As filed with the Securities and Exchange Commission on February 27, 2023.
Registration No. 333-269470
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
AMENDMENT NO. 2
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Ispire Technology Inc.
(Exact name of Registrant as specified in its charter)
| Delaware | 2111 | 84-5106049 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
19700 Magellan Drive
Los Angeles, CA 90502
(310) 742-9975
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Michael Wang, Chief Financial Officer
Ispire Technology Inc.
19700 Magellan Drive
Los Angeles, CA 90502
(310) 742-9975
(Name, address, including zip code, and telephone number, including area code, of agent for service)
| Copies to: | |
|
Richard I. Anslow, Esq. Jonathan Deblinger, Esq. Asher S. Levitsky P.C. Ellenoff Grossman & Schole LLP 1345 Avenue of the Americas; Suite 1100 New York, New York 10105 Telephone: (212) 370-1300 |
Meng Ding, Esq. Sidley Austin 39/F, Two International Finance Centre Central, Hong Kong SAR Tel: +852.2509.7858 |
Approximate date of commencement of proposed sale to the public: as soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to such Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and we are not soliciting an offer to buy these securities in any state or other jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS (Subject to Completion) | Dated February 27, 2023 |
6,000,000 Shares
Ispire Technology Inc.
Common Stock
Offered by the Company
1,750,000 Shares of Common Stock Offered by Selling Stockholders
This is the initial public offering of 6,000,000 shares of common stock on a firm commitment basis. The estimated initial public offering price is expected to be in the range of $6.00 to $8.00 per share. Currently, no public market exists for our common stock. We intend to apply to have our common stock listed on the Nasdaq Capital Market, or Nasdaq, under the symbol “ISPR.” The offering is contingent upon final approval of our Nasdaq listing, and we will not complete the offering if our common stock is not listed on Nasdaq.
In addition to the offering by us, two selling stockholders are offering an aggregate of 1,750,000 shares of common stock which they may sell at the initial public offering price of the underwritten offering until such time as our common stock is listed on the Nasdaq Capital Market, at which time they may sell such shares from time to time at prevailing market prices or at negotiated prices. The selling stockholders have not engaged any underwriter in connection with the sale of their shares, and we will not receive any proceeds from the sale by the Selling Stockholders of their shares. See “Selling Stockholders.”
We have granted the Underwriters the option, exercisable for 45 days from the date of this prospectus, to purchase up to an additional 900,000 from us at the initial public offering price less the underwriting discount and commissions to cover over-allotments.
We are an “emerging growth company,” as that term is used in the Jumpstart Our Business Startups Act of 2012, as amended, and will be subject to certain reduced public company reporting requirements for this prospectus and future filings. See “Prospectus Summary — Emerging Growth Company Status.” We will be deemed to be a “controlled company” under the Nasdaq listing rules because Tuanfang Liu, our chief executive officer and a director, and his wife, Jiangyan Zhu, who is a director, will own 63.8% of our outstanding common stock upon completion of this offering (62.8 % if the underwriter’s over-allotment option is exercised in full). As a controlled company, we are not required to comply with certain of NASDAQ’s corporate governance requirements. We do not currently intend to take advantage of any of these exceptions except that Mr. Tuanfang Liu is chairman of the nominating and corporate governance committee. See “Prospectus Summary — Controlled Company.”
Investing in our common stock is highly speculative and involves a significant degree of risk. See “Risk Factors,” which begins on Page 12.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| Initial public offering price | $ | $ | ||||||
| Underwriting discount and commissions (1) | $ | $ | ||||||
| Proceeds to us, before expenses | $ | $ | ||||||
| (1) | The Underwriters will receive compensation in addition to the underwriting discount and commission, as set forth in the section entitled “Underwriting” beginning on page 78 upon the closing of this offering. We have also agreed to reimburse Underwriters for certain expenses incurred by it and to issue to the Underwriters upon closing of this offering, warrants (the “Underwriters’ Warrants”) to purchase the number of shares of common stock in the aggregate equal to 5% of the number of shares sold in the offering. See “Underwriting” for additional information. |
If the Underwriters exercise the over-allotment option in full, the total underwriting discounts and commissions payable will be $ , and the total proceeds to us, before expenses, will be $ .
This prospectus also relates to the public offering of an aggregate of 1,750,000 shares of common stock which may be sold from time to time by the selling stockholders named in this prospectus, which is separate and apart from our underwritten public offering, We will not receive any proceeds from the sale by the selling stockholders of their shares of common stock. The selling stockholders have not engaged any underwriter in connection with the sale of their common stock. The selling stockholders may sell common stock at the initial public offering price of the underwritten offering until such time as our common stock is listed on the Nasdaq Capital Market, at which time they may sell such shares in the public market based on the market price at the time of sale or at negotiated prices or in transactions that are not in the public market in the manner set forth under “Plan of Distribution.”
The Underwriters expect to deliver the common stock to purchasers in the offering on or about [●], 2023.
| US Tiger Securities, Inc. |
TFI Securities SPDB International
The date of this prospectus is , 2023.
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus or in any related free-writing prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus. We are offering to sell, and seeking offers to buy, the common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is current only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of the common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
Neither we nor the Underwriters have done anything that would permit this offering or possession or distribution of this prospectus or any filed free writing prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus or any filed free writing prospectus must inform themselves about, and observe any restrictions relating to, the offering of the common stock and the distribution of this prospectus or any filed free writing prospectus outside of the United States.
Until , 2023 (the 25th day after the date of this prospectus), all dealers that buy, sell or trade common stock, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the obligation of dealers to deliver a prospectus when acting as an underwriter and with respect to their unsold allotments or subscriptions.
i
Introduction
We were formed on June 13, 2022. We have two operating subsidiaries, Aspire North America LLC, a California limited liability company (“Aspire North America”), and Aspire Science and Technology Limited, a Hong Kong corporation (“Aspire Science”). On July 29, 2022, we acquired 100% of the equity interest in Aspire North America from Aspire Global Inc. (“Aspire Global”), and our wholly-owned subsidiary Ispire International Limited, a British Virgin Islands corporation (“Ispire International”), acquired 100% of the equity interest in Aspire Science from a wholly-owned subsidiary of Aspire Global in connection with a restructure by Aspire Global pursuant to which the equity in Aspire North America and Aspire Science was transferred to us, and, at the time of the transfer, we had the same stockholders as Aspire Global and our stockholders held the same percentage interest in us as they had in Aspire Global at the time of the transfer. See “Business – Acquisition of Our Business from a Related Party” and “Certain Relationships and Related Party Transactions.”
Unless the context indicates otherwise, all references to “we,” “us,” “our,” the “Company,” or similar terms used in this prospectus refer to (i) Ispire Technology Inc., including its subsidiaries, and (ii) for periods prior to July 29, 2022, the date we acquired our operating subsidiaries, the operations of our subsidiaries prior to our acquisition of the equity in the subsidiaries. Our consolidated financial statements reflect the consolidated operations of us and our subsidiaries as if the acquisition of the subsidiaries occurred on July 1, 2020. See Note 1 of Notes to Consolidated Financial Statements.
Our reporting currency is the U.S. dollar. The functional currency of Aspire Science, which is located in Hong Kong, is the Hong Kong Dollar (“HKD”). For Aspire Science, results of operations and cash flows are translated at average exchange rates during the period, assets and liabilities are translated at the unified exchange rate at the end of the period, and equity is translated at historical exchange rates. As a result, amounts relating to assets and liabilities reported on the statements of cash flows may not necessarily agree with the changes in the corresponding balances on the balance sheets. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive income/loss. Transactions denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing on the transaction dates. Assets and liabilities denominated in foreign currencies are translated into the functional currencies at the exchange rates prevailing at the balance sheet date with any transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
The information presented in this prospectus that relates to the industry has been derived from industry reports prepared by Euromonitor International Limited. Euromonitor is an independent research firm. The tobacco report was commissioned by Aspire Global, and we commissioned the cannabis report. Investors are cautioned not to place any undue reliance on the information, including statistics and estimates, set forth in this section or similar information included elsewhere in this prospectus.
Overview
We are engaged in the research and development, design, commercialization, sales, marketing and distribution of branded e-cigarettes and cannabis vaping products. We sell our tobacco products worldwide except for the People’s Republic of China and Russia. Although we sold tobacco products in the United States in the years ended June 30, 2021 and 2022, we are limited in the product we may sell and, because the sales did not justify the marketing and regulatory costs, we have ceased marketing tobacco vaping products in the United States. Our tobacco products are marketed under the Aspire brand name and are sold primarily through our distribution network.
We currently sell our cannabis vaping hardware only in the United States, and we have recently commenced marketing activities in Canada and Europe, primarily in the European Union. All of our cannabis products are vaping hardware. Vaping refers to the practice of inhaling and exhaling the vapor produced by an electronic vaping device, and includes dabbing, which is the recreational inhalation of extremely concentrated tetrahydrocannabinol, the main psychotropic cannabinoid derived from the marijuana plant. Our cannabis products are marketed under the Ispire brand name, primarily on an ODM basis to other cannabis vapor companies. ODM generally involves the design and customization of core products to meet each brand’s unique image and needs, and our products are sold by our customers under their own brand names although they may also include our brand name on the products.
1
Our products use our BDC (bottom dual coil) coil technology which uses bottom dual coils to provide much higher temperature and an expanded heating that achieves much greater flavor and vapor production. We believe that the use of our dual-coil technology enhances the flavor performance of e-liquid, and the hidden wick cotton with special designed wick holes can both extend the tank e-liquid capacity and improve the speed of wicking to increase the coil life. Our BVC (bottom vertical coil) coil represents a big technological breakthrough for us in coil technology with a vertical heating wire surrounded by the cotton. This design can enable the coil heating to provide uniform temperature from the tank, together with more efficient wicking. This new technology, which Shenzhen Yi Jia introduced in 2014, enables the coil to last longer while still giving users what we believe is the purest and cleanest taste from e-liquids. The BVC coils are still very popular for MTL (mouth to lung) vapors today.
We bring a new coil technology to the tobacco vaping industry with the Cleito tank. The Cleito tank uses a revolutionary new coil design that replaces the standard chimney and, we believe, delivers maximized airflow. This design frees up even more restriction in the airflow by eliminating the need for a static chimney within the tank itself, which results in an expanded flavor profile and increased vapor production. Combined with a Clapton kanthal coil for maximum flavor, the Cleito tank delivers a rush of intense flavor and huge vapor with a broad profile. The simple top-fill design makes filling very easy, just unscrew the top cap to refill, more convenient, more enjoyable.
Our Ispire cannabis vapor products use our patented Ducore™ (Dual Coil) technology for cannabis vaporizers. This technology enables users to create massive plumes of vape without burning the cannabis oil. These products incorporate our patented dual coil technology for what we believe is best-in-class airflow and taste, and our technology for eliminating the leakage of the oil from the unit, which overcomes a major disadvantage with many existing products
At present, our products are manufactured and supplied by Shenzhen Yi Jia Technology Co., Limited, a Chinese company (“Shenzhen Yi Jia”), which is 95% owned by our chief executive officer and controlling stockholder, Tuanfang Liu. We are taking the initial steps toward the development of manufacturing operations in Vietnam and in California. Initially, our manufacturing operations will be primarily assembling from components that we purchase from other companies. Although we expect that we will commence these assembly operations in mid 2023, we cannot assure you that we will be able to meet this timetable or that we will be able to effectively and efficiently conduct such operations.
Through our global distributor network of more than 150 distributors, we sell the Aspire brand of tobacco vaporizer technology products in more than 30 countries. The main markets for our tobacco products are Europe and the Asia Pacific region, which does not include the PRC.
The following table sets forth our tobacco revenue and percentage for tobacco products by region for the years ended June 30, 2021 and 2022 and the six months ended December 31, 2021 and 2022 based on information provided to us by our distributors (dollars in thousands).
| Year Ended June 30, | Six Months Ended December 31, | |||||||||||||||||||||||||||||||
| 2021 | 2022 | 2021 | 2022 | |||||||||||||||||||||||||||||
| Revenues | % | Revenues | % | Revenues | % | Revenues | % | |||||||||||||||||||||||||
| Europe | $ | 45,568 | 74.4 | % | $ | 51,886 | 76.2 | % | $ | 29,946 | 63.4 | % | $ | 33,404 | 56.8 | % | ||||||||||||||||
| Asia Pacific (excluding China) | 11,915 | 19.4 | % | 13,213 | 19.4 | % | 7,322 | 15.5 | % | 8,117 | 13.8 | % | ||||||||||||||||||||
| North America | 3,757 | 6.1 | % | 2,849 | 4.2 | % | 9,904 | 21.0 | % | 17,163 | 29.2 | % | ||||||||||||||||||||
| Other | 30 | 0.1 | % | 169 | 0.2 | % | 61 | 0.1 | % | 156 | 0.2 | % | ||||||||||||||||||||
| $ | 61,270 | $ | 68,117 | $ | 47,233 | $ | 58,840 | |||||||||||||||||||||||||
For the years ended June 30, 2021 and 2022 and six months ended December 31, 2021 and 2022, our revenues from cannabis products was approximately $2.1 million, $20.0 million, $8.3 million and $15.8 million respectively. All sales of cannabis products to date have been in the United States, although we have recently commenced marketing efforts in Canada and Europe, primarily the European Union.
2
Industry Developments
Historically, combustible tobacco products, primarily cigarettes and cigars, have been, and continue to be, the principal tobacco products used by adult smokers. Diverse customer demands are driving the innovation in the tobacco industry. During the past few decades, a number of alternatives to combustible tobacco products have entered the market. These products can be classified in three categories – smokeless oral-use products (including moist snuff, snus, and nicotine pouches), e-vapor products and heated tobacco products.
Vapor devices are distinguished from traditional combustible tobacco products by their production of vapor through a process of heating rather than the burning associated with the consumption of cigarettes, cigars, cigarillos or smoking tobacco. In their current form, vapor devices usually include electronic circuitry and a power source supplying energy to the heating mechanism. Vapor products are not distinguished by the absence of tobacco. While the majority of current devices (e-cigarettes) are intended for use with a tobacco-derived or synthesized nicotine containing liquid the category includes tobacco products where it is heated and not combusted, such as heat-not-burn devices. Closed vaping systems designed to look like a cigarette are referred to as cigalikes.
Our products are vapor devices, a category which includes closed system vaping devices (non-cigalikes), vaping components, and open system vaping devices.
Over the past ten years, both technological advances and consumer demand resulting in large part from a desire to obtain the effects of smoking without the adverse health effects resulting from smoking cigarettes, have led to both an increase in the global popularity of vaping along with the application of anti-tobacco legislation and regulation to electronic products, including vaping. Innovation in battery and other component technologies have greatly improved product functionality and reliability. Many consumers are attracted to the discreetness that vaporizers provide in terms of size and ease of use, style/fashion, and the perception that vaping is less detrimental to health than cigarettes. The e-vapor market worldwide has experienced rapid growth through 2019. However, the growth rate decreased in 2020, in part, we believe, because of the steps taken by governments worldwide to address the COVID-19 pandemic, which was reflected in our decrease in revenue in the year ended June 30, 2020. However, government regulations, particularly in the United States, have materially impacted our revenue in the United States.
Cannabis has a long and entrenched history in the United States due to considerable popularity of medical and recreational use in addition to long-standing industrial production of hemp. While the 20th century saw the growth of increasingly negative attitudes towards cannabis from policy makers and the general public, recent decades have witnessed a significant shift in the perception of cannabis. In the late 1990s, acceptance of medical cannabis grew enough to allow for changes in regulation and the 2010s have seen rapid expansion of social acceptance for recreational use. Medical cannabis in particular has seen increasing approval as Americans seek alternatives to pharmaceutical products in the wake of a serious public health crisis related to prescription opioids. Legalization is also increasing thanks to growing movements seeking to reform the US criminal justice system. For example, on October 6, 2022, President Biden announced pardons of all prior federal convictions for simple marijuana possession, urged states to take a similar approach to state marijuana convictions, and ordered officials in his administration to revisit the Schedule I status of marijuana under the federal Controlled Substances Act. Increasing numbers of American political reformers see eliminating criminal penalties for use and sale of cannabis as a way to address various social and economic inequalities.
Adult-use cannabis has attracted mainly recreational consumers who use cannabis for relaxation and socialization. Former medical cannabis users have also been attracted to the adult-use space as it has expanded due to its lower barriers to entry (no requirement to get permission from a doctor to gain access) and significant overlap of products between medical and adult-use product line-ups. Nonetheless some medical patients do opt to continue using medical cannabis even after adult-use legalization due to preferential tax rates on medical products in some states, and social acceptance of cannabis is growing in the United States.
Cannabis vaping is the action of inhaling and exhaling vapor containing cannabis oil produced by a vaporizer technology device. We believe that vaping has become the preferred choice of many cannabis users due to its discreetness in both carrying and smell, ease of use, and perceived health benefits relative to smoking cannabis cigarettes or bowls which create smoke. Cannabis vaping products for adult recreational use is largely limited to the United States with modest use in Canada.
During the 117th Congress (which ended on January 3, 2023), legislators introduced a number of bills aimed at increasing opportunities for cannabis industry members and their service providers. The most comprehensive legislation introduced to date, the Cannabis Administration and Opportunity Act, would provide for federal regulation of cannabis products by the U.S. Food and Drug Administration (“FDA”) and taxation by the U.S. Alcohol Tobacco Tax and Trade Bureau (TTB) but would afford states continued authority to restrict and regulate such products. The States Reform Act would remove marijuana from Schedule I of the Controlled Substances Act and locate federal regulatory and taxation authority for cannabis products at the TTB while also affording states the ability to continue to restrict or prohibit such products. Passed by the House of Representatives, the Marijuana Opportunity, Reinvestment, and Expungement Act would also remove marijuana from the scope of the Controlled Substances Act and establish a federal excise and occupational tax framework for cannabis products. While none of these bills passed during the 117th Congress, we cannot predict whether or when any of these or other similar bills will be introduced in the new Congress or what specific provisions they will contain if so. However, we believe that the fact that various members of Congress in both houses have recently introduced cannabis reform bills—and that the House of Representatives has passed such legislation—suggests an openness to and momentum towards federal cannabis reform in the near term.
3
Our Strategy
We believe that we have the ability to evaluate the market need for both tobacco and cannabis vaping products and to develop products to meet the need. We believe that we have implemented systems of quality control that cover the key steps of supply chain management to provide high-quality products to adult smokers in a consistent manner.
Our strategy is to implement a multi-prong growth strategy directed at increasing our e-cigarette vaporizer technology products and developing our cannabis vaporizer technology products. Although we have ceased marketing tobacco vaping products in the United States as a result of regulator issues, we are actively marking our cannabis vaping products in the United States, primarily as ODM sales to other cannabis brands.
Historically, we have focused on building and growing our own branded business, with OEM and ODM sales accounting for a minor portion of our revenue for the years ended June 30, 2021 and 2022 and six months ended December 31, 2021 and 2022. We are looking to expand our OEM and ODM tobacco business, which accounted for approximately $1.1 million, $0.7 million, $0.6 million and $0.6 million of total revenue from tobacco products in the years ended June 30, 2021 and 2022 and six months ended December 31 2021 and 2022, respectively, and we believe that OEM/ODM will represent a key growth area for us in the future.
We plan to increase sales of our e-cigarette vaporizer technology products by increasing the number of distributors and regions as well as increasing sales to existing customers. We believe that our brands have a reputation for high quality products in the marketplace. We will seek to introduce new products to meet customer needs based on our assessment of the direction of the market. We do not market or sell nicotine vaping products in the United States and we have no plans to do so.
We will continue to expand our technology leadership by investing in vaporizer and similar technology research and development. Our research and development activities will be oriented to focus on both medical and recreational usages of cannabis products. We recognize that industry trends can change rapidly. We believe that our products must be at the forefront of technology if we are going to prosper. The cannabis vaping business is in its early stages and we will seek to develop a strong and leading position in this market. This market is currently largely in the United States, and we plan to be in the forefront as this market and other markets develop.
Through extensive research and development, our chief executive officer developed the patented Ducore technology, which is being assigned to us and which enables our cannabis vaporizer products to heat cannabis oil, which, we believe is the first leak-proof patented design, which enables the consumer to get the full flavor experience of the cannabis. Sales of our cannabis products to date are largely sales to cannabis brands on an ODM basis, and we anticipate that our cannabis sales will continue to be primarily ODM sales for the near future. It is the responsibility of our customers, cannabis brands, to manufacture the cannabis oil and load the oil into our vaping hardware product. We also sell some hardware products to end users, but our sales are primarily to ODM users. None of our products include cannabis oil or hemp oil.
Besides growing organically, we may consider strategic acquisitions, investments and business relationships if we believe it would help further our business strategy.
Effects of COVID-19 Pandemic
In December 2019, coronavirus disease 2019 (COVID-19) was first reported to have surfaced in Wuhan, China. During 2020, the disease spread to many parts of the world. The epidemic has resulted in quarantines, travel restrictions, and the temporary closure of stores and facilities in much of the world.
Measures taken by various governments to contain the virus have affected economic activity in all countries where the consumers of our product live. We took a number of measures to monitor and mitigate the effects of COVID-19, such as safety and health measures for our personnel, such as social distancing, in accordance with government policies and advice, securing the supply of materials that are essential to our production process. We will continue to follow the various government policies and advice and, in parallel, we may take further actions that we determine are in the best interests of our employees, customers, and business relationships.
Our products are manufactured in China by Shenzhen Yi Jia, which is 95% owned by our chief executive officer and controlling stockholder, Tuanfang Liu. From the middle of January 2020, the start of the Chinese New Year holiday, until the end of April 2020, its production was slowed due to the COVID-19 pandemic in China. This slowdown in production resulted in a near-total stoppage of Shenzhen Yi Jia’s supplier’s business during this nearly four-month period and our sales activities were substantially reduced. In addition, since our products are generally sold in stores such as grocery stores, convenience stores and tobacco stores, to the extent that either the stores are closed as a result of government actions or consumers are reluctant to go shopping because of the COVID-19 pandemic, retail sales of our products suffered. Following the temporary slowdown in production in the first half year of 2020, our supplier, Shenzhen Yi Jia, has recovered its production capacity, and we have not experienced significant supply chain constraints as a result of the pandemic.
4
From late 2020 to the middle of 2021, COVID-19 vaccination program had been greatly promoted around the globe, however several types of COVID-19 variants emerged in different parts of the world. Our sales in Europe and the United States continued to be affected by government actions relating to COVID-19 and COVID-19 variants, while the production was slightly reduced by the strict measures taken by the Government of China to address the small COVID-19 outbreaks in Guangdong province. China has recently ceased enforcing a zero-COVID policy. However, the COVID-19 risk in China is that deaths and hospitalizations from COVID-19 may affect the business in China and may affect Shenzhen Yi Jia’s operations as well as the operations of its suppliers. We cannot predict the effect the change from China’s zero-COVID policy will have on the business of Shenzhen Yi Jia or any of its suppliers. To the extent that the effects of the termination of China’s zero COVID policy result in closing or significantly reducing the operations of Shenzhen Yi Jia or any of its suppliers, our business will be impacted since we presently rely on Shenzhen Yi Jia for our products. We cannot predict the effect on our business, the business of Shenzhen Yi Jia and on business in the PRC in general as a result of the change in the PRC government’s zero-COVID rules, including the effect of increased infections, deaths and hospitalizations as well as the development of any new variants of COVID. To the extent that quarantine is required of any person entering mainland China, our business may be impacted. Although we do not have any operations or assets in mainland China, to the extent that any of our key personnel or any key personnel of Shenzhen Yi Jia or any of our or its suppliers are subject to quarantine on entering mainland China, our business may be impacted. Since January 8, 2023, no centralized quarantine or mass PCR testing will be undertaken on travelers entering mainland China. Travelers to mainland China are only required to take PCR test 48 hours prior to their departure and report the PCR test findings on their customs health declaration form. Only those whose test results are positive prior to departure will have to postpone their travel until the PCR results turn negative.
Supply Chain Risks
One of effects of the COVID-19 has been delays resulting from supply chain issues, which relate to the difficulty that companies have in having their products manufactured, shipped to the country of destination, and delivered from the port of entry to the customer’s location. As a result of the COVID-19 pandemic, during 2021 and early 2022, there were fewer longshoremen unloading ships and fewer truckers to deliver the products to market, which has resulted in significant delays in the delivery of products to markets. To the extent that products are shipped by sea, there are additional risks resulting from ports not being able to unload ships promptly, causing delays in getting into port, including potential damage from seawater and fire, product degradation and the possibility of containers being destroyed, damaged or falling off the ship into the water. The inability to deliver products to the ultimate vendor could impair our ability to generate revenue from our products. As the port delays have significantly decreased, we do not believe that the supply chain issues that affected our operations are currently affecting us. We cannot assure you that such delays will not affect our business in the future.
In 2021, Shenzhen Yi Jia suffered a chip shortage resulting in a slowdown in delivery of its products to us from April to August 2021. To secure the supply of chips, Shenzhen Yi Jia changed the payment terms to chip supplier from 30 days after delivery in the past to prepayment, and it engaged two new chip suppliers. Since September 2021, Shenzhen Yi Jia has obtained a supply of chips to meet its production need and a chip shortage no longer affect its production. During the six months ended December 31, 2022, a slowdown in the delivery of components to Shenzhen Yi Jia resulting from supply chain slowdowns as a result of the effects of China’s zero-COVID policy resulted in an increase in our cost of revenue during the period. We cannot assure you that we will not suffer from a chip shortage or that the effects of China’s COVID policy will not affect Shenzhen Yi Jia’s ability or the ability of its suppliers to delivery products in a timely manner.
With respect to sales to distributors other than in the United States, we are not involved in the shipping by the distributors, who arrange for shipping. However, to the extent that the distributors incur shipping delays in getting the product to market, their purchases of products from us may be delayed or reduced. We ship products for the United States market by air.
Controlled Company
A controlled company is a company of which more than 50% of the voting power for the election of directors is held by an individual, a group or another company. We are a controlled company because Mr. Tuanfang Liu, our chief executive officer, holds more than 50% of our voting power, and we expect we will continue to be a controlled company upon completion of this offering. For so long as we remain a controlled company, we are exempt from the obligation to comply with certain Nasdaq corporate governance requirements, including:
| ● | our board of directors is not required to be comprised of a majority of independent directors. | |
| ● | our board of directors is not subject to the compensation committee requirement; and | |
| ● | we are not subject to the requirements that director nominees be selected either by the independent directors or a nomination committee comprised solely of independent directors. |
The controlled company exemptions do not apply to the audit committee requirement or the requirement for executive sessions of independent directors. We are required to disclose in our annual report that we are a controlled company and the basis for that determination. Although we do not plan to take advantage of the exemptions provided to controlled companies, other than including our chief executive officer and controlling stockholder as the chairman of the nominating and corporate governance committee, we may in the future take advantage of such exemptions.
5
Implications of Being an Emerging Growth Company
As a company with less than US$1.235 billion in revenue for the last fiscal year, we qualify as an “emerging growth company” pursuant to the Jumpstart Our Business Startups Act of 2012, as amended (the “JOBS Act”). An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, in the assessment of the emerging growth company’s internal control over financial reporting. The JOBS Act also provides that an emerging growth company does not need to comply with any new or revised financial accounting standards until such date that a private company is otherwise required to comply with such new or revised accounting standards. We intend to take advantage of certain of these exemptions.
We will remain an emerging growth company until the earliest of (i) the last day of our fiscal year during which we have total annual gross revenues of at least US$1.235 billion; (ii) the last day of our fiscal year following the fifth anniversary of the completion of this offering; (iii) the date on which we have, during the previous three year period, issued more than US$1.0 billion in non-convertible debt; or (iv) the date on which we are deemed to be a “large accelerated filer” under the Exchange Act, which would occur if the market value of our common stock that are held by non-affiliates exceeds US$700 million as of the last business day of our most recently completed second fiscal quarter and we have been publicly reporting for at least 12 months. Once we cease to be an emerging growth company, we will not be entitled to the exemptions provided in the JOBS Act discussed above.
Matters Relating to PRC Laws
In this prospectus, “China” or the “PRC” refers to the People’s Republic of China; mainland China refers to the PRC, excluding Taiwan, the Hong Kong Special Administrative Region and the Macao Special Administrative Region, and PRC Laws refer to the laws, rules, regulations, statutes, notices, circulars and court judicial interpretation or the like of mainland China. Any PRC Laws refer to those currently in force, published for comments (if specifically stated) or being promulgated but have not come into effect (if specifically stated) and publicly available in mainland China as of the date of this prospectus. The majority of our operations are not in mainland China, and we do not believe we are subject to the PRC Laws applicable to those companies established in mainland China as of the date of this prospectus, based on advice from Han Kun Law Offices. We are mainly engaged in the research and development, design, commercialization, sales, marketing and distribution of branded e-cigarettes and cannabis vaping products. The sales of our tobacco products are conducted worldwide except for China and Russia. Through our global distributor network of more than 150 distributors, we sell the Aspire brand of tobacco vaporizer technology products in more than 30 countries and the main markets for such tobacco products are Europe and the Asia Pacific region, which does not include China.
We have two operating subsidiaries established, one in California and the other in Hong Kong. We do not conduct business and we do not have any employees, assets or funds in mainland China. Although most of our cash is in Hong Kong banks, a significant amount of these funds is to be paid to a related party. See “Certain Relationships and Related Party Transactions.” Our operations are primarily in the United States. Although our chief executive officer lives in mainland China, where Shenzhen Yi Jia is located, the services that he performs for us in his capacity as our chief executive officer are performed primarily in Hong Kong and the United States. In addition to serving as our chief executive officer, Tuanfang Liu is chairman of Shenzhen Yi Jia, and the services he provides in mainland China are performed in his capacity as chairman of Shenzhen Yi Jia. Our employees are largely in the United States, with 48 employees based in the United States and where our research and development activities are conducted, and eight employees in Hong Kong. Our facilities are located primarily in the United States, where we lease more than 83,600 square feet of office, manufacturing and storage space and where our research and development activities are conducted, as compared with 1,850 square feet of office space in Hong Kong. Hong Kong was established as a special administrative region of the PRC in accordance with Article 31 of the Constitution of the PRC. The Basic Law of the Hong Kong Special Administrative Region of the PRC (the “Basic Law”) was adopted and promulgated on April 4, 1990 and became effective on July 1, 1997, when the PRC resumed the exercise of sovereignty over Hong Kong. Pursuant to the Basic Law, Hong Kong is authorized by the National People’s Congress of the PRC to exercise a high degree of autonomy and the PRC Laws shall not be applied in Hong Kong, other than those relating to national defense, foreign affairs, and certain other matters that are not within the scope of autonomy of Hong Kong. While the National People’s Congress of the PRC has the power to amend the Basic Law, the Basic Law also expressly provides that no amendment to the Basic Law shall contravene the established basic policies of the PRC regarding Hong Kong. As a result, as of the date of this prospectus, national laws of the PRC that would be applicable to us if we were a corporation organized under PRC Laws or located in mainland China do not apply to our Hong Kong subsidiary. However, there is no assurance that certain PRC Laws, including existing laws and regulations and those enacted or promulgated in the future, will not be applicable to our Hong Kong subsidiary due to change in the current political arrangements between mainland China and Hong Kong or other reasons whether foreseeable or not presently foreseeable. The application of such laws and regulations may have a material adverse impact on us, as relevant PRC authorities may impose fines and penalties upon our Hong Kong subsidiary, delay or restrict the repatriation of the proceeds from this offering into Hong Kong, and any failure of us to fully comply with such new regulatory requirements may significantly limit or completely hinder our ability to offer or continue to offer our common stock, cause significant disruption to our business operations, and severely damage our reputation, which would materially and adversely affect our financial condition and results of operations and cause our common stock to significantly decline in value or in extreme cases, become worthless. See “Risk Factors – Risks Related to Our Business and Industry – Although we believe that our business is not subject to PRC Laws, our business could be materially impaired if it is determined that our business is subject to PRC Laws.” on page 27 and “Business -- Matters Relating to PRC Laws” on page 55.
At present, our products are manufactured and supplied by Shenzhen Yi Jia, a Chinese company under common control. However, we are also taking the initial steps toward the development of manufacturing operations in Vietnam and California, and our future operations will be diversified in different places of business, although we can give no assurance that we will be successful in developing and sustaining any manufacturing operations in Vietnam or California.
6
Our Organization
We are a Delaware corporation, organized on June 13, 2022. Aspire North America, LLC, a California limited liability company was formed on February 22, 2020, and 100% of its ownership was transferred to Aspire Global on September 23, 2020 and was transferred by Aspire Global to Ispire Technology on July 29, 2022. Aspire Science, a Hong Kong corporation, was incorporated on December 9, 2016 as a subsidiary of Aspire Global, and 100% of its equity was transferred to our subsidiary, Ispire International, on July 29, 2022. Ispire International was organized on July 6, 2022. Aspire North America and Aspire Science are our operating companies.
The following chart shows our corporate structure.
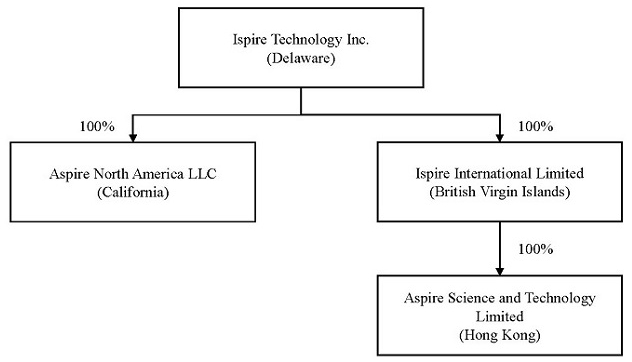
7
The Underwritten Offering
| Shares being offered by us: | 6,000,000 shares of common stock (or 6,900,000 shares of common stock if the Underwriters exercises their over-allotment option in full) on a firm commitment basis. |
| Initial offering price: | $6.00 to $8.00 per share. |
| Number of shares of common stock outstanding before the offering: | 50,000,0001 of our shares of common stock are outstanding as of the date of this prospectus. |
| Number of shares of common stock outstanding after the offering: | 56,000,0001 shares of common stock (or 56,900,0001 shares of common stock if the Underwriters exercise their over-allotment option in full). |
| Underwriter over-allotment option: | We have granted the Underwriters an option for a period of up to 45 days from the date of this prospectus to purchase up to 900,000 additional shares of common stock to cover over-allotments. |
| Use of proceeds: | We estimate that we will receive net proceeds from this offering of approximately $ 37.1 million (or approximately $43.0 million if the Underwriters exercise their over-allotment option in full), after deducting underwriting discounts, commissions and estimated offering expenses payable by us and assuming an initial offering price of $7.00 per share, the midpoint of the proposed offering price range.
We plan to use the net proceeds of this offering as follows: |
| ● | approximately 35% to develop manufacturing operations in both Vietnam and the United States; | |
| ● | approximately 25% for research and development activities, which include our efforts to develop new products and new vaping technology; | |
| ● | approximately 20% for the marketing and promotion of our branded products; | |
| ● | the balance of approximately 20%, together with any proceeds from the over-allotment option, for general administration and working capital. | |
| See “Use of Proceeds.” | ||
| Lock-up: | Our directors, officers and 5% or greater stockholders have agreed with the Underwriters not to sell, transfer or dispose of, directly or indirectly, any of our common stock or securities convertible into or exercisable or exchangeable for our common stock for a period of six months after the date of this prospectus. See “Shares Eligible for Future Sale” and “Underwriting” for more information. |
| Listing: | We intend to apply to have our common stock listed on the Nasdaq Capital Market, or Nasdaq. We cannot guarantee that we will be successful in listing our common stock on the Nasdaq; however, we will not complete this offering unless we are so listed. |
| Proposed Nasdaq symbol: | ISPR |
| Risk factors: | Investing in our common stock is highly speculative and involves a significant degree of risk. As an investor, you should be able to bear a complete loss of your investment. You should carefully consider the information set forth in the “Risk Factors” section. |
| 1 | Does not include up to 15,000,000 shares of common stock issuable pursuant to our 2022 Equity Incentive Plan. |
Offering by Selling Stockholders
Separate and apart from our underwritten public offering, two of our stockholders are offering an aggregate of 1,750,000 outstanding shares of common stock which they may sell at the initial public offering price of the underwritten offering until such time as our common stock is listed on the Nasdaq Capital Market, at which time they may sell such shares from time to time at prevailing market prices or at negotiated prices. The selling stockholders have not engaged any underwriter in connection with the sale of their shares, and we will not receive any proceeds from the sale by the Selling Stockholders of their shares. See “Selling Stockholders” and “Plan of Distribution.”
8
SUMMARY OF RISK FACTORS
Our business is subject to numerous risks described in the section titled “Risk Factors” and elsewhere in this prospectus. The main risks set forth below and others you should consider are discussed more fully in the section entitled “Risk Factors,” which you should read in its entirety.
| ● | Existing laws, regulations and policies as well as changes in existing laws, regulations and policies and the issuance of new laws, regulations, policies and any other entry barriers in relation to the e-vapor industry have materially and adversely affected and may further materially and adversely affect our business operations. | |
| ● | As a result of regulations in the United States, we are able to sell only one product line, the Nautilus Prime, in the United States. Our tobacco vaping sales in the United States were approximately $0.9 million the year ended June 30, 2022 and approximately $0.5 million for the six months ended December 31. 2022. Because the volume of sales did not justify the marketing and regulatory costs, we have ceased marketing tobacco vaping products in the United States. If any similar regulations are adopted with respect to cannabis products, our business will be severely impacted since all of our cannabis revenue for the year ended June 30, 2022 was generated from sales in the United States. | |
| ● | The recent amendments to the Prevent All Cigarette Trafficking (“PACT”) Act and regulations of the United States Postal Service extend the PACT Act to include e-cigarette and all vaping products, which include cannabis as well as tobacco products. These regulations place significant burdens on sellers of vaping products in the United States which may make it difficult to operate profitably in the United States. We use a combination of advanced accounting software and PACT Act compliant carriers to remain compliant with the tax and delivery restrictions of the PACT Act. To the extent that the carriers that we currently use change their policies and refuse to ship vaping products and we are not able to find other carriers that are PACT Act compliant, our business and prospects will be materially impaired, and we may not be able to continue in business. | |
| ● | We have no experience in the establishment and operation of manufacturing facilities in either Vietnam or the United States. In order to operate a manufacturing facility, even if it is limited to assembly from components manufactured by others, we will need to hire qualified personnel and comply with applicable laws, the failure of which could materially impair our business and operating results. | |
| ● | The market for cannabis vaping products has not developed, with the vast majority of sales being in the United States, with no assurance that a significant market will develop either in the United States or worldwide or that, if a market develops, we will be able to compete successfully with other companies that may enter the market as well as other legal and illegal forms of cannabis. | |
| ● | Because our chief executive officer and his wife will own 63.8% of our common stock after the offering ( 62.8% if the overallotment option is exercised in full) and he also owns 95% of the equity in the Chinese company that is presently our sole supplier and, as chief executive officer, he has significant authority over the conduct of our business, including the determination of price and other terms on which we purchase product from the supplier, he has a conflict of interest which may affect the development of our business and therefore the price of our common stock. | |
| ● | We sustained losses of approximately $2.0 million for the year ended June 30, 2022 and approximately $3.0 million for the six months ended December 31, 2022, and we cannot assure you that we can or will operate profitably in the future. | |
| ● | Because cannabis oil, unlike nicotine oil, is not of a uniform quality, products we design may not perform as intended, which could result in a loss of business |
| ● | If it is determined or perceived that the usage of e-vapor tobacco or cannabis products poses long-term health risks, the use of e-vapor products may decline significantly, which would materially and adversely affect our business, financial condition and results of operations. | |
| ● | Our business and the industry in which we operate are subject to inherent risks and uncertainties, including, among others, developments in regulatory landscape, medical discovery and market acceptance of vaping devices. | |
| ● | We are exposed to product liabilities and user complaints arising from the products we sell, which could have a material adverse impact on us. | |
| ● | Outbreaks of communicable diseases, natural disasters or other events may materially and adversely affect our business, results of operations and financial condition, including the effects of the COVID-19 pandemic and steps taken by governments to address the pandemic, which resulted in a four-month slowdown of our production during 2020 leading to a negative impact on our revenue and net income. | |
| ● | Misuse or abuse of our products may lead to potential adverse health effects, subjecting us to complaints, product liability claims and negative publicity. |
9
| ● | We face competition from companies in the e-vapor industry, including companies which are larger, better known and have a significantly larger market share than we do, and we may fail to compete effectively, as well as, with respect to cannabis, other legal and illegal forms of cannabis. | |
| ● | Misconduct, including illegal, fraudulent or collusive activities, by our employees, distributors, retailers or suppliers, may harm our brand and reputation and adversely affect our business and results of operations. |
| ● | We may be subject to liability if private information that we receive is not secure or if we violate privacy laws and regulations. | |
| ● | Our intellectual property rights, which were developed by our chief executive officer and Shenzhen Yi Jia and which we are acquiring from Shenzhen Yi Jia, Aspire Global and our chief executive officer with respect to cannabis and are licensing from Shenzhen Yi Jia, Aspire Global and our chief executive officer with respect to tobacco, are critical to our success. Infringement of our intellectual property rights by any third party or loss of our intellectual property rights may materially and adversely affect our business, financial condition and results of operations. | |
| ● | We and Shenzhen Yi Jia may not be able to prevent others from unauthorized use of our intellectual property, which could harm our business and competitive position. As the patents may expire and may not be extended, the patent applications may not be granted and the patent rights may be contested, circumvented, invalidated or limited in scope, our patent rights may not protect us effectively. | |
| ● | Our chief executive officer, who is also the chief executive officer and principal stockholder of Aspire Global and the 95% owner of Shenzhen Yi Jia, has potential conflicts of interest with us, which may materially and adversely affect our business and financial condition. | |
| ● | Because we are a controlled company, as defined by Nasdaq’s rules, we can take advantage of exemptions from certain Nasdaq corporate governance requirements, and, to the extent that we take advantage of these exemptions, you will not have the corporate governance protections normally provided to stockholders of a Nasdaq-listed company. | |
| ● | We may be liable for improper use or appropriation of personal information provided by our customers. | |
| ● | The sale or the anticipation of the sale by the selling stockholder may have an adverse effect upon the market price of our common stock and the Underwriters’ stabilization activities and the exercise of the Underwriters’ over-allotment option. | |
| ● | There has been no public market for our common stock prior to this offering, and you may not be able to resell our common stock at or above the price you paid, or at all. |
10
SELECTED CONSOLIDATED FINANCIAL DATA
(dollars in thousands, except per share amounts)
Consolidated Statement of Operations Data:
| Year Ended June 30, | Six Months Ended December 31, | |||||||||||||||
| 2021 | 2022 | 2021 | 2022 | |||||||||||||
| Revenue | $ | 63,415 | $ | 88,095 | $ | 47,233 | $ | 58,840 | ||||||||
| Cost of revenue | 52,999 | 74,789 | 39,921 | 48,910 | ||||||||||||
| Gross profit | 10,416 | 13,306 | 7,312 | 9,930 | ||||||||||||
| Operating expenses | 6,772 | 14,295 | 5,528 | 11,608 | ||||||||||||
| Income from operations | 3,644 | (989 | ) | 1,784 | (1,678 | ) | ||||||||||
| Other income(expense) | (20 | ) | 186 | 124 | (441 | ) | ||||||||||
| Income(loss) before income taxes | 3,624 | (803 | ) | 1,908 | (2,119 | ) | ||||||||||
| Net income(loss) | 2,938 | (1,874 | ) | 1,279 | (2,951 | ) | ||||||||||
| Net income(loss) per share | $ | 0.06 | $ | (0.04 | ) | $ | 0.03 | $ | (0.06 | ) | ||||||
| Average shares of common stock outstanding (basic and diluted) | 50,000,000 | 50,000,000 | 50,000,000 | 50,000,000 | ||||||||||||
Consolidated Balance Sheet Data
| As of June 30, | As of December 31, | |||||||||||
| 2021 | 2022 | 2022 | ||||||||||
| Assets | $ | 93,864 | $ | 100,735 | $ | 201,055 | ||||||
| Current assets | 93,421 | 99,449 | 122,808 | |||||||||
| Working capital | 13,585 | 10,481 | 7,800 | |||||||||
| Retained earnings | 13,821 | 11,946 | 8,995 | |||||||||
| Shareholders’ equity | 13,758 | 11,767 | 83,218 | |||||||||
Consolidated Cash Flows Data:
| Year Ended June 30, | Six Months
Ended December 31 | |||||||||||||||
| 2021 | 2022 | 2021 | 2022 | |||||||||||||
| Consolidated cash flow data: | ||||||||||||||||
| Net cash flows provided by (used in) operating activities | $ | 5,024 | $ | (7,558 | ) | $ | (8,484 | ) | $ | 8,825 | ||||||
| Net cash used in investing activities | (1 | ) | (122 | ) | (47 | ) | (478 | ) | ||||||||
| Net cash (used in) provided by financing activities | (228 | ) | (3,089 | ) | (2,234 | ) | 1,440 | |||||||||
| Net increase (decrease) in cash and cash equivalents | 4,795 | (10,769 | ) | (10,765 | ) | 9,787 | ||||||||||
11
An investment in our common stock involves a high degree of risks. You should carefully consider all of the information in this prospectus, including the risks and uncertainties described below, before making an investment in our common stock. Any of the following risks could have a material adverse effect on our business, financial condition and results of operations. In any such case, the market price of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Business and Industry
Existing laws, regulations and policies and the issuance of new or more stringent laws, regulations, policies and any other restrictions or limitations in relation to the tobacco vaping industry have and can materially and adversely affect our business operations.
As vaping products have become more and more popular in recent years, government authorities worldwide have imposed laws, regulations and policies to regulate nicotine vaping products and the vaping industry and may impose more stringent controls either with changes in existing laws or regulations, with new laws or regulations, or with new interpretations of existing laws or regulations. Some governments have prohibited the usage of vaping products in certain areas, imposed specific taxes on vaping products or imposed restrictions, in certain areas such as product advertising, flavorings or nicotine concentration. Governments, primarily state and municipal, have imposed restrictions or prohibitions on smoking in public and on public transportation, such as on trains, airplanes and busses. Such prohibitions have been or may in the future be extended to e-cigarettes, including vaping products, and such restrictions may be imposed by local, regional or national governments. As a result of government laws and regulations affecting tobacco products, we ceased selling nicotine vaping products in the United States.
We cannot assure you that government authorities will not impose further restrictions on vaping nicotine products in the future, including but not limited to requirements to obtain and maintain licenses, approvals or permits for relevant business operation. Such restrictions, if any, may adversely affect supplies of raw materials, production and sales activities, taxation or other aspects of our business operation. We may not be able to comply with any or all changes in existing laws and regulations or any new laws and regulations and may incur significant compliance costs. All of the above may affect our production or market demand for vaping products and thus adversely affect our business, financial condition and results of operations. To the extent that we grow in scale and significance, we expect to face increased scrutiny, which may result in increased investment in compliance and related capabilities.
The World Health Organization (“WHO”) and the United State Centers for Disease Control and Prevention (“CDC”) have been clear in their view of the harmful effects of nicotine. Although they recognize that e-cigarettes may expose users to fewer harmful chemicals than burned cigarettes, which are considered very dangerous, and that any tobacco product, including e-cigarettes, is unsafe particularly for young people and pregnant women.
Countries have taken different steps to address the dangers of nicotine and to consider the difference between e-cigarettes and burned cigarettes. However, instances of death or serious illness resulting or perceived to result from the use of e-cigarettes as well as significant reported use by certain populations, including adolescents as well as nicotine-naïve individuals, may spur governments at all levels to increase restrictions on vaping products. We cannot assure you that the actions taken by municipal, state or provincial and national governments will not materially and adversely affect the market for vaping products generally and our business in particular.
Cannabis vapor products are subject to regulations and restrictions in the United States and are prohibited in many other countries.
Cannabis products are subject to federal and state regulation in the United States, and Western Europe generally prohibits the sale and use cannabis products, although some countries permit the use of approved cannabis products for medical purposes. Although an increasing number of states in the United States permit adult use of recreational marijuana, states have restriction as to where the products can be sold and many of the states that permit recreational use of marijuana require that sales be made only at licensed stores. The U.S. federal government still prohibits non-hemp cannabis products (unless approved by the FDA) but has generally not enforced against entities and individuals operating in compliance with state laws permitting such products. Likewise, under certain circumstances, devices intended for use in consuming federally prohibited cannabis products may also technically qualify as prohibited drug paraphernalia under federal law and the laws of certain states that continue to broadly restrict production and sale of non-hemp cannabis. However, the Federal Controlled Substances Act includes an exemption for “any person authorized by local, State, or Federal law to manufacture, possess, or distribute such items.”
No country in Western Europe has yet legalized recreational cannabis, but the region has some of the most developed cannabis cultures in the world, such as in the Netherlands and Spain. However, great differences persist among consumers, with older generations typically being more reluctant to allow cannabis use. Clear generational and social gaps still exist that make legalization and development of the market a slow process, although the potential legalization of adult-use cannabis in Germany is likely to accelerate the cannabis debate within the EU and promote the development of the industry at a regional level. Our ability to expand our marketing of cannabis products in the European market is dependent upon whether recreational cannabis will become legal in Western Europe, and we cannot give any assurance that we will be able to sell products in Western Europe. These restrictions on the sale and use of cannabis could impair our ability to market and sell our products.
12
While we believe that our business and sales do not violate the Federal Paraphernalia Law, legal proceedings alleging violations of such law or changes in such law or interpretations thereof could adversely affect our business, financial condition or results of operations.
Under U.S. Code Title 21 Section 863 (the “Federal Paraphernalia Law”), the term “drug paraphernalia” means “any equipment, product or material of any kind which is primarily intended or designed for use in manufacturing, compounding, converting, concealing, producing, processing, preparing, injecting, ingesting, inhaling, or otherwise introducing into the human body a controlled substance.” That law exempts “(1) any person authorized by local, State, or Federal law to manufacture, possess, or distribute such items” and “(2) any item that, in the normal lawful course of business, is imported, exported, transported, or sold through the mail or by any other means, and traditionally intended for use with tobacco products, including any pipe, paper, or accessory.” Any non-exempt drug paraphernalia offered or sold by any person in violation of the Federal Paraphernalia Law can be subject to seizure and forfeiture upon the conviction of such person for such violation, and a convicted person can be subject to fines under the Federal Paraphernalia Law and even imprisonment.
Several states with legal cannabis programs, including California, have enacted legislation invoking this exemption to shield state-legal businesses from federal enforcement on paraphernalia grounds. In addition, a recent court decision from the U.S. Court of International Trade applied this exemption in prohibiting U.S. Customs and Border Protection from refusing import entry of cannabis paraphernalia components that the importer could legally possess in the state of importation.
We believe our sales do not violate the Federal Paraphernalia Law in any material respect. We restrict the sale of products to comply with the Federal Paraphernalia Law’s exemption for sales authorized by state law. In particular, we (a) for our direct to customer products, we do not sell those products at all into the [__] states that have maintained complete or near complete cannabis prohibition, and have the distributers we work with covenant that they will not sell our products into these states, and (b) for our custom made and white label products, we limit the sale of those products to licensed dispensaries and entities, such as licensed cultivators or manufacturers.
While we believe that our business and sales are legally compliant with the Federal Paraphernalia Law in all material respects, any legal action commenced against us under such law could result in substantial costs and could have an adverse impact on our business, financial condition or results of operations. In addition, changes in cannabis laws or interpretations of such laws are difficult to predict, and could significantly affect our business.
Because Tuanfang Liu, our chief executive officer, who is also director, and his wife, Jiangyan Zhu, who is also a director, beneficially own 71.5% of our common stock and will own 63.8% of our common stock upon completion of this offering (62.8 % if the underwriters’ over-allotment is exercised in full), and Mr. Liu owns 95% of the equity of our sole supplier, Mr. Liu has a conflict of interest.
Because our chief executive officer and his wife own 66.5% and 5%, respectively, of our common stock, they have the power to elect all of our directors and to approve any matter which is subject to stockholder approval. Mr. Liu also own 95% of the equity in Shenzhen Yi Jia, which is currently our sole supplier. Mr. Liu is chairman of Shenzhen Yi Jia and his wife, Jiangyan Zhu, is its vice president of finance. The price and other terms at which Shenzhen Yi Jia sells product to us have been largely determined by Mr. Liu. In addition, as our chief executive officer he has significant authority in the implementation of our business plan, including the commencement of our proposed manufacturing operations in Vietnam and California. He has also historically been responsible for our product development and our present products have been the result of his research and development efforts. Mr. Liu’s interests may be different from our interests. Because of Mr. Liu’s conflict of interest, there is a risk that any actions he may take may have an adverse effect upon the success and development of our business and the price of our common stock.
As a result of the voting power of Mr. Liu and his wife, Ms. Zhu, investors will have little, if any, power to influence our business or to approve any action submitted to stockholders for their approval. The fact that they will continue to have a controlling interest in us may, by itself, serve as a deterrent to any person seeking to obtain control of us or to enter into any business relationship which might be beneficial to the minority stockholders.
13
Although our supply agreements with Shenzhen Yi Jia require Shenzhen Yi Jia to sell products to us at the most favorable market price that it sells similar products to third parties, because our products are designed for us and based on technology that was either developed by Mr. Liu prior to the date of the agreement or is developed by us, we cannot determine whether another supplier would be able to provide the products at the same or a better price. However, all pricing will be designed to enable us to sell the products at a price which enables us to generate a gross margin that we consider acceptable, and Mr. Liu will have significant input as to what is an acceptable gross margin. Our supply agreements also require Shenzhen to provide us with quality products and services in a timely manner, to provide to our customers the same warrant that we provide to our customer and to give first priority to the manufacture of our products over any other manufacturing obligations. However, as our chief executive officer, Mr. Liu has the ability to determine whether to pursuant any legal action to enforce our supply agreements. Thus, we will be relying on Mr. Liu taking actions that are in our best interests, and we run the risk that he may not do so.
The recent implementation of regulations relating to e-cigarettes has resulted in our decision not to market nicotine products in the United States.
The FDA has authority to regulate e-liquids, e-cigarettes, and other vaping products that contain (or are used to consume e-liquid containing) tobacco-derived ingredients and nicotine from any source as “tobacco products” under the federal Food, Drug and Cosmetic Act (the “Food, Drug and Cosmetic Act”), as amended by Family Smoking Prevention and Tobacco Control Act of 2009 (the “Tobacco Control Act”) and subsequent legislation. Through the issuance of the “Deeming Regulation” that became effective on August 8, 2016, the FDA began regulating e-liquids, e-cigarettes, and other vaping products that qualify as “tobacco products” under the Food, Drug and Cosmetic Act’s requirements added by the Tobacco Control Act. The Food, Drug and Cosmetic Act requires that any Deemed Tobacco Product that was not commercially marketed as of the “grandfather” date of February 15, 2007, obtain premarket authorization before it can be marketed in the United States. The compliance policy generally allowed companies to market Deemed Tobacco Products that qualify as “new tobacco products” but that were on the U.S. market on August 8, 2016, until September 9, 2020, and the continued marketing of such products without otherwise-required authorization for up to one year during the FDA’s review of a pending marketing application submitted by September 9, 2020. The compliance policy did not apply to otherwise-eligible products (i) for which the manufacturer has failed to take (or is failing to take) adequate measures to prevent minors’ access and (ii) that are targeted to minors or with marketing that is likely to promote use by minors. In the absence of this policy, we would have had to obtain prior authorization from the FDA to market any of our products after August 8, 2016. Accordingly, through September 9, 2020, Aspire North America marketed tobacco vaping products in the United States pursuant to the FDA’s compliance policy based on evidence that they were on the U.S. market on August 8, 2016, and had not been physically modified since.
FDA authorization to introduce a “new tobacco product” (or to continue marketing a “new tobacco product” covered by the current compliance policy for Deemed Tobacco Products that were on the U.S. market on August 8, 2016) could be obtained via any of the following three authorization pathways: (1) submission of a premarket tobacco product application (“PMTA”) and receipt of a marketing authorization order; (2) submission of a substantial equivalence report and receipt of a substantial equivalence order; or (3) submission of a request for an exemption from substantial equivalence requirements and receipt of a substantial equivalence exemption determination.
Since there were few, if any, e-liquid, e-cigarette, or other vaping products on the market as of February 15, 2007, there is no way to utilize the less onerous substantial equivalence or substantial equivalence exemption pathways that traditional tobacco companies can utilize for cigarettes, smokeless tobacco, and other traditional tobacco products. In order to obtain marketing authorizations, manufacturers of practically all e-liquid, e-cigarette, or other vaping products would have to use the PMTA pathway, which could potentially cost $1.0 million or more per application. Furthermore, the Deeming Regulation created a significant barrier to entry for any new e-liquid, e-cigarette, or other vaping product seeking to enter the market after August 8, 2016, since any such product would require an FDA marketing authorization through one of the aforementioned pathways.
We filed a PMTA for the Nautilus Prime open system vaping products on September 9, 2020, and the FDA has not to date taken final action on our PMTA. For this reason, and based on public FDA statements, it appears that the FDA would not prioritize enforcement of the premarket review requirements against any covered Nautilus Prime products during the continued pendency of the PMTA’s review, despite the fact that the one-year compliance period closed on September 9, 2021. The PMTA application process is very expensive, and we did not submit a PMTA for any other product. The Nautilus Prime System is an enhancement of an earlier developed Nautilus line, for which we did not submit a PMTA. Our tobacco vaping sales in the United States were $2.5 million for the year ended June 30, 2021 and approximately $0.9 million for the year ended June 30, 2022, largely as a result of our inability to sell products that we sold in prior years, even though the effects of the COVID-19 pandemic in the United States were not as severe during most of the year ended June 30, 2021, as states relaxed their regulations that affected commerce and shopping. We cannot assure you that our pending PMTA (or any other PMTA filed in the future) will ultimately result in the FDA’s timely issuance of marketing orders for the Nautilus Prime product line (or other products). See “Regulations.” We have stopped marketing tobacco vapor products in the United States because our sales volume in the United States did not justify the marketing and regulatory compliance costs.
14
Further, although we are not marketing tobacco vapor products in the United States market, and we can contractually prohibit our distributors from selling our tobacco vaping products in the United States market, in the event that those products are sold in the United States market, we cannot assure you that we will not be subject to regulatory or enforcement action as a result of such products’ being sold in the United States. We may also face regulatory or enforcement action from the FDA for certain of our products that remained distributed in the United States between September 9, 2020, and April 30, 2021, and for which we did not file a PMTA by the September 9, 2020, deadline. While we have taken steps intended to ensure that no such distribution occurs, we cannot assure you that, should the FDA prioritize these violations for regulatory action, the FDA will follow its standard of approach of issuing a public warning letter and seeking voluntary corrective action rather than initiating an enforcement action under its various Food, Drug, and Cosmetic Act authorities. Such a result could materially and adversely affect our business, financial condition, and results of operations.
On March 17, 2021, the FDA issued letters to four companies operating in the e-cigarette industry, including Aspire North America, requesting documents related to their social media marketing practices. Specifically, the FDA requested the documents “to further understand the relationship between rising youth exposure to online e-cigarette marketing and youth e-cigarette use,” and the FDA asserted in each letter that each recipient had “active brand pages on multiple popular social media platforms, a large number of followers, and did not use age restriction tools to prevent youth exposure.” Under its Food, Drug, and Cosmetic Act authority requiring industry members to produce certain documents upon request, the FDA requested that we respond within 60 days but granted us a 30-day extension. On June 15, 2021, Aspire North America provided the required information to the FDA. To date, the FDA has not substantively responded or taken any further action in the matter. However, we cannot assure you that the FDA will consider the response adequate and will not initiate regulatory or enforcement action based on an alleged failure to comply with the request or that the FDA will not initiate regulatory or enforcement action on other grounds based on the contents of the documents produced in the response. Either result could materially and adversely affect our business, financial condition, and results of operations.
In the event that similar legislation or regulations are adopted with respect to cannabis products, our business will be materially impaired since all of our sales of cannabis products were in the United States.
Recently enacted legislation and regulations in the United States may make it more difficult to sell nicotine and cannabis vaping products in the United States.
Provisions of the 2021 Appropriations Act subjected e-cigarettes and other vaping devices (including, based on recent regulations, cannabis and hemp vaporization products that liquids), as well as e-liquids products, to the provisions of the Prevent All Cigarette Trafficking Act of 2009 (the “PACT Act”), which imposes stringent rules on interstate shippers and, in particular, online sellers. Under the PACT Act, interstate shippers must register with the U.S. Attorney General and the tobacco tax administrator of each jurisdiction into which they ship products as well as submit monthly reports to such tobacco tax administrators. In addition, online retailers making delivery sales to consumers must also (i) verify the age of customers using a commercially available database, (ii) use private shipping services that collect an adult signature and verify the recipient’s age using government-issued identification at the point of delivery, (iii) if shipping to jurisdictions that tax vaping products, collect and remit all applicable local and state taxes and comply with all applicable licensing requirements of the recipient’s jurisdiction, (iv) comply with shipping-package quantity restrictions and labeling requirements, and (v) maintain records for five years of any delivery interrupted because the carrier or delivery service determines or has reason to believe that the person ordering the delivery is in violation of the PACT Act. Shippers and delivery sellers who do not comply with the PACT Act are subject to civil and criminal penalties. Accordingly, compliance with the requirements of the PACT Act may significantly increase the costs of our and our customers’ online businesses, increasing the prices of our products sold online and making them less attractive to consumers as compared to products sold at local retailers. In addition, failure to comply with the PACT Act could expose us to significant penalties that could materially adversely affect our business and our financial condition and results of operations. Further, as a result of the issuance of final regulations implementing the PACT Act amendments by the United States Postal Service (the “USPS”), the USPS generally prohibits the mailing of such products, subject to potential exceptions already applicable to combusted cigarettes and smokeless tobacco (e.g., for shipments between legally operating businesses). The USPS issued these final regulations on October 21, 2021, and the regulations took effect immediately. Further, the most commonly used carriers, Federal Express and United Parcel Service, have recently announced that they would cease all deliveries of vapor products. These restrictions on use of the USPS to ship our products and the decisions by private carriers not to deliver vapor products in the United States could materially impair our ability to sell products in the United States which would adversely affect our business, financial condition and results of operations. Further, since most of our revenue from cannabis vapor product sales is from sales to other cannabis vaping brands, if our customers are not able to deliver product in the United States, which is the largest market for cannabis vaping products, our ability to generate revenue from cannabis products would be materially impaired. We use a combination of advanced accounting software and PACT Act compliant carriers to remain compliant with the tax and delivery restrictions of the PACT Act. To the extent that the carriers that we currently use change their policies and refuse to ship or are prohibited from shipping vaping products and we are not able to find other carriers that are PACT Act compliant, our business and prospects will be materially impaired, and we may not be able to continue in the cannabis vaping business.
We are exposed to risks relating to our relationship with a related party, and we may not be able to successfully establish and operate manufacturing operations.
All of our products are presently manufactured by Shenzhen Yi Jia, a related party. Due to the reliance on our business relationship with Shenzhen Yi Jia, any interruption of its operations, any failure of Shenzhen Yi Jia to accommodate our growing business demands, any termination or suspension of our cooperation terms, or any deterioration of cooperative relationships with Shenzhen Yi Jia may materially and adversely affect our operation. Failure by Shenzhen Yi Jia to provide us satisfactory products and/or services in a timely manner is likely to have a have material adverse effect on our business, financial condition and results of operations. There is a risk in relying on any third -party supplier in that we are dependent on the supplier’s ability to product a product which meets our quality standards and delivery requirements as well as being dependent upon the supplier’s priorities. These risks are present when the supplier is controlled by our chief executive officer. We do not presently have any plans to engage another supplier since Shenzhen Yi Jia is familiar with our products, and we are devoting our efforts to establishing our own production facilities with no assurance that we can successfully establish manufacturing facilities.
15
In 2021, Shenzhen Yi Jia suffered a chip shortage resulting in a slowdown in delivery of its products to us from April to August 2021. Since September 2021, Shenzhen Yi Jia has obtained a supply of chips to meet its production need and Shenzhen Yi Jia has advised us that a chip shortage no longer affect its production. However, we cannot assure you that we will not suffer from a chip shortage affecting Shenzhen Yi Jia or any other supplier. The delay in shipment and chip shortage had a negative impact on the results of our operation. In the year ended June 30, 2022, we suffered a loss of potential sales orders of approximately $2 million, around 2.3% of our total sales, which caused a decline of $0.3 million in our gross profit, resulting from delay in supply chain. Although we are not presently experiencing delays in our orders for Shenzhen Yi Jia, we cannot assure you that we will not suffer delays or shortages in the future. We cannot assure you that we will not suffer from a chip shortage affecting Shenzhen Yi Jia or any other supplier.
Although we intend to use a significant portion of the proceeds of this offering to establish manufacturing operations in Vietnam and California, we have no experience in designing or operating a manufacturing facility and we cannot assure you that we will be successful in our efforts.
We intend to use a significant portion of the proceeds from this offering to establish manufacturing operations in Vietnam and in California. We do not have any experience in manufacturing operations, and in order to establish manufacturing operations, we will have to hire personnel with experience in setting up and operating manufacturing operations. With respect to operations in Vietnam, we will need to engage personnel who have experience in managing operations in Vietnam. In any country in which we conduct manufacturing operations, even if such operations are primarily assembly operations, we will need to comply with local laws and regulations, including, but not limited to, labor, health and safety and environmental, data security and tax laws and regulations. The failure to comply with such regulations could result in penalties and restrictions on our operations. We will also need to enter into supply relationships with companies that would manufacture the components that we would assemble into products. Further, since our present products are based on the technology which is used by Shenzhen Yi Jia to manufacture our products, we may require assistance form Shenzhen Yi Jia in order to establish our manufacturing operations, and we cannot assure you that we will be able to manufacture products to the same standards as Shenzhen Yi Jia. Further, we cannot assure you that, if we are able to manufacture products, we will be able to manufacture products at a cost which is less than the price we pay Shenzhen Yi Jia for the products. We cannot assure you that we will be able to establish and operate manufacturing facilities, in which event we will continue to be dependent upon Shenzhen Yi Jia or that we will be able to manufacture products at a cost that can generate an acceptable gross margin. Our failure to establish our own manufacturing facilities would increase our dependence on Shenzhen Yi Jia. If we are not able to establish and operate our manufacturing operations efficiently and in accordance with applicable laws, our business and the results of our operations would be materially impaired. We cannot assure you that we will be able to establish and operate our manufacturing facilities profitably.
If it is determined or perceived that the usage of nicotine or cannabis vaping products poses long-term health risks, the use of vaping products may decline significantly, which may materially and adversely affect our business, financial condition and results of operations.
Since vaping products were only introduced to the market in the last two decades and are rapidly evolving, studies relating to the long-term health effects of nicotine and cannabis vaping product usage are still ongoing. Currently, there remain uncertainties regarding whether vaping products are sufficiently safe for their intended use, and health risks associated with the usage of vaping products have been under scrutiny. According to the WHO, there is no conclusive evidence that the use of nicotine vaping products facilitates smoking cessation. The WHO recommended governments to strengthen relevant laws and regulations on the sale of vaping products, including to, among others, prohibit marketing strategies targeting the underage and the non-smoking population.
Negative publicity on the health consequences of vaping products or other similar devices may also adversely affect the usage of vaping products. For example, the FDA and the CDC issued a joint statement on August 30, 2019, linking a number of cases of respiratory illnesses to nicotine vaping product use. On November 8, 2019, the CDC announced that it had preliminarily linked cases of severe respiratory illness to the presence of Vitamin E acetate, which was found in certain cannabis-derived tetrahydrocannabinol-containing vaping cartridges vaping devices not intended for use with nicotine-containing e-liquids that may have been obtained illegally. However, evidence is not sufficient to rule out the contribution of other chemicals of concern, including chemicals in either cannabis or non-cannabis products. In January 2020, after further research, the FDA and CDC recommended against the use of cannabis-containing vaping products, especially those from unofficial sources, and that the underage, pregnant women and adults who do not currently use tobacco products should not start using vaping products. On February 25, 2020, the CDC issued a final update, stating that the number of cases of severe respiratory illnesses had declined to single digits as of February 9, 2020. The CDC also reconfirmed that (i) Vitamin E acetate, which was found in some cannabis-derived vaping cartridges that were mostly obtained illegally, was strongly linked to and indicated to be the primary cause of the severe respiratory illnesses, and (ii) cannabis-derived vaping products from illicit sources were linked to most cases of severe respiratory illnesses. Furthermore, there have been recent claims that users of vaping products may suffer a greater risk of more serious COVID-19 complications. However, it remained unclear whether the exposure to toxic chemicals through vaping product usage will increase the risk of COVID-19.
Research regarding the actual causes of these illnesses is still ongoing. If vaping product usage is determined or perceived to pose long-term health risks or to be linked to illnesses, the usage of vaping products may significantly decline, which would have a material adverse effect on our business, financial condition and results of operations.
Any perceived correlation between cannabis and Vitamin E acetate may adversely affect the public’s perception of vaping products in general, regardless of whether such products contain cannabis and/or Vitamin E acetate and may impact sales of our cannabis vapor product.
We sustained losses of approximately $2.0 million for the year ended June 30, 2022 and $3.0 million for the six months ended December 31, 2022, and we cannot assure you that we can or will operate profitably in the future.
We sustained a loss of approximately $2.0 million, or $0.04 per share (basic and diluted) in the year ended June 30, 2022 and a loss of approximately $3.0 million, or $.06 per share (basic and diluted) for the six months ended December 31, 2022, following net income of approximately $2.9 million, or $0.06 per share (basic and diluted) in the year ended June 30, 2021. The losses resulted primarily because of increased operating expenses for both periods. We cannot assure you that we will be able to operate profitably in the future.
16
Because cannabis oil, unlike nicotine oil, is not of a uniform quality, products we design may not perform as intended, which could result in a loss of business.
We do not include cannabis oil in our products. The cannabis oil is provided by our customer before selling the product or a cartridge with oil is inserted in the product by the customer or the end user. Unlike nicotine oil, cannabis oil is not of a uniform quality or viscosity. Although we test our product with cannabis oil having the viscosity which is acceptable to our customer, if the end user uses cannabis oil that is too viscous for or product and does not have the desired experience from the product, our client may reject an order, cancel an order or seek a refund of the payment made to us and/or discontinue purchasing our products. These refunds and the cost of cancellation of orders are reflected as sales return, the amount for both the years ended June 30, 2021 and 2022 was not material. We cannot assure you that we will not incur significant warranty expenses and lose business as a result cannabis oil not providing the end user’s desired experience or that we will not lose significant business as a result of this problem.
The vaping market may develop more slowly or differently than we expect.
The tobacco vaping market worldwide has experienced rapid growth through 2019 and the cannabis market is developing, with the United States accounting for the overwhelming majority of sales. The growth rate for tobacco vapor products decreased in 2020 and 2021, in part, we believe, because of the steps taken by governments worldwide to address the COVID-19 pandemic, which negatively affected our revenue and industry sales in general. The growth of cannabis vaping products is largely confined to those states in the United States where recreational cannabis is legal. The growth rate may decrease or decline due to uncertainties with respect to the acceptance of vaping technologies and products, health studies relating to vaping product use, general economic conditions, disposable income growth, and pace of development of technologies and other factors. There can be no assurance that the penetration of vaping products among adult smokers will further deepen, or that the tobacco and cannabis vaping market will grow at a pace that we expect. Additionally, vapor market development is subject to the uncertainty of overall regulatory landscape for such products, which may have a material impact on the market development of vaping products, particularly in Western Europe. There can be no assurance that the regulatory regime will be favorable to nicotine or cannabis vaping products in general and us. It is also uncertain whether our products and services will achieve and sustain high levels of market acceptance and meet users’ expectation. Our ability to increase the sales of our vaping products depends on several factors, some of which may be beyond our control, including users’ receptiveness towards and adoption of vaping technologies and products, market awareness of our brand, the market acceptance of our products and services, the “word-of-mouth” effects of our products and services, our ability to attract, retain and effectively train customer representatives, our ability to develop effective relationships with distributors and expand our distribution networks and the cost, performance and functionality of our products and services and meeting consumer trends. The market for nicotine products has recently seen a change in consumer preference as closed systems are overtaking open systems in market share. If we are not successful in implementing our business strategies, developing our vaping products, anticipating consumer trends or reaching adult smokers, or if these users do not accept our vaping products, the market for our products may not develop or may develop more slowly than we expect, any of which could materially and adversely affect our profitability and growth prospects.
We are exposed to product liability and user complaints arising from the products we sell, which could have a material adverse impact on us.
Currently, we primarily sell our tobacco products to our distributors, who then supply our products to wholesale companies that in turn sell to retail outlets, and we sell our cannabis products primarily to other cannabis brands on an ODM basis, and the customers sell the products through their own distribution networks. The retail market is dominated by stores, primarily grocery stores, convenience stores and tobacco stores. Even though we generally do not sell our products directly to users, we may nevertheless be liable for defects in our products pursuant to general laws on product liability. We are exposed to potential product liability claims from users of our products in the event that the use of our products results in any personal injury, property damage or health and safety issues.
There is no assurance that we can succeed in defending ourselves, and we may be required to pay significant amounts of damages for product liability claims and, to the extent that we are able to obtain product liability coverage, product liability insurance may not provide sufficient coverage against claims of injury based on the fact that they are inhaling a nicotine product. Further, product liability claims against us, whether or not successful, are costly and time-consuming to defend. These claims, whether against us or another manufacturer, may result in negative publicity that could severely damage our reputation and affect the marketability of our products, and could result in substantial costs and diversion of our resources and management’s attention. Any of the above could in turn materially and adversely affect our business, financial condition and results of operations. Although we may seek indemnification or contribution from our suppliers in certain circumstances, we cannot assure you that we will be able to receive indemnification or contribution in full, or at all.
We maintain limited product liability insurance for claims of personal injury and property damage caused by our products. Our insurance coverage may not be adequate to cover such claims. Our insurance does not provide coverage for all liabilities (including liability for certain events involving pollution or other environmental claims). In addition, there can be no assurance that we will be able to maintain our product liability insurance on acceptable terms. If we cannot maintain our product liability insurance on reasonable terms or our insurance does not sufficiently compensate us for the losses we sustain in the event of a legal proceeding, our business, financial condition and results of operations would be adversely affected.
At present, our products are manufactured by Shenzhen Yi Jia, a Chinese company of which our chief executive officer is a 95% owner. In the event of any claim of product liability resulting from a product manufactured by Shenzhen Yi Jia, any legal action would most likely be brought against us since the plaintiff may not be willing or able to commence an action against Shenzhen Yi Jia in China. Our chief executive officer has a conflict of interest in determining the extent to which Shenzhen Yi Jia would accept responsibility for any product liability claim relating to a product manufactured by Shenzhen Yi Jia or for making changes in the manufacturing process to address the substance of any claim, whether or not such claim is valid. To the extent that that we have product liability insurance, the insurer may seek to recover any amount paid from Shenzhen Yi Jia.
17
Further, although we may have legal recourse against Shenzhen Yi Jia pursuant to applicable laws, attempts to enforce our rights against Shenzhen Yi Jia may be expensive, time-consuming and may not be successful, particularly since Shenzhen Yi Jia is located in China, and we may not be able prevail in a Chinese court.
The interests of the stockholders of Shenzhen Yi Jia in their capacities as such stockholders may differ from our interests. What is in the best interests of Shenzhen Yi Jia may not be in our best interests, including with respect to matters such as the warranty period and allocation of expenses with respect to the warranted repair or replacement. There can be no assurance that when conflicts of interest arise, the stockholders of Shenzhen Yi Jia, principally, our chairman as 95% owner, will act in our best interests of or that any conflicts of interest will be resolved in our favor. In addition, these related parties may breach or refuse to renew the existing cooperation arrangements with us.
Since our products involve inhaling nicotine or cannabis, we may be subject to claims based on the known effects of nicotine or cannabis. Because e-vaping is a relatively recent method of ingesting nicotine and cannabis and is thought by some that, for adults, it may be less toxic than cigars and cigarettes or marijuana cigarettes, it is possible that long-term effects of inhaling nicotine or cannabis may not become generally known for many years, and we cannot assure you that manufacturers and distributors of vaping products may not face liability resulting from the nature of the product – a device for inhaling nicotine or cannabis, which could materially impair our ability to operate profitably if at all.
Furthermore, negative publicity including but not limited to negative online reviews on social media and crowd-sourced review platforms, industry findings or media reports related to the quality, functionality and health concerns of vaping products, whether or not accurate, and whether or not concerning our products, can adversely affect our business, results of operations and reputation. Such negative publicity may reduce users’ confidence in us, our products and our brand, which may adversely affect our business and results of operations.
Our business, financial condition and results of operations may be adversely impacted by product defects or other quality issues.
Our products may contain defects that are not detected until after they are shipped or inspected by our users. The failure of our supplier or, when we commence manufacturing operations, our operations to maintain the consistency and quality throughout our production process could result in substandard quality or performance of our products, and product defects could cause significant damage to our market reputation and reduce our sales and market share. For example, the products we distribute may contain lithium-ion or similar types of batteries. Defects in these products could result in personal injury, property damage, pollution, release of hazardous substances or damage to equipment and facilities. As we primarily rely on one supplier, Shenzhen Yi Jia, which is a related party, to supply our products, if this supplier does not produce products that meet the industrial and our standards, we may fail to maintain our quality control over our products. Actual or alleged defects in the products we distribute may give rise to claims against us for losses and expose us to claims for damages. If we deliver any defective products, or if there is a perception that our products are of substandard quality, we may incur substantial costs associated with mass product recalls, product returns and replacements and significant warranty claims, our credibility and market reputation could be harmed and our results of operations and market share may be adversely affected.
Further, defective products may result in compliance issues that could subject us to administrative proceedings and unfavorable results such as product recall and other actions. Such proceedings and unfavorable results could have a material adverse effect on our brand, reputation and results of operations.
Our business and the industry in which we operate are subject to inherent risks and uncertainties, including, among others, developments in regulatory landscape, medical discovery and market acceptance of vaping devices.
Our business and the industry in which we operate are subject to inherent risks and uncertainties, including, among others, developments in regulatory landscape, medical discovery and market acceptance of vaping devices. Our business and the vaping industry are subject to inherent risks, challenges and uncertainties, including but not limited to the following:
| ● | the regulatory landscape in the jurisdictions to which we market our products are constantly evolving, and there may be further restrictions, bans or requirements with respect to e-cigarettes and vaping devices that may increase our cost of compliance or prevent us from marketing our products to certain jurisdictions; |
| ● | we may face unforeseen capital requirements caused by the changing industry requirements or consumer tastes and demands; demands for our vaping devices may decline significantly due to the decrease in market acceptance for our products or vaping devices generally; |
18
| ● | we may not be able to establish business relationships with customers or compete with other more established competitors as, for an evolving industry, customers generally prefer to choose more established suppliers, including Juul Labs, the largest producer of nicotine vapor products, rather than us. |
| ● | we may not be able to adjust our procurement and/or production in time to meet the changes in market demands; and |
| ● | future changes in our industry may not be consistent with our prediction. Therefore, our industrial prospects, research and development focus and business plans may not be effective in helping sustain our competitive position in the vaping industry. |
If we fail to cope with the challenges and compete with other industry players in such uncertain and evolving vaping industry, our future prospects, business, financial conditions and results of operations may be materially and adversely affected.
We may not be able to develop and introduce new products or upgrade existing products in a timely and cost-effective manner, which may adversely affect our business, results of operations and prospects.
To optimize adult smokers’ experience, we must introduce new products and upgrade our existing products to meet our users’ evolving preferences and to incorporate the latest technological developments. It is difficult to predict the preferences of users or a specific segment of users. Changes and upgrades to our existing products may not be well received by our users, and newly introduced products may not achieve expected results. Going forward, we may introduce new products with different features. Such efforts may require substantial investments of additional human capital and financial resources. However, if we are not able to develop or obtain rights to the latest technological developments, we may not be able to market a product that meets the adult consumer’s changing taste. If we fail to improve our existing products or introduce new products that meet consumer taste ones in a timely or cost-effective manner, our ability to attract and retain users may be impaired, and our results of operations and prospects may be adversely affected.
Although we endeavor to understand user preferences through surveys, sampling and other forms of interactions from time to time, we cannot assure you that we can anticipate, identify, develop or market products that respond to changes in users’ preferences and expectations. For example, our surveys may not yield accurate or useful insights on user behaviors, and feedbacks on our products may be different after such products are commercially available to a wider public. There can be no assurance that any of our new products will achieve market acceptance or generate sufficient revenues to offset the costs and expenses incurred in relation to our development and promotion efforts. There can be no assurance that each of our new products will achieve market acceptance and be successful.
Outbreaks of communicable diseases, natural disasters or other events have materially and adversely affected, and in the future, may materially and adversely affect our business, results of operations and financial condition.
Our business could be adversely affected by the effects of communicable diseases and epidemics. In recent years, there have been breakouts of epidemics in China and globally. In early 2020, there was a worldwide outbreak of a novel strain of coronavirus, later named COVID-19. The outbreak of COVID-19 has severely impacted China and the rest of the world. In response to intensifying efforts to contain the spread of the coronavirus, in 2020, the Chinese government took a number of actions, which included extending the Chinese New Year holiday, temporary closure of corporate offices, retail outlets and manufacturing facilities, quarantining individuals in China who had COVID-19, asking citizens to remain at home and to avoid gathering in public, and other actions. In light of such circumstances, our corporate office was closed for a certain period of time. As a result of these government actions, our production was affected for four months during 2020, commencing with the Chinese New Year, which led to decline in our revenue and net income. The steps taken by governments around the world impacted our distributors and the market for our product. In late 2020 and early 2021, COVID-19 vaccination program had been greatly promoted around the globe, however several types of COVID-19 variants emerged in different parts of the world. Our sales in Europe and the United States continued to be affected by government actions relating to COVID-19 and COVID-19 variants, while the production slightly slowed down by the strict measures taken by the Government of China to address the small COVID-19 outbreaks in Guangdong province. The COVID-19 global pandemic has resulted in, and may intensify, global economic distress, and the duration and extent of the impact of COVID-19 outbreak is highly uncertain at this time. The extent to which it may affect our results of operations, financial condition and cash flows will depend on the future development of the outbreak, including variants such as the delta variant, and the severity of the variants, all of which is also highly uncertain. Such uncertainty poses operational challenges to our production and inventory management. Our business and operations could be disrupted if any of our employees, distributors, retailers or manufacturers is suspected of having COVID-19, H1N1 flu, avian flu or another epidemic in our offices, within our distribution and retail channels or at the manufacturing facilities of our suppliers, since it could require all of the possible contact persons to be quarantined and/or their offices to be disinfected. In addition, since our products are presently manufactured in the PRC, our results of operations could be adversely affected to the extent that the outbreak harms the PRC economy in general or the effects of the PRC’s zero COVID policy, which has resulted in closures in a number of municipalities and provinces. If the implementation of travel restrictions is prolonged or if certain cities where our supplier’s factories are located are being restricted to certain activities due to the pandemic, there may be delays in delivery of products, which could disrupt our business operation and render us unable to deliver our products in a timely manner, or at all. We cannot predict the effect on our business, the business of Shenzhen Yi Jia and on business in the PRC in general as a result of the change in the PRC government’s zero-COVID rules, including the effect of increased infections, deaths and hospitalizations as well as the development of any new variants of COVID. To the extent that quarantine is required of any person entering mainland China, our business may be impacted. Although we do not have any operations or assets in mainland China, to the extent that any of our key personnel or any key personnel of Shenzhen Yi Jia or any of our or its suppliers are subject to quarantine on entering mainland China, our business may be impacted. Since January 8, 2023, no centralized quarantine or mass PCR testing will be undertaken on travelers entering mainland China. Travelers to mainland China are only required to take PCR test 48 hours prior to their departure and report the PCR test findings on their customs health declaration form. Only those whose test results are positive prior to departure will have to postpone their travel until the PCR results turn negative.
We are also vulnerable to natural disasters and other calamities that may affect our supplier and may affect us when we establish our own manufacturing facilities.
19
Misuse or abuse of our products may lead to potential adverse health effects, subjecting us to complaints, product liability claims and negative publicity.
We are unable to control how our users choose to use our products. For example, we cannot prevent the users from misusing or abusing our products or prevent minors from obtaining access to our products. Our users may also use our products to inhale chemicals obtained from informal sources and in other potentially hazardous applications that can result in personal injury, product liability and environmental claims.
Misuse or abuse of our products, including use of our products in combination with other products and components from third parties, may significantly and adversely affect the health of our users, subjecting us to user complaints and product liability litigation, even though such products were not used in the manner recommended by us. Applicable law may render us liable for damages without regard to negligence or fault. The FDA strongly advises against vaping during pregnancy on the ground that any products containing nicotine are not safe to use during pregnancy since nicotine is a health risk for pregnant women and developing babies and can damage a baby’s brain and lungs. We cannot assure you that we would not be subject to liability resulting from a birth defect in a baby born to a woman who used vaping products during pregnancy, notwithstanding our warnings not to use during pregnancy. Any such liability may not be covered by insurance and may materially impair our ability to operate profitably.
Regardless of whether these complaints or product liability litigation have merit, they may be costly and time-consuming to defend and resolve, bring negative publicity that could damage our reputation and result in higher scrutiny by the government or stricter regulations, all of which could materially and adversely affect our business, financial condition and results of operations.
Our business may be impacted by supply chain issues, which are affecting businesses worldwide.
One of effects of the COVID-19 has been delays resulting from supply chain issues, which relate to the difficulty that companies have in having their products manufactured, shipped to the country of destination, and delivered from the port of entry to the customer’s location. During 2021 and early 2022, there were significant delays in the delivery of products to market. To the extent that products are shipped by sea, there are additional risks resulting from ports not being able to unload ships promptly, causing delays in getting into port, including potential damage from seawater and fire, product degradation and the possibility of containers being destroyed, damaged or falling off the ship into the water. The inability to delivery products to the ultimate vendor could impair our ability to generate revenue from our products. As the port delays have significantly decreased, we do not believe that the supply chain issues that affected our operations are currently affecting us. We cannot assure you that such delays will not affect our business in the future.
In 2021, our supplier, Shenzhen Yi Jia, suffered a chip shortage resulting in a slowdown in the delivery of its products to us from April to August 2021. Since September 2021, Shenzhen Yi Jia was able to meet our requirements and a chip shortage no longer affect its production. However, we cannot assure you that Shenzhen Yi Jia and, if and when we commence manufacturing operations, any other supplier we may engage, will not suffer from a chip shortage in the future.
The delay in shipment and chip shortage had a negative impact on our results of operation. In 2022, there was a loss of potential sales orders of approximately $2 million, around 2.3% of our total sales, which caused a decline of $0.3 million in our gross profit, resulting from delay in supply chain. We believe delays in supply chain may continue in the coming year, which may affect around 3% of our total sales orders. In order to mitigate the supply chain disruptions, we are taking the initial steps toward the development of our own manufacturing operations in Vietnam and in California.
With respect to sales to distributors other than in the United States, we are not involved in the shipping by the distributors, who arrange for shipping. However, to the extent that the distributors incur shipping delays in getting the product to market, their purchases of products from us may be delayed or reduced. We ship products for the United States market by air.
Failure to manage inventory at optimal levels could adversely affect our business, financial condition and results of operations.
We are required to manage a large volume of inventory effectively for our business. We depend on our forecasts for the anticipated demand for our products to make procurement plans and manage our inventory. Our forecast for demand, however, may not accurately reflect the actual market demands, which depends on a number of factors including, without limitation, launches of new products, changes in product life cycles and pricing, product defects, changes in user spending patterns, supplier back orders and other supplier-related issues, distributors’ and retailers’ procurement plans, as well as the volatile economic environment in the markets where we sell our products. We do not have long-term contracts with some of our distributors, which makes the demands for our products from distributors unstable and unpredictable. In addition, when we launch a new product with new components or raw material, it may be difficult to establish relationships, determine appropriate raw material and product selection, and accurately forecast market demand for such product. We cannot assure you that we will be able to maintain proper inventory levels for our business at all times, and any such failure may have a material and adverse effect on our business, financial condition and results of operations.
20
Inventory levels in excess of distributor demand with respect to tobacco products and customer demand with respect to cannabis products may result in inventory write-downs, expiration of products or an increase in inventory holding costs and a potential negative effect on our liquidity. As we plan to continue expanding our product offerings, we expect to include more products in our inventory, which will make it more challenging for us to manage our inventory effectively and will put more pressure on our warehousing system. If we fail to manage our inventory effectively, we may be subject to a heightened risk of inventory obsolescence, a decline in inventory values, and significant inventory write-downs or write-offs. In addition, we may be required to lower sale prices in order to reduce inventory level, which may lead to lower gross margins. High inventory levels may also require us to commit substantial capital resources, preventing us from using that capital for other important purposes. Any of the above may materially and adversely affect our results of operations and financial condition.
Conversely, if we underestimate distributor demand, or if our supplier fails to provide products to us in a timely manner, we may experience inventory shortages, which may, in turn, require us to purchase our products at higher costs, result in unfulfilled user orders, leading to a negative impact on our financial condition and our relationships with distributors.
Additionally, the distributors largely determine the inventory levels of the retail outlets they operate or to whom they sell, based on their estimation, and such inventory levels might not correspond to actual market demands and could lead to under-stocking or over-stocking in the retail outlets. We cannot assure you that there will not be under-stocking or over-stocking in these stores which would materially impact the results of our operations and our working capital.
Under-stocking can lead to missed sales opportunities, while over-stocking could result in inventory depreciation and decreased shelf space for stocks that are in higher demands. These results could adversely affect our business, financial condition and results of operations.
One customer accounts for a significant portion of our sales.
Although we have more than 150 distributors, our largest distributor, who is a non-exclusive distributor for the United Kingdom and France, accounted for approximately 51.2%, 38.6%, 43.0% and 38.1% of our revenue for the years ended June 30, 2021 and 2022 and six months ended December 31, 2021 and 2022, respectively. On January 1, 2021, we signed a distributorship agreement with this distributor in our standard form, which does not provide any special terms or prices. No other customer accounted for 10% or more of our revenue during either year or six-month period. The loss of this distributor could have a material adverse effect upon our business. See “Business – Sales and Distribution.”
Our business may be affected by inflation.
Although inflation has not materially affected our business or the results of our operations through the years ended June 30, 2021 and 2022 or the six months ended December 31, 2022, in view of the global inflationary trends, it is possible that we may incur increased costs of manufacture and delivery which we may not be able to pass on to our customers as a result of competitive pressure which would impact the results of our operations. In order to mitigate inflationary pressures, we are taking the initial steps toward the development of manufacturing operations in Vietnam which will we believe allow us to take advantage of lower labor cost and production cost. However, we do not anticipate that such operations, if developed, will have a material effect on our costs in the near future.
We face competition from companies in the vaping industry as well as other sources of nicotine and cannabis, and we may fail to compete effectively.
Vaping products for both tobacco and cannabis compete with tobacco and marijuana cigarettes and a wide range of other tobacco and legal and illegal cannabis products. The vaping industry worldwide is intensely competitive.
21
Some of our current and potential competitors have greater financial, marketing, ordering quantities, portfolios of products and intellectual properties and other resources and some, such as JUUL Labs, Inc., which is the major seller of vaping nicotine products, and British American Tobacco Plc, another major producer of vaping nicotine products, are better known and have greater resources than we do. Certain competitors may be able to secure raw materials and products from suppliers and manufacturers on more favorable terms, devote greater resources to marketing and promotional campaigns, adopt more aggressive pricing or inventory policies, and devote substantially more resources to product development and technology. Increased competition may adversely affect our results of operations, market share and brand recognition, or force us to incur losses. There can be no assurance that we will be able to successfully compete against current and future competitors, and competitive pressures may have a material adverse effect on our business, prospects, financial condition and results of operations.
The cannabis vaping market is in the early stages and at present is mainly limited to the United States, although there is a developing market in Canada and a potential market in Europe. Our ability to be successful in this market is dependent upon our ability to develop vaping systems that attracts and retains consumer interest and the regulatory environment in the United States. Our cannabis vaping products compete with other forms of legal and illegal cannabis, marijuana cigarettes, CBD oil and other CBD products, food products and other vaping products. Since most of our revenue from cannabis is derived from sales to other brands rather than sales to distributors and consumers, we compete based on our technology and ability to work with the customers to develop a product that they can successfully market.
Misconduct, including illegal, fraudulent or collusive activities, by our employees, distributors, retailers, suppliers and manufacturers, may harm our brand and reputation and adversely affect our business and results of operations.
Misconduct, including illegal, fraudulent or collusive activities, unauthorized business conduct and behavior, or misuse of corporate authorization by our employees, contractors, distributors, retailers, suppliers and manufacturers and other business relationships could subject us to liability and negative publicity. Our employees, distributors, retailers, suppliers and manufacturers may conduct fraudulent activities or violations of the Foreign Corrupt Practices Act, such as accepting payments from or making payments to other distribution channel participants or other third parties in order to bypass our internal system and to complete shadow transactions and/or transactions outside our official or authorized distribution channels, disclosing users’ information to competitors or other third parties for personal gains, or applying for fake reimbursement. They may conduct activities in violation of unfair competition law, which may expose us to unfair competition allegations and risks. We cannot assure you that such incidents will not occur in the future. It is not always possible to identify and deter such misconduct, and the precautions we take to detect and prevent these activities may not be effective. Such misconduct could damage our brand and reputation, which could adversely affect our business and results of operations.
We may become subject to governmental regulations and other legal obligations related to privacy, information security, and data protection, and any security breaches, and our actual or perceived failure to comply with our legal obligations could harm our brand and business.
Most of our revenue is derived from sales to distributors for our tobacco products and other cannabis brands for our cannabis products, and we do not sell online. As a result, in the normal course of business we do not collect, store and process personal, transactional, statistical and behavioral data, including certain personal and other sensitive data from our users. To the extent that we market to the public and collect personal data, such as credit card information, we would face risks inherent in handling large volumes of data and in securing and protecting such data. In particular, we would face a number of data-related challenges related to our business operations, including: (i) protecting the data in and hosted on our system and cloud servers, including against attacks on our system and cloud servers by external parties or fraudulent behavior by our employees; (ii) addressing concerns related to privacy and sharing, safety, security and other factors; and (iii) complying with applicable laws, rules and regulations relating to the collection, use, disclosure or security of personal information, including any requests from regulatory and government authorities relating to such data.
22
We may be subject to liability if private information that we receive is not secure or if we violate privacy laws and regulations.
We are or may become subject to a variety of laws and regulations in the United States and abroad regarding privacy, data security, cybersecurity and data protection. These laws and regulations are continuously evolving and developing. The scope and interpretation of the laws that are or may be applicable to us are often uncertain and may be conflicting, particularly with respect to foreign laws. In particular, there are numerous United States federal, state, and local laws and regulations and foreign laws and regulations regarding privacy and the collection, sharing, use, processing, disclosure, and protection of personal information and other user data. Such laws and regulations often vary in scope, may be subject to differing interpretations, and may be inconsistent among different jurisdictions. To the extent that we deal with the public and obtain private information on our computer system including information on our system as a result of internet sales of our products, we would be subject to these laws.
In June 2018, California adopted the California Consumer Privacy Act (“CCPA”), which became effective in 2020. Under the law, any California consumer has a right to demand to see all the information a company has saved on the consumer, as well as a full list of all the third parties that data is shared with. The consumer also has the right to request that we delete the information it has on the consumer. The CCPA broadly defines “protected data.” The CCPA also has specific requirements for companies subject to the law. The CCPA provides for a private right of action for unauthorized access, theft or disclosure of personal information in certain situations, with possible damage awards of $100 to $750 per consumer per incident, or actual damages, whichever is greater. The CCPA also permits class action lawsuits. To the extent that we sell products to consumers through our website or otherwise through the Internet, we may become subject to the CCPA and any other similar consumer protection laws.
The European Union Parliament approved a new data protection regulation, known as the General Data Protection Regulation (“GDPR”), which came into effect in May 2018. The GDPR includes operational requirements for companies that receive or process personal data of residents of the European Economic Area. The GDPR imposes significant penalties for non-compliance. Although we do not conduct any business in the European Economic Area, in the event that residents of the European Economic Area access our website and input protected information, including information provided in ordering products through our website, we may become subject to provisions of the GDPR.
We are also subject to laws restricting disclosure of information relating to our employees. We strive to comply with all applicable laws, policies, legal obligations, and industry codes of conduct relating to privacy, data security, cybersecurity and data protection. However, given that the scope, interpretation, and application of these laws and regulations are often uncertain and may be conflicting, it is possible that these obligations may be interpreted and applied in a manner that is inconsistent from one jurisdiction to another and may conflict with other rules or our practices. Any failure or perceived failure by us or our third-party service-providers to comply with our privacy or security policies or privacy-related legal obligations, or any compromise of security that results in the unauthorized release or transfer of personally identifiable information or other user data, may result in governmental enforcement actions, litigation, or negative publicity, and could have an adverse effect on our business and operating results. Although we maintain cybersecurity insurance, we cannot assure you that this insurance will cover or satisfy any claim made against us or adequately cover any defense costs we may incur.
Any significant cybersecurity incident or disruption of our information technology systems or those of third-party partners could materially damage user relationships and subject us to significant reputational, financial, legal and operation consequences.
We depend on our information technology systems, as well as those of third parties, to develop new products and services, host and manage our services, store data and process transactions. Any material disruption or slowdown of our systems or those of third parties upon whom we depend could cause outages or delays in our services, particularly in the form of interruption of services delivered by our website, which could harm our brand and adversely affect our operating results. Our failure to implement adequate cybersecurity protections could subject us to claims for any breach of security, particularly if it results in disclosure of information relating to our customers. If changes in technology cause our information technology systems, or those of third parties whom we depend upon, to become obsolete, or if our or their information systems are inadequate to handle our growth, we could lose users, and our business and operating results could be adversely affected.
23
Infringement of our intellectual property by any third party or loss of our intellectual property rights may materially and adversely affect our business, financial condition and results of operations.
We, through our operating subsidiaries, either own or will own or license as an exclusive licensee patent, trademark, copyright and trade secret and other intellectual property, as well as confidentiality procedures and contractual provisions, to protect our intellectual property rights. We also enter into confidentiality agreements with our employees and any third parties who may access our proprietary information, and we control access to our proprietary technology and information.
Intellectual property protection may not be sufficient. Confidentiality agreements may be breached by counterparties, we may not be able to enforce these agreements and there may not be adequate remedies available to us for any such breach. Accordingly, we may not be able to effectively protect our intellectual property rights or to enforce our contractual rights, and, with respect to rights licensed to us, the licensor, which is a related party, may not be willing or able to enforce its intellectual property rights against alleged infringers. Policing any unauthorized use of our intellectual property, whether owned or licensed, is difficult, time-consuming and costly, and the steps we have taken may be inadequate to prevent the misappropriation of our intellectual property. In the event that we resort to litigation to enforce our intellectual property rights, such litigation could result in substantial costs and a diversion of our managerial and financial resources. We can provide no assurance that we will prevail in such litigation, and we cannot assure you that our licensor will take steps to sufficiently protect the licensed intellectual property. Furthermore, we or our licensor may be subject to the risks of losing our intellectual property rights or the intellectual property rights licensed from other third-parties due to several reasons. Certain intellectual property rights, such as patents, are subject to a limited period of time. Upon the expiry of such period of time, others may freely use such intellectual properties without any license or charges, which may impose competitive harm to us and in turn adversely affect our business and prospects. The intellectual property rights that we currently have may also be revoked, invalidated or deprived by regulatory authorities as a result of intellectual property claims or challenges successfully raised by third parties. We may also rely on certain intellectual property rights licensed from other third parties. There can be no guarantee that we will be able to maintain such licenses at all times or renew such licenses upon expiry. Moreover, our trade secrets may be leaked or otherwise become available to, or be independently discovered by, our competitors. Any failure in maintaining, protecting or enforcing our intellectual property rights could have a material adverse effect on our business, financial condition and results of operations.
We may be subject to intellectual property infringement claims from third parties, which may be expensive to defend with no assurance of success and may disrupt our business and operations.
We cannot be certain that our operations or any aspects of our business do not or will not infringe upon or otherwise violate patents, copyrights or other intellectual property rights held by third parties. Through our operating subsidiaries, we are acquiring patent, trademark and other intellectual rights from Tuanfang Liu, Aspire Global and Shenzhen Yi Jia all of their intellectual property relating to the cannabis vaping products, and we are licensing patent, trademarks and other intellectual property rights relating to the tobacco vaping products from Mr. Liu, Aspire Global and Shenzhen Yi Jia. We may, and from time to time in the future be, subject to legal proceedings and claims relating to the intellectual property rights of others. There could also be existing patents or other intellectual property of which we are not aware that we may infringe. While we do not know of any intellectual property rights on which our products or our business infringe, we cannot assure you that holders of patents or other intellectual property rights purportedly relating to some aspect of our technology or business, would not seek to enforce such patents against us or the licensor of intellectual property licensed by us, including intellectual property licensed by Shenzhen Yi Jia, or that they will not be successful in any such enforcement action. If we fail to maintain our patents or if our licensor is not able to maintain its rights, we may be subject to intellectual property infringement claims from third parties. We and Shenzhen Yi Jia have patents and patent applications in a number of jurisdictions, including the United States and the European Union. If we are found to have violated the intellectual property rights of others, we may be subject to liability for our infringement activities or may be prohibited from using such intellectual property, and we may incur licensing fees or damages or be forced to develop alternatives of our own. In addition, we may incur significant expenses, and may be forced to divert management’s time and other resources from our business and operations to defend against these third-party infringement claims, regardless of their merits. Although the intellectual property transfer agreement (the “Intellectual Property Transfer Agreement”) dated September 30, 2022, among Mr. Liu, Aspire Global, Shenzhen Yi Jia, us and Aspire North America, and the exclusive license agreement (the “Intellectual Property License Agreement”) dated September 30, 2022, among Mr. Liu, Aspire Global, Shenzhen Yi Jia, us and Aspire Science, provide that Mr. Liu, Aspire Global and Shenzhen Yi Jia will indemnify us against any liability in the event that the transferred or licensed intellectual property infringes the intellectual property rights of a third party, we cannot assure you that we will be able to enforce such indemnification. Further, since Shenzhen Yi Jia and Mr. Liu are located in the PRC, we cannot assure you that we will be able to enforce any action or any judgment we may receive from a U.S. court in a Chinese court.
24
You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing actions in China against two of our directors, one of whom is our chief executive officer, who are based in China based on foreign laws.
Our chief executive officer, Tuanfang Liu, who is also a director, and his wife Jiangyan Zhu, who is also a director, live in mainland China. As a result, it may be difficult for you to effect service of process upon either of them inside mainland China. It may also be difficult for you to enforce in U.S. courts’ judgments obtained in U.S. courts based on the civil liability provisions of the U.S. federal securities laws against these directors as they do not currently reside in the United States or have substantial assets located in the United States. In addition, there is uncertainty as to whether the courts of the PRC would recognize or enforce judgments of U.S. courts against such persons predicated upon the civil liability provisions of the securities laws of the United States or any state.
The recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedures Law. PRC courts may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedures Law based either on treaties between China and the country where the judgment is made or on principles of reciprocity between jurisdictions. China does not have any treaties or other forms of written arrangement with the United States that provide for the reciprocal recognition and enforcement of foreign judgments. In addition, according to the PRC Civil Procedures Law, the PRC courts will not enforce a foreign judgment against our directors and officers who are residents of China if they decide that the judgment violates the basic principles of PRC laws or national sovereignty, security or public interest. As a result, it is uncertain whether and on what basis a PRC court would enforce a judgment rendered by a court in the United States.
As the patents we own or are licensed may expire and may not be extended, our patent applications may not be granted and our patent rights may be contested, circumvented, invalidated or limited in scope, our patent rights and license may not protect us.
As of the date of this prospectus, our operating subsidiaries own or license more than 200 patents relating to various aspects of our operations. The rights granted under any issued patents, however, may not provide us with proprietary protection or competitive advantages. The claims under any patents that issue may not be broad enough to prevent others from developing technologies that are similar or that achieve results similar to ours. It is also possible that the intellectual property rights of others will bar us from licensing. Numerous patents owned by others exist in the fields in which we have developed and are developing our technology. These patents and patent applications might have priority over our patent applications filed by our transferor or licensor and we or our licensor may not be able to enforce these rights. Finally, in addition to those who may claim priority, any of our existing patents may also be challenged by others on the basis that they are otherwise invalid or unenforceable. Any failure in extending our existing patents, or if our patent rights were to be contested, circumvented, invalidated or limited in scope could materially and adversely affect our business, financial condition and results of operations.
If we are unable to manage our growth or execute our strategies effectively, our business and prospects may be materially and adversely affected.
To accommodate our growth, we anticipate that we will need to implement a variety of new and upgraded operational and financial systems, procedures and controls, including the improvement of our accounting and other internal management systems. We will also need to continue to expand, train, manage and motivate our workforce and manage our relationships with customers and third-party suppliers. All of these endeavors involve risks and will require substantial management effort and significant additional expenditures. We may not be able to manage our growth or execute our strategies effectively, and any failure to do so may have a material adverse effect on our business and prospects.
Our success depends on our ability to retain our core management team and other key personnel.
Our performance depends on the continued service and performance of our directors and senior management as they are expected to play an important role in guiding the implementation of our business strategies and future plans. Our chief executive officer, Tuanfang Liu, is responsible both for our operation and for our product development, since all of the patents we own or license are based on his inventions, and we anticipate that he will continue to be responsible for product development. Because of his knowledge of the market and the underlying technology for our products, the loss of Mr. Liu could have a material adverse effect on our business, financial condition and prospects. If any of our other members of senior management were to terminate his or her employment, there can be no assurance that we would be able to find suitable replacements in a timely manner, at acceptable cost or at all. The loss of services of key personnel or the inability to identify, hire, train and retain other qualified and managerial personnel in the future may materially and adversely affect our business, financial condition, results of operations and prospects. Additionally, in addition to our chief executive officer, we rely on our research and development personnel for product development and technology innovation. If any of our key research and development personnel were to leave us, we cannot assure you that we can secure equally competent research and development personnel in a timely manner, or at all. Before we can establish manufacturing facilities, we will need to hire key personnel who have experience in establishing and operating manufacturing operations, particularly in Vietnam, since each country has its own legal requirements and business customs and our failure to comply with the legal requirements and to operate in accordance with local practice could material impair our business and the results of our operations.
25
Competition for highly skilled employees is intense, and we may not be able to attract and retain the highly skilled employees needed to support our business.
As we continue to experience growth, we believe our success depends on the efforts and talents of our employees, including management team and financial personnel. Our future success depends on our continued ability to attract, develop, motivate and retain highly qualified and skilled employees. Competition for highly skilled personnel is extremely intense. We may not be able to hire and retain these personnel at compensation levels consistent with our existing compensation and salary structure. Many of the companies with which we compete for experienced employees have greater resources than we do and may be able to offer more attractive terms of employment.
In addition, we invest significant time and expense in training our employees, which increases their value to competitors who may seek to recruit them. If we fail to retain our employees, we could incur significant expenses in hiring and training their replacements, and the quality of our services and our ability to serve customers could diminish, resulting in a material adverse effect on our business.
Our business, financial condition and results of operations may be adversely affected by an economic downturn.
Because our sales may depend on customers’ levels of disposable income, perceived job prospects and willingness to spend, our business and prospects may be affected by global economic conditions. The global financial markets experienced significant disruptions in 2008 and the United States, Europe and other economies went into recession. The recovery from the lows of 2008 and 2009 was uneven and is continuously facing new challenges, including the escalation of the European sovereign debt crisis since 2011 and the slowdown of the Chinese economy in 2012 and the effects the COVID-19 pandemic and steps taken by the governments to address the pandemic, including China’s zero-COVID policy. Economic conditions in the markets in which are products are sold are sensitive to both global economic conditions, and the particular changes in each country’s economic and political policies and its expected or perceived overall economic growth rate. A decline in the economic prospects could alter current or prospective customers’ spending priorities. Therefore, a slowdown in global economy or the economy of the regions in which are products are marketed may lead to a reduction in demand for our products, which could materially and adversely affect our financial condition and results of operations.
Our internal controls over financial reporting may not be effective and our independent registered public accounting firm may not be able to certify as to their effectiveness, which could have a significant and adverse effect on our business and reputation.
As a privately-owned company, we are not subject to the reporting requirements of the Securities Act, the Exchange Act, the Sarbanes-Oxley Act and the rules and regulations of Nasdaq. Upon the completion of this offering, we will become subject to these rules and regulations. We expect that the requirements of these rules and regulations will continue to increase our legal, accounting and financial compliance costs, make some activities more difficult, time-consuming and costly, and place significant strain on our personnel, systems and resources.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. We are continuing to develop and refine our disclosure controls, internal control over financial reporting and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we will file with the SEC is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and that information required to be disclosed in reports under the Exchange Act is accumulated and communicated to our principal executive and financial officers.
Our current controls and any new controls that we develops may become inadequate because of changes in conditions in our business. Further, weaknesses in our internal controls may be discovered in the future. Any failure to develop or maintain effective controls, or any difficulties encountered in their implementation or improvement, could adversely affect our operating results or cause us to fail to meet our reporting obligations and may result in a restatement of our financial statements for prior periods. Any failure to implement and maintain effective internal controls also could adversely affect the results of periodic management evaluations and annual independent registered public accounting firm attestation reports regarding the effectiveness of our internal control over financial reporting that we are required to include in our periodic reports that we will file with the SEC under Section 404 of the Sarbanes-Oxley Act. Ineffective disclosure controls and procedures and internal control over financial reporting could also cause investors to lose confidence in our reported financial and other information.
26
In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal control over financial reporting, we have expended and anticipates that we will continue to expend significant resources, including accounting-related costs, and provide significant management oversight. Any failure to maintain the adequacy of our internal controls, or our consequent inability to produce accurate financial statements on a timely basis, could increase our operating costs and could materially and adversely affect our ability to operate our business. In the event that our internal controls are perceived as inadequate or that we are unable to produce timely or accurate financial statements, investors may lose confidence in our operating results and the stock price of the our common stock could decline. In addition, if we are unable to continue to meet these requirements, we may not be able to obtain or maintain listing on Nasdaq.
Our independent registered public accounting firm is not required to formally attest to the effectiveness of our internal control over financial reporting until after we are no longer an emerging growth company or a non-accelerated filer. At such time, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our controls are documented, designed or operating. Any failure to maintain effective disclosure controls and internal control over financial reporting could have a material and adverse effect on our company’s business and operating results.
Although we believe that our business is not subject to PRC Laws, our business could be materially impaired if it is determined that our business is subject to PRC Laws.
Based upon the nature of our existing business operations we do not believe, based on advice from counsel, that we are subject to PRC Laws. There is no assurance that certain PRC Laws, including existing laws and regulations and those enacted or promulgated in the future, will not be applicable to our Hong Kong subsidiary due to change in the current political arrangements between mainland China and Hong Kong or other unforeseeable reasons. The application of such PRC Laws may have a material adverse impact on us, as relevant PRC authorities may impose fines and penalties upon our Hong Kong subsidiary, delay or restrict the repatriation of the proceeds from this offering into Hong Kong, and any failure of us to fully comply with such new regulatory requirements may significantly limit or completely hinder our ability to offer or continue to offer our common stock, cause significant disruption to our business operations, and severely damage our reputation, which would materially and adversely affect our financial condition and results of operations and cause our Common Stock to significantly decline in value or in extreme cases, become worthless.
We have limited insurance coverage, which could expose us to significant costs and business disruption.
We are exposed to various risks associated with our business and operations, and we have limited liability insurance coverage and product liability insurance coverage, and Aspire Science does not have product liability insurance. A successful liability claim against us due to injuries or damages suffered by users of our product could materially and adversely affect our reputation, results of operations and financial conditions. Even if unsuccessful, such a claim could cause us adverse publicity, require substantial costs to defend, and divert the time and attention of our management. In addition, we do not have any business disruption insurance. Any business disruption event could result in substantial costs to us and a diversion of our resources.
The occurrence of natural disasters may adversely affect our business, financial condition and results of operations.
The occurrence of natural disasters, including hurricanes, floods, earthquakes, tornadoes, fires and other disasters disease may adversely affect our business, financial condition or results of operations. The potential impact of a natural disaster on our results of operations and financial position is speculative and would depend on numerous factors. The extent and severity of these natural disasters determines their effect on a given economy. We cannot assure you that natural disasters will not occur in the future or that our business, financial condition and results of operations will not be adversely affected.
Because we are a “controlled company” as defined in the Nasdaq Stock Market Rules, you may not have protection of certain corporate governance requirements which otherwise are required by Nasdaq’s rules.
Under Nasdaq’s rules, a controlled company is a company of which more than 50% of the voting power for the election of directors is held by an individual, group or another company. We are a controlled company because Mr. Tuanfang Liu, our chief executive officer, holds more than 50% of our voting power, and we expect we will continue to be a controlled company upon completion of this offering. For so long as we remain a controlled company, we are not required to comply with the following permitted to elect to rely, and may rely, on certain exemptions from the obligation to comply with certain corporate governance requirements, including:
| ● | our board of directors is not required to be comprised of a majority of independent directors. | |
| ● | our board of directors is not subject to the compensation committee requirement; and \ | |
| ● | we are not subject to the requirements that director nominees be selected either by the independent directors or a nomination committee comprised solely of independent directors. |
We have not taken advantage of these exemptions except that our chief executive officer and principal stockholder, Tuanfang Liu, is chairman of the nominating and corporate governance committee. As a result, to the extent that we take advantage of these exemptions, you will not have the same protections afforded to stockholders of companies that are subject to all of the Nasdaq corporate governance requirements. Although we do not currently intend to take advantage of the controlled company exemptions, except as set forth above, we cannot assure you that, in the future, we will not seek to take advantage of these exemptions.
27
You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing actions in China against two of our directors, one of whom is our chief executive officer based on foreign laws.
Although we are a Delaware corporation, two of our directors, -- our chief executive officer, director and controlling stockholder and his wife, who is also a director – live in mainland China. The PRC does not have treaties providing for the reciprocal recognition and enforcement of judgments of courts with the United States. As a result, it may not be possible for investors to serve process upon our chief executive officer, or to enforce any judgments obtained from non-PRC jurisdictions against any of them in China. As a result, it may be difficult for you to effect service of process upon us or those persons inside mainland China. It may also be difficult for you to enforce judgments obtained in U.S. courts based on the civil liability provisions of the U.S. federal securities laws against us and our officers and directors who do not reside in the United States or have substantial assets located in the United States. In addition, there is uncertainty as to whether the courts of the PRC would recognize or enforce judgments of U.S. courts against such persons predicated upon the civil liability provisions of the securities laws of the United States or any state.
Risks Related to this Offering and Ownership of Our Common Stock
There has been no public market for our common stock prior to this offering, and you may not be able to resell our common stock at or above the price you paid, or at all.
Prior to this initial public offering, there has been no public market for common stock. We have applied to list our common stock on Nasdaq under the symbol “ISPR.” If an active trading market for our common stock does not develop after this offering, the market price and liquidity of our common stock will be materially and adversely affected. The initial public offering price was determined by negotiations with the Representative of the Underwriters which may bear no relationship to their market price after the initial public offering. We cannot assure you that an active trading market for our common stock will develop or that the market price of our common stock will not decline below the initial public offering price.
The trading price of our common stock may be volatile, and you could lose all or part of your investment.
This initial public offering price does not necessarily reflect the price at which investors in the market will be willing to buy and sell shares of our common stock following this offering. In addition, the trading price of our common stock following this offering is likely to be volatile and could be subject to fluctuations in response to various factors, some of which are beyond our control. These fluctuations could cause you to lose all or part of your investment in our common stock as you might be unable to sell your shares at or above the price you paid in this offering. Factors that could cause fluctuations in the trading price of our common stock include the following:
| ● | price and volume fluctuations in the overall stock market from time to time; | |
| ● | volatility in the trading prices and trading volumes of transportation stocks; | |
| ● | changes in operating performance and stock market valuations of other transportation companies generally, or those in our industry in particular; | |
| ● | sales of shares of our common stock by us or our stockholders; |
28
| ● | failure of securities analysts to maintain coverage of us, changes in financial estimates by securities analysts who follow our Company, or our failure to meet these estimates or the expectations of investors; | |
| ● | the financial projections we may provide to the public, any changes in those projections, or our failure to meet those projections; | |
| ● | announcements by us or our competitors of new products, features, or services; | |
| ● | the public’s reaction to our press releases, other public announcements and filings with the SEC; | |
| ● | rumors and market speculation involving us or other companies in our industry; | |
| ● | actual or anticipated changes in our results of operations or fluctuations in our results of operations; | |
| ● | actual or anticipated developments in our business, our competitors’ businesses or the competitive landscape generally; | |
| ● | litigation involving us, our industry, or both, or investigations by regulators into our operations or those of our competitors; | |
| ● | developments or disputes concerning our intellectual property or other proprietary rights; | |
| ● | announced or completed acquisitions of businesses, products, services or technologies by us or our competitors; | |
| ● | new laws or regulations or new interpretations of existing laws or regulations applicable to our business; | |
| ● | changes in accounting standards, policies, guidelines, interpretations or principles; | |
| ● | any significant change in our management; and | |
| ● | general economic conditions and slow or negative growth of our markets. |
In recent years, the stock markets generally have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of listed companies. Broad market and industry factors may significantly affect the market price of our common stock, regardless of our actual operating performance. These fluctuations may be even more pronounced in the trading market for our common stock shortly after this offering. If the market price of shares of our common stock after this offering does not ever exceed the initial public offering price, you may not realize any return on your investment in us and may lose some or all of your investment.
In addition, in the past, following periods of volatility in the overall market and in the market price of a particular company’s securities, securities class action litigation has often been instituted against these companies. This litigation, if instituted against us, could result in substantial costs and a diversion of our management’s attention and resources.
Certain recent initial public offerings of companies with public floats comparable to our anticipated public float have experienced extreme volatility that was seemingly unrelated to the underlying performance of the respective company. We may experience similar volatility, which may make it difficult for prospective investors to assess the value of our Common Stock.
In addition to the risks addressed above in the preceding risk factor, our common stock may be subject to extreme volatility that is seemingly unrelated to the underlying performance of our business. Recently, companies with comparable public floats and initial public offering sizes have experienced instances of extreme stock price run-ups followed by rapid price declines, and such stock price volatility was seemingly unrelated to the respective company’s underlying performance. Although the specific cause of such volatility is unclear, our anticipated public float may amplify the impact the actions taken by a few stockholders have on the price of our common stock, which may cause the price of our common stock to deviate, potentially significantly, from a price that better reflects the underlying performance of our business. Should our common stock experience run-ups and declines that are seemingly unrelated to our actual or expected operating performance and financial condition or prospects, prospective investors may have difficulty assessing the rapidly changing value of our common stock. In addition, investors of shares of our common stock may experience losses, which may be material, if the price of our common stock declines after this offering or if such investors purchase shares of our common stock prior to any price decline.
29
Sales or the anticipation of sales of our common stock by the selling stockholders could affect the market price of our common stock and the Underwriters’ stabilization activities and the exercise of its over-allotment option.
The selling stockholders may sell or otherwise engage in transactions with respect to their common stock as described in “Plan of Distribution.” The sale or the anticipation of the sale by the selling stockholders of common stock may have a negative impact on the market for and market price of our common stock. Further, sales or the anticipation of sales by the selling stockholders may affect the exercise by Underwriters of their stabilization activity and their willingness to exercise their over-allotment option.
Nasdaq may delist our securities from trading on its exchange, which could limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions.
We have applied to have our common stock listed on Nasdaq. We cannot guarantee that our securities will be approved for listing on Nasdaq; however, we will not complete this offering unless we are so listed. Although after giving effect to this offering we expect to meet the minimum initial listing standards set forth in the Nasdaq listing standards, we cannot assure you that our securities will be, or will continue to be, listed on Nasdaq in the future. In order to continue listing our securities on Nasdaq, we must maintain certain financial, distribution and stock price levels. Generally, we must maintain a minimum amount in stockholders’ equity (generally $2,500,000) and a minimum number of holders of our securities (generally 300 public holders). Additionally, we will be required to demonstrate compliance with Nasdaq’s initial listing requirements, which are more rigorous than Nasdaq’s continued listing requirements, in order to continue to maintain the listing of our securities on Nasdaq. For instance, our share price would generally be required to be at least $4.00 per share, our stockholders’ equity would generally be required to be at least $5.0 million, and we would be required to have a minimum of 300 round lot holders of our securities (with at least 150 of such round lot holders holding securities with a market value of at least $2,500). We cannot assure you that we will continue to meet those initial listing requirements.
If a limited number of participants in this offering purchase a significant percentage of the offering, the effective public float may be smaller than anticipated and the price of our common stock may be more volatile than it otherwise would be.
As a company conducting a relatively modest public offering, we are subject to the risk that a small number of investors may hold a high percentage of common stock sold in this offering, even if the initial sales by the Underwriters are designed to comply with the Nasdaq listing requirements. If this were to happen, investors could find our shares to be more volatile than they might otherwise anticipate. Companies that experience such volatility in their stock price may be more likely to be the subject of securities litigation. In addition, if a large portion of our public float were to be held by a few investors, smaller investors may find it more difficult to sell their shares and we may cease to meet the Nasdaq public stockholder requirements.
The trading price of our common stock may be volatile, which could result in substantial losses to investors.
The trading price of our common stock is likely to be volatile and could fluctuate widely due to factors beyond our control. This may happen because of broad market and industry factors. The securities of some newly public companies have experienced significant volatility since their initial public offerings, including, in some cases, substantial increase followed by a substantial decline in their trading prices. The trading performances of other vaping companies’ securities after their offerings may affect the attitudes of investors toward vaping companies listed in the United States, which consequently may impact the trading performance of our common stock, regardless of our actual operating performance. In addition, any negative news or perceptions about inadequate corporate governance practices or fraudulent accounting, corporate structure or other matters of other vaping companies may also negatively affect the attitudes of investors towards us. In addition to the above factors, the price and trading volume of our common stock may be highly volatile due to multiple factors, including the following:
| ● | regulatory developments affecting us, our customers, or our industry; |
| ● | announcements of studies and reports relating to our service offerings or those of our competitors; |
| ● | actual or anticipated fluctuations in our results of operations and changes or revisions of our expected results; |
| ● | changes in financial estimates by securities research analysts; | |
| ● | announcements by us or our competitors of new product and service offerings, acquisitions, strategic relationships, joint ventures or capital commitments; | |
| ● | additions to or departures of our senior management; | |
| ● | detrimental negative publicity about us, our management or our industry; | |
| ● | release or expiry of lock-up or other transfer restrictions on our outstanding common stock; and | |
| ● | sales or perceived potential sales of additional common stock. |
30
Shares eligible for future sale may adversely affect the market price of our common stock, as the future sale of a substantial amount of outstanding common stock in the public marketplace could reduce the price of our common stock.
The market price of our shares could decline as a result of sales of substantial amounts of our shares in the public market, or the perception that these sales could occur. In addition, these factors could make it more difficult for us to raise through future offerings of our common stock. An aggregate of 50,000,000 shares were outstanding before the consummation of this offering and 56,000,000 shares (56,900,000 shares if the Underwriters’ over-allotment option is exercised in full) will be outstanding immediately after this offering. All of the shares sold in the offering will be freely transferable without restriction or further registration under the Securities Act. The remaining shares will be “restricted securities” as defined in Rule 144. These shares may be sold in the future without registration under the Securities Act to the extent permitted by Rule 144 or other exemptions under the Securities Act.
Our directors, executive officers and holders of 5% or more of our common stock have agreed, subject to some exceptions, not to transfer or dispose of, directly or indirectly, any of our common stock, or any securities convertible into or exchangeable or exercisable for our common stock, for a period of six months after the date of this prospectus. After the expiration of the six months period, the common stock held by our directors, executive officers and 5% stockholders may be sold subject to the restrictions under Rule 144 under the Securities Act or by means of registered public offerings. As the expiration of lock-up period approaches, the knowledge of the possible sale of the shares subject to the lock-up may have an adverse effect upon the market price of our common stock.
Because the initial public offering price is substantially higher than the net tangible book value per share of common stock at December 31, 2022, you will experience immediate and substantial dilution.
The initial public offering price of our shares is expected to be substantially higher than the net tangible book value per share of our common stock at December 31, 2022. Assuming the completion of the offering at a public offering price of $7.00 per share, the midpoint of the proposed offering price range, if you purchase shares in this offering, you will incur immediate dilution of approximately $6.16 or approximately 88.0% in the pro forma net tangible book value per share from the price per share that you pay for the shares. Accordingly, if you purchase shares in this offering, you will incur immediate and substantial dilution of your investment. See “Dilution.”
Our management has significant discretion over use of proceeds of this offering.
While we have identified the priorities to which we expect to put the proceeds of this offering, our management will have considerable discretion in the application of the net proceeds received by us. We have reserved the right to re-allocate funds currently allocated as described under “Use of Proceeds” to our general working capital. If that were to happen, then our management would have significant discretion over even more of the net proceeds to be received by us in this offering. You will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. You must rely on the judgment of our management regarding the application of the net proceeds of this offering. The net proceeds may be used for corporate purposes that do not improve our efforts to achieve profitability or increase our stock price. The net proceeds from this offering may be placed in investments that do not produce profit or increase value. See “Use of Proceeds.”
We will incur increased costs as a result of being a public company, particularly after we cease to qualify as an “emerging growth company.”
Upon completion of this offering, we will become a public company and expect to incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, as well as rules subsequently implemented by the SEC and Nasdaq, impose various requirements on the corporate governance practices of public companies. As a company with less than US$1.235 billion in revenues for our last fiscal year, we qualify as an “emerging growth company” pursuant to the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 of the Sarbanes-Oxley Act of 2002 in the assessment of the emerging growth company’s internal control over financial reporting and permission to delay adopting new or revised accounting standards until such time as those standards apply to private companies.
31
We expect these rules and regulations to increase our legal and financial compliance costs and to make some corporate activities more time-consuming and costly. After we are no longer an “emerging growth company”, we expect to incur significant expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404 of the Sarbanes-Oxley Act of 2002 and the other rules and regulations of the SEC. For example, as a result of becoming a public company, we will need to increase the number of independent directors and adopt policies regarding internal controls and disclosure controls and procedures. We also expect that operating as a public company will make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. In addition, we will incur additional costs associated with our public company reporting requirements. It may also be more difficult for us to find qualified persons to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these rules and regulations, and we cannot predict or estimate with any degree of certainty the amount of additional costs we may incur or the timing of such costs.
As an “emerging growth company” under the Jumpstart Our Business Startups Act, or JOBS Act, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements.
As an “emerging growth company” under the JOBS Act, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements. We are an emerging growth company until the earliest of:
| ● | the last day of the fiscal year during which we have total annual gross revenues of $1.235 billion or more; |
| ● | the last day of the fiscal year following the fifth anniversary of this offering; |
| ● | the date on which we have, during the previous three-year period, issued more than $1 billion in non-convertible debt; or |
| ● | the date on which we are deemed a “large accelerated issuer” as defined under the federal securities laws. |
For so long as we remain an emerging growth company, we may take advantage of certain exemptions from various reporting requirements that are applicable to public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of section 404 of the Sarbanes-Oxley Act for up to five fiscal years after the date of this offering. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and the trading price of our common stock may be more volatile. In addition, our costs of operating as a public company may increase when we cease to be an emerging growth company.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, the market price for our common stock and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. If research analysts do not establish and maintain adequate research coverage or if one or more of the analysts who cover us downgrade our common stock or publish inaccurate or unfavorable research about our business, the market price for our common stock would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, we could lose visibility in the financial markets, which, in turn, could cause the market price or trading volume for our common stock to decline.
Our by-laws include forum selection provisions which may limit your ability to commence an action against us.
Our by-laws provide that unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have jurisdiction, the federal district court for the District of Delaware) shall be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf; (ii) any action asserting a claim for breach of a fiduciary duty owed by any of our directors, officers, employees, or agents to us or our stockholders; (iii) any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation, or our by-laws; or (iv) any action asserting a claim governed by the internal affairs doctrine; in each case, subject to said court having personal jurisdiction over the indispensable parties named as defendants therein.
Our by-laws also provide that unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint for the resolution of any complaint for which such courts have exclusive jurisdiction, including, but not limited to, any complaint asserting a cause of action arising under the Securities Exchange Act of 1934. Our by-laws also provide that the exclusive forum provisions do not apply to actions arising under the Securities Act.
There is uncertainty as to whether a court would enforce these provisions, and investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder.
32
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve risks and uncertainties. All statements other than statements of historical facts are forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements.
You can identify these forward-looking statements by words or phrases such as “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “likely to” or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements include, but are not limited to, statements about:
| ● | our goals and growth strategies; |
| ● | our expectations regarding demand for and market acceptance of our brand and platforms; |
| ● | our future business development, results of operations and financial condition; |
| ● | our ability to establish manufacturing operations in Vietnam and the United States; |
| ● | our ability to establish relationships with suppliers other than Shenzhen Yi Jia; |
| ● | the effect of regulations relating to the marketing and sale of vaping products in the United States and other countries; |
| ● | our ability to maintain and improve our infrastructure necessary to operate our business; |
| ● | competition in the vaping industry; |
| ● | the expected growth of, and trends in, the markets for our products and services in the markets in which jurisdictions that we sell our products; |
| ● | the development of a market for cannabis vaping products outside of the United States, including the legalization of cannabis in certain European countries; |
| ● | the expected growth of, and trends in, the markets for our products and services in the markets in which jurisdictions that we sell our products; |
| ● | the effect of supply chain issues on our ability to manufacture and our ability and the ability of our distributors to distribute product; |
| ● | the development of a market for cannabis vaping product and our ability to market cannabis products to adult users; | |
| ● | our ability to compete successfully for both tobacco and cannabis products, the expected growth of, and trends in, the markets for our products and services jurisdictions that we sell or plan to sell our products; |
| ● | effects of the COVID-19 pandemic and steps taken by governments to address the pandemic; |
| ● | government policies and regulations relating to our operations, including regulations relating to the sale and distribution of our vaping products and those relating to manufacturing operations; |
| ● | our ability to develop and maintain effective disclosure controls and internal controls over financial reporting; |
| ● | our ability to use of proceeds from this offering as described under “Use of Proceeds”; |
33
| ● | the effect of any sales or the anticipation of sales by the selling stockholders upon the market price of our shares of common stock or the Underwriters’ stabilization activity or the exercise by the Underwriters of their over-allotment option; |
| ● | our ability to comply with the continued listing standards on the exchange or trading market on which our shares of common stock is listed for trading; |
| ● | our ability to attract and retain qualified senior management personnel and research and development staff; | |
| ● | the volatility of the Company's operating results and financial condition and the price of its common stock; |
| ● | general economic and business condition in China and elsewhere; and |
| ● | assumptions underlying or related to any of the foregoing. |
You should read thoroughly this prospectus and the documents that we refer to in this prospectus with the understanding that our actual future results may be materially different from and worse than what we expect. Other sections of this prospectus include additional factors which could adversely impact our business and financial performance. Moreover, we operate in an evolving environment. New risk factors and uncertainties emerge from time to time and it is not possible for our management to predict all risk factors and uncertainties, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. We qualify all of our forward-looking statements by these cautionary statements.
This prospectus contains certain data and information that we obtained from various government and private publications. Statistical data in these publications also include projections based on a number of assumptions. The e-vapor industry may not grow at the rate projected by market data, or at all. Failure of this market to grow at the projected rate may have a material and adverse effect on our business and the market price of the shares of common stock. In addition, the rapidly evolving nature of this industry results in significant uncertainties for any projections or estimates relating to the growth prospects or future condition of our market. Furthermore, if any one or more of the assumptions underlying the market data are later found to be incorrect, actual results may differ from the projections based on these assumptions. You should not place undue reliance on these forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. The forward-looking statements made in this prospectus relate only to events or information as of the date on which the statements are made in this prospectus. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this prospectus and the documents that we refer to in this prospectus and have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect.
After deducting the estimated Underwriters’ discount and offering expenses payable by us, we expect to receive net proceeds of approximately $37.1 million (or $43.0 million if the Underwriters exercise their over-allotment option in full) from this offering based on a public offering price of $7.00 per share, the midpoint of the proposed offering price range. We will not receive any proceeds from the sale by the selling stockholder of their common stock.
We intend to use the net proceeds of this offering as follows, after we complete the remittance process:
| ● | approximately 35% to establish manufacturing operations in Vietnam and the United States; |
| ● | approximately 25% for research and development activities, which include our efforts to develop new products and new vaping technology; |
| ● | approximately 20% for the marketing and promotion of our branded products; and |
| ● | the balance of approximately 20%, together with any proceeds from the over-allotment option, for general administration and working capital. |
34
The precise amounts and percentage of proceeds we devote to particular categories of activity, and their priority of use, will depend on prevailing market and business conditions as well as on the nature of particular opportunities that may arise from time to time. Accordingly, we reserve the right to change the use of proceeds that we presently anticipate and describe herein. Pending the use of the proceeds, we intend to invest our net proceeds in short-term, interest bearing, and investment-grade obligations. As of December 31, 2022, we owed Tuanfang Liu, our chief executive officer, approximately $3.4 million as a dividend payable, representing the unpaid amount of an approximately $3.8 million dividend declared by Aspire Science during the year ended June 30, 2020, when Mr. Liu was the sole shareholder of Aspire Science. As of December 31, 2022, Aspire Science had a balance due to Eigate (Hong Kong) Technology Co., Limited (“Eigate”), which is owned by Mr. Liu, of approximately $40.5 million, which is a non-interest bearing advance. At the time of the loan, Aspire Science and Eigate were both owned by Mr. Liu, and Eigate lent money to Aspire Science for working capital. On February 2, 2023, we made the payments to Mr. Liu and Eigate. We also owe Shenzhen Yi Jia the purchase price of products ordered from Shenzhen Yi Jia, which we pay in the normal course of business.
The foregoing is based on the order of priority of each purpose and represents our current intentions based upon our present plans and business conditions to use and allocate the net proceeds of this offering. Our management, however, will have significant flexibility and discretion to apply the net proceeds of this offering. If an unforeseen event occurs or business conditions change, we may use the proceeds of this offering differently than as described in this prospectus.
The following tables set forth our capitalization as of December 31, 2022:
| ● | on an actual basis; and |
| ● | on a pro forma as adjusted basis to reflect the issuance and sale of 6,000,000 shares at an assumed initial public offering price of $7.00 per share, the midpoint of the proposed offering price range, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. |
You should read the tables together with our consolidated financial statements and the related notes included elsewhere in this prospectus and the information under “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| As of December 31, 2022 | ||||||||
| Actual | As Adjusted | |||||||
| ($ in thousands) | ||||||||
| Equity: | ||||||||
| Shares of common stock, $0.0001 par value, 140,000,000 shares authorized, 50,000,000 shares of common stock outstanding on an actual basis; and 56,000,000 shares of common stock outstanding as adjusted | $ | 5 | $ | 6 | ||||
| Additional paid-in capital | - | 37,109 | ||||||
| Capital contribution | 74,260 | 74,260 | ||||||
| Accumulated other comprehensive loss | (42 | ) | (42 | ) | ||||
| Retained earnings | 8,995 | 8,995 | ||||||
| Total stockholders’ equity | $ | 83,218 | $ | 120,328 | ||||
| Total capitalization | $ | 83,218 | $ | 120,328 | ||||
| (1) | Pro forma additional paid in capital reflects the net proceeds we expect to receive, after deducting underwriting fee, Underwriters’ expense allowance and other expenses. We expect to receive net proceeds of approximately $37,110,000 (offering proceeds of $42,000,000, less underwriting discounts of $2,520,000, non-accountable expense of $420,000 and offering expenses of $1,950,000, which includes reimbursement of the Representative’s out-of-pocket expenses of $450,000). If the Underwriters’ over-allotment option is exercised, the net proceeds to us would be $42,969,000. The increase in additional paid in capital reflects the net proceeds we expect to receive, after deducting underwriting discounts, Underwriters’ expense allowance and other expenses of the offering. |
35
If you invest in our common stock, you will incur immediate dilution since the public offering price per share you will pay in this offering is more than the net tangible book value per share of common stock immediately after this offering.
The net tangible book value of our common stock as of December 31, 2022 was $9,730,884, or $0.19 per share, based upon 50,000,000 shares of common stock outstanding. Net tangible book value per share represents the amount of our total tangible assets reduced by the amount of our total liabilities, divided by the total number of shares of common stock outstanding.
The dilution in net tangible book value per share to new investors, represents the difference between the amount per share paid by purchasers of shares in this offering and the pro forma net tangible book value per share immediately after completion of this offering. After giving effect to the sale of the 6,000,000 shares being sold pursuant to this offering at a public offering price of $7.00 per share, the midpoint of the proposed offering price range, and after deducting Underwriters’ discount and commission payable by us in the amount of $2,520,000, non-accountable expenses of $420,000 payable to the Underwriters and estimated offering expenses in the amount of $1,950,000, our pro forma net tangible book value would be approximately $46,841,000, or $0.84 per share. This represents an immediate increase in net tangible book value of $0.65 per share to existing stockholders and an immediate decrease in net tangible book value of $6.16 per share to new investors purchasing the shares in this offering.
The following table illustrates this per share dilution:
| As of December 31, 2022 | ||||
| Assumed initial public offering price per share of common stock | $ | 7.00 | ||
| Net tangible book value per share as of December 31, 2022 | $ | 0.19 | ||
| Increase in net tangible book value per share attributable to existing stockholders | $ | 0.65 | ||
| Pro forma net tangible book value per share after this offering | $ | 0.84 | ||
| Dilution per share to new investors | $ | 6.16 | ||
Our adjusted pro forma net tangible book value after the offering, and the decrease to new investors in the offering, will change from the amounts shown above if the Underwriters’ over-allotment option is exercised.
A $1.00 increase (decrease) in the assumed public offering price would increase (decrease) our pro forma net tangible book value per share after this offering by approximately $0.1, and increase the dilution per share to new investors by approximately $0.1, after deducting the Underwriters’ discount and estimated offering expenses payable by us.
The following table sets forth, on a pro forma as adjusted basis as of June 30, 2022, the difference between the number of shares of common stock purchased from us, the total cash consideration paid, and the average price per share paid by our existing stockholders and by new public investors before deducting estimated Underwriters’ discounts and commissions and estimated offering expenses payable by us, using an assumed public offering price of $7.00 per share of common stock:
| Shares Purchased | Total Cash Consideration | Average Price Per | ||||||||||||||||||
| Number | Percent | Amount | Percent | Share | ||||||||||||||||
| Existing stockholders1 | 50,000,000 | 89.3 | % | $ | 5,000 | 0.01 | % | $ | 0.0001 | |||||||||||
| New investors from public offering | 6,000,000 | 10.7 | % | 42,000,000 | 99.9 | % | 7.00 | |||||||||||||
| Total | 100.0 | % | 100.0 | % | ||||||||||||||||
| 1 | In connection with our organization, we issued a total of 50,000,000 shares of common stock to the stockholders of Aspire Global in the same proportion as their equity interest in Aspire Global at the time of the transfer for no cash consideration as part of a restructure of Aspire Global pursuant to which the equity in Aspire North America and Aspire Science was transferred to us for no consideration. The amount shown in the table reflects the par value of the shares. |
The pro forma as adjusted information discussed above is illustrative only. Our net tangible book value following the completion of this offering is subject to adjustment based on the actual initial public offering price of our shares of common stock and other terms of this offering determined at pricing.
36
ENFORCEMENT OF CIVIL LIABILITIES
Our executive officers and directors are located in the United States except that two directors, one of whom is our chairman and chief executive officer, and one director-designee are located in mainland China. As a result, it may be difficult for a stockholder to effect service of process within the United States upon the directors and officers who are located outside of the United States.
We have been advised by Han Kun Law Offices that there is uncertainty as to whether the courts of the PRC would enforce judgments of United States courts obtained against these persons predicated upon the civil liability provisions of the United States federal and state securities laws. Han Kun Law Offices has further advised us that the recognition and enforcement of foreign judgments are provided for under PRC Civil Procedures Law. PRC courts may recognize and enforce foreign judgments in accordance with the requirements of PRC Civil Procedures Law based either on treaties between China and the country where the judgment is made or on reciprocity between jurisdictions. China does not have any treaties or other form of reciprocity with the United States that provide for the reciprocal recognition and enforcement of foreign judgments. In addition, according to the PRC Civil Procedures Law, courts in the PRC will not enforce a foreign judgment against our directors and officers if they decide that the judgment violates the basic principles of PRC law or national sovereignty, security or public interest. As a result, it is uncertain whether and on what basis a PRC court would enforce a judgment rendered by a court in the United States. Under the PRC Civil Procedures Law, foreign stockholders may originate actions based on PRC law against a company in China for disputes if they can establish sufficient nexus to the PRC for a PRC court to have jurisdiction, and meet other procedural requirements, including, among others, the plaintiff must have a direct interest in the case, and there must be a concrete claim, a factual basis and a cause for the suit. It will be, however, difficult for U.S. stockholders to originate actions in the PRC against our directors who are located in the PRC in accordance with PRC laws because we are incorporated under the laws of the State of Delaware and it will be difficult for U.S. stockholders, by virtue only of holding our common stock, to establish a connection to the PRC for a PRC court to have jurisdiction as required under the PRC Civil Procedures Law. As a result of the foregoing, it would be very expensive and time-consuming for a stockholder to either seek to enforce a U.S. judgment in China or to commence an action in a Chinese court, with a strong likelihood that the stockholder will not be successful.
The following table sets forth the names of the selling stockholders, the number of shares of common stock owned beneficially by the selling stockholders as of December 31, 2022, and the number of our shares of common stock that may be offered by the selling stockholders pursuant to this prospectus. The table and the other information contained under the captions “Selling Stockholders” and “Plan of Distribution” has been prepared based upon information furnished to us by or on behalf of the selling stockholders. The following table sets forth, as to the selling stockholders, the number of shares of common stock beneficially owned, the number of shares being sold, the number of shares beneficially owned upon completion of the offering and the percentage beneficial ownership upon completion of the offering.
| After Sale
of Shares in Offering | ||||||||||||||||
| Name | Shares Beneficially Owned | Shares Being Sold | Shares Beneficially Owned | Percent of Outstanding | ||||||||||||
| Reliance Lead Company Limited | 1,212,500 | 1,212,500 | 0 | 0 | % | |||||||||||
| Jerry Dragon Advisory LLC | 537,500 | 537,500 | 0 | 0 | % | |||||||||||
Neither selling stockholder has, and within the past three years has not had, any position, office or material relationship with us or with any of our predecessors or affiliates except as described below.
The selling stockholders, who were stockholders of Aspire Global, acquired the shares from us in connection with our organization in the same proportion as their share interest in Aspire Global. See “Business – Acquisition of Our Business from a Related Party.”
37
The selling stockholders and any of its pledgees, donees, assignees and successors-in-interest may, from time to time, sell any or all of its shares of common stock at the initial public offering price of the underwritten offering until such time as our common stock is listed on the Nasdaq Capital Market, at which time they may sell such shares on any stock exchange, market or trading facility on which the shares are traded or in private transactions or by gift. The shares offered by this prospectus may be sold by the selling stockholders at market prices prevailing at the time of sale or at negotiated prices. The selling stockholders will not sell any shares pursuant to this prospectus until such time as our common stock is traded on Nasdaq. The selling stockholders may use any one or more of the following methods when selling or otherwise transferring shares:
| ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| ● | block trades in which a broker-dealer will attempt to sell the shares as agent but may purchase a position and resell a portion of the block as principal to facilitate the transaction; |
| ● | sales to a broker-dealer as principal and the resale by the broker-dealer of the shares for its account; |
| ● | an exchange distribution in accordance with the rules of the applicable exchange if we are listed on an exchange at the time of sale; |
| ● | privately negotiated transactions, including gifts; |
| ● | covering short sales made after the date of this prospectus; |
| ● | pursuant to an arrangement or agreement with a broker-dealer to sell a specified number of such shares at a stipulated price per share; |
| ● | a combination of any such methods of sale; and |
| ● | any other method of sale permitted pursuant to applicable law. |
To the extent permitted under Rule 144, the selling stockholders may also sell shares of common stock owned by it pursuant to Rule 144 rather than pursuant to this prospectus.
Broker-dealers engaged by the selling stockholders may arrange for other broker-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling stockholders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. The selling stockholders do not expect these commissions and discounts to exceed what is customary in the types of transactions involved. None of the selling stockholders is an affiliate of any broker-dealer.
The selling stockholders may from time to time pledge or grant a security interest in some or all of the shares owned by them and, if the selling stockholders defaults in the performance of the secured obligations, the pledgees or secured parties may offer and sell the shares of common stock from time to time under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus.
In connection with the sale of our shares of common stock or interests therein, the selling stockholders may enter into hedging transactions with broker-dealers or other financial institutions which may in turn engage in short sales of our shares of common stock in the course of hedging the positions they assume. The selling stockholders may, after the date of this prospectus, also sell our shares of common stock short and deliver these securities to close out its short positions, or lend or pledge its shares of common stock to broker-dealers that in turn may sell these securities. The selling stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares of common stock offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The selling stockholders also may transfer the shares of common stock in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
The selling stockholders and any broker-dealers or agents that are involved in selling the shares may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, they will be subject to the prospectus delivery requirements of the Securities Act, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act, and federal securities laws, including Regulation M, may restrict the timing of purchases and sales of our shares of common stock by the selling stockholders and any other persons who are involved in the distribution of the shares of common stock pursuant to this prospectus. The selling stockholders has informed us that it does not have any agreement or understanding, directly or indirectly, with any person to distribute the shares of common stock.
38
We may be required to amend or supplement this prospectus in the event that (a) a selling stockholders transfers securities under conditions which require the purchaser or transferee to be named in the prospectus as a selling stockholders, in which case we will be required to amend or supplement this prospectus to name the selling stockholders, or (b) the selling stockholders sells shares to an underwriter, in which case we will be required to amend or supplement this prospectus to name the underwriter and the method of sale.
We are paying all fees and expenses incident to the registration of the shares. We have agreed to indemnify the selling stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
The following statements of operations data for the years ended June 30, 2021 and 2022, balance sheet data as of June 30, 2021 and 2022 and statements of cash flows data for the years ended June 30, 2021 and 2022 have been derived from our audited consolidated financial statements included elsewhere in this prospectus. The following consolidated statements of operations data for the six months ended December 31, 2021 and 2022, selected consolidated balance sheet data as of December 31, 2022, and consolidated statements of cash flows data for the six months ended December 31, 2021 and 2022 have been derived from our unaudited condensed consolidated financial statements included elsewhere in this prospectus and have been prepared on the same basis as our audited consolidated financial statements. You should read the Selected Financial Data together with our financial statements and the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
(dollars in thousands, except per share amounts)
Consolidated Statement of Operations Data:
| Year Ended June 30, | Six Months Ended December 31, | |||||||||||||||
| 2021 | 2022 | 2021 | 2022 | |||||||||||||
| Revenue | $ | 63,415 | $ | 88,095 | $ | 47,233 | $ | 58,840 | ||||||||
| Cost of revenue | 52,999 | 74,789 | 39,921 | 48,910 | ||||||||||||
| Gross profit | 10,416 | 13,306 | 7,312 | 9,930 | ||||||||||||
| Operating expenses | 6,772 | 14,295 | 5,528 | 11,608 | ||||||||||||
| Income(loss) from operations | 3,644 | (989 | ) | 1,784 | (1,678 | ) | ||||||||||
| Other income(expense) | (20 | ) | 186 | 124 | (441 | ) | ||||||||||
| Income(loss) before income taxes | 3,624 | (803 | ) | 1,908 | (2,119 | ) | ||||||||||
| Net income(loss) | 2,938 | (1,874 | ) | 1,279 | (2,951 | ) | ||||||||||
| Net income(loss) per share | $ | 0.06 | $ | (0.04 | ) | $ | 0.03 | $ | (0.06 | ) | ||||||
| Average shares of common stock outstanding (basic and diluted) | 50,000,000 | 50,000,000 | 50,000,000 | 50,000,000 | ||||||||||||
Consolidated Balance Sheet Data
| As of June 30, | As of December 31, | |||||||||||
| 2021 | 2022 | 2022 | ||||||||||
| Assets | $ | 93,864 | $ | 100,735 | $ | 201,055 | ||||||
| Current assets | 93,421 | 99,449 | 122,808 | |||||||||
| Working capital | 13,585 | 10,481 | 7,800 | |||||||||
| Retained earnings | 13,821 | 11,946 | 8,995 | |||||||||
| Shareholders’ equity | 13,758 | 11,767 | 83,218 | |||||||||
Consolidated Cash Flows Data:
| Year Ended June 30, | Six Months Ended December 31, | |||||||||||||||
| Consolidated cash flow data: | 2021 | 2022 | 2021 | 2022 | ||||||||||||
| Net cash flows provided by (used in) operating activities | $ | 5,024 | $ | (7,558 | ) | $ | (8,324 | ) | $ | 8,825 | ||||||
| Net cash used in investing activities | (1 | ) | (122 | ) | (47 | ) | (478 | ) | ||||||||
| Net cash (used in) provided by financing activities | (228 | ) | (3,089 | ) | (2,394 | ) | 1,440 | |||||||||
| Net increase (decrease) in cash and cash equivalents | 4,795 | (10,769 | ) | (10,765 | ) | 9,787 | ||||||||||
39
MANAGEMENT’S DISCUSSION
AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read together with “Selected Consolidated Financial Data” and our financial statements and the related notes appearing elsewhere in this prospectus. In addition to historical information, this discussion contains forward-looking statements reflecting our current expectations that involve risks, uncertainties and assumptions. See “Cautionary Note Concerning Forward-Looking Statements.” Actual results and the timing of events could differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed in “Risk Factors” and elsewhere in this prospectus.
Overview
We are engaged in the research and development, design, commercialization, sales, marketing and distribution of branded e-cigarettes and cannabis vaping products. We sell our tobacco products worldwide except for the PRC and Russia. Our tobacco products are marketed under the Aspire brand name and are sold primarily through our distribution network. We currently sell our cannabis vaping hardware only in the United States, and we have recently commenced marketing activities in Canada and Europe, primarily in the European Union. All of our products are vaping hardware. Vaping refers to the practice of inhaling and exhaling the vapor produced by an electronic vaping device, and includes dabbing, which is the recreational inhalation of extremely concentrated tetrahydrocannabinol, the main psychotropic cannabinoid derived from the marijuana plant. Our cannabis products are marketed under the Ispire brand name, primarily on an ODM basis to other cannabis vapor companies. ODM generally involves the design and customization of the core products to meet each brand’s unique image and needs, and our products are sold by our customers under their own brand names although they may also include our brand name on the products.
Regulatory Risks
The sale of tobacco and cannabis products is subject to regulations worldwide. Many countries prohibit the sale of any cannabis products, and many countries have regulations relating to tobacco products, with a particular emphasis on underage sales. As a result of regulations in the United States, we are able to sell only one tobacco vaping product line, the Nautilus Prime, in the United States. Our tobacco vaping sales in the United States were approximately $0.9 million for the year ended June 30, 2022, and we had no sales of tobacco vaping products in the United States. Because the volume of sales did not justify the marketing and regulatory costs, we have ceased marketing tobacco vaping products in the United States. If any similar regulations are adopted with respect to cannabis products, our business will be severely impacted since all of our cannabis revenue for the year ended June 30, 2022 was generated from sales in the United States. See “Regulations.”
Effects of COVID-19 Pandemic
In December 2019, coronavirus disease 2019 (COVID-19) was first reported to have surfaced in Wuhan, China. During 2020, the disease spread to many parts of the world. The epidemic has resulted in quarantines, travel restrictions, and the temporary closure of stores and facilities in much of the world.
Measures taken by various governments to contain the virus have affected economic activity in all countries where the consumers of our product live. We took a number of measures to monitor and mitigate the effects of COVID-19, such as safety and health measures for our personnel, such as social distancing, in accordance with government policies and advice, securing the supply of materials that are essential to our production process. We will continue to follow the various government policies and advice and, in parallel, we may take further actions that we determine are in the best interests of our employees, customers, and business relationships.
Our products are manufactured in China by Shenzhen Yi Jia, which is 95% owned by our chief executive officer and controlling stockholder, Tuanfang Liu. Mr. Liu is chairman of Shenzhen Yi Jia and his wife, Jiangyan Zhu, who is also one of our directors, is its vice president of finance. From the middle of January 2020, the start of the Chinese New Year holiday, until the end of April 2020, its production was slowed due to the COVID-19 pandemic in China. This slowdown in production resulted in a near-total stoppage of our supplier’s business during this nearly four-month period and our sales activities were substantially reduced. In addition, since our products are generally sold in stores such as grocery stores, convenience stores and tobacco stores, to the extent that either the stores are closed as a result of government actions or consumers are reluctant to go shopping because of the COVID-19 pandemic, retail sales of our products suffered. Following the temporary slowdown in production in the first half year of 2020, our supplier, Shenzhen Yi Jia, has recovered its production capacity, and we have not experienced significant supply chain constraints as a result of the pandemic.
40
From late 2020 to the middle of 2021, COVID-19 vaccination program had been greatly promoted around the globe, however several types of COVID-19 variants emerged in different parts of the world. Our sales in Europe and the United States continued to be affected by government actions relating to COVID-19 and COVID-19 variants, while the production was slightly reduced by the strict measures taken by the Government of China to address the small COVID-19 outbreaks in Guangdong province. To the extent that China’s zero COVID policy results in closing or significantly reducing the operations of Shenzhen Yi Jia or any of its suppliers, our business will be impacted since we presently rely on Shenzhen Yi Jia for our products. We cannot predict the effect on our business, the business of Shenzhen Yi Jia and on business in the PRC in general as a result of the change in the PRC government’s zero-COVID rules, including the effect of increased infections, deaths and hospitalizations as well as the development of any new variants of COVID. To the extent that quarantine is required of any person entering mainland China, our business may be impacted. Although we do not have any operations or assets in mainland China, to the extent that any of our key personnel or any key personnel of Shenzhen Yi Jia or any of our or its suppliers are subject to quarantine on entering mainland China, our business may be impacted. Since January 8, 2023, no centralized quarantine or mass PCR testing will be undertaken on travelers entering mainland China. Travelers to mainland China are only required to take PCR test 48 hours prior to their departure and report the PCR test findings on their customs health declaration form. Only those whose test results are positive prior to departure will have to postpone their travel until the PCR results turn negative.
The extent to which COVID-19 impacts our operations on an ongoing basis is highly uncertain. It will depend on various factors including the duration and severity of the outbreak, new information which may emerge concerning the severity of the coronavirus or any variants and the actions to contain the coronavirus or treat its impact, among others.
Supply Chain Risks
One of effects of the COVID-19 has been delays resulting from supply chain issues, which relate to the difficulty that companies have in having their products manufactured, shipped to the country of destination, and delivered from the port of entry to the customer’s location. As a result of the COVID-19 pandemic, during 2021 and early 2022 there were fewer longshoremen unloading ships and fewer truckers to deliver the products to market, which has resulted in significant delays in the delivery of products to markets. To the extent that products are shipped by sea, there are additional risks resulting from ports not being able to unload ships promptly, causing delays in getting into port, including potential damage from seawater and fire, product degradation and the possibility of containers being destroyed, damaged or falling off the ship into the water. As the port delays have significantly decreased, we do not believe that the supply chain issues that affected our operations are currently affecting us. We cannot assure you that delays will not affect our business in the future. The inability to deliver products to the ultimate vendor could impair our ability to generate revenue from our products. As the port delays have significantly decreased, we do not believe that the supply chain issues that affected our operations are currently affecting us. While restrictions have been loosened in most of the world, China continued its zero-COVID policy, which resulted in significant closures. To the extent that China’s zero-COVID policy and the recent change from the zero-COVID policy results in shutdowns that affect Shenzhen Yi Jia or its suppliers, we will be impacted.
In 2021, Shenzhen Yi Jia suffered a chip shortage resulting in a slowdown in delivery of its products to us from April to August 2021. To secure the supply of chips, Shenzhen Yi Jia changed the payment terms to chip supplier from 30 days after delivery in the past to prepayment, and it engaged two new chip suppliers. Since September 2021, Shenzhen Yi Jia has obtained a supply of chips to meet its production need and a chip shortage no longer affects its production. However, we cannot assure you that we will not suffer from a chip shortage affecting Shenzhen Yi Jia or any other supplier.
The delay in shipment and chip shortage had a negative impact on our results of operation. In 2022, there was a loss of potential sales orders of approximately $2 million, around 2.3% of our total sales, which caused a decline of $0.3 million in our gross profit, resulting from delay in supply chain. We believe delays in supply chain may continue in the coming year, which may affect around 3% of our total sales orders. In order to mitigate the supply chain disruptions, we are taking the initial steps toward the development of manufacturing operations in Vietnam and in California. With our own production facilities, we will have better control of the manufacturing process and shipment of our products to customers, as well as diversifying risks of any production shutdown. However, we do not have any experience in manufacturing operations, and in order to establish manufacturing operations, we will have to hire personnel with experience in setting up and operating manufacturing operations. With respect to operations in Vietnam, we will need to engage personnel who have experience in managing operations in Vietnam. Further, to the extent that our Vietnam or California operations rely on Chinese suppliers for any components, we will be subject to any shortages and delays as a result of any lockdowns pursuant to China’s zero-COVID policies. We cannot assure you that if our suppliers are impacted by China’s zero COVID policy, we will be able to obtain products or components from suppliers outside of China.
We are planning to establish manufacturing facilities in California and Vietnam as part of our efforts to reduce the effects of inflation because of the lower cost structure in Vietnam, and to reduce the potential impact of China’s zero COVID policy. We are not experienced in operating manufacturing facilities and we will need to hire key employees in Vietnam who understand the applicable laws and regulations, as well as local customs, in order to operate our proposed facilities. We cannot assure you that we will be able to operate efficiently in compliance with all applicable construction, environmental and other laws and regulations affecting the manufacture of our products in Vietnam. While our proposed facilities in California may provide protection from the effect of China zero COVID policy, it may not reduce the impact of inflation. In addition, our California facilities may be subject to unplanned expenses and delays as a result of compliance with local rules and regulations relating to construction. Thus, we cannot assure you that we will be able to commence manufacturing options in either location in the near future or that we will be able to reduce our costs as a result of operating our own facilities. Until we have established our own facilities, we anticipate that we will continue to rely on Shenzhen Yi Jia for our products.
41
Key Factors that Affect Our Results of Operations
We believe the following key factors may affect our financial condition and results of operations:
| ● | The effect of legislation and regulations affecting the tobacco and cannabis vaping products. | |
| ● | If we elect to market tobacco vaping products in the United States, our ability to obtain regulatory approval to market additional tobacco vaping products in the United States. | |
| ● | Our ability to develop and market tobacco and cannabis vaping products to meet the changing tastes of users. | |
| ● | The effects of competition. | |
| ● | The development of an international market for cannabis vaping products, which is presently primarily limited to certain states in the United States. | |
| ● | The effect of both the outbreak of COVID-19 or any other pandemic, which severely impacted our production for four months during 2020, and the availability, effectiveness and acceptance of vaccines. |
Results of Operations
The following table sets forth a summary of our consolidated statements of operations and comprehensive income for the years ended June 30, 2021 and 2022, and for the six months ended December 31, 2021 and 2022 (dollars in thousands except per share amounts).
| Year Ended June 30, | Six Months Ended December 31, | |||||||||||||||||||||||||||||||
| 2021 | 2022 | 2021 | 2022 | |||||||||||||||||||||||||||||
| $ | % of Revenue | $ | % of Revenue | $ | % of Revenue | $ | % of Revenue | |||||||||||||||||||||||||
| Revenue | $ | 63,415 | 100.0 | % | $ | 88,095 | 100.0 | % | $ | 47,233 | 100.0 | % | $ | 58,840 | 100.0 | % | ||||||||||||||||
| Cost of revenue | (52,999 | ) | (83.6 | )% | (74,789 | ) | (84.9 | )% | (39,921 | ) | (84.5 | )% | (48,910 | ) | (83.1 | )% | ||||||||||||||||
| Gross profit | 10,416 | 16.4 | % | 13,306 | 15.1 | % | 7,312 | 15.5 | % | 9,930 | 16.9 | % | ||||||||||||||||||||
| Operating expenses | (6,772 | ) | (10.6 | )% | (14,295 | ) | (16.2 | )% | (5,528 | ) | (11.7 | )% | (11,608 | ) | (19.7 | )% | ||||||||||||||||
| Income (loss) from operations | 3,644 | 5.7 | % | (989 | ) | (1.1 | )% | 1,784 | 3.8 | % | (1,678 | ) | 2.9 | % | ||||||||||||||||||
| Other (loss) income, net | (20 | ) | (0.0 | )% | 186 | 0.2 | % | 124 | 0.3 | % | (441 | ) | (0.7 | )% | ||||||||||||||||||
| Income (loss) before income taxes | 3,624 | 5.7 | % | (803 | ) | (0.9 | )% | 1,908 | 4.0 | % | (2,119 | ) | (3.6 | )% | ||||||||||||||||||
| Income taxes | (686 | ) | (1.1 | )% | (1,071 | ) | (1.2 | )% | 630 | 1.3 | % | 832 | 1.4 | % | ||||||||||||||||||
| Net income(loss) | 2,938 | 4.6 | % | (1,874 | ) | (2.1 | )% | 1,279 | 2.7 | % | (2,951 | ) | (5.0 | )% | ||||||||||||||||||
| Other comprehensive (loss) income | (10 | ) | (0.0 | )% | (117 | ) | (0.1 | )% | (9 | ) | 0.1 | % | 142 | 0.2 | % | |||||||||||||||||
| Comprehensive income(loss) | 2,928 | 4.6 | % | (1,991 | ) | (2.3 | )% | 1,270 | 2.7 | % | (2,809 | ) | (4.8 | )% | ||||||||||||||||||
| Net income (loss) per share (basic and diluted) | 0.06 | (0.04 | ) | 0.03 | (0.06 | ) | ||||||||||||||||||||||||||
| Weighted shares of common stock outstanding | 50,000,000 | 50,000,000 | 50,000,000 | 50,000,000 | ||||||||||||||||||||||||||||
42
Years Ended June 30, 2021 and 2022
Revenue
The following table sets out the breakdown of our revenue percentage by region based on information provided to us by our distributors.
| Years ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| Europe | 71.8 | % | 58.9 | % | ||||
| Asia Pacific | 18.8 | % | 15.0 | % | ||||
| North America | 9.3 | % | 25.9 | % | ||||
| Others | 0.1 | % | 0.2 | % | ||||
| Total | 100.0 | % | 100.0 | % | ||||
Our revenue increased by $24,680,635, or 38.9%, from $63,414,783 for the year ended June 30, 2021, to $88,095,418 for the year ended June 30, 2022. The increase in revenue is the combined effect of (i) the increase in revenue from cannabis products, which were introduced in December 2020, from $2.1 million in the year ended June 30, 2021 to approximately $20.0 million in the year ended June 30, 2022, respectively, all of which was in the North American market and (ii) increases in sales of tobacco products in Europe, offset by decreases in sales of tobacco products in the Asia Pacific and North America market. Revenue from the Europe, Asia Pacific and North America markets were still affected by government actions relating to COVID-19 in the year ended June 30, 2021. As vaccine programs were implemented, COVID-19 related government restrictions were reduced in the year ended June 30, 2022.
Cost of Revenue
Cost of revenue mainly consisted of cost of purchases of vaping products, which we purchased from Shenzhen Yi Jia. Cost of revenue increased by $21,790,450, or 41.1%, from $52,998,928 for the year ended June 30, 2021 to $74,789,378 for the year ended June 30, 2022. The increase in cost of revenue reflects the increase in year-to-year unit sales, offset by the increase in the purchase price of our products and a slowdown in the delivery of components to Shenzhen resulting from supply chain slowdowns as a result of the PRC government’s measures on COVID-19. Inflation in PRC has not materially impacted our cost of revenue. According to the National Bureau of Statistics of China, the year-over-year percent changes in the consumer price index for 2020 and 2021 were increases of 2.5% and 0.9%, respectively. Although we have not been materially affected by inflation, we can provide no assurance that we will not be affected in the future by higher rates of inflation in PRC.
Gross Profit
The following tables show the revenue, cost of revenue and gross profit of our tobacco and cannabis products (dollars in thousands).
| Year Ended June 30, 2021 | ||||||||||||||||
| Revenue | Cost of revenue | Gross profit | Gross profit % | |||||||||||||
| Tobacco products | $ | 61,271 | $ | 51,319 | $ | 9,952 | 16.2 | % | ||||||||
| Cannabis products | 2,144 | 1,680 | 464 | 21.6 | % | |||||||||||
| Total | $ | 63,415 | $ | 52,999 | $ | 10,416 | 16.4 | % | ||||||||
| Year Ended June 30, 2022 | ||||||||||||||||
| Revenue | Cost of revenue | Gross profit | Gross profit % | |||||||||||||
| Tobacco products | $ | 68,117 | $ | 57,503 | $ | 10,614 | 15.6 | % | ||||||||
| Cannabis products | 19,978 | 17,286 | 2,692 | 13.5 | % | |||||||||||
| Total | $ | 88,095 | $ | 74,789 | $ | 13,306 | 15.1 | % | ||||||||
Gross profit increased by $2,890,185, or 27.7%, from $10,415,855 for the year ended June 30, 2021 to $13,306,040 for the year ended June 30, 2022, while our gross margin declined from 16.4% to 15.1%. This decline in gross margin was primarily due to (i) the increased proportion of revenue derived from cannabis vaping products, which have a lower gross margin, (ii) tour decision to promote and develop market share by lowering price for cannabis vaping products during the year ended June 30, 2022 resulting in decline in gross margin for cannabis products, and (iii) the increase in purchase price due to a slowdown in the supply chain as a result of the PRC government’s measures on COVID-19.
Operating Expenses
Operating expenses increased $7,522,258, or 111.1%, from $6,772,453 for the year ended June 30, 2021 to $14,294,711 for the year ended June 30, 2022.
Our sales and marketing expenses mainly consist of employees’ salaries and benefits, marketing expense, travel expenses and others.
43
Sales and marketing expenses increased by $1,941,899, or 54.5%, from $3,561,731 for the year ended June 30, 2021 to $5,503,630 for the year ended June 30, 2022. The increase in sales and marketing expenses resulted from more marketing and promotion events from North America, which was with a goal of promoting our Ispire cannabis products to capture market share in 2022.
Our general and administrative expenses mainly consist of compensation and benefits, rental expense, professional fees and other administrative expenses. General and administrative expenses increased by $5,580,359, or 173.8%, from $3,210,722 for the year ended June 30, 2021, to $8,791,081 for the year ended June 30, 2022. The increase was primarily due to a significant increase in general and administrative staff, rental expenses and office expenses incurred by Aspire North America as it developed its cannabis business in the United States during the year ended June 30, 2022. The increase in our expenses is not the result of inflation. Inflation in Hong Kong, where Aspire Science is located, was relatively stable. The increase in expenses for Aspire North America results from the growth of our business. The cannabis vapor business commenced in late calendar 2021, and the increase in expenses resulted from our growth relating to this increase in business. However, inflationary pressures may affect our operations in the future.
Other income, net
Other income (expense) includes interest income, interest expense, exchange gain (loss), net and other income (expense).
Interest income was not significant in either year, $882 for the year ended June 30, 2021 and $5,078 for the year ended June 30, 2022.
Other income increased by $55,128, or 82.0%, from $67,266 for the year ended June 30, 2021 to $122,394 for the year ended June 30, 2022.
As a result of these factors, other income (expense) increased by $205,388, from other expense of $19,773 for the year ended June 30, 2021 to other income of $185,615 for the year ended June 30, 2022.
Income Taxes
Income taxes increased by $385,274, or 56.2%, from $685,823 for the year ended June 30, 2021 to $1,071,097 for the year ended June 30, 2022. Although we had a consolidated net loss for year ended June 30, 2022, the combined effect of a profit in Hong Kong and a loss in the United States resulted in a current tax expense. The increase in valuation allowance reflects our view that the taxable income in the future will not be sufficient to utilize the carryforward loss. See Note 13 of Notes to Consolidated Financial Statements.
Net Income
As a result of the foregoing, net income decreased by $4,811,959, from net income of $2,937,806, of $0.06 per share (basic and diluted) for the year ended June 30, 2021 to net loss of $1,874,153, or $(0.04) per share, for the year ended June 30, 2022.
Six Months Ended December 31, 2021 and 2022
Revenue
The following tables set out the breakdown of our revenue percentage by region based on information provided to us by our distributors.
| For the Six Months ended December 31, | ||||||||
| 2021 | 2022 | |||||||
| Europe | 63.4 | % | 56.8 | % | ||||
| Asia Pacific (excluding PRC) | 15.5 | % | 13.8 | % | ||||
| North America | 21.0 | % | 29.2 | % | ||||
| Others | 0.1 | % | 0.2 | % | ||||
| Total | 100.0 | % | 100.0 | % | ||||
Our revenue increased by $11,607,091, or 24.6%, from $47,233,358 for the six months ended December 31, 2021, to $58,840,449for the six months ended December 31, 2022. The increase in revenue is the combined effect of (i) the increase in revenue from cannabis products from $8.3 million in the six months ended December 31, 2021 to approximately $15.8 million in the six months ended December 31, 2022, all of which was in the United States and (ii) increases in sales of tobacco products in Europe, offset by decreases in sales of tobacco products in the Asia Pacific and North America market.
44
Cost of Revenue
Cost of revenue mainly consisted of cost of purchases of vaping products, which we purchased from Shenzhen Yi Jia. Cost of revenue increased by $8,988,234, or 22.5%, from $39,921,534 for the six months ended December 31, 2021 to $48,909,768 for the six months ended December 31, 2022. The increase in cost of revenue reflects both the increase in period-to-period unit sales, offset by a slowdown in the delivery of components to Shenzhen Yi Jia resulting from supply chain slowdowns as a result of the effects of mainland China’s zero-COVID policy. Inflation in PRC has not materially impacted our cost of revenue. According to the National Bureau of Statistics of China, the year-over-year percent changes in the consumer price index for 2021 and 2022 were increases of 0.9% and 2.0%, respectively. Although we have not in the past been materially affected by inflation, we can provide no assurance that we will not be affected in the future by higher rates of inflation in PRC.
Gross Profit
The following tables show the revenue, cost of revenue and gross profit of our tobacco and cannabis products (dollars in thousands).
| For the Six Months Ended December 31, 2021 | ||||||||||||||||
| Revenue | Cost of revenue | Gross profit | Gross profit % | |||||||||||||
| Tobacco products | $ | 38,938 | $ | 32,746 | $ | 6,192 | 15.9 | % | ||||||||
| Cannabis products | 8,295 | 7,175 | 1,120 | 13.5 | % | |||||||||||
| Total | $ | 47,233 | $ | 39,921 | $ | 7,312 | 15.5 | % | ||||||||
| For the Six Months Ended December 31, 2022 | ||||||||||||||||
| Revenue | Cost of revenue | Gross profit | Gross profit % | |||||||||||||
| Tobacco products | $ | 43,008 | $ | 36,307 | $ | 6,701 | 15.6 | % | ||||||||
| Cannabis products | 15,832 | 12,602 | 3,230 | 20.4 | % | |||||||||||
| Total | $ | 58,840 | $ | 48,909 | $ | 9,931 | 16.9 | % | ||||||||
Gross profit increased by $2,618,857, or 35.8%, from $7,311,824 for the six months ended December 31, 2021 to $9,930,681 for the six months ended December 31, 2022, while our gross margin increased from 15.5% to 16.9%. The gross margin for tobacco vaping products remains constant. The increase in gross margin for cannabis vaping products was primarily due to (i) a change in product mix with more higher margin products being sold during the six months ended December 31, 2022, (ii) and increase in sales volume that leads to economies of scale.
Operating Expenses
Operating expenses increased $6,080,297, or 110.0%, from $5,528,040 for the six months ended December 31, 2021 to $11,608,337 for the six months ended December 31, 2022.
Our sales and marketing expenses mainly consist of employees’ salaries and benefits, marketing expense, travel expenses and others.
Sales and marketing expenses decreased slightly by $48,631, or 2%, from $2,456,159 for the six months ended December 31, 2021 to $2,407,528 for the six months ended December 31, 2022. The sales and marketing expenses remain basically constant during the six months ended December 31, 2021 and 2022.
Our general and administrative expenses mainly consist of compensation and benefits, rental expense, professional fees and other administrative expenses. General and administrative expenses increased by $6,128,928, or 199.5%, from $3,071,881 for the six months ended December 31, 2021 to $9,200,809 for the six months ended December 31, 2022. The increase was primarily due to (i) an increase in rental and warehouse expenses of $0.7 million incurred by Aspire North America in connection with the development of its proposed manufacturing plant, (ii) an increase of $2.0 million for payroll and contract worker expenses as more employees were hired and contract workers were engaged by Aspire North America for expansion of its business and building its proposed manufacturing plant, (iii) a bad debt expense of $1.0 million recorded by Aspire North America, (iv) an increase in amortization expense of $0.8 million of intellectual properties transferred to us in September 2022, and (v) an increase in freight charges of $0.5 million resulting from an increase in sales. The increase in our expenses is not the result of inflation. Inflation in Hong Kong, where Aspire Science is located, was relatively stable. The increase in expenses for Aspire North America results from the growth of our business. The cannabis vapor business commenced in late calendar 2021, and the increase in expenses resulted from our growth relating to this increase in business. However, inflationary pressures may affect our operations in the future.
45
Other income(expense), net
Other income(expense) includes interest income, interest expense, exchange gain (loss), net and other income (expense).
Interest income was $1,067 for the six months ended December 31, 2021 and $76,811 for the six months ended December 31, 2022.
Exchange gain (loss), net decreased by $546,064, or 797.4%, from net exchange gain of $68,462 for the six months ended December 31, 2021 to net exchange loss of $477,582 for six months ended December 31, 2022. The change from exchange gain to exchange loss was from Aspire Science, primarily resulting from the change in the exchange rate of the Hong Kong Dollar to the U.S. dollar from 7.7838 for the six months ended December 31, 2021 to 7.7347 for the six months ended December 31, 2022.
As a result of these factors, other income (expense) decreased by $565,748, from other income of $124,490 for the six months ended December 31, 2021 to other expense of $441,258 for six months ended December 31, 2022.
Income Taxes
Income taxes increased by $202,414, or 32.1%, from $629,593 for the six months ended December 31, 2021 to $832,007 for the six months ended December 31, 2022. Although we had a consolidated net loss for the six months ended December 31, 2022, the combined effect of a profit by Aspire Science and a loss by Aspire North America resulted in a current tax expense. The increase in valuation allowance reflects our view that the taxable income in the future will not be sufficient to utilize the carryforward loss.
Net Income
As a result of the foregoing, net income decreased by $4,229,602, from net income of $1,278,681, of $0.03 per share (basic and diluted) for the six months ended December 31, 2021 to net loss of $2,950,921, or $(0.06) per share, for the six months ended December 31, 2022.
Liquidity and Capital Resources
The following table summarizes our changes in working capital from June 30, 2022 to December 31, 2022 (dollars in thousands).
| June 30, 2022 | December 31, 2022 | Change | % Change | |||||||||||||
| Current Assets | $ | 99,449 | $ | 122,808 | $ | 23,359 | 23.5 | % | ||||||||
| Current Liabilities | 88,968 | 115,008 | 26,040 | 29.3 | % | |||||||||||
| Working Capital | 10,481 | 7,800 | (2,681 | ) | (25.6 | )% | ||||||||||
The following table sets forth information as to consolidated cash flow information for the years ended June 30, 2021 and 2022 and the six months ended December 31, 2021 and 2022 (dollars in thousands).
| Years Ended June 30, | Six Months Ended December 31, | |||||||||||||||||||||||
| Consolidated cash flow data: | 2021 | 2022 | Increase (Decrease) | 2021 | 2022 | Increase (Decrease) | ||||||||||||||||||
| Net cash provided by(used in) operating activities | $ | 5,024 | $ | (7,558 | ) | $ | (12,582 | ) | $ | (8,324 | ) | $ | 8,825 | $ | 17,309 | ) | ||||||||
| Net cash used in investing activities | (1 | ) | (122 | ) | (121 | ) | (47 | ) | (478 | ) | (431 | ) | ||||||||||||
| Net cash (used in)provided by financing activities | (228 | ) | (3,089 | ) | (2,861 | ) | (2,394 | ) | 1,440 | 3,674 | ||||||||||||||
| Net increase (decrease) in cash and cash equivalents | 4,795 | (10,769 | ) | (15,564 | ) | (10,765 | ) | 9,787 | 20,552 | |||||||||||||||
46
Net cash flow from operating activities for the year ended June 30, 2021 of $5.0 million, reflected our net income of $2.9 million, adjusted primarily as follows: an increase in accounts payable of $9.4 million offset by an increase of account receivable of $3.9 million and an increase in inventories of $3.0 million.
Net cash flow used in operating activities for the year ended June 30, 2022 of $7.6 million, reflected our net loss of $1.9 million, adjusted primarily as follows: an increase in accounts payable of $8.9 million offset by an increase in inventories of $11.5 million, and an increase in accounts receivable of $4.0 million.
Net cash flow used in operating activities for the six months ended December 31, 2021 of $8.3 million, reflected our net income of $1.3 million, adjusted primarily as follows: offset by an increase in accounts receivable of $2.9 million, an increase in inventories of $5.9 million and an decrease of contract liabilities of $1.1 million.
Net cash flow provided by operating activities for the six months ended December 31, 2022 of $8.8 million, reflected primarily a decrease in accounts payable of $25.5 million, offset by an increase in accounts receivable of $10.8 million and an increase in inventories of $5.7 million.
Net cash flow used in investing activities for the six months ended December 31, 2022 of $0.5 million reflected primarily purchase of property, plant and equipment of $0.5 million.
Net cash flow used in financing activities for the year ended June 30, 2021 of $0.2 million reflected primarily the payment of lease liabilities of $0.3 million.
Net cash flow used in financing activities for the year ended June 30, 2022 of $3.0 million reflected primarily payments of previously declared dividends of $0.5 million and $2.4 million of repayment of advances to related parties.
Net cash flow used in financing activities for the six months ended December 31, 2021 of $2.4 million reflected primarily repayment to related parties of $1.8 million and payment made for dividends of $0.5 million.
Net cash flow provided from financing activities for the six months ended December 31, 2022 of $1.4 million reflected primarily $1.9 million of advances from related parties offset by the payment of lease liabilities of $0.4 million.
To date, we have financed our operations primarily through cash flow from operations and working capital loans from our major stockholders, who are our chief executive officer and his wife, when necessary. We plan to support our future operations primarily from cash generated from our operations and cash on hand. We believe that our current cash and cash flows provided by operating activities, and the estimated net proceeds from this offering will be sufficient to meet our working capital needs in the next 12 months. If we experience an adverse operating environment or incur unanticipated capital expenditure requirements, or if we decide to accelerate our growth, then additional financing may be required. We cannot give any assurance that additional financing will not be required or, if required, would be available on favorable terms if at all. Such financing may include the use of additional debt or the sale of additional equity securities. Any financing which involves the sale of equity securities or instruments that are convertible into equity securities could result in dilution to our stockholders which may be substantial.
The cash at bank held by our Hong Kong operating subsidiary can be freely transferred within our corporate structure without restriction. If our Hong Kong operating subsidiary were to incur additional debt on its own behalf in the future, the instruments governing the debt may restrict the ability of our operating subsidiaries to transfer cash to our U.S. investors.
Contractual Obligations
As of June 30, 2022 and December 31 2022, we had contract liabilities of $1,672,051 and $1,003,735 respectively. These liabilities are advance deposits received from customers after an order has been placed. We expect all of the contract liabilities to be settled in less than one year.
We have operating lease arrangements for office premises for Hong Kong and California which are treated as right-of-use assets. These leases typically have terms of two to five years. Leases with an initial term of 12 months or less are not presented as right-of-use assets and are expensed over the lease term. All other lease assets and lease liabilities are recognized based on the present value of lease payments over the lease term at commencement date.
The balances for our right-of-use assets where we are the lessee are presented as follow:
As of June 30, | As of December 31, | |||||||
| 2022 | 2022 | |||||||
| Right-of-use assets | $ | 295,804 | $ | 3,459,714 | ||||
| Lease liabilities - current | $ | 347,541 | $ | 784,724 | ||||
| Lease liabilities – non-current | - | 2,829,102 | ||||||
| Total | $ | 347,541 | $ | 3,613,826 | ||||
47
As of December 31, 2022, the maturities of our lease liabilities (excluding short-term leases) are as follows:
| As of December 31, 2022 | ||||
| 2023 | 1,000,428 | |||
| 2024 | 887,998 | |||
| 2025 | 919,168 | |||
| 2026 | 951,585 | |||
| 2027 | 564,731 | |||
| Total future lease payments | 4,323,910 | |||
| Less: imputed interest | (710,084 | ) | ||
| Total lease liabilities | 3,613,826 | |||
During the year ended June 30, 2020, Aspire Science declared dividends of $3.8 million, to its sole shareholder, our chief executive officer, Tuanfang Liu. The dividend was declared in June 2020 and, as of January 30, 2023, had not yet been fully paid. The unpaid balance of the dividend was paid on February 2, 2023. This dividend was declared at a time when Aspire Science was wholly owned by Mr. Liu.
Trend Information
Other than as disclosed elsewhere in this registration statement, we are not aware of any trends, uncertainties, demands, commitments, or events that are reasonably likely to have a material effect on our net revenues, income from continuing operations, profitability, liquidity or capital resources, or that would cause reported financial information not necessarily to be indicative of future operating results or financial condition.
Seasonality
Seasonality does not materially affect our business or the results of our operations.
Off-Balance Sheet Arrangements
We do not have off-balance sheet arrangements.
Critical Accounting Policies
Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Significant estimates include allowance for doubtful accounts, the useful lives of property and equipment and intangible asset, impairment of long-lived assets, and deferred cost. Actual results could differ from those estimates.
Basis of consolidation
Our consolidated financial statements include the financial statements of us and our subsidiaries. All inter-company transactions and balances have been eliminated upon consolidation. Because we acquired 100% of the equity of Aspire North America and Aspire Science from a related party for no consideration on July 29, 2022, the acquisitions are treated as the subsidiaries were acquired on July 1, 2020, the first day of the year ended June 30, 2021, and the outstanding common stock was issued on July 1, 2020.
Revenue
We sell our products to customers around the world and recognize revenue in accordance with the guidance of Accounting Standards Codification (ASC) 606, Revenue from Contracts with Customers. Revenue is recognized when control of goods has transferred to customers. For the majority of our customer arrangements, control transfers to customers at a point-in-time when goods have been delivered to the pickup location specified by the customer or a forwarder appointed by the customer, as that is generally when legal title, physical possession and risks and rewards of goods transfer to the customer.
48
Revenue is recognized at the transaction price, based on the purchase order as adjusted for the anticipated rebates, discounts and other sales incentives. When determining the transaction price, management estimates variable consideration applying the portfolio approach practical expedient under ASC 606. The main sources of variable consideration for us are customer rebates, trade promotion funds and cash discounts. These sales incentives are recorded as a reduction of revenue at the time of the initial sale using the most-likely amount estimation method. The most-likely amount method is based on the single most likely outcome from a range of possible consideration outcomes. The range of possible consideration outcomes is primarily derived from the following inputs: sales terms, historical experience, trend analysis, and projected market conditions in the various markets served. Because we serve numerous markets, the sales incentive programs offered vary across businesses, but the most common incentive relates to amounts paid or credited to customers for achieving defined volume levels or growth objectives.
There are no material instances where variable consideration is constrained and not recorded at the initial time of sale. Product returns are recorded as a reduction of revenue based on anticipated sales returns that occur in the normal course of business. We have elected to present revenue net of sales taxes and other similar taxes.
Our warranties are of an assurance-type and come standard with all of our products to cover repair or replacement should a product not perform as expected. We offer a warranty for all major products, including all types of E-vapor kits, atomizers, replacement coils and mods, but no warranty for accessories such as spare parts or packaging consumables. We generally offer a 90-day warranty period from date of purchase for products sold to all regions, but from May 2019, we offer a six-month warranty period from date of purchase for products sold in the UK and France. We offer a refund or replacement of products for manufacturer defective items, dead on arrival items and items that do not appear the same as listed on our website, and exclude damaged goods caused by misuse or unauthorized repair. Provisions for estimated expenses related to product warranties are made at the time products are sold. These estimates are established using historical information about the nature, frequency and average cost of warranty claim settlements as well as product manufacturing and recovery from suppliers. Management actively studies trends of warranty claims and takes action to improve product quality and minimize warranty costs. We estimate the actual historical warranty claims coupled with an analysis of unfulfilled claims to record a liability for specific warranty purposes. As of June 30, 2021 and 2022, products returned for repair or replacement have been immaterial. Accordingly, a warranty liability has not been deemed necessary.
Disaggregated Revenue
In accordance with ASC 606-10-50-5, we have taken into consideration the nature, amount, timing, and uncertainty of revenue and cash flows, and have determined to disaggregate our net sales by whether the products are tobacco or cannabis products, as it is important information for the Company to make resource allocation decisions. The net sales disaggregated by products for the years ended June 30, 2021 and 2022, and for the six months ended December 31, 2021 and 2022 were as follows, respectively:
| Years ended June 30, | Six months ended December 31, | |||||||||||||||
| Net sales by products branded | 2021 | 2022 | 2021 | 2022 | ||||||||||||
| Tobacco products | $ | 61,270,660 | $ | 68,116,810 | $ | 38,938,023 | 43,008,459 | |||||||||
| Cannabis products | 2,144,123 | 19,978,608 | 8,295,335 | 15,831,990 | ||||||||||||
| Total | $ | 63,414,783 | $ | 88,095,418 | $ | 47,233,358 | 58,840,449 | |||||||||
Income Tax
We account for income taxes under ASC 740. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax bases.
Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period including the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
The provisions of ASC 740-10 prescribe a more-likely-than-not threshold for consolidated financial statement recognition and measurement of a tax position taken (or expected to be taken) in a tax return. This interpretation also provides guidance on the recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, and related disclosures. For the years ended June 30, 2021 and 2022, we did not incur any interest or penalties related to an uncertain tax position. We do not believe that there were any uncertain tax positions as of June 30, 2021 and June 30, 2022.
49
Recent Accounting Pronouncements
The discussion of the recent accounting pronouncements contained in our consolidated financial statements, “Summary of Significant Accounting Policies,” is incorporated herein by reference.
As a company with less than $1.235 billion in revenue for our last fiscal year, we qualify as an “emerging growth company” pursuant to the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 of the Sarbanes-Oxley Act of 2002 in the assessment of the emerging growth company’s internal control over financial reporting. The JOBS Act also provides that an emerging growth company does not need to comply with any new or revised financial accounting standards until such date that a private company is otherwise required to comply with such new or revised accounting standards. We have elected to take advantage of such exemptions.
The information presented in this section and information elsewhere in this prospectus that relates to the industry has been derived from, to provide information regarding our industry and our market position. Neither we nor any other party involved in this offering has independently verified such information, and neither we nor any other party involved in this offering makes any representation as to the accuracy or completeness of such information. Investors are cautioned not to place any undue reliance on the information, including statistics and estimates, set forth in this section or similar information included elsewhere in this prospectus.
Tobacco Products
Historically, combustible tobacco products, primarily cigarettes and cigars, have been, and continue to be, the principal tobacco products used by adult smokers. Diverse customer demands are driving the innovation in the tobacco industry. Creative tobacco products, such as short cigarettes, king size cigarettes and flavor capsule cigarettes, have become increasingly popular among the smoking population. To profit from this rising market, tobacco manufacturers will expend more effort in its product diversification. Raising the price of tobacco products through tobacco tax increases is one of the most effective tobacco control strategies, because an increase in tobacco prices may encourage existing smokers to quit or cut down and may deter the youth population from beginning to smoke. Such trend will adversely affect the tobacco industry.
During the past few decades, a number of alternatives to combustible tobacco products have entered the market. These products can be classified in three categories – smokeless (including moist snuff and chewing tobacco), e-vapor products and heated tobacco.
Smokeless tobacco is the general term used to describe tobacco products that are utilized without combustion. Smokeless tobacco is used either in the mouth or in the nose, by chewing inhaling or sucking, and traditionally has been divided into two subcategories, snuff and chewing tobacco. Snuff was originally a nasal product but today is more commonly used in the mouth (oral snuff, moist snuff) in a manner similar to that of chewing tobacco.
Vapor devices are distinguished from traditional combustible tobacco products by their production of vapor through a process of heating rather than the burning associated with the consumption of cigarettes, cigars, cigarillos or smoking tobacco. In their current form, vapor devices usually include electronic circuitry and a power source supplying energy to the heating mechanism. Vapor products are not distinguished by the absence of tobacco. While the majority of current devices (e-cigarettes) are intended for use with a non-tobacco nicotine containing liquid, the category includes tobacco products where it is heated and not combusted, such as heat-not-burn devices. Closed vaping systems designed to look like a cigarette are referred to as cigalikes.
Heated tobacco is the consumable element of tobacco vapor products and can come in the form of tobacco pods such as PloomTech capsules or in specially designed cigarettes.
Our products are vapor devices, which are categorized as closed system vaping devices (non-cigalikes), vaping components, and open system vaping devices.
50
Over the past ten years, both technological advances and consumer demand resulting in large part from a desire to obtain the effects of smoking without the adverse health effects resulting from smoking cigarettes, have led to both an increase in the global popularity of vaping along with the application of anti-tobacco legislation and regulation to electronic products, including vaping. Innovation in battery and other component technologies have greatly improved product functionality and reliability. Many consumers are attracted to the discreetness that vaporizers provide in terms of size and ease of use, style/fashion, and the perception that vaping is less detrimental to health than cigarettes. As a result, the market for vaping devices has been growing rapidly over the past few years.
The following table shows the estimated retail sales for the years of 2017 through 2022 for all smokeless tobacco products, all vapor products, all e-vapor products, all closed vaping systems other than cigalikes and all open vaping systems (in millions of US dollars).
| 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |||||||||||||||||||
| All smokeless tobacco products | $ | 29,448 | $ | 40,213 | $ | 50,248 | $ | 55,819 | 67,917 | 79,835 | ||||||||||||||
| E-vapor products | 11,438 | 15,520 | 20,540 | 20,06 | 22,791 | 26,304 | ||||||||||||||||||
| Closed System Rechargeable and Cartridges | 3,659 | 6,599 | 11,820 | 11,434 | 13,102 | 15,725 | ||||||||||||||||||
| Open vaping systems | 7,356 | 8,402 | 8,303 | 7,852 | 8,440 | 9,063 | ||||||||||||||||||
Source: Euromonitor International Limited, Tobacco 2022, retail value RSP incl sales tax, US$, fixed 2021 exchange rates, current terms. Data extracted on 25 August, 2022.
The following table shows the estimated projected retail sales for the years of 2023 through 2026 for all smokeless tobacco products, all vapor products, all e-vapor products, all closed vaping systems other than cigalikes and all open vaping systems (in millions of US dollars).
| 2023 | 2024 | 2025 | 2026 | |||||||||||||
| All smokeless tobacco products | $ | 93,263 | $ | 108,68 | $ | 123,872 | $ | 141,658 | ||||||||
| E-vapor products | 29,911 | 33,245 | 36,742 | 40,478 | ||||||||||||
| Closed System Rechargeable and Cartridges | 18,575 | 20,941 | 23,404 | 25,949 | ||||||||||||
| Open systems | 9,622 | 10,431 | 11,313 | 12,357 | ||||||||||||
Source: Euromonitor International Limited, Tobacco 2022, retail value RSP incl sales tax, US$, fixed 2021 exchange rates, current terms. Data extracted on 25 August, 2022.
Certain health research institutions have published reports providing tentative indication that e-vapor products may serve to mitigate certain health risks that are commonly associated with use of combustible tobacco products, reduce harm of second-hand smoke and help reduce tobacco use. However, currently there remain uncertainties regarding whether e-vapor products are sufficiently safe for their intended use and health risks associated with the usage of e-vapor products have been under scrutiny. According to certain health research institutions, e-vapors products’ long-term health effects and risks are unclear given the early stage of development of the products. The United States Center for Disease Control states on its website that heated tobacco products produce emissions that are not as safe as clean air, studies of secondhand emissions from heated tobacco products suggest that the products expose both users and bystanders to some of the same chemicals found in cigarette smoke, although at lower levels than cigarette smoke, and additional research is needed to understand the health effects of heated tobacco products and their emissions. The use of any tobacco product is harmful, especially for youth, young adults, and pregnant women, as well as adults who do not currently use tobacco products. Further, regardless of whether they are heated by flame or electronically, heated tobacco products contain nicotine, which is highly addictive, and nicotine exposure can also harm the developing adolescent brain, and nicotine is toxic to developing fetuses.
The general lack of industry standards has resulted in significant variations among different brands and products in terms of product features, quality assurance and control.
51
Cannabis Products
Cannabis has a long and entrenched history in the United States due to considerable popularity of medical and recreational use in addition to long-standing industrial production of hemp. While the 20th century saw the growth of increasingly negative attitudes towards cannabis from policy makers and the general public, recent decades have witnessed a significant shift in the perception of cannabis. In the late 1990s, acceptance of medical cannabis grew enough to allow for changes in regulation and the 2010s have seen rapid expansion of social acceptance for recreational use. Medical cannabis in particular has seen increasing approval as Americans seek alternatives to pharmaceutical products in the wake of a serious public health crisis related to prescription opioids. Legalization is also increasing thanks to growing movements seeking to reform the US criminal justice system. Increasing numbers of American political reformers see eliminating criminal penalties for use and sale of cannabis as a way to address various social and economic inequalities.
An estimated 28.6 million Americans participated in the legal cannabis market in 2021 up from 23.7 million in 2019. This rapid growth is the result of growing legalization of medical and adult-use cannabis as well as consistent if somewhat diminished growth in CBD use. During the forecast period, growth will continue as consumers new to cannabis enter the market and also as illicit market users switch to legal products.
Adult-use cannabis has attracted mainly recreational consumers who use cannabis for relaxation and socialization. Former medical cannabis users have also been attracted to the adult-use space as it has expanded due to its lower barriers to entry (no requirement to get permission from a doctor to gain access) and significant overlap of products between medical and adult-use product line-ups. Nonetheless some medical patients do opt to continue using medical cannabis even after adult-use legalization due to preferential tax rates on medical products in some states. Reasons for CBD use vary widely as claims made regarding its effects have been extremely numerous, although common examples are medical and include relief from anxiety and pain. The inability of manufacturers to make specific claims about the benefits of their products leads consumers to gather information on CBD’s uses from secondary sources of varying credibility. This lack of clarity is compounded by a lack of broad scientific consensus regarding CBD’s uses and best practices for consumers. Further research and a more clearly defined framework from the FDA could be extremely valuable in ameliorating confusion regarding CBD among consumers.
Social acceptance of cannabis is growing in the US as 2020 saw a further four states legalize adult-use cannabis, including states that previously held negative attitudes towards cannabis. Euromonitor survey data indicates that in legal adult-use states, more than 40% of legal age consumers have consumed cannabis. CBD use is also widespread among Americans interested in pain relief and relaxation especially opting to try CBD. Cannabis has been legal in New York State for medical purposes through the state’s medical marijuana program since 2014, and recreational cannabis became legal in 2021.
Cannabis vaping is the action of inhaling and exhaling vapor containing cannabis oil produced by a vaporizer technology device. We believe that vaping has become the preferred choice of many cannabis users due to its discreetness in both carrying and smell, ease of use, and perceived health benefits relative to smoking cannabis cigarettes or bowls which create smoke. As the tables below show, cannabis vaping products for adult recreational use are largely limited to the United States with modest sales in Canada.
The following table shows the estimated retail volume of cannabis vapor products for adult recreational use worldwide and in the United States and Canada for 2020 and 2021 (in millions of US dollars).
| 2020 | 2021 | |||||||
| Worldwide | $ | 2,880 | $ | 4,405 | ||||
| United States | 2,572 | 3,904 | ||||||
| Canada | 308 | 501 | ||||||
Source: Euromonitor International Limited, Cannabis 2022, retail value RSP incl sales tax, US$, fixed 2021 exchange rates, current terms. Data extracted between January 12-28, 2022.
The following table shows the estimated projected retail sales of cannabis vapor products for adult recreational use worldwide and in the United States and Canada for the years of 2022 through 2025 (in millions of US dollars)
| 2022 | 2023 | 2024 | 2025 | |||||||||||||
| Worldwide | $ | 5,710 | $ | 7,313 | $ | 8,864 | $ | 10,580 | ||||||||
| United States | 5,060 | 6,467 | 7,797 | 9,318 | ||||||||||||
| Canada | 650 | 842 | 1,060 | 1,247 | ||||||||||||
Source: Euromonitor International Limited, Cannabis 2022, retail value RSP incl sales tax, US$, fixed 2021 exchange rates, current terms. Data extracted between January 12-28, 2022.
52
During the 117th Congress (which ended on January 3, 2023), legislators introduced a number of bills aimed at increasing opportunities for cannabis industry members and their service providers. The most comprehensive legislation introduced to date, the Cannabis Administration and Opportunity Act, would provide for federal regulation of cannabis products by the U.S. Food and Drug Administration (“FDA”) and taxation by the U.S. Alcohol Tobacco Tax and Trade Bureau (TTB) but would afford states continued authority to restrict and regulate such products. The States Reform Act would remove marijuana from Schedule I of the Controlled Substances Act and locate federal regulatory and taxation authority for cannabis products at the TTB while also affording states the ability to continue to restrict or prohibit such products. Passed by the House of Representatives, the Marijuana Opportunity, Reinvestment, and Expungement Act would also remove marijuana from the scope of the Controlled Substances Act and establish a federal excise and occupational tax framework for cannabis products. While none of these bills passed during the 117th Congress, we cannot predict whether or when any of these or other similar bills will be introduced in the new Congress or what specific provisions they will contain if so. However, we believe that the fact that various members of Congress in both houses have recently introduced cannabis reform bills—and that the House of Representatives has passed such legislation—suggests an openness to and momentum towards federal cannabis reform in the near term.
No country in Western Europe has yet legalized recreational cannabis, but the region has some of the most developed cannabis cultures in the world, such as in the Netherlands and Spain. However, great differences persist among consumers, with older generations typically being more reluctant to allow cannabis use. Clear generational and social gaps still exist that make legalization and development of the market a slow process, although the potential legalization of adult-use cannabis in Germany is likely to accelerate the cannabis debate within the EU and promote the development of the industry at a regional level.
Western Europe’s cannabis market is still in its infancy, with limited consumption due to restrictive legislation on recreational and medical cannabis across the region. Legalization of medical and recreational cannabis across countries will increase the consumer base and expand the availability of products rapidly in the region, with consumer awareness of its wellbeing properties rising among the European population. The coalition government in Germany stated clearly that it is introducing the controlled supply of recreational cannabis to adults in licensed shops. However, implementation is proving to be a monumental task since legalization involves almost every federal government ministry.
Overview
We are engaged in the research and development, design, commercialization, sales, marketing and distribution of branded e-cigarettes and cannabis vaping products. We sell our tobacco products worldwide except for the PRC and Russia. Our tobacco products are marketed under the Aspire brand name and are sold primarily through our distribution network.
We currently sell our cannabis vaping hardware only in the United States, and we have recently commenced marketing activities in Canada and Europe. All of our products are vaping hardware. Vaping refers to the practice of inhaling and exhaling the vapor produced by an electronic vaping device, and includes dabbing, which is the recreational inhalation of extremely concentrated tetrahydrocannabinol, the main psychotropic cannabinoid derived from the marijuana plant. Our cannabis products are marketed under the Ispire brand name, primarily on an ODM basis to other cannabis vapor companies. ODM generally involves the design and customize the core products to meet each brand’s unique image and needs, and our products are sold by our customers under their own brand names although they may also include our brand name on the products.
Our products use our BDC (bottom dual coil) coil technology which uses bottom dual coils to provide much higher temperature and an expanded heating that achieves much greater flavor and vapor production. We believe that the use of our dual-coil technology enhances the flavor performance of e-liquid, and the hidden wick cotton with special designed wick holes can both extend the tank e-liquid capacity and improve the speed of wicking to increase the coil life.
Our BVC (bottom vertical coil) coil represents a big technological breakthrough for us in coil technology with a vertical heating wire surrounded by the cotton. This design can enable the coil heating to provide uniform temperature from the tank, together with more efficient wicking. This new technology, which Aspire Global introduced in 2014, enables the coil to last longer while still giving users what we believe is the purest and cleanest taste from e-liquids. The BVC coils are still very popular for MTL (mouth to lung) vapors today.
We bring a new coil technology to the vaping industry with the Cleito tank. The Cleito uses a revolutionary new coil design that replaces the standard chimney and, we believe, delivers maximized airflow. This design frees up even more restriction in the airflow by eliminating the need for a static chimney within the tank itself, which results in an expanded flavor profile and increased vapor production. Combined with a Clapton kanthal coil for maximum flavor, the Cleito tank delivers a rush of intense flavor and huge vapor with a broad profile. The simple top-fill design makes filling very easy, just unscrew the top cap to refill, more convenient, more enjoyable.
53
Our Ispire cannabis vapor products use our patented DuCore™ (Dual Coil) technology for cannabis vaporizers. This technology enables users to create massive plumes of vape without burning the cannabis oil. These products incorporate our patented dual coil technology for what we believe is best-in-class airflow and taste, and our technology for eliminating the leakage of the oil from the unit, which overcomes a major disadvantage with many existing products
Our products are manufactured and supplied by Shenzhen Yi Jia, which is 95% owned by our chief executive officer and controlling stockholder, Tuanfang Liu. We are taking the initial steps toward the development of manufacturing operations in Vietnam and in California. Initially, our manufacturing operations will be primarily assembling from components that we purchase from other companies. Although we expect that we will commence these assembly operations in mid 2023, we cannot assure you that we will be able to meet this timetable or that we will be able to effectively and efficiently conduct such operations.
Through our global distributor network of more than 150 distributors, we sell the Aspire brand of tobacco vaporizer technology products in more than 30 countries. The main markets for our tobacco products are Europe and the Asia Pacific region, which does not include the PRC.
The following table sets forth our tobacco revenue and percentage for tobacco products by region for the years ended June 30, 2021 and 2022 and the six months ended December 31, 2021 and 2022 based on information provided to us by our distributors (dollars in thousands).
| Year Ended June 30, | Six Months Ended December 31, | |||||||||||||||||||||||||||||||
| 2021 | 2022 | 2021 | 2022 | |||||||||||||||||||||||||||||
| Revenues | % | Revenues | % | Revenues | % | Revenues | % | |||||||||||||||||||||||||
| Europe | $ | 45,568 | 74.4 | % | $ | 51,886 | 76.2 | % | $ | 29,946 | 63.4 | % | $ | 33,404 | 56.8 | % | ||||||||||||||||
| Asia Pacific (excluding China) | 11,915 | 19.4 | % | 13,213 | 19.4 | % | 7,322 | 15.5 | % | 8,117 | 13.8 | % | ||||||||||||||||||||
| North America | 3,757 | 6.1 | % | 2,849 | 4.2 | % | 9,904 | 21.0 | % | 17,163 | 29.2 | % | ||||||||||||||||||||
| Other | 30 | 0.1 | % | 169 | 0.2 | % | 61 | 0.1 | % | 156 | 0.2 | % | ||||||||||||||||||||
| $ | 61,270 | $ | 68,117 | $ | 47,233 | $ | 58,840 | |||||||||||||||||||||||||
For the years ended June 30, 2021 and 2022 and six months ended December 31, 2021 and 2022, our revenues from cannabis products was approximately $2.1 million, $20.0 million, $8.3 million and $15.8 million respectively, all of which was in the North American market. All sales of cannabis products to date have been in the United States, although we have recently commenced marketing efforts in Canada and Europe, primarily the European Union.
Acquisition of Our Business from a Related Party
We were formed on June 13, 2022. We have two operating subsidiaries, Aspire North America LLC, a California limited liability company (“Aspire North America”), and Aspire Science and Technology Limited, a Hong Kong corporation (“Aspire Science”). On July 29, 2022, we acquired 100% of the equity interest in Aspire North America from Aspire Global Inc. (“Aspire Global”), and our wholly-owned subsidiary Ispire International Limited, a British Virgin Islands corporation (“Ispire International”), acquired 100% of the equity interest in Aspire Science from a wholly-owned subsidiary of Aspire Global in connection with a restructure by Aspire Global pursuant to which the equity in Aspire North America and Aspire Science was transferred to us, and, at the time of the transfer, we had the same stockholders as Aspire Global.
Aspire North American commenced marketing cannabis vaping products in mid 2020. Aspire Science markets tobacco vaping products worldwide, except for the PRC and Russia. Since Aspire North America and Aspire Science were acquired from a related party for no consideration, our consolidated financial statements for the years ended June 30, 2021 and 2022 include the assets and liabilities of these subsidiaries on the balance sheet dates at their historic costs and the results of their operations and cash flows for the years then ended as if these subsidiaries were owned by us on July 1, 2020.
Aspire Global is a related party. Tuanfang Liu is Aspire Global’s chief executive officer and a director of both us and Aspire Global, and his wife, Jiangyan Zhu, is also a director of both companies. Mr. Liu and Ms. Zhu beneficially 66.5% and 5.0%, respectively, of our outstanding common stock and of Aspire Global’s ordinary shares and, upon completion of this offering, they will own 59.4%and 4.5%, respectively, of our common stock, assuming the Underwriters do not exercise their over-allotment option. Upon our formation we issued 50,000,000 shares of common stock to the stockholders of Aspire Global in the same proportion as their stockholdings in Aspire Global.
We presently purchase our tobacco vaping and cannabis vaping hardware from Shenzhen Yi Jia. Pursuant to agreements dated January 27, 2023, between Aspire North America and Shenzhen Yi Jia and between Aspire Science and Shenzhen Yi Jia, we purchase our cannabis and tobacco vaping products form Shenzhen Yi Jia at market prices, provided that the price, delivery, warranty and other terms are no less favorable to us that the price, delivery, warranty and other terms that are provided to any other customer of Shenzhen Yi Jia.
54
Our intellectual property was developed primarily by our chief executive officer, Tuanfang Liu. Our research and development team is headed by Mr. Liu, and was owned by Shenzhen Yi Jia, which had patents or patent application in the United States, the PRC, the European Union and elsewhere relating to various functional and ornamental aspects of our products. These patents cover both the cannabis and tobacco products. Pursuant to the Intellectual Property Transfer Agreement, Mr. Liu, Aspire Global and Shenzhen Yi Jia agreed to transfer to Aspire North America all patent and other intellectual property rights, including trademarks, Know-how and Know-how Documentation, as defined in the agreement, relating to the cannabis vaping products, and to transfer to us any new intellectual property developed or acquired by Mr. Liu, Aspire Global and Shenzhen Yi Jia which relates to cannabis vaping products. The patents, all of which are United States patents and patent applications, have been transferred to Aspire North America.
Pursuant to the Intellectual Property License Agreement, Mr. Liu, Aspire Global and Shenzhen Yi Jia granted Aspire Science a perpetual royalty free sole and exclusive right and license to use and practice all of the Licensed Technology worldwide except for the PRC and Russia. The Licensed Technology includes all patents, know-how, know-how documentation and trademarks, whether now existing or hereafter developed or acquired by, or for, Mr. Liu, Aspire Global and/or Shenzhen Yi Jia that relate, directly or indirectly, to the tobacco vaping market. Pursuant to the License Agreement, neither Mr. Liu, Aspire Global nor Shenzhen Yi Jia has any right to market or sell or grant distributors the right to market or sell tobacco vaping products in the world other than in the PRC and Russia.
Effects of COVID-19 Pandemic
In December 2019, coronavirus disease 2019 (COVID-19) was first reported to have surfaced in Wuhan, China. During 2020, the disease spread to many parts of the world. The epidemic has resulted in quarantines, travel restrictions, and the temporary closure of stores and facilities in much of the world.
Measures taken by various governments to contain the virus have affected economic activity in all countries where the consumers of our product live. We took a number of measures to monitor and mitigate the effects of COVID-19, such as safety and health measures for our personnel, such as social distancing, in accordance with government policies and advice, securing the supply of materials that are essential to our production process. We will continue to follow the various government policies and advice and, in parallel, we may take further actions that we determine are in the best interests of our employees, customers, and business relationships.
Our products are manufactured in China by Shenzhen Yi Jia, which is 95% owned by our chief executive officer and controlling stockholder, Tuanfang Liu. From the middle of January 2020, the start of the Chinese New Year holiday, until the end of April 2020, its production was slowed due to the COVID-19 pandemic in China. This slowdown in production resulted in a near-total stoppage of our supplier’s business during this nearly four-month period and our sales activities were substantially reduced. In addition, since our products are generally sold in stores such as grocery stores, convenience stores and tobacco stores, to the extent that either the stores are closed as a result of government actions or consumers are reluctant to go shopping because of the COVID-19 pandemic, retail sales of our products suffered. Following the temporary slowdown in production in the first half year of 2020, our supplier, Shenzhen Yi Jia, has recovered its production capacity, and we have not experienced significant supply chain constraints as a result of the pandemic.
From late 2020 to the middle of 2021, COVID-19 vaccination program had been greatly promoted around the globe, however several types of COVID-19 variants emerged in different parts of the world. The Company’s sales in Europe and the United States continued to be affected by government actions relating to COVID-19 and COVID-19 variants, while the production was slightly reduced by the strict measures taken by the Government of China to address the small COVID-19 outbreaks in Guangdong province. To the extent that China’s zero COVID policy results in closing or significantly reducing the operations of Shenzhen Yi Jia or any of its suppliers, our business will be impacted since we presently rely on Shenzhen Yi Jia for our products.
The extent to which COVID-19 impacts our operations on an ongoing basis is highly uncertain. It will depend on various factors including the duration and severity of the outbreak, new information which may emerge concerning the severity of the coronavirus or any variants and the actions to contain the coronavirus or treat its impact, among others.
Matters Relating to PRC Laws
The majority of our operations are in United States. We are mainly engaged in the research and development, design, commercialization, sales, marketing and distribution of branded e-cigarettes and cannabis vaping products. The sales of our tobacco products are conducted worldwide except for the PRC and Russia. Through our global distributor network of more than 150 distributors, we sell the Aspire brand of tobacco vaporizer technology products in more than 30 countries and the main markets for such tobacco products are Europe and the Asia Pacific region, which does not include China. We do not conduct business and we do not have any employees, assets or funds in mainland China. Although most of our cash is in Hong Kong banks, a significant portion of these funds is to be paid to related parties. See “Certain Relationships and Related Party Transactions.” Our operations are primarily in the United States. Although Tuanfang Liu, our chief executive officer, lives in mainland China, where Shenzhen Yi Jia is located, the services that he performs for us in his capacity as our chief executive officer are performed primarily in Hong Kong and the United States. In addition to serving as our chief executive officer, Mr. Liu is chairman of Shenzhen Yi Jia, and the services he provides in mainland China are performed in his capacity as chairman of Shenzhen Yi Jia. Our employees are largely in the United States, with 48 employees based in the United States and where our research and development activities are conducted, and eight employees in Hong Kong. Our facilities are located primarily in the United States, where we lease more than 83,600 square feet of office, manufacturing and storage space and where our research and development activities are conducted, as compared with 1,850 square feet of office space in Hong Kong. We do not have any variable interest entities arrangements or any similar agreements. As of the date of this prospectus, we do not believe we are subject to PRC Laws applicable to those Chinese companies established in mainland China, based on advice from Han Kun Law Offices.
55
We have two operating subsidiaries established in California and Hong Kong. Hong Kong was established as a special administrative region of the PRC in accordance with Article 31 of the Constitution of the PRC. The Basic Law of the Hong Kong Special Administrative Region of the PRC (the “Basic Law”) was adopted and promulgated on April 4, 1990 and became effective on July 1, 1997, when the PRC resumed the exercise of sovereignty over Hong Kong. Pursuant to the Basic Law, Hong Kong is authorized by the National People’s Congress of the PRC to exercise a high degree of autonomy and enjoy executive, legislative and independent judicial power, and the PRC laws and regulations shall not be applied to Hong Kong, other than those relating to national defense, foreign affairs, and certain other matters that are not within the scope of autonomy of Hong Kong. While the National People’s Congress of the PRC has the power to amend the Basic Law, the Basic Law also expressly provides that no amendment to the Basic Law shall contravene the established basic policies of the PRC regarding Hong Kong. As a result, as of the date of this prospectus, national laws of the PRC that would be applicable to us if we were a Chinese corporation do not apply to our Hong Kong subsidiary. However, there is no assurance that certain PRC laws and regulations, including existing laws and regulations and those enacted or promulgated in the future, will not be applicable to our Hong Kong subsidiary due to change in the current political arrangements between mainland China and Hong Kong or other unforeseeable reasons. The application of such laws and regulations may have a material adverse impact on us, as relevant PRC authorities may impose fines and penalties upon our Hong Kong subsidiary, delay or restrict the repatriation of the proceeds from this offering into Hong Kong, and any failure of us to fully comply with such new regulatory requirements may significantly limit or completely hinder our ability to offer or continue to offer our common stock, cause significant disruption to our business operations, and severely damage our reputation, which would materially and adversely affect our financial condition and results of operations and cause our common stock to significantly decline in value or in extreme cases, become worthless.
At present, our products are manufactured and supplied by Shenzhen Yi Jia, a Chinese company under common control. However, we are also taking the initial steps toward the development of manufacturing operations in Vietnam and California, and our future operations will be diversified in different places of business.
Our Organization
We are a Delaware corporation, organized on June 13, 2022. Aspire North America, a California limited liability company was formed on February 22, 2020, and 100% of its ownership was transferred to Aspire Global on September 23, 2020 and was transferred by Aspire Global to Ispire Technology on July 29, 2022. Aspire Science, a Hong Kong corporation, was incorporated on December 9, 2016 and 100% of its equity was transferred to us on July 29, 2022. Ispire International was organized on July 6, 2022. Aspire North America and Aspire Science are our operating companies.
Our principal executive offices are located at 19700 Magellan Dr, Los Angeles, CA 90502. Our telephone number is 310 742 9975. Our principal website will be www.ispiretechnology.com. The information contained on, or that can be accessed through, our website or any other website, is not a part of this prospectus.
The following chart shows our corporate structure.

56
Our Strategy
We are implementing a multi-prong growth strategy directed at increasing the sales of our e-cigarette and cannabis vaporizer technology products.
In addition to increasing sales to our existing customers, we plan to increase sales of our e-cigarette vaporizer technology products by increasing the number of distributors and regions where our products are sold. We plan to increase sales of our cannabis products by increasing sales to existing customers, increasing our customer base in the United States and seeking to penetrate the Canadian and European markets as they develop. We closely follow the legalization of cannabis globally and plan to enter markets when opportunities arise.
Research and development is at the core of our business. We will continue to innovate via our own research and development efforts. Our chief executive officer developed the patented Ducore technology, which is being assigned to us and which enables our cannabis vaporizer products to heat cannabis oil, which, we believe is the first leak-proof patented design, which enables the consumer to get the full flavor experience of the cannabis. We will continue to expand our technology leadership and invest in vaporizer and similar technology research and development. Our present products are designed for the adult recreational use. Our research and development activities will be oriented to focus on both medical and recreational usages of cannabis products. We recognize that industry trends can change rapidly. We believe that our products must be at the forefront of technology if we are going to develop our business. The cannabis vaping business is in its early stages and we will seek to develop a strong and leading position in this market. This market is currently largely in the United States and we plan to be in the forefront as other markets develop.
Through our global sales network, we have a strong understanding of all of the markets in which our products are sold. We will use forum and community groups as a means to increase engagement and collect feedback for future improvements in product research and development. We will seek to introduce new products to meet customer needs based on our assessment of the direction of the market.
We will also pursue mergers and acquisitions and strategic relationships to increase our technological human resources and technology and product portfolio. We believe that we have a strong management team adept at integrating such acquisitions and we believe that we are an attractive platform to potential acquirees.
We plan to develop manufacturing capabilities. We plan to manufacture both tobacco and cannabis vaping products. We are in the process for forming a Vietnam subsidiary which will perform our manufacturing operations in Vietnam, and we have leased a 79,512 square-foot warehouse and plan to develop assembly facilities in the Los Angeles, California area. Initially, and for at least a few years, our manufacturing operations will primarily involve the assembly of products from components manufactured for us in accordance with our specifications.
We are expanding into OEM and ODM business. OEM generally means making and selling the products as we design them and putting customers’ logos on the products. For OEM products, cost is important to the customer. ODM generally involves the design and customize the core products to meet each brand’s unique image and needs. For ODM, technology, performance and uniqueness are often more important, with cost generally being a secondary consideration. Historically, for our tobacco products, we have focused on building and growing our own branded business, with OEM and ODM sales accounting for a minor portion of our revenue. OEM and ODM sales accounted for approximately $1.1 million, $0.7 million, $0.6 million and $0.6 million, or 1.8%, 1.0%, 1.5% and 1.4% of total revenue of tobacco products in the years ended June 30, 2021 and 2022 and six months period ended December 31, 2021 and 2022, respectively. As Aspire Global continued to innovate in the last decade and the Aspire brand has become recognized as a leading innovator in the vaping industry, Aspire Science has been sought after by other brands for OEM and ODM work. We believe that OEM and ODM for our tobacco products will represent a key growth area for us in the future. In seeking to introduce new products, we will, at least initially, rely upon our chairman, Tuanfang Liu, who has been largely responsible for the development of the technology underlying our tobacco and cannabis vaping products.
Sales of our cannabis products to date are largely sales to cannabis brands on an ODM basis, and we anticipate that our cannabis sales will continue to be primarily ODM sales for the near future. It is the responsibility of our customers, cannabis brands, to manufacture the cannabis oil and load the oil into our vaping hardware product. We also sell some hardware products to end users, but our sales are primarily to ODM users. None of our products include cannabis oil or hemp oil.
57
Our Products
Tobacco Products
We develop and sell both branded and, to a significant lesser extent, OEM and ODM tobacco vaping systems and components (cartridges and batteries) to meet the needs of adult users worldwide. Such battery-powered systems and components are commonly used in tobacco (e-liquid).
There are generally two types of vaping systems – open system and closed system.
The term open system generally refers to vaping devices consisting of tanks, which include heating coils, and mods, which include the battery packs. Open system vaping devices allow end consumers to refill the tanks with their own liquid by themselves. With open systems, consumers have great flexibility in mixing different coils, mods, and e-liquid to create a more personalized experience. Our open system vaping devices are sold under our own brands, including “Nautilus,” and “Zestquest.”
The term closed system generally refers to vaping devices that consisting of cartridges, which include a heating core (sometimes referred to as atomizers) and is filled with e-liquid, and batteries, which power the cartridges. The closed system vaping devices includes rechargeable closed system vaping devices and disposable closed system vaping devices. The cartridge can last from a few days to two weeks, depending upon the frequency of use. We market a line of closed systems. Unlike the open system, the closed system includes the coils and liquid. We believe that the market for closed systems is increasing rapidly and it is becoming the dominant form of tobacco vaping.
Through our global distributor network of more than 150 distributors, our Aspire tobacco vaporizer technology products are marketed and sold in more than 30 countries.
Initially, all of our products were open systems. The first closed system was introduced in 2018.
Our vaping components include cartridges, lithium batteries, metal parts such as coils, plastic parts that are molded, circuit boards (printed circuit board assembly) and liquid cartridges for our products. The cartridges of closed system vaping device are consumable products that need to be frequently replaced.
For the years ended June 30, 2021 and 2022 and six months period ended December 31, 2021 and 2022, approximately 97.2%, 96.5%, 97.1% and 89.8% of our tobacco product sales were open systems, approximately 2.1%, 2.3%, 2.0%, and 8.4% of our sales were closed systems.
Our products use our BDC (bottom dual coil) coil technology which uses bottom dual coils to provide double temperature, and expand the heating area and achieve double flavor and vapor production. This technology allows for two separate oil tanks/cartridges to be integrated into one product/design. Each of the cartridges has its own heating coil that can be regulated separately to generate the desired heating temperatures independent of each other. This is beneficial to the consumers because one cartridge could be designed for terpenes (which has a very low evaporation temperature, typically 100-120 degree Fahrenheit), and the other can be for THC oil (which has a evaporating temperature in the range of 400-430 degree Fahrenheit). Conventional cartridge design would have the terpenes and THC oil mixed together in one cartridge and be heated to a single temperature that would typically burn the terpenes and yet under-heat the THC oil. With the double flavor design, we can optimize the heating temperature to evaporate both terpenes and THC oil without burning them. We believe that the use of our dual-coil technology enhances the flavor performance of e-liquid, and the hidden wick cotton with special designed wick holes can both extend the tank e-liquid capacity and improve the speed of wicking to increase the coil life.
The only tobacco vaping product that we may now sell in the United States under current regulations, is the Nautilus Prime product line, which is an open system. When the products on the market primarily used plastic atomizers, we created Nautilus, with a high-end and attractive appearance. It is the world’s first tank using stainless steel top and base hardware, a 5ml Pyrex glass tank, and long stainless steel drip tip, as well as our revolutionary airflow control system. This unique four-port adjustable airflow system allows the users to adjust the draw, warmth of vapor, and amount of vapor produced with the lower ring with four settings according to their vaping needs. This BVC coil can provide pure and intense flavor. We believe that all of these features make the Nautilus a special atomizer and provide the best vaping experience possible at the moment. The Nautilus Prime system is the only system that we have authorization to sell in the United States. The Nautilus Prime is an enhancement of our original Nautilus product, for which we do not have authorization to sell in the United States. Because of low sales volume for the only product that we may sell in the United States and the current regulations, in 2020, we ceased marketing tobacco vaping products in the United States.
Our BVC (bottom vertical coil) coil represents a big technological breakthrough for us in coil technology with a vertical heating wire surrounded by the cotton. This design can enable the coil heating to provide uniform temperature from the tank, together with more efficient wicking. This new technology, which Aspire Global introduced in 2014, enables the coil to last longer while still giving users what we believe is the purest and cleanest taste from e-liquids. The BVC coils are still very popular for MTL (mouth to lung) vapors today.
We bring a new coil technology to the vaping industry with the Cleito tank. The Cleito uses a revolutionary new coil design that replaces the standard chimney and, we believe, delivers maximized airflow. This design frees up even more restriction in the airflow by eliminating the need for a static chimney within the tank itself, which results in an expanded flavor profile and increased vapor production. Combined with a Clapton kanthal coil for maximum flavor, the Cleito tank delivers a rush of intense flavor and huge vapor with a broad profile. The simple top-fill design makes filling very easy, just unscrew the top cap to refill, more convenient, more enjoyable.
58
Cannabis Products

In December 2020, we introduced the Ispire line of cannabis vaping products. For the years ended June 30, 2021 and 2022, our sales of Ispire products were $2.1 million and $20.0 million, respectively, all of which was in the United States, although we have commenced marketing efforts in Canada and Europe, primarily in the European Union. Our Ispire products use our patented Ducore™ (Dual Coil) technology for cannabis vaporizers. Similar to the Nautilus series, this technology enables users to create massive plumes of vape without burning the cannabis oil. These products incorporate our patented dual coil technology for what we believe is best-in-class airflow and taste, and our technology for eliminating the leakage of the oil from the unit, which overcomes a major disadvantage with many existing products. In addition to the base unit, we offer a range of cartridges, mouthpieces and color options. In our ODM services, we work with the customer to design the product that has the desired appearance. All the products are made of stainless steel and the fluid housing is Pyrex glass. We are not involved in cannabis or hemp plant or oil business, and we do not provide cannabis or hemp oil. Our product, which is hardware only, is designed for our customers to fill the cartridge with their own cannabis or hemp oil. Cannabis oil, unlike nicotine oil which is generally of a uniform consistency, is not of a uniform consistency, with the result that if the oil is too viscous, the user will not have good experience with the product and our customer may reject or return the product. Although we test the products with cannabis oil with specific viscosity, we do not package the oil with our product and either our customer purchases the oil separately from the product it purchases from us or the end user purchases the oil independently. We have no way to ensure that the consumer will use a cannabis oil that will work in the product we have manufactured for the customer.
Sales and Distribution
Most of our revenue from tobacco products comes from sales to our distributors. We are looking to increase our OEM and ODM sales of tobacco products, which accounted for 1.9 %, 1.0%, 1.3% and 1.1% of our tobacco revenue for the years ended June 30, 2021 and 2022, and six months period ended December 31, 2021 and 2022, respectively. Most of our revenue from cannabis products is from ODM sales to other cannabis vaping brands, and we work with the customer to design the product, which is sold under the customer’s brand name and for some customers, the Ispire brand is also on the product.
Prior to our acquisition of Aspire Global sold tobacco vaping products in the United States through its distribution network. We decided not to market in the United States as a result of the effect of changes in regulations in the United States because Aspire North America would currently be able to sell one product line in the United States and that product line did not generate sufficient revenue to justify the marketing and regulatory expenses.
We believe that we have the ability to evaluate the market need for vaping products and develop products for both the tobacco and cannabis markets. We believe that we have the state-of-the-art technology, which enables us to market to other cannabis vaping brands. We believe that we have implemented systems of quality control that cover the key steps of supply chain management to provide high-quality products to adult smokers in a consistent manner. We strictly uphold our extensive internal standards for various aspects of our products and conduct thorough quality assurance and control practices throughout the entire production cycle.
59
Our cannabis products are sold directly by us, with most of our sales being to other cannabis vaping brands who purchase the product from us on an ODM basis and sell the products under their brand name, although our Ispire brand may be included on the product. We work with the customer in the design and appearance of the product. We also sell Ispire hardware online, but such sales do not generate significant volume. We do not sell cannabis or hemp oil, either as part of a product or separately.
For our tobacco products, we have a network of more than 150 distributors, whose territories cover more than 30 countries or regions. Our distributors have non-exclusive agreements and generally are not restricted from selling competing vapor products. Our largest distributor, whose territory was United Kingdom and France, is Your-Buyer International Limited, which accounted for revenue of approximately $32.5 million, or 51.2% of revenue, in the year ended June 30, 2021, and approximately $34.0 million, or 38.6% of revenue for the year ended June 30, 2022. No other distributor or customer accounted for 10% or more of our revenues for either the year ended June 30, 2021 or 2022
Typically, our distributors sell our products to wholesalers who in turn sell to retail distributors although distributors may sell products directly to retail outlets. The vast majority of sales of all classes of smokeless tobacco is sold in stores, primarily grocery stores, convenience stores and tobacco stores, which generally purchase product from wholesale distributors. Our products are also available from our distributors on the internet, including both websites and services such as Amazon. These internet distribution channels are operated by our distributors. The distributors are responsible for complying with the laws of the countries in which they sell our products. We previously sold tobacco vaping products to a distributor for Russia, and we no longer sell to that distributor.
We assist our distributors in marketing our products through websites, blogs, search engine optimization (SEO), opt-in and e-mail marketing, social media marketing, influencer, marketing and digital advertising promotions. Opt-in and email marketing strategies include newsletter sign-ups to receive new product updates and promotions, giveaway promotional activities to drive conversion, coupons and discounts promotion activities to increase sales.
We may use social media to promote our products and we market to consumers through our websites and Instagram. We use social media to educate on current and new products and offers as well as to provide real-time support to customers. Our social media strategies aim to convert and nurture leads, to increase brand awareness.
We also provide distributors with discounts and other sales incentives. From time to time, based on our sales or marketing strategy for a specific region or product, we will give distributors discounts. Although our distributors do not have sales quotas, they have sales goals and, from time to time, we may reward distributors for exceeding their sales targets. These promotions are not part of a standard plan, but developed by us from time to time based on our sales and marketing program.
All of our sales of Ispire cannabis products are made directly by our sales team in California and not through distributors. Our effort in marketing, branding and sales initiatives for this product since the product introduction in late 2020 has resulted in a significant increase in brand awareness, as reflected in our sales growth from approximately $2.1 million in the year ended June 30, 2021, the first year in which we had sales of cannabis products, to approximately $20.0 million in the year ended June 30, 2022. Our sales of Ispire cannabis products to date, which have been primarily through direct sales of Ispire branded atomizers to other cannabis brands as semi-finished products on an ODM basis. Pursuant to our agreements with our ODM customers, we design and sell these atomizers pursuant to purchase orders by the customers. Our logo is printed on some of these products. To a lesser extent we sell heating devices directly to consumers as internet sales.
Source of Supply
We purchase all of our current tobacco and cannabis vaping products from Shenzhen Yi Jia. The products that we sell are the same products that Aspire Science and Aspire North America sold prior to the transfer of the equity in these subsidiaries to us. Pursuant to agreements dated January 27, 2023, between Aspire North America and Shenzhen Yi Jia and between Aspire Science and Shenzhen Yi Jia, we purchase our cannabis and tobacco vaping products form Shenzhen Yi Jia at market prices, provided that the price, delivery, warranty and other terms are no less favorable to us than the price, delivery, warranty and other terms that are provided to any other customer of Shenzhen Yi Jia. In addition, the agreement provides that Shenzhen Yi Jia will be responsible for any warranty expenses.
We are taking the initial steps toward the development of manufacturing operations in Vietnam and in California. These operations will, at least initially consist primarily of assembling the products from components we purchase from suppliers. In this connection, we may purchase components from Shenzhen Yi Jia’s present suppliers as well as other suppliers which we may identify. Quality control will be a crucial part of our manufacturing process. We will need to include quality control checks and balances throughout our supply chain and manufacturing process. When selecting suppliers, we will have our quality control and procurement team visit potential suppliers. We will need to conduct annual inspections of the factories and we will also visit the factory if any quality issues arise. In connection with the establishment of any manufacturing facilities we will have to employ qualified manufacturing, supervisory and administrative personnel.
Warranties
We will pass on to our customers the warranties which Shenzhen Yi Jia provides to us, as a customer. These warranties are of an assurance-type and come standard with all of products we purchase from Shenzhen Yi Jia and cover repair or replacement should product not perform as expected. We offer these warranties for all major products, including all types of E-vapor kits, atomizers, replacement coils and mods, but no warranty for accessories such as spare parts or packaging consumables. Shenzhen Yi Jia generally offers 90-day warranty period from date of purchase for products sold to all regions, but Shenzhen Yi Jia offers six months warranty period from date of purchase for products sold in the United Kingdom and France. The warranty offers refund or replacement of products for manufacturer defective items, dead on arrival items and items that do not appear the same as listed on our website, and exclude damaged goods caused by misuse or unauthorized repair. We generally require our customers to test our hardware with their oils to confirm the hardware performance and approve the hardware designs, in order to minimize any hardware related discrepancy or performance issues specific to the formulation of their oils. Since we are passing on the warranties of Shenzhen Yi Jia, we do not provide for estimated expenses related to product warranties.
60
Research and Development
We believe that design and attention to detail are at the heart of our business. Historically, research and development relating to our existing products were conducted primarily by Shenzhen Yi Jia. We have commenced research and development activities independent of Shenzhen Yi Jia, which has related primarily to cannabis vaping products. This research and development effort, which is headed by our chairman, Tuanfang Liu, has eight members, who are based in Los Angeles. Prior to the transfer of the equity of Aspire North America and Aspire Science to us, the research and development activities were conducted by Shenzhen Yi Jia. As discussed under “Business – Intellectual Property” we have rights to intellectual property generated by the research and development efforts of Shenzhen Yi Jia and Mr. Liu.
During the years ended June 30, 2021 and 2022, research and development effort included the development of the Ispire cannabis vaping system, including patented dual-coil technology, a closed system for tobacco vaping that is designed to eliminate the problem of oil leaking out of the unit was conducted by Shenzhen Yi Jia under the leadership of Tuanfang Liu, who is our chief executive officer and chief executive officer of Aspire Global. Since the transfer of Aspire North America and Aspire Science to us in July 2022, we have established our research and development group independent of Aspire Global and Shenzhen Yi Jia, and the Shenzhen Yi Jia research and development activities relating to both cannabis and tobacco product are being transitioned to us. We are also entitled to the benefits of Shenzhen Yi Jia’s research and development pursuant to the Intellectual Property Transfer Agreement and the License Agreement.
Intellectual Property
Shenzhen Yi Jia has patents or patent applications in the United States, China, the European Union and elsewhere relating to various functional and ornamental aspects of our products. Pursuant to the Intellectual Property Transfer Agreement, Aspire North America, Aspire Global, Shenzhen Yi Jia and Mr. Liu have transferred to our subsidiary, Aspire North America, all their intellectual property, including patents, trademarks, brand names, know-how and know-how documentation that relate directly or indirectly to cannabis and hemp vaping products, and the patents and trademarks, all of which are United States patents, trademarks and patent and trademark application, have been transferred to Aspire North America. Pursuant to the License Agreement, Aspire Science has the right to an exclusive (to the exclusion of Shenzhen Yi Jia and Mr. Liu) right and license to any patents, trademarks and other intellectual property that relates to tobacco vaping products in the territory, which include the world except for China and Russia.
We believe that the utility patents form the core intellectual property for our electronic cigarette and vaporizer products. The utility patents primarily relate to atomizer, heating coil, and battery technologies, which we believe provide enhanced functionality and an improved smoking experience to users of our products. Our atomizer technology is directed toward enhancing the atomization of e-liquid, including by enabling the user to adjust the airflow through the atomizer to provide a customized smoking experience. Our heating coil technology is directed towards heating coil designs and arrangements that deliver heat more efficiently from the heating coil to the e-liquid, thereby producing vapor more effectively. Our battery technology is directed towards battery assemblies that are replaceable and that are controllable to help facilitate a customized smoking experience in combination with the atomizer and heating coil technologies.
We believe the design patents cover the visual aspects of certain of our products and serve to enhance the protection provide by our utility patents. We either own, with respect to cannabis vaping products, or license on an exclusive basis, with respect to tobacco products, designs patents for the ornamental appearance of the housing of certain of our electronic cigarettes and cannabis vaping products. Our design patents also extend to the ornamental appearance of certain electronic cigarette components, including certain aspects of our atomizers and heating coils.
The patents are primarily based on inventions developed by our chairman, Tuanfang Liu, who has received more than 200 patents in China, the United States, the European Union and other countries. All of these patents are being or have been assigned, licensed, or otherwise transferred to Shenzhen Yi Jia, which, in turn is either transferring to Aspire North America, with respect to intellectual property relating to cannabis products, and licensing on a sole and exclusive basis in the territory, to Aspire Science, with respect to tobacco products. The territory covered by the License Agreement is the world except for the PRC and Russia. The earliest of patents were filed in 2012. Overall, the patents expire between 2022 and 2037, depending on priority filing date, patent type, and jurisdiction. We intend to work to improve our technology and products and to seek further patent protection as warranted in connection with any new developments.
We cannot guarantee that our patent rights are sufficient to protect all aspects of our products or that we will be able to enforce those rights against third parties, as patents can be challenged, circumvented, or otherwise found to be invalid.
Shenzhen Yi Jia has obtained trademark registrations for Ispire in the countries which we believe are major markets for our products, including the United States, China, the European Union, and other countries. In addition to the Ispire mark, Shenzhen Yi Jia has also been granted trademark registrations in the United States and China for certain products and components, including the marks CLEITO, PERSEUS, PLATO, PROTEUS, and ZESTQUEST. Furthermore, Shenzhen Yi Jia has submitted trademark applications for the mark Ispire in the United States, China, the European Union, and other jurisdictions we believe are important markets. To the extent any of these trademarks were held by our chairman, Tuanfang Liu or Shenzhen Yi Jia, those trademarks have been assigned to Aspire North American with respect to cannabis products pursuant to the Intellectual Property Transfer Agreement and licensed on an exclusive license (to the exclusion of Aspire Global, Shenzhen Yi Jia and Mr. Liu) to Aspire Science pursuant to the License Agreement.
We cannot assure you that our patent and trademark rights are sufficient to protect all aspects of our brands or that we will be to enforce those rights to prevent third parties from using the same or confusingly similar marks, as trademarks can be opposed, cancelled, or otherwise challenged, especially by parties with rights to similar marks.
61
Competition
Vaping products for both tobacco and cannabis compete with tobacco and marijuana cigarettes and a wide range of other tobacco and legal and illegal cannabis products. In each case, vaping products seek to provide the user with pleasure that the user derives without the disadvantages.
The worldwide market for tobacco vaping products is highly competitive, with more than 50 companies selling products which compete with our products. In terms of volume of product sold, by far the largest worldwide producer of tobacco vapor products is Juul Labs, Inc. British American Tobacco Plc is also a major producer of tobacco vapor products.
We anticipate that the market for vaping products will evolve, with technological innovation, changing standards and changes in needs and preferences of adult vapor users. Vaping devices are more than an alternative to traditional cigarettes. Instead, they represent the user’s taste and offer them a new and fun experience, as they provide large amounts of vapor, different tastes of e-liquid and fashionable design. In light of such trend and to further differentiate their vaping devices, manufacturers are upgrading their products in terms of technology and design. Many manufacturers are now providing full-spectrum vaping devices, including closed system vaping devices, open system vaping devices and other kinds of vaping devices, so as to be more competitive in the market. In the next few years, with the technology becoming more mature, we anticipate that more differentiated vaping devices will continuously emerge to draw consumers’ attention. Our recent enhancements to our vaping products, such as the big smoke effect, have increased interest and sales of our products. We believe that our ability to remain profitable and to increase our market share is dependent upon our ability to anticipate market demand and develop and market products that address these trends.
The market for cannabis vapor products is a developing market and at present is mainly limited to the United States, although there is a developing market in Canada, and we believe that a market is developing in Europe. Our ability to be successful in this market is dependent upon our ability to develop vaping systems that attracts and retains consumer interest and the regulatory environment in the United States. Our cannabis vaping products compete with other forms of legal and illegal cannabis, marijuana cigarettes, CBD oil and other CBD products, food products and other vaping products.
Seasonality
Seasonality does not materially affect our business or the results of our operations.
Human Capital
We believe our people are central to the foundation and future of our success. Our culture and commitment to our employees are important factors in attracting, retaining, developing and progressing qualified employees. As of January 27, 2023, we had a total of 56 employees, of which 25 are operations personnel, five are general management personnel, 17 are in sales and marketing, and nine, including our chief executive officer, are in research and development relating to cannabis products.
Culture and Engagement
We value and support our people through, among other initiatives, our talent management, health and safety, employment practices and total reward programs. We are committed to fostering a culture of inclusion where differences are welcomed, appreciated and celebrated to positively impact our people and business, and where our people are engaged and encouraged to support the communities in where they live and work.
Talent Management
We are committed to providing our people with opportunities to learn, grow and be recognized for their achievements. Through our integrated talent management strategy, we strive to attract, retain, develop and progress a workforce that embraces our culture of inclusion and reflects our diversity efforts. Our talent programs play a critical role in attracting and progressing a diverse pipeline of talent. We are also committed to investing in our people by providing learning and networking opportunities and to drive retention, progression and engagement and help them excel in their current and future roles.
Health and Safety
We are committed to providing safe and healthy working environments and taking reasonable preventative measures to protect the health and safety of our employees and customers. We drive environmental, health and safety excellence across the Company and strive for incident-free workplaces – continuously assessing and developing measures that are in place to help keep our employees, customers and communities safe. In response to the COVID-19 pandemic, we have implemented significant changes to our business designed to protect the health and well-being of our employees and to support appropriate physical distancing and other health and safety protocols. These efforts continue to include: enhanced cleaning and sanitation procedures; domestic and international travel restrictions; return to work and visitor screening protocols; split shifts at facilities and the postponement or cancellation of attending large events.
62
Employment Practices and Total Rewards
We are committed to the fair, consistent and equitable treatment of our employees in relation to working conditions, wages, benefits, policies and procedures. To this end, our policies and programs are designed to respond to the needs of our employees in a manner that provides a safe, professional, efficient and rewarding workplace. Our total rewards programs are designed to offer competitive compensation, comprehensive benefits and other programs to support employees’ growth, both personally and professionally, and the diverse needs and well-being of our employees worldwide. During 2020, we enhanced certain of our benefits to support the health and well-being of our employees during the COVID-19 pandemic, including family leave and voluntary leave of absence policies and programs.
From time to time, we hire part-time employees as need in connection with our manufacturing. We consider our employee relations to be good.
We enter into labor contracts and standard confidentiality and intellectual property agreements with our key employees. We believe that maintaining good working relationships with our employees is essential, and we have not experienced any labor disputes except for the matter set forth below. None of our employees are represented by labor unions.
Property
Our headquarters are located at 19700 Magellan Dr, Los Angeles, CA 90502 and we maintain offices, manufacturing and storage facilities at the same location. We do not own any real property, and we leased an aggregate of approximately 85,483 square feet of real property. We do not expect to experience difficulties in renewing any of the leases when they expire. If we require additional space, we expect to be able to obtain additional facilities on commercially reasonable terms.
The following table sets forth information as to the real property leased by us:
| Location | Square Feet | Current Annual Rent | Expiration Date | |||||||
| 1410 Abbot Kinney Blvd., PH 1, Venice, CA 90291 | 4,121 | $ | 276,000 | June 30, 2023 | ||||||
19700 Magellan Dr, Los Angeles, CA 90502 | 79,512 | 734,580 | July 31,2027 | |||||||
| 55 King Yip Street, King Palace Plaza, Floor 31, Suite J, Kwun Tong, Hong Kong | 1,850 | 79,513 | July 14, 2023 | |||||||
Insurance
We consider our insurance coverage to be consistent with customary industry standards adopted by other companies in the same industry and of similar size although Aspire Science does not have product liability insurance.
Legal Proceedings
From time to time, we may be subject to legal proceedings, investigations and claims incidental to the conduct of our business.
Other than disclosed above, we are not a party to, nor are we aware of, any legal proceedings, investigations or claims which, in the opinion of our management, are likely to have a material adverse effect on our business, financial condition or results of operations.
On March 17, 2021, the FDA sent a letter to Aspire North America requesting that Aspire North America submit documents relating to its marketing practices for Aspire products. Specifically, the FDA requested documents related to youth exposure to Aspire North America’s social media marketing of Aspire as well as Aspire North America’s use of influencers in social media marketing. This request applied to all of Aspire electronic nicotine delivery system (ENDS) products and their components or parts. The FDA requested these documents based on the epidemic of youth ENDS use and based on Aspire North America’s marketing of Aspire products on social media platforms (e.g., Facebook, YouTube, and Instagram). The FDA requested that Aspire North America respond within 60 days but granted a 30-day extension. On June 15, 2021, Aspire North America provided the required information to the FDA. To date, the FDA has not substantively responded or taken any further action in the matter. However, we cannot assure you that the FDA will consider the response adequate and will not initiate regulatory or enforcement action based on an alleged failure to comply with the request or that the FDA will not initiate regulatory or enforcement action on other grounds based on the contents of the documents produced in the response. Either result could materially and adversely affect our business, financial condition, and results of operations.
63
United States
PMTA filings are requirements for electronic nicotine delivery systems (“ENDS”) products, including devices, components, and/or parts that deliver aerosolized e-liquid when inhaled. For existing ENDS Products ENDS products that were on the U.S. market on August 8, 2016, a PMTA was required to be submitted to the FDA by September 9, 2020. We have filed our PMTA for our Nautilus Prime open system vaping products, which are the only products we can presently sell in the United States. For new ENDS Products that were not on the U.S. market on August 8, 2016, and not the subject of a pending PMTA filed by September 9, 2020, a premarket authorization is required before introducing the product to the U.S. market. Selling ENDS products without authorization can result in civil penalties, seizures, injunctions, and even criminal prosecutions.
The PMTA pathway remains open for us to add further products, but we (and anyone else) we cannot now bring new products to the U.S. market without actual premarket authorizations. The PMTA process is an expensive procedure.
Under the FD&C Act, a PMTA includes:
| ● | Full reports of all information published or known to, or which should reasonably be known to, the applicant concerning investigations which have been made to show the health risks of such tobacco product and whether such tobacco product presents less risk than other tobacco products. |
| ● | Full statement of the components, ingredients, additives, and properties, and of the principle or principles of operation. |
| ● | Full description of the methods used in, and the facilities and controls used for, the manufacture, processing, and when relevant, packing and installation. | |
| ● | An identifying reference to any tobacco product standard, if applicable. If so, either provide (i) adequate information to show that such aspect of such tobacco product fully meets such tobacco product standard, or (ii) adequate information to justify any deviation from such standard. | |
| ● | Samples of the tobacco product as required. |
| ● | Specimens of proposed labeling. |
In adopting the Consolidated Appropriations Act, 2021, the COVID-19 relief bill that was signed on December 27, 2020, Congress amended the PACT Act to apply to e-cigarettes and all vaping products, which includes cannabis vaping products. The legislation amends the PACT Act’s definition of “cigarette” to include ENDS, which is defined to include “any electronic device that, through an aerosolized solution, delivers nicotine, flavor, or any other substance to the user inhaling from the device. The term “any other substance” has been defined by regulations to include cannabis as well as nicotine. This amendment prohibits mailing covered products through the United States Postal Service to consumers (with exceptions for certain business-to-business mailings) and requires reporting to federal and state agencies. These restrictions make it more difficult for a seller of vaping products to sell the products in the United States.
Briefly, the PACT Act requires any person who sells, transfers, or ships “cigarettes,” which is defined to include ENDS, which, as noted above, is very broadly defined, in interstate commerce for profit to, or who advertises or offers cigarettes or smokeless tobacco for such sale, transfer, or shipment to:
| ● | File a statement setting forth the name, address, phone number, email address, website address, with the U.S. Attorney General and the tobacco tax administrator of the State where shipment is being made or in which an advertisement or offer is disseminated; | |
| ● | On the 10th day of every month, file a memorandum or a copy of the invoice covering each and every shipment of “cigarettes” during the previous calendar month with the state tobacco tax administrator and, where there are also local taxes on cigarettes, with local/tribal official | |
| ● | Comply with (i) certain shipping requirements if using common carriers other than the Postal Service, such as FedEx or UPS (e.g., label requirements, weight restrictions, 21+ age verification on delivery, etc.), and (ii) recordkeeping requirements (e.g., detailed invoices covering every delivery sale, organized by the state, the city or town, and zip code into which the delivery sale is made); (iii) all state, local, tribal, and other laws generally applicable to sales of cigarettes, including: excise taxes, licensing and tax-stamping requirements; restrictions on sales to minors; and other payment obligations or legal requirements relating to the sale, distribution, or delivery of cigarettes or smokeless tobacco. |
64
Importantly, neither the mail ban nor the other PACT Act’s “delivery sale” provisions apply to business-to-business deliveries. Under an exception to the mail ban provision of the PACT Act, tobacco products may be mailed business purposes between legally operating businesses that have all applicable State and Federal Government licenses or permits and are engaged in tobacco product manufacturing, distribution, wholesale, export, import, testing, investigation, or research or for regulatory purposes between any business described above and an agency of the federal government or a state government. A business must apply for and obtain Postal Service approval of an exception to avail itself of this exception.
Except for the mail ban, the amendment to the PACT Act took effect on March 28, 2021. The mail ban took effect on October 21, 2021 pursuant to final regulations issued by the Postal Service. It applies to cannabis and hemp vaping products that aerosolize liquids only. Further, the most commonly used carriers, Federal Express and UPS, have recently announced that they would cease all deliveries of vapor products in the United States.
The other requirements of the PACT Act applicable to “delivery sellers” and “delivery sales” do not apply to business-to-business sales, as those terms involve delivery to “consumers.” The PACT Act defines “consumer” as “any person that purchases cigarettes or smokeless tobacco” and specifically excludes “any person lawfully operating as a manufacturer, distributor, wholesaler, or retailer of cigarettes or smokeless tobacco.
Starting on February 6, 2020, the FDA has prioritized for immediate enforcement against: (i) flavored, cartridge-based ENDS products (other than tobacco- or menthol-flavored ENDS products), and (ii) any flavored ENDS products (including tobacco and menthol flavors) that are targeted at minors. Several states in the United States have imposed temporary emergency flavor bans on ENDS products, and a few of these bans have been enjoined by courts while several have become permanent. Two states and the District of Columbia have also enacted permanent prohibitions on the sale of flavored ENDS products. Flavor bans are not the same as a total ban on e-cigarettes, and none of the states in the U.S. have imposed a total ban on e-cigarettes.
Our self-branded vaping systems are not affected by the flavor bans. The flavor bans are mainly aimed at ENDS products that are sold with pre-filled non-tobacco flavored or non-menthol-flavored cartridges, and our self-branded products do not contain any pre-filled cartridges.
Cannabis vaping products are governed by state laws, which vary from state to state. Most states do not permit the adult recreational use of cannabis, and no states permit the sale of recreational cannabis products to minors. As a result of the reduced revenue to states resulting from the effects of the COVID 19 pandemic, states may seek to raise revenue by permitted and taxing the use of cannabis products. We cannot predict what action states will take or the nature and amount of taxes they may impose. However, the shipping restrictions of the USPS under the PACT Act applied to cannabis products, and cannabis products cannot, with certain exceptions, be sent through the USPS. Major overnight courier services, such as Federal Express, do not ship vaping products that may not be sent using the USPS. We use a combination of advanced accounting software and PACT Act compliant carriers to remain compliant with the tax and delivery restrictions of the PACT Act.
Under federal law and the laws of certain states that continue to broadly restrict production and sale of cannabis, vaping devices intended for use in consuming cannabis products may qualify as prohibited drug paraphernalia. However, the federal Controlled Substances Act includes an exemption for “any person authorized by local, State, or Federal law to manufacture, possess, or distribute such items.” Several states with legal cannabis programs, including California, have enacted legislation invoking this exemption to shield state-legal businesses from federal enforcement on paraphernalia grounds. In addition, a recent court decision from the U.S. Court of International Trade applied this exemption in prohibiting U.S. Customs and Border Protection from refusing import entry of cannabis paraphernalia components that the importer could legally possess in the state of importation.
In distributing cannabis vaping devices in the United States, we rely on this exemption by (i) not selling our own branded cannabis vaping products directly into states that have maintained complete or near-complete cannabis prohibition, (ii) requiring distributors to whom we sell cannabis vaping products to covenant that they will not sell our products into these states, and (iii) limiting the sale of our custom made and white label cannabis vaping products to state-licensed dispensaries and entities, such as licensed cultivators or manufacturers.
During the 117th Congress (which ended on January 3, 2023), legislators introduced a number of bills aimed at increasing opportunities for cannabis industry members and their service providers. The most comprehensive legislation introduced to date, the Cannabis Administration and Opportunity Act, would provide for federal regulation of cannabis products by the U.S. Food and Drug Administration (“FDA”) and taxation by the U.S. Alcohol Tobacco Tax and Trade Bureau (TTB) but would afford states continued authority to restrict and regulate such products. The States Reform Act would remove marijuana from Schedule I of the Controlled Substances Act and locate federal regulatory and taxation authority for cannabis products at the TTB while also affording states the ability to continue to restrict or prohibit such products. Passed by the House of Representatives, the Marijuana Opportunity, Reinvestment, and Expungement Act would also remove marijuana from the scope of the Controlled Substances Act and establish a federal excise and occupational tax framework for cannabis products. While none of these bills passed during the 117th Congress, we cannot predict whether or when any of these or other similar bills will be introduced in the new Congress or what specific provisions they will contain if so. However, we believe that the fact that various members of Congress in both houses have recently introduced cannabis reform bills—and that the House of Representatives has passed such legislation—suggests an openness to and momentum towards federal cannabis reform in the near term.
To the extent that we conduct manufacturing operations in California we will be subject to federal and California laws and regulations applicable to manufacturing operations generally, including employee health and safety and environmental laws and regulations.
65
Europe
The European Commission issued the Tobacco Products Directive (the “TPD’’), which has been entered into force on May 19, 2014 and became applicable in the EU Member States on May 20, 2016. Under the TPD, an e-cigarette is widely defined as a product that can be used for, including all types of vaping devices, HNB devices and their respective components, the consumption of nicotine-containing vapor via a mouthpiece, or any component of that product. The TPD regulates e-cigarettes on five main aspects: (i) the information to be provided by the manufacturer and/or distributor, (ii) the advertising and promotion, (iii) safety issues and warnings, (iv) product presentation, and (v) provisional measures in case of suspected risk. Member states of the European Union are required to ensure that advertisements for any tobacco related product are prohibited, unless the advertisement is specifically targeted at professionals specializing in the electronic cigarettes trading. Moreover, no promotion whatsoever shall be made as to those devices with an intention (direct or indirect) to promote electronic cigarettes.
The sale of cannabis vaping products for recreational (as contrasted with medical) use is illegal in the European Union, although we believe that a market is developing, particularly in Germany, where the new coalition government stated clearly that it is introducing the controlled supply of recreational cannabis to adults in licensed shops.
United Kingdom
The Medicines and Healthcare Products Regulatory Agency (“MHRA”) is the authority for a notification scheme for e-cigarettes and refill containers in Great Britain and Northern Ireland and is responsible for implementing the majority of provisions under Part 6 of the Tobacco and related Products Regulations (TRPR) and the Tobacco Products and Nicotine Inhaling Products (Amendment) (EU Exit) Regulations 2020.
The TRPR introduced rules which ensure:
| ● | minimum standards for the safety and quality of all e-cigarettes and refill containers (otherwise known as e-liquids) | |
| ● | that information is provided to consumers so that they can make informed choices | |
| ● | an environment that protects children from starting to use these products. |
The requirements:
| ● | restrict e-cigarette tanks to a capacity of no more than 2ml | |
| ● | restrict the maximum volume of nicotine-containing e-liquid for sale in one refill container to 10ml | |
| ● | restrict e-liquids to a nicotine strength of no more than 20mg/ml | |
| ● | require nicotine-containing products or their packaging to be child-resistant and tamper evident | |
| ● | ban certain ingredients including colorant, stimulants and any carcinogenic, mutanegenic or reprotoxic elements | |
| ● | include new labelling requirements and warnings in line with the Classification, Labelling & Packaging regulations of the European Union | |
| ● | require all e-cigarettes and e-liquids be notified to the MHRA before they can be sold. |
The Tobacco Products and Nicotine Inhaling Products (Amendment) (EU Exit) Regulations 2020 explains the changes from a policy perspective:
The 2020 Regulations sets out the requirements for new products to be notified from January 1, 2021. This will mean that:
| ● | Producers placing products on the Northern Ireland market will be required to notify using the EU Common Entry Gate (EU-CEG) system for the notification of tobacco and e-cigarette products. |
| ● | Producers placing products on the Great Britain market will be required to notify on the Great Britain domestic system. |
| ● | Notifiers will be required to pay one fee if they notify in relation to placing products on one of the Great Britain or Northern Ireland markets and the same one fee if they notify in relation to placing products on the two markets. |
A producer is anyone who manufactures or imports these products or who re-brands any product as their own.
Part 6 of the Tobacco and Related Products Regulations 2016 sets out the requirements for e-cigarettes and refill containers.
Producers must submit information about their products to the MHRA through the MHRA Submission Portal and European Common Entry Gate (EU-CEG) notification portal for UK wide supply.
66
Under the TRPR, it is the responsibility of the producer to ensure that their products comply with the TRPR requirements. We check notifications submitted for completeness and verify TRPR compliance with producers. Where this review has been completed, the compliance status of products is recorded as ‘declared’ to indicate that the notification is complete, and the product has been declared compliant by the producer.
Producers of new e-cigarette and refill container products must submit a notification to the MHRA six months before they intend to put their product on the market in Great Britain and/or Northern Ireland. Once the notification has been published on the MHRA website, producers can launch the product in the notified region. A product which has been substantially modified will count as a new product and must also follow this process. Further information regarding what qualifies as a substantial modification can be found in the guidance on submission type below.
The TRPR does not include any requirements as to where testing of e-cigarettes and refill containers has to take place nor has any international testing standards been established. The notifier will need to be satisfied as to the standards of any testing carried out as they have to submit a declaration that they bear full responsibility for the quality and safety of the product when placed on the market and used under normal or reasonably foreseeable conditions.
The sale of cannabis products is illegal in the United Kingdom.
Vietnam
We are taking the initial steps to establish manufacturing operations in Vietnam. We have taken the initial steps to form a subsidiary in Vietnam. When we commence manufacturing operations in Vietnam, we will have to comply with laws and regulations relating to manufacturing operations, including regulatory approval for us to establish manufacturing operations, including satisfying the applicable government authority that we have sufficient capital to cover all of our planned activities. We are in the process of forming a Vietnam subsidiary to conduct our business in Vietnam, which is a first step if we propose to conduct business in Vietnam. The subsidiary will have to have a resident director. The first step to conducting business in Vietnam is to apply for a license to establish a foreign company, and the investors must register their investments with licensing authorities, in two steps – obtain the investment registration certificate and obtain enterprise registration certificate. We will also have to apply for the initial tax declaration at the Department of Taxation. We will also need to show that we have a lease for our subsidiary’s facilities. Transfer pricing rules in Vietnam require that the enterprise pays and Vietnam receives a reasonable rate of return on its activities as if the parties were unrelated. Vietnamese tax law does not provide special rules regarding cost plus transfer pricing. We will also be subject to wage and hour laws and laws relating to employee health and safety and environmental laws and regulations. Further, Vietnam will introduce a new law on October 1, mandating that all domestic companies and certain foreign firms providing services in areas like telecommunications, e-commerce, and online payment will have to store specific types of data in-country for a minimum period of 24 months. Since all of the sales by the Vietnam subsidiary will be to us, we do not believe that these provisions relate to our proposed business. In May 2021, Vietnam’s National Assembly passed the Revised Law on Environmental Protection. The new law will give communities a bigger role in conservation and impose responsibilities on corporations. The law, which went into effect on January 1, 2022, requires owners of factories to use the best available technology to control pollution and limit environmental impact. We will structure our Vietnam operations to comply with applicable laws and regulations.
Other requirements for e-cigarettes
Replacement e-cigarette parts that could contain nicotine only require notification if they have not already been notified as part of a device or e-cigarette kit. Identical replacement parts that have already been notified as part of another notified e-cigarette product do not need to be separately re-notified if it is clear on the labelling what notified product the part is for. Any non-identical replacement part, particularly one that alters the consumer safety profile of a product (for example by changing its refill capacity), would require a separate notification.
The Conformitè Europëenne (CE) Mark is defined as the European Union’s (EU) mandatory conformity marking for regulating the goods sold within the European Economic Area (EEA) since 1985. The CE marking represents a manufacturer’s declaration that products comply with the EU’s New Approach Directives. These directives not only apply to products within the EU but also for products that are manufactured in or designed to be sold in the EEA. This makes the CE marking recognizable worldwide even to those unfamiliar with the EEA.
Regulations Relating to Privacy and Security
We are or may become subject to a variety of laws and regulations in the United States and abroad regarding privacy, data security, cybersecurity and data protection. These laws and regulations are continuously evolving and developing. The scope and interpretation of the laws that are or may be applicable to us are often uncertain and may be conflicting, particularly with respect to foreign laws. In particular, there are numerous United States federal, state, and local laws and regulations and foreign laws and regulations regarding privacy and the collection, sharing, use, processing, disclosure, and protection of personal information and other user data. Such laws and regulations often vary in scope, may be subject to differing interpretations, and may be inconsistent among different jurisdictions. To the extent that we deal with the public and obtain private information on our computer system, we would be subject to these laws. To the extent that we conduct internet sales, we may be subject to these laws.
67
In June 2018, California adopted the California Consumer Privacy Act (“CCPA”), which became effective in 2020. Under the law, any California consumer has a right to demand to see all the information a company has saved on the consumer, as well as a full list of all the third parties that data is shared with. The consumer also has the right to request that we delete the information it has on the consumer. The CCPA broadly defines “protected data.” The CCPA also has specific requirements for companies subject to the law. The CCPA provides for a private right of action for unauthorized access, theft or disclosure of personal information in certain situations, with possible damage awards of $100 to $750 per consumer per incident, or actual damages, whichever is greater. The CCPA also permits class action lawsuits. To the extent that we sell products to consumers through our website or otherwise on the Internet, we may be subject to the CCPA as well as other consumer protection laws.
The European Union Parliament approved a new data protection regulation, known as the General Data Protection Regulation (“GDPR”), which came into effect in May 2018. The GDPR includes operational requirements for companies that receive or process personal data of residents of the European Economic Area. The GDPR imposes significant penalties for non-compliance. Although we do not conduct any business in the European Economic Area, in the event that residents of the European Economic Area access our website and input protected information, including information provided in ordering through our website, we may become subject to provisions of the GDPR.
We are also subject to laws restricting disclosure of information relating to our employees. We strive to comply with all applicable laws, policies, legal obligations, and industry codes of conduct relating to privacy, data security, cybersecurity and data protection. However, given that the scope, interpretation, and application of these laws and regulations are often uncertain and may be conflicting, it is possible that these obligations may be interpreted and applied in a manner that is inconsistent from one jurisdiction to another and may conflict with other rules or our practices. Any failure or perceived failure by us or our third-party service-providers to comply with our privacy or security policies or privacy-related legal obligations, or any compromise of security that results in the unauthorized release or transfer of personally identifiable information or other user data, may result in governmental enforcement actions, litigation, or negative publicity, and could have an adverse effect on our business and operating results. Although we maintain cybersecurity insurance, we cannot assure you that this insurance will cover or satisfy any claim made against us or adequately cover any defense costs we may incur.
Environmental Laws and Regulations
As our supplier, Shenzhen Yi Jia is responsible for compliance with Chinese environmental laws and regulations. To the extent that such compliance results in increased manufacturing costs, we anticipate that our prices will be increased, although we may not know the details of the expense of such compliance.
As a distributor of products made by third parties, we do not have any material costs in complying with environmental laws and regulations. To the extent that we establish manufacturing operations in Vietnam and California will be required to comply with applicable environmental laws and regulations. We cannot estimate the ongoing costs of such compliance. As we establish manufacturing facilities, we expect that the cost of such compliance will be included in our capital budget for any facilities we establish.
Directors and Executive Officers
The following table sets forth information regarding our executive officers and directors as of the date of this prospectus:
| Name | Age | Position/Title | ||
| Tuanfang Liu | 50 | Chief Executive Officer and Chairman | ||
| Michael Wang | 59 | Chief Financial Officer and President of Aspire North America | ||
| Tirdad Rouhani | 40 | Chief Operating Officer | ||
| Jeffrey Doiron | 50 | Chief Revenue Officer | ||
| Lauren Stower | 38 | Chief Impact Officer | ||
| Jiangyan Zhu | 47 | Director | ||
| Brent Cox | 40 | Independent Director Designee | ||
| John Fargis | 56 | Independent Director Designee | ||
| Joel Paritz | 79 | Independent Director Designee |
| Each of Brent Cox, John Fargis and Joel Paritz has agreed to serve as an independent director, effective upon the SEC’s declaration of effectiveness of the registration statement of which this prospectus is a part. |
Tuanfang Liu has been serving as our chairman of the board of directors and chief executive officer since our organization. Mr. Liu has also served as chairman of the board and chief executive officer of Aspire Global, a position he has held since its organization. Mr. Liu also serves as chairman of Shenzhen Yi Jia since he founded the company in June 2010. He is responsible for our daily operations and research and development of the e-cigarette and cannabis vaporizer technology products. Mr. Liu has served as the vice-chairman of the European Union E-cigarette Association since 2019, vice-chairman and founding member of the Canada E-cigarettes Association since 2019, vice chairman of the China Electronics Chamber of Commerce since 2017, and executive vice-chairman and founder of the Shenzhen E-Vapor Industry Association since October 2017. He received “Shenzhen High-level Professionals” award in 2019. Mr. Liu holds doctorate degrees in business management from Victoria University School of Management in Switzerland and EuroPort Business School in the Netherlands, respectively. He has more than 14 years of experience in research and development of the e-cigarette products and quality control management. Mr. Liu is the spouse of Jiangyan Zhu.
68
Michael Wang has been serving as our chief financial officer since our organization, and he has served as president of Aspire North America since its organization in 2020. Mr. Wang served chief financial officer of Aspire Global from August 2020 until his resignation in September 2022. Mr. Wang is an experienced chief executive officer, chief operating officer and president of various companies with leadership skills in profit and loss management, finance, human resources, products, technology, sales and operations. Mr. Wang has approximately 12 years of internet technology and e-commerce experience. From September 2018 through August 2020, he was the president, chief operating officer and co-chief executive officer of The Pharm/Sunday Goods (located in California and Arizona), a vertically integrated leader in the cannabis cultivation, processing, manufacturing, distribution, wholesale, and retail industry. Mr. Wang managed and transformed the cultivation, manufacturing and wholesale divisions. Mr. Wang was with Onestop Commerce, a leading e-commerce technology and service company, as president and chief operating officer from February 2013 to July 2015 and as chief executive officer from July 2015 to June 2018. Onestop Commerce managed omni-channel-commerce for major lifestyle brands and retailers. From May 2005 through June 2010, he was the chief operating and fulfillment officer and an investor in Zazzle, a leader in online customization and personalization service. He started his career in 1992 at Honeywell and also worked at Technicolor, ESS Technology and Vitec Group. Mr. Wang received bachelor of science and master of science degrees in aerospace engineering in 1983 and 1985 respectively, from the Beijing University of Aeronautics & Astronautics also known as Beihang University. In 1987, he received a master of science degree in systems engineering from Oakland University in Rochester, Michigan. In 1992, Mr. Wang received an MBA in Finance and General Management from the University of Chicago’s Booth School of Business.
Tirdad Rouhani has been the chief operating officer since July 2022. In the prior four years, Mr. Rouhani has been deeply entrenched in the cannabis industry. He held the role of chief operating officer at Touchstone (one of the largest cannabis extraction lab and co-packing businesses in California) before taking on the role of chief executive officer for Napalm Brands in March 2020. Mr. Rouhani co-founded two tech companies before converging on the cannabis industry. Between 2008 and 2015, he was a business process consultant at Live Nation. Mr. Rouhani received his undergraduate and graduate degrees from the University of Arizona where he studied business. He started his career in audit and consulting with Deloitte, expanding into tech and finance, evolving into operating roles.
Jeffrey Doiron has been the chief revenue officer of Aspire North America since March 2021 and chief revenue officer of the Company since July 2022. Mr. Doiron brings to us 22 years of marketing, sales and branding experience. Most recently he was the president of Quanta Inc., an applied science company serving brands in cannabis, anti-aging, health and wellness, stress management, pain management, fitness and brain performance enhancement between December 2017 and September 2020. Between 1999 and 2017, he was the co-founder and chief executive officer of Fuel Industries, a global creative agency focused on digital transformation. In 1992, Mr. Doiron received an oil burner technician certification in heating, ventilation, and air conditioning from Algonquin College.
Lauren Stower has been our chief impact officer since July 2022. Ms. Stower brings to us a decade of cannabis experience. Before her current role, she was the vice president of business development at Aspire North America between September 2020 and July 2022. Between 2016 and 2020, Ms. Stower held several key business development roles at Jetty Extracts and Blue River Terpenes. With a master’s degree from the University of San Francisco & BA in Social Justice from University of California at Santa Cruz, Ms. Stower blends her knowledge of the consumer retail market with a passion for social and plant-medicine justice. Her impact on drug policy has been instrumental to local decriminalization measures and first-of-its kind social equity initiatives. Named “Cannabis Culture Advocate of the Year” in 2021 by the Cannabis Chamber of Commerce, Ms. Stower is an internationally-recognized industry expert, featured on panels, podcasts and events summits and expos. She is also a renowned thought-leader and judge for the esteemed Emerald Cup Competition.
Jiangyan Zhu has been serving as our director since inception. Ms. Zhu is one of the founders of Aspire Global and is a director of Aspire Global, and, since 2013, she has served as vice president of finance of Shenzhen Yi Jia, where she is responsible for financial management, assisting in human resources management and establishing and improving the automated office system. Ms. Zhu holds a bachelor’s degree in business management from Jiangxi University of Technology. She also holds a Business Management certificate from the College of Continuing Education Graduate School of Shenzhen Tsinghua University. Ms. Zhu is the spouse of Mr. Tuanfang Liu.
69
Brent Cox serves as the managing partner of OBM Holdings, LLC, a private investment firm, a position he has held since April 2016. From September 2008 to April 2016, he served as a principal of the Yucaipa Companies, a Los Angeles, California based private equity firm where he was responsible for sourcing, analyzing and executing investment opportunities, structuring financing for investments and monitoring the performance and strategic initiatives of its portfolio companies. From 2006 to 2008, Mr. Cox served as an investment banking analyst in the Leveraged Finance Group of Jefferies & Co. a multinational independent investment bank and financial services company. Mr. Cox received a bachelor of science degree from the University of Southern California.
John Fargis has been serving as the co-founder and principal of BYG Advantage since June 2014, a Beijing-based platform that outsources business development, sales acceleration bridging best in class technology into the Asia Pacific region. Clients include Hashicorp, Trustonic, Tomorrow.io, and EF. Its services include market analysis, market entry, market acceleration, government relations and special vehicle creation across the region. Mr. Fargis founded and runs Dustybrine LLC, a market entry consulting firm in New York State. Mr. Fargis has been serving as the professor of management, strategy, and emerging markets at Hult International Business School since February 2014, where he teaches courses including strategy, management, emerging markets, leadership, operations and big data. Mr. Fargis has been also serving as the Adjunct Professor of Strategy and China History since January 2014 in Shanghai, China. Mr. Fargis has been serving as the principal Asia-Pacific of Hortonworks since 2014. From March 2010 to December 2013, Mr. Fargis served as the executive vice president and general manager at Kaseya where he incorporated, staffed and ran offices for Kaseya in Beijing, Seoul, Tokyo and Hong Kong. The company was purchased by Insight Venture Partners in June 2013. From 2007 to April 2010, Mr. Fargis served as the vice president sales and general manager of Asia of On2 Technologies which was purchased by Google in February 2010. From August 2005 to October 2007, Mr. Fargis served as the general manager Asia Pacific of Global IP Solutions (GIPS), where he oversaw sales and business development strategy for Global IP Sound (GIPS) in Asia. GIPS provides premiere quality speech processing technology for Voice Over IP (VOIP) networks, and its software enables numerous clients including application providers such as Skype, Google, AOL, Tencent, etc. From January 2004 to July 2005, Mr. Fargis served as the chief executive officer of SiMa Systems, where he oversaw funding and alliance strategy and general management for this digital clipboard solutions company. In 1998, Mr. Fargis received his master of arts in law and diplomacy degree in international consulting at The Fletcher School of Law and Diplomacy. In 1992, Mr. Fargis received his master’s degree in special education at Hunter College. In 1988, Mr. Fargis received his bachelor’s degree in medieval studies at Wesleyan University. We believe Mr. Fargis is well-qualified to serve as a member of our board of directors due to his experience in business strategy, emerging markets, and his contacts and relationships.
Joel Paritz is a certified public accountant and he has worked in the accounting industry for more than 50 years. Mr. Paritz is a partner emeritus of Prager Metis CPAs, a member of the Prager Metis International Group. Mr. Paritz worked for six years for EY. He then ran his own accounting practice for more than 30 years and sold it in 2018. He is a founder of Bancorp of New Jersey which became a public company and was sold to Connect One Bank in 2019, where he served on the board as an independent director for more than ten years. He currently provides CPA and financial services as a consultant. Mr. Paritz received his MBA in accounting from Rutgers University. We believe that Mr. Paritz is well qualified to serve as a member of our board of directors because of his accounting experience and his familiarity with the operations of public companies.
Family Relationships
Tuanfang Liu, our chairman and chief executive officer, and Jiangyan Zhu, one of our directors, are married. Other than this relationship, there are no other direct family relationships among any of our directors or executive officers.
70
Committees of the Board of Directors
Immediately prior to the date of this prospectus, we established an audit committee, a compensation committee and a nominating and corporate governance committee. We have adopted a charter for each of the three committees. Each committee’s members and functions are described below.
Audit Committee. Our audit committee will consist of Brent Cox, John Fargis and Joel Paritz, with Mr. Paritz as chair. We have determined that each of these three director nominees satisfies the “independence” requirements of the Nasdaq Listing Rules and meet the independence standards under Rule 10A-3 under the Securities Exchange Act of 1934, as amended. We have determined that Joel Paritz and Brent Cox qualify as an “audit committee financial experts.” The audit committee oversees our accounting and financial reporting processes and the audits of our financial statements. The audit committee is responsible for, among other things:
| ● | selecting the independent registered public accounting firm and pre-approving all auditing and non-auditing services permitted to be performed by the independent registered public accounting firm; |
| ● | reviewing with the independent registered public accounting firm any audit problems or difficulties and management’s response; |
| ● | reviewing and approving all proposed related party transactions, as defined in Item 404 of Regulation S-K under the Securities Act; |
| ● | discussing the annual audited financial statements with management and the independent registered public accounting firm; |
| ● | reviewing the adequacy and effectiveness of our accounting and internal control policies and procedures and any special steps taken to monitor and control major financial risk exposures; |
| ● | annually reviewing and reassessing the adequacy of our audit committee charter; |
| ● | meeting separately and periodically with management and the independent registered public accounting firm; |
| ● | monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance; and |
| ● | reporting regularly to the board. |
Following completion of this offering, our audit committee will review all proposed related party transactions on an ongoing basis and any such transactions shall be approved by the audit committee. The audit committee also approves certain pricing matters pursuant to our supply agreements with Shenzhen Yi Jia. In determining whether to approve a related party transaction, the audit committee shall consider, among other factors, the following factors to the extent relevant to the related party transaction:
| ● | whether the terms of the related party transaction are fair to the Company and on the same basis as would apply if the transaction did not involve a related party; |
| ● | whether there are business reasons for us to enter into the related party transaction; |
| ● | whether the related party transaction would impair the independence of an outside director; |
| ● | whether the related party transaction or the approval of the related party transaction would present an improper conflict of interest for any director or executive officer, taking into account the size of the transaction, the overall financial position of the director, executive officer or the related party, the direct or indirect nature of the director’s, executive officer’s or the related party’s interest in the transaction and the ongoing nature of any proposed relationship, and any other factors the audit committee deems relevant; and |
| ● | any pre-existing contractual obligations. |
Compensation Committee. Our compensation committee will consist of Joel Paritz, Brent Cox and John Fargis, with Brent Cox as chair. We have determined that each of these directors satisfies the “independence” requirements of the Nasdaq Listing Rules. The compensation committee assists the board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers. Our chief executive officer may not be present at any committee meeting during which their compensation is deliberated upon. The compensation committee is responsible for, among other things:
| ● | reviewing and approving, or recommending to the board for its approval, the compensation for our chief executive officer and other executive officers; |
| ● | reviewing and recommending to the board for determination with respect to the compensation of our non-employee directors;
| |
| ● | reviewing periodically and approving any incentive compensation or equity plans, programs or other similar arrangements; and |
| ● | selecting compensation consultant, legal counsel or other adviser only after taking into consideration all factors relevant to that person’s independence from management. |
71
Nominating and Corporate Governance Committee. Our nominating and corporate governance committee will consist of Tuanfang Liu, Brent Cox and John Fargis, with Tuanfang Liu as chair. We have determined that Mr. Cox and Mr. Fargis satisfy the “independence” requirements of the Nasdaq Listing Rules. Because we are a controlled corporation, we have included our chief executive officer, who is not an independent director, as a member and chair of the nominating and corporate governance committee. The nominating and corporate governance committee assists the board in selecting individuals qualified to become our directors and in determining the composition of the board and its committees. The nominating and corporate governance committee is responsible for, among other things:
| ● | recommending nominees to the board for election or re-election to the board, or for appointment to fill any vacancy on the board; |
| ● | reviewing annually with the board the current composition of the board with regards to characteristics such as independence, knowledge, skills, experience, expertise, diversity and availability of service to us; |
| ● | selecting and recommending to the board the names of directors to serve as members of the audit committee and the compensation committee, as well as of the nominating and corporate governance committee itself; |
| ● | developing and reviewing the corporate governance principles adopted by the board and advising the board with respect to significant developments in the law and practice of corporate governance and our compliance with such laws and practices; and |
| ● | evaluating the performance and effectiveness of the board as a whole. |
Terms of Directors
Our directors are elected for a term of one year, until the next annual meeting of stockholders and until their successors are elected and qualified. Pursuant to our bylaws, our officers serve at the pleasure of the board of directors subject to any rights they may have pursuant to employment agreements and applicable law.
The following table sets forth information regarding the compensation awarded to, earned by, or paid during the year ended June 30, 2022, to our chief executive officer and the two most highly paid executive officers other than the chief executive officer. These three officers are referred to as our “Named Executive Officers.”
Summary Compensation Table(1)
| Name and Principal Position | Year Ended June 30, | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Nonequity incentive plan compensation ($) | Nonqualified deferred compensation earnings ($) | All other compensation ($) | Total ($) | ||||||||||||||||||||||||||
| Tuanfang Liu CEO(2) | 2022 | $ | 153,757 | - | - | - | - | - | - | 153,757 | |||||||||||||||||||||||||
| Michael Wang CFO | 2022 | 350,000 | - | - | - | - | - | 350,000 | |||||||||||||||||||||||||||
| Jeff Doiron Chief Revenue Officer | 2022 | 240,000 | - | - | - | - | - | - | 240,000 | ||||||||||||||||||||||||||
| (1) | The compensation in the table reflects compensation paid by Aspire North America and/or Aspire Science prior to the date these entities were transferred to us and does not include any dividends received or accrued by Mr. Liu as a 95% stockholder in Shenzhen Yi Jia. |
| (2) | Mr. Liu’s compensation is paid in Hong Kong dollars, which are converted into U.S. dollars at the average exchange rates during the period, which was 7.8045 Hong Kong dollars to $1.00. See Note 2 of Notes to Consolidated Financial Statements. |
72
Employment Agreements
We have employment agreements dated January , 2023, with Tuanfang Liu, our chief executive officer, and Michael Wang, our chief financial officer.
The employment agreement with Mr. Liu has a term of five years and continues on year-to-year basis unless terminated by either us or Mr. Liu on notice given not later than 60 days prior to the expiration of the initial five-year term or any one-year extension. Mr. Liu receives compensation from us at the annual rate of 1,920,000 Hong Kong dollars. Any increase in his annual compensation and any bonus compensation are subject to the discretion of the Compensation Committee and Mr. Liu is also eligible for such options or other equity-based compensation, if any, as may be determined by the Compensation Committee. Mr. Liu will perform his services at such location as he may determine, and we anticipate that he will perform his services in the PRC. The agreement acknowledges that Mr. Liu is also chairman, chief executive officer and a director of Aspire Global and the chief executive officer and 95% owner of Shenzhen Yi Jia. The agreement has customary non-competition and non-solicitation provisions. Mr. Liu has agreed that we have title to all rights to any intellectual property rights which may be developed by Mr. Liu that relate to cannabis or cannabis related vaping or other products during the term of the employment agreement and he will execute such documents as may be necessary to effect our ownership of such intellectual property, including, but not limited to assignment of patents and trademarks. With respect to any intellectual property relating to tobacco vaping and other nicotine products, we shall have an exclusive license in the territory, which is worldwide except for the PRC and Russia, with respect to such intellectual property. We acknowledge the Mr. Liu is also employed as chief executive officer of Aspire Global and Shenzhen Yi Jia. Both Aspire Global and Shenzhen Yi Jia agreed to the provisions of Mr. Liu’s employment agreement relating to intellectual property developed by Mr. Liu. Although Mr. Liu does not receive any compensation from Aspire Global or Shenzhen Yi Jia, for his services as its chief executive officer of Aspire Global, as the 95% owner of Shenzhen Yi Jia, he receives dividends from Shenzhen Yi Jia.
The employment agreement with Mr. Wang has a term of three years and continues on a quarter-to-quarter basis unless terminated by either us or Mr. Wang on notice given not later than 30 days prior to the expiration of the initial three-year term or any quarterly extension. Mr. Wang receives annual compensation at the rate of $350,000. Any increase in his annual compensation and any bonus compensation are subject to the discretion of the Compensation Committee and Mr. Wang is also eligible for such options or other equity-based compensation, if any, as may be determined by the Compensation Committee. The agreement has customary assignment of invention provisions. In connection with our organization, we issued to Peak Group LLC, a limited liability company owned by Mr. Wang a 2% interest in Aspire Global for services rendered which, when our common stock was issued to the holders of the Aspire Global capital stock, resulted in the issuance to Mr. Wang of 1,000,000 shares of common stock, which were valued at $437,235. The issuance of these shares is treated as compensation for services rendered by Mr. Wang to Aspire Global, the then parent of Aspire North America and Aspire Science, as its chief financial officer.
73
Director Compensation
The following table provides information as to compensation to directors who are not Names Executive Officers:
| Name | Fees Earned or Paid in Cash ($) | Stock Awards ($) | Option Awards ($) | Nonequity incentive plan compensation ($) | Nonqualified deferred compensation earnings ($) | All other compensation ($) | Total ($) | |||||||||||||||||||||
| Jiangyan Zhu(1) | $ | 92,254 | - | - | - | - | $ | 92,254 | ||||||||||||||||||||
| (1) | Ms. Zhu’s compensation is paid in Hong Kong dollars, which are converted into U.S. dollars at the average exchange rates during the period, which was 7.8045 Hong Kong dollars to $1.00. See Note 2 of Notes to Consolidated Financial Statements. |
We have an agreement with Ms. Zhu pursuant to which we pay her annual compensation of 720,000 Hong Kong dollars. Ms. Zhu is also a director of Aspire Global and she does not receive compensation from Aspire Global.
On or before the closing of this offering, we will enter into agreements with our independent directors pursuant to which we will provide each director with cash and equity compensation. On the date of each independent director’s appointment and upon each quarter thereafter we will issue to Mr. Paritz $15,000 and $15,000 of common stock and to each of Mr. Fargis and Mr. Cox $12,000 and $12,000 of common stock. The shares issued upon their appointment will be based on the initial public offering price of this offering. Thereafter, the number of shares will be based on the market price at the time the shares become issuable.
2022 Equity Incentive Plan
In October 2022, our directors and stockholders approved the 2022 Equity Incentive Plan (the “Plan”) pursuant to which up to 15,000,000 shares of common stock may be issued pursuant to options or restricted stock grants. The Plan will be administered by the Compensation Committee. Awards under the Plan may be granted to officers, directors, employees and those consultants who qualify as a consultant or advisor under the instructions to Form S-8. The Compensation Committee has broad discretion in making awards; provided that any options shall be exercisable at the fair market value on the date of grant. As of the date of this prospectus, no awards have been granted under the Plan.
Outstanding Equity Awards at Fiscal Year-End
As of June 30, 2022 and September 30, 2022, we had no outstanding equity awards.
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following are transactions from July 1, 2020 through the date of this prospectus between us, and enterprises that directly or indirectly through one or more intermediaries, control or are controlled by, or are under common control with, (a) us, (b) our directors; (c) individuals owning, directly or indirectly, an interest in the voting power of the Company that gives them significant influence over the Company, and close members of any such individual’s family; (d) key management personnel, that is, those persons having authority and responsibility for planning, directing and controlling our activities of the Company, including senior management of companies and close members of such individuals’ families; and (e) enterprises in which a substantial interest in the voting power is owned, directly or indirectly, by any person described in (c) or (d) or over which such a person is able to exercise significant influence.
Following completion of this offering, our audit committee will review all related party transactions on an ongoing basis and all such transactions be approved by the audit committee. In determining whether to approve a related party transaction, the audit committee shall consider, among other factors, the following factors to the extent relevant to the related party transaction:
| ● | whether the terms of the related party transaction are fair to the Company and on the same basis as would apply if the transaction did not involve a related party; |
| ● | whether there are business reasons for the Company to enter into the related party transaction; |
| ● | whether the related party transaction would impair the independence of an outside director; |
| ● | whether the related party transaction or the approval of the related party transaction, would present an improper conflict of interest for any director or executive officer of the Company, taking into account the size of the transaction, the overall financial position of the director, executive officer or the related party, the direct or indirect nature of the director’s, executive officer’s or the related party’s interest in the transaction and the ongoing nature of any proposed relationship, and any other factors the audit committee deems relevant; and |
| ● | any pre-existing contractual obligations. |
74
The following are forth the major related parties and their relationships with us:
| Name of related parties and Relationship with the Company |
| - Tuanfang Liu is the chief executive officer and chairman of the Company. |
| - Jiangyan Zhu is the wife of Tuanfang Liu and a director of the Company. |
| - Eigate (Hong Kong) Technology Co., Limited (“Eigate”) is a company wholly owned and controlled by our chief executive officer. |
| - Aspire Global is a company controlled by the chief executive officer of the Company. |
| - Shenzhen Yi Jia, a Chinese company that is 95% owned by the Company’s chief executive officer and 5% by the chief executive officer’s cousin. |
Tuanfang Liu is also Aspire Global’s chief executive officer and a director of both us and Aspire Global, and his wife, Jiangyan Zhu, is also a director of both companies. Mr. Liu and Ms. Zhu beneficially own 66.5% and 5.0%, respectively, of our outstanding common stock and of the outstanding shares of Aspire Global. Michael Wang, our chief financial officer, was chief financial officer of Aspire Global from August 2020 until September 2022.
In connection with our organization in July 2022, we issued a total 50,000,000 shares to the holders of capital stock of Aspire Global in the same proportion as their share ownership in Aspire Global. Prior to the transfer of Aspire North America and Aspire Science to us, Aspire Global issued a 2% equity interest to an entity owned by Michael Wang, who was Aspire Global’s and our chief financial officer, and a 1.1% interest in Aspire Global to an entity owned by a consultant, in each case for services rendered to Aspire Global and its subsidiaries. When we issued 50,000,000 shares of common stock to the holders of Aspire Global capital stock, these issuances resulted in the entities owned by Mr. Wang and the consultant of 1,000,000 shares and 537,500 shares, respectively. Because the transfer of the equity interest in Aspire North America and Aspire Science from Aspire Global and its wholly-owned subsidiary was made for no consideration to a corporation that had identical stockholders as Aspire Global, these shares are deemed to be outstanding since July 1, 2020.
In connection with the restructure of Aspire Global, on July 29, 2022, for no consideration:
| ● | Aspire Global transferred 100% of the equity interest in Aspire North America to us. |
| ● | Aspire Holdings transferred 100% of the equity of Aspire Science to our subsidiary, Ispire International. |
In the year ended June 30, 2020, Aspire Science, declared a dividend of $3,832,272, which is payable to Tuanfang Liu, who, at the date the dividend was declared, was the sole stockholder of Aspire Science. The dividend was declared prior to the transfer of the equity interest in Aspire Science by Mr. Liu to a subsidiary of Aspire Global, which subsequently transferred the equity interest to Ispire International. During the year ended June 30, 2022, Aspire Science paid $469,633 to Mr. Liu, and the balance due to Mr. Liu was $3,362,639 and $3,384,678 at December 31, 2022, which was paid on February 2, 2023.
For the years ended June 30, 2021 and 2022, substantially all of Aspire North America’s and Aspire Science’s tobacco and cannabis products were purchased from Shenzhen Yi Jia. As of June 30, 2021 and June 30, 2022, the accounts payable to Shenzhen Yi Jia was $33,370,618 and $41,982,373 respectively. For the years ended June 30, 2021 and 2022, the purchases from Shenzhen Yi Jia were $52,998,928 and $74,787,679 respectively.
As of June 30, 2021 and 2022, Aspire Science had a balance due to Eigate of $41,172,013 and $40,672,768, respectively. These balances were all non-interest bearing, unsecured, have no due date and are repayable on demand. Prior to 2020, both Aspire Science and Eigate were owned by Mr. Liu, and Eigate lent money to Aspire Science for working capital. On February 2, 2023, we made the payments to Mr. Liu and Eigate. Although Aspire Science had the funds to make this payment and the dividend payable to Mr. Liu, payment was delayed because, as a result of the size of the transfer, in order to for Aspire Science to wire the money it was necessary for an authorized person to personally go to the bank to wire the funds. This was not possible because of COVID-19 restrictions which required Mr. Liu, who is based in mainland China, to go to the bank in Hong Kong and be subject to quarantine when he returns to mainland China. Since January 8, 2023, no centralized quarantine or mass PCR testing will be undertaken on travelers entering mainland China. Travelers to mainland China are only required to take PCR test 48 hours prior to their departure and report the PCR test findings on their customs health declaration form. Only those whose test results are positive prior to departure will have to postpone their travel until the PCR results turn negative. As a result of these changes, Mr. Liu was able to travel to Hong Kong to make the payments without beign subject to quarantine upon his return.
At June 30, 2021 and 2022, we had the following balance due from related parties:
| As of June 30, | ||||||||
| 2021 | 2022 | |||||||
| Shenzhen Yi Jia | $ | 31,297 | $ | 1,872,035 | ||||
| Tuanfang Liu | 4,924 | 62,820 | ||||||
| Total | $ | 36,221 | $ | 1,934,855 | ||||
75
The balances are payment made by Aspire Science on behalf of these related parties. These balances were all non-interest bearing, unsecured, have no due date and are repayable on demand, and were paid in full on November 28. 2022. From and after the date of this prospectus, our audit committee will review and approve all proposed related party transactions, as defined in Item 404 of Regulation S-K under the Securities Act. The audit committee will not, and prior to the appointment of the audit committee, we will not, approve any loan or extension of credit in the form of personal loans to or for the benefit of any director or executive officer.
On July 29, 2022, for no consideration:
| ● | Aspire Global transferred 100% of the equity interest in Aspire North America to the Company, and |
| ● | Aspire Holdings transferred 100% of the equity of Aspire Science to Ispire International. |
These transfers were made in connection with a restructure by Aspire Global pursuant to which the equity in Aspire North America and Aspire Science was transferred to us. At the time of the transfer, we had the same stockholders as Aspire Global and the stockholders held the same percentage equity interest in both us and Aspire Global.
Pursuant to the Intellectual Property Transfer Agreement, Mr. Liu, Aspire Global and Shenzhen Yi Jia agreed to transfer to Aspire North America all patent and other intellectual property rights, including trademarks, Know-how and Know-how Documentation, as defined in the agreement, relating to the cannabis vaping products, and to transfer to us any new intellectual property developed or acquired by Mr. Liu, Aspire Global and Shenzhen Yi Jia which relates to cannabis vaping products. The patents and patent applications, all of which are United States patents and applications, have been transferred to Aspire North America
Pursuant to the Intellectual Property License Agreement, Mr. Liu, Aspire Global and Shenzhen Yi Jia granted Aspire Science a perpetual royalty free sole and exclusive right and license to use and practice all of the Licensed Technology worldwide except for the PRC and Russia. The Licensed Technology includes all patents, know-how, know-how documentation and trademarks, whether now existing or hereafter developed or acquired by, or for, Mr. Liu, Aspire Global and/or Shenzhen Yi Jia that relate, directly or indirectly, to the tobacco vaping market. Pursuant to the License Agreement, neither Mr. Liu, Aspire Global nor Shenzhen Yi Jia has any right to market or sell or grant distributors the right to market or sell tobacco vaping products in the world other than in the PRC and Russia.
In January 2023, Aspire North America and Aspire Science entered into supply agreements with Shenzhen Yi Jia pursuant to which:
| ● | Shenzhen Yi Jia agreed to sell products to us at the most favorable market price that it sells similar products to third parties and such prices must be commercially reasonable in order to enable us to generate a gross margin based on purchase prices or a purchase price structure acceptable to our audit committee. |
| ● | Shenzhen Yi Jia is to provide us with quality products and services in a timely manner, to provide to our customers the same warrant that we provide to our customer and to honor the warranty. |
| ● | Shenzhen Yi Jia is to give us first priority to the manufacture of our products over any other manufacturing obligations it has. |
| ● | We need to provide Shenzhen Yi Jia with periodic forecasts and place orders consistent with the forecasts. |
| ● | Any intellectual property developed in connection with the manufacture of the cannabis products will be assigned, and the patents and patent applications have been assigned, to Aspire North America pursuant to the Intellectual Property Transfer Agreement and any intellectual property developed in connection with the manufacture of tobacco products will be licensed to Aspire Science pursuant to the Intellectual Property License Agreement. |
The agreement has an initial term of ten years, and automatically renews for two-year periods unless terminated by either party on not less than six months’ notice prior to the expiration of the initial term or any two-year extension.
The following table sets forth information regarding the beneficial ownership of our shares of common stock as of February 15, 2023 and as adjusted to give effect to this offering by:
| ● | Each holder of 5% or more of our common stock; |
| ● | Each member of our board of directors and each designee for director; |
| ● | Each Named Executive Officer; and |
| ● | All directors and executive officers as a group |
76
For purposes of the following table, “beneficial ownership” means the sole or shared power to vote, or to direct the voting of, a security, or sole or shared investment power with respect to a security, or any combination thereof, and the right to acquire such power (for example, through the exercise of warrants granted by us) within 60 days of February 15, 2023. Unless otherwise indicated, the person identified in this table has sole voting and investment power with respect to all shares shown as beneficially owned by him, subject to applicable community property laws. Unless otherwise noted, the mailing address of each listed beneficial owner is 19700 Magellan Dr, Los Angeles, CA 90502.
| Percentage | ||||||||||||
| Name of Beneficial Owners(1) | Number | Prior to Offering | After Offering(2) | |||||||||
| Tuanfang Liu and Jiangyan Zhu(3)(4) | 35,750,000 | 71.5 | % | 63.8 | ||||||||
| Pride Worldwide Investment Limited(3) | 33,250,000 | 66.5 | % | 59.4 | ||||||||
| Honor Epic International Limited(4) | 2,500,000 | 5.0 | % | 4.5 | ||||||||
| Fortune Genesis Enterprises Limited(5) | 2,500,000 | 5.0 | % | 4.5 | ||||||||
| Michael Wang(6) | 1,000,000 | 2.0 | % | 1.8 | ||||||||
| Jeff Doiron | 0 | 0.0 | % | 0.0 | % | |||||||
| Brent Cox | 0 | 0.0 | % | 0.0 | % | |||||||
| John Fargis | 0 | 0.0 | % | 0.0 | % | |||||||
| Joel Paritz | 0 | 0.0 | % | 0.0 | % | |||||||
| All directors and officers as a group (three individuals owning stock)(3)(4)(6) | 36,750,000 | 73.5 | % | |||||||||
| (1) | Unless otherwise noted, the business address of Pride Worldwide Investment Limited, Honor Epic Investment Limited and Fortune Genesis Enterprises Limited is 14 Jian’an Road, Tangwei Fuyong Town, Bao’an District, Shenzhen, Guangdong Province, China. |
| (2) | The percentage of ownership after the offering is based on 56,000,000 shares of common stock outstanding immediately after the offering, and assumes the over-allotment option is not exercised. |
| (3) | Tuanfang Liu, our chief executive officer, is the sole shareholder of Pride Worldwide Investment Limited and holds the voting and dispositive power over the common stock held by such entity. Mr. Liu disclaims beneficial interest in shares beneficially owned by his wife, Jiangyan Zhu. |
| (4) | Jiangyan Zhu, our director and spouse of Tuanfang Liu, our chief executive officer, is the sole shareholder of Honor Epic International Limited and holds the voting and dispositive power over the common stock held by such entity. Ms. Zhu disclaims beneficial interest in shares beneficially owned by her husband.
|
| (5) | Yuli Liu, a cousin of Tuanfang Liu, is the sole shareholder of Fortune Genesis Enterprises Limited and holds the voting and dispositive power over the common stock held by such entity.
|
| (6) | The shares beneficially owned by Michael Wang are held by Peak Group LLC. Mr. Wang has sole voting and dispositive powers over the shares of common stock owned by Peak Group LLC. |
Our authorized capital stock consists of 140,000,000 shares of common stock, par value $0.0001 per share, and 10,000,000 shares of preferred stock, par value $0.0001 per share. As of the date of this prospectus, there were 50,000,000 shares of common stock outstanding, which are held by ten stockholders. Holders of our common stock are entitled to equal voting rights, consisting of one vote per share on all matters submitted to a stockholder vote. Holders of common stock do not have cumulative voting rights. Therefore, holders of a majority of the shares of common stock voting for the election of directors can elect all of the directors. The presence, in person or by proxy duly authorized, of the holders of a majority of the outstanding shares of stock entitled to vote are necessary to constitute a quorum at any meeting of our stockholders. A vote by the holders of a majority of our outstanding shares is required to effectuate certain fundamental corporate changes such as liquidation, merger or an amendment to our articles of incorporation. In the event of liquidation, dissolution or winding up of our company, either voluntarily or involuntarily, each outstanding share of the common stock is entitled to share equally in our assets, subject to the rights of the holders of any series of preferred stock which may be created by the board of directors.
Holders of our common stock have no pre-emptive rights, no conversion rights and there are no redemption provisions applicable to our common stock. They are entitled to receive dividends when and as declared by our board of directors, out of funds legally available therefore. We have not paid cash dividends in the past and do not expect to pay any within the foreseeable future.
Preferred Stock
Our certificate of incorporation gives our board of directors the power to issue shares of preferred stock in one or more series without stockholder approval. Our board of directors has the discretion to determine the rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock. The purpose of authorizing our board of directors to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing desirable flexibility in connection with possible acquisitions and other corporate purposes, could have the effect of making it more difficult for a third party to acquire, or could discourage a third party from acquiring, a majority of our outstanding voting stock. The rights granted to the holders of a series of preferred stock could restrict payment of dividends on the common stock, dilute the voting power of the common stock, impair the liquidation rights of the holders of the common stock and delay or prevent a change in control without further action by stockholders. We have no present plans to issue any shares of preferred stock.
77
Other Provisions of Our Certificate of Incorporation
Our certificate of incorporation provides that we shall indemnify to the fullest extent permitted by law as it presently exists or may hereafter be amended any person made or threatened to be made a party to an action or proceeding, whether criminal, civil, administrative, or investigative, by reason of the fact that such person, or such person’s testator or intestate, is or was a director or officer of the Corporation or any predecessor of the Corporation, or serves or served at any other enterprise as a director or officer at the request of the Corporation or any predecessor to the Corporation. Any amendment, repeal, or modification of this provision in the certificate of incorporation shall not adversely affect any right or protection hereunder of any person in respect of any act or omission occurring prior to the time of such repeal or modification.
Our certificate of incorporation provides that, to the fullest extent permitted by the Delaware General Corporation Law as it presently exists or may hereafter be amended, a director shall not be personally liable to us or to our stockholders for monetary damages for any breach of fiduciary duty as a director. No amendment to, modification of, or repeal of this provision of the certificate of incorporation shall apply to or have any effect on the liability or alleged liability of any of our directors for or with respect to any acts or omissions of such director occurring prior to such amendment.
Our certificate of incorporation provides that where, in connection with a compromise or arrangement between us and any class of creditors or stockholders, if a majority in number and three-fourth in value of the creditors or stockholders or class of creditors or stockholders, as the case may be, approve a compromise or arrangement which is sanctioned by the court, it is binding on all of the creditors or class of creditors or stockholders or class of stockholders.
Forum Selection
Our by-laws provide that unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have jurisdiction, the federal district court for the District of Delaware) shall be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf; (ii) any action asserting a claim for breach of a fiduciary duty owed by any of our directors, officers, employees, or agents to us or our stockholders; (iii) any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation, or our by-laws; or (iv) any action asserting a claim governed by the internal affairs doctrine; in each case, subject to said court having personal jurisdiction over the indispensable parties named as defendants therein
Our by-laws also provide that unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint for the resolution of any complaint for which such courts have exclusive jurisdiction, including, but not limited to, any complaint asserting a cause of action arising under the Securities Exchange Act of 1934. The forum selection provision does not apply to actions commenced against us under the Securities Act.
Delaware Law Provisions Relating to Business Combinations with Related Persons
We are subject to the provisions of Section 203 of the Delaware General Corporation Law statute which prohibits a publicly-held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the person became an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes mergers, asset sales and other transactions resulting in a financial benefit to the interested stockholder. Subject to certain exceptions, an “interested stockholder” is a person who, together with affiliates and associates, owns, or within the prior three years did own, 15% or more of the corporation’s voting stock.
SEC Policy on Indemnification for Securities Act liabilities
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Transfer Agent
The transfer agent for the common stock is Vstock Transfer, LLC, 18 Lafayette Place, Woodmere, New York 11593, telephone (212) 828-8436.
We have entered into an underwriting agreement with US Tiger Securities, Inc., TFI Securities and Futures Limited and SPDB International Capital Limited (each an “Underwriter” and collectively the “Underwriters”). US Tiger Securities, Inc. is acting as the representative (the “Representative”) of the Underwriters of this offering. The Underwriters have severally but not jointly agreed to purchase, and we have agreed to sell to them, the number of our shares of common stock at the initial public offering price, less the underwriting discounts and commissions, as set forth on the cover page of this prospectus and as indicated below:
| Underwriter | Number of Shares | |||
| US Tiger Securities, Inc. | ||||
| TFI Securities and Futures Limited | ||||
| SPDB International Capital Limited | ||||
| Total | 6,000,000 | |||
78
The underwriting agreement provides that the obligations of the Underwriters to pay for and accept delivery of the shares of common stock offered by this prospectus are subject to the approval of certain legal matters by their counsel and to other conditions. The Underwriters are obligated to take and pay for all of the shares of common stock offered by this prospectus if any such shares are taken. However, the Underwriters are not required to take or pay for the shares covered by the Underwriters’ option to purchase additional shares described below. The underwriting agreement only relates to the underwritten shares being sold by us. The Underwriters do not have any agreement or understanding with respect to the shares being sold by the selling stockholders.
Any offers or sales in the United States will be conducted by broker-dealers registered with the SEC. Each of TFI Securities and Futures Limited and SPDB International Capital Limited is not a broker-dealer registered with the SEC and therefore, to the extent it intends to make any offers or sales of shares in the United States, it will do so only through one or more SEC-registered broker-dealers in compliance with the applicable securities laws and regulations.
We have granted to the Underwriters an option, exercisable for 45 days from the date of this prospectus, to purchase up to 900,000 shares of common stock at the initial public offering price listed on the cover page of this prospectus, less underwriting discounts and commissions. The Underwriters may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with this offering. To the extent the option is exercised, the Underwriters will become obligated, subject to certain conditions, to purchase about the same percentage of the additional shares of common stock as the number listed next to each Underwriter’s name in the preceding table bears to the total number of shares of common stock listed in the preceding table.
The Underwriters will initially offer the shares to the public at the initial public offering price set forth on the cover of this prospectus. If all of the shares are not sold at the initial offering price, the Underwriters may change the offering price and the other selling terms. The securities are offered by the Underwriters as stated herein, subject to receipt and acceptance by them and subject to their right to reject any order in whole or in part.
Discounts and Expenses
The underwriting discounts and commissions are equal to 6% of the initial public offering price set forth on the cover of this prospectus.
The following table shows the per share and total initial public offering price, underwriting discounts and commissions, and proceeds before expenses to us. These amounts are shown assuming both no exercise and full exercise of the Underwriters’ option to purchase up to 900,000 additional shares of common stock to cover over-allotments.
| Per Share | Total Without Exercise of Over- allotment Option | Total With Full Exercise of Over- allotment Option | ||||||||||
| Initial public offering price | $ | |||||||||||
| Underwriting discounts and commissions to be paid by us | $ | |||||||||||
| Proceeds, before expenses, to us | $ | |||||||||||
We will also pay to the Underwriters by deduction from the net proceeds of this offering, a non-accountable expense allowance equal to one percent (1%) of the gross proceeds received by us from the sale of our shares of common stock.
We have agreed to reimburse the Representative for its reasonable out-of-pocket accountable expenses (including the legal fees and other disbursements as disclosed below).
We agreed to pay US Tiger Securities, Inc. a total of a maximum of $450,000 for its out-of-pocket expenses, of which we paid $200,000 to US Tiger Securities, Inc. Any expense deposits in excess of the amount paid by us on account of the Representative’s out-of-pocket expenses will be refunded to us in accordance with FINRA Rule 5110(g)(4).
We estimate that the total expenses of the offering payable by us, excluding the underwriting discounts and commissions, non-accountable expense allowance and reimbursement of the Representative’s out-of-pocket expenses, will be approximately $1.5 million.
We intend to apply to list our shares of common stock on the Nasdaq Capital Market under the symbol “ISPR.” There is no assurance that such application will be approved, and if our application is not approved, this offering will not be completed.
For a period of two years from the closing of this offering, we will (i) furnish to the Underwriters and distribute to our security holders an annual report and annual financial statements; (ii) furnish to the Underwriters with copies of all filings with the SEC; and (iii) furnish to the Underwriters with special security position reports and tracking reports as prepared by Depository Trust Company for a period of two years from the closing of this offering.
79
Underwriters’ Warrants
In addition, we have agreed to issue the warrants to the Underwriters to purchase a number of shares of common stock equal to 5% of the total number of shares of common stock sold in this offering. The Underwriters’ Warrants shall have an exercise price equal to 125% of the initial public offering price of the shares of common stock sold in this offering. The Underwriters’ Warrants may be purchased in cash or via cashless exercise, will be exercisable for five years from the effective date of the registration statement of which this prospectus forms a part and will terminate on the fifth anniversary of the effective date of the registration statement of which this prospectus forms a part. The Underwriters’ Warrants and the underlying shares will be deemed compensation by FINRA, and therefore will be subject to FINRA Rule 5110(e)(1). In accordance with FINRA Rule 5110(e)(1), and except as otherwise permitted by FINRA rules, neither the Underwriters’ Warrants nor any of our shares issued upon exercise of the Underwriters’ Warrants may be sold, transferred, assigned, pledged or hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of such securities by any person, for a period of 180 days immediately following the effective date of the registration statement of which this prospectus forms a part. In addition, although the Underwriters’ Warrants and the underlying shares of common stock will be registered in the registration statement of which this prospectus forms a part, we have also agreed that the Underwriters’ Warrants will provide for registration rights in certain cases. These registration rights apply to all of the securities directly and indirectly issuable upon exercise of the Underwriters’ Warrants. The demand registration right provided consists of up to two occasions and will not be greater than five years from the effective date of the offering in compliance with FINRA Rule 5110(g)(8)(C). On the first occasion, we will bear all fees and expenses attendant to registering the shares of common stock issuable upon exercise of the Underwriters’ Warrants, other than underwriting or discounts and commissions or brokerage or other selling expenses incurred and payable by the holders of the Underwriters’ Warrants. On the second occasion, if any, the holders of the Underwriters’ Warrants shall bear all fees and expenses attendant to registering the shares of common stock issuable upon exercise of the Underwriters’ Warrants. The piggyback registration right provided will not be greater than five years from the effective date of the offering in compliance with FINRA Rule 5110(g)(8)(D). We will bear all fees and expenses attendant to registering the shares of common stock issuable upon exercise of the piggyback registration right of the Underwriters’ Warrants, other than underwriting or discounts and commissions or brokerage or other selling expenses incurred and payable by the holders of the Underwriters’ Warrants. The exercise price and number of shares of common stock issuable upon exercise of the Underwriters’ Warrants may be adjusted in certain circumstances, including in the event of a share dividend, extraordinary cash dividend or our recapitalization, reorganization, merger or consolidation.
Indemnification
We have agreed to indemnify the Underwriters against certain liabilities, including liabilities under the Securities Act and liabilities arising from breaches of representations and warranties contained in the underwriting agreement, or to contribute to payments that the Underwriters may be required to make in respect of those liabilities.
Lock-Up Agreements
Our officers, directors and 5% stockholders have agreed, subject to certain exceptions, to a “lock-up” for a period of six months after the date of this prospectus with respect to the shares of common stock that they beneficially own, including the issuance of shares upon the exercise of convertible securities and options that are currently outstanding or which may be issued. This means that, for a period of six months after the date of this prospectus, such persons may not offer, sell, pledge or otherwise transfer or dispose of, directly or indirectly, these securities without the prior written consent of the Representative. In addition, we have agreed that, during the three month period commencing on the closing date of this offering, we (and any successor) will not issue any securities, enter into swap or similar agreement, file a registration statement or publicly disclose any intention to do any of the foregoing; provided, that this restriction does not apply to the grant of equity-based incentives under an equity-based incentive plan, filing a registration statement on Form S-8, the issuance of securities in connection with an acquisition, and a registration statement in connection with an acquisition.
The Underwriters have no present intention to waive or shorten the lock-up period; however, the terms of the lock-up agreements may be waived at its discretion. In determining whether to waive the terms of the lock-up agreements, the Underwriters may base their decision on their assessment of the relative strengths of the securities markets and companies similar to ours in general, and the trading pattern of, and demand for, our securities in general.
80
Pricing of the Offering
Prior to this offering, there has been no public market for our shares of common stock. The initial public offering price of the shares has been negotiated between us and the Representative. Among the factors considered in determining the initial public offering price of the shares, in addition to the prevailing market conditions, are our historical performance, estimates of our business potential and earnings prospects, an assessment of our management and the consideration of the above factors in relation to market valuation of companies in related businesses.
No Sales of Similar Securities
We have agreed not to offer, pledge, announce the intention to offer, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock or enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of our shares of common stock, whether any such transaction is to be settled by delivery of shares of common stock or such other securities, in cash or otherwise, without the prior written consent of the Underwriters, for a period of three (3) months from the effective date of the registration statement of which this prospectus forms a part. This provision does not affect our right to file an S-8 registration statement for any equity incentive plans we may adopt.
Electronic Offer, Sale and Distribution of Securities
A prospectus in electronic format may be made available on the websites maintained by the Underwriters or selling group members, if any, participating in this offering and the Underwriters may distribute prospectuses electronically. The Underwriters may agree to allocate a number of shares of common stock to selling group members for sale to their online brokerage account holders. The shares of common stock to be sold pursuant to internet distributions will be allocated on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or the Underwriters, and should not be relied upon by investors.
Price Stabilization, Short Positions and Penalty Bids
In connection with this offering, the Underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our shares of common stock. Specifically, the Underwriters may sell more shares than they are obligated to purchase under the underwriting agreement, creating a short position. A short sale is covered if the short position is no greater than the number of shares available for purchase by the Underwriters under option to purchase additional shares. The Underwriters can close out a covered short sale by exercising the option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to close out a covered short sale, the Underwriters will consider, among other things, the open market price of shares compared to the price available under the option to purchase additional shares. The Underwriters may also sell shares in excess of the option to purchase additional shares, creating a naked short position. The Underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the Underwriters are concerned that there may be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering.
The Underwriters may also impose a penalty bid. This occurs when a particular Underwriter or dealer repays selling concessions allowed to it for distributing our shares of common stock in this offering because such Underwriter repurchases those shares in stabilizing or short covering transactions.
Finally, the Underwriters may bid for, and purchase, our shares of common stock in market making transactions, including “passive” market making transactions as described below.
These activities may stabilize or maintain the market price of our shares of common stock at a price that is higher than the price that might otherwise exist in the absence of these activities. The Underwriters are not required to engage in these activities, and may discontinue any of these activities at any time without notice. These transactions may be effected on the Nasdaq Global Market, in the over-the-counter market, or otherwise.
81
Passive Market Making
In connection with this offering, the Underwriters may engage in passive market making transactions in our shares of common stock on the Nasdaq Global Market in accordance with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement of offers or sales of the shares and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, then that bid must then be lowered when specified purchase limits are exceeded.
Potential Conflicts of Interest
The Underwriters and their affiliates may, from time to time, engage in transactions with and perform services for us in the ordinary course of their business for which they may receive customary fees and reimbursement of expenses. In the ordinary course of their various business activities, the Underwriters, their affiliates, directors, officers and employees may at any time purchase, sell, make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own accounts and for the accounts of their customers and such investment and securities activities may involve securities and/or instruments of our Company and our affiliates. The Underwriters and their affiliates, directors, officers and employees may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Other Relationships
The Underwriters are full service financial institutions engaged in various activities, which may include the sales and trading of securities, commercial and investment banking, advisory, investment management, investment research, principal investment, hedging, market making, financing, brokerage and other financial and non-financial activities and services. The Underwriters may in the future perform a variety of such activities and services for us and for persons or entities with relationships with us for which they received or will receive customary fees, commissions and expenses.
Selling Restrictions outside the United States
Notice to Prospective Investors in Australia
This prospectus is not a product disclosure statement, prospectus or other type of disclosure document for the purposes of Corporations Act 2001 (Commonwealth of Australia) (the “Act”) and does not purport to include the information required of a product disclosure statement, prospectus or other disclosure document under Chapter 6D.2 of the Act. No product disclosure statement, prospectus, disclosure document, offering material or advertisement in relation to the offer of the shares of common stock has been or will be lodged with the Australian Securities and Investments Commission or the Australian Securities Exchange.
Accordingly, (1) the offer of the shares of common stock under this prospectus may only be made to persons: (i) to whom it is lawful to offer the shares of common stock without disclosure to investors under Chapter 6D.2 of the Act under one or more exemptions set out in Section 708 of the Act, and (ii) who are “wholesale clients” as that term is defined in section 761G of the Act, (2) this prospectus may only be made available in Australia to persons as set forth in clause (1) above, and (3) by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (1) above, and the offeree agrees not to sell or offer for sale any of the shares of common stock sold to the offeree within 12 months after their issue except as otherwise permitted under the Act.
Notice to Prospective Investors in Canada
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
82
Pursuant to section 3A.3 (or, in the case of securities issued or guaranteed by the government of a non-Canadian jurisdiction, section 3A.4) of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the Underwriter is not required to comply with the disclosure requirements of NI 33-105 regarding Underwriter conflicts of interest in connection with this offering.
The shares of common stock may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the shares of common stock must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Notice to Prospective Investors in the United Kingdom
This prospectus is only being distributed to and is only directed at persons in the United Kingdom that are qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive that are also (i) to investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 within, and/or (ii) high net worth entities, and other persons to whom it may lawfully be communicated, falling with Article 49(2)(a) to (d) (all such persons together being referred to as “relevant persons”).
This prospectus and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients to any other persons in the United Kingdom. Any person in the United Kingdom who is not a relevant person should not act or rely on this prospectus or any of its contents.
Notice to Prospective Investors in Malaysia
The shares of common stock have not been and may not be approved by the securities commission Malaysia, or SC, and this document has not been and will not be registered as a prospectus with the SC under the Malaysian capital markets and services act of 2007, or CMSA. Accordingly, no securities or offer for subscription or purchase of securities or invitation to subscribe for or purchase securities are being made to any person in or from within Malaysia under this document except to persons falling within any of paragraphs 2(g)(i) to (xi) of schedule 5 of the CMSA and distributed only by a holder of a capital markets services license who carries on the business of dealing in securities and subject to the issuer having lodged this prospectus with the SC within seven days from the date of the distribution of this prospectus in Malaysia. The distribution in Malaysia of this document is subject to Malaysian laws. Save as aforementioned, no action has been taken in Malaysia under its securities laws in respect of this document. This document does not constitute and may not be used for the purpose of a public offering or an issue, offer for subscription or purchase, invitation to subscribe for or purchase any securities requiring the approval of the SC or the registration of a prospectus with the SC under the CMSA.
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares of common stock may not be circulated or distributed, nor may the shares of common stock be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”), (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to compliance with conditions set forth in the SFA.
Notice to Prospective Investors in the People’s Republic of China
This prospectus may not be circulated or distributed in China and the shares of common stock may not be offered or sold, and will not offer or sell to any person for re-offering or resale directly or indirectly to any resident of China except pursuant to applicable laws, rules and regulations of China. For the purpose of this paragraph only, China does not include Taiwan and the special administrative regions of Hong Kong and Macau.
83
Notice to Prospective Investors in Hong Kong
The shares of common stock may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap. 32, Laws of Hong Kong), (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap. 32, Laws of Hong Kong) and no advertisement, invitation or document relating to our shares of common stock be issued or may be in possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to our shares of common stock which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Notice to Prospective Investors in the Republic of China (Taiwan)
The shares of common stock have not been and will not be registered with the Financial Supervisory Commission of Taiwan, pursuant to relevant securities laws and regulations and may not be offered or sold in Taiwan through a public offering or in any manner which would constitute an offer within the meaning of the Securities and Exchange Act of Taiwan or would otherwise require registration with or the approval of the Financial Supervisory Commission of Taiwan.
Notice to Prospective Investors in Indonesia:
This prospectus does not, and is not intended to, constitute a public offering in Indonesia under Law Number 8 of 1995 regarding Capital Market. This prospectus may not be distributed in the Republic of Indonesia and the shares of common stock may not be offered or sold in the Republic of Indonesia or to Indonesian citizens wherever they are domiciled, or to Indonesia residents, in a manner which constitutes a public offering under the laws of the Republic of Indonesia.
Notice to Prospective Investors in Vietnam:
This offering of shares of common stock has not been and will not be registered with the State Securities Commission of Vietnam under the Law on Securities of Vietnam and its guiding decrees and circulars. The shares of common stock will not be offered or sold in Vietnam through a public offering and will not be offered or sold to Vietnamese persons other than those who are licensed to invest in offshore securities under the Law on Investment of Vietnam.
Notice to Prospective Investors in Thailand:
This prospectus does not, and is not intended to, constitute a public offering in Thailand. The shares of common stock may not be offered or sold to persons in Thailand, unless such offering is made under the exemptions from approval and filing requirements under applicable laws, or under circumstances which do not constitute an offer for sale of the shares to the public for the purposes of the Securities and Exchange Act of 1992 of Thailand, nor require approval from the Office of the Securities and Exchange Commission of Thailand.
Notice to Prospective Investors in Korea:
The shares of common stock may not be offered, sold and delivered directly or indirectly, or offered or sold to any person for reoffering or resale, directly or indirectly, in Korea or to any resident of Korea except pursuant to the applicable laws and regulations of Korea, including the Korea Securities and Exchange Act and the Foreign Exchange Transaction Law and the decrees and regulations thereunder. The shares of common stock have not been registered with the Financial Services Commission of Korea for public offering in Korea. Furthermore, the shares of common stock may not be resold to Korean residents unless the purchaser of the shares of common stock complies with all applicable regulatory requirements (including but not limited to government approval requirements under the Foreign Exchange Transaction Law and its subordinate decrees and regulations) in connection with the purchase of the shares of common stock.
84
Notice to Prospective Investors in Japan:
The shares of common stock offered in this prospectus have not been and will not be registered under the Financial Instruments and Exchange Law of Japan. The shares of common stock have not been offered or sold and will not be offered or sold, directly or indirectly, in Japan or to or for the account of any resident of Japan (including any corporation or other entity organized under the laws of Japan), except (1) pursuant to an exemption from the registration requirements of the Financial Instruments and Exchange Law and (2) in compliance with any other applicable requirements of Japanese law.
Notice to Prospective Investors in New Zealand:
This document has not been registered, filed with, or approved by any New Zealand regulatory authority under the Financial Markets Conduct Act 2013 (New Zealand) (“FMCA”). This document is not a product disclosure statement under New Zealand law and is not required to, and may not, contain all the information that a product disclosure statement under New Zealand law is required to contain. The Securities are not being offered or sold in New Zealand (or allotted with a view to being offered for sale in New Zealand) other than to a person who is a “wholesale investor” within the meaning of clause 3(2) of Schedule 1 of the FMCA – that is, a person who:
| ● | is an “investment business” within the meaning of clause 37 of Schedule 1 of the FMCA; |
| ● | meets the “investment activity criteria” specified in clause 38 of Schedule 1 of the FMCA; |
| ● | is “large” within the meaning of clause 39 of Schedule 1 of the FMCA; or |
| ● | is a “government agency” within the meaning of clause 40 of Schedule 1 of the FMCA. |
The Securities are not being offered or sold to retail investors in New Zealand.
Notice to Prospective Investors in the European Economic Area:
The units are not intended to be offered, sold or otherwise made available to and should not be offered, sold or otherwise made available to any retail investor in the European Economic Area (“EEA”). For these purposes, a retail investor means a person who is one (or more) of: (i) a retail client as defined in point (11) of Article 4(1) of Directive 2014/65/EU (as amended, “MiFID II”); or (ii) a customer within the meaning of Directive (EU) 2016/97 (as amended, the “Insurance Distribution Directive”), where that customer would not qualify as a professional client as defined in point (10) of Article 4(1) of MiFID II; or (iii) not a qualified investor as defined in Regulation (EU) 2017/1129 (the “Prospectus Regulation”). Consequently, no key information document required by Regulation (EU) No 1286/2014 (as amended, the “PRIIPs Regulation”) for offering or selling the units or otherwise making them available to retail investors in the EEA has been prepared and therefore offering or selling the units or otherwise making them available to any retail investor in the EEA may be unlawful under the PRIIPs Regulation.
Notice to Prospective Investors in the Cayman Islands:
No offer or invitation, whether directly or indirectly, may be made to the public in the Cayman Islands to subscribe for our securities.
85
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there was no established public trading market for our common stock. We cannot assure you that a liquid trading market for our common stock will develop on Nasdaq or be sustained after this offering. Future sales of substantial amounts of common stock in the public market, or the perception that such sales may occur, could adversely affect the market price of our common stock. Further, since a large number of our common stock will not be available for sale shortly after this offering because of the contractual and legal restrictions on resale described below, sales of substantial amounts of our common stock in the public market after these restrictions lapse, or the perception that such sales may occur, could adversely affect the prevailing market price and our ability to raise equity capital in the future.
Upon completion of this offering, we will have 56,000,000 shares of common stock outstanding (56,900,000 shares if the Underwriters’ overallotment option is exercised in full. All of the shares of common stock sold in this offering will be freely transferable by persons other than our “affiliates” without restriction or further registration under the Securities Act. Sales of substantial amounts of our shares of common stock in the public market could adversely affect prevailing market prices of our shares of common stock. Prior to this offering, there has been no public market for our shares of common stock, and while we intend to submit application for the shares of common stock to be listed on Nasdaq, we cannot assure you that a regular trading market will develop in the shares of common stock.
Lock-Up Agreements
Our officers, directors and principal stockholders (defined as owners of 5% or more of our common stock) have agreed, subject to some exceptions, not to transfer or dispose of, directly or indirectly, any of our common stock, or any securities convertible into or exchangeable or exercisable for our common stock, for a period of six months after the date of this prospectus without the consent of the Representative. After the expiration of the six month period, the common stock held by our directors, executive officers and 5% stockholders may be sold subject to the restrictions under Rule 144 under the Securities Act or by means of registered public offerings.
Rule 144
All of our common stock outstanding prior to this offering are “restricted shares” as that term is defined in Rule 144 under the Securities Act and may be sold publicly in the United States only if they are subject to an effective registration statement under the Securities Act or pursuant to an exemption from the registration requirements. Under Rule 144 as currently in effect, a person who has beneficially owned our restricted shares for at least six months is generally entitled to sell the restricted securities without registration under the Securities Act beginning 90 days after the date of this prospectus, subject to certain additional restrictions.
Our affiliates may sell within any three-month period a number of restricted shares that does not exceed the greater of the following:
| ● | 1% of the then outstanding shares of common stock of the same class, which will equal approximately 560,000 shares of common stock immediately after this offering assuming the over-allotment option is not exercised and 569,000 shares of common stock assuming the over-allotment option is exercised in full; or |
| ● | the average weekly trading volume of our shares of common stock on Nasdaq during the four calendar weeks preceding the date on which notice of the sale is filed with the SEC. |
Affiliates who sell restricted securities under Rule 144 may not solicit orders or arrange for the solicitation of orders, and they are also subject to notice requirements and the availability of current public information about us.
Persons who are not our affiliates are only subject to one of these additional restrictions, the requirement of the availability of current public information about us, and this additional restriction does not apply if they have beneficially owned our restricted shares for more than one year.
86
The validity of the common stock offered by us in this offering will be passed upon for us by Ellenoff Grossman & Schole LLP, New York, New York. The Underwriters are represented by Sidley Austin with respect to U.S. laws, and by Tian Yuan Law Firm with respect to PRC Laws.
The consolidated financial statements as of June 30, 2021 and 2022 and for each of the years then ended included in this prospectus have been so included in reliance on the report of MSPC Certified Public Accountants and Advisors, A Professional Corporation, given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
The SEC maintains an Internet site at www.sec.gov that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC.
We have filed a registration statement, including relevant exhibits, with the SEC on Form S-1 under the Securities Act with respect to the common stock to be sold in this offering. This prospectus, which constitutes a part of the registration statement on Form S-1, does not contain all of the information contained in the registration statement. You should read our registration statements and their exhibits and schedules for further information with respect to us and our common stock.
As a result of this offering, we will become subject to information requirements of the Exchange Act. We will fulfill our obligations with respect to such requirements by filing periodic reports and other information with the SEC. We intend to furnish our stockholders with annual reports containing financial statements certified by an independent public accounting firm.
87
ISPIRE TECHNOLOGY INC.
Index to Consolidated Financial Statements
F-1
UNAUDITED CONDENSED CONSOLIDATED BALANCE SHEETS
| As of June 30, | As of December 31, | |||||||
| 2022 | 2022 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 74,480,651 | $ | 84,267,154 | ||||
| Accounts receivable | 8,260,574 | 18,044,372 | ||||||
| Inventories, net | 14,580,557 | 20,305,187 | ||||||
| Prepaid expenses and other current assets | 192,499 | 191,366 | ||||||
| Due from related parties | 1,934,855 | - | ||||||
| Total current assets | 99,449,136 | 122,808,079 | ||||||
| Other assets: | ||||||||
| Property, plant and equipment, net | 114,025 | 578,838 | ||||||
| Rental deposit | 876,100 | 721,497 | ||||||
| Right-of-use assets | 295,804 | 3,459,714 | ||||||
| Intangible assets | - | 73,487,283 | ||||||
| Total other assets | 1,285,929 | 78,247,332 | ||||||
| Total assets | $ | 100,735,065 | $ | 201,055,411 | ||||
| Liabilities and stockholders’ equity | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | 290,541 | $ | 398,672 | ||||
| Accounts payable – related party | 41,982,373 | 67,323,067 | ||||||
| Contract liabilities | 1,672,051 | 1,003,735 | ||||||
| Dividends payable | 3,362,639 | 3,384,678 | ||||||
| Accrued liabilities and other payables | 159,296 | 297,443 | ||||||
| Due to related parties | 40,672,768 | 40,499,550 | ||||||
| Income tax payable | 481,113 | 1,316,273 | ||||||
| Lease liabilities | 347,541 | 784,724 | ||||||
| Total current liabilities | 88,968,322 | 115,008,142 | ||||||
| Other liabilities: | ||||||||
| Lease liabilities | - | 2,829,102 | ||||||
| Total liabilities | $ | 88,968,322 | $ | 117,837,244 | ||||
| Stockholders’ equity: | ||||||||
| Common stock, par value $0.0001 per share; 140,000,000 shares authorized; 50,000,000 shares issued and outstanding as of June 30, 2022 and December 31, 2022 | 5,000 | 5,000 | ||||||
| Preferred stock, par value $0.0001 per share, 10,000,000 shares authorized, no shares issued at June 30, 2022 and December 31, 2022 | - | - | ||||||
| Capital contribution | - | 74,259,915 | ||||||
| Accumulated other comprehensive loss | (184,664 | ) | (42,234 | ) | ||||
| Retained earnings | 11,946,407 | 8,995,486 | ||||||
| Total stockholders’ equity | 11,766,743 | 83,218,167 | ||||||
| Total liabilities and stockholders’ equity | $ | 100,735,065 | $ | 201,055,411 | ||||
See notes to unaudited condensed consolidated financial statements.
F-2
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME
| Six Months ended December 31, | ||||||||
| 2021 | 2022 | |||||||
| Revenue | $ | 47,233,358 | $ | 58,840,449 | ||||
| Cost of revenue | 39,921,534 | 48,909,768 | ||||||
| Gross profit | 7,311,824 | 9,930,681 | ||||||
| Operating expenses: | ||||||||
| Sales and marketing expenses | 2,456,159 | 2,407,528 | ||||||
| General and administrative expenses | 3,071,881 | 9,200,809 | ||||||
| Total operating expenses | 5,528,040 | 11,608,337 | ||||||
| Income(loss) from operations | 1,783,784 | (1,677,656 | ) | |||||
| Other income(expense): | ||||||||
| Interest income | 1,067 | 76,811 | ||||||
| Exchange gain(loss), net | 68,482 | (477,582 | ) | |||||
| Other income(expense), net | 54,941 | (40,487 | ) | |||||
| Total other income(expense), net | 124,490 | (441,258 | ) | |||||
| Income(loss) before income taxes | 1,908,274 | (2,118,914 | ) | |||||
| Income taxes - current | (629,593 | ) | (832,007 | ) | ||||
| Net income(loss) | $ | 1,278,681 | $ | (2,950,921 | ) | |||
| Other comprehensive loss | ||||||||
| Foreign currency translation adjustments | (9,078 | ) | 142,430 | |||||
| Comprehensive income(loss) | 1,269,603 | (2,808,491 | ) | |||||
| Net income (loss) per share | ||||||||
| Basic and diluted | $ | 0.03 | $ | (0.06 | ) | |||
| Weighted average shares outstanding: | ||||||||
| Basic and diluted | 50,000,000 | 50,000,000 | ||||||
See notes to unaudited condensed consolidated financial statements.
F-3
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
| Common Stock | Preferred Stock | Accumulated Other | ||||||||||||||||||||||||||||||
| Number of | Number of | Retained | Capital | Comprehensive | Stockholders’ | |||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Earnings | Contribution | Loss | Equity | |||||||||||||||||||||||||
| Balance, June 30, 2021 | 50,000,000 | 5,000 | - | - | 13,820,560 | - | (67,579 | ) | 13,757,981 | |||||||||||||||||||||||
| Net income | - | - | - | - | 1,278,681 | - | - | 1,278,681 | ||||||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | (9,078 | ) | (9,078 | ) | |||||||||||||||||||||||
| Balance, December 31, 2022 | 50,000,000 | $ | 5,000 | - | $ | - | 15,099,241 | $ | - | $ | (76,657 | ) | $ | 15,027,584 | ||||||||||||||||||
| Balance, June 30, 2022 | 50,000,000 | 5,000 | - | - | 11,946,407 | - | (184,664 | ) | 11,766,743 | |||||||||||||||||||||||
| Net loss | - | - | - | - | (2,950,921 | ) | - | - | (2,950,921 | ) | ||||||||||||||||||||||
| Transfer of intangible assets | 74,259,915 | 74,259,915 | ||||||||||||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | - | 142,430 | 142,430 | ||||||||||||||||||||||||
| Balance, December 31, 2022 | 50,000,000 | $ | 5,000 | - | $ | - | 8,995,486 | $ | 74,259,915 | $ | (42,234 | ) | $ | 83,218,167 | ||||||||||||||||||
See notes to unaudited condensed consolidated financial statements.
F-4
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
| Six Months ended December 31, | ||||||||
| 2021 | 2022 | |||||||
| Cash flows (used in) from operating activities: | ||||||||
| Net income(loss): | $ | 1,278,681 | $ | (2,950,921 | ) | |||
| Adjustments to reconcile net income from operations to net cash provided by operating activities: | ||||||||
| Depreciation and amortization | 981 | 786,292 | ||||||
| Depreciation of right-of-use assets | 148,142 | 552,058 | ||||||
| Accounts receivable impairment | - | 1,029,655 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (2,858,220 | ) | (10,818,728 | ) | ||||
| Inventories | (5,908,727 | ) | (5,724,630 | ) | ||||
| Prepaid expenses and other current assets | 442,619 | 134,307 | ||||||
| Accounts payable | (609,012 | ) | 25,487,786 | |||||
| Contract liabilities | (1,118,901 | ) | (665,242 | ) | ||||
| Accrued liabilities and other payables | 259,198 | 159,576 | ||||||
| Income tax payable | 41,796 | 835,160 | ||||||
| Net cash (used in)provided by operating activities | $ | (8,323,443 | ) | $ | 8,825,313 | |||
| Cash flows from investing activities: | ||||||||
| Purchase of property, plant and equipment | (47,345 | ) | (478,473 | ) | ||||
| Net cash used in investing activities | $ | (47,345 | ) | $ | (478,473 | ) | ||
| Cash flows from financing activities: | ||||||||
| Payment made for dividends | (449,026 | ) | - | |||||
| Advances from related parties | - | 1,934,855 | ||||||
| Repayment of advances from related parties | (1,804,786 | ) | (45,509 | ) | ||||
| Principal portion of lease payment | (140,619 | ) | (449,683 | ) | ||||
| Net cash (used in)provided by financing activities | $ | (2,394,431 | ) | $ | 1,439,663 | |||
| Net (decrease) increase in cash and cash equivalents | (10,765,219 | ) | 9,786,503 | |||||
| Cash and cash equivalents - beginning of period | 85,248,997 | 74,480,651 | ||||||
| Cash and cash equivalents - end of period | $ | 74,483,778 | $ | 84,267,154 | ||||
See notes to unaudited condensed consolidated financial statements.
F-5
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. ORGANIZATION AND PRINCIPAL ACTIVITIES
Ispire Technology Inc. (the “Company”) was incorporated under the laws of the State of Delaware on June 13, 2022. Through its subsidiaries, the Company is engaged in the research and development, design, commercialization, sales, marketing and distribution of branded e-cigarettes and cannabis vaping products.
Ispire owns a 100% equity interest in Ispire International Limited, a business company incorporated under the laws of the British Virgin Islands (“BVI”) (“Ispire International”) on July 6, 2022.
Prior to July 29, 2022, all of the equity of Aspire North America LLC, a California limited liability company (“Aspire North America”), was owned by Aspire Global Inc. (“Aspire Global”), and all of the equity of Aspire Science and Technology Limited, a Hong Kong corporation (“Aspire Science”), was owned by Aspire Global Holdings Limited (“Aspire Holdings”), a wholly-owned subsidiary of Aspire Global.
Aspire Global and the Company are related parties since the same individual is the chief executive officer of both companies, the chief executive officer and his wife are directors of both companies, and own 66.5% and 5.0%, respectively, of the equity of both Aspire Global and the Company. At the time of transfer of the equity in Aspire North America and Aspire Science, the Company had the same stockholders as Aspire Global and the Company’s stockholders held the same percentage interest in the Company as they had in Aspire Global. Because the transfer of the equity in Aspire North America and Aspire Science is a transfer between related parties, the historical financial information of the subsidiaries is carried forward as the historical financial information of the Company and the 50,000,000 shares that were issued at or about the time of the Company’s organization are treated as being outstanding on July 1, 2020.
On July 29, 2022:
| ● | Aspire Global transferred 100% of the equity interest in Aspire North America to the Company |
| ● | Aspire Holdings transferred 100% of the equity of Aspire Science to Ispire International. |
The following table sets forth information concerning the Company and its subsidiaries as of June 30, 2022 and December 31, 2022:
| Name of Entity | Date of Organization | Place of Organization | % of Ownership | Principal Activities | ||||
| Ispire Technology Inc. | June 13, 2022 | Delaware | Parent Company | Holding Company | ||||
| Ispire International | July 6, 2022 | BVI | 100% | Holding Company | ||||
| Aspire North America | February 22, 2020 | California | 100% | Sales and Marketing | ||||
| Aspire Science | December 9, 2016 | Hong Kong | 100% | Sales and Marketing |
Ispire is a holding company and does not engage in any active operations. Its business is conducted by its two operating subsidiaries, Aspire North America, which is engaged in the development, marketing and sales of cannabis vapor products, which were introduced in mid 2020, and Aspire Science, which is engaged in the development, marketing and sales of tobacco vaping products.
In October 2022, the directors and stockholders of the Company approved the 2022 Equity Incentive Plan (the “Plan”) pursuant to which up to 15,000,000 shares of common stock may be issued pursuant to options or restricted stock grants. The Plan will be administered by the Compensation Committee. Awards under the Plan may be granted to officers, directors, employees and those consultants who qualify as a consultant or advisor under the instructions to Form S-8. The Compensation Committee has broad discretion in making awards; provided that any options shall be exercisable at the fair market value on the date of grant. No awards have been granted since the Plan was approved.
F-6
Impact of COVID-19
In December 2019, coronavirus disease 2019 (COVID-19) was first reported to have surfaced in Wuhan, China. During 2020, the disease spread to many parts of the world. The epidemic has resulted in quarantines, travel restrictions, and the temporary closure of stores and facilities in much of the world.
Measures taken by various governments to contain the virus have affected economic activity in all countries where the consumers of our product live. We took a number of measures to monitor and mitigate the effects of COVID-19, such as safety and health measures for our personnel, such as social distancing, in accordance with government policies and advice, securing the supply of materials that are essential to our production process. We will continue to follow the various government policies and advice and, in parallel, we may take further actions that we determine are in the best interests of our employees, customers, and business relationships.
The Company’s products are manufactured in China by Shenzhen Yi Jia Technology Co., Limited, a Chinese company (“Shenzhen Yi Jia”), which is 95% owned by the Company’s chief executive officer and controlling stockholder. From the middle of January 2020, the start of the Chinese New Year holiday, until the end of April 2020, its production was slowed due to the COVID-19 pandemic in China. This slowdown in production resulted in a near-total stoppage of our supplier’s business during this nearly four-month period and our sales activities were substantially reduced. In addition, since our products are generally sold in stores such as grocery stores, convenience stores and tobacco stores, to the extent that either the stores are closed as a result of government actions or consumers are reluctant to go shopping because of the COVID-19 pandemic, retail sales of the Company’s products suffered. Following the temporary slowdown in production in first half year of 2020, Shenzhen Yi Jia has recovered its production capacity, and the Company has not experienced significant supply chain constraints as a result of the pandemic.
From late 2020 to the middle of 2021, COVID-19 vaccination program had been greatly promoted around the globe, however several types of COVID-19 variants emerged in different parts of the world. The Company’s sales in Europe and the United States continued to be affected by government actions relating to COVID-19 and COVID-19 variants, while the production was slightly reduced by the strict measures taken by the Government of China to address the small COVID-19 outbreaks in Guangdong province. To the extent that China’s zero-COVID policy results in closing or significantly reducing the operations of Shenzhen Yi Jia or any of its suppliers, our business will be impacted since the Company presently relies on Shenzhen Yi Jia for its products.
The extent to which COVID-19 impacts the Company’s operations on an ongoing basis is highly uncertain. It will depend on various factors including the duration and severity of the outbreak, new information which may emerge concerning the severity of the coronavirus or any variants and the actions to contain the coronavirus or treat its impact, among others.
Supply Chain Risks
One of effects of the COVID-19 has been delays resulting from supply chain issues, which relate to the difficulty that companies have in having their products manufactured, shipped to the country of destination, and delivered from the port of entry to the customer’s location. As a result of the COVID-19 pandemic, during 2021 and early 2022, there were fewer longshoremen unloading ships and fewer truckers to deliver the products to market, which resulted in significant delays in the delivery of products to markets. To the extent that products are shipped by sea, there are additional risks resulting from ports not being able to unload ships promptly, causing delays in getting into port, including potential damage from seawater and fire, product degradation and the possibility of containers being destroyed, damaged or falling off the ship into the water. The inability to deliver products to the ultimate vendor could impair the Company’s ability to generate revenue from its products. As the port delays have significantly decreased, we do not believe that the supply chain issues that affected our operations are currently affecting us. We cannot assure you that delays will not affect our business in the future.
F-7
In 2021, Shenzhen Yi Jia suffered a chip shortage resulting in a slowdown in delivery of its products to the Company from April to August 2021. To secure the supply of chips, Shenzhen Yi Jia changed the payment terms to chip suppliers from 30 days after delivery in the past to prepayment, and it engaged two new chip suppliers. Since September 2021, Shenzhen Yi Jia has obtained a supply of chips to meet its production needs and the chip shortage no longer affects its production. During the six months ended December 31, 2022, a slowdown in the delivery of components to Shenzhen Yi Jia resulting from supply chain slowdowns as a result of the effects of mainland China’s zero-COVID policy resulted in an increase in cost of revenue during the period. We cannot assure you that we will not suffer from a chip shortage or that the effects of China’s COVID policy will not affect Shenzhen Yi Jia’s ability or the ability of its suppliers to delivery products in a timely manner.
E-cigarette regulation
Regulation regarding e-cigarette varies across countries, from no regulation to a total ban. The legal status of e-cigarette is currently pending in many countries. But as e-cigarettes have become more and more popular recently, many countries are considering imposing more stringent law and regulations to regulate this market. Changes in existing law and regulations and the imposition of new laws, regulation in countries and regions that our major customers located in may adversely affect the Company’s business.
The Federal Food, Drug, and Cosmetic Act requires all Electronic Nicotine Delivery Systems (“ENDS”) product manufacturers that market products in the United States to submit Premarket Tobacco Product Applications (“PMTAs”) to the FDA. For ENDS products that were on the U.S. market on August 8, 2016, a PMTA was required to be submitted to the FDA by September 9, 2020; for ENDS products that were not on the U.S. market on August 8, 2016, a premarket authorization issued in response to a PMTA is required for the product to enter the U.S. market. The Company has submitted a PMTA filing for one ENDS product, and, under apparent FDA policies, the agency will not enforce the premarket review requirements for that product pending review of its PMTA. However, even with submission of the PMTA application, the FDA may reject the Company’s application and may prevent the Company’s ENDS products from being sold in the U.S., which will adversely affect the Company’s business.
Amendments to the Prevent All Cigarette Trafficking (“PACT”) Act, which became law in 2021, extend the PACT Act to include e-cigarette and all vaping products, and the amendments place significant burdens on sellers of vaping products in the United States which may make it difficult to operate profitably in the United States. Because of tighter government regulations, the Company will stop marketing tobacco products in the United States, as the volume of sales from the one tobacco vaping product which the Company may sell in the United States does not justify the marketing and regulatory costs involved.
In the United States, cannabis vaping products are governed by state laws, which vary from state to state. Most states do not permit the adult recreational use of cannabis, and no states permit the sale of recreational cannabis products to minors. As a result of the reduced revenue to states resulting from the effects of the COVID 19 pandemic, states may seek to raise revenue by permitting and taxing the use of cannabis products. The Company cannot predict what action states will take or the nature and amount of taxes they may impose. However, the extent the PACT Act applies to cannabis products that aerosolize liquids, it may be more difficult to sell our products in states that permit the sale of cannabis.
However, cannabis and its derivatives containing more than 0.3% delta-9 tetrahydrocannabinol on a dry weight basis remain Schedule I controlled substances under U.S. federal law, meaning that federal law generally prohibits their manufacture and distribution. United States federal law also deems it unlawful to sell, offer for sale, transport in interstate commerce, import, or export “drug paraphernalia,” which includes “any equipment, product, or material of any kind which is primarily intended or designed for use in manufacturing, compounding, converting, concealing, producing, processing, preparing, injecting, ingesting, inhaling, or otherwise introducing into the human body a controlled substance” the possession of which federal law prohibits, including Schedule I “marijuana.” Limited exemptions exist, most notably when state or local law authorizes these items’ manufacture, possession, or distribution.
F-8
The European Commission issued the Tobacco Products Directive (the “TPD”), which became effective on May 19, 2014 and became applicable in the European Union member states on May 20, 2016. The TPD regulates e-cigarettes on the packaging, labelling and ingredients of the products on the European Union market, the creation of smoke-free environments, tax measures and activities against illegal trade and anti-smoke campaigns. Member states of the European Union are required to ensure that advertisements for any tobacco related product are prohibited, and no promotion shall be made as to those devices with an intention to promote e-cigarettes. For the e-cigarettes released after May 20, 2016, TPD requires e-cigarette manufacturers to submit product sales applications to the regulatory market six months in advance, and ensure their products can meet the TPD requirements before they can be released. The Company has complied with TPD requirement that for all its tobacco products sold in Europe.
The sale of cannabis vaping products is illegal in the European Union and the United Kingdom.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires the Company to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Significant estimates include allowance for doubtful accounts, the useful lives of property and equipment and intangible asset, impairment of long-lived assets, and deferred cost. Actual results could differ from those estimates.
Intangible assets
The Company reviews intangible assets for impairment when events or changes in circumstances indicate the carrying amount may not be recoverable. The evaluation is performed at the lowest level of identifiable cash flows independent of other assets, The Company measure recoverability of these assets by comparing the carrying amounts to the future undiscounted cash flows that the assets are expected to generate. If the carrying value of the assets is not recoverable, the impairment recognized is measured as the amount by which the carrying value exceeds its fair value. There is no indication of impairment for the period presented.
Intangible assets with definite lives, such as patents, are amortized over their estimated useful lives on a straight-line basis generally over ten years.
Revenue recognition
The Company sells its products to customers around the world and recognizes revenue in accordance with the guidance of ASC 606, Revenue from Contracts with Customers. Revenue is recognized when control of goods has transferred to customers. For the majority of the Company’s customer arrangements, control transfers to customers at a point-in-time when goods have been delivered to the pickup location specified by the customer or a forwarder appointed by the customer, as that is generally when legal title, physical possession and risks and rewards of goods transfer to the customer.
Revenue is recognized at the transaction price based on the purchase order as adjusted for the anticipated rebates, discounts and other sales incentives. When determining the transaction price, management estimates variable consideration applying the portfolio approach practical expedient under ASC 606. The main sources of variable consideration for the Company are customer rebates, trade promotion funds, and cash discounts. These sales incentives are recorded as a reduction of revenue at the time of the initial sale using the most-likely amount estimation method. The most-likely amount method is based on the single most likely outcome from a range of possible consideration outcomes. The range of possible consideration outcomes is primarily derived from the following inputs: sales terms, historical experience, trend analysis, and projected market conditions in the various markets served. Because the Company serves numerous markets, the sales incentive programs offered vary across businesses, but the most common incentive relates to amounts paid or credited to customers for achieving defined volume levels or growth objectives.
F-9
Disaggregated Revenue
In accordance with ASC 606-10-50-5, the Company has taken into consideration the nature, amount, timing, and uncertainty of revenue and cash flows, and has determined to disaggregate its net sales of tobacco products and cannabis products. The net sales disaggregated by products for the six months period ended December 31, 2021 and 2022 were as follows:
| Six Months Period ended December 31, | ||||||||
| Net sales by products branded | 2021 | 2022 | ||||||
| Aspire - tobacco | $ | 38,938,023 | $ | 43,008,459 | ||||
| Ispire - cannabis | 8,295,335 | 15,831,990 | ||||||
| Total | $ | 47,233,358 | $ | 58,840,449 | ||||
Cost of revenue
Cost of revenue for the years ended December 31, 2021 and 2022 consisted primarily of the cost of purchasing vaping products, which were purchased from a related party. See Note 11.
Recent accounting pronouncements
As an emerging growth company, the Company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. The Company intends to take advantage of the benefits of this extended transition period.
Accounting pronouncements adopted during the six months ended December 31, 2022
In November 2018, the FASB issued ASU 2018-18, “Collaborative Arrangements (Topic 808): Clarifying the Interaction Between Topic 808 and Topic 606,” which clarifies that elements of collaborative arrangements could qualify as transactions with customers in the scope of ASC 606. The amendments require the application of existing guidance to determine the units of account in collaborative arrangement for purposes of identifying transactions with customers. For transactions outside the scope of ASC 606, companies can apply elements of ASC 606 or other relevant guidance by analogy, or apply a reasonable accounting policy if there is no appropriate analogy. ASU 2018-18 is effective retrospectively for us for the six months ended December 31, 2022. The adoption of this guidance had no material impact on our financial position, results of operations and cash flows.
Accounting pronouncements not yet effective
As the Company is an emerging growth company, the effective dates of the pronouncements applicable to us are the same as those applicable to private companies.
In June 2016, the Financial Accounting Standards Boards (“FASB”) amended guidance related to the impairment of financial instruments as part of ASU 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. The guidance replaces the incurred loss impairment methodology with an expected credit loss model for which a company recognizes an allowance based on the estimate of expected credit loss. For public business entities that meet the definition of a U.S. Securities and Exchange Commission (“SEC”) filer (“SEC filer”), excluding entities eligible to be smaller reporting companies (SRCs) as defined by the SEC, ASU No. 2016-13 is effective for fiscal years beginning after December 15, 2019, including interim periods within those fiscal years. For all other entities, including SRCs, ASU No. 2016-13 is effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years. As an emerging growth company, the Company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. The Company intends to take advantage of the benefits of this extended transition period. The Company is in the process of evaluating the impact that this guidance will have on its consolidated financial statements.
F-10
In April 2019, the FASB issued ASU 2019-04, “Codification Improvements to Topic 326, Financial Instruments – Credit Losses, Topic 815, Derivatives and Hedging, and Topic 825, Financial Instruments”, which provides narrow-scope amendments to clarify and improve guidance within the standards on credit losses, hedging, and recognition and measurement of financial instruments. Apart from the amendments to ASU 2016-13 mentioned above, the ASU also included subsequent amendments to ASU 2016-01. The effective date for Topic 815 and 825 was fiscal years beginning after December 15, 2020 and 2019, respectively, and the adoption had no material impact on our financial position, results of operations and cash flows. The effective date for Topic 326 was delayed by ASU 2019-10 to fiscal years beginning after December 15, 2022. We do not expect that the adoption of this guidance will have a material impact on the financial position, results of operations and cash flows.
In October 2018, the FASB issued ASU 2018-17, Consolidation (Topic 810): Targeted Improvements to Related Party Guidance for Variable Interest Entities, (“ASU 2018-17”). ASU 2018-17 requires reporting entities to consider indirect interests held through related parties under common control on a proportional basis rather than as the equivalent of a direct interest in its entirety for determining whether a decision-making fee is a variable interest. For entities other than private companies, the standard is effective for fiscal years beginning after December 15, 2019, and interim periods within those fiscal years. The ASU is effective for a private company for fiscal years beginning after December 15, 2020, and interim periods within fiscal years beginning after December 15, 2022. Early adoption is permitted. Entities are required to apply the amendments in ASU 2018-17 retrospectively with a cumulative-effect adjustment to retained earnings at the beginning of the earliest period presented. We do not expect that the adoption of this guidance will have a material impact on our financial position, results of operations and cash flows.
3. CASH AND CASH EQUIVALENTS
Below is a breakdown of the Company’s cash balances in banks as of June 30, 2022 and December 31, 2022, both by geography and by currencies (translated into U.S. dollars):
| As of June 30, | As of December 31 | |||||||
| By Geography: | 2022 | 2022 | ||||||
| Cash in HK | $ | 71,221,649 | $ | 80,527,138 | ||||
| Cash in U.S. | 3,259,002 | 3,740,016 | ||||||
| Total | $ | 74,480,651 | $ | 84,267,154 | ||||
| By Currency: | ||||||||
| USD | $ | 64,187,756 | $ | 74,339,265 | ||||
| HKD | 415,930 | 402,653 | ||||||
| EUR | 4,097 | 4,838 | ||||||
| GBP | 24,680 | 24,537 | ||||||
| RMB | 9,848,188 | 9,495,861 | ||||||
| Total | $ | 74,480,651 | $ | 84,267,154 | ||||
“HKD” refers to Hong Kong dollars, “GBP” refers to British pounds, and “EUR” refers to Euros.
4. FAIR VALUE MEASUREMENT
As of June 30, 2022 and December 31, 2022, information about inputs into the fair value measurement of the Company’s assets and liabilities that are measured at fair value on a recurring basis in periods subsequent to their initial recognition is as follows:
Cash and cash equivalents, accounts receivable, prepaid expenses, other receivables and due from related parties are financial assets with carrying values that approximate fair value due to their short-term nature. Accounts payable, accounts payable – related party, contract liabilities, accrued liabilities and other payables and due to related parties are financial liabilities with carrying values that approximate fair value due to their short-term nature.
F-11
5. ACCOUNTS RECEIVABLE
As of June 30, 2022 and December 31, 2022, accounts receivable consisted of the following:
| As of June 30, | As of December 31, | |||||||
| 2022 | 2022 | |||||||
| Accounts receivable – gross | $ | 8,260,574 | $ | 18,044,372 | ||||
| Allowance for doubtful accounts | - | - | ||||||
| Accounts receivables | $ | 8,260,574 | $ | 18,044,372 | ||||
The Company recorded bad debt expense of nil and $1,029,655 for the six months ended December 31, 2021 and 2022, respectively.
6. PROPERTY, PLANT AND EQUIPMENT, NET
As of June 30, 2022 and December 31, 2022, property, equipment and leasehold improvement consisted of the following:
| As of June 30, | As of December 31, | |||||||
| 2022 | 2022 | |||||||
| Leasehold improvement | $ | 433 | $ | 301,946 | ||||
| Office and other equipment | 146,798 | 146,975 | ||||||
| Furniture and fixture | - | 176,783 | ||||||
| 147,231 | 625,704 | |||||||
| Less: accumulated depreciation | (33,206 | ) | (46,866 | ) | ||||
| Total | $ | 114,025 | $ | 578,838 | ||||
For the six months ended December 31, 2021 and 2022, depreciation expense amounted to $1,782 and $13,493, respectively.
7. INTANGIBLE ASSETS
On September 30, 2022, an intellectual property transfer agreement and an exclusive license agreement was signed such that all patents, trademarks, Know-how and Know-how Documentation related to cannabis vaping products and tobacco vaping products were transferred from Tuanfang Liu, Aspire Global and Shenzhen Yi Jia to Aspire North America and Aspire Science. As the intangible assets were transferred from Tuanfang Liu, the chief executive officer and controlling stockholder, and the companies that controlled by Tuanfang Liu, the transfer was considered as capital contribution by the stockholder, which is shown as a transaction on the statements of changes in stockholders’ equity. The Company engaged a third party firm to perform a valuation to estimate the fair values of the intangible assets transferred, in accordance with ASC 350.
Information regarding transferred intangible assets is as follows:
| As of December 31, 2022 | ||||||||||||
| Gross Carrying Amount | Accumulated Amortization | Net carrying amount | ||||||||||
| Definite-lived intangible assets - Patents | $ | 30,905,261 | $ | 772,632 | $ | 30,132,629 | ||||||
| Indefinite-lived intangible assets - trademarks | 43,354,654 | - | 43,354,654 | |||||||||
| Total intangible assets | 74,259,915 | 772,632 | 73,487,283 | |||||||||
Patents acquired during the six months ended December 31, 2022 have a weighted-average useful life of 9.75 years.
Amortization expense relating to the acquired intangible assets was $0.8 million for the six months ended December 31, 2022.
F-12
8. CONTRACT LIABILITIES
As of June 30, 2022 and December 31, 2022, the Company had total contract liabilities of $1,672,051 and $1,003,735, respectively. These liabilities are advance deposits received from customers after an order has been placed. As of December 31 2022, the Company expects all of the contract liabilities to be settled in less than one year. The decrease in the balance at December 31, 2022 was due to less orders on hand on that date.
9. LEASES
The Company has operating lease arrangements for office premises in Hong Kong and California. These leases typically have terms of two to five years and are expensed on a straight-line basis.
Leases with an initial term of 12 months or less are not presented as right-of-use assets on the consolidated balance sheet and are expensed over the lease term. All other lease assets and lease liabilities are recognized based on the present value of lease payments over the lease term at commencement date.
The balances for the right-of-use assets where the Company is the lessee are presented as follow:
| As of June 30, | As of December 31, | |||||||
| 2022 | 2022 | |||||||
| Right-of-use assets | $ | 295,804 | $ | 3,459,714 | ||||
| Lease liabilities - current | $ | 347,541 | $ | 784,724 | ||||
| Lease liabilities – non-current | - | 2,829,102 | ||||||
| Total | $ | 347,541 | $ | 3,613,826 | ||||
As of December 31, 2022, the maturities of our lease liabilities (excluding short-term leases) are as follows:
| As of December 31, 2022 | |||||
| 2023 | 1,000,428 | ||||
| 2024 | 887,998 | ||||
| 2025 | 919,168 | ||||
| 2026 | 951,585 | ||||
| 2027 | 564,731 | ||||
| Total future lease payments | 4,323,910 | ||||
| Less: imputed interest | (710,084 | ) | |||
| Total lease liabilities | 3,613,826 | ||||
The Company incurred lease costs, which includes the amortization of the right-of-use assets and the payment of short-term leases, of $141,661 and $513,373 on the Company’s consolidated statements of operations and comprehensive income(loss) for the six months ended December 31, 2021 and 2022, respectively.
The Company made payments of $148,686 and $539,956 under the lease agreements during the six months ended December 31, 2021 and 2022, respectively.
The weighted-average remaining lease term related to the Company’s lease liabilities as of June 30, 2022 and December 31, 2022 was 1 and 4.38 years, respectively.
The discount rate related to the Company’s lease liabilities as of both June 30, 2022 and December 31, 2022 was 5.8% and 7.9%. The discount rates are generally based on estimates of the Company’s incremental borrowing rate, as the discount rates implicit in the Company’s leases cannot be readily determined.
F-13
10. ACCRUED LIABILITIES AND OTHER PAYABLES
As of June 30, 2022 and December 31, 2022, accrued liabilities and other payables consisted of the following:
| As of June 30, | As of December 31, | |||||||
| 2022 | 2022 | |||||||
| Accrued salaries and related benefits | $ | 43,487 | $ | 54,052 | ||||
| Other payables | 81,226 | 157,355 | ||||||
| Accrued expenses | 34,583 | 86,036 | ||||||
| Total | $ | 159,296 | $ | 297,443 | ||||
11. RELATED PARTY TRANSACTIONS
a) The table below sets forth the major related parties and their relationships with the Company:
| Name of related parties and Relationship with the Company |
| -Tuanfang Liu is the Chairman of the Company. |
| -Jiangyan Zhu is the wife of Tuanfang Liu and a director of the Company. |
| -Eigate (Hong Kong) Technology Co., Limited (“Eigate”) is a wholly-owned subsidiary of Aspire Global. |
| -Aspire Global is a company controlled by the Chairman of the Company. |
| -Shenzhen Yi Jia, a Chinese company that is 95% owned by the Company’s chairman and 5% by the chairman’s cousin. |
b) Tuanfang Liu is also Aspire Global’s chief executive officer and a director of both the Company and Aspire Global, and his wife, Jiangyan Zhu, is also a director of both companies. Mr. Liu and Ms. Zhu beneficially own 66.5% and 5.0%, respectively, of the outstanding shares of both Aspire Global and the Company.
c) The Company had the following balances due from related parties:
| As of June 30, | As of December 31, | |||||||
| 2022 | 2022 | |||||||
| Shenzhen Yi Jia | $ | 1,872,035 | $ | - | ||||
| Tuanfang Liu | 62,820 | - | ||||||
| Total | $ | 1,934,855 | $ | - | ||||
The balances represent payment on behalf of these related parties, such as freight and tariff charges and others. These balances as of June 30, 2022 were all non-interest bearing, unsecured, have no due date and are repayable on demand and the balances were fully settled in November 2022.
d) The balances in due to related parties at June 30, 2022 and December 31, 2022 represent amounts due to Eigate of $40,672,768 and $40,499,550, respectively. These balances were all non-interest bearing, unsecured, have no due date and are repayable on demand.
e) For both six months ended December 31, 2021 and 2022, substantially all of the Company’s tobacco and cannabis products were purchased from Shenzhen Yi Jia. As of June 30, 2022 and December 31, 2022, the accounts payable - related party was $41,982,373 and $67,323,067, respectively, which was payable to Shenzhen Yi Jia. For the six months ended December 31, 2021 and 2022, the purchases from Shenzhen Yi Jia were $44,833,089 and $50,801,609, respectively.
F-14
12. INCOME TAXES
For the six months ended December 30, 2021 and 2022, income(loss) before income taxes consists of:
| For the Six Months ended December 31 | ||||||||
| 2021 | 2022 | |||||||
| HK | $ | 4,355,001 | $ | 4,302,018 | ||||
| U.S. | (2,446,727 | ) | (6,420,932 | ) | ||||
| Total | $ | 1,908,274 | $ | (2,118,914 | ) | |||
The Company’s effective tax rate for the six months ended December 31, 2021 and 2022 was different from the Hong Kong statutory income tax rate due primarily to the U.S. subsidiary being in loss position. No tax benefit has been recognized for this current loss and the related carryforward losses of this subsidiary, as a full valuation allowance has been established against the deferred tax asset arising from the losses.
13. EARNINGS PER SHARE
The following table presents a reconciliation of basic net income per share:
| For the Six Months ended December 31, | ||||||||
| 2021 | 2022 | |||||||
| Net income(loss) | $ | 1,278,681 | $ | (2,950,921 | ) | |||
| Weighted average basic and diluted share of common stock outstanding | 50,000,000 | 50,000,000 | ||||||
| Net income(loss) per basic and diluted share of common stock | $ | 0.03 | $ | (0.06 | ) | |||
F-15
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Ispire Technology Inc. and Subsidiaries
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Ispire Technology Inc. and Subsidiaries (the Company) as of June 30, 2022 and 2021, and the related consolidated statements of operations and comprehensive income (loss), changes in stockholders’ equity, and cash flows for each of the years in the two-year period ended June 30, 2022, and the related notes (collectively referred to as the financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2022 and 2021, and the results of its operations and its cash flows for each of the years in the two-year period ended June 30, 2022, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to those charged with governance and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. We have determined that there are no critical matters.
| /s/ MSPC | |
| Certified Public Accountants and Advisors, | |
A Professional Corporation
We have served as the Company’s auditor since 2022. | |
New York, New York
October 7, 2022
F-16
CONSOLIDATED BALANCE SHEETS
| June 30, | ||||||||
| 2021 | 2022 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 85,248,997 | $ | 74,480,651 | ||||
| Accounts receivable | 4,305,271 | 8,260,574 | ||||||
| Inventories, net | 3,054,996 | 14,580,557 | ||||||
| Prepaid expenses and other current assets | 775,055 | 192,499 | ||||||
| Due from related parties | 36,221 | 1,934,855 | ||||||
| Total current assets | 93,420,540 | 99,449,136 | ||||||
| Other assets: | ||||||||
| Property, plant and equipment, net | 2,911 | 114,025 | ||||||
| Rental deposit | - | 876,100 | ||||||
| Right-of-use assets | 440,573 | 295,804 | ||||||
| Total other assets | 443,484 | 1,285,929 | ||||||
| Total assets | $ | 93,864,024 | $ | 100,735,065 | ||||
| Liabilities and stockholders’ equity | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | 7,945 | $ | 290,541 | ||||
| Accounts payable – related party | 33,370,618 | 41,982,373 | ||||||
| Contract liabilities | 1,125,848 | 1,672,051 | ||||||
| Dividends payable | 3,832,272 | 3,362,639 | ||||||
| Accrued liabilities and other payables | 119,235 | 159,296 | ||||||
| Due to related parties | 41,172,013 | 40,672,768 | ||||||
| Income tax payable | - | 481,113 | ||||||
| Lease liabilities | 207,972 | 347,541 | ||||||
| Total current liabilities | 79,835,903 | 88,968,322 | ||||||
| Other liabilities: | ||||||||
| Lease liabilities | 270,140 | - | ||||||
| Total liabilities | $ | 80,106,043 | $ | 88,968,322 | ||||
| Stockholders’ equity: | ||||||||
| Common stock, par value $0.0001 per share; 140,000,000 shares authorized; 50,000,000 shares issued and outstanding as of June 30, 2021 and June 30, 2022 | 5,000 | 5,000 | ||||||
| Preferred stock, par value $0,0001 per share, 10,000,000 shares authorized, no shares issued at June 30, 2021 and 2022 | - | - | ||||||
| Accumulated other comprehensive loss | (67,579 | ) | (184,664 | ) | ||||
| Retained earnings | 13,820,560 | 11,946,407 | ||||||
| Total stockholders’ equity | 13,757,981 | 11,766,743 | ||||||
| Total liabilities and stockholders’ equity | $ | 93,864,024 | $ | 100,735,065 | ||||
See notes to consolidated financial statements.
F-17
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME
| Years ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| Revenue | $ | 63,414,783 | $ | 88,095,418 | ||||
| Cost of revenue | 52,998,928 | 74,789,378 | ||||||
| Gross profit | 10,415,855 | 13,306,040 | ||||||
| Operating expenses: | ||||||||
| Sales and marketing expenses | 3,561,731 | 5,503,630 | ||||||
| General and administrative expenses | 3,210,722 | 8,791,081 | ||||||
| Total operating expenses | 6,772,453 | 14,294,711 | ||||||
| Income(loss) from operations | 3,643,402 | (988,671 | ) | |||||
| Other income (expense): | ||||||||
| Interest income | 882 | 5,078 | ||||||
| Exchange (loss)gain, net | (87,921 | ) | 58,143 | |||||
| Other income, net | 67,266 | 122,394 | ||||||
| Total other (expense) income, net | (19,773 | ) | 185,615 | |||||
| Income(loss) before income taxes | 3,623,629 | (803,056 | ) | |||||
| Income taxes - current | (685,823 | ) | (1,071,097 | ) | ||||
| Net income(loss) | $ | 2,937,806 | $ | (1,874,153 | ) | |||
| Other comprehensive loss | ||||||||
| Foreign currency translation adjustments | (9,701 | ) | (117,085 | ) | ||||
| Comprehensive income(loss) | 2,928,105 | (1,991,238 | ) | |||||
| Net income (loss) per share | ||||||||
| Basic and diluted | $ | 0.06 | $ | (0.04 | ) | |||
| Weighted average shares outstanding: | ||||||||
| Basic and diluted | 50,000,000 | 50,000,000 | ||||||
See notes to consolidated financial statements.
F-18
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
| Common Stock | Preferred Stock | Accumulated Other | ||||||||||||||||||||||||||
| Number of | Number of | Retained | Comprehensive | Stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Earnings | Loss | Equity | ||||||||||||||||||||||
| Balance, July 1, 2020 | 50,000,000 | 5,000 | - | - | 10,882,754 | (57,878 | ) | 10,829,876 | ||||||||||||||||||||
| Net income | - | - | - | - | 2,937,806 | - | 2,937,806 | |||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | (9,701 | ) | (9,701 | ) | |||||||||||||||||||
| Balance, June 30, 2021 | 50,000,000 | $ | 5,000 | - | $ | - | $ | 13,820,560 | $ | (67,579 | ) | $ | 13,757,981 | |||||||||||||||
| Net loss | - | - | - | - | (1,874,153 | ) | - | (1,874,153 | ) | |||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | (117,085 | ) | (117,085 | ) | |||||||||||||||||||
| Balance, June 30, 2022 | 50,000,000 | $ | 5,000 | - | $ | - | $ | 11,946,407 | $ | (184,664 | ) | $ | 11,766,743 | |||||||||||||||
See notes to consolidated financial statements.
F-19
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| Cash flows from operating activities: | ||||||||
| Net income(loss): | $ | 2,937,806 | $ | (1,874,153 | ) | |||
| Adjustments to reconcile net income from operations to net cash provided by operating activities: | ||||||||
| Depreciation and amortization | 3,641 | 10,402 | ||||||
| Depreciation of right-of-use assets | 316,985 | 135,141 | ||||||
| Accounts receivable impairment | 272,235 | - | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (3,869,775 | ) | (3,950,508 | ) | ||||
| Inventories | (3,054,996 | ) | (11,525,561 | ) | ||||
| Prepaid expenses and other current assets | (632,644 | ) | 29,007 | |||||
| Accounts payable | 9,391,010 | 8,875,590 | ||||||
| Contract liabilities | 150,586 | 543,890 | ||||||
| Accrued liabilities and other payables | 43,550 | (282,487 | ) | |||||
| Income tax payable | (534,069 | ) | 481,113 | |||||
| Net cash provided by/(used in) operating activities | $ | 5,024,329 | $ | (7,557,566 | ) | |||
| Cash flows from investing activities: | ||||||||
| Purchase of property, plant and equipment | (798 | ) | (121,516 | ) | ||||
| Net cash used in investing activities | $ | (798 | ) | $ | (121,516 | ) | ||
| Cash flows from financing activities: | ||||||||
| Payment made for dividends | - | (469,633 | ) | |||||
| Advances from related parties | 91,695 | - | ||||||
| Repayment of related parties | (36,221 | ) | (2,498,689 | ) | ||||
| Principal portion of lease payment | (283,323 | ) | (120,942 | ) | ||||
| Net cash used in financing activities | $ | (227,849 | ) | $ | (3,089,264 | ) | ||
| Net increase(decrease) in cash and cash equivalents | 4,795,682 | (10,768,346 | ) | |||||
| Cash and cash equivalents - beginning of year | 80,453,315 | 85,248,997 | ||||||
| Cash and cash equivalents - end of year | $ | 85,248,997 | $ | 74,480,651 | ||||
| Supplemental disclosure of cash flow information: | ||||||||
| Cash paid(refund) for income taxes | $ | 1,880,546 | $ | (69,647 | ) | |||
| Cash paid for interest | $ | - | $ | - | ||||
See notes to consolidated financial statements.
F-20
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. ORGANIZATION AND PRINCIPAL ACTIVITIES
Ispire Technology Inc. (the “Company”) was incorporated under the laws of the State of Delaware on June 13, 2022. Through its subsidiaries, the Company is engaged in the research and development, design, commercialization, sales, marketing and distribution of branded e-cigarettes and cannabis vaping products.
Ispire owns a 100% equity interest in Ispire International Limited, a business company incorporated under the laws of the British Virgin Islands (“BVI”) (“Ispire International”) on July 6, 2022.
Prior to July 29, 2022, all of the equity of Aspire North America LLC, a California limited liability company (“Aspire North America”), was owned by Aspire Global Inc. (“Aspire Global”), and all of the equity of Aspire Science and Technology Limited, a Hong Kong corporation (“Aspire Science”), was owned by Aspire Global Holdings Limited (“Aspire Holdings”), a wholly-owned subsidiary of Aspire Global.
Aspire Global and the Company are related parties since the same individual is the chief executive officer of both companies, the chief executive officer and his wife, who are both directors of both companies, own 66.5% and 5.0%, respectively, of the equity of both Aspire Global and the Company. At the time of transfer of the equity in Aspire North America and Aspire Science, the Company had the same stockholders as Aspire Global and the Company’s stockholders held the same percentage interest in the Company as they had in Aspire Global.
On July 29, 2022:
| ● | Aspire Global transferred 100% of the equity interest in Aspire North America to the Company |
| ● | Aspire Holdings transferred 100% of the equity of Aspire Science to Ispire International. |
The following table sets forth information concerning the Company and its subsidiaries as of June 30, 2022:
| Name of Entity | Date of Organization |
Place of Organization |
%
of Ownership |
Principal Activities |
|||||
| Ispire Technology Inc. | June 13, 2022 | Delaware | Parent Company | Holding Company | |||||
| Ispire International | July 6, 2022 | BVI | 100% | Holding Company | |||||
| Aspire North America | February 22, 2020 | California | 100% | Sales and Marketing | |||||
| Aspire Science | December 9, 2016 | Hong Kong | 100% | Sales and Marketing |
Ispire is a holding company and does not engage in any active operations. Its business is conducted by its two operating subsidiaries, Aspire North America, which is engaged in the development, marketing and sales of cannabis vapor products, which were introduced in mid 2020, and Aspire Science, which is engaged in the development, marketing and sales of tobacco vaping products. As of June 30, 2022, the Company has 140,000,000 authorized shares of common stock, par value $0.0001 per share, of which 50,000,000 shares are outstanding, and 10,000,000 share of preferred stock, par value $0.0001 per share, none of which are outstanding. The Company’s board of directors has the power to create one or more series of preferred stock and to determine the rights, preferences and privileges of each series. Because the transfer of the equity in Aspire North America and Aspire Science is a transfer between related parties, the historical financial information of the subsidiaries is carried forward as the historical financial information of the Company and the 50,000,000 shares that were issued at or about the time of the Company’s organization are treated as being outstanding on July 1, 2020, the first day of the two years covered by the Company’s consolidated financial statements.
In accordance with Accounting Standards Codification (“ASC”) 805-50-25, the reorganization has been accounted for as a recapitalization among entities under common control since the same controlling stockholder controls both the Company and Aspire Global before and after the reorganization. The consolidation of the Company and its subsidiaries has been accounted for at historical cost and prepared on the basis as if the aforementioned transactions had become effective as of the beginning of the first period presented in the accompanying consolidated financial statements. Furthermore, ASC 805-50-45-5 indicates that the financial statements and financial information presented for prior years shall also be retroactively adjusted to furnish comparative information.
Impact of COVID-19
In December 2019, coronavirus disease 2019 (COVID-19) was first reported to have surfaced in Wuhan, China. During 2020, the disease spread to many parts of the world. The epidemic has resulted in quarantines, travel restrictions, and the temporary closure of stores and facilities in much of the world.
Measures taken by various governments to contain the virus have affected economic activity in all countries where the consumers of our product live. We took a number of measures to monitor and mitigate the effects of COVID-19, such as safety and health measures for our personnel, such as social distancing, in accordance with government policies and advice, securing the supply of materials that are essential to our production process. We will continue to follow the various government policies and advice and, in parallel, we may take further actions that we determine are in the best interests of our employees, customers, and business relationships.
F-21
The Company’s products are manufactured in China by Shenzhen Yi Jia Technology Co., Limited, a Chinese company (“Shenzhen Yi Jia”), which is 95% owned by the Company’s chief executive officer and controlling stockholder. From the middle of January 2020, the start of the Chinese New Year holiday, until the end of April 2020, its production was slowed due to the COVID-19 pandemic in China. This slowdown in production resulted in a near-total stoppage of our supplier’s business during this nearly four-month period and our sales activities were substantially reduced. In addition, since our products are generally sold in stores such as grocery stores, convenience stores and tobacco stores, to the extent that either the stores are closed as a result of government actions or consumers are reluctant to go shopping because of the COVID-19 pandemic, retail sales of the Company’s products suffered. Following the temporary slowdown in production in first half year of 2020, Shenzhen Yi Jia has recovered its production capacity, and the Company has not experienced significant supply chain constraints as a result of the pandemic.
From late 2020 to the middle of 2021, COVID-19 vaccination program had been greatly promoted around the globe, however several types of COVID-19 variants emerged in different parts of the world. The Company’s sales in Europe and the United States continued to be affected by government actions relating to COVID-19 and COVID-19 variants, while the production was slightly reduced by the strict measures taken by the Government of China to address the small COVID-19 outbreaks in Guangdong province. To the extent that China’s zero-COVID policy results in closing or significantly reducing the operations of Shenzhen Yi Jia or any of its suppliers, our business will be impacted since the Company presently relies on Shenzhen Yi Jia for its products.
The extent to which COVID-19 impacts the Company’s operations on an ongoing basis is highly uncertain. It will depend on various factors including the duration and severity of the outbreak, new information which may emerge concerning the severity of the coronavirus or any variants and the actions to contain the coronavirus or treat its impact, among others.
Supply Chain Risks
One of effects of the COVID-19 has been delays resulting from supply chain issues, which relate to the difficulty that companies have in having their products manufactured, shipped to the country of destination, and delivered from the port of entry to the customer’s location. As a result of the COVID-19 pandemic, during 2021 and early 2022, there were fewer longshoremen unloading ships and fewer truckers to deliver the products to market, which resulted in significant delays in the delivery of products to markets. To the extent that products are shipped by sea, there are additional risks resulting from ports not being able to unload ships promptly, causing delays in getting into port, including potential damage from seawater and fire, product degradation and the possibility of containers being destroyed, damaged or falling off the ship into the water. The inability to deliver products to the ultimate vendor could impair the Company’s ability to generate revenue from its products. As the port delays have significantly decreased, we do not believe that the supply chain issues that affected our operations are currently affecting us. We cannot assure you that delays will not affect our business in the future.
In 2021, Shenzhen Yi Jia suffered a chip shortage and resulting in a slowdown in delivery of its products to the Company from April to August 2021. To secure the supply of chips, Shenzhen Yi Jia changed the payment terms to chip supplier from 30 days after delivery in the past to prepayment, and it engaged two new chip suppliers. Since September 2021, Shenzhen Yi Jia has obtained a supply of chips to meet its production need and the chip shortage no longer affects its production. However, the Company cannot assure you that it will not suffer from a chip shortage affecting Shenzhen Yi Jia or any other supplier.
E-cigarette regulation
Regulation regarding e-cigarette varies across countries, from no regulation to a total ban. The legal status of e-cigarette is currently pending in many countries. But as e-cigarettes have become more and more popular recently, many countries are considering imposing more stringent law and regulations to regulate this market. Changes in existing law and regulations and the imposition of new laws, regulation in countries and regions that our major customers located in may adversely affect the Company’s business.
The Federal Food, Drug, and Cosmetic Act requires all Electronic Nicotine Delivery Systems (“ENDS”) product manufacturers that market products in the United States to submit Premarket Tobacco Product Applications (“PMTAs”) to the FDA. For ENDS products that were on the U.S. market on August 8, 2016, a PMTA was required to be submitted to the FDA by September 9, 2020; for ENDS products that were not on the U.S. market prior on August 8, 2016, and for which a PMTA was not filed by September 9, 2020, a PMTA a premarket authorization issued in response to a PMTA is required before the subject product may enter the U.S. market. The Company has submitted a PMTA filing for one ENDS product, and, under apparent FDA policies, the agency will not enforce the premarket review requirements for that product pending review of its PMTA. However, even with submission of the PMTA application, the FDA may reject the Company’s application and may prevent the Company’s ENDS products from being sold in U.S., which will adversely affect the Company’s business.
Amendments to the Prevent All Cigarette Trafficking (“PACT”) Act, which became law in 2021, extend the PACT Act to include e-cigarette and all vaping products, and place significant burdens on sellers of vaping products in the United States which may make it difficult to operate profitably in the United States. Because of tighter government regulations, the Company will stop marketing tobacco products in the United States, as the volume of sales from the one tobacco vaping product which the Company may sell in the United States does not justify the marketing and regulatory costs involved.
F-22
In the United States, cannabis vaping products are governed by state laws, which vary from state to state. Most states do not permit the adult recreational use of cannabis, and no states permit the sale of recreational cannabis products to minors. As a result of the reduced revenue to states resulting from the effects of the COVID 19 pandemic, states may seek to raise revenue by permitting and taxing the use of cannabis products. The Company cannot predict what action states will take or the nature and amount of taxes they may impose. However, the extent the PACT Act applies to cannabis products that aerosolize liquids, it may be more difficult to sell our products in states that permit the sale of cannabis.
However, cannabis and its derivatives containing more than 0.3% delta-9 tetrahydrocannabinol on a dry weight basis remain Schedule I controlled substances under U.S. federal law, meaning that federal law generally prohibits their manufacture and distribution. United States federal law also deems it unlawful to sell, offer for sale, transport in interstate commerce, import, or export “drug paraphernalia,” which includes “any equipment, product, or material of any kind which is primarily intended or designed for use in manufacturing, compounding, converting, concealing, producing, processing, preparing, injecting, ingesting, inhaling, or otherwise introducing into the human body a controlled substance” the possession of which federal law prohibits, including Schedule I “marijuana.” Limited exemptions exist, most notably when state or local law authorizes these items’ manufacture, possession, or distribution.
The European Commission issued the Tobacco Products Directive (the “TPD”), which became effective on May 19, 2014 and became applicable in the European Union member states on May 20, 2016. The TPD regulates e-cigarettes on the packaging, labelling and ingredients of the products on the European Union market, the creation of smoke-free environments, tax measures and activities against illegal trade and anti-smoke campaigns. Member states of the European Union are required to ensure that advertisements for any tobacco related product are prohibited, and no promotion shall be made as to those devices with an intention to promote e-cigarettes. For the e-cigarettes released after May 20, 2016, TPD requires e-cigarette manufacturers to submit product sales applications to the regulatory market six months in advance, and ensure their products can meet the TPD requirements before they can be released. The Company has complied with TPD requirement that for all its tobacco products sold in Europe.
The sale of cannabis vaping products is illegal in the European Union and the United Kingdom.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation
The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Emerging growth company
The Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the independent registered public accounting firm attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that an emerging growth company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such an election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company that is neither an emerging growth company nor an emerging growth company that has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Basis of consolidation
The consolidated financial statements include the financial statements of the Company and its subsidiaries as if the subsidiaries were acquired by the Company as of July 1, 2020. All inter-company transactions and balances have been eliminated upon consolidation.
F-23
Use of estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires the Company to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Significant estimates include allowance for doubtful accounts, the useful lives of property and equipment and intangible asset, impairment of long-lived assets, and deferred cost. Actual results could differ from those estimates.
Cash and cash equivalents
Cash includes currency on hand, deposits held by banks that can be added or withdrawn without limitation and highly liquid investments with maturities of three months or less when purchased.
Fair value measurement
The Company applies ASC Topic 820, Fair Value Measurements and Disclosures, which defines fair value, establishes a framework for measuring fair value, and expands financial statement disclosure requirements for fair value measurements.
ASC Topic 820 defines fair value as the price that would be received from the sale of an asset or paid to transfer a liability (an exit price) on the measurement date in an orderly transaction between market participants in the principal or most advantageous market for the asset or liability.
ASC Topic 820 specifies a hierarchy of valuation techniques, which is based on whether the inputs into the valuation technique are observable or unobservable. The hierarchy is as follows:
Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
Level 3 inputs to the valuation methodology are unobservable and significant to the fair value. Unobservable inputs are valuation technique inputs that reflect the Company’s own assumptions about the assumptions that market participants would use in pricing an asset or liability.
Accounts receivable
Accounts receivable are recognized and carried at the original invoiced amount less an allowance for any potential uncollectible amounts. An estimate for doubtful accounts is made when collection of the full amount is no longer probable. Past due accounts are generally written off against the allowance for bad debts only after all collection attempts have been exhausted and the potential for recovery is considered remote.
The Company maintains an allowance for potential credit losses on accounts receivable. The Company reviews accounts receivable on a periodic basis, and makes provisions of 80% for accounts receivable aged between 1.5 years to 2 years, and 100% for balances aged over 2 years. Additionally, specific provisions are made when there is doubt as to collectability of individual balances. In evaluating the collectability of individual receivable balances, the Company considers many factors, including the age of the balance, the customer’s payment history, the customer’s current credit-worthiness and current economic trends.
Inventories
Inventories mainly consist of finished goods purchased from suppliers. Inventories are stated at the lower of cost or net realizable value. The cost of an inventory item is determined using the weighted average method. An allowance is established when management determines that certain inventories may not be saleable. If inventory costs exceed net realizable value, the Company will record a reserve for the difference between the cost and the net realizable value. The net realizable value is determined based on the estimated selling price, in the ordinary course of business, less estimated costs necessary to make the sale.
Property, plant and equipment, net
Property, plant and equipment are stated at cost less accumulated depreciation and depreciated on a straight-line basis over the estimated useful lives of the assets from the time the assets are placed in service. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its existing use. The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized.
F-24
When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income/loss in the year of disposition. Estimated useful lives are as follows:
| Estimated Useful Life | ||
| Office and other equipment | 3 - 5 years | |
| Leasehold improvements | Shorter of the term of the lease or the estimated useful life of the assets |
Leases
A contract is, or contains, a lease if the contract conveys a right to control the use of an identified asset for a period of time in exchange of consideration. Control is conveyed where the customer has both the right to obtain substantially all of the economic benefits from use of the identified asset and the right to direct the use of the identified asset. All leases with an initial term of more than 12 months are recognized as assets representing the right-of-use of the underlying asset and liabilities representing the obligation to make lease payments. Both the assets and the liabilities are initially measured as present value of the discounted lease payments over the lease term. As the Company’s leases typically do not provide an implicit rate, the Company uses an estimate of its incremental borrowing rate based on the information available at the lease commence date to determine the discount rate. Right-of-use assets are measured at cost less any accumulated depreciation and impairment losses, and adjusted for any re-measurement of the lease liabilities. Right-of-use assets are depreciated on a straight-line basis over the shorter of the useful lives of the assets or the lease terms. Lease liabilities are initially measured at the present value of the lease payments to be made under the lease terms and subsequently adjusted by the effect of the interest on and the settlement of the lease liabilities, and the re-measurement arising from any reassessment of the lease liabilities or lease modifications.
The Company applies the short-term lease with term of 12 months or less recognition exemption to its short-term leases of office premises and warehouse. Lease payments on short-term leases and leases of low-value assets are recognized as an expense on a straight-line basis over the lease term.
Accounts payable
Accounts payable represents payables to suppliers.
Contract liabilities
Contract liabilities represent advanced deposits received from customers after an order has been placed but before a product has been shipped. The Company’s normal policy is to require a customer deposit of around 25% to 30% of the purchase price upon placement of a sales order, although the Company exempts certain customers from this requirement. Contract liabilities are realized as revenue when control of goods has transferred to customers.
Impairment of long-lived assets
In accordance with ASC Topic 360-10, Impairment and Disposal of Long-Lived Assets, the Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. The Company recognizes an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its book value. The Company did not record any impairment charge for the years ended June 30, 2021 and 2022.
Revenue recognition
The Company sells its products to customers around the world and recognizes revenue in accordance with the guidance of ASC 606, Revenue from Contracts with Customers. Revenue is recognized when control of goods has transferred to customers. For the majority of the Company’s customer arrangements, control transfers to customers at a point-in-time when goods have been delivered to the pickup location specified by the customer or a forwarder appointed by the customer, as that is generally when legal title, physical possession and risks and rewards of goods transfer to the customer.
Revenue is recognized at the transaction price based on the purchase order as adjusted for the anticipated rebates, discounts and other sales incentives. When determining the transaction price, management estimates variable consideration applying the portfolio approach practical expedient under ASC 606. The main sources of variable consideration for the Company are customer rebates, trade promotion funds, and cash discounts. These sales incentives are recorded as a reduction of revenue at the time of the initial sale using the most-likely amount estimation method. The most-likely amount method is based on the single most likely outcome from a range of possible consideration outcomes. The range of possible consideration outcomes is primarily derived from the following inputs: sales terms, historical experience, trend analysis, and projected market conditions in the various markets served. Because the Company serves numerous markets, the sales incentive programs offered vary across businesses, but the most common incentive relates to amounts paid or credited to customers for achieving defined volume levels or growth objectives.
F-25
There are no material instances where variable consideration is constrained and not recorded at the initial time of sale. Product returns are recorded as a reduction of revenue based on anticipated sales returns that occur in the normal course of business. The Company has elected to present revenue net of sales taxes and other similar taxes.
The Company’s warranties are of an assurance-type and come standard with all Company products to cover repair or replacement should a product not perform as expected. The Company offers warranty for all major products, including all types of E-vapor kits, atomizers, replacement coils and mods, but no warranty for accessories such as spare parts or packaging consumables. The Company generally offers a 90 day warranty period from date of purchase for products sold to all regions, but from May 2019, the Company offers a six month warranty period from date of purchase for products sold in the UK and France. The warranty offers refund or replacement of products for manufacturer defective items, dead on arrival items and items that do not appear the same as listed on the Company’s or distributors’ website, and excludes damaged goods caused by misuse or unauthorized repair. Provisions for estimated expenses related to product warranties are made at the time products are sold. These estimates are established using historical information about the nature, frequency and average cost of warranty claim settlements as well as product manufacturing and recovery from suppliers. Management actively studies trends of warranty claims and takes action to improve product quality and minimize warranty costs. The Company estimates the actual historical warranty claims coupled with an analysis of unfulfilled claims to record a liability for specific warranty purposes. As of June 30, 2021 and 2022, products returned for repair or replacement have been immaterial. Accordingly, a warranty liability has not been deemed necessary.
Disaggregated Revenue
In accordance with ASC 606-10-50-5, the Company has taken into consideration the nature, amount, timing, and uncertainty of revenue and cash flows, and has determined to disaggregate its net sales of tobacco products and cannabis products. The net sales disaggregated by products for the years ended June 30, 2021 and 2022 were as follows:
| Years ended June 30, | ||||||||
| Net sales by products branded | 2021 | 2022 | ||||||
| Aspire - tobacco | $ | 61,270,660 | $ | 68,116,810 | ||||
| Ispire - cannabis | 2,144,123 | 19,978,608 | ||||||
| Total | $ | 63,414,783 | $ | 88,095,418 | ||||
Cost of revenue
Cost of revenue for the years ended June 30, 2021 and 2022 consisted primarily of the cost of purchasing vaping products, which were purchased from a related party. See Note 12.
Shipping and handling costs
Shipping and handling costs for the years ended June 30, 2021 and 2022 are included in the sales and marketing expenses.
Interest income
For the years ended June 30, 2021 and 2022, interest income related to interest on bank deposits.
Income taxes
The Company accounts for income taxes under ASC 740. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax bases.
Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period including the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
The provisions of ASC 740-10 prescribe a more-likely-than-not threshold for consolidated financial statement recognition and measurement of a tax position taken (or expected to be taken) in a tax return. This interpretation also provides guidance on the recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, and related disclosures. The Company classifies the interest and penalties, if any, as a component of income tax expense. For the years ended June 30, 2021 and 2022, the Company did not incur any interest or penalties related to an uncertain tax position. The Company does not believe that there was any uncertain tax positions as of June 30, 2021 and 2022.
F-26
Foreign currency translation
The reporting currency of the Company is the U.S. dollar (“USD”). The functional currency of Aspire Science, which is located in Hong Kong, is the Hong Kong Dollar (“HKD”). For the entities whose functional currency is the HKD, results of operations and cash flows are translated at average exchange rates during the period, assets and liabilities are translated at the unified exchange rate at the end of the period, and equity is translated at historical exchange rates. As a result, amounts relating to assets and liabilities reported on the statements of cash flows may not necessarily agree with the changes in the corresponding balances on the balance sheets. Translation adjustments resulting from the process of translating the local currency financial statements into USD are included in determining comprehensive income/loss. Transactions denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing on the transaction dates. Assets and liabilities denominated in foreign currencies are translated into the functional currencies at the exchange rates prevailing at the balance sheet date with any transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
Translations of amounts from HKD into USD were made at the following exchange rates for the respective dates and periods:
| At June 30, | ||||||||
| 2021 | 2022 | |||||||
| Consolidated balance sheets: | ||||||||
| HKD to $1.00 | 7.7638 | 7.8478 | ||||||
| Consolidated statements of operations and comprehensive income: | ||||||||
| HKD to $1.00 | 7.7562 | 7.8045 | ||||||
Earnings per share
The Company computes earnings per share (“EPS”) in accordance with ASC 260, Earnings per Share (“ASC 260”). ASC 260 requires companies with complex capital structures to present basic and diluted EPS. Basic EPS is measured as net income divided by the weighted average common shares outstanding for the period. Diluted EPS is similar to basic EPS but presents the dilutive effect on a per share basis of potential common shares (for example, convertible securities, options and warrants) as if they had been converted at the beginning of the periods presented, or issuance date, if later. The Company has no dilutive securities as of and for the years ended June 30, 2021 and 2022.
Comprehensive income
Comprehensive income consists of two components, net income and other comprehensive (loss) income. The foreign currency translation gain or loss resulting from translation of the financial statements expressed in USD is reported in other comprehensive (loss) income in the consolidated statements of income and comprehensive income.
Commitments and contingencies
In the normal course of business, the Company is subject to contingencies, such as legal proceedings and claims arising out of its business, which cover a wide range of matters. Liabilities for contingencies are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated.
If the assessment of a contingency indicates that it is probable that a material loss is incurred and the amount of the liability can be estimated, then the estimated liability is accrued in the Company’s financial statements. If the assessment indicates that a potentially material loss contingency is not probable, but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss, if determinable and material, is disclosed.
Loss contingencies considered remote are generally not disclosed unless they involve guarantees, in which case the nature of the guarantee would be disclosed.
Segment reporting
The Company uses the management approach to determine operating segments. The management approach considers the internal organization and reporting used by the Company’s chief operating decision maker (“CODM”) for making decisions, allocating resources, and assessing performance. The Company’s CODM has been identified as the chief executive officer, who reviews consolidated results when making decisions about allocating resources and assessing performance of the Company.
F-27
The Company’s CODM reviews the consolidated financial results when making decisions about allocating resources and assessing the performance of the Company as a whole and has determined that the Company has only one reportable segment. Notwithstanding that the Company has customers located around the world and the Company’s Hong Kong subsidiary serves as one of the sales and marketing centers, the Company’s long-lived assets and management are located substantially in the U.S. and management operates its business as a single segment.
Related parties
Parties are considered to be related to the Company if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management, immediate family members of principal owners of the Company and other parties with which the Company may deal with if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests. The Company discloses all significant related party transactions in Note 12.
Recent accounting pronouncements
As an emerging growth company, the Company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. The Company intends to take advantage of the benefits of this extended transition period.
Accounting pronouncements adopted during the year ended June 30, 2022
In November 2018, the FASB issued ASU 2018-18, “Collaborative Arrangements (Topic 808): Clarifying the Interaction Between Topic 808 and Topic 606,” which clarifies that elements of collaborative arrangements could qualify as transactions with customers in the scope of ASC 606. The amendments require the application of existing guidance to determine the units of account in collaborative arrangement for purposes of identifying transactions with customers. For transactions outside the scope of ASC 606, companies can apply elements of ASC 606 or other relevant guidance by analogy, or apply a reasonable accounting policy if there is no appropriate analogy. ASU 2018-18 is effective retrospectively for us for the year ending June 30, 2022. The adoption of this guidance had no material impact on our financial position, results of operations and cash flows.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842), to increase the transparency and comparability about leases among entities. The new guidance requires lessees to recognize a lease liability and a corresponding lease asset for virtually all lease contracts. It also requires additional disclosures about leasing arrangements. ASU 2016-02 is effective for fiscal years beginning after December 15, 2020, and interim periods within fiscal years beginning after December 15, 2021. FASB delayed the effective date for non-public companies (including smaller reporting companies and emerging growth companies as defined by the SEC) with ASU 2020-05 to be effective for fiscal years beginning after December 15, 2021 and interim periods within fiscal years beginning after December 15, 2022. The Company adopted the ASU on July 1, 2022 modified retrospectively. The effect on net income for June 2021 was $333,841, and the cumulative effect on retained earnings as of July 1, 2020 was $86,645.
Accounting pronouncements not yet effective
As the Company is an emerging growth company, the effective dates of the pronouncements applicable to us are the same as those applicable to private companies.
In June 2016, the Financial Accounting Standards Boards (“FASB”) amended guidance related to the impairment of financial instruments as part of ASU 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. The guidance replaces the incurred loss impairment methodology with an expected credit loss model for which a company recognizes an allowance based on the estimate of expected credit loss. For public business entities that meet the definition of a U.S. Securities and Exchange Commission (“SEC”) filer (“SEC filer”), excluding entities eligible to be smaller reporting companies (SRCs) as defined by the SEC, ASU No. 2016-13 is effective for fiscal years beginning after December 15, 2019, including interim periods within those fiscal years. For all other entities, including SRCs, ASU No. 2016-13 is effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years. As an emerging growth company, the Company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. The Company intends to take advantage of the benefits of this extended transition period. The Company is in the process of evaluating the impact that this guidance will have on its consolidated financial statements.
F-28
In January 2017, the FASB issued ASU 2017-04, “Intangibles — Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment,” which simplifies how an entity is required to test goodwill for impairment by eliminating step two from the goodwill impairment test. Step two of the goodwill impairment test measures a goodwill impairment loss by comparing the implied fair value of a reporting unit’s goodwill with its carrying amount. ASU 2017-04 was effective for public business entities that meet the definition of an SEC filer, excluding entities eligible to be SRCs as defined by the SEC, should adopt the amendments in this Update for its annual or any interim goodwill impairment tests in fiscal years beginning after December 15, 2019. All other entities should adopt the amendments in this Update for its annual or any interim goodwill impairment tests in fiscal years beginning after December 15, 2022. Early adoption is permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. We do not expect that the adoption of this guidance will have a material impact on our financial position, results of operations and cash flows.
In April 2019, the FASB issued ASU 2019-04, “Codification Improvements to Topic 326, Financial Instruments – Credit Losses, Topic 815, Derivatives and Hedging, and Topic 825, Financial Instruments”, which provides narrow-scope amendments to clarify and improve guidance within the standards on credit losses, hedging, and recognition and measurement of financial instruments. Apart from the amendments to ASU 2016-13 mentioned above, the ASU also included subsequent amendments to ASU 2016-01. We do not expect that the adoption of this guidance will have a material impact on the financial position, results of operations and cash flow.
In October 2018, the FASB issued ASU 2018-17, Consolidation (Topic 810): Targeted Improvements to Related Party Guidance for Variable Interest Entities, (“ASU 2018-17”). ASU 2018-17 requires reporting entities to consider indirect interests held through related parties under common control on a proportional basis rather than as the equivalent of a direct interest in its entirety for determining whether a decision-making fee is a variable interest. For entities other than private companies, the standard is effective for fiscal years beginning after December 15, 2019, and interim periods within those fiscal years. The ASU is effective for a private company for fiscal years beginning after December 15, 2020, and interim periods within fiscal years beginning after December 15, 2021. Early adoption is permitted. Entities are required to apply the amendments in ASU 2018-17 retrospectively with a cumulative-effect adjustment to retained earnings at the beginning of the earliest period presented. We do not expect that the adoption of this guidance will have a material impact on our financial position, results of operations and cash flows.
Concentration and risks
Risks and Uncertainties
The Company’s business, financial condition and results of operations may be negatively impacted by risks related to government regulations, natural disasters, extreme weather conditions, health epidemics and other catastrophic incidents, which could significantly disrupt the Company’s operations.
Customer and Supplier Concentration
(a) Customers
For the years ended June 30, 2021 and 2022, the Company’s major customers, who accounted for more than 10% of the Company’s consolidated revenue, were as follow:
| Year Ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| Major Customers | ||||||||
| A | 51 | % | 39 | % | ||||
(b) Suppliers
For the years ended June 30, 2021 and 2022, the Company’s suppliers, who accounted for more than 10% of the Company’s total purchases, were as follows:
| Year Ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| Major Suppliers | ||||||||
| B(1) | 99 | % | 99 | % | ||||
| (1) | Major supplier B is Shenzhen Yi Jia, a Chinese company that is 95% owned by the Company’s chief executive officer and principal stockholder. See Note 12. |
F-29
Credit Risk
The Company is subject to credit risk from cash and cash equivalents, account receivables, financial assets included in prepayments and deposits and amounts due from related parties. All the Company’s cash and cash equivalents are held in major financial institutions located in Hong Kong and the United States, which management believes are of high credit quality. At June 30, 2021 and 2022, the Company had credit risk exposure of uninsured cash in banks of $84,932,856 and $74,000,991, respectively. The Company has policies in place to evaluate credit risk when accepting new business and to limit its credit exposure to individual customers. The management considers the Company does not have a significant concentration of credit risk. The Company does not require collateral to support financial instruments that are subject to credit risk.
3. CASH AND CASH EQUIVALENTS
Below is a breakdown of the Company’s cash balances in banks for both years, both by geography and by currencies (translated into U.S. dollars):
| As of June 30, | ||||||||
| By Geography: | 2021 | 2022 | ||||||
| Cash in HK | $ | 85,050,144 | $ | 71,221,649 | ||||
| Cash in U.S. | 198,853 | 3,259,002 | ||||||
| Total | $ | 85,248,997 | $ | 74,480,651 | ||||
| By Currency: | ||||||||
| USD | $ | 84,987,908 | $ | 64,187,756 | ||||
| HKD | 176,459 | 415,930 | ||||||
| EUR | 56,456 | 4,097 | ||||||
| GBP | 28,174 | 24,680 | ||||||
| RMB | - | 9,848,188 | ||||||
| Total | $ | 85,248,997 | $ | 74,480,651 | ||||
“HKD” refers to Hong Kong dollars, “GBP” refers to British pounds, and “EUR” refers to Euros.
4. FAIR VALUE MEASUREMENT
As of June 30, 2021 and 2022, information about inputs into the fair value measurement of the Company’s assets and liabilities that are measured at fair value on a recurring basis in periods subsequent to their initial recognition is as follows:
Cash and cash equivalents, accounts receivable, prepaid expenses, other receivables and due from related parties are financial assets with carrying values that approximate fair value due to their short-term nature. Accounts payable, account payable – related party, contract liabilities, accrued liabilities and other payables and due to related parties are financial liabilities with carrying values that approximate fair value due to their short-term nature.
5. ACCOUNTS RECEIVABLE
As of June 30, 2021 and 2022, accounts receivable consisted of the following:
| As of June 30, | ||||||||
| 2021 | 2022 | |||||||
| Accounts receivable – gross | $ | 4,305,271 | $ | 8,260,574 | ||||
| Allowance for doubtful accounts | - | - | ||||||
| Accounts receivables | $ | 4,305,271 | $ | 8,260,574 | ||||
The Company recorded bad debt expense of $272,235 and nil for years ended June 30, 2021 and 2022 respectively.
6. PREPAID EXPENSES AND OTHER CURRENT ASSETS
As of June 30, 2021 and 2022, prepaid expenses and other current assets consisted of the following:
| As of June 30, | ||||||||
| 2021 | 2022 | |||||||
| Prepaid provisional tax | $ | 660,406 | $ | - | ||||
| Deposit paid | 81,626 | 14,616 | ||||||
| Prepayment | 33,023 | 50,460 | ||||||
| Other receivable | - | 127,423 | ||||||
| Total | $ | 775,055 | $ | 192,499 | ||||
Prepayments primarily consist of prepayment for raw materials and consulting services provided by suppliers.
F-30
7. PROPERTY, EQUIPMENT AND LEASEHOLD IMPROVEMENT, NET
As of June 30, 2021 and 2022, property, equipment and leasehold improvement consisted of the following:
| As of June 30, | ||||||||
| 2021 | 2022 | |||||||
| Leasehold improvement | $ | 438 | $ | 433 | ||||
| Office and other equipment | 25,277 | 146,798 | ||||||
| 25,715 | 147,231 | |||||||
| Less: accumulated depreciation | (22,804 | ) | (33,206 | ) | ||||
| Total | $ | 2,911 | $ | 114,025 | ||||
For the years ended June 30, 2021 and 2022, depreciation expense amounted to $3,634 and $11,437, respectively.
8. CONTRACT LIABILITIES
As of June 30, 2021 and 2022, the Company had total contract liabilities of $1,125,848 and $1,672,051, respectively. These liabilities are advance deposits received from customers after an order has been placed. The balance of $1,125,848 as of June 30, 2021 was recognized as revenue during 2022. As of June 30 2022, the Company expects all of the contract liabilities to be settled in less than one year. The increase in balance at June 30, 2022 was due to more orders on hand on that date.
9. LEASES
The Company has operating lease arrangements for office premises for HK and California. These leases typically have terms of two to three years.
The right-of-use assets obtained in exchange for operating lease liabilities at July 1, 2019 were $159,740.
Leases with an initial term of 12 months or less are not presented as right-of-use assets on the consolidated balance sheet and are expensed over the lease term. All other lease assets and lease liabilities are recognized based on the present value of lease payments over the lease term at commencement date.
The balances for the right-of-use assets where the Company is the lessee are presented as follow:
| As of June 30, | ||||||||
| 2021 | 2022 | |||||||
| Right-of-use assets | $ | 440,573 | $ | 295,804 | ||||
| Lease liabilities - current | $ | 207,972 | $ | 347,541 | ||||
| Lease liabilities – non-current | 270,140 | - | ||||||
| Total | $ | 478,112 | $ | 347,541 | ||||
As of June 30, 2022, the maturities of our lease liabilities (excluding short-term leases) are as follows:
| As of June 30, 2022 | ||||
| Undiscounted liabilities due in 2023 | 358,539 | |||
| Less: imputed interest | 10,998 | |||
| Total lease liabilities | 347,541 | |||
The Company incurred lease costs, which includes the amortization of the right-of-use assets and the payment of short-term leases, of $502,966 and $667,712 on the Company’s consolidated statements of operations and comprehensive income(loss) for the years ended June 30, 2021 and 2022, respectively.
The Company made payments of $298,291 and $304,291 under the lease agreements during the years ended June 30, 2021 and 2022, respectively.
The weighted-average remaining lease term related to the Company’s lease liabilities as of June 30, 2021 and 2022 was 2 and 1 years, respectively.
The discount rate related to the Company’s lease liabilities as of both June 30, 2021 and June 30, 2022 was 6% and 5.8%. The discount rate are generally based on estimates of the Company’s incremental borrowing rate, as the discount rates implicit in the Company’s leases cannot be readily determined.
F-31
As of June 30, 2022, the Company had $4.0 million of future payments under additional leases, primarily for office and warehouse, which had not yet commenced. This lease, which has a five-year term, will commence in July 2022.
The right-of-use assets obtained in exchange for new lease liabilities in the year ended June 30, 2021 are as follows:
| Years ended June 30, 2021 | ||||
| Total operating lease cost: | 502,966 | |||
| Weighted-average remaining lease term | 2 years | |||
| Weighted-average discount rate | 6 | % | ||
10. ACCRUED LIABILITIES AND OTHER PAYABLES
As of June 30, 2021 and 2022, accrued liabilities and other payables consisted of the following:
| As of June 30, | ||||||||
| 2021 | 2022 | |||||||
| Accrued salaries and related benefits | $ | 43,749 | $ | 43,487 | ||||
| Other payables | 33,958 | 81,226 | ||||||
| Accrued expenses | 32,381 | 34,583 | ||||||
| Taxes other than income tax payable | 9,147 | - | ||||||
| Total | $ | 119,235 | $ | 159,296 | ||||
11. DIVIDENDS PAYABLE
Dividends payable represent a dividend declared by the Company’s HK subsidiary, Aspire Science, in the year ended June 30, 2020, which is payable to Aspire Science’s then sole stockholder, who is the Company’s chief executive officer. The dividend was declared prior to the transfer of the equity interest in Aspire Science to Aspire Holdings, which subsequently transferred the equity interest to Ispire International. Set forth below is the information relating to the dividend at June 30, 2021 and 2022.
| As of June 30, | ||||||||
| 2021 | 2022 | |||||||
| At the beginning of the year | $ | 3,832,272 | $ | 3,832,272 | ||||
| Dividends declared | - | - | ||||||
| Dividends paid | - | (469,633 | ) | |||||
| At the end of the year | $ | 3,832,272 | $ | 3,362,639 | ||||
12. RELATED PARTY TRANSACTIONS
a) The table below sets forth the major related parties and their relationships with the Company:
| Name of related parties and Relationship with the Company |
| -Tuanfang Liu is the Chairman of the Company. |
| -Jiangyan Zhu is the wife of Tuanfang Liu and a director of the Company. |
| -Eigate (Hong Kong) Technology Co., Limited (“Eigate”) is a wholly-owned subsidiary of Aspire Global. |
| -Aspire Global is a company controlled by the Chairman of the Company. |
| -Shenzhen Yi Jia, a Chinese company that is 95% owned by the Company’s chairman and 5% by the chairman’s cousin. |
b) Tuanfang Liu is also Aspire Global’s chief executive officer and a director of both the Company and Aspire Global, and his wife, Jiangyan Zhu, is also a director of both companies. Mr. Liu and Ms. Zhu beneficially own 66.5% and 5.0%, respectively, of the outstanding shares of both Aspire Global and the Company. See Note 14.
F-32
c) The Company had the following balances due from related parties:
| As of June 30, | ||||||||
| 2021 | 2022 | |||||||
| Shenzhen Yi Jia | $ | 31,297 | $ | 1,872,035 | ||||
| Tuanfang Liu | 4,924 | 62,820 | ||||||
| Total | $ | 36,221 | $ | 1,934,855 | ||||
The balances represent payment on behalf of these related parties, such as freight and tariff charges and others. These balances were all non-interest bearing, unsecured, have no due date and are repayable on demand.
d) See Note 14 with respect to the issuance by the Company of 50,000,000 shares of common stock.
e) The balances in due to related parties at June 30, 2022 and 2021 represent amount due to Eigate of $41,172,013 and $40,672,768, respectively. These balances were all non-interest bearing, unsecured, have no due date and are repayable on demand.
f) For the years ended June 30, 2021 and 2022, substantially all of the Company’s tobacco and cannabis products were purchased from Shenzhen Yi Jia. As of June 30, 2021 and June 30, 2022, the accounts payable - related party was $ 33,370,618 and $41,982,373, respectively, which was payable to Shenzhen Yi Jia. For the years ended June 30, 2021 and 2022, the purchases from Shenzhen Yi Jia were $52,998,928 and $74,787,679, respectively.
13. INCOME TAXES
British Virgin Islands (“BVI”)
Under the current laws of the BVI, the Company’s BVI subsidiary, Ispire International, is not subject to income or capital gains taxes. In addition, dividend payments are not subject to withholding tax in the BVI.
Hong Kong
On March 21, 2018, the Hong Kong Legislative Council passed The Inland Revenue (Amendment) (No. 7) Bill 2017 (the “Bill”) which introduces the two-tiered profits tax rates regime. The Bill was signed into law on March 28, 2018 and was announced on the following day. Under the two-tiered profits tax rates regime, the first 2 million HKD of profits of the qualifying entity will be taxed at 8.25%, and profits above HKD 2 million will be taxed at 16.5%.
United States
On December 22, 2017, the United States enacted the Tax Cuts and Jobs Act, which lowered the United States statutory federal income tax rate from 35% to 21% effective January 1, 2018. The Company and Aspire North America LLC will be subject to the federal income tax rate individually if in a taxable position.
For the years ended June 30, 2021 and 2022, income(loss) before income taxes consists of:
| Years ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| HK | $ | 5,463,770 | $ | 6,679,431 | ||||
| U.S. | (1,840,141 | ) | (7,482,487 | ) | ||||
| Total | $ | 3,623,629 | $ | (803,056 | ) | |||
F-33
The reconciliation of the actual income taxes to the amount of tax computed by applying the aforementioned statutory tax rate to pre-tax income is as follows:
| Years ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| Expected taxation at HK statutory rate | $ | 592,762 | $ | (132,504 | ) | |||
| Tax effect of two-tiered profits tax regime | (21,273 | ) | (21,142 | ) | ||||
| Effect of income tax rate difference in other jurisdictions | (84,207 | ) | (336,712 | ) | ||||
| Non-deductible expenses | 33,725 | 116,287 | ||||||
| Non-taxable income | (191,365 | ) | (10,764 | ) | ||||
| Change in valuation allowance | 357,650 | 1,455,390 | ||||||
| Others | (1,469 | ) | 542 | |||||
| Income tax expense | $ | 685,823 | $ | 1,071,097 | ||||
For the years ended June 30, 2021 and 2022, there are net operating losses of $1,703,094 and $6,930,431 that arose from Aspire North America LLC, which can be carried forward indefinitely to offset up to 80% of each year’s taxable income, until fully utilized. At June 30, 2021 and 2022, these net operating loss carryforwards may result in future income tax benefits of $357,650 and $1,813,040, respectively.
Valuation allowances provided against the deferred tax assets are related to the net operating loss carryforwards, as the Company’s management does not believe that sufficient positive evidence exists to conclude that the benefits of such deferred tax assets are more likely than not to be realized in full. The amount of the valuation allowance as of June 30, 2021 and 2022 was $357,650 and $1,813,040, respectively.
Deferred tax assets and liabilities represent the future effects on income taxes that result from temporary differences and carryforwards that exist at the balance sheet date, and are measured using enacted rates and provisions of the tax law. Deferred tax assets are recognized for deductible temporary differences as well as tax attributes.
Significant components of the Company’s deferred tax liabilities and assets as of June 30, 2021 and 2022 are as follows:
| Years ended June 30, | ||||||||
| Deferred tax asset: | 2021 | 2022 | ||||||
| Net operating loss carryforward | $ | 357,650 | $ | 1,813,040 | ||||
| Valuation allowance | (357,650 | ) | (1,813,040 | ) | ||||
| Net deferred tax asset | $ | - | $ | - | ||||
Movement of valuation allowance:
| Years ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| At the beginning of the year | $ | - | $ | 357,650 | ||||
| Current year addition | 357,650 | 1,455,390 | ||||||
| At the end of the year | $ | 357,650 | $ | 1,813,040 | ||||
The Company is subject to income taxes in the U.S. federal, state, and various foreign jurisdictions. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment to apply. All of the Company’s tax years will remain open for examination by the US federal and state tax authorities from the date the returns are filed or are due, whichever is later. The Company does not have any tax audits or other issues pending.
F-34
14. STOCKHOLDERS’ EQUITY
The Company’s authorized capital stock consists of 10,000,000 shares of preferred stock, par value $0.0001 per share, and 140,000,000 shares of common stock, par value $0.0001 per share, of which 50,000,000 shares are outstanding as of June 30, 2021 and 2022. See Note 1.
15. EARNINGS PER SHARE
The following table presents a reconciliation of basic net income per share:
| Years ended June 30, | ||||||||
| 2021 | 2022 | |||||||
| Net income(loss) | $ | 2,937,806 | $ | (1,874,153 | ) | |||
| Weighted average basic and diluted share of common stock outstanding | 50,000,000 | 50,000,000 | ||||||
| Net income(loss) per basic and diluted share of common stock | $ | 0.06 | $ | (0.04 | ) | |||
16. SUBSEQUENT EVENT
In October 2022, the directors and stockholders of the Company approved the 2022 Equity Incentive Plan (the “Plan”) pursuant to which up to 15,000,000 shares of common stock may be issued pursuant to options or restricted stock grants. The Plan will be administered by the Compensation Committee. Awards under the Plan may be granted to officers, directors, employees and those consultants who qualify as a consultant or advisor under the instructions to Form S-8. The Compensation Committee has broad discretion in making awards; provided that any options shall be exercisable at the fair market value on the date of grant. As of the date of this report, no awards have been granted under the Plan.
On September 30, 2022, an intellectual property transfer agreement was signed among Tuanfang Liu, Aspire Global, Shenzhen Yi Jia, Aspire North America and the Company. Pursuant to the agreement, Tuanfang Liu, Aspire Global and Shenzhen Yi Jia agreed to transfer to Aspire North America all patent and other intellectual property rights, including trademarks, Know-how and Know-how Documentation, as defined in the agreement, relating to the cannabis vaping products, and to transfer to the Company any new intellectual property developed or acquired by Tuanfang Liu, Aspire Global and Shenzhen Yi Jia which relates to cannabis vaping products. The formal transfer of the patents has commenced, and a significant portion of the patents has been transferred. The Company expects that the transfer of the patents will be completed by December 31, 2023.
On September 30, 2022, an exclusive license agreement was signed among Tuanfang Liu, Aspire Global, Shenzhen Yi Jia, Aspire Science and the Company. Pursuant to the agreement, Tuanfang Liu, Aspire Global and Shenzhen Yi Jia granted Aspire Science a perpetual royalty free sole and exclusive right and license to use and practice all of the Licensed Technology worldwide except for the PRC and Russia. The Licensed Technology includes all patents, Know-how, Know-how documentation, as defined in the agreement, and trademarks, whether now existing or hereafter developed or acquired by, or for, Tuanfang Liu, Aspire Global and/or Shenzhen Yi Jia that relate, directly or indirectly, to the tobacco vaping market. Pursuant to the License Agreement, neither Tuanfang Liu, Aspire Global nor Shenzhen Yi Jia has any right to market or sell or grant distributors the right to market or sell tobacco vaping products in the world other than in the PRC and Russia.
F-35
PART II - INFORMATION NOT REQUIRED IN PROSPECTUS
The following table sets forth an itemized statement of the amounts of all expenses (excluding underwriting discounts and commissions and non-accountable expense allowance) payable by us in connection with the registration of the common stock offered hereby. With the exception of the SEC registration fee, the FINRA filing fee and the NASDAQ initial listing fee, the amounts set forth below are estimates.
| SEC registration fee | $ | 8,006.03 | ||
| FINRA filing fee | 88,475.00 | |||
| NASDAQ initial listing fee | 75,000.00 | |||
| Representative’s out-of-pocket expenses | 450,000.00 | |||
| Transfer agent fees | 25,000.00 | |||
| Accounting fees and expenses | 750,000.00 | |||
| Legal fees and expenses | 500,000.00 | |||
| Printing and engraving expenses | 46,000.00 | |||
| Other expenses | 7,518.97 | |||
| Total | $ | 1,950,000.00 |
Item 14. Indemnification of Directors and Officers
The Company’s certificate of incorporation provides that the Company shall indemnify to the fullest extent permitted by law as it presently exists or may hereafter be amended any person made or threatened to be made a party to an action or proceeding, whether criminal, civil, administrative, or investigative, by reason of the fact that such person, or such person’s testator or intestate, is or was a director or officer of the Company or any predecessor of the Company, or serves or served at any other enterprise as a director or officer at the request of the Company or any predecessor to the Corporation. Any amendment, repeal, or modification of this provision of the Company’s certification shall not adversely affect any right or protection hereunder of any person in respect of any act or omission occurring prior to the time of such repeal or modification.
The Company’s certificate of incorporation provides that to the fullest extent permitted by the Delaware General Corporation Law as it presently exists or may hereafter be amended, a director of the Company shall not be personally liable to the Company or to its stockholders for monetary damages for any breach of fiduciary duty as a director. No amendment to, modification of, or repeal of this provision of the certificate of incorporation shall apply to or have any effect on the liability or alleged liability of any director of the Corporation for or with respect to any acts or omissions of such director occurring prior to such amendment.
Section 145 of the Delaware General Corporation Law gives the Company broad authority to indemnify our officers and directors. under certain prescribed circumstances and subject to certain limitations against certain costs and expenses, including attorney’s fees actually and reasonably incurred in connection with any action, suit or proceeding, whether civil, criminal, administrative or investigative, to which a person is a party by reason of being a director or officer if it is determined that such person acted in accordance with the applicable standard of conduct set forth in such statutory provisions.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Item 15. Recent Sales of Unregistered Securities
In connection with the Company’s organization, the Company issued 50,000,000 shares of common stock to the stockholders of Aspire Global Inc. The shares were issued in connection with a restructure of Aspire Global, in connection with which 100% of the equity of Aspire North America LLC and Aspire Science and Technology Limited, which were wholly-owned direct or indirect subsidiaries of Aspire Global, was transferred to the Company on July 29, 2022. The issuance of the shares was exempt from registration pursuant to Section 4(a)(2) of the Securities Act of 1933, as amended, as a transaction not involving a public offering.
II-1
Item 16. Exhibits and Financial Statement Schedules
| 1 | Previously filed |
| 2 | To be filed by amendment |
| 3 | Included on the signature page of initial filing |
| 4 | Filed herewith |
| 5 | The form of independent director agreement for all three independent directors is filed as Exhibit 10.7 |
| # | Certain confidential information has been deleted from this Exhibit pursuant to both Item 601(a)(5) and Item 601(b)(10)(iv). |
| † | Compensatory plan, contract or arrangement |
The agreements included as exhibits to this registration statement contain representations and warranties by each of the parties to the applicable agreement. These representations and warranties were made solely for the benefit of the other parties to the applicable agreement and (i) were not intended to be treated as categorical statements of fact, but rather as a way of allocating the risk to one of the parties if those statements prove to be inaccurate; (ii) may have been qualified in such agreement by disclosures that were made to the other party in connection with the negotiation of the applicable agreement; (iii) may apply contract standards of “materiality” that are different from “materiality” under the applicable securities laws; and (iv) were made only as of the date of the applicable agreement or such other date or dates as may be specified in the agreement.
We acknowledge that, notwithstanding the inclusion of the foregoing cautionary statements, we are responsible for considering whether additional specific disclosures of material information regarding material contractual provisions are required to make the statements in this registration statement not misleading.
(b) Financial Statement Schedules
Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the Consolidated Financial Statements or the Notes thereto.
II-2
Item 17. Undertakings.
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
| i. | To include any prospectus required by Section 10(a)(3) of the Securities Act; |
| ii. | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective Registration Statement; |
| iii. | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof;
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(5) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(6) That, for the purpose of determining liability under the Securities Act to any purchaser:
Each prospectus filed by the registrant pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
II-3
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized in Los Angeles, California, on February 27, 2023.
| ISPIRE TECHNOLOGY INC. | ||
| By: | /s/ Tuanfang Liu | |
| Tuanfang Liu | ||
| Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the date indicated:
| Signature | Title | Date | ||
| /s/ Tuanfang Liu* | Chief executive officer and director (principal executive officer) | |||
| Tuanfang Liu | ||||
| /s/ Michael Wang* | Chief financial officer (principal financial and accounting officer) | |||
| Michael Wang | ||||
| /s/ Jiangyan Zhu* | Director | |||
| Jiangyan Zhu | ||||
| * By /s/ Tuanfang Liu | ||||
| Tuanfang Liu, Attorney in fact | February 27, 2023 |
II-4

