| PROSPECTUS | Filed Pursuant to Rule 424(b)(3) |
| Registration No. 333-271903 |
MURPHY
CANYON ACQUISITION CORP.
4995 Murphy Canyon Road, Suite 300
San Diego, CA 92123
NOTICE OF
SPECIAL
MEETING OF STOCKHOLDERS
TO BE HELD ON SEPTEMBER 7, 2023
TO THE STOCKHOLDERS OF MURPHY CANYON ACQUISITION CORP.:
NOTICE IS HEREBY GIVEN that a special meeting of stockholders of Murphy Canyon Acquisition Corp. (“MURF”), a Delaware corporation, will be held at 10:00 a.m., Eastern Time, on September 7, 2023, via webcast (the “Special Meeting”). MURF stockholders can attend, vote and examine the list of MURF stockholders entitled to vote at the live webcast of the Special Meeting by visiting www.proxyvote.com before the meeting or www.virtualshareholdermeeting.com/MURF2023SM2 during the meeting and entering the control number found on their proxy card, voting instruction form or notice they previously received. In light of public health concerns regarding the novel coronavirus (COVID-19), the Special Meeting will be held in a virtual meeting format only. You will not be able to attend the Special Meeting physically.
You are cordially invited to virtually attend the Special Meeting, which will be held for the following purposes:
| (1) | to consider and vote upon a proposal to approve the business combination (the “Business Combination”) described in this proxy statement/prospectus, including the Agreement and Plan of Merger, dated as of November 8, 2022 and as amended on January 27, 2023 and May 11, 2023 (the “Merger Agreement”), by and among MURF, Conduit Pharmaceuticals Limited, a Cayman Islands exempted company (“Conduit”) and Conduit Merger Sub, Inc., a Cayman Islands exempted company and a wholly-owned subsidiary of MURF (“Merger Sub”), which, among other things, provides for the merger of Merger Sub with and into Conduit, with Conduit surviving the merger as a wholly-owned subsidiary of MURF — we refer to this proposal as the “business combination proposal”; | |
| (2) | to consider and vote upon separate proposals to approve amendments to MURF’s current amended and restated certificate of incorporation to: |
| (i) | Charter Amendment Proposal A – change the name of the public entity from “Murphy Canyon Acquisition Corp.” to “Conduit Pharmaceuticals Inc.” (“New Conduit”); | |
| (ii) | Charter Amendment Proposal B – provide for one class of authorized common stock; | |
| (iii) | Charter Amendment Proposal C – delete the various provisions in MURF’s current amended and restated certificate of incorporation applicable only to special purpose acquisition corporations (such as the obligation to dissolve and liquidate if a business combination is not consummated within a certain period of time); | |
| (iv) | Charter Amendment Proposal D – increase the number of authorized shares of common stock to 250,000,000; and | |
| (v) | Charter Amendment Proposal E – fix the number of directors at seven (7), a majority of whom shall be independent directors in accordance with The Nasdaq Stock Market LLC’s requirements — we refer to these proposals collectively as the “charter amendment proposals”; |
| (3) | to consider and vote upon, on a non-binding advisory basis, certain governance provisions in the Proposed Charter, presented separately in accordance with U.S. Securities and Exchange Commission (“SEC”) requirements — we refer to this proposal as the “advisory charter amendments proposals”; | |
| (4) | to elect seven (7) directors who, upon consummation of the Business Combination, will be the directors of New Conduit — we refer to this proposal as the “director election proposal”; | |
| (5) | to consider and vote upon a proposal to approve the Conduit Pharmaceuticals Inc. 2023 Stock Incentive Plan (named in anticipation of the Business Combination), which is an incentive compensation plan for employees of New Conduit and its subsidiaries, including Conduit — we refer to this proposal as the “incentive plan proposal”; | |
| (6) | to consider and vote upon a proposal to approve, for purposes of complying with applicable listing rules of Nasdaq, the issuance of New Conduit common stock and warrants to purchase New Conduit common stock, par value $0.0001 per share, to Prospect Science Ventures Limited (the “Private Placement Investor”) in the Private Placement, the proceeds of which will be used to finance the Business Combination and related transactions and the costs and expenses incurred in connection therewith with any balance used for working capital purposes — we refer to this proposal as the “Nasdaq proposal”; and | |
| (7) | to consider and vote upon a proposal to adjourn the Special Meeting to a later date or dates, if necessary, to permit further solicitation and vote of proxies if MURF does not have sufficient proxies to approve one or more of the foregoing proposals — we refer to this proposal as the “adjournment proposal.” |
These items of business are described in the attached proxy statement/prospectus, which we encourage you to read in its entirety before voting. Only holders of record of MURF common stock at the close of business on August 2, 2023 (the “Record Date”), are entitled to notice of the Special Meeting and to vote and have their votes counted at the Special Meeting and any adjournments or postponements of the Special Meeting.
After careful consideration, the MURF Board has determined that the business combination proposal, the charter amendment proposals, the advisory charter amendments proposals, the director election proposal, the incentive plan proposal, the Nasdaq proposal and the adjournment proposal are fair to and in the best interests of MURF and its stockholders and unanimously recommends that you vote or give instruction to vote “FOR” the business combination proposal, “FOR” each of the charter amendment proposals, “FOR” each of the advisory charter amendments proposals, “FOR” the election of all of the persons nominated by MURF’s management for election as directors, “FOR” the incentive plan proposal, “FOR” the Nasdaq proposal and “FOR” the adjournment proposal, if presented.
Consummation of the Transactions (as described in the attached proxy statement/prospectus) is conditioned on approval of each of the business combination proposal, the charter amendment proposals, the director election proposal, the incentive plan proposal, and the Nasdaq proposal. If any of the proposals is not approved, the other proposals will not be presented to stockholders for a vote.
All MURF stockholders are cordially invited to virtually attend the Special Meeting, which will be held via live webcast. To ensure your representation at the Special Meeting, however, you are urged to complete, sign, date and return the enclosed proxy card as soon as possible. If you are a stockholder of record of MURF common stock, you may also cast your vote at the Special Meeting electronically by visiting www.proxyvote.com before the meeting or www.virtualshareholdermeeting.com/MURF2023SM2 during the meeting. If your shares are held in an account at a brokerage firm or bank, you must instruct your broker or bank on how to vote your shares or, if you wish to attend the Special Meeting virtually and vote electronically, obtain a proxy from your broker or bank.
A complete list of MURF stockholders of record entitled to vote at the Special Meeting will be available for ten days before the Special Meeting at the principal executive offices of MURF for inspection by stockholders during ordinary business hours for any purpose germane to the Special Meeting.
Your vote is important regardless of the number of shares you own. Whether you plan to virtually attend the Special Meeting or not, please sign, date and return the enclosed proxy card as soon as possible in the envelope provided. If your shares are held in “street name” or are in a margin or similar account, you should contact your broker to ensure that votes related to the shares you beneficially own are properly counted.
Thank you for your participation. We look forward to your continued support.
| By Order of the Board of Directors | |
| /s/ Jack K. Heilbron | |
| Jack K. Heilbron | |
| Chief Executive Officer, President, and Chairman | |
| August 11, 2023 |
IF YOU ARE A HOLDER OF PUBLIC SHARES AND WISH TO EXERCISE YOUR REDEMPTION RIGHTS, YOU MUST DEMAND THAT MURF REDEEM YOUR SHARES FOR CASH BY DELIVERING YOUR STOCK ELECTRONICALLY TO MURF’S TRANSFER AGENT USING DEPOSITORY TRUST COMPANY’S DWAC (DEPOSIT WITHDRAWAL AT CUSTODIAN) SYSTEM. MURF’S TRANSFER AGENT MUST RECEIVE YOUR INSTRUCTION TO REDEEM BY 5:00 PM EASTERN TIME ON SEPTEMBER 5, 2023 (TWO (2) BUSINESS DAYS BEFORE THE SPECIAL MEETING) IN ORDER FOR YOUR REDEMPTION DEMAND TO BE VALID. IN ORDER TO EXERCISE YOUR REDEMPTION RIGHT, YOU NEED TO IDENTIFY YOURSELF AS A BENEFICIAL HOLDER AND PROVIDE YOUR LEGAL NAME, PHONE NUMBER AND ADDRESS IN YOUR WRITTEN DEMAND. SEE “SPECIAL MEETING OF MURF STOCKHOLDERS — REDEMPTION RIGHTS” FOR MORE SPECIFIC INSTRUCTIONS.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES REGULATORY AGENCY HAS APPROVED OR DISAPPROVED THE TRANSACTIONS DESCRIBED IN THIS JOINT PROXY STATEMENT/PROSPECTUS, PASSED UPON THE MERITS OR FAIRNESS OF THE MERGER OR RELATED TRANSACTIONS OR PASSED UPON THE ADEQUACY OR ACCURACY OF THE DISCLOSURE IN THIS PROXY STATEMENT/PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY CONSTITUTES A CRIMINAL OFFENSE.
This proxy statement/prospectus is dated August 11, 2023 and is first being mailed to Murphy Canyon Acquisition Corp. stockholders on or about August 11, 2023.
Important Notice Regarding the Availability of Proxy Materials for the Stockholder Meeting to Be Held on September 7, 2023. MURF’s proxy statement/prospectus are available at www.proxyvote.com.
PROXY STATEMENT FOR SPECIAL MEETING OF STOCKHOLDERS OF
MURPHY CANYON ACQUISITION CORP.
PROSPECTUS
FOR 65,000,000 SHARES OF COMMON STOCK
OF
MURPHY CANYON ACQUISITION CORP.
The board of directors of Murphy Canyon Acquisition Corp., a Delaware corporation (“MURF”), has unanimously approved the Agreement and Plan of Merger, dated as of November 8, 2022 and as amended on January 27, 2023 and May 11, 2023 (the “Merger Agreement”), by and among MURF, Conduit Pharmaceuticals Limited, a Cayman Islands exempted company (“Conduit”) and Conduit Merger Sub Inc., a Cayman Islands exempted company and a wholly-owned subsidiary of MURF (“Merger Sub”). As a result of and upon consummation of the transactions contemplated by the Merger Agreement, Conduit will become a wholly-owned subsidiary of MURF, which will change its name to “Conduit Pharmaceuticals Inc.” as described in this proxy statement/prospectus, with the former shareholders of Conduit becoming stockholders of MURF (such transaction, the “Business Combination,” and the post-Business Combination entity being referred to as “MURF” or the “New Conduit”).
Pursuant to the Merger Agreement, the shareholders of Conduit will receive as merger consideration shares of New Conduit common stock that shall have an aggregate value equal to Six Hundred Fifty Million Dollars ($650,000,000). The base-case valuation of Conduit, according to a valuation advisory and consulting firm engaged by MURF, is Seven Hundred Seventy-Nine Million Dollars ($779,000,000). For further information about the valuation, see the section “Special Meeting of MURF Stockholders – Opinion of ValueScope, Inc. – Overview of Global Biotechnology Industry”. With each share of New Conduit common stock being valued at $10.00, Conduit’s outstanding ordinary shares (including the ordinary shares issued upon conversion of all outstanding convertible debt, which conversion shall have occurred prior to the consummation of the Business Combination) will be converted into an aggregate of 65,000,000 shares of New Conduit common stock (the “Closing Payment Shares”) following the Business Combination, with each such outstanding Conduit ordinary share converted into shares of New Conduit common stock on a pro rata basis. Additionally, immediately prior to the Business Combination, MURF’s Class A common stock shall automatically convert on a one-to-one basis into shares of New Conduit common stock without any action on the part of any of MURF’s Public Stockholders. Upon the consummation of the Business Combination, MURF’s Public Warrants shall, by their terms, entitle the holders to purchase shares of New Conduit common stock at a purchase price of $11.50 per share. See the section entitled “The Business Combination” for further information on the consideration being paid to Conduit shareholders.
It is anticipated that, upon completion of the Business Combination, as a result of 11,037,272 shares of MURF Class A common stock having already been redeemed (or approximately 83% of the issued and outstanding Class A common stock subject to possible redemption as of December 31, 2022) and assuming that no holders exercise their warrants to purchase shares of New Conduit common stock: (i) assuming there are no redemptions by MURF’s Public Stockholders, the Public Stockholders will own approximately 2.9% of the outstanding shares of New Conduit common stock, Conduit’s shareholders will own approximately 90% of the outstanding shares of New Conduit common stock (which reflects 65,000,000 shares of New Conduit common stock to be issued to the Conduit shareholders as consideration in the merger plus the 2,700,000 shares of New Conduit common stock to be issued to the Private Placement Investor in connection with the Private Placement as the Private Placement Investor is an affiliate of one of Conduit’s existing shareholders) and MURF’s initial stockholder, our Sponsor, will own approximately 5.3% of the outstanding shares of New Conduit common stock. Under this scenario, on a pro forma basis, it is estimated that New Conduit will have total stockholders’ equity of approximately $25.8 million, which results in a value per share of $0.34. Alternatively, assuming 100% of MURF’s Public Shares are redeemed, the Public Stockholders not hold any of the outstanding shares of New Conduit common stock, Conduit’s shareholders will own approximately 92.6% of the outstanding shares of New Conduit common stock (which reflects 65,000,000 shares of New Conduit common stock to be issued to the Conduit shareholders as consideration in the merger plus the 2,700,000 shares of New Conduit common stock to be issued to the Private Placement Investor), and our Sponsor will own approximately 5.5% of the outstanding shares of New Conduit common stock. Under this alternative scenario, on a pro forma basis, it is estimated that New Conduit will have total stockholders’ equity of approximately $3.0 million, which results in a value per share of $0.04. For more information about the ownership of New Conduit, see the question “What equity stake will current MURF stockholders and Conduit shareholders have in New Conduit after the closing of the Business Combination?” in the section entitled “Questions and Answers About the Business Combination and Proposals” and see the section entitled “Unaudited Pro Forma Condensed Combined Financial Information”.
Proposals to approve the Merger Agreement and the other matters discussed in this proxy statement/prospectus will be presented at the virtual Special Meeting of stockholders of MURF scheduled to be held on September 7, 2023.
MURF’s units, Class A Common Stock and warrants are currently listed on The Nasdaq Global Market under the symbols MURFU, MURF and MURFW, respectively. MURF intends to apply for listing under the name “Conduit Pharmaceuticals Inc.”, to be effective at the time of the Business Combination, of its shares of common stock and warrants on The Nasdaq Stock Market under the proposed symbols CDT and CDTTW, respectively. MURF will not have units traded following consummation of the Business Combination. It is a condition of the consummation of the Business Combination that MURF’s shares of common stock are approved for listing on The Nasdaq Stock Market, but there can be no assurance such listing condition will be met. If such listing condition is not met, the Transactions will not be consummated unless the listing condition set forth in the Merger Agreement is waived by the parties.
MURF is an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 and has elected to comply with certain reduced public company reporting requirements.
This proxy statement/prospectus provides you with detailed information about the Transactions and other matters to be considered at the Special Meeting of MURF’s stockholders. We encourage you to carefully read this entire document. You should also carefully consider the risk factors described in “Risk Factors” beginning on page 29 of this proxy statement/prospectus. These securities have not been approved or disapproved by the Securities and Exchange Commission or any state securities commission nor has the Securities and Exchange Commission or any state securities commission passed upon the accuracy or adequacy of this proxy statement/prospectus. Any representation to the contrary is a criminal offense.
This proxy statement/prospectus incorporates important business and financial information about MURF that is not included in or delivered with this proxy statement/prospectus. You can obtain such information by visiting the Securities and Exchange Commission’s website at www.sec.gov or requesting such information in writing or by telephone from MURF at the following address:
Jack
K. Heilbron
Murphy Canyon Acquisition Corp.
4995 Murphy Canyon Road, Suite 300
San Diego, CA 92123
Tel: 760-471-8536
You will not be charged for any of the documents that you request. Stockholders requesting documents should do so by August 30, 2023 (five business days prior to the meeting date), in order to receive them before the Special Meeting of MURF stockholders.
This proxy statement/prospectus is dated August 11, 2023, and is first being mailed to MURF stockholders on or about August 11, 2023.
TABLE OF CONTENTS
| i |
ABOUT THIS PROXY STATEMENT/PROSPECTUS
This proxy statement/prospectus, which forms part of a registration statement on Form S-4 filed with the U.S. Securities and Exchange Commission (the “SEC”) by MURF, constitutes a prospectus of MURF under Section 5 of the Securities Act of 1933, as amended (the “Securities Act”), with respect to shares of common stock to be issued by MURF to the Conduit shareholders in connection with the Business Combination. This document also constitutes a notice of meeting and a proxy statement under Section 14(a) of the U.S. Securities Exchange Act of 1934, as amended (the “Exchange Act”) with respect to the Special Meeting of MURF stockholders at which MURF stockholders will be asked to consider and vote upon a proposal to approve the Business Combination by the approval and adoption of the Merger Agreement, among other matters.
You should rely only on the information contained or incorporated by reference into this proxy statement/prospectus. No one has been authorized to provide you with information that is different from that contained in, or incorporated by reference into, this proxy statement/prospectus. This proxy statement/prospectus is dated as of the date set forth on the cover hereof. You should not assume that the information contained in this proxy statement/prospectus is accurate as of any date other than that date. You should not assume that the information incorporated by reference into this proxy statement/prospectus is accurate as of any date other than the date of such incorporated document. Neither the mailing of this proxy statement/prospectus to MURF stockholders nor the issuance by Conduit of its ordinary shares in connection with the Business Combination will create any implication to the contrary.
This proxy statement/prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities, or the solicitation of a proxy or consent, in any jurisdiction to or from any person to whom it is unlawful to make any such offer or solicitation in such jurisdiction.
TRADEMARKS, TRADE NAMES AND SERVICE MARKS
This document contains references to trademarks, trade names and service marks belonging to other entities. Solely for convenience, trademarks, trade names and service marks referred to in this proxy statement/prospectus may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that the applicable licensor will not assert, to the fullest extent under applicable law, its rights to these trademarks and trade names. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
MARKET AND INDUSTRY DATA
This proxy statement/prospectus includes industry position and industry data and forecasts that Conduit obtained or derived from internal company reports, independent third-party publications and other industry data. Some data are also based on good faith estimates, which are derived from internal company analyses or review of internal company reports as well as the independent sources referred to above.
Although Conduit believes that the information on which it has based these estimates of industry position and industry data are generally reliable, the accuracy and completeness of this information is not guaranteed and Conduit has not independently verified any of the data from third-party sources nor have they ascertained the underlying economic assumptions relied upon therein. Statements as to industry opportunities and information are based on market data currently available. While Conduit is not aware of any misstatements regarding the industry data presented herein, these estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” in this proxy statement/prospectus.
| 1 |
FREQUENTLY USED TERMS
As used in this proxy statement/prospectus:
“2023 Plan” means the Conduit Pharmaceuticals Inc. 2023 Stock Incentive Plan.
“AstraZeneca” means AstraZeneca AB (PUBL).
“Business Combination” means the Merger and the other transactions contemplated by the Merger Agreement and related agreements.
“Class A common stock” means shares of Class A common stock, par value $0.0001 per share, of Murphy Canyon Acquisition Corp., which, following an automatic conversion pursuant to the Business Combination, is referred to herein as the New Conduit common stock.
“Class B common stock” means shares of Class B common stock, par value $0.0001 per share, of Murphy Canyon Acquisition Corp.
“Closing Payment Shares” means 65,000,000 shares of New Conduit common stock.
“Code” means the Internal Revenue Code of 1986, as amended.
“Companies Act” means the Companies Act of the Cayman Islands (Revised).
“Conduit” means Conduit Pharmaceuticals Limited, a Cayman Islands exempted company.
“Conduit convertible debt” means all of the issued and outstanding indebtedness of Conduit set forth on the allocation schedule in the Merger Agreement, which indebtedness will be converted into Conduit Shares prior to consummation of the Business Combination.
“Conduit shares” means collectively, Conduit’s ordinary shares, par value GBP0.0001 per share, including ordinary shares issued upon conversion of the Conduit convertible debt, which conversion shall occur prior to consummation of the Business Combination.
“DGCL” means the General Corporation Law of the State of Delaware.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“FASB” means the Financial Accounting Standards Board.
“Founder Shares” means the 3,306,250 shares of Class B common stock, which are the remaining shares from the initial 4,312,500 shares of Class B common stock issued to the Sponsor prior to MURF’s initial public offering, of which 1,006,250 shares were forfeited for no consideration.
“HSR Act” means the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended.
“JOBS Act” means the Jumpstart Our Business Startups Act.
“Marcum” means Marcum LLP, an independent registered public accounting firm, serving as MURF and Conduit’s auditors.
“Merger” means the merger of Merger Sub with and into Conduit with Conduit surviving the merger as a wholly-owned subsidiary of MURF.
“Merger Agreement” means the Agreement and Plan of Merger, dated as of November 8, 2022, by and among MURF, Merger Sub and Conduit, a copy of which is attached to this proxy statement/prospectus as Annex A-1 and as amended by that certain Amendment to Agreement and Plan of Merger, dated as of January 27, 2023, a copy of which is attached to this proxy statement/prospectus as Annex A-2, and as further amended by that certain Second Amendment to Agreement and Plan of Merger, dated as of May 11, 2023, a copy of which is attached to this proxy statement/prospectus as Annex A-3.
“Merger Sub” means Conduit Merger Sub, Inc., an exempted company with limited liability incorporated under the laws of the Cayman Islands and wholly-owned subsidiary of MURF.
“MURF” means Murphy Canyon Acquisition Corp., a Delaware corporation.
“MURF Board” means the board of directors of MURF.
“MURF common stock” means the Class A common stock and Class B common stock.
“New Conduit” means Murphy Canyon Acquisition Corp., a Delaware corporation, after the Business Combination, which is expected to be renamed “Conduit Pharmaceuticals Inc.” upon the consummation of the Business Combination.
| 2 |
“New Conduit common stock” means shares of common stock, par value $0.0001 per share, of New Conduit, after the filing of the Proposed Charter upon the consummation of the Business Combination.
“Private Placement” or “PIPE Financing” means the private placement of an aggregate of 2,700,000 units, with each unit consisting of one share of Class A common stock together with one warrant exercisable into one share of Class A common stock, with the Private Placement Investor pursuant to Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder, for a purchase price of $10.00 per unit, or an aggregate amount of $27 million in gross cash proceeds, pursuant to the Subscription Agreement.
“Private Placement Investor” means Prospect Science Ventures Limited, a New Zealand limited company, the accredited investor with whom MURF entered into the Subscription Agreement.
“Private Shares” means (i) the Founder Shares and (ii) the 754,000 shares of Class A common stock included in the units purchased by the Sponsor in connection with MURF’s initial public offering.
“Private Warrants” means the 754,000 warrants of MURF included in the units purchased by the Sponsor in connection with MURF’s initial public offering.
“Proposed Charter” means the Second Amended and Restated Certificate of Incorporation of New Conduit, a copy of which is attached to this proxy statement/prospectus as Annex B.
“Public Shares” means the shares of Class A common stock included in the units issued in MURF’s initial public offering (excluding the Private Shares).
“Public Stockholder” means a holder of Public Shares, including the Sponsor to the extent it holds Public Shares, provided, that the Sponsor will be considered a “Public Stockholder” only with respect to any Public Shares held by it.
“Public Warrants” means the warrants included in the units issued in MURF’s initial public offering (excluding the Private Warrants).
“SEC” means the Securities and Exchange Commission.
“Securities Act” means the Securities Act of 1933, as amended.
“Special Meeting” means the special meeting of the stockholders of MURF that is the subject of this proxy statement/prospectus.
“Sponsor” means Murphy Canyon Acquisition Sponsor, LLC.
“St George Street” means St George Street Capital, a biomedical charity based in the United Kingdom.
“Subscription Agreement” means the subscription agreement, dated November 8, 2022, by and between MURF and the Private Placement Investor, as amended by that certain amendment to the Subscription Agreement, dated January 27, 2023.
“Supporting Conduit Shareholders” means Corvus Capital Limited, St George Street and Algo Holdings, Inc.
“Transactions” means the Business Combination and the Private Placement, taken together.
“U.S. GAAP” means generally accepted accounting principles in the United States.
“ValueScope” means ValueScope, Inc.
| 3 |
SUMMARY OF THE MATERIAL TERMS OF THE TRANSACTIONS
| ● | The parties to the Business Combination are MURF, Merger Sub and Conduit. Pursuant to the Merger Agreement, Merger Sub will merge with and into Conduit, with Conduit surviving as a wholly-owned subsidiary of MURF. See the section entitled “The Merger Agreement.” |
| ● | Under the Merger Agreement, the shareholders of Conduit will receive the number of New Conduit common stock that shall have an aggregate value equal to Six Hundred Fifty Million Dollars ($650,000,000). With each share of New Conduit common stock being valued at $10.00, Conduit’s outstanding ordinary shares (including the ordinary shares issued upon conversion of all outstanding convertible debt, which conversion shall have occurred prior to the consummation of the Business Combination) will be converted into an aggregate of 65,000,000 shares of New Conduit common stock following the Business Combination, with each such outstanding Conduit ordinary share converted into shares of New Conduit common stock on a pro rata basis. Additionally, immediately prior to the Business Combination, MURF’s Class A common stock shall automatically convert on a one-to-one basis into shares of New Conduit common stock without any action on the part of any of MURF’s Public Stockholders. See the section entitled “The Business Combination Proposal — Structure of the Transactions” for further information on the consideration being paid to Conduit shareholders. |
| ● | It is anticipated that, immediately after the closing of the Business Combination, as a result of 11,037,272 shares of MURF Class A common stock having already been redeemed (or approximately 83% of the issued and outstanding Class A common stock subject to possible redemption as of December 31, 2022) and assuming that no Public Stockholders exercise their redemption rights and that no warrants to purchase New Conduit common stock are exercised, MURF’s initial stockholder, our Sponsor, will retain an ownership interest of 4,015,250 shares of New Conduit common stock or approximately 5.3% of New Conduit common stock, MURF’s Public Stockholders will retain an ownership interest of 2,187,728 shares of New Conduit common stock or approximately 2.9% of New Conduit common stock, MURF’s directors will own 45,000 shares of New Conduit common stock or approximately 0.1% of New Conduit common stock, A.G.P. will own 1,300,000 shares of New Conduit common stock or approximately 1.7% of New Conduit common stock, and Conduit’s shareholders will own 67,700,000 shares of New Conduit common stock or approximately 90.0% of New Conduit common stock, which reflects 65,000,000 shares of New Conduit common stock to be issued to the Conduit shareholders as consideration in the merger plus the 2,700,000 shares of New Conduit common stock to be issued to the Private Placement Investor in connection with the Private Placement as the Private Placement Investor is an affiliate of one of Conduit’s existing shareholders.1 Under this scenario, on a pro forma basis, it is estimated that New Conduit will have total stockholders’ equity of approximately $25.8 million, which results in a value per share of $0.34, which is calculated as $25.8 million divided by 75,247,978 shares of New Conduit common stock. |
Assuming the same facts as above except that 50% of MURF’s Public Stockholders redeem their shares (a redemption of 1,093,864 shares), New Conduit will have total stockholders’ equity of approximately $14.4 million, which results in a value per share of $0.19, which is calculated as $14.4 million divided by 74,154,114 shares of New Conduit common stock. | |
Assuming the same facts as above except that 100% of MURF’s Public Stockholders redeem their shares (a redemption of 2,187,728 shares), New Conduit will have total stockholders’ equity of approximately $3.0 million, which results in a value per share of $0.04, which is calculated as $3.0 million divided by 73,060,250 shares of New Conduit common stock. | |
Immediately after the closing of the Business Combination, assuming that no Public Stockholders exercise their redemption rights and excluding all shares that will be held by our Sponsor and MURF directors and assuming that no warrants to purchase New Conduit common stock are exercised, MURF’s Public Stockholders will retain an ownership interest of 2,187,728 shares of New Conduit common stock or approximately 3.1% of New Conduit common stock, A.G.P. will own 1,300,000 shares of New Conduit common stock or approximately 1.8% of New Conduit common stock, and Conduit’s shareholders will own 67,700,000 shares of New Conduit common stock or approximately 95.1% of New Conduit common stock, which reflects 65,000,000 shares of New Conduit common stock to be issued to the Conduit shareholders as consideration in the merger plus the 2,700,000 shares of New Conduit common stock to be issued to the Private Placement Investor in connection with the Private Placement as the Private Placement Investor is an affiliate of one of Conduit’s existing shareholders.2 Under this scenario, on a pro forma basis, it is estimated that New Conduit will have total stockholders’ equity of approximately $25.8 million, which results in a value per share of $0.36, which is calculated as $25.8 million divided by 71,187,728 shares of New Conduit common stock. | |
Assuming the same facts as above except that 50% of MURF’s Public Stockholders redeem their shares (a redemption of 1,093,864 shares), New Conduit will have total stockholders’ equity of approximately $14.4 million, which results in a value per share of $0.21, which is calculated as $14.4 million divided by 70,093,864 shares of New Conduit common stock. | |
Assuming the same facts as above except that 100% of MURF’s Public Stockholders redeem their shares (a redemption of 2,187,728 shares), New Conduit will have total stockholders’ equity of approximately $3.0 million, which results in a value per share of $0.04, which is calculated as $3.0 million divided by 69,000,000 shares of New Conduit common stock. | |
Immediately after the closing of the Business Combination, assuming that no public stockholders exercise their redemption rights and that all warrants to purchase New Conduit common stock are exercised, MURF’s initial stockholder, our Sponsor, will retain an ownership interest of 4,724,250 shares of New Conduit common stock or approximately 5.1% of New Conduit common stock, MURF’s Public Stockholders will retain an ownership interest of 15,412,728 shares of New Conduit common stock or approximately 16.8% of New Conduit common stock, MURF’s Directors will own 90,000 shares of New Conduit common stock or approximately 0.1% of New Conduit common stock, A.G.P. will own 1,354,000 shares of New Conduit common stock or approximately 1.5% of New Conduit common stock, and Conduit’s shareholders will own 70,400,000 shares of New Conduit common stock or approximately 76.5% of New Conduit common stock, which reflects 65,000,000 shares of New Conduit common stock to be issued to the Conduit shareholders as consideration in the merger plus 5,400,000 shares of New Conduit common stock to be issued to the Private Placement Investor in connection with the Private Placement as the Private Placement Investor is an affiliate of one of Conduit’s existing shareholders.3 Under this scenario, on a pro forma basis, it is estimated that New Conduit will have total stockholders’ equity of approximately $25.8 million, which results in a value per share of $0.28, which is calculated as $25.8 million divided by 91,980,978 shares of New Conduit common stock. | |
Assuming the same facts as above except that 50% of MURF’s Public Stockholders redeem their shares (a redemption of 1,093,864 shares), New Conduit will have total stockholders’ equity of approximately $14.4 million, which results in a value per share of $0.16, which is calculated as $14.4 million divided by 90,887,114 shares of New Conduit common stock. | |
| Assuming the same facts as above except that 100% of MURF’s Public Stockholders redeem their shares (a redemption of 2,187,728 shares), New Conduit will have total stockholders’ equity of approximately $3.0 million, which results in a value per share of $0.03, which is calculated as $3.0 million divided by 89,793,250 shares of New Conduit common stock. |
| 4 |
| ● | The Merger Agreement may be terminated as follows: (i) by the mutual consent of MURF and Conduit; (ii) by MURF, if any of the representations or warranties of Conduit set forth in the Merger Agreement is not true and correct, or if Conduit has failed to perform any covenant or agreement set forth in the Merger Agreement (including an obligation to consummate the Business Combination), in each case such that the conditions to closing would not be satisfied and the breach or breaches causing such representations or warranties not to be true and correct, or the failure to perform any covenant or agreement, as applicable, are not cured (or waived by MURF) by the earlier of (a) the Outside Date (as defined below) or (b) 20 days after written notice thereof is delivered to Conduit; provided, however that MURF is not then in material breach of any representation, warranty, covenant, or obligation in the Merger Agreement, which breach has not been cured; (iii) by Conduit, if any of the representations or warranties of MURF or Merger Sub set forth in the Merger Agreement is not true and correct, or if MURF or Merger Sub has failed to perform any covenant or agreement set forth in the Merger Agreement (including an obligation to consummate the Business Combination), in each case such that the conditions to closing would not be satisfied and the breach or breaches causing such representations or warranties not to be true and correct, or the failure to perform any covenant or agreement, as applicable, are not cured (or waived by Conduit) by the earlier of (a) the Outside Date or (b) 20 business days after written notice thereof is delivered to MURF; provided, however that Conduit is not then in material breach of any representation, warranty, covenant, or obligation in the Merger Agreement, which breach has not been cured; (iv) by either MURF or Conduit: (a) on or after February 7, 2024 (the “Outside Date”), if the Business Combination has not been consummated prior to the Outside Date; provided, however, that this right to terminate the Merger Agreement will not be available to a party if the failure of the merger to have been consummated before the Outside Date was due to such party’s breach of or failure to perform any of its covenants or agreements set forth in the Merger Agreement, (b) if any applicable law or order that makes the transactions contemplated by the Merger Agreement illegal or otherwise prohibits consummation of such transactions becomes final and non-appealable, or (C) if MURF has not received approval from its stockholders of the Business Combination and related transactions at the Special Meeting; (v) by MURF if the Conduit shareholders’ written consent approving the Business Combination and related transactions have not have been obtained within 10 business days after the date of the Merger Agreement; and (vi) if there is a material adverse effect on behalf of the other party. See the section entitled “The Merger Agreement — Termination.” |
| ● | In addition to voting on the business combination proposal, MURF stockholders will vote on separate proposals to approve amendments to MURF’s current amended and restated certificate of incorporation to: |
| (i) | Charter Amendment Proposal A – change the name of the public entity from “Murphy Canyon Acquisition Corp.” to “Conduit Pharmaceuticals Inc.”; | |
| (ii) | Charter Amendment Proposal B – provide for one class of authorized common stock; | |
| (iii) | Charter Amendment Proposal C – delete the various provisions in MURF’s current amended and restated certificate of incorporation applicable only to special purpose acquisition corporations (such as the obligation to dissolve and liquidate if a business combination is not consummated within a certain period of time); | |
| (iv) | Charter Amendment Proposal D – increase the number of authorized shares of common stock to 250,000,000; and | |
| (v) | Charter Amendment Proposal D – fix the number of directors at seven (7), a majority of whom shall be independent directors in accordance with The Nasdaq Stock Market LLC’s requirements. See the section entitled “The Charter Amendment Proposals.” |
1 Shares outstanding at closing does not include any shares related to Cizzle PLC exercising its option to exchange its economic interest in an asset of Conduit Pharmaceuticals Limited for shares as the option is not expected to be exercised prior to or at the closing.
2 Shares outstanding at closing does not include any shares related to Cizzle PLC exercising its option to exchange its economic interest in an asset of Conduit Pharmaceuticals Limited for shares as the option is not expected to be exercised prior to or at the closing.
3 Shares outstanding at closing does not include any shares related to Cizzle PLC exercising its option to exchange its economic interest in an asset of Conduit Pharmaceuticals Limited for shares as the option is not expected to be exercised prior to or at the closing.
| 5 |
| ● | In addition to voting on the business combination proposal, MURF stockholders will vote on separate proposals to consider and vote upon, on a non-binding advisory basis, certain governance provisions in the Proposed Charter, presented separately in accordance with SEC requirements. See the section entitled “The Advisory Charter Amendments Proposals.” |
| ● | The stockholders of MURF will also consider and vote upon a proposal to approve, for purposes of complying with applicable listing rules of Nasdaq, the issuance of shares of common stock, par value $0.0001 per share, and warrants to purchase common stock, to the Private Placement Investor in the Private Placement, the proceeds of which will be used to finance the Business Combination and related transactions and the costs and expenses incurred in connection therewith with any balance used for working capital purposes. Pursuant to the Subscription Agreement, the Private Placement Investor agreed to subscribe for and purchase and MURF agreed to issue and sell to the Private Placement Investor an aggregate of 2,700,000 units, with each unit consisting of one share of Class A common stock together with one warrant exercisable into one share of Class A common stock, or an aggregate of $27 million in gross cash proceeds, in the Private Placement. See the section entitled “The Nasdaq Proposal.” |
| ● | MURF stockholders will also vote on proposals (x) to approve of the appointment of seven (7) directors who, upon consummation of the Business Combination, will become the directors of New Conduit, (y) to approve the Conduit Pharmaceuticals Inc. 2023 Stock Incentive Plan and (z) to approve, if necessary, an adjournment of the Special Meeting. See the sections entitled “The Incentive Plan Proposal” and “The Adjournment Proposal.” |
| ● | Upon completion of the Business Combination, if management’s nominees are elected, the directors of New Conduit will be (i) Dr. Freda Lewis-Hall, Chairman of New Conduit’s board of directors, (ii) Dr. David Tapolczay, a current director of Conduit and the Chief Executive Officer of New Conduit, (iii) James Bligh, an employee of Conduit, (iv) Faith L. Charles, (v) Chele Chiavacci Farley, a current director of MURF, (vi) Jennifer I. McNealey, and (vii) Dr. Andrew Regan, a current director and shareholder of Conduit (Dr. Regan also undertakes duties typically associated with an executive officer in connection with his service as a director of Conduit). |
| ● | Upon completion of the Business Combination, the executive officers of New Conduit will include Dr. David Tapolczay as Chief Executive Officer and Adam Sragovicz, MURF’s Chief Financial Officer and director, as Chief Financial Officer, and the other persons described under the section entitled “Management of New Conduit Following the Business Combination.” |
| ● | The Merger Agreement contemplates that Adam Sragovicz, MURF’s current Chief Financial Officer and a director, will be the Chief Financial Officer of New Conduit after the closing of the Business Combination. Pursuant to the terms of an employment agreement to be entered into between New Conduit and Mr. Sragovicz in connection with the closing of the Business Combination, Mr. Sragovicz is entitled to compensation consisting of (i) an annual base salary of $400,000, and (ii) a target annual bonus opportunity equal to 40% of his base salary, payable based on the achievement of performance objectives as determined by the New Conduit board of directors. In addition, the employment agreement provides that he will be entitled to receive a sign-on restricted stock unit award covering 0.10% of the shares of common stock of New Conduit pursuant to the terms of the 2023 Stock Incentive Plan, which shall vest in equal annual installments on the first three anniversaries of the Business Combination. In the future, Mr. Sragovicz could receive any additional cash compensation, stock options, stock awards or other benefits that New Conduit’s board of directors determines to pay to Mr. Sragovicz. |
| ● | Mr. Heilbron, the Chief Executive Officer and Chairman of MURF, is also the President of NetREIT Advisors LLC (“NetREIT Advisors”), which is sole and managing member of the Sponsor. NetReit Advisors is wholly-owned by Presidio Property Trust, Inc. (“Presidio”). Mr. Heilbron is the President, Chief Executive Officer and Chairman of Presidio, of which he beneficially owns 2.6% (as of March 31, 2023). In 2023, Presidio’s compensation committee of its board of directors awarded Mr. Heilbron a cash bonus equal to 1% of the value of the New Conduit common stock owned by Presidio, to be valued on the day which is six months and one day after the closing of the Business Combination.
Mr. Heilbron has entered into a consulting agreement with MURF, which is effective upon the closing of the Business Combination. The consulting agreement provides that Mr. Heilbron will provide advisory and consulting services from time to time to New Conduit for one year following the closing of the Business Combination. Mr. Heilbron will no longer serve as an officer or director of New Conduit after the closing of the Business Combination. However, pursuant to the terms of the consulting agreement, Mr. Heilbron is entitled to rights as an observer to the Board of Directors of New Conduit. Mr. Heilbron is entitled to be paid $25,000 per calendar quarter for his consulting services and is also entitled to a stock option to purchase the number of shares of the common stock of New Conduit determined by dividing (i) $300,000, by (ii) the per share Black-Scholes valuation as of the grant date, utilizing the same assumptions used in preparation of the financial statements, with the resulting quotient rounded down to the nearest whole share. |
| ● | The Sponsor and the directors and officers of MURF have each waived their redemption rights with respect to any Founder Shares and Class A common stock held by them (other than relating to liquidating distributions to Public Shares from the trust account if MURF fails to complete its initial business combination by the time required prior to MURF’s liquidation in accordance with its amended and restated certificate of incorporation), which waiver was provided in connection with the initial public offering and without any separate consideration paid in connection with providing such waiver. |
| ● | Concurrently with the execution of the Merger Agreement, the Sponsor and its affiliates, which own 4,060,250 shares of MURF common stock (representing approximately 65% of the issued and outstanding shares of MURF common stock, consisting of the Private Shares, or 754,000 shares of Class A common stock and 3,306,250 shares of Class B common stock) and certain Conduit shareholders, which own in aggregate 1,670 Conduit shares (representing 83.6% of the issued and outstanding shares of Conduit ordinary shares), have entered into support agreements with MURF and Conduit pursuant to which they have agreed to vote all of their respective shares in favor of the Business Combination, including any additional shares of MURF common stock or Conduit shares, as applicable, any may acquire ownership of or the power to vote, subject to the terms and conditions set forth in such support agreements. See the sections entitled “Certain Agreements Related to the Business Combination — Sponsor Support Agreement,” “Certain Agreements Related to the Business Combination” and “Shareholder Support Agreements.” |
| 6 |
| ● | As of August 2, 2023, the record date, the Sponsor beneficially owned and was entitled to vote an aggregate of 4,060,250 shares of MURF common stock (consisting of 754,000 shares of Class A common stock and 3,306,250 shares of Class B common stock) that were issued prior to or concurrently with MURF’s initial public offering (collectively, the “Private Shares”). As of the date of this proxy statement/prospectus, such shares constitute approximately 65% of the issued and outstanding shares of MURF common stock. Concurrently with the execution of the Merger Agreement, the Sponsor and MURF entered into a support agreement, pursuant to which the Sponsor agreed to vote all of its Private Shares, including any additional shares of MURF common stock it acquires ownership of or the power to vote, in favor of the Business Combination and related transactions. See the section entitled “Special Meeting of MURF Stockholders — Sponsor.” |
| ● | The Sponsor and its affiliates’ paid an aggregate of $7,565,000 for the Private Shares (comprised of the Founder Shares and the 754,000 units (each such unit comprised of one share of Class A common stock and one warrant to purchase one share of Class A common stock) purchased by the Sponsor in connection with MURF’s initial public offering), which has an approximate aggregate market value of $51,900,000 (assuming that the future value of New Conduit common stock and the Private Warrants will be the same as the current value of MURF’s Class A common stock). |
| ● | The Sponsor and its affiliates’ total potential ownership interest in New Conduit, as a result of 11,037,272 shares of MURF Class A common stock having already been redeemed (or approximately 83% of the issued and outstanding Class A common stock subject to possible redemption as of December 31, 2022) and assuming the exercise and conversion of all securities following the closing of the Business Combination, including the Private Shares, is estimated to comprise approximately 5.1% of the outstanding New Conduit common stock in a no redemption scenario (or a transaction value of approximately $34,021,000 or a market value of approximately $51,900,000), approximately 5.2% of outstanding New Conduit common stock in a 50% redemption scenario (or a transaction value of approximately $34,430,000 or a market value of approximately $51,900,000), and approximately 5.3% of outstanding New Conduit common stock in a 100% maximum redemption scenario (or a transaction value of approximately $34,850,000 or a market value of approximately $51,900,000). |
| ● | The Sponsor and its affiliates can earn a positive rate of return on their overall investment in MURF and Conduit after the Business Combination, even if other holders of MURF common stock experience a negative rate of return, due to having purchased the Founder Shares, as described above, for $25,000 or approximately $0.006 per share. |
| ● | On March 7, 2023, MURF issued to Sponsor an unsecured promissory note in the principal amount of $1,500,000, portions of which may be withdrawn by MURF from time to time (the “Promissory Note”). As of July 31, 2023, MURF has submitted drawdown requests in an aggregate amount of $750,000. The Promissory Note bears no interest and outstanding principal is due and payable upon the earlier of the consummation of the initial business combination or the date of the liquidation of MURF. |
| ● | The aggregate dollar amount that the Sponsor has at risk that depends on the completion of an initial business combination, including the Business Combination, is approximately $8,315,000, as of July 31, 2023, which is the current value of securities held (assuming that the current value of each of the securities held, whether publicly traded shares of Class A common stock or Private Warrants, are in each case valued at the current price of Class A common stock) and consists of (i) the Founders Shares, (ii) the 754,000 private placement units (each such unit comprised of one share of Class A common stock and one warrant to purchase one share of Class A common stock) purchased in connection with MURF’s initial public offering, and (iii) the Promissory Note. There are no fees due or out of pocket expenses for which Sponsor and its affiliates are awaiting reimbursement. |
| ● | The aggregate dollar amount that the directors of MURF have at risk that depends on the completion of an initial business combination, including the Business Combination, is approximately $970,000, as of July 31, 2023, which is the current value of the Independent Director Units. There are no loans, fees due or out of pocket expenses for which directors are awaiting reimbursement. |
| 7 |
QUESTIONS AND ANSWERS ABOUT THE BUSINESS COMBINATION AND PROPOSALS
The questions and answers below highlight only selected information from this proxy statement/prospectus and only briefly address some commonly asked questions about the Special Meeting and the proposals to be presented at the Special Meeting, including with respect to the proposed business combination. The following questions and answers do not include all the information that is important to MURF stockholders. Stockholders are urged to read carefully this entire proxy statement/prospectus, including the Annexes and the other documents referred to herein, to fully understand the proposed business combination and the voting procedures for the Special Meeting.
| Q. | Why am I receiving this proxy statement/prospectus? |
| A. | MURF and Conduit have agreed to a business combination under the terms of the Merger Agreement that is described in this proxy statement/prospectus. A copy of the Merger Agreement is attached to this proxy statement/prospectus as Annexes A-1 through A-3, and MURF encourages its stockholders to read it in its entirety. MURF’s stockholders are being asked to consider and vote upon a proposal to adopt the Merger Agreement, which, among other things, provides for Merger Sub to merge with and into Conduit, with Conduit continuing as the surviving company and a wholly-owned subsidiary of MURF. See the section entitled “The Business Combination Proposal.” |
| Q. | Are there any other matters being presented to stockholders at the meeting? |
| A. | In addition to voting on the Business Combination, the stockholders of MURF will vote on the following: |
| 1. | To consider and vote upon separate proposals to approve amendments to MURF’s current amended and restated certificate of incorporation to: |
| (i) | Charter Amendment Proposal A – change the name of the public entity from “Murphy Canyon Acquisition Corp.” to “Conduit Pharmaceuticals Inc.”; | |
| (ii) | Charter Amendment Proposal B – provide for one class of authorized common stock; | |
| (iii) | Charter Amendment Proposal C – delete the various provisions in MURF’s current amended and restated certificate of incorporation applicable only to special purpose acquisition corporations (such as the obligation to dissolve and liquidate if a business combination is not consummated within a certain period of time); | |
| (iv) | Charter Amendment Proposal D – increase the number of authorized shares of common stock to 250,000,000; and | |
| (v) | Charter Amendment Proposal E – fix the number of directors at seven (7), a majority of whom shall be independent directors in accordance with The Nasdaq Stock Market LLC’s requirements. See the section entitled “The Charter Amendment Proposals.” |
| 2. | To consider and vote upon, on a non-binding advisory basis, certain governance provisions in the Proposed Charter, presented separately in accordance with SEC requirements. See the section entitled “The Advisory Charter Amendments Proposals.” |
| 3. | To elect seven (7) directors who, upon consummation of the Transactions, will be the directors of New Conduit. See the section entitled “The Director Election Proposal.” |
| 4. | To approve the 2023 Plan. See the section entitled “The Incentive Plan Proposal.” |
| 5. | To approve the issuance of New Conduit common stock for purposes of complying with applicable Nasdaq listing rules. See the section entitled “The Nasdaq Proposal.” |
| 6. | To adjourn the meeting to a later date or dates to permit further solicitation and vote of proxies if MURF would not have been able to consummate the Business Combination. See the section entitled “The Adjournment Proposal.” |
MURF will hold the Special Meeting of its stockholders to consider and vote upon these proposals. This proxy statement/prospectus contains important information about the proposed business combination and the other matters to be acted upon at the Special Meeting. Stockholders should read it carefully.
Consummation of the Business Combination is conditioned on approval of each of the business combination proposal, the charter amendment proposals, the advisory charter amendments proposals, director election proposal, the Nasdaq proposal and the incentive plan proposal. If any of the proposals is not approved, the other proposals will not be presented to stockholders for a vote.
The vote of stockholders is important. Stockholders are encouraged to vote as soon as possible after carefully reviewing this proxy statement/prospectus.
| Q. | What will Conduit shareholders and receive in the Business Combination? |
| A. | If the Business Combination is completed, the outstanding Conduit shares (including the ordinary shares issued upon conversion of all of Conduit’s outstanding convertible debt, which conversion shall have occurred prior to the consummation of the Business Combination) shall be converted into an aggregate of 65,000,000 shares of New Conduit common stock following the Transactions. |
| 8 |
| Q. | I am a MURF warrant holder. Why am I receiving this proxy statement/prospectus? |
| A. | Upon consummation of the Transactions, MURF’s Public Warrants shall, by their terms, entitle the holders to purchase New Conduit common stock at a purchase price of $11.50 per share. This proxy statement/prospectus includes important information about MURF and the business of MURF following consummation of the Transactions. As holders of MURF warrants will be entitled to purchase shares of New Conduit common stock upon consummation of the Transactions, you are encouraged to read the information contained in this proxy statement/prospectus carefully. |
| Q. | Why is MURF proposing the Business Combination? |
| A. | MURF was organized to effect a merger, capital stock exchange, asset acquisition or other similar business combination with one or more businesses or entities. |
On February 7, 2022, MURF completed its initial public offering of units, with each unit consisting of one share of Class A common stock and one redeemable warrant, with each whole warrant entitling the holder thereof to purchase one share of Class A common stock for $11.50 per share, raising total proceeds of $132,250,000 (including a full exercise of the over-allotment option). Since the initial public offering, MURF’s activity has been limited to the evaluation of business combination candidates.
Conduit Pharmaceuticals is a clinical-stage specialty biopharmaceutical company that began operations in early 2019, to facilitate the development and commercialization of clinical assets that have not been, or are not being, prioritized by leading biopharmaceutical companies in order to develop pharmaceutical products that meet the unmet medical needs of patients.
For information about the Board’s reasons for approving the Transactions, see “The Business Combination Proposal — The MURF Board’s Reasons for Approval of the Transactions.”
| Q. | What equity stake will current MURF stockholders and Conduit shareholders have in New Conduit after the closing of the Business Combination? |
| A. | The equity stake held by non-redeeming Public Stockholders, Conduit shareholders and the Sponsor in New Conduit immediately following consummation of the Business Combination will depend on the number of redemptions from the trust account by Public Stockholders as well as various other factors, as described in the assumptions set forth below. |
| Approximate equity stakes for each of these shareholder groups upon consummation of the Business Combination, and their corresponding approximate collective voting power in New Conduit, are set forth in the table below in respect of three redemption scenarios: (1) “Scenario A,” in which there are no redemptions of Public Shares; (2) “Scenario B,” in which 50% of Public Shares are redeemed; and (3) “Scenario C,” in which 100% of Public Shares are redeemed. | |
| All else being equal, if any Public Stockholders exercise their redemption rights, then the percentage of New Conduit common stock held collectively by all non-redeeming Public Stockholders will decrease and the percentage of New Conduit common stock held by Conduit Shareholders and the Sponsor will increase, in each case, relative to the percentage held if no Public Shares are redeemed. | |
| In an effort to present possible sources of dilution and the extent of such dilution that non-redeeming Public Stockholders could experience, each of the scenarios below assumes (i) that no additional shares of MURF common stock are issued prior to the closing of the Business Combination, (ii) there is no exercise of any options to purchase New Conduit common stock that will be outstanding immediately following the Business Combination, whether such options are issued under the 2023 Incentive Plan or otherwise, (iii) the consummation of the Private Placement by the Private Placement Investor and related issuance of New Conduit common stock (iv) the issuance of Conduit’s ordinary shares upon the conversion of all outstanding convertible debt of Conduit prior to the closing of the Business Combination. | |
| The table set forth below also states the anticipated pro forma equity value of New Conduit for each of the scenarios described above. These pro forma equity values reflect an assumed price for New Conduit common stock of $10.00 per share, being the price per share negotiated with Conduit and set forth in the Merger Agreement for New Conduit common stock to be issued to the existing Conduit shareholders immediately prior to the closing of the Business Combination. | |
| The pro forma equity values include the equity consideration to be issued to Conduit shareholders at the closing of the Business Combination (being 65,000,000 share of New Conduit common stock, or approximately $650,000,000). The number of Public Shares redeemed by Public Stockholders with cash from the trust account at closing is not, all else being equal, expected to materially affect the equity value per share of New Conduit common stock held by non-redeeming Public Stockholders at the time immediately following the closing of the Business Combination, as each redemption will result in (x) the cancellation of one Public Share, and (y) the payment of approximately $10.67 to the redeeming Public Stockholder (given that, based on assumed funds in the trust account of approximately $23.3 million (after withholding funds for tax obligations) on the Record Date, the estimated per share redemption price would have been approximately $10.67) and, accordingly, such funds will not be available to New Conduit or reflected in its financial statements following the closing. You should note, however, that the level of redemptions of Public Shares from the trust account may affect the market price for New Conduit common stock following the closing of the Business Combination in ways which we cannot predict. | |
| The ownership percentages set forth below for non-redeeming Public Stockholders and all other New Conduit shareholders may be further diluted, all else being equal, in the event that options for New Conduit common stock outstanding following the closing are exercised. The issuance of any shares or other awards in connection with the 2023 Incentive Plan following the Business Combination would also have a dilutive effect on New Conduit shareholders’ ownership percentages, all else being equal, however, the magnitude of any such potential issuances is not known as of the date of this proxy statement/prospectus. |
| 9 |
| No Redemptions | 50% Redemptions (1) | 100% Redemptions (2) | ||||||||||||||||||||||
| Shares | Percentage(3) | Shares | Percentage(3) | Shares | Percentage(3) | |||||||||||||||||||
| Shares held by Conduit Shareholders(4) | 65,000,000 | 70.67 | % | 65,000,000 | 71.52 | % | 65,000,000 | 72.39 | % | |||||||||||||||
| Shares held by Public Stockholders | 2,187,728 | 2.38 | % | 1,093,864 | 1.20 | % | - | Not Applicable | ||||||||||||||||
| Shares underlying Public Warrants | 13,225,000 | 14.38 | % | 13,225,000 | 14.55 | % | 13,225,000 | 14.73 | % | |||||||||||||||
| Shares issued to Sponsor(5) | 4,015,250 | 4.37 | % | 4,015,250 | 4.42 | % | 4,015,250 | 4.47 | % | |||||||||||||||
| Shares underlying Private Warrants held by Sponsor | 709,000 | * | 709,000 | * | 709,000 | * | ||||||||||||||||||
| Shares issued to Private Placement Investor | 2,700,000 | 2.94 | % | 2,700,000 | 2.97 | % | 2,700,000 | 3.01 | % | |||||||||||||||
| Shares underlying warrants issued to Private Placement Investor | 2,700,000 | 2.94 | % | 2,700,000 | 2.97 | % | 2,700,000 | 3.01 | % | |||||||||||||||
| Shares issued to MURF Directors | 45,000 | * | 45,000 | * | 45,000 | * | ||||||||||||||||||
| Shares underlying warrants issued to MURF Directors | 45,000 | * | 45,000 | * | 45,000 | * | ||||||||||||||||||
| Shares issued to A.G.P. | 1,300,000 | 1.41 | % | 1,300,000 | 1.43 | 1,300,000 | 1.45 | % | ||||||||||||||||
| Shares underlying warrants issued to A.G.P. | 54,000 | * | 54,000 | * | 54,000 | * | ||||||||||||||||||
| Shares Outstanding at Closing(6)(7) | 91,980,978 | 100 | % | 90,887,114 | 100 | % | 89,793,250 | 100 | % | |||||||||||||||
| * | Less than 1%. |
| (1) | This scenario assumes that 1,093,864 Public Shares, or 50% of the Public Shares as of the date of this proxy statement/prospectus, are redeemed for an aggregate payment of approximately $11.7 million from the trust account as of July 31, 2023, which is a redemptions scenario that could occur. |
| (2) | This scenario assumes that 2,187,728 Public Shares, or 100% of the Public Shares as of the date of this proxy statement/prospectus, are redeemed for an aggregate payment of approximately $23.3 million from the trust account as of July 31, 2023, which is a redemptions scenario that could occur. | |
| (3) | All voting power percentages in this table are approximate and have been rounded to two decimal places. | |
| (4) | Does not include shares issued to the Private Placement Investor, who is also a Conduit shareholder. | |
| (5) | The shares issued to the Sponsor consist of (i) 3,306,250 shares of Class B common stock and (ii) 754,000 shares of Class A common stock. | |
| (6) | Shares outstanding at closing does not include any shares related to Cizzle PLC or Vela Technologies PLC exercising their options to exchange their economic interests in an asset of Conduit Pharmaceuticals Limited for shares as the options are not expected to be exercised prior to or at the closing. | |
| (7) | Shares outstanding at closing does not include any shares related to the conversion feature of the convertible notes issued in March 2023 by Conduit, as the convertible notes as the conversion feature is not expected to be exercised prior to or at the closing. |
The anticipated ownership of New Conduit’s securities set forth above, including the potential effect of any dilutive events, is accurate, subject to the assumptions and exclusions set forth above, as of the date of filing of this proxy statement/prospectus, and does not take into account any transactions that may be entered into after the date hereof unless explicitly set forth above. If the actual facts differ from these assumptions, the numbers of shares and ownership percentages set forth above, including the anticipated equity stake of non-redeeming Public Stockholders in New Conduit following the Business Combination, will be different.
You should read the section of this proxy statement/prospectus entitled “Unaudited Pro Forma Condensed Combined Financial Information” for further information.
In addition to the changes in percentage ownership depicted above, variation in the levels of redemptions will impact the dilutive effect of certain equity issuances related to the Business Combination, which would not otherwise be present in an underwritten public offering. Increasing levels of redemptions will increase the dilutive effect of these issuances on non-redeeming holders of our public shares.
The following table shows the dilutive effect and the effect on the per share value of New Conduit common stock held by non-redeeming holders under a range of scenarios at closing as described below:
| Assuming No Redemptions(1) | Assuming 50% Redemptions(2) | Assuming Maximum Redemptions(3) | ||||||||||||||||||||||
| (shares in thousands) | ||||||||||||||||||||||||
| Shares | Value Per Share (4) | Shares | Value Per Share (5) | Shares | Value Per Share (6) | |||||||||||||||||||
| Base scenario(10) | 75,248 | $ | 0.31 | 74,154 | $ | 0.16 | 73,060 | $ | 0.00 | |||||||||||||||
| Base scenario less shares held by MURF Sponsor and MURF Directors(10) | 71,188 | $ | 0.32 | 70,094 | $ | 0.17 | 69,000 | $ | 0.00 | |||||||||||||||
| Base scenario plus assumed exercise of all warrants(8)(9) | 92,941 | $ | 0.25 | 91,847 | $ | 0.13 | 90,753 | $ | 0.00 | |||||||||||||||
| (1) | Assumes that no shares of MURF Common Stock are redeemed. | |
| (2) | Assumes that 1,093,864 shares of MURF Common Stock, or 50% of our public shares outstanding are redeemed. | |
| (3) | Assumes that 2,187,728 shares of MURF Common Stock, or 100% of the shares outstanding are redeemed. | |
| (4) | Based on a post-transaction equity value of New Conduit of $23.0 million. | |
| (5) | Based on a post-transaction equity value of New Conduit of $11.5 million. | |
| (6) | Based on a post-transaction equity value of New Conduit of $0.1 million. |
| 10 |
| (7) | The Base scenario represents the post-Closing share ownership of New Conduit assuming various levels of redemption by holders of MURF Common Stock. The Base scenario (assuming no redemptions) includes 67,700,000 shares to be held by Conduit shareholders (comprised of 65,000,000 shares to be issued to Conduit shareholders and Conduit convertible noteholders and 2,700,000 shares to be issued as part of the PIPE Financing), 2,187,728 shares to be held by MURF Public Stockholders, 45,000 shares to be held by MURF Directors, 4,015,250 shares to be held by the MURF Sponsor, and 1,300,000 shares to be held by A.G.P. | |
| (8) | Represents the Base scenario plus the Public Warrants, which consist of 13,225,000 warrants issued to MURF Public Stockholders in connection with MURF’s initial public offering, 2,700,000 warrants to be issued in connection with the PIPE Financing, and 54,000 warrants to be issued to A.G.P. upon the closing of the Business Combination. In addition, this includes the Private Warrants, which consist of 709,000 warrants issued to the Sponsor in connection with MURF’s initial public offering and 45,000 warrants originally issued to the MURF Sponsor prior to MURF’s initial public offering that will be transferred to MURF Directors upon the closing of the Business Combination. In addition to the warrants, this amount also includes 402,587 options issued to Cizzle PLC and 476,503 options issued to Vela Technologies PLC, for the right to purchase MURF common stock, as well 80,526 shares related to the convertible notes payable issued in March 2023, which allow for the outstanding principal and accrued interest to be settled through conversion into shares of MURF common stock. | |
| (9) | Does not account for any proceeds being paid to New Conduit in connection with the payment of the exercise prices for any warrants. |
| Q. | How is the payment of the deferred underwriting commissions going to affect the amount left in the trust account upon the completion of the Business Combination? |
| A. | MURF anticipates paying the deferred underwriting commission with funds from the $27 million Private Placement that will be funded immediately prior to the consummation of the Business Combination. The deferred underwriting commissions in connection with MURF’s initial public offering will be released to the underwriters only on completion of the Business Combination, and is payable without regard to the number of Public Shares redeemed by MURF’s stockholders in connection with the Business Combination.
If, however, MURF turned to the trust account for funds to pay the deferred underwriting commission: after paying any redemptions to MURF’s public stockholders, funds that remain in the trust account will be applied towards tax obligations, and if funds still remain, also transaction expenses, such as the deferred underwriting commission. The following table presents the deferred underwriting commission as a percentage of the cash left in the trust account following redemptions across a range of varying redemption scenarios. |
Assuming No Redemptions | Assuming 50% Redemptions | Assuming 100% Redemptions | ||||||||||
| Deferred Underwriting Commission | $ | 4,628,750 | $ | 4,628,750 | $ | 4,628,750 | ||||||
| Deferred Underwriting Commission as a percentage of cash left in the Trust Account Following Redemptions | 19.82 | % | 39.65 | % | (1 | ) | ||||||
(1) In the event of 100% redemptions, after tax obligations, the trust account will not have enough funds to pay the deferred underwriting commission and, in such case, funds from the $27 million provided by the Private Placement Investor immediately before the closing of the Business Combination will be used for the deferred underwriting commission.
You should read the section of this proxy statement/prospectus entitled “Unaudited Pro Forma Condensed Combined Financial Information” for further information.
| 11 |
| Q. | Who will be the directors and officers of New Conduit after the closing of the Business Combination? |
| A. | Immediately following the consummation of the Business Combination, if management’s nominees are elected, the board of directors of New Conduit will consist of (i) Dr. Freda Lewis-Hall, Chairman of New Conduit’s board of directors, (ii) Dr. David Tapolczay, a current director of Conduit and the Chief Executive Officer of New Conduit, (iii) James Bligh, an employee of Conduit, (iv) Faith L. Charles, (v) Chele Chiavacci Farley, a current director of MURF, (vi) Jennifer I. McNealey, and (vii) Dr. Andrew Regan, a current director and shareholder of Conduit (Dr. Regan also undertakes duties typically associated with an executive officer in connection with his service as a director of Conduit). In addition, the executive officers of New Conduit will be Dr. David Tapolczay, Conduit’s current Chief Executive Officer and director, as Chief Executive Officer, and Adam Sragovicz, MURF’s current Chief Financial Officer and a director, as Chief Financial Officer. |
| Q. | Do I have redemption rights? |
| A. | If
you are a holder of Public Shares, then you have the right to demand that MURF redeem
such shares into a pro rata portion of the cash held in MURF’s trust account. We sometimes
refer to these rights to demand redemption of the Public Shares as “redemption rights.”
As a result of the pre-Business Combination redemption of 11,037,272 shares of MURF Class
A common stock, the redemption payment of approximately $114 million reduced the balance
of the trust account to approximately $23 million.
In connection with this Special Meeting, MURF’s public stockholders have another opportunity to redeem Public Shares. The balance of the trust account will be the following amounts in the event of these redemption scenarios (after withholding funds for tax obligations): (i) no redemptions will maintain the current balance of approximately $23.3 million in the trust account; (ii) 50% redemptions will result in approximately $11.7 million in the trust account; and (iii) 100% redemptions will result in $0 in the trust account. |
Under MURF’s amended and restated certificate of incorporation, the Business Combination may only be consummated if MURF (i) is exempt from the provisions of Rule 419 promulgated under the Securities Act other than through its net tangible assets or (ii) has at least $5,000,001 of net tangible assets either immediately prior to or upon consummation of the Business Combination. However, Conduit is not required to consummate the Transactions if there is not at least $27 million of cash available to be released from MURF’s trust account and/or received by MURF under the Subscription Agreement after giving effect to payment of amounts that MURF will be required to pay to redeeming stockholders upon consummation of the Business Combination.
| Q. | How do I exercise my redemption rights? |
| A. | If you are a holder of Public Shares and wish to exercise your redemption rights, you must, prior to 5:00 p.m., Eastern Time, on September 5, 2023 (two (2) business days before the Special Meeting), tender your shares electronically by using the Depository Trust Company’s DWAC (Deposit/Withdrawal At Custodian) System and submit a request in writing that we redeem your Public Shares for cash to Vstock Transfer, LLC, our transfer agent, at the following email address: |
| DWAC@vstocktransfer.com | |
| Attn: DWAC Team |
| MURF’S transfer agent must receive your instruction to redeem by 5:00 pm Eastern Time on September 5, 2023, in order for your redemption demand to be valid. Any holder of Public Shares, regardless of whether they vote for or against the business combination proposal or do not vote at all, will be entitled to demand that such holder’s shares be redeemed for a pro rata portion of the amount then in the trust account (which, for illustrative purposes, was $23,349,196 available for redemption, or approximately $10.67 per Public Share, as of July 31, 2023). Such amount, less any owed but unpaid taxes on the funds in the trust account, will be paid promptly upon consummation of the Business Combination. There are currently no owed but unpaid income taxes on the funds in the trust account. However, under Delaware law, the proceeds held in the trust account could be subject to claims which could take priority over those of a Public Stockholder exercising redemption rights, regardless of whether such holders vote for or against the business combination proposal or do not vote at all. Therefore, the per-share distribution from the trust account in such a situation may be less than originally anticipated due to such claims. Your vote will have no impact on the amount you will receive upon exercise of your redemption rights. |
| 12 |
Any request for redemption, once made by a Public Stockholder, may be withdrawn at any time up to 5:00 pm Eastern Time on September 5, 2023. If you deliver your shares for redemption to MURF’s transfer agent and later decide prior to the Special Meeting not to elect redemption, you may request that MURF’s transfer agent return the shares (electronically). You may make such request by contacting MURF’s transfer agent at the email address above.
Any corrected or changed proxy card or written demand of redemption rights must be received by MURF’s transfer agent prior to the Special Meeting. No demand for redemption will be honored unless the holder’s stock has been delivered electronically to the transfer agent by 5:00 pm Eastern Time on September 5, 2023.
If demand is properly made by a Public Stockholder as described above, then, if the Business Combination is consummated, MURF will redeem these shares into a pro rata portion of funds deposited in the trust account. If you exercise your redemption rights, then you will be exchanging your shares of MURF Class A common stock for cash and you will not be entitled to shares of New Conduit common stock upon consummation of the Business Transaction.
If you are a holder of Public Shares and you exercise your redemption rights, it will not result in the loss of any MURF warrants that you may also hold. Your whole warrants will become exercisable to purchase one share of New Conduit common stock for a purchase price of $11.50 upon consummation of the Business Combination.
IN ORDER TO EXERCISE YOUR REDEMPTION RIGHT, YOU NEED TO IDENTIFY YOURSELF AS A BENEFICIAL HOLDER AND PROVIDE YOUR LEGAL NAME, PHONE NUMBER AND ADDRESS IN YOUR WRITTEN DEMAND.
| Q. | Do I have appraisal rights if I object to the proposed business combination? |
| A. | No. Neither MURF stockholders nor its unit or warrant holders have appraisal rights in connection with the Business Combination under the DGCL. See the section entitled “Special Meeting of MURF Stockholders — Appraisal Rights.” |
| Q. | What happens to the funds deposited in the trust account after consummation of the Business Combination? |
| A. | Following the closing of the initial public offering on February 7, 2022, an amount of $139,790,000 from the net proceeds of the sale of the units in the initial public offering and the private placement of units to the Sponsor was placed in the trust account. This resulted in an overfunding of the trust account of $4,895,000. As such, subsequent to the initial funding of the trust account, $2,000,000 was transferred to MURF’s operating cash account and $2,895,000 was used to pay offering costs. After consummation of the Business Combination, the funds in the trust account will be used to pay holders of the Public Shares who exercise redemption rights and tax obligations, and if any funds remain, to pay fees and expenses incurred in connection with the Business Combination and for MURF’s working capital and general corporate purposes. |
| Q. | What happens if a substantial number of Public Stockholders vote in favor of the business combination proposal and exercise their redemption rights? |
| A. | MURF’s
Public Stockholders may vote in favor of the Business Combination and still exercise their
redemption rights. Accordingly, the Business Combination may be consummated even though the
funds available from the trust account and the number of Public Stockholders are substantially
reduced as a result of redemptions by Public Stockholders. As a result of the pre-Business
Combination redemption of 11,037,272 shares of MURF Class A common stock, the redemption
payment of approximately $114 million reduced the balance of the trust account to approximately
$23 million. The balance of the trust account will be the following amounts in the event
of these redemption scenarios (after withholding funds for tax obligations): (i) no redemptions will maintain the current balance of approximately $23.3
million in the trust account; (ii) 50% redemptions will result in approximately $11.7
million in the trust account; and (iii) 100% redemptions will result in $0 in the trust
account. Conduit is not required to consummate the Business Combination if there is not at least $27,000,000 of cash available for release from MURF’s trust account and/or received by MURF under the Private Placement, after giving effect to payment of amounts that MURF will be required to pay to redeeming stockholders upon consummation of the Business Combination. Also, with fewer Public Shares and Public Stockholders, the trading market for New Conduit common stock may be less liquid than the market for MURF’s shares of Class A common stock prior to the Transactions and MURF may not be able to meet the listing standards of a national securities exchange. In addition, with fewer funds available from the trust account, the capital infusion from the trust account into Conduit’s business will be reduced and Conduit may not be able to achieve its plan of reducing its outstanding indebtedness. |
| Q. | What happens if the Business Combination is not consummated? |
| A. | If MURF does not complete the Business Combination with Conduit for whatever reason, MURF would search for another target business with which to complete a business combination. If MURF does not complete the Business Combination with Conduit or another business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), MURF must redeem 100% of the outstanding Public Shares, at a per-share price, payable in cash, equal to an amount then held in the trust account. The Sponsor has no redemption rights in the event a business combination is not effected in the required time period, and, accordingly, its Private Shares will be worthless. Additionally, in the event of such liquidation, there will be no distribution with respect to the outstanding warrants. Accordingly, the warrants will expire worthless. |
| 13 |
| Q. | How does the Sponsor of MURF intend to vote on the proposals? |
| A. | As of August 2, 2023, the record date, the Sponsor beneficially owned and was entitled to vote an aggregate of 4,060,250 Private Shares that were issued prior to or concurrently with MURF’s initial public offering. Such shares currently constitute approximately 65% of the outstanding shares of MURF common stock. The Sponsor has agreed to vote such Private Shares, as well as any shares of MURF common stock acquired in the aftermarket, in favor of the business combination proposal. The Sponsor also intends to vote its Private Shares in favor of all other proposals being presented at the meeting. |
| Q. | Do any of MURF’s directors or officers have interests in the Business Combination that may differ from or be in addition to the interests of the MURF stockholders? |
| A. | The Sponsor and the directors and officers of MURF have interests in the Business Combination that are different from or in addition to (and which may conflict with) your interest. These interests include, among other things: |
| ● | If the Business Combination with Conduit or another business combination is not consummated by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), then MURF will (i) cease all operations except for the purpose of winding up, (ii) redeem the Public Shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account including interest earned on the funds held in the trust account and not previously released to MURF to pay MURF’s franchise and income taxes (less up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding Public Shares, which redemption will completely extinguish Public Stockholders’ rights as stockholders (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) subject to the approval of our remaining stockholders and our board of directors, dissolve and liquidate, subject in each case to our obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. | |
| ● | The Sponsor (including its representatives and affiliates) and MURF’s directors and officers, are, or may in the future become, affiliated with entities that are engaged in a similar business to MURF’s and the Sponsor and MURF’s directors and officers are not prohibited from sponsoring, or otherwise becoming involved with, any other blank check companies prior to MURF completing its initial business combination, and as result of which, the Sponsor and MURF’s officers and directors may become aware of business opportunities which may be appropriate for presentation to MURF, and the other entities to which they owe fiduciary or contractual duties, and may have conflicts of interests in determining to which entity a particular business opportunity should be presented (and these conflicts may include presentation to other entities prior to their presentation, if at all, to MURF, and may not always be resolved in the favor of MURF, subject to applicable fiduciary duties under Delaware law, in that MURF has provided in its amended and restated certificate of incorporation that MURF has renounced its interest in any corporate opportunity presented to MURF). | |
| ● | The Sponsor and the directors and officers of MURF have each waived their redemption rights with respect to any Founder Shares and Class A common stock held by them (other than relating to liquidating distributions to Public Shares from the trust account if MURF fails to complete its initial business combination by the time required prior to MURF’s liquidation in accordance with its amended and restated certificate of incorporation), which waiver was provided in connection with the initial public offering and without any separate consideration paid in connection with providing such waiver. | |
| ● | The Merger Agreement contemplates that Adam Sragovicz, MURF’s current Chief Financial Officer and a director, will be the Chief Financial Officer of New Conduit after the closing of the Business Combination. Pursuant to the terms of an employment agreement to be entered into between New Conduit and Mr. Sragovicz in connection with the closing of the Business Combination, Mr. Sragovicz is entitled to compensation consisting of (i) an annual base salary of $400,000, and (ii) a target annual bonus opportunity equal to 40% of his base salary, payable based on the achievement of performance objectives as determined by the New Conduit board of directors. In addition, the employment agreement provides that he will be entitled to receive a sign-on restricted stock unit award covering 0.10% of the shares of common stock of New Conduit pursuant to the terms of the 2023 Stock Incentive Plan, which shall vest in equal annual installments on the first three anniversaries of the Business Combination. In the future, Mr. Sragovicz could receive any additional cash compensation, stock options, stock awards or other benefits that New Conduit’s board of directors determines to pay to Mr. Sragovicz. | |
| ● | Mr.
Heilbron, the Chief Executive Officer and Chairman of MURF is also the President of NetREIT Advisors, which is sole and managing
member of the Sponsor. NetReit Advisors is wholly-owned by Presidio. Mr. Heilbron is the President, Chief Executive Officer and Chairman
of Presidio, of which he beneficially owns 2.6% (as of March 31, 2023). In 2023, Presidio’s compensation committee of its board
of directors awarded Mr. Heilbron a cash bonus equal to 1% of the value of the New Conduit common stock owned by Presidio, to be
valued on the day which is six months and one day after the closing of the Business Combination.
Mr. Heilbron has entered into a consulting agreement with MURF, which is effective upon the closing of the Business Combination. The consulting agreement provides that Mr. Heilbron will provide advisory and consulting services from time to time to New Conduit for one year following the closing of the Business Combination. Mr. Heilbron will no longer serve as an officer or director of New Conduit after the closing of the Business Combination. However, pursuant to the terms of the consulting agreement, Mr. Heilbron is entitled to rights as an observer to the Board of Directors of New Conduit. Mr. Heilbron is entitled to be paid $25,000 per calendar quarter for his consulting services and is also entitled to a stock option to purchase the number of shares of the common stock of New Conduit determined by dividing (i) $300,000, by (ii) the per share Black-Scholes valuation as of the grant date, utilizing the same assumptions used in preparation of the financial statements, with the resulting quotient rounded down to the nearest whole share. | |
| ● | The Sponsor paid an aggregate of approximately $25,000 for the Founder Shares and the market value of such securities as of July 31, 2023, was approximately $35,641,000, and such securities should have a significantly higher value than $25,000 at the time of the Business Combination, and if MURF does not complete an initial business combination will expire worthless. | |
| ● | The Sponsor paid $7,540,000 for the 745,000 private placement units (consisting of 745,000 shares of Class A common stock and the Private Warrants) in connection with MURF’s initial public offering, at a price of $10.00 per unit, the market value of such securities as of July 31, 2023, was approximately $15,286,000, and such securities should have a higher value at the time of the Business Combination than $7,540,000. If MURF does not complete an initial business combination by February 7, 2024, then the Private Warrants received as part of the private placement units will expire worthless. | |
| ● | The Sponsor and its affiliates’ total potential ownership interest in New Conduit, as a result of 11,037,272 shares of MURF Class A common stock having already been redeemed (or approximately 83% of the issued and outstanding Class A common stock subject to possible redemption as of December 31, 2022) and assuming the exercise and conversion of all securities following the closing of the Business Combination, including the Private Shares, is estimated to comprise approximately 5.1% of the outstanding New Conduit common stock in a no redemption scenario (or a transaction value of approximately $34,021,000 or a market value of approximately $51,900,000), approximately 5.2% of outstanding New Conduit common stock in a 50% redemption scenario (or a transaction value of approximately $34,430,000 or a market value of approximately $51,900,000), and 5.3% of outstanding New Conduit common stock in a 100% maximum redemption scenario (or a transaction value of approximately $34,850,000 or a market value of approximately $51,900,000). |
| 14 |
| ● | Immediately following the closing of the Business Combination, the independent directors of the MURF Board will receive from the Sponsor an aggregate of 45,000 private placement units, consisting of, in aggregate, 45,000 shares of MURF Class A common stock and 45,000 Private Warrants, which, assuming conversion and exercise, will be 90,000 shares of New Conduit common stock of an aggregate value of approximately $970,000 (assuming that the future value of New Conduit common stock will be the same as the current value of MURF’s Class A common stock) (the “Independent Director Units”). | |
| ● | The Sponsor and its affiliates can earn a positive rate of return on their overall investment in MURF and Conduit after the Business Combination, even if other holders of MURF common stock experience a negative rate of return, due to having purchased the Founder Shares, as described above, for $25,000 or approximately $0.006 per share. | |
| ● | On March 7, 2023, MURF has issued to Sponsor an unsecured promissory note in the principal amount of $1,500,000, portions of which may be withdrawn by MURF from time to time (the “Promissory Note”). As of July 31, 2023, MURF has submitted drawdown requests in an aggregate amount of $750,000. The Promissory Note bears no interest and outstanding principal is due and payable upon the earlier of the consummation of the initial business combination or the date of the liquidation of MURF. | |
| ● | The aggregate dollar amount that the Sponsor and its affiliates have at risk that depends on the completion of an initial business combination, including the Business Combination, is approximately $8,315,000, as of July 31, 2023, which is the current value of securities held (assuming that the current value of each of the securities held, whether publicly traded shares of MURF Class A common stock or Private Warrants, are in each case valued at the current price of MURF’s Class A common stock) and consists of (i) the Founders Shares and (ii) the 754,000 private placement units (each such unit comprised of one share of MURF’s Class A common stock and one warrant to purchase one share of MURF’s Class A common stock) purchased in connection with MURF’s initial public offering, and the Promissory Note. There are no fees due or out of pocket expenses for which Sponsor and its affiliates are awaiting reimbursement. | |
| ● | The aggregate dollar amount that the directors of MURF have at risk that depends on the completion of an initial business combination, including the Business Combination, is approximately $970,000, as of July 31, 2023, which is the current value of the Independent Director Units. There are no loans, fees due or out of pocket expenses for which directors are awaiting reimbursement. | |
| ● | As a result of the foregoing, the Sponsor, and directors and officers of MURF, including Mr. Heilbron, will benefit from the completion of an initial business combination, including the Business Combination, and may be incentivized to complete an acquisition or business combination of a less favorable target company or on terms less favorable to stockholders of MURF rather than liquidate. | |
| ● | If MURF is unable to complete a business combination within the required time period, its executive officers will be personally liable under certain circumstances described herein to ensure that the proceeds in the trust account are not reduced by the claims of target businesses or claims of vendors or other entities that are owed money by MURF for services rendered or contracted for or products sold to MURF. If MURF consummates a business combination, on the other hand, MURF will be liable for all such claims. | |
| ● | MURF’s Sponsor, including its officers and directors, and their affiliates are entitled to reimbursement of out-of-pocket expenses incurred by them in connection with certain activities on MURF’s behalf, such as identifying and investigating possible business targets and business combinations. However, if MURF fails to consummate a business combination within the required period, they will not have any claim against the trust account for reimbursement. Accordingly, MURF may not be able to reimburse these expenses if the Business Combination with Conduit, or another business combination, is not completed by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s its amended and restated certificate of incorporation). | |
| ● | The continued indemnification of current directors and officers and the continuation of directors and officers’ liability insurance. | |
| ● | Chele Chiavacci Farley, a current director of the MURF Board, will be appointed to the Board of Directors of New Conduit upon the closing of the Business Combination. |
| Q. | When do you expect the Business Combination to be completed? |
| A. | It is currently anticipated that the Business Combination will be consummated promptly following the Special Meeting, which is scheduled for September 7, 2023; however, such meeting could be adjourned or postponed, as described above. For a description of the conditions to the completion of the Business Combination, see the section entitled “The Merger Agreement — Conditions to the Closing of the Business Combination.” |
| Q. | What do I need to do now? |
| A. | MURF urges you to read carefully and consider the information contained in this proxy statement/prospectus, including the annexes, and to consider how the Business Combination will affect you as a stockholder and/or warrant holder of MURF. Stockholders should then vote as soon as possible in accordance with the instructions provided in this proxy statement/prospectus and on the enclosed proxy card. |
| Q. | How do I vote? |
| A. | If you are a holder of record of MURF common stock on the record date, you may vote in person (by virtual attendance) at the Special Meeting or by submitting a proxy for the Special Meeting. You may submit your proxy by completing, signing, dating and returning the enclosed proxy card in the accompanying pre-addressed postage paid envelope. If you hold your shares in “street name,” which means your shares are held of record by a broker, bank or nominee, you should contact your broker to ensure that votes related to the shares you beneficially own are properly counted. In this regard, you must provide the broker, bank or nominee with instructions on how to vote your shares or, if you wish to attend the virtual meeting and vote at the meeting, obtain a proxy from your broker, bank or nominee. |
| Q. | If my shares are held in “street name,” will my broker, bank or nominee automatically vote my shares for me? |
| A. | No. Your broker, bank or nominee cannot vote your shares unless you provide instructions on how to vote in accordance with the information and procedures provided to you by your broker, bank or nominee. |
| Q. | May I change my vote after I have mailed my signed proxy card? |
| A. | Yes. Stockholders may send a later-dated, signed proxy card to MURF’s transfer agent at the address set forth at the end of this section so that it is received prior to the Special Meeting or virtually attend the Special Meeting and vote. Stockholders also may revoke their proxy by sending a notice of revocation to MURF’s transfer agent, which must be received prior to the Special Meeting. You will not be able to attend the Special Meeting physically. |
| 15 |
| Q. | What happens if I fail to take any action with respect to the meeting? |
| A. | If you fail to take any action with respect to the meeting and the Business Combination is approved by stockholders and consummated, you will remain a stockholder of MURF (to be renamed Conduit Pharmaceuticals Inc., which we refer to as New Conduit) and/or your warrants will continue to entitle you to purchase shares of New Conduit common stock. |
| Q. | What should I do with my stock and/or warrants certificates? |
| A. | Those stockholders who do not elect to redeem their Class A common stock should not submit their stock certificates to MURF or our transfer agent. After the consummation of the Business Combination, MURF stockholders who do not redeem their Class A common stock will retain their shares, which will be automatically converted into New Conduit common stock. MURF stockholders who exercise their redemption rights must deliver their stock certificates to MURF’s transfer agent electronically prior to the vote at the meeting as described above. |
Upon consummation of the Transactions, MURF’s warrants, by their terms, will entitle holders to purchase shares of New Conduit common stock. Therefore, warrant holders need not deliver their warrants to MURF at any time.
| Q. | What should I do if I receive more than one set of voting materials? |
| A. | Stockholders may receive more than one set of voting materials, including multiple copies of this proxy statement/prospectus and multiple proxy cards or voting instruction cards. For example, if you hold your shares in more than one brokerage account, you will receive a separate voting instruction card for each brokerage account in which you hold shares. If you are a holder of record and your shares are registered in more than one name, you will receive more than one proxy card. Please complete, sign, date and return each proxy card and voting instruction card that you receive in order to cast a vote with respect to all of your shares of MURF common stock. |
| Q. | Who can help answer my questions? |
| A. | If you have questions about the Merger or if you need additional copies of the proxy statement/prospectus or the enclosed proxy card you should contact: |
4995 Murphy Canyon Road, Suite 300
San Diego, California 92123
Attention: Jack K. Heilbron, CEO
Email: jheilbron@presidiopt.com
or:
D.F. King & Co., Inc.
48 Wall Street, 22nd Floor
New York, NY 10005
Phone: (800) 511-9495
Email: MURF@dfking.com
You may also obtain additional information about MURF from documents filed with the SEC by following the instructions in the section entitled “Where You Can Find More Information.” If you are a holder of Public Shares and you intend to seek redemption of your shares, you will need to deliver your stock electronically to MURF’s transfer agent prior to 5:00 p.m., Eastern Time, on September 5, 2023 (two (2) business days before the Special Meeting) at the email address below. If you have questions regarding the certification of your position or delivery of your stock, please contact:
Vstock Transfer, LLC
18 Lafayette Place
Woodmere, NY 11598
Attn: DWAC Team
E-mail: DWAC@vstocktransfer.com
| 16 |
SUMMARY OF THE PROXY STATEMENT/PROSPECTUS
This summary highlights selected information from this proxy statement/prospectus and does not contain all of the information that is important to you. To better understand the proposals to be submitted for a vote at the Special Meeting, including the Business Combination, you should read this entire document carefully, including the Merger Agreement attached as Annexes A-1 through A-3 to this proxy statement/prospectus. The Merger Agreement is the legal document that governs the Transactions that will be undertaken in connection with the Business Combination. It is also described in detail in this proxy statement/prospectus in the section entitled “The Merger Agreement.”
The Parties to the Business Combination
MURF
Murphy Canyon Acquisition Corp. is a blank check company incorporated on October 19, 2021 as a Delaware corporation formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or other similar business combination with one or more businesses.
On February 7, 2022, MURF consummated its initial public offering of an aggregate of 13,225,000 units, including full exercise of the underwriters’ over-allotment option, at $10.00 per unit. The gross proceeds of the offering were $132.25 million. Simultaneously with the consummation of the initial public offering, MURF consummated the private placement of 754,000 units to the Sponsor, which amount included 69,000 private placement units purchased by the sponsor in connection with the underwriters’ exercise of their overallotment option in full, at a price of $10.00 per private placement unit, generating gross proceeds of approximately $7.54 million.
Following the closing of the initial public offering on February 7, 2022, an aggregate amount of $139,790,000 from the net proceeds of the sale of the units in the initial public offering and the private placement was placed in the trust account. This resulted in an overfunding of the trust account of $4,895,000. As such, subsequent to the initial funding of the trust account, $2,000,000 was transferred to MURF’s operating cash account and $2,895,000 was used to pay offering costs. Except with respect to interest earned on the funds held in the trust account that may be released to us to pay our taxes (less up to $100,000 interest to pay dissolution expenses), the funds held in the trust account will not be released from the trust account until the earliest of (a) the completion of our initial business combination, (b) the redemption of any Public Shares properly submitted in connection with a stockholder vote to amend our certificate of incorporation (A) to modify the substance or timing of our obligation to allow redemption in connection with MURF’s initial business combination or certain amendments to our charter prior thereto or to redeem 100% of our public shares if we do not complete our initial business combination within 12 months from the consummation of MURF’s initial public offering or up to 18 months if we extend the period of time to consummate a business combination, at MURF’s election by two separate three month extensions, subject to satisfaction of certain conditions, including the deposit of up to $1,322,500 for each three month extension, into the trust account, or as extended by MURF’s stockholders in accordance with MURF’s certificate of incorporation) or (ii) with respect to any other provision relating to stockholders’ rights or pre-initial business combination activity, and (c) the redemption of our public shares if MURF is unable to complete its initial business combination within 12 months from the consummation of its initial public offering or up to 18 months if we extend the period of time to consummate a business combination, at MURF’s election by two separate three month extensions, subject to satisfaction of certain conditions, including the deposit of up to $1,322,500 for each three month extension, into the trust account, or as extended by MURF’s stockholders in accordance with our Certificate of Incorporation), subject to applicable law.
MURF’s units, Class A common stock and warrants are traded on the Nasdaq Global Market under the symbols “MURFU,” “MURF,” and “MURFW,” respectively. Our units commenced public trading on February 3, 2022, and our Class A common stock and warrants commenced public trading on March 28, 2022. As of November 8, 2022, the date of the Merger Agreement, the closing price of MURF’s Class A common stock was $10.13 per share, the closing price of MURF’s units was $10.21 per unit and the closing price of MURF’s public warrants was $0.0566 per warrant.
The mailing address of MURF’s principal executive office is 4995 Murphy Canyon Road, Suite 300, San Diego, CA 92123, and its telephone number is 760-471-8536.
Sponsor
Murphy Canyon Acquisition Sponsor, LLC, a Delaware limited liability company, is the sponsor of MURF. The Sponsor’s executive office is located at 4995 Murphy Canyon Road, Suite 300, San Diego, CA 92123, and its telephone number is 760-471-8536.
As of August 2, 2023, the record date, the Sponsor beneficially owned and was entitled to vote an aggregate of 4,060,250 shares of MURF common stock (consisting of 754,000 shares of Class A common stock and 3,306,250 shares of Class B common stock) that were issued prior to or concurrently with MURF’s initial public offering. Such shares currently constitute approximately 65% of the outstanding shares of MURF common stock. The Sponsor has agreed to vote such Private Shares, as well as any shares of MURF common stock acquired in the aftermarket, in favor of the business combination proposal. The Sponsor also intends to vote its shares in favor of all other proposals being presented at the meeting. The Private Shares held by the Sponsor have no right to participate in any redemption distribution and will be worthless if no business combination is effected by MURF.
Merger Sub
Conduit Merger Sub, Inc. is a wholly-owned subsidiary of MURF formed solely for the purpose of effectuating the Merger described herein. Merger Sub was incorporated under the laws of the Cayman Islands on October 31, 2022. Merger Sub owns no material assets and does not operate any business.
The mailing address of Merger Sub’s principal executive office is 4995 Murphy Canyon Road, Suite 300, San Diego, CA 92123 and its telephone number is 760-471-8536. Merger Sub’s registered office is c/o Ogier Global (Cayman) Limited, 89 Nexus Way, Camana Bay, Grand Cayman, KY1-9009 Cayman Islands. After the consummation of the Business Combination, it will cease to exist as a stand-alone company and will instead be merged with and into Conduit.
| 17 |
Conduit
Conduit Pharmaceuticals Limited, a Cayman Islands exempted company, is a clinical-stage specialty biopharmaceutical company that was incorporated in 2019 to facilitate the development and commercialization of clinical assets that have not been, or are not being, prioritized by leading biopharmaceutical companies in order to develop pharmaceutical products that meet the unmet medical needs of patients.
The mailing address of Conduit’s principal executive office/registered office is c/o Ogier Global (Cayman) Limited, 89 Nexus Way, Camana Bay, Grand Cayman, KY1-9009 Cayman Islands. After the consummation of the Business Combination, Conduit will be a wholly-owned subsidiary of MURF (which will be renamed Conduit Pharmaceuticals Inc.).
Emerging Growth Company
MURF is an “emerging growth company,” as defined under the JOBS Act. As an emerging growth company, MURF is eligible to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies. These include, but are not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and the requirement to obtain stockholder approval of any golden parachute payments not previously approved.
In addition, Section 107 of the JOBS Act provides that an emerging growth company can take advantage of an extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. MURF has elected to take advantage of such extended transition period.
MURF will remain an emerging growth company until the earliest of (i) the last day of MURF’s fiscal year following February 7, 2027 (the fifth anniversary of the consummation of its initial public offering), (ii) the last day of the fiscal year in which the market value of its shares of Class A common stock that are held by non-affiliates exceeds $700 million as of June 30 of that fiscal year, (iii) the last day of the fiscal year in which it has total annual gross revenue of $1.07 billion or more during such fiscal year (as indexed for inflation) or (iv) the date on which it has issued more than $1.0 billion in non-convertible debt in the prior three-year period.
The Business Combination Proposal
Structure of the Transactions
Pursuant to the Merger Agreement, Merger Sub will merge with and into Conduit, with Conduit surviving the merger. As a result of the Transactions, Conduit will become a wholly-owned subsidiary of MURF with the shareholders of Conduit becoming stockholders of MURF, which will be renamed Conduit Pharmaceuticals Inc.
Under the Merger Agreement, the shareholders of Conduit will receive the number of New Conduit common stock that shall have an aggregate value equal to Six Hundred Fifty Million Dollars ($650,000,000). With each share of New Conduit common stock being valued at $10.00, Conduit’s outstanding ordinary shares (including the ordinary shares issued upon conversion of all outstanding convertible debt, which conversion shall have occurred prior to the consummation of the Business Combination) will be converted into an aggregate of 65,000,000 shares of the common stock of New Conduit following the Business Combination, with each such outstanding Conduit share converted into shares of the common stock of New Conduit on a pro rata basis. Accordingly, this proxy statement/prospectus covers 65,000,000 shares of New Conduit common stock. Upon the consummation of the Business Combination, MURF’s Public Warrants shall, by their terms, entitle the holders to purchase shares of New Conduit common stock at a purchase price of $11.50 per share.
In connection with the Business Combination, each outstanding share of MURF common stock immediately prior to the Merger, including each outstanding share of Class B common stock, shall automatically convert into and become one share of the common stock of New Conduit without any action on the part of any holders of MURF common stock.
Pro Forma Ownership of Former Holders of Conduit Shares and MURF Stockholders
It is anticipated that, immediately after the closing of the Business Combination, as a result of 11,037,272 shares of MURF Class A common stock having already been redeemed (or approximately 83% of the issued and outstanding Class A common stock subject to possible redemption as of December 31, 2022) and assuming that no Public Stockholders exercise their redemption rights and that no warrants to purchase New Conduit common stock are exercised, MURF’s Public Stockholders will retain an ownership interest of 2,187,728 shares of New Conduit common stock or approximately 2.9% of New Conduit common stock, and Conduit’s shareholders will own 67,700,000 shares of New Conduit common stock or approximately 90.0% of New Conduit common stock, which reflects 65,000,000 shares of New Conduit common stock to be issued to the Conduit shareholders as consideration in the merger plus the 2,700,000 shares of New Conduit common stock to be issued to the Private Placement Investor in connection with the Private Placement as the Private Placement Investor is an affiliate of one of Conduit’s existing shareholders. After consideration of the factors identified and discussed in the section entitled “The Business Combination Proposal — The MURF Board’s Reasons for Approval of the Transactions,” the MURF Board concluded that the Transactions met all of the requirements disclosed in the prospectus for its initial public offering, including that such business had a fair market value of at least 80% of the balance of the funds in the trust account at the time of execution of the Merger Agreement (excluding the deferred underwriting commissions and taxes payable on the income earned on the trust account). See the section entitled “The Business Combination Proposal — Structure of the Transactions” for more information.
| 18 |
Additional Matters Being Voted On
The Charter Amendment Proposals
In addition to voting on the business combination proposal, MURF stockholders will vote on separate proposals to approve amendments to MURF’s current amended and restated certificate of incorporation to:
| (i) | Charter Amendment Proposal A – change the name of the public entity from “Murphy Canyon Acquisition Corp.” to “Conduit Pharmaceuticals Inc.”; | |
| (ii) | Charter Amendment Proposal B – provide for one class of authorized common stock; | |
| (iii) | Charter Amendment Proposal C – delete the various provisions in MURF’s current amended and restated certificate of incorporation applicable only to special purpose acquisition corporations (such as the obligation to dissolve and liquidate if a business combination is not consummated within a certain period of time); | |
| (iv) | Charter Amendment Proposal D – increase the number of authorized shares of common stock to 250,000,000; and | |
| (v) | Charter Amendment Proposal E – fix the number of directors at seven (7), a majority of whom shall be independent directors in accordance with The Nasdaq Stock Market LLC’s requirements. See the section entitled “The Charter Amendment Proposals.” |
The Advisory Charter Amendments Proposals
In addition to voting on the business combination proposal, MURF stockholders will vote on separate proposals to consider and vote upon, on a non-binding advisory basis, certain governance provisions in the Proposed Charter, presented separately in accordance with SEC requirements. See the section entitled “The Advisory Charter Amendments Proposals”.
The Director Election Proposal
The stockholders of MURF will also vote to elect seven (7) directors who, upon consummation of the Transactions, will be the directors of New Conduit. If management’s nominees are elected, such directors will serve until the general meeting to be held in 2024 and, in each case, until their successors are elected and qualified or their earlier resignation or removal. See the section entitled “The Director Election Proposal.”
The Incentive Plan Proposal
The proposed 2023 Plan will reserve up to 11,497,622 shares of common stock of New Conduit for issuance in accordance with the plan’s terms, subject to certain adjustments. The purpose of the plan is to provide New Conduit’s and its subsidiaries’ officers, directors, employees and consultants who, by their position, ability and diligence are able to make important contributions to New Conduit’s growth and profitability, with an incentive to assist New Conduit in achieving its long-term corporate objectives, to attract and retain executive officers and other employees of outstanding competence and to provide such persons with an opportunity to acquire an equity interest in New Conduit. The 2023 Plan is attached as Annex C to this proxy statement/prospectus. You are encouraged to read the plan in its entirety. See the section entitled “The Incentive Plan Proposal.”
The Nasdaq Issuance Proposal
The stockholders will consider and vote upon a proposal to approve, for purposes of complying with applicable listing rules of Nasdaq, the issuance of New Conduit common stock, par value $0.0001 per share, to the Private Placement Investor in the Private Placement, the proceeds of which will be used to finance the Business Combination and related transactions and the costs and expenses incurred in connection therewith with any balance used for working capital purposes. See the section entitled “The Nasdaq Proposal.”
The Adjournment Proposal
If MURF does not have sufficient proxies to approve one or more of the foregoing proposals, the MURF Board may submit a proposal to adjourn the Special Meeting to a later date or dates, if necessary to permit further solicitation and vote of proxies. See the section entitled “The Adjournment Proposal.”
| 19 |
Date, Time and Place of Special Meeting of MURF’s Stockholders
The Special Meeting of stockholders of MURF will be held at 10:00 a.m., Eastern Time, on September 7, 2023, via live webcast, to consider and vote upon the business combination proposal, the charter amendment proposals, the advisory charter amendments proposals, the incentive plan proposal, the director election proposal, the Nasdaq proposal and/or if necessary, the adjournment proposal to permit further solicitation and vote of proxies if MURF does not have sufficient proxies to approve the foregoing proposals. MURF stockholders may virtually attend, vote and examine the list of MURF stockholders entitled to vote at the Special Meeting by visiting and entering the control number found on their proxy card, voting instruction form or notice they previously received. In light of public health concerns regarding the novel coronavirus (COVID-19), the Special Meeting will be held in a virtual meeting format only. You will not be able to attend the Special Meeting physically.
Voting Power; Record Date
Stockholders will be entitled to vote or direct votes to be cast at the Special Meeting if they owned shares of MURF common stock at the close of business on August 2, 2023, which is the record date for the Special Meeting. Stockholders will have one vote for each share of MURF common stock owned at the close of business on the record date. If your shares are held in “street name” or are in a margin or similar account, you should contact your broker to ensure that votes related to the shares you beneficially own are properly counted. MURF warrants do not have voting rights. On the record date, there were: (i) 2,941,728 shares of Class A common stock issued and outstanding and (ii) 3,306,250 shares of Class B common stock issued and outstanding, of which 4,060,250 shares (consisting of 754,000 shares of Class A common stock and 3,306,250 shares of Class B common stock) are held by the Sponsor.
Quorum and Vote of MURF Stockholders
A quorum of MURF stockholders is necessary to hold a valid meeting. A quorum will be present at the Special Meeting if a majority of the outstanding shares entitled to vote at the meeting are represented in person (by virtual attendance) or by proxy. Abstentions and broker non-votes will count as present for the purposes of establishing a quorum. The Sponsor holds approximately 65% of the outstanding shares of MURF common stock. Such shares, as well as any shares of Class A common stock acquired in the aftermarket by the Sponsor, will be voted in favor of the proposals presented at the Special Meeting. The proposals presented at the Special Meeting will require the following votes:
| ● | The approval of the business combination proposal will require the affirmative vote of the holders of a majority of the outstanding shares of MURF common stock on the record date. There are currently (i) 2,941,728 shares of Class A common stock issued and outstanding, and (ii) 3,306,250 shares of Class B common stock are issued and outstanding; therefore, at least 3,123,990 shares of MURF common stock must be voted in favor to pass the proposal. The Sponsor owns an aggregate of 4,060,250 shares of MURF common stock (consisting of 754,000 shares of Class A common stock and 3,306,250 shares of Class B common stock) and has agreed to vote in favor of the proposal; therefore, only 3,123,990 Public Shares are required to be voted in favor of the proposal for it to be approved. | |
| ● | The approval of the charter amendment proposals will require the affirmative vote of a majority of the issued and outstanding shares of each of the Class A common stock and Class B common stock, voting separately, as well as the vote of a majority of the issued and outstanding shares of Class A common stock and Class B common stock, voting together as a single class. |
|
| ● | The approval of each of the advisory charter amendments proposals will require the affirmative vote of the holders of a majority of the outstanding shares of MURF common stock on the record date. | |
| ● | The election of directors requires a plurality vote of the shares of MURF common stock present in person (by virtual attendance) or represented by proxy and entitled to vote at the Special Meeting. “Plurality” means that the individuals who receive the largest number of votes cast “FOR” are elected as directors. Consequently, any shares not voted “FOR” a particular nominee (whether as a result of an abstention, a direction to withhold authority or a broker non-vote) will not be counted in the nominee’s favor. | |
| ● | The approval of the incentive plan proposal will require the affirmative vote of the holders of a majority of the then outstanding shares of MURF common stock present and entitled to vote at the meeting. | |
| ● | The approval of the Nasdaq proposal will require the affirmative vote of the holders of a majority of the then outstanding shares of MURF common stock present and entitled to vote at the meeting. | |
| ● | The approval of the adjournment proposal will require the affirmative vote of the holders of a majority of the then outstanding shares of MURF common stock present and entitled to vote at the meeting. |
| 20 |
Abstentions and broker non-votes will have the same effect as a vote “against” the business combination proposal, the charter amendment proposals, and the advisory charter amendments proposals. With respect to the incentive plan proposal and adjournment proposal, if presented, abstentions will have the same effect as a vote “against” such proposals while broker non-votes will have no effect on such proposals. With respect to the director election proposal, abstentions and broker non-votes will have no effect on such proposal.
Consummation of the Transactions is conditioned on approval of each of the business combination proposal, the charter amendment proposals and director election proposal. If any proposal is not approved, the other proposals will not be presented to the stockholders for a vote.
Redemption Rights
Pursuant to MURF’s amended and restated certificate of incorporation, a holder of Public Shares may demand that MURF redeem such shares for cash if the Business Combination is consummated. Holders of Public Shares will be entitled to receive cash for these shares only if they demand that MURF redeem their shares for cash no later than 5:00pm Eastern Time on September 5, 2023, by electronically delivering their shares and a written request to redeem such shares to MURF’s transfer agent. MURF’S transfer agent must receive instruction to redeem by 5:00 pm Eastern Time on September 5, 2023, in order for such redemption demands to be valid. If the Business Combination is not completed, these shares will not be redeemed for cash. If a holder of Public Shares properly demands redemption, MURF will redeem each Public Share into a pro rata portion of the trust account, calculated as of two business days prior to the anticipated consummation of the Business Combination. As August 2, 2023, the record date, this would amount to approximately $10.67 per Public Share. If a holder of Public Shares exercises its redemption rights, then it will be exchanging its shares of MURF common stock for cash and will no longer own the shares. See the section entitled “Special Meeting of MURF Stockholders — Redemption Rights” for a detailed description of the procedures to be followed if you wish to redeem your shares for cash.
As a result of the pre-Business Combination redemption of 11,037,272 shares of MURF Class A common stock, the redemption payment of approximately $114 million reduced the balance of the trust account to approximately $23 million. In connection with this Special Meeting, MURF’s public stockholders have another opportunity to redeem Public Shares. The balance of the trust account will be the following amounts in the event of these redemption scenarios (after withholding funds for tax obligations): (i) no redemptions will maintain the current balance of approximately $23.3 million in the trust account; (ii) 50% redemptions will result in approximately $11.7 million in the trust account; and (iii) 100% redemptions will result in $0 in the trust account.
The Business Combination will not be consummated if MURF has net tangible assets of less than $5,000,001 after taking into account holders of Public Shares that have properly demanded redemption of their shares into cash or is not otherwise exempt from the provisions of Rule 419 promulgated under the Securities Act. Further, the Merger Agreement provides that Conduit is not required to consummate the Transactions if immediately prior to the consummation of the Transactions, MURF does not have at least $27 million of cash available to be released from the trust account and/or received by MURF under the Subscription Agreement after giving effect to payment of amounts that MURF will be required to pay to redeeming stockholders upon consummation of the Transactions. If Conduit does not waive its termination right and MURF has less than the required amount in trust and/or from the Subscription Agreement, the Transactions will not be consummated.
Holders of MURF warrants will not have redemption rights with respect to such securities.
Appraisal Rights
MURF stockholders and MURF warrant holders do not have appraisal rights in connection with the Transactions under the DGCL.
Proxy Solicitation
Proxies may be solicited by mail, telephone or in person. MURF has engaged D.F. King & Co., Inc. to assist in the solicitation of proxies. If a stockholder grants a proxy, it may still vote its shares at the Special Meeting if it revokes its proxy before the Special Meeting. A stockholder may also change its vote by submitting a later-dated proxy as described in the section entitled “Special Meeting of MURF Stockholders — Revoking Your Proxy.”
Interests of MURF’s Directors and Officers in the Business Combination
When you consider the recommendation of the MURF Board in favor of approval of the business combination proposal, you should keep in mind that MURF’s Sponsor and its directors and executive officers have interests in such proposal that are different from, or in addition to, your interests as a stockholder or warrant holder. These interests include, among other things:
| ● | If the Business Combination with Conduit or another business combination is not consummated by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), then MURF will (i) cease all operations except for the purpose of winding up, (ii) redeem the Public Shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account including interest earned on the funds held in the trust account and not previously released to MURF to pay MURF’s franchise and income taxes (less up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding Public Shares, which redemption will completely extinguish Public Stockholders’ rights as stockholders (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) subject to the approval of our remaining stockholders and our board of directors, dissolve and liquidate, subject in each case to our obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. | |
| ● | The Sponsor (including its representatives and affiliates) and MURF’s directors and officers, are, or may in the future become, affiliated with entities that are engaged in a similar business to MURF’s and the Sponsor and MURF’s directors and officers are not prohibited from sponsoring, or otherwise becoming involved with, any other blank check companies prior to MURF completing its initial business combination, and as result of which, the Sponsor and MURF’s officers and directors may become aware of business opportunities which may be appropriate for presentation to MURF, and the other entities to which they owe fiduciary or contractual duties, and may have conflicts of interests in determining to which entity a particular business opportunity should be presented (and these conflicts may include presentation to other entities prior to their presentation, if at all, to MURF, and may not always be resolved in the favor of MURF, subject to applicable fiduciary duties under Delaware law, in that MURF has provided in its amended and restated certificate of incorporation that MURF has renounced its interest in any corporate opportunity presented to MURF). | |
| ● | The Sponsor and the directors and officers of MURF have each waived their redemption rights with respect to any Founder Shares and Class A common stock held by them (other than relating to liquidating distributions to public shares from the trust account if MURF fails to complete its initial business combination by the time required prior to MURF’s liquidation in accordance with its amended and restated certificate of incorporation), which waiver was provided in connection with the initial public offering and without any separate consideration paid in connection with providing such waiver. |
| 21 |
| ● | Chele Chiavacci Farley, a current director of MURF, will be a director of New Conduit. | |
| ● | The Merger Agreement contemplates that Adam Sragovicz, MURF’s current Chief Financial Officer and a director, will be the Chief Financial Officer of New Conduit after the closing of the Business Combination. Pursuant to the terms of an employment agreement to be entered into between New Conduit and Mr. Sragovicz in connection with the closing of the Business Combination, Mr. Sragovicz is entitled to compensation consisting of (i) an annual base salary of $400,000, and (ii) a target annual bonus opportunity equal to 40% of his base salary, payable based on the achievement of performance objectives as determined by the New Conduit board of directors. In addition, the employment agreement provides that he will be entitled to receive a sign-on restricted stock unit award covering 0.10% of the shares of common stock of New Conduit pursuant to the terms of the 2023 Stock Incentive Plan, which shall vest in equal annual installments on the first three anniversaries of the Business Combination. In the future, Mr. Sragovicz could receive any additional cash compensation, stock options, stock awards or other benefits that New Conduit’s board of directors determines to pay to Mr. Sragovicz. | |
| ● | Mr. Heilbron, the Chief Executive Officer and Chairman of MURF is also
the President of NetREIT Advisors LLC, which is sole and managing member of the Sponsor. NetReit Advisors is wholly-owned by Presidio.
Mr. Heilbron is the President, Chief Executive Officer and Chairman of Presidio, of which he beneficially owns 2.6% (as of March
31, 2023). In 2023, Presidio’s compensation committee of its board of directors awarded Mr. Heilbron a cash bonus equal to
1% of the value of the New Conduit common stock owned by Presidio, to be valued on the day which is six months and one day after
the closing of the Business Combination.
Mr. Heilbron has entered into a consulting agreement with MURF, which is effective upon the closing of the Business Combination. The consulting agreement provides that Mr. Heilbron will provide advisory and consulting services from time to time to New Conduit for one year following the closing of the Business Combination. Mr. Heilbron will no longer serve as an officer or director of New Conduit after the closing of the Business Combination. However, pursuant to the terms of the consulting agreement, Mr. Heilbron is entitled to rights as an observer to the Board of Directors of New Conduit. Mr. Heilbron is entitled to be paid $25,000 per calendar quarter for his consulting services and is also entitled to a stock option to purchase the number of shares of the common stock of New Conduit determined by dividing (i) $300,000, by (ii) the per share Black-Scholes valuation as of the grant date, utilizing the same assumptions used in preparation of the financial statements, with the resulting quotient rounded down to the nearest whole share. | |
| ● | The Sponsor paid an aggregate of approximately $25,000 for the Founder Shares and the market value of such securities as of July 31, 2023, was approximately $35,641,000, and such securities should have a significantly higher value than $25,000 at the time of the Business Combination, and if MURF does not complete an initial business combination will expire worthless. | |
| ● | The Sponsor paid $7,540,000 for the 745,000 private placement units (consisting of 745,000 shares of Class A common stock and the Private Warrants) in connection with MURF’s initial public offering, at a price of $10.00 per unit, the market value of such securities as of July 31, 2023, was approximately $15,286,000, and such securities should have a higher value at the time of the Business Combination than $7,540,000. If MURF does not complete an initial business combination by February 7, 2024, then the Private Warrants received as part of the private placement units will expire worthless. | |
| ● | The Sponsor and its affiliates’ total potential ownership interest in New Conduit, as a result of 11,037,272 shares of MURF Class A common stock having already been redeemed (or approximately 83% of the issued and outstanding Class A common stock subject to possible redemption as of December 31, 2022) and assuming the exercise and conversion of all securities following the closing of the Business Combination, including the Private Shares, is estimated to comprise approximately 5.1% of the outstanding New Conduit common stock in a no redemption scenario (or a transaction value of approximately $34,021,000 or a market value of approximately $51,900,000), approximately 5.2% of outstanding New Conduit common stock in a 50% redemption scenario (or a transaction value of approximately $34,430,000 or a market value of approximately $51,900,000), and 5.3% of outstanding New Conduit common stock in a 100% maximum redemption scenario (or a transaction value of approximately $34,850,000 or a market value of approximately $51,900,000). | |
| ● | Immediately following the closing of the Business Combination, the independent directors of the MURF Board will receive from the Sponsor an aggregate of 45,000 private placement units, consisting of, in aggregate, 45,000 shares of MURF Class A common stock and 45,000 Private Warrants, which, assuming conversion and exercise, will be 90,000 shares of New Conduit common stock of an aggregate value of approximately $970,000 (assuming that the future value of New Conduit common stock will be the same as the current value of MURF’s Class A common stock) (the “Independent Director Units”). | |
| ● | The Sponsor and its affiliates can earn a positive rate of return on their overall investment in MURF and Conduit after the Business Combination, even if other holders of MURF common stock experience a negative rate of return, due to having purchased the Founder Shares, as described above, for $25,000 or approximately $0.006 per share. | |
| ● | On March 7, 2023, MURF has issued to Sponsor an unsecured promissory note in the principal amount of $1,500,000, portions of which may be withdrawn by MURF from time to time (the “Promissory Note”). As of July 31, 2023, MURF has submitted drawdown requests in an aggregate amount of $750,000. The Promissory Note bears no interest and is due and payable upon the earlier of the consummation of the initial business combination or the date of the liquidation of MURF. | |
| ● | The aggregate dollar amount that the Sponsor and its affiliates have at risk that depends on the completion of an initial business combination, including the Business Combination, is approximately $8,315,000, as of July 31, 2023, which is the current value of securities held (assuming that the current value of each of the securities held, whether publicly traded shares of MURF Class A common stock or Private Warrants, are in each case valued at the current price of MURF’s Class A common stock) and consists of (i) the Founders Shares and (ii) the 754,000 private placement units (each such unit comprised of one share of MURF’s Class A common stock and one warrant to purchase one share of MURF’s Class A common stock) purchased in connection with MURF’s initial public offering, and the Promissory Note. There are no fees due or out of pocket expenses for which Sponsor and its affiliates are awaiting reimbursement. |
| 22 |
| ● | The aggregate dollar amount that the directors of MURF have at risk that depends on the completion of an initial business combination, including the Business Combination, is approximately $970,000, as of July 31, 2023, which is the current value of the Independent Director Units. There are no loans, fees due or out of pocket expenses for which directors are awaiting reimbursement. | |
| ● | As a result of the foregoing, the Sponsor, and directors and officers of MURF, including Mr. Heilbron, will benefit from the completion of an initial business combination, including the Business Combination, and may be incentivized to complete an acquisition or business combination of a less favorable target company or on terms less favorable to stockholders of MURF rather than liquidate. | |
| ● | If MURF is unable to complete a business combination within the required time period, its executive officers will be personally liable under certain circumstances described herein to ensure that the proceeds in the trust account are not reduced by the claims of target businesses or claims of vendors or other entities that are owed money by MURF for services rendered or contracted for or products sold to MURF. If MURF consummates a business combination, on the other hand, MURF will be liable for all such claims. | |
| ● | MURF’s Sponsor, including its officers and directors, and their affiliates are entitled to reimbursement of out-of-pocket expenses incurred by them in connection with certain activities on MURF’s behalf, such as identifying and investigating possible business targets and business combinations. However, if MURF fails to consummate a business combination within the required period, they will not have any claim against the trust account for reimbursement. Accordingly, MURF may not be able to reimburse these expenses if the Business Combination with Conduit, or another business combination, is not completed by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation). | |
| ● | The continued indemnification of current directors and officers and the continuation of directors and officers’ liability insurance. | |
| ● | Chele Chiavacci Farley, a current director of the MURF Board, will be appointed to the Board of Directors of New Conduit upon the closing of the Business Combination. |
At any time prior to the Special Meeting, during a period when they are not then aware of any material nonpublic information regarding MURF or its securities, the Sponsor, Conduit or Conduit’s shareholders and/or their respective affiliates may purchase MURF common stock from institutional and other investors who vote, or indicate an intention to vote, against the business combination proposal, or execute agreements to purchase shares from such investors in the future, or they may enter into transactions with such investors and others to provide them with incentives to acquire shares of MURF common stock or vote their shares in favor of the business combination proposal. The purpose of such share purchases and other transactions would be to increase the likelihood of satisfaction of the requirements that the holders of a majority of MURF common stock entitled to vote at the Special Meeting to approve the business combination proposal vote in its favor and that MURF has sufficient proxies to approve the proposals set forth herein, where it appears that such requirements would otherwise not be met, or that aggregate cash proceeds available to MURF from the Private Placement and the funds remaining in the Trust Account after redemptions will not be less than $27,000,000. While the exact nature of any such incentives has not been determined as of the date of this proxy statement/prospectus, they might include, without limitation, arrangements to protect such investors or holders against potential loss in value of their shares, including the granting of put options and the transfer to such investors or holders of shares or warrants owned by the Sponsor for nominal value.
| 23 |
As of the date of this proxy statement/prospectus, with respect to the private purchase of Public Shares by the persons described above, no agreements have been entered into with any such investor or holder. In the event of any such newly purchased shares (i) the Sponsor or its affiliates will purchase the MURF common stock at a price no higher than the price offered through the redemption process; (ii) any such purchases by Sponsor or its affiliates will not be voted in favor of approving the Business Combination; and (iii) the Sponsor and its affiliates have waived their redemption rights to such shares.
Prior to the Special Meeting to approve the Business Combination, MURF will disclose in a Form 8-K (i) the amount of Public Shares purchased outside of the redemption offer by the Sponsor or its affiliates, along with the purchase price; (ii) the purpose of the purchases by the Sponsor or its affiliates; (iii) the impact, if any, of the purchases by the Sponsor or its affiliates on the likelihood that the Business Combination transaction will be approved; (iv) the identities of stockholders who sold to the Sponsor or its affiliates (if not purchased on the open market) or the nature of stockholders (e.g., 5% security holders) who sold to the Sponsor or its affiliates; and (v) the number of shares of MURF common stock for which MURF has received redemption requests pursuant to its redemption offer.
Recommendation to Stockholders
The MURF Board believes that the business combination proposal and the other proposals to be presented at the Special Meeting are fair to and in the best interest of MURF’s stockholders and unanimously recommends that its stockholders vote “FOR” the business combination proposal, “FOR” each of the charter amendment proposals, “FOR” each of the advisor charter amendments proposals, “FOR” the director election proposal, “FOR” the incentive plan proposal, “FOR” the Nasdaq proposal and “FOR” the adjournment proposal, if presented.
Conditions to the Closing of the Business Combination
General Conditions
The consummation of the Merger is conditioned upon, among other things, (i) the absence of any applicable law or order that makes the transactions contemplated by the Merger Agreement illegal or otherwise prohibits consummation of such transactions; (ii) the registration statement becoming effective under the Securities Act; (iii) the approval by MURF’s stockholders of the Business Combination and related transactions; (iv) the approval by Conduit’s shareholders of the Business Combination and related transactions; (v) the aggregate cash available to MURF at the Closing (after giving effect to any redemptions by MURF’s stockholders and the payment of all authorized transaction expenses) being at least $27,000,000; (vi) after giving effect to the Business Combination, MURF shall have at least $5,000,001 in net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act) or shall not otherwise be exempt from the provisions of Rule 419 promulgated under the Securities Act; (vii) all ancillary agreements having been executed by all parties thereto; (viii) MURF and Conduit having cooperated in good faith to negotiate and execute new employment agreements with David Tapolczay and Adam Sragovicz, the current Chief Financial Officer and a director of MURF; and (ix) all required filings under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, and any other governmental authority having been completed and cleared. For more information, please see the section entitled “The Business Combination Proposal — The Merger Agreement — Conditions to the Closing of the Business Combination.”
Conduit’s Conditions to the Closing of the Business Combination
The obligations of Conduit to consummate the Business Combination are also conditioned upon, among other things: (i) MURF and Merger Sub having duly performed or complied with all of its obligations under the Merger Agreement in all material respects; (ii) the representations and warranties of MURF and Merger Sub being true and correct in all material respects; (iii) no event having occurred that would result in a Murphy Material Adverse Effect (as defined in the Merger Agreement); (iv) MURF providing Conduit a certificate from an authorized officer of MURF as to the accuracy of the foregoing conditions; and (v) MURF’s establishment of an equity incentive plan to be in place following the closing of the Business Combination. For more information, please see the section entitled “The Business Combination Proposal — The Merger Agreement — Conditions to the Closing of the Business Combination.”
MURF’s and Merger Sub’s Conditions to the Closing of the Business Combination
The obligations of MURF and Merger Sub to consummate the Transactions are also conditioned upon, among other things, (i) Conduit having duly performed or complied with all of their obligations under the Merger Agreement in all material respects; (ii) the representations and warranties of MURF and Merger Sub being true and correct in all material respects; (iii) no event having occurred that would result in a Company Material Adverse Effect (as defined in the Merger Agreement); (iv) Conduit providing MURF a certificate from an authorized officer as to the accuracy of the foregoing conditions; and (v) Conduit UK Management Ltd. Will be transferred to Conduit and become a wholly-owned subsidiary of Conduit, and certain intellectual property having been assigned to Conduit UK Management Ltd. For more information, please see the section entitled “The Merger Agreement — Conditions to the Closing of the Business Combination.”
Waiver
If permitted under applicable law, MURF or Conduit may waive any inaccuracies in the representations and warranties made to such party and contained in the Merger Agreement and waive compliance with any agreements or conditions for the benefit of such party contained in the Merger Agreement. The parties may also waive the condition to list with Nasdaq as of the closing date, that the minimum proceeds requirement of $27,000,000 and the related condition concerning the Private Placement. However, pursuant to MURF’s existing amended and restated certificate of incorporation, the condition requiring that MURF have at least $5,000,001 of net tangible assets or otherwise be exempt from the provisions of Rule 419 promulgated under the Securities Act may not be waived.
| 24 |
Termination
The Merger Agreement may be terminated as follows: (i) by the mutual consent of MURF and Conduit; (ii) by MURF, if any of the representations or warranties of Conduit set forth in the Merger Agreement is not true and correct, or if Conduit has failed to perform any covenant or agreement set forth in the Merger Agreement (including an obligation to consummate the Business Combination), in each case such that the conditions to closing would not be satisfied and the breach or breaches causing such representations or warranties not to be true and correct, or the failure to perform any covenant or agreement, as applicable, are not cured (or waived by MURF) by the earlier of (a) the Outside Date (as defined below) or (b) 20 days after written notice thereof is delivered to Conduit; provided, however that MURF is not then in material breach of any representation, warranty, covenant, or obligation in the Merger Agreement, which breach has not been cured; (iii) by Conduit, if any of the representations or warranties of MURF or Merger Sub set forth in the Merger Agreement is not true and correct, or if MURF or Merger Sub has failed to perform any covenant or agreement set forth in the Merger Agreement (including an obligation to consummate the Business Combination), in each case such that the conditions to closing would not be satisfied and the breach or breaches causing such representations or warranties not to be true and correct, or the failure to perform any covenant or agreement, as applicable, are not cured (or waived by Conduit) by the earlier of (a) the Outside Date or (b) 20 business days after written notice thereof is delivered to MURF; provided, however that Conduit is not then in material breach of any representation, warranty, covenant, or obligation in the Merger Agreement, which breach has not been cured; (iv) by either MURF or Conduit: (a) on or after February 7, 2024 (the “Outside Date”), if the Business Combination has not been consummated prior to the Outside Date; provided, however, that this right to terminate the Merger Agreement will not be available to a party if the failure of the merger to have been consummated before the Outside Date was due to such party’s breach of or failure to perform any of its covenants or agreements set forth in the Merger Agreement, (b) if any applicable law or order that makes the transactions contemplated by the Merger Agreement illegal or otherwise prohibits consummation of such transactions becomes final and non-appealable, or (C) if MURF has not received approval from its stockholders of the Business Combination and related transactions at the Special Meeting; (v) by MURF if the Conduit shareholders’ written consent approving the Business Combination and related transactions have not have been obtained within 10 business days after the date of the Merger Agreement; and (vi) if there is a material adverse effect on behalf of the other party. See the section entitled “The Merger Agreement — Termination.”
Shareholder Support Agreements
Concurrently with the execution of the Merger Agreement, the Supporting Conduit Shareholders, who are the three largest shareholders of Conduit, have entered into support agreements with MURF and Conduit pursuant to which each Supporting Conduit Shareholder has agreed, among other things, to approve or vote in favor of the Business Combination, against any action or proposal involving MURF that is intended to, or would reasonably be expected to, prevent, impede or adversely affect the Transactions in any material respect, and promptly execute the definitive documents, agreements and filings (including with applicable governmental authorities) related to the Business Combination reasonably required to be executed by such Supporting Conduit Shareholder in furtherance of the Business Combination subject to the terms and conditions set forth therein. Under the support agreement, each Supporting Conduit Shareholder has also agreed that, with limited exceptions, prior to the termination of the applicable support agreement, such Supporting Conduit Shareholder will not transfer or otherwise enter into any agreement or understanding with respect to a transfer relating to any Claims (as defined in the applicable support agreement) owned by such Supporting Conduit Shareholder. The support agreements will terminate on the earlier of the closing of the Business Combination or the termination of the Merger Agreement. No such termination will relieve any shareholders, MURF or Conduit from any liability resulting from a breach of the support agreement occurring prior to such termination.
Tax Consequences of the Business Combination
For a description of the material U.S. federal income tax consequences of the Business Combination to holders of Conduit’s shares, please see the information set forth in the section entitled “Material U.S. Federal Income Tax Considerations — Material Tax Considerations of the Business Combination to Holders of Conduit Capital Stock.”
For a description of the material U.S. federal income tax consequences of the exercise of redemption rights, please see the information set forth in the section entitled “Material U.S. Federal Income Tax Considerations — Material Tax Considerations Related to a Redemption of Public Shares.”
Anticipated Accounting Treatment
The Business Combination will be accounted for as a reverse recapitalization in accordance with GAAP. Under this method of accounting, MURF has been treated as the “acquired” company for financial reporting purposes. Conduit was determined to be the accounting acquirer primarily because Conduit stakeholders will collectively own a majority of the outstanding shares of the combined company as of the closing of the merger, they have nominated four of the seven (7) members of the board of directors as of the closing of the merger, and Conduit’s Chief Executive Officer will continue to manage the combined company. Additionally, Conduit’s business will comprise the ongoing operations of the combined company immediately following the consummation of the Business Combination. Accordingly, for accounting purposes, the financial statements of the combined entity will represent a continuation of the financial statements of Conduit with the acquisition being treated as the equivalent of Conduit issuing stock for the net assets of MURF, accompanied by a recapitalization. The net assets of MURF will be stated at historical cost, with no goodwill or other intangible assets recorded.
Regulatory Matters
The Business Combination is not subject to any additional federal or state regulatory requirement or approval, except for the filings with the State of Delaware necessary to effectuate the Business Combination and the filing of required notifications and the expiration or termination of the required waiting periods under the HSR Act. MURF and Conduit have made the appropriate filings pursuant to the HSR Act with the DOJ and FTC. The waiting period under the HSR Act expires on December 8, 2022.
| 25 |
Risk Factor Summary
In evaluating the proposals to be presented at the Special Meeting, a stockholder should carefully read this proxy statement/prospectus and especially consider the factors discussed in the section entitled “Risk Factors.” These risks include:
| ● | Conduit’s business is dependent on the successful development, regulatory approval and commercialization of our clinical assets, in particular a glucokinase activator which we believe is active in a range of autoimmune diseases including uveitis, Hashimoto’s Thyroiditis, preterm labor and renal transplant, which we refer to as AZD1656, and a potent, irreversible inhibitor of human myeloperoxidase that has the potential to treat idiopathic male infertility, which we refer to as AZD5904. | |
| ● | Preclinical drug development for Conduit’s clinical assets AZD1656 and AZD5904 is very expensive, time-consuming and uncertain. Conduit’s preclinical trials may fail to adequately demonstrate pharmacologic activity in therapeutic areas of interest; cause unintended short- or long-term effects in other bodily systems; or produce unexpected toxicity that may alter or risk benefit assessment. | |
| ● | It is difficult to predict the time and cost of development and of subsequently obtaining regulatory approval for AZD1656 as it employs newly developed technology. | |
| ● | Conduit may not be successful in its efforts to use and expand its development platform to build a pipeline of clinical assets. | |
| ● | Clinical drug development for Conduit’s clinical assets is very expensive, time-consuming, difficult to design and implement, and uncertain. Conduit’s clinical trials may fail to adequately demonstrate the safety and efficacy of its clinical assets, which could prevent or delay regulatory approval and commercialization. | |
| ● | Conduit may be unable to obtain regulatory approval for its early-stage clinical assets under applicable regulatory requirements. The FDA and foreign regulatory bodies have substantial discretion in the approval process, including the ability to delay, limit or deny approval of clinical assets. The delay, limitation or denial of any regulatory approval would adversely impact commercialization, Conduit’s potential to generate revenue, Conduit’s business and its operating results. | |
| ● | We may face product liability exposure, and if successful claims are brought against us, we may incur substantial liability if our insurance coverage for those claims is inadequate. | |
| ● | Conduit currently relies on, and expects to continue to rely on, third-party CROs and other third parties to conduct and oversee Conduit’s clinical trials and other aspects of product development. If these third parties do not meet Conduit’s requirements or otherwise conduct the trials as required, Conduit may not be able to satisfy its contractual obligations or obtain regulatory approval for, or commercialize, its clinical assets when expected or at all. | |
| ● | Conduit currently relies on agreements with third parties for the purpose of licensing its clinical assets. In the near-term Conduit intends to rely on third parties for the licensing of clinical assets and those which may arise through future partnerships. | |
| ● | If Conduit fails to attract and retain management and other key personnel, it may be unable to continue to successfully develop or commercialize its clinical assets or otherwise implement its business plan. | |
| ● | Manufacturing and supply of the APIs and other substances and materials used in Conduit’s clinical assets is a complex and technically challenging undertaking, and there is potential for failure at many points in the manufacturing, testing, quality assurance and distribution supply chain, as well as the potential for latent defects after products have been manufactured and distributed. | |
| ● | Failure to adequately protect Conduit’s intellectual property could adversely affect Conduit’s business, financial condition, and operating results. | |
| ● | Conduit may not be able to protect its intellectual property rights throughout the world. | |
| ● | Following the consummation of the Business Combination, our only significant asset will be ownership of 100% of Conduit’s capital shares, and we do not currently intend to pay dividends on our common stock and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock. | |
| ● | The Sponsor owns shares of MURF common stock and warrants that will be worthless and has made loans that may not be reimbursed or repaid if the Business Combination is not approved. Such interests may have influenced its decision to approve the Business Combination with Conduit. | |
| ● | Adam Sragovicz, MURF’s current Chief Financial Officer and director, will be the Chief Financial Officer of New Conduit following the Business Combination and will be entitled to compensation in connection with such employment. Such personal and financial interests may have influenced his decision to approve the Business Combination with Conduit. | |
| ● | The exercise of MURF’s directors’ and officers’ discretion in agreeing to changes or waivers in the terms of the Business Combination may result in a conflict of interest when determining whether such changes to the terms of the Business Combination or waivers of conditions are appropriate and in MURF’s stockholders’ best interest. | |
| ● | If the Business Combination does not qualify as a tax-free reorganization, U.S. holders of Conduit shares will be required to recognize gain or loss for U.S. federal income tax purposes upon the exchange of their Conduit shares for the New Conduit common stock in the Business Combination which could result in such U.S. holder incurring substantially greater U.S. federal income tax liability as a result of the Business Combination. |
| 26 |
FORWARD-LOOKING STATEMENTS
MURF believes that some of the information in this proxy statement/prospectus constitutes “forward-looking statements” for purposes of the federal securities laws. Forward-looking statements include, but are not limited to, statements regarding MURF’s, MURF management team’s, Conduit’s and Conduit management team’s expectations, hopes, beliefs, intentions or strategies regarding the future. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,” “contemplate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. Forward-looking statements in this proxy statement/prospectus may include, for example, statements that:
| ● | discuss future expectations; | |
| ● | contain projections of future results of operations or financial condition; or | |
| ● | state other “forward-looking” information. |
MURF believes it is important to communicate its expectations to its security holders. However, there may be events in the future that MURF is not able to predict accurately or over which it has no control. The risk factors and cautionary language discussed in this proxy statement/prospectus provide examples of risks, uncertainties and events that may cause actual results to differ materially from the expectations described by MURF or Conduit in such forward-looking statements, including among other things:
| ● | the timing to complete the Transactions; | |
| ● | the number and percentage of its Public Stockholders voting against the business combination proposal and/or seeking redemption; | |
| ● | the occurrence of any event, change or other circumstances that could give rise to the termination of the Merger Agreement; | |
| ● | the anticipated costs associated with the proposed Business Combination; | |
| ● | the outcome of any known and unknown litigation and regulatory proceedings, including the occurrence of any event, change or other circumstances, including the outcome of any legal proceedings that may be instituted against MURF and Conduit that could give rise to the termination of the Merger Agreement; | |
| ● | the ability to maintain the listing of MURF’s securities on a national securities exchange following the Business Combination; | |
| ● | the inability to recognize the anticipated benefits of the proposed business combination, which may be affected by, among other things, the amount of cash available following any redemption of Public Shares by MURF stockholders; | |
| ● | changes adversely affecting the business in which Conduit is engaged; | |
| ● | Conduit’s ability to execute on its plans to develop and commercialize its current clinical assets, as well as any future clinical assets that it licenses, and the timing of any such commercialization; | |
| ● | Conduit’s reliance on the license agreement between AstraZeneca and St George Street; | |
| ● | Conduit’s ability to identify future clinical assets to develop and obtain licenses to such clinical assets; | |
| ● | the projected financial information, growth rate, strategies, and market opportunities for Conduit; | |
| ● | Conduit’s ability to meet its future capital requirements to fund its operations, which may involve debt and/or equity financing, and to obtain such debt and/or equity financing on favorable terms, and its sources and uses of cash; | |
| ● | the success of Conduit’s research and development strategies; | |
| ● | Conduit’s ability, assessment of and strategies to compete with its competitors; | |
| ● | Conduit’s reliance on third-party service providers; | |
| ● | Conduit’s ability to maintain and protect its intellectual property; | |
| ● | Conduit’s ability to attract and retain talent and the effectiveness of its compensation strategies and leadership; | |
| ● | the potential business or economic disruptions caused by current and future pandemics, such as the COVID-19 pandemic; |
| 27 |
| ● | Conduit’s ability to prevent and guard against cybersecurity attacks; | |
| ● | changes in applicable laws or regulations affecting Conduit and/or its business; | |
| ● | any business disruptions due to political or economic instability, pandemics, or armed hostilities (including the ongoing conflict between Russia and Ukraine); | |
| ● | geopolitical risk and general economic conditions; | |
| ● | the potential impact of climate change on Conduit, including higher regulatory and compliance costs, reputational risks, and availability of capital on attractive terms; and | |
| ● | other factors detailed under the section entitled “Risk Factors.” |
You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this proxy statement/prospectus.
All forward-looking statements included herein attributable to any of MURF, Conduit or any person acting on either party’s behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section. Except to the extent required by applicable laws and regulations, MURF and Conduit undertake no obligations to update these forward-looking statements to reflect events or circumstances after the date of this proxy statement/prospectus or to reflect the occurrence of unanticipated events.
Before a stockholder grants its proxy or instructs how its vote should be cast or vote on the business combination proposal, charter amendment proposals, advisory charter amendments proposals, director election proposal, the incentive plan proposal, the Nasdaq proposal or the adjournment proposal, it should be aware that the occurrence of the events described in the “Risk Factors” section and elsewhere in this proxy statement/prospectus may adversely affect MURF and/or Conduit.
| 28 |
RISK FACTORS
Stockholders should carefully consider the following risk factors, together with all other information in this proxy statement/prospectus, before deciding whether to vote or instruct that their vote be cast to approve the proposals described in this proxy statement/prospectus.
Risks Related to Conduit’s Business and Industry
Conduit’s business is dependent on the successful development, regulatory approval and commercialization of our clinical assets, in particular a glucokinase activator which we believe is active in a range of autoimmune diseases including uveitis, Hashimoto’s Thyroiditis, preterm labor and renal transplant, which we refer to as AZD1656, and a potent, irreversible inhibitor of human Myeloperoxidase that has the potential to treat idiopathic male infertility, which we refer to as AZD5904.
The success of Conduit’s business, including its ability to finance its operations and generate any revenue in the future, will primarily depend on the successful development, regulatory approval and commercialization or partnering of Conduit’s clinical assets. In the future, Conduit may also become dependent on just one of its clinical assets or any future clinical assets that Conduit may in-license, acquire or develop. The preclinical and clinical and commercial success of Conduit’s clinical assets will depend on a number of factors, including the following:
| ● | the ability to raise additional capital on acceptable terms, or at all; | |
| ● | the timely completion of Conduit’s clinical trials, which may be significantly slower or cost more than Conduit currently anticipates and will depend substantially upon the performance of third-party contractors; | |
| ● | whether Conduit is required by the United States Food and Drug Administration (“FDA”) or similar foreign regulatory agencies to conduct additional preclinical or clinical trials beyond those planned to support the approval and commercialization of Conduit’s clinical assets or any future clinical assets; | |
| ● | the acceptance of Conduit’s proposed indications and primary endpoint assessments relating to the proposed indications of Conduit’s clinical assets by the FDA or similar foreign regulatory authorities; | |
| ● | Conduit’s ability to demonstrate to the satisfaction of the FDA and similar foreign regulatory authorities, the safety and efficacy of Conduit’s clinical assets or any future clinical assets; | |
| ● | the prevalence, duration and severity of potential side effects experienced in connection with Conduit’s clinical assets or future approved products, if any; | |
| ● | the timely receipt of necessary marketing approvals from the FDA and similar foreign regulatory authorities; | |
| ● | achieving and maintaining, and, where applicable, ensuring that Conduit’s third-party contractors achieve and maintain, compliance with Conduit’s contractual obligations and with all regulatory requirements applicable to Conduit’s clinical assets or any future clinical assets or approved products, if any; | |
| ● | the ability of third parties with whom Conduit contracts to manufacture clinical trial and commercial supplies of Conduit’s clinical assets or any future clinical assets, remain in good standing with regulatory agencies and develop, validate and maintain commercially viable manufacturing processes that are compliant with current good manufacturing practices (“cGMP”); | |
| ● | a continued acceptable safety profile during preclinical and clinical development and following approval of Conduit’s clinical assets or any future clinical assets; | |
| ● | Conduit’s ability to successfully commercialize its clinical assets or any future clinical assets in the U.S. and internationally, if approved for marketing, sale and distribution in such countries and territories, whether alone or in collaboration with others; | |
| ● | the acceptance by physicians, patients and payors of the benefits, safety and efficacy of Conduit’s clinical assets or any future clinical assets, if approved, including relative to alternative and competing treatments; | |
| ● | Conduit’s ability to comply with numerous post-approval regulatory requirements; | |
| ● | Conduit’s and its partners’ ability to establish and enforce intellectual property rights in and to Conduit’s clinical assets or any future clinical assets; | |
| ● | Conduit’s and its partners’ ability to avoid third-party patent interference or intellectual property infringement claims; and | |
| ● | Conduit’s ability to in-license or acquire additional clinical assets or commercial-stage products that it believes that it can successfully develop and commercialize. |
| 29 |
If Conduit is unable to achieve one or more of the above factors, many of which are beyond our control, in a timely manner or at all, Conduit could experience significant delays and increased costs or an inability to obtain regulatory approvals or commercialize its clinical assets. Even if regulatory approvals are obtained, Conduit may never be able to successfully commercialize any of its clinical assets. Accordingly, Conduit cannot assure you that Conduit will be able to generate sufficient revenue through the sale of its clinical assets or any future clinical assets to continue operations.
Preclinical drug development for Conduit’s clinical assets AZD1656 and AZD5904 is very expensive, time-consuming and uncertain. Conduit’s preclinical trials may fail to adequately demonstrate pharmacologic activity in therapeutic areas of interest; cause unintended short- or long-term effects in other bodily systems; or produce unexpected toxicity that may alter or risk benefit assessment.
The scientific discoveries that form the basis for Conduit’s efforts to generate and develop its clinical assets are relatively recent. AZD1656 is a glucokinase activator that is in a number of Phase II ready autoimmune diseases including uveitis, Hashimoto’s Thyroiditis, preterm labor and renal transplant, and the successful development of AZD1656 may require additional studies and efforts to optimize its therapeutic potential. In addition, Conduit’s development pipeline includes what Conduit believes to be a potent irreversible inhibitor of human Myeloperoxidase (MPO) that has the potential to treat idiopathic male infertility, which we refer to as AZD5904. AZD5904 may not demonstrate in patients the therapeutic properties ascribed to it in the laboratory or preclinical studies, and may interact with human biological systems in unforeseen, ineffective or even harmful ways. If Conduit is not able to successfully develop and commercialize its clinical assets, including AZD1656 and AZD5904, it may never become profitable and the value of its capital stock may decline.
It is difficult to predict the time and cost of development and of subsequently obtaining regulatory approval for AZD1656 as it employs newly developed technology.
AZD1656 uses a novel mechanism to reduce inflammation in many of the immune pathways. Conduit has concentrated its research and development efforts of AZD1656 on a limited number of initial targeted disease indications for AZD1656, including the coronavirus disease. There can be no assurance that Conduit will not experience problems or delays in developing its current or future indications for AZD1656 and that such problems or delays will not cause unanticipated costs, or that any such development problems can be solved. Moreover, AZD1656 would also represent a novel approach for the treatment of uveitis as steroids are currently the most common treatment for uveitis even though there are numerous side effects associated with the use of steroids. The clinical development of these novel technologies will require review and allowance by the FDA under an Investigational New Drug Application.
Conduit may not be successful in its efforts to use and expand its development platform to build a pipeline of clinical assets.
A key element of Conduit’s strategy is to use its experienced management and scientific team to build a pipeline of clinical assets that address a broad range of human diseases in order to treat unmet medical needs. Conduit’s current clinical assets and pipeline address the areas of autoimmune disease and idiopathic male infertility. Although Conduit’s research and development efforts to date have resulted in potential clinical assets, Conduit may not be able to continue to identify and develop additional clinical assets. Even if Conduit is successful in continuing to build its pipeline, the potential clinical assets that Conduit identifies may not be suitable for clinical development. For example, these potential clinical assets may be shown to have harmful side effects or other characteristics that indicate that they are unlikely to receive marketing approval and achieve market acceptance. If Conduit does not successfully develop and commercialize clinical assets based upon its approach, Conduit will not be able to obtain product revenue in future periods, which likely would result in significant harm to its financial position. There is no assurance that Conduit will be successful in its preclinical and clinical development of its current or future clinical assets, and the process of obtaining regulatory approvals will, in any event, require the expenditure of substantial time and financial resources.
Clinical drug development for Conduit’s clinical assets is very expensive, time-consuming, difficult to design and implement, and uncertain. Conduit’s clinical trials may fail to adequately demonstrate the safety and efficacy of its clinical assets, which could prevent or delay regulatory approval and commercialization.
Clinical drug development for our clinical assets is very expensive, time-consuming, difficult to design and implement and its outcome is inherently uncertain. Before obtaining regulatory approval for the commercial sale of a clinical asset, Conduit must demonstrate through clinical trials that a clinical asset is both safe and effective for use in the target indication, which is impossible to predict. Most clinical assets that commence clinical trials are never approved by regulatory authorities for commercialization. Conduit’s clinical assets are in various stages of development and a failure of one more clinical trial can occur at any stage of testing or at any time during the trial process. Conduit expects that clinical trials for these clinical assets will continue for several years but may take significantly longer than expected to complete. Not all of Conduit’s clinical assets have been tested in humans and the first use in humans may reveal unexpected effects. Conduit has not completed all clinical trials for the approval of any of its clinical assets.
| 30 |
Conduit may experience delays in ongoing and future clinical trials for its clinical assets and Conduit does not know if future clinical trials, if any, will begin on time, need to be redesigned, enroll adequate number of patients on time or be completed on schedule, if at all. In addition, Conduit, any partner with which Conduit currently or may in the future collaborate, the FDA, an Institutional Review Board (or IRB) or other regulatory authorities, including state and local agencies and counterpart agencies in foreign countries, may suspend, delay, require modifications to or terminate Conduit’s clinical trials at any time, for various reasons, including:
| ● | discovery of safety or tolerability concerns, such as serious or unexpected toxicities or side effects or exposure to otherwise unacceptable health risks, experienced by study participants or other safety issues; | |
| ● | lack of effectiveness of any clinical asset during clinical trials or the failure of Conduit’s clinical assets to meet specified endpoints; | |
| ● | slower than expected rates of subject recruitment and enrollment rates or inability to enroll a sufficient number of patients in clinical trials resulting from numerous factors, including the prevalence of other companies’ clinical trials for their clinical assets for the same indication, or clinical trials for indications for which patients do not as commonly seek treatment; | |
| ● | delays or difficulties in Conduit’s clinical trials due to quarantines or other restrictions resulting from the COVID-19 pandemic or any other pandemic; | |
| ● | difficulty in retaining subjects who have initiated a clinical trial but may withdraw at any time due to adverse side effects from the therapy, insufficient efficacy, fatigue with the clinical trial process or for any other reason; | |
| ● | difficulty in obtaining IRB approval for studies to be conducted at each clinical trial site; | |
| ● | delays in manufacturing or obtaining, or inability to manufacture or obtain, sufficient quantities of materials for use in clinical trials; | |
| ● | inadequacy of or changes in Conduit’s manufacturing process or the product formulation or method of delivery; | |
| ● | changes in applicable laws, regulations and regulatory policies; | |
| ● | delays or failure in reaching agreement on acceptable terms in clinical trial contracts or protocols with prospective Contract Research Organizations, which we refer to as CROs, clinical trial sites and other third-party contractors; | |
| ● | inability to add a sufficient number of clinical trial sites; | |
| ● | uncertainty regarding proper formulation and dosing; | |
| ● | failure by Conduit, its employees, its CROs or their employees or other third-party contractors to comply with contractual and applicable regulatory requirements or to perform their services in a timely or acceptable manner; | |
| ● | failure by Conduit, its employees, its CROs or their employees or any partner with which Conduit may collaborate or their employees to comply with applicable FDA or other regulatory requirements relating to the conduct of clinical trials or the handling, storage, security and recordkeeping for drug and biologic products; | |
| ● | scheduling conflicts with participating clinicians and clinical institutions; | |
| ● | failure to design appropriate clinical trial protocols; | |
| ● | insufficient data to support regulatory approval; | |
| ● | inability or unwillingness of medical investigators to follow Conduit’s clinical trial protocols; or | |
| ● | difficulty in maintaining contact with subjects during or after treatment, which may result in incomplete data. |
Conduit or any partner with which Conduit may collaborate may suffer significant setbacks in their clinical trials similar to the experience of a number of other companies in the pharmaceutical and biotechnology industries, even after receiving promising results in earlier trials. In the event that Conduit or its potential partners abandon or are delayed in the clinical development efforts related to Conduit’s clinical assets, Conduit may not be able to execute on its business plan effectively and its business, financial condition, operating results and prospects would be harmed.
| 31 |
Conduit may be unable to obtain regulatory approval for its early-stage clinical assets under applicable regulatory requirements. The FDA and foreign regulatory bodies have substantial discretion in the approval process, including the ability to delay, limit or deny approval of clinical assets. The delay, limitation or denial of any regulatory approval would adversely impact commercialization, Conduit’s potential to generate revenue, Conduit’s business and its operating results.
Conduit currently has no products approved for sale, and Conduit may never obtain regulatory approval to commercialize any of its current or future clinical assets. The research, testing, manufacturing, safety surveillance, efficacy, quality control, recordkeeping, labeling, packaging, storage, approval, sale, marketing, distribution, import, export, and reporting of safety and other post-market information related to Conduit’s drug products are subject to extensive regulation by the FDA and other regulatory authorities in the U.S. and in foreign countries, and such regulations differ from country to country. Conduit is not permitted to market any of its current clinical assets in the U.S. until Conduit receives approval of a New Drug Application (“NDA”), Biologics License Application (a “BLA”), or other applicable regulatory filing from the FDA. Conduit is also not permitted to market any of its current clinical assets in any foreign countries until Conduit or its partners receive the requisite approval from the applicable regulatory authorities of such countries. To gain approval to market a new drug such as AZD1656 and AZD5904, the FDA and/or foreign regulatory authorities must receive, among other things, preclinical and clinical data that adequately demonstrate the safety, purity, potency, efficacy, and compliant manufacturing of the drug product for the intended indication applied for in a NDA, BLA or other applicable regulatory filing. The development and approval of new drug products involves a long, expensive and uncertain process, and delay or failure can occur at any stage. A number of companies in the pharmaceutical and biopharmaceutical industry have suffered significant setbacks in nonclinical development, clinical trials, including in Phase III clinical development, even after promising results in earlier preclinical studies or clinical trials. These setbacks have been caused by, among other things, findings made while clinical trials were underway and safety or efficacy observations made in clinical trials, including previously unreported adverse events. Success in clinical trials does not ensure that later clinical trials will be successful, or that nonclinical studies will be successful. The results of clinical trials by other parties may not be indicative of the results in trials that Conduit or its partners may conduct.
The FDA and foreign regulatory bodies have substantial discretion in the drug development and approval process, including the ability to delay, limit drug development or limit or deny approval of clinical assets for many reasons. The FDA or the applicable foreign regulatory body may:
| ● | disagree with the design or implementation of one or more clinical trials; | |
| ● | not deem a clinical asset safe and effective for its proposed indication, or may deem a clinical asset’s safety or other perceived risks to outweigh its clinical or other benefits; | |
| ● | not find the data from preclinical studies and clinical trials sufficient to support approval, or the results of clinical trials may not meet the level of statistical or clinical significance required by the FDA or the applicable foreign regulatory body for approval; | |
| ● | disagree with Conduit’s interpretation of data from preclinical studies or clinical trials performed by Conduit or third parties, or with the interpretation of any partner with which Conduit may collaborate; | |
| ● | determine the data collected from preclinical or clinical trials may not be sufficient to support the submission of an Investigational New Drug Application (“IND”) or NDA, or other applicable regulatory filing; | |
| ● | require additional preclinical studies or clinical trials; | |
| ● | identify deficiencies in the formulation, quality control, labeling or specifications of Conduit’s current or future clinical assets; | |
| ● | require clinical trials in pediatric patients in order to establish pharmacokinetics or safety for this more drug-sensitive population; | |
| ● | grant approval contingent on the performance of costly additional post-approval clinical trials; | |
| ● | approve Conduit’s current or any future clinical assets for a more limited indication or a narrower patient population than Conduit originally requested or with strong warnings that may affect marketability; | |
| ● | not approve the labeling that Conduit believe is necessary or desirable for the successful commercialization of Conduit’s clinical assets; | |
| ● | not approve of the manufacturing processes, controls or facilities of third-party manufacturers or testing labs with which Conduit contracts; | |
| ● | consider Conduit products a device instead of a drug requiring a different approval process and manufacturing needs; | |
| ● | consider one of Conduit’s products a combination product instead of a singular drug requiring additional clinical trials or increased number of patients per study; or | |
| ● | change its approval policies or adopt new regulations in a manner rendering Conduit’s clinical data or regulatory filings insufficient for approval. |
| 32 |
Any delay, limitation or denial in any applicable regulatory approval for any of Conduit’s clinical assets would delay or adversely impact commercialization of Conduit’s clinical assets and would harm Conduit’s business, financial condition, operating results and prospects.
The report of Conduit’s independent registered public accounting firm included a “going concern” explanatory paragraph.
The report of Conduit’s independent registered public accounting firm on Conduit’s financial statements as of and for the year ended December 31, 2022, included an explanatory paragraph indicating that there is substantial doubt about Conduit’s ability to continue as a going concern. Conduit has financed its working capital requirements to date by raising capital through private placements of its ordinary shares and issuing of short-term and convertible notes. If Conduit is unable to raise sufficient capital as and when needed, Conduit’s business, financial condition and results of operations will be materially and adversely affected, and Conduit will need to significantly modify its operational plans to continue as a going concern. If Conduit is unable to continue as a going concern, it may have to liquidate its assets, and the values Conduit may receive for its assets in liquidation or dissolution could be significantly lower than the values reflected in Conduit’s financial statements. The inclusion of a going concern explanatory paragraph by Conduit’s independent registered public accounting firm, Conduit’s lack of cash resources and Conduit’s potential inability to continue as a going concern may materially adversely affect the share price of New Conduit’s common stock following the Business Combination and New Conduit’s ability to raise new capital, enter into critical contractual relations with third parties and otherwise execute its development strategy following the Business Combination.
There is a risk that New Conduit will fail to maintain an effective system of internal controls and our ability to produce timely and accurate financial statements or comply with applicable regulations could be adversely affected. We may identify material weaknesses in our internal controls over financing reporting which we may not be able to remedy in a timely manner.
As a public company, New Conduit will operate in an increasingly demanding regulatory environment, which requires it to comply with the Sarbanes-Oxley Act, the regulations of Nasdaq, the rules and regulations of the SEC, expanded disclosure requirements, accelerated reporting requirements and more complex accounting rules. Responsibilities required by the Sarbanes-Oxley Act include establishing corporate oversight and adequate internal control over financial reporting and disclosure controls and procedures. Effective internal controls are necessary for us to produce reliable financial reports and are important to help prevent financial fraud. Prior to the closing of the Business Combination, we have never been required to test our internal controls within a specified period and, as a result, we may experience difficulty in meeting these reporting requirements in a timely manner.
We anticipate that the process of building our accounting and financial functions and infrastructure will require significant additional professional fees, internal costs and management efforts. We may need to enhance and/or implement a new internal system to combine and streamline the management of our financial, accounting, human resources and other functions. However, the enhancement and/or implementation of a system may result in substantial costs. Any disruptions or difficulties in implementing or using such a system could adversely affect our controls and harm our business. Moreover, such disruption or difficulties could result in unanticipated costs and diversion of management’s attention. In addition, we may discover additional weaknesses in our system of internal financial and accounting controls and procedures that could result in a material misstatement of our financial statements. Our internal control over financial reporting will not prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud will be detected.
If we are not able to comply with the requirements of Section 404 of the Sarbanes-Oxley Act in a timely manner, or if we are unable to maintain proper and effective internal controls, we may not be able to produce timely and accurate financial statements. If we cannot provide reliable financial reports or prevent fraud, our business and results of operations could be harmed, investors could lose confidence in our reported financial information and we could be subject to sanctions or investigations by Nasdaq, the SEC or other regulatory authorities.
We have identified material weaknesses in our internal control over financial reporting. If we fail to remedy these weaknesses or maintain an effective system of internal controls, then our ability to produce timely and accurate financial statements or comply with applicable regulations could be adversely affected. We may identify additional material weaknesses in our internal controls over financing reporting which we may not be able to remedy in a timely manner.
In connection with the preparation and audit of Conduit’s financial statements as of and for the fiscal years ended December 31, 2022 and 2021, material weaknesses were identified in Conduit’s internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis. These material weaknesses primarily relate to the following matters that are relevant to the preparation of our financial statements:
| ● | Conduit has a limited segregation of duties. For the periods under audit, Conduit did not have any internal personnel in the financial accounting and reporting department, using third party consultants to perform these activities. | |
| ● | Conduit lacked a formal process for review and approval of Conduit’s financial statements. For the periods under audit, numerous, recurring errors in account balances and disclosures were detected in the financial statements that resulted in a reasonable possibility that a material misstatement would not have been detected on a timely basis. | |
| ● | Conduit did not design adequate and appropriate internal controls, including monitoring controls, to review and evaluate the accounting implications of all material transactions that occurred in the audit period. |
If these material weaknesses are not remediated, it could result in a misstatement of account balances or disclosures that would result in a material misstatement to the annual or interim financial statements that would not be prevented or detected. We are implementing measures designed to improve our internal control over financial reporting to remediate these material weaknesses. As a part of these measures, Conduit intends to enter into an employment agreement with Mr. Sragovicz, who is the current Chief Financial Officer of MURF, which will provide that Mr. Sragovicz will serve as New Conduit’s Chief Financial Officer upon the closing of the Business Combination. In addition, Conduit anticipates hiring additional qualified accounting personnel with experience with complex GAAP and SEC rules, meanwhile, continuing to engage consultants to assist with Conduit’s financial statement close process, segregating duties among accounting personnel to enable adequate review controls, further developing and documenting our accounting policies, and designing, implementing, and/or expanding IT systems and application controls in our systems relevant to the preparation of the consolidated financial statements and as further amended by that certain Second Amendment to Agreement and Plan of Merger, dated as of May 11, 2023, a copy of which is attached to this proxy statement/prospectus as Annex A-3, to appropriately incorporate Conduit therein. Conduit also expects to engaging an external advisor to assist with evaluating and documenting the design and operating effectiveness of internal controls and assisting with the remediation of deficiencies, as necessary. The primary costs associated with such measures are corresponding recruiting and additional salary and consulting costs, which are difficult to estimate but which may be significant. These additional resources and procedures are intended to enable us to broaden the scope and quality of our internal review of underlying information related to financial reporting and to formalize and enhance our internal control procedures.
| 33 |
The material weakness will not be considered remediated until our remediation plan has been fully implemented, the applicable controls operate for a sufficient period of time, and we have concluded, through testing, that the newly implemented and enhanced controls are operating effectively. We currently expect to commence the remediation plan by documenting and implementing such plan, followed with testing such controls over time. We cannot predict the success of such efforts or the outcome of its assessment of the remediation efforts. Our efforts may not remediate this material weakness in our internal control over financial reporting, or additional material weaknesses may be identified in the future. A failure to implement and maintain effective internal control over financial reporting could result in errors in our financial statements that could result in a restatement of our financial statements and could cause us to fail to meet our reporting obligations, any of which could diminish investor confidence in us and cause a decline in the price of our common stock.
Our independent registered public accounting firm will not be required to formally attest to the effectiveness of our internal control over financial reporting until after we are no longer an “emerging growth company,” as defined in the JOBS Act. At such time, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our internal control over financial reporting is documented, designed or operating.
We may face product liability exposure, and if successful claims are brought against us, we may incur substantial liability if our insurance coverage for those claims is inadequate.
We face an inherent risk of product liability as a result of the clinical testing of our clinical assets and will face an even greater risk if we commercialize any products. This risk exists even if a product is approved for commercial sale by the FDA and manufactured in facilities licensed and regulated by the FDA or an applicable foreign regulatory authority. Our products and clinical assets are designed to affect important bodily functions and processes. Any side effects, manufacturing defects, misuse or abuse associated with our clinical assets could result in injury to a patient or even death. We cannot offer any assurance that we will not face product liability suits in the future, nor can we assure you that our insurance coverage will be sufficient to cover our liability under any such cases. In addition, a liability claim may be brought against us even if our clinical assets merely appear to have caused an injury. Product liability claims may be brought against us by consumers, health care providers, pharmaceutical companies or others selling or otherwise coming into contact with our clinical assets, among others. If we cannot successfully defend ourselves against product liability claims we will incur substantial liabilities and reputational harm.
Conduit currently relies on, and expects to continue to rely on, third-party CROs and other third parties to conduct and oversee Conduit’s clinical trials and other aspects of product development. If these third parties do not meet Conduit’s requirements or otherwise conduct the trials as required, Conduit may not be able to satisfy its contractual obligations or obtain regulatory approval for, or commercialize, its clinical assets when expected or at all.
Conduit has in the past relied and expects to continue to rely on third-party CROs to conduct and oversee its clinical trials and other aspects of product development. Conduit also relies upon various medical institutions, clinical investigators and contract laboratories to conduct its trials in accordance with its clinical trial protocols and all applicable regulatory requirements, including the FDA’s regulations and GCPs, which are an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators and monitors, and state regulations governing the handling, storage, security and recordkeeping for drug and biologic products. These CROs and other third parties play a significant role in the conduct of these trials and the subsequent collection and analysis of data from the clinical trials. Conduit relies heavily on these parties for the execution of Conduit’s clinical trials and preclinical studies, and control only certain aspects of their activities. Conduit, its CROs, and other third-party contractors are required to comply with GCP, GLP, and GACP requirements, which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities for products in clinical development. Regulatory authorities enforce these GCP, GLP and GACP requirements through periodic inspections of trial sponsors, principal investigators and trial sites. If Conduit or any of these third parties fail to comply with applicable GCP, GLP and GACP requirements, the clinical data generated in Conduit’s clinical trials may be deemed unreliable and the FDA or other regulatory authority may require Conduit to perform additional clinical trials before approving Conduit’s or its partners’ marketing applications. Conduit cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of Conduit’s clinical or preclinical trials complies with applicable GCP and GLP requirements. In addition, Conduit’s clinical trials must generally be conducted with product produced under cGMP regulations. Conduit’s failure to comply with these regulations and policies may require Conduit to repeat clinical trials, which would delay the regulatory approval process.
Conduit’s CROs are not Conduit employees, and Conduit does not control whether or not they devote sufficient time and resources to Conduit’s clinical trials. Conduit’s CROs may also have relationships with other commercial entities, including Conduit’s competitors, for whom they may also be conducting clinical trials, or other drug development activities, which could harm Conduit’s competitive position. Conduit faces the risk of potential unauthorized disclosure or misappropriation of its intellectual property by CROs, which may reduce Conduit’s trade secret protection and allow potential competitors to access and exploit Conduit’s proprietary technology. If Conduit’s CROs do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to Conduit’s clinical trial protocols or regulatory requirements or for any other reason, Conduit’s clinical trials may be extended, delayed or terminated, and Conduit may not be able to obtain regulatory approval for, or successfully commercialize any clinical asset that Conduit develops. As a result, Conduit’s financial results and the commercial prospects for any clinical asset that it develops would be harmed, Conduit’s costs could increase, and Conduit’s ability to generate revenue could be delayed.
If any of Conduit’s CROs or clinical trial sites terminate their involvement in one of Conduit’s clinical trials for any reason, Conduit may not be able to enter into arrangements with alternative CROs or clinical trial sites, or do so on commercially reasonable terms. In addition, if Conduit’s relationship with clinical trial sites is terminated, Conduit may experience the loss of follow-up information on patients enrolled in Conduit’s ongoing clinical trials unless we are able to transfer the care of those patients to another qualified clinical trial site. In addition, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and could receive cash or equity compensation in connection with such services. If these relationships and any related compensation result in perceived or actual conflicts of interest, the integrity of the data generated at the applicable clinical trial site may be questioned by the FDA.
Conduit relies completely on third-party contractors to supply, manufacture and distribute clinical drug supplies for its clinical assets, including certain sole-source suppliers and manufacturers. Conduit intends to rely on third parties for commercial supply, manufacturing and distribution if any of its clinical assets receive regulatory approval and Conduit expects to rely on third parties for supply, manufacturing and distribution of preclinical, clinical and commercial supplies of any future clinical assets.
Conduit does not currently have, nor does it plan to acquire, the infrastructure or capability to supply, manufacture or distribute preclinical, clinical or commercial quantities of drug substances or products. Conduit’s ability to develop its clinical assets depends and its ability to commercially supply our products will depend, in part, on Conduit’s ability to successfully obtain the raw materials and active pharmaceutical ingredients (“APIs”) and other substances and materials used in its clinical assets from third parties and to have finished products manufactured by third parties in accordance with regulatory requirements and in sufficient quantities for preclinical and clinical testing and commercialization. If Conduit fails to develop and maintain supply relationships with these third parties, Conduit may be unable to continue to develop or commercialize its clinical assets.
| 34 |
Conduit relies and will continue to rely on certain third parties as the sole source of the materials they supply or the finished products they manufacture. Any of Conduit’s existing suppliers or manufacturers may:
| ● | fail to supply Conduit with product on a timely basis or in the requested amount due to unexpected damage to or destruction of facilities or equipment or otherwise; | |
| ● | fail to increase manufacturing capacity and produce drug product and components in larger quantities and at higher yields in a timely or cost-effective manner, or at all, to sufficiently meet Conduit’s commercial needs; | |
| ● | be unable to meet Conduit’s production demands due to issues related to their reliance on sole-source suppliers and manufacturers; | |
| ● | supply Conduit with product that fails to meet regulatory requirements; | |
| ● | become unavailable through business interruption or financial insolvency; | |
| ● | lose regulatory status as an approved source; | |
| ● | be unable or unwilling to renew current supply agreements when such agreements expire on a timely basis, on acceptable terms or at all; or | |
| ● | discontinue production or manufacturing of necessary drug substances or products. |
In the event of any of the foregoing, if Conduit does not have an alternative supplier or manufacturer in place, Conduit would be required to expend substantial management time and expense to identify, qualify and transfer processes to alternative suppliers or manufacturers. Transferring technology to other sites may require additional processes, technologies and validation studies, which are costly, may take considerable amounts of time, may not be successful and, in most cases, require review and approval by the FDA. Any need to find and qualify new suppliers or manufacturers could significantly delay production of Conduit’s clinical assets, adversely impact our ability to market Conduit’s clinical assets and adversely affect our business. Replacements may not be available to us on a timely basis, on acceptable terms or at all. Additionally, Conduit and its manufacturers do not currently maintain significant inventory of drug substances and other materials. Any interruption in the supply of a drug substance or other material or in the manufacture of Conduit’s clinical assets could have a material adverse effect on its business, financial condition, operating results and prospects.
Conduit does not have direct control over the ability of its contract suppliers and manufacturers to maintain adequate capacity and capabilities to serve Conduit’s needs, including quality control, quality assurance and qualified personnel. Although Conduit is ultimately responsible for ensuring compliance with regulatory requirements such as cGMPs and GACP, Conduit is dependent on our contract suppliers and manufacturers for day-to-day compliance with cGMPs or GACP for production of raw materials, APIs, and finished products. Facilities used by Conduit’s contract suppliers and manufacturers to produce the APIs and other substances and materials or finished products for commercial sale must pass inspection and be approved by the FDA and other relevant regulatory authorities. Conduit’s contract suppliers and manufacturers must comply with cGMP and GACP requirements enforced by the FDA through its facilities inspection program and review of submitted technical information. If the safety of any product or clinical asset or component is compromised due to a failure to adhere to applicable laws or for other reasons, Conduit may not be able to successfully commercialize or obtain regulatory approval for the affected product or clinical asset, and Conduit may be held liable for injuries sustained as a result. Any of these factors could cause a delay or termination of preclinical studies, clinical trials or regulatory submissions or approvals of Conduit’s clinical assets, and could entail higher costs or result in Conduit being unable to effectively commercialize its approved products on a timely basis, or at all.
In addition, these contract manufacturers are engaged with other companies to supply and manufacture materials or products for such companies, which also exposes Conduit’s suppliers and manufacturers to regulatory risks for the production of such materials and products. As a result, failure to meet the regulatory requirements for the production of those materials and products may also affect the regulatory clearance of a contract supplier’s or manufacturer’s facility. If the FDA or a comparable foreign regulatory agency does not approve these facilities for the supply or manufacture of Conduit’s clinical assets, or if it withdraws its approval in the future, Conduit may need to find alternative supply or manufacturing facilities, which would negatively impact Conduit’s ability to develop, obtain regulatory approval of or market its clinical assets, if approved.
If any of Conduit’s third-party contractors terminate their involvement in the supply, manufacture or distribution of clinical drug supplies for Conduit for any reason, Conduit may not be able to enter into arrangements with alternative third party-contractors, or do so on commercially reasonable terms. In addition, if Conduit’s relationship with such third-party contractors is terminated, Conduit may experience a negative impact to the respective licenses on which Conduit relies and, therefore, on Conduit’s ability to obtain regulatory approval for, or commercialize, its clinical assets when expected or at all.
Conduit’s reliance on contract manufacturers and suppliers further exposes Conduit to the possibility that they, or third parties with access to their facilities, will have access to and may misappropriate Conduit’s trade secrets or other proprietary information.
In addition, the manufacturing facilities of certain of Conduit’s suppliers are located outside of the U.S. This may give rise to difficulties in importing Conduit’s products or clinical assets or their components into the U.S. or other countries as a result of, among other things, regulatory agency approval requirements or import inspections, incomplete or inaccurate import documentation or defective packaging.
| 35 |
Conduit currently relies on agreements with third parties for the purpose of licensing its clinical assets. In the near-term Conduit intends to rely on third parties for the licensing of clinical assets and those which may arise through future partnerships.
Conduit currently relies on agreements with third parties for the purpose of licensing clinical assets from large pharmaceutical companies. For example, Conduit has agreements with St George Street pursuant to which Conduit licenses clinical assets from St George Street and, in turn, St George Street licenses such assets from AstraZeneca. If Conduit is in breach of the agreements, the termination of such agreement(s) could materially adversely affect Conduit’s business, financial condition, operating results, and prospects. Conduit’s business strategy heavily depends on Conduit’s ability to commercialize its clinical assets and Conduit’s ability to enter into license agreements relating to such clinical assets is critical to the success of Conduit’s operations. In addition, Conduit is not a party to the license agreements between St George Street and AstraZeneca and St George Street may have other agreements with third parties relating to the development of the clinical assets that it licenses. A termination of such third-party agreements could have a material impact on or materially disrupt Conduit’s operations. While Conduit holds its own intellectual property outside of the scope of Conduit’s agreements with third parties, a termination of the agreement could adversely affect Conduit’s business and ability to commercialize its clinical assets. These third parties play a significant role in the conduct of these trials and the subsequent collection and analysis of data from the clinical trials. Conduit relies heavily on these parties for the execution of Conduit’s clinical trials and preclinical studies, and control only certain aspects of their activities.
Conduit may choose not to continue developing or commercializing any of its clinical assets at any time during development or after approval, which would reduce or eliminate our potential return on investment for those clinical assets.
Conduit may decide to discontinue the development of any of its clinical assets or not to continue commercializing one or more of its approved clinical assets for a variety of reasons, including the appearance of new technologies that make its product obsolete, competition from a competing product or changes in or failure to comply with applicable regulatory requirements at any time. If Conduit terminates a program in which Conduit has invested significant resources, Conduit will not receive any return on its investment and it will have missed the opportunity to have allocated those resources to potentially more productive uses.
If Conduit fails to attract and retain management and other key personnel, it may be unable to continue to successfully develop or commercialize its clinical assets or otherwise implement its business plan.
Conduit’s ability to compete in the highly competitive pharmaceuticals industry depends upon its ability to attract and retain highly qualified managerial, scientific, medical, sales and marketing and other personnel. Conduit is highly dependent on its management, including its Chief Executive Officer, David Tapolczay. The loss of the services of any of these individuals could impede, delay or prevent the successful development of Conduit’s product pipeline, completion of Conduit’s planned clinical trials, commercialization of Conduit’s clinical assets or in-licensing or acquisition of new assets and could negatively impact Conduit’s ability to successfully implement its business plan. If Conduit loses the services of any of these individuals, Conduit might not be able to find suitable replacements on a timely basis or at all, and its business could be harmed as a result. Conduit does not maintain “key man” insurance policies on the lives of these individuals or the lives of any of its other employees. In order to retain valuable employees at Conduit, in addition to salary and cash incentives, Conduit provides stock options that vest over time.
Conduit might not be able to attract or retain qualified management and other key personnel in the future due to the intense competition for qualified personnel among biotechnology, pharmaceutical and other businesses. Conduit could have difficulty attracting experienced personnel to Conduit and may be required to expend significant financial resources in Conduit’s employee recruitment and retention efforts. Many of the other pharmaceutical companies with whom Conduit competes for qualified personnel have greater financial and other resources, different risk profiles and longer histories in the industry than Conduit does. They also may provide more diverse opportunities and better chances for career advancement. If Conduit is not able to attract and retain the necessary personnel to accomplish its business objectives, it may experience constraints that will harm its ability to implement its business strategy and achieve its business objectives.
In addition, Conduit has scientific and clinical advisors who assist us Conduit in formulating its development and clinical strategies. These advisors are not Conduit’s employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to Conduit. In addition, Conduit’s advisors may have arrangements with other companies to assist those companies in developing products or technologies that may compete with those of Conduit.
Conduit currently has limited marketing capabilities and no sales organization. If Conduit is unable to establish sales and marketing capabilities on its own or through third parties, Conduit will be unable to successfully commercialize its clinical assets, if approved, or generate product revenue.
Conduit currently has limited marketing capabilities and no sales organization. To commercialize Conduit’s clinical assets, if approved, in the U.S., Canada, the European Union and other jurisdictions that Conduit seeks to enter, Conduit must build its marketing, sales, distribution, managerial and other non-technical capabilities or make arrangements with third parties to perform these services, and Conduit may not be successful in doing so. Although Conduit’s management team has experience in the marketing, sale and distribution of pharmaceutical products from prior employment at other companies, Conduit as a company have no prior experience in the marketing, sale and distribution of pharmaceutical products and there are significant risks involved in building and managing a sales organization, including Conduit’s ability to hire, retain and incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel and effectively manage a geographically dispersed sales and marketing team. Any failure or delay in the development of Conduit’s internal sales, marketing and distribution capabilities would adversely impact the commercialization of these products. Conduit may choose to collaborate with additional third parties that have direct sales forces and established distribution systems, either to augment Conduit’s own sales force and distribution systems or in lieu of its own sales force and distribution systems. If Conduit is unable to enter into such arrangements on acceptable terms or at all, Conduit may not be able to successfully commercialize its clinical assets. If Conduit is unable to successfully commercialize its clinical assets, either on its own or through collaborations with one or more third parties, Conduit’s business, financial condition, operating results and prospects would suffer.
| 36 |
Conduit’s failure to successfully in-license, acquire, develop, and market additional clinical assets or approved products would impair its ability to grow its business.
Conduit intends to in-license, acquire, develop and market additional products and clinical assets and Conduit may in-license or acquire commercial-stage products or engage in other strategic transactions. Because Conduit’s internal research and development capabilities are limited, Conduit may be dependent upon pharmaceutical companies, academic scientists and other researchers to sell or license products or technology to Conduit. The success of this strategy depends partly upon Conduit’s ability to identify and select promising pharmaceutical clinical assets and products, negotiate licensing or acquisition agreements with their current owners and finance these arrangements.
The process of proposing, negotiating and implementing a license or acquisition of a clinical asset or approved product is lengthy and complex. Other companies, including some with substantially greater financial, marketing, sales and other resources, may compete with Conduit for the license or acquisition of clinical assets and approved products. Conduit has limited resources to identify and execute the acquisition or in-licensing of third-party products, businesses and technologies and integrate them into Conduit’s current infrastructure. Moreover, Conduit may devote resources to potential acquisitions or licensing opportunities that are never completed, or Conduit may fail to realize the anticipated benefits of such efforts. Conduit may not be able to acquire the rights to additional clinical assets on terms that it finds acceptable, or at all.
Further, any clinical asset that Conduit acquires may require additional development efforts prior to commercial sale, including preclinical or clinical testing and approval by the FDA and applicable foreign regulatory authorities. All clinical assets are prone to risks of failure typical of pharmaceutical product development, including the possibility that a clinical asset will not be shown to be sufficiently safe and effective for approval by regulatory authorities. In addition, Conduit cannot provide assurance that any approved products that Conduit acquires will be manufactured or sold profitably or achieve market acceptance.
Additional potential transactions that Conduit may consider include a variety of different business arrangements, including spin-offs, strategic partnerships, joint ventures, restructurings, divestitures, business combinations and investments. Any such transaction may require Conduit to incur non-recurring or other charges, may increase Conduit’s near- and long-term expenditures and may pose significant integration challenges or disrupt Conduit’s management or business, which could adversely affect Conduit’s operations and financial results. For example, these transactions entail numerous potential operational and financial risks, including:
| ● | exposure to unknown liabilities; | |
| ● | disruption of Conduit’s business and diversion of our management’s time and attention in order to develop acquired products, clinical assets or technologies; | |
| ● | incurrence of substantial debt or dilutive issuances of equity securities to pay for acquisitions; | |
| ● | substantial acquisition and integration costs; | |
| ● | write-downs of assets or impairment charges; | |
| ● | increased amortization expenses; | |
| ● | difficulty and cost in combining the operations and personnel of any acquired businesses with Conduit’s operations and personnel; | |
| ● | impairment of relationships with key suppliers, partners or customers of any acquired businesses due to changes in management and ownership; and | |
| ● | inability to retain Conduit’s key employees or those of any acquired businesses. |
Accordingly, there can be no assurance that Conduit will undertake or successfully complete any transactions of the nature described above, and any transaction that Conduit does complete could harm its business, financial condition, operating results and prospects.
| 37 |
Manufacturing and supply of the APIs and other substances and materials used in Conduit’s clinical assets is a complex and technically challenging undertaking, and there is potential for failure at many points in the manufacturing, testing, quality assurance and distribution supply chain, as well as the potential for latent defects after products have been manufactured and distributed.
Manufacturing and supply of APIs, other substances and materials and finished drug products is technically challenging. Changes beyond Conduit’s direct control can impact the quality, volume, price and successful delivery of Conduit’s clinical assets and can impede, delay, limit or prevent the successful development and commercialization of Conduit’s clinical assets. Mistakes and mishandling are not uncommon and can affect successful production and supply. Some of these risks include:
| ● | failure of Conduit’s manufacturers to follow cGMP or GACP requirements or mishandling of product while in production or in preparation for transit; | |
| ● | inability of Conduit’s contract suppliers and manufacturers to efficiently and cost-effectively increase and maintain high yields and batch quality, consistency and stability; | |
| ● | Conduit’s inability to develop an FDA approved bioassay for release of any future product; | |
| ● | difficulty in establishing optimal drug delivery substances and techniques, production and storage methods and packaging and shipment processes; | |
| ● | transportation and import/export risk, particularly given the global nature of Conduit’s supply chain; | |
| ● | delays in analytical results or failure of analytical techniques that Conduit depends on for quality control and release of any future product; | |
| ● | natural disasters, pandemics, labor disputes, financial distress, lack of raw material supply, issues with facilities and equipment or other forms of disruption to business operations of Conduit’s contract manufacturers and suppliers; and | |
| ● | latent defects that may become apparent after the product has been released and which may result in recall and destruction of product. |
Any of these factors could result in delays or higher costs in connection with Conduit’s clinical trials, regulatory submissions, required approvals or commercialization of Conduit’s clinical assets, which could harm Conduit’s business, financial condition, operating results and prospects.
Conduit’s operating results may fluctuate significantly, which makes Conduit’s future operating results difficult to predict and could cause Conduit’s operating results to fall below expectations.
Conduit’s operations to date have been primarily limited to researching and developing its clinical assets and undertaking preclinical studies and clinical trials of its clinical assets. Conduit has not yet obtained regulatory approvals for any of its clinical assets. Consequently, any predictions you make about Conduit’s future success or viability may not be as accurate as they could be if Conduit had a longer operating history or approved products on the market. Furthermore, Conduit’s operating results may fluctuate due to a variety of other factors, many of which are outside of Conduit’s control and may be difficult to predict, including the following:
| ● | delays in the commencement, enrollment and the timing of clinical testing for Conduit’s clinical assets; | |
| ● | the timing and success or failure of clinical trials for Conduit’s clinical assets or competing clinical assets, or any other change in the competitive landscape of our industry, including consolidation among our competitors or partners; | |
| ● | any delays in regulatory review and approval of clinical assets in clinical development; | |
| ● | the timing and cost of, and level of investment in, research and development activities relating to Conduit’s clinical assets, which may change from time to time; | |
| ● | the cost of manufacturing Conduit’s clinical assets, which may vary depending on FDA guidelines and requirements, and the quantity of production; | |
| ● | Conduit’s ability to obtain additional funding to develop its clinical assets; | |
| ● | expenditures that Conduit will or may incur to acquire or develop additional clinical assets and technologies; | |
| ● | the level of demand for Conduit’s clinical assets, should they receive approval, which may vary significantly; | |
| ● | potential side effects of Conduit’s clinical assets that could delay or prevent commercialization or cause an approved drug to be taken off the market; | |
| ● | the ability of patients or healthcare providers to obtain coverage of or sufficient reimbursement for Conduit’s clinical assets, if approved; |
| 38 |
| ● | Conduit’s dependency on third-party manufacturers to supply or manufacture Conduit’s clinical assets; | |
| ● | Conduit’s ability to establish an effective sales, marketing and distribution infrastructure in a timely manner; | |
| ● | market acceptance of Conduit’s clinical assets, if approved, and Conduit’s ability to forecast demand for those clinical assets; | |
| ● | Conduit’s ability to receive approval and commercialize its clinical assets outside of the U.S.; | |
| ● | Conduit’s ability to establish and maintain collaborations, licensing or other arrangements; | |
| ● | Conduit’s ability and third parties’ abilities to protect intellectual property rights; | |
| ● | costs related to and outcomes of potential litigation or other disputes; | |
| ● | Conduit’s ability to adequately support future growth; | |
| ● | Conduit’s ability to attract and retain key personnel to manage Conduit’s business effectively; | |
| ● | potential liabilities associated with hazardous materials; | |
| ● | Conduit’s ability to maintain adequate insurance policies; and | |
| ● | future accounting pronouncements or changes in our accounting policies. |
Conduit’s operating results and liquidity needs could be negatively affected by market fluctuations and economic downturn.
Conduit’s operating results and liquidity could be negatively affected by economic conditions generally, both in the U.S. and elsewhere around the world. The market for discretionary medical products and procedures may be particularly vulnerable to unfavorable economic conditions. Some patients may consider certain of Conduit’s clinical assets to be discretionary, and if full reimbursement for such products is not available, demand for these products may be tied to the discretionary spending levels of Conduit’s targeted patient populations. Domestic and international equity and debt markets have experienced and may continue to experience heightened volatility and turmoil based on domestic and international economic conditions and concerns. In the event these economic conditions and concerns continue or worsen and the markets continue to remain volatile, Conduit’s operating results and liquidity could be adversely affected by those factors in many ways, including weakening demand for certain of Conduit’s products and making it more difficult for Conduit to raise funds if necessary. Additionally, although we plan to market our products primarily in the U.S., we could in the future have partners with extensive global operations, indirectly exposing us to risk.
Conduit maintains its cash and cash equivalents with high quality, accredited financial institutions. However, some of these accounts exceed the government-insured limits, and, while Conduit believes that it is not exposed to significant credit risk due to the financial strength of these depository institutions or investments, the failure or collapse of one or more of these depository institutions or default on these investments could materially adversely affect Conduit’s ability to recover these assets and/or materially harm Conduit’s financial condition.
Conduit is increasingly dependent on information technology, and Conduit’s systems and infrastructure face certain risks, including cybersecurity and data leakage risks.
Significant disruptions to Conduit’s information technology systems or breaches of information security could adversely affect Conduit’s business. In the ordinary course of business, Conduit collects, stores and transmits large amounts of confidential information, and it is critical that Conduit do so in a secure manner to maintain the confidentiality and integrity of such confidential information. The size and complexity of Conduit’s information technology systems, and those of its third-party vendors with whom it contracts, make such systems potentially vulnerable to service interruptions and security breaches from inadvertent or intentional actions by Conduit’s employees, partners or vendors, from attacks by malicious third parties, or from intentional or accidental physical damage to Conduit’s systems infrastructure maintained by Conduit or by third parties. Maintaining the secrecy of this confidential, proprietary, or trade secret information is important to Conduit’s competitive business position. While Conduit has taken steps to protect such information and invested in information technology, there can be no assurance that Conduit’s efforts will prevent service interruptions or security breaches in Conduit’s systems or the unauthorized or inadvertent wrongful use or disclosure of confidential information that could adversely affect Conduit’s business operations or result in the loss, dissemination, or misuse of critical or sensitive information. A breach of Conduit’s security measures or the accidental loss, inadvertent disclosure, unapproved dissemination, misappropriation or misuse of trade secrets, proprietary information, or other confidential information, whether as a result of theft, hacking, fraud, trickery or other forms of deception, or for any other reason, could enable others to produce competing products, use Conduit’s proprietary technology or information, or adversely affect Conduit’s business or financial condition. Further, any such interruption, security breach, loss or disclosure of confidential information, could result in financial, legal, business, and reputational harm to Conduit and could have a material adverse effect on Conduit’s business, financial position, results of operations or cash flow.
Conduit’s business and operations would suffer in the event of failures in our internal computer systems.
Despite the implementation of security measures, Conduit’s computer systems and those of Conduit’s current and any future partners, contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While Conduit has not experienced any such material system failure, accident, or security breach to date, if such an event were to occur and cause interruptions in Conduit’s operations, it could result in a material disruption of Conduit’s manufacturing activities, development programs and business operations. For example, the loss of manufacturing records or clinical trial data from completed or future clinical trials could result in delays in Conduit’s regulatory approval efforts and significantly increase Conduit’s costs to recover or reproduce the data. While Conduit has not experienced a security breach, its online sources being hacked or a data leak to date, if such an event were to occur, it could result in confidential clinical trial data being leaked to competitors and the market. To the extent that any disruption or security breach were to result in a loss of, or damage to, Conduit’s data or applications, or inappropriate disclosure of confidential or proprietary information, Conduit could incur liability and the further commercialization and development of its products and clinical assets could be delayed.
| 39 |
Failure to adequately protect Conduit’s intellectual property could adversely affect Conduit’s business, financial condition, and operating results.
Conduit’s business depends on its intellectual property and proprietary technology, the protection of which is crucial to the success of its business. Conduit relies on a combination of trademark, copyright, and trade secret laws, license agreements, intellectual property assignment agreements, and confidentiality procedures to protect its intellectual property. Additionally, Conduit relies on proprietary information (such as trade secrets, know-how and confidential information) to protect intellectual property that may not be patentable, or that Conduit believes is best protected by means that do not require public disclosure. Conduit generally attempts to protect its intellectual property, technology, and confidential information by requiring its employees and consultants who develop intellectual property on Conduit’s behalf to enter into confidentiality and invention assignment agreements and third parties that Conduit shares information with to enter into nondisclosure agreements. These agreements may not effectively prevent unauthorized use or disclosure of Conduit’s confidential information, intellectual property, or technology and may not provide an adequate remedy in the event of unauthorized use or disclosure of Conduit’s confidential information or technology, or infringement of Conduit’s intellectual property. For example, Conduit may fail to enter into the necessary agreements, and even if entered into, these agreements may be willfully breached or may otherwise fail to prevent disclosure, third-party infringement or misappropriation of Conduit’s proprietary information, may be limited as to their term and may not provide an adequate remedy in the event of unauthorized disclosure or use of proprietary information. In addition, Conduit’s proprietary information may otherwise become known or be independently developed by Conduit’s competitors or other third parties. To the extent that Conduit’s employees, consultants, contractors, and other third parties use intellectual property owned by others in their work for Conduit, disputes may arise as to the rights in related or resulting know-how and inventions. Costly and time-consuming litigation could be necessary to enforce and determine the scope of Conduit’s intellectual property rights and other proprietary rights, and failure to obtain or maintain protection for Conduit’s proprietary information could adversely affect Conduit’s competitive business position.
Despite Conduit’s efforts to protect its proprietary rights, other parties may unintentionally or willfully disclose, obtain or use Conduit’s technologies or systems, which may allow unauthorized parties to copy aspects of Conduit’s platform or other software, technology, and functionality or obtain and use information that Conduit considers proprietary. In addition, unauthorized parties may also attempt, or successfully endeavor, to obtain Conduit’s intellectual property, confidential information and trade secrets through various methods, including through scraping of public data or other content from Conduit’s website or mobile applications, cybersecurity attacks, and legal or other methods of protecting this data may be inadequate. Monitoring unauthorized use and disclosures of Conduit’s intellectual property, proprietary technology, or confidential information can be difficult and expensive and Conduit cannot be sure that the steps it has taken will prevent misappropriation or infringement of its intellectual property or proprietary rights.
Conduit has registered the domain name for the website that it uses in its business, which is www.conduitpharma.com. The inclusion of the website address in this proxy statement/prospectus does not include or incorporate by reference the information on the Conduit website into this proxy statement/prospectus.
Competitors have and may continue to adopt service names similar to Conduit’s, thereby harming Conduit’s ability to build brand identity and possibly leading to user confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other trademarks that are similar to Conduit’s trademarks. Further, litigation or proceedings before the U.S. Patent and Trademark Office or other governmental authorities and administrative bodies in the U.S. and abroad may be necessary in the future to enforce Conduit’s intellectual property rights and to determine the validity and scope of the proprietary rights of others. Any litigation initiated by Conduit concerning the violation by third parties of its intellectual property rights is likely to be expensive and time-consuming and could lead to the invalidation of, or render unenforceable, its intellectual property, or could otherwise have negative consequences for Conduit. Even when Conduit sues other parties for such infringement, that suit may have adverse consequences for Conduit’s business. In addition, Conduit may not timely or successfully apply for a patent or register its trademarks or otherwise secure its intellectual property, which could result in negative effects to its market share, financial condition and results of operations. Conduit’s efforts to protect, maintain, or enforce its proprietary rights may not be respected in the future or may be invalidated, circumvented, or challenged, and could result in substantial costs and diversion of resources, which could adversely affect Conduit’s business, financial condition, and operating results.
| 40 |
Conduit may be unable to continue to use the domain name that it uses in its business or prevent third parties from acquiring and using domain names that infringe on, are similar to, or otherwise decrease the value of Conduit’s brand, trademarks, or service marks.
Conduit has registered the domain name that it uses in its business. If Conduit loses the ability to use that domain name, whether due to trademark claims, failure to renew the applicable registration, or any other cause, Conduit may be forced to market its offerings under a new domain name, which could cause Conduit substantial harm, or to incur significant expense in order to purchase rights to the domain name in question. Conduit may not be able to obtain preferred domain names outside the U.S. due to a variety of reasons, including because they are already held by others. In addition, Conduit’s competitors and others could attempt to capitalize on Conduit’s brand recognition by using domain names similar to Conduit’s domain name. Conduit may be unable to prevent third parties from acquiring and using domain names that infringe on, are similar to, or otherwise decrease the value of Conduit’s brand or Conduit’s trademarks or service marks. Protecting, maintaining, and enforcing Conduit’s rights in its domain names may require litigation, which could result in substantial costs and diversion of resources, which could in turn adversely affect Conduit’s business, financial condition, and operating results.
Conduit may not be able to protect its intellectual property rights throughout the world.
Filing, prosecuting and defending patents on Conduit’s clinical assets in all countries throughout the world would be prohibitively expensive. The requirements for patentability may differ in certain countries, particularly developing countries. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as laws in the U.S. Consequently, Conduit may not be able to prevent third parties from practicing Conduit’s inventions in all countries outside the U.S. Competitors may use Conduit’s technologies in jurisdictions where Conduit has not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where Conduit has patent protection, but enforcement on infringing activities is inadequate. These products may compete with Conduit’s products, and Conduit’s patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to pharmaceuticals, which could make it difficult for us to stop the infringement of Conduit’s patents or marketing of competing products in violation of Conduit’s proprietary rights generally. Proceedings to enforce Conduit’s patent rights in foreign jurisdictions could result in substantial costs and divert Conduit’s efforts and attention from other aspects of its business, could put its patents at risk of being invalidated or interpreted narrowly and its patent applications at risk of not issuing, and could provoke third parties to assert claims against Conduit. Conduit may not prevail in any lawsuits that it initiates, and the damages or other remedies awarded, if any, may not be commercially meaningful. In addition, certain countries in Europe and certain developing countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In those countries, Conduit may have limited remedies if its patents are infringed or if it is compelled to grant a license to its patents to a third party, which could materially diminish the value of those patents. This could limit Conduit’s potential revenue opportunities. Accordingly, Conduit’s efforts to enforce its intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that Conduit owns or licenses. Conduit’s ability to protect and enforce its intellectual property rights may also be adversely affected by unforeseen changes in foreign intellectual property laws.
Obtaining and maintaining Conduit’s patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and Conduit’s patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance and annuity fees on any issued patent are due to be paid to the United States Patent and Trademark Office (“USPTO”) and foreign patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If Conduit or its licensors fail to maintain the patents and patent applications covering Conduit’s clinical assets, Conduit’s competitors might be able to enter the market, which would have an adverse effect on Conduit’s business.
If Conduit fails to comply with its obligations under its intellectual property license agreements, Conduit could lose license rights that are important to its business.
Conduit is a party to certain license agreements that impose various diligence, milestone, royalty, insurance and other obligations on Conduit. If Conduit fails to comply with these obligations, the respective licensors may have the right to terminate the license, in which event Conduit may not be able to develop or market the affected clinical asset. Conduit’s business strategy depends on Conduit’s ability to commercialize its clinical assets and Conduit’s ability to enter into license agreements relating to such clinical assets is critical to the success of Conduit’s operations. The loss of such rights could materially adversely affect Conduit’s business, financial condition, operating results and prospects. For more information about these license arrangements, see “Business —Strategic Alliances and Arrangements.”
| 41 |
If Conduit is sued for infringing intellectual property rights of third parties, it will be costly and time-consuming, and an unfavorable outcome in that litigation could have a material adverse effect on Conduit’s business.
Conduit’s commercial success depends upon its ability to develop, manufacture, market and sell its clinical assets and use its proprietary technologies without infringing the proprietary rights of third parties. Conduit cannot guarantee that marketing and selling such candidates and using such technologies will not infringe existing or future patents. Numerous U.S. and foreign issued patents and pending patent applications owned by third parties exist in the fields relating to Conduit’s clinical assets. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that others may assert that Conduit’s clinical assets, technologies or methods of delivery or use infringe their patent rights. Moreover, it is not always clear to industry participants, including Conduit, which patents cover various drugs, biologics, drug delivery systems or their methods of use, and which of these patents may be valid and enforceable. Thus, because of the large number of patents issued and patent applications filed in Conduit’s fields, there may be a risk that third parties may allege they have patent rights encompassing Conduit’s clinical assets, technologies or methods.
In addition, there may be issued patents of third parties that are infringed or are alleged to be infringed by Conduit’s clinical assets or proprietary technologies. Because some patent applications in the U.S. may be maintained in secrecy until the patents are issued, because patent applications in the U.S. and many foreign jurisdictions are typically not published until eighteen months after filing and because publications in the scientific literature often lag behind actual discoveries, Conduit cannot be certain that others have not filed patent applications for technology covered by Conduit’s own and in-licensed issued patents or Conduit’s pending applications. Conduit’s competitors may have filed, and may in the future file, patent applications covering Conduit’s clinical assets or technology similar to Conduit’s. Any such patent application may have priority over Conduit’s own and in-licensed patent applications or patents, which could further require Conduit to obtain rights to issued patents covering such technologies. If another party has filed a U.S. patent application on inventions similar to those owned or in-licensed to Conduit, Conduit or, in the case of in-licensed technology, the licensor may have to participate, in the U.S., in an interference proceeding to determine priority of invention.
Conduit may be exposed to, or threatened with, future litigation by third parties having patent or other intellectual property rights alleging that Conduit’s clinical assets or proprietary technologies infringe such third parties’ intellectual property rights, including litigation. These lawsuits could claim that there are existing patent rights for such drug and this type of litigation can be costly and could adversely affect Conduit’s operating results and divert the attention of managerial and technical personnel, even if Conduit does not infringe such patents or the patents asserted against Conduit are ultimately established as invalid. There is a risk that a court would decide that Conduit is infringing the third party’s patents and would order Conduit to stop the activities covered by the patents. In addition, there is a risk that a court will order Conduit to pay the other party damages for having violated the other party’s patents.
As a result of patent infringement claims, or to avoid potential claims, Conduit may choose or be required to seek licenses from third parties. These licenses may not be available on commercially acceptable terms, or at all. Even if Conduit is able to obtain a license, the license would likely obligate Conduit to pay license fees or royalties or both, and the rights granted to Conduit might be nonexclusive, which could result in Conduit’s competitors gaining access to the same intellectual property, or such rights might be restrictive and limit Conduit’s present and future activities. Ultimately, Conduit or a licensee could be prevented from commercializing a product or be forced to cease some aspect of Conduit’s business operations, if, as a result of actual or threatened patent infringement claims, Conduit is unable to enter into licenses on acceptable terms.
In addition to possible infringement claims against Conduit, Conduit may become a party to other patent litigation and other proceedings, including interference, derivation, re-examination or other post-grant proceedings declared or granted by the USPTO, and similar proceedings in foreign countries, regarding intellectual property rights with respect to Conduit’s current or future products.
There is a substantial amount of litigation involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries generally. To date, no litigation asserting infringement claims has ever been brought against Conduit. If a third-party claims that Conduit infringes its intellectual property rights, Conduit may face a number of issues, including:
| ● | infringement and other intellectual property claims which, regardless of merit, may be expensive and time-consuming to litigate and may divert Conduit’s management’s attention from Conduit’s core business; | |
| ● | substantial damages for infringement, which Conduit may have to pay if a court decides that the product or technology at issue infringes or violates the third party’s rights, and if the court finds that the infringement was willful, Conduit could be ordered to pay treble damages and the patent owner’s attorneys’ fees; | |
| ● | a court prohibiting Conduit from selling or licensing the product or using the technology unless the third party licenses its intellectual property rights to Conduit, which it is not required to do; | |
| ● | if a license is available from a third party, Conduit may have to pay substantial royalties or upfront fees or grant cross-licenses to intellectual property rights for Conduit’s products or technologies; and | |
| ● | redesigning Conduit’s products or processes so they do not infringe, which may not be possible or may require substantial monetary expenditures and time. |
| 42 |
Some of Conduit’s competitors may be able to sustain the costs of complex patent litigation more effectively than Conduit can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could harm Conduit’s ability to raise additional funds or otherwise adversely affect Conduit’s business, financial condition, operating results and prospects.
Because Conduit relies on certain third-party licensors and partners, and will continue to do so in the future, if one of Conduit’s licensors or partners is sued for infringing a third party’s intellectual property rights, Conduit’s business, financial condition, operating results and prospects could suffer in the same manner as if Conduit were sued directly. In addition to facing litigation risks, Conduit has agreed to indemnify certain third-party licensors and partners against claims of infringement caused by Conduit’s proprietary technologies, and Conduit has entered or may enter into cost-sharing agreements with some its licensors and partners that could require Conduit to pay some of the costs of patent litigation brought against those third parties whether or not the alleged infringement is caused by Conduit’s proprietary technologies. In certain instances, these cost-sharing agreements could also require Conduit to assume greater responsibility for infringement damages than would be assumed just on the basis of Conduit’s technology.
The occurrence of any of the foregoing could adversely affect Conduit’s business, financial condition or operating results.
Conduit may become involved in lawsuits to protect or enforce its patents or other intellectual property or the patents of its licensors, which could be expensive and time-consuming.
Competitors may infringe Conduit’s intellectual property, including Conduit’s patents or the patents of its licensors. As a result, Conduit may be required to file infringement claims to stop third-party infringement or unauthorized use. This can be expensive and time-consuming, particularly for a company of Conduit’s size. In addition, in an infringement proceeding, a court may decide that a patent of Conduit is not valid or is unenforceable or may refuse to stop the other party from using the technology at issue on the grounds that Conduit’s patent claims do not cover its technology or that the factors necessary to grant an injunction against an infringer are not satisfied. An adverse determination of any litigation or other proceedings could put one or more of Conduit’s patents at risk of being invalidated, interpreted narrowly or amended such that they do not cover Conduit’s clinical assets. Moreover, such adverse determinations could put Conduit’s patent applications at risk of not issuing or issuing with limited and potentially inadequate scope to cover Conduit’s clinical assets or to prevent others from marketing similar products.
Interference, derivation or other proceedings brought at the USPTO may be necessary to determine the priority or patentability of inventions with respect to Conduit’s patent applications or those of Conduit’s licensors or potential partners. Litigation or USPTO proceedings brought by Conduit may fail or may be invoked against Conduit by third parties. Even if Conduit is successful, domestic or foreign litigation or USPTO or foreign patent office proceedings may result in substantial costs and distraction to Conduit’s management. Conduit may not be able, alone or with its licensors or potential partners, to prevent misappropriation of its proprietary rights, particularly in countries where the laws may not protect such rights as fully as in the U.S.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation or other proceedings, there is a risk that some of Conduit’s confidential information could be compromised by disclosure during this type of litigation or other proceedings. In addition, during the course of this kind of litigation or proceedings, there could be public announcements of the results of hearings, motions or other interim proceedings or developments or public access to related documents.
Conduit’s reliance on third parties requires Conduit to share its trade secrets, which increases the possibility that its trade secrets will be misappropriated or disclosed, and confidentiality agreements with employees and third parties may not adequately prevent disclosure of trade secrets and protect other proprietary information.
Conduit considers proprietary trade secrets or confidential know-how and unpatented know-how to be important to its business. Conduit may rely on trade secrets or confidential know-how to protect Conduit’s technology, especially where patent protection is believed by Conduit to be of limited value.
To protect this type of information against disclosure or appropriation by competitors, Conduit’s policy is to require its employees, consultants, collaborators, contractors and advisors to enter into confidentiality agreements and, if applicable, material transfer agreements, consulting agreements or other similar agreements with Conduit prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose Conduit’s confidential information, including its trade secrets. However, current or former employees, consultants, collaborators, contractors and advisors may unintentionally or willfully disclose Conduit’s confidential information to competitors, and confidentiality agreements may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. The need to share trade secrets and other confidential information increases the risk that such trade secrets become known by Conduit’s competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that Conduit’s proprietary position is based, in part, on Conduit’s know-how and trade secrets, a competitor’s discovery of Conduit’s trade secrets or other unauthorized use or disclosure would impair Conduit’s competitive position and may have an adverse effect on Conduit’s business and results of operations. Enforcing a claim that a third party obtained illegally and is using trade secrets or confidential know-how is expensive, time consuming and unpredictable. The enforceability of confidentiality agreements may vary from jurisdiction to jurisdiction.
| 43 |
In addition, these agreements typically restrict the ability of Conduit’s employees, consultants, collaborators, contractors and advisors to publish data potentially relating to Conduit’s trade secrets, although Conduit’s agreements may contain certain limited publication rights. Despite Conduit’s efforts to protect its trade secrets, Conduit’s competitors may discover Conduit’s trade secrets, either through breach of our agreements with third parties, independent development or publication of information by any of Conduit’s third-party collaborators. A competitor’s discovery of Conduit’s trade secrets would impair Conduit’s competitive position and have an adverse impact on Conduit’s business.
Conduit may be subject to claims that its employees, consultants or independent contractors have wrongfully used or disclosed to Conduit alleged trade secrets of their former employers or their former or current customers.
As is common in the biotechnology and pharmaceutical industries, certain of Conduit’s employees were formerly employed by other biotechnology or pharmaceutical companies, including Conduit’s competitors or potential competitors. Moreover, Conduit engages the services of consultants to assist it in the development of its products and clinical assets, many of whom were previously employed at or may have previously been or are currently providing consulting services to, other biotechnology or pharmaceutical companies, including Conduit’s competitors or potential competitors. Conduit may be subject to claims that these employees and consultants or Conduit has inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers or their former or current customers. Although Conduit has no knowledge of any such claims being alleged to date, if such claims were to arise, litigation may be necessary to defend against any such claims. Even if Conduit is successful in defending against any such claims, any such litigation could be protracted, expensive, a distraction to Conduit’s management team, not viewed favorably by investors and other third parties and may potentially result in an unfavorable outcome.
If Conduit’s trademarks and trade names are not adequately protected, then Conduit may not be able to build name recognition in its markets of interest and its business may be adversely affected.
Conduit’s unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. Conduit may not be able to protect its rights to these trademarks and trade names, which Conduit needs to build name recognition among potential collaborators or customers in Conduit’s markets of interest. At times, competitors may adopt trade names or trademarks similar to those of Conduit, thereby impeding Conduit’s ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of Conduit’s unregistered trademarks or trade names. Over the long term, if Conduit is unable to successfully register its trademarks and trade names and establish name recognition based on its trademarks and trade names, then Conduit may not be able to compete effectively, and its business may be adversely affected. Conduit’s efforts to enforce or protect its proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely impact Conduit’s financial condition or results of operations.
Conduit’s proprietary information may be lost, or Conduit may suffer security breaches.
In the ordinary course of our business, Conduit collects and store sensitive data, including intellectual property, clinical trial data, proprietary business information, personal data and personally identifiable information of Conduit’s clinical trial subjects and employees, in Conduit’s data centers and on its networks. The secure processing, maintenance and transmission of this information is critical to Conduit’s operations. Despite Conduit’s security measures, Conduit’s information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Although, to Conduit’s knowledge, Conduit has not experienced any such material security breach to date, any such breach could compromise Conduit’s networks and the information stored there could be accessed, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, significant regulatory penalties, disrupt Conduit’s operations, damage Conduit’s reputation and cause a loss of confidence in Conduit and its ability to conduct clinical trials, which could adversely affect Conduit’s reputation and delay its clinical development of its clinical assets.
Conduit does not currently maintain insurance and will need to obtain insurance to cover its business and operations, including directors and officers’ insurance, property and casualty insurance, general liability, product liability insurance, and health and welfare insurance.
Since our formation, we have been operating as a virtual company and we do not currently maintain insurance coverage for our business. Prior to the closing of the Business Combination, we will have to obtain and bind suitable insurance coverage at reasonable rates on a timely basis or at all. If we are not able to bind insurance at reasonable rates or on a timely basis, it could harm our business. In addition, we will need to continue to evaluate and potentially adjust our insurance coverages as we expand our business and operations and, as a result, our costs of obtaining insurance coverage may increase over time. Any increase in the costs of our insurance coverage could negatively impact our results of operations.
| 44 |
Risks Related to the Business Combination and New Conduit Common Stock Following the Business Combination
The process of taking a company public by means of a business combination with a special purpose acquisition company (a “SPAC”) is different from taking a company public through an underwritten public offering and may create material risks for unaffiliated investors.
An underwritten offering involves a company engaging underwriters to purchase its shares and resell them to the public. An underwritten offering imposes statutory liability on the underwriters for material misstatements or omissions contained in the registration statement unless they are able to sustain the burden of providing that they did not know and could not reasonably have discovered such material misstatements or omissions. This is referred to as a “due diligence” defense and results in the underwriters undertaking a detailed review of the company’s business, financial condition and results of operations. Going public via a business combination with a SPAC does not involve any underwriters and, therefore, there is an absence of due diligence conducted by an underwriter that would be subject to liability for any material misstatements or omissions in a registration statement, and so does not generally necessitate the level of review required to establish a “due diligence” defense as would be customary in an underwritten offering.
In addition, going public via a business combination with a SPAC does not involve a book-building process as is the case in an underwritten public offering. In any underwritten public offering, the initial value of a company is set by investors who indicate the price at which they are prepared to purchase shares from the underwriters. In the case of a SPAC transaction, the value of the company is established by means of negotiations between the target company, the SPAC and, in some cases, other investors who agree to purchase shares at the time of the business combination. The process of establishing the value of a company in a SPAC business combination may be less effective than the book-building process in an underwritten public offering and also does not reflect events that may have occurred between the date of the business combination agreement and the closing of the transaction. In addition, underwritten public offerings are frequently oversubscribed resulting in additional potential demand for shares in the aftermarket following the underwritten public offering. There is no such book of demand built up in connection with a SPAC transaction and no underwriters with the responsibility of stabilizing the share price which may result in the share price being harder to sustain after the transaction.
The Sponsor, certain members of our board of directors and our officers have interests in the Business Combination that may conflict with those of other stockholders in recommending that stockholders vote in favor of approval of the Business Combination and the other proposals described in this proxy statement/prospectus.
When considering our board of directors’ recommendation that our stockholders vote in favor of the approval of the Business Combination, our stockholders should be aware that our Sponsor and certain of our directors and officers have interests in the Business Combination that may conflict with the interests of other stockholders generally. Accordingly, our Sponsor may be incentivized to complete the Business Combination, or an alternative initial business combination with a less favorable company or on terms less favorable to stockholders, rather than to liquidate, in which case our Sponsor would lose its entire investment. As a result, our Sponsor may have a conflict of interest in determining whether Conduit is an appropriate business with which to effectuate a business combination and/or in evaluating the terms of the Business Combination. Our directors were aware of and considered these interests, among other matters, in evaluating the Business Combination, and in recommending to stockholders that they approve the Business Combination. MURF”s Public Stockholders should take these interests into account in deciding whether to approve the Business Combination. For a description of these interests, see “The Business Combination Proposal — Interests of MURF’s Directors and Officers in the Business Combination”.
Following the consummation of the Business Combination, our only significant asset will be ownership of 100% of Conduit’s capital shares, and we do not currently intend to pay dividends on New Conduit common stock and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
Following the consummation of the Business Combination, New Conduit will have no direct operations and no significant assets other than the ownership of 100% of Conduit’s capital stock. We will depend on Conduit for distributions, loans and other payments to generate the funds necessary to meet our financial obligations, including our expenses as a publicly traded company, and to pay any dividends with respect to New Conduit common stock. Applicable state law and contractual restrictions, including in agreements governing the current or future indebtedness of Conduit, as well as the financial condition and operating requirements of Conduit, may limit our ability to obtain cash from Conduit. Thus, we do not expect to pay cash dividends on New Conduit common stock. Any future dividend payments are within the absolute discretion of our board of directors and will depend on, among other things, our results of operations, working capital requirements, capital expenditure requirements, financial condition, level of indebtedness, contractual restrictions with respect to payment of dividends, business opportunities, anticipated cash needs, provisions of applicable law and other factors that our board of directors may deem relevant.
Nasdaq may delist MURF’s securities from trading on its exchange prior to consummation of the Business Combination.
MURF’s Class A common stock was listed on the Nasdaq Global Market upon consummation of its initial public offering. Although MURF met the minimum initial listing standards of Nasdaq, which generally only requires that it meet certain requirements relating to shareholders’ equity, market capitalization, aggregate market value of publicly held shares and distribution requirements, MURF cannot assure you that its securities will continue to be listed on Nasdaq in the future prior to an initial business combination. The Nasdaq Global Market’s requirements for continued listing include a public float of 1,100,000, a market value of public float of $15,000,000, a market value of listed securities of $50,000,000, and 400 shareholders. The inability to comply with Nasdaq’s continued requirements or standards could result in the delisting of MURF’s Class A common stock, which could have a material adverse effect on MURF’s financial condition and could cause the value of its Class A common stock to decline.
On April 10, 2023, MURF received written notice (the “Notice”) from Nasdaq indicating that MURF was no longer in compliance with the minimum Market Value of Listed Securities (“MVLS”) of $50,000,000 required for continued listing on the Nasdaq Global Market (the “MVLS Requirement”). In accordance with Nasdaq rules, MURF has 180 calendar days, or until October 9, 2023 (the “Compliance Date”), to regain compliance with the MVLS Requirement. If, at any time before the Compliance Date, the market value of MURF’s listed securities closes at $50,000,000 or more for a minimum of 10 consecutive business days, Nasdaq will provide written notification to MURF that it has regained compliance with the MVLS Requirement. If MURF does not regain compliance with the MVLS Requirement by the Compliance Date, MURF will receive written notification that its securities are subject to delisting. At that time, MURF may appeal the delisting determination to a Nasdaq Listing Qualifications Panel. There can be no assurance that such appeal would be successful and Nasdaq would grant MURF’s request for continued listing. If MURF does not regain compliance with the MVLS Requirement by the Compliance Date, MURF may also be able to transfer the listing of its Class A common stock to the Nasdaq Capital Market, provided that MURF then meets the applicable requirements for continued listing on the Nasdaq Capital Market.
If MURF’s Class A common stock is delisted from Nasdaq, the New Conduit common stock will not be approved for listing on Nasdaq and Conduit may choose not to consummate the Business Combination.
There can be no assurance that New Conduit common stock will be approved for listing on Nasdaq or that New Conduit will be able to comply with the continued listing standards of Nasdaq.
In connection with the closing of the Business Combination, we intend to list the common stock of New Conduit on Nasdaq under the symbol “CDT.” If, after the Business Combination, Nasdaq delists New Conduit’s shares from trading on its exchange for failure to meet the applicable listing standards, we and our stockholders could face significant material adverse consequences including:
| ● | a limited availability of market quotations for our securities; | |
| ● | reduced liquidity for our securities; |
| 45 |
| ● | a determination that New Conduit common stock is a “penny stock” which will require brokers trading in New Conduit common stock to adhere to more stringent rules, possibly resulting in a reduced level of trading activity in the secondary trading market for shares of New Conduit common stock; | |
| ● | a limited amount of news and analyst coverage; and | |
| ● | a decreased ability to issue additional securities or obtain additional financing in the future. |
Subsequent to the consummation of the Business Combination, New Conduit may be required to take write-downs or write-offs, or New Conduit may be subject to restructuring, impairment or other charges that could have a significant negative effect on New Conduit’s business, prospects, financial condition, results of operations and the trading price of New Conduit’s securities, which could cause you to lose some or all of your investment.
Although MURF has conducted due diligence on Conduit, this diligence may not surface all material issues that may be present with Conduit’s business. Factors outside of Conduit’s and MURF’s control may arise at any time. As a result of these factors, New Conduit may be forced to later write-down or write-off assets, restructure its operations, or incur impairment or other charges that could result in New Conduit reporting losses. Even if MURF’s due diligence successfully identified certain risks, unexpected risks may arise, and previously known risks may materialize in a manner not consistent with MURF’s preliminary risk analysis. Even though these charges may be non-cash items and therefore not have an immediate impact on New Conduit’s liquidity, the fact that New Conduit reports charges of this nature could contribute to negative market perceptions about New Conduit or its securities. In addition, charges of this nature may cause New Conduit to be unable to obtain future financing on favorable terms, including the interest rates and fees associated with any such financing, or at all.
If the Business Combination’s benefits do not meet the expectations of investors or securities analysts, the market price of New Conduit’s securities may decline.
If the perceived benefits of the Business Combination do not meet the expectations of investors or securities analysts, the market price of MURF’s securities prior to the completion of the Business Combination may decline.
In addition, following the Business Combination, fluctuations in the trading price of New Conduit’s securities could contribute to the loss of all or part of your investment. Prior to the Business Combination, there has not been a public market for Conduit shares. Accordingly, the valuation ascribed to Conduit may not be indicative of the price that will prevail in the trading market following the Business Combination. If an active market for New Conduit’s securities develops and continues, the trading price of New Conduit’s securities following the Business Combination could be volatile and subject to wide fluctuations in response to various factors, some of which are beyond New Conduit’s control. While we are not currently aware of any litigation relating to Merger Agreement or the Business Combination, any threatened or actual litigation relating could have a material adverse effect on New Conduit’s securities.
Any of the factors listed below could have a material adverse effect on your investment in New Conduit’s securities, and New Conduit’s securities may trade at prices significantly below the price paid by you. In such circumstances, the trading price of New Conduit’s securities may not recover and may experience a further decline. Factors affecting the trading price of New Conduit’s securities may include:
| ● | actual or anticipated fluctuations in New Conduit’s financial results or the financial results of companies perceived to be similar to it; | |
| ● | changes in the market’s expectations about New Conduit’s operating results; | |
| ● | success of competitors; | |
| ● | New Conduit’s operating results failing to meet the expectation of securities analysts or investors in a particular period; | |
| ● | New Conduit’s ability to attract and retain senior management or key operating personnel, and the addition or departure of key personnel; | |
| ● | changes in financial estimates and recommendations by securities analysts concerning New Conduit or the transportation industry in general; | |
| ● | operating and share price performance of other companies that investors deem comparable to New Conduit; | |
| ● | changes in laws and regulations affecting New Conduit’s business; | |
| ● | commencement of, or involvement in, threatened or actual litigation and government investigations; | |
| ● | changes in New Conduit’s capital structure, such as future issuances of securities or the incurrence of additional debt; | |
| ● | the volume of New Conduit common stock available for public sale; | |
| ● | any change in New Conduit’s board of directors or management; | |
| ● | actions taken by New Conduit’s directors, executive officers or significant stockholders such as sales of New Conduit common stock, or the perception that such actions could occur; and | |
| ● | general economic and political conditions such as recessions, inflation, interest rates, fuel prices, international currency fluctuations and acts of war or terrorism. |
Broad market and industry factors may materially harm the market price of New Conduit’s securities irrespective of New Conduit’s operating performance. The stock markets in general have experienced price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of the particular companies affected. The trading prices and valuations of these stocks, and of New Conduit’s securities, may not be predictable. A loss of investor confidence in the market for pharmaceutical stocks or the stocks of other companies which investors perceive to be similar to New Conduit could depress the share price of New Conduit common stock regardless of New Conduit’s business, prospects, financial conditions or results of operations. A decline in the market price of New Conduit’s securities also could adversely affect New Conduit’s ability to issue additional securities and New Conduit’s ability to obtain additional financing in the future.
Fluctuations in foreign currency could have an effect on Conduit’s and New Conduit’s reported results of operations.
Conduit’s exposure to fluctuations in foreign currency rates results primarily from the translation exposure associated with the preparation of its consolidated financial statements, as well as from transaction exposure associated with transactions in currencies other than Conduit’s functional currency. While the financial statements are reported in U.S. dollars, the financial statements of Conduit are prepared using the British pound sterling as the functional currency and translated into U.S. dollars. Conduit plans to continue using the British pound sterling as its functional currency following the Business Combination. Conduit cannot accurately predict the nature or extent of future exchange rate variability of the British pound sterling or the exchange rate relative to the U.S. dollar. Foreign exchange rates are sensitive to factors beyond the Company’s control. In addition, Brexit has caused, and may continue to cause, significant volatility in currency exchange rates, especially between the U.S. dollar and the British pound sterling. These fluctuations in foreign currency exchange rates could negatively affect Conduit’s and New Conduit’s results of operations and impact reported financial results.
| 46 |
If the Business Combination does not qualify as a tax-free reorganization under Section 368(a) of the Code, U.S. Holders of Conduit shares will be required to recognize gain or loss for U.S. federal income tax purposes upon the exchange of their Conduit shares for the New Conduit common stock in the Business Combination which could result in such U.S. holder incurring substantially greater U.S. federal income tax liability as a result of the Business Combination.
In the opinion of Thompson Hine LLP, the Business Combination should qualify as a “reorganization” within the meaning of Section 368(a) of the Code. This opinion is based on the facts and representations contained in representation letters provided to Thompson Hine LLP by MURF, Merger Sub and Conduit and certain assumptions, including that the Business Combination will be completed in the manner set forth in the Merger Agreement and the Registration Statement on Form S-4 of which this proxy statement/prospectus forms a part. If any assumption or representation is or becomes inaccurate, the U.S. federal income tax consequences of the Business Combination could be adversely affected.
If the Business Combination qualifies for such tax-free treatment, U.S. Holders of Conduit shares should not recognize gain or loss upon their exchange of Conduit shares for New Conduit common stock. Neither Conduit nor MURF has requested, or intends to request, a ruling from the U.S. Internal Revenue Service (“IRS”) with respect to the U.S. federal income tax consequences of the Business Combination. A tax opinion represents the legal judgment of counsel rendering the opinion and is not binding on the IRS. Consequently, no assurance can be given that the IRS will not assert, or that a court would not sustain a position to the contrary. Accordingly, if the IRS or a court determines that the Business Combination does not qualify for such treatment and Is therefore a fully taxable transaction for U.S. federal income tax purposes, a U.S. Holder of Conduit shares would recognize gain or loss for U.S. federal income tax purposes on each share of Conduit common stock surrendered in exchange for New Conduit common stock which could result in such U.S. holder incurring substantially greater U.S. federal income tax liability as a result of the Business Combination. For a more complete discussion of the material U.S. federal income tax consequences of the Business Combination, please carefully review the information set forth in the section entitled “Material U.S. Federal Income Tax Considerations—Material Tax Considerations of the Business Combination to Holders of Conduit Capital Stock.”
Most or all of Conduit’s earnings following the merger will be taxed by the United States.
As a result of the Business Combination, Conduit will be classified as a controlled foreign corporation (“CFC”) for U.S. federal income tax purposes within the meaning of Section 957(a) of the Code. Under the applicable CFC tax rules, most or all of Conduit’s income following the Business Combination will be included as taxable income by New Conduit and therefore subject to U.S. taxation, even if the CFC has made no distributions to its shareholder.
As a result of the Business Combination, New Conduit’s tax obligations and related filings may become significantly more complex and subject to greater risk of audit or examination by taxing authorities, and outcomes resulting from such audits or examinations could adversely impact our business, prospects, financial condition and results of operations, including our after-tax profitability and financial results.
After the Business Combination, New Conduit’s operations may be subject to significant income, withholding and other tax obligations in the United States and may become subject to taxes in numerous additional state, local and non-U.S. jurisdictions with respect to our income, operations and subsidiaries related to those jurisdictions. In addition, New Conduit will have international supplier and customer relationships and may expand operations to multiple jurisdictions, including jurisdictions in which the tax laws, their interpretation or their administration may not be favorable. Additionally, future changes in tax law or regulations in any jurisdiction in which New Conduit will operate could result in changes to the taxation of New Conduit’s income and operations, which could cause our after-tax profitability to be lower than anticipated.
New Conduit’s after-tax profitability could be subject to volatility or affected by numerous factors, including (a) the availability of tax deductions, credits, exemptions, refunds (including refunds of value added taxes) and other benefits to reduce New Conduit’s tax liabilities, (b) changes in the valuation of New Conduit’s deferred tax assets and liabilities, (c) expected timing and amount of the release of any tax valuation allowances, (d) tax treatment of stock-based compensation, (e) changes in the relative amount of our earnings subject to tax in the various jurisdictions in which New Conduit operates or has subsidiaries, (f) the potential expansion of New Conduit’s business into or otherwise becoming subject to tax in additional jurisdictions, (g) changes to New Conduit’s existing intercompany structure (and any costs related thereto) and business operations, (h) the extent of New Conduit’s intercompany transactions and the extent to which taxing authorities in the relevant jurisdictions respect those intercompany transactions and (i) New Conduit’s ability to structure its operations in an efficient and competitive manner. Due to the complexity of multinational tax obligations and filings, New Conduit may have a heightened risk related to audits or examinations by U.S. federal, state, local and non-U.S. taxing authorities. Outcomes from these audits or examinations could have an adverse effect on our business, prospects, financial condition and results of operations, including our after-tax profitability and financial condition.
New Conduit’s after-tax profitability may also be adversely impacted by changes in the relevant tax laws and tax rates, treaties, regulations, administrative practices and principles, judicial decisions and interpretations thereof, in each case, possibly with retroactive effect. Additionally, the Multilateral Convention to Implement Tax Treaty Related Measures to Prevent BEPS recently entered into force among the jurisdictions that have ratified it, although the United States has not yet entered into this convention. These recent changes could negatively impact New Conduit’s taxation, especially if New Conduit expands its relationships and operations internationally.
New Conduit’s failure to timely and effectively implement controls and procedures required by Section 404(a) of the Sarbanes-Oxley Act that will be applicable to it after the Business Combination is consummated could have a material adverse effect on its business.
Conduit is currently not subject to Section 404 of the Sarbanes-Oxley Act. However, following the consummation of the Business Combination, New Conduit will be required to provide management’s attestation on internal controls commencing with New Conduit’s annual report for the year ending December 31, 2022, in accordance with applicable SEC guidance. The standards required for a public company under Section 404(a) of the Sarbanes-Oxley Act are significantly more stringent than those required of Conduit as a privately-held company. Management may not be able to effectively and timely implement controls and procedures that adequately respond to the increased regulatory compliance and reporting requirements that will be applicable after the Business Combination. If New Conduit is not able to implement the additional requirements of Section 404(a) in a timely manner or with adequate compliance, it may not be able to assess whether its internal controls over financial reporting are effective, which may subject it to adverse regulatory consequences and could harm investor confidence and the market price of its securities.
| 47 |
New Conduit will qualify as an “emerging growth company” within the meaning of the Securities Act as of the closing of the Business Combination, and if it takes advantage of certain exemptions from disclosure requirements available to emerging growth companies, it could make New Conduit’s securities less attractive to investors and may make it more difficult to compare New Conduit’s performance to the performance of other public companies.
New Conduit will qualify as an “emerging growth company” as defined in Section 2(a)(19) of the Securities Act, as modified by the JOBS Act, as of the closing of the Business Combination. As such, New Conduit will be eligible for and intends to take advantage of certain exemptions from various reporting requirements applicable to other public companies that are not emerging growth companies for as long as it continues to be an emerging growth company, including, but not limited to, (a) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes- Oxley Act, (b) reduced disclosure obligations regarding executive compensation in New Conduit’s periodic reports and proxy statements and (c) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. As a result, New Conduit’s stockholders may not have access to certain information they may deem important. New Conduit will remain an emerging growth company until the earliest of (i) the last day of the fiscal year in which the market value of New Conduit common stock held by non-affiliates exceeds $700 million as of June 30 of that fiscal year, (ii) the last day of the fiscal year in which it has total annual gross revenue of $1.07 billion or more during such fiscal year (as indexed for inflation), (iii) the date on which it has issued more than $1 billion in non-convertible debt in the prior three-year period or (iv) the last day of the fiscal year following the fifth anniversary of the date of the first sale of MURF common stock in the IPO. We cannot predict whether investors will find New Conduit’s securities less attractive because it will rely on these exemptions. If some investors find New Conduit’s securities less attractive as a result of its reliance on these exemptions, the trading prices of New Conduit’s securities may be lower than they otherwise would be, there may be a less active trading market for New Conduit’s securities and the trading prices of New Conduit’s securities may be more volatile.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non- emerging growth companies but any such an election to opt out is irrevocable. We have elected not to opt out of such extended transition period, which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of New Conduit’s financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
The unaudited pro forma condensed financial information included herein may not be indicative of what New Conduit’s actual financial position or results of operations would have been.
The unaudited pro forma condensed financial information included herein is presented for illustrative purposes only and is not necessarily indicative of what New Conduit’s actual financial position or results of operations would have been had the Business Combination been completed on the dates indicated.
Following the consummation of the Business Combination, New Conduit will incur significant increased expenses and administrative burdens as a public company, which could have an adverse effect on its business, financial condition and results of operations.
Following the consummation of the Business Combination, New Conduit will face increased legal, accounting, administrative and other costs and expenses as a public company that Conduit does not incur as a private company. The Sarbanes-Oxley Act of 2002 or the Sarbanes-Oxley Act, including the requirements of Section 404, to the extent applicable to New Conduit, as well as rules and regulations subsequently implemented by the SEC, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and the rules and regulations promulgated and to be promulgated thereunder, the PCAOB and the securities exchanges, impose additional reporting and other obligations on public companies. Compliance with public company requirements will increase costs and make certain activities more time-consuming. A number of those requirements will require that we carry out activities Conduit has not done previously. For example, New Conduit will create new board committees and adopt new internal controls and disclosure controls and procedures. In addition, expenses associated with SEC reporting requirements will be incurred. Furthermore, if any issues in complying with those requirements are identified (for example, if New Conduit identifies a material weakness or significant deficiency in the internal control over financial reporting), we could incur additional costs rectifying those issues, and the existence of those issues could adversely affect our reputation or investor perceptions of it. It may also be more expensive to obtain director and officer liability insurance. Risks associated with our status as a public company may make it more difficult to attract and retain qualified persons to serve on our board of directors or as executive officers. The additional reporting and other obligations imposed by these rules and regulations will increase legal and financial compliance costs and the costs of related legal, accounting and administrative activities. These increased costs will require us to divert a significant amount of money that could otherwise be used to expand the business and achieve strategic objectives. Advocacy efforts by stockholders and third parties may also prompt additional changes in governance and reporting requirements, which could further increase costs.
| 48 |
New Conduit may issue additional shares of common stock or preferred shares under an employee incentive plan upon or after consummation of the Business Combination, which would dilute the interest of our stockholders.
New Conduit may issue a substantial number of additional shares of common or preferred stock under an employee incentive plan after consummation of the Business Combination (although our current amended and restated certificate of incorporation provides that we may not issue securities that can vote with common stockholders on matters related to our pre-initial business combination activity). The issuance of additional shares of common or preferred stock:
| ● | may significantly dilute the equity interest of investors; | |
| ● | may subordinate the rights of holders of common stock if preferred stock is issued with rights senior to those afforded our common stock; | |
| ● | could cause a change of control if a substantial number of shares of our common stock are issued, which may affect, among other things, our ability to use our net operating loss carry forwards, if any, and could result in the resignation or removal of our present officers and directors; and | |
| ● | may adversely affect prevailing market prices for New Conduit common stock. |
Our certificate of incorporation provides, subject to limited exceptions, that the Court of Chancery of the State of Delaware will be the sole and exclusive forum for certain stockholder litigation matters, which could limit our stockholders’ ability to obtain a chosen judicial forum for disputes with us or our directors, officers, employees or stockholders.
Our certificate of incorporation requires to the fullest extent permitted by law, that derivative actions brought in our name, actions against directors, officers and employees for breach of fiduciary duty and other similar actions may be brought in the Court of Chancery in the State of Delaware or, if that court lacks subject matter jurisdiction, another federal or state court situated in the State of Delaware. Any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock shall be deemed to have notice of and consented to the forum provisions in our certificate of incorporation. In addition, our certificate of incorporation and Bylaws provide that the federal district courts of the United States shall be the exclusive forum for the resolution of any complaint asserting a cause of action under the Securities Act and the Exchange Act. Neither the exclusive forum provisions nor the federal securities laws (and the rules and regulations thereunder) may be waived by a stockholder.
In March 2020, the Delaware Supreme Court issued a decision in Salzburg et al. v. Sciabacucchi, which found that an exclusive forum provision providing for claims under the Securities Act to be brought in federal court is facially valid under Delaware law. It is unclear whether this decision will be appealed, or what the final outcome of this case will be. We intend to enforce this provision, but we do not know whether courts in other jurisdictions will agree with this decision or enforce it.
This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum of its choosing for disputes with us or any of our directors, officers, other employees or stockholders, which may discourage lawsuits with respect to such claims and, if a stockholder were to bring such a claim, the choice of forum provision may result in the stockholder incurring increased costs in connection with bring such a claim as such stockholder will be required to bring the claim in the state or federal courts located in the State of Delaware. Alternatively, if a court were to find the choice of forum provision contained in our certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm its business, operating results and financial condition.
Charter documents and Delaware law could prevent a takeover that stockholders consider favorable and could also reduce the market price of New Conduit common stock.
Our certificate of incorporation and Bylaws contain provisions that could delay or prevent a change in control of New Conduit. These provisions could also make it more difficult for stockholders to elect directors and take other corporate actions. These provisions include:
| ● | authorizing our board of directors to issue preferred stock with voting or other rights or preferences that could discourage a takeover attempt or delay changes in control; | |
| ● | prohibiting cumulative voting in the election of directors; | |
| ● | providing that vacancies on our board of directors may be filled only by a majority of directors then in office, even though less than a quorum; | |
| ● | prohibiting stockholder action by written consent; | |
| ● | limiting the persons who may call special meetings of stockholders; and | |
| ● | requiring advance notification of stockholder nominations and proposals. |
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management. These and other provisions in our certificate of incorporation and Bylaws and under Delaware law could discourage potential takeover attempts, reduce the price investors might be willing to pay in the future for shares of common stock and result in the market price of common stock being lower than it would be without these provisions. For more information, see the section of this registration statement captioned “Description of New Conduit Securities — Certain Anti-Takeover Provisions of Delaware Law and MURF’s Proposed Second Amended and Restated Certificate of Incorporation.”
| 49 |
Claims for indemnification by our directors and officers may reduce our available funds to satisfy successful third-party claims against us and may reduce the amount of money available to us.
Our certificate of incorporation and Bylaws provides that we will indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law.
In addition, as permitted by Section 145 of the DGCL, our Bylaws and our indemnification agreements that we entered into with our directors and officers provide that:
| ● | We will indemnify our directors and officers for serving New Conduit in those capacities or for serving other business enterprises at our request, to the fullest extent permitted by Delaware law. Delaware law provides that a corporation may indemnify such person if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the registrant and, with respect to any criminal proceeding, had no reasonable cause to believe such person’s conduct was unlawful; | |
| ● | We may, in our discretion, indemnify employees and agents in those circumstances where indemnification is permitted by applicable law; | |
| ● | We will be required to advance expenses, as incurred, to our directors and officers in connection with defending a proceeding, except that such directors or officers shall undertake to repay such advances if it is ultimately determined that such person is not entitled to indemnification; | |
| ● | We will not be obligated pursuant to our Bylaws to indemnify a person with respect to proceedings initiated by that person against New Conduit or our other indemnitees, except with respect to proceedings authorized by our board of directors or brought to enforce a right to indemnification; | |
| ● | the rights conferred in our Bylaws are not exclusive, and we are authorized to enter into indemnification agreements with our directors, officers, employees and agents and to obtain insurance to indemnify such persons; and | |
| ● | We may not retroactively amend our amended and restated bylaw provisions to reduce our indemnification obligations to directors, officers, employees and agents. |
The future exercise of registration rights may adversely affect the market price of New Conduit common stock.
Certain of our stockholders will have registration rights for restricted securities. We are obligated to register certain securities, including (i) all of the shares of MURF common stock and Private Warrants held by the Sponsor, and MURF Class A common stock issuable upon exercise of the Public Warrants (all of which, after the closing of the Business Combination, will automatically convert into New Conduit common stock and warrants to shares of New Conduit common stock), and (ii) the shares of New Conduit common stock and New Conduit common stock underlying warrants to be issued in the Private Placement. We are obligated to (i) to file a resale registration statement to register such securities within 15 business days after the closing of the Business Combination and (ii) use reasonable best efforts to cause such registration statement to be declared effective by the SEC within 60 business days after the closing of the Business Combination. Sales of a substantial number of shares of New Conduit common stock pursuant to the resale registration statement in the public market could occur at any time the registration statement remains effective. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of New Conduit common stock.
Concentration of ownership after the Business Combination may have the effect of delaying or preventing a change in control.
It is anticipated that, following the completion of the Business Combination and assuming (for illustrative purposes) no redemptions of outstanding Public Shares, MURF’s initial stockholder, our Sponsor, will retain an ownership interest of 4,015,250 shares of New Conduit common stock or approximately 5% of New Conduit common stock and Conduit’s shareholders will own 67,700,000 shares of New Conduit common stock or approximately 90% of the New Conduit common stock, which reflects 65,000,000 shares of New Conduit common stock to be issued to the Conduit shareholders as consideration in the merger plus the 2,700,000 shares of New Conduit common stock to be issued to the Private Placement Investor in connection with the Private Placement as the Private Placement Investor is an affiliate of one of Conduit’s existing shareholders. As a result, Conduit’s equity holders may have the ability to determine the outcome of corporate actions of New Conduit requiring stockholder approval. This concentration of ownership may have the effect of delaying or preventing a change in control and might adversely affect the market price of New Conduit common stock. See the section entitled “Unaudited Pro Forma Condensed Combined Financial Information” for further information.
| 50 |
If the Private Placement fails to close and sufficient Public Stockholders exercise their redemption rights in connection with the Business Combination, we may lack sufficient funds to consummate the Business Combination.
In connection with the merger agreement, MURF entered into the Subscription Agreement with the Private Placement Investor, which provides for the purchase of an aggregate of 2,700,000 units, with each unit consisting of one share of Class A common stock together with one warrant exercisable into one share of Class A common stock, for a purchase price of $10.00 per unit, or an aggregate amount of $27 million in gross cash proceeds immediately prior to the closing of the Business Combination. Conduit is not required to consummate the Transactions if there is not at least $27 million of cash available to be released from MURF’s trust account and/or received by MURF under the Subscription Agreement after giving effect to payment of amounts that MURF will be required to pay to redeeming stockholders upon consummation of the Business Combination.
As the trust account only had approximately $23.3 million as of July 31, 2023 (after withholding funds for tax obligations), if the Private Placement does not close by reason of the failure by the Private Placement Investor to fund the purchase price for the Private Placement securities, we may lack sufficient funds to consummate the Business Combination. The Private Placement Investor’s obligations to purchase the Private Placement Securities are subject to fulfilment of customary closing conditions, including that the Business Combination must be consummated substantially concurrently with, and immediately following, the purchase of the Private Placement securities. In the event of any such failure to fund, or if any such condition is not satisfied and not waived, we may not be able to obtain additional funds to account for such shortfall on terms favorable to us or at all. While the Private Placement Investor has informed us that it has sufficient funds to satisfy its obligations under the Subscription Agreement, we have not obligated the Private Placement Investor to reserve funds for such obligations. Further, the Private Placement Investor is based in a foreign jurisdiction and it is uncertain to what extent we can successfully enforce such investor’s commitments in the Private Placement in such foreign jurisdiction.
If securities or industry analysts do not publish research or reports about our business or publish negative reports about our business, our share price and trading volume could decline.
The trading market for New Conduit common stock will depend on the research and reports that securities or industry analysts publish about New Conduit or its business. Currently, New Conduit does not have any analyst coverage and may not obtain analyst coverage in the future. In the event New Conduit obtains analyst coverage, it will not have any control over such analysts. If one or more of the analysts who cover New Conduit downgrade New Conduit common stock or change their opinion of such shares, the share price of New Conduit common stock would likely decline. If one or more of these analysts cease coverage of New Conduit or fail to regularly publish reports on New Conduit, we could lose visibility in the financial markets, which could cause the share price or trading volume of New Conduit common stock to decline.
New Conduit’s ability to pay dividends in the future will be subject to its subsidiaries’ ability to distribute cash to it.
We do not anticipate that New Conduit’s board of directors will declare dividends in the foreseeable future. If New Conduit decides to declare dividends in the future, as a holding company, it will require dividends and other payments from its subsidiaries to meet such cash requirements. In addition, minimum capital requirements may indirectly restrict the amount of dividends paid upstream, and repatriations of cash from New Conduit’s subsidiaries may be subject to withholding, income and other taxes in various applicable jurisdictions. If New Conduit’s subsidiaries are unable to distribute cash to it and it is unable to pay dividends, New Conduit common stock may become less attractive to investors and the price of its shares of common stock may become volatile.
New Conduit, as a combined company, will incur increased costs and obligations as a result of being a public company.
As a privately held company, Conduit has not been required to comply with certain corporate governance and financial reporting practices and policies required of a publicly traded company. As a publicly traded company, New Conduit will incur significant legal, accounting and other expenses that we were not required to incur in the recent past, particularly after we are no longer an “emerging growth company” as defined under the JOBS Act. In addition, new and changing laws, regulations and standards relating to corporate governance and public disclosure, including the Dodd Frank Wall Street Reform and Consumer Protection Act and the rules and regulations promulgated and to be promulgated thereunder, as well as under the Sarbanes-Oxley Act, the JOBS Act, and the rules and regulations of the SEC and national securities exchanges have created uncertainty for public companies and increased the costs and the time that New Conduit’s board of directors and management must devote to complying with these rules and regulations. We expect these rules and regulations to increase our legal and financial compliance costs and lead to a diversion of management time and attention from revenue generating activities.
Furthermore, the need to establish the corporate infrastructure demanded of a public company may divert management’s attention from implementing our growth strategy, which could prevent us from improving our business, results of operations and financial condition. We have made, and will continue to make, changes to our internal controls and procedures for financial reporting and accounting systems to meet our reporting obligations as a publicly traded company. However, the measures we take may not be sufficient to satisfy our obligations as a publicly traded company.
For as long as we remain an “emerging growth company” as defined in the JOBS Act, we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies.” We may remain an “emerging growth company” until the earliest of (i) the last day of our fiscal year following February 7, 2027 (the fifth anniversary of the consummation of MURF’s initial public offering), (ii) the last day of the fiscal year in which the market value of our shares of common stock that are held by non-affiliates exceeds $700 million as of June 30 of that fiscal year, (iii) the last day of the fiscal year in which we have total annual gross revenue of $1.07 billion or more during such fiscal year (as indexed for inflation) or (iv) the date on which we have issued more than $1.0 billion in non-convertible debt in the prior three-year period. Further, there is no guarantee that the exemptions available to us under the JOBS Act will result in significant savings. To the extent we choose not to use exemptions from various reporting requirements under the JOBS Act, we will incur additional compliance costs, which may impact earnings.
| 51 |
As an “emerging growth company,” we cannot be certain if the reduced disclosure requirements applicable to “emerging growth companies” will make our shares of common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to obtain an assessment of the effectiveness of our internal controls over financial reporting from our independent registered public accounting firm pursuant to Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of these accounting standards until they would otherwise apply to private companies. MURF has elected not to opt out of such extended transition period. We cannot predict if investors will find our shares of common stock less attractive because we will rely on these exemptions. If some investors find our shares of common stock less attractive as a result, there may be a less active market for our shares of common stock and our share price may be more volatile.
If we do not develop and implement all required accounting practices and policies, we may be unable to provide the financial information required of a U.S. publicly traded company in a timely and reliable manner.
If we fail to develop and maintain effective internal controls and procedures and disclosure procedures and controls, we may be unable to provide financial information and required SEC reports that a U.S. publicly traded company is required to provide in a timely and reliable fashion. Any such delays or deficiencies could penalize us, including by limiting our ability to obtain financing, either in the public capital markets or from private sources and hurt our reputation and could thereby impede our ability to implement our growth strategy. In addition, any such delays or deficiencies could result in our failure to meet the requirements for listing of our shares of common stock on a national securities exchange.
If MURF’s stockholders fail to properly demand redemption rights, they will not be entitled to redeem their shares of New Conduit common stock into a pro rata portion of the trust account.
MURF stockholders holding Public Shares may demand that MURF redeem their shares into a pro rata portion of the trust account, calculated as of two business days prior to the anticipated consummation of the Business Combination. MURF stockholders who seek to exercise this conversion right must deliver their stock (either physically or electronically) to MURF’s transfer agent prior to the vote at the meeting. MURF’S transfer agent must receive instructions to redeem by 5:00 pm Eastern Time on September 5, 2023, in order for such redemption demands to be valid. Any MURF stockholder who fails to properly demand redemption rights will not be entitled to redeem his or her shares into a pro rata portion of the trust account for redemption of his shares. See the section entitled “Special Meeting of MURF Stockholders — Redemption Rights” for the procedures to be followed if you wish to redeem your shares for cash.
The Sponsor and MURF’s officers and directors own shares of MURF common stock and warrants that will be worthless and the Sponsor has made loans that may not be reimbursed or repaid if the Business Combination is not approved. Such interests may have influenced their decision to approve the Business Combination with Conduit.
The Sponsor and MURF’s officers and directors and/or their affiliates beneficially own or have a pecuniary interest in Private Shares and Private Warrants that they purchased prior to, or simultaneously with, MURF’s initial public offering. The holders have no redemption rights with respect to these securities in the event a business combination is not effected in the required time period. Therefore, if the Business Combination with Conduit, or another business combination, is not approved within the required time period, such securities held by such persons will be worthless. Such shares had an estimated aggregate market value of $43,769,495 based upon the closing price of $10.78 per Public Share on Nasdaq on July 31, 2023. Such warrants had an estimated aggregate market value of $8,128,120 based upon the closing price of $10.78 per Public Share on Nasdaq on July 31, 2023. See the section entitled “The Business Combination Proposal — Interests of MURF’s Directors and Officers in the Business Combination.”
These financial interests may have influenced the decision of MURF’s directors to approve the Business Combination with Conduit and to continue to pursue such Business Combination. In considering the recommendations of the MURF Board to vote for the business combination proposal and other proposals, its stockholders should consider these interests.
| 52 |
The exercise of MURF’s directors’ and officers’ discretion in agreeing to changes or waivers in the terms of the Business Combination may result in a conflict of interest when determining whether such changes to the terms of the Business Combination or waivers of conditions are appropriate and in MURF’s stockholders’ best interest.
In the period leading up to the closing of the Business Combination, events may occur that, pursuant to the Merger Agreement, would require MURF to agree to amend the Merger Agreement, to consent to certain actions taken by Conduit or to waive rights that MURF is entitled to under the Merger Agreement. Such events could arise because of changes in the course of Conduit’s business, a request by Conduit to undertake actions that would otherwise be prohibited by the terms of the Merger Agreement or the occurrence of other events that would have a material adverse effect on Conduit’s business and would entitle MURF to terminate the Merger Agreement. In any of such circumstances, it would be at MURF’s discretion, acting through its board of directors, to grant its consent or waive those rights. The existence of the financial and personal interests of the directors described in the preceding risk factors may result in a conflict of interest on the part of one or more of the directors between what he or they may believe is best for MURF and what he or they may believe is best for themselves in determining whether or not to take the requested action. As of the date of this proxy statement/prospectus, MURF does not believe there will be any material changes or waivers that MURF’s directors and officers would be likely to make after the mailing of this proxy statement/prospectus. MURF will circulate a new or amended proxy statement/prospectus if changes to the terms of the Transactions that would have a material impact on its stockholders are required prior to the vote on the business combination proposal.
Conduit intends to enter into an employment agreement with MURF’s Chief Financial Officer and director, Adam Sragovicz, and the prospect of such future employment may have created a conflict of interest for Mr. Sragovicz in determining whether the Business Combination is in MURF and its stockholders’ best interests.
Conduit anticipates entering into an employment agreement with Mr. Sragovicz, which will provide that Mr. Sragovicz will serve as New Conduit’s Chief Financial Officer upon the closing of the Business Combination. Pursuant to the terms of an employment agreement to be entered into between New Conduit and Mr. Sragovicz in connection with the closing of the Business Combination, Mr. Sragovicz is entitled to compensation consisting of (i) an annual base salary of $400,000, and (ii) a target annual bonus opportunity equal to 40% of his base salary, payable based on the achievement of performance objectives as determined by the New Conduit board of directors. In addition, the employment agreement provides that he will be entitled to receive a sign-on restricted stock unit award covering 0.10% of the shares of common stock of New Conduit pursuant to the terms of the 2023 Stock Incentive Plan, which shall vest in equal annual installments on the first three anniversaries of the Business Combination. In the future, Mr. Sragovicz could receive any additional cash compensation, stock options, stock awards or other benefits that New Conduit’s board of directors determines to pay to Mr. Sragovicz.
While MURF and Conduit were negotiating the terms for the Merger Agreement, they and Mr. Sragovicz also discussed the prospect of Mr. Sragovicz’s employment as the Chief Financial Officer of New Conduit, and therefore, Mr. Sragovicz’s personal and financial interests may have influenced his motivation in identifying, selecting and approving a target business for MURF. However, Mr. Sragovicz’s ability to remain with the combined company upon completion of the Business Combination was not a determining factor in his or MURF’s evaluation of the Business Combination.
A.G.P./Alliance Global Partners acted as a financial advisor to both MURF and Conduit, which potential conflict of interest could affect its advice to both parties.
A.G.P./Alliance Global Partners (“A.G.P.”) is a financial advisor to both MURF and Conduit in connection with the Business Combination transaction. Upon the closing of the Business Combination, A.G.P. is entitled to receive (i) a cash fee of $6,500,000, 1,300,000 shares of New Conduit common stock and warrants to purchase 54,000 shares of New Conduit common stock at an exercise price of $11.00 per share pursuant to its engagement agreement with Conduit entered into on August 2, 2022 and (ii) $4,628,750 of deferred underwriting fees as a result of its engagement for MURF’s initial public offering. Upon learning of Conduit’s engagement of A.G.P. and the potential conflict of interest created by such engagement, MURF took steps to mitigate any potential conflict, as further described in the section titled “Background of the Business Combination” beginning on page 72, including conducting all substantive negotiations through MURF management and MURF counsel without the substantive participation of A.G.P. and engaging an independent financial advisor to provide a fairness opinion for the Business Combination. The lead responsible individual that advises MURF in connection with the Business Combination is not the same lead responsible individual that advises Conduit in connection with the Business Combination. There can be no assurance that the fact that A.G.P. acted as the financial advisor to both parties to the Business Combination did not impact the advice that A.G.P. delivered to either or both parties, or that certain terms of the Merger were not impacted by the potential conflict of interest.
If MURF is unable to complete the Business Combination with Conduit, or another business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), MURF will cease all operations except for the purpose of winding up, redeeming 100% of the outstanding Public Shares and, subject to the approval of its remaining stockholders and its board of directors, dissolving and liquidating. In such event, third parties may bring claims against MURF and, as a result, the proceeds held in the trust account could be reduced and the per-share liquidation price received by stockholders could be less than $10.67 per Public Share.
Under the terms of MURF’s amended and restated certificate of incorporation, MURF must complete the Business Combination with Conduit, or another business combination, by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), or MURF must cease all operations except for the purpose of winding up, redeeming 100% of the outstanding Public Shares and, subject to the approval of its remaining stockholders and its board of directors, dissolving and liquidating. In such event, third parties may bring claims against MURF. Although MURF has obtained waiver agreements from the prospective target businesses it has negotiated with, whereby such parties have waived any right, title, interest or claim of any kind they may have in or to any monies held in the trust account, there is no guarantee that they or other vendors who did not execute such waivers will not seek recourse against the trust account notwithstanding such agreements. Furthermore, there is no guarantee that a court will uphold the validity of such agreements. Accordingly, the proceeds held in the trust account could be subject to claims which could take priority over those of MURF’s Public Stockholders. Therefore, the per-share distribution from the trust account in such a situation may be less than $10.67 due to such claims.
Additionally, if MURF is forced to file a bankruptcy case or an involuntary bankruptcy case is filed against it which is not dismissed, or if MURF otherwise enters compulsory or court supervised liquidation, the proceeds held in the trust account could be subject to applicable bankruptcy law, and may be included in its bankruptcy estate and subject to the claims of third parties with priority over the claims of its stockholders. To the extent any bankruptcy claims deplete the trust account, MURF may not be able to return to its Public Stockholders at least $10.67 per Public Share.
| 53 |
MURF’s Sponsor has agreed to vote in favor of the Business Combination, regardless of how our public stockholders’ vote.
Our Sponsor has agreed to vote its shares in favor of the Business Combination. The Sponsor owns approximately 65% of the outstanding shares of MURF common stock prior to the Business Combination. Accordingly, it is more likely that the necessary stockholder approval for the Business Combination will be received than would be the case if our Sponsor had agreed to vote its shares in accordance with the majority of the votes cast by our public stockholders.
The Sponsor and the directors and officers of MURF have agreed to vote in favor of MURF’s initial business combination, regardless of how Public Stockholders vote and, because the Sponsor owns approximately 65% of MURF common stock, Sponsor’s vote on most proposals will be determinative.
Unlike many other blank check companies in which the founders agree to vote their founder shares in accordance with the majority of the votes cast by the public stockholders in connection with an initial business combination, the Sponsor and the directors and officers of MURF have agreed to vote their shares of MURF common stock, including Sponsor’s Class A common stock purchased in connection with MURF’s initial public offering and its Founder Shares, in favor of the initial business combination of MURF. Our Sponsor currently owns 754,000 shares of Class A common stock and 3,306,250 shares of Class B common stock, which together represents 64.99% of the 6,247,978 issued and outstanding shares of MURF common stock. None of MURF’s directors and officers own any shares of Class A common stock or Class B common stock. Accordingly, it is likely that the necessary stockholder approval will be received than would be the case if MURF’s Sponsor, directors and officers agreed to vote their MURF common stock in accordance with the majority of the votes cast by its public stockholders on those proposals where both Class A common stock and Class B common stock vote as a single class.
However, because the Sponsor owns 754,000 shares of Class A common stock, which represents approximately 25% of the 2,941,728 issued and outstanding shares of Class A common stock, the vote of the Public Stockholders will determine the outcome of those proposals where each of Class A common stock and Class B common stock will vote separately. Those proposals are (i) the Charter Amendment Proposal B, which will remove the designations of Class A and Class B by reclassifying each outstanding share of Class A common stock and Class B common stock into a share of common stock and (ii) the Charter Amendment Proposal D, which will increase the number of (newly) authorized shares of common stock to 250,000,000. See the section of this proxy statement/prospectus titled “Charter Amendments Proposals.”
The Sponsor, MURF’s officers and directors, Conduit or Conduit’s shareholders and/or their respective affiliates may elect to purchase shares from holders of MURF public shares in connection with the Business Combination, which may influence the vote on the Business Combination or could have a depressive effect on MURF public shares.
At any time prior to the Special Meeting, during a period when they are not then aware of any material nonpublic information regarding MURF or its securities, the Sponsor, MURF’s officers and directors, Conduit or Conduit’s shareholders and/or their respective affiliates may purchase shares from institutional and other investors who vote, or indicate an intention to vote, against the business combination proposal, or execute agreements to purchase such shares from such investors in the future, or they may enter into transactions with such investors and others to provide them with incentives to acquire shares of MURF common stock or vote their shares in favor of the business combination proposal. The purpose of such share purchases and other transactions could be to increase the likelihood of MURF obtaining sufficient proxies to vote in favor of the proposals set forth herein where it appears that such proposal would otherwise not be approved.
In addition, entering into any such arrangements may have a depressive effect on MURF common stock. For example, as a result of these arrangements, an investor or holder may have the ability to effectively purchase shares at a price lower than market value and may therefore be more likely to sell shares, either prior to or immediately after the Special Meeting.
As of the date of this proxy statement/prospectus, no agreements with respect to the private purchase of public shares by the persons described above have been entered into with any such investor or holder. In the event of any such newly purchases shares (i) the Sponsor or its affiliates will purchase the MURF common stock at a price no higher than the price offered through the redemption process; (ii) any such purchases by Sponsor or its affiliates will not be voted in favor of approving the Business Combination; and (iii) the Sponsor and its affiliates have waived their redemption rights to such shares.
Prior to the special meeting to approve the Business Combination, MURF will disclose in a Form 8-K (i) the amount of public shares purchased outside of the redemption offer by the Sponsor or its affiliates, along with the purchase price; (ii) the purpose of the purchases by the Sponsor or its affiliates; (iii) the impact, if any, of the purchases by the Sponsor or its affiliates on the likelihood that the Business Combination transaction will be approved; (iv) the identities of stockholders who sold to the Sponsor or its affiliates (if not purchased on the open market) or the nature of stockholders (e.g., 5% security holders) who sold to the Sponsor or its affiliates; and (v) the number of shares of MURF common stock for which MURF has received redemption requests pursuant to its redemption offer.
Risks Related to the Redemption
There is no guarantee that a stockholder’s decision whether to redeem their shares for a pro rata portion of the trust account will put the stockholder in a better future economic position.
MURF can give no assurance as to the price at which a stockholder may be able to sell its Public Shares in the future following the completion of the Business Combination or any alternative business combination. Certain events following the consummation of any initial business combination, including the Business Combination, may cause an increase in MURF’s share price, and may result in a lower value realized now than a stockholder of MURF might realize in the future had the stockholder redeemed their shares. Similarly, if a stockholder does not redeem their shares, the stockholder will bear the risk of ownership of the Public Shares after the consummation of any initial business combination, including the Business Combination, and there can be no assurance that a stockholder can sell its shares in the future for a greater amount than the redemption price set forth in this proxy statement/prospectus. A stockholder should consult the stockholder’s own tax and/or financial advisor for assistance on how this may affect his, her or its individual situation.
| 54 |
Under certain circumstances a redemption of a MURF stockholder’s Public Shares may be treated as a corporate distribution subject to tax at regular ordinary income tax rates instead of the preferential tax rates that apply to qualified dividend income.
In the event that a MURF stockholder’s Public Shares are redeemed pursuant to the redemption provisions described in this proxy statement/prospectus, the treatment of the transaction for U.S. federal income tax purposes will depend on whether the redemption qualifies as a sale of the Public Shares or a corporate distribution under Section 302 of the Code based on the facts and circumstances applicable to such stockholder. If the redemption is treated as a corporate distribution, such a distribution generally will be includable in a U.S. Holder’s gross income, in accordance with such U.S. Holder’s method of accounting for U.S. federal income tax purposes, as dividend income, but only to the extent that such distributions are paid out of MURF’s current or accumulated earnings and profits as determined under U.S. federal income tax principles. Dividends will be taxable to a corporate U.S. Holder at regular rates and will generally be eligible for the dividends-received deduction if the requisite holding period is satisfied. With respect to non-corporate U.S. Holders and with certain exceptions, dividends may be “qualified dividend income,” which is taxed at the lower applicable long-term capital gain rate provided that the U.S. Holder satisfies certain holding period requirements and the U.S. Holder is not under an obligation to make related payments with respect to positions in substantially similar or related property. It is unclear whether the redemption rights with respect to the Public Shares may prevent a U.S. Holder from satisfying the applicable holding period requirements with respect to the dividends received deduction or the preferential tax rate on qualified dividend income, as the case may be, because there is no authority directly on-point with respect to whether such redemption rights diminish a U.S. Holder’s risk of loss with respect to Public Shares in a manner that suspends the holding period for such Public Shares under the applicable holding period requirements. If the holding period requirements are not satisfied, non-corporate U.S. Holders may be subject to tax on such dividends at regular ordinary income tax rates instead of the preferential rate that applies to qualified dividend income.
MURF may be subject to the Excise Tax included in the Inflation Reduction Act of 2022 in the event of a liquidation or in connection with redemptions of its Class A common stock after December 31, 2022.
Under the Inflation Reduction Act of 2022 (the “IRA”), adding Section 4501 to the Code, a domestic corporation whose stock is traded on an established securities market (a “covered corporation” under the IRA) is subject to an excise tax of 1% on repurchases (redemptions) of its stock after December 31, 2022 (the “Excise Tax”). Because MURF is a Delaware corporation and its securities trade on the Nasdaq Global Market, it is a “covered corporation” within the meaning of the IRA.
The IRA became law on August 16, 2022. On December 27, 2022, the IRS published Notice 2023-2, providing “Initial Guidance” regarding the application of the Excise Tax on repurchases of corporate stock under Section 4501. Under these authorities MURF expects the Excise Tax to be imposed on the fair market value of stock repurchased by us. Under a “netting rule”, the fair market value of stock repurchased by MURF may be reduced by the fair market value of securities issued by it in the same taxable year, with the 1% Excise Tax then imposed on the excess, if any, of the value of redemptions over the value of issuances. Therefore, issuances of stock by MURF in connection with its initial business combination transaction (including any PIPE transaction at the time of our initial business combination) will reduce the amount of the Excise Tax in connection with redemptions occurring in the same taxable year. However, a business combination may not be completed during 2023 and, even if a business combination is completed, the fair market value of the securities redeemed may exceed the fair market value of the securities issued in such a combination or otherwise. (The fair market value of securities that are redeemed is determined by the market price of the stock on the day of redemption, regardless of the actual redemption amount.)
Further, while the authorities indicate that as a general rule the Excise Tax does not apply in the event of a complete liquidation of a covered corporation, the availability of this exemption under circumstances that might surround the liquidation of a SPAC is not entirely clear.
While excise tax returns are generally filed on a quarterly basis, the IRS expects to issue regulations providing that reports of Excise Tax liability are due with the first quarterly excise tax return filed after the close of the taxable year. Except for franchise taxes and income taxes, the proceeds placed in the trust account and the interest earned thereon shall not be used to pay for possible excise tax or any other fees or taxes that may be levied on MURF pursuant to any current, pending or future rules or laws, including without limitation any excise tax due under the IRA on any redemptions or stock buybacks by MURF (except in the case of proceeds that are delivered to MURF following a business combination).
Stockholders who wish to redeem their Public Shares in connection with the Business Combination must comply with specific requirements for redemption that may make it more difficult for them to exercise their redemption rights prior to the deadline for exercising their rights.
Each Public Stockholder will have the right, regardless of whether he, she, or it is voting for or against the Business Combination or does not vote at all, to demand that MURF redeem such holder’s shares into a pro rata share of the trust account as of two business days prior to the consummation of the Business Combination. Public Stockholders who wish to redeem their shares must either (i) tender their certificates to MURF’s transfer agent physically or (ii) deliver their shares to the transfer agent electronically using the Depository Trust Company’s DWAC (Deposit/Withdrawal At Custodian) System, at the holders’ option, in each case prior to the Special Meeting. MURF’S transfer agent must receive instruction to redeem by 5:00 pm Eastern Time on September 5, 2023, in order for such redemption demands to be valid. In order to obtain a physical stock certificate, a stockholder’s broker and/or clearing broker, DTC and MURF’s transfer agent will need to act to facilitate this request. It is MURF’s understanding that stockholders should generally allot at least two weeks to obtain physical certificates from the transfer agent. However, because MURF does not have any control over this process or over the brokers or DTC, it may take significantly longer than two weeks to obtain a physical stock certificate. While MURF has been advised that it takes a short time to deliver shares through the DWAC System, MURF cannot assure you of this fact. Accordingly, if it takes longer than MURF anticipates for stockholders to deliver their shares, stockholders who wish to redeem may be unable to meet the deadline for exercising their redemption rights and thus may be unable to redeem their shares.
| 55 |
If Public Stockholders who wish to redeem their shares tender their certificates to MURF’s transfer agent physically, such redeeming stockholders may be unable to sell their securities when they wish to in the event that the Business Combination is not approved.
If Public Stockholders who wish to redeem their shares tender their certificates to MURF’s transfer agent physically and the Business Combination is not consummated, MURF will promptly return such certificates to the tendering Public Stockholders. Accordingly, investors who attempted to redeem their shares in such a circumstance will be unable to sell their securities after the failed acquisition until MURF has returned their securities to them. The market price for MURF’s shares of Class A common stock may decline during this time and you may not be able to sell your securities when you wish to, even while other stockholders that did not seek redemption may be able to sell their securities.
If third parties bring claims against MURF, the proceeds held in the trust account could be reduced and the per share redemption amount received by stockholders may be less than $10.67 per Public Share.
MURF’s placing of funds in trust may not protect those funds from third party claims against us. Although MURF will seek to have all vendors and service providers it engages and prospective target businesses with which it negotiates execute agreements waiving any right, title, interest or claim of any kind in or to any monies held in the trust account for the benefit of MURF’s Public Stockholders, they may not execute such agreements. Furthermore, even if such entities execute such agreements with us, they may seek recourse against the trust account. A court may not uphold the validity of such agreements. Accordingly, the proceeds held in trust could be subject to claims which could take priority over those of MURF’s Public Stockholders. If MURF is unable to complete a business combination and distribute the proceeds held in trust to MURF’s Public Stockholders, its Sponsor has agreed (subject to certain exceptions described elsewhere in this proxy statement/prospectus) that it will be liable to ensure that the proceeds in the trust account are not reduced below $10.15 per Public Share by the claims of target businesses or claims of vendors or other entities that are owed money by us for services rendered or contracted for or products sold to us. However, MURF has not asked its Sponsor to reserve for such indemnification obligations, nor has MURF independently verified whether its Sponsor has sufficient funds to satisfy its indemnity obligations and believe that its Sponsor’s only assets are securities of MURF. Therefore, MURF believes it is unlikely that its Sponsor will be able to satisfy its indemnification obligations if it is required to do so. As a result, the per-share distribution from the trust account may be less than $10.67, plus interest, due to such claims.
Additionally, if MURF is forced to file a bankruptcy case or an involuntary bankruptcy case is filed against us which is not dismissed, the proceeds held in the trust account could be subject to applicable bankruptcy law, and may be included in its bankruptcy estate and subject to the claims of third parties with priority over the claims of MURF stockholders. To the extent any bankruptcy claims deplete the trust account, MURF may not be able to return to Public Stockholders at least $10.67 per Public Share.
Risks If the Adjournment Proposal Is Not Approved
If the adjournment proposal is not approved, and an insufficient number of votes have been obtained to authorize the consummation of the Business Combination, the MURF Board will not have the ability to adjourn the Special Meeting to a later date in order to solicit further votes, and, therefore, the Business Combination will not be approved.
The MURF Board is seeking approval to adjourn the Special Meeting to a later date or dates if, at the Special Meeting, MURF does not have sufficient proxies to approve one or more of the other proposals. If the adjournment proposal is not approved, the MURF Board will not have the ability to adjourn the Special Meeting to a later date and, therefore, the Business Combination would not be completed.
| 56 |
SPECIAL MEETING OF murf STOCKHOLDERS
General
MURF is furnishing this proxy statement/prospectus to MURF’s stockholders as part of the solicitation of proxies by the MURF Board for use at the Special Meeting of MURF stockholders to be held on September 7, 2023, and at any adjournment or postponement thereof. This proxy statement/prospectus provides MURF stockholders with information they need to know to be able to vote or instruct their vote to be cast at the Special Meeting.
Date, Time and Place
The Special Meeting of stockholders will be held on September 7, 2023, at 10:00 a.m., Eastern Time, via live webcast. MURF stockholders may attend, vote and examine the list of MURF stockholders entitled to vote at the Special Meeting by visiting and entering the control number found on their proxy card, voting instruction form, or notice they previously received. In light of public health concerns regarding the novel coronavirus (COVID-19), the Special Meeting will be held in a virtual meeting format only. You will not be able to attend the Special Meeting physically.
Purpose of the Special Meeting
At the Special Meeting, MURF is asking holders of MURF common stock to:
| (1) | consider and vote upon a proposal to approve the business combination (the “Business Combination”) described in this proxy statement/prospectus, including the Agreement and Plan of Merger, dated as of November 8, 2022 (“Merger Agreement”), by and among MURF, Conduit Pharmaceuticals Limited, a Cayman Islands exempted company (“Conduit”) and Conduit Merger Sub, Inc., a Cayman Islands exempted company and a wholly-owned subsidiary of MURF (“Merger Sub”), which, among other things, provides for the merger of Merger Sub with and into Conduit, with Conduit surviving the merger as a wholly-owned subsidiary of MURF — we refer to this proposal as the “business combination proposal”; |
| (2) | consider and vote upon separate proposals to approve amendments to MURF’s current amended and restated certificate of incorporation to: |
| (i) | Charter Amendment Proposal A – change the name of the public entity from “Murphy Canyon Acquisition Corp.” to “Conduit Pharmaceuticals Inc.”; | |
| (ii) | Charter Amendment Proposal B – provide for one class of authorized common stock; | |
| (iii) | Charter Amendment Proposal C – delete the various provisions in MURF’s current amended and restated certificate of incorporation applicable only to special purpose acquisition corporations (such as the obligation to dissolve and liquidate if a business combination is not consummated within a certain period of time); | |
| (iv) | Charter Amendment Proposal D – increase the number of authorized shares of common stock to 250,000,000; and | |
| (v) | Charter Amendment Proposal E – fix the number of directors at seven (7), a majority of whom shall be independent directors in accordance with The Nasdaq Stock Market LLC’s requirements — we refer to these proposals collectively as the “charter amendment proposals”; |
| (3) | to consider and vote upon, on a non-binding advisory basis, certain governance provisions in the Proposed Charter, presented separately in accordance with U.S. Securities and Exchange Commission (“SEC”) requirements — we refer to this proposal as the “advisory charter amendments proposals”; |
| (4) | elect seven (7) directors who, upon consummation of the Business Combination, will be the directors of New Conduit — we refer to this proposal as the “director election proposal”; |
| (5) | consider and vote upon a proposal to approve the Conduit Pharmaceuticals Inc. 2023 Stock Incentive Plan, which is an incentive compensation plan for employees of New Conduit — we refer to this proposal as the “incentive plan proposal”; |
| (6) | consider and vote upon a proposal to approve, for purposes of complying with applicable listing rules of Nasdaq, the issuance of New Conduit common stock, par value $0.0001 per share, to the Private Placement Investor in connection with the Private Placement, the proceeds of which will be used to finance the Business Combination and related transactions and the costs and expenses incurred in connection therewith with any balance used for working capital purposes — we refer to this proposal as the “Nasdaq proposal”; and |
| (7) | consider and vote upon a proposal to adjourn the Special Meeting to a later date or dates, if necessary, to permit further solicitation and vote of proxies if MURF does not have sufficient proxies to approve one or more of the foregoing proposals — we refer to this proposal as the “adjournment proposal.” |
| 57 |
Recommendation of MURF Board of Directors
The MURF Board has unanimously determined that the business combination proposal, the charter amendment proposals, the advisory charter amendments proposals, the director election proposal, the incentive plan proposal, the Nasdaq proposal and the adjournment proposal are fair to and in the best interests of MURF and its stockholders and unanimously recommends that you vote or give instruction to vote “FOR” the business combination proposal, “FOR” each of the charter amendment proposals, “FOR” each of the advisory charter amendments proposals, “FOR” the election of all of the persons nominated by MURF’s management for election as directors, “FOR” the incentive plan proposal, “FOR” the Nasdaq proposal and “FOR” the adjournment proposal, if presented.
Record Date; Persons Entitled to Vote
MURF has fixed the close of business on August 2, 2023, as the record date for determining MURF stockholders entitled to notice of and to attend and vote at the Special Meeting. As of the close of business on August 2, 2023, there were 6,247,978 shares of MURF common stock outstanding and entitled to vote. Each share of MURF common stock is entitled to one vote per share at the Special Meeting.
Pursuant to agreements with MURF, the 4,060,250 Private Shares held by the Sponsor will be voted in favor of the business combination proposal.
Opinion of ValueScope, Inc.
Opinion of Financial Advisor to the MURF
ValueScope was retained by MURF to provide its opinion as to the fairness, from a financial point of view, to the stockholders of MURF regarding the Business Combination. On June 26, 2023, ValueScope delivered its draft opinion to the MURF Board, to the effect that, based on financial, business and operating information available to it as of June 26, 2023, the total consideration to be paid by MURF in the Business Combination is fair to the MURF stockholders from a financial perspective. The final opinion was delivered on June 27, 2023.
The summary of the opinion in this proxy statement/prospectus is qualified in its entirety by reference to the full text of the written opinion, which is included as Annex D to this proxy statement/prospectus and sets forth the procedures followed, assumptions made, qualifications and limitations on the review undertaken and other matters considered by ValueScope in preparing its opinion. However, neither ValueScope’s written opinion nor the summary of its opinion and the related analyses set forth in this proxy statement/prospectus are intended to be, and do not constitute, advice or a recommendation to any stockholder as to how such stockholder should act or vote with respect to any matter relating to the proposed Business Combination or otherwise, including, without limitation, whether any such stockholder should redeem its shares.
The opinion was addressed to the MURF Board for the use and benefit of the members of the MURF Board (in their capacities as such) in connection with its evaluation of the Business Combination. ValueScope’s opinion was just one of the several factors the MURF Board took into account in making its determination to approve the Business Combination, including those described elsewhere in this proxy statement/prospectus.
ValueScope’s opinion only addressed whether, as of the date of the opinion, the Merger shares to be issued in the Business Combination in the aggregate pursuant to the Merger Agreement was fair, from a financial point of view, to MURF. It did not address any other terms, aspects, or implications of the Business Combination, the Merger Agreement or any related or other transaction or agreement, including, without limitation, (i) other than assuming the consummation thereof, the Private Placement; (ii) the shareholder support agreement and the lock-up agreements to entered into in connection with the Merger Agreement, (iii) any term or aspect of the Business Combination that is not susceptible to financial analysis, (iv) the fairness of the Business Combination, or all or any portion of the Merger shares, to any security holders of MURF, Conduit or any other person or any creditors or other constituencies of MURF, Conduit or any other person, (v) the appropriate capital structure of MURF or Conduit or whether MURF should be issuing debt or equity securities or a combination of both in the Business Combination, (vi) any capital raising or financing transaction contemplated by MURF, including, without limitation, the Private Placement, nor (vi) the fairness of the amount or nature, or any other aspect, of any compensation or consideration payable to or received by any officers, directors, or employees of any parties to the Business Combination, or any class of such persons, relative to the Merger shares, or otherwise. ValueScope did not express any opinion as to what the value of shares of MURF’s Class A common stock, MURF’s Class B common stock or any other security of MURF actually will be when issued in the Business Combination or the prices at which shares of MURF Class A common stock, MURF Class B common stock, or any other securities of MURF or Conduit could trade, be purchased or sold at any time.
ValueScope’s opinion did not address the relative merits of the Business Combination as compared to any alternative transaction or business strategy that might have existed for MURF, or the merits of the underlying decision by the MURF Board or MURF to engage in or consummate the Business Combination. The financial and other terms of the Business Combination were determined pursuant to negotiations between the parties to the Merger Agreement and were not determined by or pursuant to any recommendation from ValueScope. In addition, ValueScope was not authorized to, and did not, solicit indications of interest from third parties regarding a potential transaction involving MURF.
ValueScope was not requested to, and did not, (a) initiate or participate in any discussions or negotiations with respect to the Merger, the securities, assets, businesses or operations of MURF, Conduit or any other party, or any alternatives to the Merger, (b) negotiate the terms of the Business Combination, or (c) advise the MURF Board, MURF or any other party with respect to alternatives to the Business Combination. ValueScope’s analyses and opinion were necessarily based upon market, economic, and other conditions as they existed on, and could be evaluated as of, the date of ValueScope’s opinion and upon certain assumptions regarding such financial, economic, market and other conditions, which were subject to unusual volatility and which, if different than assumed, could have a material impact on ValueScope’s analyses and opinion. Accordingly, although subsequent developments could arise that would otherwise affect its opinion, ValueScope did not assume any obligation to update, review, or reaffirm its opinion to MURF or any other person or otherwise to comment on or consider events occurring or coming to ValueScope’s attention after the date of its opinion.
ValueScope received a fee of $32,000 for rendering its opinion, no portion of which was contingent upon the completion of the Business Combination. In addition, MURF agreed to reimburse certain ValueScope expenses and to indemnify ValueScope and certain related parties for certain liabilities that may arise out of its engagement or the rendering of its opinion. ValueScope prepared a prior fairness opinion with a Valuation Date of September 30, 2022 which they received additional fees for.
| 58 |
In connection with its analysis, ValueScope has made such reviews, analyses, and inquiries as it deemed necessary and appropriate under the circumstances. ValueScope also took into account its assessment of general economic, market, and financial conditions, as well as its experience in business valuation in general, and with respect to similar transactions, in particular. ValueScope’s procedures, investigations, and financial analyses included, but were not limited to a review of the letter of intent entered into on July 29, 2022, between MURF and Conduit, forward-looking projections provided by Conduit, industry and market research, discussions with the management teams of MURF and the Conduit, and other documents related to the Business Combination and Conduit. ValueScope performed certain valuation analyses using generally accepted valuation and analysis techniques, including a discounted cash flow analysis. ValueScope conducted such other analyses and considered such other factors as it deemed appropriate.
ValueScope
ValueScope is a valuation advisory and consulting firm based in Southlake, Texas. As a firm, ValueScope completes over 300 projects per year, most requiring valuation opinions or conclusions of value. Additionally, ValueScope’s principals and senior staff have been retained over 100 times by federal agencies to assist in valuation matters and dispute resolutions.
ValueScope’s principals and senior staff have issued numerous fairness opinions (as well as solvency opinions) for boards of directors and company shareholders for a period of 35 years. Within the last few years ValueScope has issued multiple opinions relating to SPAC and “de-SPAC” transactions. Additionally, ValueScope has extensive experience with SPACs outside of fairness opinions and has recently conducted valuations on warrants for approximately a dozen SPACs.
Overview of Global Biotechnology Industry
ValueScope estimated the probability of each of Conduit’s indications success based on a review of academic studies, which analyzed historical data.
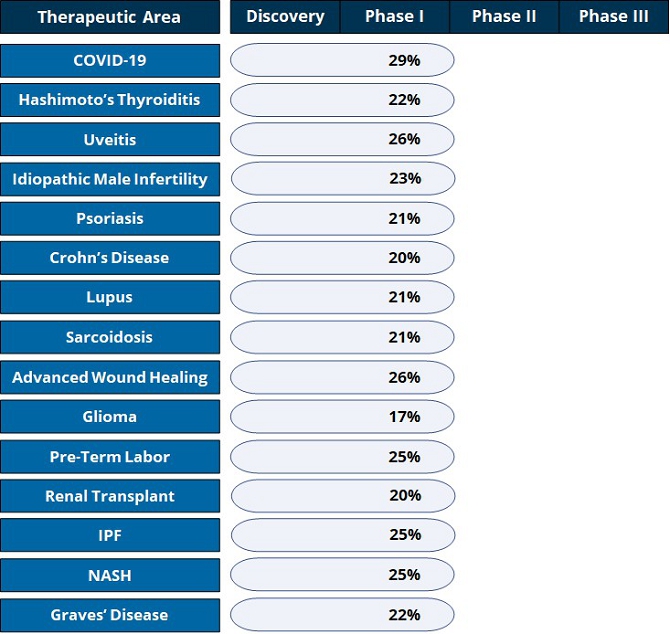
| 59 |
Base-Case probability of success figures for each phase calculated based on average of selected datapoints from two studies: Chi Heem Wong, Kien Wei Siah, Andrew W. Lo, Estimation of clinical trial success rates and related parameters, Biostatistics, Volume 20, Issue 2, April 2019, Pages 273–286
Biotechnology Innovation Organization, Informa Pharma Intelligence, QLS, Clinical Development Success Rates and Contributing Factors 2011-2020, February 2021
ValueScope considered the global biotechnology industry, which comprises a large range of companies engaged in diverse activities, such as biopharmaceutical development. Industry companies also span across a wide spectrum of operational models. Some small, dedicated biotechnology companies are research and development intensive and operate primarily with venture capital, grants, initial public offerings and collaborative agreements. Conversely, large, diversified companies hold significant in-house research and development resources and well-established production, commercialization and distribution processes. Industry revenue is expected to increase at an annualized rate of 3.1% to $358.2 billion over the next five years, with more profound growth tempered by the pandemic. Increased research and development funding and consumer spending in 2022 are expected to catalyze revenue growing 2.9% the same year. Global investor confidence has fallen during the period, which served to somewhat subdue revenue growth. However, global investment in research and development has grown strongly and consistently in recent years, with much of this funding funneled into medical biotechnology development, aimed at providing better care for the aging global population, thus bolstering industry revenue.
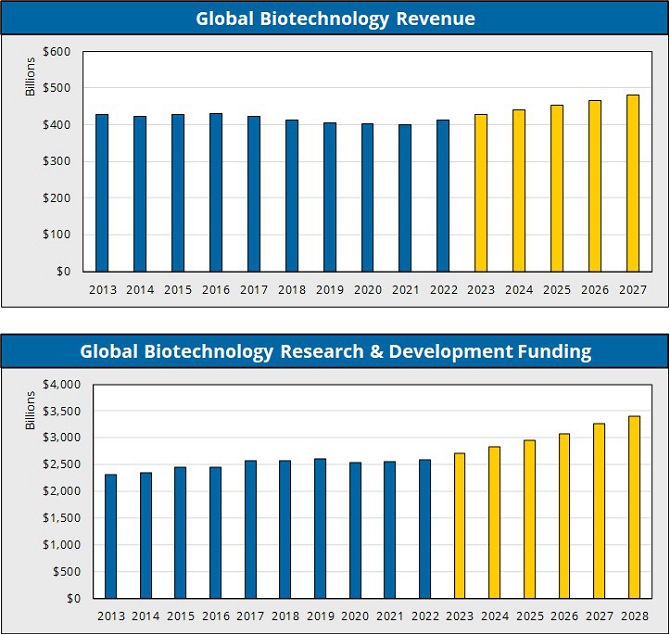
IBISWorld Industry Report L6724-GL – Global Biotechnology, May 2021
Over the previous five years, pharmaceutical companies have benefited from an aging population in developed economies and a growing middle class in emerging economies. Many companies have also tapped into regional demand for pharmaceuticals that may differ from developed markets and have expanded their global presence to tap into regional market needs. To this end, the global spread of COVID-19 (coronavirus) offers pharmaceutical and medicine manufacturers an opportunity for growth moving forward should operators successfully develop medications and vaccines to treat the virus, which several companies have successfully done.
| 60 |
Patent cliffs occur when the patent expires on a product and its market share faces a steep and rapid decline. Patent cliffs have continued to hamper industry revenue during the current period. When drugs lose patent exclusivity, the market is inundated with low-cost generic drugs. As manufacturers contend with more price-based competition from generics, many operators respond by lowering their research & development expenditures, which limits the industry’s drug pipelines. Additionally, many governments and health insurance organizations have reduced their drug reimbursements to control healthcare costs, such as implementing incentives for patients to use generic drugs.
ValueScope considered that industry revenue is estimated to grow an annualized 3.2% to $1.3 trillion over the next five years amid an anticipated persistence of global demand for industry products.
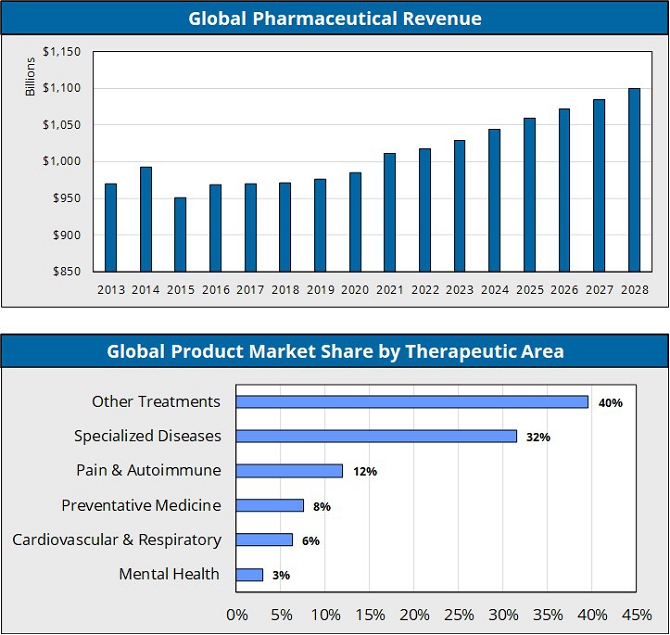
Valuation Methodology – Income Approach
The income and market approaches are most appropriate when conducting a valuation of a going-concern. Due to the stage of Conduit and reliance on future cash flows from new products, ValueScope believed the market approach would not provide a meaningful estimate of value. Therefore, ValueScope relied on the income approach to determine the intrinsic value of Conduit.
ValueScope assessed the enterprise value of the Conduit utilizing the Sum of the Parts (SOTP) approach to the application of a discounted cash flow (DCF) analysis. We determined a SOTP approach was appropriate as each product of the Conduit is subject to unique risks and projections. The DCF methodology is based on a multi-period analysis that seeks to approximate the present value of projected free cash flows. Under the SOTP approach, each product’s performance and value was forecasted on a stand-alone basis. Each therapeutic application’s revenue was forecasted using development and launch timing assumptions, market size forecasts, market share and penetration rates, and licensing royalty rates. The forecasted revenue was then adjusted based on each application’s probability of success (PoS). Each potential product’s associated development costs were forecasted as well, based on the expected development timeline. The resulting future net cash flow was then discounted to the present value, using an appropriate discount rate to arrive at stand-alone product values. Corporate overhead and operating expenses (G&A) were also projected and discounted to present value. The G&A value was netted against the sum of the valuations of each product, resulting in an enterprise valuation of the Conduit as a whole.
| 61 |
Overview of Key Assumptions and Inputs
The current development stage of each application was provided by Management. Management estimated that Phase I would take about 1 year, Phase II would take about 2 years, Phase III would take about 1 year, and approval would take an additional 1 year. ValueScope researched development timelines by Phase in the industry and determined that Management’s estimates provided by Conduit management were reasonable.
ValueScope reviewed industry and market research reports for each of the 15 applications to determine the current total addressable market sizes for each, as well as forecasted compound annual growth rates (CAGR). ValueScope forecasted the market sizes to grow at the appropriate CAGR through the market research report’s projection period. Thereafter, we forecasted the markets to grow in line with long-term inflation expectations, 2.5% per the latest Federal Reserve projections as of June 26, 2023.
ValueScope estimated peak market share percentages for each indication based on its total addressable market size, market growth rate, existing treatments available, product uniqueness, and input from Conduit and MURF. ValueScope assumed straight-line market share growth upon approval based on a 5-year to peak market penetration rate as this was consistent with industry research we reviewed. ValueScope forecasted an exclusivity cliff 5 years after the launch of each indication.
ValueScope reviewed two industry studies to determine the appropriate probabilities of success (PoS) for each application by stage. ValueScope selected the most appropriate PoS data from each study based on the therapeutic area involved and averaged the two data points to form our base-case PoS assumptions for each indication. ValueScope estimated the PoS of each indication based on the base-case assumption and the indication’s current stage.
Conduit intends to leverage its clinical assets to enter into advantageous licensing agreements with large global pharmaceutical companies. Conduit intends on licensing the products upon successful Phase II completion. The licensing agreements will provide Conduit with development success driven milestone payments and a royalty on future revenue generated by the products.
Development cost estimates were provided by Conduit per stage for each product. ValueScope distributed these expected costs per phase on an annual basis based upon its development timeline assumptions for each stage.
Conduit expects future licensing agreements to contain a royalty rate of 15.0% of revenue. ValueScope reviewed industry data and observed a range of 12.5% to 18.0% within similar licensing agreements and used a 15.0% royalty rate in its base case. The royalty rates were applied to all forecasted product revenue, excluding milestone payments.
ValueScope forecasted certain milestone payments for each product including an initial upfront payment and two additional success milestone awards based on development success. Conduit intends to license the products upon successful completion of Phase II, whereupon Conduit will receive an initial payment. The first milestone is contingent upon successful completion of Phase III trials. The second milestone is contingent upon the product being approved and successfully launched to market.
ValueScope determined milestone payments through discussions with Conduit and a review of industry milestone payments in historical licensing deals with large pharmaceutical companies. The upfront payment was estimated at $115,000,000. Milestone 1 was estimated at $30,000,000 Milestone 2 was estimated at $60,000,000. ValueScope assumed all milestones were achieved in its base case and adjusted the resulting revenue for PoS.
ValueScope reviewed industry data to determine the effect of the patent cliff on pharmaceutical products. ValueScope estimated that 70% of the product’s existing market share would be ceded within the first year to new entrants. ValueScope forecasted that 85% of existing market share would be lost within two years and 90% would be permanently lost beyond two years.
To determine an appropriate discount rate, ValueScope calculated the weighted average cost of capital (“WACC”) of Conduit as a whole. The WACC approximates the expected return required by debt and equity holders on invested capital within the industry. Equity return assumptions were determined using the capital asset pricing model (“CAPM”) and debt assumptions were based on the yield of Moody’s Baa corporate bonds as of the Valuation Date.
ValueScope selected its terminal growth rate based on a review of long-term inflation expectations. The Philadelphia Federal Reserve’s Survey of Professional Forecasters, published in May 2023, indicated a 2.5% long-term inflation forecast. As a mature product in a stable market, ValueScope believes this is an appropriate expectation of long-term performance.
| 62 |
Comparable Public Companies Selected for Beta Analysis
In order to determine the industry beta to use in the WACC calculation, ValueScope selected eighteen publicly traded companies that operate in the biopharmaceutical product development industry. ValueScope determined that these companies have similar suppliers, customers, and face similar business and economic risks relative to Conduit:
U.S. Pharmaceutical Companies:
| ● | Bristol Myers Squibb Company | |
| ● | Eli Lilly and Company | |
| ● | Pfizer Inc. | |
| ● | Merck & Co., Inc. | |
| ● | Royalty Pharma PLC | |
| ● | Vertex, Inc. |
Global Pharmaceutical Companies
| ● | AstraZeneca PLC | |
| ● | GSK PLC | |
| ● | Roche Holding Ltd (Roche) | |
| ● | Takeda Pharmaceutical Company Limited | |
| ● | Novartis AG | |
| ● | Sanofi S.A. |
Biotechnology and Therapeutics Companies
| ● | SpringWorks Therapeutics, Inc. | |
| ● | Fate Therapeutics, Inc. | |
| ● | Relay Therapeutics, Inc. | |
| ● | Nuvation Bio, Inc. | |
| ● | Replimune Group, Inc. | |
| ● | Xencor, Inc. |

Market data source: S&P Capital IQ as of June 26, 2023.
Levered beta data based on 2-year weekly performance of comparable companies relative to the S&P 500 Index.
Unlevered betas calculated as levered beta / [1 + (1 - tax rate) x (Debt / Equity)].
ValueScope believes that typically smaller companies have more risk and uncertainty than similar larger companies, and ValueScope believes that, consistent with its observations of historical returns, this greater risk and uncertainty may result in a required higher return on investment. ValueScope included a 1.21% small stock risk premium to Conduit’s cost of equity to account for the smaller size of Conduit compared to comparable publicly traded companies. The small stock risk premium was obtained from the Kroll (previously Duff & Phelps) equity risk premium referenced in the Weighted Average Cost of Capital (WACC) table on page 64.
| 63 |
Weighted Average Cost of Capital (WACC)
ValueScope conducted a sensitivity of the WACC inputs of cost of equity, cost of debt, and debt/capital ratio. ValueScope then recalculated the equity value of Conduit at a WACC of 8.30%, 1.70% below the selected WACC of 10.00% and at 11.90%, 1.90% above the selected WACC of 10.00%. The chart below shows the concluded values of Conduit at different WACC conclusions:

The “General & Administrative” line in the chart above represents the present value of forecasted corporate expenses.


Probability of Success (“PoS”)
ValueScope reviewed two studies to determine the appropriate PoS for each application by stage. ValueScope selected the most appropriate PoS data from each study based on the therapeutic area involved and averaged the two data points to form its base-case PoS assumptions for each indication. ValueScope estimated the PoS of each indication based on the base-case assumption and the indication’s current stage.

| 64 |
Chi Heem Wong, Kien Wei Siah, Andrew W. Lo, Estimation of clinical trial success rates and related parameters, Biostatistics, Volume 20, Issue 2, April 2019, Pages 273–286
Biotechnology Innovation Organization, Informa Pharma Intelligence, QLS, Clinical Development Success Rates and Contributing Factors 2011-2020, February 2021

| 65 |
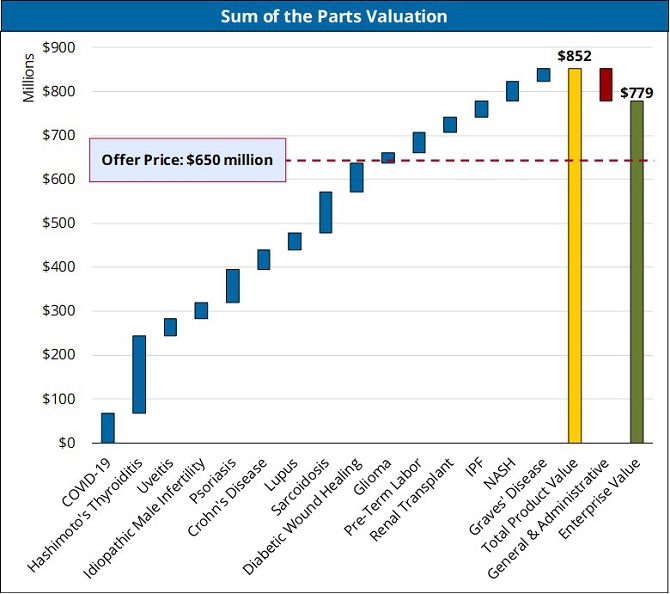
| ● | Valuation Date as of June 26, 2023 |
| ● | Stub period plus 19 years discounted cash flow model |
| ● | Base-case assumptions |
| ● | Discount rate = WACC of 10.0% |
| ● | U.S. corporate income tax rate of 21.0% assumed |
| ● | Concluded product portfolio value of $852 million |
| ● | G&A expenses projected to grow annually with inflation at 2.5% and valued at negative $73 million |
| ● | Base-case enterprise value of $779 million |
| ● | Supplementary information is presented in the appendices. |
| 66 |
To determine a reasonable range of value for Conduit, ValueScope sensitized the WACC and conducted a low case and a high case analysis. WACC has an inverse relationship with present value as a greater required return reduces the value to investors. The WACC used in the low-case was 11.9% and the WACC used in the high-case was 8.3%. Based on this analysis, ValueScope concluded that the enterprise value range of Conduit is approximately $690.8 million to $826.4 million.

| 67 |
In ValueScope’s industry researched, Phase II licensing deals with big pharma had royalty rates which ranged from approximately 12.5% to 18.0%. ValueScope used these values for our low and high cases, respectively, resulting an enterprise value range of $719.3 million to $850.9 million.

Low case values calculated solely using BIO study data. High case values calculated solely using MIT/Wong study data. This results in an enterprise value range of $498.4 million. to $1.0 billion.
Conclusion & Opinion
ValueScope’s analysis provided a fair market valuation range of Conduit of approximately $690.8 million to $826.4 million, exclusive of debt and cash. ValueScope’s base-case valuation is $779.0 million, and the offer price is $650.0 million. Based on ValueScope’s analysis, it is their opinion that the Business Combination is “fair” to shareholders of MURF from a financial perspective.
| 68 |
The above estimates are based on the assumption that Conduit would complete the Business Combination and become a publicly traded company in fiscal year 2023.
The inclusion of the above information should not be regarded as an indication that MURF, Conduit, or any of their respective financial advisors or any other recipient of this information, considered — or now considers — it to be predictive of actual future results. The information regarding projected expenses and operating losses is speculative in many respects. As a result, there can be no assurance that the actual results will not be significantly lower than estimated.
This information was not prepared with a view toward public disclosure, or with a view toward complying with the guidelines of the SEC, or the guidelines established by the American Institute of Certified Public Accountants with respect to projected financial information. This information was prepared exclusively by Conduit. Neither MURF’s nor Conduit’s independent auditors, independent accountants nor any other financial advisors of MURF or Conduit, have compiled, examined or performed any procedures with respect to the information contained in this section, nor have they expressed any opinion, representation or any other form of assurance on such information or its achievability, and assume no responsibility for, and disclaim any association with, this information. The audit reports included in this proxy statement/prospectus relate to historical financial information. They do not extend to the projected information and should not be read to do so.
The summary of the information included above is not being included to influence your decision whether to vote to approve the Business Combination. It is being provided solely because it was made available by Conduit in connection with the Business Combination.
Quorum
The presence, in person (by virtual attendance) or by proxy, of a majority of all the outstanding shares of MURF common stock entitled to vote constitutes a quorum at the Special Meeting.
Abstentions and Broker Non-Votes
Proxies that are marked “abstain” and proxies relating to “street name” shares that are returned to MURF but marked by brokers as “not voted” will be treated as shares present for purposes of determining the presence of a quorum on all matters. The latter will not be treated as shares entitled to vote on the matter as to which authority to vote is withheld from the broker. If a stockholder does not give the broker voting instructions, under applicable self-regulatory organization rules, its broker may not vote its shares on “non-routine” proposals, such as the business combination proposal, the charter amendment proposals, the advisory charter amendments proposals, and the incentive plan proposal.
Required Vote for Approval
The approval of the business combination proposal, and the advisory charter amendments proposals will require the affirmative vote for the proposal by the holders of a majority of the then outstanding shares of MURF common stock. The approval of the charter amendment proposals requires the affirmative vote of a majority of the issued and outstanding shares of each of the Class A common stock and Class B common stock, voting separately, as well as the vote of a majority of the issued and outstanding shares of Class A common stock and Class B common stock, voting together as a single class. Abstentions and broker non-votes have the same effect as a vote against the proposals.
The approval of the incentive plan proposal, the Nasdaq proposal and adjournment proposal, if presented, will require the affirmative vote of the holders of a majority of MURF common stock represented and entitled to vote thereon at the meeting. Abstentions are deemed entitled to vote on such proposals. Therefore, they have the same effect as a vote against the proposals. Broker non-votes are not deemed entitled to vote on such proposals and, therefore, they will have no effect on the vote on such proposals.
Directors are elected by a plurality. “Plurality” means that the individuals who receive the largest number of votes cast “FOR” are elected as directors. Consequently, any shares not voted “FOR” a particular nominee (whether as a result of an abstention, a direction to withhold authority or a broker non-vote) will not be counted in the nominee’s favor.
Voting Your Shares
Each share of MURF common stock that you own in your name entitles you to one vote. Your proxy card shows the number of shares of MURF common stock that you own. If your shares are held in “street name” or are in a margin or similar account, you should contact your broker to ensure that votes related to the shares you beneficially own are properly counted.
| 69 |
MURF stockholders may vote electronically at the Special Meeting by proxy or by visiting www.proxyvote.com before the meeting or www.virtualshareholdermeeting.com/MURF2023SM2 during the meeting and entering the control number found on their proxy card, voting instruction form, or notice they previously received. If you vote by proxy, you may change your vote by submitting a later dated proxy before the deadline or by voting electronically at the Special Meeting.
Revoking Your Proxy
If you are a stockholder and you give a proxy, you may revoke it at any time before it is exercised by doing any one of the following:
● you may send another proxy card with a later date;
● you may notify MURF’s Secretary in writing before the Special Meeting that you have revoked your proxy; or
● you may virtually attend the Special Meeting, revoke your proxy, and vote at the meeting, as indicated above.
Who Can Answer Your Questions About Voting Your Shares
If you are a stockholder and have any questions about how to vote or direct a vote in respect of your shares of MURF common stock, you may call D.F. King & Co., Inc., MURF’s proxy solicitor, at (800) 511-9495, or Jack K. Heilbron, MURF’s Chief Executive Officer, at (760) 471-8536.
Redemption Rights
Holders of Public Shares may seek to redeem their shares to cash, regardless of whether they vote for or against the business combination proposal or do not vote at all. Any stockholder holding Public Shares as of the record date may demand that MURF redeem such shares into a pro rata portion of the trust account (which, for illustrative purposes, was approximately $10.67 per Public Share as of August 2, 2023, the record date), calculated as of two business days prior to the anticipated consummation of the Business Combination. If a holder properly seeks redemption as described in this section and the Business Combination is consummated, MURF will redeem these shares into a pro rata portion of funds deposited in the trust account and the holder will no longer own these shares following the Business Combination.
MURF’s Sponsor will not have redemption rights with respect to any shares of MURF common stock owned by them, directly or indirectly.
Holders may demand redemption by delivering their stock, either physically or electronically using Depository Trust Company’s DWAC System, to MURF’s transfer agent prior to the vote at the meeting. MURF’S transfer agent must receive instruction to redeem by 5:00 pm Eastern Time on September 5, 2023, in order for such redemption demands to be valid. If you hold the shares in street name, you will have to coordinate with your broker to have your shares certificated or delivered electronically. Certificates that have not been tendered (either physically or electronically) in accordance with these procedures will not be redeemed for cash. There is a nominal cost associated with this tendering process and the act of certificating the shares or delivering them through the DWAC system. The transfer agent will typically charge the tendering broker $45 and it would be up to the broker whether or not to pass this cost on to the redeeming stockholder. In the event the proposed business combination is not consummated this may result in an additional cost to stockholders for the return of their shares.
Any request to redeem such shares, once made, may be withdrawn at any time up to the vote on the business combination proposal. Furthermore, if a holder of a Public Share delivered its certificate in connection with an election of its redemption and subsequently decides prior to the applicable date not to elect to exercise such rights, it may simply request that the transfer agent return the certificate (physically or electronically).
If the Business Combination is not approved or completed for any reason, then MURF’s Public Stockholders who elected to exercise their redemption rights will not be entitled to redeem their shares into a pro rata portion of the trust account, as applicable. In such case, MURF will promptly return any shares delivered by public holders. If MURF would be left with less than $5,000,001 of net tangible assets as a result of the holders of Public Shares properly demanding redemption of their shares for cash, or otherwise not be exempt from the provisions of Rule 419 promulgated under the Securities Act, MURF will not be able to consummate the Business Combination.
The closing price of MURF common stock on August 2, 2023, the record date, was $10.78. The cash held in the trust account (after withholding funds for tax obligations) on such date was approximately $23,300,000 (or $10.67 per Public Share). Prior to exercising redemption rights, stockholders should verify the market price of MURF common stock as they may receive higher proceeds from the sale of their MURF common stock in the public market than from exercising their redemption rights if the market price per share is higher than the redemption price. MURF cannot assure its stockholders that they will be able to sell their shares of MURF common stock in the open market, even if the market price per share is higher than the redemption price stated above, as there may not be sufficient liquidity in its securities when its stockholders wish to sell their shares.
If a holder of Public Shares exercises its redemption rights, then it will be exchanging its shares of MURF common stock for cash and will no longer own those shares. You will be entitled to receive cash for these shares only if you properly demand redemption no later than 5:00 p.m., Eastern Time, on September 5, 2023 (two (2) business days before the Special Meeting) by tendering your shares electronically using the Depository Trust Company’s DWAC (Deposit/Withdrawal At Custodian) System and submitting a request in writing that MURF redeem your Public Shares for cash to Vstock Transfer, LLC, our transfer agent, at the following email address:
DWAC@vstocktransfer.com
Attn: DWAC Team
MURF’S transfer agent must receive your instruction to redeem by 5:00 pm Eastern Time on September 5, 2023, in order for your redemption demand to be valid.
IN ORDER TO EXERCISE YOUR REDEMPTION RIGHT, YOU NEED TO IDENTIFY YOURSELF AS A BENEFICIAL HOLDER AND PROVIDE YOUR LEGAL NAME, PHONE NUMBER AND ADDRESS IN YOUR WRITTEN DEMAND.
| 70 |
Appraisal Rights
Neither stockholders, unitholders nor warrant holders of MURF have appraisal rights in connection with the Business Combination under the DGCL.
Proxy Solicitation Costs
MURF is soliciting proxies on behalf of the MURF Board. This solicitation is being made by mail but also may be made by telephone or in person. MURF and its directors, officers and employees may also solicit proxies in person, by telephone or by other electronic means. MURF will bear the cost of the solicitation.
MURF has hired D.F. King & Co., Inc. to assist in the proxy solicitation process. MURF will pay the firm a fee of $25,000 and a discretionary fee, if warranted in MURF’s sole discretion. In addition, MURF agreed to pay the firm additional charges for any incoming/outgoing stockholder telephone calls on a per unit basis of $5.00. Such payments will be made from non-trust account funds.
MURF will ask banks, brokers and other institutions, nominees and fiduciaries to forward the proxy materials to their principals and to obtain their authority to execute proxies and voting instructions. MURF will reimburse them for their reasonable expenses.
Sponsor
As of August 2, 2023, the record date, the Sponsor beneficially owned and was entitled to vote an aggregate of 4,060,250 Private Shares that were issued prior to or concurrently with MURF’s initial public offering. Such shares currently constitute approximately 23.49% of the outstanding shares of MURF common stock. The Sponsor has agreed to vote such Private Shares, as well as any shares of MURF common stock acquired in the aftermarket, in favor of the business combination proposal. The Sponsor also intends to vote its shares in favor of all other proposals being presented at the meeting. The Private Shares held by the Sponsor have no right to participate in any redemption distribution and will be worthless if no business combination is effected by MURF. In connection with MURF’s initial public offering, the Sponsor entered into a letter agreement containing certain lock-up provisions which provide that the Founder Shares may not be transferred (subject to limited exceptions) until the earlier to occur of: (A) six months after the completion of MURF’s initial business combination, and (B) subsequent to the initial business combination if MURF completes a liquidation, merger, stock exchange or other similar transaction that results in all of our public stockholders having the right to exchange their public shares for cash, securities or other property. Also, and notwithstanding the foregoing, if subsequent to MURF’s initial business combination the reported last sale price of its Class A common stock equals or exceeds $12.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 90 days after MURF’s initial business combination, all of the founder shares will be released from the lock-up. The Sponsor agreed not to transfer any Private Shares or MURF common stock issuable upon exercise of the Private Warrants until 30 days after completion of MURF’s initial business combination.
At any time prior to the Special Meeting, during a period when they are not then aware of any material nonpublic information regarding MURF or its securities, the Sponsor, MURF’s officers or directors, Conduit or its shareholders and/or their respective affiliates may purchase shares from institutional and other investors who vote, or indicate an intention to vote, against the business combination proposal, or execute agreements to purchase such shares from such investors in the future, or they may enter into transactions with such investors and others to provide them with incentives to acquire shares of MURF common stock or vote their shares in favor of the business combination proposal. The purpose of such share purchases and other transactions could be to increase the likelihood of satisfaction of the requirements to complete the Business Combination where it appears that such requirements would otherwise not be met. While the exact nature of any such incentives has not been determined as of the date of this proxy statement/prospectus, they might include, without limitation, arrangements to protect such investors or holders against potential loss in value of their shares, including the granting of put options and the transfer to such investors or holders of shares or rights owned by the Sponsor for nominal value.
Entering into any such arrangements may have a depressive effect on MURF common stock. For example, as a result of these arrangements, an investor or holder may have the ability to effectively purchase shares at a price lower than market value and may therefore be more likely to sell shares, either prior to or immediately after the Special Meeting.
If such transactions are effected, the consequence could be to cause the Business Combination to be approved in circumstances where such approval could not otherwise be obtained. Purchases of shares by the persons described above would allow them to exert more influence over the approval of the business combination proposal and other proposals and would likely increase the chances that such proposals would be approved.
As of the date of this proxy statement/prospectus, no agreements with respect to the private purchase of public shares by the persons described above have been entered into with any such investor or holder. In the event of any such newly purchases shares (i) the Sponsor or its affiliates will purchase the MURF common stock at a price no higher than the price offered through the redemption process; (ii) any such purchases by Sponsor or its affiliates will not be voted in favor of approving the Business Combination; and (iii) the Sponsor and its affiliates have waived their redemption rights to such shares. Prior to the special meeting to approve the Business Combination, MURF will disclose in a Form 8-K (i) the amount of public shares purchased outside of the redemption offer by the Sponsor or its affiliates, along with the purchase price; (ii) the purpose of the purchases by the Sponsor or its affiliates; (iii) the impact, if any, of the purchases by the Sponsor or its affiliates on the likelihood that the Business Combination transaction will be approved; (iv) the identities of stockholders who sold to the Sponsor or its affiliates (if not purchased on the open market) or the nature of stockholders (e.g., 5% security holders) who sold to the Sponsor or its affiliates; and (v) the number of shares of MURF common stock for which MURF has received redemption requests pursuant to its redemption offer.
| 71 |
THE BUSINESS COMBINATION PROPOSAL
The discussion in this proxy statement/prospectus of the Business Combination and the principal terms of the Merger Agreement is subject to, and is qualified in its entirety by reference to, the Merger Agreement. A copy of the Merger Agreement is attached as Annexes A-1 through A-3 to this proxy statement/prospectus.
Structure of the Transactions
The Merger Agreement provides, among other things, for Merger Sub to merge with and into Conduit, with Conduit surviving as a wholly-owned subsidiary of MURF.
Under the Merger Agreement, the shareholders of Conduit will receive the number of New Conduit common stock that shall have an aggregate value equal to Six Hundred Fifty Million Dollars ($650,000,000). With each share of New Conduit common stock being valued at $10.00, Conduit’s outstanding ordinary shares (including the ordinary shares issued upon conversion of all outstanding convertible debt, which conversion shall have occurred prior to the consummation of the Business Combination) will be converted into an aggregate of 65,000,000 shares of the common stock of New Conduit following the Business Combination, with each such outstanding Conduit ordinary share converted into shares of the common stock of New Conduit on a pro rata basis. Each outstanding share of MURF common stock immediately prior to the Business Combination shall automatically convert into and become one share of the common stock of New Conduit without any action on the part of any holders of such common stock.
Accordingly, this proxy statement/prospectus covers up to an aggregate of 65,000,000 shares of common stock of New Conduit.
In connection with the Business Combination, each outstanding share of MURF common stock, by its terms, will automatically convert into one share of common stock of New Conduit upon consummation of the Business Combination. Each outstanding warrant of MURF entitles the holder thereof to purchase shares of New Conduit common stock beginning on the later of 30 days after the consummation of a business combination and 12 months from the closing of the initial public offering.
Immediately after the closing of the Business Combination, assuming no Public Stockholder exercises its redemption rights, MURF’s initial stockholder, our Sponsor, will retain an ownership interest of 17,285,250 shares of New Conduit common stock or approximately 20% of New Conduit common stock and Conduit’s shareholders (including holders of ordinary shares issued upon conversion of all outstanding convertible debt, which conversion shall have occurred prior to the consummation of the Business Combination) will own 67,700,000 shares of New Conduit common stock or approximately 80% of the New Conduit common stock, which reflects 65,000,000 shares of New Conduit common stock to be issued to the Conduit shareholders as consideration in the merger plus the 2,700,000 shares of New Conduit common stock to be issued to the Private Placement Investor in connection with the Private Placement as the Private Placement Investor is an affiliate of one of Conduit’s existing shareholders, in each case, based on the number of shares of MURF common stock outstanding as of July 31, 2023.
Conduit Headquarters; Stock Symbols
After completion of the Business Combination:
| ● | the corporate headquarters and principal executive offices of New Conduit will be located at 4995 Murphy Canyon Road, Suite 300, San Diego, CA 92123, which is MURF’s corporate headquarters; and | |
| ● | if the parties’ application for listing is approved, New Conduit common stock and warrants will be traded on the Nasdaq Stock Market under the symbols “CDT” and “CDTTW”, respectively. |
Background of the Business Combination
MURF is a blank check company formed in order to effect a merger, share exchange, asset acquisition, stock purchase, recapitalization, reorganization or other similar business combination with one or more businesses or entities. MURF was incorporated under the laws of the State of Delaware on October 19, 2021.
On February 7, 2022, MURF closed its initial public offering. Prior to the consummation of its initial public offering, neither MURF, nor anyone on its behalf, contacted any prospective target businesses or had any substantive discussions, formal or otherwise, with respect to a transaction with MURF.
The Business Combination with Conduit is the result of an extensive search for a potential transaction and business combination utilizing the network and investing and transaction experience of MURF’s management team and the MURF Board. The terms of the Merger Agreement are the result of arm’s-length negotiations between representatives of MURF and Conduit. The following is a brief discussion of the background of these negotiations, the Merger Agreement and the Business Combination.
| 72 |
From the date of the initial public offering through the signing of the Merger Agreement with Conduit on November 8, 2022, representatives of MURF contacted and were contacted by a number of individuals and entities with respect to business combination opportunities and engaged in discussions with several possible target businesses regarding potential transactions. During that period, MURF’s officers and directors identified and met with over 100 potential target businesses from a wide range of industry segments and had in person and/or virtual and telephonic meetings with many target management teams, owners, and their representatives. MURF executed 11 non-disclosure agreements to learn about targets’ financial conditions, state of corporate governance, and business prospects in greater detail. The decision not to pursue any particular target business that MURF analyzed was generally the result of one or more of (i) MURF’s determination that such business did not have a realistic sense of its likely public market valuation, (ii) a potential target’s unwillingness to abide by public company disclosure, compliance, and governance requirements, and (iii) a potential target’s lack of team members with public market experience, including their inability to produce audited financial statements in a timely manner. This list is not considered to be exhaustive but rather to enumerate the most common reasons for MURF not pursuing a particular target. MURF conducted weekly meetings with the MURF Board to review target information and analysis, which included A.G.P., the underwriter of its initial public offering and its investment banking advisor.
On July 27, 2022, A.G.P. asked MURF if its management was available to speak with representatives of Conduit, and scheduled a call for July 29, 2022, on which day a non-disclosure agreement between MURF and Conduit was executed. The non-disclosure agreement contained customary agreements by MURF and Conduit to hold information provided by the other in confidence and use such information solely to explore their collaboration on a business combination. On July 29, 2022, Conduit conducted a management presentation for MURF and that same day began uploading diligence items to MURF’s data room.
Conduit entered into an engagement letter with A.G.P. on August 2, 2022, pursuant to which Conduit engaged A.G.P. as its exclusive financial advisor relating to the purchase or sale of all, or substantially all, of Conduit’s securities to any Nasdaq or NYSE listed vehicle introduced to Conduit by A.G.P. At the time that A.G.P. introduced Conduit to MURF, A.G.P. informed Conduit that A.G.P. had a prior relationship with MURF and its Sponsor, relating to an initial underwritten public offering of MURF. In addition, Conduit informed its legal counsel of A.G.P.’s past relationship with MURF. As exclusive financial advisor to Conduit in connection with the consummation of the Business Combination, it is anticipated that A.G.P. will be paid a cash fee of $6,500,000 and reimbursement of certain costs and expenses and will be issued 1,300,000 shares of New Conduit common stock and warrants to purchase 54,000 shares of New Conduit common stock at an exercise price of $11.00 per share.
A.G.P. does not have a written engagement letter with MURF relating to the Business Combination; however, in connection with the consummation of the Business Combination, A.G.P. will be entitled to the payment of $4,628,750 in deferred underwriting fees that it was entitled to as a result of its engagement in connection with MURF’s initial underwritten public offering. On August 17, 2022, MURF learned of the financial advisory relationship between Conduit and A.G.P. and the cash fees due to A.G.P. from Conduit. On or around April 25, 2023, the MURF Board reviewed and approved the A.G.P. additional compensation of 1,300,000 shares of New Conduit common stock and warrants to purchase 54,000 shares of New Conduit common stock at an exercise price of $11.00 per share.
Throughout the negotiation and drafting of the Merger Agreement and private placement documents and continuing through the date of this proxy statement/prospectus, MURF took steps to mitigate the risks resulting from potential conflicts of interest relating to A.G.P.’s role as an advisor to both MURF and Conduit by engaging ValueScope to prepare the fairness opinion, tasking MURF’s independent legal, tax, intellectual property and other advisors with due diligence responsibilities, ensuring that MURF’s legal counsel was present during planning and strategy calls with A.G.P., discussing with its legal counsel the reasonableness of A.G.P.’s advice, and discussing the Merger with the MURF Board on a weekly basis, relying on the MURF Board’s commercial experience in evaluating financial advice. The lead responsible individual that advises MURF in connection with the Business Combination is not the same lead responsible individual that advises Conduit in connection with the Business Combination.
Given Conduit’s prompt population of the data room, institutional support, and experienced management team, MURF proposed a letter of intent on August 1, 2022, which was fully executed by all parties on August 5, 2022, for exclusivity of negotiations between the parties until September 15, 2022. Material terms of the letter of intent provided for (i) a valuation of Conduit equal to the greater of $650 million or the valuation derived by investors in the private placement, (ii) a $50 million private placement to close concurrently with the closing of the Business Combination, and (iii) registration rights for the Sponsor’s MURF common stock and Private Warrants. Additionally, certain material terms in the original draft version of the letter of intent, including the Sponsor’s agreement to waive its anti-dilution protection with respect to its MURF common stock provided by MURF’s Amended and Restated Certificate of Incorporation, the exclusion of holders of less than 5% of Conduit’s shares from the 180 day lockup period, the Sponsor’s designation of two directors to the board of New Conduit (instead of one director as set forth in the final letter of intent), and MURF’s establishment of an equity incentive plan to be in place following the closing of the Business Combination, were not included in the final version of the letter of intent. Subsequently, the parties amended the letter of intent several times to extend the expiration date.
On August 11, 2022, MURF and Conduit, together with their respective legal, accounting, and financial advisors, held an “all hands” organizational call. Attending the call were representatives from A.G.P., MURF’s auditor Marcum LLP, MURF’s accounting advisor Brio Financial Group, MURF’s corporate counsel Sichenzia Ross Ference LLP (“SRF”), Conduit’s former auditor RSM, Conduit’s financial advisors CFGI and Conduit’s corporate counsel Thompson Hine LLP (“Thompson Hine”). At that point, MURF began more intensive due diligence, including its legal, tax, intellectual property and other advisors to review information being uploaded to the data room and shared by Conduit.
These diligence efforts included a review of the operations, corporate governance documents, regulatory and other healthcare matters, financial condition, intellectual property, material contracts, pre-clinical data, clinical study reports, investigators’ brochures, peer-reviewed and other publications, clinical development plans, a clinical study protocol and clinical study report, technology, suppliers, team members, and corporate structure of Conduit and such other customary areas of due diligence for a potential business combination. MURF’s legal advisor, Sichenzia Ross Ference LLP, and intellectual property counsel, commenced legal and intellectual property diligence on Conduit on behalf of MURF.
The initial draft of the Merger Agreement was provided by SRF to Thompson Hine on September 8, 2022 and provided for, among other things: (i) a transaction structure which required soliciting and obtaining the approval of MURF and Conduit shareholders after the execution of the merger agreement, (ii) the issuance of MURF common stock as consideration in the merger pursuant to a registration statement, (iii) a mutual closing condition in favor of both MURF and Conduit providing that MURF’s cash at closing (the funds contained in the trust account as of immediately prior to the effective time, plus all other cash and cash equivalents of MURF, minus the aggregate amount of cash proceeds that would be required to satisfy the redemption of any shares of MURF common stock pursuant to the redemption offer (to the extent not already paid), plus the cash proceeds from the private placement) would equal or exceed or $27 million, (iv) regulatory efforts and covenants requiring the parties to use reasonable best efforts to take all actions necessary in order to obtain regulatory clearance, (v) the entry into certain ancillary agreements concurrently with the execution of the merger agreement, and (vi) representations, warranties and covenants customary for transactions of this type.
Thompson Hine sent comments back on the Merger Agreement on September 22, 2022, primarily relating to Conduit’s employee benefit plans, real property, intellectual property, taxation, environmental matters, insurance and Conduit’s organizational structure.
On September 16, 2022, SRF sent a diligence request list to Conduit and Thompson Hine.
| 73 |
On September 23, 2022, MURF requested additional diligence items related to Conduit’s products, intellectual property, clinical investigation and trial record, correspondence with regulators and ongoing, planned or terminated clinical research.
The MURF Board discussed the need to obtain additional financing for the combined company at regular intervals since the introduction to Conduit, viewing Conduit as an early-stage company which will require funding to advance its assets through clinical trials.
The negotiation and marketing of the private placement was led by Conduit’s management, with minimal input from A.G.P. or MURF, other than MURF’s approval of the proposed terms. The Private Placement Investor, an owner of certain of Conduit’s outstanding convertible debt, was identified by Conduit on or around September 18, 2022. The initial terms of the private placement (a unit comprised of one share of common stock and one warrant to purchase a share of common stock at $10.00 per unit with a warrant exercise price of $11.50 per share) were proposed by A.G.P. and were intended to be substantially similar to the terms of MURF’s initial public offering. The MURF Board viewed the private placement terms as described in the term sheet favorably, as they were substantially similar to the terms of the units sold in the initial public offering, despite worsening market conditions since the initial public offering that favored investor-friendly terms, and have no repricing for future lower-priced issuances.
On September 26, 2022, MURF, Conduit and the Private Placement Investor who signed a term sheet for the private placement, setting the amount to be raised at $27 million at a purchase price of $10.00 per share together with 100% warrant coverage with the warrants having an exercise price of $11.50 per share.
On September 29, 2022, Conduit responded to SRF’s diligence questions related to investor brochures, pre-clinical pharmacology, pre-clinical safety pharmacology study reports, GLP toxicology (pre-clinical) study reports and minutes of meetings with regulatory agencies. In addition, Conduit provided the documentation requested by the due diligence request list that was sent on September 16, 2022.
On or around the first two weeks of October 2022, Mr. Sragovicz discussed remaining with MURF as Chief Financial Officer following the closing the Business Combination first with A.G.P. and then with Conduit. Mr. Sragovicz and Conduit discussed that his compensation should be commensurate with the compensation of similarly situated public companies in the same industry. On May 1, 2023, Conduit proposed compensation consisting of (i) an annual base salary of $400,000, and (ii) a target annual bonus opportunity equal to 40% of the base salary, payable based on the achievement of performance objectives as determined by the New Conduit board of directors. In addition, Conduit’s employment agreement provides that he will be entitled to receive a sign-on restricted stock unit award covering 0.10% of the shares of common stock of New Conduit pursuant to the terms of the 2023 Stock Incentive Plan, which shall vest in equal annual installments on the first three anniversaries of the Merger. The employment agreement between Conduit and Mr. Sragovicz will not be entered into until the closing of the Merger.
On October 6, 2022, MURF requested additional diligence information and circulated a revised Merger Agreement with changes primarily relating to Conduit’s convertible debt, the fairness opinion, and the Outside Date. In addition. Thompson Hine circulated drafts of the private placement subscription agreement and warrant.
On October 12, 2022, MURF, Conduit, St George Street Capital and CNF Pharma, LLC, MURF’s scientific advisors, executed a mutual non-disclosure agreement and St George Street Capital commenced providing diligence material to MURF and its advisors.
Throughout October 2022 and through early November 2022 until the signing of the Merger Agreement, MURF and its advisors conducted due diligence on Conduit and engaged in several phone calls with Conduit’s management relating to Conduit’s products, material products and intellectual property.
On October 6, 2022, Thompson Hine circulated initial drafts of the subscription agreement and warrant for the private placement, with terms substantially similar to those set forth in the September 26, 2022 term sheet.
On October 11, 2022, SRF circulated revised drafts of the subscription agreement and warrant for the private placement, with changes primarily related to descriptions of MURF’s capital structure, public information failure penalties, the Private Placement Investor’s registration rights and, in the warrant, a 4.99% ownership blocker.
On October 14, 2022, Thompson Hine circulated revised drafts of the subscription agreement and warrant for the private placement, incorporating SRF’s changes.
On October 14, 2022, Thompson Hine circulated a revised Merger Agreement with changes primarily relating to defined terms, the truthfulness of statements included in the disclosure schedules, and the timing for delivery of the fairness opinion.
On October 28, 2022, SRF circulated drafts of the each of the Sponsor Support Agreement and the Shareholder Support Agreement, as well as drafts of the Proposed Charter and bylaws for New Conduit.
On November 3, 2022, Thompson Hine circulated a revised Merger Agreement with changes primarily relating to defined terms and Conduit’s and Merger Sub’s capitalizations, and circulated revised ancillary documents.
On November 3, 2022, Thompson Hine circulated a revised subscription agreement and warrant for the private placement with minor, non-substantive changes.
On November 4, 2022, SRF circulated a revised Merger Agreement with changes primarily related to closing conditions and revised ancillary documents.
MURF and its advisors arrived at the proposed valuation for Conduit by using a discounted cash flow sum of the parts valuation. This analysis included assumptions about development timeline, addressable market size, market share and launch curve, probability of success, licensing agreements, development costs, royalty rates, milestone hurdles, milestone payments, patent cliffs, and applicable discount and terminal growth rates. MURF also considered qualitative differences, based on the experience of its management team, in the operating and financial characteristics of similar companies, as well as other factors that could affect the public trading values of the companies reviewed, to provide a context in which to consider the results of the quantitative analysis.
When compared to the pro forma enterprise value determined in accordance with MURF’s analysis, and using market information available at the time, the comparative analysis showed that Conduit’s pro forma enterprise value was reasonable.
On October 6, 2022, MURF engaged ValueScope Inc. (“ValueScope”) to evaluate the proposed transaction and render to the MURF Board an opinion as to whether the consideration to be paid or issued by MURF in the transaction is fair, from a financial point of view to MURF’s stockholders. The MURF Board determined to seek this opinion in order to obtain a view from a third party with experience in de-SPAC transactions regarding the fairness, from a financial point of view, to MURF stockholders of the consideration paid by MURF to Conduit pursuant to the Merger Agreement.
During the fall of 2022, the draft Merger Agreement and progress on due diligence was discussed at an all weekly MURF Board meetings, held virtually on each Thursdays.
| 74 |
At the November 3, 2022 meeting, the full MURF Board had a robust discussion about the transaction, valuation, closing conditions, potential conflicts of interest posed by A.G.P.’s role as a financial advisor to both parties, and by Mr. Sragovicz’s employment with New Conduit following the closing and due diligence process, which included a number of MURF’s advisors, including A.G.P., as well as representatives of ValueScope. Messrs. Benjamin Westcott, Martin Hanan, Nathan Hoelscher, and Jason Wainwright each from ValueScope provided ValueScope’s draft opinion to the MURF Board that the 65,000,000 new shares of common stock to be issued by MURF (under the new corporate name of Conduit Pharmaceuticals Inc.) in the Business Combination pursuant to the Merger Agreement was fair to MURF’s stockholders from a financial point of view. The representatives of ValueScope confirmed that ValueScope’s signed opinion would be identical to the draft opinion. After further discussion of the Merger Agreement, the Private Placement, the ValueScope fairness opinion and related documents and agreements, the MURF Board approved a motion giving MURF management authority to finalize all transaction documentation and to circulate all final documentation to the MURF Board. Following distribution of final transaction documentation later on November 3, 2022, the MURF Board executed a written consent dated November 4, 2022, approving the Private Placement, the Merger Agreement, all ancillary agreements and resolving to submit for approval of its stockholders the Merger Agreement and the transactions contemplated thereby, including the issuance of common stock of MURF necessary to consummate the Private Placement and the Business Combination. On November 4, 2022, ValueScope issued its fairness opinion, which was identical to the draft opinion.
The Merger Agreement was signed on the evening of November 8, 2022. Also on November 8, 2022, MURF and Conduit jointly issued a press release announcing the signing of the Merger Agreement, and MURF filed a Current Report on Form 8-K announcing the execution of the Merger Agreement and discussing the key terms of the Merger Agreement. On November 14, 2022, MURF filed a Current Report on Form 8-K disclosing the materials terms of the Merger Agreement and ancillary agreements, including filing such agreements as exhibits.
On or around April 20, 2023 Conduit clarified to the MURF Board that Conduit itself does not intend to fund or otherwise develop further AZD1656 for Covid-19. In the event that any third party were to fund and develop further AZD1656 for the treatment of Covid-19, Conduit would be entitled to receive compensation, if any, from those development activities through its funding agreement with St George.
In June 2023, the MURF Board commenced discussions relating to updating ValueScope’s fairness opinion to address the de-prioritization of funding and developing AZD1656 for Covid-19 and any developments in the therapeutics areas of interest to Conduit. On June 9, 2023 the MURF Board engaged ValueScope to update its fairness opinion for a fee of $32,000.
On June 16, 2023, representatives from the MURF Board and Conduit, together with SRF, Thompson Hine, MURF’s intellectual property counsel and Conduit’s intellectual property counsel, held a diligence call relating to Conduit’s co-crystals of AZD1656.
On June 26, 2023, ValueScope delivered its updated draft fairness opinion to the MURF Board. On June 27, 2023, ValueScope issued its updated fairness opinion to the MURF Board. On June 29, 2023, the full MURF Board convened to review and discuss the updated fairness opinion. The members of the MURF Board had a robust discussion about the updated valuation. Messrs. Benjamin Westcott, Martin Hanan, Nathan Hoelscher, and Jason Wainwright, each from ValueScope, provided ValueScope’s draft updated fairness opinion to the MURF Board that the 65,000,000 new shares of common stock to be issued by MURF (under the new corporate name of Conduit Pharmaceuticals Inc.) in the Business Combination pursuant to the Merger Agreement was fair to MURF’s stockholders from a financial point of view. It was noted that the comparable beta companies identified by ValueScope in its analysis, while significantly larger in size and product offerings, had similar development, regulatory, customer, and supply risks, and that ValueScope included a 1.21% small stock risk premium to Conduit’s cost of equity in its analysis. Also discussed was ValueScope’s base-case valuation of Conduit increasing to $779,000,000, from the $682,000,000 base-case valuation provided November 2022. Key reasons identified for the increase in valuation include the addition of Graves’ disease as a new therapeutic, the determination that certain projected expenses were no longer applicable, decreased projected time to future cash in-flows resulting in the present value of future cash inflows being higher, a larger total addressable market size (including for Hashimoto’s Thyroiditis and Sarcoidosis), higher probability of success for certain indications such as IPF and NASH, and additional market data for the worst-case scenario numbers. After further discussion of the updated fairness opinion, the MURF Board approved ValueScope’s updated fairness opinion and reaffirmed its earlier approval of the Business Combination, the transaction documentation and transactions contemplated thereby, including the issuance of common stock of MURF necessary to consummate the Private Placement and the Business Combination.
Recommendation of the MURF Board and Reasons for the Business Combination
The MURF management and Board, in evaluating the Business Combination, reviewed a number of materials, including the investor presentation and analyses therein, the transaction documentation, and certain due diligence summary materials prepared by MURF’s management team, and consulted with MURF’s management team and legal advisors. The investor presentation included a summary of the transaction structure as well as a description of Conduit, its clinical asset pipeline, market opportunity, and management.
In reaching its resolution (i) that the Merger Agreement and the transactions contemplated thereby are advisable and in the best interests of MURF and its shareholders and (ii) to recommend that MURF public stockholders adopt the Merger Agreement and approve the Business Combination and the transactions contemplated thereby, the MURF Board considered a range of factors, including, but not limited to, the factors discussed below.
In light of the number and wide variety of factors considered in connection with its evaluation of the Business Combination, the MURF Board did not consider it practicable to, and did not attempt to, quantify or otherwise assign relative weights to the specific factors that it considered in reaching its determination and supporting its decision. The MURF Board viewed its decision as being based on all of the information available and the factors presented to and considered by it. In addition, individual directors may have given different weight to different factors. This explanation of MURF’s reasons for the Business Combination and all other information presented in this section is forward-looking in nature and, therefore, should be read in light of the factors discussed under “Cautionary Note Regarding Forward-Looking Statements.”
In approving the Business Combination, MURF Board obtained a fairness opinion from ValueScope, Inc., which was updated upon the request of the board in connection with the de-prioritization of funding and developing AZD1656 for Covid-19 and developments in the therapeutics areas of interest to Conduit. The officers and directors of MURF have substantial experience in evaluating the operating and financial merits of companies from a wide range of industries and concluded that their experience and background enabled them to make the necessary analyses and determinations regarding the Business Combination.
In addition, MURF’s officers, directors and advisors have substantial experience with mergers and acquisitions across a variety of different industries.
In evaluating the Business Combination, the MURF Board considered criteria and guidelines to evaluate prospective business opportunities including, but not limited to these general guidelines:
| ● | Accomplished leadership; | |
| ● | Scalable platform with public company readiness; | |
| ● | Potential of value accretion via the infusion of capital/access to the capital markets; | |
| ● | Compelling underlying value proposition(s) and a compelling risk/reward proposition; | |
| ● | Attractiveness of the company’s assets to other larger companies/acquirors/licensees; | |
| ● | Addressing unmet market need; | |
| ● | Significant growth potential; | |
| ● | Competitive advantages; | |
| ● | Barriers to entry; | |
| ● | Leveraging MURF’s management team’s experiences; and | |
| ● | Other criteria. |
The criteria above are not intended to be exhaustive. Any evaluation relating to the merits of a particular initial business combination may be based, to the extent relevant, on these general guidelines as well as other considerations, factors and criteria that MURF’s management and the MURF Board deemed relevant.
| 75 |
Following a presentation from Conduit’s management team and due diligence, the MURF Board determined that Conduit meets many of the above criteria, including having:
| ● | Accomplished leadership. The MURF Board considered the fact that New Conduit’s Chair of the board of directors, Dr. Freda Lewis-Hall, previously served as Pfizer’s Chief Medical Officer and Executive Vice President, experience which the MURF Board believes will be of significant value to MURF shareholders. The MURF Board also considered the background of Dr. David Tapolczay, Conduit’s CEO, and his experience in the pharmaceutical industry as well as his tenure leading LifeArc, a United-Kingdom based charity advancing lab-based scientific discoveries to a point at which they can be developed into the next generation of diagnostics, treatments and cures. Dr. Lewis-Hall and Dr. Tapolczay were both instrumental in the successful launch of SpringWorks Therapeutics (Nasdaq: SWTX), a clinical-stage biopharmaceutical company. |
| ● | Scalable platform with public company readiness. The MURF Board considered the fact that Conduit’s leadership team has experience licensing deprioritized assets from large pharmaceutical companies and developing them through the clinical trial process, creating a platform, or “Conduit,” suited to drug development with great potential for scale. Conduit’s proposed leadership, including its proposed board of directors, has extensive public company experience. | |
| ● | Potential of value accretion via the infusion of capital/access to the capital markets. The MURF Board reviewed a list of proposed indications set forth below to be addressed by Conduit’s current assets and evaluated the estimates of Phase II investments compared to possible returns. While there can be no assurance that any of these returns will be realized, additional investments for clinical investigation are required to realize potential value, and the MURF Board believes that Conduit would be able to fund those additional investments more efficiently as a publicly traded company with capital markets access. |
| Licensed indication | Conduit Asset | |
| Idiopathic male infertility | AZD5904 | |
| Renal Transplant | AZD1656 | |
| COVID-19 | AZD1656 | |
| Uveitis | AZD1656 | |
| Premature Labor | AZD1656 | |
| Hashimoto’s Thyroiditis | AZD1656 | |
| Psoriasis | ||
| Crohn’s Disease | ||
| Lupus | ||
| Sarcoidosis | ||
| Diabetic Wound Healing | ||
| Glioma | ||
| Idiopathic Pulmonary Fibrosis (IPF) | ||
| Nonalcoholic Steatohepatitis (NASH) |
| ● | Addressing unmet market need. The MURF Board reviewed the indications for which Conduit has licensed assets, and determined that there are still significant market opportunities that are not currently being fully addressed, especially in the areas of idiopathic male infertility and uveitis. |
| ● | Barriers to entry. The MURF Board considered that Conduit has licensing rights to the above-referenced clinical-stage pharmaceuticals for significant major pharmaceutical markets. |
| ● | Financial Condition. The MURF Board also considered factors such as Conduit’s historical financial results, outlook and expansion opportunities, and financial plan. In considering those factors, the MURF Board reviewed Conduit’s historical finances and its current prospects for growth if Conduit achieves its business plan. In reviewing those factors, the MURF Board determined that Conduit has significant growth prospects. |
The MURF Board also considered a number of other factors pertaining to the Business Combination as generally supporting its decision to enter into the Merger Agreement and the transactions contemplated thereby, including, but not limited to, the following material factors:
| ● | Due Diligence. Due diligence examinations of Conduit, and discussions with Conduit’s management and MURF’s management team, business advisors and legal advisors concerning MURF’s due diligence examination of Conduit; |
| ● | Negotiated Transaction. The financial and other terms of the Merger Agreement and the fact that such terms and conditions are reasonable and were the product of arm’s-length negotiations between MURF and Conduit; |
| ● | Lock-Up. The Conduit founders and senior management of Conduit have agreed to be subject to a 180-day lock-up in respect of their Conduit shares subject to early release upon achievement of a specified price target for New Conduit shares (see “The Merger Agreement”, “Certain Agreements Related to the Business Combination” and “Description of New Conduit Securities”); |
| ● | Fairness Opinion. The financial analyses of ValueScope, reviewed with the MURF Board on November 3, 2022 and June 29, 2023, and opinions dated as of November 4, 2022 and updated as of June 26, 2023, as to the fairness, from a financial point of view, of the shares to be issued by MURF, in the aggregate, in the Business Combination pursuant to the Merger Agreement; and |
| 76 |
| ● | Other Alternatives. The MURF Board believes, after a thorough review of other business combination opportunities reasonably available to MURF, that the proposed Business Combination represents the best potential business combination for MURF and the most attractive opportunity for MURF’s management to accelerate its business plan based upon the process utilized to evaluate and assess other potential acquisition targets, and the MURF Board believes that such process has not presented a better alternative. |
The MURF Board also considered a variety of uncertainties and risks and other potentially negative factors concerning the Business Combination, including, but not limited to, the following:
| ● | Early-Stage Company and Limited Operating History. The fact that Conduit is an early-stage company with a history of losses and a limited operating history; |
| ● | Growth Initiatives May Not be Achieved. The risk that Conduit’s growth initiatives may not be fully achieved or may not be achieved within the expected timeframe; |
| ● | Intellectual Property Risk. The risk that Conduit may not have adequate intellectual property rights to carry out its business, may need to defend itself against patent, copyright, trademark, trade secret or other intellectual property infringement or misappropriation claims, and may need to enforce its intellectual property rights from unauthorized use by third parties; |
| ● | Clinical Development Risk. The risks that are associated with clinical development. Clinical development involves a lengthy and expensive process, with an uncertain outcome. Conduit may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of its product candidates; |
| ● | Regulatory Risk. The risks that are associated with Conduit operating in the highly regulated healthcare industry. Failure to comply with regulations or laws could subject Conduit to significant regulatory risk, including the risk of litigation, regulatory actions and compliance issues that could subject Conduit to significant fines, penalties, judgments, remediation costs, negative publicity and requirements resulting in increased expenses; |
| ● | Macroeconomic Risks. Macroeconomic uncertainty and the effects it could have on the Post-Combination Company’s revenues; |
| ● | Redemption Risk. The potential that a significant number of MURF public stockholders elect to redeem their shares prior to the consummation of the Business Combination and pursuant to the existing organizational documents, which would potentially make the Business Combination more difficult or impossible to complete; |
| ● | Stockholder Vote. The risk that MURF public stockholders may fail to provide the respective votes necessary to effect the Business Combination; |
| ● | Closing Conditions. The fact that the completion of the Business Combination is conditioned on the satisfaction of certain closing conditions that are not within MURF’s control; |
| ● | Litigation. The possibility of litigation challenging the Business Combination or that an adverse judgment granting permanent injunctive relief could indefinitely enjoin consummation of the Business Combination; |
| ● | Listing Risks. The challenges associated with preparing Conduit, a private entity, for the applicable disclosure and listing requirements to which New Conduit will be subject as a publicly traded company on Nasdaq; |
| ● | Benefits May Not Be Achieved. The risks that the potential benefits of the Business Combination may not be fully achieved or may not be achieved within the expected timeframe; |
| ● | Liquidation of MURF. The risks and costs to MURF if the Business Combination is not completed, including the risk of diverting management focus and resources from other business combination opportunities; |
| ● | Costs Savings and Growth Initiatives May Not be Achieved. The risk that the cost savings and growth initiatives may not be fully achieved or may not be achieved within the expected timeframe; |
| ● | MURF Public Stockholders Receiving a Minority Position in New Conduit. The risk that MURF public stockholders will hold a minority position in New Conduit; |
| ● | Evolving Regulatory Regime Governing Special Purpose Acquisition Companies. The risk that regulation of special purpose acquisition companies continues to evolve, and the SEC, Nasdaq and other regulators may revisit and update their laws, regulations and policies; and |
| 77 |
| ● | Fees and Expenses. The fees and expenses that are associated with completing the Business Combination. |
In addition to considering the factors described above, the MURF Board also considered other factors including, without limitation:
| ● | Interests of Certain Persons. Some officers and directors of MURF may have interests in the Business Combination (see “— Interests of MURF’s Directors and Officers in the Business Combination” and “Risk Factors — Risks Related to the Business Combination and New Conduit Common Stock Following the Business Combination” ); and | |
| ● | Other Risk Factors. Various other risk factors associated with the business of Conduit, as described in the section entitled “Risk Factors” appearing elsewhere in this proxy statement/prospectus. |
The MURF Board concluded that the potential benefits that it expects MURF and its shareholders to achieve as a result of the Business Combination outweighed the potentially negative and other factors associated with the Business Combination. The MURF Board also noted that MURF public stockholders would have a substantial economic interest in Conduit (depending on the level of MURF public stockholders that sought redemption of their public shares for cash). Accordingly, the MURF Board determined that the Business Combination and the transactions contemplated by the Merger Agreement were advisable and in the best interests of MURF and its shareholders.
Approval of the Transactions by Conduit’s Board of Directors
In connection with signing the Merger Agreement, Conduit’s board of directors unanimously approved, under written resolutions, the Merger Agreement and the Merger, upon the terms and subject to the conditions set forth in the Merger Agreement.
Interests of MURF’s Directors and Officers in the Business Combination
In considering the recommendation of the MURF Board to vote in favor of approval of the business combination proposal, the charter amendments proposal, the advisory charter amendments proposals, and the other proposals, stockholders should keep in mind that MURF’s Sponsor and its directors and executive officers have interests in such proposals that are different from, or in addition to, those of MURF stockholders generally. In particular:
| ● | If the Business Combination with Conduit, or another business combination, is not consummated by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), then MURF will (i) cease all operations except for the purpose of winding up, (ii) redeem the Public Shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account including interest earned on the funds held in the trust account and not previously released to MURF to pay MURF’s franchise and income taxes (less up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding Public Shares, which redemption will completely extinguish Public Stockholders’ rights as stockholders (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) subject to the approval of our remaining stockholders and our board of directors, dissolve and liquidate, subject in each case to our obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. | |
| ● | The Sponsor (including its representatives and affiliates) and MURF’s directors and officers, are, or may in the future become, affiliated with entities that are engaged in a similar business to MURF’s and the Sponsor and MURF’s directors and officers are not prohibited from sponsoring, or otherwise becoming involved with, any other blank check companies prior to MURF completing its initial business combination, and as result of which, the Sponsor and MURF’s officers and directors may become aware of business opportunities which may be appropriate for presentation to MURF, and the other entities to which they owe fiduciary or contractual duties, and may have conflicts of interests in determining to which entity a particular business opportunity should be presented (and these conflicts may include presentation to other entities prior to their presentation, if at all, to MURF, and may not always be resolved in the favor of MURF, subject to applicable fiduciary duties under Delaware law, in that MURF has provided in its amended and restated certificate of incorporation that MURF has renounced its interest in any corporate opportunity presented to MURF). | |
| ● | The Sponsor and the directors and officers of MURF have each waived their redemption rights with respect to any Founder Shares and Class A common stock held by them (other than relating to liquidating distributions to public shares from the trust account if MURF fails to complete its initial business combination by the time required prior to MURF’s liquidation in accordance with its amended and restated certificate of incorporation), which waiver was provided in connection with the initial public offering and without any separate consideration paid in connection with providing such waiver. | |
| ● | The Merger Agreement contemplates that Adam Sragovicz, MURF’s current Chief Financial Officer and a director, will be the Chief Financial Officer of New Conduit after the closing of the Business Combination. Pursuant to the terms of an employment agreement to be entered into between New Conduit and Mr. Sragovicz in connection with the closing of the Business Combination, Mr. Sragovicz is entitled to compensation consisting of (i) an annual base salary of $400,000, and (ii) a target annual bonus opportunity equal to 40% of his base salary, payable based on the achievement of performance objectives as determined by the New Conduit board of directors. In addition, the employment agreement provides that he will be entitled to receive a sign-on restricted stock unit award covering 0.10% of the shares of common stock of New Conduit pursuant to the terms of the 2023 Stock Incentive Plan, which shall vest in equal annual installments on the first three anniversaries of the Business Combination. In the future, Mr. Sragovicz could receive any additional cash compensation, stock options, stock awards or other benefits that New Conduit’s board of directors determines to pay to Mr. Sragovicz. | |
| ● | Mr.
Heilbron, the Chief Executive Officer and Chairman of MURF, is also the President of NetREIT Advisors, which is sole and
managing member of the Sponsor. NetReit Advisors is wholly-owned by Presidio. Mr. Heilbron is the President, Chief Executive
Officer and Chairman of Presidio, of which he beneficially owns 2.6% (as of March 31, 2023). In
2023, Presidio’s compensation committee of its board of directors awarded Mr. Heilbron a cash bonus equal to 1% of the value
of the New Conduit common stock owned by Presidio, to be valued on the day which is six months and one day after the closing
of the Business Combination.
Mr. Heilbron has entered into a consulting agreement with MURF, which is effective upon the closing of the Business Combination. The consulting agreement provides that Mr. Heilbron will provide advisory and consulting services from time to time to New Conduit for one year following the closing of the Business Combination. Mr. Heilbron will no longer serve as an officer or director of New Conduit after the closing of the Business Combination. However, pursuant to the terms of the consulting agreement, Mr. Heilbron is entitled to rights as an observer to the Board of Directors of New Conduit. Mr. Heilbron is entitled to be paid $25,000 per calendar quarter for his consulting services and is also entitled to a stock option to purchase the number of shares of the common stock of New Conduit determined by dividing (i) $300,000, by (ii) the per share Black-Scholes valuation as of the grant date, utilizing the same assumptions used in preparation of the financial statements, with the resulting quotient rounded down to the nearest whole share. | |
| ● | The Sponsor paid an aggregate of approximately $25,000 for the Founder Shares and the market value of such securities as of July 31, 2023, was approximately $35,641,000, and such securities should have a significantly higher value than $25,000 at the time of the Business Combination, and if MURF does not complete an initial business combination will expire worthless. |
| 78 |
| ● | The Sponsor paid $7,540,000 for the 745,000 private placement units (consisting of 745,000 shares of Class A common stock and the Private Warrants) in connection with MURF’s initial public offering, at a price of $10.00 per unit, the market value of such securities as of July 31, 2023, was approximately $15,286,000, and such securities should have a higher value at the time of the Business Combination than $7,540,000. If MURF does not complete an initial business combination by February 7, 2024, then the Private Warrants received as part of the private placement units will expire worthless. | |
| ● | The Sponsor and its affiliates’ total potential ownership interest in New Conduit, as a result of 11,037,272 shares of MURF Class A common stock having already been redeemed (or approximately 83% of the issued and outstanding Class A common stock subject to possible redemption as of December 31, 2022) and assuming the exercise and conversion of all securities following the closing of the Business Combination, including the Private Shares, is estimated to comprise approximately 5.1% of the outstanding New Conduit common stock in a no redemption scenario (or a transaction value of approximately $34,021,000 or a market value of approximately $51,900,000), approximately 5.2% of outstanding New Conduit common stock in a 50% redemption scenario (or a transaction value of approximately $34,430,000 or a market value of approximately $51,900,000), and 5.3% of outstanding New Conduit common stock in a 100% maximum redemption scenario (or a transaction value of approximately $34,850,000 or a market value of approximately $51,900,000). | |
| ● | Immediately following the closing of the Business Combination, the independent directors of the MURF Board will receive from the Sponsor an aggregate of 45,000 private placement units, consisting of, in aggregate, 45,000 shares of MURF Class A common stock and 45,000 Private Warrants, which, assuming conversion and exercise, will be 90,000 shares of New Conduit common stock of an aggregate value of approximately $970,000 (assuming that the future value of New Conduit common stock will be the same as the current value of MURF’s Class A common stock) (the “Independent Director Units”). | |
| ● | The Sponsor and its affiliates can earn a positive rate of return on their overall investment in MURF and Conduit after the Business Combination, even if other holders of MURF common stock experience a negative rate of return, due to having purchased the Founder Shares, as described above, for $25,000 or approximately $0.006 per share. | |
| ● | On March 7, 2023, MURF has issued to Sponsor an unsecured promissory note in the principal amount of $1,500,000, portions of which may be withdrawn by MURF from time to time (the “Promissory Note”). As of July 31, 2023, MURF has submitted drawdown requests in an aggregate amount of $750,000. The Promissory Note bears no interest and is due and payable upon the earlier of the consummation of the initial business combination or the date of the liquidation of MURF. | |
| ● | The aggregate dollar amount that the Sponsor and its affiliates have at risk that depends on the completion of an initial business combination, including the Business Combination, is approximately $8,315,000, as of July 31, 2023, which is the current value of securities held (assuming that the current value of each of the securities held, whether publicly traded shares of MURF Class A common stock or Private Warrants, are in each case valued at the current price of MURF’s Class A common stock) and consists of (i) the Founders Shares and (ii) the 754,000 private placement units (each such unit comprised of one share of MURF’s Class A common stock and one warrant to purchase one share of MURF’s Class A common stock) purchased in connection with MURF’s initial public offering, and the Promissory Note. There are no fees due or out of pocket expenses for which Sponsor and its affiliates are awaiting reimbursement. | |
| ● | The aggregate dollar amount that the directors of MURF have at risk that depends on the completion of an initial business combination, including the Business Combination, is approximately $970,000, as of July 31, 2023, which is the current value of the Independent Director Units. There are no loans, fees due or out of pocket expenses for which directors are awaiting reimbursement. | |
| ● | As a result of the foregoing, the Sponsor, and directors and officers of MURF, including Mr. Heilbron, will benefit from the completion of an initial business combination, including the Business Combination, and may be incentivized to complete an acquisition or business combination of a less favorable target company or on terms less favorable to stockholders of MURF rather than liquidate. | |
| ● | If MURF is unable to complete a business combination within the required time period, its executive officers will be personally liable under certain circumstances described herein to ensure that the proceeds in the trust account are not reduced by the claims of target businesses or claims of vendors or other entities that are owed money by MURF for services rendered or contracted for or products sold to MURF. If MURF consummates a business combination, on the other hand, MURF will be liable for all such claims. | |
| ● | MURF’s Sponsor, including its officers and directors, and their affiliates are entitled to reimbursement of out-of-pocket expenses incurred by them in connection with certain activities on MURF’s behalf, such as identifying and investigating possible business targets and business combinations. However, if MURF fails to consummate a business combination within the required period, they will not have any claim against the trust account for reimbursement. Accordingly, MURF may not be able to reimburse these expenses if the Business Combination with Conduit, or another business combination, is not completed by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation). |
| 79 |
| ● | The continued indemnification of current directors and officers and the continuation of directors and officers liability insurance. | |
| ● | Chele Chiavacci Farley, a current director of the MURF Board, will be appointed to the Board of Directors of New Conduit upon the closing of the Business Combination. |
When determining how to vote in relation to the Merger Agreement, the MURF Board concluded that Mr. Heilbron’s control of the Sponsor, through his role as the President of its sole and managing member, NetREIT Advisors, and as the President, Chief Executive Officer and Chairman of Presidio, the Sponsor’s ultimate owner, did not entitle him to benefits different from those that would be enjoyed by the Sponsor, and that he had no conflicts of interest except as related to his position with the Sponsor or identical to those of the other officers and directors of MURF. In evaluating the Business Combination, the MURF Board did not consider the 1% cash bonus to be payable to Mr. Heilbron from Presidio, or the compensation payable to Mr. Heilbron pursuant to the terms of the consulting agreement with New Conduit, because the Merger Agreement was executed prior to the determination of the cash bonus and entry into the consulting agreement.
MURF’s existing charter waives the corporate opportunities doctrine with respect to MURF or any of its officers, directors or respective affiliates. While this may result in a potential conflict of interest as between the fiduciary duties or contractual obligations of our officers or directors and the interests of MURF and its stockholders, the MURF Board determined that it did not impact MURF’s search for an initial business combination target, including Conduit, as no MURF Board member was presented with a potential initial business combination target that it did not introduce to MURF, and MURF was not prevented from reviewing any opportunities as a result of such waiver.
Recommendation of MURF Board
After careful consideration of the matters described above, particularly Conduit’s accomplished leadership, scalable platform with public company readiness, potential of value accretion, addressing of unmet market need and significant growth prospects, the MURF Board determined unanimously that each of the business combination proposal, the charter amendment proposals, the advisory charter amendments proposals, the director election proposal, the incentive plan proposal, the Nasdaq proposal and the adjournment proposal, if presented, is fair to and in the best interests of MURF and its stockholders. The MURF Board has approved and declared advisable and unanimously recommend that you vote or give instructions to vote “FOR” each of these proposals.
The foregoing discussion of the information and factors considered by the MURF Board is not meant to be exhaustive but includes the material information and factors considered by the MURF Board.
Anticipated Accounting Treatment
The Business Combination will be accounted for as a reverse recapitalization in accordance with GAAP. Under this method of accounting, MURF has been treated as the “acquired” company for financial reporting purposes. Conduit was determined to be the accounting acquirer primarily because Conduit stakeholders will collectively own a majority of outstanding shares of the combined company as of the closing of the merger, they have nominated four of the seven (7) board of directors as of the closing of the merger and Conduit’s Chief Executive Officer will continue to manage the combined company. Additionally, Conduit’s business will comprise the ongoing operations of the combined company immediately following the consummation of the Business Combination. Accordingly, for accounting purposes, the financial statements of the combined entity will represent a continuation of the financial statements of Conduit with the acquisition being treated as the equivalent of Conduit issuing stock for the net assets of MURF, accompanied by a recapitalization. The net assets of MURF will be stated at historical cost, with no goodwill or other intangible assets recorded.
Regulatory Matters
The Business Combination is not subject to any additional federal or state regulatory requirement or approval, except for the filings with the State of Delaware necessary to effectuate the Business Combination.
Required Vote for Approval
The approval of the business combination proposal will require the affirmative vote of the holders of a majority of the then outstanding shares of MURF common stock entitled to vote at the meeting. Additionally, the Business Combination will not be consummated if MURF has less than $5,000,001 of net tangible assets after taking into account the holders of Public Shares that properly demanded that MURF convert their Public Shares into their pro rata share of the trust account, or is otherwise not exempt from the provisions of Rule 419 promulgated under the Securities Act.
The approval of the business combination proposal is a condition to the consummation of the Business Combination. If the business combination proposal is not approved, the other proposals (except an adjournment proposal, as described below) will not be presented to the stockholders for a vote.
THE MURF BOARD UNANIMOUSLY RECOMMENDS THAT THE MURF STOCKHOLDERS VOTE “FOR” THE APPROVAL OF THE BUSINESS COMBINATION PROPOSAL.
THE MERGER AGREEMENT
For a discussion of the merger structure and merger consideration provisions of the Merger Agreement, see the section entitled “The Business Combination Proposal.” Such discussion and the following summary of other material provisions of the Merger Agreement is qualified by reference to the complete text of the Merger Agreement, a copy of which is attached as Annexes A-1, A-2 and A-3 to this proxy statement/prospectus. All stockholders are encouraged to read the Merger Agreement in its entirety for a more complete description of the terms and conditions of the Business Combination.
| 80 |
On November 8, 2022, MURF entered into a Merger Agreement by and among MURF, Merger Sub and Conduit, the terms of which are reflected in the summary of the Merger Agreement below. Pursuant to and subject to the terms and conditions of the Merger Agreement, Merger Sub will merge with and into Conduit, with Conduit surviving the merger. As a result of the Business Combination, Conduit will become a wholly-owned subsidiary of MURF, with the shareholders of Conduit becoming stockholders of MURF.
Pursuant to the Merger Agreement, the shareholders of Conduit will receive the number of New Conduit common stock that shall have an aggregate value equal to Six Hundred Fifty Million Dollars ($650,000,000). With each share of New Conduit common stock being valued at $10.00, Conduit’s outstanding ordinary shares (including the shares issued upon conversion of all outstanding convertible debt, which conversion shall have occurred prior to the consummation of the Business Combination) will be converted into an aggregate of 65,000,000 shares of the common stock of New Conduit following the Business Combination, with each such outstanding Conduit share converted into shares of the common stock of New Conduit on a pro rata basis. Additionally, each outstanding share of MURF common stock immediately prior to the Merger, including all outstanding shares of Class B common stock, shall automatically convert into and become one share of the common stock of New Conduit without any action on the part of any holders of such common stock.
The Business Combination is expected to be consummated in the second quarter of 2023, following the receipt of the required approval by the stockholders of MURF and the shareholders of Conduit and the satisfaction of certain other customary closing conditions.
Closing and Effective Time of the Business Combination
The closing of the Business Combination will take place on a date no later than the third business day following the satisfaction or waiver of the conditions described below under the subsection entitled “— Conditions to the Closing of the Business Combination,” or waiver of all the conditions that are required to be satisfied prior to the closing. The Business Combination is expected to be consummated as soon as practicable after the Special Meeting of MURF stockholders described in this proxy statement/prospectus.
Representations and Warranties
The Merger Agreement contains customary representations and warranties of Conduit with respect to, among other things: (i) organization and qualification; (ii) organizational documents; (iii) capitalization; (iv) authorization to enter into the Merger Agreement and related transactions; (v) no conflicts and non-contravention; (vi) permits and compliance; (vii) financial statements; (viii) no undisclosed liabilities; (ix) absence of certain changes; (x) absence of litigation; (xi) employee benefit plans; (xii) labor matters; (xiii) real property and title to and sufficiency of assets; (xiv) intellectual property; (xv) taxes; (xvi) environmental matters; (xvii) material contracts; (xviii) insurance; (xix) internal controls; (xx) accuracy of statements; (xxi) delivery of support agreement; (xxii) board approval; (xxiii) brokers and finders’ fees; (xxiv) takeover laws; (xxv) international trade matters and anti-bribery compliance; (xxvi) related party transactions; (xxvii) that Conduit is not an investment company; (xxviii) exclusivity of representations and warranties; and (xxix) full disclosure.
The Merger Agreement contains customary representations and warranties of MURF and Merger Sub with respect to, among other things: (i) corporate organization and power; (ii) organizational documents; (iii) capitalization; (iv) authorization to enter into the Merger Agreement and related transactions; (v) no conflicts and non-contravention; (vi) compliance; (vii) MURF publicly filed documents and financial statements; (viii) absence of certain changes; (ix) absence of litigation; (x) board approval; (xi) no prior operations of Merger Sub; (xii) amount in the trust account; (xiii) employees; (xiv) taxes; (xv) listing of MURF securities; (xvi) that MURF is not an investment company; (xvii) statements in public filings; (xviii) contracts; (xix) brokers and finders’ fees; (xx) delivery of support agreement; (xxi) MURF and Merger Sub’s investigation and reliance; and (xxii) full disclosure.
| 81 |
Covenants
The Merger Agreement includes customary covenants of the parties with respect to operation of their respective businesses prior to consummation of the Business Combination and efforts to satisfy conditions to consummation of the Business Combination. The Merger Agreement also contains additional covenants of the parties, including, among others, access to information, cooperation in the preparation of the registration statement on Form S-4 and proxy statement required to be filed in connection with the Business Combination and to obtain all requisite approvals of MURF’s stockholders. MURF has also agreed to include in the proxy statement the recommendation of its board that its stockholders approve all of the proposals to be presented at the special meeting of MURF’s stockholders that will be called in order to approve the Merger and related transactions. Conduit has agreed that, unless otherwise required or permitted under the Merger Agreement, and subject to certain exceptions, neither it nor its subsidiaries will take the following actions, among others, without the prior written consent of MURF (which consent will not be unreasonably conditioned, withheld, delayed or denied):
| ● | amend or otherwise change its or any subsidiary’s certificate of incorporation or bylaws or equivalent organizational documents; | |
| ● | issue, sell, pledge, dispose of, grant or encumber, or authorize the issuance, sale, pledge, disposition, grant or encumbrance of, any shares of any class of capital stock or other securities of Conduit or of any subsidiary or any options, warrants, convertible securities or other rights of any kind to acquire any shares of such capital stock, or any other ownership interest (including any phantom interest), of Conduit or any subsidiary; | |
| ● | (a) fail to maintain its or any subsidiary’s existence; or (b) adopt or enter into a plan of complete or partial liquidation, dissolution, merger, consolidation, restructuring, recapitalization or other reorganization of Conduit or any subsidiary (other than the transactions contemplated by the Merger Agreement); | |
| ● | declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, property or otherwise; | |
| ● | reclassify, combine, split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly; | |
| ● | (a) acquire (including by merger, consolidation, or acquisition of stock or assets or any other business combination), whether in whole or in part or via an equity or asset acquisition, any other corporation, limited liability company, partnership, other business organization or any division thereof; (b) incur or guarantee any indebtedness for borrowed money or issue any debt securities or assume, guarantee or endorse, or otherwise become responsible for, the obligations of any person, or make any loans or advances, or intentionally grant any security interest in any of its assets, other than additional extensions or borrowings as permitted under existing credit facilities or (c) enter into any new line of business; | |
| ● | make any loans, advances or capital contributions to, or investments in, any other person (including to any of its officers, directors, agents or consultants), make any material change in its existing borrowing or lending arrangements for or on behalf of such persons, or enter into any “keep well” or similar agreement to maintain the financial condition of any other person, except as required under any indemnification agreement to which Conduit is a party as of the date of the Merger Agreement and which has been disclosed in Conduit’s disclosure schedules to the Merger Agreement; | |
| ● | except as otherwise permitted by the Merger Agreement, (a) adopt, enter into or materially amend any employee benefit plan or any collective bargaining or similar agreement (including agreements with works councils and trade unions and side letters) to which Conduit is a party or by which it is bound, (b) grant or provide any severance, termination payments, bonus, change of control, retention, or benefits to any employee of Conduit, except in connection with the promotion or hiring (to the extent permitted under the Merger Agreement) or separation of any employee in the ordinary course of business, (c) hire any employee of Conduit or any other individual who is providing or will provide services to the Conduit other than any employee with an annual base salary of less than $150,000 or any employee hired to replace terminated employees in the ordinary course of business, (d) adopt, enter into or materially amend contracts with any consultants or natural person independent contractors that involve consideration of more than $150,000 in the aggregate or (e) take any action to accelerate the vesting, payment or funding of any cash or equity-based compensation, payment or benefit other than as contemplated by the Merger Agreement; | |
| ● | grant any material increase in the compensation, incentives or benefits payable or to become payable to any current or former director, officer, employee or consultant of Conduit as of the date of the Merger Agreement, other than (a) increases in the ordinary course of business, or (b) increases required by the terms of an employee benefit plan or applicable law; |
| 82 |
| ● | make any change in any method of financial accounting or financial accounting principles, policies, procedures or practices, except as required by a concurrent amendment in GAAP or applicable law made subsequent to the date of the Merger Agreement, as agreed to by its independent accountants; | |
| ● | make, change or revoke any material tax election, adopt or change any material tax accounting method or period, file any amendment to a material tax return, enter into any agreement with a governmental authority with respect to a material amount of taxes, settle or compromise any examination, audit or other action with a governmental authority of or relating to any material United States federal, state, local or non-United States income tax liability, consent to any extension or waiver of the statutory period of limitations applicable to any claim or assessment in respect of taxes, or enter into any tax sharing or similar agreement (excluding any commercial contract not primarily related to taxes) ; | |
| ● | take any action, or knowingly fail to take any action, which action or failure to act would reasonably be expected to prevent or impede the transactions from qualifying for the intended tax treatment; | |
| ● | enter into, modify in any material respect or terminate any contract that is (or would be if entered into prior to the date of the Merger Agreement) a material contract), other than in the ordinary course of business; | |
| ● | acquire any fee interest in real property; | |
| ● | settle any cause of action, litigation, suit, hearing, arbitration or other similar proceeding of any nature, civil, criminal, regulatory, administrative or otherwise, whether in equity or at law, in contract, in tort or otherwise (a) if such settlement would require payment by Conduit in an amount greater than $200,000 or (b) to the extent such settlement is adverse to Conduit and involves an action brought by a governmental authority or alleged criminal wrongdoing; | |
| ● | make or authorize any payment of, or accrual or commitment for, capital expenditures in excess of $100,000 for any individual capital expenditure or series of related capital expenditures or $300,000 in the aggregate; | |
| ● | enter into, renew, or materially amend, any agreement between Conduit and a related party; | |
| ● | make any payment, distribution, loan or other transfer of value to any related party, other than (a) payments to employees in the ordinary course of business, and (b) payments pursuant to agreements between Conduit and a related party set forth on Conduit’s disclosure schedule to the Merger Agreement; | |
| ● | fail to maintain, cancel or materially change coverage under any insurance policy in form and amount equivalent in all material respects to the insurance coverage currently maintained with respect to Conduit and its assets, properties and businesses; | |
| ● | permit any material item of Conduit’s intellectual property rights to lapse or to be abandoned, invalidated, dedicated to the public, or disclaimed, or otherwise become unenforceable; | |
| ● | fail to conduct Conduit’s business in the ordinary course of business consistent with past practice; and | |
| ● | enter into any agreement or otherwise make a binding commitment to do any of the foregoing. |
The Merger Agreement also contains additional covenants of the parties, including covenants in connection with:
| ● | the preparation and filing by MURF of this registration statement on Form S-4 and including the proxy statement in connection with the registration under the Securities Act of MURF’s common stock to be issued in connection with the Business Combination; | |
| ● | the protection of confidential information of the parties and, subject to confidentiality, attorney-client privilege and legal requirements, the provision of reasonable access to information; | |
| ● | the exclusivity of discussions or negotiations, directly or indirectly, any inquiries, proposals or offers with respect to acquire or purchase any equity ownership in Conduit or any of its controlled affiliates or all or a material portion of assets or businesses of Conduit; |
| 83 |
| ● | the cooperation in good faith to negotiate certain employment agreements, to be effective on the closing date of the Business Combination; | |
| ● | survival of all exculpation and indemnification rights for all current and former directors and officers of MURF, Merger Sub and Conduit in effect as of the date of the Merger Agreement in accordance with their respective terms; Conduit maintaining a six-year directors’ and officers’ liability policy covering directors and officers currently covered by liability insurance, and covering directors of Conduit following the effective time of the Merger; and MURF and Conduit being permitted to obtain “tail” insurance policy that provides coverage up to six years from the effective time of the Merger for events occurring prior thereto; | |
| ● | the required notification of any litigation against MURF or its directors or officers by any of MURF’s stockholders or by a governmental authority prior to the closing of the Business Combination; | |
| ● | each party’s obligation to use reasonable best efforts to effect the intended tax treatment of the Business Combination; | |
| ● | that MURF will use reasonable best efforts to keep MURF’s Class A common stock and warrants listed on Nasdaq until the closing of the Business Combination and cause New Conduit common stock issued in connection with the Business Combination to be approved for listing on Nasdaq; | |
| ● | that MURF use reasonable best efforts to keep current and timely file all reports required to be filed or furnished with the SEC and otherwise to comply in all material respects with its reporting obligations under applicable securities laws; | |
| ● | each party’s obligation to use its best efforts to cause to be done such things as are necessary, proper or advisable under applicable laws to consummate and make effective the Business Combination; | |
| ● | MURF’s obligation to provide notice to make appropriate arrangements to cause the funds in the trust account to be disbursed in accordance with the terms of the Business Combination; | |
| ● | MURF’s obligation to adopt a resolution to make the acquisition of MURF common stock pursuant to the Merger Agreement an exempt transaction for purposes of Section 16(b) of the Exchange Act pursuant to Rule 16b-3 thereunder; and | |
| ● | Conduit’s obligation to as soon as reasonably practicable following the date of the Merger Agreement, deliver to MURF audited financial statements for the years ending December 31, 2020 and 2021 and unaudited financial statements for subsequent interim periods. |
Conditions to the Closing of the Business Combination
General Conditions
The consummation of the Merger is conditioned upon, among other things, (i) the absence of any applicable law or order that makes the transactions contemplated by the Merger Agreement illegal or otherwise prohibits consummation of such transactions; (ii) the registration statement becoming effective under the Securities Act; (iii) the approval by MURF’s stockholders of the Business Combination and related transactions; (iv) the approval by Conduit’s shareholders of the Business Combination and related transactions; (v) the aggregate cash available to MURF at the Closing (after giving effect to any redemptions by MURF’s stockholders and the payment of all authorized transaction expenses) being at least $27,000,000; (vi) after giving effect to the Business Combination, MURF shall have at least $5,000,001 in net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange Act) or shall otherwise be exempt from the provisions of Rule 419 promulgated under the Securities Act; (vii) all ancillary agreements having been executed by all parties thereto; (viii) MURF and Conduit having cooperated in good faith to negotiate and execute new employment agreements with David Tapolczay and Adam Sragovicz, the current Chief Financial Officer and a director of MURF; and (ix) all required filings under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, and any other governmental authority having been completed and cleared.
Conduit’s Conditions to the Closing of the Business Combination
The obligations of Conduit to consummate the Business Combination are subject to the satisfaction, among other things, of the following at or prior to the Closing of the Business Combination:
| ● | MURF and Merger Sub having duly performed or complied with all of its obligations under the Merger Agreement in all material respects; (ii) the representations and warranties of MURF and Merger Sub being true and correct in all material respects; |
| 84 |
| ● | no event having occurred that would result in a Murphy Material Adverse Effect; |
| ● | MURF providing Conduit a certificate from an authorized officer of MURF as to the accuracy of the foregoing conditions; |
| ● | MURF’s establishment of an equity incentive plan to be in place following the closing of the Business Combination; |
| ● | MURF and Conduit having cooperated in good faith to negotiate and execute new employment agreements with David Tapolczay and Adam Sragovicz, the current Chief Financial Officer and a director of MURF; and |
| ● | certain intellectual property having been assigned to Conduit UK Management Ltd, an entity that will be a wholly-owned subsidiary of Conduit as of the consummation of the Business Combination. |
MURF’s and Merger Sub’s Conditions to the Closing of the Business Combination
The obligations of MURF and Merger Sub to consummate the Business Combination are subject to the satisfaction, among other things, of the following at or prior to the Closing of the Business Combination:
| ● | Conduit having duly performed or complied with all of their obligations under the Merger Agreement in all material respects; |
| ● | the representations and warranties of Conduit being true and correct in all material respects; |
| ● | no event having occurred that would result in a Company Material Adverse Effect; and |
| ● | Conduit providing MURF a certificate from an authorized officer as to the accuracy of the foregoing conditions. |
| ● | Conduit UK Management Ltd. will be transferred to the Company and become a wholly-owned subsidiary of the Company, and certain intellectual property having been assigned to Conduit UK Management Ltd. |
Waiver
If permitted under applicable law, MURF or Conduit may waive any inaccuracies in the representations and warranties made to such party and contained in the Merger Agreement and waive compliance with any agreements or conditions for the benefit of such party contained in the Merger Agreement. However, pursuant to MURF’s existing amended and restated certificate of incorporation, the condition requiring that MURF have at least $5,000,001 of net tangible assets or otherwise be exempt from the provisions of Rule 419 promulgated under the Securities Act may not be waived.
Termination
The Merger Agreement may be terminated at any time, but not later than the closing of the Business Combination, as follows:
| ● | by the mutual consent of MURF and Conduit; |
| ● | by MURF, if any of the representations or warranties of Conduit set forth in the Merger Agreement is not true and correct, or if Conduit has failed to perform any covenant or agreement set forth in the Merger Agreement (including an obligation to consummate the Business Combination), in each case such that the conditions to closing would not be satisfied and the breach or breaches causing such representations or warranties not to be true and correct, or the failure to perform any covenant or agreement, as applicable, are not cured (or waived by MURF) by the earlier of (i) the Outside Date (as defined below) or (ii) 20 days after written notice thereof is delivered to Conduit; provided, however that MURF is not then in material breach of any representation, warranty, covenant, or obligation in the Merger Agreement, which breach has not been cured; |
| ● | by Conduit, if any of the representations or warranties of MURF or Merger Sub set forth in the Merger Agreement is not true and correct, or if MURF or Merger Sub has failed to perform any covenant or agreement set forth in the Merger Agreement (including an obligation to consummate the Business Combination), in each case such that the conditions to closing would not be satisfied and the breach or breaches causing such representations or warranties not to be true and correct, or the failure to perform any covenant or agreement, as applicable, are not cured (or waived by Conduit) by the earlier of (i) the Outside Date or (ii) 20 business days after written notice thereof is delivered to MURF; provided, however that Conduit is not then in material breach of any representation, warranty, covenant, or obligation in the Merger Agreement, which breach has not been cured; |
| 85 |
| ● | by either MURF or Conduit: (A) on or after February 7, 2024 (the “Outside Date”), if the Business Combination has not been consummated prior to the Outside Date; provided, however, that this right to terminate the Merger Agreement will not be available to a party if the failure of the merger to have been consummated before the Outside Date was due to such party’s breach of or failure to perform any of its covenants or agreements set forth in the Merger Agreement; or; (B) if any applicable law or order that makes the transactions contemplated by the Merger Agreement illegal or otherwise prohibits consummation of such transactions becomes final and non-appealable; (C) if MURF has not received approval from its stockholders of the Business Combination and related transactions at the Special Meeting; |
| ● | by MURF if the Conduit shareholders’ written consent approving the Business Combination and related transactions have not been obtained within 10 business days after the date of the Merger Agreement; and |
| ● | if there is a material adverse effect on behalf of the other party. |
Effect of Termination
In the event of proper termination by any of the parties, the Merger Agreement will be of no further force or effect (other than with respect to certain surviving obligations specified in the Merger Agreement), without any liability on the part of any party thereto or its respective affiliates, officers, directors or stockholders, other than liability of any party thereto for any intentional and willful breach of the Merger Agreement by such party occurring prior to such termination.
Fees and Expenses
Except as otherwise set forth in the Merger Agreement, all fees and expenses incurred in connection with the Merger Agreement and the transactions contemplated thereby will be paid by the party incurring such expenses whether or not the transactions are consummated.
Tax Matters
Each of MURF, Merger Sub and Conduit agreed that, for U.S. federal income tax purposes, (a) the Merger Agreement shall be adopted as a “plan of reorganization” within the meaning of Section 368 of the Code and Treasury Regulations Section 1.368-2(g) and (b) they shall report the Business Combination as an integrated transaction that qualifies as a “reorganization” within the meaning of Section 368(a) of the Code and the Treasury Regulations to which the MURF, Merger Sub and Conduit are parties within the meaning of Section 368(b) of the Code.
Confidentiality; Access to Information
Each party to the Merger Agreement will afford to the other parties and their financial advisors, accountants, counsel and other representatives reasonable access during normal business hours, upon reasonable notice, to all of their respective properties, books, records and personnel during the period prior to the closing of the Business Combination to obtain all information concerning the business, including the status of business development efforts, properties, results of operations and personnel, as each party may reasonably request. The parties agree to maintain in confidence any non-public information received from the other party, and to use such non-public information only for purposes of consummating the transactions contemplated by the Merger Agreement.
Amendments
The Merger Agreement may be amended by the parties thereto at any time prior to the closing of the Business Combination by execution of an instrument in writing signed on behalf of each of the parties.
Governing Law; Consent to Jurisdiction
The Merger Agreement is governed by and construed in accordance with the law of the state of Delaware, regardless of the law that might otherwise govern under applicable principles of the conflicts of laws of Delaware. However, the following matters arising out of or relating to Merger Agreement shall be construed, performed and enforced in accordance with the Companies Act: the Business Combination, the vesting of the rights, property, choses in action, business, undertaking, goodwill, benefits, immunities and privileges, contracts, obligations, claims, debts and liabilities of Merger Sub and Conduit in Conduit, the cancellation of the shares of Conduit, the rights provided in Section 238 of the Companies Act, the fiduciary or other duties of the Conduit board of directors and the board of directors of Merger Sub and the internal corporate affairs of Conduit and Merger Sub.
| 86 |
CERTAIN AGREEMENTS RELATED TO THE BUSINESS COMBINATION
This section describes the material provisions of certain additional agreements entered into or to be entered into pursuant to or in connection with the transactions contemplated by the Merger Agreement, which are referred to as the “Related Agreements.” Such discussion and the following summary of other material provisions of the Related Agreements are qualified by reference to the complete texts of the Related Agreement, copies of which are filed as exhibits to this proxy statement/prospectus. All stockholders are encouraged to read the Related Agreements in their entirety.
Sponsor Support Agreement
Concurrently with the execution of the Merger Agreement, the Sponsor and MURF entered into a certain sponsor support agreement dated November 8, 2022, pursuant to which the Sponsor agreed to vote all shares of MURF common stock beneficially owned by it, including any additional shares of MURF it acquires ownership of or the power to vote, in favor of the Business Combination and related transactions. Under the support agreement, the Sponsor also agreed that, prior to the termination of the support agreement, the Sponsor will not transfer or otherwise enter into any agreement or understanding with respect to a transfer relating to any shares of MURF common stock legally and beneficially owned by it. The support agreement will terminate upon the earliest to occur of: (a) the effective time of the Business Combination; (b) the termination of the Merger Agreement in accordance with its terms; and (c) the mutual agreement of Conduit and the Sponsor.
Shareholder Support Agreements
Concurrently with the execution of the Merger Agreement, MURF, Conduit, and certain shareholders of Conduit entered into a certain shareholder support agreement dated November 8, 2022, pursuant to which the Conduit Shareholders agreed to vote all Conduit shares beneficially owned by them, including any additional shares of Conduit they acquire ownership of or the power to vote, in favor of the Business Combination and related transactions. Under the support agreements, each Conduit shareholder has also agreed that, prior to the termination of the applicable support agreement, such shareholder will not transfer or otherwise enter into any agreement or understanding with respect to a transfer relating to any ordinary shares of Conduit owned by such shareholder. The support agreements will terminate automatically without any further required actions or notice upon the earliest to occur: (a) the effective time of the Business Combination; (b) the termination of the Merger Agreement in accordance with its terms; and (c) the mutual agreement of Conduit and the shareholder.
Lock-up Agreements
In connection with the Merger Agreement, certain shareholders of Conduit entered into lock-up agreements with MURF, pursuant to which the shareholders are subject to a lock-up period commencing from the date of the closing of the Business Combination and ending on the earlier of (x) one hundred and eighty (180) days after the date of the closing of the Business Combination, and (y) the date on which MURF consummates a liquidation, merger, share exchange or other similar transaction with an unaffiliated third party that results in all of MURF’s shareholders having the right to exchange their equity holdings in MURF for cash, securities or other property.
Subscription Agreement
In connection with the transactions contemplated by the Merger Agreement, MURF entered into a subscription agreement with the Private Placement Investor for units that are materially the same as those purchased by the Sponsor at the time of the initial public offering. MURF’s directors, officers, Sponsor or their affiliates will not be participating in the private placement.
Pursuant to the Subscription Agreement, the Private Placement Investor has agreed to purchase 2,700,000 units of MURF, with each unit consisting of (i) one share of MURF’s Class A common stock and (ii) one warrant to purchase one share of Class A common stock, for a purchase price of $10.00 per unit in the Private Placement. The Subscription Agreement contains registration rights, pursuant to which within fifteen (15) business days after the closing of the Private Placement, MURF will use reasonable best efforts to file with the SEC a registration statement registering the resale of shares of MURF common stock included in the Units and the shares of MURF common stock issued and issuable upon exercise of the warrants. The closing of the Private Placement is conditioned on there not being a suspension of the qualification of MURF common stock for offering or sale or trading in any jurisdiction, or the initiation or threatening of any legal proceeding, no legal prohibitions to consummate the Business Combination, and all conditions precedent to the closing of the Business Combination set forth in the Merger Agreement having been satisfied or waived.
The warrant will be exercisable for a period of five years after the completion of the Business Combination and will have an exercise price of $11.50 per share, subject to adjustment as set forth in the Warrant for stock splits, stock dividends, recapitalizations and similar customary adjustments. The Private Placement Investor may exercise each warrant on a cashless basis if the shares underlying the warrants are not then registered pursuant to an effective registration statement. The Private Placement Investor has contractually agreed to restrict its ability to exercise the warrants such that the number of shares of MURF common stock held by the investor and its affiliates after such exercise does not exceed the beneficial ownership limitation set forth in the warrant which may not exceed 4.99% of the then issued and outstanding shares of MURF common stock.
In connection with the amendment to the Subscription Agreement dated January 27, 2023, the Private Placement Investor agreed to receive and be issued shares of New Conduit common stock. Upon the closing of the Business Combination, and specifically the filing of the Proposed Charter, MURF’s Class A common stock and Class B common stock will be reclassified as a single class of common stock, and MURF’s company name will change to “Conduit Pharmaceuticals Inc.” also referred to as New Conduit. The underlying terms of the Class A common stock and the single class of common stock are materially the same as each has or will have the same voting right of one vote per each share, same dividend rights and same liquidation rights.
The closing of the Private Placement will occur immediately prior to the consummation of the Business Combination. The common stock to be issued pursuant to the Subscription Agreement has not been registered under the Securities Act, and will be issued in reliance upon the exemption provided under Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder. The Subscription Agreement will terminate and be void and of no further force and effect, and all rights and obligations of the parties without any further liability on the part of any party in respect thereof, upon the earlier to occur of: (a) the mutual written agreement of each of the parties to terminate the Subscription Agreement; (b) such date and time as the Merger Agreement is terminated in accordance with its terms; (c) if any of the conditions to the closing of the Private Placement are not satisfied or waived on or prior to the closing of the Private Placement and, as a result thereof, the transactions contemplated by the Subscription Agreement are not consummated at the closing of the Private Placement or (d) written notice by either party to the other party to terminate the Subscription Agreement if the transactions contemplated by the Subscription Agreement are not consummated on or prior to February 7, 2024.
| 87 |
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following discussion is a summary of the material U.S. federal income tax considerations relevant to (i) Non-U.S. Holders (as defined below) of shares of Conduit that hold New Conduit Common Stock in connection with the Business Combination and (ii) U.S. Holders and Non-U.S. Holders (as defined below) of Public Shares electing to have their Public Shares redeemed for cash upon the closing of the Business Combination. This discussion applies only to Conduit shares and Public Shares that are held as “capital assets” within the meaning of Section 1221 of the Code (generally, property held for investment).
The following discussion does not address the effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local or non-U.S. tax laws. This discussion is based on the Code, Treasury regulations promulgated thereunder, judicial decisions and published rulings and administrative pronouncements of the IRS, in each case in effect as of the date hereof. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect the tax consequences discussed below. Neither Conduit nor MURF has sought or will seek any rulings from that the IRS regarding the matters discussed below. There can be no assurance the IRS or a court will not take a position contrary to that set forth below in this discussion.
This discussion does not address the tax considerations for any beneficial owners of Private Warrants or Founder Shares. Further, this discussion does not address all U.S. federal income tax consequences relevant to a holder’s particular circumstances, including the impact of the Medicare contribution tax on net investment income. In addition, it does not address consequences relevant to holders subject to special rules, including, without limitation:
| ● | banks, insurance companies and certain other financial institutions; | |
| ● | regulated investment companies and real estate investment trusts; | |
| ● | brokers, dealers or traders in securities; | |
| ● | traders in securities who elect to apply a mark-to-market method of accounting; | |
| ● | tax-exempt organizations or governmental organizations; | |
| ● | persons subject to the alternative minimum tax; | |
| ● | U.S. expatriates and former citizens or long-term residents of the United States; | |
| ● | persons holding shares as part of a hedge, straddle, constructive sale or other risk reduction strategy or as part of a conversion transaction or other integrated investment; | |
| ● | persons subject to special tax accounting rules as a result of any item of gross income with respect to Public Shares being taken into account in an applicable financial statement; | |
| ● | persons deemed to sell shares under the constructive sale provisions of the Code; | |
| ● | persons who elect to apply the provisions of Section 1400Z-2 of the Code to any gain realized; | |
| ● | “controlled foreign corporations,” “passive foreign investment companies” and corporations that accumulate earnings to avoid U.S. federal income tax; | |
| ● | S corporations, partnerships or other entities or arrangements treated as partnerships or other flow-through entities for U.S. federal income tax purposes (and investors therein); | |
| ● | U.S. Holders having a functional currency other than the U.S. dollar; | |
| ● | persons who hold or received shares pursuant to the exercise of any employee stock option, through a tax qualified retirement plan or otherwise as compensation; | |
| ● | tax-qualified retirement plans; and | |
| ● | pension plans, including any “qualified foreign pension funds” as defined in Section 897(l)(2) of the Code and entities all of the interests of which are held by qualified foreign pension funds. |
| 88 |
For purposes of this discussion, a “U.S. Holder” is any beneficial owner of shares of Public Shares that is for U.S. federal income tax purposes:
| ● | an individual who is a citizen or resident of the United States; | |
| ● | a corporation (or other entity taxable as a corporation) created or organized under the laws of the United States, any state thereof, or the District of Columbia; | |
| ● | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or | |
| ● | a trust that (1) is subject to the primary supervision of a U.S. court and the control of one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code), or (2) has a valid election in effect under applicable Treasury Regulations to be treated as a United States person for U.S. federal income tax purposes. |
For purposes of this discussion, a “Non-U.S. Holder” is any beneficial owner of shares of Conduit or Public Shares that is not a U.S. Holder for U.S. federal income tax purposes including:
| ● | a non-resident alien individual; | |
| ● | a foreign corporation; or | |
| ● | a foreign estate or trust. |
If an entity or arrangement that is treated as a partnership for U.S. federal income tax purposes holds shares, the U.S. federal income tax treatment of the entity or an owner of such entity will depend on the status of the owners, the activities of the entity and certain determinations made at the owner level. Accordingly, entities treated as partnerships for U.S. federal income tax purposes and the partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences to them of the Business Combination or a redemption of Public Shares, as applicable.
INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
Material U.S. Tax Considerations of the Business Combination to Holders of Conduit Capital Stock
Treatment of the Business Combination to U.S. Holders
In the opinion of Thompson Hine LLP, the Business Combination should qualify as a “reorganization” within the meaning of Section 368(a) of the Code. This opinion is based on the facts and representations contained in representation letters provided to Thompson Hine LLP by MURF, Merger Sub and Conduit and certain assumptions, including that the Business Combination will be completed in the manner set forth in the Merger Agreement and the Registration Statement on Form S-4 of which this proxy statement/prospectus forms a part. The accuracy of such facts, representations and assumptions could affect the conclusions set forth in the opinion. The opinion will not be binding on the IRS or the courts.
The material U.S. federal income tax consequences of a U.S. Holder of Conduit should be as follows: (1) a U.S. Holder should not recognize gain or loss upon the exchange of Conduit shares for shares of New Conduit Common Stock; (2) a U.S. Holder should obtain an aggregate tax basis in the shares of New Conduit Common Stock that such U.S. Holder receives in the Business Combination equal to such U.S. Holder’s aggregate tax basis in the Conduit shares exchanged therefor; and (3) the holding period of the shares of New Conduit Common Stock received by a U.S. Holder in the Business Combination should include the holding period of the Conduit shares surrendered in exchange therefor. Treasury Regulations provide detailed rules for allocating the tax basis and holding period of the Conduit shares surrendered to the shares of New Conduit Common Stock received. U.S. Holders of Conduit shares acquired at different dates and at different prices should consult their tax advisors regarding the allocation of the tax basis and holding period of such shares.
If the Business Combination does not qualify as a “reorganization” within the meaning of Section 368(a) of the Code, each U.S. Holder of Conduit shares would recognize gain or loss on the exchange of the Conduit shares for shares of New Conduit Common Stock in the Business Combination equal to the difference between (a) the fair market value of the shares of New Conduit Common Stock received in exchange for the Conduit shares and (b) such U.S. Holder’s adjusted tax basis in the Conduit shares surrendered. U.S. Holders should consult their own tax advisors regarding the application of the foregoing rules in light of their particular facts and circumstances.
Treatment of the Business Combination to Non-U.S. Holders
Assuming that the Business Combination qualifies as a “reorganization” within the meaning of Section 368(a) of the Code, a Non-U.S. Holder of Conduit shares is not expected to recognize any gain or loss under U.S. federal income tax laws upon the exchange of their Conduit shares for New Conduit Common Stock. It is expected that each such holder would have the same basis in its New Conduit Common Stock as that holder has in their Conduit shares exchanged therefor, and such holder’s holding period in the New Conduit Common Stock would include the holder’s holding period in the Conduit shares surrendered in exchange therefor.
Consequences if the Business Combination Does Not Qualify as a Tax-Free Reorganization
If the Business Combination does not qualify as a “reorganization” within the meaning of Section 368(a) of the Code, Non-U.S. Holders should not be subject to U.S. federal income tax on any gain recognized as a result of the Business Combination unless any of the conditions described below in the bullets under the section entitled “Material Tax Considerations Related to a Redemption of Public Shares—Non U.S. Holders—Gain on Redemption Treated as a Sale of Public Shares” are satisfied with respect to gain recognized by a Non-U.S. Holder on the Conduit shares. With respect to the third bullet, Conduit believes that it is not, and has not been at any time since its formation, a U.S. real property holding corporation and neither Conduit nor New Conduit expects to be a United States real property holding corporation immediately after the Business Combination is completed. Non-U.S. Holders should consult their own tax advisors regarding the application of the foregoing rules in light of their particular facts and circumstances.
| 89 |
HOLDERS OF CONDUIT SHARES ARE ENCOURAGED TO CONSULT THEIR TAX ADVISORS TO DETERMINE THE SPECIFIC TAX CONSEQUENCES TO THEM OF THE BUSINESS COMBINATION, INCLUDING THE EFFECT OF ANY U.S. FEDERAL, STATE, LOCAL, NON-U.S. OR OTHER TAX LAWS.
Ownership and Disposition of New Conduit Common Stock by a Non-U.S. Holder
Distributions, if any, made on New Conduit Common Stock will constitute dividends for U.S. federal income tax purposes to the extent they are paid out of New Conduit’s accumulated or current earnings and profits, as determined for U.S. federal income tax purposes. Non-U.S. Holders will be treated in the same manner as described below under “Material Tax Considerations Related to a Redemption of Public Shares—Non U.S. Holders—Taxation of Redemption Treated as a Distribution” with respect to distributions made on New Conduit Common Stock that constitute dividends.
The U.S. federal income tax consequences of a sale, exchange or other disposition of New Conduit Common Stock by a Non-U.S. Holder are generally the same as those discussed above concerning a Non-U.S. Holder’s disposition of Conduit shares pursuant to the Business Combination under the heading “—Consequences if the Business Combination Does Not Qualify as a Tax-Free Reorganization”.
Information Reporting and Backup Withholding
Generally, any taxable proceeds received by a Non-U.S. Holder in connection with the Business Combination, any distributions made by New Conduit (or its paying agent) to a Non-U.S. Holder with respect to its New Conduit Common Stock, and any payments of the proceeds of the sale of New Conduit Common Stock by a Non-U.S. Holder are generally subject to the same information reporting and backup withholding rules as described below under “Material Tax Considerations Related to a Redemption of Public Shares—Non U.S. Holders—Information Reporting and Backup Withholding”.
THE U.S. FEDERAL INCOME TAX CONSEQUENCES DESCRIBED ABOVE ARE NOT INTENDED TO CONSTITUTE A COMPLETE DESCRIPTION OF ALL TAX CONSEQUENCES RELATING TO THE BUSINESS COMBINATION OR THE HOLDING AND DISPOSING OF NEW CONDUIT COMMON STOCK. BECAUSE INDIVIDUAL CIRCUMSTANCES MAY DIFFER, EACH SHAREHOLDER SHOULD CONSULT THE SHAREHOLDER’S TAX ADVISOR REGARDING THE APPLICABILITY OF THE RULES DISCUSSED ABOVE TO THE SHAREHOLDER AND THE PARTICULAR TAX EFFECTS TO THE SHAREHOLDER OF THE BUSINESS COMBINATION AND THE HOLDING OR DISPOSING OF NEW CONDUIT COMMON STOCK IN LIGHT OF SUCH SHAREHOLDER’S PARTICULAR CIRCUMSTANCES AND THE APPLICATION OF STATE, LOCAL AND FOREIGN TAX LAWS.
Material Tax Considerations Related to a Redemption of Public Shares
U.S. Holders
Redemption of Public Shares.
In the event that a U.S. Holder’s Public Shares are redeemed pursuant to the redemption provisions described in this proxy statement/prospectus under the section entitled “Special Meeting of MURF Stockholders — Redemption Rights,” the treatment of the transaction for U.S. federal income tax purposes will depend on whether the redemption qualifies as a sale of the Public Shares under Section 302 of the Code. If the redemption qualifies as a sale of the Public Shares, the U.S. Holder will be treated as described under “— U.S. Holders — Gain or Loss on Redemption Treated as Sale of Public Shares” below. Whether a redemption qualifies for sale treatment will depend largely on the total number of shares of MURF common stock treated as held by the U.S. Holder (including any stock constructively owned by the U.S. Holder as a result of owning warrants) relative to all of MURF’s shares outstanding both before and after the redemption. The redemption of MURF common stock generally will be treated as a sale of the Public Shares (rather than a corporate distribution) if the redemption or purchase by MURF (i) is “substantially disproportionate” with respect to the U.S. Holder, (ii) results in a “complete termination” of the U.S. Holder’s interest in MURF or (iii) is “not essentially equivalent to a dividend” with respect to the U.S. Holder. These tests are explained more fully below.
| 90 |
In determining whether any of the foregoing tests are satisfied, a U.S. Holder takes into account not only stock actually owned by the U.S. Holder, but also shares of MURF common stock that are constructively owned by it. A U.S. Holder may constructively own, in addition to stock owned directly, stock owned by certain related individuals and entities in which the U.S. Holder has an interest or that have an interest in such U.S. Holder, as well as any stock the U.S. Holder has a right to acquire by exercise of an option, which would generally include MURF common stock which could be acquired pursuant to the exercise of the warrants. In order to meet the substantially disproportionate test, the percentage of MURF’s outstanding voting stock actually and constructively owned by the U.S. Holder immediately following the redemption of Public Shares must, among other requirements, be less than 80% of the percentage of MURF’s outstanding voting stock actually and constructively owned by the U.S. Holder immediately before the redemption. There will be a complete termination of a U.S. Holder’s interest if either (i) all of the shares of MURF common stock actually and constructively owned by the U.S. Holder are redeemed or (ii) all of the shares of MURF common stock actually owned by the U.S. Holder are redeemed and the U.S. Holder is eligible to waive, and effectively waives in accordance with specific rules, the attribution of stock owned by certain family members and the U.S. Holder does not constructively own any other MURF common stock. The redemption of Public Shares will not be essentially equivalent to a dividend if a U.S. Holder’s conversion results in a “meaningful reduction” of the U.S. Holder’s proportionate interest in MURF. Whether the redemption will result in a meaningful reduction in a U.S. Holder’s proportionate interest in MURF will depend on the particular facts and circumstances. However, the IRS has indicated in a published ruling that even a small reduction in the proportionate interest of a small minority stockholder in a publicly held corporation who exercises no control over corporate affairs may constitute such a “meaningful reduction.” A U.S. Holder should consult with its own tax advisors as to the tax consequences of a redemption or purchase by MURF.
If none of the foregoing tests is satisfied, then the redemption will be treated as a corporate distribution and the tax effects will be as described under “— U.S. Holders — Taxation of Redemption Treated as a Distribution” below. After the application of those rules, any remaining tax basis of the U.S. Holder in the redeemed Public Shares will be added to the U.S. Holder’s adjusted tax basis in its remaining stock, or, if it has none, to the U.S. Holder’s adjusted tax basis in its warrants or possibly in other stock constructively owned by it.
Gain or Loss on Redemption Treated as Sale of Public Shares.
If the redemption qualifies as a sale or other taxable disposition of Public Shares, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized and the U.S. Holder’s adjusted tax basis in the Public Shares. Any such capital gain or loss generally will be long-term capital gain or loss if the U.S. Holder’s holding period for the Public Shares so disposed of exceeds one year. The initial public offering of the Public Shares closed on February 7, 2022, and any redemption is expected to occur prior to the one-year anniversary of such date. If that is the case, any such capital gain will be short-term capital gain which will be taxed at regular ordinary income tax rates.
Generally, the amount of gain or loss recognized by a U.S. Holder is an amount equal to the difference between (i) the sum of the amount of cash and the fair market value of any property received in such disposition and (ii) the U.S. Holder’s adjusted tax basis in its Public Shares so disposed of. A U.S. Holder’s adjusted tax basis in its Public Shares generally will equal the U.S. Holder’s adjusted cost less any prior distributions treated as a return of capital for U.S. federal income tax purposes.
Taxation of Redemption Treated as a Distribution.
If the redemption does not qualify as a sale of Public Shares, a U.S. Holder will generally be treated as receiving a distribution. Such distributions generally will be included in a U.S. Holder’s gross income, in accordance with such U.S. Holder’s method of accounting for U.S. federal income tax purposes, as dividend income, but only to the extent that such distributions are paid out of MURF’s current or accumulated earnings and profits as determined under U.S. federal income tax principles. Dividends will be taxable to a corporate U.S. Holder at regular rates and will generally be eligible for the dividends received deduction if the requisite holding period is satisfied. Distributions in excess of such earnings and profits generally will be applied against and reduce the U.S. Holder’s basis in its MURF common stock (but not below zero) and, to the extent in excess of such basis, will be treated as gain from the sale or exchange of such MURF common stock in the manner described above under “— U.S. Holders — Gain or Loss on Redemption Treated as a Sale of Public Shares.”
With respect to non-corporate U.S. Holders and with certain exceptions, dividends may be “qualified dividend income,” which is taxed at the lower applicable long-term capital gain rate provided that the U.S. Holder satisfies certain holding period requirements and the U.S. Holder is not under an obligation to make related payments with respect to positions in substantially similar or related property. It is unclear whether the redemption rights with respect to the Public Shares may prevent a U.S. Holder from satisfying the applicable holding period requirements with respect to the dividends received deduction or the preferential tax rate on qualified dividend income, as the case may be, because there is no authority directly on-point with respect to whether such redemption rights diminish a U.S. Holder’s risk of loss with respect to Public Shares in a manner that suspends the holding period for such Public Shares under the applicable holding period requirements. If the holding period requirements are not satisfied, then non-corporate U.S. Holders may be subject to tax on such dividends at regular ordinary income tax rates instead of the preferential rate that applies to qualified dividend income.
U.S. Information Reporting and Backup Withholding.
Distributions with respect to the MURF common stock to a U.S. Holder, whether or not such distributions qualify as dividends for U.S. federal income tax purposes, and proceeds from the sale, exchange or redemption of the MURF common stock by a U.S. Holder generally are subject to information reporting to the IRS and possible U.S. backup withholding, unless the U.S. Holder is an exempt recipient. Backup withholding may apply to such payments if a U.S. Holder fails to furnish a correct taxpayer identification number, a certification of exempt status or has been notified by the IRS that it is subject to backup withholding (and such notification has not been withdrawn).
| 91 |
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a U.S. Holder’s U.S. federal income tax liability, and such holder may obtain a refund of any excess amounts withheld under the backup withholding rules by timely filing the appropriate claim for refund with the IRS and furnishing any required information.
Non-U.S. Holders
Redemption of Public Shares.
The characterization for U.S. federal income tax purposes of the redemption of a Non-U.S. Holder’s Public Shares pursuant to the redemption provisions described in the section of this proxy statement/prospectus entitled “Special Meeting of MURF Stockholders — Redemption Rights” generally will follow the U.S. federal income tax characterization of such a redemption of a U.S. Holder’s Public Shares, as described under “— U.S. Holders — Redemption of Public Shares” above, and the consequences of the redemption to the Non-U.S. Holder will be as described below under “— Non-U.S. Holders — Gain on Redemption Treated as a Sale of Public Shares” and “— Non-U.S. Holders — Taxation of Redemption Treated as a Distribution,” as applicable. It is possible that because the applicable withholding agent may not be able to determine the proper characterization of a redemption of a Non-U.S. Holder’s Public Shares, the withholding agent might treat the redemption as a distribution subject to withholding tax.
Gain on Redemption Treated as a Sale of Public Shares.
A Non-U.S. Holder generally will not be subject to U.S. federal income or withholding tax in respect of any gain realized upon the redemption of Public Shares unless:
| ● | the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the United States to which such gain is attributable); | |
| ● | the Non-U.S. Holder is a nonresident alien individual present in the United States for 183 days or more during the taxable year of the disposition and certain other requirements are met; or | |
| ● | the Public Shares constitute a U.S. real property interest (“USRPI”) by reason of MURF’s status as a U.S. real property holding corporation (“USRPHC”) for U.S. federal income tax purposes. |
Gain described in the first bullet point above generally will be subject to U.S. federal income tax on a net income basis at the regular graduated rates applicable to a U.S. Holder, unless an applicable tax treaty provides otherwise. A Non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on such effectively connected gain, as adjusted for certain items.
Gain described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty), which may be offset by U.S. source capital losses of the Non-U.S. Holder (even though the individual is not considered a resident of the United States), provided the Non-U.S. Holder has timely filed U.S. federal income tax returns with respect to such losses.
With respect to the third bullet above, MURF believes that it is not and has not been at any time since its formation, and does not expect to be immediately after the Business Combination is completed, a USRPHC.
Non-U.S. Holders should consult their tax advisors regarding potentially applicable income tax treaties that may provide for different rules.
Taxation of Redemption Treated as a Distribution.
If a redemption does not qualify as a sale of Public Shares, Non-U.S. Holders will generally be treated as receiving a distribution. Such distributions to the extent paid out of MURF’s current or accumulated earnings and profits (as determined under U.S. federal income tax principles) will constitute dividends for U.S. federal income tax purposes. Amounts not treated as dividends for U.S. federal income tax purposes will constitute a return of capital and first be applied against and reduce a Non-U.S. Holder’s adjusted tax basis in its MURF common stock, but not below zero. Any excess will be treated as capital gain and will be treated as described above under “— Non-U.S. Holders — Gain on Redemption Treated as a Sale of Public Shares.”
Subject to the discussion below on effectively connected income, dividends paid to a Non-U.S. Holder of MURF common stock will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends (or such lower rate specified by an applicable income tax treaty, provided the Non-U.S. Holder furnishes a valid IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) certifying qualification for the lower treaty rate). A Non-U.S. Holder that does not timely furnish the required documentation, but that qualifies for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS.
| 92 |
If dividends paid to a Non-U.S. Holder are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the United States to which such dividends are attributable), the Non-U.S. Holder will be exempt from the U.S. federal withholding tax described above. To claim the exemption, the Non-U.S. Holder must furnish to the applicable withholding agent a valid IRS Form W-8ECI, certifying that the dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States.
Any such effectively connected dividends will be subject to U.S. federal income tax on a net income basis at the same regular graduated U.S. tax rates that apply to United States persons. A Non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on such effectively connected dividends, as adjusted for certain items. Non-U.S. Holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
Information Reporting and Backup Withholding.
Payments of dividends on MURF common stock will not be subject to backup withholding, provided the applicable withholding agent does not have actual knowledge or reason to know that the holder is a United States person and the holder either certifies its non-U.S. status, such as by furnishing a valid IRS Form W-8BEN, W-8BEN-E or W-8ECI, or otherwise establishes an exemption. However, information returns are required to be filed with the IRS in connection with any dividends on MURF common stock paid to the Non-U.S. Holder, regardless of whether any tax was actually withheld. In addition, proceeds from a sale or other taxable disposition of MURF common stock within the United States or conducted through certain U.S.-related brokers generally will not be subject to backup withholding or information reporting if the applicable withholding agent receives the certification described above and does not have actual knowledge or reason to know that such holder is a United States person, or the holder otherwise establishes an exemption. Proceeds from a disposition of MURF common stock conducted through a non-U.S. office of a non-U.S. broker generally will not be subject to backup withholding or information reporting.
Copies of information returns that are filed with the IRS may also be made available under the provisions of an applicable treaty or agreement to the tax authorities of the country in which the Non-U.S. Holder resides or is established. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a Non-U.S. Holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS.
Additional Withholding Tax on Payments Made to Foreign Accounts.
Withholding taxes may be imposed under Sections 1471 to 1474 of the Code (such Sections commonly referred to as the Foreign Account Tax Compliance Act, or “FATCA”) on certain types of payments made to non-U.S. financial institutions and certain other non-U.S. entities. Specifically, a 30% withholding tax may be imposed on dividends on, or (subject to the proposed Treasury Regulations discussed below) gross proceeds from the sale or disposition of MURF common stock paid to a “foreign financial institution” or a “non-financial foreign entity” (each as defined in the Code), unless (1) the foreign financial institution undertakes certain diligence and reporting obligations, (2) the non-financial foreign entity either certifies it does not have any “substantial United States owners” (as defined in the Code) or furnishes identifying information regarding each substantial United States owner, or (3) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from these rules. If the payee is a foreign financial institution and is subject to the diligence and reporting requirements in (1) above, it must enter into an agreement with the U.S. Department of the Treasury requiring, among other things, that it undertake to identify accounts held by certain “specified United States persons” or “United States-owned foreign entities” (each as defined in the Code), annually report certain information about such accounts, and withhold 30% on certain payments to non-compliant foreign financial institutions and certain other account holders. Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules.
Under the applicable Treasury regulations and administrative guidance, withholding under FATCA generally applies to payments of dividends on MURF common stock. While withholding under FATCA would have applied also to payments of gross proceeds from the sale or other disposition of MURF common stock on or after January 1, 2019, proposed Treasury Regulations eliminate FATCA withholding on payments of gross proceeds entirely. Taxpayers generally may rely on these proposed Treasury Regulations until final Treasury Regulations are issued.
Non-U.S. Holders should consult their tax advisors regarding the potential application of withholding under FATCA.
| 93 |
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Defined terms included below have the same meaning as terms defined and included elsewhere in this proxy statement/prospectus. All dollar amounts are expressed in thousands of United States dollars (“$”), unless otherwise indicated.
Introduction
The following unaudited pro forma condensed combined financial statements are provided to aid you in your analysis of the financial aspects of the Transactions and adjustments for other material events. These other material events are referred to herein as “Material Events” and the pro forma adjustments for the Material Events are referred to herein as “Adjustments for Material Events.”
The unaudited pro forma condensed combined financial statements have been prepared based on MURF’s historical financial statements and Conduit’s historical financial statements as adjusted to give effect to the Transactions and Material Events. The unaudited pro forma condensed combined balance sheet as of March 31, 2023 gives pro forma effect to the Transactions and Material Events as if they had occurred on March 31, 2023. The unaudited pro forma condensed combined statement of operations for the year ended December 31, 2022 gives effect to the Transactions and Material Events as if they had occurred on January 1, 2022. The unaudited pro forma condensed combined statement of operations for the three months ended March 31, 2023 gives effect to the Transactions and Material Events as if they had occurred on January 1, 2022.
The unaudited pro forma condensed combined financial statements have been presented for illustrative purposes only and do not necessarily reflect what New Conduit’s financial condition or results of operations would have been had the Transactions and Material Events occurred on the dates indicated. Further, the pro forma condensed combined financial information may not be useful in predicting the future financial condition and results of operations of New Conduit. The actual financial position and results of operations may differ significantly from the pro forma amounts reflected herein due to a variety of factors.
The unaudited pro forma condensed combined financial statements have been derived from and should be read in conjunction with:
| ● | the accompanying notes to the unaudited pro forma condensed combined financial statements; |
| ● | the historical audited financial statements of MURF as of and for the year ended December 31, 2022, and the historical unaudited financial statements of MURF as of and for the three months ended March 31, 2023, and the related notes included elsewhere in this proxy statement/prospectus; |
| ● | the historical audited financial statements of Conduit as of and for the year ended December 31, 2022, and the historical unaudited financial statements of Conduit as of and for the three months ended March 31, 2023, and the related notes included elsewhere in this proxy statement/prospectus; and |
| ● | the sections entitled “MURF’s Management’s Discussion and Analysis of Financial Condition and Results of Operations”, “Conduit’s Management’s Discussion and Analysis of Financial Condition and Results of Operations”, and other financial information relating to each of MURF and Conduit included elsewhere in this proxy statement/prospectus. |
Description of the Business Combination
On November 8, 2022, Murphy Canyon Acquisition Corp., a Delaware corporation, Conduit Pharmaceuticals Limited, a Cayman Islands exempt company, and Conduit Merger Sub, Inc., a Cayman Islands exempt company and wholly-owned subsidiary of MURF (“Merger Sub”), entered into the Merger Agreement, which contains customary representations and warranties, covenants, closing conditions, termination provisions and other terms relating to the transactions contemplated thereby. Pursuant to the Merger Agreement, Merger Sub will merge with and into Conduit with Conduit surviving the merger (the “Merger”). As a result of the Merger, Conduit will become a wholly-owned subsidiary of MURF, with the shareholders of Conduit becoming stockholders of MURF.
| 94 |
As a result of the Merger, all of the issued and outstanding MURF Class A common stock (including the MURF Class B common stock that is automatically converted on a one-for-one basis into MURF Class A common stock upon the consummation of the Business Combination) will be converted into one class of New Conduit common stock and each issued and outstanding warrant to purchase a share of MURF Class A common stock will become exercisable for one share of New Conduit common stock.
Pursuant to the terms of the Merger Agreement, the aggregate number of shares of New Conduit common stock that are to be delivered as consideration in the Merger is capped at 65,000,000 shares, which includes the consideration to be delivered to holders of the Conduit convertible notes on an as converted basis. Accordingly, in the Merger, each holder of Conduit convertible notes, on an as-converted basis, and each holder of Conduit ordinary shares will be entitled to receive such holder’s pro rata portion of the 65,000,000 shares of New Conduit common stock to be delivered as merger consideration. Please see “Exchange of Conduit Shares for Shares of New Conduit” below.
For additional information about the Business Combination, please see the section entitled “The Business Combination Proposal.”
Basis of Pro Forma Presentation
The unaudited pro forma condensed combined financial statements were prepared in accordance with Article 11 of SEC Regulation S-X, as amended by the final rule, Release No. 33-10786, Amendments to Financial Disclosures about Acquired and Disposed Businesses. Release No. 33-10786 replaces the historical pro forma adjustments criteria with simplified requirements to depict the accounting for the transaction (“Transaction Accounting Adjustments”) and present the reasonably estimable synergies and other transaction effects that have occurred or are reasonably expected to occur (“Management’s Adjustments”). MURF has elected not to present Management’s Adjustments and will only be presenting Transaction Accounting Adjustments in the unaudited pro forma condensed combined financial information. The adjustments presented in the unaudited pro forma condensed combined financial statements have been identified and presented to provide relevant information necessary for an understanding of the combined company upon consummation of the Transactions.
The unaudited pro forma condensed combined balance sheet as of March 31, 2023, was derived from the unaudited historical balance sheet of MURF as of March 31, 2023, and the unaudited historical balance sheet of Conduit as of March 31, 2023 and gives effect to the Transactions and Material Events as if they had occurred on March 31, 2023. The unaudited pro forma condensed combined statement of operations for the year ended December 31, 2022, combines the historical statement of operations of MURF for the year ended December 31, 2022, and the historical statement of operations of Conduit for the year ended December 31, 2022, and gives effect to the Transactions and Material Events as if they had occurred on January 1, 2022. The unaudited pro forma condensed combined statement of operations for the three months ended March 31, 2023, combines the historical statement of operations of MURF for the three months ended March 31, 2023, and the historical statement of operations of Conduit for the three months ended March 31, 2023, and gives effect to the Transactions and Material Events as if they had occurred on January 1, 2022.
Management has made significant estimates and assumptions in its determination of the pro forma adjustments. As the unaudited pro forma condensed combined financial information has been prepared based on these preliminary estimates, the final amounts recorded may differ materially from the information presented.
The pro forma adjustments reflecting the consummation of the Transactions and Material Events are based on certain currently available information and certain assumptions and methodologies that each of MURF and Conduit believes are reasonable under the circumstances. The pro forma adjustments, which are described in the accompanying notes, may be revised as additional information becomes available and is evaluated. Therefore, it is likely that the actual adjustments will differ from the pro forma adjustments, and it is possible the differences may be material. Each of MURF and Conduit believes that its assumptions and methodologies provide a reasonable basis for presenting all the significant effects of the Transactions and Material Events based on information available to management at this time and that the pro forma adjustments give appropriate effect to those assumptions and are properly applied in the unaudited pro forma condensed combined financial information.
| 95 |
The unaudited pro forma condensed combined financial information does not give effect to any anticipated synergies, operating efficiencies, tax savings, or cost savings that may be associated with the Transactions. MURF and Conduit have not had any historical relationship prior to the Transactions. Accordingly, no pro forma adjustments were required to eliminate activities between the companies.
The unaudited pro forma condensed combined financial information presents two redemption scenarios as follows:
| ● | Assuming No Redemption Scenario: This scenario, which we refer to as the “No Redemption Scenario” assumes that no MURF Public Stockholders exercise their right to have their MURF Class A common stock converted into their pro rata share of the Trust Account and thus the full amount held in the Trust Account as of the closing is available for the Business Combination; and |
| ● | Assuming Maximum Redemption Scenario: This scenario, which we refer to as the “Maximum Redemption Scenario” assumes that all 2,187,728 shares of MURF Class A common stock subject to possible are redeemed, resulting in an aggregate cash payment of approximately $22.9 million out of the Trust Account based on an assumed redemption price of $10.45 per share. The Maximum Redemption Scenario includes all adjustments contained in the No Redemption Scenario and presents additional adjustments to reflect the effect of the Maximum Redemption Scenario. The 2,187,728 shares assumed redeemed in the maximum redemption scenario is calculated as the 13,225,000 shares of MURF Class A common stock subject to possible redemption outstanding as of December 31, 2022 less the redemption in February 2023 of 11,037,272 shares of MURF Class A common stock subject to possible redemption. |
The foregoing scenarios are for illustrative purposes as MURF does not have, as of the date of this proxy statement/prospectus, a meaningful way of providing any certainty regarding the number of redemptions by Public Stockholders that may occur.
Included in the shares outstanding and weighted-average shares outstanding as presented in the unaudited pro forma condensed combined financial statements are the shares of New Conduit common stock to be issued to legacy Conduit shareholders under the No Redemption Scenario and the Maximum Redemption Scenario on the closing date of the Business Combination, the shares of New Conduit common stock that are held by existing MURF investors (as adjusted, where applicable, for the Maximum Redemption Scenario), and the shares of New Conduit common stock to be issued in respect of the PIPE Shares.
Upon consummation of the Transactions, assuming no Public Stockholders elect to redeem their shares for cash, Conduit’s shareholders will own approximately 90.0% of the shares of New Conduit common stock, MURF’s Public Stockholders will own approximately 2.9% of the shares of New Conduit common stock, MURF Directors will own approximately 0.1% of the shares of New Conduit common stock, MURF’s Sponsor will own approximately 5.3% of the shares of New Conduit common stock, and A.G.P. will own approximately 1.7% of the shares of New Conduit common stock, based on the number of shares of MURF Class A common stock and MURF Class B common stock outstanding as of March 31, 2023 (in each case, not giving effect to any shares of New Conduit common stock issuable upon the exercise of any warrants or options).
| 96 |
| Scenario 1 | ||||||||
| Assuming No Redemptions | ||||||||
| Number of Shares Owned | % Ownership | |||||||
| (Shares in thousands) | ||||||||
| Conduit shareholders(2) | 65,000 | 86.4 | % | |||||
| Private Placement Investor(2) | 2,700 | 3.6 | % | |||||
| MURF Public stockholders | 2,188 | 2.9 | % | |||||
| MURF Directors(1) | 45 | 0.1 | % | |||||
| MURF Sponsor(1) | 4,015 | 5.3 | % | |||||
| A.G.P. | 1,300 | 1.7 | % | |||||
| Total(3) | 75,248 | 100.0 | % | |||||
1) In connection with the Business Combination, 3,306,250 shares of MURF Class B common stock held by the Sponsor will be converted into 3,306,250 shares of MURF Class A common stock. The 4,015,250 shares of New Conduit common stock that will be held by the MURF Sponsor (as reflected in the table above) will come from the MURF Sponsor exchanging the 3,306,250 shares of MURF Class A common stock for 3,306,250 shares of New Conduit common stock upon the closing of the Business Combination and from the MURF Sponsor exchanging an additional 709,000 shares of MURF Class A common stock that it will hold (prior to the closing of the Business Combination) for 709,000 shares of New Conduit common stock upon the closing of the Business Combination. The 709,000 shares taking part in the above-mentioned exchange were originally issued to the MURF Sponsor as part of the Private Placement and the 3,306,250 shares taking part in the above-mentioned exchange were originally issued to the MURF Sponsor prior to MURF’s initial public offering. The 45,000 shares of New Conduit common stock that will be held by MURF Directors will come from the MURF Sponsor transferring (in connection with the Business Combination) to the MURF Directors 45,000 shares of MURF Class A common stock. The 45,000 shares of MURF Class A common stock will be exchanged for 45,000 shares of New Conduit common stock upon the closing of the Business Combination.
2) In connection with the Business Combination, 2,000 Conduit ordinary shares will be exchanged for 64,616,510 shares of New Conduit common stock and Conduit convertible notes will be redeemed for 383,490 shares of New Conduit common stock (refer to the Exchange of Conduit Shares for Shares of New Conduit section below for details). The remaining 2,700,000 shares will be issued in connection with the PIPE Financing to a related party of a Conduit shareholder.
3) Note that the shares as presented above do not include any shares related to a member of management of New Conduit who will be granted restricted stock units (“RSUs”) 0.10% of the shares of common stock of New Conduit outstanding immediately as of the closing of the Business Combination. No shares related to this grant are included because the RSUs will vest in equal annual installments on the first three anniversaries of the closing of the Business Combination.
If, upon consummation of the Transactions, 2,187,728 shares of MURF Class A common stock (which represents 100.0% of the shares of MURF Class A common stock subject to possible redemption) are redeemed for cash, Conduit’s shareholders will own approximately 92.6% of the shares of New Conduit common stock, MURF’s Public Stockholders will not own any of the shares of New Conduit common stock, MURF Directors will own approximately 0.1% of the shares of New Conduit common stock, MURF’s Sponsor will own approximately 5.5% of the shares of New Conduit common stock, and A.G.P. will own approximately 1.8% of the shares of New Conduit common stock, based on the number of shares of MURF Class A common stock and MURF Class B common stock outstanding as of March 31, 2023 (in each case, not giving effect to any shares of New Conduit common stock issuable upon the exercise of any warrants or options).
| 97 |
| Scenario 2 | ||||||||
| Assuming Maximum Redemptions | ||||||||
| Number of Shares Owned | % Ownership | |||||||
| (Shares in thousands) | ||||||||
| Conduit shareholders(2) | 65,000 | 89.0 | % | |||||
| Private Placement Investor(2) | 2,700 | 3.6 | % | |||||
| MURF Public stockholders | - | 0.0 | % | |||||
| MURF Directors(1) | 45 | 0.1 | % | |||||
| MURF Sponsor(1) | 4,015 | 5.5 | % | |||||
| A.G.P. | 1,300 | 1.8 | % | |||||
| Total(3) | 73,060 | 100.0 | % | |||||
1) In connection with the Business Combination, 3,306,250 shares of MURF Class B common stock held by the Sponsor will be converted into 3,306,250 shares of MURF Class A common stock. The 4,015,250 shares of New Conduit common stock that will be held by the MURF Sponsor (as reflected in the table above) will come from the MURF Sponsor exchanging the 3,306,250 shares of MURF Class A common stock for 3,306,250 shares of New Conduit common stock upon the closing of the Business Combination and from the MURF Sponsor exchanging an additional 709,000 shares of MURF Class A common stock that it will hold (prior to the closing of the Business Combination) for 709,000 shares of New Conduit common stock upon the closing of the Business Combination. The 709,000 shares taking part in the above-mentioned exchange were originally issued to the MURF Sponsor as part of the Private Placement and the 3,306,250 shares taking part in the above-mentioned exchange were originally issued to the MURF Sponsor prior to MURF’s initial public offering. The 45,000 shares of New Conduit common stock that will be held by MURF Directors will come from the MURF Sponsor transferring (in connection with the Business Combination) to the MURF Directors 45,000 shares of MURF Class A common stock. The 45,000 shares of MURF Class A common stock will be exchanged for 45,000 shares of New Conduit common stock upon the closing of the Business Combination.
2) In connection with the Business Combination, 2,000 Conduit ordinary shares will be exchanged for 64,616,510 shares of New Conduit common stock and Conduit convertible notes will be redeemed for 383,490 shares of New Conduit common stock (refer to the Exchange of Conduit Shares for Shares of New Conduit section below for details). The remaining 2,700,000 shares will be issued in connection with the PIPE Financing to a related party of a Conduit shareholder.
3) Note that the shares as presented above do not include any shares related to a member of management of New Conduit who will be granted restricted stock units (“RSUs”) 0.10% of the shares of common stock of New Conduit outstanding immediately as of the closing of the Business Combination. No shares related to this grant are included because the RSUs will vest in equal annual installments on the first three anniversaries of the closing of the Business Combination.
Material Events and Background Relevant to Material Events
On April 20, 2023, Conduit entered into an agreement with Vela Technologies PLC (“Vela”) which granted Vela the right, but not the obligation, to sell its 8% royalty interest in the Covid Asset back to Conduit (the “Vela Put Option”). In accordance with the Vela Put Option, Vela paid a one-time, non-refundable option fee to Conduit of $0.5 million (£0.4 million). Total consideration payable to Vela upon exercise of the Vela Put Option is $4.8 million (£4.0 million) worth of shares of New Conduit common stock, following the consummation of the Merger at a price per share equal to the volume-weighted average price per share over the ten (10) business days prior to the date of the notice of the exercise of the option. The Vela Put Option contains a provision that in no event shall the price per share for the consideration shares be lower than $5 or higher than $15. The option is exercisable in whole at any time from the close of the Merger (the “Effective Time”) until the earlier of (i) the date that is six (6) months from the Effective Time, and (ii) February 7, 2024.
| 98 |
On April 5, 2023, MURF’s sponsor advanced $0.2 million to MURF under a promissory note with a maximum borrowable amount of $1.5 million that was originally issued on March 7, 2023. The purpose of the note is to fund the Trust Account and MURF’s operating expenses. On April 6, 2023 and May 4, 2023 MURF deposited $79 thousand and $78 thousand, respectively, into the Trust Account. In addition, $43 thousand from the $0.2 million advance was deposited into cash and cash equivalents.
Exchange of Conduit Shares for Shares of New Conduit
Based on 2,000 Conduit ordinary shares outstanding immediately prior to the closing of the Business Combination, the estimated Exchange Ratio determined in accordance with the terms of the Merger Agreement is approximately 32,308.2548, subject to variation related to an increase (or decrease) in shares outstanding prior to Closing. We are not expecting an increase (or decrease) in shares outstanding prior to Closing. New Conduit expects to issue 65,000,000 shares of New Conduit common stock to legacy Conduit shareholders (including holders of Conduit’s convertible notes payable) in the Business Combination, determined as follows:
Conduit shares outstanding as of March 31, 2023 (Historical) | ||||
| Ordinary shares, par value £0.0001 per share | 2,000 | |||
| Assumed Exchange Ratio | 32,308.2548 | |||
| Estimated shares of New Conduit common stock issued to Conduit shareholders upon Closing(1) | 64,616,510 | |||
1) In addition to the shares issued to legacy Conduit shareholders noted above, an additional 383,490 shares of New Conduit common stock will be issued to Conduit convertible note holders, resulting in a total of 65,000,000 shares of New Conduit common stock being issued to Conduit shareholders and holders of Conduit convertible notes payable. The 383,490 shares resulting from the redemption of the convertible notes payable was calculated by taking the principal of the convertible notes payable outstanding in Great British Pounds (“GBP”) of £2.4 million and applying the GBP-to-United States Dollars (“USD”) exchange rate as of June 30, 2023 to convert the principal to approximately $3.1 million USD. The Conversion Price is equal to the $10.00 price per share per the PIPE Financing discounted by 20% for the Change of Control ($10.00 * 20% discount = $8.00). The 383,490 shares resulting from the redemption of the convertible notes payable is calculated by taking the approximately $3.1 million outstanding principal and dividing this amount by $8.00.
Accounting for the Business Combination
Notwithstanding the legal form, the Business Combination will be accounted for as a reverse recapitalization in accordance with U.S. GAAP. Under this method of accounting, MURF will be treated as the acquired company for accounting purposes, whereas Conduit will be treated as the accounting acquirer. In accordance with this method of accounting, the Business Combination will be treated as the equivalent of Conduit issuing shares for the net assets of MURF, accompanied by a recapitalization. The net assets of Conduit will be stated at historical cost, with no goodwill or other intangible assets recorded, and operations prior to the Business Combination will be those of Conduit. Conduit has been determined to be the accounting acquirer for purposes of the Business Combination based on an evaluation of the following facts and circumstances:
| ● | Conduit shareholders will have a majority of the voting interest in New Conduit under both the No Redemption Scenario and the Maximum Redemption Scenario; | |
| ● | The largest single shareholder of New Conduit will be a legacy shareholder of Conduit; | |
| ● | Conduit will designate a majority of the governing body of New Conduit. | |
| ● | An individual from Conduit will be designated as the chairman of the governing body of New Conduit and the Chief Executive Officer of New Conduit; and | |
| ● | Conduit’s operations will comprise the ongoing operations of New Conduit. |
| 99 |
UNAUDITED PRO FORMA CONDENSED COMBINED BALANCE SHEET
AS OF MARCH 31, 2023
(in thousands)
| Scenario 1 Assuming No Redemptions into Cash | Scenario 2 Assuming Maximum Redemptions into Cash | |||||||||||||||||||||||||||||||||||||||
Transaction Accounting Adjustments | Transaction Accounting Adjustments | |||||||||||||||||||||||||||||||||||||||
| MURF Historical | Conduit Historical | Adjustments for Material Events | Notes | Other Transaction Accounting Adjustments | Notes | Pro Forma Balance Sheet | Other Transaction | Notes | Pro Forma Balance Sheet | |||||||||||||||||||||||||||||||
| ASSETS | ||||||||||||||||||||||||||||||||||||||||
| Current assets: | ||||||||||||||||||||||||||||||||||||||||
| Cash and cash equivalents | $ | 91 | $ | 8 | $ | 498 | a | $ | (4,629 | ) | d | $ | 31,705 | $ | (22,862 | ) | o | $ | 8,843 | |||||||||||||||||||||
| - | - | 43 | b | 23,816 | c | - | - | - | ||||||||||||||||||||||||||||||||
| - | - | - | 27,000 | f | - | - | - | |||||||||||||||||||||||||||||||||
| - | - | - | (14,622 | ) | k | - | - | - | ||||||||||||||||||||||||||||||||
| - | - | - | (500 | ) | n | - | - | - | ||||||||||||||||||||||||||||||||
| Prepaid expenses - current | 219 | - | - | - | 219 | - | 219 | |||||||||||||||||||||||||||||||||
| Total current assets | 310 | 8 | 541 | $ | 31,065 | 31,924 | (22,862 | ) | 9,062 | |||||||||||||||||||||||||||||||
| Non-current assets: | ||||||||||||||||||||||||||||||||||||||||
| Investments held in Trust Account | 23,659 | - | 157 | b | (23,816 | ) | c | - | - | - | ||||||||||||||||||||||||||||||
| Intangible assets - research and development | - | 5 | - | - | 5 | - | 5 | |||||||||||||||||||||||||||||||||
| Total non-current assets | 23,659 | 5 | 157 | (23,816 | ) | 5 | - | 5 | ||||||||||||||||||||||||||||||||
| Total assets | $ | 23,969 | $ | 13 | $ | 698 | $ | 7,249 | $ | 31,929 | $ | (22,862 | ) | $ | 9,067 | |||||||||||||||||||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||||||||||||||||||||||||||||||||||
| Current liabilities: | ||||||||||||||||||||||||||||||||||||||||
| Accrued expenses and other current liabilities | $ | 1,433 | $ | - | $ | - | $ | (7 | ) | k | $ | 285 | $ | - | $ | 285 | ||||||||||||||||||||||||
| - | - | - | (1,141 | ) | m | - | - | - | ||||||||||||||||||||||||||||||||
| Accrued professional fees | - | 2,419 | - | (2,204 | ) | k | 215 | - | 215 | |||||||||||||||||||||||||||||||
| Accrued payroll and other current liabilities | - | 25 | - | - | 25 | - | 25 | |||||||||||||||||||||||||||||||||
| Option liability | - | 1,309 | 1,402 | a | - | 2,711 | - | 2,711 | ||||||||||||||||||||||||||||||||
| Income taxes payable | 224 | - | - | - | k | 224 | - | 224 | ||||||||||||||||||||||||||||||||
| Note payable - Sponsor | 300 | - | 200 | b | (500 | ) | n | - | - | - | ||||||||||||||||||||||||||||||
| Total current liabilities | 1,958 | 3,753 | 1,602 | (3,852 | ) | 3,460 | - | 3,460 | ||||||||||||||||||||||||||||||||
| Non-current liabilities: | ||||||||||||||||||||||||||||||||||||||||
| Convertible notes payable, carried at fair value | - | 3,620 | - | (3,077 | ) | h | - | - | - | |||||||||||||||||||||||||||||||
| - | - | - | (543 | ) | j | - | - | - | ||||||||||||||||||||||||||||||||
| Liability related to the sale of future revenue | - | 4,173 | - | - | 4,173 | - | 4,173 | |||||||||||||||||||||||||||||||||
| Convertible promissory note payable | - | 805 | - | - | 805 | - | 805 | |||||||||||||||||||||||||||||||||
| Notes payable | - | 179 | - | - | 179 | - | 179 | |||||||||||||||||||||||||||||||||
| Derivative warrant liability | - | - | - | 243 | f | 243 | - | 243 | ||||||||||||||||||||||||||||||||
| Deferred commission payable | 4,629 | - | - | (4,629 | ) | d | - | - | - | |||||||||||||||||||||||||||||||
| Total non-current liabilities | 4,629 | 8,777 | - | (8,006 | ) | 5,400 | - | 5,400 | ||||||||||||||||||||||||||||||||
See accompanying notes to the unaudited pro forma condensed combined financial information.
| 100 |
UNAUDITED PRO FORMA CONDENSED COMBINED BALANCE SHEET
AS OF MARCH 31, 2023
(in thousands)
| Scenario 1 Assuming No Redemptions into Cash | Scenario 2 Assuming Maximum Redemptions into Cash | |||||||||||||||||||||||||||||||||||||||
Transaction Accounting Adjustments | Transaction Accounting Adjustments | |||||||||||||||||||||||||||||||||||||||
| MURF Historical | Conduit Historical | Adjustments for Material Events | Notes | Other Transaction Accounting Adjustments | Notes | Pro Forma Balance Sheet | Other Transaction Accounting Adjustments | Notes | Pro Forma Balance Sheet | |||||||||||||||||||||||||||||||
| Common stock subject to possible redemption at redemption value (2,187,728 shares) | 22,862 | - | - | (22,862 | ) | g | - | - | - | |||||||||||||||||||||||||||||||
| Stockholders’ equity/(deficit): | ||||||||||||||||||||||||||||||||||||||||
| Conduit ordinary shares | - | - | - | - | h | - | - | - | ||||||||||||||||||||||||||||||||
| - | - | - | - | i | - | |||||||||||||||||||||||||||||||||||
| MURF Class A common stock | - | - | - | - | e | - | - | - | ||||||||||||||||||||||||||||||||
| - | - | - | - | l | - | - | - | |||||||||||||||||||||||||||||||||
| MURF Class B common stock | - | - | - | - | e | - | - | - | ||||||||||||||||||||||||||||||||
| New Conduit common stock | - | - | - | - | f | 7 | - | o | 7 | |||||||||||||||||||||||||||||||
| - | - | - | - | g | - | - | - | |||||||||||||||||||||||||||||||||
| - | - | - | - | k | - | - | - | |||||||||||||||||||||||||||||||||
| - | - | - | 6 | i | - | - | - | |||||||||||||||||||||||||||||||||
| - | - | - | - | l | - | - | - | |||||||||||||||||||||||||||||||||
| Additional paid-in capital | - | - | - | 26,649 | f | 38,079 | (22,862 | ) | o | 15,217 | ||||||||||||||||||||||||||||||
| - | - | - | 22,862 | g | - | - | - | |||||||||||||||||||||||||||||||||
| - | - | - | 3,156 | h | - | - | - | |||||||||||||||||||||||||||||||||
| - | - | - | (5,486 | ) | i | - | - | - | ||||||||||||||||||||||||||||||||
| - | - | - | (9,781 | ) | k | - | - | - | ||||||||||||||||||||||||||||||||
| - | - | - | 679 | j | - | - | - | |||||||||||||||||||||||||||||||||
| Accumulated deficit | (5,480 | ) | (12,929 | ) | (904 | ) | a | (79 | ) | h | (15,429 | ) | - | (15,429 | ) | |||||||||||||||||||||||||
| - | - | - | 5,480 | i | - | - | - | |||||||||||||||||||||||||||||||||
| - | - | - | (2,630 | ) | k | - | - | - | ||||||||||||||||||||||||||||||||
| - | - | - | (136 | ) | j | - | - | - | ||||||||||||||||||||||||||||||||
| - | - | - | 108 | f | - | - | - | |||||||||||||||||||||||||||||||||
| - | - | - | 1,141 | m | - | - | - | |||||||||||||||||||||||||||||||||
| Accumluated other comprehensive income | - | 412 | - | - | 412 | - | 412 | |||||||||||||||||||||||||||||||||
| Total stockholders’ equity (deficit): | (5,480 | ) | (12,517 | ) | (904 | ) | 41,969 | 23,069 | (22,862 | ) | 207 | |||||||||||||||||||||||||||||
| Total liabilities and stockholders’ equity (deficit) | $ | 23,969 | $ | 13 | $ | 698 | $ | 7,249 | $ | 31,929 | $ | (22,862 | ) | $ | 9,067 | |||||||||||||||||||||||||
See accompanying notes to the unaudited pro forma condensed combined financial information.
| 101 |
UNAUDITED
PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
FOR THE YEAR ENDED DECEMBER 31, 2022
(in thousands, except share and per share amounts)
| Scenario
1 Assuming No Redemptions into Cash |
Scenario 2 Assuming Maximum Redemptions into Cash | |||||||||||||||||||||||||||||||||||||||||||||
Transaction Accounting Adjustments |
Transaction Accounting Adjustments |
|||||||||||||||||||||||||||||||||||||||||||||
| (In thousands, except per share and weighted-average share data) | MURF Historical | Conduit Historical | Adjustments for Material Events | Notes | Other
Transaction Accounting Adjustments |
Notes | Pro Forma Statement of Operations | Other
Transaction Accounting Adjustments |
Notes | Pro
Forma Statement of Operations |
Notes | |||||||||||||||||||||||||||||||||||
| Operating expenses (income): | ||||||||||||||||||||||||||||||||||||||||||||||
| Research and devlopment expenses | $ | - | $ | 37 | $ | - | $ | - | $ | 37 | $ | - | $ | 37 | ||||||||||||||||||||||||||||||||
| General and administrative expenses | 111 | 3,049 | - | 2,630 | c | 6,039 | (7 | ) | f | 6,032 | ||||||||||||||||||||||||||||||||||||
| - | - | - | 249 | f | - | - | - | |||||||||||||||||||||||||||||||||||||||
| Administration fee - related party | 1,093 | - | - | - | 1,093 | - | 1,093 | |||||||||||||||||||||||||||||||||||||||
| Funding expenses | - | 74 | - | - | 74 | - | 74 | |||||||||||||||||||||||||||||||||||||||
| Total operating expenses (income) | 1,204 | 3,160 | - | 2,879 | 7,243 | (7 | ) | 7,236 | ||||||||||||||||||||||||||||||||||||||
| Income (loss) from operations | (1,204 | ) | (3,160 | ) | - | (2,879 | ) | (7,243 | ) | 7 | (7,236 | ) | ||||||||||||||||||||||||||||||||||
| Other income (expense): | ||||||||||||||||||||||||||||||||||||||||||||||
| Loss on extinguishment of debt | - | - | - | (215 | ) | d | (215 | ) | - | (215 | ) | |||||||||||||||||||||||||||||||||||
| Interest income - Investments held in Trust Account | 1,976 | - | - | (1,976 | ) | b | - | - | - | |||||||||||||||||||||||||||||||||||||
| Other income (expense), net | - | (1,727 | ) | 3,618 | a | 162 | e | 2,053 | - | 2,053 | ||||||||||||||||||||||||||||||||||||
| Total other income (expense), net | 1,976 | (1,727 | ) | 3,618 | (2,029 | ) | 1,838 | - | 1,838 | |||||||||||||||||||||||||||||||||||||
| Income (loss) before taxes | 772 | (4,887 | ) | 3,618 | (4,908 | ) | (5,405 | ) | 7 | (5,398 | ) | |||||||||||||||||||||||||||||||||||
| Income tax expense | (375 | ) | - | - | - | (375 | ) | - | (375 | ) | ||||||||||||||||||||||||||||||||||||
| Net income (loss) | $ | 397 | $ | (4,887 | ) | $ | 3,618 | $ | (4,908 | ) | $ | (5,780 | ) | $ | 7 | $ | (5,773 | ) | ||||||||||||||||||||||||||||
| Basic and diluted net loss per share, Conduit ordinary shares | $ | (2,444 | ) | |||||||||||||||||||||||||||||||||||||||||||
| Basic and diluted weighted average shares outstanding, Conduit ordinary shares | 2,000 | |||||||||||||||||||||||||||||||||||||||||||||
| Basic and diluted earnings attributable to MURF Class A common stock | $ | 316 | ||||||||||||||||||||||||||||||||||||||||||||
| Basic and diluted weighted average shares outstanding, MURF Class A common stock | 12,558,058 | |||||||||||||||||||||||||||||||||||||||||||||
| Basic and diluted earnings per share attributable to MURF Class A common stock | $ | 0.03 | ||||||||||||||||||||||||||||||||||||||||||||
| Basic and diluted earnings attributable to MURF Class B common stock | $ | 83 | ||||||||||||||||||||||||||||||||||||||||||||
| Basic and diluted weighted average shares outstanding, MURF Class B common stock | 3,306,250 | |||||||||||||||||||||||||||||||||||||||||||||
| Basic and diluted earnings per share attributable to MURF Class B common stock | $ | 0.03 | ||||||||||||||||||||||||||||||||||||||||||||
| Basic and diluted net loss per share attributable to New Conduit common stock | $ | (0.08 | ) | $ | (0.08 | ) | ||||||||||||||||||||||||||||||||||||||||
| Basic and diluted weighted average shares outstanding attributable to New Conduit common stock | 75,247,978 | g | 73,060,250 | g | ||||||||||||||||||||||||||||||||||||||||||
See accompanying notes to the unaudited pro forma condensed combined financial information
| 102 |
UNAUDITED
PRO FORMA CONDENSED COMBINED STATEMENT OF OPERATIONS
FOR THE THREE MONTHS ENDED MARCH 31, 2023
(in thousands, except share and per share amounts)
| Scenario 1 Assuming No Redemptions into Cash | Scenario 2 Assuming Maximum Redemptions into Cash | |||||||||||||||||||||||||||||||||||
| (In thousands, except per share and weighted-average share data) | MURF Historical | Conduit Historical | Transaction Accounting Adjustments | Notes | Pro Forma Statement of Operations | Transaction Accounting Adjustments | Notes | Pro Forma Statement of Operations | Notes | |||||||||||||||||||||||||||
| Operating expenses (income): | ||||||||||||||||||||||||||||||||||||
| General and administrative expenses | $ | 477 | $ | 2,008 | $ | 62 | f | $ | 2,547 | $ | (2 | ) | f | $ | 2,545 | |||||||||||||||||||||
| Administration fee - related party | 30 | - | - | 30 | - | 30 | ||||||||||||||||||||||||||||||
| Total operating expenses (income) | 507 | 2,008 | 62 | 2,577 | (2 | ) | 2,575 | |||||||||||||||||||||||||||||
| Income (loss) from operations | (507 | ) | (2,008 | ) | (62 | ) | (2,577 | ) | 2 | (2,575 | ) | |||||||||||||||||||||||||
| Other income (expense): | ||||||||||||||||||||||||||||||||||||
| Interest income - Investments held in Trust Account | 664 | - | (664 | ) | b | - | - | - | ||||||||||||||||||||||||||||
| Other expense, net | - | (157 | ) | (54 | ) | e | (211 | ) | - | (211 | ) | |||||||||||||||||||||||||
| Total other income (expense), net | 664 | (157 | ) | (718 | ) | (211 | ) | - | (211 | ) | ||||||||||||||||||||||||||
| Income (loss) before taxes | 157 | (2,165 | ) | (780 | ) | (2,788 | ) | 2 | (2,786 | ) | ||||||||||||||||||||||||||
| Income tax expense | (37 | ) | - | - | (37 | ) | - | (37 | ) | |||||||||||||||||||||||||||
| Net income (loss) | $ | 120 | $ | (2,165 | ) | $ | (780 | ) | $ | (2,825 | ) | 2 | $ | (2,823 | ) | |||||||||||||||||||||
| Basic and diluted net loss per share, Conduit ordinary shares | $ | (1,083 | ) | |||||||||||||||||||||||||||||||||
| Basic and diluted weighted average shares outstanding, Conduit ordinary shares | 2,000 | |||||||||||||||||||||||||||||||||||
| Basic and diluted earnings attributable to MURF Class A common stock | $ | 76 | ||||||||||||||||||||||||||||||||||
| Basic and diluted weighted average shares outstanding, MURF Class A common stock | 5,762,364 | |||||||||||||||||||||||||||||||||||
| Basic and diluted earnings per share attributable to MURF Class A common stock | $ | 0.01 | ||||||||||||||||||||||||||||||||||
| Basic and diluted earnings attributable to MURF Class B common stock | $ | 44 | ||||||||||||||||||||||||||||||||||
| Basic and diluted weighted average shares outstanding, MURF Class B common stock | 3,306,250 | |||||||||||||||||||||||||||||||||||
| Basic and diluted earnings per share attributable to MURF Class B common stock | $ | 0.01 | ||||||||||||||||||||||||||||||||||
| Basic and diluted net loss per share attributable to New Conduit common stock | $ | (0.04 | ) | $ | (0.04 | ) | ||||||||||||||||||||||||||||||
| Basic and diluted weighted average shares outstanding attributable to New Conduit common stock | 75,273,061 | g | 73,084,603 | g | ||||||||||||||||||||||||||||||||
See accompanying notes to the unaudited pro forma condensed combined financial information
| 103 |
Adjustments to Unaudited Pro Forma Condensed Combined Balance Sheet as of March 31, 2023
The pro forma notes and adjustments, based on preliminary estimates that could change materially as additional information is obtained, are as follows:
Pro Forma Adjustments for Material Events:
| a) | To reflect the issuance of the Vela Put Option as if the closing of the Business Combination had occurred and the option had been issued on March 31, 2023. The adjustment reflects an increase to cash of $0.5 million (£0.4 million) from the receipt of the option fee, an increase to option liability of $1.4 million to recognize the option at its estimated fair value, and an increase to accumulated deficit of $0.9 million. The option is classified as a liability under Accounting Standard Codification (“ASC”) 480 as it represents an obligation to issue a variable number of shares for which the monetary value is predominantly fixed. The fair value of the option liability was estimated using a two-step approach; 1) using a market approach to determine the underlying asset value; and 2) using a Monte-Carlo simulation to ultimately determine the fair value. The inputs used in the market approach to estimate the underlying asset value were the consideration received from the sale of future revenue of £1.9 million and a blended success rate of respiratory and cardiovascular studies advancing from Phase I to Phase II of 52.7%, resulting in an underlying asset value used in the Monte-Carlo simulation of £3.6 million. The inputs used in the simulation to estimate the fair value were a discount period of 0.50 years, a risk-free interest rate of 4.9%, an estimated stock volatility after the Merger of 93.0%, and an estimated volatility of the Covid Asset of 85.0%. | |
| b) | To reflect the MURF sponsor advancing $0.2 million to MURF under a promissory note with a maximum borrowable amount of $1.5 million that was originally issued on March 7, 2023 and MURF depositing $79 thousand and $78 thousand into the Trust Account on April 6, 2023, and May 4, 2023, respectively. In addition, $43 thousand from the $0.2 million advance was deposited into cash and cash equivalents. |
Pro Forma Other Transaction Accounting Adjustments:
| c) | To reflect the release of the investments held in the Trust Account to cash and cash equivalents, assuming no Public Stockholders exercise their right to have their Common stock subject to possible redemption redeemed for their pro rata share of the Trust Account. | |
| d) | To reflect the payment of deferred underwriting fees incurred in connection with MURF’s initial public offering. | |
| e) | To reflect the conversion of 3,306,250 shares of MURF Class B common stock to MURF Class A common stock. | |
| f) | To reflect the issuance of an aggregate of 2,700,000 shares of New Conduit common stock to the Subscription Investors at a price of $10.00 per share, for an aggregate purchase price of $27.0 million. The adjustment reflects the recording of a derivative warrant liability of $0.2 million for the warrant to purchase up to 2,700,000 shares of New Conduit issued to the Private Placement Investor. The warrant is classified as a derivative liability because it does not meet the criteria in ASC 815-40 to be considered indexed to the entity’s own stock as the warrant could be settled for an amount that is not equal to the difference between the fair value of a fixed number of the entity’s shares and a fixed monetary amount. In addition, the adjustment reflects a reduction to accumulated deficit of $0.1 million. The reduction to accumulated deficit is due to remeasurement of the derivative warrant liability for the unaudited pro forma condensed combined statement of operations as if the Transactions had occurred and the warrant was issued on January 1, 2022. The derivative warrant liability was measured based on the quoted market price for MURF’s publicly traded warrants, which, for valuation purposes, have materially similar terms as the warrant issued to the Private Placement Investor. The adjustment also increases additional paid-in capital by approximately $26.7 million and increases New Conduit common stock by an immaterial dollar amount (2,700,000 shares multiplied by par value of $0.0001 is less than $1 thousand). |
| 104 |
Certain terms of the warrant issued to the Private Placement Investor include the following1:
| ● | Exercise price of $11.50; | |
| ● | Exercisable from 30 days after completion of the Business Combination through 5 years after the completion of the Business Combination. | |
| ● | Within 30 days of the occurrence of a Fundamental Transaction that is within the control of New Conduit, the holder of the warrant can exercise and as compensation for exercise receive a cash payment equal to the Black Scholes Value. The inputs to the Black Scholes Value are as follows: |
| ○ | Risk-free interest rate corresponding to the U.S. Treasury rate for a period equal to the time between the date of the public announcement of the applicable Fundamental Transaction and the Termination Date; | |
| ○ | An expected volatility equal to the greater of 100% and the 100-day volatility obtained from the HVT function on Bloomberg (determined utilizing a 365-day annualization factor) as of the Trading Day immediately following the public announcement of the applicable Fundamental Transaction; | |
| ○ | The underlying price per share used in such calculation shall be the greater of (i) the sum of the price per share being offered in cash, if any, plus the value of any non-cash consideration, if any, being offered in such Fundamental Transaction and (ii) the highest VWAP during the period beginning on the Trading Day immediately preceding the announcement of the applicable Fundamental Transaction (or the consummation of the applicable Fundamental Transaction, if earlier) and ending on the Trading Day of the Holder’s request pursuant to Section 3(d) of the warrant agreement; | |
| ○ | A remaining option time equal to the time between the date of the public announcement of the applicable Fundamental Transaction and the Termination Date; | |
| ○ | Zero cost of borrow. |
Since the volatility input is based on the greater of 1) a fixed volatility assumption (100%) and 2) an estimate of current volatility at the time of settlement (the 100-day volatility obtained from the HVT function on Bloomberg), this input could result in a settlement amount that is greater than the settlement amount of a fixed-for-fixed option on the entity’s equity shares. Therefore, the settlement amount is not considered fixed-for-fixed and thus the warrant is not considered indexed to the entity’s own stock under ASC 815-40. As the warrant is not considered indexed to the entity’s own stock, it cannot be equity-classified and is thus classified as a derivative liability.
| g) | To reflect the reclassification of common stock subject to possible redemption of $22.9 million to an immaterial dollar amount (2,187,728 shares multiplied by par value of $0.0001 is less than $1 thousand) of New Conduit common stock and $22.9 million of additional paid-in capital. | |
| h) | To reflect the automatic redemption of $3.1 million of convertible notes at a 20% discount in accordance with the terms of the 2022 Convertible Loan Note Instrument and 2021 Convertible Loan Note Instrument. | |
| i) | To reflect the recapitalization of Conduit through the Business Combination and the issuance of 65,000,000 shares of New Conduit common stock and the elimination of the accumulated deficit of MURF, the accounting acquiree. As a result of the recapitalization, MURF’s accumulated deficit of $5.5 million and Conduit’s ordinary shares consisting of an immaterial dollar amount (2,000 shares multiplied by par value of £0.0001 is less than $1 thousand) were derecognized. The shares of New Conduit common stock issued in exchange for Conduit’s capital were recorded as an increase to New Conduit common stock of $6 thousand and a decrease to additional paid-in capital of $5.5 million. |
1 Capitalized terms in the bullet points below are defined in the warrant agreement. For all of the terms of the warrant, refer to the warrant agreement, which is attached as an exhibit to this registration statement.
| 105 |
| j) | To reflect the automatic redemption of $0.5 million of convertible notes issued to a related party at a 20% discount in accordance with the terms of the 2022 Convertible Loan Note Instrument and the 2021 Convertible Loan Note Instrument. | |
| k) | To reflect the payment of MURF’s and Conduit’s total estimated transaction costs payable in cash of $14.6 million. The adjustment reflects estimated advisory, legal, and other professional fees of $9.8 million which are deemed to be direct and incremental costs of the Business Combination, and additional estimated transaction costs of $2.6 million that are not deemed to be direct and incremental to the Business Combination and are not expected to recur, and the payment of transaction costs of $2.2 million that were incurred and expensed in MURF’s and Conduit’s historical financial statements and that are not expected to recur. These expenses were recorded as accrued professional fees of $2.2 million in Conduit’s historical balance sheet and as accrued expenses and other current liabilities of $7 thousand dollars in MURF’s historical balance sheet. The $9.8 million in total estimated costs that are direct and incremental to the Business Combination are recorded as a reduction to additional paid-in capital, and the $2.6 million in estimated costs that are not direct and incremental to the Business Combination are recorded as an increase to accumulated deficit. The total transaction costs are comprised of $9.4 million of Conduit transaction costs recorded as a reduction to additional paid-in capital, $0.3 million of Conduit transaction costs recorded as expense, $0.4 million of MURF transaction costs recorded as a reduction to additional paid-in capital, and $2.3 million of MURF transaction costs recorded as expense. The adjustment also increases New Conduit common stock for an immaterial dollar amount (1,300,000 shares multiplied by par value of $0.0001 is less than $1 thousand) as a result of the issuance of 1,300,000 shares of New Conduit common stock to A.G.P. upon the closing of the Business Combination. | |
| l) | To reflect the conversion of MURF Class A common stock into New Conduit common stock. | |
| m) | To reflect the elimination of accrued excise tax liability and additional paid-in capital of $1.1 million as the excise tax is not due if the Business Combination closes prior to December 31, 2023 and it is expected that the Business Combination will close prior to December 31, 2023. | |
| n) | To reflect the repayment of the note payable – sponsor upon closing of the Business Combination in accordance with its terms. | |
| o) | To reflect, in Scenario 2, the assumption that MURF Public Stockholders exercise their redemption rights with respect to a maximum of 2,187,728 shares of common stock subject to possible redemption prior to the consummation of the Transactions at a redemption price of $10.45 per share, or $22.9 million in cash. |
Adjustments to Unaudited Pro Forma Condensed Combined Statement of Operations for the year ended December 31, 2022 and the three months ended March 31, 2023
The pro forma notes and adjustments, based on preliminary estimates that could change materially as additional information is obtained, are as follows:
Pro Forma Adjustments for Material Events:
| (a) | To reflect an increase to other income of $3.6 million as a result of measuring the change in fair value of the Vela Put Option from its assumed issuance on January 1, 2022 to December 31, 2022. The fair value of the option liability was estimated using a Monte-Carlo simulation. The inputs used in the simulation to estimate the fair value of the option liability at January 1, 2022 were a discount period of 0.50 years, a risk-free interest rate of 0.2%, an estimated stock volatility after the Merger of 80.0%, and an estimated volatility of the Covid Asset of 75.0%. The estimated fair value of the option at January 1, 2022 was $3.6 million and the option had no fair value at December 31, 2022. The option had no fair value at December 31, 2022 because its terms specify that it expires upon the earlier of i) six months from the closing of the Business Combination, and ii) February 7, 2024. As the Business Combination is assumed to have occurred on January 1, 2022, it is assumed that the Vela Put Option will expire on June 30, 2022, which is six months from the assumed closing of the Business Combination. As such, the adjustment increases other income by $3.6 million as a result of the reduction in the fair value of the option liability from $3.6 million at January 1, 2022 to no fair value at December 31, 2022. |
| 106 |
Pro Forma Other Transaction Accounting Adjustments:
| (b) | To reflect the elimination of interest income earned on the investments held in the Trust Account. | |
| (c) | Reflects estimated transaction costs for MURF and Conduit for certain accounting, auditing, and other professional fees that are not deemed to be direct and incremental costs of the Business Combination. | |
| (d) | To reflect a loss on extinguishment of convertible notes payable, carried at fair value. The convertible notes are automatically redeemed at a 20% discount in accordance with the terms of the 2021 Convertible Note Loan Instrument and the 2022 Convertible Note Loan instrument as a result of the Business Combination. | |
| (e) | To reflect remeasurement of the derivative warrant liability issued to the Private Placement Investor as if the Transactions had occurred and the warrant was issued on January 1, 2022. | |
| (f) | To reflect stock-based compensation expense for restricted stock units (“RSUs”) that will be granted to a member of management of New Conduit upon the closing of the Business Combination as if the Business Combination had closed and the RSUs had been issued on January 1, 2022. The number of RSUs granted will be equal to 0.10% of the shares of common stock of New Conduit that will be outstanding immediately upon the closing of the Business Combination. The RSUs will vest in equal annual installments on the first three anniversaries of the closing of the Business Combination. The adjustment to reduce expense in Scenario 2 is due to the number of RSUs assumed granted in Scenario 2 being lower (as compared to Scenario 1) as the number of shares of common stock of New Conduit outstanding immediately upon the closing of the Business Combination is lower assuming maximum redemptions (as is assumed in Scenario 2). | |
| (g) | The pro forma basic and diluted net loss per share amounts presented in the unaudited pro forma condensed combined statements of operations are based on the number of New Conduit shares outstanding as if the Business Combination had occurred on January 1, 2022. |
Pro Forma weighted-average shares outstanding—basic and diluted is calculated as follows for the year ended December 31, 2022:
| Scenario 1 | Scenario 2 | |||||||
| Weighted-average shares calculation - basic and diluted | Assuming No Redemptions into Cash | Assuming Maximum Redemptions into Cash | ||||||
| Assume conversion of MURF Class B common stock into New Conduit common stock effective January 1, 2022, as a result of assuming closing of the Business Combination on January 1, 2022 | 3,306,250 | 3,306,250 | ||||||
| Assume reclassification of common stock subject to possible redemption to New Conduit common stock effective January 1, 2022 as a result of assuming closing of the Business Combination on January 1, 2022 | 2,187,728 | - | ||||||
| Assume January 1, 2022 issuance of New Conduit common stock in connection with the closing of the PIPE Financing | 2,700,000 | 2,700,000 | ||||||
| Assume January 1, 2022 issuance of New Conduit common stock to A.G.P. as a result of assuming closing of the Business Combination on January 1, 2022 | 1,300,000 | 1,300,000 | ||||||
| Assume conversion of MURF Class A common stock into New Conduit common stock effective January 1, 2022 as a result of assuming closing of the Business Combination on January 1, 2022 | 754,000 | 754,000 | ||||||
| Assume January 1, 2022 issuance of New Conduit common stock to Conduit shareholders as a result of assuming closing of the Business Combination on January 1, 2022 | 65,000,000 | 65,000,000 | ||||||
| Pro forma weighted-average shares outstanding—basic and diluted | 75,247,978 | 73,060,250 | ||||||
Pro Forma weighted-average shares outstanding—basic and diluted is calculated as follows for the three months ended March 31, 2023:
| Scenario 1 | Scenario 2 | |||||||
| Weighted-average shares calculation - basic and diluted | Assuming No Redemptions into Cash | Assuming Maximum Redemptions into Cash | ||||||
| Assume conversion of MURF Class B common stock into New Conduit common stock effective January 1, 2022 as a result of assuming closing of the Business Combination on January 1, 2022 | 3,306,250 | 3,306,250 | ||||||
| Assume reclassification of common stock subject to possible redemption to New Conduit common stock effective January 1, 2022 as a result of assuming closing of the Business Combination on January 1, 2022 | 2,187,728 | - | ||||||
| Assume January 1, 2022 issuance of New Conduit common stock in connection with the closing of the PIPE Financing | 2,700,000 | 2,700,000 | ||||||
| Assume January 1, 2022 issuance of New Conduit common stock to A.G.P. as a result of assuming closing of the Business Combination on January 1, 2022 | 1,300,000 | 1,300,000 | ||||||
| Assume conversion of MURF Class A common stock into New Conduit common stock effective January 1, 2022 as a result of assuming closing of the Business Combination on January 1, 2022 | 754,000 | 754,000 | ||||||
| Assume January 1, 2022 issuance of New Conduit common stock to Conduit shareholders as a result of assuming closing of the Business Combination on January 1, 2022 | 65,000,000 | 65,000,000 | ||||||
| Assume one-third vesting on January 1, 2023 of restricted stock units awarded to a member of New Conduit management as a result of assuming issuance on January 1, 2022 (closing of the Business Combination) of the award which vests in equal annual installments on the first three anniversaries of the closing of the Business Combination | 25,083 | 24,353 | ||||||
| Pro forma weighted-average shares outstanding—basic and diluted | 75,273,061 | 73,084,603 | ||||||
| 107 |
The following potentially dilutive instruments were not included in the calculation of weighted-average shares outstanding for the year ended December 31, 2022 as their effects would have been anti-dilutive:
| Scenario 1 | Scenario 2 | |||||||
Assuming No Redemptions into Cash | Assuming Maximum Redemptions into Cash | |||||||
| Public Warrants(1) | 15,979,000 | 15,979,000 | ||||||
| Private Warrants(2) | 754,000 | 754,000 | ||||||
| Cizzle PLC option(3) | 391,831 | 391,831 | ||||||
| Vela Put Option(4) | 471,895 | 471,895 | ||||||
| Unvested restricted stock units(5) | 75,248 | 73,060 | ||||||
| Total | 17,671,974 | 17,669,786 | ||||||
1) The Public Warrants will consist of 13,225,000 warrants issued to MURF Public Stockholders in connection with MURF’s initial public offering, 2,700,000 warrants to be issued in connection with the PIPE Financing, and 54,000 warrants to be issued to A.G.P. upon the closing of the Business Combination. Assuming that New Conduit were in a net income position, the Public Warrants would have resulted in 15,979,000 potentially dilutive shares for the year ended December 31, 2022.
2) The Private Warrants will consist of 709,000 warrants issued to the Sponsor in connection with MURF’s initial public offering and 45,000 warrants originally issued to the MURF Sponsor prior to MURF’s initial public offering that will be transferred to MURF Directors upon the closing of the Business Combination. Assuming that New Conduit were in a net income position, the Private Warrants would have resulted in 754,000 potentially dilutive shares for the year ended December 31, 2022.
3) Cizzle PLC holds an option whereby it can exchange its economic interest in an asset of Conduit Pharmaceuticals Limited for shares worth £3.25 million valued at $10.00 per share at the GBP-to-USD exchange rate in effect on the exercise date. Assuming that New Conduit were in a net income position, the option would have resulted in 391,831 potentially dilutive shares for the year ended December 31, 2022.
4) Vela holds an option whereby it can exchange its economic interest in an asset of Conduit Pharmaceuticals Limited for shares worth £4.0 million valued at a price per share equal to the New Conduit volume-weighted average price per share over the ten business days prior to the date of the notice of exercise of the option, at the exchange rate in effect on the exercise date. Assuming that New Conduit were in a net income position, the option would have resulted in 471,895 potentially dilutive shares for the year ended December 31, 2022.
5) Assuming the closing of the Business Combination had occurred on January 1, 2022, a member of management of New Conduit would have been granted restricted stock units equal to 0.10% of the shares of common stock of New Conduit outstanding immediately as of the closing of the Business Combination. The restricted stock units vest in equal annual installments on the first three anniversaries of the closing of the Business Combination. The numbers of shares presented in the table above are calculated based on 75,247,978 shares outstanding immediately upon the Closing of the Business Combination in Scenario 1 and 73,060,250 shares outstanding immediately upon the closing of the Business Combination in Scenario 2. These share amounts are multiplied by 0.10% to arrive at the number of unvested restricted stock units as of December 31, 2022. All of the restricted stock units granted are unvested as of December 31, 2022 because the first of the three equal annual installments of vesting occurs on January 1, 2023. Assuming that New Conduit were in a net income position, the unvested restricted stock units would have resulted in 75,248 and 73,060 potentially dilutive shares for Scenario 1 and Scenario 2, respectively, as of December 31, 2022.
| 108 |
The following potentially dilutive instruments were not included in the calculation of weighted-average shares outstanding for the three months ended March 31, 2023 as their effects would have been anti-dilutive:
| Scenario 1 | Scenario 2 | |||||||
Assuming No Redemptions into Cash | Assuming Maximum Redemptions into Cash | |||||||
| Public Warrants(1) | 15,979,000 | 15,979,000 | ||||||
| Private Warrants(2) | 754,000 | 754,000 | ||||||
| Cizzle PLC option(3) | 402,587 | 402,587 | ||||||
| Vela Put Option(4) | 476,503 | 476,503 | ||||||
| March 2023 convertible note issued by Conduit(5) | 80,526 | 80,526 | ||||||
| Unvested restricted stock units(6) | 50,165 | 48,707 | ||||||
| Total | 17,742,781 | 17,741,323 | ||||||
1) The Public Warrants will consist of 13,225,000 warrants issued to MURF Public Stockholders in connection with MURF’s initial public offering, 2,700,000 warrants to be issued in connection with the PIPE Financing, and 54,000 warrants to be issued to A.G.P. upon the closing of the Business Combination. Assuming that New Conduit were in a net income position, the Public Warrants would have resulted in 15,979,000 potentially dilutive shares for the three months ended March 31, 2023.
2) The Private Warrants will consist of 709,000 warrants issued to the Sponsor in connection with MURF’s initial public offering and 45,000 warrants originally issued to the MURF Sponsor prior to MURF’s initial public offering that will be transferred to MURF Directors upon the closing of the Business Combination. Assuming that New Conduit were in a net income position, the Private Warrants would have resulted in 754,000 potentially dilutive shares for the three months ended March 31, 2023.
3) Cizzle PLC holds an option whereby it can exchange its economic interest in an asset of Conduit Pharmaceuticals Limited for shares worth £3.25 million valued at $10.00 per share at the GBP-to-USD exchange rate in effect on the exercise date. Assuming that New Conduit were in a net income position, the option would have resulted in 402,587 potentially dilutive shares for the three months ended March 31, 2023.
4) Vela holds an option whereby it can exchange its economic interest in an asset of Conduit Pharmaceuticals Limited for shares worth £4.0 million valued at a price per share equal to the New Conduit volume-weighted average price per share over the ten business days prior to the date of the notice of exercise of the option, at the exchange rate in effect on the exercise date. Assuming that New Conduit were in a net income position, the option would have resulted in 476,503 potentially dilutive shares for the three months ended March 31, 2023.
5) A non-related third party holds an option whereby it can redeem the remaining outstanding principal and interest on a convertible note with an original principal balance of $0.8 million for New Conduit common stock at a price of $10.00 per share. Assuming that New Conduit were in a net income position, the option would have resulted in 80,526 potentially dilutive shares for the three months ended March 31, 2023.
6) Assuming the closing of the Business Combination had occurred on January 1, 2022, a member of management of New Conduit would have been granted restricted stock units equal to 0.10% of the shares of common stock of New Conduit outstanding immediately as of the closing of the Business Combination. The restricted stock units vest in equal annual installments on the first three anniversaries of the closing of the Business Combination. The numbers of shares presented in the table above are calculated based on 75,247,978 shares outstanding immediately upon the Closing of the Business Combination in Scenario 1 and 73,060,250 shares outstanding immediately upon the closing of the Business Combination in Scenario 2. These share amounts are multiplied by 0.10% to arrive at the total number of restricted stock units assumed granted on January 1, 2022 of 75,248 and 73,060 in Scenario 1 and Scenario 2, respectively. Two-thirds of the 75,248 and 73,060 restricted stock units (in Scenario 1 and Scenario 2, respectively) remain unvested as of March 31, 2023 because one-third of the units vested on January 1, 2023 (the first anniversary of the assumed closing of the Business Combination on January 1, 2022). Assuming that New Conduit were in a net income position, the unvested restricted stock units would have resulted in 50,165 and 48,707 potentially dilutive shares for Scenario 1 and Scenario 2, respectively, as of March 31, 2023.
| 109 |
THE CHARTER AMENDMENTS PROPOSAL
The following table sets forth a summary of the principal changes proposed to be made between MURF’s existing amended and restated certificate of incorporation and New Conduit’s Proposed Charter. This summary is qualified by reference to the complete text of the Proposed Charter, a copy of which is attached to this proxy statement/prospectus as Annex B. All stockholders are encouraged to read the Proposed Charter in its entirety for a more complete description of its terms.
| MURF Amended and Restated Certificate of Incorporation | MURF Second Amended and Restated Certificate of Incorporation | |||
| Name | “Murphy Canyon Acquisition Corp.” | “Conduit Pharmaceuticals Inc.” | ||
| Common Stock | The existing amended and restated certificate of incorporation authorizes two classes of common stock – Class A common stock and Class B common stock. MURF has 111,000,000 authorized shares of common stock, par value $0.0001 per share comprised of 100,000,000 shares of Class A common stock and 11,000,000 shares of Class B common stock | New Conduit will have 250,000,000 authorized shares of common stock, par value $0.0001 per share. Upon the filing and effectiveness of the Second Amended and Restated Certificate, or the effective time, each share of MURF common stock issued and outstanding immediately prior to the Effective Time shall, automatically and without any further action by the public entity or any stockholder, be reclassified into one fully paid and nonassessable share of New Conduit common stock. | ||
| Number of Directors | The number of directors, other than those who may be elected by the holders of one or more series of preferred stock voting separately by class or series, shall be fixed from time to time exclusively by the board pursuant to a resolution adopted by a majority of the board. | The total number constituting the board of directors of New Conduit following the Closing will initially consist of seven (7) individuals, a majority of whom shall be independent directors in accordance with Nasdaq requirements. | ||
| Stockholder Actions | The existing amended and restated certificate of incorporation provides that any action required or permitted to be taken by the stockholders of MURF must be effected by a duly called annual or special meeting of such stockholders and may not be effected by written consent of the stockholders other than with respect to the Class B common stock; action with respect to such stock may be taken by written consent. | New Conduit stockholders may not act by written consent in lieu of a meeting. | ||
| Provisions Specific to a Special Purpose Acquisition Company | The existing amended and restated certificate of incorporation sets forth various provisions related to its operations as a blank check special purpose acquisition company prior to the consummation of an initial business combination. | These provisions are not applicable to an operating company, and so the Proposed Charter does not include the blank check special purpose acquisition company provisions. |
Each of the amendments above is referred to as a “charter amendment” and collectively, the “charter amendments.” These consist of the following separable proposals:
| Charter Amendment Proposal A — To change MURF’s name to “Conduit Pharmaceuticals Inc.”; | |
| Charter Amendment Proposal B — To provide for the reclassification of each outstanding share of MURF common stock, including each outstanding share of Class A common stock and Class B common stock, into one fully paid and nonassessable share of common stock and to remove the designations of Class A and Class B common stock entirely; | |
| Charter Amendment Proposal C — To establish that the board of directors of MURF following the closing of the Business Combination shall be initially fixed at seven (7), a majority of whom shall be independent directors in accordance with Nasdaq requirements; | |
| Charter Amendment Proposal D — To increase the number of authorized shares of common stock to 250,000,000; |
| 110 |
| Charter Amendment Proposal E — To require that stockholders only act at annual and special meetings of the corporation and not by written consent; and | |
| Charter Amendment Proposal F — To remove various provisions applicable to special purpose acquisition corporations. |
Pursuant to the Merger Agreement, upon the closing of the Business Combination, MURF’s bylaws will be amended and restated promptly to:
| ● | reflect necessary changes and to be consistent with the proposed charter amendments described herein; and |
| ● | make certain other changes that the board of directors of MURF deems appropriate for a public operating company. |
Reasons for the Charter Amendments
Name
Changing the post-Business Combination corporate name from “Murphy Canyon Acquisition Corp.” to “Conduit Pharmaceuticals Inc.” is desirable to reflect the Business Combination with Conduit. Additionally, the MURF Board believes the name of New Conduit should more closely align with the name of the existing operating business of Conduit.
Common Stock
The principal purpose of this charter amendment is to reclassify each outstanding share of MURF common stock, including each outstanding share of Class A common stock and Class B common stock, into one fully paid and nonassessable share of common stock. The MURF Board believes that a single class of common stock provides a cleaner capital structure and that this suits New Conduit’s requirements following the consummation of the Business Combination.
Number of Directors
This charter amendment is being made to ensure stockholders understand the exact number of directors of New Conduit as of the consummation of the Business Combination.
MURF’s directors and officers have interests in the Business Combination that may conflict with your interests as a stockholder. See the section entitled “Business Combination Proposal — Interests of MURF’s Directors and Officers in the Business Combination” for a further discussion of these considerations.
Number of Shares of Authorized Common Stock
The principal purpose of this charter amendment is to approve an increase in the number of authorized shares of common stock available for issuance following the consummation of the Business Combination to 250,000,000. Under MURF’s existing amended and restated certificate of incorporation, there are 100,000,000 shares of Class A common stock authorized and 10,000,000 shares of Class B common stock authorized. The Proposed Charter provides for 250,000,000 shares of common stock to be authorized. The MURF Board believes that the greater number of authorized shares of capital stock is desirable for New Conduit to have sufficient shares to issue in order to complete the Business Combination and have additional authorized shares for financing its business, for acquiring other businesses, for forming strategic partnerships and alliances and for stock dividends and stock splits.
Stockholder Actions
The Proposed Charter provides that any action to be taken by MURF’s stockholders may not be taken by written consent. The MURF Board believes that each decision of the stockholders should be made by all stockholders and only after thoughtful consideration of complete information. Information is provided to stockholders through a proxy statement, and the period between delivery of the proxy statement and the stockholder meeting provides time for consideration of stockholder proposals. The MURF Board believes that all stockholders, not just stockholders executing a written consent, should have the opportunity to participate in the decision-making process. This allows minority stockholders to take whatever action they deem appropriate to protect their interests, including seeking to persuade majority stockholders to follow a different course, or selling their shares.
| 111 |
The proposed charter amendment will have the effect of preventing MURF stockholders from taking action at any time other than an annual meeting or a special meeting to replace directors or take any other action authorized to be taken by stockholders under the DGCL. This charter amendment may make more difficult, or delay, actions by a person or a group seeking to acquire a substantial percentage of MURF common stock, to replace directors or to take other action to influence or control its management or policies, even though the holders of a majority of the outstanding shares of MURF common stock might desire those actions.
Provisions Specific to a Special Purpose Acquisition Company
The elimination of certain provisions related to MURF’s status as a special purpose acquisition company is desirable because these provisions will serve no purpose following the Business Combination. For example, the Proposed Charter does not include the requirement to dissolve MURF after a certain time period and allows it to continue as a corporate entity with perpetual existence following consummation of the Business Combination. Perpetual existence is the usual period of existence for corporations, and the MURF Board believes it is the most appropriate period for MURF. In addition, certain other provisions in the existing amended and restated certificate of incorporation require that proceeds from MURF’s initial public offering be held in the trust account until the completion of a business combination or redemption of 100% of the outstanding public shares has occurred. These provisions cease to apply once the Business Combination is consummated and are therefore not included in the Proposed Charter.
A copy of the Proposed Charter, as will be in effect assuming approval of the charter amendments Proposal and upon consummation of the Business Combination and filing with the Delaware Secretary of State, is attached to this proxy statement/prospectus as Annex B.
Vote Required for Approval
If the business combination proposal is not approved, the charter amendment proposals will not be presented at the Special Meeting.
The approval of each of Charter Amendment Proposal A, Charter Amendment Proposal B, Charter Amendment Proposal C, Charter Amendment Proposal E and Charter Amendment Proposal F will require the affirmative vote of the majority of the issued and outstanding shares of Class A common stock and Class B common stock , voting as a single class. The approval of Charter Amendment Proposal D will require the affirmative vote of a majority of the issued and outstanding shares of each of the Class A common stock and Class B common stock, voting separately. Accordingly, a MURF public stockholder’s failure to vote by proxy or to vote in person (which would include presence at a virtual meeting) at the Special Meeting or an abstention will have the same effect as a vote “AGAINST” the Charter Amendment Proposal.
Under the Merger Agreement, the approval of the charter amendment proposals is a condition to the adoption of the business combination proposal.
THE MURF BOARD RECOMMENDS THAT STOCKHOLDERS VOTE “FOR” THE APPROVAL OF EACH OF THE CHARTER AMENDMENT PROPOSALS.
| 112 |
THE ADVISORY CHARTER AMENDMENTS PROPOSALS
Overview
In connection with the Business Combination, MURF is asking its stockholders to vote upon, on a non-binding advisory basis, proposals to approve certain governance provisions contained in the Proposed Charter. This separate vote is not otherwise required by Delaware law separate and apart from the charter amendments proposal but, pursuant to SEC guidance, MURF is required to submit these provisions to its stockholders separately for approval, allowing stockholders the opportunity to present their separate views on important governance provisions. However, the stockholder votes regarding these proposals are advisory votes, and are not binding on MURF or the MURF Board (separate and apart from the approval of the charter amendment proposals). In the judgment of the MURF Board, these provisions are necessary to adequately address the needs of MURF. Furthermore, the Business Combination is not conditioned on the separate approval of the advisory charter amendments proposals (separate and apart from approval of the charter amendments proposal).
MURF stockholders will be asked to approve, on a non-binding advisory basis, the following material differences between the Proposed Charter of New Conduit and the existing amended and restated certificate of incorporation, which are being presented in accordance with the requirements of the SEC as six separate sub-proposals (the “Advisory charter amendments Proposals”):
| Advisory Charter Amendment Proposal A — To change MURF’s name to “Conduit Pharmaceuticals Inc.”; | |
| Advisory Charter Amendment Proposal B — To provide for the reclassification of each outstanding share of MURF common stock, including each outstanding share of Class A common stock and Class B common stock, into one fully paid and nonassessable share of common stock and to remove the designations of Class A and Class B common stock entirely; | |
| Advisory Charter Amendment Proposal C — To establish that the board of directors of MURF following the closing of the Business Combination shall be initially fixed at seven (7), a majority of whom shall be independent directors in accordance with Nasdaq requirements; | |
| Advisory Charter Amendment Proposal D — To increase the number of authorized shares of common stock to 250,000,000; | |
| Advisory Charter Amendment Proposal E —To require that stockholders only act at annual and special meetings of the corporation and not by written consent; and | |
| Advisory Charter Amendment Proposal F — To remove various provisions applicable to special purpose acquisition corporations. |
Reasons for the Advisory Charter Amendments
Advisory Charter Amendment Proposal A
Changing the post-Business Combination corporate name from “Murphy Canyon Acquisition Corp.” to “Conduit Pharmaceuticals Inc.” is desirable to reflect the Business Combination with Conduit. Additionally, the MURF Board believes the name of New Conduit should more closely align with the name of the existing operating business of Conduit.
Advisory Charter Amendment Proposal B
The principal purpose of this charter amendment is to reclassify each outstanding share of common stock, including each outstanding share of Class A common stock and Class B common stock, into one fully paid and nonassessable share of common stock. The MURF Board believes that a single class of common stock provides a cleaner capital structure and that this suits New Conduit’s requirements following the consummation of the Business Combination.
Advisory Charter Amendment Proposal C
The total number constituting the MURF Board following the closing of the Business Combination will initially consist of seven (7) individuals, a majority of whom shall be independent directors in accordance with Nasdaq requirements. It is in the interests of stockholders of New Conduit to know the size of its board of directors, and such provisions are also in accordance with the DGCL.
MURF’s directors and officers have interests in the Business Combination that may conflict with your interests as a stockholder. See the section entitled “Business Combination Proposal — Interests of MURF’s Directors and Officers in the Business Combination” for a further discussion of these considerations.
| 113 |
Advisory Charter Amendment Proposal D
The principal purpose of this charter amendment is to approve an increase in the number of authorized shares of common stock available for issuance following the consummation of the Business Combination to 250,000,000. Under MURF’s existing amended and restated certificate of incorporation, there are 100,000,000 shares of Class A common stock authorized and 10,000,000 shares of Class B common stock authorized. The Proposed Charter provides for 250,000,000 shares of common stock to be authorized. The MURF Board believes that the greater number of authorized shares of capital stock is desirable for New Conduit to have sufficient shares to issue in order to complete the Business Combination and have additional authorized shares for financing its business, for acquiring other businesses, for forming strategic partnerships and alliances and for stock dividends and stock splits.
Advisory Charter Amendment Proposal E
The Proposed Charter provides that any action to be taken by the MURF stockholders may not be taken by written consent. The MURF Board believes that each decision of the stockholders should be made by all stockholders and only after thoughtful consideration of complete information. Information is provided to stockholders through a proxy statement, and the period between delivery of the proxy statement and the stockholder meeting provides time for consideration of stockholder proposals. The MURF Board believes that all stockholders, not just stockholders executing a written consent, should have the opportunity to participate in the decision-making process. This allows minority stockholders to take whatever action they deem appropriate to protect their interests, including seeking to persuade majority stockholders to follow a different course, or selling their shares.
The proposed charter amendment will have the effect of preventing MURF stockholders from taking action at any time other than an annual meeting or a special meeting to replace directors or take any other action authorized to be taken by stockholders under the DGCL. This charter amendment may make more difficult, or delay, actions by a person or a group seeking to acquire a substantial percentage of MURF common stock, to replace directors or to take other action to influence or control its management or policies, even though the holders of a majority of the outstanding shares of MURF common stock might desire those actions.
Advisory Charter Amendment Proposal F
The elimination of certain provisions related to MURF’s status as a special purpose acquisition company is desirable because these provisions will serve no purpose following the Business Combination. For example, the Proposed Charter does not include the requirement to dissolve MURF after a certain time period and allows it to continue as a corporate entity with perpetual existence following consummation of the Business Combination. Perpetual existence is the usual period of existence for corporations, and the MURF Board believes it is the most appropriate period for MURF. In addition, certain other provisions in the existing amended and restated certificate of incorporation require that proceeds from MURF’s initial public offering be held in the trust account until the completion of a business combination or redemption of 100% of the outstanding public shares has occurred. These provisions cease to apply once the Business Combination is consummated and are therefore not included in the Proposed Charter.
A copy of the Proposed Charter, as will be in effect assuming approval of the charter amendments Proposal and upon consummation of the Business Combination and filing with the Delaware Secretary of State, is attached to this proxy statement/prospectus as Annex B.
Vote Required for Approval
These advisory charter amendments proposals require the affirmative vote of a majority of the votes cast by stockholders present in person (by virtual attendance) or represented by proxy and entitled to vote thereon at the Special Meeting (which would include presence by virtual attendance at the Special Meeting). An abstention will be counted towards the quorum requirement but will not count as a vote cast at the Special Meeting. A broker non-vote will neither be counted towards the quorum requirement (as the proposals we believe will be considered as non-discretionary) nor count as a vote cast in the Special Meeting.
The approval and adoption of the advisory charter amendments proposals is non-binding and not conditioned on any other proposal at the Special Meeting.
THE MURF BOARD RECOMMENDS THAT STOCKHOLDERS VOTE “FOR” THE APPROVAL OF EACH OF THE ADVISORY CHARTER PROPOSALS.
| 114 |
THE DIRECTOR ELECTION PROPOSAL
Election of Directors
At the Special Meeting, seven (7) directors will be elected who will be the directors of New Conduit upon consummation of the Transactions. New Conduit’s board of directors will be of a single class serving a term of one year. If management’s nominees are elected, such nominees will serve as directors until the general meeting to be held in 2024 and, in each case, until their successors are elected and qualified or their earlier resignation or removal. New Conduit’s board of directors will consist of (i) Dr. Freda Lewis-Hall, Chairman of New Conduit’s board of directors, (ii) Dr. David Tapolczay, a current director of Conduit and the Chief Executive Officer of New Conduit, (iii) James Bligh, an employee of Conduit, (iv) Faith L. Charles, (v) Chele Chiavacci Farley, a current director of MURF, (vi) Jennifer I. McNealey, and (vii) Dr. Andrew Regan, a current director and shareholder of Conduit (Dr. Regan also undertakes duties typically associated with an executive officer in connection with his service as a director of Conduit). Information regarding each nominee is set forth in the section entitled “Management of New Conduit Following the Business Combination.”
Vote Required for Approval
Under Delaware law, the election of directors requires a plurality vote of the shares of common stock present in person (by virtual attendance) or represented by proxy and entitled to vote at the Special Meeting. “Plurality” means that the individuals who receive the largest number of votes cast “FOR” are elected as directors. Consequently, any shares not voted “FOR” a particular nominee (whether as a result of an abstention, a direction to withhold authority or a broker non-vote) will not be counted in the nominee’s favor.
Unless authority is withheld or the shares are subject to a broker non-vote, the proxies solicited by the MURF Board will be voted “FOR” the election of these nominees. In case any of the nominees becomes unavailable for election to the MURF Board, due to an event that is not anticipated, the persons named as proxies, or their substitutes, will have full discretion and authority to vote or refrain from voting for any other candidate in accordance with their judgment.
If the business combination proposal is not approved or any of the charter amendment proposals is not approved and the applicable condition in the Merger Agreement is not waived, the director election proposal will not be presented at the meeting.
Following consummation of the Transactions, the election of directors of New Conduit will be governed by its charter documents, the bylaws, and the DGCL of New Conduit.
THE MURF BOARD UNANIMOUSLY RECOMMENDS THAT MURF’S STOCKHOLDERS VOTE “FOR” EACH OF THE NOMINEES LISTED IN THIS PROXY STATEMENT/PROSPECTUS.
| 115 |
MANAGEMENT OF NEW CONDUIT FOLLOWING THE BUSINESS COMBINATION
In connection with the Business Combination, the existing members of the board of directors of MURF, other than Chele Chiavacci Farley, will resign and, if management’s nominees are elected, the board of directors of New Conduit will consist of a total of seven (7) individuals, which will initially be (i) Dr. Freda Lewis-Hall, Chairman of New Conduit’s board of directors, (ii) Dr. David Tapolczay, a current director of Conduit and the Chief Executive Officer of New Conduit, (iii) James Bligh, an employee of Conduit, (iv) Faith L. Charles, (v) Chele Chiavacci Farley, a current director of MURF, (vi) Jennifer I. McNealey, and (vii) Dr. Andrew Regan, a current director and shareholder of Conduit (Dr. Regan also undertakes duties typically associated with an executive officer in connection with his service as a director of Conduit).
Upon completion of the Business Combination, the executive officers of New Conduit will include Dr. David Tapolczay as the Chief Executive Officer of New Conduit and Adam Sragovicz, MURF’s Chief Financial Officer and director, as the Chief Financial Officer of New Conduit.
Accordingly, following the Business Combination, the directors and executive officers of New Conduit will be:
| Name | Age | Position | ||
| David Tapolczay | 63 | Chief Executive Officer and Director | ||
| Adam Sragovicz | 54 | Chief Financial Officer | ||
| Freda Lewis-Hall | 67 | Chairman of the Board of Directors | ||
| James Bligh | 35 | Director | ||
| Faith L. Charles | 61 | Director | ||
| Chele Chiavacci Farley | 55 | Director | ||
| Jennifer I. McNealey | 49 | Director | ||
| Andrew Regan | 58 | Director |
David Tapolczay
Dr. Tapolczay has more than 20 years of experience in research and development management. He was a co-founder and has been the Chief Executive Officer of Conduit since 2019 and a member of Conduit’s board of directors since 2019. He also currently serves as Chief Executive Officer of St George Street Capital, a United Kingdom-based medical research charity that is also a scientific advisor to MURF and a business partner to Conduit, a position which he has held since July 2018, and as Chief Executive Officer of Medeor Pharma Ltd, a pharmaceutical consultancy company, a position which he has held since 2006.
From February 2008 to December 2018, he served as Chief Executive Officer at LifeArc (formerly the Medical Research Counsel Technology Group (MRCT)), a United-Kingdom based charity advancing lab-based scientific discoveries to a point at which they can be developed into the next generation of diagnostics, treatments and cures. He previously served as joint worldwide head of chemistry for Zeneca Agrochemicals, a research and development unit of AstraZeneca, and as senior manager of chemical development for GlaxoSmithKlein plc, a pharmaceutical and biotechnology company. Dr. Tapolczay served as Executive Vice President at Cambridge Discovery Chemistry, where he was responsible for the rapid growth of Cambridge Discovery Chemistry and was a key figure in two successful sales of that company, the first to Oxford Molecular and the second to Millennium Pharmaceuticals. After this last acquisition, Dr. Tapolczay was Senior Vice President of Pharmaceutical Sciences at Millennium Pharmaceuticals, with responsibility for over 230 scientists. On leaving Millennium, Dr. Tapolczay was a founder and Chairman of Pharmorphix Ltd., which was acquired by Sigma Aldrich Fine Chemicals in August 2006. He has also been involved with the start-up of five companies, all of which are still trading and one of which has been AIM listed. He was VP of Technology Development for GSK Pharmaceuticals from December 2005 to April 2007. He was awarded visiting Professorial Chair in Chemistry at Sussex University from August 1999 to May 2007 and has previously held the position of visiting lecturer at Nottingham, Reading and Durham Universities and a member of both the Technical Opportunities Panel and the User Panel of the EPSRC. He holds a BSc Hons and PhD in Chemistry from the University of Southampton. Dr. Tapolczay also completed his Post-Doctoral Experience in Organic Chemistry from the University of Oxford. Dr. Tapolczay was selected to serve on the board of New Conduit following the Business Combination based on his deep knowledge of Conduit, his extensive experience in research and development of clinical assets, and his in-depth knowledge of the pharmaceutical industry.
Adam Sragovicz
Mr. Sragovicz has been MURF’s Chief Financial Officer since MURF’s inception. Mr. Sragovicz has been a director of the Company since December 2021. Mr. Sragovicz has been the Chief Financial Officer of Presidio Property Trust, Inc. since January 11, 2018. He previously served as Senior Vice President, Finance of Presidio Property Trust, Inc. since May 2017. Before joining Presidio Property Trust, Inc., Mr. Sragovicz served as Treasurer of Encore Capital Group from 2011 to 2017, where he was responsible for global capital raising, foreign exchange risk management and cash management. Mr. Sragovicz has also held capital markets, finance, and treasury management positions with KPMG, Union Bank of California / MUFG and Bank of America Merrill Lynch. Mr. Sragovicz is the Director of the Yale Alumni Schools Committee in San Diego and previously sat on the board of Congregation Adat Yeshurun. Mr. Sragovicz is a graduate of Yale University with a Bachelor of Arts degree in Soviet and Eastern European Studies, with a concentration in Economics. Upon the consummation of the Business Combination, Mr. Sragovicz will be appointed Chief Financial Officer of New Conduit.
| 116 |
Freda Lewis-Hall, M.D., DFAPA
Dr. Lewis-Hall served as Senior Medical Advisor to the CEO of Pfizer Inc., or Pfizer, from December 2019 until her retirement in March 2020. Before assuming that responsibility, from January 2019, Dr. Lewis-Hall served as Chief Patient Officer and Executive Vice President of Pfizer, beginning January 2019. Dr. Lewis-Hall began her service with Pfizer as its Chief Medical Officer from 2009 to January 2019. Prior to joining Pfizer in 2009, Dr. Lewis-Hall held various senior leadership positions including Chief Medical Officer and Executive Vice President, Medicines Development at Vertex Pharmaceuticals Incorporated from June 2008 to May 2009; Senior Vice President, U.S. Pharmaceuticals, Medical Affairs for Bristol-Myers Squibb Company from 2003 until May 2008; Vice President Research and Development at Pharmacia Corporation from 2002-2003; Product Team Leader at Pharmacia and Eli Lilly and Company from 1998 to 2002; Director of Lilly Center for Women’s Health from 1996-1999, and Clinical Research Physician at Eli Lilly from 1994 through 1996. In October 2021, Dr. Lewis-Hall became a member of the board of directors for Pyxis Oncology (PYXS), (where she serves as a member of the Nominating and Corporate Governance Committee. She has been a member of the board of directors for Exact Sciences Corporation (EXAS) since April 2020, where she serves as a member of the Human Capital and Innovation, Technology and Pipeline Committees; a member of 1LifeHealthCare, Inc.(ONEM) board since November 2019, serving as a member of the Nominating and Corporate Governance Committee; a member of the board of directors for Milliken & Company since July 2019, as a member of the Audit and HR and Compensation Committees;, and as a member of the board of directors of SpringWorks Therapeutics, Inc. (SWTX) since 2017, serving as the chair of the Nominating and Governance Committee. Dr. Lewis-Hall served as a member of the board of directors for Tenet Healthcare Corporation (THC) from 2014 to 2017. Dr. Lewis-Hall holds an M.D. from Howard University College of Medicine and a B.A. in natural sciences from the Johns Hopkins University. Conduit believes Dr. Lewis-Hall is qualified to serve on the board of directors based on her expertise and experience in the biopharmaceutical industry and her leadership experience as a senior executive at various biopharmaceutical companies.
James (“Jamie”) Bligh
Mr. Bligh was a co-founder of Conduit Pharmaceuticals Limited in 2019. From 2008 to 2019, Mr. Bligh worked closely with investment vehicle Corvus Capital Limited, including as a Partner, where he led a number of reverse takeover transactions, stock market listings, initial public offerings, secondary fundraisings, and merger transactions. Mr. Bligh’s prior transaction experience includes advising several special purpose acquisition vehicles in listing on the London Stock Exchange, including the listing of Bermele Plc, a special purpose acquisition vehicle, and the subsequent acquisition of Bermele by East Imperial Pte. Ltd., a global purveyor of ultra-premium beverages, in June 2019; the listing of Leverett Plc, which subsequently acquired Nuformix Plc, a pharmaceutical development company targeting unmet medical needs in fibrosis and oncology via drug repurposing; and Cizzle Biotechnology Holdings Plc, a UK-based diagnostics developer. Jamie previously served as a director of Bermele Plc from June 2021 through February 2022; Mertz Plc from January 2021 through March 2022; and East Imperial Pte. Ltd. from September 2017 through April 2018. Jamie graduated from the University of Bristol with a BSc in Economics & Finance. Mr. Bligh was selected to serve on the board of New Conduit following the Business Combination based on his past experience with business development, capital raising, financings, public offerings and other strategic transactions, including mergers and acquisitions.
Faith L. Charles
Faith L. Charles, has been a corporate transactions and securities partner at the law firm of Thompson Hine LLP, since 2010. She leads Thompson Hine’s Life Sciences practice and co-heads the securities practice, advising public and emerging biotech and pharmaceutical companies in the U.S. and internationally. Ms. Charles negotiates complex private and public financing transactions, mergers and acquisitions, licensing transactions and strategic collaborations. She serves as outside counsel to a myriad of life sciences companies and is known in the industry as an astute business advisor, providing valuable insights into capital markets, corporate governance and strategic development. Ms. Charles has been a member of the board of directors of: CNS Pharmaceuticals, Inc. (Nasdaq: CNSP), a biotechnology company developing novel treatments for cancers of the brain and central nervous system, since December 2022; Avenue Therapeutics, Inc. (Nasdaq: ATXI), a specialty pharmaceutical company specializing in developing and commercializing therapies for the treatment of the central nervous system, since May 2022; and Abeona Therapeutics, Inc. (Nasdaq: ABEO), a fully integrated gene and cell therapy company, since March 2021. Ms. Charles serves as Chair of CNS Pharmaceuticals, on the Audit Committee of Avenue Therapeutics and on the Audit Committee and as the Chair of the Nominating and Governance Committee of Abeona Therapeutics. From 2018 until October 2021, Ms. Charles served on the Board of Directors and as a member of the Audit Committee and Chair of the Compensation Committee of Entera Bio Ltd., a publicly-traded biotechnology company. Ms. Charles founded the Women in Bio Metro New York chapter and chaired the chapter for five years. She also served on the national board of Women in Bio. Ms. Charles is also a member of the board of Red Door Community (formerly Gilda’s Club New York City.) She has been recognized as a Life Sciences Star by Euromoney’s LMG Life Sciences, has been named a BTI Client Service All-Star, and was named by Crain’s New York Business to the list of 2020 Notable Women in the Law. Ms. Charles holds a J.D degree from The George Washington University Law School and a B.A. in Psychology from Barnard College, Columbia University. Ms. Charles is a graduate of Women in Bio’s Boardroom Ready Program, an Executive Education Program taught by The George Washington University School of Business. Ms. Charles’ qualifications to serve on our Board include her leadership skills and her vast legal experience representing companies in the biotech and pharmaceutical field.
Chele Chiavacci Farley
Chele Chiavacci Farley has served on the MURF Board since the closing of its initial public offering and currently serves as a partner and managing director of Mistral Capital International (“Mistral”), a private equity firm, since 1995. In her role as Partner and Managing Director of Mistral, Ms. Farley originates, evaluates and executes equity investment opportunities, creates and implements deal and financial structures, negotiates with banks for credit facilities, and oversees management. Ms. Farley is the President and a member of the Board of Directors and Management Committee of Palmilla San Jose Inmobiliaria, the Master Developer of the luxury Palmilla resort development in Cabo San Lucas, Mexico. Prior to Mistral, Ms. Farley was Vice President of Tricap International from 1994 to 1995. From 1992 to 1994, Ms. Farley was an Associate at UBS Capital Corporation, and analyzed and evaluated principal investment and financing opportunities for the firm’s internal $1 billion fund. Ms. Farley began her career as a Financial Analyst in the Global Finance department - Energy and Telecom Group of Goldman, Sachs & Co. Ms. Farley has also had an active political career. In 2020, Ms. Farley ran for election to the U.S. House of Representatives to represent New York’s 18th Congressional district. In 2018, Ms. Farley ran for election to the U.S. Senate to represent New York. Ms. Farley graduated from Stanford University with a B.S. and M.S. in Industrial Engineering. She is a member of YPO - Young Presidents’ Organization. Ms. Farley was selected to serve on the board of New Conduit following the Business Combination based on her past experience with business development, capital raising, financings, and banking.
Jennifer I. McNealey
Jennifer I. McNealey has served as the Chief Financial Officer of Abdera Therapeutics Inc., a biotechnology company developing targeted radiotherapeutics since January 2023. Prior to Abdera, Ms. McNealey served as CFO of Codex DNA, Inc. (now Telesis Bio Inc.) from March 2021 until July 2022, and assisted that company through its initial public offering in 2021. From February 2015 to March 2021, Ms. McNealey served as Vice President of Investor Relations and Strategy at Calithera Biosciences, Inc., a development stage biotechnology company. Ms. McNealey guided Calithera through multiple equity raises including its IPO and secondary raises. Ms. McNealey has served as a director at Antibe Therapeutics Inc. since 2020, where she serves on the Audit and Governance committees. Antibe Therapeutics is a development-stage biotechnology company building a pipeline of safer, non-addictive therapeutics for pain and inflammation. Previously she served on the board of Enzon Pharmaceuticals, Inc. from November 2013 to November 2021. In 2005, Ms. McNealey founded and launched Laurient, an equity research and competitive intelligence tool for the biotechnology investment community. Prior to founding Laurient, Ms. McNealey served as an equity analyst and portfolio manager at Franklin Templeton and Morgan Stanley, each with a focus in investing in public biotechnology companies. Ms. McNealey earned an MHA from the Sloan Program in healthcare administration and a BA in psychology from Cornell University. Ms. McNealey was selected to serve on the board of New Conduit following the Business Combination based on her service as a member of the management team of another public company, as well as her extensive experience in the biotechnology and pharmaceutical industries.
| 117 |
Andrew Regan
Dr. Regan is a British born polar explorer and entrepreneur. He was a co-founder of Conduit Pharmaceuticals Limited and has served as a Board member since 2019. Dr. Regan also founded Corvus Capital Limited and has been its Chief Executive Officer since 2008. Corvus Capital is an investment vehicle that was previously listed on the London Stock Exchange prior to being taken private in 2008. Corvus Capital continues to invest in a number of industries and sectors. Dr. Regan also has experience as an investor in a number of public and private companies, including ASOS.com Ltd, a global online fashion and beauty retailer, Virtual Internet, an IT services company that specializes in hosting infrastructure such as VMWare cloud hosting and Managed and Dedicated Servers, and Imperial Energy Corporation plc, an upstream oil and gas exploration and production company. Prior to that, Dr. Regan was the Chief Executive Officer of Hobson Plc, which was listed on the London Stock Exchange, until its sale in 1996 through a cash takeover. Dr. Regan has a strong interest in the use of bio-inspired science to create solutions for present day problems. In 2014, he was awarded a PhD from Oxford Brookes University for his research in writing and developing a bio-inspired algorithm for forecasting the financial markets. He is passionate about the polar regions and is an accomplished polar explorer having led a number of expeditions to both the Arctic and Antarctica. Dr. Regan was selected to serve on the board of New Conduit following the Business Combination based on his knowledge of Conduit and his extensive experience in investing, financing, overseeing and developing companies. In 2003, Dr. Regan, in his capacity as Chief Executive Officer of Hobson Plc, was prosecuted in the United Kingdom in relation to his conduct during the failed £1.2 Billion takeover bid of Co-operative Wholesale Society by Hobson Plc in 1997. Dr. Regan was acquitted by a jury and the judge awarded him his costs and expenses. Civil claims relating to certain costs associated with the failed takeover by Dr. Regan were settled in 2005.
Executive Compensation Arrangements
Employment Arrangements with Executive Officers of New Conduit
New Conduit will enter into employment agreements with each of Dr. Tapolczay and Mr. Sragovicz, which will become effective upon completion the consummation of the Business Combination.
Dr. Tapolczay
The employment agreement with Dr. Tapolczay provides that he will serve as the Chief Executive Officer of New Conduit and shall be appointed to the New Conduit board of directors.
Under the agreement, Dr. Tapolczay will be entitled to (i) an annual base salary of $550,000, and (ii) a target annual bonus opportunity equal to 50% of his base salary, payable based on the achievement of performance objectives as determined by the New Conduit board of directors. In addition, the employment agreement provides that he will be entitled to receive a sign-on stock option award to purchase 0.40% of the shares of common stock of New Conduit pursuant to the terms of the 2023 Stock Incentive Plan, which shall vest in equal annual installments on the first four anniversaries of the Merger.
The employment agreement provides that if New Conduit terminates Dr. Tapolczay’s employment other than for cause or disability, or if he terminates his employment for good reason, in either case other than the change in control protection period (described below), he would be entitled to receive (i) continued payment of his annual base salary for 12 months following the date of termination, (ii) a lump sum payment of his annual cash performance bonus that had been earned by him for a completed fiscal year or other measuring period but that had not yet been paid to him as of the date of termination, (iii) a lump sum payment equal to his then target annual bonus opportunity, pro-rated based on the total number of days elapsed in the calendar year through the date of termination, (iv) payment or reimbursement of the COBRA premiums for him and his eligible dependents, or if COBRA is not available under our group health plan, a cash amount equal to such payments or reimbursements (in either case, less the premiums he was paying for such coverage while employed), until the earliest of (x) the last day of the applicable salary continuation period specified above, or (y) the date he becomes eligible for comparable health insurance coverage under a subsequent employer’s group health plan; and (v) accelerated vesting of such number of his unvested equity awards as would have vested had he remained employed during the 12-month period following his date of termination (provided, however, that, any equity awards that vest in whole or in part based on the attainment of performance-vesting conditions shall be governed by the terms of the applicable award agreement).
The employment agreement provides that if New Conduit terminates Dr. Tapolczay’s employment other than for cause or disability, or if he terminates his employment for good reason, in either case within three months prior to or 12 months after a change in control (such period, the change in control period), he would be entitled to receive (i) continued payment of his annual base salary for 18 months following the date of termination, (ii) a lump sum payment of his annual cash performance bonus that had been earned by him for a completed fiscal year or other measuring period but that had not yet been paid to him as of the date of termination, (iii) a lump sum payment equal to 150% of his then target annual bonus opportunity (without pro-ration), (iv) payment or reimbursement of the COBRA premiums for him and his eligible dependents, or if COBRA is not available under our group health plan, a cash amount equal to such payments or reimbursements (in either case, less the premiums he was paying for such coverage while employed), until the earliest of (x) the last day of the applicable salary continuation period specified above, or (y) the date he becomes eligible for comparable health insurance coverage under a subsequent employer’s group health plan; and (v) accelerated vesting of 100% of his unvested equity awards (provided, however, that, any equity awards that vest in whole or in part based on the attainment of performance-vesting conditions shall be governed by the terms of the applicable award agreement).
Additionally, to the extent that any payment or benefit received in connection with a change in control would be subject to an excise tax under Section 4999 of the Code, such payments and/or benefits will be subject to a “best pay cap” reduction if such reduction would result in a greater net after-tax benefit to the executive than receiving the full amount of such payments.
In exchange for the severance benefits described above, Dr. Tapolczay must (i) sign and not revoke a release of claims in favor of New Conduit, (ii) comply with his proprietary information and inventions assignment agreement, (iii) refrain from soliciting employees of New Conduit for a period of 1 year after his termination of employment, and (iv) comply with the other provisions of his employment agreement.
Mr. Sragovicz
The employment agreement under negotiation with Mr. Sragovicz provides that he will serve as the Chief Financial Officer of New Conduit.
Under the letter agreement, Mr. Sragovicz will be entitled to (i) an annual base salary of $400,000, and (ii) a target annual bonus opportunity equal to 40% of his base salary, payable based on the achievement of performance objectives as determined by the New Conduit board of directors. In addition, the proposed letter agreement provides that he will be entitled to receive a sign-on restricted stock unit award covering 0.10% of the shares of New Conduit’s common stock pursuant to the terms of the 2023 Stock Incentive Plan, which shall vest in equal annual installments on each of the first three anniversaries of the Merger.
The employment agreement provides that if New Conduit terminates Mr. Sragovicz’s employment other than for cause or disability, or if he terminates his employment for good reason, in either case other than the change in control protection period (described below), he would be entitled to receive (i) continued payment of his annual base salary for 9 months following the date of termination, (ii) a lump sum payment of his annual cash performance bonus that had been earned by him for a completed fiscal year or other measuring period but that had not yet been paid to him as of the date of termination, (iii) a lump sum payment equal to his then target annual bonus opportunity, pro-rated based on the total number of days elapsed in the calendar year through the date of termination, (iv) payment or reimbursement of the COBRA premiums for him and his eligible dependents, or if COBRA is not available under our group health plan, a cash amount equal to such payments or reimbursements (in either case, less the premiums he was paying for such coverage while employed), until the earliest of (x) the last day of the applicable salary continuation period specified above, or (y) the date he becomes eligible for comparable health insurance coverage under a subsequent employer’s group health plan; and (v) accelerated vesting of such number of his unvested equity awards as would have vested had he remained employed during the 9-month period following his date of termination (provided, however, that, any equity awards that vest in whole or in part based on the attainment of performance-vesting conditions shall be governed by the terms of the applicable award agreement).
| 118 |
The employment agreement provides that if New Conduit terminates Mr. Sragovicz’s employment other than for cause or disability, or if he terminates his employment for good reason, in either case within three months prior to or 12 months after a change in control (such period, the change in control period), he would be entitled to receive (i) continued payment of his annual base salary for 12 months following the date of termination, (ii) a lump sum payment of his annual cash performance bonus that had been earned by him for a completed fiscal year or other measuring period but that had not yet been paid to him as of the date of termination, (iii) a lump sum payment equal to 100% of his then target annual bonus opportunity (without pro-ration), (iv) payment or reimbursement of the COBRA premiums for him and his eligible dependents, or if COBRA is not available under our group health plan, a cash amount equal to such payments or reimbursements (in either case, less the premiums he was paying for such coverage while employed), until the earliest of (x) the last day of the applicable salary continuation period specified above, or (y) the date he becomes eligible for comparable health insurance coverage under a subsequent employer’s group health plan; and (v) accelerated vesting of 100% of his unvested equity awards (provided, however, that, any equity awards that vest in whole or in part based on the attainment of performance-vesting conditions shall be governed by the terms of the applicable award agreement).
Additionally, to the extent that any payment or benefit received in connection with a change in control would be subject to an excise tax under Section 4999 of the Code, such payments and/or benefits will be subject to a “best pay cap” reduction if such reduction would result in a greater net after-tax benefit to the executive than receiving the full amount of such payments.
In exchange for the severance benefits described above, Mr. Sragovicz must (i) sign and not revoke a release of claims in favor of New Conduit, (ii) comply with his proprietary information and inventions assignment agreement, (iii) refrain from soliciting employees of New Conduit for a period of 1 year after his termination of employment, and (iv) comply with the other provisions of his employment agreement.
Director Compensation Arrangements
Compensation Program for the New Conduit Board of Directors
New Conduit will adopt a compensation program for the New Conduit board of directors, which will become effective upon completion the consummation of the Business Combination.
Under the new program, the non-employee directors will receive the following annual cash retainers for their service on the New Conduit board of directors and its committees:
| ● | $35,000 for each non-employee director; | |
| ● | $30,000 for the Chairman of the New Conduit board of directors; | |
| ● | $15,000 for the chair of the Audit Committee and $7,500 for each of the other members of that committee; | |
| ● | $10,000 for the chair of the Compensation Committee and $5,000 for each of the other members of that committee; and | |
| ● | $8,000 for the chair of the Nominating and Corporate Governance Committee and $4,000 for each of the other members of that committee. |
In addition, each non-employee director who is initially elected or appointed to the board on or after the completion of the Business Combination shall automatically be granted on the day of such first election or appointment a stock option to purchase 65,000 shares of New Conduit common stock (the “Initial Award”) (provided that the Initial Award with respect to each non-employee director who initially is elected or appointed to the board at the closing of the Business Combination shall be granted upon the effectiveness of the Form S-8 with respect to the New Conduit common stock issuable under the 2023 Stock Incentive Plan). Each Initial Award shall vest and become exercisable in substantially equal installments on each of the first three anniversaries of the date of grant, subject to the non-employee director continuing in service on the New Conduit board of directors through each such vesting date.
A non-employee director who is serving on the board as of the date of any annual meeting after the effective date of the new program, and who will continue to serve as a non-employee director immediately following such meeting, shall automatically be granted on the date of such annual meeting a stock option to purchase 32,500 shares of New Conduit common stock, which amount is pro-rated for new directors to reflect their service since the last annual meeting (the “Annual Award”). Each Annual Award shall vest and become exercisable on the earlier of (i) the first anniversary of the date of grant, or (ii) the date immediately prior to the next annual meeting of the Company’s stockholders following the date of grant, subject to the non-employee director continuing in service on the New Conduit board through such vesting date.
Upon a change in control, all outstanding equity awards that are held by a non-employee director shall become fully vested and exercisable.
Board Composition
New Conduit’s business and affairs will be organized under the direction of its board of directors. The board of directors of New Conduit will meet on a regular basis and additionally as required. In accordance with the terms of the amended and restated certificate of incorporation, which will be effective upon the consummation of the Business Combination, New Conduit’s board of directors may establish the authorized number of directors from time to time by resolution. New Conduit’s board of directors will consist of seven (7) directors upon the consummation of the Business Combination.
Director Independence
Under the Nasdaq listing standards, a majority of the members of New Conduit’s board of directors must qualify as “independent,” as affirmatively determined by the board of directors. The MURF Board affirmatively determined that all of the presently expected directors, except for Messrs. Bligh, Tapolczay, and Regan are independent directors within the meaning of the applicable Nasdaq listing standards. A majority of the members of the board of New Conduit and all members of the New Conduit’s Audit Committee, Compensation Committee, and Nominating and Corporate Governance Committee will be independent directors under the applicable Nasdaq listing standards.
Board Leadership Structure
The New Conduit’s board will be responsible for the control and direction of New Conduit, the combined company following the Business Combination. It is anticipated that New Conduit will separate the positions of Chairman and Chief Executive Officer. Dr. Freda Lewis-Hall will serve as the Chairman of New Conduit’s board of directors and Dr. David Tapolczay will serve as the Chief Executive Officer of New Conduit and as a member of New Conduit’s board of directors. The MURF Board and the Conduit board believe that this structure will serve New Conduit well by maintaining a link between management, through Dr. Tapolczay’s membership on New Conduit’s board of directors, and the non-executive directors led by Dr. Lewis-Hall in her role as a non-executive Chairman.
Board Oversight of Risk
Upon the consummation of the Business Combination, one of the key functions of New Conduit’s board of directors will be to conduct informed oversight of New Conduit’s risk management process. New Conduit’s board of directors does not anticipate having a standing risk management committee, but rather anticipates administering this oversight function directly through New Conduit’s board of directors as a whole, as well as through various standing committees of the board of directors that address risks inherent in their respective areas of oversight. In particular, the board of directors will be responsible for monitoring and assessing strategic risk exposure and New Conduit’s Audit Committee will have the responsibility to consider and discuss New Conduit’s major financial risk exposures and the steps its management will take to monitor and control such exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. The Audit Committee will also monitor compliance with legal and regulatory requirements. New Conduit’s Compensation Committee will also assess and monitor whether New Conduit’s compensation plans, policies and programs comply with applicable legal and regulatory requirements.
| 119 |
Board Committees
Audit Committee. Upon the consummation of the Business Combination, New Conduit will establish an Audit Committee. The Audit Committee assists the board of directors with its oversight of the integrity of the financial statements; the compliance with legal and regulatory requirements; the qualifications, independence and performance of the independent registered public accounting firm; the design and implementation of the financial risk assessment and risk management. Among other things, the Audit Committee is responsible for reviewing and discussing with management the adequacy and effectiveness of disclosure controls and procedures. The Audit Committee also discusses with management and independent registered public accounting firm the annual audit plan and scope of audit activities, scope and timing of the annual audit of the financial statements, and the results of the audit, quarterly reviews of the financial statements and, as appropriate, initiates inquiries into certain aspects of the financial affairs.
The Audit Committee is responsible for establishing and overseeing procedures for the receipt, retention and treatment of any complaints regarding accounting, internal accounting controls or auditing matters, as well as for the confidential and anonymous submissions by employees of concerns regarding questionable accounting or auditing matters. In addition, the Audit Committee has direct responsibility for the appointment, compensation, retention and oversight of the work of the independent registered public accounting firm. The Audit Committee has sole authority to approve the hiring and discharging of the independent registered public accounting firm, all audit engagement terms and fees and all permissible non-audit engagements with the independent auditor. The Audit Committee reviews and oversees all related person transactions in accordance with policies and procedures.
The Audit Committee of New Conduit will undertake these responsibilities following completion of the Business Combination.
New Conduit’s Audit Committee will be comprised of three members, Ms. Farley (Chairman), Ms. Lewis-Hall and Ms. McNealey. Each member of the Audit Committee of New Conduit will meet the requirements for independence under the current Nasdaq and SEC rules and regulations and each member is financially literate. In addition, the board of directors has determined that each of Ms. Farley and Ms. McNealey is an “audit committee financial expert” as defined in Item 407(d)(5)(ii) of Regulation S-K promulgated under the Securities Act.
Compensation Committee. Upon the consummation of the Business Combination, New Conduit will establish a Compensation Committee. The Compensation Committee assists New Conduit’s board of directors with its oversight of the forms and amount of compensation for executive officers (including officers reporting under Section 16 of the Exchange Act), the administration of equity and non-equity incentive plans for employees and other service providers and certain other matters related to compensation programs. The Compensation Committee, among other responsibilities, evaluates the performance of the New Conduit’s chief executive officer and, in consultation with him, evaluates the performance of other executive officers (including officers reporting under Section 16 of the Exchange Act). The Compensation Committee of the New Conduit will undertake these responsibilities following completion of the Business Combination. New Conduit’s Compensation Committee will be comprised of three members, Ms. Charles (Chairperson), Ms. Farley, and Ms. McNealy. The composition of the Compensation Committee of New Conduit will meet the requirements for independence under the current Nasdaq and SEC rules and regulations. Each member of the Compensation Committee of New Conduit will be a “non-employee” director within the meaning of Rule 16b-3 promulgated under the Exchange Act.
Nominating and Governance Committee. Upon the consummation of Business Combination, New Conduit will establish a Nominating and Corporate Governance Committee. The Nominating and Corporate Governance Committee assists the board of directors with its oversight of and identification of individuals qualified to become members of the board of directors, consistent with criteria approved by the board of directors, and selects, or recommends that the board of directors selects, director nominees; develops and recommends to the board of directors a set of corporate governance guidelines; oversees the evaluation of the board of directors; and reviews the environmental, safety, sustainability and corporate social responsibility policies, objectives and practices on a periodic basis.
The Nominating and Corporate Governance Committee of the New Conduit will undertake these responsibilities following completion of the Business Combination. New Conduit’s Nominating and Corporate Governance Committee will be comprised of two members, Ms. Lewis-Hall (Chairman) and Ms. Charles. The composition of the Nominating and Corporate Governance Committee will meet the requirements for independence under the current Nasdaq and SEC rules and regulations.
The board of directors of New Conduit may from time to time establish other committees.
Compensation Committee Interlocks and Insider Participation
None of the proposed executive officers of New Conduit will serve as a member of the board of directors or compensation committee of any entity that has one or more executive officers who is proposed to serve on New Conduit’s board of directors or compensation committee following the Business Combination.
Family Relationships
There are no family relationships among the New Conduit directors and executive officers.
Code of Ethics
In connection with the consummation of the Business Combination, New Conduit will adopt a written Code of Business Conduct and Ethics (the “Code of Conduct”) applicable to all of its employees, executive officers and directors. The Code of Conduct will cover fundamental ethical and compliance-related principles and practices such as accurate accounting records and financial reporting, avoiding conflicts of interest, the protection and use of its property and information and compliance with legal and regulatory.
Director and Officer Liability and Indemnification
New Conduit will purchase directors’ and officers’ liability insurance and will enter into indemnification agreements with each of its directors and executive officers. The indemnification agreements and New Conduit’s amended and restated certificate of incorporation and amended and restated bylaws will require it to indemnify the directors and officers to the fullest extent permitted by Delaware law.
| 120 |
THE INCENTIVE PLAN PROPOSAL
Overview
MURF is asking its stockholders to approve the Conduit Pharmaceuticals Inc. 2023 Stock Incentive Plan (named in anticipation of the Business Combination) (the “2023 Plan”). The MURF Board approved the 2023 Plan, prior to the Special Meeting, subject to stockholder approval at the Special Meeting. The 2023 Plan will become effective as of the date immediately prior to the Closing, subject to approval by the MURF stockholders.
The 2023 Plan is described in more detail below. A copy of the 2023 Plan is attached to this proxy statement/prospectus as Annex C.
The 2023 Plan
The principal purpose of the 2023 Plan is to attract, retain and motivate selected employees, consultants and directors through the granting of stock-based compensation awards and cash-based awards. Equity awards and equity-linked compensatory opportunities are intended to motivate high levels of performance and align the interests of directors, employees and consultants with those of stockholders by giving directors, employees and consultants the perspective of an owner with an equity or equity-linked stake in our company and providing a means of recognizing their contributions to our success. The MURF Board believes that equity awards are necessary for New Conduit to remain competitive in its industry and are essential to recruiting and retaining highly qualified employees.
Summary of the 2023 Plan
This section summarizes certain principal features of the 2023 Plan. The summary is qualified in its entirety by reference to the complete text of the 2023 Plan.
Eligibility and Administration
Options, restricted stock units and other stock-based and cash-based awards under the 2023 Plan may be granted to individuals who are then officers, employees or consultants or are the officers, employees or consultants of New Conduit of certain of New Conduit’s subsidiaries. Such awards also may be granted to directors of the New Conduit Board. Only employees of our company or certain of our subsidiaries may be granted incentive stock options (“ISOs”). Immediately following the consummation of the Business Combination, New Conduit is expected to have approximately 3 employees, 2 consultants and 6 non-employee directors who will be eligible to receive awards under the 2023 Plan.
The compensation committee of the New Conduit Board is expected to administer the 2023 Plan unless the New Conduit Board assumes authority for administration. The 2023 Plan provides that the board or compensation committee may delegate its authority to grant awards to employees other than executive officers and certain senior executives of New Conduit to a committee consisting of one or more members of New Conduit Board or one or more of our officers, other than awards made to our non-employee directors, which must be approved by our full board of directors.
Subject to the terms and conditions of the 2023 Plan, the plan administrator has the authority to select the persons to whom awards are to be made, to determine the number of shares to be subject to awards and the terms and conditions of awards, and to make all other determinations and to take all other actions necessary or advisable for the administration of the 2023 Plan. The plan administrator is also authorized to adopt, amend or rescind rules relating to administration of the 2023 Plan.
Shares Available for Awards
The aggregate number of shares of New Conduit common stock initially reserved for issuance pursuant to awards under the 2023 Plan will be 11,497,622 shares, which is intended to represent approximately 12.5% of the fully diluted shares of New Conduit common stock outstanding immediately after Closing, plus an annual increase on the first day of each calendar year beginning in 2024 and ending in 2033 equal to the lesser of (i) 5% of the shares of New Conduit common stock outstanding on the last day of the immediately preceding calendar year and (ii) such smaller number of shares of common stock as determined by the New Conduit Board; provided, however, that no more than 57,488,110 shares of common stock may be issued upon the exercise of ISOs.
| 121 |
The following counting provisions will be in effect for the share reserve under the 2023 Plan:
| ● | to the extent that an award terminates, expires or lapses for any reason or an award is settled in cash without the delivery of shares, any shares subject to the award at such time will be available for future grants under the 2023 Plan; |
| ● | the payment of dividend equivalents in cash in conjunction with any outstanding awards will not be counted against the shares available for issuance under the 2023 Plan; and |
| ● | to the extent permitted by applicable law or any exchange rule, shares issued in assumption of, or in substitution for, any outstanding awards of any entity acquired in any form of combination by us or any of our subsidiaries will not be counted against the shares available for issuance under the 2023 Plan. |
However, the following shares issued or delivered under the 2023 Plan shall not again be available for future grants of awards: (i) shares that are tendered or withheld to satisfy the exercise price or tax withholding obligation with respect to any award under the 2023 Plan; (ii) shares that are repurchased by us with option proceeds; and (iii) shares subject to a stock appreciation right that are not issued in connection with the stock settlement of the stock appreciation right on exercise thereof.
The 2023 Plan also provides that the sum of the grant date fair value of all equity-based awards and the maximum amount of cash that may become payable to any individual for services as a non-employee director during any calendar year may not exceed $750,000, increased to $1,000,000 in the calendar year of a non-employee director’s initial service as a non-employee director of the New Conduit Board. The plan administrator may make exceptions to this limit for individual non-employee directors in extraordinary circumstances, as the plan administrator may determine in its discretion, provided that the non-employee director receiving such additional compensation may not participate in the decision to award such compensation or in other contemporaneous compensation decisions involving non-employee directors.
Types of Awards
The 2023 Plan provides that the plan administrator may grant or issue stock options, including ISOs and nonqualified stock options (“NSOs”), stock appreciation rights (“SARs”), restricted stock, restricted stock units (“RSUs”), performance stock units (“PSUs”), other stock- or cash-based awards and dividend equivalents, or any combination thereof. Certain awards under the 2023 Plan may constitute or provide for payment of “nonqualified deferred compensation” under Section 409A of the Code, which may impose additional requirements on the terms and conditions of such awards. All awards under the 2023 Plan will be evidenced by award agreements, which will detail the terms and conditions of awards, including any applicable vesting and payment terms and post-termination exercise limitations. Awards other than cash awards generally will be settled in shares of New Conduit common stock, but the applicable award agreement may provide for cash settlement of any award. A brief description of each award type follows.
| ● | Stock Options and SARs. Stock options provide for the purchase of shares of New Conduit common stock at a specified price set on the grant date. ISOs, in contrast to NSOs, may provide tax deferral beyond exercise and favorable capital gains tax treatment to their holders if certain holding period and other requirements of the Code are satisfied. Upon exercise, holders of SARs will receive from New Conduit an amount equal to the appreciation of the shares subject to the award between the grant date and the exercise date. Unless otherwise determined by the plan administrator, the exercise price of a stock option or SAR may not be less than 100% of the fair market value of the underlying share of common stock on the grant date (or 110% in the case of ISOs granted to certain significant stockholders), except with respect to certain substitute awards granted in connection with a corporate transaction. Unless otherwise determined by the plan administrator, the term of a stock option or SAR may not be longer than ten years (or five years in the case of ISOs granted to certain significant stockholders). |
| ● | Restricted Stock. Restricted stock is an award of non-transferable shares of New Conduit common stock that are subject to certain vesting conditions and other restrictions as may be determined by the plan administrator. Restricted stock, typically, may be forfeited for no consideration or repurchased by us at the original purchase price if the conditions or restrictions on vesting are not met. In general, restricted stock may not be sold or otherwise transferred until restrictions are removed or expire. Holders of restricted stock, unlike recipients of stock options, will have voting rights and will have the right to receive dividends, if any, prior to the time when the restrictions lapse, however, dividends will not be released until restrictions are removed or expire. |
| ● | RSUs. RSUs are contractual promises to deliver shares of New Conduit common stock in the future or an equivalent in cash and other consideration determined by the plan administrator, which may also remain forfeitable unless and until specified conditions are met and may be accompanied by the right to receive the equivalent value of dividends paid on shares of New Conduit common stock prior to the delivery of the underlying shares (i.e., dividend equivalent rights). Like restricted stock, restricted stock units may not be sold, or otherwise transferred or hypothecated, until vesting conditions are removed or expire. Unlike restricted stock, stock underlying restricted stock units will not be issued until the restricted stock units have vested, and recipients of restricted stock units generally will have no voting or dividend rights prior to the time when vesting conditions are satisfied. |
| 122 |
| ● | PSUs. PSUs are performance awards denominated in cash or shares/unit equivalents, respectively, and may be linked to one or more performance or other criteria as determined by the plan administrator. | |
| ● | Other Stock or Cash Based Awards. Other stock or cash-based awards are awards of cash, fully vested shares of New Conduit common stock and other awards valued wholly or partially by referring to, or otherwise based on, shares of New Conduit common stock. Other stock or cash-based awards may be granted to participants and may also be available as a payment form in the settlement of other awards, as standalone payments and as payment in lieu of compensation to which a participant is otherwise entitled. The plan administrator will determine the terms and conditions of other stock or cash based awards, which may include vesting conditions based on continued service, performance and/or other conditions. |
| ● | Dividend Equivalents. Dividend equivalents represent the right to receive the equivalent value of dividends paid on shares of New Conduit common stock and may be granted alone or in tandem with awards other than stock options or SARs. Dividend equivalents are converted to cash or shares by such formula and such time as determined by the plan administrator. In addition, dividend equivalents with respect to an award subject to vesting will either (i) to the extent permitted by applicable law, not be paid or credited or (ii) be accumulated and subject to vesting to the same extent as the related award. |
Any award may be granted as a performance award, meaning that the award will be subject to vesting and/or payment based on the attainment of specified performance goals. Performance goals or performance criteria established by the plan administrator for any award may be described in terms of company-wide objectives or objectives that are related to the performance of a subsidiary, division, business unit, department, region or function in which the participant is employed or in terms of the performance of the individual participant and may be based on the following criteria: revenues, earnings from operations, operating income, income before taxes, net income, cash flow, earnings per share, return on total capital, return on invested capital, return on equity, return on assets, total return to shareholders, earnings before or after interest, taxes, depreciation, amortization or extraordinary or special items, return on investment, free cash flow, cash conversion cycle, cash flow return on investment (discounted or otherwise), net cash provided by operations, cash flow in excess of cost of capital, operating margin, profit margin, contribution margin, stock price, new customers, order intake, cost controls, operating efficiencies, strategic partnerships or transactions (including in-licensing and out-licensing of intellectual property, milestones related to research development (including, but not limited to, preclinical and clinical studies), product development and manufacturing, implementation or completion of projects or processes (including, without limitation, clinical trial initiation, clinical trial enrollment and dates, clinical trial results, regulatory filing submissions, regulatory filing acceptances, regulatory or advisory committee interactions, regulatory approvals, and product supply), submission to, or approval by, a regulatory body (including, but not limited to the U.S. Food and Drug Administration) of an applicable filing or a product, market penetration, geographic business expansion, cost targets, productivity, corporate value and sustainability metrics (including, without limitation, environmental, social and governance matters), human capital metrics (including, without limitation, employee satisfaction, management of employment practices, employee benefits, retention and safety), supervision of litigation or labor negotiations, dealings with regulatory bodies, acquisitions or divestitures, customer satisfaction, strategic business criteria related to a participant’s area or areas of responsibility, or other criteria established by the plan administrator.
Certain Transactions
In the event of any stock dividend or other distribution, stock split, reverse stock split, reorganization, combination or exchange of shares, merger, consolidation, split-up, spin-off, recapitalization, repurchase or any other corporate event affecting the number of outstanding shares of New Conduit common stock or the share price of New Conduit common stock that would require adjustments to the 2023 Plan or any awards under the 2023 Plan in order to prevent the dilution or enlargement of the potential benefits intended to be made available thereunder, the plan administrator will make appropriate, proportionate adjustments to: (i) the aggregate number and type of shares subject to the 2023 Plan; (ii) the number and kind of shares subject to outstanding awards and terms and conditions of outstanding awards (including, without limitation, any applicable performance targets or criteria with respect to such awards); and (iii) the grant or exercise price per share of any outstanding awards under the 2023 Plan; as well as granting new awards or making cash payments to participants. The administrator is also authorized to provide for the cancellation, exchange, accelerated vesting or exercisability, replacement or termination of outstanding awards in the event of a corporate transaction or event affecting New Conduit or any change in applicable law or accounting principles.
| 123 |
In the event of a change in control (as defined in the 2023 Plan), to the extent that the successor entity does not assume or substitute outstanding awards, the administrator will cause all such awards to become fully vested and, if applicable, exercisable immediately prior to the consummation of such transaction, and with respect to awards with performance-based vesting, all performance goals or other vesting criteria will be deemed achieved at the greater of (i) one hundred percent (100%) of target levels, or (ii) the actual level of achievement of all relevant performance goals against target measured through the date immediately prior to the change in control (or as close to such date as administratively practicable), unless specifically provided otherwise under the applicable award agreement or otherwise determined by the administrator, and all forfeiture restrictions on such awards will lapse and, to the extent unexercised upon the consummation of such transaction, will be terminated in exchange for cash, rights or other property.
Repricing
The plan administrator will, without the approval from the New Conduit stockholders, have the authority to amend any outstanding stock option or SAR to reduce its exercise price per share or cancel any outstanding stock option or SAR in exchange for cash or another award.
Amendment and Termination
The New Conduit Board may terminate, amend or suspend the 2023 Plan at any time and from time to time. However, we must generally obtain stockholder approval to the extent required by applicable law, rule or regulation (including any applicable stock exchange rule). No amendments to outstanding awards that materially and adversely affect a participant’s rights under the award may be made without the affected participant’s consent, except in connection with certain transactions (such as equity restructurings, corporate transactions, or a change in control) or to preserve the intended tax treatment of the participant’s award.
No awards may be granted pursuant to the 2023 Plan on or after the tenth anniversary of the date the New Conduit Board approved the 2023 Plan. Any award that is outstanding on the termination date of the 2023 Plan will remain in force according to the terms of the 2023 Plan and the applicable award agreement.
Foreign Participants, Claw-Back Provisions, Transferability and Participant Payments
The plan administrator may modify awards granted to participants who are foreign nationals or employed outside the United States or establish subplans or procedures to address, among other things, differences in laws, rules, regulations or customs of such foreign jurisdictions. All awards will be subject to any New Conduit claw-back policy as set forth in such claw-back policy or the applicable award agreement. Except as the plan administrator may determine or provide in an award agreement, awards under the 2023 Plan are generally non-transferrable, except by will or the laws of descent and distribution, or, subject to the plan administrator’s consent, pursuant to a domestic relations order, and are generally exercisable only by the participant. With regard to tax withholding obligations arising in connection with awards under the 2023 Plan, and exercise price obligations arising in connection with the exercise of stock options under the 2023 Plan, the plan administrator may, in its discretion, accept cash, wire transfer or check, shares of common stock that meet specified conditions, a “market sell order,” or such other consideration as the plan administrator deems suitable or any combination of the foregoing.
U.S. Federal Income Tax Consequences Related to Awards Under the 2023 Plan
The following summary is based on an analysis of the Code as currently in effect, existing laws, judicial decisions, administrative rulings, regulations and proposed regulations, all of which are subject to change. Moreover, the following is only a summary of United States federal income tax consequences. Actual tax consequences to participants in the 2023 Plan may be either more or less favorable than those described below depending on the participants’ particular circumstances. State and local tax consequences may in some cases differ from the U.S. federal income tax consequences. The following summary of the income tax consequences in respect of the 2023 Plan is for general information only. Interested parties should consult their own advisors as to the specific tax consequences of their awards, including the applicability and effect of state, local and foreign laws.
Incentive Stock Options
No income will be recognized by a participant for United States federal income tax purposes upon the grant or exercise of an ISO under the 2023 Plan. The basis of shares transferred to a participant upon exercise of an ISO is the price paid for the shares. If the participant holds the shares for at least one year after the transfer of the shares to the participant and two years after the grant of the ISO, the participant generally will recognize capital gain or loss upon sale of the shares received upon exercise equal to the difference between the amount realized on the sale and the basis of the stock. Generally, if the shares are not held for that period, the participant will recognize ordinary income upon disposition in an amount equal to the excess of the fair market value of the shares on the date of exercise over the amount paid for the shares, or if less (and if the disposition is a transaction in which loss, if any, will be recognized), the gain on disposition. Any additional gain realized by the participant upon the disposition will be a capital gain. The excess of the fair market value of shares received upon the exercise of an incentive stock option over the option price for the shares is an item of adjustment for the participant for purposes of the alternative minimum tax. Therefore, although no income is recognized upon exercise of an incentive stock option, a participant may be subject to alternative minimum tax as a result of the exercise.
| 124 |
Non-statutory Stock Options
No income is expected to be recognized by a participant for United States federal income tax purposes upon the grant of a NSO. Upon exercise of a NSO, the participant will recognize ordinary income in an amount equal to the excess of the fair market value of the shares on the date of exercise over the amount paid for the shares. Income recognized upon the exercise of a NSO will be considered compensation subject to withholding at the time the income is recognized, and, therefore, the participant’s employer must make the necessary arrangements with the participant to ensure that the amount of the tax required to be withheld is available for payment. NSOs are designed to provide the employer with a deduction equal to the amount of ordinary income recognized by the participant at the time of the recognition by the participant, subject to the deduction limitations described below.
Stock Appreciation Rights
There are expected to be no United States federal income tax consequences to either the participant or the employer upon the grant of SARs. Generally, the participant will recognize ordinary income subject to withholding upon the receipt of payment pursuant to SARs in an amount equal to the aggregate amount of cash and the fair market value of any common stock received. Subject to the deduction limitations described below, the employer generally will be entitled to a corresponding tax deduction equal to the amount includible in the participant’s income.
Restricted Stock
If the restrictions on an award of shares of restricted stock are of a nature that the shares are both subject to a substantial risk of forfeiture and are not freely transferable (within the meaning of Section 83 of the Code), the participant will not recognize income for United States federal income tax purposes at the time of the award unless the participant affirmatively elects to include the fair market value of the shares of restricted stock on the date of the award, less any amount paid for the shares, in gross income for the year of the award pursuant to Section 83(b) of the Code. In the absence of this election, the participant will be required to include in income for United States federal income tax purposes on the date the shares either become freely transferable or are no longer subject to a substantial risk of forfeiture (within the meaning of Section 83 of the Code), the fair market value of the shares of restricted stock on such date, less any amount paid for the shares. The employer will be entitled to a deduction at the time of income recognition to the participant in an amount equal to the amount the participant is required to include in income with respect to the shares, subject to the deduction limitations described below. If a Section 83(b) election is made within 30 days after the date the restricted stock is received, the participant will recognize ordinary income at the time of the receipt of the restricted stock, and the employer will be entitled to a corresponding deduction, equal to the fair market value of the shares at the time, less the amount paid, if any, by the participant for the restricted stock. If a Section 83(b) election is made, no additional income will be recognized by the participant upon the lapse of restrictions on the restricted stock, but, if the restricted stock is subsequently forfeited, the participant may not deduct the income that was recognized pursuant to the Section 83(b) election at the time of the receipt of the restricted stock.
Dividends paid to a participant holding restricted stock before the expiration of the restriction period will be additional compensation taxable as ordinary income to the participant subject to withholding, unless the participant made an election under Section 83(b) of the Code. Subject to the deduction limitations described below, the employer generally will be entitled to a corresponding tax deduction equal to the dividends includible in the participant’s income as compensation. If the participant has made a Section 83(b) election, the dividends will be dividend income, rather than additional compensation, to the participant.
If the restrictions on an award of restricted stock are not of a nature that the shares are both subject to a substantial risk of forfeiture and not freely transferable, within the meaning of Section 83 of the Code, the participant will recognize ordinary income for United States federal income tax purposes at the time of the transfer of the shares in an amount equal to the fair market value of the shares of restricted stock on the date of the transfer, less any amount paid therefor. The employer will be entitled to a deduction at that time in an amount equal to the amount the participant is required to include in income with respect to the restricted shares, subject to the deduction limitations described below.
Restricted Stock Units
There generally will be no United States federal income tax consequences to either the participant or the employer upon the grant of RSUs. Generally, the participant will recognize ordinary income subject to withholding upon the receipt of cash and/or transfer of shares of common stock in payment of the RSUs in an amount equal to the aggregate of the cash received and the fair market value of the common stock so transferred. Subject to the deduction limitations described below, the employer generally will be entitled to a corresponding tax deduction equal to the amount includible in the participant’s income.
| 125 |
Generally, a participant will recognize ordinary income subject to withholding upon the payment of any dividend equivalents paid with respect to an award in an amount equal to the cash and the fair market value of any common stock the participant receives. Subject to the deduction limitations described below, the employer generally will be entitled to a corresponding tax deduction equal to the amount includible in the participant’s income.
Limitation on the Employer’s Compensation Deduction
Section 162(m) of the Code limits the deduction certain employers may take for otherwise deductible compensation payable to certain executive officers of the employer to the extent the compensation paid to such an officer for the year exceeds $1 million.
Excess Parachute Payments
Section 280G of the Code limits the deduction that the employer may take for otherwise deductible compensation payable to certain individuals if the compensation constitutes an “excess parachute payment.” Excess parachute payments arise from payments made to disqualified individuals that are in the nature of compensation and are contingent on changes in ownership or control of the employer or certain affiliates. Accelerated vesting or payment of awards under the 2023 Plan upon a change in ownership or control of the employer or its affiliates could result in excess parachute payments. In addition to the deduction limitation applicable to the employer, a disqualified individual receiving an excess parachute payment is subject to a 20% excise tax on the amount thereof.
Section 409A of the Code
Certain types of awards under the 2023 Plan may constitute, or provide for, a deferral of compensation subject to Section 409A of the Code. Unless certain requirements set forth in Section 409A of the Code are complied with, holders of such awards may be taxed earlier than would otherwise be the case (e.g., at the time of vesting instead of the time of payment) and may be subject to an additional 20% penalty tax (and, potentially, certain interest, penalties and additional state taxes). To the extent applicable, the 2023 Plan and awards granted under the 2023 Plan are intended to be structured and interpreted in a manner intended to either comply with or be exempt from Section 409A of the Code and the Department of Treasury regulations and other interpretive guidance that may be issued under Section 409A of the Code. To the extent determined necessary or appropriate by the plan administrator, the 2023 Plan and applicable award agreements may be amended to further comply with Section 409A of the Code or to exempt the applicable awards from Section 409A of the Code.
New Plan Benefits
The benefits or amounts that may be received by or allocated to participants under the 2023 Plan will be determined at the discretion of the plan administrator and are not currently determinable. The value of future awards granted under the 2023 Plan will depend on a number of factors, including the fair market value of the common stock on future dates, the exercise decisions made by the participants and the extent to which any applicable performance goals necessary for vesting or payment are achieved.
Registration Statement
New Conduit intends to file with the SEC a registration statement on Form S-8 covering some or all of the shares of New Conduit Common Stock issuable under the 2023 Plan.
Vote Required for Approval
If the Business Combination Proposal is not approved, the Incentive Plan Proposal will not be presented at the Special Meeting. The Incentive Plan Proposal will be approved and adopted only if a majority of the votes cast by the MURF stockholders present in person (by virtual attendance) or represented by proxy at the Special Meeting and entitled to vote thereon, assuming a quorum is present, vote “FOR” the Incentive Plan Proposal.
Failure to submit a proxy or to vote virtually at the Special Meeting, an abstention from voting or a broker non-vote will have no effect on the Incentive Plan Proposal.
The Business Combination is conditioned upon the approval of the Incentive Plan Proposal. Notwithstanding the approval of the Incentive Plan Proposal, if the Business Combination is not consummated for any reason, the actions contemplated by the Incentive Plan Proposal will not be effected. Each of the Condition Precedent Proposals is cross-conditioned on the approval of each other. The Sponsor agreed to vote such shares in favor of the business combination proposal. See the section entitled “Special Meeting of MURF Stockholders — Sponsor.”
THE MURF BOARD UNANIMOUSLY RECOMMENDS THAT THE MURF’S STOCKHOLDERS VOTE “FOR” THE INCENTIVE PLAN PROPOSAL.
| 126 |
EXECUTIVE AND DIRECTOR COMPENSATION
New Conduit Executive and Director Compensation
In connection with the Business Combination, the existing members of the board of directors of MURF, other than Chele Chiavacci Farley, will resign and, if management’s nominees are elected, the board of directors of New Conduit will consist of a total of seven (7) individuals, which will initially be: (i) Dr. Freda Lewis-Hall, Chairman of New Conduit’s board of directors, (ii) Dr. David Tapolczay, a current director of Conduit and the Chief Executive Officer of New Conduit, (iii) James Bligh, an employee of Conduit, (iv) Faith L. Charles, (v) Chele Chiavacci Farley, a current director of MURF, (vi) Jennifer I. McNealey, and (vii) Dr. Andrew Regan, a current director and shareholder of Conduit (Dr. Regan also undertakes duties typically associated with an executive officer in connection with his service as a director of Conduit).
Upon completion of the Business Combination, the executive officers of New Conduit will include Dr. David Tapolczay as Chief Executive Officer and Adam Sragovicz, MURF’s Chief Financial Officer and director, as Chief Financial Officer.
MURF Executive and Director Compensation
No executive officer of MURF has received any cash compensation for services rendered to us. Starting in February 2022, MURF pays to Murphy Canyon Management Group, Inc., an affiliate of the Sponsor, $10,000 per month for providing us with office space and certain office and secretarial services. However, this arrangement is solely for MURF’s benefit and is not intended to provide MURF’s officers or directors compensation in lieu of a salary.
Other than the $10,000 per month administrative fee, the payment of consulting, success or finder fees to the Sponsor, officers, directors or their affiliates in connection with the consummation of MURF’s initial business combination, and the transfer of 15,000 placement units to each of the three independent directors following the closing of our initial public offering, no compensation or fees of any kind will be paid to the Sponsor, members of our management team or their respective affiliates, for services rendered prior to or in connection with the consummation of MURF’s initial business combination (regardless of the type of transaction that it is). However, they will receive reimbursement for any out-of-pocket expenses incurred by them in connection with activities on our behalf, such as identifying potential target businesses, performing business due diligence on suitable target businesses and business combinations as well as traveling to and from the offices, plants or similar locations of prospective target businesses to examine their operations. There is no limit on the amount of consulting, success or finder fees payable by MURF upon consummation of an initial business combination. Additionally, there is no limit on the amount of out-of-pocket expenses reimbursable by MURF; provided, however, that to the extent such expenses exceed the available proceeds not deposited in the Trust Account, such expenses would not be reimbursed unless MURF consummates an initial business combination.
| 127 |
THE NASDAQ PROPOSAL
Overview
In connection with the Business Combination, MURF intends to issue (subject to customary terms and conditions) up to 2,700,000 units, with each unit consisting of one share of New Conduit common stock together with one warrant exercisable into one share of New Conduit common stock, to the Private Placement Investor pursuant to the Subscription Agreement and 65,000,000 shares of New Conduit common stock pursuant to the Merger Agreement.
Why MURF Needs Stockholder Approval
We are seeking stockholder approval in order to comply with Nasdaq Listing Rules 5635(a), (b) and (d).
Under Nasdaq Listing Rule 5635(a), stockholder approval is required prior to the issuance of securities in connection with the acquisition of another company if such securities are not issued in a public offering and (A) have, or will have upon issuance, voting power equal to or in excess of 20% of the voting power outstanding before the issuance of common stock (or securities convertible into or exercisable for common stock) or (B) the number of shares of common stock to be issued is or will be equal to or in excess of 20% of the number of shares of common stock outstanding before the issuance of the stock or securities. MURF will issue shares (including shares exercisable upon warrants) representing 20% or more of the number of outstanding shares of MURF common stock prior to the issuance, or 20% or more of its voting power prior to the issuance, pursuant to the Merger Agreement and the Private Placement.
Under Nasdaq Listing Rule 5635(b), stockholder approval is required prior to the issuance of securities when the issuance or potential issuance will result in a change of control of the registrant.
Under Nasdaq Listing Rule 5635(d), stockholder approval is required for a transaction other than a public offering involving the sale, issuance or potential issuance by an issuer of common stock (or securities convertible into or exercisable for common stock) at a price that is less than the lower of: (i) the Nasdaq official closing price immediately preceding the signing of the binding agreement; or (ii) the average Nasdaq official closing price of the common stock for the five trading days immediately preceding the signing of the binding agreement if the number of shares of common stock to be issued is or may be equal to 20% or more of the common stock, or 20% or more of the voting power, outstanding before the issuance.
Effect of Proposal on Current Stockholders
If the Nasdaq proposal is adopted, up to 70,400,000 shares of New Conduit common stock, (including up to 2,700,000 shares of New Conduit common stock issuable upon exercise of warrants) may be issued pursuant to the terms of the Merger Agreement and the Subscription Agreement, which would result in significant dilution to MURF’s stockholders, and would afford stockholders a smaller percentage interest in the voting power, liquidation value and aggregate book value of MURF after the closing of the Business Combination
In the event that this proposal is not approved by MURF stockholders, the Business Combination may not be consummated.
Required Vote for Approval
Approval of the Nasdaq proposal requires the affirmative vote of a majority in voting power of the outstanding shares of MURF common stock present in person (by virtual attendance) or by proxy at the Special Meeting. Assuming a valid quorum is otherwise established, failure to vote and broker non-votes will have no effect on the outcome of any vote on the Nasdaq proposal. Abstentions are deemed entitled to vote on such proposals. Therefore, they have the same effect as a vote against the proposals.
The Nasdaq proposal is conditioned upon the approval and completion of the business combination proposal. If the business combination proposal is not approved, the Nasdaq proposal will have no effect, even if approved by the MURF stockholders.
THE MURF BOARD UNANIMOUSLY RECOMMENDS THAT MURF STOCKHOLDERS VOTE “FOR” THE NASDAQ PROPOSAL.
| 128 |
THE ADJOURNMENT PROPOSAL
The adjournment proposal allows the MURF Board to submit a proposal to adjourn the Special Meeting to a later date or dates, if necessary, to permit further solicitation and vote of proxies in the event MURF does not have sufficient proxies to approve one or more of the foregoing proposals. In no event will MURF solicit proxies to adjourn the Special Meeting or consummate the Business Combination beyond the date by which it may properly do so under its amended and restated certificate of incorporation and Delaware law. See the section entitled “The Business Combination Proposal — Interests of MURF’s Directors and Officers in the Business Combination.”
In addition to an adjournment of the Special Meeting upon approval of an adjournment proposal, the MURF Board is empowered under Delaware law to postpone the meeting at any time prior to the meeting being called to order. In such event, MURF will issue a press release and take such other steps as it believes are necessary and practical in the circumstances to inform its stockholders of the postponement.
Consequences if the Adjournment Proposal is not Approved
If an adjournment proposal is presented to the meeting and is not approved by the stockholders, the MURF Board may not be able to adjourn the Special Meeting to a later date if MURF is unable to consummate the Business Combination (because either the business combination proposal is not approved or the conditions to consummating the Business Combination have not been met). In such event, the Business Combination would not be completed.
Required Vote for Approval
Adoption of the adjournment proposal requires the affirmative vote of a majority of the issued and outstanding shares of MURF common stock represented in person (by virtual attendance) or by proxy at the meeting and entitled to vote thereon. Adoption of the adjournment proposal is not conditioned upon the adoption of any of the other proposals.
THE MURF BOARD UNANIMOUSLY RECOMMENDS THAT MURF STOCKHOLDERS VOTE “FOR” THE APPROVAL OF THE ADJOURNMENT PROPOSAL.
| 129 |
INFORMATION ABOUT murf
Introduction
MURF was incorporated on October 19, 2021, for the purpose of entering into a merger, share exchange, asset acquisition, stock purchase, recapitalization, reorganization or other similar business combination with one or more businesses or entities. MURF’s efforts to identify a prospective target business were not limited to any particular industry or geographic region. Prior to executing the Merger Agreement, MURF’s efforts were limited to organizational activities, completion of its initial public offering and the evaluation of possible business combinations.
Initial Public Offering and Simultaneous Private Placement
On February 7, 2022, MURF closed its initial public offering of 13,225,000 units, with each unit consisting of one share of its Class A common stock and one warrant, with each whole warrant entitling the holder thereof to purchase one share of its Class A common stock at a purchase price of $11.50. The units from the initial public offering were sold at an offering price of $10.00 per unit, generating total gross proceeds of $132.25 million. Simultaneously with the consummation of the initial public offering, MURF consummated the private sale of 754,000 private units at $10.00 per unit for gross proceeds of approximately $7.54 million. A total of $139,790,000 was deposited into the trust account. This resulted in an overfunding of the trust account by $4,895,000. As such, subsequent to the initial funding of the trust account, $2,000,000 was transferred to MURF’s operating cash account and $2,895,000 was used to pay offering costs.
Except as described in the prospectus for MURF’s initial public offering and described in the subsections below entitled “— January 2023 Extension” and “— MURF’s Management’s Discussion and Analysis of Financial Condition and Results of Operations,” these proceeds will not be released until the earlier of the completion of an initial business combination and MURF’s redemption of 100% of the outstanding Public Shares upon its failure to consummate a business combination within the required time period.
January 2023 Extension
Initially, MURF was required to complete its initial business combination transaction by 12 months from the consummation of its initial public offering or up to 18 months if it extended the period of time to consummate a business combination in accordance with its amended and restated certificate of incorporation. On January 26, 2023, at a special meeting of its stockholders, MURF’s stockholders approved a proposal to amend the amended and restated certificate of incorporation to allow it to extend the date by which it has to consummate a business combination up to 12 times, each such extension for an additional one month period, from February 7, 2023, to February 7, 2024. MURF’s stockholders also approved a related proposal to amend the trust agreement allowing MURF to deposit into the trust account, for each one-month extension, one-third of 1% of the funds remaining in the trust account following the redemptions made in connection with the approval of the extension proposal at the special meeting. At the special meeting MURF’s stockholders also approved a proposal to amend its amended and restated certificate of incorporation to expand the methods that it may employ to not become subject to the “penny stock” rules of the SEC.
In connection with such proposals, MURF’s public stockholders had the right to redeem their shares for cash equal to their pro rata share of the aggregate amount on deposit in the trust account as of two days prior to such stockholder vote. The public stockholders holding 11,037,272 shares of Class A common stock (out of a total of 13,979,000 shares of Class A common stock) exercised their right to redeem such shares at a redemption price of approximately $10.33 per share. Approximately $114 million in cash was removed from the trust account to pay such stockholders and, accordingly, after giving effect to such redemptions, the balance in the trust account was approximately $23 million.
As a result of the approval of such proposals, MURF agreed to deposit into the trust account one-third of 1% of the funds then on deposit in the trust account for each month of the extension period, resulting in a monthly contribution of approximately $0.035 per share that was not redeemed in connection with the Special Meeting (approximately $77,000 in the aggregate per month), or an aggregate of $924,000 (the “Maximum Contribution”) if the date MURF has to consummate a business combination is extended 12 times, each assuming no interest is earned on the funds in the trust account.
Fair Market Value of Target Business
The target business or businesses that MURF acquires must collectively have a fair market value equal to at least 80% of the balance of the funds in the trust account (excluding the deferred underwriting commissions and taxes payable on the income earned on the trust account) at the time of the execution of a definitive agreement for its initial business combination, although MURF may acquire a target business whose fair market value significantly exceeds 80% of the trust account balance. The MURF Board determined that this test was met in connection with the proposed business combination with Conduit as described in the section titled “The Business Combination Proposal” above.
Redemption Rights for Public Stockholders upon Completion of MURF’s Initial Business Combination
MURF will provide its public stockholders with the opportunity to redeem all or a portion of their shares of Class A common stock upon the completion of MURF’s initial business combination at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account as of two business days prior to the consummation of the initial business combination including interest earned on the funds held in the trust account and not previously released to MURF to pay MURF’s taxes, divided by the number of then outstanding public shares, subject to the limitations described herein. The amount in the trust account (after withholding funds for tax obligations) is anticipated to be approximately $23.3 million (or $10.67 per public share). MURF’s Sponsor, officers and directors entered into a letter agreement with MURF, pursuant to which they agreed to waive their redemption rights with respect to any founder shares and any public shares held by them in connection with the completion of MURF’s initial business combination.
As a result of the pre-Business Combination redemption of 11,037,272 shares of MURF Class A common stock, the redemption payment of approximately $114 million reduced the balance of the trust account to approximately $23 million. In connection with this Special Meeting, MURF’s public stockholders have another opportunity to redeem Public Shares. The balance of the trust account will be the following amounts in the event of these redemption scenarios (after withholding funds for tax obligations): (i) no redemptions will maintain the current balance of approximately $23.3 million in the trust account; (ii) 50% redemptions will result in approximately $11.7 million in the trust account; and (iii) 100% redemptions will result in $0 in the trust account.
Manner of Conducting Redemptions
MURF will provide its public stockholders with the opportunity to redeem all or a portion of their public shares of Class A common stock upon the completion of MURF’s initial business combination either (i) in connection with a stockholder meeting called to approve the initial business combination or (ii) by means of a tender offer. The decision as to whether MURF will seek stockholder approval of a proposed initial business combination or conduct a tender offer will be made by MURF, in its sole discretion, and will be based on a variety of factors such as the timing of the transaction and whether the terms of the transaction would require MURF to seek stockholder approval under the law or stock exchange listing requirement. Under Nasdaq rules, asset acquisitions and stock purchases would not typically require stockholder approval while direct mergers with the company where it does not survive and any transactions in which the company issues more than 20% of its outstanding common stock or seeks to amend its certificate of incorporation would require stockholder approval. MURF may conduct redemptions without a stockholder vote pursuant to the tender offer rules of the SEC unless stockholder approval is required by law or stock exchange listing requirements or MURF chooses to seek stockholder approval for business or other legal reasons. So long as MURF obtain and maintain a listing for MURF’s securities on the Nasdaq, it will be required to comply with such rules.
| 130 |
If stockholder approval of the transaction is required by law or stock exchange listing requirement, or MURF decides to obtain stockholder approval for business or other legal reasons, MURF will, pursuant to its certificate of incorporation:
| ● | conduct the redemptions in conjunction with a proxy solicitation pursuant to Regulation 14A of the Exchange Act, which regulates the solicitation of proxies, and not pursuant to the tender offer rules, and | |
| ● | file proxy materials with the SEC. |
In the event that MURF seeks stockholder approval of its initial business combination, it will distribute proxy materials and, in connection therewith, provide its public stockholders with the redemption rights described above upon completion of the initial business combination.
If MURF seeks stockholder approval, it will complete its initial business combination only if a majority of the outstanding shares of MURF common stock present and entitled to vote at the meeting to approve the initial business combination when a quorum is present are voted in favor of the initial business combination. A quorum for such meeting will consist of the holders present in person (by virtual attendance) or by proxy of shares of outstanding capital stock of MURF representing a majority of the voting power of all outstanding shares of capital stock of MURF entitled to vote at such meeting. MURF’s initial stockholder, our Sponsor, will count toward this quorum and pursuant to the letter agreement, its Sponsor, officers and directors have agreed to vote any founder shares held by them and any public shares acquired during or after its initial public offering (including in open market and privately negotiated transactions) in favor of its initial business combination. For purposes of seeking approval of the majority of the outstanding shares of MURF common stock voted, non-votes will have no effect on the approval of its initial business combination once a quorum is obtained. MURF intends to give approximately 30 days (but not less than 10 days nor more than 60 days) prior written notice of any such meeting, if required, at which a vote shall be taken to approve its initial business combination. These quorum and voting thresholds, and the support agreement between MURF and the Sponsor, may make it more likely that MURF will consummate its initial business combination. Each public stockholder may elect to redeem its public shares irrespective of whether they vote for or against the proposed transaction.
If a stockholder vote is not required and MURF does not decide to hold a stockholder vote for business or other legal reasons, MURF will, pursuant to its certificate of incorporation:
| ● | conduct the redemptions pursuant to Rule 13e-4 and Regulation 14E of the Exchange Act, which regulate issuer tender offers, and | |
| ● | file tender offer documents with the SEC prior to completing its initial business combination which contain substantially the same financial and other information about the initial business combination and the redemption rights as is required under Regulation 14A of the Exchange Act, which regulates the solicitation of proxies. |
Upon the public announcement of MURF’s initial business combination, MURF or its Sponsor will terminate any plan established in accordance with Rule 10b5-1 to purchase shares of Class A common stock in the open market if MURF elects to redeem its public shares through a tender offer, to comply with Rule 14e-5 under the Exchange Act.
In the event MURF conducts redemptions pursuant to the tender offer rules, MURF’s offer to redeem will remain open for at least 20 business days, in accordance with Rule 14e-1(a) under the Exchange Act, and MURF will not be permitted to complete its initial business combination until the expiration of the tender offer period. In addition, so that MURF is not subject to the SEC’s “penny stock” rules, it will not redeem any public shares unless (i) its net tangible assets will be at least $5,000,001 either immediately prior to or upon consummation of the initial business combination and after payment of underwriters’ fees and commissions or (ii) it is otherwise exempt from the provisions of Rule 419 promulgated under the Securities Act. If public stockholders tender more shares than MURF has offered to purchase, it will withdraw the tender offer and not complete the initial business combination.
MURF’s certificate of incorporation provides that MURF may not redeem its public shares unless (i) it is exempt from the provisions of Rule 419 promulgated under the Securities Act other than through its net tangible assets or (ii) its net tangible assets are at least $5,000,001 either immediately prior to or upon consummation of its initial business combination and after payment of underwriters’ fees and commissions (so that it is not subject to the SEC’s “penny stock” rules) or any greater net tangible asset or cash requirement which may be contained in the agreement relating to the initial business combination. For example, the proposed initial business combination may require: (i) cash consideration to be paid to the target or its owners, (ii) cash to be transferred to the target for working capital or other general corporate purposes or (iii) the retention of cash to satisfy other conditions in accordance with the terms of the proposed initial business combination. In the event the aggregate cash consideration MURF would be required to pay for all shares of Class A common stock that are validly submitted for redemption plus any amount required to satisfy cash conditions pursuant to the terms of the proposed initial business combination exceeds the aggregate amount of cash available to MURF, it will not complete the initial business combination or redeem any shares, and all shares of Class A common stock submitted for redemption will be returned to the holders thereof.
| 131 |
Limitation on Redemption upon Completion of MURF’s Initial Business Combination if MURF Seeks Stockholder Approval
Notwithstanding the foregoing, if MURF seeks stockholder approval of its initial business combination and MURF does not conduct redemptions in connection with its initial business combination pursuant to the tender offer rules, MURF’s certificate of incorporation provides that a public stockholder, together with any affiliate of such stockholder or any other person with whom such stockholder is acting in concert or as a “group” (as defined under Section 13 of the Exchange Act), will be restricted from seeking redemption rights with respect to more than an aggregate of 15% of the shares sold in MURF’s initial public offering, which MURF refers to as the “Excess Shares.” Such restriction shall also be applicable to MURF’s affiliates. MURF believes this restriction will discourage stockholders from accumulating large blocks of shares, and subsequent attempts by such holders to use their ability to exercise their redemption rights against a proposed initial business combination as a means to force MURF or its management to purchase their shares at a significant premium to the then-current market price or on other undesirable terms. By limiting MURF’s stockholders’ ability to redeem no more than 15% of the shares sold in its initial public offering without MURF’s prior consent, MURF believes it will limit the ability of a small group of stockholders to unreasonably attempt to block MURF’s ability to complete its initial business combination, particularly in connection with an initial business combination with a target that requires as a closing condition that MURF has a minimum net worth or a certain amount of cash. However, MURF would not be restricting its stockholders’ ability to vote all of their shares (including Excess Shares) for or against its initial business combination.
Tendering Stock Certificates in Connection with Redemption Rights
MURF may require public stockholders seeking to exercise their redemption rights, whether they are record holders or hold their shares in “street name,” to either tender their certificates to MURF’s transfer agent up to two business days prior to the vote on the proposal to approve the initial business combination, or to deliver their shares to the transfer agent electronically using the Depository Trust Company’s DWAC (Deposit/Withdrawal At Custodian) System, at the holder’s option. The proxy materials that MURF will furnish to holders of public shares in connection with its initial business combination will indicate whether MURF is requiring public stockholders to satisfy such delivery requirements. Accordingly, a public stockholder would have up to two days prior to the vote on the initial business combination to tender its shares if it wishes to seek to exercise its redemption rights. Given the relatively short exercise period, it is advisable for stockholders to use electronic delivery of their public shares.
There is a nominal cost associated with the above-referenced tendering process and the act of certificating the shares or delivering them through the DWAC System. The transfer agent will typically charge the tendering broker $80.00 and it would be up to the broker whether or not to pass this cost on to the redeeming holder. However, this fee would be incurred regardless of whether or not MURF requires holders seeking to exercise redemption rights to tender their shares. The need to deliver shares is a requirement of exercising redemption rights regardless of the timing of when such delivery must be effectuated.
The foregoing is different from the procedures used by many special purpose acquisition companies. In order to perfect redemption rights in connection with their business combinations, many blank check companies would distribute proxy materials for the stockholders’ vote on an initial business combination, and a holder could simply vote against a proposed initial business combination and check a box on the proxy card indicating such holder was seeking to exercise his or her redemption rights. After the initial business combination was approved, the company would contact such stockholder to arrange for him or her to deliver his or her certificate to verify ownership. As a result, the stockholder then had an “option window” after the completion of the initial business combination during which he or she could monitor the price of the company’s stock in the market. If the price rose above the redemption price, he or she could sell his or her shares in the open market before actually delivering his or her shares to the company for cancellation. As a result, the redemption rights, to which stockholders were aware they needed to commit before the stockholder meeting, would become “option” rights surviving past the completion of the initial business combination until the redeeming holder delivered its certificate. The requirement for physical or electronic delivery prior to the meeting ensures that a redeeming holder’s election to redeem is irrevocable once the initial business combination is approved.
Any request to redeem such shares, once made, may be withdrawn at any time up to the date of the stockholder meeting. Furthermore, if a holder of a public share delivered its certificate in connection with an election of redemption rights and subsequently decides prior to the applicable date not to elect to exercise such rights, such holder may simply request that the transfer agent return the certificate (physically or electronically). It is anticipated that the funds to be distributed to holders of MURF’s public shares electing to redeem their shares will be distributed promptly after the completion of MURF’s initial business combination.
If MURF’s initial business combination is not approved or completed for any reason, then its public stockholders who elected to exercise their redemption rights would not be entitled to redeem their shares for the applicable pro rata share of the trust Account. In such case, MURF will promptly return any certificates delivered by public holders who elected to redeem their shares.
If MURF’s initial proposed initial business combination is not completed, MURF may continue to try to complete an initial business combination with a different target until February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation).
| 132 |
Redemption of Public Shares and Liquidation if No Business Combination
MURF’s amended and restated certificate of incorporation provides that it will have until February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), to complete its initial business combination. If MURF is unable to complete its initial business combination by then, it will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account including interest earned on the funds held in the trust account and not previously released to MURF to pay its taxes (less up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of MURF’s remaining stockholders and board of directors, dissolve and liquidate, subject in the case of clauses (ii) and (iii) above to MURF’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. There will be no redemption rights or liquidating distributions with respect to MURF’s warrants, which will expire worthless if MURF fails to complete its initial business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation).
MURF’s Sponsor, officers and directors entered into a letter agreement with MURF, pursuant to which they waived their rights to liquidating distributions from the trust account with respect to any founder shares held by them if MURF fails to complete its initial business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation). However, if MURF’s Sponsor, officers or directors acquired public shares in or after MURF’s initial public offering, they will be entitled to liquidating distributions from the trust account with respect to such public shares if MURF fails to complete its initial business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation).
MURF’s Sponsor, officers and directors will agree, pursuant to a written agreement, that they will not propose any amendment to MURF’s amended and restated certificate of incorporation (i) to modify the substance or timing of MURF’s obligation to allow redemption in connection with MURF’s initial business combination or certain amendments to MURF’s charter prior thereto or to redeem 100% of MURF’s public shares if it does not complete its initial business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), or (ii) with respect to any other provision relating to stockholders’ rights or pre-initial business combination activity, unless MURF provides its public stockholders with the opportunity to redeem their shares of Class A common stock upon approval of any such amendment at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account including interest earned on the funds held in the trust account and not previously released to MURF to pay its taxes divided by the number of then outstanding public shares. However, MURF may not redeem its public shares unless its net tangible assets are at least $5,000,001 either immediately prior to or upon consummation of the initial business combination and after payment of underwriters’ fees and commissions or is not otherwise exempt from the provisions of Rule 419 promulgated under the Securities Act (so that MURF is not subject to the SEC’s “penny stock” rules). If this optional redemption right is exercised with respect to an excessive number of public shares such that MURF cannot satisfy the net tangible asset requirement (described above), MURF would not proceed with the amendment or the related redemption of its public shares at such time.
MURF expects that all costs and expenses associated with implementing its plan of dissolution, as well as payments to any creditors, will be funded from amounts remaining out of the funds held outside the trust account, although it cannot assure that there will be sufficient funds for such purpose. As of December 31, 2022, MURF had approximately $346,000 in cash remaining. It will depend on sufficient interest being earned on the proceeds held in the trust account to pay any tax obligations it may owe. However, if those funds are not sufficient to cover the costs and expenses associated with implementing MURF’s plan of dissolution, to the extent that there is any interest accrued in the trust account not required to pay taxes, MURF may request the trustee to release to it an additional amount of up to $100,000 of such accrued interest to pay those costs and expenses.
If MURF were to expend all of the net proceeds of its initial public offering and the sale of the placement units, other than the proceeds deposited in the trust account, and without taking into account interest, if any, earned on the trust account between July 31, 2023, and the date of distribution, the per-share redemption amount received by stockholders upon MURF’s dissolution would be approximately $10.62. The proceeds deposited in the trust account could, however, become subject to the claims of MURF’s creditors who would have higher priority than the claims of MURF’s public stockholders. MURF cannot assure you that the actual per-share redemption amount received by stockholders will not be substantially less than $10.62. Under Section 281(b) of the DGCL, MURF’s plan of dissolution must provide for all claims against it to be paid in full or make provision for payments to be made in full, as applicable, if there are sufficient assets. These claims must be paid or provided for before MURF makes any distribution of its remaining assets to its stockholders. While MURF intends to pay such amounts, if any, MURF cannot assure you that it will have funds sufficient to pay or provide for all creditors’ claims.
Although MURF will seek to have all vendors, service providers, prospective target businesses or other entities with which it does business execute agreements waiving any right, title, interest or claim of any kind in or to any monies held in the trust account for the benefit of MURF’s public stockholders, there is no guarantee that they will execute such agreements or even if they execute such agreements that they would be prevented from bringing claims against the trust account including but not limited to fraudulent inducement, breach of fiduciary responsibility or other similar claims, as well as claims challenging the enforceability of the waiver, in each case in order to gain an advantage with respect to a claim against MURF’s assets, including the funds held in the trust account. If any third party refuses to execute an agreement waiving such claims to the monies held in the trust account, MURF’s management will perform an analysis of the alternatives available to it and will only enter into an agreement with a third party that has not executed a waiver if management believes that such third party’s engagement would be significantly more beneficial to MURF than any alternative. Examples of possible instances where MURF may engage a third party that refuses to execute a waiver include the engagement of a third party consultant whose particular expertise or skills are believed by management to be significantly superior to those of other consultants that would agree to execute a waiver or in cases where management is unable to find a service provider willing to execute a waiver. MURF’s independent registered public accounting firm and the underwriters of the initial public offering did not execute agreements with MURF waiving such claims to the monies held in the trust account.
| 133 |
In addition, there is no guarantee that such entities will agree to waive any claims they may have in the future as a result of, or arising out of, any negotiations, contracts or agreements with MURF and will not seek recourse against the trust account for any reason. MURF’s Sponsor has agreed that it will be liable to MURF if and to the extent any claims by a third party for services rendered or products sold to MURF, or a prospective target business with which MURF has entered into a written letter of intent, confidentiality or similar agreement or business combination agreement, reduce the amount of funds in the trust account to below the lesser of (i) $10.15 per public share and (ii) the actual amount per public share held in the trust account as of the date of the liquidation of the trust account, if less than $10.15 per share due to reductions in the value of the trust assets, less taxes payable, provided that such liability will not apply to any claims by a third party or prospective target business who executed a waiver of any and all rights to the monies held in the trust account (whether or not such waiver is enforceable) nor will it apply to any claims under MURF’s indemnity of the underwriters of this offering against certain liabilities, including liabilities under the Securities Act. However, MURF has not asked its Sponsor to reserve for such indemnification obligations, nor has MURF independently verified whether the Sponsor has sufficient funds to satisfy its indemnity obligations. Further, MURF believes that its Sponsor’s only assets are MURF securities. Therefore, MURF cannot assure you that the Sponsor would be able to satisfy those obligations. None of MURF’s officers or directors will indemnify MURF for claims by third parties including, without limitation, claims by vendors and prospective target businesses.
In the event that the proceeds in the trust account are reduced below (i) $10.15 per public share or (ii) such lesser amount per public share held in the trust account as of the date of the liquidation of the trust account, due to reductions in value of the trust assets, in each case net of the amount of interest which may be withdrawn to pay taxes, and the Sponsor asserts that it is unable to satisfy its indemnification obligations or that it has no indemnification obligations related to a particular claim, MURF’s independent directors would determine whether to take legal action against the Sponsor to enforce its indemnification obligations. While MURF currently expects that its independent directors would take legal action on its behalf against the Sponsor to enforce its indemnification obligations to MURF, it is possible that MURF’s independent directors in exercising their business judgment may choose not to do so if, for example, the cost of such legal action is deemed by the independent directors to be too high relative to the amount recoverable or if the independent directors determine that a favorable outcome is not likely. MURF has not asked the Sponsor to reserve for such indemnification obligations and MURF cannot assure you that the Sponsor would be able to satisfy those obligations. Accordingly, MURF cannot assure you that due to claims of creditors the actual value of the per-share redemption price will not be less than $10.15 per public share.
MURF will seek to reduce the possibility that its Sponsor will have to indemnify the trust account due to claims of creditors by endeavoring to have all vendors, service providers, prospective target businesses or other entities with which MURF does business execute agreements with MURF waiving any right, title, interest or claim of any kind in or to monies held in the trust account. The Sponsor will also not be liable as to any claims under our indemnity of the underwriters of MURF’s initial public offering against certain liabilities, including liabilities under the Securities Act. MURF will have access to up to approximately $1,500,000 from loans from the Sponsor with which to pay any such potential claims (including costs and expenses incurred in connection with our liquidation, currently estimated to be no more than approximately $100,000). In the event that MURF liquidates and it is subsequently determined that the reserve for claims and liabilities is insufficient, stockholders who received funds from MURF’s trust account could be liable for claims made by creditors.
MURF will seek to reduce the possibility that its Sponsor will have to indemnify the trust account due to claims of creditors by endeavoring to have all vendors, service providers, prospective target businesses or other entities with which MURF does business execute agreements with MURF waiving any right, title, interest or claim of any kind in or to monies held in the trust account. MURF’s Sponsor will also not be liable as to any claims under MURF’s indemnity of the underwriters of this offering against certain liabilities, including liabilities under the Securities Act. In the event that MURF liquidates and it is subsequently determined that the reserve for claims and liabilities is insufficient, stockholders who received funds from MURF’s trust account could be liable for claims made by creditors.
Under the DGCL, stockholders may be held liable for claims by third parties against a corporation to the extent of distributions received by them in a dissolution. The pro rata portion of MURF’s trust account distributed to its stockholders upon the redemption of MURF’s public shares in the event MURF does not complete its initial business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), may be considered a liquidating distribution under Delaware law. If the corporation complies with certain procedures set forth in Section 280 of the DGCL intended to ensure that it makes reasonable provision for all claims against it, including a 60-day notice period during which any third-party claims can be brought against the corporation, a 90-day period during which the corporation may reject any claims brought, and an additional 150-day waiting period before any liquidating distributions are made to stockholders, any liability of stockholders with respect to a liquidating distribution is limited to the lesser of such stockholder’s pro rata share of the claim or the amount distributed to the stockholder, and any liability of the stockholder would be barred after the third anniversary of the dissolution.
Furthermore, if the pro rata portion of MURF’s trust account distributed to MURF’s public stockholders upon the redemption of MURF’s public shares in the event MURF does not complete its initial business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), is not considered a liquidating distribution under Delaware law and such redemption distribution is deemed to be unlawful (potentially due to the imposition of legal proceedings that a party may bring or due to other circumstances that are currently unknown), then pursuant to Section 174 of the DGCL, the statute of limitations for claims of creditors could then be six years after the unlawful redemption distribution, instead of three years, as in the case of a liquidating distribution. If MURF is unable to complete its initial business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), it will: (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the public shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the trust account including interest earned on the funds held in the trust account and not previously released to MURF to pay its taxes (less up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding public shares, which redemption will completely extinguish public stockholders’ rights as stockholders (including the right to receive further liquidating distributions, if any), subject to applicable law, and (iii) as promptly as reasonably possible following such redemption, subject to the approval of MURF’s remaining stockholders and the MURF Board, dissolve and liquidate, subject in the case of clauses (ii) and (iii) above to MURF’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. Accordingly, it is MURF’s intention to redeem its public shares as soon as reasonably possible following February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), and, therefore, MURF does not intend to comply with those procedures. As such, MURF’s stockholders could potentially be liable for any claims to the extent of distributions received by them (but no more) and any liability of MURF’s stockholders may extend well beyond the third anniversary of such date.
| 134 |
Because MURF will not be complying with Section 280, Section 281(b) of the DGCL requires it to adopt a plan, based on facts known to MURF at such time that will provide for MURF’s payment of all existing and pending claims or claims that may be potentially brought against MURF within the subsequent 10 years. However, because MURF is a blank check company, rather than an operating company and its operations will be limited to searching for prospective target businesses to acquire, the only likely claims to arise would be from MURF’s vendors (such as lawyers, investment bankers, etc.) or prospective target businesses. As described above, pursuant to the obligation contained in MURF’s underwriting agreement, MURF will seek to have all vendors, service providers, prospective target businesses or other entities with which MURF does business execute agreements with MURF waiving any right, title, interest or claim of any kind in or to any monies held in the trust account. As a result of this obligation, the claims that could be made against MURF are significantly limited and the likelihood that any claim that would result in any liability extending to the trust account is remote. Further, MURF’s Sponsor may be liable only to the extent necessary to ensure that the amounts in the trust account are not reduced below (i) $10.15 per public share or (ii) such lesser amount per public share held in the trust account as of the date of the liquidation of the trust account, due to reductions in value of the trust assets, in each case net of the amount of interest withdrawn to pay taxes and will not be liable as to any claims under MURF’s indemnity of the underwriters of its initial public offering against certain liabilities, including liabilities under the Securities Act. In the event that an executed waiver is deemed to be unenforceable against a third party, the Sponsor will not be responsible to the extent of any liability for such third-party claims.
If MURF files a bankruptcy petition or an involuntary bankruptcy petition is filed against MURF that is not dismissed, the proceeds held in the trust account could be subject to applicable bankruptcy law, and may be included in MURF’s bankruptcy estate and subject to the claims of third parties with priority over the claims of MURF stockholders. To the extent any bankruptcy claims deplete the trust account, MURF cannot assure you it will be able to return $10.15 per share to its public stockholders. Additionally, if MURF files a bankruptcy petition or an involuntary bankruptcy petition is filed against MURF that is not dismissed, any distributions received by stockholders could be viewed under applicable debtor/creditor and/or bankruptcy laws as either a “preferential transfer” or a “fraudulent conveyance.” As a result, a bankruptcy court could seek to recover some or all amounts received by MURF stockholders. Furthermore, the MURF Board may be viewed as having breached its fiduciary duty to MURF’s creditors and/or may have acted in bad faith, thereby exposing itself and MURF to claims of punitive damages, by paying public stockholders from the trust account prior to addressing the claims of creditors. MURF cannot assure you that claims will not be brought against MURF for these reasons.
MURF’s public stockholders will be entitled to receive funds from the trust account only upon the earlier to occur of: (i) the completion of MURF’s initial business combination, (ii) the redemption of any public shares properly tendered in connection with a stockholder vote to amend any provisions of MURF’s amended and restated certificate of incorporation (A) to modify the substance or timing of its obligation to allow redemption in connection with its initial business combination or certain amendments to MURF’s charter prior thereto or to redeem 100% of MURF’s public shares if MURF does not complete its initial business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), or (B) with respect to any other provision relating to stockholders’ rights or pre-initial business combination activity, and (iii) the redemption of all of MURF public shares if MURF is unable to complete its business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation), subject to applicable law. In no other circumstances will a stockholder have any right or interest of any kind to or in the trust account. In the event MURF seeks stockholder approval in connection with its initial business combination, a stockholder’s voting in connection with the initial business combination alone will not result in a stockholder’s redeeming its shares to MURF for an applicable pro rata share of the trust account. Such stockholder must have also exercised its redemption rights as described above. These provisions of MURF’s amended and restated certificate of incorporation, like all provisions of its amended and restated certificate of incorporation, may be amended with a stockholder vote.
Facilities
MURF’s executive offices are located at 4995 Murphy Canyon Road, Suite 300 San Diego, CA 92123, and our telephone number is 760-471-8536. Its executive offices are provided to it by the Sponsor. MURF pays Murphy Canyon Management Group, Inc., an affiliate of the Sponsor, a total of $10,000 per month for office space, utilities and secretarial and administrative support.
| 135 |
Employees
MURF has three executive officers. These individuals are not obligated to devote any specific number of hours to MURF’s matters and intend to devote only as much time as they deem necessary to its affairs.
Directors and Executive Officers
MURF’s current directors and executive officers are as follows:
| Name | Age | Position | ||
| Jack K. Heilbron | 72 | Chief Executive Officer, President, and Chairman | ||
| Adam Sragovicz | 54 | Chief Financial Officer, Treasurer, and Director | ||
| Ed Bentzen | 46 | Chief Accounting Officer | ||
| Francis Knuettel II | 56 | Director | ||
| Chele Chiavacci Farley | 55 | Director | ||
| Richard E. Feinberg | 75 | Director |
Jack K. Heilbron
Mr. Heilbron has been MURF’s Chief Executive Officer since its inception. Mr. Heilbron has served as a director and Chief Executive Officer and President of Presidio Property Trusty Inc. since its inception in February 2010. Mr. Heilbron also has served as Chairman, CEO and President of NetREIT Dubose since its inception, and has served as CEO and/or President of NetREIT Advisors, LLC, Dubose Advisors, LLC, and NTR Property Management, Inc. since their inceptions, all of which are Company affiliated entities. Mr. Heilbron was a founding officer, director, and stockholder of the former CI Holding Group, Inc. and of its subsidiary corporations (Centurion Counsel, Inc., Bishop Crown Investment Research Inc., PIM Financial Securities Inc., Centurion Institutional Services Inc. and CHG Properties, Inc.) and currently serves as Chairman and CEO of Centurion Counsel, Inc., a licensed investment advisor. He also served as a director of the Centurion Counsel Funds, an investment company registered under the Investment Company Act of 1940, from 2001 until 2005. From 1994 until its dissolution in 1999, Mr. Heilbron served as the Chairman and/or director of Clover Income and Growth REIT. Mr. Heilbron graduated with a B.S. degree in Business Administration from California Polytechnic College, San Luis Obispo, California. Based on his experience as a director and his experience with other REITs, the Nominating and Corporate Governance Committee determined that Mr. Heilbron is qualified to serve on the MURF Board.
Adam Sragovicz
Mr. Sragovicz has been MURF’s Chief Financial Officer since MURF’s inception. Mr. Sragovicz has been a director of the Company since December 2021. Mr. Sragovicz has been the Chief Financial Officer of Presidio Property Trust, Inc. since January 11, 2018. He previously served as Senior Vice President, Finance of Presidio Property Trust, Inc. since May 2017. Before joining Presidio Property Trust, Inc., Mr. Sragovicz served as Treasurer of Encore Capital Group from 2011 to 2017, where he was responsible for global capital raising, foreign exchange risk management and cash management. Mr. Sragovicz has also held capital markets, finance, and treasury management positions with KPMG, Union Bank of California / MUFG and Bank of America Merrill Lynch. Mr. Sragovicz is the Director of the Yale Alumni Schools Committee in San Diego and previously sat on the board of Congregation Adat Yeshurun. Mr. Sragovicz is a graduate of Yale University with a Bachelor of Arts degree in Soviet and Eastern European Studies, with a concentration in Economics. Upon the consummation of the Business Combination, Mr. Sragovicz will be appointed Chief Financial Officer of New Conduit.
Ed Bentzen
Mr. Bentzen has been MURF’s Chief Accounting Officer since its inception. Mr. Bentzen has been the Chief Accounting Officer of Presidio Property Trust, Inc. March 2021. Prior to that, Mr. Bentzen served as Chief Financial Officer and Chief Operations Officer for Crystal View Capital Management in 2020, as a Chief Financial Officer / Finance consultant for various clients (including real estate development companies) from 2018 to 2020, and as Chief Financial Officer for The Parking REIT (formerly MVP REIT and MVP REIT II) from 2016 to 2018. Prior to these roles, Mr. Bentzen held senior and/or accounting roles at Western Funding, Inc., Vestin Group, Inc., and a local CPA firm in Las Vegas, Nevada. In addition, Mr. Bentzen worked as a Senior Internal Auditor at Ameristar Casinos, Inc. (formerly Nasdaq: ASCA). He holds a Bachelor of Science degree in Hotel Administration, with an emphasis in Gaming, and a Master of Science degree in Accountancy, from University of Nevada, Las Vegas, and is licensed as a Certified Internal Auditor (inactive).
| 136 |
Francis Knuettel II
Mr. Knuettel II has served on the MURF Board since the closing of its initial public offering. From December 2020 to 2022, he served as the Chief Executive Officer and board member of Unrivaled Brands, Inc. (OTCQX: UNRV). In 2022, Mr. Knuettel joined Chromocell Therapeutics Corp., where he serves as the Chief Financial and Strategy Officer. Mr. Knuettel was formerly a Restructuring Advisory Consultant at Viridian Capital Advisors from May 2020 to November 2020. Mr. Knuettel joined Viridian while at One Cannabis Group (“OCG”) where Mr. Knuettel was the Chief Financial Officer from June 2019 to January 2021 and was integral to the sale of the company to Item 9 Labs Corp. (OTCQX: INLB). Prior to OCG, Mr. Knuettel was CFO at MJardin, a Denver-based cannabis cultivation and dispensary management company, from August 2018 to June 2019 where he led the company’s IPO on the Canadian Securities Exchange. Following the IPO, Mr. Knuettel managed MJardin’s merger with GrowForce, a Toronto-based cannabis cultivator, after which he moved over to the Chief Strategy Role. In his role as CSO, he managed the acquisition of several private companies before recommending and executing the consolidation of management and other operations to Toronto and the closure of the executive office in Denver. Prior to MJardin, Mr. Knuettel held numerous CFO and CEO positions at early-stage and NASDAQ-listed companies where he had significant experience both building and restructuring businesses. Mr. Knuettel serves on several corporate boards, including on the Board of Directors of 180 Life Sciences, an early-stage therapeutic biotech company, since July 2021, on the Board of Directors of Sanatio BioScience Corp., an early-stage anti-viral platform, since September 2020 (where he is the chair of the company’s audit committee) and on the Board of Directors of ECOM Medical, Inc., a developer of endotracheal patient monitoring systems, since July 2019 (where he is the chair of the company’s audit committee). Mr. Knuettel has advised that he will be named as a director nominee of a special purpose acquisition company Relativity Acquisition Corp., and may be named a director nominee of additional special purpose acquisition companies. Each such appointment will not take effect until the consummation of the initial public offering for the applicable company. If such appointment becomes effective, Mr. Knuettel will have fiduciary duties equivalent to and on the same level of priority as those obligations owed to MURF. MURF do not believe this gives rise to any theoretical or actual conflict of interest with respect to such other special purpose acquisition companies and MURF, since each of these entities intends to target business combinations in a different industry than those targeted by us. Accordingly, while any such companies, businesses or investments may present additional conflicts of interest in pursuing an initial business combination, MURF does not believe that any such potential conflicts would materially affect its ability to complete its initial business combination. Mr. Knuettel graduated cum laude from Tufts University with a B.A. degree in Economics and from The Wharton School of Business at the University of Pennsylvania with an MBA in Finance and Entrepreneurial Management.
Chele Chiavacci Farley
Chele Chiavacci Farley has served on the MURF Board since the closing of its initial public offering and currently serves as a partner and managing director of Mistral Capital International (“Mistral”), a private equity firm, since 1995. In her role as Partner and Managing Director of Mistral, Ms. Farley originates, evaluates and executes equity investment opportunities, creates and implements deal and financial structures, negotiates with banks for credit facilities, and oversees management. Ms. Farley is the President and a member of the Board of Directors and Management Committee of Palmilla San Jose Inmobiliaria, the Master Developer of the luxury Palmilla resort development in Cabo San Lucas, Mexico. Prior to Mistral, Ms. Farley was Vice President of Tricap International from 1994 to 1995. From 1992 to 1994, Ms. Farley was an Associate at UBS Capital Corporation, and analyzed and evaluated principal investment and financing opportunities for the firm’s internal $1 billion fund. Ms. Farley began her career as a Financial Analyst in the Global Finance department - Energy and Telecom Group of Goldman, Sachs & Co. Ms. Farley has also had an active political career. In 2020, Ms. Farley ran for election to the U.S. House of Representatives to represent New York’s 18th Congressional district. In 2018, Ms. Farley ran for election to the U.S. Senate to represent New York. Ms. Farley graduated from Stanford University with a B.S. and M.S. in Industrial Engineering. She is a member of YPO - Young Presidents’ Organization.
| 137 |
Richard E. Feinberg
Richard E. Feinberg has served on the MURF Board since the closing of its initial public offering. Mr. Feinberg has been a Professor of International Political Economy at the University of California, San Diego, since 1996 and an Emeritus Professor since 2021. Previously, Mr. Feinberg served as Special Assistant to the President for National Security Affairs and as a Senior Director for the Office of Inter-American Affairs, National Security Council, the White House, from 1993 to 1996. Mr. Feinberg was integral to architecture of the 1994 Miami Summit of the Americas and of the proposed Free Trade Area of the Americas (FTAA). Mr. Feinberg has worked in various other governmental roles including: (i) Member of the Policy Planning Staff of the Department of State (1977-1980); and (ii) international economist for the U.S. Treasury Department (1975-1977). Mr. Feinberg served in executive positions at various public policy institutes including: (i) as president of the Inter-American Dialogue (1992-1993); (ii) as executive vice president and director of studies of the Overseas Development Council (1982-1991). Mr. Feinberg taught a graduate-level course on international financial institutions as an adjunct professor at Georgetown University School of Foreign Service from 1980 to 1985. Mr. Feinberg holds a Ph.D. in international economics from Stanford University and a B.A. in European history from Brown University. Mr. Feinberg is well-qualified to serve on the MURF Board due to his expertise in political relations, public policies, financial and economic management internationally, which enable him to provide accurate and reliable expertise on international economic policies and trends including as they may relate to international acquisitions.
Executive Compensation and Director Compensation
None of MURF’s executive officers or directors have received any cash compensation for services rendered to MURF. MURF pays Murphy Canyon Management Group, Inc., an affiliate of the Sponsor, $10,000 per month for office space, utilities and secretarial and administrative support. Upon completion of our initial business combination or our liquidation, we will cease paying these monthly fees. No compensation of any kind, including any finder’s fee, reimbursement, consulting fee or monies in respect of any payment of a loan, will be paid by us to our sponsor, officers or directors or any affiliate of our sponsor, officers or directors, prior to, or in connection with any services rendered in order to effectuate, the consummation of our initial business combination (regardless of the type of transaction that it is). However, these individuals will be reimbursed for any out-of-pocket expenses incurred in connection with activities on our behalf such as identifying potential target businesses and performing due diligence on suitable business combinations. Our audit committee reviews on a quarterly basis all payments that were made to our Sponsor, officers or directors or our or their affiliates.
After the completion of the Business Combination, officers or directors who remain with MURF may be paid consulting, management or other fees from the combined company, New Conduit. The directors of New Conduit will be responsible for determining officer and director compensation. Any compensation to be paid will be determined by a compensation committee constituted solely by independent directors.
MURF is not party to any agreements with its officers and directors that provide for benefits upon termination of employment.
Legal Proceedings
There is no material litigation, arbitration, governmental proceeding or any other legal proceeding currently pending or known to be contemplated against MURF, and MURF has not been subject to any such proceeding in the 10 years preceding the date of this proxy statement/prospectus.
Periodic Reporting and Audited Financial Statements
MURF has registered its securities under the Exchange Act and has reporting obligations, including the requirement to file annual and quarterly reports with the SEC. In accordance with the requirements of the Exchange Act, MURF’s annual reports contain financial statements audited and reported on by MURF’s independent registered public accounting firm.
| 138 |
Management’s Discussion and Analysis of Financial Condition and Results of Operations OF MURPHY CANYON ACQUISITION CORP.
The following discussion of MURF’s financial condition and results of operations should be read in conjunction with MURF’s consolidated financial statements and notes to those statements included in this proxy statement/prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. Please see the sections entitled “Forward-Looking Statements” and “Risk Factors” in this proxy statement/prospectus.
Overview
MURF is a blank check company incorporated as a Delaware corporation and formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses. MURF intends to effectuate its initial business combination using cash from the proceeds of the initial public offering and the sale of the placement units, the proceeds of the sale of its shares in connection with its initial business combination (including pursuant to backstop agreements MURF may enter into), shares issued to the owners of the target, debt issued to bank or other lenders or the owners of the target, or a combination of the foregoing.
The issuance of additional shares in connection with an initial business combination to the owners of the target or other investors:
| ● | may significantly dilute the equity interest of existing shareholders; | |
| ● | may subordinate the rights of holders of its common stock if preferred stock is issued with rights senior to those afforded our common stock; | |
| ● | could cause a change in control if a substantial number of shares of its common stock is issued, which may affect, among other things, our ability to use our net operating loss carry forwards, if any, and could result in the resignation or removal of its present officers and directors; | |
| ● | may have the effect of delaying or preventing a change of control of MURF by diluting the stock ownership or voting rights of a person seeking to obtain control of us; and | |
| ● | may adversely affect prevailing market prices for its common stock and warrants. |
Similarly, if MURF issues debt securities or otherwise incurs significant debt to bank or other lenders or the owners of a target, it could result in:
| ● | default and foreclosure on its assets if its operating revenues after an initial business combination are insufficient to repay our debt obligations; |
| ● | acceleration of its obligations to repay the indebtedness even if MURF make all principal and interest payments when due if MURF breach certain covenants that require the maintenance of certain financial ratios or reserves without a waiver or renegotiation of that covenant; | |
| ● | its immediate payment of all principal and accrued interest, if any, if the debt is payable on demand; | |
| ● | its inability to obtain necessary additional financing if the debt contains covenants restricting its ability to obtain such financing while the debt is outstanding; | |
| ● | its inability to pay dividends on its common stock; | |
| ● | using a substantial portion of its cash flow to pay principal and interest on its debt, which will reduce the funds available for dividends on our common stock if declared, our ability to pay expenses, make capital expenditures and acquisitions, and fund other general corporate purposes; |
| ● | limitations on its flexibility in planning for and reacting to changes in its business and in the industry in which MURF operate; |
| ● | increased vulnerability to adverse changes in general economic, industry and competitive conditions and adverse changes in government regulation; |
| ● | limitations on its ability to borrow additional amounts for expenses, capital expenditures, acquisitions, debt service requirements, and execution of our strategy; and | |
| ● | other purposes and other disadvantages compared to its competitors who have less debt. |
| 139 |
As indicated in the accompanying financial statements, at March 31, 2023 and December 31, 2022, MURF had $90,606 and $345,777 in cash, respectively.
At December 31, 2022, and December 31, 2021, MURF had $345,777 and $48,555 in cash, respectively, and deferred offering costs of $0 and $108,962, respectively. Additionally, the underwriters are entitled to a deferred fee of $0.35 per Unit, or $4,628,750. The deferred fee will become payable to the underwriters from the amounts held in the trust account solely in the event that the Company completes a Business Combination, subject to the terms of the underwriting agreement. Further, MURF expects to continue to incur significant costs in the pursuit of its initial business combination plans. We cannot assure you that its plans to raise capital or to complete its initial business combination will be successful.
Merger Agreement
On November 8, 2022, MURF entered into the Merger Agreement by and among MURF, Merger Sub and Conduit. If the Merger Agreement is approved by MURF’s stockholders and the transactions under the Merger Agreement are consummated, Merger Sub will merge with and into Conduit, with Conduit surviving the merger as its wholly owned subsidiary. Upon the closing of the Merger, it is anticipated that MURF will change its name to “Conduit Pharmaceuticals Inc.” The MURF Board has (i) approved and declared advisable the Merger Agreement, the related ancillary agreements thereto and the transactions contemplated thereby and (ii) resolved to recommend approval of the Merger Agreement and related transactions by its stockholders.
Pursuant to the Merger Agreement, the shareholders of Conduit will receive the number of New Conduit common stock that shall have an aggregate value equal to Six Hundred Fifty Million Dollars ($650,000,000). With each share of New Conduit common stock being valued at $10.00, Conduit’s outstanding ordinary shares (including the shares issued upon conversion of all outstanding convertible debt, which conversion shall have occurred prior to the consummation of the Business Combination) will be converted into an aggregate of 65,000,000 shares of the common stock of New Conduit following the Business Combination, with each such outstanding Conduit share converted into shares of the common stock of New Conduit on a pro rata basis. Additionally, each outstanding share of MURF common stock immediately prior to the Merger, including all outstanding shares of Class B common stock, shall automatically convert into and become one share of the common stock of New Conduit without any action on the part of any holders of such common stock.
In connection with the transactions contemplated by the Merger Agreement, MURF entered into a subscription agreement with the Private Placement Investor for units that are materially the same as those purchased by the Sponsor at the time of the initial public offering. MURF’s directors, officers, Sponsor or their affiliates will not be participating in the private placement.
Pursuant to the Subscription Agreement, the Private Placement Investor has agreed to purchase 2,700,000 units of MURF, with each unit consisting of (i) one share of MURF’s Class A common stock and (ii) one warrant to purchase one share of Class A common stock, for a purchase price of $10.00 per unit in the Private Placement. The Subscription Agreement contains registration rights, pursuant to which within fifteen (15) business days after the closing of the Private Placement, MURF will use reasonable best efforts to file with the SEC a registration statement registering the resale of shares of MURF common stock included in the Units and the shares of MURF common stock issued and issuable upon exercise of the warrants. The closing of the Private Placement is conditioned on there not being a suspension of the qualification of MURF common stock for offering or sale or trading in any jurisdiction, or the initiation or threatening of any legal proceeding, no legal prohibitions to consummate the Business Combination, and all conditions precedent to the closing of the Business Combination set forth in the Merger Agreement having been satisfied or waived.
The warrant will be exercisable for a period of five years after the completion of the Business Combination and will have an exercise price of $11.50 per share, subject to adjustment as set forth in the Warrant for stock splits, stock dividends, recapitalizations and similar customary adjustments. The Private Placement Investor may exercise each warrant on a cashless basis if the shares underlying the warrants are not then registered pursuant to an effective registration statement. The Private Placement Investor has contractually agreed to restrict its ability to exercise the warrants such that the number of shares of MURF common stock held by the investor and its affiliates after such exercise does not exceed the beneficial ownership limitation set forth in the warrant which may not exceed 4.99% of the then issued and outstanding shares of MURF common stock.
| 140 |
In connection with the amendment to the Subscription Agreement dated January 27, 2023, the Private Placement Investor agreed to receive and be issued shares of New Conduit common stock. Upon the closing of the Business Combination, and specifically the filing of the Proposed Charter, MURF’s Class A common stock and Class B common stock will be reclassified as a single class of common stock, and MURF’s company name will change to “Conduit Pharmaceuticals Inc.” also referred to as New Conduit. The underlying terms of the Class A common stock and the single class of common stock are materially the same as each has or will have the same voting right of one vote per each share, same dividend rights and same liquidation rights.
January 2023 Extension
Initially, MURF was required to complete its initial business combination transaction by 12 months from the consummation of its initial public offering or up to 18 months if it extended the period of time to consummate a business combination in accordance with its amended and restated certificate of incorporation. On January 26, 2023, at a special meeting of its stockholders, MURF’s stockholders approved a proposal to amend the amended and restated certificate of incorporation to allow it to extend the date by which it has to consummate a business combination up to 12 times, each such extension for an additional one month period, from February 7, 2023, to February 7, 2024. MURF’s stockholders also approved a related proposal to amend the trust agreement allowing MURF to deposit into the Trust Account, for each one-month extension, one-third of 1% of the funds remaining in the trust account following the redemptions made in connection with the approval of the extension proposal at the special meeting. At the special meeting MURF’s stockholders also approved a proposal to amend its certificate of incorporation to expand the methods that it may employ to not become subject to the “penny stock” rules of the SEC.
In connection with such proposals, MURF’s public stockholders had the right to redeem their shares for cash equal to their pro rata share of the aggregate amount on deposit in the trust account as of two days prior to such stockholder vote. The public stockholders holding 11,037,272 shares of Class A common stock (out of a total of 13,979,000 shares of Class A common stock) exercised their right to redeem such shares at a redemption price of approximately $10.33 per share. Approximately $114 million in cash was removed from the trust account to pay such stockholders and, accordingly, after giving effect to such redemptions, the balance in the trust account was approximately $23 million.
As a result of the approval of such proposals, MURF agreed to deposit into the trust account one-third of 1% of the funds then on deposit in the trust account for each month of the extension period, resulting in a monthly contribution of approximately $0.035 per share that was not redeemed in connection with the Special Meeting (approximately $77,000 in the aggregate per month), or an aggregate of $924,000 (the “Maximum Contribution”) if the date MURF has to consummate a business combination is extended 12 times, each assuming no interest is earned on the funds in the trust account. During the three months ended March 31, 2023, the Company funded $155,403 in monthly extension payments into the Trust Account.
Results of Operations and Known Trends or Future Events
MURF’s entire activity since inception up to March 31, 2023, relates to its formation, its initial public offering and, since the closing of the initial public offering, a search for a business combination candidate. MURF will not be generating any operating revenues until the closing and completion of its initial business combination, at the earliest.
For the three months ended March 31, 2023, and 2022, MURF had net income of $120,269 and a net loss of $253,536, respectively. For the three months ended March 31, 2023, this consisted primarily of general and administrative expenses of $507,406 and income tax expense of $36,557. This was offset by interest income of $664,232 earned on trust assets during the three months March 31, 2023. For the three months ended March 31, 2022, this consisted primarily of general and administrative expenses of $243,718 and related party administration fees of $20,000, offset by $10,182 of interest income on trust assets. The increase in general and administrative expense for the three months ended March 31, 2023, as compared to the three months ended March 31, 2022 mainly consists of increased costs for D&O insurance expense, and professional fees for legal and accounting costs associated with the special meeting held in January 2023.
For the year ended December 31, 2022, and during the period from October 19, 2021 (inception) through December 31, 2021, MURF had net income and a loss of $398,639 and $4,381, respectively. For the year ended December 31, 2022 this consisted primarily of general and administrative expenses of approximately $1.20 million and income tax expense of $374,862. This was offset by interest income of approximately $1.98 million earned on Trust assets during the year ended December 31, 2022. There was no interest income earned and during the period from October 19, 2021 (inception) through December 31, 2021, and the net loss consisted of formation costs.
| 141 |
In January 2023, its public stockholders had the right to redeem their shares for cash equal to their pro rata share of the aggregate amount on deposit in the trust account. Its public stockholders holding 11,037,272 shares of Class A common stock (out of a total of 13,979,000 shares of Class A common stock) exercised their right to redeem such shares at a redemption price of approximately $10.33 per share. Approximately $114 million in cash was removed from the trust account to pay such stockholders and, accordingly, after giving effect to such redemptions, the balance in the trust account was approximately $23 million. As a result of less funds in the trust account, MURF does not expect the same level of interest income in 2023 as MURF experienced during the year ended December 31, 2022.
Liquidity, Capital Resources and Going Concern
As indicated in the accompanying financial statements, at March 31, 2023, MURF had $90,606 in cash and at December 31, 2022, MURF had $345,777 in cash.
For the three months ended March 31, 2023, the net decrease in cash was $255,171. Cash used in operating activities was $599,818 and was mainly the result of a net income of $120,269, interest income earned on trust assets $664,232, cash used in accrued expenses of $13,143, and cash used in prepaid expenses of $81,445 offset by change in income taxes payable of $150,443. Cash provided by investing activities mainly consisted of $113,831,930 from the trust account for redemptions, and $200,050 for tax payments. This was offset by $155,403 of cash deposited into the trust account for monthly extension payments in February and March 2023. Cash used in financing activities was $113,831,930 for redemptions of Class A common stock, offset by $300,000 in funds from our Sponsor for operating activities in connection with a note payable.
For the year ended December 31, 2022, the net increase in cash was $297,222. Cash used in operating activities was $1,311,310 and was mainly the result of a net income of $398,639, interest income earned on trust assets $1,976,183, cash used in accrued expenses of $27,664, and cash used in prepaid expenses of $245,254 partially offset by change in deferred offering costs of $108,962 and accrued income taxes payable of $374,862. Cash used in investing activities was $134,895,000 and was the result of funds deposited into the trust account. Cash provided by financing activities was $136,503,532 and was primarily related to the initial public offering.
On February 7, 2022, MURF consummated its initial public offering of 11,500,000 units (the “Units”). Each Unit consists of one share of Class A common stock of the Company, par value $0.0001 per share (“Class A Common Stock”), and one redeemable warrant of the Company (“Warrant”), with each whole Warrant entitling the holder thereof to purchase one share of Class A Common Stock for $11.50 per share. The Units were sold at a price of $10.00 per Unit, generating gross proceeds to the Company of $115,000,000 the Company granted the Underwriters in the Offering a 45-day option to purchase up to 1,725,000 additional Units solely to cover over-allotments, if any (the “Option”). The Underwriters exercised the Option in full, resulting in the sale of 13,225,000 Units in total and total gross proceeds of $132.25 million, which were placed in a U.S.-based trust account, maintained by Wilmington Trust Company, acting as trustee.
On February 7, 2022, simultaneously with the consummation of the Offering, the Company consummated the private placement of 754,000 units (the “Private Placement Units”) to the Sponsor, which amount includes 69,000 Private Placement Units purchased by the Sponsor in connection with the Underwriters’ exercise of the Option in full, at a price of $10.00 per Private Placement Unit, generating gross proceeds of approximately $7.54 million (the “Private Placement”) a portion of the proceeds of were placed in the trust account and a portion was used to pay offering expenses including the non-deferred underwriting discount related to the Offering. See “January 2023 Extension” as noted above for additional information regarding proceeds currently in the trust account.
In connection with the Company’s assessment of going concern considerations in accordance with Accounting Standards Update (“ASU”) 2014-15, “Disclosures of Uncertainties about an Entity’s Ability to Continue as a Going Concern,” management has determined that the Combination Period is less than one year from the date of the issuance of the financial statements. There is no assurance that the Company’s plans to consummate a Business Combination will be successful within the Combination Period. As a result, these factors raise substantial doubt about the Company’s ability to continue as a going concern for the next twelve months from the issuance of these financial statements. The financial statements do not include any adjustments that might result from the outcome of the uncertainty.
Nasdaq Delisting Notice
As previously disclosed, on April 10, 2023, MURF received a written notice from the staff of Nasdaq’s Listing Qualifications Department stating that MURF was no longer in compliance with the minimum Market Value of Listed Securities of $50,000,000 required for continued listing on the Nasdaq Global Market (the “MVLS Requirement”). In accordance with Nasdaq rules, MURF has 180 calendar days, or until October 9, 2023, to regain compliance with the MVLS Requirement. If MURF fails to satisfy Nasdaq’s continued listing requirements, such as the MVLS Requirement, Nasdaq may take steps to delist MURF’s Class A common stock. Such a delisting would likely have a negative effect on the price of MURF’s Class A common stock and may, among other things, adversely impact its ability to raise additional capital or enter into strategic transactions. See “Risk Factors - Risks Related to the Business Combination and New Conduit Common Stock Following the Business Combination - Nasdaq may delist MURF’s securities from trading on its exchange prior to consummation of the Business Combination” for additional information.
| 142 |
Related Party Transactions
On November 16, 2021, the Sponsor purchased 4,312,500 founder shares for an aggregate purchase price of $25,000, or approximately $0.006 per share. On January 26, 2022, the sponsor surrendered and forfeited 1,006,250 Founder Shares for no consideration, following which the sponsor holds 3,306,250 founder shares at approximately $0.008 per share. The founder shares (including the Class A common stock issuable upon exercise thereof) may not, subject to certain limited exceptions, be transferred, assigned or sold by the holder.
Commencing on the date of its initial public offering, MURF pays an affiliate of the Sponsor a total of $10,000 per month for office space, utilities and secretarial and administrative support. For the three months ended March 31, 2023, and 2022, total payments to Murphy Canyon Management Group, Inc. were $30,000 and $20,000 respectively. For the year ended December 31, 2022, total payments to Murphy Canyon Management Group were $110,000. Upon completion of its initial business combination or its liquidation, MURF will cease paying these monthly fees.
Its sponsor, officers and directors, or any of their respective affiliates, will be reimbursed for any out-of-pocket expenses incurred in connection with activities on its behalf such as identifying potential target businesses and performing due diligence on suitable business combinations. Its audit committee will review on a quarterly basis all payments that were made to the Sponsor, officers or directors or its or their affiliates and will determine which expenses and the amount of expenses that will be reimbursed. There is no cap or ceiling on the reimbursement of out-of-pocket expenses incurred by such persons in connection with activities on its behalf.
On November 4, 2021, the Sponsor loaned MURF $300,000 to be used for a portion of the expenses of the initial public offering. These loans are non-interest bearing, unsecured and were repaid upon the closing of the initial public offering in February 2022.
In addition, in order to finance transaction costs in connection with an intended initial business combination, the Sponsor or an affiliate of the Sponsor or certain of its officers and directors may, but are not obligated to, loan MURF funds as may be required. If MURF complete its initial business combination, MURF would repay such loaned amounts. In the event that its initial business combination does not close, MURF may use a portion of the working capital held outside the trust account to repay such loaned amounts but no proceeds from its trust account would be used for such repayment. Up to $1,500,000 of such loans may be convertible into units, at a price of $10.00 per unit at the option of the lender, upon consummation of its initial business combination. The units would be identical to the placement units. The terms of such loans by its officers and directors, if any, have not been determined and no written agreements exist with respect to such loans. MURF does not expect to seek loans from parties other than the Sponsor or an affiliate of the Sponsor as MURF does not believe third parties will be willing to loan such funds and provide a waiver against any and all rights to seek access to funds in its trust account.
In connection with the initial public offering, the Sponsor purchased 754,000 placement units for an aggregate purchase price of $7,540,000. Each whole warrant is exercisable to purchase one whole share of Class A common stock at $11.50 per share. Its Sponsor has agreed to transfer, but has not yet transferred, an aggregate of 45,000 placement units (15,000 each) to each of its three independent directors. There will be no redemption rights or liquidating distributions from the trust account with respect to the founder shares, or placement units, which will expire worthless if MURF does not consummate a business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation). The placement units are identical to the units sold in the initial public offering except that the placement units and their component securities will not be transferable, assignable or saleable until 30 days after the consummation of its initial business combination except to permitted transferees, the purchasers of the placement units waive any and all rights and claims that they may have to any proceeds, and any interest thereon, held in the trust account in respect of the common stock underlying such placement units in the event that a business combination is not consummated. The placement units are entitled registration rights. Additionally, the warrants underlying the placement units contain a cashless exercise provision and shall be non-redeemable while held by the initial purchasers thereof or their permitted assignees. There will be no underwriting fees or commissions due with the respect to the private placement.
| 143 |
The Sponsor has agreed to waive its redemption rights with respect to its founder shares (i) in connection with the consummation of a business combination, (ii) in connection with a stockholder vote to amend its amended and restated certificate of incorporation to modify the substance or timing of its obligation to allow redemption in connection with its initial business combination or certain amendments to its charter prior thereto or to redeem 100% of its public shares if MURF does not complete its initial business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation) and (iii) if MURF fails to consummate a business combination by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation)or if MURF liquidates prior to the expiration of such period. However, its initial stockholders will be entitled to redemption rights with respect to any public shares held by them if MURF fails to consummate a business combination or liquidate by February 7, 2024 (or such later date as may be extended by means of amendment to MURF’s amended and restated certificate of incorporation).
Pursuant to a registration rights agreement MURF entered into with its initial stockholders, MURF may be required to register certain securities for sale under the Securities Act. These holders, and holders of units issued upon conversion of working capital loans, if any, are entitled under the registration rights agreement to make up to three demands that MURF register certain of its securities held by them for sale under the Securities Act and to have the securities covered thereby registered for resale pursuant to Rule 415 under the Securities Act. In addition, these holders have the right to include their securities in other registration statements filed by us. MURF will bear the costs and expenses of filing any such registration statements.
On March 7, 2023, MURF entered into a $1.5 million promissory note with the Sponsor. The promissory note is to fund the Trust Account and MURF’s operating expenses. On March 7, 2023, the Sponsor advanced $300,000 and an additional $200,000 on April 5, 2023. The Sponsor will provide additional funds as necessary under the promissory note. This loan is non-interest bearing, unsecured and will be repayable in cash in full upon the earlier of (i) the date on which the Company consummates an initial business combination and (ii) the date that the Company’s winding up is effective.
Off-Balance Sheet Arrangements; Commitments and Contractual Obligations; Quarterly Results
As of March 31, 2023, and December 31, 2022, MURF did not have any off-balance sheet arrangements as defined in Item 303(a)(4)(ii) of Regulation S-K and did not have any commitments or contractual obligations.
JOBS Act
On April 5, 2012, the JOBS Act was signed into law. The JOBS Act contains provisions that, among other things, relax certain reporting requirements for qualifying public companies. MURF will qualify as an “emerging growth company” and under the JOBS Act will be allowed to comply with new or revised accounting pronouncements based on the effective date for private (not publicly traded) companies. MURF is electing to delay the adoption of new or revised accounting standards, and as a result, MURF may not comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. As a result, its financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
Additionally, MURF is in the process of evaluating the benefits of relying on the other reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, if, as an “emerging growth company”, MURF chooses to rely on such exemptions MURF may not be required to, among other things, (i) provide an independent registered public accounting firm’s attestation report on its system of internal controls over financial reporting pursuant to Section 404, (ii) provide all of the compensation disclosure that may be required of non-emerging growth public companies under the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) comply with any requirement that may be adopted by the PCAOB regarding mandatory audit firm rotation or a supplement to the report of independent registered public accounting firm providing additional information about the audit and the financial statements (auditor discussion and analysis), and (iv) disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of the Chief Executive Officer’s compensation to median employee compensation. These exemptions will apply for a period of five years following the completion of the initial public offering or until MURF is no longer an “emerging growth company,” whichever is earlier.
| 144 |
BUSINESS OF Conduit PHARMACEUTICALS LIMITED
“We,” “us,” and “our” in this section generally refer to Conduit Pharmaceuticals Limited (“Conduit”) prior to the consummation of the Business Combination, which will be the business of the post-combination company following the consummation of the Business Combination. Unless otherwise indicated or the context otherwise requires, references in this Conduit’s Business section to “Conduit,” “we,” “us,” “our” and other similar terms refer to Conduit prior to the Business Combination and to the Company after giving effect to the Business Combination.
Overview and Strategy
Conduit Pharmaceuticals is a clinical-stage specialty biopharmaceutical company that began operations in early 2019, to facilitate the development and commercialization of clinical assets to address the unmet medical needs of patients, through our exclusive relationships. Our unique approach allows us to act as a “conduit” whereby we focus on clinical assets from pharmaceutical companies that have otherwise been deprioritized and fulfil our mission to develop new treatments for patients.
Conduit is led by highly experienced pharmaceutical executives: Dr. Freda Lewis-Hall, former Chief Medical Officer of Pfizer Inc., and Dr. David Tapolczay, former Chief Executive Officer of the United Kingdom-based medical research charity LifeArc. Our management team includes active senior clinicians who have an extensive understanding of the pharmaceuticals market, which supports our strategy of developing clinical assets in a cost-efficient manner while focusing on therapeutic efficacy and patient safety.
Conduit has an exclusive relationship and partnership with St George Street Capital (“St George Street”), a biomedical charity based in the United Kingdom. Conduit has the option to fund 100% of the development of clinical assets that are initially licensed by St George Street from AstraZeneca AB (PUBL) (“AstraZeneca”). In most instances, AstraZeneca has conducted initial pre-clinical and, in some instances, clinical trials on these assets, but has decided to license them for further development.
Conduit evaluates the clinical assets held by St George Street to determine which assets to fund for further development. In connection with the funding and development of clinical assets, Conduit evaluates and selects the specific clinical assets to be developed and collaborates with external contract research organizations (“CROs”) to run clinical trials that are managed, funded and overseen by Conduit. We intend to leverage our comprehensive clinical and scientific expertise in order to facilitate development of clinical assets through Phase IIb trials in an efficient manner by using CROs and third-party service providers. Conduit believes that successful Phase IIb trials of the clinical assets in its pipeline will increase the value of such assets. There is no assurance that any clinical trials on the assets owned or licensed by Conduit will be successful.
Conduit will seek to license assets following potentially successful clinical trials (of which there can be no assurance that the outcomes of such trials will be successful) to large biotech or pharmaceutical companies, typically for milestone payments and retain a royalty income stream for the life of the asset patent. Conduit anticipates using any future royalty income stream to develop its asset portfolio in order to balance its potential future income streams with new debt or equity financings.
Outside of our relationship with St George Street, Conduit is free, and intends, to pursue additional relationships and/or partnerships with third parties for the licensing of further assets which are currently deprioritized. We plan to focus our efforts on developing clinical assets to address diseases that impact a large population where there is no present treatment or the present treatment, carries significant unwanted side effects.
Our initial licensed clinical assets are focused on idiopathic male infertility and autoimmune diseases or immunodeficient conditions like uveitis, premature labor, renal transplant rejection and Hashimotos Thyroiditis. Our initial assets which were licensed from Astra Zeneca to St George that will be developed by Conduit are known as AZD5904 (a Myeloperoxidase Inhibitor) and AZD1656 (a Glucokinase Activator). As the clinical assets have undergone initial pre-clinical and clinical testing conducted by AstraZeneca, Conduit is able to assess the safety data generated in these clinical trials to assess which clinical assets to further develop and for which indications.
Initially, Conduit will focus on assets to which it has access, through its relationship with St George Street and St George Street’s relationship with AstraZeneca, to the active pharmaceutical ingredients (“API”) that were used by AstraZeneca in conducting its clinical trials. As a result, Conduit does not have to develop the API, which is often a time consuming and expensive process, and benefits from the fact that the API was produced by a reputable pharmaceutical company, which is subject to rigorous quality control measures so that the API that is used by Conduit for additional clinical trials is likely to be more consistent in terms of the quality and performance.
Strategic Alliances and Arrangements
Global Funding Agreement – St George Street
Conduit and St George Street entered into an Exclusive Funding Agreement on March 26, 2021 (the “Global Funding Agreement”), pursuant to which St George Street granted Conduit the exclusive first right to provide to St George Street, or procure the provision of, all funding for the performance of a drug discovery and/or development project in consideration for a share of the net revenue in respect of such project.
Conduit and St George Street have entered into five project funding agreements, which are subject to the terms of the Global Funding Agreement, to develop certain clinical assets that have been licensed to St George Street by AstraZeneca. The project funding agreements relate to:
| ● | AZD1656 for use in renal transplant, | |
| ● | AZD1656 for use in pre-term labor, | |
| ● | AZD1656 for use in Hashimoto’s Thyroiditis and Graves’ Disease, | |
| ● | AZD1656 for use in uveitis, and | |
| ● | AZD5904 for use in idiopathic male infertility. |
| 145 |
Subject to the terms of the Global Funding Agreement, St George Street may seek funding for projects from third parties (other than Conduit).
There may be additional opportunities for us to partner with St George Street to fund the development of additional clinical assets in the future, licensed from Astra Zeneca.
As of June 15, 2023, Conduit has provided approximately GBP £220,000.00 in aggregate project funding pursuant to the Global Funding Agreement and the project funding agreements and has received aggregate revenues totalling GBP £0 pursuant to the Global Funding Agreement and the project funding agreements. Conduit is entitled to receive 100% of the Net Receipts (as defined in the relevant project funding agreement) under each of the project funding agreements.
Pursuant to its terms, the Global Funding Agreement remains effective in respect of each project until the expiry of the right of a party to receive a share of the Net Revenue (as defined in the Global Funding Agreement) pursuant to the Global Funding Agreement. Under certain circumstances, St George Street may terminate a project (i) in the event of a material or persistent breach of the Global Funding Agreement by Conduit, subject to a cure period if the breach is capable of remedy, or (ii) in the event that St George Street decides to cease development of a project. If an event of force majeure occurs and continues for a certain period of time, the innocent party may terminate the Global Funding Agreement after a notice period.
Either party may terminate a project if a voluntary arrangement is proposed or approved or an administration order is made, or a receiver or administrative receiver is appointed of any of the other party’s assets or undertakings or a winding-up resolution or petition is passed (otherwise than for the purpose of solvent reconstruction or amalgamation, in particular with respect to any reorganization of the structure of that party) or if any circumstances arise which entitle a court or a creditor to appoint a receiver, administrative receiver or administrator or make a winding-up order or similar or equivalent action is taken against or by that other party by reason of its insolvency or in consequence of debt. Generally, each project funding agreement may be terminated by Conduit if at any time St George Street ceases the conduct of development or commercialization of the relevant products in accordance with the relevant development plan for a certain period of time, provided that the termination is only effective with respect to the specified project and the Global Funding Agreement continues in effect for all other projects. They may also be terminated by either party upon written notice to other party if the other party materially breaches the project funding agreement and does not fully cure the breach to the non-breaching party’s satisfaction within 90 days.
The Global Funding Agreement also contains customary representations and warranties. Each party also agreed to keep secret and confidential certain confidential information of the other party.
The foregoing summary does not purport to be a complete description of all of the provisions of the Global Funding Agreement and the project funding agreements and is qualified by reference to the full text of the Global License Agreement and the project funding agreements, which are filed as exhibits to the registration statement of which this proxy statement/prospectus is a part, and which are incorporated by reference in their entirety.
Our Strategy
Our strategy is to generate value through the development of new medicines, or clinical assets, for patients where our research indicates that there are not effective pharmaceutical treatments available or such existing pharmaceutical treatments are not adequate due to, among other things, cost of such pharmaceuticals and side effects. We are working to develop new medicines in diseases where competitive treatments carry a high incidence of unacceptable side effects.
We believe that our relationship with St George Street, which in turn has a relationship with AstraZeneca, allows us to bypass certain traditional hurdles to development of clinical assets. For example, through St George Street’s existing relationship with AstraZeneca, the clinical assets that we have licensed from St George Street have already undergone initial pre-clinical and, in some instances, clinical testing conducted by AstraZeneca, which means that Conduit is able to use the safety data generated in the prior trials in order to assess which assets to continue to develop. Conduit regularly assesses its asset portfolio to identify potential risks and take steps to mitigate those risks, such as the repurposing of assets, which reduces development costs and timelines, as the clinical asset has already undergone safety and toxicity testing in humans. If a clinical asset is used for a new indication, the early stages of drug development, including preclinical studies and Phase I clinical trials, do not need to be conducted again, which allows for Conduit to begin its development of such clinical assets at a later stage.
The prior preclinical and clinical studies conducted by AstraZeneca may allow Conduit to reduce the costs, expenses and time associated with the early stages of drug development by allowing Conduit to continue the development of the clinical assets at the Phase Ib or Phase II stage, rather than the preclinical or Phase I stage, even if Conduit is investigating the assets for a new indication. For example, if a clinical asset was subject to a Phase I trial, such clinical asset may be advanced to a Phase II trial even if the clinical asset is being investigated for a different indication.
In addition, Conduit has access, through its relationship with St George Street and St George Street’s relationship with AstraZeneca, to the API that were used by AstraZeneca in conducting its clinical trials. As a result, Conduit does not have to develop the API, which is often a time consuming and expensive process, and benefits from the fact that the API was produced by a reputable pharmaceutical company, which is subject to rigorous quality control measures so that the API that is used by Conduit for additional clinical trials is likely to be more consistent in terms of the quality and performance.
Through its relationship with St George Street, Conduit believes that it will be able to efficiently advance the development of its clinical assets because pre-clinical and, in some instances, clinical studies have already been completed and it has access to existing API. Conduit intends to enter into other agreements with third parties (other than St George Street) to fund and develop additional clinical assets in the future.
Conduit’s development plan is to conduct clinical trials and if those trials are successful, Conduit will then seek to enter into a transaction with a third party with respect to the clinical asset for the particular indication. Conduit does not intend to continue development of its clinical assets beyond Phase II clinical trials. Accordingly, Conduit anticipates developing clinical assets that have undergone pre-clinical and clinical trials through the Phase II stage and then to monetize those clinical assets through a license, royalty or other transaction. Conduit does not expect that it will commercialize any clinical assets or seek marketing approval from the FDA (or similar organizations) as Conduit intends to enter into agreements with third parties following Phase II clinical trials for each such clinical asset that would provide that such third party would pursue the further development, commercialization, and marketing of such assets. In order for Conduit to monetize its clinical assets, Conduit, in partnership with a CRO, intends to conduct additional clinical trials on its clinical assets in order to generate clinical data to support the further development of its clinical assets beyond the Phase II stage. In the event successful clinical trial data is generated for a clinical asset with a particular indication, at that point, Conduit will seek to enter into a license, royalty, or other transaction with a third party whereby the third party would continue to pursue the development of the clinical asset in Phase III clinical trials. There is no assurance that any clinical trials on the assets owned or licensed by Conduit will be successful.
Following potentially successful clinical trials, of which there can be no assurance that the outcomes of such trials will be successful, Conduit anticipates that the clinical assets in its pipeline will be commercialized through licensing arrangements with larger pharmaceuticals companies, which may include up-front milestone payments and/or royalties during the time at which such clinical asset is subject to patents. Conduit intends to use the income received from licensing clinical assets in its pipeline to fund the development of additional clinical assets, which will allow Conduit to use the existing income stream from clinical assets that have been licensed to fund our on-going operations, including the development and commercialization of additional clinical assets, without having to rely solely on debt and/or equity financing.
| 146 |
License Agreement – St George Street and AstraZeneca
In August 2019, St George Street entered into a license agreement with AstraZeneca (the “AZ License Agreement”), pursuant to which AstraZeneca granted an exclusive worldwide license to St George Street, under certain AstraZeneca patents and know-how, to exploit the pharmaceutical compounds known individually and together as AZD5904 (Myeloperoxidase Inhibitor) and AZD1656 (Glucokinase Activator). The AZ License Agreement also included any additional compounds to be developed by St George Street and any product that is comprised of or contains any such licensed compound pertaining to the field of idiopathic male infertility for the licensed compound AZD5904 and in the field of renal transplant for the licensed compound for AZD1656.
Under the AZ License Agreement, for a period of sixty (60) days following AstraZeneca’s receipt of a proof of concept study for any licensed compound, AstraZeneca retains an exclusive right of first negotiation to transfer all development, commercialization, or other ongoing planned activities related to such licensed compound, to AstraZeneca or any of its affiliates, and to undertake future exploitation of such licensed compound. Subject to the foregoing negotiation right, St George Street has the right and obligation to develop each licensed compound at its sole cost and expense in accordance with the development plan set forth in the AZ License Agreement, and the right to grant sublicenses to its affiliates and other persons with respect to each licensed compound. Any sublicense shall be consistent with, and expressly made subject and subordinate to, the terms and conditions of the AZ License Agreement, and St George Street shall cause each sublicensee to comply with the applicable terms and conditions of the AZ License Agreement. The development plan for each licensed compound shall be managed by a joint coordination committee consisting of representatives from each party to the agreement.
St George Street is required to pay AstraZeneca a share of any revenue payable to St George Street by any sublicensee according to the relevant sublicense (the “Sublicense Revenue”), which shall be calculated based on the amounts payable to St George Street by the sublicensee gross of tax, and shall include any upfront, milestone, or royalty payments payable. The percentage of Sublicense Revenue payable to AstraZeneca is sixty percent (60%) for Sublicense Revenue that is less than $10 million; fifty percent (50%) for Sublicense Revenue that is greater than $10 million but less than $15 million; and forty percent (40%) for Sublicense Revenue that is greater than $15 million.
The term of the AstraZeneca License Agreement commences on the effective date of that agreement and, unless earlier terminated in accordance therewith, continues until the date of expiration of the last royalty term for the last licensed product. Following the expiration (but not earlier termination) of the royalty term for a licensed product in a country, the license grant set forth in this agreement shall become non-exclusive, fully-paid, and irrevocable for such licensed product.
The AZ Agreement is terminable by either party if the other party is in material breach of the agreement, and has not cured the breach within ninety (90) days of notice (or ten (10) days of notice with respect to a payment breach).
AstraZeneca may immediately terminate the agreement, including the rights of any sublicensees, upon written notice if St George Street or any of its affiliates or sublicensees, anywhere in the territory, institutes, prosecutes or otherwise participates in any claim, demand, action or cause of action for declaratory relief, damages or any other remedy or for an enjoinment, injunction or any other equitable remedy alleging that any claim in an AstraZeneca patent is invalid, unenforceable or otherwise not patentable or would not be infringed by St George Street’s activities absent the rights and licenses granted under the agreement. AstraZeneca may also terminate the agreement upon thirty (30) days’ prior written notice if St George Street ceases development of all licensed compounds and all licensed products and a licensed product is not being commercialized in the territory by or on behalf of St George Street.
St George Street may terminate its activities under the agreement for convenience, on a project-by-project basis, upon reasonable notice to AstraZeneca. St George Street may also cease its activities under any development plan of a licensed compound if the joint commercialization committee determines that it is in appropriate to continue such plan for scientific, safety, or for ethical reasons, or that a licensed product no longer meets an unmet medical need.
In 2020, St George Street and AstraZeneca entered into an amendment to the AZ License Agreement to add Covid-19 to the field for licensed compound AZD1656. In 2021, St George Street and AstraZeneca entered into a second amendment and a third amendment to the AZ License Agreement. The second amendment to the AZ License Agreement added Schedule 1.36(a) to the AZ License Agreement, which describes additional terms and conditions that apply only to the parties with respect to Covid-19 for the licensed compound AZD1656. The third amendment added Hashimoto’s thyroiditis, uveitis, preterm labor, and Covid-19 to the field for AZD1656 and added Schedule 1.42(a) to the AZ License Agreement, which describes additional terms and conditions that apply to the parties (i) only with respect to Hashimoto’s thyroiditis, uveitis, and preterm labor for the licensed compound AZD1656, and (ii) with respect to all other indications and Licensed Compounds (as defined in the AZ License Agreement) as set forth in the AZ License Agreement, except with respect to Covid-19 for AZD1656, for which Schedule 1.36(a) of the AZ License Agreement applies. The terms and conditions contained in the second and third amendments to the AZ License Agreement also sets forth the obligations and responsibilities of the parties regarding supply of study drugs, conducting studies, and other matters. The foregoing summary does not purport to be a complete description of all of the provisions of the AZ License Agreement and the amendments to the AZ License Agreement, and is qualified by reference to the full text of the AZ License Agreement and its amendments, which are filed as exhibits to the registration statement of which this proxy statement/prospectus is a part, and which are incorporated by reference in their entirety.
2 Reference is made to the section “Special Meeting of MURF Stockholders – Opinion of ValueScope, Inc. – Overview of Global Biotechnology Industry”.
Conduit Asset Development
Conduit’s development plan for each of AZD1656 and AZD5904 is to conduct clinical trials and if those trials are successful, of which there can be no assurance that the outcomes of such trials will be successful, Conduit will then seek to enter into a transaction with a third party with respect to AZD1656 orAZD5904, as applicable, for the particular indication. Conduit does not intend to continue development of such clinical assets beyond Phase II clinical trials. Accordingly, Conduit anticipates developing clinical assets that have undergone pre-clinical and clinical trials through the Phase II stage and then to monetize such clinical assets through a license, royalty or other transaction. Conduit does not expect that it will commercialize any clinical assets or seek marketing approval from the FDA (or similar organizations) as Conduit intends to enter into agreements with third parties following Phase II clinical trials for each such clinical asset that would provide that such third party would pursue the further development, commercialization, and marketing of such assets. In order for Conduit to monetize its clinical assets, Conduit, in partnership with a CRO, intends to conduct additional clinical trials on its clinical assets in order to generate clinical data to support the further development of its clinical assets beyond the Phase II stage. In the event successful clinical trial data is generated for a clinical asset with a particular indication, at that point, Conduit will seek to enter into a license, royalty, or other transaction with a third party whereby the third party would continue to pursue the development of the clinical asset in Phase III clinical trials. There is no assurance that any clinical trials on the assets owned or licensed by Conduit will be successful.
In addition to the exclusive rights to develop AZD1656 and AZD5904 through our Global Funding Agreement with St George Street, we also wholly own the intellectual property and the rights to further develop certain co-crystals of AZD1656 (AZD1656 Co-Crystal PCT/IB2022/00075 - Patent Expires 02/09/2042). We will seek to develop the AZD1656 Co-Crystal in
| ● | Psoriasis, |
| ● | Crohn’s disease, |
| ● | Lupus, |
| ● | Sarcoidosis, |
| ● | Diabetic wound healing, |
| ● | Idiopathic pulmonary fibrosis, and |
| ● | Nonalcoholic steatohepatitis (NASH). |
| 147 |
Market Overview
Global Biotechnology Industry
The global biotechnology industry comprises a large range of companies engaged in diverse activities, such as biopharmaceutical development. The industry companies also span across a wide spectrum of operational models. Some small, dedicated biotechnology companies are research and development (“R&D”) intensive and operate primarily with venture capital, grants, initial public offerings and collaborative agreements. Conversely, large, diversified companies hold significant in-house R&D resources and well-established production, commercialization, and distribution processes.
The industry revenue is expected to increase at an annualized rate of 3.1% to $358.2 billion over the next five years, with more profound growth tempered by the pandemic. Increased R&D funding and consumer spending in 2022 are expected to catalyze revenue growing 2.9% the same year.1
Global investor confidence has fallen during the period, which served to somewhat subdue revenue growth. However, global investment in R&D has grown strongly and consistently in recent years, with much of this funding funneled into medical biotechnology development, aimed at providing better care for the aging global population, thus bolstering industry revenue.

1 Reference is made to the section “Special Meeting of MURF Stockholders – Opinion of ValueScope, Inc. – Overview of Global Biotechnology Industry”.
| 148 |
Global Pharmaceutical Industry
Over the previous five years, pharmaceutical companies have benefited from an aging population in developed economies and a growing middle class in emerging economies. Many companies have also tapped into regional demand for pharmaceuticals that may differ from developed markets and have expanded their global presence to tap into regional market needs.
Patent cliffs have continued to hamper industry revenue during the current period. When drugs lose patent exclusivity, the market is inundated with low-cost generic drugs. As manufacturers contend with more price-based competition from generics, many operators respond by lowering their research & development expenditures, which limits the industry’s drug pipelines. Additionally, many governments and health insurance organizations have reduced their drug reimbursements to control healthcare costs, such as implementing incentives for patients to use generic drugs.
Moving forward, revenue is forecast to grow an annualized 3.2% to $1.3 trillion over the next five years amid an anticipated persistence of global demand for industry products.2

2 Reference is made to the section “Special Meeting of MURF Stockholders – Opinion of ValueScope, Inc. – Overview of Global Biotechnology Industry”.
| 149 |
Our Initial Pipeline: AZD1656 and AZD5904
We currently have the exclusive rights to develop clinical assets, AZD1656 and AZD5904, which are licensed to St George Street by AstraZeneca, in six indications.
Due to its relationship with St George Street, Conduit intends to leverage the data generated from these historical trials in order to investigate the efficacy and safety to AZD1656 to treat HT, uveitis, preterm labor, and renal transplant patients, and the efficacy and safety of AZD5904 to treat IMI. AZD1656 has undergone testing in a total of 20 Phase I clinical trials and 5 Phase II clinical trials conducted by AstraZeneca since 2008 and 19 of which were conducted in the U.S. Additional information about those clinical trials is available at the U.S. National Library of Medicine’s website at www.clinicaltrials.gov. AZD5904 has undergone testing in 5 Phase I clinical trials conducted by AstraZeneca, one of which was conducted in the U.S. While a significant amount of clinical trial data has already been generated for both AZD1656 and AZD5904, some of this data was generated outside of the U.S. and accordingly may not be accepted by the FDA. In the event that such data is not accepted by the FDA, additional clinical trials may be required, which would result in additional costs and time to develop these clinical assets.
The table below sets forth the pre-clinical or clinical trials that have been conducted by or at the direction of AstraZeneca to date on the particular clinical asset. All of these pre-clinical or clinical trials were conducted by AstraZeneca prior to AstraZeneca entering into its license agreement with St George Street. None of the pre-clinical or clinical trials that have taken place to date were conducted by or at the direction of Conduit.
| Asset | Therapeutic Area | Stage of Development | Location of Trials | |||
AZD1656 |
Type II Diabetes |
Preliminary, Phase I and Phase II | United Kingdom; United States | |||
| AZD1656 | Renal Transplant Patients with Type II Diabetes |
Preliminary, Phase I and Phase II | United Kingdom | |||
| AZD1656 | Covid-19 | Preliminary, Phase I | United Kingdom | |||
AZD5904 |
Idiopathic Male Infertility |
Preliminary, Phase I | European Union; United States |
The following table sets forth the current asset development stage for each of AZD1656 and AZD5904 for the indications noted below.
| Asset | Therapeutic Area | Assets at Their Present Stage of Readiness* | Next Stage of Development to be Conducted by Conduit | Anticipated Exit Stage for Monetization(4) | ||||||
| Phase I | Phase II | Phase III | ||||||||
| AZD1656 | Covid-19, Long Covid | ✓ | N/A(3) | N/A(3) | ||||||
| AZD1656 | Uveitis | ✓ | Phase II | Following completion of Phase II | ||||||
| AZD1656 | Hashimoto’s Thyroiditis | ✓ | Phase II | Following completion of Phase II | ||||||
| AZD1656 | Preterm Labor | ✓ | Phase II | Following completion of Phase II | ||||||
| AZD1656 | Renal Transplant | ✓ | Phase II | Following completion of Phase II | ||||||
| AZD5904 | Idiopathic Male Infertility | ✓ | Phase II
|
Following completion of Phase II | ||||||
* Indicates that the asset is considered ready for this Phase. For example, if an asset is listed under Phase II, this means that the asset has already completed Phase I trials and is therefore considered Phase II ready.
(3) Conduit does not intend to provide additional funding to develop AZD1656 for Covid-19. However, Conduit is entitled to a portion of the revenues in the event that AZD1656 is further developed by St. George Street or another third party and is monetized, whether through a sale, license agreement, or otherwise.
(4) Reflects the stage at which Conduit anticipates that it will seek to monetize such assets through a license, royalty or other transaction with a third party, who would then seek to continue the development of such clinical asset until its potential commercialization after Phase III clinical trials were completed. There is no assurance that Conduit will be able to monetize such assets by entering into a license, royalty or other transaction with a third party. In addition, there is no assurance that any of the clinical assets licensed or owned by Conduit will successfully complete Phase II or Phase III clinical trials or obtain regulatory approvals, or that such assets will be monetized or commercialized.
AZD1656 was subject to Phase I and Phase IIa clinical trials consisting of 23 studies in 526 subjects, 446 of whom were dozed with AZD1656. Other than for the intended effect of lowering glucose, there were no difference identified between the AZD1656-treated and placebo-treated subjects relating to adverse events. All of cases where low glucose levels were identified were managed by the patients and resolved. Based on these clinical trials, no safety signals were identified regarding vital signs, safety laboratory values or electrocardiogram data. No deaths occurred in any studies with healthy volunteers or patients. AZD1656 was also subject to Phase IIb clinical trials consisting of 2 studies where AZD1656 was given to patients with Type 2 Diabetes Mellitus for 4 months or longer. In total, there were 754 randomized patients, 516 of whom were exposed to AZD1656 (316 men and 200 women). There were no clinically important differences in the adverse effects profile between the AZD1656 treatment group and the AZD1656 placebo group and there were no deaths in either of the Phase IIb studies. The efficacy of AZD1656 as a potential treatment for diabetes was also assessed during the Phase IIb clinical trials, including whether the efficacy was statistically significant. Clinically relevant and statistically significant reductions in HbA1c were seen after 4 months; however, the initial improvement in glucose control deteriorated over time and the change in HbA1c levels after 4 months were not statistically different than the placebo. This decreasing efficacy over time was seen in both Phase IIb studies.
AZD5904 was subject to 5 Phase I clinical studies, with a total of 1181 subjects being exposed to AZD5904. Single doses of up to 1200 mg and multiple doses of up to 325 mg for up to three times per day for 21 days have been administered as an oral solution in the completed clinical studies. In addition, single doses of up to 1400 mg and multiple doses of up to 600 mg for 10 days have been administered as an “extended release” formulation. The data from these studies did not identify any expected adverse drug reactions for AZD5904 and no adverse effects were reported as related to AZD5904. In addition, the data revealed no clinically significant changes in blood pressure or pulse rate related to AZD5904 and electrocardiogram data was within the physiological range for the population studied. The effect of AZD5904 on human myeloperoxidase, which we refer to as MPO, activity was evaluated by determination in an ex vivo assay of MPO activity in plasma. The correlation between MPO activity and plasma concentrations was assessed for single and multiple doses of AZD5904. A relationship between plasma concentrations of AZD5904 and MPO activity was demonstrated, which indicates that AZD5904 may be an effective inhibitor of MPO activity in humans. However, Phase I trials do not assess statistical significance so additional Phase II trials are necessary to determine if the inhibition of MPO activity as a result of AZD5904 is statistically significant.
Conduit intends to conduct clinical trials on the AZD1656 and AZD5904 in the therapeutic areas listed below. The following table does not reflect the pre-clinical or clinical trials that have been conducted by third parties to date, but instead reflects the next anticipated stage of clinical trials, if any, that are intended to be conducted by Conduit. There can be no assurances that the clinical trials that Conduit intends to conduct will be successful.
| Asset | Therapeutic Area | Next Stage of Development to be Conducted by Conduit | ||
| AZD1656 | Covid-19, Long Covid | N/A(5) | ||
| AZD1656 | Uveitis | Phase II | ||
| AZD1656 | Hashimoto’s Thyroiditis | Phase II | ||
| AZD1656 | Preterm Labor | Phase II | ||
| AZD1656 | Renal Transplant | Phase II | ||
| AZD5904 | Idiopathetic Male Infertility | Phase Ib |
| (5) | Conduit does not intend to provide additional funding to develop AZD1656 for Covid-19. However, Conduit is entitled to a portion of the revenues in the event that AZD1656 is further developed by St George Street or another third party and is monetized, whether through a sale, license agreement, or otherwise. |
| 150 |
AZ1656 in Autoimmune Diseases.
Autoimmune diseases refers to a broad group of diseases and conditions that arise from an abnormal immune response to a functioning body part. For example, autoimmune diseases may arise from an abnormal immune response of major organs (i.e., the heart, kidneys, bladder, liver, lungs, and skin), glands (i.e., the adrenal gland, pancreas, thyroid or reproductive organs), digestive system, and tissue (i.e., blood, connective tissue, muscle, eyes, ears, or vascular system). Management believes that there are over 80 types of autoimmune diseases that have been identified, including lupus, celiac disease, multiple sclerosis, rheumatoid arthritis, psoriasis, and inflammatory bowel disease. Autoimmune diseases are often difficult to diagnose and often the cause of the disease is not known.
It is estimated by the American Autoimmune Related Diseases Association (“AARDA”) that as many as 50 million Americans are living with an autoimmune disease – at a cost of $86 billion a year and there is presently no totally effective treatment known to management. The currently available treatments for autoimmune diseases include non-steroidal anti-inflammatory drugs (“NSAIDS”) or immune suppressants. These treatments often improve the symptoms but ultimately do not cure the disease and often involve side effects.
AZD1656 is a highly specific glucokinase activator; originally developed by AstraZeneca for use in diabetes mellitus. It has now been tested in over 1,000 patients with both type I and II diabetes and no significant safety concerns have been raised. It was most recently tested in the ARCADIA phase II trial in diabetic patients hospitalized with Covid-19 on the basis of new research into immunometabolic modulation. We believe that AZD1656 may be used to activate a patient’s own immune system in order to limit harmful inflation. We have identified several autoimmune diseases, which reflects good market potential, with a high level of need that may be treatable using AZD1656. We believe that our clinical assets have the potential to treat numerous autoimmune diseases. We intend to initially focus on the indications below in order to maximize the commercial potential of our clinical assets.
Thyroid Disease: Hashimoto’s Thyroiditis and Graves’ Disease
Both Hashimoto’s Thyroiditis (“HT”) and Graves’ disease are autoimmune diseases involving the improper functioning of the thyroid. HT is an autoimmune disease driven by T cells, which are one of the types of white blood cells, where the immune system attacks the thyroid gland. Graves’ disease is an autoimmune disorder in which antibodies result in the hyperfunction of the tissue and an increase in the amount of thyroid hormones. Patients with Graves’ disease may develop an enlargement of the thyroid called a goiter and often have eye problems due to lymphocyte, a type of immune cell, infiltrating into the muscles of the eye.
Management believes that HT is the most prevalent autoimmune thyroid disease worldwide and anticipates that the prevalence of HT will continue to increase due to rising obesity and the rising prevalence of other autoimmune disorders that made patients more susceptible to HT. Graves’ disease is the most common cause of persistent hyperthyroidism, which is an over production of hormones by the thyroid gland located in the front of the neck, in adults.
The current treatment for HT involves hormone replacement therapy with levothyroxine. However, determining the appropriate dose for each individual is complex with the individual needing to continue hormone replacement therapy for the rest of his or her life while still suffering with some symptoms of HT. Under the current treatment, the patient is monitored by measuring Thyroid-Stimulating Hormone levels (“TSH”). Studies have shown up to 27% of patients have TSH that are too high and 41% of patients have TSH levels that are too low, which reflects the difficulty in treating the disease. In addition, this difficulty in titrating the appropriate dose of levothyroxine leads to a high burden of medical appointments and the risk of development of comorbidities, including cardiovascular disease. Graves’ disease is also difficult to control with carbimazole and steroids, both of which have numerous side effects. Another common treatment is using radioactive iodine (“RAI”) to destroy the thyroid gland. After using RAI, patients are left dependent upon levothyroxine hormone replacement treatment. It is also believed that the use of RAI has also been associated with the development or worsening of thyroid eye disease in approximately 15% to 20% of patients. The alternative treatment is surgical removal of the thyroid gland following which the patient will need lifelong replacement therapy with levothyroxine which involves the same problems described above.
As published by news sources, management believes that the thyroid gland disorder treatment market is set to grow by approximately $632 million at a compound annual growth rate (“CAGR”) of 4.63% from 2020 to 2025.
AZD1656 was previously subject to preclinical and clinical trials, including Phase I and Phase II trials, conducted by AstraZeneca relating to its potential to treat type 2 diabetes. As of the date hereof, no preclinical or clinical trials have been conducted on the use of AZD1656 to treat either HT or Graves’ disease.
Conduit intends to conduct further trials on AZD1656 relating to HT and Graves’ disease. Conduit plans to conduct further research on AZD1656 to investigate if AZD1656 is a treatment option for HT and Graves’ disease, including investigating any negative side effects in the use of AZD1656 as compared to the currently available treatment options for HT and Graves’ disease. Conduit, in connection with a CRO, has prepared clinical trial protocols for the use of AZD1656 in HT in a PhaseIIb clinical trial: a Phase II, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of AZD1656 in patients with HT with an anticipated enrollment of 200 patients.
Pharmaceutical companies typically find market entry for HT clinical assets challenging due to the manufacturing complexities and careful consideration of manufacturing product, which are usually patented or trade secrets of companies. Due to its relationship with St George Street, Conduit has sufficient API to conduct Phase II clinical trials on AZD1656 for the treatment of HT and Graves’ disease. There can be no assurances that the clinical trials that Conduit intends to conduct on AZD1656 to treat HT and Graves’ disease will be successful.
Uveitis
Uveitis is an autoimmune disease of the eye that refers to a number of intraocular inflammatory conditions and involves the swelling of the uvea, the colored portion of the eyes. Management believes that in the U.S. uveitis causes an estimated approximately 30,000 new cases of blindness per year and may be the third leading cause of blindness worldwide. (6) Unlike other leading causes of blindness, uveitis is particularly prevalent in younger working-age people. Uveitis has a prevalence of around 40-100 per 100,000 persons, and can be subdivided into specific conditions, so it qualifies as a rare disease. (7) We believe that a treatment for non-infectious uveitis would be eligible for orphan drug designation, which provides for market exclusivity of 10 years in the European Union and 7 years in the United States.
| (6) | “Epidemiology of uveitis in a US population-based study,” by Marta Mora Gonzalez, Marisee Masis Solano, Travis C. Porco, Catherine E. Oldenburg, Nisha R. Acharya, Shan C. Lin, and Matilda F. Chan (Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5904090/) | |
| (7) | “Epidemiology and risk factors in non-infectious uveitis: a systemic review,” by Katherine A. Joltikov and Anne-Marie Lobo-Chan (Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8461013/). |
| 151 |
Steroids, which can cause elevated intraocular pressures and cataracts, are often used to manage uveitis. Most patients develop elevated intraocular pressures and/or cataracts after long-term treatment with steroids and may have to switch therapies or the disease may become resistant to steroid treatment. Biological drugs have been developed but these are expensive and not always effective as many patients still go blind every year.
AZD1656 was previously subject to preclinical and clinical trials, including Phase I and Phase II trials, conducted by AstraZeneca relating to its potential to treat type 2 diabetes. As of the date hereof, no preclinical or clinical trials have been conducted on the use of AZD1656 to treat uveitis. Conduit, in connection with a CRO, has prepared clinical trial protocols relating to the use of AZD1656 in uveitis in a Phase IIb clinical trial: a Phase IIb, double-blind, placebo-controlled study to evaluate the efficacy and safety of ADZ1656 in patients with non-infectious uveitis with an anticipated enrollment of 120 patients. Conduit intends to conduct further trials on AZD1656 in order to investigate if AZD1656 is an option to treat uveitis without the side effects involved in the current treatment using steroids. There can be no assurances that the clinical trials that Conduit intends to conduct on AZD1656 to treat uveitis will be successful.
Renal Transplant Failure
Renal transplant failure occurs when a patient’s body rejects a kidney transplant and involves the gradual decrease in kidney function that starts following a kidney transplant surgery and often results in organ failure. According to the United Network for Organ Sharing, there are over 100,000 patients waiting for a kidney transplant in the U.S. The United Network for Organ Sharing reports that the prevalence of chronic kidney disease is rising due to other conditions, such as diabetes, and as a result of an aging population. The United Network for Organ Sharing reported that during 2021, over 24,000 individuals received a kidney transplant. According to the Organ Procurement & Transplantation Network, approximately 13 people die each day in the U.S. while waiting for a life-saving kidney transplant.
The current treatment for renal transplant failure involves using immunosuppressives to suppress the patient’s immune system, which has numerous side effects including high blood pressure, weight gain, diabetes, dyslipidemia and some cancers. Malignancy, which refers to uncontrolled growth and division of abnormal cells, is one of the most common causes of death in kidney transplant recipients. Immunosuppressives are a major contributing factor to malignancy.
AZD1656 was previously subject to preclinical and clinical trials, including Phase I and Phase II trials, conducted by AstraZeneca relating to its potential to impact on renal transplant patients with type 2 diabetes. We believe that AZD1656 may facilitate the immune system in tolerating or accepting the transplanted kidney. Conduit intends to conduct Phase II studies on AZD1656 to investigate if AZD1656 decreases the rejection in kidney transplant patients. Conduit is currently working with a CRO to prepare protocols for clinical trials to investigate the use of AZD1656 to reduce the rejection in kidney transplant patients. There can be no assurances that the clinical trials that Conduit intends to conduct on AZD1656 to treat renal transplant patients will be successful.
Preterm Labor
Preterm labor refers to giving birth to babies that are born alive before 37 weeks of pregnancy. Preterm labor may result in premature birth and the earlier premature birth happens, the great of health risks for the baby. Preterm labor is a condition that may result in the death of the baby and/or the mother. There is no effective treatment for preterm labor that is known to us. Management believes that approximately 60,000 babies per year in the U.K. according to the Mums and Midwives Awareness Academy and approximately 380,000 per year in the U.S. are born preterm according to the Preeclampsia Foundation. Globally, prematurity is the leading cause of death in children under the age of 5 years, and preterm labor rates are increasing. For example, according to the Centers for Disease Control and Prevention, in the U.S., the preterm labor rate rose for the fifth straight year in 2019. For 2021, the preterm labor rate in the U.S. was approximately 10.5%. According to the World Health Organization, the rates of preterm labor by country range from approximately 5% to approximately 18%. Management believes that the preterm birth and PROM testing market size was valued at approximately $1.6 billion in 2021 and is anticipated to be over $2.2 billion by 2030 with a CAGR of 3.2% from 2022 to 2030. Preterm labor results in increases costs, both higher costs of labor and neonatal care, and often results in additional medical care during the child’s lifetime for those that are born prematurely. Accordingly, the reduction in preterm labor would have a significant health and economic impact.
AZD1656 was previously subject to preclinical and clinical trials, including Phase I and Phase II trials, conducted by AstraZeneca relating to its potential to treat type 2 diabetes. As of the date hereof, no preclinical or clinical trials have been conducted on the use of AZD1656 to treat preterm labor. Specially, Conduit intends to conduct a Phase II study on the use of AZD1656 to assist in maintaining pregnancy beyond 37 weeks.
Conduit, in connection with a CRO, has prepared clinical trial protocols relating to the use of AZD1656 in preterm labor in a Phase IIb clinical trial: a multicenter, randomized, double-bind, placebo-controlled Phase II clinical trial evaluating the efficacy and safety of AZD1656 in the prevent of preterm labor with an anticipated enrollment of 200 patients. In the event that AZD1656 is shown to be able to effectively treat preterm labor (of which there can be no assurance), AZD1656 could potentially maintain a pregnancy for longer, reduce the number of babies that are born prematurely and reduce the costs associated with preterm labor. There can be no assurances that the clinical trials that Conduit intends to conduct on AZD1656 to treat preterm labor will be successful.
Most drugs for preterm labor are only used for about 24-48 hours once a woman is already in labor, so that the patients can be treated with corticosteroids to promote the functioning of the baby’s lungs. These drugs are unable to sustain a pregnancy beyond this and are not safe to be used for prolonged periods. We believe that, in the event that AZD1656 is shown to be able to effectively treat preterm labor (of which there can be no assurance), AZD1656 could potentially maintain a pregnancy for longer, reduce the number of babies that are born prematurely and reduce the costs associated with preterm labor.
AZD1656 in Infectious Diseases – Covid-19 and Long Covid
Covid-19 is a disease caused by a virus named SARS-CoV-2, which refers to severe acute respiratory syndrome coronavirus 2, and is a strain of the coronavirus, which is a respiratory illness. Conduit continues to have an economic interest in AZD1656 for treatment of Covid-19 and has included AZD1656 for the treatment of Covid-19 in its pipeline. However, at this time, Conduit does not intend to provide additional funding to develop AZD1656 for Covid-19. However, Conduit is entitled to a portion of the revenues in the event that AZD1656 is further developed by St George Street or other third parties and is monetized, whether through a sale, license agreement, or otherwise. While Conduit does not intend to further fund the research and development of the use of AZD1656 in Covid, Conduit retains an economic interest in the clinical asset and if such asset is further developed through funding provided by other third parties, then Conduit may be entitled to receive compensation from those development activities conducted by third parties. There can be no assurances that AZD1656 will be further developed or commercialized for the treatment of Covid-19 or Long Covid.
| 152 |
AZD5904 in Idiopathic Male Infertility
Idiopathic Male Infertility (“IMI”) is defined as failure of a couple to conceive after one year of regular sexual intercourse where the physical examination and endocrine laboratory testing of the male are normal, but semen analysis reveals sperm abnormalities. Approximately 15% of couples globally, or 48.5 million couples globally, are infertile and that 30% of infertility cases can be attributed solely to the female, 30% can be attributed solely to the male, 30% can be attributed to a combination of both partners, and 10% of cases have an unknown cause.(8) Conduit’s management believes that male sperm counts have declined in Western men and will continue to decline due, in part, to increasing rates of diseases such as obesity and diabetes that can reduce fertility.
IMI affects families worldwide and is inherent in problems of reproduction. Currently, there are no specific treatment for male infertility, and we are not aware of any other company that is developing a treatment for male infertility. There are no approved pharmacotherapies for idiopathic male infertility. Lifestyle medicine and unproven supplements are often used. Intracytoplasmic sperm injection, a form of in vitro fertilization, is the only treatment currently available for male infertility. This process is not a treatment of male infertility but rather is an alternative means of fertilizing the egg. In vitro fertilization places a significant burden on the woman as it requires the induction of egg production and harvesting of eggs. In vitro fertilization is costly and time consuming and has modest success rates. Management believes that the male infertility market (including diagnostic testing) is predicted to reach approximately $6 billion by 2030.
Damaged sperm are unable to successfully fertilize eggs due to factors including impaired motility, impaired ability to penetrate and/or DNA damaged sperm that is unable to form a viable fetus. Our development pipeline for AZD5904 includes a potent, irreversible inhibitor of human myeloperoxidase, which we refer to as MPO, that has the potential to treat idiopathic male infertility.
AZD5904 was investigated by AstraZeneca for the treatment of idiopathic male infertility in Phase I trials, which confirmed the suitability to progress to Phase II trials. While AZD 5904 is Phase II ready, Conduit’s management intends to conduct a Phase Ib “proof of mechanism” trial to verify AZD5904 has the intended biological effect in semen (as well as in blood) prior to commencing a Phase II trial for the use of AZD5904 to treat idiopathic male infertility. Specifically, Conduit’s management intends to conduct the Phase Ib study in order to see if the trial will provide evidence that AZD5904 has its intended effect of inhibiting myeloperoxidase and reduce oxidative stress in semen. We believe that AZD5904 has the potential to be used to create a tablet that could treat IMI and would be the first drug developed to directly treat IMI. Conduit, in connection with a CRO, has prepared clinical trial protocols relating to the use of AZD5904 to treat IMI in a Phase Ib clinical trial: a Phase Ib, randomized, double-blind, placebo-controlled, dose escalation study to evaluate the safety, tolerability and preliminary efficacy of AZD5905 in adult men with IMI with an anticipated enrollment of 60 patients, and a Phase IIb clinical trial: a Phase IIb, randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of AZD5904 in the treatment of IMI with an anticipated enrollment of 200 patients. There can be no assurances that the clinical trials that Conduit intends to conduct on AZD5904 to treat idiopathic male infertility will be successful.
| (8) | “A unique view on male infertility around the globe,” by Ashok Agarwal, Aditi Mulgund, Alaa Hamada, and Michelle Renee Chyatte (Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4424520/). |
| 153 |
Future Product Candidates
As part of our strategic planning process, we intend to explore the efficacy of using AZD1656 to treat other diseases. Specifically, Conduit intends to conduct research on whether AZD1656 may be effective treating other autoimmune diseases, include systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, motor neuron disease and amyotrophic lateral sclerosis. As part of our strategic planning process, we intend to explore the efficacy of using AZD1656 to treat other diseases. We also plan to further develop the co-crystals that we own from our prior development work on AZD1656, including to research the ability of the co-crystals developed from AZD1656 to treat psoriasis, Crohn’s disease, lupus, sarcoidosis, diabetic wound healing, idiopathic pulmonary fibrosis and nonalcoholic steatohepatitis. In addition, we currently intend to explore the use of AZD5904 for the treatment of glioma. Due to our on-going relationship with St George Street, from time to time, there may be additional clinical assets that we are able to partner with St George Street to develop. We expect to seek to develop other clinical assets and determine based on pre-clinical and clinical data which clinical assets in order to determine which assets in our pipeline to continue to develop. Accordingly, we believe that our management team will be able to effectively allocate resources to the development of clinical assets that we believe show the most promise. However, there can be not guarantee that the clinical trials conducted by Conduit of its clinical assets will be successful. If we are unable to commercialize our clinical assets or experience significant delays in doing so, our business will be materially harmed.
Manufacturing
Conduit does not currently own or operate any facilities to formulate, manufacture, test, store, package or distribute any of the product candidates that we are developing or may seek to develop and do not currently have the capabilities to conduct such activities. We currently rely on third parties to manufacture, store and test the product candidates that we seek to develop. We will depend on third-party suppliers and manufacturing organizations for all our required raw materials and drug substance and to formulate, manufacture, test, store, package and distribute clinical trial quantities of product candidates that we may seek to develop. We plan to continue to use third-party suppliers and manufacturing organizations and we anticipate expanding our network of third-party suppliers and manufacturing organizations as our operations expand.
We have internal personnel and utilizes consultants with extensive technical, manufacturing, analytical and quality experience to oversee our contract manufacturing and testing activities. Manufacturing is subject to extensive regulations that impose procedural and documentation requirements, including, but not limited to, record-keeping, manufacturing processes and controls, personnel, quality control, and quality assurance. Our systems, procedures, and contractors are required to be in compliance with these regulations and are assessed through regular monitoring and formal audits.
Conduit entered into a master services agreement with a CRO to provide services relating to conducting clinical trials on those clinical assets that are designated from time to time by Conduit. In collaboration with the CRO, Conduit has prepared clinical trial protocols for AZD1656 in Hashimoto’s Thyroiditis, AZD1656 in Uveitis, AZD1656 in Preterm Labor, and AZD5904 in Idiopathic Male Infertility. These protocols serve as comprehensive plans outlining the methodology, objectives, and procedures for conducting clinical trials specific to each indication. As part of its business model, Conduit intends to continue to seek to contract with third parties in order to conduct clinical trial related services with respect to the clinical assets in its pipeline.
Research and Development
We spent approximately $28,000 on research and development activities during the year ended December 31, 2021, and $38,000 during the year ended December 31, 2022. Our research and development activities were wholly focused on developing co-crystals of AZD1656 to increase patent life. Some of this work was completed by third-party CROs but all intellectual property is retained by Conduit. We currently have one pending international patent application and two pending national patent applications. The successful completion of clinical trials increases the value of clinical assets and may lead to the commercialization and/or licensing of such assets to other pharmaceutical companies. There is no assurance that any clinical trials on the assets owned or licensed by Conduit will be successful.
Conduit does not intend to further fund the research and development of the use of AZD1656 in Covid; however, Conduit retains an economic interest in the clinical asset and if such asset is further developed through funding provided by other third parties, then Conduit may be entitled to receive compensation from those development activities conducted by third parties due to its economic interest in AZD1656 in Covid.
Sales and Marketing
We do not currently have marketing, sales, or distribution capabilities. In order to commercialize any clinical asset that is approved for commercial sale, we must either develop our own sales, marketing, and distribution infrastructure or collaborate with third parties that have such commercial infrastructure and relevant marketing and sales experience. We anticipate relying on licensing, co-sale, co-promotion, and distribution agreements with strategic partners for the commercialization of our products. We do not currently anticipate that we would develop our own internal sales force organization.
Competition
We operate in the highly competitive pharmaceutical and biotechnology industry. Our competitors may include public and private companies, universities, governmental agencies, and other research organizations actively engaged in the research and development of clinical assets and biopharmaceutical products. Our competitors may have greater financial, technical, and human resources than we currently have and/or may be better equipped to develop, manufacture, and market their products. Our competitors may be developing product candidates for products for similar indications. However, we believe that we have an unprecedented advantage in novelty. As discussed above AZD1656 is an activator (not an inhibitor) of a metabolic process. We anticipate that the number of companies seeking to develop clinical assets, biopharmaceutical products and therapies will continue to increase. As a result, the competition we face may also increase, however both in the treatment of autoimmune disease and idiopathic male infertility the competition is currently expected to come in years, even if biopharmaceutical products that we develop and/or commercialize were not to compete with products of our competitors based on the product efficacy, safety, ease of use, price, demonstrated cost-effectiveness, marketing effectiveness, service, reputation, and access to technical information. However, we believe that our ability to focus on clinical assets that have been deprioritized by larger pharmaceutical companies is a competitive advantage.
Intellectual Property
We hold exclusive rights to develop AZD1656 and AZD5904 through our Global Funding Agreement with St George Street and we also own the intellectual property and the rights to further develop co-crystals resulting from our prior development work on AZD1656.
We currently have one pending international patent application and two pending national patent applications. Even though we have filed patent applications, there is no guarantee that the validity of the patents will be upheld if challenged by a third party, that patents will be granted on the applications filed in the respective jurisdictions, or that once granted, the patents will contain claims that encompass our commercial products. There can be no assurance that any of our intellectual property rights will afford us any protection from competition.
| 154 |
The following patent applications are relevant to the operation of Conduit’s business:
| Related Product Candidate | Mechanism of Action | Patent Information and Number | Patent Ownership/Licensing Status; Patent Status | Jurisdictions Protected | Expiration | |||||
| AZD1656 | Glucokinase Activator | Composition of Matter Patent; 101901 (family number) | Licensed to St George Street Capital from AstraZeneca for use in thyroiditis, uveitis, pre-term labor, renal transplant failure. Granted and In force. | Australia, Brazil, Canada, Switzerland, China, Germany, European Procedure, Spain, France, United Kingdom, Hong Kong, India, Japan, South Korea, Mexico, Netherlands, Russian Federation, Sweden, Turkey, United States | Expires July 3, 2026.
| |||||
| AZD1656 | Glucokinase Activator | Polymorph Patent; 103631 (family number) | Licensed to St George Street Capital from AstraZeneca for use in thyroiditis, uveitis, pre-term labor, renal transplant failure. Granted and In force. |
China and United States | Expires February 2030. | |||||
| AZD1656 | Glucokinase Activator | Co-crystal PCT/IB2022/00075 | Owned by Conduit Pharmaceuticals. Filed September 2, 2022. |
Global | Filing date September 2, 2022. If granted, will expire September 2, 2042.
| |||||
| AZD5904 | MPO Inhibitor | Idiopathic Male Infertility; AZD5904 use patent; 200644 (family number) [WO/2019/016074] |
Licensed to St George Street Capital from AstraZeneca. | International Description | Expires July 12, 2038.
|
We have not filed any application for trademark protection of any names or logos for products or technologies in development. We plan to seek trademark protection inside and outside of the United States where and when appropriate and if available. We intend to use these registered marks in connection with our pharmaceutical research and development, including proprietary technologies, as well as our product candidates.
We expect to seek to protect our products and technologies through a combination of patents, regulatory exclusivity, potentially confidential and proprietary know-how. We intend to actively seek to obtain, where appropriate, the broadest commercially reasonable intellectual property protection possible for our product candidates and technologies, including any future product candidates and technologies under development, our proprietary information, and our proprietary technology through a combination of contractual arrangements and patents, in the United States and abroad. However, we cannot guarantee that patent protection will provide complete protection against competitors who seek to circumvent our patents.
Government Regulation and Product Approval
Government authorities in the United States, at the federal, state and local level, and in other countries, extensively regulate, among other things, the research, development, clinical trials, testing, manufacture, including any manufacturing changes, authorization, pharmacovigilance, adverse event reporting, recalls, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, import and export of pharmaceutical products and product candidates, including clinical assets such as those we are developing. The processes for obtaining regulatory approvals in the United States and in foreign countries, along with subsequent compliance with applicable statutes and regulations have no guaranteed outcomes and require the expenditure of substantial time and financial resources.
Conduit’s development plan for each of AZD1656 and AZD5904 is to conduct clinical trials and if those trials are successful, Conduit will then seek to enter into a transaction with a third party with respect to AZD1656 orAZD5904, as applicable, for the particular indication. Conduit does not intend to continue development of such clinical assets beyond Phase II clinical trials. Accordingly, Conduit anticipates developing clinical assets, which it owns or licenses from third parties, that have undergone pre-clinical and clinical trials through the Phase II stage and then to monetize such clinical assets through a license, royalty or other transaction. Conduit does not expect that it will commercialize any clinical assets or seek marketing approval from the FDA (or similar organizations) as Conduit intends to enter into agreements with third parties following Phase II clinical trials for each such clinical asset that would provide that such third party would pursue the further development, commercialization and marketing of such assets. The following description of the process relating to obtaining regulatory approvals in the United States and in foreign countries is intended for informational purposes only as Conduit does not expect to continue the development of any of the clinical assets beyond the Phase II stage. There is no assurance that any clinical trials on the assets owned or licensed by Conduit will be successful.
United States Government Regulation
In the United States, the U.S. Food and Drug Administration (“FDA”) regulates drugs under the Federal Food, Drug, and Cosmetic Act (“FDCA”) and implementing regulations. Failure to comply with the applicable United States requirements at any time during the product development process, approval process or after approval, may subject an applicant to a variety of administrative or judicial sanctions, such as the FDA’s refusal to approve pending New Drug Applications (“NDAs”), withdrawal of an approval, imposition of a clinical hold, issuance of warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil and/or criminal penalties.
The process required by the FDA before a drug may be marketed in the United States generally involves the following steps, each of which requires the expenditure of substantial time and financial resources:
| ● | completion of preclinical laboratory tests, animal studies and formulation studies in compliance with good laboratory practices (“GLPs”) and other applicable regulations; | |
| ● | submission to the FDA of an Investigational New Drug Application (“IND”), which must become effective before human clinical trials may begin; | |
| ● | approval by an independent institutional review board (“IRB”) at each clinical site before each trial may be initiated; | |
| ● | performance of well-controlled human clinical trials in accordance with good clinical practices (“GCPs”), which may include placebo controls, to establish the safety and efficacy of the proposed drug product for each indication; | |
| ● | submission to the FDA of a New Drug Application (“NDA”) and payment of fees; |
| 155 |
| ● | satisfactory completion of an FDA advisory committee review, if applicable; | |
| ● | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with current good manufacturing practices (“cGMPs”) and to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; | |
| ● | satisfactory completion of audits of clinical trial sites conducted by FDA to assure compliance with GCPs and the integrity of clinical data; and | |
| ● | FDA review and approval of the NDA. |
Preclinical Studies
Preclinical studies include laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies to assess potential safety and efficacy. Preclinical tests intended for submission to the FDA to support the safety of a product candidate must be conducted in compliance with GLP regulations and the U.S. Department of Agriculture’s Animal Welfare Act. A drug sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data and any available ex-U.S. clinical data or relevant literature, among other things, to the FDA as part of an IND. Some nonclinical testing may continue even after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to one or more proposed clinical trials and places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence. A clinical hold may occur at any time during the life of an IND and may affect one or more specific studies or all studies conducted under the IND.
Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an Institutional Review Board (“IRB”) can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug candidate has been associated with unexpected serious harm to patients.
Clinical Trials
Clinical trials involve the administration of the investigational new drug to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include the requirement that all research subjects provide their informed consent in writing for their participation in any clinical trial along with the requirement to ensure that the data and results reported from the clinical trials are credible and accurate. Clinical trials are conducted under protocols detailing, among other things, the objectives of the trial, the criteria for determining subject eligibility, the dosing plan, the parameters to be used in monitoring safety, the procedure for timely reporting of adverse events, and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. In addition, an IRB at each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution.
Information about certain clinical trials and clinical trial results must be submitted within specific timeframes to the National Institutes of Health for public dissemination on the Clinicaltrials.gov registry. Failure to timely register a covered clinical study or to submit study results as provided for in the law can give rise to civil monetary penalties and also prevent the non-compliant party from receiving future grant funds from the federal government. The government has recently begun enforcing these registration and results reporting requirements against non-compliant clinical trial sponsors.
Human clinical trials are typically conducted in at least three sequential phases and occasionally four or more, which may require repetition, or overlap or be combined:
Phase I: The drug candidate is initially introduced into healthy human subjects or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness. During Phase I clinical trials, sufficient information about the investigational drug’s pharmacokinetics and pharmacological effects may be obtained to permit the design of well-controlled and scientifically valid Phase II clinical trials.
Phase II: The drug candidate is administered to a larger, but still limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted indications and to determine dosage tolerance and optimal dosage. Phase II clinical trials are typically well-controlled and closely monitored.
Phase III: The drug candidate is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product. Phase 3III clinical trials usually involve a larger number of participants than a Phase II clinical trial.
There is no guarantee that a product candidate will successfully complete any such clinical trials. There is no assurance that any clinical trials on the assets owned or licensed by Conduit will be successful.
Interactions with FDA During the Clinical Development Program
Following the clearance of an IND and the commencement of clinical trials, the sponsor of such trial will continue to have interactions with the FDA. Progress reports detailing the results of clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. In addition, IND safety reports must be submitted to the FDA for any of the following: serious and unexpected suspected adverse reactions; findings from other studies or animal or in vitro testing that suggest a significant risk in humans exposed to the product; and any clinically important increase in the occurrence of a serious suspected adverse reaction over that listed in the protocol or investigator brochure.
| 156 |
In addition, sponsors are given opportunities to meet with the FDA at certain points in the clinical development program. Specifically, sponsors may meet with the FDA prior to the submission of an IND (“pre-IND meeting”), at the end of Phase II clinical trial (“EOP2” meeting) and before an NDA is submitted (“pre-NDA meeting”). Meetings at other times may also be requested. These meetings provide an opportunity for the sponsor to share information about the data gathered to date with the FDA and for the FDA to provide advice on the next phase of development. For example, at an EOP2, a sponsor may discuss its Phase II clinical results and present its plans for the pivotal Phase III clinical trial(s) that it believes will support the approval of the new product. Such meetings may be conducted in person, via teleconference/videoconference or written response only with minutes reflecting the questions that the sponsor posed to the FDA and the agency’s responses. The FDA has indicated that its responses, as conveyed in meeting minutes and advice letters, only constitute recommendations and/or advice made to a sponsor and, as such, sponsors are not bound by such recommendations and/or advice. Nonetheless, from a practical perspective, a sponsor’s failure to follow the FDA’s recommendations for design of a clinical program may put the program at significant risk of failure.
Acceptance of NDAs
Assuming successful completion of the required clinical testing, the results of the preclinical studies and clinical trials, along with information relating to the product’s chemistry, manufacturing, controls, safety updates, patent information, abuse information and proposed labeling, are submitted to the FDA as part of an application requesting approval to market the product candidate for one or more indications. Data may come from company-sponsored clinical trials intended to test the safety and efficacy of a product’s use or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and efficacy of a drug product. The fee required for the submission and review of an application under the Prescription Drug User Fee Act (“PDUFA”) is substantial, and the sponsor of an approved application is also subject to an annual program fee assessed based on eligible prescription drug products. These fees are typically adjusted annually, and exemptions and waivers may be available under certain circumstances, such as where a waiver is necessary to protect the public health, where the fee would present a significant barrier to innovation, or where the applicant is a small business submitting its first human therapeutic application for review.
The FDA conducts a preliminary review of all applications within 60 days of receipt and must inform the sponsor at that time or before whether an application is sufficiently complete to permit substantive review. In pertinent part, the FDA’s regulations state that an application “shall not be considered as filed until all pertinent information and data have been received” by the FDA. In the event that the FDA determines that an application does not satisfy this standard, it will issue a Refuse to File (“RTF”) determination to the applicant. Typically, an RTF will be based on administrative incompleteness, such as clear omission of information or sections of required information; scientific incompleteness, such as omission of critical data, information or analyses needed to evaluate safety and efficacy or provide adequate directions for use; or inadequate content, presentation, or organization of information such that substantive and meaningful review is precluded. The FDA may request additional information rather than accept an application for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing.
Review of NDAs
After the submission is accepted for filing, the FDA begins an in-depth substantive review of the application. The FDA reviews the application to determine, among other things, whether the proposed product is safe and effective for its intended use, whether it has an acceptable purity profile and whether the product is being manufactured in accordance with cGMP.
Under the goals and policies agreed to by the FDA under PDUFA, the FDA has ten months from the filing date in which to complete its initial review of a standard application that is a new molecular entity, and six months from the filing date for an application with “priority review.” The review process may be extended by the FDA for three additional months to consider new information or in the case of a clarification provided by the applicant to address an outstanding deficiency identified by the FDA following the original submission. Despite these review goals, the NDA review process can be very lengthy, and it is not uncommon for FDA review of an application to extend beyond the PDUFA target action date. Most innovative drug products (other than biological products) obtain FDA marketing approval pursuant to an NDA submitted under Section 505(b)(1) of the FDCA, commonly referred to as a traditional or “full NDA.” In 1984, with passage of the Drug Price Competition and Patent Term Restoration Act, informally known as the Hatch-Waxman Act, that established an abbreviated regulatory scheme authorizing the FDA to approve generic drugs based on an innovator or “reference” product, Congress also enacted Section 505(b)(2) of the FDCA, which provides a hybrid pathway combining features of a traditional NDA and a generic drug application. Section 505(b)(2) enables the applicant to rely, in part, on the FDA’s prior findings of safety and efficacy data for an existing product, or published literature, in support of its application. Section 505(b)(2) NDAs may provide an alternate path to FDA approval for new or improved formulations or new uses of previously approved products that would require new clinical data to demonstrate safety or effectiveness. Section 505(b)(2) permits the filing of an NDA in which the applicant relies, at least in part, on information from studies made to show whether a drug is safe or effective that were not conducted by or for the applicant and for which the applicant has not obtained a right of reference or use. A Section 505(b)(2) applicant may eliminate or reduce the need to conduct certain preclinical or clinical studies, if it can establish that reliance on studies conducted for a previously approved product is scientifically appropriate. The FDA may also require companies to perform additional studies or measurements, including nonclinical and clinical studies, to support the change from the approved product. The FDA may then approve the new product candidate for all or some of the labeled indications for which the referenced product has been approved, as well as for any new indication for which the Section 505(b)(2) NDA applicant has submitted data.
| 157 |
In connection with its review of an application, the FDA will typically submit information requests to the applicant and set deadlines for responses thereto. The FDA will also conduct a pre-approval inspection of the manufacturing facilities for the new product to determine whether the manufacturing processes and facilities comply with cGMPs. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and are adequate to assure consistent production of the product within required specifications.
The FDA also may inspect the sponsor and one or more clinical trial sites to assure compliance with IND and GCP requirements and the integrity of the clinical data submitted to the FDA. To ensure cGMP and GCP compliance by its employees and third-party contractors, an applicant may incur significant expenditure of time, money and effort in the areas of training, record keeping, production and quality control. The FDA generally accepts data from foreign clinical trials in support of an NDA if the trials were conducted under an IND. If a foreign clinical trial is not conducted under an IND, the FDA nevertheless may accept the data in support of an NDA if the study was conducted in accordance with GCPs and the FDA is able to validate the data through an on-site inspection, if deemed necessary. Although the FDA generally requests that marketing applications be supported by some data from domestic clinical trials, the FDA may accept foreign data as the sole basis for marketing approval if (1) the foreign data are applicable to the United States population and United States medical practice, (2) the studies were performed by clinical investigators with recognized competence, and (3) the data may be considered valid without the need for an on-site inspection or, if the FDA considers the inspection to be necessary, the FDA is able to validate the data through an on-site inspection or other appropriate means.
The FDA may also refer an application, including applications for novel product candidates which present difficult questions of safety or efficacy, to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendation of an advisory committee, but it considers such recommendations when making final decisions on approval.
Data from clinical trials are not always conclusive, and the FDA or its advisory committee may interpret data differently than the sponsor interprets the same data. The FDA may also re-analyze the clinical trial data, which could result in extensive discussions between the FDA and the applicant during the review process or delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis or at all.
The FDA also may require submission of a risk evaluation and mitigation strategy (“REMS”) if it determines that a REMS is necessary to ensure that the benefits of the drug product outweigh its risks and to assure the safe use of the product. The REMS could include medication guides, physician communication plans, assessment plans and/or elements to assure safe use, such as restricted distribution methods, patient registries or other risk minimization tools. The FDA determines the requirement for a REMS, as well as the specific REMS provisions, on a case-by-case basis. If the FDA concludes a REMS is needed, the sponsor of the application must submit a proposed REMS and the FDA will not approve the application without a REMS.
Decisions on NDAs
The FDA reviews an applicant to determine, among other things, whether the product is safe and whether it is effective for its intended use(s), with the latter determination being made on the basis of substantial evidence. The term “substantial evidence” is defined under the FDCA as “evidence consisting of adequate and well-controlled investigations, including clinical investigations, by experts qualified by scientific training and experience to evaluate the effectiveness of the product involved, on the basis of which it could fairly and responsibly be concluded by such experts that the product will have the effect it purports or is represented to have under the conditions of use prescribed, recommended, or suggested in the labeling or proposed labeling thereof.”
The FDA has interpreted this evidentiary standard to require at least two adequate and well-controlled clinical investigations to establish effectiveness of a new product. Under certain circumstances, however, the FDA has indicated that a single trial with certain characteristics and additional information may satisfy this standard. This approach was subsequently endorsed by Congress in 1998 with legislation providing, in pertinent part, that “If [the FDA] determines, based on relevant science, that data from one adequate and well-controlled clinical investigation and confirmatory evidence (obtained prior to or after such investigation) are sufficient to establish effectiveness, the FDA may consider such data and evidence to constitute substantial evidence.” This modification to the law recognized the potential for the FDA to find that one adequate and well controlled clinical investigation with confirmatory evidence, including supportive data outside of a controlled trial, is sufficient to establish effectiveness. In December 2019, the FDA issued draft guidance further explaining the studies that are needed to establish substantial evidence of effectiveness. It has not yet finalized that guidance.
| 158 |
After evaluating the application and all related information, including the advisory committee recommendations, if any, and inspection reports of manufacturing facilities and clinical trial sites, the FDA will issue either a Complete Response Letter (“CRL”) or an approval letter. To reach this determination, the FDA must determine that the drug is effective and that its expected benefits outweigh its potential risks to patients. This “benefit-risk” assessment is informed by the extensive body of evidence about the product’s safety and efficacy in the NDA. This assessment is also informed by other factors, including: the severity of the underlying condition and how well patients’ medical needs are addressed by currently available therapies; uncertainty about how the premarket clinical trial evidence will extrapolate to real-world use of the product in the post-market setting; and whether risk management tools are necessary to manage specific risks. In connection with this assessment, the FDA review team will assemble all individual reviews and other documents into an “action package,” which becomes the record for FDA review. The review team then issues a recommendation, and a senior FDA official makes a decision.
A CRL indicates that the review cycle of the application is complete, and the application will not be approved in its present form. A CRL generally outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. The CRL may require additional clinical or other data, additional pivotal Phase III clinical trial(s) and/or other significant and time- consuming requirements related to clinical trials, preclinical studies or manufacturing. If a CRL is issued, the applicant will have one year to respond to the deficiencies identified by the FDA, at which time the FDA can deem the application withdrawn or, in its discretion, grant the applicant an additional six-month extension to respond. The FDA has committed to reviewing resubmissions in response to an issued CRL in either two or six months depending on the type of information included. Even with the submission of this additional information, however, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
An approval letter, on the other hand, authorizes commercial marketing of the product with specific prescribing information for specific indications. That is, the approval will be limited to the conditions of use (e.g., patient population, indication) described in the FDA-approved labeling. Further, depending on the specific risk(s) to be addressed, the FDA may require that contraindications, warnings or precautions be included in the product labeling, require that post-approval trials, including Phase 4 clinical trials, be conducted to further assess a product’s safety after approval, require testing and surveillance programs to monitor the product after commercialization or impose other conditions, including distribution and use restrictions or other risk management mechanisms under a REMS which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-marketing trials or surveillance programs. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Special FDA Expedited Review Programs
The FDA is authorized to designate certain products for expedited development or review if they are intended to address an unmet medical need in the treatment of a serious or life-threatening disease or condition. These programs include fast track designation, breakthrough therapy designation, and priority review designation. The purpose of these programs is to provide important new drugs to patients earlier than under standard FDA review procedures.
To be eligible for a fast-track designation, the FDA must determine, based on the request of a sponsor, that a product is intended to treat a serious or life-threatening disease or condition and demonstrates the potential to address an unmet medical need. The FDA will determine that a product will fill an unmet medical need if it will provide a therapy where none exists or provide a therapy that may be potentially superior to existing therapy based on efficacy or safety factors. Fast track designation provides additional opportunities for interaction with the FDA’s review team and may allow for a rolling review of NDA components before the completed application is submitted, if the sponsor provides a schedule for the submission of the sections of the NDA, the FDA agrees to accept sections of the NDA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the NDA. In addition, fast track designation may be withdrawn by the sponsor or rescinded by the FDA if the designation is no longer supported by data emerging in the clinical trial process.
In addition, with the enactment of the FDA Safety and Innovation Act (“FDASIA”) in 2012, Congress created a new regulatory program for therapeutic candidates designated by FDA as “breakthrough therapies” upon a request made by the IND sponsors. A breakthrough therapy is defined as a drug that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA must take certain actions with respect to breakthrough therapies, such as holding timely meetings with and providing advice to the product sponsor, intended to expedite the development and review of an application for approval of a breakthrough therapy.
Finally, the FDA may designate a product for priority review if it is a drug that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. The FDA determines at the time that the marketing application is submitted, on a case-by-case basis, whether the proposed drug represents a significant improvement in treatment, prevention or diagnosis of disease when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment-limiting drug reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes, or evidence of safety and effectiveness in a new subpopulation. A priority review designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA’s goal for taking action on a marketing application from ten months to six months for an NDA for a new molecular entity from the date of filing.
| 159 |
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened. Furthermore, fast track designation, breakthrough therapy designation and priority review do not change the standards for approval and may not ultimately expedite the development or approval process.
Accelerated Approval Pathway
In addition, a product studied for its safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval, meaning that it may be approved on (i) the basis of adequate and well-controlled clinical trials establishing that the drug product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or (ii) on an intermediate clinical endpoint that can be measured earlier than irreversible morbidity or mortality (“IMM”) and that is reasonably likely to predict an effect on IMM or other clinical benefits, taking into account the severity, rarity or prevalence of the condition and the availability or lack of alternative treatments. As a condition of approval, the FDA may require a sponsor of a drug receiving accelerated approval to perform post-marketing studies to verify and describe the predicted effect on IMM or other clinical endpoints, and the drug may be subject to expedited withdrawal procedures. Drugs granted accelerated approval must meet the same statutory standards for safety and effectiveness as those granted traditional approval.
The accelerated approval pathway is usually contingent on a sponsor’s agreement to conduct, in a diligent manner, additional post-approval confirmatory studies to verify and describe the drug’s clinical benefit. As a result, a therapeutic candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or to confirm the predicted clinical benefit of the product during post-marketing studies, would allow the FDA to withdraw approval of the drug. All promotional materials for drug products being considered and approved under the accelerated approval program are subject to prior review by the FDA. Lawmakers, FDA officials, and other stakeholders have recently been evaluating the accelerated approval program and have proposed potential reforms to improve certain aspects. Scrutiny of the accelerated approval pathway is likely to continue and may lead to legislative and/or administrative changes in the future.
Post-Approval Requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. Certain modifications to the product, including changes in indications or manufacturing processes or facilities, may require the applicant to develop additional data or conduct additional preclinical studies and clinical trials to support the submission to FDA. As previously noted, there also are continuing, annual user fee requirements for any marketed products, as well as new application fees for supplemental applications with clinical data.
The FDA may impose a number of post-approval requirements as a condition of approval of an NDA. For example, the FDA may require post-marketing testing, including Phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization.
In addition, FDA regulations require that products be manufactured in specific approved facilities and in accordance with cGMPs. The cGMP regulations include requirements relating to the organization of personnel, buildings and facilities, equipment, control of components and drug product containers and closures, production and process controls, packaging and labeling controls, holding and distribution, laboratory controls, records and reports and returned or salvaged products. Drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and some state agencies and are subject to periodic unannounced inspections by the FDA for compliance with cGMP requirements and other laws. Changes to the manufacturing process are strictly regulated and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers. Accordingly, manufacturers must continue to expend time, money, and effort in production and quality control to maintain compliance with cGMP and other aspects of quality control and quality assurance.
The FDA strictly regulates the marketing, labeling, advertising and promotion of drug products that are placed on the market. A product cannot be commercially promoted before it is approved, and approved drugs may generally be promoted only for their approved indications and for use in patient populations described in the product’s approved labeling. Promotional claims must also be consistent with the product’s FDA-approved label, including claims related to safety and effectiveness. The government closely scrutinizes the promotion of prescription drugs in specific contexts such as direct-to-consumer advertising, industry-sponsored scientific and educational activities, and promotional activities involving the Internet and social media. Although physicians may prescribe legally available products for off-label uses, manufacturers may not market or promote such uses.
| 160 |
Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in mandatory revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences of regulatory non-compliance include, among other things:
| ● | restrictions on, or suspensions of, the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; | |
| ● | interruption of production processes, including the shutdown of manufacturing facilities or production lines or the imposition of new manufacturing requirements; | |
| ● | fines, warning letters or other enforcement letters or clinical holds on post-approval clinical trials; | |
| ● | mandated modification of promotional materials and labeling and the issuance of corrective information; | |
| ● | refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product approvals; | |
| ● | product seizure or detention, or refusal to permit the import or export of products; | |
| ● | injunctions or the imposition of civil or criminal penalties; or | |
| ● | consent decrees, corporate integrity agreements, debarment, or exclusion from federal healthcare programs. |
In addition, the distribution of prescription pharmaceutical products is subject to the Prescription Drug Marketing Act (“PDMA”) which regulates the distribution of drugs and drug samples at the federal level and sets minimum standards for the registration and regulation of drug distributors by the states. Both the PDMA and state laws limit the distribution of prescription pharmaceutical product samples and impose requirements to ensure accountability in distribution. More recently, the Drug Supply Chain Security Act (the “DSCSA”), was enacted with the aim of building an electronic system to identify and trace certain prescription drugs distributed in the United States. The DSCSA mandates phased-in and resource-intensive obligations for pharmaceutical manufacturers, wholesale distributors, and dispensers over a 10-year period that is expected to culminate in November 2023. From time to time, new legislation and regulations may be implemented that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA. For example, FDA released proposed regulations in February 2022 to amend the national standards for licensing of wholesale drug distributors by the states; establish new minimum standards for state licensing third-party logistics providers; and create a federal system for licensure for use in the absence of a state program, each of which is mandated by the DSCSA. It is impossible to predict whether further legislative or regulatory changes will be enacted, or FDA regulations, guidance or interpretations will be changed or what the impact of such potential changes, if any, may be.
Regulatory Exclusivity and Approval of Follow-on Products
Hatch-Waxman Exclusivity
In addition to enacting Section 505(b)(2) of the FDCA as part of the Hatch-Waxman Amendments to the FDCA, Congress also established an abbreviated regulatory scheme authorizing the FDA to approve generic drugs that are shown to contain the same active ingredients as, and to be bioequivalent to, drugs previously approved by the FDA pursuant to NDAs. To obtain approval of a generic drug, an applicant must submit an abbreviated new drug application (“ANDA”) to the agency. An ANDA is a comprehensive submission that contains, among other things, data and information pertaining to the active pharmaceutical ingredient, bioequivalence, drug product formulation, specifications and stability of the generic drug, as well as analytical methods, manufacturing process validation data and quality control procedures. ANDAs are “abbreviated” because they cannot include preclinical and clinical data to demonstrate safety and effectiveness. Instead, in support of such applications, a generic manufacturer must rely on the preclinical and clinical testing previously conducted for a drug product previously approved under an NDA, known as the reference listed drug (“RLD”).
In order for an ANDA to be approved, the FDA must find that the generic version is identical to the RLD with respect to the active ingredients, the route of administration, the dosage form, the strength of the drug and the conditions of use of the drug. At the same time, the FDA must also determine that the generic drug is “bioequivalent” to the innovator drug. Under the statute, a generic drug is bioequivalent to an RLD if “the rate and extent of absorption of the drug do not show a significant difference from the rate and extent of absorption of the listed drug.” Unlike the 505(b)(2) NDA pathway that permits a follow-on applicant to conduct and submit data from additional clinical trials or nonclinical studies in order to support the proposed change(s) to the reference product, the ANDA regulatory pathway does not allow applicants to submit new clinical data other than bioavailability or bioequivalence data.
| 161 |
Upon approval of an ANDA, the FDA indicates whether the generic product is “therapeutically equivalent” to the RLD in its publication “Approved Drug Products with Therapeutic Equivalence Evaluations,” also referred to as the “Orange Book.” Physicians and pharmacists consider a therapeutic equivalent generic drug to be fully substitutable for the RLD. In addition, by operation of certain state laws and numerous health insurance programs, the FDA’s designation of therapeutic equivalence often results in substitution of the generic drug without the knowledge or consent of either the prescribing physician or patient.
As part of the NDA review and approval process, applicants are required to list with the FDA each patent that has claims that cover the applicant’s product or method of therapeutic use. Upon approval of a new drug, each of the patents listed in the application for the drug is then published in the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential follow-on competitors in support of approval of an ANDA or 505(b)(2) NDA.
When an ANDA applicant submits its application to the FDA, it is required to certify to the FDA concerning any patents listed for the reference product in the FDA’s Orange Book. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. Moreover, to the extent that the Section 505(b)(2) NDA applicant is relying on studies conducted for an already approved product, the applicant also is required to certify to the FDA concerning any patents listed for the NDA-approved product in the Orange Book to the same extent that an ANDA applicant would.
If the follow-on applicant does not challenge the innovator’s listed patents, the FDA will not approve the ANDA or 505(b)(2) application until all the listed patents claiming the referenced product have expired. A certification that the new product will not infringe the already approved product’s listed patents, or that such patents are invalid, is called a Paragraph IV certification. If the follow-on applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA or 505(b)(2) NDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit, or a decision in the infringement case that is favorable to the ANDA or 505(b)(2) applicant.
An ANDA or 505(b)(2) application also will not be approved until any applicable non-patent exclusivities listed in the Orange Book for the referenced product have expired. The Hatch-Waxman Amendments to the FDCA provided a five-year period of non-patent data exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity (“NCE”). For the purposes of this provision, an NCE is a drug that contains no active moiety that has previously been approved by the FDA in any other NDA. An active moiety is the molecule or ion responsible for the physiological or pharmacological action of the drug substance. In cases where such NCE exclusivity has been granted, an ANDA or 505(b)(2) NDA may not be filed with the FDA until the expiration of five years unless the submission is accompanied by a Paragraph IV certification, in which case the applicant may submit its application four years following the original product approval.
The FDCA also provides for a period of three years of data exclusivity if an NDA or NDA supplement includes reports of one or more new clinical investigations, other than bioavailability or bioequivalence studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application. This three-year exclusivity period often protects changes to a previously approved drug product, such as new indications, dosage forms, route of administration or combination of ingredients. Three-year exclusivity would be available for a drug product that contains a previously approved active moiety, provided the statutory requirement for a new clinical investigation is satisfied. Unlike five-year NCE exclusivity, an award of three-year exclusivity does not block the FDA from accepting ANDAs or 505(b)(2) NDAs seeking approval for generic versions of the drug as of the date of approval of the original drug product; rather, this three-year exclusivity covers only the conditions of use associated with the new clinical investigations and, as a general matter, does not prohibit the FDA from approving follow-on applications for drugs containing the original active ingredient.
Five-year and three-year exclusivity also will not delay the submission or approval of a traditional NDA filed under Section 505(b)(1) of the FDCA; however, an applicant submitting a traditional NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Orphan Drug Designation and Exclusivity
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects either (i) fewer than 200,000 individuals in the United States, or (ii) more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for this type of disease or condition will be recovered from sales in the United States for that drug. Legislative proposals are currently being considered that would revise or revoke the second option available for a drug candidate to receive an orphan designation, the so-called “cost recovery” pathway. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use will be disclosed publicly by the FDA; the posting will also indicate whether a drug is no longer designated as an orphan drug.
| 162 |
More than one product candidate may receive an orphan drug designation for the same indication, and the same product candidate can be designated for more than one qualified orphan indication. The benefits of orphan drug designation include research and development tax credits and exemption from FDA prescription drug user fees. Orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process if or when an NDA for the drug candidate is filed.
If a product that has orphan drug designation subsequently receives the first FDA approval for the indication for which it has such designation, the product is entitled to orphan product exclusivity, which means that for seven years, the FDA may not approve any other marketing applications for the same drug for the same indication, except under limited circumstances described further below. Orphan exclusivity does not block the approval of a different drug for the same rare disease or condition, nor does it block the approval of the same drug for different conditions. As a result, the FDA can still approve different drugs for use in treating the same indication or disease. Additionally, if a drug designated as an orphan product receives marketing approval for an indication broader than what was designated, it may not be entitled to orphan drug exclusivity.
Orphan exclusivity will not bar approval of another product with the same drug for the same condition under certain circumstances, including if a subsequent product with the same drug for the same condition is shown to be clinically superior to the approved product on the basis of greater efficacy or safety or a major contribution to patient care, or if the company with orphan drug exclusivity cannot assure the availability of sufficient quantities of the drug to meet the needs of persons with the disease or condition for which the drug was designated. The FDA is now required to publish a summary of the clinical superiority findings when a drug is eligible for orphan product exclusivity on the basis of a demonstration of clinical superiority.
Patent Term Extension
A patent claiming a prescription drug for which FDA approval is granted may be eligible for a limited patent term extension under the FDCA, which permits a patent restoration of up to five years for patent term lost during product development and the FDA regulatory review provided that certain statutory and regulatory requirements are met. The length of the patent term extension is related to the length of time the drug is under regulatory review while the patent is in force. The restoration period granted on a patent covering a new FDA-regulated medical product is typically one-half the time between the date a clinical investigation on human beings is begun and the submission date of an application for premarket approval of the product, plus the time between the submission date of an application for approval of the product and the ultimate approval date. Patent term restoration cannot be used to extend the remaining term of a patent past a total of 14 years from the product’s approval date. Only one patent applicable to an approved drug product is eligible for the extension, and the application for the extension must be submitted prior to the expiration of the patent in question. A patent that covers multiple products for which approval is sought can only be extended in connection with one of the marketing approvals. The USPTO reviews and approves the application for any patent term extension or restoration in consultation with the FDA.
Other U.S. Healthcare Laws and Regulations
Manufacturing, sales, promotion and other activities following product approval may also be subject to regulation by other regulatory authorities in the United States in addition to the FDA. Depending on the nature of the product, those authorities may include the Centers for Medicare and Medicaid Services (“CMS”), other divisions of the Department of Health and Human Services (“HHS”), the Department of Justice, the Drug Enforcement Administration, the Federal Trade Commission, the Occupational Safety and Health Administration, and state and local governments.
For example, in the United States, sales and marketing for prescription biopharmaceutical products must comply with state and federal fraud and abuse laws. These laws include the federal Anti-Kickback Statute, which makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf), to knowingly and willfully solicit, receive, offer or pay any remuneration that is intended to induce or reward referrals, including the purchase, recommendation, order or prescription of a particular drug, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. Violations of this law are punishable by up to ten years in prison, criminal fines, administrative civil money penalties and exclusion from participation in federal healthcare programs. In addition, the Patient Protection and Affordable Care Act, or ACA, among other things, amended the intent requirement of the federal Anti-Kickback Statute and two of the five criminal healthcare fraud statutes created by the Health Insurance Portability and Accountability Act of 1996, or HIPAA. A person or entity no longer needs to have actual knowledge of these two provisions in the statute or specific intent to violate them; specifically with respect to the prohibition on executing or attempting to execute a scheme or artifice to defraud or to fraudulently obtain money or property of any healthcare benefit program and the prohibition on disposing of assets to enable a person to become eligible for Medicaid. Moreover, the government may now assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
| 163 |
Pricing and rebate programs must comply with the Medicaid rebate requirements of the U.S. Omnibus Budget Reconciliation Act of 1990 and more recent requirements in the ACA. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. There also are federal transparency requirements under the Physician Payments Sunshine Act that require manufacturers of FDA-approved drugs, devices, biologics and medical supplies covered by Medicare or Medicaid to report, on an annual basis, to CMS information related to payments and other transfers of value to physicians, teaching hospitals, and certain advanced non-physician healthcare practitioners and physician ownership and investment interests. Prescription drug products also must meet applicable child-resistant packaging requirements under the U.S. Poison Prevention Packaging Act.
Manufacturing, sales, promotion and other activities also are potentially subject to federal and state consumer protection and unfair competition laws. Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines, or the relevant compliance guidance promulgated by the federal government, in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures to the extent that those laws impose requirements that are more stringent than the Physician Payments Sunshine Act. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
The failure to comply with any of these laws or regulatory requirements subjects firms to possible legal or regulatory action. Depending on the circumstances, failure to meet applicable regulatory requirements can result in criminal prosecution, fines or other penalties, injunctions, requests for recall, seizure of products, total or partial suspension of production, denial or withdrawal of product approvals or refusal to allow a firm to enter into supply contracts, including government contracts.
Government Regulation Outside the U.S.
In addition to regulations in the United States, we will be subject to a variety of foreign regulations that govern, among other things, clinical trials and any commercial sales and distribution of our products, if approved, either directly or through distribution partners. Whether or not we obtain FDA approval for a product candidate, we must obtain the requisite approvals from regulatory authorities in foreign countries or economic areas, such as the European Union and the United Kingdom, among other foreign countries, before we may commence clinical trials or market products in those countries or areas. The foreign regulatory approval process includes all of the risks associated with the FDA approval described above, and the time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Some foreign jurisdictions have a drug product approval process similar to that in the U.S., which requires the submission of a clinical trial application much like the IND prior to the commencement of clinical studies. In Europe, for example, a clinical trial application, or CTA, must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed. To obtain regulatory approval of a medicinal product candidate under European Union regulatory systems, we would be required to submit a Marketing Authorisation Application, or MAA, which is similar to the NDA, except that, among other things, there are country-specific document requirements. For countries outside of the European Union, such as countries in Eastern Europe, Latin America or Asia, and recently the United Kingdom, the requirements governing the conduct of clinical trials, product approval, pricing and reimbursement vary from country to country. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others. Moreover, some nations may not accept clinical studies performed for U.S. approval to support approval in their countries or require that additional studies be performed on natives of their countries. In addition, in certain foreign markets, the pricing of drug products is subject to government control and reimbursement may in some cases be unavailable or insufficient. If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions, and criminal prosecution.
As of January 31, 2020, the United Kingdom is no longer a member state of the European Union, and therefore a separate marketing authorization application and approval will be required to market a medicinal product in the U.K. The Medicines and Healthcare products Regulatory Agency, or the MHRA, is the U.K.’s standalone pharmaceutical regulator.
Clinical Trials and Regulation of Medicinal Products in Europe
As in the United States, medicinal products can be marketed in the European Union only if a marketing authorization from the competent regulatory agencies has been obtained. Similar to the United States, the various phases of preclinical and clinical research in the European Union are subject to significant regulatory controls.
| 164 |
Pursuant to the European Clinical Trials Directive, a system for the approval of clinical trials in the European Union has been implemented through national legislation of the member states. Under this system, an applicant must obtain approval from the competent national authority of an European Union member state in which the clinical trial is to be conducted. Furthermore, the applicant may only start a clinical trial after a competent ethics committee has issued a favorable opinion. Clinical trial applications must be accompanied by an investigational medicinal product dossier with supporting information prescribed by the European Clinical Trials Directive and corresponding national laws of the member states and further detailed in applicable guidance documents. In April 2014, the new Clinical Trials Regulation, (EU) No 536/2014 (Clinical Trials Regulation) was adopted and became effective on January 31, 2022. The Clinical Trials Regulation is directly applicable in all the European Union Member States, repealing the prior Clinical Trials Directive 2001/20/EC. The extent to which ongoing clinical trials will be governed by the Clinical Trials Regulation will depend on the duration of the individual clinical trial; if a clinical trial continues for more than three years from the day on which the Clinical Trials Regulation becomes applicable the Clinical Trials Regulation will at that time begin to apply to the clinical trial.
The new Clinical Trials Regulation aims to simplify and streamline the approval of clinical trials in the European Union. The main characteristics of the regulation include: a streamlined application procedure via a single entry point; a single set of documents to be prepared and submitted for the application as well as simplified reporting procedures for clinical trial sponsors; and a harmonized procedure for the assessment of applications for clinical trials.
To obtain marketing approval of a drug in the European Union, an applicant must submit a MAA either under a centralized or decentralized procedure. The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid for all European Union member states, Iceland, Lichtenstein and Norway. The centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy products (such as gene-therapy, somatic cell-therapy or tissue-engineered medicines) and products with a new active substance indicated for the treatment of certain diseases. For products with a new active substance indicated for the treatment of certain diseases and products that are highly innovative or for which a centralized process is in the interest of patients, the centralized procedure may be optional. Under the centralized procedure the maximum timeframe for the evaluation of an MAA by the European Medicines Agency (“EMA”) is 210 days, excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the Committee for Medicinal Products for Human Use (“CHMP”). Accelerated assessment might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest, particularly from the point of view of therapeutic innovation. The timeframe for the evaluation of an MAA under the accelerated assessment procedure is of 150 days, excluding stop-clocks.
The decentralized procedure is available to applicants who wish to market a product in specific European Union member states where such product has not received marketing approval in any European Union member states before. The decentralized procedure provides for an applicant to apply to one-member state to assess the application (the reference member state) and specifically list other member states in which it wishes to obtain approval (concerned member states).
In the European Union, only products for which marketing authorizations have been granted may be promoted. A marketing authorization is valid for five years in principle and the marketing authorization may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the authorizing member state. To this end, the marketing authorization holder must provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variations introduced since the marketing authorization was granted, at least six months before the marketing authorization ceases to be valid. Once renewed, the marketing authorization is valid for an unlimited period, unless the European Commission or the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal. Any authorization which is not followed by the actual placing of the drug on the European Union market (in case of centralized procedure) or on the market of the authorizing member state within three years after authorization ceases to be valid (the so-called sunset clause).
Moreover, even if authorized to be marketed in the European Union, prescription medicines may only be promoted to healthcare professionals, not the general public. All promotion should be in accordance with the particulars listed in the summary of product characteristics. Promotional materials must also comply with various laws, and codes of conduct developed by pharmaceutical industry bodies in the European Union which govern (among other things) the training of sales staff, promotional claims and their justification, comparative advertising, misleading advertising, endorsements, and (where permitted) advertising to the general public. Failure to comply with these requirements could lead to the imposition of penalties by the competent authorities of the European Union member states. The penalties could include warnings, orders to discontinue the promotion of the drug product, seizure of promotional materials, fines and possible imprisonment.
Regulation of New Drugs in the United Kingdom
The United Kingdom left the European Union on January 31, 2020 (commonly referred to as “Brexit”), with a transitional period that expired on December 31, 2020. The United Kingdom and the European Union entered into a trade agreement known as the Trade and Cooperation Agreement, which went into effect on January 1, 2021. It remains to be seen how, if at all, Brexit and the Trade and Cooperation Agreement will impact regulatory requirements for product candidates and products in the United Kingdom. We are currently evaluating the potential impacts on our business of the Trade and Cooperation Agreement and guidance issued to date by the United Kingdom’s MHRA regarding the requirements for licensing and marketing medicinal products in the United Kingdom.
| 165 |
Since the regulatory framework for pharmaceutical products in the United Kingdom covering the quality, safety and efficacy of pharmaceutical products, clinical trials, marketing authorization, commercial sales and distribution of medicinal products is derived from EU Directives and Regulations, Brexit could materially impact the future regulatory regime which applies to such products and the approval of product candidates in the United Kingdom. Such outcomes could make it more difficult and expensive for us to do business in Europe, complicate our clinical, manufacturing and regulatory strategies and impair our ability to obtain and maintain regulatory approval for, and, if approved, commercialize, our products and product candidates in Europe.
Pharmaceutical Coverage, Pricing and Reimbursement & Healthcare Reform
Sales of our products, if approved for marketing, will depend, in part, on the availability and extent of coverage and reimbursement by third-party payors, such as government health programs, including Medicare and Medicaid, commercial insurance and managed healthcare organizations. These third-party payors are increasingly challenging the price and limiting the coverage and reimbursement amounts for medical products and services. There may be significant delays in obtaining coverage and reimbursement for approved products, and coverage may be more limited than the purposes for which the product is approved by the FDA or regulatory authorities in other countries. It is time-consuming and expensive to seek reimbursement from third-party payors. Moreover, eligibility for reimbursement does not imply that any product will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim payments for new products, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Payment rates may vary according to the use of the product and the clinical setting in which it is used, may be based on payments allowed for lower-cost products that are already reimbursed and may be incorporated into existing payments for other services. Net prices for products may be reduced by mandatory discounts or rebates required by third-party payors and by any future relaxation of laws that presently restrict imports of products from countries where they may be sold at lower prices than in the United States. In the United States, third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies, but they also have their own methods and approval process apart from Medicare coverage and reimbursement determinations. Accordingly, one third-party payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage for the product.
In addition, the containment of healthcare costs has become a priority for federal and state governments, and the prices of drugs have been a focus in this effort. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on coverage and reimbursement, and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Decreases in third-party reimbursement for our product candidates or a decision by a third-party payor to not cover our product candidates could reduce physician usage of the product candidate and have a material adverse effect on our sales, results of operations and financial condition. Moreover, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. Individual states in the United States have also increasingly passed legislation and implemented regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In December 2020, the U.S. Supreme Court held unanimously that federal law does not preempt the states’ ability to regulate pharmaceutical benefit managers (“PBMs”) and other members of the healthcare and pharmaceutical supply chain, an important decision that has led to further and more aggressive efforts by states in this area.
Most recently, on August 16, 2022, President Biden signed into the law the Inflation Reduction Act of 2022, or the IRA. Among other things, the IRA has multiple provisions that may impact the prices of drug products that are both sold into the Medicare program and throughout the United States. Starting in 2023, a manufacturer of drugs covered by Medicare Parts B or D must pay a rebate to the federal government if their drug product’s price increases faster than the rate of inflation. This calculation is made on a drug product by drug product basis and the amount of the rebate owed to the federal government is directly dependent on the volume of a drug product that is paid for by Medicare Parts B or D. Additionally, starting for payment year 2026, CMS will negotiate drug prices annually for a select number of single source Part D drugs without generic or biosimilar competition. CMS will also negotiate drug prices for a select number of Part B drugs starting for payment year 2028. If a drug product is selected by CMS for negotiation, it is expected that the revenue generated from such drug will decrease.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, in the European Union, the sole legal instrument at the European Union level governing the pricing and reimbursement of medicinal products is Council Directive 89/105/EEC (the “Price Transparency Directive”). The aim of the Price Transparency Directive is to ensure that pricing and reimbursement mechanisms established in the European Union Member States are transparent and objective, do not hinder the free movement of and trade in medicinal products in the European Union, and do not hinder, prevent or distort competition on the market. The Price Transparency Directive does not provide any guidance concerning the specific criteria on the basis of which pricing and reimbursement decisions are to be made in the individual European Union Member States, nor does it have any direct consequence for pricing or reimbursement levels in the individual European Union Member States. The European Union Member States are free to restrict the range of medicinal products for which their national health insurance systems provide reimbursement, and to control the prices and/or reimbursement levels of medicinal products for human use. A European Union Member State may approve a specific price or level of reimbursement for the medicinal product, or alternatively adopt a system of direct or indirect controls on the profitability of the company responsible for placing the medicinal product on the market, including volume-based arrangements, caps and reference pricing mechanisms.
| 166 |
Health Technology Assessment (“HTA”) of medicinal products is becoming an increasingly common part of the pricing and reimbursement procedures in some European Union Member States, including France, Germany, Ireland, Italy and Sweden. The HTA process in the European Union Member States is governed by the national laws of these countries. HTA is the procedure according to which the assessment of the public health impact, therapeutic impact, and the economic and societal impact of the use of a given medicinal product in the national healthcare systems of the individual country is conducted. HTA generally focuses on the clinical efficacy and effectiveness, safety, cost, and cost-effectiveness of individual medicinal products as well as their potential implications for the healthcare system. Those elements of medicinal products are compared with other treatment options available on the market. The outcome of HTA regarding specific medicinal products will often influence the pricing and reimbursement status granted to these medicinal products by the competent authorities of individual European Union Member States. The extent to which pricing and reimbursement decisions are influenced by the HTA of the specific medicinal product vary between the European Union Member States. For example, European Union Member States that have not yet developed HTA mechanisms could rely to some extent on the HTA performed in countries with a developed HTA framework when adopting decisions concerning the pricing and reimbursement of a specific medicinal product.
Separately from cost containment efforts, in the United States and some foreign jurisdictions, there also have been, and continue to be, several legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of product candidates or restrict or regulate post-approval activities. The FDA’s and other regulatory authorities’ policies may change, and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our current or future product candidates.
Data Privacy and the Protection of Personal Information
We are subject to laws and regulations governing data privacy and the protection of personal information including health information. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing focus on privacy and data protection issues which will continue to affect our business. In the United States, we may be subject to state security breach notification laws, state laws protecting the privacy of health and personal information and federal and state consumer protections laws that regulate the collection, use, disclosure and transmission of personal information. These laws overlap and often conflict and each of these laws is subject to varying interpretations by courts and government agencies, creating complex compliance issues. If we fail to comply with applicable laws and regulations we could be subject to penalties or sanctions, including criminal penalties. Our customers and research partners must comply with laws governing the privacy and security of health information, including HIPAA and state health information privacy laws. If we knowingly obtain health information that is protected under HIPAA, called “protected health information,” our customers or research collaborators may be subject to enforcement, and we may have direct liability for the unlawful receipt of protected health information or for aiding and abetting a HIPAA violation.
State laws protecting health and personal information are becoming increasingly stringent. For example, California has implemented the California Confidentiality of Medical Information Act that imposes restrictive requirements regulating the use and disclosure of health information and other personally identifiable information, and California has recently adopted the California Consumer Privacy Act of 2018 (“CCPA”). The CCPA mirrors a number of the key provisions of the EU General Data Protection Regulation (“GDPR”) described below. The CCPA establishes a new privacy framework for covered businesses by creating an expanded definition of personal information, establishing new data privacy rights for consumers in the State of California, imposing special rules on the collection of consumer data from minors, and creating a new and potentially severe statutory damages framework for violations of the CCPA and for businesses that fail to implement reasonable security procedures and practices to prevent data breaches. Since passage of the CCPA, several other states (Connecticut, Colorado, Virginia, and Utah) have also enacted comprehensive consumer privacy laws that include key differences from California’s law, further complicating compliance by industry and other stakeholders. Other states in the U.S. are considering privacy laws similar to the CCPA.
In Europe, the GDPR went into effect in May 2018, implementing a broad data protection framework that expanded the scope of European Union data protection law, including to non- European Union entities that process, or control the processing of, personal data relating to individuals located in the European Union, including clinical trial data. The GDPR sets out a number of requirements that must be complied with when handling the personal data of European Union-based data subjects including: providing expanded disclosures about how their personal data will be used; higher standards for organizations to demonstrate that they have obtained valid consent or have another legal basis in place to justify their data processing activities; the obligation to appoint data protection officers in certain circumstances; new rights for individuals to be “forgotten” and rights to data portability, as well as enhanced current rights (e.g. access requests); the principal of accountability and demonstrating compliance through policies, procedures, training and audit; and a new mandatory data breach regime. In particular, medical or health data, genetic data and biometric data where the latter is used to uniquely identify an individual are all classified as “special category” data under the GDPR and afforded greater protection and require additional compliance obligations. Further, European Union member states have a broad right to impose additional conditions – including restrictions – on these data categories. This is because the GDPR allows European Union member states to derogate from the requirements of the GDPR mainly in regard to specific processing situations (including special category data and processing for scientific or statistical purposes). As the European Union states continue to reframe their national legislation to harmonize with the GDPR, we will need to monitor compliance with all relevant European Union member states’ laws and regulations, including where permitted derogations from the GDPR are introduced. We will also be subject to evolving European Union laws on data export, if we transfer data outside the European Union to ourselves or third parties outside of the European Union.
| 167 |
The Cayman Islands Government enacted the Data Protection Act on May 18, 2017 (as amended, the “DPA”). The DPA regulates the processing of personal data in the Cayman Islands. Under the DPA, Conduit is a “data controller” and Conduit’s affiliates and/or its delegates may be “data processors” (or, in some circumstances, data controllers in their own right), in respect of such personal data.
U.S. Foreign Corrupt Practices Act and Anti-bribery Regulations
In general, the Foreign Corrupt Practices Act of 1977, as amended, or the FCPA, prohibits offering to pay, paying, promising to pay, or authorizing the payment of money or anything of value to a foreign official in order to influence any act or decision of the foreign official in his or her official capacity or to secure any other improper advantage in order to obtain or retain business for or with, or in order to direct business to, any person. The prohibitions apply not only to payments made to “any foreign official,” but also those made to “any foreign political party or official thereof,” to “any candidate for foreign political office” or to any person, while knowing that all or a portion of the payment will be offered, given, or promised to anyone in any of the foregoing categories. “Foreign officials” under the FCPA include officers or employees of a department, agency, or instrumentality of a foreign government. The term “instrumentality” is broad and can include state-owned or state-controlled entities. Importantly, United States authorities deem most healthcare professionals and other employees of foreign hospitals, clinics, research facilities and medical schools in countries with public healthcare and/or public education systems to be “foreign officials” under the FCPA. When we interact with foreign healthcare professionals and researchers in testing and marketing our products abroad, should any of our product candidates receive foreign regulatory approval in the future, we must have policies and procedures in place sufficient to prevent us and agents acting on our behalf from providing any bribe, gift or gratuity, including excessive or lavish meals, travel or entertainment in connection with marketing our products and services or securing required permits and approvals. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring us to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
We are also subject to U.K. Bribery Act of 2010, which prohibits both domestic and international bribery, as well as bribery across both private and public sectors. In addition, an organization that “fails to prevent bribery” committed by anyone associated with the organization can be charged under the U.K. Bribery Act unless the organization can establish the defense of having implemented “adequate procedures” to prevent bribery. As we expand our operations, we are likely to be subject to additional laws and restrictions relating to anti-bribery.
Environmental, Health and Safety Regulation
We are subject to numerous federal, state and local environmental, health and safety (“EHS”) laws and regulations relating to, among other matters, safe working conditions, product stewardship, environmental protection, and handling or disposition of products, including those governing the generation, storage, handling, use, transportation, release, and disposal of hazardous or potentially hazardous materials, medical waste, and infectious materials that may be handled by our partner research laboratories. Some of these laws and regulations also require us to obtain licenses or permits to conduct our operations. If we fail to comply with such laws or obtain and comply with the applicable permits, we could face substantial fines or possible revocation of our permits or limitations on our ability to conduct our operations. Certain of our development and manufacturing activities may involve, from time to time, use of hazardous materials, and we believe we are in compliance with the applicable environmental laws, regulations, permits, and licenses. However, we cannot ensure that EHS liabilities will not develop in the future. EHS laws and regulations are complex, change frequently and have tended to become more stringent over time. Although the costs to comply with applicable laws and regulations, have not been material, we cannot predict the impact on our business of new or amended laws or regulations or any changes in the way existing and future laws and regulations are interpreted or enforced, nor can we ensure we will be able to obtain or maintain any required licenses or permits.
Properties
We currently operate as a virtual company and do not own or lease any real property. Instead, our employees work remotely. We anticipate that we will need to enter into one or more leases for office space in both the United Kingdom and United States in connection with the anticipated expansion of our staff.
Employees
We currently expect to have a total of 16 full or part-time employees on completion of the Business Combination.
We currently rely on several consultants who provide services to our Company. None of our employees are represented by a labor union or covered by collective bargaining agreements. We consider our relationship with our employees to be good. We anticipate that the number of employees will increase as we continue to develop the assets in our pipeline and other product candidates that we seek to develop. Additionally, we utilize and expect to continue to utilize clinical research organizations and third parties to perform our pre-clinical studies, clinical studies and manufacturing.
Legal Proceedings
We are not currently party to or aware of being subject to any material legal proceedings. However, we may from time to time become a party to various legal proceedings arising in the ordinary course of our business, which could have a material adverse effect on our business, financial condition or results of operations. Regardless of outcome, litigation could impact our business due to defense and settlement costs, diversion of management resources and other factors.
Corporate Information
Conduit Pharmaceuticals Limited was incorporated under the laws of the Cayman Islands in 2019. Our registered office is currently located at c/o Ogier Global (Cayman) Limited 89 Nexus Way, Camana Bay Grand Cayman, KY1-9009 Cayman Islands. Our website can be found at https://www.conduitpharma.com. Following the Business Combination, our principal executive offices will be located at MURF’s current principal executive offices, which are located at 4995 Murphy Canyon Road, Suite 300, San Diego, CA 92123.
| 168 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS OF CONDUIT PHARMACEUTICALS LIMITED
You should read the following discussion and analysis of our financial condition and results of operations with our audited financial statements for the year ended December 31, 2022, together with related notes thereto, and unaudited financial statements for the nine months ended March 31, 2023, together with related notes thereto, included elsewhere in this proxy statement/prospectus. The discussion and the analysis should also be read together with the section of this proxy statement/prospectus entitled “Business of Conduit Pharmaceuticals Limited” and the unaudited pro forma combined financial information as of and for the nine months ended March 31, 2023, and for the year ended December 31, 2022 (in the section of this proxy statement/prospectus entitled “Summary Unaudited Pro Forma Condensed Combined Financial Information”). The following discussion contains forward-looking statements based upon current expectations that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under the section titled “Risk factors” or in other parts of this proxy statement/prospectus. Our historical results are not necessarily indicative of the results that may be expected for any period in the future. In this section, unless otherwise specified, the terms “we”, “our”, “us” and “Conduit” refer to Conduit Pharmaceuticals Limited. All dollar amounts are expressed in thousands of United States dollars (“$”), unless otherwise indicated.
Overview
Conduit Pharmaceuticals Limited formerly SGS Global Limited (“Conduit” or the “Company”) was incorporated in the Cayman Islands as an Exempted Company with Limited Liability in December 2018.
We are a clinical-stage specialty biopharmaceutical company that was formed to facilitate the development and commercialization of clinical assets that have not been, or are not being, prioritized by leading biopharmaceutical companies in order to develop pharmaceutical products that meet the unmet medical needs of patients.
We are led by highly experienced pharma executives, Dr. Freda Lewis-Hall, former Chief Medical Officer of Pfizer Inc., and Dr. David Tapolczay, former Chief Executive Officer of the United Kingdom-based medical research charity LifeArc.
Our current development pipeline includes a glucokinase activator, which is Phase II ready in autoimmune diseases including uveitis, Hashimoto’s Thyroiditis, preterm labor and renal transplant rejection. Our development pipeline also includes a potent, irreversible inhibitor of human Myeloperoxidase (MPO) that has the potential to treat idiopathic male infertility.
We have an exclusive relationship and partnership with St George Street Capital (“SGSC”), a biomedical charity based in the United Kingdom. We fund the development of clinical assets that are initially licensed by SGSC from its existing relationship with AstraZeneca AB (PUBL). We conduct clinical trials on the clinical assets that we license from AstraZeneca in collaboration with SGSC. In doing so, we are able to leverage the comprehensive clinical and scientific expertise in order to develop assets through Phase IIb trials in an efficient manner. We expect that successful Phase IIb trials on the assets in our pipeline will increase the value of the assets. There is no assurance that any clinical trials on the assets owned or licensed by Conduit will be successful.
Following potentially successful clinical trials, of which there can be no assurance that the outcomes of such trials will be successful, we expect to commercialize the clinical assets in our pipeline through licensing arrangements with larger pharmaceuticals companies, which may include up-front milestone payments and/or royalties during the time at which such clinical asset is subject to patents.
We intend to use the income received from licensing our clinical assets to fund the development of additional clinical assets. This will allow us to use the existing income stream from clinical assets that have been licenses to fund our on-going operations without having to rely solely on debt and/or equity financing. We initially intend to focus on assets that have been deprioritized by larger pharmaceuticals companies and that have a high unmet medical need in order to develop these assets through the Phase IIb stage. We also believe that we may be able to apply our business strategy and approach to arrangements to pharmaceutical companies in addition to AstraZeneca.
We have incurred significant operating losses since inception. For the three months ended March 31, 2023 and 2022, our net loss was $2.2 million and $0.3 million, respectively. For the year ended December 31, 2022 and 2021, our net loss was $4.9 million and $3.7 million, respectively and we expect to continue to incur significant losses for the foreseeable future as we continue to invest in a number of research and development programs. As of March 31, 2023 and December 31, 2022, we had an accumulated deficit of $12.9 million and $10.8 million, respectively.
The Merger Agreement and PIPE Financing
After a comprehensive review of strategic alternatives, including identifying and reviewing potential candidates for a strategic transaction, on November 8, 2022, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) by and among Conduit, Murphy Canyon Acquisition Corp (“MURF”), and Conduit Merger Sub, Inc., a Cayman Islands exempted company and a wholly-owned subsidiary of MURF (“Merger Sub”), which, among other things, provides for the merger of Merger Sub with and into Conduit, with Conduit surviving the merger as a wholly-owned subsidiary of MURF (the “Business Combination”). Following the Business Combination, MURF will be renamed “Conduit Pharmaceuticals Inc.” and will continue as a public company and a registrant with the SEC (“New Conduit”). Conduit and MURF believe that the Business Combination and related proceeds will result in the ability for combined business, through New Conduit, to access the capital markets, increased exposure for the clinical assets that Conduit is currently developing, and an enhanced ability to attract collaboration, partnering and/or license arrangements as more information will be readily available about New Conduit.
| 169 |
The Business Combination will be accounted for a reverse recapitalization in accordance with U.S. GAAP. Under the reverse recapitalization method, MURF will be treated as the acquired company for financial reporting purposes, and Conduit, the accounting acquirer, will be assumed to have issued shares for the net assets of MURF, with no goodwill or other intangible assets recorded. This determination is primarily based on the following predominant factors: (i) post-closing, our shareholders are expected to have a majority of the voting power of the combined company and ability to elect the members of the combined company Board of Directors; (ii) the on-going operations post-merger will comprise those of Conduit; and (iii) all of the senior management of the combined company except for the Chief Financial Officer will be members of the management of Conduit. The board of directors of each of MURF and Conduit have approved the Business Combination. The completion of the Business Combination, which is expected to occur in the third quarter of 2023, is subject to approval of the shareholders of Conduit and the stockholders of MURF and the satisfaction or waiver of certain other customary closing conditions.
Following the consummation of the Business Combination and the closing of the PIPE Financing, the most significant change in our future reported financial position and result of operations is expected to be an estimated increase in cash (as compared to our balance sheet as of March 31, 2023) to approximately $14.5 million, assuming the Maximum Redemption Scenario, or to $141.5 million, assuming the No Redemption Scenario, including $27.0 million in gross proceeds from the PIPE Financing. Total direct transaction costs of MURF and Conduit are estimated at approximately $18.1 million, substantially all of which will be recorded as a reduction to additional paid-in-capital as costs related to the reverse recapitalization. For additional information refer to the section of this proxy statement/prospectus titled “Unaudited Pro Forma Condensed Combined Financial Information.”
Upon the closing of the Business Combination, MURF will change its name to “Conduit Pharmaceuticals Inc.” which we refer to as New Conduit and New Conduit will be an SEC registrant and its common stock and Public Warrants will be listed on the Nasdaq Stock Market (“Nasdaq”) under the proposed symbols “CDT” and “CDTTW”, respectively. As a public company, New Conduit will need to hire additional personnel and implement procedures and processes to address applicable regulatory requirements and customary practices. We expect that New Conduit will incur additional annual expenses as a public company for, among other things, directors’ and officers’ liability insurance, director fees and additional internal and external accounting, legal and administrative resources, including increased audit, legal and filing fees.
Impact of COVID-19, the Russia and Ukraine Conflict, and Global Economic Conditions
As a result of the spread of the COVID-19 pandemic, economic uncertainties have arisen which may negatively affect our financial position, results of operations and cash flows. We have assessed that the COVID-19 pandemic has not so far had a material or direct impact on our operations or financial position. Nevertheless, in light of the ongoing COVID-19 pandemic, we have implemented measures to protect employees and take social responsibilities while at the same time attempting to limit any negative effects on our business.
The full impact of the COVID-19 pandemic continues to evolve as of the date of this proxy statement/prospectus. As such, the full magnitude of the pandemic’s effect on our financial condition, liquidity and future results of operations is uncertain. Management continues to actively monitor our financial condition, liquidity, operations, suppliers, industry and workforce. See “Risk Factors—Risks Related to Conduit’s Business and Industry—We may in the future be adversely affected by continuation or worsening of the global COVID-19 pandemic, its various strains or future pandemics.”
Due to the pandemic, all clinical trials in the United Kingdom that were not related to COVID-19 were put on hiatus for significant portions of the year ended December 31, 2021. As a result, during the hiatus, the Company shifted its activities to focus on clinical trials related to COVID-19. The hiatus on clinical trials not related to COVID-19 was lifted at the end of the year ended December 31, 2021.
The conflict between Russia and Ukraine has caused major macroeconomic disruptions that have impacted the global trade and economies. As such increasing inflation around the globe has forced national banks to increase their interest rates, consequently impacting interest yields around the globe. We have assessed the impact of these measures and concluded that as of today, no material impact has been identified on our business or our ability to continue as a going concern.
Key Component of Result of Operations
Operating Expenses
Research and Development Expenses
Research and development expenses consist primarily of costs incurred in connection with the research and development of our product candidates and programs. We expense research and development costs and intangible assets acquired that have no alternative future use as incurred. These expenses include:
| ● | personnel-related expenses, including salaries, bonuses, benefits and stock-based compensation for employees engaged in research and development functions; |
| ● | expenses incurred in connection with the clinical development and regulatory approval of our product candidates, including under agreements with third parties, such as consultants, contractors and CROs; |
| 170 |
| ● | license fees with no alternative use; and |
| ● | other expenses related to research and development. |
We expense research and development costs as incurred. Advance payments that we make for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses. The prepaid amounts are expensed as the benefits are consumed.
To date, we do not track our research and development expenses on a program-by-program basis as we only worked on one program related to COVID-19 treatment. Moving forward, we do not expect further research and development expense for clinical research into COVID-19 as we explore broader applications of our research to date. Our direct external research and development expenses consist primarily of external costs, such as fees paid to outside consultants, CROs, CMOs and research laboratories in connection with our preclinical development, process development, manufacturing and clinical development activities. We do not allocate employee costs, costs associated with our discovery efforts, laboratory supplies, and facilities, including depreciation or other indirect costs, to specific programs because these costs are deployed across multiple programs and, as such, are not separately classified. We use internal resources primarily to conduct its research and discovery as well as for managing its preclinical development, process development, manufacturing and clinical development activities. These employees work across multiple programs and, therefore, we do not track their costs by program.
Research and development activities have historically been central to our business model. We anticipate that our research and development expenses will increase for the foreseeable future in connection with our planned clinical development activities.
At this time, we cannot reasonably estimate or know the nature, timing and costs of the efforts that would be necessary to complete the preclinical and clinical development of any of our product candidates or when, if ever, material net cash inflows may commence from any of our product candidates. The successful development and commercialization of any of our product candidates is highly uncertain. This uncertainty is due to the numerous risks and uncertainties associated with product development and commercialization, including the uncertainty of the following:
| ● | the scope, progress, timing, outcome and costs of any continued preclinical development activities, clinical trials and other related development activities; | |
| ● | delays, suspensions, or other setbacks or interruptions encountered, including as a result of the ongoing COVID-19 pandemic; | |
| ● | successful patient enrollment in and the initiation and completion of any clinical trials; | |
| ● | the timing, receipt and terms of any marketing approvals from applicable regulatory authorities including the U.S. Food and Drug Administration (“FDA”) and non-U.S. regulatory authorities; | |
| ● | the extent of any required post-marketing approval commitments to applicable regulatory authorities; | |
| ● | establishing clinical and commercial manufacturing capabilities or making arrangements with third-party manufacturers in order to ensure that us or our third-party manufacturers are able to make and scale our products successfully; | |
| ● | development and timely delivery of clinical-grade and commercial-grade drug formulations that can be used in Gemini’s clinical trials and for commercial launch; | |
| ● | obtaining, maintaining, defending and enforcing patent claims and other intellectual property rights; | |
| ● | significant and changing government regulation; | |
| ● | launching commercial sales of Gemini’s product candidates, if and when approved, whether alone or in collaboration with others; and | |
| ● | maintaining a continued acceptable safety profile of Gemini’s product candidates following approval, if any, of Gemini’s product candidates. |
A change in any of these variables with respect to any of Gemini’s programs would significantly change the costs, timing and viability associated with that program.
General and Administrative Expenses
General and administrative expenses consist of salaries and other related costs, legal fees relating to intellectual property and corporate matters, professional fees for accounting, auditing, tax and consulting services, insurance costs, travel, and other operating costs.
| 171 |
We anticipate that our general and administrative expenses will increase substantially for the foreseeable future as we increase our administrative headcount to operate as a public company and as we advance product candidates through clinical development. We also will incur additional expenses as a result of operating as a public company, including expenses related to compliance with the rules and regulations of the SEC and the Nasdaq listing rules, additional insurance expenses, investor relations activities and other administrative and professional services. In addition, if regulatory approval is obtained for product candidates, we expect to incur expenses associated with building a sales and marketing team.
Other Expense
Other expense consists of proceeds from the sale of equity securities, unrealized foreign currency transaction loss, loss on the change in fair value of convertible notes, write-off of long-term debt- related party, donations to a related party and commission on the placement of equity securities.
Results of Operations
The following table set forth our results of operations for the periods indicated:
| Three months ended March 31, | Year ended December 31, | |||||||||||||||
| (In thousands, except share and per share data) | 2023 | 2022 | 2022 | 2021 | ||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development expenses | $ | - | $ | - | $ | 37 | $ | 28 | ||||||||
| General and administrative expenses | 2,008 | 291 | 3,049 | 2,890 | ||||||||||||
| Funding expenses | - | - | 74 | 255 | ||||||||||||
| Total operating expenses | 2,008 | 291 | 3,160 | 3,173 | ||||||||||||
| Loss from operations | (2,008 | ) | (291 | ) | (3,160 | ) | (3,173 | ) | ||||||||
| Other expense: | ||||||||||||||||
| Other expense, net | (157 | ) | (16 | ) | (1,727 | ) | (484 | ) | ||||||||
| Total other expense, net | (157 | ) | (307 | ) | (1,727 | ) | (484 | ) | ||||||||
| Net loss | $ | (2,165 | ) | $ | (307 | ) | $ | (4,887 | ) | $ | (3,657 | ) | ||||
| Foreign currency translation adjustment | (263 | ) | 140 | 740 | 79 | |||||||||||
| Net comprehensive loss | $ | (2,428 | ) | $ | (167 | ) | $ | (4,147 | ) | $ | (3,578 | ) | ||||
Comparison of the Three Months Ended March 31, 2023 and 2022
General and administrative expenses
| Three months ended March 31, | Change | |||||||||||||||
| (In thousands) | 2023 | 2022 | Amount | % | ||||||||||||
| General and administrative expenses | $ | 2,008 | $ | 291 | $ | 1,716 | 590 | % | ||||||||
General and administrative expenses increased by $1.7 million, or 590%, to $2.0 million for the three months ended March 31, 2023, as compared to $0.3 million for the three months ended March 31, 2022. The increase was primarily driven by an increase of $0.7 million in listing fees, $0.3 million change in the fair value of convertible notes, $0.2 million allowance for uncollectible loans receivable, $0.2 million in audit fees and $0.3 million for discretionary directors bonuses paid.
Other expense, net
| Three months ended March 31, | Change | |||||||||||||||
| (In thousands) | 2023 | 2022 | Amount | % | ||||||||||||
| Other expense, net | $ | (157 | ) | $ | (16 | ) | $ | (141 | ) | 907 | % | |||||
Other expense, net increased by $0.1 million, or 874%, to $0.2 million for the three months ended March 31, 2023, as compared to $16 thousand for the three months ended March 31, 2022. The increase was primarily driven by an increase by a loss of $0.3 million on the change in fair value of convertible notes, partially offset by a gain of $0.1 on the change in fair value of the Cizzle option and $48 thousand decrease attributable to the loss on sale of investment in equity securities.
For further details refer to Note 11 – Other Expense, net in the unaudited financial statements as of March 31, 2023 and December 31, 2022 and for the three months ended March 31, 2023 and 2022 included elsewhere in this proxy statement/prospectus.
| 172 |
Comparison of the Years Ended December 31, 2022 and 2021
Research and development expenses
| Year ended December 31, | Change | |||||||||||||||
| (In thousands) | 2022 | 2021 | Amount | % | ||||||||||||
| Research and development expenses | $ | 37 | $ | 28 | $ | 9 | 32 | % | ||||||||
Research and development expenses increased by $9,000, or 100%, to $37,000 for the year ended December 31, 2022, as compared to $28,000 for the year ended December 31, 2021. The increase was primarily driven by expenses incurred for our clinical research into COVID-19 treatment during the year ended December 31, 2022.
General and administrative expenses
| Year ended December 31, | Change | |||||||||||||||
| (In thousands) | 2022 | 2021 | Amount | % | ||||||||||||
| General and administrative expenses | $ | 3,049 | $ | 2,890 | $ | 159 | 6 | % | ||||||||
General and administrative expenses increased by $0.2 million, or 6%, to $3.0 million for the year ended December 31, 2022, as compared to $2.9 million for the year ended December 31, 2021. The increase was primarily driven by an increase of $0.3 million in the reserve for loans receivable, partially offset by a decrease of $0.1 million in legal fees.
Funding expenses
| Year ended December 31, | Change | |||||||||||||||
| (In thousands) | 2022 | 2021 | Amount | % | ||||||||||||
| Funding expenses | $ | 74 | $ | 255 | $ | (181 | ) | (71 | )% | |||||||
Funding expenses decreased by $0.2 million, or 71%, to $0.1 million for the year ended December 31, 2022, as compared to $0.3 million for the year ended December 31, 2021. The decrease was primarily driven by a decrease of $0.2 million in funding requirements from SGSC for research and development expenses incurred which we agreed to fund.
Other expense, net
| Year ended December 31, | Change | |||||||||||||||
| (In thousands) | 2022 | 2021 | Amount | % | ||||||||||||
| Other expense | $ | (1,727 | ) | $ | (484 | ) | $ | (1,243 | ) | 257 | % | |||||
Other expense increased by $1.2 million, or 257%, to other expense of $1.7 million for the year ended December 31, 2022, as compared to other expense, net for $0.5 million for the year ended December 31, 2021. The decrease was driven primarily by an increase of $1.3 million on the loss incurred through the issuance of the Option to Cizzle and a $0.2 million increase in the loss on the change in fair value of convertible notes payable, partially offset by a decrease in placement fees on the sale of equity securities of $0.3 million.
For further details refer to Note 11 – Other Expense in the audited financial statements as of and for the year ended December 31, 2022 and 2021 included elsewhere in this proxy statement/prospectus.
Liquidity and Capital Resources
Management assesses liquidity in terms of our ability to generate cash to fund operating, investing and financing activities. Since our inception, and in line with our growth strategy, we have prepared our financial statements assuming we will continue as a going concern. Since our inception, we have incurred net losses and experienced negative cash flows from operations. To date, our primary sources of capital have been through private placements of equity securities and convertible debt. During the three months ended March 31, 2023 and 2022, we had net losses of $2.2 million and $0.2 million, respectively. During the years ended December 31, 2022 and 2021, we incurred net losses of $4.9 million and $3.7 million, respectively. We expect to incur additional losses and higher operating expenses for the foreseeable future as we continue to invest in research and development programs. We have determined that additional financing will be required to fund our operations for the next 12 months and our ability to continue as a going concern is dependent upon obtaining additional capital and financing, including through the consummation of the Business Combination. In the fourth quarter of 2022 the Company approved approximately $3 million of Convertible Notes, of which approximately $1.8 million was issued and funded in full.
| 173 |
Sources and Uses of Liquidity
Our primary uses of cash are to fund our operations as we continue to grow our business. We will require a significant amount of cash for expenditures as we invest in ongoing research and development and business operations. Until such time as we can generate significant revenue from commercialization of our product, we expect to finance our cash needs for ongoing research and development and business operations through public or private equity or debt financings or other capital sources, including strategic partnerships. However, we may be unable to raise additional funds or enter into such other arrangements, when needed, on favorable terms or at all. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our shareholders will be, or could be, diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common shareholders. Debt financing and equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, or substantially reduce research and development efforts. While the Company believes in the viability of its ability to raise additional funds, there can be no assurances to that effect. There is currently no public market for the Company’s ordinary shares. These matters raise substantial doubt about the Company’s ability to continue as a going concern for a period of twelve months from the issue date of this report. These financial statements have been prepared assuming the Company will continue as a going concern and do not include adjustments to reflect the possible effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
Cash Requirements
Our material cash requirements include the following contractual and other obligations.
2021 Convertible Notes
Between June 2021 and October 2022 we issued an aggregate principal amount of $1.2 million of convertible notes to various investors.
The convertible notes issuable under the 2021 Convertible Loan Note Instrument mature three years after issuance to the respective noteholders and bear 5% interest, only to be paid to the noteholders in the event we effect a material breach. In the event of a Change of Control, the convertible notes issued under the 2021 Convertible Loan Note Instrument automatically convert into ordinary shares of our common stock. We may prepay the convertible notes payable without penalty. The convertible notes are general, unsecured obligations.
2022 Convertible Notes
Between November 2022 and February 2023 we issued an aggregate principal amount of $1.8 million of convertible notes to various investors and a related party.
The convertible notes issuable under the 2022 Convertible Loan Note Instrument mature three years after issuance to the respective noteholders and bear 5% interest, only to be paid to the noteholders in the event we effect a material breach. In the event of a Change of Control, the convertible notes issued under the 2021 Convertible Loan Note Instrument automatically convert into ordinary shares of our common stock. We may prepay the convertible notes payable without penalty. The convertible notes are general, unsecured obligations.
Promissory Convertible Note
In March 2023, we issued an aggregate principal amount of $0.8 million convertible promissory note payable to an investor.
The promissory convertible note matures and is payable in full 18 months from the date of the note. The note carries 20% interest and is payable every six (6) months from the date of the note until the maturity date. The note is subject to conversion of MURF common stock upon consummation of the Business Combination prior to the maturity date.
As of March 31, 2023 the aggregate principal amount of our convertible notes was $3.8 million. For additional information regarding our convertible notes, see Notes 2 and 5 of the notes to the unaudited financial statements.
Capital Expenditures
We currently anticipate that cash required for capital expenditures for the next 12 months is approximately $6.9 million, which includes accrued expenses of $2.4 million and a note payable of $0.2 million that matures within the next 12 months. We do not anticipate being able to fund required capital expenditures for the next 12 months with cash and cash equivalents on hand as we have a history of limited cash on hand. Management believes that we will be able to fund cash required for the next 12 months through the issuance of existing convertible loan note instruments and the issuance of new convertible loan note instruments. We have historically been able to access funds through the issuance of our convertible notes and believe we can continue to obtain funding through these debt financing agreements as needed to meet cash requirements for the next 12 months.
Our future capital requirements and the adequacy of available funds will depend on many factors, including those set forth in the section titled “Risk Factors—Risks Related to Conduit’s Business and Industry.”
| 174 |
Cash Flows
The following table set forth our cash flows for the period indicated (in thousands):
| Three Months Ended March 31, | Year Ended December 31, | |||||||||||||||
| 2023 | 2022 | 2022 | 2021 | |||||||||||||
| Net cash (used in) provided by: | ||||||||||||||||
| Operating Activities | $ | (1,969 | ) | $ | (788 | ) | $ | (2,266 | ) | $ | (2,151 | ) | ||||
| Investing Activities | (243 | ) | - | (183 | ) | - | ||||||||||
| Financing Activities | 2,220 | 789 | 2,448 | 1,898 | ||||||||||||
| Effect of exchange rate changes on cash and cash equivalents | - | 1 | 1 | 1 | ||||||||||||
| Net (decrease) increase in cash and cash equivalents | $ | 8 | $ | 2 | $ | - | $ | (252 | ) | |||||||
Cash Flows (Used) Provided by Operating Activities
Net cash used in operating activities for the three months ended March 31, 2023 was $2.0 million, resulting primarily from a net loss of $2.2 million, adjusted for non-cash charges of $0.4 million and working capital adjustments of $0.2 million.
Net cash used in operating activities for the three months ended March 31, 2022 was $0.8 million, resulting primarily from a net loss of $0.3 million, working capital adjustments of $0.5 million.
Net cash used in operating activities during the year ended December 31, 2023 was $2.3 million, resulting primarily from a net loss of $4.9 million, adjusted for non-cash charges of $2.0 million and working capital adjustments of $0.6 million.
Net cash used in operating activities during the year ended December 31, 2022 was $2.2 million, resulting primarily from a net loss of $3.7 million, adjusted for non-cash charges of $0.1 million and working capital adjustments of $1.4 million.
Cash Flows (Used) Provided by Investing Activities
Net cash used in investing activities for the three months ended March 31, 2023 was $0.2 million resulting from the issuance of a loan to a related party of $0.2 million.
Net cash used in investing activities for the year ended December 31, 2022 was $0.2 million resulting from the issuance of a loan to a related party of $0.3 million, partially offset by an option fee received from Cizzle of $0.1 million .
Cash Flows (Used) Provided by Financing Activities
Net cash provided by financing activities for the three months ended March 31, 2023 was $2.2 million, resulting from the issuance of our convertible debt of $1.4 million and proceeds from the issuance of a promissory convertible note payable of $0.8 million.
Net cash provided by financing activities for the three months ended March 31, 2022 was $0.8 million resulting from the sale equity securities received from the sale of future revenue of $0.8 million.
Net cash provided by financing activities during the year ended December 31, 2022 was $2.4 million. resulting from the proceeds from the sale of shares received for the sale of future revenue of $1.3 million, proceeds from notes payable of $0.2 million and the issuance of our convertible debt of $0.9 million.
Net cash provided by financing activities during the year ended December 31, 2021 was $1.9 million, resulting from the proceeds from the sale of shares received for the sale of future revenue of $1.2 million and the issuance of our convertible debt of $0.7 million.
Contractual Obligations and Other Commitments
As of March 31, 2023, we had no non-cancellable commitments for the purchase of clinical materials, contract manufacturing, maintenance and committed funding which we expect to pay within one year.
Critical Accounting Policies and Significant Judgments
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which we have prepared in accordance with accounting principles generally accepted in the United States. The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and related disclosures of contingent assets and liabilities at the date of the financial statements as well as the reported amounts of revenues and expenses during the reporting period. Estimates are based on several factors including the facts and circumstances available at the time the estimates are made, historical experience, risk of loss, general economic conditions and trends and the assessment of the probable future outcome. Subjective and significant estimates include, but are not limited to, research and development accruals. These estimates and assumptions are most significant where they involve levels of subjectivity and judgment necessary to account for highly uncertain matters or matters susceptible to change, and where they can have a material impact on our financial statements and operating performance. Actual results could differ from those estimates. Estimates and assumptions are reviewed periodically and the effects of changes, if any, are reflected in the statements of operations in the period that they are determined.
| 175 |
We believe that the accounting policies described below involve a significant degree of judgment and complexity. Accordingly, we believe these are the most critical to aid in fully understanding and evaluating our financial condition and results of operations. For further information, refer to Note 1 “Nature of the Business and Basis of Presentation and Summary of Significant Accounting Policies” to our financial statements included elsewhere in this proxy statement/prospectus.
Fair Value Measurements
Accounting Standards Codification (“ASC”) Topic 820, Fair Value Measurements and Disclosures, defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements. Fair value is to be determined based on the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. In determining fair value, the Company used various valuation approaches. A fair value hierarchy has been established for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are those that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Company.
Unobservable inputs reflect the Company’s assumption about the inputs that market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. The fair value hierarchy is categorized into three levels, based on the inputs, as follows:
| ● | Level 1—Valuations based on quoted prices for identical instruments in active markets. Since valuations are based on quoted prices that are readily and regularly available in an active market, valuation of these instruments does not entail a significant degree of judgment. | |
| ● | Level 2— Valuations based on observable inputs other than quoted prices included in Level 1, such as quoted prices for either similar instruments in active markets, identical or similar instruments in markets that are not active, or model-derived valuations whose inputs or significant value drivers are observable or can be corroborated by observable market data. | |
| ● | Level 3—Valuations based on inputs that are unobservable. These valuations require significant judgment. |
The Company’s Level 1 assets consist of cash and cash equivalents in the accompanying balance sheets and the value of accrued expenses and other current liabilities approximate fair value due to the short-term nature of these assets and liabilities.
As of March 31, 2023, the Company has two financial liabilities, a convertible note and liability for the Cizzle Option (the “Option”) that are adjusted to fair value on a recurring basis for which the fair value is determined based on Level 3 inputs as such inputs are not readily observable. For further information, refer to Notes 2 “Fair Value”, 4”Liability Related to the Sale of Future Revenue” and 5 “Convertible Notes Payable” to our financial statements included elsewhere in this proxy statement/prospectus.
Investments in Equity Securities
Investments in the equity securities of Cizzle that have a readily determinable fair value are reported at fair value. Changes in fair value between accounting periods are recorded in other income, net, in the statements of operations and comprehensive loss. Realized gains or losses upon sale are recorded in other income, net, in the statements of operations and comprehensive loss. We assess the fair value of our equity securities periodically, a large fluctuation in the fair value of the shares may lead to a material adjustment of our investment in equity securities.
Cizzle Option Agreement
We account for the Cizzle Option at fair value in order to measure the liability at an amount that more accurately reflects the current economic environment in which we operate. We recorded the convertible notes at fair value with changes in fair value recorded in earnings at each reporting period through settlement. The significant assumptions used to estimate the fair value of the option liability involved inherent uncertainties and the application of significant judgment and included the time to maturity and the underlying asset price based on the probability of the Covid Asset successfully moving from Phase I to Phase II. The sensitivity of these inputs to the fair value of the convertible notes is assessed on a periodic basis.
The fair value of the option liability was estimated using the Black-Scholes-Merton Model, where the value of the Cizzle option was estimated based on an analysis of six inputs. Valuation models require the input of highly subjective assumptions, including the expected volatility of the underlying asset. If any of the assumptions used in the Black-Scholes-Merton Model changes significantly, the option liability may differ materially from that recorded in the current period.
Fair Value Option for Convertible Notes
We elected to account for our convertible notes at fair value in order to measure those liabilities at amounts that more accurately reflect the current economic environment in which we operate. We recorded the convertible notes at fair value with changes in fair value recorded in earnings at each reporting period through settlement. The fair value of the convertible notes was determined using a probability-weighted income approach as the convertible notes contained various settlement outcomes. The significant assumptions used to estimate the fair value of the convertible notes involved inherent uncertainties and the application of significant judgment, and included the time to maturity and the probability of the various settlement outcomes. The sensitivity of these inputs to the fair value of the convertible notes is assessed on a periodic basis.
| 176 |
Fair values of the derivative liabilities related to the convertible notes were estimated using a probability-weighted expected return method, where the values of various instruments were estimated based on an analysis of future values of our business, assuming various future outcomes. The resulting instruments’ values were based upon the probability-weighted present value of expected future investment returns, considering each of the possible future outcomes available to us, as well as the economic benefits attributable to each class of instruments. The expected future investment returns were estimated using a variety of methodologies, including both the market approach and the income approach, where an observable quoted market does not exist, and were generally classified as Level 3. Such methodologies included reviewing values ascribed to our most recent financing, comparing the subject instrument with similar instruments of publicly traded companies in similar lines of business, and reviewing our underlying financial performance and subject instrument, including estimating discounted cash flows. If any of the assumptions used in the probability-weighted expected return method changes significantly, the convertible notes may differ materially from that recorded in the current period.
Functional Currency
Transaction amounts denominated in foreign currencies are translated into their U.S. dollar equivalents at exchange rates prevailing at the transaction dates. Foreign currency gains and losses on transactions or settlements are recognized in the statement of loss and comprehensive loss. Our functional currency of is the British pound sterling. Assets and liabilities are translated at the period end foreign exchange rate and revenue and expenses are translated at the average rate for the period.
The financial statements are translated into U.S. dollars with assets and liabilities translated at the current rate on the financial statements date and revenue and expense items translated at the average rates for the period. Translation adjustments are recorded as accumulated other comprehensive loss in shareholders’ deficit.
Recent Accounting Pronouncements
A discussion of recent accounting pronouncements is included in Note 1 - Nature of the Business and Basis of Presentation and Summary of Significant Accounting Policies to our financial statements included elsewhere in this proxy statement/ prospectus.
Qualitative and quantitative disclosures about market risk
We are exposed to market risk related to changes in interest rates. As of March 31, 2023, we had cash of approximately $8,000. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates. Due to the low interest rates available on cash investments and the immaterial amount of cash as of March 31, 2023, we believe an immediate 10% change in interest rates would not have a material effect on our operating results until maturity, and therefore, we would not expect our operating results or cash flows to be affected to any significant degree by the effect of a change in market interest rates on our investment portfolio.
We are currently exposed to market risk related to changes in foreign currency exchange rates; however, we do contract with vendors that are located primarily in the United Kingdom and may be subject to fluctuations in foreign currency rates. We may enter into additional contracts with vendors located outside the United States in the future, which may increase our foreign currency exchange risk. We do not believe that foreign currency risk had a material effect on our business, financial condition, or results of operations during the periods presented.
We do not believe that inflation had a significant impact on our results of operations for any periods presented in our financial statements. Nonetheless, if our costs were to become subject to significant inflationary pressures, we may not be able to fully offset such higher costs, and our inability or failure to do so could harm our business, financial condition and results of operations.
Emerging Growth Company Status and Smaller Reporting Company Status
In April 2012, the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” may take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. Therefore, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. New Conduit has elected to avail itself of this extended transition period and, as a result, New Conduit will not adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies. In addition, as an emerging growth company, New Conduit may take advantage of certain reduced disclosure and other requirements that are otherwise applicable generally to public companies. New Conduit will take advantage of these exemptions until such earlier time that it is no longer an emerging growth company. New Conduit would cease to be an emerging growth company on the date that is the earliest of (i) the last day of the fiscal year following the fifth anniversary of the date of the completion of this offering; (ii) the last day of the fiscal year in which its total annual gross revenue is equal to or more than $1.235 billion; (iii) the date on which it has issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the date on which it is deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission.
In addition, New Conduit will be a smaller reporting company as defined in the Exchange Act. New Conduit may continue to be a smaller reporting company even after we are no longer an emerging growth company. New Conduit may take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as (i) New Conduit’s voting and non-voting common stock held by non-affiliates is less than $250.0 million measured on the last business day of our second fiscal quarter or (ii) New Conduit’s annual revenue is less than $100.0 million during the most recently completed fiscal year and its voting and non-voting common stock held by non-affiliates is less than $700.0 million measured on the last business day of its second fiscal quarter.
| 177 |
BENEFICIAL OWNERSHIP OF SECURITIES
The following table sets forth information regarding the beneficial ownership regarding: (i) the actual beneficial ownership of MURF common stock as of July 31, 2023; (ii) the actual beneficial ownership of Conduit ordinary shares as of July 31, 2023; and (iii) expected beneficial ownership of the common stock of New Conduit immediately following the closing of the Business Combination, assuming that no holder of MURF’s Public Shares exercises redemption rights as described in this proxy statement/prospectus, by:
| ● | each of MURF’s current executive officers and directors; | |
| ● | MURF’s current executive officers and directors as a group; | |
| ● | each of Conduit’s current executive officers and directors; | |
| ● | Conduit’s current executive officers and directors as a group; | |
| ● | each person who will become an executive officer or a director of New Conduit upon consummation of the Business Combination; | |
| ● | all of the executive officers and directors of New Conduit as a group after the consummation of the Business Combination; | |
| ● | each person known to be the beneficial owner of more than 5% of the outstanding shares of MURF common stock as of July 31, 2023; | |
| ● | each person known to be the beneficial owner of more than 5% of Conduit ordinary shares as of July 31, 2023; and | |
| ● | each person who may become the beneficial owner of more than 5% of outstanding shares of New Conduit common stock immediately following the Business Combination. |
Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power over that security. Under those rules, beneficial ownership includes securities that the individual or entity has the right to acquire, such as through the exercise of warrants or stock options or the vesting of restricted stock units, within 60 days of the closing of the Business Combination for purposes of the calculations set forth below. Shares subject to warrants or options that are currently exercisable or exercisable within 60 days of the closing of the Business Combination or subject to restricted stock units that vest within 60 days of the closing of the Business Combination are considered outstanding and beneficially owned by the person holding such warrants, options or restricted stock units for the purpose of computing the percentage ownership of that person but are not treated as outstanding for the purpose of computing the percentage ownership of any other person. As of the closing of the Business Combination, New Conduit is not expected to have any options that will be exercisable within 60 days or restricted stock units that vest within 60 days.
The beneficial ownership of shares of MURF common stock prior to the Business Combination is based on 6,247,978 shares of common stock of MURF (consisting of 2,941,728 shares of Class A common stock and 3,306,250 shares of Class B common stock) issued and outstanding as of July 31, 2023.
The beneficial ownership of Conduit ordinary shares prior to the Business Combination is based on 2,000 ordinary shares of Conduit issued and outstanding as of July 31, 2023.
The expected beneficial ownership of shares of New Conduit common stock after the Business Combination assuming none of the public shares are redeemed has been determined based upon the following: (i) that no additional public stockholders exercise their Redemption Rights; (ii) that none of the investors set forth in the table below has purchased or purchases additional shares of common stock (prior to or after the Business Combination); (iii) that 65,000,000 shares of New Conduit common stock are issued to the former Conduit shareholders as Merger Consideration including the shares of New Conduit common stock issued upon conversion of the Conduit convertible notes (excluding shares issued to the Private Placement Investor and shares issuable upon exercise of the warrants issued to the Private Placement Investor); (iv) that the aggregate unpaid principal amount on Conduit’s convertible notes is £2.4 million; (v) that the £2.4 million in Conduit’s convertible notes are converted into $3.1 million U.S. dollars; (vi) that the Conduit shares to be issued upon conversion of the convertible notes are issued at $8.00 per share (which reflects the 20% discount as required by the terms of each convertible note); and (vii) that there will be an aggregate of 75,247,978 shares of New Conduit common stock issued and outstanding after the closing of the Business Combination.
The Private Placement Investor is not listed below because the Private Placement Investor has contractually agreed to restrict its ability to exercise the warrants such that the number of shares of New Conduit common stock held by such investor and its affiliates after such exercise does not exceed the beneficial ownership limitation set forth in the warrant which may not exceed 4.99% of the then issued and outstanding shares of New Conduit common stock.
The share numbers and ownership percentages set forth herein do not take into account the issuance of any shares of New Conduit common stock upon completion of the Business Combination under the 2023 Stock Incentive Plan. In addition, the calculations of the expected number of shares of New Conduit common stock to be issued in the Merger has been determined based upon the number of Conduit shares and convertible notes of Conduit that were issued and outstanding as of July 31, 2023, after giving effect to the conversion of each of Conduit’s convertible notes into Conduit ordinary shares based on the assumptions set forth above. If the facts are different from the assumptions set forth above, which they are likely to be, the share numbers and ownership percentages upon completion of the Business Combination will be different.
Except as noted by footnote, and subject to community property laws where applicable, based on the information provided to MURF and Conduit, respectively, the persons and entities named in the table below have sole voting and investment power with respect to all shares shown as beneficially owned by them.
| 178 |
| Before the Business Combination (1) | After the Business Combination (2) | |||||||||||||||
| Amount and Nature of Beneficial Ownership | Approximate Percentage of Outstanding Shares | Amount and Nature of Beneficial Ownership | Approximate Percentage of Outstanding Shares | |||||||||||||
| Name and Address of Beneficial Owner (3) | ||||||||||||||||
| MURF Directors and Executive Officers Before the Business Combination | ||||||||||||||||
| Chele Chiavacci Farley | - | - | 30,000 | (4) | * | |||||||||||
| Richard Feinberg | - | - | 30,000 | (5) | * | |||||||||||
| Jack K. Heilbron | 4,814,250 | (6) | 68.8 | % | 4,724,250 | (7) | 6.2 | % | ||||||||
| Francis Knuettel II | - | - | 30,000 | (8) | * | |||||||||||
| Adam Sragovicz | - | - | - | (9) | - | |||||||||||
| All directors and executive officers of MURF as a group (5 individuals) | 4,814,250 | 68.8 | % | 4,814,250 | 6.3 | % | ||||||||||
| Conduit Directors and Executive Officers Before the Business Combination | ||||||||||||||||
| Andrew Regan (10) | 1,409 | 70.5 | % | 45,678,573 | (11) | 60.7 | % | |||||||||
| David Tapolczay (12) | 62 | 3.1 | % | 2,003,177 | (13) | 2.7 | % | |||||||||
| All directors and executive officers of Conduit as a group (2 individuals) | 1,471 | 73.6 | % | 47,681,750 | 63.4 | % | ||||||||||
| Directors and Executive Officers After the Business Combination (14) | ||||||||||||||||
| James Bligh | - | - | - | - | ||||||||||||
| Faith L. Charles | - | - | - | - | ||||||||||||
| Chele Chiavacci Farley | - | - | 30,000 | (15) | * | |||||||||||
| Freda Lewis-Hall | 62 | 3.1 | % | 2,003,177 | (16) | 2.7 | % | |||||||||
| Jennifer McNealey | - | - | - | - | ||||||||||||
| Andrew Regan | 1,409 | 70.5 | % | 45,678,573 | (17) | 60.7 | % | |||||||||
| Adam Sragovicz | - | - | - | (18) | - | |||||||||||
| David Tapolczay | 62 | 3.1 | % | 2,003,177 | (19) | 2.7 | % | |||||||||
| All directors and executive officers of New Conduit as a group (8 individuals) | - | - | 49,714,927 | (20) | 66.1 | % | ||||||||||
| Five-Percent Holders of MURF Before the Business Combination | ||||||||||||||||
| Murphy Canyon Acquisition Sponsor, LLC (the “Sponsor”) | 4,814,250 | (21) | 68.8 | % | 4,724,250 | (22) | 6.2 | % | ||||||||
| Five-Percent Holders of Conduit Before the Business Combination | ||||||||||||||||
| Corvus Capital Limited | 1,409 | 70.5 | % | 45,678,573 | (23) | 60.7 | % | |||||||||
| St George Street Capital | 225 | 11.3 | % | 7,269,594 | (24) | 9.7 | % | |||||||||
| Five-Percent Holders After the Business Combination | ||||||||||||||||
| Corvus Capital Limited | 1,409 | 70.5 | % | 45,678,573 | (25) | 60.7 | % | |||||||||
| Murphy Canyon Acquisition Sponsor, LLC (the “Sponsor”) | 4,814,250 | (26) | 68.8 | % | 4,724,250 | (27) | 6.2 | % | ||||||||
| St George Street Capital | 225 | 11.3 | % | 7,269,594 | (28) | 9.7 | % | |||||||||
* Less than 1%.
(1) The percentage of beneficial ownership of MURF before the Business Combination is calculated based on 2,941,728 shares Class A common stock and 3,306,250 shares Class B common stock outstanding as of July 31, 2023. Unless otherwise indicated, MURF believes that all persons named in the table have sole voting and investment power with respect to all shares beneficially owned by them prior to the Business Combination.
| 179 |
(2) The “Amount and Nature of Beneficial Ownership” and “Approximate Percentage of Outstanding Shares” after the Business Combination is calculated based on 75,247,978 shares of New Conduit common stock expected to be outstanding immediately following consummation of the Business Combination whereby all Class B common stock will be converted into Class A common stock, all of which will be converted into New Conduit common stock. Such expected number of shares outstanding amount: (i) assumes that no additional Public Stockholders properly elect to redeem their shares of MURF Class A common stock for cash and (ii) includes the 2,700,000 shares issued in the Private Placement to the Private Placement Investor but excludes shares issuable upon exercise of the warrants issued to the Private Placement Investor. The amount of beneficial ownership for each individual or entity after the Business Combination does not include shares issuable upon exercise of the warrants included in the units offered in the initial public offering. Unless otherwise indicated, MURF believes that all persons named in the table have sole voting and investment power with respect to all of the shares shown to be beneficially owned by them after giving effect to the Business Combination.
(3) Unless otherwise indicated, the business address of each of the entities and individuals below is c/o Murphy Canyon Acquisition Corp., 4995 Murphy Canyon Road, Suite 300, San Diego, California 92123.
(4) Reflects 15,000 shares of common stock and 15,000 redeemable warrants underlying 15,000 units (consisting of one share of Class A common stock and one warrant to purchase one share of Class A common stock) that the Sponsor has agreed to transfer to each of MURF’s independent directors immediately after the closing of the Business Combination.
(5) Reflects 15,000 shares of common stock and 15,000 redeemable warrants underlying 15,000 units (consisting of one share of Class A common stock and one warrant to purchase one share of Class A common stock) that the Sponsor has agreed to transfer to each of MURF’s independent directors immediately after the closing of the Business Combination.
(6) Includes the following securities held by Murphy Canyon Acquisition Sponsor, LLC: (i) 3,306,250 shares of Class B common stock, which at the time of the Business Combination, are automatically convertible into shares of Class A common stock, which will then automatically convert into New Conduit common stock; (ii) 754,000 shares of Class A common stock underlying units (each consisting of one share of Class A common stock and one redeemable warrant); and (iii) 754,000 shares of Class A common stock issuable upon exercise of 754,000 private placement warrants underlying units. Mr. Heilbron is the President of NetREIT Advisors LLC (“NetREIT Advisors”), which is the Managing Member of Murphy Canyon Acquisition Sponsor, LLC. By virtue of his executive role at NetREIT Advisors, Mr. Heilbron may be deemed to share beneficial ownership of the securities held of record by Murphy Canyon Acquisition Sponsor, LLC. Mr. Heilbron disclaims any such beneficial ownership except to the extent of his pecuniary interest therein.
(7) Includes the following securities held by Murphy Canyon Acquisition Sponsor, LLC: (i) 3,306,250 shares of New Conduit common stock due to the conversion of the shares of Class B common stock into shares of Class A common stock and finally into shares of New Conduit common stock at the time of the Business Combination; (ii) 709,000 shares of New Conduit common stock, which prior to the Business Combination were part of the MURF units; and (iii) 709,000 shares of New Conduit common stock converted from an equal number of shares of Class A common stock issuable upon exercise of 709,000 private placement warrants, which prior to the Business Combination were part of the MURF units. Mr. Heilbron is the President of NetREIT Advisors LLC (“NetREIT Advisors”), which is the Managing Member of Murphy Canyon Acquisition Sponsor, LLC. By virtue of his executive role at NetREIT Advisors, Mr. Heilbron may be deemed to share beneficial ownership of the securities held of record by Murphy Canyon Acquisition Sponsor, LLC. Mr. Heilbron disclaims any such beneficial ownership except to the extent of his pecuniary interest therein.
(8) Reflects 15,000 shares of common stock and 15,000 redeemable warrants underlying 15,000 units (consisting of one share of Class A common stock and one warrant to purchase one share of Class A common stock) that the Sponsor has agreed to transfer to each of MURF’s independent directors immediately after the closing of the Business Combination.
(9) It is anticipated that Mr. Sragovicz will serve as New Conduit’s Chief Financial Officer following the closing of the Business Combination and that as part of his employment, Mr. Sragovicz will receive a sign-on restricted stock unit award covering 0.10% of the shares of common stock of New Conduit pursuant to the terms of the 2023 Stock Incentive Plan, which shall vest in equal annual installments on the first three anniversaries of the Business Combination.
(10) Dr. Andrew Regan is currently a director of Conduit (Dr. Regan also undertakes duties typically associated with an executive officer in connection with his service as a director of Conduit).
(11) Assumes that 31,164,171 shares of New Conduit common stock are issued to Corvus Capital Limited and 14,377,641 shares of New Conduit common stock are issued to Algo Holdings, Inc., in each case as consideration in the Merger for the cancellation of the Conduit shares held by it and 68,380 shares of New Conduit common stock are issued to Dr. Regan as consideration for the Conduit shares that will be issued to Dr. Regan upon the conversion of the convertible promissory notes held by him. Dr. Regan is the Chief Executive Officer of Corvus Capital Limited and Algo Holdings, Inc. is a wholly owned subsidiary of Corvus Capital Limited. By virtue of this relationship, Dr. Regan may be deemed to share beneficial ownership of the securities held of record by Corvus Capital Limited and Algo Holdings, Inc. Dr. Regan disclaims any such beneficial ownership except to the extent of his pecuniary interest therein. The business address of Corvus Capital Limited is Floor 2, Willow House, Cricket Square PO Box 709 Grand Cayman KY1-1107, Cayman Islands.
(12) David Tapolczay is currently a director of Conduit.
(13) Assumes that 2,003,177 shares of New Conduit common stock are issued to Mr. Tapolczay as consideration in the Merger for the cancellation of the Conduit shares held by him.
| 180 |
(14) Unless otherwise indicated, the business address of each of the entities and individuals below is c/o Murphy Canyon Acquisition Corp., 4995 Murphy Canyon Road, Suite 300, San Diego, California 92123.
(15) See footnote 4 above.
(16) Assumes that 2,003,177 shares of New Conduit common stock are issued to Intelmed LLC as consideration in the Merger for the cancellation of the Conduit shares held by it. Ms. Lewis-Hall is the Managing Director of Intelmed LLC. By virtue of this relationship, Ms. Lewis-Hall may be deemed to share beneficial ownership of the securities held of record by Intelmed LLC. Ms. Lewis-Hall disclaims any such beneficial ownership except to the extent of her pecuniary interest therein. The business address of Intelmed LLC is 11421 Golden Eagle Court Naples, Florida 34120.
(17) See footnote 11 above.
(18) See footnote 9 above.
(19) See footnote 13 above.
(20) Includes (i) an aggregate of 49,714,927 shares of New Conduit common stock and (ii) an aggregate of 15,000 shares of New Conduit common stock underlying warrants.
(21) See footnote 6 above.
(22) See footnote 7 above.
(23) See footnote 11 above.
(24) Assumes that 7,269,594 shares of New Conduit common stock are issued to St George Street Capital as consideration in the Merger for the cancellation of the Conduit shares held by it. The business address of St George Street Capital is 10 Queen Street Place London, England EC4R 1BE, United Kingdom.
(25) See footnote 11 above.
(26) See footnote 6 above.
(27) See footnote 7 above.
(28) See footnote 24 above.
| 181 |
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS
Related Person Policy
MURF’s Code of Ethics requires MURF to avoid, wherever possible, all conflicts of interests, except under guidelines or resolutions approved by the MURF Board (or the appropriate committee of its board) or as disclosed in its public filings with the SEC. Under MURF’s code of ethics, conflict of interest situations will include any financial transaction, arrangement or relationship (including any indebtedness or guarantee of indebtedness) involving the company. MURF’s conflict of interest policy provides that a committee of independent members of the MURF Board may, among other things, cause any officer or director who has a direct or indirect interest in a transaction to recuse him or herself from the consideration of such transaction and, to the extent necessary, the committee may retain appropriately qualified, non-conflicted personnel to advise the company in connection with such transaction.
In addition, MURF’s audit committee is responsible for reviewing and approving related party transactions to the extent that it enters into such transactions. An affirmative vote of a majority of the members of the audit committee present at a meeting at which a quorum is present will be required in order to approve a related party transaction. A majority of the members of the entire audit committee will constitute a quorum. Without a meeting, the unanimous written consent of all of the members of the audit committee will be required to approve a related party transaction. MURF also requires each of its directors and executive officers to complete a directors’ and officers’ questionnaire that elicits information about related party transactions.
These procedures are intended to determine whether any such related party transaction impairs the independence of a director or presents a conflict of interest on the part of a director, employee or officer.
MURF Related Person Transactions
Founder Shares
On November 16, 2021, the Sponsor purchased an aggregate of 4,312,500 shares of the MURF common stock for the aggregate price of $25,000 (the “Founder Shares”). The Founder Shares included an aggregate of up to 750,000 shares subject to forfeiture by the Sponsor to the extent that the underwriters’ over-allotment was not exercised in full or in part, so that the Sponsor would collectively own 20% of MURF’s issued and outstanding shares after the initial public offering (assuming the Sponsor did not purchase any Public Shares in the initial public offering and excluding the Private Shares). As a result of the underwriters’ election to exercise their over-allotment option, on January 26, 2022, the Sponsor surrendered and forfeited 1,006,250 Founder Shares. Following such forfeiture, the Sponsor now holds 3,306,250 Founder Shares.
The Sponsor has agreed, subject to certain limited exceptions, not to transfer, assign or sell any of the Founder Shares until the earlier to occur of: (A) six months after the completion of our initial business combination, and (B) subsequent to the initial business combination if we complete a liquidation, merger, stock exchange or other similar transaction that results in all of our public stockholders having the right to exchange their public shares for cash, securities or other property. Notwithstanding the foregoing, the Sponsor shall have the right to transfer its ownership in the founder shares at any time to the extent that it determines, in good faith, that such transfer is necessary to ensure that it and/or any of its parents, subsidiaries or affiliates are in compliance with the Investment Company Act of 1940. Also, and notwithstanding the foregoing, if subsequent to our initial business combination the reported last sale price of our common stock equals or exceeds $12.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 90 days after our initial business combination, all of the Founder Shares will be released from the lock-up, subject to certain exceptions.
Private Units
Contemporaneously with the closing of the initial public offering and the exercise of the overallotment option, the Sponsor purchased an aggregate of 754,000 private units in a private placement at a price of $10.00 per private unit. Each private unit consists of one Private Share and one Private Warrant. The private units are identical to the units sold in the initial public offering except that the (a) the placement units and their component securities will not be transferable, assignable or saleable until 30 days after the consummation of the Company’s initial business combination except to permitted transferees and (b) the warrants and rights included as a component of the placement units, so long as they are held by the Sponsor or its permitted transferees, will be entitled to registration rights, respectively. Additionally, the warrants underlying the placement units contain a cashless exercise provision and shall be non-redeemable while held by the initial purchasers thereof or their permitted assignees. The Sponsor has agreed not to transfer, assign or sell any of the private units and underlying securities (except in connection with the same limited exceptions that the Private Shares may be transferred as described above) until after the completion of the initial business combination. The Sponsor has agreed to transfer 45,000 placement units (15,000 each) to each of MURF’s three independent directors.
Promissory Notes
On November 4, 2021, the Sponsor issued an unsecured promissory note to MURF pursuant to which MURF could borrow up to an aggregate principal amount of $300,000. The promissory note is non-interest bearing and payable on the earlier of (i) the consummation of the Proposed Public Offering or (ii) the decision not to execute the Proposed Public Offering. As of December 31, 2021, there was $177,057 outstanding under the promissory note. The balance was paid in full on February 10, 2022.
Administrative Services Agreement
MURF entered into an agreement whereby, starting February 2, 2022, through the earlier of MURF’s consummation of a business combination or its liquidation, MURF will pay Murphy Canyon Management Group, Inc., an affiliate of MURF’s sponsor, a total of $10,000 per month for office space, utilities and secretarial and administrative support. For the period from February 2, 2022, through September 30, 2022, MURF incurred and paid $80,000 for these services.
| 182 |
Sponsor Support Agreement
Concurrently with the execution of the Merger Agreement, MURF entered into a support agreement with the Sponsor pursuant to which the Sponsor has agreed to, among other things, vote all of the shares of MURF common stock legally and beneficially owned by it in favor of the Business Combination.
Subscription Agreement
In connection with the transactions contemplated by the Merger Agreement, MURF entered into a subscription agreement, subsequently amended on January 27, 2023, with the Private Placement Investor. Pursuant to the Subscription Agreement, the Private Placement Investor agreed to purchase 2,700,000 units, with each unit consisting of (i) one share of Class A common stock and (ii) one warrant to purchase one share of Class A common stock, for a purchase price of $10.00 per unit in the Private Placement. The Subscription Agreement contains registration rights, pursuant to which within fifteen (15) business days after the closing of the Private Placement, MURF will use reasonable best efforts to file with the SEC a registration statement registering the resale of shares of MURF common stock included in the Units and the shares of Class A common stock issued and issuable upon exercise of the warrants. The closing of the Private Placement is conditioned on there not being a suspension of the qualification of MURF common stock for offering or sale or trading in any jurisdiction, or the initiation or threatening of any legal proceeding, no legal prohibitions to consummate the Business Combination, and all conditions precedent to the closing of the Business Combination set forth in the Merger Agreement having been satisfied or waived.
The warrant will be exercisable for a period of five years after the completion of the Business Combination and will have an exercise price of $11.50 per share, subject to adjustment as set forth in the Warrant for stock splits, stock dividends, recapitalizations and similar customary adjustments. The Private Placement Investor may exercise each warrant on a cashless basis if the shares underlying the warrants are not then registered pursuant to an effective registration statement. The Private Placement Investor has contractually agreed to restrict its ability to exercise the warrants such that the number of shares of MURF common stock held by the investor and its affiliates after such exercise does not exceed the beneficial ownership limitation set forth in the warrant which may not exceed 4.99% of the then issued and outstanding shares of MURF common stock.
In connection with the amendment to the Subscription Agreement dated January 27, 2023, the Private Placement Investor agreed to receive and be issued shares of New Conduit common stock. Upon the closing of the Business Combination, and specifically the filing of the Proposed Charter, MURF’s Class A common stock and Class B common stock will be reclassified as a single class of common stock, and MURF’s company name will change to “Conduit Pharmaceuticals Inc.” also referred to as New Conduit. The underlying terms of the Class A common stock and the single class of common stock are materially the same as each has or will have the same voting right of one vote per each share, same dividend rights and same liquidation rights.
The closing of the Private Placement will occur immediately prior to consummation of the Business Combination and is conditioned thereon and on other customary closing conditions. The common stock to be issued pursuant to the Subscription Agreement has not been registered under the Securities Act, and will be issued in reliance upon the exemption provided under Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder. The Subscription Agreement will terminate and be void and of no further force and effect, and all rights and obligations of the parties without any further liability on the part of any party in respect thereof, upon the earlier to occur of: (a) the mutual written agreement of each of the parties to terminate the Subscription Agreement; (b) such date and time as the Merger Agreement is terminated in accordance with its terms; (c) if any of the conditions to the closing of the Private Placement are not satisfied or waived on or prior to the closing of the Private Placement and, as a result thereof, the transactions contemplated by the Subscription Agreement are not consummated at the closing of the Private Placement or (d) written notice by either party to the other party to terminate the Subscription Agreement if the transactions contemplated by the Subscription Agreement are not consummated on or prior to February 7, 2024.
Consulting Agreement with Jack K. Heilbron
Jack K. Heilbron, who is currently the Chief Executive Officer, President and Chairman of the Board of Directors of Murphy Canyon Acquisition Corp., has entered into a Consulting Agreement with Murphy Canyon Acquisition Corp., which is effective upon the closing of the Business Combination. The Consulting Agreement provides that Mr. Heilbron will provide advisory and consulting services from time to time to Conduit Pharmaceuticals Inc., the post-closing combined entity, for one year following the closing of the Business Combination. Mr. Heilbron will no longer serve as an officer or director of Conduit Pharmaceuticals Inc., the post-closing combined entity, after the closing of the Business Combination. However, pursuant to the terms of the Consulting Agreement, Mr. Heilbron is entitled to rights as an observer to the Board of Directors of Conduit Pharmaceuticals Inc., the post-closing combined entity. Mr. Heilbron is entitled to be paid $25,000 per calendar quarter for his consulting services and is also entitled to a stock option to purchase the number of shares of the common stock of Conduit Pharmaceuticals Inc., the post-closing combined entity, determined by dividing (i) $300,000, by (ii) the per share Black-Scholes valuation as of the grant date, utilizing the same assumptions used in preparation of the financial statements, with the resulting quotient rounded down to the nearest whole share.
Conduit Related Person Transactions
The following is a summary of transactions since January 1, 2020, to which Conduit has been a participant and in which the amount involved exceeded or will exceed $120,000, and in which any of Conduit’s directors, executive officers or holders of more than 5% of any class of Conduit’s equity interests at the time of such transaction, or any members of their immediate family, had or will have a direct or indirect material interest.
Shareholder Support Agreements
Concurrently with the execution of the Merger Agreement, MURF, Conduit, and certain shareholders of Conduit entered into a certain shareholder support agreement dated November 8, 2022, pursuant to which the Conduit Shareholders agreed to vote all Conduit shares beneficially owned by them, including any additional shares of Conduit they acquire ownership of or the power to vote, in favor of the Business Combination and related transactions. Under the support agreements, each Conduit shareholder has also agreed that, prior to the termination of the applicable support agreement, such shareholder will not transfer or otherwise enter into any agreement or understanding with respect to a transfer relating to any shares of Conduit owned by such shareholder. The support agreements will terminate automatically without any further required actions or notice upon the earliest to occur: (a) the effective time of the Business Combination; (b) the termination of the Merger Agreement in accordance with its terms; and (c) the mutual agreement of Conduit and the shareholder.
Conduit Shareholder Lockup Agreements
Under the Merger Agreement, as a condition to receiving New Conduit common stock after the closing of the Business Combination in respect of their Conduit shares, certain Conduit shareholders are required to execute lockup agreements pursuant to which such shareholders must agree not to sell, transfer or take certain other actions with respect to such shares of New Conduit common stock for a period of 180 days after the closing of the Business Combination, subject to certain customary exceptions.
| 183 |
Corvus Capital Limited
Corvus Capital Limited (“Corvus”) is a significant investor in Conduit due to its ownership of 1,000 ordinary shares of Conduit as of the year ended December 31, 2022. Dr. Andrew Regan, the Chief Executive Officer of Corvus, is a member of Conduit’s board of directors. For the year ended December 31, 2021, Conduit incurred $1.6 million (£1.3 million) in advisory fees for funding and review of a potential acquisition candidates to Corvus. For the nine months ended September 30, 2022, Conduit incurred director’s fees payable to the CEO of Corvus of approximately $0.2 million.
During January 2023, under the terms of the 2022 Convertible Loan Note Instrument, Conduit issued convertible notes payable with an aggregate principal amount of $0.2 million (£0.2 million) to Dr. Regan. The convertible notes payable generally mature three years after issuance and bear 5% annual interest, only to be paid in the event of a material breach by Conduit of the terms of the 2022 Convertible Loan Note Instrument or an Event of Default (as defined in the 2022 Convertible Loan Note Instrument). In the event of a Change of Control (as defined in the 2022 Convertible Loan Note Instrument), the convertible notes payable automatically convert into ordinary shares of Conduit at a conversion price equal to a 20% discount to the price per share paid for the most senior class of shares in respect of such Change of Control.
St George Street Capital
St George Street Capital is a significant investor in Conduit due to its ownership of 225 ordinary shares of Conduit. Dr. David Tapolczay, the Chief Executive Officer of St George Street Capital is also the Chief Executive Officer of Conduit. Further, Conduit has a Global Funding Agreement (as defined below) with St George Street Capital. On March 26, 2021, Conduit entered into the Exclusive Funding Agreement (“Global Funding Agreement”) with St George Street Capital. Under the Global Funding Agreement, Conduit has the exclusive first right, but not the obligation, to provide or procure funding for the performance drug discovery and/or development project that St George Street Capital wishes to undertake. The Global Funding Agreement entitles Conduit to 100% of the net revenue on projects that Conduit funds by itself. For additional information regarding the Global Funding Agreement and related agreements, see the “Business of Conduit Pharmaceuticals Limited — Global Funding Agreement — St George Street” section of this proxy statement/prospectus.
A.G.P./Alliance Global Partners
A.G.P./Alliance Global Partners (“A.G.P.”) is a financial advisor to both MURF and Conduit in connection with the Business Combination transaction. Upon the closing of the Business Combination, A.G.P. is entitled to receive (i) a cash fee of $6,500,000, 1,300,000 shares of New Conduit common stock and warrants to purchase 54,000 shares of New Conduit common stock at an exercise price of $11.00 per share pursuant to its engagement agreement with Conduit entered into on August 2, 2022 and (ii) $4,628,750 of deferred underwriting fees as a result of its engagement for MURF’s initial public offering. There can be no assurance that the fact that A.G.P. acted as the financial advisor to both parties to the Business Combination did not impact the advice that A.G.P. delivered to either or both parties, or that certain terms of the Merger were not impacted by the potential conflict of interest.
New Conduit’s Directors and Officers
As noted above, certain of the individuals that will serve as members of the board of directors of New Conduit upon completion of the Business Combination have relationships with MURF, Conduit and/or one of their respective stockholders. Dr. Freda Lewis-Hall, who will serve as the Chairman of New Conduit’s board of directors, is a shareholder of Conduit and will receive shares of New Conduit’s common stock in the Business Combination. Dr. David Tapolczay, who will serve as a director of New Conduit and as the Chief Executive Officer of New Conduit, is currently a stockholder of Conduit and will receive shares of New Conduit’s common stock in the Business Combination and he is also a director of Conduit and the Chief Executive Officer of St George Street Capital. Dr. Andrew Regan, who will serve as a director of New Conduit, is currently a director of Conduit and a shareholder of Conduit and will receive shares of New Conduit’s common stock in the Business Combination. James Bligh, who will serve as a director of New Conduit, is an employee of Conduit. Faith L. Charles, who will serve as a director of New Conduit, is a partner at Thompson Hine LLP, a law firm that provides legal services to Conduit. Chele Chiavacci Farley, who will serve as a director of New Conduit, is currently a director of MURF.
In addition, upon completion of the Business Combination, the executive officers of New Conduit will include Dr. David Tapolczay, as Chief Executive Officer of New Conduit, and Adam Sragovicz, as Chief Financial Officer of New Conduit. Dr. Tapolczay is currently a director of Conduit and the Chief Executive Officer of St George Street Capital. Mr. Sragovicz is currently MURF’s Chief Financial Officer and a director of MURF.
Policies and Procedures for Related Person Transactions
Prior to the consummation of the Business Combination, New Conduit’s board of directors intends to adopt a policy with respect to the review, approval and ratification of related party transactions. Under the policy, New Conduit’s audit committee is responsible for reviewing and approving related person transactions. In the course of its review and approval of related party transactions, New Conduit’s audit committee will consider the relevant facts and circumstances to decide whether to approve such transactions. In particular, New Conduit’s policy requires New Conduit’s audit committee to consider, among other factors it deems appropriate:
| ● | the related person’s relationship to New Conduit and interest in the transaction; | |
| ● | the material facts of the proposed transaction, including the proposed aggregate value of the transaction; | |
| ● | the impact on a director’s or a director nominee’s independence in the event the related person is a director or director nominee or an immediate family member of the director or director nominee; | |
| ● | the benefits to New Conduit of the proposed transaction; | |
| ● | if applicable, the availability of other sources of comparable products or services; and | |
| ● | an assessment of whether the proposed transaction is on terms that are comparable to the terms available to an unrelated third party or to employees generally. |
The audit committee may only approve those transactions that are in, or are not inconsistent with, New Conduit’s best interests and those of New Conduit’s shareholders, as the audit committee determines in good faith.
In addition, under New Conduit’s code of business conduct and ethics, which will be adopted prior to the consummation of the Business Combination, New Conduit’s employees, officers, directors and director nominees will have an affirmative responsibility to disclose any transaction or relationship that reasonably could be expected to give rise to a conflict of interest.
All of the transactions described above were entered into prior to the adoption of the New Conduit’s written related party transactions policy (which policy will be adopted prior to the consummation of Business Combination), but all were approved by Conduit’s board of directors or management considering similar factors to those described above.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires MURF’s directors, officers and persons owning more than 10% of MURF common stock to file reports of ownership and changes of ownership with the SEC. Based on its review of the copies of such reports furnished to MURF, or representations from certain reporting persons that no other reports were required, MURF believes that all applicable filing requirements were complied with to date.
| 184 |
COMPARISON OF STOCKHOLDERS’ RIGHTS
If the Business Combination is consummated, shareholders of Conduit will receive and become holders of shares of new common stock of New Conduit. Conduit is incorporated under the laws of the Cayman Islands and New Conduit is organized under the laws of Delaware. Differences in the rights of holders of Conduit shares and holders of New Conduit common stock will arise due to differences between the laws of their respective jurisdictions and their respective constitutional documents, including their certificates of incorporation, bylaws or memorandum and articles of association, as applicable. As holders of shares of New Conduit common stock, your rights with respect thereto will be governed by Delaware law, including the DGCL, as well as the second amended and restated certificate of incorporation of New Conduit in the form attached to this proxy statement/prospectus as Annex B and the second amended and restated bylaws of New Conduit. This section summarizes the material differences between the rights of Conduit shareholders and holders of shares of New Conduit common stock.
The following summary is not a complete statement of the rights of the shareholders or stockholders of either of the two companies or a complete description of the specific provisions referred to below. The identification of specific differences is not intended to indicate that other equally significant or more significant differences do not exist. The form of the second amended and restated certificate of incorporation of New Conduit that will be in effect upon the completion of the Business Combination is attached to this proxy statement/prospectus as Annex B. We urge you to read the second amended and restated certificate of incorporation in its entirety for a complete description of the rights and preferences of the post-combination company’s securities following the business combination.
For more information on the charter amendment proposals, see the section entitled “The Charter Amendment Proposals.”
| New Conduit | Conduit | |
| Organizational Documents | ||
| Following the Business Combination, the rights of the stockholders of New Conduit will be governed by the second amended and restated certificate of incorporation of New Conduit (the “New Conduit Charter”), the second amended and restated bylaws of New Conduit (the “New Conduit Bylaws”), and Delaware law, including the DGCL. | The rights of the shareholders of Conduit are governed by the memorandum of association and the articles of association of Conduit (the “Conduit M&A”) and Cayman Islands law, including the Companies Act (as Revised) of the Cayman Islands (the “Companies Act”). | |
| Number of Authorized Shares | ||
| New Conduit will have 250,000,000 authorized shares of capital stock, each with a par value of $0.0001 per share, consisting of 250,000,000 authorized shares of common stock, and 1,000,000 authorized shares of preferred stock. | The authorized share capital of Conduit is GBP40,000 divided into 400,000,000 Ordinary shares of par value GBP0.0001 each. | |
| Common Stock of New Conduit v. Ordinary Shares of Conduit | ||
| New Conduit stockholders will have no preemptive or other subscription rights and there will be no sinking fund or redemption provisions applicable to the common stock. | Holders of ordinary shares have no preemptive or other subscription rights (other than applying for a subscription for consideration by the directors of Conduit). There is no sinking fund or redemption provisions applicable to the ordinary shares that Conduit’s shareholders may exercise. The Company may, by its directors, issue redeemable shares, with the consent by a special resolution of members, vary the rights attaching to a certain class of shares or purchase all or any of its own shares, including any redeemable shares. | |
| 185 |
| Preferred Stock | ||
The New Conduit board of directors will be authorized to issue shares of preferred stock in one or more series, and to fix voting powers (if any), designations, powers, preferences and relative, participating, optional, special and other rights for each series and any qualifications, limitations or restrictions applicable to the shares of each series. The number of authorized shares of preferred stock may be increased or decreased (but not below the number of shares thereof then outstanding) without stockholder approval.
The New Conduit board of directors will be able to, subject to limitations prescribed by Delaware law, without stockholder approval, issue preferred stock with voting and other rights that could adversely affect the voting power and other rights of the holders of the common stock and could have anti-takeover effects. The ability of the New Conduit board of directors to issue preferred stock without stockholder approval, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change of control of New Conduit or the removal of New Conduit’s management and may adversely affect the market price of New Conduit common stock and the voting and other rights of the holders of New Conduit. New Conduit will have no preferred stock outstanding at the date the second amended and restated certificate of incorporation becomes effective. Although the Conduit board of directors does not currently intend to issue any shares of preferred stock, we cannot assure you that the New Conduit board of directors will not do so in the future. |
Not applicable. | |
| Rights, Warrants, and Options | ||
| New Conduit will have the authority to create and issue rights, warrants, and options entitling the holders thereof to purchase shares of any class or series of New Conduit’s capital stock or other securities of New Conduit, and such rights, warrants and options shall be evidenced by or in instrument(s) approved by the New Conduit board of directors. The New Conduit board of directors will be empowered to set the exercise price, duration, times for exercise and other terms and conditions of such rights, warrants or options; provided, however, that the consideration to be received for any shares of capital stock subject thereto may not be less than the par value thereof. | Subject to the provisions of the Companies Act and the Conduit M&A about the redemption and purchase of Conduit’s own shares, the directors have general and unconditional authority to allot (with or without confirming rights of renunciation), grant options over or otherwise deal with any unissued shares of Conduit to such persons, at such times and on such terms and conditions as they may decide.
No Share of Conduit may be issued at a discount except in accordance with the Companies Act. | |
| Voting Power | ||
| Except as otherwise required by law or by the second amended and restated certificate of incorporation, the holders of common stock will possess all voting power for the election of New Conduit’s directors and all other matters requiring stockholder action. Holders of common stock are entitled to one vote per share. | The holders of ordinary shares are entitled to one vote per share. | |
| Director Elections | ||
| Subject to the special rights of the holders of one or more series of preferred stock to elect directors, at each succeeding annual meeting of stockholders, a director shall be elected and hold office until the next annual meeting of stockholders. There is no cumulative voting with respect to the election of directors, with the result that the holders of more than 50% of the shares voted for the election of directors can elect all of the directors. | Conduit may, by resolutions passed by a simple majority of the voting powers of Conduit shareholders as, being entitled to do so, voting at a general meeting of Conduit or approved in writing, appoint any person to be a director of Conduit. The board of directors of Conduit may also appoint any person to be a director. Each appointment of directors may be to either to fill a vacancy or as an addition to the existing directors, subject to any upper limit on the number of directors prescribed pursuant to the Conduit M&A. | |
| Dividends | ||
| Subject to applicable law and the rights, if any, of the holders of any outstanding series of the preferred stock, the holders of the shares of the common stock shall be entitled to receive such dividends and other distributions (payable in cash, property or capital stock of New Conduit) when, as and if declared thereon by the New Conduit board of directors from time to time out of any assets or funds of New Conduit legally available therefor, and shall share equally on a per share basis in such dividends and distributions. | Subject to the Companies Act, Conduit may by ordinary resolution declare dividends in accordance with the respective rights of the members but no dividend shall exceed the amount recommended by the directors.
The directors may pay interim dividends or declare final dividends in accordance with the respective rights of the members if it appears to them that they are justified by the financial position of Conduit and that such dividends may lawfully be paid.
Dividends may be paid in cash or wholly or partly by the distribution of assets. | |
| 186 |
| Exclusive Forum | ||
Unless New Conduit consents in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall, to the fullest extent permitted by the applicable law, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for any stockholder (including a beneficial owner) to bring (i) any derivative action or proceeding brought on behalf of New Conduit, (ii) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of New Conduit to New Conduit or New Conduit’s stockholders, (iii) any action asserting a claim against New Conduit, its directors, officers or employees arising pursuant to any provision of the DGCL or the second amended and restated certificate of incorporation or the bylaws, or (iv) any action asserting a claim against New Conduit, its directors, officers or employees governed by the internal affairs doctrine and, if brought outside of Delaware, the stockholder bringing the suit will be deemed to have consented to service of process on such stockholder’s counsel except any action (A) as to which the Court of Chancery in the State of Delaware determines that there is an indispensable party not subject to the jurisdiction of the Court of Chancery (and the indispensable party does not consent to the personal jurisdiction of the Court of Chancery within ten days following such determination), (B) which is vested in the exclusive jurisdiction of a court or forum other than the Court of Chancery, or (C) for which the Court of Chancery does not have subject matter jurisdiction. Notwithstanding the foregoing sentence will not apply to suits brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction, and (ii) to the fullest extent permitted by the applicable law, the federal district courts of the United States of America for the District of Delaware and the Court of Chancery of the State of Delaware shall have concurrent jurisdiction for the resolution of any complaint asserting a cause of action arising under the Securities Act or the rules and regulations promulgated thereunder.
If any action the subject matter of which is within the scope described above is filed in a court other than a court located within the State of Delaware (a “Foreign Action”) in the name of any stockholder, such stockholder shall be deemed to have consented to (i) the personal jurisdiction of the state and federal courts located within the State of Delaware in connection with any action brought in any such court to enforce Section 12.1 of the New Conduit Bylaws immediately above (an “FSC Enforcement Action”) and (ii) having service of process made upon such stockholder in any such FSC Enforcement Action by service upon such stockholder’s counsel in the Foreign Action as agent for such stockholder. |
Not applicable. | |
| Liquidation, Dissolution, and Winding Up | ||
| Subject to applicable law and the rights, if any, of the holders of any outstanding series of the preferred stock, in the event of any voluntary or involuntary liquidation, dissolution or winding-up of New Conduit, after payment or provision for payment of the debts and other liabilities of New Conduit, the holders of the shares of the common stock shall be entitled to receive all the remaining assets of New Conduit available for distribution to its stockholders, ratably in proportion to the number of shares of the common stock held by them. | In the event of any voluntary or involuntary liquidation, dissolution or winding-up of Conduit, after payment or provision for payment of the debts and other liabilities of Conduit, Conduit will distribute any remaining proceeds or assets to holders of ordinary shares ratably in proportion to the number of shares then held by them.
Furthermore, if Conduit is wound up, the members may, subject to the Conduit M&A and any other sanction required by the Companies Act, pass a special resolution allowing the liquidator to do either or both of: (a) to divide in specie among the members the whole or any part of the assets of Conduit and, for that purpose, to value any assets and to determine how the division shall be carried out as between the members or different classes of members; (b) to vest the whole or any part of the assets in trustees for the benefit of members and those liable to contribute to the winding up. | |
| 187 |
DESCRIPTION OF NEW CONDUIT SECURITIES
The following description of the material terms of the share capital of New Conduit following the Transactions includes a summary of specified provisions of the charter documents of New Conduit that will be in effect upon completion of the Transactions. This description is qualified by reference to New Conduit’s charter documents as will be in effect upon consummation of the Transactions, copies of which are attached to this proxy statement/prospectus and are incorporated in this proxy statement/prospectus by reference.
General
After the Business Combination, New Conduit’s second amended and restated certificate of incorporation will provide for 251,000,000 authorized shares, consisting of (a) 250,000,000 shares of common stock, $0.0001 par value per share, and (b) 1,000,000 shares of preferred stock, $0.0001 par value per share.
Common Stock
The holders of the common stock will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
There is no cumulative voting with respect to the election of directors, with the result that the holders of more than 50% of the voting power represented by shares of New Conduit common stock voted for the election of directors can elect all of the directors.
Holders of New Conduit common stock will not have any conversion, preemptive or other subscription rights and there will be no sinking fund or redemption provisions applicable to the common stock.
Preferred Stock
New Conduit’s second amended and restated certificate of incorporation will authorize the issuance of 1,000,000 shares of preferred stock with such designations, rights and preferences as may be determined from time to time by the New Conduit board of directors. The New Conduit board of directors will be empowered, without stockholder approval, to issue the preferred stock with dividend, liquidation, conversion, voting or other rights which could adversely affect the voting power or other rights of the holders of New Conduit common stock. In addition, the preferred stock could be utilized as a method of discouraging, delaying or preventing a change in control of New Conduit.
Warrants
Upon consummation of the Transactions, New Conduit will have warrants outstanding to purchase an aggregate of 16,733,000 shares of common stock (assuming no holders of public shares of New Conduit seek to redeem such shares). Each outstanding whole warrant of New Conduit shall continue to represent the right to purchase one share of common stock upon closing of the Transactions at a price of $11.00 per share, subject to adjustment as discussed below, at any time commencing on the later of 30 days after the consummation of a business combination and 12 months from the closing of the initial public offering.
We will not be obligated to deliver any shares of common stock pursuant to the exercise of a warrant and will have no obligation to settle such warrant exercise unless a registration statement under the Securities Act with respect to the shares of common stock underlying the warrants is then effective and a prospectus relating thereto is current, subject to our satisfying our obligations described below with respect to registration. No warrant will be exercisable and we will not be obligated to issue shares of common stock upon exercise of a warrant unless the common stock issuable upon such warrant exercise has been registered, qualified or deemed to be exempt under the securities laws of the state of residence of the registered holder of the warrants. In the event that the conditions in the two immediately preceding sentences are not satisfied with respect to a warrant, the holder of such warrant will not be entitled to exercise such warrant and such warrant may have no value and expire worthless. In no event will we be required to net cash settle any warrant. In the event that a registration statement is not effective for the exercised warrants, the purchaser of a unit containing such warrant will have paid the full purchase price for the unit solely for the share of common stock underlying such unit.
The Private Warrants, as well as any warrants underlying additional units issued to the Sponsor or New Conduit’s officers, directors or their affiliates in payment of working capital loans, are identical to the warrants underlying the units offered in the initial public offering except that such warrants will be exercisable for cash or on a cashless basis, at the holder’s option, and will not be redeemable by New Conduit, in each case so long as they are still held by the Sponsor or its permitted transferees. The Private Warrants will become worthless if MURF does not consummate a business combination by February 7, 2024.
| 188 |
New Conduit may call the warrants for redemption (excluding the Private Warrants and any warrants underlying additional units issued to the Sponsor, New Conduit’s officers, directors or their affiliates in payment of working capital loans made to New Conduit), in whole and not in part, at a price of $0.01 per warrant,
| ● | upon not less than 30 days’ prior written notice of redemption to each warrant holder; and | |
| ● | if, and only if, the reported last sale price of the common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period commencing once the warrants become exercisable and ending three business days before we send the notice of redemption to the warrant holders. |
If and when the warrants become redeemable by New Conduit, New Conduit may not exercise its redemption right if the issuance of shares of common stock upon exercise of the warrants is not exempt from registration or qualification under applicable state blue sky laws or New Conduit are unable to effect such registration or qualification. New Conduit will use its best efforts to register or qualify such shares of common stock under the blue sky laws of the state of residence in those states in which the warrants were offered by New Conduit in the offering.
If New Conduit calls the warrants for redemption as described above, New Conduit’s management will have the option to require any holder that wishes to exercise its warrant to do so on a “cashless basis.” In determining whether to require all holders to exercise their warrants on a “cashless basis,” New Conduit’s management will consider, among other factors, New Conduit’s cash position, the number of warrants that are outstanding and the dilutive effect on New Conduit stockholders of issuing the maximum number of shares of common stock issuable upon the exercise of our warrants. If New Conduit’s management takes advantage of this option, all holders of warrants would pay the exercise price by surrendering their warrants for that number of shares of common stock equal to the quotient obtained by dividing (x) the product of the number of shares of common stock underlying the warrants, multiplied by the difference between the exercise price of the warrants and the “fair market value” (defined below) by (y) the fair market value. The “fair market value” for this purpose shall mean the average reported last sale price of the common stock for the 10 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants. If New Conduit’s management takes advantage of this option, the notice of redemption will contain the information necessary to calculate the number of shares of common stock to be received upon exercise of the warrants, including the “fair market value” in such case. Requiring a cashless exercise in this manner will reduce the number of shares to be issued and thereby lessen the dilutive effect of a warrant redemption. New Conduit believes this feature is an attractive option to New Conduit if it does not need the cash from the exercise of the warrants after its initial business combination.
A holder of a warrant may notify New Conduit in writing in the event it elects to be subject to a requirement that such holder will not have the right to exercise such warrant, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates), to the warrant agent’s actual knowledge, would beneficially own in excess of 4.9% (or such other amount as a holder may specify) of the shares of common stock outstanding immediately after giving effect to such exercise.
If the number of outstanding shares of common stock is increased by a stock dividend payable in shares of common stock, or by a split-up of shares of common stock or other similar event, then, on the effective date of such stock dividend, split-up or similar event, the number of shares of common stock issuable on exercise of each whole warrant will be increased in proportion to such increase in the outstanding shares of common stock.
In addition, if New Conduit, at any time while the warrants are outstanding and unexpired, pays a dividend or makes a distribution in cash, securities or other assets to the holders of common stock on account of such shares of common stock (or other shares of New Conduit’s capital stock into which the warrants are convertible), other than (a) as described above, (b) certain ordinary cash dividends, (c) to satisfy the redemption rights of the holders of common stock in connection with a proposed initial business combination, (d) to satisfy the redemption rights of the holders of common stock in connection with a stockholder vote to amend New Conduit’s amended and restated certificate of incorporation (i) to modify the substance or timing of New Conduit’s obligation to allow redemption in connection with New Conduit’s initial business combination or certain amendments to New Conduit’s charter prior thereto or to redeem 100% of New Conduit’s common stock if New Conduit does not complete its initial business combination within 12 months from the closing of this offering (or up to 18 months from the closing of the offering at the election of New Conduit subject to satisfaction of certain conditions or as extended by the New Conduit stockholders in accordance with New Conduit’s amended and restated certificate of incorporation) or (ii) with respect to any other provision relating to stockholders’ rights or pre-initial business combination activity, or (e) in connection with the redemption of our public shares upon New Conduit’s failure to complete its initial business combination, then the warrant exercise price will be decreased, effective immediately after the effective date of such event, by the amount of cash and/or the fair market value of any securities or other assets paid on each share of common stock in respect of such event.
If the number of outstanding shares of New Conduit’s common stock is decreased by a consolidation, combination, reverse stock split or reclassification of shares of common stock or other similar event, then, on the effective date of such consolidation, combination, reverse stock split, reclassification or similar event, the number of shares of common stock issuable on exercise of each warrant will be decreased in proportion to such decrease in outstanding shares of common stock.
| 189 |
In case of any reclassification or reorganization of the outstanding shares of common stock (other than those described above or that solely affects the par value of such shares of common stock), or in the case of any merger or consolidation of us with or into another corporation (other than a consolidation or merger in which New Conduit is the continuing corporation and that does not result in any reclassification or reorganization of New Conduit’s outstanding shares of common stock), or in the case of any sale or conveyance to another corporation or entity of the assets or other property of New Conduit as an entirety or substantially as an entirety in connection with which New Conduit is dissolved, the holders of the warrants will thereafter have the right to purchase and receive, upon the basis and upon the terms and conditions specified in the warrants and in lieu of the shares of New Conduit’s common stock immediately theretofore purchasable and receivable upon the exercise of the rights represented thereby, the kind and amount of shares of stock or other securities or property (including cash) receivable upon such reclassification, reorganization, merger or consolidation, or upon a dissolution following any such sale or transfer, that the holder of the warrants would have received if such holder had exercised their warrants immediately prior to such event. However, if less than 70% of the consideration receivable by the holders of common stock in such a transaction is payable in the form of common stock in the successor entity that is listed for trading on a national securities exchange or is quoted in an established over-the-counter market, or is to be so listed for trading or quoted immediately following such event, and if the registered holder of the warrant properly exercises the warrant within 30 days following public disclosure of such transaction, the warrant exercise price will be reduced as specified in the warrant agreement based on the Black-Scholes value (as defined in the warrant agreement) of the warrant. The purpose of such exercise price reduction is to provide additional value to holders of the warrants when an extraordinary transaction occurs during the exercise period of the warrants pursuant to which the holders of the warrants otherwise do not receive the full potential value of the warrants in order to determine and realize the option value component of the warrant. This formula is to compensate the warrant holder for the loss of the option value portion of the warrant due to the requirement that the warrant holder exercise the warrant within 30 days of the event. The Black-Scholes model is an accepted pricing model for estimating fair market value where no quoted market price for an instrument is available.
The warrants will be issued in registered form under a warrant agreement between Vstock Transfer, LLC, as warrant agent, and New Conduit. You should review a copy of the warrant agreement, which will be filed as an exhibit to the registration statement of which this proxy statement/prospectus is a part, for a complete description of the terms and conditions applicable to the warrants. The warrant agreement provides that the terms of the warrants may be amended without the consent of any holder to cure any ambiguity or correct any mistake, including to conform the provisions of the warrant agreement to the description of the terms of the warrants and the warrant agreement set forth in this proxy statement/prospectus, or defective provision, but requires the approval by the holders of at least a majority of the then outstanding public warrants to make any change that adversely affects the interests of the registered holders of public warrants.
In addition, if (x) New Conduit issues additional shares of common stock or equity-linked securities for capital raising purposes in connection with the closing of the initial business combination at a Newly Issued Price of less than $9.20 per share of common stock (with such issue price or effective issue price to be determined in good faith by the New Conduit board of directors and, in the case of any such issuance to New Conduit’s sponsor or its affiliates, without taking into account any founder shares held by New Conduit’s sponsor such affiliates, as applicable, prior to such issuance), (y) the aggregate gross proceeds from such issuances represent more than 60% of the total equity proceeds, and interest thereon, available for the funding of the initial business combination on the date of the consummation of the initial business combination (net of redemptions), and (z) the Market Value is below $9.20 per share, then the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115% of the greater of the Market Value and the Newly Issued Price, and the $18.00 per share redemption trigger price described above will be adjusted (to the nearest cent) to be equal to 180% of the greater of the Market Value and the Newly Issued Price.
The warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the warrant agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price (or on a cashless basis, if applicable), by certified or official bank check payable to us, for the number of warrants being exercised. The warrant holders do not have the rights or privileges of holders of common stock and any voting rights until they exercise their warrants and receive shares of common stock. After the issuance of shares of common stock upon exercise of the warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
No fractional shares will be issued upon exercise of the warrants. If, upon exercise of the warrants, a holder would be entitled to receive a fractional interest in a share, New Conduit will, upon exercise, round down to the nearest whole number of shares of common stock to be issued to the warrant holder.
Certain Anti-Takeover Provisions of Delaware Law and New Conduit’s Proposed Second Amended and Restated Certificate of Incorporation
Upon consummation of the Business Combination and assuming approval of the New Conduit charter amendment proposals, New Conduit will have certain anti-takeover provisions in place as follows:
| 190 |
Special Meeting of Stockholders
New Conduit’s bylaws will provide that, subject to the rights of the holders of any outstanding series of the preferred stock of New Conduit and to the requirements of applicable law, special meetings of stockholders, for any purpose or purposes, may be called only by (i) the chairperson of the board of directors, (ii) the chief executive officer, or (iii) a majority vote of New Conduit’s board of directors.
Advance Notice Requirements for Stockholder Proposals and Director Nominations
New Conduit’s bylaws will provide that, in addition to any other applicable requirements, for a nomination to be made by a stockholder, such stockholder must have given timely notice thereof in proper written form to the Secretary. To be timely, a stockholder’s notice to the Secretary must be received by the Secretary at the principal New Conduit’s executive offices (i) in the case of an annual meeting, not later than the close of business on the 90th day nor earlier than the close of business on the 120th day before the anniversary date of the immediately preceding annual meeting of stockholders; provided, however, that in the event that the annual meeting is more than 30 days before or more than 60 days after such anniversary date, notice by the stockholder to be timely must be so received no earlier than the close of business on the 120th day before the meeting and not later than the later of (x) the close of business on the 90th day before the meeting, or (y) the close of business on the 10th day following the day on which public announcement of the date of the annual meeting was first made by New Conduit; and (ii) in the case of a special meeting of stockholders called for the purpose of electing directors, not later than the close of business on the 10th day following the day on which public announcement of the date of the special meeting is first made by New Conduit.
Authorized but Unissued Shares
New Conduit’s authorized but unissued common stock and preferred stock will be available for future issuances without stockholder approval and could be utilized for a variety of corporate purposes, including future offerings to raise additional capital, acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved common stock and preferred stock could render more difficult or discourage an attempt to obtain control of New Conduit by means of a proxy contest, tender offer, merger or otherwise.
Exclusive Forum Selection
New Conduit’s second amended and restated certificate of incorporation will require that, to the fullest extent permitted by the applicable law, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for any stockholder (including a beneficial owner) to bring (i) any derivative action or proceeding brought on behalf of New Conduit, (ii) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of New Conduit to New Conduit or New Conduit’s stockholders, (iii) any action asserting a claim against New Conduit, its directors, officers or employees arising pursuant to any provision of the DGCL or the second amended and restated certificate of incorporation or the bylaws, or (iv) any action asserting a claim against New Conduit, its directors, officers or employees governed by the internal affairs doctrine and, if brought outside of Delaware, the stockholder bringing the suit will be deemed to have consented to service of process on such stockholder’s counsel except any action (A) as to which the Court of Chancery in the State of Delaware determines that there is an indispensable party not subject to the jurisdiction of the Court of Chancery (and the indispensable party does not consent to the personal jurisdiction of the Court of Chancery within ten days following such determination), (B) which is vested in the exclusive jurisdiction of a court or forum other than the Court of Chancery, or (C) for which the Court of Chancery does not have subject matter jurisdiction. Notwithstanding the foregoing, (i) the foregoing will not apply to suits brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction and (ii) to the fullest extent permitted by the applicable law, the federal district courts of the United States of America for the District of Delaware and the Court of Chancery of the State of Delaware shall have concurrent jurisdiction for the resolution of any complaint asserting a cause of action arising under the Securities Act or the rules and regulations promulgated thereunder.
This choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with New Conduit or any of New Conduit’s directors, officers, other employees or stockholders, which may discourage lawsuits with respect to such claims. New Conduit cannot be certain that a court will decide that this provision is either applicable or enforceable, and if a court were to find the choice of forum provision contained in New Conduit’s second amended and restated certificate of incorporation to be inapplicable or unenforceable in an action, New Conduit may incur additional costs associated with resolving such action in other jurisdictions, which could harm New Conduit’s business, operating results and financial condition.
Limitation on Liability and Indemnification of Directors and Officers
New Conduit’s second amended and restated certificate of incorporation will provide that directors and officers will be indemnified by New Conduit to the fullest extent authorized by Delaware law as it now exists or may in the future be amended.
New Conduit’s bylaws will also permit New Conduit to secure insurance on behalf of any officer, director or employee for any liability arising out of his or her actions, regardless of whether Delaware law would permit indemnification. Upon consummation of the Business Combination, New Conduit will have purchased a policy of directors’ and officers’ liability insurance that insures New Conduit’s directors and officers against the cost of defense, settlement or payment of a judgment in some circumstances and insures New Conduit against its obligations to indemnify the directors and officers.
These provisions may discourage stockholders from bringing a lawsuit against New Conduit’s directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit New Conduit and New Conduit stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent New Conduit pays the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. We believe that these provisions, the insurance and the indemnity agreements are necessary to attract and retain talented and experienced directors and officers.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to New Conduit’s directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, New Conduit has been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
| 191 |
MURF SECURITIES AND DIVIDENDS
Murphy Canyon Acquisition Corp.
Market Price of Units, Common Stock and Warrants
MURF’s units, warrants and Class A common stock are traded on the Nasdaq Stock Market LLC under the symbols MURFU, MURFW and MURF, respectively. The shares of MURF Class A common stock and warrants underlying the units began trading separately on the Nasdaq Global Market on February 3, 2022.
Holders
As of August 2, 2023, there were two holders of record of units, one holder of record of shares of MURF Class A common stock and Class B common stock and one holder of record of public warrants. Management believes MURF has approximately 300 beneficial holders of its securities as of the record date.
Dividends
MURF has not paid any dividends to its security holders to date.
Transfer Agent and Warrant Agent
The transfer agent for MURF common stock and warrant agent for its warrants upon consummation of the Business Combination will be VStock Transfer, LLC, 18 Lafayette Place, Woodmere, NY 11598.
Conduit
Market Price of Ordinary Shares
Historical market price information regarding Conduit is not provided because there is no public market for its securities.
Holders
As of August 2, 2023, there were 24 holders of record of Conduit shares.
| 192 |
OTHER STOCKHOLDER COMMUNICATIONS
Stockholders and interested parties may communicate with the MURF Board, any committee chairperson or the non-management directors as a group by writing to the board or committee chairperson in care of Murphy Canyon Acquisition Corp., 4995 Murphy Canyon Road, Suite 300, San Diego, CA 92123. Following the Business Combination, such communications should directed to Jack K. Heilbron, Chief Executive Officer. Each communication will be forwarded, depending on the subject matter, to the board of directors, the appropriate committee chairperson or all non-management directors.
LEGAL MATTERS
Sichenzia Ross Ference LLP will pass upon the validity of the shares of New Conduit common stock to be issued in connection with the Business Combination. The material U.S. federal income tax consequences of the Business Combination will be passed upon for Conduit by Thompson Hine LLP.
EXPERTS
The financial statements of Murphy Canyon Acquisition Corp. as of December 31, 2022 and 2021, and for the year ended December 31, 2022 and the period from October 19, 2021 (inception) through December 31, 2021, included in this proxy statement/prospectus, have been audited by Marcum LLP, independent registered public accounting firm, as set forth in its report thereon, (which contains an explanatory paragraph relating to substantial doubt about the ability of Murphy Canyon Acquisition Corp. to continue as a going concern as described in Note 1 to the financial statements) appearing elsewhere in this proxy statement/prospectus, and are included in reliance upon such report given on the authority of said firm as experts in auditing and accounting.
The financial statements of Conduit Pharmaceuticals Limited as of December 31, 2022, and 2021, and for the years then ended included in this proxy statement/prospectus (which contain an explanatory paragraph relating to the substantial doubt about the abilities of Conduit Pharmaceuticals Limited to continue as a going concern as described in Note 1 to the financial statements) have been so included in reliance on the report of Marcum LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
DELIVERY OF DOCUMENTS TO STOCKHOLDERS
Pursuant to the rules of the SEC, MURF and services that it employs to deliver communications to its stockholders are permitted to deliver to two or more stockholders sharing the same address a single copy of MURF’s proxy statement. Upon written or oral request, MURF will deliver a separate copy of the proxy statement to any stockholder at a shared address to which a single copy of each document was delivered and who wishes to receive separate copies of such documents. Stockholders receiving multiple copies of such documents may likewise request that MURF deliver single copies of such documents in the future. Stockholders may notify MURF of their requests by calling or writing MURF at its principal executive offices at 4995 Murphy Canyon Road, Suite 300, San Diego, CA 92123 or (760) 471-8536. Following the Business Combination, such requests should be made by calling or writing New Conduit at 4995 Murphy Canyon Road, Suite 300, San Diego, CA 92123, or (760) 471-8536.
WHERE YOU CAN FIND MORE INFORMATION
MURF files reports, proxy statements and other information with the SEC as required by the Exchange Act. You may access information on MURF at the SEC web site containing reports, proxy statements and other information at: http://www.sec.gov.
Information and statements contained in this proxy statement/prospectus or any annex to this proxy statement/prospectus are qualified in all respects by reference to the copy of the relevant contract or other annex filed as an exhibit to this proxy statement/prospectus.
All information contained in this document relating to MURF has been supplied by MURF, and all such information relating to Conduit has been supplied by Conduit. Information provided by one another does not constitute any representation, estimate or projection of the other.
If you would like additional copies of this document or if you have questions about the Business Combination, you should contact via phone or in writing:
Jack K. Heilbron
4995 Murphy Canyon Road, Suite 300
San Diego, CA 92123
(760) 471-8536
| 193 |
INDEX TO FINANCIAL STATEMENTS
| Unaudited Condensed Financial Statements of Conduit Pharmaceuticals Limited: | |
| Page(s) | |
| Condensed Balance Sheets | F-2 |
| Condensed Statements of Operations and Comprehensive Loss | F-3 |
| Condensed Statements of Changes in Shareholders’ Deficit | F-4 |
| Condensed Statements of Cash Flows | F-5 |
| Notes to Financial Statements | F-6 |
| Audited Financial Statements of Conduit Pharmaceuticals Limited: | |
| Page(s) | |
| Report of Independent Registered Public Accounting Firm | F-17 |
| Balance Sheets | F-18 |
| Statements of Operations and Comprehensive Loss | F-19 |
| Statements of Changes in Shareholders’ Deficit | F-20 |
| Statements of Cash Flows | F-21 |
| Notes to Financial Statements | F-22 |
| F-1 |
CONDUIT PHARMACEUTICALS LIMITED
BALANCE SHEETS
(unaudited, in thousands, except share amounts)
| As of March 31, | As of December 31, | |||||||
| 2023 | 2022 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 8 | $ | - | ||||
| Total current assets | 8 | - | ||||||
| Intangible assets – research and development | 5 | 5 | ||||||
| Total assets | $ | 13 | $ | 5 | ||||
| Liabilities and shareholders’ deficit | ||||||||
| Current liabilities: | ||||||||
| Accrued professional fees | $ | 2,419 | $ | 2,246 | ||||
| Accrued payroll and other current liabilities | 25 | 338 | ||||||
| Option liability | 1,309 | 1,417 | ||||||
| Total current liabilities | 3,753 | 4,001 | ||||||
| Convertible notes payable, carried at fair value | 3,620 | 1,835 | ||||||
| Deferred revenue | 4,173 | 4,083 | ||||||
| Convertible promissory note payable, carried at cost | 805 | - | ||||||
| Notes payable | 179 | 175 | ||||||
| Total liabilities | 12,530 | 10,094 | ||||||
| Commitments and Contingencies (Note 9) | - | - | ||||||
| Ordinary shares, par value $0.0001 (£0.0001) per share; 400,000,000 shares authorized; 2,000 shares issued and outstanding as of March 31, 2023 and December 31, 2022, respectively | - | - | ||||||
| Accumulated deficit | (12,929 | ) | (10,764 | ) | ||||
| Accumulated other comprehensive income (loss) | 412 | 675 | ||||||
| Total shareholders’ deficit | (12,517 | ) | (10,089 | ) | ||||
| Total liabilities and shareholders’ deficit | $ | 13 | $ | 5 | ||||
The accompanying notes are an integral part of these condensed financial statements.
| F-2 |
CONDUIT PHARMACEUTICALS LIMITED
STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(unaudited, in thousands, except share amounts and per share data)
| Three Months Ended March 31, | ||||||||
| 2023 | 2022 | |||||||
| Operating expenses: | ||||||||
| General and administrative expenses | $ | 2,008 | $ | 291 | ||||
| Total operating expenses | 2,008 | 291 | ||||||
| Loss from operation | (2,008 | ) | (291 | ) | ||||
| Other expense, net: | ||||||||
| Interest income – Investments held in Trust Account | ||||||||
| Other expense, net | (157 | ) | (16 | ) | ||||
| Total other expense, net | (157 | ) | (16 | ) | ||||
| Net loss | $ | (2,165 | ) | $ | (307 | ) | ||
| Net loss per share attributable to ordinary shareholders – basic and diluted | $ | (1,082 | ) | $ | (307 | ) | ||
| Weighted-average shares outstanding used in calculating net loss per share – basic and diluted | 2,000 | 1,000 | ||||||
| Comprehensive income (loss): | ||||||||
| Foreign currency translation adjustment | (263 | ) | 140 | |||||
| Total comprehensive loss | $ | (2,428 | ) | $ | (167 | ) | ||
The accompanying notes are an integral part of these condensed financial statements.
| F-3 |
CONDUIT PHARMACEUTICALS LIMITED
STATEMENTS OF CHANGES IN SHAREHOLDERS’ DEFICIT
(unaudited, in thousands, except share amounts)
| Ordinary shares | Accumulated | Accumulated other comprehensive | Total shareholders’ | |||||||||||||||||
| Shares | Amount | deficit | income | deficit | ||||||||||||||||
| Balance at January 1, 2023 | 2,000 | $ | - | $ | (10,764 | ) | $ | 675 | $ | (10,089 | ) | |||||||||
| Foreign currency translation adjustment | - | - | - | (263 | ) | (263 | ) | |||||||||||||
| Net loss | - | - | (2,165 | ) | - | (2,165 | ) | |||||||||||||
| Balance at March 31, 2023 | 2,000 | $ | - | $ | (12,929 | ) | $ | 412 | $ | (12,517 | ) | |||||||||
The accompanying notes are an integral part of these condensed financial statements.
| F-4 |
CONDUIT PHARMACEUTICALS LIMITED
STATEMENTS OF CASH FLOWS
(unaudited, in thousands)
| Three Months Ended March 31, | ||||||||
| 2023 | 2022 | |||||||
| Cash flows from operating activities | ||||||||
| Net Loss | $ | (2,165 | ) | $ | (307 | ) | ||
| Adjustments to reconcile net loss to net cash flows from operating activities: | ||||||||
| Gain on sale of equity securities received for sale of future revenue | - | (33 | ) | |||||
| Gain on change in fair value of option liability | (136 | ) | - | |||||
| Loss on equity securities receivable | - | 48 | ||||||
| Change in fair value of convertible notes payable | 280 | 1 | ||||||
| Change in reserve for uncollectible loan | 243 | - | ||||||
| Noncash interest expense | 5 | - | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accrued expenses and other current liabilities | (196 | ) | (497 | ) | ||||
| Net cash flows used in operating activities | (1,969 | ) | (788 | ) | ||||
| Cash flows from investing activities | ||||||||
| Issuance of loan – related party | (243 | ) | - | |||||
| Net cash flows (used in) provided by investing activities | (243 | ) | - | |||||
| Cash flows from financing activities | ||||||||
| Issuance of convertible promissory note payable, carried at cost | 786 | - | ||||||
| Issuance of convertible notes payable, carried at fair value | 1,434 | - | ||||||
| Proceeds from sale of equity securities | - | 789 | ||||||
| Net cash flows provided by financing activities | 2,220 | 789 | ||||||
| Net change in cash and cash equivalents before effect of exchange rates | 8 | 1 | ||||||
| Effect of exchange rate changes on cash and cash equivalents | - | 1 | ||||||
| Net increase in cash and cash equivalents | 8 | 2 | ||||||
| Cash and cash equivalents, at beginning of period | $ | - | $ | - | ||||
| Cash and cash equivalents, ending | $ | 8 | $ | 2 | ||||
| Supplemental schedule of noncash financing activities | ||||||||
| FV of shares received and receivable related to the sale of future revenue | $ | - | $ | 1,422 | ||||
The accompanying notes are an integral part of these condensed financial statements.
| F-5 |
CONDUIT PHARMACEUTICALS LIMITED
NOTES TO CONDENSED FINANCIAL STATEMENTS
1. Nature of the Business, Basis of Presentation and Summary of Significant Accounting Policies
Conduit Pharmaceuticals Limited, formerly known as SGS Global Limited (“Conduit” or the “Company”), was incorporated in the Cayman Islands as an exempted company in December 2018.
Conduit is a clinical-stage specialty biopharmaceutical company that was formed to facilitate the development and commercialization of clinical assets that have not been, or are not being, prioritized by leading biopharmaceutical companies in order to develop pharmaceutical products that meet the unmet medical needs of patients.
The Company’s current development pipeline includes a glucokinase activator, which is Phase II ready in autoimmune diseases including uveitis, Hashimoto’s Thyroiditis, preterm labor and renal transplant rejection. The Company’s development pipeline also includes a potent, irreversible inhibitor of human Myeloperoxidase (MPO) that has the potential to treat idiopathic male infertility.
Merger Agreement
On November 8, 2022, the Company entered into a Merger Agreement (the “Merger Agreement”) with Conduit Merger Sub, Inc. (“Merger Sub”) and Murphy Canyon Acquisition Corp. (“MURF”), a publicly traded blank check special purpose acquisition company.
Under the terms of the Merger proposed in the Merger Agreement, a wholly owned subsidiary of Murphy will merge with the Company, with the Company being the surviving company and therefore becoming, upon closing of the Merger Agreement, a wholly owned subsidiary of Murphy.
The Business Combination will be accounted for a reverse recapitalization in accordance with U.S. GAAP. Under the reverse recapitalization method, MURF will be treated as the acquired company for financial reporting purposes, and the Company, the accounting acquirer, will be assumed to have issued shares of stock for the net assets of MURF, with no goodwill or other intangible assets recorded. This determination is primarily based on the following predominant factors: (i) post-closing, the Company’s shareholders are expected to have a majority of the voting power of the combined company and ability to elect the members of the combined company’s Board of Directors (“Board”); (ii) the on-going operations post-merger will comprise those of Conduit; and (iii) all of the senior management of the combined company, except for the Chief Financial Officer, will be members of the management of the Company. As a result of the Business Combination, MURF will be renamed “Conduit Pharmaceuticals Inc.” The board of directors of each MURF and Conduit have each approved the Business Combination. The completion of the Business Combination, which is expected to occur in the second quarter of 2023, is subject to a special resolution of the Company’s shareholders and approval of MURF’s stockholders of the respective entities and the satisfaction or waiver of certain other customary closing conditions.
Basis of Presentation
The accompanying condensed financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and the rules and regulations of the Securities and Exchange Commission. The functional currency of the Company is the British pound sterling. The accompanying financial statements are reported in United States Dollars, the reporting currency of the Company. Operating results for the three months ended March 31, 2023 are not necessarily indicative of the results that may be expected through December 31, 2023. The accompanying unaudited condensed financial statements should be read in conjunction with the audited financial statements and notes thereto included in the Registration Statement.
Liquidity and Going Concern
Since its inception, and in line with its growth strategy, the Company has prepared its financial statements assuming it will continue as a going concern. As of March 31, 2023, and December 31, 2022, the Company had an accumulated deficit of $12.9 million and $10.8 million, respectively. For the three months ended March 31, 2023 and March 31, 2022, the Company had net losses of $2.2 million and $0.3 million, respectively, and cash used in operating activities of $2.0 million and $0.8 million, respectively. To date, the Company has not generated sufficient liquidity to fund its operations and has relied on funding through a combination of debt and equity financing. The Company has determined additional financing will be required to fund its operations for the next twelve months and the ability of the Company to continue as a going concern is dependent upon obtaining such additional financing.
| F-6 |
As further discussed in Note 5 – Convertible Notes Payable, in the fourth quarter of 2022 the Company approved approximately $3.7 million of Convertible Notes, of which approximately $1.8 million was issued and funded in full through the date of issuance of the financial statements. Additionally, as further discussed in the preceding section, on November 8, 2022, the Company entered into a definitive Agreement and Plan of Merger with Merger Sub and MURF, that included a private placement of an aggregate amount of $27 million of MURF’s shares of common stock which will be settled upon closing (referred to as the “PIPE”).
The Company has raised and plans on raising further funds as set out above including through the above-mentioned convertible debt as well as through the transactions contemplated by the Merger Agreement and the related PIPE transaction. There is currently no public market for the Company’s ordinary shares. While the Company believes in the viability of its ability to raise additional funds, there can be no assurances to that effect. These matters raise substantial doubt about the Company’s ability to continue as a going concern for a period of twelve months from the issue date of this report. These financial statements have been prepared assuming the Company will continue as a going concern and do not include adjustments to reflect the possible effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
Risks and Uncertainties
The Company is subject to risks common to companies in the pharmaceutical industry including, but not limited to, uncertainties related to commercialization of competitor products, regulatory approvals, dependence on key products, dependence on key customers and suppliers, and protection of intellectual property rights. Clinical assets currently under development will require significant additional research and development efforts, including extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts will require significant amounts of additional capital, adequate personnel, infrastructure, and extensive compliance and reporting capabilities. Even if the Company’s efforts are successful, it is uncertain when, if ever, the Company will realize significant revenue from product sales.
In addition, on March 11, 2020, the World Health Organization declared the Coronavirus Disease 2019 (“COVID-19”) a global pandemic. The pandemic has been a highly disruptive economic and societal event that remains unpredictable. Its duration and extent continue to be dependent on various developments, such as the emergence of variants to the virus that may cause additional strains of COVID-19, the administration and ultimate effectiveness of vaccines, and the eventual timeline to achieve a sufficient level of herd immunity among the general population. Accordingly, the COVID-19 pandemic may continue to have negative effects on the health of the U.S. and other economies for the foreseeable future.
As this crisis has unfolded, the Company has continued to monitor conditions and adapt its operations to meet federal, state, and local standards. The Company cannot predict the duration or severity of the COVID-19 pandemic or its ultimate impact on the broader economy or the Company’s operations and liquidity.
Due to the pandemic, all clinical trials in the United Kingdom that were not related to COVID-19 were put on hiatus for significant portions of the three months ended March 31, 2022. As a result, during the hiatus, the Company shifted its activities to focus on clinical trials related to COVID-19.
Summary of Significant Accounting Policies
Cash and Cash Equivalents
Cash and cash equivalents are primarily maintained with major financial institutions in the United Kingdom and Switzerland. The Company considers cash equivalents to be short-term, highly liquid investments that (a) are readily convertible into known amounts of cash, (b) are traded and held for cash management purposes, and (c) have original maturities of three months or less at the time of purchase. The Switzerland bank accounts holding cash balances are uninsured. The Company has not experienced any losses on this account through the period ended March 31, 2023.
As of March 31, 2023, the Company held immaterial cash equivalents. As of December 31, 2022, the Company did not hold any cash equivalents.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and related disclosures of contingent assets and liabilities at the date of the financial statements as well as the reported amounts of revenues and expenses during the reporting period. Estimates are based on several factors including the facts and circumstances available at the time the estimates are made, historical experience, risk of loss, general economic conditions and trends, and the assessment of the probable future outcome. Subjective and significant estimates include, but are not necessarily limited to, the inputs used to determine the fair value of convertible notes payable, the Cizzle option and the amount to reserve for the related party loan receivable. Actual results could differ materially from such estimates. Estimates and assumptions are reviewed periodically by management and changes in estimates are made as management becomes aware of changes in circumstances surrounding the estimates. The effects of changes are reflected in the financial statements in the period that they are determined.
| F-7 |
Fair Value Measurements
Accounting Standards Codification (“ASC”) Topic 820, Fair Value Measurements and Disclosures, defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements. Fair value is to be determined based on the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. In determining fair value, the Company used various valuation approaches. A fair value hierarchy has been established for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are those that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Company.
Unobservable inputs reflect the Company’s assumption about the inputs that market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. The fair value hierarchy is categorized into three levels, based on the inputs, as follows:
| ● | Level 1—Valuations based on quoted prices for identical instruments in active markets. Since valuations are based on quoted prices that are readily and regularly available in an active market, valuation of these instruments does not entail a significant degree of judgment. | |
| ● | Level 2— Valuations based on observable inputs other than quoted prices included in Level 1, such as quoted prices for either similar instruments in active markets, identical or similar instruments in markets that are not active, or model-derived valuations whose inputs or significant value drivers are observable or can be corroborated by observable market data. | |
| ● | Level 3—Valuations based on inputs that are unobservable. These valuations require significant judgment. |
The Company’s Level 1 assets consist of cash and cash equivalents in the accompanying balance sheets and the value of accrued expenses and other current liabilities approximate fair value due to the short-term nature of these assets and liabilities.
As of March 31, 2023, the Company has two financial liabilities, convertible notes that are adjusted to fair value on a recurring basis as well as an option liability for which the fair value is determined based on Level 3 inputs as such inputs are not readily observable. See Note 2, Note 4 and Note 5 for further information on the Company’s financial liability held at fair value.
Research and Development and Funding
Research and development expenses consist primarily of costs incurred in connection with the research and development of our product candidates and programs. Funding expenses consist primarily of costs incurred in connection with the Company providing funding to St George Street Capital (“SGSC”) to carry out its research and development activities. SGSC holds all licenses to conduct clinical research through third party pharmaceutical companies. The Company expenses research and development costs and intangible assets acquired that have no alternative future use as incurred. These expenses include:
| ● | expenses incurred under agreements with organizations that support the Company’s drug discovery and development activities; | |
| ● | expenses incurred in connection with the preclinical and clinical development of the Company’s product candidates and programs, including under agreements with contract research organizations, or CROs; | |
| ● | costs related to contract manufacturing organizations, or CMOs, that are primarily engaged to provide drug substance and product for our clinical trials, research and development programs, as well as investigative sites and consultants that conduct the Company’s clinical trials, nonclinical studies and other scientific development services; | |
| ● | the costs of acquiring and manufacturing nonclinical and clinical trial materials, including manufacturing registration and validation batches; | |
| ● | employee-related expenses, including salaries, related benefits and equity-based compensation expense, for employees engaged in research and development functions; | |
| ● | costs related to compliance with quality and regulatory requirements; | |
| ● | payments made under third-party licensing agreements; and | |
| ● | direct and allocated costs related to facilities, information technology, personnel and other overhead. |
| F-8 |
Advance payments that we make for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses. Such amounts are recognized as an expense as the goods are delivered or consumed or the related services are performed, or until it is no longer expected that the goods will be delivered, or the services rendered.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs for personnel in executive management, finance, corporate and business development, and administrative functions. General and administrative expenses also include legal fees relating to patent and corporate matters; professional fees for accounting, auditing, tax, and administrative consulting services; insurance costs; administrative travel expenses and other operating costs
The Company has incurred increased accounting, audit, legal, regulatory, compliance and director and officer insurance costs as well as investor and public relations expenses associated with being a public company. The Company anticipates that its general and administrative expenses will increase in the future as it increases its headcount to support development of its product candidates and programs and with continued research and development activities.
Income Taxes
ASC Topic 740, Income Taxes, sets forth standards for financial presentation and disclosure of income tax liabilities and expense. Interest and penalties recognized have been classified in the statements of operations and comprehensive loss as income taxes. Deferred tax assets and liabilities are recognized for future tax consequences attributable to temporary differences between the financial statement carrying amount of existing assets and liabilities and their respective tax bases and operating losses carried forward. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the statements of operations in the period that includes the enactment date. The measurement of deferred tax assets is reduced, if necessary, by a valuation allowance for any tax benefits of which future realization is uncertain.
The Company is exempt from income tax in the Cayman Islands.
Net Loss per Ordinary Share Attributable to Ordinary Shareholders
The Company calculates net loss per share attributable to ordinary shareholders – basic and diluted using the two-class method under ASC Topic 260, Earnings Per Share. Basic net loss per share attributable to ordinary shareholders is computed by dividing the net loss attributable to ordinary shareholders by the number of weighted-average ordinary shares outstanding for the period. Diluted net loss attributable to ordinary shareholders is computed by adjusting net loss attributable to ordinary shareholders to reallocate undistributed earnings based on the potential impact of any dilutive securities. Diluted net loss per share attributable to ordinary shareholders is computed by dividing the diluted net loss attributable to ordinary shareholders by the number of weighted-average ordinary shares outstanding for the period including potential dilutive ordinary shares. All potentially dilutive securities have been excluded from the diluted net loss per share calculations for the periods ended March 31, 2023 and March 31, 2022, respectively, as the assumed issuance of all such potentially dilutive shares would have had an anti-dilutive effect.
Investments in Equity Securities
Investments in equity securities that have a readily determinable fair value are reported at fair value. Changes in fair value between accounting periods are recorded in other expense, net, in the statements of operations and comprehensive loss. Realized gains or losses upon sale are recorded in other expense, net, in the statements of operations and comprehensive loss. The Company did not hold any available for sale or trading securities at the period ended March 31, 2023, or at the year ended December 31, 2022.
Intangible Assets
Intangible assets subject to amortization include a developed patent. The Company qualitatively evaluates intangible assets for impairment annually or whenever events or changes in circumstances indicate that it is more likely than not the carrying amount of intangible assets may excess their implied fair values. The company recorded an immaterial amount for the intangible asset and an immaterial amount of amortization expense for the periods ended March 31, 2023 and March 31, 2022. As of March 31, 2023 and December 31, 2022, no indicators of impairment of the intangible asset were identified.
| F-9 |
Related Party Loan
The loan made to a related party is stated at the principal amount of $0.6 million outstanding. The Company recorded an allowance in full of $0.6 million for potential loan losses, resulting in no balance on the face of the balance sheet as of the period ended March 31, 2023 and year ended December 31, 2022. The loan carries no interest, and as such, no interest receivable is recorded. The Company has recorded a full reserve against the loan as the related party did not have the ability to repay the loans as of March 31, 2023 and December 31, 2022. The Company will analyze the reserve periodically to determine whether an adjustment to the allowance is appropriate.
Foreign Currency Translation
Monetary assets and liabilities in the functional currency are re-measured into the reporting currency at the rates of exchange prevailing at the reporting date. Income and expense transactions in the functional currency are re-measured into the reporting currency at the average exchange rate prevailing during the reporting period. Non-monetary items in functional currency are re-measured into the reporting currency at the historical exchange rate (i.e., the rate of exchange at the date of the transaction). The gains or losses resulting from foreign currency translation are included in the statements of operations and comprehensive loss.
Emerging Growth Company Status
The Company is an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. The Company has elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that: (i) is no longer an emerging growth company or (ii) affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. As a result, these financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates.
If the Business Combination were to be consummated, the surviving company will remain an emerging growth company, as defined by the Jumpstart Our Business Startups act of 2012, until the earliest of (i) the last day of the combined entity’s first fiscal year following the fifth anniversary of the completion of MURF’s initial public offering, (ii) the last day of the fiscal year in which the combined entity has total annual gross revenue of at least $1.235 billion, (iii) the last day of the fiscal year in which the combined entity is deemed to be a large accelerated filer, which means the market value of the combined entity’s common stock that is held by non-affiliates exceeds $700.0 million as of the prior December 31st or (iv) the date on which the combined entity has issued more than $1.0 billion in non-convertible debt securities during the prior three year period.
2. Fair Value
The following table presents as of March 31, 2023 the Company’s liabilities subject to measurement at fair value on a recurring basis (in thousands):
Schedule of Liabilities Subject to Measurement at Fair Value on Recurring Basis
| Fair Value Measurements as of March 31, 2023 | ||||||||||||||||
| In thousands | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
| Liabilities: | ||||||||||||||||
| Convertible notes payable | $ | - | $ | - | $ | 3,620 | $ | 3,620 | ||||||||
| Option liability | $ | - | $ | - | $ | 1,309 | $ | 1,309 | ||||||||
| Total Liabilities | $ | - | $ | - | $ | 4,929 | $ | 4,929 | ||||||||
The following table presents as of December 31, 2022 the Company’s liabilities subject to measurement at fair value on a recurring basis (in thousands):
| Fair Value Measurements as of December 31, 2022 | ||||||||||||||||
| In thousands | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
| Liabilities: | ||||||||||||||||
| Convertible notes payable | $ | - | $ | - | $ | 1,835 | $ | 1,835 | ||||||||
| Option liability | $ | - | $ | - | $ | 1,417 | $ | 1,417 | ||||||||
| Total Liabilities | $ | - | $ | - | $ | 3,252 | $ | 3,252 | ||||||||
| F-10 |
The following table presents additional information about the financial liabilities subject to measurement at fair value on a recurring basis for which the Company used significant unobservable inputs (Level 3) (in thousands):
Schedule of Additional Information About the Financial Liabilities Subject To Measurement at Fair Value
| Amount | ||||
| Balance as of December 31, 2022 | $ | 1,835 | ||
| Issuance of debt | 1,459 | |||
| Change in fair value | 280 | |||
| Foreign currency exchange impact | 46 | |||
| Balance as of March 31, 2023 | $ | 3,620 | ||
The convertible notes payable were valued using the fair value option and are considered Level 3 measured instruments. See Note 5 for additional information. Due to the embedded derivatives included in the convertible notes payable, the Company elected to use the fair value option. The fair value was determined based upon a probability-weighted present value approach under three scenarios that consider the provisions of the convertible notes payable. The following table outlines the range of significant unobservable inputs as of March 31, 2023 and December 31, 2022, respectively:
Schedule of Fair Value Significant Unobservable Inputs
| Assumption | ||||||||
| Unobservable input - Change of control | 2023 | 2022 | ||||||
| Probabilities of conversion provisions | 10 - 90 | % | 10 - 90 | % | ||||
| Estimated timing of conversion | 0.33 - 1.59 years | 0.25 - 1.41 years | ||||||
| Time period to maturity | 1.41 years | 1.41 years | ||||||
| Risk-adjusted discount rate | 6.3 | % | 6.1 | % | ||||
The option liability was valued using public market research to determine the probability of success that similar studies in the respiratory and cardiovascular disease areas and a Black-Scholes pricing model. In reviewing the public market research, the Company determined the phase transition success rates for trials similar to the Covid Asset from Phase I to Phase II was 52.7%. In applying this rate to the sale of future revenue consideration realized , the Company determined the underlying Covid Asset value to be $2.8 million. The Company used the underlying Covid Asset value within a Black-Scholes model to determine the fair value was $1.3 million and $1.4 million at March 31, 2023 and December 31, 2022, respectively. In accordance with ASC 815, the fair value of the option will be remeasured at the end of each reporting period, with changes in fair value recorded to the statement of operations and total comprehensive loss. For the three months ended March 31, 2023, the Company recorded an option liability of $1.3 million on the balance sheet.
The following table presents additional information about the option liability subject to measurement at fair value on a recurring basis for which the Company used significant unobservable inputs (Level 3) (in thousands):
Schedule of Additional Information About the Option Liability Subject to Measurement at Fair Value
| Amount | ||||
| Balance, beginning of period | $ | 1,417 | ||
| Option issued | - | |||
| Change in fair value | (136 | ) | ||
| Foreign currency exchange impact | 28 | |||
| Balance, end of period | $ | 1,309 | ||
During the period ended March 31, 2023, there were no transfers between Level 1 and Level 2, nor into or out of Level 3.
| F-11 |
3. Accrued Expenses
Accrued expenses consisted of the following as of March 31, 2023 and December 31, 2022 (in thousands):
Schedule of Accrued Expenses
| March 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| Accrued expenses: | ||||||||
| Professional service fees | $ | 2,419 | $ | 2,246 | ||||
| Payroll | 25 | 338 | ||||||
| Total accrued expenses and other current liabilities | $ | 2,444 | $ | 2,584 | ||||
4. Liability Related to the Sale of Future Revenue
Indirect Investment Regarding the Covid Asset
On June 3, 2020, the Company entered into an agreement with SGSC for an indirect investment in the AZ Covid Asset. Under the terms of the agreement, SGSC agreed to pay the Company a royalty of 30% of sales in excess of $24.5 million (£19.2 million) of the Covid Asset should it reach the commercialization stage and generate revenue in exchange for the Company funding SGSC’s research and development efforts. As of March 31, 2023, the Company provided $0.3 million in funding to SGSC.
Vela Technologies PLC
The Company entered into an Agreement with SGSC to approve an Indirect Investment from Vela Technologies PLC (“Vela”) on October 20, 2020, whereby Vela agreed to provide funding to the Company for an indirect investment in an asset related to COVID-19 (the “Covid Asset”) for use in the field in exchange for 8% of future revenue earned if the Covid Asset is commercialized (the “Vela Agreement”). Total consideration under the Vela Agreement was $2.9 million (£2.35 million), consisting of $1.6 million (£1.25 million) cash and the issuance of 1.1 billion ordinary shares in Vela, which based on the fair value of stock at the September 10, 2021, was $1.3 million. The Company received the $1.6 million (£1.25) million cash consideration during the year ended December 31, 2020. This consideration was recorded in deferred income as a liability on the balance sheet in accordance with ASC 470-10.
Cizzle PLC
On February 11, 2022, the Company entered into an agreement with Cizzle PLC (“Cizzle”) whereby Cizzle agreed to purchase a percentage of future revenue earned in the Covid Asset, should it reach the commercialization stage. Total consideration under the agreement is specified as $1.6 million (£1.2 million), consisting of the issuance of fair value of 25.0 million new ordinary shares in Cizzle on the date of the agreement and the fair value of 22.0 million shares to be issued at the earlier of Cizzle’s shareholder approval or one year from the date of the agreement. The 22.0 million shares were received by the Company in the fourth quarter of 2022 and were subsequently sold within the fourth quarter of 2022. The Company recorded a liability related to deferred revenue of $1.4 million for the consideration received from Cizzle.
The payments received for the sale of future revenue will be classified as deferred income. Under ASC 470-10-25, a seller of future revenue should evaluate whether the proceeds received should be accounted for as debt or deferred income. In assessing the factors that created rebuttable presumption of debt within the guidance, the Company determined that there were factors present to overcome the debt presumption and deferred income classification to be appropriate. The main factors the Company considered were that the transactions in form were sales, and not debt transactions. Each agreement does not guarantee a return to each purchaser, the return is based solely on future performance of the AZ Covid Asset should it reach commercialization, with neither purchaser having an involvement in generating future cash flows from the AZ Covid Asset.
The following table presents as of March 31, 2023 the Company’s liability for the sale of future revenue (in thousands):
Schedule of Liability for the Sale of Future Revenue
| Liability related to the sale of future royalties | ||||
| December 31, 2022 | $ | 4,083 | ||
| Sale of future royalties | - | |||
| Foreign currency exchange impact | 90 | |||
| March 31, 2023 | $ | 4,173 | ||
| F-12 |
On December 15, 2022, the Company entered into an agreement with Cizzle whereby the Company granted Cizzle the option, but not the obligation, to sell its economic interest in the Covid Asset back to the Company. The agreement contained an option period of nine months from the date of the agreement for Cizzle to notify the Company of its intent to exercise the option to sell its economic interest in the Covid Asset. Upon closing of the agreement, Cizzle agreed to pay the Company an option fee of $0.1 million (£0.1 million). If Cizzle does exercise the right to sell its interest in the Covid Asset, consideration payable by the Company to Cizzle is $3.9 million (£3.25) million through the issuance of new Ordinary Shares in the combined entity post-Merger at the same price per share of the proposed PIPE investment in the Company by new and existing investors.
5. Convertible Notes Payable
On May 27, 2021, the Company approved a Master Convertible Loan Note Instrument (the “2021 Convertible Loan Note Instrument”), permitting the Company to issue convertible notes in a maximum aggregate principal amount of up to $1.4 million (£1.0 million). The convertible notes issuable under the 2021 Convertible Loan Note Instrument mature three years after issuance to the respective noteholders and bear 5% interest, only to be paid to the noteholders in the event of a material breach by the Company of the terms of the 2021 Convertible Loan Note Instrument. In the event of a Change of Control (as defined in the 2021 Convertible Loan Note Instrument), the convertible notes issued under the 2021 Convertible Loan Note Instrument automatically convert into ordinary shares of the Company at a conversion price equal to a 20% discount to the price per share paid for the most senior class of shares in respect of such Change of Control. The Company, with consent from the noteholders, may prepay the convertible notes payable issued under the 2021 Convertible Loan Note Instrument without penalty. The convertible notes payable issued under the 2021 Convertible Loan Note Instrument are general, unsecured obligations of the Company.
On August 26, 2022, under the terms of the 2021 Convertible Loan Note Instrument, the Company issued a $0.5 million (£0.4 million) convertible note payable to an investor.
On October 6, 2022, under the terms of the 2021 Convertible Loan Note Instrument, the Company issued a $67 thousand (£50 thousand) convertible note payable to an investor. As of October 6, 2022, $1.3 million (£950,000) 2021 Convertible Loan Notes were issued and outstanding.
On November 1, 2022, the Company approved a master Convertible Loan Note Instrument (the “2022 Convertible Loan Note Instrument”), permitting the Company to issue convertible notes payable for a maximum aggregate principal amount of up to $3.3 million (£3.0 million). The convertible notes payable issuable under the 2022 Convertible Loan Note Instrument mature three years after issuance to the respective noteholders and bear 5% interest, only to be paid to the noteholders in the event of a material breach by the Company of the terms of the 2022 Convertible Loan Note Instrument. In the event of a Change of Control (as defined in the 2022 Convertible Loan Note Instrument), the convertible notes payable issued under the 2022 Convertible Loan Note Instrument automatically convert into ordinary shares of the Company at a conversion price equal to a 20% discount to the price per share paid for the most senior class of shares in respect of such Change of Control. The Company, with consent from the noteholders, may prepay the convertible notes payable issued under the 2022 Convertible Loan Note Instrument without penalty. The convertible notes payable issued under the 2022 Convertible Loan Note Instrument are general, unsecured obligations of the Company.
On November 16, 2022, under the terms of the 2022 Convertible Loan Note Instrument, the Company issued convertible notes payable with an aggregate principal amount of $0.4 million (£0.3 million) to an investor.
During January and February 2023, under the terms of the 2022 Convertible Loan Note Instrument, the Company issued convertible notes payable with an aggregate principal amount of $0.9 million (£0.8 million) to non-related third parties.
As discussed in Note 10 – Related Party Transactions, during January and February 2023, under the terms of the 2022 Convertible Loan Note Instrument, the Company issued convertible notes payable with an aggregate principal amount of $0.5 million (£0.4 million) to the CEO of Corvus.
| F-13 |
The Company elected to fair value the convertible notes payable issued under the 2021 and 2022 Convertible Loan Note Instruments. At the end of each reporting period, the Company calculates the fair value of the convertible notes payable, and any changes in fair value are reported in other income, net, in the current period’s statement of operations and comprehensive loss. There has been no change in fair value from a change in credit quality. For the year period ended March 31, 2023, the Company recorded a loss from the change in fair value of convertible notes payable of $0.3 million in other income, net, in its statement of operations and comprehensive less. For the year ended March 31, 2022, the Company recorded an immaterial loss from the change in fair value of convertible notes payable in other income, net, in its statement of operations and comprehensive loss. See Note 2 for additional information regarding the fair value measurement of convertible notes payable. Accrued interest
Convertible Promissory Notes Payable
During March 2023, the Company issued a promissory convertible note payable with an aggregate principal amount of $0.8 million to a non-related third party. The note matures and is payable in full 18 months from the date of the note. The note carries 20% interest and is payable every six (6) months from the date of the note until the maturity date. The note is subject to conversion of MURF common stock upon consummation of the Business Combination prior to the maturity date. Issuance costs associated with the note were immaterial and expensed as incurred on the Company’s statement of operations and comprehensive loss. The Company has not elected the fair value option and will account for the promissory convertible note payable as a liability in accordance with ASC 480 on the Company’s balance sheet. The only subsequent measurement impact on a recurring basis until conversion (if conversion occurs) or prepayment (if prepayment occurs) will be to record the accrued interest as a liability and reduce the balances of the Note and its accrued interest for cash payments made against these balances. As of March 31, 2023, accrued interest on the convertible promissory note was immaterial.
The Company notes that this issuance was outside of the terms of the 2022 Convertible Loan Note Instrument.
6. Loans Payable
On May 1, 2022, the Company entered into Loan Agreements (the “Loans”) with two lenders, totaling $0.2 million. The Loans mature two years from the date of the agreement and bear no interest. Each loan was made available to the Company by the lenders in three tranches of (i) $33 thousand (£30 thousand); (ii) $33 thousand (£30 thousand) and (iii) $28 thousand (£25 thousand), totaling $0.2 million. The Loans provided for events of default, including, among others, failure to make payment, bankruptcy and non-compliance with the terms of the Loans. As of March 31, 2023 the Company utilized all three tranches of the first loan and two out of three tranches of the second loan, with total loans payable at March 31, 2023 and December 31, 2022 of $0.2 million and $0.2 million, respectively.
7. Ordinary Shares
As of March 31, 2023 and December 31, 2022, the Company has authorized the issuance of up to 400,000,000 Ordinary Shares (the “Ordinary Shares”), par value £0.0001 per share.
On November 4, 2022, the Company issued 1,000 Ordinary Shares to Corvus Capital Limited. Corvus Capital Limited subsequently transferred 775 Ordinary Shares to other investors.
As of March 31, 2023 and December 31, 2022, 2,000 Ordinary Shares were issued and outstanding. As of March 31, 2023 and March 31, 2022, no cash dividends have been declared or paid.
Holders of the Ordinary Shares are entitled to one vote per share, and to receive dividends, on and if declared by the board of directors and, upon liquidation or dissolution, are entitled to receive all assets available for distribution, subordinate to the rights, preferences, and privileges of any outstanding preferred shares (if any) with respect to dividends and in connection with liquidation, winding up and dissolution of the Company. The holders have no preemptive or other subscription rights.
8. Net Loss Per Share Attributable to Ordinary Shareholders
The following table presents the calculation of basic and diluted net loss per share attributable to holders of the Ordinary Shares (in thousands, except share amounts and per share data):
Schedule of Basic and Diluted Net Loss Per Share
| March 31, | ||||||||
| 2023 | 2022 | |||||||
| Numerator: | ||||||||
| Net loss and comprehensive loss | $ | (2,165 | ) | $ | (307 | ) | ||
| Denominator: | ||||||||
| Weighted average number of ordinary shares outstanding - basic and diluted | 2,000 | 1,000 | ||||||
| Net loss per share - basic and diluted | $ | (1,082 | ) | $ | (307 | ) | ||
| F-14 |
Since the Company was in a loss position for all periods presented, basic net loss per share attributable to holders of the Ordinary Shares is the same as diluted net loss per share attributable to holders of the Ordinary Shares for all periods presented as the inclusion of all potential Ordinary Shares outstanding would have been anti-dilutive.
Potentially dilutive securities (upon conversion) that were not included in the diluted per share calculations because they would have been anti-dilutive were as follows:
Schedule of Potentially Dilutive Securities
| March 31, | ||||
| 2023 | ||||
| Ordinary Shares potentially issuable upon conversion of convertible notes payable | 3,070,000 | |||
| Total | 3,070,000 | |||
| March 31, | ||||
| 2022 | ||||
| Ordinary Shares potentially issuable upon conversion of convertible notes payable | 500,000 | |||
| Total | 500,000 | |||
9. Commitments and Contingencies
Legal Proceedings
From time to time, the Company may become involved in legal proceedings or other litigation relating to claims arising in the ordinary course of business. The Company intends to accrue a liability for such matters when it is probable that future expenditures will be made and that such expenditures can be reasonably estimated. Significant judgment is required to determine both probability and estimated exposure amount. Legal fees and other costs associated with such proceedings are expensed as incurred. The Company is not presently a party to any litigation such that the ultimate resolution is reasonably expected to have a material effect on the Company’s financial statements.
10. Related Party Transactions
Corvus Capital Limited
Corvus Capital Limited (“Corvus”) is a significant investor in the Company through subscribing to 1,000 Ordinary Shares as of the period ended March 31, 2023. The Chief Executive Officer of Corvus is a member of Conduit’s board of directors. During the period ended September 30, 2021, the Company incurred $1.7 million (£1.3 million) in advisory fees for funding and review of a potential acquisition candidates to Corvus, which are included in general and administrative expenses in the statement of operations and comprehensive loss. As of March 31, 2023 and December 31, 2022, approximately $0.6 million of the advisory fees were not paid and were recorded to accrued expenses on the balance sheet. For the periods ended March 31, 2023 and March 31, 2022, the Company incurred director’s fees payable to the CEO of Corvus of approximately $0.3 million and $0.1 million, respectively. As of March 31, 2023 and December 31, 2022, the Company owed the CEO of Corvus director’s fees of approximately $0.2 million and $0.4 million, respectively. These amounts owed to the CEO of Corvus are included in accrued expenses and other current liabilities in the balance sheets. During the period ended March 31, 2022, the Company paid a family member of the CEO of Corvus approximately $33 thousand. The Company did not make any payments to the family member of the CEO of Corvus for the period ended March 31, 2023.
During January and February 2023, under the terms of the 2022 Convertible Loan Note Instrument, the Company issued convertible notes payable with an aggregate principal amount of $0.5 million (£0.4 million) to the CEO of Corvus. The convertible notes payable mature three years after issuance and bear 5% interest, only to be paid in the event of a material breach by the Company of the terms of the 2022 Convertible Loan Note Instrument. In the event of a Change of Control, the convertible notes payable automatically convert into ordinary shares of the Company at a conversion price equal to a 20% discount to the price per share paid for the most senior class of shares in respect of such Change of Control.
| F-15 |
St George Street Capital
St George Street Capital is a significant investor in the Company through subscribing to 225 Ordinary Shares. The Chief Executive Officer of St George Street Capital is also the Chief Executive Officer of Conduit. Further, the Company has an Exclusive Funding Agreement (as defined below) with St George Street Capital. For the periods ended March 31, 2023 and March 31, 2022, the Company did not incur expenses to St George Street Capital. As of March 31, 2023 and December 31, 2022, the Company did not owe any amounts to St George Street Capital.
On March 26, 2021, the Company entered into the Exclusive Funding Agreement (“Funding Agreement”) with St George Street Capital. Under the agreement, the Company has the first exclusive right, but not the obligation, to provide or procure funding for the performance or research and development projects undertaken by St George Street Capital. The Funding Agreement entitles the Company to 100% of the net revenue on projects that the Company funds by itself. As of March 31, 2023, the Company has not recognized any net revenue from the Funding Agreement. See Note 1 for discussion on research and development costs.
11. Other Expense, net
The following table presents other expense, net, for the periods ended March 31, 2023 and 2022 (in thousands):
Schedule of Other Expense, Net
| For the three months ended March 31, | ||||||||
| 2023 | 2022 | |||||||
| Other income: | ||||||||
| Change in fair value of option liability |
|
$ | 136 | $ | - | |||
| Gain on sale of investment in equity securities | - | 33 | ||||||
| Total other income | $ | 136 | $ | 33 | ||||
| Other expense: | ||||||||
| Change in fair value of equity securities receivable | - | 48 | ||||||
| Change in fair value of convertible notes payable | 280 | 1 | ||||||
| Realized foreign currency transaction loss | 8 | - | ||||||
| Interest expense on convertible promissory note | 5 | - | ||||||
| Total other expense | $ | 293 | $ | 49 | ||||
| Total other income (expense), net | $ | (157 | ) | $ | (16 | ) | ||
12. Subsequent Events
Vela Option Agreement
In April 2023, the Conduit entered into an agreement with Vela which granted Vela the right, but not the obligation, to sell its 8% royalty interest in the Covid Asset back to Conduit. Vela paid a one-time, non-refundable option fee to Conduit of $0.5 million (£0.4 million). Total consideration payable to Vela upon exercise of the option is $4.8 million (£4.0 million) worth of MURF common stock, following the consummation of the Merger, at a price per share equal to the volume-weighted average price per share over the ten (10) business days prior to the date of the notice of exercise. The option contains a provision stating that in no event shall the price per share for the consideration shares be lower than $5 or higher than $15. The option is exercisable in whole at any time from the close of the Merger (the “Effective Time”) until the earlier of (i) the date that is six (6) months from the Effective Time, and (ii) February 7, 2024, the expiration date of the term.
| F-16 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
Conduit Pharmaceutical Limited
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Conduit Pharmaceuticals Limited (the “Company”) as of December 31, 2022 and 2021, the related statements of operations and comprehensive loss, changes in shareholders’ deficit and cash flows for each of the two years in the period ended December 31, 2021, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 1, the Company has a significant working capital deficiency, has incurred significant losses and needs to raise additional funds to meet its obligations and sustain its operations. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB and in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Marcum llp
Marcum llp
We have served as the Company’s auditor since 2022
New York, NY
May 12, 2023
| F-17 |
December
31, 2022
CONDUIT PHARMACEUTICALS LIMITED
BALANCE SHEETS
(In thousands, except share amounts)
As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Assets | ||||||||
| Intangible assets – research and development | $ | 5 | $ | - | ||||
| Total assets | $ | 5 | $ | - | ||||
| Liabilities and shareholders’ deficit | ||||||||
| Current liabilities: | ||||||||
| Accrued professional fees | $ | 2,246 | $ | 1,308 | ||||
| Accrued payroll and other current liabilities | 338 | 927 | ||||||
| Option liability | 1,404 | - | ||||||
| Total current liabilities | 3,988 | 2,235 | ||||||
| Convertible notes payable, carried at fair value | 1,835 | 749 | ||||||
| Deferred revenue | 4,083 | 2,958 | ||||||
| Notes payable | 175 | - | ||||||
| Total liabilities | 10,081 | 5,942 | ||||||
| Commitments and Contingencies (Note 8) | - | - | ||||||
| Ordinary shares, par value $0.0001 (£0.0001) per share; 400,000,000 shares authorized; 2,000 and 1,000 shares issued and outstanding as of December 31, 2022 and 2021, respectively | - | - | ||||||
| Accumulated deficit | (10,764 | ) | (5,877 | ) | ||||
| Accumulated other comprehensive loss | 688 | (65 | ) | |||||
| Total shareholders’ deficit | (10,076 | ) | (5,942 | ) | ||||
| Total liabilities and shareholders’ deficit | $ | 5 | $ | - | ||||
The accompanying notes are an integral part of these financial statements.
| F-18 |
CONDUIT PHARMACEUTICALS LIMITED
STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except share amounts and per share data)
| Year Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Operating expenses: | ||||||||
| Research and development expenses | $ | 37 | $ | 28 | ||||
| General and administrative expenses | 3,049 | 2,890 | ||||||
| Funding expenses | 74 | 255 | ||||||
| Total operating expenses | 3,160 | 3,173 | ||||||
| Loss from operation | (3,160 | ) | (3,173 | ) | ||||
| Other expense: | ||||||||
| Other expense | (1,727 | ) | (484 | ) | ||||
| Total other expense | (1,727 | ) | (484 | ) | ||||
| Net loss | $ | (4,887 | ) | $ | (3,657 | ) | ||
| Net loss per share attributable to ordinary shareholders – basic and diluted | $ | (2,444 | ) | $ | (3,657 | ) | ||
| Weighted-average shares outstanding used in calculating net loss per share – basic and diluted | 2,000 | 1,000 | ||||||
| Comprehensive loss: | ||||||||
| Net loss | (4,887 | ) | (3,657 | ) | ||||
| Foreign currency translation adjustment | 753 | 79 | ||||||
| Total comprehensive loss | $ | (4,134 | ) | $ | (3,578 | ) | ||
The accompanying notes are an integral part of these financial statements.
| F-19 |
CONDUIT PHARMACEUTICALS LIMITED
STATEMENTS OF CHANGES IN SHAREHOLDERS’ DEFICIT
(In thousands, except share amounts)
Accumulated other | Total | |||||||||||||||||||
| Ordinary shares | Accumulated | comprehensive | shareholders’ | |||||||||||||||||
| Shares | Amount | deficit | income/(loss) |
deficit | ||||||||||||||||
| Balance at January 1, 2021 | 1,000 | $ | - | $ | (2,220 | ) | $ | (144 | ) | $ | (2,364 | ) | ||||||||
| Foreign currency translation adjustment | - | - | - | 79 | 79 | |||||||||||||||
| Net loss | - | - | (3,657 | ) | - | (3,657 | ) | |||||||||||||
| Balance at December 31, 2021 | 1,000 | $ | - | $ | (5,877 | ) | $ | (65 | ) | $ | (5,942 | ) | ||||||||
| Issuance of common shares | 1,000 | - | ||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | 753 | 753 | |||||||||||||||
| Net loss | (4,887 | ) | - | (4,887 | ) | |||||||||||||||
| Balance at December 31, 2022 | 2,000 | $ | - | $ | (10,764 | ) | $ | 688 | $ | (10,076 | ) | |||||||||
The accompanying notes are an integral part of these financial statements.
| F-20 |
CONDUIT PHARMACEUTICALS LIMITED
STATEMENTS OF CASH FLOWS
(In thousands)
| Year Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Cash flows from operating activities | ||||||||
| Net Loss | $ | (4,887 | ) | $ | (3,657 | ) | ||
| Adjustments to reconcile net loss to net cash flows from operating activities: | ||||||||
| Loss on option | 1,300 | |||||||
| Loss on sale of equity securities | 129 | 76 | ||||||
| Change in fair value of convertible notes payable | 265 | 73 | ||||||
| Change in loan allowance | 331 | - | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accrued expenses and other current liabilities | 601 | 1,357 | ||||||
| Intangible assets | (5 | ) | - | |||||
| Net cash flows used in operating activities | (2,266 | ) | (2,151 | ) | ||||
| Cash flows from investing activities | ||||||||
| Proceeds from issuance of option | 148 | - | ||||||
| Loans to related party | (331 | ) | - | |||||
| Net cash flows used in investing activities | (183 | ) | - | |||||
| Cash flows from financing activities | ||||||||
| Issuance of convertible notes payable | 928 | 688 | ||||||
| Proceeds from sale of equity securities | 1,341 | 1,210 | ||||||
| Proceeds from related party long-term debt | 179 | - | ||||||
| Net cash flows provided by financing activities | 2,448 | 1,898 | ||||||
| Net change in cash and cash equivalents before effect of exchange rate changes | (1 | ) | (253 | ) | ||||
| Effect of exchange rate changes on cash and cash equivalents | 1 | 1 | ||||||
| Net decrease in cash and cash equivalents | - | (252 | ) | |||||
| Cash and cash equivalents, at beginning of period | $ | - | $ | 252 | ||||
| Cash and cash equivalents, ending | $ | - | $ | - | ||||
| Supplemental schedule of noncash financing activities | ||||||||
| FV of shares received related to the sale of future revenue | $ | 1,471 | $ | 1,286 | ||||
The accompanying notes are an integral part of these financial statements.
| F-21 |
CONDUIT PHARMACEUTICALS LIMITED
NOTES TO FINANCIAL STATEMENTS
| 1. | Nature of the Business, Basis of Presentation and Summary of Significant Accounting Policies |
Conduit Pharmaceuticals Limited, formerly known as SGS Global Limited (“Conduit” or the “Company”), was incorporated in the Cayman Islands as an exempted company in December 2018.
Conduit is a clinical-stage specialty biopharmaceutical company that was formed to facilitate the development and commercialization of clinical assets that have not been, or are not being, prioritized by leading biopharmaceutical companies in order to develop pharmaceutical products that meet the unmet medical needs of patients.
The Company’s current development pipeline includes a glucokinase activator, which is Phase II ready in autoimmune diseases including uveitis, Hashimoto’s Thyroiditis, preterm labor and renal transplant rejection. The Company’s development pipeline also includes a potent, irreversible inhibitor of human Myeloperoxidase (MPO) that has the potential to treat idiopathic male infertility.
Merger Agreement
On November 8, 2022, the Company entered into a Merger Agreement (the “Merger Agreement”) with Conduit Merger Sub, Inc. (“Merger Sub”) and Murphy Canyon Acquisition Corp. (“MURF”), a publicly traded blank check special purpose acquisition company.
Under the terms of the Merger proposed in the Merger Agreement, a wholly owned subsidiary of Murphy will merge with the Company, with the Company being the surviving company and therefore becoming, upon closing of the Merger Agreement, a wholly owned subsidiary of Murphy.
The Business Combination will be accounted for a reverse recapitalization in accordance with U.S. GAAP. Under the reverse recapitalization method, MURF will be treated as the acquired company for financial reporting purposes, and the Company, the accounting acquirer, will be assumed to have issued shares of stock for the net assets of MURF, with no goodwill or other intangible assets recorded. This determination is primarily based on the following predominant factors: (i) post-closing, the Company’s shareholders are expected to have a majority of the voting power of the combined company and ability to elect the members of the combined company’s Board of Directors (“Board”); (ii) the on-going operations post-merger will comprise those of Conduit; and (iii) all of the senior management of the combined company, except for the Chief Financial Officer, will be members of the management of the Company. As a result of the Business Combination, MURF will be renamed “Conduit Pharmaceuticals Inc.” The board of directors of each MURF and Conduit have each approved the Business Combination. The completion of the Business Combination, which is expected to occur in the second quarter of 2023, is subject to a special resolution of the Company’s shareholders and approval of MURF’s stockholders of the respective entities and the satisfaction or waiver of certain other customary closing conditions.
Basis of Presentation
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and the rules and regulations of the Securities and Exchange Commission. The functional currency of the Company is the British pound sterling. The accompanying financial statements are reported in United States Dollars, the reporting currency of the Company.
| F-22 |
Liquidity and Going Concern
Since its inception, and in line with its growth strategy, the Company has prepared its financial statements assuming it will continue as a going concern. Since its inception, the Company has incurred net losses and negative cash flows from operations. As of December 31, 2022, and 2021, the Company had an accumulated deficit of $10.8 million and $5.9 million, respectively. For the years ended December 31, 2022 and 2021, the Company had net losses of $4.9 million and $3.7 million, respectively, and cash used in operating activities of $2.3 million and $2.2 million, respectively. To date, the Company has not generated sufficient liquidity to fund its operations and has relied on funding through a combination of debt and equity financing. The Company has determined additional financing will be required to fund its operations for the next twelve months and the ability of the Company to continue as a going concern is dependent upon obtaining such additional financing.
As further discussed in Note 5 – Convertible Notes Payable, in the fourth quarter of 2022 the Company approved approximately $3.6 million of Convertible Notes, of which approximately $1.8 million was issued and funded in full through the date of issuance of the financial statements. Additionally, as further discussed in the preceding section, on November 8, 2022, the Company entered into a definitive Agreement and Plan of Merger with Merger Sub and MURF, that included a private placement of an aggregate amount of $27 million of MURF’s shares of common stock which will be settled upon closing (referred to as the “PIPE”).
The Company has raised and plans on raising further funds as set out above including through the above-mentioned convertible debt as well as through the transactions contemplated by the Merger Agreement and the related PIPE transaction. There is currently no public market for the Company’s ordinary shares. While the Company believes in the viability of its ability to raise additional funds, there can be no assurances to that effect. These matters raise substantial doubt about the Company’s ability to continue as a going concern for a period of twelve months from the issue date of this report. These financial statements have been prepared assuming the Company will continue as a going concern and do not include adjustments to reflect the possible effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
Risks and Uncertainties
The Company is subject to risks common to companies in the pharmaceutical industry including, but not limited to, uncertainties related to commercialization of competitor products, regulatory approvals, dependence on key products, dependence on key customers and suppliers, and protection of intellectual property rights. Clinical assets currently under development will require significant additional research and development efforts, including extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts will require significant amounts of additional capital, adequate personnel, infrastructure, and extensive compliance and reporting capabilities. Even if the Company’s efforts are successful, it is uncertain when, if ever, the Company will realize significant revenue from product sales.
In addition, on March 11, 2020, the World Health Organization declared the Coronavirus Disease 2019 (“COVID-19”) a global pandemic. The pandemic has been a highly disruptive economic and societal event that remains unpredictable. Its duration and extent continue to be dependent on various developments, such as the emergence of variants to the virus that may cause additional strains of COVID-19, the administration and ultimate effectiveness of vaccines, and the eventual timeline to achieve a sufficient level of herd immunity among the general population. Accordingly, the COVID-19 pandemic may continue to have negative effects on the health of the U.S. and other economies for the foreseeable future.
As this crisis has unfolded, the Company has continued to monitor conditions and adapt its operations to meet federal, state, and local standards. The Company cannot predict the duration or severity of the COVID-19 pandemic or its ultimate impact on the broader economy or the Company’s operations and liquidity.
Due to the pandemic, all clinical trials in the United Kingdom that were not related to COVID-19 were put on hiatus through a significant portion of the year ended December 31, 2021. During the year ended December 31, 2022, the hiatus As a result, during the hiatus, the Company shifted its activities to focus on clinical trials related to COVID-19.
| F-23 |
Summary of Significant Accounting Policies
Cash and Cash Equivalents
Cash and cash equivalents are primarily maintained with major financial institutions in the United Kingdom and Switzerland. The Company considers cash equivalents to be short-term, highly liquid investments that (a) are readily convertible into known amounts of cash, (b) are traded and held for cash management purposes, and (c) have original maturities of three months or less at the time of purchase. The Switzerland bank accounts holding cash balances are uninsured. The Company has not experienced any losses on this account through the year ended December 31, 2022.
As of December 31, 2022 and 2021, the Company did not hold any cash equivalents.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and related disclosures of contingent assets and liabilities at the date of the financial statements as well as the reported amounts of revenues and expenses during the reporting period. Estimates are based on several factors including the facts and circumstances available at the time the estimates are made, historical experience, risk of loss, general economic conditions and trends, and the assessment of the probable future outcome. Subjective and significant estimates include, but are not necessarily limited to, the inputs used to determine the fair value of convertible notes payable and the amount to reserve for the related party loan receivable. Actual results could differ materially from such estimates. Estimates and assumptions are reviewed periodically by management and changes in estimates are made as management becomes aware of changes in circumstances surrounding the estimates. The effects of changes are reflected in the financial statements in the period that they are determined.
Fair Value Measurements
Accounting Standards Codification (“ASC”) Topic 820, Fair Value Measurements and Disclosures, defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements. Fair value is to be determined based on the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants. In determining fair value, the Company used various valuation approaches. A fair value hierarchy has been established for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are those that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Company.
Unobservable inputs reflect the Company’s assumption about the inputs that market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. The fair value hierarchy is categorized into three levels, based on the inputs, as follows:
| ● | Level 1—Valuations based on quoted prices for identical instruments in active markets. Since valuations are based on quoted prices that are readily and regularly available in an active market, valuation of these instruments does not entail a significant degree of judgment. |
| F-24 |
| ● | Level 2— Valuations based on observable inputs other than quoted prices included in Level 1, such as quoted prices for either similar instruments in active markets, identical or similar instruments in markets that are not active, or model-derived valuations whose inputs or significant value drivers are observable or can be corroborated by observable market data. | |
| ● | Level 3—Valuations based on inputs that are unobservable. These valuations require significant judgment. |
The Company’s Level 1 assets consist of cash and cash equivalents in the accompanying balance sheets and the value of accrued expenses and other current liabilities approximate fair value due to the short-term nature of these assets and liabilities.
As of December 31, 2022 and 2021, the Company has two financial liabilities, convertible notes that are adjusted to fair value on a recurring basis as well as an option liability for which the fair value is determined based on Level 3 inputs as such inputs are not readily observable. See Note 2 and Note 5 for further information on the Company’s financial liability held at fair value.
Research and Development and Funding
Research and development expenses consist primarily of costs incurred in connection with the research and development of our product candidates and programs. Funding expenses consist primarily of costs incurred in connection with the Company providing funding to St George Street Capital (“St George Street”) to carry out its research and development activities. St George Street holds all licenses to conduct clinical research through third party pharmaceutical companies. The Company expenses research and development costs and intangible assets acquired that have no alternative future use as incurred. These expenses include:
| ● | expenses incurred under agreements with organizations that support the Company’s drug discovery and development activities; | |
| ● | expenses incurred in connection with the preclinical and clinical development of the Company’s product candidates and programs, including under agreements with contract research organizations, or CROs; | |
| ● | costs related to contract manufacturing organizations, or CMOs, that are primarily engaged to provide drug substance and product for our clinical trials, research and development programs, as well as investigative sites and consultants that conduct the Company’s clinical trials, nonclinical studies and other scientific development services; | |
| ● | the costs of acquiring and manufacturing nonclinical and clinical trial materials, including manufacturing registration and validation batches; | |
| ● | employee-related expenses, including salaries, related benefits and equity-based compensation expense, for employees engaged in research and development functions; | |
| ● | costs related to compliance with quality and regulatory requirements; | |
| ● | payments made under third-party licensing agreements; and | |
| ● | direct and allocated costs related to facilities, information technology, personnel and other overhead. |
Advance payments that we make for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses. Such amounts are recognized as an expense as the goods are delivered or consumed or the related services are performed, or until it is no longer expected that the goods will be delivered, or the services rendered.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs for personnel in executive management, finance, corporate and business development, and administrative functions. General and administrative expenses also include legal fees relating to patent and corporate matters; professional fees for accounting, auditing, tax, and administrative consulting services; insurance costs; administrative travel expenses and other operating costs.
| F-25 |
The Company has incurred increased accounting, audit, legal, regulatory, compliance and director and officer insurance costs as well as investor and public relations expenses associated with being a public company. The Company anticipates that its general and administrative expenses will increase in the future as it increases its headcount to support the development of its product candidates and programs and with continued research and development activities.
Income Taxes
ASC Topic 740, Income Taxes, sets forth standards for financial presentation and disclosure of income tax liabilities and expense. Interest and penalties recognized have been classified in the statements of operations and comprehensive loss as income taxes. Deferred tax assets and liabilities are recognized for future tax consequences attributable to temporary differences between the financial statement carrying amount of existing assets and liabilities and their respective tax bases and operating losses carried forward. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the statements of operations in the period that includes the enactment date. The measurement of deferred tax assets is reduced, if necessary, by a valuation allowance for any tax benefits of which future realization is uncertain.
The Company is exempt from income tax in the Cayman Islands.
Net Loss per Ordinary Share Attributable to Ordinary Shareholders
The Company calculates net loss per share attributable to ordinary shareholders – basic and diluted using the two-class method under ASC Topic 260, Earnings Per Share. Basic net loss per share attributable to ordinary shareholders is computed by dividing the net loss attributable to ordinary shareholders by the number of weighted-average ordinary shares outstanding for the period. Diluted net loss attributable to ordinary shareholders is computed by adjusting net loss attributable to ordinary shareholders to reallocate undistributed earnings based on the potential impact of any dilutive securities. Diluted net loss per share attributable to ordinary shareholders is computed by dividing the diluted net loss attributable to ordinary shareholders by the number of weighted-average ordinary shares outstanding for the period including potential dilutive ordinary shares. All potentially dilutive securities have been excluded from the diluted net loss per share calculations for the years ended December 31, 2022 and 2021, respectively, as the assumed issuance of all such potentially dilutive shares would have had an anti-dilutive effect.
Intangible Assets
Intangible assets subject to amortization include a developed patent. The Company qualitatively evaluates intangible assets for impairment annually or whenever events or changes in circumstances indicate that it is more likely than not the carrying amount of intangible assets may excess their implied fair values. The company recorded an immaterial amount for the intangible asset and an immaterial amount of amortization expense for the year ended December 31, 2022. As of December 31, 2022, no indicators of impairment of the intangible asset were identified.
Related Party Loan
The loan made to a related party is stated at the principal amount of $0.3 million outstanding. The Company recorded an allowance in full of $0.3 million for potential loan losses, resulting in no balance on the face of the balance sheet at December 31, 2022. The loan carries no interest, and as such, no interest receivable is recorded. The Company has recorded a full reserve against the loan as the related party did not have the ability to repay the loans as of December 31, 2022. The Company will analyze the reserve periodically to determine whether an adjustment to the allowance is appropriate.
| F-26 |
Investments in Equity Securities
Investments in equity securities that have a readily determinable fair value are reported at fair value. Changes in fair value between accounting periods are recorded in other income, net, in the statements of operations and comprehensive loss. Realized gains or losses upon sale are recorded in other income, net, in the statements of operations and comprehensive loss. The Company did not hold any available for sale or trading securities at the years ended December 31, 2022 and December 31, 2021.
Foreign Currency Translation
Monetary assets and liabilities in the functional currency are re-measured into the reporting currency at the rates of exchange prevailing at the reporting date. Income and expense transactions in the functional currency are re-measured into the reporting currency at the average exchange rate prevailing during the reporting period. Non-monetary items in functional currency are re-measured into the reporting currency at the historical exchange rate (i.e., the rate of exchange at the date of the transaction). The gains or losses resulting from foreign currency translation are included in the statements of operations and comprehensive loss.
Emerging Growth Company Status
The Company is an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. The Company has elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that: (i) is no longer an emerging growth company or (ii) affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. As a result, these financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates.
If the Business Combination (see Note 1) were to be consummated, the surviving company will remain an emerging growth company, as defined by the Jumpstart Our Business Startups act of 2012, until the earliest of (i) the last day of the combined entity’s first fiscal year following the fifth anniversary of the completion of MURF’s initial public offering, (ii) the last day of the fiscal year in which the combined entity has total annual gross revenue of at least $1.235 billion, (iii) the last day of the fiscal year in which the combined entity is deemed to be a large accelerated filer, which means the market value of the combined entity’s common stock that is held by non-affiliates exceeds $700.0 million as of the prior December 31st or (iv) the date on which the combined entity has issued more than $1.0 billion in non-convertible debt securities during the prior three year period
Recently Adopted Accounting Pronouncements
Effective January 1, 2021, the Company adopted ASU No. 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40) (“ASU No. 2020-06”). ASU No. 2020-06 was issued to address issues identified as a result of the complexity associated with applying generally accepted accounting principles for certain financial instruments with characteristics of liabilities and equity. For convertible instruments, the FASB decided to reduce the number of accounting models for convertible debt instruments and convertible preferred stock. In addition to eliminating certain accounting models, the FASB also decided to enhance information transparency by making targeted improvements to the disclosures for convertible instruments and earnings-per-share guidance. The adoption of ASU No. 2020-06 did not have a material impact on the Company’s financial statements.
| F-27 |
In February 2016, the FASB issued ASU 2016-02, “Leases (Topic 842).” Topic 842 was subsequently amended by ASU 2018-10, “Codification Improvements to Topic 842, Leases” and ASU 2018-11, “Leases (Topic 842)”. The amendments in this update increase transparency and comparability among organizations by recognizing lease assets and lease liabilities on the balance sheet and disclosing key information about leasing arrangements. For leases with a term of 12 months or less, the amendments permit lessees to make an accounting policy election by class of underlying assets not to recognize lease assets and lease liabilities. For finance leases, the amendments in this update require a lessee to (1) recognize a right-of-use asset and lease liability, initially measured at the present value of the lease payments, on the balance sheet; (2) recognize interest on the lease liability separately from amortization of the right-of-use asset in the statement of operations; (3) classify repayments of the principal portion of the lease liability within financing activities and payments of interest on the lease liability and variable lease payments within operating activities in the statement of cash flows. For operating leases, the amendments in this update require a lessee to (1) recognize a right-of-use asset and a lease liability, initially measured at the present value of the lease payments, on the balance sheet; (2) recognize a single lease cost, calculated so that the cost of the lease is allocated over the lease term on a generally straight-line basis; (3) classify all cash payments within operating activities in the statement of cash flows. On October 16, 2019, the FASB approved the proposal to delay the effective date for this standard for private and all other entities. Due to the Company’s extended transition period election, the amendments are effective for fiscal years beginning after December 15, 2020. In June 2020, the FASB issued ASU No. 2020-05, Coronavirus Disease 2019 (“COVID-19”) in response to the pandemic which has adversely affected the global economy and caused significant and widespread business and capital market disruptions. The FASB is committed to supporting and assisting stakeholders during this difficult time. The FASB issued ASU 2020-05 as a limited deferral of the effective dates of certain ASUs, including ASU 2016-02 (including amendments issued after the issuance of the original) to provide immediate, near-term relief for certain entities for whom these ASUs are either currently effective or imminently effective. This update is effective for annual periods beginning January 1, 2022, and interim periods beginning January 1, 2023, with early adoption permitted. The Company is in the process of evaluating its impact. The Company did not have any lease agreements as of the years ended December 31, 2022 and 2021 and does not believe the new standard will have a material impact on the financial statements. The Company adopted the standard on January 1, 2022. The adoption of ASU No. 2016-02 did not have a material impact on the Company’s financial statements.
Recent Accounting Pronouncements Not Yet Adopted
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740) (“ASU No. 2019-12”). ASU No. 2019-12 was issued to simplify the accounting for income taxes. The amendments in this update remove certain exceptions and add other requirements to govern the accounting for income taxes. This update is effective for public business entities for fiscal years beginning after December 15, 2020. For all other entities, this update is effective for fiscal years beginning after December 15, 2021, and interim periods within fiscal years beginning after December 15, 2022. The Company is exempt from tax in the Cayman Islands and therefore does not believe that the adoption of ASU No. 2019-12 will have a material effect on its financial statements or related disclosures.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. The ASU adds to US GAAP an impairment model known as the current expected credit loss (CECL) model, which is based on expected losses rather than incurred losses. The objectives of the CECL model are to: (1) reduce the complexity in US GAAP by decreasing the number of credit impairment models that entities use to account for debt instruments, (2) eliminate the barrier to timely recognition of credit losses by using an expected loss model instead of an incurred loss model, (3) require an entity to recognize an allowance of lifetime expected credit losses, and (4) not require a specific method for entities to use in estimating expected credit losses. This ASU will be effective for fiscal years beginning after December 15, 2022. An entity should apply the amendment on a prospective basis at the beginning of the interim period that includes the adoption date. The Company is currently evaluating the new standard to determine the potential impact on its financial condition, results of operations, cash flows, and financial statement disclosures.
| F-28 |
2. Fair Value
The following tables present as of December 31, 2022 and 2021 respectively, the Company’s liabilities subject to measurement at fair value on a recurring basis (in thousands):
Schedule of Liabilities Subject to Measurement at Fair Value on Recurring Basis
| Fair Value Measurements as of December 31, 2022 | ||||||||||||||||
| In thousands | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
| Liabilities: | ||||||||||||||||
| Convertible notes payable | $ | - | $ | - | $ | 1,835 | $ | 1,835 | ||||||||
| Option liability | $ | - | $ | - | $ | 1,404 | $ | 1,404 | ||||||||
| Total Liabilities | $ | - | $ | - | $ | 3,239 | $ | 3,239 | ||||||||
| Fair Value Measurements as of December 31, 2021 | ||||||||||||||||
| In thousands | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
| Liabilities: | ||||||||||||||||
| Convertible notes payable | $ | - | $ | - | $ | 749 | $ | 749 | ||||||||
| Total Liabilities | $ | - | $ | - | $ | 749 | $ | 749 | ||||||||
The following table presents additional information about the convertible notes payable subject to measurement at fair value on a recurring basis for which the Company used significant unobservable inputs (Level 3) (in thousands):
Schedule of Additional Information About the Financial Liabilities Subject To Measurement at Fair Value
| Amount | ||||
| Balance, beginning of period | $ | 749 | ||
| Notes issued | 908 | |||
| Change in fair value | 264 | |||
| Foreign currency exchange impact | (86 | ) | ||
| Balance, end of period | $ | 1,835 | ||
| F-29 |
The convertible notes payable were valued using the fair value option and are considered Level 3 measured instruments. See Note 5 for additional information. Due to the embedded derivatives included in the convertible notes payable, the Company elected to use the fair value option. The fair value was determined based upon a probability-weighted present value approach under three scenarios that consider the provisions of the convertible notes payable. The following table outlines the range of significant unobservable inputs used in the third-party valuation of the convertible notes payable as of December 31, 2022 and December 31, 2021, respectively:
Schedule of Fair Value Significant Unobservable Inputs
| Assumption | ||||||||
| Unobservable input - Change of control | 2022 | 2021 | ||||||
| Probabilities of conversion provisions | 10 - 90% | 5 - 67.5% | ||||||
| Estimated timing of conversion | 0.25 - 1.41 years | 0.75 - 2.41 years | ||||||
| Time period to maturity | 1.41 years | 2.41 years | ||||||
| Risk-adjusted discount rate | 6.1 | % | 2.9 | % | ||||
The option liability was valued using public market research to determine the probability of success that similar studies in the respiratory and cardiovascular disease areas and a Black-Scholes pricing model. In reviewing the public market research, the Company determined the phase transition success rates for trials similar to the Covid Asset from Phase I to Phase II was 52.7%. In applying this rate to the sale of future revenue consideration realized , the Company determined the underlying Covid Asset value to be $2.8 million. The Company used the underlying Covid Asset value within a Black-Scholes model to determine the fair value was $1.4 million at December 31, 2022. In accordance with ASC 815, the fair value of the option will be remeasured at the end of each reporting period, with changes in fair value recorded to the statement of operations and total comprehensive loss. For the year ended December 31, 2022, the Company recorded an option liability of $1.4 million on the balance sheet.
The following table presents additional information about the option liability subject to measurement at fair value on recurring basis for which the Company used significant unobservable inputs (Level 3) (in thousands):
Schedule of Additional Information About the Option Liability Subject to Measurement at Fair Value
| Amount | ||||
| Balance, beginning of period | $ | - | ||
| Option issued | 1,404 | |||
| Change in fair value | - | |||
| Foreign currency exchange impact | - | |||
| Balance, end of period | $ | 1,404 | ||
The following table presents the inputs to the Black-Scholes model as of December 31, 2022 to determine the fair value of the Covid Asset option (in thousands where applicable):
Schedule of Fair Value of the Covid Asset Option
| Black-Scholes Inputs | Assumption | |||
| Underlying asset price | $ | 2,812 | ||
| Strike Price | $ | 3,960 | ||
| Volatility | 90 | % | ||
| Risk-free rate | 4.68 | % | ||
| Dividend yield | 0 | % | ||
| Option term | .71 years | |||
During the years ended December 31, 2022 and 2021, there were no transfers between 1evel 1 and Level 2, nor into or out of Level 3.
3. Accrued Expenses
Accrued expenses consisted of the following as of December 31, 2022 and 2021 (in thousands):
Schedule of Accrued Expenses
| December 31, | ||||||||
| 2022 | 2021 | |||||||
| Accrued expenses: | ||||||||
| Professional service fees | $ | 2,246 | $ | 1,308 | ||||
| Payroll | 338 | 921 | ||||||
| Other current liabilities: | ||||||||
| Other | - | 6 | ||||||
| Total accrued expenses and other current liabilities | $ | 2,584 | $ | 2,235 | ||||
| F-30 |
4. Liability Related to the Sale of Future Royalties
Indirect Investment Regarding the Covid Asset
On June 3, 2020, the Company entered into an agreement with St George Street for an indirect investment in the AZ Covid Asset. Under the terms of the agreement, St George Street agreed to pay the Company a royalty of 30% of sales of the Covid Asset should it reach the commercialization stage and generate revenue in exchange for the Company funding St George Street’s research and development efforts. This funding for this agreement differed from other projects under the Funding Agreement as the Company was investing in the future revenues of the AZ Covid Asset and not funding 100% of the total project. The Company has the right of first of refusal to fund projects. Should the Company choose not to fund a project as requested by St George Street, St George Street has the right to seek third party funding at which point the Company will not receive the 30% royalty per the agreement for future revenues generated from that specific project. St George Street holds all intellectual rights received from third party pharmaceutical companies and performs all of the research and development and clinical trials necessary to move each asset toward commercialization. As of December 31, 2022, the Company provided $0.3 million in funding to St George Street.
Vela Technologies PLC
The Company entered into an Agreement with St George Street to approve funding from Vela Technologies PLC (“Vela”) on October 20, 2020, whereby Vela agreed to provide funding to the Company via a purchase of future royalties an asset related to COVID-19 (the “Covid Asset”) totaling 8% of future revenue earned if the Covid Asset is commercialized (the “Vela Agreement”). Total consideration under the Vela Agreement was $2.9 million (£2.35 million), consisting of $1.6 million (£1.25 million) cash and the issuance of 1.1 billion ordinary shares in Vela, which based on the fair value of stock at the September 10, 2021, was $1.3 million. The Company received the $1.6 million (£1.25) million cash consideration during the year ended December 31, 2020. This consideration was recorded as deferred revenue in the balance sheet in accordance with ASC 470-10. The shares were received by St George Street and subject to lock-up agreement.
During the year ended December 31, 2021, the Company received the 1.1 billion ordinary shares in Vela from St George Street, upon expiration of the lock-up agreement, that were issuable as consideration under the agreement. On the date the Company received the shares, Vela’s equity securities had a fair value of $1.3 million and were recorded as a liability on the balance sheet. During the year ended December 31, 2021, the Company sold all 1.1 billion of its Vela shares for $1.2 million. The Company recorded a loss on the sale of Vela shares of $76 thousand to the statement of operations and comprehensive loss. The Company incurred placement fees of $0.3 million in conjunction with the sale of its investment in the Vela shares, which the Company recorded with the loss on sale of shares as other expense in the statement of operations and comprehensive loss. During the year ended December 31, 2021, the Company did not pay any net sums to Vela for the commercialization of the Covid Asset.
Share Consideration in Cizzle PLC
On February 11, 2022, the Company entered into an agreement with Cizzle PLC (“Cizzle”) whereby Cizzle agreed to provide funding to the Company for the Covid Asset for use in the field through a purchase of a 5% of future revenue earned if the Covid Asset is commercialized. Total consideration under the agreement is specified as $1.4 million (£1.2 million), consisting of the issuance of fair value of 25.0 million new ordinary shares in Cizzle on the date of the agreement and the fair value of 22.0 million shares to be issued at the earlier of Cizzle’s shareholder approval or one year from the date of the agreement. The 22.0 million shares were received in October 2022. The Company recorded a liability related to deferred revenue of $1.4 million for the consideration received from Cizzle.
The payments received for the sale of future revenue will be classified as deferred income. Under ASC 470-10-25, a seller of future revenue should evaluate whether the proceeds received should be accounted for as debt or deferred income. In assessing the factors that created rebuttable presumption of debt within the guidance, the Company determined that there were factors present to overcome the debt presumption and deferred income classification to be appropriate. The main factors the Company considered were that the transactions in form were sales, and not debt transactions. Each agreement does not guarantee a return to each purchaser, the return is based solely on future performance of the AZ Covid Asset should it reach commercialization, with neither purchaser having an involvement in generating future cash flows from the AZ Covid Asset.
| F-31 |
The following table presents as of December 31, 2022 the Company’s liability for the sale of future revenue (in thousands):
Schedule of Liability for the Sale of Future Revenue
| Liability related to the sale of future royalties | ||||
| December 31, 2021 | $ | 2,958 | ||
| Sale of future royalties | 1,439 | |||
| Foreign currency exchange impact | (314 | ) | ||
| December 31, 2022 | $ | 4,083 | ||
On December 15, 2022, the Company entered into an agreement with Cizzle whereby the Company granted Cizzle the option, but not the obligation, to sell its economic interest in the Covid Asset back to the Company. The agreement contained an option period of nine months from the date of the agreement for Cizzle to notify the Company of its intent to exercise the option to sell its economic interest in the Covid Asset. Upon closing of the agreement, Cizzle agreed to pay the Company an option fee of $0.1 million (£0.1 million). If Cizzle does exercise the right to sell its interest in the Covid Asset, consideration payable by the Company to Cizzle is $3.9 million (£3.25) million through the issuance of new Ordinary Shares in the combined entity post-Merger at the same price per share of the proposed PIPE investment in the Company by new and existing investors.
5. Convertible Notes Payable
On May 27, 2021, the Company approved a Master Convertible Loan Note Instrument (the “2021 Convertible Loan Note Instrument”), permitting the Company to issue convertible notes in a maximum aggregate principal amount of up to $1.4 million (£1.0 million). The convertible notes issuable under the 2021 Convertible Loan Note Instrument mature three years after issuance to the respective noteholders and bear 5% interest, only to be paid to the noteholders in the event of a material breach by the Company of the terms of the 2021 Convertible Loan Note Instrument. In the event of a Change of Control (as defined in the 2021 Convertible Loan Note Instrument), the convertible notes issued under the 2021 Convertible Loan Note Instrument automatically convert into ordinary shares of the Company at a conversion price equal to a 20% discount to the price per share paid for the most senior class of shares in respect of such Change of Control. The Company, with consent from the noteholders, may prepay the convertible notes payable issued under the 2021 Convertible Loan Note Instrument without penalty. The convertible notes payable issued under the 2021 Convertible Loan Note Instrument are general, unsecured obligations of the Company.
The Company issued $0.3 million (£0.25 million) convertible notes payable to two separate investors on June 11, 2021 and June 12, 2021, $0.5 million (£0.4 million) on August 26, 2022 and $67 thousand (£50 thousand) on October 6, 2022. As of December 31, 2022, $1.1 million (£950,000) 2021 Convertible Loan Notes were issued and outstanding. As of December 31, 2022, $67 thousand (£50 thousand) convertible notes were available for issuance.
On November 1, 2022, the Company approved a master Convertible Loan Note Instrument (the “2022 Convertible Loan Note Instrument”), permitting the Company to issue convertible notes payable for a maximum aggregate principal amount of up to $3.3 million (£3.0 million). The convertible notes payable issuable under the 2022 Convertible Loan Note Instrument mature three years after issuance to the respective noteholders and bear 5% interest, only to be paid to the noteholders in the event of a material breach by the Company of the terms of the 2022 Convertible Loan Note Instrument. In the event of a Change of Control (as defined in the 2022 Convertible Loan Note Instrument), the convertible notes payable issued under the 2022 Convertible Loan Note Instrument automatically convert into ordinary shares of the Company at a conversion price equal to a 20% discount to the price per share paid for the most senior class of shares in respect of such Change of Control. The Company, with consent from the noteholders, may prepay the convertible notes payable issued under the 2022 Convertible Loan Note Instrument without penalty. The convertible notes payable issued under the 2022 Convertible Loan Note Instrument are general, unsecured obligations of the Company.
| F-32 |
On November 16, 2022, under the terms of the 2022 Convertible Loan Note Instrument, the Company issued convertible notes payable with an aggregate principal amount of $0.4 million (£0.3 million) to an investor.
The Company elected to fair value the convertible notes payable issued under the 2021 and 2022 Convertible Loan Note Instruments. At the end of each reporting period, the Company calculates the fair value of the convertible notes payable, and any changes in fair value are reported in other income, net, in the current period’s statement of operations and comprehensive loss. There has been no change in fair value from a change in credit quality. For the year ended December 31, 2022, the Company recorded a loss from the change in fair value of convertible notes payable of $0.3 million in other income, net, in its statement of operations and comprehensive less. For the year ended December 31, 2021, the Company recorded a loss from the change in fair value of convertible notes payable of $0.1 million in other income, net, in its statement of operations and comprehensive loss. See Note 2 for additional information regarding the fair value measurement of convertible notes payable.
6. Loans Payable
On May 1, 2022, the Company entered into Loan Agreements (the “Loans”) with two lenders, totaling $0.2 million. The Loans mature two years from the date of the agreement and bear no interest. Each loan was made available to the Company by the lenders in three tranches of (i) $33 thousand (£30 thousand); (ii) $33 thousand (£30 thousand) and (iii) $28 thousand (£25 thousand), totaling $0.2 million. The Loans provided for events of default, including, among others, failure to make payment, bankruptcy and non-compliance with the terms of the Loans. As of December 31, 2022 the Company utilized all three tranches of the first loan and two out of three tranches of the second loan, with total loans payable at December 31, 2022 of $0.2 million.
7. Ordinary Shares
As of December 31, 2022 and 2021, the Company has authorized the issuance of up to 400,000,000 ordinary shares, par value £0.0001 per share (the “Ordinary Shares”).
As of December 31, 2022 and December 31, 2021, 2,000 and 1,000 Ordinary Shares (the “Ordinary Shares”) were issued and outstanding, respectively. The Company issued 1,000 ordinary shares during the year ended December 31, 2022. No shares were issued during the year ended December 31, 2021. As of December 31, 2022 and 2021, no cash dividends have been declared or paid.
Holders of the Ordinary Shares are entitled to one vote per share, and to receive dividends, on and if declared by the board of directors and, upon liquidation or dissolution, are entitled to receive all assets available for distribution, subordinate to the rights, preferences, and privileges of any outstanding preferred shares (if any) with respect to dividends and in connection with liquidation, winding up and dissolution of the Company. The holders have no preemptive or other subscription rights.
8. Net Loss Per Share Attributable to Ordinary Shareholders
The following table presents the calculation of basic and diluted net loss per share attributable to holders of the Ordinary Shares (in thousands, except share amounts and per share data):
Schedule of Basic and Diluted Net Loss Per Share
| December 31, | ||||||||
| 2022 | 2021 | |||||||
| Numerator: | ||||||||
| Net loss | $ | (4,887 | ) | $ | (3,657 | ) | ||
| Denominator: | ||||||||
| Weighted-average shares outstanding used in calculating net loss per share – basic and diluted | 2,000 | 1,000 | ||||||
| Net loss per share attributable to ordinary shareholders – basic and diluted | $ | (2,444 | ) | $ | (3,657 | ) | ||
| F-33 |
Since the Company was in a loss position for all periods presented, basic net loss per share attributable to holders of the Ordinary Shares is the same as diluted net loss per share attributable to holders of the Ordinary Shares for all periods presented as the inclusion of all potential Ordinary Shares outstanding would have been anti-dilutive.
Potentially dilutive securities (upon conversion) that were not included in the diluted per share calculations because they would have been anti-dilutive were as follows:
Schedule of Potentially Dilutive Securities
| December 31, | ||||
| 2022 | ||||
| Ordinary Shares potentially issuable upon conversion of convertible notes payable | 1,250,000 | |||
| Total | 1,250,000 | |||
| December 31, | ||||
| 2021 | ||||
| Ordinary Shares potentially issuable upon conversion of convertible notes payable | 500,000 | |||
| Total | 500,000 | |||
9. Commitments and Contingencies
Legal Proceedings
From time to time, the Company may become involved in legal proceedings or other litigation relating to claims arising in the ordinary course of business. The Company intends to accrue a liability for such matters when it is probable that future expenditures will be made and that such expenditures can be reasonably estimated. Significant judgment is required to determine both probability and estimated exposure amount. Legal fees and other costs associated with such proceedings are expensed as incurred.
10. Related Party Transactions
Corvus Capital Limited
Corvus Capital Limited (“Corvus”) is a significant investor in the Company through holding 1,000 and 775 Ordinary Shares as of the years ended December 31, 2022 and December 31, 2021, respectively. On November 4, 2022, the Company issued 1,000 Ordinary Shares to Corvus Capital Limited. Corvus Capital Limited subsequently transferred 775 Ordinary Shares to other investors. The Chief Executive Officer of Corvus is a member of Conduit’s board of directors. For the year ended December 31, 2021, the Company incurred $1.6 million (£1.3 million) in advisory fees for funding and review of a potential acquisition candidates to Corvus, which are included in general and administrative expenses in the statement of operations and comprehensive loss, the Company did not incur advisory fees during the year ended December 31, 2022. As of December 31, 2022 and December 31, 2021, approximately $0.6 million and $0.7 million, respectively, of the advisory fees were not paid and were recorded to accrued expenses on the balance sheet. For the years ended December 31, 2022 and 2021, the Company incurred director’s fees payable to the CEO of Corvus of approximately $0.4 million and $0.4 million, respectively. As of December 31, 2022 and 2021, the Company owed the CEO of Corvus director’s fees of approximately $0.4 million and $0.4 million, respectively. These amounts owed to the CEO of Corvus are included in accrued expenses and other current liabilities in the balance sheets.
During January and February 2023, under the terms of the 2022 Convertible Loan Note Instrument, the Company issued convertible notes payable with an aggregate principal amount of $0.5 million (£0.4 million) to the CEO of Corvus. The convertible notes payable mature three years after issuance and bear 5% interest, only to be paid in the event of a material breach by the Company of the terms of the 2022 Convertible Loan Note Instrument. In the event of a Change of Control, the convertible notes payable automatically convert into ordinary shares of the Company at a conversion price equal to a 20% discount to the price per share paid for the most senior class of shares in respect of such Change of Control.
| F-34 |
St George Street Capital
St George Street Capital is a significant investor in the Company through holding 225 Ordinary shares. The Chief Executive Officer of St George Street Capital is also the Chief Executive Officer of Conduit. Further, the Company has an Exclusive Funding Agreement (as defined below) with St George Street Capital. For the years ended December 31, 2022 and December 31, 2021, the Company incurred expenses to St George Street Capital totaling$0.3 million and $0.1 million, respectively. As of the years ended December 31, 2022 and December 31, 2021, the Company did not owe any amounts to St George Street Capital.
On March 26, 2021, the Company entered into the Exclusive Funding Agreement (“Funding Agreement”) with St George Street Capital. Under the agreement, the Company has the first exclusive right, but not the obligation, to provide or procure funding for the performance or research and development projects undertaken by St George Street Capital. The Funding Agreement entitles the Company to 100% of the net revenue on projects that the Company funds by itself. As of December 31, 2022, the Company has not recognized any net revenue from the Funding Agreement. See Note 1 for discussion on research and development costs.
11. Other Expense
The following table presents other expense, for the years ended December 31, 2022 and 2021 (in thousands):
Schedule of Other Expense, Net
| For the year ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Other expense: | ||||||||
| Loss on Cizzle option | $ | 1,300 | $ | - | ||||
| Realized foreign currency transaction loss | - | 8 | ||||||
| Loss on sale of investment in equity securities | 129 | 76 | ||||||
| Placement fees on sale of investment in equity securities | 33 | 327 | ||||||
| Change in fair value of convertible notes payable | 265 | 73 | ||||||
| Total other expense | $ | 1,727 | $ | 484 | ||||
12. Subsequent Events
The Company has evaluated all events occurring through May 12, 2023, the date on which the financial statements were available to be issued. The Company did not identify any subsequent events that would have required adjustment or disclosure in the financial statements, except for the ones described below.
Convertible Loan Note Instruments
As discussed in Note 10 – Related Party Transactions, during January and February 2023, under the terms of the 2022 Convertible Loan Note Instrument, the Company issued convertible notes payable with an aggregate principal amount of $0.5 million (£0.4 million) to the CEO of Corvus.
During January and February 2023, under the terms of the 2022 Convertible Loan Note Instrument, the Company issued convertible notes payable with an aggregate principal amount of $1.3 million (£1.1 million) to non-related third parties.
During March 2023, the Company issued promissory convertible notes payable with an aggregate principal amount of $0.8 million to a non-related third party. The note matures and is payable in full 18 months from the date of the note. The note carries 20% interest and is payable every six months from the date of the note until the maturity date. The note is subject to conversion into shares of the Company’s common stock prior to the consummation of the Business Combination, which shares will then convert into MURF common stock. The Company notes that this issuance was outside of the terms of the 2022 Convertible Loan Note Instrument.
Vela Option Agreement
On April 2023, the Company entered into an agreement with Vela which granted Vela the right, but not the obligation, to sell its 8% royalty interest in the Covid Asset back to the Company. Vela paid a one-time, non-refundable option fee to the Company of $0.5 million (£0.4 million). Total consideration payable to Vela upon exercise is $4.8 million (£4.0 million) through the issuance of shares of the Company, following the consummation of the Merger at a price per share equal to the volume-weighted average price per share over the ten (10) business days prior to the date of the notice of the exercise of the Option. The option is exercisable in whole at any time from the close of the Merger (the “Effective Time”) until the earlier of (i) the date that is six (6) months from the Effective Time, and (ii) February 7, 2024, the expiration of the term.
| F-35 |
MURPHY CANYON ACQUISITION CORP.
CONDENSED BALANCE SHEETS
| March 31, 2023 | December 31, 2022 | |||||||
| (unaudited) | ||||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 90,606 | $ | 345,777 | ||||
| Prepaid expenses | 219,417 | 300,862 | ||||||
| Total current assets | 310,023 | 646,639 | ||||||
| Investments held in Trust Account | 23,658,838 | 136,871,183 | ||||||
| Total Assets | $ | 23,968,861 | $ | 137,517,822 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| Current liabilities: | ||||||||
| Accrued expenses | $ | 1,433,289 | $ | 43,113 | ||||
| Income taxes payable | 224,419 | 374,862 | ||||||
| Note payable – Sponsor | 300,000 | - | ||||||
| Total current liabilities | 1,957,708 | 417,975 | ||||||
| Deferred commission payable | 4,628,750 | 4,628,750 | ||||||
| Total Liabilities | 6,586,458 | 5,046,725 | ||||||
| Commitments and Contingencies (Note 6) | - | - | ||||||
| Common stock subject to possible redemption at redemption value (2,187,728 shares at $10.45 per share and 13,225,000 shares at $10.34 per share, as of March 31, 2023 and December 31, 2022, respectively) | 22,861,803 | 136,771,183 | ||||||
| Stockholders’ Deficit | ||||||||
| Preferred stock, $0.0001 par value; 1,000,000 shares authorized; none issued and outstanding | - | - | ||||||
| Class A common stock, $0.0001 par value; 100,000,000 shares authorized; 754,000 (excluding 2,187,728 shares and 13,225,000 shares subject to possible redemption) issued and outstanding at March 31, 2023, and December 31, 2022, respectively | 75 | 75 | ||||||
| Class B common stock, $0.0001 par value; 10,000,000 shares authorized; 3,306,250 shares issued and outstanding | 331 | 331 | ||||||
| Additional paid-in capital | - | - | ||||||
| Accumulated deficit | (5,479,806 | ) | (4,300,492 | ) | ||||
| Total Stockholders’ Deficit | (5,479,400 | ) | (4,300,086 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 23,968,861 | $ | 137,517,822 | ||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
| F-36 |
MURPHY CANYON ACQUISITION CORP.
CONDENSED STATEMENTS OF OPERATIONS
(Unaudited)
| For the Three Months Ended March 31, 2023 | For the Three Months Ended March 31, 2022 | |||||||
| General and administrative expenses | $ | 477,406 | $ | 243,718 | ||||
| Administration fee – related party | 30,000 | 20,000 | ||||||
| Total expenses | 507,406 | 263,718 | ||||||
| Other Income | ||||||||
| Interest income – Investments held in Trust Account | 664,232 | 10,182 | ||||||
| Total other income | 664,232 | 10,182 | ||||||
| Net income (loss) before income taxes | 156,826 | (253,536 | ) | |||||
| Income tax expense | (36,557 | ) | - | |||||
| Net income (loss) | $ | 120,269 | $ | (253,536 | ) | |||
| Class A common stock – weighted average shares outstanding, basic and diluted | 5,762,364 | 8,167,506 | ||||||
| Class A common stock – Basic and diluted net income (loss) per share | $ | 0.01 | $ | (0.02 | ) | |||
| Class B common stock – weighted average shares outstanding, basic and diluted | 3,306,250 | 3,306,250 | ||||||
| Class B common stock – Basic and diluted net income (loss) per share | $ | 0.01 | $ | (0.02 | ) | |||
The accompanying notes are an integral part of these unaudited condensed financial statements.
| F-37 |
MURPHY CANYON ACQUISITION CORP.
CONDENSED STATEMENTS OF CHANGES IN STOCKHOLDERS’ DEFICIT
FOR THE THREE MONTHS ENDED MARCH 31, 2023 AND 2022 (Unaudited)
| Class A | Class B | Additional Paid-in | Accumulated | Total Stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||||||||
| Balance, January 1, 2023 | 754,000 | $ | 75 | 3,306,250 | $ | 331 | $ | — | $ | (4,300,492 | ) | $ | (4,300,086 | ) | ||||||||||||||
| Remeasurement of Class A common stock subject to possible redemption | - | - | - | - | - | (158,900 | ) | (158,900 | ) | |||||||||||||||||||
| Accrued excise tax on January 24, 2023 redemptions | (1,140,683 | ) | (1,140,683 | ) | ||||||||||||||||||||||||
| Net income | - | - | - | - | - | 120,269 | 120,269 | |||||||||||||||||||||
| Balance, March 31, 2023 | 754,000 | $ | 75 | 3,306,250 | $ | 331 | $ | — | $ | (5,479,806 | ) | $ | (5,479,400 | ) | ||||||||||||||
| Class A | Class B | Additional Paid-in | Accumulated | Total Stockholders’ Equity | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | (Deficit) | ||||||||||||||||||||||
| Balance, January 1, 2022 | - | $ | - | 3,306,250 | $ | 331 | $ | 24,669 | $ | (4,381 | ) | $ | 20,619 | |||||||||||||||
| Proceeds allocated to Public Warrants, net of offering costs | - | - | - | - | 21,917,543 | - | 21,917,543 | |||||||||||||||||||||
| Sale of private placement units, net of offering costs | 754,000 | 75 | - | - | 7,520,275 | - | 7,520,350 | |||||||||||||||||||||
| Remeasurement of Class A common stock subject to possible redemption | - | - | - | - | (29,462,487 | ) | (2,818,567 | ) | (32,281,054 | ) | ||||||||||||||||||
| Net loss | - | - | - | - | - | (253,536 | ) | (253,536 | ) | |||||||||||||||||||
| Balance, March 31, 2022 | 754,000 | $ | 75 | 3,306,250 | $ | 331 | $ | - | $ | (3,076,484 | ) | $ | (3,076,078 | ) | ||||||||||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
| F-38 |
MURPHY CANYON ACQUISITION CORP.
CONDENSED STATEMENT OF CASH FLOWS
(Unaudited)
| For the Three Months Ended March 31, 2023 | For the Three Months Ended March 31, 2022 | |||||||
| Cash flows from operating activities: | ||||||||
| Net income (loss) | $ | 120,269 | $ | (253,536 | ) | |||
| Adjustments to reconcile net income (loss) to net cash used in operating activities | ||||||||
| Interest earned on investments held in Trust Account | (664,232 | ) | (10,182 | ) | ||||
| Changes in operating assets and liabilities: | ||||||||
| Deferred offering costs | - | 108,962 | ||||||
| Prepaid expenses | 81,445 | (600,637 | ) | |||||
| Accrued expenses | 13,143 | 59,033 | ||||||
| Related party payable | - | 3,875 | ||||||
| Accrued income taxes payable | (150,443 | ) | - | |||||
| Net cash used in operating activities | (599,818 | ) | (692,485 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Extension deposit into the Trust Account | (155,403 | ) | - | |||||
| Withdrawals from Trust Account for redemptions | 113,831,930 | - | ||||||
| Withdrawals from the Trust Account for taxes | 200,050 | - | ||||||
| Cash deposited into Trust Account | - | (134,895,000 | ) | |||||
| Net cash provided by (used in) investing activities | 113,876,577 | (134,895,000 | ) | |||||
| Cash flows from financing activities: | ||||||||
| Proceeds from note payable - Sponsor | 300,000 | - | ||||||
| Redemptions of Class A common stock | (113,831,930 | ) | - | |||||
| Sale of units in public offering | - | 132,250,000 | ||||||
| Sale of private placement units | - | 7,540,000 | ||||||
| Payment of offering costs | - | (3,109,411 | ) | |||||
| Repayment of note payable - Sponsor | - | (177,057 | ) | |||||
| Net cash (used in) provided by financing activities | (113,531,930 | ) | 136,503,532 | |||||
| Net change in cash | (255,171 | ) | 916,047 | |||||
| Cash at beginning of period | 345,777 | 48,555 | ||||||
| Cash at end of period | $ | 90,606 | $ | 964,602 | ||||
| Non-cash financing activities: | ||||||||
| Deferred commission payable | $ | - | $ | 4,628,750 | ||||
| Accrued excise tax on January 24, 2023 redemptions | $ | 1,140,683 | - | |||||
| Remeasurement of Class A common stock subject to possible redemption | $ | (158,900 | ) | $ | - | |||
The accompanying notes are an integral part of these unaudited condensed financial statements.
| F-39 |
MURPHY CANYON ACQUISITION CORP.
Notes to financial statements
NOTE 1 — DESCRIPTION OF ORGANIZATION, BUSINESS OPERATIONS AND GOING CONCERN
Murphy Canyon Acquisition Corp. (the “Company”) was incorporated in Delaware on October 19, 2021. The Company was formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses (the “Business Combination”). The Company is not limited to a particular industry or sector for purposes of consummating a Business Combination. The Company is an early stage and emerging growth company and, as such, the Company is subject to all of the risks associated with early stage and emerging growth companies.
As of March 31, 2023, the Company had not commenced any operations. All activity for the period from October 19, 2021 (inception), through March 31, 2023, relates to the Company’s formation, the proposed initial public offering (“Initial Public Offering”), which is described below, and searching for an initial Business Combination, as defined below. The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest. The Company will generate non-operating income in the form of interest income from the proceeds derived from the Initial Public Offering. The Company has selected December 31 as its fiscal year end.
The registration statement for the Company’s Initial Public Offering (the “Registration Statement”) was declared effective on February 2, 2022. On February 7, 2022, the Company consummated the Initial Public Offering of 13,225,000 units (“Units”), generating gross proceeds of $132,250,000, which is described in Note 3.
Simultaneously with the closing of the Initial Public Offering, the Company consummated the sale of 754,000 units (the “Private Placement Units”) at a price of $10.00 per Private Placement Unit in private placements to Murphy Canyon Acquisition Sponsor LLC (the ‘Sponsor” or “our Sponsor”), with gross proceeds of $7,540,000.
Following the closing of the Initial Public Offering on February 7, 2022, an amount of $139,790,000 from the net proceeds of the sale of the Units in the Initial Public Offering and the Private Placement Units was placed in the Trust Account, as defined below. This resulted in an overfunding of the Trust Account of $4,895,000. As such, subsequent to the initial funding of the Trust Account, $2,000,000 was transferred to the Company’s operating cash account and $2,895,000 was used to pay offering costs. The funds held in the Trust Account may be invested in U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act of 1940, as amended (the “Investment Company Act”), with a maturity of 185 days or less or in any open-ended investment company that holds itself out as a money market fund selected by the Company meeting the conditions of Rule 2a-7 of the Investment Company Act, as determined by the Company, until the earlier of: (i) the completion of a Business Combination or (ii) the distribution of the Trust Account, as described below.
On November 8, 2022, the Company entered into a definitive Business Combination Agreement (the “BCA”) with Conduit Pharmaceuticals Limited, a Cayman Islands exempted company (“Conduit”), and Conduit Merger Sub, Inc., a Cayman Islands exempted company (“Merger Sub”). Merger Sub is a wholly owned subsidiary of the Company. Conduit is a pharmaceutical company led by experienced pharma executives, established to fund the development of successful deprioritized clinical assets licensed from large pharmaceutical companies through its exclusive relationships. The BCA was amended in January 2023 and May 2023.
Upon the consummation of the transactions contemplated by the BCA, Merger Sub will merge with and into Conduit, with Conduit surviving as a wholly owned subsidiary of the Company (the “Business Combination”). The Company is expected to be renamed Conduit Pharmaceuticals Inc. at the closing of the Business Combination.
Pursuant to the BCA, at the closing, the Company shall issue and deliver to the shareholders of Conduit an aggregate number of shares of the Company’s common stock with an aggregate value equal to $650,000,000, with each share valued at $10.00 per share. A private placement transaction shall be conducted by the Company contemporaneously with the Business Combination (the “PIPE Financing”), pursuant to which the Company has entered into subscription agreements providing for aggregate investments in the Company’s securities of $27,000,000.
| F-40 |
There can be no assurance that the Business Combination or PIPE Financing will occur as planned or at all.
The Company’s management has broad discretion with respect to the specific application of the net proceeds of the Initial Public Offering and the sale of Private Placement Warrants, although substantially all of the net proceeds are intended to be applied generally toward consummating a Business Combination. There is no assurance that the Company will be able to complete a Business Combination successfully. The Company must complete one or more initial Business Combinations with one or more operating businesses or assets with a fair market value equal to at least 80% of the net assets held in the Trust Account (as defined below) (excluding the deferred underwriting commissions and taxes payable on the interest earned on the Trust Account). The Company will only complete a Business Combination if the post-transaction company owns or acquires 50% or more of the outstanding voting securities of the target or otherwise acquires a controlling interest in the target business sufficient for it not to be required to register as an investment company under the Investment Company Act of 1940, as amended (the “Investment Company Act”). Upon the closing of the Initial Public Offering, management has agreed that an amount equal to at least $10.00 per Unit sold in the Initial Public Offering, including proceeds of the Private Placement Warrants, will be held in a trust account (“Trust Account”), located in the United States and invested only in U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with a maturity of 185 days or less or in any open-ended investment company that holds itself out as a money market fund selected by the Company meeting certain conditions of Rule 2a-7 of the Investment Company Act, as determined by the Company, until the earlier of: (i) the completion of a Business Combination and (ii) the distribution of the funds held in the Trust Account, as described below.
The Company will provide the holders of the outstanding Public Shares (the “Public Stockholders”) with the opportunity to redeem all or a portion of their Public Shares either (i) in connection with a stockholder meeting called to approve the Business Combination or (ii) by means of a tender offer in connection with the Business Combination. The decision as to whether the Company will seek stockholder approval of a Business Combination or conduct a tender offer will be made by the Company. The Public Stockholders will be entitled to redeem their Public Shares for a pro rata portion of the amount then in the Trust Account (net of taxes payable). There will be no redemption rights upon the completion of a Business Combination with respect to the Company’s warrants. All of the Public Shares contain a redemption feature which allows for the redemption of such Public Shares in connection with our liquidation, if there is a stockholder vote or tender offer in connection with our initial business combination and in connection with certain amendments to our amended and restated certificate of incorporation. In accordance with U.S. Securities and Exchange Commission (“SEC”) and its guidance on redeemable equity instruments, which has been codified in ASC 480-10-S99, redemption provisions not solely within the control of a company require common stock subject to redemption to be classified outside of permanent equity. Given that the Public Shares will be issued with other freestanding instruments (i.e., public warrants), the initial carrying value of Class A common stock classified as temporary equity will be the allocated proceeds determined in accordance with ASC 470-20. The Class A common stock is subject to ASC 480-10-S99. If it is probable that the equity instrument will become redeemable, we have the option to either (i) accrete changes in the redemption value over the period from the date of issuance (or from the date that it becomes probable that the instrument will become redeemable, if later) to the earliest redemption date of the instrument or (ii) recognize changes in the redemption value immediately as they occur and adjust the carrying amount of the instrument to equal the redemption value at the end of each reporting period. We have elected to recognize the changes immediately. The accretion or remeasurement will be treated as a deemed dividend (i.e., a reduction to retained earnings, or in absence of retained earnings, additional paid-in capital). The Public Shares are redeemable and will be classified as such on the balance sheet until such date that a redemption event takes place.
If the Company seeks stockholder approval of the Business Combination, the Company will proceed with a Business Combination if a majority of the outstanding shares voted are voted in favor of the Business Combination, or such other vote as required by law or stock exchange rule. If a stockholder vote is not required by applicable law or stock exchange listing requirements and the Company does not decide to hold a stockholder vote for business or other reasons, the Company will, pursuant to its amended and restated certificate of incorporation (the “Certificate of Incorporation”), conduct the redemptions pursuant to the tender offer rules of the (“SEC”) and file tender offer documents with the SEC prior to completing a Business Combination. If, however, stockholder approval of the transaction is required by applicable law or stock exchange listing requirements, or the Company decides to obtain stockholder approval for business or other reasons, the Company will offer to redeem shares in conjunction with a proxy solicitation pursuant to the proxy rules and not pursuant to the tender offer rules. If the Company seeks stockholder approval in connection with a Business Combination, the Sponsor has agreed to vote its Founder Shares (as defined in Note 5) and any Public Shares purchased during or after the Initial Public Offering in favor of approving a Business Combination. Additionally, each Public Stockholder may elect to redeem their Public Shares without voting, and if they do vote, irrespective of whether they vote for or against the proposed transaction.
| F-41 |
Notwithstanding the foregoing, if the Company seeks stockholder approval of a Business Combination and it does not conduct redemptions pursuant to the tender offer rules, the Certificate of Incorporation provides that a Public Stockholder, together with any affiliate of such stockholder or any other person with whom such stockholder is acting in concert or as a “group” (as defined under Section 13 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), will be restricted from redeeming its shares with respect to more than an aggregate of 15% of the Public Shares, without the prior consent of the Company.
The holders of the Founder Shares have agreed (a) to waive their redemption rights with respect to the Founder Shares and Public Shares held by them in connection with the completion of a Business Combination and (b) not to propose an amendment to the Certificate of Incorporation (i) to modify the substance or timing of the Company’s obligation to allow redemptions in connection with a Business Combination or to redeem 100% of its Public Shares if the Company does not complete a Business Combination within the Combination Period (as defined below) or (ii) with respect to any other provision relating to stockholders’ rights or pre-business combination activity, unless the Company provides the Public Stockholders with the opportunity to redeem their Public Shares in conjunction with any such amendment.
If the Company has not completed a Business Combination within 12 months from the consummation of our initial public offering (or up to February 7, 2024 at the election of the Company subject to satisfaction of certain conditions, as amended at the January 2023 Special Meeting, see Note 10) (“Business Combination Period”), the Company will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the Public Shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including interest earned on the funds held in the Trust Account and not previously released to pay taxes (less up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding Public Shares, which redemption will completely extinguish Public Stockholders’ rights as stockholders (including the right to receive further liquidating distributions, if any), and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the Company’s remaining stockholders and the Company’s board of directors, dissolve and liquidate, subject in each case to the Company’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. Our Sponsor has committed to provide additional funds if needed to make such a deposit for the extensions. There will be no redemption rights or liquidating distributions with respect to the Company’s warrants, which will expire worthless if the Company fails to complete a Business Combination within the Combination Period.
The holders of the Founders Shares have agreed to waive their liquidation rights with respect to the Founder Shares if the Company fails to complete a Business Combination within the Combination Period. However, if the holders of Founder Shares acquire Public Shares in or after the Initial Public Offering, such Public Shares will be entitled to liquidating distributions from the Trust Account if the Company fails to complete a Business Combination within the Combination Period. The underwriters have agreed to waive their rights to their deferred underwriting commission (see Note 6) held in the Trust Account in the event the Company does not complete a Business Combination within the Combination Period and, in such event, such amounts will be included with the other funds held in the Trust Account that will be available to fund the redemption of the Public Shares. In the event of such distribution, it is possible that the per share value of the assets remaining available for distribution will be less than the Initial Public Offering price per Unit ($10.00).
In order to protect the amounts held in the Trust Account, the Sponsor has agreed to be liable to the Company if and to the extent any claims by a third party for services rendered or products sold to the Company, or a prospective target business with which the Company has discussed entering into a transaction agreement, reduce the amount of funds in the Trust Account to below (i) $10.00 per Public Share or (ii) such lesser amount per Public Share held in the Trust Account as of the date of the liquidation of the Trust Account, if less than $10.00 per public Share due to reductions in the value of the trust assets, in each case net of the amount of interest which may be withdrawn to pay taxes, except as to any claims by a third party who executed a waiver of any and all rights to seek access to the Trust Account and except as to any claims under the Company’s indemnity of the underwriters of the Initial Public Offering against certain liabilities, including liabilities under the Securities Act of 1933, as amended (the “Securities Act”). Moreover, in the event that an executed waiver is deemed to be unenforceable against a third party, the Sponsor will not be responsible to the extent of any liability for such third-party claims. The Company will seek to reduce the possibility that the Sponsor will have to indemnify the Trust Account due to claims of creditors by endeavoring to have all vendors, service providers (except for the Company’s independent registered accounting firm), prospective target businesses and other entities with which the Company does business, execute agreements with the Company waiving any right, title, interest or claim of any kind in or to monies held in the Trust Account.
| F-42 |
Initially, the Company was required to complete an initial business combination transaction by 12 months from the consummation of initial public offering or up to 18 months if the Company extended the period of time to consummate a business combination in accordance with the Company’s Certificate of Incorporation. On January 26, 2023, at a special meeting of the Company’s stockholders (the “Special Meeting”), the Company’s stockholders approved a proposal to amend the Company’s certificate of incorporation to allow the Company to extend, at the Company’s election, the date by which the Company has to consummate a business combination up to 12 times, each such extension for an additional one month period, from February 7, 2023, to February 7, 2024. The Company’s stockholders also approved a related proposal to amend the trust agreement allowing the Company to deposit into the Trust Account, for each one-month extension, one-third of 1% of the funds remaining in the Trust Account following the redemptions made in connection with the approval of the extension proposal at the Special Meeting. At the Special Meeting the Company’s stockholders also approved a proposal to amend the Company’s certificate of incorporation to expand the methods that the Company may employ to not become subject to the “penny stock” rules of the SEC.
In connection with such proposals, the Company’s public stockholders had the right to redeem their shares for cash equal to their pro rata share of the aggregate amount on deposit in the Trust Account as of two days prior to such stockholder vote. The Company’s public stockholders holding 11,037,272 shares of Class A common stock (out of a total of 13,979,000 shares of Class A common stock) exercised their right to redeem such shares at a redemption price of approximately $10.33 per share. Approximately $114 million in cash was removed from the Trust Account to pay such stockholders and, accordingly, after giving effect to such redemptions, the balance in the Trust Account was approximately $24 million.
As a result of the approval of such proposals, the Company agreed to deposit into the Trust Account one-third of 1% of the funds then on deposit in the Trust Account for each month of the extension period, resulting in a monthly contribution of approximately $0.035 per share that was not redeemed in connection with the Special Meeting, or an aggregate of approximately $77,000 per month, and an aggregate of $924,000 if the date the Company has to consummate a business combination is extended 12 times, each assuming no interest is earned on the funds in the Trust Account. During the three months ended March 31, 2023, the Company funded $155,403 in monthly extension payments into the Trust Account.
Management’s Plan and Going Concern
In connection with the Company’s assessment of going concern considerations in accordance with Accounting Standards Update (“ASU”) 2014-15, “Disclosures of Uncertainties about an Entity’s Ability to Continue as a Going Concern,” management has determined that the Combination Period is less than one year from the date of the issuance of the financial statements. There is no assurance that the Company’s plans to consummate a Business Combination will be successful within the Combination Period. Additionally, the Company has incurred and expects to continue to incur significant costs in pursuit of its acquisition plans. As a result, these factors raise substantial doubt about the Company’s ability to continue as a going concern for the next twelve months from the issuance of these financial statements. The financial statements do not include any adjustments that might result from the outcome of the uncertainty.
Risks and Uncertainties
Management is currently evaluating the impact of the COVID-19 pandemic and has concluded that while it is reasonably possible that the virus could have a negative effect on the Company’s financial position, results of its operations, and search for a target company, the specific impact is not readily determinable as of the date of these financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| F-43 |
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) as contained within the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”).
Emerging Growth Company
The Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012, as amended (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the independent registered public accounting firm attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Use of Estimates
The preparation of financial statements in conformity with US GAAP requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period.
Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. Accordingly, the actual results could differ significantly from those estimates.
Cash and Cash Equivalents
The Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents. The Company had $90,606 and $345,777 in cash as of March 31, 2023 and December 31, 2022, respectively. The Company did not have any cash equivalents as of March 31, 2023 and December 31, 2022.
Investments Held in Trust Account
At March 31, 2023, and December 31, 2022, the Company had $23,658,838 and $136,871,183, respectively, in investments held in the Trust Account.
Offering Costs Associated With a Public Offering
The Company complies with the requirements of FASB ASC 340-10-S99-1 and SEC Staff Accounting Bulletin (“SAB”) Topic 5A — “Expenses of Offering.” Offering costs of $7,738,161, consisting of $2,645,000 of underwriting fees, $4,628,750 of deferred underwriting fee (which are held in the Trust Account with Wilmington Trust Company acting as trustee), and $464,411 of Initial Public Offering costs. Of these costs, $1,358,457 and $19,656 were allocated to Public Warrants (as defined in Note 3) and Private Placement Warrants (as defined in Note 4), respectively.
| F-44 |
Class A Common Stock Subject to Possible Redemption
The Company accounts for its shares of Class A common stock subject to possible redemption in accordance with the guidance enumerated in ASC 480 “Distinguishing Liabilities from Equity”. Common stock subject to mandatory redemption is classified as a liability instrument and is measured at fair value. Conditionally redeemable common stock (including common stock that feature redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s control) are classified as temporary equity. At all other times, common stock is classified as stockholders’ equity. The shares of the Company’s Class A common stock feature certain redemption rights that are considered by the Company to be outside of the Company’s control and subject to the occurrence of uncertain future events.
During the three months ended March 31, 2023, 11,037,272 shares of the Class A common stock were redeemed. See Note 1 – Description of Organization, Business Operations and Going Concern for additional information. Accordingly, as of March 31, 2023, the shares of Class A common stock subject to possible redemption in the amount of $22,861,803 are presented as temporary equity, outside of the stockholders’ deficit section of the Company’s balance sheet.
The following table provides a reconciliation of the shares of Class A common stock subject to possible redemption reflected on the balance sheet as of March 31, 2023:
SCHEDULE OF COMMON STOCK SUBJECT TO POSSIBLE REDEMPTION
| Gross proceeds from IPO | $ | 132,250,000 | ||
| Less: | ||||
| Proceeds allocated to Public Warrants | (23,276,000 | ) | ||
| Class A common stock issuance costs | (6,360,054 | ) | ||
| Plus: | ||||
| Remeasurement adjustment of carrying value to redemption value | 32,281,054 | |||
| Class A common stock subject to possible redemption, as of March 31, 2022 | 134,895,000 | |||
| Plus: | ||||
| Remeasurement adjustment of carrying value to redemption value | 1,876,183 | |||
| Class A common stock subject to possible redemption as of December 31, 2022 | $ | 136,771,183 | ||
| Less: | ||||
| Redemptions during the three months ended March 31, 2023 * | (114,068,280 | ) | ||
| Plus: | ||||
| Remeasurement adjustment of carrying value to redemption value | 158,900 | |||
| Class A common stock subject to possible redemption as of March 31, 2023 | 22,861,803 |
| * | As of March 31, 2023, $236,350 of cash was still in the Trust account from funds that were returned and are awaiting redistribution, which were paid in April 2023. |
Excise Tax
In accordance with the Inflation Reduction Act of 2022, the Company accrues the expected excise tax obligation at the end of each reporting period as a cost of redeeming any shares as of that date. In connection with the vote to amend the Company’s certificate of incorporation, holders of 11,037,272 shares of Class A common stock properly exercised their right to redeem their shares of Class A common stock for the aggregate redemption amount of $114,068,280. As such the Company has recorded a 1% excise tax liability in the amount of $1,140,683 on the condensed balance sheet as of March 31, 2023. The liability does not impact the condensed statements of operations or condensed statement cash flows and is an offset against additional paid in capital, to the extent available, and accumulated deficit. This excise tax liability can be offset by future shares of issuance which will be evaluated and adjusted in the period in which the issuances occur. Should the Company liquidate prior to December 31, 2023, the excise tax liability will not be due.
Net Income (Loss) per Common Share
The Company complies with accounting and disclosure requirements of FASB ASC Topic 260, “Earnings Per Share”. Net income (loss) per share of common stock is computed by dividing net income (loss) by the weighted average number of shares of common stock outstanding for the period. Accretion associated with the redeemable shares of Class A common stock is excluded from income (loss) per common share as the redemption value approximates fair value.
The calculation of diluted income (loss) per share of common stock does not consider the effect of the warrants issued in connection with the (i) Initial Public Offering, and (ii) the private placement since the exercise of the warrants is contingent upon the occurrence of future events. As of March 31, 2023, the Company’s outstanding warrants (13,979,000) have been excluded from diluted net loss as their inclusion would be anti-dilutive. As a result, diluted net income (loss) per common share is the same as basic net income (loss) per common share for the periods presented.
| F-45 |
The following table reflects the calculation of basic and diluted net income (loss) per common share (in dollars, except per share amounts):
SCHEDULE OF BASIC AND DILUTED NET INCOME (LOSS) PER COMMON SHARE
| Three Months Ended | ||||||||
| March 31, 2023 | ||||||||
Class A common stock | Class B common stock | |||||||
| Basic and diluted net income per common share | ||||||||
| Numerator: | ||||||||
| Allocation of net loss | $ | 76,421 | $ | 43,848 | ||||
| Denominator: | ||||||||
| Basic and diluted weighted average shares outstanding | 5,762,364 | 3,306,250 | ||||||
| Basic and diluted net income per common share | $ | 0.01 | $ | 0.01 | ||||
| For the Three Months Ended | ||||||||
| March 31, 2022 | ||||||||
| Class A common stock | Class B common stock | |||||||
| Basic and diluted net loss per common share | ||||||||
| Numerator: | ||||||||
| Allocation of net loss | $ | (180,478 | ) | $ | (73,058 | ) | ||
| Denominator: | ||||||||
| Basic and diluted weighted average shares outstanding | 8,167,506 | 3,306,250 | ||||||
| Basic and diluted net loss per common share | $ | (0.02 | ) | $ | (0.02 | ) | ||
Income Taxes
The Company follows the asset and liability method of accounting for income taxes under ASC 740, “Income Taxes.” Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that included the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
ASC 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. There were no unrecognized tax benefits and no amounts accrued for interest and penalties as of March 31, 2023 or December 31, 2022. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception.
The Company has identified the United States and California as its only tax jurisdictions. The Company is subject to income taxation by major taxing authorities since inception. All tax periods are open to examination by tax authorities. These examinations may include questioning the timing and amount of deductions, the nexus of income among various tax jurisdictions and compliance with federal and state tax laws. The Company’s management does not expect that the total amount of unrecognized tax benefits will materially change over the next twelve months. The Company actual tax expense differs from the expected tax expense due to the change in the valuation allowance.
| F-46 |
Concentration of Credit Risk
Financial instruments that potentially subject the Company to credit risk consist principally of cash and investments held in the Trust Account. Cash is maintained in accounts with financial institutions, which, at times may exceed the Federal Depository Insurance Corporation coverage limit of $250,000, and investments held in the Trust Account. As of March 31, 2023 and December 31, 2022, the Company had not experienced losses on these accounts and management believes the Company is not exposed to significant risks on such accounts.
Investments Held in Trust Account
The Company’s Investments held in the Trust Account were $23,658,838 at March 31, 2023, and $136,871,183 at December 31, 2022.
The Company’s portfolio of investments is comprised of U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with a maturity of 185 days or less, or investments in money market funds that invest in U.S. government securities and generally have a readily determinable fair value, or a combination thereof. When the Company’s investments held in the Trust Account are comprised of U.S. government securities, the investments are classified as trading securities. When the Company’s investments held in the Trust Account are comprised of money market funds, the investments are recognized at fair value. Trading securities and investments in money market funds are presented on the balance sheets at fair value at the end of each reporting period. Gains and losses resulting from the change in fair value of these securities is included in income on investments held in the Trust Account in the accompanying statements of operations. The estimated fair values of investments held in the Trust Account are determined using available market information.
Fair Value of Financial Instruments
The fair value of the Company’s assets and liabilities, which qualify as financial instruments under ASC 820, “Fair Value Measurements” approximates the carrying amounts represented in the balance sheet, partially due to their short-term nature.
Fair value is defined as the price that would be received for sale of an asset or paid to transfer of a liability, in an orderly transaction between market participants at the measurement date. US GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
| ● | Level 1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets; | |
| ● | Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and | |
| ● | Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. |
Derivative Financial Instruments
The Company evaluates its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with ASC Topic 815, “Derivatives and Hedging”. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value on the grant date and is then re-valued at each reporting date, with changes in the fair value reported in the statements of operations. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period. Derivative liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement or conversion of the instrument could be required within 12 months of the balance sheet date.
| F-47 |
Warrant Instruments
The Company accounts for warrants in accordance with the guidance contained in FASB ASC 815, “Derivatives and Hedging”. Under ASC 815-40 warrants that meet the criteria for equity treatment are recorded in stockholders’ equity (deficit). The warrants are subject to re-evaluation of the proper classification and accounting treatment at each reporting period. If the warrants no longer meet the criteria for equity treatment, they will be recorded as a liability and remeasured each period with changes recorded in the statement of operations.
Recent Accounting Standards
Management does not believe that any recently issued, but not yet effective, accounting standards, if currently adopted, would have a material effect on the Company’s financial statements.
NOTE 3 — INITIAL PUBLIC OFFERING
Pursuant to the Initial Public Offering, the Company sold 13,225,000 Units at a price of $10.00 per Unit. Each Unit consists of one share of Class A common stock and one redeemable warrant (“Public Warrant”). Each whole Public Warrant entitles the holder to purchase one share of Class A common stock at a price of $11.50 per share, subject to adjustment (see Note 7).
NOTE 4 — PRIVATE PLACEMENTS
Simultaneously with the closing of the Initial Public Offering, the Company consummated the private sale to the Sponsor of 754,000 Private Placement Units at a price of $10.00 per Private Placement Unit ($7,540,000). Each Private Placement Unit is comprised of one Class A share and one warrant. Each Private Placement Warrant is exercisable to purchase one share of Class A common stock at a price of $11.50 per share, subject to adjustment (see Note 7). Our Sponsor has agreed to transfer, but has not yet transferred, 15,000 placement units to each of our director nominees. The proceeds from the sale of the Private Placement Units will be added to the net proceeds from the Initial Public Offering held in the Trust Account. If the Company does not complete a Business Combination within the Combination Period, the proceeds from the sale of the Private Placement Units held in the Trust Account will be used to fund the redemption of the Public Shares (subject to the requirements of applicable law) and the securities comprising the Private Placement Units will expire worthless. The Private Placement Units (including the Class A common stock issuable upon exercise of the warrants included in the Private Placement Units) will not be transferable, assignable or saleable until 30 days after the completion of an Initial Business Combination, subject to certain exceptions.
NOTE 5 — RELATED PARTY TRANSACTIONS
Related Party Transactions
Founder Shares
On November 16, 2021, the Sponsor received 4,312,500 shares of the Company’s Class B common stock (the “Founder Shares”) for $25,000. On January 26, 2022, the Sponsor surrendered and forfeited 1,006,250 Founder Shares for no consideration, following which the Sponsor holds 3,306,250 Founder Shares.
The holders of the Founder Shares have agreed, subject to limited exceptions, not to transfer, assign or sell any of the Founder Shares until the earlier to occur of: (A) one year after the completion of a Business Combination and (B) subsequent to a Business Combination, (x) if the last reported sale price of the Class A common stock equals or exceeds $12.00 per share (as adjusted for stock splits, stock capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 150 days after a Business Combination, or (y) the date on which the Company completes a liquidation, merger, capital stock exchange or other similar transaction that results in all of the Public Stockholders having the right to exchange their shares of common stock for cash, securities or other property. However, our sponsor has agreed to transfer, but has not yet transferred, 15,000 placement units to each of our director nominees.
Promissory Note — Related Party
On November 4, 2021, the Sponsor issued an unsecured promissory note to the Company (the “Promissory Note”), pursuant to which the Company may borrow up to an aggregate principal amount of $300,000. The Promissory Note is non-interest bearing and payable on the earlier of (i) the consummation of the Initial Public Offering or (ii) the decision not to execute the Initial Public Offering. The balance of this Promissory Note in the amount of $177,057 was paid in full on February 10, 2022.
| F-48 |
On March 7, 2023, the Company entered into a $1.5 million promissory note with the Company’s Sponsor. The promissory note is to fund the Trust Account and the Company’s operating expenses. On March 7, 2023 the Company’s Sponsor advanced $300,000 and an additional $200,000 on April 5, 2023 (see Note 9). The Company’s Sponsor will provide additional funds as necessary under the promissory note. This loan is non-interest bearing, unsecured and will be repayable in cash in full upon the earlier of (i) the date on which the Company consummates an initial business combination and (ii) the date that the Company’s winding up is effective.
General and Administrative Services
Commencing on the date the Units are first listed on the Nasdaq, the Company has agreed to pay the Sponsor a total of $10,000 per month for office space, utilities and secretarial and administrative support. Upon completion of the Initial Business Combination or the Company’s liquidation, the Company will cease paying these monthly fees. During the three months ended March 31, 2023 and March 31, 2022, the Company incurred $30,000 and $20,000, respectively, of such expenses.
Related Party Loans
In order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required (“Working Capital Loans”). Such Working Capital Loans would be evidenced by promissory notes. The notes may be repaid upon completion of a Business Combination, without interest, or, at the lender’s discretion, up to $1,500,000 of the notes may be converted upon completion of a Business Combination into units at a price of $10.00 per unit. Such units would be identical to the Private Placement Units. In the event that a Business Combination does not close, the Company may use a portion of proceeds held outside the Trust Account to repay the Working Capital Loans but no proceeds held in the Trust Account would be used to repay the Working Capital Loans.
NOTE 6 — COMMITMENTS AND CONTINGENCIES
Commitments and Contingencies
Registration Rights
The holders of the Founder Shares, Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans (and any shares of common stock issuable upon the exercise of the Private Placement Warrants or warrants issued upon conversion of the Working Capital Loans and upon conversion of the Founder Shares) will be entitled to registration rights pursuant to a registration rights agreement to be signed prior to or on the effective date of Initial Public Offering requiring the Company to register such securities for resale. The holders of these securities will be entitled to make up to three demands, excluding short form registration demands, that the Company register such securities. In addition, the holders have certain “piggy-back” registration rights with respect to registration statements filed subsequent to completion of a Business Combination and rights to require the Company to register for resale such securities pursuant to Rule 415 under the Securities Act. However, the registration rights agreement provides that the Company will not be required to effect or permit any registration or cause any registration statement to become effective until the securities covered thereby are released from their lock-up restrictions. The Company will bear the expenses incurred in connection with the filing of any such registration statements.
Underwriting Agreement
The Company granted the underwriters a 45-day option from the date of Initial Public Offering to purchase up to 1,725,000 additional Units to cover over-allotments, if any, at the Initial Public Offering price less the underwriting discounts and commissions. The option was fully exercised on February 7, 2022.
The underwriters were paid a cash underwriting discount of $0.20 per Unit, or $2,645,000, payable upon the closing of the Initial Public Offering. In addition, the underwriters are entitled to a deferred fee of $0.35 per Unit, or $4,628,750. The deferred fee will become payable to the underwriters from the amounts held in the Trust Account solely in the event that the Company completes a Business Combination, subject to the terms of the underwriting agreement.
| F-49 |
In addition to the underwriting discount, the Company paid the underwriters $50,000 as an advance against out-of-pocket accountable expenses actually anticipated to be incurred by the underwriters, which advance will be returned to the Company to the extent not actually incurred. The Company agreed to pay or reimburse the underwriters for travel, lodging and other “road show” expenses, expenses of the underwriters’ legal counsel and certain diligence and other fees, which such fees and expenses are capped at an aggregate of $150,000 (less the $50,000 advance previously paid).
NOTE 7 — STOCKHOLDERS’ DEFICIT
Preferred Stock — The Company is authorized to issue 1,000,000 shares of preferred stock with a par value of $0.0001 per share. As of March 31, 2023 and December 31, 2022, there were no shares of preferred stock issued or outstanding.
Class A Common Stock — The Company is authorized to issue 100,000,000 shares of Class A common stock with a par value of $0.0001 per share. Holders of Class A common stock are entitled to one vote for each share. As of March 31, 2023 and December 31, 2022, there were 754,000 and zero, respectively, shares of Class A common stock issued and outstanding (excluding the 2,187,728 shares and 13,225,000 shares subject to possible redemption as of March 31, 2023 and December 31, 2022, respectively). During the three months ended March 31, 2023, a total of 11,037,272, shares of Class A common stock were redeemed for $114,068,280 or approximately $10.33 per share.
Class B Common Stock — The Company is authorized to issue 10,000,000 shares of Class B common stock with a par value of $0.0001 per share. Holders of Class B common stock are entitled to one vote for each share. As of each March 31, 2023 and December 31, 2022, there were 3,306,250 shares of Class B common stock issued and outstanding.
Only holders of the Class B common stock will have the right to vote on the election of directors prior to the Business Combination. Holders of Class A common stock and holders of Class B common stock will vote together as a single class on all matters submitted to a vote of our shareholders except as otherwise required by law. In connection with our initial business combination, we may enter into a stockholders agreement or other arrangements with the stockholders of the target or other investors to provide for voting or other corporate governance arrangements that differ from those in effect upon completion of this offering.
The shares of Class B common stock will automatically convert into Class A common stock at the time of a Business Combination. In the case that additional shares of Class A common stock, or equity-linked securities, are issued or deemed issued in excess of the amounts issued in the Initial Public Offering and related to the closing of a Business Combination, the ratio at which shares of Class B common stock shall convert into shares of Class A common stock will be adjusted (unless the holders of a majority of the then-outstanding shares of Class B common stock agree to waive such adjustment with respect to any such issuance or deemed issuance) so that the number of shares of Class A common stock issuable upon conversion of all shares of Class B common stock will equal, in the aggregate, on an as-converted basis, 20% of the sum of the total number of all shares of common stock outstanding upon the completion of Initial Public Offering plus all shares of Class A common stock and equity-linked securities issued or deemed issued in connection with a Business Combination (net of the number of shares of Class A common stock redeemed in connection with a Business Combination), excluding any shares or equity-linked securities issued or issuable to any seller of an interest in the target to us in a Business Combination.
Only holders of the Class B common stock will have the right to vote on the election of directors prior to the Business Combination. Holders of Class B common stock will vote together as a single class on all matters submitted to a vote of our shareholders except as otherwise required by law. In connection with our initial business combination, we may enter into a stockholders agreement or other arrangements with the stockholders of the target or other investors to provide for voting or other corporate governance arrangements that differ from those in effect upon completion of this offering.
Warrants — Public Warrants may only be exercised for a whole number of shares. No fractional warrants will be issued upon separation of the Units and only whole warrants will trade. The Public Warrants will become exercisable on the later of (a) 30 days after the completion of a Business Combination and (b) 12 months from the closing of the Initial Public Offering. The Public Warrants will expire five years after the completion of a Business Combination or earlier upon redemption or liquidation.
| F-50 |
The Company will not be obligated to deliver any shares of Class A common stock pursuant to the exercise of a warrant and will have no obligation to settle such warrant exercise unless a registration statement under the Securities Act covering the issuance of the shares of Class A common stock issuable upon exercise of the warrants is then effective and a current prospectus relating to those shares of Class A common stock is available, subject to the Company satisfying its obligations with respect to registration, or a valid exemption from registration is available. No warrant will be exercisable for cash or on a cashless basis, and the Company will not be obligated to issue any shares to holders seeking to exercise their warrants, unless the issuance of the shares upon such exercise is registered or qualified under the securities laws of the state of residence of the exercising holder, or an exemption from registration is available.
The Company has agreed that as soon as practicable, but in no event later than 15 business days after the closing of a Business Combination, the Company will use its commercially reasonable efforts to file, and within 60 business days following a Business Combination to have declared effective, a registration statement covering the issuance of the shares of Class A common stock issuable upon exercise of the warrants and to maintain a current prospectus relating to those shares of Class A common stock until the warrants expire or are redeemed. Notwithstanding the above, if the Class A common stock is at the time of any exercise of a warrant not listed on a national securities exchange such that it satisfies the definition of a “covered security” under Section 18(b)(1) of the Securities Act, the Company may, at its option, require holders of Public Warrants who exercise their warrants to do so on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act and, in the event the Company so elects, the Company will not be required to file or maintain in effect a registration statement, but will use its commercially reasonable efforts to register or qualify the shares under applicable blue sky laws to the extent an exemption is not available.
Redemption of Warrants When the Price per Share of Class A Common Stock Equals or Exceeds $18.00 — Once the warrants become exercisable, the Company may redeem the outstanding Public Warrants:
| ● | in whole and not in part; | |
| ● | at a price of $0.01 per Public Warrant; | |
| ● | upon a minimum of 30 days’ prior written notice of redemption, or the 30-day redemption period to each warrant holder; and | |
| ● | if, and only if, the last reported sale price of the Class A common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganization, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending on the third trading day prior to the date on which the Company sends the notice of redemption to warrant holders. |
If and when the warrants become redeemable by the Company, the Company may exercise its redemption right even if it is unable to register or qualify the underlying securities for sale under all applicable state securities laws.
If the Company calls the Public Warrants for redemption, as described above, its management will have the option to require any holder that wishes to exercise the Public Warrants to do so on a “cashless basis,” as described in the warrant agreement. The exercise price and number of common stock issuable upon exercise of the Public Warrants may be adjusted in certain circumstances including in the event of a stock dividend, extraordinary dividend or recapitalization, reorganization, merger or consolidation. However, except as described below, the Public Warrants will not be adjusted for issuances of common stock at a price below its exercise price. Additionally, in no event will the Company be required to net cash settle the Public Warrants. If the Company is unable to complete a Business Combination within the Combination Period and the Company liquidates the funds held in the Trust Account, holders of Public Warrants will not receive any of such funds with respect to their Public Warrants, nor will they receive any distribution from the Company’s assets held outside of the Trust Account with respect to such Public Warrants. Accordingly, the Public Warrants may expire worthless.
The Private Placement Warrants are identical to the Public Warrants underlying the Units sold in the Initial Public Offering.
| F-51 |
The Private Placement Warrants and Public Warrants are recorded in stockholders’ equity (deficit) as they qualify for equity treatment under ASC 815.
The key assumptions used to value the Public Warrants, which was determined to be $23,276,000, were as follows:
| ● | Term – 5 years | |
| ● | Volatility – 22% | |
| ● | Dividends – 0% | |
| ● | Discount rate – 1.76% |
NOTE 8 — FAIR VALUE MEASUREMENTS
The Company follows the guidance in ASC 820 for its financial assets and liabilities that are re-measured and reported at fair value at each reporting period, and non-financial assets and liabilities that are re-measured and reported at fair value at least annually.
The fair value of the Company’s financial assets and liabilities reflects management’s estimate of amounts that the Company would have received in connection with the sale of the assets or paid in connection with the transfer of the liabilities in an orderly transaction between market participants at the measurement date. In connection with measuring the fair value of its assets and liabilities, the Company seeks to maximize the use of observable inputs (market data obtained from independent sources) and to minimize the use of unobservable inputs (internal assumptions about how market participants would price assets and liabilities). The following fair value hierarchy is used to classify assets and liabilities based on the observable inputs and unobservable inputs used in order to value the assets and liabilities:
| Level 1: | Quoted prices in active markets for identical assets or liabilities. An active market for an asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis. | |
| Level 2: | Observable inputs other than Level 1 inputs. Examples of Level 2 inputs include quoted prices in active markets for similar assets or liabilities and quoted prices for identical assets or liabilities in markets that are not active. | |
| Level 3: | Unobservable inputs based on our assessment of the assumptions that market participants would use in pricing the asset or liability. |
The following table presents information about the Company’s assets and liabilities that are measured at fair value at March 31, 2023 and December 31, 2022, and indicates the fair value hierarchy of the valuation inputs the Company utilized to determine such fair value:
SCHEDULE OF FAIR VALUE OF ASSETS AND LIABILITIES
| Description | Level | March 31, 2023 | ||||||
| Assets: | ||||||||
| Investments held in Trust Account | 1 | $ | 23,658,838 | |||||
| Description | Level | December 31, 2022 | ||||||
| Assets: | ||||||||
| Investments held in Trust Account | 1 | $ | 136,871,183 | |||||
NOTE 9 — TAXES
The expected tax expense based on the statutory rate is reconciled with actual tax expense as follows:
The effective tax rate differs from the statutory tax rate of 21% for the year ended December 31, 2022, due to the valuation allowance recorded on the Company’s net operating losses and unrealized income on the Trust Account. During the three months ended March 31, 2023, the estimated effect tax rates is approximately 23.3% due to the change in the valuation allowance and prior year tax true up. The Company files income tax returns in the U.S. federal jurisdiction and is subject to examination by the various taxing authorities. The Company’s tax returns since inception remain open to examination by the taxing authorities. The Company considers California to be a significant state tax jurisdiction.
NOTE 10 — SUBSEQUENT EVENTS
Subsequent Events
The Company evaluated subsequent events and transactions that occurred after the balance sheet date through the date the financial statements were issued. Based upon this review, except as disclosed below, the Company did not identify any subsequent events that would have required adjustment or disclosure in the financial statements.
On April 5, 2023, the Company’s Sponsor advanced $200,000 to the Company under the Company’s $1.5 million promissory note issued March 7, 2023.
On April 6, 2023, and May 4, 2023 the Company deposited into the Trust Account $78,863 and $77,688, respectively, to extend the period of time to consummate a business combination each month.
On May 11, 2023, the Company, Conduit, and Merger Sub entered into a second amendment to the Merger Agreement to provide for (i) removal of the provision that indicates that no tax opinion would be delivered in connection with the closing, (ii) a closing obligation that that the Company either (a) be exempt from the provisions of Rule 419 promulgated under the Securities Act other than through its net tangible assets or (b) have at least $5,000,001 of net tangible assets either immediately prior to or upon consummation of the Merger, and (iii) extension of the outside date for the closing of the Merger from May 31, 2023, to February 7, 2024.
| F-52 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
Murphy Canyon Acquisition Corp.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Murphy Canyon Acquisition Corp. (the “Company”) as of December 31, 2022 and 2021, the related statements of operations, stockholders’ equity (deficit) and cash flows for the year ended December 31, 2022 and for the period from October 19, 2021 (inception) through December 31, 2021, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for the year ended December 31, 2022 and for the period from October 19, 2021 (inception) through December 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 1 to the financial statements, the Company’s business plan is dependent on the completion of a business combination which is less than one year form the date of the issuance of the financial statements. Additionally, the Company has incurred and expects to continue to incur significant costs in pursuit of its acquisition plans. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Marcum LLP
Marcum llp
We have served as the Company’s auditor since 2021.
New
York, NY
March 28, 2023
| F-53 |
MURPHY CANYON ACQUISITION CORP.
BALANCE SHEETS
| December 31, 2022 | December 31, 2021 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 345,777 | $ | 48,555 | ||||
| Prepaid expenses | 300,862 | 55,608 | ||||||
| Total current assets | 646,639 | 104,163 | ||||||
| Investments held in Trust Account | 136,871,183 | - | ||||||
| Deferred offering costs | - | 108,962 | ||||||
| Total Assets | $ | 137,517,822 | $ | 213,125 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| Current liabilities: | ||||||||
| Accrued expenses | $ | 43,113 | $ | 15,449 | ||||
| Income taxes payable | 374,862 | - | ||||||
| Note payable – Sponsor | - | 177,057 | ||||||
| Total current liabilities | 417,975 | 192,506 | ||||||
| Deferred commission payable | 4,628,750 | - | ||||||
| Total Liabilities | 5,046,725 | 192,506 | ||||||
| Commitments and Contingencies (Note 6) | - | |||||||
| Common stock subject to possible redemption at redemption value (13,225,000 shares at $10.34 per share) | 136,771,183 | - | ||||||
| Stockholders’ Equity (Deficit) | ||||||||
| Preferred stock, $0.0001 par value; 1,000,000 shares authorized; none issued and outstanding | - | - | ||||||
| Class A common stock, $0.0001 par value; 100,000,000 shares authorized; 754,000 (excluding 13,225,000 subject to possible redemption) and none issued and outstanding at December 31, 2022 and December 31, 2021, respectively | 75 | - | ||||||
| Class B common stock, $0.0001 par value; 10,000,000 shares authorized; 3,306,250 shares issued and outstanding | 331 | 331 | ||||||
| Additional paid-in capital | - | 24,669 | ||||||
| Accumulated deficit | (4,300,492 | ) | (4,381 | ) | ||||
| Total Stockholders’ Equity (Deficit) | (4,300,086 | ) | 20,619 | |||||
| Total Liabilities and Stockholders’ Equity (Deficit) | $ | 137,517,822 | $ | 213,125 | ||||
The accompanying notes are an integral part of these financial statements.
| F-54 |
MURPHY CANYON ACQUISITION CORP.
STATEMENTS OF OPERATIONS
| For
the Year December 31, 2022 | FOR THE PERIOD FROM OCTOBER 19, 2021 (INCEPTION) THROUGH DECEMBER 31, 2021 | |||||||
| General and administrative expenses | $ | 1,092,682 | $ | 4,381 | ||||
| Administration fee – related party | 110,000 | - | ||||||
| Total expenses | 1,202,682 | 4,381 | ||||||
| Other Income | ||||||||
| Interest income – Investments held in Trust Account | 1,976,183 | - | ||||||
| Total other income | 1,976,183 | - | ||||||
| Net income (loss) before income taxes | 773,501 | (4,381 | ) | |||||
| Income tax expense | (374,862 | ) | - | |||||
| Net income (loss) | $ | 398,639 | $ | (4,381 | ) | |||
| Class A common stock – weighted average shares outstanding, basic and diluted | 12,558,058 | - | ||||||
| Class A common stock – Basic and diluted net income (loss) per share | $ | 0.03 | $ | - | ||||
| Class B common stock – weighted average shares outstanding, basic and diluted | 3,306,250 | 2,875,000 | (1)(2) | |||||
| Class B common stock – Basic and diluted net income (loss) per share | $ | 0.03 | $ | (0.00 | ) | |||
| (1) | Excludes an aggregate of up to 431,250 shares of Class B common stock subject to forfeiture if the over-allotment option is not exercised in full or in part by the underwriters. The underwriters exercised the over-allotment option in full on February 7, 2022. As such, the Class B common stock is no longer subject to forfeiture (see Notes 5 and 6). | |
| (2) | On January 26, 2022, the Sponsor surrendered and forfeited 1,006,250 founder shares for no consideration following which the Sponsor holds 3,306,250 founder shares. All share amounts have been retroactively restated to reflect this surrender (see Note 5). |
The accompanying notes are an integral part of these financial statements.
| F-55 |
MURPHY CANYON ACQUISITION CORP.
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE YEAR ENDED DECEMBER 31, 2022 AND FOR THE PERIOD FROM OCTOBER 19, 2021 (INCEPTION) TO DECEMBER 31, 2021
| Class A | Class B | Additional Paid-in | Accumulated | Total Stockholders’ Equity | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | (Deficit) | ||||||||||||||||||||||
| Balance, October 19, 2021 (inception) | - | - | - | - | ||||||||||||||||||||||||
| Issuance of Class B common stock to Sponsor(1) (2) | - | - | 3,306,250 | 331 | 24,669 | - | 25,000 | |||||||||||||||||||||
| Net loss | - | - | - | (4,381 | ) | (4,381 | ||||||||||||||||||||||
| Balance, December 31, 2022 | - | $ | - | 3,306,250 | $ | 331 | $ | 24,669 | $ | (4,381 | ) | $ | 20,619 | |||||||||||||||
| Proceeds allocated to Public Warrants, net of offering costs | - | - | - | - | 21,917,543 | - | 21,917,543 | |||||||||||||||||||||
| Sale of Private Placement Units, net of offering costs | 754,000 | 75 | - | - | 7,520,275 | - | 7,520,350 | |||||||||||||||||||||
| Remeasurement of Class A common stock subject to possible redemption upon IPO | - | - | - | - | (29,462,487 | ) | (2,818,567 | ) | (32,281,054 | ) | ||||||||||||||||||
| Remeasurement of Class A shares to redemption value | (1,876,183 | ) | (1,876,183 | ) | ||||||||||||||||||||||||
| Net income | - | - | - | - | - | 398,639 | 398,639 | |||||||||||||||||||||
| Balance, December 31, 2022 | 754,000 | $ | 75 | 3,306,250 | $ | 331 | $ | - | $ | (4,300,492 | ) | $ | (4,300,086 | ) | ||||||||||||||
| (1) | Includes an aggregate of up to 431,250 shares of Common stock subject to forfeiture if the over-allotment option is not exercised in full or in part by the underwriters. The underwriters exercised the over-allotment option in full on February 7, 2022. As such, the Class B common stock is no longer subject to forfeiture (see Notes 5 and 6). | |
| (2) | On January 26, 2022, the Sponsor surrendered and forfeited 1,006,250 founder shares for no consideration following which the Sponsor holds 3,306,250 founder shares. All share amounts have been retroactively restated to reflect this surrender (see Note 5). |
The accompanying notes are an integral part of these financial statements.
| F-56 |
MURPHY CANYON ACQUISITION CORP.
STATEMENTS OF CASH FLOWS
| For the Year Ended December 31, 2022 | FOR THE PERIOD FROM OCTOBER 19, 2021 (INCEPTION) THROUGH DECEMBER 31, 2021 | |||||||
| Cash flows from operating activities: | ||||||||
| Net income (loss) | $ | 398,639 | $ | (4,381 | ) | |||
| Adjustments to reconcile net income (loss) to net cash used in operating activities | ||||||||
| Formation costs paid by note payable – Sponsor | 3,994 | |||||||
| Interest earned on investments held in Trust Account | (1,976,183 | ) | - | |||||
| Changes in operating assets and liabilities: | ||||||||
| Deferred offering costs | 108,962 | - | ||||||
| Prepaid expenses | (245,254 | ) | (5,608 | ) | ||||
| Accrued expenses | 27,664 | - | ||||||
| Accrued income taxes payable | 374,862 | - | ||||||
| Net cash used in operating activities | (1,311,310 | ) | (5,995 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Cash deposited into Trust Account | (134,895,000 | ) | - | |||||
| Net cash used in investing activities | (134,895,000 | ) | - | |||||
| Cash flows from financing activities: | ||||||||
| Deferred offering costs | - | (45,450 | ) | |||||
| Proceeds from note payable - Sponsor | - | 75,000 | ||||||
| Proceeds from issuance of Class B common stock to Sponsor | - | 25,000 | ||||||
| Sale of units in public offering | 132,250,000 | - | ||||||
| Sale of private placement units | 7,540,000 | - | ||||||
| Payment of offering costs | (3,109,411 | ) | - | |||||
| Repayment of note payable - Sponsor | (177,057 | ) | - | |||||
| Net cash provided by financing activities | 136,503,532 | 54,550 | ||||||
| Net change in cash | 297,222 | 48,555 | ||||||
| Cash at beginning of period | 48,555 | - | ||||||
| Cash at end of period | $ | 345,777 | $ | 48,555 | ||||
| Non-cash financing activities: | ||||||||
| Deferred offering costs included in accrued offering costs | $ | - | $ | 15,449 | ||||
| Prepaid expenses paid by note payable - Sponsor | $ | - | $ | 50,000 | ||||
| Deferred offering costs paid by note payable - Sponsor | $ | - | $ | 47,875 | ||||
| Deferred commission payable | $ | 4,628,750 | $ | - | ||||
| Class A shares subject to redemption | $ | 136,771,183 | $ | - | ||||
The accompanying notes are an integral part of these financial statements.
| F-57 |
MURPHY CANYON ACQUISITION CORP.
FOR THE YEAR ENDED DECEMBER 31, 2022 AND FOR THE PERIOD FROM OCTOBER 19, 2021 (INCEPTION) THROUGH DECEMBER 31, 2021
Notes to financial statements
NOTE 1 — DESCRIPTION OF ORGANIZATION, BUSINESS OPERATIONS AND GOING CONCERN
Murphy Canyon Acquisition Corp. (the “Company”) was incorporated in Delaware on October 19, 2021. The Company was formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, reorganization or similar business combination with one or more businesses (the “Business Combination”). The Company is not limited to a particular industry or sector for purposes of consummating a Business Combination. The Company is an early stage and emerging growth company and, as such, the Company is subject to all of the risks associated with early stage and emerging growth companies.
As of December 31, 2022, the Company had not commenced any operations. All activity for the period from October 19, 2021 (inception) through December 31, 2022 relates to the Company’s formation, the proposed initial public offering (“Initial Public Offering”), which is described below, and searching for an initial Business Combination, as defined below. The Company will not generate any operating revenues until after the completion of its initial Business Combination, at the earliest. The Company will generate non-operating income in the form of interest income from the proceeds derived from the Initial Public Offering. The Company has selected December 31 as its fiscal year end.
The registration statement for the Company’s Initial Public Offering (the “Registration Statement”) was declared effective on February 2, 2022. On February 7, 2022, the Company consummated the Initial Public Offering of 13,225,000 units (“Units”), generating gross proceeds of $132,250,000, which is described in Note 3.
Simultaneously with the closing of the Initial Public Offering, the Company consummated the sale of 754,000 units (the “Private Placement Units”) at a price of $10.00 per Private Placement Unit in private placements to Murphy Canyon Acquisition Sponsor LLC (the “Sponsor”), with gross proceeds of $7,540,000.
Following the closing of the Initial Public Offering on February 7, 2022, an amount of $139,790,000 from the net proceeds of the sale of the Units in the Initial Public Offering and the Private Placement Units was placed in the Trust Account, as defined below. This resulted in an overfunding of the Trust Account of $4,895,000. As such, subsequent to the initial funding of the Trust Account, $2,000,000 was transferred to the Company’s operating cash account and $2,895,000 was used to pay offering costs. The funds held in the Trust Account may be invested in U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act of 1940, as amended (the “Investment Company Act”), with a maturity of 185 days or less or in any open-ended investment company that holds itself out as a money market fund selected by the Company meeting the conditions of Rule 2a-7 of the Investment Company Act, as determined by the Company, until the earlier of: (i) the completion of a Business Combination or (ii) the distribution of the Trust Account, as described below.
On November 8, 2022, the Company entered into a definitive Business Combination Agreement (the “BCA”) with Conduit Pharmaceuticals Limited, a Cayman Islands exempted company (“Conduit”), and Conduit Merger Sub, Inc., a Cayman Islands exempted company (“Merger Sub”). Merger Sub is a wholly owned subsidiary of the Company. Conduit is a pharmaceutical company led by experienced pharma executives, established to fund the development of successful deprioritized clinical assets licensed from large pharmaceutical companies through its exclusive relationships. The BCA was amended in January 2023, see Note 10 for additional information.
Upon the consummation of the transactions contemplated by the BCA, Merger Sub will merge with and into Conduit, with Conduit surviving as a wholly owned subsidiary of the Company (the “Business Combination”). The Company is expected to be renamed Conduit Pharmaceuticals Inc. at the closing of the Business Combination.
Pursuant to the BCA, at the closing, the Company shall issue and deliver to the shareholders of Conduit an aggregate number of shares of the Company’s common stock with an aggregate value equal to $650,000,000, with each share valued at $10.00 per share. A private placement transaction shall be conducted by the Company contemporaneously with the Business Combination (the “PIPE Financing”), pursuant to which the Company has entered into subscription agreements providing for aggregate investments in the Company’s securities of $27,000,000.
| F-58 |
There can be no assurance that the Business Combination or PIPE Financing will occur as planned or at all.
The Company’s management has broad discretion with respect to the specific application of the net proceeds of the Initial Public Offering and the sale of Private Placement Warrants, although substantially all of the net proceeds are intended to be applied generally toward consummating a Business Combination. There is no assurance that the Company will be able to complete a Business Combination successfully. The Company must complete one or more initial Business Combinations with one or more operating businesses or assets with a fair market value equal to at least 80% of the net assets held in the Trust Account (as defined below) (excluding the deferred underwriting commissions and taxes payable on the interest earned on the Trust Account). The Company will only complete a Business Combination if the post-transaction company owns or acquires 50% or more of the outstanding voting securities of the target or otherwise acquires a controlling interest in the target business sufficient for it not to be required to register as an investment company under the Investment Company Act of 1940, as amended (the “Investment Company Act”). Upon the closing of the Initial Public Offering, management has agreed that an amount equal to at least $10.00 per Unit sold in the Initial Public Offering, including proceeds of the Private Placement Warrants, will be held in a trust account (“Trust Account”), located in the United States and invested only in U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with a maturity of 185 days or less or in any open-ended investment company that holds itself out as a money market fund selected by the Company meeting certain conditions of Rule 2a-7 of the Investment Company Act, as determined by the Company, until the earlier of: (i) the completion of a Business Combination and (ii) the distribution of the funds held in the Trust Account, as described below.
The Company will provide the holders of the outstanding Public Shares (the “Public Stockholders”) with the opportunity to redeem all or a portion of their Public Shares either (i) in connection with a stockholder meeting called to approve the Business Combination or (ii) by means of a tender offer in connection with the Business Combination. The decision as to whether the Company will seek stockholder approval of a Business Combination or conduct a tender offer will be made by the Company. The Public Stockholders will be entitled to redeem their Public Shares for a pro rata portion of the amount then in the Trust Account (net of taxes payable). There will be no redemption rights upon the completion of a Business Combination with respect to the Company’s warrants. All of the Public Shares contain a redemption feature which allows for the redemption of such Public Shares in connection with our liquidation, if there is a stockholder vote or tender offer in connection with our initial business combination and in connection with certain amendments to our amended and restated certificate of incorporation. In accordance with U.S. Securities and Exchange Commission (“SEC”) and its guidance on redeemable equity instruments, which has been codified in ASC 480-10-S99, redemption provisions not solely within the control of a company require common stock subject to redemption to be classified outside of permanent equity. Given that the Public Shares will be issued with other freestanding instruments (i.e., public warrants), the initial carrying value of Class A common stock classified as temporary equity will be the allocated proceeds determined in accordance with ASC 470-20. The Class A common stock is subject to ASC 480-10-S99. If it is probable that the equity instrument will become redeemable, we have the option to either (i) accrete changes in the redemption value over the period from the date of issuance (or from the date that it becomes probable that the instrument will become redeemable, if later) to the earliest redemption date of the instrument or (ii) recognize changes in the redemption value immediately as they occur and adjust the carrying amount of the instrument to equal the redemption value at the end of each reporting period. We have elected to recognize the changes immediately. The accretion or remeasurement will be treated as a deemed dividend (i.e., a reduction to retained earnings, or in absence of retained earnings, additional paid-in capital). The Public Shares are redeemable and will be classified as such on the balance sheet until such date that a redemption event takes place.
If the Company seeks stockholder approval of the Business Combination, the Company will proceed with a Business Combination if a majority of the outstanding shares voted are voted in favor of the Business Combination, or such other vote as required by law or stock exchange rule. If a stockholder vote is not required by applicable law or stock exchange listing requirements and the Company does not decide to hold a stockholder vote for business or other reasons, the Company will, pursuant to its amended and restated certificate of incorporation (the “Certificate of Incorporation”), conduct the redemptions pursuant to the tender offer rules of the (“SEC”) and file tender offer documents with the SEC prior to completing a Business Combination. If, however, stockholder approval of the transaction is required by applicable law or stock exchange listing requirements, or the Company decides to obtain stockholder approval for business or other reasons, the Company will offer to redeem shares in conjunction with a proxy solicitation pursuant to the proxy rules and not pursuant to the tender offer rules. If the Company seeks stockholder approval in connection with a Business Combination, the Sponsor has agreed to vote its Founder Shares (as defined in Note 5) and any Public Shares purchased during or after the Initial Public Offering in favor of approving a Business Combination. Additionally, each Public Stockholder may elect to redeem their Public Shares without voting, and if they do vote, irrespective of whether they vote for or against the proposed transaction.
| F-59 |
Notwithstanding the foregoing, if the Company seeks stockholder approval of a Business Combination and it does not conduct redemptions pursuant to the tender offer rules, the Certificate of Incorporation provides that a Public Stockholder, together with any affiliate of such stockholder or any other person with whom such stockholder is acting in concert or as a “group” (as defined under Section 13 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), will be restricted from redeeming its shares with respect to more than an aggregate of 15% of the Public Shares, without the prior consent of the Company.
The holders of the Founder Shares have agreed (a) to waive their redemption rights with respect to the Founder Shares and Public Shares held by them in connection with the completion of a Business Combination and (b) not to propose an amendment to the Certificate of Incorporation (i) to modify the substance or timing of the Company’s obligation to allow redemptions in connection with a Business Combination or to redeem 100% of its Public Shares if the Company does not complete a Business Combination within the Combination Period (as defined below) or (ii) with respect to any other provision relating to stockholders’ rights or pre-business combination activity, unless the Company provides the Public Stockholders with the opportunity to redeem their Public Shares in conjunction with any such amendment.
If the Company has not completed a Business Combination within 12 months from the consummation of our initial public offering (or up to February 7, 2024 at the election of the Company subject to satisfaction of certain conditions, as amended at the January 2023 Special Meeting, see Note 10) (“Business Combination Period”), the Company will (i) cease all operations except for the purpose of winding up, (ii) as promptly as reasonably possible but not more than ten business days thereafter, redeem the Public Shares, at a per-share price, payable in cash, equal to the aggregate amount then on deposit in the Trust Account, including interest earned on the funds held in the Trust Account and not previously released to pay taxes (less up to $100,000 of interest to pay dissolution expenses), divided by the number of then outstanding Public Shares, which redemption will completely extinguish Public Stockholders’ rights as stockholders (including the right to receive further liquidating distributions, if any), and (iii) as promptly as reasonably possible following such redemption, subject to the approval of the Company’s remaining stockholders and the Company’s board of directors, dissolve and liquidate, subject in each case to the Company’s obligations under Delaware law to provide for claims of creditors and the requirements of other applicable law. Our Sponsor has committed to provide additional funds if needed to make such a deposit for the extensions. There will be no redemption rights or liquidating distributions with respect to the Company’s warrants, which will expire worthless if the Company fails to complete a Business Combination within the Combination Period.
The holders of the Founders Shares have agreed to waive their liquidation rights with respect to the Founder Shares if the Company fails to complete a Business Combination within the Combination Period. However, if the holders of Founder Shares acquire Public Shares in or after the Initial Public Offering, such Public Shares will be entitled to liquidating distributions from the Trust Account if the Company fails to complete a Business Combination within the Combination Period. The underwriters have agreed to waive their rights to their deferred underwriting commission (see Note 6) held in the Trust Account in the event the Company does not complete a Business Combination within the Combination Period and, in such event, such amounts will be included with the other funds held in the Trust Account that will be available to fund the redemption of the Public Shares. In the event of such distribution, it is possible that the per share value of the assets remaining available for distribution will be less than the Initial Public Offering price per Unit ($10.00).
In order to protect the amounts held in the Trust Account, the Sponsor has agreed to be liable to the Company if and to the extent any claims by a third party for services rendered or products sold to the Company, or a prospective target business with which the Company has discussed entering into a transaction agreement, reduce the amount of funds in the Trust Account to below (i) $10.00 per Public Share or (ii) such lesser amount per Public Share held in the Trust Account as of the date of the liquidation of the Trust Account, if less than $10.00 per public Share due to reductions in the value of the trust assets, in each case net of the amount of interest which may be withdrawn to pay taxes, except as to any claims by a third party who executed a waiver of any and all rights to seek access to the Trust Account and except as to any claims under the Company’s indemnity of the underwriters of the Initial Public Offering against certain liabilities, including liabilities under the Securities Act of 1933, as amended (the “Securities Act”). Moreover, in the event that an executed waiver is deemed to be unenforceable against a third party, the Sponsor will not be responsible to the extent of any liability for such third-party claims. The Company will seek to reduce the possibility that the Sponsor will have to indemnify the Trust Account due to claims of creditors by endeavoring to have all vendors, service providers (except for the Company’s independent registered accounting firm), prospective target businesses and other entities with which the Company does business, execute agreements with the Company waiving any right, title, interest or claim of any kind in or to monies held in the Trust Account.
| F-60 |
Management’s Plan and Going Concern
In connection with the Company’s assessment of going concern considerations in accordance with Accounting Standards Update (“ASU”) 2014-15, “Disclosures of Uncertainties about an Entity’s Ability to Continue as a Going Concern,” management has determined that the Combination Period is less than one year from the date of the issuance of the financial statements. There is no assurance that the Company’s plans to consummate a Business Combination will be successful within the Combination Period. Additionally, the Company has incurred and expects to continue to incur significant costs in pursuit of its acquisition plans. As a result, these factors raise substantial doubt about the Company’s ability to continue as a going concern for the next twelve months from the issuance of these financial statements. The financial statements do not include any adjustments that might result from the outcome of the uncertainty.
Risks and Uncertainties
Management is currently evaluating the impact of the COVID-19 pandemic and has concluded that while it is reasonably possible that the virus could have a negative effect on the Company’s financial position, results of its operations, and search for a target company, the specific impact is not readily determinable as of the date of these financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) as contained within the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”).
Emerging Growth Company
The Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012, as amended (the “JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the independent registered public accounting firm attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
| F-61 |
Use of Estimates
The preparation of financial statements in conformity with US GAAP requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period.
Making estimates requires management to exercise significant judgment. It is at least reasonably possible that the estimate of the effect of a condition, situation or set of circumstances that existed at the date of the financial statements, which management considered in formulating its estimate, could change in the near term due to one or more future confirming events. Accordingly, the actual results could differ significantly from those estimates.
Cash and Cash Equivalents
The Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents. The Company had $345,777 and $48,555 in cash as of December 31, 2022 and 2021. The Company did not have any cash equivalents as of December 31, 2022 or December 31, 2021.
Investments Held in Trust Account
At December 31, 2022 and 2021, the Company had $136,871,183 and zero, respectively, in investments held in the Trust Account.
Offering Costs Associated With a Public Offering
The Company complies with the requirements of FASB ASC 340-10-S99-1 and SEC Staff Accounting Bulletin (“SAB”) Topic 5A — “Expenses of Offering.” Offering costs of $7,738,161 consisting of $2,645,000 of underwriting fees, $4,628,750 of deferred underwriting fee (which are held in the Trust Account with Wilmington Trust Company acting as trustee), and $464,411 of Initial Public Offering costs. Of these costs, $1,358,457 and $19,656 were allocated to Public Warrants (as defined in Note 3) and Private Placement Warrants (as defined in Note 4), respectively.
Class A Common Stock Subject to Possible Redemption
The Company accounts for its shares of Class A common stock subject to possible redemption in accordance with the guidance enumerated in ASC 480 “Distinguishing Liabilities from Equity”. Common stock subject to mandatory redemption is classified as a liability instrument and is measured at fair value. Conditionally redeemable common stock (including common stock that feature redemption rights that are either within the control of the holder or subject to redemption upon the occurrence of uncertain events not solely within the Company’s control) are classified as temporary equity. At all other times, common stock is classified as stockholders’ equity. The shares of the Company’s Class A common stock feature certain redemption rights that are considered by the Company to be outside of the Company’s control and subject to the occurrence of uncertain future events. Accordingly, as of December 31, 2022, the shares of Class A common stock subject to possible redemption in the amount of $136,771,183 are presented as temporary equity, outside of the stockholders’ equity (deficit) section of the Company’s balance sheet.
As of December 31, 2022, the Class A common stock subject to possible redemption reflected on the balance sheet are reconciled in the following table:
SCHEDULE OF COMMON STOCK SUBJECT TO POSSIBLE REDEMPTION
| Gross proceeds from IPO | $ | 132,250,000 | ||
| Less: | ||||
| Proceeds allocated to Public Warrants | (23,276,000 | ) | ||
| Class A common stock issuance costs | (6,360,054 | ) | ||
| Plus: | ||||
| Remeasurement adjustment of carrying value to redemption value | 32,281,054 | |||
| Class A common stock subject to possible redemption, as of March 31, 2022 | 134,895,000 | |||
| Plus: | ||||
| Remeasurement adjustment of carrying value to redemption value | 101,767 | |||
| Class A common stock subject to possible redemption as of June 30, 2022 | 134,996,767 | |||
| Plus: | ||||
| Remeasurement adjustment of carrying value to redemption value | 609,920 | |||
| Class A common stock subject to possible redemption as of September 30, 2022 | 135,606,687 | |||
| Plus: | ||||
| Remeasurement adjustment of carrying value to redemption value | 1,164,496 | |||
| Class A common stock subject to possible redemption as of December 31, 2022 | $ | 136,771,183 |
| F-62 |
Net Income (Loss) per Common Share
The Company complies with accounting and disclosure requirements of FASB ASC Topic 260, “Earnings Per Share”. Net income (loss) per share of common stock is computed by dividing net income (loss) by the weighted average number of shares of common stock outstanding for the period. Accretion associated with the redeemable shares of Class A common stock is excluded from income (loss) per common share as the redemption value approximates fair value.
The calculation of diluted income (loss) per share of common stock does not consider the effect of the warrants issued in connection with the (i) Initial Public Offering, and (ii) the private placement since the exercise of the warrants is contingent upon the occurrence of future events. As of December 31, 2022, the Company’s outstanding warrants (13,979,000) have been excluded from diluted net loss as their inclusion would be anti-dilutive. As a result, diluted net income (loss) per common share is the same as basic net income (loss) per common share for the periods presented.
The following table reflects the calculation of basic and diluted net income (loss) per common share (in dollars, except per share amounts):
SCHEDULE OF BASIC AND DILUTED NET INCOME (LOSS) PER COMMON SHARE
| For the Period Since Inception to | ||||||||
| December 31, 2021 | ||||||||
Class A common stock | Class B common stock | |||||||
| Basic and diluted net income per common share | ||||||||
| Numerator: | ||||||||
| Allocation of net loss | $ | - | $ | (4,381 | ) | |||
| Denominator: | ||||||||
| Basic and diluted weighted average shares outstanding | - | 2,875,000 | ||||||
| Basic and diluted net income per common share | $ | - | $ | (0.00 | ) | |||
| For the Year Ended | ||||||||
| December 31, 2022 | ||||||||
| Class A common stock | Class B common stock | |||||||
| Basic and diluted net loss per common share | ||||||||
| Numerator: | ||||||||
| Allocation of net income | $ | 315,560 | $ | 83,079 | ||||
| Denominator: | ||||||||
| Basic and diluted weighted average shares outstanding | 12,558,058 | 3,306,250 | ||||||
| Basic and diluted net loss per common share | $ | 0.03 | $ | 0.03 | ||||
| F-63 |
Income Taxes
The Company follows the asset and liability method of accounting for income taxes under ASC 740, “Income Taxes.” Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that included the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
ASC 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities. The Company recognizes accrued interest and penalties related to unrecognized tax benefits as income tax expense. There were no unrecognized tax benefits and no amounts accrued for interest and penalties as of December 31, 2022 or December 31, 2021. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position. The Company is subject to income tax examinations by major taxing authorities since inception.
The Company has identified the United States and California as its only tax jurisdictions. The Company is subject to income taxation by major taxing authorities since inception. All tax periods are open to examination by tax authorities. These examinations may include questioning the timing and amount of deductions, the nexus of income among various tax jurisdictions and compliance with federal and state tax laws. The Company’s management does not expect that the total amount of unrecognized tax benefits will materially change over the next twelve months. The Company actual tax expense differs from the expected tax expense due to the change in the valuation allowance.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to credit risk consist principally of cash and investments held in the Trust Account. Cash is maintained in accounts with financial institutions, which, at times may exceed the Federal Depository Insurance Corporation coverage limit of $250,000, and investments held in the Trust Account. As of December 31, 2022 and 2021, the Company had not experienced losses on these accounts and management believes the Company is not exposed to significant risks on such accounts.
Investments Held in Trust Account
The Company’s Investments held in the Trust Account were $136,871,183 at December 31, 2022 and zero as of December 31, 2021.
The Company’s portfolio of investments is comprised of U.S. government securities, within the meaning set forth in Section 2(a)(16) of the Investment Company Act, with a maturity of 185 days or less, or investments in money market funds that invest in U.S. government securities and generally have a readily determinable fair value, or a combination thereof. When the Company’s investments held in the Trust Account are comprised of U.S. government securities, the investments are classified as trading securities. When the Company’s investments held in the Trust Account are comprised of money market funds, the investments are recognized at fair value. Trading securities and investments in money market funds are presented on the balance sheets at fair value at the end of each reporting period. Gains and losses resulting from the change in fair value of these securities is included in income on investments held in the Trust Account in the accompanying statements of operations. The estimated fair values of investments held in the Trust Account are determined using available market information.
| F-64 |
Fair Value of Financial Instruments
The fair value of the Company’s assets and liabilities, which qualify as financial instruments under ASC 820, “Fair Value Measurements” approximates the carrying amounts represented in the balance sheet, partially due to their short-term nature.
Fair value is defined as the price that would be received for sale of an asset or paid to transfer of a liability, in an orderly transaction between market participants at the measurement date. US GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
| ● | Level 1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets; | |
| ● | Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and | |
| ● | Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. |
Derivative Financial Instruments
The Company evaluates its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with ASC Topic 815, “Derivatives and Hedging”. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value on the grant date and is then re-valued at each reporting date, with changes in the fair value reported in the statements of operations. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is evaluated at the end of each reporting period. Derivative liabilities are classified in the balance sheet as current or non-current based on whether or not net-cash settlement or conversion of the instrument could be required within 12 months of the balance sheet date.
Warrant Instruments
The Company accounts for warrants in accordance with the guidance contained in FASB ASC 815, “Derivatives and Hedging”. Under ASC 815-40 warrants that meet the criteria for equity treatment are recorded in stockholders’ equity (deficit). The warrants are subject to re-evaluation of the proper classification and accounting treatment at each reporting period. If the warrants no longer meet the criteria for equity treatment, they will be recorded as a liability and remeasured each period with changes recorded in the statement of operations.
Recent Accounting Standards
Management does not believe that any recently issued, but not yet effective, accounting standards, if currently adopted, would have a material effect on the Company’s financial statements.
NOTE 3 — INITIAL PUBLIC OFFERING
Pursuant to the Initial Public Offering, the Company sold 13,225,000 Units at a price of $10.00 per Unit. Each Unit consists of one share of Class A common stock (“Public Shares”) and one redeemable warrant (“Public Warrant”). Each whole Public Warrant entitles the holder to purchase one share of Class A common stock at a price of $11.50 per share, subject to adjustment (see Note 7).
| F-65 |
NOTE 4 — PRIVATE PLACEMENTS
Simultaneously with the closing of the Initial Public Offering, the Company consummated the private sale to the Sponsor of 754,000 Private Placement Units at a price of $10.00 per Private Placement Unit ($7,540,000). Each Private Placement Unit is comprised of one Class A share and one warrant (“Private Placement Warrant”). Each Private Placement Warrant is exercisable to purchase one share of Class A common stock at a price of $11.50 per share, subject to adjustment (see Note 7). The Company’s Sponsor has agreed to transfer, but has not yet transferred, 15,000 Private Placement Units to each of our director nominees. The proceeds from the sale of the Private Placement Units were added to the net proceeds from the Initial Public Offering held in the Trust Account. If the Company does not complete a Business Combination within the Combination Period, the proceeds from the sale of the Private Placement Units held in the Trust Account will be used to fund the redemption of the Public Shares (subject to the requirements of applicable law) and the securities comprising the Private Placement Units will expire worthless. The Private Placement Units (including the Class A common stock issuable upon exercise of the warrants included in the Private Placement Units) will not be transferable, assignable or saleable until 30 days after the completion of an Initial Business Combination, subject to certain exceptions.
NOTE 5 — RELATED PARTY TRANSACTIONS
Related Party Transactions
Founder Shares
On November 16, 2021, the Sponsor received 4,312,500 shares of the Company’s Class B common stock (the “Founder Shares”) for $25,000. On January 26, 2022, the Sponsor surrendered and forfeited 1,006,250 Founder Shares for no consideration, following which the Sponsor holds 3,306,250 Founder Shares.
The holders of the Founder Shares have agreed, subject to limited exceptions, not to transfer, assign or sell any of the Founder Shares until the earlier to occur of: (A) one year after the completion of a Business Combination and (B) subsequent to a Business Combination, (x) if the last reported sale price of the Class A common stock equals or exceeds $12.00 per share (as adjusted for stock splits, stock capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period commencing at least 150 days after a Business Combination, or (y) the date on which the Company completes a liquidation, merger, capital stock exchange or other similar transaction that results in all of the Public Stockholders having the right to exchange their shares of common stock for cash, securities or other property.
Promissory Note — Related Party
On November 4, 2021, the Sponsor issued an unsecured promissory note to the Company (the “Promissory Note”), pursuant to which the Company may borrow up to an aggregate principal amount of $300,000. The Promissory Note is non-interest bearing and payable on the earlier of (i) the consummation of the Initial Public Offering or (ii) the decision not to execute the Initial Public Offering. As of December 31, 2021, there was $177,057 outstanding under the Promissory Note. The balance was paid in full on February 10, 2022. See Note 10, regarding a new promissory note issued by the Sponsor to the Company for $1.5 million, subsequent to December 31, 2022.
General and Administrative Services
Commencing on the date the Units are first listed on the Nasdaq, the Company has agreed to pay the Sponsor a total of $10,000 per month for office space, utilities and secretarial and administrative support. Upon completion of the Initial Business Combination or the Company’s liquidation, the Company will cease paying these monthly fees. During the year ended December 31, 2022, the Company incurred $110,000, of such expenses.
Related Party Loans
In order to finance transaction costs in connection with a Business Combination, the Sponsor or an affiliate of the Sponsor, or certain of the Company’s officers and directors may, but are not obligated to, loan the Company funds as may be required (“Working Capital Loans”). Such Working Capital Loans would be evidenced by promissory notes. The notes may be repaid upon completion of a Business Combination, without interest, or, at the lender’s discretion, up to $1,500,000 of the notes may be converted upon completion of a Business Combination into units at a price of $10.00 per unit. Such units would be identical to the Private Placement Units. In the event that a Business Combination does not close, the Company may use a portion of proceeds held outside the Trust Account to repay the Working Capital Loans but no proceeds held in the Trust Account would be used to repay the Working Capital Loans. As of December 31, 2022 and 2021, there were no amounts outstanding under the Working Capital Loans.
| F-66 |
NOTE 6 — COMMITMENTS AND CONTINGENCIES
Commitments and Contingencies
Registration Rights
The holders of the Founder Shares, Private Placement Warrants and warrants that may be issued upon conversion of Working Capital Loans (and any shares of common stock issuable upon the exercise of the Private Placement Warrants or warrants issued upon conversion of the Working Capital Loans and upon conversion of the Founder Shares) will be entitled to registration rights pursuant to a registration rights agreement to be signed prior to or on the effective date of Initial Public Offering requiring the Company to register such securities for resale. The holders of these securities will be entitled to make up to three demands, excluding short form registration demands, that the Company register such securities. In addition, the holders have certain “piggy-back” registration rights with respect to registration statements filed subsequent to completion of a Business Combination and rights to require the Company to register for resale such securities pursuant to Rule 415 under the Securities Act. However, the registration rights agreement provides that the Company will not be required to effect or permit any registration or cause any registration statement to become effective until the securities covered thereby are released from their lock-up restrictions. The Company will bear the expenses incurred in connection with the filing of any such registration statements.
Underwriting Agreement
The Company granted the underwriters a 45-day option from the date of Initial Public Offering to purchase up to 1,725,000 additional Units to cover over-allotments, if any, at the Initial Public Offering price less the underwriting discounts and commissions. The option was fully exercised on February 7, 2022.
The underwriters were paid a cash underwriting discount of $0.20 per Unit, or $2,645,000, payable upon the closing of the Initial Public Offering. In addition, the underwriters are entitled to a deferred fee of $0.35 per Unit, or $4,628,750. The deferred fee will become payable to the underwriters from the amounts held in the Trust Account solely in the event that the Company completes a Business Combination, subject to the terms of the underwriting agreement.
In addition to the underwriting discount, the Company paid the underwriters $50,000 as an advance against out-of-pocket accountable expenses actually anticipated to be incurred by the underwriters, which advance will be returned to the Company to the extent not actually incurred. The Company agreed to pay or reimburse the underwriters for travel, lodging and other “road show” expenses, expenses of the underwriters’ legal counsel and certain diligence and other fees, which such fees and expenses are capped at an aggregate of $150,000 (less the $50,000 advance previously paid).
NOTE 7 — STOCKHOLDERS’ EQUITY (DEFICIT)
Preferred Stock — The Company is authorized to issue 1,000,000 shares of preferred stock with a par value of $0.0001 per share. As of December 31, 2022 and December 31, 2021, there were no shares of preferred stock issued or outstanding.
Class A Common Stock — The Company is authorized to issue 100,000,000 shares of Class A common stock with a par value of $0.0001 per share. Holders of Class A common stock are entitled to one vote for each share. As of December 31, 2022 and 2021, there were 754,000 and zero, respectively, shares of Class A common stock issued and outstanding (excluding the 13,225,000 shares subject to possible redemption).
Class B Common Stock — The Company is authorized to issue 10,000,000 shares of Class B common stock with a par value of $0.0001 per share. Holders of Class B common stock are entitled to one vote for each share. As of each December 31, 2022 and 2021, there were 3,306,250 shares of Class B common stock issued and outstanding.
| F-67 |
Only holders of the Class B common stock will have the right to vote on the election of directors prior to the Business Combination. Holders of Class A common stock and holders of Class B common stock will vote together as a single class on all matters submitted to a vote of our shareholders except as otherwise required by law. In connection with our initial business combination, we may enter into a stockholders agreement or other arrangements with the stockholders of the target or other investors to provide for voting or other corporate governance arrangements that differ from those in effect upon completion of this offering.
The shares of Class B common stock will automatically convert into Class A common stock at the time of a Business Combination. In the case that additional shares of Class A common stock, or equity-linked securities, are issued or deemed issued in excess of the amounts issued in the Initial Public Offering and related to the closing of a Business Combination, the ratio at which shares of Class B common stock shall convert into shares of Class A common stock will be adjusted (unless the holders of a majority of the then-outstanding shares of Class B common stock agree to waive such adjustment with respect to any such issuance or deemed issuance) so that the number of shares of Class A common stock issuable upon conversion of all shares of Class B common stock will equal, in the aggregate, on an as-converted basis, 20% of the sum of the total number of all shares of common stock outstanding upon the completion of Initial Public Offering plus all shares of Class A common stock and equity-linked securities issued or deemed issued in connection with a Business Combination (net of the number of shares of Class A common stock redeemed in connection with a Business Combination), excluding any shares or equity-linked securities issued or issuable to any seller of an interest in the target to us in a Business Combination.
Only holders of the Class B common stock will have the right to vote on the election of directors prior to the Business Combination. Holders of Class B common stock will vote together as a single class on all matters submitted to a vote of our shareholders except as otherwise required by law. In connection with our initial business combination, we may enter into a stockholders agreement or other arrangements with the stockholders of the target or other investors to provide for voting or other corporate governance arrangements that differ from those in effect upon completion of this offering.
Warrants — Public Warrants may only be exercised for a whole number of shares. No fractional warrants will be issued upon separation of the Units and only whole warrants will trade. The Public Warrants will become exercisable on the later of (a) 30 days after the completion of a Business Combination and (b) 12 months from the closing of the Initial Public Offering. The Public Warrants will expire five years after the completion of a Business Combination or earlier upon redemption or liquidation.
The Company will not be obligated to deliver any shares of Class A common stock pursuant to the exercise of a warrant and will have no obligation to settle such warrant exercise unless a registration statement under the Securities Act covering the issuance of the shares of Class A common stock issuable upon exercise of the warrants is then effective and a current prospectus relating to those shares of Class A common stock is available, subject to the Company satisfying its obligations with respect to registration, or a valid exemption from registration is available. No warrant will be exercisable for cash or on a cashless basis, and the Company will not be obligated to issue any shares to holders seeking to exercise their warrants, unless the issuance of the shares upon such exercise is registered or qualified under the securities laws of the state of residence of the exercising holder, or an exemption from registration is available.
The Company has agreed that as soon as practicable, but in no event later than 15 business days after the closing of a Business Combination, the Company will use its commercially reasonable efforts to file, and within 60 business days following a Business Combination to have declared effective, a registration statement covering the issuance of the shares of Class A common stock issuable upon exercise of the warrants and to maintain a current prospectus relating to those shares of Class A common stock until the warrants expire or are redeemed. Notwithstanding the above, if the Class A common stock is at the time of any exercise of a warrant not listed on a national securities exchange such that it satisfies the definition of a “covered security” under Section 18(b)(1) of the Securities Act, the Company may, at its option, require holders of Public Warrants who exercise their warrants to do so on a “cashless basis” in accordance with Section 3(a)(9) of the Securities Act and, in the event the Company so elects, the Company will not be required to file or maintain in effect a registration statement, but will use its commercially reasonable efforts to register or qualify the shares under applicable blue sky laws to the extent an exemption is not available.
| F-68 |
Redemption of Warrants When the Price per Share of Class A Common Stock Equals or Exceeds $18.00 — Once the warrants become exercisable, the Company may redeem the outstanding Public Warrants:
| ● | in whole and not in part; | |
| ● | at a price of $0.01 per Public Warrant; | |
| ● | upon a minimum of 30 days’ prior written notice of redemption, or the 30-day redemption period to each warrant holder; and | |
| ● | if, and only if, the last reported sale price of the Class A common stock equals or exceeds $18.00 per share (as adjusted for stock splits, stock dividends, reorganization, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending on the third trading day prior to the date on which the Company sends the notice of redemption to warrant holders. |
If and when the warrants become redeemable by the Company, the Company may exercise its redemption right even if it is unable to register or qualify the underlying securities for sale under all applicable state securities laws.
If the Company calls the Public Warrants for redemption, as described above, its management will have the option to require any holder that wishes to exercise the Public Warrants to do so on a “cashless basis,” as described in the warrant agreement. The exercise price and number of common stock issuable upon exercise of the Public Warrants may be adjusted in certain circumstances including in the event of a stock dividend, extraordinary dividend or recapitalization, reorganization, merger or consolidation. However, except as described below, the Public Warrants will not be adjusted for issuances of common stock at a price below its exercise price. Additionally, in no event will the Company be required to net cash settle the Public Warrants. If the Company is unable to complete a Business Combination within the Combination Period and the Company liquidates the funds held in the Trust Account, holders of Public Warrants will not receive any of such funds with respect to their Public Warrants, nor will they receive any distribution from the Company’s assets held outside of the Trust Account with respect to such Public Warrants. Accordingly, the Public Warrants may expire worthless.
The Private Placement Warrants are identical to the Public Warrants underlying the Units sold in the Initial Public Offering.
The Private Placement Warrants and Public Warrants are recorded in stockholders’ equity (deficit) as they qualify for equity treatment under ASC 815.
The key assumptions used to value the Public Warrants, which was determined to be $23,276,000, were as follows:
| ● | Term – 5 years | |
| ● | Volatility – 22% | |
| ● | Dividends – 0% | |
| ● | Discount rate – 1.76% |
NOTE 8 — FAIR VALUE MEASUREMENTS
The following table presents information about the Company’s assets and liabilities that are measured at fair value at December 31, 2022, and indicates the fair value hierarchy of the valuation inputs the Company utilized to determine such fair value:
SCHEDULE OF FAIR VALUE OF ASSETS AND LIABILITIES
| Description | Level | December 31, 2022 | ||||||
| Assets: | ||||||||
| Investments held in Trust Account | 1 | $ | 136,871,183 | |||||
As of December 31, 2021, there were no assets requiring fair value measurement.
| F-69 |
NOTE 9 — TAXES
The expected tax expense based on the statutory rate is reconciled with actual tax expense as follows:
SCHEDULE OF EFFECTIVE INCOME TAX RATE RECONCILIATION
| For the Year Ended | ||||
| December 31, | ||||
| 2022 | ||||
| U.S. federal statutory rate | 21.0 | % | ||
| State taxes, net of federal benefit | 0.1 | % | ||
| Change in valuation allowance | 27.36 | % | ||
| Income tax provision | 48.46 | % | ||
The effective tax rate differs from the statutory tax rate of 21% for the year ended December 31, 2021, due to the valuation allowance recorded on the Company’s net operating losses. The Company files income tax returns in the U.S. federal jurisdiction and is subject to examination by the various taxing authorities. The Company’s tax returns since inception remain open to examination by the taxing authorities. The Company considers California to be a significant state tax jurisdiction.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets and liabilities and the change in valuation are as follows at December 31:
SCHEDULE OF DEFERRED TAX ASSETS AND LIABILITIES
| 2022 | ||||
| Deferred tax assets: | ||||
| Startup costs | 211,627 | |||
| Total deferred tax assets | 211,627 | |||
| Valuation Allowance | (211,627 | ) | ||
| Net deferred tax asset | $ | — | ||
SCHEDULE OF CHANGE IN VALUATION
| 2022 | ||||
| Federal | ||||
| Current | $ | 374,862 | ||
| Deferred | (211,627 | ) | ||
| State and Local | ||||
| Current | - | |||
| Deferred | - | |||
| Change in valuation | 211,627 | |||
| Income Tax Provision | $ | 374,862 | ||
In assessing the realization of the deferred tax assets, management considers whether it is more likely than not that some portion of all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is independent upon the generation of future taxable income during the periods in which temporary differences representing net future deductible amounts become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. After consideration of all of the information available, management believes that significant uncertainty exists with respect to future realization of the deferred tax assets and has therefore established a full valuation allowance. For the year ended December 31,2021, the change in the valuation allowance was zero.
NOTE 10 — SUBSEQUENT EVENTS
Subsequent Events
The Company evaluated subsequent events and transactions that occurred after the balance sheet date through the date the financial statements were issued. Based upon this review, except as disclosed below, the Company did not identify any subsequent events that would have required adjustment or disclosure in the financial statements.
Initially, the Company was required to complete an initial business combination transaction by 12 months from the consummation of initial public offering or up to 18 months if the Company extended the period of time to consummate a business combination in accordance with the Company’s Certificate of Incorporation. On January 26, 2023, at a special meeting of the Company’s stockholders (the “Special Meeting”), the Company’s stockholders approved a proposal to amend the Company’s certificate of incorporation to allow the Company to extend, at the Company’s election, the date by which the Company has to consummate a business combination up to 12 times, each such extension for an additional one month period, from February 7, 2023, to February 7, 2024. The Company’s stockholders also approved a related proposal to amend the trust agreement allowing the Company to deposit into the Trust Account, for each one-month extension, one-third of 1% of the funds remaining in the Trust Account following the redemptions made in connection with the approval of the extension proposal at the Special Meeting. At the Special Meeting the Company’s stockholders also approved a proposal to amend the Company’s certificate of incorporation to expand the methods that the Company may employ to not become subject to the “penny stock” rules of the SEC.
In connection with such proposals, the Company’s public stockholders had the right to redeem their shares for cash equal to their pro rata share of the aggregate amount on deposit in the Trust Account as of two days prior to such stockholder vote. The Company’s public stockholders holding 11,037,272 shares of Class A common stock (out of a total of 13,979,000 shares of Class A common stock) exercised their right to redeem such shares at a redemption price of approximately $10.33 per share. Approximately $114 million in cash was removed from the Trust Account to pay such stockholders and, accordingly, after giving effect to such redemptions, the balance in the Trust Account was approximately $23 million.
As a result of the approval of such proposals, the Company agreed to deposit into the Trust Account one-third of 1% of the funds then on deposit in the Trust Account for each month of the extension period, resulting in a monthly contribution of approximately $0.035 per share that was not redeemed in connection with the Special Meeting, or an aggregate of approximately $77,000 per month, and an aggregate of $924,000 if the date the Company has to consummate a business combination is extended 12 times, each assuming no interest is earned on the funds in the Trust Account.
On March 7, 2023, the Company entered into a $1.5 million promissory note with the Company’s Sponsor to fund the Trust Account and for the Company’s operating expenses. On March 7, 2023 the Company’s Sponsor advanced $300,000 and will provide additional funds as necessary under the promissory note. These loans are non-interest bearing, unsecured and will be repayable in full upon the earlier of (i) the date on which the Company consummates an initial business combination and (ii) the date that the Company’s winding up is effective.
| F-70 |
Annex A-1
AGREEMENT AND PLAN OF MERGER
by and among
MURPHY CANYON ACQUISITION CORP.
CONDUIT MERGER SUB, INC.
and
CONDUIT PHARMACEUTICALS LIMITED
Dated as of November 8, 2022
TABLE OF CONTENTS
| ARTICLE I DEFINITIONS | A-1-2 | |
| Section 1.1 | Certain Definitions | A-1-2 |
| Section 1.2 | Construction | A-1-16 |
| ARTICLE II THE MERGER; CLOSING | A-1-17 | |
| Section 2.1 | The Merger | A-1-17 |
| Section 2.2 | Closing | A-1-17 |
| Section 2.3 | Effective Time | A-1-18 |
| Section 2.4 | Organizational Documents of Murphy and the Surviving | |
| Corporation | A-1-18 | |
| Section 2.5 | Directors | A-1-18 |
| Section 2.6 | Chief Financial Officer | A-1-18 |
| Section 2.7 | No Further Ownership Rights in Company Capital Stock. | A-1-18 |
| Section 2.8 | Rights Not Transferable | A-1-19 |
| Section 2.9 | Taking of Necessary Action; Further Action | A-1-19 |
| ARTICLE III ACTIONS PRIOR TO THE MERGER; CONVERSION OF SECURITIES; CONSIDERATION | ||
| A-1-19 | ||
| Section 3.1 | CONVERSION OF SECURITIES | A-1-19 |
| Section 3.2 | Payment of Merger Consideration | A-1-21 |
| Section 3.3 | Withholding Rights | A-1-21 |
| Section 3.4 | Register of Members | A-1-22 |
| Section 3.5 | Payment of Expenses | A-1-22 |
| Section 3.6 | Dissenter’s Rights | A-1-23 |
| ARTICLE IV REPRESENTATIONS AND WARRANTIES OF THE COMPANY | A-1-23 | |
| Section 4.1 | Organization and Qualification | A-1-24 |
| Section 4.2 | Organizational Documents. | A-1-24 |
| Section 4.3 | Capitalization | A-1-24 |
| Section 4.4 | Authority Relative to this Agreement | A-1-25 |
| Section 4.5 | No Conflict; Required Filings and Consents | A-1-26 |
| Section 4.6 | Permits; Compliance | A-1-26 |
| Section 4.7 | Financial Statements | A-1-26 |
| Section 4.8 | Undisclosed Liabilities. | A-1-27 |
| Section 4.9 | Absence of Certain Changes or Events | A-1-28 |
| Section 4.10 | Absence of Litigation | A-1-28 |
| Section 4.11 | Employee Benefit Plans | A-1-28 |
| Section 4.12 | Labor Matters | A-1-29 |
| Section 4.13 | Real Property; Title to and Sufficiency of Assets | A-1-30 |
| Section 4.14 | Intellectual Property | A-1-30 |
| Section 4.15 | Taxes. | A-1-31 |
| Section 4.16 | Environmental Matters | A-1-33 |
| Section 4.17 | Material Contracts | A-1-33 |
| Section 4.18 | Insurance | A-1-35 |
| Section 4.19 | Internal Controls | A-1-36 |
| i |
| Section 4.20 | Registration Statement | A-1-36 |
| Section 4.21 | Support Agreement | A-1-36 |
| Section 4.22 | Board Approval; Vote Required | A-1-36 |
| Section 4.23 | Brokers | A-1-36 |
| Section 4.24 | Takeover Laws | A-1-37 |
| Section 4.25 | International Trade Matters; Anti-Bribery Compliance | A-1-37 |
| Section 4.26 | Related Party Transactions | A-1-38 |
| Section 4.27 | Not An Investment Company | A-1-38 |
| Section 4.28 | Exclusivity of Representations and Warranties | A-1-38 |
| Section 4.29 | Full Disclosure | A-1-38 |
| ARTICLE V REPRESENTATIONS AND WARRANTIES OF MURPHY AND MERGER SUB | A-1-39 | |
| Section 5.1 | Corporate Organization | A-1-39 |
| Section 5.2 | Murphy and Merger Sub Organizational Documents | A-1-39 |
| Section 5.3 | Capitalization | A-1-40 |
| Section 5.4 | Authority Relative to This Agreement | A-1-40 |
| Section 5.5 | No Conflict; Required Filings and Consents | A-1-41 |
| Section 5.6 | Compliance | A-1-41 |
| Section 5.7 | Murphy SEC Documents and Financial Statements | A-1-42 |
| Section 5.8 | Absence of Certain Changes or Events | A-1-43 |
| Section 5.9 | Absence of Litigation | A-1-43 |
| Section 5.10 | Board Approval; Vote Required | A-1-43 |
| Section 5.11 | No Prior Operations of Merger Sub | A-1-43 |
| Section 5.12 | Murphy Trust Fund | A-1-44 |
| Section 5.13 | Employees | A-1-44 |
| Section 5.14 | Taxes | A-1-44 |
| Section 5.15 | Listing | A-1-47 |
| Section 5.16 | Investment Company Act | A-1-47 |
| Section 5.17 | Registration Statement | A-1-47 |
| Section 5.18 | Contracts | A-1-47 |
| Section 5.19 | Brokers | A-1-47 |
| Section 5.20 | Sponsor Support Agreement | A-1-47 |
| Section 5.21 | Murphy’s and Merger Sub’s Investigation and Reliance | A-1-48 |
| Section 5.22 | Full Disclosure | A-1-48 |
| ARTICLE VI CONDUCT OF BUSINESS PENDING THE MERGER | A-1-48 | |
| Section 6.1 | Conduct of Business by the Company Pending the Merger | A-1-48 |
| Section 6.2 | Conduct of Business by Murphy Pending the Merger | A-1-51 |
| Section 6.3 | Claims Against Trust Account | A-1-51 |
| Section 6.4 | Applicable Per Share Merger Consideration | A-1-52 |
| ARTICLE VII ADDITIONAL AGREEMENTS | A-1-52 | |
| Section 7.1 | Preparation of Registration Statement; Special Meeting; Company Requisite Approval | A-1-52 |
| Section 7.2 | Access to Information; Confidentiality; Publicity | A-1-54 |
| Section 7.3 | Exclusivity | A-1-55 |
| ii |
| Section 7.4 | Employment Agreements. | A-1-57 |
| Section 7.5 | Directors’ and Officers’ Indemnification | A-1-57 |
| Section 7.6 | Transaction Litigation | A-1-57 |
| Section 7.7 | Tax Matters | A-1-59 |
| Section 7.8 | Stock Exchange Listing | A-1-60 |
| Section 7.9 | Murphy Public Filings | A-1-60 |
| Section 7.10 | Efforts to Consummate; Antitrust; Regulatory Approvals | A-1-60 |
| Section 7.11 | Trust Account | A-1-62 |
| Section 7.12 | Section 16 Matters | A-1-62 |
| Section 7.13 | Preparation and Delivery of PCAOB Audited Financial Statements and Interim Financial Statements. | A-1-62 |
| Section 7.14 | Support of Transaction | A-1-63 |
| Section 7.15 | Notice of Certain Events | A-1-63 |
| ARTICLE VIII CONDITIONS TO THE MERGER | A-1-63 | |
| Section 8.1 | Conditions to the Obligations of Each Party | A-1-63 |
| Section 8.2 | Conditions to the Obligations of Murphy and Merger Sub | A-1-65 |
| Section 8.3 | Conditions to the Obligations of the Company. | A-1-66 |
| Section 8.4 | Frustration of Conditions | A-1-67 |
| ARTICLE IX TERMINATION, AMENDMENT AND WAIVER | A-1-67 | |
| Section 9.1 | Termination | A-1-67 |
| Section 9.2 | Effect of Termination | A-1-68 |
| Section 9.3 | Expenses | A-1-68 |
| Section 9.4 | Amendment | A-1-68 |
| Section 9.5 | Waiver | A-1-68 |
| ARTICLE X NON-SURVIVAL OF REPRESENTATIONS AND WARRANTIES | A-1-69 | |
| Section 10.1 | Non-Survival | A-1-69 |
| ARTICLE XI GENERAL PROVISIONS | A-1-69 | |
| Section 11.1 | Notices | A-1-69 |
| Section 11.2 | Severability | A-1-71 |
| Section 11.3 | Entire Agreement; Assignment | A-1-71 |
| Section 11.4 | Parties in Interest | A-1-71 |
| Section 11.5 | Governing Law | A-1-71 |
| Section 11.6 | WAIVER OF JURY TRIAL | A-1-72 |
| Section 11.7 | Headings | A-1-72 |
| Section 11.8 | Counterparts | A-1-72 |
| Section 11.9 | Specific Performance | A-1-72 |
| Section 11.10 | Legal Representation | A-1-72 |
| iii |
MERGER AGREEMENT AND PLAN OF MERGER
This AGREEMENT AND PLAN OF MERGER, dated as of November 8, 2022 (this “Agreement”), is entered into by and among (i) Murphy Canyon Acquisition Corp., a Delaware corporation (“Murphy”), (ii) Conduit Merger Sub, Inc., a Cayman Islands exempted company (“Merger Sub”), and (iii) Conduit Pharmaceuticals Limited, a Cayman Islands exempted company (the “Company”). Murphy, Merger Sub, and the Company are sometimes referred to individually herein as a “Party” and, collectively, the “Parties”.
WHEREAS, Murphy is a special purpose acquisition company incorporated in Delaware and formed to acquire one or more operating businesses through a business combination;
WHEREAS, Merger Sub is a newly formed, wholly owned, direct subsidiary of Murphy and was formed for the sole purpose of the Merger;
WHEREAS, subject to the terms and conditions of this Agreement and in accordance with the Companies Act (Revised) of the Cayman Islands (the “Companies Act”), at the Effective Time, Merger Sub will merge with and into the Company pursuant to the Merger, with the Company surviving as the Surviving Corporation;
WHEREAS, in connection with the Merger, the Shareholders will be entitled to receive the Merger Consideration, as described in this Agreement;
WHEREAS, the Sponsor, certain of Sponsor’s Affiliates and Murphy have entered into a Sponsor Support Agreement, dated as of the date hereof (the “Sponsor Support Agreement”), providing that, among other things, the Principal Shareholders will vote in favor of this Agreement and the Transactions (including the Merger);
WHEREAS, Murphy and the Principal Shareholders, contemporaneously with the execution and delivery of this Agreement, have entered into the Shareholder Support Agreement, dated as of the date hereof (the “Shareholder Support Agreement”), providing that, among other things, the Principal Shareholders will vote in favor of this Agreement and the Transactions (including the Merger);
WHEREAS, at the Closing, Murphy, certain stockholders of Murphy and certain Shareholders will enter into an Amended and Restated Registration Rights Agreement with Murphy (the “Registration Rights Agreement”), which will, among other things, govern the registration of certain Company Ordinary Shares for resale and also provide for a lock-up pertaining to certain Company Ordinary Shares owned by such Shareholders and which shall be effective as of the Closing.
WHEREAS, in connection with the Merger, Murphy shall adopt the second amended and restated certificate of incorporation (the “Amended Charter”) in the form attached hereto as Exhibit B, to provide for, among other things, an increase in the number of authorized shares of Murphy Common Stock;
WHEREAS, in connection with the Merger, Murphy shall adopt the amended and restated bylaws (the “Amended Bylaws”) in the form attached hereto as Exhibit C;
| A-1-1 |
WHEREAS, in connection with the transactions contemplated by this Agreement, Murphy will enter into subscription agreements (each, as amended or modified from time to time, a “Subscription Agreement”), with certain persons providing for aggregate investments in Murphy Common Stock in a private placement of an amount not less than $27,000,000 and valued in an amount of $10.00 per share (the “PIPE Financing”);
WHEREAS, the respective boards of directors of each of Murphy, Merger Sub and the Company have each (a) unanimously approved and declared advisable this Agreement and the Transactions upon the terms and subject to the conditions of this Agreement and in accordance with the DGCL and the Companies Act, as applicable, and (b) adopted a resolution recommending to their respective stockholders or shareholders, as the case may be, the approval and adoption of this Agreement and the Transactions;
WHEREAS, the sole shareholder of Merger Sub has approved and declared advisable this Agreement and the Transactions upon the terms and subject to the conditions of this Agreement and in accordance with the Companies Act;
WHEREAS, each of the Parties intends that, for United States federal income tax purposes, (a) this Agreement shall be adopted as a “plan of reorganization” within the meaning of Section 368 of the Code and Treasury Regulations Section 1.368-2(g) and (b) the Merger shall constitute an integrated transaction that qualifies as a “reorganization” within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as amended (the “Code”) and the Treasury Regulations to which the Murphy, Merger Sub and the Company are parties within the meaning of Section 368(b) of the Code (collectively, the “Intended Tax Treatment”); and
WHEREAS, all capitalized terms not defined in these Recitals shall have the respective meanings ascribed to them in this Agreement;
NOW, THEREFORE, in consideration of the foregoing and the mutual covenants and agreements herein contained, and intending to be legally bound hereby, the Parties hereto hereby agree as follows:
ARTICLE I
DEFINITIONS
Section 1.1 Certain Definitions. For purposes of this Agreement:
“Action” has the meaning set forth in Section 4.10.
“Additional Murphy SEC Documents” has the meaning set forth in Section 5.7(a).
“Affiliate” of a specified Person means a Person who, directly or indirectly through one or more intermediaries, controls, is controlled by, or is under common control with, such specified Person. For the avoidance of doubt, Merger Sub shall be deemed to be an Affiliate of Murphy.
“Affiliate Transaction” has the meaning set forth in Section 4.26.
| A-1-2 |
“Agreement” has the meaning set forth in the preamble of this Agreement.
“Amended Bylaws” has the meaning set forth in the recitals of this Agreement.
“Amended Charter” has the meaning set forth in the recitals of this Agreement.
“Ancillary Agreements” means the Amended Charter, the Amended Bylaws, the Shareholder Support Agreement, the Sponsor Support Agreement, the Registration Rights Agreement, the Subscription Agreements, and all other agreements, certificates and instruments executed and delivered by the Parties in connection with the Transactions and specifically contemplated by this Agreement.
“Anti-Corruption Laws” means any applicable Laws relating to anti-bribery or anti- corruption (governmental or commercial), including the U.S. Foreign Corrupt Practices Act of 1977, as amended (the “FCPA”), the U.S. Travel Act, 18 U.S.C. § 1952, and the U.K. Bribery Act 2010, and any other equivalent or comparable Laws of other countries in which the Company has conducted and/or currently conducts business, when applicable.
“Antitrust Laws” has the meaning set forth in Section 7.10(c).
“Applicable Per Share Merger Consideration” means, with respect to each holder of Company Ordinary Shares, including Company Ordinary Shares issued upon the Company Convertible Debt Conversion, such holder’s pro rata portion of the Closing Payment Shares for each Company Ordinary Share issued and outstanding as of the Effective Time after giving effect to the Company Convertible Debt Conversion as specified on Exhibit A as updated pursuant to Section 6.4.
“Blue Sky Laws” has the meaning set forth in Section 4.5(b).
“Books and Records” means books and records (whether written, electronic, or otherwise embodied) in which a Person’s assets, the business or its transactions are otherwise reflected, other than stock books and minute books.
“Business Data” means all business information and data, including Personal Information (whether of employees, contractors, consultants, customers, consumers, or other Persons and whether in electronic or any other form or medium) that is accessed, collected, used, processed, stored, shared, distributed, transferred, disclosed, destroyed, or disposed of by any of the Business Systems or otherwise in the course of the conduct of the business of the Company.
“Business Day” means any day on which the principal offices of the SEC in Washington, D.C. are customarily open to accept filings, or, in the case of determining a date when any payment is due, any day on which banks are not required or authorized to close in New York, NY.
“Business Systems” means all Software, computer hardware (whether general or special purpose), electronic data processing, information, record keeping, communications, telecommunications, networks, interfaces, platforms, servers, peripherals, and computer systems, including any outsourced systems and processes, that are owned or used or held for use in the conduct of the Company Business.
| A-1-3 |
“Claims” has the meaning set forth in Section 6.3.
“Closing” has the meaning set forth in Section 2.2.
“Closing Date” has the meaning set forth in Section 2.2.
“Closing Payment Shares” means sixty-five million (65,000,000) shares of Murphy Common Stock.
“Code” has the meaning set forth in the recitals of this Agreement.
“Companies Act” has the meaning set forth in the recitals of this Agreement.
“Company” has the meaning set forth in the preamble of this Agreement.
“Company Acquisition Proposal” means any proposal or offer from a Person or a “group” (as defined in the Exchange Act) relating to (a) any direct or indirect acquisition or purchase, in a single transaction or series of related transactions, of (i) any equity ownership in the Company or any of its controlled Affiliates or (ii) all or a material portion of assets or businesses of the Company or any of its controlled Affiliates (in the case of each of clause (i) and (ii), whether by merger, consolidation, recapitalization, purchase or issuance of equity securities, tender offer or otherwise), or (b) any equity or similar investment in the Company or any of its controlled Affiliates. Notwithstanding the foregoing or anything to the contrary herein, none of this Agreement, the Ancillary Agreements or the Transactions shall constitute a Company Acquisition Proposal.
“Company Affiliate Agreement” means any Contract between the Company, on the one hand, and a Related Party, on the other hand.
“Company Board Recommendation” has the meaning set forth in Section 7.1(f).
“Company Business” means acquiring, developing, researching, commercializing, licensing, sublicensing, out-licensed, in-licensed, distributing, selling, funding, investing in, and the management of pharmaceutical, therapeutic, biologic and/or medicinal product candidates or devices, including, but not limited to, entering into strategic partnerships, joint ventures, development, royalty, commercialization, licensing, funding, investment and/or collaboration agreements related thereto.
“Company Capital Stock” means the Company Ordinary Shares and includes Company Ordinary Shares issued upon the Company Convertible Debt Conversion.
“Company Convertible Debt” means, collectively, (i) the convertible notes issued by the Company prior to the date hereof that are listed on Schedule 4.3(d) of the Company Disclosure Schedule, and (ii) the convertibles notes that may be issued by the Company following the date hereof but prior to the Closing Date pursuant to Section 6.1(a), provided that the Company shall promptly provide Murphy with notice, including all information that is required by Section 4.3(d), after any such issuance.
| A-1-4 |
“Company D&O Insurance” has the meaning set forth in Section 7.5(b).
“Company Debt” means the following consolidated obligations of the Company: (a) all indebtedness for borrowed money or in respect of loans or advances of any kind or for the deferred purchase price of property or services, including “earn-out” payments; (b) all liabilities evidenced by bonds, debentures, promissory notes, mortgages or other debt instruments and debt securities; (c) all guarantees of the debt of other Persons on assets or properties of such Person, whether or not the obligations secured thereby have been assumed; (d) contingent reimbursement obligations with respect to letters of credit, bankers’ acceptance or similar facilities (in each case to the extent drawn); (e) obligations under capitalized leases, (f) any unfunded or underfunded liabilities pursuant to any pension or nonqualified deferred compensation plan or arrangement and any earned but unpaid compensation (including salary, bonuses and paid time off) for any period prior to the Closing Date; and (g) guarantees, make-whole agreements, hold harmless agreements or other similar arrangements with respect to any amounts of a type described in clauses (a) through (f) above, and with respect to each of the foregoing, any unpaid interest, breakage costs, prepayment or redemption penalties or premiums, or other unpaid fees or obligations. For the avoidance of doubt, Company Debt shall not include any trade payables or other accounts payable incurred in the ordinary course of business to suppliers or other service providers. The amount of Company Debt as of the date hereof is set forth on Schedule 1.01(b) of the Company Disclosure Schedules.
“Company Disclosure Schedules” has the meaning set forth in the preamble to Article IV.
“Company Employees” has the meaning set forth in Section 4.11(a).
“Company IP” means, collectively, all Company-Owned IP and Company-Licensed IP.
“Company-Licensed IP” means all Intellectual Property rights owned or purported to be owned by a third party and licensed to the Company or to which the Company otherwise has a right to use.
| A-1-5 |
“Company Material Adverse Effect” means any event, circumstance, change, effect or occurrence (collectively “Effect”) that, individually or in the aggregate with all other Effects, (a) has had, or would reasonably be expected to have, a material adverse effect on the business, financial condition, assets or results of operations of the Company or (b) has a material adverse effect on the ability of the Company to consummate the Transactions in accordance with the terms of this Agreement; provided, however, that for purposes of clause (a) above, any Effects directly or indirectly attributable to, resulting from, relating to or arising out of the following (by themselves or when aggregated with any other, changes or effects) shall not be deemed to be, constitute, or be taken into account when determining whether there has or may, would or could have occurred a Company Material Adverse Effect: (i) any change or proposed change in the interpretation of any Law (including any COVID-19 Measures) after the date of this Agreement; (ii) events or conditions generally affecting the industries or geographic areas or markets in which the Company operates; (iii) any material downturn in general economic conditions, including material changes in the credit, debt, securities, financial or capital markets (including changes in interest or exchange rates, prices of any security or market index or commodity or any disruption of such markets); (iv) acts of war, sabotage, civil unrest, terrorism, cyberterrorism (including ransomware attacks), epidemics, pandemics or disease outbreaks (including COVID-19), or any escalation or worsening of any of the foregoing; (v) any hurricane, tornado, flood, earthquake, wild fire, natural disaster, or other acts of God or other force majeure event, including, for the avoidance of doubt, COVID-19 and any COVID-19 Measures, (vi) any actions taken or not taken by the Company as required by this Agreement or any Ancillary Agreement, (vii) any fact, event, circumstance, change or effect attributable to the announcement or execution, pendency, negotiation or consummation of the Merger or any of the other Transactions (including the impact thereof on relationships with customers, suppliers, distributors, licensors, partners, providers, employees or Governmental Authorities), provided, however, that the exceptions in clauses (vi) or (vii) shall not be deemed to apply to references to “Material Adverse Effect” in the representations and warranties set forth in Section 4.9(a) and, to the extent related thereto, the condition in Section 8.2(a), or (viii) any failure to meet any projections, forecasts, guidance, estimates, milestones, budgets or financial or operating predictions of revenue, earnings, cash flow or cash position (provided, that clause (viii) shall not prevent or otherwise affect a determination that any event, change, fact or circumstance underlying such failure to meet projections or forecasts has resulted in, or contributed to, or would reasonably be expected to result in or contribute to, a Company Material Adverse Effect, and provided, further, that any event, change, fact or circumstance referred to in clauses (i), (ii), (iii), and (v) may be taken into account in determining if a “Company Material Adverse Effect” occurred to the extent it has a disproportionate impact on the Company as compared to similarly situated companies in the industry in which the Company conducts its operations.
“Company Ordinary Shares” means the Company’s ordinary shares, with a par value of GBP0.0001 per share.
“Company Organizational Documents” means the Certificate of Incorporation of the Company, as issued by with the Assistant Registrar of Companies, Cayman Islands, on December 21, 2018, together with the Memorandum and Articles of Association of the Company, as filed with the Assistant Registrar of Companies, Cayman Islands, on December 21, 2018, as each may have been amended, supplemented, designated or modified from time to time.
“Company-Owned IP” means all Intellectual Property rights owned or purported to be owned by the Company.
“Company Permits” has the meaning set forth in Section 4.6.
“Company Requisite Approval” means the affirmative vote of the holders of at least a two- thirds majority of the outstanding shares of the Company Ordinary Shares, voting together as a single class.
“Confidential Information” means any information, knowledge or data concerning the businesses and affairs of the Company, or any Suppliers or customers of the Company or Murphy or its subsidiaries (as applicable) that is not already generally available to the public.
“Confidentiality Agreement” has the meaning set forth in Section 7.2(b). “Contribution” has the meaning set forth in Section 4.14(d).
| A-1-6 |
“Contributor” has the meaning set forth in Section 4.14(d).
“control” (including the terms “controlled by” and “under common control with”) means the possession, directly or indirectly, or as trustee or executor, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, as trustee or executor, by contract or otherwise.
“Copyright License” means any license that requires, as a condition of use, modification or distribution of software or other Intellectual Property subject to such license, that such software or other Intellectual Property subject to such license, or other software or other Intellectual Property incorporated into, derived from, used or distributed with such software or other Intellectual Property subject to such license (a) in the case of software, be made available or distributed in a form other than binary (e.g., source code form), (b) be licensed for the purpose of preparing derivative works, (c) be licensed under terms that allow the Products or portions thereof or interfaces therefor to be reverse engineered, reverse assembled or disassembled (other than by operation of Law) or (d) be redistributable at no license fee.
“COVID-19” shall mean SARS-CoV-2 or COVID-19, and any evolutions or mutations thereof or related or associated epidemics, pandemics or disease outbreaks.
“COVID-19 Measures” means any quarantine, “shelter in place,” “stay at home,” workforce reduction, social distancing, shut down, closure, sequester, workplace safety or similar Law promulgated by any Governmental Authority, including the Centers for Disease Control and Prevention and the World Health Organization, in each case, in connection with or in response to COVID-19, including the CARES Act and Families First Act.
“D&O Indemnified Persons” has the meaning set forth in Section 7.5(a).
“D&O Insurance” has the meaning set forth in Section 7.5(b).
“D&O Tail Insurance” has the meaning set forth in Section 7.5(c).
“DGCL” means the General Corporation Law of the State of Delaware.
“Dissenting Shares” has the meaning set forth in Section 3.6(a).
“Effective Time” has the meaning set forth in Section 2.3.
“Environmental Laws” means any applicable Law, and any United States federal, state or local or non-United States laws relating to: (a) pollution (or the cleanup thereof) or the protection of natural resources, endangered or threatened species, human health or safety, or the environment (including ambient or indoor air, soil, soil gas, surface water, groundwater, sediments, surface or subsurface strata); or (b) concerning the release, threatened release, presence of, exposure to, contamination of, or any injury or threat of injury to persons or property relating to, or the management, manufacture, use, containment, storage, recycling, reclamation, reuse, treatment, generation, discharge, transportation, processing, production, sale, distribution, labeling, disposal or remediation of any Hazardous Substances or materials containing Hazardous Substances. The term “Environmental Law” includes the following (including their implementing regulations and any state analogs): the Comprehensive Environmental Response, Compensation, and Liability Act of 1980, as amended by the Superfund Amendments and Reauthorization Act of 1986, 42 U.S.C. §§ 9601 et seq.; the Solid Waste Disposal Act, as amended by the Resource Conservation and Recovery Act of 1976, as amended by the Hazardous and Solid Waste Amendments of 1984, 42 U.S.C. §§ 6901 et seq.; the Federal Water Pollution Control Act of 1972, as amended by the Clean Water Act of 1977, 33 U.S.C. §§ 1251 et seq.; the Toxic Substances Control Act of 1976, as amended, 15 U.S.C. §§ 2601 et seq.; the Emergency Planning and Community Right-to-Know Act of 1986, 42 U.S.C. §§ 11001 et seq.; and the Clean Air Act of 1966, as amended by the Clean Air Act Amendments of 1990, 42 U.S.C. §§ 7401 et seq.; the Occupational Safety and Health Act of 1970, as amended, 29 U.S.C. §§ 651 et seq; the Safe Drinking Water and Toxic Enforcement Act of 1986, 27 Cal. Code Regs., §§ 25249 et seq, and Federal Insecticide, Fungicide, and Rodenticide Act.
| A-1-7 |
“Environmental Claim” means any action, suit, claim, investigation or other proceeding by any person alleging liability of whatever kind or nature (including liability or responsibility for the costs of enforcement proceedings, investigations, cleanup, governmental response, removal or remediation, natural resources assessments and damages, property damages, personal injuries, medical monitoring, penalties, contribution, indemnification and injunctive relief) arising out of, based on or resulting from: (a) the actual or alleged presence, generation, use, handling, transportation, storage, treatment, disposal, threatened Release or Release of, or exposure to, any Hazardous Substances; (b) any actual or alleged non-compliance with any Environmental Law or term or condition of any Environmental Permit; or (c) any contract, agreement or other consensual arrangement pursuant to which liability is assumed or imposed with respect to any of the foregoing.
“ERISA” has the meaning set forth in Section 4.11(a).
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Exchange Ratio” means the quotient of (a) the Merger Consideration, divided by (b) the number of shares of Company Capital Stock.
“Export Control Laws” has the meaning set forth in Section 4.25(a).
“GAAP” has the meaning set forth in Section 4.7(a).
“Governmental Authority” has the meaning set forth in Section 4.5(b).
“Hazardous Substance(s)” means: (a) any material, substance, chemical, waste, product, derivative, compound, mixture, solid, liquid, mineral or gas, in each case, whether naturally occurring or man-made, that is hazardous, acutely hazardous, toxic, carcinogenic, mutagenic, radioactive, a pollutant, a contaminant or is otherwise characterized by words of similar import or regulatory effect or that could give rise to Liability under Environmental Laws; and (b) any petroleum or petroleum-derived products, radon, radioactive materials or wastes, natural gas, synthetic gas, asbestos in any form, lead or lead-containing materials, urea formaldehyde foam insulation, polychlorinated biphenyls and per- and polyfluoroalkyl substances.
“HSR Act” means the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended.
| A-1-8 |
“Immediate Family” means, with respect to any natural person, such person’s spouse or domestic partner, lineal ancestor or descendant, or sibling, including any adoptive relationship and relationships through marriage.
“Intellectual Property” means any and all of the following in any jurisdiction throughout the world (a) patents and patent applications, together with all reissues, continuations, continuations-in-part, divisionals, extensions or reexaminations thereof; (b) trademarks and service marks, trade dress, trade names, corporate names, any other indicia of source or origin, and all applications, registrations, and renewals in connection therewith, together with all of the goodwill associated with the foregoing; (c) copyrights and other works of authorship (whether or not copyrightable), and moral rights, and registrations and applications for registration, renewals and extensions thereof; (d) trade secrets, know-how and other confidential information, in each instance, that derive independent economic value from not being generally known to the public and not being readily ascertainable by other Persons; (e) Internet domain names, social media accounts, websites and content; (f) Software and rights in Software.
“Intended Tax Treatment” has the meaning set forth in the recitals of this Agreement.
“Interim Balance Sheet” has the meaning set forth in Section 4.7(b).
“Interim Financial Statements” has the meaning set forth in Section 4.7(b).
“International Trade Control Laws” has the meaning set forth in Section 4.25(a).
“International Trade Laws” means all applicable laws, regulations, rules and licenses of the United States and other governments, including but not limited to, the sanctions, embargoes and restrictions administered by the U.S. Department of the Treasury’s Office of Foreign Assets Control (“OFAC”) and the Foreign Trade Regulations administered by the U.S. Department of Commerce’s Bureau of Census, the anti-boycott regulations administered by the U.S. Department of Commerce and the U.S. Department of the Treasury.
“Investment Company Act” has the meaning set forth in Section 4.27.
“Knowledge” or “to the Knowledge” of a Person shall mean (i) in the case of the Company, the actual knowledge of the Persons listed on Schedule 1.01(c) of the Company Disclosure Schedules after reasonable inquiry, or (ii) in the case of Murphy, the actual knowledge of the Persons listed on Schedule 1.01(c) of the Murphy Disclosure Schedules after reasonable inquiry.
“Law” means any federal, state, local or foreign (including the general principles of common law, civil law and equity) statute, law, ordinance, regulation, code, executive order, injunction, judgment, decree, constitution, convention, treaty, common law, act, code, edict, determination, binding interpretation, subpoena, decision, verdict, judgment, award, administrative requirement, decree and the rules and regulations promulgated thereunder, in each case enacted, promulgated or imposed by any Governmental Authority, and to the extent they have the force of law, any policies, guidelines and notices of any Governmental Authority.
| A-1-9 |
“Leased Real Property” means the real property leased by the Company as tenant, together with, to the extent leased by the Company, all buildings and other structures, facilities or Improvements located thereon.
“Liabilities” means any and all liabilities, Indebtedness, Actions or obligations of any nature (whether absolute, accrued, contingent or otherwise, whether known or unknown, whether direct or indirect, whether matured or unmatured, whether due or to become due and whether or not required to be recorded or reflected on a balance sheet under GAAP or other applicable accounting standards), including Tax liabilities due or to become due.
“Lien” means any lien, security interest, mortgage, pledge, adverse claim or other encumbrance of any kind that secures the payment or performance of an obligation (other than those created under applicable Securities Laws).
“Material Contracts” has the meaning set forth in Section 4.17(a).
“Merger Consideration” means six hundred and fifty million dollars ($650,000,000).
“Merger Sub” has the meaning set forth in the preamble of this Agreement.
“Merger Sub Ordinary Shares” has the meaning set forth in Section 5.3(b).
“Merger Sub Organizational Documents” means the certificate of incorporation and memorandum and articles of association of Merger Sub, as amended, modified or supplemented from time to time.
“Murphy” has the meaning set forth in the preamble of this Agreement.
“Murphy Acquisition Proposal” means any proposal or offer from a Person or a “group” (as defined in the Exchange Act) relating to (a) any direct or indirect acquisition or purchase, in a single transaction or series of related transactions, of (i) any equity ownership in Murphy or any of its controlled Affiliates or (ii) all or a material portion of assets or businesses of Murphy or any of its controlled Affiliates (in the case of each of clause (i) and (ii), whether by merger, consolidation, recapitalization, purchase or issuance of equity securities, tender offer or otherwise), or (b) any equity or similar investment in Murphy or any of its controlled Affiliates. Notwithstanding the foregoing or anything to the contrary herein, none of this Agreement, the Ancillary Agreements or the Transactions shall constitute a Murphy Acquisition Proposal.”
“Murphy Board” has the meaning set forth in Section 5.10(a).
“Murphy Board Recommendation” has the meaning set forth in Section 7.1(e).
“Murphy Certificate of Incorporation” means the Amended and Restated Certificate of Incorporation of Murphy, dated February 2, 2022.
“Murphy Class A Common Stock” means, at all times prior to the Effective Time, Murphy’s Class A Common Stock, par value $0.0001 per share.
| A-1-10 |
“Murphy Class B Common Stock” means, at all times prior to the Effective Time, Murphy’s Class B Common Stock, par value $0.0001 per share.
“Murphy Common Stock” means, at all times prior to the Effective Time, Murphy Class A Common Stock and Murphy Class B Common Stock, collectively.
“Murphy Disclosure Schedules” has the meaning set forth in the preamble to Section 4.29.
“Murphy Financial Statements” has the meaning set forth in Section 5.7(b).
“Murphy Material Adverse Effect” means any Effect that, individually or in the aggregate with all other Effects, (a) has had, or would reasonably be expected to have, a material adverse effect on the business, financial condition, assets or results of operations of Murphy or (b) has a material adverse effect on the ability of Murphy and/or Merger Sub to consummate the Transactions in accordance with the terms of this Agreement; provided, however, that for purposes of clause (a) above, any Effects directly or indirectly attributable to, resulting from, relating to or arising out of the following (by themselves or when aggregated with any other, changes or effects) shall not be deemed to be, constitute, or be taken into account when determining whether there has or may, would or could have occurred a Murphy Material Adverse Effect: (i) any change or proposed change in the interpretation of any Law (including any COVID-19 Measures) after the date of this Agreement; (ii) events or conditions generally affecting the industries or geographic areas or markets in which Murphy operates; (iii) any material downturn in general economic conditions, including material changes in the credit, debt, securities, financial or capital markets (including changes in interest or exchange rates, prices of any security or market index or commodity or any disruption of such markets); (iv) acts of war, sabotage, civil unrest, terrorism, cyberterrorism (including ransomware attacks), epidemics, pandemics or disease outbreaks (including COVID-19), or any escalation or worsening of any of the foregoing; (v) any hurricane, tornado, flood, earthquake, wild fire, natural disaster, or other acts of God or other force majeure event, including, for the avoidance of doubt, COVID-19 and any COVID-19 Measures, (vi) any actions taken or not taken by Murphy as required by this Agreement or any Ancillary Agreement, (vii) any fact, event, circumstance, change or effect attributable to the announcement or execution, pendency, negotiation or consummation of the Merger or any of the other Transactions (including the impact thereof on relationships with customers, suppliers, distributors, licensors, partners, providers, employees or Governmental Authorities), provided, however, that the exceptions in clauses (vi) or (vii) shall not be deemed to apply to references to “Murphy Material Adverse Effect” in the representations and warranties set forth in Section 5.8 and, to the extent related thereto, the condition in Section 8.3(a), or (viii) any failure to meet any projections, forecasts, guidance, estimates, milestones, budgets or financial or operating predictions of revenue, earnings, cash flow or cash position (provided, that clause (viii) shall not prevent or otherwise affect a determination that any event, change, fact or circumstance underlying such failure to meet projections or forecasts has resulted in, or contributed to, or would reasonably be expected to result in or contribute to, a Murphy Material Adverse Effect, and provided, further, that any event, change, fact or circumstance referred to in clauses (i), (ii), (iii), and (v) may be taken into account in determining if a “Murphy Material Adverse Effect” occurred to the extent it has a disproportionate impact on Murphy as compared to similarly situated companies in the industry in which Murphy conducts its operations.
| A-1-11 |
“Murphy Organizational Documents” means the Murphy Certificate of Incorporation and bylaws, in each case as amended, modified or supplemented from time to time, which will be amended and restated in connection with the Merger as provided herein.
“Murphy Preferred Stock” has the meaning set forth in Section 5.3(a).
“Murphy Private Placement Warrants” has the meaning ascribed to “Private Warrant” in the Murphy SEC Reports as of the date of this Agreement.
“Murphy Proposals” has the meaning set forth in Section 7.1(b).
“Murphy Public Warrant” has the meaning ascribed to “Public Warrant” in the Murphy SEC Reports as of the date of this Agreement.
“Murphy SEC Documents” has the meaning set forth in Section 5.7(a).
“Murphy Units” means the units of Murphy issued in connection with its initial public offering, which such units are comprised of one share of Murphy Class A Common Stock and one Murphy Public Warrant, which ceased trading and were separated into their component parts as of March 28, 2022.
“Murphy Warrants” means, collectively, Murphy Public Warrants and Murphy Private Placement Warrants.
“Nasdaq” means the Nasdaq Global Select Market or The Nasdaq Global Market.
“Open Source License” means any license meeting the Open Source Definition (as promulgated by the Open Source Initiative) or the Free Software Definition (as promulgated by the Free Software Foundation), or any substantially similar license, including any license approved by the Open Source Initiative or any Creative Commons License. For the avoidance of doubt, Open Source Licenses include Copyright Licenses.
“Outside Date” has the meaning set forth in Section 9.1(b).
“Outstanding Company Transaction Expenses” has the meaning set forth in Section 3.5(a).
“Outstanding Murphy Transaction Expenses” has the meaning set forth in Section 3.5(b).
“Owned Real Property” means the land owned by the Company (collectively, the “Land”), together with all buildings and other structures, facilities, and other improvements located thereon (collectively, the “Improvements”); all right, title and interest of the Company if any, in and to any and all appurtenances, strips or gores, roads, easements, streets, alleys, drainage facilities and rights-of-way bounding any of the Land; all utility capacity, utilities, water rights, licenses, permits, entitlements, and bonds, if any, and all other rights and benefits attributable to the Land; and all rights of ingress and egress thereto; all transferable consents, authorizations, variances or waivers, licenses, permits and approvals from any Governmental Authority in connection with the Land or the Improvements held by or granted to the Company any of its respective predecessors in title, and/or the agents thereof with respect to the Land or the Improvements; all right, title and interest of the Company in and to all site plans, surveys, soil and substratus studies, and engineering and architectural drawings, plans and specifications, in the possession or control of the Company relating to the Land or Improvements; all equipment and other personal property owned by the Company located on and/or exclusively used in connection with the operation of the Land or Improvements; and all written service and maintenance contracts and other written contracts, if any, relating to the Land or Improvements.
| A-1-12 |
“Party” and “Parties” have the meaning set forth in the preamble of this Agreement.
“PCAOB Audited Financial Statements” has the meaning set forth in Section 4.7(c).
“Permitted Liens” means: (a) such imperfections of title, easements, encumbrances, Liens or restrictions that do not materially impair the current use of the Company’s assets that are subject thereto; (b) Liens for Taxes not yet due and payable, or being contested in good faith for which adequate reserves have been set aside according to GAAP; (c) zoning, entitlement, conservation restriction and other land use and environmental regulations promulgated by Governmental Authorities, (d) non-exclusive licenses, sublicenses or other rights to Intellectual Property owned by or licensed to the Company granted to any licensee in the ordinary course of business; (e) non- monetary Liens, encumbrances and restrictions on real property (including easements, covenants, rights of way and similar restrictions of record) that do not materially interfere with the present uses of such real property; and (f) materialmen’s, mechanics’, carriers’, workmen’s, warehousemen’s, repairmen’s, landlord’s, and other similar Liens arising in the ordinary course of business, or deposits to obtain the release of such Liens.
“Person” means an individual, corporation, partnership, limited partnership, limited liability company, syndicate, person (including a “person” as defined in Section 13(d)(3) of the Exchange Act), trust, association or entity or government, political subdivision, agency or instrumentality of a government.
“Personal Information” means (a) information related to an identified or identifiable individual (e.g., name, address telephone number, email address, financial account number, health information, government-issued identifier), (b) any other data used or intended to be used or which allows one to identify, contact, or precisely locate an individual, device or household, including any internet protocol address or other persistent identifier, and (c) any other, similar information or data regulated by Privacy/Data Security Laws.
“PIPE Financing” has the meaning set forth in the recitals of this Agreement.
“Plan” has the meaning set forth in Section 4.11(a).
“Plan of Merger” has the meaning set forth in Section 2.3.
“Principal Shareholders” means (i) Corvus Capital Limited, (ii) St. George Street Capital Limited, and (iii) Algo Holdings, Inc.
“Prior Financial Statements” has the meaning set forth in Section 4.7(a).
| A-1-13 |
“Privacy/Data Security Laws” means all Laws, self-regulatory standards, third party system and platform requirements, and industry regulations governing (a) the receipt, collection, use, storage, processing, sharing, security, disclosure, transfer, sale, unauthorized access or modification, theft, loss, inaccessibility, breach, or transfer of Personal Information, Confidential Information, the Company’s Business Systems or Business Data and (b) unfair and deceptive practices, accessibility, advertising communications (e.g., text messages, emails, calls), PCI-DSS, location tracking and marketing.
“Products” mean any products or services, developed, manufactured, performed, out- licensed, sold, distributed other otherwise made available by or on behalf of the Company from which the Company has derived previously, is currently deriving or is scheduled to derive, revenue from the sale or provision thereof.
“Prohibited Party” has the meaning set forth in Section 4.25(b).
“Proxy Statement” means the proxy statement to be filed by Murphy as part of the Registration Statement with respect to the Special Meeting for the purpose of soliciting proxies from stockholders of Murphy to approve Murphy Proposals.
“Redeeming Shareholder” means a stockholder of Murphy who properly demands that Murphy redeem its Class A Common Stock for cash in connection with the Murphy Proposals and in accordance and in compliance with Murphy Organizational Documents.
“Registered Company IP” means patents, patent applications, registrations and applications for registration of trademarks, service marks, and trade dress, internet domains, and copyrights owned or licensed by the Company.
“Registration Rights Agreement” has the meaning set forth in the recitals of this Agreement.
“Registration Statement” has the meaning set forth in Section 7.1(a).
“Related Party” means, with respect to the Company, (a) any Affiliate of the Company or any Person who serves as a director, officer, general partner or managing member of such Affiliate;
(b) any Person who serves as a director, officer, general partner or managing member of the Company; (c) any Person that beneficially owns, directly or indirectly, at least 5% of the Company Capital Stock or any Affiliate described in clause (i) of such Person; or (d) any Immediate Family member of any Person described in clause (a), (b) or (c). For purposes of this definition, “Affiliate” shall not include the Company.
“Remedies Exceptions” has the meaning set forth in Section 4.4.
“Representatives” has the meaning set forth in Section 7.2.
“Sanctions Laws” has the meaning set forth in Section 4.25(a).
“Securities Act” means the Securities Exchange Act of 1933, as amended.
| A-1-14 |
“Securities Laws” means the securities laws of any state, federal or foreign entity and the rules and regulations promulgated thereunder.
“Shareholder” means a holder of Company Ordinary Shares, including holders of shares of Company Ordinary Shares issued upon the Company Convertible Debt Conversion.
“Shareholder Support Agreement” has the meaning set forth in the recitals of this Agreement.
“Software” means all computer software (in object code or source code format), data and databases, and related documentation and materials.
“Special Meeting” means a meeting of the holders of Murphy Common Stock to be held for the purpose of approving the Murphy Proposals.
“Sponsor” means Murphy Canyon Acquisition Sponsor LLC, a Delaware limited liability company.
“Sponsor Support Agreement” has the meaning set forth in the recitals of this Agreement.
“Subscription Agreement” has the meaning set forth in the recitals of this Agreement.
“Subsidiary” means, with respect to a Person, any other Person, of which (a) an amount of the voting securities, other voting ownership or voting partnership interests of which is sufficient to elect at least a majority of its board of directors or other governing body (or, if there are no such voting interests, fifty percent (50%) or more of the equity interests of which) is owned directly or indirectly by such first Person or (b) such Persons controls any other Person. For the purposes hereof, the term “Subsidiary” shall include all subsidiaries of such Subsidiary.
“Supplier” means any Person that supplies inventory or other materials or personal property, components, or other goods or services that are utilized in or comprise the Products of the Company.
“Surviving Corporation” has the meaning set forth in Section 2.1.
“Surviving Corporation Organizational Documents” means the form of certificate of incorporation and articles and memorandum of association set forth on Exhibit D to this Agreement.
“Surviving Provisions” has the meaning set forth in Section 9.2.
“Tax” and “Taxes” means any U.S. federal, state, local, foreign, and other income, gross income, adjusted gross income or gross receipts, franchise, estimated, alternative or add-on minimum, sales, use, transfer, value added, excise, stamp, customs, duties, ad valorem, real property, personal property (tangible and intangible), capital stock, social security, unemployment, payroll, wage, employment, severance, occupation, registration, communication, mortgage, profits, license, lease, service, goods and services, withholding, disability, estimated, escheat, premium, turnover, windfall profits or other taxes of any kind whatsoever, together with any interest, penalties, additions to tax, or additional amounts imposed by any Governmental Authority with respect thereto.
| A-1-15 |
“Tax Return” means any return, information return, statement, declaration, claim for refund, estimate, report, or other document relating to Taxes that is filed or required to be filed with any Governmental Authority, including any schedule or attachment thereto and including any amendments thereof.
“Terminating Company Breach” has the meaning set forth in Section 9.1(d).
“Terminating Murphy Breach” has the meaning set forth in Section 9.1(e).
“Transactions” means the transactions contemplated by this Agreement, including the
Merger and the Ancillary Agreements.
“Treasury Regulations” means the final and temporary United States Treasury regulations issued pursuant to the Code.
“Treasury Shares” has the meaning set forth in Section 3.1(b)(ii). “Trust Account” has the meaning set forth in Section 5.12.
“Trust Agreement” means the Investment Management Trust Agreement, dated December 2, 2022, between Murphy and Wilmington Trust Company.
“Trust Fund” has the meaning set forth in Section 5.12.
“Trustee” has the meaning set forth in Section 5.12.
“Willful Breach” means, with respect to any agreement, a Party’s knowing and intentional material breach of any of its representations or warranties as set forth in such agreement, or such Party’s material breach of any of its covenants or other agreements set forth in such agreement, which material breach constitutes, or is a consequence of, a purposeful act or failure to act by such Party with the Knowledge that the taking of such act or failure to take such act would cause a material breach of this Agreement.
“Withholding Agent” has the meaning set forth in Section 3.3.
Section 1.2 Construction.
(a) Unless the context of this Agreement otherwise requires, (i) words of any gender include all other genders, (ii) words using the singular or plural number also include the plural or singular number, respectively, (iii) the terms “hereof,” “herein,” “hereby,” “hereto” and derivative or similar words refer to this entire Agreement, (iv) the terms “Article,” “Section,” “Schedule” and “Exhibit” refer to the specified Article, Section, Schedule or Exhibit of or to this Agreement, (v) the word “including”, “include” or similar derivations means “including without limitation,” (vi) the word “or” shall be disjunctive but not exclusive, (vii) references to agreements and other documents shall be deemed to include all subsequent amendments and other modifications thereto, (viii) the word “extent” in the phrase “to the extent” means the degree to which a subject or other thing extends, and such phrase shall not mean simply “if”; and (ix) references to statutes shall include all regulations promulgated thereunder and references to statutes or regulations shall be construed as including all statutory and regulatory provisions consolidating, amending or replacing the statute or regulation.
| A-1-16 |
(b) The phrases “delivered,” “provided to,” “furnished to,” “made available” and phrases of similar import when used herein, unless the context otherwise requires, means that a copy of the information or material referred to has been (i) provided no later than five days prior to the date of this Agreement to the Party to which such information or material is to be provided or furnished (A) in the “virtual data room” set up by the Company in connection with this Agreement or (B) by delivery to such Party or its legal counsel via electronic mail or hard copy form, or (ii) with respect to Murphy, filed with the SEC by Murphy no later than five days prior to the date hereof.
(c) When used herein, “ordinary course of business” means an action taken, or omitted to be taken, in the ordinary and usual course of the Company’s or Murphy’s business, as applicable, consistent with past practice (including, for the avoidance of doubt, recent past practice in light of COVID-19 or COVID-19 Measures).
(d) The language used in this Agreement shall be deemed to be the language chosen by the Parties to express their mutual intent and no rule of strict construction shall be applied against any Party.
(e) Whenever this Agreement refers to a number of days, such number shall refer to calendar days unless Business Days are specified. If any action is to be taken or given on or by a particular calendar day, and such calendar day is not a Business Day, then such action may be deferred until the next Business Day.
(f) All accounting terms used herein and not expressly defined herein shall have the meanings given to them under GAAP.
ARTICLE II
THE MERGER; CLOSING
Section 2.1 The Merger. On the terms and subject to the conditions set forth in this Agreement, at the Effective Time, (a) Merger Sub shall be merged with and into the Company in accordance with the Companies Act, and the separate corporate existence of Merger Sub shall thereupon cease, (b) the Company shall be the surviving corporation in the Merger (sometimes hereinafter referred to as the “Surviving Corporation”) and become a wholly owned Subsidiary of Murphy, and the separate corporate existence of the Company with all of its rights, privileges, immunities, powers and franchises shall continue unaffected by the Merger as provided in the Companies Act, and (c) the Merger shall have such other effects as provided in the Companies Act and this Agreement.
Section 2.2 Closing. Subject to the terms and conditions of this Agreement, the closing of the Merger (the “Closing”) shall take place at the offices of Sichenzia Ross Ference LLP, 1185 Avenue of the Americas, 31st Floor, New York, New York 10036 on a date no later than three (3) Business Days after the satisfaction or waiver of all the conditions set forth in Article VIII that are required to be satisfied prior to the Closing Date, or at such other place and time as Murphy and the Company may mutually agree upon. The parties may participate in the Closing via electronic means. The date on which the Closing actually occurs is referred to in this Agreement as the “Closing Date.”
| A-1-17 |
Section 2.3 Effective Time. Subject to the satisfaction or waiver of all of the conditions set forth in Article VIII of this Agreement, as soon as practicable following the Closing on the Closing Date the Company, Murphy and Merger Sub will cause a plan of merger relating to the Merger and other documents required by the Companies Act (collectively, the “Plan of Merger”) to be executed, acknowledged and filed with the Registrar of Companies of the Cayman Islands in accordance with the relevant provisions of the Companies Act. The Merger shall become effective at the time when the Plan of Merger has been duly filed with and accepted by the Registrar of Companies of the Cayman Islands or at such later date and time as may be agreed by the Parties in writing and specified in the Plan of Merger (such date and time, the “Effective Time”).
Section 2.4 Organizational Documents of Murphy and the Surviving Corporation.
(a) At the Closing and immediately prior to the Effective Time, subject to obtaining the affirmative vote of the stockholders of Murphy for the Murphy Proposals in accordance with the Proxy Statement, Murphy shall cause the Murphy Organizational Documents to be amended and restated in their entirety to be the Amended Charter and the Amended Bylaws, respectively, until thereafter supplemented or amended in accordance with their respective terms and the DGCL. Without limiting the generality of the foregoing, as of the Effective Time, Murphy will change its corporate name to “Conduit Pharmaceuticals Inc.”
(b) At the Effective Time, by virtue of the Merger, the Company Organizational Documents, as in effect immediately prior to the Effective Time, shall be amended and restated in their entirety to read in the forms of the Surviving Corporation Organizational Documents, and as so amended and restated, will be the certificate of incorporation and articles and memorandum of association of the Surviving Corporation until thereafter supplemented or amended in accordance with their respective terms and the Companies Act.
Section 2.5 Directors. Immediately after the Closing, the board of directors of the Surviving Corporation and of Murphy each shall consist of five (5) directors, consisting of (a) one (1) director designated prior to Closing by the Sponsor and reasonably acceptable by the Company and (b) the remaining designated prior to the Closing by the Company; with at a least three (3) of the five (5) directors who will serve as independent directors satisfying the independence requirements of the Securities Act and the Nasdaq rules.
Section 2.6 Chief Financial Officer. Immediately after the Closing, the Chief Financial Officer of Murphy, Adam Sragovicz shall be appointed Chief Financial Officer of the Company.
Section 2.7 No Further Ownership Rights in Company Capital Stock. At the Effective Time, the register of members of the Company shall be closed and thereafter there shall be no further registration of transfers of Company Ordinary Shares on the records of the Company.
| A-1-18 |
Section 2.8 Rights Not Transferable. The rights of the Shareholders as of immediately prior to the Effective Time are personal to each such Shareholder and shall not be assignable or otherwise transferable for any reason (except (i) in the case of an entity, by operation of Law or
(ii) in the case of a natural person, by will or the Laws of descent and distribution). Any attempted transfer of such right by any holder thereof (otherwise than as permitted by the immediately preceding sentence) shall be null and void.
Section 2.9 Taking of Necessary Action; Further Action. If, at any time after the Effective Time, any further action is necessary or desirable to carry out the purposes of this Agreement and to vest the Surviving Corporation with full right, title and interest in, to and under, and/or possession of, all assets, property, rights, privileges, powers and franchises of Merger Sub and the Company, the officers and directors of Merger Sub and the Company are fully authorized in the name of their respective corporations or otherwise to take, and will take, all such lawful and necessary action, so long as such action is not inconsistent with this Agreement.
ARTICLE III
ACTIONS PRIOR TO THE MERGER; CONVERSION OF SECURITIES;
CONSIDERATION
Section 3.1 CONVERSION OF SECURITIES
(a) Company Convertible Debt. Prior to the Effective Time, all holders of Company Convertible Debt shall have converted their full principal amount of Company Convertible Debt that is issued and outstanding immediately prior to the Effective Time into a number of shares of Company Ordinary Shares at the then-effective conversion rate as calculated pursuant to the senior secured convertible promissory note agreements that govern the terms of the Company Convertible Debt (the “Company Convertible Debt Conversion”). After the Company Convertible Debt Conversion, the Company Convertible Debt shall no longer be outstanding and shall cease to exist, and each holder of Company Convertible Debt shall thereafter cease to have any rights with respect to such securities, except for its pro rata portion of the Closing Payment Shares.
(b) Cancellation of Company Capital Stock. At the Effective Time, by virtue of the Merger and without any action on the part of Murphy, Merger Sub or the Company, each Company Ordinary Share issued and outstanding immediately prior to the Effective Time shall be canceled and automatically converted into the right to receive, without interest, the Applicable Per Share Merger Consideration. For avoidance of any doubt, each Shareholder will cease to have any rights with respect to his, her or its Company Ordinary Shares, except the right to receive the Applicable Per Share Merger Consideration. At the Effective Time (and, for the avoidance of doubt, following the Company Convertible Debt Conversion), by virtue of the Merger and without any action on the part of any holder thereof:
(i) Company Ordinary Shares. Each Company Ordinary Share (including Company Ordinary Shares issued as a result of the Company Convertible Debt Conversion) that is issued and outstanding immediately prior to the Effective Time, other than the Dissenting Shares, shall thereupon be converted into the right to receive, and the holder of such share of Company Stock shall be entitled to receive, the Applicable Per Share Merger Consideration;
| A-1-19 |
(ii) Company Treasury Stock. Each Company Ordinary Share held in the treasury of the Company (“Treasury Shares”) immediately prior to the Effective Time shall be cancelled without any conversion thereof and no payment or distribution shall be made with respect thereto; and
(iii) Company Dissenting Share. Each of the Dissenting Shares issued and outstanding immediately prior to the Effective Time shall be cancelled and cease to exist in accordance with Section 3.6(a) and shall thereafter represent only the right to receive the applicable payments set forth in Section 3.6(a).
(c) Murphy Class B Common Stock. Each share of Murphy Class B Common Stock shall automatically convert into and become one validly issued, fully paid and non- assessable share of Murphy Class A Common Stock.
(d) Merger Sub Shares. Each ordinary share of Merger Sub issued and outstanding immediately prior to the Effective Time shall no longer be outstanding and shall thereupon be converted into and become one validly issued, fully paid and non-assessable ordinary share of the Surviving Corporation, and all such shares shall constitute the only outstanding shares of the Surviving Corporation as of immediately following the Effective Time.
(e) Surrender of Certificates. All securities issued upon the surrender of Company Ordinary Shares in accordance with the terms hereof, shall be deemed to have been issued in full satisfaction of all rights pertaining to such securities, provided that any restrictions on the sale and transfer of such Company Ordinary Shares shall also apply to the Closing Payment Shares so issued in exchange.
(f) Lost, Stolen or Destroyed Certificates. In the event any certificates for Company Ordinary Shares shall have been lost, stolen or destroyed, Murphy shall cause to be issued in exchange for such lost, stolen or destroyed certificates and for each such share, upon the making of an affidavit of that fact by the holder thereof; provided, however, that Murphy may, in its discretion and as a condition precedent to the issuance thereof, require the owner of such lost, stolen or destroyed certificates to deliver a bond in such sum as it may reasonably direct as indemnity against any claim that may be made against the Company or Murphy with respect to the certificates alleged to have been lost, stolen or destroyed.
(g) Adjustments. Without limiting the other provisions of this Agreement, if at any time during the period between the date of this Agreement and the Effective Time, any change in the outstanding securities of the Company or Murphy shall occur (other than the issuance of additional shares of the Company or Murphy as permitted by this Agreement), including by reason of any reclassification, recapitalization, share split (including a reverse share split), or combination, exchange, readjustment of shares, or similar transaction, or any share dividend or distribution paid in shares, the Exchange Ratio and the number of the Closing Payment Shares (as well as the Applicable Per Share Merger Consideration) payable to the Shareholders pursuant to this Agreement shall be appropriately adjusted to reflect such change; provided, however, that this sentence shall not be construed to permit Murphy or the Company to take any action with respect to its securities that is prohibited by the terms of this Agreement.
| A-1-20 |
Section 3.2 Payment of Merger Consideration.
(a) Upon and subject to the terms and conditions of this Agreement, on the Closing Date, Murphy shall issue to each Shareholder such number of Closing Payment Shares as set forth opposite such Shareholder’s name on Exhibit A.
(b) No certificates or scrip representing fractional Murphy Class A Common Stock will be issued pursuant to the Merger, and such fractional share interests will not entitle the owner thereof to vote or to any rights of a shareholder of Murphy.
(c) Each certificate issued pursuant to the Merger to any Shareholder shall bear the legend set forth below, or a legend substantially equivalent thereto, together with any other legends that may be required by any securities laws at the time of the issuance of the Closing Payment Shares:
“THE SHARES REPRESENTED BY THIS CERTIFICATE HAVE NOT BEEN REGISTERED UNDER THE UNITED STATES SECURITIES ACT OF 1933, AS AMENDED (THE “ACT”), AND MAY NOT BE OFFERED, SOLD OR OTHERWISE TRANSFERRED, PLEDGED OR HYPOTHECATED UNLESS AND UNTIL (I) SUCH OFFER, SALE, TRANSFER, PLEDGE OR HYPOTHECATION HAS BEEN REGISTERED UNDER THE ACT OR (II) THE ISSUER OF THE SHARES HAS RECEIVED AN OPINION OF COUNSEL IN FORM AND SUBSTANCE SATISFACTORY TO THE ISSUER THAT SUCH OFFER, SALE OR TRANSFER, PLEDGE OR HYPOTHECATION IS IN COMPLIANCE WITH THE ACT.”
Section 3.3 Withholding Rights. Each of Murphy and Merger Sub (each, a “Withholding Agent”) shall be entitled to deduct and withhold from the consideration otherwise payable pursuant to this Agreement to any Person such amounts as it is required to deduct and withhold with respect to the making of such payment under applicable Law; provided that prior to making any deduction or withholding pursuant to this Section 3.3, the Withholding Agent shall use reasonable best efforts to provide the Shareholders with advance written notice of any such intended deduction or withholding (other than any withholding on amounts properly treated as compensation to employees for U.S. federal income Tax purposes) at least ten (10) days before the making of such payment, and the Withholding Agent shall consult with the Company and/or its Shareholders in good faith to determine whether such deduction and withholding is required under applicable Law and cooperate in good faith with the Shareholders to obtain any available exception from, or reduction in, such deduction or withholding (including through the request and provision of any statements, forms, or other documents to reduce or eliminate any such deduction or withholding) pursuant to this Section 3.3 to the extent permitted under applicable Law. To the extent that amounts are so withheld by the Withholding Agent or any of their respective Affiliates with respect to any Person and are properly remitted to the applicable Governmental Authority, such withheld amounts shall be treated for all purposes of this Agreement as having been paid on behalf of such Person in respect of which such deduction and withholding was made. In the case of any such payment payable to employees of the Company or its Affiliates in connection with the Merger treated as compensation, the parties shall cooperate to pay such amounts through the Company’s payroll to facilitate applicable withholding.
| A-1-21 |
Section 3.4 Register of Members. At the Effective Time, the register of members of the Company shall be closed and there shall be no further registration of transfers of Company Capital Stock thereafter on the records of the Company. From and after the Effective Time, the holders of Certificates representing Company Capital Stock outstanding immediately prior to the Effective Time shall cease to have any rights with respect to such Company Capital Stock, except as otherwise provided in this Agreement or by Law. On or after the Effective Time, any Certificates presented to Murphy for any reason shall be converted into the Merger Consideration in accordance with the provisions of Section 3.1.
Section 3.5 Payment of Expenses.
(a) No sooner than five nor later than two Business Days prior to the Closing Date, the Company shall provide to Murphy a written report setting forth a list of all of the following fees and expenses incurred by or on behalf of the Company and/or the Shareholders in connection with the preparation, negotiation and execution of this Agreement and the consummation of the Transactions (together with written invoices and wire transfer instructions for the payment thereof), solely to the extent such fees and expenses are incurred and expected to remain unpaid as of the close of business on the Business Day immediately preceding the Closing Date: (i) the fees and disbursements of outside counsel to the Company incurred in connection with the Transactions and (ii) the fees and expenses of any other agents, advisors, consultants, experts, financial advisors and other service providers engaged by the Company in connection with the Transactions (collectively, the “Outstanding Company Transaction Expenses”). On the Closing Date concurrent with the Closing, Murphy shall pay or cause to be paid by wire transfer of immediately available funds all such Outstanding Company Transaction Expenses.
(b) No sooner than five nor later than two Business Days prior to the Closing Date, Murphy shall provide to the Company a written report setting forth a list of all of the following fees and expenses incurred by or on behalf of Murphy and/or Merger Sub in connection with the preparation, negotiation and execution of this Agreement and the consummation of the Transactions (together with written invoices and wire transfer instructions for the payment thereof), solely to the extent such fees and expenses are incurred and expected to remain unpaid as of the close of business on the Business Day immediately preceding the Closing Date: (i) the fees and disbursements of outside counsel to the Company incurred in connection with the Transactions and (ii) the fees and expenses of any other agents, advisors, consultants, experts, financial advisors and other service providers engaged by the Company in connection with the Transactions (collectively, the “Outstanding Murphy Transaction Expenses”). On the Closing Date concurrent with the Closing, Murphy shall pay or cause to be paid by wire transfer of immediately available funds all such Outstanding Murphy Transaction Expenses.
(c) Neither Murphy nor the Surviving Corporation shall be liable to any holder of Company Capital Stock for any such Company Capital Stock (or dividends or distributions with respect thereto) or cash delivered to a public official pursuant to any abandoned property, escheat or similar Law.
| A-1-22 |
(d) Murphy shall not pay or cause to be paid any Outstanding Murphy Transaction Expenses or Outstanding Company Transaction Expenses other than in accordance with this Section 3.5.
Section 3.6 Dissenter’s Rights.
(a) Notwithstanding any provision of this Agreement to the contrary and to the extent available under the Companies Act, shares of Company Capital Stock that are outstanding immediately prior to the Effective Time and that are held by Shareholders who shall have neither voted in favor of the Merger nor consented thereto in writing and who shall have demanded properly in writing dissenter’s rights for such Company Capital Stock in accordance with the Companies Act and otherwise complied at all times with all of the provisions of the Companies Act relevant to the exercise and perfection of dissenters’ rights (collectively, the “Dissenting Shares”) shall not be converted into, and such Shareholders shall have no right to receive, the applicable Merger Consideration in accordance with the provisions of Section 3.1, unless and until such Shareholder fails to perfect or withdraws or otherwise loses his, her or its dissenter’s rights and payment under the Companies Act. Any Shareholder who fails to perfect or who effectively withdraws or otherwise loses his, her or its dissenter’s rights as to such shares of Company Capital Stock under the Companies Act shall thereupon be deemed to have such shares of Company Capital Stock converted into, and to have become exchangeable for, as of the Effective Time, the right to receive the applicable Merger Consideration in accordance with the provisions of Section 3.1, without any interest thereon, upon surrender of the Certificate or Certificates that formerly evidenced such shares of Company Capital Stock.
(b) Prior to the Closing, the Company shall give Murphy (i) prompt notice of any demands for dissenter’s rights received by the Company (which demands must, for the avoidance of doubt, be received prior to the passing of the Company Requisite Approval in accordance with the Companies Act) and any withdrawals of such demands, and (ii) the opportunity to participate in all negotiations and proceedings with respect to demands for dissenter’s rights under the Companies Act. The Company shall not, except with the prior written consent of Murphy (which consent shall not be unreasonably withheld, conditioned or delayed), make any payment with respect to any demands for dissenter’s rights or offer to settle or settle any such demands.
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF THE COMPANY
Except as set forth in the disclosure schedules delivered as of the date hereof by the Company to Murphy in connection with this Agreement (the “Company Disclosure Schedules”), the Company hereby represents and warrants to Murphy that each of the following representations and warranties is true, correct and complete as of the date of this Agreement (or, if such representations and warranties are made with respect to a certain date, as of such date). Any disclosure made with respect to a section of this Article IV shall be deemed to qualify any other section of this Article IV specifically referenced or cross-referenced therein or to the extent that it is readily apparent that such disclosure is applicable thereto.
| A-1-23 |
Section 4.1 Organization and Qualification.
(a) The Company is an exempted company duly organized, validly existing and in good standing under the laws of the Cayman Islands and has the requisite corporate or other organizational power and authority and all necessary governmental approvals to own, lease and operate its properties and to carry on its business as it is now being conducted. The Company is duly qualified or licensed as a foreign corporation or other organization to do business, and is in good standing, in each jurisdiction where the character of the properties owned, leased or operated by it or the nature of its business makes such qualification or licensing necessary, except for such failures to be so qualified or licensed and in good standing that do not constitute a Company Material Adverse Effect.
(b) Except as provided in Schedule 4.1(b) of the Company Disclosure Schedules, the Company does not directly or indirectly own any Subsidiaries. The Company does not directly or indirectly own any equity or similar interest in, or any interest convertible into or exchangeable or exercisable for any equity or similar interest in, any other corporation, partnership, joint venture or business association or other entity.
Section 4.2 Organizational Documents. The Company has prior to the date of this Agreement made available a complete and correct copy of the Company Organizational Documents. Such Company Organizational Documents are in full force and effect. The Company is not in violation of any of the provisions of the Company Organizational Documents. The Company has prior to the date of this Agreement made available a complete and correct copy of the organization documents of its Subsidiary. The organizational documents of such Subsidiary are in full force and effect. The Company’s Subsidiary is not in violation of any of the provisions of the Company Organizational Documents.
Section 4.3 Capitalization.
(a) The authorized share capital of the Company is GBP40,000 made up of 400,000,000 Company Ordinary Shares. As of the date of this Agreement, (i) 2,000 Company Ordinary Shares are issued and outstanding, (ii) no Company Ordinary Shares are held in the treasury of the Company, and (iii) other than with respect to the Company Convertible Debt, there are no Ordinary Shares or other equity or voting interests of the Company reserved for issuance. Exhibit A sets forth the current capitalization table of the Company, including all of the Company Ordinary Shares, the holders thereof, and each such holder’s pro rata percentage of the issued and outstanding Company Ordinary Shares.
(b) The aggregate outstanding and unpaid Company Convertible Debt as of the date of this Agreement is set forth on Exhibit A.
(c) Other than this Agreement, the Ancillary Agreements and the Company Convertible Debt, there are no options, restricted shares, restricted share units, phantom equity awards, warrants, preemptive rights, calls, convertible securities, conversion rights or other rights, agreements, arrangements or commitments of any character relating to the issued or unissued shares of the Company or obligating the Company to issue or sell any shares of, or other equity interests in, the Company. The Company is not a party to, or otherwise bound by, and the Company has not granted, any equity appreciation rights, participations, phantom equity or similar rights. Other than this Agreement and any Ancillary Agreements, there are no voting trusts, voting agreements, proxies, shareholder agreements or other agreements with respect to the voting or transfer of the Company Ordinary Shares, the Company Convertible Debt or any of the equity interests or other securities of the Company.
| A-1-24 |
(d) Schedule 4.3(d) of the Company Disclosure Schedules sets forth, as of the date of this Agreement, the following information with respect to each instrument evidencing Company Convertible Debt outstanding (i) the name of the holder of such Company Convertible Debt and (ii) the date on which such Company Convertible Debt was incurred. The Company has made available to Murphy an accurate and complete copy of each promissory note evidencing Company Convertible Debt. All Company Ordinary Shares subject to issuance pursuant to the Company Convertible Debt Conversion upon issuance on the terms and conditions specified therein, will be and has been duly authorized, validly issued, fully paid and nonassessable.
(e) Except as contemplated by or as otherwise set forth in this Agreement, there are no outstanding contractual obligations of the Company to repurchase, redeem or otherwise acquire any shares of the Company or to provide funds to or make any investment (in the form of a loan, capital contribution or otherwise) in any Person.
(f) All outstanding shares of the Company Capital Stock have been issued and granted in compliance with (A) all applicable Securities and other Laws and (B) all pre-emptive rights and other requirements set forth in applicable contracts to which the Company is a party.
(g) The Shareholders collectively own directly and beneficially and of record, all of the equity of the Company (which are represented by the issued and outstanding shares of Company Capital Stock). Except for the Company Capital Stock held by the Shareholders and the Company Convertible Debt, no shares or other equity or voting interest of the Company, or options, warrants, convertible debt or other rights to acquire any such shares or other equity or voting interest, of the Company is issued and outstanding.
Section 4.4 Authority Relative to this Agreement. The Company has all necessary power and authority to execute and deliver this Agreement, to perform its obligations hereunder and, subject to receiving the Company Requisite Approval, to consummate the Transactions. The execution and delivery of this Agreement by the Company and the consummation by the Company of the Transactions have been duly and validly authorized by all necessary corporate action, and no other corporate proceedings on the part of the Company are necessary to authorize this Agreement or to consummate the Transactions (other than, with respect to the Merger, the Company Requisite Approval, which the written consent delivered in connection with Section 7.1(f) shall satisfy, and the filing and recordation of appropriate merger documents as required by the Companies Act). This Agreement has been duly and validly executed and delivered by the Company and, assuming the due authorization, execution and delivery by each of Murphy and Merger Sub, constitutes a legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, except as limited by applicable bankruptcy, insolvency, reorganization, moratorium and other laws of general application affecting enforcement of creditors’ rights generally, by general equitable principles (the “Remedies Exceptions”).
| A-1-25 |
Section 4.5 No Conflict; Required Filings and Consents.
(a) Except as set forth in Schedule 4.5(a) of the Company Disclosure Schedules, the execution and delivery of this Agreement by the Company does not, and the performance of this Agreement by the Company will not, (i) conflict with or violate the Company Organizational Documents or (iii) result in any breach of, or constitute a default (or an event which, with notice or lapse of time or both, would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, or result in the creation of a Lien on any property or asset of the Company pursuant to, any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which the Company is a party or by which the Company or any of its property or assets is bound or affected.
(b) The execution and delivery of this Agreement, and the transactions contemplated hereby, by the Company do not, and the performance of this Agreement by the Company will not, require any consent, approval, authorization or permit of, or filing with or notification to, any United States federal, state, county or local or non-United States government, foreign government, governmental or quasi-governmental, regulatory or administrative authority or office, any political or other subdivision thereof, agency, instrumentality, bureau, authority, body or commission or any court, tribunal, judicial or arbitral body or body exercising, or entitled to exercise, any judicial, quasi-judicial, legislative, executive, police, regulatory, taxing or other administrative instrumentality (a “Governmental Authority”), or conflict with or violate any Law, rule, regulation, order, judgment or decree applicable to the Company or by which any of its property or assets are bound or affected, except for applicable requirements, if any, of the Securities Act, the Exchange Act, state securities or “blue sky” laws (“Blue Sky Laws”) and takeover laws of the Cayman Islands, the pre-merger notification requirements of the HSR Act, and filing and recordation of appropriate merger documents as required by the Companies Act.
Section 4.6 Permits; Compliance. The Company is in possession of all material franchises, grants, authorizations, licenses, permits, easements, variances, exceptions, consents, certificates, approvals and orders of any Governmental Authority necessary for the Company to own, lease and operate its properties or to carry on its business as it is now being conducted (the “Company Permits”), except where the failure to have such Company Permits does not constitute a Company Material Adverse Effect. To the Company’s Knowledge, no suspension or cancellation of any of the Company Permits is pending or threatened in writing. Except (i) with respect to compliance with Environmental Laws (as to which certain representations and warranties are made pursuant to Section 4.16) and compliance with Laws related to Taxes (which are the subject of Section 4.15), and (ii) where the failure to be, or to have been, in compliance with such Laws would not, individually or in the aggregate, reasonably be expected to constitute a Company Material Adverse Effect, the Company is, and since January 1, 2020, has been, in compliance with all applicable Laws.
Section 4.7 Financial Statements.
(a) The Company has made available to Murphy true and complete, in all material respects, except for the absence of footnotes and subject to any audit adjustments, copies of the unaudited consolidated balance sheets as of and the related audited consolidated statements of operations and cash flows of the Company for the periods ended December 31, 2020 and December 31, 2021 (collectively, the “Prior Financial Statements”). The Prior Financial Statements (including any notes thereto), except for the absence of footnotes and subject to any audit adjustments, (i) were prepared on an accrual basis in accordance with United States generally accepted accounting principles (“GAAP”) applied on a consistent basis throughout the periods indicated (except as may be indicated in any notes thereto), (ii) were derived from the Books and Records of the Company, and (iii) fairly present, in all material respects, the financial position, results of operations, income (loss), changes in shareholders’ equity, and cash flows of the Company as at the date thereof and for the period indicated therein.
| A-1-26 |
(b) Prior to Closing, the Company will deliver to Murphy a true and complete, in all material respects, except for the absence of footnotes and subject to any yearend and/or audit adjustments, copy of the consolidated unaudited balance sheet of the Company as of September 30, 2022 (the “Interim Balance Sheet”), and the related unaudited consolidated statements of operations and cash flows of the Company for the nine-month period then ended (such financial statements, including the Interim Balance Sheet, the “Interim Financial Statements”). The Interim Financial Statements, except for the absence of footnotes and subject to any yearend and/or audit adjustments, will be (i) prepared on an accrual basis in accordance with GAAP applied on a consistent basis throughout the periods indicated, (ii) derived from the Books and Records of the Company, and (iii) fairly present, in all material respects, the financial position, results of operations, income (loss), changes in shareholders’ equity, and cash flows of the Company as at the date thereof and for the period indicated therein.
(c) Prior to Closing, the Company will deliver to Murphy true and complete, in all material respects, copies of the audited consolidated balance sheets of the Company as of December 31, 2020 and December 31, 2021 and the related audited consolidated statements of operations, cash flows and shareholders’ equity for the years ended December 31, 2020 and December 31, 2021, together with the auditor’s reports thereon, which will comply in all material respects with the applicable accounting requirements and with the rules and regulations of the SEC, the Exchange Act and the Securities Act for financial statements required to be included in the Registration Statement (collectively, the “PCAOB Audited Financial Statements”).
(d) The PCAOB Audited Financial Statements (i) will be prepared from, and will reflect in all material respects, the Books and Records of the Company, (ii) will present fairly, in all material respects, the consolidated financial position, results of operations, income (loss), changes in shareholders’ equity, and cash flows of the Company as of the dates and for the periods indicated therein in conformity with GAAP consistently applied throughout the periods covered thereby, and (iii) when delivered by the Company for inclusion in the Registration Statement for filing with the SEC in accordance with Section 7.1, will comply in all material respects with the applicable accounting requirements and with the rules and regulations of the SEC, the Exchange Act and the Securities Act for financial statements required to be included in the Registration Statement, in effect as of the respective dates thereof.
Section 4.8 Undisclosed Liabilities. The Company has no liabilities, obligations or commitments of any nature whatsoever, asserted or unasserted, known or unknown, absolute or contingent, accrued or unaccrued, matured or unmatured or otherwise, except (a) those which are adequately reflected or reserved against in the Interim Balance Sheet as of the date thereof, and (b) those which have been incurred in the ordinary course of business consistent with past practice since the date of the Interim Balance Sheet and which are not, individually or in the aggregate, material in amount. Except for the Company Convertible Debt, the Company does not have any Indebtedness for borrowed money.
| A-1-27 |
Section 4.9 Absence of Certain Changes or Events. Since inception, or as expressly contemplated by this Agreement and the Transactions, (a) there has not been any Company Material Adverse Effect, and (b) the Company has conducted its business in the ordinary course consistent with past practices.
Section 4.10 Absence of Litigation. Except as set forth on Schedule 4.10 of the Company Disclosure Schedules, there is no cause of action, litigation, suit, hearing, arbitration or other similar proceeding of any nature, civil, criminal, regulatory, administrative or otherwise, whether in equity or at law, in contract, in tort or otherwise (an “Action”) pending against or, to the Knowledge of the Company, threatened in writing against the Company, any directors, or officers thereof, or any property or asset of the Company. Neither the Company nor any material property or asset of the Company is subject to any continuing order of, consent decree, settlement agreement or other similar written agreement with any Governmental Authority, or any order, writ, judgment, injunction, decree, determination or award of any Governmental Authority that would constitute a Company Material Adverse Effect. There is no unsatisfied judgment or any injunction binding upon the Company which would, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect.
Section 4.11 Employee Benefit Plans.
(a) Schedule 4.11(a) of the Company Disclosure Schedules sets forth a true and complete list of each company benefit plan (“Plan”). For purposes of this Agreement, “Plan” means each “employee benefit plan” as defined in Section 3(3) of the Employee Retirement Income Security Act of 1974, as amended (“ERISA”), and any stock purchase, stock option, equity or equity-based or phantom equity compensation, severance, retirement, employment, individual consulting, retention, change-in-control, fringe benefit, collective bargaining, bonus, incentive, deferred compensation, employee loan and all other employee benefit plans, agreements, programs, policies or other arrangements, whether or not subject to ERISA, whether formal or informal, oral or written, in each case, (a) which are contributed to (or required to be contributed to), sponsored by or maintained by the Company for the benefit of any current or former employee, officer, director or individual consultant of the Company (the “Company Employees”) or (b) pursuant to which the Company could have any liability, other than any statutory plan, program or arrangement that is required under applicable Laws and maintained by any Governmental Authority.
(b) With respect to each Plan, the Company has delivered or made available to Murphy copies, to the extent applicable of each Plan and any current trust agreement or other funding instrument relating to such plan and the most recent summary plan description, with respect to such Plan. Each Plan has been established, administered and funded in accordance with its terms, and in compliance in all material respects with all applicable Laws.
(c) Except as set forth on Schedule 4.11(c) of the Company Disclosure Schedules, neither the execution and delivery of this Agreement by the Company nor the consummation of the Transactions will (whether alone or in connection with any subsequent event(s)) (i) result in the payment, acceleration, vesting, funding or creation of any compensatory rights of any Company Employee to payments or benefits or increases in any compensation or benefits (including any loan forgiveness) under any Plan (or under any plan, agreement or arrangement that would be a Plan if in effect as of the date of this Agreement), (ii) result in severance pay or any increase in severance pay upon any termination of employment, or (iii) require any contributions or payments to fund any obligations under any Plan, or cause the Company to transfer or set aside any assets to fund any Plan.
| A-1-28 |
Section 4.12 Labor Matters.
(a) The Company is not a party to any collective bargaining agreement or other agreement with a labor union or like organization, and to the Knowledge of the Company, there are no activities or Actions by any individual or group of individuals, including representatives of any labor organizations or labor unions, to organize any employees of the Company.
(b) As of the date hereof, the Company does not have any employees located in the United States.
(c) The Company is, and since January 1, 2020, has been, in compliance in all material respects with all applicable Laws regarding employment and employment practices, including all laws respecting terms and conditions of employment, health and safety, employee classification, non-discrimination, wages and hours, immigration, disability rights or benefits, equal opportunity, plant closures and layoffs, affirmative action, workers’ compensation, labor relations, pay equity, overtime pay, employee leave issues, the proper classification of employees and independent contractors, the proper classification of exempt and non-exempt employees, and unemployment insurance.
(d) The Company is not delinquent in any material respect in payments to any of its current or former employees, officers, directors, consultants or other service providers for any services rendered or amounts required to be reimbursed or otherwise paid.
(e) To the knowledge of the Company, no employee of the Company at the level of senior vice president or above is in violation of any term of any employment agreement, nondisclosure agreement, noncompetition agreement, restrictive covenant or other obligation with or to: (i) the Company; or (ii) a former employer of any such employee relating (A) to the right of any such employee to be employed by the Company or (B) to the knowledge or use of trade secrets or proprietary information.
(f) All employees of the Company are legally permitted to be employed by the Company in the jurisdiction in which such employees are employed in their current job capacities.
(g) Since January 1, 2020, the Company has not entered into any settlement agreement related to allegations of sexual harassment or sexual misconduct by any director, officer or employee.
| A-1-29 |
Section 4.13 Real Property; Title to and Sufficiency of Assets.
(a) Since its inception, the Company has not, and as of the date of this Agreement, does not, own or hold any Owned Real Property or lease any Leased Real Property.
(b) The Company has legal and valid leasehold or subleasehold interests in, all of its material properties and material assets, tangible and intangible, personal and mixed, used or held for use in its business, free and clear of all encumbrances, liens or other restrictions other than Permitted Liens.
Section 4.14 Intellectual Property.
(a) Schedule 4.14(a) of the Company Disclosure Schedules contains a true, correct and complete list of the Registered Company IP (showing in each, as applicable, the jurisdiction, filing date, date of issuance, expiration date, owner, and registration or application number, and registrar). Each item on Schedule 4.14(a) is subsisting, in full force and effect, and has not expired, been cancelled, abandoned or otherwise terminated and the registered, issued and/or patented items on Schedule 4.14(a) are valid, subsisting and enforceable.
(b) Except as provided on Schedule 4.14(b), the Company solely and exclusively owns and possesses, free and clear of all Liens (other than Permitted Liens), all right, title and interest in and to the Company-Owned IP and the Company has the right to use pursuant to a valid and enforceable written license, all Company-Licensed IP used by it in the Company Business. Except as provided on Schedule 4.14(b), no funding, facilities or personnel of any governmental authority or any university, college, research institute or other educational institution have been or are being used by the Company to develop or create, in whole or in part, any Company-Owned IP. All documents and instruments necessary to record the ownership rights (if applicable) of the Company in the registrations, patents and applications for the Company-Owned IP have been validly executed and filed with the appropriate governmental authority.
(c) The Company has taken reasonable actions to maintain, protect and enforce rights in the Company-Owned IP, including the secrecy, confidentiality and value of its trade secrets and other Confidential Information. To the Knowledge of the Company, the Company has not disclosed any trade secrets or other Confidential Information that is material to the business of the Company to any other Person other than pursuant to a written confidentiality agreement under which such other Person agrees to maintain the confidentiality and protect such trade secrets and Confidential Information.
(d) Except as provided on Schedule 4.14(d) of the Company Disclosure Schedules, all Persons who have contributed, developed or conceived (each, a “Contributor”) any Intellectual Property (i) for or on behalf of the Company, or (ii) in the course of and related to his, her or its relationship with the Company (in each case a “Contribution”) have an obligation to maintain the confidentiality of the Company’s proprietary information and have either assigned all rights, title and interest to such Company-Owned IP to the Company or is a party to a “work-for- hire” agreement under which the Company is deemed to be the owner or author of all property rights therein.
| A-1-30 |
(e) The Company owns, leases, licenses, or otherwise has the legal right to use all Business Systems. In the past year, there has not been a material failure with respect to any of the Business Systems that has not been remedied. Except for commercially available, “off-the- shelf Software or shrink-wrap licenses, the Company does not hold any licenses for any Software, including Open Source Licenses.
(f) The Company is in compliance in all material respects with all applicable Privacy/Data Security Laws. Since January 1, 2020, except as set forth on Schedule 4.14(f) of the Company Disclosure Schedules, to the Knowledge of the Company, there have been no material incidents of security breaches or intrusions or unauthorized access, distribution, disclosure, destruction, disposal or use of any of the Business Systems or Personal Information that are in the possession of and controlled by the Company.
Section 4.15 Taxes.
(a) The Company: (i) has duly and timely filed (taking into account any extension of time within which to file) all material Tax Returns required to be filed by Law by the Company and all such filed Tax Returns are complete and accurate in all material respects; (ii) has timely paid all material Taxes, whether or not shown as due on such filed Tax Returns, except with respect to current Taxes that are not yet due and payable or otherwise being contested in good faith and are disclosed in Schedule 4.15(a) of the Company Disclosure Schedules or that are described in clause (a)(v) below; (iii) with respect to all material Tax Returns filed by or with respect to them, has not waived any statute of limitations with respect to Taxes or agreed to any extension of time with respect to a Tax assessment or deficiency which such waiver or extension remains in effect, and no written request for any such waiver or extension is currently pending; (iv) does not have any deficiency, assessment, claim, audit, examination, investigation, litigation or other proceeding in respect of material Taxes or material Tax matters pending or proposed or threatened in writing, for a Tax period for which the statute of limitations for assessments remains open; and (v) has provided adequate reserves in accordance with GAAP in the most recent consolidated financial statements of the Company, for any material Taxes of the Company that have not been paid, whether or not shown as being due on any Tax Return.
(b) The Company is not a party to, bound by or has an obligation to any Governmental Authority or other Person under any Tax sharing agreement, Tax indemnification agreement, Tax allocation agreement or similar contract or arrangement (excluding agreements, contracts, arrangements or commitments the primary purpose of which do not relate to Taxes).
(c) The Company has (i) withheld or collected all material Taxes required to have been withheld and paid in connection with amounts paid or owing to any current or former employee, independent contractor, creditor, shareholder or other third party, and (ii) reported and timely remitted such amounts required to have been withheld or collected, reported and remitted to the appropriate Governmental Authority. All Tax Returns required with respect thereto have been properly completed and timely filed.
(d) The Company has not been a member of an affiliated group filing a consolidated, combined or unitary U.S. federal, state, local or foreign income Tax Return (other than a group of which the Company was the common parent).
| A-1-31 |
(e) The Company does not have any material liability for the Taxes of any Person (other than the Company) under Treasury Regulation Section 1.1502-6 (or any similar provision of state, local or foreign Law), as a transferee or successor, by contract (excluding contracts, the primary purpose of which do not relate to Taxes), or otherwise.
(f) The Company (i) does not have any request for a material ruling in respect of Taxes pending between the Company and any Tax authority; and (ii) has not entered into any closing agreement, private letter ruling technical advice memoranda or similar agreements with any Tax authority in respect of material Taxes, in each case, that will be in effect after the Closing.
(g) The Company has not in any year for which the applicable statute of limitations remains open distributed stock of another Person, or has had its stock distributed by another Person, in a transaction that was purported or intended to be governed in whole or in part by Section 355 or Section 361 of the Code.
(h) The Company has not engaged in or entered into a “listed transaction” within the meaning of Treasury Regulation Section 1.6011-4(b)(2).
(i) There are no Tax liens upon any assets of the Company except for Permitted
Liens.
(j) The Company is not, and has not been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code. The Company has not been a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise has an office or fixed place of business in a country other than the country in which it is organized.
(k) The unpaid Taxes of the Company (i) did not, as of the date of the Financial Statements, exceed the reserve for Taxes (rather than any reserve for deferred Taxes established to reflect timing difference between book and Tax income) set forth on the face of the Financial Statements (rather than in any notes thereto), and (ii) do not exceed that reserve as adjusted for the passage of time through the Effective Time in accordance with the past custom and practice of Company in filing its Tax Returns.
(l) To the Knowledge of the Company, the Company does not expect (i) any jurisdiction to assess any additional Taxes for any period for which Tax Returns have been filed, and (ii) any jurisdiction in which Tax Returns are not filed to require the filing of Tax Returns. To the Knowledge of the Company, within the last three (3) years, no written claim has been made by any Governmental Authority in a jurisdiction where the Company does not file a Tax Return that such entity is or may be subject to material Taxes by that jurisdiction in respect of Taxes that would be the subject of such Tax Return, which claim has not been resolved.
(m) The Company is organized outside the United States and has no operations in the United States.
(n) None of the shareholders of the Company are “United States persons” within the meaning of Section 7701(a)(30) of the Code.
| A-1-32 |
(o) The Company will not be required to include any item or income, or exclude any item or deduction or loss from, taxable income for any taxable period (or portion thereof) ending after the Effective Time as a result of any:
(i) change in method of accounting for a taxable period (or portion thereof) ending on or prior to the Effective Time;
(ii) use of an improper method of accounting for a taxable period ending on or prior to the Effective Time;
(iii) ruling by, or written agreement with, a Governmental Authority (including any “closing agreement” as described in Section 7121 of the Code (or any corresponding provision of state, local, or non-U.S. Tax law)) for a taxable period ending on or prior to the Effective Time;
(iv) installment sale or open transaction disposition made on or prior to the Effective Time;
(v) prepaid amount received on or prior to the Effective Time;
(vi) intercompany transaction or excess loss accounts described in the Treasury Regulations promulgated under Section 1502 of the Code (or any corresponding or similar provision of state, local or foreign income Tax Law) that existed prior to the Effective Time;
(vii) election under Section 965(h) of the Code; or
(viii) election under Section 108(i) of the Code.
Nothing in this Section 4.15 shall be construed as providing a representation or warranty with respect to any taxable period (or portion thereof) beginning after the Effective Time or (ii) the existence, amount, expiration date or limitations on (or availability of) any Tax attribute.
Section 4.16 Environmental Matters. The Company is currently, and has been, in compliance with all Environmental Laws, and there are no actions or Environmental Claims pending or threatened in writing against the Company.
Section 4.17 Material Contracts.
(a) Schedule 4.17(a) of the Company Disclosure Schedules lists, as of the date of this Agreement, the following types of contracts and agreements (other than purchase orders) to which the Company is a party (such contracts and agreements as are required to be set forth in Schedule 4.17(a) of the Company Disclosure Schedules, the “Material Contracts”):
(i) each employee collective bargaining Contract;
(ii) each contract and agreement with consideration paid or payable to the Company of more than $100,000, in the aggregate, over any 12-month period;
| A-1-33 |
(iii) each contract and agreement with suppliers to the Company for expenditures paid or payable by the Company of more than $100,000, in the aggregate, over any 12-month period;
(iv) any Contract that is a definitive merger, acquisition, purchase and sale or similar agreement entered into in connection with an acquisition or disposition by the Company since January 1, 2020, involving consideration in excess of $250,000 of any Person or of any business entity or division or business of any Person (including through merger or consolidation or the purchase of a controlling equity interest in or substantially all of the assets of such Person or by any other manner), but excluding any Contracts in which the applicable acquisition or disposition has been consummated and there are no material obligations of the Company ongoing;
(v) all management and employment contracts (excluding at-will contracts for employment that do not contain any severance or change of control provisions) and all contracts with natural person consultants and natural person independent contractors that cannot be terminated with less than 30 days’ prior notice and that provide for base salary or compensation in excess of $125,000, in either case to which the Company is a party;
(vi) any Contract evidencing or guaranteeing Company Debt or under which the Company has created, incurred, assumed or guaranteed any other Person’s indebtedness, has the right to draw upon credit that has been extended for indebtedness, or has granted a Lien on its assets, whether tangible or intangible, to secure any indebtedness, in each case, in an amount in excess of $50,000;
(vii) all partnership agreements, collaboration agreements or other joint venture agreements that are material to the business of the Company;
(viii) all contracts and agreements with any Governmental Authority to which the Company is a party, other than any Company Permits and Environmental Permits;
(ix) all contracts and agreements that materially limit, or purport to materially limit, the ability of the Company to compete in any line of business or with any Person or entity or in any geographic area or during any period of time, excluding customary confidentiality agreements and agreements that contain customary confidentiality clauses;
(x) any Contract that (A) grants to any Person any preferred pricing, “most favored nation” or similar rights or (B) grants exclusivity to any Person in respect of any geographic location, any customer, or any product or service;
(xi) each Company Affiliate Agreement, excluding those that are employee confidentiality and invention assignment agreements, equity or incentive equity documents, and employment agreements;
| A-1-34 |
(xii) all contracts or agreements for any Company-Licensed IP that are material to the Company Business, including for Intellectual Property rights incorporated in or necessary for any Products, and the Business Systems of any other person (excluding both unmodified, commercially available, “off-the-shelf” Software or shrink-wrap licenses and Open Source Licenses);
(xiii) all contracts relating to registration rights, drag-along, tag along, right of first refusal or put rights or any other agreements with, among or between Shareholders;
(xiv) all contracts or agreements providing for revenues to the Company, whether currently, in the future, or since January 1, 2020, in an amount exceeding $25,000;
(xv) all leases or master leases of personal property reasonably likely to result in annual payments of $25,000 or more in a 12-month period by the Company, or for any facility material to the operations of the Company; and
(xvi) any commitment to enter into any Contract of the type described in clauses (i) through (xiv) of this Section 4.17(a).
(b) Each Material Contract is a legal, valid and binding obligation of the Company and, to the Knowledge of the Company, the other parties thereto, and the Company is not in breach or violation of, or default under, any Material Contract nor, to the Knowledge of the Company, has any Material Contract been canceled by the other party. To the Company’s Knowledge, no other party is in breach or violation of, or default under, any Material Contract. The Company has not received any written claim of breach or default under any such Material Contract. The Company has furnished or made available to Murphy true and complete copies of all Material Contracts in effect as of the date of this Agreement, including amendments thereto that are material in nature. Each Material Contract sets forth the entire agreement and understanding between the Company and the other parties thereto. Since January 1, 2020, the Company has not received any notice or request, in each case, in writing, from or on behalf of any other party to a Material Contract to terminate, cancel or not renew such Material Contract, or to renegotiate any material term thereof that would reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, or alleging or disputing any breach or default under such Material Contract.
Section 4.18 Insurance. As of the Closing Date, the insurance policies maintained by the Company are in full force and effect and provide insurance in such amounts and against such risks as the Company reasonably has determined to be prudent, taking into account the industries in which the Company operates, and as is sufficient to comply with applicable Law. With respect to each such insurance policy and as of the Closing Date, the Company is not in material breach or default (including any such breach or default with respect to the payment of premiums or the giving of notice) and no written notice of cancellation, non-renewal, disallowance or reduction in coverage or claim or termination has been received other than in connection with ordinary renewals.
| A-1-35 |
Section 4.19 Internal Controls. As of the Closing Date, except as set forth in the Registration Statement or in any notification from the Company’s auditors provided to Murphy, the Company will maintain a system of internal accounting controls sufficient to provide reasonable assurance that: (a) transactions are executed in accordance with management’s general or specific authorizations; (b) transactions are recorded as necessary to permit preparation of financial statements in conformity with GAAP and to maintain asset accountability; (c) access to assets is permitted only in accordance with management’s general or specific authorization; and (d) the recorded accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences. Since January 1, 2020, the Company has not identified and has not been advised by the Company’s auditors of any fraud or allegation of fraud, whether or not material, that involves management or other employees who have a role in the Company’s internal controls over financial reporting.
Section 4.20 Registration Statement. None of the information relating to the Company supplied by the Company, or by any other Person acting on behalf of the Company, in writing specifically for inclusion or incorporation by reference in the Registration Statement will, as of (a) the time the Registration Statement becomes effective under the Securities Act, (b) the date of mailing of the Proxy Statement to stockholders of Murphy or (c) the time of the Special Meeting (including any adjournment thereof), contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements therein, in light of the circumstances under which they were made, not misleading; provided, however, notwithstanding the foregoing provisions of this Section 4.20, no representation or warranty is made by the Company with respect to information or statements made or incorporated by reference in the Registration Statement that were not supplied by or on behalf of the Company for use therein.
Section 4.21 Support Agreement. The Company will deliver to Murphy a true, correct and complete copy of the Shareholder Support Agreement. The Shareholder Support Agreement is in full force and effect and has not been withdrawn or terminated, or otherwise amended or modified, in any respect, and, to the Knowledge of the Company, no withdrawal, termination, amendment or modification is contemplated.
Section 4.22 Board Approval; Vote Required. The Board of Directors of the Company, by resolutions duly adopted by unanimous vote of those voting at a meeting duly called and held and not subsequently rescinded or modified in any way, or by unanimous written consent, has duly
(a) determined that this Agreement and the Merger are fair to and in the best interests of the Company and its Shareholders, (b) approved this Agreement and the Transactions (including the Merger) and declared their advisability, and (c) recommended that the Shareholders approve and adopt this Agreement and approve the Merger and directed that this Agreement and the Transactions (including the Merger) be submitted for consideration by the Shareholders. The Company Requisite Approval is the only vote of the holders of any class or series of capital stock of the Company necessary to adopt this Agreement and approve the Transactions.
Section 4.23 Brokers. Except for those Persons set forth on Schedule 4.23 of the Company Disclosure Schedules, no broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission in connection with the Transactions based upon arrangements made by or on behalf of the Company.
| A-1-36 |
Section 4.24 Takeover Laws. Assuming the accuracy of the representations set forth in Section 5.3(e), no anti-takeover, “fair price”, “moratorium”, “control share acquisition”, “business combination” or other similar statute or regulation is applicable to this Agreement or the transactions contemplated hereby. The Company does not have any shareholder rights plan or “poison pill” in effect, including any agreement with a third-party trust or fiduciary entity with respect thereto.
Section 4.25 International Trade Matters; Anti-Bribery Compliance.
(a) The Company currently is and, since its inception has been, in compliance with applicable Laws related to (i) Anti-Corruption Laws, (ii) economic sanctions administered, enacted or enforced by U.S. Governmental Authorities (including, but not limited to, OFAC, the U.S. Department of State and the U.S. Department of Commerce), the United Nations Security Council, the European Union, Her Majesty’s Treasure, or any other relevant Governmental Authority (collectively, “Sanctions Laws”), (iii) export controls, including the U.S. Export Administration Regulations, 15 C.F.R. §§ 730, et seq., and any other equivalent or comparable Laws of other countries in which the company has conducted and/or currently conducts business (collectively, “Export Control Laws”), (iv) anti-money laundering, including the Money Laundering Control Act of 1986, 18 U.S.C. §§ 1956, 1957, and any other equivalent or comparable Laws of other countries; (v) anti-boycott regulations, as administered by the U.S. Department of Commerce; and (vi) importation of goods, including Laws administered by the U.S. Customs and Border Protection, Title 19 of the U.S.C. and C.F.R., and any other equivalent or comparable Laws of other countries in which the Company has conducted and/or currently conducts business (collectively, “International Trade Control Laws”).
(b) The Company, its directors and officers and, to the Knowledge of the Company, the employees or agents of the Company (acting on behalf of the Company), is not and is not acting under the direction of, on behalf of or for the benefit of a Person that is, (i) the subject of Sanctions Laws or identified on any sanctions-related lists administered by the U.S. Department of State, the U.S. Department of the Treasury, including the OFAC specially Designated Nationals List, the U.S. Department of Commerce, including the Bureau of Industry and Security’s Denied Persons List and Entity List, Her Majesty’s Treasury, including the Consolidated List of Financial Sanctions Targets and the Investment Bank List, or any similar list enforced by any other relevant Governmental Authority, as amended from time to time, or any Person owned or controlled by any of the foregoing (collectively, “Prohibited Party”); or (ii) located, organized or resident in a country or territory that is, or whose government is, the target of comprehensive trade sanctions under Sanctions Laws, including, as of the date of this Agreement, Crimea, Cuba, Iran, North Korea, Sudan and Syria. Neither the Company, nor any director or officer, nor, to the Knowledge of the Company, any employee or agent of the Company (acting on behalf of the Company) has since the Company’s inception, engaged in any transaction involving a Prohibited Party, or any country or territory that was during such period or is, or whose government was during such period or is, the target of comprehensive trade sanctions under Sanctions Laws.
(c) To the Knowledge of the Company, the Company has not exported (including deemed exportation) or re-exported, directly or indirectly, any commodity, software, technology, or services in violation of any applicable Export Control Laws or has participated in any transaction in violation of or connected with any purpose prohibited by Anti-Corruption Laws or any applicable International Trade Control Laws, including support for international terrorism and nuclear, chemical, or biological weapons proliferation.
| A-1-37 |
(d) Since its inception, the Company has not received written notice of, nor, to the Knowledge of the Company, none of its officers, employees, agents or third-party representatives is or has been the subject of, any investigation, inquiry or enforcement proceedings by any Governmental Authority regarding any offense or alleged offense under Anti-Corruption Laws or International Trade Control Laws.
Section 4.26 Related Party Transactions. Schedule 4.26 of the Company Disclosure Schedules sets forth a true, complete and correct list of the following (each such arrangement of the type required to be set forth thereon, whether or not actually set forth thereon, an “Affiliate Transaction”): (i) each Contract entered into prior to the date hereof, between the Company, on the one hand, and any Shareholder or any other current or former Affiliate of the Company on the other hand; and (ii) all Indebtedness (for monies actually borrowed or lent) owed by any Shareholder or any other current or former Affiliate to the Company. Other than the Affiliate Transactions, no Shareholder or Affiliate thereof owns any right in or to any of the material Assets or properties belonging to the Company.
Section 4.27 Not An Investment Company. The Company is not an “investment company” within the meaning of the Investment Company Act of 1940, as amended (the “Investment Company Act”), and the rules and regulations promulgated thereunder.
Section 4.28 Exclusivity of Representations and Warranties. Except as otherwise expressly provided in this Article IV (as modified by the Company Disclosure Schedules), the Company hereby expressly disclaims and negates, any express or implied representation or warranty whatsoever (whether at Law or in equity) with respect to the Company and its Affiliates, and any matter relating to any of them, including their affairs, the condition, value or quality of the assets, liabilities, financial condition or results of operations, or with respect to the accuracy or completeness of any other information made available to Murphy, its Affiliates or any of their respective Representatives by, or on behalf of, Company, and any such representations or warranties are expressly disclaimed. Without limiting the generality of the foregoing, neither the Company nor any other Person on behalf of Company has made or makes, any representation or warranty, whether express or implied, with respect to any projections, forecasts, estimates or budgets made available to Murphy, its affiliates or any of their respective Representatives of future revenues, future results of operations (or any component thereof), future cash flows or future financial condition (or any component thereof) of the Company (including the reasonableness of the assumptions underlying any of the foregoing), whether or not included in any management presentation or in any other information made available to Murphy, its Affiliates or any of their respective Representatives or any other Person, and that any such representations or warranties are expressly disclaimed.
Section 4.29 Full Disclosure. No representation or warranty by the Company in this Agreement and no statement contained in the Company Disclosure Schedules to this Agreement or any certificate or other document furnished or to be furnished to Murphy pursuant to this Agreement, including all information furnished in writing to Murphy by or on behalf of the Company specifically for inclusion in the Registration Statement, contains any untrue statement of a material fact, or omits to state a material fact necessary to make the statements contained therein, in light of the circumstances in which they are made, not misleading.
| A-1-38 |
ARTICLE V
REPRESENTATIONS AND WARRANTIES OF MURPHY AND MERGER SUB
Except as set forth in (a) the disclosure schedules, delivered as of the date hereof, by Murphy and Merger Sub in connection with this Agreement (the “Murphy Disclosure Schedules”); (provided, however, that any disclosures made with respect to a section of this Section 4.29 shall be deemed to qualify any other section of this Section 4.29 specifically referenced or cross- referenced) and (b) the Murphy SEC Reports filed at least five days prior to the date hereof (to the extent the qualifying nature of such disclosure is readily apparent from the content of such Murphy SEC Reports, but excluding disclosures referred to in “Forward-Looking Statements”, “Risk Factors” and any other disclosures therein to the extent they are of a predictive or cautionary nature or related to forward-looking statements), Murphy and Merger Sub hereby represent and warrant to the Company as follows:
Section 5.1 Corporate Organization.
(a) Each of Murphy and Merger Sub is a corporation duly organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation and has the requisite corporate power and authority and all necessary governmental approvals to own, lease and operate its properties and to carry on its business as it is now being conducted.
(b) Merger Sub is the only Subsidiary of Murphy. Except for Merger Sub, Murphy does not directly or indirectly own any equity or similar interest in, or any interest convertible into or exchangeable or exercisable for any equity or similar interest in, any corporation, partnership, joint venture or business association or other Person.
Section 5.2 Murphy and Merger Sub Organizational Documents. Each of Murphy and Merger Sub has heretofore furnished to the Company complete and correct copies of the Murphy Organizational Documents and the Merger Sub Organizational Documents. The Murphy Organizational Documents and the Merger Sub Organizational Documents are in full force and effect. Neither Murphy nor Merger Sub is in violation of any of the provisions of the Murphy Organizational Documents and the Merger Sub Organizational Documents.
| A-1-39 |
Section 5.3 Capitalization.
(a) The authorized capital stock of Murphy consists of (x) 100,000,000 shares of Murphy Class A Common Stock, (y) 10,000,000 shares of Murphy Class B Common Stock and (z) 1,000,000 shares of preferred stock, par value $0.0001 per share (“Murphy Preferred Stock”). As of the date of this Agreement (i) 13,979,000 shares of Murphy Class A Common Stock are issued and outstanding, all of which are duly authorized, validly issued, fully paid and non- assessable and not subject to any preemptive rights, (ii) 3,306,250 shares of Murphy Class B Common Stock are issued and outstanding, all of which are validly issued, fully paid and non- assessable and not subject to any preemptive rights, (iii) no shares of Murphy Class A Common Stock or Murphy Class B Common Stock are held in the treasury of Murphy, and (iv) 13,979,000 Murphy Warrants are issued and outstanding, all of which constitute binding obligations of Murphy under the law of the jurisdiction governing the Murphy Warrants, and consisting of (A) 13,225,000 Murphy Public Warrants and (B) 754,000 Murphy Private Placement Warrants, and (v) 13,979,000 shares of Murphy Class A Common Stock are reserved for future issuance pursuant to the Murphy Warrants. There are no shares of Murphy Preferred Stock issued and outstanding. Each Murphy Public Warrant is exercisable for one share of Murphy Class A Common Stock at an exercise price of $11.50. Each Murphy Private Placement Warrant is exercisable for one share of Murphy Class A Common Stock at an exercise price of $11.50.
(b) As of the date of this Agreement, the authorized capital stock of Merger Sub is U.S. $50,000 made up of 50,000 ordinary shares (the “Merger Sub Ordinary Shares”). As of the date hereof, one Merger Sub Ordinary Share is issued and outstanding. The outstanding Merger Sub Ordinary Share has been duly authorized, validly issued, fully paid and is non- assessable and is not subject to preemptive rights, and is held by Murphy free and clear of all Liens, other than transfer restrictions under applicable Securities Laws and the Merger Sub Organizational Documents.
(c) All of the outstanding Murphy Units, shares of Murphy Common Stock and Murphy Warrants have been issued and granted in compliance with all applicable Securities Laws and other applicable Laws and were issued free and clear of all Liens other than transfer restrictions under applicable Securities Laws and the Murphy Organizational Documents.
(d) The Closing Payment Shares to be delivered by Murphy in the Merger shall be duly and validly issued, fully paid and nonassessable, and each Closing Payment Share shall be issued free and clear of preemptive rights and all Liens, other than transfer restrictions under applicable Securities Laws and the Murphy Organizational Documents. The Closing Payment Shares will be issued in compliance with all applicable securities Laws and other applicable Laws and without contravention of any other Person’s rights therein or with respect thereto.
(e) Except in connection with this Agreement and the Ancillary Agreements, no anti-takeover, “fair price”, “moratorium”, “control share acquisition”, “business combination” or other similar statute or regulation is applicable to this Agreement or the transactions contemplated hereby. Murphy does not have any shareholder rights plan or “poison pill” in effect, including any agreement with a third-party trust or fiduciary entity with respect thereto. As of the date hereof, neither Murphy nor Merger Sub own any of the capital stock of the Company.
Section 5.4 Authority Relative to This Agreement. Each of Murphy and Merger Sub has all necessary power and authority to execute and deliver this Agreement, to perform its obligations hereunder and, subject to the approval of the Murphy Proposals, to consummate the Transactions. The execution and delivery of this Agreement by each of Murphy and Merger Sub and the consummation by each of Murphy and Merger Sub of the Transactions, have been duly and validly authorized by all necessary action, and no other proceedings on the part of Murphy or Merger Sub are necessary to authorize this Agreement or to consummate the Transactions (other than with respect to the Murphy Proposals, the approval of a majority of the then-outstanding shares of Murphy Common Stock). This Agreement has been duly and validly executed and delivered by Murphy and Merger Sub and, assuming due authorization, execution and delivery by the Company, constitutes a legal, valid and binding obligation of Murphy and Merger Sub, enforceable against Murphy and Merger Sub in accordance with its terms subject to the Remedies Exceptions.
| A-1-40 |
Section 5.5 No Conflict; Required Filings and Consents.
(a) The execution and delivery of this Agreement by each of Murphy and Merger Sub do not, and the performance of this Agreement by each of Murphy and Merger Sub will not, (i) conflict with or violate the Murphy Organizational Documents or the Merger Sub Organizational Documents or (ii) result in any breach of, or constitute a default (or an event which, with notice or lapse of time or both, would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, or result in the creation of a Lien on any property or asset of each of Murphy or Merger Sub pursuant to, any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which each of Murphy or Merger Sub is a party or by which each of Murphy or Merger Sub or any of their property or assets is bound or affected.
(b) The execution and delivery of this Agreement by each of Murphy and Merger Sub do not, and the performance of this Agreement by each of Murphy and Merger Sub will not, require any consent, approval, authorization or permit of, or filing with or notification to, any Governmental Authority or conflict with or violate any Law, rule, regulation, order, judgment or decree applicable to each of Murphy or Merger Sub or by which any of their property or assets is bound or affected, except (i) for applicable requirements, if any, of the Exchange Act, Blue Sky Laws and state takeover laws, the pre-merger notification requirements of the HSR Act, and filing and recordation of appropriate merger documents as required by the Companies Act and (ii) where the failure to obtain such consents, approvals, authorizations or permits, or to make such filings or notifications, would not, individually or in the aggregate, prevent or materially delay consummation of any of the Transactions or otherwise prevent any of Murphy or Merger Sub from performing its material obligations under this Agreement.
Section 5.6 Compliance. Neither Murphy nor Merger Sub is in breach or violation of, (a) any Law applicable to Murphy or Merger Sub or by which any material property or asset of Murphy or Merger Sub is bound or affected, or (b) any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which Murphy or Merger Sub is a party or by which Murphy or Merger Sub or any property or asset of Murphy or Merger Sub is bound, except, in each case, for any such breaches or violations that do not constitute a Murphy Material Adverse Effect. Each of Murphy and Merger Sub is in possession of all material franchises, grants, authorizations, licenses, permits, easements, variances, exceptions, consents, certificates, approvals and orders of any Governmental Authority necessary for Murphy or Merger Sub to own, lease and operate its properties or to carry on its business as it is now being conducted.
| A-1-41 |
Section 5.7 Murphy SEC Documents and Financial Statements.
(a) Murphy has filed all forms, reports, schedules, statements and other documents, including any exhibits thereto, required to be filed or furnished by Murphy with the SEC since Murphy’s formation under the Exchange Act or the Securities Act, together with any amendments, restatements or supplements thereto, and will file all such forms, reports, schedules, statements and other documents required to be filed subsequent to the date of execution hereof by the Parties (the “Additional Murphy SEC Documents”). Murphy has made available to the Company copies in the form filed with the SEC of all of the following, except to the extent available in full without redaction on the SEC’s website through EDGAR for at least two (2) days prior to the date of this Agreement: (i) Murphy’s Quarterly Reports on Form 10-Q for each fiscal quarter of Murphy beginning with the first quarter Murphy was required to file such a form, (ii) all proxy statements relating to Murphy’s meetings of shareholders (whether annual or special) held, and all information statements relating to shareholder consents, since the beginning of the first fiscal quarter referred to in clause (i) above, (iii) its Form 8-Ks filed since the beginning of the first fiscal quarter referred to in clause (i) above, and (iv) all other forms, reports, registration statements and other documents (other than preliminary materials if the corresponding definitive materials have been provided to the Company pursuant to this Section 5.7(a)) filed by Murphy with the SEC since Murphy’s formation (the forms, reports, registration statements and other documents referred to in clauses (i), (ii), (iii), and (iv) above, whether or not available through EDGAR, are, collectively, the “Murphy SEC Documents”). The Murphy SEC Documents were, and the Additional Murphy SEC Documents will be, prepared in all material respects in accordance with the requirements of the Securities Act, the Exchange Act and the Sarbanes-Oxley Act, as the case may be, and the rules and regulations thereunder. The Murphy SEC Documents did not, and the Additional Murphy SEC Documents will not, at the time they were or are to be filed, as the case may be, with the SEC (except to the extent that information contained in any Murphy SEC Document or Additional Murphy SEC Document has been or is revised or superseded by a later filed Murphy SEC Document or Additional Murphy SEC Document, then on the date of such filing) contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading. As used in this Section 5.7(a), the term “file” shall be broadly construed to include any manner in which a document or information is furnished, supplied or otherwise made available to the SEC.
(b) The financial statements and notes contained or incorporated by reference in the Murphy SEC Documents and the Additional Murphy SEC Documents (collectively, the “Murphy Financial Statements”) are or will be, as the case may be, complete and accurate and fairly present in all material respects, in conformity with U.S. GAAP applied on a consistent basis in all material respects and Regulation S-X or Regulation S-K, as applicable, the financial position of Murphy as of the dates thereof and the results of operations of Murphy for the periods reflected therein. The Murphy Financial Statements are or will, as the case may be (i) prepared from the Books and Records of Murphy; (ii) prepared on an accrual basis in accordance with U.S. GAAP consistently applied; and they contain and reflect or will contain and reflect, as the case may be;
(iii) all necessary adjustments and accruals for a fair presentation of Murphy’s financial condition as of their dates; and (iv) adequate provisions for all material Liabilities for all material Taxes applicable to Murphy with respect to the periods then ended.
(c) Except as specifically disclosed, reflected or fully reserved against in the Murphy Financial Statements, and except for liabilities and obligations of a similar nature and in similar amounts incurred in the ordinary course of business since Murphy’s formation, there are no material liabilities, debts or obligations (whether accrued, fixed or contingent, liquidated or unliquidated, asserted or unasserted or otherwise) relating to Murphy. All debts and Liabilities, fixed or contingent, which should be included under U.S. GAAP on a balance sheet are or will be (as the case may be) included in Murphy Financial Statements.
| A-1-42 |
Section 5.8 Absence of Certain Changes or Events. Since its inception, except as expressly contemplated by this Agreement, there has not been any Murphy Material Adverse Effect.
Section 5.9 Absence of Litigation. As of the date of this Agreement, there is no Action pending or, to the Knowledge of Murphy, threatened in writing against Murphy, or any property or asset of Murphy, before any Governmental Authority. As of the date hereof, neither Murphy nor any material property or asset of Murphy is subject to any continuing order of, consent decree, settlement agreement or other similar written agreement with, or, to the Knowledge of Murphy, continuing investigation by, any Governmental Authority.
Section 5.10 Board Approval; Vote Required.
(a) The Board of Directors of Murphy (the “Murphy Board”), by resolutions duly adopted by majority vote of those voting at a meeting duly called and held and not subsequently rescinded or modified in any way, has duly (i) determined that this Agreement and the Transactions are fair to and in the best interests of Murphy and its stockholders, (ii) approved this Agreement and the Transactions (including the Merger) and declared their advisability, (iii) recommended that the stockholders of Murphy approve and adopt this Agreement and Merger and the other Murphy Proposals, and (iv) directed that this Agreement and the Merger and the other Murphy Proposals be submitted for consideration by the stockholders of Murphy at the Special Meeting.
(b) The only vote of the holders of any class or series of capital stock of Murphy necessary to approve the Murphy Proposals is the affirmative vote of the holders of a majority of the outstanding shares of Murphy Common Stock.
(c) The board of directors of Merger Sub by resolutions duly adopted by written consent and not subsequently rescinded or modified in any way, have duly (i) determined that this Agreement and the Merger are fair to and in the best interests of Merger Sub and its sole shareholder, (ii) approved this Agreement and the Merger and declared their advisability, and (iii) recommended that the sole shareholder of Merger Sub approve and adopt this Agreement and approve the Merger and directed that this Agreement and the Transactions be submitted for consideration by the sole shareholder of Merger Sub.
(d) The only vote of the holders of any class or series of capital stock of Merger Sub that is necessary to approve this Agreement, the Merger and the other Transactions is the affirmative vote of the holders of a majority of the outstanding Merger Sub Ordinary Shares.
Section 5.11 No Prior Operations of Merger Sub. Merger Sub was formed solely for the purpose of engaging in the Transactions and has not engaged in any business activities or conducted any operations or incurred any obligation or liability and holds no assets, other than as specifically contemplated by this Agreement.
| A-1-43 |
Section 5.12 Murphy Trust Fund. As of the date of this Agreement, Murphy has at least $134,895,000, subject to deferred underwriting fees of $4,628,750 in the trust fund established by Murphy for the benefit of its public shareholders (the “Trust Fund”) in a United States-based account maintained by Wilmington Trust (the “Trustee”) acting as trustee (the “Trust Account”), and such monies are invested in “government securities” (as such term is defined in the Investment Company Act) and held in trust by the Trustee pursuant to the Trust Agreement. There are no separate agreements, side letters or other agreements or understandings (whether written, unwritten, express or implied) that would cause the description of the Trust Agreement in the Murphy SEC Documents to be inaccurate in any material respect or, to the Parent Parties’ Knowledge, that would entitle any Person to any portion of the funds in the Trust Account. Prior to the Closing, none of the funds held in the Trust Account are permitted to be released, except in the circumstances described in the Organizational Documents of Murphy and the Trust Agreement. Murphy has performed all material obligations required to be performed by it to date under, and is not in material default or delinquent in performance or any other respect (claimed or actual) in connection with the Trust Agreement, and, to the Knowledge of Murphy, no event has occurred which, with due notice or lapse of time or both, would constitute such a material default thereunder. As of the date of this Agreement, there are no claims or proceedings pending with respect to the Trust Account. Since February 2, 2022, Murphy has not released any money from the Trust Account (other than interest income earned on the funds held in the Trust Account as permitted by the Trust Agreement). Upon the consummation of the transactions contemplated hereby, Murphy shall have no further obligation under either the Trust Agreement or the Organizational Documents of Parent to liquidate or distribute any assets held in the Trust Account, and the Trust Agreement shall terminate in accordance with its terms.
Section 5.13 Employees. Other than any officers as described in the Murphy SEC Reports, Murphy and Merger Sub have never employed any employees. Other than reimbursement of any out-of-pocket expenses incurred by Murphy’s officers and directors in connection with activities on Murphy’s behalf in an aggregate amount not in excess of the amount of cash held by Murphy outside of the Trust Account, Murphy and Merger Sub have no unsatisfied material liability with respect to any employee, officer or director.
Section 5.14 Taxes.
(a) Murphy and Merger Sub (i) have duly and timely filed (taking into account any extension of time within which to file) all material Tax Returns required by Law to be filed by Murphy or Merger Sub, and all such filed Tax Returns are complete and accurate in all material respects; (ii) have timely paid all material Taxes, whether or not shown as due on such filed Tax Returns and any other material Taxes that Murphy and Merger Sub are otherwise obligated to pay, except with respect to current Taxes not yet due and payable or otherwise being contested in good faith or that are described in clause (a)(v) below; (iii) with respect to all material Tax Returns filed by or with respect to them, have not waived any statute of limitations with respect to Taxes or agreed to any extension of time with respect to a Tax assessment or deficiency which such waiver or extension remains in effect, and no written request for any such waiver or extension is currently pending; (iv) do not have any deficiency, assessment, claim, audit, examination, investigation, litigation or other proceeding in respect of a material amount of Taxes or material Tax matters pending or proposed or threatened in writing, for a Tax period for which the statute of limitations for assessments remains open; and (v) have provided adequate reserves in accordance with GAAP in the most recent consolidated financial statements of Murphy, for any material Taxes of Murphy and Merger Sub that have not been paid, whether or not shown as being due on any Tax Return.
| A-1-44 |
(b) Neither Murphy nor Merger Sub is a party to, is bound by or has an obligation to any Governmental Authority or other Person under any Tax sharing agreement, Tax indemnification agreement, Tax allocation agreement or similar contract or arrangement (excluding agreements, contracts, arrangements or commitments the primary purpose of which do not relate to Taxes).
(c) Each of Murphy and Merger Sub has (i) withheld or collected all material Taxes required to have been withheld and paid in connection with amounts paid or owing to any current or former employee, independent contractor, creditor, shareholder or other third party, and
(ii) reported and timely remitted such amounts required to have been withheld or collected, reported and remitted to the appropriate Governmental Authority. All Forms W-2 or 1099 or other Tax Returns required with respect thereto have been properly completed and timely filed.
(d) Neither Murphy nor Merger Sub has been a member of an affiliated group filing a consolidated, combined or unitary U.S. federal, state, local or foreign income Tax Return.
(e) Neither Murphy nor Merger Sub has any material liability for the Taxes of any Person under Treasury Regulation section 1.1502-6 (or any similar provision of state, local or foreign Law), as a transferee or successor, by contract (excluding contracts the primary purpose of which do not relate to Taxes), or otherwise.
(f) Neither Murphy nor Merger Sub (i) has any request for a material ruling in respect of Taxes pending between Murphy and/or Merger Sub, on the one hand, and any Tax authority, on the other hand, or (ii) has entered into any closing agreement, private letter ruling technical advice memoranda or similar agreements with any Tax authority in respect of material Taxes.
(g) Neither Murphy nor Merger Sub has in any year for which the applicable statute of limitations remains open distributed stock of another Person, or has had its stock distributed by another Person, in a transaction that was purported or intended to be governed in whole or in part by Section 355 or Section 361 of the Code.
(h) Neither Murphy nor Merger Sub has engaged in or entered into a “listed transaction” within the meaning of Treasury Regulation Section 1.6011-4(b)(2).
(i) There are no Tax liens upon any assets of Murphy or Merger Sub except for Permitted Liens.
(j) Neither Murphy nor Merger Sub has been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code. Neither Murphy nor Merger Sub has a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise has an office or fixed place of business in a country other than the country in which it is organized.
| A-1-45 |
(k) The unpaid Taxes of Murphy and Merger Sub (i) did not, as of the date of the Financial Statements, exceed the reserve for Taxes (rather than any reserve for deferred Taxes established to reflect timing difference between book and Tax income) set forth on the face of the Financial Statements (rather than in any notes thereto), and (ii) do not exceed that reserve as adjusted for the passage of time through the Effective Time in accordance with the past custom and practice of Murphy and Merger Sub in filing its Tax Returns.
(l) To the Knowledge of Murphy and Merger Sub, Murphy and Merger Sub do not expect (i) any jurisdiction to assess any additional Taxes for any period for which Tax Returns have been filed, and (ii) any jurisdiction in which Tax Returns are not filed to require the filing of Tax Returns. To the Knowledge of Murphy and Merger Sub, within the last three (3) years, no written claim has been made by any Governmental Authority in a jurisdiction where Murphy or Merger Sub does not file a Tax Return that such entity is or may be subject to material Taxes by that jurisdiction in respect of Taxes that would be the subject of such Tax Return, which claim has not been resolved.
(m) Neither Murphy nor Merger Sub will be required to include any item or income, or exclude any item or deduction or loss from, taxable income for any taxable period (or portion thereof) ending after the Effective Time as a result of any:
(i) change in method of accounting for a taxable period (or portion thereof) ending on or prior to the Effective Time;
(ii) use of an improper method of accounting for a taxable period ending on or prior to the Effective Time;
(iii) ruling by, or written agreement with, a Governmental Authority (including any “closing agreement” as described in Section 7121 of the Code (or any corresponding provision of state, local, or non-U.S. Tax law)) for a taxable period ending on or prior to the Effective Time;
(iv) installment sale or open transaction disposition made on or prior to the Effective Time;
(v) prepaid amount received on or prior to the Effective Time;
(vi) intercompany transaction or excess loss accounts described in the Treasury Regulations promulgated under Section 1502 of the Code (or any corresponding or similar provision of state, local or foreign income Tax Law) that existed prior to the Effective Time;
(vii) election under Section 965(h) of the Code; or
(viii) election under Section 108(i) of the Code.
(n) Each of Murphy and the Merger Sub is classified as a domestic C corporation for U.S. federal income tax purposes.
| A-1-46 |
(o) Murphy has no Subsidiaries (and has not had any Subsidiary) other than
Merger Sub.
Nothing in this Section 5.14 shall be construed as providing a representation or warranty with respect to (i) any taxable period (or portion thereof) beginning after the Effective Time or (ii) the existence, amount, expiration date or limitations on (or availability of) any Tax attribute.
Section 5.15 Listing. As of the date hereof, the Murphy Units, Murphy Common Stock and Murphy Warrants are listed on the Nasdaq Stock Market, with trading symbols “MURFU”, “MURF” and “MURFW,” respectively.
Section 5.16 Investment Company Act. Murphy is not an “investment company” within the meaning of the Investment Company Act and the rules and regulations promulgated thereunder.
Section 5.17 Registration Statement. As of the time the Registration Statement becomes effective under the Securities Act, the Registration Statement (together with any amendments or supplements thereto) will not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements therein, in the light of the circumstances under which they were made, not misleading; provided, however, that Murphy makes no representations or warranties as to the information contained in or omitted from the Registration Statement in reliance upon and in conformity with information furnished in writing to Murphy by or on behalf of the Company specifically for inclusion in the Registration Statement.
Section 5.18 Contracts. Except for those contracts filed (or incorporated by reference) as exhibits to the Murphy SEC Reports, neither of Murphy or Merger Sub is party to any contract that would be required to be filed (or incorporated by reference) as an exhibit to Murphy’s Annual Report on Form 10-K pursuant to Item 601(b)(10) of Regulation S-K.
Section 5.19 Brokers. Except as set forth on Schedule 5.19 of the Murphy Disclosure Schedules, no broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission (including any deferred underwriting commission) in connection with the Transactions (including the Private Placement) based upon arrangements made by or on behalf of Murphy, Merger Sub or any other their respective Affiliates, including Sponsor.
Section 5.20 Sponsor Support Agreement. Concurrently with the execution of this Agreement, Murphy has delivered to the Company a true, correct and complete copy of the Sponsor Support Agreement. The Sponsor Support Agreement is in full force and effect and has not been withdrawn or terminated, or otherwise amended or modified, in any respect, and no withdrawal, termination, amendment or modification is contemplated by the Company or Sponsor. The Sponsor Support Agreement is a legal, valid and binding obligation of Murphy and Sponsor and neither the execution or delivery by any party thereto of, nor the performance of any party’s obligations under, the Sponsor Support Agreement violates any provision of, or results in the breach of or default under, or requires any filing, registration or qualification under, any applicable Law. No event has occurred that, with or without notice, lapse of time or both, would constitute a default or breach on the part of Murphy or Sponsor under any term or condition of the Sponsor Support Agreement.
| A-1-47 |
Section 5.21 Murphy’s and Merger Sub’s Investigation and Reliance. Each of Murphy and Merger Sub is a sophisticated purchaser and has made its own independent investigation, review and analysis regarding the Company and the Transactions, which investigation, review and analysis were conducted by Murphy and Merger Sub together with expert advisors, including legal counsel that they have engaged for such purpose. Murphy, Merger Sub and their Representatives have been provided with full and complete access to the Representatives, properties, offices, plants and other facilities, Books and Records of the Company and other information that they have requested in connection with their investigation of the Company and the Transactions. Neither of Murphy nor Merger Sub is relying on any statement, representation or warranty, oral or written, express or implied, made by the Company or any of its Representatives, except as expressly set forth in Article IV (as modified by the Company Disclosure Schedules). Neither the Company nor any of its respective Shareholders, Affiliates or Representatives shall have any liability to Murphy, Merger Sub or any of their respective Shareholders, Affiliates or Representatives resulting from the use of, and Murphy, Merger Sub and their Representatives shall not have relied upon, any information, documents or materials made available to Murphy or Merger Sub or any of their Representatives, whether orally or in writing, in any confidential information memoranda, “data rooms,” management presentations, due diligence discussions or in any other form in expectation of the Transactions. Neither the Company nor any of its Shareholders, Affiliates or Representatives is making, directly or indirectly, any representation or warranty with respect to any estimates, projections or forecasts involving the Company.
Section 5.22 Full Disclosure. No representation or warranty by Murphy or Merger Sub in this Agreement and no statement contained in the Murphy Disclosure Schedules to this Agreement or any certificate or other document furnished or to be furnished to the Company pursuant to this Agreement, including all information furnished in writing to the Company by or on behalf of Murphy or Merger Sub specifically for inclusion in the Registration Statement, contains any untrue statement of a material fact, or omits to state a material fact necessary to make the statements contained therein, in light of the circumstances in which they are made, not misleading.
ARTICLE VI
CONDUCT OF BUSINESS PENDING THE MERGER
Section 6.1 Conduct of Business by the Company Pending the Merger.
(a) Except as (i) contemplated by any other provision of this Agreement or any Ancillary Agreement, (ii) set forth in Schedule 6.1 of the Company Disclosure Schedules, (iii) taken (or omitted to be taken), as reasonably necessary, in response to COVID-19 Measures, (iv) may be requested or compelled by any Governmental Authority or (v) required by applicable Law (provided, however, that Company shall provide to the extent practicable reasonable advance notice in writing to Murphy of any action taken as a result of clauses (iii)-(v)), the Company shall not, between the date of this Agreement and the Effective Time or the earlier termination of this Agreement, directly or indirectly, do any of the following without the prior written consent of Murphy, which consent shall not be unreasonably withheld, conditioned or delayed:
(i) amend or otherwise change its or any Subsidiary’s certificate of incorporation or bylaws or equivalent organizational documents;
| A-1-48 |
(ii) issue, sell, pledge, dispose of, grant or encumber, or authorize the issuance, sale, pledge, disposition, grant or encumbrance of, any shares of any class of capital stock or other securities of the Company or of any Subsidiary or any options, warrants, convertible securities or other rights of any kind to acquire any shares of such capital stock, or any other ownership interest (including any phantom interest), of the Company or any Subsidiary;
(iii) (A) fail to maintain its or any Subsidiary’s existence; or (B) adopt or enter into a plan of complete or partial liquidation, dissolution, merger, consolidation, restructuring, recapitalization or other reorganization of the Company or any Subsidiary (other than the transactions contemplated by this Agreement);
(iv) declare, set aside, make or pay any dividend or other distribution, payable in cash, stock, property or otherwise, with respect to any Company Capital Stock;
(v) reclassify, combine, split, subdivide or redeem, or purchase or otherwise acquire, directly or indirectly, any Company Capital Stock;
(vi) (A) acquire (including by merger, consolidation, or acquisition of stock or assets or any other business combination), whether in whole or in part or via an equity or asset acquisition, any other corporation, limited liability company, partnership, other business organization or any division thereof; (B) incur or guarantee any indebtedness for borrowed money or issue any debt securities or assume, guarantee or endorse, or otherwise become responsible for, the obligations of any Person, or make any loans or advances, or intentionally grant any security interest in any of its assets, other than additional extensions or borrowings as permitted under existing credit facilities or (C) enter into any new line of business;
(vii) make any loans, advances or capital contributions to, or investments in, any other Person (including to any of its officers, directors, agents or consultants), make any material change in its existing borrowing or lending arrangements for or on behalf of such Persons, or enter into any “keep well” or similar agreement to maintain the financial condition of any other Person, except as required under any indemnification agreement to which the Company is a party as of the date hereof and which has been disclosed in the Company Disclosure Schedules;
(viii) except to the extent otherwise permitted pursuant to this Section 6.1, ( A) adopt, enter into or materially amend any Plan or any collective bargaining or similar agreement (including agreements with works councils and trade unions and side letters) to which the Company is a party or by which it is bound, (B) grant or provide any severance, termination payments, bonus, change of control, retention, or benefits to any employee of the Company, except in connection with the promotion or hiring (to the extent permitted by clause (C) of this paragraph) or separation of any employee in the ordinary course of business, (C) hire any employee of the Company or any other individual who is providing or will provide services to the Company other than any employee with an annual base salary of less than $150,000 or any employee hired to replace terminated employees in the ordinary course of business, (D) adopt, enter into or materially amend Contracts with any consultants or natural person independent contractors that involve consideration of more than $150, 000 in the aggregate or (E) take any action to accelerate the vesting, payment or funding of any cash or equity-based compensation, payment or benefit other than as contemplated by this Agreement;
| A-1-49 |
(ix) grant any material increase in the compensation, incentives or benefits payable or to become payable to any current or former director, officer, employee or consultant of the Company as of the date of this Agreement, other than (A) increases in the ordinary course of business, or (B) increases required by the terms of a Plan or applicable Law;
(x) make any change in any method of financial accounting or financial accounting principles, policies, procedures or practices, except as required by a concurrent amendment in GAAP or applicable Law made subsequent to the date hereof, as agreed to by its independent accountants;
(xi) make, change or revoke any material Tax election, adopt or change any material Tax accounting method or period, file any amendment to a material Tax Return, enter into any agreement with a Governmental Authority with respect to a material amount of Taxes, settle or compromise any examination, audit or other Action with a Governmental Authority of or relating to any material United States federal, state, local or non-United States income Tax liability, consent to any extension or waiver of the statutory period of limitations applicable to any claim or assessment in respect of Taxes, or enter into any Tax sharing or similar agreement (excluding any commercial contract not primarily related to Taxes);
(xii) take any action, or knowingly fail to take any action, which action or failure to act would reasonably be expected to prevent or impede the Transactions from qualifying for the Intended Tax Treatment;
(xiii) enter into, modify in any material respect or terminate any Contract that is (or would be if entered into prior to the date of this Agreement) a Material Contract of the type described in Section 4.17(a), other than in the ordinary course of business;
(xiv) acquire any fee interest in real property;
(xv) settle any Action (A) if such settlement would require payment by the Company in an amount greater than $200,000 or (B) to the extent such settlement is adverse to the Company and involves an Action brought by a Governmental Authority or alleged criminal wrongdoing;
(xvi) make or authorize any payment of, or accrual or commitment for, capital expenditures in excess of $100,000 for any individual capital expenditure or series of related capital expenditures or $300,000 in the aggregate;
| A-1-50 |
(xvii) enter into, renew, or materially amend, any Company Affiliate Agreement;
(xviii) make any payment, distribution, loan or other transfer of value to any Related Party, other than (A) payments to employees in the ordinary course of business, and (B) payments pursuant to Company Affiliate Agreements set forth on Schedule 4.26 of the Company Disclosure Schedules;
(xix) fail to maintain, cancel or materially change coverage under any insurance policy in form and amount equivalent in all material respects to the insurance coverage currently maintained with respect to the Company and its assets, properties and businesses;
(xx) permit any material item of Company IP to lapse or to be abandoned, invalidated, dedicated to the public, or disclaimed, or otherwise become unenforceable;
(xxi) in all respects not already set forth in this Section 6.1, fail to conduct the Company Business in the ordinary course of business consistent with past practice; or
(xxii) enter into any agreement or otherwise make a binding commitment to do any of the foregoing.
Section 6.2 Conduct of Business by Murphy Pending the Merger. From the date hereof through the Closing Date, Murphy shall remain a “blank check company” as defined under the Securities Act, and without the Company’s prior written consent (which shall not be unreasonably withheld, conditioned or delayed) shall not conduct any business operations other than in connection with this Agreement and ordinary course operations to maintain its status as a Nasdaq-listed special purpose acquisition company pending the completion of the transactions contemplated hereby. Without limiting the generality of the foregoing, from the date hereof through the Closing Date, other than in connection with the transactions contemplated by this Agreement, without the Company’s prior written consent (which shall not be unreasonably withheld, conditioned or delayed), Murphy shall not amend, waive or otherwise change the Trust Agreement in any manner adverse to Murphy.
Section 6.3 Claims Against Trust Account. The Company (on its own behalf and on behalf of the Shareholders) agrees that, notwithstanding any other provision contained in this Agreement, the Company does not now have, and shall not at any time prior to the Effective Time have, any claim to, or make any claim against, the Trust Fund, regardless of whether such claim arises as a result of, in connection with or relating in any way to, the business relationship between the Company on the one hand, and Murphy and Merger Sub on the other hand, this Agreement, or any other agreement or any other matter, and regardless of whether such claim arises based on contract, tort, equity or any other theory of legal liability (any and all such claims are collectively referred to in this Section 6.3 as the “Claims”). Notwithstanding any other provision contained in this Agreement, the Company hereby irrevocably waives any Claim it may have, now or in the future and will not seek recourse against the Trust Fund, any monies held in the Trust Account (or any distributions therefrom directly or indirectly to Murphy’s stockholders) for any reason whatsoever in respect thereof. This Section 6.3 shall survive the termination of this Agreement for any reason.
| A-1-51 |
Section 6.4 Applicable Per Share Merger Consideration. At least three (3) Business Days prior to the Closing Date, the Company shall provide Murphy with an updated version of Exhibit A that sets for the Applicable Per Share Merger Consideration for each holder of the Company Ordinary Shares, including (a) Company Ordinary Shares issued upon the Company Convertible Debt Conversion and (b) the conversion of any convertible notes that were issued following the date hereof but prior to the Closing Date pursuant to Section 6.1(a).
ARTICLE VII
ADDITIONAL AGREEMENTS
Section 7.1 Preparation of Registration Statement; Special Meeting; Company Requisite Approval.
(a) As promptly as practicable after the execution of this Agreement, (i) Murphy (with the assistance and cooperation of the Company as reasonably requested by Murphy) shall use reasonable best efforts to cause to be filed with the SEC a registration statement on Form S-4 (as amended or supplemented from time to time, and including the Proxy Statement contained therein, the “Registration Statement”) in connection with the registration under the Securities Act of the Murphy Common Stock to be issued in connection with the Merger. Murphy shall use reasonable best efforts to (i) cause the Registration Statement when filed with the SEC to comply in all material respects with all legal requirements applicable thereto, (ii) respond as promptly as reasonably practicable to and resolve all comments received from the SEC concerning the Registration Statement, and (iii) cause the Registration Statement to be declared effective under the Securities Act as promptly as practicable and (iv) keep the Registration Statement effective as long as is necessary to consummate the Transactions unless this Agreement is terminated in accordance with Article IX. The Company shall promptly provide to Murphy such information concerning the Company (including, for the avoidance of doubt, any Company financial statements) and the Shareholders as is required by the Securities Laws. As promptly as practicable after the Registration Statement is declared effective under the Securities Act, Murphy will cause the Proxy Statement to be mailed to stockholders of Murphy.
(b) Murphy agrees to include provisions in the Proxy Statement and to take reasonable action related thereto with respect to approval and adoption of (i) this Agreement, the Merger and the other Transactions, (ii) the Amended Charter, (iii) the issuance of the Murphy Common Stock in connection with the issuance of the Merger Consideration, including any required approvals under Nasdaq rules, (iv) the election of directors effective as of the Closing as contemplated by Section 2.5, and (v) any other proposals the Parties deem necessary or advisable to effectuate the Merger and the other Transactions (collectively, the “Murphy Proposals”).
| A-1-52 |
(c) No filing of, or amendment or supplement to the Proxy Statement or the Registration Statement will be made by Murphy without the approval of the Company, which shall not be unreasonably withheld, conditioned or delayed. Murphy will advise the Company, promptly after it receives notice thereof, of the time when the Registration Statement has become effective or any supplement or amendment has been filed, of the issuance of any stop order, of the suspension of the qualification of the Murphy Common Stock to be issued or issuable to the Shareholders in connection with this Agreement for offering or sale in any jurisdiction, or of any request by the SEC for amendment of the Proxy Statement or the Registration Statement or comments thereon and responses thereto or requests by the SEC for additional information. Each of Murphy and the Company shall cooperate and mutually agree upon (such agreement not to be unreasonably withheld, conditioned or delayed), any response to comments of the SEC or its staff with respect to the Registration Statement and any amendment to the Registration Statement filed in response thereto.
(d) If Murphy or the Company becomes aware that the Registration Statement, (i) as of the Effective Time, contains any untrue statement of a material fact or omits to state a material fact necessary in order to make the statements, in light of the circumstances under which they were made, not misleading, or, (ii) at any time prior to the Effective Time, information contained in the Registration Statement shall have become false or misleading in any material respect or that the Registration Statement is required to be amended in order to comply with applicable Law (including the Securities Laws), then (x) such Party shall promptly inform the other Parties and (y) Murphy, on the one hand, and the Company, on the other hand, shall cooperate and mutually agree upon (such agreement not to be unreasonably withheld, conditioned or delayed) an amendment or supplement to the Registration Statement. Murphy shall use reasonable best efforts to cause the Registration Statement, as so amended or supplemented, to be filed with the SEC and the Proxy Statement to be disseminated to the holders of shares of Murphy Common Stock, as applicable, in each case pursuant to applicable Law and subject to the terms and conditions of this Agreement and Murphy Organizational Documents.
(e) Murphy shall use reasonable best efforts to, as promptly as practicable, after the Registration Statement is declared effective by the SEC, (i) establish the record date for, duly call, give notice of, convene and hold the Special Meeting in accordance with the DGCL, (ii) cause the Proxy Statement to be disseminated to the stockholders of Murphy and (iii) solicit proxies from the stockholders of Murphy to vote in favor of each of the Murphy Proposals. Murphy shall, through the Murphy Board, recommend to its stockholders that they approve each of the Murphy Proposals (the “Murphy Board Recommendation”) and shall include the unqualified Murphy Board Recommendation in the Proxy Statement. The Murphy Board shall not (and no committee or subgroup thereof shall) change, withdraw, withhold, qualify or modify, or publicly propose to change, withdraw, withhold, qualify or modify, the Murphy Board Recommendation, except as required by Law; provided, however, that, the Murphy Board may make a withdrawal of such recommendation or an amendment, qualification or modification of such recommendation to the extent that (i) after consultation with counsel, the Murphy Board determines that a failure to make such a change would reasonably be likely to be inconsistent with its fiduciary duties under applicable Law, (ii) Murphy promptly delivers to the Company a written notice advising the Company that the Murphy Board proposes to take such action and specifying the reasons therefor, (iii) until 5:00 pm on the third Business day following the date such notice was delivered, if requested by the Company, Murphy will engage in good faith negotiations to make adjustments to the terms of this Agreement so that the need to make such change in the Murphy Board Recommendation is obviated and (iv) following such time referred to in clause (iii) above, the Murphy Board determines in good faith (after consultation with its counsel, and taking into account any modifications to this Agreement proposed by the Company prior to such time) that the failure to take such action would be inconsistent with its fiduciary duties under applicable Law. Notwithstanding the foregoing provisions of this Section 7.1(e), if on a date for which the Special Meeting is scheduled, Murphy has not received proxies representing a sufficient number of shares of Murphy Common Stock to approve, in consultation with the Company, the Murphy Proposals, whether or not a quorum is present, Murphy shall have the right to make one or more successive postponements or adjournments of the Special Meeting.
| A-1-53 |
(f) As promptly as reasonably practicable, and in any event within 10 Business Days, the Company shall use reasonable best efforts to obtain and deliver to Murphy a true, complete and correct copy of a written consent (in form and substance reasonably satisfactory to Murphy) evidencing the Company Requisite Approval that is duly executed by the Shareholders that hold at least the requisite number and class of issued and outstanding shares of Company Capital Stock required to obtain the Company Requisite Approval. If the Company Requisite Approval is obtained, then promptly following the receipt of the Company Requisite Approval, the Company will prepare and deliver to its Shareholders who have not consented the notice required by the Companies Act. Unless this Agreement has been terminated in accordance with its terms, the Company’s obligation to solicit written consents from the Shareholders to give the Company Requisite Approval in accordance with this Section 7.1(f) shall not be limited or otherwise affected by the making, commencement, disclosure, announcement or submission of any Company Acquisition Proposal. The Company shall, through the Company Board, recommend to the Shareholders that they adopt this Agreement (the “Company Board Recommendation”). The Company Board shall not (and no committee or subgroup thereof shall) change, withdraw, withhold, qualify or modify, or publicly propose to change, withdraw, withhold, qualify or modify, the Company Board Recommendation, except as required by Law.
Section 7.2 Access to Information; Confidentiality; Publicity.
(a) From the date of this Agreement until the Effective Time, the Company and Murphy shall (and shall cause their respective subsidiaries, Affiliates and Representatives to): (i) provide to the other Party (and the other Party’s officers, directors, employees, accountants, consultants, legal counsel, agents and other representatives, collectively, “Representatives”) reasonable access at reasonable times upon prior notice to the officers, employees, agents, properties, offices and other facilities of such Party and its subsidiaries and to the Books and Records thereof; and (ii) furnish promptly to the other Party such information concerning the business, properties, contracts, assets, liabilities, personnel and other aspects of such party and its subsidiaries as the other Party or its Representatives may reasonably request. Notwithstanding the foregoing, neither the Company nor Murphy shall be required to provide access to or disclose information where the access or disclosure would (i) jeopardize the protection of attorney-client privilege or contravene applicable Law or (ii) require providing access that such Party reasonably determines, in light of COVID-19 or COVID-19 Measures, would jeopardize the health and safety of any employee of such Party (it being agreed that the Parties shall use their reasonable best efforts to cause such information to be provided in a manner that would not result in such jeopardy or contravention).
(b) All information obtained by the Parties pursuant to this Section 7.2 shall be kept confidential in accordance with the confidentiality agreement, dated July 29, 2022 (the “Confidentiality Agreement”), between Murphy and the Company.
| A-1-54 |
(c) No Party hereto shall make any public announcement or issue any public communication regarding this Agreement or the Transactions, or any matter related to the foregoing, without first obtaining the prior written consent of the Company or Murphy, as applicable (which consent shall not be unreasonably withheld, conditioned or delayed), except if such announcement or other communication is required by applicable Law or legal process (including pursuant to the Securities Laws or the rules of any national securities exchange), in which case Murphy or the Company, as applicable, shall use their reasonable best efforts to coordinate such announcement or communication with the other Party, prior to announcement or issuance and allow the other Party a reasonable opportunity to comment thereon (which shall be considered by Murphy or the Company, as applicable, in good faith); provided, however, that, the foregoing shall not prohibit any Party from communicating with third parties, under confidentiality terms no less restrictive than the Confidentiality Agreement, to the extent necessary for the purpose of seeking any third party consent.
Section 7.3 Exclusivity.
(a) From the date of this Agreement until the earlier of the Closing or the termination of this Agreement in accordance with its terms, the Company shall not, and shall cause its Representatives not to, directly or indirectly: (i) solicit, initiate, encourage (including by means of furnishing or disclosing information), facilitate, discuss or negotiate, directly or indirectly, any inquiry, proposal or offer (written or oral) with respect to a Company Acquisition Proposal; (ii) furnish or disclose any non-public information to any Person in connection with, or that could reasonably be expected to lead to, a Company Acquisition Proposal; (iii) enter into any Contract or other arrangement or understanding regarding a Company Acquisition Proposal; (iv) prepare or take any steps in connection with a public or private offering of any equity securities of the Company (or any Affiliate or successor of the Company); or (v) otherwise cooperate in any way with, or assist or participate in, or knowingly facilitate or encourage any effort or attempt by any Person to do or seek to do any of the foregoing. The Company shall, and shall cause its Affiliates and Representatives to, immediately cease any and all existing discussions or negotiations with any Person (other than with Murphy, its stockholders and their Representatives) conducted prior to the date hereof with respect to, or which is reasonably likely to give rise to or result in, a Company Acquisition Proposal. The Company agrees to (A) notify Murphy promptly upon receipt of any Company Acquisition Proposal by the Company, and to describe the material terms and conditions of any such Company Acquisition Proposal in reasonable detail (including the identity of the Persons making such Company Acquisition Proposal) and (B) keep Murphy reasonably informed on a current basis of any modifications to such offer or information.
| A-1-55 |
(b) From the date of this Agreement until the earlier of the Closing or the termination of this Agreement in accordance with its terms, Murphy and Merger Sub shall not, and shall cause their Representatives not to, directly or indirectly: (i) solicit, initiate, encourage (including by means of furnishing or disclosing information), facilitate, discuss or negotiate, directly or indirectly, any inquiry, proposal or offer (written or oral) with respect to a Murphy Acquisition Proposal; (ii) furnish or disclose any non-public information to any Person in connection with, or that could reasonably be expected to lead to, a Murphy Acquisition Proposal; (iii) enter into any Contract or other arrangement or understanding regarding a Murphy Acquisition Proposal; (iv) prepare or take any steps in connection with a public or private offering of any equity securities of Murphy (or any Affiliate or successor of Murphy); or (v) otherwise cooperate in anyway with, or assist or participate in, or knowingly facilitate or encourage any effort or attempt by any Person to do or seek to do any of the foregoing. Murphy and Merger Sub shall, and shall cause their Affiliates and Representatives to, immediately cease any and all existing discussions or negotiations with any Person (other than with the Company, its stockholders and their Representatives) conducted prior to the date hereof with respect to, or which is reasonably likely to give rise to or result in, a Murphy Acquisition Proposal. Murphy and Merger Sub agree to (A) notify the Company promptly upon receipt of any Murphy Acquisition Proposal by Murphy (or any Affiliate or successor of Murphy), and to describe the material terms and conditions of any such Murphy Acquisition Proposal in reasonable detail (including the identity of the Persons making such Murphy Acquisition Proposal) and (B) keep the Company reasonably informed on a current basis of any modifications to such offer or information.
| A-1-56 |
Section 7.4 Employment Agreements. The Company and Murphy shall cooperate in good faith to negotiate new employment agreements with the persons listed on Schedule 7.4 of the Company Disclosure Schedule, to be effective on the Closing Date, and subject to the terms identified thereon.
Section 7.5 Directors’ and Officers’ Indemnification.
(a) The Parties agree that all rights to exculpation, indemnification and advancement of expenses existing in favor of the current or former directors and officers of Murphy, Merger Sub or the Company and each Person who served as a director, officer, member, trustee or fiduciary of another corporation, partnership, joint venture, trust, pension or other employee benefit plan or enterprise at the request of Murphy, Merger Sub or the Company (the “D&O Indemnified Persons”) as provided in their respective organizational documents or under any indemnification, employment or other similar agreements between any D&O Indemnified Person and Murphy, Merger Sub or the Company, in each case as in effect on the date of this Agreement, shall survive the Closing and continue in full force and effect in accordance with their respective terms to the extent permitted by applicable Law. Such rights shall include indemnification against any costs or expenses (including reasonable attorneys’ fees), judgments, fines, losses, claims, damages, or liabilities, whether asserted or claimed prior to or after the Effective Time and whether the claim involves the enforcement of the terms of this Section 7.5(a). For a period of six (6) years after the Effective Time, Murphy shall cause the Amended Charter and the Surviving Corporation Articles of Incorporation to contain provisions no less favorable with respect to exculpation and indemnification of and advancement of expenses to D&O Indemnified Persons than are set forth as of the date of this Agreement in the Murphy Organizational Documents and Company Organizational Documents to the extent permitted by applicable Law. The provisions of this Section 7.5 shall survive the consummation of the Merger and are intended to be for the benefit of, and shall be enforceable by, each of the D&O Indemnified Persons and their respective heirs and representatives.
(b) For a period of six (6) years after the Effective Time, the Surviving Corporation shall maintain in effect directors’ and officers’ liability (“D&O Insurance”) covering those persons who are (i) currently covered by the Company’s and officers’ liability insurance policy and (ii) at or after the Closing Date on the board of directors of the Surviving Corporation (true, correct and complete copies of which have been heretofore made available to the Company or its agents and Representatives) (the “Company D&O Insurance”) on terms no less favorable than the terms of such current insurance coverage.
| A-1-57 |
(c) For the benefit of Murphy’s, Merger Sub’s and the Company’s directors and officers, Murphy and the Company shall be permitted prior to the Effective Time to obtain and fully pay the premium for a “tail” insurance policy that provides coverage for up to a six-year period from and after the Effective Time for events occurring prior to the Effective Time (the “D&O Tail Insurance”) that is substantially equivalent to and in any event not less favorable in the aggregate than Murphy’s existing policy or, if substantially equivalent insurance coverage is unavailable, the best available coverage. If obtained, Murphy shall maintain the D&O Tail Insurance in full force and effect, and continue to honor the obligations thereunder, and Murphy shall timely pay or caused to be paid all premiums with respect to the D&O Tail Insurance.
(d) Notwithstanding anything contained in this Agreement to the contrary, this Section 7.5 shall survive the consummation of the Merger indefinitely and shall be binding, jointly and severally, on Murphy and the Surviving Corporation and all successors and assigns of Murphy and the Surviving Corporation. In the event that Murphy, the Surviving Corporation or any of their respective successors or assigns consolidates with or merges into any other Person and shall not be the continuing or surviving corporation or entity of such consolidation or merger or transfers or conveys all or substantially all of its properties and assets to any Person or effects any division transaction, then, and in each such case, Murphy and the Surviving Corporation shall ensure that proper provision shall be made so that the successors and assigns of Murphy or the Surviving Corporation, as the case may be, shall succeed to the obligations set forth in this Section 7.5. The obligations of Murphy and the Surviving Corporation under this Section 7.5 shall not be terminated or modified in such a manner as to materially and adversely affect any present and former director or officer of the Company or Murphy, or other Person that may be a director or officer of the Company or Murphy prior to the Effective Time, to whom this Section 7.5 applies without the consent of the affected Person. The rights of each Person entitled to indemnification or advancement hereunder shall be in addition to, and not in limitation of, any other rights such Person may have under the Company Organizational Documents, the Murphy Organizational Documents, or any other indemnification arrangement, any applicable law, rule or regulation or otherwise. The provisions of this Section 7.5 are expressly intended to benefit, and are enforceable by, each Person entitled to indemnification or advancement hereunder and their respective successors, heirs and representatives, each of whom is an intended third-party beneficiary of this Section 7.5.
Section 7.6 Transaction Litigation. In the event that any Action related to this Agreement, any Ancillary Agreement or the Transactions is brought, or to the Knowledge of Murphy, threatened in writing, against Murphy or its directors or officers by any of Murphy’s stockholders or by a Governmental Authority prior to the Closing, Murphy shall promptly notify the Company of any such Action and keep the Company reasonably informed with respect to the status thereof. Murphy shall provide the Company the opportunity to participate in (subject to a customary joint defense agreement), but not control, the defense of any such Action, shall give due consideration to the Company’s advice with respect to such litigation and shall provide the Company with a meaningful opportunity to review and give due consideration to the Company’s concerns regarding the settlement of any such Action.
| A-1-58 |
Section 7.7 Tax Matters.
(a) Each of Murphy, Merger Sub and the Company shall use their respective reasonable best efforts to cause the Merger to qualify, and agree not to, and not to permit or cause any of their Affiliates or subsidiaries to, take any action which to its Knowledge could reasonably be expected to prevent or impede the Transactions from qualifying for the Intended Tax Treatment. This Agreement is intended to constitute, and the Parties hereto hereby adopt this Agreement as, a “plan of reorganization” within the meaning of Treasury Regulation Sections 1.368-2(g) and 1.368-3(a). Each of Murphy, Merger Sub and the Company shall report the Transactions in a manner consistent with the Intended Tax Treatment unless otherwise required pursuant to a “determination” within the meaning of Section 1313(a) of the Code, including attaching the statement described in Treasury Regulations Section 1.368-3(a) on or with its U.S. federal income Tax Return for the taxable year of the Merger.
(b) The Company, Murphy and Merger Sub hereby adopt this Agreement as a “plan of reorganization” within the meaning of Treasury Regulation Sections 1.368-2(g) and 1.368-3(a).
(c) Murphy and the Merger Sub will cause the Company to continue the Company’s historic business or use a significant portion of the Company’s historic business assets in a business within the meaning of Section 1.368-1(d) of the Treasury Regulations.
(d) If, in connection with the preparation and filing of the Registration Statement/Proxy Statement, if the SEC requires that an opinion of counsel be provided with respect to the tax treatment of the Transactions, Murphy and the Company shall deliver to counsel for both Parties, respectively, customary Tax representation letters satisfactory to each counsel, dated and executed as of the date the Registration Statement shall have been declared effective by the SEC and such other date(s) as determined reasonably necessary by such counsel. Notwithstanding the foregoing or anything to the contrary herein, neither Sichenzia Ross Ference LLP nor Thompson Hine LLP shall be required to provide a tax opinion with respect to the Intended Tax Treatment. In addition, as the Company is a non-U.S. corporation without a sufficient nexus to the United States, including having no U.S. shareholders or operations, notwithstanding anything in this Agreement or any Ancillary Document to the contrary, the Company and its Affiliates, representatives, and advisors shall not be required to provide a Tax opinion with respect to the Intended Tax Treatment.
(e) Murphy and the Company acknowledge that the Merger is not conditioned on the receipt of a Tax opinion that the Merger qualifies as a reorganization within the meaning of Section 368(a) of the Code. Any filings with the SEC in connection with the Merger, including the Registration Statement/Proxy Statement, shall include a statement indicating that the Merger is not conditioned on the receipt of a Tax opinion that opines that the Merger qualifies as a reorganization within the meaning of Section 368(a) of the Code. The Company is organized outside the United States and has no operations in the United States. None of the shareholders of the Company are “United States persons” within the meaning of Section 7701(a)(30) of the Code. Thus, even if the Merger were a taxable transaction for U.S. tax purposes, neither the Company nor any of its shareholders would have any U.S. tax liability resulting from the Merger.
(f) Each of the Parties shall (and shall cause their respective Affiliates to) cooperate fully, as and to the extent reasonably requested by another Party, in connection with the filing of any relevant Tax Returns, and any audit, examination or other proceeding with respect to Taxes.
| A-1-59 |
(g) Murphy shall be responsible for any sales, use, real property transfer, stamp or other similar transfer Taxes imposed in connection with the Transactions and the preparation and filing of any Tax Returns required to be filed with respect thereto.
Section 7.8 Stock Exchange Listing. Murphy will use reasonable best efforts to cause the Murphy Common Stock issued in connection with the Transactions to be approved for listing on Nasdaq (and the Company shall reasonably cooperate in connection therewith), subject to official notice of issuance, in each case, as promptly as reasonably practicable after the date of this Agreement, and in any event prior to the Effective Time. During the period from the date hereof until the Closing, Murphy shall use reasonable best efforts to keep the Murphy Common Stock and Murphy Warrants listed for trading on Nasdaq.
Section 7.9 Murphy Public Filings. From the date hereof through the Closing, Murphy will use reasonable best efforts to keep current and timely file all reports required to be filed or furnished with the SEC and otherwise to comply in all material respects with its reporting obligations under applicable Securities Laws.
Section 7.10 Efforts to Consummate; Antitrust; Regulatory Approvals.
(a) Upon the terms and subject to the conditions of this Agreement, each of the Parties hereto shall use reasonable best efforts to take, or cause to be taken, appropriate action, and to do, or cause to be done, such things as are necessary, proper or advisable under applicable Laws or otherwise to consummate and make effective the Transactions, including using reasonable best efforts to obtain all permits, consents, approvals, authorizations, qualifications and orders of Governmental Authorities and parties to contracts with the Company necessary for the consummation of the Transactions and to fulfill the conditions to the Merger. In case, at any time after the Effective Time, any further action is necessary or desirable to carry out the purposes of this Agreement, the proper officers and directors of each Party shall use their reasonable best efforts to take all such action.
(b) Each of the Parties shall keep each other apprised of the status of matters relating to the Transactions, including promptly notifying the other Parties of any communication it or any of its Affiliates receives from any Governmental Authority relating to the matters that are the subject of this Agreement and permitting the other Parties to review in advance, and to the extent practicable consult about, any proposed communication by such Party to any Governmental Authority in connection with the Transactions. No Party shall agree to participate in any meeting with any Governmental Authority in respect of any filings, investigation or other inquiry unless it consults with the other Parties in advance and, to the extent permitted by such Governmental Authority, gives the other Parties the opportunity to attend and participate at such meeting. Subject to the terms of the Confidentiality Agreement, the Parties will coordinate and cooperate fully with each other in exchanging such information and providing such assistance as the other Parties may reasonably request in connection with the foregoing. Subject to the terms of the Confidentiality Agreement, the Parties will provide each other with copies of all material correspondence, filings or communications, including any documents, information and data contained therewith, between them or any of their Representatives, on the one hand, and any Governmental Authority or members of its staff, on the other hand, with respect to this Agreement and the Transactions. No Party shall take or cause to be taken any action before any Governmental Authority that is inconsistent with or intended to delay its action on requests for a consent or the consummation of the Transactions.
| A-1-60 |
(c) To the extent required under any Laws that are designed to prohibit, restrict or regulate actions having the purpose or effect of monopolization or restraint of trade, including the HSR Act (“Antitrust Laws”), each Party agrees to promptly, but in no event more than fifteen (15) Business Days after the date of this Agreement, make any required filing or application under Antitrust Laws, as applicable. The Parties agree to supply as promptly as reasonably practicable any additional information and documentary material that may be requested pursuant to Antitrust Laws and to take all other actions necessary, proper or advisable to cause the expiration or termination of the applicable waiting periods or obtain required approvals, as applicable under Antitrust Laws as soon as practicable, including by requesting early termination of the waiting period provided for under the HSR Act.
(d) Each Party shall, in connection with its efforts to obtain all requisite approvals and authorizations for the Transactions under any Antitrust Law, use reasonable best efforts to: (i) cooperate in all respects with each other Party or its Affiliates in connection with any filing or submission and in connection with any investigation or other inquiry, including any proceeding initiated by a private person; (ii) keep the other Parties reasonably informed of any communication received by such Party or its Representatives from, or given by such Party or its Representatives to, any Governmental Authority and of any communication received or given in connection with any proceeding by a private person, in each case regarding any of the Transactions; (iii) permit a Representative of the other Parties and their respective outside counsel to review any communication given by it to, and consult with each other in advance of any meeting or conference with, any Governmental Authority or, in connection with any proceeding by a private person, with any other Person, and to the extent permitted by such Governmental Authority or other Person, give a Representative or Representatives of the other Parties the opportunity to attend and participate in such meetings and conferences; (iv) in the event a Party’s Representative is prohibited from participating in or attending any meetings or conferences, the other Parties shall keep such Party promptly and reasonably apprised with respect thereto; and (v) use reasonable best efforts to cooperate in the filing of any memoranda, white papers, filings, correspondence or other written communications explaining or defending the Transactions, articulating any regulatory or competitive argument, and/or responding to requests or objections made by any Governmental Authority.
(e) Each of Murphy and the Company shall not, and shall cause its respective subsidiaries (as applicable) not to, acquire or agree to acquire, by merging with or into or consolidating with, or by purchasing a portion of the assets of or equity in, or by any other manner, any business or any corporation, partnership, association or other business organization or division thereof, or otherwise acquire or agree to acquire any assets, or take any other action, if the entering into of a definitive agreement relating to, or the consummation of such acquisition, merger or consolidation, or the taking of any other action, would reasonably be expected to: (i) impose any delay in the obtaining of, or increase the risk of not obtaining, any authorizations, consents, orders or declarations of any Governmental Authorities or the expiration or termination of any applicable waiting period; (ii) increase the risk of any Governmental Authority entering an order prohibiting the consummation of the Transactions; (iii) increase the risk of not being able to remove any such order on appeal or otherwise; or (iv) delay or prevent the consummation of the Transactions.
| A-1-61 |
(f) The Parties further covenant and agree, with respect to a threatened or pending preliminary or permanent injunction or other order, decree or ruling or statute, rule, regulation or executive order that would adversely affect the ability of the Parties to consummate the Transactions, to use reasonable best efforts to prevent or lift the entry, enactment or promulgation thereof, as the case may be.
(g) The Company will pay all filing fees related to Antitrust Laws required by the Transactions.
Section 7.11 Trust Account. Prior to or at the Closing (subject to the satisfaction or waiver of the conditions set forth in Article VIII), Murphy shall provide notice to the Trustee in accordance with the Trust Agreement and shall deliver any other documents, opinions or notices required to be delivered to the Trustee pursuant to the Trust Agreements so that the Trustee shall make appropriate arrangements to cause the funds in the Trust Account to be disbursed in accordance with the Trust Agreement, for the following uses: (a) the redemption of any shares of Murphy Class A Common Stock held by a Redeeming Shareholder in connection with the Offer;
(b) the payment of the Outstanding Company Transaction Expenses and Outstanding Murphy Transaction Expenses pursuant to Section 3.5; and (c) the balance after payment and disbursement of the amounts required under the foregoing clauses (a)-(b), to be disbursed to Murphy, and thereafter shall cause the Trust Account and the Trust Agreement to terminate.
Section 7.12 Section 16 Matters. Prior to the Closing, the Murphy Board, or an appropriate committee of “non-employee directors” (as defined in Rule 16b-3 under the Exchange Act) thereof, shall adopt a resolution consistent with the interpretive guidance of the SEC so that the acquisition of Murphy Common Stock pursuant to this Agreement and the other agreements contemplated hereby, by any Person owning securities of the Company who is expected to become a director or officer (as defined under Rule 16a-1(f) under the Exchange Act) of Murphy following the Closing shall be an exempt transaction for purposes of Section 16(b) of the Exchange Act pursuant to Rule 16b-3 thereunder. Murphy shall provide the Company and such individuals with copies of any resolutions proposed to be adopted by the Murphy Board in connection with the foregoing prior to such adoption.
Section 7.13 Preparation and Delivery of PCAOB Audited Financial Statements and Interim Financial Statements. As soon as reasonably practicable following the date of this Agreement, the Company shall deliver to Murphy the PCAOB Audited Financial Statements and Interim Financial Statements.
| A-1-62 |
Section 7.14 Support of Transaction. Subject to Section 7.3, without limiting any covenant contained herein, including the obligations of the Company and Murphy with respect to the notifications, filings and applications described in Section 7.10, which obligations shall control to the extent of any conflict with the succeeding provisions of this Section 7.14, Murphy and the Company shall each, and shall each cause their respective Subsidiaries to: (a) use reasonable best efforts to assemble, prepare and file any information (and, as needed, to supplement such information) as may be reasonably necessary to obtain as promptly as reasonably practicable all governmental and regulatory consents required to be obtained in connection with the Transactions, (b) use reasonable best efforts to obtain all material consents and approvals of third parties that any of Murphy, the Company or their respective Affiliates are required to obtain in order to consummate the Transactions, including any required approvals of parties to Material Contracts with the Company, and (c) take such other action as may reasonably be necessary or as another party may reasonably request to satisfy the conditions of Article VIII or otherwise to comply with this Agreement and to consummate the Transactions as soon as practicable. Notwithstanding the foregoing, in no event shall Murphy, Merger Sub or the Company be obligated to bear (and without the consent of Murphy the Company shall not agree to bear) any material expense or pay any material fee or grant any material concession, in connection with obtaining any consents, authorizations or approvals pursuant to the terms of any Contract to which the Company is a party or otherwise in connection with the consummation of the Transactions.
Section 7.15 Notice of Certain Events. Each Party shall promptly notify the other Party of:
(a) any notice or other communication from any Person alleging that the consent of such Person is or may be required in connection with the transactions contemplated by this Agreement or any Ancillary Agreement, or that the transactions contemplated by this Agreement or the Ancillary Agreements might give rise to any Action by or on behalf of such Person or result in the creation of any Lien on any Company capital stock, any share capital or capital stock of Murphy or any of the Company’s or Murphy’s assets;
(b) any notice or other communication from any Authority in connection with the transactions contemplated by this Agreement or the Ancillary Agreements;
(c) any Actions commenced or, to such Party’s Knowledge, threatened against, relating to or involving or otherwise affecting the consummation of the transactions contemplated by this Agreement or the Ancillary Agreements;
(d) the occurrence of any fact or circumstance which constitutes or results, or might reasonably be expected to constitute or result, in a Material Adverse Change; and
(e) the occurrence of any fact or circumstance which results, or might reasonably be expected to result, in any representation or warranty made hereunder by such Party to be false or misleading in any material respect or to omit or fail to state a material fact.
ARTICLE VIII
CONDITIONS TO THE MERGER
Section 8.1 Conditions to the Obligations of Each Party. The obligations of the Company, Murphy and Merger Sub to consummate the Transactions, including the Merger, are subject to the satisfaction or waiver (where permissible) at or prior to the Closing of the following conditions:
(a) Murphy Stockholders’ Approval. The Murphy Proposals shall have been approved and adopted by the requisite affirmative vote of the stockholders of Murphy in accordance with the Proxy Statement, the DGCL, the Murphy Organizational Documents and the rules and regulations of Nasdaq.
| A-1-63 |
(b) Company Requisite Approval. The Company Requisite Approval shall have been obtained.
(c) No Order. No Governmental Authority shall have enacted, issued, promulgated, enforced or entered any Law, rule, regulation, judgment, decree, executive order or award which is then in effect and has the effect of making the Transactions, including the Merger, illegal or otherwise prohibiting consummation of the Transactions, including the Merger.
(d) No Litigation. There shall not be pending any Action by any Governmental Entity in any court of competent jurisdiction seeking to prohibit the consummation of the Merger or any other transaction contemplated by this Agreement or that would otherwise cause a Murphy Material Adverse Effect or a Company Material Adverse Effect.
(e) Antitrust Approvals and Waiting Periods. If applicable, all required filings under the HSR Act shall have been completed and any applicable waiting period (and any extension thereof) applicable to the consummation of the Transactions under the HSR Act shall have expired or been terminated, and any pre-Closing approvals or clearances reasonably required thereunder shall have been obtained.
(f) Registration Statement. The Registration Statement shall have been declared effective under the Securities Act. No stop order suspending the effectiveness of the Registration Statement shall be in effect, and no proceedings for purposes of suspending the effectiveness of the Registration Statement shall have been initiated or be threatened by the SEC.
(g) Stock Exchange Listing. The shares of Murphy Common Stock to be issued in connection with the Transactions shall have been approved for listing on Nasdaq as of the Closing Date.
(h) Ancillary Agreements. The Ancillary Agreements shall have been executed and delivered by all parties thereto.
(i) Minimum Proceeds. The aggregate cash available to Murphy at the Closing from the Trust Account and its corporate account (after giving effect to the redemption of any shares of Murphy Class A Common Stock in connection with the Murphy Proposals, but before giving effect to (i) the payment of the Outstanding Murphy Transaction Expenses, and (ii) the payment of the Outstanding Company Transaction Expenses), shall equal or exceed twenty-seven million dollars ($27,000,000).
(j) Net Tangible Assets Test. Upon the Closing, Murphy shall not have redeemed shares of Murphy Class A Common Stock in the Offer in an amount that would cause Murphy to have less than $5,000,001 of net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) under the Exchange Act).
| A-1-64 |
(k) PIPE Financing. The aggregate cash proceeds available to Murphy from the PIPE Financing and the funds remaining in the Trust Account after redemptions shall be not less than an aggregate of $27,000,000.
(l) Lock-Up. The holders of the Company Capital Stock set forth on Schedule 8.1(l) shall have entered into lock-up agreements in a form that is reasonably satisfactory to Murphy and the Company.
(m) Non-Continuing Directors and Officers. Each of the Company and Murphy shall have delivered resignation letters, in a form that is reasonably satisfactory to Murphy and the Company, for the directors and officers of each of the Company and Murphy that shall not continue in such positions following the Closing (it being understood that each of the Company and Murphy shall notify the other party of all such individuals at least three (3) Business Days prior to the Closing).
Section 8.2 Conditions to the Obligations of Murphy and Merger Sub. The obligations of Murphy and Merger Sub to consummate the Transactions, including the Merger, are subject to the satisfaction or waiver (where permissible) at or prior to the Closing of the following additional conditions:
(a) Representations and Warranties. The representations and warranties of the Company shall be true and correct in all respects (without giving effect to any limitation as to “materiality” or “Company Material Adverse Effect” or any similar limitation contained herein) on and as of the date of this Agreement and on as of the Closing Date as though made on and as of the Closing Date (except to the extent that any such representation or warranty expressly is made as of an earlier date, in which case such representation and warranty shall be so true and correct as of such specified date), except where the failures of any such representations and warranties to be so true and correct, individually or in the aggregate, have not had a Company Material Adverse Effect.
(b) Agreements and Covenants. The Company shall have performed or complied in all material respects with all agreements and covenants required by this Agreement to be performed or complied with by it on or prior to the Effective Time.
(c) Officer Certificate. The Company shall have delivered to Murphy a certificate, dated the date of the Closing, signed by an officer of the Company, certifying as to the satisfaction of the conditions specified in Section 8.2(a), Section 8.2(b) and Section 8.2(e).
(d) Consents. All approvals, consents and waivers that are listed on Schedule 8.2(d) of the Company Disclosure Schedules shall have been received, and executed counterparts thereof shall have been delivered to Murphy at or prior to the Closing.
(e) No Company Material Adverse Effect. No Company Material Adverse Effect shall have occurred between the date of this Agreement and the Closing Date that is continuing.
| A-1-65 |
(f) Shareholder Support Agreement. The Shareholder Support Agreement shall be in full force and effect, and no signatory to the Shareholder Support Agreement shall have attempted to repudiate or disclaim any of its or his obligations thereunder.
(g) Company Convertible Securities. Murphy shall have received evidence acceptable to Murphy that the Company shall have converted, terminated, extinguished and cancelled in full the Company Convertible Debt, and any other securities convertible into Company Ordinary Shares.
(h) Conduit UK Management Ltd. Conduit UK Management Ltd. will be transferred to the Company and become a wholly owned subsidiary of the Company.
(i) Assignment of Certain Intellectual Property. Application No. JP 2022- 176753 shall have been assigned to Conduit UK Management Ltd.
Section 8.3 Conditions to the Obligations of the Company. The obligations of the Company to consummate the Transactions, including the Merger, are subject to the satisfaction or waiver (where permissible) at or prior to Closing of the following additional conditions:
(a) Representations and Warranties. The representations and warranties of Murphy and Merger Sub shall be true and correct in all respects (without giving effect to any limitation as to “materiality” or “Murphy Material Adverse Effect” or any similar limitation contained herein) on and as of the date of this Agreement and on as of the Closing Date as though made on and as of the Closing Date (except to the extent that any such representation or warranty expressly is made as of an earlier date, in which case such representation and warranty shall be so true and correct as of such specified date), except where the failures of any such representations and warranties to be so true and correct, individually or in the aggregate, have not had a Murphy Material Adverse Effect.
(b) Agreements and Covenants. Murphy and Merger Sub shall have performed or complied in all material respects with all agreements and covenants required by this Agreement to be performed or complied with by it on or prior to the Effective Time.
(c) Officer Certificate. Murphy shall have delivered to the Company a certificate, dated the date of the Closing, signed by the President of Murphy, certifying as to the satisfaction of the conditions specified in Section 8.3(a), Section 8.3(b) and Section 8.3(d).
(d) Material Adverse Effect. No Murphy Material Adverse Effect shall have occurred between the date of this Agreement and the Closing Date that is continuing.
(e) Equity Incentive Plan. Murphy, in consultation with, and based on the recommendations of, a compensation consultant, shall have agreed to a new equity incentive plan for Murphy to be in effect following the Closing Date with customary market terms for comparable public companies and with an initial share reserve of no more than ten percent (10%) of Murphy’s fully diluted common stock immediately outstanding after Closing.
Section 8.4 Frustration of Conditions. None of the Parties may rely on the failure of any condition set forth in this Article VIII to be satisfied if such failure was caused by such Party’s breach of a covenant or agreement contained herein.
| A-1-66 |
ARTICLE IX
TERMINATION, AMENDMENT AND WAIVER
Section 9.1 Termination. This Agreement may be terminated and the Merger and the other Transactions may be abandoned at any time prior to the Effective Time, notwithstanding any requisite approval and adoption of this Agreement and the Transactions by the Shareholders or Murphy, as follows:
(a) by mutual written consent of Murphy and the Company;
(b) by either Murphy or the Company if the Effective Time shall not have occurred prior to May 31, 2023 (the “Outside Date”); provided, however, that this Agreement may not be terminated under this Section 9.1(b) by or on behalf of any Party that either directly or indirectly through its Affiliates is in breach or violation of any representation, warranty, covenant, agreement or obligation contained herein and such breach or violation is the principal cause of the failure of a condition set forth in Article VIII on or prior to the Outside Date;
(c) by either Murphy or the Company if any Governmental Authority in the United States or a foreign government shall have enacted, issued, promulgated, enforced or entered any permanent injunction, order, decree or ruling which has become final and nonappealable and has the effect of making consummation of the Transactions, including the Merger, illegal or otherwise preventing or prohibiting consummation of the Transactions or the Merger;
(d) by Murphy upon a breach of any representation, warranty, covenant or agreement on the part of the Company set forth in this Agreement, or if any representation or warranty of the Company shall have become untrue, in either case such that the conditions set forth in Sections 8.2(a) or 8.2(b), as applicable, would not be satisfied (“Terminating Company Breach”); provided, however, that Murphy and Merger Sub are not then in material breach of their representations, warranties, covenants or agreements in this Agreement; and provided further, that if such Terminating Company Breach is curable by the Company, Murphy may not terminate this Agreement under this Section 9.1(d) for so long as the Company continues to exercise its reasonable efforts to cure such breach, unless such breach is not cured within the earlier of (i) 20 Business Days after notice of such breach is provided by Murphy to the Company and (ii) the Outside Date;
| A-1-67 |
(e) by the Company upon a breach of any representation, warranty, covenant or agreement on the part of Murphy or Merger Sub set forth in this Agreement, or if any representation or warranty of Murphy or Merger Sub shall have become untrue, in either case such that the conditions set forth in Sections 8.3(a) or 8.3(b), as applicable, would not be satisfied (“Terminating Murphy Breach”); provided, however, that the Company has not waived such Terminating Murphy Breach and the Company is not then in material breach of its representations, warranties, covenants or agreements in this Agreement; and provided further, that, if such Terminating Murphy Breach is curable by Murphy and Merger Sub, the Company may not terminate this Agreement under this Section 9.1(e) for so long as Murphy and Merger Sub continue to exercise their reasonable efforts to cure such breach, unless such breach is not cured within the earlier of (i) 20 Business Days after notice of such breach is provided by the Company to Murphy and (ii) the Outside Date;
(f) by Murphy if there is a Company Material Adverse Effect;
(g) by the Company if there is a Murphy Material Adverse Effect;
(h) by either Murphy or the Company if any of the Murphy Proposals shall fail to receive the requisite vote for approval at the Special Meeting; or
(i) by written notice from Murphy if the Company Requisite Approval is not obtained within 10 Business Days after the date of this Agreement.
Section 9.2 Effect of Termination. In the event of the termination of this Agreement pursuant to Section 9.1, this Agreement shall forthwith become void, and there shall be no liability under this Agreement on the part of any Party, except as set forth in this Section 9.2, Article XI, and any corresponding definitions set forth in Article I, or in the case of termination subsequent to a Willful Breach of this Agreement by a Party. The provisions of Section 6.3 (Claims Against Trust Account), Section 7.2 (Access to Information; Confidentiality; Publicity), this Section 9.2 (Effect of Termination) and Article XI (General Provisions) (collectively, the “Surviving Provisions”) and the Confidentiality Agreement, and any other Section or Article of this Agreement referenced in the Surviving Provisions which is required to survive in order to give appropriate effect to the Surviving Provisions, shall in each case survive any termination of this Agreement.
Section 9.3 Expenses. Except for the Outstanding Company Transaction Expenses and the Outstanding Murphy Transaction Expenses paid at the Closing, and the expenses paid by the Parties as contemplated in Section 7.10(g), all expenses incurred in connection with this Agreement and the Transactions shall be paid by the Party incurring such expenses, whether or not the Merger or any other Transactions are consummated.
Section 9.4 Amendment. This Agreement may be amended in writing by the Parties hereto at any time prior to the Effective Time. This Agreement may not be amended except by an instrument in writing signed by each of the Parties hereto.
Section 9.5 Waiver. At any time prior to the Effective Time, (a) Murphy may (i) extend the time for the performance of any obligation or other act of the Company, (ii) waive any inaccuracy in the representations and warranties of the Company contained herein or in any document delivered by the Company pursuant hereto and (iii) waive compliance with any agreement of the Company or any condition to its own obligations contained herein, and (b) the Company may (i) extend the time for the performance of any obligation or other act of Murphy or Merger Sub, (ii) waive any inaccuracy in the representations and warranties of Murphy or Merger Sub contained herein or in any document delivered by Murphy and/or Merger Sub pursuant hereto and (iii) waive compliance with any agreement of Murphy or Merger Sub or any condition to its own obligations contained herein. Any such extension or waiver shall be valid if set forth in an instrument in writing signed by the Party or Parties to be bound thereby.
| A-1-68 |
ARTICLE X
NON-SURVIVAL OF REPRESENTATIONS AND WARRANTIES
Section 10.1 Non-Survival. All representations and warranties of Murphy, Merger Sub and the Company contained in this Agreement shall terminate as of the Closing, and no such representation or warranty shall survive the Closing.
ARTICLE XI
GENERAL PROVISIONS
Section 11.1 Notices. All notices, requests, claims, demands and other communications hereunder shall be in writing and shall be given (and shall be deemed to have been duly given upon receipt) by delivery in person, by email or by registered or certified mail (postage prepaid, return receipt requested) to the respective Parties at the following addresses (or at such other address for a Party as shall be specified in a notice given in accordance with this Section 11.1):
If to Murphy or Merger Sub, at or prior to the Closing:
4995 Murphy Canyon Road, Suite 300
San Diego, California 92123
Attention: Jack K. Heilbron, CEO
Email: jheilbron@presidiopt.com
with a copy (which shall not constitute notice) to:
Sichenzia Ross Ference LLP
1185 Avenue of the Americas, 31st Floor
New York, NY 10036
Attention: Darrin Ocasio, Esq.
E-mail: dmocasio@srf.law
If to the Company, at or prior to the Closing:
Conduit Pharmaceuticals Limited
c/o Ogier Global (Cayman) Limited
89 Nexus Way,
Camana Bay
Grand Cayman, KY1-9009 Cayman Islands
Attention: Dr. Andrew Regan; James Bligh
Email: ar@corvus.com; jb@conduitpharma.com
| A-1-69 |
with a copy (which shall not constitute notice) to:
Thompson Hine LLP 335
Madison Avenue
12th Floor
New York, NY 10017-4611
Attention: Faith Charles, Todd Mason, and Corby Baumann
Email:faith.charles@thompsonhine.com;todd.mason@thompsonhine.com;
corby.baumann@thompsonhine.com
If to the Surviving Corporation or the Company following the Closing:
Murphy Canyon Acquisition Corp.
4995 Murphy Canyon Road, Suite 300
San Diego, California 92123
Attention: Jack K. Heilbron, CEO
Email: jheilbron@presidiopt.com
Conduit Pharmaceuticals Limited
c/o Ogier Global (Cayman) Limited
89 Nexus Way, Camana Bay
Grand Cayman, KY1-9009
Cayman Islands
Attention: Dr. Andrew Regan; James Bligh
Email: ar@corvus.com; jb@conduitpharma.com
with copies (which shall not constitute notice) to:
Sichenzia Ross Ference LLP
1185 Avenue of the Americas, 31st Floor
New York, NY 10036
Attention: Darrin Ocasio, Esq.
E-mail: dmocasio@srf.law
Thompson Hine LLP
335 Madison Avenue
12th Floor
New York, NY 10017-4611
Attention: Faith Charles, Todd Mason, and Corby Baumann
Email:faith.charles@thompsonhine.com;todd.mason@thompsonhine.com;
corby.baumann@thompsonhine.com
| A-1-70 |
Section 11.2 Severability. If any term or other provision of this Agreement is invalid, illegal or incapable of being enforced by any rule of Law, or public policy, all other conditions and provisions of this Agreement shall nevertheless remain in full force and effect so long as the economic or legal substance of the Transactions is not affected in any manner materially adverse to any Party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the Parties hereto shall negotiate in good faith to modify this Agreement so as to effect the original intent of the Parties as closely as possible in a mutually acceptable manner in order that the Transactions be consummated as originally contemplated to the fullest extent possible.
Section 11.3 Entire Agreement; Assignment. This Agreement and the Ancillary Agreements constitute the entire agreement among the Parties with respect to the subject matter hereof and supersede, except as set forth in Section 7.2(b), all prior agreements and undertakings, both written and oral, among the Parties, or any of them, with respect to the subject matter hereof, except for the Confidentiality Agreement. This Agreement shall not be assigned (whether pursuant to a merger, by operation of law or otherwise) by any Party without the prior express written consent of the other Parties hereto.
Section 11.4 Parties in Interest. This Agreement shall be binding upon and inure solely to the benefit of each Party hereto, and nothing in this Agreement, express or implied, is intended to or shall confer upon any other Person any right, benefit or remedy of any nature whatsoever under or by reason of this Agreement, other than Section 7.5 (which is intended to be for the benefit of the Persons covered thereby and may be enforced by such Persons).
Section 11.5 Governing Law. This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware, without regard to any conflict of law rule or principle that would result in the application of any laws other than the laws of the State of Delaware. All Actions and proceedings arising out of or relating to this Agreement (whether in contract, tort or otherwise and whether seeking monetary or equitable relief) shall be heard and determined exclusively in the Delaware Court of Chancery (or, only if the Delaware Court of Chancery declines to accept jurisdiction over a particular matter, the Delaware Supreme Court or the United States District Court for the District of Delaware), and any appellate court from any thereof. The Parties hereto hereby (a) irrevocably submit to the exclusive jurisdiction of the aforesaid courts for themselves and with respect to their respective properties for the purpose of any Action arising out of or relating to this Agreement brought by any Party, and (b) agree not to commence any Action relating thereto except in the courts described above in Delaware, other than Actions in any court of competent jurisdiction to enforce any judgment, decree or award rendered by any such court in Delaware as described herein. Each of the Parties further agrees that notice as provided herein shall constitute sufficient service of process and the Parties further waive any argument that such service is insufficient. Each of the Parties hereby irrevocably and unconditionally waives, and agrees not to assert, by way of motion or as a defense, counterclaim or otherwise, in any Action arising out of or relating to this Agreement or the Transactions, (a) any claim that it is not personally subject to the jurisdiction of the courts in Delaware as described herein for any reason, (b) that it or its property is exempt or immune from jurisdiction of any such court or from any legal process commenced in such courts (whether through service of notice, attachment prior to judgment, attachment in aid of execution of judgment, execution of judgment or otherwise) and (c) that (i) the Action in any such court is brought in an inconvenient forum, (ii) the venue of such Action is improper or (iii) this Agreement, or the subject matter hereof, may not be enforced in or by such courts.
| A-1-71 |
Section 11.6 WAIVER OF JURY TRIAL. EACH OF THE PARTIES HERETO HEREBY WAIVES TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW ANY RIGHT IT MAY HAVE TO A TRIAL BY JURY WITH RESPECT TO ANY LITIGATION DIRECTLY OR INDIRECTLY ARISING OUT OF, UNDER OR IN CONNECTION WITH THIS AGREEMENT OR THE TRANSACTIONS. EACH OF THE PARTIES HERETO (A) CERTIFIES THAT NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THAT FOREGOING WAIVER AND (B) ACKNOWLEDGES THAT IT AND THE OTHER HERETO HAVE BEEN INDUCED TO ENTER INTO THIS AGREEMENT AND THE TRANSACTIONS, AS APPLICABLE, BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 11.6.
Section 11.7 Headings. The descriptive headings contained in this Agreement are included for convenience of reference only and shall not affect in any way the meaning or interpretation of this Agreement.
Section 11.8 Counterparts. This Agreement may be executed and delivered (including by facsimile or portable document format (.pdf) transmission) in counterparts, and by the different Parties hereto in separate counterparts, each of which when executed shall be deemed to be an original but all of which taken together shall constitute one and the same agreement.
Section 11.9 Specific Performance. The Parties agree that irreparable damage would occur if any provision of this Agreement were not performed in accordance with the terms hereof, and, accordingly, that the Parties shall, prior to a valid termination of this Agreement, be entitled to seek an injunction or injunctions to prevent breaches of this Agreement or to enforce specifically the performance of the terms and provisions hereof (including the Parties’ obligation to consummate the Merger) in the Delaware Court of Chancery (or, only if the Delaware Court of Chancery declines to accept jurisdiction over a particular matter, the Delaware Supreme Court or the United States District Court for the District of Delaware), and any appellate court from any thereof, without proof of actual damages or otherwise, in addition to any other remedy to which they are entitled at law or in equity as expressly permitted in this Agreement. Each of the Parties hereby further waives (a) any defense in any action for specific performance that a remedy at law would be adequate and (b) any requirement under any Law to post security or a bond as a prerequisite to obtaining equitable relief.
Section 11.10 Legal Representation. The Parties agree that, notwithstanding the fact that Sichenzia Ross Ference LLP may have, prior to Closing, jointly represented Murphy and/or Merger Sub in connection with this Agreement, the Ancillary Documents and the transactions contemplated hereby and thereby, and has also represented Murphy and/or its Affiliates in connection with matters other than the transaction that is the subject of this Agreement, Sichenzia Ross Ference LLP will be permitted in the future, after Closing, to represent Sponsor or its Affiliates in connection with matters in which such Persons are adverse to Murphy or any of its Affiliates, including any disputes arising out of, or related to, this Agreement. The Company, who is or has the right to be represented by independent counsel in connection with the transactions contemplated by this Agreement, hereby agree, in advance, to waive (and to cause their Affiliates to waive) any actual or potential conflict of interest that may hereafter arise in connection with Sichenzia Ross Ference LLP’s future representation of one or more of Sponsor or their respective Affiliates in which the interests of such Person are adverse to the interests of Murphy, the Company or any of their respective Affiliates, including any matters that arise out of this Agreement or that are substantially related to this Agreement or to any prior representation by Sichenzia Ross Ference LLP of Murphy, Merger Sub, or any of their respective Affiliates. The Parties acknowledge and agree that, for the purposes of the attorney-client privilege, Sponsor and Murphy shall be deemed the clients of Sichenzia Ross Ference LLP with respect to the negotiation, execution and performance of this Agreement and the Ancillary Documents. All such communications shall remain privileged after the Closing and the privilege and the expectation of client confidence relating thereto shall belong solely to Sponsor and Murphy, shall be controlled by Sponsor and Murphy and shall not pass to or be claimed by Murphy post-Closing; provided, further, that nothing contained herein shall be deemed to be a waiver by Murphy or any of its Affiliates (including, after the Effective Time) of any applicable privileges or protections that can or may be asserted to prevent disclosure of any such communications to any third party.
[signature Page Follows]
| A-1-72 |
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed as of the date first written above by their respective officers thereunto duly authorized.
| MURPHY CANYON ACQUISITION CORP. | ||
| By: | /s Jack K. Heilbron | |
| Name: | Jack K. Heilbron | |
| Title: | CEO | |
| CONDUIT MERGER SUB, INC. | ||
| By: | /s/ Jack K. Heilbron | |
| Name: | Jack K. Heilbron | |
| Title: | Director | |
| CONDUIT PHARMACEUTICALS LIMITED | ||
| By: | /s/ Dr. Andrew Regan | |
| Name: | Dr. Andrew Regan | |
| A-1-73 |
LIST OF EXHIBITS
| EXHIBIT A | Capitalization Table |
| EXHIBIT B | Form of Amended Charter |
| EXHIBIT C | Form of Amended Bylaws |
| EXHIBIT D | Form of Surviving Corporation Certificate of Incorporation and Articles and Memorandum of Association |
| A-1-74 |
Annex A-2
AMENDMENT TO AGREEMENT AND PLAN OF MERGER
THIS AMENDMENT TO AGREEMENT AND PLAN OF MERGER (this “Amendment”), dated as of January 27, 2023 (the “Effective Date”), is made and entered into by and among Murphy Canyon Acquisition Corp., a Delaware corporation (“Murphy”), Conduit Merger Sub, Inc., a Cayman Islands exempted company (“Merger Sub”), and Conduit Pharmaceuticals Limited, a Cayman Islands exempted company (the “Company”). Capitalized terms used herein and not otherwise defined will have the meanings ascribed to such terms in the Merger Agreement (as defined below).
RECITALS
A. The Parties entered into that certain Agreement and Plan of Merger on November 8, 2022 (the “Merger Agreement”).
B. Pursuant to Section 9.4 of the Merger Agreement, the Parties may amend the Merger Agreement by an agreement in writing signed by the Parties at any time prior to the Effective Time.
C. The Parties desire to amend the Merger Agreement as set forth in this Amendment.
NOW, THEREFORE, in consideration of the mutual covenants and agreements set forth herein and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, intending to be legally bound hereby, the Parties agree as follows, as of the Effective Date:
1. Amendments to the Merger Agreement.
(a) The definitions of “Exchange Ratio” and “Murphy Common Stock” are amended and restated in their entirety as follows:
“‘Exchange Ratio’ means the quotient of (a) the Merger Consideration, divided by (b) the number of shares of Company Capital Stock, divided by (c) ten dollars ($10).”
“‘Murphy Common Stock’ means, at all times prior to the Effective Time, Murphy Class A Common Stock and Murphy Class B Common Stock, collectively, and at all times as of and from the Effective Time, Murphy’s common stock, par value $0.0001 per share, as set forth in Article IV of the Amended Charter.”
(b) Section 2.5 is amended and restated in its entirety as follows:
“Section 2.5 Directors. Immediately after the Closing, the size and composition of the board of directors of each of the Surviving Corporation and Murphy shall be mutually determined by Murphy and the Company, provided that the board of directors of each of Murphy and the Surviving Corporation shall consist of no more than nine (9) directors, at least a majority of whom shall be independent directors satisfying the independence requirements of the Securities Act and the Nasdaq rules.”
(c) Section 3.1(c) is amended and restated in its entirety as follows:
“(c) Murphy Class A Common Stock and Murphy Class B Common Stock. At the Closing and immediately prior to the Effective Time, in connection with filing the Amended Charter pursuant to Section 2.4(a) hereof, each share of Murphy Class B Common Stock shall automatically convert into and become one validly issued, fully paid and non-assessable share of Murphy Class A Common Stock, and by virtue of the Merger and without any action on the part of Murphy, Merger Sub or the Company, each share of Murphy Class A Common Stock shall automatically convert into and become one validly issued, fully paid and non-assessable share of one class of Murphy common stock, par value $0.001.”
(d) Section 3.2(b) is hereby amended by deleting the reference to “Class A” such that Section 3.2(b) is amended and restated in its entirety as follows:
“(b) No certificates or scrip representing fractional Murphy Common Stock will be issued pursuant to the Merger, and such fractional share interests will not entitle the owner thereof to vote or to any rights of a shareholder of Murphy. If a holder would be entitled to receive a fractional interest in a share of Murphy Common Stock, the amount of shares of Murphy Common Stock will be rounded down to the nearest whole number of the number of shares of Murphy Common Stock to be issued to such holder.”
(e) Section 8.3(e) is hereby amended and restated in its entirety as follows:
“(e) Equity Incentive Plan. Murphy, in consultation with, and based on the recommendations of, a compensation consultant, shall have agreed to a new equity incentive plan for Murphy to be in effect following the Closing Date with customary market terms for comparable public companies and with an initial share reserve of no more than twelve and one-half percent (12.5%) of Murphy’s fully diluted common stock immediately outstanding after Closing.”
| A-2-2 |
(f) Section 7 is hereby amended by inserting the following provision at the end of such section:
“Section 7.16 Murphy shall, within fifteen (15) Business Days after the Closing, use reasonable best efforts to file with the SEC (at its sole cost and expense) a registration statement (the “Resale Registration Statement”) registering the resale of 4,060,250 shares of Murphy Common Stock held by the Sponsor or its designees and 754,000 shares of Murphy Common Stock issuable upon exercise of the Murphy Private Placement Warrants held by the Sponsor or its designees, and Murphy shall use reasonable best efforts to cause such Resale Registration Statement to become effective within 60 Business Days following the Closing. Murphy agrees that it will use its reasonable best efforts to cause such Resale Registration Statement or another registration statement (which may be a “shelf” registration statement) to remain effective until the earlier of (i) the date on which the Sponsor or its designees cease to hold the Registrable Securities covered by such Resale Registration Statement, or (iii) on the first date on which the Sponsor or its designees can sell all of its Registrable Securities under Rule 144 promulgated under the Securities Act (“Rule 144”) without limitation as to the manner of sale or the amount of such equity interests that may be sold. If the SEC prevents Murphy from including any or all of the Registrable Securities proposed to be registered for resale under the Resale Registration Statement due to limitations on the use of Rule 415 of the Securities Act for the resale of Murphy’s Registrable Securities by the applicable stockholders or otherwise, (i) such Resale Registration Statement shall register for resale such number of Murphy registrable securities which is equal to the maximum number of Murphy registrable securities as is permitted by the SEC and (ii) the number of Murphy registrable securities to be registered for each selling stockholder named in the Resale Registration Statement shall be reduced pro rata among all such selling stockholders. “Registrable Securities” shall include the shares of Murphy Common Stock referenced in the first sentence of this Section 7.16 and any other equity security of the of Murphy issued or issuable with respect to the Registrable Securities by way of share split, dividend, distribution, recapitalization, merger, exchange, replacement or similar event or otherwise, but not, for the avoidance of doubt, any other equity security of Murphy owned or acquired by the Sponsor or its designees. For as long as the Sponsor or its designees holds the Registrable Securities, Murphy shall (A) make and keep public information available, as those terms are understood and defined in Rule 144, (B) file in a timely manner all reports and other documents with the SEC required under the Exchange Act, as long as Murphy remains subject to such requirements, and (C) provide all customary and reasonable cooperation necessary, in each case, to enable Subscriber or its designees to resell the Registrable Securities pursuant to the Resale Registration Statement or Rule 144 (when Rule 144 becomes available to the Sponsor or its designees), as applicable.
(g) The Amended Charter attached as Exhibit B to the Merger Agreement is here by amended and restated in its entirety as set forth in Exhibit A to this Amendment. All references to the Amended Charter in the Merger Agreement shall refer to the Amended Charter attached hereto as Exhibit A.
2. No Further Amendments. Except as expressly modified by this Amendment, the Merger Agreement will remain unmodified and in full force and effect.
3. Counterparts. This Agreement may be executed and delivered (including by facsimile or portable document format (.pdf) transmission) in counterparts, and by the different Parties hereto in separate counterparts, each of which when executed shall be deemed to be an original but all of which taken together shall constitute one and the same agreement.
4. Conflict. To the extent there is a conflict between the terms and provisions of this Amendment and the Merger Agreement, the terms and provisions of this Amendment will govern as to such conflict.
[SIGNATURES BEGIN ON FOLLOWING PAGE]
| A-2-3 |
IN WITNESS WHEREOF, the Parties have caused this Amendment to be executed and delivered as of the Effective Date.
| MURPHY CANYON ACQUISITION CORP. | ||
| /s/ Jack K. Heilbron | ||
| By: | Jack K. Heilbron | |
| Title: | CEO | |
| CONDUIT MERGER SUB, INC. | ||
| /s/ Jack K. Heilbron | ||
| By: | Jack K. Heilbron | |
| Title: | Director | |
| CONDUIT PHARMACEUTICALS LIMITED | ||
| /s/ Andrew Regan | ||
| By: | Dr. Andrew Regan | |
| Title: | Director | |
[Signature Page to Amendment to Agreement and Plan of Merger]
| A-2-4 |
Exhibit A
Form of Amended Charter
Annex A-3
SECOND AMENDMENT TO AGREEMENT AND PLAN OF MERGER
THIS SECOND AMENDMENT TO AGREEMENT AND PLAN OF MERGER (this “Amendment”), dated as of May 11, 2023 (the “Effective Date”), is made and entered into by and among Murphy Canyon Acquisition Corp., a Delaware corporation (“Murphy”), Conduit Merger Sub, Inc., a Cayman Islands exempted company (“Merger Sub”), and Conduit Pharmaceuticals Limited, a Cayman Islands exempted company (the “Company”). Capitalized terms used herein and not otherwise defined will have the meanings ascribed to such terms in the Merger Agreement (as defined below).
RECITALS
A. The Parties entered into that certain Agreement and Plan of Merger on November 8, 2022 (the “Original Merger Agreement”).
B. The Original Merger Agreement was amended by the Amendment to Agreement and Plan of Merger (the “First Amendment”) dated as of January 27, 2023 (the Original Merger Agreement as amended by the First Amendment is referred to herein as the “Merger Agreement”).
C. Pursuant to Section 9.4 of the Merger Agreement, the Parties may amend the Merger Agreement by an agreement in writing signed by the Parties at any time prior to the Effective Time.
D. The Parties desire to amend the Merger Agreement as set forth in this Amendment.
NOW, THEREFORE, in consideration of the mutual covenants and agreements set forth herein and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, intending to be legally bound hereby, the Parties agree as follows, as of the Effective Date:
1. Amendments to the Merger Agreement.
(a) Section 7.7(d) is amended and restated in its entirety as follows:
“(d) If, in connection with the preparation and filing of the Registration Statement/Proxy Statement, if the SEC requires that an opinion of counsel be provided with respect to the tax treatment of the Transactions, Murphy and the Company shall deliver to counsel for both Parties, respectively, customary Tax representation letters satisfactory to each counsel, dated and executed as of the date the Registration Statement shall have been declared effective by the SEC and such other date(s) as determined reasonably necessary by such counsel.”
(b) Section 8.1(j) is amended and restated in its entirety as follows:
“(j) Net Tangible Assets Test. Upon the Closing, Murphy (i) shall not have redeemed shares of Murphy Class A Common Stock in the Offer in an amount that would cause Murphy to have less than $5,000,001 of net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) under the Exchange Act) or (ii) shall otherwise be exempt from the provisions of Rule 419 promulgated under the Securities Act.”
(c) Section 9.1(b) is amended and restated in its entirety as follows:
“(b) by either Murphy or the Company if the Effective Time shall not have occurred prior to February 7, 2024 (the “Outside Date”); provided, however, that this Agreement may not be terminated under this Section 9.1(b) by or on behalf of any Party that either directly or indirectly through its Affiliates is in breach or violation of any representation, warranty, covenant, agreement or obligation contained herein and such breach or violation is the principal cause of the failure of a condition set forth in Article VIII on or prior to the Outside Date;”
2. No Further Amendments. Except as expressly modified by this Amendment, the Merger Agreement will remain unmodified and in full force and effect.
3. Counterparts. This Amendment may be executed and delivered (including by facsimile or portable document format (.pdf) transmission) in counterparts, and by the different Parties hereto in separate counterparts, each of which when executed shall be deemed to be an original but all of which taken together shall constitute one and the same agreement.
4. Conflict. To the extent there is a conflict between the terms and provisions of this Amendment and the Merger Agreement, the terms and provisions of this Amendment will govern as to such conflict.
[SIGNATURES BEGIN ON FOLLOWING PAGE]
| A-3-1 |
IN WITNESS WHEREOF, the Parties have caused this Amendment to be executed and delivered as of the Effective Date.
| MURPHY CANYON ACQUISITION CORP. | ||
| /s/ Jack K. Heilbron | ||
| By: | Jack K. Heilbron | |
| Title: | CEO | |
| CONDUIT MERGER SUB, INC. | ||
| /s/ Jack K. Heilbron | ||
| By: | Jack K. Heilbron | |
| Title: | Director | |
| CONDUIT PHARMACEUTICALS LIMITED | ||
| /s/ Andrew Regan | ||
| By: | Dr. Andrew Regan | |
| Title: | Director | |
[Signature Page to Second Amendment to Agreement and Plan of Merger]
| A-3-2 |
Annex B
SECOND AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION
OF
MURPHY CANYON ACQUISITION CORP.
[ ], 2023
Murphy Canyon Acquisition Corp., a corporation organized and existing under the laws of the State of Delaware (the “Corporation”), DOES HEREBY CERTIFY AS FOLLOWS:
1. The present name of the Corporation is “Murphy Canyon Acquisition Corp”. The original certificate of incorporation of the Corporation was filed with the Secretary of State of the State of Delaware on October 19, 2021 (the “Original Certificate”). The Corporation amended and restated the Original Certificate, which was filed with the Secretary of State of the State of Delaware on February 2, 2022 (“First Amended and Restated Certificate”).
2. This Second Amended and Restated Certificate of Incorporation (the “Second Amended and Restated Certificate”), which both restates and amends the provisions of the First Amended and Restated Certificate, was duly adopted in accordance with Sections 228, 242 and 245 of the General Corporation Law of the State of Delaware, as amended from time to time (the “DGCL”).
3. This Second Amended and Restated Certificate shall become effective on the date of filing with the Secretary of State of Delaware.
4. The text of the First Amended and Restated Certificate is hereby restated and amended in its entirety to read as follows:
ARTICLE I
NAME
The name of the corporation is Conduit Pharmaceuticals Inc. (the “Corporation”).
ARTICLE II
PURPOSE
The purpose of the Corporation is to engage in any lawful act or activity for which corporations may be organized under the DGCL. The Corporation is to have a perpetual existence.
ARTICLE III
REGISTERED AGENT
The address of the Corporation’s registered office in the State of Delaware is 1209 Orange Street, Wilmington, Delaware 19801, New Castle County. The name of the Corporation’s registered agent at such address is National Registered Agents, Inc.
ARTICLE IV
CAPITALIZATION
Section 4.1 Authorized Capital Stock. The total number of shares of all classes of capital stock, each with a par value of $0.0001 per share, which the Corporation is authorized to issue is 251,000,000 shares, consisting of (a) 250,000,000 shares of common stock (the “Common Stock”), and (b) 1,000,000 shares of preferred stock (the “Preferred Stock”). Upon the filing and effectiveness of the Second Amended and Restated Certificate (the “Effective Time”), each share of Class A Common Stock, par value $0.0001 per share, of the Corporation issued and outstanding immediately prior to the Effective Time shall, automatically and without any further action by the Corporation or any stockholder, be reclassified into one fully paid and nonassessable share of Common Stock. The number of authorized shares of Common Stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority in voting power of the stock of the Corporation with the power to vote thereon irrespective of the provisions of Section 242(b)(2) of the DGCL or any successor provision thereof, and no vote of the holders of any of the Common Stock or Preferred Stock voting separately as a class shall be required therefor.
Section 4.2 Preferred Stock. The Board of Directors of the Corporation (the “Board”) is hereby expressly authorized to provide out of the unissued shares of the Preferred Stock for one or more series of Preferred Stock and to establish from time to time the number of shares to be included in each such series and to fix the voting rights, if any, designations, powers, preferences and relative, participating, optional, special and other rights, if any, of each such series and any qualifications, limitations and restrictions thereof, as shall be stated in the resolution or resolutions adopted by the Board providing for the issuance of such series and included in a certificate of designation (a “Preferred Stock Designation”) filed pursuant to the DGCL, and the Board is hereby expressly vested with the authority to the full extent provided by law, now or hereafter, to adopt any such resolution or resolutions.
Section 4.3 Common Stock.
(a) Voting.
(i) Except as otherwise required by law or this Second Amended and Restated Certificate (including any Preferred Stock Designation), the holders of the Common Stock shall exclusively possess all voting power with respect to the Corporation.
(ii) Except as otherwise required by law or this Second Amended and Restated Certificate (including any Preferred Stock Designation), the holders of shares of Common Stock shall be entitled to one vote for each such share on each matter properly submitted to the stockholders on which the holders of the Common Stock are entitled to vote.
(iii) Except as otherwise required by law or this Second Amended and Restated Certificate (including any Preferred Stock Designation), at any annual or special meeting of the stockholders of the Corporation, holders of Common Stock, shall have the exclusive right to vote for the election of directors and on all other matters properly submitted to a vote of the stockholders. Notwithstanding the foregoing, except as otherwise required by law or this Second Amended and Restated Certificate (including any Preferred Stock Designation), holders of shares of Common Stock shall not be entitled to vote on any amendment to this Second Amended and Restated Certificate (including any amendment to any Preferred Stock Designation) that relates solely to the terms of one or more outstanding series of Preferred Stock if the holders of such affected series of Preferred Stock are entitled exclusively, either separately or together with the holders of one or more other such series, to vote thereon pursuant to this Second Amended and Restated Certificate (including any Preferred Stock Designation) or the DGCL.
(b) Dividends. Subject to applicable law and the rights, if any, of the holders of any outstanding series of the Preferred Stock, the holders of shares of Common Stock shall be entitled to receive such dividends and other distributions (payable in cash, property or capital stock of the Corporation) when, as and if declared thereon by the Board from time to time out of any assets or funds of the Corporation legally available therefor and shall share equally on a per share basis in such dividends and distributions.
(c) Liquidation, Dissolution or Winding Up of the Corporation. Subject to applicable law and the rights, if any, of the holders of any outstanding series of the Preferred Stock, in the event of any voluntary or involuntary liquidation, dissolution or winding up of the Corporation, after payment or provision for payment of the debts and other liabilities of the Corporation, the holders of shares of Common Stock shall be entitled to receive all the remaining assets of the Corporation available for distribution to its stockholders, ratably in proportion to the number of shares of Common Stock held by them.
Section 4.4 Rights and Options. The Corporation has the authority to create and issue rights, warrants and options entitling the holders thereof to acquire from the Corporation any shares of its capital stock of any class or classes, with such rights, warrants and options to be evidenced by or in instrument(s) approved by the Board. The Board is empowered to set the exercise price, duration, times for exercise and other terms and conditions of such rights, warrants or options; provided, however, that the consideration to be received for any shares of capital stock issuable upon exercise thereof may not be less than the par value thereof.
| B-2 |
ARTICLE V
BOARD OF DIRECTORS
Section 5.1 Board Powers. The business and affairs of the Corporation shall be managed by, or under the direction of, the Board. In addition to the powers and authority expressly conferred upon the Board by statute, this Second Amended and Restated Certificate or the By-Laws of the Corporation (“By-Laws”), the Board is hereby empowered to exercise all such powers and do all such acts and things as may be exercised or done by the Corporation, subject, nevertheless, to the provisions of the DGCL, this Second Amended and Restated Certificate, and any By-Laws adopted by the stockholders; provided, however, that no By-Laws hereafter adopted by the stockholders shall invalidate any prior act of the Board that would have been valid if such By-Laws had not been adopted.
Section 5.2 Number, Election and Term.
(a) The number of directors which shall constitute the entire Board shall be the number of directors as fixed from time to time in accordance with the By-Laws, a majority of whom shall be independent directors in accordance with The Nasdaq Stock Market LLC’s requirements.
(b) A director shall hold office until the annual meeting for the year in which his or her term expires and until his or her successor has been elected and qualified, subject, however, to such director’s earlier death, resignation, retirement, disqualification or removal.
(c) Unless and except to the extent that the By-Laws shall so require, the election of directors need not be by written ballot. The holders of shares of Common Stock shall not have cumulative voting rights.
Section 5.3 Newly Created Directorships and Vacancies. Subject to Section 5.5 hereof, newly created directorships resulting from an increase in the number of directors and any vacancies on the Board resulting from death, resignation, retirement, disqualification, removal or other cause may be filled solely and exclusively by a majority vote of the remaining directors then in office, even if less than a quorum, or by a sole remaining director (and not by stockholders), and any director so chosen shall hold office for the remainder of the term of the class of directors to which the new directorship was added or in which the vacancy occurred and until his or her successor has been elected and qualified, subject, however, to such director’s earlier death, resignation, retirement, disqualification or removal.
Section 5.4 Removal. Subject to Sections 5.5 hereof and except as otherwise required by this Second Amended and Restated Certificate, any or all of the directors may be removed from office at any time, but only for cause and only by the affirmative vote of holders of a majority of the voting power of all then outstanding shares of capital stock of the Corporation entitled to vote generally in the election of directors, voting together as a single class.
Section 5.5 Preferred Stock - Directors. Notwithstanding any other provision of this Article V, and except as otherwise required by law, whenever the holders of one or more series of the Preferred Stock shall have the right, voting separately by class or series, to elect one or more directors, the term of office, the filling of vacancies, the removal from office and other features of such directorships shall be governed by the terms of such series of the Preferred Stock as set forth in this Second Amended and Restated Certificate (including any Preferred Stock Designation) and such directors shall not be included in any of the classes created pursuant to this Article V unless expressly provided by such terms.
| B-3 |
ARTICLE VI
BYLAWS
In furtherance and not in limitation of the powers conferred upon it by law, the Board shall have the power and is expressly authorized to adopt, amend, alter or repeal the By-Laws. The affirmative vote of a majority of the Board shall be required to adopt, amend, alter or repeal the By-Laws. The By-Laws also may be adopted, amended, altered or repealed by the stockholders; provided, however, that in addition to any vote of the holders of any class or series of capital stock of the Corporation required by law or by this Second Amended and Restated Certificate (including any Preferred Stock Designation), the affirmative vote of the holders of at least a majority of the voting power of all then outstanding shares of capital stock of the Corporation entitled to vote generally in the election of directors, voting together as a single class, shall be required for the stockholders to adopt, amend, alter or repeal the By-Laws; and provided further, however, that no By-Laws hereafter adopted by the stockholders shall invalidate any prior act of the Board that would have been valid if such By-Laws had not been adopted.
ARTICLE VII
MEETINGS OF STOCKHOLDERS; ACTION BY WRITTEN CONSENT
Section 7.1 Meetings. Subject to the rights, if any, of the holders of any outstanding series of the Preferred Stock, and to the requirements of applicable law, special meetings of stockholders of the Corporation may be called only by the Chairman of the Board, the Chief Executive Officer of the Corporation, or the Board pursuant to a resolution adopted by a majority of the Board, and the ability of the stockholders of the Corporation to call a special meeting is hereby specifically denied. Except as provided in the foregoing sentence, special meetings of stockholders of the Corporation may not be called by another person or persons.
Section 7.2 Advance Notice. Advance notice of stockholder nominations for the election of directors and of business to be brought by stockholders before any meeting of the stockholders of the Corporation shall be given in the manner provided in the By-Laws.
Section 7.3 No Action by Written Consent. Any action required or permitted to be taken by the stockholders of the Corporation shall be taken at an annual or special meeting of stockholders of the Corporation and shall not be taken by any consent in writing by such stockholders.
ARTICLE VIII
LIMITED LIABILITY; INDEMNIFICATION
Section 8.1 Limitation of Director Liability. A director of the Corporation shall not be personally liable to the Corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except to the extent such exemption from liability or limitation thereof is not permitted under the DGCL as the same exists or may hereafter be amended unless they violated their duty of loyalty to the Corporation or its stockholders, acted in bad faith, knowingly or intentionally violated the law, authorized unlawful payments of dividends, unlawful stock purchases or unlawful redemptions, or derived improper personal benefit from their actions as directors. Any amendment, modification or repeal of the foregoing sentence shall not adversely affect any right or protection of a director of the Corporation hereunder in respect of any act or omission occurring prior to the time of such amendment, modification or repeal.
| B-4 |
Section 8.2 Indemnification and Advancement of Expenses.
(a) To the fullest extent permitted by applicable law, as the same exists or may hereafter be amended, the Corporation shall indemnify and hold harmless each person who is or was made a party or is threatened to be made a party to or is otherwise involved in any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (a “proceeding”) by reason of the fact that he or she is or was a director or officer of the Corporation or, while a director or officer of the Corporation, is or was serving at the request of the Corporation as a director, officer, employee or agent of another corporation or of a partnership, joint venture, trust, other enterprise or nonprofit entity, including service with respect to an employee benefit plan (an “indemnitee”), whether the basis of such proceeding is alleged action in an official capacity as a director, officer, employee or agent, or in any other capacity while serving as a director, officer, employee or agent, against all liability and loss suffered and expenses (including, without limitation, attorneys’ fees, judgments, fines, ERISA excise taxes and penalties and amounts paid in settlement) reasonably incurred by such indemnitee in connection with such proceeding. The Corporation shall to the fullest extent not prohibited by applicable law pay the expenses (including attorneys’ fees) incurred by an indemnitee in defending or otherwise participating in any proceeding in advance of its final disposition; provided, however, that, to the extent required by applicable law, such payment of expenses in advance of the final disposition of the proceeding shall be made only upon receipt of an undertaking, by or on behalf of the indemnitee, to repay all amounts so advanced if it shall ultimately be determined that the indemnitee is not entitled to be indemnified under this Section 8.2 or otherwise. The rights to indemnification and advancement of expenses conferred by this Section 8.2 shall be contract rights and such rights shall continue as to an indemnitee who has ceased to be a director, officer, employee or agent and shall inure to the benefit of his or her heirs, executors and administrators. Notwithstanding the foregoing provisions of this Section 8.2(a), except for proceedings to enforce rights to indemnification and advancement of expenses, the Corporation shall indemnify and advance expenses to an indemnitee in connection with a proceeding (or part thereof) initiated by such indemnitee only if such proceeding (or part thereof) was authorized by the Board.
(b) The rights to indemnification and advancement of expenses conferred on any indemnitee by this Section 8.2 shall not be exclusive of any other rights that any indemnitee may have or hereafter acquire under law, this Second Amended and Restated Certificate, the By-Laws, an agreement, vote of stockholders or disinterested directors, or otherwise.
(c) Any repeal or amendment of this Section 8.2 by the stockholders of the Corporation or by changes in law, or the adoption of any other provision of this Second Amended and Restated Certificate inconsistent with this Section 8.2, shall, unless otherwise required by law, be prospective only (except to the extent such amendment or change in law permits the Corporation to provide broader indemnification rights on a retroactive basis than permitted prior thereto), and shall not in any way diminish or adversely affect any right or protection existing at the time of such repeal or amendment or adoption of such inconsistent provision in respect of any proceeding (regardless of when such proceeding is first threatened, commenced or completed) arising out of, or related to, any act or omission occurring prior to such repeal or amendment or adoption of such inconsistent provision.
(d) This Section 8.2 shall not limit the right of the Corporation, to the extent and in the manner authorized or permitted by law, to indemnify and to advance expenses to persons other than indemnitees.
ARTICLE IX
BUSINESS COMBINATIONS
The Corporation will not be subject to Section 203 of the DGCL.
ARTICLE X
CORPORATE OPPORTUNITY
To the extent allowed by law, the doctrine of corporate opportunity, or any other analogous doctrine, shall not apply with respect to the Corporation or any of its officers or directors, or any of their respective affiliates, in circumstances where the application of any such doctrine would conflict with any fiduciary duties or contractual obligations they may have as of the date of this Second Amended and Restated Certificate or in the future, and the Corporation renounces any expectancy that any of the directors or officers of the Corporation will offer any such corporate opportunity of which he or she may become aware to the Corporation, except, the doctrine of corporate opportunity shall apply with respect to any of the directors or officers of the Corporation with respect to a corporate opportunity that was offered to such person solely in his or her capacity as a director or officer of the Corporation and (i) such opportunity is one the Corporation is legally and contractually permitted to undertake and would otherwise be reasonable for the Corporation to pursue and (ii) the director or officer is permitted to refer that opportunity to the Corporation without violating any legal obligation.
ARTICLE XI
AMENDMENT OF SECOND AMENDED AND RESTATED CERTIFICATE OF INCORPORATION
The Corporation reserves the right at any time and from time to time to amend, alter, change or repeal any provision contained in this Second Amended and Restated Certificate (including any Preferred Stock Designation), and other provisions authorized by the laws of the State of Delaware at the time in force that may be added or inserted, in the manner now or hereafter prescribed by this Second Amended and Restated Certificate and the DGCL; and, except as set forth in Article VIII, all rights, preferences and privileges of whatever nature herein conferred upon stockholders, directors or any other persons by and pursuant to this Second Amended and Restated Certificate in its present form or as hereafter amended are granted subject to the right reserved in this Article XI.
| B-5 |
ARTICLE XII
EXCLUSIVE FORUM FOR CERTAIN LAWSUITS; CONSENT TO JURISDICTION
Section 12.1 Forum. Subject to the last sentence in this Section 12.1, and unless the Corporation consents in writing to the selection of an alternative forum, to the fullest extent permitted by the applicable law, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for any stockholder (including a beneficial owner) to bring (i) any derivative action or proceeding brought on behalf of the Corporation, (ii) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of the Corporation to the Corporation or the Corporation’s stockholders, (iii) any action asserting a claim against the Corporation, its directors, officers or employees arising pursuant to any provision of the DGCL or this Second Amended and Restated Certificate or the By-Laws, or (iv) any action asserting a claim against the Corporation, its directors, officers or employees governed by the internal affairs doctrine and, if brought outside of Delaware, the stockholder bringing the suit will be deemed to have consented to service of process on such stockholder’s counsel except any action (A) as to which the Court of Chancery in the State of Delaware determines that there is an indispensable party not subject to the jurisdiction of the Court of Chancery (and the indispensable party does not consent to the personal jurisdiction of the Court of Chancery within ten days following such determination), (B) which is vested in the exclusive jurisdiction of a court or forum other than the Court of Chancery, or (C) for which the Court of Chancery does not have subject matter jurisdiction. Notwithstanding the foregoing, (i) the provisions of this Section 12.1 will not apply to suits brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction and (ii) to the fullest extent permitted by the applicable law, the federal district courts of the United States of America for the District of Delaware and the Court of Chancery of the State of Delaware shall have concurrent jurisdiction for the resolution of any complaint asserting a cause of action arising under the Securities Act of 1933, as amended, or the rules and regulations promulgated thereunder.
Section 12.2 Consent to Jurisdiction. If any action the subject matter of which is within the scope of Section 12.1 immediately above is filed in a court other than a court located within the State of Delaware (a “Foreign Action”) in the name of any stockholder, such stockholder shall be deemed to have consented to (i) the personal jurisdiction of the state and federal courts located within the State of Delaware in connection with any action brought in any such court to enforce Section 12.1 immediately above (an “FSC Enforcement Action”) and (ii) having service of process made upon such stockholder in any such FSC Enforcement Action by service upon such stockholder’s counsel in the Foreign Action as agent for such stockholder.
Section 12.3 Severability. If any provision or provisions of this Article XII shall be held to be invalid, illegal or unenforceable as applied to any person or entity or circumstance for any reason whatsoever, then, to the fullest extent permitted by law, the validity, legality and enforceability of such provisions in any other circumstance and of the remaining provisions of this Article XII (including, without limitation, each portion of any sentence of this Article XII containing any such provision held to be invalid, illegal or unenforceable that is not itself held to be invalid, illegal or unenforceable) and the application of such provision to other persons or entities and circumstances shall not in any way be affected or impaired thereby. Any person or entity purchasing or otherwise acquiring any interest in shares of capital stock of the Corporation shall be deemed to have notice of and consented to the provisions of this Article XII.
[Remainder of page intentionally left blank]
[Signature Page Follows]
| B-6 |
IN WITNESS WHEREOF, the Corporation has caused this Second Amended and Restated Certificate to be duly executed and acknowledged in its name and on its behalf by an authorized officer as of the date first set forth above.
| By: | ||
| Name: | ||
| Title: |
[Signature Page to Second Amended and Restated Certificate of Incorporation]
| B-7 |
Annex C
CONDUIT
PHARMACEUTICALS INC.
2023 STOCK INCENTIVE PLAN
ARTICLE I.
PURPOSE
The Plan’s purpose is to enhance the Company’s ability to attract, retain and motivate persons who make (or are expected to make) important contributions to the Company by providing these individuals with equity ownership opportunities.
ARTICLE
II.
DEFINITIONS
As used in the Plan, the following words and phrases have the meanings specified below, unless the context clearly indicates otherwise:
2.1 “Administrator” means the Board or a Committee to the extent that the Board’s powers or authority under the Plan have been delegated to such Committee. With reference to the Board’s or a Committee’s powers or authority under the Plan that have been delegated to one or more officers pursuant to Section 4.2, the term “Administrator” shall refer to such officer(s) unless and until such delegation has been revoked.
2.2 “Applicable Law” means any applicable law, including without limitation: (a) provisions of the Code, the Securities Act, the Exchange Act and any rules or regulations thereunder; (b) corporate, securities, tax or other laws, statutes, rules, requirements or regulations, whether federal, state, local or foreign; and (c) rules of any securities exchange or automated quotation system on which the Shares are listed, quoted or traded.
2.3 “Award” means an Option, Stock Appreciation Right, Restricted Stock award, Restricted Stock Unit award, Performance Stock Unit award, Dividend Equivalents award or Other Stock or Cash Based Award granted to a Participant under the Plan.
2.4 “Award Agreement” means an agreement evidencing an Award, which may be written or electronic, that contains such terms and conditions as the Administrator determines, consistent with and subject to the terms and conditions of the Plan.
2.5 “Board” means the Board of Directors of the Company.
2.6 “Cause” shall have the meaning ascribed to such term, or term of similar effect, in any offer letter, employment, severance or similar agreement, including any Award Agreement, between the Participant and the Company or any Subsidiary; provided, that in the absence of an offer letter, employment, severance or similar agreement containing such definition, Cause means, with respect to a Participant, the occurrence of any of the following: (a) an act of dishonesty made by the Participant in connection with the Participant’s responsibilities as a Service Provider; (b) the Participant’s conviction of, or plea of nolo contendere to, a felony or any crime involving fraud, embezzlement or any other act of moral turpitude, or a material violation of federal or state law (or equivalent in any non-U.S. jurisdiction) by the Participant that the Administrator reasonably determines has had or will have a material detrimental effect on the Company’s reputation or business; (c) the Participant’s gross misconduct; (d) the Participant’s unauthorized use or disclosure of any proprietary information or trade secrets of the Company or any other party to whom the Participant owes an obligation of nondisclosure as a result of the Participant’s relationship with the Company or any Subsidiary; (e) the Participant’s breach of any material obligations under any written agreement or covenant with the Company or any Subsidiary; (f) the Participant’s continued substantial failure to perform the Participant’s duties as a Service Provider (other than as a result of the Participant’s physical or mental incapacity) after the Participant has received a written demand for performance that specifically sets forth the factual basis for the determination that the Participant has not substantially performed the Participant’s duties and has failed to cure such non-performance to the Administrator’s reasonable satisfaction within 30 business days after receiving such notice; (g) the Participant’s violation of the Company’s code of ethics; or (h) the Participant’s disregard of the policies of the Company or any Subsidiary so as to cause loss, harm, damage or injury to the property, reputation or employees of the Company or a Subsidiary.
2.7 “Change in Control” means any of the following:
(a) The consummation of a transaction or series of transactions (other than an offering of Common Stock to the general public through a registration statement filed with the Securities and Exchange Commission) whereby any “person” or related “group” of “persons” (as such terms are used in Sections 13(d) and 14(d)(2) of the Exchange Act) directly or indirectly acquires beneficial ownership (within the meaning of Rules 13d-3 and 13d-5 under the Exchange Act) of the Company’s securities possessing more than 50% of the total combined voting power of the Company’s securities outstanding immediately after such acquisition; provided, however, that the following acquisitions shall not constitute a Change in Control: (i) any acquisition by the Company or any of its Subsidiaries; (ii) any acquisition by an employee benefit plan maintained by the Company or any of its Subsidiaries, (iii) any acquisition which complies with clauses (c)(i), (c)(ii) and (c)(iii) of this definition; or (iv) in respect of an Award held by a particular Participant, any acquisition by the Participant or any group of persons including the Participant (or any entity controlled by the Participant or any group of persons including the Participant);
(b) The Incumbent Directors cease for any reason to constitute a majority of the Board;
| 2 |
(c) The consummation by the Company (whether directly involving the Company or indirectly involving the Company through one or more intermediaries) of (x) a merger, consolidation, reorganization, or business combination, (y) a sale or other disposition of all or substantially all of the Company’s assets in any single transaction or series of related transactions or (z) the acquisition of assets or stock of another entity, in each case other than a transaction:
(i) which results in the Company’s voting securities outstanding immediately before the transaction continuing to represent (either by remaining outstanding or by being converted into voting securities of the Company or the person that, as a result of the transaction, controls, directly or indirectly, the Company or owns, directly or indirectly, all or substantially all of the Company’s assets or otherwise succeeds to the business of the Company (the Company or such person, the “Successor Entity”)) directly or indirectly, at least a majority of the combined voting power of the Successor Entity’s outstanding voting securities immediately after the transaction;
(ii) after which no person or group beneficially owns voting securities representing 50% or more of the combined voting power of the Successor Entity; provided, however, that no person or group shall be treated for purposes of this clause (c)(ii) as beneficially owning 50% or more of the combined voting power of the Successor Entity solely as a result of the voting power held in the Company prior to the consummation of the transaction; and
(iii) after which at least a majority of the members of the board of directors (or the analogous governing body) of the Successor Entity were Board members at the time of the Board’s approval of the execution of the initial agreement providing for such transaction; or
(d) The completion of a liquidation or dissolution of the Company.
Notwithstanding the foregoing, in no event will the transactions contemplated by the Merger Agreement or the transactions occurring in connection therewith constitute a Change in Control, and if a Change in Control constitutes a payment event with respect to any Award (or any portion of an Award) that provides for the deferral of compensation that is subject to Section 409A, to the extent required to avoid the imposition of additional taxes under Section 409A, the transaction or event described in subsection (a), (b), (c) or (d) of this definition with respect to such Award (or portion thereof) shall only constitute a Change in Control for purposes of the payment timing of such Award if such transaction also constitutes a “change in control event,” as defined in Treasury Regulation Section 1.409A-3(i)(5).
The Administrator shall have full and final authority, which shall be exercised in its sole discretion, to determine conclusively whether a Change in Control has occurred pursuant to the above definition, the date of such Change in Control and any incidental matters relating thereto; provided that any exercise of authority in conjunction with a determination of whether a Change in Control is a “change in control event” as defined in Treasury Regulation Section 1.409A-3(i)(5) shall be consistent with such regulation.
2.8 “Code” means the U.S. Internal Revenue Code of 1986, as amended, and all regulations, guidance, compliance programs and other interpretative authority issued thereunder.
| 3 |
2.9 “Committee” means one or more committees or subcommittees of the Board, which may include one or more Company directors or executive officers, to the extent permitted by Applicable Law. To the extent required to comply with the provisions of Rule 16b-3, it is intended that each member of the Committee will be, at the time the Committee takes any action with respect to an Award that is subject to Rule 16b-3, a “non-employee director” within the meaning of Rule 16b-3; however, a Committee member’s failure to qualify as a “non-employee director” within the meaning of Rule 16b-3 will not invalidate any Award granted by the Committee that is otherwise validly granted under the Plan.
2.10 “Common Stock” means the common stock of the Company.
2.11 “Company” means Conduit Pharmaceuticals Inc., a Delaware corporation, or any successor.
2.12 “Consultant” means any person, including any adviser, engaged by the Company or a Subsidiary to render services to such entity if the consultant or adviser: (i) renders bona fide services to the Company or a Subsidiary; (ii) renders services not in connection with the offer or sale of securities in a capital-raising transaction and does not directly or indirectly promote or maintain a market for the Company’s securities; and (iii) is a natural person.
2.13 “Designated Beneficiary” means, if permitted by the Company, the beneficiary or beneficiaries the Participant designates, in a manner the Company determines, to receive amounts due or exercise the Participant’s rights if the Participant dies. Without a Participant’s effective designation, “Designated Beneficiary” will mean the Participant’s estate or legal heirs.
2.14 “Director” means a Board member.
2.15 “Disability” means a permanent and total disability under Section 22(e)(3) of the Code.
2.16 “Dividend Equivalents” means a right granted to a Participant to receive the equivalent value (in cash or Shares) of dividends paid on a specified number of Shares. Such Dividend Equivalent shall be converted to cash or additional Shares, or a combination of cash and Shares, by such formula and at such time and subject to such limitations as may be determined by the Administrator.
2.17 “DRO” means a “domestic relations order” as defined by the Code or Title I of the Employee Retirement Income Security Act of 1974, as amended, or the rules thereunder.
2.18 “Effective Date” has the meaning set forth in Section 11.3.
2.19 “Employee” means any employee of the Company or any of its Subsidiaries.
2.20 “Equity Restructuring” means a nonreciprocal transaction between the Company and its stockholders, such as a stock dividend, stock split (including a reverse stock split), spin-off or recapitalization through a large, nonrecurring cash dividend, that affects the number or kind of Shares (or other Company securities) or the share price of Common Stock (or other Company securities) and causes a change in the per share value of the Common Stock underlying outstanding Awards.
| 4 |
2.21 “Exchange Act” means the U.S. Securities Exchange Act of 1934, as amended, and all regulations, guidance and other interpretative authority issued thereunder.
2.22 “Fair Market Value” means, as of any date, the value of a Share determined as follows: (i) if the Common Stock is listed on any established stock exchange, the value of a Share will be the closing sales price for a Share as quoted on such exchange for such date, or if no sale occurred on such date, the last day preceding such date during which a sale occurred, as reported in The Wall Street Journal or another source the Administrator deems reliable; (ii) if the Common Stock is not listed on an established stock exchange but is quoted on a national market or other quotation system, the value of a Share will be the closing sales price for a Share on such date, or if no sales occurred on such date, then on the last date preceding such date during which a sale occurred, as reported in The Wall Street Journal or another source the Administrator deems reliable; or (iii) if the Common Stock is not listed on any established stock exchange or quoted on a national market or other quotation system, the value established by the Administrator in its sole discretion.
2.23 “Greater Than 10% Stockholder” means an individual then owning (within the meaning of Section 424(d) of the Code) more than 10% of the total combined voting power of all classes of stock of the Company or any parent corporation or subsidiary corporation of the Company, as determined in accordance with Section 424(e) and (f) of the Code, respectively.
2.24 “Incentive Stock Option” means an Option that meets the requirements to qualify as an “incentive stock option” as defined in Section 422 of the Code.
2.25 “Incumbent Directors” means, for any period of 12 consecutive months, individuals who, at the beginning of such period, constitute the Board together with any new Director(s) (other than a Director designated by a person who shall have entered into an agreement with the Company to effect a transaction described in clause (a) or (c) of the Change in Control definition) whose election or nomination for election to the Board was approved by a vote of at least a majority (either by a specific vote or by approval of the proxy statement of the Company in which such person is named as a nominee for Director without objection to such nomination) of the Directors then still in office who either were Directors at the beginning of the 12-month period or whose election or nomination for election was previously so approved. No individual initially elected or nominated as a director of the Company as a result of an actual or threatened election contest with respect to Directors or as a result of any other actual or threatened solicitation of proxies by or on behalf of any person other than the Board shall be an Incumbent Director.
2.26 “Merger Agreement” means the Agreement and Plan of Merger by and among Murphy Canyon Acquisition Corp. Conduit Merger Sub, Inc. and Conduit Pharmaceuticals Limited dated as of November 8, 2022.
| 5 |
2.27 “Non-Employee Director” means a Director who is not an Employee.
2.28 “Nonqualified Stock Option” means an Option that is not an Incentive Stock Option.
2.29 “Option” means a right granted under Article VI to purchase a specified number of Shares at a specified price per Share during a specified time period. An Option may be either an Incentive Stock Option or a Nonqualified Stock Option.
2.30 “Other Stock or Cash Based Awards” means cash awards, awards of Shares, and other awards valued wholly or partially by referring to, or are otherwise based on, Shares or other property.
2.31 “Overall Share Limit” has the meaning set forth in Section 5.1 below.
2.32 “Participant” means a Service Provider who has been granted an Award.
2.33 “Performance Stock Unit” means a right granted to a Participant pursuant to Section 8.1 and subject to Section 8.2, to receive Shares, the payment of which is contingent upon achieving certain performance goals or other performance-based targets established by the Administrator.
2.34 “Permitted Transferee” means, with respect to a Participant, any “family member” of the Participant, as defined in the General Instructions to Form S-8 Registration Statement under the Securities Act (or any successor form thereto), or any other transferee specifically approved by the Administrator after taking into account Applicable Law.
2.35 “Plan” means this 2023 Stock Incentive Plan.
2.36 “Restricted Stock” means Shares awarded to a Participant under Article VII, subject to certain vesting conditions and other restrictions.
2.37 “Restricted Stock Unit” means an unfunded, unsecured right granted to a Participant under Article VII to receive, on the applicable settlement date, one Share or an amount in cash or other consideration determined by the Administrator to be of equal value as of such settlement date, subject to certain vesting conditions and other restrictions.
2.38 “Rule 16b-3” means Rule 16b-3 promulgated under the Exchange Act.
2.39 “Section 409A” means Section 409A of the Code and the regulations promulgated thereunder by the United States Treasury Department, as amended or as may be amended from time to time.
2.40 “Securities Act” means the Securities Act of 1933, as amended, and all regulations, guidance and other interpretative authority issued thereunder.
| 6 |
2.41 “Service Provider” means an Employee, Consultant or Director.
2.42 “Shares” means shares of Common Stock.
2.43 “Stock Appreciation Right” or “SAR” means a right granted under Article VI to receive a payment equal to the excess of the Fair Market Value of a specified number of Shares on the date the right is exercised over the exercise price set forth in the applicable Award Agreement.
2.44 “Subsidiary” means any entity (other than the Company), whether U.S. or non-U.S., in an unbroken chain of entities beginning with the Company if each of the entities other than the last entity in the unbroken chain beneficially owns, at the time of the determination, securities or interests representing at least 50% of the total combined voting power of all classes of securities or interests in one of the other entities in such chain.
2.45 “Substitute Awards” means Awards granted or Shares issued by the Company in assumption of, or in substitution or exchange for, awards previously granted, or the right or obligation to make future awards, in each case by a company or other entity acquired by the Company or any Subsidiary or with which the Company or any Subsidiary combines.
2.46 “Tax-Related Items” means any U.S. and non-U.S. federal, state and/or local taxes (including, without limitation, income tax, social insurance contributions, fringe benefit tax, employment tax, stamp tax and any employer tax liability which has been transferred to a Participant) for which a Participant is liable in connection with Awards and/or Shares.
2.47 “Termination of Service” means:
(a) As to a Consultant, the time when the engagement of a Participant as a Consultant to the Company or a Subsidiary is terminated for any reason, with or without cause, including, without limitation, by resignation, discharge, death or retirement, but excluding terminations where the Consultant simultaneously commences or remains in employment or service with the Company or any Subsidiary.
(b) As to a Non-Employee Director, the time when a Participant who is a Non-Employee Director ceases to be a Director for any reason, including, without limitation, a termination by resignation, failure to be elected, death or retirement, but excluding terminations where the Participant simultaneously commences or remains in employment or service with the Company or any Subsidiary.
(c) As to an Employee, the time when the employee-employer relationship between a Participant and the Company or any Subsidiary is terminated for any reason, including, without limitation, a termination by resignation, discharge, death, disability or retirement; but excluding terminations where the Participant simultaneously commences or remains in employment or service with the Company or any Subsidiary.
| 7 |
The Company, in its sole discretion, shall determine the effect of all matters and questions relating to any Termination of Service, including, without limitation, whether a Termination of Service has occurred, whether a Termination of Service resulted from a discharge for Cause and all questions of whether particular leaves of absence constitute a Termination of Service. For purposes of the Plan, a Participant’s employee-employer relationship or consultancy relationship shall be deemed to be terminated in the event that the Subsidiary employing or contracting with such Participant ceases to remain a Subsidiary following any merger, sale of stock or other corporate transaction or event (including, without limitation, a spin-off), even though the Participant may subsequently continue to perform services for that entity.
ARTICLE
III.
ELIGIBILITY
Service Providers are eligible to be granted Awards under the Plan, subject to the limitations described herein. No Service Provider shall have any right to be granted an Award pursuant to the Plan and neither the Company nor the Administrator is obligated to treat Service Providers, Participants or any other persons uniformly.
ARTICLE
IV.
ADMINISTRATION AND DELEGATION
4.1 Administration.
(a) The Plan is administered by the Administrator. The Administrator has authority to determine which Service Providers receive Awards, grant Awards and set Award terms and conditions, subject to the conditions and limitations in the Plan. The Administrator also has the authority to take all actions and make all determinations under the Plan, to interpret the Plan and Award Agreements and to adopt, amend and repeal Plan administrative rules, guidelines and practices as it deems advisable. The Administrator may correct defects and ambiguities, supply omissions, reconcile inconsistencies in the Plan or any Award and make all other determinations that it deems necessary or appropriate to administer the Plan and any Awards. The Administrator (and each member thereof) is entitled to, in good faith, rely or act upon any report or other information furnished to it, him or her by any officer or other employee of the Company or any Subsidiary, the Company’s independent certified public accountants, or any executive compensation consultant or other professional retained by the Company to assist in the administration of the Plan. The Administrator’s determinations under the Plan are in its sole discretion and will be final, binding and conclusive on all persons having or claiming any interest in the Plan or any Award.
| 8 |
(b) Without limiting the foregoing, the Administrator has the exclusive power, authority and sole discretion to: (i) designate Participants; (ii) determine the type or types of Awards to be granted to each Participant; (iii) determine the number of Awards to be granted and the number of Shares to which an Award will relate; (iv) subject to the limitations in the Plan, determine the terms and conditions of any Award and related Award Agreement, including, but not limited to, the exercise price, grant price, purchase price, any performance criteria, any restrictions or limitations on the Award, any schedule for vesting, lapse of forfeiture restrictions or restrictions on the exercisability of an Award, and accelerations, waivers or amendments thereof; (v) determine whether, to what extent, and under what circumstances an Award may be settled in, or the exercise price of an Award may be paid in cash, Shares, or other property, or an Award may be canceled, forfeited, or surrendered; and (vi) make all other decisions and determinations that may be required pursuant to the Plan or as the Administrator deems necessary or advisable to administer the Plan.
4.2 Delegation of Authority. To the extent permitted by Applicable Law, the Board or any Committee may delegate any or all of its powers under the Plan to one or more Committees or officers of the Company or any of its Subsidiaries; provided, however, that in no event shall an officer of the Company or any of its Subsidiaries be delegated the authority to grant Awards to, or amend Awards held by, the following individuals: (a) individuals who are subject to Section 16 of the Exchange Act, or (b) officers of the Company or any of its Subsidiaries or Directors to whom authority to grant or amend Awards has been delegated hereunder. Any delegation hereunder shall be subject to the restrictions and limits that the Board or Committee specifies at the time of such delegation or that are otherwise included in the applicable organizational documents, and the Board or Committee, as applicable, may at any time rescind the authority so delegated or appoint a new delegatee. At all times, the delegatee appointed under this Section 4.2 shall serve in such capacity at the pleasure of the Board or the Committee, as applicable, and the Board or the Committee may abolish any committee at any time and re-vest in itself any previously delegated authority. Further, regardless of any delegation, the Board or a Committee may, in its discretion, exercise any and all rights and duties as the Administrator under the Plan delegated thereby, except with respect to Awards that are required to be determined in the sole discretion of the Committee under the rules of any securities exchange or automated quotation system on which the Shares are listed, quoted or traded.
ARTICLE
V.
STOCK AVAILABLE FOR AWARDS
5.1 Number of Shares. Subject to adjustment under Article IX and the terms of this Article V, Awards may be made under the Plan covering up to the sum of (i) 11,497,622 Shares; and (ii) an increase commencing on January 1, 2024 and continuing annually on each anniversary thereof through (and including) January 1, 2033, equal to the lesser of (A) 5% of the Shares outstanding on the last day of the immediately preceding fiscal year and (B) such smaller number of Shares as determined by the Board or the Committee (the “Overall Share Limit”). Shares issued or delivered under the Plan may consist of authorized but unissued Shares, Shares purchased on the open market or treasury Shares.
| 9 |
5.2 Share Recycling.
(a) If all or any part of an Award expires, lapses or is terminated, exchanged for or settled in cash, surrendered, repurchased, canceled without having been fully exercised or forfeited, in any case, in a manner that results in the Company acquiring Shares covered by the Award at a price not greater than the price (as adjusted to reflect any Equity Restructuring) paid by the Participant for such Shares or not issuing any Shares covered by the Award, the unused Shares covered by the Award will, as applicable, become or again be available for Awards under the Plan. The payment of Dividend Equivalents in cash in conjunction with any outstanding Awards shall not count against the Overall Share Limit.
(b) Notwithstanding the foregoing, the following Shares issued or delivered under this Plan shall not again be available for future grants of Awards: (i) Shares tendered by a Participant or withheld by the Company in payment of the exercise price of an Option; (ii) Shares tendered by the Participant or withheld by the Company to satisfy any tax withholding obligation with respect to an Award; (iii) Shares that are repurchased by the Company with Option proceeds; and (iv) Shares subject to a Stock Appreciation Right that are not issued in connection with the stock settlement of the Stock Appreciation Right on exercise thereof. Notwithstanding the provisions of this Section 5.2(b), no Shares may again be optioned, granted or awarded pursuant to an Incentive Stock Option if such action would cause such Option to fail to qualify as an incentive stock option under Section 422 of the Code.
5.3 Incentive Stock Option Limitations. Notwithstanding anything to the contrary herein, no more than 57,488,110 Shares (as adjusted to reflect any Equity Restructuring) may be issued pursuant to the exercise of Incentive Stock Options.
5.4 Substitute Awards. In connection with an entity’s merger or consolidation with the Company or any Subsidiary or the Company’s or any Subsidiary’s acquisition of an entity’s property or stock, the Administrator may grant Awards in substitution for any options or other stock or stock-based awards granted before such merger or consolidation by such entity or its affiliate. Substitute Awards may be granted on such terms and conditions as the Administrator deems appropriate, notwithstanding limitations on Awards in the Plan. Substitute Awards will not count against the Overall Share Limit (nor shall Shares subject to a Substitute Award be added to the Shares available for Awards under the Plan as provided above), except that Shares acquired by exercise of substitute Incentive Stock Options will count against the maximum number of Shares that may be issued pursuant to the exercise of Incentive Stock Options under the Plan. Additionally, in the event that a company acquired by the Company or any Subsidiary or with which the Company or any Subsidiary combines has shares available under a pre-existing plan approved by stockholders and not adopted in contemplation of such acquisition or combination, the shares available for grant pursuant to the terms of such pre-existing plan (as appropriately adjusted to reflect the transaction) may be used for Awards under the Plan and shall not reduce the Shares authorized for grant under the Plan (and Shares subject to such Awards may again become available for Awards under the Plan as provided under Section 5.2 above); provided that Awards using such available shares shall not be made after the date awards or grants could have been made under the terms of the pre-existing plan, absent the acquisition or combination, and shall only be made to individuals who were not Employees, Directors or Consultants prior to such acquisition or combination.
| 10 |
5.5 Non-Employee Director Award Limit. Notwithstanding any provision to the contrary in the Plan or in any policy of the Company regarding non-employee director compensation, the sum of the grant date fair value (determined as of the grant date in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718, or any successor thereto) of all equity-based Awards and the maximum amount of cash that may become payable to a Service Provider as compensation for services as a Non-Employee Director during any calendar year shall not exceed $750,000, increased to $1,000,000 in the calendar year of a non-employee Director’s initial service as a non-employee Director. The Administrator may make exceptions to this limit for Non-Employee Directors in extraordinary circumstances, as the Administrator may determine in its discretion, provided that the Non-Employee Director receiving such additional compensation may not participate in the decision to award such compensation or in other contemporaneous compensation decisions involving Non-Employee Directors.
ARTICLE
VI.
STOCK OPTIONS AND STOCK APPRECIATION RIGHTS
6.1 General. The Administrator may grant Options or Stock Appreciation Rights to one or more Service Providers, subject to such terms and conditions not inconsistent with the Plan as the Administrator shall determine. The Administrator will determine the number of Shares covered by each Option and Stock Appreciation Right, the exercise price of each Option and Stock Appreciation Right and the conditions and limitations applicable to the exercise of each Option and Stock Appreciation Right. A Stock Appreciation Right will entitle the Participant (or other person entitled to exercise the Stock Appreciation Right) to receive from the Company upon exercise of the exercisable portion of the Stock Appreciation Right an amount determined by multiplying the excess, if any, of the Fair Market Value of one Share on the date of exercise over the exercise price per Share of the Stock Appreciation Right by the number of Shares with respect to which the Stock Appreciation Right is exercised, subject to any limitations of the Plan or that the Administrator may impose and payable in cash, Shares valued at Fair Market Value on the date of exercise or a combination of the two as the Administrator may determine or provide in the Award Agreement.
6.2 Exercise Price. The Administrator will establish each Option’s and Stock Appreciation Right’s exercise price and specify the exercise price in the Award Agreement. Subject to Section 6.6, the exercise price will not be less than 100% of the Fair Market Value on the grant date of the Option or Stock Appreciation Right. Notwithstanding the foregoing, in the case of an Option or Stock Appreciation Right that is a Substitute Award, the exercise price per share of the Shares subject to such Option or Stock Appreciation Right, as applicable, may be less than the Fair Market Value per share on the date of grant; provided that the exercise price of any Substitute Award shall be determined in accordance with the applicable requirements of Sections 424 and 409A of the Code.
| 11 |
6.3 Duration of Options. Subject to Section 6.6, each Option or Stock Appreciation Right will be exercisable at such times and as specified in the Award Agreement, provided that the term of an Option or Stock Appreciation Right will not exceed ten years; provided, further, that, unless otherwise determined by the Administrator or specified in the Award Agreement, (a) no portion of an Option or Stock Appreciation Right which is unexercisable at a Participant’s Termination of Service shall thereafter become exercisable and (b) the portion of an Option or Stock Appreciation Right that is unexercisable at a Participant’s Termination of Service shall automatically expire on the date of such Termination of Service. In addition, in no event shall an Option or Stock Appreciation Right granted to an Employee who is a non-exempt employee for purposes of overtime pay under the U.S. Fair Labor Standards Act of 1938 be exercisable earlier than six months after its date of grant. Notwithstanding the foregoing, if the Participant, prior to the end of the term of an Option or Stock Appreciation Right, commits an act of Cause (as determined by the Administrator prior to or after the Participant’s Termination of Service), or violates any non-competition, non-solicitation or confidentiality provisions of any employment contract, confidentiality and nondisclosure agreement or other agreement between the Participant and the Company or any of its Subsidiaries, the right to exercise the Option or Stock Appreciation Right, as applicable, may be terminated by the Company and the Company may suspend the Participant’s right to exercise the Option or Stock Appreciation Right when it reasonably believes that the Participant may have participated in any such act or violation.
6.4 Exercise. Options and Stock Appreciation Rights may be exercised by delivering to the Company (or such other person or entity designated by the Administrator) a notice of exercise, in a form and manner the Company approves (which may be written, electronic or telephonic and may contain representations and warranties deemed advisable by the Administrator), signed or authenticated by the person authorized to exercise the Option or Stock Appreciation Right, together with, as applicable, (a) payment in full of the exercise price for the number of Shares for which the Option is exercised in a manner specified in Section 6.5 and (b) satisfaction in full of any withholding obligation for Tax-Related Items in a manner specified in Section 10.5. The Administrator may, in its discretion, limit exercise with respect to fractional Shares and require that any partial exercise of an Option or Stock Appreciation Right be with respect to a minimum number of Shares.
6.5 Payment Upon Exercise. The Administrator shall determine the methods by which payment of the exercise price of an Option shall be made, including, without limitation:
(a) Cash, check or wire transfer of immediately available funds;
(b) If there is a public market for Shares at the time of exercise, unless the Company otherwise determines, (i) delivery (including electronically or telephonically to the extent permitted by the Company) of a notice that the Participant has placed a market sell order with a broker acceptable to the Company with respect to Shares then issuable upon exercise of the Option and that the broker has been directed to deliver promptly to the Company funds sufficient to pay the exercise price, or (ii) the Participant’s delivery to the Company of a copy of irrevocable and unconditional instructions to a broker acceptable to the Company to deliver promptly to the Company an amount sufficient to pay the exercise price by cash, wire transfer of immediately available funds or check; provided that such amount is paid to the Company at such time as may be required by the Company;
| 12 |
(c) To the extent permitted by the Administrator, delivery (either by actual delivery or attestation) of Shares owned by the Participant valued at their Fair Market Value on the date of delivery;
(d) To the extent permitted by the Administrator, surrendering Shares then issuable upon the Option’s exercise valued at their Fair Market Value on the exercise date;
(e) Any other lawful consideration permitted by the Administrator; or
(f) To the extent permitted by the Administrator, any combination of the above payment forms.
6.6 Additional Terms of Incentive Stock Options. The Administrator may grant Incentive Stock Options only to employees of the Company, any of its present or future parent or subsidiary corporations, as defined in Sections 424(e) or (f) of the Code, respectively. If an Incentive Stock Option is granted to a Greater Than 10% Stockholder, the exercise price will not be less than 110% of the Fair Market Value on the Option’s grant date, and the term of the Option will not exceed five years. All Incentive Stock Options (and Award Agreements related thereto) will be subject to and construed consistently with Section 422 of the Code. By accepting an Incentive Stock Option, if requested the Participant agrees to give prompt notice to the Company of dispositions or other transfers (other than in connection with a Change in Control) of Shares acquired under the Option made within (a) two years from the grant date of the Option or (b) one year after the transfer of such Shares to the Participant, specifying the date of the disposition or other transfer and the amount the Participant realized, in cash, other property, assumption of indebtedness or other consideration, in such disposition or other transfer. Neither the Company nor the Administrator will be liable to a Participant, or any other party, if an Incentive Stock Option fails or ceases to qualify as an “incentive stock option” under Section 422 of the Code. Any Incentive Stock Option or portion thereof that fails to qualify as an “incentive stock option” under Section 422 of the Code for any reason, including becoming exercisable with respect to Shares having a fair market value exceeding the $100,000 limitation under Treasury Regulation Section 1.422-4, will be a Nonqualified Stock Option.
ARTICLE
VII.
RESTRICTED STOCK; RESTRICTED STOCK UNITS
7.1 General. The Administrator may grant Restricted Stock, or the right to purchase Restricted Stock, to any Service Provider, subject to forfeiture or the Company’s right to repurchase all or part of the underlying Shares at their issue price or other stated or formula price from the Participant if conditions the Administrator specifies in the Award Agreement are not satisfied before the end of the applicable restriction period or periods that the Administrator establishes for such Award. In addition, the Administrator may grant Restricted Stock Units, which may be subject to vesting and forfeiture conditions during the applicable restriction period or periods, as set forth in an Award Agreement, to Service Providers. The Administrator shall establish the purchase price, if any, and form of payment for Restricted Stock and Restricted Stock Units; provided, however, that if a purchase price is charged, such purchase price shall be no less than the par value, if any, of the Shares to be purchased, unless otherwise permitted by Applicable Law. In all cases, legal consideration shall be required for each issuance of Restricted Stock and Restricted Stock Units to the extent required by Applicable Law. The Award Agreement for each Award of Restricted Stock and Restricted Stock Units shall set forth the terms and conditions not inconsistent with the Plan as the Administrator shall determine.
| 13 |
7.2 Restricted Stock.
(a) Stockholder Rights. Unless otherwise determined by the Administrator, each Participant holding shares of Restricted Stock will be entitled to all the rights of a stockholder with respect to such Shares, subject to the restrictions in the Plan and the applicable Award Agreement, including the right to receive all dividends and other distributions paid or made with respect to the Shares to the extent such dividends and other distributions have a record date that is on or after the date on which such Participant becomes the record holder of such Shares; provided, however, that with respect to a share of Restricted Stock subject to restrictions or vesting conditions, except in connection with a spin-off or other similar event as otherwise permitted under Section 9.2, dividends which are paid to Company stockholders prior to the removal of restrictions and satisfaction of vesting conditions shall only be paid to the Participant to the extent that the restrictions are subsequently removed and the vesting conditions are subsequently satisfied and the share of Restricted Stock vests.
(b) Stock Certificates. The Company may require that the Participant deposit in escrow with the Company (or its designee) any stock certificates issued in respect of shares of Restricted Stock, together with a stock power endorsed in blank.
(c) Section 83(b) Election. If a Participant makes an election under Section 83(b) of the Code to be taxed with respect to the Restricted Stock as of the date of transfer of the Restricted Stock rather than as of the date or dates upon which such Participant would otherwise be taxable under Section 83(a) of the Code, such Participant shall be required to deliver a copy of such election to the Company promptly after filing such election with the Internal Revenue Service along with proof of the timely filing thereof.
7.3 Restricted Stock Units. The Administrator may provide that settlement of Restricted Stock Units will occur upon or as soon as reasonably practicable after the Restricted Stock Units vest or will instead be deferred, on a mandatory basis or at the Participant’s election, subject to compliance with Applicable Law.
ARTICLE
VIII.
OTHER TYPES OF AWARDS
8.1 General. The Administrator may grant Performance Stock Unit awards, Dividend Equivalents or Other Stock or Cash Based Awards, to one or more Service Providers, in such amounts and subject to such terms and conditions not inconsistent with the Plan as the Administrator shall determine.
| 14 |
8.2 Performance Stock Unit Awards. Each Performance Stock Unit award shall be denominated in a number of Shares or in unit equivalents of Shares or units of value (including a dollar value of Shares) and may be linked to any one or more of performance or other specific criteria, including service to the Company or Subsidiaries, determined to be appropriate by the Administrator, in each case on a specified date or dates or over any period or periods determined by the Administrator. In making such determinations, the Administrator may consider (among such other factors as it deems relevant in light of the specific type of award) the contributions, responsibilities and other compensation of the particular Participant.
8.3 Dividend Equivalents. If the Administrator provides, an Award (other than an Option or Stock Appreciation Right) may provide a Participant with the right to receive Dividend Equivalents. Dividend Equivalents may be paid currently or credited to an account for the Participant, settled in cash or Shares and subject to the same restrictions on transferability and forfeitability as the Award with respect to which the Dividend Equivalents are granted and subject to other terms and conditions as set forth in the Award Agreement. Notwithstanding anything to the contrary herein, Dividend Equivalents with respect to an Award subject to vesting shall either (i) to the extent permitted by Applicable Law, not be paid or credited or (ii) be accumulated and subject to vesting to the same extent as the related Award. All such Dividend Equivalents shall be paid at such time as the Administrator shall specify in the applicable Award Agreement.
8.4 Other Stock or Cash Based Awards. Other Stock or Cash Based Awards may be granted to Participants, including Awards entitling Participants to receive cash or Shares to be delivered in the future and annual or other periodic or long-term cash bonus awards (whether based on specified performance criteria or otherwise), in each case subject to any conditions and limitations in the Plan. Such Other Stock or Cash Based Awards will also be available as a payment form in the settlement of other Awards, as standalone payments and as payment in lieu of compensation to which a Participant is otherwise entitled. Other Stock or Cash Based Awards may be paid in Shares, cash or other property, as the Administrator determines. Subject to the provisions of the Plan, the Administrator will determine the terms and conditions of each Other Stock or Cash Based Award, including any purchase price, performance goal(s), transfer restrictions, and vesting conditions, which will be set forth in the applicable Award Agreement. Except in connection with a spin-off or other similar event as otherwise permitted under Article IX, dividends that are paid prior to vesting of any Other Stock or Cash Based Award shall only be paid to the applicable Participant to the extent that the vesting conditions are subsequently satisfied and the Other Stock or Cash Based Award vests.
| 15 |
8.5 Performance Goals and Criteria. Performance goals or performance criteria established by the Administrator for any Award may be described in terms of Company-wide objectives or objectives that are related to the performance of a Subsidiary, division, business unit, department, region or function within the Company or Subsidiary in which the Participant is employed or in terms of the performance of the individual Participant and may be based on the following criteria: revenues, earnings from operations, operating income, income before taxes, net income, cash flow, earnings per share, return on total capital, return on invested capital, return on equity, return on assets, total return to shareholders, earnings before or after interest, taxes, depreciation, amortization or extraordinary or special items, return on investment, free cash flow, cash conversion cycle, cash flow return on investment (discounted or otherwise), net cash provided by operations, cash flow in excess of cost of capital, operating margin, profit margin, contribution margin, stock price, new customers, order intake, cost controls, operating efficiencies, strategic partnerships or transactions (including in-licensing and out-licensing of intellectual property, milestones related to research development (including, but not limited to, preclinical and clinical studies), product development and manufacturing, implementation or completion of projects or processes (including, without limitation, clinical trial initiation, clinical trial enrollment and dates, clinical trial results, regulatory filing submissions, regulatory filing acceptances, regulatory or advisory committee interactions, regulatory approvals, and product supply), submission to, or approval by, a regulatory body (including, but not limited to the U.S. Food and Drug Administration) of an applicable filing or a product, market penetration, geographic business expansion, cost targets, productivity, corporate value and sustainability metrics (including, without limitation, environmental, social and governance matters), human capital metrics (including, without limitation, employee satisfaction, management of employment practices, employee benefits, retention and safety), supervision of litigation or labor negotiations, dealings with regulatory bodies, acquisitions or divestitures, customer satisfaction, strategic business criteria related to a Participant’s area or areas of responsibility, or any other criteria established by the Administrator.
ARTICLE
IX.
ADJUSTMENTS FOR CHANGES IN COMMON STOCK
AND CERTAIN OTHER EVENTS
9.1 Equity Restructuring. In connection with any Equity Restructuring, notwithstanding anything to the contrary in this Article IX, the Administrator will equitably adjust the terms of the Plan and each outstanding Award as it deems appropriate to reflect the Equity Restructuring, which may include (a) adjusting the number and type of securities subject to each outstanding Award or with respect to which Awards may be granted under the Plan (including, but not limited to, adjustments of the limitations in Article V hereof on the maximum number and kind of shares that may be issued); (b) adjusting the terms and conditions of (including the grant or exercise price), and the performance goals or other criteria included in, outstanding Awards; and (iii) granting new Awards or making cash payments to Participants. The adjustments provided under this Section 9.1 will be nondiscretionary and final and binding on all interested parties, including the affected Participant and the Company; provided that the Administrator will determine whether an adjustment is equitable.
| 16 |
9.2 Corporate Transactions. In the event of any extraordinary dividend or other distribution (whether in the form of cash, Common Stock, other securities, or other property), reorganization, merger, consolidation, split-up, spin off, combination, amalgamation, repurchase, recapitalization, liquidation, dissolution, or sale, transfer, exchange or other disposition of all or substantially all of the assets of the Company, or sale or exchange of Common Stock or other securities of the Company, Change in Control, issuance of warrants or other rights to purchase Common Stock or other securities of the Company, other similar corporate transaction or event (including the sale, merger or other corporate transaction involving a Subsidiary), other unusual or nonrecurring transaction or event affecting the Company or its financial statements or any change in any Applicable Law or accounting principles, the Administrator, on such terms and conditions as it deems appropriate, either by the terms of the Award or by action taken prior to the occurrence of such transaction or event (except that action to give effect to a change in Applicable Law or accounting principles may be made within a reasonable period of time after such change), is hereby authorized to take any one or more of the following actions whenever the Administrator determines that such action is appropriate in order to (x) prevent dilution or enlargement of the benefits or potential benefits intended by the Company to be made available under the Plan or with respect to any Award granted or issued under the Plan, (y) to facilitate such transaction or event or (z) give effect to such changes in Applicable Law or accounting principles:
(a) To provide for the cancellation of any such Award in exchange for either an amount of cash or other property with a value equal to the amount that could have been obtained upon the exercise or settlement of the vested portion of such Award or realization of the Participant’s rights under the vested portion of such Award, as applicable; provided that, if the amount that could have been obtained upon the exercise or settlement of the vested portion of such Award or realization of the Participant’s rights, in any case, is equal to or less than zero, then the Award may be terminated without payment;
(b) To provide that such Award shall vest and, to the extent applicable, be exercisable as to all Shares (or other property) covered thereby, notwithstanding anything to the contrary in the Plan or the provisions of such Award;
(c) To provide that such Award be assumed by the successor or survivor corporation or entity, or a parent or subsidiary thereof, or shall be substituted for by awards covering the stock of the successor or survivor corporation or entity, or a parent or subsidiary thereof, with appropriate adjustments as to the number and kind of shares and applicable exercise or purchase price, in all cases, as determined by the Administrator;
(d) To make adjustments in the number and type of shares of Common Stock (or other securities or property) subject to outstanding Awards or with respect to which Awards may be granted under the Plan (including, but not limited to, adjustments of the limitations in Article V hereof on the maximum number and kind of shares which may be issued) or in the terms and conditions of (including the grant or exercise price), and the criteria included in, outstanding Awards;
(e) To replace such Award with other rights or property selected by the Administrator; or
| 17 |
(f) To provide that the Award will terminate and cannot vest, be exercised or become payable after the applicable event.
9.3 Change in Control.
(a) Notwithstanding any other provision of the Plan, in the event of a Change in Control, unless the Administrator elects to (i) terminate an Award in exchange for cash, rights or property, pursuant to Section 9.2, or (ii) cause an Award to become fully exercisable and no longer subject to any forfeiture restrictions prior to the consummation of a Change in Control, pursuant to Section 9.2, (A) such Award (other than any portion subject to performance-based vesting) shall continue in effect or be assumed or an equivalent Award substituted by the successor corporation or a parent or subsidiary of the successor corporation and (B) the portion of such Award subject to performance-based vesting shall be subject to the terms and conditions of the applicable Award Agreement and, in the absence of applicable terms and conditions, the Administrator’s discretion.
(b) In the event that the successor corporation in a Change in Control refuses to assume or substitute for an Award, the Administrator shall cause such Award to become fully vested and, if applicable, exercisable immediately prior to the consummation of such transaction, and with respect to Awards with performance-based vesting, all performance goals or other vesting criteria will be deemed achieved at the greater of: (i) one hundred percent (100%) of target levels, or (ii) the actual level of achievement of all relevant performance goals against target measured through the date immediately prior to the Change in Control (or as close to such date as administratively practicable), unless specifically provided otherwise under the applicable Award Agreement or otherwise determined by the Administrator, and all forfeiture restrictions on such Award to lapse and, to the extent unexercised upon the consummation of such transaction, to terminate in exchange for cash, rights or other property. The Administrator shall notify the Participant of any Award that becomes exercisable pursuant to the preceding sentence that such Award shall be fully exercisable for a period of at least 10 days from the date of such notice (or such shorter period as required to permit a timely closing of the transaction), contingent upon the occurrence of the Change in Control, or that such Award shall be settled for an amount of cash or securities equal to the in-the-money spread value (if any) of such Award, and such Award shall terminate upon the consummation of the Change in Control in accordance with the preceding sentence.
(c) For the purposes of this Section 9.3, an Award shall be considered assumed if, following the Change in Control, the Award confers the right to purchase or receive, for each Share subject to the Award immediately prior to the Change in Control, the consideration (whether stock, cash, or other securities or property) received in the Change in Control by holders of Common Stock for each Share held on the effective date of the transaction (and if holders were offered a choice of consideration, the type of consideration chosen by the holders of a majority of the outstanding Shares); provided, however, that if such consideration received in the Change in Control was not solely common stock of the successor corporation or its parent, the Administrator may, with the consent of the successor corporation, provide for the consideration to be received upon the exercise of the Award, for each Share subject to an Award, to be solely common stock of the successor corporation or its parent equal in fair market value to the per-share consideration received by holders of Common Stock in the Change in Control.
| 18 |
9.4 Administrative Stand Still. In the event of any pending stock dividend, stock split, combination or exchange of shares, merger, consolidation or other distribution (other than normal cash dividends) of Company assets to stockholders, or any other extraordinary transaction or change affecting the Shares or the share price of Common Stock (including any Equity Restructuring or any securities offering or other similar transaction) or for reasons of administrative convenience or to facilitate compliance with any Applicable Law, the Company may refuse to permit the exercise or settlement of one or more Awards for such period of time as the Company may determine to be reasonably appropriate under the circumstances.
9.5 General. Except as expressly provided in the Plan or the Administrator’s action under the Plan, no Participant will have any rights due to any subdivision or consolidation of Shares of any class, dividend payment, increase or decrease in the number of Shares of any class or dissolution, liquidation, merger, or consolidation of the Company or other corporation. Except as expressly provided with respect to an Equity Restructuring under Section 9.1 above or the Administrator’s action under the Plan, no issuance by the Company of Shares of any class, or securities convertible into Shares of any class, will affect, and no adjustment will be made regarding, the number of Shares subject to an Award or the Award’s grant or exercise price. The existence of the Plan, any Award Agreements and the Awards granted hereunder will not affect or restrict in any way the Company’s right or power to make or authorize (i) any adjustment, recapitalization, reorganization or other change in the Company’s capital structure or its business, (ii) any merger, consolidation, spin-off, dissolution or liquidation of the Company or sale of Company assets or (iii) any sale or issuance of securities, including securities with rights superior to those of the Shares or securities convertible into or exchangeable for Shares.
ARTICLE
X.
PROVISIONS APPLICABLE TO AWARDS
10.1 Transferability.
(a) No Award may be sold, assigned, transferred, pledged or otherwise encumbered, either voluntarily or by operation of law, except by will or the laws of descent and distribution, or, subject to the Administrator’s consent, pursuant to a DRO, unless and until such Award has been exercised or the Shares underlying such Award have been issued, and all restrictions applicable to such Shares have lapsed. During the life of a Participant, Awards will be exercisable only by the Participant, unless it has been disposed of pursuant to a DRO. After the death of a Participant, any exercisable portion of an Award may, prior to the time when such portion becomes unexercisable under the Plan or the applicable Award Agreement, be exercised by the Participant’s personal representative or by any person empowered to do so under the deceased Participant’s will or under the then-Applicable Law of descent and distribution. References to a Participant, to the extent relevant in the context, will include references to a transferee approved by the Administrator.
| 19 |
(b) Notwithstanding Section 10.1(a), the Administrator, in its sole discretion, may determine to permit a Participant or a Permitted Transferee of such Participant to transfer an Award other than an Incentive Stock Option (unless such Incentive Stock Option is intended to become a Nonqualified Stock Option) to any one or more Permitted Transferees of such Participant, subject to the following terms and conditions: (i) an Award transferred to a Permitted Transferee shall not be assignable or transferable by the Permitted Transferee other than (A) to another Permitted Transferee of the applicable Participant or (B) by will or the laws of descent and distribution or, subject to the consent of the Administrator, pursuant to a domestic relations order; (ii) an Award transferred to a Permitted Transferee shall continue to be subject to all the terms and conditions of the Award as applicable to the original Participant (other than the ability to further transfer the Award to any Person other than another Permitted Transferee of the applicable Participant); (iii) the Participant (or transferring Permitted Transferee) and the receiving Permitted Transferee shall execute any and all documents requested by the Administrator, including, without limitation documents to (A) confirm the status of the transferee as a Permitted Transferee, (B) satisfy any requirements for an exemption for the transfer under Applicable Law and (C) evidence the transfer; and (iv) any transfer of an Award to a Permitted Transferee shall be without consideration, except as required by Applicable Law. In addition, and further notwithstanding Section 10.1(a), the Administrator, in its sole discretion, may determine to permit a Participant to transfer Incentive Stock Options to a trust that constitutes a Permitted Transferee if, under Section 671 of the Code and other Applicable Law, the Participant is considered the sole beneficial owner of the Incentive Stock Option while it is held in the trust.
(c) Notwithstanding Section 10.1(a), if permitted by the Administrator, a Participant may, in the manner determined by the Administrator, designate a Designated Beneficiary. A Designated Beneficiary, legal guardian, legal representative, or other person claiming any rights pursuant to the Plan is subject to all terms and conditions of the Plan and any Award Agreement applicable to the Participant and any additional restrictions deemed necessary or appropriate by the Administrator. If the Participant is married or a domestic partner in a domestic partnership qualified under Applicable Law and resides in a community property state, a designation of a person other than the Participant’s spouse or domestic partner, as applicable, as the Participant’s Designated Beneficiary with respect to more than 50% of the Participant’s interest in the Award shall not be effective without the prior written or electronic consent of the Participant’s spouse or domestic partner. Subject to the foregoing, a beneficiary designation may be changed or revoked by a Participant at any time; provided that the change or revocation is delivered in writing to the Administrator prior to the Participant’s death.
10.2 Documentation. Each Award will be evidenced in an Award Agreement in such form as the Administrator determines in its discretion. Each Award may contain such terms and conditions as are determined by the Administrator in its sole discretion, to the extent not inconsistent with those set forth in the Plan.
| 20 |
10.3 Discretion. Except as the Plan otherwise provides, each Award may be made alone or in addition or in relation to any other Award. The terms of each Award to a Participant need not be identical, and the Administrator need not treat Participants or Awards (or portions thereof) uniformly.
10.4 Changes in Participant’s Status. The Administrator will determine how the disability, death, retirement, authorized leave of absence or any other change or purported change in a Participant’s Service Provider status affects an Award and the extent to which, and the period during which, the Participant, the Participant’s legal representative, conservator, guardian or Designated Beneficiary may exercise rights under the Award, if applicable. Except to the extent otherwise required by Applicable Law or expressly authorized by the Company or by the Company’s written policy on leaves of absence, no service credit shall be given for vesting purposes for any period the Participant is on a leave of absence.
10.5 Withholding. Each Participant must pay the Company or a Subsidiary, as applicable, or make provision satisfactory to the Administrator for payment of, any Tax-Related Items required by Applicable Law to be withheld in connection with such Participant’s Awards and/or Shares by the date of the event creating the liability for Tax-Related Items. At the Company’s discretion and subject to any Company insider trading policy (including black-out periods), any withholding obligation for Tax-Related Items may be satisfied by (i) deducting an amount sufficient to satisfy such withholding obligation from any payment of any kind otherwise due to a Participant; (ii) accepting a payment from the Participant in cash, by wire transfer of immediately available funds, or by check made payable to the order of the Company or a Subsidiary, as applicable; (iii) accepting the delivery of Shares, including Shares delivered by attestation; (iv) retaining Shares from the Award creating the withholding obligation for Tax-Related Items, valued on the date of delivery, (v) if there is a public market for Shares at the time the withholding obligation for Tax-Related Items is satisfied, selling Shares issued pursuant to the Award creating the withholding obligation for Tax-Related Items, either voluntarily by the Participant or mandatorily by the Company; (vi) any other lawful consideration permitted by the Administrator; or (vii) any combination of the foregoing payment forms. The amount withheld pursuant to any of the foregoing payment forms shall be determined by the Company and may be up to, but no greater than, the aggregate amount of such obligations based on the maximum statutory withholding rates in the applicable Participant’s jurisdiction for all Tax-Related Items that are applicable to such taxable income. If any tax withholding obligation will be satisfied under clause (v) of the preceding paragraph, each Participant’s acceptance of an Award under the Plan will constitute the Participant’s authorization to the Company and instruction and authorization to any brokerage firm selected by the Company to effect the sale to complete the transactions described in clause (v).
10.6 Amendment of Award; Repricing. The Administrator may amend, modify or terminate any outstanding Award, including by substituting another Award of the same or a different type, changing the exercise or settlement date, and converting an Incentive Stock Option to a Nonqualified Stock Option. The Participant’s consent to such action will be required unless (i) the action, taking into account any related action, does not materially and adversely affect the Participant’s rights under the Award, or (ii) the change is permitted under Article IX or pursuant to Section 11.6. In addition, the Administrator shall, without the approval of the stockholders of the Company, have the authority to (a) amend any outstanding Option or Stock Appreciation Right to reduce its exercise price per Share, or (b) cancel any Option or Stock Appreciation Right in exchange for cash or another Award.
| 21 |
10.7 Conditions on Delivery of Stock. The Company will not be obligated to deliver any Shares under the Plan or remove restrictions from Shares previously delivered under the Plan until (i) all Award conditions have been met or removed to the Company’s satisfaction, (ii) as determined by the Company, all other legal matters regarding the issuance and delivery of such Shares have been satisfied, including, without limitation, any applicable securities laws and stock exchange or stock market rules and regulations, (iii) any approvals from governmental agencies that the Company determines are necessary or advisable have been obtained, and (iv) the Participant has executed and delivered to the Company such representations or agreements as the Administrator deems necessary or appropriate to satisfy Applicable Law. The inability or impracticability of the Company to obtain or maintain authority from any regulatory body having jurisdiction, which authority is deemed by the Company’s counsel to be necessary to the lawful issuance and sale of any Shares hereunder, shall relieve the Company of any liability in respect of the failure to issue or sell such Shares as to which such requisite authority shall not have been obtained, and shall constitute circumstances in which the Administrator may determine to amend or cancel Awards pertaining to such Shares, with or without consideration to the Participant.
10.8 Acceleration. The Administrator may at any time provide that any Award will become immediately vested and fully or partially exercisable, free of some or all restrictions or conditions, or otherwise fully or partially realizable.
ARTICLE
XI.
MISCELLANEOUS
11.1 No Right to Employment or Other Status. No person will have any claim or right to be granted an Award, and the grant of an Award will not be construed as giving a Participant the right to continue employment or any other relationship with the Company or a Subsidiary. The Company and its Subsidiaries expressly reserve the right at any time to dismiss or otherwise terminate its relationship with a Participant free from any liability or claim under the Plan or any Award, except as expressly provided in an Award Agreement or other written agreement between the Participant and the Company or any Subsidiary.
11.2 No Rights as Stockholder; Certificates. Subject to the Award Agreement, no Participant or Designated Beneficiary will have any rights as a stockholder with respect to any Shares to be distributed under an Award until becoming the record holder of such Shares. Notwithstanding any other provision of the Plan, unless the Administrator otherwise determines or Applicable Law requires, the Company will not be required to deliver to any Participant certificates evidencing Shares issued in connection with any Award and instead such Shares may be recorded in the books of the Company (or, as applicable, its transfer agent or stock plan administrator). The Company may place legends on any share certificate or book entry to reference restrictions applicable to the Shares (including, without limitation, restrictions applicable to Restricted Stock).
| 22 |
11.3 Effective Date. The Plan was approved by the Board on [●], 2023. The Plan will become effective on the date immediately prior to the date of the closing (the “Effective Date”) of the transactions contemplated by the Merger Agreement, provided that it is approved by the Company’s stockholders on or prior to the Effective Date and such approval occurs within 12 months following the date the Board approved the Plan. If the Plan is not approved by the Company’s stockholders within the foregoing time frame, or if the Merger Agreement is terminated prior to the consummation of the transactions contemplated thereby, the Plan will not become effective. No Award may be granted pursuant to the Plan on or after the tenth (10th) anniversary of the Effective Date.
11.4 Amendment of Plan. The Board may amend, suspend or terminate the Plan at any time and from time to time; provided that (a) no amendment requiring stockholder approval to comply with Applicable Law shall be effective unless approved by the Board, and (b) no amendment, other than an increase to the Overall Share Limit or pursuant to Article IX or Section 11.6, may materially and adversely affect any Award outstanding at the time of such amendment without the affected Participant’s consent. No Awards may be granted under the Plan during any suspension period or after Plan termination. Awards outstanding at the time of any Plan suspension or termination will continue to be governed by the Plan and the Award Agreement, as in effect before such suspension or termination. The Board will obtain stockholder approval of any Plan amendment to the extent necessary to comply with Applicable Law.
11.5 Provisions for Foreign Participants. The Administrator may modify Awards granted to Participants who are nationals of a country other than the United States or employed or residing outside the United States, establish subplans or procedures under the Plan or take any other necessary or appropriate action to address Applicable Law, including (a) differences in laws, rules, regulations or customs of such jurisdictions with respect to tax, securities, currency, employee benefit or other matters, (b) listing and other requirements of any non-U.S. securities exchange, and (c) any necessary local governmental or regulatory exemptions or approvals.
11.6 Section 409A.
(a) General. The Company intends that all Awards be structured to comply with, or be exempt from, Section 409A, such that no adverse tax consequences, interest, or penalties under Section 409A apply. Notwithstanding anything in the Plan or any Award Agreement to the contrary, the Administrator may, without a Participant’s consent, amend this Plan or Awards, adopt policies and procedures, or take any other actions (including amendments, policies, procedures and retroactive actions) as are necessary or appropriate to preserve the intended tax treatment of Awards, including any such actions intended to (A) exempt this Plan or any Award from Section 409A, or (B) comply with Section 409A, including regulations, guidance, compliance programs and other interpretative authority that may be issued after an Award’s grant date. The Company makes no representations or warranties as to an Award’s tax treatment under Section 409A or otherwise. The Company will have no obligation under this Section 11.6 or otherwise to avoid the taxes, penalties or interest under Section 409A with respect to any Award and will have no liability to any Participant or any other person if any Award, compensation or other benefits under the Plan are determined to constitute noncompliant “nonqualified deferred compensation” subject to taxes, penalties or interest under Section 409A.
| 23 |
(b) Separation from Service. If an Award constitutes “nonqualified deferred compensation” under Section 409A, any payment or settlement of such Award upon a Participant’s Termination of Service will, to the extent necessary to avoid taxes under Section 409A, be made only upon the Participant’s “separation from service” (within the meaning of Section 409A), whether such “separation from service” occurs upon or after the Participant’s Termination of Service. For purposes of this Plan or any Award Agreement relating to any such payments or benefits, references to a “termination,” “termination of employment” or like terms means a “separation from service.”
(c) Payments to Specified Employees. Notwithstanding any contrary provision in the Plan or any Award Agreement, any payment(s) of “nonqualified deferred compensation” required to be made under an Award to a “specified employee” (as defined under Section 409A and as the Administrator determines) due to his or her “separation from service” will, to the extent necessary to avoid taxes under Section 409A(a)(2)(B)(i) of the Code, be delayed for the six-month period immediately following such “separation from service” (or, if earlier, until the specified employee’s death) and will instead be paid (as set forth in the Award Agreement) on the day immediately following such six-month period or as soon as administratively practicable thereafter (without interest). Any payments of “nonqualified deferred compensation” under such Award payable more than six months following the Participant’s “separation from service” will be paid at the time or times the payments are otherwise scheduled to be made.
11.7 Limitations on Liability. Notwithstanding any other provisions of the Plan, no individual acting as a director, officer or other employee of the Company or any Subsidiary will be liable to any Participant, former Participant, spouse, beneficiary, or any other person for any claim, loss, liability, or expense incurred in connection with the Plan or any Award, and such individual will not be personally liable with respect to the Plan because of any contract or other instrument executed in his or her capacity as an Administrator, director, officer or other employee of the Company or any Subsidiary. The Company will indemnify and hold harmless each director, officer or other employee of the Company or any Subsidiary that has been or will be granted or delegated any duty or power relating to the Plan’s administration or interpretation, against any cost or expense (including attorneys’ fees) or liability (including any sum paid in settlement of a claim with the Administrator’s approval) arising from any act or omission concerning this Plan unless arising from such person’s own fraud or bad faith; provided that he or she gives the Company an opportunity, at its own expense, to handle and defend the same before he or she undertakes to handle and defend it on his or her own behalf.
| 24 |
11.8 Data Privacy. As a condition for receiving any Award, each Participant explicitly and unambiguously consents to the collection, use and transfer, in electronic or other form, of personal data as described in this Section 11.8 by and among the Company and its Subsidiaries and affiliates exclusively for implementing, administering and managing the Participant’s participation in the Plan. The Company and its Subsidiaries and affiliates may hold certain personal information about a Participant, including the Participant’s name, address and telephone number; birthdate; social security, insurance number or other identification number; salary; nationality; job title(s); any Shares held in the Company or its Subsidiaries and affiliates; and Award details, to implement, manage and administer the Plan and Awards (the “Data”). The Company and its Subsidiaries and affiliates may transfer the Data amongst themselves as necessary to implement, administer and manage a Participant’s participation in the Plan, and the Company and its Subsidiaries and affiliates may transfer the Data to third parties assisting the Company with Plan implementation, administration and management. These recipients may be located in the Participant’s country, or elsewhere, and the Participant’s country may have different data privacy laws and protections than the recipients’ country. By accepting an Award, each Participant authorizes such recipients to receive, possess, use, retain and transfer the Data, in electronic or other form, to implement, administer and manage the Participant’s participation in the Plan, including any required Data transfer to a broker or other third party with whom the Company or the Participant may elect to deposit any Shares. The Data related to a Participant will be held only as long as necessary to implement, administer, and manage the Participant’s participation in the Plan. A Participant may, at any time, view the Data that the Company holds regarding such Participant, request additional information about the storage and processing of the Data regarding such Participant, recommend any necessary corrections to the Data regarding the Participant or refuse or withdraw the consents in this Section 11.8 in writing, without cost, by contacting the local human resources representative. The Company may cancel Participant’s ability to participate in the Plan and, in the Administrator’s sole discretion, the Participant may forfeit any outstanding Awards if the Participant refuses or withdraws the consents in this Section 11.8. For more information on the consequences of refusing or withdrawing consent, Participants may contact their local human resources representative.
11.9 Severability. If any portion of the Plan or any action taken under it is held illegal or invalid for any reason, the illegality or invalidity will not affect the remaining parts of the Plan, and the Plan will be construed and enforced as if the illegal or invalid provisions had been excluded, and the illegal or invalid action will be null and void.
11.10 Governing Documents. If any contradiction occurs between the Plan and any Award Agreement or other written agreement between a Participant and the Company (or any Subsidiary), the Plan will govern, unless such Award Agreement or other written agreement was approved by the Administrator and expressly provides that a specific provision of the Plan will not apply.
11.11 Governing Law. The Plan and all Awards will be governed by and interpreted in accordance with the laws of the State of Delaware, without regard to the conflict of law rules thereof or of any other jurisdiction.
| 25 |
11.12 Clawback Provisions. All Awards (including the gross amount of any proceeds, gains or other economic benefit the Participant actually or constructively receives upon receipt or exercise of any Award or the receipt or resale of any Shares underlying the Award) will be subject to recoupment by the Company to the extent required to comply with Applicable Law or any policy of the Company providing for the reimbursement of incentive compensation, whether or not such policy was in place at the time of grant of an Award.
11.13 Titles and Headings. The titles and headings in the Plan are for convenience of reference only and, if any conflict, the Plan’s text, rather than such titles or headings, will control.
11.14 Conformity to Applicable Law. Participant acknowledges that the Plan is intended to conform to the extent necessary with Applicable Law. Notwithstanding anything herein to the contrary, the Plan and all Awards will be administered only in a manner intended to conform with Applicable Law. To the extent Applicable Law permits, the Plan and all Award Agreements will be deemed amended as necessary to conform to Applicable Law.
11.15 Relationship to Other Benefits. No payment under the Plan will be taken into account in determining any benefits under any pension, retirement, savings, profit sharing, group insurance, welfare or other benefit plan of the Company or any Subsidiary, except as expressly provided in writing in such other plan or an agreement thereunder.
11.16 Unfunded Status of Awards. The Plan is intended to be an “unfunded” plan for incentive compensation. With respect to any payments not yet made to a Participant pursuant to an Award, nothing contained in the Plan or Award Agreement shall give the Participant any rights that are greater than those of a general creditor of the Company or any Subsidiary.
11.17 Limitations Applicable to Section 16 Persons. Notwithstanding any other provision of the Plan, the Plan and any Award granted or awarded to any individual who is then subject to Section 16 of the Exchange Act shall be subject to any additional limitations set forth in any applicable exemptive rule under Section 16 of the Exchange Act (including Rule 16b-3 of the Exchange Act and any amendments thereto) that are requirements for the application of such exemptive rule. To the extent permitted by Applicable Law, the Plan and Awards granted or awarded hereunder shall be deemed amended to the extent necessary to conform to such applicable exemptive rule.
11.18 Prohibition on Executive Officer Loans. Notwithstanding any other provision of the Plan to the contrary, no Participant who is a Director or an “executive officer” of the Company within the meaning of Section 13(k) of the Exchange Act shall be permitted to make payment with respect to any Awards granted under the Plan, or continue any extension of credit with respect to such payment, with a loan from the Company or a loan arranged by the Company in violation of Section 13(k) of the Exchange Act.
* * * * *
| 26 |
Annex D

















