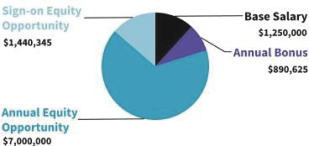
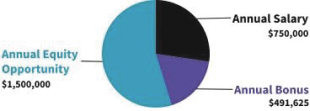
Our business is comprised of four segments that are aligned with the industries we serve:

Imaging Business
GE HealthCare is a global leader in medical imaging with a comprehensive portfolio of scanning devices, clinical applications, service capabilities, and digital solutions. Our Imaging portfolio spans the care continuum and provides critical tools for physicians from initial screening and diagnosis, through therapeutic decision-making, to monitoring of patient progression. Our products are essential in the delivery of care for a broad spectrum of clinical specialties, including oncology, cardiology, neurology, nuclear medicine, orthopedics, women’s health, pediatrics, and surgery.
Our Imaging portfolio is comprised of six product lines and associated service capabilities: Molecular Imaging (“MI”), Computed Tomography (“CT”), Magnetic Resonance (“MR”), Image-Guided Therapies, Women’s Health (“WH”), and X-ray. We manage our MI and CT product lines together (“MI/CT”) and our Women’s Health and X-ray product lines together (“WH/XR”).
| • | MI enables the visualization, characterization, and quantification of functional processes taking place at the cellular and subcellular levels within patients. The images produced by MI systems allow clinicians to study the cellular and molecular pathways and mechanisms of disease in patients. We offer a complete MI solution from cyclotrons, chemistry synthesis, positron emission tomography (“PET”), computed tomography (“PET/CT”), PET/MR, and nuclear medicine to advanced digital solutions. Our MI team works closely with the Pharmaceutical Diagnostics (“PDx”) segment and their innovations and collaborations with pharmaceutical companies. |
| • | CT scans render 3D anatomical images of structures such as bone, soft tissue, and air cavities using an X-ray tube that rotates around a patient. The images are used in a wide variety of applications, including the detection of tumors or lesions, blocked blood vessels in the brain, abnormal heart conditions, complex bone fractures, and internal injuries from trauma. Our comprehensive CT portfolio includes multi-purpose and specialty scanners. |
| • | MR is a sophisticated, non-invasive imaging technology that produces detailed anatomical images of almost every internal structure in the human body, such as the brain, spinal cord, heart, breast, kidneys, muscles, ligaments, and tendons. MR can also be used for functional imaging, and it is well-suited for disease detection, diagnosis, and treatment monitoring of a variety of conditions, including stroke, cancer, trauma, aneurysm, multiple sclerosis, cardiomyopathy, and congenital disorders. Our MR |
94