Table of Contents
FILED PURSUANT TO RULE 424(b)(3)
REG. STATEMENT NO. 333-185713
PROSPECTUS

DJO Finance LLC
DJO Finance Corporation
Offers to Exchange (the “exchange offers”)
$330,000,000 aggregate principal amount of their 8.75% Second Priority Senior Secured Notes due 2018 (the “exchange senior secured notes”) and $440,000,000 aggregate principal amount of their 9.875% Senior Notes due 2018 (the “exchange senior notes” and, together with the exchange senior secured notes, the “exchange notes”), each of which have been registered under the Securities Act of 1933, as amended (the “Securities Act”), for any and all of their outstanding unregistered 8.75% Second Priority Senior Secured Notes due 2018 (the “outstanding senior secured notes”) and for any and all of their outstanding unregistered 9.875% Senior Notes due 2018 (the “outstanding senior notes” and, together with the outstanding senior secured notes, the “outstanding notes”), respectively.
We are conducting the exchange offers in order to provide you with an opportunity to exchange your unregistered notes for freely tradable notes that have been registered under the Securities Act.
The exchange offers
| • | We will exchange all outstanding notes that are validly tendered and not validly withdrawn for an equal principal amount of exchange notes that are freely tradable. |
| • | You may withdraw tenders of outstanding notes at any time prior to the expiration date of the applicable exchange offer. |
| • | The exchange offers expire at 12:00 a.m. midnight, New York City time, on February 6, 2013, unless extended. We do not currently intend to extend the expiration date. |
| • | The exchange of the relevant outstanding notes for the relevant exchange notes in the exchange offers will not be a taxable event for United States federal income tax purposes. |
| • | The terms of the relevant exchange notes to be issued in the exchange offers are substantially identical to the relevant outstanding notes, except that the exchange notes will be freely tradable. |
Results of the exchange offers
| • | The exchange notes may be sold in the over-the-counter market, in negotiated transactions or through a combination of such methods. We do not plan to list the exchange notes on a national market. |
All untendered outstanding notes will continue to be subject to the restrictions on transfer set forth in the outstanding notes and in the applicable indenture. In general, the outstanding notes may not be offered or sold, unless registered under the Securities Act, except pursuant to an exemption from, or in a transaction not subject to, the Securities Act and applicable state securities laws. Other than in connection with the exchange offers, we do not currently anticipate that we will register the outstanding notes under the Securities Act.
See “Risk Factors” beginning on page 17 for a discussion of certain risks that you should consider before participating in the applicable exchange offer.
Neither the Securities and Exchange Commission (the “SEC”) nor any state securities commission has approved or disapproved of the exchange notes to be distributed in the exchange offers or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is January 8, 2013.
Table of Contents
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with different information. The prospectus may be used only for the purposes for which it has been published and no person has been authorized to give any information not contained herein. If you receive any other information, you should not rely on it. We are not making an offer of these securities in any state where the offer is not permitted.
i
Table of Contents
MARKET, RANKING AND OTHER INDUSTRY DATA
The data included in this prospectus regarding the markets and the industry in which we operate, including the size of certain markets and our position and the position of our competitors within these markets, are based on reports of government agencies, independent industry sources and our own estimates relying on our management’s knowledge and experience in the markets in which we operate. Our management’s knowledge and experience is based on information obtained from our customers, distributors, suppliers, trade and business organizations and other contacts in the markets in which we operate. We believe these estimates to be accurate as of the date of this prospectus. However, this information may prove to be inaccurate because of the method by which we obtained some of the data for our estimates or because this information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties. As a result, you should be aware that market, ranking and other industry data included in this prospectus, and our estimates and beliefs based on that data, may not be reliable. We cannot guarantee the accuracy or completeness of any such information contained in this prospectus.
TRADEMARKS
Each of the following trademarks, trade names or service marks, which is used in this prospectus, is either (1) our registered trademark, (2) a trademark for which we have a pending application or (3) a trademark or service mark for which we claim common law rights: Cefar®, Empi®, Ormed®, Compex®, Aircast®, DonJoy®, OfficeCare®, ProCare®, SpinaLogic®, Dr. Comfort™, CMF™, OL1000™ and OL1000 SC™. All other trademarks, trade names or service marks of any other company appearing in this prospectus belong to their respective owners.
FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which are intended to be covered by the safe harbors created thereby. To the extent that any statements are not recitations of historical fact, such statements constitute forward-looking statements that, by definition, involve risks and uncertainties. Specifically, the sections entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business” may contain forward-looking statements. These statements can be identified because they contain words like “anticipates,” “believes,” “estimates,” “expects,” “forecasts,” “future,” “intends,” “plans,” and similar terms. These statements reflect only our current expectations. Forward-looking statements include statements concerning our plans, objectives, goals, strategies, future events, capital expenditures, future results, our competitive strengths, our business strategy, the trends in our industry and the benefits of and the anticipated cost savings related to our recent acquisitions.
Although we do not make forward-looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy, and actual results may differ materially from those we anticipated due to a number of uncertainties, many of which are unforeseen, including, among others, the risks we face as described under the “Risk Factors” section and elsewhere in this prospectus. You should not place undue reliance on these forward-looking statements, which apply only as of the date of this prospectus. In any forward-looking statement where we express an expectation or belief as to future results or events, such expectation or belief is expressed in good faith and is believed to have a reasonable basis, but there can be no assurance that any future results or events expressed by the statement of expectation or belief will be achieved or accomplished. Our actual results, performance, or achievements could differ materially from those expressed in, or implied by, forward-looking statements. The events anticipated by forward-looking statements may not occur or, if any of them do, we cannot predict what impact they will have on our results of operations and financial condition. Some of the factors that we believe could affect our results include the risks discussed in the “Risk Factors” section.
ii
Table of Contents
We caution you that in light of the risks and uncertainties described in the “Risk Factors” section and elsewhere in this prospectus, the matters referred to in the forward-looking statements contained in this prospectus may not in fact occur. We undertake no obligation to update or revise any forward-looking statement as a result of new information, future events, or otherwise, except as otherwise required by law.
iii
Table of Contents
This summary highlights selected information in this prospectus and may not contain all of the information that is important to you. You should carefully read this entire prospectus, including the information set forth under the heading “Risk Factors” and the financial statements included elsewhere in this prospectus, before making an investment decision. Unless the context otherwise requires, references in this prospectus to “we,” “our,” “us,” “the Company,” “DJOFL” and “our Company” for the purposes of this section refer to DJO Finance LLC and its consolidated subsidiaries (which include all operations of DJO Global, Inc.).
Our Company
We are a global developer, manufacturer and distributor of high-quality medical devices that provide solutions for musculoskeletal health, vascular health and pain management. Our products address the continuum of patient care from injury prevention to rehabilitation after surgery, injury or from degenerative disease, enabling people to regain or maintain their natural motion.
Our products are used by orthopedic specialists, spine surgeons, primary care physicians, pain management specialists, physical therapists, podiatrists, chiropractors, athletic trainers and other healthcare professionals. In addition, many of our medical devices and related accessories are used by athletes and patients for injury prevention and at-home physical therapy treatment. Our product lines include rigid and soft orthopedic bracing, hot and cold therapy, bone growth stimulators, vascular therapy systems and compression garments, therapeutic shoes and inserts, electrical stimulators used for pain management and physical therapy products. Our surgical implant business offers a comprehensive suite of reconstructive joint products for the hip, knee and shoulder.
Our products are marketed under a portfolio of brands including Aircast®, DonJoy®, ProCare®, CMF™, Empi®, Chattanooga, DJO Surgical, Dr. Comfort™ and Compex®.
Operating Segments
We currently develop, manufacture and distribute our products through four operating segments.
Bracing and Vascular Segment
Our Bracing and Vascular segment, which generates its revenues in the United States, offers our rigid knee bracing products, orthopedic soft goods, cold therapy products, vascular systems, therapeutic shoes and inserts and compression therapy products, primarily under our DonJoy, ProCare, Aircast and Dr. Comfort brands. This segment also includes our OfficeCare business, through which we maintain an inventory of soft goods and other products at healthcare facilities, primarily orthopedic practices, for immediate distribution to patients. The Bracing and Vascular segment primarily sells its products to orthopedic and sports medicine professionals, hospitals, podiatry practices, orthotic and prosthetic centers, home medical equipment providers and independent pharmacies.
Recovery Sciences Segment
Our Recovery Sciences segment, which generates its revenues in the United States, is divided into four main businesses:
| • | Empi. Our Empi business unit offers our home electrotherapy, iontophoresis, and home traction products. We primarily sell these products directly to patients or to physical therapy clinics. For products sold to patients, we arrange billing to the patients and their third party payors. |
| • | Regeneration. Our Regeneration business unit sells our bone growth stimulation products. We sell these products either directly to patients or to independent distributors. For products sold to patients, we arrange billing to the patients and their third party payors. |
1
Table of Contents
| • | Chattanooga. Our Chattanooga business unit offers products in the clinical rehabilitation market in the category of clinical electrotherapy devices, clinical traction devices, and other clinical products and supplies such as treatment tables, continuous passive motion (“CPM”) devices and dry heat therapy. |
| • | Athlete Direct. Our Athlete Direct business unit offers consumers ranging from fitness enthusiasts to competitive athletes our Compex electrostimulation devices, which are used in athletic training programs to aid muscle development and to accelerate muscle recovery after training sessions. |
International Segment
Our International segment, which generates most of its revenues in Europe, sells all of our products and certain third party products through a combination of direct sales representatives and independent distributors.
Surgical Implant Segment
Our Surgical Implant segment, which generates its revenues in the United States, develops, manufactures and markets a wide variety of knee, hip and shoulder implant products that serve the orthopedic reconstructive joint implant market.
Our four operating segments enable us to reach a diverse customer base through multiple distribution channels and give us the opportunity to provide a wide range of medical devices and related products to orthopedic specialists and other healthcare professionals operating in a variety of patient treatment settings. These four segments constitute our reportable segments.
Market Opportunities
We participate globally in the rehabilitation, pain management, bone growth stimulation and reconstruction segments of the orthopedic device market. In the United States, we estimate these segments accounted for $8.6 billion of total industry sales in 2010. We believe that several factors are driving growth in the orthopedic products industry, including the following:
| • | Favorable demographics. An aging population is driving growth in the orthopedic products market. Many conditions that result in rehabilitation, physical therapy or orthopedic surgery are more likely to affect people in middle age or later in life. According to a 2011 United States Census Bureau—International Data Base projection, the aging baby boomer generation will result in the percentage of the North American population aged 65 and over to grow from 13.4% in 2011 to 16.5% in 2020 and to 20.0% by 2030. In Western Europe, the population aged 65 and over is expected to grow from 18.5% in 2011 to 21.2% in 2020 and to 25.3% by 2030. In addition, according to the 2011 United States Census Bureau—International Data Base projection, the average life expectancy in North America is 78.6 years in 2011 and is expected to grow to 80.9 years by 2030. In Western Europe, the average life expectancy is 80.6 years in 2011 and is expected to grow to 82.4 years by 2030. As life expectancy increases, we believe people will remain active longer, causing the number of injuries requiring orthopedic rehabilitation, bone growth stimulation and reconstructive implants to increase. |
| • | Shift toward non-surgical rehabilitation devices and at-home physical therapy. We believe the growing awareness and clinical acceptance by healthcare professionals of the benefits of non-surgical, non-pharmaceutical treatment and rehabilitation products, combined with the increasing interest by patients in rehabilitation solutions that minimize risk and recuperation time and provide greater convenience, will continue to drive demand for these products. In addition, we believe that orthopedic surgeons are increasingly utilizing braces that assist in rehabilitation and bone growth stimulators that enable in-home treatment as viable alternatives to surgery. We design many of our orthopedic rehabilitation products for at-home use, which we believe should allow us to benefit from the market shift toward these treatment alternatives. |
2
Table of Contents
| • | Lower cost alternatives appeal to third party payors. With the cost of healthcare rising in the United States and internationally, third party payors are seeking more cost-effective therapies without reducing quality of care. For example, third party payors seek to reduce clinic visits and accommodate patients’ preference for therapies that can be conveniently administered at home. We believe that many of our orthopedic rehabilitation products offer cost-effective alternatives to surgery, pharmaceutical and other traditional forms of physical therapy and pain management. |
| • | Increased need for rehabilitation due to increased orthopedic surgical volume. The combination of increased prevalence of degenerative joint disease (such as osteoarthritis), an increased number of sports-related injuries, an aging population and improvements in orthopedic surgical technique (such as arthroscopy) has contributed to an increase in the number of orthopedic surgeries. We believe that orthopedic surgical volume will continue to increase, which should result in an increase in the need for our products. |
Competitive Strengths
We believe that we have a number of competitive strengths that will enable us to further enhance our position in the markets we serve:
| • | Leading market positions. We believe we have leading market positions for many of our products. We believe our orthopedic and physical therapy rehabilitation products marketed under the Aircast, DonJoy, ProCare, CMF, Empi, Chattanooga, DJO Surgical, Dr. Comfort and Compex brands have a reputation for quality, durability and reliability among healthcare professionals. We believe the strength of our brands and our focus on customer service have allowed us to establish market leading positions in the highly fragmented and growing orthopedic rehabilitation market. |
| • | Comprehensive range of orthopedic products. We offer a diverse range of medical devices for musculoskeletal health, vascular health and pain management, including rigid and soft orthopedic bracing, hot and cold therapy, bone growth stimulators, vascular therapy systems and compression garments, therapeutic shoes and inserts, electrical stimulators used for pain management and physical therapy products. Our surgical implant business offers a comprehensive suite of reconstructive joint products for the hip, knee and shoulder. Our broad product offering meets many of the needs of healthcare professionals and patients and enables us to leverage our brand loyalty with our customer and distributor base. Our products are available across various stages of the patient’s continuum of care. |
| • | Extensive and diverse distribution network. We use multiple channels to distribute our products to our customers. We use approximately 6,000 dealers and distributors and a direct sales force of approximately 700 employed sales representatives and approximately 1,200 independent sales representatives to supply our products to orthopedic specialists, spine surgeons, primary care physicians, pain management specialists, physical therapists, podiatrists, chiropractors, athletic trainers and other healthcare professionals. We believe that our distribution network provides us with a significant competitive advantage in selling our existing products and in introducing new products. |
| • | Strong relationships with managed care organizations and rehabilitation healthcare providers. Our leading market positions in many of our product lines and the breadth of our product offerings have enabled us to secure important preferred provider and managed care contracts. Our database includes approximately 8,250 different insurance companies and other payors, including approximately 1,530 active payor contracts. We have developed a proprietary third party billing system that is designed to reduce our reimbursement cycles, improve relationships with managed care organizations and physicians and track patients to improve quality of care and create subsequent selling opportunities. Further, our OfficeCare business maintains inventory at over 1,500 healthcare facilities, primarily orthopedic practices, which further strengthens our relationships with these healthcare providers. |
3
Table of Contents
| • | National contracts with group purchasing organizations. We enjoy strong relationships with a number of group purchasing organizations due to our significant scale. We believe that our broad range of products is well suited to the goals of these buying groups. Under these national contracts, we provide favorable pricing to the buying group and are designated a preferred purchasing source for the members of the buying group for specified products. As we have made acquisitions and expanded our product range, we have been able to add incremental products to our national contracts. During 2011, we signed or renewed approximately 30 national contracts. |
| • | Low cost, high quality manufacturing capabilities. We have a major manufacturing facility in Tijuana, Mexico that has been recognized for operational excellence. The Mexico facility and our other manufacturing facilities employ lean manufacturing, Six Sigma concepts and continuous improvement processes to drive manufacturing efficiencies and lower costs. |
| • | Ability to generate significant cash flow. Historically, our strong competitive position, brand awareness and high quality products and service as well as our low cost manufacturing have allowed us to generate attractive operating margins before non-cash amortization expense and certain non-recurring charges. These operating margins, together with limited capital expenditures and modest working capital requirements, significantly benefit our ability to generate cash flow. |
| • | Experienced management team. The members of our management team have an average of 29 years of relevant experience. This team has successfully integrated a number of acquisitions in the last several years. |
Strategy
Our strategy is to increase our leading position in key products and markets, increase revenues and profitability and enhance cash flow. Our key initiatives to implement this strategy include the following:
| • | Increase our leading market positions. We believe we are the market leader in many of the markets in which we compete. We intend to continue to increase our market share by leveraging the cross-selling and other opportunities created by the DJO Merger and by implementing the initiatives described below. The DJO Merger has allowed us to offer customers a more comprehensive range of products to better meet their evolving needs. We believe our size, scale, brand recognition, comprehensive and integrated product offerings and leading market positions enable us to capitalize on the growth in the orthopedic product industry. |
| • | Focus sales force on entire range of DJO products. Our products address the continuum of patient care from injury prevention to rehabilitation after surgery, injury or from degenerative disease. Our strategy is to train and incentivize our sales force, which consists of agents and representatives familiar with a particular set of products, to work cooperatively and collaboratively with all segments of our sales force to introduce their customers to the full range of our products of which the customer is typically using only a portion. We believe that this represents a significant opportunity to expand our business through existing customers. |
| • | Continue to develop and launch new products and product enhancements. We have a history of developing and introducing innovative products into the marketplace, and we expect to continue future product launches by leveraging our internal research and development platforms. We believe our ability to develop new technology and to advance existing technology to create new products will position us to further diversify our revenues and to expand our target markets by providing viable alternatives to surgery or medication. We believe that product innovation through effective and focused research and development, as well as our relationships with a number of widely recognized orthopedic surgeons and professionals who assist us in product research, development and marketing, will provide a significant competitive advantage. During 2011, sales of new products, which include products that have been on the market less than one year, were $44.7 million. |
4
Table of Contents
| • | Maximize existing and secure additional national accounts. We plan to capitalize on the growing practice in healthcare in which hospitals and other large healthcare providers seek to consolidate their purchasing activities to national buying groups. Contracts with these national accounts represent a significant opportunity for revenue growth. We believe that our existing relationships with national buying groups and our broad range of products position us to not only pursue additional national contracts, but also to expand the scope of our existing contracts. |
| • | Expand international sales. In recent years, we have successfully established direct distribution capabilities in several major international markets. We believe that sales to European and other markets outside the United States continue to represent a significant growth opportunity, and we intend to continue to expand our direct and independent distribution capabilities in attractive foreign markets. Several of the acquisitions we have made in recent years have substantially increased our international revenues and operating infrastructure and have provided us with opportunities to expand our international product offerings. |
| • | Drive operating efficiency. We plan to continue to apply the principles of lean operations to our manufacturing sites as well as in our operating and administrative functions to increase speed and efficiency and reduce waste. We have instilled a culture of continuous improvement throughout the Company and are pursuing a regular schedule of addressing operations and processes in the Company to improve efficiency. We believe these lean principles and continuous improvement efforts will enhance our operating efficiencies and our ability to compete in an increasingly price-sensitive healthcare industry. |
5
Table of Contents
Corporate Structure
The following diagram illustrates our corporate structure as of September 29, 2012, with debt amounts shown as adjusted to reflect (1) the issuance of $100.0 million aggregate principal amount of outstanding senior secured notes and $440.0 million aggregate principal amount of outstanding senior notes, (2) the repurchase or redemption of $465.0 million aggregate principal amount of outstanding 10.875% Senior Notes due 2014 (the “10.875% Senior Notes”) and (3) the repayment of borrowings under our senior secured revolving credit facilities.
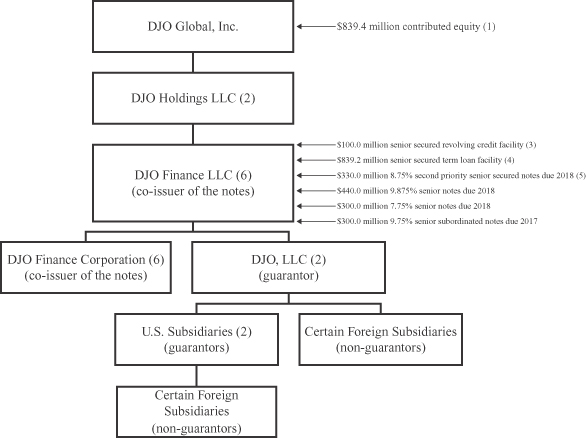
| (1) | Represents equity contributed by investment funds affiliated with The Blackstone Group, L.P. (“Blackstone”) and the contribution of equity by certain members of DJO Global, Inc. management primarily through the rollover of existing stock options in connection with the DJO Merger and the sales of common stock through private offerings to certain accredited investors comprised of employees, directors and independent sales agents. |
| (2) | The obligations under our senior secured credit facilities are guaranteed by DJO Holdings LLC and all of our existing and future direct and indirect wholly owned domestic restricted subsidiaries, subject to certain exceptions. The exchange notes will be guaranteed by all of our subsidiaries, other than DJO Finance Corporation, that guarantee the obligations under our senior secured credit facilities. The exchange notes will not be guaranteed by DJO Holdings LLC. The guarantors of the exchange notes are also guarantors of our 7.75% Senior Notes due 2018 (the “7.75% Senior Notes”) and our 9.75% Senior Subordinated Notes due 2017 (the “Senior Subordinated Notes” and, together with the 7.75% Senior Notes, the “Existing Notes”). |
6
Table of Contents
| (3) | As of September 29, 2012, we had $38.0 million of borrowings outstanding under our senior secured revolving credit facility. |
| (4) | As of September 29, 2012, we had $839.2 million of borrowings outstanding under our senior secured term loan facilities. Does not reflect unamortized original issue discount as of September 29, 2012 of $9.3 million. |
| (5) | Represents $100.0 million of outstanding senior secured notes (exclusive of an original issue premium associated with the exchange senior secured notes of $6.75 million) that we issued on October 1, 2012 and $230.0 million of outstanding senior secured notes that we issued on March 20, 2012. |
| (6) | DJO Finance LLC and DJO Finance Corporation are co-issuers of the Existing Notes and the outstanding notes. DJO Finance Corporation was formed solely to act as co-issuer of the Existing Notes, has only nominal assets and does not conduct any operations. See “Description of Senior Secured Notes” and “Description of Senior Notes.” |
Corporate Information
On November 3, 2006, affiliates of Blackstone acquired all of the outstanding shares of capital stock of ReAble (the “Blackstone Acquisition”). The total purchase price for the Blackstone Acquisition was approximately $529.2 million and was financed through a combination of equity contributed by affiliates of Blackstone, cash on hand of ReAble and a combination of borrowings under a prior senior secured credit facility and the issuance of senior subordinated notes.
On July 15, 2007, we entered into an agreement and plan of merger with DJO Opco pursuant to which DJO Opco became a wholly owned subsidiary of DJO Finance LLC. The total purchase price for the DJO Merger, which was consummated on November 20, 2007, was approximately $1.3 billion and was financed through a combination of equity contributed by affiliates of Blackstone, borrowings under our senior secured credit facilities and proceeds from the issuance of a portion of certain unsecured senior notes, which were redeemed in November 2012.
DJO Finance LLC was formed and DJO Finance Corporation was incorporated under the laws of the State of Delaware in September 2006 (as ReAble Therapeutics Finance LLC (formerly Encore Medical Finance LLC) and ReAble Therapeutics Finance Corporation (formerly Encore Medical Finance Corp.), respectively. DJO Global, Inc. (formerly DJO Incorporated and originally Healthcare Acquisition Corporation), the indirect parent company of DJO Finance LLC, was incorporated under the laws of the State of Delaware in March 1995. Our principal executive offices are located at 1430 Decision Street, Vista, California 92081, and our telephone number is (760) 727-1280.
Blackstone
Blackstone, one of the world’s leading investment and advisory firms, was founded in 1985. Through its different businesses, as of September 30, 2012, Blackstone had total fee-earning assets under management of approximately $168.6 billion. Blackstone’s alternative asset management businesses include the management of private equity funds, real estate funds, funds of hedge funds, credit-oriented funds, collateralized loan obligations vehicles and closed end mutual funds. Blackstone also provides various financial advisory services, including mergers and acquisition advisory, restructuring and reorganizational advisory and fund placement services.
7
Table of Contents
The Exchange Offers
$230.0 million aggregate principal amount of outstanding senior secured notes were issued in a private offering on March 20, 2012 and an additional $100.0 million aggregate principal amount of outstanding senior secured notes were issued in a private offering on October 1, 2012 (the “2012 additional senior secured notes offering”). $440.0 million aggregate principal amount of outstanding senior notes were issued in a private offering on October 1, 2012 (the “2012 senior notes offering”) and, together with the 2012 additional senior secured notes offering, the “2012 offering”. The term “notes” refers collectively to the outstanding notes and the exchange notes.
| General |
In connection with the private offerings, DJOFL and DJO Finance Corporation and the guarantors of the outstanding notes entered into registration rights agreements with the initial purchasers in which they agreed, among other things, to deliver this prospectus to you and to complete the applicable exchange offer within 360 days after the date of original issuance of the applicable outstanding notes. You are entitled to exchange in the applicable exchange offer your outstanding notes for the exchange notes which are identical in all material respects to the outstanding notes except: |
| • | the exchange notes have been registered under the Securities Act; |
| • | the exchange notes are not entitled to any registration rights which are applicable to the outstanding notes under the registration rights agreements; and |
| • | the liquidated damages provisions of the registration rights agreements are no longer applicable. |
| The Exchange Offers |
DJOFL and DJO Finance Corporation are offering to exchange: |
| • | $330.0 million aggregate principal amount of their exchange senior secured notes which have been registered under the Securities Act for any and all of the outstanding senior secured notes; and |
| • | $440.0 million aggregate principal amount of their exchange senior notes which have been registered under the Securities Act for any and all of the outstanding senior notes. |
| You may only exchange outstanding notes in denominations of $2,000 and integral multiples of $1,000, in excess thereof. |
| Resale |
Based on an interpretation by the staff of the SEC set forth in no-action letters issued to third parties, we believe that the exchange notes issued pursuant to the exchange offers in exchange for outstanding notes may be offered for resale, resold and otherwise transferred by you (unless you are our “affiliate” within the meaning of Rule 405 under the Securities Act) without compliance with the registration and prospectus delivery provisions of the Securities Act; provided that: |
| • | you are acquiring the exchange notes in the ordinary course of your business; and |
8
Table of Contents
| • | you have not engaged in, do not intend to engage in, and have no arrangement or understanding with any person to participate in, a distribution of the exchange notes. |
| If you are a broker-dealer and receive exchange notes for your own account in exchange for outstanding notes that you acquired as a result of market-making activities or other trading activities, you must acknowledge that you will deliver this prospectus in connection with any resale of the exchange notes. See “Plan of Distribution.” |
| Any holder of outstanding notes who: |
| • | is our affiliate; |
| • | does not acquire exchange notes in the ordinary course of its business; or |
| • | tenders its outstanding notes in the exchange offers with the intention to participate, or for the purpose of participating, in a distribution of exchange notes cannot rely on the position of the staff of the SEC enunciated in Morgan Stanley & Co. Incorporated (available June 5, 1991) and Exxon Capital Holdings Corporation (available May 13, 1988), as interpreted in the SEC’s letter to Shearman & Sterling, dated available July 2, 1993, or similar no-action letters and, in the absence of an exemption therefrom, must comply with the registration and prospectus delivery requirements of the Securities Act in connection with any resale of the exchange notes. |
| Expiration Date |
The exchange offers will expire at 12:00 a.m. midnight, New York City time, on February 6, 2013, unless extended by DJOFL and DJO Finance Corporation. DJOFL and DJO Finance Corporation do not currently intend to extend the expiration date. |
| Withdrawal |
You may withdraw the tender of your outstanding notes at any time prior to the expiration of the applicable exchange offer. DJOFL and DJO Finance Corporation will return to you any of your outstanding notes that are not accepted for any reason for exchange, without expense to you, promptly after the expiration or termination of the applicable exchange offer. |
| Conditions to the Exchange Offers |
Each exchange offer is subject to customary conditions, which DJOFL and DJO Finance Corporation may waive. See “The Exchange Offers—Conditions to the Exchange Offers.” |
| Procedures for Tendering Outstanding Notes |
If you wish to participate in the exchange offers, you must complete, sign and date the accompanying letter of transmittal, or a facsimile of such letter of transmittal, according to the instructions contained in this prospectus and the letter of transmittal. You must then mail or otherwise deliver the letter of transmittal, or a facsimile of such letter of transmittal, together with the outstanding notes and any other required documents, to the exchange agent at the address set forth on the cover page of the letter of transmittal. |
9
Table of Contents
| If you hold outstanding notes through The Depository Trust Company (“DTC”) and wish to participate in the exchange offers, you must comply with the Automated Tender Offer Program procedures of DTC by which you will agree to be bound by the letter of transmittal. By signing, or agreeing to be bound by, the letter of transmittal, you will represent to us that, among other things: |
| • | you are not our “affiliate” within the meaning of Rule 405 under the Securities Act; |
| • | you do not have an arrangement or understanding with any person or entity to participate in the distribution of the exchange notes; |
| • | you are acquiring the exchange notes in the ordinary course of your business; and |
| • | if you are a broker-dealer that will receive exchange notes for your own account in exchange for outstanding notes that were acquired as a result of market-making activities, that you will deliver a prospectus, as required by law, in connection with any resale of such exchange notes. |
| Special Procedures for Beneficial Owners |
If you are a beneficial owner of outstanding notes that are registered in the name of a broker, dealer, commercial bank, trust company or other nominee, and you wish to tender those outstanding notes in the applicable exchange offer, you should contact the registered holder promptly and instruct the registered holder to tender those outstanding notes on your behalf. If you wish to tender on your own behalf, you must, prior to completing and executing the letter of transmittal and delivering your outstanding notes, either make appropriate arrangements to register ownership of the outstanding notes in your name or obtain a properly completed bond power from the registered holder. The transfer of registered ownership may take considerable time and may not be able to be completed prior to the expiration date. |
| Guaranteed Delivery Procedures |
If you wish to tender your outstanding notes and your outstanding notes are not immediately available or you cannot deliver your outstanding notes, the letter of transmittal or any other required documents, or you cannot comply with the procedures under DTC’s Automated Tender Offer Program for transfer of book-entry interests, prior to the expiration date, you must tender your outstanding notes according to the guaranteed delivery procedures set forth in this prospectus under “The Exchange Offers—Guaranteed Delivery Procedures.” |
| Effect on Holders of Outstanding Notes |
As a result of the making of, and upon acceptance for exchange of, all validly tendered outstanding notes pursuant to the terms of the exchange offers, DJOFL and DJO Finance Corporation and the guarantors of the notes will have fulfilled a covenant under each registration rights agreement. Accordingly, there will be no increase |
10
Table of Contents
| in the interest rate on the outstanding notes under the circumstances described in the registration rights agreements. If you do not tender your outstanding notes in the applicable exchange offer, you will continue to be entitled to all the rights and limitations applicable to the outstanding notes as set forth in the applicable indenture; however, DJOFL and DJO Finance Corporation and the guarantors of the notes will not have any further obligation to you to provide for the exchange and registration of the outstanding notes under the applicable registration rights agreement. To the extent that the outstanding notes are tendered and accepted in the exchange offers, the trading market for the outstanding notes that are not so tendered and accepted could be adversely affected. |
| Consequences of Failure to Exchange |
All untendered outstanding notes will continue to be subject to the restrictions on transfer set forth in the outstanding notes and in the applicable indenture. In general, the outstanding notes may not be offered or sold, unless registered under the Securities Act, except pursuant to an exemption from, or in a transaction not subject to, the Securities Act and applicable state securities laws. Other than in connection with the exchange offers, DJOFL and DJO Finance Corporation and the guarantors of the notes do not currently anticipate that they will register the outstanding notes under the Securities Act. |
| United States Federal Income Tax Consequences |
The exchange of outstanding notes for exchange notes in the exchange offers will not be a taxable event to holders for United States federal income tax purposes. See “United States Federal Income Tax Consequences of the Exchange Offers.” |
| Use of Proceeds |
We will not receive any cash proceeds from the issuance of the exchange notes in the exchange offers. |
| Exchange Agent |
The Bank of New York Mellon is the exchange agent for the exchange offers. The addresses and telephone numbers of the exchange agent are set forth under “The Exchange Offers—Exchange Agent.” |
11
Table of Contents
The Exchange Notes
The summary below describes the principal terms of the exchange notes. Certain of the terms and conditions described below are subject to important limitations and exceptions. The “Description of Senior Secured Notes” and the “Description of Senior Notes” sections of this prospectus contain a more detailed description of the terms and conditions of each series of notes. The exchange notes will have terms identical in all material respects to the corresponding outstanding notes, except that the exchange notes will not contain terms with respect to transfer restrictions, registration rights and additional interest for failure to observe certain obligations in the applicable registration rights agreement.
| Issuers |
DJO Finance LLC, an indirect wholly owned subsidiary of DJO Global, Inc., and DJO Finance Corporation, a wholly owned subsidiary of DJO Finance LLC, will jointly and severally issue the notes. |
Securities Offered
| Exchange Senior Secured Notes |
$330,000,000 aggregate principal amount of 8.75% Second Priority Senior Secured Notes due 2018. |
| Exchange Senior Notes |
$440,000,000 aggregate principal amount of 9.875% Senior Notes due 2018. |
Maturity Date
| Exchange Senior Secured Notes |
March 15, 2018. |
| Exchange Senior Notes |
April 15, 2018. |
Interest
| Exchange Senior Secured Notes |
8.75% per annum, payable semi-annually on March 15 and September 15 of each year, commencing March 15, 2013. Interest on the exchange senior secured notes accrued from and including September 15, 2012. |
| Exchange Senior Notes |
9.875% per annum, payable semi-annually on April 15 and October 15 of each year, commencing April 15, 2013. Interest on the exchange senior notes accrued from and including October 1, 2012. |
Guarantees
| Exchange Senior Secured Notes |
The exchange senior secured notes will be jointly and severally and fully and unconditionally guaranteed on a senior secured basis by each of our existing and future direct and indirect wholly owned domestic subsidiaries that guarantees the senior secured credit facilities. |
| Exchange Senior Notes |
The exchange senior notes will be jointly and severally and fully and unconditionally guaranteed on a senior unsecured basis by each of our existing and future direct and indirect wholly owned domestic subsidiaries that guarantees the senior secured credit facilities. |
12
Table of Contents
| Our non-guarantor subsidiaries accounted for: |
| • | $236.1 million, or approximately 8.3%, of our total assets and $47.3 million, or approximately 1.8%, of our total liabilities, in each case as of September 29, 2012; and |
| • | $265.8 million, or 23.7%, of our net sales and $7.0 million, or 2.5%, of our Adjusted EBITDA, in each case for the twelve months ended September 29, 2012. |
Ranking
| Exchange Senior Secured Notes |
The exchange senior secured notes and the guarantees of such exchange senior secured notes will be the issuers’ and the guarantors’ senior secured obligations. Accordingly, they will: |
| • | rank contractually senior in right of payment to any existing and future subordinated indebtedness, including our existing Senior Subordinated Notes; |
| • | rank effectively senior in right of payment with all of our existing and future senior unsecured indebtedness; |
| • | rank equally in right of payment to any senior secured indebtedness to the extent of the collateral securing such indebtedness on a second-priority basis; |
| • | be effectively junior to the indebtedness under our senior secured credit facilities and any capital leases to the extent of the collateral securing such indebtedness on a first-priority basis; and |
| • | be structurally subordinated to all existing and future indebtedness and other liabilities of our non-guarantor subsidiaries (other than indebtedness and liabilities owed to us or one of our subsidiary guarantors). |
| As of September 29, 2012, as adjusted to give effect to the 2012 offering, exchange senior secured notes and related guarantees: |
| • | would have ranked effectively senior in right of payment to $1,040.0 million of senior unsecured indebtedness under our Existing Notes and the outstanding senior notes to the extent of the collateral securing such secured indebtedness on a second-priority basis; |
| • | would have ranked contractually senior in right of payment to $300.0 million of our Senior Subordinated Notes; |
| • | would have ranked effectively junior in right of payment to $839.2 million, exclusive of unamortized original issue discount of $9.3 million, of senior secured indebtedness under our senior secured credit facilities to the extent of the collateral securing such indebtedness on a first-priority basis; we had an additional $100.0 million of availability under the senior secured revolving credit facility; and |
13
Table of Contents
| • | would have been structurally junior to $47.3 million of the total liabilities of our non-guarantor subsidiaries. |
| Exchange Senior Notes |
The exchange senior notes and the guarantees of such exchange senior notes will be the issuers’ and the guarantors’ senior unsecured obligations. Accordingly, they will: |
| • | rank contractually senior in right of payment to any existing and future subordinated indebtedness, including our existing Senior Subordinated Notes; |
| • | rank equally in right of payment with all of our existing and future senior indebtedness; |
| • | be effectively subordinated in right of payment to all of the existing and future secured debt of the issuers and the guarantors (including our senior secured credit facilities and the outstanding senior secured notes) to the extent of the value of the assets securing such debt; and |
| • | be structurally subordinated to all existing and future indebtedness and other liabilities of our non-guarantor subsidiaries (other than indebtedness and liabilities owed to us or one of our subsidiary guarantors). |
| As of September 29, 2012, as adjusted to give effect to the 2012 offering, the exchange senior notes: |
| • | would have ranked contractually senior in right of payment to $300.0 million of our Senior Subordinated Notes; |
| • | would have ranked equal in right of payment to $300.0 million of our 7.75% Senior Notes; |
| • | would have ranked effectively junior in right of payment to $1,169.2 million, exclusive of unamortized original issue discounts and premiums aggregating to a net discount of $2.6 million, of senior secured indebtedness under our senior secured credit facilities and the outstanding senior secured notes to the extent of the value of the assets securing such debt; we had an additional $100.0 million of availability under the senior secured revolving credit facility; and |
| • | would have been structurally junior to $47.3 million of the total liabilities of our non-guarantor subsidiaries. |
Security
| Exchange Senior Secured Notes |
The exchange senior secured notes and related guarantees will be secured by second-priority liens, subject to permitted liens, on certain of our assets and the assets of the subsidiary guarantors that secure our senior secured credit facilities including: |
| • | substantially all the capital stock of our wholly owned subsidiaries and each subsidiary guarantor of the notes (but |
14
Table of Contents
| limited to 65% of the voting stock of any such wholly owned subsidiary that is a foreign subsidiary); and |
| • | substantially all tangible and intangible assets of our company and each subsidiary guarantor, other than certain customary exceptions. See “Description of Senior Secured Notes—Security for the exchange senior secured notes.” |
| Exchange Senior Notes |
The exchange senior notes and related guarantees will be unsecured. |
Optional Redemption
| Exchange Senior Secured Notes |
We are entitled to redeem some or all of the exchange senior secured notes at any time on or after March 15, 2015 at the redemption prices set forth in this prospectus. Prior to March 15, 2015, we are entitled to redeem some or all of the exchange senior secured notes at a price equal to 100% of the principal amount thereof, plus accrued and unpaid interest, if any, plus the “make-whole” premium set forth in this prospectus. In addition, we are entitled to redeem up to 35% of the aggregate principal amount of the exchange senior secured notes until March 15, 2015 with the net proceeds from certain equity offerings at the redemption price set forth in this prospectus. |
| Exchange Senior Notes |
We are entitled to redeem some or all of the exchange senior notes at any time on or after April 15, 2015 at the redemption prices set forth in this prospectus. Prior to April 15, 2015, we are entitled to redeem some or all of the exchange senior notes at a price equal to 100% of the principal amount thereof, plus accrued and unpaid interest, if any, plus the “make-whole” premium set forth in this prospectus. In addition, we are entitled to redeem up to 35% of the aggregate principal amount of the exchange senior notes until April 15, 2015 with the net proceeds from certain equity offerings at the redemption price set forth in this prospectus. |
| Change of Control Offer |
Upon the occurrence of a change of control, we must give holders of the exchange notes an opportunity to sell to us some or all of their exchange notes at a price equal to 101% of their principal amount, plus accrued and unpaid interest to the repurchase date. See “Description of Senior Secured Notes—Repurchase at the Option of Holders—Change of Control” and “Description of Senior Notes—Repurchase at the Option of Holders—Change of Control.” |
| Certain Covenants |
The indentures governing the exchange senior secured notes and the exchange senior notes contain covenants limiting our ability and the ability of our restricted subsidiaries to: |
| • | incur additional debt or issue certain preferred and convertible shares; |
| • | pay dividends on, redeem, repurchase or make distributions in respect of our capital stock or make other restricted payments; |
| • | make certain investments; |
15
Table of Contents
| • | sell certain assets; |
| • | create liens on certain assets to secure debt; |
| • | consolidate, merge, sell or otherwise dispose of all or substantially all of our assets; |
| • | enter into certain transactions with our affiliates; and |
| • | designate our subsidiaries as unrestricted subsidiaries. |
| These covenants are subject to a number of important limitations and exceptions. In addition, during any period of time that the applicable series of Notes have investment grade ratings from both Moody’s Investors Service, Inc. and Standard & Poor’s, many of these covenants will cease to apply. See “Description of Senior Secured Notes” and “Description of Senior Notes.” |
| Public Market |
The exchange notes will be freely transferrable but will be new securities for which there will not initially be a market. Accordingly, we cannot assure you that a liquid market for the exchange notes will develop. See “Risk Factors—Risks Related to Our Indebtedness and the Exchange Notes—Your ability to transfer the exchange notes may be limited by the absence of an active trading market, and there is no assurance that any active trading market will be maintained for the exchange notes.” |
You should carefully consider all the information in the prospectus prior to exchanging your outstanding notes. In particular, we urge you to carefully consider the factors set forth under the “Risk Factors” section.
16
Table of Contents
You should carefully consider the following risk factors and all other information contained in this prospectus before deciding to tender your outstanding notes in the exchange offers. The risks and uncertainties described below are not the only ones we face. Our ability to achieve our operating and financial goals is subject to a number of risks, including risks relating to our business operations, our debt level and government regulations. If any of the following risks actually occur, our business, operating results, prospects or financial condition could be materially and adversely affected. The risks described below are not the only ones that we face. Additional risks not presently known to us or that we currently deem immaterial may also affect our business operations.
Risks Related to the Exchange Offers
There may be adverse consequences if you do not exchange your outstanding notes.
If you do not exchange your outstanding notes for exchange notes in the exchange offers, you will continue to be subject to restrictions on transfer of your outstanding notes as set forth in the applicable offering circular distributed in connection with the private offerings of the outstanding notes. In general, the outstanding notes may not be offered or sold unless they are registered or exempt from registration under the Securities Act and applicable state securities laws. Except as required by the applicable registration rights agreement, we do not intend to register resales of the outstanding notes under the Securities Act. You should refer to “Prospectus Summary—The Exchange Offers” and “The Exchange Offers” for information about how to tender your outstanding notes.
The tender of outstanding notes under the exchange offers will reduce the outstanding amount of the outstanding notes, which may have an adverse effect upon, and increase the volatility of, the market price of the outstanding notes not exchanged in the exchange offers due to a reduction in liquidity.
Certain persons who participate in the exchange offers must deliver a prospectus in connection with resales of the exchange notes.
Based on interpretations of the staff of the SEC contained in Exxon Capital Holdings Corp., SEC no-action letter (April 13, 1988), Morgan Stanley & Co. Inc., SEC no-action letter (June 5, 1991) and Shearman & Sterling, SEC no-action letter (July 2, 1983), we believe that you may offer for resale, resell or otherwise transfer the exchange notes without compliance with the registration and prospectus delivery requirements of the Securities Act. However, in some instances described in this prospectus under “Plan of Distribution,” certain holders of exchange notes will remain obligated to comply with the registration and prospectus delivery requirements of the Securities Act to transfer the exchange notes. If such a holder transfers any exchange notes without delivering a prospectus meeting the requirements of the Securities Act or without an applicable exemption from registration under the Securities Act, such a holder may incur liability under the Securities Act. We do not and will not assume, or indemnify such a holder against, this liability.
Risks Related to Our Indebtedness
Our substantial leverage could adversely affect our ability to raise additional capital to fund our operations, limit our ability to react to changes in the economy or our industry, expose us to interest rate risk to the extent of our variable rate debt and prevent us from meeting our obligations under the indebtedness.
We are highly leveraged. As of September 29, 2012, our total indebtedness was $2,165.5 million, exclusive of unamortized original issue discounts and premiums aggregating to a net discount of $2.6 million. We also had an additional $62.0 million available for borrowing under our senior secured revolving credit facility. Our high degree of leverage could have important consequences for you, including:
| • | making it difficult for us to make payments on our Existing Notes, the notes and other debt, |
| • | increasing our vulnerability to general economic and industry conditions, |
17
Table of Contents
| • | requiring a substantial portion of cash flow from operations to be dedicated to the payment of principal and interest on our indebtedness, therefore reducing our ability to use our cash flow to fund our operations, capital expenditures and future business opportunities, |
| • | exposing us to the risk of increased interest rates as certain of our borrowings, including certain borrowings under our senior secured credit facilities, will be subject to variable rates of interest, |
| • | limiting our ability to make strategic acquisitions or causing us to make non-strategic divestitures, |
| • | limiting our ability to obtain additional financing for working capital, capital expenditures, product development, debt service requirements, acquisitions and general corporate or other purposes, and |
| • | limiting our ability to adjust to changing market conditions and placing us at a competitive disadvantage compared to our competitors who are less highly leveraged. |
We, and our subsidiaries, will be able to incur substantial additional indebtedness in the future. Although our senior secured credit facilities and the indentures governing the Existing Notes and the indentures that govern the notes (collectively, the “Indentures”) contain restrictions on the incurrence of additional indebtedness, these restrictions are subject to a number of significant qualifications and exceptions, and under certain circumstances, the amount of indebtedness that could be incurred in compliance with these restrictions could be substantial. If we add new borrowings to our current debt levels, the related risks that we now face could intensify. In addition, the Indentures will not prevent us from incurring obligations that do not constitute indebtedness under such Indentures.
Our cash paid for interest for the nine months ended September 29, 2012 and the years ended December 31, 2009, 2010 and 2011 was $103.4 million, $144.2 million, $139.1 million, and $151.2 million, respectively. As of September 29, 2012, we had $839.2 million of debt subject to floating interest rates under the senior secured credit facilities, exclusive of $9.3 million of unamortized original issue discount.
Our debt agreements contain restrictions that limit our flexibility in operating our business.
Our senior secured credit facilities and the Indentures contain various covenants that limit our ability to engage in specified types of transactions. These covenants limit our and our restricted subsidiaries’ ability to, among other things:
| • | incur additional indebtedness or issue certain preferred shares, |
| • | pay dividends on, repurchase or make distributions in respect of our capital stock or make other restricted payments, |
| • | make certain investments, |
| • | sell certain assets, |
| • | create liens, |
| • | consolidate, merge, sell or otherwise dispose of all or substantially all of our assets, and |
| • | enter into certain transactions with our affiliates. |
In addition, we are required to satisfy and maintain a specified senior secured leverage ratio, which becomes more restrictive over time. This covenant could materially adversely affect our ability to finance our future operations or capital needs. Furthermore, it may restrict our ability to conduct and expand our business and pursue our business strategies. Our ability to meet this senior secured leverage ratio can be affected by events beyond our control, including changes in general economic and business conditions, and we cannot assure you that we will meet the senior secured leverage ratio in the future or at all.
18
Table of Contents
A breach of any of these covenants could result in a default under one or more of these agreements, including as a result of cross default provisions. Upon the occurrence of an event of default under the senior secured credit facilities, the lenders could elect to declare all amounts outstanding under the senior secured credit facilities to be immediately due and payable and terminate all commitments to extend further credit. Such actions by those lenders could cause cross defaults under our other indebtedness. If we were unable to repay those amounts, the lenders under the senior secured credit facilities could proceed against the collateral granted to them to secure that indebtedness. We have pledged substantially all of our assets as collateral under the senior secured credit facilities. If the lenders under the senior secured credit facilities accelerate the repayment of borrowings, we cannot assure you that we will have sufficient assets to repay the amounts borrowed under the senior secured credit facilities, as well as our unsecured indebtedness.
Risks Related to the Notes
We may not be able to generate sufficient cash to service all of our indebtedness, including the notes, and may be forced to take other actions to satisfy our obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments on or to refinance our debt obligations depends on our financial condition and operating performance, which is subject to prevailing economic and competitive conditions and to certain financial, business and other factors beyond our control. We cannot assure you that we will maintain a level of cash flows from operating activities sufficient to permit us to pay the principal, premium, if any, and interest on our indebtedness, including the notes.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay investments and capital expenditures, or to sell assets, seek additional capital or restructure or refinance our indebtedness, including the notes. These alternative measures could affect the operation and growth of our business and may not be successful and may not permit us to meet our scheduled debt service obligations. If our operating results and available cash are insufficient to meet our debt service obligations, we could face substantial liquidity problems and might be required to dispose of material assets or operations to meet our debt service and other obligations. In that case, we may not be able to consummate those dispositions or to obtain the proceeds that we could realize from them, and the proceeds from those dispositions may not be adequate to meet any debt service obligations then due. Additionally, our senior secured credit facilities and the Indentures limit the use of the proceeds from dispositions of assets; as a result, we may not be permitted to use the proceeds from such dispositions to satisfy all current debt service obligations.
Claims of noteholders will be structurally subordinate to claims of creditors of all of our non-U.S. subsidiaries because they will not guarantee the notes.
The notes will not be guaranteed by our non-U.S. subsidiaries. Accordingly, claims of holders of the notes will be structurally subordinate to the claims of creditors of these non-guarantor subsidiaries, including trade creditors. All obligations of our non-guarantor subsidiaries will have to be satisfied before any of the assets of such subsidiaries would be available for distribution, upon a liquidation or otherwise, to us or a guarantor of the notes.
If we default on our obligations to pay our indebtedness, we may not be able to make payments on the exchange notes.
Any default under the agreements governing our indebtedness, including a default under our senior secured credit facilities, that is not waived by the required lenders, and the remedies sought by the holders of such indebtedness could make us unable to pay principal, premium, if any, and interest on the exchange notes and substantially decrease the market value of the exchange notes. If we are unable to generate sufficient cash flow and are otherwise unable to obtain funds necessary to meet required payments of principal, premium, if any, and interest on our indebtedness, or if we otherwise fail to comply with the various covenants, including financial and
19
Table of Contents
operating covenants, in the instruments governing our indebtedness (including covenants in our senior secured credit facilities and the Indentures), we could be in default under the terms of the agreements governing such indebtedness, including our senior secured credit facilities and the Indentures. In the event of such default, the holders of such indebtedness could elect to declare all the funds borrowed thereunder to be due and payable, together with accrued and unpaid interest, the lenders under our senior secured credit facilities could elect to terminate their commitments thereunder, cease making further loans and institute foreclosure proceedings against our assets, and we could be forced into bankruptcy or liquidation. If our operating performance declines, we may in the future need to obtain waivers from the required lenders under our senior secured credit facilities to avoid being in default. If we breach our covenants under our senior secured credit facilities and seek a waiver, we may not be able to obtain a waiver from the required lenders. If this occurs, we would be in default under our senior secured credit facilities, the lenders could exercise their rights, as described above, and we could be forced into bankruptcy or liquidation.
We may not be able to repurchase the notes upon a change of control.
Upon the occurrence of specific kinds of change of control events, we will be required to offer to repurchase all outstanding Notes at 101% of their principal amount plus accrued and unpaid interest. The source of funds for any such purchase of the notes will be our available cash or cash generated from our subsidiaries’ operations or other sources, including borrowings, sales of assets, or sales of equity. We may not be able to repurchase the notes upon a change of control because we may not have sufficient financial resources to purchase all of the notes that are tendered upon a change of control. Further, we are contractually restricted under the terms of our senior secured credit facilities from repurchasing all of the notes tendered by holders upon a change of control. Accordingly, we may not be able to satisfy our obligations to purchase the notes unless we are able to refinance or obtain waivers under our senior secured credit facilities. Our failure to repurchase the notes upon a change of control would cause a default under the indentures that will govern the notes and a cross default under our senior secured credit facilities and the indentures governing our Existing Notes. The senior secured credit facilities also provide that a change of control will be a default that permits lenders to accelerate the maturity of borrowings thereunder and, if such debt is not paid, to enforce security interests in the collateral securing such debt, thereby limiting our ability to raise cash to purchase the notes, and reducing the practical benefit of the offer-to-purchase provisions to the holders of the notes. Any of our future debt agreements may contain similar provisions.
In addition, the change of control provisions in the indentures that will govern the notes may not protect you from certain important corporate events, such as a leveraged recapitalization (which would increase the level of our indebtedness), reorganization, restructuring, merger or other similar transaction. Such a transaction may not involve a change in voting power or beneficial ownership or, even if it does, may not involve a change that constitutes a “Change of Control” as defined in such indentures that would trigger our obligation to repurchase the notes. If an event occurs that does not constitute a “Change of Control” as defined in such indentures, we will not be required to make an offer to repurchase the notes and you may be required to continue to hold your Notes despite the event.
The lenders under our senior secured credit facilities will have the discretion to release the guarantors under our senior secured credit facilities in a variety of circumstances, which will cause those guarantors to be released from their guarantees of the notes.
While any obligations under the senior secured credit facilities remain outstanding, any guarantor of the notes may be released without action by, or consent of, any holder of the notes or the trustee under the indentures that will govern the notes, at the discretion of lenders under the senior secured credit facilities, if the guarantor is no longer a guarantor of obligations under the senior secured credit facilities or any other indebtedness. See “Description of Senior Secured Notes” and “Description of Senior Notes.” The lenders under the senior secured credit facilities will have the discretion to release the guarantors under the senior secured credit facilities in a variety of circumstances. You will not have a claim as a creditor against any subsidiary that is no longer a guarantor of the notes, and the indebtedness and other liabilities, including trade payables, whether secured or unsecured, of those subsidiaries will effectively be senior to claims of noteholders.
20
Table of Contents
Federal and state fraudulent transfer laws may permit a court to void the notes and the guarantees, and, if that occurs, you may not receive any payments on the notes.
Federal and state fraudulent transfer and conveyance statutes may apply to the issuance of the notes and the incurrence of any guarantees. Under federal bankruptcy law and comparable provisions of state fraudulent transfer or conveyance laws, which may vary from state to state, the notes or guarantees could be voided as a fraudulent transfer or conveyance if (1) we or any of the guarantors, as applicable, issued the notes or incurred the guarantees with the intent of hindering, delaying, or defrauding creditors or (2) we or any of the guarantors, as applicable, received less than reasonably equivalent value or fair consideration in return for either issuing the notes or incurring the guarantees and, in the case of (2) only, one of the following is also true at the time thereof:
| • | we or any of the guarantors, as applicable, were insolvent or rendered insolvent by reason of the issuance of the notes or the incurrence of the guarantees; |
| • | the issuance of the notes or the incurrence of the guarantees left us or any of the guarantors, as applicable, with an unreasonably small amount of capital to carry on the business; |
| • | we or any of the guarantors intended to, or believed that we or such guarantor would, incur debts beyond our or such guarantor’s ability to pay such debts as they mature; or |
| • | we or any of the guarantors was a defendant in an action for money damages, or had a judgment for money damages docketed against us or such guarantor if, in either case, after final judgment, the judgment is unsatisfied. |
If a court were to find that the issuance of the notes or the incurrence of the guarantee was a fraudulent transfer or conveyance, the court could void the payment obligations under the notes or such guarantee or subordinate the notes or such guarantee to presently existing and future indebtedness of ours or of the related guarantor, or require the holders of the notes to repay any amounts received with respect to the notes or such guarantee. In the event of a finding that a fraudulent transfer or conveyance occurred, you may not receive any repayment on the notes. In addition, each guarantee will contain a provision intended to limit the guarantor’s liability to the maximum amount that it could incur without causing the incurrence of obligations under its guarantee to be a fraudulent conveyance. This provision may not be effective to protect the guarantees from being voided under fraudulent conveyance laws or may eliminate the guarantor’s obligations or reduce the guarantor’s obligations to an amount that effectively makes the guarantee worthless. In a recent Florida bankruptcy case, this kind of provision was found to be ineffective to protect the guarantees. Further, the voidance of the notes could result in an event of default with respect to our and our subsidiaries’ other debt that could result in acceleration of such debt.
As a general matter, value is given for a transfer or an obligation if, in exchange for the transfer or obligation, property is transferred or an antecedent debt is secured or satisfied. A debtor will generally not be considered to have received value in connection with a debt offering if the debtor uses the proceeds of that offering to make a dividend payment or otherwise retire or redeem equity securities issued by the debtor.
We cannot be certain as to the standards a court would use to determine whether or not we or the guarantors were solvent at the relevant time or, regardless of the standard that a court uses, that the issuance of the notes and the guarantees would not be subordinated to our or any of our guarantors’ other debt. Generally, however, an entity would be considered insolvent if, at the time it incurred indebtedness:
| • | the sum of its debts, including contingent liabilities, was greater than the fair saleable value of all its assets; |
| • | the present fair saleable value of its assets was less than the amount that would be required to pay its probable liability on its existing debts, including contingent liabilities, as they become absolute and mature; or |
| • | it could not pay its debts as they become due. |
21
Table of Contents
If the guarantees were legally challenged, any guarantee could also be subject to the claim that, since the guarantee was incurred for our benefit, and only indirectly for the benefit of the guarantor, the obligations of the applicable guarantor were incurred for less than fair consideration. A court could thus void the obligations under the guarantees, subordinate them to the applicable guarantor’s other debt or take other action detrimental to the holders of the notes.
Your ability to transfer the notes may be limited by the absence of an active trading market, and there is no assurance that any active trading market will develop for the notes.
There is no established public market for the notes.
The initial purchasers have advised us that they intend to make a market in the notes, as permitted by applicable laws and regulations; however, the initial purchasers are not obligated to make a market in the notes, and they may discontinue their market-making activities at any time without notice. Therefore, we cannot assure you that an active market for the notes will develop or, if developed, that it will continue. Historically, the market for non-investment grade debt has been subject to disruptions that have caused substantial volatility in the prices of securities similar to the notes. We cannot assure you that the market, if any, for the notes or exchange notes will be free from similar disruptions or that any such disruptions may not adversely affect the prices at which you may sell your Notes. In addition, subsequent to their initial issuance, the notes may trade at a discount from their initial offering price, depending upon prevailing interest rates, the market for similar notes, our performance and other factors.
The trading price of the exchange notes may be volatile.
The trading price of the exchange notes could be subject to significant fluctuation in response to, among other factors, changes in our operating results, interest rates, the market for non-investment grade securities, general economic conditions and securities analysts’ recommendations regarding our securities.
Risks Related to the Exchange Senior Secured Notes
Other secured indebtedness, including our senior secured credit facilities, will be effectively senior to the exchange senior secured notes to the extent of the value of the collateral securing such facility on a first-priority basis.
Certain of our senior secured credit facilities are collateralized by a first-priority lien, subject to permitted liens, in, among other things, the capital stock of the Company, the capital stock of any material wholly owned first-tier subsidiary of the Company or of any U.S. subsidiary guarantor and substantially all of our and the U.S. subsidiary guarantors’ other tangible and intangible assets, subject to exceptions. The Indentures will also permit us to incur additional indebtedness secured on a first-priority basis by such assets in the future. The first-priority liens in the collateral securing indebtedness under our senior secured credit facilities and any such future indebtedness will be higher in priority as to such collateral than the security interests securing the exchange senior secured notes and the related guarantees. The exchange senior secured notes and the related guarantees will be secured, subject to permitted liens, by a second-priority lien, in the assets that secure our senior secured credit facilities on a first-priority basis. Holders of the indebtedness under our senior secured credit facilities and any other indebtedness collateralized by a higher-priority lien in such collateral will be entitled to receive proceeds from the realization of value of such collateral to repay such indebtedness in full before the holders of the exchange senior secured notes will be entitled to any recovery from such collateral. As a result, holders of the exchange senior secured notes will only be entitled to receive proceeds from the realization of value of assets securing our senior secured credit facilities on a higher-priority basis after all indebtedness and other obligations under our senior secured credit facilities and any other obligations secured by higher-priority liens on such assets are repaid in full. The exchange senior secured notes will be effectively junior in right of payment to indebtedness under our senior secured credit facilities and any other indebtedness collateralized by a higher-priority lien in our assets, to the extent of the realizable value of such collateral. In addition, the indenture
22
Table of Contents
governing the exchange senior secured notes permits us to incur additional indebtedness secured by a lien that ranks equally with the exchange senior secured notes. Any such indebtedness may further limit the recovery from the realization of the value of such collateral available to satisfy holders of the exchange senior secured notes.
As of September 29, 2012, we had outstanding an aggregate principal amount of $1,169.2 million of senior secured indebtedness, exclusive of unamortized original issue discounts and premiums aggregating to a net discount of $2.6 million. We also had an additional $62.0 million available for borrowing under our senior secured revolving credit facility.
The lien ranking provisions of the indenture governing the exchange senior secured notes and other agreements relating to the collateral securing the exchange senior secured notes will limit the rights of holders of the exchange senior secured notes with respect to that collateral, even during an event of default.
The rights of the holders of the exchange senior secured notes with respect to the collateral that will secure the exchange senior secured notes on a second-priority basis will be substantially limited by the terms of the lien ranking agreements set forth in the indenture governing the exchange senior secured notes and the intercreditor agreement, even during an event of default. Under the indenture governing the exchange senior secured notes and the intercreditor agreement, at any time that obligations that have the benefit of the higher-priority liens are outstanding, any actions that may be taken with respect to such collateral, including the ability to cause the commencement of enforcement proceedings against such collateral and to control the conduct of such proceedings, and the approval of amendments to, releases of such collateral from the lien of, and waivers of past defaults under, such documents relating to such collateral, will be at the direction of the holders of the obligations secured by the first-priority liens and the holders of the exchange senior secured notes secured by lower-priority liens may be adversely affected.
In addition, the indenture governing the exchange senior secured notes and the intercreditor agreement contain certain provisions benefiting holders of indebtedness under our senior secured credit facilities, including provisions requiring the trustee and the second lien agent not to object following the filing of a bankruptcy petition to a number of important matters regarding the collateral. After such filing, the value of this collateral could materially deteriorate, and holders of the exchange senior secured notes would be unable to raise an objection. In addition, the right of holders of obligations secured by first-priority liens to foreclose upon and sell such collateral upon the occurrence of an event of default also would be subject to limitations under applicable bankruptcy laws if we or any of our subsidiaries become subject to a bankruptcy proceeding.
The collateral that will secure the exchange senior secured notes and related guarantees on a lower-priority basis will also be subject to any and all exceptions, defects, encumbrances, liens and other imperfections as may be accepted by the lenders under our senior secured credit facilities and other creditors that have the benefit of higher-priority liens on such collateral from time to time, whether on or after the date the exchange senior secured notes and related guarantees are issued. The existence of any such exceptions, defects, encumbrances, liens and other imperfections could adversely affect the value of the collateral securing the exchange senior secured notes as well as the ability of the second lien agent to realize or foreclose on such collateral. The initial purchasers have neither analyzed the effect of, nor participated in any negotiations relating to, such exceptions, defects, encumbrances, liens and imperfections, and the existence thereof could adversely affect the value of the collateral that will secure the exchange senior secured notes as well as the ability of the second lien agent to realize or foreclose on such collateral.
In the event of our bankruptcy, the ability of the holders of the exchange senior secured notes to realize upon the collateral will be subject to certain bankruptcy law limitations.
The ability of holders of the exchange senior secured notes to realize upon the collateral will be subject to certain bankruptcy law limitations in the event of our bankruptcy. Under applicable U.S. federal bankruptcy laws, secured creditors are prohibited from, among other things, repossessing their security from a debtor in a
23
Table of Contents
bankruptcy case without bankruptcy court approval and may be prohibited from retaining security repossessed by such creditor without bankruptcy court approval. Moreover, applicable federal bankruptcy laws generally permit the debtor to continue to retain collateral, including cash collateral, even though the debtor is in default under the applicable debt instruments, provided that the secured creditor is given “adequate protection.”
The secured creditor is entitled to “adequate protection” to protect the value of the secured creditor’s interest in the collateral as of the commencement of the bankruptcy case but the adequate protection actually provided to a secured creditor may vary according to the circumstances. Adequate protection may include cash payments or the granting of additional security if and at such times as the court, in its discretion and at the request of such creditor, determines after notice and a hearing that the collateral has diminished in value as a result of the imposition of the automatic stay of repossession of such collateral or the debtor’s use, sale or lease of such collateral during the pendency of the bankruptcy case. In view of the lack of a precise definition of the term “adequate protection” and the broad discretionary powers of a U.S. bankruptcy court, we cannot predict whether or when the trustee under the indenture governing the exchange senior secured notes could foreclose upon or sell the collateral or whether or to what extent holders of exchange senior secured notes would be compensated for any delay in payment or loss of value of the collateral through the requirement of “adequate protection.”
Moreover, the controlling collateral agent and the applicable authorized representative under the intercreditor agreement among all holders of obligations secured by that collateral on a first-priority basis may need to evaluate the impact of the potential liabilities before determining to foreclose on collateral consisting of real property, if any, because secured creditors that hold a security interest in real property may be held liable under environmental laws for the costs of remediating or preventing the release or threatened releases of hazardous substances at such real property. Consequently, the controlling collateral agent may decline to foreclose on such collateral or exercise remedies available in respect thereof if it does not receive indemnification to its satisfaction from the holders of the exchange senior secured notes or holders of other obligations secured by that collateral on a first-priority basis. See “Description of Senior Secured Notes.”
The value of the collateral securing the exchange senior secured notes may not be sufficient to satisfy our obligations under the exchange senior secured notes.
No appraisal of the value of the collateral has been made in connection with the issuances of the outstanding senior secured notes, and the fair market value of the collateral is subject to fluctuations based on factors that include, among others, general economic conditions and similar factors. The amount to be received upon a sale of the collateral would be dependent on numerous factors, including, but not limited to, the actual fair market value of the collateral at such time, the timing and the manner of the sale and the availability of buyers. By its nature, portions of the collateral may be illiquid and may have no readily ascertainable market value. In the event of a foreclosure, liquidation, bankruptcy or similar proceeding, the collateral may not be sold in a timely or orderly manner, and the proceeds from any sale or liquidation of this collateral may not be sufficient to pay our obligations under the exchange senior secured notes.
To the extent that liens securing obligations under the senior secured credit facilities, pre-existing liens, liens permitted under the indenture governing the exchange senior secured notes and other rights, including liens on excluded assets, such as those securing purchase money obligations and capital lease obligations granted to other parties (in addition to the holders of any other obligations secured by higher priority liens), encumber any of the collateral securing the exchange senior secured notes and the related guarantees, those parties have or may exercise rights and remedies with respect to the collateral that could adversely affect the value of the collateral and the ability of the second lien agent, the trustee under the indenture governing the exchange senior secured notes or the holders of the exchange senior secured notes to realize or foreclose on the collateral.
There may not be sufficient collateral to pay off all amounts we may borrow under our senior secured credit facilities, the exchange senior secured notes and additional notes that we may offer that would be secured on the same basis as the exchange senior secured notes. Liquidating the collateral securing the exchange senior secured notes may not result in proceeds in an amount sufficient to pay any amounts due under the exchange senior
24
Table of Contents
secured notes after also satisfying the obligations to pay any creditors with prior liens. In addition, we may be required to repay obligations under our senior secured credit facilities with proceeds from a sale of assets before repaying the exchange senior secured notes. If the proceeds of any sale of collateral are not sufficient to repay all amounts due on the exchange senior secured notes, the holders of the exchange senior secured notes (to the extent not repaid from the proceeds of the sale of the collateral) would have only a senior unsecured, unsubordinated claim against our and the subsidiary guarantors’ remaining assets.
In the event of a bankruptcy of us or any of the subsidiary guarantors, holders of the exchange senior secured notes may be deemed to have an unsecured claim to the extent that our obligations in respect of the exchange senior secured notes exceed the fair market value of the collateral securing the exchange senior secured notes.
In any bankruptcy proceeding with respect to us or any of the guarantors, it is possible that the bankruptcy trustee, the debtor-in-possession or competing creditors will assert that the fair market value of the collateral with respect to the exchange senior secured notes on the date of the bankruptcy filing was less than the then-current principal amount of the exchange senior secured notes. Upon a finding by the bankruptcy court that the exchange senior secured notes are under-collateralized, the claims in the bankruptcy proceeding with respect to the exchange senior secured notes would be bifurcated between a secured claim and an unsecured claim, and the unsecured claim would not be entitled to the benefits of security in the collateral. Other consequences of a finding of under-collateralization would be, among other things, a lack of entitlement on the part of the exchange senior secured notes to receive post-petition interest and a lack of entitlement on the part of the unsecured portion of the exchange senior secured notes to receive “adequate protection” under federal bankruptcy laws. In addition, if any payments of post-petition interest had been made at any time prior to such a finding of under-collateralization, those payments would be recharacterized by the bankruptcy court as a reduction of the principal amount of the secured claim with respect to the exchange senior secured notes.
The value of the collateral securing the exchange senior secured notes may not be sufficient to secure post-petition interest.
In the event of a bankruptcy, liquidation, dissolution, reorganization or similar proceeding against us, holders of the exchange senior secured notes will only be entitled to post-petition interest under the U.S. Bankruptcy Code to the extent that the value of their security interest in the collateral is greater than their pre-bankruptcy claim. Holders of the exchange senior secured notes that have a security interest in collateral with a value equal or less than their pre-bankruptcy claim will not be entitled to post-petition interest under the U.S. Bankruptcy Code. No appraisal of the fair market value of the collateral has been prepared in connection with the issuances of the outstanding senior secured notes and we therefore cannot assure you that the value of the noteholders’ interest in the collateral equals or exceeds the principal amount of the exchange senior secured notes.
We will in most cases have control over the collateral, and the sale of particular assets by us could reduce the pool of assets securing the exchange senior secured notes and the guarantees in respect thereof.
The collateral documents allow us to remain in possession of, retain exclusive control over, freely operate, and collect, invest and dispose of any income from, the collateral securing the exchange senior secured notes and the related guarantees, except, under certain circumstances, cash transferred to accounts controlled by the administrative agent under our senior secured credit facilities.
There are circumstances other than repayment or discharge of the exchange senior secured notes under which the collateral securing the exchange senior secured notes and guarantees in respect thereof will be released automatically, without your consent or the consent of the trustee.
Under various circumstances, collateral securing the exchange senior secured notes will be released automatically, including:
| • | a sale, transfer or other disposal of such collateral in a transaction not prohibited under the indenture governing the exchange senior secured notes; |
25
Table of Contents
| • | with respect to collateral held by a guarantor, upon the release of such guarantor from its guarantee; |
| • | with respect to collateral that is capital stock, upon the dissolution of the issuer of such capital stock in accordance with the indenture governing the exchange senior secured notes; and |
| • | with respect to any collateral in which the exchange senior secured notes have a second-priority lien, upon any release by the lenders under our senior secured credit facilities of their first-priority or second-priority security interest in such collateral unless such release occurs in connection with a discharge in full in cash of first lien obligations, which discharge is not in connection with a foreclosure of, or other exercise of remedies with respect to, non-receivables collateral by the first lien secured parties (such discharge not in connection with any such foreclosure or exercise of remedies, a “Payment Discharge”); provided that, in the case of a Payment Discharge, the lien on any non-receivables collateral disposed of in satisfaction in whole or in part of first lien obligations shall be automatically released but any proceeds thereof not used for purposes of the discharge of first lien obligations in full in cash or otherwise in accordance with the indenture governing the exchange senior secured notes shall be subject to lien in favor of the second lien agent for the noteholders. |
In addition, the guarantee of a subsidiary guarantor will be automatically released to the extent it is released under the senior secured credit facilities or in connection with a sale of such subsidiary guarantor in a transaction not prohibited by the indenture governing the exchange senior secured notes.
The indenture governing the exchange senior secured notes also permits us to designate one or more of our restricted subsidiaries that is a guarantor of the exchange senior secured notes as an unrestricted subsidiary. If we designate a subsidiary guarantor as an unrestricted subsidiary for purposes of the indenture governing the exchange senior secured notes, all of the liens on any collateral owned by such subsidiary or any of its subsidiaries and any guarantees of the exchange senior secured notes by such subsidiary or any of its subsidiaries will be released under the indenture governing the exchange senior secured notes but not necessarily under the credit agreement governing our senior secured credit facilities. Designation of an unrestricted subsidiary will reduce the aggregate value of the collateral securing the exchange senior secured notes to the extent that liens on the assets of the unrestricted subsidiary and its subsidiaries are released. In addition, the creditors of the unrestricted subsidiary and its subsidiaries will have a senior claim on the assets of such unrestricted subsidiary and its subsidiaries. See “Description of Senior Secured Notes.”
The imposition of certain permitted liens will cause the assets on which such liens are imposed to be excluded from the collateral securing the exchange senior secured notes and the guarantees in respect thereof. There are also certain other categories of property that are excluded from the collateral.
The indenture governing the exchange senior secured notes permits liens in favor of third parties to secure additional debt, including purchase money indebtedness and capital lease obligations, and any assets subject to such liens will be automatically excluded from the collateral securing the exchange senior secured notes and the guarantees. Our ability to incur purchase money indebtedness and capital lease obligations is subject to the limitations as described in “Description of Senior Secured Notes.” In addition, certain categories of assets are excluded from the collateral securing the exchange senior secured notes and the related guarantees. Excluded assets include the assets of our non-guarantor subsidiaries and equity investees, certain capital stock and other securities of our subsidiaries and equity investees, certain properties that do not secure our senior secured credit facilities, deposit accounts, other bank or securities accounts, cash, leaseholds and motor vehicles, and the proceeds from any of the foregoing. If an event of default occurs and the exchange senior secured notes are accelerated, the exchange senior secured notes and the related guarantees will rank equally with the holders of other unsubordinated and unsecured indebtedness of the relevant entity with respect to such excluded property.
Our non-guarantor subsidiaries accounted for $236.1 million, or approximately 8.3%, of our total assets as of September 29, 2012.
26
Table of Contents
The pledge of the capital stock, other securities and similar items of our subsidiaries that secure the exchange senior secured notes will automatically be released from the lien on them and no longer constitute collateral for so long as the pledge of such capital stock or such other securities would require the filing of separate financial statements with the SEC for that subsidiary.
The Secured Notes and the related guarantees are secured by a pledge of the stock of some of our subsidiaries. Under the SEC regulations in effect as of the issue date of the exchange senior secured notes, if the par value, book value as carried by us or market value (whichever is greatest) of the capital stock, other securities or similar items of a subsidiary pledged as part of the collateral is greater than or equal to 20% of the aggregate principal amount of the exchange senior secured notes then outstanding, such a subsidiary would be required to provide separate financial statements to the SEC. Therefore, the indenture governing the exchange senior secured notes and the collateral documents provide that any capital stock and other securities of any of our subsidiaries will be excluded from the collateral for so long as the pledge of such capital stock or other securities to secure the exchange senior secured notes would cause such subsidiary to be required to file separate financial statements with the SEC pursuant to Rule 3-16 of Regulation S-X (as in effect from time to time).
As a result, holders of the exchange senior secured notes could lose a portion or all of their security interest in the capital stock or other securities of those subsidiaries during such period. It may be more difficult, costly and time-consuming for holders of the exchange senior secured notes to foreclose on the assets of a subsidiary than to foreclose on its capital stock or other securities, so the proceeds realized upon any such foreclosure could be significantly less than those that would have been received upon any sale of the capital stock or other securities of such subsidiary. See “Description of Senior Secured Notes—Security for the notes.”
Your rights in the collateral may be adversely affected by the failure to perfect security interests in certain collateral in the future.
Applicable law requires that certain property and rights acquired after the grant of a general security interest, such as equipment subject to a certificate and certain proceeds, can only be perfected at the time such property and rights are acquired and identified. In addition, the trustee or the second lien agent may not monitor, or we may fail to inform the trustee or the second lien agent of, the future acquisition of property and rights that constitute collateral, and necessary action may not be taken to properly perfect the security interest in such after-acquired collateral. The second lien agent has no obligation to monitor the acquisition of additional property or rights that constitute collateral or the perfection of any security interest in favor of the exchange senior secured notes against third parties. Such failure may result in the loss of the security interest therein or the priority of the security interest in favor of the exchange senior secured notes against third parties.
The collateral is subject to casualty risks.
We intend to maintain insurance or otherwise insure against hazards in a manner appropriate and customary for our business. There are, however, certain losses that may be either uninsurable or not economically insurable, in whole or in part. Insurance proceeds may not compensate us fully for our losses. If there is a complete or partial loss of any of the pledged collateral, the insurance proceeds may not be sufficient to satisfy all of the secured obligations, including obligations under the senior secured credit facilities, the exchange senior secured notes and the guarantees in respect thereof.
If the obligations under the exchange senior secured notes were voided, subordinated or reduced there may be no way to distinguish between the exchange senior secured notes and the exchange senior secured notes.
The exchange senior secured notes, together with the exchange senior secured notes, will be part of a single class under the indenture governing the exchange senior secured notes. If any of the exchange senior secured notes were to be tainted by a court finding of a fraudulent conveyance, the exchange senior secured notes may be tainted as well, particularly if it is not possible to distinguish the exchange senior secured notes from the
27
Table of Contents
exchange senior secured notes. The exchange senior secured notes may have been or may be issued under circumstances in which there is an increased risk that a court would determine that the issuance of such exchange senior secured notes or any guarantee thereof constitutes fraudulent conveyance. In such a scenario, because the exchange senior secured notes will share the same CUSIP number as the exchange senior secured notes, there may be no way to distinguish between the exchange senior secured notes and the exchange senior secured notes if the obligations under the exchange senior secured notes or the related guarantees were voided, subordinated or reduced, and therefore the exchange senior secured notes and the guarantees may be similarly voided, subordinated or reduced.
In addition, in the event that a bankruptcy proceeding were commenced with respect to us or the guarantors within 90 days following this offering (or, in certain circumstances, a longer period) and the exchange senior secured notes were found to be distinguishable from the exchange senior secured notes, in such a circumstance the payment obligation under the exchange senior secured notes could be voided or reduced, and you may not receive any repayment on the exchange senior secured notes.
Risks Related to the Exchange Senior Notes
Your right to receive payments on the exchange senior notes is effectively junior to those lenders who have a security interest in our and our subsidiaries’ assets.
Our obligations under the exchange senior notes and our guarantors’ obligations under their guarantees of the exchange senior notes are unsecured, but our obligations under our senior secured credit facilities and our Secured Notes and each guarantor’s obligations under their respective guarantees of the senior secured credit facilities and our Secured Notes are secured by a security interest in substantially all of our domestic tangible and intangible assets, including the stock of our wholly owned U.S. subsidiaries and a portion of the stock of our non-U.S. subsidiaries. If we are declared bankrupt or insolvent, or if we default under our senior secured credit facilities and our Secured Notes, the lenders and/or the holders could declare all of the funds borrowed or issued thereunder, together with accrued interest, immediately due and payable. If we were unable to repay such indebtedness, the lenders or holders could foreclose on the pledged assets to the exclusion of holders of the exchange senior notes, even if an event of default exists under the indenture governing the exchange senior notes at such time. Furthermore, if the lenders or holders foreclose on and sell the pledged equity interests in any subsidiary guarantor under the exchange senior notes, then that guarantor will be released from its guarantee of the exchange senior notes automatically and immediately upon such sale. In any such event, because the exchange senior notes will not be secured by any of our assets or the equity interests in subsidiary guarantors, it is possible that there would be no assets remaining from which your claims could be satisfied or, if any assets remained, they might be insufficient to satisfy your claims fully.
As of September 29, 2012, we had $1,169.2 million of senior secured indebtedness, exclusive of net unamortized original issue discount, of which $839.2 million was indebtedness under our senior secured credit facilities and $330.0 million was indebtedness under our outstanding senior secured notes. The $1,169.2 million of senior secured indebtedness did not include availability of $62.0 million under our revolving credit facility, all of which would be secured if borrowed. The Indentures permit the incurrence of substantial additional indebtedness by us and our restricted subsidiaries in the future, including senior secured indebtedness.
Risks Related to Our Business
The current U.S. and global economic downturn and related credit and financial market problems may pose additional risks and exacerbate existing risks to our business.
The serious slowdown in the U.S. and global economy, as well as the dramatic problems in the current credit and financial markets, especially the European credit markets, had and may continue to have a negative impact on demand for our products, availability and reliability of vendors and third party contract manufacturers, our ability to timely collect our accounts receivable and the availability of financing for acquisitions and working capital requirements. Continued or renewed deterioration of general economic conditions in the United States and overseas could contribute to those trends remaining a problem or becoming worse.
28
Table of Contents
The slowing of economic activity and lack of available financing has affected and could continue to affect our business in a variety of ways, including the following:
| • | loss of jobs and lack of health insurance as a result of the economic slowdown could depress demand for healthcare services and demand for our products, |
| • | weakened demand for healthcare services, reduction in the number of insured patients and lack of available credit could result in the inability of private insurers to satisfy their reimbursement obligations, lead to delays in payment or cause the insurers to increase their scrutiny of our claims, |
| • | shortage of available credit for working capital could lead customers who buy capital goods from us to curtail their purchases or have difficulty meeting payment obligations, |
| • | tightening of credit and disruption in the financial markets could disrupt or delay performance by our third party vendors and contractors and adversely affect our business, or |
| • | problems in the credit and financial markets could limit the availability and size of alternative or additional financing for our working capital or other corporate needs and could make it more difficult and expensive to obtain waivers under or make changes to our existing credit arrangements. |
Any of these risks, among others, could adversely affect our business and operating results, and the risks could become more pronounced if the problems in the U.S. and global economies and the credit and financial markets continue or become worse.
If adequate levels of reimbursement and coverage from third party payors for our products are not obtained, healthcare providers and patients may be reluctant to use our products, and our revenues and profits may decline.
Our sales depend largely on whether there is adequate reimbursement and coverage by government healthcare programs, such as Medicare and Medicaid, and by private payors. We believe that surgeons, hospitals, physical therapists and other healthcare providers may not use, purchase or prescribe our products and patients may not purchase our products if these third party payors do not provide satisfactory coverage of and reimbursement for the costs of our products or the procedures involving the use of our products.
Third party payors continue to review their coverage policies carefully and can, without notice, reduce or eliminate reimbursement for our products or treatments that use our products. For instance, they may attempt to control costs by (i) authorizing fewer elective surgical procedures, including joint reconstructive surgeries, (ii) requiring the use of the least expensive product available, (iii) reducing the reimbursement for or limiting the number of authorized visits for rehabilitation procedures or (iv) otherwise restricting coverage or reimbursement of our products or procedures using our products.
In the United States, Congress and CMS frequently engage in efforts to contain costs, which may result in more restrictive Medicare or Medicaid coverage, reduced reimbursement, or selective contracting for our products. As discussed under “Government Regulations—Third Party Reimbursement” in the “Business” section below, CMS has recently determined that coverage for TENS for the treatment of chronic low back pain would not be available under Medicare unless the patient is enrolled in a clinical study meeting certain defined standards. This coverage decision will have the effect of reducing the available market for TENS devices. If our Empi business is unsuccessful in offsetting the adverse effects of these revenue losses, the long-term revenue and profit impact of this coverage decision could be materially adverse to the Empi business and could have an adverse impact on our business and results of operations as a whole.
Medicare payment for durable medical equipment, prosthetics, orthotics and supplies (“DMEPOS”) also can be impacted by the DMEPOS competitive bidding program, under which Medicare rates are based on bid amounts for certain products in designated geographic areas, rather than the Medicare fee schedule amount. Only
29
Table of Contents
those suppliers selected through the competitive bidding process within each designated competitive bidding area (“CBA”) are eligible to have their products reimbursed by Medicare. Competitive bidding went into effect January 1, 2011 in nine CBAs and nine product categories, with reimbursement to contract suppliers averaging 32% below the Medicare DMEPOS fee schedule amount. The second round of competitive bidding is underway in 100 CBAs (in addition to national mail order competition for diabetic testing supplies). While none of our products was included in the first two rounds, CMS has announced that TENS units will be included in a recompetition of round one as part of a new “General Home Equipment and Related Supplies and Accessories” product category. Bidding for the new three-year contracts will be conducted in late 2012, and implementation of the contracts and prices is schedules to take place on January 1, 2014. In addition, CMS recently released a listing of codes that it considers to be off-the-shelf orthotics and subject to competitive bidding in the future. When our products become subject to competitive bidding, if we are not selected as a contract supplier (or subcontractor) in a particular region, or if contract prices are significantly below Medicare fee schedule reimbursement levels, it could have an adverse impact on our sales and profitability.
Because many private payors model their coverage and reimbursement policies on Medicare, other third party payors’ coverage of, and reimbursement for, our products also could be negatively impacted by legislative, regulatory or other measures that restrict Medicare coverage or reduce Medicare reimbursement.
Our international sales also depend in part upon the coverage and eligibility for reimbursement of our products through government-sponsored healthcare payment systems and third party payors, the amount of reimbursement and the cost allocation of payments between the patient and government-sponsored healthcare payment systems and third party payors. Coverage and reimbursement practices vary significantly by country, with certain countries requiring products to undergo a lengthy regulatory review in order to be eligible for third party coverage and reimbursement. In addition, healthcare cost containment efforts similar to those we face in the United States are prevalent in many of the foreign countries in which our products are sold, and these efforts are expected to continue in the future, possibly resulting in the adoption of more stringent reimbursement standards relating to our international operations.
The loss of the services of our key management and personnel could adversely affect our ability to operate our business.
Our executive officers have substantial experience and expertise in our industry. Our future success depends, to a significant extent, on the abilities and efforts of our executive officers and other members of our management team. We compete for such personnel with other companies, academic institutions, government entities and other organizations, and our failure to hire and retain qualified individuals for senior executive positions could have a material adverse impact on our business.
Federal and state health reform and cost control efforts include provisions that could adversely impact our business and results of operations.
The Patient Protection and Affordable Care Act as amended by the Health Care and Education Reconciliation Act (collectively referred to as the “Affordable Care Act” or “ACA”) is a sweeping measure designed to expand access to affordable health insurance, control health care spending, and improve health care quality. On June 28, 2012, the United States Supreme Court upheld the vast majority of this landmark health reform law. Several provisions of the ACA specifically affect the medical equipment industry. In addition to changes in Medicare DMEPOS reimbursement and an expansion of the DMEPOS competitive bidding program, the ACA provides that for sales on or after January 1, 2013, manufacturers, producers, and importers of taxable medical devices must pay an annual excise tax of 2.3% of the price for which the devices are sold.
The ACA also establishes enhanced Medicare and Medicaid program integrity provisions, including expanded documentation requirements for Medicare DMEPOS orders, more stringent procedures for screening Medicare and Medicaid DMEPOS suppliers, and new disclosure requirements regarding manufacturer payments
30
Table of Contents
to physicians and teaching hospitals, along with broader expansion of federal fraud and abuse authorities. CMS has issued regulations to implement certain of these provisions. For instance, on July 30, 2012, CMS issued a proposed rule that would implement an ACA provision requiring a face-to-face encounter as a condition of Medicare payment for certain DME items. The proposed rule would require a physician to document that the physician or a physician assistant, a nurse practitioner, or a clinical nurse specialist has had a face-to-face encounter with the beneficiary no more than 90 days before or within 30 days after the order is written for specified DME items, including certain of our products. If the rule is finalized as proposed, or if other of our products are added to the requirement, it would impose new administrative burdens and could increase our costs of operation.
Although the eventual impact of the ACA is still uncertain, it is possible that the legislation will have a material adverse impact on our business. Likewise, most states have adopted or are considering policies to reduce Medicaid spending as a result of state budgetary shortfalls, which in some cases include reduced reimbursement for DMEPOS items and/or other Medicaid coverage restrictions. As states continue to face significant financial pressures, it is possible that state health policy changes will adversely affect our profitability.
If we fail to meet Medicare accreditation and surety bond requirements or DMEPOS supplier standards, it could negatively affect our business operations.
Medicare DMEPOS suppliers (other than certain exempted professionals) must be accredited by an approved accreditation organization as meeting DMEPOS quality standards adopted by CMS including specific requirements for suppliers of custom-fabricated and custom-fitted orthoses and certain prosthetics. Medicare suppliers also are required to meet surety bond requirements. In addition, Medicare DMEPOS suppliers must comply with Medicare supplier standards in order to obtain and retain billing privileges, including meeting all applicable federal and state licensure and regulatory requirements. CMS periodically expands or otherwise clarifies the Medicare DMEPOS supplier standards. We believe we are in compliance with these requirements. If we fail to maintain our Medicare accreditation status and/or do not comply with Medicare surety bond or supplier standard requirements in the future, or if these requirements are changed or expanded, it could adversely affect our profits and results of operations.
If we fail to comply with the Food and Drug Administration’s (the “FDA”) Quality System Regulation, our manufacturing could be delayed, and our product sales and profitability could suffer.
Our manufacturing processes are required to comply with the FDA’s Quality System Regulation, which covers current Good Manufacturing Practice requirements including procedures concerning (and documentation of) the design, testing, production processes, controls, quality assurance, labeling, packaging, storage and shipping of our devices. We also are subject to state requirements and licenses applicable to manufacturers of medical devices. In addition, we must engage in extensive recordkeeping and reporting and must make available our manufacturing facilities and records for periodic unscheduled inspections by governmental agencies, including the FDA, state authorities and comparable agencies in other countries. Moreover, if we fail to pass a Quality System Regulation inspection or to comply with these and other applicable regulatory requirements, we may receive a notice of a violation in the form of inspectional observations on Form FDA-483, a warning letter, or could otherwise be required to take corrective action and, in severe cases, we could suffer a disruption of our operations and manufacturing delays. If we fail to take adequate corrective actions, we could be subject to certain enforcement actions, including, among other things, significant fines, suspension of approvals, seizures or recalls of products, operating restrictions and criminal prosecutions. We cannot assure you that the FDA or other governmental authorities would agree with our interpretation of applicable regulatory requirements or that we have in all instances fully complied with all applicable requirements. Any notice or communication from the FDA regarding a failure to comply with applicable requirements could adversely affect our product sales and profitability. We have received FDA warnings letters in the past and we cannot assure you that the FDA will not take further action in the future.
31
Table of Contents
We may not be able to successfully integrate businesses that we have recently acquired, or businesses we may acquire in the future, and we may not be able to realize the anticipated cost savings, revenue enhancements or other synergies from such acquisitions.
Our ability to successfully implement our business plan and achieve targeted financial results is highly dependent on our ability to successfully integrate businesses that we have recently acquired and other businesses we may acquire in the future. The process of integrating such acquired businesses involves risks. These risks include, but are not limited to:
| • | demands on management related to the significant increase in the size of our business, |
| • | diversion of management’s attention from the management of daily operations to the integration of newly acquired operations, |
| • | difficulties in the assimilation of different corporate cultures, practices and sales and distribution methodologies, |
| • | difficulties in conforming the acquired company’s accounting, books and records, internal accounting controls, and procedures and policies to ours, |
| • | increased exposure to risks relating to business operations outside the United States, |
| • | retaining the loyalty and business of the customers of acquired businesses, |
| • | retaining employees who may be vital to the integration of the acquired business or to the future prospects of the combined businesses, |
| • | difficulties and unanticipated expenses related to the integration of departments and information technology systems, including accounting systems, |
| • | difficulties integrating technologies and maintaining uniform standards, such as internal accounting controls, procedures and policies, and |
| • | unanticipated costs and expenses associated with any undisclosed or potential liabilities. |
If we fail to realize anticipated cost savings, synergies or revenue enhancements from recent or future acquisitions, our financial results will be adversely affected, and we may not generate the cash flow from operations that we anticipated, or that is sufficient to repay our indebtedness.
We may pursue, but may not be able to identify, finance, or successfully complete, other strategic acquisitions.
Our growth strategy may include the pursuit of acquisitions, both domestically and internationally. However, we may not be able to identify acceptable opportunities or complete acquisitions of targets in a timely manner or on acceptable terms. To the extent we are unable to consummate acquisitions, we will experience slower than expected growth.
In addition, we may require additional debt or equity financing for future acquisitions, and such financing may not be available on favorable terms, if available at all. If we complete acquisitions, or obtain financing for them on unfavorable terms, or if we fail to properly integrate an acquired business, our financial condition and results of operations would be adversely affected.
We may experience substantial fluctuations in our quarterly operating results and you should not rely on them as an indication of our future results.
Our quarterly operating results may vary significantly due to a combination of factors, many of which are beyond our control. These factors include:
| • | demand for many of our products, which historically has been higher in the fourth quarter when scholastic sports and ski injuries are more frequent, |
| • | our ability to meet the demand for our products, |
32
Table of Contents
| • | the direct distribution of our products in foreign countries that have seasonal variations, |
| • | the number, timing and significance of new products and product introductions and enhancements by us and our competitors, including delays in obtaining government review and clearance of medical devices, |
| • | our ability to develop, introduce and market new and enhanced versions of our products on a timely basis, |
| • | the impact of any acquisitions that occur in a quarter, |
| • | the impact of any changes in generally accepted accounting principles, |
| • | changes in pricing policies by us and our competitors and reimbursement rates by third party payors, including government healthcare agencies and private insurers, |
| • | the loss of any of our significant distributors, |
| • | changes in the treatment practices of orthopedic and spine surgeons, primary care physicians, and pain-management specialists, and their allied healthcare professionals, and |
| • | the timing of significant orders and shipments. |
Accordingly, our quarterly sales and operating results may vary significantly in the future and period-to-period comparisons of our results of operations may not be meaningful and should not be relied upon as indications of future performance. We cannot assure you that our sales will increase or be sustained in future periods or that we will be profitable in any future period.
We operate in a highly competitive business environment, and our inability to compete effectively could adversely affect our business prospects and results of operations.
We operate in highly competitive and fragmented markets. Our Bracing and Vascular, Recovery Sciences and International segments compete with both large and small companies, including several large, diversified companies with significant market share and numerous smaller niche companies, particularly in the physical therapy products market. Our Surgical Implant segment competes with a small number of very large companies that dominate the market, as well as other companies similar to our size. We may not be able to offer products similar to, or more desirable than, those of our competitors or at a price comparable to that of our competitors. Compared to us, many of our competitors have:
| • | greater financial, marketing and other resources, |
| • | more widely accepted products, |
| • | a larger number of endorsements from healthcare professionals, |
| • | a larger product portfolio, |
| • | superior ability to maintain new product flow, |
| • | greater research and development and technical capabilities, |
| • | patent portfolios that may present an obstacle to the conduct of our business, |
| • | stronger name recognition, |
| • | larger sales and distribution networks, and/or |
| • | international manufacturing facilities that enable them to avoid the transportation costs and foreign import duties associated with shipping our products manufactured in the United States to international customers. |
Accordingly, we may be at a disadvantage with respect to our competitors. These factors may materially impair our ability to develop and sell our products.
33
Table of Contents
If we are unable to develop or license new products or product enhancements or find new applications for our existing products, we will not remain competitive.
The markets for our products are characterized by continued new product development and the obsolescence of existing products. Our future success and our ability to increase revenues and make payments on our indebtedness will depend, in part, on our ability to develop, license, acquire and distribute new and innovative products, enhance our existing products with new technology and find new applications for our existing products. However, we may not be successful in developing, licensing or introducing new products, enhancing existing products or finding new applications for our existing products. We also may not be successful in manufacturing, marketing and distributing products in a cost-effective manner, establishing relationships with marketing partners, obtaining coverage of and satisfactory reimbursement for our future products or product enhancements or obtaining required regulatory clearances and approvals in a timely fashion or at all. If we fail to keep pace with continued new product innovation or enhancement or fail to successfully commercialize our new or enhanced products, our competitive position, financial condition and results of operations could be materially adversely affected.
In addition, if any of our new or enhanced products contain undetected errors or design defects, especially when first introduced, or if new applications that we develop for existing products do not work as planned, our ability to market these and other products could be substantially delayed, and we could ultimately become subject to product liability litigation, resulting in lost revenues, potential damage to our reputation and/or delays in regulatory clearance. In addition, approval of our products or obtaining acceptance of our products by physicians, physical therapists and other healthcare professionals that recommend and prescribe our products could be adversely affected.
The success of our surgical implant products depends on our relationships with leading surgeons who assist with the development and testing of our products, and our ability to comply with enhanced disclosure requirements regarding payments to physicians.
A key aspect of the development and sale of our surgical implant products is the use of designing and consulting arrangements with orthopedic surgeons who are well recognized in the healthcare community. These surgeons assist in the development and clinical testing of new surgical implant products. They also participate in symposia and seminars introducing new surgical implant products and assist in the training of healthcare professionals in using our new products. We may not be successful in maintaining or renewing our current designing and consulting arrangements with these surgeons or in developing similar arrangements with new surgeons. In that event, our ability to develop, test and market new surgical implant products could be adversely affected.
In addition, the ACA establishes new disclosure requirements (sometimes referred to as the Physician Payment Sunshine Act) regarding financial arrangements between medical device and supplies manufacturers and physicians, including physicians who serve as consultants, effective March 31, 2013. On December 19, 2011, CMS proposed regulations to implement these requirements. The proposed rule would require us to report annually to CMS beginning in 2013 all payments and other transfers of value to physicians and teaching hospitals for products payable under federal health care programs, as well as ownership or investments held by physicians or their family members. Several states also have enacted specific marketing and payment disclosure requirements and others may do so in the future. Likewise, voluntary industry guidelines have been adopted regarding device manufacturer financial arrangements with physicians and other healthcare professionals. While we believe we are in compliance with current requirements, we cannot determine at this time the impact, if any, of new requirements or voluntary guidelines on our relationships with surgeons, and there can be no assurances that such requirements and guidelines would not impose additional costs on us and/or adversely impact our consulting and other arrangements with surgeons.
Proposed laws that would limit the types of orthopedic professionals who can fit, sell or seek reimbursement for our products could, if adopted, adversely affect our business.
In response to pressure from certain groups (mostly orthotists), federal and state legislatures have periodically considered proposals to limit the types of orthopedic professionals who can fit or sell our orthotic
34
Table of Contents
device products or who can seek reimbursement for them. Several states have adopted legislation imposing certification or licensing requirements on the measuring, fitting and adjusting of certain orthotic devices. Although some of these state laws exempt manufacturers’ representatives, others do not. Additional states may be considering similar legislation. Such laws could reduce the number of potential customers by restricting our sales representatives’ activities in those jurisdictions and/or reduce demand for our products by reducing the number of professionals who fit and sell them. The adoption of such policies could have a material adverse impact on our business.
In addition, legislation has been adopted, but not implemented to date, requiring that certain certification or licensing requirements be met for individuals and suppliers furnishing certain custom-fabricated orthotic devices as a condition of Medicare payment. Medicare currently follows state policies in those states that require the use of an orthotist or prosthetist for furnishing of orthotics or prosthetics. We cannot predict whether additional restrictions will be implemented at the state or federal level or the impact of such policies on our business.
If we fail to establish new sales and distribution relationships or maintain our existing relationships, or if our third party distributors and independent sales representatives fail to commit sufficient time and effort or are otherwise ineffective in selling our products, our results of operations and future growth could be adversely impacted.
The sale and distribution of certain of our orthopedic products, regeneration products and our surgical implant products depend, in part, on our relationships with a network of third party distributors and independent commissioned sales representatives. These third party distributors and independent sales representatives maintain the customer relationships with the hospitals, orthopedic surgeons, physical therapists and other healthcare professionals that purchase, use and recommend the use of our products. Although our internal sales staff trains and manages these third party distributors and independent sales representatives, we do not directly monitor the efforts that they make to sell our products. In addition, some of the independent sales representatives that we use to sell our surgical implant products also sell products that directly compete with our core product offerings. These sales representatives may not dedicate the necessary effort to market and sell our products. If we fail to attract and maintain relationships with third party distributors and skilled independent sales representatives or fail to adequately train and monitor the efforts of the third party distributors and sales representatives that market and sell our products, or if our existing third party distributors and independent sales representatives choose not to carry our products, our results of operations and future growth could be adversely affected.
We rely on our own direct sales force for certain of our products, which may result in higher fixed costs than our competitors and may slow our ability to reduce costs in the face of a sudden decline in demand for our products.
We rely on our own direct sales force of approximately 550 representatives in the United States and approximately 150 representatives in Europe to market and sell certain of the orthopedic rehabilitation products which are intended for use in the home and in rehabilitation clinics. Some of our competitors rely predominantly on independent sales agents and third party distributors. A direct sales force may subject us to higher fixed costs than those of companies that market competing products through independent third parties, due to the costs that we will bear associated with employee benefits, training, and managing sales personnel. As a result, we could be at a competitive disadvantage. Additionally, these fixed costs may slow our ability to reduce costs in the face of a sudden decline in demand for our products, which could have a material adverse impact on our results of operations.
The success of all of our products depends heavily on acceptance by healthcare professionals who prescribe and recommend our products, and our failure to maintain a high level of confidence by key healthcare professionals in our products could adversely affect our business.
We have maintained customer relationships with numerous orthopedic surgeons, primary care physicians, other specialist physicians, physical therapists, athletic trainers, chiropractors and other healthcare professionals.
35
Table of Contents
We believe that sales of our products depend significantly on their confidence in, and recommendations of, our products. Acceptance of our products depends on educating the healthcare community as to the distinctive characteristics, perceived benefits, clinical efficacy and cost-effectiveness of our products compared to the products offered by our competitors and on training healthcare professionals in the proper use and application of our products. Failure to maintain these customer relationships and develop similar relationships with other leading healthcare professionals could result in a less frequent recommendation of our products, which may adversely affect our sales and profitability.
Our international operations expose us to risks related to conducting business in multiple jurisdictions outside the United States.
The international scope of our operations exposes us to economic, regulatory and other risks in the countries in which we operate. We generated 26.0% of our net revenues from customers outside the United States for the year ended December 31, 2011. Doing business in foreign countries exposes us to a number of risks, including the following:
| • | fluctuations in currency exchange rates, |
| • | imposition of investment, currency repatriation and other restrictions by foreign governments, |
| • | potential adverse tax consequences, including the imposition or increase of withholding and other taxes on remittances and other payments by foreign subsidiaries, which, among other things, may preclude payments or dividends from foreign subsidiaries from being used for our debt service, and exposure to adverse tax regimes, |
| • | difficulty in collecting accounts receivable and longer collection periods, |
| • | the imposition of additional foreign governmental controls or regulations on the sale of our products, |
| • | intellectual property protection difficulties, |
| • | changes in political and economic conditions, including the recent political changes in Tunisia in which we maintain a small manufacturing facility and security issues in Mexico in which we maintain a significant manufacturing facility, |
| • | difficulties in attracting high-quality management, sales and marketing personnel to staff our foreign operations, |
| • | labor disputes, |
| • | import and export restrictions and controls, tariffs and other trade barriers, |
| • | increased costs of transportation or shipping, |
| • | exposure to different approaches to treating injuries, |
| • | exposure to different legal, regulatory and political standards, and |
| • | difficulties of local governments in responding to severe weather emergencies, natural disasters or other such similar events. |
In addition, as we grow our operations internationally, we will become increasingly dependent on foreign distributors and sales agents for our compliance and adherence to foreign laws and regulations that we may not be familiar with, and we cannot assure you that these distributors and sales agents will adhere to such laws and regulations or adhere to our own business practices and policies. Any violation of laws and regulations by foreign distributors or sales agents or a failure of foreign distributors or sales agents to comply with our business practices and policies could result in legal or regulatory sanctions against us or potentially damage our reputation in that respective international market. If we fail to manage these risks effectively, we may not be able to grow our international operations, and our business and results of operations may be materially adversely affected.
36
Table of Contents
We may fail to comply with customs and import/export laws and regulations
Our business is conducted world-wide, with raw material and finished goods imported from and exported to a substantial number of countries. In particular, a significant portion of our products are manufactured in our plant in Tijuana, Mexico and imported to the United States before shipment to domestic customers or export to other countries. We are subject to customs and import/export rules in the U.S., including FDA regulatory requirements applicable to medical devices, detailed below, and in other countries, and to requirements for payment of appropriate duties and other taxes as goods move between countries. Customs authorities monitor our shipments and payments of duties, fees and other taxes and can perform audits to confirm compliance with applicable laws and regulations. Our failure to comply with import/export rules and restrictions or to properly classify our products under tariff regulations and pay the appropriate duty could expose us to fines and penalties and adversely affect our financial condition and business operations.
Fluctuations in foreign exchange rates may adversely affect our financial condition and results of operations and may affect the comparability of our results between financial periods.
Our foreign operations expose us to currency fluctuations and exchange rate risks. We are exposed to the risk of currency fluctuations between the U.S. Dollar and the Euro, Pound Sterling, Canadian Dollar, Mexican Peso, Swiss Franc, Australian Dollar, Japanese Yen, Norwegian Krone, Danish Krone, Swedish Krona, South African Rand and Tunisian Dinar. Sales denominated in foreign currencies accounted for 30.2% of our consolidated net sales for the year ended December 31, 2011, of which 22.3% were denominated in the Euro. Our exposure to fluctuations in foreign currencies arises because certain of our subsidiaries’ results are recorded in these currencies and then translated into U.S. Dollars for inclusion in our consolidated financial statements, and certain of our subsidiaries enter into purchase or sale transactions using a currency other than our functional currency. We utilize Mexican Peso (“MXN”) foreign exchange forward contracts to hedge a portion of our exposure to fluctuations in foreign exchange rates, as our Mexico-based manufacturing operations incur costs that are largely denominated in MXN. As of December 31, 2011, we had outstanding MXN forward contracts to purchase an aggregate U.S. dollar equivalent of $14.6 million. As we continue to distribute and manufacture our products in selected foreign countries, we expect that future sales and costs associated with our activities in these markets will continue to be denominated in the applicable foreign currencies, which could cause currency fluctuations to materially impact our operating results. Changes in currency exchange rates may adversely affect our financial condition and results of operations and may affect the comparability of our results between reporting periods.
We may not be able to effectively manage our currency translation risks, and volatility in currency exchange rates may adversely affect our financial condition and results of operations.
Our success depends on receiving regulatory approval for our products, and failure to do so could adversely affect our growth and operating results.
Our products are subject to extensive regulation in the United States by the FDA and by similar governmental authorities in the foreign countries where we do business. The FDA regulates virtually all aspects of a medical device’s development, testing, manufacturing, labeling, promotion, distribution and marketing. In general, unless an exemption applies, a medical device must receive either pre-market approval or pre-market clearance from the FDA before it can be marketed in the United States. While in the past we have received such approvals and clearances, we may not be successful in the future in receiving such approvals and clearances in a timely manner or at all. The FDA asked the Institute of Medicine (“IOM”) to conduct a two-year study of the clearance process for devices under § 510(k) of the Food Drug, and Cosmetic Act, as amended, and to provide recommendations for changes, if necessary. The IOM released its report, “Medical Devices and the Public Health, the FDA 510(k) Clearance Process at 35 Years,” in July 2011. In addition, the FDA is implementing recommendations from its own internal review of the 510(k) clearance process. Many of our products are cleared for marketing under the 510(k) process. If we begin to have significant difficulty obtaining such FDA approvals or clearances in a timely manner or at all, it could have a material adverse impact on our revenues and growth.
37
Table of Contents
If we fail to obtain regulatory approval for the modification of, or new uses for, our products, our growth and operating results could suffer.
In order to market modifications to our existing products or market our existing products for new indications, we may be required to obtain pre-market approvals, pre-market supplement approvals or pre-market clearances. The FDA requires device manufacturers themselves to make and document a determination of whether or not a modification requires a new approval or clearance; however, the FDA can review and disagree with a manufacturer’s decision. We may not be successful in receiving such approvals or clearances or the FDA may not agree with our decisions not to seek approvals or clearances for any particular device modification. The FDA may require an approval or clearance for any past or future modification or a new indication for our existing products. Any new product introduction or existing product modification could be subjected to a lengthier, more rigorous FDA examination process. The FDA may also require additional clinical or preclinical data in such submissions, which may be time consuming and costly, and it may not ultimately approve or clear one or more of our products for marketing. If the FDA requires us to obtain pre-market approvals, pre-market supplement approvals or pre-market clearances for any modification to a previously cleared or approved device, we may be required to cease manufacturing and marketing the modified device or to recall such modified device until we obtain FDA clearance or approval, and we may be subject to significant regulatory fines or penalties. In addition, the FDA may not clear or approve such submissions in a timely manner, if at all. Because a significant portion of our revenues is generated by products that are modified or used for new treatments, delays or failures in obtaining such approvals could reduce our revenue and adversely affect our operating results.
As a result of the FDA’s recent internal review of the 510(k) process, the FDA may consider requiring manufacturers to provide regular, periodic updates of device modifications; provide a list and brief description of all scientific information related to the safety and effectiveness of a new device; issue guidance to clarify when manufacturing data should be submitted as part of a 510(k); and clarify when it will withhold clearance for failure to comply with good manufacturing practices (i.e., when the FDA will conduct a pre-clearance inspection). These or other revisions to the FDA’s 510(k) clearance process, when fully implemented, could impose additional regulatory requirements upon us that could delay our ability to obtain new clearances, increase the costs of compliance, or restrict our ability to maintain current clearances. The requirements of the more rigorous premarket approval process and/or significant changes to the Section 510(k) clearance process could delay product introductions and increase our costs associated with FDA compliance.
We may fail to receive positive clinical results for our products in development that require clinical trials, and even if we receive positive clinical results, we may still fail to receive the necessary clearance or approvals to market our products.
In the development of new products or new indications for, or modifications to, existing products, we may conduct or sponsor clinical trials. Clinical trials are expensive and require significant investment of time and resources and may not generate the data we need to support a submission to the FDA. Clinical trials are subject to regulation by the FDA and, if federal funds are involved or if an investigator or site has signed a federal assurance, are subject to further regulation by the Office for Human Research Protections and the National Institutes of Health. Failure to comply with such regulation, including, but not limited to, failure to obtain adequate consent of subjects, failure to adequately disclose financial conflicts or failure to report data or adverse events accurately, could result in fines, penalties, suspension of trials, and the inability to use the data to support an FDA submission. In addition, the American Recovery and Reinvestment Act expands federal efforts to compare the effectiveness of different medical treatments, which could include some element of explicit cost or cost-effectiveness comparisons; research supported by these efforts eventually could be used to guide public and private coverage and reimbursement policies. In the international market, we are subject to regulations for clinical studies in each respective country.
If we fail to comply with the various regulatory regimes for the foreign markets in which we operate, our operational results could be adversely affected.
In many of the foreign countries in which we market our products, we are subject to extensive regulations, including those in Europe. The regulation of our products in the European Economic Area (which consists of the
38
Table of Contents
twenty-seven member states of the European Union, as well as Iceland, Liechtenstein and Norway) is governed by various directives and regulations promulgated by the European Commission and national governments. Only medical devices that comply with certain conformity requirements are allowed to be marketed within the European Economic Area. In addition, the national health or social security organizations of certain foreign countries, including certain countries outside Europe, require our products to be qualified before they can be marketed in those countries. Failure to receive or delays in the receipt of, relevant foreign qualifications in the European Economic Area or other foreign countries could have a material adverse impact on our business.
The FDA regulates the export of medical devices from the U.S. to foreign countries and certain foreign countries may require FDA certification that our products are in compliance with U.S. law. If we fail to obtain or maintain export certificates required for the export of our products, we could suffer a material adverse impact on our revenues and growth.
We are subject to laws concerning our marketing activities in foreign countries where we conduct business. For example, within the EU, the control of unlawful marketing activities is a matter of national law in each of the member states of the EU. The member states of the EU closely monitor perceived unlawful marketing activity by companies. We could face civil, criminal and administrative sanctions if any member state determines that we have breached our obligations under its national laws. In particular, as a result of conducting business in the U.K. through our subsidiary in that country, we are, in certain circumstances, subject to the anti-corruption provisions of the U.K. Bribery Act in our activities conducted in any country in the world. Industry associations also closely monitor the activities of member companies. If these organizations or authorities name us as having breached our obligations under their regulations, rules or standards, our reputation would suffer and our business and financial condition could be adversely affected. We are also subject to the U.S. Foreign Corrupt Practices Act (the “FCPA”), antitrust and anti-competition laws, and similar laws in foreign countries, any violation of which could create a substantial liability for us and also cause a loss of reputation in the market. The FCPA prohibits U.S. companies and their officers, directors, employees, shareholders acting on their behalf and agents from corruptly offering, promising, authorizing or making payments, or giving anything of value, directly or indirectly, to foreign officials for the purpose of obtaining or retaining business abroad or otherwise obtaining favorable treatment. Companies must also maintain records that fairly and accurately reflect transactions and maintain an adequate system of internal accounting controls. In many countries, hospitals and clinics are government-owned and healthcare professionals employed by such hospitals and clinics, with whom we regularly interact, may meet the definition of a foreign official for purposes of the FCPA. If we are found to have violated the FCPA, we may face sanctions including fines, criminal penalties, disgorgement of profits and suspension or debarment of our ability to contract with government agencies or receive export licenses. From time to time, we may face audits or investigations by one or more domestic or foreign government agencies, compliance with which could be costly and time-consuming, and could divert our management and key personnel from our business operations. An adverse outcome under any such investigation or audit could subject us to fines or other penalties, which could adversely affect our business and financial results.
If the Department of Health and Human Services (“HHS”), the Office of Inspector General (“OIG”), the FDA or another regulatory agency determines that we have promoted off-label use of our products, we may be subject to various penalties, including civil or criminal penalties, and the off-label use of our products may result in injuries that lead to product liability suits, which could be costly to our business.
The OIG, the FDA and other regulatory agencies actively enforce regulations prohibiting the promotion of a medical device for a use that has not been cleared or approved by the FDA. Use of a device outside its cleared or approved indications is known as “off-label” use. Physicians may use our products for off-label uses, as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. However, if the OIG or the FDA, or another regulatory agency determines that our promotional materials, training, or activities constitute improper promotion of an off-label use, the regulatory agency could request that we modify our promotional materials; training, or activities, or subject us to regulatory enforcement actions, including the issuance of a warning letter, injunction, seizure, civil fine and criminal penalties. Although our policy is to
39
Table of Contents
refrain from statements and activities that could be considered off-label promotion of our products, the FDA, another regulatory agency, or the U.S. Department of Justice could disagree and conclude that we have engaged in off-label promotion and, potentially, caused the submission of false claims. In addition, the off-label use of our products may increase the risk of injury to patients, and, in turn, the risk of product liability claims. Product liability claims are expensive to defend and could divert our management’s attention and result in substantial damage awards against us.
Our compensation, marketing and sales practices may contain certain risks with respect to the manner in which these practices were historically conducted that could have a material adverse impact on us.
We have entered into written agreements for designing and consulting services with physicians for surgical implant products, and we compensate them under our designing physician agreements for services in developing products sold by us. We also seek the assistance of physicians in the design and evaluation of bracing and other rehabilitative products. The form of compensation for such services has historically been a royalty on the sale of our products in the cases where the physician has contributed to the design of the product. We may also compensate the physicians under consulting agreements for assistance with product development and clinical efforts. We believe that in each instance remuneration paid to physicians represents fair market value for the services provided and is otherwise in compliance with applicable laws. For some products, we also use an independent sales force to which we provide compliance-related training. The sales force has generally been compensated on a commission basis, based on a percentage of revenues generated by products sold, as is typical in our industry. We also pay physicians certain rental and office support fees under our OfficeCare program. Under applicable federal and state healthcare fraud and abuse, anti-kickback, false claims and self-referral laws, it could be determined that our designing and consulting arrangements with surgeons, our marketing and sales practices, and our OfficeCare program fall outside permitted arrangements, thereby subjecting us to possible civil and/or criminal sanctions (including exclusion from the Medicare and Medicaid programs), which could have a material adverse impact on our Surgical Implant segment and possibly on our other lines of business. The federal government has significantly increased investigations of medical device manufacturers with regards to alleged kickbacks and other forms of remuneration to physicians who use and prescribe their products and recently has entered into settlement, deferred prosecution and corporate integrity agreements with such manufacturers. Such investigations and enforcement activities often arise based on allegations of violations of the federal Anti-Kickback Statute, and sometimes of the civil False Claims Act. Although we believe we maintain a satisfactory compliance program, it may not be adequate in the detection or prevention of violations. The form and effectiveness of our compliance program may be taken into account by the government in assessing sanctions, if any, should it be determined that violations of laws have occurred.
Audits or denials of our claims by government agencies could reduce our revenues or profits.
As part of our business operations, we submit claims on behalf of patients directly to, and receive payments directly from, the Medicare and Medicaid programs and private payors. Therefore, we are subject to extensive government regulation, including detailed requirements for submitting reimbursement claims under appropriate codes and maintaining certain documentation to support our claims. Medicare contractors and Medicaid agencies periodically conduct pre- and post-payment reviews and other audits of claims and are under increasing pressure to more closely scrutinize healthcare claims and supporting documentation. We have historically been subject to pre-payment and post-payment reviews as well as audits of claims and may experience such reviews and audits of claims in the future. Such reviews and/or similar audits of our claims including by Recovery Audit Contractors (private companies operating on a contingent fee basis to identify and recoup Medicare overpayments) could result in material delays in payment, as well as material recoupments or denials, which would reduce our net sales and profitability, or in exclusion from participation in the Medicare or Medicaid programs. Private payors may from time to time conduct similar reviews and audits.
Additionally, we participate in the government’s Federal Supply Schedule program for medical equipment, whereby we contract with the government to supply certain of our products. Participation in this program
40
Table of Contents
requires us to follow certain pricing practices and other contract requirements. Failure to comply with such pricing practices and/or other contract requirements could result in delays in payment or fines or penalties, which could reduce our revenues or profits.
Federal and state agencies have become increasingly vigilant in recent years in their investigation of various business practices under various healthcare “fraud and abuse” laws with respect to our business arrangements with prescribing physicians and other healthcare professionals, as well as our filing of DMEPOS claims for reimbursement.
We are, directly or indirectly through our customers, subject to various federal and state laws pertaining to healthcare fraud and abuse. These laws, which directly or indirectly affect our ability to operate our business include, but are not limited to, the following:
| • | the federal Anti-Kickback Statute, which prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce either the referral of an individual, or the furnishing or arranging for or recommending of a good or service, for which payment may be made under federal healthcare programs, such as Medicare and Medicaid, Veterans Administration health programs, and TRICARE, |
| • | several federal False Claims statutes, which have been expanded by recent legislation and impose civil and criminal liability on individuals and entities who submit, or cause to be submitted, false or fraudulent claims for payment to the government, |
| • | the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which prohibits executing a scheme to defraud any healthcare benefit program, and also prohibits false statements, defined as knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services, |
| • | the federal physician self-referral prohibition, commonly known as the Stark Law, which, in the absence of a statutory or regulatory exception, prohibits the referral of Medicare and Medicaid patients by a physician to an entity for the provision of certain designated healthcare services, if the physician or a member of the physician’s immediate family has a direct or indirect financial relationship, including an ownership interest in, or a compensation arrangement with, the entity and also prohibits that entity from submitting a bill to a federal payor for services rendered pursuant to a prohibited referral, and |
| • | state law equivalents to the Anti-Kickback Statute, the false claims provisions, the Stark Law and the physician self-referral prohibitions, some of which may apply even more broadly than their federal counterparts because they are not limited to government reimbursed items and include items or services reimbursed by any payor. |
The federal government has significantly increased investigations of and enforcement activity involving medical device manufacturers with regard to alleged kickbacks and other forms of remuneration to physicians who use and prescribe their products. Such investigations often arise based on allegations of violations of the federal Anti-Kickback Statute and sometimes allege violations of the civil False Claims Act, in connection with off-label marketing of products to physicians and others. In addition, significant state and federal investigative and enforcement activity addresses alleged improprieties in the filings of claims for payment or reimbursement by Medicare, Medicaid, and other payors.
We are both a device manufacturer and a supplier of DMEPOS, and like other companies in the orthopedic industry, are involved in ongoing governmental investigations (civil and criminal) and litigation, the results of which may adversely impact our business and results of operations. Defendants determined to be liable under the civil False Claims Act may be required to pay three times the actual damages sustained by the government, plus
41
Table of Contents
mandatory civil penalties ranging between $5,500 and $11,000 for each false claim. We are also subject to allegations by private whistleblowers under state or federal false claims act provisions. In addition, we are subject to a variety of civil monetary penalty and exclusion provisions.
The fraud and abuse laws and regulations are complex and even minor, inadvertent irregularities in submissions can potentially give rise to investigations and claims that the law has been violated. Any violations of these laws or regulations could result in a material adverse impact on our business, financial condition and results of operations. If there is a change in law, regulation or administrative or judicial interpretations, we may have to change one or more of our business practices to be in compliance with these laws. Required changes could be costly and time consuming. Any failure to make required changes could result in our losing business or our existing business practices being challenged as unlawful.
Our activities are subject to Federal Privacy and Transaction Law and Regulations, which could have an impact on our operations.
HIPAA impacts the transmission, maintenance, use and disclosure of certain individually identifiable health information (referred to as “protected health information” or “PHI”). Since HIPAA was enacted in 1996, numerous implementing regulations have been issued, including, but not limited to: (1) standards for the privacy of individually identifiable health information (the “Privacy Rule”), (2) standards to protect the confidentiality, integrity and security of electronic protected health information (the “Security Rule”), (3) standards for electronic transactions, (4) a standard unique national identifier for providers and health plans, and (5) the HHS Breach Notification Rule. We refer to these rules, as well as similar state laws applicable to our operations as the “HIPAA Rules.” HHS has also issued regulations governing the enforcement of the HIPAA Rules, the violation of which potentially includes significant criminal and civil penalties. Furthermore, many states have similar laws and regulations applicable to our operations, including but not limited to state data security breach requirements.
The HIPAA Rules apply to “covered entities,” which includes healthcare providers who conduct certain transactions electronically, including but not limited to the electronic submission of health care claims to an insurance carrier. We also provide services to customers that are directly regulated entities under HIPAA and the HIPAA Rules, and we are required to provide satisfactory written assurances to these customers through our written agreements that we will provide our services in accordance with HIPAA and the HIPAA Rules. As such, HIPAA and the HIPAA Rules apply to certain aspects of our business. To the extent applicable to our operations, we believe we are currently in compliance with HIPAA and the applicable HIPAA Rules. Any failure to comply with applicable requirements could adversely affect our profitability.
On February 17, 2009, President Obama signed into law the Health Information Technology for Economic and Clinical Health (“HITECH”) Act as part of the American Recovery and Reinvestment Act. This law includes strengthened federal privacy and security provisions to protect personally-identifiable health information, such as the notification requirements set forth in the Breach Notification Rule. These new statutorily mandated changes to the HIPAA Rules are being implemented through regulations, which, aside from the Breach Notification Rule (an interim final rule), have been released only in proposed form. We are reviewing these proposed changes to the HIPAA Rules to assess the potential impact on our operations. There are costs and administrative burdens associated with ongoing compliance with the HIPAA Rules and similar state law requirements. Any failure to comply with current and applicable future requirements could adversely affect our profitability.
Like many health care providers, we maintain personal information concerning our patients. Such information is subject to increasing regulation designed to prevent or mitigate the effects of financial and medical identity theft. There can be no assurance that the loss or improper exposure of such personal data will not adversely impact the business and prospects of our operations, nor result in possible civil litigation by customers and affected individuals.
Managed care and buying groups have put downward pressure on the prices of our products.
The growth of managed care and the advent of buying groups in the United States have caused a shift toward coverage and payments based on more cost-effective treatment alternatives. Buying groups enter into
42
Table of Contents
preferred supplier arrangements with one or more manufacturers of medical products in return for price discounts to members of these buying groups. Our failure to obtain new preferred supplier commitments from major group purchasing organizations or our failure to retain our existing preferred supplier commitments could adversely affect our sales and profitability. In international markets where we sell our products, we have historically experienced downward pressure on product pricing and other effects of healthcare cost control efforts that are similar to that which we have experienced in the United States. We expect a continued emphasis on healthcare cost controls and managed care in the United States and in these international markets, which could put further downward pressure on product pricing, which, in turn may adversely affect our sales and profitability.
Our marketed, approved, or cleared products are subject to the recall authority of U.S. and foreign regulatory bodies. Product recalls could harm our reputation and business.
We are subject to ongoing medical device reporting regulations that require us to report to the FDA and similar governmental authorities in other countries if we receive a report or otherwise learn that any of our products may have caused, or contributed to death or serious injury, or that any of our products has malfunctioned in a way that would be likely to cause, or contribute to, death or serious injury if the malfunction were to recur. The FDA and similar governmental authorities in other countries have the authority to require us to recall our products in the event of actual or potential material deficiencies or defects in design manufacturing, or labeling, and we have been subject to product recalls in the past. In addition, in light of an actual or potential material deficiency or defect in design, manufacturing, or labeling, we may voluntarily elect to recall our products. A government mandated recall or a voluntary recall initiated by us could occur as a result of actual or potential component failures, manufacturing errors, or design defects, including defects in labeling. Any recall would divert managerial and financial resources and could harm our reputation with our customers and with the healthcare professionals that use, prescribe and recommend our products. We could have product recalls that result in significant costs to us in the future, and such recalls could have a material adverse impact on our business.
Product liability claims may harm our business, particularly if the number of claims increases significantly or our product liability insurance proves inadequate.
The manufacture and sale of orthopedic devices and related products exposes us to a significant risk of product liability claims. From time to time, we have been, and we are currently, subject to a number of product liability claims alleging that the use of our products resulted in adverse effects. Even if we are successful in defending against any liability claims, such claims could nevertheless distract our management, result in substantial costs, harm our reputation, adversely affect the sales of all our products and otherwise harm our business. If there is a significant increase in the number of product liability claims, our business could be adversely affected. Further, a significant increase in claims or adverse outcomes could prove our product liability insurance inadequate.
Our concentration of manufacturing operations in Mexico increases our business and competitive risks.
Our most significant manufacturing facility is our facility in Tijuana, Mexico, and we also have a relatively small manufacturing operation in Tunisia. Our current and future foreign operations are subject to risks of political and economic instability inherent in activities conducted in foreign countries. Because there are no readily accessible alternatives to these facilities, any event that disrupts manufacturing at or distribution or transportation from these facilities would materially adversely affect our operations. In addition, as a result of this concentration of manufacturing activities, our sales in foreign markets may be at a competitive disadvantage to products manufactured locally due to freight costs, custom and import duties and favorable tax rates for local businesses.
If we lose one of our key suppliers or one of our contract manufacturers stops making the raw materials and components used in our products, we may be unable to meet customer orders for our products in a timely manner or within our budget.
We rely on a limited number of foreign and domestic suppliers for the raw materials and components used in our products. One or more of our suppliers may decide to cease supplying us with raw materials and
43
Table of Contents
components for reasons beyond our control. FDA regulations may require additional testing of any raw materials or components from new suppliers prior to our use of those materials or components. In addition, in the case of a device which is the subject of a pre-market approval, we may be required to obtain prior FDA permission (which may or may not be given), which could delay or prevent our access or use of such raw materials or components. If we are unable to obtain materials we need from our suppliers or our agreements with our suppliers are terminated, and we cannot obtain these materials from other sources, we may be unable to manufacture our products to meet customer orders in a timely manner or within our manufacturing budget. In that event, our business and results of operations could be adversely affected.
In addition, we rely on third parties to manufacture some of our products. For example, Medireha, which is 50% owned by us, has been a supplier for a significant portion of our CPM devices. CPM devices represented 4% of our net sales for the year ended December 31, 2011. If we encounter a cessation, interruption or delay in the supply of the products purchased from Medireha, we may be unable to obtain such products through other sources on acceptable terms, within a reasonable amount of time or at all. We also use a single source for many of the devices Cefar and Compex distribute. In addition, if our agreements with the manufacturing companies were terminated, we may not be able to find suitable replacements within a reasonable amount of time or at all. Any such cessation, interruption or delay may impair our ability to meet scheduled deliveries of our products to our customers and may cause our customers to cancel orders. In that event, our reputation and results of operations may be adversely affected.
Some of our important suppliers are in China and other parts of Asia and provide predominately finished soft goods products. In the year ended December 31, 2011, we obtained 31.6% of our total purchased materials from suppliers in China and other parts of Asia. Political and economic instability and changes in government regulations in these areas could affect our ability to continue to receive materials from suppliers there. The loss of suppliers in China and other parts of Asia, any other interruption or delay in the supply of required materials or our inability to obtain these materials at acceptable prices and within a reasonable amount of time could impair our ability to meet scheduled product deliveries to our customers and could hurt our reputation and cause customers to cancel orders.
In addition, we purchase the microprocessor used in the OL1000 and SpinaLogic devices from a single manufacturer. Although there are feasible alternate microprocessors that might be used immediately, all are produced by a single supplier. In addition, there are single suppliers for other components used in the OL1000 and SpinaLogic devices and only two suppliers for the magnetic field sensor employed in them. Establishment of additional or replacement suppliers for these components cannot be accomplished quickly.
If our patents and other intellectual property rights do not adequately protect our products, we may lose market share to our competitors and may not be able to operate our business profitably.
We rely on a combination of patents, trade secrets, copyrights, trademarks, license agreements and contractual provisions to establish and protect our intellectual property rights in our products and the processes for the development, manufacture and marketing of our products.
We use non-patented, proprietary know-how, trade secrets, processes and other proprietary information and currently employ various methods to protect this proprietary information, including confidentiality agreements, invention assignment agreements and proprietary information agreements with vendors, employees, independent sales agents, distributors, consultants, and others. However, these agreements may be breached. The FDA or another governmental agency may require the disclosure of such information in order for us to have the right to market a product. The FDA may also disclose such information on its own initiative if it should decide that such information is not confidential business or trade secret information. Trade secrets, know-how and other unpatented proprietary technology may also otherwise become known to or independently developed by our competitors.
44
Table of Contents
In addition, we also hold U.S. and foreign patents relating to a number of our components and products and have patent applications pending with respect to other components and products. We also apply for additional patents in the ordinary course of our business, as we deem appropriate. However, these precautions offer only limited protection, and our proprietary information may become known to, or be independently developed by, competitors, or our proprietary rights in intellectual property may be challenged, any of which could have a material adverse impact on our business, financial condition and results of operations. Additionally, we cannot assure you that our existing or future patents, if any, will afford us adequate protection or any competitive advantage, that any future patent applications will result in issued patents or that our patents will not be circumvented, invalidated or declared unenforceable. In addition, certain of our subsidiaries have not always taken commercially reasonable measures to protect their ownership of some of their patents. While such measures are currently employed and have been employed by us in the past, disputes may arise as to the ownership, or co-ownership, of certain of our patents. We do not consider patent protection to be a significant competitive advantage in the marketplace for electrotherapy devices. However, patent protection may be of significance with respect to our orthopedic technology.
Any proceedings before the U.S. Patent and Trademark Office could result in adverse decisions as to the priority of our inventions and the narrowing or invalidation of claims in issued or pending patents. We could also incur substantial costs in any such proceedings. In addition, the laws of some of the countries in which our products are or may be sold may not protect our products and intellectual property to the same extent as U.S. laws, if at all. We may also be unable to protect our rights in trade secrets, trademarks and unpatented proprietary technology in these countries.
In addition, we hold patent, trademark and other intellectual property licenses from third parties for some of our products and on technologies that are necessary in the design and manufacture of some of our products. The loss of such licenses could prevent us from manufacturing, marketing and selling these products, which in turn could harm our business.
Our operating results and financial condition could be adversely affected if we become involved in litigation regarding our patents or other intellectual property rights.
Litigation involving patents and other intellectual property rights is common in our industry, and companies in our industry have used intellectual property litigation in an attempt to gain a competitive advantage. We may become a party to lawsuits involving patents or other intellectual property. Such litigation is costly and time consuming. If we lose any of these proceedings, a court or a similar foreign governing body could invalidate or render unenforceable our owned or licensed patents, require us to pay significant damages, seek licenses and/or pay ongoing royalties to third parties (which may not be available under terms acceptable to us, or at all), require us to redesign our products, or prevent us from manufacturing, using or selling our products, any of which would have an adverse impact on our results of operations and financial condition.
We have brought, and may in the future also bring, actions against third parties for infringement of our intellectual property rights. We may not succeed in such actions. The defense and prosecution of intellectual property suits, proceedings before the U.S. Patent and Trademark Office or its foreign equivalents and related legal and administrative proceedings are both costly and time consuming. Protracted litigation to defend or enforce our intellectual property rights could seriously detract from the time our management would otherwise devote to running our business. Intellectual property litigation relating to our products could cause our customers or potential customers to defer or limit their purchase or use of the affected products until resolution of the litigation.
Our business strategy relies on certain assumptions concerning demographic and other trends that impact the market for our products. If these assumptions prove to be incorrect, demand for our products may be lower than we currently expect.
Our ability to achieve our business objectives is subject to a variety of factors, including the relative increase in the aging of the general population and an increase in participation in exercise and sports and more
45
Table of Contents
active lifestyles. In addition, our business strategy relies on an increasing awareness and clinical acceptance of non-invasive, non-systemic treatment and rehabilitation products, such as electrotherapy. We believe that these trends will increase the need for our orthopedic, physical therapy, regenerative and surgical implant products. The projected demand for our products could materially differ from actual demand if our assumptions regarding these trends and acceptance of our products by healthcare professionals and patients prove to be incorrect or do not materialize. If our assumptions regarding these factors prove to be incorrect, we may not be able to successfully implement our business strategy, which could adversely affect our results of operations. In addition, the perceived benefits of these trends may be offset by competitive or business factors, such as the introduction of new products by our competitors or the emergence of other countervailing trends.
Consolidation in the healthcare industry could have an adverse impact on our revenues and results of operations.
Many healthcare industry companies, including medical device, orthopedic and physical therapy products companies, are consolidating to create larger companies. As the healthcare industry consolidates, competition to provide products and services to industry participants may become more intense. In addition, many of our customers are also consolidating, and our customers and other industry participants may try to use their purchasing power to negotiate price concessions or reductions for the products that we manufacture and market. If we are forced to reduce our prices because of consolidation in the healthcare industry, our revenues could decrease, and our business, financial condition and results of operations could be adversely affected.
We could incur significant costs complying with environmental and health and safety requirements, or as a result of liability for contamination or other harm caused by hazardous materials that we use.
Our research and development and manufacturing processes involve the use of hazardous materials. We are subject to federal, state, local and foreign environmental requirements, including regulations governing the use, manufacture, handling, storage and disposal of hazardous materials, discharge to air and water, the clean up of contamination and occupational health and safety matters. We cannot eliminate the risk of contamination or injury resulting from hazardous materials, and we may incur liability as a result of any contamination or injury. Under some environmental laws and regulations, we could also be held responsible for costs relating to any contamination at our past or present facilities and at third party waste disposal sites where we have sent wastes. These could include costs relating to contamination that did not result from any violation of law, and in some circumstances, contamination that we did not cause. We may incur significant expenses in the future relating to any failure to comply with environmental laws. Any such future expenses or liability could have a significant negative impact on our financial condition. The enactment of stricter laws or regulations, the stricter interpretation of existing laws and regulations or the requirement to undertake the investigation or remediation of currently unknown environmental contamination at our own or third party sites may require us to make additional expenditures, which could be material.
Our reported results may be adversely affected by increases in reserves for contractual allowances, rebates, product returns, rental credits, uncollectible accounts receivable and inventory.
We have established reserves to account for contractual allowances, rebates, product returns and reserves for rental credits. Significant management judgment must be used and estimates must be made in connection with establishing the reserves for contractual allowances, rebates, product returns, rental credits and other allowances in any accounting period. Any increase in our reserves for such items could adversely affect our reported financial results by reducing our net revenues and/or profitability for the reporting period.
If a natural or man-made disaster strikes our manufacturing facilities, we will be unable to manufacture our products for a substantial amount of time and our sales will decline.
A significant portion of our rehabilitation products are manufactured in a facility in Tijuana, Mexico, with a number of products for the European market manufactured in a Tunisian facility. In Vista, California we
46
Table of Contents
manufacture our custom rigid bracing products, which remain in the United States to facilitate quick turnaround on custom orders, vascular products, and our regeneration product line. Our clinical electrotherapy devices, patient care products, physical therapy and certain CPM devices are now manufactured in our facilities located in Tijuana, Mexico, following the closure of our Chattanooga facility during the first half of 2010. Our home electrotherapy devices sold in the United States as well as some components and related accessories are manufactured at our facility in Clear Lake, South Dakota. In our Surgical Implant business, we manufacture our products in our manufacturing facility at Austin, Texas. These facilities and the manufacturing equipment we use to produce our products would be difficult to repair or replace. Our facilities may be affected by natural or man-made disasters. If one of our facilities were affected by a disaster, we would be forced to rely on third party manufacturers or shift production to another manufacturing facility. In such an event, we would face significant delays in manufacturing which would prevent us from being able to sell our products. In addition, our insurance may not be sufficient to cover all of the potential losses and may not continue to be available to us on acceptable terms, or at all.
If we do not effectively manage our growth, our existing infrastructure may become strained, and we may be unable to increase sales of our products or generate revenue growth.
The growth that we have experienced, and in the future may experience, including due to acquisitions, may provide challenges to our organization, requiring us to expand our personnel, manufacturing and distribution operations. Future growth may strain our infrastructure, operations, product development and other managerial and operating resources. If our business resources become strained, we may be unable to increase sales of our products or generate revenue growth.
Affiliates of Blackstone own substantially all of the equity interest in us and may have conflicts of interest with us or investors in the future.
Affiliates of Blackstone collectively beneficially own 98.1% of DJO’s issued and outstanding capital stock and Blackstone designees hold a majority of the seats on DJO’s board of directors. As a result, affiliates of Blackstone have control over our decisions to enter into any corporate transaction and have the ability to prevent any transaction that requires the approval of stockholders regardless of whether holders of the notes believe that any such transactions are in their own best interests. For example, affiliates of Blackstone could collectively cause us to make acquisitions that increase the amount of indebtedness or to sell assets, or could cause us to issue additional capital stock or declare dividends. So long as affiliates of Blackstone continue to directly or indirectly own a significant amount of the outstanding shares of our common stock, they will continue to be able to strongly influence or effectively control our decisions. In addition, Blackstone has no obligation to provide us with any additional debt or equity financing.
Additionally, Blackstone and its affiliates are in the business of making investments in companies and may from time to time acquire and hold interests in businesses that compete directly or indirectly with us. Blackstone and its affiliates may also pursue acquisition opportunities that may be complementary to our business and, as a result, those acquisition opportunities may not be available to us.
If we do not achieve and maintain effective internal controls over financial reporting, we could fail to accurately report our financial results.
During the course of the preparation of our financial statements, we evaluate our internal controls to identify and correct deficiencies in our internal controls over financial reporting. In the event we are unable to identify and correct deficiencies in our internal controls in a timely manner, we may not record, process, summarize and report financial information accurately and within the time periods required for our financial reporting under the terms of the agreements governing our indebtedness.
We have completed a significant number of acquisitions in the past several years, and may continue to pursue growth through strategic acquisitions. Among the risks associated with acquisitions are the risks of control deficiencies that result from the integration of the acquired business. In connection with the integration of
47
Table of Contents
our recent acquisitions and our continuous assessment of internal controls, including with respect to acquired foreign operations, we have identified certain internal control deficiencies that we have remedied or for which we have undertaken steps to remediate.
It is possible that control deficiencies could be identified by our management or independent registered public accounting firm in the future or may occur without being identified. Such a failure could negatively impact the market price and liquidity of the notes, causing holders of our notes to lose confidence in our reported financial condition, lead to a default under our senior secured credit facilities and the Indentures and otherwise materially adversely affect our business and financial condition.
48
Table of Contents
We will not receive any cash proceeds from the issuance of the exchange notes pursuant to the exchange offers. In consideration for issuing the exchange notes as contemplated in this prospectus, we will receive in exchange a like principal amount of outstanding notes, the terms of which are identical in all material respects to the exchange notes. The outstanding notes surrendered in exchange for the exchange notes will be retired and cancelled and cannot be reissued. Accordingly, the issuance of the exchange notes will not result in any change in our capitalization.
49
Table of Contents
The following table sets forth DJO Finance LLC’s cash and cash equivalents and capitalization as of September 29, 2012 on a historical basis and on an as adjusted basis after giving effect to the 2012 offering. The information in this table should be read in conjunction with the historical financial statements and related notes incorporated by reference in this offering circular. See “Use of Proceeds.”
| As of September 29, 2012 | ||||||||
| (in millions) |
Historical | As adjusted | ||||||
| Cash and cash equivalents (1)(2)(3) |
$ | 38.2 | $ | 52.5 | ||||
|
|
|
|
|
|||||
| Debt: |
||||||||
| Senior secured credit facilities: |
||||||||
| Revolving credit facility (2)(4)(9) |
$ | 38.0 | $ | — | ||||
| New Term Loans due 2017 (5) |
452.7 | 452.7 | ||||||
| Extended Term Loans due 2016 (5) |
386.5 | 386.5 | ||||||
| 8.75% Second priority senior secured notes due 2018 (6) |
230.0 | 330.0 | ||||||
| 10.875% Senior unsecured notes due 2014 (2)(7)(8) |
465.0 | — | ||||||
| 9.875% Senior notes due 2018 offered hereby |
— | 440.0 | ||||||
| 7.75% Senior unsecured notes due 2018 |
300.0 | 300.0 | ||||||
| 9.75% Senior subordinated notes due 2017 |
300.0 | 300.0 | ||||||
| Capital lease obligations |
— | — | ||||||
|
|
|
|
|
|||||
| Total debt |
2,172.2 | 2,209.2 | ||||||
| Membership equity |
228.4 | 200.9 | ||||||
|
|
|
|
|
|||||
| Total capitalization |
$ | 2,400.6 | $ | 2,410.1 | ||||
|
|
|
|
|
|||||
| (1) | As of November 24, 2012, our cash and cash equivalents were $30.6 million. |
| (2) | Does not reflect $20.1 million of accrued interest that was repaid with respect to the indebtedness refinanced in the 2012 offering. |
| (3) | Reflects original issue premium of $6.75 million on the $100.0 million of outstanding secured notes offered in the 2012 offering. |
| (4) | As of November 24, 2012, we had $2,225.8 million aggregate principal amount of borrowings outstanding under our senior secured revolving credit facility. |
| (5) | Does not reflect unamortized original issue discount as of September 29, 2012 of $9.3 million. |
| (6) | Includes $100.0 million aggregate principal amount of 8.75% Second priority senior secured notes being offered hereby, which will issue with $6.75 million of premium that will be amortized and included as a reduction of interest expense as the Second priority senior secured notes mature. |
| (7) | Does not reflect unamortized original issue premium as of September 29, 2012 of $2.5 million. |
| (8) | On November 20, 2007, we issued $575 million aggregate principal amount of 10.875% Senior unsecured notes in connection with the DJO Merger. On January 20, 2010 we issued an additional $100 million aggregate principal amount of 10.875% Senior unsecured notes. On March 20, 2012, we repurchased $210 million aggregate principal amount of 10.875% Senior unsecured notes. On October 1, 2012 and November 15, 2012, we repurchased and redeemed, respectively, all $465 million aggregate principal amount of the outstanding 10.875% Senior unsecured notes. See “Use of Proceeds.” |
| (9) | As of October 1, 2012, the outstanding balance of the Revolving credit facility of $38 million was paid with the net proceeds from the offering of the outstanding notes. |
50
Table of Contents
SELECTED HISTORICAL CONSOLIDATED AND COMBINED FINANCIAL DATA
The following table presents data as of and for the periods indicated and has been derived from the audited historical consolidated financial statements. The data reported for all periods includes the results of operations attributable to businesses acquired from the date of acquisition. This selected financial data should be read in conjunction with the audited consolidated financial statements and related notes thereto, and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
| Three Months Ended September 29, 2012 |
Nine Months Ended September 29, 2012 |
Year Ended December 31, | ||||||||||||||||||||||||||
| ($ in thousands) |
2011 | 2010 | 2009 | 2008 | 2007 | |||||||||||||||||||||||
| Statement of Operations Data (1)(2): |
||||||||||||||||||||||||||||
| Net sales |
$ | 273,986 | $ | 790,615 | $ | 1,074,770 | $ | 965,973 | $ | 946,126 | $ | 948,469 | $ | 464,811 | ||||||||||||||
| Gross profit |
165,689 | 478,906 | 656,632 | 620,703 | 607,407 | 598,292 | 279,613 | |||||||||||||||||||||
| Loss from continuing operations (3) |
(22,535 | ) | (65,590 | ) | (213,587 | ) | (51,675 | ) | (49,391 | ) | (97,683 | ) | (83,455 | ) | ||||||||||||||
| Net loss attributable to DJOFL (3) |
(22,562 | ) | (66,258 | ) | (214,469 | ) | (52,532 | ) | (50,433 | ) | (97,786 | ) | (82,422 | ) | ||||||||||||||
| Other Financial Data: |
||||||||||||||||||||||||||||
| Depreciation and amortization (2) |
32,171 | 96,178 | 121,251 | 103,519 | 105,150 | 122,561 | 48,240 | |||||||||||||||||||||
| Balance Sheet Data (at period end): |
||||||||||||||||||||||||||||
| Cash and cash equivalents |
$ | 38,225 | $ | 38,225 | $ | 38,169 | $ | 38,132 | $ | 44,611 | $ | 30,483 | $ | 63,471 | ||||||||||||||
| Total assets |
2,852,041 | 2,852,041 | 2,894,860 | 2,779,790 | 2,850,179 | 2,940,130 | 3,086,272 | |||||||||||||||||||||
| Long-term debt, net of current portion |
2,156,863 | 2,156,863 | 2,159,091 | 1,816,291 | 1,796,944 | 1,832,044 | 1,818,598 | |||||||||||||||||||||
| DJOFL membership equity |
228,359 | 228,359 | 295,813 | 504,139 | 555,860 | 598,366 | 704,988 | |||||||||||||||||||||
| (1) | For additional information about our acquisitions in the past three years, see Note 3 of the notes to the audited consolidated financial statements included elsewhere in this prospectus. |
| (2) | We sold our Empi Therapy Solutions catalog business on June 12, 2009 and its results have been excluded from continuing operations for all periods presented. See Note 4 of the notes to the audited consolidated financial statements included elsewhere in this prospectus. |
| (3) | Results for the year ended December 31, 2011 include aggregate goodwill and intangible asset impairment charges of $141.0 million. Results for the year ended December 31, 2009 include aggregate intangible asset impairment charges of $7.0 million. |
51
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
This management’s discussion and analysis of financial condition and results of operations is intended to provide an understanding of our results of operations, financial condition and where appropriate, factors that may affect future performance.
You should read the following discussion of our results of operations and financial condition with “Selected Historical Consolidated and Combined Financial Data” and our audited historical consolidated financial statements and related notes thereto included elsewhere in this prospectus. The following management’s discussion and analysis contains “forward-looking statements” within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act that represent our expectations or beliefs concerning future events, including, but not limited to, statements regarding growth in sales of our products, profit margins and the sufficiency of our cash flow for future liquidity and capital resource needs. These forward-looking statements are further qualified by important factors that could cause actual results to differ materially from those in the forward-looking statements. These factors are described under the heading “Risk Factors” above. Results actually achieved may differ materially from expected results included in these statements as a result of these or other factors.
Overview of Business
We are a global developer, manufacturer and distributor of high-quality medical devices that provide solutions for musculoskeletal health, vascular health and pain management. Our products address the continuum of patient care from injury prevention to rehabilitation after surgery, injury or from degenerative disease, enabling people to regain or maintain their natural motion. Our products are used by orthopedic specialists, spine surgeons, primary care physicians, pain management specialists, physical therapists, podiatrists, chiropractors, athletic trainers and other healthcare professionals. In addition, many of our medical devices and related accessories are used by athletes and patients for injury prevention and at-home physical therapy treatment. Our product lines include rigid and soft orthopedic bracing, hot and cold therapy, bone growth stimulators, vascular therapy systems and compression garments, therapeutic shoes and inserts, electrical stimulators used for pain management and physical therapy products. Our surgical implant business offers a comprehensive suite of reconstructive joint products for the hip, knee and shoulder.
Our products are marketed under a portfolio of brands including Aircast®, DonJoy®, ProCare®, CMF™, Empi®, Chattanooga, DJO Surgical, Dr. Comfort ™ and Compex®.
Operating Segments
We currently develop, manufacture and distribute our products through the following four operating segments:
Bracing and Vascular Segment
Our Bracing and Vascular segment, which generates its revenues in the United States, offers our rigid knee bracing products, orthopedic soft goods, cold therapy products, vascular systems, therapeutic shoes and inserts and compression therapy products, primarily under our DonJoy, ProCare, Aircast and Dr. Comfort brands. This segment also includes our OfficeCare business, through which we maintain an inventory of soft goods and other products at healthcare facilities, primarily orthopedic practices, for immediate distribution to patients. The Bracing and Vascular segment primarily sells its products to orthopedic and sports medicine professionals, hospitals, podiatry practices, orthotic and prosthetic centers, home medical equipment providers and independent pharmacies.
52
Table of Contents
Recovery Sciences Segment
Our Recovery Sciences segment, which generates its revenues in the United States, is divided into four main businesses:
| • | Empi. Our Empi business unit offers our home electrotherapy, iontophoresis, and home traction products. We primarily sell these products directly to patients or to physical therapy clinics. For products sold to patients, we arrange billing to the patients and their third party payors. |
| • | Regeneration. Our Regeneration business unit sells our bone growth stimulation products. We sell these products either directly to patients or to independent distributors. For products sold to patients, we arrange billing to the patients and their third party payors. |
| • | Chattanooga. Our Chattanooga business unit offers products in the clinical rehabilitation market in the category of clinical electrotherapy devices, clinical traction devices, and other clinical products and supplies such as treatment tables, CPM devices and dry heat therapy. |
| • | Athlete Direct. Our Athlete Direct business unit offers consumers ranging from fitness enthusiasts to competitive athletes our Compex electrostimulation devices, which are used in athletic training programs to aid muscle development and to accelerate muscle recovery after training sessions. |
International Segment
Our International segment, which generates most of its revenues in Europe, sells all of our products and certain third party products through a combination of direct sales representatives and independent distributors.
Surgical Implant Segment
Our Surgical Implant segment, which generates its revenues in the United States, develops, manufactures and markets a wide variety of knee, hip and shoulder implant products that serve the orthopedic reconstructive joint implant market.
Our four operating segments enable us to reach a diverse customer base through multiple distribution channels and give us the opportunity to provide a wide range of medical devices and related products to orthopedic specialists and other healthcare professionals operating in a variety of patient treatment settings. These four segments constitute our reportable segments.
Recent Acquisitions, Dispositions and Other Transactions
Acquisitions
On April 7, 2011, we acquired all of the LLC membership interests of Rikco International, LLC, D/B/A Dr. Comfort (Dr. Comfort), for a total purchase price of $257.5 million. Dr. Comfort is a provider of therapeutic footwear, which serves the diabetes care market in podiatry practices, orthotic and prosthetic centers, home medical equipment providers and independent pharmacies.
On March 10, 2011, we acquired substantially all of the assets of Circle City Medical, Inc. (“Circle City”) for a total purchase price of $11.7 million. Circle City markets orthopedic soft goods and medical compression therapy products to independent pharmacies and home healthcare dealers.
On February 4, 2011, we purchased certain assets of an e-commerce business (“BetterBraces.com”), which offers various bracing, cold therapy and electrotherapy products, for total consideration of $3.0 million.
On January 4, 2011, we acquired all of the outstanding shares of capital stock of Elastic Therapy, Inc. (“ETI”), a designer and manufacturer of private label medical compression therapy products used to treat and prevent a wide range of venous disorders. The purchase price was $46.4 million.
53
Table of Contents
We completed the following acquisitions during the years ended December 31, 2010 and 2009, each of which represents an expansion of our international business:
On September 20, 2010, we acquired certain assets and contractual rights from an independent South African distributor of DonJoy products for total consideration of $1.9 million.
On August 4, 2009, we acquired Chattanooga Group Inc. and Empi Canada Inc., independent Canadian distributors of certain of our products for total consideration of $14.6 million.
On February 3, 2009, we acquired DonJoy Orthopaedics Pty., Ltd., an independent Australian distributor of DonJoy products for total consideration of $3.4 million.
See Note 3 of the notes to the audited consolidated financial statements included elsewhere in this prospectus for additional information regarding our acquisitions.
Sale of Empi Therapy Solutions (“ETS”)
On June 12, 2009 we sold a physical therapy catalog business to Patterson Medical Supply, Inc. for $21.8 million. As such, results of the ETS business for periods prior to the date of sale are presented as discontinued operations. See Note 4 of the notes to the audited consolidated financial statements included elsewhere in this prospectus.
Sale and Discontinuation of Other Product Lines
During the fourth quarter of 2009 we sold all rights, title and interest to our spinal implant business and related property for $2.9 million. In addition, also during the fourth quarter of 2009, we sold our line of chiropractic tables known as the Ergostyle line, and the TE-CH3 product (together referred to as product line) and other assets used in or otherwise related to the manufacture, sale and marketing of the product line for $0.8 million. We also discontinued certain other non-core product lines in our Recovery Sciences and Bracing and Vascular segments in 2009.
Impairment of Goodwill and Intangible Assets
In the fourth quarter of 2011, we determined that the carrying value of goodwill and intangible assets related to our Empi and Surgical Implant reporting units was in excess of their estimated fair value. As a result, we recorded a goodwill impairment charges for the Empi and Surgical Implant reporting units of $76.7 million and $47.4 million, respectively. Additionally, in the fourth quarter of 2011, we determined that the carrying value of our Empi trade name was in excess of its estimated fair value, and recorded an impairment charge of $16.9 million. See Note 8 of the notes to the audited consolidated financial statements included elsewhere in this prospectus for further discussion and the factors that contributed to these impairment charges.
54
Table of Contents
The tables below present financial information for our reportable segments for the periods presented. Segment results exclude the impact of amortization of intangible assets, certain general corporate expenses, and charges related to various integration activities, as defined by management.
| Three Months Ended | Nine Months Ended | |||||||||||||||
| ($ in thousands) |
September 29, 2012 | October 1, 2011 | September 29, 2012 | October 1, 2011 | ||||||||||||
| Bracing and Vascular: |
||||||||||||||||
| Net sales |
$ | 111,212 | $ | 101,452 | $ | 329,094 | $ | 281,687 | ||||||||
| Gross profit |
$ | 57,306 | $ | 52,997 | $ | 169,148 | $ | 149,593 | ||||||||
| Gross profit margin |
51.5 | % | 52.2 | % | 51.4 | % | 53.1 | % | ||||||||
| Operating income |
$ | 22,146 | $ | 18,844 | $ | 64,551 | $ | 54,339 | ||||||||
| Operating income as a percent of net segment sales |
19.9 | % | 18.6 | % | 19.6 | % | 19.3 | % | ||||||||
| Recovery Sciences: |
||||||||||||||||
| Net sales |
$ | 80,906 | $ | 81,956 | $ | 249,997 | $ | 253,335 | ||||||||
| Gross profit |
$ | 61,656 | $ | 61,573 | $ | 189,167 | $ | 191,836 | ||||||||
| Gross profit margin |
76.2 | % | 75.1 | % | 75.7 | % | 75.7 | % | ||||||||
| Operating income |
$ | 23,054 | $ | 21,392 | $ | 66,920 | $ | 68,892 | ||||||||
| Operating income as a percent of net segment sales |
28.5 | % | 26.1 | % | 26.8 | % | 27.2 | % | ||||||||
| International: |
||||||||||||||||
| Net sales |
$ | 64,671 | $ | 64,475 | $ | 206,649 | $ | 207,423 | ||||||||
| Gross profit |
$ | 34,815 | $ | 36,472 | $ | 114,696 | $ | 118,376 | ||||||||
| Gross profit margin |
53.8 | % | 56.6 | % | 55.5 | % | 57.1 | % | ||||||||
| Operating income |
$ | 10,604 | $ | 11,723 | $ | 39,502 | $ | 40,790 | ||||||||
| Operating income as a percent of net segment sales |
16.4 | % | 18.2 | % | 19.1 | % | 19.7 | % | ||||||||
| Surgical Implant: |
||||||||||||||||
| Net sales |
$ | 17,197 | $ | 15,235 | $ | 53,170 | $ | 48,170 | ||||||||
| Gross profit |
$ | 12,670 | $ | 10,698 | $ | 39,858 | $ | 34,239 | ||||||||
| Gross profit margin |
73.7 | % | 70.2 | % | 75.0 | % | 71.1 | % | ||||||||
| Operating income |
$ | 812 | $ | 827 | $ | 4,746 | $ | 2,230 | ||||||||
| Operating income as a percent of net segment sales |
4.7 | % | 5.4 | % | 8.9 | % | 4.6 | % | ||||||||
Results of Operations
We operate our business on a manufacturing calendar, with our fiscal year always ending on December 31. Each quarter is 13 weeks, consisting of two four-week periods and one five-week period. Our first and fourth quarters may have more or fewer shipping days from year to year based on the days of the week on which holidays and December 31 fall. For our domestic business segments, the three months ended September 29, 2012 and October 1, 2011 each included 63 shipping days and the nine months ended September 29, 2012 and October 1, 2011 each included 191 shipping days.
Changes in our financial results include the impact of changes in foreign currency exchange rates and acquisitions made in the current year. We provide “constant currency” and “pro forma” calculations to remove the impact of these items from net sales.
When we use the term “constant currency,” it means that we have translated financial data for a period into U.S. Dollars using the same foreign currency exchange rates that we used to translate financial data for the previous period. We believe that this calculation is a useful measure, indicating the actual growth of our operations.
55
Table of Contents
When we use the term “pro forma,” it means that we have included the impact of the pre-acquisition results of Dr. Comfort and Circle City Medical (Circle City), as if they had closed at the beginning of the prior year period. We believe that this calculation is a useful measure, providing a more meaningful comparison of the company’s year-over-year performance.
The constant currency and pro forma financial measures are used to supplement measures that are in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). These financial measures are not measures of financial performance under GAAP, and should not be considered as alternatives to measures presented in accordance with GAAP.
The following table sets forth our statement of operations as a percentage of net sales ($ in thousands):
| Three Months Ended | Nine Months Ended | |||||||||||||||||||||||||||||||
| September 29, 2012 |
October 1, 2011 |
September 29, 2012 |
October 1, 2011 |
|||||||||||||||||||||||||||||
| Net sales |
$ | 273,986 | 100.0 | % | $ | 263,118 | 100.0 | % | $ | 838,910 | 100.0 | % | $ | 790,615 | 100.0 | % | ||||||||||||||||
| Cost of sales (exclusive of amortization of intangible assets (1)) |
108,297 | 39.5 | 107,463 | 40.8 | 328,334 | 39.1 | 311,709 | 39.4 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Gross profit |
165,689 | 60.5 | 155,655 | 59.2 | 510,576 | 60.9 | 478,906 | 60.6 | ||||||||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||||||
| Selling, general and administrative |
110,735 | 40.4 | 115,854 | 44.0 | 342,617 | 40.8 | 361,761 | 45.8 | ||||||||||||||||||||||||
| Research and development |
7,938 | 2.9 | 6,477 | 2.5 | 21,695 | 2.6 | 19,721 | 2.5 | ||||||||||||||||||||||||
| Amortization of intangible assets |
24,487 | 8.9 | 24,435 | 9.3 | 73,505 | 8.8 | 69,373 | 8.8 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| 143,160 | 52.2 | 146,766 | 55.8 | 437,817 | 52.2 | 450,855 | 57.1 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Operating income |
22,529 | 8.3 | 8,889 | 3.4 | 72,759 | 8.7 | 28,051 | 3.5 | ||||||||||||||||||||||||
| Other income (expense): |
||||||||||||||||||||||||||||||||
| Interest expense |
(46,411 | ) | (16.9 | ) | (42,764 | ) | (16.2 | ) | (134,899 | ) | (16.1 | ) | (126,320 | ) | (16.0 | ) | ||||||||||||||||
| Interest income |
46 | — | 77 | — | 151 | — | 240 | — | ||||||||||||||||||||||||
| Loss on modification of debt |
— | — | — | — | (9,398 | ) | (1.1 | ) | (2,065 | ) | (0.2 | ) | ||||||||||||||||||||
| Other income (expense), net |
1,870 | 0.7 | (6,004 | ) | (2.3 | ) | 2,931 | 0.3 | (1,551 | ) | (0.2 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| (44,495 | ) | (16.2 | ) | (48,691 | ) | (18.5 | ) | (141,215 | ) | (16.9 | ) | (129,696 | ) | (16.4 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Loss before income taxes |
(21,966 | ) | (7.9 | ) | (39,802 | ) | (15.1 | ) | (68,456 | ) | (8.2 | ) | (101,645 | ) | (12.9 | ) | ||||||||||||||||
| Income tax (provision) benefit |
(569 | ) | (0.2 | ) | 14,096 | 5.3 | (3,044 | ) | (0.4 | ) | 36,055 | 4.6 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss |
(22,535 | ) | (8.1 | ) | (25,706 | ) | (9.8 | ) | (71,500 | ) | (8.6 | ) | (65,590 | ) | (8.3 | ) | ||||||||||||||||
| Net income attributable to noncontrolling interests |
(27 | ) | — | (58 | ) | — | (614 | ) | (0.1 | ) | (668 | ) | (0.1 | ) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net loss attributable to DJO Finance LLC |
$ | (22,562 | ) | (8.1 | )% | $ | (25,764 | ) | (9.8 | )% | $ | (72,114 | ) | (8.7 | )% | $ | (66,258 | ) | (8.4 | )% | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| (1) | Cost of sales is exclusive of amortization of intangible assets of $9,837 and $29,513 for the three and nine months ended September 29, 2012, respectively, and $9,688 and $28,831 for the three and nine months ended October 1, 2011, respectively. |
Three Months Ended September 29, 2012 (third quarter 2012) compared to Three Months Ended October 1, 2011 (third quarter 2011)
Net Sales. Net sales for third quarter 2012 were $274.0 million, increasing 4.1% from net sales of $263.1 million for third quarter 2011. This increase was driven primarily by sales of our new products and improved sales execution, partially offset by unfavorable changes in foreign currency exchange rates in effect in third quarter 2012 as compared to foreign currency exchange rates in effect in third quarter 2011. Excluding the
56
Table of Contents
impact of the changes in foreign currency exchange rates in the third quarter 2012 (constant currency) of $5.3 million, net sales in constant currency increased by $16.2 million, or 6.2%, to $279.3 million for the third quarter 2012 from $263.1 million for the third quarter 2011. The following table sets forth the mix of our net sales by business segment ($ in thousands):
| Third Quarter 2012 |
% of Net Sales |
Third Quarter 2011 |
% of Net Sales |
Increase (Decrease) |
% Increase (Decrease) |
|||||||||||||||||||
| Bracing and Vascular |
$ | 111,212 | 40.6 | % | $ | 101,452 | 38.6 | % | $ | 9,760 | 9.6 | % | ||||||||||||
| Recovery Sciences |
80,906 | 29.5 | 81,956 | 31.1 | (1,050 | ) | (1.3 | ) | ||||||||||||||||
| International |
64,671 | 23.6 | 64,475 | 24.5 | 196 | 0.3 | ||||||||||||||||||
| Surgical Implant |
17,197 | 6.3 | 15,235 | 5.8 | 1,962 | 12.9 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| $ | 273,986 | 100.0 | % | $ | 263,118 | 100.0 | % | 10,868 | 4.1 | % | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
Net sales in our Bracing and Vascular segment were $111.2 million for third quarter 2012, increasing 9.6% from net sales of $101.5 million for third quarter 2011. Growth in net sales in this segment is being driven by sales from new products and improving sales execution.
Net sales in our Recovery Sciences segment were $80.9 million for third quarter 2012, decreasing 1.3% from net sales of $82.0 million for third quarter 2011. The decrease was primarily driven by changes in reimbursement for certain products in our Empi business unit and by slow market conditions for capital equipment sold by our Chattanooga business unit.
Net sales in our International segment were $64.7 million for third quarter 2012, increasing 0.3% from net sales of $64.5 million for third quarter 2011. The increase was primarily driven by the impact of changes in foreign currency exchange rates in effect in during third quarter 2012 as compared to foreign currency exchange rates in effect during third quarter 2011. Excluding the impact of the changes in foreign currency exchange rates in the third quarter 2012 of $5.3 million, net sales in constant currency in this segment increased by $5.5 million, or 8.6%, to $70.0 million for the third quarter 2012 from $64.5 million for the third quarter 2011. Growth in net sales in this segment is being driven by sales from new products, improvements in sales execution and increases in sales penetration in certain geographies.
Net sales in our Surgical Implant segment were $17.2 million for third quarter 2012, increasing 12.9% from net sales of $15.2 million for third quarter 2011. The increase was driven primarily by strong sales of new products and improving sales execution.
Gross Profit. Consolidated gross profit as a percentage of net sales was 60.5% for third quarter 2012, compared to 59.2% for third quarter 2011.
Gross profit in our Bracing and Vascular segment as a percentage of net sales was 51.5% for third quarter 2012, compared to 52.2% for third quarter 2011. The decrease was primarily due to a lower margin mix of products sold.
Gross profit in our Recovery Sciences segment as a percentage of net sales increased to 76.2% for third quarter 2012, from 75.1% for third quarter 2011. The increase was primarily due to a higher margin mix of products sold.
Gross profit in our International segment as a percentage of net sales decreased to 53.8% for third quarter 2012, from 56.6% for third quarter 2011. The decrease was primarily driven by a lower margin mix of products sold.
Gross profit in our Surgical Implant segment as a percentage of net sales increased to 73.7% for third quarter 2012, compared to 70.2% for third quarter 2011. The increase was driven by a higher margin mix of products sold.
57
Table of Contents
Selling, General and Administrative (SG&A). SG&A decreased to $110.7 million for third quarter 2012, from $115.9 million for third quarter 2011. As a percentage of sales, SG&A expense decreased to 40.4% in third quarter 2012 from 44.0% in third quarter 2011.
Our SG&A expenses are impacted by significant non-recurring integration charges and other adjustments related to our ongoing restructuring activities and acquisitions. We incurred the following SG&A expenses in connection with such activities during the periods presented (in thousands):
| Third Quarter 2012 |
Third Quarter 2011 |
|||||||
| Integration charges: |
||||||||
| Employee severance and relocation |
$ | 84 | $ | 2,345 | ||||
| U.S commercial sales and marketing reorganization |
1,031 | 243 | ||||||
| Acquisition related expenses and integration |
193 | 642 | ||||||
| CEO transition |
— | 700 | ||||||
| Other non-recurring and integration charges |
2,190 | 1,133 | ||||||
| Litigation costs and settlements, net |
1,920 | 1,321 | ||||||
| ERP implementation and other automation projects |
723 | 5,217 | ||||||
|
|
|
|
|
|||||
| $ | 6,141 | $ | 11,601 | |||||
|
|
|
|
|
|||||
Research and Development (R&D). R&D expense was $7.9 million and $6.5 million for third quarter 2012 and third quarter 2011, respectively. As a percentage of sales, R&D expense increased to 2.9% in third quarter 2012 from 2.5% in third quarter 2011, reflecting an increase in our new product development activities.
Amortization of Intangible Assets. In both periods presented, amortization of intangible assets was $24.4 million.
Interest Expense. Interest expense was $46.4 million and $42.8 million for third quarter 2012 and third quarter 2011, respectively. The increase is due to an increase in the total amount of outstanding borrowings during third quarter 2012, as compared to third quarter 2011.
Other Income (Expense), Net. Other income (expense) was $1.9 million and $(6.0) million for third quarter 2012 and third quarter 2011, respectively. Results for both periods were primarily associated with net realized and unrealized foreign currency transaction gains (losses).
Income Tax (Provision) Benefit. For third quarter 2012, we recorded income tax expense of $0.6 million on pre-tax losses of $22.0 million, resulting in a negative effective tax rate of 2.6%. For third quarter 2011, we recorded an income tax benefit of $14.1 million, on pre-tax losses of $39.8 million, resulting in an effective tax rate of 35.4%. The change in our effective tax rates from 2011 to 2012 primarily relates to U.S federal and state valuation allowances provided against deferred tax assets beginning in the first quarter of 2012 and differences in our projected annualized effective tax rates for each year. Given the relationship between fixed dollar tax items and pre-tax financial results, the projected annual effective tax rate can change materially based on small variations of income.
Nine Months Ended September 29, 2012 (nine months 2012) compared to Nine Months Ended October 1, 2011 (nine months 2011)
Net Sales. Net sales for nine months 2012 were $838.9 million, increasing 6.1% from net sales of $790.6 million for nine months 2011. This increase was driven primarily by sales of our new products, improved sales execution and sales from our 2011 acquisitions of Dr. Comfort and Circle City, partially offset by unfavorable changes in foreign currency exchange rates in effect during nine months 2012 as compared to foreign currency exchange rates in effect during nine months 2011. Excluding the impact of the changes in
58
Table of Contents
foreign currency exchange rates in the nine months 2012 (constant currency) of $13.8 million, and including pre-acquisition sales in the nine months 2011 of $20.7 million, pro forma net sales in constant currency increased by $41.4 million, or 5.1%, to $852.7 million for the nine months 2012 from $811.3 million for the nine months 2011. The following table sets forth the mix of our net sales by business segment ($ in thousands):
| Nine Months 2012 |
% of Net Sales |
Nine Months 2011 |
% of Net Sales |
Increase (Decrease) |
% Increase (Decrease) |
|||||||||||||||||||
| Bracing and Vascular |
$ | 329,094 | 39.2 | % | $ | 281,687 | 35.7 | % | $ | 47,407 | 16.8 | % | ||||||||||||
| Recovery Sciences |
249,997 | 29.8 | 253,335 | 32.0 | (3,338 | ) | (1.3 | ) | ||||||||||||||||
| International |
206,649 | 24.6 | 207,423 | 26.2 | (774 | ) | (0.4 | ) | ||||||||||||||||
| Surgical Implant |
53,170 | 6.4 | 48,170 | 6.1 | 5,000 | 10.4 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| $ | 838,910 | 100.0 | % | $ | 790,615 | 100.0 | % | $ | 48,295 | 6.1 | % | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
Net sales in our Bracing and Vascular segment were $329.1 million for nine months 2012, increasing 16.8% from net sales of $281.7 million for nine months 2011. Our Bracing and Vascular segment benefited from sales from Dr. Comfort and Circle City, which we acquired on April 7, 2011 and March 4, 2011, respectively. Including the impact of pre-acquisition sales in the nine months 2011 of $19.9 million, pro forma net sales in this segment increased by $27.4 million, or 9.1%, from $301.7 million for the nine months 2011. Growth in net sales in this segment is being driven by sales from new products and improving sales execution.
Net sales in our Recovery Sciences segment were $250.0 million for nine months 2012, decreasing 1.3% from net sales of $253.3 million for nine months 2011. The decrease was primarily driven by changes in reimbursement levels for certain products in our Empi business unit and by slow market conditions for the capital equipment sold by our Chattanooga business unit.
Net sales in our International segment were $206.6 million for nine months 2012, decreasing 0.4% from net sales of $207.4 million for nine months 2011. The decrease was primarily driven by unfavorable changes in foreign currency exchange rates in effect during nine months 2012 as compared to foreign currency exchange rates in effect during nine months 2011. Excluding the impact of the changes in foreign currency exchange rates in the nine months 2012 (constant currency) of $13.8 million, and including pre-acquisition sales in the nine months 2011 of $0.7 million, pro forma net sales in constant currency in this segment increased by $12.3 million, or 5.9%, to $220.4 million for nine months 2012 from $208.1 million for the nine months 2011. Growth in net sales in this segment is being driven by sales from new products and increasing sales penetration in certain geographies.
Net sales in our Surgical Implant segment were $53.2 million for nine months 2012, increasing 10.4% from net sales of $48.2 million for nine months 2011. The increase was primarily driven by strong sales of new products and improving sales execution.
Gross Profit. Consolidated gross profit as a percentage of net sales was 60.9%, for nine months 2012, compared to 60.6% for nine months 2011.
Gross profit in our Bracing and Vascular segment as a percentage of net sales was 51.4% for nine months 2012, compared to 53.1% for nine months 2011. The decrease was primarily due to a lower margin mix of products sold, including sales from our recently acquired businesses. Also impacting gross profit in nine months 2011 were $10.0 million of purchase accounting adjustments related to the fair market value step-up of acquired inventory.
Gross profit in our Recovery Sciences segment as a percentage of net sales remained consistent at 75.7% for nine months 2012 and nine months 2011.
59
Table of Contents
Gross profit in our International segment as a percentage of net sales decreased to 55.5% for nine months 2012, from 57.1% for nine months 2011. The decrease was primarily driven by a lower margin mix of products sold, including sales from our recently acquired businesses.
Gross profit in our Surgical Implant segment as a percentage of net sales increased to 75.0% for nine months 2012, compared to 71.1% for nine months 2011. The increase was driven by a higher margin mix of products sold.
Selling, General and Administrative (SG&A). SG&A decreased to $342.6 million for nine months 2012, from $361.8 million for nine months 2011. As a percentage of sales, SG&A expense decreased to 40.8% for nine months 2012 from 45.8% for nine months 2011.
Our SG&A expenses were impacted by significant non-recurring integration charges and other adjustments related to our ongoing restructuring activities and acquisitions. We incurred the following SG&A expenses in connection with such activities during the periods presented (in thousands):
| Nine Months 2012 |
Nine Months 2011 |
|||||||
| Integration charges: |
||||||||
| Employee severance and relocation |
$ | 1,515 | $ | 5,374 | ||||
| U.S commercial sales and marketing reorganization |
1,126 | 1,446 | ||||||
| Chattanooga integration |
— | 119 | ||||||
| Acquisition related expenses and integration |
748 | 7,987 | ||||||
| CEO transition |
173 | 2,327 | ||||||
| Other non-recurring and integration charges |
5,100 | 3,336 | ||||||
| Litigation costs and settlements, net |
4,745 | 4,735 | ||||||
| Additional product liability insurance |
— | 3,107 | ||||||
| ERP implementation and other automation projects |
4,620 | 19,387 | ||||||
|
|
|
|
|
|||||
| $ | 18,027 | $ | 47,818 | |||||
|
|
|
|
|
|||||
Research and Development (R&D). R&D expense was $21.7 million, or 2.6% of net sales, and $19.7 million, or 2.5% of sales, for nine months 2012 and nine months 2011, respectively. R&D expense in nine months 2011 included $0.9 million related to the write-off of an abandoned product under development in our Surgical Implant segment.
Amortization of Intangible Assets. Amortization of intangible assets was $73.5 million and $69.4 million for nine months 2012 and nine months 2011, respectively. The increase is due to amortization of intangible assets acquired with our 2011 acquisitions of Dr. Comfort, Circle City and BetterBraces.com.
Interest Expense. Interest expense was $134.9 million for nine months 2012, and $126.3 million for nine months 2011. The increase is due to an increase in the total amount of outstanding borrowings during nine months 2012 as compared to nine months 2011.
Loss on Modification and Extinguishment of Debt. During nine months 2012 and nine months 2011, we recognized $9.4 million and $2.1 million respectively, of arrangement and lender consent fees related to amendments to our Senior Secured Credit Facility.
Other Income (Expense), Net. Other income (expense) was $2.9 million and $(1.6) million for nine months 2012 and nine months 2011, respectively. Results for both periods were primarily associated with net realized and unrealized foreign currency transaction gains (losses).
60
Table of Contents
Income Tax (Provision) Benefit. For nine months 2012, we recorded an income tax expense of $3.0 million on a pre-tax loss of $68.5 million, resulting in a negative effective tax rate of 4.4%. For nine months 2011, we recorded income tax benefit of $36.1 million on a pre-tax loss of $101.6 million, resulting in an effective tax rate of 35.5%. The change in our effective tax rates from 2011 to 2012 primarily relates to U.S federal and state valuation allowances provided against deferred tax assets beginning in the first quarter of 2012 and differences in our projected annualized effective tax rates for each year. Given the relationship between fixed dollar tax items and pre-tax financial results, the projected annual effective tax rate can change materially based on small variations of income.
Results of Operations
The following table sets forth our statements of operations as a percentage of net sales ($ in thousands):
| Year Ended December 31, | ||||||||||||||||||||||||
| 2011 | 2010 | 2009 | ||||||||||||||||||||||
| Net sales |
$ | 1,074,770 | 100.0 | % | $ | 965,973 | 100.0 | % | $ | 946,126 | 100.0 | % | ||||||||||||
| Cost of sales (exclusive of amortization of intangible assets (1)) |
418,138 | 38.9 | 345,270 | 35.7 | 338,719 | 35.8 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Gross profit |
656,632 | 61.1 | 620,703 | 64.3 | 607,407 | 64.2 | ||||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Selling, general and administrative |
487,084 | 45.3 | 433,408 | 44.9 | 420,758 | 44.5 | ||||||||||||||||||
| Research and development |
26,850 | 2.5 | 21,892 | 2.3 | 23,540 | 2.5 | ||||||||||||||||||
| Amortization of intangible assets |
93,957 | 8.7 | 77,523 | 8.0 | 77,254 | 8.2 | ||||||||||||||||||
| Impairment of goodwill and intangible assets |
141,006 | 13.1 | — | 0.0 | 6,998 | 0.7 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| 748,897 | 69.7 | 532,823 | 55.2 | 528,550 | 55.9 | |||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Operating (loss) income |
(92,265 | ) | (8.6 | ) | 87,880 | 9.1 | 78,857 | 8.3 | ||||||||||||||||
| Other income (expense): |
||||||||||||||||||||||||
| Interest expense |
(169,332 | ) | (15.8 | ) | (155,181 | ) | (16.1 | ) | (157,032 | ) | (16.6 | ) | ||||||||||||
| Interest income |
345 | 0.0 | 310 | 0.0 | 1,033 | 0.1 | ||||||||||||||||||
| Loss on modification and extinguishment of debt |
(2,065 | ) | (0.2 | ) | (19,798 | ) | (2.0 | ) | — | 0.0 | ||||||||||||||
| Other income (expense), net |
(2,814 | ) | (0.3 | ) | 859 | 0.1 | 6,073 | 0.6 | ||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| (173,866 | ) | (16.2 | ) | (173,810 | ) | (18.0 | ) | (149,926 | ) | (15.9 | ) | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Loss from continuing operations before income taxes |
(266,131 | ) | (24.8 | ) | (85,930 | ) | (8.9 | ) | (71,069 | ) | (7.5 | ) | ||||||||||||
| Income tax benefit |
52,544 | 4.9 | 34,255 | 3.5 | 21,678 | 2.3 | ||||||||||||||||||
| Loss from discontinued operations, net |
— | 0.0 | — | 0.0 | (319 | ) | 0.0 | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Net loss |
(213,587 | ) | (19.9 | ) | (51,675 | ) | (5.3 | ) | (49,710 | ) | (5.2 | ) | ||||||||||||
| Net income attributable to noncontrolling interests |
(882 | ) | (0.1 | ) | (857 | ) | (0.1 | ) | (723 | ) | (0.1 | ) | ||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Net loss attributable to DJOFL |
$ | (214,469 | ) | (20.0 | )% | $ | (52,532 | ) | (5.4 | )% | $ | (50,433 | ) | (5.3 | )% | |||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| (1) | Cost of sales is exclusive of amortization of intangible assets of $38,668, $36,343 and $37,884 for the years ended December 31, 2011, 2010 and 2009, respectively. |
Year Ended December 31, 2011 (2011) Compared to Year Ended December 31, 2010 (2010)
Net Sales. Our net sales for 2011 were $1,074.8 million, compared to net sales of $966.0 million for 2010, representing an 11.3% increase year over year. This increase was driven primarily by sales from our 2011 acquisitions of Dr. Comfort, ETI and Circle City and favorable changes in foreign currency exchange rates.
61
Table of Contents
The following table sets forth the mix of our net sales by business segment ($ in thousands):
| 2011 | % of Net Sales |
2010 | % of Net Sales |
Increase (Decrease) |
% Increase (Decrease) |
|||||||||||||||||||
| Bracing and Vascular |
$ | 387,928 | 36.1 | % | $ | 311,620 | 32.3 | % | $ | 76,308 | 24.5 | % | ||||||||||||
| Recovery Sciences |
342,599 | 31.9 | 347,139 | 35.9 | (4,540 | ) | (1.3 | ) | ||||||||||||||||
| International |
279,299 | 26.0 | 244,493 | 25.3 | 34,806 | 14.2 | ||||||||||||||||||
| Surgical Implant |
64,944 | 6.0 | 62,721 | 6.5 | 2,223 | 3.5 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| $ | 1,074,770 | 100.0 | % | $ | 965,973 | 100.0 | % | $ | 108,797 | 11.3 | % | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
Net sales in our Bracing and Vascular segment were $387.9 million for 2011 and $311.6 million for 2010. The increase was primarily due to $75.6 million of net sales attributable to our newly acquired Dr. Comfort, ETI and Circle City businesses. In addition; our Bracing and Vascular segment continued to benefit from sales of our VenaFlow Elite dynamic compression therapy pump used to combat Deep Vein Thrombosis, as well as sales of our newer bracing and supports products for knee and upper extremity.
Net sales in our Recovery Sciences segment were $342.6 million for 2011 and $347.1 million for 2010. The decrease was primarily attributable to decreased sales at our Empi business unit due primarily to certain reimbursement price changes.
Net sales in our International segment were $279.3 million for 2011 and $244.5 million for 2010. The increase was driven primarily by $10.5 million of net sales from our newly acquired Dr. Comfort and ETI businesses, sales of new products, and the favorable impact of foreign exchange rates in effect during 2011 as compared to 2010, which increased net sales by $11.8 million.
Net sales in our Surgical Implant segment were $64.9 million for 2011 and $62.7 million for 2010. The increase was driven by strong sales of our Reverse Shoulder products as well as our newly launched Turon shoulder product, offset by decreases in sales of hip and knee products.
Gross Profit. Consolidated gross profit as a percentage of net sales was 61.1% for 2011 and 64.3% for 2010. The decrease was driven by several factors including the impact of sales from our acquired businesses which have lower margins, reduced average selling prices of certain of our products and purchase accounting adjustments related to the fair market value step-up of acquired inventory. These decreases were partially offset by the favorable impact of an adjustment made by the Company to reduce deferred gross profit from intercompany sales of inventory.
Gross profit in our Bracing and Vascular segment as a percentage of net sales was 52.4% for 2011 and 54.8% for 2010. The decrease was primarily due to a lower margin mix of products sold, including sales from our recently acquired businesses. In addition, gross profit for 2011 was impacted by $12.3 million of purchase accounting adjustments related to the fair market value step-up of acquired inventory.
Gross profit in our Recovery Sciences segment as a percentage of net sales was 75.6% for 2011 and 76.4% for 2010. The decrease was primarily due to reduced average selling prices of certain products, primarily in our Empi business unit.
Gross profit in our International segment as a percentage of net sales was 57.7% for 2011 and 58.7% for 2010. The decrease was primarily driven by a lower margin mix of products sold, including sales from our recently acquired Dr. Comfort and ETI businesses.
Gross profit in our Surgical Implant segment as a percentage of net sales was 72.2% for 2011 and 73.4% for 2010.
62
Table of Contents
Selling, General and Administrative (SG&A). SG&A expenses were $487.1 million for 2011 and $433.4 million in 2010. As a percentage of net sales, SG&A expenses increased slightly to 45.3% in 2011 from 44.9% in 2010. Our SG&A expenses for both years were impacted by non-recurring charges, including significant amounts related to our global ERP implementation and other adjustments related to ongoing restructuring activities and acquisitions. We incurred the following SG&A expenses in connection with such activities during the periods presented:
| Year Ended December 31, | ||||||||
| (in thousands) |
2011 | 2010 | ||||||
| Integration charges: |
||||||||
| Employee severance and relocation |
$ | 5,452 | $ | 2,781 | ||||
| U.S. commercial sales and marketing reorganization |
1,568 | 8,195 | ||||||
| Chattanooga integration |
175 | 4,106 | ||||||
| Acquisition related expenses and integration |
8,487 | — | ||||||
| DJO Merger and other integration |
2,283 | 3,564 | ||||||
| CEO transition |
2,544 | — | ||||||
| International integration |
3,506 | 191 | ||||||
| Litigation costs and settlements, net |
6,971 | 7,561 | ||||||
| Additional product liability insurance premiums |
3,342 | 11,138 | ||||||
| ERP implementation |
24,083 | 16,916 | ||||||
| Impairment of fixed assets |
7,116 | — | ||||||
|
|
|
|
|
|||||
| $ | 65,527 | $ | 54,452 | |||||
|
|
|
|
|
|||||
In the fourth quarter of 2011, we determined that certain capitalized ERP assets would not be used and we recorded an impairment of $7.1 million in our consolidated statement of operations.
Research and Development (R&D). R&D expenses were $26.9 million for 2011 and $21.9 million for 2010, increasing slightly to 2.5% of net sales in 2011 from 2.3% of net sales in 2010. R&D expense for the year ended December 31, 2011 included $0.9 million related to the write off of an abandoned product under development in our Surgical Implant segment.
Amortization of Intangible Assets. Amortization of intangible assets was $94.0 million in 2011 and $77.5 million for 2010. The increase is attributable to intangible assets acquired through our 2011 acquisitions of Dr. Comfort, ETI, Circle City and BetterBraces.com.
Impairment of Goodwill and Intangible Assets. During the year ended December 31, 2011 we determined that the carrying value of our Empi and Surgical Implant reporting units was in excess of its estimated fair value. As a result, we recorded aggregate goodwill impairment charges of $124.1 million consisting of $76.7 million for the Empi reporting unit and $47.4 million for the Surgical Implant reporting unit. In addition, during the year ended December 31, 2011 we recorded intangible asset impairment charges of $16.9 million, related to our Empi trade name. There were no goodwill or intangible asset impairment charges recognized during 2010.
Interest Expense. Our interest expense was $169.3 million for 2011 and $155.2 million for 2010. An increase in the total amount of outstanding borrowings was partially offset by lower weighted average interest rates on outstanding borrowings. For 2010, interest expense included $4.5 million of accelerated amortization of debt discount and issuance costs related to $182.5 million of early prepayments of our term loans in conjunction with certain debt modification and extinguishment activities.
Loss on Modification and Extinguishment of Debt. In 2011, we recognized $2.1 million of arrangement and lender consent fees related to amendments to our Senior Secured Credit Facility. In 2010, we recognized a loss on extinguishment of debt of $19.8 million, including $13.0 million of premiums, $4.3 million for a non-cash
63
Table of Contents
write-off of unamortized debt issuance costs, $1.4 million of fees and expenses associated with the redemption of our $200 million of 11.75% senior subordinated notes in October 2010, and $1.1 million of fees and expenses related to the prepayment of $101.5 million of our term loan in January 2010.
Other Income (Expense), Net. Other income (expense), net was $(2.8) million for 2011 and $0.9 million for 2010. Results for both periods presented were primarily attributable to net realized and unrealized foreign currency translation gains and losses.
Income Tax Benefit. We recorded an income tax benefit of $52.5 million on a pre-tax loss of $266.1 million, resulting in an effective tax rate of 19.7% in 2011. In 2010 we recorded a tax benefit of $34.3 million on a pre-tax loss of $85.9 million, resulting in an effective tax rate of 39.9%. Income tax benefit for both years is net of tax expense related to foreign operations, deferred taxes on the assumed repatriation of foreign earnings, and other non-deductible items.
Year Ended December 31, 2010 (2010) Compared to Year Ended December 31, 2009 (2009)
Net Sales. Our net sales for 2010 were $966.0 million, compared to net sales of $946.1 million for 2009, representing a 2.1% increase year over year. Sales growth for 2010 was negatively impacted by $4.1 million of unfavorable changes in foreign exchange rates compared to the rates in effect for 2009. On the basis of constant currency rates, net sales increased 2.5% for 2010 compared to 2009. Product lines sold or discontinued in 2009 generated revenue of $9.5 million in 2009. Excluding 2009 revenue from these product lines, net sales increased 3.1% for 2010 compared to 2009.
The following table sets forth the mix of our net sales ($ in thousands):
| 2010 | % of Net Sales |
2009 | % of Net Sales |
Increase (Decrease) |
% Increase (Decrease) |
|||||||||||||||||||
| Bracing and Vascular |
$ | 311,620 | 32.3 | % | $ | 298,759 | 31.6 | % | $ | 12,861 | 4.3 | % | ||||||||||||
| Recovery Sciences |
347,139 | 35.9 | 342,026 | 36.1 | 5,113 | 1.5 | ||||||||||||||||||
| International |
244,493 | 25.3 | 241,464 | 25.5 | 3,029 | 1.3 | ||||||||||||||||||
| Surgical Implant |
62,721 | 6.5 | 63,877 | 6.8 | (1,156 | ) | (1.8 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| $ | 965,973 | 100.0 | % | $ | 946,126 | 100.0 | % | $ | 19,847 | 2.1 | % | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
Net sales in our Bracing and Vascular segment were $311.6 million for 2010, reflecting an increase of 4.3% over net sales of $298.8 million for 2009. The increase was driven primarily by increased unit sales across most product lines, and increased revenue under our new soft goods contract with the Novation group purchasing organization. Growth in this segment was negatively impacted due to conversions wherein a greater percentage of clinics handled their own insurance reimbursement billing as opposed to billing for the reimbursement through our OfficeCare program. While we generally retain the unit sales in these conversions, the lower average selling price per unit negatively impacts sales for 2010 as compared to 2009. In addition, the pain pump product line contributed revenue of $0.3 million in 2009 before sales of this product were discontinued.
Net sales in our Recovery Sciences segment were $347.1 million for 2010, reflecting an increase of 1.5% over net sales of $342.0 million for 2009. Increases in sales of new products in our Empi business unit, and improved sales of the products in our Chattanooga business unit, were partially offset by the impact of discontinuing certain Chattanooga products which contributed revenue of $3.8 million in 2009.
Net sales in our International segment were $244.5 million for 2010, reflecting an increase of $3.0 million, or 1.3% over net sales of $241.5 million for 2009. Strong sales of our bracing and supports products across all major international markets, and continued improvement in sales of our Chattanooga products were partially offset by the impact of the discontinuation of certain Chattanooga products sold in international markets, which contributed revenue of $3.5 million in 2009. On the basis of constant currency rates, net sales in our International segment increased 3.0% for 2010 compared to 2009.
64
Table of Contents
Net sales in our Surgical Implant segment were $62.7 million for 2010, as compared to $63.9 million for 2009, representing a decrease of 1.8%. The decrease was primarily attributable to the loss of a few key customers of our hip and knee products, and the impact of the 2009 sale of a non-core spine product line which contributed revenue of $1.9 million in 2009. These decreases were partially offset by increased sales of our shoulder products, and strong sales of our hip revision system, a new product offered under our partnership with Lima corporation.
Gross Profit. Consolidated gross profit was 64.3% of net sales for 2010, a slight increase compared to gross profit of 64.2% of net sales for 2009. Gross profit margin for 2010 was favorably impacted by cost savings achieved in connection with the Chattanooga integration and various other integration activities, and unfavorably impacted by a lower margin mix of products sold, and unfavorable changes in foreign currency exchange rates compared to the rates in effect in 2009.
Gross profit in our Bracing and Vascular segment was 54.8% of net sales for 2010 compared to 56.2% for 2009. The decrease in our gross profit as a percentage of net sales was primarily attributable to a lower margin mix of products sold, including the impact attributable to clinics choosing to do their own insurance reimbursement billing, as opposed to billing for the reimbursement through our OfficeCare program. While we generally retain the unit sales in these conversions, lower average selling price per unit negatively impacts gross margin.
Gross profit in our Recovery Sciences segment was 76.4% of net sales for 2010 compared to 75.3% for 2009. The increase as a percentage of net sales was primarily driven by cost savings resulting from the integration of our Chattanooga business operations.
Gross profit in our International segment was 58.7% of net sales for 2010 compared to 56.8% for 2009. The increase as a percentage of net sales was primarily driven by the impact of a higher margin mix of products sold, and cost savings associated with the integration of our Chattanooga business operations, partially offset by unfavorable changes in foreign exchange rates compared to the rates in effect for 2009.
Gross profit in our Surgical Implant segment was 73.4% for 2010 compared to 78.0% for 2009. The decrease was primarily driven by lower sales volume and the unfavorable impact of certain non-recurring inventory adjustments in 2010.
Selling, General and Administrative (SG&A). Our SG&A expenses were $432.3 in 2010, compared to $420.8 million in 2009. SG&A expenses for both years were impacted by non-recurring charges, including significant amounts related to our global ERP implementation, and other adjustments related to ongoing restructuring activities and acquisitions. We incurred the following SG&A expenses in connection with such activities during the periods presented:
| Year Ended December 31, | ||||||||
| (in thousands) |
2010 | 2009 | ||||||
| Integration charges: |
||||||||
| Employee severance and relocation |
$ | 2,781 | $ | 7,938 | ||||
| U.S commercial sales and marketing reorganization |
8,195 | — | ||||||
| Chattanooga integration |
4,106 | 620 | ||||||
| DJO Merger and other integration |
3,564 | 13,386 | ||||||
| International integration |
191 | 5,142 | ||||||
| Litigation costs and settlements, net |
7,561 | 2,845 | ||||||
| Additional product liability insurance premiums |
11,138 | — | ||||||
| Reversal of reimbursement claims |
— | (6,000 | ) | |||||
| ERP implementation |
16,916 | 18,163 | ||||||
|
|
|
|
|
|||||
| $ | 54,452 | $ | 42,094 | |||||
|
|
|
|
|
|||||
65
Table of Contents
During 2010, we commenced a U.S. commercial sales and marketing reorganization in which we integrated the U.S. marketing and sales operations under new leadership. In connection with this reorganization, we incurred $8.2 million of expenses in 2010. In addition during 2010, we paid insurance premiums of $11.1 million related to a supplemental five-year extended reporting period for product liability claims related to our discontinued pain pump products, for which annual insurance coverage was not renewed.
Research and Development (R&D). Our R&D expense decreased to $21.9 million for 2010 from $23.5 million for 2009, primarily reflecting cost savings initiatives from integration related activities. As a percentage of net sales, R&D expense for 2010 decreased to 2.3% compared to 2.5% for 2009.
Amortization of Intangible Assets. Amortization of intangible assets was $77.5 million for 2010 and $77.3 million for 2009.
Impairment of Intangible Assets. During the year ended December 31, 2009, we recorded aggregate intangible asset impairment charges of $7.0 million related to two indefinite lived intangible assets of which $3.9 million was related to our Bracing and Vascular segment, and $3.1 million was related to our Recovery Sciences segment. There were no intangible asset impairment charges recognized during 2010.
Interest Expense. Our interest expense was $155.2 million for 2010 compared to $157.0 million for 2009. Overall, we benefited from lower weighted average interest rates on outstanding borrowings during 2010, as compared to 2009. This benefit was partially offset by $4.5 million of accelerated amortization of debt discount and issuance costs related to $182.5 million of early prepayments of our term loans in conjunction with certain debt modification and extinguishment activities during 2010.
Loss on Modification and Extinguishment of Debt. In 2010, we recognized a loss on extinguishment of debt of $19.8 million, including $13.0 million of premiums, $4.3 million for a non-cash write-off of unamortized debt issuance costs, $1.4 million of fees and expenses associated with the redemption of our $200 million of 11.75% senior subordinated notes in October 2010, and $1.1 million of fees and expenses related to the prepayment of $101.5 million of our term loan in January 2010.
Other Income (Expense), Net. Other income, net totaled $0.9 million for 2010 as compared to $6.1 million for 2009. Results for 2009 included a $3.1 million gain related to the sales of certain non-core product lines. The remaining activity for 2009 was primarily attributable to net realized and unrealized foreign currency translation gains. Results for 2010 were primarily attributable to net realized and unrealized foreign currency translation gains.
Income Tax Benefit. We recorded an income tax benefit of $34.3 million for 2010 compared to $21.7 million for 2009. Our effective tax rate for 2010 was 39.9% as compared to 30.5% for 2009. Income tax benefit for both years is net of tax expense related to foreign operations, deferred taxes on the assumed repatriation of foreign earnings, and other non-deductible items.
Recent Accounting Pronouncements
During the year ended December 31, 2011, there we no accounting pronouncements adopted which had a material impact on our financial position, results of operations, or cash flows.
In July 2012, the FASB issued an accounting standard update regarding testing of intangible assets for impairment. This standard update allows companies the option to perform a qualitative assessment to determine whether it is more likely than not that an indefinite-lived intangible asset is impaired. An entity is not required to calculate the fair value of an indefinite-lived intangible asset and perform the quantitative impairment test unless the entity determines that it is more likely than not the asset is impaired. We will adopt this standard update during the first quarter of 2013. The adoption of this standard is not expected to have a significant impact on the Company’s consolidated financial statements.
66
Table of Contents
Liquidity and Capital Resources
Three Months Ended September 29, 2012 (third quarter 2012) compared to Three Months Ended October 1, 2011 (third quarter 2011)
As of September 29, 2012, our primary sources of liquidity consisted of cash and cash equivalents totaling $38.2 million and our $100.0 million revolving credit facility, of which $62.0 million was available. Working capital at September 29, 2012 was $211.5 million. We believe that our existing cash, plus the amounts we expect to generate from operations and amounts available through our revolving credit facility, will be sufficient to meet our operating needs for the next twelve months, including working capital requirements, capital expenditures and debt repayment and interest obligations. While we currently believe that we will be able to meet all of our financial covenants imposed by our Senior Secured Credit Facility, we may not in fact be able to do so or be able to obtain waivers of default or amendments to the Senior Secured Credit Facility in the future. We and our subsidiaries, affiliates or significant shareholders (including Blackstone and its affiliates) may from time to time, in our or their sole discretion, purchase, repay, redeem or retire any of our outstanding debt or equity securities (including any publicly issued debt securities), in privately negotiated or open market transactions, by tender offer or otherwise.
A summary of our cash flow activity is presented below (in thousands):
| Nine Months 2012 |
Nine Months 2011 |
|||||||
| Cash provided by operating activities |
$ | 51,303 | $ | 40,118 | ||||
| Cash used in investing activities |
(24,104 | ) | (348,365 | ) | ||||
| Cash (used in) provided by financing activities |
(27,358 | ) | 309,262 | |||||
| Effect of exchange rate changes on cash and cash equivalents |
215 | 176 | ||||||
|
|
|
|
|
|||||
| Net increase in cash and cash equivalents |
$ | 56 | $ | 1,191 | ||||
|
|
|
|
|
|||||
Cash Flows
Operating activities provided cash of $51.3 million and $40.1 million in nine months 2012 and nine months 2011, respectively, reflecting our net loss adjusted for non-cash expenses. Cash paid for interest was $103.4 million and $78.5 million in nine months 2012 and nine months 2011, respectively.
Investing activities used $24.1 million and $348.4 million of cash in nine months 2012 and nine months 2011, respectively. Cash used in investing activities for nine months 2012 primarily consisted of purchases of property and equipment. Cash used in investing activities for nine months 2011 included $317.7 million of net cash paid for the acquisitions of Dr. Comfort, Elastic Therapy, Inc. (ETI) and Circle City and $29.2 million for purchases of property and equipment.
Financing activities used $27.4 million of cash for nine months 2012 and provided $309.3 million of cash for nine months 2011. Cash used in financing activities in nine months 2012 consisted of proceeds from the borrowings under our Amended Senior Secured Credit Facility and our new 8.75% Notes, offset by repayments of the Original Senior Secured Credit Facility and a portion of our 10.875% Notes and the payment of $25.2 million of debt issuance costs. Cash provided by financing activities in nine months 2011 was primarily related to net proceeds from our issuance of $300.0 million aggregate principal of 7.75% Notes and net borrowings of $23.0 million from our revolving credit facility which, together with cash on hand, were primarily used to fund the acquisitions of Dr. Comfort, ETI and Circle City.
For the remainder of 2012, we expect to spend cash of approximately $604.6 million for the following:
| • | $15.1 million for scheduled principal and estimated interest payments on our Amended Senior Secured Credit Facility; |
67
Table of Contents
| • | $27.6 million for scheduled interest payments on our 8.75% Notes, 10.875% Notes, 7.75% Notes and 9.75% Notes; and |
| • | $9.4 million for capital expenditures, |
| • | $392.2 million for the early tender and redemption of $363.2 million principal of our 10.875% Notes, including aggregate tender premium costs of $14.1 million and accrued interest of $14.9 million, |
| • | $110.1 million for the remaining tender and redemption of $101.8 million principal of our 10.875% Notes, including aggregate tender premium costs of $2.8 million and accrued interest of $5.5 million, |
| • | $38.0 million for the payment of the balance outstanding on the Revolving Credit Facility as of October 1, 2012, |
| • | $12.2 million for the payment of debt issuance costs related to the issuance of 9.875% Senior Notes and the Additional 8.75% Notes. |
Years Ended December 31, 2011, 2010 and 2009
As of December 31, 2011, our primary source of liquidity consisted of cash and cash equivalents totaling $38.2 million and $49.0 million of available borrowings under our revolving credit facility, as described below. Working capital at December 31, 2011 was $218.7 million. We believe that our existing cash, plus the amounts we expect to generate from operations and amounts available through our revolving credit facility, will be sufficient to meet our operating needs for the next twelve months, including working capital requirements, capital expenditures, and debt and interest repayment obligations. While we currently believe that we will be able to meet all of the financial covenants imposed by our Senior Secured Credit Facility, there is no assurance that we will in fact be able to do so or that, if we do not, we will be able to obtain from our lenders waivers of default or amendments to the Senior Secured Credit Facility in the future. We and our subsidiaries, affiliates, or significant shareholders (including Blackstone and its affiliates) may from time to time, in our or their sole discretion, purchase, repay, redeem or retire any of our outstanding debt or equity securities (including any publicly issued debt securities), in privately negotiated or open market transactions, by tender offer or otherwise.
A summary of our cash flow activity is presented below (in thousands):
| 2011 | 2010 | 2009 | ||||||||||
| Cash provided by operating activities |
$ | 23,605 | $ | 25,594 | $ | 67,794 | ||||||
| Cash used in investing activities |
(358,662 | ) | (30,195 | ) | (16,000 | ) | ||||||
| Cash provided by (used in) financing activities |
334,290 | 413 | (35,261 | ) | ||||||||
| Effect of exchange rate changes on cash and cash equivalents |
804 | (2,291 | ) | (2,405 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
$ | 37 | $ | (6,479 | ) | $ | 14,128 | |||||
|
|
|
|
|
|
|
|||||||
Cash Flows
Operating activities provided $23.6 million, $25.6 million and $67.8 million of cash for 2011, 2010 and 2009, respectively. Cash provided by operating activities for all years presented primarily represented our net loss, adjusted for non-cash expenses. For 2011, 2010 and 2009, cash paid for interest was $151.2 million, $139.1 million, and $144.2 million, respectively.
Investing activities used $358.7 million, $30.2 million and $16.0 million of cash for 2011, 2010, and 2009 respectively. Cash used in investing activities for 2011 primarily consisted of $317.7 million of net cash paid for acquisitions and $39.4 million of cash paid for purchases of property and equipment, including $3.8 million paid for our ERP system. Cash used in investing activities for 2010 primarily consisted of $27.2 million of purchases
68
Table of Contents
of property and equipment, including $13.8 million for our new ERP system, $1.2 million related to the acquisition of assets from an independent South African distributor, and the payment of $0.8 million related to an earn-out provision associated with the 2009 acquisition of an independent Australian distributor. Cash used in investing activities for 2009 primarily consisted of $28.9 million of purchases of property and equipment, including $7.8 million for our new ERP system, and the acquisition of businesses for a total of $13.1 million, partially offset by $25.7 million of proceeds from sales of assets, including $21.8 million attributable to our sale of ETS.
Financing activities provided $334.3 million and $0.4 million of cash for 2011 and 2010, respectively, and used $35.3 million of cash for 2009. Cash provided by financing activities in 2011 was primarily related to net proceeds from our issuance of $300.0 million aggregate principal of 7.75% Senior Notes and net borrowings from our revolving credit facility, which together with cash on hand were used to fund acquisitions. In connection with the issuance of our $300.0 million aggregate principal of 7.75% Senior Notes, we paid $7.7 million in debt issuance costs. In addition, during 2011, our indirect parent, DJO, sold shares of its common stock, and contributed net proceeds of $3.2 million to us. During 2010, cash provided by financing activities primarily consisted of cash received from issuances of $100.0 million aggregate principal of 10.875% Notes, and $300.0 million aggregate principal of 9.75% Notes, offset by cash paid for the redemption of our $200.0 million aggregate principal of 11.75% Notes, prepayments of $182.5 million of term loans under the Senior Secured Credit Facility, and payment of $10.3 million of capitalized debt issuance costs in connection with the issuance and registered exchange offer of our $100.0 million 10.875% Notes and the issuance of our $300.0 million of 9.75% Notes. In addition, during 2010 we received an investment of $1.5 million from DJO, our indirect parent, related to proceeds from the issuance of DJO common stock to certain accredited investors. Cash used in financing activities for 2009 primarily represented net payments on long-term debt and revolving lines of credit.
Indebtedness
As of September 29, 2012, our total indebtedness was $2,165.5 million, net of unamortized original issue discounts and premiums of $6.8 million, net.
Senior Secured Credit Facility
Overview. On November 20, 2007, we entered into a Senior Secured Credit Facility (Original Senior Secured Credit Facility) consisting of a $1,065.0 million term loan facility maturing in May 2014 (Original Term Loans) and a $100.0 million revolving credit facility maturing in November 2013 (Original Revolving Credit Facility). We issued the Original Term Loans at a 1.2% discount.
On March 20, 2012, we entered into an Amendment and Restatement Agreement to amend and restate our Original Senior Secured Credit Facility (Amended Senior Secured Credit Facility), which (1) permitted the issuance of $230.0 million aggregate principal of 8.75% Notes; (2) extended the maturity of $564.7 million of term loans outstanding under the Original Senior Secured Credit Facility to the earlier of November 1, 2016 or August 15, 2014, if on such date the aggregate principal amount of our 10.875% Notes is not less than $150.0 million (Extended Term Loans); (3) provided for the issuance of a new tranche of $350.0 million of term loans that will mature on the earlier of September 15, 2017 or August 15, 2014 if on such date the aggregate outstanding principal amount of our 10.875% Notes is not less than $150.0 million; (4) deemed certain previous acquisitions and investments to be permitted under the terms of the Amended Senior Secured Credit Facility; (5) increased the total net leverage ratio limitation in the permitted acquisitions covenant from 7.0x to 7.5x; (6) changed the financial maintenance covenant from a senior secured leverage ratio covenant to a new Senior Secured first lien leverage ratio covenant; and (7) replaced our Original Revolving Credit Facility with a new $100.0 million revolving credit facility (Revolving Credit Facility) which matures on the earlier of March 14, 2017 or August 15, 2014, if on such date the aggregate principal amount of 10.875% Notes is not less than $150.0 million.
69
Table of Contents
On March 30, 2012, we entered into Amendment No. 1 to our Amended Senior Secured Credit Facility which, among other things, provided for the issuance of an additional $105.0 million of new term loans that will mature on the earlier of September 15, 2017 or August 15, 2014, if on such date the outstanding aggregate principal amount of our 10.875% Notes is not less than $150.0 million. The net proceeds from the issuance were used to repay $103.5 million aggregate principal of existing term loans under the Original Senior Secured Credit Facility and to pay related fees, premiums and expenses.
Collectively, the $350.0 million of new term loans issued on March 20, 2012 and the $105.0 million of new term loans issued on March 30, 2012 are referred to as the New Term Loans.
The New Term Loans were issued at a discount as follows: $350.0 million of the New Term Loans were issued at a 1.5% discount and $105.0 million of the New Term Loans were issued at a 1.0% discount. We are amortizing the $6.3 million aggregate original issue discount using the effective interest method.
The net proceeds from the New Term Loans were used to repay the remaining portion of the Original Term Loans which would have matured in May 2014, and to repay a portion of the Extended Term Loans.
As of September 29, 2012, the balance of term loans outstanding under the Amended Senior Secured Credit Facility was $839.2 million (exclusive of $9.3 million of unamortized original issue discount) and there were $38.0 million of borrowings outstanding under the Revolving Credit Facility.
Interest Rates. The interest rate margins applicable to borrowings under the Revolving Credit Facility are, at our option, either (a) the Eurodollar rate plus 475 basis points or (b) a base rate plus 375 basis points. The interest rate margins applicable to the Extended Term Loans and the New Term Loans are, at our option, either (a) the Eurodollar rate plus 500 basis points or (b) a base rate plus 400 basis points. There is a minimum LIBOR rate applicable with respect to the Eurodollar component of interest rates on New Term Loan borrowings of 1.25%. The applicable margin for borrowings under the Revolving Credit Facility, the Extended Term Loans and the New Term Loans may be reduced, subject to our attaining certain leverage ratios. As of September 29, 2012, our weighted average interest rate for all borrowings under the Amended Senior Secured Credit Facility was 5.74%.
Fees. In addition to paying interest on outstanding principal under the Amended Senior Secured Credit Facility, we are required to pay a commitment fee to the lenders under the Revolving Credit Facility with respect to the unutilized commitments thereunder. The current commitment fee rate is 0.50% per annum, subject to step-downs based upon the achievement of certain leverage ratios. We must also pay customary letter of credit fees.
Principal Payments. We are required to pay annual payments in equal quarterly installments on the New Term Loans in an amount equal to 1.00% of the funded total principal amount through June 2017, with the remaining amount payable in full at maturity in September 2017. We are required to pay annual payments in equal quarterly installments on the Extended Term Loans in an amount equal to 1.00% of the funded total principal amount through September 2016, with any remaining amount payable in full at maturity in November 2016.
Certain Covenants and Events of Default. The Amended Senior Secured Credit Facility contains a number of covenants that, among other things, restrict, subject to certain exceptions, our and our subsidiaries’ ability to:
| • | incur additional indebtedness; |
| • | create liens on assets; |
| • | change fiscal years; |
| • | enter into sale and leaseback transactions; |
| • | engage in mergers or consolidations; |
| • | sell assets; |
70
Table of Contents
| • | pay dividends and make other restricted payments; |
| • | make investments, loans or advances; |
| • | repay subordinated indebtedness; |
| • | make certain acquisitions; |
| • | engage in certain transactions with affiliates; |
| • | restrict the ability of restricted subsidiaries that are not Guarantors to pay dividends or make distributions; |
| • | amend material agreements governing our subordinated indebtedness; and |
| • | change our lines of business. |
Pursuant to the terms of the credit agreement relating to the Amended Senior Secured Credit Facility, we are required to maintain a maximum Senior Secured first lien leverage ratio of consolidated first lien net debt to Adjusted EBITDA of 4.25:1 for the trailing twelve months ended September 29, 2012. Adjusted EBITDA is defined as net income (loss) attributable to DJOFL, plus interest expense, net, income tax (provision) benefit and depreciation and amortization, further adjusted for certain non-cash items, non-recurring items and other adjustment items, as permitted in calculating covenant compliance under our Amended Senior Secured Credit Facility and the Indentures governing our 8.75% Notes, 10.875% Notes, 7.75% Notes, 9.75% Notes and 9.875% Notes (collectively, the notes). Adjusted EBITDA is a material component of these covenants. As of September 29, 2012, our actual Senior Secured first lien leverage ratio was within the required ratio at 3.00:1.
Adjusted EBITDA should not be considered as an alternative to net (loss) income or other performance measures presented in accordance with GAAP, or as an alternative to cash flow from operations as a measure of our liquidity. Adjusted EBITDA does not represent net (loss) income or cash flow from operations as those terms are defined by GAAP and does not necessarily indicate whether cash flows will be sufficient to fund cash needs. In particular, the definition of Adjusted EBITDA in the Indentures and our Amended Senior Secured Credit Facility allows us to add back certain non-cash, extraordinary, unusual or non-recurring charges that are deducted in calculating net income (loss). However, these are expenses that may recur, vary greatly and are difficult to predict. While Adjusted EBITDA and similar measures are frequently used as measures of operations and the ability to meet debt service requirements, Adjusted EBITDA is not necessarily comparable to other similarly titled captions of other companies due to the potential inconsistencies in the method of calculation.
Note indentures
The Indentures governing the notes limit our (and most or all of our subsidiaries’) ability to:
| • | incur additional debt or issue certain preferred shares; |
| • | pay dividends on or make other distributions in respect of our capital stock or make other restricted payments; |
| • | make certain investments; |
| • | sell certain assets; |
| • | create liens on certain assets to secure debt; |
| • | consolidate, merge, sell or otherwise dispose of all or substantially all of our assets; |
| • | enter into certain transactions with our affiliates; and |
| • | designate our subsidiaries as unrestricted subsidiaries. |
Under the Indentures governing the notes, our ability to incur additional debt, subject to specified exceptions, is tied to either improving the ratio of our Adjusted EBITDA to fixed charges or having this ratio be
71
Table of Contents
at least 2.00:1 on a pro forma basis after giving effect to such incurrence. Additionally, our ability to make certain restricted payments is also tied to having an Adjusted EBITDA to fixed charges ratio of at least 2.00:1 on a pro forma basis, as defined, subject to specified exceptions. Our pro forma ratio of Adjusted EBITDA to fixed charges for the twelve months ended September 29, 2012, measured on that date, was below the required ratio for such additional incurrence or payments at 1.64:1. Notwithstanding these limitations, the aggregate amount of term loan increases and revolving commitment increases shall not exceed the greater of (i) $150.0 million and (ii) the additional aggregate amount of secured indebtedness which would be permitted to be incurred as of any date of determination (assuming for this purpose that the full amount of any revolving credit increase had been utilized as of such date) such that, after giving pro forma effect to such incurrence (and any other transactions consummated on such date), the Senior Secured first lien leverage ratio for the immediately preceding test period would not be greater than 4.25:1. Fixed charges is defined in the Indentures as consolidated interest expense plus all cash dividends or other distributions paid on any series of preferred stock of any restricted subsidiary and all dividends or other distributions accrued on any series of disqualified stock.
Covenant Compliance
The following is a summary of our covenant requirement ratios as of September 29, 2012:
| Covenant Requirements |
Actual Ratios |
|||||||
| Amended Senior Secured Credit Facility: |
||||||||
| Maximum ratio of Senior Secured first lien leverage to Adjusted EBITDA |
4.25:1 | 3.00:1 | ||||||
| Notes: |
||||||||
| Minimum ratio of Adjusted EBITDA to fixed charges required to incur additional debt pursuant to ratio provision, pro forma |
2.00:1 | 1.64:1 | ||||||
As described above, our Amended Senior Secured Credit Facility and the Indentures governing the notes represent significant components of our capital structure. Under our Amended Senior Secured Credit Facility, we are required to maintain specified Senior Secured first lien leverage ratios, which become more restrictive over time, and which are determined based on our Adjusted EBITDA. If we fail to comply with the Senior Secured first lien leverage ratio under our Amended Senior Secured Credit Facility, we would be in default under the credit facility. Upon the occurrence of an event of default under the Amended Senior Secured Credit Facility, the lenders could elect to declare all amounts outstanding under the Amended Senior Secured Credit Facility to be immediately due and payable and terminate all commitments to extend further credit. If we were unable to repay those amounts, the lenders under the Amended Senior Secured Credit Facility could proceed against the collateral granted to them to secure that indebtedness. We have pledged substantially all of our assets as collateral under the Amended Senior Secured Credit Facility. Any acceleration under the Amended Senior Secured Credit Facility would also result in a default under the Indentures governing the notes, which could lead to the note holders electing to declare the principal, premium, if any, and interest on the then outstanding Notes immediately due and payable. In addition, under the Indentures governing the notes, our ability to engage in activities such as incurring additional indebtedness, making investments, refinancing subordinated indebtedness, paying dividends and entering into certain merger transactions is governed, in part, by our ability to satisfy tests based on Adjusted EBITDA.
Our ability to meet the covenants specified above will depend on future events, many of which are beyond our control, and we cannot assure you that we will meet those covenants. A breach of any of these covenants in the future could result in a default under our Amended Senior Secured Credit Facility and the Indentures, at which time the lenders could elect to declare all amounts outstanding under our Amended Senior Secured Credit Facility to be immediately due and payable. Any such acceleration would also result in a default under the Indentures.
72
Table of Contents
The following table provides a reconciliation from our net loss to Adjusted EBITDA for the three and twelve months ended September 29, 2012 (in thousands). The terms and related calculations are defined in the credit agreement relating to our Amended Senior Secured Credit Facility and the Indentures.
| Three Months Ended September 29, 2012 |
Twelve Months Ended September 29, 2012 |
|||||||
| Net loss attributable to DJO Finance LLC |
$ | (22,562 | ) | $ | (220,325 | ) | ||
| Interest expense, net |
46,365 | 177,655 | ||||||
| Income tax provision (benefit) |
569 | (13,445 | ) | |||||
| Depreciation and amortization |
32,170 | 126,750 | ||||||
| Non-cash charges (a) |
1,317 | 155,428 | ||||||
| Non-recurring and integration charges (b) |
7,011 | 31,803 | ||||||
| Other adjustment items, before adjustments applicable for the twelve month period only (c) |
33 | 15,956 | ||||||
|
|
|
|
|
|||||
| Adjusted EBITDA before other adjustment items applicable to the twelve month period only |
64,903 | 273,822 | ||||||
| Other adjustment items applicable to the twelve month period only (d): |
||||||||
| Future cost savings related to recent acquisitions |
— | 2,343 | ||||||
|
|
|
|
|
|||||
| Adjusted EBITDA |
$ | 64,903 | $ | 276,165 | ||||
|
|
|
|
|
|||||
| (a) | Non-cash charges are comprised of the following (in thousands): |
| Three Months Ended September 29, 2012 |
Twelve Months Ended September 29, 2012 |
|||||||
| Stock based compensation expense |
$ | 1,310 | $ | 4,564 | ||||
| Impairment of Chattanooga assets held for sale |
— | 380 | ||||||
| Loss on disposal of assets, net |
7 | (2 | ) | |||||
| Impairment of goodwill and intangible assets |
— | 141,006 | ||||||
| Purchase accounting adjustments |
— | 2,364 | ||||||
| Impairment of fixed assets |
— | 7,116 | ||||||
|
|
|
|
|
|||||
| Total non-cash charges |
$ | 1,317 | $ | 155,428 | ||||
|
|
|
|
|
|||||
| (b) | Non-recurring and integration charges are comprised of the following (in thousands): |
| Three Months Ended September 29, 2012 |
Twelve Months Ended September 29, 2012 |
|||||||
| Integration charges: |
||||||||
| U.S. commercial sales and marketing reorganization |
$ | 1,031 | $ | 2,030 | ||||
| Acquisition related expenses and integration (1) |
580 | 1,990 | ||||||
| CEO transition |
— | 266 | ||||||
| Other non-recurring and integration expenses |
2,757 | 10,244 | ||||||
| Litigation costs and settlements, net |
1,920 | 7,216 | ||||||
| ERP implementation and other automation projects |
723 | 10,057 | ||||||
|
|
|
|
|
|||||
| Total non-recurring and integration charges |
$ | 7,011 | $ | 31,803 | ||||
|
|
|
|
|
|||||
| (1) | Direct acquisition costs and integration expenses related to the Dr. Comfort, ETI and Circle City acquisitions. |
73
Table of Contents
| (c) | Other adjustment items are comprised of the following (in thousands): |
| Three Months Ended September 29, 2012 |
Twelve Months Ended September 29, 2012 |
|||||||
| Blackstone monitoring fees |
1,750 | 7,000 | ||||||
| Noncontrolling interests |
27 | 827 | ||||||
| Loss on modification and extinguishment of debt (1) |
— | 9,398 | ||||||
| Other (2) |
(1,744 | ) | (1,269 | ) | ||||
|
|
|
|
|
|||||
| Total other adjustment items |
$ | 33 | $ | 15,956 | ||||
|
|
|
|
|
|||||
| (1) | Loss on modification and extinguishment of debt for the twelve months ending September 29, 2012 consists of $8.6 million of arrangement and amendment fees and other fees and expenses incurred in connection with the March 2012 amendment of our Senior Secured Credit Facility and $0.8 million related to the non-cash write off of unamortized debt issuance costs and original issue discount associated with the portion of the term loans which were extinguished. |
| (2) | Other adjustments consist primarily of net realized and unrealized foreign currency transaction gains and losses. |
| (d) | Other adjustment items applicable for the twelve month period only include future cost savings related to the acquisitions of Dr. Comfort, ETI and Circle City. |
Contractual Commitments
As of December 31, 2011, our consolidated contractual commitments are as follows (in thousands):
| Payment due: | ||||||||||||||||||||
| Total | 2012 | 2013-2014 | 2015-2016 | Thereafter | ||||||||||||||||
| Long-term debt obligations |
$ | 2,169,029 | $ | 8,782 | $ | 1,560,247 | $ | — | $ | 600,000 | ||||||||||
| Interest payments (1) |
612,031 | 156,950 | 294,456 | 105,000 | 55,625 | |||||||||||||||
| Capital lease obligations |
38 | 38 | — | — | — | |||||||||||||||
| Operating lease obligations |
67,640 | 11,749 | 21,489 | 14,865 | 19,537 | |||||||||||||||
| Purchase obligations |
74,499 | 21,956 | 17,543 | 14,000 | 21,000 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| $ | 2,923,237 | $ | 199,475 | $ | 1,893,735 | $ | 133,865 | $ | 696,162 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | $1,275.0 million principal amount of long-term debt is subject to fixed interest rates and $843.0 million of principal amount of long-term debt is subject to a floating interest rate. Interest payments for the floating rate debt were determined using an average assumed effective interest rate of 3.7%, which is equal to the average assumed effective interest rate for the term loans under the Senior Secured Credit Facility over the remainder of their term. |
As of December 31, 2011, we had entered into purchase commitments for inventory, capital expenditures and other services totaling $74.5 million in the ordinary course of business. In addition, under the amended transaction and monitoring fee agreement entered into in connection with the DJO Merger, the purchase obligations shown above include DJO’s obligation to pay a $7.0 million annual monitoring fee to Blackstone Management Partners V L.L.C. through 2019.
The amounts presented in the table above may not necessarily reflect our actual future cash funding requirement because the actual timing of future payments made may vary from the stated contractual obligation.
74
Table of Contents
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates, including those related to reserves for contractual allowances, doubtful accounts, rebates, product returns and rental credits, goodwill and intangible assets, deferred tax assets and liabilities and inventory. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. To the extent that actual events differ from our estimates and assumptions, there could be a material adverse effect on our consolidated financial statements.
We believe the following critical accounting policies reflect our more significant judgments and estimates used in the preparation of our consolidated financial statements and this discussion and analysis of our financial condition and results of operations.
Reserves for Contractual Allowances, Doubtful Accounts, Rebates, Product Returns and Rental Credits
We have established reserves to account for contractual allowances, doubtful accounts, rebates, product returns and rental credits. Significant management judgment must be used and estimates must be made in connection with establishing these reserves.
We maintain provisions for estimated contractual allowances for reimbursement amounts from our third party payor customers based on negotiated contracts and historical experience for non-contracted payors. We report these allowances as reductions in our gross revenue. We estimate the amount of the reduction based on historical experience and invoices generated in the period, and we consider the impact of new contract terms or modifications of existing arrangements with our customers. We have contracts with certain third party payors for our third party reimbursement billings, which call for specified reductions in reimbursement of billed amounts based upon contractual reimbursement rates. For the years ended December 31, 2011, 2010, and 2009, we reserved for and reduced gross revenues from third party payors by estimated allowances of 33%, 32%, and 31%; respectively, related to these contractual reductions.
Our reserve for doubtful accounts is based upon estimated losses from customers who are billed directly and the portion of third party reimbursement billings that ultimately become the financial responsibility of the end user patients. Direct-billed customers represented approximately 71% of our net revenues for the year ended December 31, 2011 and approximately 67% of our net revenues for each of the years ended December 31, 2010 and 2009. Direct-billed customers represented approximately 71% and 66% of our net accounts receivable at December 31, 2011 and 2010, respectively. We experienced write-offs related to direct-billed customers of less than 1% of related net revenues in each of the years ended December 31, 2011, 2010, and 2009.
Our third party reimbursement customers including insurance companies, managed care companies and certain governmental payors, such as Medicare, include all of our OfficeCare customers, most of our Empi customers, and certain other customers of our Recovery Sciences and Bracing and Vascular segments. Our third party payor customers represented approximately 29% of our net revenues for the year ended December 31, 2011 and approximately 33% of our net revenues for each of the years ended December 31, 2010 and 2009. Third party payor customers represented approximately 29% and 34%, respectively, of our net accounts receivable at December 31, 2011 and 2010. For the years ended December 31, 2011, 2010, and 2009, we estimate bad debt expense to be approximately 5%, 6% and 7%, respectively, of gross revenues from these third party reimbursement customers. If the financial condition of our customers were to deteriorate resulting in an impairment of their ability to make payments or if third party payors were to deny claims for late filings,
75
Table of Contents
incomplete information or other reasons, additional provisions may be required. Additions to this reserve are reflected as selling, general and administrative expense in our consolidated statements of operations.
Our reserve for rebates accounts for incentives that we offer to certain of our distributors. These rebates are substantially attributable to sales volume, sales growth or to reimburse the distributor for certain discounts. We record estimated reductions to revenue for customer rebate programs based upon historical experience and estimated revenue levels.
Our reserve for product returns accounts for estimated customer returns of our products after purchase. These returns are mainly attributable to a third party payor’s refusal to provide reimbursement for the product or the inability of the product to adequately address the patient’s condition. We provide for this reserve by reducing gross revenue based on our historical rate of returns.
Our reserve for rental credit recognizes a timing difference between billing for a sale and processing a rental credit associated with some of our rehabilitation devices. Many insurance providers require patients to rent our rehabilitation devices for a period of one to three months prior to purchase. If the patient has a long-term need for the device, these insurance companies may authorize purchase of the device after such time period. When the device is purchased, most providers require that rental payments previously made on the device be credited toward the purchase price. These credits are processed at the time the payment is received for the purchase of the device, which creates a time lag between billing for a sale and processing the rental credit. Our rental credit reserve estimates unprocessed rental credits based on the number of devices converted to purchase. The reserve is calculated by first assessing the number of our products being rented during the relevant period and our historical conversion rate of rentals to sales, and then reducing our revenue by the applicable amount. We provide for these reserves by reducing our gross revenue. The cost to refurbish rented products is expensed as incurred to cost of sales in our consolidated statements of operations.
Inventory Reserves
We provide reserves for estimated excess and obsolete inventories equal to the difference between the costs of inventories on hand plus future purchase commitments and the estimated market value based upon assumptions about future demand. If future demand is less favorable than currently projected by management, additional inventory write-downs may be required. We also provide reserves for newer product inventories, as appropriate, based on any minimum purchase commitments and our level of sales of the new products.
We consign a portion of our inventory to allow our products to be immediately dispensed to patients. This requires a large amount of inventory to be on hand for the products we sell through consignment arrangements. It also increases the sensitivity of these products to obsolescence reserve estimates. As this inventory is not in our possession, we maintain additional reserves for estimated shrinkage of these inventories based on the results of periodic inventory counts and historical trends.
Goodwill and Intangible Assets
We evaluate the carrying value of goodwill and indefinite life intangible assets annually on the first day of the fourth quarter or whenever events or circumstances indicate the carrying value may not be recoverable. We evaluate the carrying value of finite life intangible assets whenever events or circumstances indicate the carrying value may not be recoverable. Significant assumptions are required to estimate the fair value of goodwill and intangible assets, most notably estimated future cash flows generated by these assets. As such, these fair valuation measurements use significant unobservable inputs. Changes to these assumptions could require us to record impairment charges on these assets.
In performing our 2011 goodwill impairment test, we estimated the fair values of our reporting units using the income approach valuation methodology which includes the discounted cash flow method and the market
76
Table of Contents
approach valuation methodology which includes the use of market multiples. The discounted cash flows for each reporting unit were based on discrete financial forecasts developed by management for planning purposes, and required significant judgment with respect to forecasted sales, gross margin, selling, general and administrative expenses, EBITDA, capital expenditures, and the selection and use of an appropriate discount rate. For purposes of calculating the discounted cash flows of our reporting units, we used estimated revenue growth rates between 0% and 11%. Cash flows beyond the discrete forecasts were estimated using a terminal value calculation, which incorporated historical and forecasted financial trends for each identified reporting unit and considered long-term earnings growth rates for publicly traded peer companies. Future cash flows were then discounted to present value at discount rates ranging from 10.7% to 12.5%, and terminal value growth rates of 3%. Publicly available information regarding comparable market capitalization was also considered in assessing the reasonableness of the cumulative fair values of our reporting units estimated using the discounted cash flow methodology.
In the fourth quarter of 2011, we determined that the carrying value of our Empi and Surgical Implant reporting units was in excess of their estimated fair value. As a result, we recorded a goodwill impairment charges for the Empi and Surgical Implant reporting units of $76.7 million and $47.4 million, respectively. See Note 8 of the notes to the audited consolidated financial statements included elsewhere in this prospectus for further discussion and the factors that contributed to these impairment charges.
We have five other reporting units with goodwill assigned to them. For each of those five reporting units, the estimated fair values substantially exceed their carrying value.
Additionally, on the first day of the fourth quarter of 2011 we tested for impairment, our indefinite lived intangible assets, consisting of trade names. This test work compares the fair value of the asset with its carrying amount. To determine the fair value we applied the relief from royalty (RFR) method. Under the RFR method, the value of the trade name is determined by calculating the present value of the after-tax cost savings associated with owning the asset and therefore not being required to pay royalties for its use during the asset’s indefinite life. Significant judgments inherent in this analysis include the selection of appropriate discount rates, estimating future cash flows and the identification of appropriate terminal growth rate assumptions. Discount rate assumptions are based on an assessment of the risk inherent in the projected future cash generated by the respective intangible assets. Also subject to judgment are assumptions about royalty rates, which are based on the estimated rates at which similar brands and trademarks are being licensed in the marketplace.
In the fourth quarter of 2011, we determined that the carrying value of our Empi trade name was in excess of its estimated fair value, and recorded an impairment charge of $16.9 million. See Note 8 of the notes to the audited consolidated financial statements included elsewhere in this prospectus for further discussion and the factors that contributed to this impairment charge.
The estimates we have used are consistent with the plans and estimates that we use to manage our business, however, it is possible that the plans may change and estimates used may prove to be inaccurate. If our actual results, or the plans and estimates used in future impairment analyses, are lower than the original estimates used to assess the recoverability of these assets, we could incur significant impairment charges.
Deferred Tax Asset Valuation Allowance
We recognize deferred tax assets and liabilities based on the differences between the financial statement carrying amount and the tax bases of assets, liabilities and net operating loss carryforwards (NOLs). We establish valuation allowances when the recovery of a deferred tax asset is not likely based on historical income, projected future income, the expected timing of the reversals of temporary differences and the implementation of tax-planning strategies. Beginning in the first quarter of June 30, 2012, we were unable to determine that it was more likely than not that certain U.S federal and state deferred tax assets would be realized. We therefore established a valuation allowance against those deferred tax assets.
77
Table of Contents
Our gross deferred tax asset balance was approximately $235.2 million at September 29, 2012 and primarily related to reserves for accounts receivable and inventory, accrued expenses, and NOLs (see Note 9 to our unaudited condensed consolidated financial statements). As of September 29, 2012, we maintained a valuation allowance of $30.5 million due to uncertainties related to our ability to realize certain NOLs.
78
Table of Contents
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to certain market risks as part of our ongoing business operations, primarily from fluctuating interest rates and foreign currency exchange rates that could impact our financial condition, results of operations, and cash flows.
Interest Rate Risk
Our primary exposure is to fluctuating interest rates. We have historically managed our interest rate risk by balancing the amounts of our fixed and variable rate debt. For our fixed rate debt, interest rate changes may affect the market value of the debt but do not impact our earnings or cash flow. Conversely, for our variable rate debt, interest rate changes generally do not affect the fair market value of the debt but do impact future earnings and cash flow, assuming other factors are constant. We have $1070.0 million aggregate principal amount of fixed rate senior notes and $300 million senior subordinated notes. Our borrowings under the Amended Senior Secured Credit Facility bear interest at floating rates based on LIBOR or the prime rate, as defined. As of September 29, 2012, we had $839.2 million of borrowings outstanding under the Amended Senior Secured Credit Facility, exclusive of $9.3 million of unamortized original issue discount. A hypothetical 1% increase in variable interest rates for the Amended Senior Secured Credit Facility would have impacted our earnings and cash flow for the nine months ended September 29, 2012 by approximately $6.6 million. We may use financial instruments where appropriate to manage our interest rate risk, although we had no such instruments in effect at September 29, 2012. As a matter of policy, we do not enter into derivative or other financial investments for trading or speculative purposes.
Foreign Currency Risk
Due to the global reach of our business, we are exposed to market risk from changes in foreign currency exchange rates, particularly with respect to the U.S. dollar compared to the Euro and the Mexican Peso (MXN). Our wholly owned foreign subsidiaries are consolidated into our financial results and are subject to risks typical of an international business including, but not limited to, differing economic conditions, changes in political climate, differing tax structures, other regulations and restrictions and foreign exchange volatility. To date, we have not used international currency derivatives to hedge against our investment in our European subsidiaries or their operating results, which are converted into U.S. Dollars at period-end and average foreign exchange rates, respectively.
As we continue to expand our business, the sales of our products that are denominated in foreign currencies has increased, as have the costs associated with our foreign subsidiaries which operate in currencies other than the U.S. dollar. Accordingly, our future results could be materially impacted by changes in these or other factors. For the three and nine months ended September 29, 2012, sales denominated in foreign currencies accounted for approximately 19.7% and 20.9% of our consolidated net sales, respectively, of which 13.8% and 14.9%, respectively, were denominated in the Euro. In addition, our exposure to fluctuations in foreign currencies may arise because certain of our subsidiaries enter into purchase or sale transactions using a currency other than its functional currency. Accordingly, our future results could be materially impacted by changes in foreign exchange rates or other factors.
Occasionally, we seek to reduce the potential impact of currency fluctuations on our business through hedging transactions. During the nine months ended September 29, 2012, we utilized MXN foreign exchange forward contracts to hedge a portion of our exposure to fluctuations in foreign exchange rates, as our Mexico-based manufacturing operations incur costs that are largely denominated in MXN (see Note 7 to our unaudited condensed consolidated financial statements). Foreign exchange forward contracts held as of September 29, 2012 expire weekly through June 2013.
79
Table of Contents
Overview
We are a global developer, manufacturer and distributor of high-quality medical devices that provide solutions for musculoskeletal health, vascular health and pain management. Our products address the continuum of patient care from injury prevention to rehabilitation after surgery, injury or from degenerative disease, enabling people to regain or maintain their natural motion.
Our products are used by orthopedic specialists, spine surgeons, primary care physicians, pain management specialists, physical therapists, podiatrists, chiropractors, athletic trainers and other healthcare professionals. In addition, many of our medical devices and related accessories are used by athletes and patients for injury prevention and at-home physical therapy treatment. Our product lines include rigid and soft orthopedic bracing, hot and cold therapy, bone growth stimulators, vascular therapy systems and compression garments, therapeutic shoes and inserts, electrical stimulators used for pain management and physical therapy products. Our surgical implant business offers a comprehensive suite of reconstructive joint products for the hip, knee and shoulder.
Our products are marketed under a portfolio of brands including Aircast®, DonJoy®, ProCare®, CMF™, Empi®, Chattanooga, DJO Surgical, Dr. Comfort ™ and Compex®.
Operating Segments
We currently develop, manufacture and distribute our products through four operating segments.
Bracing and Vascular Segment
Our Bracing and Vascular segment, which generates its revenues in the United States, offers our rigid knee bracing products, orthopedic soft goods, cold therapy products, vascular systems, therapeutic shoes and inserts and compression therapy products, primarily under our DonJoy, ProCare, Aircast and Dr. Comfort brands. This segment also includes our OfficeCare business, through which we maintain an inventory of soft goods and other products at healthcare facilities, primarily orthopedic practices, for immediate distribution to patients. The Bracing and Vascular segment primarily sells its products to orthopedic and sports medicine professionals, hospitals, podiatry practices, orthotic and prosthetic centers, home medical equipment providers and independent pharmacies.
Recovery Sciences Segment
Our Recovery Sciences segment, which generates its revenues in the United States, is divided into four main businesses:
| • | Empi. Our Empi business unit offers our home electrotherapy, iontophoresis, and home traction products. We primarily sell these products directly to patients or to physical therapy clinics. For products sold to patients, we arrange billing to the patients and their third party payors. |
| • | Regeneration. Our Regeneration business unit sells our bone growth stimulation products. We sell these products either directly to patients or to independent distributors. For products sold to patients, we arrange billing to the patients and their third party payors. |
| • | Chattanooga. Our Chattanooga business unit offers products in the clinical rehabilitation market in the category of clinical electrotherapy devices, clinical traction devices, and other clinical products and supplies such as treatment tables, CPM devices and dry heat therapy. |
| • | Athlete Direct. Our Athlete Direct business unit offers consumers ranging from fitness enthusiasts to competitive athletes our Compex electrostimulation devices, which are used in athletic training programs to aid muscle development and to accelerate muscle recovery after training sessions. |
80
Table of Contents
International Segment
Our International segment, which generates most of its revenues in Europe, sells all of our products and certain third party products through a combination of direct sales representatives and independent distributors.
Surgical Implant Segment
Our Surgical Implant segment, which generates its revenues in the United States, develops, manufactures and markets a wide variety of knee, hip and shoulder implant products that serve the orthopedic reconstructive joint implant market.
Our four operating segments enable us to reach a diverse customer base through multiple distribution channels and give us the opportunity to provide a wide range of medical devices and related products to orthopedic specialists and other healthcare professionals operating in a variety of patient treatment settings. These four segments constitute our reportable segments.
Acquisitions
Our growth has been driven both by the introduction of products facilitated by our research and development efforts and by selected acquisitions of businesses or products.
On April 7, 2011, we acquired all of the LLC membership interests of Rikco International, LLC, D/B/A Dr. Comfort (Dr. Comfort), for a total purchase price of $257.5 million. Dr. Comfort is a provider of therapeutic footwear, which serves the diabetes care market in podiatry practices, orthotic and prosthetic centers, home medical equipment providers and independent pharmacies.
On March 10, 2011, we acquired substantially all of the assets of Circle City Medical, Inc. (Circle City) for a total purchase price of $11.7 million. Circle City markets orthopedic soft goods and medical compression therapy products to independent pharmacies and home healthcare dealers.
On February 4, 2011, we purchased certain assets of an e-commerce business (BetterBraces.com), which offers various bracing, cold therapy and electrotherapy products, for total consideration of $3.0 million.
On January 4, 2011, we acquired all of the outstanding shares of capital stock of Elastic Therapy, Inc. (ETI), a designer and manufacturer of private label medical compression therapy products used to treat and prevent a wide range of venous disorders. The purchase price was $46.4 million.
We completed the following acquisitions during the years ended December 31, 2010 and 2009, each of which represents an expansion of our international business:
On September 20, 2010, we acquired certain assets and contractual rights from an independent South African distributor of DonJoy products for total consideration of $1.9 million.
On August 4, 2009, we acquired Chattanooga Group Inc. and Empi Canada Inc., independent Canadian distributors of certain of our products, for total consideration of $14.6 million.
On February 3, 2009, we acquired DonJoy Orthopaedics Pty., Ltd., an independent Australian distributor of DonJoy products, for total consideration of $3.4 million.
81
Table of Contents
Industry Background
Market Opportunities
We participate globally in the rehabilitation, pain management, bone growth stimulation and reconstruction segments of the orthopedic device market. In the United States, we estimate these segments accounted for $8.6 billion of total industry sales in 2010. We believe that several factors are driving growth in the orthopedic products industry, including the following:
| • | Favorable demographics. An aging population is driving growth in the orthopedic products market. Many conditions that result in rehabilitation, physical therapy or orthopedic surgery are more likely to affect people in middle age or later in life. According to a 2011 United States Census Bureau—International Data Base projection, the aging baby boomer generation will result in the percentage of the North American population aged 65 and over to grow from 13.4% in 2011 to 16.5% in 2020 and to 20.0% by 2030. In Western Europe, the population aged 65 and over is expected to grow from 18.5% in 2011 to 21.2% in 2020 and to 25.3% by 2030. In addition, according to the 2011 United States Census Bureau—International Data Base projection, the average life expectancy in North America is 78.6 years in 2011 and is expected to grow to 80.9 years by 2030. In Western Europe, the average life expectancy is 80.6 years in 2011 and is expected to grow to 82.4 years by 2030. As life expectancy increases, we believe people will remain active longer, causing the number of injuries requiring orthopedic rehabilitation, bone growth stimulation and reconstructive implants to increase. |
| • | Shift toward non-surgical rehabilitation devices and at-home physical therapy. We believe the growing awareness and clinical acceptance by healthcare professionals of the benefits of non-surgical, non-pharmaceutical treatment and rehabilitation products, combined with the increasing interest by patients in rehabilitation solutions that minimize risk and recuperation time and provide greater convenience, will continue to drive demand for these products. In addition, we believe that orthopedic surgeons are increasingly utilizing braces that assist in rehabilitation and bone growth stimulators that enable in-home treatment as viable alternatives to surgery. We design many of our orthopedic rehabilitation products for at-home use, which we believe should allow us to benefit from the market shift toward these treatment alternatives. |
| • | Lower cost alternatives appeal to third party payors. With the cost of healthcare rising in the United States and internationally, third party payors are seeking more cost-effective therapies without reducing quality of care. For example, third party payors seek to reduce clinic visits and accommodate patients’ preference for therapies that can be conveniently administered at home. We believe that many of our orthopedic rehabilitation products offer cost-effective alternatives to surgery, pharmaceutical and other traditional forms of physical therapy and pain management. |
| • | Increased need for rehabilitation due to increased orthopedic surgical volume. The combination of increased prevalence of degenerative joint disease (such as osteoarthritis), an increased number of sports-related injuries, an aging population and improvements in orthopedic surgical technique (such as arthroscopy) has contributed to an increase in the number of orthopedic surgeries. We believe that orthopedic surgical volume will continue to increase, which should result in an increase in the need for our products. |
Competitive Strengths
We believe that we have a number of competitive strengths that will enable us to further enhance our position in the markets we serve:
| • | Leading market positions. We believe we have leading market positions for many of our products. We believe our orthopedic and physical therapy rehabilitation products marketed under the Aircast, DonJoy, ProCare, CMF, Empi, Chattanooga, DJO Surgical, Dr. Comfort and Compex brands have a reputation for quality, durability and reliability among healthcare professionals. We believe the |
82
Table of Contents
| strength of our brands and our focus on customer service have allowed us to establish market leading positions in the highly fragmented and growing orthopedic rehabilitation market. |
| • | Comprehensive range of orthopedic products. We offer a diverse range of medical devices for musculoskeletal health, vascular health and pain management, including rigid and soft orthopedic bracing, hot and cold therapy, bone growth stimulators, vascular therapy systems and compression garments, therapeutic shoes and inserts, electrical stimulators used for pain management and physical therapy products. Our surgical implant business offers a comprehensive suite of reconstructive joint products for the hip, knee and shoulder. Our broad product offering meets many of the needs of healthcare professionals and patients and enables us to leverage our brand loyalty with our customer and distributor base. Our products are available across various stages of the patient’s continuum of care. |
| • | Extensive and diverse distribution network. We use multiple channels to distribute our products to our customers. We use approximately 6,000 dealers and distributors and a direct sales force of approximately 700 employed sales representatives and approximately 1,200 independent sales representatives to supply our products to orthopedic specialists, spine surgeons, primary care physicians, pain management specialists, physical therapists, podiatrists, chiropractors, athletic trainers and other healthcare professionals. We believe that our distribution network provides us with a significant competitive advantage in selling our existing products and in introducing new products. |
| • | Strong relationships with managed care organizations and rehabilitation healthcare providers. Our leading market positions in many of our product lines and the breadth of our product offerings have enabled us to secure important preferred provider and managed care contracts. Our database includes approximately 8,250 different insurance companies and other payors, including approximately 1,530 active payor contracts. We have developed a proprietary third party billing system that is designed to reduce our reimbursement cycles, improve relationships with managed care organizations and physicians and track patients to improve quality of care and create subsequent selling opportunities. Further, our OfficeCare business maintains inventory at over 1,500 healthcare facilities, primarily orthopedic practices, which further strengthens our relationships with these healthcare providers. |
| • | National contracts with group purchasing organizations. We enjoy strong relationships with a number of group purchasing organizations due to our significant scale. We believe that our broad range of products is well suited to the goals of these buying groups. Under these national contracts, we provide favorable pricing to the buying group and are designated a preferred purchasing source for the members of the buying group for specified products. As we have made acquisitions and expanded our product range, we have been able to add incremental products to our national contracts. During 2011, we signed or renewed approximately 30 national contracts. |
| • | Low cost, high quality manufacturing capabilities. We have a major manufacturing facility in Tijuana, Mexico that has been recognized for operational excellence. The Mexico facility and our other manufacturing facilities employ lean manufacturing, Six Sigma concepts and continuous improvement processes to drive manufacturing efficiencies and lower costs. |
| • | Ability to generate significant cash flow. Historically, our strong competitive position, brand awareness and high quality products and service as well as our low cost manufacturing have allowed us to generate attractive operating margins before non-cash amortization expense and certain non-recurring charges. These operating margins, together with limited capital expenditures and modest working capital requirements, significantly benefit our ability to generate cash flow. |
| • | Experienced management team. The members of our management team have an average of 29 years of relevant experience. This team has successfully integrated a number of acquisitions in the last several years. |
83
Table of Contents
Strategy
Our strategy is to increase our leading position in key products and markets, increase revenues and profitability and enhance cash flow. Our key initiatives to implement this strategy include the following:
| • | Increase our leading market positions. We believe we are the market leader in many of the markets in which we compete. We intend to continue to increase our market share by leveraging the cross-selling and other opportunities created by the DJO Merger and by implementing the initiatives described below. The DJO Merger has allowed us to offer customers a more comprehensive range of products to better meet their evolving needs. We believe our size, scale, brand recognition, comprehensive and integrated product offerings and leading market positions enable us to capitalize on the growth in the orthopedic product industry. |
| • | Focus sales force on entire range of DJO products. Our products address the continuum of patient care from injury prevention to rehabilitation after surgery, injury or from degenerative disease. Our strategy is to train and incentivize our sales force, which consists of agents and representatives familiar with a particular set of products, to work cooperatively and collaboratively with all segments of our sales force to introduce their customers to the full range of our products of which the customer is typically using only a portion. We believe that this represents a significant opportunity to expand our business through existing customers. |
| • | Continue to develop and launch new products and product enhancements. We have a history of developing and introducing innovative products into the marketplace, and we expect to continue future product launches by leveraging our internal research and development platforms. We believe our ability to develop new technology and to advance existing technology to create new products will position us to further diversify our revenues and to expand our target markets by providing viable alternatives to surgery or medication. We believe that product innovation through effective and focused research and development, as well as our relationships with a number of widely recognized orthopedic surgeons and professionals who assist us in product research, development and marketing, will provide a significant competitive advantage. During 2011, sales of new products, which include products that have been on the market less than one year, were $44.7 million. |
| • | Maximize existing and secure additional national accounts. We plan to capitalize on the growing practice in healthcare in which hospitals and other large healthcare providers seek to consolidate their purchasing activities to national buying groups. Contracts with these national accounts represent a significant opportunity for revenue growth. We believe that our existing relationships with national buying groups and our broad range of products position us to not only pursue additional national contracts, but also to expand the scope of our existing contracts. |
| • | Expand international sales. In recent years, we have successfully established direct distribution capabilities in several major international markets. We believe that sales to European and other markets outside the United States continue to represent a significant growth opportunity, and we intend to continue to expand our direct and independent distribution capabilities in attractive foreign markets. Several of the acquisitions we have made in recent years have substantially increased our international revenues and operating infrastructure and have provided us with opportunities to expand our international product offerings. |
| • | Drive operating efficiency. We plan to continue to apply the principles of lean operations to our manufacturing sites as well as in our operating and administrative functions to increase speed and efficiency and reduce waste. We have instilled a culture of continuous improvement throughout the Company and are pursuing a regular schedule of addressing operations and processes in the Company to improve efficiency. We believe these lean principles and continuous improvement efforts will enhance our operating efficiencies and our ability to compete in an increasingly price-sensitive healthcare industry. |
84
Table of Contents
Our Products
Our products are used by orthopedic specialists, spine surgeons, primary care physicians, pain management specialists, physical therapists, podiatrists, chiropractors, athletic trainers and other healthcare professionals to treat patients with musculoskeletal conditions resulting from degenerative diseases, deformities, traumatic events and sports related injuries. In addition, many of our non-surgical medical devices and related accessories are used by athletes and patients for injury prevention and at-home physical therapy treatment.
Bracing and Vascular Segment
Our Bracing and Vascular segment generated net sales of $329.1 million, $387.9 million, $311.6 million and $298.8 million for the nine months ended September 29, 2012 and the years ended December 31, 2011, 2010 and 2009, respectively. The following table summarizes our Bracing and Vascular segment product categories:
| Product Category |
Description | |
| Rigid bracing and soft goods | Soft goods Lower extremity fracture boots Dynamic splinting Ligament braces Post-operative braces Osteoarthritis braces Ankle bracing Shoulder, elbow and wrist braces Back braces Neck braces | |
| Cold and compression therapy | Cold and compression therapy products | |
| Vascular therapy | Vascular system pumps Compression hosiery | |
| Therapeutic shoes and inserts | Therapeutic footwear and related medical and comfort products |
Recovery Sciences Segment
Our Recovery Sciences segment generated net sales of $250.0 million, $342.6 million, $347.1 million and $342.0 million for the nine months ended September 29, 2012 and the years ended December 31, 2011, 2010 and 2009, respectively. The following table summarizes our Recovery Sciences segment product categories:
| Product Category |
Description | |
| Home electrotherapy devices | Transcutaneous electrical nerve stimulation (TENS) Neuromuscular electrical stimulation (NMES) Interferential electrical nerve stimulation | |
| Clinical electrotherapy | TENS NMES Ultrasound Laser Light therapy Shortwave Diathermy Shockwave | |
| Patient care | Nutritional supplements Patient safety devices Pressure care products Continuous passive motion devices |
85
Table of Contents
| Product Category |
Description | |
| Hot, cold and compression therapy | Dry heat therapy Hot/cold therapy Paraffin wax therapy Moist heat therapy Cold therapy Compression therapy | |
| Physical therapy tables and traction products | Treatment tables Traction tables Cervical traction for home use Lumbar traction for home use | |
| Iontophoresis | Needle-free transdermal drug delivery | |
| Regeneration | Non-union fracture bone growth stimulation devices Spine bone growth stimulation devices Back braces |
International Segment
Our International segment generated net sales of $206.6 million, $279.3 million, $244.5 million and $241.5 million for the nine months ended September 29, 2012 and the years ended December 31, 2011, 2010 and 2009, respectively. The product categories for our International segment are similar to the product categories for our domestic segments except certain products are tailored to international market requirements and preferences. In addition, our International segment sells a number of product categories, none of which is individually significant, that we do not sell domestically.
Surgical Implant Segment
Our Surgical Implant segment generated net sales of $53.2 million, $64.9 million, $62.7 million and $63.9 million for the nine months ended September 29, 2012 and the years ended December 31, 2011, 2010 and 2009, respectively. The following table summarizes our Surgical Implant segment product categories:
| Product Category |
Description | |
| Knee implants | Primary total joint replacement Revision total joint replacement Unicondylar joint replacement | |
| Hip implants | Primary replacement stems Acetabular cup system Revision joint replacement | |
| Shoulder implants | Primary total joint replacement Fracture repair system Revision total joint replacement (including reverse shoulder) |
Research and Development
Our research and development programs focus on the development of new products, as well as the enhancement of existing products with the latest technology and updated designs. We seek to develop new technologies to improve durability, performance and usability of existing products, and to develop our manufacturing process to improve product performance and reduce manufacturing costs. In addition to our own research and development, we receive new product and invention ideas from orthopedic surgeons and other healthcare professionals. We also seek to obtain rights to ideas we consider promising from a clinical and commercial perspective through entering into either assignment or licensing agreements.
86
Table of Contents
We conduct research and development programs at our facilities in Vista, California; Austin, Texas; and Ecublens, Switzerland. We spent $26.9 million, $21.9 million, and $23.5 million in 2011, 2010 and 2009, respectively, for research and development activities. As of December 31, 2011, we had approximately 30 employees in our research and development departments.
Marketing and Sales
Our products reach our customers, including hospitals and other healthcare facilities, physicians and other healthcare providers and end user patients, through several sales and distribution channels.
No particular customer or distributor accounted for 10% or more of product sales in any of our segments for the year ended December 31, 2011. Medicare and Medicaid together accounted for approximately 6.5% of our consolidated 2011 net sales.
Bracing and Vascular Segment
We market and sell our Bracing and Vascular segment products in several different ways. The DonJoy channel is primarily dedicated to the sale of our bracing and supports products to orthopedic surgeons, podiatrists, orthotic and prosthetic centers, hospitals, surgery centers, physical therapists, athletic trainers and other healthcare professionals. Certain DonJoy sales representatives also sell our Regeneration products. The DonJoy channel consists of approximately 270 independent commissioned sales representatives who are employed by approximately 35 independent sales agents and approximately 25 employed sales representatives. Because the DonJoy product lines generally require customer education in the application and use of the product, DonJoy sales representatives are technical specialists who receive extensive training both from us and the agent, and use their expertise to help fit the patient with the product and assist the orthopedic professional in choosing the appropriate product to meet the patient’s needs. After a sales representative receives a product order, we generally ship and bill the product directly to the orthopedic professional, and pay a sales commission to the agent. For certain custom rigid braces and other products, we sell directly to the patient and bill a third party payor, if applicable, on behalf of the patient. We enjoy long-standing relationships with most of our agents and sales representatives. Under the arrangements with the agents, each agent is granted an exclusive geographic territory for sales of our products and is not permitted to market products, or represent competitors who sell or distribute products, that compete with our existing products. The agents receive a commission, which varies based on the type of product being sold. If an agent fails to achieve specified sales quotas, we have the right to terminate our relationship with the agent.
The ProCare/Aircast channel consists of approximately 115 direct and independent sales representatives that manage approximately 610 distributors focused on selling our bracing and supports products to primary and acute care facilities. Eight vascular systems specialists are also included in this channel. Products in this channel are generally sold in non-exclusive territories to third party distributors as well as through our direct sales force. Our distributors include large, national third party distributors such as Owens & Minor Inc., McKesson/HBOC, Allegiance Healthcare and Physician Sales and Service Inc., regional medical and surgical distributors, outpatient surgery centers and medical products buying groups that consist of a number of healthcare providers who make purchases through the buying group. These distributors and our direct sales force generally sell our products to large hospital chains, primary care networks and orthopedic physicians for use by the patients. In addition, we sell our products through GPOs that are a preferred purchasing source for members of a buying group. With the exception of our vascular systems, products sold by our ProCare/Aircast channel generally do not require significant customer education for their use. Our vascular systems pumps and related equipment are typically consigned to hospitals, and the hospitals then purchase the cuffs that are applied to each patient.
Through our Dr. Comfort business, we market and distribute our therapeutic footwear and related medical and comfort products primarily through the podiatry, home medical equipment (“HME”), pharmacy, and orthotic and prosthetic (“O&P”) channels through our sales force of approximately 30 direct and independent sales representatives.
87
Table of Contents
Our OfficeCare business provides stock and bill arrangements for physician practices. Through OfficeCare, we maintain an inventory of bracing and supports products at approximately 1,500 orthopedic practices and other healthcare facilities for immediate distribution to patients. We then bill the patient or, if applicable, a third party payor. For certain facilities, we provide on-site technical representatives. The OfficeCare channel is managed by our DonJoy sales force.
Recovery Sciences Segment
We market and sell our Recovery Sciences segment products in several different ways. Through our Empi channel, we market our prescription-based home therapy products primarily to physicians and physical therapy clinics, which include hospital physical therapy departments, sports medicine clinics and pain management centers, through our sales force of approximately 200 direct and independent sales representatives. A physician such as an orthopedic surgeon generally prescribes our electrotherapy and orthotics products to patients. The physician will typically direct the patient to a physical therapy clinic to meet with a trained physical therapist who provides the patient with the prescribed product from our consigned inventory at the clinic. This sales process is facilitated by our relationships with third party payors, such as managed care organizations, who ultimately pay us for the products prescribed to patients. We currently have approximately 690 related managed care contracts. For these reasons, we view physical therapists, physicians and third party payors as key decision makers in product selection and patient referral. Our home therapy products generally are eligible for third party reimbursement by government payors, such as Medicare and Medicaid, and private payors.
Through our Regeneration channels, our non-union fracture bone growth stimulator devices (OL1000) are sold primarily by approximately 250 employed and independent sales representatives specially trained to sell the product. A few of our direct sales representatives and a network of independent spine product distributors sell the spine bone growth stimulator device (“SpinaLogic”). Most of our bone growth stimulator products are sold directly to the patient and a third party payor is billed, if applicable, on behalf of the patient.
Through our Chattanooga business, we sell our clinical rehabilitation product lines to physical therapy clinics, primarily through a national network of over 1,600 independent distributors, which are managed by our employed sales managers. These distributors sell our clinical rehabilitation products to a variety of healthcare professionals, including physical therapists, athletic trainers, chiropractors, and sports medicine physicians. Except for distributors outside of the United States, we do not maintain formal distribution contracts for our clinical rehabilitation products. These distributors purchase products from us at discounts off our published list price. We maintain an internal marketing and sales support program to support our distributor network. This program comprises a group of individuals who provide distributor and end-user training, develop promotional materials, and attend trade shows each year.
International Segment
We sell our products internationally through a network of wholly owned subsidiaries and independent distributors. In Europe, we use sales forces aggregating approximately 180 direct and independent salespersons and a network of independent distributors who call on healthcare professionals, as well as consumer retail stores, such as sporting equipment providers, and pharmacies, to sell our products.
We intend to continue to expand our direct and indirect distribution capabilities in attractive foreign markets. Recent examples of our strategy to expand our international sales are our 2010 acquisition of an independent South African distributor of DonJoy products, and our 2009 acquisitions of two independent Canadian distributors of Empi and Chattanooga products and an independent Australian distributor of DonJoy products. Our 2011 acquisitions of ETI and Dr. Comfort also increased our product offerings internationally.
Surgical Implant Segment
We currently market and sell the products of our Surgical Implant segment to hospitals and orthopedic surgeons through a network of approximately 170 independent commissioned sales representatives who are
88
Table of Contents
employed by approximately 45 sales agents. Generally, our independent sales representatives sell a range of reconstructive joint products, including our products. We usually enter into agreements with sales agents for a term of one to five years. Agents are typically paid a sales commission and are eligible for bonuses if sales exceed certain preset objectives. Our independent sales representatives work for these agents. We assign our sales agents to an exclusive sales territory. Substantially all of our sales agents agree not to sell competitive products. Typically, we can only terminate our agreements with sales agents prior to the expiration of the agreements for cause, which includes failure to meet specified periodic sales targets. We provide our sales agents with product inventories, on consignment, for their use in marketing and filling customer orders.
To a significant extent, sales of our surgical implant products depend on the preference of orthopedic surgeons. We maintain contractual relationships with orthopedic surgeons who assist us in developing our products and provide consulting services in connection with our products. In addition to providing design input into our new products, some of these orthopedic surgeons may give demonstrations using our products, speak about our products at medical seminars, train other orthopedic surgeons in the use of our products, and provide us with feedback on the acceptance of our products. We have also established relationships with surgeons who conduct clinical studies on various products, establish protocols for use of the products and participate at various symposia. Surgeons who assist us in developing our products are generally compensated with a royalty payment. We pay consulting surgeons fees for their services.
Manufacturing
We use both in-house manufacturing capabilities and relationships with third party vendors to supply our products. Generally, we use third party vendors only when they have special manufacturing capabilities or when we believe it is appropriate based on certain factors, including our in-house capacity, lead-time control and cost. Although we have certain sole source supply agreements, we believe alternate vendors are available, and we believe that adequate capacity exists at our current vendors to meet our anticipated needs.
Our manufacturing facilities are generally certified by the International Organization for Standardization (ISO) and generally comply with the U.S. Food and Drug Administration (the “FDA”) current Good Manufacturing Practice and Quality System Regulations (“QSRs”) requirements, which provide standards for safe and consistent manufacturing of medical devices and appropriate documentation of the manufacturing and distribution process. Many of our products carry the European Community Medical Device Directive (“CE”) certification mark.
Our manufacturing facility in Tijuana, Mexico is our largest manufacturing facility. Our Mexico facility has achieved ISO 9001 and ISO 13485 certification. These certifications are internationally recognized quality standards for manufacturing and assist us in marketing our products in certain foreign markets. Our Vista, California facility has achieved ISO 9001 certification, and certification to the Canadian Medical Device Regulation (ISO 13485) and the European Medical Device Directive. Products manufactured at the Vista, California facility include our custom rigid knee bracing products, the pump portion of our vascular systems products, and our Regeneration products. Products manufactured at our Tijuana, Mexico facility include most of our bracing and supports product lines, and our Chattanooga products including electrotherapy devices, patient care products, physical therapy treatment tables and CPM devices. Within both our Vista and Tijuana facilities, we operate vertically integrated manufacturing and cleanroom packaging operations and many subassemblies and components are produced in-house. These include metal stamped parts, injection molded components and fabric-strapping materials. We also have extensive in-house tool and die fabrication capabilities, which typically provide savings in the development of tools and molds as well as flexibility to respond to and capitalize on market opportunities as they are identified.
Our home electrotherapy devices sold in the United States and certain components and related accessories are manufactured at our Clear Lake, South Dakota facility. Manufacturing activities at the Clear Lake facility include electronic and mechanical assembly, electrode fabrication and assembly and fabric sewing processes. Our electrotherapy products comprise a variety of components, including die cast metal parts, injection molded
89
Table of Contents
plastic parts, printed circuit boards, electronic components, lead wires, electrodes and other components. Parts for these components are purchased from outside suppliers or, in certain instances, manufactured on a custom basis. Our Clear Lake facility has achieved the ISO 13485:2003 certification. Our home electrotherapy devices sold outside the United States are primarily manufactured by third party vendors.
Many of the component parts and raw materials we use in our manufacturing and assembly operations are available from more than one supplier and are generally available on the open market. We source some of our finished products from manufacturers in China as well as other third party manufacturers. We also currently purchase certain CPM devices from a single supply source, Medireha, which is 50% owned by us. Our distribution agreement with Medireha grants us exclusive rights to the distribution of products that Medireha manufactures. The distribution agreement also requires that we purchase a certain amount of product annually and that we seek Medireha’s approval if we choose to manufacture or distribute products that are identical or similar, or otherwise compete with the products that are the subject of the distribution agreement.
In our Surgical Implant segment, we manufacture our products in our Austin, Texas facility. This manufacturing facility includes computer controlled machine tools, belting, polishing, cleaning, packaging and quality control. Our Austin facility has achieved the ISO 13485:2003 certification. The primary raw materials used in the manufacture of our surgical implant products are cobalt chromium alloy, stainless steel alloys, titanium alloy and ultra high molecular weight polyethylene. All products in our Surgical Implant segment go through in-house quality control, cleaning and packaging operations.
Many of the products for our International segment are manufactured in the same facilities as our domestic segments. We operate a manufacturing facility in Tunisia that provides bracing and supports products for the French and other European markets. In addition, our Ormed and Cefar-Compex businesses source certain of the products they sell from third party suppliers. Cefar-Compex currently utilizes a single vendor for many of its home electrotherapy devices.
Intellectual Property
We own or have licensing rights to U.S. and foreign patents covering a wide range of our products and have filed applications for additional patents. We have numerous trademarks registered in the United States, a number of which are also registered in countries around the world. We also assert ownership of numerous unregistered trademarks, some of which have been submitted for registration in the United States and foreign countries. In the future, we will continue to apply for such additional patents and trademarks as we deem appropriate. Additionally, we seek to protect our non-patented know-how, trade secrets, processes and other proprietary confidential information, through a variety of methods; including having our vendors, employees and consultants sign invention assignment agreements, proprietary information agreements and confidentiality agreements and having our independent sales agents and distributors sign confidentiality agreements. Because many of our products are regulated, proprietary information created during our development of a new or improved product may have to be disclosed to the FDA or another U.S. or foreign regulatory agency in order for us to have the lawful right to market such product. We have distribution rights for certain products that are manufactured by others and hold both exclusive and nonexclusive licenses under third party patents and trade secrets that cover some of our existing products and products under development.
The validity of any of the patents or other intellectual property owned by or licensed to us may not be upheld if challenged by others in litigation. Due to these and other risks described in this Prospectus, we do not rely solely on our patents and other intellectual property to maintain our competitive position. We believe that the development and marketing of new products and improvement of existing ones is, and will continue to be, more important to our competitive position than relying solely on existing products and intellectual property.
Competition
The markets we compete in are highly competitive and fragmented. Some of our competitors, either alone or in conjunction with their respective corporate parent groups, have greater research and development, sales and
90
Table of Contents
marketing, and manufacturing capabilities than we do, and thus may have a competitive advantage over us. Although we believe that the design and quality of our products compare favorably with those of our competitors, if we are unable to offer products with the latest technological advances at competitive prices, our ability to compete successfully could be materially adversely affected.
Given our sales history, our history of product development and the experience of our management team, we believe we are capable of effectively competing in our markets in the future. Further, we believe the comprehensive range of products we offer enables us to reach a diverse customer base and to use multiple distribution channels in an attempt to increase our growth across our markets. In addition, we believe the various company and product line acquisitions we have made in recent years continue to improve the name recognition of our company and our products. Our ability to compete is affected by, among other things, our ability to:
| • | develop new products and innovative technologies, |
| • | obtain regulatory clearance and compliance for our products, |
| • | manufacture and sell our products cost-effectively, |
| • | meet all relevant quality standards for our products and their markets, |
| • | respond to competitive pressures specific to each of our geographic markets, including our ability to enforce non-compete agreements, |
| • | protect the proprietary technology of our products and manufacturing processes, |
| • | market our products, |
| • | attract and retain skilled employees and sales representatives, and |
| • | establish and maintain distribution relationships. |
All of our segments compete with large, diversified corporations and companies that are part of corporate groups that have significantly greater financial, marketing and other resources than we do, as well as numerous smaller niche companies.
Bracing and Vascular Segment
Our primary competitors in the rigid knee bracing market include companies such as Össur hf., Orthofix International, N.V. (“Orthofix”), Bledsoe Brace Systems (“Bledsoe”), and Townsend Design. Competition in the rigid knee brace market is primarily based on product technology, quality and reputation, relationships with customers, service and price.
In the soft goods products market, our competitors include Biomet Inc. (“Biomet”), DeRoyal Industries, Össur hf. and Zimmer Holdings, Inc. (“Zimmer”). In the cold therapy products market, our competitors include Orthofix, Bledsoe and Stryker Corporation (“Stryker”). Competition in the soft goods and pain management markets is less dependent on innovation and technology and is primarily based on product range, quality, service and price.
Our primary competitor in the dynamic splinting market is Dynasplint Systems, Inc.
The therapeutic footwear and related medical and comfort products market is highly fragmented with multiple channels, such as DPM, HME, O&P, retail pharmacy and numerous other service categories. Our competitors include several multi-product companies and numerous smaller niche competitors. Competition in the therapeutic footwear market tends to be based on product technology, quality and reputation, relationships with customers, service and price.
Several competitors have initiated stock and bill programs similar to our OfficeCare program, and there are numerous regional stock and bill competitors.
91
Table of Contents
Recovery Sciences Segment
The primary competitors of our Empi and Chattanooga products are Dynatronics Corporation, Mettler Electronics Corporation, Rich-Mar, Patterson Medical, Enraf-Nonius, Gymna-Uniphy, Acorn Engineering, International Rehabilitation Sciences, Inc. (d/b/a RS Medical) and Care Rehab. The physical therapy products market is highly competitive and fragmented. Our competitors in the CPM devices market include several multi-product companies with significant market share and numerous smaller niche competitors. Competition in these markets is based primarily on the quality and technical features of products, product pricing and contractual arrangements with third party payors and national accounts.
Our competitors for Regeneration products are large, diversified orthopedic companies. In the non-union bone growth stimulation market, our competitors include Orthofix, Biomet and Smith & Nephew plc (Smith & Nephew), and in the spinal fusion market, we compete with Biomet and Orthofix. Competition in bone growth stimulation devices is limited as higher regulatory thresholds provide a barrier to market entry.
International Segment
Competition for the products in our International segment arises from many of the companies and types of companies that compete with our domestic segments and from foreign manufacturers whose costs may be lower due to their ability to manufacture products within their respective countries. Competition is based primarily on quality, innovative design and technical capability, breadth of product line, availability of and qualification for reimbursement, and price.
Surgical Implant Segment
The market for orthopedic products similar to those produced by our surgical implant business is dominated by a number of large companies, including Biomet, DePuy, Inc. (a Johnson & Johnson company), Smith & Nephew, Stryker, and Zimmer, which are much larger and have significantly greater financial resources than we do. Our Surgical Implant segment also faces competition from U.S.-based companies similar in size to ours, such as Wright Medical Group, Inc. and Exactech, Inc. Competition in the market in which our Surgical Implant segment participates is based primarily on price, quality, innovative design and technical capability, breadth of product line, scale of operations and distribution capabilities. Our current and future competitors may have greater resources, more widely accepted and innovative products, less-invasive therapies, greater technical capabilities, and stronger name recognition than we do.
Government Regulation
FDA and Similar Foreign Government Regulations
Our products are subject to rigorous government agency regulation in the United States and in other countries. In the United States, the FDA regulates the development, testing, labeling, manufacturing, storage, recordkeeping, pre-market clearance or approval, promotion, distribution and marketing of medical devices to ensure that medical products distributed in the United States are safe and effective for their intended uses. The FDA also regulates the export of medical devices manufactured in the United States to international markets. Our medical devices are subject to such FDA regulation.
Under the Food, Drug and Cosmetic Act, as amended, medical devices are generally classified into one of three classes depending on the degree of risk to patients using the device. Class I is the lowest risk classification. Class I devices are those for which safety and effectiveness can be assured by adherence to General Controls, which include compliance with FDA QSRs, facility and device registrations and listings, reporting of adverse medical events, and appropriate truthful and non-misleading labeling, advertising, and promotional materials. Most Class I devices are exempt from pre-market submission requirements. Some Class I devices require a pre-market notification to and clearance from FDA as set forth under § 510(k) of the Food, Drug and Cosmetic Act, as amended, also known as a “510(k)” submission. The 510(k) process is described more fully below.
92
Table of Contents
Class II devices are subject to General Controls, as well as pre-market demonstration of adherence to certain performance standards or other special controls as specified by the FDA. Although some Class II medical devices are exempt from 510(k) requirements, most Class II devices are subject to 510(k) review and clearance by FDA prior to marketing.
By way of 510(k) submission, a manufacturer provides certain required information to the FDA to establish that the device is “substantially equivalent” to a device that was legally marketed prior to May 28, 1976, the date upon which the Medical Device Amendments of 1976 were enacted. A device legally marketed before May 28, 1976 is called a “pre-amendment device.” A manufacturer may also obtain marketing clearance by showing that its medical device is substantially equivalent to a commercially available “post-amendment device” which is a device cleared through the 510(k) process after May 28, 1976. Upon establishment of such substantial equivalence, the FDA may grant clearance to commercially market the device. If the FDA determines that the device, or its intended use, is not “substantially equivalent,” the FDA will automatically place the device into Class III.
A Class III device is a product that has a new intended use or is based on technology that is not substantially equivalent to a use or technology of a legally marketed device and for which the safety and effectiveness of the device cannot be assured solely by the General Controls, performance standards and special controls applied to Class I and II devices. These devices generally require clinical trials involving human subjects to assess their safety and effectiveness. A Pre-Market Application (“PMA”) must be submitted to and approved by the FDA before the manufacturer of a Class III product can proceed in marketing the product. The PMA process is much more extensive and takes longer than the 510(k) process. In order to obtain approval of a PMA, the manufacturer generally must first conduct clinical trials of a Class III device for its intended use pursuant to an FDA-approved Investigational Device Exemption (“IDE”) application. An IDE allows the manufacturer to test an unapproved device in a clinical study for a specific intended use in order to collect safety and effectiveness data to support a PMA application or a 510(k) submission to the FDA. The PMA process can take up to several years. In approving a PMA application, the FDA may require additional clinical data and may also require some form of post-market surveillance or clinical study whereby the manufacturer follows certain patient groups for a number of years, making periodic reports to the FDA on the clinical status of those patients.
Our products include both pre-amendment and post-amendment Class I, II and III medical devices. All our currently marketed devices are either exempt from the FDA clearance and approval process (based on our interpretation of those regulations) or we have obtained the requisite clearances or approvals (including all modifications, amendments and changes), as appropriate, required under federal medical device law. The FDA may disagree with our conclusion that clearances or approvals were not required for specific products and may require clearances or approval for such products. In these circumstances, we may be required to cease distribution of the product, the devices may be subject to seizure by the FDA or to a voluntary or mandatory recall, and we could be subject to significant fines and penalties.
The FDA has asked the Institute of Medicine (the “IOM”) to conduct a two-year study of the clearance process for devices under § 510(k) of the Food Drug, and Cosmetic Act, as amended, and to provide recommendations for changes, if necessary. The IOM report “Medical Devices and the Public Health, the FDA 510(k) Clearance Process at 35 Years,” was released July 29, 2011. Recently, the FDA also completed an internal review of the 510(k) clearance process, and issued a report with recommendations that include: streamlining the de novo reclassification process, issuing more guidance to provide greater clarity about the 510(k) program, improving training for Center for Devices and Radiological Health (CDRH) staff and industry, making greater use of external experts, and making process improvements within CDRH, such as establishing a Center Science Council. Based on these recommendations, CDRH is expected to explore the feasibility of requiring manufacturers to provide regular, periodic updates of device modifications; consider requiring 510(k) submitters to provide a list and brief description of all scientific information related to the safety and effectiveness of a new device known or reasonably known to the submitter; issue guidance to clarify when manufacturing data should be submitted as part of a 510(k); and clarify when it will withhold clearance for failure to comply with good
93
Table of Contents
manufacturing practices (i.e., when FDA will conduct a pre-clearance inspection). FDA issued draft guidance on December 27, 2011 clarifying the circumstances under which it is appropriate to use multiple predicate devices to demonstrate substantial equivalence, a practice FDA supports.
The recommended, expected and completed FDA actions could lead to changes in the review, including the length of review of medical device products seeking clearance for marketing. Many of our products are cleared for marketing under the 510(k) process. If we begin to have significant difficulty obtaining such FDA approvals or clearances in a timely manner or at all, it could have a material adverse impact on our revenues and growth.
Our manufacturing processes are also required to comply with the FDA’s current Good Manufacturing Practice requirements for medical devices, which are specified in FDA QSRs. The QSRs cover the methods and documentation of the design, testing, production processes, control, quality assurance, labeling, packaging and shipping of our products. Furthermore, our facilities, records and manufacturing processes are subject to periodic unscheduled inspections by the FDA and other agencies. Failure to comply with applicable QSR or other U.S. medical device regulatory requirements could result in, among other things, warning letters, fines, injunctions, civil penalties, repairs, replacements, refunds, recalls or seizures of products, total or partial suspensions of production, refusal of the FDA to grant future pre-market clearances or PMA approvals, withdrawals or suspensions of current clearances or approvals, and criminal prosecution. We are also required to report to the FDA if our products cause or contribute to death or serious injury or malfunction in a way that would likely cause or contribute to death or serious injury were the malfunction to recur; the FDA or other agencies may require the recall of products in the event of material defects or deficiencies in design or manufacturing. The FDA can also withdraw or limit our product approvals or clearances in the event of serious unanticipated health or safety concerns.
In the third quarter of 2009, we received a Form FDA-483 “Inspectional Observations” in connection with an FDA inspection of our Surgical Implant segment, stating that: (1) we failed to follow our standard operating procedures to ensure that the designs of certain products were correctly transferred into production; (2) we failed to adequately analyze certain quality data to identify existing and potential causes of nonconforming product and quality problems, resulting in disposal or reworking of certain nonconforming parts in the later stages of our production processes; (3) our complaint handling procedures were not well defined to ensure that all complaints are processed in a uniform and timely manner; and (4) we failed to follow our standard operating procedures related to procurement to minimize receipt of nonconforming materials from suppliers. We promptly implemented corrective actions that we believe adequately address each Inspectional Observation and submitted a timely response to the FDA. We have not received any further communications from the FDA regarding this inspection and the Inspectional Observations. We cannot assure you that the FDA will not take further action in the future, however.
The State of California Health and Human Services, Food and Drug Branch (“FDB”) inspected our Vista manufacturing site in October 2010, and issued a Notice of Violation for this site stating that: (1) the type and extent of control to be exercised over suppliers was not clearly defined in our written standard operating procedures; (2) lack of evidence that certain employees had been adequately been trained on certain specific work instructions; and (3) certain corrective and preventive actions taken had not been verified or validated to ensure that the action was effective and did not adversely affect the finished device. We promptly implemented corrective and preventive actions that we believe are acceptable to the FDB. We have notified the FDB that this has occurred and we have not received any information from the FDB indicating objection to the remedial action taken.
In the third quarter of 2011, we received a Form FDA-483 “Inspectional Observations” in connection with an FDA inspection of our Vista manufacturing site, stating that (1) we did not take any action when certain testing equipment used during the manufacturing process was reported to be out of tolerance, and no equipment calibration deviation form was completed to document the calibration deviation, nor was there an evaluation of product impact according to internal procedures; (2) procedures to ensure that all purchased or otherwise received product and services conform to specified requirements have not been adequately established; (3) we
94
Table of Contents
did not submit an MDR report within 30 days of receiving or otherwise becoming aware of additional information that reasonably suggested that one of our knee braces may have caused or contributed to a serious injury; and (4) a supplemental MDR report was not submitted to FDA within one month following receipt of information that was not provided when the initial report was submitted. During the close of the inspection, all Inspectional Observations were annotated as “corrected” by the FDA. We have not received any further communications from the FDA regarding this inspection or the Inspectional Observations.
Even if regulatory approval or clearance of a medical device is granted, the FDA may impose limitations or restrictions on the use and indications for which the device may be labeled or promoted. Medical devices may be marketed only for the uses and indications for which they are cleared or approved. FDA regulations prohibit a manufacturer from promotion for an unapproved or off-label use.
The FDA has broad regulatory and enforcement powers. If the FDA determines we have failed to comply with applicable regulatory requirements, it can impose a variety of enforcement actions, from warning letters, fines, injunctions, consent decrees, and civil penalties, to suspension or delayed issuance of applications, seizure or recall of our products, total or partial shutdowns, withdrawals of approvals or clearances already granted, and criminal prosecution. The FDA can also require us to repair, replace, or refund the costs of devices we manufactured or distributed.
We must obtain export certificates from the FDA before we can export certain of our products. We are also subject to extensive regulations that are similar to those of the FDA in many of the foreign countries in which we sell our products, including those in Europe, our largest foreign market. These include product standards, packaging requirements, labeling requirements, import restrictions, tariff regulations, duties and tax requirements. The regulation of our products in the European Economic Area (which consists of the twenty-seven member states of the European Union, as well as Iceland, Liechtenstein and Norway) is governed by various directives and regulations promulgated by the European Commission and national governments. Only medical devices that comply with certain conformity requirements are allowed to be marketed within the European Economic Area. In addition, the national health or social security organizations of certain countries, including certain countries outside Europe, require our products to be qualified before they can be marketed in those countries.
We have also implemented policies and procedures allowing us to position ourselves for the changing international regulatory environment. Our international surgical implant activities received an ISO 13485:2003 certification for its facilities and an EC Certificate for its many products. Receiving ISO 13485:2003 certification assists us in meeting international regulatory requirements to allow for export of products to Japan, countries in Europe, Australia and Canada. Our international surgical implant activities have also met the requisites for the Canadian Medical Device Requirements. Our International segment has received ISO 9001 certification, EN46001 certification and certification to the Canadian Medical Device Regulation (ISO 13485) and the European Medical Device Directive.
Third Party Reimbursement
Our home therapy products, rigid knee braces, Regeneration products, and certain of our soft goods are generally prescribed by physicians and are eligible for third party reimbursement by government payors, such as Medicare and Medicaid, and private payors. Customer selection of our products depends, in part, on coverage of our products and whether third party payment amounts will be adequate. We believe that Medicare and other third party payors will continue to focus on measures to contain or reduce their costs through managed care and other methods. Medicare policies are important to our business because private payors often model their policies after the Medicare program’s coverage and reimbursement policies.
In recent years, Congress has enacted a number of laws that affect Medicare reimbursement for and coverage of durable medical equipment (“DME”), prosthetics, orthotics and supplies (“DMEPOS”), including many of our products. These laws have included temporary freezes or reductions in Medicare fee schedule
95
Table of Contents
updates. Most recently, in March 2010, President Obama signed into law the Patient Protection and Affordable Care Act, which was amended by a second bill signed into law on March 30, 2010, known as the Health Care and Education Reconciliation Act (collectively referred to as the “Affordable Care Act” or “ACA”). The ACA is a sweeping measure designed to expand access to affordable health insurance, control health care spending, and improve health care quality. Several provisions of the ACA specifically impact the medical equipment industry. Among other things, the ACA eliminates the full inflation update to the DMEPOS fee schedule for the years 2011 through 2014. Instead, beginning in 2011, the ACA reduces the inflation update for DMEPOS by a “productivity adjustment” factor intended to reflect productivity gains in delivering health care services. For 2012, the inflation update is 3.6% and the productivity adjustment is 1.2%, resulting in a 2.4% update factor.
Medicare payment for DMEPOS also can be impacted by the DMEPOS competitive bidding program, under which Medicare rates are based on bid amounts for certain products in designated geographic areas, rather than the Medicare fee schedule amount. Only those suppliers selected through the competitive bidding process within each designated competitive bidding area (“CBA”) are eligible to have their products reimbursed by Medicare. Competitive bidding went into effect January 1, 2011 in nine CBA’s and nine product categories, with reimbursement to contract suppliers averaging 32% below the Medicare DMEPOS fee schedule amount. Bidding for the second round of competitive bidding is underway in 100 CBAs (in addition to national mail order competition for diabetic testing supplies). While none of our products is included in the first two rounds, there is no assurance they will not be included in the future. The Centers for Medicare & Medicaid Services (“CMS”) recently released a listing of codes that it considers to be off-the-shelf (“OTS”) orthotics and subject to competitive bidding in the future. Should our products be subject to competitive bidding, if we are not selected as a contract supplier in a particular region or if contract prices are significantly below Medicare fee schedule reimbursement levels, it could have a material adverse impact on our sales and profitability. Further, the ACA requires the Secretary to use competitive bidding payment information to adjust DMEPOS payments in areas outside of competitive bidding areas beginning in 2016. Additional reforms to Medicare DMEPOS payment amounts are proposed periodically. Any changes in the basis for Medicare reimbursement of our products could have a material adverse impact on our results of operations.
On June 8, 2012, the Centers for Medicare & Medicaid Services (“CMS”) issued a final decision memorandum in which CMS determined that coverage for transcutaneous electrical nerve stimulation (“TENS”) for the treatment of chronic low back pain (other than chronic low back pain due to certain diseases) would not be available under Medicare unless the patient is enrolled in a clinical study meeting certain defined standards. On August 3, 2012, CMS published a National Coverage Determination and listed the diagnostic codes for TENS prescriptions that will no longer be covered under Medicare outside of a clinical study. Our Empi business unit in our Recovery Sciences Segment provides TENS devices that are prescribed for patients to use in the home (and for patients with diagnoses that fall within one of the diagnostic codes for which Medicare coverage is no longer available). As a result, this coverage decision by CMS will have the effect of reducing the available market for these TENS devices. In addition to eliminating a common application for TENS for Medicare beneficiaries, this coverage policy may be adopted by some or all of the private health insurers with which Empi does business. We have no plans to conduct a clinical study at this time. We are in the process of adapting our business procedures to reflect this coverage policy and to cease billing Medicare for TENS units prescribed for the applicable diagnostic codes. We also are in the process of determining how and when this coverage position may impact available reimbursement for supplies provided by our Empi business unit to Medicare beneficiaries in connection with TENS devices prescribed to those beneficiaries prior to the CMS decision. While the full effect of this coverage decision will not be known for some time, it could reduce the revenue of our Empi business for Medicare billing of TENS and related supplies by an estimated $9 million to $14 million annually, and over time by a similar or greater amount if the Medicare coverage policy is adopted by private insurers. We believe Empi can offset, at least partially, the impact of these revenue losses by (i) offering these devices as self-pay items to Medicare beneficiaries, (ii) convincing private insurers that they should continue to cover TENS because of its effectiveness for chronic low back pain, its ability to reduce the use of pharmaceuticals and its favorable impact on health economics, (iii) focusing on growth initiatives related to other TENS indications for which reimbursement is available and on other Empi products and (iv) adjusting operating expenses where possible.
96
Table of Contents
Nevertheless, the long-term revenue and profit impact of this coverage decision could be materially adverse to the Empi business and as a result could have an adverse effect on our business and results of operations as a whole.
Medicare suppliers must meet a variety of program criteria. Medicare DMEPOS suppliers (other than certain exempted professionals) must be accredited by an approved accreditation organization as meeting DMEPOS Quality Standards adopted by CMS, including specific requirements for suppliers of custom fabricated and custom fitted orthoses and certain prosthetics. The portion of our business serving in a Medicare supplier capacity has been accredited. Most Medicare DMEPOS suppliers also must post a $50,000 surety bond from an authorized surety, with higher amounts required for certain “high-risk” suppliers. We believe we are in compliance with current surety bond requirements. If in the future we fail to maintain our Medicare accreditation status and/or do not comply with Medicare surety bond or supplier standard requirements, or if these requirements are expanded or if additional conditions for coverage or payment are adopted in the future, it could adversely impact our profits and results of operation.
Likewise, Medicare establishes standards that items must meet to qualify as DME. In November 2011, CMS finalized a rule to establish a 3-year minimum lifetime requirement for an item or device to be considered “durable” under the Medicare DME benefit category. Items already categorized as DME on the date of this new standard are exempted from the minimum lifetime requirement. Certain items of DME also are subject to verification by Medicare’s contractors that they meet the standards for particular DMEPOS product codes. Failure of our products to meet applicable standards could adversely impact our business.
In October 2011, CMS proposed allowing Medicare Advantage managed care plans to limit coverage of DME to specific manufacturers or brands. This rule has not yet been finalized. If adopted and our products were subject to selective contracting, unless we are successful in competing for such contracts, the use of our products by Medicare Advantage plan enrollees could be restricted, which could have an adverse impact on our profitability.
The ACA imposes a new annual federal excise tax on certain medical device manufacturers and importers. Specifically, for sales on or after January 1, 2013, manufacturers, producers, and importers of taxable medical devices must pay as an excise tax 2.3% of the price for which the devices are sold. Treasury regulations governing, among other things, the specific medical devices that will be subject to the tax are in proposed form subject to public comment, and the impact of this excise tax on the Company is not yet certain.
The ACA also establishes new Medicare and Medicaid program integrity provisions, including expanded documentation requirements for Medicare DMEPOS orders and more stringent procedures for screening Medicare and Medicaid DMEPOS suppliers, along with broader expansion of federal fraud and abuse authorities. On February 2, 2011, CMS published a final rule implementing the ACA provider and supplier screening provisions, effective March 25, 2011. Under the final rule, DMEPOS suppliers could be subject to verification of compliance with enrollment and licensure requirements, database checks, unannounced site visits, and, for newly-enrolling suppliers, fingerprint-based criminal history record checks of law enforcement repositories. The rule also imposes application fees on providers and suppliers; authorizes CMS and states to impose moratoria on new provider enrollment to protect against a high risk of fraud; authorizes the suspension of payments pending an investigation of a credible allegation of fraud; and expands health program termination authority. There can be no assurances that the new policy will not increase compliance costs or otherwise adversely impact our results of operation.
In addition, the ACA establishes new disclosure requirements regarding financial arrangements between medical device and supply manufacturers and physicians, including physicians who serve as consultants, effective March 31, 2013. CMS has proposed regulations to implement the new requirement, but the policy has not yet been finalized. A number of states also have enacted specific marketing and payment disclosure requirements and other states may do so in the future. Likewise, in recent years, voluntary industry guidelines have been adopted regarding device manufacturer financial arrangements with physicians and other health care
97
Table of Contents
professionals. We cannot determine at this time the impact, if any, of such requirements or voluntary guidelines on our relationships with surgeons, but there can be no assurances that such requirements and guidelines would not impose additional costs on us and/or adversely affect our consulting and other arrangements with surgeons.
On August 27, 2010, CMS published a final rule that, among other things, prohibits suppliers from sharing a practice location in certain circumstances, imposes new physical facility requirements on suppliers, clarifies the prohibition on the direct solicitation of Medicare beneficiaries, generally prohibits suppliers from contracting with another individual to perform licensed services, and clarifies a number of other supplier operational requirements. The rule generally is effective September 27, 2010 (although there are separate deadlines for compliance with the physical facility standards for existing suppliers with leases that expire after that date). We believe we are in compliance with the requirements of the new rule.
In response to pressure from certain groups (primarily orthotists), the United States Congress and state legislatures have periodically considered proposals that limit the types of orthopedic professionals who can fit or sell our orthotic device products or who can seek reimbursement for them. Several states have adopted legislation which imposes certification or licensing requirements on the measuring, fitting and adjusting of certain orthotic devices. Although some of these state laws exempt manufacturers’ representatives, other states’ laws subject the activities of such representatives to certification or licensing requirements. The state of Texas has adopted such a licensure law without an exemption for manufacturer’s sales representatives acting under the supervision of a physician and has issued a cease and desist letter directed to the fitting activities of our sales representatives in that state. We are in communication with the Texas authorities to respond to such letter. Additional states may be considering similar legislation. Such laws could reduce the number of potential customers by restricting the activities of our sales representatives in jurisdictions where such policies are enacted. Furthermore, because the sales of orthotic devices are driven in part by the number of professionals who fit and sell them, laws that limit these activities potentially could reduce demand for these products. We may not be successful in opposing the adoption of such legislation or regulations and, therefore, such laws could have a material adverse impact on our business.
In addition, efforts have been made to establish similar requirements at the federal level for the Medicare program. For example, in 2000, Congress passed the Medicare, Medicaid and SCHIP Benefits Improvement and Protection Act of 2000 (BIPA). BIPA contained a provision requiring, as a condition for payment by the Medicare program, that certain certification or licensing requirements be met for individuals and suppliers furnishing certain, but not all, custom-fabricated orthotic devices. Although CMS has not implemented this requirement to date, Medicare follows state requirements in those states that require the use of an orthotist or prosthetist for furnishing of orthotics or prosthetics. We cannot predict the effect of implementation of BIPA or implementation of other such laws will have on our business.
Our business also can be impacted by changes in state health care legislative and regulatory policies being adopted as a result of state budgetary shortfalls. These changes have included reductions in provider and supplier reimbursement levels under state Medicaid programs, including in some cases reduced reimbursement for DMEPOS items, and/or other Medicaid coverage restrictions. In addition, on February 13, 2012, President Obama released his proposed federal fiscal year 2013 budget, which would, if enacted, reduce federal reimbursement to states for their Medicaid DME expenditures by basing aggregate reimbursement on what the federal government would have paid under the Medicare DMEPOS competitive bidding program. While the proposal requires Congressional approval, if enacted it is expected to reduce Medicaid reimbursement for DME by $3 billion over ten years. As states continue to face significant financial pressures, it is possible that state health policy changes will adversely affect our profitability.
Our international sales also depend in part upon the eligibility of our products for reimbursement through third party payors, the amount of reimbursement and the allocation of payments between the patient and third party payors. Reimbursement practices vary significantly by country, with certain countries requiring products to undergo a lengthy regulatory review in order to be eligible for third party reimbursement. In addition, healthcare cost containment efforts similar to those we face in the United States are prevalent in many of the foreign countries in
98
Table of Contents
which our products are sold, and these efforts are expected to continue in the future, possibly resulting in the adoption of more stringent reimbursement standards. For example, in Germany, our largest foreign market, new regulations generally require adult patients to pay a portion of the cost of each medical technical device prescribed. This may adversely affect our sales and profitability by making it more difficult for patients in Germany to pay for our products. Any developments in our foreign markets that eliminate, reduce or materially modify coverage of, and reimbursement rates for, our products could have an adverse impact on our ability to sell our products.
Fraud and Abuse
We are subject to various federal and state laws and regulations pertaining to healthcare fraud and abuse. Violations of these laws are punishable by criminal and civil sanctions, including, in some instances, exclusion from participation in federal and state healthcare programs, including Medicare, Medicaid, Veterans Administration health programs and TRICARE (the health care program for active duty military, retirees and their families managed by the Department of Defense). We have no reason to believe that our operations are not in material compliance with such laws. However, because these laws and regulations are broad in scope and may change, we may be required to alter one or more of our practices to be in compliance with these laws. In addition, the occurrence of one or more violations of these laws or regulations, a challenge to our operations by a governmental authority under these laws or regulations or a change in the laws or regulations may have a material adverse impact on our financial condition and results of operations.
Anti-Kickback and Other Fraud Laws
Our operations are subject to federal and state anti-kickback laws. Certain provisions of the Social Security Act, commonly referred to as the Anti-Kickback Statute, prohibit persons from knowingly and willfully soliciting, receiving, offering or providing remuneration directly or indirectly to induce either the referral of an individual, or the furnishing, recommending, or arranging for a good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid. The definition of remuneration has been broadly interpreted to include anything of value, including such items as gifts, discounts, waiver of payments, and providing anything at less than its fair market value. The U.S. Department of Health and Human Services (“HHS”) has issued regulations, commonly known as safe harbors, which set forth certain conditions, which if fully met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. Although full compliance with these provisions ensures against prosecution under the Anti-Kickback Statute, the failure of a transaction or arrangement to fit within a specific safe harbor does not necessarily mean that the transaction or arrangement is illegal or that prosecution under the Anti-Kickback Statute will be pursued. The penalties for violating the Anti-Kickback Statute include imprisonment for up to five years, fines of up to $25,000 per violation and possible exclusion from federal healthcare programs such as Medicare and Medicaid. Many states have adopted prohibitions similar to the Anti-Kickback Statute, some of which apply to the referral of patients for healthcare services reimbursed by any source, not only by the Medicare and Medicaid programs.
Recently, certain manufacturers of implant products entered into monetary settlement agreements, corporate integrity agreements and deferred prosecution agreements with the U.S. Department of Justice (“DOJ”) based upon allegations that, among other things, they entered into a variety of consulting and other agreements with physicians as improper inducements to those physicians to use the manufacturers’ products in violation of the federal Anti-Kickback Statute. We believe that remuneration paid to surgeons with whom we have agreements represents fair market value for legitimate designing, consulting and advisory services rendered on our behalf.
Our OfficeCare program is a stock and bill arrangement through which we make products and services available in the offices of physicians or other providers. In conjunction with the OfficeCare program, we may pay participating physicians a fee for rental space and support services provided by such physicians to us. In a February 2000 Special Fraud Alert, the Office of Inspector General (“OIG”) indicated that it may scrutinize stock and bill programs involving excessive rental payments or rental space for possible violation of the Anti-Kickback
99
Table of Contents
Statute, but noted that legitimate arrangements, including fair market value rental arrangements, will not be considered violations of the statute. We believe that we have structured our OfficeCare program to comply with the Anti-Kickback Statute.
HIPAA
The Health Insurance Portability and Accountability Act of 1995 (“HIPAA”) created two new federal crimes effective as of August 21, 1996, relating to healthcare fraud and false statements regarding healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing or attempting to execute a scheme or artifice to defraud any healthcare benefit program, including private payors. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation in connection with the delivery of or payment for healthcare benefits, items or services. HIPAA applies to any healthcare benefit plan, not just Medicare and Medicaid. Additionally, HIPAA granted expanded enforcement authority to HHS and the DOJ and provided enhanced resources to support the activities and responsibilities of the HHS, OIG and DOJ by authorizing large increases in funding for investigating fraud and abuse violations relating to healthcare delivery and payment. In addition, HIPAA mandates the adoption of standards for the electronic exchange of health information, as described below in greater detail under “Federal Privacy and Transaction Law and Regulations.”
Physician Self-Referral Laws
We may also be subject to federal and state physician self-referral laws. Federal physician self-referral legislation, commonly known as the Stark Law, prohibits, subject to certain exceptions, physician referrals of Medicare and Medicaid patients to an entity providing certain “designated health services” if the physician or an immediate family member of the physician or a physician organization in which the physician participates has any financial relationship with the entity. DME and orthotics are included as designated health services. The Stark Law also prohibits the entity receiving the referral from billing any good or service furnished pursuant to an unlawful referral, and any person collecting any amounts in connection with an unlawful referral is obligated to refund such amounts. A person who engages in a scheme to circumvent the Stark Law’s referral prohibition may be fined up to $100,000 for each such arrangement or scheme. The penalties for violating the Stark Law also include civil monetary penalties of up to $15,000 per referral and possible exclusion from federal healthcare programs such as Medicare and Medicaid. Various states have corollary laws to the Stark Law, including laws that require physicians to disclose any financial interest they may have with a healthcare provider to their patients when referring patients to that provider. Both the scope and exceptions for such laws vary from state to state.
False Claims Laws
Under multiple state and federal statutes, submissions of claims for payment that are “not provided as claimed” may lead to civil money penalties, criminal fines and imprisonment and/or exclusion from participation in Medicare, Medicaid and other federally funded state health programs. These false claims statutes include the federal False Claims Act, which prohibits the knowing filing of a false claim or the knowing use of false statements to obtain payment from the federal government. When an entity is determined to have violated the False Claims Act, it must pay three times the actual damages sustained by the government, plus mandatory civil penalties of between $5,500 and $11,000 for each separate false claim. Suits filed under the False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government and such individuals, commonly known as whistleblowers, may share in any amounts paid by the entity to the government in fines or settlement. In addition, certain states have enacted laws modeled after the federal False Claims Act. Qui tam actions have increased significantly in recent years, causing greater numbers of healthcare companies to have to defend a false claim action, pay fines or be excluded from Medicare, Medicaid or other federal or state healthcare programs as a result of an investigation arising out of such action. A number of states have enacted false claims acts that are similar to the federal False Claims Act.
The federal government has used the federal False Claims Act to prosecute a wide variety of alleged false claims and fraud allegedly perpetrated against Medicare and state healthcare programs. The government and a
100
Table of Contents
number of courts also have taken the position that claims presented in violation of certain other statutes, including the federal Anti-Kickback Statute or the Stark Law, can be considered a violation of the federal False Claims Act, based on the theory that a provider impliedly certifies compliance with all applicable laws, regulations and other rules when submitting claims for reimbursement.
On May 20, 2009, President Obama signed into law the Fraud Enforcement and Recovery Act of 2009 (“FERA”). Among other things, FERA modifies the federal False Claims Act by expanding liability to contractors and subcontractors who do not directly present claims to the federal government. FERA also expands False Claims Act liability for what is referred to as a “reverse false claim” by explicitly making it unlawful to knowingly conceal or knowingly and improperly avoid or decrease an obligation owed to the federal government. FERA also seeks to clarify that liability exists for attempts to avoid repayment of overpayments, including improper retention of federal funds. FERA also expands the government’s ability to use the Civil Investigative Demand process to investigate defendants, and permits government complaints in intervention to relate back to the filing of the whistleblower’s original complaint. FERA is likely to increase both the volume and liability exposure of False Claims Act cases brought against healthcare entities.
Additional fraud and abuse measures were adopted as part of the ACA. Specifically, the ACA increases funding for program integrity initiatives, modifies screening procedures for providers and suppliers before and after granting Medicare billing privileges and establishes new and enhanced penalties and procedures to deter fraud and abuse. The ACA also specifically adds a requirement that physician orders for covered items of DME must be written by a physician and must document that a physician, a physician assistant, a nurse practitioner, or a clinical nurse specialist has had a face-to-face encounter (including through the use of telehealth) with the individual involved during the six-month period preceding such written order, or other reasonable timeframe as determined by the Secretary of Health and Human Services. The scope of these new provisions will be identified in future rulemaking.
In March 2006, the U.S. Attorney’s Office for the Eastern District of Wisconsin (“U.S. Attorney’s Office”) and the Office of the Inspector General of the Department of Health and Human Services (“OIG” and, together with the U.S. Attorney’s Office, “Federal Authorities”) began an investigation of Dr. Comfort, regarding allegations filed by two whistleblowers that from 2004 through 2006, Dr. Comfort sold custom diabetic shoe inserts as Medicare approved custom inserts that were not, in fact, custom as defined by Medicare because they were not created with a unique image of each foot; and Dr. Comfort sold heat moldable diabetic shoe inserts that did not comply with Medicare requirements for the inserts and did not conform to the heat moldable diabetic inserts that Dr. Comfort submitted to Medicare for coding verification, allegedly in violation of the federal False Claims Act (collectively, the Covered Conduct).
As a condition to DJO’s acquisition of Dr. Comfort in April 2011, Dr. Comfort has entered into a settlement agreement for the Covered Conduct (Settlement Agreement) with the Federal Authorities resolving alleged violations of the federal False Claims Act which were the subject of an investigation triggered by two whistleblower actions. Dr. Comfort also entered into a Corporate Integrity Agreement (“CIA”) with the OIG-HHS. As required by the CIA, Dr. Comfort has established a compliance program and has and will submit required reports to the OIG at least annually on the status of implementation of the requirements of the CIA and compliance activities. Although we conducted healthcare regulatory and related due diligence efforts concerning Dr. Comfort’s operations and business practices prior to our acquisition of Dr. Comfort in April, 2011, and we believe the activities that were the subject of the Covered Conduct described in the Settlement Agreement were isolated and have been addressed through Dr. Comfort’s compliance efforts, including those required by CIA, we cannot assure you that we will not identify additional healthcare regulatory issues in the future or that the Covered Conduct will not be reviewed or investigated by other parties which purchased or reimbursed products of Dr. Comfort that allegedly did not comply with Medicare requirements. Even if we cause Dr. Comfort to take corrective actions to remedy such alleged violations of healthcare regulatory laws, Dr. Comfort could be subject to certain enforcement actions, including, among other things, significant fines, suspension of approvals, seizures or recalls of products, operating restrictions and criminal prosecutions. Failure of Dr. Comfort to comply with certain obligations set forth in the CIA may result in the imposition of monetary (stipulated) penalties and/or
101
Table of Contents
Dr. Comfort’s exclusion from participation in the Federal health care programs. We cannot assure you that relevant governmental authorities would agree with our interpretation of Dr. Comfort’s obligations under applicable healthcare regulatory laws and under the CIA to which Dr. Comfort is subject for five years, or that Dr. Comfort has in all instances fully complied with all applicable healthcare regulatory laws. Any enforcement action could adversely affect Dr. Comfort’s business and results of operations.
Customs and Import/Export Laws and Regulations
Our business is conducted world-wide, with raw material and finished goods imported from and exported to a substantial number of countries. In particular, a significant portion of our products are manufactured in our plant in Tijuana, Mexico and imported to the United States before shipment to domestic customers or export to other countries. We are subject to customs and import/export rules in the U.S. and other countries and to requirements for payment of appropriate duties and other taxes as goods move between countries. Customs authorities monitor our shipments and payments of duties, fees and other taxes and can perform audits to confirm compliance with applicable laws and regulations. After receiving a series of inquiries from U.S. Customs and Border Protection (“CBP”) regarding the tariff classification we were using for some of our bracing products, primarily orthopedic soft goods, made in our Mexico plant and imported to the U.S., we submitted a Prior Disclosure to CBP on January 4, 2012, in which we indicated that we may have misclassified certain imported products as orthopedic devices, rather than textiles. We may also have done the reverse and misclassified orthopedic devices as textiles. Any products reclassified as textiles will also have to be examined to determine whether the applicable duty should be reduced or eliminated under provisions of the North American Free Trade Agreement (“NAFTA”). We committed to CBP that we would undertake an investigation of the potential misclassification issues, and if we determine that products were misclassified, we will correct the classification for entries made during the five-year period included in the Prior Disclosure letter, determine the impact of NAFTA and pay the appropriate duties as a result of the corrected classification. CBP also has the authority to impose penalties for such misclassification in certain cases. We expect that it will take at least several months to complete the investigation and submit a perfected disclosure to CBP.
Governmental Audits
Because we participate in governmental programs as a supplier of medical devices, our operations are subject to periodic surveys and audits by governmental entities or contractors to assure compliance with Medicare and Medicaid standards and requirements. To maintain our billing privileges, we are required to comply with certain supplier standards, including licensure and documentation requirements for our claims submissions. From time to time in the ordinary course of business, we, like other healthcare companies, are audited by, or receive claims documentation requests from, governmental entities, which may identify certain deficiencies based on our alleged failure to comply with applicable supplier standards or other requirements. Medicare contractors and Medicaid agencies periodically conduct pre-payment and post-payment reviews and other audits of claims and are under increasing pressure to more closely scrutinize healthcare claims and supporting documentation. Among other things, the ACA expanded the Recovery Audit Contractors (“RAC”) program, an audit tool that utilizes private companies operating on a contingent fee basis to identify and recoup Medicare overpayments. We have historically been subject to pre and post-payment reviews as well as audits of claims and may experience such reviews and audits of claims in the future. We review and assess such audits or reports and attempt to take appropriate corrective action. We are also subject to surveys of our facilities for compliance with the supplier standards.
We have also been subject to periodic audits of our compliance with other federal requirements for our facilities and related quality and manufacturing processes. Our Surgical Implant facility in Austin, Texas received an FDA warning letter received in 2009, which is described above in the section “FDA and Similar Foreign Government Regulations”.
Federal Privacy and Transaction Law and Regulations
HIPAA impacts the transmission, maintenance, use and disclosure of certain individually identifiable health information (referred to as “protected health information,” or “PHI”). Since HIPAA was enacted in 1996, numerous
102
Table of Contents
implementing regulations have been issued, including, but not limited to: (1) standards for the privacy of individually identifiable health information (the Privacy Rule), (2) the Security Rule, (3) standards for electronic transactions, (4) standard unique national provider identifier, and (5) the HHS Breach Notification Rule. We refer to these rules as the HIPAA Rules. Sanctions for violation of HIPAA and /or the HIPAA Rules include criminal and civil penalties.
HIPAA applies to covered entities, which includes certain health care providers who conduct certain transactions electronically. As such, HIPAA and the HIPAA Rules apply to certain aspects of our business. The effective date for all of the HIPAA Rules outlined above has passed, and, as such, all of the HIPAA Rules are in effect. To the extent applicable to our operations, we are currently in compliance with HIPAA and the applicable Administrative Simplification Rules. Any failure to comply with applicable requirements could adversely affect our profitability.
On February 17, 2009, President Obama signed into law the Health Information Technology for Economic and Clinical Health Act (“HITECH Act”) as part of the American Recovery and Reinvestment Act. This economic stimulus package includes many health care policy provisions, including strengthened federal privacy and security provisions to protect personally-identifiable health information, such as notification requirements for health data security breaches. Many of the details of the new requirements are being implemented through regulations, which have been released in proposed form. We are reviewing these proposed changes to the HIPAA Rules to assess the potential impact on our operations. Any failure to comply with applicable requirements could adversely affect our profitability.
Employees
As of December 31, 2011, we had approximately 5,110 employees. Of these, approximately 3,680 were engaged in production and production support, approximately 30 in research and development, approximately 1,050 in sales and support, and approximately 350 in various administrative capacities including third party billing. Of these employees, approximately 2,120 were located in the United States, approximately 2,170 were located in Mexico and approximately 820 were located in various other countries, primarily in Europe. We have not experienced any strikes or work stoppages, and our management considers our relationship with our employees to be good.
Segment and Geographic Information
Information about our segments and geographic areas can be found in Note 19 of the notes to the audited consolidated financial statements included elsewhere in this prospectus.
103
Table of Contents
Properties
Information about our facilities is set forth in the following table:
| Location |
Use |
Status | Lease Termination Date |
Square Feet (in thousands) | ||||
| Vista, California |
Corporate headquarters, operations, manufacturing facility, research and development |
Leased | August 2021 | 112 | ||||
| Tijuana, Mexico |
Manufacturing and distribution facility |
Leased | September 2016 | 286 | ||||
| Asheboro, North Carolina |
Manufacturing and distribution facility |
Owned | N/A | 115 | ||||
| Indianapolis, Indiana |
Distribution facility | Leased | October 2016 | 110 | ||||
| Mequon, Wisconsin |
Office, manufacturing and distribution facility |
Leased | June 2024 | 95 | ||||
| Shoreview, Minnesota |
Office, operations, medical billing | Leased | October 2018 (a) | 94 | ||||
| Milwaukee, Wisconsin |
Warehouse and distribution facility | Leased | Month-to-month | 86 | ||||
| Clear Lake, South Dakota |
Manufacturing, distribution and refurbishment, and repair facility |
Owned | N/A | 54 | ||||
| Sfax, Tunisia |
Manufacturing facility | Leased | December 2013 | 62 | ||||
| Austin, Texas |
Operations and manufacturing facility, warehouse, research and development |
Leased | March 2019 (b) | 53 | ||||
| Vista, California |
Manufacturing facility | Leased | December 2018 | 53 | ||||
| Freiburg, Germany |
Research and development, distribution facility |
Leased | December 2014 | 47 | ||||
| Mouguerre, France |
Office and distribution | Leased | October 2016 | 43 | ||||
| Mississauga, Canada |
Office and distribution | Leased | March 2015 | 30 | ||||
| Herentals, Belgium |
Distribution facility | Leased | December 2013 | 26 | ||||
| Freiburg, Germany |
Distribution facility | Leased | December 2020 | 22 | ||||
| Malmo, Sweden |
Operations, warehouse and distribution facility |
Leased | March 2014 | 16 | ||||
| Asheboro, North Carolina |
Retail and storage | Owned | N/A | 16 | ||||
| Guildford, United Kingdom |
International headquarters, office, operations |
Leased | January 2015 | 12 | ||||
| Guildford, United Kingdom |
Warehouse | Leased | May 2016 | 12 | ||||
| Hixson, Tennessee (c) |
N/A | Owned | N/A | 226 | ||||
| Other various locations |
Various | Leased | Various | 45 |
| (a) | Renewable, at our option, for one additional five-year term. |
| (b) | Renewable, at our option, for two additional five-year terms. |
| (c) | Our buildings in Hixson, Tennessee are currently held for sale. |
Legal Proceedings
From time to time, we are plaintiffs or defendants in various litigation matters in the ordinary course of our business, some of which involve claims for damages that are substantial in amount. We believe that the disposition of claims currently pending will not have a material adverse impact on our financial position or results of operations.
The manufacture and sale of orthopedic devices and related products exposes us to a significant risk of product liability claims. From time to time, we have been, and we are currently, subject to a number of product liability claims alleging that the use of our products resulted in adverse effects. Even if we are successful in
104
Table of Contents
defending against any liability claims, such claims could nevertheless distract our management, result in substantial costs, harm our reputation, adversely affect the sales of all our products and otherwise harm our business. If there is a significant increase in the number of product liability claims, our business could be adversely affected.
Pain Pump Litigation
We are currently named as one of several defendants in a number of product liability lawsuits involving approximately 60 plaintiffs in U.S. cases and a lawsuit in Canada which has been granted class action status, related to a disposable drug infusion pump product (pain pump) manufactured by two third party manufacturers that we distributed through our Bracing and Vascular segment. We sold pumps manufactured by one manufacturer from 1999 to 2003 and then sold pumps manufactured by a second manufacturer from 2003 to 2009. We discontinued our sale of these products in the second quarter of 2009. These cases have been brought against the manufacturers and certain distributors of these pumps, and in some cases, the manufacturers of the anesthetics used in these pumps. All of these lawsuits allege that the use of these pumps with certain anesthetics for prolonged periods after certain shoulder surgeries has resulted in cartilage damage to the plaintiffs. The lawsuits allege damages ranging from unspecified amounts to claims of up to $10 million. Many of the lawsuits which have been filed in the past three years have named multiple pain pump manufacturers and distributors without having established which manufacturer manufactured or sold the pump in issue. In the past three years, we have been dismissed from more than 400 cases when product identification was later established showing that we did not sell the pump in issue. At present, we are named in approximately 25 lawsuits in which product identification has yet to be determined and, as a result, we believe that we will be dismissed from a meaningful number of such cases in the future. In the past two years, we have entered into settlements with plaintiffs in approximately 75 pain pump lawsuits. Of these, we have settled approximately 40 cases in joint settlements involving our first manufacturer and we have settled approximately 35 cases involving our second manufacturer in which the manufacturer’s carrier has made some contribution to our settlement amount or any joint settlement, but for which we are seeking indemnity for the balance of our costs.
Indemnity and Insurance Coverage Related to Pain Pump Claims
We have sought indemnity and tendered the defense of the pain pump cases to the two manufacturers who supplied these pumps to us, to their products liability carriers and to our products liability carriers. These lawsuits are about equally divided between the two manufacturers. Both manufacturers have rejected our tenders of indemnity. The base policy for one of the manufacturers contributed to our defense, but that policy has been exhausted by defense costs and settlements, as has a second policy of that manufacturer. This manufacturer has ceased operations, has little assets and no additional insurance coverage. The Company has asserted indemnification rights against the successor to this manufacturer and is pursuing claims against the manufacturer, its owners and its successor. The base policy for the other manufacturer has been exhausted and the excess liability carriers for that manufacturer have not accepted coverage for the Company and are not expected to provide for its defense. The Company and this manufacturer have been cooperating in jointly negotiating settlements of those lawsuits in which both parties are named. Our products liability carriers have accepted coverage of these cases, subject to a reservation of the right to deny coverage for customary matters, including punitive damages and off-label promotion. In August 2010, one of our excess carriers for the period ending July 1, 2010 and for the supplemental extended reporting period (SERP) discussed below, which is insuring $10 million in excess of $25 million, informed us that it has reserved its right to rescind the policy based on an alleged failure by us and our insurance broker to disclose material information. We disagree with this allegation and are seeking to resolve the issue with this carrier. We could be exposed to material liabilities if our insurance coverage is not available or inadequate and the resources of the two manufacturers, including their respective products liability insurance policies, are unavailable or insufficient to pay the defense costs and settlements or judgments in these cases.
105
Table of Contents
Pain Pump-Related HIPAA Subpoena
On August 2, 2010, we were served with a subpoena under HIPAA seeking numerous documents related to our activities involving the pain pumps discussed above. The subpoena which was issued by the United States Attorney’s Office for the Central District of California, refers to an official investigation by the DOJ and the FDA of Federal health care offenses. We have produced documents that are responsive to the subpoena. We believe that our actions related to our prior distribution of these pain pumps have been in compliance with applicable legal standards. We can make no assurance as to the resources that will be needed to respond to any follow-up requests related to the subpoena or the final outcome of any investigation or further action.
Pain Pump Investigation—U.S. Attorney’s Office for the Western District of Missouri
In January 2012 the Company became aware of a civil investigation by the United States Attorney’s Office for the Western District of Missouri regarding the Company’s previous sale and marketing of pain pump devices. The investigation relates to whether the Company caused false claims to be filed with government payors as a result of alleged off-label promotion of the pain pumps. The Company denies that it improperly promoted the pain pump devices and believes that its marketing and sales activities were in compliance with applicable legal standards.
Cold Therapy Litigation
Since mid-2010, we have been named in nine multi-plaintiff lawsuits involving a total of 210 plaintiffs, alleging that the plaintiffs had been injured following the use of certain cold therapy products manufactured by the Company. The complaints are not specific as to the nature of the injuries, but allege various product liability theories, including inadequate warnings regarding the risks associated with the use of cold therapy and failure to incorporate certain safety features into the design. No specific dollar amounts of damages are alleged and as of September 29, 2012, we cannot estimate a range of potential loss. These cases have been included in a coordinated proceeding in San Diego Superior Court with a similar number of cases filed against one of our competitors. A total of nine of the plaintiffs included in the cases filed against us have been identified as the first “bellwether” cases to be tried, of which four have been set for trial in July 2013. Discovery is proceeding on these bellweather cases.
Our Product Liability Insurance Coverage
We maintain product liability insurance that is subject to annual renewal. Our current policy covers claims reported between July 1, 2012 and June 30, 2013. This policy excludes coverage for claims related to both pain pump products and cold therapy products. As described below, we have other insurance which provides coverage for these excluded products. For the current policy year, we maintain coverage limits (together with excess policies) of up to $50 million, with deductibles of $500,000 per claim for claims relating to invasive products (principally our surgical implant products) and $50,000 per claim for claims relating to all other covered products, with an aggregate self-insured retention of $2 million. Starting with the 2010-2011 policy period, our products liability policy excluded claims related to pain pump products. We purchased supplemental extended reporting period (“SERP”) coverage for the $80 million limit product liability policy that expired on June 30, 2010, and this supplemental coverage allows us to report pain pump claims beyond the end of the prior policy. Except for the additional excess coverage mentioned below, this SERP coverage does not provide additional limits to the aggregate $80 million limits on the prior policy but it does provide that these limits will remain available for pain pump claims reported for an extended period of time. We also purchased additional coverage for pain pump claims of $25 million in excess of the $80 million limits with a five year reporting period. Thus, the SERP coverage for current and future pain pump claims has a total limit of $105 million (less amounts paid for claims reported to date). Starting with the 2011-2012 policy period, our primary products liability coverage excluded claims related to cold therapy products. Concurrently with the exclusion of our cold therapy products from the 2011-2012 primary policy, we purchased SERP coverage for cold therapy product claims for injuries alleged to have occurred prior to July 1, 2011. This SERP allows us to report such cold therapy claims under our
106
Table of Contents
expired 2010-2011 policy which had a total limit of $50 million. We have also purchased separate primary and excess policies providing for a total of $5 million of coverage for claims related to cold therapy products arising from injuries alleged to have occurred after June 30, 2011, with a deductible of $250,000 per claim and an aggregate deductible of $3 million. We believe we have adequate insurance coverage for our product liability claims. However, if a product liability claim or series of claims is brought against us for uninsured liabilities or there is an increase in claims which is in excess of our available insurance coverage, our business could suffer materially.
BGS Qui Tam Action and HIPAA Subpoena
On April 15, 2009, we became aware of a qui tam action filed in Federal Court in Boston, Massachusetts in March 2005 and amended in December 2007 that names us as a defendant along with each of the other companies that manufactures and sells external bone growth stimulators, as well as The Blackstone Group L.P., an affiliate of DJO’s principal stockholder, and the principal stockholder of one of the other companies in the bone growth stimulation business. This case is captioned United States ex rel. Beirman v. Orthofix International, N.V., et al., Civil Action No. 05-10557 (D. Mass.). The case was sealed when originally filed and unsealed in March 2009. The plaintiff, or relator, alleges that the defendants have engaged in Medicare fraud and violated Federal and state false claims acts from the time of the original introduction of the devices by each defendant to the present by seeking reimbursement for bone growth stimulators as a purchased item rather than a rental item. The relator also alleges that the defendants are engaged in other marketing practices constituting violations of the Federal and various state anti-kickback statutes. On December 4, 2009, we filed a motion to dismiss the relator’s complaint. The relator filed a second amended complaint in May 2010 that, among other things, dropped The Blackstone Group as a defendant. We filed another motion to dismiss directed at the second amended complaint, and that motion was denied. The case is proceeding to the discovery phase. Shortly before becoming aware of the qui tam action, we were advised that our bone growth stimulator business was the subject of an investigation by the DOJ, and on April 10, 2009, we were served with a subpoena under HIPAA seeking numerous documents relating to the marketing and sale by us of bone growth stimulators. On September 21, 2009, we were served with a second HIPAA subpoena related to this DOJ investigation seeking additional documents relating to the marketing and sale by us of bone growth stimulators. We believe that these subpoenas are related to the DOJ’s investigation of the allegations in the qui tam action, although the DOJ has decided not to intervene in the qui tam action at this time. We believe that our marketing practices in the bone growth stimulation business are in compliance with applicable legal standards and we intend to defend this case and investigation vigorously. We can make no assurance as to the resources that will be needed to respond to these matters or the final outcome of such action and as of September 29, 2012, we cannot estimate a range of potential loss, fines or damages.
107
Table of Contents
The following table sets forth information about the directors and executive officers of our indirect parent, DJO. The executive officers of DJO are also the executive officers of DJOFL.
| Name | Age | Position | ||
| Michael P. Mogul |
46 | President, Chief Executive Officer and Director; Manager of DJOFL | ||
| Vickie L. Capps |
50 | Executive Vice President, Chief Financial Officer and Treasurer; Manager of DJOFL | ||
| Donald M. Roberts |
63 | Executive Vice President, General Counsel and Secretary; Manager of DJOFL | ||
| Thomas A. Capizzi |
53 | Executive Vice President, Global Human Resources | ||
| Stephen J. Murphy |
47 | Executive Vice President, Sales and Marketing, International Commercial Businesses | ||
| Mike S. Zafirovski |
58 | Chairman of the Board | ||
| John Chiminski |
48 | Director | ||
| Chinh E. Chu |
45 | Director | ||
| Julia Kahr |
33 | Director | ||
| Sidney Braginsky |
74 | Director | ||
| James R. (Ron) Lawson |
68 | Director | ||
| John R. Murphy |
61 | Director | ||
| James Quella |
62 | Director |
Michael P. Mogul—President, Chief Executive Officer and Director. Mr. Mogul was appointed President, Chief Executive Officer and Director of DJO and Manager of DJOFL in June 2011. Prior to joining DJO, Mr. Mogul served as President and subsequently Group President of Orthopaedics for Stryker Corp. from 2005 until his transition to DJO in 2011. Prior to that, he served as Managing Director of Stryker Germany, Austria and Switzerland, where he led the rebuilding of those organizations following the Howmedica acquisition. From 1994 to 2000, he served as Vice President, Sales for the Osteonics Division of Stryker, where he led the successful integration of the U.S. Osteonics and Howmedica sales teams. Mr. Mogul served as General Manager of the Osteonics Instrument Business Unit, Assistant to the Chairman and Regional Sales Manager of Stryker Instruments. He joined Stryker in 1989 as Sales Representative for Stryker Instruments after starting his career in 1986 as an Account Manager for NCR Corp. Mr. Mogul received a Bachelor of Science Degree from the University of Colorado and has attended the Advanced Management Program at the Harvard Business School.
Vickie L. Capps—Executive Vice President, Chief Financial Officer and Treasurer. Ms. Capps was appointed Executive Vice President, Chief Financial Officer and Treasurer of DJO and DJOFL as of the effective date of the DJO Merger. Ms. Capps became one of DJOFL’s managers in 2010. Prior to the DJO Merger, Ms. Capps served as the Executive Vice President, Chief Financial Officer and Treasurer of DJO Opco since July 2002. From September 2001 until July 2002, Ms. Capps was employed by AirFiber, a privately held provider of broadband wireless solutions, where she served as Senior Vice President, Finance and Administration and Chief Financial Officer. From July 1999 to July 2001, Ms. Capps served as Vice President of Finance and Administration and Chief Financial Officer for Maxwell Technologies, Inc., a publicly traded technology company. From 1992 to 1999, Ms. Capps served in various positions, including Chief Financial Officer, with Wavetek Wandel Goltermann, Inc., a multinational communications equipment company. Ms. Capps also served as a senior audit and accounting professional for Ernst & Young LLP from 1982 to 1992. Ms. Capps is a California Certified Public Accountant and received a B.S. degree in business administration/accounting from San Diego State University. Ms Capps served on the board of directors and was a member of the audit committee and chairperson of the nominating and governance committee of SenoRx, Inc., a publicly traded medical device company, until the company was sold in July, 2010.
Donald M. Roberts—Executive Vice President, General Counsel and Secretary. Mr. Roberts was appointed Executive Vice President, General Counsel and Secretary of DJO and DJOFL as of the effective date of the DJO
108
Table of Contents
Merger. Mr. Roberts became one of DJOFL’s managers in 2010. Prior to the DJO Merger, Mr. Roberts served as Senior Vice President, General Counsel and Secretary of DJO Opco since December 2002. From 1994 to December 2002, Mr. Roberts served as Vice President, Secretary and General Counsel for Maxwell Technologies, Inc., a publicly held technology company. Previous to that, he was with the Los Angeles—based law firm of Parker, Milliken, Clark, O’Hara & Samuelian for 21 years. Mr. Roberts was a shareholder in the firm, having served as partner in a predecessor partnership. Mr. Roberts received his undergraduate degree in political science from Yale University and earned his J.D. at the University of California, Berkeley, Boalt Hall School of Law.
Thomas A. Capizzi—Executive Vice President, Global Human Resources. Mr. Capizzi was appointed Executive Vice President, Global Human Resources of DJO and DJOFL as of the effective date of the DJO Merger. Prior to the DJO Merger, Mr. Capizzi served as Senior Vice President, Human Resources of DJO Opco since July 2007. From 2001 to July 2007, Mr. Capizzi served as Vice President, Worldwide Human Resources & Administration for Magellan GPS, a Consumer Electronics Company. Previous to that, from 1999 to 2001, he was Vice President, HR, Chief Administrative Officer for PCTEL a publicly held Telecommunications and Modem Technology Company. From 1997 to 1999 he served as Corporate Vice President, Human Resources for McKesson, a Medical Distribution and Pharmaceutical Solution company. Mr. Capizzi has held various other Human Resources Management positions in companies such as Charles Schwab, Genentech, PepsiCo and The Hertz Corporation. Mr. Capizzi brings well over 25 years of Human Resources experience. Mr. Capizzi received his undergraduate degree in Psychology and Philosophy from Cathedral College/St. John ‘s University and his post graduate work in Organizational Development from the New School.
Stephen J. Murphy—Executive Vice President, Sales and Marketing, International Commercial Business. Mr. Murphy was appointed Executive Vice President, Sales and Marketing, International Commercial Business of DJO in September 2009. Prior to September 2009, Mr. Murphy served as Senior Vice President, International Sales and Marketing of DJO since the DJO Merger and before that in various international positions with DJO Opco since August 2001. Prior to this, Mr. Murphy served in similar positions with DonJoy, LLC, since June 1999 and served in various international sales and marketing positions since 1992 with affiliates of DonJoy, LLC’s predecessor, Smith & Nephew, Inc., assuming responsibility first for the Medical Business of Smith & Nephew in Ireland and later for the international business of the S&N Homecraft Rehabilitation business, based in England. Mr. Murphy began his career as an accountant with Smith & Nephew Ireland in 1991. He is a Chartered Management Accountant and completed his studies at the Accountancy and Business College in Dublin in 1991.
Mike S. Zafirovski—Chairman of the Board. Mr. Zafirovski became one of DJO’s directors and was named as non-executive Chairman of the Board of DJO in January 2012. Mr. Zafirovski is currently a Senior Advisor to The Blackstone Group, L.P., an affiliate of DJO’s primary shareholder. He served as Director, President and Chief Executive Officer of Nortel Networks Corporation from November 2005 to August 2009. Previously, Mr. Zafirovski was Director, President and Chief Operating Officer of Motorola, Inc. from July 2002 to January 2005, and remained a consultant to and a director of Motorola until May 2005. He served as Executive Vice President and President of the Personal Communications Sector of Motorola from June 2000 to July 2002. Prior to joining Motorola, Mr. Zafirovski spent nearly 25 years with General Electric Company, where he served in management positions, including 13 years as President and Chief Executive Officer of five businesses in the industrial and financial services arenas, his most recent being President and Chief Executive Officer of GE Lighting from July 1999 to May 2000. Mr. Zafirovski also serves on the boards of the Boeing Company and Apria Healthcare Group Inc.
Sidney Braginsky—Director. Mr. Braginsky became one of DJO’s directors in December 2006. Mr. Braginsky has been President, Chief Executive Officer and Chairman of the Board of Atropos Technology, LLC since July 2000. Mr. Braginsky also serves a director of Double D (Devices and Diagnostics), a Venture Capital Fund and is Chairman and CEO of Digilab LLC, a molecular spectroscopy division acquired by Atropos in 2001. Double D and Digilab LLC are both affiliated with Atropos Technology, LLC. Before joining Atropos,
109
Table of Contents
Mr. Braginsky served as President of Olympus America, Inc. where he built a large business focused on optical products. Prior to Olympus America, Mr. Braginsky served as President and Chief Operating Officer of Mediscience Technology Corp., a designer and developer of diagnostic medical devices for cancer detection. Mr. Braginsky currently serves on the board of directors and audit committees of MELA Sciences, Inc (formerly Electro-Optical Sciences, Inc.), Invendo Medical GmbH and Endogene Pty., Ltd. Mr. Braginsky formerly served on the board of directors of Diomed Holdings, Inc., Geneva Acquisition Corp, and Noven Pharmaceuticals, Inc.
John Chiminski—Director. Mr. Chiminiski became one of DJO’s directors in March 2012. Mr. Chiminski currently serves as President and Chief Executive Officer of Catalent Pharma Solutions. From 2007 until March 2009 when he joined Catalent Pharma Solutions, Mr. Chiminski served as President and Chief Executive Officer of GE Medical Diagnostics, a global business with sales of $1.9 billion. From 2005 to 2007, he served as Vice President and General Manager of GE Healthcare’s Global Magnetic Resonance Business, and from 2001 to 2005, Mr. Chiminski served as Vice President and General Manager of Global Healthcare Services. Mr. Chiminski holds a B.S. from Michigan State University and an M.S. from Purdue University, both in electrical engineering, as well as a Master in Management degree from the Kellogg School of Management at Northwestern University. He is on the Board of Trustees for HealthCare Institute of New Jersey.
Chinh E. Chu—Director. Mr. Chu became one of DJO’s directors immediately after the completion of the acquisition of DJO by an affiliate of The Blackstone Group L.P. in November 2006, and became Chairman of the Board in January 2009. He served as Chairman of the Board until the appointment of Leslie H. Cross as Chairman of the Board in June 2011. Mr. Chu is a senior managing director of The Blackstone Group. An affiliate of The Blackstone Group owns substantially all of the capital stock of DJO. Since joining Blackstone in 1990, Mr. Chu has led the execution of The Blackstone Group’s investments in Healthmarkets, Inc., SunGuard Data Systems Inc., Nalco, Celanese, Nycomed and LIFFE. He has also been involved in the execution of Blackstone’s investments in Graham Packaging, Sirius Satellite Radio, StorageApps, Haynes International, Prime Succession/Rose Hills, Interstate Hotels, HFS and Alco Holdings. Before joining The Blackstone Group, Mr. Chu worked at Salomon Brothers in the Mergers & Acquisitions Department. Mr. Chu currently serves on the boards of directors of Catalent, SunGard Data Systems Inc., Healthmarkets, Inc, Bayview Financial Holdings, Bank United Financial Corporation and Freescale Semiconductor. Mr. Chu was formerly a director of Celanese Corporation, Graham Packaging, Financial Guaranty Insurance Company and Nalco Holding Company.
Julia Kahr—Director. Ms. Kahr became one of DJO’s directors immediately after the completion of the acquisition of DJO by an affiliate of The Blackstone Group L.P. in November 2006. Ms. Kahr is currently a managing director of The Blackstone Group. Before joining The Blackstone Group in 2004, Ms. Kahr was a Project Leader at the Boston Consulting Group, where she worked with companies in a variety of industries, including financial services, pharmaceuticals, media and entertainment, and consumer goods. Ms. Kahr is a director of Summit Materials. Ms. Kahr is also the sole author of Working Knowledge, a book published by Simon & Schuster in 1998.
James R. Lawson—Director. Mr. Lawson became one of DJO’s directors in September 2012. Mr. Lawson has over 35 years of experience in the orthopedic medical device industry. He is currently Chairman of the Board of IMDS, an orthopedic contract manufacturing and innovation company, a member of the Health Care Advisory Board of Arsenal Capital Partners and a member of the board of directors of Cold Plasma Medical Technologies, a startup company specializing in the field of plasma medicine. Mr. Lawson has served in several senior management positions, including as Senior Vice President of Howmedica’s Worldwide Sales and Customer Service (prior to its acquisition by Stryker Corporation) and at Stryker as Senior Vice President of Sales, Marketing and Product Development, President EMEA, and Group President, International and Global Orthopedics. Mr. Lawson has also been involved as an entrepreneur in several privately held businesses. Mr. Lawson retired from Stryker in 2007 and in 2008 he formed Lawson Group LLC which provides strategic consulting services specializing in the orthopedic medical technology field.
110
Table of Contents
John R. Murphy—Director. Mr. Murphy became one of DJO’s directors and was named as Chairman of the Audit Committee in January, 2012. Since 2003, Mr. Murphy has served on the Board of Directors, the Governance Committee and as Chairman of the Audit Committee of O’Reilly Automotive, Inc. Mr. Murphy was elected as a director and audit committee member of Graham Packaging in February 2011. Graham Packaging was subsequently sold in September 2011. He was Senior Vice President and Chief Financial Officer of Smurfit-Stone Container Corporation from 2009 to 2010, and prior thereto from 1998 to 2008 he served in various senior management roles, including Chief Financial Officer and Chief Operating Officer and ending as President and Chief Executive Officer of Accuride Corporation. Accuride Corporation filed for Chapter 11 bankruptcy protection in October 2009, and emerged in 2010. In February 2012, Mr. Murphy was elected as a director and Audit Committee Chairman of Summit Materials, LLC.
James Quella—Director. Mr. Quella became one of DJO’s directors in September 2012. Mr. Quella is a Senior Managing Director and Senior Operating Partner in the Corporate Private Equity group of The Blackstone Group, LP. Affiliates of The Blackstone Group, LP own substantially all of the capital stock of DJO. Prior to joining The Blackstone Group, LP in 2004, Mr. Quella was a Managing Director and Senior Operating Partner with DLJ Merchant Banking Partners and CSFB Private Equity. Prior to that, Mr. Quella worked at Mercer Management Consulting and Strategic Planning Associates, its predecessor firm, where he served as a senior consultant to CEOs and senior management teams, and was Co-Vice Chairman with shared responsibility for overall management of the firm. Mr. Quella has been a member of various Private Equity company boards and currently serves as a director of Catalent, Freescale Semiconductor and Michaels Stores.
Corporate Governance Matters
Background and Experience of Directors. When considering whether directors and nominees have the experience, qualifications, attributes or skills, taken as a whole, to enable the DJO Board of Directors to satisfy its oversight responsibilities effectively in light of DJO’s business and structure, the DJO Board of Directors focused primarily on each person’s background and experience as reflected in the information discussed in each of the directors’ individual biographies set forth immediately above. We believe that our directors provide an appropriate mix of experience and skills relevant to the size and nature of DJO’s business. In particular, the members of the DJO Board of Directors considered the following important characteristics: (i) Mr. Chu, Ms. Kahr, Mr. McEvoy and Mr. Quella are representatives appointed by The Blackstone Group L.P., an affiliate of our principal stockholder, and have significant financial and investment experience from their involvement in The Blackstone Group’s investment in numerous portfolio companies and have played active roles in overseeing those businesses, (ii) our Chief Executive Officer, has extensive experience in the orthopedic device industry and in executive management; (iii) our Chairman of the Board has significant experience as a CEO, COO and other senior management positions with large multi-national companies; and (iv) our outside directors have a diverse background of management, accounting and financial experience from the healthcare and medical device industries, as well as other industries. Specifically, Mr. Zafirovski brings extensive financial management and board experience; Mr. Murphy is the Chairman of our Audit Committee and is an audit committee financial expert, as defined in Item 407(d)(5)(ii) of Regulation S-K under the Exchange Act, by virtue of his years of experience in various senior financial management and board positions; Mr. Braginsky brings both financial and management experience in a diverse range of businesses, as well as audit and board service; Mr. Chiminiski has served in numerous senior management roles with medical device and pharmaceutical companies; and Mr. Lawson has over 35 years of senior management and board of directors experience in the orthopedic medical device industry.
In recommending directors, our Board of Directors considers the specific background and experience of the Board members and other personal attributes in an effort to provide a diverse mix of capabilities, contributions and viewpoints which the Board believes enables it to function effectively as the Board of Directors of a company with our size and nature of business. In January 2009, Nortel Networks Corporation, for which Mr. Zafirovski served as Director, President and Chief Executive Officer, and subsidiary companies filed for bankruptcy protection in the United States, Canada and Europe. Mr. Zafirovski resigned from Nortel on
111
Table of Contents
August 9, 2009. In October 2009, Accuride Corporation, for which Mr. Murphy served in various senior management roles, including as Chief Financial Officer, President and Chief Executive Officer, filed for bankruptcy protection in the United States. Mr. Murphy resigned from Accuride in September 2008. The Board has concluded that neither of these events impair either Mr. Zafirovski’s or Mr. Murphy’s ability to serve as a director.
Board Leadership Structure. Mr. Chu served as Chairman of the Board from January 2009 until the appointment of Mr. Cross as Chairman upon his retirement in June 2011. Mr. Cross served as Chairman until December 30, 2011. Effective January 5, 2012, the Board of Directors elected Mr. Zafirovski as a member of the Board and as non-executive Chairman of the Board. The Chief Executive Officer position is and will remain separate from the Chairman position. We believe that the separation of the Chairman and CEO positions is appropriate for a company of the size and nature of DJO.
Role of Board in Risk Oversight. The Board of Directors has extensive involvement in the oversight of risk related to the company and its business. The Audit Committee of the Board plays a key role in representing and assisting the Board in discharging its oversight responsibility relating to the accounting, reporting and financial practices of the company, including the integrity of our financial statements, the surveillance of administrative and financial controls and the company’s compliance with legal and regulatory requirements. Through its regular meetings with management, including legal, regulatory, compliance and internal audit functions, the Audit Committee reviews and discusses all of the principal functions of our business and updates the Board of Directors on all material matters.
Audit Committee. Our Audit Committee currently consists of three appointed Directors, Mr. Murphy (Chairman), Mr. Braginsky and Ms. Kahr. As a privately held company, our Audit Committee is not required to be composed of only independent directors. We believe that Messrs. Murphy and Braginsky each meet the definition of an independent director under the Rules of the New York Stock Exchange. Our Board of Directors has determined that Mr. Murphy is an audit committee financial expert, as defined in SEC Regulation S-K Item 407 (d)(5)(ii). Our Board of Directors also believes that the other members of the Audit Committee have requisite levels of financial literacy and financial sophistication to enable the Audit Committee to be effective in relation to the purposes outlined in its charter and in light of the scope and nature of our business and financial statements.
Compensation Committee. The Compensation Committee of the DJO Board currently consists of three appointed Directors, Mr. Chu, Ms. Kahr, and Mr. Quella. Because DJO is a privately held company, the Compensation Committee is not required to be composed of independent directors.
Code of Ethics. Our Business Ethics Policy and Code of Conduct, Code of Conduct for the Board of Directors, and Code of Ethics for the Chief Executive Officer and Senior Executives and Financial Officers are available, free of charge, on the Company’s website at www.DJOglobal.com. Please note, however, that the information contained on the website is not incorporated by reference in, or considered part of, this Prospectus. We will post any amendments to the Code of Ethics, and any waivers that are required to be disclosed by the SEC rules on our website within the required time period. We will also provide copies of these documents, free of charge, to any security holder upon written request to: Investor Relations, DJO Global, Inc., 1430 Decision Street, Vista, California 92081-8553.
112
Table of Contents
Compensation Discussion and Analysis
The following Compensation Discussion and Analysis describes the objectives of our executive Compensation Program and the material elements of compensation for our executive officers identified under Item 11. “Executive Compensation—Summary Compensation Table” (the Named Executive Officers or NEOs), along with the role of the Compensation Committee of the DJO Board of Directors (the Compensation Committee) in reviewing and making decisions regarding our executive compensation program.
Role of the Compensation Committee in Establishing Compensation
The Compensation Committee establishes salaries and reviews benefit programs for the Chief Executive Officer (CEO) and each of our other executive officers; reviews and approves our annual incentive compensation for management employees; reviews, administers and grants stock options under our stock option plan; advises the DJO Board and makes recommendations with respect to plans that require Board approval; and approves employment agreements with our executive officers. The Compensation Committee establishes and maintains our executive compensation program through internal evaluations of performance, and analysis of compensation practices in industries where we compete for experienced senior management. The Compensation Committee reviews our compensation programs and philosophy regularly, particularly in connection with its evaluation and approval of changes in the compensation structure for a given year. The Compensation Committee met five times during 2011. The CEO makes recommendations for the salaries for executive officers other than himself and reviews such recommendations with the Compensation Committee.
Objectives of Our Compensation Program
Our executive compensation program is designed to attract, retain, and reward talented senior management who can contribute to our growth and success and thereby build long-term value for our stockholders. We believe that an effective executive compensation program is critical to our long-term success. By having an executive compensation program that is competitive with current market practice and focused on driving superior and enduring performance, we believe we can align the interests of our executive officers with the interests of stockholders and reward our executive officers for successfully improving stockholder returns. Our compensation program has the following objectives:
| • | Attract and retain talented senior management to ensure our future success, |
| • | Encourage a pay-for-performance mentality by directly relating variable compensation elements to the achievement of financial and strategic objectives, |
| • | Promote a direct relationship between executive compensation and the interests of our stockholders, with long-term incentive compensation that links a significant portion of executive compensation to our sustained performance through stock option awards, and |
| • | Structure a compensation program that appropriately rewards our executive officers for their skills and contributions to our company based on competitive market practice. |
The Elements of Our Executive Compensation Program
The elements of our executive compensation program are as follows:
| • | Base salary, |
| • | Annual and quarterly cash incentive compensation (performance-based bonuses, with bonus of up to 70% of base salary for the executive officers (other than the CEO) for achieving target goals and with a supplemental bonus of up to the same percentage of base salary for achieving enhanced goals and a target bonus of 100% of base salary for the CEO with a supplemental bonus of up to 50% of his base salary), |
113
Table of Contents
| • | Equity-based awards (stock options), |
| • | Retention and severance agreements where appropriate, and |
| • | Other benefits. |
Base Salary.
Base salaries provide a fixed form of compensation designed to reward an executive officer’s core competence in his or her role. The Compensation Committee determines base salaries by taking into consideration such factors as competitive industry salaries, the nature of the position, the contribution and experience of the officers and the length of service. In connection with the hiring of Mr. Mogul as CEO in June 2011, we entered into an employment agreement with Mr. Mogul which provides for payment of an annual base salary of $750,000. See “Employment Agreement with CEO” below.
Annual and Quarterly Cash Incentive Compensation.
Performance-based cash incentive compensation is provided to motivate our executive officers for each quarter and for the full year to pursue objectives that the Compensation Committee believes are consistent with the overall goals and long-term strategic direction that the DJO Board has set for our company. Over the past four years, the Compensation Committee has adopted annual bonus plans which have several basic features which have carried over from year to year, with some modifications and the establishment of specific financial targets for each year.
In February 2011, the Compensation Committee approved the management incentive bonus plan for 2011 (2011 Bonus Plan) for the executive officers of the Company other than for Mr. Cross. Pursuant to his Director Arrangement, Seperation Agreement and General Release, Mr. Cross was paid a monthly payment until July 13, 2011 consisting of one-twelfth of his base salary, plus one twelfth of his target bonus (80% of his base salary), The 2011 Bonus Plan was based upon the structure of the bonus plans that were approved for 2008-2010. Under the 2011 Bonus Plan, each executive officer (other than the CEO) had an opportunity to earn up to 70% of such executive’s annual base salary as a target bonus (Target Bonus), with 50% of the Target Bonus earned based on the Company’s achievement of certain quarterly financial results and the remaining 50% earned based on the Company’s overall 2011 financial results. The portion of the bonus that could be earned in each of the fiscal quarters was divided equally among the four quarters. The 2011 Bonus Plan contained quarterly and annual revenue goals that determined whether 50% of the Target Bonus was earned and quarterly and annual Adjusted EBITDA goals that determined whether the remaining 50% of the Target Bonus was earned. At the end of each quarter and the full year, the bonus opportunity was determined based on whether the applicable financial targets were met, and if one or more such targets were met, the applicable portion of the target bonus would be paid. The Compensation Committee retains the discretion to reduce an executive’s bonus if the executive fails to achieve individual performance goals.
The Compensation Committee selected revenue and Adjusted EBITDA as the relevant company-wide performance criteria for the bonus plans because the Compensation Committee believes that these criteria are consistent with the metrics by which the DJO Board measures the overall goals and long-term strategic direction for DJO. Further, these criteria are closely related to or reflective of DJO’s financial and operational improvements, growth and return to shareholders. Revenue growth is a critical metric for enhancing the value of our Company. Adjusted EBITDA is an important non-GAAP valuation tool that potential investors use to measure our Company’s profitability and liquidity against other companies in our industry. Adjusted EBITDA, for the purposes of the 2011 Bonus Plan, was calculated as earnings before interest, income taxes, depreciation and amortization, further adjusted for non-cash items, non-recurring items and other adjustment items pursuant to the definition of consolidated EBITDA contained in the credit agreement for our Senior Secured Credit Facility, excluding forward cost savings as determined by the Board of Directors.
114
Table of Contents
The 2011 Bonus Plan provided for the payment of as little as 60% of the Target Bonus if the Company’s financial performance fell short of the applicable target by less than 2.0% for revenue and 2.0% for Adjusted EBITDA (Threshold Bonus). Likewise, the 2011 Plan provided for the payment of an additional supplemental bonus (Supplemental Bonus) of up to 60% of base salary if the Company’s financial performance exceeded the applicable target by up to 2.0% of revenue and 2.0% of Adjusted EBITDA. As with prior bonus plans, the effects of foreign currency translation were excluded from the financial calculations under the 2011 Bonus Plan. In establishing the specific financial performance goals for the 2011 Bonus Plan, the Compensation Committee set the annual revenue and Adjusted EBITDA targets to reflect growth over 2010 of 5.5% and 3.2%, respectively. Following the acquisition of Dr. Comfort in April 2011, the Compensation Committee revised the revenue and Adjusted EBITDA targets to reflect growth of 2011 revenue and Adjusted EBITDA over pro forma revenue and Adjusted EBITDA as if the acquisitions had been completed on January 1, 2010. As a result of the Company’s financial performance in 2011, the adjusted targets for annual revenue and annual Adjusted EBITDA were not met and no annual bonuses were awarded. Based on the quarterly results in 2011, a full quarterly bonus was earned on both the revenue and Adjusted EBITDA targets in the first quarter of 2011 and no quarterly bonuses were earned in the second, third, or fourth quarter of 2011.
Pursuant to the terms of his Employment Agreement, Mr. Mogul was paid an annual bonus of $652,000 for 2011 in lieu of the annual performance-based incentive plan described above. In addition to the other compensation terms described below, Mr. Mogul’s Employment Agreement provides that for each full fiscal year during the term of his Employment Agreement beginning in 2012, Mr. Mogul will be eligible to earn an annual target bonus of 100% of his base salary based on achievement of the same quarterly and annual revenue and Adjusted EBITDA targets as are established each year for the Company’s management incentive bonus plan. Mr. Mogul is also eligible for an additional annual bonus of up to 50% of his base salary upon achievement of the same performance criteria for the Supplemental Bonus.
On January 6, 2012, the Compensation Committee approved the management incentive bonus plan for 2012 (2012 Bonus Plan) for the executive officers of the Company other than the CEO. The Target Bonus percentages remained at 70% of base salary for the executive officers other than the CEO. As with the 2011 Bonus Plan, 50% of the Target Bonus is based on meeting revenue targets and 50% of the Target Bonus is based on meeting Adjusted EBITDA targets. The revenue and Adjusted EBITDA performance metrics for the 2012 Bonus Plan reflect the Compensation Committee’s desire to focus management exclusively on growth in revenue and Adjusted EBITDA. As with the 2011 Bonus Plan, 50% of the Target Bonus will be based on full-year performance and 50% on quarterly performance, with each quarter representing 25% of the quarterly bonus opportunity. A minimum bonus of 25% of the Target Bonus can be earned if the Company’s financial performance falls short of the applicable targets by less than 3.0% for revenue and 3.0% for Adjusted EBITDA. As with prior bonus plans, the 2012 Plan provides for a Supplemental Bonus for the full year only pursuant to which the executive officers may earn an additional bonus of up to 100% of their applicable Target Bonus if the Company’s financial performance exceeds the applicable target by up to 103% for revenue and 103% for Adjusted EBITDA.
Equity Compensation Awards.
In November 2007, the Compensation Committee adopted the DJO 2007 Incentive Stock Plan (the 2007 Plan). The purpose of the 2007 Plan is to promote the interests of the Company and its shareholders by enabling selected key employees to participate in our long-term growth by receiving the opportunity to acquire shares of DJO common stock and to provide for additional compensation based on appreciation in DJO common stock. The 2007 Plan provides for the grant of stock options and other stock-based awards to key employees, directors and distributor-principals. The Compensation Committee determines whether to grant options and the exercise price of the options granted. The Committee has broad discretion in determining the terms, restrictions and conditions of each award granted under the 2007 Plan, provided that no options may be granted after November 20, 2017 and no option may be exercisable after ten years from the date of grant. All option awards granted under the 2007 Plan have an exercise price equal to the fair market value of DJO’s common stock on the
115
Table of Contents
date of grant. Fair market value is defined under the 2007 Plan to be the closing market price of a share of DJO’s common stock on the date of grant or if no market price is available, the fair market value as determined by the Board of Directors. The Compensation Committee retains the discretion to make equity awards at any time in connection with the initial hiring of a new employee, for retention purposes, or otherwise. We do not have any program, plan or practice to time annual or ad hoc grants of stock options or other equity-based awards in coordination with the release of material non-public information or otherwise. The 2007 Plan may be amended or terminated at any time by the DJO Board. However, any amendment that would require shareholder approval in order for the 2007 Plan to continue to meet any applicable legal or regulatory requirements will be effective only if it is approved by DJO’s shareholders. In June 2011, DJO’s Board and majority shareholder approved an amendment to the 2007 Plan to increase the number of shares available for equity awards from 7,500,000 to 7,925,529. Equity awards under the 2007 Plan may be in the form of options or other stock-based awards. Options can be either incentive stock options or non-qualified stock options.
The initial options granted under the 2007 Plan provided that one-third of the stock options would vest over a five year period contingent solely upon the optionee’s continued employment with us, with the remaining two-thirds of the options based on achievement of pre-determined performance targets over a five year period, consisting of Adjusted EBITDA and free cash flow metrics. As described below, amendments in March 2009, March 2010 and June 2011 to the options granted in 2008, 2009 and 2010, have replaced the original financial performance vesting metrics with metrics based upon the achievement of a minimum return of money on invested capital (MOIC), by Blackstone following a liquidation of all or a portion of its investment in DJO’s capital stock.
2008 Option Grants. In February 2008, we granted options for a total of 1,459,812 shares (2008 Options) under the 2007 Plan to Messrs. Cross, Faulstick and Roberts and to Ms. Capps. The 2008 Options have a term of ten years from the date of grant and an exercise price of $16.46 per share. As a result of the several option amendments referred to above, the 2008 options vest in accordance with the following schedule: (a) one-third of each stock option grant (Time-Based Tranche) vests in increments at the end of each calendar year after the grant date if the optionee remains employed with us or any of our subsidiaries as of each vesting date, (b) one-third of each stock option grant vests upon achievement of a specified MOIC to be achieved by Blackstone following the sale of all or a portion of its shares of DJO capital stock (the Market Return Tranche) and (c) one-third of each stock option grant vests upon achievement of a greater MOIC by Blackstone following the sale of all or a portion of its shares of DJO capital stock (the Enhanced Market Return Tranche). The MOIC conditions are required to be achieved or none of the options in the Market Return or the Enhanced Market Return Tranche will vest.
In March 2009, we granted options for 44,228, 33,202 and 22,114 shares under the 2007 Plan to Messrs. Faulstick, Roberts and Murphy respectively (2009 Options). These options have a term of 10 years from the date of grant and an exercise price of $16.46 per share. As with the 2008 Options, following the option amendments described above one-third of each option grant consists of a five year vesting Time-Based Tranche, a Market Return Tranche and an Enhanced Market Return Tranche.
In October 2009, we granted options for 200,000 shares under the 2007 Plan to Mr. Holman in connection with his hiring as Executive Vice President, Sales and Marketing, U.S. Commercial Businesses. These options contained the same terms as the 2009 Options, except that the Time-Based Tranche would vest on the anniversary of the grant date. As a result of the Company’s termination of Mr. Holman’s employment in September 2011, the unvested portion of the Time-Based Tranche was forfeited upon his termination, the vested portion of his options expired 90 days after his termination, and the unvested portion of the Market Return Tranche and the Enhanced Market Return Tranche will terminate in September 2012 unless a vesting event has occurred, in which case these options will terminate 90 days after vesting.
In connection with the hiring of Mr. Mogul as CEO in June 2011, we granted options to Mr. Mogul to purchase 800,000 shares under the 2007 Plan. The options were granted at an exercise price of $16.46. One-third of these options will vest in equal annual installments over four years, contingent on Mr. Mogul’s continued
116
Table of Contents
employment through each vesting date. The other two-thirds of the stock options will vest based upon Blackstone achieving the same minimum MOIC levels included in the outstanding options described in the preceding paragraph.
2012 Amendments to Plan and Option Grants. On February 16, 2012, DJO’s Board and majority shareholder approved an amendment to the 2007 Plan to increase the number of shares of DJO common stock available for award under the 2007 Plan by 2,650,000 (from 7,925,529 to 10,575,529). On February 16, 2012, the Compensation Committee approved the grant of options under the 2007 Plan to key members of management, including a grant of 100,000 options to each of Ms. Capps, Mr. Roberts and Mr. Murphy. These options have a term of 10 years from the date of grant and an exercise price of $16.46 per share. The 2012 Options will vest in four equal installments for 2012 and for each of the three calendar years following 2012, with each such installment vesting only if the final reported financial results for such year show that the Adjusted EBITDA for such year equaled or exceeded the Adjusted EBITDA amount in the financial plan for such year as adopted by the Board of Directors. In the event that Adjusted EBITDA in any of such four years falls short of the amount of Adjusted EBITDA in the financial plan for that year, the installment that did not vest for such year shall be eligible for subsequent vesting at the end of the four year vesting period if the cumulative Adjusted EBITDA achieved over such four period equals or exceeds the cumulative Adjusted EBITDA in the financial plans for such four years and the Adjusted EBITDA in the fourth vesting year equals or exceeds the Adjusted EBITDA in the financial plan for such year. In the event that Blackstone meets its 2.25x MOIC target during the four year vesting period under the options, any unvested installments from prior years and all installments for future years shall thereupon vest.
Change in Control Provisions in Option Awards. All options granted under the 2007 Plan prior to 2012 contain change-in-control provisions that cause the options in the Time-Based Tranche to become immediately vested and exercisable upon the occurrence of a change-in-control if the optionee remains in continuous employment of the Company until the consummation of the change-in-control. These change-in-control provisions will not result in accelerated vesting of the Market Return Tranche or the Enhanced Market Return Tranche, the vesting of which require the achievement of the MOIC target following a liquidation by Blackstone of all or a portion of its equity investment in DJO.
Management Rollover Options. In connection with the acquisition of DJO Opco by DJO in November 2007, certain members of DJO Opco management were permitted to exchange a portion of their DJO Opco stock options for options to purchase an aggregate of 1,912,577 shares of DJO common stock granted under the 2007 Plan on a tax-deferred basis (the DJO Management Rollover Options). The exercise price and number of shares underlying such options were each adjusted in proportion to the relative market values of DJO Opco’s and DJO’s common stock upon the closing of the DJO Merger. All of the DJO Management Rollover Options were fully vested and remained subject to the same terms as were applicable to the original options. During the year ended December 31, 2011, in connection with Mr. Cross’s retirement, we cancelled 355,155 of his DJO Management Rollover Options. As of December 31, 2011, there were 1,557,422 DJO Management Rollover Options outstanding.
Employment Agreement with CEO.
On May 31, 2011, we entered into an Employment Agreement with Mr. Mogul, pursuant to which he will be entitled to receive an annual base salary of $750,000 and an annual bonus at a target rate of 100% of his base salary with a maximum bonus of 150% of his base salary, contingent on his achieving target and maximum performance objectives established by the Company’s Board of Directors. 50% of such bonus shall be earned and paid quarterly based on the Company’s achievement of the established quarterly financial results and the remaining 50% of the target bonus plus any supplemental bonus shall be paid annually based on the Company’s overall financial results for the year. For the 2011 fiscal year, Mr. Mogul’s annual bonus was set at $652,000 in lieu of the foregoing formulaic bonus. The Employment Agreement has a four year term, with automatic one-year extensions unless prior notice of termination is given by either party. Following a termination without
117
Table of Contents
cause (as defined in the Employment Agreement), Mr. Mogul will be entitled to (i) a pro rata portion of his annual bonus based on the percentage of the fiscal year which has elapsed (ii) subject to Mr. Mogul’s compliance with certain non-competition and confidentiality provisions, an amount equal to 1.5 times the sum of his base salary plus his Target Bonus for the year of termination, payable in equal installments in accordance with DJO’s standard pay practices over a period of 18 months, and (iii) continued coverage under the Company’s benefit plans for up to 18 months. The Employment Agreement contains a covenant not to compete during the term of the agreement and for 18 months after termination of the agreement. The Employment Agreement also provided that contingent on Mr. Mogul’s purchase of $2,600,000 in shares of DJO’s common stock at fair market value, the Company would award Mr. Mogul 60,753 restricted shares or restricted share units. Mr. Mogul consummated the purchase of $2,600,000 in DJO shares and elected to receive the 60,753 restricted shares. The restricted shares will vest 50% on June 13, 2012 and 50% on June 13, 2013, in each case contingent on his continued employment through the applicable vesting date.
Retention and Severance Agreements
Retention Agreement for Mr. Holman. In April 2010, DJO, LLC entered into a Retention and Relocation Bonus Agreement (2010 Retention Agreement) with Mr. Holman which provided for the payment to Mr. Holman of certain retention and relocation bonuses. The 2010 Retention Agreement provided for payment of a $300,000 retention bonus which was required to be repaid if Mr. Holman’s employment with us terminated prior to January 1, 2012, other than by reason of death, disability, or a termination without cause. The 2010 Retention Agreement also provided for payment of a $100,000 bonus which was to be credited against future bonus payments to which Mr. Holman would otherwise be entitled under the management incentive bonus plan; provided, however, that other than the crediting of such bonus payment against future bonus payments, such bonus payment was not otherwise required to be repaid upon Mr. Holman’s termination or otherwise. As a result of the Company’s termination of Mr. Holman’s employment without cause in September 2011, the previously paid retention and relocation bonuses became vested and were no longer subject to forfeiture.
2011 Retention and Severance Agreements. On February 25, 2011, the Compensation Committee approved forms of retention bonus (2011 Retention Agreement) and severance agreements (2011 Severance Agreement) for the NEOs, other than for Mr. Cross or Mr. Mogul. The Compensation Committee felt that the assurances offered by these arrangements were necessary in light of the uncertainty surrounding the recent announcement of the retirement of Mr. Cross as CEO and the search for a new CEO.
The 2011 Retention Agreements provide for payment of a cash bonus (the Retention Amount), subject to certain time and performance conditions described herein. The total Retention Amount is $500,000 for Ms. Capps and Mr. Faulstick and $250,000 for Mr. Roberts, Mr. Holman and Mr. Murphy. The 2011 Retention Agreements provide that sixty-five percent (65%) of the executive’s applicable Retention Amount will be paid to the executive on the first payroll date after January 31, 2012 if the executive is continuously employed by the Company through that date, or will be paid upon the earlier termination of the executive’s employment due to death, disability or termination without cause (as defined in the retention agreement). This portion was paid to Mr. Holman following his termination without cause in September 2011 and to Ms. Capps and Messrs. Faulstick, Roberts, Capizzi and Murphy on February 10, 2012. The remaining 35% of the Retention Amount is payable as follows: (a) 17.5% of the Retention Amount will be paid to the executive if the executive is employed through January 31, 2012 and the Company achieves the revenue target for 2011 under the 2011 Bonus Plan as originally adopted, and (b) 17.5% of the Retention Amount will be paid to the executive if the executive is employed through January 31, 2012 and the Company achieves the Adjusted EBITDA target for 2011 under the 2011 Bonus Plan as originally adopted. As a result of the Company’s 2011 financial performance, the following amounts are scheduled to be paid in February 2012 to the following executives: Ms. Capps $87,500, Mr. Faulstick $87,500, Mr. Roberts $43,750, Mr. Murphy $43,750 and Mr. Holman $43,750.
The 2011 Severance Agreements provide that if the executive’s employment is terminated by the Company without “cause” (as defined in the severance agreement) and for so long as the executive is in compliance with
118
Table of Contents
the restrictive covenants described below, the executive will be paid certain amounts as described below in “Potential Payments Upon Termination or Change-in-Control.” As a result of the termination without cause of Mr. Holman’s employment, he is receiving severance payments pursuant to his 2011 Severance Agreement. As a result of Mr. Faulstick’s voluntary resignation effective in February 2012, he is not entitled to any benefits under the 2011 Severance Agreement.
Retirement of Mr. Cross as CEO. On January 17, 2011, Mr. Cross announced his intention to retire and resign as President and CEO of DJO effective the earlier of June 30, 2011 or the date his successor is hired (Resignation Date). Mr. Cross’ employment terminated with the hiring of Mr. Mogul as CEO on June 13, 2011. Mr. Cross agreed to serve in the position of Chairman of the Board of Directors following the Resignation Date through December 31, 2011. On January 21, 2011, Mr. Cross and the Company entered into a Director Arrangement, Separation Agreement and General Release (the Separation Agreement) pursuant to which Mr. Cross is entitled to receive:
| • | A monthly salary of $98,437.66 over the period beginning on January 1, 2011 and ending on the Resignation Date. This amount represents a pro-rated portion of Mr. Cross’s base salary and a pro-rated bonus for 2011. |
| • | A cash severance payment of $1,181,250, paid in installments over a 12-month period following the Resignation Date. |
| • | Payment of his 2010 bonus under the 2010 Bonus Plan at the same time as amounts are paid to other participants in such plan, based on the Company’s actual achievement of performance goals through December 31, 2010. |
| • | Eighteen (18) months of continued medical coverage with the Company agreeing to be responsible for the full COBRA premium with respect to such continuation of medical coverage for Mr. Cross and his beneficiaries. |
| • | An extended option exercise period for 117,940 of his Company stock options that were vested as of December 31, 2010. Mr. Cross will have until the earlier of the date of a “change in control” (as defined in the applicable grant agreement) and the original date of expiration of the option term to exercise the vested stock options that remain outstanding. |
| • | An extended option exercise period for an additional 30,000 Company stock options that were vested as of December 31, 2010. Mr. Cross will have until the earlier of the date of a “change in control” (as defined in the applicable grant agreement) or January 2, 2012 to exercise these stock options. |
| • | A cash payment of $1,999,758.75 for the cancellation of 355,155 vested DJO Management Rollover Options granted to Mr. Cross on November 20, 2007. Mr. Cross may exercise his remaining 177,577 vested DJO Management Rollover Options granted to him on November 20, 2007 until the date of expiration of the applicable option term. |
Effective on the Resignation Date, 20,546 vested and 59,523 unvested time-based stock options were forfeited. In addition, Mr. Cross’s unvested stock options that were subject to the First Market Return Tranche and the Second Market Return Tranche (as defined in the applicable grant agreement) were forfeited on January 1, 2012.
In consideration for the receipt of the payments and benefits provided under the Separation Agreement, Mr. Cross agreed to certain restrictive covenants. Mr. Cross will not compete with the Company for one year following the Resignation Date either by engaging in a competitive business, entering the employ of a competitive business or interfering with any business relationship between the Company and its customers. Mr. Cross will also agree not to solicit or hire any Company employees for one year following the Resignation Date. In addition, certain of the payments in the Separation Agreement are conditioned upon the execution of a general release by Mr. Cross of all claims, liabilities and causes of action which he may have or ever claim to have against the Company and its affiliates.
119
Table of Contents
The Separation Agreement also set forth Mr. Cross’s compensation as Chairman of the Board, when he assumed such position on the Resignation Date. Mr. Cross received a monthly fee of $49,218.77 for his service as Chairman of the Board for the period beginning on the Resignation Date and ending on December 30, 2011. Mr. Cross resigned as Chairman of the Board on December 30, 2011 and Mr. Zafirovski was appointed the new Chairman on January 5, 2012.
Tax Considerations
Section 162(m) of the Internal Revenue Code of 1986, as amended, provides that compensation in excess of $1,000,000 paid to the CEO or to other executive officers of a public company will not be deductible for federal income tax purposes unless such compensation is paid pursuant to one of the enumerated exceptions set forth in Section 162(m). As a privately held company, we are not required to comply with Section 162(m) to ensure tax deductibility of executive compensation.
Summary Compensation Table
The following table sets forth summary information about the compensation during 2011, 2010, and 2009 for services rendered in all capacities by our Chief Executive Officer, Chief Financial Officer and each of our three other most highly compensated executive officers. Also included in the table below is information regarding Mr. Cross, who was Chief Executive Officer until June 13, 2011, and Mr. Holman, who was not serving as executive officer as of the end of 2011 but for whom disclosure would have been provided but for the fact that he was not serving as executive officers as of the end of 2011. All of the individuals listed in the following table are referred herein collectively as the Named Executive Officers or NEOs.
| Name and Principal Position |
Year | Salary | Bonus (2) |
Stock Awards |
Option Awards (11) |
Non-Equity Incentive Plan Compensation (14) |
All
Other Compensation (15) |
Total | ||||||||||||||||||||||||
| Michael P. Mogul (1) |
2011 | $ | 418,269 | $ | 652,000 | (3) | $ | 999,995 | (10) | $ | 1,662,775 | (12) | $ | — | $ | 259,352 | $ | 3,992,391 | ||||||||||||||
| President, Chief Executive Officer and Director |
||||||||||||||||||||||||||||||||
| Vickie L. Capps |
2011 | 472,500 | — | — | — | 38,163 | 8,575 | 519,238 | ||||||||||||||||||||||||
| Executive Vice President, Chief Financial Officer and Treasurer |
2010 | 450,000 | — | — | — | 95,436 | 8,575 | 554,011 | ||||||||||||||||||||||||
| 2009 | 453,461 | — | — | — | 178,546 | 8,575 | 640,582 | |||||||||||||||||||||||||
| Luke T. Faulstick (4) |
2011 | 470,000 | — | — | — | 37,288 | 8,575 | 515,863 | ||||||||||||||||||||||||
| Former Executive Vice President, Chief Operating Officer |
2010 | 400,000 | — | — | — | 84,832 | 8,575 | 493,407 | ||||||||||||||||||||||||
| 2009 | 403,077 | — | — | 185,650 | (13) | 158,708 | 8,575 | 756,010 | ||||||||||||||||||||||||
| Donald M. Roberts |
2011 | 315,000 | — | — | — | 25,442 | 8,575 | 349,017 | ||||||||||||||||||||||||
| Executive Vice President, General Counsel and Secretary |
2010 | 300,000 | — | — | — | 63,624 | 8,575 | 372,199 | ||||||||||||||||||||||||
| 2009 | 302,308 | — | — | 139,366 | (13) | 119,031 | 8,575 | 569,280 | ||||||||||||||||||||||||
| Stephen J. Murphy (5) |
2011 | 304,760 | — | — | — | 60,870 | 21,565 | 387,195 | ||||||||||||||||||||||||
| Executive Vice President, Sales and Marketing, International Commercial Businesses |
2010 | 297,312 | — | — | — | 102,410 | 49,302 | 449,024 | ||||||||||||||||||||||||
| 2009 | 247,344 | — | — | 54,567 | (13) | 178,956 | 44,019 | 524,886 | ||||||||||||||||||||||||
| Leslie H. Cross (6) |
2011 | 527,020 | — | — | — | — | 3,165,353 | 3,692,373 | ||||||||||||||||||||||||
| Former President and Chief Executive Officer: Former Chairman of the Board: Director |
2010 | 625,000 | — | — | — | 132,550 | 8,575 | 766,125 | ||||||||||||||||||||||||
| 2009 | 629,808 | — | — | — | 247,981 | 8,575 | 886,364 | |||||||||||||||||||||||||
| Andrew P. Holman (7) |
2011 | 259,808 | 206,250 | (8) | — | — | 28,269 | 199,225 | 693,552 | |||||||||||||||||||||||
| Former Executive Vice President, Sales and Marketing, U.S. Commercial Businesses |
2010 | 300,000 | 479,430 | (9) | — | — | 63,624 | 8,575 | 851,629 | |||||||||||||||||||||||
| 2009 | 92,308 | 15,000 | (9) | — | 849,884 | (13) | 31,611 | 1,154 | 989,957 | |||||||||||||||||||||||
| (1) | Mr. Mogul commenced employment with the Company on June 13, 2011 as President, Chief Executive Officer and Director. |
| (2) | Excluded from the table are amounts payable under retention bonus agreements entered into in January 2011 with each of our executive officers (other than Mr. Holman who vested in his retention bonus upon his termination without cause in September 2011, and Mr. Mogul who was hired in June 2011). 65% of the retention bonus is payable in 2012 contingent on the executive’s continuous employment until January 31, 2012, and 35% of the retention bonus is payable following the achievement of revenue and Adjusted EBITDA performance conditions under the 2011 |
120
Table of Contents
| Bonus Plan, contingent on the executive’s continued employment until January 31, 2012. See “2011 Retention and Severance Agreements” in “Compensation Discussion and Analysis” above. |
| (3) | Pursuant to his Employment Agreement dated June 13, 2011, Mr. Mogul was entitled to receive a bonus of $652,000 for 2011, which was paid in February 2012. |
| (4) | On December 9, 2011, Mr. Faulstick announced his resignation as executive officer and employee. His employment ended on February 3, 2012. |
| (5) | Mr. Murphy’s Salary, Non-Equity Incentive Plan Compensation and All Other Compensation for each fiscal year have been converted from pounds sterling at an average annual exchange rate for the year as follows: for fiscal year 2011 at $1.60 per pound, for fiscal year 2010 at $1.56 per pound and for fiscal year 2009 at $1.55 per pound. |
| (6) | Mr. Cross’s employment and status as President and Chief Executive Officer terminated on June 13, 2011. Mr. Cross continued as Chairman of the Board until December 30, 2011 and he remains a director. |
| (7) | Mr. Holman’s employment terminated effective September 27, 2011. |
| (8) | Mr. Holman’s employment was terminated without cause, resulting in payment to him of 65% the Retention Amount under his 2011 Retention Agreement, plus an additional $43,750 based on achievement of the revenue component of the Retention Agreement. Pursuant to the 2011 Severance Agreement, Mr. Holman was paid $160,192 in restrictive covenant payments in 2011. See “2011 Severance and Retention Agreements” in “Retention and Severance Agreements” in the “Compensation Discussion and Analysis” above. |
| (9) | Amounts consist of retention and relocation bonuses paid to Mr. Holman pursuant to his 2010 Retention Agreement. See “Retention Agreement for Mr. Holman” in “Retention and Severance Agreements” in “Compensation Discussion and Analysis” above. |
| (10) | This amount shown in this column represents the aggregate grant date fair value of 60,753 restricted shares awarded to Mr. Mogul on September 9, 2011 computed in accordance with FASB ASC Topic 718. These shares vest 50% on June 13, 2012 and 50% on June 12, 2013, contingent upon his continued employment with the Company. The fair value of the restricted stock award is estimated using the fair market value of our stock on the grant date ($16.46 per share). We are required to reflect the total grant date fair value of this award in the year of award, rather than the portion of this amount that is recognized for financial statement reporting purposes in a given fiscal year. This amount has not been paid to and may not correspond to the actual value that is ultimately realized by Mr. Mogul. See Note 15 of the notes to the audited consolidated financial statements included in this Prospectus for a discussion of the relevant assumptions used in calculating the aggregate grant date fair value. |
| (11) | The amounts shown in this column reflect the aggregate grant date fair value of the option awards granted in the respective years. Pursuant to SEC rules, the amounts shown exclude the impact of estimated forfeitures related to service-based vesting conditions. We are required to reflect the total grant date fair values of the option grants in the year of grant, rather than the portion of this amount that was recognized for financial statement reporting purposes in a given fiscal year. These amounts may not correspond to the actual value that is ultimately realized by the NEOs. See Note 15 of the notes to the audited consolidated financial statements included in this Prospectus for a discussion of the relevant assumptions used in calculating the aggregate grant date fair value. See “Equity Compensation Awards” in the “Compensation Discussion and Analysis” above for a description of the vesting conditions for these options. |
| (12) | The amount shown for Mr. Mogul’s 2011 option award includes only the amount related to the Time-Based Tranche of options granted, as achievement of the performance conditions of the Market Return Tranche and Enhanced Market Return Tranche was not deemed probable. If the satisfaction of the performance component to the Market Return Tranche and Enhanced Market Return Tranche was determined to be probable, the aggregate grant date fair value of the 2011 option awards would have been: $2,265,442. |
| (13) | The amounts shown for the 2009 option awards include an amount related to the Market Return Tranche (when it was referred to as the Performance-Based Tranche, and prior to giving effect to the 2010 and 2011 modifications) because achievement of the performance conditions related to this tranche at the grant date was determined to be probable. However, the value attributable to the portion of the 2009 option awards included in the Enhanced Market Return Tranche (when it was referred to as the Enhanced Performance-Based Tranche), which had both a performance component and a market component, was excluded because achievement of the performance component related to this tranche at the grant date was not deemed probable. If the satisfaction of the performance component to the Enhanced Market Return Tranche was determined to be probable, the aggregate grant date fair value of the 2009 option awards would have been: $206,878 for Mr. Faulstick, $155,303 for Mr. Roberts, $61,644 for Mr. Murphy and $945,883 for Mr. Holman. |
| (14) | The amounts shown in this column represent amounts earned in the respective year based on the results of the Bonus Plan, some of which was paid in the subsequent year. See “Annual and Quarterly Cash Incentive Compensation” in the “Compensation Discussion and Analysis” above for terms of bonus plans. |
| (15) | Perquisites and other personal benefits are valued on the basis of the aggregate incremental cost to the Company of such perquisites and other personal benefits. The amounts shown in this column for 2011 for each of the NEOs is set forth in the following table: |
For the Year Ended December 31, 2011
| Mr. Mogul | Ms. Capps | Mr. Faulstick | Mr. Roberts | Mr. Murphy | Mr. Cross | Mr. Holman | ||||||||||||||||||||||
| Severance (a) |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | 2,681,249 | $ | 160,192 | ||||||||||||||
| Director compensation (b) |
321,563 | |||||||||||||||||||||||||||
| Taxable relocation |
240,082 | — | — | — | — | — | 10,104 | |||||||||||||||||||||
| Accrued vacation |
— | — | — | — | — | 153,966 | 6,790 | |||||||||||||||||||||
| Housing reimbursement |
10,695 | — | — | — | — | — | 13,564 | |||||||||||||||||||||
| 401(k) matching contribution |
8,575 | 8,575 | 8,575 | 8,575 | — | 8,575 | 8,575 | |||||||||||||||||||||
| Vehicle allowance |
— | — | — | — | 15,529 | — | — | |||||||||||||||||||||
| Medical insurance, Life insurance and Income protection |
— | — | — | — | 6,036 | — | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total |
$ | 259,352 | $ | 8,575 | $ | 8,575 | $ | 8,575 | $ | 21,565 | $ | 3,165,353 | $ | 199,225 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| (a) | Mr. Cross resigned as president and chief executive officer on June 13, 2011. The Severance amount for Mr. Cross includes $1,999,759 paid to him to repurchase 355,155 DJO Management Rollover Options. See “Retirement of Mr. Cross as CEO” in “Compensation Discussion and Analysis” above. Mr. Holman’s employment was terminated by the Company without cause on September 27, 2011. |
| (b) | Represents fees paid to Mr. Cross for his service as Chairman of the Board from June 14, 2011 through December 30, 2011. |
121
Table of Contents
Grants of Plan-Based Awards in 2011
The following table sets forth certain information with respect to grants of plan-based awards made to the NEOs during 2011.
| Estimated
Future Payouts Under Non-Equity Incentive Plan Awards (1) |
Estimated
Future Payouts Under Equity Incentive Plan Awards |
All Other Stock Awards: Number of Shares of Stock or Units (#) |
All Other Option Awards: Number of Securities Underlying Options (#) |
Exercise or Base Price of Option Awards ($/Share) |
Grant Date Fair Value of Stock and Option Awards ($) |
|||||||||||||||||||||||||||||||||||||||
| Name |
Grant Date |
Threshold ($) |
Target ($) |
Maximum ($) |
Threshold (#) |
Target (#) |
Maximum (#) |
|||||||||||||||||||||||||||||||||||||
| Michael P. Mogul |
6/13/2011 | (2) | $ | — | $ | — | $ | — | — | — | — | — | 800,000 | $ | 16.46 | $ | 2,265,442 | |||||||||||||||||||||||||||
| 6/13/2011 | (3) | — | — | — | — | — | — | 60,753 | — | 16.46 | 999,995 | |||||||||||||||||||||||||||||||||
| Vickie L. Capps |
1/1/2011 | 198,450 | 330,750 | 614,250 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||
| Luke T. Faulstick |
1/1/2011 | 197,400 | 329,000 | 611,000 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||
| Donald M. Roberts |
1/1/2011 | 132,300 | 220,500 | 409,500 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||
| Stephen J. Murphy (4) |
1/1/2011 | 127,999 | 213,332 | 396,188 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||
| Leslie H. Cross |
1/1/2011 | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||
| Andrew P. Holman |
1/1/2011 | 126,000 | 210,000 | 390,000 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||
| (1) | The amounts set forth in these columns under “Estimated Future Payouts Under Non-Equity Incentive Plan Awards” represent the threshold, target and maximum bonus potential under the 2011 Bonus Plan. See discussion of “Threshold Bonus”, “Target Bonus” and “Supplemental Bonus” in “Annual and Quarterly Cash Incentive Compensation” in “Compensation Discussion and Analysis” above for a description of the conditions for the 2011 Bonus Plan. |
| (2) | Upon commencement of his employment on June 13, 2011, Mr. Mogul was granted 800,000 options to acquire shares of common stock, pursuant to the DJO Form Option Agreement. |
| (3) | As provided in his Employment Agreement, upon Mr. Mogul’s purchase of $2,600,000 of shares of common stock, the Company awarded Mr. Mogue 60,753 restricted shares, which vest 50% on June 13, 2012 and 50% on June 13, 2013, contingent upon his continued employment with the Company. |
| (4) | Amounts shown for Mr. Murphy have been translated from pounds sterling at an average annual rate of $1.60 per pound. |
122
Table of Contents
Outstanding Equity Awards at 2011 Fiscal Year-End
The following table sets forth certain information regarding equity-based awards held by each of the NEOs as of December 31, 2011.
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||
| Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) |
Option Exercise Price ($) |
Option Expiration Date |
Number of Shares or Units of Stock That Have Not Vested (#) |
Market Value of Shares or Units That Have Not Vested ($) |
Equity Incentive Plan Awards: Number of Unearned Shares, Units, or Other Rights That Have Not Vested (#) |
Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units, or Other Rights That Have Not Vested ($) |
|||||||||||||||||||||||||||
| Michael P. Mogul |
— | 266,667 | (11) | 533,333 | (6) | 16.46 | 6/13/2021 | — | — | — | — | |||||||||||||||||||||||||
| — | — | — | 60,753 | (8) | 999,995 | — | — | |||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| — | 266,667 | 533,333 | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| Vickie L. Capps |
162,920 | (1) | 24,468 | (4) | 206,791 | (6) | 16.46 | 2/21/2018 | — | — | — | — | ||||||||||||||||||||||||
| 91,586 | (2) | — | — | 13.10 | 5/11/2017 | — | — | — | — | |||||||||||||||||||||||||||
| 48,846 | (2) | — | — | 12.91 | 4/3/2016 | — | — | — | — | |||||||||||||||||||||||||||
| 76,321 | (2) | — | — | 7.00 | 12/8/2014 | — | — | — | — | |||||||||||||||||||||||||||
| 5,343 | (2) | — | — | 7.18 | 2/26/2014 | — | — | — | — | |||||||||||||||||||||||||||
| 69,785 | (2) | — | — | 8.29 | 12/9/2013 | — | — | — | — | |||||||||||||||||||||||||||
| 6,075 | (2) | — | — | 8.29 | 12/9/2013 | — | — | — | — | |||||||||||||||||||||||||||
| 15,725 | (2) | — | — | 8.29 | 12/9/2013 | — | — | — | — | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| 476,601 | 24,468 | 206,791 | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| Luke T. Faulstick (10) |
9,949 | (3) | — | 25,799 | (6) | 16.46 | 3/7/2019 | — | — | — | — | |||||||||||||||||||||||||
| 144,818 | (1) | — | 183,815 | (6) | 16.46 | 2/21/2018 | — | — | — | — | ||||||||||||||||||||||||||
| 91,586 | (2) | — | — | 13.10 | 5/11/2017 | — | — | — | — | |||||||||||||||||||||||||||
| 48,846 | (2) | — | — | 12.91 | 4/3/2016 | — | — | — | — | |||||||||||||||||||||||||||
| 2,552 | (2) | — | — | 7.00 | 12/8/2014 | — | — | — | — | |||||||||||||||||||||||||||
| 7,480 | (2) | — | — | 7.00 | 12/8/2014 | — | — | — | — | |||||||||||||||||||||||||||
| 6,075 | (2) | — | — | 8.29 | 12/9/2013 | — | — | — | — | |||||||||||||||||||||||||||
| 4,753 | (2) | — | — | 8.29 | 12/9/2013 | — | — | — | — | |||||||||||||||||||||||||||
| 34,964 | (2) | — | — | 8.29 | 12/9/2013 | — | — | — | — | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| 351,023 | — | 209,614 | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| Donald M. Roberts |
7,470 | (3) | 6,086 | (5) | 19,367 | (6) | 16.46 | 3/7/2019 | — | — | — | — | ||||||||||||||||||||||||
| 88,248 | (1) | 13,253 | (4) | 112,012 | (6) | 16.46 | 2/21/2018 | — | — | — | — | |||||||||||||||||||||||||
| 91,586 | (2) | — | — | 13.10 | 5/12/2017 | — | — | — | — | |||||||||||||||||||||||||||
| 48,846 | (2) | — | — | 12.91 | 4/3/2016 | — | — | — | — | |||||||||||||||||||||||||||
| 1,557 | (2) | — | — | 8.84 | 5/25/2015 | — | — | — | — | |||||||||||||||||||||||||||
| 6,075 | (2) | — | — | 8.84 | 5/25/2015 | — | — | — | — | |||||||||||||||||||||||||||
| 22,896 | (2) | — | — | 8.84 | 5/25/2015 | — | — | — | — | |||||||||||||||||||||||||||
| 10,810 | (2) | — | — | 8.29 | 12/9/2013 | — | — | — | — | |||||||||||||||||||||||||||
| 24,270 | (2) | — | — | 8.29 | 12/9/2013 | — | — | — | — | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| 301,758 | 19,339 | 131,379 | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| Stephen J. Murphy |
3,318 | (3) | 4,054 | (5) | 14,742 | (6) | 16.46 | 12/9/2019 | — | — | — | — | ||||||||||||||||||||||||
| 72,410 | (1) | 10,874 | (5) | 91,907 | (6) | 16.46 | 2/21/2018 | — | — | — | — | |||||||||||||||||||||||||
| 36,634 | (2) | — | — | 13.10 | 5/11/2017 | — | — | — | — | |||||||||||||||||||||||||||
| 30,529 | (2) | — | — | 12.91 | 4/3/2016 | — | — | — | — | |||||||||||||||||||||||||||
| 27,476 | (2) | — | — | 7.00 | 12/8/2014 | — | — | — | — | |||||||||||||||||||||||||||
| 8,395 | (2) | — | — | 8.29 | 12/9/2013 | — | — | — | — | |||||||||||||||||||||||||||
| 6,075 | (2) | — | — | 1.33 | 12/19/2012 | — | — | — | — | |||||||||||||||||||||||||||
| 5,373 | (2) | — | — | 1.33 | 12/19/2012 | — | — | — | — | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| 190,210 | 14,928 | 106,649 | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| Leslie H. Cross |
147,940 | (9) | — | 251,620 | (6) | 16.46 | 2/21/2018 | — | — | — | — | |||||||||||||||||||||||||
| 71,233 | (2) | — | — | 13.10 | 5/12/2017 | — | — | — | — | |||||||||||||||||||||||||||
| 27,476 | (2) | — | — | 12.91 | 4/3/2016 | — | — | — | — | |||||||||||||||||||||||||||
| 14,450 | (2) | — | — | 7.00 | 12/8/2014 | — | — | — | — | |||||||||||||||||||||||||||
| 64,418 | (2) | — | — | 8.29 | 12/9/2013 | — | — | — | — | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| 325,517 | — | 251,620 | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| Andrew P. Holman |
— | — | 133,333 | (7) | 16.46 | 10/5/2019 | — | — | — | — | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
| — | — | 133,333 | ||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||
123
Table of Contents
| (1) | These amounts reflect (a) the number of shares underlying the Time-Based Tranche of options that are vested and exercisable which were granted in 2008 under the 2007 Plan, and (b) the number of shares underlying the Market Return Tranche (which, prior to the March 2010 option modification, was referred to as the Performance-Based Tranche) of options that were granted in 2008 under the 2007 Plan, and were earned (i.e., their performance conditions were satisfied) during 2008 and 2009. |
| (2) | These amounts reflect the number of shares underlying the DJO Management Rollover Options which were fully vested upon issuance in connection with the DJO Merger. |
| (3) | These amounts reflect (a) the number of shares underlying the Time-Based Tranche of options that are vested and exercisable which were granted in 2009 under the 2007 Plan, and (b) the number of shares underlying the Market Return Tranche (which, prior to the March 2010 option modification, was referred to as the Performance-Based Tranche) of options that were granted in 2009 under the 2007 Plan, and were earned (i.e., their performance conditions were satisfied) in 2009. |
| (4) | These amounts reflect the number of shares underlying the Time-Based Tranche of options that are not vested and not exercisable which were granted in 2008 under the 2007 Plan. These options reflect the remaining portion of the grant made in 2008 and vest on December 31, 2012. |
| (5) | These amounts reflect the number of shares underlying the Time-Based Tranche of options that are not vested and not exercisable which were granted in 2009 under the 2007 Plan. These options reflect the remaining portion of the grant made in 2009 and vest as follows: 18.33% of the original grant vests on each of March 7, 2012, and March 7, 2013 and 18.34% of the original grant vests on March 7, 2014. |
| (6) | The amounts set forth in this column reflect the number of shares underlying the Market Return Tranche and the Enhanced Market Return Tranches of options that have not been earned (i.e., their performance conditions have not been satisfied). See “Equity Compensation Awards” in “Compensation Discussion and Analysis” above. |
| (7) | Mr. Holman’s employment terminated on September 27, 2011. Pursuant to the DJO Form Option Agreement, upon termination without cause, unvested options from the Time-Based Tranche were forfeited and unvested options from the Market Return and Enhanced Market Return Tranches shall remain outstanding for the 12 month period following the date of such termination. If the options in the Market Return Tranche and Enhanced Market Return Tranche do not vest in this 12 month period, then they will be forfeited and if they vest in this 12 month period, then they must be exercised within 90 days after vesting or they will be forfeited. |
| (8) | Upon Mr. Mogul’s purchase of $2,600,000 of shares of common stock, the Company granted 60,753 restricted shares, which vest 50% on the first anniversary date and 50% on the second anniversary date, contingent upon his continued employment with the Company. |
| (9) | Pursuant to the Director Arrangement, Separation Agreement and General Release, 30,000 of these options expired on January 2, 2012. The remainder of these options expire on the earlier of a change of control or the original option expiration date. |
| (10) | Pursuant to Mr. Faulstick’s resignation, all of his unvested options terminated on the date of termination of his employment on February 3, 2012 and the vested Time-Based Tranche expires 90 days after termination of his employment. Mr. Faulstick’s vested Rollover Options were amended to provide that such options will not expire until the original expiration date for such options. |
| (11) | This amount reflects the number of shares underlying the Time-Based Tranche of options that are not vested and not exercisable which were granted on June 13, 2011 under the 2007 Plan. These options vest 25% on each of June 13, 2012, June 13, 2013, June 13, 2014 and June 13, 2015. |
Option Exercises During 2011
No options were exercised during the year ended December 31, 2011 by or for our NEOs.
Non-Qualified Deferred Compensation for 2011
Certain executives may defer receipt of part or all of their cash compensation under the DJO, LLC Executive Deferred Compensation Plan (the Deferred Plan), a plan sponsored by DJO, LLC, a subsidiary of DJOFL. The Deferred Plan allows executives to save for retirement in a tax-effective way at minimal cost to DJO, LLC. Under this program, amounts deferred by the executive are deposited into a trust for investment and eventual benefit payment. The obligations of DJO, LLC under the Deferred Plan are unsecured obligations to pay deferred compensation in the future from the assets of the trust. Participants will have the status of unsecured general creditors with respect to the benefit obligations of the Deferred Plan, and the assets set aside in the trust for those benefits will be available to creditors of DJO, LLC in the event of bankruptcy or insolvency. Each participant may elect to defer under the Deferred Plan all or a portion of his or her cash compensation that may otherwise be payable in a calendar year. A participant’s compensation deferrals are credited to the participant’s account under the Deferred Plan and the trust. Each participant may elect to have the amounts in such participant’s account invested in one or more investment options available under the Deferred Plan, which investment options are substantially the same investment options available to participants in DJO, LLC’s 401(k) Savings Plan. The Deferred Plan also permits DJO, LLC to make contributions to the Deferred Plan, including matching contributions, at its discretion, but no such contributions have been made to date. To the extent that Company contributions are made to the Deferred Plan, the Committee may impose vesting criteria to aid in the employment retention of participants. A participant’s eventual benefit will depend on his or her level of contributions, DJO, LLC’s contributions, if any, and the investment performance of the particular investment options selected.
124
Table of Contents
Certain employees of our U.K. subsidiary, including Mr.Murphy, are participants in Personal Pension Plan contracts which provide for the deferral of part or all of their cash compensation (UK Deferral Plan). The UK Deferral Plan allows the UK employees to save for retirement in a tax-effective way. Neither DJO nor its affiliates have any obligations under the UK Deferral Plan. Each participant may elect to defer under the UK Deferral Plan all or a portion of his or her cash compensation that may otherwise be payable in a calendar year. A participant’s compensation deferrals are credited to the participant’s account under the UK Deferral Plan. Each participant may elect to have the amounts in such participant’s account invested in one or more investment options available under the UK Deferral Plan. The UK Deferral Plan also permits DJO’s UK subsidiary to make contributions to the Deferred Plan at its discretion and monthly contributions have been made. A participant’s eventual benefit will depend on his or her level of contributions, the Company’s contributions and the investment performance of the particular investment options selected.
The following table sets forth information for each of the NEO’s who participate in DJO LLC’s non-qualified deferred compensation plan or the U.K. deferral plan.
| Name |
Executive Contributions in 2011 |
Registrant Contributions in 2011 |
Aggregate Earnings in 2011 |
Aggregate Withdrawals/ Distributions |
Aggregate Balance at December 31, 2011 |
|||||||||||||||
| Michael P. Mogul |
$ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
| Vickie L. Capps |
— | — | — | — | — | |||||||||||||||
| Luke T. Faulstick |
— | — | — | — | — | |||||||||||||||
| Donald M. Roberts (1) |
23,825 | — | 2,259 | — | 218,869 | |||||||||||||||
| Stephen J. Murphy (2) |
15,200 | 48,579 | — | — | 343,262 | |||||||||||||||
| Leslie H. Cross |
— | — | — | — | — | |||||||||||||||
| Andrew P. Holman |
— | — | — | — | — | |||||||||||||||
| (1) | Amounts deferred by Mr. Roberts under the Deferred Plan have been reported as 2011 compensation to Mr. Roberts in the “Salary” column in the Summary Compensation Table above. Amounts in the aggregate balance for Mr. Roberts include amounts earned as compensation prior to the DJO Merger when Mr. Roberts was an executive officer of DJO Opco. Amounts included in aggregate earnings are not required to be included in Summary Compensation Table above. |
| (2) | Amounts deferred by Mr. Murphy under the UK Deferral Plan have been reported as compensation to Mr Murphy in the “Salary” column in the Summary Compensation Table above. Amounts in the aggregate balance for Mr. Murphy include amounts earned as compensation prior to the DJO Merger when Mr. Murphy was an executive officer of DJO Opco, as well as additional amounts transferred by Mr. Murphy from other sources. Amounts included in aggregate earnings are not required to be included in the Summary Compensation Table above. The amounts for Mr. Murphy have been converted from pounds sterling at an average annual exchange rate for 2011 of $1.60 per pound. The registrant is unable to determine the Aggregate Earnings in 2011 due to the inclusion of transfers which were not part of Mr. Murphy’s compensation. |
Potential Payments Upon Termination or Change-in-Control
The 2011 Severance Agreements provide that if the executive’s employment is terminated by the Company without “cause” (as defined in the severance agreement) and for so long as the executive is in compliance with the restrictive covenants described below, the executive will be paid the following amounts: (a) a monthly payment equal to the executive’s monthly base salary for 18 months, in the case of Mr. Faulstick and Ms. Capps, or 12 months, in the case of Mr. Roberts, Mr. Holman and Mr. Murphy; (b) a monthly payment equal to one-twelfth of the executive’s target annual bonus amount under the management incentive bonus plan for the year of termination for the 18 or 12 month period, as applicable; (c) a pro-rata share of any quarterly bonus for the quarter in which the executive’s employment is terminated plus a pro-rata share of the annual bonus that the executive would have received for the year of termination but for the termination of employment; and (d) Company-paid COBRA benefits for the 18 month or 12 month period, as applicable. In addition, if the
125
Table of Contents
executive holds DJO Management Rollover Options, the severance agreement provides that the Company will purchase the Management Rollover Options held by the executive on the termination date at a price equal to the difference, if any, between the fair market value of the underlying common stock and the per share exercise price of the Management Rollover Options. Payment of the benefits under the severance agreement is contingent on compliance with a covenant not to compete against the Company and a covenant not to solicit customers or employees for either 18 or 12 months, as applicable. Such payments will not be made if the executive’s employment terminates due to death or disability. As a result of the termination without cause of Mr. Holman’s employment, he is receiving the severance payments described in this paragraph. As a result of Mr. Faulstick’s voluntary resignation effective in February 2012, he is not entitled to any benefits under the 2011 Severance Agreement.
On May 31, 2011, the Company entered into an Employment Agreement with Mr. Mogul which contains severance provisions which provide that following a termination without cause (as defined in the Employment Agreement), Mr, Mogul will be entitled to (i) a pro rata portion of his annual bonus based on the percentage of the fiscal year which has elapsed, (ii) subject to Mr. Mogul’s compliance with certain non-competition and confidentiality provisions, an amount equal to 1.5 times the sum of his base salary plus his Target Bonus for the year of termination, payable in equal installments in accordance with DJO’s standard pay practices over a period of 18 months, and (iii) continued coverage under the Company’s benefit plans for up to 18 months.
As a result of the termination of Mr. Holman’s employment without cause, he is receiving the monthly severance payments described above in twelve monthly payments since his termination date on September 27, 2011. If Mr. Holman had been paid his total severance benefit in a lump sum on his termination date he would have received: $259,808 in salary; $181,866 for his Target Bonus and $11,357 in health benefits.
The following table shows the amount of potential cash payments and the value of other severance benefits each of the NEOs would be entitled to if his or her employment were terminated “without cause” or if he or she resigned for “good reason” as of December 31, 2011:
| Name |
Base Salary Payment |
Bonus Payment |
Health Benefits |
Total | ||||||||||||
| Michael P. Mogul |
$ | 1,125,000 | $ | 750,000 | $ | 28,204 | $ | 1,903,204 | ||||||||
| Vickie L. Capps |
708,750 | 496,125 | 27,396 | 1,232,271 | ||||||||||||
| Luke T. Faulstick |
705,000 | 329,000 | 23,742 | 1,057,742 | ||||||||||||
| Donald M. Roberts |
315,000 | 220,500 | 13,152 | 548,652 | ||||||||||||
| Stephen J. Murphy |
304,760 | 213,332 | 28,032 | 546,124 | ||||||||||||
| (1) | Mr. Faulstick’s employment terminated on February 3, 2012. Because Mr. Faulstick resigned, he was not entitled to receive any of the compensation provided for in his 2011 Severance Agreement. |
The options granted to our executive officers and other members of management contain change-in-control provisions that would result in accelerated vesting of the Time-Based Tranche upon the occurrence of a change-in-control. Specifically, the Time-Based Tranche would become immediately exercisable upon the occurrence of a change-in-control if the optionee remains in continuous employment of the Company until the consummation of the change-in-control. However, this change-in-control provision does not apply to the Market Return or Enhanced Market Return Tranches, the vesting of which requires the achievement of the minimum return on MOIC targets following a liquidation by Blackstone of all or a portion of its equity interest in DJO.
Compensation of Directors
The Compensation Committee of the DJO Board reviews the compensation of our Directors on an annual basis. During 2011, our Board of Directors consisted of nine persons: Leslie H. Cross, Chinh E. Chu, Julia Kahr, Bruce McEvoy, Michael P. Mogul (from June 13, 2011), Sidney Braginsky, Lesley Howe, Phillip J. Hildebrand,
126
Table of Contents
and Paul LaViolette. Mr. Howe resigned effective December 30, 2011. During 2012, our Board of Directors consisted of up to eleven persons: Leslie H. Cross (until December 4, 2012), Chinh E. Chu, Julia Kahr, Bruce McEvoy (until September 11, 2012), Michael P. Mogul, Mike Zafirovski (since January 5, 2012), John R. Murphy (since January 5, 2012), James A. Quella (since September 11, 2012), Sidney Braginsky, John Chiminski (since March 22, 2012), Phillip J. Hildebrand (until June 29, 2012), James R. (Ron) Lawson (since September 11, 2012), and Paul LaViolette (until June 29, 2012). Mr. Chu, Ms. Kahr and Mr. Quella are affiliated with Blackstone and are not compensated for serving as members of our Board of Directors. Mr. Mogul is our Chief Executive Officer and is not separately compensated for serving as a member of the Board of Directors.
The standard compensation package for directors who are not employed by the Company or by any Blackstone-controlled entity (Eligible Directors), namely Messrs. Howe, Braginsky, Hildebrand, and LaViolette during 2011, and Messrs. Braginsky, Chiminski, Hildebrand, Lawson and LaViolette during 2012, consisted of an annual fee for each such director, a per meeting fee and stock option grants. Each of the Eligible Directors is paid an annual fee of $75,000. In addition, the Chairman of the Audit Committee receives an annual fee of $25,000 and the other members of the Audit Committee (who are Eligible Directors) receive an annual fee of $15,000. The Eligible Directors were also eligible for annual option awards under the 2007 Plan. Although it has been the practice to grant 4,600 options to the directors on an annual basis, no options were granted to Messrs. Braginsky, Hildebrand, Howe and LaViolette in 2011.
On January 5, 2012, the Compensation Committee approved Mr. Zafirovski’s compensation as Chairman of the Board of DJO, consisting of annual cash compensation of $400,000 per year, and options to acquire 303,767 shares of the Company’s common stock at an exercise price of $16.46 per share. One-third of these options will vest in equal annual installments over three years, contingent on Mr. Zafirovski’s continued service as Chairman on the Board on each vesting date. The other two-thirds of the stock options will vest based upon Blackstone achieving a minimum return of money on invested capital (MOIC) as defined in the stock option agreement. Mr. Zafirovski also was granted the right to purchase approximately $1,000,000 in shares of the Company’s common stock, also at a price of $16.46 per share and he completed that purchase in January 2012.
On September 11,2012, the Board’s Compensation Committee approved Mr. Lawson’s compensation as a director of DJO, consisting of annual cash compensation of $200,000 per year. In addition to his responsibilities as a member of the Board, Mr. Lawson will devote up to 40 days per year to assist the Company in building its surgical business in the US and all of its businesses outside the US, with special focus on assisting in the building of a stronger distribution network outside the US. The Board’s Compensation Committee also granted Mr. Lawson options to acquire 100,000 shares of the Company’s common stock at an exercise price per share equal to $16.46. One-third of these options will vest in equal annual installments over three years, contingent on Mr. Lawson’s continued service as a director on each vesting date. The other two-thirds of the stock options will vest based upon Blackstone achieving a minimum MOIC as defined in the stock option agreement. Mr. Lawson was also granted the right to purchase up to $1,000,000 in shares of the Company’s common stock at a price of $16.46 per share and he completed that purchase in December 2012.
127
Table of Contents
The following table sets forth the compensation earned by our non-employee directors for their services in 2011:
| Directors Compensation for 2011 | ||||||||||||
| Name |
Fees Earned or Paid in Cash |
Option Awards (1) |
Total | |||||||||
| Chinh E. Chu |
$ | — | $ | — | $ | |||||||
| Julia Kahr |
— | — | — | |||||||||
| Bruce McEvoy (2) |
— | — | — | |||||||||
| Leslie H. Cross (3) |
— | — | — | |||||||||
| Sidney Braginsky |
90,000 | — | 90,000 | |||||||||
| Phillip Hildebrand (4) |
75,000 | — | 75,000 | |||||||||
| Lesley Howe (5) |
100,000 | — | 100,000 | |||||||||
| Paul LaViolette (4) |
75,000 | — | 75,000 | |||||||||
| (1) | As of December 31, 2011, Mr. Braginsky had a total of 16,050 stock options outstanding, and each of Messrs. Hildebrand, Howe and LaViolette had 9,200 stock options outstanding. |
| (2) | Mr. McEvoy resigned as director effective September 11, 2012. |
| (3) | Mr. Cross served as director and CEO until June 13, 2011, at which time he retired as CEO and became non-executive Chairman of the Board. Mr. Cross did not receive separate compensation for his service as a director while serving as CEO. Mr. Cross resigned as Chairman (but remains as a director) effective December 31, 2011. The table above excluded $321,563 paid to Mr. Cross for his services as Chairman from June 13, 2011 to December 30, 2011. Such amount is included in “All Other Compensation” in “Summary Compensation Table” above. Mr. Cross resigned as director effective December 4, 2012. |
| (4) | Messrs. Hilderbrand and LaViolette resigned as director effective June 29, 2012. |
| (5) | Mr. Howe resigned as director effective December 30, 2011. |
Compensation Committee Interlocks and Insider Participation
During 2011, our Compensation Committee consisted of three designees of Blackstone, Mr. Chu, Ms. Kahr and Mr. McEvoy. During 2012, our Compensation Committee consisted of three designees of Blackstone, Mr. Chu, Ms. Kahr, Mr. McEvoy (until September 11, 2012), and Mr. Quella (from September 11, 2012). None of the members of the Compensation Committee is or has been an officer or employee of DJO. See “Item 13. Certain Relationships and Related Transactions, and Director Independence” below for a description of certain agreements with Blackstone and its affiliates. None of our executive officers has served as a director or a member of the compensation committee (or other committee serving an equivalent function) of any other entity, which has one or more executive officers serving as a director of DJO or member of our Compensation Committee.
128
Table of Contents
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
DJOFL is a wholly owned subsidiary of DJO, which owns all of our issued and outstanding capital stock. The following table sets forth as of December 15, 2012, certain information regarding the beneficial ownership of the voting securities of DJO by each person who beneficially owns more than five percent of DJO’s common stock, and by each of the directors and NEOs of DJO, individually, and by our directors and executive officers as a group.
| Aggregate Number of Shares Beneficially Owned (1) | ||||||||||||
| Name and Address of Beneficial Owner |
Number of Issued Shares |
Acquirable within 60 days (2) |
Percent of Class | |||||||||
| Grand Slam Holdings, LLC (3) |
48,098,209 | — | 98.1 | % | ||||||||
| Directors and Executive Officers: |
||||||||||||
| Michael P. Mogul (4) |
||||||||||||
| President, Chief Executive Officer and Director |
188,716 | 66,667 | * | |||||||||
| Vickie L. Capps |
||||||||||||
| Executive Vice President, Chief Financial Officer and Treasurer |
— | 476,601 | * | |||||||||
| Donald M. Roberts |
||||||||||||
| Executive Vice President, General Counsel and Secretary |
— | 303,787 | * | |||||||||
| Luke T. Faulstick (5) |
||||||||||||
| Former Executive Vice President and Chief Operating Officer |
— | 196,256 | * | |||||||||
| Stephen J. Murphy |
||||||||||||
| Executive Vice President, Sales and Marketing, International Commercial Business |
6,076 | 191,561 | * | |||||||||
| Mike S. Zafirovski |
||||||||||||
| Chairman of the Board |
60,753 | — | * | |||||||||
| Chinh E. Chu |
||||||||||||
| Director (6) |
48,098,209 | — | 98.1 | % | ||||||||
| Julia Kahr |
||||||||||||
| Director (7) |
— | — | — | |||||||||
| Sidney Braginsky |
||||||||||||
| Director |
6,076 | 14,486 | * | |||||||||
| John Chiminski |
||||||||||||
| Director |
— | — | — | |||||||||
| John R. Murphy |
||||||||||||
| Director |
— | — | — | |||||||||
| James R. (Ron) Lawson |
||||||||||||
| Director |
60,753 | — | * | |||||||||
| James Quella |
||||||||||||
| Director (7) |
— | — | — | |||||||||
| Andrew Holman |
||||||||||||
| Former Executive Vice President, Sales and Marketing, U.S. Commercial Businesses |
— | — | * | |||||||||
| All Directors and executive officers as a group |
48,420,583 | 1,347,105 | 98.8 | % | ||||||||
| * | Less than 1% |
| (1) | Includes shares held in the beneficial owner’s name or jointly with others, or in the name of a bank, nominee or trustee for the beneficial owner’s account. Unless otherwise indicated in the footnotes to this table and |
129
Table of Contents
| subject to community property laws where applicable, we believe that each stockholder named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned. |
| (2) | Includes the number of shares that could be purchased by exercise of options on or within 60 days after December 15, 2012 under DJO’s stock option plan. For the NEOs, this number includes the DJO Management Rollover Options which are fully vested, the portion of the Time Based Tranche and the portion of the Market Return Tranche (when it was referred to as the Performance-Based Tranche) of options that have vested or will vest in 60 days, but no portion of the Enhanced Market Return Tranche. |
| (3) | Shares of common stock of DJO held by Grand Slam Holdings, LLC (“BCP Holdings”) may also be deemed to be beneficially owned by the following entities and persons: (i) Blackstone Capital Partners V L.P., a Delaware limited partnership (“BCP V”), Blackstone Family Investment Partnership V L.P., a Delaware limited partnership (“BFIP”), Blackstone Family Investment Partnership V-A L.P., a Delaware limited partnership (“BFIP-A”), and Blackstone Participation Partnership V L.P., a Delaware limited partnership (together with BCP V, BFIP and BFIP-A, the “Blackstone Partnerships”), which collectively own all of the equity in BCP Holdings; (ii) Blackstone Management Associates V L.L.C., a Delaware limited liability company (“BMA”), the general partner of the Blackstone Partnerships; (iii) BMA V L.L.C., a Delaware limited liability company (“BMA V”), the sole member of BMA; and (iv) Peter G. Peterson and Stephen A. Schwarzman, the founding members and controlling persons of BMA V. Each of Messrs. Peterson and Schwarzman disclaims beneficial ownership of such shares, except to the extent of his pecuniary interest therein. The address of BCP Holdings and each of the entities and individuals listed in this footnote is c/o The Blackstone Group, L.P., 345 Park Avenue, New York, New York 10154. |
| (4) | Excludes 30,376 of the 60,753 restricted shares awarded to Mr. Mogul on September 9, 2011 which will vest on June 13, 2013, contingent on his continued employment with the Company. |
| (5) | Mr. Faulstick’s employment terminated on February 3, 2012. Mr. Faulstick’s 196,256 Management Rollover Options were amended to provide that such options will not terminate early due to his termination of employment, but will remain outstanding until their original expiration dates. All unvested options held by Mr. Faulstick terminated upon termination of his employment. Vested options in the Time-Based Tranche expired 90 days after termination of his employment. |
| (6) | Mr. Chu, a director of DJO, is a member of BMA V and a senior managing director of The Blackstone Group, L.P. The number of shares disclosed for Mr. Chu are also included in the above table in the number of shares disclosed for Grand Slam Holdings, LLC. Mr. Chu disclaims beneficial ownership of any shares owned or controlled by BCP Holdings, except to the extent of his pecuniary interest therein. |
| (7) | Ms. Kahr and Mr. Quella are employees of The Blackstone Group, L.P. but do not have any investment or voting control over the shares beneficially owned by BCP Holdings. |
Securities Authorized for Issuance Under Equity Compensation Plans
The following table provides information as of December 31, 2011 with respect to the number of shares to be issued upon the exercise of outstanding stock options under our 2007 Plan, which is our only equity compensation plan and has been approved by the stockholders:
| Plan Category |
(a) Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted-average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|||||||||
| Equity compensation plans approved by stockholders |
7,547,838 | $ | 15.10 | 377,691 | ||||||||
130
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Management Stockholder’s Agreement
All members of DJO’s management who own shares of DJO common stock or options to purchase DJO common stock are parties to a Management Stockholders Agreement, dated November 3, 2006, among DJO, Grand Slam Holdings, LLC (“BCP Holdings”), Blackstone Capital Partners V L.P. (“Blackstone”), certain of its affiliates (BCP Holdings and Blackstone and its affiliates are referred to as “Blackstone Parent Stockholders”), and such members of DJO’s management, as amended by the First Amendment to Management Stockholders Agreement (the “Management Stockholders Agreement”). The Management Stockholders Agreement provides that upon termination of a management stockholder’s employment for any reason, DJO and a Blackstone Parent Stockholder may collectively exercise the right to purchase all of the shares of DJO common stock held by such management stockholder within one year after such termination (or, with respect to shares purchased upon exercise of options after termination of employment, one year following such exercise). If a management stockholder is terminated for cause (as defined in the Agreement), or voluntarily terminates their employment and such termination would have constituted a termination for cause if it would have been initiated by DJO, and DJO or a Blackstone Parent Stockholder exercises its call rights after such termination, the management stockholder would receive the lower of fair market value or cost for the management stockholder’s callable shares. In the case of all other terminations of employment, the management stockholder would receive fair market value for such shares.
The Management Stockholders Agreement imposes significant restrictions on transfers of shares of DJO’s common stock held by management stockholders and provides a right of first refusal to DJO or Blackstone, if DJO fails to exercise such right, on any proposed sale of DJO’s common stock held by a management stockholder following the lapse of the transfer restrictions and prior to the occurrence of a qualified public offering (as such term is defined in that agreement) of DJO. In addition, prior to a qualified public offering, Blackstone will have drag-along rights, and management stockholders will have tag-along rights, in the event of a sale of DJO’s common stock by Blackstone to a third party (or in the event of a sale of BCP Holdings’ equity interests to a third party) in the same proportion as the shares or equity interests sold by Blackstone. The Management Stockholders Agreement also provides that, after the occurrence of a qualified public offering, the management stockholders will receive customary piggyback registration rights with respect to shares of DJO common stock held by them.
All parties receiving an award of stock options, including all DJO directors who have been granted options, as well as all purchasers of common stock in DJO’s private stock offering in 2010, are parties to a Stockholders Agreement which has the same material terms and conditions as the Management Stockholders Agreement.
Transaction and Monitoring Fee Agreement
In connection with the DJO Merger, on November 20, 2007, DJO and Blackstone Management Partners V L.L.C. (“BMP”) amended and restated the transaction and monitoring fee agreement in existence at that time (the “Old Transaction and Monitoring Fee Agreement”) between them, with effect from and after the closing of the DJO Merger (such agreement, as amended and restated, the “New Transaction and Monitoring Fee Agreement”).
Under the New Transaction and Monitoring Fee Agreement, DJO paid BMP, at the closing of the DJO Merger, a $15.0 million transaction fee and $0.6 million for related expenses. Also pursuant to this agreement, at the closing of the DJO Merger, DJO paid Blackstone Advisory Services, L.P., an affiliate of BMP (“BAS”), a $3.0 million advisory fee in consideration of the provision of certain strategic and other advice and assistance by BAS on behalf of BMP.
Under the New Transaction and Monitoring Fee Agreement, BMP (including through its affiliates and representatives) will continue to provide certain monitoring, advisory and consulting services to DJO, on
131
Table of Contents
substantially the same terms and conditions as the Old Transaction and Monitoring Fee Agreement, for an annual monitoring fee which has been increased from $3.0 million to the greater of $7.0 million or 2.0% of consolidated EBITDA (as defined in the New Transaction and Monitoring Fee Agreement).
The New Transaction and Monitoring Fee Agreement also provides, on substantially the same terms and conditions as the Old Transaction and Monitoring Fee Agreement, that:
| • | at any time in connection with or in anticipation of a change of control of DJO, a sale of all or substantially all of its assets or an initial public offering of common stock of DJO or its successor, BMP may elect to receive, in lieu of remaining annual monitoring fee payments, a single lump sum cash payment equal to the then-present value of all then-current and future annual monitoring fees payable under the agreement, assuming a hypothetical termination date of the agreement to be November 2019; |
| • | the New Transaction and Monitoring Fee Agreement will continue until the earlier of November 2019, or such date as DJO and BMP may mutually agree; and |
| • | DJO will indemnify BMP and its affiliates, and their respective partners, members, shareholders, directors, officers, employees, agents and representatives from and against all liabilities relating to the services performed under the Old Transaction and Monitoring Fee Agreement or by the New Transaction and Monitoring Fee Agreement and the engagement of BMP pursuant to, and the performance of BMP and its affiliates and their respective representatives of the services contemplated by, each such agreement. |
Other Related Party Transactions
During the year ended December 31, 2011, in connection with the Dr. Comfort acquisition, we paid $5.0 million of transaction and advisory fees to Blackstone Advisory Partners, L.P., an affiliate of our major shareholder.
Policy and Procedures with Respect to Related Person Transactions
The Board of Directors has not adopted a formal written policy for the review and approval of transactions with related persons. However, all such transactions will be reviewed by the Board on an as-needed basis.
Director Independence
As a privately held company, the DJO Board is not required to have a majority of its directors be independent. We believe that Messrs. Braginsky, Murphy and LaViolette would be deemed independent directors according to the independence definition promulgated under the New York Stock Exchange listing standards.
132
Table of Contents
DESCRIPTION OF OTHER INDEBTEDNESS
Senior Secured Credit Facilities
On November 20, 2007, we entered into senior secured credit facilities (the “original senior secured credit facilities”) consisting of a $1,065.0 million term loan facility maturing in May 2014 (the “original term loans”) and a $100.0 million revolving credit facility maturing in November 2013 (the “original senior secured revolving credit facility”). We issued the original term loans at a 1.2% discount.
On March 20, 2012, we entered into an amendment and restatement agreement to amend and restate our original senior secured credit facilities, as amended (the “senior secured credit facilities”), which (1) permitted the issuance of $230.0 million aggregate principal of exchange senior secured notes; (2) extended the maturity of $564.7 million of the original term loans outstanding under the original senior secured credit facilities to the earlier of November 1, 2016 or August 15, 2014, if on such date the aggregate outstanding principal amount of our 10.875% Senior Notes is not less than $150.0 million (the “extended term loans”; such maturity accelerating condition, the “accelerating condition”); (3) provided for the issuance of a new tranche of $350.0 million of term loans that will mature on September 15, 2017, subject to the acceleration condition; (4) deemed certain previous acquisitions and investments to be permitted under the terms of the senior secured credit facilities; (5) increased the total net leverage ratio limitation in the permitted acquisitions covenant from 7.0x to 7.5x; (6) changed the financial maintenance covenant from a senior secured leverage ratio covenant to a senior secured first lien leverage ratio covenant; and (7) replaced our original senior secured revolving credit facility with a new $100.0 million revolving credit facility (the “senior secured revolving credit facility”) which matures on March 15, 2017, subject to the acceleration condition.
On March 30, 2012, we entered into Amendment No. 1 to the senior secured credit facilities which, among other things, provided for the issuance of an additional $105.0 million of new term loans that will mature on the earlier of September 15, 2017 or August 15, 2014, if on such date the outstanding aggregate principal amount of our 10.875% Senior Notes is not less than $150.0 million. The net proceeds from the issuance were used to repay $103.5 million aggregate principal of original term loans under the original senior secured credit facilities and to pay related fees, premiums and expenses.
Collectively, the $350.0 million of new term loans issued on March 20, 2012 and the $105.0 million of new term loans issued on March 30, 2012 are referred to as the “new term loans”.
The new term loans were issued at a discount as follows: the $350.0 million of new term loans issued on March 20, 2012 were issued at a 1.5% discount and the $105.0 million of the term loans issued on March 30, 2012 were issued at a 1.0% discount. The original issue discounts are being amortized over the term of the new term loans using the effective interest method.
The net proceeds from the new term loans were used to repay the remaining portion of the original term loans maturing in May 2014 and to repay a portion of the extended term loans.
As of September 29, 2012, the market values of our senior secured credit facilities and senior secured revolving credit facility were $845.5 million and $35.0 million, respectively. We determined market value using trading prices for the senior secured credit facilities on or near that date. As of September 29, 2012, we had $839.2 million aggregate principal amount of indebtedness remaining under our senior secured credit facilities, exclusive of unamortized original issue discount of $9.3 million.
Interest Rates. The interest rate margins applicable to borrowings under the senior secured revolving credit facility are, at our option, either (a) the Eurodollar rate, plus 475 basis points or (b) a base rate determined by reference to the highest of (1) the prime rate, (2) the federal funds rate, plus 0.50% and (3) the Eurodollar rate for a one-month interest period, plus 375 basis points. The interest rate margins applicable to the extended term
133
Table of Contents
loans and the new term loans are, at our option, either (a) the Eurodollar rate plus 500 basis points or (b) a base rate plus 400 basis points. There is a minimum LIBOR rate applicable to the Eurodollar component of interest rates on new term loan borrowings of 1.25%. The applicable margin for borrowings under the senior secured revolving credit facility, the extended term loans and the new term loans may be reduced, subject to our attaining certain leverage ratios. As of September 29, 2012, our weighted average interest rate for all borrowings under the senior secured credit facilities was 5.74%.
Fees. In addition to paying interest on outstanding principal under the senior secured credit facilities, we are required to pay a commitment fee to the lenders under the senior secured revolving credit facility with respect to the unutilized commitments thereunder. The current commitment fee rate is 0.50% per annum, subject to step-downs based upon the achievement of certain leverage ratios. We must also pay customary letter of credit fees.
Principal Payments. We are required to pay annual payments in equal quarterly installments on the new term loans in an amount equal to 1.00% of the funded total principal amount through June 2017, with any remaining amount payable in full at maturity in September 2017, subject to the acceleration condition. We are required to pay annual payments in equal quarterly installments on the extended term loans in an amount equal to 1.00% of the funded total principal amount through September 2016, with any remaining amount payable in full at maturity in November 2016, subject to the acceleration condition.
Prepayments. The senior secured credit facilities require us to prepay outstanding term loans, subject to certain exceptions, with (1) 50% (which percentage can be reduced to 25% or 0% upon our attaining certain leverage ratios) of our annual excess cash flow, as defined; (2) 100% of the net cash proceeds above an annual amount of $25.0 million from non-ordinary course asset sales (including insurance and condemnation proceeds) by us and our restricted subsidiaries, subject to certain exceptions, including a 100% reinvestment right if reinvested or committed to reinvest within 15 months of such sale or disposition so long as reinvestment is completed within 180 days thereafter; and (3) 100% of the net cash proceeds from issuance or incurrence of debt by us and our restricted subsidiaries, other than proceeds from debt permitted to be incurred under the senior secured credit facilities and related amendments. Any mandatory prepayments are applied to the term loan facility in direct order of maturity. We were not required to make any prepayments in 2012 related to our 2011 excess cash flow calculation.
Subject to certain exceptions, voluntary prepayments of the extended term loans and the new term loans within one year of the effective date of Amendment No. 1 are subject to a 1.0% “soft call” premium, while other voluntary prepayments of outstanding loans under the senior secured credit facilities may be made at any time without premium or penalty, provided that voluntary prepayments of Eurodollar loans made on a date other than the last day of an interest period applicable thereto shall be subject to customary breakage costs.
Guarantee and Security. All obligations under the senior secured credit facilities are unconditionally guaranteed by DJO Holdings LLC (“DJO Holdings”) and each of our existing and future direct and indirect wholly-owned domestic subsidiaries other than immaterial subsidiaries, unrestricted subsidiaries and subsidiaries that are precluded by law or regulation from guaranteeing the obligations (collectively, the “Guarantors”).
All obligations under the senior secured credit facilities, and the guarantees of those obligations, are secured by pledges of 100% of our capital stock, 100% of the capital stock of each wholly-owned domestic subsidiary and 65% of the capital stock of each wholly-owned foreign subsidiary that is, in each case, directly owned by us or one of the Guarantors, and a security interest in, and mortgages on, substantially all tangible and intangible assets of DJO Holdings, us and each Guarantor.
Certain Covenants and Events of Default. The senior secured credit facilities contain covenants that, among other things, restrict, subject to certain exceptions, our and our subsidiaries’ ability to:
| • | incur additional indebtedness; |
| • | create liens on assets; |
134
Table of Contents
| • | change fiscal years; |
| • | enter into sale and leaseback transactions; |
| • | engage in mergers or consolidations; |
| • | sell assets; |
| • | pay dividends and other restricted payments; |
| • | make investments, loans or advances; |
| • | repay subordinated indebtedness; |
| • | make certain acquisitions; |
| • | engage in certain transactions with affiliates; |
| • | restrict the ability of restricted subsidiaries that are not Guarantors to pay dividends or make distributions; |
| • | amend material agreements governing our subordinated indebtedness; and |
| • | change our lines of business. |
In addition, the senior secured credit facilities require us to maintain a maximum senior secured first lien leverage ratio of consolidated senior secured first lien debt to Adjusted EBITDA (as defined in the senior secured credit facilities) of 4.25:1 for the trailing twelve months ended September 29, 2012, stepping down periodically to 3.25 at the end of 2016. The senior secured credit facilities also contain certain customary affirmative covenants and events of default. As of September 29, 2012, our actual senior secured first lien net leverage ratio was 3.00:1, and we were in compliance with all other applicable covenants.
7.75% Senior Notes
On April 7, 2011, the Issuers issued $300.0 million aggregate principal amount of 7.75% Senior Notes maturing on April 15, 2018. The 7.75% Senior Notes are guaranteed jointly and severally and on an unsecured senior basis by each of our existing and future direct and indirect wholly-owned domestic subsidiaries that guarantee any of our indebtedness, or any indebtedness of our domestic subsidiaries, or is an obligor under our senior secured credit facilities.
As of September 29, 2012, the market value of the 7.75% Senior Notes was $276.8 million. We determined market value using trading prices for the 7.75% Senior Notes on or near that date.
Optional Redemption. Under the agreement governing the 7.75% Senior Notes (the “7.75% Indenture”), prior to April 15, 2014, the Issuers have the option to redeem some or all of the 7.75% Senior Notes for cash at a redemption price equal to 100% of the then outstanding principal balance plus an applicable make-whole premium, plus accrued and unpaid interest. Beginning on April 15, 2014, the Issuers may redeem some or all of the 7.75% Senior Notes at a redemption price of 105.813% of the then outstanding principal balance plus accrued and unpaid interest. The redemption price decreases to 103.875%, 101.938% and 100% of the then outstanding principal balance at April 15, 2015, 2016 and 2017, respectively, in each case plus accrued and unpaid interest. Additionally, from time to time, before April 15, 2014, the Issuers may redeem up to 35% of the 7.75% Senior Notes at a redemption price equal to 107.75% of the then outstanding principal balance, plus accrued and unpaid interest, in each case, with proceeds we raise, or a direct or indirect parent company raises, in certain offerings of equity of us or our direct or indirect parent companies, as long as at least 65% of the aggregate principal amount of the 7.75% Senior Notes issued remains outstanding.
Change of Control. Upon the occurrence of a change of control, unless we have previously sent or concurrently send a notice exercising our optional redemption rights with respect to all of the then outstanding 7.75% Senior Notes, we will be required to make an offer to repurchase all of the then outstanding 7.75% Senior Notes at 101% of the then outstanding principal balance, plus accrued and unpaid interest.
135
Table of Contents
Covenants. The 7.75% Indenture contains covenants limiting, among other things, our and our restricted subsidiaries’ ability to (i) incur additional indebtedness or issue certain preferred and convertible shares, pay dividends on, redeem, repurchase or make distributions in respect of the capital stock of DJO or make other restricted payments, (ii) make certain investments, (iii) sell certain assets, (iv) create liens on certain assets to secure debt, (v) consolidate, merge, sell or otherwise dispose of all or substantially all of our assets, (vi) enter into certain transactions with affiliates, or (vii) designate our subsidiaries as unrestricted subsidiaries. As of September 29, 2012, we were in compliance with all applicable covenants.
9.75% Senior Subordinated Notes
On October 18, 2010, the Issuers issued $300.0 million aggregate principal amount of the Senior Subordinated Notes maturing on October 15, 2017. The Senior Subordinated Notes are guaranteed jointly and severally and on an unsecured senior subordinated basis by each of our existing and future direct and indirect wholly-owned domestic subsidiaries that guarantee any of our indebtedness, or any indebtedness of our domestic subsidiaries, or by any of our subsidiaries that are an obligor under our senior secured credit facilities.
As of September 29, 2012, the market value of the Senior Subordinated Notes was $252.0 million. We determined market value using trading prices for the Senior Subordinated Notes on or near that date.
Optional Redemption. Under the agreement governing the Senior Subordinated Notes (the “9.75% Indenture”), prior to October 15, 2013, the Issuers have the option to redeem some or all of the Senior Subordinated Notes for cash at a redemption price equal to 100% of the then outstanding principal balance plus an applicable make-whole premium, plus accrued and unpaid interest. Beginning on October 15, 2013, the Issuers may redeem some or all of the Senior Subordinated Notes at a redemption price of 107.313% of the then outstanding principal balance, plus accrued and unpaid interest. The redemption price decreases to 104.875%, 102.438% and 100% of the then outstanding principal balance at October 15, 2014, 2015 and 2016, respectively, in each case plus accrued and unpaid interest. Additionally, from time to time, before October 15, 2013, the Issuers may redeem up to 35% of the Senior Subordinated Notes at a redemption price equal to 109.75% of the principal amount then outstanding, plus accrued and unpaid interest, in each case, with proceeds we raise, or a direct or indirect parent company raises, in certain offerings of equity of us or our direct or indirect parent companies, as long as at least 65% of the aggregate principal amount of the Senior Subordinated Notes issued remains outstanding.
Change of Control. Upon the occurrence of a change of control, unless we have previously sent or concurrently send a notice exercising its optional redemption rights with respect to all of the Senior Subordinated Notes, we will be required to make an offer to repurchase all of the Senior Subordinated Notes at 101% of the then outstanding principal balance, plus accrued and unpaid interest.
Covenants. The 9.75% Indenture contains covenants limiting, among other things, our and our restricted subsidiaries’ ability to (i) incur additional indebtedness or issue certain preferred and convertible shares, pay dividends on, redeem, repurchase or make distributions in respect of the capital stock of DJO or make other restricted payments, (ii) make certain investments, (iii) sell certain assets, (iv) create liens on certain assets to secure debt, (v) consolidate, merge, sell or otherwise dispose of all or substantially all of our assets, (vi) enter into certain transactions with affiliates, or (vii) designate our subsidiaries as unrestricted subsidiaries. As of September 29, 2012, we were in compliance with all applicable covenants.
136
Table of Contents
Purpose and Effect of the Exchange Offers
DJOFL, DJO Finance Corporation and the guarantors of the outstanding notes entered into registration rights agreements with the initial purchasers of the outstanding notes in which they agreed, under certain circumstances, to use their reasonable best efforts to file a registration statement relating to an offer to exchange the outstanding notes for exchange notes and thereafter cause the registration statement to become effective under the Securities Act no later than 360 days following the closing date of the issuance of the applicable outstanding notes. The exchange notes will have terms identical in all material respects to the related outstanding notes, except that the exchange notes will not contain terms with respect to transfer restrictions, registration rights and additional interest for failure to observe certain obligations in the registration rights agreement. $230 million of outstanding senior secured notes were issued on March 20, 2012, an additional $100 million of outstanding senior secured notes were issued on October 1, 2012 and $440 million of outstanding senior notes were issued on October 1, 2012.
Under the circumstances set forth below, DJOFL, DJO Finance Corporation and the guarantors will use their reasonable best efforts to cause the SEC to declare effective a shelf registration statement with respect to the resale of the outstanding notes within the time periods specified in the registration rights agreements and keep the statement effective for up to two years after the effective date of the shelf registration statement. These circumstances include:
| • | if any changes in law, SEC rules or regulations or applicable interpretations thereof by the SEC do not permit us to effect an exchange offer as contemplated by the applicable registration rights agreement; |
| • | if an exchange offer is not consummated within 360 days after the date of issuance of the applicable outstanding notes; |
| • | if any initial purchaser so requests with respect to the outstanding notes not eligible to be exchanged for the exchange notes and held by it within 30 days after the consummation of the applicable exchange offer; or |
| • | if any holder that participates in an exchange offer does not receive freely transferable exchange notes in exchange for tendered outstanding notes. |
Under the registration rights agreements, if DJOFL and DJO Finance Corporation fails to complete the exchange offer (other than in the event we file a shelf registration statement) or the shelf registration statement, if required thereby, is not declared effective, in either case on or prior to 360 days after the applicable issue date (the “target registration date”), the interest rate on the related outstanding notes will be increased by (x) 0.25% per annum for the first 90-day period immediately following the target registration date and (y) an additional 0.25% per annum with respect to each subsequent 90-day period, in each case, until the applicable exchange offer is completed or the shelf registration statement, if required, is declared effective by the SEC or the outstanding notes cease to constitute transfer restricted notes, up to a maximum of 1.00% per annum of additional interest. Copies of the registration rights agreements have been filed as an exhibit to the registration statement of which this prospectus is a part.
If you wish to exchange your outstanding notes for exchange notes in the exchange offers, you will be required to make the following written representations:
| • | you are not an affiliate of DJOFL and DJO Finance Corporation or an affiliate of any guarantor within the meaning of Rule 405 of the Securities Act; |
| • | you have no arrangement or understanding with any person to participate in a distribution (within the meaning of the Securities Act) of the exchange notes in violation of the provisions of the Securities Act; |
137
Table of Contents
| • | you are not engaged in, and do not intend to engage in, a distribution of the exchange notes; and |
| • | you are acquiring the exchange notes in the ordinary course of your business. |
Each broker-dealer that receives exchange notes for its own account in exchange for outstanding notes, where the broker-dealer acquired the outstanding notes as a result of market-making activities or other trading activities, must acknowledge that it will deliver a prospectus in connection with any resale of such exchange notes. Please see “Plan of Distribution.”
Resale of Exchange Notes
Based on interpretations by the SEC set forth in no-action letters issued to third parties, we believe that you may resell or otherwise transfer exchange notes issued in the exchange offers without complying with the registration and prospectus delivery provisions of the Securities Act, if:
| • | you are not our affiliate or an affiliate of any guarantor within the meaning of Rule 405 under the Securities Act; |
| • | you do not have an arrangement or understanding with any person to participate in a distribution of the exchange notes; |
| • | you are not engaged in, and do not intend to engage in, a distribution of the exchange notes; and |
| • | you are acquiring the exchange notes in the ordinary course of your business. |
If you are an affiliate of DJOFL and DJO Finance Corporation or an affiliate of any guarantor, or are engaging in, or intend to engage in, or have any arrangement or understanding with any person to participate in, a distribution of the exchange notes, or are not acquiring the exchange notes in the ordinary course of your business:
| • | you cannot rely on the position of the SEC set forth in Morgan Stanley & Co. Incorporated (available June 5, 1991) and Exxon Capital Holdings Corporation (available May 13, 1988), as interpreted in the SEC’s letter to Shearman & Sterling, dated July 2, 1993, or similar no-action letters; and |
| • | in the absence of an exception from the position stated immediately above, you must comply with the registration and prospectus delivery requirements of the Securities Act in connection with any resale of the exchange notes. |
This prospectus may be used for an offer to resell, resale or other transfer of exchange notes only as specifically set forth in this prospectus. With regard to broker-dealers, only broker-dealers that acquired the outstanding notes as a result of market-making activities or other trading activities may participate in the exchange offers. Each broker-dealer that receives exchange notes for its own account in exchange for outstanding notes, where such outstanding notes were acquired by such broker-dealer as a result of market-making activities or other trading activities, must acknowledge that it will deliver a prospectus in connection with any resale of the exchange notes. Please read “Plan of Distribution” for more details regarding the transfer of exchange notes.
Terms of the Exchange Offers
On the terms and subject to the conditions set forth in this prospectus and in the accompanying letters of transmittal, DJOFL and DJO Finance Corporation will accept for exchange in the exchange offers any outstanding notes that are validly tendered and not validly withdrawn prior to the expiration date. Outstanding notes may only be tendered in multiples of $2,000 and in integral multiples of $1,000 in excess thereof. DJOFL and DJO Finance Corporation will issue $2,000 and integral multiples of $1,000, in excess thereof, principal amount of exchange notes in exchange for each $2,000 and integral multiples of $1,000, in excess thereof, principal amount of outstanding notes surrendered in the exchange offers.
138
Table of Contents
The form and terms of the exchange notes will be identical in all material respects to the form and terms of the outstanding notes except the exchange notes will be registered under the Securities Act, will not bear legends restricting their transfer and will not provide for any additional interest upon our failure to fulfill our obligations under the registration rights agreements to complete the exchange offers, or file, and cause to be effective, a shelf registration statement, if required thereby, within the specified time period. The exchange notes will evidence the same debt as the outstanding notes. The exchange notes will be issued under and entitled to the benefits of the indenture that authorized the issuance of the outstanding notes. For a description of the indentures, see “Description of Senior Secured Notes” and “Description of Senior Notes.”
The exchange offers are not conditioned upon any minimum aggregate principal amount of outstanding notes being tendered for exchange.
As of the date of this prospectus, $330.0 million aggregate principal amount of the 8.75% Second Priority Senior Secured Notes due 2018 that were issued in private offerings on March 20, 2012 and October 1, 2012 are outstanding and unregistered and $440.0 million aggregate principal amount of the 9.875% Senior Notes due 2018 that were issued in a private offering on October 1, 2012 are outstanding and unregistered. This prospectus and the applicable letter of transmittal are being sent to all registered holders of outstanding notes. There will be no fixed record date for determining registered holders of outstanding notes entitled to participate in the exchange offers. DJOFL and DJO Finance Corporation intend to conduct the exchange offers in accordance with the provisions of the registration rights agreements, the applicable requirements of the Securities Act and the Exchange Act and the rules and regulations of the SEC. Outstanding notes that are not tendered for exchange in the exchange offers will remain outstanding and continue to accrue interest and will be entitled to the rights and benefits such holders have under the applicable indenture relating to such holders’ series of outstanding notes and the applicable registration rights agreement except we will not have any further obligation to you to provide for the registration of the outstanding notes under the applicable registration rights agreement.
DJOFL and DJO Finance Corporation will be deemed to have accepted for exchange properly tendered outstanding notes when it has given written notice of the acceptance to the exchange agent. The exchange agent will act as agent for the tendering holders for the purposes of receiving the exchange notes from us and delivering exchange notes to holders. Subject to the terms of the applicable registration rights agreement, DJOFL and DJO Finance Corporation expressly reserve the right to amend or terminate the exchange offers and to refuse to accept the occurrence of any of the conditions specified below under “—Conditions to the Exchange Offers.”
If you tender your outstanding notes in the exchange offers, you will not be required to pay brokerage commissions or fees or, subject to the instructions in the letter of transmittal, transfer taxes with respect to the exchange of outstanding notes. We will pay all charges and expenses, other than certain applicable taxes described below in connection with the exchange offers. It is important that you read “—Fees and Expenses” below for more details regarding fees and expenses incurred in the exchange offers.
Expiration Date; Extensions, Amendments
As used in this prospectus, the term “expiration date” means 12:00 a.m. midnight, New York City time, on February 6, 2013. However, if we, in our sole discretion, extend the period of time for which an exchange offer is open, the term “expiration date” will mean the latest time and date to which we shall have extended the expiration of such exchange offer.
To extend the period of time during which an exchange offer is open, we will notify the exchange agent of any extension by written notice, followed by notification by press release or other public announcement to the registered holders of the outstanding notes no later than 9:00 a.m., New York City time, on the next business day after the previously scheduled expiration date.
DJOFL and DJO Finance Corporation reserve the right, in their sole discretion:
| • | to delay accepting for exchange any outstanding notes (only in the case that we amend or extend an exchange offer); |
139
Table of Contents
| • | to extend an exchange offer or to terminate an exchange offer if any of the conditions set forth below under “—Conditions to the Exchange Offers” have not been satisfied, by giving written notice of such delay, extension or termination to the exchange agent; and |
| • | subject to the terms of the applicable registration rights agreement, to amend the terms of an exchange offer in any manner. In the event of a material change in an exchange offer, including the waiver of a material condition, we will extend the offer period, if necessary, so that at least five business days remain in such offer period following notice of the material change. |
Any delay in acceptance, extension, termination or amendment will be followed as promptly as practicable by written notice to the registered holders of the outstanding notes. If DJOFL and DJO Finance Corporation amend an exchange offer in a manner that we determine to constitute a material change, they will promptly disclose the amendment in a manner reasonably calculated to inform the holders of applicable outstanding notes of that amendment.
Conditions to the Exchange Offers
Despite any other term of the exchange offers, DJOFL and DJO Finance Corporation will not be required to accept for exchange, or to issue exchange notes in exchange for, any outstanding notes and they may terminate or amend an exchange offer as provided in this prospectus prior to the expiration date if in their reasonable judgment:
| • | an exchange offer or the making of any exchange by a holder violates any applicable law or interpretation of the SEC; or |
| • | any action or proceeding has been instituted or threatened in writing in any court or by or before any governmental agency with respect to an exchange offer that, in our judgment, would reasonably be expected to impair our ability to proceed with such exchange offer. |
In addition, DJOFL and DJO Finance Corporation will not be obligated to accept for exchange the outstanding notes of any holder that has not made to us:
| • | the representations described under “—Purpose and Effect of the Exchange Offers,” “—Procedures for Tendering” and “Plan of Distribution;” or |
| • | any other representations as may be reasonably necessary under applicable SEC rules, regulations, or interpretations to make available to us an appropriate form for registration of the exchange notes under the Securities Act. |
DJOFL and DJO Finance Corporation expressly reserve the right at any time or at various times to extend the period of time during which an exchange offer is open. Consequently, DJOFL and DJO Finance Corporation may delay acceptance of any outstanding notes by giving written notice of such extension to their holders. DJOFL and DJO Finance Corporation will return any outstanding notes that they do not accept for exchange for any reason without expense to their tendering holder promptly after the expiration or termination of an exchange offer.
DJOFL and DJO Finance Corporation expressly reserve the right to amend or terminate an exchange offer and to reject for exchange any outstanding notes not previously accepted for exchange, upon the occurrence of any of the conditions of an exchange offer specified above. DJOFL and DJO Finance Corporation will give written notice of any extension, amendment, non-acceptance or termination to the holders of the outstanding notes as promptly as practicable. In the case of any extension, such notice will be issued no later than 9:00 a.m., New York City time, on the next business day after the previously scheduled expiration date.
These conditions are for our sole benefit and DJOFL and DJO Finance Corporation may assert them regardless of the circumstances that may give rise to them or waive them in whole or in part at any or at various
140
Table of Contents
times prior to the expiration date in our sole discretion. If DJOFL and DJO Finance Corporation fail at any time to exercise any of the foregoing rights, this failure will not constitute a waiver of such right. Each such right will be deemed an ongoing right that they may assert at any time or at various times prior to the expiration date.
In addition, DJOFL and DJO Finance Corporation will not accept for exchange any outstanding notes tendered, and will not issue exchange notes in exchange for any such outstanding notes, if at such time any stop order is threatened or in effect with respect to the registration statement of which this prospectus constitutes a part or the qualification of the relevant indenture under the Trust Indenture Act of 1939 (the “TIA”).
Procedures for Tendering Outstanding Notes
To tender your outstanding notes in the exchange offers, you must comply with either of the following:
| • | complete, sign and date the applicable letter of transmittal, or a facsimile of the letter of transmittal, have the signature(s) on the applicable letter of transmittal guaranteed if required by such letter of transmittal and mail or deliver such letter of transmittal or facsimile thereof to the exchange agent at the address set forth below under “—Exchange Agent—Notes” prior to the expiration date; or |
| • | comply with DTC’s Automated Tender Offer Program procedures described below. |
In addition, either:
| • | the exchange agent must receive certificates for outstanding notes along with the applicable letter of transmittal prior to the expiration date; |
| • | the exchange agent must receive a timely confirmation of book-entry transfer of outstanding notes into the exchange agent’s account at DTC according to the procedures for book-entry transfer described below or a properly transmitted agent’s message prior to the expiration date; or |
| • | you must comply with the guaranteed delivery procedures described below. |
Your tender, if not withdrawn prior to the expiration date, constitutes an agreement between us and you upon the terms and subject to the conditions described in this prospectus and in the applicable letter of transmittal.
The method of delivery of outstanding notes, letters of transmittal, and all other required documents to the exchange agent is at your election and risk. We recommend that instead of delivery by mail, you use an overnight or hand delivery service, properly insured. In all cases, you should allow sufficient time to assure timely delivery to the exchange agent before the expiration date. You should not send letters of transmittal or certificates representing outstanding notes to us. You may request that your broker, dealer, commercial bank, trust company or nominee effect the above transactions for you.
If you are a beneficial owner whose outstanding notes are registered in the name of a broker, dealer, commercial bank, trust company, or other nominee and you wish to tender your outstanding notes, you should promptly contact the registered holder and instruct the registered holder to tender on your behalf. If you wish to tender the outstanding notes yourself, you must, prior to completing and executing the applicable letter of transmittal and delivering your outstanding notes, either:
| • | make appropriate arrangements to register ownership of the outstanding notes in your name; or |
| • | obtain a properly completed bond power from the registered holder of outstanding notes. |
The transfer of registered ownership may take considerable time and may not be able to be completed prior to the expiration date.
141
Table of Contents
Signatures on the letter of transmittal or a notice of withdrawal, as the case may be, must be guaranteed by a member firm of a registered national securities exchange or of the National Association of Securities Dealers, Inc., a commercial bank or trust company having an office or correspondent in the United States or another “eligible guarantor institution” within the meaning of Rule 17A(d)-15 under the Exchange Act unless the outstanding notes surrendered for exchange are tendered:
| • | by a registered holder of the outstanding notes who has not completed the box entitled “Special Registration Instructions” or “Special Delivery Instructions” on the letter of transmittal; or |
| • | for the account of an eligible guarantor institution. |
If the letter of transmittal is signed by a person other than the registered holder of any outstanding notes listed on the outstanding notes, such outstanding notes must be endorsed or accompanied by a properly completed bond power. The bond power must be signed by the registered holder as the registered holder’s name appears on the outstanding notes and an eligible guarantor institution must guarantee the signature on the bond power.
If the letter of transmittal or any certificates representing outstanding notes, or bond powers are signed by trustees, executors, administrators, guardians, attorneys-in-fact, officers of corporations, or others acting in a fiduciary or representative capacity, those persons should also indicate when signing and, unless waived by us, they should also submit evidence satisfactory to us of their authority to so act.
The exchange agent and DTC have confirmed that any financial institution that is a participant in DTC’s system may use DTC’s Automated Tender Offer Program to tender. Participants in the program may, instead of physically completing and signing the letter of transmittal and delivering it to the exchange agent, electronically transmit their acceptance of the exchange by causing DTC to transfer the outstanding notes to the exchange agent in accordance with DTC’s Automated Tender Offer Program procedures for transfer. DTC will then send an agent’s message to the exchange agent. The term “agent’s message” means a message transmitted by DTC, received by the exchange agent and forming part of the book-entry confirmation, which states that:
| • | DTC has received an express acknowledgment from a participant in its Automated Tender Offer Program that is tendering outstanding notes that are the subject of the book-entry confirmation; |
| • | the participant has received and agrees to be bound by the terms of the letter of transmittal, or in the case of an agent’s message relating to guaranteed delivery, that such participant has received and agrees to be bound by the notice of guaranteed delivery; and |
| • | we may enforce that agreement against such participant. |
DTC is referred to herein as a “book-entry transfer facility.”
Acceptance of Exchange Notes
In all cases, DJOFL and DJO Finance Corporation will promptly issue exchange notes for outstanding notes that it has accepted for exchange under the exchange offers only after the exchange agent timely receives:
| • | outstanding notes or a timely book-entry confirmation of such outstanding notes into the exchange agent’s account at the book-entry transfer facility; and |
| • | a properly completed and duly executed letter of transmittal and all other required documents or a properly transmitted agent’s message. |
By tendering outstanding notes pursuant to the exchange offers, you will represent to us that, among other things:
| • | you are not our affiliate or an affiliate of any guarantor within the meaning of Rule 405 under the Securities Act; |
142
Table of Contents
| • | you do not have an arrangement or understanding with any person or entity to participate in a distribution of the exchange notes; and |
| • | you are acquiring the exchange notes in the ordinary course of your business. |
In addition, each broker-dealer that is to receive exchange notes for its own account in exchange for outstanding notes must represent that such outstanding notes were acquired by that broker-dealer as a result of market-making activities or other trading activities and must acknowledge that it will deliver a prospectus that meets the requirements of the Securities Act in connection with any resale of the exchange notes. The letter of transmittal states that by so acknowledging and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act. See “Plan of Distribution.”
DJOFL and DJO Finance Corporation will interpret the terms and conditions of the exchange offers, including the letters of transmittal and the instructions to the letters of transmittal, and will resolve all questions as to the validity, form, eligibility, including time of receipt, and acceptance of outstanding notes tendered for exchange. Our determinations in this regard will be final and binding on all parties. DJOFL and DJO Finance Corporation reserves the absolute right to reject any and all tenders of any particular outstanding notes not properly tendered or to not accept any particular outstanding notes if the acceptance might, in their or their counsel’s judgment, be unlawful. We also reserve the absolute right to waive any defects or irregularities as to any particular outstanding notes prior to the expiration date.
Unless waived, any defects or irregularities in connection with tenders of outstanding notes for exchange must be cured within such reasonable period of time as we determine. Neither DJOFL, DJO Finance Corporation, the exchange agent, nor any other person will be under any duty to give notification of any defect or irregularity with respect to any tender of outstanding notes for exchange, nor will any of them incur any liability for any failure to give notification. Any outstanding notes received by the exchange agent that are not properly tendered and as to which the irregularities have not been cured or waived will be returned by the exchange agent to the tendering holder, unless otherwise provided in the letter of transmittal, promptly after the expiration date.
Book-Entry Delivery Procedures
Promptly after the date of this prospectus, the exchange agent will establish an account with respect to the outstanding notes at DTC and, as the book-entry transfer facility, for purposes of the exchange offers. Any financial institution that is a participant in the book-entry transfer facility’s system may make book-entry delivery of the outstanding notes by causing the book-entry transfer facility to transfer those outstanding notes into the exchange agent’s account at the facility in accordance with the facility’s procedures for such transfer. To be timely, book-entry delivery of outstanding notes requires receipt of a confirmation of a book-entry transfer, a “book-entry confirmation,” prior to the expiration date. In addition, although delivery of outstanding notes may be effected through book-entry transfer into the exchange agent’s account at the book-entry transfer facility, the letter of transmittal or a manually signed facsimile thereof, together with any required signature guarantees and any other required documents, or an “agent’s message,” as defined below, in connection with a book-entry transfer, must, in any case, be delivered or transmitted to and received by the exchange agent at its address set forth on the cover page of the letter of transmittal prior to the expiration date to receive exchange notes for tendered outstanding notes, or the guaranteed delivery procedure described below must be complied with. Tender will not be deemed made until such documents are received by the exchange agent. Delivery of documents to the book-entry transfer facility does not constitute delivery to the exchange agent.
Holders of outstanding notes who are unable to deliver confirmation of the book-entry tender of their outstanding notes into the exchange agent’s account at the book-entry transfer facility or all other documents required by the letter of transmittal to the exchange agent on or prior to the expiration date must tender their outstanding notes according to the guaranteed delivery procedures described below.
143
Table of Contents
Guaranteed Delivery Procedures
If you wish to tender your outstanding notes but your outstanding notes are not immediately available or you cannot deliver your outstanding notes, the letter of transmittal or any other required documents to the exchange agent or comply with the procedures under DTC’s Automatic Tender Offer Program in the case of outstanding notes, prior to the expiration date, you may still tender if:
| • | the tender is made through an eligible guarantor institution; |
| • | prior to the expiration date, the exchange agent receives from such eligible guarantor institution either a properly completed and duly executed notice of guaranteed delivery, by facsimile transmission, mail, or hand delivery or a properly transmitted agent’s message and notice of guaranteed delivery, that (1) sets forth your name and address, the certificate number(s) of such outstanding notes and the principal amount of outstanding notes tendered; (2) states that the tender is being made thereby; and (3) guarantees that, within three New York Stock Exchange trading days after the expiration date, the letter of transmittal, or facsimile thereof, together with the outstanding notes or a book-entry confirmation, and any other documents required by the letter of transmittal, will be deposited by the eligible guarantor institution with the exchange agent; and |
| • | the exchange agent receives the properly completed and executed letter of transmittal or facsimile thereof, as well as certificate(s) representing all tendered outstanding notes in proper form for transfer or a book-entry confirmation of transfer of the outstanding notes into the exchange agent’s account at DTC all other documents required by the letter of transmittal within three New York Stock Exchange trading days after the expiration date. |
Upon request, the exchange agent will send to you a notice of guaranteed delivery if you wish to tender your outstanding notes according to the guaranteed delivery procedures.
Withdrawal Rights
Except as otherwise provided in this prospectus, you may withdraw your tender of outstanding notes at any time prior to 12:00 a.m. midnight, New York City time, on the expiration date.
For a withdrawal to be effective:
| • | the exchange agent must receive a written notice, which may be by telegram, telex, facsimile or letter, of withdrawal at its address set forth below under “—Exchange Agent”; or |
| • | you must comply with the appropriate procedures of DTC’s Automated Tender Offer Program system. |
Any notice of withdrawal must:
| • | specify the name of the person who tendered the outstanding notes to be withdrawn; |
| • | identify the outstanding notes to be withdrawn, including the certificate numbers and principal amount of the outstanding notes; and |
| • | where certificates for outstanding notes have been transmitted, specify the name in which such outstanding notes were registered, if different from that of the withdrawing holder. |
| • | If certificates for outstanding notes have been delivered or otherwise identified to the exchange agent, then, prior to the release of such certificates, you must also submit: |
| • | the serial numbers of the particular certificates to be withdrawn; and |
| • | a signed notice of withdrawal with signatures guaranteed by an eligible institution unless you are an eligible guarantor institution. |
If outstanding notes have been tendered pursuant to the procedures for book-entry transfer described above, any notice of withdrawal must specify the name and number of the account at the book-entry transfer facility to
144
Table of Contents
be credited with the withdrawn outstanding notes and otherwise comply with the procedures of the facility. We will determine all questions as to the validity, form, and eligibility, including time of receipt of notices of withdrawal and our determination will be final and binding on all parties. Any outstanding notes so withdrawn will be deemed not to have been validly tendered for exchange for purposes of the exchange offers. Any outstanding notes that have been tendered for exchange but that are not exchanged for any reason will be returned to their holder, without cost to the holder, or, in the case of book-entry transfer, the outstanding notes will be credited to an account at the book-entry transfer facility, promptly after withdrawal, rejection of tender or termination of the exchange offers. Properly withdrawn outstanding notes may be retendered by following the procedures described under “—Procedures for Tendering Outstanding Notes” above at any time on or prior to the expiration date.
Exchange Agent
The Bank of New York Mellon has been appointed as the exchange agent for the exchange offers. The Bank of New York Mellon also acts as trustee under the indentures governing the notes. You should direct all executed letters of transmittal and all questions and requests for assistance, requests for additional copies of this prospectus or of the letters of transmittal, and requests for notices of guaranteed delivery to the exchange agent addressed as follows:
| By Registered or Certified Mail: | By Regular Mail: | By Overnight Courier or Hand Delivery: | ||
| The Bank of New York Mellon | The Bank of New York Mellon | The Bank of New York Mellon | ||
| Reorganization Unit | Reorganization Unit | Reorganization Unit | ||
| 111 Sanders Creek Parkway | 111 Sanders Creek Parkway | 111 Sanders Creek Parkway | ||
| East Syracuse, NY 13057 | East Syracuse, NY 13057 | East Syracuse, NY 13057 | ||
| Attention: Dacia Brown-Jones | Attention: Dacia Brown-Jones | Attention: Dacia Brown-Jones | ||
| By Facsimile Transmission: (eligible institutions only): (732) 667-9408 |
||||
| Telephone Inquiries: (315) 414-3349 |
||||
Note: Delivery of this instrument to an address other than as set forth above, or transmission of instructions other than as set forth above, will not constitute a valid delivery.
If you deliver the letter of transmittal to an address other than the one set forth above or transmit instructions via facsimile other than the one set forth above, that delivery or those instructions will not be effective.
Fees and Expenses
Each registration rights agreement provides that we will bear all expenses in connection with the performance of our obligations relating to the registration of the exchange notes and the conduct of the exchange offers. These expenses include registration and filing fees, accounting and legal fees and printing costs, among others. We will pay the exchange agent reasonable and customary fees for its services and reasonable out-of-pocket expenses. We will also reimburse brokerage houses and other custodians, nominees and fiduciaries for customary mailing and handling expenses incurred by them in forwarding this prospectus and related documents to their clients that are holders of outstanding notes and for handling or tendering for such clients.
We have not retained any dealer-manager in connection with the exchange offers and will not pay any fee or commission to any broker, dealer, nominee or other person, other than the exchange agent, for soliciting tenders of outstanding notes pursuant to the exchange offers.
145
Table of Contents
Accounting Treatment
We will record the exchange notes in our accounting records at the same carrying value as the outstanding notes, which is the aggregate principal amount as reflected in our accounting records on the date of exchanges, as the terms of the exchange notes are substantially identical to the terms of the outstanding notes. Accordingly, we will not recognize any gain or loss for accounting purposes upon the consummation of the exchange offers. We will capitalize the expenses relating to the exchange offers.
Transfer Taxes
We will pay all transfer taxes, if any, applicable to the exchanges of outstanding notes under the exchange offers. The tendering holder, however, will be required to pay any transfer taxes, whether imposed on the registered holder or any other person, if:
| • | certificates representing outstanding notes for principal amounts not tendered or accepted for exchange are to be delivered to, or are to be issued in the name of, any person other than the registered holder of outstanding notes tendered; |
| • | tendered outstanding notes are registered in the name of any person other than the person signing the letter of transmittal; or |
| • | a transfer tax is imposed for any reason other than the exchange of outstanding notes under the exchange offers. |
If satisfactory evidence of payment of such taxes is not submitted with the letter of transmittal, the amount of such transfer taxes will be billed to that tendering holder.
Holders who tender their outstanding notes for exchange will not be required to pay any transfer taxes. However, holders who instruct us to register exchange notes in the name of, or request that outstanding notes not tendered or not accepted in the exchange offers be returned to, a person other than the registered tendering holder will be required to pay any applicable transfer tax.
Consequences of Failure to Exchange
If you do not exchange your outstanding notes for exchange notes under the exchange offers, your outstanding notes will remain subject to the restrictions on transfer of such outstanding notes:
| • | as set forth in the legend printed on the outstanding notes as a consequence of the issuance of the outstanding notes pursuant to the exemptions from, or in transactions not subject to, the registration requirements of the Securities Act and applicable state securities laws; and |
| • | as otherwise set forth in the offering circular distributed in connection with the private offerings of the outstanding notes. |
In general, you may not offer or sell your outstanding notes unless they are registered under the Securities Act or if the offer or sale is exempt from registration under the Securities Act and applicable state securities laws. Except as required by the registration rights agreements, we do not intend to register resales of the outstanding notes under the Securities Act.
Other
Participating in the exchange offers are voluntary, and you should carefully consider whether to accept. You are urged to consult your financial and tax advisors in making your own decision on what action to take.
We may in the future seek to acquire untendered outstanding notes in open market or privately negotiated transactions, through subsequent exchange offers or otherwise. We have no present plans to acquire any outstanding notes that are not tendered in the exchange offers or to file a registration statement to permit resales of any untendered outstanding notes.
146
Table of Contents
DESCRIPTION OF SENIOR SECURED NOTES
General
Certain terms used in this description are defined under the subheading “Certain Definitions.” In this description, (a) the terms “we,” “our,” “us,” and “Company” refer only to DJO Finance LLC and not any of its Affiliates, (b) the terms “DJO Finance Corp.” and “Co-Issuer” refer only to DJO Finance Corporation and not any of its Affiliates and (c) the term “Issuers” refers to the Company and the Co-Issuer.
On March 20, 2012, the Issuers issued $230.0 million in aggregate principal amount of 8.75% second priority senior secured notes due 2018 (the “Senior Secured Notes”) under an indenture dated as of March 20, 2012 (as amended from time to time, the “Secured Notes Indenture”) among the Issuers, the Guarantors and The Bank of New York Mellon, a New York banking corporation, as trustee (the “Secured Notes Trustee”) and The Bank of New York Mellon, a New York banking corporation, as second lien collateral agent (the “Second Lien Agent”). On October 1, 2012, the Issuers issued an additional $100.0 million in aggregate principal amount of the Senior Secured Notes under the Secured Notes Indenture.
The following description is only a summary of the material provisions of the Senior Secured Notes Indenture and the Security Documents, does not purport to be complete and is qualified in its entirety by reference to the provisions of those agreements, including the definitions therein of certain terms used below. We urge you to read the Senior Secured Notes Indenture and the Security Documents because they, and not this description, will define your rights as Holders of the Senior Secured Notes. You may request copies of the Senior Secured Notes Indenture and the Security Documents at our address set forth on the cover page of the Registration Statement.
The Issuers are jointly and severally liable for all obligations under the Senior Secured Notes. The Co-Issuer is a Wholly-Owned Subsidiary of the Company that has been incorporated in Delaware as a special purpose finance subsidiary to facilitate the offering of the Senior Secured Notes and other debt securities of the Company. The Company believes that some prospective purchasers of the Senior Secured Notes may be restricted in their ability to purchase debt securities of partnerships or limited liability companies, such as the Company, unless the securities are jointly issued by a corporation. The Co-Issuer will not have any substantial operations or assets and will not have any revenues. Accordingly, you should not expect the Co-Issuer to participate in servicing the principal and interest obligations on the Senior Secured Notes.
Brief Description of Senior Secured Notes
The Senior Secured Notes:
| • | are general senior secured obligations of the Issuers; |
| • | without giving effect to security interests, are pari passu in right of payment with all existing and future Senior Indebtedness (including the Senior Credit Facilities) of the Issuers; |
| • | are secured on a second priority basis (together with the Senior Secured Notes and Permitted Additional Pari Passu Obligations) by the Collateral, subject to the first priority liens securing the First Lien Obligations and permitted liens; |
| • | are effectively subordinated to all First Lien Obligations (including the Senior Credit Facilities) to the extent of the value of the assets securing such indebtedness; |
| • | are structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of the Company’s Subsidiaries that are not guaranteeing the Senior Secured Notes; |
| • | are senior in right of payment to any Subordinated Indebtedness (including the Existing Senior Subordinated Notes) of the Issuers; |
147
Table of Contents
| • | are effectively senior in right of payment to any existing and future unsecured Indebtedness of the Issuers to the extent of the value of the Collateral securing the Senior Secured Notes (after giving effect to any senior Lien on the Collateral); |
| • | are effectively subordinated to any existing or future Indebtedness of the Issuers that is secured by liens on assets that do not constitute a part of the Collateral securing the Senior Secured Notes to the extent of the value of such assets; |
| • | are guaranteed on a senior secured basis by the Guarantors, as described under “—Guarantees”; and |
| • | are subject to registration with the SEC pursuant to the Senior Secured Notes Registration Rights Agreement, as described under “Exchange Offer; Registration Rights”. |
All of the Company’s subsidiaries are currently “Restricted Subsidiaries.” However, under certain circumstances, we are permitted to designate certain of our subsidiaries as “Unrestricted Subsidiaries.” Any Unrestricted Subsidiaries are not subject to any of the restrictive covenants in the Senior Secured Notes Indenture and will not guarantee the Senior Secured Notes.
Guarantees
The Guarantors, as primary obligors and not merely as sureties, have initially jointly and severally, fully and unconditionally guaranteed, on a senior secured basis, the performance and full and punctual payment when due, whether at maturity, by acceleration or otherwise, of all obligations, of the Issuers under the Senior Secured Notes Indenture and the Senior Secured Notes, whether for payment of principal of, any premium or interest on or Additional Interest in respect of the Senior Secured Notes, expenses, indemnification or otherwise, on the terms set forth in the Senior Secured Notes Indenture.
The Restricted Subsidiaries (other than as detailed below) have initially guaranteed the Senior Secured Notes. None of our Foreign Subsidiaries have guaranteed the Senior Secured Notes. Each of the Guarantees of the Senior Secured Notes:
| • | are a general secured obligation of each Guarantor; |
| • | without giving effect to security interests, are pari passu in right of payment with all existing and future Senior Indebtedness of each such Guarantor; |
| • | are secured on a second-priority lien basis (together with Permitted Additional Pari Passu Obligations) by substantially all of the assets of such Guarantor that constitute Collateral, subject to the first priority liens securing the First Lien Obligations and permitted liens; |
| • | are effectively subordinated to all First Lien Obligations of each such Guarantor to the extent of the collateral securing such Indebtedness; |
| • | are structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of the Company’s Subsidiaries that are not guaranteeing the Senior Secured Notes; |
| • | are senior in right of payment to all existing and future Subordinated Indebtedness (including guarantees of the Existing Senior Subordinated Notes) of each such entity; |
| • | are effectively senior in right of payment to any existing and future unsecured Indebtedness of the Guarantor to the extent of the value of the Collateral securing the Senior Secured Notes (after giving effect to any senior Lien on the Collateral); and |
| • | are effectively subordinated to any existing or future Indebtedness of the Guarantor that is secured by liens on assets that do not constitute a part of the Collateral securing the Senior Secured Notes to the extent of the value of such assets. |
148
Table of Contents
The Senior Secured Notes are structurally subordinated to Indebtedness, claims of holders of Preferred Stock and other liabilities of Subsidiaries of the Issuers that do not guarantee the Senior Secured Notes.
Not all of the Company’s Subsidiaries have guaranteed the Senior Secured Notes. In the event of a bankruptcy, liquidation or reorganization of any of these non-guarantor Subsidiaries, the non-guarantor Subsidiaries will pay the holders of their debt and their trade creditors before they are able to distribute any of their assets to the Company. For the twelve months ended September 29, 2012, our Subsidiaries that are not Guarantors accounted for approximately $265.8 million, or 23.7%, of the Company’s net sales, and approximately $7.0 million, or 2.5%, of the Company’s total Adjusted EBITDA, and, as of September 29, 2012, our Subsidiaries that are not Guarantors accounted for approximately $236.1 million, or 8.3%, of the total assets, and approximately $47.3 million, or 1.8%, of the Company’s total liabilities.
The obligations of each Guarantor under its Guarantee are limited as necessary to prevent the Guarantee from constituting a fraudulent conveyance under applicable law.
Any entity that makes a payment under its Guarantee are entitled upon payment in full of all guaranteed obligations under the Senior Secured Notes Indenture to a contribution from each other Guarantor in an amount equal to such other Guarantor’s pro rata portion of such payment based on the respective net assets of all the Guarantors at the time of such payment determined in accordance with GAAP.
If a Guarantee were rendered voidable, it could be subordinated by a court to all other indebtedness (including guarantees and other contingent liabilities) of the Guarantor, and, depending on the amount of such indebtedness, a Guarantor’s liability on its Guarantee could be reduced to zero. See “Risk Factors—Risks Related to the Notes—Federal and state fraudulent transfer laws may permit a court to void the note and the guarantees, and, if that occurs, you may not receive any payments on the notes.”
A Guarantee by a Guarantor provides by its terms that it shall be automatically and unconditionally released and discharged upon:
(1) (a) any sale, exchange or transfer (by merger or otherwise) of the Capital Stock of such Guarantor (including any sale, exchange or transfer, after which the applicable Guarantor is no longer a Restricted Subsidiary) if such sale, exchange or transfer is made in compliance with the applicable provisions of the Senior Secured Notes Indenture;
(b) the release or discharge of the guarantee by such Guarantor of the Senior Credit Facilities or the guarantee which resulted in the creation of such Guarantee, except a discharge or release by or as a result of payment under such guarantee;
(c) the proper designation of any Restricted Subsidiary that is a Guarantor as an Unrestricted Subsidiary in accordance with the provisions set forth under “—Certain Covenants—Limitation on Restricted Payments” and the definition of “Unrestricted Subsidiary”; or
(d) the Issuers exercising their legal defeasance option or covenant defeasance option as described under “—Legal Defeasance and Covenant Defeasance” or the Issuers’ obligations under the Indenture being discharged in accordance with the terms of the Indenture; and
(2) such Guarantor delivering to the Senior Secured Notes Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for in the Senior Secured Notes Indenture relating to such transaction have been complied with.
Ranking
The payment of the principal of, premium, if any, and interest on the Senior Secured Notes and the payment of any Guarantee are pari passu in right of payment with all Senior Indebtedness of the Issuers or the relevant Guarantor, as the case may be, including the obligations of the Issuers and such Guarantor under the Senior
149
Table of Contents
Credit Facilities, the Senior Secured Notes, the Existing Senior Notes and the 9.875% Senior Notes due 2018 that are being issued by us in this offering (the “Senior Notes”), subject to the collateral and intercreditor arrangements described below.
The Secured Notes are effectively subordinated to the First Lien Obligations to the extent of the value of the assets securing such Indebtedness. In the event of bankruptcy, liquidation, reorganization or other winding up of the Issuers or the Guarantors or upon a default in payment with respect to, or the acceleration of any Indebtedness under, the Senior Credit Facilities or other First Lien Obligations, the assets of the Issuers and the Guarantors that secure such Indebtedness and other First Lien Obligations are available to pay obligations on the Senior Secured Notes and the Guarantees only after all such Indebtedness has been repaid in full from such assets and there may not be sufficient assets remaining to pay amounts due on any or all of the Senior Secured Notes and the Guarantees then outstanding. As of September 29, 2012, the Issuers and the Guarantors had $839.2 million principal amount of First Lien Obligations consisting entirely of First Lien Obligations under the Senior Credit Facilities, and the Issuers had $62.0 million of available borrowings under the revolving credit facility of the Senior Credit Facilities. As of September 29, 2012, we also had the option to increase the amount available under the Senior Credit Facilities by an amount not to exceed the greater of $150.0 million (subject to pro forma compliance with the senior first lien secured leverage ratio financial maintenance covenant) and the amount of indebtedness the Issuers could incur to the extent the senior first lien secured leverage ratio remains below a certain threshold, a certain portion of which availability are reduced in connection with the issuance of the Senior Secured Notes.
Although the Senior Secured Notes Indenture contains limitations on the amount of additional Indebtedness that the Issuers and the Guarantors may incur, under certain circumstances the amount of such Indebtedness could be substantial and in any case, such Indebtedness may be Senior Indebtedness. See “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.” In addition, under certain circumstances, such Indebtedness could be Secured Indebtedness. See “Certain Covenants—Liens.”
Security for the Senior Secured Notes
General
The Secured Notes, the Guarantees and all obligations with respect thereto under the Senior Secured Notes Indenture, as well as any Permitted Additional Pari Passu Obligations, are secured by second priority security interests in the Collateral. The Senior Secured Notes, the Senior Secured Notes and any other Permitted Additional Pari Passu Obligations will share in the second priority security interests in the Collateral. The security interests securing the Senior Secured Notes, the Guarantees and the Permitted Additional Pari Passu Obligations are second in priority to any and all security interests at any time granted to secure the First Lien Obligations. The First Lien Obligations include our Credit Agreement and related obligations. A Person holding such First Lien Obligations may have rights and remedies with respect to the property subject to such Liens that, if exercised, could adversely affect the value of the Collateral or the ability of the Second Lien Agent to realize or foreclose on the Collateral on behalf of Holders of the Senior Secured Notes.
Pursuant to the Security Documents, the Issuers and each Grantor (as defined in the Security Documents) granted to the Second Lien Agent, for the benefit of the Senior Secured Notes Trustee and the Holders of the Senior Secured Notes, second priority liens and security interests in all of the following (the “Collateral”), subject to certain exceptions in addition to those specified below:
(a) the following (the “Security Collateral”): (i) all Equity Interests from time to time acquired, owned or held by such Grantor (the “Pledged Equity”); provided that the Pledged Equity shall not include (A) more than 65% of the issued and outstanding voting Equity Interests of any Foreign Subsidiary, (B) Equity Interests of Unrestricted Subsidiaries, (C) Equity Interests of any Subsidiary of a Foreign Subsidiary, (D) Equity Interests of any Subsidiary acquired pursuant to a Permitted Acquisition (as defined in the Credit
150
Table of Contents
Agreement) financed with Indebtedness incurred pursuant to Section 7.03(g) of the Credit Agreement if such Equity Interests serve as security for such Indebtedness or if the terms of such Indebtedness prohibit the creation of any other lien on such Equity Interests, (E) Equity Interests of any Person (other than the Company) that is not a direct or indirect, material Wholly-Owned Subsidiary of the Company and (F) Equity Interests of any Subsidiary with respect to which the Administrative Agent and the Company determine in their reasonable judgments that the costs or other consequences (including adverse tax consequences) of providing a pledge of its Equity Interests is excessive in view of the benefits to be obtained; (ii) promissory notes or other instruments evidencing indebtedness owned from time to time or obtained in the future by the Grantor (“Pledged Debt”); (iii) all other property that may be delivered to and held by the Second Lien Agent pursuant to the terms of the Security Documents; (iv) all payments of principal or interest, dividends, cash, instruments and other property from time to time received, receivable or otherwise distributed in respect of the Pledged Equity and the Pledged Debt; and (v) all rights and privileges of such Grantor with respect to the securities and other property referred to in clauses (i), (ii), (iii) and (iv) above;
(b) the following (the “Article 9 Collateral”), in each case of each Grantor: intellectual property; accounts (including deposit accounts); chattel paper; commercial tort claims; documents; equipment; general intangibles; instruments; inventory; investment property; all books and records relating to the Article 9 Collateral; and to the extent not otherwise included, all supporting obligations, collateral security and guarantees given by any person with respect to any of the foregoing; and
(c) all proceeds and products of the property and assets described in clauses (a) and (b) above.
The benefits of such second priority liens, security interests and pledges are subject to the first priority liens securing the First Lien Obligations.
Each Security Document is governed by New York law, except that the pledges of Security Collateral issued by certain material Foreign Subsidiaries may be governed by the law of the jurisdiction applicable to such Foreign Subsidiary. Since the Holders are not parties to the Security Documents, Holders may not, individually or collectively, take any direct action to enforce any rights in their favor under the Security Documents. The Holders may only act by instruction to the Senior Secured Notes Trustee, which shall instruct the Second Lien Agent.
Notwithstanding the foregoing, the Collateral does not and will not in the future include any of the following:
(i) motor vehicles and other assets subject to certificates of title;
(ii) Equity Interests in any Unrestricted Subsidiary or any Equity Interests of any Subsidiary acquired pursuant to an acquisition financed with Indebtedness permitted under the Senior Secured Notes Indenture, to the extent such Equity Interests serve as security for such Indebtedness and the terms of such Indebtedness prohibits the creation of any other Lien on such Equity Interest;
(iii) more than 65% of the issued and outstanding voting Equity Interests of a Foreign Subsidiary or of a Subsidiary of a Foreign Subsidiary;
(iv) Equity Interests of any Person (other than the Company) that is not a direct or indirect material Wholly-Owned Subsidiary of the Company;
(v) any assets (including Equity Interests) with respect to which the Administrative Agent and the Company determine in their reasonable judgments that the costs or other consequences (including adverse tax consequences) of obtaining such security interest are excessive in relation to the value of the security to be afforded thereby; or
(vi) any general intangible assets, investment property or other rights of a Grantor arising under any contract, lease, instrument, license or other document to the extent that the grant of a security interest therein would (x) constitute a violation of a valid and enforceable restriction in respect of such general intangible
151
Table of Contents
assets, investment property or other such rights in favor of a third party or under any law, regulation, permit, order or decree of any governmental authority, unless and until all required consents shall have been obtained (for the avoidance of doubt, the restrictions described herein shall not include negative pledges or similar undertakings in favor of a lender or other financial counterparty) or (y) expressly give any other party in respect of any such contract, lease, instrument, license or other document, the right to terminate its obligations thereunder; provided, however, that the limitation set forth above, shall not affect, limit, restrict or impair the grant by a grantor of a security interest in any such Article 9 Collateral to the extent that an otherwise applicable prohibition or restriction on such grant is rendered ineffective by any applicable law, including the Uniform Commercial Code.
In addition, the Collateral does not and will not in the future include Capital Stock or other securities of any direct or indirect Subsidiary of the Company to the extent necessary for such Subsidiary not to be subject to any requirement pursuant to Rule 3-16 of Regulation S-X under the Securities Act (or any other law, rule or regulation) to file separate financial statements with the SEC (or any other governmental agency). Any property described in this paragraph is referred to collectively or individually as “Excluded Property.” In the event that Rule 3-16 of Regulation S-X under the Securities Act requires or is amended, modified or interpreted by the SEC to require (or is replaced with another rule or regulation, or any other law, rule or regulation is adopted, which would require) the filing with the SEC (or any other governmental agency) of separate financial statements of any Subsidiary of the Company due to the fact that such Subsidiary’s Capital Stock and other securities secure the Senior Secured Notes and Permitted Additional Pari Passu Obligations, then the Capital Stock and other securities of such Subsidiary shall automatically be deemed not to be part of the Collateral (to the extent necessary to not be subject to such requirement). In such event, the Security Documents may be amended or modified, without the consent of any Holder of Secured Notes or a holder of Permitted Additional Pari Passu Obligations, to the extent necessary to release the security interests in the shares of Capital Stock and other securities that are so deemed to no longer constitute part of the Collateral.
The Issuers were required to perfect on the Issue Date the security interests in the Collateral solely to the extent they can be perfected by the filing of UCC-1 financing statements, filings with the US Patent and Trademark Office or Copyright Office, or the delivery of Capital Stock or instruments.
From and after the Issue Date, if the Issuers or any Guarantor creates any additional Lien upon any property to secure First Lien Obligations, they must concurrently grant at least a second priority Lien upon such property (subject to Permitted Liens) as security for the Senior Secured Notes and Guarantees (and any Permitted Additional Pari Passu Obligations) substantially concurrently with granting any such additional Lien.
The Liens on the Collateral securing the Senior Secured Notes and the Guarantees under the Security Documents rank junior in priority to any and all security interests and Liens in and on the Collateral at any time granted to secure the First Lien Obligations and certain Permitted Liens and rank equally in priority with the security interest and Liens in and on the Collateral securing any Permitted Additional Pari Passu Obligations. In addition, the Senior Secured Notes are not and will not in the future be secured by the Equity Interests in the Company or by any of the assets of any Subsidiary that is not a Guarantor. See “Risk Factors—Risks Related to the Senior Secured Notes.”
The Company and its Restricted Subsidiaries are able to incur additional Indebtedness in the future which could share in the Collateral, including additional First Lien Obligations, additional Second Lien Obligations, obligations secured by junior liens and obligations secured by Permitted Liens. The amount of such additional Indebtedness could be significant. Subject to certain conditions, including compliance with the covenants described under “—Impairment of Security Interest” and “—Certain Covenants—Liens,” the Issuers are permitted to pledge the Collateral in connection with future issuances of Permitted Additional Pari Passu Obligations.
No appraisals of any of the Collateral have been prepared by or on behalf of the Issuers in connection with the issuance of the Senior Secured Notes and the Guarantees. By its nature, some or all of the Collateral are
152
Table of Contents
illiquid and may have no readily ascertainable market value. Accordingly, there can be no assurance that the Collateral are able to be sold in a short period of time or at all.
The Issuers estimate that the fair market value of the Collateral on the issue date of the Senior Secured Notes are less than the amounts due under our Secured Indebtedness, including the Senior Secured Notes. We expect that this will also be the case in the future. See “Risk Factors—Risks Relating to the Senior Secured Notes —In the event of a bankruptcy of us or any of the subsidiary guarantors, holders of the Senior Secured Notes may be deemed to have an unsecured claim to the extent that our obligations in respect of the Senior Secured Notes exceed the fair market value of the collateral securing the Senior Secured Notes.”
Administration of Security
Subject to the terms of the Intercreditor Agreement, the Collateral are administered by the Administrative Agent for the benefit of the First Lien Secured Parties and by the Second Lien Agent for the benefit of the Senior Secured Notes Trustee and the Holders of the Senior Secured Notes and any Permitted Additional Pari Passu Obligations. The ability of Holders of the Senior Secured Notes to realize upon the Collateral are subject, among other things, to various bankruptcy law limitations in the event of the Issuers’ bankruptcy. See “Risk Factors—Risks Related to the Notes—Federal and state fraudulent transfer laws may permit a court to void the note and the guarantees, and, if that occurs, you may not receive any payments on the notes.”
Subject to the terms of the Security Documents, the Issuers and the Guarantors will have the right to remain in possession and retain exclusive control over the Collateral securing the Senior Secured Notes (other than as set forth in the Security Documents), to freely operate the Collateral and to collect, invest and dispose of any income therefrom.
By accepting a Secured Note, each Holder thereof is deemed to have:
(i) irrevocably appointed the Second Lien Agent to act as its agent under the Security Documents; and
(ii) irrevocably authorized the Second Lien Agent to (A) perform the duties and exercise the rights, powers and discretions that are specifically given to it under the Security Documents or other documents to which it is a party, together with any other incidental rights, powers and discretions and (B) execute each document expressed to be executed by the Second Lien Agent on its behalf.
Intercreditor Agreement
The Collateral securing the Senior Secured Notes and the Guarantees will also serve as collateral to secure the obligations (including reimbursement obligations in respect of letters of credit) of the Issuers and the Guarantors under the Senior Credit Facilities (and may serve as collateral to secure other Credit Facilities) and other First Lien Obligations on a first-priority basis. On the Issue Date, the Issuers, the Guarantors, the Second Lien Agent, on behalf of itself and the Holders of the Senior Secured Notes, and the Administrative Agent entered into an intercreditor agreement (the “Intercreditor Agreement”) to define the rights under the Senior Credit Facilities and related agreements of the First Lien Secured Parties and the Holders of the Senior Secured Notes with respect to the Collateral.
153
Table of Contents
The Intercreditor Agreement provides, among other things, that
| (1) | Liens on the Collateral securing the Senior Secured Notes are junior to the Liens securing the First Lien Obligations, and consequently, the First Lien Secured Parties or Holders of First Lien Obligations are entitled to receive the proceeds from the disposition of any Collateral prior to the Holders of the Senior Secured Notes; |
| (2) | the Administrative Agent and the Second Lien Agent will not contest or support any other Person in contesting, in any proceedings (including any insolvency proceedings), the perfection, priority, validity or enforceability of a Lien held by or for the benefit or on behalf of any First Lien Secured Party or by or on behalf of any of the Holders of the Senior Secured Notes and any Permitted Additional Pari Passu Obligations in any Collateral; |
| (3) | so long as the Discharge of First Lien Debt (as such term is defined in the Intercreditor Agreement) has not occurred, if the Second Lien Agent or any holder of the Senior Secured Notes or any Permitted Additional Pari Passu Obligations holds any Lien on any assets of the Issuers or any Guarantor securing any Second Lien Obligations that are not also subject to the first priority Lien of the Administrative Agent under the First Lien Documents, the Issuers or such Guarantor, as the case may be, are required to grant a Lien on such assets to the Administrative Agent for the benefit of the First Lien Secured Parties; |
| (4) | so long as the Senior Secured Notes or any Permitted Additional Pari Passu Obligations remain outstanding, if any First Lien Secured Party holds any Lien on any assets of the Issuers or any Guarantor securing any First Lien Obligations that are not also subject to the second priority Lien of the Second Lien Agent under the Security Documents (except for any assets that are expressly not required to be subject to a Lien of the Second Lien Agent under the Senior Secured Notes Indenture or the Security Documents), the Issuers or such Guarantor, as the case may be, are required to grant a Lien on such assets to the Second Lien Agent for the benefit of the Holders of the Senior Secured Notes and any Permitted Additional Pari Passu Obligations; |
| (5) | during any insolvency proceedings, the Administrative Agent and the Second Lien Agent will coordinate their efforts to give effect to the relative priority of their security interests in the Collateral; and |
| (6) | certain procedures for enforcing the second priority Liens on the Collateral shall be followed. |
The Intercreditor Agreement defines “Discharge of First Lien Debt” to mean, subject to certain provisions of the Intercreditor Agreement,
| (1) | the termination of the commitments of the First Lien Lenders and the financing arrangements provided by the First Lien Lenders and the other First Lien Secured Parties to the Issuers and the Guarantors under the First Lien Documents, |
| (2) | the final payment in full in cash of the First Lien Obligations (other than the First Lien Obligations described in clause (3) of this definition and any First Lien Obligations consisting of unasserted contingent indemnity obligations), and |
| (3) | payment in full in cash or cash collateral, or at the issuer’s option, the delivery to the Administrative Agent of a letter of credit payable to the issuer, in either case to the extent required under the terms of the Credit Agreement, in respect of letters of credit issued under the Senior Credit Facilities and Bank Product Obligations. |
If after receipt of any payment of, or proceeds of Collateral applied to the payment of, the First Lien Obligations, the Administrative Agent or any other First Lien Secured Party is required to surrender or return such payment or proceeds to any person for any reason, then the First Lien Obligations intended to be satisfied by such payment or proceeds shall be reinstated and continue and the Intercreditor Agreement shall continue in
154
Table of Contents
full force and effect as if such payment and proceeds had just been received by the Administrative Agent or other First Lien Secured Party as the case may be, and no Discharge of First Lien Debt shall be deemed to have occurred.
In addition, the Senior Secured Notes Indenture provides that
| (1) | if Indebtedness is incurred under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and such Indebtedness is secured on a first-priority basis by any Collateral held or released by the Administrative Agent and the Senior Secured Notes and the Guarantees are secured by any such asset that qualifies as Collateral, then, at the request of the Issuers, the Second Lien Agent and the representative of the Holders of such Indebtedness arecome party to an intercreditor agreement with terms substantially similar to the Intercreditor Agreement (or a supplement to the Intercreditor Agreement), |
| (2) | if Indebtedness is incurred that is, and is permitted to be pursuant to the terms of the Senior Secured Notes Indenture, secured on a second-priority basis by any Collateral held or released by the Administrative Agent and the Senior Secured Notes and the Senior Secured Notes and Guarantees are secured by any such asset that qualifies as Collateral, then the Second Lien Agent, at the request of the Issuers, will enter into an intercreditor agreement with customary terms and provisions, or an amendment or supplement to the Intercreditor Agreement, with the representative of Holders of such Indebtedness and the Administrative Agent (if the Administrative Agent so agrees) and |
| (3) | if Indebtedness is incurred that is, and is permitted to be pursuant to the terms of the Senior Secured Notes Indenture, secured on a junior-priority basis by any Collateral, then the Second Lien Agent, at the request of the Issuers, will agree to enter into an intercreditor agreement, with customary terms and provisions, or a supplement to the Intercreditor Agreement, with the representative of the Holders of such Indebtedness and the Administrative Agent (if the Administrative Agent so agrees). |
Pursuant to the terms of the Intercreditor Agreement, so long as First Lien Obligations are secured, the Administrative Agent (or representative of any other First Lien Obligations) will determine the time and method by which the security interests in the Collateral are enforced. The Second Lien Agent are not permitted to enforce the security interests and certain other rights related to the Senior Secured Notes on the Collateral even if an Event of Default has occurred and the Senior Secured Notes have been accelerated except in any insolvency or liquidation proceeding as necessary to file a claim or statement of interest with respect to the Senior Secured Notes or any Guarantee and in certain other limited situations. After the discharge of the First Priority Liens securing the First Lien Obligations, the Second Lien Agent, acting at the instruction of the Holders of a majority in principal amount of the Senior Secured Notes and Holders of any Permitted Additional Pari Passu Obligations, voting as one class, in accordance with the provisions of the Senior Secured Notes Indenture and the Security Documents, will determine the time and method by which its Lien in the Collateral are enforced and, if applicable, will distribute proceeds (after payment of the costs of enforcement and collateral administration) of the Collateral received by it under the Security Documents for the ratable benefit of the Holders of the Senior Secured Notes and Holders of the Permitted Additional Pari Passu Obligations.
In the event of any sale or other disposition of any Collateral permitted, or consented to by the Administrative Agent, under the terms of the Senior Credit Facilities that results in the release of any of the Administrative Agent’s Liens on any Collateral (excluding any sale or other disposition that is expressly prohibited by the Senior Secured Notes Indenture and the Security Documents (as in effect on the Issue Date) unless such sale or other disposition is consummated in connection with the exercise of the Administrative Agent’s remedies in respect of Collateral or consummated after the commencement of any insolvency or liquidation proceeding or consummated upon the occurrence or during the existence of an event of default under the Senior Credit Facilities), the second priority Liens on the Collateral securing the Senior Secured Notes (the “Second Priority Liens”) are automatically released to the same extent as the Administrative Agent’s Lien and the Second Lien Agent are required to take such actions (and are deemed to have authorized such actions) as necessary to effect such release.
155
Table of Contents
Upon either the acceleration of all the First Lien Obligations or receipt by the Second Lien Agent of written notice from the Administrative Agent of its intention to foreclose or take any similar action to realize upon the Collateral (such notice to be provided within five Business Days before any such action is taken), the Second Lien Agent and holders of the Senior Secured Notes will have the option, by notice to the representative under the First Lien Obligations, to be exercised within 30 days of acceleration or delivery of the notice referred to in the preceding clause, to purchase all (but not less than all) First Lien Obligations in full in cash. Such purchase of the First Lien Obligations shall be consummated within not less than five Business Days and not more than the later of (a) 10 days after receipt by the Administrative Agent of the notice of the election to exercise of the purchase option or (b) 30 days after such acceleration of the First Lien Obligations or receipt of written notice from the Administrative Agent of its intent to commence enforcement actions.
In the event a bankruptcy proceeding shall be commenced by or against the Issuers or any Guarantor and the Administrative Agent shall desire to permit the Issuers or any Guarantor the use of cash collateral which constitutes Collateral securing First Lien Obligations or to enter into certain debtor-in-possession financings with a holder of First Lien Obligations (a “DIP Financing”) in such proceeding, the Second Priority Liens on the Collateral may, without any further action or consent by the Second Lien Agent, be made junior and subordinated to Liens granted to secure such DIP Financings, provided that
(1) such cash collateral use or DIP Financing is on commercially reasonable terms and, if required by applicable law, is approved by the governmental authority having jurisdiction over such bankruptcy proceeding and
(2) such DIP Financing does not compel the Issuers or any Guarantor to seek confirmation of a specific plan of reorganization for which all or substantially all of the material terms are set forth in the documents for the DIP Financing, except that such DIP Financing may (a) provide that the plan of reorganization require the Discharge of First Lien Debt and (b) require the Issuers and any Guarantor to seek confirmation of a plan acceptable to the Administrative Agent and Holders of First Lien Obligations or entities providing the DIP Financing and contain milestones relating to such plan.
To the extent the Liens securing the First Lien Obligations are subordinated or pari passu with such DIP Financing, the Second Lien Agent will subordinate their Second Priority Liens on the Collateral to the Liens securing such DIP Financing (and all obligations relating thereto and to a “carve-out” agreed to by the Administrative Agent or otherwise applicable thereto) and will not request adequate protection or any other relief in connection with its rights as a Holder of Liens on the Collateral (other than as expressly agreed by the Administrative Agent or to the extent described as permitted in the following paragraph).
The Second Lien Agent will only be permitted to seek adequate protection without the prior written consent of the Administrative Agent
(1) to obtain adequate protection in the form of the benefit of additional or replacement Liens on the Collateral, or additional or replacement collateral to secure the Senior Secured Notes, as long as in each case, the Administrative Agent is also granted such additional or replacement Liens or additional or replacement collateral and such Liens are subordinated to the Liens securing the First Lien Obligations to the same extent as the Second Priority Liens on the Collateral are subordinated to the Liens securing the First Lien Obligations,
(2) to obtain adequate protection in the form of reports, notices, inspection rights and similar forms of adequate protection to the extent granted to the Administrative Agent and
(3) to seek and receive, subject to the provisions of the Intercreditor Agreement, adequate protection of its junior interest in the Collateral solely in the form of a superpriority administrative expense claim, including a claim arising under Section 507(b) of the United States Bankruptcy Code (the “Bankruptcy Code”); provided that any such superpriority administrative expense claim of the Second Lien Agent shall be junior in all respects to
156
Table of Contents
any superpriority administrative expense claim granted to the Administrative Agent with respect to such Collateral and in the event that the Second Lien Agent seeks or receives protection of its junior security interest in the Collateral and is granted a superpriority administrative expense claim, including a claim arising under Section 507(b) of the Bankruptcy Code then the Second Lien Agent agrees that the Administrative Agent and Holders of First Lien Obligations shall receive a superpriority administrative expense claims which shall be senior in all respect to the superpriority administrative expense claim granted to the Second Lien Agent with respect to the Collateral.
The Intercreditor Agreement limits the right of the Second Lien Agent and the Holders of the Senior Secured Notes to seek relief from the “automatic stay.” The Intercreditor Agreement provides that the Second Lien Agent may not assert any right of marshalling, appraisal, valuation or other similar right that may be available under applicable law with respect to the Collateral. Until the Discharge of First Lien Debt has occurred, in the event of any insolvency or liquidation proceeding, the Second Lien Agent and the Holders of the Senior Secured Notes or any Permitted Additional Pari Passu Obligations are not permitted to object or oppose (or support any Person in objecting or opposing) a motion to any sale, lease, license, exchange, transfer or other disposition of any Collateral free and clear of the Liens of the Second Lien Agent, Holders of the Senior Secured Notes or any Permitted Additional Pari Passu Obligations or other claims under Section 363 of the Bankruptcy Code, or any comparable provision of any bankruptcy law and shall be deemed to have consented to any such sale, lease, license, exchange, transfer or other disposition of any Collateral under Section 363(f) of the Bankruptcy Code that has been consented to by the Administrative Agent.
Exercise of Remedies Under Security Documents
The holders of the majority in principal outstanding amount of the Second Lien Obligations will have the right to direct the Second Lien Agent, following the occurrence of an Event of Default under the Senior Secured Notes Indenture or an event of default under any agreement or instrument representing such additional Second Lien Obligations, to foreclose on, or exercise its other rights with respect to, the Collateral (or exercise other remedies with respect to the Collateral).
Any action taken or not taken without the vote of any holder of Second Lien Obligations will nevertheless be binding on such holder.
Except as provided in the succeeding sentence, in the case of an Event of Default under the Senior Secured Notes Indenture, or an event of default under any agreement or instrument representing other Second Lien Obligations where such remedies arise, the Second Lien Agent will only be permitted, subject to applicable law, to exercise remedies and sell the Collateral at the direction of the applicable holders of Second Lien Obligations as set forth above. If the Second Lien Agent has asked the holders of Second Lien Obligations for instruction and the applicable holders have not yet responded to such request, the Second Lien Agent are authorized to take, but are not required to take, and will in no event have any liability for the taking, any delay in taking or the failure to take, such actions with regard to a default or event which the Second Lien Agent, in good faith, believes to be reasonably required to promote and protect the interests of the holders of Second Lien Obligations and to preserve the value of the Collateral; provided that once instructions with respect to such request have been received by the Second Lien Agent from the applicable holders of Second Lien Obligations, the actions of the Second Lien Agent are governed thereby and the Second Lien Agent will not take any further action which would be contrary thereto.
Holders of the Senior Secured Notes are deemed to have agreed and accepted the terms of the Intercreditor Agreement by their acceptance of the Senior Secured Notes.
Control over Collateral and Enforcement of Liens; Application of Proceeds
The Security Documents provide that, prior to the Discharge of First Lien Debt, the Administrative Agent will have the sole power to exercise remedies against the Collateral (subject to the right of the Second Lien Agent and the holders of Second Lien Obligations to take limited protective measures with respect to the Second
157
Table of Contents
Priority Liens) and to foreclose upon and dispose of the Collateral. Proceeds realized by the Administrative Agent from the Collateral are applied to amounts owing to the holders of First Lien Obligations until the Discharge of First Lien Debt.
After the Discharge of First Lien Debt, proceeds realized by the Second Lien Agent from the Collateral (including proceeds of Collateral in any insolvency or liquidation proceeding) are applied:
| • | first, to amounts owing to the Second Lien Agent in its capacity as such in accordance with the terms of the Security Documents; |
| • | second, ratably to amounts owing to any representatives for Permitted Additional Pari Passu Obligations in their capacity as such in accordance with the terms of such Permitted Additional Pari Passu Obligations and to amounts owing to the Senior Secured Notes Trustee in its capacity as such in accordance with the terms of the Senior Secured Notes Indenture; |
| • | third, ratably to amounts owing to the holders of Second Lien Obligations in accordance with the terms of the Security Documents, the Senior Secured Notes Indenture and documents governing Permitted Additional Pari Passu Obligations; and |
| • | fourth, to the Company or the relevant Guarantor, as applicable, their successors and assigns or as a court of competent jurisdiction may otherwise direct. |
Certain Bankruptcy Limitations
The right of the Administrative Agent or the Second Lien Agent to repossess and dispose of the Collateral upon the occurrence of an Event of Default would be significantly impaired by bankruptcy law in the event that a bankruptcy case were to be commenced by or against the Issuers or any Guarantor prior to the Administrative Agent’s or the Second Lien Agent’s having repossessed and disposed of the Collateral. Upon the commencement of a case for relief under the Bankruptcy Code, a secured creditor such as the Second Lien Agent is prohibited from repossessing its security from a debtor in a bankruptcy case, or from disposing of security without bankruptcy court approval. Moreover, the Bankruptcy Code permits the debtor to continue to retain and use Collateral even though the debtor is in default under the applicable debt instruments provided that the secured creditor is given adequate protection. The meaning of the term “adequate protection” may vary according to the circumstances, but it is intended in general to protect the value of the secured creditor’s interest in the Collateral and may include cash payments or the granting of additional security, if and at such times as the court in its discretion determines, for any diminution in the value of the Collateral as a result of the stay of repossession or disposition as a result of the automatic stay under the Bankruptcy Code or any use of the Collateral by the debtor during the pendency of the bankruptcy case. A bankruptcy court may determine that a secured creditor may not require compensation for a diminution in the value of the Collateral if the value of the Collateral exceeds the debt it secures. In addition, a bankruptcy court may determine not to provide cash payments as adequate protection to a secured creditor if (among other reasons) the bankruptcy court determines that the amount due under the Senior Secured Notes exceeds the value of the Collateral.
In view of the broad equitable powers of a U.S. bankruptcy court, it is impossible to predict how long payments under the Senior Secured Notes could be delayed following commencement of a bankruptcy case, whether or when the Administrative Agent or the Second Lien Agent could repossess or dispose of the Collateral, the value of the Collateral at any time during a bankruptcy case or whether or to what extent holders of the Senior Secured Notes would be compensated for any delay in payment or loss of value of the Collateral. Any disposition of the Collateral during a bankruptcy case would also require permission from the bankruptcy court. The Bankruptcy Code permits only the payment and/or accrual of post-petition interest, costs and attorneys’ fees to a secured creditor during a debtor’s bankruptcy case to the extent the value of such creditor’s interest in the Collateral is determined by the bankruptcy court to exceed the aggregate outstanding principal amount of the obligations secured by the Collateral.
158
Table of Contents
Furthermore, in the event a bankruptcy court determines that the value of the Collateral is not sufficient to repay all amounts due on the Senior Secured Notes, the holders of the Senior Secured Notes would hold secured claims only to the extent of the value of the Collateral to which the holders of the Senior Secured Notes are entitled, and unsecured claims with respect to such shortfall.
Release of Liens
The Security Documents and the Senior Secured Notes Indenture provide that the Liens securing the Guarantee of any Guarantor are automatically released when such Guarantor’s Guarantee is released in accordance with the terms of the Senior Secured Notes Indenture. In addition, the Liens securing the Obligations under the Senior Secured Notes and the Senior Secured Notes Indenture are released:
| • | in whole, upon a legal defeasance or a covenant defeasance of the Senior Secured Notes as set forth below under “—Legal Defeasance and Covenant Defeasance;” |
| • | in whole, upon satisfaction and discharge of the Senior Secured Notes Indenture; |
| • | in whole, upon payment in full of principal, interest and all other Obligations on the Senior Secured Notes issued under the Senior Secured Notes Indenture; |
| • | in whole or in part, with the consent of the requisite holders of the Senior Secured Notes in accordance with the provisions under “—Amendments, Supplement and Waiver,” including consents obtained in connection with a tender offer or exchange offer for, or purchase of, Secured Notes; and |
| • | in part, as to any asset constituting Collateral (A) that is sold or otherwise disposed of by the Issuers or any of the Guarantors (other than to the Issuers or another Guarantor) in a transaction permitted by the Senior Secured Notes Indenture (to the extent of the interest sold or disposed of); (B) if all other Liens on that asset securing the First Lien Obligations and Permitted Additional Pari Passu Obligations then secured by that asset (including all commitments thereunder) are released or are released simultaneously therewith unless such release occurs in connection with a discharge in full in cash of the First Lien Obligations, which discharge is not in connection with a foreclosure of, or other exercise of remedies with respect to, non-receivables collateral by the First Lien Secured Parties (such discharge not in connection with any such foreclosure or exercise of remedies, a “Payment Discharge”); provided that, in the case of a Payment Discharge, the lien on any non-receivables collateral disposed of in satisfaction in whole or in part of First Lien Obligations shall be automatically released but any proceeds thereof not used for purposes of the discharge of First Lien Obligations in full in cash or otherwise in accordance with the Senior Secured Notes Indenture shall be subject to Lien in favor of the Second Lien Agent; (C) that is cash or Net Proceeds used for any one or more purposes permitted under the “—Repurchase at the Option of Holders—Asset Sales”; or (D) that is otherwise released in accordance with the Senior Secured Notes Indenture or the Security Documents. |
To the extent applicable, following qualification of the Senior Secured Notes Indenture under the Trust Indenture Act, the Company will comply with Section 313(b) of the Trust Indenture Act, relating to reports, and Section 314(d) of the Trust Indenture Act, relating to the release of property and to the substitution therefor of any property to be pledged as Collateral for the Senior Secured Notes. Any certificate or opinion required by Section 314(d) of the Trust Indenture Act may be made by an officer or legal counsel of the Company except in cases where Section 314(d) requires that such certificate or opinion be made by an independent engineer, appraiser or other expert, who shall be reasonably satisfactory to the Senior Secured Notes Trustee. Notwithstanding anything to the contrary in this paragraph, the Company are not required to comply with all or any portion of Section 314(d) of the Trust Indenture Act if it determines, in good faith based on the written advice of counsel, a copy of which written advice shall be provided to the Senior Secured Notes Trustee, that under the terms of Section 314(d) of the Trust Indenture Act or any interpretation or guidance as to the meaning thereof of the SEC and its staff, including “no action” letters or exemptive orders, all or any portion of Section 314(d) of the Trust Indenture Act is inapplicable to any release or series of releases of Collateral.
159
Table of Contents
Impairment of Security Interest
Subject to the following paragraph and the terms of the Security Documents, the Issuers shall not, and shall not permit any of their Restricted Subsidiaries to, take or knowingly or negligently omit to take, any action which action or omission might or would have the result of impairing the security interest with respect to a material portion of the Collateral for the benefit of the Senior Secured Notes Trustee and the holders of the Senior Secured Notes (including the priority thereof). The foregoing shall not be deemed to prohibit any action or inaction that is otherwise permitted by the Senior Secured Notes Indenture or the Security Documents.
The Secured Notes Indenture provides that, at the direction of the Issuers and without the consent of the Holders, the Senior Secured Notes Trustee and the Second Lien Agent shall from time to time enter into one or more amendments to the Security Documents to: (i) cure any ambiguity, omission, defect or inconsistency therein (which may include a release of Collateral), (ii) add to the Collateral or (iii) make any other change thereto that does not adversely affect the Holders, the Senior Secured Notes Trustee or the Second Lien Agent.
After-Acquired Property
If the Issuers or a Guarantor acquires property that is not automatically subject to a perfected security interest or Lien under the Security Documents and such property would be of the type that is required to be pledged as Collateral under the Senior Secured Notes Indenture and the Security Documents, or a Restricted Subsidiary becomes a Guarantor, then the Company or Guarantor will reasonably promptly provide security interests in and liens on such property (or, in the case of a new Guarantor, all of its assets constituting Collateral under the Senior Secured Notes Indenture and the Security Documents), subject to Permitted Liens, in favor of the Second Lien Agent for its benefit and the benefit of the Senior Secured Notes Trustee and the Holders of the Senior Secured Notes and deliver certain joinder agreements and certificates in respect thereof as required by the Senior Secured Notes Indenture and the Security Documents and take all actions required by the Security Documents to perfect the liens created, except as described in the next sentence.
Further Assurances
Subject to the limitations described above, the Security Documents and the Senior Secured Notes Indenture provide that the Issuers and the Guarantors shall, at their expense, execute and deliver, or cause to be executed and delivered, such further agreements, documents and instruments, and do or cause to be done such further acts as may be necessary or proper to evidence, perfect, maintain and enforce the security interests and the priority thereof in the Collateral for the benefit of the Holders of the Senior Secured Notes and the Senior Secured Notes Trustee and the holders of any Permitted Additional Pari Passu Obligations, in each case, to the extent required by the Senior Secured Notes Indenture, the Security Documents and the agreements governing the Permitted Additional Pari Passu Obligations, and to otherwise effectuate the provisions or purposes of the Senior Secured Notes Indenture and the Security Documents.
Paying Agent and Registrar for the Senior Secured Notes
The Issuers maintain one or more paying agents for the Senior Secured Notes in the Borough of Manhattan, City of New York. The initial paying agent for the Senior Secured Notes is the Senior Secured Notes Trustee.
The Issuers also maintain a registrar with offices in the Borough of Manhattan, City of New York. The initial registrar is the Senior Secured Notes Trustee. The registrar maintains a register reflecting ownership of the Senior Secured Notes outstanding from time to time and makes payments on and facilitate transfer of Secured Notes on behalf of the Issuers.
The Company may change the paying agents or the registrars without prior notice to the Holders. The Company or any of its Subsidiaries may act as a paying agent or registrar.
160
Table of Contents
Transfer and Exchange
A Holder may transfer or exchange Notes in accordance with the Senior Secured Notes Indenture. The registrar and the Senior Secured Notes Trustee may require a Holder to furnish appropriate endorsements and transfer documents in connection with a transfer of Secured Notes. Holders are required to pay all taxes due on transfer. The Issuers are not required to transfer or exchange any Secured Note selected for redemption. Also, the Issuers are not required to transfer or exchange any Secured Note for a period of 15 days before a selection of Secured Notes to be redeemed.
Principal, Maturity and Interest
The Issuers issued $230.0 million of Senior Secured Notes on March 20, 2012, and issued $100.0 million of Senior Secured Notes on October 1, 2012. The Secured Notes will mature on March 15, 2018. Subject to compliance with the covenant described below under the caption “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” the Issuers may issue an unlimited principal amount of additional Secured Notes from time to time under the Senior Secured Notes Indenture (“Additional Secured Notes”). The Senior Secured Notes offered by the Issuers, the Senior Secured Notes and any Additional Secured Notes subsequently issued under the Senior Secured Notes Indenture are treated as a single class for all purposes under the Senior Secured Notes Indenture, including waivers, amendments, redemptions and offers to purchase. Unless the context requires otherwise, references to “Secured Notes” for all purposes of the Senior Secured Notes Indenture and this “Description of Senior Secured Notes” include the Senior Secured Notes, the Senior Secured Notes and any Additional Secured Notes that are actually issued. The Issuers issued the Senior Secured Notes in denominations of $2,000 and integral multiples of $1,000, in excess thereof.
Interest on the Senior Secured Notes accrues at the rate of 8.75% per annum and is payable semi-annually in arrears on March 15 and September 15. The first interest payment date on the Senior Secured Notes are March 15, 2013. Interest on the Senior Secured Notes will accrue from September 15, 2012. We makes each interest payment to the Holders of Secured Notes of record on the immediately preceding March 1 and September 1. Interest on the Senior Secured Notes are computed on the basis of a 360-day year comprised of twelve 30-day months.
Additional Interest may accrue on the Senior Secured Notes in certain circumstances pursuant to the Senior Secured Notes Registration Rights Agreement. All references in the Senior Secured Notes Indenture, in any context, to any interest or other amount payable on or with respect to the Senior Secured Notes shall be deemed to include any Additional Interest pursuant to the Senior Secured Notes Registration Rights Agreement.
Principal of, premium, if any, and interest on the Senior Secured Notes are payable at the office or agency of the Issuers maintained for such purpose within the City and State of New York or, at the option of the Issuers, payment of interest may be made by check mailed to the Holders of the Senior Secured Notes at their respective addresses set forth in the register of Holders; provided that all payments of principal, premium, if any, and interest with respect to the Senior Secured Notes represented by one or more global notes registered in the name of or held by The Depository Trust Company (“DTC”) or its nominee are made by wire transfer of immediately available funds to the accounts specified by the Holder or Holders thereof. Until otherwise designated by the Issuers, the Issuers’ office or agency in New York are the office of the Senior Secured Notes Trustee maintained for such purpose.
Mandatory Redemption; Offers to Purchase; Open Market Purchases
The Issuers are not required to make any mandatory redemption or sinking fund payments with respect to the Senior Secured Notes. However, under certain circumstances, the Issuers may be required to make an offer to purchase Secured Notes as described under the caption “—Repurchase at the Option of Holders.” In addition, we may, at our discretion, at any time and from time to time purchase Secured Notes in the open market or otherwise.
161
Table of Contents
Optional Redemption
Except as set forth below, the Issuers are not entitled to redeem the Senior Secured Notes at their option prior to March 15, 2015.
At any time prior to March 15, 2015, the Issuers may redeem all or a part of the Senior Secured Notes, upon not less than 30 nor more than 60 days prior notice mailed by first-class mail to the registered address of each Holder or otherwise delivered in accordance with the procedures of DTC, at a redemption price equal to 100% of the principal amount of Secured Notes redeemed plus the Applicable Premium as of, and accrued and unpaid interest and Additional Interest, if any, to the date of redemption (the “Redemption Date”), subject to the rights of Holders on the relevant record date to receive interest due on the relevant interest payment date.
On and after March 15, 2015, the Issuers may redeem the Senior Secured Notes, in whole or in part, upon notice as described under the heading “—Repurchase at the Option of Holders—Selection and Notice” at the redemption prices (expressed as percentages of principal amount of the Senior Secured Notes to be redeemed) set forth below, plus accrued and unpaid interest thereon and Additional Interest, if any, to the applicable Redemption Date, subject to the right of Holders of record, on the relevant record date to receive interest due on the relevant interest payment date, if redeemed during the twelve-month period beginning on March 15 of each of the years indicated below:
| Year |
Percentage | |||
| 2015 |
104.375 | % | ||
| 2016 |
102.188 | |||
| 2017 |
100.000 | |||
In addition, until March 15, 2015, the Issuers may, at their option, redeem up to 35% of the aggregate principal amount of Secured Notes issued by them at a redemption price equal to 108.750% of the aggregate principal amount thereof, plus accrued and unpaid interest thereon and Additional Interest, if any, to the applicable Redemption Date, subject to the right of Holders of Secured Notes of record on the relevant record date to receive interest due on the relevant interest payment date, with the net cash proceeds of one or more Equity Offerings; provided that at least 65% of the aggregate principal amount of Secured Notes originally issued under the Senior Secured Notes Indenture and any Additional Secured Notes (including the Senior Secured Notes) issued under the Senior Secured Notes Indenture after the Issue Date (excluding Secured Notes held by the Issuers or Subsidiaries or Affiliates of the Issuers) remains outstanding immediately after the occurrence of each such redemption; provided further that each such redemption occurs within 90 days of the date of closing of each such Equity Offering.
Any notice of redemption may be subject to one or more conditions precedent, including, but not limited to, completion of an Equity Offering or other corporate transaction.
The Secured Notes Trustee shall select the Senior Secured Notes to be purchased in the manner described under “Repurchase at the Option of Holders—Selection and Notice.”
Repurchase at the Option of Holders
Change of Control
The Secured Notes Indenture provides that if a Change of Control occurs, unless the Issuers have previously or concurrently mailed a redemption notice with respect to all the outstanding Secured Notes as described under “—Optional Redemption,” the Issuers makes an offer to purchase all of the Senior Secured Notes pursuant to the offer described below (the “Change of Control Offer”) at a price in cash (the “Change of Control Payment”) equal to 101% of the aggregate principal amount thereof plus accrued and unpaid interest and Additional Interest, if any, to the date of purchase, subject to the right of Holders of the Senior Secured Notes of record on the relevant record date to receive interest due on the relevant interest payment date. Within 60 days following any
162
Table of Contents
Change of Control, the Issuers will send notice of such Change of Control Offer by first-class mail, with a copy to the Senior Secured Notes Trustee, to each Holder of Secured Notes to the address of such Holder appearing in the security register or otherwise in accordance with the procedures of DTC with a copy to the Senior Secured Notes Trustee, with the following information:
(1) that a Change of Control Offer is being made pursuant to the covenant entitled “Change of Control” under the Senior Secured Notes Indenture and that all Secured Notes properly tendered pursuant to such Change of Control Offer are accepted for payment by the Issuers;
(2) the purchase price and the purchase date, which are no earlier than 30 days nor later than 60 days from the date such notice is mailed (the “Change of Control Payment Date”);
(3) that any Secured Note not properly tendered will remain outstanding and continue to accrue interest;
(4) that unless the Issuers default in the payment of the Change of Control Payment, all Secured Notes accepted for payment pursuant to the Change of Control Offer will cease to accrue interest on the Change of Control Payment Date;
(5) that Holders electing to have any Secured Notes purchased pursuant to a Change of Control Offer are required to surrender such Secured Notes, with the form entitled “Option of Holder to Elect Purchase” on the reverse of such Secured Notes completed, to the paying agent specified in the notice at the address specified in the notice prior to the close of business on the third Business Day preceding the Change of Control Payment Date;
(6) that Holders are entitled to withdraw their tendered Secured Notes and their election to require the Issuers to purchase such Secured Notes; provided that the paying agent receives, not later than the close of business on the expiration date of the Change of Control Offer, a telegram, telex, facsimile transmission or letter setting forth the name of the Holder of the Senior Secured Notes, the principal amount of Secured Notes tendered for purchase, and a statement that such Holder is withdrawing its tendered Secured Notes and its election to have such Secured Notes purchased;
(7) that if the Issuers are repurchasing less than all of the Senior Secured Notes, the remaining Secured Notes are equal in principal amount to the unpurchased portion of the Senior Secured Notes surrendered. The unpurchased portion of the Senior Secured Notes must be equal to $2,000 or an integral multiple of $1,000 in excess thereof;
(8) the other instructions, as determined by the Issuers, consistent with the covenant described hereunder, that a Holder must follow; and
(9) if such notice is mailed prior to the occurrence of a Change of Control, stating that the Change of Control Offer is conditional upon the occurrence of such Change of Control.
The Issuers will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of Secured Notes pursuant to a Change of Control Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Senior Secured Notes Indenture, the Issuers will comply with the applicable securities laws and regulations and shall not be deemed to have breached their obligations described in the Senior Secured Notes Indenture by virtue thereof.
On the Change of Control Payment Date, the Issuers will, to the extent permitted by law,
(1) accept for payment all Secured Notes issued by them or portions thereof properly tendered pursuant to the Change of Control Offer,
163
Table of Contents
(2) deposit with the paying agent an amount equal to the aggregate Change of Control Payment in respect of all Secured Notes or portions thereof so tendered, and
(3) deliver, or cause to be delivered, to the Senior Secured Notes Trustee for cancellation the Senior Secured Notes so accepted together with an Officer’s Certificate to the Senior Secured Notes Trustee stating that such Secured Notes or portions thereof have been tendered to, and purchased by, the Issuers.
The Senior Credit Facilities will, and future credit agreements, or other agreements relating to Senior Indebtedness to which the Issuers become a party may, provide that certain change of control events with respect to the Issuers, would constitute a default thereunder (including events that would constitute a Change of Control under the Senior Secured Notes Indenture). If we experience a change of control that triggers a default under our Senior Credit Facilities or any such future Indebtedness, we could seek a waiver of such default or seek to refinance our Senior Credit Facilities or such future Indebtedness. In the event we do not obtain such a waiver or refinance the Senior Credit Facilities or such future Indebtedness, such default could result in amounts outstanding under our Senior Credit Facilities or such future Indebtedness being declared due and payable.
The Existing Senior Notes and the Existing Senior Subordinated Notes have similar requirements regarding our obligation to offer to purchase such notes upon the occurrence of a Change of Control.
Our ability to pay cash to the Holders of Secured Notes following the occurrence of a Change of Control may be limited by our then existing financial resources. Therefore, sufficient funds may not be available when necessary to make any required repurchases.
The Change of Control purchase feature of the Senior Secured Notes may in certain circumstances make more difficult or discourage a sale or takeover of us and, thus, the removal of incumbent management. We have no present intention to engage in a transaction involving a Change of Control, although it is possible that we could decide to do so in the future. Subject to the limitations discussed below, we could, in the future, enter into certain transactions, including acquisitions, refinancings or other recapitalizations, that would not constitute a Change of Control under the Senior Secured Notes Indenture, but that could increase the amount of indebtedness outstanding at such time or otherwise affect our capital structure or credit ratings. Restrictions on our ability to incur additional Indebtedness are contained in the covenants described under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Certain Covenants—Liens.” Such restrictions in the Senior Secured Notes Indenture can be waived only with the consent of the Holders of a majority in principal amount of the Senior Secured Notes then outstanding. Except for the limitations contained in such covenants, however, the Senior Secured Notes Indenture will not contain any covenants or provisions that may afford Holders of the Senior Secured Notes protection in the event of a highly leveraged transaction.
We are not required to make a Change of Control Offer following a Change of Control if a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the Senior Secured Notes Indenture applicable to a Change of Control Offer made by us and purchases all Secured Notes validly tendered and not withdrawn under such Change of Control Offer. Notwithstanding anything to the contrary herein, a Change of Control Offer may be made in advance of a Change of Control, conditional upon such Change of Control, if a definitive agreement is in place for the Change of Control at the time of making of the Change of Control Offer.
The definition of “Change of Control” includes a disposition of all or substantially all of the assets of the Company to any Person. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law. Accordingly, in certain circumstances there may be a degree of uncertainty as to whether a particular transaction would involve a disposition of “all or substantially all” of the assets of the Company. As a result, it may be unclear as to whether a Change of Control has occurred and whether a Holder of Secured Notes may require the Issuers to make an offer to repurchase the Senior Secured Notes as described above.
164
Table of Contents
The provisions under the Senior Secured Notes Indenture relating to the Issuers’ obligation to make an offer to repurchase the Senior Secured Notes as a result of a Change of Control may be waived or modified with the written consent of the Holders of a majority in principal amount of the Senior Secured Notes.
Asset Sales
The Secured Notes Indenture provides that the Company will not, and will not permit any of its Restricted Subsidiaries to consummate an Asset Sale, unless:
(1) the Company or such Restricted Subsidiary, as the case may be, receives consideration at the time of such Asset Sale at least equal to the fair market value (as determined in good faith by the Company) of the assets sold or otherwise disposed of; and
(2) except in the case of a Permitted Asset Swap, at least 75% of the consideration therefor received by the Company or such Restricted Subsidiary, as the case may be, is in the form of cash or Cash Equivalents; provided that the following shall be deemed to be cash for purposes of this provision and for no other purpose:
(a) any liabilities (as reflected in the Company’s or such Restricted Subsidiary’s most recent balance sheet or in the footnotes thereto, or if incurred or accrued subsequent to the date of such balance sheet, such liabilities that would have been shown on the Company’s or such Restricted Subsidiary’s balance sheet or in the footnotes thereto if such incurrence or accrual had taken place on the date of such balance sheet) of the Company or such Restricted Subsidiary (other than liabilities that are by their terms subordinated to the Senior Secured Notes or liabilities to the extent owed to the Company or any Affiliate of the Company) that are assumed by the transferee of any such assets and for which the Company and all of its Restricted Subsidiaries have been validly released by all creditors in writing,
(b) any securities, notes or other similar obligations received by the Company or such Restricted Subsidiary from such transferee that are converted by the Company or such Restricted Subsidiary into cash (to the extent of the cash received) within 180 days following the closing of such Asset Sale, and
(c) any Designated Non-cash Consideration received by the Company or such Restricted Subsidiary in such Asset Sale having an aggregate fair market value, taken together with all other Designated Non-cash Consideration received pursuant to this clause (c) that is at that time outstanding, not to exceed 2.5% of Total Assets at the time of the receipt of such Designated Non-cash Consideration, with the fair market value of each item of Designated Non-cash Consideration being measured at the time received and without giving effect to subsequent changes in value.
Within 450 days after the receipt of any Net Proceeds of any Asset Sale, the Company or such Restricted Subsidiary, at its option, may apply the Net Proceeds from such Asset Sale,
(1) to permanently reduce:
(a) if the assets subject of such Asset Sale constitute Collateral, First Lien Obligations (and to correspondingly reduce commitments with respect thereto) and/or to permanently reduce (or offer to reduce) Obligations under the Senior Secured Notes and under any other Permitted Additional Pari Passu Obligations on a pro rata basis; provided that all reductions of Obligations under the Senior Secured Notes shall be made as provided under “Optional Redemption,” through open-market purchases (to the extent such purchases are at or above 100% of the principal amount thereof) or by making an offer (in accordance with the procedures set forth below for an Asset Sale Offer) to all Holders to purchase their Notes at 100% of the principal amount thereof, plus the amount of accrued but unpaid interest and Additional Interest, if any, on the amount of the Senior Secured Notes that would otherwise be prepaid;
(b) if the assets subject of such Asset Sale do not constitute Collateral, Obligations under Senior Indebtedness that is secured by a Lien, which Lien is permitted by the Senior Secured Notes Indenture, and to correspondingly reduce commitments with respect thereto;
165
Table of Contents
(c) if the assets subject of such Asset Sale do not constitute Collateral, Obligations under other Senior Indebtedness (and to correspondingly reduce commitments with respect thereto), provided that the Issuers shall equally and ratably reduce (or offer to reduce, as applicable) Obligations under the Senior Secured Notes (and may elect to reduce Permitted Additional Pari Passu Obligations) on a pro rata basis; provided further that all reductions of Obligations under the Senior Secured Notes shall be made as provided under “Optional Redemption,” through open-market purchases (to the extent such purchases are at or above 100% of the principal amount thereof plus accrued unpaid interest) or by making an offer (in accordance with the procedures set forth below for an Asset Sale Offer) to all Holders of Secured Notes to purchase their Notes at 100% of the principal amount thereof, plus the amount of accrued but unpaid interest, if any, on the amount of Secured Notes that would otherwise be prepaid; or
(d) Indebtedness of a Restricted Subsidiary that is not a Guarantor, other than Indebtedness owed to the Company or any Affiliate of the Company,
(2) to make (a) an Investment in any one or more businesses; provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Company or another of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) capital expenditures or (c) acquisitions of other assets, in each of (a), (b) and (c), used or useful in a Similar Business, or
(3) to make an Investment in (a) any one or more businesses; provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Company or another of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) properties or (c) acquisitions of other assets that, in each of (a), (b) and (c), replace the businesses, properties and/or assets that are the subject of such Asset Sale;
provided that, in the case of clauses (2) and (3) above, (A) a binding commitment shall be treated as a permitted application of the Net Proceeds from the date of such commitment so long as the Company, or such other Restricted Subsidiary enters into such commitment with the good faith expectation that such Net Proceeds are applied to satisfy such commitment within 180 days of such commitment (an “Acceptable Commitment”) and, in the event any Acceptable Commitment is later cancelled or terminated for any reason before the Net Proceeds are applied in connection therewith, the Company or such Restricted Subsidiary enters into another Acceptable Commitment (a “Second Commitment”) within 180 days of such cancellation or termination; provided further that if any Second Commitment is later cancelled or terminated for any reason before such Net Proceeds are applied, then such Net Proceeds shall constitute Excess Proceeds; and (B) to the extent the assets or property that were the subject of such Asset Sale were included in the Collateral, then, consistent with the terms of the Security Documents, the Second Lien Agent shall receive from the Issuers a valid and perfected second priority lien on any business, property, assets or Capital Stock so acquired.
Any Net Proceeds from the Asset Sale that are not invested or applied as provided and within the time period set forth in the first sentence of the preceding paragraph are deemed to constitute “Excess Proceeds.” When the aggregate amount of Excess Proceeds exceeds $20.0 million, the Issuers shall make an offer to all Holders of the Senior Secured Notes (x) in the case of Net Proceeds from Collateral, to the holders of any other Permitted Additional Pari Passu Obligations if required by the terms of such Permitted Additional Pari Passu Obligations and (y) in the case of any other Net Proceeds, to all holders of other Indebtedness that is pari passu with the Senior Secured Notes or any Guarantee if required by the terms of such Indebtedness (“Pari Passu Indebtedness”), to the holders of such Pari Passu Indebtedness (an “Asset Sale Offer”), to purchase the maximum aggregate principal amount of the Senior Secured Notes and such Pari Passu Indebtedness that is an integral multiple of $1,000 (but in minimum amounts of $2,000) that may be purchased out of the Excess Proceeds at an offer price in cash in an amount equal to 100% of the principal amount thereof, plus accrued and unpaid interest and Additional Interest, if any, to the date fixed for the closing of such offer, in accordance with the procedures set forth in the Senior Secured Notes Indenture. The Issuers will commence an Asset Sale Offer with respect to
166
Table of Contents
Excess Proceeds within ten Business Days after the date that Excess Proceeds exceed $20.0 million by mailing the notice required pursuant to the terms of the Senior Secured Notes Indenture, with a copy to the Senior Secured Notes Trustee.
To the extent that the aggregate amount of Secured Notes and such Permitted Additional Pari Passu Obligations or such Pari Passu Indebtedness, as applicable, tendered pursuant to an Asset Sale Offer is less than the Excess Proceeds, the Company may use any remaining Excess Proceeds for general corporate purposes, subject to other covenants contained in the Senior Secured Notes Indenture. If the aggregate principal amount of Secured Notes and other Permitted Additional Pari Passu Obligations (in the case of Net Proceeds from Collateral) or other Pari Passu Indebtedness (in the case of any other Net Proceeds) surrendered by such holders thereof exceeds the amount of Excess Proceeds, the Senior Secured Notes Trustee shall select the Senior Secured Notes and the Permitted Additional Pari Passu Obligations or the Pari Passu Indebtedness, as the case may be, to be purchased on a pro rata basis based on the accreted value or principal amount of the Senior Secured Notes and such Permitted Additional Pari Passu Obligations or such Pari Passu Indebtedness tendered. Additionally, the Issuers may, at their option, make an Asset Sale Offer using proceeds from any Asset Sale at any time after consummation of such Asset Sale. Upon consummation of any Asset Sale Offer, any Net Proceeds not used to purchase Secured Notes in such Asset Sale Offer shall not be deemed Excess Proceeds and the Company may use any Net Proceeds not required to be used for general corporate purposes, subject to other covenants contained in the Senior Secured Notes Indenture.
Pending the final application of any Net Proceeds pursuant to this covenant, the holder of such Net Proceeds may apply such Net Proceeds temporarily to reduce Indebtedness outstanding under a revolving credit facility or otherwise invest such Net Proceeds in any manner not prohibited by the Senior Secured Notes Indenture.
The Issuers will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of the Senior Secured Notes pursuant to an Asset Sale Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Senior Secured Notes Indenture, the Issuers will comply with the applicable securities laws and regulations and shall not be deemed to have breached their obligations described in the Senior Secured Notes Indenture by virtue thereof.
Events of Loss
Subject to the Intercreditor Agreement and the other Security Documents, in the case of an Event of Loss with respect to any Collateral, the Issuers or the affected Restricted Subsidiary, as the case may be, will apply the Net Loss Proceeds from such Event of Loss, within 450 days after receipt, at its option to:
| (1) | reduce its First Lien Obligations (and to correspondingly reduce commitments with respect thereto) and/or to permanently reduce (or offer to reduce) Obligations under the Senior Secured Notes and under any other Permitted Additional Pari Passu Obligations on a pro rata basis; |
| (2) | rebuild, repair, replace or construct improvements to the affected property or facility (or enter into a binding agreement to do so, provided that (x) such rebuilding, repair, replacement or construction has been completed within the later of (i) 450 days after the receipt of the Net Loss Proceeds and (ii) 180 days after the date of such binding agreement and (y) if such rebuilding, repair, replacement or construction is not consummated within the period set forth in subclause (x), the Net Loss Proceeds not so applied are deemed to be Excess Loss Proceeds (as defined below)); or |
| (3) | invest in assets and properties as described in clause (2) of the first paragraph under “Asset Sales,” substituting the term “Event of Loss” for the term “Asset Sale,” the term “Net Loss Proceeds” for the term “Net Proceeds” and the term “Excess Loss Proceeds” for the term “Excess Proceeds.” |
In the case of clause (2) or (3) above, any replacement assets or property shall be pledged as Collateral for the Senior Secured Notes, if required under the Security Documents.
167
Table of Contents
Any Net Loss Proceeds from an Event of Loss that are not applied or invested as provided in the prior paragraph are deemed to constitute “Excess Loss Proceeds.” When the aggregate amount of Excess Loss Proceeds exceeds $25.0 million, the Issuers makes an offer (a “Loss Proceeds Offer”) to all Holders of the Senior Secured Notes and to any holders of Permitted Additional Pari Passu Obligations to the extent required by the terms thereof to purchase the maximum principal amount of Secured Notes and such Permitted Additional Pari Passu Obligations that may be purchased out of such Excess Loss Proceeds, at an offer price in cash in an amount equal to 100% of the principal amount of the Senior Secured Notes, plus accrued and unpaid interest thereon, if any, to the date of purchase and in the case of any Permitted Additional Pari Passu Obligations at the offer price required by the terms thereof but not to exceed 100% of the principal amount thereof, plus accrued and unpaid interest, if any. If any Excess Loss Proceeds remain after consummation or expiration of a Loss Proceeds Offer, such Excess Loss Proceeds may be used for any purpose not otherwise prohibited by the Senior Secured Notes Indenture. If the aggregate principal amount of the Senior Secured Notes and Permitted Additional Pari Passu Obligations tendered into such Loss Proceeds Offer exceeds the amount of Excess Loss Proceeds, then such Secured Notes and Permitted Additional Pari Passu Obligations are purchased on a pro rata basis based on the accreted value or principal amount of such Secured Notes and such Permitted Additional Pari Passu Obligations tendered (and the Senior Secured Notes Trustee will select the tendered Secured Notes of tendering holders on a pro rata basis based on the amount of Secured Notes tendered). The Issuers may satisfy the foregoing obligations with respect to any Net Loss Proceeds from an Event of Loss by making a Loss Proceeds Offer with respect to such Net Loss Proceeds prior to the expiration of the relevant 450 days or with respect to Net Loss Proceeds of $25.0 million or less.
The Issuers will comply with the applicable tender offer rules, including Rule 14e-1 under the Exchange Act, and any other applicable securities laws or regulations in connection with a Loss Proceeds Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Senior Secured Notes Indenture, the Issuers shall comply with the applicable securities laws and regulations and are not deemed to have breached its obligations under the Senior Secured Notes Indenture by virtue of this compliance.
Selection and Notice
If the Issuers are redeeming less than all of the Senior Secured Notes issued by them at any time, the Senior Secured Notes Trustee will select the Senior Secured Notes to be redeemed (a) if the Senior Secured Notes are listed on any national securities exchange, in compliance with the requirements of the principal national securities exchange on which the Senior Secured Notes are listed or (b) on a pro rata basis to the extent practicable or, to the extent that selection on a pro rata basis is not practicable, by lot or such other similar method in accordance with the procedures of DTC.
Notices of purchase or redemption shall be mailed by first-class mail, postage prepaid, at least 30 but not more than 60 days before the purchase or redemption date to each Holder of Secured Notes at such Holder’s registered address or otherwise in accordance with the procedures of DTC, except that redemption notices may be mailed more than 60 days prior to a redemption date if the notice is issued in connection with a defeasance of the Senior Secured Notes or a satisfaction and discharge of the Senior Secured Notes Indenture. If any Secured Note is to be purchased or redeemed in part only, any notice of purchase or redemption that relates to such Secured Note shall state the portion of the principal amount thereof that has been or is to be purchased or redeemed.
The Issuers will issue a new Secured Note in a principal amount equal to the unredeemed portion of the original Secured Note in the name of the Holder upon cancellation of the original Secured Note. Secured Notes called for redemption become due on the date fixed for redemption. On and after the redemption date, interest ceases to accrue on Secured Notes or portions thereof called for redemption.
Certain Covenants
Set forth below are summaries of certain covenants contained in the Senior Secured Notes Indenture. During any period of time that (i) the Senior Secured Notes have Investment Grade Ratings from both Rating Agencies,
168
Table of Contents
and (ii) no Default has occurred and is continuing under the Senior Secured Notes Indenture (the occurrence of the events described in the foregoing clauses (i) and (ii) being collectively referred to as a “Covenant Suspension Event”), the Company and the Restricted Subsidiaries are not subject to the following covenants (the “Suspended Covenants”):
(1) “—Repurchase at the Option of Holders—Asset Sales” and “—Repurchase at the Option of Holders—Change of Control”;
(2) “—Limitation on Restricted Payments”;
(3) “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(4) clause (4) of the first paragraph of “—Merger, Consolidation or Sale of All or Substantially All Assets”;
(5) “—Transactions with Affiliates”;
(6) “—Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries”; and
(7) “—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries.”
In the event that the Company and the Restricted Subsidiaries are not subject to the Suspended Covenants under the Senior Secured Notes Indenture for any period of time as a result of the foregoing, and on any subsequent date (the “Reversion Date”) one or both of the Rating Agencies withdraw their Investment Grade Rating or downgrade the rating assigned to the Senior Secured Notes below an Investment Grade Rating, then the Company and the Restricted Subsidiaries will thereafter again be subject to the Suspended Covenants under the Senior Secured Notes Indenture with respect to future events. The period of time between the Suspension Date and the Reversion Date is referred to in this description as the “Suspension Period.” The Guarantees of the Guarantors are suspended during the Suspension Period. Additionally, upon the occurrence of a Covenant Suspension Event, the amount of Excess Proceeds from Net Proceeds shall be reset at zero.
During any Suspension Period, the Company will not, and will not permit any Restricted Subsidiary to, enter into any Sale and Lease-Back Transaction; provided, that the Company or any Restricted Subsidiary may enter into a Sale and Lease-Back Transaction if (i) the Company or such Restricted Subsidiary could have incurred a Lien to secure the Indebtedness attributable to such Sale and Lease-Back Transaction pursuant to “—Liens” below without equally and ratably securing the Senior Secured Notes pursuant to the covenant described under such covenant; and (ii) the consideration received by the Company or such Restricted Subsidiary in that Sale and Lease-Back Transaction is at least equal to the fair market value of the property sold and otherwise complies with “—Repurchase at the Option of Holders—Asset Sales” above; provided, further, that the foregoing provisions shall cease to apply on and subsequent to the Reversion Date following such Suspension Period.
During the Suspension Period, the Company and its Restricted Subsidiaries are entitled to incur Liens to the extent provided for under “—Liens” (including Permitted Liens) to the extent provided for in such covenant and any Permitted Liens which may refer to one or more Suspended Covenants shall be interpreted as though such applicable Suspended Covenant(s) continued to be applicable during the Suspension Period (but solely for purposes of the “—Liens” covenant and for no other covenant).
Notwithstanding the foregoing, in the event of any such reinstatement, no action taken or omitted to be taken by the Company or any of its Restricted Subsidiaries prior to such reinstatement will give rise to a Default or Event of Default under the Senior Secured Notes Indenture with respect to Secured Notes; provided that (1) with respect to Restricted Payments made after any such reinstatement, the amount of Restricted Payments made are calculated as though the covenant described above under the caption “Limitation on Restricted Payments” had been in effect prior to but not during the Suspension Period, provided that any Subsidiaries designated as Unrestricted Subsidiaries during the Suspension Period shall automatically become Restricted
169
Table of Contents
Subsidiaries on the Reversion Date (subject to the Company’s right to subsequently designate them as Unrestricted Subsidiaries in compliance with the covenants set out below) and (2) all Indebtedness incurred, or Disqualified Stock issued, during the Suspension Period are classified to have been incurred or issued pursuant to clause (3) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.” In addition, for purposes of clause (3) of the first paragraph under the caption “—Limitation on Restricted Payments,” all events (including the accrual of Consolidated Net Income) set forth in such clause (3) occurring during a Suspension Period shall be disregarded for purposes of determining the amount of Restricted Payments the Company or any Restricted Subsidiary is permitted to make pursuant to such clause (3).
There can be no assurance that the Senior Secured Notes will ever achieve or maintain Investment Grade Ratings.
Limitation on Restricted Payments
The Company will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly:
(I) declare or pay any dividend or make any payment or distribution on account of the Company’s, or any of its Restricted Subsidiaries’ Equity Interests, including, without limitation, payable in connection with any merger or consolidation other than:
(a) dividends or distributions by the Company payable solely in Equity Interests (other than Disqualified Stock) of the Company; or
(b) dividends, payments or distributions by a Restricted Subsidiary so long as, in the case of any dividend, payment or distribution payable on or in respect of any class or series of securities issued by a Restricted Subsidiary other than a Wholly-Owned Subsidiary, the Company or a Restricted Subsidiary receives at least its pro rata share of such dividend or distribution in accordance with its Equity Interests in such class or series of securities;
(II) purchase, redeem, defease or otherwise acquire or retire for value any Equity Interests of the Company, or any direct or indirect parent of the Company, including, without limitation, in connection with any merger or consolidation;
(III) make any principal payment on, or redeem, repurchase, defease or otherwise acquire or retire for value in each case, prior to any scheduled repayment, sinking fund payment or maturity, any Subordinated Indebtedness, other than (a) Indebtedness permitted under clauses (7) and (8) of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” (other than, in the event any Default has occurred and is continuing, Indebtedness owing to any Restricted Subsidiary that is not a Guarantor) or (b) the purchase, repurchase or other acquisition of Subordinated Indebtedness purchased in anticipation of satisfying a sinking fund obligation, principal installment or final maturity, in each case due within one year of the date of purchase, repurchase or acquisition; or
(IV) make any Restricted Investment
(all such payments and other actions set forth in clauses (I) through (IV) above (other than any exceptions thereof) being collectively referred to as “Restricted Payments”), unless, at the time of such Restricted Payment:
(1) no Default or Event of Default shall have occurred and be continuing or would occur as a consequence thereof;
(2) immediately after giving effect to such transaction on a pro forma basis, the Company could incur $1.00 of additional Indebtedness under the provisions of the first paragraph of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; and
170
Table of Contents
(3) such Restricted Payment, together with the aggregate amount of all other Restricted Payments made by the Company and its Restricted Subsidiaries after the Issue Date (including Restricted Payments permitted by clauses (1), (2) (with respect to the payment of dividends on Refunding Capital Stock (as defined below) pursuant to clause (b) thereof only), (6)(c), (9) and (14) (to the extent not deducted in calculating Consolidated Net Income) of the next succeeding paragraph, but excluding all other Restricted Payments permitted by the next succeeding paragraph), is less than the sum of (without duplication):
(a) 50% of the Consolidated Net Income of the Company for the period (taken as one accounting period) beginning September 30, 2007 to the end of the Company’s most recently ended fiscal quarter for which internal financial statements are available at the time of such Restricted Payment, or, in the case such Consolidated Net Income for such period is a deficit, minus 100% of such deficit; plus
(b) 100% of the aggregate net cash proceeds and the fair market value, as determined in good faith by the Company, of marketable securities or other property received by the Company since immediately after November 20, 2007 (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness, Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) from the issue or sale of:
(i) (A) Equity Interests of the Company, including Treasury Capital Stock (as defined below), but excluding cash proceeds and the fair market value, as determined in good faith by the Company, of marketable securities or other property received from the sale of:
(x) Equity Interests to employees, directors or consultants of the Company, any direct or indirect parent company of the Company and the Company’s Subsidiaries after November 20, 2007, to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph; and
(y) Designated Preferred Stock; and
(B) to the extent such net cash proceeds are actually contributed to the Company as equity (other than Disqualified Stock), Equity Interests of any of the Company’s direct or indirect parent companies (excluding contributions of the proceeds from the sale of Designated Preferred Stock of any such companies or contributions to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph); or
(ii) debt securities of the Company that have been converted into or exchanged for such Equity Interests of the Company;
provided, however, that this clause (b) shall not include the proceeds from (W) Refunding Capital Stock, (X) Equity Interests or debt securities of the Company sold to a Restricted Subsidiary, as the case may be, (Y) Disqualified Stock or debt securities that have been converted into Disqualified Stock or (Z) Excluded Contributions; plus
(c) 100% of the aggregate amount of cash and the fair market value, as determined in good faith by the Company, of marketable securities or other property contributed to the capital of the Company (other than as Disqualified Stock) after November 20, 2007 (other than (i) net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness, Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”, (ii) contributions from a Restricted Subsidiary or (iii) any Excluded Contribution); plus
171
Table of Contents
(d) 100% of the aggregate amount received in cash and the fair market value, as determined in good faith by the Company, of marketable securities or other property received by the Issuer or any Restricted Subsidiary by means of:
(i) the sale or other disposition (other than to the Company or a Restricted Subsidiary) of Restricted Investments made by the Company or its Restricted Subsidiaries and repurchases and redemptions of such Restricted Investments from the Company or its Restricted Subsidiaries and repayments of loans or advances, and releases of guarantees, which constitute Restricted Investments by the Company or its Restricted Subsidiaries, in each case after November 20, 2007; or
(ii) the sale (other than to the Company or a Restricted Subsidiary) of the stock of an Unrestricted Subsidiary or a distribution or dividend from an Unrestricted Subsidiary (other than in each case to the extent the Investment in such Unrestricted Subsidiary was made by the Company or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment) after November 20, 2007; plus
(e) in the case of the redesignation of an Unrestricted Subsidiary as a Restricted Subsidiary after November 20, 2007, the fair market value of the Investment in such Unrestricted Subsidiary, as determined by the Company in good faith or if, in the case of an Unrestricted Subsidiary, such fair market value may exceed $20.0 million, in writing by an Independent Financial Advisor, at the time of the redesignation of such Unrestricted Subsidiary as a Restricted Subsidiary other than an Unrestricted Subsidiary to the extent the Investment in such Unrestricted Subsidiary was made by the Company or a Restricted Subsidiary pursuant to clause (7) of the paragraph following the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment.
As of September 29, 2012, the amount available for Restricted Payments pursuant to this clause (3) was approximately $175.2 million, although we are unable to utilize this capacity until we would be able to incur $1.00 of additional Indebtedness pursuant to the provisions of the first paragraph of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” on a pro forma basis after giving effect to any such Restricted Payment.
The foregoing provisions will not prohibit:
(1) the payment of any dividend or distribution within 60 days after the date of declaration thereof, if at the date of declaration such payment would have complied with the provisions of the Senior Secured Notes Indenture;
(2) (a) the redemption, repurchase, retirement or other acquisition of any Equity Interests (“Treasury Capital Stock”) or Subordinated Indebtedness of the Company or any Equity Interests of any direct or indirect parent company of the Company, in exchange for, or out of the proceeds of the substantially concurrent sale (other than to a Restricted Subsidiary) of, Equity Interests of the Company or any direct or indirect parent company of the Company to the extent contributed to the Company (in each case, other than any Disqualified Stock or Designated Preferred Stock) (“Refunding Capital Stock”) and (b) if immediately prior to the retirement of Treasury Capital Stock, the declaration and payment of dividends thereon was permitted under clause (6) of this paragraph, the declaration and payment of dividends on the Refunding Capital Stock (other than Refunding Capital Stock the proceeds of which were used to redeem, repurchase, retire or otherwise acquire any Equity Interests of any direct or indirect parent company of the Company) in an aggregate amount no greater than the aggregate amount per year of dividends per annum that were declarable and payable on such Treasury Capital Stock immediately prior to such retirement;
172
Table of Contents
(3) the redemption, repurchase, defeasance or other acquisition or retirement of Subordinated Indebtedness of the Issuers or a Guarantor made in exchange for, or out of the proceeds of the substantially concurrent sale of, new Indebtedness of the Issuers or a Guarantor, as the case may be, which is incurred in compliance with “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” so long as:
(a) the principal amount (or accreted value, if applicable) of such new Indebtedness does not exceed the principal amount of (or accreted value, if applicable), plus any accrued and unpaid interest on, the Subordinated Indebtedness being so redeemed, repurchased, acquired or retired for value, plus the amount of any reasonable premium to be paid (including reasonable premiums) and any reasonable fees and expenses incurred in connection with the issuance of such new Indebtedness;
(b) such new Indebtedness is subordinated to the Senior Secured Notes or the applicable Guarantee at least to the same extent as such Subordinated Indebtedness so purchased, exchanged, redeemed, repurchased, defeased, acquired or retired for value;
(c) such new Indebtedness has a final scheduled maturity date equal to or later than the final scheduled maturity date of the Subordinated Indebtedness being so redeemed, repurchased, defeased, acquired or retired; and
(d) such new Indebtedness has a Weighted Average Life to Maturity equal to or greater than the remaining Weighted Average Life to Maturity of the Subordinated Indebtedness being so redeemed, repurchased, defeased, acquired or retired;
(4) a Restricted Payment to pay for the repurchase, redemption or other acquisition or retirement for value of Equity Interests (other than Disqualified Stock) of the Company or any of its direct or indirect parent companies held by any future, present or former employee, director or consultant of the Company, any of its Restricted Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement, including any Equity Interests rolled over by management of the Company in connection with the DJO Acquisition; provided, however, that the aggregate Restricted Payments made under this clause (4) do not exceed in any calendar year $10.0 million (which shall increase to $20.0 million subsequent to the consummation of an underwritten public Equity Offering by the Company or any direct or indirect parent corporation of the Company) (with unused amounts in any calendar year being carried over to succeeding calendar years subject to a maximum (without giving effect to the following proviso) of $20.0 million in any calendar year (which shall increase to $40.0 million subsequent to the consummation of an underwritten public Equity Offering by the Company or any direct or indirect parent of the Company)); provided further that such amount in any calendar year may be increased by an amount not to exceed:
(a) the cash proceeds from the sale of Equity Interests (other than Disqualified Stock) of the Company and, to the extent contributed to the Company, Equity Interests of any of the Company’s direct or indirect parent companies, in each case to members of management, directors or consultants of the Company, any of its Subsidiaries or any of its direct or indirect parent companies that occurs after the Issue Date, to the extent the cash proceeds from the sale of such Equity Interests have not otherwise been and are not thereafter applied to the payment of Restricted Payments by virtue of clause (3) of the preceding paragraph or otherwise; plus
(b) the cash proceeds of key man life insurance policies received by the Company or its Restricted Subsidiaries after the Issue Date; less
(c) the amount of any Restricted Payments previously made with the cash proceeds described in clauses (a) and (b) of this clause (4); and provided further that cancellation of Indebtedness owing to the Company or any of its Restricted Subsidiaries from members of management of the Company, any of the Company’s direct or indirect parent companies or any of the Company’s Subsidiaries in connection with a repurchase of Equity Interests of the Company or any of its direct or indirect parent companies are not deemed to constitute a Restricted Payment for purposes of this covenant or any other provision of the Senior Secured Notes Indenture;
173
Table of Contents
(5) the declaration and payment of dividends to holders of any class or series of Disqualified Stock of the Company or any of its Restricted Subsidiaries and of Preferred Stock of any Restricted Subsidiary issued in accordance with the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” to the extent such dividends are included in the definition of “Fixed Charges”;
(6) (a) the declaration and payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by the Company after the Issue Date;
(b) the declaration and payment of dividends to a direct or indirect parent company of the Company, the proceeds of which are used to fund the payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) of such parent corporation issued after the Issue Date, provided that the amount of dividends paid pursuant to this clause (b) shall not exceed the aggregate amount of cash actually contributed to the Company from the sale of such Designated Preferred Stock; or
(c) the declaration and payment of dividends on Refunding Capital Stock that is Preferred Stock in excess of the dividends declarable and payable thereon pursuant to clause (2) of this paragraph;
provided, however, in the case of each of (a) and (c) of this clause (6), that for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date of issuance of such Designated Preferred Stock or the declaration of such dividends on Refunding Capital Stock that is Preferred Stock, after giving effect to such issuance or declaration on a pro forma basis, the Company and its Restricted Subsidiaries on a consolidated basis had a Fixed Charge Coverage Ratio of at least 2.00 to 1.00;
(7) Investments in Unrestricted Subsidiaries having an aggregate fair market value, taken together with all other Investments made pursuant to this clause (7) that are at the time outstanding, without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities, not to exceed 2.0% of Total Assets at the time of such Investment (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
(8) repurchases of Equity Interests deemed to occur upon exercise of stock options or warrants if such Equity Interests represent a portion of the exercise price of such options, or warrants;
(9) the declaration and payment of dividends on the Company’s common stock (or payments of dividends to any-direct or indirect parent entity to fund payments of dividends on such entity’s common stock), following the consummation of an underwritten public offering of the Company’s common stock or the common stock of any of its direct or indirect parent companies after the Issue Date, of up to 6% per annum of the net cash proceeds received by or contributed to the Company in or from any such public offering, other than public offerings with respect to common stock registered on Form S-8 and other than any public sale constituting an Excluded Contribution;
(10) Restricted Payments in any amount that do not in the aggregate exceed all Excluded Contributions made since the Issue Date;
(11) other Restricted Payments in an aggregate amount taken together with all other Restricted Payments made pursuant to this clause (11) not to exceed 1.75% of Total Assets at the time made;
(12) distributions or payments of Receivables Fees;
(13) any Restricted Payment made as part of the Transactions, and the fees and expenses related thereto, or used to fund amounts owed to Affiliates (including dividends to any direct or indirect parent of the Company to permit payment by such parent of such amounts), in each case to the extent permitted by (or, in the case of a dividend to fund such payment, to the extent such payment, if made by the Company, would be permitted by) the covenant described under “—Transactions with Affiliates”;
174
Table of Contents
(14) the repurchase, redemption or other acquisition or retirement for value of any Subordinated Indebtedness in accordance with the provisions similar to those described under the captions “Repurchase at the Option of Holders—Change of Control” and “Repurchase at the Option of Holders—Asset Sales”; provided that all Secured Notes tendered by Holders in connection with a Change of Control Offer, Asset Sale Offer or Loss Proceeds Offer, as applicable, have been repurchased, redeemed or acquired for value;
(15) the declaration and payment of dividends by the Company to, or the making of loans to, any direct or indirect parent in amounts required for any direct or indirect parent companies to pay, in each case without duplication:
(a) franchise and excise taxes and other fees, taxes and expenses in each case to the extent required to maintain their corporate existence;
(b) federal, state, foreign and local income taxes, to the extent such income taxes are attributable to the income of the Company and its Restricted Subsidiaries and, to the extent of the amount actually received from its Unrestricted Subsidiaries, in amounts required to pay such taxes to the extent attributable to the income of such Unrestricted Subsidiaries; provided that in each case the amount of such payments in any fiscal year does not exceed the amount that the Company and its Restricted Subsidiaries would be required to pay in respect of federal, state and local taxes for such fiscal year were the Company, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes separately from any such parent entity;
(c) customary salary, bonus and other benefits payable to officers and employees of any direct or indirect parent company of the Company to the extent such salaries, bonuses and other benefits are attributable to the ownership or operation of the Company and its Restricted Subsidiaries;
(d) general corporate operating and overhead costs and expenses of any direct or indirect parent company of the Company to the extent such costs and expenses are attributable to the ownership or operation of the Company and its Restricted Subsidiaries; and
(e) fees and expenses other than to Affiliates of the Company related to any unsuccessful equity or debt offering of such parent entity; and
(16) the distribution, by dividend or otherwise, of shares of Capital Stock of, or Indebtedness owed to the Company or a Restricted Subsidiary by, Unrestricted Subsidiaries (other than Unrestricted Subsidiaries, the primary assets of which are cash and/or Cash Equivalents or were contributed to such Unrestricted Subsidiary in anticipation of such distribution, dividend or other payment);
provided, however, that at the time of, and after giving effect to, any Restricted Payment permitted under clauses (7), (11) and (16), no Default shall have occurred and be continuing or would occur as a consequence thereof.
As of the issue date of the Senior Secured Notes, all of the Company’s Subsidiaries are Restricted Subsidiaries. The Company will not permit any Unrestricted Subsidiary to become a Restricted Subsidiary except pursuant to the last sentence of the definition of “Unrestricted Subsidiary.” For purposes of designating any Restricted Subsidiary as an Unrestricted Subsidiary, all outstanding Investments by the Company and its Restricted Subsidiaries (except to the extent repaid) in the Subsidiary so designated are deemed to be Restricted Payments in an amount determined as set forth in the last sentence of the definition of “Investment.” Such designation are permitted only if a Restricted Payment in such amount would be permitted at such time, whether pursuant to the first paragraph of this covenant or under clause (7), (10) or (11) of the second paragraph of this covenant, or pursuant to the definition of “Permitted Investments,” and if such Subsidiary otherwise meets the definition of an Unrestricted Subsidiary. Unrestricted Subsidiaries are not subject to any of the restrictive covenants set forth in the Senior Secured Notes Indenture.
175
Table of Contents
Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock
The Company will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable, contingently or otherwise (collectively, “incur” and collectively, an “incurrence”) with respect to any Indebtedness (including Acquired Indebtedness) and the Company will not issue any shares of Disqualified Stock and will not permit any Restricted Subsidiary to issue any shares of Disqualified Stock or Preferred Stock; provided, however, that the Company may incur Indebtedness (including Acquired Indebtedness) or issue shares of Disqualified Stock, and any of its Restricted Subsidiaries may incur Indebtedness (including Acquired Indebtedness), issue shares of Disqualified Stock and issue shares of Preferred Stock, if the Fixed Charge Coverage Ratio on a consolidated basis for the Company and its Restricted Subsidiaries’ most recently ended four fiscal quarters for which internal financial statements are available immediately preceding the date on which such additional Indebtedness is incurred or such Disqualified Stock or Preferred Stock is issued would have been at least 2.00 to 1.00, determined on a pro forma basis (including a pro forma application of the net proceeds therefrom), as if the additional Indebtedness had been incurred, or the Disqualified Stock or Preferred Stock had been issued, as the case may be, and the application of proceeds therefrom had occurred at the beginning of such four-quarter period.
The foregoing limitations will not apply to:
(1) the incurrence of Indebtedness under Credit Facilities by the Company or any of its Restricted Subsidiaries and the issuance and creation of letters of credit and bankers’ acceptances thereunder (with letters of credit and bankers’ acceptances being deemed to have a principal amount equal to the face amount thereof), up to an aggregate principal amount of $1,325.0 million outstanding at any one time;
(2) the incurrence by the Company and any Guarantor of Indebtedness represented by the Senior Secured Notes issued on March 20, 2012 (including any Guarantee) and any notes (including guarantees thereof) issued in exchange for such Senior Secured Notes pursuant to the Senior Secured Notes Registration Rights Agreement or similar agreement;
(3) Indebtedness of the Company and its Restricted Subsidiaries in existence on the Issue Date (other than Indebtedness described in clauses (1) and (2));
(4) Indebtedness (including Capitalized Lease Obligations), Disqualified Stock and Preferred Stock incurred by the Company or any of its Restricted Subsidiaries, to finance the purchase, lease or improvement of property (real or personal) or equipment (other than software) that is used or useful in a Similar Business, whether through the direct purchase of assets or the Capital Stock of any Person owning such assets, in an aggregate principal amount at the date of such incurrence (including all Refinancing Indebtedness incurred to refinance any other Indebtedness incurred pursuant to this clause (4)) not to exceed 4.0% of Total Assets; provided, however, that such Indebtedness exists at the date of such purchase or transaction or is created within 270 days thereafter (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (4) shall cease to be deemed incurred or outstanding for purposes of this clause (4) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Company or such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (4));
(5) Indebtedness incurred by the Company or any of its Restricted Subsidiaries constituting reimbursement obligations with respect to letters of credit issued in the ordinary course of business, including letters of credit in respect of workers’ compensation claims, or other Indebtedness with respect to reimbursement type obligations regarding workers’ compensation claims; provided, however, that upon the drawing of such letters of credit or the incurrence of such Indebtedness, such obligations are reimbursed within 30 days following such drawing or incurrence;
(6) Indebtedness arising from agreements of the Company or its Restricted Subsidiaries providing for indemnification, adjustment of purchase price or similar obligations, in each case, incurred or assumed in connection with the disposition of any business, assets or a Subsidiary, other than guarantees of Indebtedness
176
Table of Contents
incurred by any Person acquiring all or any portion of such business, assets or a Subsidiary for the purpose of financing such acquisition; provided, however, that such Indebtedness is not reflected on the balance sheet of the Company, or any of its Restricted Subsidiaries (contingent obligations referred to in a footnote to financial statements and not otherwise reflected on the balance sheet are not deemed to be reflected on such balance sheet for purposes of this clause (6));
(7) Indebtedness of the Company to a Restricted Subsidiary; provided that any such Indebtedness owing to a Restricted Subsidiary that is not the Co-Issuer or a Guarantor is expressly subordinated in right of payment to the Senior Secured Notes; provided further that any subsequent issuance or transfer of any Capital Stock or any other event which results in the Restricted Subsidiary holding such Indebtedness ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such Indebtedness (except to the Company or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(8) Indebtedness of a Restricted Subsidiary to the Company or another Restricted Subsidiary; provided that if a Guarantor incurs such Indebtedness to a Restricted Subsidiary that is not the Co-Issuer or a Guarantor, such Indebtedness is expressly subordinated in right of payment to the Guarantee of the Senior Secured Notes of such Guarantor; provided further that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Indebtedness being held by a Person other than the Company or a Restricted Subsidiary or any subsequent transfer of any such Indebtedness (except to the Company or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(9) shares of Preferred Stock of a Restricted Subsidiary issued to the Company or another Restricted Subsidiary, provided that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such shares of Preferred Stock (except to the Company or another Restricted Subsidiary) shall be deemed in each case to be an issuance of such shares of Preferred Stock not permitted by this clause;
(10) Hedging Obligations (excluding Hedging Obligations entered into for speculative purposes) for the purpose of limiting interest rate risk with respect to any Indebtedness of the Company or any Restricted Subsidiary permitted to be incurred pursuant to “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” exchange rate risk or commodity pricing risk;
(11) obligations in respect of performance, bid, appeal and surety bonds and completion guarantees provided by the Company or any of its Restricted Subsidiaries in the ordinary course of business;
(12) (a) Indebtedness or Disqualified Stock of the Company and Indebtedness, Disqualified Stock or Preferred Stock of the Company or any Restricted Subsidiary equal to 200.0% of the net cash proceeds received by the Company since immediately after the Issue Date from the issue or sale of Equity Interests of the Company or cash contributed to the capital of the Company (in each case, other than proceeds of Disqualified Stock or sales of Equity Interests to the Company or any of its Subsidiaries) as determined in accordance with clauses (3)(b) and (3)(c) of the first paragraph of “—Limitation on Restricted Payments” to the extent such net cash proceeds or cash have not been applied to make Restricted Payments or to make other Investments, payments or exchanges pursuant to such clauses or pursuant to the second paragraph of “—Limitation on Restricted Payments” or to make Permitted Investments (other than Permitted Investments specified in clauses (1), (2) and (3) of the definition thereof) and (b) Indebtedness or Disqualified Stock of the Company and Indebtedness, Disqualified Stock or Preferred Stock of the Company or any Restricted Subsidiary not otherwise permitted hereunder in an aggregate principal amount or liquidation preference, which when aggregated with the principal amount and liquidation preference of all other Indebtedness, Disqualified Stock and Preferred Stock then outstanding and incurred pursuant to this clause (12)(b), does not at any one time outstanding exceed $175.0 million (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (12)(b) shall cease to be deemed incurred or outstanding for purposes of this clause (12)(b) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which
177
Table of Contents
the Company or such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (12)(b));
(13) the incurrence or issuance by the Company or any Restricted Subsidiary of Indebtedness, Disqualified Stock or Preferred Stock which serves to refund, refinance, replace, renew, extend or defease any Indebtedness, Disqualified Stock or Preferred Stock of the Company or any Restricted Subsidiary incurred as permitted under the first paragraph of this covenant and clauses (2), (3), (4) and (12)(a) above, this clause (13) and clause (14) below or any Indebtedness, Disqualified Stock or Preferred Stock issued to so refund, refinance, replace, renew, extend or defease such Indebtedness, Disqualified Stock or Preferred Stock including additional Indebtedness, Disqualified Stock or Preferred Stock incurred to pay premiums (including reasonable tender premiums), defeasance costs and fees in connection therewith (the “Refinancing Indebtedness”) prior to its respective maturity; provided, however, that such Refinancing Indebtedness:
(a) has a Weighted Average Life to Maturity at the time such Refinancing Indebtedness is incurred which is not less than the remaining Weighted Average Life to Maturity of the Indebtedness, Disqualified Stock or Preferred Stock being refunded, refinanced, replaced, renewed, extended or defeased,
(b) to the extent such Refinancing Indebtedness refinances (i) Indebtedness subordinated or pari passu to the Senior Secured Notes or any Guarantee thereof, such Refinancing Indebtedness is subordinated or pari passu to the Senior Secured Notes or the Guarantee at least to the same extent as the Indebtedness being refinanced or refunded or (ii) Disqualified Stock or Preferred Stock, such Refinancing Indebtedness must be Disqualified Stock or Preferred Stock, respectively, and
(c) shall not include Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Company that is not a Co-Issuer or a Guarantor that refinances Indebtedness, Disqualified Stock or Preferred Stock of the Company, the Co-Issuer or a Guarantor;
provided further that subclause (a) of this clause (13) will not apply to any refunding or refinancing of any Secured Indebtedness;
(14) Indebtedness, Disqualified Stock or Preferred Stock of (x) the Company or a Restricted Subsidiary incurred to finance an acquisition or (y) Persons that are acquired by the Company or any Restricted Subsidiary or merged into the Company or a Restricted Subsidiary in accordance with the terms of the Senior Secured Notes Indenture; provided that after giving effect to such acquisition or merger, either (a) the Company would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first sentence of this covenant or (b) the Fixed Charge Coverage Ratio of the Company and the Restricted Subsidiaries is greater than immediately prior to such acquisition or merger;
(15) Indebtedness arising from the honoring by a bank or other financial institution of a check, draft or similar instrument drawn against insufficient funds in the ordinary course of business, provided that such Indebtedness is extinguished within two Business Days of its incurrence;
(16) Indebtedness of the Company or any of its Restricted Subsidiaries supported by a letter of credit issued pursuant to the Credit Facilities, in a principal amount not in excess of the stated amount of such letter of credit;
(17) (a) any guarantee by the Company or a Restricted Subsidiary of Indebtedness or other obligations of any Restricted Subsidiary so long as the incurrence of such Indebtedness incurred by such Restricted Subsidiary is permitted under the terms of the Senior Secured Notes Indenture,
(b) any guarantee by a Restricted Subsidiary of Indebtedness of the Company provided that such guarantee is incurred in accordance with the covenant described below under “—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries,” or
(c) any incurrence by the Co-Issuer of Indebtedness as a co-issuer of Indebtedness of the Company that was permitted to be incurred by another provision of this covenant;
178
Table of Contents
(18) Indebtedness of Foreign Subsidiaries of the Company in an amount not to exceed, at any one time outstanding and together with any other Indebtedness incurred under this clause (18), 10.0% of the Total Assets of the Foreign Subsidiaries (it being understood that any Indebtedness incurred pursuant to this clause (18) shall cease to be deemed incurred or outstanding for purposes of this clause (18) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Company or its Restricted Subsidiaries could have incurred such Indebtedness under the first paragraph of this covenant without reliance on this clause (18));
(19) Indebtedness of the Company or any of its Restricted Subsidiaries consisting of (i) the financing of insurance premiums or (ii) take-or-pay obligations contained in supply arrangements in each case, incurred in the ordinary course of business; and
(20) Indebtedness consisting of Indebtedness issued by the Company or any of its Restricted Subsidiaries to current or former officers, directors and employees thereof, their respective estates, spouses or former spouses, in each case to finance the purchase or redemption of Equity Interests of the Company or any direct or indirect parent company of the Company to the extent described in clause (4) of the second paragraph under the caption “—Limitation on Restricted Payments.”
For purposes of determining compliance with this covenant:
(1) in the event that an item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) meets the criteria of more than one of the categories of permitted Indebtedness, Disqualified Stock or Preferred Stock described in clauses (1) through (20) above or is entitled to be incurred pursuant to the first paragraph of this covenant, the Company, in its sole discretion, will classify or reclassify such item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) and will only be required to include the amount and type of such Indebtedness, Disqualified Stock or Preferred Stock in one of the above clauses; and
(2) at the time of incurrence, the Company are entitled to divide and classify an item of Indebtedness in more than one of the types of Indebtedness described in the first and second paragraphs above; provided that all Indebtedness outstanding under the Senior Credit Facilities on the Issue Date are treated as incurred on the Issue Date under clause (1) of the preceding paragraph.
Accrual of interest or dividends, the accretion of accreted value, the accretion or amortization of original issue discount, the payment of interest in the form of additional Indebtedness and the payment of dividends in the form of additional Disqualified Stock or Preferred Stock, as applicable, will in each case not be deemed to be an incurrence of Indebtedness, Disqualified Stock or Preferred Stock for purposes of this covenant.
For purposes of determining compliance with any U.S. dollar-denominated restriction on the incurrence of Indebtedness, the U.S. dollar-equivalent principal amount of Indebtedness denominated in a foreign currency shall be calculated based on the relevant currency exchange rate in effect on the date such Indebtedness was incurred, in the case of term debt, or first committed, in the case of revolving credit debt; provided that if such Indebtedness is incurred to refinance other Indebtedness denominated in a foreign currency, and such refinancing would cause the applicable U.S. dollar denominated restriction to be exceeded if calculated at the relevant currency exchange rate in effect on the date of such refinancing, such U.S. dollar-denominated restriction shall be deemed not to have been exceeded so long as the principal amount of such refinancing Indebtedness does not exceed the principal amount of such Indebtedness being refinanced.
The principal amount of any Indebtedness incurred to refinance other Indebtedness, if incurred in a different currency from the Indebtedness being refinanced, shall be calculated based on the currency exchange rate applicable to the currencies in which such respective Indebtedness is denominated that is in effect on the date of such refinancing.
179
Table of Contents
The Secured Notes Indenture does not treat (1) unsecured Indebtedness as subordinated or junior to Secured Indebtedness merely because it is unsecured or (2) Senior Indebtedness as subordinated or junior to any other Senior Indebtedness merely because it has a junior priority with respect to the same collateral.
Liens
The Company will not, and will not permit the Co-Issuer or any Guarantor to, directly or indirectly, create, incur, assume or otherwise cause or suffer to exist any Lien (except Permitted Liens) that secures Obligations under any Indebtedness or any related guarantee, on any asset or property of the Company, the Co-Issuer or any Guarantor, or any income or profits therefrom, or assign or convey any right to receive income therefrom, unless:
(1) in the case of Liens securing Subordinated Indebtedness, the Senior Secured Notes and related Guarantees are secured by a Lien on such property, assets or proceeds that is senior in priority to such Liens; or
(2) in all other cases, the Senior Secured Notes or the Guarantees are equally and ratably secured, except that the foregoing shall not apply to (a) Liens securing the Senior Secured Notes and the related Guarantees, (b) Liens securing Indebtedness permitted to be incurred under Credit Facilities, including any letter of credit facility relating thereto, that was permitted by the terms of the Senior Secured Notes Indenture to be incurred pursuant to clause (1) of the second paragraph under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” (including, during any Suspension Period, Indebtedness of the type and in the amounts specified under such clause) and (c) Liens securing Indebtedness under Credit Facilities permitted to be incurred under the covenant described above under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that, with respect to Liens securing Indebtedness permitted under this subclause (c), at the time of incurrence and after giving pro forma effect thereto, the Consolidated Secured Debt Ratio would be no greater than 4.00 to 1.00.
Merger, Consolidation or Sale of All or Substantially All Assets
Company. The Company may not, directly or indirectly, consolidate or merge with or into or wind up into (whether or not the Company is the surviving corporation), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of the Company’s properties or assets, in one or more related transactions, to any Person unless:
(1) the Company is the surviving corporation or the Person formed by or surviving any such consolidation or merger (if other than the Company) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made is a corporation, partnership (including a limited partnership), trust or limited liability company organized or existing under the laws of the jurisdiction of organization of the Company or the laws of the United States, any state thereof, the District of Columbia or any territory thereof (such Person, as the case may be, being herein called the “Successor Company”);
(2) the Successor Company, if other than the Company, expressly assumes all the obligations of the Company under the Senior Secured Notes and the Security Documents, pursuant to supplemental indentures or other documents or instruments in form reasonably satisfactory to the Senior Secured Notes Trustee, and the Senior Secured Notes Registration Rights Agreement, in each case if the exchange offers contemplated thereby have not been consummated or if the Issuers continue to have an obligation to file or maintain the effectiveness of a shelf registration statement as provided under such agreements;
(3) immediately after such transaction, no Default or Event of Default exists;
(4) immediately after giving pro forma effect to such transaction and any related financing transactions, as if such transactions had occurred at the beginning of the applicable four-quarter period,
(a) the Company or the Successor Company, as applicable, would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first sentence of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” or
180
Table of Contents
(b) the Fixed Charge Coverage Ratio for the Company (or, if applicable, the Successor Company) and its Restricted Subsidiaries would be greater than such Ratio for the Company and its Restricted Subsidiaries immediately prior to such transaction;
(5) each Guarantor, unless it is the other party to the transactions described above, in which case subclause (b) of the second succeeding paragraph shall apply, shall have by supplemental indenture confirmed that its Guarantee shall apply to such Person’s obligations under the Senior Secured Notes Indenture, the Senior Secured Notes and the Senior Secured Notes Registration Rights Agreement, in each case if the exchange offers contemplated thereby have not been consummated or if the Issuers continue to have an obligation to file or maintain the effectiveness of a shelf registration statement as provided under such agreements;
(6) the Co-Issuer, unless it is the party to the transactions described above, in which case clause (3) of the third succeeding paragraph shall apply, shall have by supplemental indenture confirmed that it continues to be a co-obligor of the Senior Secured Notes;
(7) the Company (or, if applicable, the Successor Company) shall have delivered to the Senior Secured Notes Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Senior Secured Notes Indenture;
(8) the Successor Company causes such amendments, supplements or other instruments to be executed, delivered, filed and recorded, as applicable, in such jurisdictions as may be required by applicable law under the Security Documents to preserve and protect the Lien of the Security Documents on the Collateral owned by or transferred to the Successor Company;
(9) the Collateral owned by or transferred to the Successor Company shall (a) continue to constitute Collateral under the Senior Secured Notes Indenture and the Security Documents, (b) be subject to the Lien in favor of the Second Lien Agent for the benefit of the Senior Secured Notes Trustee and the Holders of the Senior Secured Notes, and (c) not be subject to any Lien other than Liens permitted under the Senior Secured Notes Indenture; and
(10) the property and assets of the Person which is merged or consolidated with or into the Company, to the extent that they are property or assets of the types which would constitute Collateral under the Security Documents, shall be treated as After-Acquired Property and the Company shall take such action as may be reasonably necessary to cause such property and assets to be made subject to the Lien of the Security Documents in the manner and to the extent required under the Security Documents.
The Successor Company will succeed to, and be substituted for the Company, as the case may be, under the Senior Secured Notes Indenture, the Guarantees and the Senior Secured Notes, as applicable. Notwithstanding the foregoing clauses (3) and (4),
(1) any Restricted Subsidiary may consolidate with or merge into or transfer all or part of its properties and assets to the Company, and
(2) the Company may merge with an Affiliate of the Company, as the case may be, solely for the purpose of reincorporating the Company in the United States, any state thereof, the District of Columbia or any territory thereof so long as the amount of Indebtedness of the Company and its Restricted Subsidiaries is not increased thereby.
Guarantors. Subject to certain limitations described in the Senior Secured Notes Indenture governing release of a Guarantee, upon the sale, disposition or transfer of a Guarantor, no Guarantor will, and the Company will not permit any Guarantor to, consolidate or merge with or into or wind up into (whether or not the Company
181
Table of Contents
or Guarantor is the surviving corporation), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) (a) such Guarantor is the surviving corporation or the Person formed by or surviving any such consolidation or merger (if other than such Guarantor) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made is a corporation, partnership, trust or limited liability company organized or existing under the laws of the jurisdiction of organization of such Guarantor, as the case may be, or the laws of the United States, any state thereof, the District of Columbia or any territory thereof (such Guarantor or such Person, as the case may be, being herein called the “Successor Person”);
(b) the Successor Person, if other than such Guarantor, expressly assumes all the obligations of such Guarantor under the Senior Secured Notes Indenture and the Security Documents and such Guarantor’s related Guarantee pursuant to supplemental indentures or other documents or instruments in form reasonably satisfactory to the Senior Secured Notes Trustee;
(c) immediately after such transaction, no Default or Event of Default exists;
(d) the Company shall have delivered to the Senior Secured Notes Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Senior Secured Notes Indenture;
(e) the Successor Person causes such amendments, supplements or other instruments to be executed, delivered, filed and recorded, as applicable, in such jurisdictions as may be required by applicable law under the Security Documents to preserve and protect the Lien of the Security Documents on the Collateral owned by or transferred to the Successor Person;
(f) the Collateral owned by or transferred to the Successor Person shall (a) continue to constitute Collateral under the Senior Secured Notes Indenture and the Security Documents, (b) be subject to the Lien in favor of the Second Lien Agent for the benefit of the Senior Secured Notes Trustee and the Holders of the Senior Secured Notes, and (c) not be subject to any Lien other than Liens permitted under the Senior Secured Notes Indenture; and
(g) the property and assets of the Person which is merged or consolidated with or into the Guarantor, to the extent that they are property or assets of the types which would constitute Collateral under the Security Documents, shall be treated as After-Acquired Property and the Company shall take such action as may be reasonably necessary to cause such property and assets to be made subject to the Lien of the Security Documents in the manner and to the extent required under the Security Documents.
(2) the transaction is made in compliance with the covenant described under “Repurchase at the Option of Holders—Asset Sales.”
Subject to certain limitations described in the Senior Secured Notes Indenture, the Successor Person will succeed to, and be substituted for, such Guarantor under the Senior Secured Notes Indenture and such Guarantor’s Guarantee. Notwithstanding the foregoing, any Guarantor may (i) merge into or transfer all or part of its properties and assets to another Guarantor or the Company, (ii) merge with an Affiliate of the Company solely for the purpose of reincorporating the Guarantor in the United States, any state thereof, the District of Columbia or any territory thereof or (iii) convert into a corporation, partnership, limited partnership, limited liability company or trust organized under the laws of the jurisdiction of organization of such Guarantor, in each case without regard to the requirements set forth in the preceding paragraph.
Co-Issuer. The Co-Issuer may not, directly or indirectly, consolidate or merge with or into or wind up into (whether or not the Co-Issuer is the surviving corporation), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of the Co-Issuer’s properties or assets, in one or more related transactions, to any Person unless:
(1) (a) concurrently therewith, a corporate Wholly-Owned Subsidiary that is a Restricted Subsidiary of the Company organized and validly existing under the laws of the United States, any state thereof, the District of
182
Table of Contents
Columbia or any territory thereof (which may be the continuing Person as a result of such transaction) expressly assumes all the obligations of the Co-Issuer under the Senior Secured Notes and the Security Documents, pursuant to supplemental indentures or other documents or instruments in form reasonably satisfactory to the Senior Secured Notes Trustee, and the Senior Secured Notes Registration Rights Agreement, in each case if the exchange offers contemplated thereby have not been consummated or if the Issuers continue to have an obligation to file or maintain the effectiveness of a shelf registration statement as provided under such agreements; or
(b) after giving effect thereto, at least one obligor on the Senior Secured Notes shall be a corporation organized and validly existing under the laws of the United States, any state thereof, the District of Columbia or any territory thereof;
(2) immediately after such transaction, no Default or Event of Default will have occurred and be continuing; and
(3) the Co-Issuer shall have delivered to the Senior Secured Notes Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indenture, if any, comply with the Senior Secured Notes Indenture.
Transactions with Affiliates
The Company will not, and will not permit any of its Restricted Subsidiaries to, make any payment to, or sell, lease, transfer or otherwise dispose of any of its properties or assets to, or purchase any property or assets from, or enter into or make or amend, any transaction, contract, agreement, understanding, loan, advance or guarantee with, or for the benefit of any Affiliate of the Company (each of the foregoing, an “Affiliate Transaction”) involving aggregate payments or consideration in excess of $12.5 million, unless:
(1) such Affiliate Transaction is on terms that are not materially less favorable to the Company or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Company or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis; and
(2) the Company delivers to the Senior Secured Notes Trustee, with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate payments or consideration in excess of $25.0 million, a resolution adopted by the majority of the board of directors of the Company approving such Affiliate Transaction and set forth in an Officer’s Certificate certifying that such Affiliate Transaction complies with clause (1) above.
The foregoing provisions will not apply to the following:
(1) transactions between or among the Company or any of its Restricted Subsidiaries;
(2) Restricted Payments permitted by the provisions of the Senior Secured Notes Indenture described above under the covenant “—Limitation on Restricted Payments” and the definition of “Permitted Investments”;
(3) the payment of management, consulting, monitoring and advisory fees and related expenses to the Investors pursuant to the Sponsor Management Agreement in an aggregate amount in any fiscal year not to exceed the greater of $7.0 million and 2.0% of EBITDA for such fiscal year (calculated, solely for the purpose of this clause (3), assuming (a) that such fees and related expenses had not been paid, when calculating Net Income, and (b) without giving effect to clause (h) of the definition of EBITDA) (plus any unpaid management, consulting, monitoring and advisory fees and related expenses within such amount accrued in any prior year) and the termination fees pursuant to the Sponsor Management Agreement not to exceed the amount set forth in the Sponsor Management Agreement as in effect on the Issue Date or any amendment thereto (so long as any such amendment is not disadvantageous to the Holders when taken as a whole as compared to the Sponsor Management Agreement in effect on the Issue Date);
183
Table of Contents
(4) the payment of reasonable and customary fees paid to, and indemnities provided for the benefit of, former, current or future officers, directors, employees or consultants of Company, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
(5) transactions in which the Company or any of its Restricted Subsidiaries, as the case may be, delivers to the Senior Secured Notes Trustee a letter from an Independent Financial Advisor stating that such transaction is fair to the Company or such Restricted Subsidiary from a financial point of view or stating that such terms are not materially less favorable to the Company or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Company or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis;
(6) any agreement as in effect as of the Issue Date, or any amendment thereto (so long as any such amendment is not disadvantageous to the Holders when taken as a whole as compared to the applicable agreement as in effect on the Issue Date);
(7) the existence of, or the performance by the Company or any of its Restricted Subsidiaries of its obligations under the terms of, any stockholders agreement (including any registration rights agreement or purchase agreement related thereto) to which it is a party as of the Issue Date and any similar agreements which it may enter into thereafter; provided, however, that the existence of, or the performance by the Company or any of its Restricted Subsidiaries of obligations under any future amendment to any such existing agreement or under any similar agreement entered into after the Issue Date shall only be permitted by this clause (7) to the extent that the terms of any such amendment or new agreement are not otherwise disadvantageous to the Holders when taken as a whole;
(8) the Transactions and the payment of all fees and expenses related to the Transactions;
(9) transactions with customers, clients, suppliers, or purchasers or sellers of goods or services, in each case in the ordinary course of business and otherwise in compliance with the terms of the Senior Secured Notes Indenture which are fair to the Company and its Restricted Subsidiaries, in the reasonable determination of the board of directors of the Company or the senior management thereof, or are on terms at least as favorable as might reasonably have been obtained at such time from an unaffiliated party;
(10) the issuance of Equity Interests (other than Disqualified Stock) of the Company to any Permitted Holder or to any director, officer, employee or consultant (or their respective estates, investment funds, investment vehicles, spouses or former spouses) of the Company or any direct or indirect parent companies of any of its Subsidiaries;
(11) sales of accounts receivable, or participations therein, in connection with any Receivables Facility;
(12) payments by the Company or any of its Restricted Subsidiaries to any of the Investors made for any financial advisory, financing, underwriting or placement services or in respect of other investment banking activities, including, without limitation, in connection with acquisitions or divestitures which payments are approved by a majority of the board of directors of the Company in good faith;
(13) payments or loans (or cancellation of loans) to employees or consultants of the Company, any of its direct or indirect parent companies or any of its Restricted Subsidiaries and employment agreements, stock option plans and other similar arrangements with such employees or consultants which, in each case, are approved by the Company in good faith; and
(14) Investments by the Investors in securities of the Company or any of its Restricted Subsidiaries so long as (i) the Investment is being offered generally to other investors on the same or more favorable terms and (ii) the Investment constitutes less than 5.0% of the proposed or outstanding issue amount of such class of securities.
184
Table of Contents
Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries
The Company will not, and will not permit any of its Restricted Subsidiaries that are not Guarantors to, directly or indirectly, create or otherwise cause or suffer to exist or become effective any consensual encumbrance or consensual restriction on the ability of any Restricted Subsidiary that is not a Guarantor to:
(1) (a) pay dividends or make any other distributions to the Company or any of its Restricted Subsidiaries on its Capital Stock or with respect to any other interest or participation in, or measured by, its profits, or
(b) pay any liabilities owed to the Company or any of its Restricted Subsidiaries;
(2) make loans or advances to the Company or any of its Restricted Subsidiaries; or
(3) sell, lease or transfer any of its properties or assets to the Company or any of its Restricted Subsidiaries, except (in each case) for such encumbrances or restrictions existing under or by reason of:
(a) contractual encumbrances or restrictions in effect on the Issue Date, including pursuant to the Senior Credit Facilities and the related documentation and Hedging Obligations and pursuant to the terms of the Existing Senior Subordinated Notes and the Existing Senior Notes;
(b) the Senior Secured Notes Indenture and the Senior Secured Notes;
(c) purchase money obligations for property acquired in the ordinary course of business that impose restrictions of the nature discussed in clause (3) above on the property so acquired;
(d) applicable law or any applicable rule, regulation or order;
(e) any agreement or other instrument of a Person acquired by the Company or any Restricted Subsidiaries in existence at the time of such acquisition (but not created in contemplation thereof), which encumbrance or restriction is not applicable to any Person, or the properties or assets of any Person, other than the Person and its Subsidiaries, or the property or assets of the Person and its Subsidiaries, so acquired;
(f) contracts for the sale of assets, including customary restrictions with respect to a Subsidiary of the Company pursuant to an agreement that has been entered into for the sale or disposition of all or substantially all of the Capital Stock or assets of such Subsidiary;
(g) Secured Indebtedness otherwise permitted to be incurred pursuant to the covenants described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Liens” that limit the right of the debtor to dispose of the assets securing such Indebtedness;
(h) restrictions on cash or other deposits or net worth imposed by customers under contracts entered into in the ordinary course of business;
(i) other Indebtedness, Disqualified Stock or Preferred Stock of Foreign Subsidiaries permitted to be incurred subsequent to the Issue Date pursuant to the provisions of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(j) customary provisions in joint venture agreements and other similar agreements relating solely to such joint venture;
(k) customary provisions contained in leases or licenses of intellectual property and other agreements, in each case, entered into in the ordinary course of business;
(l) any encumbrances or restrictions of the type referred to in clauses (1), (2) and (3) above imposed by any amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings of the contracts, instruments or obligations referred to in clauses (a) through (k) above; provided that such amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings are, in the good faith judgment of the Company, no more restrictive with respect to such encumbrance and other restrictions taken as a whole than those prior to such amendment, modification, restatement, renewal, increase, supplement, refunding, replacement or refinancing; and
185
Table of Contents
(m) restrictions created in connection with any Receivables Facility that, in the good faith determination of the Company are necessary or advisable to effect the transactions contemplated under such Receivables Facility.
Limitation on Guarantees of Indebtedness by Restricted Subsidiaries
The Company will not permit any of its Wholly-Owned Subsidiaries that are Restricted Subsidiaries (and non-Wholly Owned Subsidiaries if such non-Wholly Owned Subsidiaries guarantee other capital markets debt securities), other than a Guarantor, the Co-Issuer or a Foreign Subsidiary guaranteeing Indebtedness of another Foreign Subsidiary, to guarantee the payment of any Indebtedness of the Company, the Co-Issuer or any other Guarantor unless:
(1) such Restricted Subsidiary within 30 days executes and delivers (x) joinders to, or additional Security Documents, as applicable, and (y) a supplemental indenture to the Senior Secured Notes Indenture providing for a Guarantee by such Restricted Subsidiary, except that with respect to a guarantee of Indebtedness of the Company, the Co-Issuer or any Guarantor:
(a) if the Senior Secured Notes or such Guarantor’s Guarantee are subordinated in right of payment to such Indebtedness, the Guarantee under the supplemental indenture shall be subordinated to such Restricted Subsidiary’s guarantee with respect to such Indebtedness substantially to the same extent as the Senior Secured Notes are subordinated to such Indebtedness; and
(b) if such Indebtedness is by its express terms subordinated in right of payment to the Senior Secured Notes or such Guarantor’s Guarantee, any such guarantee by such Restricted Subsidiary with respect to such Indebtedness shall be subordinated in right of payment to such Guarantee substantially to the same extent as such Indebtedness is subordinated to the Senior Secured Notes; and
(c) such Restricted Subsidiary waives and will not in any manner whatsoever claim or take the benefit or advantage of, any rights of reimbursement, indemnity or subrogation or any other rights against the Company or any other Restricted Subsidiary as a result of any payment by such Restricted Subsidiary under its Guarantee;
provided that this covenant shall not be applicable to any guarantee of any Restricted Subsidiary that existed at the time such Person became a Restricted Subsidiary and was not incurred in connection with, or in contemplation of, such Person becoming a Restricted Subsidiary.
Reports and Other Information
Notwithstanding that the Company may not be subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act or otherwise report on an annual and quarterly basis on forms provided for such annual and quarterly reporting pursuant to rules and regulations promulgated by the SEC, the Senior Secured Notes Indenture will require the Company to file with the SEC (and make available to the Senior Secured Notes Trustee and Holders of the Senior Secured Notes (without exhibits), without cost to any Holder, within 15 days after it files them with the SEC) from and after the Issue Date,
(1) within 90 days (or any other time period then in effect under the rules and regulations of the Exchange Act with respect to the filing of a Form 10-K by a non-accelerated filer) after the end of each fiscal year, annual reports on Form 10-K, or any successor or comparable form, containing the information required to be contained therein, or required in such successor or comparable form;
(2) within 45 days after the end of each of the first three fiscal quarters of each fiscal year, reports on Form 10-Q containing all quarterly information that would be required to be contained in Form 10-Q, or any successor or comparable form;
(3) promptly from time to time after the occurrence of an event required to be therein reported, such other reports on Form 8-K, or any successor or comparable form; and
186
Table of Contents
(4) any other information, documents and other reports which the Company would be required to file with the SEC if it were subject to Section 13 or 15(d) of the Exchange Act;
in each case, in a manner that complies in all material respects with the requirements specified in such form; provided that the Company shall not be so obligated to file such reports with the SEC if the SEC does not permit such filing, in which event the Company makes available such information to prospective purchasers of Secured Notes, in addition to providing such information to the Senior Secured Notes Trustee and the Holders of the Senior Secured Notes, in each case within 15 days after the time the Company would be required to file such information with the SEC, if it were subject to Section 13 or 15(d) of the Exchange Act. In addition, to the extent not satisfied by the foregoing, the Company will agree that, for so long as any Secured Notes are outstanding, it will furnish to Holders and to securities analysts and prospective investors, upon their request, the information required to be delivered pursuant to Rule 144A(d)(4) under the Securities Act.
In the event that any direct or indirect parent company of the Company becomes a Guarantor of the Senior Secured Notes, the Senior Secured Notes Indenture permits the Company to satisfy its obligations in this covenant with respect to financial information relating to the Company by furnishing financial information relating to such parent company; provided that the same is accompanied by consolidating information that explains in reasonable detail the differences between the information relating to such parent, on the one hand, and the information relating to the Company and its Restricted Subsidiaries on a standalone basis, on the other hand.
Notwithstanding the foregoing, such requirements shall be deemed satisfied prior to the commencement of the exchange offer or the effectiveness of the shelf registration statement (1) by the filing with the SEC of the exchange offer registration statement or shelf registration statement (or any other registration statement), and any amendments thereto, with such financial information that satisfies Regulation S-X of the Securities Act or (2) by posting reports that would be required to be filed substantially in the form required by the SEC on the Company’s website (or on the website of any of its parent companies) or providing such reports to the Senior Secured Notes Trustee, with financial information that satisfied Regulation S-X of the Securities Act, subject to exceptions consistent with the presentation of financial information in this prospectus, to the extent filed within the times specified above.
Limitation on Business Activities of the Co-Issuer
The Co-Issuer may not hold any assets, become liable for any obligations or engage in any business activities; provided that it may be a co-obligor with respect to the Senior Secured Notes or any other Indebtedness issued by the Company, and may engage in any activities directly related thereto or necessary in connection therewith. The Co-Issuer shall be a Wholly-Owned Subsidiary of the Company at all times.
Events of Default and Remedies
The Secured Notes Indenture provides that each of the following is an “Event of Default”:
(1) default in payment when due and payable (whether at maturity, upon redemption, acceleration or otherwise), of principal of, or premium, if any, on the Senior Secured Notes;
(2) default for 30 days or more in the payment when due of interest or Additional Interest on or with respect to the Senior Secured Notes;
(3) failure by the Company, the Co-Issuer or any Guarantor for 60 days after receipt of written notice given by the Senior Secured Notes Trustee or the Holders of not less than 25% in principal amount of the Senior Secured Notes to comply with any of its obligations, covenants or agreements (other than a default referred to in clauses (1) and (2) above) contained in the Senior Secured Notes Indenture or the Senior Secured Notes;
187
Table of Contents
(4) default under any mortgage, indenture or instrument under which there is issued or by which there is secured or evidenced any Indebtedness for money borrowed by the Company or any of its Restricted Subsidiaries or the payment of which is guaranteed by the Company or any of its Restricted Subsidiaries, other than Indebtedness owed to the Company or a Restricted Subsidiary, whether such Indebtedness or guarantee now exists or is created after the issuance of the Senior Secured Notes, if both:
(a) such default either results from the failure to pay any principal of such Indebtedness at its stated final maturity (after giving effect to any applicable grace periods) or relates to an obligation other than the obligation to pay principal of any such Indebtedness at its stated final maturity and results in the holder or holders of such Indebtedness causing such Indebtedness to become due prior to its stated maturity; and
(b) the principal amount of such Indebtedness, together with the principal amount of any other such Indebtedness in default for failure to pay principal at stated final maturity (after giving effect to any applicable grace periods), or the maturity of which has been so accelerated, aggregate $50.0 million or more at any one time outstanding;
(5) failure by the Company or any Significant Subsidiary (including the Co-Issuer) to pay final judgments aggregating in excess of $50.0 million, which final judgments remain unpaid, undischarged and unstayed for a period of more than 60 days after such judgment becomes final, and in the event such judgment is covered by insurance, an enforcement proceeding has been commenced by any creditor upon such judgment or decree which is not promptly stayed;
(6) certain events of bankruptcy or insolvency with respect to the Company or any Significant Subsidiary;
(7) the Guarantee of any Significant Subsidiary shall for any reason cease to be in full force and effect or be declared null and void or any responsible officer of any Guarantor that is a Significant Subsidiary, as the case may be, denies that it has any further liability under its Guarantee or gives notice to such effect, other than by reason of the termination of the Senior Secured Notes Indenture or the release of any such Guarantee in accordance with the Senior Secured Notes Indenture; or
(8) unless all of the Collateral has been released from the Second Priority Liens in accordance with the provisions of the Security Documents, (i) default by the Company or any Significant Subsidiary in the performance of the Security Documents which adversely affects the enforceability, validity, perfection or priority of the Second Priority Liens on a material portion of the Collateral granted to the Collateral Agent for its benefit and the benefit of the Holders of the Senior Secured Notes, (ii) the repudiation in writing by the Company or any Significant Subsidiary of its material obligations under the Security Documents or (iii) the final determination in a judicial proceeding that the Security Documents are unenforceable or invalid against the Company or any Significant Subsidiary party thereto for any reason with respect to a material portion of the Collateral, in each case of (i), (ii) or (iii), which default, repudiation or determination is not rescinded, stayed, or waived by the Persons having such authority pursuant to the Security Documents or otherwise cured within 60 days after the Issuers receive written notice thereof specifying such occurrence from the Senior Secured Notes Trustee or the Holders of at least 25% of the outstanding principal amount of the Senior Secured Notes and demanding that such default be remedied.
If any Event of Default (other than of a type specified in clause (6) above) occurs and is continuing under the Senior Secured Notes Indenture, the Senior Secured Notes Trustee or the Holders of at least 25% in principal amount of the then total outstanding Secured Notes may declare the principal, premium, if any, interest and any other monetary obligations on all the then outstanding Secured Notes to be due and payable immediately.
Upon the effectiveness of such declaration, such principal and interest are due and payable immediately. Notwithstanding the foregoing, in the case of an Event of Default arising under clause (6) of the first paragraph of this section, all outstanding Secured Notes arecome due and payable without further action or notice. The Secured Notes Indenture provides that the Senior Secured Notes Trustee may withhold from the Holders notice of any continuing Default, except a Default relating to the payment of principal, premium, if any, or interest, if it
188
Table of Contents
determines that withholding notice is in their interest. In addition, the Senior Secured Notes Trustee shall have no obligation to accelerate the Senior Secured Notes if in the best judgment of the Senior Secured Notes Trustee acceleration is not in the best interest of the Holders of the Senior Secured Notes.
The Secured Notes Indenture provides that the Holders of a majority in aggregate principal amount of the then outstanding Secured Notes by notice to the Senior Secured Notes Trustee may on behalf of the Holders of all of the Senior Secured Notes waive any existing Default and its consequences under the Senior Secured Notes Indenture except a continuing Default in the payment of interest on, premium, if any, or the principal of any Secured Note held by a non-consenting Holder and rescind any acceleration with respect to the Senior Secured Notes and its consequences (provided such rescission would not conflict with any judgment of a court of competent jurisdiction). In the event of any Event of Default specified in clause (4) above, such Event of Default and all consequences thereof (excluding any resulting payment default, other than as a result of acceleration of the Senior Secured Notes) shall be annulled, waived and rescinded, automatically and without any action by the Senior Secured Notes Trustee or the Holders, if within 20 days after such Event of Default arose:
(1) the Indebtedness or guarantee that is the basis for such Event of Default has been discharged;
(2) holders thereof have rescinded or waived the acceleration, notice or action (as the case may be) giving rise to such Event of Default; or
(3) the Default that is the basis for such Event of Default has been cured.
Subject to the provisions of the Senior Secured Notes Indenture relating to the duties of the Senior Secured Notes Trustee thereunder, in case an Event of Default occurs and is continuing, the Senior Secured Notes Trustee are under no obligation to exercise any of the rights or powers under the Senior Secured Notes Indenture at the request or direction of any of the Holders of the Senior Secured Notes unless the Holders have offered to the Senior Secured Notes Trustee reasonable indemnity or security against any loss, liability or expense. Except to enforce the right to receive payment of principal, premium (if any) or interest when due, no Holder of a Secured Note may pursue any remedy with respect to the Senior Secured Notes Indenture or the Senior Secured Notes unless:
(1) such Holder has previously given the Senior Secured Notes Trustee notice that an Event of Default is continuing;
(2) Holders of at least 25% in principal amount of the total outstanding Secured Notes have requested the Senior Secured Notes Trustee to pursue the remedy;
(3) Holders of the Senior Secured Notes have offered the Senior Secured Notes Trustee reasonable security or indemnity against any loss, liability or expense;
(4) the Senior Secured Notes Trustee has not complied with such request within 60 days after the receipt thereof and the offer of security or indemnity; and
(5) Holders of a majority in principal amount of the total outstanding Secured Notes have not given the Senior Secured Notes Trustee a direction inconsistent with such request within such 60-day period.
Subject to certain restrictions, under the Senior Secured Notes Indenture, the Holders of a majority in principal amount of the total outstanding Secured Notes are given the right to direct the time, method and place of conducting any proceeding for any remedy available to the Senior Secured Notes Trustee or of exercising any trust or power conferred on the Senior Secured Notes Trustee. The Secured Notes Trustee, however, may refuse to follow any direction that conflicts with law or the Senior Secured Notes Indenture or that the Senior Secured Notes Trustee determines is unduly prejudicial to the rights of any other Holder of a Secured Note or that would involve the Senior Secured Notes Trustee in personal liability.
189
Table of Contents
The Secured Notes Indenture provides that the Company is required to deliver to the Senior Secured Notes Trustee annually a statement regarding compliance with the Senior Secured Notes Indenture, and the Company is required, within 30 days, upon becoming aware of any Default, to deliver to the Senior Secured Notes Trustee a statement specifying such Default.
No Personal Liability of Directors, Officers, Employees and Stockholders
No director, officer, employee, incorporator or stockholder of the Issuers or any Guarantor or any of their parent companies shall have any liability for any obligations of the Issuers or the Guarantors under the Senior Secured Notes, the Guarantees or the Senior Secured Notes Indenture, the Security Documents or for any claim based on, in respect of, or by reason of such obligations or their creation. Each Holder by accepting the Senior Secured Notes waives and releases all such liability. The waiver and release are part of the consideration for issuance of the Senior Secured Notes. Such waiver may not be effective to waive liabilities under the federal securities laws and it is the view of the SEC that such waiver is against public policy.
Legal Defeasance and Covenant Defeasance
The obligations of the Issuers and the Guarantors under the Senior Secured Notes Indenture, the Senior Secured Notes, the Guarantees and the Security Documents, as the case may be, will terminate (other than certain obligations) and are released upon payment in full of all of the Senior Secured Notes. The Issuers may, at their option and at any time, elect to have all of their obligations discharged with respect to the Senior Secured Notes and have each Guarantor’s obligation discharged with respect to its Guarantee (“Legal Defeasance”) and cure all then existing Events of Default except for:
(1) the rights of Holders of Secured Notes to receive payments in respect of the principal of, premium, if any, and interest on the Senior Secured Notes when such payments are due solely out of the trust created pursuant to the Senior Secured Notes Indenture;
(2) the Issuers’ obligations with respect to Secured Notes concerning issuing temporary Secured Notes, registration of such Secured Notes, mutilated, destroyed, lost or stolen Secured Notes and the maintenance of an office or agency for payment and money for security payments held in trust;
(3) the rights, powers, trusts, duties and immunities of the Senior Secured Notes Trustee and the Second Lien Agent, and the Issuers’ obligations in connection therewith; and
(4) the Legal Defeasance provisions of the Senior Secured Notes Indenture.
In addition, the Issuers may, at their option and at any time, elect to have their obligations and those of each Guarantor released with respect to substantially all the restrictive covenants that are described in the Senior Secured Notes Indenture (“Covenant Defeasance”) and thereafter any omission to comply with such obligations shall not constitute a Default with respect to the Senior Secured Notes. In the event Covenant Defeasance occurs, certain events (not including bankruptcy, receivership, rehabilitation and insolvency events pertaining to the Issuers) described under “Events of Default and Remedies” will no longer constitute an Event of Default with respect to the Senior Secured Notes.
In order to exercise either Legal Defeasance or Covenant Defeasance with respect to the Senior Secured Notes:
(1) the Issuers must irrevocably deposit with the Senior Secured Notes Trustee, in trust, for the benefit of the Holders of the Senior Secured Notes, cash in U.S. dollars, Government Securities, or a combination thereof, in such amounts as are sufficient, in the opinion of a nationally recognized firm of independent public accountants, to pay the principal of, premium, if any, and interest due on the Senior Secured Notes on the stated maturity date or on the redemption date, as the case may be, of such principal, premium, if any, or interest on such Secured Notes and the Company must specify whether such Secured Notes are being defeased to maturity or to a particular redemption date;
190
Table of Contents
(2) in the case of Legal Defeasance, the Company shall have delivered to the Senior Secured Notes Trustee an Opinion of Counsel reasonably acceptable to the Senior Secured Notes Trustee confirming that, subject to customary assumptions and exclusions,
(a) the Issuers have received from, or there has been published by, the United States Internal Revenue Service a ruling, or
(b) since the issuance of the Senior Secured Notes, there has been a change in the applicable U.S. federal income tax law,
in either case to the effect that, and based thereon such Opinion of Counsel shall confirm that, subject to customary assumptions and exclusions, the Holders of the Senior Secured Notes will not recognize income, gain or loss for U.S. federal income tax purposes, as applicable, as a result of such Legal Defeasance and are subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Legal Defeasance had not occurred;
(3) in the case of Covenant Defeasance, the Issuers shall have delivered to the Senior Secured Notes Trustee an Opinion of Counsel reasonably acceptable to the Senior Secured Notes Trustee confirming that, subject to customary assumptions and exclusions, the Holders of the Senior Secured Notes will not recognize income, gain or loss for U.S. federal income tax purposes as a result of such Covenant Defeasance and are subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Covenant Defeasance had not occurred;
(4) no Default (other than that resulting from borrowing funds to be applied to make the deposit required to effect such Legal Defeasance or Covenant Defeasance and any similar and simultaneous deposit relating to other Indebtedness and, in each case, the granting of Liens in connection therewith) shall have occurred and be continuing on the date of such deposit;
(5) such Legal Defeasance or Covenant Defeasance shall not result in a breach or violation of, or constitute a default under the Senior Credit Facilities or any other material agreement or instrument (other than the Senior Secured Notes Indenture) to which, the Issuers or any Guarantor is a party or by which the Issuers or any Guarantor is bound (other than that resulting with respect to any Indebtedness being defeased from any borrowing of funds to be applied to make the deposit required to effect such Legal Defeasance or Covenant Defeasance and any similar and simultaneous deposit relating to such Indebtedness, and the granting of Liens in connection therewith);
(6) the Issuers shall have delivered to the Senior Secured Notes Trustee an Opinion of Counsel to the effect that, as of the date of such opinion and subject to customary assumptions and exclusions following the deposit, the trust funds are not subject to the effect of Section 547 of Title 11 of the United States Code;
(7) the Issuers shall have delivered to the Senior Secured Notes Trustee an Officer’s Certificate stating that the deposit was not made by the Issuers with the intent of defeating, hindering, delaying or defrauding any creditors of the Issuers or any Guarantor or others; and
(8) the Issuers shall have delivered to the Senior Secured Notes Trustee an Officer’s Certificate and an Opinion of Counsel (which Opinion of Counsel may be subject to customary assumptions and exclusions) each stating that all conditions precedent provided for or relating to the Legal Defeasance or the Covenant Defeasance, as the case may be, have been complied with.
191
Table of Contents
Satisfaction and Discharge
The Secured Notes Indenture and the Security Documents are discharged and will cease to be of further effect as to all Secured Notes, when:
(1) either
(a) all Secured Notes theretofore authenticated and delivered, except lost, stolen or destroyed Secured Notes which have been replaced or paid and Secured Notes for whose payment money has theretofore been deposited in trust, have been delivered to the Senior Secured Notes Trustee for cancellation; or
(b) all Secured Notes not theretofore delivered to the Senior Secured Notes Trustee for cancellation have become due and payable by reason of the making of a notice of redemption or otherwise, arecome due and payable within one year or are to be called for redemption within one year under arrangements satisfactory to the Senior Secured Notes Trustee for the giving of notice of redemption by the Senior Secured Notes Trustee in the name, and at the expense, of the Issuers and the Issuers or any Guarantor have irrevocably deposited or caused to be deposited with the Senior Secured Notes Trustee as trust funds in trust solely for the benefit of the Holders of the Senior Secured Notes, cash in U.S. dollars, Government Securities, or a combination thereof, in such amounts as are sufficient without consideration of any reinvestment of interest to pay and discharge the entire Indebtedness on the Senior Secured Notes not theretofore delivered to the Senior Secured Notes Trustee for cancellation for principal, premium, if any, and accrued interest to the date of maturity or redemption;
(2) no Default (other than that resulting from borrowing funds to be applied to make such deposit or any similar or simultaneous deposit relating to other Indebtedness) with respect to the Senior Secured Notes Indenture or the Senior Secured Notes shall have occurred and be continuing on the date of such deposit or shall occur as a result of such deposit and such deposit will not result in a breach or violation of, or constitute a default under the Senior Credit Facilities, the indentures governing the Existing Senior Subordinated Notes and the Existing Senior Notes or any other material agreement or instrument (other than the Senior Secured Notes Indenture) to which the Issuers or any Guarantor is a party or by which the Issuers or any Guarantor is bound (other than that resulting from borrowing funds to be applied to make such deposit and any similar and simultaneous deposit relating to other Indebtedness and, in each case, the granting of Liens in connection therewith);
(3) the Issuers have paid or caused to be paid all sums payable by it under the Senior Secured Notes Indenture; and
(4) the Issuers have delivered irrevocable instructions to the Senior Secured Notes Trustee to apply the deposited money toward the payment of the Senior Secured Notes at maturity or the redemption date, as the case may be.
In addition, the Issuers must deliver an Officer’s Certificate and an Opinion of Counsel to the Senior Secured Notes Trustee stating that all conditions precedent to satisfaction and discharge have been satisfied.
Amendment, Supplement and Waiver
Except as provided in the next three succeeding paragraphs, the Senior Secured Notes Indenture, any Guarantee and the Senior Secured Notes may be amended or supplemented with the consent of the Holders of at least a majority in principal amount of the Senior Secured Notes then outstanding, including consents obtained in connection with a purchase of, or tender offer or exchange offer for, Secured Notes and any existing Default or compliance with any provision of the Senior Secured Notes Indenture or the Senior Secured Notes issued thereunder may be waived with the consent of the Holders of a majority in principal amount of the then outstanding Secured Notes, other than Secured Notes beneficially owned by the Issuers or their Affiliates (including consents obtained in connection with a purchase of or tender offer or exchange offer for the Senior Secured Notes).
192
Table of Contents
In addition, except as provided in the next two succeeding paragraphs, the Security Documents may be amended or supplemented or otherwise modified in any manner with the consent of the holders of at least a majority in aggregate principal amount of the outstanding Secured Notes and the Permitted Additional Pari Passu Obligations, voting as one class.
The Secured Notes Indenture provides that, without the consent of each affected Holder of Secured Notes, an amendment or waiver may not, with respect to any Secured Notes held by a non-consenting Holder:
(1) reduce the principal amount of such Secured Notes whose Holders must consent to an amendment, supplement or waiver;
(2) reduce the principal of or change the fixed final maturity of any such Secured Note or alter or waive the provisions with respect to the redemption of such Secured Notes (other than provisions relating to the covenants described above under the caption “Repurchase at the Option of Holders”);
(3) reduce the rate of or change the time for payment of interest on any Secured Note;
(4) waive a Default in the payment of principal of or premium, if any, or interest on the Senior Secured Notes, except a rescission of acceleration of the Senior Secured Notes by the Holders of at least a majority in aggregate principal amount of the Senior Secured Notes and a waiver of the payment default that resulted from such acceleration, or in respect of a covenant or provision contained in the Senior Secured Notes Indenture or any Guarantee which cannot be amended or modified without the consent of all Holders;
(5) make any Secured Note payable in money other than U.S. dollars;
(6) make any change in the provisions of the Senior Secured Notes Indenture relating to waivers of past Defaults or the rights of Holders to receive payments of principal of or premium, if any, or interest or Additional Interest on the Senior Secured Notes;
(7) make any change in these amendment and waiver provisions;
(8) impair the right of any Holder to receive payment of principal of, or interest on such Holder’s Secured Notes on or after the due dates therefor or to institute suit for the enforcement of any payment on or with respect to such Holder’s Secured Notes or the Guarantees;
(9) make any change to or modify the ranking of the Senior Secured Notes that would adversely affect the Holders; or
(10) except as expressly permitted by the Senior Secured Notes Indenture, modify the Guarantee of any Significant Subsidiary in any manner adverse to the Holders of the Senior Secured Notes or release the Co-Issuer from its obligations under the Senior Secured Notes Indenture.
Notwithstanding the foregoing, the Issuers, any Guarantor (with respect to a Guarantee or the Senior Secured Notes Indenture to which it is a party) and the Senior Secured Notes Trustee may amend or supplement the Senior Secured Notes Indenture, the Security Documents and any Guarantee or Secured Notes without the consent of any Holder:
(1) to cure any ambiguity, omission, mistake, defect or inconsistency;
(2) to provide for uncertificated Notes of such series in addition to or in place of certificated Notes;
(3) to comply with the covenant relating to mergers, consolidations and sales of assets;
(4) to provide for the assumption of the Issuers’ or any Guarantor’s obligations to the Holders;
193
Table of Contents
(5) to make any change that would provide any additional rights or benefits to the Holders or that does not adversely affect the legal rights under the Senior Secured Notes Indenture of any such Holder;
(6) to add assets to the Collateral or to release Collateral from any Lien pursuant to the Senior Secured Notes Indenture and the Security Documents when permitted or required by the Senior Secured Notes Indenture, to the extent necessary to provide for the granting of a security interest for the benefit of any Person; provided that the granting of such security interest is not prohibited under “—Impairment of Security Interest” or otherwise under the Senior Secured Notes Indenture;
(7) to add parties to the Security Documents, including Guarantors, or successors, including successor trustees or other representatives;
(8) to make provision for equal and ratable pledges of any collateral to secure the Senior Secured Notes;
(9) to add covenants for the benefit of the Holders or to surrender any right or power conferred upon the Issuers or any Guarantor;
(10) to comply with requirements of the SEC in order to effect or maintain the qualification of the Senior Secured Notes Indenture under the Trust Indenture Act;
(11) to evidence and provide for the acceptance and appointment under the Senior Secured Notes Indenture of a successor Trustee or a successor Second Lien Agent thereunder pursuant to the requirements thereof;
(12) to provide for the issuance of exchange notes or private exchange notes, which are identical to exchange notes except that they are not freely transferable;
(13) to provide for the issuance of Additional Secured Notes in accordance with the Senior Secured Notes Indenture;
(14) to add a Guarantor under the Senior Secured Notes Indenture or to release a Guarantor in accordance with the terms of the Senior Secured Notes Indenture;
(15) to conform the text of the Senior Secured Notes Indenture, Guarantees, the Security Documents or the Senior Secured Notes to any provisions of the “Description of Notes” in the offering circular related to the Senior Secured Notes to the extent that such provision in such “Description of Notes” was intended to be a verbatim recitation of a provision of the Senior Secured Notes Indenture, Guarantee, the Security Documents or Secured Notes;
(16) to make any amendment to the provisions of the Senior Secured Notes Indenture relating to the transfer and legending of Secured Notes as permitted by the Senior Secured Notes Indenture, including, without limitation to facilitate the issuance and administration of the Senior Secured Notes; provided, however, that (i) compliance with the Senior Secured Notes Indenture as so amended would not result in Secured Notes being transferred in violation of the Securities Act or any applicable securities law and (ii) such amendment does not materially and adversely affect the rights of Holders to transfer Secured Notes;
(17) to make any other modifications to the Senior Secured Notes or the Senior Secured Notes Indentures of a formal, minor or technical nature or necessary to correct a manifest error, so long as such modification does not adversely affect the rights of any Holders of the Senior Secured Notes in any material respect;
(18) to mortgage, pledge, hypothecate or grant any other Lien in favor of the Second Lien Agent for the benefit of the Senior Secured Notes Trustee on behalf of the Holders of the Senior Secured Notes, as additional security for the payment and performance of all or any portion of the Second Lien Obligations, in any property or assets, including any which are required to be mortgaged, pledged or hypothecated, or in which a Lien is required to be granted to or for the benefit of the Senior Secured Notes Trustee or the Second Lien Agent pursuant to the Senior Secured Notes Indenture, any of the Security Documents or otherwise;
194
Table of Contents
(19) to secure any First Lien Obligations or any Permitted Additional Pari Passu Obligations under the Security Documents and to include the same in the Intercreditor Agreement;
(20) to enter into any intercreditor arrangements with respect to Indebtedness secured by junior Liens on the collateral; or
(21) to provide for (i) the succession of any parties to the Security Documents or the Intercreditor Agreement (and other amendments that are administrative or ministerial in nature) in connection with an amendment, renewal, extension, substitution, refinancing, restructuring, replacement, supplementing or other modification from time to time of the Senior Credit Facilities or any other agreement that is not prohibited by the Senior Secured Notes Indenture, or (ii) the succession of the Second Lien Agent as collateral agent under the Senior Secured Notes Indenture, the Intercreditor Agreement and the Security Documents.
Any amendment to, or waiver of, the provisions of the Senior Secured Notes Indenture or any Security Document that has the effect of releasing all or substantially all of the Collateral from the Liens securing the Senior Secured Notes other than in accordance with the Senior Secured Notes Indenture and the Security Documents, or modifying the Intercreditor Agreement in any manner adverse in any material respect to the Holders of the Senior Secured Notes, will require the consent of the holders of at least 66-2/3% in aggregate principal amount of the Senior Secured Notes and Permitted Additional Pari Passu Obligations then outstanding, voting as one class.
Prior to the repayment of the First Lien Obligations, the Administrative Agent or holders of the First Lien Obligations may amend, waive or modify the security documents of such holders and, pursuant to the Intercreditor Agreement, such changes will automatically apply to comparable provisions of the Security Documents. The Administrative Agent shall give the Senior Secured Notes Trustee and the Second Lien Agent notice of such amendment, waiver or consent, but any failure to provide such notice will not affect the validity or effectiveness of any such amendment, waiver or consent.
No amendment of, or supplement or waiver to, the Security Documents (other than the Intercreditor Agreement) shall be permitted to be effected in violation of or inconsistent with the terms of the Intercreditor Agreement without the prior written consent of the Administrative Agent. No amendment of, or supplement or waiver to, the Intercreditor Agreement shall be permitted to be effected without the consent of the Administrative Agent and the Second Lien Agent.
The Secured Notes Indenture also provides that each Holder of Secured Notes, by accepting a Secured Note, shall be deemed to have agreed to and accepted the terms and conditions of the Security Documents and the performance by the Senior Secured Notes Trustee and the Second Lien Agent of their respective obligations and the exercise of their respective rights thereunder and in connection therewith. A copy of the Security Documents shall be made available for inspection during normal business hours on any Business Day upon prior written request at the offices of the Senior Secured Notes Trustee.
The consent of the Holders is not necessary under the Senior Secured Notes Indenture to approve the particular form of any proposed amendment. It is sufficient if such consent approves the substance of the proposed amendment.
Notices
Notices given by publication are deemed given on the first date on which publication is made and notices given by first-class mail, postage prepaid, are deemed given five calendar days after mailing.
Concerning the Senior Secured Notes Trustee
The Secured Notes Indenture contains certain limitations on the rights of the Senior Secured Notes Trustee thereunder, should it become a creditor of the Issuers, to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim as security or otherwise. The Secured Notes Trustee is
195
Table of Contents
permitted to engage in other transactions; however, if it acquires any conflicting interest, it must eliminate such conflict within 90 days, apply to the SEC for permission to continue or resign.
The Secured Notes Indenture provides that the Holders of a majority in principal amount of the outstanding Secured Notes will have the right to direct the time, method and place of conducting any proceeding for exercising any remedy available to the Senior Secured Notes Trustee, subject to certain exceptions. The Secured Notes Indenture provides that in case an Event of Default shall occur (which shall not be cured), the Senior Secured Notes Trustee is required, in the exercise of its power, to use the degree of care of a prudent person in the conduct of his own affairs. Subject to such provisions, the Senior Secured Notes Trustee is under no obligation to exercise any of its rights or powers under the Senior Secured Notes Indenture at the request of any Holder of the Senior Secured Notes, unless such Holder shall have offered to the Senior Secured Notes Trustee security and indemnity satisfactory to it against any loss, liability or expense.
Concerning the Second Lien Agent
The Bank of New York Mellon is the Second Lien Agent under the Security Documents. The Second Lien Agent are under no obligation to exercise any of its rights or powers under the Security Documents at the request of any Holder of the Senior Secured Notes unless such Holder shall have offered to the Second Lien Agent security and indemnity satisfactory to it against any loss, liability or expense.
Governing Law
The Secured Notes Indenture, the Senior Secured Notes and any Guarantee are governed by and construed in accordance with the laws of the State of New York.
Certain Definitions
Set forth below are certain defined terms used in the Senior Secured Notes Indenture. For purposes of the Senior Secured Notes Indenture, unless otherwise specifically indicated, the term “consolidated” with respect to any Person refers to such Person consolidated with its Restricted Subsidiaries, and excludes from such consolidation any Unrestricted Subsidiary as if such Unrestricted Subsidiary were not an Affiliate of such Person.
“Acquired Indebtedness” means, with respect to any specified Person,
(1) Indebtedness of any other Person existing at the time such other Person is merged with or into or became a Restricted Subsidiary of such specified Person, including Indebtedness incurred in connection with, or in contemplation of, such other Person merging with or into or becoming a Restricted Subsidiary of such specified Person, and
(2) Indebtedness secured by a Lien encumbering any asset acquired by such specified Person.
“Additional Interest” means all additional interest then owing pursuant to the Senior Secured Notes Registration Rights Agreement.
“Additional Notes” means Secured Notes (other than the Senior Secured Notes and Secured Notes issued in exchange therefor) issued as permitted under the Senior Secured Notes Indenture.
“Administrative Agent” means Credit Suisse AG, in its capacities as administrative agent and collateral agent under the Credit Agreement and any successor administrative agent thereunder.
“Affiliate” of any specified Person means any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such specified Person. For purposes of this definition, “control” (including, with correlative meanings, the terms “controlling,” “controlled by” and “under common
196
Table of Contents
control with”), as used with respect to any Person, shall mean the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such Person, whether through the ownership of voting securities, by agreement or otherwise.
“After-Acquired Property” means any property of the Issuers or any Guarantor acquired after the Issue Date that is required to secure the obligations of the Issuers and the Guarantors under the Senior Secured Notes and the Guarantees pursuant to the Senior Secured Notes Indenture and the Security Documents.
“Applicable Premium” means, with respect to any Secured Note on any Redemption Date, the greater of:
(1) 1.0% of the principal amount of such Secured Note; and
(2) the excess, if any, of (a) the present value at such Redemption Date of (i) the redemption price of such Secured Note at March 15, 2015 (each such redemption price being set forth in the table appearing above under the caption “Optional Redemption”), plus (ii) all required interest payments due on such Secured Note through March 15, 2015 (excluding accrued but unpaid interest to the Redemption Date), computed using a discount rate equal to the Treasury Rate as of such Redemption Date plus 50 basis points; over (b) the principal amount of such Secured Note.
Calculation of the Applicable Premium shall be made by the Company or on behalf of the Company by such Person as the Company shall designate; provided that such calculation or the correctness thereof shall not be a duty or obligation of the Senior Secured Notes Trustee.
“Asset Sale” means:
(1) the sale, conveyance, transfer or other disposition, whether in a single transaction or a series of related transactions, of property or assets (including by way of a Sale and Lease-Back Transaction) of the Company or any of its Restricted Subsidiaries (each referred to in this definition as a “disposition”); or
(2) the issuance or sale of Equity Interests of any Restricted Subsidiary (other than Preferred Stock of Restricted Subsidiaries issued in compliance with the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”), whether in a single transaction or a series of related transactions;
in each case, other than:
(a) any disposition of Cash Equivalents or Investment Grade Securities or obsolete or worn-out equipment in the ordinary course of business or any disposition of inventory or goods (or other assets) held for sale in the ordinary course of business;
(b) the disposition of all or substantially all of the assets of the Company governed by, and in a manner permitted pursuant to, the provisions described above under “Certain Covenants—Merger, Consolidation or Sale of All or Substantially All Assets” or any disposition that constitutes a Change of Control pursuant to the Senior Secured Notes Indenture;
(c) the making of any Restricted Payment or Permitted Investment that is permitted to be made, and is made, under the covenant described above under “Certain Covenants—Limitation on Restricted Payments”;
(d) any disposition of assets or issuance or sale of Equity Interests of any Restricted Subsidiary in any transaction or series of transactions with an aggregate fair market value of less than $10.0 million;
(e) any disposition of property or assets or issuance of securities by a Restricted Subsidiary of the Company to the Company or by the Company or a Restricted Subsidiary of the Company to another Restricted Subsidiary of the Company;
197
Table of Contents
(f) to the extent allowable under Section 1031 of the Internal Revenue Code of 1986 or comparable law or regulation, any exchange of like property (excluding any boot thereon) for use in a Similar Business;
(g) the lease, assignment or sub-lease of any real or personal property in the ordinary course of business;
(h) any issuance or sale of Equity Interests in, or Indebtedness or other securities of, an Unrestricted Subsidiary;
(i) foreclosures on assets;
(j) sales of accounts receivable, or participations therein, in connection with any Receivables Facility; and
(k) any financing transaction with respect to the acquisition or construction of property by the Company or any Restricted Subsidiary after the Issue Date, including Sale and Lease-Back Transactions and asset securitizations permitted by the Senior Secured Notes Indenture.
“Bank Product Obligations” means Cash Management Obligations and Hedging Obligations.
“Business Day” means each day which is not a Legal Holiday.
“Capital Stock” means:
(1) in the case of a corporation, corporate stock;
(2) in the case of an association or business entity, any and all shares, interests, participations, rights or other equivalents (however designated) of corporate stock;
(3) in the case of a partnership or limited liability company, partnership or membership interests (whether general or limited); and
(4) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distributions of assets of, the issuing Person.
“Capitalized Lease Obligation” means, at the time any determination thereof is to be made, the amount of the liability in respect of a capital lease that would at such time be required to be capitalized and reflected as a liability on a balance sheet (excluding the footnotes thereto) in accordance with GAAP.
“Cash Equivalents” means:
(1) United States dollars;
(2) (a) €, or any national currency of any participating member state of the EMU; or
(b) such local currencies held by the Company or any Restricted Subsidiary from time to time in the ordinary course of business;
(3) securities issued or directly and fully and unconditionally guaranteed or insured by the U.S. government (or any agency or instrumentality thereof the securities of which are unconditionally guaranteed as a full faith and credit obligation of the U.S. government), with maturities of 24 months or less from the date of acquisition;
(4) certificates of deposit, time deposits and eurodollar time deposits with maturities of one year or less from the date of acquisition, bankers’ acceptances with maturities not exceeding one year and overnight bank deposits,
198
Table of Contents
in each case with any commercial bank having capital and surplus of not less than $500.0 million in the case of U.S. banks and $100.0 million (or the U.S. dollar equivalent as of the date of determination) in the case of non-U.S. banks;
(5) repurchase obligations for underlying securities of the types described in clauses (3) and (4) entered into with any financial institution meeting the qualifications specified in clause (4) above;
(6) commercial paper rated at least P-1 by Moody’s or at least A-1 by S&P and in each case maturing within 24 months after the date of creation thereof;
(7) marketable short-term money market and similar securities having a rating of at least P-2 or A-2 from either Moody’s or S&P, respectively (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) and in each case maturing within 24 months after the date of creation thereof;
(8) investment funds investing 95% of their assets in securities of the types described in clauses (1) through (7) above;
(9) readily marketable direct obligations issued by any state, commonwealth or territory of the United States or any political subdivision or taxing authority thereof having an Investment Grade Rating from either Moody’s or S&P with maturities of 24 months or less from the date of acquisition;
(10) Indebtedness or Preferred Stock issued by Persons with a rating of “A” or higher from S&P or “A2” or higher from Moody’s with maturities of 24 months or less from the date of acquisition; and
(11) Investments with average maturities of 24 months or less from the date of acquisition in money market funds rated AAA- (or the equivalent thereof) or better by S&P or Aaa3 (or the equivalent thereof) or better by Moody’s.
Notwithstanding the foregoing, Cash Equivalents shall include amounts denominated in currencies other than those set forth in clauses (1) and (2) above, provided that such amounts are converted into any currency listed in clauses (1) and (2) as promptly as practicable and in any event within ten Business Days following the receipt of such amounts.
“Cash Management Obligations” means obligations owed by the Issuers or any Restricted Subsidiary to any agent or a lender under the Credit Agreement or any Affiliate of any such agent or lender in respect of any treasury, depository and cash management services (including in respect of liabilities arising from purchase card, travel and entertainment cards or other card services) or any automated clearing house transfers of funds.
“Change of Control” means the occurrence of any of the following:
(1) the sale, lease or transfer, in one or a series of related transactions, of all or substantially all of the assets of the Company and its Subsidiaries, taken as a whole, to any Person other than a Permitted Holder; or
(2) the Company becomes aware (by way of a report or any other filing pursuant to Section 13(d) of the Exchange Act, proxy, vote, written notice or otherwise) of the acquisition by any Person or group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act, or any successor provision), including any group acting for the purpose of acquiring, holding or disposing of securities (within the meaning of Rule 13d-5(b)(1) under the Exchange Act), other than the Permitted Holders, in a single transaction or in a related series of transactions, by way of merger, consolidation or other business combination or purchase of beneficial ownership (within the meaning of Rule 13d-3 under the Exchange
199
Table of Contents
Act, or any successor provision) of 50% or more of the total voting power of the Voting Stock of the Company or any of its direct or indirect parent companies holding directly or indirectly 100% of the total voting power of the Voting Stock of the Company.
“Consolidated Depreciation and Amortization Expense” means with respect to any Person for any period, the total amount of depreciation and amortization expense, including the amortization of deferred financing fees of such Person and its Restricted Subsidiaries for such period on a consolidated basis and otherwise determined in accordance with GAAP.
“Consolidated Interest Expense” means, with respect to any Person for any period, without duplication, the sum of:
(1) consolidated interest expense of such Person and its Restricted Subsidiaries for such period, to the extent such expense was deducted (and not added back) in computing Consolidated Net Income (including (a) amortization of original issue discount or premium resulting from the issuance of Indebtedness at less than or greater than par, as applicable, (b) all commissions, discounts and other fees and charges owed with respect to letters of credit or bankers acceptances, (c) non-cash interest payments (but excluding any non-cash interest expense attributable to the movement in the mark to market valuation of Hedging Obligations or other derivative instruments pursuant to GAAP), (d) the interest component of Capitalized Lease Obligations and (e) net payments, if any, pursuant to interest rate Hedging Obligations with respect to Indebtedness and excluding (t) accretion or accrual of discounted liabilities not constituting Indebtedness, (u) interest expense attributable to Indebtedness of a parent entity resulting from push-down accounting to the extent such Person and its Restricted Subsidiaries are not liable for the payment of such Indebtedness, (v) any expense resulting from the discounting of any outstanding Indebtedness in connection with the application of purchase accounting in connection with any acquisition, (w) any Additional Interest and any comparable “additional interest” with respect to other securities, (x) amortization of deferred financing fees, debt issuance costs, commissions, fees and expenses, (y) any expensing of bridge, commitment and other financing fees and (z) commissions, discounts, yield and other fees and charges (including any interest expense) related to any Receivables Facility); plus
(2) consolidated capitalized interest of such Person and its Restricted Subsidiaries for such period, whether paid or accrued; less
(3) interest income for such period.
For purposes of this definition, interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by such Person to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP.
“Consolidated Net Income” means, with respect to any Person for any period, the aggregate of the Net Income, of such Person and its Restricted Subsidiaries for such period, on a consolidated basis, and otherwise determined in accordance with GAAP; provided, however, that, without duplication,
(1) any after-tax effect of extraordinary, non-recurring or unusual gains or losses (less all fees and expenses relating thereto) or expenses (including relating to the Transactions to the extent incurred on or prior to April 15, 2012), severance, relocation costs and curtailments or modifications to pension and post-retirement employee benefit plans and other restructuring costs shall be excluded,
(2) the cumulative effect of a change in accounting principles during such period shall be excluded,
(3) any after-tax effect of income (loss) from disposed, abandoned, transferred, closed or discontinued operations and any net after-tax gains or losses on disposal of disposed, abandoned, transferred, closed or discontinued operations shall be excluded,
200
Table of Contents
(4) any after-tax effect of gains or losses (less all fees and expenses relating thereto) attributable to asset dispositions other than in the ordinary course of business, as determined in good faith by the Company, shall be excluded,
(5) the Net Income for such period of any Person that is not a Subsidiary or is an Unrestricted Subsidiary or that is accounted for by the equity method of accounting, shall be excluded; provided that Consolidated Net Income of the Company shall be increased by the amount of dividends or distributions or other payments that are actually paid in cash (or to the extent converted into cash) to the referent Person or a Restricted Subsidiary thereof in respect of such period,
(6) solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of “Certain Covenants—Limitation on Restricted Payments,” the Net Income for such period of any Restricted Subsidiary (other than any Guarantor) shall be excluded to the extent that the declaration or payment of dividends or similar distributions by that Restricted Subsidiary of its Net Income is not at the date of determination permitted without any prior governmental approval (which has not been obtained) or, directly or indirectly, by the operation of the terms of its charter or any agreement, instrument, judgment, decree, order, statute, rule, or governmental regulation applicable to that Restricted Subsidiary or its stockholders, unless such restriction with respect to the payment of dividends or similar distributions has been legally waived, provided that Consolidated Net Income of the Company are increased by the amount of dividends or other distributions or other payments actually paid in cash (or to the extent converted into cash) or Cash Equivalents to the Company or a Restricted Subsidiary thereof in respect of such period, to the extent not already included therein,
(7) effects of adjustments (including the effects of such adjustments pushed down to the Company and its Restricted Subsidiaries) in the property and equipment, inventory and other intangible assets, deferred revenue and debt line items in such Person’s consolidated financial statements pursuant to GAAP resulting from the application of purchase accounting in relation to the DJO Acquisition, the Transactions or any consummated acquisition or the amortization or write-off of any amounts thereof, net of taxes, shall be excluded,
(8) any after-tax effect of income (loss) from the early extinguishment of Indebtedness or Hedging Obligations or other derivative instruments shall be excluded,
(9) any impairment charge or asset write-off, in each case, pursuant to GAAP and the amortization of intangibles arising pursuant to GAAP shall be excluded,
(10) any non-cash compensation expense recorded from grants of stock appreciation or similar rights, stock options, restricted stock or other rights shall be excluded,
(11) any fees and expenses incurred during such period, or any amortization thereof for such period, in connection with any acquisition, disposition, recapitalization, Investment, Asset Sale, issuance or repayment of Indebtedness, issuance of Equity Interests, refinancing transaction or amendment or modification of any debt instrument (in each case, including any such transaction consummated prior to the Issue Date and any such transaction undertaken but not completed) and any charges or non-recurring merger costs incurred during such period as a result of any such transaction shall be excluded,
(12) accruals and reserves that are established or adjusted within twelve months after the Issue Date that are so required to be established or adjusted as a result of the Transactions in accordance with GAAP or changes as a result of a modification of accounting policies shall be excluded and
(13) to the extent covered by insurance and actually reimbursed, or, so long as the Issuers have made a determination that there exists reasonable evidence that such amount will in fact be reimbursed by the insurer and only to the extent that such amount is (a) not denied by the applicable carrier in writing within 180 days and (b) in fact reimbursed within 365 days of the date of such evidence (with a deduction for any amount so added back to the extent not so reimbursed within 365 days), expenses with respect to liability or casualty events or business interruption shall be excluded.
201
Table of Contents
Notwithstanding the foregoing, for the purpose of the covenant described under “Certain Covenants—Limitation on Restricted Payments” only (other than clause (3)(d) thereof), there shall be excluded from Consolidated Net Income any income arising from any sale or other disposition of Restricted Investments made by the Company and its Restricted Subsidiaries, any repurchases and redemptions of Restricted Investments from the Company and its Restricted Subsidiaries, any repayments of loans and advances which constitute Restricted Investments by the Company or any of its Restricted Subsidiaries, any sale of the stock of an Unrestricted Subsidiary or any distribution or dividend from an Unrestricted Subsidiary, in each case only to the extent such amounts increase the amount of Restricted Payments permitted under such covenant pursuant to clause (3)(d) thereof.
“Consolidated Secured Debt Ratio” as of any date of determination means, the ratio of (1) Consolidated Total Indebtedness of the Company and its Restricted Subsidiaries that is secured by Liens (other than Liens that are junior in priority to the Liens securing the Senior Secured Notes) as of the end of the most recent fiscal quarter for which internal financial statements are available immediately preceding the date on which such event for which such calculation is being made shall occur to (2) the Company’s EBITDA for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date on which such event for which such calculation is being made shall occur, in each case with such pro forma adjustments to Consolidated Total Indebtedness EBITDA as are appropriate and consistent with the pro forma adjustment provisions set forth in the definition of Fixed Charge Coverage Ratio.
“Consolidated Total Indebtedness” means, as at any date of determination, an amount equal to the sum of (1) the aggregate amount of all outstanding Indebtedness of the Company and its Restricted Subsidiaries on a consolidated basis consisting of Indebtedness for borrowed money, Obligations in respect of Capitalized Lease Obligations and debt obligations evidenced by promissory notes and similar instruments and (2) the aggregate amount of all outstanding Disqualified Stock of the Company and all Preferred Stock of its Restricted Subsidiaries on a consolidated basis, with the amount of such Disqualified Stock and Preferred Stock equal to the greater of their respective voluntary or involuntary liquidation preferences and maximum fixed repurchase prices, in each case, determined on a consolidated basis in accordance with GAAP. For purposes hereof, the “maximum fixed repurchase price” of any Disqualified Stock or Preferred Stock that does not have a fixed repurchase price shall be calculated in accordance with the terms of such Disqualified Stock or Preferred Stock as if such Disqualified Stock or Preferred Stock were purchased on any date on which Consolidated Total Indebtedness shall be required to be determined pursuant to the Senior Secured Notes Indenture, and if such price is based upon, or measured by, the fair market value of such Disqualified Stock or Preferred Stock, such fair market value shall be determined reasonably and in good faith by the Company.
“Contingent Obligations” means, with respect to any Person, any obligation of such Person guaranteeing any leases, dividends or other obligations that do not constitute Indebtedness (“primary obligations”) of any other Person (the “primary obligor”) in any manner, whether directly or indirectly, including, without limitation, any obligation of such Person, whether or not contingent,
(1) to purchase any such primary obligation or any property constituting direct or indirect security therefor,
(2) to advance or supply funds
(a) for the purchase or payment of any such primary obligation, or
(b) to maintain working capital or equity capital of the primary obligor or otherwise to maintain the net worth or solvency of the primary obligor, or
(3) to purchase property, securities or services primarily for the purpose of assuring the owner of any such primary obligation of the ability of the primary obligor to make payment of such primary obligation against loss in respect thereof.
“Credit Agreement” means the Credit Agreement entered into as of November 20, 2007 by and among the Company, the Co-Issuer, the Guarantors, the lenders party thereto in their capacities as lenders thereunder and
202
Table of Contents
Credit Suisse, Cayman Islands Branch, as Administrative Agent, including any guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements, refundings or refinancings thereof and any indentures or credit facilities or commercial paper facilities with banks or other institutional lenders or investors that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” above).
“Credit Facilities” means, with respect to the Company or any of its Restricted Subsidiaries, one or more debt facilities, including the Senior Credit Facilities, or other financing arrangements (including, without limitation, commercial paper facilities or indentures) providing for revolving credit loans, term loans, letters of credit or other long-term Indebtedness, including any notes, mortgages, guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements or refundings thereof and any indentures or credit facilities or commercial paper facilities that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount permitted to be borrowed thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) or adds Restricted Subsidiaries as additional borrowers or guarantors thereunder and whether by the same or any other agent, lender or group of lenders.
“Default” means any event that is, or with the passage of time or the giving of notice or both would be, an Event of Default.
“Designated Non-cash Consideration” means the fair market value of non-cash consideration received by the Company or a Restricted Subsidiary in connection with an Asset Sale that is so designated as Designated Non-cash Consideration pursuant to an Officer’s Certificate, setting forth the basis of such valuation, executed by the principal financial officer of the Company, less the amount of cash or Cash Equivalents received in connection with a subsequent sale of or collection on such Designated Non-cash Consideration.
“Designated Preferred Stock” means Preferred Stock of the Company or any parent corporation thereof (in each case other than Disqualified Stock) that is issued for cash (other than to a Restricted Subsidiary or an employee stock ownership plan or trust established by the Company or any of its Subsidiaries) and is so designated as Designated Preferred Stock, pursuant to an Officer’s Certificate executed by the principal financial officer of the Company or the applicable parent corporation thereof, as the case may be, on the issuance date thereof, the cash proceeds of which are excluded from the calculation set forth in clause (3) of the first paragraph of the “Certain Covenants—Limitation on Restricted Payments” covenant and are not otherwise applied to make any other Restricted Payment.
“Disqualified Stock” means, with respect to any Person, any Capital Stock of such Person which, by its terms, or by the terms of any security into which it is convertible or for which it is putable or exchangeable, or upon the happening of any event, matures or is mandatorily redeemable (other than solely as a result of a change of control or asset sale) pursuant to a sinking fund obligation or otherwise, or is redeemable at the option of the holder thereof (other than solely as a result of a change of control or asset sale), in whole or in part, in each case prior to the date 91 days after the maturity date of the Senior Secured Notes; provided, however, that if such Capital Stock is issued to any plan for the benefit of employees of the Company or its Subsidiaries or by any such plan to such employees, such Capital Stock shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Company or its Subsidiaries in order to satisfy applicable statutory or regulatory obligations.
203
Table of Contents
“DJO Acquisition” means the acquisition contemplated by the Agreement and Plan of Merger, dated as of July 15, 2007, by and among ReAble Therapeutics Finance LLC, Reaction Acquisition Merger Sub, Inc. and DJO Opco Holdings Inc. (f/k/a DJO Incorporated), and related financings.
“EBITDA” means, with respect to any Person for any period, the Consolidated Net Income of such Person for such period
(1) increased (without duplication) by:
(a) provision for taxes based on income or profits or capital gains, including, without limitation, federal, state, foreign, franchise and similar taxes and foreign withholding taxes (including penalties and interest related to such taxes or arising from tax examinations) of such Person paid or accrued during such period to the extent the same was deducted (and not added back) in computing Consolidated Net Income; plus
(b) Fixed Charges of such Person for such period (including (x) net losses or Hedging Obligations or other derivative instruments entered into for the purpose of hedging interest rate risk and (y) costs of surety bonds in connection with financing activities, in each case, to the extent included in Fixed Charges), together with items excluded from the definition of “Consolidated Interest Expense” pursuant to clauses 1(t) through 1(z) thereof, to the extent the same were deducted (and not added back) in calculating such Consolidated Net Income; plus
(c) Consolidated Depreciation and Amortization Expense of such Person for such period to the extent the same were deducted (and not added back) in computing Consolidated Net Income; plus
(d) any expenses or charges (other than depreciation or amortization expense) related to any Equity Offering, Permitted Investment, acquisition, disposition, recapitalization or the incurrence of Indebtedness permitted to be incurred by the Senior Secured Notes Indenture (including a refinancing thereof) (whether or not successful), including (i) such fees, expenses or charges related to the offering of the Senior Secured Notes and the Credit Facilities and (ii) any amendment or other modification of the Senior Secured Notes, and, in each case, deducted (and not added back) in computing Consolidated Net Income; plus
(e) the amount of any restructuring charges, integration costs or other business optimization expenses or reserves deducted (and not added back) in such period in computing Consolidated Net Income, including any one-time costs incurred in connection with acquisitions after the Issue Date and costs related to the closure and/or consolidation of facilities; plus
(f) any other non-cash charges, including any write-offs or write-downs, reducing Consolidated Net Income for such period (provided that if any such non-cash charges represent an accrual or reserve for potential cash items in any future period, the cash payment in respect thereof in such future period shall be subtracted from EBITDA to such extent, and excluding amortization of a prepaid cash item that was paid in a prior period); plus
(g) the amount of any minority interest expense consisting of Subsidiary income attributable to minority equity interests of third parties in any non-Wholly-Owned Subsidiary deducted (and not added back) in such period in calculating Consolidated Net Income; plus
(h) the amount of management, monitoring, consulting and advisory fees and related expenses paid in such period to the Investors to the extent otherwise permitted under “Certain Covenants—Transactions with Affiliates”; plus
(i) [RESERVED]; plus
(j) the amount of loss on sale of receivables and related assets to the Receivables Subsidiary in connection with a Receivables Facility; plus
(k) any costs or expense incurred by the Company or a Restricted Subsidiary pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement or any stock subscription or shareholder agreement, to the extent that such cost or expenses are
204
Table of Contents
funded with cash proceeds contributed to the capital of the Company or net cash proceeds of an issuance of Equity Interest of the Company (other than Disqualified Stock) solely to the extent that such net cash proceeds are excluded from the calculation set forth in clause (3) of the first paragraph under “Certain Covenants—Limitation on Restricted Payments”;
(2) decreased by (without duplication) non-cash gains increasing Consolidated Net Income of such Person for such period, excluding any non-cash gains to the extent they represent the reversal of an accrual or reserve for a potential cash item that reduced EBITDA in any prior period; and
(3) increased or decreased by (without duplication):
(a) any net gain or loss resulting in such period from Hedging Obligations and the application of Financial Accounting Standards Codification No. 815—Derivatives and Hedging; plus or minus, as applicable,
(b) any net gain or loss resulting in such period from currency translation gains or losses related to currency remeasurements of Indebtedness (including any net loss or gain resulting from hedge agreements for currency exchange risk and revaluations of intercompany balances).
“EMU” means economic and monetary union as contemplated in the Treaty on European Union.
“Equity Interests” means Capital Stock and all warrants, options or other rights to acquire Capital Stock, but excluding any debt security that is convertible into, or exchangeable for, Capital Stock.
“Equity Offering” means any public or private sale of common stock or Preferred Stock of the Company (excluding Disqualified Stock) or any of its direct or indirect parent companies to the extent contributed to the Company as Equity (other than Disqualified Stock), other than:
(1) public offerings with respect to the Company’s or any direct or indirect parent company’s common stock registered on Form S-8;
(2) issuances to any Subsidiary of the Company; and
(3) any such public or private sale that constitutes an Excluded Contribution.
“€” means the single currency of participating member states of the EMU.
“Event of Loss” means, with respect to any property or asset (tangible or intangible, real or personal) constituting Collateral, any of the following:
(i) any loss, destruction or damage of such property or asset;
(ii) any institution of any proceeding for the condemnation or seizure of such property or asset or for the exercise of any right of eminent domain;
(iii) any actual condemnation, seizure or taking by exercise of the power of eminent domain or otherwise of such property or asset, or confiscation of such property or asset or the requisition of the use of such property or asset; or
(iv) any settlement in lieu of clauses (ii) or (iii) above.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Excluded Contribution” means net cash proceeds, marketable securities or Qualified Proceeds received by the Company from
(1) contributions to its common equity capital, and
205
Table of Contents
(2) the sale (other than to a Subsidiary of the Company or to any management equity plan or stock option plan or any other management or employee benefit plan or agreement of the Company) of Capital Stock (other than Disqualified Stock and Designated Preferred Stock) of the Company,
in each case after the Issue Date and in each case designated as Excluded Contributions pursuant to an Officer’s Certificate executed by the principal financial officer of the Company on the date such capital contributions are made or the date such Equity Interests are sold, as the case may be, which are excluded from the calculation set forth in clause (3) of the first paragraph under “Certain Covenants—Limitation on Restricted Payments.”
“Existing Senior Notes” means the (i) 10.875% Senior Notes due 2014 issued by the Issuers, pursuant to an indenture, dated November 20, 2007, among the Issuers, certain Subsidiaries of the Company, as guarantors, and The Bank of New York Mellon (formerly known as The Bank of New York), as trustee and the (ii) 7.75% Senior Notes due 2018 issued by the Issuers, pursuant to an indenture, dated April 4, 2011, among the Issuers, certain Subsidiaries of the Company, as guarantors, and The Bank of New York Mellon, as trustee.
“Existing Senior Subordinated Notes” means the 9.75% Senior Subordinated Notes due 2017 issued by the Issuers, pursuant to an indenture, dated October 18, 2010, among the Issuers, certain Subsidiaries of the Company, as guarantors, and The Bank of New York Mellon, as trustee.
“First Lien Documents” shall mean, collectively, the Credit Agreement and all agreements, documents and instruments at any time executed and/or delivered by the Issuers or any Guarantor or any other Person to, with or in favor of any First Lien Secured Party in connection therewith or related thereto, as all of the foregoing now exist or may hereafter be amended, modified, supplemented, extended, renewed, restated, refinanced, replaced or restructured (in whole or in part and including any agreements with, to or in favor of any other lender or group of lenders that at any time refinances, replaces or succeeds to all or any portion of the First Lien Obligations on the terms set forth in the Intercreditor Agreement).
“First Lien Lenders” shall mean, collectively, any Person party to the First Lien Documents as a lender (and including any other Person that at any time refinances, replaces or succeeds to all or any portion of the First Lien Obligations or is otherwise party to the First Lien Documents).
“First Lien Obligations” means all (x) obligations under the Credit Agreement and any other obligations which are secured by a Lien senior in priority to the Liens securing the Senior Secured Notes and the Guarantees, (y) Hedging Obligations owed to a Hedge Bank and (z) Cash Management Obligations.
“First Lien Secured Parties” means (a) with respect to the Credit Agreement, collectively, the Administrative Agent, the lenders and their Affiliates (including lenders and their Affiliates to whom Cash Management Obligations are owed), the Hedge Banks, the supplemental administrative agent and each co-agent or sub-agent appointed by the Administrative Agent from time to time and (b) with respect to any other First Lien Document, all lenders, holders or agents thereunder to which any First Lien Obligations are owing.
“Fixed Charge Coverage Ratio” means, with respect to any Person for any period, the ratio of EBITDA of such Person for such period to the Fixed Charges of such Person for such period. In the event that the Company or any Restricted Subsidiary incurs, assumes, guarantees, redeems, retires or extinguishes any Indebtedness (other than Indebtedness incurred under any revolving credit facility unless such Indebtedness has been permanently repaid and has not been replaced) or issues or redeems Disqualified Stock or Preferred Stock subsequent to the commencement of the period for which the Fixed Charge Coverage Ratio is being calculated but prior to or simultaneously with the event for which the calculation of the Fixed Charge Coverage Ratio is made (the “Fixed Charge Coverage Ratio Calculation Date”), then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect to such incurrence, assumption, guarantee, redemption, retirement or extinguishment of Indebtedness, or such issuance or redemption of Disqualified Stock or Preferred Stock, as if the same had occurred at the beginning of the applicable four-quarter period.
206
Table of Contents
For purposes of making the computation referred to above, Investments, acquisitions, dispositions, mergers, consolidations and disposed operations (as determined in accordance with GAAP) that have been made by the Company or any of its Restricted Subsidiaries during the four-quarter reference period or subsequent to such reference period and on or prior to or simultaneously with the Fixed Charge Coverage Ratio Calculation Date shall be calculated on a pro forma basis, assuming that all such Investments, acquisitions, dispositions, mergers, consolidations and disposed operations (and the change in any associated fixed charge obligations and the change in EBITDA resulting therefrom) had occurred on the first day of the four-quarter reference period. If since the beginning of such period any Person that subsequently became a Restricted Subsidiary or was merged with or into the Company or any of its Restricted Subsidiaries since the beginning of such period shall have made any Investment, acquisition, disposition, merger, consolidation or disposed operation that would have required adjustment pursuant to this definition, then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect thereto for such period as if such Investment, acquisition, disposition, merger, consolidation or disposed operation had occurred at the beginning of the applicable four-quarter period.
For purposes of this definition, whenever pro forma effect is to be given to a transaction, the pro forma calculations shall be made in good faith by a responsible financial or accounting officer of the Company. If any Indebtedness bears a floating rate of interest and is being given pro forma effect, the interest on such Indebtedness shall be calculated as if the rate in effect on the Fixed Charge Coverage Ratio Calculation Date had been the applicable rate for the entire period (taking into account any Hedging Obligations applicable to such Indebtedness). Interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by a responsible financial or accounting officer of the Company to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP. For purposes of making the computation referred to above, interest on any Indebtedness under a revolving credit facility computed on a pro forma basis shall be computed based upon the average daily balance of such Indebtedness during the applicable period except as set forth in the first paragraph of this definition. Interest on Indebtedness that may optionally be determined at an interest rate based upon a factor of a prime or similar rate, a eurocurrency interbank offered rate, or other rate, shall be deemed to have been based upon the rate actually chosen, or, if none, then based upon such optional rate chosen as the Company may designate.
“Fixed Charges” means, with respect to any Person for any period, the sum of:
(1) Consolidated Interest Expense of such Person for such period;
(2) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Preferred Stock of any Restricted Subsidiary during such period; and
(3) all dividends or other distributions accrued (excluding items eliminated in consolidation) on any series of Disqualified Stock during such period.
“Foreign Subsidiary” means, with respect to any Person, any Restricted Subsidiary of such Person that is not organized or existing under the laws of the United States, any state thereof, or the District of Columbia and any Restricted Subsidiary of such Foreign Subsidiary.
“GAAP” means generally accepted accounting principles in the United States which are in effect on the Issue Date.
“Government Securities” means securities that are:
(1) direct obligations of the United States of America for the timely payment of which its full faith and credit is pledged; or
(2) obligations of a Person controlled or supervised by and acting as an agency or instrumentality of the United States of America the timely payment of which is unconditionally guaranteed as a full faith and credit obligation by the United States of America,
207
Table of Contents
which, in either case, are not callable or redeemable at the option of the issuers thereof, and shall also include a depository receipt issued by a bank (as defined in Section 3(a)(2) of the Securities Act), as custodian with respect to any such Government Securities or a specific payment of principal of or interest on any such Government Securities held by such custodian for the account of the holder of such depository receipt; provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the Government Securities or the specific payment of principal of or interest on the Government Securities evidenced by such depository receipt.
“guarantee ” means a guarantee (other than by endorsement of negotiable instruments for collection in the ordinary course of business), direct or indirect, in any manner (including letters of credit and reimbursement agreements in respect thereof), of all or any part of any Indebtedness or other obligations.
“Guarantee” means the guarantee by any Guarantor of the Issuers’ Obligations under the Senior Secured Notes Indenture.
“Guarantor ” means, each Restricted Subsidiary that Guarantees the Senior Secured Notes in accordance with the terms of the Senior Secured Notes Indenture and its successors and assigns, until released from its obligations under its Guarantee in accordance with the terms of the Senior Secured Notes Indenture.
“Hedge Bank” means any Person that is a revolving credit lender under the Credit Agreement or an Affiliate (determined as of the date of entry into the Secured Hedge Agreement) of a revolving credit lender under the Credit Agreement, in its capacity as a party to a Secured Hedge Agreement.
“Hedging Obligations” means, with respect to any Person, the obligations of such Person under any interest rate swap agreement, interest rate cap agreement, interest rate collar agreement, commodity swap agreement, commodity cap agreement, commodity collar agreement, foreign exchange contract, currency swap agreement or similar agreement providing for the transfer or mitigation of interest rate or currency risks either generally or under specific contingencies.
“Holder” means the Person in whose name a Secured Note is registered on the registrar’s books.
“Indebtedness” means, with respect to any Person, without duplication:
(1) any indebtedness of such Person, whether or not contingent:
(a) in respect of borrowed money;
(b) evidenced by bonds, notes, debentures or similar instruments or letters of credit or bankers’ acceptances (or, without duplication, reimbursement agreements in respect thereof);
(c) representing the balance deferred and unpaid of the purchase price of any property (including Capitalized Lease Obligations), except (i) any such balance that constitutes a trade payable or similar obligation to a trade creditor, in each case accrued in the ordinary course of business and (ii) any earn-out obligations until, after 30 days of becoming due and payable, has not been paid and such obligation becomes a liability on the balance sheet of such Person in accordance with GAAP; or
(d) representing any Hedging Obligations; or
(e) during a Suspension Period only, obligations of the lessee for rental payments in respect of Sale and Lease-Back Transactions in an amount equal to the present value of such obligations during the remaining term of the lease using a discount rate equal to the rate of interest implicit in such transaction determined in accordance with GAAP.
208
Table of Contents
if and to the extent that any of the foregoing Indebtedness (other than letters of credit and Hedging Obligations) would appear as a liability upon a balance sheet (excluding the footnotes thereto) of such Person prepared in accordance with GAAP;
(2) to the extent not otherwise included, any obligation by such Person to be liable for, or to pay, as obligor, guarantor or otherwise on, the obligations of the type referred to in clause (1) of a third Person (whether or not such items would appear upon the balance sheet of the such obligor or guarantor), other than by endorsement of negotiable instruments for collection in the ordinary course of business; and
(3) to the extent not otherwise included, the obligations of the type referred to in clause (1) of a third Person secured by a Lien on any asset owned by such first Person, whether or not such Indebtedness is assumed by such first Person;
provided, however, that notwithstanding the foregoing, Indebtedness shall be deemed not to include (a) Contingent Obligations incurred in the ordinary course of business or (b) obligations under or in respect of Receivables Facilities.
“Independent Financial Advisor” means an accounting, appraisal, investment banking firm or consultant to Persons engaged in Similar Businesses of nationally recognized standing that is, in the good faith judgment of the Company, qualified to perform the task for which it has been engaged.
“Initial Purchasers” means Credit Suisse Securities (USA) LLC, Goldman, Sachs & Co., UBS Securities LLC, Wells Fargo Securities, LLC, RBC Capital Markets, LLC, Macquarie Capital (USA) Inc. and Natixis Securities Americas LLC.
“Investment Grade Rating” means a rating equal to or higher than Baa3 (or the equivalent) by Moody’s and BBB- (or the equivalent) by S&P, or an equivalent rating by any other Rating Agency.
“Investment Grade Securities” means:
(1) securities issued or directly and fully guaranteed or insured by the United States government or any agency or instrumentality thereof (other than Cash Equivalents);
(2) debt securities or debt instruments with an Investment Grade Rating, but excluding any debt securities or instruments constituting loans or advances among the Company and its Subsidiaries;
(3) investments in any fund that invests exclusively in investments of the type described in clauses (1) and (2) which fund may also hold immaterial amounts of cash pending investment or distribution; and
(4) corresponding instruments in countries other than the United States customarily utilized for high quality investments.
“Investments” means, with respect to any Person, all investments by such Person in other Persons (including Affiliates) in the form of loans (including guarantees), advances or capital contributions (excluding accounts receivable, trade credit, advances to customers, commission, travel and similar advances to officers and employees, in each case made in the ordinary course of business), purchases or other acquisitions for consideration of Indebtedness, Equity Interests or other securities issued by any other Person and investments that are required by GAAP to be classified on the balance sheet (excluding the footnotes) of the Company in the same manner as the other investments included in this definition to the extent such transactions involve the transfer of cash or other property. For purposes of the definition of “Unrestricted Subsidiary” and the covenant described under “Certain Covenants—Limitation on Restricted Payments”:
(1) “Investments” shall include the portion (proportionate to the Company’s equity interest in such Subsidiary) of the fair market value of the net assets of a Subsidiary of the Company at the time that such Subsidiary is designated an Unrestricted Subsidiary; provided, however, that upon a redesignation of such
209
Table of Contents
Subsidiary as a Restricted Subsidiary, the Company shall be deemed to continue to have a permanent “Investment” in an Unrestricted Subsidiary in an amount (if positive) equal to:
(a) the Company “Investment” in such Subsidiary at the time of such redesignation; less
(b) the portion (proportionate to the Company’s equity interest in such Subsidiary) of the fair market value of the net assets of such Subsidiary at the time of such redesignation; and
(2) any property transferred to or from an Unrestricted Subsidiary shall be valued at its fair market value at the time of such transfer, in each case as determined in good faith by the Company.
“Investors ” means The Blackstone Group and each of its Affiliates, but not including any of its portfolio companies.
“Issue Date” means, solely for purposes of this “Description of Senior Secured Notes,” (i) March 20, 2012, in respect of the $230.0 million offering of Senior Secured Notes thereon, and (ii) October 1, 2012, in respect of the $100.0 million offering of Senior Secured Notes thereon, as applicable.
“Legal Holiday” means a Saturday, a Sunday or a day on which commercial banking institutions are not required to be open in the State of New York.
“Lien” means, with respect to any asset, any mortgage, lien (statutory or otherwise), pledge, hypothecation, charge, security interest, preference, priority or encumbrance of any kind in respect of such asset, whether or not filed, recorded or otherwise perfected under applicable law, including any conditional sale or other title retention agreement, any lease in the nature thereof, any option or other agreement to sell or give a security interest in and any filing of or agreement to give any financing statement under the Uniform Commercial Code (or equivalent statutes) of any jurisdiction; provided that in no event shall an operating lease be deemed to constitute a Lien.
“Moody’s” means Moody’s Investors Service, Inc. and any successor to its rating agency business.
“Net Income” means, with respect to any Person, the net income (loss) of such Person, determined in accordance with GAAP and before any reduction in respect of Preferred Stock dividends.
“Net Loss Proceeds” means the aggregate cash proceeds received by the Issuers or any Guarantor in respect of any Event of Loss, including insurance proceeds, condemnation awards or damages awarded by any judgment, net of (i) the direct cost in recovery of such Net Loss Proceeds (including legal, accounting, appraisal and insurance adjuster fees and any relocation expenses incurred as a result thereof), (ii) amounts required to be applied to the repayment of Indebtedness secured by any Permitted Lien on the asset or assets that were the subject of such Event of Loss (other than any such Permitted Lien that does not rank prior to the Second Priority Liens), and (iii) any taxes paid or payable as a result thereof.
“Net Proceeds” means the aggregate cash proceeds received by the Company or any of its Restricted Subsidiaries in respect of any Asset Sale, including any cash received upon the sale or other disposition of any Designated Non-cash Consideration received in any Asset Sale, net of (i) the direct costs relating to such Asset Sale and the sale or disposition of such Designated Non-cash Consideration, including legal, accounting and investment banking fees, and brokerage and sales commissions, (ii) any relocation expenses incurred as a result thereof, (iii) taxes paid or payable as a result thereof (after taking into account any available tax credits or deductions and any tax sharing arrangements), (iv) to the extent such Asset Sale does not involve Collateral, amounts required to be applied to the repayment of principal, premium, if any, and interest on Senior Indebtedness required (other than required by clause (1) of the second paragraph of “Repurchase at the Option of Holders—Asset Sales”) to be paid as a result of such transaction and (v) any deduction of appropriate amounts to be provided by the Company or any of its Restricted Subsidiaries as a reserve in accordance with GAAP against any liabilities associated with the asset disposed of in such transaction and retained by the Company or any of its Restricted Subsidiaries after such sale or other disposition thereof, including pension and other post-employment benefit liabilities and liabilities related to environmental matters or against any indemnification obligations associated with such transaction.
210
Table of Contents
“Obligations” means any principal, interest (including any interest accruing subsequent to the filing of a petition in bankruptcy, reorganization or similar proceeding at the rate provided for in the documentation with respect thereto, whether or not such interest is an allowed claim under applicable state, federal or foreign law), penalties, fees, indemnifications, reimbursements (including reimbursement obligations with respect to letters of credit and banker’s acceptances), damages and other liabilities, and guarantees of payment of such principal, interest, penalties, fees, indemnifications, reimbursements, damages and other liabilities, payable under the documentation governing any Indebtedness.
“Officer” means the Chairman of the Board, the Chief Executive Officer, Chief Financial Officer, Chief Operating Officer, the President, any Executive Vice President, Senior Vice President or Vice President, the Treasurer or the Secretary of the applicable Issuer or Guarantor.
“Officer’s Certificate” means a certificate signed on behalf of an Issuer by an Officer of such Issuer or on behalf of a Guarantor by an Officer of such Guarantor, who must be the principal executive officer, the principal financial officer, the treasurer or the principal accounting officer of such Issuer or Guarantor, as applicable, that meets the requirements set forth in the Senior Secured Notes Indenture.
“Opinion of Counsel” means a written opinion from legal counsel who is acceptable to the Senior Secured Notes Trustee. The counsel may be an employee of or counsel to the Company, a Subsidiary of the Company or the Senior Secured Notes Trustee.
“Permitted Additional Pari Passu Obligations” means Additional Notes issued pursuant to the Senior Secured Notes Indenture or other Indebtedness, in each case, that are secured by Second Priority Liens; provided that (i) the representative of such Permitted Additional Pari Passu Obligation executes a joinder agreement to the Security Agreement and the Intercreditor Agreement, in each case in the form attached thereto, agreeing to be bound thereby and (ii) the Company has designated such Indebtedness as “Second Lien Debt” under the Security Agreement and the Intercreditor Agreement.
“Permitted Asset Swap” means the concurrent purchase and sale or exchange of Related Business Assets or a combination of Related Business Assets and cash or Cash Equivalents between the Company or any of its Restricted Subsidiaries and another Person; provided, that any cash or Cash Equivalents received must be applied in accordance with the “Repurchase at the Option of Holders—Asset Sales” covenant.
“Permitted Holders” means each of the Investors and members of management of the Company (or its direct parent) on the Issue Date who are holders of Equity Interests of the Company (or any of its direct or indirect parent companies) and any group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members; provided that, in the case of such group and without giving effect to the existence of such group or any other group, such Investors and members of management, collectively have beneficial ownership of more than 50% of the total voting power of the Voting Stock of the Company or any of its direct or indirect parent companies. Any Person or group whose acquisition of beneficial ownership constitutes a Change of Control in respect of which a Change of Control Offer is made in accordance with the requirements of the Senior Secured Notes Indenture will thereafter, together with its Affiliates, constitute an additional Permitted Holder.
“Permitted Investment” means:
(1) any Investment in the Company or any of its Restricted Subsidiaries;
(2) any Investment in cash and Cash Equivalents or Investment Grade Securities;
(3) any Investment by the Company or any of its Restricted Subsidiaries in a Person that is engaged in a Similar Business if as a result of such Investment:
(a) such Person becomes a Restricted Subsidiary; or
211
Table of Contents
(b) such Person, in one transaction or a series of related transactions, is merged or consolidated with or into, or transfers or conveys substantially all of its assets to, or is liquidated into, the Company or a Restricted Subsidiary,
and, in each case, any Investment held by such Person; provided, that such Investment was not acquired by such Person in contemplation of such acquisition, merger, consolidation or transfer;
(4) any Investment in securities or other assets, including earnouts, not constituting cash and Cash Equivalents and received in connection with an Asset Sale made pursuant to the provisions of “Repurchase at the Option of Holders—Asset Sales” or any other disposition of assets not constituting an Asset Sale;
(5) any Investment existing on the Issue Date;
(6) any Investment acquired by the Company or any of its Restricted Subsidiaries:
(a) in exchange for any other Investment or accounts receivable held by the Company or any such Restricted Subsidiary in connection with or as a result of a bankruptcy, workout, reorganization or recapitalization of the issuer of such other Investment or accounts receivable; or
(b) as a result of a foreclosure by the Company or any of its Restricted Subsidiaries with respect to any secured Investment or other transfer of title with respect to any secured Investment in default;
(7) Hedging Obligations permitted under clause (10) of the covenant described in “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(8) any Investment in a Similar Business having an aggregate fair market value, taken together with all other Investments made pursuant to this clause (8) that are at that time outstanding, not to exceed 2.5% of Total Assets at the time of such Investment (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
(9) Investments the payment for which consists of Equity Interests (exclusive of Disqualified Stock) of the Company, or any of its direct or indirect parent companies; provided, however, that such Equity Interests will not increase the amount available for Restricted Payments under clause (3) of the first paragraph under the covenant described in “Certain Covenants—Limitations on Restricted Payments”;
(10) guarantees of Indebtedness of the Company and any Restricted Subsidiary permitted under the covenant described in “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(11) any transaction to the extent it constitutes an Investment that is permitted and made in accordance with the provisions of the second paragraph of the covenant described under “Certain Covenants—Transactions with Affiliates” (except transactions described in clauses (2), (5) and (9) of such paragraph);
(12) Investments consisting of purchases and acquisitions of inventory, supplies, material or equipment;
(13) additional Investments having an aggregate fair market value, taken together with all other Investments made pursuant to this clause (13) that are at that time outstanding (without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities), not to exceed 3.5% of Total Assets at the time of such Investment (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
(14) Investments relating to a Receivables Subsidiary that, in the good faith determination of the Company are necessary or advisable to effect any Receivables Facility;
(15) advances to, or guarantees of Indebtedness of, employees not in excess of $10.0 million outstanding at any one time, in the aggregate;
212
Table of Contents
(16) loans and advances to officers, directors and employees for business-related travel expenses, moving expenses and other similar expenses, in each case incurred in the ordinary course of business or consistent with past practices or to fund such Person’s purchase of Equity Interests of the Company or any direct or indirect parent company thereof; and
(17) loans and advances to independent sales persons against commissions not in excess of $15.0 million outstanding at any one time, in the aggregate.
“Permitted Liens” means, with respect to any Person (and, in each case, including but not limited to Liens on Collateral):
(1) pledges or deposits by such Person under workmen’s compensation laws, unemployment insurance laws or similar legislation, or good faith deposits in connection with bids, tenders, contracts (other than for the payment of Indebtedness) or leases to which such Person is a party, or deposits to secure public or statutory obligations of such Person or deposits of cash or U.S. government bonds to secure surety or appeal bonds to which such Person is a party, or deposits as security for contested taxes or import duties or for the payment of rent, in each case incurred in the ordinary course of business;
(2) Liens imposed by law, such as carriers’, warehousemen’s and mechanics’ Liens, in each case for sums not yet overdue for a period of more than 30 days or being contested in good faith by appropriate proceedings or other Liens arising out of judgments or awards against such Person with respect to which such Person shall then be proceeding with an appeal or other proceedings for review if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(3) Liens for taxes, assessments or other governmental charges not yet overdue for a period of more than 30 days or payable or subject to penalties for nonpayment or which are being contested in good faith by appropriate proceedings diligently conducted, if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(4) Liens in favor of issuers of performance and surety bonds or bid bonds or with respect to other regulatory requirements or letters of credit issued pursuant to the request of and for the account of such Person in the ordinary course of its business;
(5) minor survey exceptions, minor encumbrances, easements or reservations of, or rights of others for, licenses, rights-of-way, sewers, electric lines, telegraph and telephone lines and other similar purposes, or zoning or other restrictions as to the use of real properties or Liens incidental, to the conduct of the business of such Person or to the ownership of its properties which were not incurred in connection with Indebtedness and which do not in the aggregate materially adversely affect the value of said properties or materially impair their use in the operation of the business of such Person;
(6) Liens securing Indebtedness permitted to be incurred pursuant to clause (4) of the second paragraph under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” (including, during any Suspension Period, Indebtedness of the type and in the amounts specified under such clause);
(7) Liens existing on the Issue Date;
(8) Liens on property or shares of stock of a Person at the time such Person becomes a Subsidiary; provided, however, such Liens are not created or incurred in connection with, or in contemplation of, such other Person becoming such a Subsidiary; provided further, however, that such Liens may not extend to any other property owned by the Company or any of its Restricted Subsidiaries;
(9) Liens on property at the time the Company or a Restricted Subsidiary acquired the property, including any acquisition by means of a merger or consolidation with or into the Company or any of its Restricted Subsidiaries; provided, however, that such Liens are not created or incurred in connection with, or in contemplation of, such acquisition; provided further, however, that the Liens may not extend to any other property owned by the Company or any of its Restricted Subsidiaries;
213
Table of Contents
(10) Liens securing Indebtedness or other obligations of a Restricted Subsidiary owing to the Company or another Restricted Subsidiary permitted to be incurred in accordance with the covenant described under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(11) customary Liens securing Hedging Obligations entered into in the ordinary course of business by the Issuer or its Restricted Subsidiaries;
(12) Liens on specific items of inventory of other goods and proceeds of any Person securing such Person’s obligations in respect of bankers’ acceptances issued or created for the account of such Person to facilitate the purchase, shipment or storage of such inventory or other goods;
(13) leases, subleases, licenses or sublicenses granted to others in the ordinary course of business which do not materially interfere with the ordinary conduct of the business of the Company or any of its Restricted Subsidiaries and do not secure any Indebtedness;
(14) Liens arising from Uniform Commercial Code financing statement filings regarding operating leases entered into by the Company and its Restricted Subsidiaries in the ordinary course of business;
(15) Liens in favor of the Company, the Co-Issuer or any Guarantor;
(16) Liens on equipment of the Company or any of its Restricted Subsidiaries granted in the ordinary course of business to the Company’s clients;
(17) Liens on accounts receivable and related assets incurred in connection with a Receivables Facility;
(18) Liens to secure any refinancing, refunding, extension, renewal or replacement (or successive refinancing, refunding, extensions, renewals or replacements) as a whole, or in part, of any Indebtedness secured by any Lien referred to in the foregoing clauses (7), (8) and (9); provided, however, that (a) such new Lien shall be limited to all or part of the same property that secured the original Lien (plus improvements on such property), and (b) the Indebtedness secured by such Lien at such time is not increased to any amount greater than the sum of (i) the outstanding principal amount or, if greater, committed amount of the Indebtedness described under clauses (7), (8) and (9) at the time the original Lien became a Permitted Lien under the Senior Secured Notes Indenture, and (ii) an amount necessary to pay any fees and expenses, including premiums, related to such refinancing, refunding, extension, renewal or replacement;
(19) deposits made in the ordinary course of business to secure liability to insurance carriers;
(20) other Liens securing obligations incurred in the ordinary course of business which obligations do not exceed $65.0 million at any one time outstanding;
(21) Liens securing Indebtedness of any Foreign Subsidiary permitted to be incurred under the Senior Secured Notes Indenture, to the extent such Liens relate only to the assets and properties of such Foreign Subsidiary;
(22) Liens securing judgments for the payment of money not constituting an Event of Default under clause (5) under the caption “Events of Default and Remedies” so long as such Liens are adequately bonded and any appropriate legal proceedings that may have been duly initiated for the review of such judgment have not been finally terminated or the period within which such proceedings may be initiated has not expired;
(23) Liens in favor of customs and revenue authorities arising as a matter of law to secure payment of customs duties in connection with the importation of goods in the ordinary course of business;
(24) Liens (i) of a collection bank arising under Section 4-210 of the Uniform Commercial Code or any comparable or successor provision on items in the course of collection, (ii) attaching to commodity trading accounts or other commodity brokerage accounts incurred in the ordinary course of business, and (iii) in favor of banking institutions arising as a matter of law encumbering deposits (including the right of set-off) and which are within the general parameters customary in the banking industry;
214
Table of Contents
(25) Liens deemed to exist in connection with Investments in repurchase agreements permitted under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that such Liens do not extend to any assets other than those that are the subject of such repurchase agreement;
(26) Liens encumbering reasonable customary initial deposits and margin deposits and similar Liens attaching to commodity trading accounts or other brokerage accounts incurred in the ordinary course of business and not for speculative purposes;
(27) Liens that are contractual rights of set-off (i) relating to the establishment of depository relations with banks not given in connection with the issuance of Indebtedness, (ii) relating to pooled deposit or sweep accounts of the Company or any of its Restricted Subsidiaries to permit satisfaction of overdraft or similar obligations incurred in the ordinary course of business of the Company and its Restricted Subsidiaries or (iii) relating to purchase orders and other agreements entered into with customers of the Company or any of its Restricted Subsidiaries in the ordinary course of business;
(28) during a Suspension Period only, Liens securing Indebtedness (other than Indebtedness that is secured equally and ratably with (or on a basis subordinated to) the Senior Secured Notes), including Indebtedness represented by Sale and Lease-Back Transactions, in an amount not to exceed 5.0% of Total Assets at any one time outstanding;
(29) Liens on Collateral securing any Indebtedness incurred pursuant to the covenant described under “Certain Covenants—Limitations on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that such Liens on Collateral described in this clause (29) are junior in priority to the Liens securing the Senior Secured Notes and the Guarantees; and
(30) Liens on the Collateral granted under the Security Documents in favor of the Second Lien Agent to secure the Senior Secured Notes and the Guarantees.
For purposes of this definition, the term “Indebtedness” shall be deemed to include interest on such Indebtedness.
“Person” means any individual, corporation, limited liability company, partnership, joint venture, association, joint stock company, trust, unincorporated organization, government or any agency or political subdivision thereof or any other entity.
“Preferred Stock” means any Equity Interest with preferential rights of payment of dividends or upon liquidation, dissolution, or winding up.
“Qualified Proceeds” means assets that are used or useful in, or Capital Stock of any Person engaged in, a Similar Business; provided that the fair market value of any such assets or Capital Stock shall be determined by the Company in good faith.
“Rating Agencies” means Moody’s and S&P or if Moody’s or S&P or both shall not make a rating on the Senior Secured Notes publicly available, a nationally recognized statistical rating agency or agencies, as the case may be, selected by the Issuers which shall be substituted for Moody’s or S&P or both, as the case may be.
“Receivables Facility” means any of one or more receivables financing facilities as amended, supplemented, modified, extended, renewed, restated or refunded from time to time, the Obligations of which are non-recourse (except for customary representations, warranties, covenants and indemnities made in connection with such facilities) to the Company or any of its Restricted Subsidiaries (other than a Receivables Subsidiary) pursuant to which the Company or any of its Restricted Subsidiaries sells its accounts receivable to either (a) a Person that is not a Restricted Subsidiary or (b) a Receivables Subsidiary that in turn sells its accounts receivable to a Person that is not a Restricted Subsidiary.
215
Table of Contents
“Receivables Fees” means distributions or payments made directly or by means of discounts with respect to any accounts receivable or participation interest therein issued or sold in connection with, and other fees paid to a Person that is not a Restricted Subsidiary in connection with, any Receivables Facility.
“Receivables Subsidiary” means any Subsidiary formed for the purpose of, and that solely engages only in one or more Receivables Facilities and other activities reasonably related thereto.
“Related Business Assets” means assets (other than cash or Cash Equivalents) used or useful in a Similar Business, provided that any assets received by the Company or a Restricted Subsidiary in exchange for assets transferred by the Company or a Restricted Subsidiary shall not be deemed to be Related Business Assets if they consist of securities of a Person, unless upon receipt of the securities of such Person, such Person would become a Restricted Subsidiary.
“Restricted Investment ” means an Investment other than a Permitted Investment.
“Restricted Subsidiary ” means, at any time, any direct or indirect Subsidiary of the Company (including the Co-Issuer and any Foreign Subsidiary) that is not then an Unrestricted Subsidiary; provided, however, that upon the occurrence of an Unrestricted Subsidiary ceasing to be an Unrestricted Subsidiary, such Subsidiary shall be included in the definition of “Restricted Subsidiary.”
“S&P” means Standard & Poor’s Rating Services and any successor to its rating agency business.
“Sale and Lease-Back Transaction” means any arrangement providing for the leasing by the Company or any of its Restricted Subsidiaries of any real or tangible personal property, which property has been or is to be sold or transferred by the Company or such Restricted Subsidiary to a third Person in contemplation of such leasing.
“SEC” means the U.S. Securities and Exchange Commission.
“Second Lien Obligations” means the Indebtedness incurred and Obligations under the Senior Secured Notes Indenture and any Permitted Additional Pari Passu Obligations.
“Secured Hedge Agreement” has the meaning given to it in the Credit Agreement.
“Secured Indebtedness” means any Indebtedness of the Company or any of its Restricted Subsidiaries secured by a Lien.
“Securities Act ”means the Securities Act of 1933, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Security Agreement” means the security agreement to be dated as of the Issue Date among the Second Lien Agent, the Issuers and the Guarantors granting, among other things, a Second Priority Lien on the Collateral, subject to Permitted Liens, in each case in favor of the Second Lien Agent for its benefit and for the benefit of the Senior Secured Notes Trustee and the Holders of the Senior Secured Notes and the holders of any Permitted Additional Pari Passu Obligations, as amended, modified, restated, supplemented or replaced from time to time in accordance with its terms.
“Security Documents” means the Intercreditor Agreement and the agreements pursuant to which security interests in the Collateral are granted to secure the Senior Secured Notes and the Guarantees from time to time, including the Security Agreement.
“Senior Credit Facilities” means the Credit Facility under the Credit Agreement.
216
Table of Contents
“Senior Indebtedness” means:
(1) all Indebtedness of the Issuers or any Guarantor outstanding under the Senior Credit Facilities (including interest accruing on or after the filing of any petition in bankruptcy or similar proceeding or for reorganization of the Issuers or any Guarantor (at the rate provided for in the documentation with respect thereto, regardless of whether or not a claim for post-filing interest is allowed in such proceedings), and any and all other fees, expense reimbursement obligations, indemnification amounts, penalties, and other amounts (whether existing on the Issue Date or thereafter created or incurred) and all obligations of the Issuers or any Guarantor to reimburse any bank or other Person in respect of amounts paid under letters of credit, acceptances or other similar instruments;
(2) all Hedging Obligations (and guarantees thereof) owing to a Lender (as defined in the Senior Credit Facilities) or any Affiliate of such Lender (or any Person that was a Lender or an Affiliate of such Lender at the time the applicable agreement giving rise to such Hedging Obligation was entered into), provided that such Hedging Obligations are permitted to be incurred under the terms of the Senior Secured Notes Indenture;
(3) any other Indebtedness of the Issuers or any Guarantor permitted to be incurred under the terms of the Senior Secured Notes Indenture, unless the instrument under which such Indebtedness is incurred expressly provides that it is subordinate in right of payment to the Senior Secured Notes or any related Guarantee; and
(4) all Obligations with respect to the items listed in the preceding clauses (1), (2) and (3); provided, however, that Senior Indebtedness shall not include:
(a) any obligation of such Person to the Issuers or any of their Subsidiaries;
(b) any liability for federal, state, local or other taxes owed or owing by such Person;
(c) any accounts payable or other liability to trade creditors arising in the ordinary course of business;
(d) any Indebtedness or other Obligation of such Person which is subordinate or junior in right of payment to any other Indebtedness or other Obligation of such Person; or
(e) that portion of any Indebtedness which at the time of incurrence is incurred in violation of the Senior Secured Notes Indenture; provided, however, that such Indebtedness shall be deemed not to have been incurred in violation of the Senior Secured Notes Indenture for purposes of this clause if such Indebtedness consists of Senior Credit Facilities, and the holder(s) of such Indebtedness or their agent or representative (i) had no actual knowledge at the time of incurrence that the incurrence of such Indebtedness violated the Senior Secured Notes Indenture and (ii) shall have received a certificate from an Officer of the Company to the effect that the incurrence of such Indebtedness does not violate the provisions of the Senior Secured Notes Indenture.
“Senior Secured Notes Registration Rights Agreement” means, as applicable, (i) the Registration Rights Agreement with respect to the Senior Secured Notes dated as of March 20, 2012, among the Issuers, the Guarantors and Credit Suisse Securities (USA) LLC, Goldman, Sachs & Co., UBS Securities LLC, Wells Fargo Securities, LLC, Macquarie Capital (USA) Inc., RBC Capital Markets, LLC and Natixis Securities Americas LLC or (ii) the Registration Rights Agreement with respect to the Senior Secured Notes dated as of October 1, 2012, among the Issuers, the Guarantors and Credit Suisse Securities (USA) LLC, Goldman, Sachs & Co., UBS Securities LLC, Wells Fargo Securities, LLC, Macquarie Capital (USA) Inc., RBC Capital Markets, LLC and Natixis Securities Americas LLC.
“Significant Subsidiary” means (i) the Co-Issuer and (ii) any Restricted Subsidiary that would be a “significant subsidiary” as defined in Article 1, Rule 1-02 of Regulation S-X, promulgated pursuant to the Securities Act, as such regulation is in effect on the Issue Date.
217
Table of Contents
“Similar Business” means any business conducted or proposed to be conducted by the Company and its Restricted Subsidiaries on the Issue Date or any business that is similar, reasonably related, incidental or ancillary thereto.
“Sponsor Management Agreement” means the management agreement between certain of the management companies associated with the Investors and the Company and/or one of its direct or indirect parent companies as in effect on the Issue Date.
“Subordinated Indebtedness” means, with respect to the Senior Secured Notes,
(1) any Indebtedness of the Issuers which is by its terms subordinated in right of payment to the Senior Secured Notes, and
(2) any Indebtedness of any Guarantor which is by its terms subordinated in right of payment to the Guarantee of such entity of the Senior Secured Notes.
“Subsidiary” means, with respect to any Person:
(1) any corporation, association or other business entity (other than a partnership, joint venture, limited liability company or similar entity) of which more than 50% of the total voting power of shares of Capital Stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time of determination on owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof or is consolidated under GAAP with such Person at such time; and
(2) any partnership, joint venture, limited liability company or similar entity of which
(a) more than 50% of the capital accounts, distribution rights, total equity and voting interests or general or limited partnership interests, as applicable, are owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof whether in the form of membership, general, special or limited partnership or otherwise, and
(b) such Person or any Restricted Subsidiary of such Person is a general partner or otherwise controls such entity.
“Total Assets” means the total assets of the Company, except where expressly provided otherwise, and its Restricted Subsidiaries on a consolidated basis, as shown on the most recent balance sheet of such other Person.
“Transactions” means the acquisition contemplated by the Transaction Agreement and related financings.
“Transaction Agreement” means the Equity Interest Purchase Agreement, dated as of March 14, 2011, by and among Rikco International, LLC d/b/a Dr. Comfort, Rikco Holding Corporation, Merit Mezzanine Fund IV, L.P., Merit Mezzanine Parallel Fund IV, L.P., the members of Ricko International, LLC parties thereto and DJO LLC, as the same may be amended prior to the Issue Date.
“Treasury Rate” means, as of any Redemption Date, the yield to maturity as of such Redemption Date of United States Treasury securities with a constant maturity (as compiled and, published in the most recent Federal Reserve Statistical Release H.15 (519) that has become publicly available at least two Business Days prior to the Redemption Date (or, if such Statistical Release is no longer published, any publicly available source of similar market data)) most nearly equal to the period from the Redemption Date to March 15, 2015; provided, however, that if the period from the Redemption Date to March 15, 2015 is less than one year, the weekly average yield on actually traded United States Treasury securities adjusted to a constant maturity of one year are used.
“Trust Indenture Act” means the Trust Indenture Act of 1939, as amended (15 U.S.C. §§ 77aaa-777bbbb).
218
Table of Contents
“Unrestricted Subsidiary” means:
(1) any Subsidiary of the Company which at the time of determination is an Unrestricted Subsidiary (as designated by the Company, as provided below); and
(2) any Subsidiary of an Unrestricted Subsidiary.
The Company may designate any Subsidiary of the Company, other than the Co-Issuer (including any existing Subsidiary and any newly acquired or newly formed Subsidiary) to be an Unrestricted Subsidiary unless such Subsidiary or any of its Subsidiaries owns any Equity Interests or Indebtedness of, or owns or holds any Lien on any property of, the Company or any Subsidiary of the Company (other than solely any Subsidiary of the Subsidiary to be so designated); provided that
(1) any Unrestricted Subsidiary must be an entity of which the Equity Interests entitled to cast at least a majority of the votes that may be cast by all Equity Interests having ordinary voting power for the election of directors or Persons performing a similar function are owned, directly or indirectly, by the Company;
(2) such designation complies with the covenants described under “Certain Covenants—Limitation on Restricted Payments”; and
(3) each of:
(a) the Subsidiary to be so designated; and
(b) its Subsidiaries has not at the time of designation, and does not thereafter, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable with respect to any Indebtedness pursuant to which the lender has recourse to any of the assets of the Company or any Restricted Subsidiary.
The Company may designate any Unrestricted Subsidiary to be a Restricted Subsidiary; provided that, immediately after giving effect to such designation, no Default shall have occurred and be continuing and either:
(1) the Company could incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test described in the first paragraph under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; or
(2) the Fixed Charge Coverage Ratio for the Company and its Restricted Subsidiaries would be greater than such ratio for the Company and its Restricted Subsidiaries immediately prior to such designation, in each case on a pro forma basis taking into account such designation.
Any such designation by the Company shall be notified by the Company to the Senior Secured Notes Trustee by promptly filing with the Senior Secured Notes Trustee a copy of the resolution of the board of directors of the Company or any committee thereof giving effect to such designation and an Officer’s Certificate certifying that such designation complied with the foregoing provisions.
“Voting Stock” of any Person as of any date means the Capital Stock of such Person that is at the time entitled to vote in the election of the board of directors of such Person.
“Weighted Average Life to Maturity” means, when applied to any Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, at any date, the quotient obtained by dividing:
(1) the sum of the products of the number of years from the date of determination to the date of each successive scheduled principal payment of such Indebtedness or redemption or similar payment with respect to such Disqualified Stock or Preferred Stock multiplied by the amount of such payment; by
(2) the sum of all such payments.
“Wholly-Owned Subsidiary” of any Person means a Subsidiary of such Person, 100% of the outstanding Equity Interests of which (other than directors’ qualifying shares) shall at the time be owned by such Person or by one or more Wholly-Owned Subsidiaries of such Person.
219
Table of Contents
General
Certain terms used in this description are defined under the subheading “Certain Definitions.” In this description, (a) the terms “we,” “our,” “us,” and “Company” refer only to DJO Finance LLC and not any of its Affiliates, (b) the terms “DJO Finance Corp.” and “Co-Issuer” refer only to DJO Finance Corporation and not any of its Affiliates and (c) the term “Issuers” refers to the Company and the Co-Issuer.
On October 1, 2012, the Issuers issued $440.0 million in aggregate principal amount of 9.875% senior notes due 2018 (the “Senior Notes”) under an indenture dated as of October 1, 2012 (the “Senior Notes Indenture”) among the Issuers, the Guarantors and The Bank of New York Mellon, a New York banking corporation, as trustee (the “Senior Notes Trustee”).
The following description is only a summary of the material provisions of the Senior Notes Indenture, does not purport to be complete and is qualified in its entirety by reference to the provisions of the Senior Notes Indenture, including the definitions therein of certain terms used below. We urge you to read the Senior Notes Indenture because it, and not this description, will define your rights as Holders of the Senior Notes. You may request copies of the Senior Notes Indenture at our address set forth on the cover page of the Registration Statement.
The Issuers are jointly and severally liable for all obligations under the Senior Notes. The Co-Issuer is a Wholly-Owned Subsidiary of the Company that has been incorporated in Delaware as a special purpose finance subsidiary to facilitate the offering of the Senior Notes and other debt securities of the Company. The Company believes that some prospective purchasers of the Senior Notes may be restricted in their ability to purchase debt securities of partnerships or limited liability companies, such as the Company, unless the securities are jointly issued by a corporation. The Co-Issuer will not have any substantial operations or assets and will not have any revenues. Accordingly, you should not expect the Co-Issuer to participate in servicing the principal and interest obligations on the Senior Notes.
Brief Description of Senior Notes
The Senior Notes:
| • | are general unsecured senior obligations of the Issuers; |
| • | are pari passu in right of payment with all existing and future Senior Indebtedness (including the Senior Credit Facilities, the Senior Secured Notes and the Existing Senior Notes) of the Issuers; |
| • | are effectively subordinated to all secured Indebtedness of the Issuers (including the Senior Credit Facilities and the Senior Secured Notes) to the extent of the value of the assets securing such indebtedness; and are structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of the Company’s Subsidiaries that are not guaranteeing the Senior Notes; |
| • | are senior in right of payment to any Subordinated Indebtedness (including the Senior Subordinated Notes) of the Issuers; |
| • | are guaranteed on an unsecured senior basis by the Guarantors, as described under “—Guarantees”; and |
| • | are subject to registration with the SEC pursuant to the Senior Notes Registration Rights Agreement, as described under “Exchange Offer; Registration Rights”. |
As of the date of the Senior Notes Indenture, all of the Company’s subsidiaries are “Restricted Subsidiaries.” However, under certain circumstances, we are permitted to designate certain of our subsidiaries as “Unrestricted Subsidiaries.” Any Unrestricted Subsidiaries are not subject to any of the restrictive covenants in the Senior Notes Indenture and will not guarantee the Senior Notes.
220
Table of Contents
Guarantees
The Guarantors, as primary obligors and not merely as sureties, have initially jointly and severally, fully and unconditionally guaranteed, on an unsecured senior basis, the performance and full and punctual payment when due, whether at maturity, by acceleration or otherwise, of all obligations, of the Issuers under the Senior Notes Indenture and the Senior Notes, whether for payment of principal of, any premium or interest on or Additional Interest in respect of the Senior Notes, expenses, indemnification or otherwise, on the terms set forth in the Senior Notes Indenture by executing the Senior Notes Indenture.
The Restricted Subsidiaries (other than as detailed below) have initially guaranteed the Senior Notes. None of our Foreign Subsidiaries have guaranteed the Senior Notes. Each of the Guarantees of the Senior Notes are a general unsecured obligation of each Guarantor, are pari passu in right of payment with all existing and future Senior Indebtedness of each such Guarantor and are effectively subordinated to all secured Indebtedness of each such Guarantor to the extent of the collateral securing such Indebtedness, and are senior in right of payment to all existing and future Subordinated Indebtedness (including guarantees of the Senior Subordinated Notes) of each such entity. The Senior Notes are structurally subordinated to Indebtedness, claims of holders of Preferred Stock and other liabilities of Subsidiaries of the Issuers that do not guarantee the Senior Notes.
Not all of the Company’s Subsidiaries have guaranteed the Senior Notes. In the event of a bankruptcy, liquidation or reorganization of any of these non-guarantor Subsidiaries, the non-guarantor Subsidiaries will pay the holders of their debt and their trade creditors before they are able to distribute any of their assets to the Company. For the twelve months ended September 29, 2012, our Subsidiaries that are not Guarantors accounted for approximately $265.8 million, or 23.7%, of the Company’s net sales, and approximately $7.0 million, or 2.5%, of the Company’s total Adjusted EBITDA, and, as of September 29, 2012, our Subsidiaries that are not Guarantors accounted for approximately $236.1 million, or 8.3%, of the total assets, and approximately $47.3 million, or 1.8%, of the Company’s total liabilities.
The obligations of each Guarantor under its Guarantee are limited as necessary to prevent the Guarantee from constituting a fraudulent conveyance under applicable law.
Any entity that makes a payment under its Guarantee are entitled upon payment in full of all guaranteed obligations under the Senior Notes Indenture to a contribution from each other Guarantor in an amount equal to such other Guarantor’s pro rata portion of such payment based on the respective net assets of all the Guarantors at the time of such payment determined in accordance with GAAP.
If a Guarantee was rendered voidable, it could be subordinated by a court to all other indebtedness (including guarantees and other contingent liabilities) of the Guarantor, and, depending on the amount of such indebtedness, a Guarantor’s liability on its Guarantee could be reduced to zero. See “Risk Factors—Risks Related to the Notes—Federal and state fraudulent transfer laws may permit a court to void the notes and the guarantees, and, if that occurs, you may not receive any payments on the notes.”
A Guarantee by a Guarantor provides by its terms that it shall be automatically and unconditionally released and discharged upon:
(1) (a) any sale, exchange or transfer (by merger or otherwise) of the Capital Stock of such Guarantor (including any sale, exchange or transfer, after which the applicable Guarantor is no longer a Restricted Subsidiary) if such sale, exchange or transfer is made in compliance with the applicable provisions of the Senior Notes Indenture;
(b) the release or discharge of the guarantee by such Guarantor of the Senior Credit Facilities or the guarantee which resulted in the creation of such Guarantee, except a discharge or release by or as a result of payment under such guarantee;
(c) the proper designation of any Restricted Subsidiary that is a Guarantor as an Unrestricted Subsidiary in accordance with the provisions set forth under “—Certain Covenants—Limitation on Restricted Payments” and the definition of “Unrestricted Subsidiary”; or
221
Table of Contents
(d) the Issuers exercising their legal defeasance option or covenant defeasance option as described under “—Legal Defeasance and Covenant Defeasance” or the Issuers’ obligations under the Senior Notes Indenture being discharged in accordance with the terms of the Senior Notes Indenture; and
(2) such Guarantor delivering to the Senior Notes Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for in the Senior Notes Indenture relating to such transaction have been complied with.
Ranking
The payment of the principal of, premium, if any, and interest on the Senior Notes and the payment of any Guarantee are pari passu in right of payment with all Senior Indebtedness of the Issuers or the relevant Guarantor, as the case may be, including the obligations of the Issuers and such Guarantor under the Senior Credit Facilities, the Senior Secured Notes and the Existing Senior Notes.
The Senior Notes are effectively subordinated to all of the Issuers’ and each Guarantor’s existing and future Secured Indebtedness to the extent of the value of the assets securing such Indebtedness. As of September 29, 2012, the Issuers and the Guarantors had $1,169.2 million principal amount of secured Indebtedness consisting of $839.2 million of secured Indebtedness under the Senior Credit Facilities and $330.0 million aggregate principal amount of Senior Secured Notes, and the Issuers had $62.0 million of available borrowings under the revolving credit facility of the Senior Credit Facilities. As of September 29, 2012, we also had the option to increase the amount available under the Senior Credit Facilities by an amount not to exceed the greater of $150.0 million (subject to pro forma compliance with the senior first lien secured leverage ratio financial maintenance covenant) and the amount of indebtedness the Issuers could incur to the extent the senior first lien secured leverage ratio remains below a certain threshold, a certain portion of which availability are reduced in connection with the issuance of Senior Secured Notes.
Although the Senior Notes Indenture will contain limitations on the amount of additional Indebtedness that the Issuers and the Guarantors may incur, under certain circumstances the amount of such Indebtedness could be substantial and in any case, such Indebtedness may be Senior Indebtedness. See “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.”
Paying Agent and Registrar for the Senior Notes
The Issuers maintain one or more paying agents for the Senior Notes in the Borough of Manhattan, City of New York. The initial paying agent for the Senior Notes is the Senior Notes Trustee.
The Issuers also maintain a registrar with offices in the Borough of Manhattan, City of New York. The initial registrar is the Senior Notes Trustee. The registrar maintains a register reflecting ownership of the Senior Notes outstanding from time to time and makes payments on and facilitates transfer of Senior Notes on behalf of the Issuers.
The Company may change the paying agents or the registrars without prior notice to the Holders. The Company or any of its Subsidiaries may act as a paying agent or registrar.
Transfer and Exchange
A Holder may transfer or exchange Senior Notes in accordance with the Senior Notes Indenture. The registrar and the Senior Notes Trustee may require a Holder to furnish appropriate endorsements and transfer documents in connection with a transfer of Senior Notes. Holders are required to pay all taxes due on transfer. The Issuers are not required to transfer or exchange any Senior Notes selected for redemption. Also, the Issuers are not required to transfer or exchange any Senior Note for a period of 15 days before a selection of Senior Notes to be redeemed.
222
Table of Contents
Principal, Maturity and Interest
The Issuers issued $440.0 million of Senior Notes on October 1, 2012. The Senior Notes will mature on April 15, 2018. Subject to compliance with the covenant described below under the caption “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” the Issuers may issue an unlimited principal amount of additional Senior Notes from time to time under the Senior Notes Indenture (“Additional Senior Notes”). The Senior Notes offered by the Issuers and any Additional Senior Notes subsequently issued under the Senior Notes Indenture are treated as a single class for all purposes under the Senior Notes Indenture, including waivers, amendments, redemptions and offers to purchase. Unless the context requires otherwise, references to “Senior Notes” for all purposes of the Senior Notes Indenture and this “Description of Senior Notes” include any Additional Senior Notes that are actually issued. The Issuers will issue the Senior Notes in denominations of $2,000 and integral multiples of $1,000, in excess thereof.
Interest on the Senior Notes will accrue at the rate of 9.875% per annum and are payable semi-annually in arrears on April 15 and October 15, commencing on April 15, 2013 to the Holders of Senior Notes of record on the immediately preceding and . Interest on the Senior Notes will accrue from the most recent date to which interest has been paid or, if no interest has been paid, from and including the Issue Date. Interest on the Senior Notes are computed on the basis of a 360-day year comprised of twelve 30-day months.
Additional Interest may accrue on the Senior Notes in certain circumstances pursuant to the Senior Notes Registration Rights Agreement. All references in the Senior Notes Indenture, in any context, to any interest or other amount payable on or with respect to the Senior Notes shall be deemed to include any Additional Interest pursuant to the Senior Notes Registration Rights Agreement.
Principal of, premium, if any, and interest on the Senior Notes are payable at the office or agency of the Issuers maintained for such purpose within the City and State of New York or, at the option of the Issuers, payment of interest may be made by check mailed to the Holders of the Senior Notes at their respective addresses set forth in the register of Holders; provided that all payments of principal, premium, if any, and interest with respect to the Senior Notes represented by one or more global notes registered in the name of or held by The Depository Trust Company (“DTC”) or its nominee are made by wire transfer of immediately available funds to the accounts specified by the Holder or Holders thereof. Until otherwise designated by the Issuers, the Issuers’ office or agency in New York are the office of the Senior Notes Trustee maintained for such purpose.
Mandatory Redemption; Offers to Purchase; Open Market Purchases
The Issuers are not required to make any mandatory redemption or sinking fund payments with respect to the Senior Notes. However, under certain circumstances, the Issuers may be required to make an offer to purchase Senior Notes as described under the caption “—Repurchase at the Option of Holders.” In addition, we may, at our discretion, at any time and from time to time purchase Senior Notes in the open market or otherwise.
Optional Redemption
Except as set forth below, the Issuers are not entitled to redeem the Senior Notes at their option prior to April 15, 2015.
At any time prior to April 15, 2015, the Issuers may redeem all or a part of the Senior Notes, upon not less than 30 nor more than 60 days prior notice mailed by first-class mail to the registered address of each Holder or otherwise delivered in accordance with the procedures of DTC, at a redemption price equal to 100% of the principal amount of Senior Notes redeemed plus the Applicable Premium as of, and accrued and unpaid interest and Additional Interest, if any, to the date of redemption (the “Redemption Date”), subject to the rights of Holders on the relevant record date to receive interest due on the relevant interest payment date.
223
Table of Contents
On and after April 15, 2015, the Issuers may redeem the Senior Notes, in whole or in part, upon notice as described under the heading “—Repurchase at the Option of Holders—Selection and Notice”, at the redemption prices (expressed as percentages of principal amount of the Senior Notes to be redeemed) set forth below, plus accrued and unpaid interest thereon and Additional Interest, if any, to the applicable Redemption Date, subject to the right of Holders of record, on the relevant record date to receive interest due on the relevant interest payment date, if redeemed during the twelve-month period beginning on of April 15 each of the years indicated below:
| Year |
Percentage | |||
| 2015 |
104.938 | % | ||
| 2016 |
102.469 | % | ||
| 2017 and thereafter |
100.000 | % | ||
In addition, until April 15, 2015, the Issuers may, at their option, redeem up to 35% of the aggregate principal amount of Senior Notes issued by them at a redemption price equal to 109.875% of the aggregate principal amount thereof, plus accrued and unpaid interest thereon and Additional Interest, if any, to the applicable Redemption Date, subject to the right of Holders of Senior Notes of record on the relevant record date to receive interest due on the relevant interest payment date, with the net cash proceeds of one or more Equity Offerings; provided that at least 65% of the aggregate principal amount of Senior Notes originally issued under the Senior Notes Indenture and any Additional Senior Notes issued under the Senior Notes Indenture after the Issue Date (excluding Senior Notes and Additional Senior Notes held by the Issuers or Subsidiaries or Affiliates of the Issuers) remains outstanding immediately after the occurrence of each such redemption; provided further that each such redemption occurs within 90 days of the date of closing of each such Equity Offering.
Any notice of redemption may be subject to one or more conditions precedent, including, but not limited to, completion of an Equity Offering or other corporate transaction.
The Senior Notes Trustee shall select the Senior Notes to be purchased in the manner described under “Repurchase at the Option of Holders—Selection and Notice.”
Repurchase at the Option of Holders
Change of Control
The Senior Notes Indenture provides that if a Change of Control occurs, unless the Issuers have previously or concurrently mailed a redemption notice with respect to all the outstanding Senior Notes as described under “—Optional Redemption,” the Issuers makes an offer to purchase all of the Senior Notes pursuant to the offer described below (the “Change of Control Offer”) at a price in cash (the “Change of Control Payment”) equal to 101% of the aggregate principal amount thereof plus accrued and unpaid interest and Additional Interest, if any, to the date of purchase, subject to the right of Holders of the Senior Notes of record on the relevant record date to receive interest due on the relevant interest payment date. Within 60 days following any Change of Control, the Issuers will send notice of such Change of Control Offer by first-class mail, with a copy to the Senior Notes Trustee, to each Holder of Senior Notes to the address of such Holder appearing in the security register or otherwise in accordance with the procedures of DTC with a copy to the Senior Notes Trustee, with the following information:
(1) that a Change of Control Offer is being made pursuant to the covenant entitled “Change of Control” under the Senior Notes Indenture and that all Senior Notes properly tendered pursuant to such Change of Control Offer are accepted for payment by the Issuers;
(2) the purchase price and the purchase date, which are no earlier than 30 days nor later than 60 days from the date such notice is mailed (the “Change of Control Payment Date”);
(3) that any Senior Notes not properly tendered will remain outstanding and continue to accrue interest;
224
Table of Contents
(4) that unless the Issuers default in the payment of the Change of Control Payment, all Senior Notes accepted for payment pursuant to the Change of Control Offer will cease to accrue interest on the Change of Control Payment Date;
(5) that Holders electing to have any Senior Notes purchased pursuant to a Change of Control Offer are required to surrender such Senior Notes, with the form entitled “Option of Holder to Elect Purchase” on the reverse of such Senior Notes completed, to the paying agent specified in the notice at the address specified in the notice prior to the close of business on the third Business Day preceding the Change of Control Payment Date;
(6) that Holders are entitled to withdraw their tendered Senior Notes and their election to require the Issuers to purchase such Senior Notes; provided that the paying agent receives, not later than the close of business on the expiration date of the Change of Control Offer, a telegram, telex, facsimile transmission or letter setting forth the name of the Holder of the Senior Notes, the principal amount of Senior Notes tendered for purchase, and a statement that such Holder is withdrawing its tendered Senior Notes and its election to have such Senior Notes purchased;
(7) that if the Issuers are repurchasing less than all of the Senior Notes, the remaining Senior Notes are equal in principal amount to the unpurchased portion of the Senior Notes surrendered. The unpurchased portion of the Senior Notes must be equal to $2,000 or an integral multiple of $1,000 in excess thereof;
(8) the other instructions, as determined by the Issuers, consistent with the covenant described hereunder, that a Holder must follow; and
(9) if such notice is mailed prior to the occurrence of a Change of Control, stating that the Change of Control Offer is conditional upon the occurrence of such Change of Control.
The Issuers will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of Senior Notes pursuant to a Change of Control Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Senior Notes Indenture, the Issuers will comply with the applicable securities laws and regulations and shall not be deemed to have breached their obligations described in the Senior Notes Indenture by virtue thereof.
On the Change of Control Payment Date, the Issuers will, to the extent permitted by law,
(1) accept for payment all Senior Notes issued by them or portions thereof properly tendered pursuant to the Change of Control Offer,
(2) deposit with the paying agent an amount equal to the aggregate Change of Control Payment in respect of all Senior Notes or portions thereof so tendered, and
(3) deliver, or cause to be delivered, to the Senior Notes Trustee for cancellation the Senior Notes so accepted together with an Officer’s Certificate to the Senior Notes Trustee stating that such Senior Notes or portions thereof have been tendered to, and purchased by, the Issuers.
The Senior Credit Facilities will, and future credit agreements, or other agreements relating to Senior Indebtedness to which the Issuers become a party may, provide that certain change of control events with respect to the Issuers, would constitute a default thereunder (including events that would constitute a Change of Control under the Senior Notes Indenture). If we experience a change of control that triggers a default under our Senior Credit Facilities or any such future Indebtedness, we could seek a waiver of such default or seek to refinance our Senior Credit Facilities or such future Indebtedness. In the event we do not obtain such a waiver or refinance the
225
Table of Contents
Senior Credit Facilities or such future Indebtedness, such default could result in amounts outstanding under our Senior Credit Facilities or such future Indebtedness being declared due and payable.
The Existing Senior Notes, the Senior Secured Notes and the Senior Subordinated Notes have similar requirements regarding our obligation to offer to purchase such notes upon the occurrence of a Change of Control.
Our ability to pay cash to the Holders of Senior Notes following the occurrence of a Change of Control may be limited by our then existing financial resources. Therefore, sufficient funds may not be available when necessary to make any required repurchases.
The Change of Control purchase feature of the Senior Notes may in certain circumstances make more difficult or discourage a sale or takeover of us and, thus, the removal of incumbent management. The Change of Control purchase feature is a result of negotiations between the Initial Purchasers and us. After the Issue Date, we have no present intention to engage in a transaction involving a Change of Control, although it is possible that we could decide to do so in the future. Subject to the limitations discussed below, we could, in the future, enter into certain transactions, including acquisitions, refinancings or other recapitalizations, that would not constitute a Change of Control under the Senior Notes Indenture, but that could increase the amount of indebtedness outstanding at such time or otherwise affect our capital structure or credit ratings. Restrictions on our ability to incur additional Indebtedness are contained in the covenants described under “—Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Certain Covenants—Liens.” Such restrictions in the Senior Notes Indenture can be waived only with the consent of the Holders of a majority in principal amount of the Senior Notes then outstanding. Except for the limitations contained in such covenants, however, the Senior Notes Indenture will not contain any covenants or provisions that may afford Holders of the Senior Notes protection in the event of a highly leveraged transaction.
We are not required to make a Change of Control Offer following a Change of Control if a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the Senior Notes Indenture applicable to a Change of Control Offer made by us and purchases all Senior Notes validly tendered and not withdrawn under such Change of Control Offer. Notwithstanding anything to the contrary herein, a Change of Control Offer may be made in advance of a Change of Control, conditional upon such Change of Control, if a definitive agreement is in place for the Change of Control at the time of making of the Change of Control Offer.
The definition of “Change of Control” includes a disposition of all or substantially all of the assets of the Company to any Person. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law. Accordingly, in certain circumstances there may be a degree of uncertainty as to whether a particular transaction would involve a disposition of “all or substantially all” of the assets of the Company. As a result, it may be unclear as to whether a Change of Control has occurred and whether a Holder of Senior Notes may require the Issuers to make an offer to repurchase the Senior Notes as described above.
The provisions under the Senior Notes Indenture relating to the Issuers’ obligation to make an offer to repurchase the Senior Notes as a result of a Change of Control may be waived or modified with the written consent of the Holders of a majority in principal amount of the Senior Notes.
Asset Sales
The Senior Notes Indenture provides that the Company will not, and will not permit any of its Restricted Subsidiaries to consummate an Asset Sale, unless:
(1) the Company or such Restricted Subsidiary, as the case may be, receives consideration at the time of such Asset Sale at least equal to the fair market value (as determined in good faith by the Company) of the assets sold or otherwise disposed of; and
226
Table of Contents
(2) except in the case of a Permitted Asset Swap, at least 75% of the consideration therefor received by the Company or such Restricted Subsidiary, as the case may be, is in the form of cash or Cash Equivalents; provided that the following shall be deemed to be cash for purposes of this provision and for no other purpose:
(a) any liabilities (as reflected in the Company’s or such Restricted Subsidiary’s most recent balance sheet or in the footnotes thereto, or if incurred or accrued subsequent to the date of such balance sheet, such liabilities that would have been shown on the Company’s or such Restricted Subsidiary’s balance sheet or in the footnotes thereto if such incurrence or accrual had taken place on the date of such balance sheet) of the Company or such Restricted Subsidiary (other than liabilities that are by their terms subordinated to the Senior Notes or liabilities to the extent owed to the Company or any Affiliate of the Company) that are assumed by the transferee of any such assets and for which the Company and all of its Restricted Subsidiaries have been validly released by all creditors in writing,
(b) any securities, notes or other similar obligations received by the Company or such Restricted Subsidiary from such transferee that are converted by the Company or such Restricted Subsidiary into cash (to the extent of the cash received) within 180 days following the closing of such Asset Sale, and
(c) any Designated Non-cash Consideration received by the Company or such Restricted Subsidiary in such Asset Sale having an aggregate fair market value, taken together with all other Designated Non-cash Consideration received pursuant to this clause (c) that is at that time outstanding, not to exceed 2.5% of Total Assets at the time of the receipt of such Designated Non-cash Consideration, with the fair market value of each item of Designated Non-cash Consideration being measured at the time received and without giving effect to subsequent changes in value.
Within 450 days after the receipt of any Net Proceeds of any Asset Sale, the Company or such Restricted Subsidiary, at its option, may apply the Net Proceeds from such Asset Sale,
(1) to permanently reduce:
(a) Obligations under the Senior Credit Facilities, and to correspondingly reduce commitments with respect thereto;
(b) Obligations under Senior Indebtedness that is secured by a Lien, which Lien is permitted by the Senior Notes Indenture, and to correspondingly reduce commitments with respect thereto;
(c) Obligations under other Senior Indebtedness (and to correspondingly reduce commitments with respect thereto), provided that the Issuers shall equally and ratably reduce (or offer to reduce, as applicable) Obligations under the Senior Notes; provided further that all reductions of Obligations under the Senior Notes shall be made as provided under “Optional Redemption,” through open-market purchases (to the extent such purchases are at or above 100% of the principal amount thereof plus accrued unpaid interest) or by making an offer (in accordance with the procedures set forth below for an Asset Sale Offer) to all Holders of Senior Notes to purchase their Senior Notes at 100% of the principal amount thereof, plus the amount of accrued but unpaid interest, if any, on the amount of Senior Notes that would otherwise be prepaid; or
(d) Indebtedness of a Restricted Subsidiary that is not a Guarantor, other than Indebtedness owed to the Company or any Affiliate of the Company,
(2) to make (a) an Investment in any one or more businesses; provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Company or another of its Restricted Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) capital expenditures or (c) acquisitions of other assets, in each of (a), (b) and (c), used or useful in a Similar Business, or
(3) to make an Investment in (a) any one or more businesses; provided that such Investment in any business is in the form of the acquisition of Capital Stock and results in the Company or another of its Restricted
227
Table of Contents
Subsidiaries, as the case may be, owning an amount of the Capital Stock of such business such that it constitutes a Restricted Subsidiary, (b) properties or (c) acquisitions of other assets that, in each of (a), (b) and (c), replace the businesses, properties and/or assets that are the subject of such Asset Sale;
provided that, in the case of clauses (2) and (3) above, a binding commitment shall be treated as a permitted application of the Net Proceeds from the date of such commitment so long as the Company, or such other Restricted Subsidiary enters into such commitment with the good faith expectation that such Net Proceeds are applied to satisfy such commitment within 180 days of such commitment (an “Acceptable Commitment”) and, in the event any Acceptable Commitment is later cancelled or terminated for any reason before the Net Proceeds are applied in connection therewith, the Company or such Restricted Subsidiary enters into another Acceptable Commitment (a “Second Commitment”) within 180 days of such cancellation or termination; provided further that if any Second Commitment is later cancelled or terminated for any reason before such Net Proceeds are applied, then such Net Proceeds shall constitute Excess Proceeds.
Any Net Proceeds from the Asset Sale that are not invested or applied as provided and within the time period set forth in the first sentence of the preceding paragraph are deemed to constitute “Excess Proceeds.” When the aggregate amount of Excess Proceeds exceeds $20.0 million, the Issuers shall make an offer to all Holders of the Senior Notes and, if required by the terms of any Indebtedness that is pari passu with the Senior Notes or any Guarantee (“Pari Passu Indebtedness”), to the holders of such Pari Passu Indebtedness (an “Asset Sale Offer”), to purchase the maximum aggregate principal amount of the Senior Notes and such Pari Passu Indebtedness that is an integral multiple of $1,000 (but in minimum amounts of $2,000) that may be purchased out of the Excess Proceeds at an offer price in cash in an amount equal to 100% of the principal amount thereof, plus accrued and unpaid interest and Additional Interest, if any, to the date fixed for the closing of such offer, in accordance with the procedures set forth in the Senior Notes Indenture. The Issuers will commence an Asset Sale Offer with respect to Excess Proceeds within ten Business Days after the date that Excess Proceeds exceed $20.0 million by mailing the notice required pursuant to the terms of the Senior Notes Indenture, with a copy to the Senior Notes Trustee.
To the extent that the aggregate amount of Senior Notes and such Pari Passu Indebtedness tendered pursuant to an Asset Sale Offer is less than the Excess Proceeds, the Company may use any remaining Excess Proceeds for general corporate purposes, subject to other covenants contained in the Senior Notes Indenture. If the aggregate principal amount of Senior Notes or the Pari Passu Indebtedness surrendered by such holders thereof exceeds the amount of Excess Proceeds, the Senior Notes Trustee shall select the Senior Notes and such Pari Passu Indebtedness to be purchased on a pro rata basis based on the accreted value or principal amount of the Senior Notes or such Pari Passu Indebtedness tendered. Additionally, the Issuers may, at their option, make an Asset Sale Offer using proceeds from any Asset Sale at any time after consummation of such Asset Sale. Upon consummation of any Asset Sale Offer, any Net Proceeds not used to purchase Senior Notes in such Asset Sale Offer shall not be deemed Excess Proceeds and the Company may use any Net Proceeds not required to be used for general corporate purposes, subject to other covenants contained in the Senior Notes Indenture.
Pending the final application of any Net Proceeds pursuant to this covenant, the holder of such Net Proceeds may apply such Net Proceeds temporarily to reduce Indebtedness outstanding under a revolving credit facility or otherwise invest such Net Proceeds in any manner not prohibited by the Senior Notes Indenture.
The Issuers will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the repurchase of the Senior Notes pursuant to an Asset Sale Offer. To the extent that the provisions of any securities laws or regulations conflict with the provisions of the Senior Notes Indenture, the Issuers will comply with the applicable securities laws and regulations and shall not be deemed to have breached their obligations described in the Senior Notes Indenture by virtue thereof.
228
Table of Contents
Selection and Notice
If the Issuers are redeeming less than all of the Senior Notes issued by them at any time, the Senior Notes Trustee will select the Senior Notes to be redeemed (a) if the Senior Notes are listed on any national securities exchange, in compliance with the requirements of the principal national securities exchange on which the notes are listed or (b) on a pro rata basis to the extent practicable or, to the extent that selection on a pro rata basis is not practicable, by lot or such other similar method in accordance with the procedures of DTC.
Notices of purchase or redemption shall be mailed by first-class mail, postage prepaid, at least 30 but not more than 60 days before the purchase or redemption date to each Holder of Senior Notes at such Holder’s registered address or otherwise in accordance with the procedures of DTC, except that redemption notices may be mailed more than 60 days prior to a redemption date if the notice is issued in connection with a defeasance of the notes or a satisfaction and discharge of the Senior Notes Indenture. If any Senior Note is to be purchased or redeemed in part only, any notice of purchase or redemption that relates to such Senior Note shall state the portion of the principal amount thereof that has been or is to be purchased or redeemed.
Certain Covenants
Set forth below are summaries of certain covenants contained in the Senior Notes Indenture. During any period of time that (i) the Senior Notes have Investment Grade Ratings from both Rating Agencies, and (ii) no Default has occurred and is continuing under the Senior Notes Indenture (the occurrence of the events described in the foregoing clauses (i) and (ii) being collectively referred to as a “Covenant Suspension Event”), the Company and the Restricted Subsidiaries are not subject to the following covenants (the “Suspended Covenants”):
(1) “—Repurchase at the Option of Holders—Asset Sales” and “—Repurchase at the Option of Holders—Change of Control”;
(2) “—Limitation on Restricted Payments”;
(3) “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(4) clause (4) of the first paragraph of “—Merger, Consolidation or Sale of All or Substantially All Assets”;
(5) “—Transactions with Affiliates”;
(6) “—Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries”; and
(7) “—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries.”
In the event that the Company and the Restricted Subsidiaries are not subject to the Suspended Covenants under the Senior Notes Indenture for any period of time as a result of the foregoing, and on any subsequent date (the “Reversion Date”) one or both of the Rating Agencies withdraw their Investment Grade Rating or downgrade the rating assigned to the Senior Notes below an Investment Grade Rating, then the Company and the Restricted Subsidiaries will thereafter again be subject to the Suspended Covenants under the Senior Notes Indenture with respect to future events. The period of time between the Suspension Date and the Reversion Date is referred to in this description as the “Suspension Period.” The Guarantees of the Guarantors are suspended during the Suspension Period. Additionally, upon the occurrence of a Covenant Suspension Event, the amount of Excess Proceeds from Net Proceeds shall be reset at zero.
During any Suspension Period, the Company will not, and will not permit any Restricted Subsidiary to, enter into any Sale and Lease-Back Transaction; provided, that the Company or any Restricted Subsidiary may enter into a Sale and Lease-Back Transaction if (i) the Company or such Restricted Subsidiary could have incurred a Lien to secure the Indebtedness attributable to such Sale and Lease-Back Transaction pursuant to “—Liens” below without equally and ratably securing the Senior Notes pursuant to the covenant described under such covenant; and (ii) the consideration received by the Company or such Restricted Subsidiary in that Sale and
229
Table of Contents
Lease-Back Transaction is at least equal to the fair market value of the property sold and otherwise complies with “—Repurchase at the Option of Holders—Asset Sales” above; provided, further, that the foregoing provisions shall cease to apply on and subsequent to the Reversion Date following such Suspension Period.
During the Suspension Period, the Company and its Restricted Subsidiaries are entitled to incur Liens to the extent provided for under “—Liens” (including Permitted Liens) to the extent provided for in such covenant and any Permitted Liens which may refer to one or more Suspended Covenants shall be interpreted as though such applicable Suspended Covenant(s) continued to be applicable during the Suspension Period (but solely for purposes of the “—Liens” covenant and for no other covenant).
Notwithstanding the foregoing, in the event of any such reinstatement, no action taken or omitted to be taken by the Company or any of its Restricted Subsidiaries prior to such reinstatement will give rise to a Default or Event of Default under the Senior Notes Indenture with respect to Senior Notes; provided that (1) with respect to Restricted Payments made after any such reinstatement, the amount of Restricted Payments made are calculated as though the covenant described above under the caption “Limitation on Restricted Payments” had been in effect prior to but not during the Suspension Period, provided that any Subsidiaries designated as Unrestricted Subsidiaries during the Suspension Period shall automatically become Restricted Subsidiaries on the Reversion Date (subject to the Company’s right to subsequently designate them as Unrestricted Subsidiaries in compliance with the covenants set out below) and (2) all Indebtedness incurred, or Disqualified Stock issued, during the Suspension Period are classified to have been incurred or issued pursuant to clause (3) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.” In addition, for purposes of clause (3) of the first paragraph under the caption “—Limitation on Restricted Payments,” all events (including the accrual of Consolidated Net Income) set forth in such clause (3) occurring during a Suspension Period shall be disregarded for purposes of determining the amount of Restricted Payments the Company or any Restricted Subsidiary is permitted to make pursuant to such clause (3).
There can be no assurance that the Senior Notes will ever achieve or maintain Investment Grade Ratings.
Limitation on Restricted Payments
The Company will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly:
(I) declare or pay any dividend or make any payment or distribution on account of the Company’s, or any of its Restricted Subsidiaries’ Equity Interests, including, without limitation, payable in connection with any merger or consolidation other than:
(a) dividends or distributions by the Company payable solely in Equity Interests (other than Disqualified Stock) of the Company; or
(b) dividends, payments or distributions by a Restricted Subsidiary so long as, in the case of any dividend, payment or distribution payable on or in respect of any class or series of securities issued by a Restricted Subsidiary other than a Wholly-Owned Subsidiary, the Company or a Restricted Subsidiary receives at least its pro rata share of such dividend or distribution in accordance with its Equity Interests in such class or series of securities;
(II) purchase, redeem, defease or otherwise acquire or retire for value any Equity Interests of the Company, or any direct or indirect parent of the Company, including, without limitation, in connection with any merger or consolidation;
(III) make any principal payment on, or redeem, repurchase, defease or otherwise acquire or retire for value in each case, prior to any scheduled repayment, sinking fund payment or maturity, any Subordinated Indebtedness, other than (a) Indebtedness permitted under clauses (7) and (8) of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” (other than, in the event any Default has occurred and is continuing, Indebtedness owing to any Restricted Subsidiary
230
Table of Contents
that is not a Guarantor) or (b) the purchase, repurchase or other acquisition of Subordinated Indebtedness purchased in anticipation of satisfying a sinking fund obligation, principal installment or final maturity, in each case due within one year of the date of purchase, repurchase or acquisition; or
(IV) make any Restricted Investment
(all such payments and other actions set forth in clauses (I) through (IV) above (other than any exceptions thereof) being collectively referred to as “Restricted Payments”), unless, at the time of such Restricted Payment:
(1) no Default or Event of Default shall have occurred and be continuing or would occur as a consequence thereof;
(2) immediately after giving effect to such transaction on a pro forma basis, the Company could incur $1.00 of additional Indebtedness under the provisions of the first paragraph of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; and
(3) such Restricted Payment, together with the aggregate amount of all other Restricted Payments made by the Company and its Restricted Subsidiaries after the Issue Date (including Restricted Payments permitted by clauses (1), (2) (with respect to the payment of dividends on Refunding Capital Stock (as defined below) pursuant to clause (b) thereof only), (6)(c), (9) and (14) (to the extent not deducted in calculating Consolidated Net Income) of the next succeeding paragraph, but excluding all other Restricted Payments permitted by the next succeeding paragraph), is less than the sum of (without duplication):
(a) 50% of the Consolidated Net Income of the Company for the period (taken as one accounting period) beginning September 30, 2007 to the end of the Company’s most recently ended fiscal quarter for which internal financial statements are available at the time of such Restricted Payment, or, in the case such Consolidated Net Income for such period is a deficit, minus 100% of such deficit; plus
(b) 100% of the aggregate net cash proceeds and the fair market value, as determined in good faith by the Company, of marketable securities or other property received by the Company since immediately after November 20, 2007 (other than net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness, Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) from the issue or sale of:
(i) (A) Equity Interests of the Company, including Treasury Capital Stock (as defined below), but excluding cash proceeds and the fair market value, as determined in good faith by the Company, of marketable securities or other property received from the sale of:
(x) Equity Interests to employees, directors or consultants of the Company, any direct or indirect parent company of the Company and the Company’s Subsidiaries after November 20, 2007, to the extent such amounts have been applied to Restricted Payments made in accordance with clause (4) of the next succeeding paragraph; and
(y) Designated Preferred Stock; and
(B) to the extent such net cash proceeds are actually contributed to the Company as equity (other than Disqualified Stock), Equity Interests of any of the Company’s direct or indirect parent companies (excluding contributions of the proceeds from the sale of Designated Preferred Stock of any such companies or contributions to the extent such amounts have been applied to Restricted Payments made in accordance with-clause (4) of the next succeeding paragraph); or
(ii) debt securities of the Company that have been converted into or exchanged for such Equity Interests of the Company;
provided, however, that this clause (b) shall not include the proceeds from (W) Refunding Capital Stock, (X) Equity Interests or debt securities of the Company sold to a Restricted Subsidiary, as the case may be, (Y) Disqualified Stock or debt securities that have been converted into Disqualified Stock or (Z) Excluded Contributions; plus
231
Table of Contents
(c) 100% of the aggregate amount of cash and the fair market value, as determined in good faith by the Company, of marketable securities or other property contributed to the capital of the Company (other than as Disqualified Stock) after November 20, 2007 (other than (i) net cash proceeds to the extent such net cash proceeds have been used to incur Indebtedness, Disqualified Stock or Preferred Stock pursuant to clause (12)(a) of the second paragraph of “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”, (ii) contributions from a Restricted Subsidiary or (iii) any Excluded Contribution); plus
(d) 100% of the aggregate amount received in cash and the fair market value, as determined in good faith by the Company, of marketable securities or other property received by the Issuer or any Restricted Subsidiary by means of:
(i) the sale or other disposition (other than to the Company or a Restricted Subsidiary) of Restricted Investments made by the Company or its Restricted Subsidiaries and repurchases and redemptions of such Restricted Investments from the Company or its Restricted Subsidiaries and repayments of loans or advances, and releases of guarantees, which constitute Restricted Investments by the Company or its Restricted Subsidiaries, in each case after November 20, 2007; or
(ii) the sale (other than to the Company or a Restricted Subsidiary) of the stock of an Unrestricted Subsidiary or a distribution or dividend from an Unrestricted Subsidiary (other than in each case to the extent the Investment in such Unrestricted Subsidiary was made by the Company or a Restricted Subsidiary pursuant to clause (7) of the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment) after November 20, 2007; plus
(e) in the case of the redesignation of an Unrestricted Subsidiary as a Restricted Subsidiary after November 20, 2007, the fair market value of the Investment in such Unrestricted Subsidiary, as determined by the Company in good faith or if, in the case of an Unrestricted Subsidiary, such fair market value may exceed $20.0 million, in writing by an Independent Financial Advisor, at the time of the redesignation of such Unrestricted Subsidiary as a Restricted Subsidiary other than an Unrestricted Subsidiary to the extent the Investment in such Unrestricted Subsidiary was made by the Company or a Restricted Subsidiary pursuant to clause (7) of the paragraph following the next succeeding paragraph or to the extent such Investment constituted a Permitted Investment.
As of September 29, 2012, the amount available for Restricted Payments pursuant to this clause (3) was approximately $175.2 million, although we are unable to utilize this capacity until we would be able to incur $1.00 of additional Indebtedness pursuant to the provisions of the first paragraph of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” on a pro forma basis after giving effect to any such Restricted Payment.
The foregoing provisions will not prohibit:
(1) the payment of any dividend or distribution within 60 days after the date of declaration thereof, if at the date of declaration such payment would have complied with the provisions of the Senior Notes Indenture;
(2) (a) the redemption, repurchase, retirement or other acquisition of any Equity Interests (“Treasury Capital Stock”) or Subordinated Indebtedness of the Company or any Equity Interests of any direct or indirect parent company of the Company, in exchange for, or out of the proceeds of the substantially concurrent sale (other than to a Restricted Subsidiary) of, Equity Interests of the Company or any direct or indirect parent company of the Company to the extent contributed to the Company (in each case, other than any Disqualified Stock or Designated Preferred Stock) (“Refunding Capital Stock”) and (b) if immediately prior to the retirement of Treasury Capital Stock, the declaration and payment of dividends thereon was permitted under clause (6) of this paragraph, the declaration and payment of dividends on the Refunding Capital Stock (other than Refunding Capital Stock the proceeds of which were used to redeem, repurchase, retire or otherwise acquire any Equity Interests of any direct or indirect parent company of the Company) in an aggregate amount no greater than the aggregate amount per year of dividends per annum that were declarable and payable on such Treasury Capital Stock immediately prior to such retirement;
232
Table of Contents
(3) the redemption, repurchase, defeasance or other acquisition or retirement of Subordinated Indebtedness of the Issuers or a Guarantor made in exchange for, or out of the proceeds of the substantially concurrent sale of , new Indebtedness of the Issuers or a Guarantor, as the case may be, which is incurred in compliance with “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” so long as:
(a) the principal amount (or accreted value, if applicable) of such new Indebtedness does not exceed the principal amount of (or accreted value, if applicable), plus any accrued and unpaid interest on, the Subordinated Indebtedness being so redeemed, repurchased, acquired or retired for value, plus the amount of any reasonable premium to be paid (including reasonable premiums) and any reasonable fees and expenses incurred in connection with the issuance of such new Indebtedness;
(b) such new Indebtedness is subordinated to the Senior Notes or the applicable Guarantee at least to the same extent as such Subordinated Indebtedness so purchased, exchanged, redeemed, repurchased, defeased, acquired or retired for value;
(c) such new Indebtedness has a final scheduled maturity date equal to or later than the final scheduled maturity date of the Subordinated Indebtedness being so redeemed, repurchased, defeased, acquired or retired; and
(d) such new Indebtedness has a Weighted Average Life to Maturity equal to or greater than the remaining Weighted Average Life to Maturity of the Subordinated Indebtedness being so redeemed, repurchased, defeased, acquired or retired;
(4) a Restricted Payment to pay for the repurchase, redemption or other acquisition or retirement for value of Equity Interests (other than Disqualified Stock) of the Company or any of its direct or indirect parent companies held by any future, present or former employee, director or consultant of the Company, any of its Restricted Subsidiaries or any of its direct or indirect parent companies pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement, including any Equity Interests rolled over by management of the Company in connection with the DJO Acquisition; provided, however, that the aggregate Restricted Payments made under this clause (4) do not exceed in any calendar year $10.0 million (which shall increase to $20.0 million subsequent to the consummation of an underwritten public Equity Offering by the Company or any direct or indirect parent corporation of the Company) (with unused amounts in any calendar year being carried over to succeeding calendar years subject to a maximum (without giving effect to the following proviso) of $20.0 million in any calendar year (which shall increase to $40.0 million subsequent to the consummation of an underwritten public Equity Offering by the Company or any direct or indirect parent of the Company)); provided further that such amount in any calendar year may be increased by an amount not to exceed:
(a) the cash proceeds from the sale of Equity Interests (other than Disqualified Stock) of the Company and, to the extent contributed to the Company, Equity Interests of any of the Company’s direct or indirect parent companies, in each case to members of management, directors or consultants of the Company, any of its Subsidiaries or any of its direct or indirect parent companies that occurs after the Issue Date, to the extent the cash proceeds from the sale of such Equity Interests have not otherwise been and are not thereafter applied to the payment of Restricted Payments by virtue of clause (3) of the preceding paragraph or otherwise; plus
(b) the cash proceeds of key man life insurance policies received by the Company or its Restricted Subsidiaries after the Issue Date; less
(c) the amount of any Restricted Payments previously made with the cash proceeds described in clauses (a) and (b) of this clause (4); and provided further that cancellation of Indebtedness owing to the Company or any of its Restricted Subsidiaries from members of management of the Company, any of the Company’s direct or indirect parent companies or any of the Company’s Subsidiaries in connection with a repurchase of Equity Interests of the Company or any of its direct or indirect parent companies are not deemed to constitute a Restricted Payment for purposes of this covenant or any other provision of the Senior Notes Indenture;
233
Table of Contents
(5) the declaration and payment of dividends to holders of any class or series of Disqualified Stock of the Company or any of its Restricted Subsidiaries and of Preferred Stock of any Restricted Subsidiary issued in accordance with the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” to the extent such dividends are included in the definition of “Fixed Charges”;
(6) (a) the declaration and payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) issued by the Company after the Issue Date;
(b) the declaration and payment of dividends to a direct or indirect parent company of the Company, the proceeds of which are used to fund the payment of dividends to holders of any class or series of Designated Preferred Stock (other than Disqualified Stock) of such parent corporation issued after the Issue Date, provided that the amount of dividends paid pursuant to this clause (b) shall not exceed the aggregate amount of cash actually contributed to the Company from the sale of such Designated Preferred Stock; or
(c) the declaration and payment of dividends on Refunding Capital Stock that is Preferred Stock in excess of the dividends declarable and payable thereon pursuant to clause (2) of this paragraph;
provided, however, in the case of each of (a) and (c) of this clause (6), that for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date of issuance of such Designated Preferred Stock or the declaration of such dividends on Refunding Capital Stock that is Preferred Stock, after giving effect to such issuance or declaration on a pro forma basis, the Company and its Restricted Subsidiaries on a consolidated basis had a Fixed Charge Coverage Ratio of at least 2.00 to 1.00;
(7) Investments in Unrestricted Subsidiaries having an aggregate fair market value, taken together with all other Investments made pursuant to this clause (7) that are at the time outstanding, without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities, not to exceed 2.0% of Total Assets at the time of such Investment (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
(8) repurchases of Equity Interests deemed to occur upon exercise of stock options or warrants if such Equity Interests represent a portion of the exercise price of such options, or warrants;
(9) the declaration and payment of dividends on the Company’s common stock (or payments of dividends to any-direct or indirect parent entity to fund payments of dividends on such entity’s common stock), following the consummation of an underwritten public offering of the Company’s common stock or the common stock of any of its direct or indirect parent companies after the Issue Date, of up to 6% per annum of the net cash proceeds received by or contributed to the Company in or from any such public offering, other than public offerings with respect to common stock registered on Form S-8 and other than any public sale constituting an Excluded Contribution;
(10) Restricted Payments in any amount that do not in the aggregate exceed all Excluded Contributions made since the Issue Date;
(11) other Restricted Payments in an aggregate amount taken together with all other Restricted Payments made pursuant to this clause (11) not to exceed 1.75% of Total Assets at the time made;
(12) distributions or payments of Receivables Fees;
(13) any Restricted Payment made as part of the Transactions, and the fees and expenses related thereto, or used to fund amounts owed to Affiliates (including dividends to any direct or indirect parent of the Company to permit payment by such parent of such amounts), in each case to the extent permitted by (or, in the case of a dividend to fund such payment, to the extent such payment, if made by the Company, would be permitted by) the covenant described under “—Transactions with Affiliates”;
234
Table of Contents
(14) the repurchase, redemption or other acquisition or retirement for value of any Subordinated Indebtedness in accordance with the provisions similar to those described under the captions “Repurchase at the Option of Holders—Change of Control” and “Repurchase at the Option of Holders—Asset Sales”; provided that all Senior Notes tendered by Holders in connection with a Change of Control Offer or Asset Sale Offer, as applicable, have been repurchased, redeemed or acquired for value;
(15) the declaration and payment of dividends by the Company to, or the making of loans to, any direct or indirect parent in amounts required for any direct or indirect parent companies to pay, in each case without duplication:
(a) franchise and excise taxes and other fees, taxes and expenses in each case to the extent required to maintain their corporate existence;
(b) federal, state, foreign and local income taxes, to the extent such income taxes are attributable to the income of the Company and its Restricted Subsidiaries and, to the extent of the amount actually received from its Unrestricted Subsidiaries, in amounts required to pay such taxes to the extent attributable to the income of such Unrestricted Subsidiaries; provided that in each case the amount of such payments in any fiscal year does not exceed the amount that the Company and its Restricted Subsidiaries would be required to pay in respect of federal, state and local taxes for such fiscal year were the Company, its Restricted Subsidiaries and its Unrestricted Subsidiaries (to the extent described above) to pay such taxes separately from any such parent entity;
(c) customary salary, bonus and other benefits payable to officers and employees of any direct or indirect parent company of the Company to the extent such salaries, bonuses and other benefits are attributable to the ownership or operation of the Company and its Restricted Subsidiaries;
(d) general corporate operating and overhead costs and expenses of any direct or indirect parent company of the Company to the extent such costs and expenses are attributable to the ownership or operation of the Company and its Restricted Subsidiaries; and
(e) fees and expenses other than to Affiliates of the Company related to any unsuccessful equity or debt offering of such parent entity; and
(16) the distribution, by dividend or otherwise, of shares of Capital Stock of, or Indebtedness owed to the Company or a Restricted Subsidiary by, Unrestricted Subsidiaries (other than Unrestricted Subsidiaries, the primary assets of which are cash and/or Cash Equivalents or were contributed to such Unrestricted Subsidiary in anticipation of such distribution, dividend or other payment);
provided, however, that at the time of, and after giving effect to, any Restricted Payment permitted under clauses (7), (11) and (16), no Default shall have occurred and be continuing or would occur as a consequence thereof.
As of the Issue Date, all of the Company’s Subsidiaries were Restricted Subsidiaries. The Company will not permit any Unrestricted Subsidiary to become a Restricted Subsidiary except pursuant to the last sentence of the definition of “Unrestricted Subsidiary.” For purposes of designating any Restricted Subsidiary as an Unrestricted Subsidiary, all outstanding Investments by the Company and its Restricted Subsidiaries (except to the extent repaid) in the Subsidiary so designated are deemed to be Restricted Payments in an amount determined as set forth in the last sentence of the definition of “Investment.” Such designation are permitted only if a Restricted Payment in such amount would be permitted at such time, whether pursuant to the first paragraph of this covenant or under clause (7), (10) or (11) of the second paragraph of this covenant, or pursuant to the definition of “Permitted Investments,” and if such Subsidiary otherwise meets the definition of an Unrestricted Subsidiary. Unrestricted Subsidiaries are not subject to any of the restrictive covenants set forth in the Senior Notes Indenture.
235
Table of Contents
Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock
The Company will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable, contingently or otherwise (collectively, “incur” and collectively, an “incurrence”) with respect to any Indebtedness (including Acquired Indebtedness) and the Company will not issue any shares of Disqualified Stock and will not permit any Restricted Subsidiary to issue any shares of Disqualified Stock or Preferred Stock; provided, however, that the Company may incur Indebtedness (including Acquired Indebtedness) or issue shares of Disqualified Stock, and any of its Restricted Subsidiaries may incur Indebtedness (including Acquired Indebtedness), issue shares of Disqualified Stock and issue shares of Preferred Stock, if the Fixed Charge Coverage Ratio on a consolidated basis for the Company and its Restricted Subsidiaries’ most recently ended four fiscal quarters for which internal financial statements are available immediately preceding the date on which such additional Indebtedness is incurred or such Disqualified Stock or Preferred Stock is issued would have been at least 2.00 to 1.00, determined on a pro forma basis (including a pro forma application of the net proceeds therefrom), as if the additional Indebtedness had been incurred, or the Disqualified Stock or Preferred Stock had been issued, as the case may be, and the application of proceeds therefrom had occurred at the beginning of such four-quarter period.
The foregoing limitations will not apply to:
(1) the incurrence of Indebtedness under Credit Facilities by the Company or any of its Restricted Subsidiaries and the issuance and creation of letters of credit and bankers’ acceptances thereunder (with letters of credit and bankers’ acceptances being deemed to have a principal amount equal to the face amount thereof), up to an aggregate principal amount of $1,325.0 million outstanding at any one time;
(2) the incurrence by the Company and any Guarantor of Indebtedness represented by the Senior Notes (including any Guarantee) (other than any Additional Senior Notes) and any notes (including guarantees thereof) issued in exchange for such Senior Notes pursuant to the Senior Notes Registration Rights Agreement or similar agreement;
(3) Indebtedness of the Company and its Restricted Subsidiaries in existence on the Issue Date (other than Indebtedness described in clauses (1) and (2)), including, without limitation, the Senior Secured Notes;
(4) Indebtedness (including Capitalized Lease Obligations), Disqualified Stock and Preferred Stock incurred by the Company or any of its Restricted Subsidiaries, to finance the purchase, lease or improvement of property (real or personal) or equipment (other than software) that is used or useful in a Similar Business, whether through the direct purchase of assets or the Capital Stock of any Person owning such assets, in an aggregate principal amount at the date of such incurrence (including all Refinancing Indebtedness incurred to refinance any other Indebtedness incurred pursuant to this clause (4)) not to exceed 4.0% of Total Assets; provided, however, that such Indebtedness exists at the date of such purchase or transaction or is created within 270 days thereafter (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (4) shall cease to be deemed incurred or outstanding for purposes of this clause (4) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Company or such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (4));
(5) Indebtedness incurred by the Company or any of its Restricted Subsidiaries constituting reimbursement obligations with respect to letters of credit issued in the ordinary course of business, including letters of credit in respect of workers’ compensation claims, or other Indebtedness with respect to reimbursement type obligations regarding workers’ compensation claims; provided, however, that upon the drawing of such letters of credit or the incurrence of such Indebtedness, such obligations are reimbursed within 30 days following such drawing or incurrence;
(6) Indebtedness arising from agreements of the Company or its Restricted Subsidiaries providing for indemnification, adjustment of purchase price or similar obligations, in each case, incurred or assumed in connection with the disposition of any business, assets or a Subsidiary, other than guarantees of Indebtedness
236
Table of Contents
incurred by any Person acquiring all or any portion of such business, assets or a Subsidiary for the purpose of financing such acquisition; provided, however, that such Indebtedness is not reflected on the balance sheet of the Company, or any of its Restricted Subsidiaries (contingent obligations referred to in a footnote to financial statements and not otherwise reflected on the balance sheet are not deemed to be reflected on such balance sheet for purposes of this clause (6));
(7) Indebtedness of the Company to a Restricted Subsidiary; provided that any such Indebtedness owing to a Restricted Subsidiary that is not the Co-Issuer or a Guarantor is expressly subordinated in right of payment to the Senior Notes; provided further that any subsequent issuance or transfer of any Capital Stock or any other event which results in the Restricted Subsidiary holding such Indebtedness ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such Indebtedness (except to the Company or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(8) Indebtedness of a Restricted Subsidiary to the Company or another Restricted Subsidiary; provided that if a Guarantor incurs such Indebtedness to a Restricted Subsidiary that is not the Co-Issuer or a Guarantor, such Indebtedness is expressly subordinated in right of payment to the Guarantee of the Senior Notes of such Guarantor; provided further that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Indebtedness being held by a Person other than the Company or a Restricted Subsidiary or any subsequent transfer of any such Indebtedness (except to the Company or another Restricted Subsidiary) shall be deemed, in each case, to be an incurrence of such Indebtedness not permitted by this clause;
(9) shares of Preferred Stock of a Restricted Subsidiary issued to the Company or another Restricted Subsidiary, provided that any subsequent issuance or transfer of any Capital Stock or any other event which results in any such Restricted Subsidiary ceasing to be a Restricted Subsidiary or any other subsequent transfer of any such shares of Preferred Stock (except to the Company or another Restricted Subsidiary) shall be deemed in each case to be an issuance of such shares of Preferred Stock not permitted by this clause;
(10) Hedging Obligations (excluding Hedging Obligations entered into for speculative purposes) for the purpose of limiting interest rate risk with respect to any Indebtedness of the Company or any Restricted Subsidiary permitted to be incurred pursuant to “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” exchange rate risk or commodity pricing risk;
(11) obligations in respect of performance, bid, appeal and surety bonds and completion guarantees provided by the Company or any of its Restricted Subsidiaries in the ordinary course of business;
(12) (a) Indebtedness or Disqualified Stock of the Company and Indebtedness, Disqualified Stock or Preferred Stock of the Company or any Restricted Subsidiary equal to 200.0% of the net cash proceeds received by the Company since immediately after the Issue Date from the issue or sale of Equity Interests of the Company or cash contributed to the capital of the Company (in each case, other than proceeds of Disqualified Stock or sales of Equity Interests to the Company or any of its Subsidiaries) as determined in accordance with clauses (3)(b) and (3)(c) of the first paragraph of “—Limitation on Restricted Payments” to the extent such net cash proceeds or cash have not been applied to make Restricted Payments or to make other Investments, payments or exchanges pursuant to such clauses or pursuant to the second paragraph of “—Limitation on Restricted Payments” or to make Permitted Investments (other than Permitted Investments specified in clauses (1), (2) and (3) of the definition thereof) and (b) Indebtedness or Disqualified Stock of the Company and Indebtedness, Disqualified Stock or Preferred Stock of the Company or any Restricted Subsidiary not otherwise permitted hereunder in an aggregate principal amount or liquidation preference, which when aggregated with the principal amount and liquidation preference of all other Indebtedness, Disqualified Stock and Preferred Stock then outstanding and incurred pursuant to this clause (12)(b), does not at any one time outstanding exceed $175.0 million (it being understood that any Indebtedness, Disqualified Stock or Preferred Stock incurred pursuant to this clause (12)(b) shall cease to be deemed incurred or outstanding for purposes of this clause (12)(b) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after
237
Table of Contents
the first date on which the Company or such Restricted Subsidiary could have incurred such Indebtedness, Disqualified Stock or Preferred Stock under the first paragraph of this covenant without reliance on this clause (12)(b));
(13) the incurrence or issuance by the Company or any Restricted Subsidiary of Indebtedness, Disqualified Stock or Preferred Stock which serves to refund, refinance, replace, renew, extend or defease any Indebtedness, Disqualified Stock or Preferred Stock of the Company or any Restricted Subsidiary incurred as permitted under the first paragraph of this covenant and clauses (2), (3), (4) and (12)(a) above, this clause (13) and clause (14) below or any Indebtedness, Disqualified Stock or Preferred Stock issued to so refund, refinance, replace, renew, extend or defease such Indebtedness, Disqualified Stock or Preferred Stock including additional Indebtedness, Disqualified Stock or Preferred Stock incurred to pay premiums (including reasonable tender premiums), defeasance costs and fees in connection therewith (the “Refinancing Indebtedness”) prior to its respective maturity; provided, however, that such Refinancing Indebtedness:
(a) has a Weighted Average Life to Maturity at the time such Refinancing Indebtedness is incurred which is not less than the remaining Weighted Average Life to Maturity of the Indebtedness, Disqualified Stock or Preferred Stock being refunded, refinanced, replaced, renewed, extended or defeased,
(b) to the extent such Refinancing Indebtedness refinances (i) Indebtedness subordinated or pari passu to the Senior Notes or any Guarantee thereof, such Refinancing Indebtedness is subordinated or pari passu to the Senior Notes or the Guarantee at least to the same extent as the Indebtedness being refinanced or refunded or (ii) Disqualified Stock or Preferred Stock, such Refinancing Indebtedness must be Disqualified Stock or Preferred Stock, respectively, and
(c) shall not include Indebtedness, Disqualified Stock or Preferred Stock of a Subsidiary of the Company that is not a Co-Issuer or a Guarantor that refinances Indebtedness, Disqualified Stock or Preferred Stock of the Company, the Co-Issuer or a Guarantor;
provided further that subclause (a) of this clause (13) will not apply to any refunding or refinancing of any Secured Indebtedness;
(14) Indebtedness, Disqualified Stock or Preferred Stock of (x) the Company or a Restricted Subsidiary incurred to finance an acquisition or (y) Persons that are acquired by the Company or any Restricted Subsidiary or merged into the Company or a Restricted Subsidiary in accordance with the terms of the Senior Notes Indenture; provided that after giving effect to such acquisition or merger, either (a) the Company would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first sentence of this covenant or (b) the Fixed Charge Coverage Ratio of the Company and the Restricted Subsidiaries is greater than immediately prior to such acquisition or merger;
(15) Indebtedness arising from the honoring by a bank or other financial institution of a check, draft or similar instrument drawn against insufficient funds in the ordinary course of business, provided that such Indebtedness is extinguished within two Business Days of its incurrence;
(16) Indebtedness of the Company or any of its Restricted Subsidiaries supported by a letter of credit issued pursuant to the Credit Facilities, in a principal amount not in excess of the stated amount of such letter of credit;
(17) (a) any guarantee by the Company or a Restricted Subsidiary of Indebtedness or other obligations of any Restricted Subsidiary so long as the incurrence of such Indebtedness incurred by such Restricted Subsidiary is permitted under the terms of the Senior Notes Indenture,
(b) any guarantee by a Restricted Subsidiary of Indebtedness of the Company provided that such guarantee is incurred in accordance with the covenant described below under “—Limitation on Guarantees of Indebtedness by Restricted Subsidiaries,” or
(c) any incurrence by the Co-Issuer of Indebtedness as a co-issuer of Indebtedness of the Company that was permitted to be incurred by another provision of this covenant;
238
Table of Contents
(18) Indebtedness of Foreign Subsidiaries of the Company in an amount not to exceed, at any one time outstanding and together with any other Indebtedness incurred under this clause (18), 10.0% of the Total Assets of the Foreign Subsidiaries (it being understood that any Indebtedness incurred pursuant to this clause (18) shall cease to be deemed incurred or outstanding for purposes of this clause (18) but shall be deemed incurred for the purposes of the first paragraph of this covenant from and after the first date on which the Company or its Restricted Subsidiaries could have incurred such Indebtedness under the first paragraph of this covenant without reliance on this clause (18));
(19) Indebtedness of the Company or any of its Restricted Subsidiaries consisting of (i) the financing of insurance premiums or (ii) take-or-pay obligations contained in supply arrangements in each case, incurred in the ordinary course of business; and
(20) Indebtedness consisting of Indebtedness issued by the Company or any of its Restricted Subsidiaries to current or former officers, directors and employees thereof, their respective estates, spouses or former spouses, in each case to finance the purchase or redemption of Equity Interests of the Company or any direct or indirect parent company of the Company to the extent described in clause (4) of the second paragraph under the caption “—Limitation on Restricted Payments.”
For purposes of determining compliance with this covenant:
(1) in the event that an item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) meets the criteria of more than one of the categories of permitted Indebtedness, Disqualified Stock or Preferred Stock described in clauses (1) through (20) above or is entitled to be incurred pursuant to the first paragraph of this covenant, the Company, in its sole discretion, will classify or reclassify such item of Indebtedness, Disqualified Stock or Preferred Stock (or any portion thereof) and will only be required to include the amount and type of such Indebtedness, Disqualified Stock or Preferred Stock in one of the above clauses; and
(2) at the time of incurrence, the Company are entitled to divide and classify an item of Indebtedness in more than one of the types of Indebtedness described in the first and second paragraphs above; provided that all Indebtedness outstanding under the Senior Credit Facilities on the Issue Date are treated as incurred on the Issue Date under clause (1) of the preceding paragraph.
Accrual of interest or dividends, the accretion of accreted value, the accretion or amortization of original issue discount, the payment of interest in the form of additional Indebtedness and the payment of dividends in the form of additional Disqualified Stock or Preferred Stock, as applicable, will in each case not be deemed to be an incurrence of Indebtedness, Disqualified Stock or Preferred Stock for purposes of this covenant.
For purposes of determining compliance with any U.S. dollar-denominated restriction on the incurrence of Indebtedness, the U.S. dollar-equivalent principal amount of Indebtedness denominated in a foreign currency shall be calculated based on the relevant currency exchange rate in effect on the date such Indebtedness was incurred, in the case of term debt, or first committed, in the case of revolving credit debt; provided that if such Indebtedness is incurred to refinance other Indebtedness denominated in a foreign currency, and such refinancing would cause the applicable U.S. dollar denominated restriction to be exceeded if calculated at the relevant currency exchange rate in effect on the date of such refinancing, such U.S. dollar-denominated restriction shall be deemed not to have been exceeded so long as the principal amount of such refinancing Indebtedness does not exceed the principal amount of such Indebtedness being refinanced.
The principal amount of any Indebtedness incurred to refinance other Indebtedness, if incurred in a different currency from the Indebtedness being refinanced, shall be calculated based on the currency exchange rate applicable to the currencies in which such respective Indebtedness is denominated that is in effect on the date of such refinancing.
The Senior Notes Indenture does not treat (1) unsecured Indebtedness as subordinated or junior to Secured Indebtedness merely because it is unsecured or (2) Senior Indebtedness as subordinated or junior to any other Senior Indebtedness merely because it has a junior priority with respect to the same collateral.
239
Table of Contents
Liens
The Company will not, and will not permit the Co-Issuer or any Guarantor to, directly or indirectly, create, incur, assume or otherwise cause or suffer to exist any Lien (except Permitted Liens) that secures Obligations under any Indebtedness or any related guarantee, on any asset or property of the Company, the Co-Issuer or any Guarantor, or any income or profits therefrom, or assign or convey any right to receive income therefrom, unless:
(1) in the case of Liens securing Subordinated Indebtedness, the Senior Notes and related Guarantees are secured by a Lien on such property, assets or proceeds that is senior in priority to such Liens; or
(2) in all other cases, the Senior Notes or the Guarantees are equally and ratably secured,
except that the foregoing shall not apply to (a) Liens securing (x) the Senior Notes and the related Guarantees and (y) the Senior Secured Notes and any related guarantees provided for under the Senior Secured Notes Indenture, (b) Liens securing Indebtedness permitted to be incurred under Credit Facilities, including any letter of credit facility relating thereto, that was permitted by the terms of the Senior Notes Indenture to be incurred pursuant to clause (1) of the second paragraph under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” (including, during any Suspension Period, Indebtedness of the type and in the amounts specified under such clause) and (c) Liens securing Indebtedness under Credit Facilities permitted to be incurred under the covenant described above under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that, with respect to Liens securing Indebtedness permitted under this subclause (c), at the time of incurrence and after giving pro forma effect thereto, the Consolidated Secured Debt Ratio would be no greater than 4.00 to 1.00.
Merger, Consolidation or Sale of All or Substantially All Assets
Company. The Company may not, directly or indirectly, consolidate or merge with or into or wind up into (whether or not the Company is the surviving corporation), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of the Company’s properties or assets, in one or more related transactions, to any Person unless:
(1) the Company is the surviving corporation or the Person formed by or surviving any such consolidation or merger (if other than the Company) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made is a corporation, partnership (including a limited partnership), trust or limited liability company organized or existing under the laws of the jurisdiction of organization of the Company or the laws of the United States, any state thereof, the District of Columbia or any territory thereof (such Person, as the case may be, being herein called the “Successor Company”);
(2) the Successor Company, if other than the Company, expressly assumes all the obligations of the Company under the Senior Notes, pursuant to supplemental indentures or other documents or instruments in form reasonably satisfactory to the Senior Notes Trustee, and the Senior Notes Registration Rights Agreement if the exchange offer contemplated therein has not been consummated or if the Issuers continue to have an obligation to file or maintain the effectiveness of a shelf registration statement as provided under such agreement;
(3) immediately after such transaction, no Default or Event of Default exists;
(4) immediately after giving pro forma effect to such transaction and any related financing transactions, as if such transactions had occurred at the beginning of the applicable four-quarter period,
(a) the Company or the Successor Company, as applicable, would be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first sentence of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” or
240
Table of Contents
(b) the Fixed Charge Coverage Ratio for the Company (or, if applicable, the Successor Company) and its Restricted Subsidiaries would be greater than such Ratio for the Company and its Restricted Subsidiaries immediately prior to such transaction;
(5) each Guarantor, unless it is the other party to the transactions described above, in which case subclause (b) of the second succeeding paragraph shall apply, shall have by supplemental indenture confirmed that its Guarantee shall apply to such Person’s obligations under the Senior Notes Indenture, the Senior Notes and the Senior Notes Registration Rights Agreement if the exchange offer contemplated therein has not been consummated or if the Issuers continue to have an obligation to file or maintain the effectiveness of a shelf registration statement as provided under such agreement;
(6) the Co-Issuer, unless it is the party to the transactions described above, in which case clause (3) of the third succeeding paragraph shall apply, shall have by supplemental indenture confirmed that it continues to be a co-obligor of the Senior Notes; and
(7) the Company (or, if applicable, the Successor Company) shall have delivered to the Senior Notes Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Senior Notes Indenture.
The Successor Company will succeed to, and be substituted for the Company, as the case may be, under the Senior Notes Indenture, the Guarantees and the Senior Notes, as applicable. Notwithstanding the foregoing clauses (3) and (4),
(1) any Restricted Subsidiary may consolidate with or merge into or transfer all or part of its properties and assets to the Company, and
(2) the Company may merge with an Affiliate of the Company, as the case may be, solely for the purpose of reincorporating the Company in the United States, any state thereof, the District of Columbia or any territory thereof so long as the amount of Indebtedness of the Company and its Restricted Subsidiaries is not increased thereby.
Guarantors. Subject to certain limitations described in the Senior Notes Indenture governing release of a Guarantee upon the sale, disposition or transfer of a Guarantor, no Guarantor will, and the Company will not permit any Guarantor to, consolidate or merge with or into or wind up into (whether or not the Company or Guarantor is the surviving corporation), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of its properties or assets, in one or more related transactions, to any Person unless:
(1) (a) such Guarantor is the surviving corporation or the Person formed by or surviving any such consolidation or merger (if other than such Guarantor) or to which such sale, assignment, transfer, lease, conveyance or other disposition will have been made is a corporation, partnership, trust or limited liability company organized or existing under the laws of the jurisdiction of organization of such Guarantor, as the case may be, or the laws of the United States, any state thereof, the District of Columbia or any territory thereof (such Guarantor or such Person, as the case may be, being herein called the “Successor Person”);
(b) the Successor Person, if other than such Guarantor, expressly assumes all the obligations of such Guarantor under the Senior Notes Indenture and such Guarantor’s related Guarantee pursuant to supplemental indentures or other documents or instruments in form reasonably satisfactory to the Senior Notes Trustee;
(c) immediately after such transaction, no Default or Event of Default exists; and
(d) the Company shall have delivered to the Senior Notes Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indentures, if any, comply with the Senior Notes Indenture; or
241
Table of Contents
(2) the transaction is made in compliance with the covenant described under “Repurchase at the Option of Holders—Asset Sales.”
Subject to certain limitations described in the Senior Notes Indenture, the Successor Person will succeed to, and be substituted for, such Guarantor under the Senior Notes Indenture and such Guarantor’s Guarantee. Notwithstanding the foregoing, any Guarantor may (i) merge into or transfer all or part of its properties and assets to another Guarantor or the Company, (ii) merge with an Affiliate of the Company solely for the purpose of reincorporating the Guarantor in the United States, any state thereof, the District of Columbia or any territory thereof or (iii) convert into a corporation, partnership, limited partnership, limited liability company or trust organized under the laws of the jurisdiction of organization of such Guarantor, in each case without regard to the requirements set forth in the preceding paragraph.
Co-Issuer. The Co-Issuer may not, directly or indirectly, consolidate or merge with or into or wind up into (whether or not the Co-Issuer is the surviving corporation), or sell, assign, transfer, lease, convey or otherwise dispose of all or substantially all of the Co-Issuer’s properties or assets, in one or more related transactions, to any Person unless:
(1) (a) concurrently therewith, a corporate Wholly-Owned Subsidiary that is a Restricted Subsidiary of the Company organized and validly existing under the laws of the United States, any state thereof, the District of Columbia or any territory thereof (which may be the continuing Person as a result of such transaction) expressly assumes all the obligations of the Co-Issuer under the Senior Notes, pursuant to supplemental indentures or other documents or instruments in form reasonably satisfactory to the Senior Notes Trustee, and the Senior Notes Registration Rights Agreement if the exchange offer contemplated therein has not been consummated or if the Issuers continue to have an obligation to file or maintain the effectiveness of a shelf registration statement as provided under such agreement; or
(b) after giving effect thereto, at least one obligor on the Senior Notes shall be a corporation organized and validly existing under the laws of the United States, any state thereof, the District of Columbia or any territory thereof;
(2) immediately after such transaction, no Default or Event of Default will have occurred and be continuing; and
(3) the Co-Issuer shall have delivered to the Senior Notes Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that such consolidation, merger or transfer and such supplemental indenture, if any, comply with the Senior Notes Indenture.
Transactions with Affiliates
The Company will not, and will not permit any of its Restricted Subsidiaries to, make any payment to, or sell, lease, transfer or otherwise dispose of any of its properties or assets to, or purchase any property or assets from, or enter into or make or amend, any transaction, contract, agreement, understanding, loan, advance or guarantee with, or for the benefit of any Affiliate of the Company (each of the foregoing, an “Affiliate Transaction”) involving aggregate payments or consideration in excess of $12.5 million, unless:
(1) such Affiliate Transaction is on terms that are not materially less favorable to the Company or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Company or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis; and
242
Table of Contents
(2) the Company delivers to the Senior Notes Trustee, with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate payments or consideration in excess of $25.0 million, a resolution adopted by the majority of the board of directors of the Company approving such Affiliate Transaction and set forth in an Officer’s Certificate certifying that such Affiliate Transaction complies with clause (1) above.
The foregoing provisions will not apply to the following:
(1) transactions between or among the Company or any of its Restricted Subsidiaries;
(2) Restricted Payments permitted by the provisions of the Senior Notes Indenture described above under the covenant “—Limitation on Restricted Payments” and the definition of “Permitted Investments”;
(3) the payment of management, consulting, monitoring and advisory fees and related expenses to the Investors pursuant to the Sponsor Management Agreement in an aggregate amount in any fiscal year not to exceed the greater of $7.0 million and 2.0% of EBITDA for such fiscal year (calculated, solely for the purpose of this clause (3), assuming (a) that such fees and related expenses had not been paid, when calculating Net Income, and (b) without giving effect to clause (h) of the definition of EBITDA) (plus any unpaid management, consulting, monitoring and advisory fees and related expenses within such amount accrued in any prior year) and the termination fees pursuant to the Sponsor Management Agreement not to exceed the amount set forth in the Sponsor Management Agreement as in effect on the Issue Date or any amendment thereto (so long as any such amendment is not disadvantageous to the Holders when taken as a whole as compared to the Sponsor Management Agreement in effect on the Issue Date);
(4) the payment of reasonable and customary fees paid to, and indemnities provided for the benefit of, former, current or future officers, directors, employees or consultants of Company, any of its direct or indirect parent companies or any of its Restricted Subsidiaries;
(5) transactions in which the Company or any of its Restricted Subsidiaries, as the case may be, delivers to the Senior Notes Trustee a letter from an Independent Financial Advisor stating that such transaction is fair to the Company or such Restricted Subsidiary from a financial point of view or stating that such terms are not materially less favorable to the Company or its relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Company or such Restricted Subsidiary with an unrelated Person on an arm’s-length basis;
(6) any agreement as in effect as of the Issue Date, or any amendment thereto (so long as any such amendment is not disadvantageous to the Holders when taken as a whole as compared to the applicable agreement as in effect on the Issue Date);
(7) the existence of, or the performance by the Company or any of its Restricted Subsidiaries of its obligations under the terms of, any stockholders agreement (including any registration rights agreement or purchase agreement related thereto) to which it is a party as of the Issue Date and any similar agreements which it may enter into thereafter; provided, however, that the existence of, or the performance by the Company or any of its Restricted Subsidiaries of obligations under any future amendment to any such existing agreement or under any similar agreement entered into after the Issue Date shall only be permitted by this clause (7) to the extent that the terms of any such amendment or new agreement are not otherwise disadvantageous to the Holders when taken as a whole;
(8) the Transactions and the payment of all fees and expenses related to the Transactions;
(9) transactions with customers, clients, suppliers, or purchasers or sellers of goods or services, in each case in the ordinary course of business and otherwise in compliance with the terms of the Senior Notes Indenture which are fair to the Company and its Restricted Subsidiaries, in the reasonable determination of the board of directors of the Company or the senior management thereof, or are on terms at least as favorable as might reasonably have been obtained at such time from an unaffiliated party;
243
Table of Contents
(10) the issuance of Equity Interests (other than Disqualified Stock) of the Company to any Permitted Holder or to any director, officer, employee or consultant (or their respective estates, investment funds, investment vehicles, spouses or former spouses) of the Company or any direct or indirect parent companies of any of its Subsidiaries;
(11) sales of accounts receivable, or participations therein, in connection with any Receivables Facility;
(12) payments by the Company or any of its Restricted Subsidiaries to any of the Investors made for any financial advisory, financing, underwriting or placement services or in respect of other investment banking activities, including, without limitation, in connection with acquisitions or divestitures which payments are approved by a majority of the board of directors of the Company in good faith;
(13) payments or loans (or cancellation of loans) to employees or consultants of the Company, any of its direct or indirect parent companies or any of its Restricted Subsidiaries and employment agreements, stock option plans and other similar arrangements with such employees or consultants which, in each case, are approved by the Company in good faith; and
(14) Investments by the Investors in securities of the Company or any of its Restricted Subsidiaries so long as (i) the Investment is being offered generally to other investors on the same or more favorable terms and (ii) the Investment constitutes less than 5.0% of the proposed or outstanding issue amount of such class of securities.
Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries
The Company will not, and will not permit any of its Restricted Subsidiaries that are not Guarantors to, directly or indirectly, create or otherwise cause or suffer to exist or become effective any consensual encumbrance or consensual restriction on the ability of any Restricted Subsidiary that is not a Guarantor to:
(1) (a) pay dividends or make any other distributions to the Company or any of its Restricted Subsidiaries on its Capital Stock or with respect to any other interest or participation in, or measured by, its profits, or
(b) pay any liabilities owed to the Company or any of its Restricted Subsidiaries;
(2) make loans or advances to the Company or any of its Restricted Subsidiaries; or
(3) sell, lease or transfer any of its properties or assets to the Company or any of its Restricted Subsidiaries, except (in each case) for such encumbrances or restrictions existing under or by reason of:
(a) contractual encumbrances or restrictions in effect on the Issue Date, including pursuant to the Senior Credit Facilities and the related documentation and Hedging Obligations and pursuant to the terms of the Senior Subordinated Notes, the Existing Senior Notes and the Senior Secured Notes Indenture;
(b) the Senior Notes Indenture and the Senior Notes;
(c) purchase money obligations for property acquired in the ordinary course of business that impose restrictions of the nature discussed in clause (3) above on the property so acquired;
(d) applicable law or any applicable rule, regulation or order;
(e) any agreement or other instrument of a Person acquired by the Company or any Restricted Subsidiaries in existence at the time of such acquisition (but not created in contemplation thereof), which encumbrance or restriction is not applicable to any Person, or the properties or assets of any Person, other than the Person and its Subsidiaries, or the property or assets of the Person and its Subsidiaries, so acquired;
(f) contracts for the sale of assets, including customary restrictions with respect to a Subsidiary of the Company pursuant to an agreement that has been entered into for the sale or disposition of all or substantially all of the Capital Stock or assets of such Subsidiary;
(g) Secured Indebtedness otherwise permitted to be incurred pursuant to the covenants described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Liens” that limit the right of the debtor to dispose of the assets securing such Indebtedness;
244
Table of Contents
(h) restrictions on cash or other deposits or net worth imposed by customers under contracts entered into in the ordinary course of business;
(i) other Indebtedness, Disqualified Stock or Preferred Stock of Foreign Subsidiaries permitted to be incurred subsequent to the Issue Date pursuant to the provisions of the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(j) customary provisions in joint venture agreements and other similar agreements relating solely to such joint venture;
(k) customary provisions contained in leases or licenses of intellectual property and other agreements, in each case, entered into in the ordinary course of business;
(l) any encumbrances or restrictions of the type referred to in clauses (1), (2) and (3) above imposed by any amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings of the contracts, instruments or obligations referred to in clauses (a) through (k) above; provided that such amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings are, in the good faith judgment of the Company, no more restrictive with respect to such encumbrance and other restrictions taken as a whole than those prior to such amendment, modification, restatement, renewal, increase, supplement, refunding, replacement or refinancing; and
(m) restrictions created in connection with any Receivables Facility that, in the good faith determination of the Company are necessary or advisable to effect the transactions contemplated under such Receivables Facility.
Limitation on Guarantees of Indebtedness by Restricted Subsidiaries
The Company will not permit any of its Wholly-Owned Subsidiaries that are Restricted Subsidiaries (and non-Wholly Owned Subsidiaries if such non-Wholly Owned Subsidiaries guarantee other capital markets debt securities), other than a Guarantor, the Co-Issuer or a Foreign Subsidiary guaranteeing Indebtedness of another Foreign Subsidiary, to guarantee the payment of any Indebtedness of the Company, the Co-Issuer or any other Guarantor unless:
(1) such Restricted Subsidiary within 30 days executes and delivers a supplemental indenture to the Senior Notes Indenture providing for a Guarantee by such Restricted Subsidiary, except that with respect to a guarantee of Indebtedness of the Company, the Co-Issuer or any Guarantor:
(a) if the Senior Notes or such Guarantor’s Guarantee are subordinated in right of payment to such Indebtedness, the Guarantee under the supplemental indenture shall be subordinated to such Restricted Subsidiary’s guarantee with respect to such Indebtedness substantially to the same extent as the Senior Notes are subordinated to such Indebtedness; and
(b) if such Indebtedness is by its express terms subordinated in right of payment to the Senior Notes or such Guarantor’s Guarantee, any such guarantee by such Restricted Subsidiary with respect to such Indebtedness shall be subordinated in right of payment to such Guarantee substantially to the same extent as such Indebtedness is subordinated to the Senior Notes; and
(c) such Restricted Subsidiary waives and will not in any manner whatsoever claim or take the benefit or advantage of, any rights of reimbursement, indemnity or subrogation or any other rights against the Company or any other Restricted Subsidiary as a result of any payment by such Restricted Subsidiary under its Guarantee;
provided that this covenant shall not be applicable to any guarantee of any Restricted Subsidiary that existed at the time such Person became a Restricted Subsidiary and was not incurred in connection with, or in contemplation of, such Person becoming a Restricted Subsidiary.
245
Table of Contents
Reports and Other Information
Notwithstanding that the Company may not be subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act or otherwise report on an annual and quarterly basis on forms provided for such annual and quarterly reporting pursuant to rules and regulations promulgated by the SEC, the Senior Notes Indenture will require the Company to file with the SEC (and make available to the Senior Notes Trustee and Holders of the Senior Notes (without exhibits), without cost to any Holder, within 15 days after it files them with the SEC) from and after the Issue Date,
(1) within 90 days (or any other time period then in effect under the rules and regulations of the Exchange Act with respect to the filing of a Form 10-K by a non-accelerated filer) after the end of each fiscal year, annual reports on Form 10-K, or any successor or comparable form, containing the information required to be contained therein, or required in such successor or comparable form;
(2) within 45 days after the end of each of the first three fiscal quarters of each fiscal year, reports on Form 10-Q containing all quarterly information that would be required to be contained in Form 10-Q, or any successor or comparable form;
(3) promptly from time to time after the occurrence of an event required to be therein reported, such other reports on Form 8-K, or any successor or comparable form; and
(4) any other information, documents and other reports which the Company would be required to file with the SEC if it were subject to Section 13 or 15(d) of the Exchange Act;
in each case, in a manner that complies in all material respects with the requirements specified in such form; provided that the Company shall not be so obligated to file such reports with the SEC if the SEC does not permit such filing, in which event the Company makes available such information to prospective purchasers of Senior Notes, in addition to providing such information to the Senior Notes Trustee and the Holders of the Senior Notes, in each case within 15 days after the time the Company would be required to file such information with the SEC, if it were subject to Section 13 or 15(d) of the Exchange Act. In addition, to the extent not satisfied by the foregoing, the Company will agree that, for so long as any Senior Notes are outstanding, it will furnish to Holders and to securities analysts and prospective investors, upon their request, the information required to be delivered pursuant to Rule 144A(d)(4) under the Securities Act.
In the event that any direct or indirect parent company of the Company becomes a Guarantor of the Senior Notes, the Senior Notes Indenture permits the Company to satisfy its obligations in this covenant with respect to financial information relating to the Company by furnishing financial information relating to such parent company; provided that the same is accompanied by consolidating information that explains in reasonable detail the differences between the information relating to such parent, on the one hand, and the information relating to the Company and its Restricted Subsidiaries on a standalone basis, on the other hand.
Notwithstanding the foregoing, such requirements shall be deemed satisfied prior to the commencement of the exchange offer or the effectiveness of the shelf registration statement (1) by the filing with the SEC of the exchange offer registration statement or shelf registration statement (or any other registration statement), and any amendments thereto, with such financial information that satisfies Regulation S-X of the Securities Act or (2) by posting reports that would be required to be filed substantially in the form required by the SEC on the Company’s website (or on the website of any of its parent companies) or providing such reports to the Senior Notes Trustee, with financial information that satisfied Regulation S-X of the Securities Act, subject to exceptions consistent with the presentation of financial information in this prospectus, to the extent filed within the times specified above.
246
Table of Contents
Limitation on Business Activities of the Co-Issuer
The Co-Issuer may not hold any assets, become liable for any obligations or engage in any business activities; provided that it may be a co-obligor with respect to the Senior Notes or any other Indebtedness issued by the Company, and may engage in any activities directly related thereto or necessary in connection therewith. The Co-Issuer shall be a Wholly-Owned Subsidiary of the Company at all times.
Events of Default and Remedies
The Senior Notes Indenture provides that each of the following is an Event of Default:
(1) default in payment when due and payable (whether at maturity, upon redemption, acceleration or otherwise), of principal of, or premium, if any, on the Senior Notes;
(2) default for 30 days or more in the payment when due of interest or Additional Interest on or with respect to the Senior Notes;
(3) failure by the Company, the Co-Issuer or any Guarantor for 60 days after receipt of written notice given by the Senior Notes Trustee or the Holders of not less than 25% in principal amount of the Senior Notes to comply with any of its obligations, covenants or agreements (other than a default referred to in clauses (1) and (2) above) contained in the Senior Notes Indenture or the Senior Notes;
(4) default under any mortgage, indenture or instrument under which there is issued or by which there is secured or evidenced any Indebtedness for money borrowed by the Company or any of its Restricted Subsidiaries or the payment of which is guaranteed by the Company or any of its Restricted Subsidiaries, other than Indebtedness owed to the Company or a Restricted Subsidiary, whether such Indebtedness or guarantee now exists or is created after the issuance of the Senior Notes, if both:
(a) such default either results from the failure to pay any principal of such Indebtedness at its stated final maturity (after giving effect to any applicable grace periods) or relates to an obligation other than the obligation to pay principal of any such Indebtedness at its stated final maturity and results in the holder or holders of such Indebtedness causing such Indebtedness to become due prior to its stated maturity; and
(b) the principal amount of such Indebtedness, together with the principal amount of any other such Indebtedness in default for failure to pay principal at stated final maturity (after giving effect to any applicable grace periods), or the maturity of which has been so accelerated, aggregate $50.0 million or more at any one time outstanding;
(5) failure by the Company or any Significant Subsidiary (including the Co-Issuer) to pay final judgments aggregating in excess of $50.0 million, which final judgments remain unpaid, undischarged and unstayed for a period of more than 60 days after such judgment becomes final, and in the event such judgment is covered by insurance, an enforcement proceeding has been commenced by any creditor upon such judgment or decree which is not promptly stayed;
(6) certain events of bankruptcy or insolvency with respect to the Company or any Significant Subsidiary; or
(7) the Guarantee of any Significant Subsidiary shall for any reason cease to be in full force and effect or be declared null and void or any responsible officer of any Guarantor that is a Significant Subsidiary, as the case may be, denies that it has any further liability under its Guarantee or gives notice to such effect, other than by reason of the termination of the Senior Notes Indenture or the release of any such Guarantee in accordance with the Senior Notes Indenture.
If any Event of Default (other than of a type specified in clause (6) above) occurs and is continuing under the Senior Notes Indenture, the Senior Notes Trustee or the Holders of at least 25% in principal amount of the then total outstanding Senior Notes may declare the principal, premium, if any, interest and any other monetary obligations on all the then outstanding Senior Notes to be due and payable immediately.
247
Table of Contents
Upon the effectiveness of such declaration, such principal and interest are due and payable immediately. Notwithstanding the foregoing, in the case of an Event of Default arising under clause (6) of the first paragraph of this section, all outstanding Senior Notes arecome due and payable without further action or notice. The Senior Notes Indenture provides that the Senior Notes Trustee may withhold from the Holders notice of any continuing Default, except a Default relating to the payment of principal, premium, if any, or interest, if it determines that withholding notice is in their interest. In addition, the Senior Notes Trustee shall have no obligation to accelerate the Senior Notes if in the best judgment of the Senior Notes Trustee acceleration is not in the best interest of the Holders of the Senior Notes.
The Senior Notes Indenture provides that the Holders of a majority in aggregate principal amount of the then outstanding Senior Notes by notice to the Senior Notes Trustee may on behalf of the Holders of all of the Senior Notes waive any existing Default and its consequences under the Senior Notes Indenture except a continuing Default in the payment of interest on, premium, if any, or the principal of any Senior Note held by a non-consenting Holder and rescind any acceleration with respect to the Senior Notes and its consequences (provided such rescission would not conflict with any judgment of a court of competent jurisdiction). In the event of any Event of Default specified in clause (4) above, such Event of Default and all consequences thereof (excluding any resulting payment default, other than as a result of acceleration of the Senior Notes) shall be annulled, waived and rescinded, automatically and without any action by the Senior Notes Trustee or the Holders, if within 20 days after such Event of Default arose:
(1) the Indebtedness or guarantee that is the basis for such Event of Default has been discharged;
(2) holders thereof have rescinded or waived the acceleration, notice or action (as the case may be) giving rise to such Event of Default; or
(3) the Default that is the basis for such Event of Default has been cured.
Subject to the provisions of the Senior Notes Indenture relating to the duties of the Senior Notes Trustee thereunder, in case an Event of Default occurs and is continuing, the Senior Notes Trustee are under no obligation to exercise any of the rights or powers under the Senior Notes Indenture at the request or direction of any of the Holders of the Senior Notes unless the Holders have offered to the Senior Notes Trustee reasonable indemnity or security against any loss, liability or expense. Except to enforce the right to receive payment of principal, premium (if any) or interest when due, no Holder of a Senior Note may pursue any remedy with respect to the Senior Notes Indenture or the Senior Notes unless:
(1) such Holder has previously given the Senior Notes Trustee notice that an Event of Default is continuing;
(2) Holders of at least 25% in principal amount of the total outstanding Senior Notes have requested the Senior Notes Trustee to pursue the remedy;
(3) Holders of the Senior Notes have offered the Senior Notes Trustee reasonable security or indemnity against any loss, liability or expense;
(4) the Senior Notes Trustee has not complied with such request within 60 days after the receipt thereof and the offer of security or indemnity; and
(5) Holders of a majority in principal amount of the total outstanding Senior Notes have not given the Senior Notes Trustee a direction inconsistent with such request within such 60-day period.
Subject to certain restrictions, under the Senior Notes Indenture, the Holders of a majority in principal amount of the total outstanding Senior Notes are given the right to direct the time, method and place of conducting any proceeding for any remedy available to the Senior Notes Trustee or of exercising any trust or power conferred on the Senior Notes Trustee. The Senior Notes Trustee, however, may refuse to follow any direction that conflicts with law or the Senior Notes Indenture or that the Senior Notes Trustee determines is unduly prejudicial to the rights of any other Holder of a Senior Note or that would involve the Senior Notes Trustee in personal liability.
248
Table of Contents
The Senior Notes Indenture provides that the Company is required to deliver to the Senior Notes Trustee annually a statement regarding compliance with the Senior Notes Indenture, and the Company is required, within 30 days, upon becoming aware of any Default, to deliver to the Senior Notes Trustee a statement specifying such Default.
No Personal Liability of Directors, Officers, Employees and Stockholders
No director, officer, employee, incorporator or stockholder of the Issuers or any Guarantor or any of their parent companies shall have any liability for any obligations of the Issuers or the Guarantors under the Senior Notes, the Guarantees or the Senior Notes Indenture or for any claim based on, in respect of, or by reason of such obligations or their creation. Each Holder by accepting the Senior Notes waives and releases all such liability. The waiver and release are part of the consideration for issuance of the Senior Notes. Such waiver may not be effective to waive liabilities under the federal securities laws and it is the view of the SEC that such waiver is against public policy.
Legal Defeasance and Covenant Defeasance
The obligations of the Issuers and the Guarantors under the Senior Notes Indenture, the Senior Notes and the Guarantees, as the case may be, will terminate (other than certain obligations) and are released upon payment in full of all of the Senior Notes. The Issuers may, at their option and at any time, elect to have all of their obligations discharged with respect to the Senior Notes and have each Guarantor’s obligation discharged with respect to its Guarantee (“Legal Defeasance”) and cure all then existing Events of Default except for:
(1) the rights of Holders of Senior Notes to receive payments in respect of the principal of, premium, if any, and interest on the Senior Notes when such payments are due solely out of the trust created pursuant to the Senior Notes Indenture;
(2) the Issuers’ obligations with respect to Senior Notes concerning issuing temporary Senior Notes, registration of such Senior Notes, mutilated, destroyed, lost or stolen Senior Notes and the maintenance of an office or agency for payment and money for security payments held in trust;
(3) the rights, powers, trusts, duties and immunities of the Senior Notes Trustee, and the Issuers’ obligations in connection therewith; and
(4) the Legal Defeasance provisions of the Senior Notes Indenture.
In addition, the Issuers may, at their option and at any time, elect to have their obligations and those of each Guarantor released with respect to substantially all the restrictive covenants that are described in the Senior Notes Indenture (“Covenant Defeasance”) and thereafter any omission to comply with such obligations shall not constitute a Default with respect to the Senior Notes. In the event Covenant Defeasance occurs, certain events (not including bankruptcy, receivership, rehabilitation and insolvency events pertaining to the Issuers) described under “Events of Default and Remedies” will no longer constitute an Event of Default with respect to the Senior Notes.
In order to exercise either Legal Defeasance or Covenant Defeasance with respect to the Senior Notes:
(1) the Issuers must irrevocably deposit with the Senior Notes Trustee, in trust, for the benefit of the Holders of the Senior Notes, cash in U.S. dollars, Government Securities, or a combination thereof, in such amounts as are sufficient, in the opinion of a nationally recognized firm of independent public accountants, to pay the principal of, premium, if any, and interest due on the Senior Notes on the stated maturity date or on the redemption date, as the case may be, of such principal, premium, if any, or interest on such Senior Notes and the Company must specify whether such Senior Notes are being defeased to maturity or to a particular redemption date;
249
Table of Contents
(2) in the case of Legal Defeasance, the Company shall have delivered to the Senior Notes Trustee an Opinion of Counsel reasonably acceptable to the Senior Notes Trustee confirming that, subject to customary assumptions and exclusions,
(a) the Issuers have received from, or there has been published by, the United States Internal Revenue Service a ruling, or
(b) since the issuance of the Senior Notes, there has been a change in the applicable U.S. federal income tax law,
in either case to the effect that, and based thereon such Opinion of Counsel shall confirm that, subject to customary assumptions and exclusions, the Holders of the Senior Notes will not recognize income, gain or loss for U.S. federal income tax purposes, as applicable, as a result of such Legal Defeasance and are subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Legal Defeasance had not occurred;
(3) in the case of Covenant Defeasance, the Issuers shall have delivered to the Senior Notes Trustee an Opinion of Counsel reasonably acceptable to the Senior Notes Trustee confirming that, subject to customary assumptions and exclusions, the Holders of the Senior Notes will not recognize income, gain or loss for U.S. federal income tax purposes as a result of such Covenant Defeasance and are subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Covenant Defeasance had not occurred;
(4) no Default (other than that resulting from borrowing funds to be applied to make the deposit required to effect such Legal Defeasance or Covenant Defeasance and any similar and simultaneous deposit relating to other Indebtedness and, in each case, the granting of Liens in connection therewith) shall have occurred and be continuing on the date of such deposit;
(5) such Legal Defeasance or Covenant Defeasance shall not result in a breach or violation of, or constitute a default under the Senior Credit Facilities or any other material agreement or instrument (other than the Senior Notes Indenture) to which, the Issuers or any Guarantor is a party or by which the Issuers or any Guarantor is bound (other than that resulting with respect to any Indebtedness being defeased from any borrowing of funds to be applied to make the deposit required to effect such Legal Defeasance or Covenant Defeasance and any similar and simultaneous deposit relating to such Indebtedness, and the granting of Liens in connection therewith);
(6) the Issuers shall have delivered to the Senior Notes Trustee an Opinion of Counsel to the effect that, as of the date of such opinion and subject to customary assumptions and exclusions following the deposit, the trust funds are not subject to the effect of Section 547 of Title 11 of the United States Code;
(7) the Issuers shall have delivered to the Senior Notes Trustee an Officer’s Certificate stating that the deposit was not made by the Issuers with the intent of defeating, hindering, delaying or defrauding any creditors of the Issuers or any Guarantor or others; and
(8) the Issuers shall have delivered to the Senior Notes Trustee an Officer’s Certificate and an Opinion of Counsel (which Opinion of Counsel may be subject to customary assumptions and exclusions) each stating that all conditions precedent provided for or relating to the Legal Defeasance or the Covenant Defeasance, as the case may be, have been complied with.
250
Table of Contents
Satisfaction and Discharge
The Senior Notes Indenture are discharged and will cease to be of further effect as to all Senior Notes, when:
(1) either
(a) all Senior Notes theretofore authenticated and delivered, except lost, stolen or destroyed Senior Notes which have been replaced or paid and Senior Notes for whose payment money has theretofore been deposited in trust, have been delivered to the Senior Notes Trustee for cancellation; or
(b) all Senior Notes not theretofore delivered to the Senior Notes Trustee for cancellation have become due and payable by reason of the making of a notice of redemption or otherwise, arecome due and payable within one year or are to be called for redemption within one year under arrangements satisfactory to the Senior Notes Trustee for the giving of notice of redemption by the Senior Notes Trustee in the name, and at the expense, of the Issuers and the Issuers or any Guarantor have irrevocably deposited or caused to be deposited with the Senior Notes Trustee as trust funds in trust solely for the benefit of the Holders of the Senior Notes, cash in U.S. dollars, Government Securities, or a combination thereof, in such amounts as are sufficient without consideration of any reinvestment of interest to pay and discharge the entire Indebtedness on the Senior Notes not theretofore delivered to the Senior Notes Trustee for cancellation for principal, premium, if any, and accrued interest to the date of maturity or redemption;
(2) no Default (other than that resulting from borrowing funds to be applied to make such deposit or any similar or simultaneous deposit relating to other Indebtedness) with respect to the Senior Notes Indenture or the Senior Notes shall have occurred and be continuing on the date of such deposit or shall occur as a result of such deposit and such deposit will not result in a breach or violation of, or constitute a default under the Senior Credit Facilities, the Senior Secured Notes Indenture, the indentures governing the Senior Subordinated Notes and the Existing Senior Notes or any other material agreement or instrument (other than the Senior Notes Indenture) to which the Issuers or any Guarantor is a party or by which the Issuers or any Guarantor is bound (other than that resulting from borrowing funds to be applied to make such deposit and any similar and simultaneous deposit relating to other Indebtedness and, in each case, the granting of Liens in connection therewith);
(3) the Issuers have paid or caused to be paid all sums payable by it under the Senior Notes Indenture; and
(4) the Issuers have delivered irrevocable instructions to the Senior Notes Trustee to apply the deposited money toward the payment of the Senior Notes at maturity or the redemption date, as the case may be.
In addition, the Issuers must deliver an Officer’s Certificate and an Opinion of Counsel to the Senior Notes Trustee stating that all conditions precedent to satisfaction and discharge have been satisfied.
Amendment, Supplement and Waiver
Except as provided in the next two succeeding paragraphs, the Senior Notes Indenture, any Guarantee and the Senior Notes may be amended or supplemented with the consent of the Holders of at least a majority in principal amount of the Senior Notes then outstanding, including consents obtained in connection with a purchase of, or tender offer or exchange offer for, Senior Notes and any existing Default or compliance with any provision of the Senior Notes Indenture or the Senior Notes issued thereunder may be waived with the consent of the Holders of a majority in principal amount of the then outstanding Senior Notes, other than Senior Notes beneficially owned by the Issuers or their Affiliates (including consents obtained in connection with a purchase of or tender offer or exchange offer for the Senior Notes).
251
Table of Contents
The Senior Notes Indenture provides that, without the consent of each affected Holder of Senior Notes, an amendment or waiver may not, with respect to any Senior Notes held by a non-consenting Holder:
(1) reduce the principal amount of such Senior Notes whose Holders must consent to an amendment, supplement or waiver;
(2) reduce the principal of or change the fixed final maturity of any such Senior Note or alter or waive the provisions with respect to the redemption of such Senior Notes (other than provisions relating to the covenants described above under the caption “Repurchase at the Option of Holders”);
(3) reduce the rate of or change the time for payment of interest on any Senior Note;
(4) waive a Default in the payment of principal of or premium, if any, or interest on the Senior Notes, except a rescission of acceleration of the Senior Notes by the Holders of at least a majority in aggregate principal amount of the Senior Notes and a waiver of the payment default that resulted from such acceleration, or in respect of a covenant or provision contained in the Senior Notes Indenture or any Guarantee which cannot be amended or modified without the consent of all Holders;
(5) make any Senior Note payable in money other than U.S. dollars;
(6) make any change in the provisions of the Senior Notes Indenture relating to waivers of past Defaults or the rights of Holders to receive payments of principal of or premium, if any, or interest or Additional Interest on the Senior Notes;
(7) make any change in these amendment and waiver provisions;
(8) impair the right of any Holder to receive payment of principal of, or interest on such Holder’s Senior Notes on or after the due dates therefor or to institute suit for the enforcement of any payment on or with respect to such Holder’s Senior Notes or the Guarantees;
(9) make any change to or modify the ranking of the Senior Notes that would adversely affect the Holders;
(10) except as expressly permitted by the Senior Notes Indenture, modify the Guarantee of any Significant Subsidiary in any manner adverse to the Holders of the Senior Notes or release the Co-Issuer from its obligations under the Senior Notes Indenture.
Notwithstanding the foregoing, the Issuers, any Guarantor (with respect to a Guarantee or the Senior Notes Indenture to which it is a party) and the Senior Notes Trustee may amend or supplement the Senior Notes Indenture and any Guarantee or Senior Notes without the consent of any Holder;
(1) to cure any ambiguity, omission, mistake, defect or inconsistency;
(2) to provide for uncertificated Senior Notes of such series in addition to or in place of certificated Senior Notes;
(3) to comply with the covenant relating to mergers, consolidations and sales of assets;
(4) to provide for the assumption of the Issuers’ or any Guarantor’s obligations to the Holders;
(5) to make any change that would provide any additional rights or benefits to the Holders or that does not adversely affect the legal rights under the Senior Notes Indenture of any such Holder;
(6) to add covenants for the benefit of the Holders or to surrender any right or power conferred upon the Issuers or any Guarantor;
252
Table of Contents
(7) to comply with requirements of the SEC in order to effect or maintain the qualification of the Senior Notes Indenture under the Trust Indenture Act;
(8) to evidence and provide for the acceptance and appointment under the Senior Notes Indenture of a successor Trustee thereunder pursuant to the requirements thereof;
(9) to provide for the issuance of exchange notes or private exchange notes, which are identical to exchange notes except that they are not freely transferable;
(10) to provide for the issuance of Additional Senior Notes in accordance with the Senior Notes Indenture;
(11) to add a Guarantor under the Senior Notes Indenture or to release a Guarantor in accordance with the terms of the Senior Notes Indenture;
(12) to conform the text of the Senior Notes Indenture, Guarantees or the Senior Notes to any provisions of this “Description of Senior Notes” to the extent that such provision in this “Description of Senior Notes” was intended to be a verbatim recitation of a provision of the Senior Notes Indenture, Guarantee or Senior Notes;
(13) to make any amendment to the provisions of the Senior Notes Indenture relating to the transfer and legending of Senior Notes as permitted by the Senior Notes Indenture, including, without limitation to facilitate the issuance and administration of the Senior Notes; provided, however, that (i) compliance with the Senior Notes Indenture as so amended would not result in Senior Notes being transferred in violation of the Securities Act or any applicable securities law and (ii) such amendment does not materially and adversely affect the rights of Holders to transfer Senior Notes; or
(14) to make any other modifications to the Senior Notes or the Senior Notes Indentures of a formal, minor or technical nature or necessary to correct a manifest error, so long as such modification does not adversely affect the rights of any Holders of the Senior Notes in any material respect.
The consent of the Holders is not necessary under the Senior Notes Indenture to approve the particular form of any proposed amendment. It is sufficient if such consent approves the substance of the proposed amendment.
Notices
Notices given by publication are deemed given on the first date on which publication is made and notices given by first-class mail, postage prepaid, are deemed given five calendar days after mailing.
Concerning the Senior Notes Trustee
The Senior Notes Indenture contains certain limitations on the rights of the Senior Notes Trustee thereunder, should it become a creditor of the Issuers, to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim as security or otherwise. The Senior Notes Trustee is permitted to engage in other transactions; however, if it acquires any conflicting interest, it must eliminate such conflict within 90 days, apply to the SEC for permission to continue or resign.
The Senior Notes Indenture provides that the Holders of a majority in principal amount of the outstanding Senior Notes will have the right to direct the time, method and place of conducting any proceeding for exercising any remedy available to the Senior Notes Trustee, subject to certain exceptions. The Senior Notes Indenture provides that in case an Event of Default shall occur (which shall not be cured), the Senior Notes Trustee is required, in the exercise of its power, to use the degree of care of a prudent person in the conduct of his own affairs. Subject to such provisions, the Senior Notes Trustee is under no obligation to exercise any of its rights or powers under the Senior Notes Indenture at the request of any Holder of the Senior Notes, unless such Holder shall have offered to the Senior Notes Trustee security and indemnity satisfactory to it against any loss, liability or expense.
253
Table of Contents
Governing Law
The Senior Notes Indenture, the Senior Notes and any Guarantee are governed by and construed in accordance with the laws of the State of New York.
Certain Definitions
Set forth below are certain defined terms used in the Senior Notes Indenture. For purposes of the Senior Notes Indenture, unless otherwise specifically indicated, the term “consolidated” with respect to any Person refers to such Person consolidated with its Restricted Subsidiaries, and excludes from such consolidation any Unrestricted Subsidiary as if such Unrestricted Subsidiary were not an Affiliate of such Person.
“Acquired Indebtedness” means, with respect to any specified Person,
(1) Indebtedness of any other Person existing at the time such other Person is merged with or into or became a Restricted Subsidiary of such specified Person, including Indebtedness incurred in connection with, or in contemplation of, such other Person merging with or into or becoming a Restricted Subsidiary of such specified Person, and
(2) Indebtedness secured by a Lien encumbering any asset acquired by such specified Person.
“Additional Interest” means all additional interest then owing pursuant to the Senior Notes Registration Rights Agreement.
“Affiliate” of any specified Person means any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such specified Person. For purposes of this definition, “control” (including, with correlative meanings, the terms “controlling,” “controlled by” and “under common control with”), as used with respect to any Person, shall mean the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such Person, whether through the ownership of voting securities, by agreement or otherwise.
“Applicable Premium” means, with respect to any Senior Note on any Redemption Date, the greater of:
(1) 1.0% of the principal amount of such Senior Note; and
(2) the excess, if any, of (a) the present value at such Redemption Date of (i) the redemption price of such Senior Note at April 15, 2015 (each such redemption price being set forth in the table appearing above under the caption “Optional Redemption”), plus (ii) all required interest payments due on such Senior Note through April 15, 2015 (excluding accrued but unpaid interest to the Redemption Date), computed using a discount rate equal to the Treasury Rate as of such Redemption Date plus 50 basis points; over (b) the principal amount of such Senior Note.
Calculation of the Applicable Premium shall be made by the Company or on behalf of the Company by such Person as the Company shall designate; provided that such calculation or the correctness thereof shall not be a duty or obligation of the Senior Notes Trustee.
“Asset Sale” means:
(1) the sale, conveyance, transfer or other disposition, whether in a single transaction or a series of related transactions, of property or assets (including by way of a Sale and Lease-Back Transaction) of the Company or any of its Restricted Subsidiaries (each referred to in this definition as a “disposition”); or
(2) the issuance or sale of Equity Interests of any Restricted Subsidiary (other than Preferred Stock of Restricted Subsidiaries issued in compliance with the covenant described under “—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”), whether in a single transaction or a series of related transactions;
254
Table of Contents
in each case, other than:
(a) any disposition of Cash Equivalents or Investment Grade Securities or obsolete or worn-out equipment in the ordinary course of business or any disposition of inventory or goods (or other assets) held for sale in the ordinary course of business;
(b) the disposition of all or substantially all of the assets of the Company governed by, and in a manner permitted pursuant to, the provisions described above under “Certain Covenants—Merger, Consolidation or Sale of All or Substantially All Assets” or any disposition that constitutes a Change of Control pursuant to the Senior Notes Indenture;
(c) the making of any Restricted Payment or Permitted Investment that is permitted to be made, and is made, under the covenant described above under “Certain Covenants—Limitation on Restricted Payments”;
(d) any disposition of assets or issuance or sale of Equity Interests of any Restricted Subsidiary in any transaction or series of transactions with an aggregate fair market value of less than $10.0 million;
(e) any disposition of property or assets or issuance of securities by a Restricted Subsidiary of the Company to the Company or by the Company or a Restricted Subsidiary of the Company to another Restricted Subsidiary of the Company;
(f) to the extent allowable under Section 1031 of the Internal Revenue Code of 1986 or comparable law or regulation, any exchange of like property (excluding any boot thereon) for use in a Similar Business;
(g) the lease, assignment or sub-lease of any real or personal property in the ordinary course of business;
(h) any issuance or sale of Equity Interests in, or Indebtedness or other securities of, an Unrestricted Subsidiary;
(i) foreclosures on assets;
(j) sales of accounts receivable, or participations therein, in connection with any Receivables Facility; and
(k) any financing transaction with respect to the acquisition or construction of property by the Company or any Restricted Subsidiary after the Issue Date, including Sale and Lease-Back Transactions and asset securitizations permitted by the Senior Notes Indenture.
“Business Day” means each day which is not a Legal Holiday.
“Capital Stock” means:
(1) in the case of a corporation, corporate stock;
(2) in the case of an association or business entity, any and all shares, interests, participations, rights or other equivalents (however designated) of corporate stock;
(3) in the case of a partnership or limited liability company, partnership or membership interests (whether general or limited); and
(4) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distributions of assets of, the issuing Person.
“Capitalized Lease Obligation” means, at the time any determination thereof is to be made, the amount of the liability in respect of a capital lease that would at such time be required to be capitalized and reflected as a liability on a balance sheet (excluding the footnotes thereto) in accordance with GAAP.
255
Table of Contents
“Cash Equivalents” means:
(1) United States dollars;
(2) (a) €, or any national currency of any participating member state of the EMU; or
(b) such local currencies held by the Company or any Restricted Subsidiary from time to time in the ordinary course of business;
(3) securities issued or directly and fully and unconditionally guaranteed or insured by the U.S. government (or any agency or instrumentality thereof the securities of which are unconditionally guaranteed as a full faith and credit obligation of the U.S. government), with maturities of 24 months or less from the date of acquisition;
(4) certificates of deposit, time deposits and eurodollar time deposits with maturities of one year or less from the date of acquisition, bankers’ acceptances with maturities not exceeding one year and overnight bank deposits, in each case with any commercial bank having capital and surplus of not less than $500.0 million in the case of U.S. banks and $100.0 million (or the U.S. dollar equivalent as of the date of determination) in the case of non-U.S. banks;
(5) repurchase obligations for underlying securities of the types described in clauses (3) and (4) entered into with any financial institution meeting the qualifications specified in clause (4) above;
(6) commercial paper rated at least P-1 by Moody’s or at least A-1 by S&P and in each case maturing within 24 months after the date of creation thereof;
(7) marketable short-term money market and similar securities having a rating of at least P-2 or A-2 from either Moody’s or S&P, respectively (or, if at any time neither Moody’s nor S&P shall be rating such obligations, an equivalent rating from another Rating Agency) and in each case maturing within 24 months after the date of creation thereof;
(8) investment funds investing 95% of their assets in securities of the types described in clauses (1) through (7) above;
(9) readily marketable direct obligations issued by any state, commonwealth or territory of the United States or any political subdivision or taxing authority thereof having an Investment Grade Rating from either Moody’s or S&P with maturities of 24 months or less from the date of acquisition;
(10) Indebtedness or Preferred Stock issued by Persons with a rating of “A” or higher from S&P or “A2” or higher from Moody’s with maturities of 24 months or less from the date of acquisition; and
(11) Investments with average maturities of 24 months or less from the date of acquisition in money market funds rated AAA– (or the equivalent thereof) or better by S&P or Aaa3 (or the equivalent thereof) or better by Moody’s.
Notwithstanding the foregoing, Cash Equivalents shall include amounts denominated in currencies other than those set forth in clauses (1) and (2) above, provided that such amounts are converted into any currency listed in clauses (1) and (2) as promptly as practicable and in any event within ten Business Days following the receipt of such amounts.
“Change of Control” means the occurrence of any of the following:
(1) the sale, lease or transfer, in one or a series of related transactions, of all or substantially all of the assets of the Company and its Subsidiaries, taken as a whole, to any Person other than a Permitted Holder; or
(2) the Company becomes aware (by way of a report or any other filing pursuant to Section 13(d) of the Exchange Act, proxy, vote, written notice or otherwise) of the acquisition by any Person or group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act, or any successor provision), including any group acting for the purpose of acquiring, holding or disposing of securities (within the
256
Table of Contents
meaning of Rule 13d-5(b)(1) under the Exchange Act), other than the Permitted Holders, in a single transaction or in a related series of transactions, by way of merger, consolidation or other business combination or purchase of beneficial ownership (within the meaning of Rule 13d-3 under the Exchange Act, or any successor provision) of 50% or more of the total voting power of the Voting Stock of the Company or any of its direct or indirect parent companies holding directly or indirectly 100% of the total voting power of the Voting Stock of the Company.
“Consolidated Depreciation and Amortization Expense” means with respect to any Person for any period, the total amount of depreciation and amortization expense, including the amortization of deferred financing fees of such Person and its Restricted Subsidiaries for such period on a consolidated basis and otherwise determined in accordance with GAAP.
“Consolidated Interest Expense” means, with respect to any Person for any period, without duplication, the sum of:
(1) consolidated interest expense of such Person and its Restricted Subsidiaries for such period, to the extent such expense was deducted (and not added back) in computing Consolidated Net Income (including (a) amortization of original issue discount or premium resulting from the issuance of Indebtedness at less than or greater than par, as applicable, (b) all commissions, discounts and other fees and charges owed with respect to letters of credit or bankers acceptances, (c) non-cash interest payments (but excluding any non-cash interest expense attributable to the movement in the mark to market valuation of Hedging Obligations or other derivative instruments pursuant to GAAP), (d) the interest component of Capitalized Lease Obligations and (e) net payments, if any, pursuant to interest rate Hedging Obligations with respect to Indebtedness and excluding (t) accretion or accrual of discounted liabilities not constituting Indebtedness, (u) interest expense attributable to Indebtedness of a parent entity resulting from push-down accounting to the extent such Person and its Restricted Subsidiaries are not liable for the payment of such Indebtedness, (v) any expense resulting from the discounting of any outstanding Indebtedness in connection with the application of purchase accounting in connection with any acquisition, (w) any Additional Interest and any comparable “additional interest” with respect to other securities, (x) amortization of deferred financing fees, debt issuance costs, commissions, fees and expenses, (y) any expensing of bridge, commitment and other financing fees and (z) commissions, discounts, yield and other fees and charges (including any interest expense) related to any Receivables Facility); plus
(2) consolidated capitalized interest of such Person and its Restricted Subsidiaries for such period, whether paid or accrued; less
(3) interest income for such period.
For purposes of this definition, interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by such Person to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP.
“Consolidated Net Income” means, with respect to any Person for any period, the aggregate of the Net Income, of such Person and its Restricted Subsidiaries for such period, on a consolidated basis, and otherwise determined in accordance with GAAP; provided, however, that, without duplication,
(1) any after-tax effect of extraordinary, non-recurring or unusual gains or losses (less all fees and expenses relating thereto) or expenses (including relating to the Transactions to the extent incurred on or prior to April 15, 2012), severance, relocation costs and curtailments or modifications to pension and post-retirement employee benefit plans and other restructuring costs shall be excluded,
(2) the cumulative effect of a change in accounting principles during such period shall be excluded,
(3) any after-tax effect of income (loss) from disposed, abandoned, transferred, closed or discontinued operations and any net after-tax gains or losses on disposal of disposed, abandoned, transferred, closed or discontinued operations shall be excluded,
257
Table of Contents
(4) any after-tax effect of gains or losses (less all fees and expenses relating thereto) attributable to asset dispositions other than in the ordinary course of business, as determined in good faith by the Company, shall be excluded,
(5) the Net Income for such period of any Person that is not a Subsidiary or is an Unrestricted Subsidiary or that is accounted for by the equity method of accounting, shall be excluded; provided that Consolidated Net Income of the Company shall be increased by the amount of dividends or distributions or other payments that are actually paid in cash (or to the extent converted into cash) to the referent Person or a Restricted Subsidiary thereof in respect of such period,
(6) solely for the purpose of determining the amount available for Restricted Payments under clause (3)(a) of the first paragraph of “Certain Covenants—Limitation on Restricted Payments,” the Net Income for such period of any Restricted Subsidiary (other than any Guarantor) shall be excluded to the extent that the declaration or payment of dividends or similar distributions by that Restricted Subsidiary of its Net Income is not at the date of determination permitted without any prior governmental approval (which has not been obtained) or, directly or indirectly, by the operation of the terms of its charter or any agreement, instrument, judgment, decree, order, statute, rule, or governmental regulation applicable to that Restricted Subsidiary or its stockholders, unless such restriction with respect to the payment of dividends or similar distributions has been legally waived, provided that Consolidated Net Income of the Company are increased by the amount of dividends or other distributions or other payments actually paid in cash (or to the extent converted into cash) or Cash Equivalents to the Company or a Restricted Subsidiary thereof in respect of such period, to the extent not already included therein,
(7) effects of adjustments (including the effects of such adjustments pushed down to the Company and its Restricted Subsidiaries) in the property and equipment, inventory and other intangible assets, deferred revenue and debt line items in such Person’s consolidated financial statements pursuant to GAAP resulting from the application of purchase accounting in relation to the DJO Acquisition, the Transactions or any consummated acquisition or the amortization or write-off of any amounts thereof, net of taxes, shall be excluded,
(8) any after-tax effect of income (loss) from the early extinguishment of Indebtedness or Hedging Obligations or other derivative instruments shall be excluded,
(9) any impairment charge or asset write-off, in each case, pursuant to GAAP and the amortization of intangibles arising pursuant to GAAP shall be excluded,
(10) any non-cash compensation expense recorded from grants of stock appreciation or similar rights, stock options, restricted stock or other rights shall be excluded,
(11) any fees and expenses incurred during such period, or any amortization thereof for such period, in connection with any acquisition, disposition, recapitalization, Investment, Asset Sale, issuance or repayment of Indebtedness, issuance of Equity Interests, refinancing transaction or amendment or modification of any debt instrument (in each case, including any such transaction consummated prior to the Issue Date and any such transaction undertaken but not completed) and any charges or non-recurring merger costs incurred during such period as a result of any such transaction shall be excluded,
(12) accruals and reserves that are established or adjusted within twelve months after the Issue Date that are so required to be established or adjusted as a result of the Transactions in accordance with GAAP or changes as a result of a modification of accounting policies shall be excluded and
(13) to the extent covered by insurance and actually reimbursed, or, so long as the Issuers have made a determination that there exists reasonable evidence that such amount will in fact be reimbursed by the insurer and only to the extent that such amount is (a) not denied by the applicable carrier in writing within 180 days and (b) in fact reimbursed within 365 days of the date of such evidence (with a deduction for any amount so added back to the extent not so reimbursed within 365 days), expenses with respect to liability or casualty events or business interruption shall be excluded.
258
Table of Contents
Notwithstanding the foregoing, for the purpose of the covenant described under “Certain Covenants—Limitation on Restricted Payments” only (other than clause (3)(d) thereof), there shall be excluded from Consolidated Net Income any income arising from any sale or other disposition of Restricted Investments made by the Company and its Restricted Subsidiaries any repurchases and redemptions of Restricted Investments from the Company and its Restricted Subsidiaries, any repayments of loans and advances which constitute Restricted Investments by the Company or any of its Restricted Subsidiaries, any sale of the stock of an Unrestricted Subsidiary or any distribution or dividend from an Unrestricted Subsidiary, in each case only to the extent such amounts increase the amount of Restricted Payments permitted under such covenant pursuant to clause (3)(d) thereof.
“Consolidated Secured Debt Ratio” as of any date of determination means, the ratio of (1) Consolidated Total Indebtedness of the Company and its Restricted Subsidiaries that is secured by Liens as of the end of the most recent fiscal quarter for which internal financial statements are available immediately preceding the date on which such event for which such calculation is being made shall occur to (2) the Company’s EBITDA for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date on which such event for which such calculation is being made shall occur, in each case with such pro forma adjustments to Consolidated Total Indebtedness EBITDA as are appropriate and consistent with the pro forma adjustment provisions set forth in the definition of Fixed Charge Coverage Ratio.
“Consolidated Total Indebtedness” means, as at any date of determination, an amount equal to the sum of (1) the aggregate amount of all outstanding Indebtedness of the Company and its Restricted Subsidiaries on a consolidated basis consisting of Indebtedness for borrowed money, Obligations in respect of Capitalized Lease Obligations and debt obligations evidenced by promissory notes and similar instruments and (2) the aggregate amount of all outstanding Disqualified Stock of the Company and all Preferred Stock of its Restricted Subsidiaries on a consolidated basis, with the amount of such Disqualified Stock and Preferred Stock equal to the greater of their respective voluntary or involuntary liquidation preferences and maximum fixed repurchase prices, in each, case, determined on a consolidated basis in accordance with GAAP. For purposes hereof, the “maximum fixed repurchase price” of any Disqualified Stock or Preferred Stock that does not have a fixed repurchase price shall be calculated in accordance with the terms of such Disqualified Stock or Preferred Stock as if such Disqualified Stock or Preferred Stock were purchased on any date on which Consolidated Total Indebtedness shall be required to be determined pursuant to the Senior Notes Indenture, and if such price is based upon, or measured by, the fair market value of such Disqualified Stock or Preferred Stock, such fair market value shall be determined reasonably and in good faith by the Company.
“Contingent Obligations” means, with respect to any Person, any obligation of such Person guaranteeing any leases, dividends or other obligations that do not constitute Indebtedness (“primary obligations”) of any other Person (the “primary obligor”) in any manner, whether directly or indirectly, including, without limitation, any obligation of such Person, whether or not contingent,
(1) to purchase any such primary obligation or any property constituting direct or indirect security therefor,
(2) to advance or supply funds
(a) for the purchase or payment of any such primary obligation, or
(b) to maintain working capital or equity capital of the primary obligor or otherwise to maintain the net worth or solvency of the primary obligor, or
(3) to purchase property, securities or services primarily for the purpose of assuring the owner of any such primary obligation of the ability of the primary obligor to make payment of such primary obligation against loss in respect thereof.
“Credit Agreement” means the Credit Agreement entered into as of November 20, 2007 by and among the Company, the Co-Issuer, the Guarantors, the lenders party thereto in their capacities as lenders thereunder and Credit Suisse, Cayman Islands Branch, as Administrative Agent, including any guarantees, collateral documents,
259
Table of Contents
instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements, refundings or refinancings thereof and any indentures or credit facilities or commercial paper facilities with banks or other institutional lenders or investors that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount borrowable thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” above).
“Credit Facilities” means, with respect to the Company or any of its Restricted Subsidiaries, one or more debt facilities, including the Senior Credit Facilities, or other financing arrangements (including, without limitation, commercial paper facilities or indentures) providing for revolving credit loans, term loans, letters of credit or other long-term Indebtedness, including any notes, mortgages, guarantees, collateral documents, instruments and agreements executed in connection therewith, and any amendments, supplements, modifications, extensions, renewals, restatements or refundings thereof and any indentures or credit facilities or commercial paper facilities that replace, refund or refinance any part of the loans, notes, other credit facilities or commitments thereunder, including any such replacement, refunding or refinancing facility or indenture that increases the amount permitted to be borrowed thereunder or alters the maturity thereof (provided that such increase in borrowings is permitted under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”) or adds Restricted Subsidiaries as additional borrowers or guarantors thereunder and whether by the same or any other agent, lender or group of lenders.
“Default” means any event that is, or with the passage of time or the giving of notice or both would be, an Event of Default.
“Designated Non-cash Consideration” means the fair market value of non-cash consideration received by the Company or a Restricted Subsidiary in connection with an Asset Sale that is so designated as Designated Non-cash Consideration pursuant to an Officer’s Certificate, setting forth the basis of such valuation, executed by the principal financial officer of the Company, less the amount of cash or Cash Equivalents received in connection with a subsequent sale of or collection on such Designated Non-cash Consideration.
“Designated Preferred Stock” means Preferred Stock of the Company or any parent corporation thereof (in each case other than Disqualified Stock) that is issued for cash (other than to a Restricted Subsidiary or an employee stock ownership plan or trust established by the Company or any of its Subsidiaries) and is so designated as Designated Preferred Stock, pursuant to an Officer’s Certificate executed by the principal financial officer of the Company or the applicable parent corporation thereof, as the case may be, on the issuance date thereof, the cash proceeds of which are excluded from the calculation set forth in clause (3) of the first paragraph of the “Certain Covenants—Limitation on Restricted Payments” covenant and are not otherwise applied to make any other Restricted Payment.
“Disqualified Stock” means, with respect to any Person, any Capital Stock of such Person which, by its terms, or by the terms of any security into which it is convertible or for which it is putable or exchangeable, or upon the happening of any event, matures or is mandatorily redeemable (other than solely as a result of a change of control or asset sale) pursuant to a sinking fund obligation or otherwise, or is redeemable at the option of the holder thereof (other than solely as a result of a change of control or asset sale), in whole or in part, in each case prior to the date 91 days after the maturity date of the Senior Notes; provided, however, that if such Capital Stock is issued to any plan for the benefit of employees of the Company or its Subsidiaries or by any such plan to such employees, such Capital Stock shall not constitute Disqualified Stock solely because it may be required to be repurchased by the Company or its Subsidiaries in order to satisfy applicable statutory or regulatory obligations.
“DJO Acquisition” means the acquisition contemplated by the Agreement and Plan of Merger, dated as of July 15, 2007, by and among ReAble Therapeutics Finance LLC, Reaction Acquisition Merger Sub, Inc. and DJO Opco Holdings Inc. (f/k/a DJO Incorporated), and related financings.
260
Table of Contents
“EBITDA” means, with respect to any Person for any period, the Consolidated Net Income of such Person for such period
(1) increased (without duplication) by:
(a) provision for taxes based on income or profits or capital gains, including, without limitation, federal, state, foreign, franchise and similar taxes and foreign withholding taxes (including penalties and interest related to such taxes or arising from tax examinations) of such Person paid or accrued during such period to the extent the same was deducted (and not added back) in computing Consolidated Net Income; plus
(b) Fixed Charges of such Person for such period (including (x) net losses or Hedging Obligations or other derivative instruments entered into for the purpose of hedging interest rate risk and (y) costs of surety bonds in connection with financing activities, in each case, to the extent included in Fixed Charges), together with items excluded from the definition of “Consolidated Interest Expense” pursuant to clauses 1(t) through 1(z) thereof, to the extent the same were deducted (and not added back) in calculating such Consolidated Net Income; plus
(c) Consolidated Depreciation and Amortization Expense of such Person for such period to the extent the same were deducted (and not added back) in computing Consolidated Net Income; plus
(d) any expenses or charges (other than depreciation or amortization expense) related to any Equity Offering, Permitted Investment, acquisition, disposition, recapitalization or the incurrence of Indebtedness permitted to be incurred by the Senior Notes Indenture (including a refinancing thereof) (whether or not successful), including (i) such fees, expenses or charges related to the offering of the Senior Notes and the Credit Facilities and (ii) any amendment or other modification of the Senior Notes, and, in each case, deducted (and not added back) in computing Consolidated Net Income; plus
(e) the amount of any restructuring charges, integration costs or other business optimization expenses or reserves deducted (and not added back) in such period in computing Consolidated Net Income, including any one-time costs incurred in connection with acquisitions after the Issue Date and costs related to the closure and/or consolidation of facilities; plus
(f) any other non-cash charges, including any write-offs or write-downs, reducing Consolidated Net Income for such period (provided that if any such non-cash charges represent an accrual or reserve for potential cash items in any future period, the cash payment in respect thereof in such future period shall be subtracted from EBITDA to such extent, and excluding amortization of a prepaid cash item that was paid in a prior period); plus
(g) the amount of any minority interest expense consisting of Subsidiary income attributable to minority equity interests of third parties in any non-Wholly-Owned Subsidiary deducted (and not added back) in such period in calculating Consolidated Net Income; plus
(h) the amount of management, monitoring, consulting and advisory fees and related expenses paid in such period to the Investors to the extent otherwise permitted under “Certain Covenants—Transactions with Affiliates”; plus
(i) [RESERVED]; plus
(j) the amount of loss on sale of receivables and related assets to the Receivables Subsidiary in connection with a Receivables Facility; plus
(k) any costs or expense incurred by the Company or a Restricted Subsidiary pursuant to any management equity plan or stock option plan or any other management or employee benefit plan or agreement or any stock subscription or shareholder agreement, to the extent that such cost or expenses are funded with cash proceeds contributed to the capital of the Company or net cash proceeds of an issuance of Equity Interest of the Company (other than Disqualified Stock) solely to the extent that such net cash proceeds are excluded from the calculation set forth in clause (3) of the first paragraph under “Certain Covenants—Limitation on Restricted Payments”;
261
Table of Contents
(2) decreased by (without duplication) non-cash gains increasing Consolidated Net Income of such Person for such period, excluding any non-cash gains to the extent they represent the reversal of an accrual or reserve for a potential cash item that reduced EBITDA in any prior period; and
(3) increased or decreased by (without duplication):
(a) any net gain or loss resulting in such period from Hedging Obligations and the application of Financial Accounting Standards Codification No. 815—Derivatives and Hedging; plus or minus, as applicable,
(b) any net gain or loss resulting in such period from currency translation gains or losses related to currency remeasurements of Indebtedness (including any net loss or gain resulting from hedge agreements for currency exchange risk and revaluations of intercompany balances).
“EMU” means economic and monetary union as contemplated in the Treaty on European Union.
“Equity Interests” means Capital Stock and all warrants, options or other rights to acquire Capital Stock, but excluding any debt security that is convertible into, or exchangeable for, Capital Stock.
“Equity Offering” means any public or private sale of common stock or Preferred Stock of the Company (excluding Disqualified Stock) or any of its direct or indirect parent companies to the extent contributed to the Company as Equity (other than Disqualified Stock), other than:
(1) public offerings with respect to the Company’s or any direct or indirect parent company’s common stock registered on Form S-8;
(2) issuances to any Subsidiary of the Company; and
(3) any such public or private sale that constitutes an Excluded Contribution.
“€” means the single currency of participating member states of the EMU.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Excluded Contribution” means net cash proceeds, marketable securities or Qualified Proceeds received by the Company from
(1) contributions to its common equity capital, and
(2) the sale (other than to a Subsidiary of the Company or to any management equity plan or stock option plan or any other management or employee benefit plan or agreement of the Company) of Capital Stock (other than Disqualified Stock and Designated Preferred Stock) of the Company,
in each case after the Issue Date and in each case designated as Excluded Contributions pursuant to an Officer’s Certificate executed by the principal financial officer of the Company on the date such capital contributions are made or the date such Equity Interests are sold, as the case may be, which are excluded from the calculation set forth in clause (3) of the first paragraph under “Certain Covenants—Limitation on Restricted Payments.”
“Existing Senior Notes” means the (i) 10.875% Senior Notes due 2014 issued by the Issuers, pursuant to an indenture, dated November 20, 2007, among the Issuers, certain Subsidiaries of the Company, as guarantors, and The Bank of New York Mellon (formerly known as The Bank of New York), as trustee and (ii) 7.75% Senior Notes due 2018 issued by the Issuers, pursuant to an indenture, dated April 4, 2011, among the Issuers, certain Subsidiaries of the Company, as guarantors, and The Bank of New York Mellon, as trustee, in each case outstanding on the Issue Date.
262
Table of Contents
“Fixed Charge Coverage Ratio” means, with respect to any Person for any period, the ratio of EBITDA of such Person for such period to the Fixed Charges of such Person for such period. In the event that the Company or any Restricted Subsidiary incurs, assumes, guarantees, redeems, retires or extinguishes any Indebtedness (other than Indebtedness incurred under any revolving credit facility unless such Indebtedness has been permanently repaid and has not been replaced) or issues or redeems Disqualified Stock or Preferred Stock subsequent to the commencement of the period for which the Fixed Charge Coverage Ratio is being calculated but prior to or simultaneously with the event for which the calculation of the Fixed Charge Coverage Ratio is made (the “Fixed Charge Coverage Ratio Calculation Date”), then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect to such incurrence, assumption, guarantee, redemption, retirement or extinguishment of Indebtedness, or such issuance or redemption of Disqualified Stock or Preferred Stock, as if the same had occurred at the beginning of the applicable four-quarter period.
For purposes of making the computation referred to above, Investments, acquisitions, dispositions, mergers, consolidations and disposed operations (as determined in accordance with GAAP) that have been made by the Company or any of its Restricted Subsidiaries during the four-quarter reference period or subsequent to such reference period and on or prior to or simultaneously with the Fixed Charge Coverage Ratio Calculation Date shall be calculated on a pro forma basis, assuming that all such Investments, acquisitions, dispositions, mergers, consolidations and disposed operations (and the change in any associated fixed charge obligations and the change in EBITDA resulting therefrom) had occurred on the first day of the four-quarter reference period. If since the beginning of such period any Person that subsequently became a Restricted Subsidiary or was merged with or into the Company or any of its Restricted Subsidiaries since the beginning of such period shall have made any Investment, acquisition, disposition, merger, consolidation or disposed operation that would have required adjustment pursuant to this definition, then the Fixed Charge Coverage Ratio shall be calculated giving pro forma effect thereto for such period as if such Investment, acquisition, disposition, merger, consolidation or disposed operation had occurred at the beginning of the applicable four-quarter period.
For purposes of this definition, whenever pro forma effect is to be given to a transaction, the pro forma calculations shall be made in good faith by a responsible financial or accounting officer of the Company. If any Indebtedness bears a floating rate of interest and is being given pro forma effect, the interest on such Indebtedness shall be calculated as if the rate in effect on the Fixed Charge Coverage Ratio Calculation Date had been the applicable rate for the entire period (taking into account any Hedging Obligations applicable to such Indebtedness). Interest on a Capitalized Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by a responsible financial or accounting officer of the Company to be the rate of interest implicit in such Capitalized Lease Obligation in accordance with GAAP. For purposes of making the computation referred to above, interest on any Indebtedness under a revolving credit facility computed on a pro forma basis shall be computed based upon the average daily balance of such Indebtedness during the applicable period except as set forth in the first paragraph of this definition. Interest on Indebtedness that may optionally be determined at an interest rate based upon a factor of a prime or similar rate, a eurocurrency interbank offered rate, or other rate, shall be deemed to have been based upon the rate actually chosen, or, if none, then based upon such optional rate chosen as the Company may designate.
“Fixed Charges” means, with respect to any Person for any period, the sum of:
(1) Consolidated Interest Expense of such Person for such period;
(2) all cash dividends or other distributions paid (excluding items eliminated in consolidation) on any series of Preferred Stock of any Restricted Subsidiary during such period; and
(3) all dividends or other distributions accrued (excluding items eliminated in consolidation) on any series of Disqualified Stock during such period.
“Foreign Subsidiary” means, with respect to any Person, any Restricted Subsidiary of such Person that is not organized or existing under the laws of the United States, any state thereof, or the District of Columbia and any Restricted Subsidiary of such Foreign Subsidiary.
263
Table of Contents
“GAAP” means generally accepted accounting principles in the United States which are in effect on the Issue Date.
“Government Securities” means securities that are:
(1) direct obligations of the United States of America for the timely payment of which its full faith and credit is pledged; or
(2) obligations of a Person controlled or supervised by and acting as an agency or instrumentality of the United States of America the timely payment of which is unconditionally guaranteed as a full faith and credit obligation by the United States of America,
which, in either case, are not callable or redeemable at the option of the issuers thereof, and shall also include a depository receipt issued by a bank (as defined in Section 3(a)(2) of the Securities Act), as custodian with respect to any such Government Securities or a specific payment of principal of or interest on any such Government Securities held by such custodian for the account of the holder of such depository receipt; provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depository receipt from any amount received by the custodian in respect of the Government Securities or the specific payment of principal of or interest on the Government Securities evidenced by such depository receipt.
“guarantee” means a guarantee (other than by endorsement of negotiable instruments for collection in the ordinary course of business), direct or indirect, in any manner (including letters of credit and reimbursement agreements in respect thereof), of all or any part of any Indebtedness or other obligations.
“Guarantee” means the guarantee by any Guarantor of the Issuers’ Obligations under the Senior Notes Indenture.
“Guarantor” means, each Restricted Subsidiary that Guarantees the Senior Notes in accordance with the terms of the Senior Notes Indenture and its successors and assigns, until released from its obligations under its Guarantee in accordance with the terms of the Senior Notes Indenture.
“Hedging Obligations” means, with respect to any Person, the obligations of such Person under any interest rate swap agreement, interest rate cap agreement, interest rate collar agreement, commodity swap agreement, commodity cap agreement, commodity collar agreement, foreign exchange contract, currency swap agreement or similar agreement providing for the transfer or mitigation of interest rate or currency risks either generally or under specific contingencies.
“Holder” means the Person in whose name a Senior Note is registered on the registrar’s books.
“Indebtedness” means, with respect to any Person, without duplication:
(1) any indebtedness of such Person, whether or not contingent:
(a) in respect of borrowed money;
(b) evidenced by bonds, notes, debentures or similar instruments or letters of credit or bankers’ acceptances (or, without duplication, reimbursement agreements in respect thereof);
(c) representing the balance deferred and unpaid of the purchase price of any property (including Capitalized Lease Obligations), except (i) any such balance that constitutes a trade payable or similar obligation to a trade creditor, in each case accrued in the ordinary course of business and (ii) any earn-out obligations until, after 30 days of becoming due and payable, has not been paid and such obligation becomes a liability on the balance sheet of such Person in accordance with GAAP; or
(d) representing any Hedging Obligations; or
264
Table of Contents
(e) during a Suspension Period only, obligations of the lessee for rental payments in respect of Sale and Lease-Back Transactions in an amount equal to the present value of such obligations during the remaining term of the lease using a discount rate equal to the rate of interest implicit in such transaction determined in accordance with GAAP.
if and to the extent that any of the foregoing Indebtedness (other than letters of credit and Hedging Obligations) would appear as a liability upon a balance sheet (excluding the footnotes thereto) of such Person prepared in accordance with GAAP;
(2) to the extent not otherwise included, any obligation by such Person to be liable for, or to pay, as obligor, guarantor or otherwise on, the obligations of the type referred to in clause (1) of a third Person (whether or not such items would appear upon the balance sheet of the such obligor or guarantor), other than by endorsement of negotiable instruments for collection in the ordinary course of business; and
(3) to the extent not otherwise included, the obligations of the type referred to in clause (1) of a third Person secured by a Lien on any asset owned by such first Person, whether or not such Indebtedness is assumed by such first Person;
provided, however, that notwithstanding the foregoing, Indebtedness shall be deemed not to include (a) Contingent Obligations incurred in the ordinary course of business or (b) obligations under or in respect of Receivables Facilities.
“Independent Financial Advisor” means an accounting, appraisal, investment banking firm or consultant to Persons engaged in Similar Businesses of nationally recognized standing that is, in the good faith judgment of the Company, qualified to perform the task for which it has been engaged.
“Initial Purchasers” means Credit Suisse Securities (USA) LLC, Goldman, Sachs & Co., UBS Securities LLC, Wells Fargo Securities, LLC, RBC Capital Markets, LLC, Macquarie Capital (USA) Inc. and Natixis Securities Americas LLC.
“Investment Grade Rating” means a rating equal to or higher than Baa3 (or the equivalent) by Moody’s and BBB- (or the equivalent) by S&P, or an equivalent rating by any other Rating Agency.
“Investment Grade Securities” means:
(1) securities issued or directly and fully guaranteed or insured by the United States government or any agency or instrumentality thereof (other than Cash Equivalents);
(2) debt securities or debt instruments with an Investment Grade Rating, but excluding any debt securities or instruments constituting loans or advances among the Company and its Subsidiaries;
(3) investments in any fund that invests exclusively in investments of the type described in clauses (1) and (2) which fund may also hold immaterial amounts of cash pending investment or distribution; and
(4) corresponding instruments in countries other than the United States customarily utilized for high quality investments.
265
Table of Contents
“Investments” means, with respect to any Person, all investments by such Person in other Persons (including Affiliates) in the form of loans (including guarantees), advances or capital contributions (excluding accounts receivable, trade credit, advances to customers, commission, travel and similar advances to officers and employees, in each case made in the ordinary course of business), purchases or other acquisitions for consideration of Indebtedness, Equity Interests or other securities issued by any other Person and investments that are required by GAAP to be classified on the balance sheet (excluding the footnotes) of the Company in the same manner as the other investments included in this definition to the extent such transactions involve the transfer of cash or other property. For purposes of the definition of “Unrestricted Subsidiary” and the covenant described under “Certain Covenants—Limitation on Restricted Payments”:
(1) “Investments” shall include the portion (proportionate to the Company’s equity interest in such Subsidiary) of the fair market value of the net assets of a Subsidiary of the Company at the time that such Subsidiary is designated an Unrestricted Subsidiary; provided, however, that upon a redesignation of such Subsidiary as a Restricted Subsidiary, the Company shall be deemed to continue to have a permanent “Investment” in an Unrestricted Subsidiary in an amount (if positive) equal to:
(a) the Company “Investment” in such Subsidiary at the time of such redesignation; less
(b) the portion (proportionate to the Company equity interest in such Subsidiary) of the fair market value of the net assets of such Subsidiary at the time of such redesignation; and
(2) any property transferred to or from an Unrestricted Subsidiary shall be valued at its fair market value at the time of such transfer, in each case as determined in good faith by the Company.
“Investors” means The Blackstone Group and each of its Affiliates, but not including any of its portfolio companies.
“Issue Date” means October 1, 2012.
“Legal Holiday” means a Saturday, a Sunday or a day on which commercial banking institutions are not required to be open in the State of New York.
“Lien” means, with respect to any asset, any mortgage, lien (statutory or otherwise), pledge, hypothecation, charge, security interest, preference, priority or encumbrance of any kind in respect of such asset, whether or not filed, recorded or otherwise perfected under applicable law, including any conditional sale or other title retention agreement, any lease in the nature thereof, any option or other agreement to sell or give a security interest in and any filing of or agreement to give any financing statement under the Uniform Commercial Code (or equivalent statutes) of any jurisdiction; provided that in no event shall an operating lease be deemed to constitute a Lien.
“Moody’s” means Moody’s Investors Service, Inc. and any successor to its rating agency business.
“Net Income” means, with respect to any Person, the net income (loss) of such Person, determined in accordance with GAAP and before any reduction in respect of Preferred Stock dividends.
“Net Proceeds” means the aggregate cash proceeds received by the Company or any of its Restricted Subsidiaries in respect of any Asset Sale, including any cash received upon the sale or other disposition of any Designated Non-cash Consideration received in any Asset Sale, net of (i) the direct costs relating to such Asset Sale and the sale or disposition of such Designated Non-cash Consideration, including legal, accounting and investment banking fees, and brokerage and sales commissions, (ii) any relocation expenses incurred as a result thereof, (iii) taxes paid or payable as a result thereof (after taking into account any available tax credits or deductions and any tax sharing arrangements), (iv) amounts required to be applied to the repayment of principal, premium, if any, and interest on Senior Indebtedness required (other than required by clause (1) of the second paragraph of “Repurchase at the Option of Holders—Asset Sales”) to be paid as a result of such transaction and (v) any deduction of appropriate amounts to be provided by the Company or any of its Restricted Subsidiaries as
266
Table of Contents
a reserve in accordance with GAAP against any liabilities associated with the asset disposed of in such transaction and retained by the Company or any of its Restricted Subsidiaries after such sale or other disposition thereof, including pension and other post-employment benefit liabilities and liabilities related to environmental matters or against any indemnification obligations associated with such transaction.
“Obligations” means any principal, interest (including any interest accruing subsequent to the filing of a petition in bankruptcy, reorganization or similar proceeding at the rate provided for in the documentation with respect thereto, whether or not such interest is an allowed claim under applicable state, federal or foreign law), penalties, fees, indemnifications, reimbursements (including reimbursement obligations with respect to letters of credit and banker’s acceptances), damages and other liabilities, and guarantees of payment of such principal, interest, penalties, fees, indemnifications, reimbursements, damages and other liabilities, payable under the documentation governing any Indebtedness.
“Officer” means the Chairman of the Board, the Chief Executive Officer, Chief, Financial Officer, Chief Operating Officer, the President, any Executive Vice President, Senior Vice President or Vice President, the Treasurer or the Secretary of the applicable Issuer or Guarantor.
“Officer’s Certificate” means a certificate signed on behalf of an Issuer by an Officer of such Issuer or on behalf of a Guarantor by an Officer of such Guarantor, who must be the principal executive officer, the principal financial officer, the treasurer or the principal accounting officer of such Issuer or Guarantor, as applicable, that meets the requirements set forth in the Senior Notes Indenture.
“Opinion of Counsel” means a written opinion from legal counsel who is acceptable to the Senior Notes Trustee. The counsel may be an employee of or counsel to the Company, a Subsidiary of the Company or the Senior Notes Trustee.
“Permitted Asset Swap” means the concurrent purchase and sale or exchange of Related Business Assets or a combination of Related Business Assets and cash or Cash Equivalents between the Company or any of its Restricted Subsidiaries and another Person; provided, that any cash or Cash Equivalents received must be applied in accordance with the “Repurchase at the Option of Holders—Asset Sales” covenant.
“Permitted Holders” means each of the Investors and members of management of the Company (or its direct parent) on the Issue Date who are holders of Equity Interests of the Company(or any of its direct or indirect parent companies) and any group (within the meaning of Section 13(d)(3) or Section 14(d)(2) of the Exchange Act or any successor provision) of which any of the foregoing are members; provided that, in the case of such group and without giving effect to the existence of such group or any other group, such Investors and members of management, collectively have beneficial ownership of more than 50% of the total voting power of the Voting Stock of the Company or any of its direct or indirect parent companies. Any Person or group whose acquisition of beneficial ownership constitutes a Change of Control in respect of which a Change of Control Offer is made in accordance with the requirements of the Senior Notes Indenture will thereafter, together with its Affiliates, constitute an additional Permitted Holder.
“Permitted Investment” means:
(1) any Investment in the Company or any of its Restricted Subsidiaries;
(2) any Investment in cash and Cash Equivalents or Investment Grade Securities;
(3) any Investment by the Company or any of its Restricted Subsidiaries in a Person that is engaged in a Similar Business if as a result of such Investment:
(a) such Person becomes a Restricted Subsidiary; or
(b) such Person, in one transaction or a series of related transactions, is merged or consolidated with or into, or transfers or conveys substantially all of its assets to, or is liquidated into, the Company or a Restricted Subsidiary,
267
Table of Contents
and, in each case, any Investment held by such Person; provided, that such Investment was not acquired by such Person in contemplation of such acquisition, merger, consolidation or transfer;
(4) any Investment in securities or other assets, including earnouts, not constituting cash and Cash Equivalents and received in connection with an Asset Sale made pursuant to the provisions of “Repurchase at the Option of Holders—Asset Sales” or any other disposition of assets not constituting an Asset Sale;
(5) any Investment existing on the Issue Date;
(6) any Investment acquired by the Company or any of its Restricted Subsidiaries:
(a) in exchange for any other Investment or accounts receivable held by the Company or any such Restricted Subsidiary in connection with or as a result of a bankruptcy, workout, reorganization or recapitalization of the issuer of such other Investment or accounts receivable; or
(b) as a result of a foreclosure by the Company or any of its Restricted Subsidiaries with respect to any secured Investment or other transfer of title with respect to any secured Investment in default;
(7) Hedging Obligations permitted under clause (10) of the covenant described in “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(8) any Investment in a Similar Business having an aggregate fair market value, taken together with all other Investments made pursuant to this clause (8) that are at that time outstanding, not to exceed 2.5% of Total Assets at the time of such Investment (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
(9) Investments the payment for which consists of Equity Interests (exclusive of Disqualified Stock) of the Company, or any of its direct or indirect parent companies; provided, however, that such Equity Interests will not increase the amount available for Restricted Payments under clause (3) of the first paragraph under the covenant described in “Certain Covenants—Limitations on Restricted Payments”;
(10) guarantees of Indebtedness of the Company and any Restricted Subsidiary permitted under the covenant described in “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(11) any transaction to the extent it constitutes an Investment that is permitted and made in accordance with the provisions of the second paragraph of the covenant described under “Certain Covenants—Transactions with Affiliates” (except transactions described in clauses (2), (5) and (9) of such paragraph);
(12) Investments consisting of purchases and acquisitions of inventory, supplies, material or equipment;
(13) additional Investments having an aggregate fair market value, taken together with all other Investments made pursuant to this clause (13) that are at that time outstanding (without giving effect to the sale of an Unrestricted Subsidiary to the extent the proceeds of such sale do not consist of cash or marketable securities), not to exceed 3.5% of Total Assets at the time of such Investment (with the fair market value of each Investment being measured at the time made and without giving effect to subsequent changes in value);
(14) Investments relating to a Receivables Subsidiary that, in the good faith determination of the Company are necessary or advisable to effect any Receivables Facility;
(15) advances to, or guarantees of Indebtedness of, employees not in excess of $10.0 million outstanding at any one time, in the aggregate;
(16) loans and advances to officers, directors and employees for business-related travel expenses, moving expenses and other similar expenses, in each case incurred in the ordinary course of business or consistent with past practices or to fund such Person’s purchase of Equity Interests of the Company or any direct or indirect parent company thereof; and
268
Table of Contents
(17) loans and advances to independent sales persons against commissions not in excess of $15.0 million outstanding at any one time, in the aggregate.
“Permitted Liens” means, with respect to any Person:
(1) pledges or deposits by such Person under workmen’s compensation laws, unemployment insurance laws or similar legislation, or good faith deposits in connection with bids, tenders, contracts (other than for the payment of Indebtedness) or leases to which such Person is a party, or deposits to secure public or statutory obligations of such Person or deposits of cash or U.S. government bonds to secure surety or appeal bonds to which such Person is a party, or deposits as security for contested taxes or import duties or for the payment of rent, in each case incurred in the ordinary course of business;
(2) Liens imposed by law, such as carriers’, warehousemen’s and mechanics’ Liens, in each case for sums not yet overdue for a period of more than 30 days or being contested in good faith by appropriate proceedings or other Liens arising out of judgments or awards against such Person with respect to which such Person shall then be proceeding with an appeal or other proceedings for review if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(3) Liens for taxes, assessments or other governmental charges not yet overdue for a period of more than 30 days or payable or subject to penalties for nonpayment or which are being contested in good faith by appropriate proceedings diligently conducted, if adequate reserves with respect thereto are maintained on the books of such Person in accordance with GAAP;
(4) Liens in favor of issuers of performance and surety bonds or bid bonds or with respect to other regulatory requirements or letters of credit issued pursuant to the request of and for the account of such Person in the ordinary course of its business;
(5) minor survey exceptions, minor encumbrances, easements or reservations of, or rights of others for, licenses, rights-of-way, sewers, electric lines, telegraph and telephone lines and other similar purposes, or zoning or other restrictions as to the use of real properties or Liens incidental, to the conduct of the business of such Person or to the ownership of its properties which were not incurred in connection with Indebtedness and which do not in the aggregate materially adversely affect the value of said properties or materially impair their use in the operation of the business of such Person;
(6) Liens securing Indebtedness permitted to be incurred pursuant to clause (4) of the second paragraph under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” (including, during any Suspension Period, Indebtedness of the type and in the amounts specified under such clause);
(7) Liens existing on the Issue Date;
(8) Liens on property or shares of stock of a Person at the time such Person becomes a Subsidiary; provided, however, such Liens are not created or incurred in connection with, or in contemplation of, such other Person becoming such a Subsidiary; provided further, however, that such Liens may not extend to any other property owned by the Company or any of its Restricted Subsidiaries;
(9) Liens on property at the time the Company or a Restricted Subsidiary acquired the property, including any acquisition by means of a merger or consolidation with or into the Company or any of its Restricted Subsidiaries; provided, however, that such Liens are not created or incurred in connection with, or in contemplation of, such acquisition; provided further, however, that the Liens may not extend to any other property owned by the Company or any of its Restricted Subsidiaries;
(10) Liens securing Indebtedness or other obligations of a Restricted Subsidiary owing to the Company or another Restricted Subsidiary permitted to be incurred in accordance with the covenant described under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
269
Table of Contents
(11) customary Liens securing Hedging Obligations entered into in the ordinary course of business by the Issuer or its Restricted Subsidiaries;
(12) Liens on specific items of inventory of other goods and proceeds of any Person securing such Person’s obligations in respect of bankers’ acceptances issued or created for the account of such Person to facilitate the purchase, shipment or storage of such inventory or other goods;
(13) leases, subleases, licenses or sublicenses granted to others in the ordinary course of business which do not materially interfere with the ordinary conduct of the business of the Company or any of its Restricted Subsidiaries and do not secure any Indebtedness;
(14) Liens arising from Uniform Commercial Code financing statement filings regarding operating leases entered into by the Company and its Restricted Subsidiaries in the ordinary course of business;
(15) Liens in favor of the Company, the Co-Issuer or any Guarantor;
(16) Liens on equipment of the Company or any of its Restricted Subsidiaries granted in the ordinary course of business to the Company’s clients;
(17) Liens on accounts receivable and related assets incurred in connection with a Receivables Facility;
(18) Liens to secure any refinancing, refunding, extension, renewal or replacement (or successive refinancing, refunding, extensions, renewals or replacements) as a whole, or in part, of any Indebtedness secured by any Lien referred to in the foregoing clauses (7), (8) and (9); provided, however, that (a) such new Lien shall be limited to all or part of the same property that secured the original Lien (plus improvements on such property), and (b) the Indebtedness secured by such Lien at such time is not increased to any amount greater than the sum of (i) the outstanding principal amount or, if greater, committed amount of the Indebtedness described under clauses (7), (8) and (9) at the time the original Lien became a Permitted Lien under the Senior Notes Indenture, and (ii) an amount necessary to pay any fees and expenses, including premiums, related to such refinancing, refunding, extension, renewal or replacement;
(19) deposits made in the ordinary course of business to secure liability to insurance carriers;
(20) other Liens securing obligations incurred in the ordinary course of business which obligations do not exceed $65.0 million at any one time outstanding;
(21) Liens securing Indebtedness of any Foreign Subsidiary permitted to be incurred under the Senior Notes Indenture, to the extent such Liens relate only to the assets and properties of such Foreign Subsidiary;
(22) Liens securing judgments for the payment of money not constituting an Event of Default under clause (5) under the caption “Events of Default and Remedies” so long as such Liens are adequately bonded and any appropriate legal proceedings that may have been duly initiated for the review of such judgment have not been finally terminated or the period within which such proceedings may be initiated has not expired;
(23) Liens in favor of customs and revenue authorities arising as a matter of law to secure payment of customs duties in connection with the importation of goods in the ordinary course of business;
(24) Liens (i) of a collection bank arising under Section 4-210 of the Uniform Commercial Code or any comparable or successor provision on items in the course of collection, (ii) attaching to commodity trading accounts or other commodity brokerage accounts incurred in the ordinary course of business, and (iii) in favor of banking institutions arising as a matter of law encumbering deposits (including the right of set-off) and which are within the general parameters customary in the banking industry;
(25) Liens deemed to exist in connection with Investments in repurchase agreements permitted under” Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; provided that such Liens do not extend to any assets other than those that are the subject of such repurchase agreement;
270
Table of Contents
(26) Liens encumbering reasonable customary initial deposits and margin deposits and similar Liens attaching to commodity trading accounts or other brokerage accounts incurred in the ordinary course of business and not for speculative purposes;
(27) Liens that are contractual rights of set-off (i) relating to the establishment of depository relations with banks not given in connection with the issuance of Indebtedness, (ii) relating to pooled deposit or sweep accounts of the Company or any of its Restricted Subsidiaries to permit satisfaction of overdraft or similar obligations incurred in the ordinary course of business of the Company and its Restricted Subsidiaries or (iii) relating to purchase orders and other agreements entered into with customers of the Company or any of its Restricted Subsidiaries in the ordinary course of business; and
(28) during a Suspension Period only, Liens securing Indebtedness (other than Indebtedness that is secured equally and ratably with (or on a basis subordinated to) the Senior Notes), including Indebtedness represented by Sale and Leaseback Transactions, in an amount not to exceed 5.0% of Total Assets at any one time outstanding.
For purposes of this definition, the term “Indebtedness” shall be deemed to include interest on such Indebtedness.
“Person” means any individual, corporation, limited liability company, partnership, joint venture, association, joint stock company, trust, unincorporated organization, government or any agency or political subdivision thereof or any other entity.
“Preferred Stock” means any Equity Interest with preferential rights of payment of dividends or upon liquidation, dissolution, or winding up.
“Qualified Proceeds” means assets that are used or useful in, or Capital Stock of any Person engaged in, a Similar Business; provided that the fair market value of any such assets or Capital Stock shall be determined by the Company in good faith.
“Rating Agencies” means Moody’s and S&P or if Moody’s or S&P or both shall not make a rating on the Senior Notes publicly available, a nationally recognized statistical rating agency or agencies, as the case may be, selected by the Issuers which shall be substituted for Moody’s or S&P or both, as the case may be.
“Receivables Facility” means any of one or more receivables financing facilities as amended, supplemented, modified, extended, renewed, restated or refunded from time to time, the Obligations of which are non-recourse (except for customary representations, warranties, covenants and indemnities made in connection with such facilities) to the Company or any of its Restricted Subsidiaries (other than a Receivables Subsidiary) pursuant to which the Company or any of its Restricted Subsidiaries sells its accounts receivable to either (a) a Person that is not a Restricted Subsidiary or (b) a Receivables Subsidiary that in turn sells its accounts receivable to a Person that is not a Restricted Subsidiary.
“Receivables Fees” means distributions or payments made directly or by means of discounts with respect to any accounts receivable or participation interest therein issued or sold in connection with, and other fees paid to a Person that is not a Restricted Subsidiary in connection with, any Receivables Facility.
“Receivables Subsidiary” means any Subsidiary formed for the purpose of, and that solely engages only in one or more Receivables Facilities and other activities reasonably related thereto.
“Related Business Assets” means assets (other than cash or Cash Equivalents) used or useful in a Similar Business, provided that any assets received by the Company or a Restricted Subsidiary in exchange for assets transferred by the Company or a Restricted Subsidiary shall not be deemed to be Related Business Assets if they consist of securities of a Person, unless upon receipt of the securities of such Person, such Person would become a Restricted Subsidiary.
271
Table of Contents
“Representative” means any trustee, agent or representative (if any) for an issue of Senior Indebtedness of the Issuers.
“Restricted Investment” means an Investment other than a Permitted Investment.
“Restricted Subsidiary” means, at any time, any direct or indirect Subsidiary of the Company (including the Co-Issuer and any Foreign Subsidiary) that is not then an Unrestricted Subsidiary; provided, however, that upon the occurrence of an Unrestricted Subsidiary ceasing to be an Unrestricted Subsidiary, such Subsidiary shall be included in the definition of “Restricted Subsidiary.”
“S&P” means Standard & Poor’s Rating Services and any successor to its rating agency business.
“Sale and Lease-Back Transaction” means any arrangement providing for the leasing by the Company or any of its Restricted Subsidiaries of any real or tangible personal property, which property has been or is to be sold or transferred by the Company or such Restricted Subsidiary to a third Person in contemplation of such leasing.
“SEC” means the U.S. Securities and Exchange Commission.
“Secured Indebtedness” means any Indebtedness of the Company or any of its Restricted Subsidiaries secured by a Lien.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations of the SEC promulgated thereunder.
“Senior Credit Facilities” means the Credit Facility under the Credit Agreement.
“Senior Indebtedness” means:
(1) all Indebtedness of the Issuers or any Guarantor outstanding under the Senior Credit Facilities (including interest accruing on or after the filing of any petition in bankruptcy or similar proceeding or for reorganization of the Issuers or any Guarantor (at the rate provided for in the documentation with respect thereto, regardless of whether or not a claim for post-filing interest is allowed in such proceedings), and any and all other fees, expense reimbursement obligations, indemnification amounts, penalties, and other amounts (whether existing on the Issue Date or thereafter created or incurred) and all obligations of the Issuers or any Guarantor to reimburse any bank or other Person in respect of amounts paid under letters of credit, acceptances or other similar instruments;
(2) all Hedging Obligations (and guarantees thereof) owing to a Lender (as defined in the Senior Credit Facilities) or any Affiliate of such Lender (or any Person that was a Lender or an Affiliate of such Lender at the time the applicable agreement giving rise to such Hedging Obligation was entered into), provided that such Hedging Obligations are permitted to be incurred under the terms of the Senior Notes Indenture;
(3) any other Indebtedness of the Issuers or any Guarantor permitted to be incurred under the terms of the Senior Notes Indenture, unless the instrument under which such Indebtedness is incurred expressly provides that it is subordinate in right of payment to the Senior Notes or any related Guarantee; and
(4) all Obligations with respect to the items listed in the preceding clauses (1), (2) and (3); provided, however, that Senior Indebtedness shall not include:
(a) any obligation of such Person to the Issuers or any of their Subsidiaries;
(b) any liability for federal, state, local or other taxes owed or owing by such Person;
(c) any accounts payable or other liability to trade creditors arising in the ordinary course of business;
272
Table of Contents
(d) any Indebtedness or other Obligation of such Person which is subordinate or junior in right of payment to any other Indebtedness or other Obligation of such Person; or
(e) that portion of any Indebtedness which at the time of incurrence is incurred in violation of the Senior Notes Indenture; provided, however, that such Indebtedness shall be deemed not to have been incurred in violation of the Senior Notes Indenture for purposes of this clause if such Indebtedness consists of Senior Credit Facilities, and the holder(s) of such Indebtedness or their agent or representative (i) had no actual-knowledge at the time of incurrence that the incurrence of such Indebtedness violated the Senior Notes Indenture and (ii) shall have received a certificate from an Officer of the Company to the effect that the incurrence of such Indebtedness does not violate the provisions of the Senior Notes Indenture.
“Senior Notes Registration Rights Agreement” means the Registration Rights Agreement with respect to the Senior Notes dated as of the Issue Date, among the Issuers, the Guarantors and the Initial Purchasers.
“Senior Secured Notes” means the (i) outstanding 8.75% Second Priority Senior Secured Notes due 2018 of the Issuers issued prior to the Issue Date pursuant to the Senior Secured Notes Indenture and (ii) 8.75% Second Priority Senior Secured Notes due 2018 of the Issuers issued on the Issue Date pursuant to the Senior Secured Notes Indenture.
“Senior Secured Notes Indenture” means the indenture, dated as of March 20, 2012, among the Issuers, certain Subsidiaries of the Company, as guarantors, and The Bank of New York Mellon, as trustee.
“Senior Subordinated Notes” means the 9.75% Senior Subordinated Notes due 2017 issued by the Issuers, pursuant to an indenture, dated October 18, 2010, among the Issuers, certain Subsidiaries of the Company, as guarantors, and The Bank of New York Mellon, as trustee.
“Significant Subsidiary” means (i) the Co-Issuer and (ii) any Restricted Subsidiary that would be a “significant subsidiary” as defined in Article 1, Rule 1-02 of Regulation S-X, promulgated pursuant to the Securities Act, as such regulation is in effect on the Issue Date.
“Similar Business” means any business conducted or proposed to be conducted by the Company and its Restricted Subsidiaries on the Issue Date or any business that is similar, reasonably related, incidental or ancillary thereto.
“Sponsor Management Agreement” means the management agreement between certain of the management companies associated with the Investors and the Company and/or one of its direct or indirect parent companies as in effect on the Issue Date.
“Subordinated Indebtedness” means, with respect to the Senior Notes,
(1) any Indebtedness of the Issuers which is by its terms subordinated in right of payment to the Senior Notes, and
(2) any Indebtedness of any Guarantor which is by its terms subordinated in right of payment to the Guarantee of such entity of the Senior Notes.
“Subsidiary” means, with respect to any Person:
(1) any corporation, association or other business entity (other than a partnership, joint venture, limited liability company or similar entity) of which more than 50% of the total voting power of shares of Capital Stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time of determination on owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof or is consolidated under GAAP with such Person at such time; and
273
Table of Contents
(2) any partnership, joint venture, limited liability company or similar entity of which
(a) more than 50% of the capital accounts, distribution rights, total equity and voting interests or general or limited partnership interests, as applicable, are owned or controlled, directly or indirectly, by such Person or one or more of the other Subsidiaries of that Person or a combination thereof whether in the form of membership, general, special or limited partnership or otherwise, and
(b) such Person or any Restricted Subsidiary of such Person is a general partner or otherwise controls such entity.
“Total Assets” means the total assets of the Company, except where expressly provided otherwise, and its Restricted Subsidiaries on a consolidated basis, as shown on the most recent balance sheet of such other Person.
“Transactions” means the acquisition contemplated by the Transaction Agreement and related financings.
“Transaction Agreement” means the Equity Interest Purchase Agreement, dated as of March 14, 2011, by and among Rikco International, LLC d/b/a Dr. Comfort, Rikco Holding Corporation, Merit Mezzanine Fund IV, L.P., Merit Mezzanine Parallel Fund IV, L.P., the members of Ricko International, LLC parties thereto and DJO LLC, as the same may have been amended prior to April 7, 2011.
“Treasury Rate” means, as of any Redemption Date, the yield to maturity as of such Redemption Date of United States Treasury securities with a constant maturity (as compiled and, published in the most recent Federal Reserve Statistical Release H.15 (519) that has become publicly available at least two Business Days prior to the Redemption Date (or, if such Statistical Release is no longer published, any publicly available source of similar market data)) most nearly equal to the period from the Redemption Date to April 15, 2015; provided, however, that if the period from the Redemption Date to April 15, 2015 is less than one year, the weekly average yield on actually traded United States Treasury securities adjusted to a constant maturity of one year are used.
“Trust Indenture Act” means the Trust Indenture Act of 1939, as amended (15 U.S.C §§ 77aaa-777bbbb).
“Unrestricted Subsidiary” means:
(1) any Subsidiary of the Company which at the time of determination is an Unrestricted Subsidiary (as designated by the Company, as provided below); and
(2) any Subsidiary of an Unrestricted Subsidiary.
The Company may designate any Subsidiary of the Company, other than the Co-Issuer (including any existing Subsidiary and any newly acquired or newly formed Subsidiary) to be an Unrestricted Subsidiary unless such Subsidiary or any of its Subsidiaries owns any Equity Interests or Indebtedness of, or owns or holds any Lien on any property of, the Company or any Subsidiary of the Company (other than solely any Subsidiary of the Subsidiary to be so designated); provided that
(1) any Unrestricted Subsidiary must be an entity of which the Equity Interests entitled to cast at least a majority of the votes that may be cast by all Equity Interests having ordinary voting power for the election of directors or Persons performing a similar function are owned, directly or indirectly, by the Company;
(2) such designation complies with the covenants described under “Certain Covenants—Limitation on Restricted Payments”; and
(3) each of:
(a) the Subsidiary to be so designated; and
(b) its Subsidiaries has not at the time of designation, and does not thereafter, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable with respect to any Indebtedness pursuant to which the lender has recourse to any of the assets of the Company or any Restricted Subsidiary.
274
Table of Contents
The Company may designate any Unrestricted Subsidiary to be a Restricted Subsidiary; provided that, immediately after giving effect to such designation, no Default shall have occurred and be continuing and either:
(1) the Company could incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test described in the first paragraph under “Certain Covenants—Limitation on Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; or
(2) the Fixed Charge Coverage Ratio for the Company and its Restricted Subsidiaries would be greater than such ratio for the Company and its Restricted Subsidiaries immediately prior to such designation, in each case on a pro forma basis taking into account such designation.
Any such designation by the Company shall be notified by the Company to the Senior Notes Trustee by promptly filing with the Senior Notes Trustee a copy of the resolution of the board of directors of the Company or any committee thereof giving effect to such designation and an Officer’s Certificate certifying that such designation complied with the foregoing provisions.
“Voting Stock” of any Person as of any date means the Capital Stock of such Person that is at the time entitled to vote in the election of the board of directors of such Person.
“Weighted Average Life to Maturity” means, when applied to any Indebtedness, Disqualified Stock or Preferred Stock, as the case may be, at any date, the quotient obtained by dividing:
(1) the sum of the products of the number of years from the date of determination to the date of each successive scheduled principal payment of such Indebtedness or redemption or similar payment with respect to such Disqualified Stock or Preferred Stock multiplied by the amount of such payment; by
(2) the sum of all such payments.
“Wholly-Owned Subsidiary” of any Person means a Subsidiary of such Person, 100% of the outstanding Equity Interests of which (other than directors’ qualifying shares) shall at the time be owned by such Person or by one or more Wholly-Owned Subsidiaries of such Person.
275
Table of Contents
UNITED STATES FEDERAL INCOME TAX CONSEQUENCES OF THE EXCHANGE OFFERS
The exchange of outstanding notes for exchange notes in the exchange offers will not constitute a taxable event to holders for United States federal income tax purposes. Consequently, no gain or loss will be recognized by a holder upon receipt of an exchange note, the holding period of the exchange note will include the holding period of the outstanding unregistered note exchanged therefor and the basis of the exchange note will be the same as the basis of the outstanding unregistered note immediately before the exchange.
In any event, persons considering the exchange of outstanding notes for exchange notes should consult their own tax advisors concerning the United States federal income tax consequences in light of their particular situations as well as any consequences arising under the laws of any other taxing jurisdiction.
The following is a summary of certain considerations associated with the acquisition and holding of the notes by employee benefit plans that are subject to Title I of the Employee Retirement Income Security Act of 1974, as amended (“ERISA”), plans, individual retirement accounts and other arrangements that are subject to Section 4975 of the Internal Revenue Code of 1986, as amended (the “Code”) or provisions under any other federal, state, local, non-U.S. or other laws or regulations that are similar to such provisions of the Code or ERISA (collectively, “Similar Laws”), and entities whose underlying assets are considered to include “plan assets” of any such plan, account or arrangement (each, a “Plan”).
General Fiduciary Matters
ERISA and the Code impose certain duties on persons who are fiduciaries of a Plan subject to Title I of ERISA or Section 4975 of the Code (an “ERISA Plan”) and prohibit certain transactions involving the assets of an ERISA Plan and its fiduciaries or other interested parties. Under ERISA and the Code, any person who exercises any discretionary authority or control over the administration of such an ERISA Plan or the management or disposition of the assets of such an ERISA Plan, or who renders investment advice for a fee or other compensation to such an ERISA Plan, is generally considered to be a fiduciary of the ERISA Plan.
In considering an investment in the notes of a portion of the assets of any Plan, a fiduciary should determine whether the investment is in accordance with the documents and instruments governing the Plan and the applicable provisions of ERISA, the Code or any Similar Law relating to a fiduciary’s duties to the Plan including, without limitation, the prudence, diversification, delegation of control and prohibited transaction provisions of ERISA, the Code and any other applicable Similar Laws.
Prohibited Transaction Issues
Section 406 of ERISA and Section 4975 of the Code prohibit ERISA Plans from engaging in specified transactions involving plan assets with persons or entities who are “parties in interest,” within the meaning of ERISA, or “disqualified persons,” within the meaning of Section 4975 of the Code, unless an exemption is available. A party in interest or disqualified person who engaged in a non-exempt prohibited transaction may be subject to excise taxes and other penalties and liabilities under ERISA and the Code. In addition, the fiduciary of the ERISA Plan that engaged in such a non-exempt prohibited transaction may be subject to penalties and liabilities under ERISA and the Code. The acquisition and/or holding of notes (including the exchange of outstanding notes for exchange notes) by an ERISA Plan with respect to which DJOFL, DJO Finance Corporation or a guarantor is considered a party in interest or a disqualified person may constitute or result in a direct or indirect prohibited transaction under Section 406 of ERISA and/or Section 4975 of the Code, unless the investment is acquired and is held in accordance with an applicable statutory, class or individual prohibited transaction exemption. In this regard, the U.S. Department of Labor has issued prohibited transaction class exemptions, or “PTCEs,” that may apply to the acquisition and holding of the notes. These class exemptions
276
Table of Contents
include, without limitation, PTCE 84-14 respecting transactions determined by independent qualified professional asset managers, PTCE 90-1 respecting insurance company pooled separate accounts, PTCE 91-38 respecting bank collective investment funds, PTCE 95-60 respecting life insurance company general accounts and PTCE 96-23 respecting transactions determined by in-house asset managers. In addition, Section 408(b)(17) of ERISA and Section 4975(d)(20) of the Code provide relief from the prohibited transaction provisions of ERISA and Section 4975 of the Code for certain transactions, provided that neither the issuer of the securities nor any of its affiliates (directly or indirectly) have or exercise any discretionary authority or control or render any investment advice with respect to the assets of any ERISA Plan involved in the transaction and provided further that the ERISA Plan pays no more than adequate consideration in connection with the transaction. There can be no assurance that all of the conditions of any such exemptions will be satisfied.
Because of the foregoing, the notes should not be acquired or held by any person investing “plan assets” of any Plan, unless such acquisition and holding (and the exchange of outstanding notes for exchange notes) will not constitute a non-exempt prohibited transaction under ERISA and the Code or a similar violation of any applicable Similar Laws.
Representation
Accordingly, by acceptance of a note (including an exchange of outstanding notes for exchange notes), each purchaser and subsequent transferee of a note will be deemed to have represented and warranted that either (i) no portion of the assets used by such purchaser or transferee to acquire or hold the notes constitutes assets of any Plan or (ii) the acquisition, holding and disposition of the notes by such purchaser or transferee will not constitute a non-exempt prohibited transaction under Section 406 of ERISA or Section 4975 of the Code or a similar violation under any applicable Similar Laws.
The foregoing discussion is general in nature and is not intended to be all inclusive. Due to the complexity of these rules and the penalties that may be imposed upon persons involved in non-exempt prohibited transactions, it is particularly important that fiduciaries, or other persons considering acquiring any notes on behalf of, or with the assets of, any Plan, consult with their counsel regarding the potential applicability of ERISA, Section 4975 of the Code and any Similar Laws to such investment and whether an exemption would be applicable to any such acquisition or holding of the notes.
277
Table of Contents
Each broker-dealer that receives exchange notes for its own account pursuant to an exchange offer must acknowledge that it will deliver a prospectus in connection with any resale of such exchange notes. This prospectus, as it may be amended or supplemented from time to time, may be used by a broker-dealer in connection with resales of exchange notes received in exchange for outstanding notes where such outstanding notes were acquired as a result of market-making activities or other trading activities. We have agreed that, for a period ending on the earlier of (i) 90 days from the date on which the registration statement for the exchange offers are declared effective, (ii) the date on which a broker-dealer is no longer required to deliver a prospectus in connection with market-making or other trading activities and (iii) the date on which all the notes covered by such registration statement have been sold pursuant to the exchange offers, we will make this prospectus, as amended or supplemented, available to any broker-dealer for use in connection with any such resale, and will promptly send additional copies of this prospectus and any amendments or supplements to this prospectus to any broker-dealer that requests such documents in the letter of transmittal. In addition, all dealers effecting transactions in the exchange notes may be required to deliver a prospectus.
We will not receive any proceeds from any sale of exchange notes by broker-dealers. Exchange notes received by broker-dealers for their own account pursuant to an exchange offer may be sold from time to time in one or more transactions in the over-the-counter market, in negotiated transactions, through the writing of options on the exchange notes or a combination of such methods of resale, at market prices prevailing at the time of resale, at prices related to such prevailing market prices or at negotiated prices. Any such resale may be made directly to purchasers or through brokers or dealers who may receive compensation in the form of commissions or concessions from any such broker-dealer and/or the purchasers of any such exchange notes. Any broker-dealer that resells exchange notes that were received by it for its own account pursuant to an exchange offer and any broker or dealer that participates in a distribution of such exchange notes may be deemed to be an “underwriter” within the meaning of the Securities Act and any profit of any such resale of exchange notes and any commission or concessions received by any such persons may be deemed to be underwriting compensation under the Securities Act. The letter of transmittal states that, by acknowledging that it will deliver and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act.
We have agreed to pay all expenses incident to the exchange offers (including the expenses of one counsel for the holders of the outstanding notes) other than commissions or concessions of any broker-dealers and will indemnify you (including any broker-dealers) against certain liabilities, including liabilities under the Securities Act.
278
Table of Contents
The validity and enforceability of the exchange notes and the related guarantees will be passed upon for us by Simpson Thacher & Bartlett LLP, New York, New York. In rendering its opinion, Simpson Thacher & Bartlett LLP will rely upon the opinion of Faegre Baker Daniels LLP as to all matters governed by the laws of the State of Minnesota, the opinion of Rice Silbey Reuther & Sullivan, LLP as to all matters governed by the laws of the State of Nevada, the opinion of Moore & Van Allen, PLLC as to all matters governed by the laws of the State of North Carolina and the opinion of Reinhart Boerner Van Deuren s.c. as to all matters governed by the laws of the State of Wisconsin. An investment vehicle comprised of several partners of Simpson Thacher & Bartlett LLP, members of their families, related persons and others own interests representing less than 1% of the capital commitments of funds affiliated with Blackstone.
The consolidated financial statements and schedule of DJOFL at December 31, 2011 and 2010, and for each of the three years in the period ended December 31, 2011, appearing in this prospectus and registration statement, have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We and our guarantor subsidiaries have filed with the SEC a registration statement on Form S-4 under the Securities Act with respect to the exchange notes. This prospectus, which forms a part of the registration statement, does not contain all of the information set forth in the registration statement. For further information with respect to us, our guarantor subsidiaries and the exchange notes, reference is made to the registration statement. Statements contained in this prospectus as to the contents of any contract or other document are not necessarily complete, and, where such contract or other document is an exhibit to the registration statement, each such statement is qualified by the provisions in such exhibit, to which reference is hereby made. We have historically filed annual, quarterly and current reports and other information with the SEC. The registration statement, such reports and other information can be inspected and copied at the Public Reference Room of the SEC located at Room 1580, 100 F Street, N.E., Washington D.C. 20549. Copies of such materials, including copies of all or any portion of the registration statement, can be obtained from the Public Reference Room of the SEC at prescribed rates. You can call the SEC at 1-800-SEC-0330 to obtain information on the operation of the Public Reference Room. Such materials may also be accessed electronically by means of the SEC’s home page on the Internet (http://www.sec.gov). However, any such information filed with the SEC does not constitute a part of this prospectus.
So long as we are subject to the periodic reporting requirements of the Exchange Act, we are required to furnish the information required to be filed with the SEC to the trustee and the holders of the outstanding notes. We have agreed that, even if we are not required under the Exchange Act to furnish such information to the SEC, we will nonetheless continue to furnish information that would be required to be furnished by us by Section 13 or 15(d) of the Exchange Act.
279
Table of Contents
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page No. | ||||
| Audited Consolidated Financial Statements |
||||
| F-2 | ||||
| F-3 | ||||
| Consolidated Statements of Operations for the Years Ended December 31, 2011, 2010 and 2009 |
F-4 | |||
| Consolidated Statements of Equity for the Years Ended December 31, 2011, 2010 and 2009 |
F-5 | |||
| Consolidated Statements of Comprehensive Loss for the Years Ended December 31, 2011, 2010 and 2009 |
F-6 | |||
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2011, 2010 and 2009 |
F-7 | |||
| F-8 | ||||
| Unaudited Consolidated Financial Statements |
||||
| Unaudited Condensed Consolidated Balance Sheets as of September 29, 2012 and December 31, 2011 |
F-53 | |||
| F-54 | ||||
| F-55 | ||||
| Unaudited Condensed Consolidated Statement of Equity for the nine months ended September 29, 2012 |
F-56 | |||
| F-57 | ||||
| Notes to Unaudited Condensed Consolidated Financial Statements |
F-58 | |||
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Managers of DJO Finance LLC
We have audited the accompanying consolidated balance sheets of DJO Finance LLC as of December 31, 2011 and 2010, and the related consolidated statements of operations, equity, comprehensive loss, and cash flows for each of the three years in the period ended December 31, 2011. Our audits also included the financial statement schedule listed in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of DJO Finance LLC at December 31, 2011 and 2010 and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2011, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
/s/ ERNST & YOUNG LLP
San Diego, California
February 21, 2012
F-2
Table of Contents
DJO Finance LLC
(in thousands)
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Assets | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 38,169 | $ | 38,132 | ||||
| Accounts receivable, net |
158,982 | 145,523 | ||||||
| Inventories, net |
128,699 | 94,460 | ||||||
| Deferred tax assets, net |
43,458 | 48,061 | ||||||
| Prepaid expenses and other current assets |
18,791 | 23,419 | ||||||
|
|
|
|
|
|||||
| Total current assets |
388,099 | 349,595 | ||||||
| Property and equipment, net |
107,108 | 93,660 | ||||||
| Goodwill |
1,228,778 | 1,188,887 | ||||||
| Intangible assets, net |
1,132,694 | 1,110,841 | ||||||
| Other assets |
38,181 | 36,807 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 2,894,860 | $ | 2,779,790 | ||||
|
|
|
|
|
|||||
| Liabilities and Equity | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 57,926 | $ | 48,947 | ||||
| Accrued interest |
20,928 | 15,578 | ||||||
| Current portion of debt and capital lease obligations |
8,820 | 8,821 | ||||||
| Other current liabilities |
81,771 | 81,709 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
169,445 | 155,055 | ||||||
| Long-term debt and capital lease obligations |
2,159,091 | 1,816,291 | ||||||
| Deferred tax liabilities, net |
252,194 | 289,913 | ||||||
| Other long-term liabilities |
16,174 | 11,712 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
2,596,904 | 2,272,971 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
||||||||
| Equity: |
||||||||
| DJO Finance LLC membership equity: |
||||||||
| Member capital |
834,871 | 830,994 | ||||||
| Accumulated deficit |
(539,276 | ) | (324,807 | ) | ||||
| Accumulated other comprehensive income (loss) |
218 | (2,048 | ) | |||||
|
|
|
|
|
|||||
| Total membership equity |
295,813 | 504,139 | ||||||
| Noncontrolling interests |
2,143 | 2,680 | ||||||
|
|
|
|
|
|||||
| Total equity |
297,956 | 506,819 | ||||||
|
|
|
|
|
|||||
| Total liabilities and equity |
$ | 2,894,860 | $ | 2,779,790 | ||||
|
|
|
|
|
|||||
See accompanying notes to consolidated financial statements.
F-3
Table of Contents
DJO Finance LLC
Consolidated Statements of Operations
(in thousands)
| Year ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Net sales |
$ | 1,074,770 | $ | 965,973 | $ | 946,126 | ||||||
| Cost of sales (exclusive of amortization of intangible assets of $38,668, $36,343 and $37,884 for the year ended December 31, 2011, 2010 and 2009, respectively) |
418,138 | 345,270 | 338,719 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit |
656,632 | 620,703 | 607,407 | |||||||||
| Operating expenses: |
||||||||||||
| Selling, general and administrative |
487,084 | 433,408 | 420,758 | |||||||||
| Research and development |
26,850 | 21,892 | 23,540 | |||||||||
| Amortization of intangible assets |
93,957 | 77,523 | 77,254 | |||||||||
| Impairment of goodwill and intangible assets |
141,006 | — | 6,998 | |||||||||
|
|
|
|
|
|
|
|||||||
| 748,897 | 532,823 | 528,550 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Operating (loss) income |
(92,265 | ) | 87,880 | 78,857 | ||||||||
| Other income (expense): |
||||||||||||
| Interest expense |
(169,332 | ) | (155,181 | ) | (157,032 | ) | ||||||
| Interest income |
345 | 310 | 1,033 | |||||||||
| Loss on modification and extinguishment of debt |
(2,065 | ) | (19,798 | ) | — | |||||||
| Other income (expense), net |
(2,814 | ) | 859 | 6,073 | ||||||||
|
|
|
|
|
|
|
|||||||
| (173,866 | ) | (173,810 | ) | (149,926 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Loss from continuing operations before income taxes |
(266,131 | ) | (85,930 | ) | (71,069 | ) | ||||||
| Income tax benefit |
52,544 | 34,255 | 21,678 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from continuing operations |
(213,587 | ) | (51,675 | ) | (49,391 | ) | ||||||
| Loss from discontinued operations, net of tax |
— | — | (319 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(213,587 | ) | (51,675 | ) | (49,710 | ) | ||||||
| Net income attributable to noncontrolling interests |
(882 | ) | (857 | ) | (723 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net loss attributable to DJO Finance LLC |
$ | (214,469 | ) | $ | (52,532 | ) | $ | (50,433 | ) | |||
|
|
|
|
|
|
|
|||||||
See accompanying notes to consolidated financial statements.
F-4
Table of Contents
DJO Finance LLC
Consolidated Statements of Equity
(in thousands)
| DJO Finance LLC | ||||||||||||||||||||||||
| Member capital |
Accumulated deficit |
Accumulated other comprehensive income (loss) |
Total membership equity |
Noncontrolling interests |
Total equity |
|||||||||||||||||||
| Balance at December 31, 2008 |
$ | 824,235 | $ | (221,842 | ) | $ | (4,027 | ) | $ | 598,366 | $ | 1,743 | $ | 600,109 | ||||||||||
| Net (loss) income |
— | (50,433 | ) | — | (50,433 | ) | 723 | (49,710 | ) | |||||||||||||||
| Other comprehensive income, net of taxes |
— | — | 4,545 | 4,545 | 43 | 4,588 | ||||||||||||||||||
| Stock-based compensation |
3,382 | — | — | 3,382 | — | 3,382 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2009 |
827,617 | (272,275 | ) | 518 | 555,860 | 2,509 | 558,369 | |||||||||||||||||
| Net (loss) income |
— | (52,532 | ) | — | (52,532 | ) | 857 | (51,675 | ) | |||||||||||||||
| Other comprehensive loss, net of taxes |
— | — | (2,566 | ) | (2,566 | ) | (129 | ) | (2,695 | ) | ||||||||||||||
| Investment by parent |
1,489 | — | — | 1,489 | — | 1,489 | ||||||||||||||||||
| Stock-based compensation |
1,888 | — | — | 1,888 | — | 1,888 | ||||||||||||||||||
| Dividend paid by subsidiary to owners of noncontrolling interests |
— | — | — | — | (557 | ) | (557 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2010 |
830,994 | (324,807 | ) | (2,048 | ) | 504,139 | 2,680 | 506,819 | ||||||||||||||||
| Net (loss) income |
— | (214,469 | ) | — | (214,469 | ) | 882 | (213,587 | ) | |||||||||||||||
| Other comprehensive income (loss), net of taxes |
— | — | 2,266 | 2,266 | (53 | ) | 2,213 | |||||||||||||||||
| Investment by parent |
3,176 | — | — | 3,176 | — | 3,176 | ||||||||||||||||||
| Stock-based compensation |
2,701 | — | — | 2,701 | — | 2,701 | ||||||||||||||||||
| Cancellation of vested options |
(2,000 | ) | — | — | (2,000 | ) | — | (2,000 | ) | |||||||||||||||
| Dividend paid by subsidiary to owners of noncontrolling interests |
— | — | — | — | (1,366 | ) | (1,366 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2011 |
$ | 834,871 | $ | (539,276 | ) | $ | 218 | $ | 295,813 | $ | 2,143 | $ | 297,956 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
See accompanying notes to consolidated financial statements.
F-5
Table of Contents
DJO Finance LLC
Consolidated Statements of Comprehensive Loss
(in thousands)
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Net loss |
$ | (213,587 | ) | $ | (51,675 | ) | $ | (49,710 | ) | |||
| Other comprehensive income (loss), net of taxes: |
||||||||||||
| Foreign currency translation adjustments, net of tax benefit (provision) of $1,681, $942 and $(2,153) for the year ended December 31, 2011, 2010, and 2009, respectively |
(1,896 | ) | (5,435 | ) | 3,353 | |||||||
| Unrealized loss on cash flow hedges, net of tax benefit of $175, $2,965, and $6,309 for the year ended December 31, 2011, 2010, and 2009, respectively |
(272 | ) | (4,708 | ) | (9,827 | ) | ||||||
| Reclassification adjustment for losses on cash flow hedges included in net loss, net of tax (provision) of $(2,773), $(4,764) and $(7,102) for the year ended December 31, 2011, 2010, and 2009, respectively |
4,381 | 7,448 | 11,062 | |||||||||
|
|
|
|
|
|
|
|||||||
| Other comprehensive income (loss) |
2,213 | (2,695 | ) | 4,588 | ||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
(211,374 | ) | (54,370 | ) | (45,122 | ) | ||||||
| Comprehensive income attributable to noncontrolling interests |
(829 | ) | (728 | ) | (766 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss attributable to DJO Finance LLC |
$ | (212,203 | ) | $ | (55,098 | ) | $ | (45,888 | ) | |||
|
|
|
|
|
|
|
|||||||
See accompanying notes to consolidated financial statements.
F-6
Table of Contents
DJO Finance LLC
Consolidated Statements of Cash Flows
(in thousands)
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Cash Flows From Operating Activities: |
||||||||||||
| Net loss |
$ | (213,587 | ) | $ | (51,675 | ) | $ | (49,710 | ) | |||
| Adjustments to reconcile net loss to net cash provided by operating activities: |
||||||||||||
| Depreciation |
27,294 | 25,996 | 27,896 | |||||||||
| Amortization of intangible assets |
93,957 | 77,523 | 77,254 | |||||||||
| Amortization of debt issuance costs and non-cash interest expense |
8,476 | 13,272 | 12,679 | |||||||||
| Stock-based compensation expense |
2,701 | 1,888 | 3,382 | |||||||||
| Impairment of goodwill and intangible assets |
141,006 | — | 6,998 | |||||||||
| Loss (gain) on disposal of assets, net of depreciation and adjustments |
4,385 | 2,067 | (2,094 | ) | ||||||||
| Deferred income tax benefit |
(60,620 | ) | (39,687 | ) | (23,690 | ) | ||||||
| Provisions for doubtful accounts and sales returns |
31,673 | 33,077 | 34,904 | |||||||||
| Inventory reserves |
7,706 | 6,596 | 7,462 | |||||||||
| Loss on modification and extinguishment of debt |
— | 19,798 | — | |||||||||
| Gain on sale of discontinued operations |
— | — | (393 | ) | ||||||||
| Changes in operating assets and liabilities, net of acquired assets and liabilities: |
||||||||||||
| Accounts receivable |
(32,231 | ) | (33,105 | ) | (15,156 | ) | ||||||
| Inventories |
(13,190 | ) | (13,908 | ) | (1,868 | ) | ||||||
| Prepaid expenses and other assets |
8,435 | (4,837 | ) | 3,438 | ||||||||
| Accrued interest |
5,351 | 4,610 | 2 | |||||||||
| Accounts payable and other current liabilities |
12,249 | (16,021 | ) | (13,310 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by operating activities |
23,605 | 25,594 | 67,794 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash Flows From Investing Activities: |
||||||||||||
| Purchases of property and equipment |
(39,397 | ) | (27,247 | ) | (28,872 | ) | ||||||
| Cash paid in connection with acquisitions, net of cash acquired |
(317,669 | ) | (2,045 | ) | (13,086 | ) | ||||||
| Proceeds received upon sale of discontinued operations, net |
— | — | 21,846 | |||||||||
| Other investing activities, net |
(1,596 | ) | (903 | ) | 4,112 | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
(358,662 | ) | (30,195 | ) | (16,000 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash Flows From Financing Activities: |
||||||||||||
| Proceeds from issuance of debt |
439,000 | 447,130 | 68,260 | |||||||||
| Repayments of debt and capital lease obligations |
(96,826 | ) | (437,367 | ) | (103,521 | ) | ||||||
| Payment of debt issuance costs |
(7,694 | ) | (10,282 | ) | — | |||||||
| Investment by parent |
3,176 | 1,489 | — | |||||||||
| Cash paid in connection with the cancellation of vested options |
(2,000 | ) | — | — | ||||||||
| Dividend paid by subsidiary to owners of noncontrolling interests |
(1,366 | ) | (557 | ) | — | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) financing activities |
334,290 | 413 | (35,261 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Effect of exchange rate changes on cash and cash equivalents |
804 | (2,291 | ) | (2,405 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
37 | (6,479 | ) | 14,128 | ||||||||
| Cash and cash equivalents, beginning of year |
38,132 | 44,611 | 30,483 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of year |
$ | 38,169 | $ | 38,132 | $ | 44,611 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosures of cash flow information: |
||||||||||||
| Cash paid for interest |
$ | 151,207 | $ | 139,095 | $ | 144,215 | ||||||
| Cash (refunded) paid for taxes, net |
$ | (956 | ) | $ | 4,515 | $ | 3,777 | |||||
| Non-cash investing and financing activities: |
||||||||||||
| Increases in property and equipment and in other liabilities in connection with capitalized software costs |
$ | — | $ | 1,934 | $ | 3,876 | ||||||
| Issuance of notes payable in connection with acquisitions |
$ | — | $ | — | $ | 2,860 | ||||||
See accompanying notes to consolidated financial statements.
F-7
Table of Contents
Notes to Consolidated Financial Statements
| 1. | ORGANIZATION AND BASIS OF PRESENTATION |
Organization and Business
We are a global developer, manufacturer and distributor of medical devices that provide solutions for musculoskeletal health, vascular health and pain management. Our products address the continuum of patient care from injury prevention to rehabilitation after surgery, injury or from degenerative disease, enabling people to regain or maintain their natural motion. Our products are used by orthopedic specialists, spine surgeons, primary care physicians, pain management specialists, physical therapists, podiatrists, chiropractors, athletic trainers and other healthcare professionals. Our product lines include rigid and soft orthopedic bracing, hot and cold therapy, bone growth stimulators, vascular therapy systems and compression garments, therapeutic shoes and inserts, electrical stimulators used for pain management and physical therapy products. Our surgical implant business offers a comprehensive suite of reconstructive joint products for the hip, knee and shoulder.
Our current business activities are the result of a combination of ReAble Therapeutics, Inc. (ReAble), which was acquired by an affiliate of Blackstone Capital Partners V L.P. (Blackstone), and DJO Opco Holdings, Inc. (DJO Opco), formerly named DJO Incorporated. On November 20, 2007, a subsidiary of ReAble was merged with DJO Opco, with DJO Opco continuing as the surviving corporation (the DJO Merger). As a result of the DJO Merger, DJO Opco became a subsidiary of ReAble Therapeutics Finance LLC (RTFL), which is itself a wholly owned indirect subsidiary of ReAble. Following the DJO Merger, ReAble was renamed DJO Incorporated, RTFL was renamed DJO Finance LLC (DJOFL) and ReAble Finance Corporation, the co-issuer of the 10.875% Notes, 9.75% Notes and 7.75% Notes (see Note 13), was renamed DJO Finance Corporation (DJO Finco). Effective December 31, 2009, DJO Opco was merged with DJO, LLC, a wholly owned subsidiary of DJOFL. Effective February 10, 2011, DJO Incorporated changed its name to DJO Global, Inc. (DJO). Substantially all business activities of DJO are conducted by DJOFL and its wholly owned subsidiaries. Except as otherwise indicated, references to “us,” “we,” “DJOFL,” “our,” or “the Company,” refers to DJOFL and its consolidated subsidiaries.
Segment Reporting
During the second quarter of 2011, we changed the name of our Bracing and Supports segment to Bracing and Vascular to reflect the addition of our recent acquisitions, which have increased our focus on the vascular market. This segment includes the U.S. results of operations attributable to Dr. Comfort, ETI, and Circle City from their respective dates of acquisition (see Note 3). This change had no impact on previously reported segment information.
We market and distribute our products through four operating segments, Recovery Sciences, Bracing and Vascular, Surgical Implant, and International. Our Recovery Sciences, Bracing and Vascular and Surgical Implant segments generate their revenues within the United States.
Our Bracing and Vascular segment offers rigid knee braces, orthopedic soft goods, cold therapy products, vascular systems, compression therapy, and therapeutic footwear for the diabetes care market. Our Recovery Sciences segment offers home electrotherapy, inotophoresis, home traction products, bone growth stimulation products, and clinical therapy equipment. Our Surgical Implant segment offers a comprehensive suite of reconstructive joint products for the knee, hip and shoulder. Our International segment offers all of our products to customers outside the United States. See Note 19 for additional information about our reportable segments.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (GAAP) requires management to make estimates and assumptions that affect the reported amounts
F-8
Table of Contents
of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Estimates and assumptions are used in accounting for, among other things, contractual allowances, rebates, product returns, warranty obligations, allowances for doubtful accounts, valuation of inventories, self-insurance reserves, income taxes, loss contingencies, fair values of derivative instruments, fair values of long-lived assets and any related impairments, capitalization of costs associated with internally developed software and stock-based compensation. Actual results could differ from those estimates.
Basis of Presentation
We consolidate the results of operations of our 50% owned subsidiary Medireha GmbH (Medireha) and reflect the 50% share of results not owned by us as noncontrolling interests in our consolidated statements of operations. We maintain control of Medireha through certain rights that enable us to prohibit certain business activities that are not consistent with our plans for the business and provide us with exclusive distribution rights for products manufactured by Medireha.
The accompanying consolidated financial statements include our accounts and all voting interest entities where we exercise a controlling financial interest through the ownership of a direct or indirect majority voting interest. All significant intercompany accounts and transactions have been eliminated in consolidation.
Reclassifications and prior period adjustments
Certain reclassifications have been made to prior year amounts to conform to the current year presentation.
In the fourth quarter of fiscal year 2011, we made a reclassification in our balance sheet of the replacement parts for our instrument sets used by our Surgical Implant business. Complete instrument sets are generally loaned to surgeons to enable them to perform implant surgeries with our products. These complete sets are classified as property and equipment, net of depreciation in our consolidated balance sheet. Historically, we had presented replacement parts used for these instrument kits as finished goods in inventory, net of reserves. During the current year, we determined that the replacement parts were more appropriately classified as a non-current asset in property and equipment rather than a current asset in inventory. Accordingly, we have reclassified the balance of these replacement parts in our consolidated balance sheets as of December 31, 2011 and 2010. This change resulted in an increase to property and equipment and a decrease to inventory of $8.6 million as of December 31, 2010.
In addition, during the fourth quarter of fiscal year 2011, we identified and corrected an immaterial error which impacted the consolidated financial statements for the years ended December 31, 2010 and 2009, related to the elimination of intercompany profits on the sale of products between subsidiaries. This error resulted in an overstatement of cost of goods sold of $1.1 million and $3.1 million for the years ended December 31, 2009 and 2010, respectively.
Based on a quantitative and qualitative analysis of the error as required by SEC Staff Accounting Bulletin No. 108 Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements (SAB 108), we determined that correcting the cumulative impact of this error, which decreased cost of goods sold and increased property and equipment by $4.2 million in the fourth quarter of the year ended December 31, 2011, was not material to the results for the year ended December 31, 2011 or any prior period.
| 2. | SIGNIFICANT ACCOUNTING POLICIES |
Cash and Cash Equivalents. Cash consists of deposits with financial institutions. We consider all short-term, highly liquid investments and investments in money market funds and commercial paper with remaining maturities of less than three months at the time of purchase to be cash equivalents. While our cash and cash equivalents are on deposit with high-quality institutions, such deposits exceed Federal Deposit Insurance Corporation insured limits.
F-9
Table of Contents
Allowance for Doubtful Accounts. We make estimates of the collectability of accounts receivable. Management analyzes accounts receivable historical collection rates and bad debts write-offs, customer concentrations, customer credit-worthiness and current economic trends when evaluating the adequacy of the allowance for doubtful accounts.
Sales Returns and Allowances. We make estimates of the amount of sales returns and allowances that will eventually be incurred. Management analyzes sales programs that are in effect, contractual arrangements, market acceptance and historical trends when evaluating the adequacy of sales returns and allowance accounts. We estimate contractual discounts and allowances for reimbursement amounts from our third party payor customers based on negotiated contracts and historical experience.
Inventories. We state our inventories at the lower of cost or market. We use standard cost methodology to determine cost basis for our inventories. This methodology approximates actual cost on a first-in, first-out basis. We establish reserves for slow moving and excess inventory, product obsolescence, shrinkage and other valuation impairments based on future demand and historical experience.
Property and Equipment. Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets that range from three to 25 years. Leasehold improvements and equipment under capital leases are amortized on a straight-line basis over the shorter of the lease term or estimated useful life of the asset. We capitalize surgical implant instruments that we provide to surgeons, free of charge, for use while implanting our products and the related depreciation expense is recorded as a component of selling, general and administrative expense. We also capitalize electrotherapy devices that we rent to patients and record the related depreciation expense in cost of sales.
Software Developed For Internal Use. Software is stated at cost less accumulated amortization and is amortized on a straight-line basis over estimated useful lives ranging from three to ten years. We capitalize costs of internally developed software during the development stage, including external consulting costs, cost of software licenses, and internal payroll and payroll-related costs for employees who are directly associated with a software project. Software assets are reviewed for impairment when events or circumstances indicate that the carrying value may not be recoverable. Upgrades and enhancements are capitalized if they result in added functionality. Amortization expense related to internally developed software was $2.4 million and $1.0 million for the years ended December 31, 2011 and 2010 respectively. There was no amortization expense related to internally developed software during the year ended December 31, 2009 as the assets had not yet been placed in service.
In 2008, we began implementing a new ERP system to replace six legacy accounting and finance systems and numerous other software systems with a single-entry ERP system that will be used by most of our businesses. During the year ended December 31, 2011, we determined that certain capitalized ERP assets would not be used and we recorded an impairment charge of $7.1 million which is included in selling and general administrative expense in our consolidated statement of operations. As of December 31, 2011 and 2010, we had $17.4 million and $23.5 million respectively, of unamortized internally developed software costs included within property and equipment in our consolidated balance sheets.
Intangible Assets. Our primary intangible assets are goodwill, customer relationships, patents and technology and trademarks and trade names. Goodwill represents the excess purchase price over the fair value of the identifiable net assets acquired in business combinations. Goodwill and intangible assets with indefinite useful lives are not amortized, but instead are tested for impairment at least annually. Intangible assets with definite lives are amortized over their respective estimated useful lives and reviewed for impairment when circumstances warrant. Our identifiable intangible assets subject to amortization include customer relationships, patents, intellectual property, distributor contracts and relationships, trademarks and trade names, and non-compete agreements and are being amortized using the straight-line method over their remaining weighted
F-10
Table of Contents
average useful lives of 7.7 years for customer relationships, 10.4 years for patents and technology, 5.0 years for distributor contracts and relationships, 9.2 years for trademarks and trade names, and 3.5 years for non-compete agreements.
Goodwill and Intangible Assets
We evaluate the carrying value of goodwill and indefinite life intangible assets annually on the first day of the fourth quarter or whenever events or circumstances indicate the carrying value may not be recoverable. We evaluate the carrying value of finite life intangible assets whenever events or circumstances indicate the carrying value may not be recoverable. Significant assumptions are required to estimate the fair value of goodwill and intangible assets, most notably estimated future cash flows generated by these assets. As such, these fair valuation measurements use significant unobservable inputs. Changes to these assumptions could require us to record impairment charges on these assets.
In performing our 2011 goodwill impairment test, we estimated the fair values of our reporting units using the income approach valuation methodology which includes the discounted cash flow method and the market approach valuation methodology which includes the use of market multiples. The discounted cash flows for each reporting unit were based on discrete financial forecasts developed by management for planning purposes, and required significant judgment with respect to forecasted sales, gross margin, selling, general and administrative expenses, EBITDA, capital expenditures, and the selection and use of an appropriate discount rate. For purposes of calculating the discounted cash flows of our reporting units we used estimated revenue growth rates between 0% and 11%. Cash flows beyond the discrete forecasts were estimated using a terminal value calculation, which incorporated historical and forecasted financial trends for each identified reporting unit and considered long-term earnings growth rates for publicly traded peer companies. Future cash flows were then discounted to present value at discount rates ranging from 10.7% to 12.5%, and terminal value growth rates of 3%. Publicly available information regarding comparable market capitalization was also considered in assessing the reasonableness of the cumulative fair values of our reporting units estimated using the discounted cash flow methodology.
In the fourth quarter of 2011, we determined that the carrying value of our Empi and Surgical Implant reporting units were in excess of their estimated fair values. As a result, we recorded goodwill impairment charges for the Empi and Surgical Implant reporting units of $76.7 million and $47.4 million, respectively. See Note 8 for further discussion and the factors that contributed to these impairment charges.
We have five other reporting units with goodwill assigned to them. For each of those five reporting units, the estimated fair values exceed their carrying value.
Additionally, on the first day of the fourth quarter of 2011 we tested our indefinite lived intangible assets, consisting of trade names for impairment. This test work compares the fair value of the asset with its carrying amount. To determine the fair value we applied the relief from royalty (RFR) method. Under the RFR method, the value of the trade name is determined by calculating the present value of the after-tax cost savings associated with owning the asset and therefore not being required to pay royalties for its use during the asset’s indefinite life. Significant judgments inherent in this analysis include the selection of appropriate discount rates, estimating future cash flows and the identification of appropriate terminal growth rate assumptions. Discount rate assumptions are based on an assessment of the risk inherent in the projected future cash generated by the respective intangible assets. Also subject to judgment are assumptions about royalty rates, which are based on the estimated rates at which similar brands and trademarks are being licensed in the marketplace.
In the fourth quarter of 2011, we determined that the carrying value of our Empi trade name was in excess of its estimated fair value, and recorded an impairment charge of $16.9 million. See Note 8 for further discussion and the factors that contributed to this impairment charge.
The estimates we have used are consistent with the plans and estimates that we use to manage our business, however, it is possible that the plans may change and estimates used may prove to be inaccurate. If our actual
F-11
Table of Contents
results, or the plans and estimates used in future impairment analyses, are lower than the original estimates used to assess the recoverability of these assets, we could incur significant impairment charges.
Warranty Costs. We provide expressed warranties on certain products for periods typically ranging from one to three years. We estimate our warranty obligations at the time of sale based upon historical experience and known product issues, if any.
A summary of the activity in our warranty reserves is as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Balance, beginning of year |
$ | 2,222 | $ | 1,936 | $ | 1,761 | ||||||
| Amount charged to expense for estimated warranty costs |
105 | 1,283 | 952 | |||||||||
| Deductions for actual costs incurred |
(571 | ) | (997 | ) | (777 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Balance, end of year |
$ | 1,756 | $ | 2,222 | $ | 1,936 | ||||||
|
|
|
|
|
|
|
|||||||
Self Insurance. We are partially self insured for certain employee health benefits and product liability claims. Accruals for losses are provided based upon claims experience and actuarial assumptions, including provisions for incurred but not reported losses.
Revenue Recognition. Revenue is recognized when all four of the following criteria are met: (i) persuasive evidence that an arrangement exists; (ii) shipment of goods and passage of title; (iii) the selling price is fixed or determinable; and (iv) collectibility is reasonably assured.
We sell our Bracing and Vascular, Recovery Sciences, and International segment products through a variety of distribution channels. We sell our home therapy products to wholesale customers and directly to patients. We recognize wholesale revenue when we ship our products to our wholesale customers. We recognize home therapy retail revenue, both rental and purchase, when our product has been dispensed to the patient and the patient’s insurance has been verified. We recognize revenue for product shipped directly to the patient at the time of shipment. For retail products that are sold from our inventories consigned at clinic locations, we recognize revenue when we receive notice that the device has been prescribed and dispensed to the patient and the insurance has been verified or preauthorization from the insurance company has been obtained, when required.
We sell our DonJoy products through a network of independent sales representatives. We record revenues from sales made by sales representatives, who are paid commissions, when the product is shipped to the customer. For certain of our other products, we sell directly to the patient and bill a third party payor, if applicable, on behalf of the patient.
We sell our ProCare, Aircast and clinical rehabilitation products to distributors. We record revenue at the time product is shipped to the distributor. Distributors take title to the products, assume credit and product obsolescence risks, must pay within specified periods regardless of when they sell or use the products and have no price protection except for distributors who participate in our rebate program.
We sell our products to customers outside the United States through wholly owned subsidiaries or independent distributors. We record revenue from sales to distributors at the time product is shipped to the distributor. Our international distributors take title to the products, assume credit and product obsolescence risks, must pay within specified periods regardless of when they sell the products and have no price protection. We record revenue from sales made by our wholly owned subsidiaries at the time product is shipped to the customer.
We sell our Surgical Implant products through a network of independent sales representatives. We record revenues from sales made by sales representatives, who are paid commissions at the time the product is used in a surgical procedure (implanted in a patient) and a purchase order is received from the hospital. We include amounts billed to customers for freight in revenue.
F-12
Table of Contents
We reduce revenue by estimates of potential future product returns and other allowances. Revenues are also reduced by rebates related to sales transacted through distribution agreements that provide the distributors with a right to return inventory or take certain pricing adjustments based on sales mix or volume. Provisions for product returns and other allowances are recorded as a reduction to revenue in the period sales are recognized.
Advertising Costs. We expense advertising costs as they are incurred. For the years ended December 31, 2011, 2010 and 2009, advertising costs were $17.1 million, $10.4 million, and $5.4 million, respectively.
Shipping and Handling Expenses. Shipping and handling expenses are included within cost of sales in our consolidated statements of operations.
Stock Based Compensation. We maintain a stock option plan under which stock options have been granted to both employees and non-employees. All share based payments to employees are recognized in the financial statements based on their grant date fair values and our estimates of forfeitures. We amortize stock-based compensation for service-based awards granted on a straight-line basis over the requisite service (vesting) period for the entire award. Other awards vest upon the achievement of certain pre-determined performance targets, and compensation expense is recognized to the extent the achievement of the performance targets is deemed probable.
Income Taxes. Income taxes are accounted for under the asset and liability method, whereby deferred tax assets and liabilities are recognized and measured using enacted tax rates in effect for each taxing jurisdiction in which we operate for the year in which those temporary differences are expected to be recognized. Net deferred tax assets are then reduced by a valuation allowance if we believe it more-likely-than-not such net deferred tax assets will not be realized.
Foreign Currency Translation and Transactions. The reporting currency of DJOFL is the U.S. Dollar. Assets and liabilities of foreign subsidiaries (including intercompany balances for which settlement is not anticipated in the foreseeable future) are translated at the spot rate in effect at the applicable reporting date, and our consolidated statement of operations is translated at the average exchange rates in effect during the applicable period. The resulting unrealized cumulative translation adjustment, net of applicable income taxes, is recorded as a component of accumulated other comprehensive income (loss) in our consolidated statement of equity. Cash flows from our operations in foreign countries are translated at the average rate for the applicable period. The effect of exchange rates on cash balances held in foreign currencies are separately reported in our consolidated statements of cash flows.
Transactions denominated in currencies other than our or our subsidiaries’ functional currencies are recorded based on exchange rates at the time such transactions arise. Changes in exchange rates with respect to amounts recorded in our consolidated balance sheets related to such transactions result in transaction gains and losses that are reflected in our consolidated statements of operations as either unrealized (based on the applicable period end translation) or realized (upon settlement of the transactions).
Derivative Financial Instruments. All derivative instruments are recognized in the financial statements and measured at fair value regardless of the purpose or intent for holding them.
We make use of debt financing as a source of funds and are therefore exposed to interest rate fluctuations in the normal course of business. Our credit facilities are subject to floating interest rates. Historically, we used interest rate swaps to manage the risk of unfavorable movements in interest rates on a portion of the outstanding loan balance, thereby locking in a fixed rate on a portion of the principal, reducing the effect of possible rising interest rates and making interest expense more predictable. Our interest rate swaps expired on December 31, 2011. These interest rate swap agreements were designated as cash flow hedges for accounting purposes, and therefore, changes in the fair values of the derivatives were recorded in accumulated other comprehensive income (loss) and are subsequently recognized in earnings when the hedged item affects earnings.
F-13
Table of Contents
We use foreign exchange forward contracts to hedge expense commitments that are denominated in currencies other than the U.S. dollar. The purpose of our foreign currency hedging activities is to fix the dollar value of specific commitments and payments to foreign vendors. Before acquiring a derivative instrument to hedge a specific risk, potential natural hedges are evaluated. While our foreign exchange contracts act as economic hedges, we have not designated such instruments as hedges for accounting purposes. Therefore, gains and losses resulting from changes in the fair values of these derivative instruments are recorded in other income (expense), net, in our consolidated statements of operations.
The fair value of our derivative instruments has been determined through the use of models that consider various assumptions, including time value and yield curves, as well as other relevant economic measures, which are inputs that are classified as Level 2 in the valuation hierarchy (see Notes 11 and 12).
Comprehensive Income (Loss). Comprehensive income (loss) includes net income (loss) as per our consolidated statement of operations and other comprehensive income (loss). Other comprehensive income (loss), which is comprised of unrealized gains and losses on foreign currency translation adjustments and cash flow hedges, net of tax, is included in our consolidated statement of equity as accumulated other comprehensive income (loss).
Concentration of Credit Risk. We sell the majority of our products in the United States to orthopedic professionals, hospitals, distributors, specialty dealers, insurance companies, managed care companies and certain governmental payors such as Medicare. International sales comprised 26.0%, 25.3%, and 25.5% of our net sales for the years ended December 31, 2011, 2010, and 2009, respectively. International sales are generated from a diverse group of customers through our wholly owned subsidiaries and certain independent distributors. Credit is extended based on an evaluation of the customer’s financial condition and generally collateral is not required. We provide a reserve for estimated bad debts. Management reviews and revises its estimates for credit losses from time to time and such credit losses have generally been within management’s estimates. In each of the years ended December 31, 2011, 2010 and 2009, we had no individual customer or distributor that accounted for 10% or more of our total annual net sales.
Fair Value of Financial Instruments. The carrying amounts of our short-term financial instruments, including cash and cash equivalents, accounts receivable and accounts payable, approximate fair values due to their short-term nature. The fair values of our variable rate debt, including borrowings under our Senior Secured Credit Facility, approximate carrying value due to the variable interest rate features on these instruments. See Note 13 for information concerning the fair value of our fixed rate debt.
Recent Accounting Standards. We do not believe that any recently issued accounting standards will have a material impact on our consolidated financial statements.
| 3. | ACQUISITIONS |
During 2011, we made the following acquisitions, all of which are included in our Bracing and Vascular segment with the exception of the international activities of Dr. Comfort and ETI, which are included in our International segment:
On April 7, 2011, we acquired all of the LLC membership interests of Rikco International, LLC, D/B/A Dr. Comfort (Dr. Comfort), for a total purchase price of $257.5 million. Dr. Comfort is a provider of therapeutic footwear, which serves the diabetes care market in podiatry practices, orthotic and prosthetic centers, home medical equipment providers and independent pharmacies.
Of the total purchase price, $24.5 million was paid to a third party escrow agent to secure the indemnity obligations of the seller and to secure any post closing obligations resulting from the final determination of working capital and cash on hand as of the closing date. Following a post-closing purchase price adjustment which was deducted from the escrow account, the balance in the escrow account at December 31, 2011 was $23.5 million.
F-14
Table of Contents
The acquisition was funded using proceeds from $300.0 million of new 7.75% senior notes (7.75% Notes) issued in April 2011 (see Note 13). In connection with the acquisition of Dr. Comfort, we incurred $11.3 million of direct acquisition costs during the year ended December 31, 2011, which are included in selling and general administrative expense in our consolidated statement of operations. Fees and expenses related to the acquisition of Dr. Comfort and the issuance of the 7.75% Notes included $3.9 million of bridge financing fees paid to Credit Suisse. In addition, we paid $5.0 million of transaction and advisory fees paid to Blackstone Advisory Partners, L.P., an affiliate of our major shareholder (see Note 18).
On March 10, 2011, we acquired substantially all of the assets of Circle City Medical, Inc. (Circle City). Circle City markets orthopedic soft goods and medical compression therapy products to independent pharmacies and home healthcare dealers. The purchase price was $11.7 million, of which $1.3 million was withheld from the closing date payment and was paid to a third party escrow agent to secure the indemnity obligations of the seller. An additional $1.3 million was deposited into escrow for the retention of a key employee union will be recognized as compensation expense over the retention period of 24 months. Direct acquisition costs associated with the Circle City acquisition of $0.1 million are included in selling, general and administrative expense in our consolidated statement of operations. We financed the acquisition with cash on hand and a draw of $7.0 million on our revolving line of credit. Up to an additional $2.0 million may be earned by the sole shareholder of Circle City as a royalty payment based on future sales of a specific product line over the next six years. This potential royalty payment was evaluated separately from the acquisition of the assets and liabilities of Circle City, and the royalty payments will be expensed as they are earned. For the year ended December 31, 2011, royalty payments made to the seller for sales of this product line were not significant to the Company.
On February 4, 2011, we purchased certain assets of an e-commerce business (BetterBraces.com), which offers various bracing, cold therapy and electrotherapy products, for total consideration of $3.0 million. Of the total purchase price, $1.8 million was paid in cash at closing, $0.4 million was offset against accounts receivable due from the seller, $0.5 million was retained to fully repay outstanding principal and accrued interest due from the seller under a revolving convertible promissory note, and $0.3 million was held back until February 4, 2012 as security for potential indemnification claims. No claims were made and the holdback was paid to the seller in February 2012. The acquisition was financed using cash on hand.
On January 4, 2011, we acquired all of the outstanding shares of capital stock of Elastic Therapy, Inc. (ETI), a designer and manufacturer of private label medical compression therapy products used to treat and prevent a wide range of venous disorders. The purchase price was $46.4 million, of which a total of $3.6 million was deposited in escrow for up to one year to fund potential indemnity of claims. No claims were made and the holdback was paid in January 2012. An additional $1.0 million was deposited in escrow for the retention of certain key employees to be paid in installments six months, nine months and twelve months after the closing date, with the first installment paid to the sellers in July 2011. This balance was expensed over the period it was earned. Direct acquisition costs associated with the ETI acquisition of $0.3 million are included in selling, general and administrative expense in our consolidated statement of operations. The acquisition was financed using cash on hand and a draw of $35.0 million on our revolving line of credit. On January 5, 2011, we converted ETI to a limited liability company.
During the years ended December 31, 2010 and 2009, we acquired businesses from four independent international distributors of our products. Our primary reason for these acquisitions was to improve the profitability of our sales and to expand the range of our products sold in these markets, which we believe we can accomplish more successfully by participating directly in the markets, instead of through independent distributors.
On September 20, 2010, we acquired certain assets and contractual rights from an independent South African distributor of DonJoy products for total consideration of $1.9 million, which included a cash payment of $1.2 million on the closing date, forgiveness of $0.4 million of accounts receivable from the distributor and
F-15
Table of Contents
holdbacks of $0.3 million related primarily to potential indemnification claims, which was to be paid in September 2011 if there are no such claims. We have withheld this payment pending resolution of certain post-closing adjustments.
On August 4, 2009, we acquired Chattanooga Group Inc. (Chattanooga Canada), an independent Canadian distributor of Chattanooga products, for $7.2 million. Pursuant to the terms of the acquisition agreement and included within the purchase price, was a $1.4 million indemnification holdback, which accrued interest at an annual rate of 2.5% for the first 18 months and a variable rate thereafter; and a $1.4 million promissory note, which accrued interest at an annual rate of 6%. We paid the promissory note and related interest thereon in August 2010. The holdback provides security for potential indemnification claims and, if not used for that purpose, is payable to the sellers. The first half of the holdback amount not used to cover indemnification claims, including interest thereon, was paid in May 2011. The second half of the holdback amount, including interest thereon, will be payable in 2012 if not used to cover indemnification claims.
On August 4, 2009, we acquired Empi Canada Inc. (Empi Canada), an independent Canadian distributor of Empi products, for $7.4 million. Pursuant to the terms of the acquisition agreement and included within the purchase price was a $1.4 million indemnification holdback, which accrued interest at an annual rate of 2.5% for the first 18 months and a variable rate thereafter; and a $1.4 million promissory note, which accrued interest at an annual rate of 6%. We paid the promissory note and related interest thereon in August 2010. The holdback provides security for potential indemnification claims and, if not used for that purpose, is payable to the sellers. The first half of the holdback amount not used to cover indemnification claims, including interest thereon, was paid in May 2011. The second half of the holdback amount, including interest thereon, will be payable in 2012 if not used to cover indemnification claims.
On February 3, 2009, we acquired DonJoy Orthopaedics Pty., Ltd. (DJO Australia), an independent Australian distributor of DonJoy products, for $3.4 million. Pursuant to the terms of the acquisition agreement, and included within the purchase price, was $0.8 million, representing the acquisition date fair value of the additional amount payable to the selling shareholder if certain revenue targets were met by December 31, 2009. We attained these revenue targets and paid the $0.8 million to the selling shareholder in the first quarter of 2010.
The purchase price for each of these acquisitions was allocated to the fair values of the net tangible and intangible assets acquired as follows (in thousands):
| (in thousands): |
Dr. Comfort | Circle City | BetterBraces.com | ETI | DJO South Africa |
Chattanooga Canada |
Empi Canada |
DJO Australia |
||||||||||||||||||||||||
| Cash |
$ | 59 | $ | — | $ | — | $ | 817 | $ | — | $ | 59 | $ | 29 | $ | 912 | ||||||||||||||||
| Accounts receivable |
9,187 | 572 | — | 3,690 | — | 423 | 300 | 397 | ||||||||||||||||||||||||
| Inventory |
27,241 | 1,736 | — | 2,133 | 435 | 261 | 536 | 725 | ||||||||||||||||||||||||
| Other current assets |
2,108 | — | — | 1,542 | — | — | 19 | 12 | ||||||||||||||||||||||||
| Property and equipment |
2,183 | — | — | 7,230 | 310 | — | — | |||||||||||||||||||||||||
| Other non-current assets |
1,607 | — | — | 394 | — | — | — | |||||||||||||||||||||||||
| Liabilities assumed |
(25,965 | ) | (406 | ) | — | (11,485 | ) | — | (2,254 | ) | (1,033 | ) | (1,120 | ) | ||||||||||||||||||
| Identifiable intangible assets (1): |
||||||||||||||||||||||||||||||||
| Customer relationships |
72,100 | 3,700 | 75 | 13,400 | 1,103 | 5,058 | 2,512 | 1,614 | ||||||||||||||||||||||||
| Technology |
7,000 | — | 1,120 | 6,000 | — | — | — | — | ||||||||||||||||||||||||
| Non-compete |
1,200 | 200 | 185 | 1,600 | — | 253 | 174 | — | ||||||||||||||||||||||||
| Trademarks and trade names |
22,200 | 1,400 | 50 | — | — | — | — | — | ||||||||||||||||||||||||
| Goodwill |
138,548 | 4,469 | 1,570 | 21,085 | 64 | 3,354 | 4,902 | 899 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total purchase price |
$ | 257,468 | $ | 11,671 | $ | 3,000 | $ | 46,406 | $ | 1,912 | $ | 7,154 | $ | 7,439 | $ | 3,439 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
F-16
Table of Contents
| (1) | The fair value of customer relationships was assigned to relationships with major pharmaceutical, medical and home healthcare distributors and certain other customers existing on the acquisition date based upon an estimate of the future discounted cash flows that would be derived from those customers, after deducting contributory asset charges. |
The fair value of technology was determined primarily by estimating the present value of future royalty costs that will be avoided due to our ownership of the patents and technology acquired.
The fair value of non-compete agreements was assigned to non-compete agreements entered into with certain executive officers and senior management. The values were determined by estimating the present value of the cash flows associated with having these agreements in place, less the present value of the cash flows assuming the non-compete agreements were not in place.
The fair value of trademarks and trade names was determined primarily by estimating the present value of future royalty costs that will be avoided due to our ownership of the trade names and trademarks acquired.
The useful lives of the intangible assets acquired were estimated based on the underlying agreements and/or the future economic benefit expected to be received from the assets.
| (2) | Goodwill represents the excess purchase price over the fair value of the identifiable net assets acquired. We anticipate future cost savings as a result of the Chattanooga Canada and Empi Canada acquisitions, driven by estimated synergies from operating efficiencies as we combine these businesses with our existing business in Canada. This is the primary reason the purchase prices for Chattanooga Canada and Empi Canada resulted in the recognition of goodwill. We acquired Dr. Comfort to expand our product offerings and increase our addressable market. We also believe there are certain cost reduction synergies that may be realized when certain portions of the Dr. Comfort business are integrated with our existing businesses and as we implement lean principles in Dr. Comfort’s supply chain and distribution activities. We acquired ETI in order to expand our product offerings and vertically integrate into ETI products which we currently acquire from a third party manufacturer. In addition, we believe there are cost reduction synergies to be realized with the implementation of lean manufacturing methodology. Among the factors which resulted in the recognition of goodwill for Circle City were consolidation of warehouse facilities and expected cost savings from reduction of redundant general and administrative expenses. Among the factors which resulted in the recognition of goodwill for BetterBraces.com were expected cost savings resulting from production and distribution efficiencies and from reduction of redundant general and administrative expenses. |
Goodwill related to our Circle City and BetterBraces.com acquisitions is expected to be deductible for tax purposes.
The goodwill arising from our 2011 acquisitions was allocated to our reportable segments as follows (in thousands):
| December 31, 2011 |
||||
| Bracing and Vascular |
$ | 140,656 | ||
| International |
25,016 | |||
|
|
|
|||
| $ | 165,672 | |||
|
|
|
|||
The results of operations attributable to each acquisition are included in our condensed consolidated financial statements from the date of acquisition. The pro forma financial results presented below (in thousands) give effect to the acquisitions of Dr. Comfort, ETI, and Circle City as if such acquisitions had been completed as of the beginning of each of the periods presented. These pro forma results are not necessarily indicative of the operating results that would have been achieved had these acquisitions occurred on such date.
| Year Ended December 31, | ||||||||
| 2011 | 2010 | |||||||
| Net sales |
$ | 1,095,433 | $ | 1,070,757 | ||||
|
|
|
|
|
|||||
| Net loss attributable to DJOFL |
$ | (209,163 | ) | $ | (94,836 | ) | ||
|
|
|
|
|
|||||
F-17
Table of Contents
Our condensed consolidated statements of operations for the year ended December 31, 2011 include $86.0 million of aggregate net sales and $1.5 million of aggregate net loss attributable to our acquisitions of Dr. Comfort, ETI, and Circle City.
| 4. | DISCONTINUED OPERATIONS |
On June 12, 2009 we sold our Empi Therapy Solutions (ETS) catalog business, formerly known as Rehab Medical Equipment, or RME, to Patterson Medical Supply, Inc. for $21.8 million. Our ETS business, which was included within our Recovery Sciences segment, sold a wide range of proprietary and third party rehabilitation products to physical therapists and chiropractors through printed catalogs and an on-line e-commerce site. As such, results of the ETS business for periods prior to the date of sale are presented as discontinued operations. The operating results of ETS that are classified as discontinued operations in our consolidated statements of operations are summarized in the following table (in thousands):
| Year Ended December 31, 2009 |
||||
| Net sales |
$ | 13,450 | ||
| Pre-tax income |
6,590 | |||
| Income tax provision |
(6,909 | ) | ||
|
|
|
|||
| Net income (loss) |
$ | (319 | ) | |
|
|
|
|||
Included within discontinued operations for the year ended December 31, 2009 is a pre-tax gain on disposal of discontinued operations of $6.6 million, which includes $12.0 million of goodwill associated with the ETS business, based on the relative fair values of ETS and the portion of the reporting unit that remained. The effective tax rate for the discontinued operations for the year ended December 31, 2009 was 105%. This rate differs from the amount which would have been recorded using the U.S. Federal statutory income tax rate of 35% due primarily to a large difference in the book and tax basis of goodwill disposed of.
| 5. | ACCOUNTS RECEIVABLE RESERVES |
A summary of activity in our accounts receivable reserves for doubtful accounts and sales returns is presented below (in thousands):
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Balance, beginning of year |
$ | 53,076 | $ | 48,306 | $ | 36,521 | ||||||
| Provision for doubtful accounts and sales returns |
31,673 | 33,077 | 34,904 | |||||||||
| Write-offs, net of recoveries |
(46,434 | ) | (28,307 | ) | (23,119 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Balance, end of year |
$ | 38,315 | $ | 53,076 | $ | 48,306 | ||||||
|
|
|
|
|
|
|
|||||||
| 6. | INVENTORIES |
Inventories consist of the following (in thousands):
| December 31, 2011 |
December 31, 2010 |
|||||||
| Components and raw materials |
$ | 50,322 | $ | 27,287 | ||||
| Work in process |
4,681 | 5,478 | ||||||
| Finished goods |
60,839 | 51,956 | ||||||
| Inventory held on consignment |
27,003 | 22,592 | ||||||
|
|
|
|
|
|||||
| 142,845 | 107,313 | |||||||
| Inventory reserves |
(14,146 | ) | (12,853 | ) | ||||
|
|
|
|
|
|||||
| $ | 128,699 | $ | 94,460 | |||||
|
|
|
|
|
|||||
F-18
Table of Contents
A summary of the activity in our reserves for estimated slow moving, excess, obsolete and otherwise impaired inventory is presented below (in thousands):
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Balance, beginning of year |
$ | 12,853 | $ | 13,063 | $ | 17,798 | ||||||
| Provision charged to costs of sales |
7,706 | 6,596 | 7,462 | |||||||||
| Write-offs, net of recoveries |
(6,413 | ) | (6,806 | ) | (12,197 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Balance, end of year |
$ | 14,146 | $ | 12,853 | $ | 13,063 | ||||||
|
|
|
|
|
|
|
|||||||
The write-offs to the reserve were principally related to the disposition of fully reserved inventory.
| 7. | PROPERTY AND EQUIPMENT, NET |
Property and equipment consists of the following (in thousands):
| December 31, 2011 |
December 31, 2010 |
Depreciable lives (years) | ||||||||
| Land |
$ | 266 | $ | 100 | Indefinite | |||||
| Buildings and improvements |
22,646 | 18,832 | 3 to 25 | |||||||
| Equipment |
92,816 | 73,225 | 2 to 7 | |||||||
| Software |
29,314 | 21,260 | 3 to 10 | |||||||
| Furniture and fixtures |
17,335 | 14,031 | 3 to 8 | |||||||
| Surgical implant instrumentation |
40,739 | 33,231 | 5 | |||||||
| Construction in progress |
8,753 | 15,572 | N/A | |||||||
|
|
|
|
|
|||||||
| 211,869 | 176,251 | |||||||||
| Accumulated depreciation and amortization |
(104,761 | ) | (82,591 | ) | ||||||
|
|
|
|
|
|||||||
| Property and equipment, net |
$ | 107,108 | $ | 93,660 | ||||||
|
|
|
|
|
|||||||
Depreciation and amortization expense relating to property and equipment (including equipment under capital leases) was $27.3 million, $26.0 million, and $27.9 million for the years ended December 31, 2011, 2010, and 2009, respectively.
| 8. | LONG-LIVED ASSETS |
Goodwill
Changes in the carrying amount of goodwill are presented in the table below (in thousands):
| Year Ended December 31, | ||||||||
| 2011 | 2010 | |||||||
| Balance, beginning of year |
$ | 1,188,887 | $ | 1,191,497 | ||||
| Acquisitions (see Note 3) |
165,672 | 64 | ||||||
| Impairment of goodwill |
(124,106 | ) | — | |||||
| Foreign currency translation |
(1,675 | ) | (2,674 | ) | ||||
|
|
|
|
|
|||||
| Balance, end of year |
$ | 1,228,778 | $ | 1,188,887 | ||||
|
|
|
|
|
|||||
During the fourth quarter of 2011, we conducted our annual goodwill impairment test. As a result of this test, we determined that the carrying value of our Empi and Surgical Implant reporting units was in excess of its estimated fair value. Fair value was estimated using the income approach valuation methodology which includes the discounted cash flow method and the market approach valuation methodology which includes the use of market multiples.
F-19
Table of Contents
As a result, we recorded an aggregate goodwill impairment charge of $124.1 million, consisting of $76.7 million for the Empi reporting unit, within the Recovery Sciences segment and $47.4 million for the Surgical Implant reporting unit. This impairment charge was included in impairment of goodwill and intangible assets in our consolidated statement of operations.
The goodwill impairment in our Empi reporting unit resulted primarily from reductions in our projected operating results due to unfavorable decisions made by certain third party payors related to insurance pricing for certain products sold by the Empi business.
The goodwill impairment in our Surgical Implant reporting unit resulted primarily from reductions in our projected operating results and estimated future cash flows for the business.
We have five other reporting units with goodwill assigned to them. For each of those five reporting units, the estimated fair values exceed their carrying value.
Identifiable intangible assets consisted of the following (in thousands):
| December 31, 2011 |
Gross Carrying Amount |
Accumulated Amortization |
Intangible Assets, Net |
|||||||||
| Definite lived intangible assets: |
||||||||||||
| Customer relationships |
$ | 569,528 | $ | (181,396 | ) | $ | 388,132 | |||||
| Patents and technology |
460,624 | (156,290 | ) | 304,334 | ||||||||
| Trademarks and trade names |
23,650 | (1,807 | ) | 21,843 | ||||||||
| Distributor contracts and relationships |
5,089 | (1,206 | ) | 3,883 | ||||||||
| Non-compete agreements |
3,636 | (883 | ) | 2,753 | ||||||||
|
|
|
|
|
|
|
|||||||
| $ | 1,062,527 | $ | (341,582 | ) | 720,945 | |||||||
|
|
|
|
|
|||||||||
| Indefinite lived intangible assets: |
||||||||||||
| Trademarks and trade names |
411,749 | |||||||||||
|
|
|
|||||||||||
| Net identifiable intangible assets |
$ | 1,132,694 | ||||||||||
|
|
|
|||||||||||
| December 31, 2010 |
Gross Carrying Amount |
Accumulated Amortization |
Intangible Assets, Net |
|||||||||
| Definite lived intangible assets: |
||||||||||||
| Customer relationships |
$ | 484,115 | $ | (130,362 | ) | $ | 353,753 | |||||
| Patents and technology |
447,437 | (119,985 | ) | 327,452 | ||||||||
| Distributor contracts and relationships |
789 | (482 | ) | 307 | ||||||||
| Non-compete agreements |
459 | (129 | ) | 330 | ||||||||
|
|
|
|
|
|
|
|||||||
| $ | 932,800 | $ | (250,958 | ) | 681,842 | |||||||
|
|
|
|
|
|||||||||
| Indefinite lived intangible assets: |
||||||||||||
| Trademarks and trade names |
428,999 | |||||||||||
|
|
|
|||||||||||
| Net identifiable intangible assets |
$ | 1,110,841 | ||||||||||
|
|
|
|||||||||||
During the fourth quarter of 2011, as a result of our annual impairment test of our indefinite lived intangible assets, we determined that the carrying value of our Empi trade name was in excess of its estimated fair value and recorded an impairment charge of $16.9 million. This impairment charge was included in impairment of goodwill and intangible assets in our consolidated statement of operations.
Our definite lived intangible assets are being amortized using the straight line method over their remaining weighted average useful lives of 7.7 years for customer relationships, 10.4 years for patents and technology,
F-20
Table of Contents
5.0 years for distributor contracts and relationships, 9.2 years for trademarks and trade names, and 3.5 years for non-compete agreements. Based on our amortizable intangible asset balance as of December 31, 2011, we estimate that amortization expense will be as follows for the next five years and thereafter (in thousands):
| 2012 |
$ | 97,004 | ||
| 2013 |
91,116 | |||
| 2014 |
89,098 | |||
| 2015 |
84,679 | |||
| 2016 |
80,808 | |||
| Thereafter |
278,240 | |||
|
|
|
|||
| $ | 720,945 | |||
|
|
|
Our goodwill and intangible assets by segment are as follows (in thousands):
| December 31, 2011 |
Goodwill | Intangible Assets, Net |
||||||
| Bracing and Vascular |
$ | 705,954 | $ | 949,335 | ||||
| Recovery Sciences |
419,299 | 134,693 | ||||||
| International |
103,525 | 31,380 | ||||||
| Surgical Implant |
— | 17,286 | ||||||
|
|
|
|
|
|||||
| $ | 1,228,778 | $ | 1,132,694 | |||||
|
|
|
|
|
|||||
| December 31, 2010 |
Goodwill | Intangible Assets, Net |
||||||
| Bracing and Vascular |
$ | 565,298 | $ | 700,953 | ||||
| Recovery Sciences |
495,999 | 353,323 | ||||||
| International |
80,184 | 36,453 | ||||||
| Surgical Implant |
47,406 | 20,112 | ||||||
|
|
|
|
|
|||||
| $ | 1,188,887 | $ | 1,110,841 | |||||
|
|
|
|
|
|||||
| 9. | OTHER CURRENT LIABILITIES |
Other current liabilities consist of the following (in thousands):
| December 31, 2011 |
December 31, 2010 |
|||||||
| Accrued wages and related expenses |
$ | 29,856 | $ | 24,154 | ||||
| Accrued commissions |
12,831 | 10,402 | ||||||
| Income taxes payable |
2,878 | 2,656 | ||||||
| Accrued rebates |
6,027 | 6,006 | ||||||
| Accrued other taxes |
4,195 | 2,322 | ||||||
| Accrued professional expenses |
3,927 | 3,881 | ||||||
| Derivative liabilities |
545 | 6,707 | ||||||
| Other accrued liabilities |
21,512 | 25,581 | ||||||
|
|
|
|
|
|||||
| $ | 81,771 | $ | 81,709 | |||||
|
|
|
|
|
|||||
| 10. | EMPLOYEE BENEFIT PLANS |
We have multiple qualified defined contribution plans, which allow for voluntary pre-tax contributions by employees. We pay all general and administrative expenses of the plans and may make contributions to the plans. Based on 100% of the first 1% and 50% of the next 5% of compensation deferred by employees (subject to IRS
F-21
Table of Contents
limits and non-discrimination testing), we made matching contributions of $3.7 million, $3.4 million, and $3.7 million, to the plans for the years ended December 31, 2011, 2010 and 2009, respectively. The plans provide for discretionary contributions by us, as approved by the Board of Directors. There have been no such discretionary contributions through December 31, 2011. In addition, we made contributions to our international pension plans of $0.8 million for the year ended December 31, 2011 and $0.4 million for each of the year ended December 31, 2010 and 2009.
| 11. | DERIVATIVE INSTRUMENTS |
We use derivative financial instruments to manage interest rate risk related to our variable rate credit facilities and risk related to foreign currency exchange rates. Our objective is to reduce the risk to earnings and cash flows associated with changes in interest rates and changes in foreign currency exchange rates. Before acquiring a derivative instrument to hedge a specific risk, we evaluate potential natural hedges. Factors considered in the decision to hedge an underlying market exposure include the materiality of the risk, the volatility of the market, the duration of the hedge, and the availability, effectiveness and cost of derivative instruments. We do not use derivative instruments for speculative or trading purposes.
All derivatives, whether designated as hedging relationships or not, are recorded on the balance sheet at fair value. The fair value of our derivatives is determined through the use of models that consider various assumptions, including time value, yield curves and other relevant economic measures which are inputs that are classified as Level 2 in the valuation hierarchy. The classification of gains and losses resulting from changes in the fair values of derivatives is dependent on the intended use of the derivative and its resulting designation. Our interest rate swap agreements were designated as cash flow hedges, and accordingly, effective portions of changes in the fair value of the derivatives were recorded in accumulated other comprehensive income (loss) and subsequently reclassified into our consolidated statement of operations when the hedged forecasted transaction affects income (loss). Ineffective portions of changes in the fair value of cash flow hedges are recognized in income (loss). Our foreign exchange contracts have not been designated as hedges, and accordingly, changes in the fair value of the derivatives are recorded in income (loss).
Interest Rate Swap Agreements. Our Senior Secured Credit Facility is subject to floating interest rates. Historically, we used interest rate swaps to manage the risk of unfavorable movements in interest rates on a portion of the outstanding loan balance, thereby locking in a fixed rate on a portion of the principal, reducing the effect of possible rising interest rates and making interest expense more predictable. In August 2009, we entered into four interest swap agreements with notional amounts aggregating $300.0 million, and a weighted average fixed LIBOR rate of 2.5825%. These interest rate swap agreements expired on December 31, 2011.
Our interest rate swap agreements were designated as cash flow hedges for accounting purposes, and the hedges were considered effective. As such, the effective portion of the gain or loss on the derivative instrument was reported as a component of accumulated other comprehensive income (loss) and reclassified into interest expense in our consolidated statement of operations in the period in which it affected income (loss).
Foreign Exchange Rate Contracts. We utilize Mexican Peso (MXN) foreign exchange forward contracts to hedge a portion of our exposure to fluctuations in foreign exchange rates, as our Mexico-based manufacturing operations incur costs that are largely denominated in MXN. These foreign exchange forward contracts expire weekly throughout fiscal year 2012. While our foreign exchange forward contracts act as economic hedges, we have not designated such instruments as hedges for accounting purposes. Therefore, gains and losses resulting from changes in the fair values of these derivative instruments are recorded in other income (expense), net, in our accompanying consolidated statements of operations.
F-22
Table of Contents
Information regarding the notional amounts of our foreign exchange forward contracts is presented in the table below (in thousands):
| Notional Amount (MXN) | Notional Amount (USD) | |||||||||||||||
| December 31, 2011 |
December 31, 2010 |
December 31, 2011 |
December 31, 2010 |
|||||||||||||
| Foreign exchange contracts not designated as hedges |
197,900 | 116,910 | $ | 14,576 | $ | 9,428 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The following table summarizes the fair value of derivative instruments in our consolidated balance sheets (in thousands):
| Balance Sheet Location |
December 31, 2011 |
December 31, 2010 |
||||||||
| Derivative Assets: |
||||||||||
| Foreign exchange forward contracts not designated as hedges |
Other current assets | $ | — | $ | 374 | |||||
|
|
|
|
|
|||||||
| Derivative Liabilities: |
||||||||||
| Interest rate swap agreements designated as cash flow hedges |
Other current liabilities | $ | — | $ | 6,707 | |||||
| Foreign exchange forward contracts not designated as hedges |
Other current liabilities | 545 | — | |||||||
|
|
|
|
|
|||||||
| $ | 545 | $ | 6,707 | |||||||
|
|
|
|
|
|||||||
The following table summarizes the effect our derivative instruments have on our consolidated statements of operations (in thousands):
| Year Ended December 31, | ||||||||||||||
| Location of gain (loss) |
2011 | 2010 | 2009 | |||||||||||
| Interest rate swap agreements designated as cash flow hedges |
Interest expense (1) | $ | (7,154 | ) | $ | (12,211 | ) | $ | (18,164 | ) | ||||
| Foreign exchange forward contracts not designated as hedges |
Other income (expense), net | (830 | ) | 285 | 2,913 | |||||||||
|
|
|
|
|
|
|
|||||||||
| $ | (7,984 | ) | $ | (11,926 | ) | $ | (15,251 | ) | ||||||
|
|
|
|
|
|
|
|||||||||
| (1) | Represents the loss on derivative instruments designated as cash flow hedges, reclassified from accumulated other comprehensive income (loss) into interest expense during the periods presented. |
The pre-tax loss on derivative instruments designated as cash flow hedges recognized in other comprehensive income (loss) is presented below (in thousands):
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Interest rate swap agreements designated as cash flow hedges |
$ | 447 | $ | 7,674 | $ | 16,136 | ||||||
|
|
|
|
|
|
|
|||||||
| 12. | FAIR VALUE MEASUREMENTS |
Financial assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurements. Our assessment of the significance of a particular input to the fair value measurements requires judgment and may affect the valuation of the assets and liabilities being measured and their placement within the fair value hierarchy.
F-23
Table of Contents
The following tables present the balances of financial assets and liabilities measured at fair value on a recurring basis (in thousands):
| As of December 31, 2011 |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Recorded Balance |
||||||||||||
| Liabilities: |
||||||||||||||||
| Foreign exchange forward contracts not designated as hedges |
$ | — | $ | 545 | $ | — | $ | 545 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | — | $ | 545 | $ | — | $ | 545 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| As of December 31, 2010 |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Recorded Balance |
||||||||||||
| Assets: |
||||||||||||||||
| Foreign exchange forward contracts not designated as hedges |
$ | — | $ | 374 | $ | — | $ | 374 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities: |
||||||||||||||||
| Interest rate swap agreements designated as cash flow hedges |
$ | — | $ | 6,707 | $ | — | $ | 6,707 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The following table presents the balances of nonfinancial assets measured at fair value on a non recurring basis (in thousands):
| As of December 31, 2011 |
Recorded Balance |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total Losses | |||||||||||||||
| Assets: |
||||||||||||||||||||
| Goodwill |
$ | 169,798 | $ | — | $ | — | $ | 169,798 | $ | 124,106 | ||||||||||
| Intangible assets |
20,700 | — | — | 20,700 | 16,900 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| $ | 190,498 | $ | — | $ | — | $ | 190,498 | $ | 141,006 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
During 2011, goodwill relating to our Empi and Surgical Implant reporting units with an aggregate carrying amount of $293.9 million was written down to its implied fair value of $169.8 million, resulting in an aggregate impairment charge of $124.1 million. The implied fair value of goodwill equals the estimated fair value of the reporting unit minus the fair value of the reporting unit’s net assets. In determining the implied fair value of the goodwill of the Empi and Surgical Implant reporting units, we used unobservable inputs to estimate the fair value of the reporting unit and its assets and liabilities.
In performing our 2011 goodwill impairment test, we estimated the fair values of our reporting units using the income approach valuation methodology which includes the discounted cash flow method and the market approach valuation methodology which includes the use of market multiples. The discounted cash flows for each reporting unit were based on discrete financial forecasts developed by management for planning purposes and required significant judgment with respect to forecasted sales, gross margin, selling, general and administrative expenses, EBITDA, capital expenditures and the selection and use of an appropriate discount rate. Cash flows beyond the discrete forecasts were estimated using a terminal value calculation, which incorporated historical and forecasted financial trends for each identified reporting unit and considered long-term earnings growth rates
F-24
Table of Contents
for publicly traded peer companies. Future cash flows were then discounted to present value at discount rates ranging from 10.7% to 12.5%, and terminal value growth rates of 3%. Publicly available information regarding comparable market capitalization was also considered in assessing the cumulative fair values of our reporting units estimated using the discounted cash flow methodology.
The fair value of the reporting unit’s assets and liabilities was determined by using the same methods that are used in business combination purchase accounting. See Note 8 for further discussion and the factors that contributed to this impairment charge.
Also during 2011, we estimated the fair value of our indefinite lived intangible assets, consisting of trade names. To determine the fair value we applied the relief from royalty (RFR) method. Under the RFR method, the value of the trade name is determined by calculating the present value of the after-tax cost savings associated with owning the asset and therefore not being required to pay royalties for its use during the asset’s indefinite life. Significant judgments inherent in this analysis include the selection of appropriate discount rates, estimating future cash flows and the identification of appropriate terminal growth rate assumptions. Discount rate assumptions are based on an assessment of the risk inherent in the projected future cash generated by the respective intangible assets. Also subject to judgment are assumptions about royalty rates, which are based on the estimated rates at which similar brands and trademarks are being licensed in the marketplace.
Based on the results of our testwork, we determined that the carrying value of the Empi trade name was in excess of its fair value and we recorded an impairment charge of $16.9 million to write it down to its revised fair value. We determined the fair value of the Empi trade name using unobservable inputs.
| 13. | DEBT AND CAPITAL LEASES |
Debt and capital lease obligations consists of the following (in thousands):
| December 31, 2011 |
December 31, 2010 |
|||||||
| Senior Secured Credit Facility: |
||||||||
| $100 million revolving credit facility |
$ | 51,000 | $ | — | ||||
| Term loan, net of unamortized original issue discount ($4.4 million and $6.0 million at December 31, 2011 and 2010, respectively) |
838,591 | 845,792 | ||||||
| 10.875% Senior Notes, including unamortized original issue premium ($3.3 million and $4.2 million at December 31, 2011 and 2010, respectively) |
678,282 | 679,239 | ||||||
| 9.75% Senior Subordinated Notes |
300,000 | 300,000 | ||||||
| 7.75% Senior Notes |
300,000 | — | ||||||
| Capital lease obligations and other |
38 | 81 | ||||||
|
|
|
|
|
|||||
| Total debt and capital lease obligations |
2,167,911 | 1,825,112 | ||||||
| Current maturities |
(8,820 | ) | (8,821 | ) | ||||
|
|
|
|
|
|||||
| Long-term debt and capital lease obligations |
$ | 2,159,091 | $ | 1,816,291 | ||||
|
|
|
|
|
|||||
Senior Secured Credit Facility
On November 20, 2007, we entered into the Senior Secured Credit Facility consisting of a $1,065.0 million term loan facility maturing May 2014 and a $100.0 million revolving credit facility maturing November 2013. We issued the term loan facility at a 1.2% discount, resulting in net proceeds of $1,052.4 million. We are amortizing the $12.6 million discount using the effective interest method through the maturity date of the term loan facility.
F-25
Table of Contents
We have subsequently entered into three amendments to the Senior Secured Credit Facility. The first amendment, entered into in January 2010 permitted us to issue $100.0 million in aggregate principal amount of new 10.875% senior notes, as long as the net cash proceeds were used to make a voluntary prepayment of the term loans.
The second amendment, entered into in October 2010, permitted us to issue $300.0 million of new senior subordinated notes and repurchase or redeem all of our then outstanding 11.75% senior subordinated notes, prepay a portion of the term loans under our Senior Secured Credit Facility and pay related premiums, fees and expenses, all without utilizing existing debt incurrence capacity under our Senior Secured Credit Facility.
The third amendment, entered into in February 2011, increased the Maximum Total Leverage Ratio limitation in the Permitted Acquisitions covenant from 6.0x to 7.0x, and deemed the ETI acquisition to have been made as a Permitted Acquisition. The Permitted Acquisitions covenant has no limit on the dollar amount of acquisitions we are permitted to make, as long as the acquired entity becomes a loan party under the Senior Secured Credit Facility, and we are in compliance with this 7.0x maximum total net leverage ratio requirement, our senior secured leverage ratio requirement, and are not in default.
As of December 31, 2011, the market values of our term loan facility and revolving credit facility were $811.4 million and $46.9 million, respectively. We determine market value using trading prices for our term loan on or near that date.
Interest Rates. Borrowings under the Senior Secured Credit Facility bear interest at a rate equal to an applicable margin plus, at our option, either (a) a base rate determined by reference to the higher of (1) the prime rate, as defined, and (2) the federal funds rate plus 0.50% or (b) the Eurodollar rate determined by reference to the costs of funds for deposits in U.S. dollars for the interest period relevant to each borrowing adjusted for required reserves. The initial applicable margin for borrowings under the term loan facility and the revolving credit facility is 2.00% with respect to base rate borrowings and 3.00% with respect to Eurodollar borrowings. The applicable margin for borrowings under the term loan facility and the revolving credit facility may be reduced subject to us attaining certain leverage ratios. We use interest rate swap agreements in an effort to hedge our exposure to fluctuating interest rates related to a portion of our Senior Secured Credit Facility (see Note 11). As of December 31, 2011, our weighted average interest rate for all borrowings under the Senior Secured Credit Facility was 3.30%.
Fees. In addition to paying interest on outstanding principal under the Senior Secured Credit Facility, we are required to pay a commitment fee to the lenders under the revolving credit facility with respect to the unutilized commitments thereunder. The current commitment fee rate is 0.50% per annum. The commitment fee rate may be reduced subject to us attaining certain leverage ratios. We must also pay customary letter of credit fees.
Principal Payments. We are required to pay annual payments in equal quarterly installments on the loans under the term loan facility in an amount equal to 1.00% of the funded total principal amount through February 2014, with any remaining amount payable in May 2014.
Prepayments. The Senior Secured Credit Facility requires us to prepay outstanding term loans, subject to certain exceptions, with (1) 50% (which percentage can be reduced to 25% or 0% upon our attaining certain leverage ratios) of our annual excess cash flow, as defined; (2) 100% of the net cash proceeds above an annual amount of $25.0 million from non-ordinary course asset sales (including insurance and condemnation proceeds) by DJOFL and its restricted subsidiaries, subject to certain exceptions, including a 100% reinvestment right if reinvested or committed to reinvest within 15 months of such sale or disposition so long as reinvestment is completed within 180 days thereafter; and (3) 100% of the net cash proceeds from issuances or incurrences of debt by DJOFL and its restricted subsidiaries, other than proceeds from debt permitted to be incurred under the Senior Secured Credit Facility and related amendments. Any mandatory prepayments are applied to the term loan facilities in direct order of maturity. We may voluntarily prepay outstanding loans under the Senior Secured
F-26
Table of Contents
Credit Facility at any time without premium or penalty, provided that voluntary prepayments of Eurodollar loans made on a date other than the last day of an interest period applicable thereto shall be subject to customary breakage costs. We are not required to make any prepayments in 2012 related to our 2011 excess cash flow calculation.
Guarantee and Security. All obligations under the Senior Secured Credit Facility are unconditionally guaranteed by DJO Holdings LLC (DJO Holdings) and each existing and future direct and indirect wholly owned domestic subsidiary of DJOFL other than immaterial subsidiaries, unrestricted subsidiaries and subsidiaries that are precluded by law or regulation from guaranteeing the obligations (collectively, the Guarantors).
All obligations under the Senior Secured Credit Facility, and the guarantees of those obligations, are secured by pledges of 100% of the capital stock of DJOFL, 100% of the capital stock of each wholly owned domestic subsidiary and 65% of the capital stock of each wholly owned foreign subsidiary that is, in each case, directly owned by DJOFL or one of the Guarantors; and a security interest in, and mortgages on, substantially all tangible and intangible assets of DJO Holdings, DJOFL and each Guarantor.
Certain Covenants and Events of Default. The Senior Secured Credit Facility contains a number of covenants that, among other things, restrict, subject to certain exceptions, our and our subsidiaries’ ability to:
| • | incur additional indebtedness, |
| • | create liens on assets, |
| • | change fiscal years, |
| • | enter into sale and leaseback transactions, |
| • | engage in mergers or consolidations, |
| • | sell assets, |
| • | pay dividends and other restricted payments, |
| • | make investments, loans or advances, |
| • | repay subordinated indebtedness, |
| • | make certain acquisitions, |
| • | engage in certain transactions with affiliates, |
| • | restrict the ability of restricted subsidiaries that are not Guarantors to pay dividends or make distributions, |
| • | amend material agreements governing our subordinated indebtedness, and |
| • | change our lines of business. |
In addition, the Senior Secured Credit Facility requires us to maintain a maximum senior secured leverage ratio of 3.25:1 as of the twelve months ended December 31, 2011. The Senior Secured Credit Facility also contains certain customary affirmative covenants and events of default. As of December 31, 2011, our senior secured leverage ratio was 3.06:1, and we were in compliance with all other applicable covenants.
10.875% Senior Notes
On November 20, 2007, DJOFL and DJO Finance Corporation (DJO Finco) (collectively, the Issuers) issued $575.0 million aggregate principal amount of 10.875% Senior Notes under an agreement dated as of November 20, 2007 (the 10.875% Indenture) among the Issuers, the guarantors party thereto and The Bank of New York Mellon (formerly known as The Bank of New York), as trustee. We refer to the 10.875% Senior Notes, individually, or collectively, as the 10.875% Notes.
F-27
Table of Contents
On January 20, 2010, the Issuers issued $100.0 million aggregate principal amount of new 10.875% Notes, pursuant to the 10.875% Indenture that governs our existing 10.875% Notes due 2014. We issued the new 10.875% Notes at a 5.0% premium, resulting in gross proceeds of $105.0 million. We are amortizing the premium over the term of the new 10.875% Notes using the effective interest method, thereby decreasing the reported outstanding balance through the maturity date. Net proceeds from the issuance along with cash on hand, were used to repay $101.5 million of existing term loans under the Senior Secured Credit Facility.
As of December 31, 2011, the market value of the 10.875% Notes was $627.8 million. We determined market value using trading prices for the 10.875% Notes on or near that date.
Optional Redemption. Under the 10.875% Indenture, beginning on November 15, 2011, the Issuers may redeem some or all of the 10.875% Notes at a redemption price of 105.438% of the then outstanding principal balance plus accrued and unpaid interest. The redemption price decreases to 102.719% and 100% of the then outstanding principal balance at November 2012 and November 2013, respectively.
Change of Control. Upon the occurrence of a change of control, unless DJOFL has previously sent or concurrently sends a notice exercising its optional redemption rights with respect to all of the then-outstanding 10.875% Notes, DJOFL will be required to make an offer to repurchase all of the then-outstanding 10.875% Notes at 101% of their principal amount, plus accrued and unpaid interest.
Covenants. The 10.875% Indenture contains covenants limiting, among other things, our and our restricted subsidiaries’ ability to incur additional indebtedness or issue certain preferred and convertible shares, pay dividends on, redeem, repurchase or make distributions in respect of the capital stock of DJO or make other restricted payments, make certain investments, sell certain assets, create liens on certain assets to secure debt, consolidate, merge, sell or otherwise dispose of all or substantially all of our assets, enter into certain transactions with affiliates, and designate our subsidiaries as unrestricted subsidiaries. As of December 31, 2011, we were in compliance with all applicable covenants.
9.75% Senior Subordinated Notes
On October 18, 2010, the Issuers issued $300.0 million aggregate principal amount of 9.75% senior subordinated notes (9.75% Notes) maturing on October 15, 2017. The 9.75% Notes are guaranteed jointly and severally and on an unsecured senior subordinated basis by each of DJOFL’s existing and future direct and indirect wholly owned domestic subsidiaries that guarantee any of DJOFL’s indebtedness or any indebtedness of DJOFL’s domestic subsidiaries or by any of DJOFL’s subsidiaries that are an obligor under DJOFL’s Senior Secured Credit Facility.
As of December 31, 2011, the market value of the 9.75% Notes was $232.5 million. We determined market value using trading prices for the 9.75% Notes on or near that date.
Optional Redemption. Under the Indenture to the 9.75% Notes (the 9.75% Indenture), prior to October 15, 2013, the Issuers have the option to redeem some or all of the 9.75% Notes for cash at a redemption price equal to 100% of the then outstanding principal balance plus an applicable make-whole premium plus accrued and unpaid interest. Beginning on October 15, 2013, the Issuers may redeem some or all of the 9.75% Notes at a redemption price of 107.313% of the then outstanding principal balance plus accrued and unpaid interest. The redemption price decreases to 104.875%, 102.438% and 100% of the then outstanding principal balance at October 15, 2014, 2015 and 2016, respectively. Additionally, from time to time, before October 15, 2013, the Issuers may redeem up to 35% of the 9.75% Notes at a redemption price equal to 109.75% of the principal amount then outstanding, plus accrued and unpaid interest, in each case, with proceeds we raise, or a direct or indirect parent company raises, in certain offerings of equity of DJOFL or its direct or indirect parent companies, as long as at least 65% of the aggregate principal amount of the notes issued remains outstanding.
F-28
Table of Contents
Change of Control. Upon the occurrence of a change of control, unless DJOFL has previously sent or concurrently sends a notice exercising its optional redemption rights with respect to all of the then-outstanding 9.75% Notes, DJOFL will be required to make an offer to repurchase all of the then-outstanding 9.75% Notes at 101% of their principal amount, plus accrued and unpaid interest.
Covenants. The 9.75% Indenture contains covenants limiting, among other things, our and our restricted subsidiaries’ ability to incur additional indebtedness or issue certain preferred and convertible shares, pay dividends on, redeem, repurchase or make distributions in respect of the capital stock of DJO or make other restricted payments, make certain investments, sell certain assets, create liens on certain assets to secure debt, consolidate, merge, sell or otherwise dispose of all or substantially all of our assets, enter into certain transactions with affiliates, and designate our subsidiaries as unrestricted subsidiaries. As of December 31, 2011, we were in compliance with all applicable covenants.
7.75% Senior Notes
On April 7, 2011, the Issuers issued $300.0 million aggregate principal amount of 7.75% Senior Notes (7.75% Notes) maturing on April 15, 2018. The 7.75% Notes are guaranteed jointly and severally and on an unsecured senior basis by each of DJOFL’s existing and future direct and indirect wholly owned domestic subsidiaries that guarantee any of DJOFL’s indebtedness or any indebtedness of DJOFL’s domestic subsidiaries or is an obligor under DJOFL’s Senior Secured Credit Facility.
As of December 31, 2011, the market value of the 7.75% Notes was $237.0 million. We determined market value using trading prices for the 7.75% Notes on or near that date.
Optional Redemption. Under the Indenture to the 7.75% Notes (the 7.75% Indenture), prior to April 15, 2014, the Issuers have the option to redeem some or all of the 7.75% Notes for cash at a redemption price equal to 100% of the then outstanding principal balance plus an applicable make-whole premium plus accrued and unpaid interest. Beginning on April 15, 2014, the Issuers may redeem some or all of the 7.75% Notes at a redemption price of 105.813% of the then outstanding principal balance plus accrued and unpaid interest. The redemption price decreases to 103.875%, 101.938% and 100% of the then outstanding principal balance at April 15, 2015, 2016 and 2017, respectively. Additionally, from time to time, before April 15, 2014, the Issuers may redeem up to 35% of the 7.75% Notes at a redemption price equal to 107.75% of the principal amount then outstanding, plus accrued and unpaid interest, in each case, with proceeds we raise, or a direct or indirect parent company raises, in certain offerings of equity of DJOFL or its direct or indirect parent companies, as long as at least 65% of the aggregate principal amount of the notes issued remains outstanding.
Change of Control. Upon the occurrence of a change of control, unless DJOFL has previously sent or concurrently sends a notice exercising its optional redemption rights with respect to all of the then-outstanding 7.75% Notes, DJOFL will be required to make an offer to repurchase all of the then-outstanding 7.75% Notes at 101% of their principal amount, plus accrued and unpaid interest.
Covenants. The 7.75% Indenture contains covenants limiting, among other things, our and our restricted subsidiaries’ ability to incur additional indebtedness or issue certain preferred and convertible shares, pay dividends on, redeem, repurchase or make distributions in respect of the capital stock of DJO or make other restricted payments, make certain investments, sell certain assets, create liens on certain assets to secure debt, consolidate, merge, sell or otherwise dispose of all or substantially all of our assets, enter into certain transactions with affiliates, and designate our subsidiaries as unrestricted subsidiaries. As of December 31, 2011, we were in compliance with all applicable covenants.
Our ability to continue to meet the covenants related to our indebtedness specified above in future periods will depend, in part, on events beyond our control, and we may not continue to meet those ratios. A breach of any of these covenants in the future could result in a default under the Senior Secured Credit Facility, the 10.875%
F-29
Table of Contents
Indenture, the 9.75% Indenture and the 7.75% Indenture (collectively, the Indentures), at which time the lenders could elect to declare all amounts outstanding under the Senior Secured Credit Facility to be immediately due and payable. Any such acceleration would also result in a default under the Indentures.
At December 31, 2011, the aggregate amounts of annual principal maturities of long-term debt and capital leases for the next five years and thereafter are as follows (in thousands):
| Years Ending December 31, |
||||
| 2012 |
$ | 8,782 | ||
| 2013 |
8,782 | |||
| 2014 |
1,551,465 | |||
| 2015 |
— | |||
| 2016 |
— | |||
| Thereafter |
600,000 | |||
|
|
|
|||
| $ | 2,169,029 | |||
|
|
|
|||
Loss on Modification and Extinguishment of Debt
During the year ended December 31, 2011, in connection with the third amendment to the Senior Secured Credit Facility, we incurred $2.1 million of arrangement and lender consent fees which are included in loss on modification and extinguishment of debt in our consolidated statement of operations.
During the year ended December 31, 2010, we recognized a loss on modification and extinguishment of debt of $19.8 million. This loss includes $13.0 million of premiums, a $4.3 million non-cash write-off of unamortized debt issuance costs, and $1.4 million of fees and expenses associated with the amendment of our Senior Secured Credit Facility, issuance of $300.0 million of 9.75% Notes and redemption of our $200.0 million of 11.75% Notes in October 2010. In addition, this loss includes $1.1 million of arrangement and lender consent fees related to the amendment of our Senior Secured Credit Facility in connection with the issuance of $100.0 million 10.875% Notes in January 2010.
Debt Issuance Costs
As of December 31, 2011 and 2010, we had $34.0 million and $34.1 million, respectively, of unamortized debt issuance costs, which are included in other assets in our consolidated balance sheets. During the year ended December 31, 2011, we capitalized $7.7 million of debt issuance costs incurred in connection with the issuance of the 7.75% Notes in April 2011. During the year ended December 31, 2010, we capitalized $10.3 million of debt issuance costs, in connection with the issuance of $100.0 million of new 10.875% Notes in January 2010, and the issuance of the 9.75% Notes in October 2010.
For each the years ended December 31, 2011 and 2010, amortization of debt issuance costs was $7.7 million, and for the year ended December 31, 2009, amortization of debt issuance costs was $12.7 million. Amortization of debt issuance costs was included in interest expense in our consolidated statements of operations for each of the periods presented.
| 14. | MEMBERSHIP EQUITY |
In connection with the DJO Merger in November 2007, certain members of DJO management elected to rollover certain options held by them that had not been exercised at or prior to the effective time of the DJO Merger. Such rollover options were converted to options to purchase 1,912,577 shares of DJO’s common stock under the 2007 Plan on a tax-deferred basis (Rollover Options). The fair value of these vested Rollover Options was $15.2 million and was recorded as a component of the cost of the DJO Merger.
F-30
Table of Contents
During the year ended December 31, 2011, we paid cash of $2.0 million to our former chief executive officer, upon his retirement, to cancel 355,155 shares of vested Rollover Options held by him. The amount paid represents the excess of the fair market value of the shares over their exercise price. This amount is included as a reduction to member capital in our consolidated balance sheet as of December 31, 2011.
In addition, during the year ended December 31, 2011, DJO sold 192,959 shares of its common stock at $16.46 per share, consisting of 157,959 shares purchased by our new chief executive officer, and 35,000 shares purchased by another member of senior management. The share purchases were subject to execution of a stockholder agreement including certain rights and restrictions (see Note 18). Net proceeds of $3.2 million from the share purchases were contributed by DJO to us, and are included in member capital in our consolidated balance sheet as of December 31, 2011.
During the year ended December 31, 2010, DJO, sold 93,128 shares of its common stock, subject to a stockholders agreement (See Note 18), at $16.46 per share, in an offering to certain accredited investors comprised of employees, directors and independent sales agents. Net proceeds of $1.5 million from the share purchases were contributed by DJO to us and are included in member capital in our consolidated balance sheet as of December 31, 2011.
The proceeds from the share purchases in the years ended December 31, 2011 and 2010 were used for working capital purposes.
| 15. | STOCK OPTION PLANS AND STOCK-BASED COMPENSATION |
Stock Option Plan
We have one active equity compensation plan, the DJO 2007 Incentive Stock Plan (the 2007 Plan) under which we are authorized to grant awards of stock, options, and other stock-based awards of shares of Common Stock of DJO, subject to adjustment in certain events. In June 2011, we amended the 2007 Plan to increase the number of shares available to grant from 7,500,000 to 7,925,529.
Options issued under the 2007 Plan can be either incentive stock options or non-qualified stock options. The exercise price of stock options granted will not be less than 100% of the fair market value of the underlying shares on the date of grant and will expire no more than ten years from the date of grant. We adopted a form of non-statutory stock option agreement (the DJO Form Option Agreement) for employee stock option awards under the 2007 Plan, as amended.
Under the DJO Form Option Agreement, one-third of each stock option grant will vest over a specified period of time (typically five years from the date of grant) contingent solely upon the awardees’ continued employment with us (Time-Based Tranche). Prior to the June 2011 amendment described below, another one-third of the stock option grant would have vested based upon achieving a minimum internal rate of return (IRR) and a minimum return of money on invested capital (MOIC), as defined, each with respect to Blackstone’s aggregate investment in DJO’s capital stock, to be achieved by Blackstone following a liquidation of all or a portion of its investment in DJO’s capital stock (Market Return Tranche). The final one-third of the stock option grant would have vested based upon achieving an increased minimum IRR and an increased minimum return of MOIC, as defined, each with respect to Blackstone’s aggregate investment in DJO’s capital stock, to be achieved by Blackstone following a liquidation of all or a portion of its investment in DJO’s capital stock (Enhanced Market Return Tranche).
In June 2011, the compensation committee approved further modifications to the terms of the options in the Market Return Tranche and Enhanced Market Return Tranche and the DJO Form Option Agreement. As amended, vesting of the options in the Market Return Tranche are no longer subject to the achievement of a minimum IRR and the options will vest based upon achieving a minimum return of MOIC, and vesting of the
F-31
Table of Contents
options in the Enhanced Market Return Tranche are also no longer subject to achievement of a minimum IRR and the options will vest based on achieving an increased minimum return of MOIC, as defined, each with respect to Blackstone’s aggregate investment in DJO’s capital stock, to be achieved by Blackstone following a liquidation of all or a portion of its investment in DJO’s capital stock.
Stock-Based Compensation
During the year ended December 31, 2011, we granted a total of 983,000 options to employees including 800,000 options granted to Michael P. Mogul, our new president and chief executive officer. The weighted average grant date fair value of the options granted was $6.23 per share, for options in the Time-Based Tranche. In addition, during the year ended December 31, 2011, we granted 60,753 restricted shares to Michael P. Mogul. The shares will vest 50% per year on each of the anniversary dates of his employment commencement date, subject to his continued employment through the applicable anniversary dates.
During the year ended December 31, 2010, we granted 645,050 stock options to employees with a weighted average grant date fair value of $6.68 per share, for options in the Time-Based Tranche. In addition, during the year ended December 31, 2010, we granted 24,600 options, to non-employee distributors.
The fair value of each option award is estimated on the date of grant, or modification, using the Black-Scholes option pricing model for service based awards, and a binomial model for market based awards. In estimating fair value for options issued under the 2007 Plan, expected volatility was based on historical volatility of comparable publicly-traded companies. As our historical share option exercise experience does not provide a reasonable basis upon which to estimate the expected term, we used the simplified method. Expected life is calculated in two tranches based on the employment level defined as executive or employee. The risk-free rate used in calculating fair value of service based stock options for periods within the expected term of the option is based on the U.S. Treasury yield bond curve in effect on the date of grant.
The following table summarizes certain assumptions we used to estimate the fair value of the Time-Based Tranche of stock options granted during the years ended December 31, 2011, 2010, and 2009:
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Expected volatility |
34.0 - 34.4 | % | 34.2 - 35.8 | % | 34.4 - 34.7 | % | ||||||
| Risk-free interest rate |
1.3 - 2.1 | % | 2.0 -3.0 | % | 2.3 - 2.8 | % | ||||||
| Expected years until exercise |
6.4 - 6.6 | 6.4 -7.0 | 5.8 - 6.3 | |||||||||
| Expected dividend yield |
0.0 | % | 0.0 | % | 0.0 | % | ||||||
We recorded non-cash stock-based compensation expense during the periods presented as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Cost of goods sold |
$ | 149 | $ | 50 | $ | 167 | ||||||
| Operating expenses: |
||||||||||||
| Selling, general and administrative |
2,493 | 1,764 | 3,062 | |||||||||
| Research and development |
59 | 74 | 153 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 2,701 | $ | 1,888 | $ | 3,382 | |||||||
|
|
|
|
|
|
|
|||||||
We are required to reassess at each reporting period whether the achievement of any performance condition is probable, at which time we would recognize the related compensation expense over the remaining performance or service period, if any. In each of the periods presented, we only recognized stock-based compensation expense
F-32
Table of Contents
for options granted to employees in the Time-Based Tranche, as the performance and market components of the Market Return and Enhanced Market Return Tranches are not deemed probable at this time.
Stock based compensation expense for options granted to non-employees was not significant to the Company for all periods presented, and was included in selling, general and administrative expense in our consolidated statements of operations.
Included in stock-based compensation expense for the year ended December 31, 2011 is $0.8 million of incremental expense associated with the modification of the terms of options previously granted.
A summary of option activity under the 2007 Plan is presented below:
| Number of Shares |
Weighted- Average Exercise Price |
Weighted- Average Remaining Contractual Term (Years) |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding at December 31, 2010 |
7,188,284 | $ | 14.77 | 6.7 | $ | 12,221,730 | ||||||||||
| Granted |
983,000 | $ | 16.46 | |||||||||||||
| Exercised |
— | |||||||||||||||
| Forfeited or expired |
(684,199 | ) | $ | 13.57 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding at December 31, 2011 |
7,487,085 | $ | 15.10 | 5.9 | $ | 10,221,968 | ||||||||||
|
|
|
|||||||||||||||
| Exercisable at December 31, 2011 |
3,491,204 | $ | 13.55 | 4.7 | $ | 10,221,968 | ||||||||||
|
|
|
|||||||||||||||
As of December 31, 2011, total unrecognized stock-based compensation expense related to unvested stock options granted under the 2007 Plan, excluding options subject to the performance and market components of the Market Return and Enhanced Market Return Tranches, was $2.3 million, net of expected forfeitures. We anticipate this expense to be recognized over a weighted-average period of approximately two years. Compensation expense associated with the Market Return and Enhanced Market Return Tranches of options granted under the 2007 Plan, with the exception of those that were issued in connection with a modification, will be recognized only to the extent achievement of the performance and market components are deemed probable.
| 16. | INCOME TAXES |
DJO files consolidated tax returns in the U.S. The income taxes of domestic and foreign subsidiaries not included within the consolidated U.S. tax group are presented in our financial statements based on a separate return basis for each tax-paying entity or group.
The components of loss from continuing operations before income tax benefit consist of the following (in thousands):
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| U.S. operations |
$ | (283,137 | ) | $ | (92,599 | ) | $ | (76,881 | ) | |||
| Foreign operations |
17,006 | 6,669 | 5,812 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | (266,131 | ) | $ | (85,930 | ) | $ | (71,069 | ) | ||||
|
|
|
|
|
|
|
|||||||
F-33
Table of Contents
The income tax benefit consists of the following (in thousands):
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Current income taxes: |
||||||||||||
| U.S. Federal |
$ | (1,175 | ) | $ | (305 | ) | $ | 1,144 | ||||
| U.S. State |
1,619 | 1,308 | 2,321 | |||||||||
| Foreign |
6,019 | 4,429 | (2,147 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total current income taxes |
6,463 | 5,432 | 1,318 | |||||||||
|
|
|
|
|
|
|
|||||||
| Deferred income taxes: |
||||||||||||
| U.S. Federal |
(54,875 | ) | (28,231 | ) | (19,708 | ) | ||||||
| U.S. State |
(2,889 | ) | (8,879 | ) | (8,627 | ) | ||||||
| Foreign |
(1,243 | ) | (2,577 | ) | 5,339 | |||||||
|
|
|
|
|
|
|
|||||||
| Total deferred income taxes |
(59,007 | ) | (39,687 | ) | (22,996 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total income tax benefit |
$ | (52,544 | ) | $ | (34,255 | ) | $ | (21,678 | ) | |||
|
|
|
|
|
|
|
|||||||
The difference between the income tax benefit derived by applying the U.S. Federal statutory income tax rate of 35% to loss from continuing operations before income tax and the income tax benefit recognized in the consolidated financial statements is as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Benefit derived by applying the U.S. Federal statutory income tax rate to loss from continuing operations before income taxes |
$ | (93,144 | ) | $ | (30,075 | ) | $ | (24,874 | ) | |||
| Add (deduct) the effect of: |
||||||||||||
| State tax benefit, net |
(5,315 | ) | (2,594 | ) | (1,071 | ) | ||||||
| Change in state effective tax rates |
— | (2,350 | ) | (3,859 | ) | |||||||
| Change in German tax laws |
— | — | (379 | ) | ||||||||
| Gain on subsidiary stock sale |
— | — | 2,609 | |||||||||
| Unrecognized tax benefits |
(344 | ) | 706 | 2,460 | ||||||||
| Goodwill impairment |
39,513 | — | — | |||||||||
| Valuation allowance |
2,100 | (470 | ) | 519 | ||||||||
| Foreign exchange gain |
— | (37 | ) | 1,816 | ||||||||
| Permanent differences and other, net |
4,646 | 565 | 1,101 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | (52,544 | ) | $ | (34,255 | ) | $ | (21,678 | ) | ||||
|
|
|
|
|
|
|
|||||||
The components of deferred income tax assets and liabilities are as follows (in thousands):
| December 31, 2011 |
December 31, 2010 |
|||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards |
$ | 149,142 | $ | 124,810 | ||||
| Receivables reserve |
22,689 | 25,615 | ||||||
| Other |
37,702 | 35,175 | ||||||
|
|
|
|
|
|||||
| Gross deferred tax assets |
209,533 | 185,600 | ||||||
| Valuation allowance |
(6,163 | ) | (4,664 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
203,370 | 180,936 | ||||||
|
|
|
|
|
|||||
| Deferred tax liabilities: |
||||||||
| Intangible assets |
(392,113 | ) | (398,509 | ) | ||||
| Foreign earnings repatriation |
(12,024 | ) | (14,073 | ) | ||||
| Other |
(7,969 | ) | (10,206 | ) | ||||
|
|
|
|
|
|||||
| Gross deferred tax liabilities |
(412,106 | ) | (422,788 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax liabilities |
$ | (208,736 | ) | $ | (241,852 | ) | ||
|
|
|
|
|
|||||
F-34
Table of Contents
At December 31, 2011, we maintain $625 million of net operating loss carryforwards in the U.S., which expire over a period of one to 20 years. Our European net operating loss carryforwards of $6 million generally are not subject to expiration dates, unless we trigger certain events.
At December 31, 2011 and 2010, we recorded gross deferred tax assets of $209.5 million, and $185.6 million, respectively, which we reduced by valuation allowances of $6.2 million, and $4.7 million, respectively. We have recorded a valuation allowance against certain European and domestic net operating loss carryforwards due to uncertainties regarding our ability to realize these deferred tax assets.
We do not intend to permanently reinvest the earnings of foreign operations. Accordingly, we recorded a deferred tax expense of $1.5 million, $1.1 million and $0.5 million for the years ended December 31, 2011, 2010 and 2009, respectively, for unrepatriated foreign earnings in those years.
We and our subsidiaries file income tax returns in the U.S. federal, state and local, and foreign jurisdictions. With few exceptions, we are no longer subject to U.S. federal, state and local, or non-U.S. income tax examinations by tax authorities for years before 2007. The Internal Revenue Service (IRS) completed its field examination of the 2005 and 2006 tax years during the first half of 2010. The IRS has proposed material adjustments related to transaction cost, stock option, and bad debt deductions included in our 2006 tax return. We intend to appeal each of the proposed adjustments vigorously through the IRS appeals process. However, should the IRS’ proposed adjustments be upheld in appeals, a material reduction in our currently unreserved net operating losses could result.
A reconciliation of the beginning and ending amount of gross unrecognized tax benefits is as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Balance, beginning of year |
$ | 17,659 | $ | 17,495 | $ | 14,294 | ||||||
| Additions based on tax positions related to current year |
777 | 372 | 2,660 | |||||||||
| Additions for tax positions related to prior years |
3,097 | 477 | 1,114 | |||||||||
| Reductions for tax positions of prior years |
— | — | — | |||||||||
| Reduction due to lapse of statute of limitations |
(2,065 | ) | (685 | ) | (474 | ) | ||||||
| Reductions for settlements of tax positions |
(25 | ) | — | (99 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Balance, end of year |
$ | 19,443 | $ | 17,659 | $ | 17,495 | ||||||
|
|
|
|
|
|
|
|||||||
To the extent our gross unrecognized tax benefits are recognized in the future, a reduction of $2.0 million of U.S. Federal tax benefit for related state income tax deductions would result. There is a reasonable possibility that the closing of the IRS appeals process could result in a material reduction to our unrecognized tax benefits within the next twelve months. Due to the fact that the appeals process has not been finalized, the amount of the unrecognized tax benefits that may be reduced cannot be reasonably estimated. We anticipate that approximately $0.8 million of uncertain tax positions related to transfers of intellectual property and $0.6 million of unrecognized tax positions, each of which are individually immaterial, will decrease in the next twelve months due to the expiration of the statute of limitations. The majority of our unrecognized tax benefits will impact the effective tax rate upon recognition; however, $0.5 million related to prior acquisitions will impact other balance sheet accounts due to various indemnification provisions. We recognized interest and penalties of $0.2 million, $0.6 million and $0.5 million in the years ended December 31, 2011, 2010 and 2009, respectively, which was included as a component of income tax benefit in our consolidated statements of operations. As of December 31, 2011 and 2010, we have $2.4 million and $2.2 million, respectively, accrued for interest and penalties.
F-35
Table of Contents
| 17. | COMMITMENTS AND CONTINGENCIES |
Operating Leases. We lease building space, manufacturing facilities and equipment under non-cancelable operating lease agreements that expire at various dates. We record rent incentives as deferred rent and amortize as reductions to lease expense over the lease term. The aggregate minimum rental commitments under non-cancelable leases for the next five years and thereafter, as of December 31, 2011, are as follows (in thousands):
| Years Ending December 31, |
||||
| 2012 |
$ | 11,749 | ||
| 2013 |
11,036 | |||
| 2014 |
10,453 | |||
| 2015 |
7,664 | |||
| 2016 |
7,201 | |||
| Thereafter |
19,537 | |||
|
|
|
|||
| $ | 67,640 | |||
|
|
|
|||
Rental expense under operating leases totaled $14.3 million, $12.0 million, and $13.4 million for the years ended December 31, 2011, 2010, and 2009 respectively. Scheduled increases in rent expense are amortized on a straight line basis over the life of the lease.
Litigation
From time to time, we are plaintiffs or defendants in various litigation matters in the ordinary course of our business, some of which involve claims for damages that are substantial in amount. We believe that the disposition of claims currently pending will not have a material adverse impact on our financial position or results of operations.
The manufacture and sale of orthopedic devices and related products exposes us to a significant risk of product liability claims. From time to time, we have been, and we are currently, subject to a number of product liability claims alleging that the use of our products resulted in adverse effects.
Pain Pump Litigation
We are currently named as one of several defendants in a number of product liability lawsuits involving approximately 87 plaintiffs in U.S. cases and a lawsuit in Canada which has been granted class action status, related to a disposable drug infusion pump product (pain pump) manufactured by two third party manufacturers that we distributed through our Bracing and Vascular segment. We sold pumps manufactured by one manufacturer from 1999 to 2003 and then sold pumps manufactured by a second manufacturer from 2003 to 2009. We discontinued our sale of these products in the second quarter of 2009. These cases have been brought against the manufacturers and certain distributors of these pumps, and in some cases, the manufacturers of the anesthetics used in these pumps. All of these lawsuits allege that the use of these pumps with certain anesthetics for prolonged periods after certain shoulder surgeries has resulted in cartilage damage to the plaintiffs. The lawsuits allege damages ranging from unspecified amounts to claims of up to $10 million. Many of the lawsuits which have been filed in the past three years have named multiple pain pump manufacturers and distributors without having established which manufacturer manufactured or sold the pump in issue. In the past three years, we have been dismissed from more than 350 cases when product identification was later established showing that we did not sell the pump in issue. At present, we are named in approximately 60 lawsuits in which product identification has yet to be determined and, as a result, we believe that we will be dismissed from a meaningful number of such cases in the future. In the past two years, we have entered into settlements with plaintiffs in approximately 60 pain pump lawsuits. Of these, we have settled approximately 34 cases in joint settlements involving our first manufacturer and we have settled approximately 26 cases involving our second manufacturer
F-36
Table of Contents
in which the manufacturer’s carrier has made some contribution to our settlement amount or any joint settlement, but for which we are seeking indemnity for the balance of our costs. As of December 31, 2011, the range of potential loss is not estimable.
Indemnity and Insurance Coverage Related to Pain Pump Claims
We have sought indemnity and tendered the defense of the pain pump cases to the two manufacturers who supplied these pumps to us, to their products liability carriers and to our products liability carriers. These lawsuits are about equally divided between the two manufacturers. Both manufacturers have rejected our tenders of indemnity. The base policy for one of the manufacturers contributed to our defense, but that policy has been exhausted by defense costs and settlements, as has a second policy of that manufacturer. This manufacturer has ceased operations, has little assets and no additional insurance coverage. The Company has asserted indemnification rights against the successor to this manufacturer and is pursuing claims against the manufacturer, its owners and its successor. The base policy for the other manufacturer has been exhausted and the excess liability carriers for that manufacturer have not accepted coverage for the Company and are not expected to provide for its defense. The Company and this manufacturer have been cooperating in jointly negotiating settlements of those lawsuits in which both parties are named. Our products liability carriers have accepted coverage of these cases, subject to a reservation of the right to deny coverage for customary matters, including punitive damages and off-label promotion. In August 2010, one of our excess carriers for the period ending July 1, 2010 and for the supplemental extended reporting period (SERP) discussed below, which is insuring $10 million in excess of $25 million, informed us that it has reserved its right to rescind the policy based on an alleged failure by us and our insurance broker to disclose material information. We disagree with this allegation and are seeking to resolve the issue with this carrier.
Pain Pump-Related HIPAA Subpoena
On August 2, 2010, we were served with a subpoena under HIPAA seeking numerous documents related to our activities involving the pain pumps discussed above. The subpoena which was issued by the United States Attorney’s Office for the Central District of California, refers to an official investigation by the DOJ and the FDA of Federal health care offenses. We have produced documents that are responsive to the subpoena. We believe that our actions related to our prior distribution of these pain pumps have been in compliance with applicable legal standards.
Pain Pump Investigation—U.S. Attorney’s Office for the Western District of Missouri
In January 2012 the Company became aware of a civil investigation by the United States Attorney’s Office for the Western District of Missouri regarding the Company’s previous sale and marketing of pain pump devices. The investigation relates to whether the Company caused false claims to be filed with government payors as a result of alleged off-label promotion of the pain pumps. The Company believes that this investigation is related to the investigation by the United States Attorney’s Office for the Central District of California that is described above. The Company denies that it improperly promoted the pain pump devices and believes that its marketing and sales activities were in compliance with applicable legal standards.
Cold Therapy Litigation
Since mid-2010, we have been named in nine multi-plaintiff lawsuits involving a total of 210 plaintiffs, alleging that the plaintiffs had been injured following use of certain cold therapy products manufactured by the Company. The complaints are not specific as to the nature of the injuries, but allege various product liability theories, including inadequate warnings regarding the risks associated with the use of cold therapy and failure to incorporate certain safety features into the design. No specific dollar amounts of damages are alleged and as of December 31, 2011, we cannot estimate a range of potential loss. These cases have been included in a coordinated proceeding in San Diego Superior Court with a similar number of cases filed against our competitor.
F-37
Table of Contents
A total of 10 of the plaintiffs included in the cases filed against us have been identified as the first “bellwether” cases to be tried, of which four will go to trial in September 2012. Discovery is proceeding on these bellweather cases.
Our Product Liability Insurance Coverage
We maintain product liability insurance that is subject to annual renewal. Our current policy covers claims reported between July 1, 2011 and June 30, 2012. This policy excludes coverage for claims related to both pain pump products and cold therapy products. As described below, we have other insurance which provides coverage for these excluded products. For the current policy year, we maintain coverage limits (together with excess policies) of up to $50 million, with deductibles of $500,000 per claim for claims relating to invasive products (principally our surgical implant products) and $50,000 per claim for claims relating to all other covered products, with an aggregate self-insured retention of $2 million. Starting with the 2010-2011 policy period, our products liability policy excluded claims related to pain pump products. We purchased supplemental extended reporting period (SERP) coverage for the $80 million limit product liability policy that expired on June 30, 2010, and this supplemental coverage allows us to report pain pump claims beyond the end of the prior policy. Except for the additional excess coverage mentioned below, this SERP coverage does not provide additional limits to the aggregate $80 million limits on the prior policy but it does provide that these limits will remain available for pain pump claims reported for an extended period of time. We also purchased additional coverage of $25 million in excess of the $80 million limits with a five year reporting period. Thus, the SERP coverage for current and future pain pump claims has a total limit of $105 million (less amounts paid for claims reported to date). Concurrently with the exclusion of our cold therapy products from the current primary products coverage, we purchased SERP coverage for cold therapy product claims for injuries alleged to have occurred prior to July 1, 2011. This SERP allows us to report such cold therapy claims under our expired 2010-2011 policy which had total limits of $50 million. We also purchased separate primary and excess policies providing for a total of $5 million of coverage for claims related to cold therapy products arising from injuries alleged to have occurred after June 30, 2011, with a deductible of $300,000 per claim and an aggregate deductible of $4 million. We believe we have adequate insurance coverage for our product liability claims.
BGS Qui Tam Action and HIPAA Subpoena
On April 15, 2009, we became aware of a qui tam action filed in Federal Court in Boston, Massachusetts in March 2005 and amended in December 2007 that names us as a defendant along with each of the other companies that manufactures and sells external bone growth stimulators, as well as The Blackstone Group L.P., an affiliate of DJO’s principal stockholder, and the principal stockholder of one of the other companies in the bone growth stimulation business. This case is captioned United States ex rel. Beirman v. Orthofix International, N.V., et al., Civil Action No. 05-10557 (D. Mass.). The case was sealed when originally filed and unsealed in March 2009. The plaintiff, or relator, alleges that the defendants have engaged in Medicare fraud and violated Federal and state false claims acts from the time of the original introduction of the devices by each defendant to the present by seeking reimbursement for bone growth stimulators as a purchased item rather than a rental item. The relator also alleges that the defendants are engaged in other marketing practices constituting violations of the Federal and various state anti-kickback statutes. On December 4, 2009, we filed a motion to dismiss the relator’s complaint. The relator filed a second amended complaint in May 2010 that, among other things, dropped The Blackstone Group as a defendant. We filed another motion to dismiss directed at the second amended complaint, and that motion was denied. The case is proceeding to the discovery phase. Shortly before becoming aware of the qui tam action, we were advised that our bone growth stimulator business was the subject of an investigation by the DOJ, and on April 10, 2009, we were served with a subpoena under HIPAA seeking numerous documents relating to the marketing and sale by us of bone growth stimulators. On September 21, 2009, we were served with a second HIPAA subpoena related to this DOJ investigation seeking additional documents relating to the marketing and sale by us of bone growth stimulators. We believe that these subpoenas are related to the DOJ’s investigation of the allegations in the qui tam action, although the DOJ has decided not to intervene in the qui tam action at this time. We believe that our marketing practices in the bone growth stimulation business are in
F-38
Table of Contents
compliance with applicable legal standards and we intend to defend this case and investigation vigorously. We can make no assurance as to the resources that will be needed to respond to these matters or the final outcome of such action and as of December 31, 2011, we cannot estimate a range of potential loss, fines or damages.
| 18. | RELATED PARTY TRANSACTIONS |
Management Stockholder’s Agreement
All members of DJO’s management who own shares of DJO common stock or options to purchase DJO common stock are parties to a Management Stockholders Agreement, dated November 3, 2006, among DJO, Grand Slam Holdings, LLC (BCP Holdings), Blackstone, certain of its affiliates (BCP Holdings and Blackstone and its affiliates are referred to as Blackstone Parent Stockholders), and such members of DJO’s management, as amended by the First Amendment to Management Stockholders Agreement (the Management Stockholders Agreement). The Management Stockholders Agreement provides that upon termination of a management stockholder’s employment for any reason, DJO and a Blackstone Parent Stockholder may collectively exercise the right to purchase all of the shares of DJO common stock held by such management stockholder within one year after such termination (or, with respect to shares purchased upon exercise of options after termination of employment, one year following such exercise). If a management stockholder is terminated for cause (as defined in the Agreement), or voluntarily terminates their employment and such termination would have constituted a termination for cause if it would have been initiated by DJO, and DJO or a Blackstone Parent Stockholder exercises its call rights after such termination, the management stockholder would receive the lower of fair market value or cost for the management stockholder’s callable shares. In the case of all other terminations of employment, the management stockholder would receive fair market value for such shares.
The Management Stockholders Agreement imposes significant restrictions on transfers of shares of DJO’s common stock held by management stockholders and provides a right of first refusal to DJO or Blackstone, if DJO fails to exercise such right, on any proposed sale of DJO’s common stock held by a management stockholder following the lapse of the transfer restrictions and prior to the occurrence of a qualified public offering (as such term is defined in that agreement) of DJO. In addition, prior to a qualified public offering, Blackstone will have drag-along rights, and management stockholders will have tag-along rights, in the event of a sale of DJO’s common stock by Blackstone to a third party (or in the event of a sale of BCP Holdings’ equity interests to a third party) in the same proportion as the shares or equity interests sold by Blackstone. The Management Stockholders Agreement also provides that, after the occurrence of a qualified public offering, the management stockholders will receive customary piggyback registration rights with respect to shares of DJO common stock held by them.
All parties receiving an award of stock options, including all DJO directors who have been granted options, as well as all purchasers of common stock in DJO’s offerings in the years ended December 31, 2011 and 2010, are parties to a Stockholders Agreement which has the same material terms and conditions as the Management Stockholders Agreement.
Transaction and Monitoring Fee Agreement
Under the Transaction and Monitoring Fee Agreement, at the closing of the DJO Merger, we paid BMP, an affiliate of our primary shareholder, a $15.0 million transaction fee and $0.6 million for related expenses. Also, pursuant to this agreement, at the closing of the DJO Merger, we paid Blackstone Advisory Services, L.P. (BAS), an affiliate of BMP, a $3.0 million advisory fee in consideration of the provision of certain strategic and other advice and assistance by BAS on behalf of BMP.
In connection with the DJO Merger, BMP has agreed to provide certain monitoring, advisory and consulting services to us for an annual monitoring fee equal to the greater of $7.0 million or 2% of consolidated EBITDA as defined in the Transaction and Monitoring Fee Agreement, payable in the first quarter of each year. The monitoring fee agreement will continue until the earlier of November 2019, or such date as DJO and BMP may
F-39
Table of Contents
mutually determine. DJO has agreed to indemnify BMP and its affiliates, directors, officers, employees, agents and representatives from and against all liabilities relating to the services contemplated by the Transaction and Monitoring Fee Agreement and the engagement of BMP pursuant to, and the performance of BMP and its affiliates of the services contemplated by, the Transaction and Monitoring Fee Agreement. At any time in connection with or in anticipation of a change of control of DJO, a sale of all or substantially all of DJO’s assets or an initial public offering of common stock of DJO, BMP may elect to receive, in lieu of remaining annual monitoring fee payments, a single lump sum cash payment equal to the then-present value of all then-current and future annual monitoring fees payable under the Transaction and Monitoring Fee Agreement, assuming a hypothetical termination date of the agreement to be November 2019. For each of the years ended December 31, 2011, 2010 and 2009, we expensed $7.0 million related to the annual monitoring fee, which is recorded as a component of selling, general and administrative expense in the consolidated statements of operations.
Other Related Party Transactions
During the year ended December 31, 2011, in connection with the Dr. Comfort acquisition (see Note 3), we paid $5.0 million of transaction and advisory fees to Blackstone Advisory Partners, L.P., an affiliate of our major shareholder, which was recorded as a component of selling, general and administrative expense in the consolidated statement of operations.
| 19. | SEGMENT AND GEOGRAPHIC INFORMATION |
We provide a broad array of orthopedic rehabilitation and regeneration products, as well as surgical implants to customers in the United States and abroad.
During the first quarter of 2011, we changed the name of our Bracing and Supports segment to Bracing and Vascular to reflect the addition of our recent acquisitions, which have increased our focus on the vascular market. This segment also includes the U.S. results of operations attributable to Dr. Comfort, ETI and Circle City, from their respective dates of acquisition (see Note 3). This change had no impact on previously reported segment information.
We currently develop, manufacture and distribute our products through the following four operating segments:
Bracing and Vascular Segment
Our Bracing and Vascular segment, which generates its revenues in the United States, offers our rigid knee bracing products, orthopedic soft goods, cold therapy products, vascular systems, and compression therapy products, primarily under our DonJoy, ProCare and Aircast brands. The U.S. results of our recent Circle City and ETI acquisitions are included within this segment. This segment also includes our OfficeCare business, through which we maintain an inventory of soft goods and other products at healthcare facilities, primarily orthopedic practices, for immediate distribution to patients. In addition, included within this segment is our newly acquired Dr. Comfort business, which develops and manufactures therapeutic footwear and related medical and comfort products serving the diabetes care market in podiatry practices, orthotic and prosthetic centers, home medial equipment providers and independent pharmacies.
Recovery Sciences Segment
Our Recovery Sciences segment, which generates its revenues in the United States, is divided into four main businesses:
| • | Empi. Our Empi business unit offers our home electrotherapy, iontophoresis, and home traction products. We primarily sell these products directly to patients or to physical therapy clinics. For products sold to patients, we arrange billing to the patients and their third party payors. |
F-40
Table of Contents
| • | Regeneration. Our Regeneration business unit primarily sells our bone growth stimulation products. We sell these products either directly to patients or to independent distributors. For products sold to patients, we arrange billing to the patients and their third party payors. |
| • | Chattanooga. Our Chattanooga business unit offers products in the clinical rehabilitation market in the categories of clinical electrotherapy devices, clinical traction devices, and other clinical products and supplies such as treatment tables, continuous passive motion (CPM) devices and dry heat therapy. |
| • | Athlete Direct. Our Athlete Direct business unit offers consumers ranging from fitness enthusiasts to competitive athletes our Compex electrostimulation device, which is used in athletic training programs to aid muscle development and to accelerate muscle recovery after training sessions. |
International Segment
Our International segment, which generates most of its revenues in Europe, sells all of our products and certain third party products through a combination of direct sales representatives and independent distributors.
Surgical Implant Segment
Our Surgical Implant segment develops, manufactures and markets a wide variety of knee, hip and shoulder implant products that serve the orthopedic reconstructive joint implant market in the United States.
Information regarding our reportable business segments is presented in the table below (in thousands). Segment results exclude the impact of amortization and impairment of goodwill and intangible assets, certain general corporate expenses, and charges related to various integration activities, as defined by management. The accounting policies of the reportable segments are the same as the accounting policies of the Company. We allocate resources and evaluate the performance of segments based on net sales, gross profit, operating income and other non-GAAP measures, as defined. Moreover, we do not allocate assets to reportable segments because a significant portion of assets are shared by the segments.
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Net sales: |
||||||||||||
| Bracing and Vascular |
$ | 387,928 | $ | 311,620 | $ | 298,759 | ||||||
| Recovery Sciences |
342,599 | 347,139 | 342,026 | |||||||||
| International |
279,299 | 244,493 | 241,464 | |||||||||
| Surgical Implant |
64,944 | 62,721 | 63,877 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 1,074,770 | $ | 965,973 | $ | 946,126 | |||||||
|
|
|
|
|
|
|
|||||||
| Gross profit: |
||||||||||||
| Bracing and Vascular |
$ | 203,217 | $ | 170,786 | $ | 168,009 | ||||||
| Recovery Sciences |
258,920 | 265,196 | 257,466 | |||||||||
| International |
161,142 | 143,562 | 137,142 | |||||||||
| Surgical Implant |
46,860 | 46,031 | 49,799 | |||||||||
| Expenses not allocated to segments and eliminations |
(13,507 | ) | (4,872 | ) | (5,009 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| $ | 656,632 | $ | 620,703 | $ | 607,407 | |||||||
|
|
|
|
|
|
|
|||||||
| Operating (loss) income: |
||||||||||||
| Bracing and Vascular |
$ | 75,095 | $ | 68,058 | $ | 70,805 | ||||||
| Recovery Sciences |
93,394 | 117,656 | 107,157 | |||||||||
| International |
57,501 | 56,356 | 49,051 | |||||||||
| Surgical Implant |
4,323 | 7,121 | 12,955 | |||||||||
| Expenses not allocated to segments and eliminations |
(322,578 | ) | (161,311 | ) | (161,111 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| $ | (92,265 | ) | $ | 87,880 | $ | 78,857 | ||||||
|
|
|
|
|
|
|
|||||||
F-41
Table of Contents
Geographic Area
Following are our net sales by geographic area (in thousands):
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| United States |
$ | 795,471 | $ | 718,601 | $ | 704,954 | ||||||
| Germany |
90,000 | 74,441 | 74,185 | |||||||||
| Other Europe, Middle East, and Africa |
109,768 | 98,502 | 110,140 | |||||||||
| Asia Pacific |
8,952 | 20,426 | 15,541 | |||||||||
| Other |
70,579 | 54,003 | 41,306 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 1,074,770 | $ | 965,973 | $ | 946,126 | |||||||
|
|
|
|
|
|
|
|||||||
Net sales are attributed to countries based on location of customer. In each of the years ended December 31, 2011, 2010 and 2009, no individual customer or distributor accounted for 10% or more of total annual net sales.
Following are our long-lived assets by geographic area (in thousands):
| December 31, 2011 |
December 31, 2010 |
|||||||
| United States |
$ | 2,353,410 | $ | 2,269,213 | ||||
| International |
153,351 | 160,982 | ||||||
|
|
|
|
|
|||||
| $ | 2,506,761 | $ | 2,430,195 | |||||
|
|
|
|
|
|||||
| 20. | UNAUDITED QUARTERLY CONSOLIDATED FINANCIAL DATA |
We operate our business on a manufacturing calendar, with our fiscal year always ending on December 31. Each quarter is 13 weeks, consisting of two four-week periods and one five-week period. Our first and fourth quarters may have more or fewer shipping days from year to year based on the days of the week on which holidays and December 31 fall.
In addition, during the fourth quarter of fiscal year 2011, we identified and corrected an immaterial error which impacted the consolidated financial statements for the years ended December 31, 2010 and 2009, related to the elimination of intercompany profits on the sale of products between subsidiaries. This error resulted in an overstatement of cost of goods sold of $1.1 million and $3.1 million for the years ended December 31, 2009 and 2010, respectively.
Based on a quantitative and qualitative analysis of the error as required by SAB 108, we determined that correcting the cumulative impact of this error, which decreased cost of goods sold and increased property and equipment by $4.2 million in the fourth quarter of the year ended December 31, 2011, was not material to the results for the year ended December 31, 2011.
In the fourth quarter of 2011, we determined that the carrying value of our Empi and Surgical Implant reporting units was in excess of their estimated fair value. As a result, we recorded an aggregate goodwill impairment charge of $124.1 million.
In addition, during the fourth quarter of 2011, we determined that the carrying value of our Empi trade name was in excess of its estimated fair value and recorded an impairment charge of $16.9 million.
F-42
Table of Contents
The following table presents our unaudited quarterly consolidated financial data (in thousands):
| Three months ended | ||||||||||||||||
| April 2, 2011 |
July 2, 2011 |
October 1, 2011 |
December 31, 2011 |
|||||||||||||
| Net sales |
$ | 249,711 | $ | 277,786 | $ | 263,118 | $ | 284,155 | ||||||||
| Gross profit |
156,555 | 166,696 | 155,655 | 177,726 | ||||||||||||
| Operating income (loss) |
11,919 | 7,243 | 8,889 | (120,316 | ) | |||||||||||
| Net loss |
(20,924 | ) | (18,960 | ) | (25,706 | ) | (147,997 | ) | ||||||||
| Net loss attributable to DJOFL |
(21,239 | ) | (19,255 | ) | (25,764 | ) | (148,211 | ) | ||||||||
| Three months ended | ||||||||||||||||
| April 3, 2010 |
July 3, 2010 |
October 2, 2010 |
December 31, 2010 |
|||||||||||||
| Net sales |
$ | 240,076 | $ | 242,527 | $ | 233,559 | $ | 249,811 | ||||||||
| Gross profit |
152,722 | 157,962 | 149,412 | 160,607 | ||||||||||||
| Operating income |
17,571 | 15,969 | 21,445 | 32,895 | ||||||||||||
| Net (loss) income |
(33,336 | ) | 564 | (7,531 | ) | (11,372 | ) | |||||||||
| Net (loss) income attributable to DJOFL |
(33,658 | ) | 243 | (7,715 | ) | (11,402 | ) | |||||||||
| 21. | SUPPLEMENTAL GUARANTOR CONDENSED CONSOLIDATING FINANCIAL STATEMENTS |
DJOFL and its direct wholly owned subsidiary, DJO Finco, issued the 10.875% Notes with an aggregate principal amount of $575.0 million and $100.0 million on November 20, 2007 and January 20, 2010, respectively. On October, 2010, DJOFL and DJO Finco issued $300.0 million aggregate principal amount of 9.75% Notes, and used a portion of the proceeds to repurchase $200.0 million aggregate principal amount of 11.75% Notes, which were issued on November 3, 2006. DJO Finco was formed solely to act as a co-issuer of the notes, has only nominal assets and does not conduct any operations. The Indentures generally prohibit DJO Finco from holding any assets, becoming liable for any obligations, or engaging in any business activity. The 10.875% Notes are jointly and severally, fully and unconditionally guaranteed, on an unsecured senior basis by all of DJOFL’s domestic subsidiaries (other than the co-issuer) that are 100% owned, directly or indirectly, by DJOFL (the Guarantors). The 9.75% Notes are jointly and severally, fully and unconditionally guaranteed, on an unsecured senior subordinated basis by the Guarantors. Our foreign subsidiaries (the Non-Guarantors) do not guarantee our notes. The Guarantors also unconditionally guarantee the Senior Secured Credit Facility.
F-43
Table of Contents
The following tables present the financial position, results of operations and cash flows of DJOFL, the Guarantors, the Non-Guarantors and certain eliminations as of December 31, 2011 and 2010 and for the years ended December 31, 2011, 2010, and 2009.
DJO Finance LLC
Condensed Consolidating Balance Sheets
As of December 31, 2011
(in thousands)
| DJOFL | Guarantors | Non- Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Assets | ||||||||||||||||||||
| Current assets: |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 13,773 | $ | 1,778 | $ | 22,617 | $ | 1 | $ | 38,169 | ||||||||||
| Accounts receivable, net |
— | 125,097 | 33,885 | — | 158,982 | |||||||||||||||
| Inventories, net |
— | 97,516 | 20,719 | 10,464 | 128,699 | |||||||||||||||
| Deferred tax assets, net |
— | 43,190 | 268 | — | 43,458 | |||||||||||||||
| Prepaid expenses and other current assets |
160 | 15,001 | 3,186 | 444 | 18,791 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current assets |
13,933 | 282,582 | 80,675 | 10,909 | 388,099 | |||||||||||||||
| Property and equipment, net |
— | 94,904 | 13,070 | (866 | ) | 107,108 | ||||||||||||||
| Goodwill |
— | 1,150,269 | 107,344 | (28,835 | ) | 1,228,778 | ||||||||||||||
| Intangible assets, net |
— | 1,101,314 | 31,380 | — | 1,132,694 | |||||||||||||||
| Investment in subsidiaries |
1,297,699 | 1,686,366 | 72,514 | (3,056,579 | ) | — | ||||||||||||||
| Intercompany receivables |
1,138,947 | — | — | (1,138,947 | ) | — | ||||||||||||||
| Other assets |
33,971 | 2,655 | 1,557 | (2 | ) | 38,181 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total assets |
$ | 2,484,550 | $ | 4,318,090 | $ | 306,540 | $ | (4,214,320 | ) | $ | 2,894,860 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Liabilities and Equity | ||||||||||||||||||||
| Current liabilities: |
||||||||||||||||||||
| Accounts payable |
$ | — | $ | 47,049 | $ | 10,872 | $ | 5 | $ | 57,926 | ||||||||||
| Current portion of debt and capital lease obligations |
8,782 | 38 | — | — | 8,820 | |||||||||||||||
| Other current liabilities |
20,864 | 56,509 | 24,805 | 521 | 102,699 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current liabilities |
29,646 | 103,596 | 35,677 | 526 | 169,445 | |||||||||||||||
| Long-term debt and capital leases obligations |
2,159,091 | — | — | — | 2,159,091 | |||||||||||||||
| Deferred tax liabilities, net |
— | 242,237 | 9,957 | — | 252,194 | |||||||||||||||
| Intercompany payables, net |
— | 996,889 | 142,058 | (1,138,947 | ) | — | ||||||||||||||
| Other long-term liabilities |
— | 14,689 | 1,485 | — | 16,174 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities |
2,188,737 | 1,357,411 | 189,177 | (1,138,421 | ) | 2,596,904 | ||||||||||||||
| Noncontrolling interests |
— | — | 2,143 | — | 2,143 | |||||||||||||||
| Total membership equity |
295,813 | 2,960,679 | 115,220 | (3,075,899 | ) | 295,813 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities and equity |
$ | 2,484,550 | $ | 4,318,090 | $ | 306,540 | $ | (4,214,320 | ) | $ | 2,894,860 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-44
Table of Contents
DJO Finance LLC
Condensed Consolidating Statements of Operations
For the Year Ended December 31, 2011
(in thousands)
| DJOFL | Guarantors | Non- Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Net sales |
$ | — | $ | 893,036 | $ | 263,908 | $ | (82,174 | ) | $ | 1,074,770 | |||||||||
| Cost of sales (exclusive of amortization of intangible assets of $38,668) |
— | 360,601 | 168,307 | (110,770 | ) | 418,138 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
— | 532,435 | 95,601 | 28,596 | 656,632 | |||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Selling, general and administrative |
— | 394,588 | 92,496 | — | 487,084 | |||||||||||||||
| Research and development |
— | 23,050 | 3,800 | — | 26,850 | |||||||||||||||
| Amortization of intangible assets |
— | 89,637 | 4,320 | — | 93,957 | |||||||||||||||
| Impairment of goodwill and intangible assets |
— | 141,006 | — | — | 141,006 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| — | 648,281 | 100,616 | — | 748,897 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating (loss) income |
— | (115,846 | ) | (5,015 | ) | 28,596 | (92,265 | ) | ||||||||||||
| Other income (expense): |
||||||||||||||||||||
| Interest expense |
(169,117 | ) | (22 | ) | (193 | ) | — | (169,332 | ) | |||||||||||
| Interest income |
14 | 127 | 204 | — | 345 | |||||||||||||||
| Loss on modification of debt |
(2,065 | ) | — | — | — | (2,065 | ) | |||||||||||||
| Other income (expense), net |
— | 986 | (3,745 | ) | (55 | ) | (2,814 | ) | ||||||||||||
| Intercompany income (expense), net |
10,625 | 18,126 | (18,381 | ) | (10,370 | ) | — | |||||||||||||
| Equity in loss of subsidiaries, net |
(53,926 | ) | — | — | 53,926 | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (214,469 | ) | 19,217 | (22,115 | ) | 43,501 | (173,866 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (Loss) income before income taxes |
(214,469 | ) | (96,629 | ) | (27,130 | ) | 72,097 | (266,131 | ) | |||||||||||
| Income tax benefit (provision) |
— | 57,173 | 4,775 | (146 | ) | 52,544 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income |
(214,469 | ) | (39,456 | ) | (31,905 | ) | 72,243 | (213,587 | ) | |||||||||||
| Net income attributable to noncontrolling interests |
— | — | (882 | ) | — | (882 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income (loss) attributable to DJOFL |
$ | (214,469 | ) | $ | (39,456 | ) | $ | (32,787 | ) | $ | 72,243 | $ | (214,469 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-45
Table of Contents
DJO Finance LLC
Condensed Consolidating Statements of Cash Flows
For the Year Ended December 31, 2011
(in thousands)
| DJOFL | Guarantors | Non- Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Cash Flows From Operating Activities: |
||||||||||||||||||||
| Net income (loss) |
$ | (214,469 | ) | $ | (39,456 | ) | $ | (31,905 | ) | $ | 72,243 | $ | (213,587 | ) | ||||||
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: |
||||||||||||||||||||
| Depreciation |
— | 23,231 | 5,083 | (1,020 | ) | 27,294 | ||||||||||||||
| Amortization of intangible assets |
— | 89,637 | 4,320 | — | 93,957 | |||||||||||||||
| Amortization of debt issuance costs and non-cash interest expense |
8,476 | — | — | — | 8,476 | |||||||||||||||
| Stock-based compensation expense |
— | 2,701 | — | — | 2,701 | |||||||||||||||
| Loss on disposal of assets, net |
— | 7,434 | 438 | (3,487 | ) | 4,385 | ||||||||||||||
| Impairment of goodwill and intangible assets |
— | 141,006 | — | — | 141,006 | |||||||||||||||
| Deferred income tax (benefit) expense |
(2,599 | ) | (56,651 | ) | (1,224 | ) | (146 | ) | (60,620 | ) | ||||||||||
| Equity in loss of subsidiaries, net |
53,926 | — | — | (53,926 | ) | — | ||||||||||||||
| Provision for doubtful accounts and sales returns |
— | 31,000 | 673 | — | 31,673 | |||||||||||||||
| Inventory reserves |
— | 6,798 | 908 | — | 7,706 | |||||||||||||||
| Changes in operating assets and liabilities, net of acquired assets and liabilities: |
||||||||||||||||||||
| Accounts receivable |
— | (30,398 | ) | (1,833 | ) | — | (32,231 | ) | ||||||||||||
| Inventories |
— | (7,631 | ) | 6,402 | (11,961 | ) | (13,190 | ) | ||||||||||||
| Prepaid expenses and other assets |
(17 | ) | 7,351 | (1,090 | ) | 2,191 | 8,435 | |||||||||||||
| Accounts payable and other current liabilities |
5,336 | 3,877 | 13,062 | (4,675 | ) | 17,602 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash provided by (used in) operating activities |
(149,347 | ) | 178,899 | (5,166 | ) | (781 | ) | 23,605 | ||||||||||||
| Cash Flows From Investing Activities: |
||||||||||||||||||||
| Cash paid in connection with acquisitions, net of cash acquired |
— | (317,669 | ) | — | — | (317,669 | ) | |||||||||||||
| Purchases of property and equipment |
— | (33,673 | ) | (5,536 | ) | (188 | ) | (39,397 | ) | |||||||||||
| Other investing activities, net |
— | (1,603 | ) | 7 | — | (1,596 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash used in investing activities |
— | (352,945 | ) | (5,529 | ) | (188 | ) | (358,662 | ) | |||||||||||
| Cash Flows From Financing Activities: |
||||||||||||||||||||
| Intercompany |
(191,181 | ) | 177,245 | 12,966 | 970 | — | ||||||||||||||
| Proceeds from issuance of debt |
439,000 | — | — | — | 439,000 | |||||||||||||||
| Repayments of debt and capital lease obligations |
(96,782 | ) | (42 | ) | (2 | ) | — | (96,826 | ) | |||||||||||
| Payment of debt issuance costs |
(7,694 | ) | — | — | — | (7,694 | ) | |||||||||||||
| Investment by parent |
3,176 | — | — | — | 3,176 | |||||||||||||||
| Cancellation of vested options |
— | (2,000 | ) | — | — | (2,000 | ) | |||||||||||||
| Dividend paid by subsidiary to owners of noncontrolling interests |
— | — | (1,366 | ) | — | (1,366 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash provided by (used in) financing activities |
146,519 | 175,203 | 11,598 | 970 | 334,290 | |||||||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
— | — | 804 | — | 804 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net increase (decrease) in cash and cash equivalents |
(2,828 | ) | 1,157 | 1,707 | 1 | 37 | ||||||||||||||
| Cash and cash equivalents, beginning of year |
16,601 | 621 | 20,910 | — | 38,132 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents, end of year |
$ | 13,773 | $ | 1,778 | $ | 22,617 | $ | 1 | $ | 38,169 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-46
Table of Contents
DJO Finance LLC
Condensed Consolidating Balance Sheets
As of December 31, 2010
(in thousands)
| DJOFL | Guarantors | Non- Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Assets | ||||||||||||||||||||
| Current assets: |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 16,601 | $ | 621 | $ | 20,910 | $ | — | $ | 38,132 | ||||||||||
| Accounts receivable, net |
— | 112,250 | 33,273 | — | 145,523 | |||||||||||||||
| Inventories, net |
— | 75,929 | 29,611 | (11,080 | ) | 94,460 | ||||||||||||||
| Deferred tax assets, net |
— | 47,805 | 402 | (146 | ) | 48,061 | ||||||||||||||
| Prepaid expenses and other current assets |
162 | 18,199 | 2,418 | 2,640 | 23,419 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current assets |
16,763 | 254,804 | 86,614 | (8,586 | ) | 349,595 | ||||||||||||||
| Property and equipment, net |
— | 85,856 | 13,357 | (5,553 | ) | 93,660 | ||||||||||||||
| Goodwill |
— | 1,108,703 | 109,693 | (29,509 | ) | 1,188,887 | ||||||||||||||
| Intangible assets, net |
— | 1,074,388 | 36,453 | — | 1,110,841 | |||||||||||||||
| Investment in subsidiaries |
1,296,776 | 1,663,969 | 127,148 | (3,087,893 | ) | — | ||||||||||||||
| Intercompany receivables |
1,003,751 | — | — | (1,003,751 | ) | — | ||||||||||||||
| Other assets |
34,115 | 1,177 | 1,479 | 36 | 36,807 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total assets |
$ | 2,351,405 | $ | 4,188,897 | $ | 374,744 | $ | (4,135,256 | ) | $ | 2,779,790 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Liabilities and Equity | ||||||||||||||||||||
| Current liabilities: |
||||||||||||||||||||
| Accounts payable |
$ | — | $ | 40,893 | $ | 8,054 | $ | — | $ | 48,947 | ||||||||||
| Current portion of debt and capital lease obligations |
8,782 | 39 | — | — | 8,821 | |||||||||||||||
| Other current liabilities |
22,234 | 52,150 | 20,263 | 2,640 | 97,287 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current liabilities |
31,016 | 93,082 | 28,317 | 2,640 | 155,055 | |||||||||||||||
| Long-term debt and capital leases obligations |
1,816,250 | 41 | — | — | 1,816,291 | |||||||||||||||
| Deferred tax liabilities, net |
— | 277,135 | 11,657 | 1,121 | 289,913 | |||||||||||||||
| Intercompany payables, net |
— | 825,647 | 178,104 | (1,003,751 | ) | — | ||||||||||||||
| Other long-term liabilities |
— | 10,160 | 1,552 | — | 11,712 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities |
1,847,266 | 1,206,065 | 219,630 | (999,990 | ) | 2,272,971 | ||||||||||||||
| Noncontrolling interests |
— | — | 2,680 | — | 2,680 | |||||||||||||||
| Total membership equity |
504,139 | 2,982,832 | 152,434 | (3,135,266 | ) | 504,139 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities and equity |
$ | 2,351,405 | $ | 4,188,897 | $ | 374,744 | $ | (4,135,256 | ) | $ | 2,779,790 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-47
Table of Contents
DJO Finance LLC
Condensed Consolidating Statements of Operations
For the Year Ended December 31, 2010
(in thousands)
| DJOFL | Guarantors | Non- Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Net sales |
$ | — | $ | 830,186 | $ | 276,295 | $ | (140,508 | ) | $ | 965,973 | |||||||||
| Cost of sales (exclusive of amortization of intangible assets of $36,343) |
— | 304,206 | 177,592 | (136,528 | ) | 345,270 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
— | 525,980 | 98,703 | (3,980 | ) | 620,703 | ||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Selling, general and administrative |
— | 353,854 | 79,554 | — | 433,408 | |||||||||||||||
| Research and development |
— | 18,062 | 3,830 | — | 21,892 | |||||||||||||||
| Amortization of intangible assets |
— | 73,560 | 3,963 | — | 77,523 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| — | 445,476 | 87,347 | — | 532,823 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating income |
— | 80,504 | 11,356 | (3,980 | ) | 87,880 | ||||||||||||||
| Other income (expense): |
||||||||||||||||||||
| Interest expense |
(154,823 | ) | (51 | ) | (307 | ) | — | (155,181 | ) | |||||||||||
| Interest income |
9 | 191 | 110 | — | 310 | |||||||||||||||
| Loss on modification and extinguishment of debt |
(19,798 | ) | — | — | — | (19,798 | ) | |||||||||||||
| Other income (expense), net |
— | 2,567 | (1,708 | ) | — | 859 | ||||||||||||||
| Intercompany income (expense), net |
75,099 | (34,980 | ) | 2,302 | (42,421 | ) | — | |||||||||||||
| Equity in income of subsidiaries, net |
46,981 | — | — | (46,981 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (52,532 | ) | (32,273 | ) | 397 | (89,402 | ) | (173,810 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (Loss) income before income taxes |
(52,532 | ) | 48,231 | 11,753 | (93,382 | ) | (85,930 | ) | ||||||||||||
| Income tax benefit (provision) |
— | 39,791 | (5,536 | ) | — | 34,255 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income |
(52,532 | ) | 88,022 | 6,217 | (93,382 | ) | (51,675 | ) | ||||||||||||
| Net income attributable to noncontrolling interests |
— | — | (857 | ) | — | (857 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income attributable to DJOFL |
$ | (52,532 | ) | $ | 88,022 | $ | 5,360 | $ | (93,382 | ) | $ | (52,532 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-48
Table of Contents
DJO Finance LLC
Condensed Consolidating Statements of Cash Flows
For the Year Ended December 31, 2010
(in thousands)
| DJOFL | Guarantors | Non- Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Cash Flows From Operating Activities: |
||||||||||||||||||||
| Net (loss) income |
$ | (52,532 | ) | $ | 88,022 | $ | 6,217 | $ | (93,382 | ) | $ | (51,675 | ) | |||||||
| Adjustments to reconcile net (loss) income to net cash provided by (used in) operating activities: |
||||||||||||||||||||
| Depreciation |
— | 21,403 | 4,713 | (120 | ) | 25,996 | ||||||||||||||
| Amortization of intangible assets |
— | 73,560 | 3,963 | — | 77,523 | |||||||||||||||
| Amortization of debt issuance costs and non-cash interest expense |
13,272 | — | — | — | 13,272 | |||||||||||||||
| Stock-based compensation expense |
— | 1,888 | — | — | 1,888 | |||||||||||||||
| Loss on disposal of assets, net |
— | 1,918 | 551 | (402 | ) | 2,067 | ||||||||||||||
| Deferred income tax (benefit) expense |
— | (85,634 | ) | 45,947 | — | (39,687 | ) | |||||||||||||
| Equity in income of subsidiaries, net |
(46,981 | ) | — | — | 46,981 | — | ||||||||||||||
| Provision for doubtful accounts and sales returns |
— | 31,918 | 1,159 | — | 33,077 | |||||||||||||||
| Inventory reserves |
— | 5,890 | 706 | — | 6,596 | |||||||||||||||
| Loss on modification and extinguishment of debt |
19,798 | — | — | — | 19,798 | |||||||||||||||
| Changes in operating assets and liabilities, net of acquired assets and liabilities: |
||||||||||||||||||||
| Accounts receivable |
— | (31,619 | ) | (1,486 | ) | — | (33,105 | ) | ||||||||||||
| Inventories |
— | (4,081 | ) | (7,449 | ) | (2,378 | ) | (13,908 | ) | |||||||||||
| Prepaid expenses and other assets |
— | 25,457 | (30,294 | ) | — | (4,837 | ) | |||||||||||||
| Accounts payable and other current liabilities |
(12,653 | ) | 101 | 1,141 | — | (11,411 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash (used in) provided by operating activities |
(79,096 | ) | 128,823 | 25,168 | (49,301 | ) | 25,594 | |||||||||||||
| Cash Flows From Investing Activities: |
||||||||||||||||||||
| Purchases of property and equipment |
— | (26,111 | ) | (4,233 | ) | 3,097 | (27,247 | ) | ||||||||||||
| Cash paid in connection with acquisitions, net of cash acquired |
— | (2,045 | ) | — | — | (2,045 | ) | |||||||||||||
| Other investing activities, net |
— | 1,180 | (2,083 | ) | — | (903 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash used in investing activities |
— | (26,976 | ) | (6,316 | ) | 3,097 | (30,195 | ) | ||||||||||||
| Cash Flows From Financing Activities: |
||||||||||||||||||||
| Intercompany |
85,871 | (102,826 | ) | (29,249 | ) | 46,204 | — | |||||||||||||
| Proceeds from issuance of debt |
447,000 | — | 130 | — | 447,130 | |||||||||||||||
| Repayments of debt and capital lease obligations |
(433,891 | ) | (17,278 | ) | 13,802 | — | (437,367 | ) | ||||||||||||
| Payment of debt issuance costs |
(10,282 | ) | — | — | — | (10,282 | ) | |||||||||||||
| Investment by parent |
— | 1,489 | — | — | 1,489 | |||||||||||||||
| Dividend paid by subsidiary to owners of noncontrolling interests |
— | — | (557 | ) | — | (557 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash provided by (used in) financing activities |
88,698 | (118,615 | ) | (15,874 | ) | 46,204 | 413 | |||||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
— | — | (2,291 | ) | — | (2,291 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net increase (decrease) in cash and cash equivalents |
9,602 | (16,768 | ) | 687 | — | (6,479 | ) | |||||||||||||
| Cash and cash equivalents, beginning of year |
6,999 | 17,389 | 20,223 | — | 44,611 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents, end of year |
$ | 16,601 | $ | 621 | $ | 20,910 | $ | — | $ | 38,132 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-49
Table of Contents
DJO Finance LLC
Condensed Consolidating Statements of Operations
For the Year Ended December 31, 2009
(in thousands)
| DJOFL | Guarantors | Non- Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Net sales |
$ | — | $ | 804,912 | $ | 268,644 | $ | (127,430 | ) | $ | 946,126 | |||||||||
| Cost of sales (exclusive of amortization of intangible assets of $37,884) |
— | 290,097 | 175,464 | (126,842 | ) | 338,719 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
— | 514,815 | 93,180 | (588 | ) | 607,407 | ||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Selling, general and administrative |
— | 343,574 | 76,201 | 983 | 420,758 | |||||||||||||||
| Research and development |
— | 20,712 | 2,828 | — | 23,540 | |||||||||||||||
| Amortization of intangible assets |
— | 74,433 | 2,821 | — | 77,254 | |||||||||||||||
| Impairment of intangible assets |
— | 6,998 | — | — | 6,998 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| — | 445,717 | 81,850 | 983 | 528,550 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating income |
— | 69,098 | 11,330 | (1,571 | ) | 78,857 | ||||||||||||||
| Other income (expense): |
||||||||||||||||||||
| Interest expense |
(156,228 | ) | (635 | ) | (169 | ) | — | (157,032 | ) | |||||||||||
| Interest income |
7 | 677 | 349 | 1,033 | ||||||||||||||||
| Other income, net |
— | 4,817 | 1,256 | — | 6,073 | |||||||||||||||
| Intercompany income (expense), net |
131,911 | 38,335 | (2,778 | ) | (167,468 | ) | — | |||||||||||||
| Equity in loss of subsidiaries, net |
(26,123 | ) | — | — | 26,123 | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (50,433 | ) | 43,194 | (1,342 | ) | (141,345 | ) | (149,926 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (Loss) income from continuing operations before income taxes |
(50,433 | ) | 112,292 | 9,988 | (142,916 | ) | (71,069 | ) | ||||||||||||
| Income tax benefit |
— | 23,555 | 1,876 | (3,753 | ) | 21,678 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (Loss) income from continuing operations |
(50,433 | ) | 135,847 | 11,864 | (146,669 | ) | (49,391 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from discontinued operations, net |
— | (319 | ) | — | — | (319 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income |
(50,433 | ) | 135,528 | 11,864 | (146,669 | ) | (49,710 | ) | ||||||||||||
| Net income attributable to noncontrolling interests |
— | — | (723 | ) | — | (723 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income attributable to DJOFL |
$ | (50,433 | ) | $ | 135,528 | $ | 11,141 | $ | (146,669 | ) | $ | (50,433 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-50
Table of Contents
DJO Finance LLC
Condensed Consolidating Statements of Cash Flows
For the Year Ended December 31, 2009
(in thousands)
| DJOFL | Guarantors | Non- Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Cash Flows From Operating Activities: |
||||||||||||||||||||
| Net (loss) income |
$ | (50,433 | ) | $ | 135,528 | $ | 11,864 | $ | (146,669 | ) | $ | (49,710 | ) | |||||||
| Adjustments to reconcile net (loss) income to net cash provided by (used in) operating activities: |
||||||||||||||||||||
| Depreciation |
— | 23,400 | 4,992 | (496 | ) | 27,896 | ||||||||||||||
| Amortization of intangible assets |
— | 74,433 | 2,821 | — | 77,254 | |||||||||||||||
| Amortization of debt issuance costs and non-cash interest expense |
12,679 | — | — | — | 12,679 | |||||||||||||||
| Stock-based compensation expense |
— | 3,382 | — | — | 3,382 | |||||||||||||||
| Loss on disposal of assets, net |
— | 455 | 651 | (142 | ) | 964 | ||||||||||||||
| Impairment of intangible assets |
— | 6,998 | — | — | 6,998 | |||||||||||||||
| Deferred income tax benefit |
— | (26,474 | ) | (3,228 | ) | 6,012 | (23,690 | ) | ||||||||||||
| Equity in loss of subsidiaries, net |
26,123 | — | — | (26,123 | ) | — | ||||||||||||||
| Provision for doubtful accounts and sales returns |
— | 34,175 | 729 | — | 34,904 | |||||||||||||||
| Inventory reserves |
— | 6,894 | 568 | — | 7,462 | |||||||||||||||
| Gain on sales of product lines |
— | (3,058 | ) | — | — | (3,058 | ) | |||||||||||||
| Gain on disposal of discontinued operations |
— | (496 | ) | — | 103 | (393 | ) | |||||||||||||
| Changes in operating assets and liabilities, net of acquired assets and liabilities: |
||||||||||||||||||||
| Accounts receivable |
— | (17,962 | ) | 2,806 | — | (15,156 | ) | |||||||||||||
| Inventories |
— | (3,958 | ) | (705 | ) | 2,795 | (1,868 | ) | ||||||||||||
| Prepaid expenses and other assets |
141 | 17,353 | (13,718 | ) | (338 | ) | 3,438 | |||||||||||||
| Accounts payable and other current liabilities |
(2,962 | ) | (6,143 | ) | (4,456 | ) | 253 | (13,308 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash provided by (used in) operating activities |
(14,452 | ) | 244,527 | 2,324 | (164,605 | ) | 67,794 | |||||||||||||
| Cash Flows From Investing Activities: |
||||||||||||||||||||
| Purchases of property and equipment |
— | (24,601 | ) | (5,961 | ) | 1,690 | (28,872 | ) | ||||||||||||
| Cash paid in connection with acquisitions, net of cash acquired |
— | (2,580 | ) | (10,506 | ) | — | (13,086 | ) | ||||||||||||
| Proceeds received upon disposition of discontinued operations, net |
— | 21,846 | — | — | 21,846 | |||||||||||||||
| Other investing activities, net |
— | 4,112 | — | — | 4,112 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash used in investing activities |
— | (1,223 | ) | (16,467 | ) | 1,690 | (16,000 | ) | ||||||||||||
| Cash Flows From Financing Activities: |
||||||||||||||||||||
| Intercompany |
56,101 | (240,224 | ) | 21,208 | 162,915 | — | ||||||||||||||
| Proceeds from issuance of debt |
68,000 | 12 | 248 | — | 68,260 | |||||||||||||||
| Repayments of debt and capital lease obligations |
(102,650 | ) | (76 | ) | (795 | ) | — | (103,521 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash provided by (used in) financing activities |
21,451 | (240,288 | ) | 20,661 | 162,915 | (35,261 | ) | |||||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
— | — | (2,405 | ) | — | (2,405 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net increase in cash and cash equivalents |
6,999 | 3,016 | 4,113 | — | 14,128 | |||||||||||||||
| Cash and cash equivalents, beginning of year |
— | 14,373 | 16,110 | — | 30,483 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents, end of year |
$ | 6,999 | $ | 17,389 | $ | 20,223 | $ | — | $ | 44,611 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-51
Table of Contents
| 22. | SUBSEQUENT EVENTS |
On January 5, 2012, DJO announced that Mike S. Zafirovski had been elected to the Board of Directors as a member and as non-executive Chairman of the Board. In connection with his election to the Board of Directors, on January 5, 2012, the Compensation Committee granted Mr. Zafirovski the right to purchase 60,753 shares of the Company’s stock at a price of $16.46 per share, subject to execution of a stockholder agreement including certain rights and restrictions (see Note 18). Mr. Zafirovski purchased such shares on January 10, 2012, and the proceeds were contributed by DJO to us and will be used for working capital purposes.
In addition, on January 5, 2012, the Compensation Committee granted Mr. Zafirovski options to acquire 303,767 shares of the Company’s common stock at an exercise price of $16.46 per share.
F-52
Table of Contents
DJO Finance LLC
Unaudited Condensed Consolidated Balance Sheets
(in thousands)
| September 29, 2012 |
December 31, 2011 |
|||||||
| Assets | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 38,225 | $ | 38,169 | ||||
| Accounts receivable, net |
160,156 | 158,982 | ||||||
| Inventories, net |
143,801 | 128,699 | ||||||
| Deferred tax assets, net |
43,770 | 43,458 | ||||||
| Prepaid expenses and other current assets |
23,134 | 18,791 | ||||||
|
|
|
|
|
|||||
| Total current assets |
409,086 | 388,099 | ||||||
| Property and equipment, net |
104,930 | 107,108 | ||||||
| Goodwill |
1,229,941 | 1,228,778 | ||||||
| Intangible assets, net |
1,059,936 | 1,132,694 | ||||||
| Other assets |
48,148 | 38,181 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 2,852,041 | $ | 2,894,860 | ||||
|
|
|
|
|
|||||
| Liabilities and Equity | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 58,075 | $ | 57,926 | ||||
| Accrued interest |
44,829 | 20,928 | ||||||
| Current portion of debt and capital lease obligations |
8,614 | 8,820 | ||||||
| Other current liabilities |
86,088 | 81,771 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
197,606 | 169,445 | ||||||
| Long-term debt and capital lease obligations |
2,156,863 | 2,159,091 | ||||||
| Deferred tax liabilities, net |
251,284 | 252,194 | ||||||
| Other long-term liabilities |
15,187 | 16,174 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
2,620,940 | 2,596,904 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
||||||||
| Equity: |
||||||||
| DJO Finance LLC membership equity: |
||||||||
| Member capital |
839,434 | 834,871 | ||||||
| Accumulated deficit |
(611,390 | ) | (539,276 | ) | ||||
| Accumulated other comprehensive income |
315 | 218 | ||||||
|
|
|
|
|
|||||
| Total membership equity |
228,359 | 295,813 | ||||||
| Noncontrolling interests |
2,742 | 2,143 | ||||||
|
|
|
|
|
|||||
| Total equity |
231,101 | 297,956 | ||||||
|
|
|
|
|
|||||
| Total liabilities and equity |
$ | 2,852,041 | $ | 2,894,860 | ||||
|
|
|
|
|
|||||
See accompanying notes to unaudited condensed consolidated financial statements.
F-53
Table of Contents
DJO Finance LLC
Unaudited Condensed Consolidated Statements of Operations
(in thousands)
| Three Months Ended | Nine Months Ended | |||||||||||||||
| September 29, 2012 |
October 1, 2011 |
September 29, 2012 |
October 1, 2011 |
|||||||||||||
| Net sales |
$ | 273,986 | $ | 263,118 | $ | 838,910 | $ | 790,615 | ||||||||
| Cost of sales (exclusive of amortization of intangible assets of $9,837 and $29,513 for the three and nine months ended September 29, 2012 and $9,688 and $28,831 for the three and nine months ended October 1, 2011, respectively) |
108,297 | 107,463 | 328,334 | 311,709 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
165,689 | 155,655 | 510,576 | 478,906 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Selling, general and administrative |
110,735 | 115,854 | 342,617 | 361,761 | ||||||||||||
| Research and development |
7,938 | 6,477 | 21,695 | 19,721 | ||||||||||||
| Amortization of intangible assets |
24,487 | 24,435 | 73,505 | 69,373 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| 143,160 | 146,766 | 437,817 | 450,855 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating income |
22,529 | 8,889 | 72,759 | 28,051 | ||||||||||||
| Other income (expense): |
||||||||||||||||
| Interest expense |
(46,411 | ) | (42,764 | ) | (134,899 | ) | (126,320 | ) | ||||||||
| Interest income |
46 | 77 | 151 | 240 | ||||||||||||
| Loss on modification and extinguishment of debt |
— | — | (9,398 | ) | (2,065 | ) | ||||||||||
| Other income (expense), net |
1,870 | (6,004 | ) | 2,931 | (1,551 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (44,495 | ) | (48,691 | ) | (141,215 | ) | (129,696 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before income taxes |
(21,966 | ) | (39,802 | ) | (68,456 | ) | (101,645 | ) | ||||||||
| Income tax (provision) benefit |
(569 | ) | 14,096 | (3,044 | ) | 36,055 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(22,535 | ) | (25,706 | ) | (71,500 | ) | (65,590 | ) | ||||||||
| Net income attributable to noncontrolling interests |
(27 | ) | (58 | ) | (614 | ) | (668 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to DJO Finance LLC |
$ | (22,562 | ) | $ | (25,764 | ) | $ | (72,114 | ) | $ | (66,258 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
See accompanying notes to unaudited condensed consolidated financial statements.
F-54
Table of Contents
DJO Finance LLC
Unaudited Condensed Consolidated Statements of Comprehensive Loss
(in thousands)
| Three Months Ended | Nine Months Ended | |||||||||||||||
| September 29, 2012 |
October 1, 2011 |
September 29, 2012 |
October 1, 2011 |
|||||||||||||
| Net loss |
$ | (22,535 | ) | $ | (25,706 | ) | $ | (71,500 | ) | $ | (65,590 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other comprehensive (loss) income, net of taxes: |
||||||||||||||||
| Foreign currency translation adjustments, net of tax benefit (provision) of $(1,488) and $(546) for the three and nine months ended September 29, 2012 and $3,173 and $(450) for the three and nine months ended October 1, 2011, respectively |
2,119 | (5,790 | ) | 82 | 817 | |||||||||||
| Unrealized loss on cash flow hedges, net of tax benefit of $4 and $171 for the three and nine months ended October 1, 2011, respectively |
— | (6 | ) | — | (265 | ) | ||||||||||
| Reclassification adjustment for losses on cash flow hedges included in net loss, net of tax (provision) of $(693) and $(2,095) for the three and nine months ended October 1, 2011, respectively |
— | 1,115 | — | 3,290 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other comprehensive (loss) income |
2,119 | (4,681 | ) | 82 | 3,842 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Comprehensive loss |
(20,416 | ) | (30,387 | ) | (71,418 | ) | (61,748 | ) | ||||||||
| Comprehensive loss (income) attributable to noncontrolling interests |
(97 | ) | 139 | (599 | ) | (700 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Comprehensive loss attributable to DJO Finance LLC |
$ | (20,513 | ) | $ | (30,248 | ) | $ | (72,017 | ) | $ | (62,448 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
See accompanying notes to unaudited condensed consolidated financial statements.
F-55
Table of Contents
DJO Finance LLC
Unaudited Condensed Consolidated Statement of Equity
(in thousands)
| DJO Finance LLC | ||||||||||||||||||||||||
| Member capital |
Accumulated deficit |
Accumulated other comprehensive income |
Total membership equity |
Noncontrolling interests |
Total equity |
|||||||||||||||||||
| Balance at December 31, 2011 |
$ | 834,871 | $ | (539,276 | ) | $ | 218 | $ | 295,813 | $ | 2,143 | $ | 297,956 | |||||||||||
| Net (loss) income |
— | (72,114 | ) | — | (72,114 | ) | 614 | (71,500 | ) | |||||||||||||||
| Other comprehensive income (loss), net of taxes |
— | — | 97 | 97 | (15 | ) | 82 | |||||||||||||||||
| Stock-based compensation |
3,563 | — | — | 3,563 | — | 3,563 | ||||||||||||||||||
| Investment by parent |
1,000 | — | — | 1,000 | — | 1,000 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at September 29, 2012 |
$ | 839,434 | $ | (611,390 | ) | $ | 315 | $ | 228,359 | $ | 2,742 | $ | 231,101 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
See accompanying notes to unaudited condensed consolidated financial statements.
F-56
Table of Contents
DJO Finance LLC
Unaudited Condensed Consolidated Statements of Cash Flows
(in thousands)
| Nine Months Ended | ||||||||
| September 29, 2012 |
October 1, 2011 |
|||||||
| Cash Flows From Operating Activities: |
||||||||
| Net loss |
$ | (71,500 | ) | $ | (65,590 | ) | ||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: |
||||||||
| Depreciation |
22,673 | 21,305 | ||||||
| Amortization of intangible assets |
73,505 | 69,373 | ||||||
| Amortization of debt issuance costs and non-cash interest expense |
7,729 | 6,270 | ||||||
| Stock-based compensation expense |
3,563 | 1,700 | ||||||
| Loss on modification and extinguishment of debt |
9,398 | — | ||||||
| Loss on disposal of assets, net |
936 | 683 | ||||||
| Deferred income tax benefit |
(1,836 | ) | (43,074 | ) | ||||
| Provision for doubtful accounts and sales returns |
16,442 | 22,455 | ||||||
| Inventory reserves |
4,440 | 5,812 | ||||||
| Changes in operating assets and liabilities, net of acquired assets and liabilities: |
||||||||
| Accounts receivable |
(17,677 | ) | (18,831 | ) | ||||
| Inventories |
(16,568 | ) | (11,450 | ) | ||||
| Prepaid expenses and other assets |
(3,670 | ) | 2,277 | |||||
| Accrued interest |
23,897 | 37,280 | ||||||
| Accounts payable and other current liabilities |
(29 | ) | 11,908 | |||||
|
|
|
|
|
|||||
| Net cash provided by operating activities |
51,303 | 40,118 | ||||||
| Cash Flows From Investing Activities: |
||||||||
| Cash paid in connection with acquisitions, net of cash acquired |
— | (317,669 | ) | |||||
| Purchases of property and equipment |
(23,448 | ) | (29,217 | ) | ||||
| Other investing activities, net |
(656 | ) | (1,479 | ) | ||||
|
|
|
|
|
|||||
| Net cash used in investing activities |
(24,104 | ) | (348,365 | ) | ||||
| Cash Flows from Financing Activities: |
||||||||
| Proceeds from issuance of debt |
751,700 | 400,000 | ||||||
| Repayments of debt and capital lease obligations |
(754,824 | ) | (83,620 | ) | ||||
| Payment of debt issuance costs |
(25,234 | ) | (7,612 | ) | ||||
| Investment by parent |
1,000 | 3,176 | ||||||
| Cancellation of vested options |
— | (2,000 | ) | |||||
| Dividend paid by subsidiary to owners of noncontrolling interests |
— | (682 | ) | |||||
|
|
|
|
|
|||||
| Net cash (used in) provided by financing activities |
(27,358 | ) | 309,262 | |||||
| Effect of exchange rate changes on cash and cash equivalents |
215 | 176 | ||||||
|
|
|
|
|
|||||
| Net increase in cash and cash equivalents |
56 | 1,191 | ||||||
| Cash and cash equivalents at beginning of period |
38,169 | 38,132 | ||||||
|
|
|
|
|
|||||
| Cash and cash equivalents at end of period |
$ | 38,225 | $ | 39,323 | ||||
|
|
|
|
|
|||||
| Supplemental disclosures of cash flow information: |
||||||||
| Cash paid for interest |
$ | 103,351 | $ | 78,549 | ||||
| Cash paid (refunded) for taxes, net |
$ | 3,198 | $ | (3,932 | ) | |||
| Non-cash investing and financing activities: |
||||||||
| Increases in property and equipment and in other current liabilities in connection with capitalized software costs |
$ | — | $ | 258 | ||||
See accompanying notes to unaudited condensed consolidated financial statements.
F-57
Table of Contents
DJO Finance LLC
Notes to Unaudited Condensed Consolidated Financial Statements
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Organization and Business
We are a global developer, manufacturer and distributor of high-quality medical devices that provide solutions for musculoskeletal health, vascular health and pain management. Our products address the continuum of patient care from injury prevention to rehabilitation after surgery, injury or from degenerative disease, enabling people to regain or maintain their natural motion. Our products are used by orthopedic specialists, spine surgeons, primary care physicians, pain management specialists, physical therapists, podiatrists, chiropractors, athletic trainers and other healthcare professionals. In addition, many of our medical devices and related accessories are used by athletes and patients for injury prevention and at-home physical therapy treatment. Our product lines include rigid and soft orthopedic bracing, hot and cold therapy, bone growth stimulators, vascular therapy systems and compression garments, therapeutic shoes and inserts, electrical stimulators used for pain management and physical therapy products. Our surgical implant business offers a comprehensive suite of reconstructive joint products for the hip, knee and shoulder. Our products are marketed under a portfolio of brands including Aircast®, DonJoy®, ProCare®, CMF™, Empi®, Chattanooga, DJO Surgical, Dr. Comfort ™ and Compex®. Substantially all business activities of DJO Global, Inc. (DJO) are conducted by DJO Finance LLC (DJOFL) and its wholly owned subsidiaries. Except as otherwise indicated, references to “us,” “we,” “DJOFL,” “our,” or “the Company,” refers to DJOFL and its consolidated subsidiaries.
Fiscal Year
We operate our business on a manufacturing calendar, with our fiscal year always ending on December 31. Each quarter is 13 weeks, consisting of two four-week periods and one five-week period. Our first and fourth quarters may have more or fewer shipping days from year to year based on the days of the week on which holidays and December 31 fall. For our domestic business segments, the three months ended September 29, 2012 and October 1, 2011 each included 63 shipping days and the nine months ended September 29, 2012 and October 1, 2011 each included 191 shipping days.
Segment Reporting
We market and distribute our products through four operating segments, Bracing and Vascular, Recovery Sciences, Surgical Implant, and International. Our Bracing and Vascular, Recovery Sciences, and Surgical Implant segments generate their revenues within the United States. Our Bracing and Vascular segment offers rigid knee braces, orthopedic soft goods, cold therapy products, vascular systems, compression therapy products and therapeutic footwear for the diabetes care market. Our Recovery Sciences segment offers home electrotherapy, iontophoresis, home traction products, bone growth stimulation products and clinical therapy equipment. Our Surgical Implant segment offers a comprehensive suite of reconstructive joint products for the knee, hip and shoulder. Our International segment offers all of our products to customers outside the United States. See Note 14 for additional information about our reportable segments.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Estimates and assumptions are used in accounting for, among other things, contractual allowances, rebates, product returns, warranty obligations, allowances for doubtful accounts, valuation of inventories, self-insurance reserves, income taxes, loss contingencies, fair values of derivative instruments, fair values of long-lived assets and any related impairments, capitalization of costs associated with internally developed software and stock-based compensation. Actual results could differ from those estimates.
F-58
Table of Contents
Basis of Presentation
We consolidate the results of operations of our 50% owned subsidiary, Medireha GmbH (Medireha), and reflect the 50% share of results not owned by us as noncontrolling interests in our consolidated statements of operations. We maintain control of Medireha through certain rights that enable us to prohibit certain business activities that are not consistent with our plans for the business and provide us with exclusive distribution rights for products manufactured by Medireha.
The accompanying unaudited condensed consolidated financial statements include our accounts and all voting interest entities where we exercise a controlling financial interest through the ownership of a direct or indirect majority voting interest. All significant intercompany accounts and transactions have been eliminated in consolidation.
Our unaudited condensed consolidated financial statements have been prepared in accordance with GAAP and with the instructions to Form 10-Q and Article 10 of Regulation S-X for interim financial information. Accordingly, these financial statements do not include all of the information required by GAAP or Securities and Exchange Commission (SEC) rules and regulations for complete financial statements. In the opinion of management, these financial statements reflect all adjustments (consisting of normal recurring adjustments) necessary for a fair presentation of the results of operations for the interim periods presented. The results of operations for any interim period are not necessarily indicative of results for the full year. These unaudited condensed consolidated financial statements should be read in conjunction with our consolidated financial statements and notes thereto included in our Annual Report on Form 10-K for the year ended December 31, 2011.
Certain prior period amounts have been reclassified to conform to the current year presentation.
Recent Accounting Standards
In May 2011, the FASB issued guidance to amend the requirements related to fair value measurement which changes the wording used to describe many requirements in GAAP for measuring fair value and for disclosing information about fair value measurements. Additionally, the amendments clarify the FASB’s intent about the application of existing fair value measurement requirements. The amended guidance was effective for interim and annual periods beginning after December 15, 2011 and was applied prospectively. The Company adopted this guidance during the first quarter of fiscal year 2012. Adoption of this guidance did not have a material impact on the Company’s consolidated financial statements.
In June 2011, the FASB issued guidance to amend the presentation of comprehensive income to allow an entity the option to present the total of comprehensive income, the components of net income and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. In both choices, an entity is required to present each component of net income along with total net income, each component of other comprehensive income along with a total for other comprehensive income and a total amount for comprehensive income. The guidance eliminates the option to present the components of other comprehensive income as part of the statement of changes in stockholders’ equity. The amended guidance was effective for interim and annual periods beginning after December 15, 2011, and was applied retrospectively. The Company adopted this guidance during the first quarter of fiscal year 2012. Adoption of this guidance did not have a material impact on the Company’s consolidated financial statements.
In July 2012, the FASB issued an accounting standard update regarding testing of intangible assets for impairment. This standard update allows companies the option to perform a qualitative assessment to determine whether it is more likely than not that an indefinite-lived intangible asset is impaired. An entity is not required to calculate the fair value of an indefinite-lived intangible asset and perform the quantitative impairment test unless the entity determines that it is more likely than not the asset is impaired. We will adopt this standard update during the first quarter of 2013. The adoption of this standard is not expected to have a significant impact on the Company’s consolidated financial statements.
F-59
Table of Contents
2. ACCOUNTS RECEIVABLE RESERVES
A summary of activity in our accounts receivable reserves for doubtful accounts and sales returns is presented below (in thousands):
| Nine Months Ended | ||||||||
| September 29, 2012 |
October 1, 2011 |
|||||||
| Balance, beginning of period |
$ | 38,315 | $ | 53,076 | ||||
| Provision for doubtful accounts and sales returns |
16,442 | 22,455 | ||||||
| Write-offs, net of recoveries |
(23,666 | ) | (24,288 | ) | ||||
|
|
|
|
|
|||||
| Balance, end of period |
$ | 31,091 | $ | 51,243 | ||||
|
|
|
|
|
|||||
3. INVENTORIES
Inventories consist of the following (in thousands):
| September 29, 2012 |
December 31, 2011 |
|||||||
| Components and raw materials |
$ | 47,411 | $ | 50,322 | ||||
| Work in process |
5,310 | 4,681 | ||||||
| Finished goods |
83,966 | 60,839 | ||||||
| Inventory held on consignment |
23,846 | 27,003 | ||||||
|
|
|
|
|
|||||
| 160,533 | 142,845 | |||||||
| Less inventory reserves |
(16,732 | ) | (14,146 | ) | ||||
|
|
|
|
|
|||||
| $ | 143,801 | $ | 128,699 | |||||
|
|
|
|
|
|||||
A summary of the activity in our reserves for estimated slow moving, excess, obsolete and otherwise impaired inventory is presented below (in thousands):
| Nine Months Ended | ||||||||
| September 29, 2012 |
October 1, 2011 |
|||||||
| Balance, beginning of period |
$ | 14,146 | $ | 12,853 | ||||
| Provision charged to cost of sales |
4,440 | 5,812 | ||||||
| Write-offs, net of recoveries |
(1,854 | ) | (3,539 | ) | ||||
|
|
|
|
|
|||||
| Balance, end of period |
$ | 16,732 | $ | 15,126 | ||||
|
|
|
|
|
|||||
The write-offs to the reserve were primarily related to the disposition of fully reserved inventory.
4. LONG-LIVED ASSETS
Goodwill
Changes in the carrying amount of our goodwill for the nine months ended September 29, 2012 are presented below (in thousands):
| Balance, beginning of period |
$ | 1,228,778 | ||
| Foreign currency translation adjustments |
1,163 | |||
|
|
|
|||
| Balance, end of period |
$ | 1,229,941 | ||
|
|
|
F-60
Table of Contents
Intangible assets, net
Identifiable intangible assets consisted of the following (in thousands):
| September 29, 2012 |
Gross Carrying Amount |
Accumulated Amortization |
Intangible Assets, Net |
|||||||||
| Definite-lived intangible assets: |
||||||||||||
| Customer relationships |
$ | 570,037 | $ | (222,422 | ) | $ | 347,615 | |||||
| Patents and technology |
460,961 | (186,158 | ) | 274,803 | ||||||||
| Trademarks and trade names |
23,650 | (3,565 | ) | 20,085 | ||||||||
| Distributor contracts and relationships |
5,226 | (1,825 | ) | 3,401 | ||||||||
| Non-compete agreements |
3,654 | (1,518 | ) | 2,136 | ||||||||
|
|
|
|
|
|
|
|||||||
| $ | 1,063,528 | $ | (415,488 | ) | 648,040 | |||||||
|
|
|
|
|
|||||||||
| Indefinite-lived intangible assets: |
||||||||||||
| Trademarks and trade names |
411,896 | |||||||||||
|
|
|
|||||||||||
| Net identifiable intangible assets |
$ | 1,059,936 | ||||||||||
|
|
|
|||||||||||
| December 31, 2011 |
Gross Carrying Amount |
Accumulated Amortization |
Intangible Assets, Net |
|||||||||
| Definite-lived intangible assets: |
||||||||||||
| Customer relationships |
$ | 569,528 | $ | (181,396 | ) | $ | 388,132 | |||||
| Patents and technology |
460,624 | (156,290 | ) | 304,334 | ||||||||
| Trademarks and trade names |
23,650 | (1,807 | ) | 21,843 | ||||||||
| Distributor contracts and relationships |
5,089 | (1,206 | ) | 3,883 | ||||||||
| Non-compete agreements |
3,636 | (883 | ) | 2,753 | ||||||||
|
|
|
|
|
|
|
|||||||
| $ | 1,062,527 | $ | (341,582 | ) | 720,945 | |||||||
|
|
|
|
|
|||||||||
| Indefinite-lived intangible assets: |
||||||||||||
| Trademarks and trade names |
411,749 | |||||||||||
|
|
|
|||||||||||
| Net identifiable intangible assets |
$ | 1,132,694 | ||||||||||
|
|
|
|||||||||||
Our definite-lived intangible assets are being amortized using the straight-line method over their remaining weighted average useful lives of 7.0 years for customer relationships, 10.0 years for patents and technology, 4.2 years for distributor rights, 8.5 years for trademarks and trade names, and 2.8 years for non-compete agreements. Based on our amortizable intangible asset balance as of September 29, 2012, we estimate that amortization expense will be as follows for the next five years and thereafter (in thousands):
| Remaining 2012 |
$ | 23,589 | ||
| 2013 |
91,216 | |||
| 2014 |
89,150 | |||
| 2015 |
84,690 | |||
| 2016 |
80,836 | |||
| Thereafter |
278,559 | |||
|
|
|
|||
| $ | 648,040 | |||
|
|
|
F-61
Table of Contents
5. OTHER CURRENT LIABILITIES
Other current liabilities consist of the following (in thousands):
| September 29, 2012 |
December 31, 2011 |
|||||||
| Accrued wages and related expenses |
$ | 33,378 | $ | 29,856 | ||||
| Accrued commissions |
12,737 | 12,831 | ||||||
| Income taxes payable |
4,205 | 2,878 | ||||||
| Accrued rebates |
6,141 | 6,027 | ||||||
| Accrued other taxes |
3,995 | 4,195 | ||||||
| Accrued professional expenses |
4,248 | 3,927 | ||||||
| Derivative liabilities |
— | 545 | ||||||
| Other accrued liabilities |
21,384 | 21,512 | ||||||
|
|
|
|
|
|||||
| $ | 86,088 | $ | 81,771 | |||||
|
|
|
|
|
|||||
6. DERIVATIVE INSTRUMENTS
From time to time, we use derivative financial instruments to manage interest rate risk related to our variable rate credit facilities and risk related to foreign currency exchange rates. Our objective is to reduce the risk to earnings and cash flows associated with changes in foreign currency exchange rates. Before acquiring a derivative instrument to hedge a specific risk, we evaluate potential natural hedges. Factors considered in the decision to hedge an underlying market exposure include the materiality of the risk, the volatility of the market, the duration of the hedge, and the availability, effectiveness and cost of derivative instruments. We do not use derivative instruments for speculative or trading purposes.
All derivatives, whether designated as hedging relationships or not, are recorded on the balance sheet at fair value. The fair value of our derivatives are determined through the use of models that consider various assumptions, including time value, yield curves and other relevant economic measures which are inputs that are classified as Level 2 in the valuation hierarchy. The classification of gains and losses resulting from changes in the fair values of derivatives is dependent on the intended use of the derivative and its resulting designation.
Interest Rate Swap Agreements. Historically, we have used interest rate swaps from time to time to manage the risk of unfavorable movements in interest rates on a portion of the then outstanding loan balance under our senior secured credit facility. In August 2009, we entered into four interest rate swap agreements with notional amounts aggregating $300.0 million, and a weighted average fixed LIBOR rate of 2.5825%.
Our interest rate swap agreements were designated as cash flow hedges for accounting purposes, and the hedges were considered effective. As such, the effective portion of the gain or loss on the derivative instrument was reported as a component of accumulated other comprehensive income (loss) and reclassified into interest expense in our consolidated statement of operations in the period in which it affected income (loss).
These interest rate swap agreements expired on December 31, 2011. We currently have no interest rate swap agreements in place.
Foreign Currency Exchange Rate Contracts. We utilize Mexican Peso (MXN) foreign exchange forward contracts to hedge a portion of our exposure to fluctuations in foreign currency exchange rates, as our Mexico-based manufacturing operations incur costs that are largely denominated in MXN. Foreign currency exchange forward contracts held as of September 29, 2012 expire weekly through June 2013. While our foreign currency exchange forward contracts act as economic hedges, we have not designated such instruments as hedges for accounting purposes. Therefore, gains and losses resulting from changes in the fair values of these derivative instruments are recorded in other income (expense), net, in our unaudited condensed consolidated statements of operations.
F-62
Table of Contents
Information regarding the notional amounts of our foreign exchange forward contracts is presented in the table below (in thousands):
| Notional Amount (MXN) | Notional Amount (USD) | |||||||||||||||
| September 29, 2012 |
December 31, 2011 |
September 29, 2012 |
December 31, 2011 |
|||||||||||||
| Foreign currency exchange contracts not designated as hedges |
186,230 | 197,900 | $ | 12,874 | $ | 14,576 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The following table summarizes the location and fair value of derivative instruments in our unaudited condensed consolidated balance sheets (in thousands):
| Balance Sheet Location | September 29, 2012 |
December 31, 2011 |
||||||||
| Derivative Assets: |
||||||||||
| Foreign currency exchange forward contracts not designated as hedges |
Other current assets | $ | 1,459 | $ | — | |||||
|
|
|
|
|
|||||||
| Derivative Liabilities: |
||||||||||
| Foreign currency exchange forward contracts not designated as hedges |
Other current liabilities | $ | — | $ | 545 | |||||
|
|
|
|
|
|||||||
The following table summarizes the effect of derivative instruments on our unaudited condensed consolidated statements of operations (in thousands):
| Three Months Ended | Nine Months Ended | |||||||||||||||||
| Location of gain (loss) |
September 29, 2012 |
October 1, 2011 |
September 29, 2012 |
October 1, 2011 |
||||||||||||||
| Interest rate swap agreements designated as cash flow hedges |
Interest expense (1) | $ | — | $ | (1,807 | ) | $ | — | $ | (5,384 | ) | |||||||
| Foreign currency exchange forward contracts not designated as hedges |
Other income (expense), net | 281 | (2,121 | ) | 2,004 | (2,088 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| $ | 281 | $ | (3,928 | ) | $ | 2,004 | $ | (7,472 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Represents the loss on derivative instruments designated as cash flow hedges, which has been reclassified from accumulated other comprehensive income (loss) into interest expense during the periods presented. |
The pre-tax loss on derivative instruments designated as cash flow hedges recognized in other comprehensive income (loss) is presented below (in thousands):
| Three Months Ended | Nine Months Ended | |||||||||||||||
| September 29, 2012 |
October 1, 2011 |
September 29, 2012 |
October 1, 2011 |
|||||||||||||
| Interest rate swaps designated as cash flow hedges |
$ | — | $ | 9 | $ | — | $ | 435 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-63
Table of Contents
7. FAIR VALUE MEASUREMENTS
Financial assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurements. Our assessment of the significance of a particular input to the fair value measurements requires judgment and may affect the valuation of the assets and liabilities being measured and their placement within the fair value hierarchy. The following tables present the balances of assets and liabilities measured at fair value on a recurring basis (in thousands):
| As of September 29, 2012 |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Recorded Balance |
||||||||||||
| Assets: |
||||||||||||||||
| Foreign currency exchange forward contracts not designated as hedges |
$ | — | $ | 1,459 | $ | — | $ | 1,459 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| As of December 31, 2011 |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Recorded Balance |
||||||||||||
| Liabilities: |
||||||||||||||||
| Foreign currency exchange forward contracts not designated as hedges |
$ | — | $ | 545 | $ | — | $ | 545 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
8. DEBT AND CAPITAL LEASE OBLIGATIONS
Debt and capital lease obligations consists of the following (in thousands):
| September 29, 2012 |
December 31, 2011 |
|||||||
| Amended Senior Secured Credit Facility: |
||||||||
| Revolving Credit Facility |
$ | 38,000 | $ | — | ||||
| $452.7 million New Term Loans, net of unamortized original issue discount of $7.7 million |
445,062 | — | ||||||
| $386.5 million Extended Term Loans, net of unamortized original issue discount of $1.6 million |
384,868 | — | ||||||
| Original Senior Secured Credit Facility: |
||||||||
| Original Revolving Credit Facility |
— | 51,000 | ||||||
| Original Term Loans, net of unamortized original issue discount of $4.4 million |
— | 838,591 | ||||||
| 8.75% second priority senior secured notes |
230,000 | — | ||||||
| 10.875% senior unsecured notes, including unamortized original issue premium of $2.5 million and $3.3 million, respectively |
467,541 | 678,282 | ||||||
| 7.75% senior unsecured notes |
300,000 | 300,000 | ||||||
| 9.75% senior subordinated notes |
300,000 | 300,000 | ||||||
| Capital lease obligations and other |
6 | 38 | ||||||
|
|
|
|
|
|||||
| Total debt and capital lease obligations |
2,165,477 | 2,167,911 | ||||||
| Current maturities |
(8,614 | ) | (8,820 | ) | ||||
|
|
|
|
|
|||||
| Long-term debt and capital lease obligations |
$ | 2,156,863 | $ | 2,159,091 | ||||
|
|
|
|
|
|||||
F-64
Table of Contents
Senior Secured Credit Facility
On November 20, 2007, we entered into a Senior Secured Credit Facility (Original Senior Secured Credit Facility) consisting of a $1,065.0 million term loan facility maturing in May 2014 (Original Term Loans) and a $100.0 million revolving credit facility maturing in November 2013 (Original Revolving Credit Facility). We issued the Original Term Loans at a 1.2% discount.
On March 20, 2012, we entered into an Amendment and Restatement Agreement to amend and restate our Original Senior Secured Credit Facility, as amended (Amended Senior Secured Credit Facility), which (1) permitted the issuance of $230.0 million aggregate principal of 8.75% second priority senior secured notes (as defined and further described below) (2) extended the maturity of $564.7 million of term loans outstanding under the Original Senior Secured Credit Facility to the earlier of November 1, 2016 or August 15, 2014, if on such date the aggregate principal amount of our 10.875% senior unsecured notes (as defined and further described below) is not less than $150.0 million (Extended Term Loans); (3) provided for the issuance of a new tranche of $350.0 million of term loans that will mature on the earlier of September 15, 2017 or August 15, 2014, if on such date the aggregate outstanding principal amount of our 10.875% senior unsecured notes is not less than $150.0 million; (4) deemed certain previous acquisitions and investments to be permitted under the terms of the Amended Senior Secured Credit Facility; (5) increased the total net leverage ratio limitation in the permitted acquisitions covenant from 7.0x to 7.5x; (6) changed the financial maintenance covenant from a senior secured leverage ratio covenant to a new Senior Secured first lien leverage ratio covenant; and (7) replaced our Original Revolving Credit Facility with a new $100.0 million revolving credit facility (Revolving Credit Facility) which matures on the earlier of March 14, 2017 or August 15, 2014, if on such date the aggregate principal amount of 10.875% senior unsecured notes is not less than $150.0 million.
On March 30, 2012, we entered into Amendment No. 1 to our Amended Senior Secured Credit Facility which among other things, provided for the issuance of an additional $105.0 million of new term loans that will mature on the earlier of September 15, 2017 or August 15, 2014, if on such date the outstanding aggregate principal amount of our 10.875% senior unsecured notes is not less than $150.0 million. The net proceeds from the issuance were used to repay $103.5 million aggregate principal of Original Term Loans under the Original Senior Secured Credit Facility and pay related fees, premiums and expenses.
Collectively, the $350.0 million of new term loans issued on March 20, 2012 and the $105.0 million of new term loans issued on March 30, 2012 are referred to as the New Term Loans.
The New Term Loans were issued at a discount as follows: $350.0 million of the New Term Loans were issued at a 1.5% discount and $105.0 million of the New Term Loans were issued at a 1.0% discount. The original issue discounts are being amortized over the term of the loans using the effective interest method.
The net proceeds from the New Term Loans were used to repay the remaining portion of the Original Term Loans maturing in May 2014 and to repay a portion of the Extended Term Loans.
As of September 29, 2012, the market values of our Amended Senior Secured Credit Facility and Revolving Credit Facility were $845.5 million and $35.0 million, respectively. We determined market value using trading prices for our credit facilities on or near that date.
Interest Rates. The interest rate margins applicable to borrowings under the Revolving Credit Facility are, at our option, either (a) the Eurodollar rate plus 475 basis points or (b) a base rate plus 375 basis points. The interest rate margins applicable to the Extended Term Loans and the New Term Loans are, at our option, either (a) the Eurodollar rate plus 500 basis points or (b) a base rate plus 400 basis points. There is a minimum LIBOR rate applicable to the Eurodollar component of interest rates on New Term Loan borrowings of 1.25%. The applicable margin for borrowings under the Revolving Credit Facility, the Extended Term Loans and the New Term Loans may be reduced, subject to our attaining certain leverage ratios. As of September 29, 2012, our weighted average interest rate for all borrowings under the Amended Senior Secured Credit Facility was 5.74%.
F-65
Table of Contents
Fees. In addition to paying interest on outstanding principal under the Amended Senior Secured Credit Facility, we are required to pay a commitment fee to the lenders under the Revolving Credit Facility with respect to the unutilized commitments thereunder. The current commitment fee rate is 0.50% per annum, subject to step-downs based upon the achievement of certain leverage ratios. We must also pay customary letter of credit fees.
Principal Payments. We are required to pay annual payments in equal quarterly installments on the New Term Loans in an amount equal to 1.00% of the funded total principal amount through June 2017, with any remaining amount payable in full at maturity in September 2017. We are required to pay annual payments in equal quarterly installments on the Extended Term Loans in an amount equal to 1.00% of the funded total principal amount through September 2016, with any remaining amount payable in full at maturity in November 2016.
Prepayments. The Amended Senior Secured Credit Facility requires us to prepay outstanding term loans, subject to certain exceptions, with (1) 50% (which percentage can be reduced to 25% or 0% upon our attaining certain leverage ratios) of our annual excess cash flow, as defined; (2) 100% of the net cash proceeds above an annual amount of $25.0 million from non-ordinary course asset sales (including insurance and condemnation proceeds) by DJOFL and its restricted subsidiaries, subject to certain exceptions to be agreed upon, including a 100% reinvestment right if reinvested or committed to reinvest within 15 months of such sale or disposition so long as reinvestment is completed within 180 days thereafter; and (3) 100% of the net cash proceeds from issuance or incurrence of debt by DJOFL and its restricted subsidiaries, other than proceeds from debt permitted to be incurred under the Amended Senior Secured Credit Facility and related amendments. Any mandatory prepayments are applied to the term loan facility in direct order of maturity. We were not required to make any prepayments in 2012 related to our 2011 excess cash flow calculation.
Subject to certain exceptions, voluntary prepayments of the Extended Term Loans and the New Term Loans within one year of the effective date of Amendment No. 1 are subject to a 1.0% “soft call” premium, while other voluntary prepayments of outstanding loans under the Amended Senior Secured Credit Facility may be made at any time without premium or penalty, provided that voluntary prepayments of Eurodollar loans made on a date other than the last day of an interest period applicable thereto shall be subject to customary breakage costs.
Guarantee and Security. All obligations under the Amended Senior Secured Credit Facility are unconditionally guaranteed by DJO Holdings LLC (DJO Holdings) and each existing and future direct and indirect wholly-owned domestic subsidiary of DJOFL other than immaterial subsidiaries, unrestricted subsidiaries and subsidiaries that are precluded by law or regulation from guaranteeing the obligations (collectively, the Guarantors).
All obligations under the Amended Senior Secured Credit Facility, and the guarantees of those obligations, are secured by pledges of 100% of the capital stock of DJOFL, 100% of the capital stock of each wholly-owned domestic subsidiary and 65% of the capital stock of each wholly-owned foreign subsidiary that is, in each case, directly owned by DJOFL or one of the Guarantors, and a security interest in, and mortgages on, substantially all tangible and intangible assets of DJO Holdings, DJOFL and each Guarantor.
Certain Covenants and Events of Default. The Amended Senior Secured Credit Facility contains covenants that, among other things, restrict, subject to certain exceptions, our and our subsidiaries’ ability to:
| • | incur additional indebtedness; |
| • | create liens on assets; |
| • | change fiscal years; |
| • | enter into sale and leaseback transactions; |
| • | engage in mergers or consolidations; |
| • | sell assets; |
F-66
Table of Contents
| • | pay dividends and other restricted payments; |
| • | make investments, loans or advances; |
| • | repay subordinated indebtedness; |
| • | make certain acquisitions; |
| • | engage in certain transactions with affiliates; |
| • | restrict the ability of restricted subsidiaries that are not Guarantors to pay dividends or make distributions; |
| • | amend material agreements governing our subordinated indebtedness; and |
| • | change our lines of business. |
In addition, the Amended Senior Secured Credit Facility requires us to maintain a maximum Senior Secured first lien leverage ratio of consolidated Senior Secured first lien debt to Adjusted EBITDA (as defined) of 4.25:1 for the trailing twelve months ended September 29, 2012, stepping down periodically to 3.25:1 at the end of 2016. The Amended Senior Secured Credit Facility also contains certain customary affirmative covenants and events of default. As of September 29, 2012, our actual Senior Secured first lien net leverage ratio was 3.00:1, and we were in compliance with all other applicable covenants.
8.75% Second Priority Senior Secured Notes
On March 20, 2012, DJOFL and DJO Finance Corporation (DJO Finco) (collectively, the Issuers) issued $230.0 million aggregate principal amount of 8.75% second priority senior secured notes (8.75% Notes) maturing on March 15, 2018. The 8.75% Notes are guaranteed jointly and severally and on a senior secured basis by each of DJOFL’s existing and future direct and indirect wholly-owned domestic subsidiaries that guarantee any of DJOFL’s indebtedness, or any indebtedness of DJOFL’s domestic subsidiaries, or is an obligor under the Amended Senior Secured Credit Facility.
Pursuant to a second lien security agreement among the Issuers, the guarantor party thereto and The Bank of New York Mellon, as second lien collateral agent, the 8.75% Notes are secured by second priority liens, subject to permitted liens, on certain of the Issuers’ subsidiary guarantor assets that secure borrowings under the Amended Senior Secured Credit Facility.
As of September 29, 2012, the market value of the 8.75% Notes was $244.4 million. We determined market value using trading prices for the 8.75% Notes on or near that date.
Optional Redemption. Under the agreement governing the 8.75% Notes (8.75% Indenture), prior to March 15, 2015, the Issuers have the option to redeem some or all of the 8.75% Notes for cash at a redemption price equal to 100% of the then outstanding principal balance plus an applicable make-whole premium, plus accrued and unpaid interest. Beginning on March 15, 2015, the Issuers may redeem some or all of the 8.75% Notes at a redemption price of 104.375% of the then outstanding principal balance, plus accrued and unpaid interest. The redemption price decreases to 102.188% and 100% of the then outstanding principal balance at March 15, 2016 and 2017, respectively, plus accrued and unpaid interest. Additionally, from time to time, before March 15, 2015, the Issuers may redeem up to 35% of the 8.75% Notes at a redemption price equal to 108.75% of the then outstanding principal balance, plus accrued and unpaid interest, in each case, with proceeds we raise, or a direct or indirect parent company raises, in certain offerings of equity of DJOFL or its direct or indirect parent companies, as long as at least 65% of the aggregate principal amount of the notes issued remains outstanding.
Change of Control. Upon the occurrence of a change of control, unless DJOFL has previously sent or concurrently sends a notice exercising its optional redemption rights with respect to all of the 8.75% Notes, DJOFL will be required to make an offer to repurchase all of the 8.75% Notes at 101% of the then outstanding principal balance, plus accrued and unpaid interest.
F-67
Table of Contents
Covenants. The 8.75% Indenture contains covenants limiting, among other things, our and our restricted subsidiaries’ ability to (i) incur additional indebtedness or issue certain preferred and convertible shares, pay dividends on, redeem, repurchase or make distributions in respect of the capital stock of DJO or make other restricted payments, (ii) make certain investments, (iii) sell certain assets, (iv) create liens on certain assets to secure debt, (v) consolidate, merge, sell or otherwise dispose of all or substantially all of our assets, (vi) enter into certain transactions with affiliates, or (vii) designate our subsidiaries as unrestricted subsidiaries. As of September 29, 2012, we were in compliance with all applicable covenants.
10.875% Senior Unsecured Notes
On November 20, 2007 and January 20, 2010, the Issuers issued 10.875% senior unsecured notes maturing on November 15, 2014, with aggregate principal amounts of $575.0 million and $100.0 million, respectively (10.875% Notes). The 10.875% Notes are guaranteed jointly and severally and on an unsecured senior basis by each of DJOFL’s existing and future direct and indirect wholly-owned domestic subsidiaries that guarantee any of DJOFL’s indebtedness, or any indebtedness of DJOFL’s domestic subsidiaries, or is an obligor under DJOFL’s Amended Senior Secured Credit Facility.
The $100.0 million of 10.875% Notes were issued at a 5.0% premium and we are amortizing the premium over the term of the notes using the effective interest method.
On March 20, 2012, we repurchased $210.0 million aggregate principal amount of 10.875% Notes from existing holders, using proceeds from our issuance of the $230.0 million aggregate principal amount of the 8.75% Notes. The notes were repurchased at a redemption price of 102% of the then outstanding principal balance, resulting in a total purchase price of $214.2 million. In addition, accrued and unpaid interest through the redemption date of $7.9 million was paid to the existing holders in connection with the redemption.
As of September 29, 2012, the market value of the remaining $465.0 million of aggregate principal amount of the 10.875% Notes was $481.9 million. We determined market value using trading prices for the 10.875% Notes on or near that date.
Optional Redemption. Under the agreement governing the 10.875% Notes (10.875% Indenture), prior to November 15, 2011, the Issuers had the option to redeem some or all of the 10.875% Notes for cash at a redemption price equal to 100% of the then outstanding principal balance plus an applicable make-whole premium, plus accrued and unpaid interest. Beginning on November 15, 2011, the Issuers gained the option to redeem some or all of the 10.875% Notes at a redemption price of 105.438% of the then outstanding principal balance, plus accrued and unpaid interest. The redemption price decreases to 102.719% and 100% of the then outstanding principal balance on November 15, 2012 and 2013, respectively, plus accrued and unpaid interest.
Change of Control. Upon the occurrence of a change of control, unless DJOFL has previously sent or concurrently sends a notice exercising its optional redemption rights with respect to all of the 10.875% Notes, DJOFL will be required to make an offer to repurchase all of the 10.875% Notes at 101% of the then outstanding principal balance, plus accrued and unpaid interest.
Covenants. The 10.875% Indenture contains covenants limiting, among other things, our and our restricted subsidiaries’ ability to (i) incur additional indebtedness or issue certain preferred and convertible shares, pay dividends on, redeem, repurchase or make distributions in respect of the capital stock of DJO, or make other restricted payments, (ii) make certain investments, (iii) sell certain assets, (iv) create liens on certain assets to secure debt, (v) consolidate, merge, sell or otherwise dispose of all or substantially all of our assets, (vi) enter into certain transactions with affiliates, or (vii) designate our subsidiaries as unrestricted subsidiaries. As of September 29, 2012, we were in compliance with all applicable covenants.
F-68
Table of Contents
7.75% Senior Unsecured Notes
On April 7, 2011, the Issuers issued $300.0 million aggregate principal amount of 7.75% senior unsecured notes (7.75% Notes) maturing on April 15, 2018. The 7.75% Notes are guaranteed jointly and severally and on an unsecured senior basis by each of DJOFL’s existing and future direct and indirect wholly-owned domestic subsidiaries that guarantee any of DJOFL’s indebtedness, or any indebtedness of DJOFL’s domestic subsidiaries, or is an obligor under the Amended Senior Secured Credit Facility.
As of September 29, 2012, the market value of the 7.75% Notes was $276.8 million. We determined market value using trading prices for the 7.75% Notes on or near that date.
Optional Redemption. Under the agreement governing the 7.75% Notes (7.75% Indenture), prior to April 15, 2014, the Issuers have the option to redeem some or all of the 7.75% Notes for cash at a redemption price equal to 100% of the then outstanding principal balance plus an applicable make-whole premium, plus accrued and unpaid interest. Beginning on April 15, 2014, the Issuers may redeem some or all of the 7.75% Notes at a redemption price of 105.813% of the then outstanding principal balance plus accrued and unpaid interest. The redemption price decreases to 103.875%, 101.938% and 100% of the then outstanding principal balance at April 15, 2015, 2016 and 2017, respectively, plus accrued and unpaid interest. Additionally, from time to time, before April 15, 2014, the Issuers may redeem up to 35% of the 7.75% Notes at a redemption price equal to 107.75% of the then outstanding principal balance, plus accrued and unpaid interest, in each case, with proceeds we raise, or a direct or indirect parent company raises, in certain offerings of equity of DJOFL or its direct or indirect parent companies, as long as at least 65% of the aggregate principal amount of the notes issued remains outstanding.
Change of Control. Upon the occurrence of a change of control, unless DJOFL has previously sent or concurrently sends a notice exercising its optional redemption rights with respect to all of the then outstanding 7.75% Notes, DJOFL will be required to make an offer to repurchase all of the then outstanding 7.75% Notes at 101% of the then outstanding principal balance, plus accrued and unpaid interest.
Covenants. The 7.75% Indenture contains covenants limiting, among other things, our and our restricted subsidiaries’ ability to (i) incur additional indebtedness or issue certain preferred and convertible shares, pay dividends on, redeem, repurchase or make distributions in respect of the capital stock of DJO or make other restricted payments, (ii) make certain investments, (iii) sell certain assets, (iv) create liens on certain assets to secure debt, (v) consolidate, merge, sell or otherwise dispose of all or substantially all of our assets, (vi) enter into certain transactions with affiliates, or (vii) designate our subsidiaries as unrestricted subsidiaries. As of September 29, 2012, we were in compliance with all applicable covenants.
9.75% Senior Subordinated Notes
On October 18, 2010, the Issuers issued $300.0 million aggregate principal amount of 9.75% senior subordinated notes (9.75% Notes) maturing on October 15, 2017. The 9.75% Notes are guaranteed jointly and severally and on an unsecured senior subordinated basis by each of DJOFL’s existing and future direct and indirect wholly-owned domestic subsidiaries that guarantee any of DJOFL’s indebtedness, or any indebtedness of DJOFL’s domestic subsidiaries, or by any of DJOFL’s subsidiaries that are an obligor under the Amended Senior Secured Credit Facility.
As of September 29, 2012, the market value of the 9.75% Notes was $252.0 million. We determined market value using trading prices for the 9.75% Notes on or near that date.
Optional Redemption. Under the agreement governing the 9.75% Notes (9.75% Indenture), prior to October 15, 2013, the Issuers have the option to redeem some or all of the 9.75% Notes for cash at a redemption price equal to 100% of the then outstanding principal balance plus an applicable make-whole premium, plus
F-69
Table of Contents
accrued and unpaid interest. Beginning on October 15, 2013, the Issuers may redeem some or all of the 9.75% Notes at a redemption price of 107.313% of the then outstanding principal balance, plus accrued and unpaid interest. The redemption price decreases to 104.875%, 102.438% and 100% of the then outstanding principal balance at October 15, 2014, 2015 and 2016, respectively, plus accrued and unpaid interest. Additionally, from time to time, before October 15, 2013, the Issuers may redeem up to 35% of the 9.75% Notes at a redemption price equal to 109.75% of the principal amount then outstanding, plus accrued and unpaid interest, in each case, with proceeds we raise, or a direct or indirect parent company raises, in certain offerings of equity of DJOFL or its direct or indirect parent companies, as long as at least 65% of the aggregate principal amount of the notes issued remains outstanding.
Change of Control. Upon the occurrence of a change of control, unless DJOFL has previously sent or concurrently sends a notice exercising its optional redemption rights with respect to all of the 9.75% Notes, DJOFL will be required to make an offer to repurchase all of the 9.75% Notes at 101% of the then outstanding principal balance, plus accrued and unpaid interest.
Covenants. The 9.75% Indenture contains covenants limiting, among other things, our and our restricted subsidiaries’ ability to (i) incur additional indebtedness or issue certain preferred and convertible shares, pay dividends on, redeem, repurchase or make distributions in respect of the capital stock of DJO or make other restricted payments, (ii) make certain investments, (iii) sell certain assets, (iv) create liens on certain assets to secure debt, (v) consolidate, merge, sell or otherwise dispose of all or substantially all of our assets, (vi) enter into certain transactions with affiliates, or (vii) designate our subsidiaries as unrestricted subsidiaries. As of September 29, 2012, we were in compliance with all applicable covenants.
Our ability to continue to meet the covenants related to our indebtedness specified above in future periods will depend, in part, on events beyond our control, and we may not continue to meet the ratios required by certain of those covenants. A breach of any of these covenants in the future could result in a default under the Amended Senior Secured Credit Facility or the 8.75% Indenture, the 10.875% Indenture, the 9.75% Indenture or the 7.75% Indenture (collectively, the Indentures), at which time the lenders thereunder could elect to declare all amounts outstanding under the Amended Senior Secured Credit Facility to be immediately due and payable. Any such acceleration would also result in a default under the Indentures.
Debt Issuance Costs
As of September 29, 2012 and December 31, 2011, we had incurred $42.9 million and $34.0 million, respectively, of unamortized debt issuance costs which were included in other assets in our unaudited condensed consolidated balance sheets. During the nine months ended September 29, 2012, we capitalized $15.9 million of debt issuance costs incurred in connection with the March 2012 issuance of the 8.75% Notes and the New Term Loans and the amendment and extension of our Amended Senior Secured Credit Facility.
For the three and nine months ended September 29, 2012, amortization of debt issuance costs was $2.5 million and $7.1 million, respectively. For the three and nine months ended October 1, 2011, amortization of debt issuance costs was $2.0 million and $5.8 million, respectively. Amortization of debt issuance costs is included in interest expense in our unaudited condensed consolidated statements of operations.
Loss on Modification and Extinguishment of Debt
During the nine months ended September 29, 2012, we recognized a loss on modification and extinguishment of debt of $9.4 million, consisting of $8.6 million of arrangement and amendment fees and other fees and expenses incurred in connection with the March 2012 amendment of our senior secured credit facilities and $0.8 million related to the write off of unamortized debt issuance costs and original issue discount associated with the portion of the Original Term Loans which were extinguished.
F-70
Table of Contents
Subsequent Event
During the fourth quarter we entered into transactions to repay the $465.0 million outstanding 10.875% Notes and issue $100.0 million of 8.75% Second Priority Senior Secured Notes and $440.0 million of 9.875% Senior Notes as described in Note 16.
9. INCOME TAXES
Income taxes for the interim periods presented have been included in our unaudited condensed consolidated financial statements on the basis of an estimated annual effective tax rate, adjusted for discrete items. The income tax expense for these periods differed from the amount which would have been recorded using the U.S. statutory tax rate due primarily to certain foreign and U.S. federal and state valuation allowances provided against deferred tax assets, the impact of nondeductible expenses, foreign taxes, and deferred taxes on the assumed repatriation of foreign earnings.
For the three and nine months ended September 29, 2012, we recorded income tax expense of approximately $0.6 million and $3.0 million, respectively, on pre-tax losses of $22.0 million and $68.5 million, respectively, resulting in negative effective tax rates of 2.6% and 4.5%, respectively. For the three and nine months ended October 1, 2011, we recorded income tax benefits of approximately $14.1 million and $36.1 million, respectively, on pre-tax losses of approximately $39.8 million and $101.6 million, respectively, resulting in effective tax rates of 35.4% and 35.5%, respectively. The change in our effective tax rates from 2011 to 2012 primarily relates to U.S federal and state valuation allowances provided against deferred tax assets beginning in the first quarter of 2012 and differences in our projected annualized effective tax rates for each year. Given the relationship between fixed dollar tax items and pre-tax financial results, the projected annual effective tax rate can change materially based on small variations of income.
We have recorded valuation allowances against a portion of the deferred tax assets related to our 2012 U.S. federal and state net operating losses. We record net deferred tax assets to the extent we conclude that it is more likely than not that the related deferred tax assets will be realized. In making such determination, we consider all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income, tax planning strategies and recent financial operations. At this time, we cannot conclude that it is more likely than not that the benefit from certain U.S. federal and state net operating loss carryforwards will be realized. Accordingly, we have provided valuation allowances of $7.8 million and $24.7 million, respectively, on the deferred tax assets related to the net operating loss carryforwards generated in the three and nine months ended September 29, 2012. If our assumptions change and we determine that it is more likely than not that we will be able to realize the deferred tax assets related to these net operating losses, reversal of the valuation allowances we have recorded against those deferred tax assets will be recognized as a reduction of income tax expense. The establishment of valuation allowances does not preclude us from utilizing our loss carryforwards or other deferred tax assets in the future and does not impact our cash resources.
We and our subsidiaries file income tax returns in the U.S. federal jurisdiction and various states and foreign jurisdictions. With few exceptions, we are no longer subject to U.S. federal, state and local, or foreign income tax examinations by tax authorities for years before 2008. The Internal Revenue Service (IRS) completed its field examination of our 2005 and 2006 tax years during the first half of 2010. The IRS had originally proposed material adjustments related to deductions for transaction costs, stock options expense and bad debt expense included in our 2006 tax return. During the second quarter of 2012, we engaged in discussions and negotiations with IRS appeals officers with respect to the adjustments proposed by the IRS. In the fourth quarter of 2012, we received documentation from the IRS signifying the completion of the audit. As anticipated, the closing of the audit will not result in a material reduction to our currently unreserved net operating losses and will result in a reduction to our unrecognized tax benefits of approximately $7.5 million in the fourth quarter.
At September 29, 2012, our gross unrecognized tax benefits were $18.6 million, reflecting a reduction of $0.8 million, due to the expiration of certain statutes of limitation related to transfers of intellectual property,
F-71
Table of Contents
from the unrecognized amount of $19.4 million at December 31, 2011. As of September 29, 2012, we have $2.4 million accrued for interest and penalties related to these unrecognized tax benefits. To the extent all or a portion of our gross unrecognized tax benefits are recognized in the future, no U.S. federal tax benefit for related state income tax deductions would result due to the existence of the U.S. federal valuation allowance. In addition to the reduction of unrecognized tax benefits related to the completion of the IRS appeals process, we anticipate that approximately $0.6 million of unrecognized tax benefits related to transfers of intellectual property and $0.2 million of aggregate of other individually immaterial unrecognized tax benefits will decrease in the next twelve months due to the expiration of the statutes of limitation. As of September 29, 2012, we have unrecognized various foreign and U.S. state tax benefits of approximately $4.0 million, which, if recognized, would impact our effective tax rate in future periods.
10. STOCK OPTION PLANS AND STOCK-BASED COMPENSATION
Stock Option Plan
We have one active equity compensation plan, the DJO 2007 Incentive Stock Plan (2007 Plan) under which we are authorized to grant awards of stock, options, and other stock-based awards of shares of common stock of our indirect parent, DJO, subject to adjustment in certain events. In February 2012, we amended the 2007 Plan to increase the number of shares available to grant from 7,925,529 to 10,575,529.
Options issued under the 2007 Plan can be either incentive stock options or non-qualified stock options. The exercise price of stock options granted will not be less than 100% of the fair market value of the underlying shares on the date of grant and will expire no more than ten years from the date of grant.
Options granted prior to 2012 vest as follows: one-third of each stock option grant vests over a specified period of time contingent solely upon the awardees’ continued employment with us (Time-Based Tranche). Another one-third of each stock option grant will vest upon achieving a minimum return of money on invested capital (MOIC), as defined, with respect to Blackstone’s aggregate investment in DJO’s capital stock, to be achieved by Blackstone following a liquidation of all or a portion of its investment in DJO’s capital stock (Market Return Tranche). The final one-third of each stock option grant will vest based upon achieving an increased minimum return of MOIC, as defined, with respect to Blackstone’s aggregate investment in DJO’s capital stock, to be achieved by Blackstone following a liquidation of all or a portion of its investment in DJO’s capital stock (Enhanced Market Return Tranche).
Stock-Based Compensation
During the nine months ended September 29, 2012, the compensation committee granted 2,477,500 options which vest in four equal installments beginning in 2012 and for each of the three calendar years following 2012, with each such installment vesting only if the final reported financial results for such year show that the Adjusted EBITDA for such year equaled or exceeded the Adjusted EBITDA amount in the financial plan approved by DJO’s Board of Directors for such year (Performance Options). In the event that the Adjusted EBITDA in any of such four years falls short of the amount of Adjusted EBITDA in the financial plan for that year, the installment that did not therefore vest at the end of such year shall be eligible for subsequent vesting at the end of the four year vesting period if the cumulative Adjusted EBITDA for such four years equals or exceeds the cumulative Adjusted EBITDA in the financial plans for such four years and the Adjusted EBITDA in the fourth vesting year equals or exceeds the Adjusted EBITDA in the financial plan for such year. In addition, in the event of Blackstone achieving a minimum return of MOIC, as defined, with respect to Blackstone’s aggregate investment in DJO’s capital stock, following a liquidation of all or a portion of its investment in DJO’s capital stock, any unvested installments from prior years and all installments for future years shall thereupon vest.
In addition to the 2,477,500 Performance Options granted during the nine months ended September 29, 2012, we granted 426,767 stock options to members of DJO’s Board of Directors. Of these options, 303,767 were granted to DJO’s Chairman of the Board and 100,000 were granted to a newly elected board member, who has
F-72
Table of Contents
agreed to perform certain additional services to assist the Company in building its surgical business in the US and all of its businesses outside of the US. The 403,767 options vest as follows: one-third of the stock option grant will vest in increments of 33 1/3% per year on each of the first through third anniversary dates from the grant date contingent upon the optionee’s continued service; one-third of the stock option grant will vest in the same manner as the Market Return Tranche; and one-third of the stock option grant will vest in the same manner as the Enhanced Market Return Tranche. The remaining 23,000 options were granted to the other five outside directors on our Board of Directors and vest in increments of 33 1/3% per year on each of the first through third anniversary dates from the grant date, contingent upon the optionee’s continued service as a director. The time-based vesting options granted to all directors are referred to herein as Director Service Options.
The weighted average grant date fair value of the Performance Options and the Director Service Options granted during the nine months ended September 29, 2012 was $6.09.
During the nine months ended October 1, 2011, we granted 983,000 stock options to employees, including 800,000 options granted to Michael P. Mogul, our new President and Chief Executive Officer. The weighted average grant date fair value of the options in the Time-Based Tranche was $6.23 per share.
For the Performance Options granted during the nine months ended September 29, 2012, we only recognized expense for the options which have the potential to vest based on achievement of the 2012 Adjusted EBITDA target, as we believe that it is probable that this target will be met. We have not recognized expense for any of the options which have the potential to vest based on Adjusted EBITDA targets for years 2013-2015, as these targets have not yet been established and therefore we are unable to assess the probability of achieving such targets.
In each of the periods presented below, for the options granted prior to 2012 we only recognized expense for options granted to employees in the Time-Based Tranche, as achievement of the vesting targets for the performance and market components of the Market Return Tranche and Enhanced Market Return Tranche are not deemed probable at this time.
The following table summarizes certain assumptions we used to estimate the fair value of the Time-Based Tranche of stock options granted:
| Three Months Ended | Nine Months Ended | |||||||||||||||
| September 29, 2012 |
October 1, 2011 |
September 29, 2012 |
October 1, 2011 |
|||||||||||||
| Expected volatility |
37.7 | % | 34.4 | % | 35.3-37.7 | % | 34.0-34.4 | % | ||||||||
| Risk-free interest rate |
0.8 | % | 1.3 | % | 0.8-1.5 | % | 1.3-2.1 | % | ||||||||
| Expected years until exercise |
6.1 | 6.6 | 6.1-6.2 | 6.4-6.6 | ||||||||||||
| Expected dividend yield |
0.0 | % | 0.0 | % | 0.0 | % | 0.0 | % | ||||||||
We recorded non-cash stock-based compensation expense during the periods presented as follows (in thousands):
| Three Months Ended | Nine Months Ended | |||||||||||||||
| September 29, 2012 |
October 1, 2011 |
September 29, 2012 |
October 1, 2011 |
|||||||||||||
| Cost of goods sold |
$ | 66 | $ | 19 | $ | 162 | $ | 130 | ||||||||
| Operating Expenses: |
||||||||||||||||
| Selling, general and administrative |
1,215 | 341 | 3,309 | 1,533 | ||||||||||||
| Research and development |
29 | 9 | 92 | 37 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 1,310 | $ | 369 | $ | 3,563 | $ | 1,700 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-73
Table of Contents
Stock-based compensation expense for options granted to non-employees was not significant to the Company for any period presented and is included in selling, general and administrative expense in our unaudited consolidated statements of operations.
11. EQUITY
During the nine months ended September 29, 2012, DJO sold 60,753 shares of its common stock at $16.46 per share to the new Chairman of its Board of Directors, subject to execution of a stockholder agreement including certain rights and restrictions. Net proceeds of $1.0 million from the share purchases were contributed by DJO to us, and are included in member capital in our unaudited condensed consolidated balance sheet as of September 29, 2012. The proceeds were used for working capital purposes.
12. RELATED PARTY TRANSACTIONS
Blackstone Management Partners V L.L.C. (BMP), an affiliate of our major shareholder, provides certain monitoring, advisory and consulting services to us for an annual monitoring fee equal to the greater of $7.0 million or 2% of consolidated EBITDA as defined in the Transaction and Monitoring Fee Agreement, payable in the first quarter of each year. The monitoring fee agreement will continue until the earlier of November 2019, or such date as DJO and BMP may mutually determine. DJO has agreed to indemnify BMP and its affiliates, directors, officers, employees, agents and representatives from and against all liabilities relating to the services contemplated by the Transaction and Monitoring Fee Agreement and the engagement of BMP pursuant to, and the performance of BMP and its affiliates of the services contemplated by, the Transaction and Monitoring Fee Agreement. At any time in connection with or in anticipation of a change of control of DJO, a sale of all or substantially all of DJO’s assets or an initial public offering of common stock of DJO, BMP may elect to receive, in lieu of remaining annual monitoring fee payments, a single lump sum cash payment equal to the then-present value of all then-current and future annual monitoring fees payable under the Transaction and Monitoring Fee Agreement, assuming a hypothetical termination date of the agreement to be November 2019. For each of the three and nine month periods presented, we recognized $1.75 million and $5.25 million, respectively, related to the annual monitoring fee, which was recorded as a component of selling, general and administrative expense in our unaudited condensed consolidated statements of operations.
13. COMMITMENTS AND CONTINGENCIES
The manufacture and sale of orthopedic devices and related products exposes us to a significant risk of product liability claims. From time to time, we have been, and we are currently, subject to a number of product liability claims alleging that the use of our products resulted in adverse effects. Even if we are successful in defending against any liability claims, such claims could nevertheless distract our management, result in substantial costs, harm our reputation, adversely affect the sales of all our products and otherwise harm our business. If there is a significant increase in the number of product liability claims, our business could be adversely affected.
Pain Pump Litigation
We are currently named as one of several defendants in a number of product liability lawsuits involving approximately 60 plaintiffs in U.S. cases and a lawsuit in Canada which has been granted class action status, related to a disposable drug infusion pump product (pain pump) manufactured by two third party manufacturers that we distributed through our Bracing and Vascular segment. We sold pumps manufactured by one manufacturer from 1999 to 2003 and then sold pumps manufactured by a second manufacturer from 2003 to 2009. We discontinued our sale of these products in the second quarter of 2009. These cases have been brought against the manufacturers and certain distributors of these pumps. All of these lawsuits allege that the use of these pumps with certain anesthetics for prolonged periods after certain shoulder surgeries has resulted in cartilage damage to the plaintiffs. The lawsuits allege damages ranging from unspecified amounts to claims of up
F-74
Table of Contents
to $10 million. Many of the lawsuits which have been filed in the past three years have named multiple pain pump manufacturers and distributors without having established which manufacturer manufactured or sold the pump in issue. In the past three years, we have been dismissed from more than 400 cases when product identification was later established showing that we did not sell the pump in issue. At present, we are named in approximately 25 lawsuits in which product identification has yet to be determined and, as a result, we believe that we will be dismissed from a meaningful number of such cases in the future. In the past two years, we have entered into settlements with plaintiffs in approximately 70 pain pump lawsuits. Of these, we have settled approximately 40 cases in joint settlements involving our first manufacturer and we have settled approximately 30 cases involving our second manufacturer. As of September 29, 2012, the range of potential loss is not estimable.
Indemnity and Insurance Coverage Related to Pain Pump Claims
We have sought indemnity and tendered the defense of the pain pump cases to the two manufacturers who supplied these pumps to us, to their products liability carriers and to our products liability carriers. These lawsuits are about equally divided between the two manufacturers. Both manufacturers have rejected our tenders of indemnity. The base policy for one of the manufacturers contributed to our defense, but that policy has been exhausted by defense costs and settlements, as has a second policy of that manufacturer. This manufacturer has ceased operations, has little assets and no additional insurance coverage. We have asserted indemnification rights against the successor to this manufacturer and are pursuing claims against the manufacturer, its owners and its successor. This manufacturer has asserted a counterclaim for indemnity against us, alleging that we are responsible for any liability incurred by the manufacturer in connection with our sale of pain pumps supplied by this manufacturer. The base policy for the other manufacturer has been exhausted and the excess liability carriers for that manufacturer have not accepted coverage for us and are not expected to provide for our defense. We and this manufacturer have been cooperating in jointly negotiating settlements of those lawsuits in which both parties are named. Our products liability carriers have accepted coverage of these cases, subject to a reservation of the right to deny coverage for customary matters, including punitive damages and off-label promotion. In August 2010, one of our excess carriers for the period ending July 1, 2010 and for the supplemental extended reporting period (SERP) discussed below, which is insuring $10 million in excess of $25 million, informed us that it has reserved its right to rescind the policy based on an alleged failure by us and our insurance broker to disclose material information regarding the pain pump claims prior to the binding of coverage by this carrier. We disagree with this allegation and are seeking to resolve the issue with this carrier.
Pain Pump-Related Health Insurance Portability and Accountability Act (HIPAA) Subpoena
In August 2010, we were served with a subpoena under HIPAA seeking numerous documents related to our activities involving the pain pumps discussed above. The subpoena, which was issued by the United States Attorney’s Office for the Central District of California, refers to an official investigation by the DOJ and the FDA of Federal health care offenses. We have produced documents that are responsive to the subpoena. We believe that our actions related to our prior distribution of these pain pumps have been in compliance with applicable legal standards.
Pain Pump Investigation—U.S. Attorney’s Office for the Western District of Missouri
In January 2012, we became aware of a civil investigation by the United States Attorney’s Office for the Western District of Missouri regarding our previous sale and marketing of pain pump devices. The investigation relates to whether we caused false claims to be filed with government payors as a result of alleged off-label promotion of the pain pumps. We deny that we improperly promoted the pain pump devices and believe that our marketing and sales activities were in compliance with applicable legal standards.
Cold Therapy Litigation
Since mid-2010, we have been named in nine multi-plaintiff lawsuits involving a total of 210 plaintiffs, alleging that the plaintiffs had been injured following use of certain cold therapy products manufactured by the
F-75
Table of Contents
Company. The complaints are not specific as to the nature of the injuries, but allege various product liability theories, including inadequate warnings regarding the risks associated with the use of cold therapy and failure to incorporate certain safety features into the design. No specific dollar amounts of damages are alleged and as of September 29, 2012, we cannot estimate a range of potential loss. These cases have been included in a coordinated proceeding in San Diego Superior Court with a similar number of cases filed against one of our competitors. A total of ten of the plaintiffs included in the cases filed against us have been selected as the first cases to be tried, of which four of these “bellwether” cases will go to trial in April 2013. Discovery is proceeding on these bellwether cases.
Our Product Liability Insurance Coverage
We maintain product liability insurance that is subject to annual renewal. Our current policy covers claims reported between July 1, 2012 and June 30, 2013. This policy excludes coverage for claims related to both pain pump products and cold therapy products. As described below, we have other insurance which provides coverage for these excluded products. For the current policy year, we maintain coverage limits (together with excess policies) of up to $50 million, with deductibles of $500,000 per claim for claims relating to invasive products (principally our surgical implant products) and $50,000 per claim for claims relating to all other covered products, with an aggregate self-insured retention of $2 million. Starting with the 2010-2011 policy period, our products liability policy excluded claims related to pain pump products. We purchased supplemental extended reporting period (SERP) coverage for the $80 million limit product liability policy that expired on June 30, 2010, and this supplemental coverage allows us to report pain pump claims for an additional five years beyond the end of that policy period. Except for the additional excess coverage mentioned below, this SERP coverage does not provide additional limits to the aggregate $80 million limits on the 2009-2010 policy but it does provide that these limits will remain available for pain pump claims reported for an extended period of time. We also purchased additional coverage of $25 million in excess of the $80 million limits with a five year reporting period. Thus, the SERP coverage for current and future pain pump claims has a total limit of $105 million (less amounts paid for claims reported to date). Starting with the 2011-2012 policy period, our primary products liability coverage excluded claims related to cold therapy products. Concurrently with the exclusion of our cold therapy products from the 2011-2012 primary policy, we purchased SERP coverage for cold therapy product claims for injuries alleged to have occurred prior to July 1, 2011. This SERP allows us to report such cold therapy claims under our expired 2010-2011 policy which had total limits of $50 million. At that time, we also purchased separate primary and excess policies providing for a total of $5 million of coverage for claims related to cold therapy products arising from injuries alleged to have occurred after June 30, 2011, with a deductible of $250,000 per claim and an aggregate deductible of $3 million. We continued this $5 million in coverage on similar terms for the policy period commencing July 1, 2012. We believe we have adequate insurance coverage for our product liability claims.
BGS Qui Tam Action and HIPAA Subpoena
On April 15, 2009, we became aware of a qui tam action filed in Federal Court in Boston, Massachusetts in March 2005 and amended in December 2007 that names us as a defendant along with each of the other companies that manufactures and sells external bone growth stimulators, as well as The Blackstone Group L.P., an affiliate of DJO’s principal stockholder, and the principal stockholder of one of the other companies in the bone growth stimulation business. This case is captioned United States ex rel. Beirman v. Orthofix International, N.V., et al., Civil Action No. 05-10557 (D. Mass.). The case was sealed when originally filed and unsealed in March 2009. The plaintiff, or relator, alleges that the defendants have engaged in Medicare fraud and violated Federal and state false claims acts from the time of the original introduction of the devices by each defendant to the present by seeking reimbursement for bone growth stimulators as a purchased item rather than a rental item. The relator also alleges that the defendants are engaged in other marketing practices constituting violations of the Federal and various state anti-kickback statutes. On December 4, 2009, we filed a motion to dismiss the relator’s complaint. The relator filed a second amended complaint in May 2010 that, among other things, dropped The Blackstone Group as a defendant. We filed another motion to dismiss directed at the second amended complaint,
F-76
Table of Contents
and that motion was denied. The case is proceeding to the discovery phase. Shortly before becoming aware of the qui tam action, we were advised that our bone growth stimulator business was the subject of an investigation by the DOJ, and on April 10, 2009, we were served with a subpoena under HIPAA seeking numerous documents relating to the marketing and sale by us of bone growth stimulators. On September 21, 2009, we were served with a second HIPAA subpoena related to this DOJ investigation seeking additional documents relating to the marketing and sale by us of bone growth stimulators. We believe that these subpoenas are related to the DOJ’s investigation of the allegations in the qui tam action, although the DOJ has decided not to intervene in the qui tam action at this time. We believe that our marketing practices in the bone growth stimulation business are in compliance with applicable legal standards and we intend to defend this case and investigation vigorously. We can make no assurance as to the resources that will be needed to respond to these matters or the final outcome of such action and as of September 29, 2012, we cannot estimate a range of potential loss, fines or damages.
14. SEGMENT AND GEOGRAPHIC INFORMATION
We provide a broad array of orthopedic rehabilitation and regeneration products, as well as surgical implants to customers in the United States and abroad.
We currently develop, manufacture and distribute our products through the following four operating segments:
Bracing and Vascular Segment
Our Bracing and Vascular segment, which generates its revenues in the United States, offers our rigid knee bracing products, orthopedic soft goods, cold therapy products, vascular systems, therapeutic shoes and inserts and compression therapy products, primarily under our DonJoy, ProCare, Aircast and Dr. Comfort brands. This segment also includes our OfficeCare business, through which we maintain an inventory of soft goods and other products at healthcare facilities, primarily orthopedic practices, for immediate distribution to patients. The Bracing and Vascular segment primarily sells its products to orthopedic and sports medicine professionals, hospitals, podiatry practices, orthotic and prosthetic centers, home medical equipment providers and independent pharmacies.
Recovery Sciences Segment
Our Recovery Sciences segment, which generates its revenues in the United States, is divided into four main businesses:
| • | Empi. Our Empi business unit offers our home electrotherapy, iontophoresis, and home traction products. We primarily sell these products directly to patients or to physical therapy clinics. For products sold to patients, we arrange billing to the patients and their third party payors. |
| • | Regeneration. Our Regeneration business unit primarily sells our bone growth stimulation products. We sell these products either directly to patients or to independent distributors. For products sold to patients, we arrange billing to the patients and their third party payors. |
| • | Chattanooga. Our Chattanooga business unit offers products in the clinical rehabilitation market in the categories of clinical electrotherapy devices, clinical traction devices, and other clinical products and supplies such as treatment tables, continuous passive motion (CPM) devices and dry heat therapy. |
| • | Athlete Direct. Our Athlete Direct business unit offers consumers ranging from fitness enthusiasts to competitive athletes our Compex electrostimulation devices, which are used in athletic training programs to aid muscle development and to accelerate muscle recovery after training sessions. |
International Segment
Our International segment, which generates most of its revenues in Europe, sells all of our products and certain third party products through a combination of direct sales representatives and independent distributors.
F-77
Table of Contents
Surgical Implant Segment
Our Surgical Implant segment, which generates its revenues in the United States, develops, manufactures and markets a wide variety of knee, hip and shoulder implant products that serve the orthopedic reconstructive joint implant market.
Information regarding our reportable business segments is presented below (in thousands). Segment results exclude the impact of amortization of intangible assets, certain general corporate expenses and various non-recurring and integration charges, as defined by management. The accounting policies of the reportable segments are the same as the accounting policies of the Company. We allocate resources and evaluate the performance of segments based on net sales, gross profit, operating income and other non-GAAP measures, as defined. We do not allocate assets to reportable segments because a significant portion of our assets are shared by segments.
| Three Months Ended | Nine Months Ended | |||||||||||||||
| September 29, 2012 |
October 1, 2011 |
September 29, 2012 |
October 1, 2011 |
|||||||||||||
| Net sales: |
||||||||||||||||
| Bracing and Vascular |
$ | 111,212 | $ | 101,452 | $ | 329,094 | $ | 281,687 | ||||||||
| Recovery Sciences |
80,906 | 81,956 | 249,997 | 253,335 | ||||||||||||
| International |
64,671 | 64,475 | 206,649 | 207,423 | ||||||||||||
| Surgical Implant |
17,197 | 15,235 | 53,170 | 48,170 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 273,986 | $ | 263,118 | $ | 838,910 | $ | 790,615 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit: |
||||||||||||||||
| Bracing and Vascular |
$ | 57,306 | $ | 52,997 | $ | 169,148 | $ | 149,593 | ||||||||
| Recovery Sciences |
61,656 | 61,573 | 189,167 | 191,836 | ||||||||||||
| International |
34,815 | 36,472 | 114,696 | 118,376 | ||||||||||||
| Surgical Implant |
12,670 | 10,698 | 39,858 | 34,239 | ||||||||||||
| Expenses not allocated to segments and eliminations |
(758 | ) | (6,085 | ) | (2,293 | ) | (15,138 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 165,689 | $ | 155,655 | $ | 510,576 | $ | 478,906 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating income: |
||||||||||||||||
| Bracing and Vascular |
$ | 22,146 | $ | 18,844 | $ | 64,551 | $ | 54,339 | ||||||||
| Recovery Sciences |
23,054 | 21,392 | 66,920 | 68,892 | ||||||||||||
| International |
10,604 | 11,723 | 39,502 | 40,790 | ||||||||||||
| Surgical Implant |
812 | 827 | 4,746 | 2,230 | ||||||||||||
| Expenses not allocated to segments and eliminations |
(34,087 | ) | (43,897 | ) | (102,960 | ) | (138,200 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 22,529 | $ | 8,889 | $ | 72,759 | $ | 28,051 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-78
Table of Contents
Geographic Area
Following are our net sales by geographic area, based on location of customer (in thousands):
| Three Months Ended | Nine Months Ended | |||||||||||||||
| September 29, 2012 |
October 1, 2011 |
September 29, 2012 |
October 1, 2011 |
|||||||||||||
| Net sales: |
||||||||||||||||
| United States |
$ | 209,314 | $ | 198,643 | $ | 632,261 | $ | 583,193 | ||||||||
| Other Europe, Middle East and Africa |
29,729 | 30,533 | 98,146 | 98,967 | ||||||||||||
| Germany |
19,790 | 20,712 | 64,209 | 68,203 | ||||||||||||
| Australia and Asia Pacific |
6,506 | 5,708 | 19,418 | 17,333 | ||||||||||||
| Canada |
6,301 | 5,605 | 18,264 | 16,909 | ||||||||||||
| Latin America |
2,346 | 1,917 | 6,612 | 6,010 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 273,986 | $ | 263,118 | $ | 838,910 | $ | 790,615 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
15. SUPPLEMENTAL GUARANTOR CONDENSED CONSOLIDATING FINANCIAL STATEMENTS
DJOFL and its direct wholly owned subsidiary, DJO Finco, issued $575.0 million and $100.0 million of 10.875% Notes in November 2007 and January 2010, respectively, $300.0 million of 9.75% Notes in October 2010, $300.0 million of 7.75% Notes in April 2011 and $230.0 million of 8.75% Notes in March 2012. DJO Finco was formed solely to act as a co-issuer of the notes, has only nominal assets and does not conduct any operations. The Indentures generally prohibit DJO Finco from holding any assets, becoming liable for any obligations or engaging in any business activity.
The 8.75% Notes are jointly and severally, fully and unconditionally guaranteed, on a senior secured basis by all of DJOFL’s domestic subsidiaries (other than the co-issuer) that are 100% owned, directly or indirectly, by DJOFL (the Guarantors). The 10.875% Notes and 7.75% Notes are jointly and severally, fully and unconditionally guaranteed, on an unsecured senior basis by the Guarantors. The 9.75% Notes are jointly and severally, fully and unconditionally guaranteed, on an unsecured senior subordinated basis by the Guarantors. Our foreign subsidiaries (the Non-Guarantors) do not guarantee the notes. The Guarantors also unconditionally guarantee the Amended Senior Secured Credit Facility.
F-79
Table of Contents
The following tables present the financial position, results of operations and cash flows of DJOFL, the Guarantors, the Non-Guarantors and certain eliminations for the periods presented.
DJO Finance LLC
Unaudited Condensed Consolidating Balance Sheets
As of September 29, 2012
(in thousands)
| DJOFL | Guarantors | Non- Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Assets | ||||||||||||||||||||
| Current assets: |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 20,513 | $ | 1,680 | $ | 16,056 | $ | (24 | ) | $ | 38,225 | |||||||||
| Accounts receivable, net |
— | 125,112 | 35,044 | — | 160,156 | |||||||||||||||
| Inventories, net |
— | 119,944 | 29,628 | (5,771 | ) | 143,801 | ||||||||||||||
| Deferred tax assets, net |
— | 43,502 | 268 | — | 43,770 | |||||||||||||||
| Prepaid expenses and other current assets |
29 | 18,558 | 4,035 | 512 | 23,134 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current assets |
20,542 | 308,796 | 85,031 | (5,283 | ) | 409,086 | ||||||||||||||
| Property and equipment, net |
— | 93,240 | 12,270 | (580 | ) | 104,930 | ||||||||||||||
| Goodwill |
— | 1,150,269 | 108,413 | (28,741 | ) | 1,229,941 | ||||||||||||||
| Intangible assets, net |
— | 1,031,162 | 28,774 | — | 1,059,936 | |||||||||||||||
| Investment in subsidiaries |
1,297,699 | 1,686,366 | 78,501 | (3,062,566 | ) | — | ||||||||||||||
| Intercompany receivables |
1,077,460 | — | — | (1,077,460 | ) | — | ||||||||||||||
| Other non-current assets |
42,859 | 3,657 | 1,636 | (4 | ) | 48,148 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total assets |
$ | 2,438,560 | $ | 4,273,490 | $ | 314,625 | $ | (4,174,634 | ) | $ | 2,852,041 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Liabilities and Equity | ||||||||||||||||||||
| Current liabilities: |
||||||||||||||||||||
| Accounts payable |
$ | — | $ | 48,397 | $ | 9,667 | $ | 11 | $ | 58,075 | ||||||||||
| Current portion of debt and capital lease obligations |
8,608 | 6 | — | — | 8,614 | |||||||||||||||
| Other current liabilities |
44,730 | 58,817 | 26,259 | 1,111 | 130,917 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current liabilities |
53,338 | 107,220 | 35,926 | 1,122 | 197,606 | |||||||||||||||
| Long-term debt and capital lease obligations |
2,156,863 | — | — | — | 2,156,863 | |||||||||||||||
| Deferred tax liabilities, net |
— | 241,888 | 9,923 | (527 | ) | 251,284 | ||||||||||||||
| Intercompany payables, net |
— | 863,971 | 142,934 | (1,006,905 | ) | — | ||||||||||||||
| Other long-term liabilities |
— | 13,712 | 1,475 | — | 15,187 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities |
2,210,201 | 1,226,791 | 190,258 | (1,006,310 | ) | 2,620,940 | ||||||||||||||
| Noncontrolling interests |
— | — | 2,742 | — | 2,742 | |||||||||||||||
| Total membership equity |
228,359 | 3,046,699 | 121,625 | (3,168,324 | ) | 228,359 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities and equity |
$ | 2,438,560 | $ | 4,273,490 | $ | 314,625 | $ | (4,174,634 | ) | $ | 2,852,041 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-80
Table of Contents
DJO Finance LLC
Unaudited Condensed Consolidating Statements of Operations
For the Three Months Ended September 29, 2012
(in thousands)
| DJOFL | Guarantors | Non - Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Net sales |
$ | — | $ | 241,368 | $ | 60,905 | $ | (28,287 | ) | $ | 273,986 | |||||||||
| Cost of sales (exclusive of amortization of intangible assets of $9,837) |
— | 97,277 | 41,764 | (30,744 | ) | 108,297 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
— | 144,091 | 19,141 | 2,457 | 165,689 | |||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Selling, general and administrative |
— | 91,661 | 19,080 | (6 | ) | 110,735 | ||||||||||||||
| Research and development |
— | 6,735 | 1,203 | — | 7,938 | |||||||||||||||
| Amortization of intangible assets |
— | 23,507 | 980 | — | 24,487 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| — | 121,903 | 21,263 | (6 | ) | 143,160 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating income (loss) |
— | 22,188 | (2,122 | ) | 2,463 | 22,529 | ||||||||||||||
| Other income (expense): |
||||||||||||||||||||
| Interest expense |
(46,386 | ) | — | (25 | ) | — | (46,411 | ) | ||||||||||||
| Interest income |
4 | 36 | 6 | — | 46 | |||||||||||||||
| Loss on modification and extinguishment of debt |
— | — | — | — | — | |||||||||||||||
| Other income (expense), net |
— | 625 | 1,245 | — | 1,870 | |||||||||||||||
| Intercompany income (expense) |
— | 366 | (407 | ) | 41 | — | ||||||||||||||
| Equity in income (loss) of subsidiaries, net |
23,821 | — | — | (23,821 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (22,561 | ) | 1,027 | 819 | (23,780 | ) | (44,495 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (Loss) income before income taxes |
(22,561 | ) | 23,215 | (1,303 | ) | (21,317 | ) | (21,966 | ) | |||||||||||
| Income tax (provision) benefit |
— | 605 | (1,174 | ) | — | (569 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income |
(22,561 | ) | 23,820 | (2,477 | ) | (22,317 | ) | (22,535 | ) | |||||||||||
| Net income attributable to noncontrolling interests |
— | — | (27 | ) | — | (27 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income attributable to DJOFL |
$ | (22,561 | ) | $ | 23,820 | $ | (2,504 | ) | $ | (21,317 | ) | $ | (22,562 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-81
Table of Contents
DJO Finance LLC
Unaudited Condensed Consolidating Statements of Operations
For the Nine Months Ended September 29, 2012
(in thousands)
| DJOFL | Guarantors | Non - Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Net sales |
$ | — | $ | 726,719 | $ | 195,903 | $ | (83,712 | ) | $ | 838,910 | |||||||||
| Cost of sales (exclusive of amortization of intangible assets of $29,513) |
— | 290,889 | 132,130 | (94,685 | ) | 328,334 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
— | 435,830 | 63,773 | 10,973 | 510,576 | |||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Selling, general and administrative |
— | 281,024 | 61,589 | 4 | 342,617 | |||||||||||||||
| Research and development |
— | 18,346 | 3,349 | — | 21,695 | |||||||||||||||
| Amortization of intangible assets |
— | 70,523 | 2,982 | — | 73,505 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| — | 369,893 | 67,920 | 4 | 437,817 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating income (loss) |
— | 65,937 | (4,147 | ) | 10,969 | 72,759 | ||||||||||||||
| Other income (expense): |
||||||||||||||||||||
| Interest expense |
(134,829 | ) | — | (70 | ) | — | (134,899 | ) | ||||||||||||
| Interest income |
14 | 72 | 65 | — | 151 | |||||||||||||||
| Loss on modification and extinguishment of debt |
(9,398 | ) | — | — | (9,398 | ) | ||||||||||||||
| Other income (expense), net |
— | 2,493 | 438 | — | 2,931 | |||||||||||||||
| Intercompany income (expense) |
— | 728 | (661 | ) | (67 | ) | — | |||||||||||||
| Equity in income (loss) of subsidiaries, net |
72,099 | — | — | (72,099 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (72,114 | ) | 3,293 | (228 | ) | (72,116 | ) | (141,215 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (Loss) income before income taxes |
(72,114 | ) | 69,230 | (4,375 | ) | (61,197 | ) | (68,456 | ) | |||||||||||
| Income tax benefit (provision) |
— | 1,381 | (4,425 | ) | — | (3,044 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income |
(72,114 | ) | 70,611 | (8,800 | ) | (61,197 | ) | (71,500 | ) | |||||||||||
| Net income attributable to noncontrolling interests |
— | — | (614 | ) | — | (614 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income attributable to DJOFL |
$ | (72,114 | ) | $ | 70,611 | $ | (9,414 | ) | $ | (61,197 | ) | $ | (72,114 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-82
Table of Contents
DJO Finance LLC
Unaudited Condensed Consolidating Statements of Cash Flows
For the Nine Months Ended September 29, 2012
(in thousands)
| DJOFL | Guarantors | Non- Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Cash Flows from Operating Activities: |
||||||||||||||||||||
| Net (loss) income |
$ | (72,114 | ) | $ | 70,611 | $ | (8,800 | ) | $ | (61,197 | ) | $ | (71,500 | ) | ||||||
| Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities: |
||||||||||||||||||||
| Depreciation |
— | 19,063 | 3,883 | (273 | ) | 22,673 | ||||||||||||||
| Amortization of intangible assets |
— | 70,523 | 2,982 | — | 73,505 | |||||||||||||||
| Amortization of debt issuance costs and non-cash interest expense |
7,729 | — | — | — | 7,729 | |||||||||||||||
| Stock-based compensation expense |
— | 3,563 | — | — | 3,563 | |||||||||||||||
| Loss on modification and extinguishment of debt |
9,398 | — | — | — | 9,398 | |||||||||||||||
| Loss on disposal of assets, net |
— | 485 | 451 | — | 936 | |||||||||||||||
| Deferred income tax benefit |
— | (1,247 | ) | (62 | ) | (527 | ) | (1,836 | ) | |||||||||||
| Provision for doubtful accounts and sales returns |
— | 16,281 | 161 | — | 16,442 | |||||||||||||||
| Inventory reserves |
— | 4,198 | 242 | — | 4,440 | |||||||||||||||
| Equity in (income) loss of subsidiaries, net |
(72,099 | ) | — | — | 72,099 | — | ||||||||||||||
| Changes in operating assets and liabilities: |
||||||||||||||||||||
| Accounts receivable |
— | (16,296 | ) | (1,381 | ) | — | (17,677 | ) | ||||||||||||
| Inventories |
— | (12,871 | ) | 6,175 | (9,872 | ) | (16,568 | ) | ||||||||||||
| Prepaid expenses and other assets |
131 | (2,856 | ) | (886 | ) | (59 | ) | (3,670 | ) | |||||||||||
| Accounts payable and other current liabilities |
23,867 | 345 | (3,533 | ) | 3,189 | 23,868 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash (used in) provided by operating activities |
(103,088 | ) | 151,799 | (768 | ) | 3,360 | 51,303 | |||||||||||||
| Cash Flows from Investing Activities: |
||||||||||||||||||||
| Purchases of property and equipment |
— | (19,921 | ) | (3,519 | ) | (8 | ) | (23,448 | ) | |||||||||||
| Other investing activities, net |
— | (656 | ) | — | — | (656 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash used in investing activities |
— | (20,577 | ) | (3,519 | ) | (8 | ) | (24,104 | ) | |||||||||||
| Cash Flows from Financing Activities: |
||||||||||||||||||||
| Intercompany |
137,154 | (131,288 | ) | (2,489 | ) | (3,377 | ) | — | ||||||||||||
| Proceeds from issuance of debt |
751,700 | — | — | — | 751,700 | |||||||||||||||
| Repayments of debt and capital lease obligations |
(754,792 | ) | (32 | ) | — | — | (754,824 | ) | ||||||||||||
| Payment of debt issuance costs |
(25,234 | ) | — | — | — | (25,234 | ) | |||||||||||||
| Investment by parent |
1,000 | — | — | — | 1,000 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash provided by (used in) financing activities |
109,828 | (131,320 | ) | (2,489 | ) | (3,377 | ) | (27,358 | ) | |||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
— | — | 215 | — | 215 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (decrease) increase in cash and cash equivalents |
6,740 | (98 | ) | (6,561 | ) | (25 | ) | 56 | ||||||||||||
| Cash and cash equivalents at beginning of period |
13,773 | 1,778 | 22,617 | 1 | 38,169 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents at end of period |
$ | 20,513 | $ | 1,680 | $ | 16,056 | $ | (24 | ) | $ | 38,225 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-83
Table of Contents
DJO Finance LLC
Unaudited Condensed Consolidating Statements of Operations
For the Three Months Ended October 1, 2011
(in thousands)
| DJOFL | Guarantors | Non - Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Net sales |
$ | — | $ | 222,120 | $ | 60,319 | $ | (19,321 | ) | $ | 263,118 | |||||||||
| Cost of sales (exclusive of amortization of intangible assets of $9,688) |
— | 90,555 | 37,788 | (20,880 | ) | 107,463 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
— | 131,565 | 22,531 | 1,559 | 155,655 | |||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Selling, general and administrative |
— | 94,502 | 21,352 | — | 115,854 | |||||||||||||||
| Research and development |
— | 5,546 | 931 | — | 6,477 | |||||||||||||||
| Amortization of intangible assets |
— | 23,328 | 1,107 | — | 24,435 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| — | 123,376 | 23,390 | — | 146,766 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating income (loss) |
— | 8,189 | (859 | ) | 1,559 | 8,889 | ||||||||||||||
| Other income (expense): |
||||||||||||||||||||
| Interest expense |
(42,756 | ) | — | (8 | ) | — | (42,764 | ) | ||||||||||||
| Interest income |
3 | 17 | 57 | — | 77 | |||||||||||||||
| Other income, net |
— | (1,820 | ) | (4,184 | ) | — | (6,004 | ) | ||||||||||||
| Intercompany income (expense) |
— | 664 | 1,211 | (1,875 | ) | — | ||||||||||||||
| Equity in income of subsidiaries, net |
16,989 | — | — | (16,989 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (25,764 | ) | (1,139 | ) | (2,924 | ) | (18,864 | ) | (48,691 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (Loss) income before income taxes |
(25,764 | ) | 7,050 | (3,783 | ) | (17,305 | ) | (39,802 | ) | |||||||||||
| Income tax benefit (provision) |
— | 15,306 | (875 | ) | (335 | ) | 14,096 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income |
(25,764 | ) | 22,356 | (4,658 | ) | (17,640 | ) | (25,706 | ) | |||||||||||
| Net income attributable to noncontrolling interests |
— | — | (58 | ) | — | (58 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income attributable to DJOFL |
$ | (25,764 | ) | $ | 22,356 | $ | (4,716 | ) | $ | (17,640 | ) | $ | (25,764 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-84
Table of Contents
DJO Finance LLC
Unaudited Condensed Consolidating Statements of Operations
For the Nine Months Ended October 1, 2011
(in thousands)
| DJOFL | Guarantors | Non- Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Net sales |
$ | — | $ | 654,999 | $ | 194,502 | $ | (58,886 | ) | $ | 790,615 | |||||||||
| Cost of sales (exclusive of amortization of intangible assets of $28,831) |
— | 264,678 | 124,189 | (77,158 | ) | 311,709 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
— | 390,321 | 70,313 | 18,272 | 478,906 | |||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Selling, general and administrative |
— | 292,764 | 68,997 | — | 361,761 | |||||||||||||||
| Research and development |
— | 16,496 | 3,225 | — | 19,721 | |||||||||||||||
| Amortization of intangible assets |
— | 66,129 | 3,244 | — | 69,373 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| — | 375,389 | 75,466 | — | 450,855 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating income (loss) |
— | 14,932 | (5,153 | ) | 18,272 | 28,051 | ||||||||||||||
| Other income (expense): |
||||||||||||||||||||
| Interest expense |
(126,173 | ) | (10 | ) | (137 | ) | — | (126,320 | ) | |||||||||||
| Interest income |
10 | 111 | 119 | — | 240 | |||||||||||||||
| Loss on modification of debt |
(2,065 | ) | — | — | — | (2,065 | ) | |||||||||||||
| Other income, net |
— | 396 | (1,947 | ) | — | (1,551 | ) | |||||||||||||
| Intercompany income (expense) |
10,625 | (8,134 | ) | (468 | ) | (2,023 | ) | — | ||||||||||||
| Equity in income of subsidiaries, net |
51,345 | — | — | (51,345 | ) | — | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (66,258 | ) | (7,637 | ) | (2,433 | ) | (53,368 | ) | (129,696 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (Loss) income before income taxes |
(66,258 | ) | 7,295 | (7,586 | ) | (35,096 | ) | (101,645 | ) | |||||||||||
| Income tax benefit (provision) |
— | 40,559 | (4,650 | ) | 146 | 36,055 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income |
(66,258 | ) | 47,854 | (12,236 | ) | (34,950 | ) | (65,590 | ) | |||||||||||
| Net income attributable to noncontrolling interests |
— | — | (668 | ) | — | (668 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income attributable to DJOFL |
$ | (66,258 | ) | $ | 47,854 | $ | (12,904 | ) | $ | (34,950 | ) | $ | (66,258 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-85
Table of Contents
DJO Finance LLC
Unaudited Condensed Consolidating Statements of Cash Flows
For the Nine Months Ended October 1, 2011
(in thousands)
| DJOFL | Guarantors | Non- Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Cash Flows from Operating Activities: |
||||||||||||||||||||
| Net (loss) income |
$ | (66,258 | ) | $ | 47,854 | $ | (12,236 | ) | $ | (34,950 | ) | $ | (65,590 | ) | ||||||
| Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities: |
||||||||||||||||||||
| Depreciation |
— | 17,281 | 4,091 | (67 | ) | 21,305 | ||||||||||||||
| Amortization of intangible assets |
— | 66,129 | 3,244 | — | 69,373 | |||||||||||||||
| Amortization of debt issuance costs and non-cash interest expense |
6,270 | — | — | — | 6,270 | |||||||||||||||
| Stock-based compensation expense |
— | 1,700 | — | — | 1,700 | |||||||||||||||
| Loss on disposal of assets, net |
— | 391 | 292 | — | 683 | |||||||||||||||
| Deferred income tax benefit |
(1,925 | ) | (40,267 | ) | (736 | ) | (146 | ) | (43,074 | ) | ||||||||||
| Provision for doubtful accounts and sales returns |
— | 20,105 | 334 | — | 20,439 | |||||||||||||||
| Inventory reserves |
— | 4,609 | 878 | — | 5,487 | |||||||||||||||
| Equity in income of subsidiaries, net |
(51,345 | ) | — | — | 51,345 | — | ||||||||||||||
| Changes in operating assets and liabilities: |
||||||||||||||||||||
| Accounts receivable |
— | (15,822 | ) | (993 | ) | — | (16,815 | ) | ||||||||||||
| Inventories |
— | (7,759 | ) | 6,263 | (9,629 | ) | (11,125 | ) | ||||||||||||
| Prepaid expenses and other assets |
79 | 3,295 | (1,239 | ) | 142 | 2,277 | ||||||||||||||
| Accounts payable and other current liabilities |
37,273 | 4,014 | 7,399 | 502 | 49,188 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash (used in) provided by operating activities |
(75,906 | ) | 101,530 | 7,297 | 7,197 | 40,118 | ||||||||||||||
| Cash Flows from Investing Activities: |
||||||||||||||||||||
| Cash paid in connection with acquisitions, net of cash acquired |
— | (317,669 | ) | — | — | (317,669 | ) | |||||||||||||
| Purchases of property and equipment |
— | (24,917 | ) | (4,087 | ) | (213 | ) | (29,217 | ) | |||||||||||
| Other investing activities, net |
— | (1,484 | ) | 5 | — | (1,479 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash used in investing activities |
— | (344,070 | ) | (4,082 | ) | (213 | ) | (348,365 | ) | |||||||||||
| Cash Flows from Financing Activities: |
||||||||||||||||||||
| Intercompany |
(237,398 | ) | 243,762 | 609 | (6,973 | ) | — | |||||||||||||
| Proceeds from issuance of debt |
400,000 | — | — | — | 400,000 | |||||||||||||||
| Repayments of debt and capital lease obligations |
(83,586 | ) | (32 | ) | (2 | ) | — | (83,620 | ) | |||||||||||
| Payment of debt issuance costs |
(7,612 | ) | — | — | — | (7,612 | ) | |||||||||||||
| Investment by parent |
3,176 | — | — | — | 3,176 | |||||||||||||||
| Repurchase of vested options |
— | (2,000 | ) | — | — | (2,000 | ) | |||||||||||||
| Dividend paid by subsidiary to owners of noncontrolling interests |
— | — | (682 | ) | — | (682 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net cash provided by (used in) financing activities |
74,580 | 241,730 | (75 | ) | (6,973 | ) | 309,262 | |||||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
— | — | 176 | — | 176 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (decrease) increase in cash and cash equivalents |
(1,326 | ) | (810 | ) | 3,316 | 11 | 1,191 | |||||||||||||
| Cash and cash equivalents at beginning of period |
16,601 | 621 | 20,910 | — | 38,132 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash and cash equivalents at end of period |
$ | 15,275 | $ | (189 | ) | $ | 24,226 | $ | 11 | $ | 39,323 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-86
Table of Contents
DJO Finance LLC
Condensed Consolidating Balance Sheets
As of December 31, 2011
(in thousands)
| DJOFL | Guarantors | Non- Guarantors |
Eliminations | Consolidated | ||||||||||||||||
| Assets | ||||||||||||||||||||
| Current assets: |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 13,773 | $ | 1,778 | $ | 22,617 | $ | 1 | $ | 38,169 | ||||||||||
| Accounts receivable, net |
— | 125,097 | 33,885 | — | 158,982 | |||||||||||||||
| Inventories, net |
— | 97,516 | 20,719 | 10,464 | 128,699 | |||||||||||||||
| Deferred tax assets, net |
— | 43,190 | 268 | — | 43,458 | |||||||||||||||
| Prepaid expenses and other current assets |
160 | 15,001 | 3,186 | 444 | 18,791 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current assets |
13,933 | 282,582 | 80,675 | 10,909 | 388,099 | |||||||||||||||
| Property and equipment, net |
— | 94,904 | 13,070 | (866 | ) | 107,108 | ||||||||||||||
| Goodwill |
— | 1,150,269 | 107,344 | (28,835 | ) | 1,228,778 | ||||||||||||||
| Intangible assets, net |
— | 1,101,314 | 31,380 | — | 1,132,694 | |||||||||||||||
| Investment in subsidiaries |
1,297,699 | 1,686,366 | 72,514 | (3,056,579 | ) | — | ||||||||||||||
| Intercompany receivables |
1,138,947 | — | — | (1,138,947 | ) | — | ||||||||||||||
| Other assets |
33,971 | 2,655 | 1,557 | (2 | ) | 38,181 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total assets |
$ | 2,484,550 | $ | 4,318,090 | $ | 306,540 | $ | (4,214,320 | ) | $ | 2,894,860 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Liabilities and Equity | ||||||||||||||||||||
| Current liabilities: |
||||||||||||||||||||
| Accounts payable |
$ | — | $ | 47,049 | $ | 10,872 | $ | 5 | $ | 57,926 | ||||||||||
| Current portion of debt and capital lease obligations |
8,782 | 38 | — | — | 8,820 | |||||||||||||||
| Other current liabilities |
20,864 | 56,509 | 24,805 | 521 | 102,699 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total current liabilities |
29,646 | 103,596 | 35,677 | 526 | 169,445 | |||||||||||||||
| Long-term debt and capital leases obligations |
2,159,091 | — | — | — | 2,159,091 | |||||||||||||||
| Deferred tax liabilities, net |
— | 242,237 | 9,957 | — | 252,194 | |||||||||||||||
| Intercompany payables, net |
— | 996,889 | 142,058 | (1,138,947 | ) | — | ||||||||||||||
| Other long-term liabilities |
— | 14,689 | 1,485 | — | 16,174 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities |
2,188,737 | 1,357,411 | 189,177 | (1,138,421 | ) | 2,596,904 | ||||||||||||||
| Noncontrolling interests |
— | — | 2,143 | — | 2,143 | |||||||||||||||
| Total membership equity |
295,813 | 2,960,679 | 115,220 | (3,075,899 | ) | 295,813 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total liabilities and equity |
$ | 2,484,550 | $ | 4,318,090 | $ | 306,540 | $ | (4,214,320 | ) | $ | 2,894,860 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| 16. | SUBSEQUENT EVENTS |
On September 11, 2012, we commenced a cash tender offer for any and all of our $465.0 million of outstanding 10.875% Notes (see Note 8) due 2014 with a final tender expiration date of October 9, 2012. The total tender offer consideration was $1,038.75 for each $1,000 principal amount of 10.875% Notes, including an early tender premium of $30 per $1,000 principal amount of 10.875% Notes validly tendered by the early tender deadline of September 24, 2012. In addition, holders who validly tendered their 10.875% Notes were entitled to receive accrued interest from and including the last interest payment date through the applicable settlement date. Holders of 10.875% Notes aggregating $363.2 million of principal tendered their notes on or before the early tender deadline. On October 1, 2012, we purchased from the holders the 10.875% Notes tendered by the early
F-87
Table of Contents
tender deadline. The aggregate payment to these holders was $392.2 million, including aggregate tender premium cost of $14.1 million and accrued interest of $14.9 million. We intend to redeem all 10.875% Notes that are not previously tendered on November 15, 2012 at the applicable redemption price of 102.719% of the remaining principal amount outstanding, or an aggregate total of $104.5 million, plus accrued interest. In connection with the tender and the redemption, we will record a loss on extinguishment of debt during the fourth quarter. The loss is preliminarily estimated to include $18.5 million in tender and redemption premium costs and $10.2 million in unamortized original issuance costs, net of $2.5 million in unamortized original issuance premium.
On October 1, 2012, we issued $100.0 million in additional 8.75% Second Priority Senior Secured Notes (Additional 8.75% Notes). This issuance increases the aggregate principal amount of existing 8.75% Notes from $230.0 million to $330.0 million. The Additional 8.75% Notes are fungible with the original 8.75% Notes and mature on March 15, 2018. Interest on the Additional 8.75% Notes will be payable on March 15 and September 15 of each year, commencing on March 15, 2013. Gross proceeds of $106.75 million from the Additional 8.75% Notes included a $6.75 million issuance premium. This premium will be amortized over the term of the Additional 8.75% Notes using the effective interest method.
The 8.75% Notes are guaranteed jointly and severally and on a senior secured basis by each of DJOFL’s existing and future direct and indirect wholly-owned domestic subsidiaries that guarantee the Amended Senior Secured Credit Facility. The 8.75% Notes are secured by second priority liens, subject to permitted liens, on certain of the Issuers’ subsidiary guarantor assets that secure borrowings under the Amended Senior Secured Credit Facility.
On October 1, 2012, we issued $440.0 million aggregate principal amount of new 9.875% Senior Notes (9.875% Notes) maturing on April 15, 2018. Interest will be payable on April 15 and October 15 of each year, commencing on April 15, 2013.
The 9.875% Notes are guaranteed jointly and severally and on an unsecured senior basis by each of DJOFL’s existing and future direct and indirect wholly owned domestic subsidiaries that guarantee any of DJOFL’s indebtedness or any indebtedness of DJOFL’s domestic subsidiaries or is an obligor under DJOFL’s Senior Secured Credit Facility.
The net proceeds from the issuances were or will be used to repay the 10.875% Notes and the balance outstanding on our Revolving Credit Facility as of October 1, 2012, as well as fees and expenses, including tender premiums, in connection with the 10.875% Notes repayment and issuance of the Additional 8.75% Notes and the 9.875% Notes.
F-88
Table of Contents
DJO Finance LLC
DJO Finance Corporation
Offers to Exchange
$330,000,000 aggregate principal amount of their 8.75% Second Priority Senior Secured Notes due 2018 and $440,000,000 aggregate principal amount of their 9.875% Senior Notes due 2018, each of which have been registered under the Securities Act of 1933, as amended, for any and all of their outstanding unregistered 8.75% Second Priority Senior Secured Notes due 2018 and for any and all of their outstanding unregistered 9.875% Senior Notes due 2018, respectively.
Until the date that is 90 days from the date of this prospectus, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters with respect to their unsold allotments or subscriptions.