better positioned to leverage the Actavis Specialty Brands portfolio to a broader physician base in the United States, as a result of Forest’s pre-eminent position in primary care sales. |
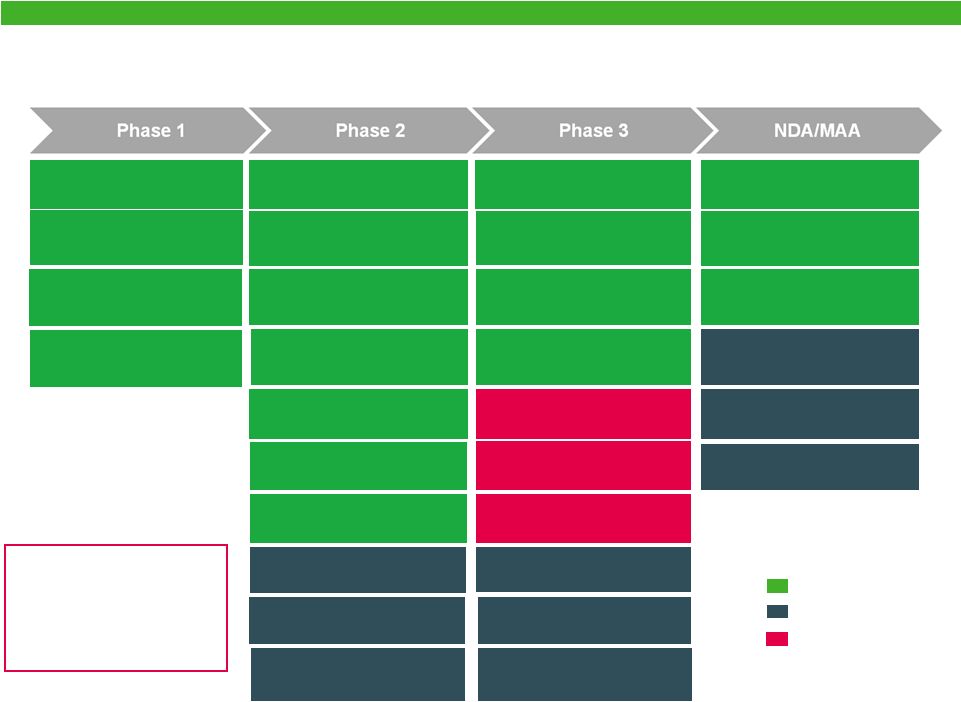
Expanded Specialty Pharmaceuticals R&D Pipeline
| • | The combined company will have investment in new product development in excess of $1 billion on an annual basis. |
| • | The combination of Actavis and Forest will add more than a half dozen near- and mid-term R&D products to Actavis’ robust development portfolio. |
| • | Five Forest products are at the NDA stage of development, including treatments for Alzheimer’s disease, cardiovascular disease, infectious disease, as well as Schizophrenia and bipolar disorders and treatments for COPD. |
Transaction Details
In the proposed transaction, shareholders of Forest will receive 0.3306 shares of ACT common stock and $26.04 in cash for each share of Forest. The transaction will include an election mechanism for Forest shareholders to elect all-stock or all-cash consideration, subject to proration in accordance with the terms of the merger agreement. The stock component of the consideration is expected to represent a tax-free exchange. The aggregate purchase consideration represents a premium of approximately 25 percent above the closing price of Forest shares on February 14, 2014. Forest shareholders are expected to own approximately 35% of the combined company on a pro forma basis.
Greenhill & Co. is serving as financial advisor to Actavis, and Latham & Watkins LLP is serving as Actavis’ legal advisor. J.P. Morgan is serving as financial advisor to Forest, and Wachtell, Lipton, Rosen & Katz is serving as Forest’s legal advisor.
Actavis currently has bridge loan commitments from BofA Merrill Lynch and Mizuho Bank pending execution of its final financing plans.
Conference Call
Actavis and Forest management will host a conference call to discuss the transaction today at 8:00 AM EST. The number to call from within the United States is (877) 251-7980, conference ID 97009455. From international locations, the conference call can be accessed at (706) 643-1573 using the same conference ID. The call will also be webcast and can be accessed through the companies’ websites at www.frx.com] and www.actavis.com. To access the slides go to Actavis’ Investor Relations Web site at http://ir.actavis.com, or directly at http://www.videonewswire.com/event.asp?id=98186. A replay of the conference call will also be available by calling (855) 859-2056 in the U.S. or (404) 537-3406 outside of the U.S., conference ID 97009455.
About Actavis
Actavis plc (NYSE: ACT) is a global, integrated specialty pharmaceutical company focused on developing, manufacturing and distributing generic, brand and biosimilar products. Actavis has global headquarters in Dublin, Ireland and U.S. administrative headquarters in Parsippany, New Jersey, USA.
Actavis develops and manufactures generic, brand, branded generic, legacy brands and Over-the-Counter (OTC) pharmaceutical products and has commercial operations in approximately 60 countries. The Company’s North American branded pharmaceuticals business is focused principally in the Women’s Health, Urology, Gastroenterology and Dermatology therapeutic categories with a strong pipeline of products in various stages of development. Actavis also has a portfolio of five biosimilar products in development in Women’s Health and Oncology. Actavis Global Operations has more than 30 manufacturing and distribution facilities around the world, and includes Anda, Inc., a U.S. pharmaceutical product distributor.
For press release and other company information, visit Actavis’ Web site at http://www.actavis.com.
About Forest
Forest Laboratories, Inc. (NYSE: FRX) is a leading, fully integrated, specialty pharmaceutical company largely focused on the United States market. The Company markets a portfolio of branded drug products and develops new medicines to treat patients suffering from diseases principally in the following therapeutic areas: central nervous system, cardiovascular, gastrointestinal, respiratory, anti-infective, and cystic fibrosis. Our strategy of acquiring product rights for development and commercialization through licensing, collaborative partnerships, and targeted mergers and acquisitions allows us to take advantage of attractive late-stage development and commercial opportunities, thereby managing the risks inherent in drug development. The Company is headquartered in New York, NY.
To learn more, visit Forest’s Web Site at www.frx.com.
Important Information for Investors and Shareholders
This communication does not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote or approval, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. In connection with the proposed merger between Actavis and Forest, Actavis will file with the Securities and Exchange Commission (the “SEC”) a registration statement on Form S-4 that will include a joint proxy statement of Actavis and Forest that also constitutes a prospectus of Actavis. The definitive joint proxy statement/prospectus will be delivered to shareholders of Actavis and Forest. INVESTORS AND SECURITY HOLDERS OF ACTAVIS AND FOREST ARE URGED TO READ THE DEFINITIVE JOINT PROXY STATEMENT/PROSPECTUS AND OTHER DOCUMENTS THAT WILL BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. Investors and security holders will be able to obtain free copies of the registration statement and the definitive joint proxy statement/prospectus (when available) and other documents filed with the SEC by Actavis and Forest through the website maintained by the SEC at http://www.sec.gov. Copies of the documents filed with the SEC by Actavis will be available free of charge on Actavis’ internet website at www.actavis.com or by contacting Actavis’ Investor Relations Department at (862) 261-7488. Copies of the documents filed with the SEC by Forest will be available free of charge on Forest’s internet website at www.frx.com or by contacting Forest’s Investor Relations Department at (212) 224-6713.
Participants in the Merger Solicitation
Actavis, Forest, their respective directors and certain of their executive officers and employees may be considered participants in the solicitation of proxies in connection with the proposed transaction. Information regarding the persons who may, under the rules of the SEC, be deemed participants in the solicitation of the Actavis and Forest shareholders in connection with the proposed merger will be set forth in the joint proxy statement/prospectus when it is filed with the SEC. Information about the directors and executive officers of Forest is set forth in its proxy statement for its 2013 annual meeting of stockholders, which was filed with the SEC on July 8, 2013 and certain of its Current Reports on Form 8-K. Information about the directors and executive officers of Actavis is set forth in its proxy statement for its 2013 annual meeting of stockholders, which was filed with the SEC on March 29, 2013 and certain of its Current Reports on Form 8-K. Additional information regarding the participants in the proxy solicitations and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the joint proxy statement/prospectus filed with the above-referenced registration statement on Form S-4 and other relevant materials to be filed with the SEC when they become available.
Actavis Cautionary Statement Regarding Forward-Looking Statements
Statements contained in this communication that refer to Actavis’ estimated or anticipated future results, including estimated synergies, or other non-historical facts are forward-looking statements that reflect Actavis’ current perspective of existing trends and information as of the date of this communication. Forward looking statements generally will be accompanied by words such as “anticipate,” “believe,” “plan,” “could,” “should,” “estimate,” “expect,” “forecast,” “outlook,” “guidance,” “intend,” “may,” “might,” “will,” “possible,” “potential,” “predict,” “project,” or other similar words, phrases or expressions. Such forward-looking statements include, but are not limited to, statements about the benefits of the Forest acquisition, including future financial and operating results, Actavis’ or Forest’s plans, objectives, expectations and intentions and the expected timing of completion of the transaction. It is important to note that Actavis’ goals and expectations are not predictions of actual performance. Actual results may differ materially from Actavis’ current expectations depending upon a number of factors affecting Actavis’ business, Forest’s business and risks associated with acquisition transactions. These factors include, among others, the inherent uncertainty associated with financial projections; restructuring in connection with, and successful closing of, the Forest acquisition; subsequent integration of the Forest acquisition and the ability to recognize the anticipated synergies and benefits of the Forest acquisition; the ability to obtain required regulatory approvals for the transaction (including the approval of antitrust authorities necessary to complete the acquisition), the timing of obtaining such approvals and the risk that such approvals may result in the imposition of conditions that could adversely affect the combined company or the expected benefits of the transaction; the ability to obtain the requisite Forest and Actavis shareholder approvals; the risk that a condition to closing of the Forest acquisition may not be satisfied on a timely basis or at all; the failure of the proposed transaction to close for any other reason; risks relating to the value of the Actavis shares to be issued in the transaction; the anticipated size of the markets and continued demand for Actavis’ and Forest’s products; the impact of competitive products and pricing; access to available financing (including financing for the acquisition or refinancing of Actavis or Forest debt) on a timely basis and on reasonable terms; the risks of fluctuations in foreign currency exchange rates; the risks and uncertainties normally incident to the pharmaceutical industry, including product liability claims and the availability of product liability insurance on reasonable terms; the difficulty of predicting the timing or outcome of pending or future litigation or government
investigations; periodic dependence on a small number of products for a material source of net revenue or income; variability of trade buying patterns; changes in generally accepted accounting principles; risks that the carrying values of assets may be negatively impacted by future events and circumstances; the timing and success of product launches; the difficulty of predicting the timing or outcome of product development efforts and regulatory agency approvals or actions, if any; market acceptance of and continued demand for Actavis’ and Forest’s products; costs and efforts to defend or enforce intellectual property rights; difficulties or delays in manufacturing; the availability and pricing of third party sourced products and materials; successful compliance with governmental regulations applicable to Actavis’ and Forest’s facilities, products and/or businesses; changes in the laws and regulations affecting, among other things, pricing and reimbursement of pharmaceutical products; changes in tax laws or interpretations that could increase Actavis’ consolidated tax liabilities; the loss of key senior management or scientific staff; and such other risks and uncertainties detailed in Actavis’ periodic public filings with the Securities and Exchange Commission, including but not limited to Actavis’ Annual Report on form 10-K for the year ended December 31, 2012 and from time to time in Actavis’ other investor communications. Except as expressly required by law, Actavis disclaims any intent or obligation to update or revise these forward-looking statements.
Forest Cautionary Statement Regarding Forward-Looking Statements
This release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements include, but are not limited to, statements about the benefits of the acquisition of Forest by Actavis, including future financial and operating results, Forest’s or Actavis’ plans, objectives, expectations and intentions and the expected timing of completion of the transaction. It is important to note that Forest’s goals and expectations are not predictions of actual performance. Actual results may differ materially from Forest’s current expectations depending upon a number of factors affecting Forest’s business, Actavis’ business and risks associated with acquisition transactions. These factors include, among others, the inherent uncertainty associated with financial projections; restructuring in connection with, and successful closing of, the acquisition; subsequent integration of the companies and the ability to recognize the anticipated synergies and benefits of the acquisition; the ability to obtain required regulatory approvals for the transaction (including the approval of antitrust authorities necessary to complete the acquisition), the timing of obtaining such approvals and the risk that such approvals may result in the imposition of conditions that could adversely affect the combined company or the expected benefits of the transaction; the ability to obtain the requisite Forest and Actavis shareholder approvals; the risk that a condition to closing of the acquisition may not be satisfied on a timely basis or at all; the failure of the proposed transaction to close for any other reason; risks relating to the value of the Actavis shares to be issued in the transaction; access to available financing (including financing for the acquisition or refinancing of Forest or Actavis debt) on a timely basis and on reasonable terms; the difficulty of predicting FDA approvals, the acceptance and demand for new pharmaceutical products, the impact of competitive products and pricing, the timely development and launch of new products, and the risk factors listed from time to time in Forest Laboratories’ Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and any subsequent SEC filings. Forest assumes no obligation to update forward-looking statements contained in this release to reflect new information or future events or developments.